
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 117
METHYL ISOBUTYL KETONE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr. K. Chipman,
University of Birmingham, United Kingdom
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Methyl isobutyl ketone.
(Environmental health criteria ; 117)
1.Ketones - adverse effects 2.Ketones - toxicity
I.Series
ISBN 92 4 157117 9 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL ISOBUTYL KETONE
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Environmental media
2.4.1.1 Air
2.4.1.2 Water
2.4.1.3 Tissues, body fluids, and skin washings
2.4.1.4 Food
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Photochemical smog reactivity
4.3. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Food
5.1.3. Water
5.1.4. Soil
5.2. Occupational exposure
5.2.1. Exposure limit values
6. KINETICS AND METABOLISM
6.1. Experimental animals
6.1.1. Effect on liver alcohol dehydrogenase in vitro
6.2. Humans
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.3. Terrestrial organisms
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Short-term exposure
8.2.1. Inhalation
8.2.2. Oral
8.2.3. Parenteral
8.2.4. Skin application
8.3. Skin, eye, and respiratory irritation; sensitization
8.3.1. Skin irritation
8.3.2. Eye irritation
8.3.3. Respiratory irritation
8.3.4. Skin sensitization
8.4. Long-term exposure
8.5. Reproduction, embryotoxicity, and teratogenicity
8.6. Mutagenicity and related end-points
8.6.1. Bacterial assays
8.6.2. Yeast assay for mitotic gene conversions
8.6.3. L5178Y TK+/- mouse lymphoma assay
8.6.4. Unscheduled DNA synthesis in primary rat
hepatocytes in vitro
8.6.5. Mouse micronucleus assay
8.6.6. Assay for structural chromosome damage
8.6.7. Cell transformation assay
8.7. Carcinogenicity
8.8. Neurotoxicity
8.9. In vitro toxicity assays
9. EFFECTS ON MAN
9.1. Acute toxicity
9.2. Short-term exposure
9.3. Eye and respiratory irritation
9.4. Long-term exposure
9.5. Placental transfer
9.6. Neurotoxicity
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of effects on the environment
10.2. Evaluation of health risks for humans
11. RECOMMENDATIONS
12. FURTHER RESEARCH
REFERENCES
RESUME
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS
SUR L'ENVIRONNEMENT
RECOMMANDATIONS
RECHERCHES A EFFECTUER
RESUMEN
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS
EFECTOS EN EL MEDIO AMBIENTE
RECOMENDACIONES
OTRAS INVESTIGACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL
ISOBUTYL KETONE (MIBK)
Participants
Professor E.A. Bababunmi, Department of Tropical Paediatrics,
Liverpool School of Tropical Medicine, Liverpool, United
Kingdom (Rapporteur)
Dr M. Cikrt, Centre of Industrial Hygiene and Occupational
Diseases, Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia (Vice-Chairman)
Dr S. Dobson, Pollution and Ecotoxicology Section, Institute of
Terrestrial Ecology, Monks Wood Experimental Station,
Huntingdon, United Kingdom
Professor C.L. Galli, Toxicology Laboratory, Institute of
Pharmacological Sciences, University of Milan, Milan, Italy
(Chairman)
Dr S.D. Gangolli, British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Dr C. Konantakieti, Technical Division, Food and Drug
Administration, Ministry of Public Health, Bangkok, Thailand
Dr O. Ladefoged, Laboratory of Pathology, Institute of Toxicology,
National Food Agency of Denmark, Ministry of Health, Soborg,
Denmark
Professor A. Massoud, Department of Community Environmental and
Occupational Medicine, Ainshams Faculty of Medicine, Cairo,
Egypt
Dr V. Riihimäki, Department of Industrial Hygiene and Toxicology,
Institute of Occupational Health, Helsinki, Finland
Secretariat
Dr P.G. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Ms B. Labarthe, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850)
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL ISOBUTYL KETONE
A WHO Task Group on Environmental Health Criteria for Methyl
Isobutyl Ketone met at Carshalton, United Kingdom, from 12 to 16
March 1990. Dr E.M. Smith, IPCS, opened the meeting on behalf of
the heads of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the health risks of exposure to
methyl isobutyl ketone.
The first draft of this document was prepared by Dr K.
Chipman, University of Birmingham, United Kingdom. The second draft
was also prepared by Dr Chipman following circulation of the first
draft to IPCS contact points for Environmental Health Criteria
monographs. Particularly valuable comments on the draft were made
by the United Kingdom Department of Health, the European Chemical
Industry Ecology and Toxicology Centre (ECETOC), and the US
Environmental Protection Agency, National Institute of
Environmental Health Sciences, and National Institute of
Occupational Safety and Health.
Dr E.M. Smith and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the technical development and
editing, respectively, of this monograph.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the
United Kingdom Department of Health as part of its contributions to
IPCS.
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
ABBREVIATIONS
CLV ceiling value
GC gas chromatography
GRAS generally regarded as safe
HMP 4-hydroxy-4-methyl-2-pentanone
ip intraperitoneal
MIBK methyl isobutyl ketone
NOEL no-observed-effect level
QSAR quantitative structure activity relationship
TLV threshold limit value
1. SUMMARY
Methyl isobutyl ketone (MIBK) is a clear liquid with a sweet
odour and is produced commercially for wide use as a solvent. It
can be measured by gas chromatography with flame ionization
detection. It rapidly evaporates into the atmosphere, where it is
rapidly phototransformed. MIBK is readily biodegradable, and this,
together with its moderate water solubility and low octanol/water
partition coefficient, suggests that it has a low bioaccumulation
potential. Occupational exposure limits range from 100-410 mg/m3
time-weighted average, TWA) and 5-300 mg/m3 (ceiling value, CLV)
in different countries.
MIBK is readily metabolized to water-soluble excretory products
and is of low acute systemic toxicity in animals by the oral and
inhalation routes of exposure. Peripheral axonopathy has not been
reported in animal studies. There are no accurate LC50 data. A
4-h exposure to 16 400 mg/m3 (4000 ppm) was lethal in rats.
Liquid MIBK and vapour concentrations in the range 10-410 mg/m3
(2.4-100 ppm) are irritant to the eyes and the upper respiratory
tract. Concentrations up to 200 mg/m3 (50 ppm) produced no
significant effects on humans in a simple reaction time task or a
test of mental arithmetic. Prolonged or repeated skin contact may
cause drying and flaking of the skin. Accidental aspiration of
liquid MIBK can cause chemical pneumonitis.
In a 90-day gavage study on rats, a NOEL of 50 mg/kg per day
was found. In 90-day inhalation studies on rats and mice,
concentrations of up to 4100 mg/m3 (1000 ppm) did not result in
any life-threatening signs of toxicity. However, compound-related
reversible morphological changes in the liver and kidney were
reported. In a number of studies, MIBK concentrations as low as
1025 mg/m3 (250 ppm) were capable of increasing liver size. With
exposure to 4100 mg/m3 (1000 ppm) for 50 days, microsomal enzyme
metabolism was induced in the livers of chickens. Effects at higher
doses (up to 8180 mg/m3, 1996 ppm) were limited to increased
liver weight with no histological damage. In 90-day studies with
mice, rats, dogs, and monkeys, only male rats developed hyaline
droplets in the proximal tubules of the kidney (hyaline droplet
toxic tubular nephrosis). This effect in male rats was reversible
and of doubtful significance for humans. Enzyme induction may be
the basis of potentiation of haloalkane toxicity by MIBK. MIBK was
also able to potentiate the cholestatic effect of manganese given
with or without bilirubin.
In baboons exposed for 7 days to 205 mg/m3 (50 ppm), effects
on neurobehaviour were reported.
MIBK is fetotoxic at a concentration that produces definite
maternal toxicity (12 300 mg/m3, 3000 ppm) but is not embryotoxic
or teratogenic at this concentration. At a concentration of 4100
mg/m3 (1000 ppm), it was neither embryotoxic, fetotoxic, nor
teratogenic in rats or mice.
MIBK has been studied for genotoxicity in a number of
short-term assays, including in vitro bacterial, yeast, and
mammalian cell tests and a micronucleus assay in mice. These
studies indicate that MIBK is not genotoxic. No reports of
long-term or carcinogenicity studies are available.
At 410 mg/m3 (100 ppm) MIBK can induce in humans symptoms
such as eye irritation, headaches, nausea, dizziness, and fatigue
consistent with a reversible depressant effect on the central
nervous system, but there is no evidence that it produces permanent
damage to the nervous system.
MIBK has low toxicity for aquatic organisms and microorganisms.
The relatively high volatility, rapid atmospheric
hototransformation, ready biodegradability, and low mammalian and
aquatic toxicity of MIBK indicate that adverse environmental
effects of this substance are only likely to occur after accidental
spills or from uncontrolled industrial effluents.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Common name: Methyl isobutyl ketone (MIBK)
Chemical structure:
O H CH3
" ' '
H3C - C - C - C - CH3
' '
H H
Chemical formula: C6H12O
Relative molecular 100.16
mass:
Common synonyms: MIBK, MIK, 4-methyl-2-pentanone,
2-methyl-4-pentanone, hexanone, hexone,
isopropyl-acetone, 4-methyl pentan-2-one,
4-methyl-2-oxopentane, 2-methyl propyl
methyl ketone, isobutylmethyl ketone
CAS registry number: 108-10-1
A typical sample of MIBK has a purity of 99% (by mass); it may
contain the following impurities: dimethyl heptane (< 0.3%), water
(< 0.1%), methyl isobutyl carbinol (< 0.06%), mesityloxide
(< 0.03%), acidity as acetic acid (< 0.002%), and non-volatiles
(< 0.002%).
2.2 Physical and chemical properties
MIBK is a clear liquid with a sweet odour.
Some physical and chemical properties of MIBK are given in
Table 1. The partition coefficients of MIBK are 79 for water/air,
90 for blood/air, and 926 for oil/air (Sato & Nakajima, 1979). MIBK
can react violently with oxidizing agents such as peroxides,
nitrates, and perchlorates, reducing agents, or with potassium
tert -butoxide. When heated, MIBK may form peroxides by
auto-oxidation and these may explode spontaneously (Sax,1979).
Table 1. Some physical and chemical properties of MIBK a
Physical form liquid
Colour colourless
Odour/taste sweet
Odour threshold limit (mg/m3) 1.64 (0.4 ppm) b
1.68 (0.41 ppm) a
Boiling point (°C at 101 KPa) 116.2 (range, 116 to 119) c
Freezing point (°C) -80.26 (range, -80 to -85) c
Specific gravity (20°C/4°C) 0.8017
Refractive index (nD20) 1.395 to 1.397
Viscosity (mPa.s) (20°C) 0.58 to 0.61
Vapour density (air = 1) 3.45
Vapour pressure (KPa) (20°C) 1.99
Concentration in saturated air 27
(g/m3) (20°C) (101 KPa)
Flashpoint (°C) (closed cup) 14
Auto-ignition temperature (°C) 460
Explosion limits in air 1.4 to 7.5
(101 KPa) (% vol.)
Solubility in water 17
(20°C) (g/litre)
Octanol/water partition 1.38 d
coefficient (log Pow)
a From: Verschueren (1983).
b From: Ruth (1986).
c For commercial products.
d From: Leo & Weininger (1984).
2.3 Conversion factors
1 ppm = 4.1 mg/m3
1 mg/m3 = 0.244 ppm
2.4 Analytical methods
2.4.1 Environmental media
Gas chromatography (GC) is suitable for analysing trace
quantities of MIBK (Analytical Quality Control, 1972; Webb et al.,
1973) and the use of fused capillary columns is advantageous. The
use of bonded-phase capillary columns may overcome the need for
solid or liquid phase extraction of samples. Flame ionization
detection (FID) is very sensitive (Webb et al., 1973), while mass
spectroscopy is particularly useful for identifying MIBK in complex
media.
2.4.1.1 Air
Measurement of MIBK in air involves sampling (10-12 litres at
a rate of 0.2 litre/min) on charcoal, silica gel, or some
chromatographic column packings, followed by desorption with carbon
disulfide and further analysis by gas chromatography with flame
ionization detection (Tomczyk & Rogaczewska, 1979; NIOSH, 1984;
Moshlakova & Indina, 1986). The method has been validated over the
range of 208-836 mg/m3(52-209 ppm) and the probable useful
concentration range is 40-1230 mg/m3 (10-300 ppm) (NIOSH, 1984).
No interferences were reported. Using this technique, Bamberger et
al. (1978) reported an average desorption efficiency of
approximately 81% following the exposure of a dosimeter for 5 h to
103 or 410 or 820 mg MIBK/m3 (25 or 100 or 200 ppm). Storage in
a covered dosimeter for 2 weeks reduced the recovery to 69%. Levin
& Carleborg (1987) investigated a range of adsorbents for work-room
air sampling of MIBK. The retention capacity was as follows (for 5
mg generated at 85% relative humidity at 0.2 litres/min in 5 litres
air): XAD-2, 21%; XAD-4, 65%; XAD-7, 39%; activated charcoal, 97%;
Ambersorb XE-348, 98%. Although recovery was reduced to 61%
following storage of samples on charcoal, recoveries were not
reduced under these conditions for Ambersorb XE-348.
MIBK can also be sampled efficiently by the use of
Tenax-GC(R), a polymer of 2,6-diphenyl- p -phenylene oxide. Its
main advantages are its high temperature stability and low affinity
for water vapour. For MIBK, collection efficiency is 99% using 135
g Tenax-GC(R)/17 litres of air. This method is more sensitive
than the charcoal/solvent desorption technique and produces samples
that remain stable for 6 months (Brown & Purnell, 1979).
2.4.1.2 Water
Techniques such as head-space sampling when there is no
interference (Corwin, 1969), liquid-liquid extraction (Keith, 1974;
Austern et al., 1975), distillation, or stripping with an inert gas
stream (Webb et al., 1973; Ellison & Wallbank, 1974) have been
used, because water is not a suitable solvent for gas
chromatographic analysis. The use of synthetic resin gas
chromatographic columns gives low detection limits (µg/ml range)
and high recovery; for example, they have been used in the analysis
of traces of MIBK in drinking-water (Burnham et al., 1972). Ellison
& Wallbank (1974) removed MIBK from waste water and waste sludges
by steam distillation and partitioning into cyclohexanone before
gas chromatographic analysis.
2.4.1.3 Tissues, body fluids, and skin washings
The presence of MIBK and other 2-pentanones in 24-h urine
samples of unexposed human beings has been demonstrated using gas
chromatography and mass spectrometry (Zlatkis & Liebich, 1971).
Bellanca et al. (1982) used both gas chromatography and mass
spectroscopy in the electron ionization mode and, for more
sensitive detection, employed select ion monitoring coupled with
capillary gas chromatography. These methods were used to detect
MIBK liberated into head-space gas from samples of brain, liver,
lung, vitreous fluid, kidney, and blood. MIBK and its metabolites
have been detected by gas chromatography in the serum of
guinea-pigs administered MIBK (DiVincenzo et al., 1976), and
Moshlakova & Indina (1986) have detected MIBK (0.006 to 0.06
mg/cm2) in skin washings of workers using gas chromatography and
flame ionization detection.
2.4.1.4 Food
Residual MIBK in food packaging films can be analysed by gas
chromatography (Raccio & Widomski, 1981; Fernandes, 1985). For milk
analyses, gas chromatography can be combined with mass spectroscopy
(Weller & Wolf, 1989).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
MIBK occurs naturally in food, is a permitted flavouring agent
with GRAS status in the USA, and is used in food contact packaging
materials. It is found in a wide range of foods, e.g., fruits,
baked potatoes, cheese, milk, some meats, and some alcoholic
beverages. The following values have been reported: papaya, 8
µg/kg; beer, 10 to 120 µg/kg; coffee, 6.5 mg/kg (TNO 1983a,b; 1986;
1987).
3.2 Man-made sources
MIBK is produced commercially by acetone condensation, followed
by catalytic hydrogenation in a one-step catalytic process. The
annual production in the USA in 1975 was estimated to be 80 500
tonnes (Lande et al., 1976), and the annual global production in
1975 was estimated to be 250 000 tonnes (OECD, 1977). Consumption
in the European Economic Community (EEC) in 1981 was estimated to
be 45 000 tonnes (ECDIN, 1990).
3.3 Uses
MIBK is used as one of the component ketones in lacquers, such
as cellulose and polyurethane lacquers (Sabroe & Olsen, 1979), and
as a minor component of paint solvents, including car and
industrial spray paints (Hänninen et al., 1976; Elofsson et al.,
1980). It also has uses as an extraction solvent, e.g., in
pharmaceutical products and in the manufacture of methyl amyl
alcohol and as a denaturant for ethyl alcohol (Zakhari et al.,
1977).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
There are no experimental data on the transport, mobility, and
concentration of MIBK in the environment.
MIBK is moderately soluble in water and volatilizes only slowly
from soil and surface waters. A theoretical half-life of 33 days,
in a body of water with a depth of 1 m, can be calculated according
to the Fugacity Model of MacKay & Wolkoff (1973). Based on its
moderate water solubility and low soil adsorption coefficient, MIBK
is a potential contaminant of ground water (see section 5.1.3).
4.2 Biotransformation
4.2.1 Biodegradation
Using the standard dilution method with sludge from a
waste-treatment plant, Bridié et al. (1979b) found a biological
oxygen demand after 5 days at 20°C (BOD5) for MIBK of 76% of the
theoretical oxygen demand (ThOD). MITI (1978) confirmed that MIBK
was readily biodegradable in fresh water and sea water. Price et
al. (1974) studied the biodegradability of MIBK and found that the
nonacclimated extent of bio-oxidation was 56, 66, 69, and 69% at 5,
10, 15, and 20 days, respectively, in fresh water. The respective
values in synthetic salt water were 15, 46, 50, and 53%. The
measured chemical oxygen demand (COD) was 2.4 mg/mg.
Data are not available on the biodegradation of MIBK in soil.
4.2.2 Abiotic degradation
MIBK is degraded in the atmosphere by OH radicals. Cox et al.
(1980) and Atkinson et al. (1982) found kOH reactivity constants
of 12.4 x 10-12 and 14.5 x 10-12 cm3/mol per sec,
respectively, which corresponded to half-lives of 0.57 and 0.55
days. MIBK is also photodegraded. The major phototransformation
product is acetone, which has a kOH of 0.5 x 10-12 cm3/mol
per sec, corresponding to a half-life of 16 days (Cox et al.,
1980).
4.2.3 Photochemical smog reactivity
There is some experimental evidence indicating the
participation of ketones in the photochemical smog cycle as
free-radical chain initiators. However, their contribution to
overall smog generation has not been established, but is thought to
be minor (Lande et al., 1976).
4.3 Bioaccumulation
There are no data on the ability of MIBK to accumulate in
biological material. However, its moderate water solubility and low
octanol/water partition coefficient (log Pow) suggest that it has
low bioaccumulation potential (OECD, 1984). MIBK is not expected to
be persistent. It will probably volatilize fairly readily except in
wet environments and may be oxidized in the atmosphere. Due to its
low log Pow, it is unlikely that it will be appreciably absorbed.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
MIBK release into the atmosphere may occur during its
production through fugitive emissions and incomplete removal of
vapours from reaction gases before they are vented or disposed of
in a scrubber. In the Federal Republic of Germany, MIBK belongs to
class III, air emissions of which must not exceed (as the sum of
all compounds in any class) 150 mg/m3 (37 ppm) at a mass flow of
3 kg/h or more. The maximum recommended ambient concentrations are
0.2 mg/m3 (0.05 ppm) in Czechoslovakia and must not exceed 0.1
mg/m3 (0.025 ppm) in the USSR (IRPTC, 1990). MIBK has been
detected in automotive exhaust emissions (Hampton et al., 1982).
5.1.2 Food
MIBK is allowed as a component of food packaging and is allowed
to come in contact with food materials in the USA and the EEC. The
EEC limit for thesumof all permitted solvents is 60 mg/m2 on the
side with food contact. The intake via food flavourings based on a
1970 survey of usage in the USA was estimated to be 3.35 mg/person
per day (NTIS, 1985). The levels in particular foods were: baked
goods, 10.9 mg/kg; frozen dairy products 11.5 mg/kg; meat products,
2.6 mg/kg; soft candy, 12.3 mg/kg; gelatins, puddings, 10.9 mg/kg;
beverages, 10.2 mg/kg.
MIBK has also been detected in human breast milk (Pellizzari
et al., 1982).
5.1.3 Water
MIBK may be released during the discharge of spent scrubbing
water from industrial production processes. Traces of MIBK have
been found in tap water in the USA (CEC, 1976) and in the United
Kingdom (Fawell & Hunt, 1981). MIBK is included in the Code of
Federal Regulations (CFR, 1987), which lays down methods for the
analysis of organic chemicals in ground water at hazardous waste
sites. MIBK has frequently been detected in leachates from waste
sites (Francis et al., 1980; Sawhney & Kozloski, 1984; Garman et
al., 1987; Brown & Donnelly, 1988).
5.1.4 Soil
MIBK can contaminate soil as a result of accidental spillage
or disposal of solid wastes or sludges (Basu et al., 1968), but
there are no data on levels in soil.
5.2 Occupational exposure
Closed production systems should ensure that occupational
exposures are below recommended occupational exposure limits.
However, emissions that occur when MIBK is used as a solvent, e.g.,
in paints and lacquers, are less easily controlled. Hänninen et al.
(1976) reported a mean time-weighted average (TWA) concentration of
7 mg/m3 (range, 4-160 mg/m3) (1.7 ppm; range, 1-39 ppm) in the
breathing zone of spray painters in car repair shops. Residual MIBK
contained in plastic products can outgas under reduced pressure
conditions and may appear as a contaminant in the environment of
spacecraft (MacEwen et al., 1971). It has been detected at levels
of < 0.005 to 0.02 mg/m3 in the atmosphere of spacecraft
(Rippstein & Coleman, 1984). MIBK has also been found as a volatile
degradation product of polypropylene at temperatures of 220 or 280
°C (Frostling et al., 1984). According to a study by Kristensson &
Beving (1987), exposure measurements for workers painting indoors
for periods of 6-8 h indicated that the concentrations of probe
solvents (including MIBK) were usually well below prescribed
threshold limit values.
5.2.1 Exposure limit values
Exposure limit values for various countries are given in Table
2. The USSR requires special skin and eye protection for workers
exposed to MIBK.
Table 2. Some national occupational air exposure limits used in various
countries a
Country/ Exposure limit description b Value Effective
organization (mg/m3) date
Australia Recommended threshold limit value (TLV)
- Time-weighted average (TWA) 205 1985(r)
- Short-term exposure limit (STEL) 300
Belgium Recommended threshold limit value (TLV)
- Time-weighted average (TWA) 205 1988(r)
- Short-term exposure limit (STEL) 300
Finland Occupational exposure limit (MPC)
- Time-weighted average (TWA) 210 1987
- Short-term exposure limit (STEL) 315
- Ceiling value (CLV) 300
Table 2 (contd).
Country/ Exposure limit description b Value Effective
organization (mg/m3) date
Germany, Recommended threshold limit value (MAK)
Federal - Time-weighted average (TWA) 400 1988(r)
Republic of - Short-term exposure limit (STEL) 2000
Japan Administrative concentration
- Time-weghted average (TWA) 205 1990(n)
Netherlands Recommended threshold limit value (MXL)
- Time-weighted average (TWA) 240 1989(r)
Poland Permissible exposure limit (MPC)
- Time-weighted average (TWA) 200 1982(r)
Romania Permissible exposure limit (MPC)
- Time-weighted average (TWA) 200 1984(r)
Switzerland Permissible exposure limit (MAK)
- Time-weighted average (TWA) 205 1987(r)
Sweden Permissible exposure limit (HLV)
- Time-weighted average (TWA) 100 1990(n)
- Short-term exposure limit (STEL) 200
United Occupational exposure standard (OES)
Kingdom - Time-weighted average (TWA) 205 1990(n)
- Short-term exposure limit (STEL) 300
USA (ACGIH) Recommended threshold limit value (TLV)
- Time-weighted average (TWA) 205 1987(r)
- Short-term exposure limit (STEL) 300
(OSHA) Permissible exposure limit (PEL)
- Time-weighted average (TWA) 205 1990(n)
- Short-term exposure limit (STEL) 300
USSR Temporary exposure limit (TSEL)
- Ceiling value (CLV) 5 1989
Yugoslavia Permissible exposure limit (MAC)
- Time-weighted average (TWA) 410 1971(r)
Table 2 (contd).
a From IRPTC, 1990
b TWA = a maximum mean exposure limit based generally over the period of a working day.
STEL = a maximum concerntration of exposure for a specified time duration (generally 10-30 min.)
(n) = directly notified by countries
Where no effective date appears in the IRPTC legal file, the year of the reference from which the data
are taken is shown, indicated by (r).
6. KINETICS AND METABOLISM
6.1 Experimental animals
Following the intraperitoneal (ip) injection of 450 mg MIBK/kg
body weight to guinea-pigs, two metabolites were found in the serum
(DiVincenzo et al., 1976). The major metabolite,
4-hydroxy-4-methyl-2-pentanone (HMP), was formed by oxidation of
MIBK, while a minor metabolite, 4-methyl-2-pentanol, was formed by
reduction of MIBK (Fig. 1). The serum half-life and total clearance
time for parent MIBK were calculated as 66 min and 6 h,
respectively, whereas 4-hydroxy-4-methyl-2-pentanone (HMP) was
cleared in 16 h. The hydroxylation products of MIBK, such as
4-methyl-2-pentanol, are expected either to be conjugated with
sulfate or glucuronic acid and excreted in the urine or to enter
intermediary metabolism to be converted to carbon dioxide.
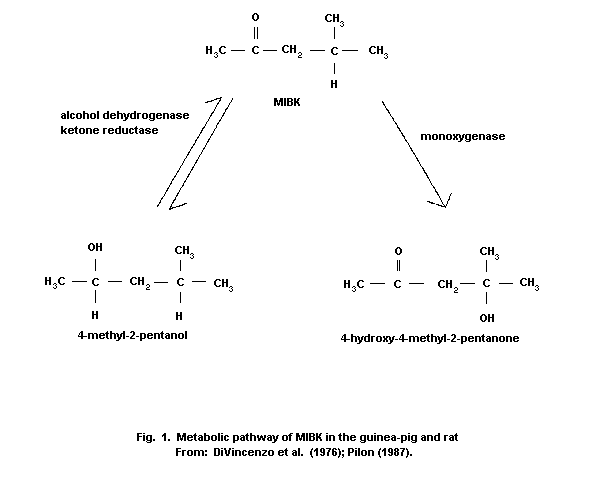 HMP and 4-methyl-2-pentanone have also been identified in rats
(Pilon, 1987). MIBK increased aniline hydroxylase activity and
cytochrome P-450 concentration in chicken liver microsomes after
inhalation (4100 mg/m3, 1000 ppm, for 50 days) (Abou-Donia et
al., 1985b). The induction by MIBK was apparently of similar
capacity to that of methyl n -butyl ketone (Abou-Donia et al.,
1985a). It is likely that MIBK can induce its own oxidative
metabolism as well as that of other substances. The study of
Malyscheva (1988) (section 8.2.4) suggested that MIBK is absorbed
through the skin as well as via the oral and inhalation routes
(section 8.1). A comparison of intraperitoneal and oral LD50
values (Zakhari et al., 1977) suggests an oral absorption of 30% or
more. Malyscheva (1988) showed dermal absorption followed by
extensive distribution (toxic signs in many organs). Likewise,
inhalation studies producing liver and kidney changes are
suggestive of extensive distribution (MacEwen et al., 1971; Vernot
et al., 1971).
The structure of MIBK precludes the metabolic production of
2,5-hexane-dione, the neurotoxic agent formed from both hexane and
methyl n -butyl ketone.
6.1.1 Effect on liver alcohol dehydrogenase in vitro
MIBK in N,N -dimethylacetamide has been shown to reduce the
activity of mouse liver alcohol dehydrogenase in vitro
(Cunningham et al., 1989).
6.2 Humans
MIBK and other substituted 2-pentanones have been reported in
urine samples from unexposed humans (Zlatkis & Liebich, 1971). MIBK
was detected in various tissues and body fluids (section 2.4.1.3)
of two individuals who suffered fatal exposure to a mixture of
organic solvents. The concentration range of MIBK in the tissues
and body fluids of the two decedents was 1.4-2.5 and 0.2-0.8 mg/kg,
respectively. The tissue distribution differed markedly between the
two individuals (Bellanca et al., 1982). The inhalation study
carried out by Dick et al. (1990) with human volunteers suggested
that exposures to 410 mg MIBK/m3 (100 ppm) for 4 h causes steady
state blood levels to be attained. Blood and breath samples
collected 90 min after exposure indicated essentially complete
clearance of the absorbed MIBK. Hjelm et al. (1990) exposed human
volunteers for 2 h during light physical excercise to MIBK (10,
100, and 200 mg/m3; 2.4, 24.4, and 48.8 ppm). The concentration
of MIBK in blood rose rapidly after the onset of exposure, during
which no plateau level was reached. No tendency towards saturation
kinetics was observed over the dose range, the apparent blood
clearance being 1.6 litres/h per kg throughout. Only 0.04% of the
total MIBK dose was eliminated unchanged via the kidneys within 3
h after exposure.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Toxicity data reported in this chapter should be interpreted
with caution, since the tests were conducted under static
conditions using nominal rather than measured concentrations.
Actual concentrations experienced by the test organisms cannot be
determined for these tests.
7.1 Microorganisms
MIBK has low toxicity for microorganisms as indicated by the
threshold concentrations required for inhibition of growth (Table
3).
Table 3. Toxicity of MIBK for microorganisms
Species Threshold Duration of Reference
concentration for study
growth inhibition
(mg/litre)
Protozoa
Saprozoic > 800 48 h Bringmann & Kühn (1981)
flagellate
(Chilomonas
paramaecium)
Bacteriovorous 450 72 h Bringmann & Kühn (1981)
flagellate
(Entosiphon
sulcatum)
Bacteriovorous 950 20 h Bringmann & Kühn (1981)
ciliate ( Uronema
parduczi)
Bacterium
Pseudomonas putida 275 16 h Bringmann & Kühn (1977b)
Table 4. Acute toxicity of MIBK for aquatic organisms
Species LC50 Duration Reference
(mg/litre) of study
Freshwater fish
Golden orfe 672-744 48 h Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 460 24 h Bridié et al. (1979b)
(Carassius auratus)
Fathead minnow approx. 525 96 h Call et al. (1985)
(Pimephales promelas)
Invertebrates
Freshwater
Water flea 4280 24 h Bringmann & Kühn (1977a)
(Daphnia magna) 1550 24 h Bringmann & Kühn (1982)
Marine
Brine shrimp 1230 24 h Price et al. (1974)
(Artemia salina)
Freshwater algae
Green algae 725 a 8 days Bringmann & Kühn (1977b)
(Scenedesmus
quadricauda)
Bluegreen algae 136 a 8 days Bringmann & Kühn (1978)
(Microcystis
aeruginosa)
a Threshold concentration for reduction of total biomass
7.2 Aquatic organisms
MIBK appears to have low toxicity for aquatic organisms (Table
4). The maximum zero lethality concentration (LC0) is in the
range of 480-720 mg/litre (Juhnke & Lüdemann, 1978). Using a QSAR
model, Lipnick et al. (1987) showed that the 24-h LC50 of 460
mg/litre reported by Bridié et al. (1979a) in goldfish (Carassius
auratus) fitted a narcotic mechanism of action.
Aquatic invertebrates are less sensitive than fish to the
toxicity of MIBK, 24-h LC50 values of 4280 mg/litre (Bringmann &
Kuhn, 1977a) and 1550 mg/litre (Bringmann & Kuhn, 1982) having been
reported for the water flea Daphnia magna and 1230 mg/litre for
the brine shrimp Artemia salina (Price et al., 1974). The
LC100 and LC0 values for Daphnia magna were 5000 and 2280
mg/litre, respectively (Bringmann & Kuhn, 1977a).
The toxicity of MIBK was also measured in the green alga
Scenedesmus quadricauda , in which the 8-day threshold for
toxicity was 725 mg/litre (Bringmann & Kuhn, 1977b), and in the
relatively more sensitive cyanobacterium (blue-green alga)
Microcystis aeruginosa , in which the toxicity threshold was 136
mg/litre (Bringmann & Kuhn, 1978).
7.3 Terrestrial organisms
Experimental data are not available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
MIBK is of low acute toxicity by the oral and inhalation routes
of exposure (Table 5).
The maximum time for which rats could be exposed to a saturated
atmosphere of MIBK without dying was 15 min (Smyth et al., 1951).
In one study, six rats survived a 4-h exposure to 8200 mg MIBK/m3
(2000 ppm), but, following a 4-h exposure to 16 400 mg/m3 (4000
ppm), all six animals died within 14 days.
In studies by Specht (1938) and Specht et al. (1940), female
guinea-pigs were exposed to MIBK concentrations of 4100, 69 000,
and 115 000 mg/m3 (1000, 16 800, and 28 000 ppm, respectively)
for up to 24 h. In view of the method used for generating the
atmosphere (allowing measured amounts of MIBK to evaporate freely
to one cubic meter volume of air at 25-26 °C), the two higher
levels must be greatly exaggerated because the saturation
concentration in air for MIBK at 25 °C is 40 000 mg/m3. At 4100
mg/m3 there was minimal eye or nasal irritation. However, there
was a decreased respiratory rate during the first 6 h of exposure,
which was attributed to a narcotic effect. The higher levels
produced obvious signs of eye and nose irritation, followed by
salivation, lacrimation, ataxia, progressive narcosis, and death.
The highest level killed 50% of the animals within 45 min. Autopsy
and histopathological investigations in some animals showed fatty
livers and congestion of the brain, lungs, and spleen, but no
damage to the heart and kidneys was observed.
A single ip injection of 500 mg MIBK/kg body weight in
guinea-pigs did not induce changes in the serum ornithine-carbamyl
transferase level, and there were no histopathological changes in
the liver. However, an injection of 1000 mg/kg body weight killed
one out of four animals, and a slight increase in the serum
ornithine-carbamyl transferase level was seen in the survivors 24
h after dosing. Histopathologically, there was possible lipid
accumulation in liver cells but no evidence of liver damage
(Divincenzo & Krasavage, 1974). It should be noted that an ip
injection of 560 mg/kg produced minimal effects in rats (Vezina et
al., 1985), suggesting that mice are more sensitive than rats (the
ip LD50 is 590 mg/kg in mice, see Table 5).
Table 5. LD50 and LC50 values for MIBK in rats and mice
Route of Species Duration LD50 or LC50 Reference
exposure of exposure
Oral (LD50) rat 4570 mg/kg body weight Smyth et al. (1951)
rat 4600 mg/kg body weight Batyrova (1973)
rat 2080 mg/kg body weight RTECS (1987)
mouse 2850 mg/kg body weight Batyrova (1973)
mouse 1900 mg/kg body weight Zakhari et al. (1977)
Intraperitoneal (LD50) mouse 590 mg/kg body weight Zakhari et al. (1977)
Inhalation (LC50) rat 4 h 8.2-16.4 g/m3 Smyth et al. (1951); Smyth (1956)
mouse 2 h 20.5 g/m3 Batyrova (1973)
mouse 45 mins 74.2 g/m3 Zakhari et al. (1977)
(18 105 ppm)
In male Sprague-Dawley rats, a single oral dose of MIBK
enhanced the hepatotoxicity of a single ip dose of chloroform given
24 h later. The no-observed-effect and minimal-effect levels of
MIBK were 375 and 560 mg/kg body weight, respectively (Vezina et
al., 1985). The ketone potentiation of haloalkane-induced
hepatonecrosis has been attributed to enhanced bioactivation of the
haloalkane, which is mediated by the increased cytochrome P-450
activity induced by the ketone (Branchflower et al., 1983). The
extent of potentiation of carbon tetrachloride liver toxicity (as
shown by an increase in plasma alanine transaminase activity and
bilirubin concentration) was found to depend on the concentration
of both MIBK and carbon tetrachloride in male rats. The minimum
effective MIBK dose decreased 10-fold when the carbon tetrachloride
dose was increased from 0.01 ml/kg to 0.1 ml/kg. These findings
suggest that liver injury is determined by the product of MIBK and
carbon tetrachloride doses (Pilon et al., 1988). Attention should
be paid to this when working in environments containing mixtures of
solvents.
8.2 Short-term exposure
8.2.1 Inhalation
In rats exposed to 410 mg/m3 (100 ppm) for 2 weeks, there was
an increase in kidney weight. An increase in both liver and kidney
weights was observed after exposure to 820 mg/m3 (200 ppm) for 2
weeks or 410 mg/m3 (100 ppm) for 90 days. Histopathological
investigations showed hyaline droplets in the proximal tubules of
the kidney and this finding was named hyaline droplet toxic tubular
nephrosis (MacEwen et al., 1971; Vernot et al., 1971). In rats,
dogs, and monkeys exposed continuously for 90 days to MIBK at 410
mg/m3 (100 ppm) under reduced oxygen tension and reduced
atmospheric pressure (65% oxygen at 34.7 k Pa), liver and kidney
weights were increased in rats after 90 days. Hyaline droplets were
observed in rat kidney epithelium after 15 days, but this effect
was reversible after a 3- to 4-week recovery period. No
histopathological changes were reported in monkeys or dogs and no
sex differences were reported for any parameter (MacEwen et al.,
1971).
Four groups of six male and six female F-344 rats and six male
and six female B6C3F1 mice were exposed to 0, 44, 2050, or 8180
mg MIBK/m3 (0, 10.1, 501, or 1996 ppm, respectively) for 6 h/day
(for 5 days with 2 days off followed by 4 more consecutive days of
exposure) (Dodd et al., 1982; Phillips et al., 1987). Lacrimation
was observed in the highest dose group, but no ophthalmological
lesions or alterations in body weight gain were found. Liver
weight, expressed as a percentage of body weight, was increased in
male and female rats and in female mice exposed to 8180 mg/m3. A
statistically significant increase in liver weight was also
observed in male rats exposed to 2050 mg/m3. Male and female rats
and female mice showed a significant increase in both absolute and
relative kidney weights when exposed to 8180 mg/m3. However, male
mice at this exposure level exhibited a significant decrease in
relative kidney weight. Of the animals exposed to 2050 mg/m3,
only male rats exhibited an increase in kidney weight, but this was
not statistically significantly different from the control value.
Hyaline droplet formation was seen in the kidneys of male rats
exposed to 2050 and 8180 mg/m3. Epithelial regeneration of the
proximal convoluted tubules was also seen at 8180 mg/m3. There
were no histopathological abnormalities in rats and mice exposed to
414 mg/m3, and this concentration was considered a clear
no-observed-adverse-effect level (Dodd et al., 1982). In a
subsequent study, four groups of 14 male and 14 female F-344 rats
and 14 male and 14 female B6C3F1 mice were exposed to 0, 205,
1025, or 4100 mg MIBK/m3 (0, 50, 250, or 1000 ppm, respectively)
for 6 h/day (5 days/week for 90 days). No growth retardation or
clinical effects were observed in either rats or mice. Clinical
observations were made in addition to measurements of body and
organ weights (heart, kidneys, liver, lungs, and testes).
Haematology, ophthalmology, gross pathology, and histology were
also investigated and, in rats, water consumption and urine and
serum chemistry analyses were made. Male rats and mice exposed to
4100 mg/m3 (1000 ppm) showed a slight increase in liver weight
(approximately 11%) and in liver weight per body weight ratio, and
liver weight was also slightly increased in male mice exposed to
1025 mg/m3 (250 ppm). However, neither gross nor microscopic
hepatic lesions were observed, and urinalysis and serum chemistry
values were normal. In male rats exposed to 1025-4100 mg/m3
(250-1000 ppm), there was an increase in the number of hyaline
droplets within the proximal tubular cells of the kidney. No other
gross or microscopic renal changes were observed (Dodd & Eisler,
1983; Phillips et al., 1987). It was considered that the hyaline
droplet effects produced by MIBK may be specific to the male rat
due to the presence of alpha-2-µglobulin (Phillips et al., 1987).
In studies by Brondeau et al. (1989), groups of five hens were
continuously exposed for 50 days by inhalation to either 4100 mg
MIBK/m3 (1000 ppm) or 3520 mg n -hexane/m3 (1000 ppm) or for
30 days to a mixture of 4100 mg MIBK/m3 and 3520 mg n
-hexane/m3. Inhalation of n -hexane alone had no effect on
hepatic microsomal enzymes, but inhalation of MIBK or the MIBK/ n
-hexane mixture increased significantly the aniline hydroxylase
activity and cytochrome P-450 content of the liver (Abou-Donia et
al., 1985b). Inhalation of 2440 to 12 400 mg MIBK/m3 (595 to 3020
ppm) in rats produced enhancement of the liver cytochrome P-450
content and glutathione- S -transferase activity and also enhanced
the ability of 1,2-dichlorobenzene to increase serum glutamate
dehydrogenase activity.
Experiments in open-chest cats demonstrated that significant
pulmonary hypertension and vasoconstriction, with reduced pulmonary
arterial flow, occurred as a result of MIBK inhalation for 5 min at
all concentrations tested (410 to 41 000 mg/m3 (100 to 10 000
ppm)). Systemic arterial pressure and vascular resistance were not
significantly affected (Zakhari et al., 1977). Broncho-constriction
was also produced by MIBK inhalation for 5 min, the effect being
statistically significant at concentrations at or above 2050
mg/m3 (500 ppm) or 4100 mg/m3 (1000 ppm) for pulmonary
resistance or transpulmonary pressure, respectively. Adult mongrel
dogs with open-chest surgery showed pulmonary hypertension at an
inhalation concentration of 20.5 mg MIBK/m3 (5 ppm) for 5 min. At
41 mg/m3 (10 ppm), myocardial contractility occurred (Zakhari et
al., 1977).
8.2.2 Oral
Three daily doses of 375 or 1500 mg MIBK/kg body weight given
by gavage to rats reduced the bile flow produced by an intravenous
injection of taurocholate (20 mg per kg body weight) (Plaa &
Ayotte, 1985). The effect of MIBK on the cholestatic activity of
manganese, with or without bilirubin, has also been investigated in
male Sprague-Dawley rats. MIBK was administered by gavage in corn
oil at doses ranging from 188 to 1502 mg/kg body weight once a day
for 1, 3, or 7 days (Vezina et al., 1985; Vezina and Plaa, 1987).
MIBK was not cholestatic but, at doses of 375 mg/kg or more, was
found to potentiate the cholestasis induced by a
manganese-bilirubin combination when this was given 18 h after the
1-day treatment with MIBK. When given for 3 or 7 days, MIBK
produced a dose-related enhancement of the cholestasis induced by
a manganese-bilirubin combination. A 3-day treatment with MIBK (750
mg/kg) was also shown to potentiate the cholestasis induced by
manganese alone. Two known metabolites of MIBK (HMP and
4-methyl-2-pentanol) were also able to potentiate the cholestatic
effect of the manganese-bilirubin combination or of manganese alone
in male Sprague-Dawley rats. When the metabolite was given by
gavage 18 h prior to the administration of the manganese-bilirubin
combination, cholestasis was potentiated by 375 mg/kg or 1502 mg/kg
(expressed as equivalents of MIBK) of 4-methyl-2-pentanol or HMP,
respectively. Lower doses did not decrease bile flow rate.
4-Methyl-2-pentanol was also more effective than HMP as a
potentiator following daily treatment for 3 days prior to
manganese-bilirubin administration. However, with manganese alone,
HMP was more effective. It was suggested that the potentiation of
cholestasis may be associated with metabolic induction or might
reflect a separate mechanism of action involving a membrane
interaction (Vezina & Plaa, 1988).
The toxic effects of MIBK in Sprague-Dawley rats (groups of 30
animals of each sex) were examined following 13 weeks of oral
gavage administration at levels of 0, 59, 250, or 1000 mg/kg daily.
Body weight, food consumption, organ weight, morbidity, clinical
chemistry, haematology, and histopathology evaluations were
performed. All surviving animals were killed after 90 days (13
weeks) and 10 animals of each sex per group were examined.
Nephrotoxicity was seen as a general nephropathy for both male and
female rats administered 1000 mg/kg per day. Although increased
liver and kidney weights were observed for males and females at
1000 mg/kg per day, there were no corresponding histopathological
lesions present in the liver. The effects seen at 1000 mg/kg per
day were present to a significantly lesser extent in the females
and males fed 250 mg/kg per day. No effects were observed at 50
mg/kg per day, identifying a no-observed-effect level
(Microbiological Associates, 1986).
8.2.3 Parenteral
In studies by Krasavage et al. (1982), rats (strain and number
not specified) were given intraperitoneal injections of MIBK or a
mixture of methyl ethyl ketone (MEK) and MIBK (9:1 by volume), 5
times/week, for 35 weeks. The dose levels for the first 2 weeks
were 10, 30, and 100 mg per kg body weight, and these were then
doubled for the remainder of the treatment period. Body weight gain
suppression was seen after 3-4 weeks of treatment. The only other
effect noted was transient narcosis during the first 4 weeks at the
highest dose. Pulmonary vascular effects were observed following an
intraperitoneal administration of MIBK to cats (threshold dose 8
mg/kg), but bronchoconstriction was not seen following an
intraperitoneal administration of 4-32 mg/kg (Zakhari et al.,
1977).
8.2.4 Skin application
In rats exposed dermally to 300-600 mg MIBK/kg per day for 4
months, dose- and time-dependent morphological changes were
observed in the skin, brain, liver, adrenals, spleen, and testis.
Body temperature decreased and oxygen consumption increased
(Malyscheva, 1988).
8.3 Skin, eye, and respiratory irritation; sensitization
8.3.1 Skin irritation
A single 10-h occluded application of MIBK to the shaved skin
of rabbits produced erythema, which occurred immediately after the
application and persisted for up to 24 h. Daily applications of 10
ml on 10 cm2 skin for 7 days caused drying and flaking of the
surface (Krasavage et al., 1982).
8.3.2 Eye irritation
Undiluted MIBK (0.1 ml) produced some irritation within 10 min
when instilled in the rabbit eye. Inflammation and conjunctival
swelling occurred within 8 h; the inflammation, swelling, and
exudate present at 24 h had disappeared by 60 h (Krasavage et al.,
1982).
8.3.3 Respiratory irritation
De Ceaurriz et al. (1981) measured the reflex decrease in
respiratory rate in male Swiss OF1 mice as an index of sensory
irritation. MIBK caused a concentration-dependent decrease in
respiratory rate during a 5-min exposure, and a 50% decrease in
respiratory rate (RD50) was seen at 13 100 mg/m3 (3195 ppm).
Specht et al. (1940) attributed the decreased respiratory rate to
a narcotic effect. It should be recognized that the reduction may
not be due to sensory irritation.
8.3.4 Skin sensitization
There are no reports of skin sensitization studies.
8.4 Long-term exposure
No long-term toxicity studies have been reported.
8.5 Reproduction, embryotoxicity, and teratogenicity
In studies by Tyl (1984) and Tyl et al. (1987), groups of 35
pregnant Fischer-344 rats and 30 pregnant CD-1 mice were exposed to
1230, 4100, or 12 300 mg MIBK/m3 (300, 1000, or 3000 ppm) on days
6-15 (inclusive) of gestation. The animals were sacrificed on days
21 (rats) or 18 (mice) and fetuses examined for external, visceral,
and skeletal alterations. In rats, exposure to 12 300 mg/m3
resulted in maternal toxicity with decreased body weight gain,
increased relative kidney weight, decreased food consumption, and
fetotoxicity (reduced fetal body weight per litter and delays in
skeletal ossification). Clinical signs in dams included loss of
coordination, negative toe pinch, paresis, muscular weakness,
piloerection, lacrimation, and perioral encrustation. No increase
in fetal malformation was observed in any group. At 1230 and 4100
mg/m3, there was no maternal, embryo, or fetal toxicity, or
malformations. However, reduced fetal body weight and delay in some
ossification parameters were observed at the lowest dose but not at
the intermediate dose level. These effects were attributed to the
larger litter size (average 10.8) in this group compared to that in
controls (9.5).
In mice, exposure to 12 300 mg/m3 produced maternal toxicity
with increased mortality (3/25), increased absolute and relative
liver weights, and fetotoxicity (increased incidence of dead
fetuses, reduced fetal body weight per litter, and delayed or
reduced ossification). Clinical signs in dams included irregular
gait, paresis, hypoactivity, ataxia, negative toe pinch, and
lacrimation. There was no treatment-related increase in
embryotoxicity or fetal malformations at any exposure concentration
tested. No significant treatment-related maternal, embryo, or fetal
toxicity (including malformations) was observed at 1230 or 4100
mg/m3.
Table 6. Mutagenicity and related end-points
System Dose Response Reference
Bacterial Assays
Salmonella typhimurium a 0.04-4 µg/plate negative Chemical
strains TA98, TA100, Manufacturers
TA1537, TA1538 Association
(1984);
O'Donoghue et
al. (1988)
Salmonella typhimurium a
strains TA1535, TA1537 Up to 8000 µg/ml negative Brooks et al.
TA1538, TA98, TA100 (1988)
Eschericia coli
strains WP2 and Up to 8000 µg/ml negative Brooks et al.
WP2 uvr A (1988)
Yeast Assays
Saccharomyces cerevisiae JDI Up to 5 mg/ml negative Brooks et al.
mitotic gene conversion (1988)
assay ± rat liver S9
Mammalian cell assays in vitro :
L51784 TK +/- mouse 0.001-100 µl/ml negative Chemical
lymphoma mutation assay (preliminary assay) Manufacturers
(± rat liver S9) 0.4-6 µl/ml negative Association
(1984);
O'Donoghue et
al. (1988)
Primary rat hepatocytes; 0.01-100 µl/ml negative Chemical
unscheduled DNA synthesis Manufacturers
(DNA repair) Association
(1984);
O'Donoghue et
al. (1988)
Cultured rat liver cells Up to 1000 µl/ml negative Brooks et al.
chromosomal damage assay (half the dose for (1988)
RL4 cells 50% inhibition of
cell growth)
Table 6 (contd).
System Dose Response Reference
Balb/3T3; cell transformation 2-5 µl/ml (-S9) inconclusive Chemical
assay ± rat liver S9 1-7 µl/ml (+S9) Manufacturers
Association
(1984);
O'Donoghue et
al. (1988)
Mammalian in vivo assay
Mouse (male and female) 0.73 ml/kg ip negative Chemical
micronucleus assay (maximum tolerated Manufacturers
(polychromatic dose level) Association
erythrocytes) (1984);
O'Donoghue et
al. (1988).
a Preincubation mutation assay incorporating Arochlor-induced rat liver S9
8.6 Mutagenicity and related end-points
A summary of the reported data on MIBK mutagenicity is given
in Table 6.
8.6.1 Bacterial assays
A pre-incubation assay with Salmonella typhimurium (strains
TA98, TA100, TA1537, TAl538) was conducted at dose levels of 0.04
to 4 µg/plate both in the presence and absence of a metabolic
activation system prepared from Aroclor-induced rat liver
homogenate (S9 fraction). Precautions were taken to prevent the
escape of MIBK vapour and assure prolonged exposure of the bacteria
to the test substance. MIBK did not cause an increase in reverse gene
mutation (Chemical Manufacturers Association 1984; O'Donoghue et
al., 1988).
Both MIBK and the oxidative metabolite 4-hydroxy-4-methyl-2-
pentanone (HMP) (see section 6.1) were tested for mutagenicity in
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538, and in Eschericia coli strains WP2 and WP2 uvr A
(Brooks et al., 1988). Aroclor-induced rat liver S9 fraction was
included. No induction of reverse gene mutation was observed up to
a maximum concentration of 8000 µg MIBK/ml (pre-incubation assay in
a sealed container) or 4000 µg HMP/plate (plate incorporation
assay).
8.6.2 Yeast assay for mitotic gene conversions
MIBK and the metabolite HMP were assayed for mitotic gene
conversion using log-phase cultures of the yeast Saccharomyces
cerevisiae JD1 (Brooks et al., 1988). Compounds were tested up to
a concentration of 5 mg/ml in the presence and absence of rat liver
S9 fraction in a sealed container for 18 h. Neither compound
induced mitotic gene conversion.
8.6.3 L5178Y TK+/- mouse lymphoma assay
A preliminary assay was carried out in the presence and absence
of a metabolic activation system at doses of 0.001-100 µl/ml. The
non-activated cultures showed 3 to 157% total relative growth,
while the cultures containing the rat liver S9 fraction had a
relative growth of 23-95% compared with untreated control cultures.
No increase in mutation frequencies was observed in cultures
containing the metabolic activation system, but in the
non-activated cultures, a 2-fold increase above controls was seen
at two non-consecutive doses. An increase in mutation frequency of
approximately 5 times the concurrent control occurred at one test
concentration, but this concentration also caused 97% cell death.
In the absence of a dose-related effect, this result was considered
equivocal. A repeat assay was performed using duplicate cultures
and a narrower range of doses (0.4-6 µl/ml). The total relative
growth ranged from 1 to 80% in non-metabolically activated cells
and from 28 to 63% in cultures that contained the S9 fraction. None
of the activated cultures revealed increased mutation frequencies.
A borderline positive result was found at 6 µl/ml, but different
mutation frequencies occurred in the duplicate cultures and 96-99%
of the cells were killed (Chemical Manufacturers Association, 1984;
O'Donoghue et al., 1988).
8.6.4 Unscheduled DNA synthesis in primary rat hepatocytes in vitro
When MIBK was tested at five dose levels ranging from 0.01
µl/ml to 100 µl/ml in a single assay, there was an increase of less
than 5 fold in labelled nuclear grains in cells treated with MIBK
compared with cells of the solvent control plates (Chemical
Manufacturers Association, 1984; O'Donoghue et al., 1988). Since
the value did not exceed that of the negative control by two
standard deviations of the control value, it was considered that,
under the conditions tested, MIBK did not cause a significant
increase in the nuclear grain count.
8.6.5 Mouse micronucleus assay
Male and female mice were administered MIBK by ip injection at
the maximum tolerated dose level of 0.73 ml/kg body weight, and
bone marrow polychromatic erythrocytes were estimated 12, 24, and
48 h later. There were no significant differences between the
treated and control animals in the ratio of polychromatic to
normochromatic erythrocytes. The number of micronucleated
polychromatic erythrocytes per 1000 cells was not significantly
increased in the MIBK-treated animals (Chemical Manufacturers
Association, 1984; O'Donoghue et al., 1988).
8.6.6 Assay for structural chromosome damage
MIBK (purity > 98.5%) and the metabolite HMP were tested (24-h
exposure) in cultured rat liver cells (RL4) for the ability to
induce chromosomal damage. Metabolic activation with S9 mix was not
used because RL4 cells are metabolically competent. The
concentrations of MIBK employed were 0.125, 0.25, and 0.5 times the
concentration required for 50% inhibition of cell growth. Maximum
concentrations tested were thus 1000 µg MIBK/ml and 4000µg HMP/ml
(this HMP level was equivalent to the concentration required for
>60% growth inhibition). Incubations with MIBK were sealed to
prevent loss by evaporation. MIBK did not produce chromosomal
damage, but HMP gave a small increase (which was not dose related)
in chromatid damage within the concentration range 2000-4000 µg/ml.
It should be noted that HMP did not induce reverse gene mutation in
bacteria or mitotic gene conversion in yeast (Brooks et al., 1988).
8.6.7 Cell transformation assay
In studies reported by the Chemical Manufacturers Association
(1984) and O'Donoghue et al. (1988), MIBK was tested in the
Balb/3T3 (clone A31-1) morphological transformation assay. Doses of
2.4, 3.6, and 4.8 µl MIBK/ml were added to the culture medium in
the absence of a metabolic activation system, and 1, 2, and 4 µl
MIBK/ml were added in the presence of such a system (Aroclorinduced
rat liver S9 fraction). MIBK produced a positive response in the
non-activated cultures only (4.8 µl MIBK per ml gave 3 type III
foci in 15 dishes). A confirmatory study was conducted with doses
of 2, 3, 4, and 5 µl/ml and 4, 5, 6, and 7 µl/ml, respectively, in
the presence and absence of S9 fraction. No significant increase in
the number of transformed foci was found in this study, either in
the presence or absence of the metabolic activation system. Thus,
the effect of MIBK on cell transformation was not reproducible in
the two assays and the ambiguity of the results makes them
unreliable.
8.7 Carcinogenicity
No carcinogenicity studies have been reported.
8.8 Neurotoxicity
In a study by Krasavage et al. (1982), rats were given ip
injections of MIBK, or a mixture of methyl ethyl ketone and MIBK
(9:1 by volume), 5 times/week, for 35 weeks. The dose levels of 10,
30, and 100 mg/kg body weight were doubled after 2 weeks of
treatment. Transient anaesthesia was noted during the first 4 weeks
in the highest dose group, but there was no evidence of peripheral
neuropathy. In dogs administered 300 mg MIBK/kg body weight per day
subcutaneously (sc) for 11 months, electromyographic examination
showed no evidence of neurotoxicity.
Cats treated subcutaneously with 150 mg MIBK/kg body weight per
day or a mixture of methyl ethyl ketone/MIBK (9:1) twice daily, 5
times/week, for up to 8.5 months showed no evidence of nervous
system damage (Spencer & Schaumburg, 1976). In beagle dogs
receiving similar treatment, there were no neurotoxic changes
(Krasavage et al., 1982).
Groups of male rats were exposed to 5330 mg/m3 (1300 ppm)
methyl n -butyl ketone for 4 months or 6150 mg/m3 (1500 ppm)
MIBK for 5 months (Spencer et al., 1975). Methyl n -butyl ketone
produced a toxic distal axonopathy. MIBK produced minimal distal
axonal changes, but 3% methyl n -butyl ketone was present as a
contaminant in the MIBK, and the design of the cages used may have
caused compression neuropathy (Spencer et al., 1975; Spencer &
Schaumburg, 1976). Animals exposed to MIBK showed slight signs of
narcosis, but body weight gain was normal, and, at 5 months, there
were no clinical signs of neurological dysfunction. Rats exposed
for 3 h to 102 mg MIBK/m3 (25 ppm) showed a 58% increase in
pressor lever response, which had not returned to control levels 7
days after the end of exposure (Geller et al., 1978). The maximum
motor-fibre conduction velocity in the tail nerve decreased
markedly when male rats were treated with methyl n -butyl ketone
(401 mg/kg, 5 times/week for 55 weeks) but not when they were
treated with MIBK (601 mg/kg, 5 times/week for 55 weeks). However,
treatment with MIBK (201 mg/kg) facilitated the neurotoxic effect
of methyl n -butyl ketone (401 mg/kg) possibly due to the
demonstrated ability of MIBK to increase the metabolic activity of
10 000 g liver supernatants towards both MIBK and methyl n -butyl
ketone (Nagano et al., 1988).
Discriminatory behaviour and memory in baboons was not affected
by exposures of 82-164 mg/m3 (20-40 ppm) (Geller et al., 1978).
Geller et al. (1979) reported an effect on accuracy of performance
of tasks in a ``delayed match-to-sample discrimination-test'' in
baboons exposed for 7 days to 205 mg/m3 (50 ppm) MIBK, but there
was no change in response when MIBK was combined with methyl ethyl
ketone at 295 mg/m3 (100 ppm).
De Ceaurriz et al. (1984) exposed male Swiss OF1 mice to an
atmosphere containing MIBK and measured the total duration of
immobility during a 3-min ``behavioural despair'' swimming test. At
concentrations of 2714, 3104, 3309, and 3657 mg/m3 (662, 757,
807, and 892 ppm), a dose-dependent decrease of mobility was
observed (25, 38, 46, and 70% respectively). The authors noted that
the mean active level of MIBK was lower for this neurobehavioural
effect (3292 mg/m3 (893 ppm) for 50% inhibition of immobility)
than for an effect on sensory irritation (13 100 mg/m3 (3195 ppm)
for 50% inhibition of respiratory rate) (De Ceaurriz et al., 1981,
section 8.3.3).
In inhalation studies on hens (five per group) into the effect
of MIBK on n -hexane-induced neurotoxicity, a continuous exposure
period of 90 days was followed by a 30-day observation period
(Abou-Donia et al., 1985b). One group of hens was exposed to 3520
mgnw-hexane/m3 (1000 ppm) and another group to 4100 mg MIBK/m3
(1000 ppm). Four additional groups were exposed simultaneously to
3520 mg n -hexane/m3 (1000 ppm) and 410, 1025, 2050, or 4100 mg
MIBK/m3 (100, 250, 500, or 1000 ppm, respectively). A control
group was exposed to ambient air in an exposure chamber. Hens
continuously exposed to 4100 mg MIBK/m3 developed weakness of the
legs with subsequent recovery. Inhalation of 3520 mg n
-hexane/m3 produced mild ataxia. Exposure to 3520 mg n
-hexane/m3 together with 1025, 2050, or 4100 mg MIBK/m3
resulted in signs of neurotoxicity including paralysis, the
severity of which depended on the MIBK concentration. Hens
continuously exposed to the 3520/410 mg/m3 n -hexane/MIBK
mixture exhibited severe ataxia throughout the observation period.
Histopathological examination of hens exposed to the n
-hexane/MIBK mixture showed large swollen axons and degeneration
of the axon and myelin of the spinal cord and peripheral nerves.
There were no histopathological abnormalities in the central
nervous system of hens exposed only to MIBK. This demonstrates that
MIBK potentiates the neurotoxic action of n -hexane. In a
subsequent study, in which hens were exposed for 50 days to MIBK at
4100 mg/m3 (1000 ppm) or to n -hexane, it was suggested that
the potentiation by MIBK of n -hexane neurotoxicity is related to
the induction by MIBK of liver microsomal cytochrome P-450,
resulting in increased metabolism of n -hexane to its neurotoxic
metabolites (sections 6.1, 8.2.1). The findings also suggest that
the neurotoxicity of technical methyl butyl-ketone (methyl n
-butyl ketone/MIBK (7/3)) was correctly attributed to the methyl
n -butyl ketone component (Abdo et al., 1982). Simultaneous
treatment of hens with 41, 205, or 410 mg/m3 (10, 50, or 100 ppm)
technical methyl butyl ketone, 5 days/week for 90 days, with a
dermal application of technical grade 0 -ethyl- 0 -4-nitrophenyl
phenylphosphonothionate (EPN, 1.0 mg/kg, 85%) greatly enhanced the
neurotoxic effects. It was proposed that MIBK partially contributed
to this potentiation by inducing cytochrome P-450 and thus
enhancing the formation of neurotoxic products from methyl- n
-butyl ketone and EPN (Abou-Donia et al., 1985a).
The effect of MIBK on the duration of ethanol-induced loss of
righting reflex and on ethanol elimination has been studied in
mice. MIBK was dissolved in corn oil and injected ip 30 min before
ethanol (4 g/kg ip). At a dose of 501 mg/kg, MIBK significantly
prolonged the duration of ethanol-induced loss of righting reflex.
The concentrations of ethanol in blood and brain on return of the
righting reflex were similar in MIBK-treated and control animals
(Cunningham et al., 1989).
8.9 In vitro toxicity assays
In contrast to methyl n -butyl ketone and n -hexane, MIBK
caused little or no cytopathological or growth-inhibiting effects
in cultured mouse neuroblastoma cells (Selkoe et al., 1978). MIBK
in N,N -dimethylacetamide reduced the activity of mouse liver
alcohol dehydrogenase in vitro (Cunningham et al., 1989). MIBK
was found to inhibit the sulfhydryl-dependent creatine kinase and
adenylate kinase enzymes in vitro but not to the same extent as
did the neurotoxic agent, methyl- n -butyl ketone (Lapin et al.,
1982).
9. EFFECTS ON MAN
9.1 Acute toxicity
In a study on the sensory threshold, Silverman et al. (1946)
exposed 12 volunteers of both sexes to various concentrations of
MIBK for a 15-min period. This period permitted an accurate
observation of olfactory fatigue and increasing or decreasing
irritation of mucous membranes. The sensory response limit was 410
mg/m3 (100 ppm). The majority of the subjects found the odour
objectionable at 820 mg/m3 (200 ppm), and the vapour irritated
the eyes. The low odour threshold (1.64 mg/m3) (Ruth, 1986) and
the irritant effects can provide warning of high concentrations.
Because of its low viscosity, MIBK may, when swallowed, also be
aspirated into the lungs causing a chemical pneumonitis (Panson &
Winek, 1980).
9.2 Short-term exposure
Workers exposed to 410 mg MIBK/m3 (100 ppm) complained either
of headache and nausea or of respiratory irritation (Elkins, 1959).
Tolerance was said to be acquired during the working week but was
lost over the weekend. Reduction of the exposure to 82 mg/m3 (20
ppm) largely eliminated the complaints. In the study of Hjelm et
al. (1990) (see section 6.3) on human volunteers, CNS symptoms
(headache and/or vertigo and/or nausea) were reported at 2-h
exposure levels of 10-200 mg MIBK/m3 (2.4-48.8 ppm). There were
no significant effects from exposure on the performance of a
reaction time task or a test of mental arithmetic.
9.3 Eye and respiratory irritation
Exposure to a concentration of 820 mg MIBK/m3 (200 ppm) for
a 15-min period caused eye irritation in 12 human volunteers
(Silverman et al., 1946). Undiluted MIBK splashed in the eyes may
cause painful irritation (Shell, 1957). A group of workers exposed
to 410 mg MIBK/m3 (100 ppm) complained of respiratory tract
irritation, but there were no complaints at 82 mg/m3 (20 ppm)
(Elkins, 1959). In the study of Hjelm et al. (1990) (see section
6.3) on human volunteers, irritation particularly of the nose and
throat was reported at 2-h exposure levels of 10, 100, and 200
mg/m3 (2.4, 24.4, and 48.8 ppm).
9.4 Long-term exposure
In workers exposed to up to 2050 mg MIBK/m3 (500 ppm) for
20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, over half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomiting, and sore throat. A few of the
workers experienced insomnia, somnolence, heartburn, intestinal
pain, and some unsteadiness. Four workers had slightly enlarged
livers and six had a nonspecific colitis. Clinical chemistry
examination revealed no abnormalities in any of the workers. Five
years later, work practices had greatly improved, the highest MIBK
concentration was 410-430 mg/m3 (100-105 ppm), and the general
concentration was 205 mg/m3 (50 ppm). A few workers still
complained of gastrointestinal and central nervous system effects,
and slight liver enlargement had persisted in two workers, but
other symptoms had disappeared (Armeli et al., 1968).
9.5 Placental transfer
MIBK was detected in maternal and umbilical cord blood samples
from 11 patients (Dowty et al., 1976).
9.6 Neurotoxicity
A few isolated cases of peripheral neuropathy have been
reported after exposure to spray paint or lacquer thinner that
apparently contained MIBK and other hydrocarbon solvents, including
neurotoxic agents (Oh & Kim, 1976; Aubuchon et al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of effects on the environment
MIBK is not likely to persist in the environment. It will
slowly volatilize from soil and water and is readily biodegraded in
fresh and salt water. In the atmosphere, MIBK is estimated to be
degraded by OHÊ radicals with a half-life of approximately 14 h.
MIBK is not expected to bioaccumulate and has a low toxicity for
microorganisms, fish, algae, and aquatic invertebrates. Only in
cases of accidental spillage or inappropriate disposal of wastes
into the environment are levels of MIBK likely to cause toxicity to
organisms in the environment.
10.2 Evaluation of health risks for humans
The general population is exposed to low levels of MIBK. Only
small quantities have been detected in food, drinking-water, and
other beverages (baked goods, 10.9 mg/kg; frozen dairy products,
11.5 mg/kg; gelatins, puddings, 10.9 mg/kg; beverages, 10.2 mg/kg).
For general population exposure, maximum ambient air concentrations
in the range of 0.1 to 0.2 mg/m3 have been defined by two
countries.
Occupational exposure occurs particularly in the production and
use of lacquers, paints, and extraction solvents. The major route
of entry is by inhalation. The low odour threshold (1.64 mg/m3)
and the irritant effects can provide warning of high
concentrations. Exposure to levels of 10-410 mg/m3 (2.4-100 ppm)
produced perceptible irritation of either the eyes, nose, or
throat, and 820 mg/m3 (200 ppm) produced discomfort. Symptoms
such as headache, nausea, and vertigo also occurred at a level of
10-410 mg/m3 (2.4-100 ppm). There were no significant effects
from a 2-h exposure of up to 200 mg/m3 (50 ppm) on a simple
reaction time task or test of mental arithmetic.
In the single report concerning long-term occupational
exposure, where workers were exposed to 2050 mg MIBK/m3 (500 ppm)
for 20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, more than half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomitting, and sore throat. A few workers
experienced insomnia, somnolence, and some unsteadiness. Four had
slightly enlarged livers and six had nonspecific colitis. Five
years later, work practices had greatly improved and the highest
concentrations were reduced to about one fifth of the previous
level. A few workers still complained of irritation of the eyes and
upper respiratory tract as well as gastrointestinal and central
nervous system symptoms. Prolonged skin contact with MIBK caused
irritation and flaking of the skin.
In animal studies, acute systemic MIBK toxicity is low by the
oral and inhalation routes. In a 90-day study, Sprague-Dawley rats
were given MIBK by gavage at doses of 50, 250, or 1000 mg/kg body
weight per day. Lethargy was noted in the highest-dose group and
males showed reduced body weight gain. In this group there was
generalized nephropathy, with an increase in relative kidney weight
and hepatomegaly. Relative kidney weight was also increased in the
animals fed 250 mg/kg per day, and slight hepatomegaly was reported
in male rats only. There were no histopathological lesions in the
liver or other tissues at any dose level. It was concluded that the
NOEL was 50 mg/kg per day. In 90-day inhalation studies on rats and
mice, concentrations of up to 4100 mg/m3 (1000 ppm) did not
result in any life-threatening signs of toxicity. However,
compound-related reversible morphological changes in the liver and
kidney were reported. Levels of 4100 mg/m3 produced evidence of
central nervous system depression. MIBK was capable of increasing
liver weight (at > 1025 mg/m3 (250 ppm)) and inducing hepatic
microsomal metabolism. This may be the explanation for the
exacerbation of haloalkane toxicity and the potentiation of the
neurotoxicity of n -hexane. In 90-day studies with mice, rats,
dogs, and monkeys, only male rats developed hyaline droplets in the
proximal tubules of the kidney (hyaline droplet toxic tubular
nephrosis). This effect in male rats was reversible and of doubtful
significance for humans. MIBK reduces the activity of mouse liver
alcohol dehydrogenase in vitro . It has also been found to
potentiate the cholestatic effects of manganese given with or
without bilirubin.
In rats and mice exposed to MIBK by inhalation at
concentrations of 1230, 4100, or 12 300 mg/m3 (300, 1000, or 3000
ppm) on days 6 to 15 of gestation and sacrificed on day 21 (rats)
or day 18 (mice), marked maternal toxicity was observed at the
highest concentration in both species. This concentration produced
fetotoxicity (reduced fetal body weight and delayed ossification)
but was not embryotoxic or teratogenic. At 4100 and 1230 mg/m3
there was no maternal toxicity and no evidence of embryotoxicity,
fetotoxicity, or teratogenicity.
MIBK did not induce gene mutation in bacterial test systems
(Salmonella typhimurium and Escherichia coli ) either with or
without metabolic activation. Negative results were also obtained
in tests (both with and without metabolic activation) for mitotic
gene conversion in yeast ( Saccharomyces cerevisiae ) and in gene
mutation tests using cultured mammalian cells (mouse lymphoma). In
vitro assays for unscheduled DNA synthesis in primary rat
hepatocytes and for structural chromosome damage in cultured rat
liver cells (RL4) were negative. An in vivo micronucleus test in
mice was negative. These data indicate that MIBK is not genotoxic.
11. RECOMMENDATIONS
At the levels of MIBK to which the general human population is
exposed, there is unlikely to be any hazard. In the occupational
health context, where the major route of exposure is by inhalation,
atmospheric levels should be kept below the recommended
occupational exposure limits by suitably designed work processes
and engineering controls, including ventilation. Skin and eye
contamination should be avoided. Suitable protective clothing and
respiratory protection should be readily available for use in
enclosed spaces, in emergencies, and for certain maintenance
operations. MIBK is inflammable and should be handled accordingly.
MIBK has low toxicity for microorganisms and fish, and its
half-life in the environment is short. Consequently, there is no
risk to the environment provided there are adequate controls to
minimize emissions. Large-scale release could have local adverse
effects on the environment.
12. FURTHER RESEARCH
1. MIBK affects a number of enzyme systems. Therefore, it can
significantly influence the biotransformation of xenobiotics that
are metabolized by these enzymes. Since humans are usually exposed
to more than one compound, studies on the combined effects of
mixtures containing MIBK should be undertaken.
2. There is very little information available on the dose-response
relationships for the effects of MIBK on the human central nervous
system (e.g., reaction time, behavioural effects), on the upper
airways and mucous membranes, or on kidney function. More
information on toxico-kinetics is needed for MIBK alone and in
mixture with other solvents. The skin penetration of MIBK should be
assessed.
3. Epidemiological studies should be undertaken to elucidate the
effects on the nervous system of long-term exposure to moderate
concentrations of MIBK alone or in mixture with other solvents.
REFERENCES
ABDO, K.M., GRAHAM, D.G., TIMMINS, P.R., & ABOU-DONIA, M.B. (1982)
Neurotoxicity of continuous (90 days) inhalation of technical grade
methyl butyl ketone in hens. J. Toxicol. environ. Health, 9:
199-215.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & ABDO, K.M.
(1985a) The joint neurotoxic action of inhaled methyl butyl ketone
vapour and dermally applied 0-ethyl 0-4-nitrophenyl
phenylphosphonothioate in hens: potentiating effect. Toxicol. appl.
Pharmacol., 79: 69-82.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & TIMMONS, P.R.
(1985b) The synergism of n -hexane-induced neurotoxicity by
methyl isobutyl ketone following subchronic (90 days) inhalation in
hens: induction of hepatic microsomal cytochrome P-450. Toxicol.
appl. Pharmacol., 81: 1-16.
ANALYTICAL QUALITY CONTROL (1972) Handbook for analytical quality
control in water and waste water laboratories, Cincinnati, Ohio,
National Environmental Research Centre.
ARMELI, G., LINARI, F., & MARTORANO, G. (1968) [Clinical and
haematochemical examinations in workers exposed to the action of a
higher ketone (MIBK) repeated after 5 years.] Lav. Um., 20:
418-424 (in Italian).
ATKINSON, R., ASCHMANN, S.M., CARTER, W.P.L., & PITTS, J.N., Jr
(1982) Rate constants for the gas-phase reaction of OH radicals
with a series of ketones at 299 ± 2 ° K. Int. J. chem. Kinet.,
14: 839-847.
AUBUCHON, J., ROBINS, H.I., & VISESKUL, C. (1979) Peripheral
neuropathy after exposure to methyl isobutyl ketone in spray paint.
Lancet, August 18: 363-364.
AUSTERN, B.M., DOBBS, R.A., & COHEN, J.M. (1975)
Gas-chromatographic determination of selected organic compounds
added to wastewater. Environ. Sci. Technol., 9: 588-590.
BAMBERGER, R.L., ESPOSITO, G.G., JACOBS, B.W., PODOLAK, G.E., &
MAZUR, J.F. (1978) A new personal sampler for organic vapors. Am.
Ind. Hyg. Assoc. J., 39: 701-708.
BASU, P., CARPENTER, J., CHEN, C., NELSON, H., PERRY, W., SHOCLET,
A., TAYLOR, D., & ZAFRAN, F. (1968) Human exposure assessment:
Methyl isobutyl ketone, Washington, DC, US Environmental Protection
Agency (EPA contract 68-01.4839).
BATYROVA, T.F. (1973) Substantiation of the maximum permissible
concentration of methylisobutyl ketone in air or workrooms. Gig.
Tr. prof. Zabol., 17(11): 52-53.
BELLANCA, J.A., DAVIS, P.L., DONNELLY, B., DAL CORTIVO, L.A., &
WEINBERG, S.B. (1982) Detection and quantitation of multiple
volatile compounds in tissues by GC and GC/MS. J. anal. Toxicol.,
6: 238-240.
BRANCHFLOWER, R.V., SCHULICK, R.D., GEORGE, J.W., & POHL, L.R.
(1983) Comparison of the effects of methyl n -butyl ketone and
phenobarbital on rat liver cytochromes P-450 and the metabolism of
chloroform to phosgene. Toxicol. appl. Pharmacol., 71: 414-421.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER M. (1979a) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUHN, R. (1977a) [Findings concerning the harmful
effect of water-endangering substances on, Daphnia magna .] Z.
Wasser Abwasser Forsch., 10: 161-166 (in German).
BRINGMANN, G. & KUHN, R. (1977b) [Limit values for the harmful
effect of water-endangering substances on bacteria ( Pseudomonas
putida ) and green algae ( Scenedesmus quadricauda ).] Z. Wasser
Abwasser Forsch., 10: 87-98 (in German).
BRINGMANN, G. & KUHN, R. (1978) [Limit values for the harmful
effect of water-endangering substances on blue-green algae
( Microcystis aeruginosa ) and green algae ( Scenedesmus
quadricauda ) in the cell multiplication inhibitor test.] Vom
Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effects of
pollutants on flagellates and ciliates, and/or on holozoic
bacteria-eating and saprozoic protozoa.] GWF-Wasser Abwasser,
122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna straus tested by an approved
standardized procedure.] Z. Wasser Abwasser Forsch., 15: 1-6 (in
German).
BRONDEAU, M.T., BAN, M., BONNET, P., GUENIER, J.P., & DE CEAURRIZ,
J. (1989) Acetone compared to other ketones in modifying the
hepatotoxicity of inhaled 1,2-dichlorobenzene in rats and mice.
Toxicol. Lett. 49: 69-78.
BROOKS, T.M., MEYER, A.L., & HUTSON, D.H. (1988) The genetic
toxicology of some hydrocarbon and oxygenated solvents.
Mutagenesis, 3: 227-232.
BROWN, K.W. & DONNELLY, K.C. (1988) An estimation of the risk
associated with the organic constituents of hazardous and municipal
waste landfill leachates. Hazardous Waste Hazardous Mater., 5(1):
1-30.
BROWN, R.H. & PURNELL, C.J. (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. J. Chromatogr.,
178: 79-90.
BURNHAM, A.K., CALDER, G.V., FRITZ, J.S., JUNK, G.A., SVEC, H.J.,
& WILLIS, R. (1972) Identification and estimation of neutral
organic contaminants in potable water. Anal. Chem., 44: 139-142.
CALL, D.J., BROOKE, L.T., KNUTH, M.L., POIRIER, S.H., & HOGLUND,
M.D. (1985) Fish subchronic toxicity prediction model for
industrial organic chemicals that produce narcosis. Environ.
Toxicol. Chem., 4: 335-341.
CEC (1976) Analysis of organic micropollutants in water,
Luxembourg, Commission of the European Communities (Cost 64b bis).
CFR (1987) Code of Federal Regulations. Methods of analysis for
organic chemicals in groundwater at hazardous waste sites,
Washington, DC, US Government Printing Office (Appendix IX, 40 CFR,
Part 264).
CHEMICAL MANUFACTURERS ASSOCIATION (1984) Ketones Program Panel,
Vol. 1 - Methyl isobutyl ketone: Mutagenicity and teratology
studies, Washington, DC, Chemical Manufacturers Association.
CORWIN, J.F. (1969) Volatile oxygen-containing organic compounds in
sea water: determination. Bull. mar. Sci., 19: 504-509.
COX, R.A., DERWENT, R.G., & WILLIAMS, M.R. (1980) Atmospheric
photooxidation reactions. Rates, reactivity and mechanism for
reaction of organic compounds with hydroxyl radicals. Environ. Sci.
Technol., 14: 57-61.
CUNNINGHAM, J., SHARKAWI, M., & PLAA, G.L. (1989) Pharmacological
and metabolic interactions between ethanol and methyl n -butyl
ketone, methyl isobutyl ketone, methyl ethyl ketone, or acetone in
mice. Fundam. appl. Toxicol., 13: 102-9.
DE CEAURRIZ, J., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J., MICILLINO, J.C., MARIGNAC, B., BONNET, P., MULLER,
J., & GUENIER, J.P. (1984) Quantitative evolution of sensory
irritating and neurobehavioural properties of aliphatic ketones in
mice. Food Chem. Toxicol., 22, 545-549.
DICK, R., DANKOVIC, D., SETZER, J., PHIPPS, F., & LOWRY, L. (1990)
Body burden profiles of methyl ethyl ketone and methyl isobutyl
ketone exposure in human subjects. Toxicologist, 10: 122.
DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic
solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
DIVINCENZO, G.D., KAPLAN, C.J., & DEDINAS, J. (1976)
Characterization of the metabolites of methyl n -butyl ketone,
methyl iso-butyl ketone and methyl ethyl ketone in guinea pig serum
and their clearance. Toxicol. appl. Pharmacol., 36: 511-522.
DODD, D.E. & EISLER, D.L. (1983) Methyl isobutyl ketone ninety-day
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 46-504
submitted to US EPA).
DODD, D.E., LONGO, L.C., & EISLER, D.L. (1982) Nine-day vapour
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 45-501
submitted to US EPA).
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic
constituents. Pediatr. Res., 10: 696-701.
ECDIN (1990) Data bank on environmental chemicals, Ispra (Varese),
Establishment, Joint Research Centre of the Commission of the
European Communities.
ELKINS, H.B. (1959) Chemistry of industrial toxicology, New York,
John Wiley and Sons, p. 121.
ELLISON, W.K. & WALLBANK, T.E. (1974) Solvents in sewage and
industrial waste waters. Identification and determination. Water
Pollut. Control, 73: 656-672.
ELOFSSON, S.A., GAMBERALE, F., HINDMARSH, T., IREGREN, A.,
ISAKSSON, A., JOHNSSON, I., KNAVE, B., LYDAHL, E., MINDUS, P.,
PERSSON, H.E., PHILIPSON, B., STEBY, M., STRUWE, G., SODERMAN, E.,
WENNBERG, A., & WIDEN, L. (1980) Exposure to organic solvents: a
cross-sectional epidemiologic investigation on occupationally
exposed car and industrial spray painters with special reference to
the nervous system. Scand. J. Work Environ. Health, 6: 239-273.
FAWELL, J.K. & HUNT, S. (1981) Organic micropollutants in drinking
water, Medmenham, Water Research Centre (Technical Report No. 159).
FERNANDES, M. (1985) Methodology for the analysis of volatile
compounds in food packaging materials. Coletanea Inst. Technol.
Aliment., 15: 49-59.
FIRE PREVENTION (1981) Information sheets on hazardous materials,
London, London Fire Protection Association, p. 47 (H97 No. 140).
FRANCIS, A.J., IDEN, G.T., NINE, B.J., & CHANG, C.K. (1980)
Characterization of organics in leachates from low level
radioactive waste disposal sites. Nucl. Technol., 50: 158-163.
FROSTLING, H., HOFF, A., JACOBSSON, S., PFAFFLI, P., VAINIOTALO,
S., ZITTING, A., & TECHN, D. (1984) Analytical, occupational and
toxicologic aspects of the degradation products of polypropylene
plastics. Scand. J. Work Environ. Health., 10: 163-169.
GARMAN, J.R., FREUND, T., & LAWLESS, E.W. (1987) Testing for
groundwater contamination at hazardous waste sites. Chromatogr.
Sci., 25: 328-344.
GELLER, I., ROWLANDS, J.R., & KAPLAN, H.L. (1978) Effects of
ketones on operant behaviour of laboratory animals. In: Voluntary
inhalation of industrial solvents, Washington, DC, US Department of
Health, Education and Welfare, p. 363 (DHEW Publication No.
79-779).
GELLER I., GAUSE, E., KAPLAN, H., & HARTMANN, R.J. (1979) Effects
of acetone, methyl ethyl ketone, and methyl isobutyl ketone on a
match-to-sample task in the baboon. Pharmacol. Biochem. Behav.,
11: 401-406.
HAMPTON, C.V., PIERSON, W.R., HARVEY, T.M., UPDEGROVE, W.S., &
MARANO, R.S. (1982) Hydrocarbon gases emitted from vehicles on the
road. 1. A qualitative gas chromatography/mass spectroscopy survey.
Environ. Sci. Technol., 16: 287-298.
HANNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioural effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HJELM, E.W., HAGBERG, M., IREGREN, A., & LÖF, A. (1990) Exposure to
methyl isobutyl ketone: toxicokinetics and occurrence of irritative
and CNS symptoms in man. Int. Arch. occup. environ. Health, 62:
19-26.
IRPTC (1990) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JUHNKE, I. & LÜDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute fish toxicity in the orfe test.] Z.
Wasser Abwasser Forsch., 11(5): 161-164 (in German).
KEITH, L.H. (1974) Chemical characterization of industrial waste
waters by gas chromatography-mass spectrometry. Sci. total
Environ., 3: 87-102.
KRASAVAGE, W.J., O'DONOGHUE, J.L., & DIVINCENZO, G.D. (1982) Methyl
isobutyl ketone. In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, New York, John Wiley and Sons,
Vol. 2e, pp. 4747-4751.
KRISTENSSON, J. & BEVING, H. (1987) A study of painters
occupationally exposed to water and solvent based paints,
Luxembourg, Commission of the European Communities, pp. 71-72 (EUR.
10555).
LANDE, S.S., DURKIN, P.R., CHRISTOPHER, D.H., HOWARD, P.H., &
SAXENA, J. (1976) Investigation of selected potential environmental
contamination: ketonic solvents, Syracuse, New York, Center for
Chemical Hazard Assessment Research Corporation, p. 252.
LAPIN, E.P., WEISSBARTH, S., MAKER, H.S., & LEHRER, G.M. (1982) The
sensitivities of creatine and adenylate kinases to the neurotoxins
acrylamide and metyl n-butyl ketone. Environ. Res., 28: 21-31.
LEO, A. & WEININGER, D. (1984) Medicinal chemistry report,
Claremont, California, Pomona College.
LEVIN, J.-O. & CARLEBORG, L. (1987) Evaluation of solid sorbents
for sampling ketones in work-room air. Ann. occup. Hyg., 31:
31-38.
LIPNICK, R.L., WATSON, K.R., & STRAUSZ, A.K. (1987) A QSAR study of
the acute toxicity of some industrial organic chemicals to
goldfish. Narcosis, electrophile and proelectrophile mechanisms.
Xenobiotica, 17, 1011-1025.
MACEWEN, J.D., VERNOT, E.H., & HAUN, C.C. (1971) Effects of 90-day
continous exposure to methyl isobutyl ketone on dogs, monkeys, and
rats, Ohio, Wright-Patterson AFB, Aerospace Medical Research
Laboratory (Report No. AMRL TR-71-65).
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 7: 611- 614.
MALYSCHEVA, M.V. (1988) [The effect of the skin route of
administration of methyl isobutyl ketone on its toxicity.] Gig. i
Sanit., 10: 79-80 (in Russian).
MICROBIOLOGICAL ASSOCIATES (1986) Subchronic toxicity of methyl
isobutyl ketone in Sprague-Dawley rats. Preliminary report,
Research Triangle Park, North Carolina, Research Triangle Park
Institute, (Study No. 5221.04).
MITI (1978) The biodegradability and bioaccumulation of new and
existing chemical substances, Tokyo, Ministry of International
Trade and Industry, Chemical Products Safety Division, Basic
Industries Bureau.
MOSHLAKOVA, L.A., & INDINA, T.V. (1986) [Gas chromatographic
determination of ketones present simultaneously in the air of the
work area and in the washings from workers' skin. Gig. i Sanit.,
2: 90-91 (in Russian).
NAGANO, M., HARADA, K., MISUMI, J., & NOMURA, S. (1988) [Effect of
methyl isobutyl ketone on methyl n-butyl ketone neurotoxicity in
rats.] Sangyo Igaku, 30: 50-51 (in Japanese).
NIOSH (1984) NIOSH manual of analytical methods: ketones I, 3rd
ed., Cincinnati, Ohio, US National Institute for Occupational
Safety and Health,Vol. 2 (No. 1300).
NTIS (1985) Scientific literature review of aliphatic ketones,
secondary alcohols and related esters in flavour usage. Volume 1 -
Introduction and summary, tables of data bibliography: Al,
Washington, DC, National Technical Information Service, Part 2
(PB85-141059).
O'DONOGHUE, J.L., HAWORTH, S.R., CURREN, R.D., KIRBY, P.E., LAWLOR,
T., MORAN, E.J., PHILLIPS, R.D., PUTNAM, D.L., ROGERS-BACK, A.M.,
SLESINSKI, R.S., & THILAGAR, A. (1988) Mutagenicity studies on
ketone solvents: methyl ethyl ketone, methyl isobutyl ketone and
isophorone. Mutat. Res., 206: 149-161.
OECD (1977) The asessment of environmental chemicals: production
figures and use patterns for some high volume chemicals, Paris,
Organization for Economic Cooperation and Development
(ENV/Chem./77.6).
OECD (1984) Data interpretation guides for initial hazard
assessment of chemicals (provisional), Paris, Organisation for
Economic Cooperation and Development, p. 31.
OH, S.J. & KIM, J.M. (1976) Giant axonal swelling in "Huffer's"
neuropathy. Arch. Neurol., 33: 583-586.
PANSON, R.D. & WINEK, C.L. (1980) Aspiration toxicity of ketones.
Clin. Toxicol., 17: 271-317.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERICKSON, M.D. (1982) Purgeable organic compounds
in mothers' milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PHILLIPS, R.D., MORAN, E.J., DODD, D.E., FOWLER, E.H., KARY, C.D.,
&O'DONOGHUE, J. (1987) A 14-week vapor inhalation toxicity study of
methyl isobutyl ketone. Fundam. appl. Toxicol., 9: 380-388.
PILON, D. (1987) Interaction cétones/hydrocarbures halogènes:
Utilization des métabolites cétoniques comme indices d'exposition
aux cétones, Montreal, University of Montreal (Ph.D. Thesis).
PILON, D., BRODEUR, J., & PLAA, G.L. (1988) Potentiation of carbon
tetrachloride-induced liver injury by ketonic and ketogenic
compounds: role of the CCl4 dose. Toxicol. appl. Pharmacol.,
94: 183-190.
PLAA, G.L. & AYOTTE, D. (1985) Taurolithocholate-induced
intrahepatic cholestasis: potentiation by methyl isobutyl ketone
and methyl n-butylketone in rats. Toxicol. appl. Pharmacol., 80:
228-234.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimps
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77
RACCIO, J.M. & WIDOMSKI, J.R. (1981) Quality control of flavors in
soft drinks and the analysis of residual solvents in food packaging
films utilizing headspace sampling with open tubular columns.
Chromatogr. Newsl., 9(2): 42-45.
RIPPSTEIN, W.J. & COLEMAN, M.E. (1984) [Toxicological evaluation on
the Columbian spacecraft.] Kosmet. Biol. Aviakosm. Med., 18:
87-96 (in Russian).
RTECS (1987) Registry of toxic effects of chemical substances,
1985-86 ed., Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Vol. 1-6 (DHSS (NIOSH) Publication No. 87-114).
RUTH, J.H. (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am. Ind. Hyg. Assoc. J., 47:
A142-A151.
SABROE, S. & OLSEN, J. (1979) Health complaints and work conditions
among lacquerers in the Danish furniture industry. Scand. J. soc.
Med., 7: 97-104
SATO, A. & NAKAJIMA, T. (1979) Partition coefficients of some
aromatic hydrocarbons and ketones in water, blood and oil. Brit. J.
ind. Med., 36, 231-234.
SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13: 349-352.
SAX, N.I. (1979) Dangerous properties of industrial materials, 6th
ed, New York, Van Norstrand Reinhold Company, p. 750.
SELKOE, D.J., LUCKENBILL-EDDS, L., & SHELANSKI, M.L. (1978) Effects
of neurotoxic industrial solvents on cultured neuroblastoma cells:
methyl n -butyl ketone, n -hexane, and derivatives. J.
Neuropathol. exp. Neurol., 37: 768-789.
SHELL (1957) Methyl isobutyl ketone: industrial hygiene bulletin,
New York, Shell Chemical Corporation, pp. 57-112.
SILVERMAN, L., SCHULTE, H.F., & FIRST, M.W. (1946) Further studies
on sensory response to certain industrial solvent vapors. J. ind.
Hyg. Toxicol., 28: 262-266.
SMYTH, H.F. (1956) Hygienic standards for daily inhalation. Am.
Ind. Hyg. Assoc. J., 17: 129-266.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SPECHT, H. (1938) Acute response of guinea pigs to inhalation of
methyl isobutyl ketone, Washington, DC, US Public Health Service,
pp. 292-300 (US Public Health Report No. 53).
SPECHT, H., MILLER, J.W., VALAER, P.J., & SAYERS, R.R. (1940) Acute
response of guinea pigs to the inhalation of ketone vapours,
Washington, DC, US Public Health Service, Division of Industrial
Hygiene (NIH Bulletin No. 176).
SPENCER, P.S. & SCHAUMBURG, H.H. (1976) Feline nervous system
response to chronic intoxication with commercial grades of methyl
n -butyl ketone, methyl isobutyl ketone and methyl ethyl ketone.
Toxicol. appl. Pharmacol., 37: 301-311.
SPENCER, P.S., SCHAUMBURG, H.H., RALEIGH, R.L., & TERHAAR, C.J.
(1975) Nervous system degeneration produced by the industrial
solvent methyl n -butyl ketone. Arch. Neurol., 32: 219-222.
TNO (1983a) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 2.
TNO (1983b) Volatile compounds in food: Qualitative data, Zeist,
Netherlands, Organization for Applied Scientific Research.
TNO (1986) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 5.
TNO (1987) Volatile compounds in food: Supplement 4, Zeist,
Netherlands, Organization for Applied Scientific Research.
TOMCZYK, H. & ROGACZEWSKA, T. (1979) [Gas chromatographic
determination of airborne methyl isobutyl ketone, methyl isobutyl
carbinol, acetone, toluene and o-xylene.] Med. Pr., XXX(6):
417-423 (in Polish).
TYL, R.W. (1984) A teratologic evaluation of methyl isobutyl ketone
in Fischer 344 rats and CD-1 mice following inhalation exposure,
Washington, DC, Chemical Manufacturers Association (Bushy Run
Research Center Report No. 47.505).
TYL, R.W., FRANCE, K.A., FISHER, L.C., PRITTS, I.M., TYLER, T.R.,
PHILLIPS, R.D., & MORAN, E.J. (1987) Developmental toxicity
evaluation of inhaled methyl isobutyl ketone in Fischer 344 rats
and CD-l mice. Fundam. appl. Toxicol., 8: 319-327.
VERNOT, E.H., MACEWEN, J.D., & HARRIS, E.S. (1971) Continuous
exposure of animals to methyl isobutyl ketone, Ohio, Wright
Patterson AFB, Aerospace Medical Research Laboratory (US NTIS AD
Report No. 751443).
VERSCHUEREN, K. (1983) Handbook of environmental data on organic
chemicals, 2nd ed., New York, Van Nostrand Reinhold Company, pp.
459-461.
VEZINA, M. & PLAA, G.L. (1987) Potentiation by methyl isobutyl
ketone of the cholestasis induced in rats by a manganese-bilirubin
combination or manganese alone. Toxicol. appl. Pharmacol. 91:
477-483.
VEZINA, M. & PLAA, G.L. (1988) Methyl isobutyl ketone metabolites
and potentiation of the cholestasis induced in rats by a
manganese-bilirubin combination or manganese alone. Toxicol. appl.
Pharmacol. 92: 419-427.
VEZINA, M., AYOTTE, P., & PLAA, G.L. (1985) Potentiation of
necrogenic and cholestatic liver injury by 4-methyl-2-pentanone.
Can. Fed. Biol. Soc., 28: 221.
WEBB, R.G., GARRISON, A.W., KEITH, L.H., & MCGUIRE, J.H. (1973)
Current practice in GC-MS analysis of organics in water,
Washington, DC, US Environmental Protection Agency (EPA Report No.
R2-73-277) (NTIS PB 224 947/2).
WELLER, J.P. & WOLF, M. (1989) Mass spectroscopy and headspace gas
chromatography. Beitr. gerichtl. Med., 47: 525-532.
ZAKHARI, S., LEVY, P., LIEBOWITZ, M., & AVIADO, D.M. (1977) Acute
oral, intraperitoneal, and inhalation toxicity of methyl isobutyl
ketone in the mouse. In: Goldberg, L., ed. Isopropanol and ketones
in the environment, Cleveland, Ohio, CRC Press, Part 3, Chapter
10-14, pp. 93-133.
ZLATKIS, A. & LIEBICH, H.M. (1971) Profile of volatile metabolites
in human urine. Clin. Chem., 17: 592-594.
RESUME
La méthylisobutylcétone est un liquide limpide d'odeur
douceâtre produite en vue d'une vaste utilisation commerciale comme
solvant. On peut la doser par chromatographie en phase gazeuse avec
détection par ionisation de flamme. Elle s'évapore rapidement dans
l'atmosphère où elle subit une photoconversion à brève échéance. La
méthylisobutylcétone est facilement biodégradable et, compte tenu
de sa solubilité moyenne dans l'eau et de son faible coefficient de
partage entre l'octanol et l'eau, elle devrait présenter un faible
potentiel de bioaccumulation. Les limites d'exposition
professionnelle sont de 100-400 mg/m3 (moyenne pondérée par
rapport au temps: TWA) et de 5-300 mg/m3 (valeur plafond: CLV)
selon les pays.
La méthylisobutylcétone est rapidement métabolisée en produits
d'excrétion solubles dans l'eau et sa toxicité aiguë générale est
faible chez l'animal après exposition par voie orale ou
respiratoire. L'expérimentation animale n'a pas révélé
d'axonopathie périphérique. On ne dispose pas de données précises
sur la CL50. Une exposition de 4 heures à une concentration de 16
400 mg/m3 (4000 ppm) a été mortelle pour des rats. La
méthylisobutylcétone liquide ou sous forme de vapeurs à la
concentration de 10 à 410 mg/m3 (2,4 à 100 ppm) est irritante
pour les yeux et les voies respiratoires supérieures. Des
concentrations allant jusqu'à 200 mg/m3 (50 ppm) n'ont produit
aucun effet sensible sur l'homme lors d'épreuves portant sur le
temps de réaction et le calcul mental. Un contact prolongé ou
répété avec la peau peut produire un dessèchement et des crevasses.
L'aspiration accidentelle de méthylisobutylcétone liquide peut
provoquer une pneumonie chimique.
Lors d'une étude de 90 jours effectuée en gavant des rats, on
a obtenu une dose sans effet observable de 50 mg/kg par jour. Des
études d'inhalation de 90 jours portant sur des rats et des souris
à des concentrations allant jusqu'à 4100 mg/m3 (1000 ppm) n'ont
pas révélé de signes de toxicité engageant le pronostic vital.
Toute fois des altérations morphologiques réversibles liées à
l'administration de ce composé ont été observées au niveau du foie
et des reins. Dans un certain nombre d'études, on a observé une
hypertrophie du foie dès la dose de 1025 mg/m3 (250 ppm). Exposés
à 4100 mg/m3 (1000 ppm) pendant 50 jours, des poulets ont
présenté une induction des enzymes microsomiques. A doses plus
élevées (jusqu'à 8180 mg/m3, 1996 ppm) les effets se limitaient
à un accroissement du poids du foie sans lésion histologique. Lors
d'études de 90 jours effectuées sur des souris, des rats, des
chiens et des singes, seuls les rats mâles ont présenté des
altérations histologiques: présence de gouttelettes hyalines dans
les tubules proximaux des reins (néphrose tubulaire toxique à
inclusions hyalines). Cet effet s'est révélé réversible et sa
portée en toxicologie humaine demeure incertaine. Il est possible
que la potentialisation de la toxicité des alcanes halogénés par la
méthylisobutylcétone repose sur une induction enzymatique. La
méthylisobutylcétone potentialise également l'effet cholestatique
du manganèse administré avec ou sans bilirubine.
Des babouins exposés pendant sept jours à une dose de 205
mg/m3 (50 ppm) ont présenté des troubles neuro-comportementaux.
La méthylisobutylcétone est foetotoxique à une concentration
manifestement toxique pour la mère (12 300 mg par m3, 3000 ppm)
mais elle n'est pas embryotoxique ni tératogène à cette
concentration. A la concentration de 4100 mg/m3 (1000 ppm), on
n'a pas observé d'embryotoxicité, de foetotoxicité ni de
tératogénicité chez les rats et les souris.
On a recherché la génotoxicité éventuelle de la
méthylisobutylcétone en pratiquant un certain nombre d'épreuves à
court terme, consistant notamment en tests sur des cellules
mammaliennes, des bactéries et des levures ainsi que dans la
recherche de micro-noyaux chez la souris. Les résultats indiquent
que la méthyliso-butylcétone n'est pas génotoxique. En ce qui
concerne la génotoxicité à long terme ou la cancérogénicité, on ne
dispose d'aucune donnée.
A la concentration de 410 mg/m3 (100 ppm), la
méthylisobutylcétone peut produire des symptômes chez l'homme
consistant en irritation occulaire, migraines, nausées, vertiges et
fatigue et qui correspondent à un effet dépresseur réversible sur
le système nerveux central. Toutefois, rien n'indique l'existence
de lésions permanentes.
La toxicité pour les organismes et micro-organismes aquatiques
est faible.
Compte tenu de la volatilité relativement forte de la
méthylisobutylcétone, de sa photoconversion rapide dans
l'atmosphère, de sa biodégradabilité et de sa faible toxicité pour
les mammifères et la faune aquatique, il est vraisemblable que
cette substance ne peut exercer d'effets néfastes sur
l'environnement qu'à la suite de déversements accidentels ou de la
décharge incontrôlée d'effluents industriels.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des effets sur l'environnement
La méthylisobutylcétone ne devrait pas persister dans
l'environnement. Elle se volatilise lentement à partir du sol et de
l'eau et subit une biodégradation rapide dans l'eau douce et l'eau
salée. Dans l'atmosphère, on pense qu'elle est décomposée par les
radicaux libres OH avec une demi-vie d'environ 14 heures. Elle ne
s'accumule probablement pas et présente une faible toxicité pour
les micro-organismes, les poissons, les algues et les invertébrés
aquatiques. Ce n'est qu'en cas de déversement accidentel ou de
rejet incontrôlé de déchets que cette substance est susceptible
d'atteindre des concentrations toxiques pour les êtres vivants.
2. Evaluation des risques pour la santé humaine
La population générale n'est exposée qu'à de faibles
concentrations de méthylisobutylcétone. On en a décelé de faibles
quantités dans les denrées alimentaires et dans l'eau de
consommation ou autres boissons (produits panifiés, 10,9 mg/kg;
produits laitiers, 11,5 mg/kg; gélatines, puddings, 10,9 mg/kg;
boissons diverses, 10,2 mg/kg). En ce qui concerne la population
générale, deux pays ont fixé des concentrations maximales dans
l'air ambiant qui se situent dans les limites de 0,1 à 0,2 mg/m3.
L'exposition professionnelle se produit notamment lors de la
production et de l'utilisation de vernis, de peintures et de
solvants d'extraction. La principale voie de pénétration est
l'inhalation. Le faible seuil olfactif (1,64 mg/m3) et les effets
irritants de cette substance peuvent avertir de la présence de
fortes concentrations. L'exposition à ces concentrations de 10 à
410 mg/m3 (2,4 à 100 ppm) a produit une irritation perceptible au
niveau des yeux, du nez et de la gorge; à 820 mg/m3 (200 ppm), on
éprouve une sensation de malaise. Entre 10 et 410 mg par m3 (2,4
à 100 ppm), on a également observé des céphalées, des nausées et
des vertiges. Une exposition de deux heures à des concentrations
allant jusqu'à 200 mg/m3 (50 ppm) n'a pas produit d'effets
sensibles à en juger par un simple test de temps de réaction et de
calcul mental.
On ne dispose que d'un seul rapport faisant état d'une
exposition professionnelle de longue durée à une dose de 2050
mg/m3 (500 ppm), 20 à 30 minutes par jour et à 328 mg/m3 (80
ppm) pour la majeure partie du reste de la journée. Plus de la
moitié des 19 ouvriers exposés se sont plaints de faiblesse, de
perte d'appétit, de maux de tête, d'irritation oculaire, de maux
d'estomac, de nausées, de vomissements et de maux de gorge.
Quelques ouvriers ont éprouvé de l'insomnie, de la somnolence et
une perte d'équilibre. Chez quatre d'entre eux on a constaté une
légère hypertrophie du foie et chez six autres une colite non
spécifique. Cinq années plus tard, les méthodes de travail
s'étaient considérablement améliorées et les concentrations
maximales réduites au cinquième des teneurs précédentes. Quelques
ouvriers se sont encore plaints d'irritation au niveau des yeux et
des voies respiratoires supérieures ainsi que de symptômes
digestifs et neurologiques. La contact prolongé avec la peau a
provoqué une irritation cutanée et des crevasses.
Il ressort de l'expérimentation animale que la toxicité
générale aiguë de la méthylisobutylcétone est faible, par voie
orale ou par inhalation. Lors d'une étude de 90 jours, des rats
Sprague-Dawley ont reçu par gavage de la méthylisobutylcétone à des
doses quotidiennes de 50, de 250 et 1000 mg/kg de poids corporel.
On a noté une léthargie chez des animaux du groupe soumis à la dose
la plus élevée et, chez les mâles, une réduction du gain de poids.
Les animaux de ce groupe présentaient une néphropathie généralisée,
avec augmentation du poids relatif des reins et une hypertrophie du
foie. Cette augmentation du poids relatif des reins était également
notable chez les animaux soumis à la dose de 250 mg/kg, mais à
cette dose, on ne constatait qu'une légère hypertrophie du foie
chez les mâles. Quelle que soit la dose, on n'a pas constaté de
lésion histopathologique au niveau du foie ou des autres tissus. La
dose sans effet observable a été évaluée à 60 mg/kg et par jour.
Lors d'une étude de même durée au cours de laquelle on a fait
inhaler à des rats et des souris des concentrations allant jusqu'à
4100 mg/m3 (1000 ppm) on n'a pas relevé de signes de toxicité
engageant le pronostic vital. Toute fois des altérations
morphologiques réversibles liées à l'administration de cette
substance ont été observées au niveau du foie et des reins. A la
concentration de 4100 mg par m3, on observait des signes de
dépression du système nerveux central. La méthylisobutylcétone a
provoqué une augmentation du poids du foie (à une dose supérieure
à 1025 mg/m3, soit 250 ppm) et provoqué l'induction des enzymes
microsomiques du foie. C'est ce dernier mécanisme qui serait à la
base de l'exacerbation de la toxicité des alcanes halogénés et de
la potentialisation de la neurotoxicité dunw-hexane. Lors d'études
de 90 jours sur des souris, des rats, des chiens et des singes, on
a observé, chez les rats seulement, l'apparition d'inclusions
hyalines dans les tubules proximaux des reins (néphrose tubulaire
à inclusions hyalines d'origine toxique). Cet effet observé chez
les rats mâles était réversible et il est douteux qu'il ait une
signification quelconque en toxicologie humaine. La
méthylisobutylcétone réduit l'activité de l'alcool-déshydrogénase
hépatique chez la souris in vitro . On a également constaté
qu'elle potentialisait les effets cholestatiques du manganèse en
présence ou en l'absence de bilirubine.
Des rats et des souris exposés par inhalation à des
concentrations de 1230, 4100 ou 12 300 mg/m3 (300, 1000 ou 3000
ppm) du sixième au quinzième jours de la gestation puis sacrifiés
le vingt-et-unième jour (rats) ou le dixhuitième jour (souris), ont
présenté des signes marqués d'intoxication à la concentration la
plus forte. Cette concentration était foetotoxique (réduction du
poids foetal et ossification retardée) mais n'était ni
embryotoxique ni tératogène. Aux concentrations de 4100 et 1230
mg/m3, on n'a constaté aucune toxicité pour les mères ni signe
d'embryotoxicité, de foetotoxicité ou de tératogénicité.
La méthylisobutylcétone n'a pas produit de mutation génique
dans des systèmes d'épreuve bactériens (Salmonella typhimurium et
Escherichia coli ), qu'il y ait ou non activation métabolique. On
a également obtenu des résultats négatifs dans différentes épreuves
(avec ou sans activation métabolique) à la recherche de conversions
géniques mitotiques dans des levures ( Saccharomyces cerevisiae )
ou lors d'épreuves de mutation génique sur des cellules
mammaliennes en culture (lymphome murin). La recherche in vitro
d'une synthèse anarchique de l'ADN dans des hépatocytes primaires
de rat et de lésions chromosomiques structurales dans des cellules
de foie de rat en culture (RL4) s'est révélée négative. Chez la
souris, la recherche in vivo de micro-noyaux s'est également
révélée négative. Toutes ces données montrent que la
méthyl-isobutylcétone n'est pas génotoxique.
RECOMMANDATIONS
Les concentrations de méthylisobutylcétone auxquelles la
population, dans son ensemble, est susceptible d'être exposée, ne
présentent vraisemblablement aucun danger. La principale voie
d'exposition professionnelle est la voie respiratoire, aussi les
concentrations atmosphériques devront être maintenues en dessous
des limites recommandées d'exposition professionnelle, grâce à un
aménagement convenable des procédés de production et à des moyens
mécaniques tels que la ventilation. Il convient d'éviter toute
contamination de la peau et des yeux. Des vêtements protecteurs
appropriés ainsi que des masques respiratoires doivent être placés
dans les ateliers confinés; ils seront utilisés en cas d'urgence ou
pour effectuer certaines opérations d'entretien. La
méthylisobutylcétone est inflammable et doit donc être manipulée
avec les précautions d'usage.
La méthylisobutylcétone présente une faible toxicité pour les
micro-organismes et pour les poissons et sa demivie dans
l'environnement est courte. Il s'ensuit qu'elle ne présente aucun
risque pour l'environnement, dans la mesure où des mesures
appropriées sont prises pour réduire les émissions au minimum. La
décharge de quantités importantes dans l'environnement pourrait
avoir localement des effets indésirables.
RECHERCHES A EFFECTUER
1. La méthylisobutylcétone affecte un certain nombre de systèmes
enzymatiques. Elle peut donc avoir une influence sensible sur
la biotransformation des produits xénobiotiques métabolisés
par ces enzymes. Etant donné que l'homme est généralement
exposé à plusieurs composés différents, il faudrait
entre-prendre des études sur les effets combinés de mélanges
contenant de la méthylisobutylcétone.
2. On ne dispose que de très peu d'informations sur la relation
dose-réponse relative aux effets toxiques de la
méthylisobutylcétone sur le système nerveux central (par
exemple temps de réaction, effets comportementaux), sur les
voies respiratoires supérieures, sur les muqueuses et sur la
fonction rénale. Il faudrait obtenir davantage de
renseignements sur la toxico-cinétique de cette cétone, soit
seule soit associée à d'autres solvants. Il faudra également
étudier la pénétration percutanée de la méthylisobutylcétone.
3. Il faudrait entreprendre des études épidémiologiques pour
élucider les effets exercés à long terme sur le système
nerveux central par des concentrations moyennes de
méthylisobutylcétone soit seule soit associée à d'autres
solvants.
RESUMEN
La metil isobutil acetona (MIBA) es un líquido transparente de
buen olor que se produce a escala comercial y tiene un uso muy
extendido como disolvente. Puede medirse mediante cromatografía de
gases con detección de ionización de llama. Se evapora rápidamente
a la atmósfera, donde se fototransforma en poco tiempo. La MIBA es
fácilmente biodegradable, lo que, junto con su moderada solubilidad
en el agua y su reducido coeficiente de partición octanol/agua,
sugiere que tiene un bajo potencial de bioacumulación. Los límites
de exposición profesional varían entre 100-410 mg/m3 (promedio
ponderado en el tiempo) y 5-300 mg/m3 (valor máximo) en distintos
países.
La MIBA se metaboliza fácilmente para dar productos de
excreción hidrosolubles y su toxicidad sistémica aguda en animales
es baja por las vías de exposición oral y de inhalación. No se ha
observado axonopatía periférica en estudios realizados en animales.
No se dispone de datos exactos sobre la CL50. La exposición a 16
400 mg/m3 (4000 ppm) durante 4 horas resultó letal para la rata.
Las concentraciones de MIBA líquida y en vapor comprendidas entre
10 y 410 mg/m3 (2,4-100 ppm) son irritantes para los ojos y las
vías respiratorias superiores. Con concentraciones de hasta 200
mg/m3 (50 ppm) no se observaron en el hombre efectos
significativos en una prueba sencilla de tiempo de reacción ni en
una prueba de aritmética mental. El contacto cutáneo prolongado o
repetido puede desecar y descamar la piel. La aspiración accidental
de MIBA líquida puede provocar pneumonitis química.
En un estudio de ceba de ratas durante 90 días, se determinó
un nivel sin efecto observado de 50 mg/kg. En estudios de
inhalación durante 90 días en ratas y ratones, concentraciones de
hasta 4100 mg/m3 (1000 ppm) no produjeron ningún signo de
toxicidad con peligro para la vida. No obstante, se notificaron
cambios morfológicos reversibles relacionados con el compuesto en
el hígado y el riñón. En varios estudios se observó que
concentraciones de MIBA tan bajas como 1025 mg/m3 (250 ppm) eran
capaces de aumentar el tamaño del hígado. Mediante la exposición a
4100 mg/m3 (1000 ppm) durante 50 días, se indujo actividad
metabólica en las enzimas microsómicas del hígado del pollo. A
dosis más elevadas (hasta 8180 mg/m3, 1996 ppm) los efectos se
limitaron a un aumento del peso del hígado sin lesiones
histológicas. En estudios durante 90 días con ratones, ratas,
perros y monos, sólo las ratas macho presentaron corpúsculos
hialinos en los túbulos proximales del riñón (nefrosis tubulotóxica
de corpúsculos hialinos). Este efecto en la rata macho resultó ser
reversible y de dudosa importancia para el hombre. La inducción
enzimática puede ser la base de la potenciación de la toxicidad de
los haloalcanos por la MIBA. Se observó también que la MIBA era
capaz de potenciar el efecto colestático del manganeso administrado
con o sin bilirrubina.
En papiones expuestos durante 7 días a 205 mg/m3 (50 ppm),
se observaron efectos en el neurocomportamiento.
La MIBA resulta fetotóxica a una concentración que produce sin
lugar a dudas toxicidad materna (12 300 mg/m3, 3000 ppm) pero no
es embriotóxica ni teratogénica en esa concentración. En una
concentración de 4100 mg/m3 (1000 ppm), no resultó ni
embriotóxica, ni fetotóxica ni teratogénica en la rata ni en el
ratón.
Se ha estudiado la genotoxicidad de la MIBA en varios ensayos
a corto plazo, inclusive pruebas in vitro con bacterias, levaduras
y células de mamíferos y un ensayo de micronúcleos en el ratón.
Esos estudios indican que la MIBA no es genotóxica. No se dispone
de informes sobre estudios a largo plazo ni estudios de
carcinogenicidad.
Aunque con una concentración de 410 mg/m3 (100 ppm) la MIBA
puede inducir en el hombre síntomas como irritación ocular, dolores
de cabeza, náuseas, mareos y fatiga, que corresponden a un efecto
reversible de depresión del sistema nervioso central, no existen
pruebas de que produzca lesiones permanentes en el sistema
nervioso.
La toxicidad de la MIBA para organismos y micro-organismos
acuáticos es baja.
La volatilidad relativamente alta de la MIBA, su rápida
fototransformación en la atmósfera, su fácil biodegradación y su
baja toxicidad para mamíferos y organismos acuáticos indican que
los efectos medioambientales adversos de esta sustancia
probablemente sólo se producirán como consecuencia de vertidos
accidentales o de efluentes industriales no controlados.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS
EFECTOS EN EL MEDIO AMBIENTE
1. Evaluación de los efectos en el medio ambiente
La MIBA tiene pocas probabilidades de persistir en el medio
ambiente. Se volatiliza poco a poco desde el suelo y el agua y se
biodegrada fácilmente en agua dulce y salada. En la atmósfera, se
ha calculado que la MIBA es degradada por los radicales OHÊ una
semivida de aproximadamente 14 horas. En principio, la MIBA no se
bioacumula y tiene una toxicidad baja para micro-organismos, peces,
algas e invertebrados acuáticos. Sólo en los casos de vertido
accidental o de evacuación inadecuada de desechos en el medio
ambiente es probable que los niveles de MIBA provoquen toxicidad en
los organismos del entorno.
2. Evaluación de los riesgos para la salud humana
La población general está expuesta a niveles reducidos de MIBA.
Se han detectado sólo pequeñas cantidades en los alimentos, el agua
potable y otras bebidas (alimentos horneados, 10,9 mg/kg; productos
lácteos congelados, 11,5 mg/kg; gelatinas y budines, 10,9 mg/kg;
bebidas, 10,2 mg/kg). En cuanto a la exposición de la población
general, dos países han definido concentraciones máximas en el aire
entre 0,1 y 0,2 mg/m3.
La exposición profesional tiene lugar especialmente en la
producción y utilización de lacas, pinturas y disolventes de
extracción. La principal vía de entrada es por inhalación. El bajo
umbral olfativo (1,64 mg/m3) y los efectos irritantes pueden
servir como indicadores de las concentraciones elevadas. La
exposición a niveles de 10-410 mg/m3 (2,4-100 ppm) produjo
irritación perceptible de los ojos, la nariz, o la garganta y 820
mg/m3 (200 ppm) produjeron molestias. Con un nivel de 10-410
mg/m3 (2,4-100 ppm) también se produjeron síntomas como dolor de
cabeza, náuseas y vértigos. No se observaron efectos significativos
debidos a una exposición durante 2 horas a hasta 200 mg/m3 (50
ppm) al realizar una prueba sencilla de tiempo de reacción ni una
prueba de aritmética mental.
En el único informe sobre un estudio de la exposición
profesional a largo plazo, en el que se expuso a trabajadores a
2050 mg MIBA/m3 (500 ppm) durante 20-30 minutos al día y a 328
mg/m3 (80 ppm) durante la mayor parte del resto de la jornada
laboral, más de la mitad de los 19 trabajadores se quejaron de
debilidad, pérdida del apetito, dolores de cabeza, irritación
ocular, dolor de estómago, náuseas, vómitos y dolor de garganta.
Algunos trabajadores sufrieron insomio, somnolencia y cierta
inestabilidad. En cuatro se observó un ligero agrandamiento del
hígado y en seis se observó colitis no específica. Al cabo de 5
años, las prácticas laborales habían mejorado en gran medida y las
concentraciones más elevadas se redujeron a aproximadamente la
quinta parte del nivel anterior. Algunos trabajadores siguieron
quejándose de irritación en los ojos y de las vías respiratorias
superiores así como de síntomas gastrointestinales y del sistema
nervioso central. El contacto cutáneo prolongado con la MIBA
provocó irritación y descamación de la piel.
En estudios en animales, la toxicidad sistémica aguda de la
MIBA es baja por las vías oral y respiratoria. En un estudio de 90
días de duración, se cebó con MIBA a ratas Sprague-Dawley en dosis
diarias de 50, 250 ó 1000 mg/kg de peso corporal. Se observó
letargo en el grupo que recibió la dosis más alta y en los machos
se observó una reducción del ritmo de aumento del peso corporal. En
este grupo se observó nefropatía generalizada, con aumento del peso
relativo del riñón y hepatomegalia. El peso relativo del riñón
también aumentó en los animales alimentados con 250 mg/kg al día,
y se observó ligera hepatomegalia sólo en los machos. No
aparecieron lesiones histopatológicas en el hígado ni en otros
tejidos con ninguna de las dosis administradas. Se concluyó que el
nivel de efecto no observado era de 50 mg/kg al día. En estudios de
inhalación durante 90 días realizados en ratas y ratones, las
concentraciones de hasta 4100 mg/m3 (1000 ppm) no originaron
ningún signo de toxicidad que pusiera en peligro la vida. No
obstante, se comunicó la observación de cambios morfológicos
reversibles relacionados con el compuesto en el hígado y el riñón.
Con niveles de 4100 mg/m3 se observaron signos de depresión del
sistema nervioso central. La MIBA fue capaz de aumentar el peso del
hígado (concentración > 1025 mg/m3 (250 ppm)) y de inducir el
metabolismo microsómico hepático. Esto puede explicar la
exacerbación de la toxicidad de los haloalcanos y la potenciación
de la neurotoxicidad del n‹hexano. En estudios de 90 días con
ratones, ratas, perros y monos, sólo las ratas macho desarrollaron
corpúsculos hialinos en los túbulos proximales del riñón (nefrosis
tubulotóxica de corpúsculos hialinos). Este efecto en ratas macho
resultó ser reversible y de dudosa importancia para el hombre. In
vitro, la MIBA reduce la actividad de la deshidrogenasa alcohólica
en el hígado del ratón. También se ha observado que potencia los
efectos colestáticos del manganeso administrado con o sin
bilirrubina.
En ratas y ratones expuestos a la inhalación de MIBA en
concentraciones de 1230, 4100 ó 12 300 mg/m3 (300, 1000 ó 3000
ppm) en los días 6 a 15 de la gestación y sacrificados el día 21
(ratas) o el día 18 (ratones), se observó una notable toxicidad
materna a la concentración más elevada en ambas especies. Esta
concentración produjo fetotoxicidad (peso del cuerpo fetal reducido
y retraso en la osificación) pero no resultó embriotóxico ni
teratogénico. A 4100 y 1230 mg/m3 no se observó ni toxicidad
materna ni síntomas de embriotoxicidad, fetotoxicidad o
teratogenicidad.
La MIBA no indujo mutación genética en sistemas de ensayo
bacterianos (Salmonella typhimurium y Escherichia coli), con o sin
activación metabólica. También se obtuvieron resultados negativos
en los ensayos (tanto con y sin activación metabólica) para la
conversión mitótica de genes en levaduras (Saccharomyces
cerevisiae) y en pruebas de mutación génica con cultivos de células
de mamíferos (linfoma de ratón). Los ensayos in vitro sobre
síntesis no controlada de ADN en hepatocitos primarios de rata y
sobre lesiones cromosómicas estructurales en cultivos de células
hepáticas de rata (RL4) resultaron negativos. En el ratón, un
ensayo de micronúcleo in vivo resultó negativo. Estos datos indican
que la MIBA no es genotóxica.
RECOMENDACIONES
En los niveles de MIBA a que está expuesta la población humana
general, es poco probable que se plantee riesgo alguno. En el medio
laboral, donde la principal vía de exposición es por inhalación,
los niveles atmosféricos deben mantenerse por debajo de los límites
de exposición profesional recomendados mediante procesos de trabajo
y controles de ingeniería, inclusive la ventilación, de diseño
adecuado. Debe evitarse la contaminación de la piel y de los ojos.
Debe facilitarse el uso de prendas protectoras adecuadas y de
protección respiratoria en los espacios cerrados, en casos de
emergencia y para ciertas operaciones de mantenimiento. La MIBA es
inflamable y debe manipularse teniendo esta característica en
cuenta.
La MIBA tiene baja toxicidad para los microorganismos y los
peces, y su semivida en el medio ambiente es corta. Por
consiguiente, no presenta riesgos para el medio ambiente siempre
que se apliquen las medidas de control adecuadas para reducir al
mínimo las emisiones. El vertido en gran escala podría ejercer
efectos adversos en el medio ambiente a escala local.
OTRAS INVESTIGACIONES
1. LA MIBA afecta a varios sistemas enzimáticos. Por lo tanto,
puede influir de modo significativo en la bio-transformación
de sustancias biológicas externas que son metabolizadas por
estas enzimas. Como las personas suelen estar expuestas a más
de un compuesto, deben llevarse a cabo estudios sobre los
efectos combinados de muestras que contengan MIBA.
2. Se dispone de muy poca información sobre las relaciones
dosis‹respuesta en cuanto a los efectos de la MIBA en el
sistema nervioso central humano (por ejemplo, tiempo de
reacción, efectos conductuales) en las vías respiratorias
superiores y las mucosas, o en la función renal. Se necesita
más información sobre la toxicocinética de la MIBA por sí sola
y mezclada con otros disolventes. Debe evaluarse la
penetración cutánea de la MIBA.
3. Deben emprenderse estudios epidemiológicos para dilucidar los
efectos que tiene en el sistema nervioso la exposición a largo
plazo a concentraciones moderadas de MIBA, por sí sola o
mezclada con otros disolventes.
HMP and 4-methyl-2-pentanone have also been identified in rats
(Pilon, 1987). MIBK increased aniline hydroxylase activity and
cytochrome P-450 concentration in chicken liver microsomes after
inhalation (4100 mg/m3, 1000 ppm, for 50 days) (Abou-Donia et
al., 1985b). The induction by MIBK was apparently of similar
capacity to that of methyl n -butyl ketone (Abou-Donia et al.,
1985a). It is likely that MIBK can induce its own oxidative
metabolism as well as that of other substances. The study of
Malyscheva (1988) (section 8.2.4) suggested that MIBK is absorbed
through the skin as well as via the oral and inhalation routes
(section 8.1). A comparison of intraperitoneal and oral LD50
values (Zakhari et al., 1977) suggests an oral absorption of 30% or
more. Malyscheva (1988) showed dermal absorption followed by
extensive distribution (toxic signs in many organs). Likewise,
inhalation studies producing liver and kidney changes are
suggestive of extensive distribution (MacEwen et al., 1971; Vernot
et al., 1971).
The structure of MIBK precludes the metabolic production of
2,5-hexane-dione, the neurotoxic agent formed from both hexane and
methyl n -butyl ketone.
6.1.1 Effect on liver alcohol dehydrogenase in vitro
MIBK in N,N -dimethylacetamide has been shown to reduce the
activity of mouse liver alcohol dehydrogenase in vitro
(Cunningham et al., 1989).
6.2 Humans
MIBK and other substituted 2-pentanones have been reported in
urine samples from unexposed humans (Zlatkis & Liebich, 1971). MIBK
was detected in various tissues and body fluids (section 2.4.1.3)
of two individuals who suffered fatal exposure to a mixture of
organic solvents. The concentration range of MIBK in the tissues
and body fluids of the two decedents was 1.4-2.5 and 0.2-0.8 mg/kg,
respectively. The tissue distribution differed markedly between the
two individuals (Bellanca et al., 1982). The inhalation study
carried out by Dick et al. (1990) with human volunteers suggested
that exposures to 410 mg MIBK/m3 (100 ppm) for 4 h causes steady
state blood levels to be attained. Blood and breath samples
collected 90 min after exposure indicated essentially complete
clearance of the absorbed MIBK. Hjelm et al. (1990) exposed human
volunteers for 2 h during light physical excercise to MIBK (10,
100, and 200 mg/m3; 2.4, 24.4, and 48.8 ppm). The concentration
of MIBK in blood rose rapidly after the onset of exposure, during
which no plateau level was reached. No tendency towards saturation
kinetics was observed over the dose range, the apparent blood
clearance being 1.6 litres/h per kg throughout. Only 0.04% of the
total MIBK dose was eliminated unchanged via the kidneys within 3
h after exposure.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Toxicity data reported in this chapter should be interpreted
with caution, since the tests were conducted under static
conditions using nominal rather than measured concentrations.
Actual concentrations experienced by the test organisms cannot be
determined for these tests.
7.1 Microorganisms
MIBK has low toxicity for microorganisms as indicated by the
threshold concentrations required for inhibition of growth (Table
3).
Table 3. Toxicity of MIBK for microorganisms
Species Threshold Duration of Reference
concentration for study
growth inhibition
(mg/litre)
Protozoa
Saprozoic > 800 48 h Bringmann & Kühn (1981)
flagellate
(Chilomonas
paramaecium)
Bacteriovorous 450 72 h Bringmann & Kühn (1981)
flagellate
(Entosiphon
sulcatum)
Bacteriovorous 950 20 h Bringmann & Kühn (1981)
ciliate ( Uronema
parduczi)
Bacterium
Pseudomonas putida 275 16 h Bringmann & Kühn (1977b)
Table 4. Acute toxicity of MIBK for aquatic organisms
Species LC50 Duration Reference
(mg/litre) of study
Freshwater fish
Golden orfe 672-744 48 h Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 460 24 h Bridié et al. (1979b)
(Carassius auratus)
Fathead minnow approx. 525 96 h Call et al. (1985)
(Pimephales promelas)
Invertebrates
Freshwater
Water flea 4280 24 h Bringmann & Kühn (1977a)
(Daphnia magna) 1550 24 h Bringmann & Kühn (1982)
Marine
Brine shrimp 1230 24 h Price et al. (1974)
(Artemia salina)
Freshwater algae
Green algae 725 a 8 days Bringmann & Kühn (1977b)
(Scenedesmus
quadricauda)
Bluegreen algae 136 a 8 days Bringmann & Kühn (1978)
(Microcystis
aeruginosa)
a Threshold concentration for reduction of total biomass
7.2 Aquatic organisms
MIBK appears to have low toxicity for aquatic organisms (Table
4). The maximum zero lethality concentration (LC0) is in the
range of 480-720 mg/litre (Juhnke & Lüdemann, 1978). Using a QSAR
model, Lipnick et al. (1987) showed that the 24-h LC50 of 460
mg/litre reported by Bridié et al. (1979a) in goldfish (Carassius
auratus) fitted a narcotic mechanism of action.
Aquatic invertebrates are less sensitive than fish to the
toxicity of MIBK, 24-h LC50 values of 4280 mg/litre (Bringmann &
Kuhn, 1977a) and 1550 mg/litre (Bringmann & Kuhn, 1982) having been
reported for the water flea Daphnia magna and 1230 mg/litre for
the brine shrimp Artemia salina (Price et al., 1974). The
LC100 and LC0 values for Daphnia magna were 5000 and 2280
mg/litre, respectively (Bringmann & Kuhn, 1977a).
The toxicity of MIBK was also measured in the green alga
Scenedesmus quadricauda , in which the 8-day threshold for
toxicity was 725 mg/litre (Bringmann & Kuhn, 1977b), and in the
relatively more sensitive cyanobacterium (blue-green alga)
Microcystis aeruginosa , in which the toxicity threshold was 136
mg/litre (Bringmann & Kuhn, 1978).
7.3 Terrestrial organisms
Experimental data are not available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
MIBK is of low acute toxicity by the oral and inhalation routes
of exposure (Table 5).
The maximum time for which rats could be exposed to a saturated
atmosphere of MIBK without dying was 15 min (Smyth et al., 1951).
In one study, six rats survived a 4-h exposure to 8200 mg MIBK/m3
(2000 ppm), but, following a 4-h exposure to 16 400 mg/m3 (4000
ppm), all six animals died within 14 days.
In studies by Specht (1938) and Specht et al. (1940), female
guinea-pigs were exposed to MIBK concentrations of 4100, 69 000,
and 115 000 mg/m3 (1000, 16 800, and 28 000 ppm, respectively)
for up to 24 h. In view of the method used for generating the
atmosphere (allowing measured amounts of MIBK to evaporate freely
to one cubic meter volume of air at 25-26 °C), the two higher
levels must be greatly exaggerated because the saturation
concentration in air for MIBK at 25 °C is 40 000 mg/m3. At 4100
mg/m3 there was minimal eye or nasal irritation. However, there
was a decreased respiratory rate during the first 6 h of exposure,
which was attributed to a narcotic effect. The higher levels
produced obvious signs of eye and nose irritation, followed by
salivation, lacrimation, ataxia, progressive narcosis, and death.
The highest level killed 50% of the animals within 45 min. Autopsy
and histopathological investigations in some animals showed fatty
livers and congestion of the brain, lungs, and spleen, but no
damage to the heart and kidneys was observed.
A single ip injection of 500 mg MIBK/kg body weight in
guinea-pigs did not induce changes in the serum ornithine-carbamyl
transferase level, and there were no histopathological changes in
the liver. However, an injection of 1000 mg/kg body weight killed
one out of four animals, and a slight increase in the serum
ornithine-carbamyl transferase level was seen in the survivors 24
h after dosing. Histopathologically, there was possible lipid
accumulation in liver cells but no evidence of liver damage
(Divincenzo & Krasavage, 1974). It should be noted that an ip
injection of 560 mg/kg produced minimal effects in rats (Vezina et
al., 1985), suggesting that mice are more sensitive than rats (the
ip LD50 is 590 mg/kg in mice, see Table 5).
Table 5. LD50 and LC50 values for MIBK in rats and mice
Route of Species Duration LD50 or LC50 Reference
exposure of exposure
Oral (LD50) rat 4570 mg/kg body weight Smyth et al. (1951)
rat 4600 mg/kg body weight Batyrova (1973)
rat 2080 mg/kg body weight RTECS (1987)
mouse 2850 mg/kg body weight Batyrova (1973)
mouse 1900 mg/kg body weight Zakhari et al. (1977)
Intraperitoneal (LD50) mouse 590 mg/kg body weight Zakhari et al. (1977)
Inhalation (LC50) rat 4 h 8.2-16.4 g/m3 Smyth et al. (1951); Smyth (1956)
mouse 2 h 20.5 g/m3 Batyrova (1973)
mouse 45 mins 74.2 g/m3 Zakhari et al. (1977)
(18 105 ppm)
In male Sprague-Dawley rats, a single oral dose of MIBK
enhanced the hepatotoxicity of a single ip dose of chloroform given
24 h later. The no-observed-effect and minimal-effect levels of
MIBK were 375 and 560 mg/kg body weight, respectively (Vezina et
al., 1985). The ketone potentiation of haloalkane-induced
hepatonecrosis has been attributed to enhanced bioactivation of the
haloalkane, which is mediated by the increased cytochrome P-450
activity induced by the ketone (Branchflower et al., 1983). The
extent of potentiation of carbon tetrachloride liver toxicity (as
shown by an increase in plasma alanine transaminase activity and
bilirubin concentration) was found to depend on the concentration
of both MIBK and carbon tetrachloride in male rats. The minimum
effective MIBK dose decreased 10-fold when the carbon tetrachloride
dose was increased from 0.01 ml/kg to 0.1 ml/kg. These findings
suggest that liver injury is determined by the product of MIBK and
carbon tetrachloride doses (Pilon et al., 1988). Attention should
be paid to this when working in environments containing mixtures of
solvents.
8.2 Short-term exposure
8.2.1 Inhalation
In rats exposed to 410 mg/m3 (100 ppm) for 2 weeks, there was
an increase in kidney weight. An increase in both liver and kidney
weights was observed after exposure to 820 mg/m3 (200 ppm) for 2
weeks or 410 mg/m3 (100 ppm) for 90 days. Histopathological
investigations showed hyaline droplets in the proximal tubules of
the kidney and this finding was named hyaline droplet toxic tubular
nephrosis (MacEwen et al., 1971; Vernot et al., 1971). In rats,
dogs, and monkeys exposed continuously for 90 days to MIBK at 410
mg/m3 (100 ppm) under reduced oxygen tension and reduced
atmospheric pressure (65% oxygen at 34.7 k Pa), liver and kidney
weights were increased in rats after 90 days. Hyaline droplets were
observed in rat kidney epithelium after 15 days, but this effect
was reversible after a 3- to 4-week recovery period. No
histopathological changes were reported in monkeys or dogs and no
sex differences were reported for any parameter (MacEwen et al.,
1971).
Four groups of six male and six female F-344 rats and six male
and six female B6C3F1 mice were exposed to 0, 44, 2050, or 8180
mg MIBK/m3 (0, 10.1, 501, or 1996 ppm, respectively) for 6 h/day
(for 5 days with 2 days off followed by 4 more consecutive days of
exposure) (Dodd et al., 1982; Phillips et al., 1987). Lacrimation
was observed in the highest dose group, but no ophthalmological
lesions or alterations in body weight gain were found. Liver
weight, expressed as a percentage of body weight, was increased in
male and female rats and in female mice exposed to 8180 mg/m3. A
statistically significant increase in liver weight was also
observed in male rats exposed to 2050 mg/m3. Male and female rats
and female mice showed a significant increase in both absolute and
relative kidney weights when exposed to 8180 mg/m3. However, male
mice at this exposure level exhibited a significant decrease in
relative kidney weight. Of the animals exposed to 2050 mg/m3,
only male rats exhibited an increase in kidney weight, but this was
not statistically significantly different from the control value.
Hyaline droplet formation was seen in the kidneys of male rats
exposed to 2050 and 8180 mg/m3. Epithelial regeneration of the
proximal convoluted tubules was also seen at 8180 mg/m3. There
were no histopathological abnormalities in rats and mice exposed to
414 mg/m3, and this concentration was considered a clear
no-observed-adverse-effect level (Dodd et al., 1982). In a
subsequent study, four groups of 14 male and 14 female F-344 rats
and 14 male and 14 female B6C3F1 mice were exposed to 0, 205,
1025, or 4100 mg MIBK/m3 (0, 50, 250, or 1000 ppm, respectively)
for 6 h/day (5 days/week for 90 days). No growth retardation or
clinical effects were observed in either rats or mice. Clinical
observations were made in addition to measurements of body and
organ weights (heart, kidneys, liver, lungs, and testes).
Haematology, ophthalmology, gross pathology, and histology were
also investigated and, in rats, water consumption and urine and
serum chemistry analyses were made. Male rats and mice exposed to
4100 mg/m3 (1000 ppm) showed a slight increase in liver weight
(approximately 11%) and in liver weight per body weight ratio, and
liver weight was also slightly increased in male mice exposed to
1025 mg/m3 (250 ppm). However, neither gross nor microscopic
hepatic lesions were observed, and urinalysis and serum chemistry
values were normal. In male rats exposed to 1025-4100 mg/m3
(250-1000 ppm), there was an increase in the number of hyaline
droplets within the proximal tubular cells of the kidney. No other
gross or microscopic renal changes were observed (Dodd & Eisler,
1983; Phillips et al., 1987). It was considered that the hyaline
droplet effects produced by MIBK may be specific to the male rat
due to the presence of alpha-2-µglobulin (Phillips et al., 1987).
In studies by Brondeau et al. (1989), groups of five hens were
continuously exposed for 50 days by inhalation to either 4100 mg
MIBK/m3 (1000 ppm) or 3520 mg n -hexane/m3 (1000 ppm) or for
30 days to a mixture of 4100 mg MIBK/m3 and 3520 mg n
-hexane/m3. Inhalation of n -hexane alone had no effect on
hepatic microsomal enzymes, but inhalation of MIBK or the MIBK/ n
-hexane mixture increased significantly the aniline hydroxylase
activity and cytochrome P-450 content of the liver (Abou-Donia et
al., 1985b). Inhalation of 2440 to 12 400 mg MIBK/m3 (595 to 3020
ppm) in rats produced enhancement of the liver cytochrome P-450
content and glutathione- S -transferase activity and also enhanced
the ability of 1,2-dichlorobenzene to increase serum glutamate
dehydrogenase activity.
Experiments in open-chest cats demonstrated that significant
pulmonary hypertension and vasoconstriction, with reduced pulmonary
arterial flow, occurred as a result of MIBK inhalation for 5 min at
all concentrations tested (410 to 41 000 mg/m3 (100 to 10 000
ppm)). Systemic arterial pressure and vascular resistance were not
significantly affected (Zakhari et al., 1977). Broncho-constriction
was also produced by MIBK inhalation for 5 min, the effect being
statistically significant at concentrations at or above 2050
mg/m3 (500 ppm) or 4100 mg/m3 (1000 ppm) for pulmonary
resistance or transpulmonary pressure, respectively. Adult mongrel
dogs with open-chest surgery showed pulmonary hypertension at an
inhalation concentration of 20.5 mg MIBK/m3 (5 ppm) for 5 min. At
41 mg/m3 (10 ppm), myocardial contractility occurred (Zakhari et
al., 1977).
8.2.2 Oral
Three daily doses of 375 or 1500 mg MIBK/kg body weight given
by gavage to rats reduced the bile flow produced by an intravenous
injection of taurocholate (20 mg per kg body weight) (Plaa &
Ayotte, 1985). The effect of MIBK on the cholestatic activity of
manganese, with or without bilirubin, has also been investigated in
male Sprague-Dawley rats. MIBK was administered by gavage in corn
oil at doses ranging from 188 to 1502 mg/kg body weight once a day
for 1, 3, or 7 days (Vezina et al., 1985; Vezina and Plaa, 1987).
MIBK was not cholestatic but, at doses of 375 mg/kg or more, was
found to potentiate the cholestasis induced by a
manganese-bilirubin combination when this was given 18 h after the
1-day treatment with MIBK. When given for 3 or 7 days, MIBK
produced a dose-related enhancement of the cholestasis induced by
a manganese-bilirubin combination. A 3-day treatment with MIBK (750
mg/kg) was also shown to potentiate the cholestasis induced by
manganese alone. Two known metabolites of MIBK (HMP and
4-methyl-2-pentanol) were also able to potentiate the cholestatic
effect of the manganese-bilirubin combination or of manganese alone
in male Sprague-Dawley rats. When the metabolite was given by
gavage 18 h prior to the administration of the manganese-bilirubin
combination, cholestasis was potentiated by 375 mg/kg or 1502 mg/kg
(expressed as equivalents of MIBK) of 4-methyl-2-pentanol or HMP,
respectively. Lower doses did not decrease bile flow rate.
4-Methyl-2-pentanol was also more effective than HMP as a
potentiator following daily treatment for 3 days prior to
manganese-bilirubin administration. However, with manganese alone,
HMP was more effective. It was suggested that the potentiation of
cholestasis may be associated with metabolic induction or might
reflect a separate mechanism of action involving a membrane
interaction (Vezina & Plaa, 1988).
The toxic effects of MIBK in Sprague-Dawley rats (groups of 30
animals of each sex) were examined following 13 weeks of oral
gavage administration at levels of 0, 59, 250, or 1000 mg/kg daily.
Body weight, food consumption, organ weight, morbidity, clinical
chemistry, haematology, and histopathology evaluations were
performed. All surviving animals were killed after 90 days (13
weeks) and 10 animals of each sex per group were examined.
Nephrotoxicity was seen as a general nephropathy for both male and
female rats administered 1000 mg/kg per day. Although increased
liver and kidney weights were observed for males and females at
1000 mg/kg per day, there were no corresponding histopathological
lesions present in the liver. The effects seen at 1000 mg/kg per
day were present to a significantly lesser extent in the females
and males fed 250 mg/kg per day. No effects were observed at 50
mg/kg per day, identifying a no-observed-effect level
(Microbiological Associates, 1986).
8.2.3 Parenteral
In studies by Krasavage et al. (1982), rats (strain and number
not specified) were given intraperitoneal injections of MIBK or a
mixture of methyl ethyl ketone (MEK) and MIBK (9:1 by volume), 5
times/week, for 35 weeks. The dose levels for the first 2 weeks
were 10, 30, and 100 mg per kg body weight, and these were then
doubled for the remainder of the treatment period. Body weight gain
suppression was seen after 3-4 weeks of treatment. The only other
effect noted was transient narcosis during the first 4 weeks at the
highest dose. Pulmonary vascular effects were observed following an
intraperitoneal administration of MIBK to cats (threshold dose 8
mg/kg), but bronchoconstriction was not seen following an
intraperitoneal administration of 4-32 mg/kg (Zakhari et al.,
1977).
8.2.4 Skin application
In rats exposed dermally to 300-600 mg MIBK/kg per day for 4
months, dose- and time-dependent morphological changes were
observed in the skin, brain, liver, adrenals, spleen, and testis.
Body temperature decreased and oxygen consumption increased
(Malyscheva, 1988).
8.3 Skin, eye, and respiratory irritation; sensitization
8.3.1 Skin irritation
A single 10-h occluded application of MIBK to the shaved skin
of rabbits produced erythema, which occurred immediately after the
application and persisted for up to 24 h. Daily applications of 10
ml on 10 cm2 skin for 7 days caused drying and flaking of the
surface (Krasavage et al., 1982).
8.3.2 Eye irritation
Undiluted MIBK (0.1 ml) produced some irritation within 10 min
when instilled in the rabbit eye. Inflammation and conjunctival
swelling occurred within 8 h; the inflammation, swelling, and
exudate present at 24 h had disappeared by 60 h (Krasavage et al.,
1982).
8.3.3 Respiratory irritation
De Ceaurriz et al. (1981) measured the reflex decrease in
respiratory rate in male Swiss OF1 mice as an index of sensory
irritation. MIBK caused a concentration-dependent decrease in
respiratory rate during a 5-min exposure, and a 50% decrease in
respiratory rate (RD50) was seen at 13 100 mg/m3 (3195 ppm).
Specht et al. (1940) attributed the decreased respiratory rate to
a narcotic effect. It should be recognized that the reduction may
not be due to sensory irritation.
8.3.4 Skin sensitization
There are no reports of skin sensitization studies.
8.4 Long-term exposure
No long-term toxicity studies have been reported.
8.5 Reproduction, embryotoxicity, and teratogenicity
In studies by Tyl (1984) and Tyl et al. (1987), groups of 35
pregnant Fischer-344 rats and 30 pregnant CD-1 mice were exposed to
1230, 4100, or 12 300 mg MIBK/m3 (300, 1000, or 3000 ppm) on days
6-15 (inclusive) of gestation. The animals were sacrificed on days
21 (rats) or 18 (mice) and fetuses examined for external, visceral,
and skeletal alterations. In rats, exposure to 12 300 mg/m3
resulted in maternal toxicity with decreased body weight gain,
increased relative kidney weight, decreased food consumption, and
fetotoxicity (reduced fetal body weight per litter and delays in
skeletal ossification). Clinical signs in dams included loss of
coordination, negative toe pinch, paresis, muscular weakness,
piloerection, lacrimation, and perioral encrustation. No increase
in fetal malformation was observed in any group. At 1230 and 4100
mg/m3, there was no maternal, embryo, or fetal toxicity, or
malformations. However, reduced fetal body weight and delay in some
ossification parameters were observed at the lowest dose but not at
the intermediate dose level. These effects were attributed to the
larger litter size (average 10.8) in this group compared to that in
controls (9.5).
In mice, exposure to 12 300 mg/m3 produced maternal toxicity
with increased mortality (3/25), increased absolute and relative
liver weights, and fetotoxicity (increased incidence of dead
fetuses, reduced fetal body weight per litter, and delayed or
reduced ossification). Clinical signs in dams included irregular
gait, paresis, hypoactivity, ataxia, negative toe pinch, and
lacrimation. There was no treatment-related increase in
embryotoxicity or fetal malformations at any exposure concentration
tested. No significant treatment-related maternal, embryo, or fetal
toxicity (including malformations) was observed at 1230 or 4100
mg/m3.
Table 6. Mutagenicity and related end-points
System Dose Response Reference
Bacterial Assays
Salmonella typhimurium a 0.04-4 µg/plate negative Chemical
strains TA98, TA100, Manufacturers
TA1537, TA1538 Association
(1984);
O'Donoghue et
al. (1988)
Salmonella typhimurium a
strains TA1535, TA1537 Up to 8000 µg/ml negative Brooks et al.
TA1538, TA98, TA100 (1988)
Eschericia coli
strains WP2 and Up to 8000 µg/ml negative Brooks et al.
WP2 uvr A (1988)
Yeast Assays
Saccharomyces cerevisiae JDI Up to 5 mg/ml negative Brooks et al.
mitotic gene conversion (1988)
assay ± rat liver S9
Mammalian cell assays in vitro :
L51784 TK +/- mouse 0.001-100 µl/ml negative Chemical
lymphoma mutation assay (preliminary assay) Manufacturers
(± rat liver S9) 0.4-6 µl/ml negative Association
(1984);
O'Donoghue et
al. (1988)
Primary rat hepatocytes; 0.01-100 µl/ml negative Chemical
unscheduled DNA synthesis Manufacturers
(DNA repair) Association
(1984);
O'Donoghue et
al. (1988)
Cultured rat liver cells Up to 1000 µl/ml negative Brooks et al.
chromosomal damage assay (half the dose for (1988)
RL4 cells 50% inhibition of
cell growth)
Table 6 (contd).
System Dose Response Reference
Balb/3T3; cell transformation 2-5 µl/ml (-S9) inconclusive Chemical
assay ± rat liver S9 1-7 µl/ml (+S9) Manufacturers
Association
(1984);
O'Donoghue et
al. (1988)
Mammalian in vivo assay
Mouse (male and female) 0.73 ml/kg ip negative Chemical
micronucleus assay (maximum tolerated Manufacturers
(polychromatic dose level) Association
erythrocytes) (1984);
O'Donoghue et
al. (1988).
a Preincubation mutation assay incorporating Arochlor-induced rat liver S9
8.6 Mutagenicity and related end-points
A summary of the reported data on MIBK mutagenicity is given
in Table 6.
8.6.1 Bacterial assays
A pre-incubation assay with Salmonella typhimurium (strains
TA98, TA100, TA1537, TAl538) was conducted at dose levels of 0.04
to 4 µg/plate both in the presence and absence of a metabolic
activation system prepared from Aroclor-induced rat liver
homogenate (S9 fraction). Precautions were taken to prevent the
escape of MIBK vapour and assure prolonged exposure of the bacteria
to the test substance. MIBK did not cause an increase in reverse gene
mutation (Chemical Manufacturers Association 1984; O'Donoghue et
al., 1988).
Both MIBK and the oxidative metabolite 4-hydroxy-4-methyl-2-
pentanone (HMP) (see section 6.1) were tested for mutagenicity in
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538, and in Eschericia coli strains WP2 and WP2 uvr A
(Brooks et al., 1988). Aroclor-induced rat liver S9 fraction was
included. No induction of reverse gene mutation was observed up to
a maximum concentration of 8000 µg MIBK/ml (pre-incubation assay in
a sealed container) or 4000 µg HMP/plate (plate incorporation
assay).
8.6.2 Yeast assay for mitotic gene conversions
MIBK and the metabolite HMP were assayed for mitotic gene
conversion using log-phase cultures of the yeast Saccharomyces
cerevisiae JD1 (Brooks et al., 1988). Compounds were tested up to
a concentration of 5 mg/ml in the presence and absence of rat liver
S9 fraction in a sealed container for 18 h. Neither compound
induced mitotic gene conversion.
8.6.3 L5178Y TK+/- mouse lymphoma assay
A preliminary assay was carried out in the presence and absence
of a metabolic activation system at doses of 0.001-100 µl/ml. The
non-activated cultures showed 3 to 157% total relative growth,
while the cultures containing the rat liver S9 fraction had a
relative growth of 23-95% compared with untreated control cultures.
No increase in mutation frequencies was observed in cultures
containing the metabolic activation system, but in the
non-activated cultures, a 2-fold increase above controls was seen
at two non-consecutive doses. An increase in mutation frequency of
approximately 5 times the concurrent control occurred at one test
concentration, but this concentration also caused 97% cell death.
In the absence of a dose-related effect, this result was considered
equivocal. A repeat assay was performed using duplicate cultures
and a narrower range of doses (0.4-6 µl/ml). The total relative
growth ranged from 1 to 80% in non-metabolically activated cells
and from 28 to 63% in cultures that contained the S9 fraction. None
of the activated cultures revealed increased mutation frequencies.
A borderline positive result was found at 6 µl/ml, but different
mutation frequencies occurred in the duplicate cultures and 96-99%
of the cells were killed (Chemical Manufacturers Association, 1984;
O'Donoghue et al., 1988).
8.6.4 Unscheduled DNA synthesis in primary rat hepatocytes in vitro
When MIBK was tested at five dose levels ranging from 0.01
µl/ml to 100 µl/ml in a single assay, there was an increase of less
than 5 fold in labelled nuclear grains in cells treated with MIBK
compared with cells of the solvent control plates (Chemical
Manufacturers Association, 1984; O'Donoghue et al., 1988). Since
the value did not exceed that of the negative control by two
standard deviations of the control value, it was considered that,
under the conditions tested, MIBK did not cause a significant
increase in the nuclear grain count.
8.6.5 Mouse micronucleus assay
Male and female mice were administered MIBK by ip injection at
the maximum tolerated dose level of 0.73 ml/kg body weight, and
bone marrow polychromatic erythrocytes were estimated 12, 24, and
48 h later. There were no significant differences between the
treated and control animals in the ratio of polychromatic to
normochromatic erythrocytes. The number of micronucleated
polychromatic erythrocytes per 1000 cells was not significantly
increased in the MIBK-treated animals (Chemical Manufacturers
Association, 1984; O'Donoghue et al., 1988).
8.6.6 Assay for structural chromosome damage
MIBK (purity > 98.5%) and the metabolite HMP were tested (24-h
exposure) in cultured rat liver cells (RL4) for the ability to
induce chromosomal damage. Metabolic activation with S9 mix was not
used because RL4 cells are metabolically competent. The
concentrations of MIBK employed were 0.125, 0.25, and 0.5 times the
concentration required for 50% inhibition of cell growth. Maximum
concentrations tested were thus 1000 µg MIBK/ml and 4000µg HMP/ml
(this HMP level was equivalent to the concentration required for
>60% growth inhibition). Incubations with MIBK were sealed to
prevent loss by evaporation. MIBK did not produce chromosomal
damage, but HMP gave a small increase (which was not dose related)
in chromatid damage within the concentration range 2000-4000 µg/ml.
It should be noted that HMP did not induce reverse gene mutation in
bacteria or mitotic gene conversion in yeast (Brooks et al., 1988).
8.6.7 Cell transformation assay
In studies reported by the Chemical Manufacturers Association
(1984) and O'Donoghue et al. (1988), MIBK was tested in the
Balb/3T3 (clone A31-1) morphological transformation assay. Doses of
2.4, 3.6, and 4.8 µl MIBK/ml were added to the culture medium in
the absence of a metabolic activation system, and 1, 2, and 4 µl
MIBK/ml were added in the presence of such a system (Aroclorinduced
rat liver S9 fraction). MIBK produced a positive response in the
non-activated cultures only (4.8 µl MIBK per ml gave 3 type III
foci in 15 dishes). A confirmatory study was conducted with doses
of 2, 3, 4, and 5 µl/ml and 4, 5, 6, and 7 µl/ml, respectively, in
the presence and absence of S9 fraction. No significant increase in
the number of transformed foci was found in this study, either in
the presence or absence of the metabolic activation system. Thus,
the effect of MIBK on cell transformation was not reproducible in
the two assays and the ambiguity of the results makes them
unreliable.
8.7 Carcinogenicity
No carcinogenicity studies have been reported.
8.8 Neurotoxicity
In a study by Krasavage et al. (1982), rats were given ip
injections of MIBK, or a mixture of methyl ethyl ketone and MIBK
(9:1 by volume), 5 times/week, for 35 weeks. The dose levels of 10,
30, and 100 mg/kg body weight were doubled after 2 weeks of
treatment. Transient anaesthesia was noted during the first 4 weeks
in the highest dose group, but there was no evidence of peripheral
neuropathy. In dogs administered 300 mg MIBK/kg body weight per day
subcutaneously (sc) for 11 months, electromyographic examination
showed no evidence of neurotoxicity.
Cats treated subcutaneously with 150 mg MIBK/kg body weight per
day or a mixture of methyl ethyl ketone/MIBK (9:1) twice daily, 5
times/week, for up to 8.5 months showed no evidence of nervous
system damage (Spencer & Schaumburg, 1976). In beagle dogs
receiving similar treatment, there were no neurotoxic changes
(Krasavage et al., 1982).
Groups of male rats were exposed to 5330 mg/m3 (1300 ppm)
methyl n -butyl ketone for 4 months or 6150 mg/m3 (1500 ppm)
MIBK for 5 months (Spencer et al., 1975). Methyl n -butyl ketone
produced a toxic distal axonopathy. MIBK produced minimal distal
axonal changes, but 3% methyl n -butyl ketone was present as a
contaminant in the MIBK, and the design of the cages used may have
caused compression neuropathy (Spencer et al., 1975; Spencer &
Schaumburg, 1976). Animals exposed to MIBK showed slight signs of
narcosis, but body weight gain was normal, and, at 5 months, there
were no clinical signs of neurological dysfunction. Rats exposed
for 3 h to 102 mg MIBK/m3 (25 ppm) showed a 58% increase in
pressor lever response, which had not returned to control levels 7
days after the end of exposure (Geller et al., 1978). The maximum
motor-fibre conduction velocity in the tail nerve decreased
markedly when male rats were treated with methyl n -butyl ketone
(401 mg/kg, 5 times/week for 55 weeks) but not when they were
treated with MIBK (601 mg/kg, 5 times/week for 55 weeks). However,
treatment with MIBK (201 mg/kg) facilitated the neurotoxic effect
of methyl n -butyl ketone (401 mg/kg) possibly due to the
demonstrated ability of MIBK to increase the metabolic activity of
10 000 g liver supernatants towards both MIBK and methyl n -butyl
ketone (Nagano et al., 1988).
Discriminatory behaviour and memory in baboons was not affected
by exposures of 82-164 mg/m3 (20-40 ppm) (Geller et al., 1978).
Geller et al. (1979) reported an effect on accuracy of performance
of tasks in a ``delayed match-to-sample discrimination-test'' in
baboons exposed for 7 days to 205 mg/m3 (50 ppm) MIBK, but there
was no change in response when MIBK was combined with methyl ethyl
ketone at 295 mg/m3 (100 ppm).
De Ceaurriz et al. (1984) exposed male Swiss OF1 mice to an
atmosphere containing MIBK and measured the total duration of
immobility during a 3-min ``behavioural despair'' swimming test. At
concentrations of 2714, 3104, 3309, and 3657 mg/m3 (662, 757,
807, and 892 ppm), a dose-dependent decrease of mobility was
observed (25, 38, 46, and 70% respectively). The authors noted that
the mean active level of MIBK was lower for this neurobehavioural
effect (3292 mg/m3 (893 ppm) for 50% inhibition of immobility)
than for an effect on sensory irritation (13 100 mg/m3 (3195 ppm)
for 50% inhibition of respiratory rate) (De Ceaurriz et al., 1981,
section 8.3.3).
In inhalation studies on hens (five per group) into the effect
of MIBK on n -hexane-induced neurotoxicity, a continuous exposure
period of 90 days was followed by a 30-day observation period
(Abou-Donia et al., 1985b). One group of hens was exposed to 3520
mgnw-hexane/m3 (1000 ppm) and another group to 4100 mg MIBK/m3
(1000 ppm). Four additional groups were exposed simultaneously to
3520 mg n -hexane/m3 (1000 ppm) and 410, 1025, 2050, or 4100 mg
MIBK/m3 (100, 250, 500, or 1000 ppm, respectively). A control
group was exposed to ambient air in an exposure chamber. Hens
continuously exposed to 4100 mg MIBK/m3 developed weakness of the
legs with subsequent recovery. Inhalation of 3520 mg n
-hexane/m3 produced mild ataxia. Exposure to 3520 mg n
-hexane/m3 together with 1025, 2050, or 4100 mg MIBK/m3
resulted in signs of neurotoxicity including paralysis, the
severity of which depended on the MIBK concentration. Hens
continuously exposed to the 3520/410 mg/m3 n -hexane/MIBK
mixture exhibited severe ataxia throughout the observation period.
Histopathological examination of hens exposed to the n
-hexane/MIBK mixture showed large swollen axons and degeneration
of the axon and myelin of the spinal cord and peripheral nerves.
There were no histopathological abnormalities in the central
nervous system of hens exposed only to MIBK. This demonstrates that
MIBK potentiates the neurotoxic action of n -hexane. In a
subsequent study, in which hens were exposed for 50 days to MIBK at
4100 mg/m3 (1000 ppm) or to n -hexane, it was suggested that
the potentiation by MIBK of n -hexane neurotoxicity is related to
the induction by MIBK of liver microsomal cytochrome P-450,
resulting in increased metabolism of n -hexane to its neurotoxic
metabolites (sections 6.1, 8.2.1). The findings also suggest that
the neurotoxicity of technical methyl butyl-ketone (methyl n
-butyl ketone/MIBK (7/3)) was correctly attributed to the methyl
n -butyl ketone component (Abdo et al., 1982). Simultaneous
treatment of hens with 41, 205, or 410 mg/m3 (10, 50, or 100 ppm)
technical methyl butyl ketone, 5 days/week for 90 days, with a
dermal application of technical grade 0 -ethyl- 0 -4-nitrophenyl
phenylphosphonothionate (EPN, 1.0 mg/kg, 85%) greatly enhanced the
neurotoxic effects. It was proposed that MIBK partially contributed
to this potentiation by inducing cytochrome P-450 and thus
enhancing the formation of neurotoxic products from methyl- n
-butyl ketone and EPN (Abou-Donia et al., 1985a).
The effect of MIBK on the duration of ethanol-induced loss of
righting reflex and on ethanol elimination has been studied in
mice. MIBK was dissolved in corn oil and injected ip 30 min before
ethanol (4 g/kg ip). At a dose of 501 mg/kg, MIBK significantly
prolonged the duration of ethanol-induced loss of righting reflex.
The concentrations of ethanol in blood and brain on return of the
righting reflex were similar in MIBK-treated and control animals
(Cunningham et al., 1989).
8.9 In vitro toxicity assays
In contrast to methyl n -butyl ketone and n -hexane, MIBK
caused little or no cytopathological or growth-inhibiting effects
in cultured mouse neuroblastoma cells (Selkoe et al., 1978). MIBK
in N,N -dimethylacetamide reduced the activity of mouse liver
alcohol dehydrogenase in vitro (Cunningham et al., 1989). MIBK
was found to inhibit the sulfhydryl-dependent creatine kinase and
adenylate kinase enzymes in vitro but not to the same extent as
did the neurotoxic agent, methyl- n -butyl ketone (Lapin et al.,
1982).
9. EFFECTS ON MAN
9.1 Acute toxicity
In a study on the sensory threshold, Silverman et al. (1946)
exposed 12 volunteers of both sexes to various concentrations of
MIBK for a 15-min period. This period permitted an accurate
observation of olfactory fatigue and increasing or decreasing
irritation of mucous membranes. The sensory response limit was 410
mg/m3 (100 ppm). The majority of the subjects found the odour
objectionable at 820 mg/m3 (200 ppm), and the vapour irritated
the eyes. The low odour threshold (1.64 mg/m3) (Ruth, 1986) and
the irritant effects can provide warning of high concentrations.
Because of its low viscosity, MIBK may, when swallowed, also be
aspirated into the lungs causing a chemical pneumonitis (Panson &
Winek, 1980).
9.2 Short-term exposure
Workers exposed to 410 mg MIBK/m3 (100 ppm) complained either
of headache and nausea or of respiratory irritation (Elkins, 1959).
Tolerance was said to be acquired during the working week but was
lost over the weekend. Reduction of the exposure to 82 mg/m3 (20
ppm) largely eliminated the complaints. In the study of Hjelm et
al. (1990) (see section 6.3) on human volunteers, CNS symptoms
(headache and/or vertigo and/or nausea) were reported at 2-h
exposure levels of 10-200 mg MIBK/m3 (2.4-48.8 ppm). There were
no significant effects from exposure on the performance of a
reaction time task or a test of mental arithmetic.
9.3 Eye and respiratory irritation
Exposure to a concentration of 820 mg MIBK/m3 (200 ppm) for
a 15-min period caused eye irritation in 12 human volunteers
(Silverman et al., 1946). Undiluted MIBK splashed in the eyes may
cause painful irritation (Shell, 1957). A group of workers exposed
to 410 mg MIBK/m3 (100 ppm) complained of respiratory tract
irritation, but there were no complaints at 82 mg/m3 (20 ppm)
(Elkins, 1959). In the study of Hjelm et al. (1990) (see section
6.3) on human volunteers, irritation particularly of the nose and
throat was reported at 2-h exposure levels of 10, 100, and 200
mg/m3 (2.4, 24.4, and 48.8 ppm).
9.4 Long-term exposure
In workers exposed to up to 2050 mg MIBK/m3 (500 ppm) for
20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, over half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomiting, and sore throat. A few of the
workers experienced insomnia, somnolence, heartburn, intestinal
pain, and some unsteadiness. Four workers had slightly enlarged
livers and six had a nonspecific colitis. Clinical chemistry
examination revealed no abnormalities in any of the workers. Five
years later, work practices had greatly improved, the highest MIBK
concentration was 410-430 mg/m3 (100-105 ppm), and the general
concentration was 205 mg/m3 (50 ppm). A few workers still
complained of gastrointestinal and central nervous system effects,
and slight liver enlargement had persisted in two workers, but
other symptoms had disappeared (Armeli et al., 1968).
9.5 Placental transfer
MIBK was detected in maternal and umbilical cord blood samples
from 11 patients (Dowty et al., 1976).
9.6 Neurotoxicity
A few isolated cases of peripheral neuropathy have been
reported after exposure to spray paint or lacquer thinner that
apparently contained MIBK and other hydrocarbon solvents, including
neurotoxic agents (Oh & Kim, 1976; Aubuchon et al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of effects on the environment
MIBK is not likely to persist in the environment. It will
slowly volatilize from soil and water and is readily biodegraded in
fresh and salt water. In the atmosphere, MIBK is estimated to be
degraded by OHÊ radicals with a half-life of approximately 14 h.
MIBK is not expected to bioaccumulate and has a low toxicity for
microorganisms, fish, algae, and aquatic invertebrates. Only in
cases of accidental spillage or inappropriate disposal of wastes
into the environment are levels of MIBK likely to cause toxicity to
organisms in the environment.
10.2 Evaluation of health risks for humans
The general population is exposed to low levels of MIBK. Only
small quantities have been detected in food, drinking-water, and
other beverages (baked goods, 10.9 mg/kg; frozen dairy products,
11.5 mg/kg; gelatins, puddings, 10.9 mg/kg; beverages, 10.2 mg/kg).
For general population exposure, maximum ambient air concentrations
in the range of 0.1 to 0.2 mg/m3 have been defined by two
countries.
Occupational exposure occurs particularly in the production and
use of lacquers, paints, and extraction solvents. The major route
of entry is by inhalation. The low odour threshold (1.64 mg/m3)
and the irritant effects can provide warning of high
concentrations. Exposure to levels of 10-410 mg/m3 (2.4-100 ppm)
produced perceptible irritation of either the eyes, nose, or
throat, and 820 mg/m3 (200 ppm) produced discomfort. Symptoms
such as headache, nausea, and vertigo also occurred at a level of
10-410 mg/m3 (2.4-100 ppm). There were no significant effects
from a 2-h exposure of up to 200 mg/m3 (50 ppm) on a simple
reaction time task or test of mental arithmetic.
In the single report concerning long-term occupational
exposure, where workers were exposed to 2050 mg MIBK/m3 (500 ppm)
for 20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, more than half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomitting, and sore throat. A few workers
experienced insomnia, somnolence, and some unsteadiness. Four had
slightly enlarged livers and six had nonspecific colitis. Five
years later, work practices had greatly improved and the highest
concentrations were reduced to about one fifth of the previous
level. A few workers still complained of irritation of the eyes and
upper respiratory tract as well as gastrointestinal and central
nervous system symptoms. Prolonged skin contact with MIBK caused
irritation and flaking of the skin.
In animal studies, acute systemic MIBK toxicity is low by the
oral and inhalation routes. In a 90-day study, Sprague-Dawley rats
were given MIBK by gavage at doses of 50, 250, or 1000 mg/kg body
weight per day. Lethargy was noted in the highest-dose group and
males showed reduced body weight gain. In this group there was
generalized nephropathy, with an increase in relative kidney weight
and hepatomegaly. Relative kidney weight was also increased in the
animals fed 250 mg/kg per day, and slight hepatomegaly was reported
in male rats only. There were no histopathological lesions in the
liver or other tissues at any dose level. It was concluded that the
NOEL was 50 mg/kg per day. In 90-day inhalation studies on rats and
mice, concentrations of up to 4100 mg/m3 (1000 ppm) did not
result in any life-threatening signs of toxicity. However,
compound-related reversible morphological changes in the liver and
kidney were reported. Levels of 4100 mg/m3 produced evidence of
central nervous system depression. MIBK was capable of increasing
liver weight (at > 1025 mg/m3 (250 ppm)) and inducing hepatic
microsomal metabolism. This may be the explanation for the
exacerbation of haloalkane toxicity and the potentiation of the
neurotoxicity of n -hexane. In 90-day studies with mice, rats,
dogs, and monkeys, only male rats developed hyaline droplets in the
proximal tubules of the kidney (hyaline droplet toxic tubular
nephrosis). This effect in male rats was reversible and of doubtful
significance for humans. MIBK reduces the activity of mouse liver
alcohol dehydrogenase in vitro . It has also been found to
potentiate the cholestatic effects of manganese given with or
without bilirubin.
In rats and mice exposed to MIBK by inhalation at
concentrations of 1230, 4100, or 12 300 mg/m3 (300, 1000, or 3000
ppm) on days 6 to 15 of gestation and sacrificed on day 21 (rats)
or day 18 (mice), marked maternal toxicity was observed at the
highest concentration in both species. This concentration produced
fetotoxicity (reduced fetal body weight and delayed ossification)
but was not embryotoxic or teratogenic. At 4100 and 1230 mg/m3
there was no maternal toxicity and no evidence of embryotoxicity,
fetotoxicity, or teratogenicity.
MIBK did not induce gene mutation in bacterial test systems
(Salmonella typhimurium and Escherichia coli ) either with or
without metabolic activation. Negative results were also obtained
in tests (both with and without metabolic activation) for mitotic
gene conversion in yeast ( Saccharomyces cerevisiae ) and in gene
mutation tests using cultured mammalian cells (mouse lymphoma). In
vitro assays for unscheduled DNA synthesis in primary rat
hepatocytes and for structural chromosome damage in cultured rat
liver cells (RL4) were negative. An in vivo micronucleus test in
mice was negative. These data indicate that MIBK is not genotoxic.
11. RECOMMENDATIONS
At the levels of MIBK to which the general human population is
exposed, there is unlikely to be any hazard. In the occupational
health context, where the major route of exposure is by inhalation,
atmospheric levels should be kept below the recommended
occupational exposure limits by suitably designed work processes
and engineering controls, including ventilation. Skin and eye
contamination should be avoided. Suitable protective clothing and
respiratory protection should be readily available for use in
enclosed spaces, in emergencies, and for certain maintenance
operations. MIBK is inflammable and should be handled accordingly.
MIBK has low toxicity for microorganisms and fish, and its
half-life in the environment is short. Consequently, there is no
risk to the environment provided there are adequate controls to
minimize emissions. Large-scale release could have local adverse
effects on the environment.
12. FURTHER RESEARCH
1. MIBK affects a number of enzyme systems. Therefore, it can
significantly influence the biotransformation of xenobiotics that
are metabolized by these enzymes. Since humans are usually exposed
to more than one compound, studies on the combined effects of
mixtures containing MIBK should be undertaken.
2. There is very little information available on the dose-response
relationships for the effects of MIBK on the human central nervous
system (e.g., reaction time, behavioural effects), on the upper
airways and mucous membranes, or on kidney function. More
information on toxico-kinetics is needed for MIBK alone and in
mixture with other solvents. The skin penetration of MIBK should be
assessed.
3. Epidemiological studies should be undertaken to elucidate the
effects on the nervous system of long-term exposure to moderate
concentrations of MIBK alone or in mixture with other solvents.
REFERENCES
ABDO, K.M., GRAHAM, D.G., TIMMINS, P.R., & ABOU-DONIA, M.B. (1982)
Neurotoxicity of continuous (90 days) inhalation of technical grade
methyl butyl ketone in hens. J. Toxicol. environ. Health, 9:
199-215.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & ABDO, K.M.
(1985a) The joint neurotoxic action of inhaled methyl butyl ketone
vapour and dermally applied 0-ethyl 0-4-nitrophenyl
phenylphosphonothioate in hens: potentiating effect. Toxicol. appl.
Pharmacol., 79: 69-82.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & TIMMONS, P.R.
(1985b) The synergism of n -hexane-induced neurotoxicity by
methyl isobutyl ketone following subchronic (90 days) inhalation in
hens: induction of hepatic microsomal cytochrome P-450. Toxicol.
appl. Pharmacol., 81: 1-16.
ANALYTICAL QUALITY CONTROL (1972) Handbook for analytical quality
control in water and waste water laboratories, Cincinnati, Ohio,
National Environmental Research Centre.
ARMELI, G., LINARI, F., & MARTORANO, G. (1968) [Clinical and
haematochemical examinations in workers exposed to the action of a
higher ketone (MIBK) repeated after 5 years.] Lav. Um., 20:
418-424 (in Italian).
ATKINSON, R., ASCHMANN, S.M., CARTER, W.P.L., & PITTS, J.N., Jr
(1982) Rate constants for the gas-phase reaction of OH radicals
with a series of ketones at 299 ± 2 ° K. Int. J. chem. Kinet.,
14: 839-847.
AUBUCHON, J., ROBINS, H.I., & VISESKUL, C. (1979) Peripheral
neuropathy after exposure to methyl isobutyl ketone in spray paint.
Lancet, August 18: 363-364.
AUSTERN, B.M., DOBBS, R.A., & COHEN, J.M. (1975)
Gas-chromatographic determination of selected organic compounds
added to wastewater. Environ. Sci. Technol., 9: 588-590.
BAMBERGER, R.L., ESPOSITO, G.G., JACOBS, B.W., PODOLAK, G.E., &
MAZUR, J.F. (1978) A new personal sampler for organic vapors. Am.
Ind. Hyg. Assoc. J., 39: 701-708.
BASU, P., CARPENTER, J., CHEN, C., NELSON, H., PERRY, W., SHOCLET,
A., TAYLOR, D., & ZAFRAN, F. (1968) Human exposure assessment:
Methyl isobutyl ketone, Washington, DC, US Environmental Protection
Agency (EPA contract 68-01.4839).
BATYROVA, T.F. (1973) Substantiation of the maximum permissible
concentration of methylisobutyl ketone in air or workrooms. Gig.
Tr. prof. Zabol., 17(11): 52-53.
BELLANCA, J.A., DAVIS, P.L., DONNELLY, B., DAL CORTIVO, L.A., &
WEINBERG, S.B. (1982) Detection and quantitation of multiple
volatile compounds in tissues by GC and GC/MS. J. anal. Toxicol.,
6: 238-240.
BRANCHFLOWER, R.V., SCHULICK, R.D., GEORGE, J.W., & POHL, L.R.
(1983) Comparison of the effects of methyl n -butyl ketone and
phenobarbital on rat liver cytochromes P-450 and the metabolism of
chloroform to phosgene. Toxicol. appl. Pharmacol., 71: 414-421.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER M. (1979a) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUHN, R. (1977a) [Findings concerning the harmful
effect of water-endangering substances on, Daphnia magna .] Z.
Wasser Abwasser Forsch., 10: 161-166 (in German).
BRINGMANN, G. & KUHN, R. (1977b) [Limit values for the harmful
effect of water-endangering substances on bacteria ( Pseudomonas
putida ) and green algae ( Scenedesmus quadricauda ).] Z. Wasser
Abwasser Forsch., 10: 87-98 (in German).
BRINGMANN, G. & KUHN, R. (1978) [Limit values for the harmful
effect of water-endangering substances on blue-green algae
( Microcystis aeruginosa ) and green algae ( Scenedesmus
quadricauda ) in the cell multiplication inhibitor test.] Vom
Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effects of
pollutants on flagellates and ciliates, and/or on holozoic
bacteria-eating and saprozoic protozoa.] GWF-Wasser Abwasser,
122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna straus tested by an approved
standardized procedure.] Z. Wasser Abwasser Forsch., 15: 1-6 (in
German).
BRONDEAU, M.T., BAN, M., BONNET, P., GUENIER, J.P., & DE CEAURRIZ,
J. (1989) Acetone compared to other ketones in modifying the
hepatotoxicity of inhaled 1,2-dichlorobenzene in rats and mice.
Toxicol. Lett. 49: 69-78.
BROOKS, T.M., MEYER, A.L., & HUTSON, D.H. (1988) The genetic
toxicology of some hydrocarbon and oxygenated solvents.
Mutagenesis, 3: 227-232.
BROWN, K.W. & DONNELLY, K.C. (1988) An estimation of the risk
associated with the organic constituents of hazardous and municipal
waste landfill leachates. Hazardous Waste Hazardous Mater., 5(1):
1-30.
BROWN, R.H. & PURNELL, C.J. (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. J. Chromatogr.,
178: 79-90.
BURNHAM, A.K., CALDER, G.V., FRITZ, J.S., JUNK, G.A., SVEC, H.J.,
& WILLIS, R. (1972) Identification and estimation of neutral
organic contaminants in potable water. Anal. Chem., 44: 139-142.
CALL, D.J., BROOKE, L.T., KNUTH, M.L., POIRIER, S.H., & HOGLUND,
M.D. (1985) Fish subchronic toxicity prediction model for
industrial organic chemicals that produce narcosis. Environ.
Toxicol. Chem., 4: 335-341.
CEC (1976) Analysis of organic micropollutants in water,
Luxembourg, Commission of the European Communities (Cost 64b bis).
CFR (1987) Code of Federal Regulations. Methods of analysis for
organic chemicals in groundwater at hazardous waste sites,
Washington, DC, US Government Printing Office (Appendix IX, 40 CFR,
Part 264).
CHEMICAL MANUFACTURERS ASSOCIATION (1984) Ketones Program Panel,
Vol. 1 - Methyl isobutyl ketone: Mutagenicity and teratology
studies, Washington, DC, Chemical Manufacturers Association.
CORWIN, J.F. (1969) Volatile oxygen-containing organic compounds in
sea water: determination. Bull. mar. Sci., 19: 504-509.
COX, R.A., DERWENT, R.G., & WILLIAMS, M.R. (1980) Atmospheric
photooxidation reactions. Rates, reactivity and mechanism for
reaction of organic compounds with hydroxyl radicals. Environ. Sci.
Technol., 14: 57-61.
CUNNINGHAM, J., SHARKAWI, M., & PLAA, G.L. (1989) Pharmacological
and metabolic interactions between ethanol and methyl n -butyl
ketone, methyl isobutyl ketone, methyl ethyl ketone, or acetone in
mice. Fundam. appl. Toxicol., 13: 102-9.
DE CEAURRIZ, J., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J., MICILLINO, J.C., MARIGNAC, B., BONNET, P., MULLER,
J., & GUENIER, J.P. (1984) Quantitative evolution of sensory
irritating and neurobehavioural properties of aliphatic ketones in
mice. Food Chem. Toxicol., 22, 545-549.
DICK, R., DANKOVIC, D., SETZER, J., PHIPPS, F., & LOWRY, L. (1990)
Body burden profiles of methyl ethyl ketone and methyl isobutyl
ketone exposure in human subjects. Toxicologist, 10: 122.
DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic
solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
DIVINCENZO, G.D., KAPLAN, C.J., & DEDINAS, J. (1976)
Characterization of the metabolites of methyl n -butyl ketone,
methyl iso-butyl ketone and methyl ethyl ketone in guinea pig serum
and their clearance. Toxicol. appl. Pharmacol., 36: 511-522.
DODD, D.E. & EISLER, D.L. (1983) Methyl isobutyl ketone ninety-day
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 46-504
submitted to US EPA).
DODD, D.E., LONGO, L.C., & EISLER, D.L. (1982) Nine-day vapour
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 45-501
submitted to US EPA).
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic
constituents. Pediatr. Res., 10: 696-701.
ECDIN (1990) Data bank on environmental chemicals, Ispra (Varese),
Establishment, Joint Research Centre of the Commission of the
European Communities.
ELKINS, H.B. (1959) Chemistry of industrial toxicology, New York,
John Wiley and Sons, p. 121.
ELLISON, W.K. & WALLBANK, T.E. (1974) Solvents in sewage and
industrial waste waters. Identification and determination. Water
Pollut. Control, 73: 656-672.
ELOFSSON, S.A., GAMBERALE, F., HINDMARSH, T., IREGREN, A.,
ISAKSSON, A., JOHNSSON, I., KNAVE, B., LYDAHL, E., MINDUS, P.,
PERSSON, H.E., PHILIPSON, B., STEBY, M., STRUWE, G., SODERMAN, E.,
WENNBERG, A., & WIDEN, L. (1980) Exposure to organic solvents: a
cross-sectional epidemiologic investigation on occupationally
exposed car and industrial spray painters with special reference to
the nervous system. Scand. J. Work Environ. Health, 6: 239-273.
FAWELL, J.K. & HUNT, S. (1981) Organic micropollutants in drinking
water, Medmenham, Water Research Centre (Technical Report No. 159).
FERNANDES, M. (1985) Methodology for the analysis of volatile
compounds in food packaging materials. Coletanea Inst. Technol.
Aliment., 15: 49-59.
FIRE PREVENTION (1981) Information sheets on hazardous materials,
London, London Fire Protection Association, p. 47 (H97 No. 140).
FRANCIS, A.J., IDEN, G.T., NINE, B.J., & CHANG, C.K. (1980)
Characterization of organics in leachates from low level
radioactive waste disposal sites. Nucl. Technol., 50: 158-163.
FROSTLING, H., HOFF, A., JACOBSSON, S., PFAFFLI, P., VAINIOTALO,
S., ZITTING, A., & TECHN, D. (1984) Analytical, occupational and
toxicologic aspects of the degradation products of polypropylene
plastics. Scand. J. Work Environ. Health., 10: 163-169.
GARMAN, J.R., FREUND, T., & LAWLESS, E.W. (1987) Testing for
groundwater contamination at hazardous waste sites. Chromatogr.
Sci., 25: 328-344.
GELLER, I., ROWLANDS, J.R., & KAPLAN, H.L. (1978) Effects of
ketones on operant behaviour of laboratory animals. In: Voluntary
inhalation of industrial solvents, Washington, DC, US Department of
Health, Education and Welfare, p. 363 (DHEW Publication No.
79-779).
GELLER I., GAUSE, E., KAPLAN, H., & HARTMANN, R.J. (1979) Effects
of acetone, methyl ethyl ketone, and methyl isobutyl ketone on a
match-to-sample task in the baboon. Pharmacol. Biochem. Behav.,
11: 401-406.
HAMPTON, C.V., PIERSON, W.R., HARVEY, T.M., UPDEGROVE, W.S., &
MARANO, R.S. (1982) Hydrocarbon gases emitted from vehicles on the
road. 1. A qualitative gas chromatography/mass spectroscopy survey.
Environ. Sci. Technol., 16: 287-298.
HANNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioural effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HJELM, E.W., HAGBERG, M., IREGREN, A., & LÖF, A. (1990) Exposure to
methyl isobutyl ketone: toxicokinetics and occurrence of irritative
and CNS symptoms in man. Int. Arch. occup. environ. Health, 62:
19-26.
IRPTC (1990) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JUHNKE, I. & LÜDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute fish toxicity in the orfe test.] Z.
Wasser Abwasser Forsch., 11(5): 161-164 (in German).
KEITH, L.H. (1974) Chemical characterization of industrial waste
waters by gas chromatography-mass spectrometry. Sci. total
Environ., 3: 87-102.
KRASAVAGE, W.J., O'DONOGHUE, J.L., & DIVINCENZO, G.D. (1982) Methyl
isobutyl ketone. In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, New York, John Wiley and Sons,
Vol. 2e, pp. 4747-4751.
KRISTENSSON, J. & BEVING, H. (1987) A study of painters
occupationally exposed to water and solvent based paints,
Luxembourg, Commission of the European Communities, pp. 71-72 (EUR.
10555).
LANDE, S.S., DURKIN, P.R., CHRISTOPHER, D.H., HOWARD, P.H., &
SAXENA, J. (1976) Investigation of selected potential environmental
contamination: ketonic solvents, Syracuse, New York, Center for
Chemical Hazard Assessment Research Corporation, p. 252.
LAPIN, E.P., WEISSBARTH, S., MAKER, H.S., & LEHRER, G.M. (1982) The
sensitivities of creatine and adenylate kinases to the neurotoxins
acrylamide and metyl n-butyl ketone. Environ. Res., 28: 21-31.
LEO, A. & WEININGER, D. (1984) Medicinal chemistry report,
Claremont, California, Pomona College.
LEVIN, J.-O. & CARLEBORG, L. (1987) Evaluation of solid sorbents
for sampling ketones in work-room air. Ann. occup. Hyg., 31:
31-38.
LIPNICK, R.L., WATSON, K.R., & STRAUSZ, A.K. (1987) A QSAR study of
the acute toxicity of some industrial organic chemicals to
goldfish. Narcosis, electrophile and proelectrophile mechanisms.
Xenobiotica, 17, 1011-1025.
MACEWEN, J.D., VERNOT, E.H., & HAUN, C.C. (1971) Effects of 90-day
continous exposure to methyl isobutyl ketone on dogs, monkeys, and
rats, Ohio, Wright-Patterson AFB, Aerospace Medical Research
Laboratory (Report No. AMRL TR-71-65).
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 7: 611- 614.
MALYSCHEVA, M.V. (1988) [The effect of the skin route of
administration of methyl isobutyl ketone on its toxicity.] Gig. i
Sanit., 10: 79-80 (in Russian).
MICROBIOLOGICAL ASSOCIATES (1986) Subchronic toxicity of methyl
isobutyl ketone in Sprague-Dawley rats. Preliminary report,
Research Triangle Park, North Carolina, Research Triangle Park
Institute, (Study No. 5221.04).
MITI (1978) The biodegradability and bioaccumulation of new and
existing chemical substances, Tokyo, Ministry of International
Trade and Industry, Chemical Products Safety Division, Basic
Industries Bureau.
MOSHLAKOVA, L.A., & INDINA, T.V. (1986) [Gas chromatographic
determination of ketones present simultaneously in the air of the
work area and in the washings from workers' skin. Gig. i Sanit.,
2: 90-91 (in Russian).
NAGANO, M., HARADA, K., MISUMI, J., & NOMURA, S. (1988) [Effect of
methyl isobutyl ketone on methyl n-butyl ketone neurotoxicity in
rats.] Sangyo Igaku, 30: 50-51 (in Japanese).
NIOSH (1984) NIOSH manual of analytical methods: ketones I, 3rd
ed., Cincinnati, Ohio, US National Institute for Occupational
Safety and Health,Vol. 2 (No. 1300).
NTIS (1985) Scientific literature review of aliphatic ketones,
secondary alcohols and related esters in flavour usage. Volume 1 -
Introduction and summary, tables of data bibliography: Al,
Washington, DC, National Technical Information Service, Part 2
(PB85-141059).
O'DONOGHUE, J.L., HAWORTH, S.R., CURREN, R.D., KIRBY, P.E., LAWLOR,
T., MORAN, E.J., PHILLIPS, R.D., PUTNAM, D.L., ROGERS-BACK, A.M.,
SLESINSKI, R.S., & THILAGAR, A. (1988) Mutagenicity studies on
ketone solvents: methyl ethyl ketone, methyl isobutyl ketone and
isophorone. Mutat. Res., 206: 149-161.
OECD (1977) The asessment of environmental chemicals: production
figures and use patterns for some high volume chemicals, Paris,
Organization for Economic Cooperation and Development
(ENV/Chem./77.6).
OECD (1984) Data interpretation guides for initial hazard
assessment of chemicals (provisional), Paris, Organisation for
Economic Cooperation and Development, p. 31.
OH, S.J. & KIM, J.M. (1976) Giant axonal swelling in "Huffer's"
neuropathy. Arch. Neurol., 33: 583-586.
PANSON, R.D. & WINEK, C.L. (1980) Aspiration toxicity of ketones.
Clin. Toxicol., 17: 271-317.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERICKSON, M.D. (1982) Purgeable organic compounds
in mothers' milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PHILLIPS, R.D., MORAN, E.J., DODD, D.E., FOWLER, E.H., KARY, C.D.,
&O'DONOGHUE, J. (1987) A 14-week vapor inhalation toxicity study of
methyl isobutyl ketone. Fundam. appl. Toxicol., 9: 380-388.
PILON, D. (1987) Interaction cétones/hydrocarbures halogènes:
Utilization des métabolites cétoniques comme indices d'exposition
aux cétones, Montreal, University of Montreal (Ph.D. Thesis).
PILON, D., BRODEUR, J., & PLAA, G.L. (1988) Potentiation of carbon
tetrachloride-induced liver injury by ketonic and ketogenic
compounds: role of the CCl4 dose. Toxicol. appl. Pharmacol.,
94: 183-190.
PLAA, G.L. & AYOTTE, D. (1985) Taurolithocholate-induced
intrahepatic cholestasis: potentiation by methyl isobutyl ketone
and methyl n-butylketone in rats. Toxicol. appl. Pharmacol., 80:
228-234.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimps
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77
RACCIO, J.M. & WIDOMSKI, J.R. (1981) Quality control of flavors in
soft drinks and the analysis of residual solvents in food packaging
films utilizing headspace sampling with open tubular columns.
Chromatogr. Newsl., 9(2): 42-45.
RIPPSTEIN, W.J. & COLEMAN, M.E. (1984) [Toxicological evaluation on
the Columbian spacecraft.] Kosmet. Biol. Aviakosm. Med., 18:
87-96 (in Russian).
RTECS (1987) Registry of toxic effects of chemical substances,
1985-86 ed., Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Vol. 1-6 (DHSS (NIOSH) Publication No. 87-114).
RUTH, J.H. (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am. Ind. Hyg. Assoc. J., 47:
A142-A151.
SABROE, S. & OLSEN, J. (1979) Health complaints and work conditions
among lacquerers in the Danish furniture industry. Scand. J. soc.
Med., 7: 97-104
SATO, A. & NAKAJIMA, T. (1979) Partition coefficients of some
aromatic hydrocarbons and ketones in water, blood and oil. Brit. J.
ind. Med., 36, 231-234.
SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13: 349-352.
SAX, N.I. (1979) Dangerous properties of industrial materials, 6th
ed, New York, Van Norstrand Reinhold Company, p. 750.
SELKOE, D.J., LUCKENBILL-EDDS, L., & SHELANSKI, M.L. (1978) Effects
of neurotoxic industrial solvents on cultured neuroblastoma cells:
methyl n -butyl ketone, n -hexane, and derivatives. J.
Neuropathol. exp. Neurol., 37: 768-789.
SHELL (1957) Methyl isobutyl ketone: industrial hygiene bulletin,
New York, Shell Chemical Corporation, pp. 57-112.
SILVERMAN, L., SCHULTE, H.F., & FIRST, M.W. (1946) Further studies
on sensory response to certain industrial solvent vapors. J. ind.
Hyg. Toxicol., 28: 262-266.
SMYTH, H.F. (1956) Hygienic standards for daily inhalation. Am.
Ind. Hyg. Assoc. J., 17: 129-266.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SPECHT, H. (1938) Acute response of guinea pigs to inhalation of
methyl isobutyl ketone, Washington, DC, US Public Health Service,
pp. 292-300 (US Public Health Report No. 53).
SPECHT, H., MILLER, J.W., VALAER, P.J., & SAYERS, R.R. (1940) Acute
response of guinea pigs to the inhalation of ketone vapours,
Washington, DC, US Public Health Service, Division of Industrial
Hygiene (NIH Bulletin No. 176).
SPENCER, P.S. & SCHAUMBURG, H.H. (1976) Feline nervous system
response to chronic intoxication with commercial grades of methyl
n -butyl ketone, methyl isobutyl ketone and methyl ethyl ketone.
Toxicol. appl. Pharmacol., 37: 301-311.
SPENCER, P.S., SCHAUMBURG, H.H., RALEIGH, R.L., & TERHAAR, C.J.
(1975) Nervous system degeneration produced by the industrial
solvent methyl n -butyl ketone. Arch. Neurol., 32: 219-222.
TNO (1983a) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 2.
TNO (1983b) Volatile compounds in food: Qualitative data, Zeist,
Netherlands, Organization for Applied Scientific Research.
TNO (1986) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 5.
TNO (1987) Volatile compounds in food: Supplement 4, Zeist,
Netherlands, Organization for Applied Scientific Research.
TOMCZYK, H. & ROGACZEWSKA, T. (1979) [Gas chromatographic
determination of airborne methyl isobutyl ketone, methyl isobutyl
carbinol, acetone, toluene and o-xylene.] Med. Pr., XXX(6):
417-423 (in Polish).
TYL, R.W. (1984) A teratologic evaluation of methyl isobutyl ketone
in Fischer 344 rats and CD-1 mice following inhalation exposure,
Washington, DC, Chemical Manufacturers Association (Bushy Run
Research Center Report No. 47.505).
TYL, R.W., FRANCE, K.A., FISHER, L.C., PRITTS, I.M., TYLER, T.R.,
PHILLIPS, R.D., & MORAN, E.J. (1987) Developmental toxicity
evaluation of inhaled methyl isobutyl ketone in Fischer 344 rats
and CD-l mice. Fundam. appl. Toxicol., 8: 319-327.
VERNOT, E.H., MACEWEN, J.D., & HARRIS, E.S. (1971) Continuous
exposure of animals to methyl isobutyl ketone, Ohio, Wright
Patterson AFB, Aerospace Medical Research Laboratory (US NTIS AD
Report No. 751443).
VERSCHUEREN, K. (1983) Handbook of environmental data on organic
chemicals, 2nd ed., New York, Van Nostrand Reinhold Company, pp.
459-461.
VEZINA, M. & PLAA, G.L. (1987) Potentiation by methyl isobutyl
ketone of the cholestasis induced in rats by a manganese-bilirubin
combination or manganese alone. Toxicol. appl. Pharmacol. 91:
477-483.
VEZINA, M. & PLAA, G.L. (1988) Methyl isobutyl ketone metabolites
and potentiation of the cholestasis induced in rats by a
manganese-bilirubin combination or manganese alone. Toxicol. appl.
Pharmacol. 92: 419-427.
VEZINA, M., AYOTTE, P., & PLAA, G.L. (1985) Potentiation of
necrogenic and cholestatic liver injury by 4-methyl-2-pentanone.
Can. Fed. Biol. Soc., 28: 221.
WEBB, R.G., GARRISON, A.W., KEITH, L.H., & MCGUIRE, J.H. (1973)
Current practice in GC-MS analysis of organics in water,
Washington, DC, US Environmental Protection Agency (EPA Report No.
R2-73-277) (NTIS PB 224 947/2).
WELLER, J.P. & WOLF, M. (1989) Mass spectroscopy and headspace gas
chromatography. Beitr. gerichtl. Med., 47: 525-532.
ZAKHARI, S., LEVY, P., LIEBOWITZ, M., & AVIADO, D.M. (1977) Acute
oral, intraperitoneal, and inhalation toxicity of methyl isobutyl
ketone in the mouse. In: Goldberg, L., ed. Isopropanol and ketones
in the environment, Cleveland, Ohio, CRC Press, Part 3, Chapter
10-14, pp. 93-133.
ZLATKIS, A. & LIEBICH, H.M. (1971) Profile of volatile metabolites
in human urine. Clin. Chem., 17: 592-594.
RESUME
La méthylisobutylcétone est un liquide limpide d'odeur
douceâtre produite en vue d'une vaste utilisation commerciale comme
solvant. On peut la doser par chromatographie en phase gazeuse avec
détection par ionisation de flamme. Elle s'évapore rapidement dans
l'atmosphère où elle subit une photoconversion à brève échéance. La
méthylisobutylcétone est facilement biodégradable et, compte tenu
de sa solubilité moyenne dans l'eau et de son faible coefficient de
partage entre l'octanol et l'eau, elle devrait présenter un faible
potentiel de bioaccumulation. Les limites d'exposition
professionnelle sont de 100-400 mg/m3 (moyenne pondérée par
rapport au temps: TWA) et de 5-300 mg/m3 (valeur plafond: CLV)
selon les pays.
La méthylisobutylcétone est rapidement métabolisée en produits
d'excrétion solubles dans l'eau et sa toxicité aiguë générale est
faible chez l'animal après exposition par voie orale ou
respiratoire. L'expérimentation animale n'a pas révélé
d'axonopathie périphérique. On ne dispose pas de données précises
sur la CL50. Une exposition de 4 heures à une concentration de 16
400 mg/m3 (4000 ppm) a été mortelle pour des rats. La
méthylisobutylcétone liquide ou sous forme de vapeurs à la
concentration de 10 à 410 mg/m3 (2,4 à 100 ppm) est irritante
pour les yeux et les voies respiratoires supérieures. Des
concentrations allant jusqu'à 200 mg/m3 (50 ppm) n'ont produit
aucun effet sensible sur l'homme lors d'épreuves portant sur le
temps de réaction et le calcul mental. Un contact prolongé ou
répété avec la peau peut produire un dessèchement et des crevasses.
L'aspiration accidentelle de méthylisobutylcétone liquide peut
provoquer une pneumonie chimique.
Lors d'une étude de 90 jours effectuée en gavant des rats, on
a obtenu une dose sans effet observable de 50 mg/kg par jour. Des
études d'inhalation de 90 jours portant sur des rats et des souris
à des concentrations allant jusqu'à 4100 mg/m3 (1000 ppm) n'ont
pas révélé de signes de toxicité engageant le pronostic vital.
Toute fois des altérations morphologiques réversibles liées à
l'administration de ce composé ont été observées au niveau du foie
et des reins. Dans un certain nombre d'études, on a observé une
hypertrophie du foie dès la dose de 1025 mg/m3 (250 ppm). Exposés
à 4100 mg/m3 (1000 ppm) pendant 50 jours, des poulets ont
présenté une induction des enzymes microsomiques. A doses plus
élevées (jusqu'à 8180 mg/m3, 1996 ppm) les effets se limitaient
à un accroissement du poids du foie sans lésion histologique. Lors
d'études de 90 jours effectuées sur des souris, des rats, des
chiens et des singes, seuls les rats mâles ont présenté des
altérations histologiques: présence de gouttelettes hyalines dans
les tubules proximaux des reins (néphrose tubulaire toxique à
inclusions hyalines). Cet effet s'est révélé réversible et sa
portée en toxicologie humaine demeure incertaine. Il est possible
que la potentialisation de la toxicité des alcanes halogénés par la
méthylisobutylcétone repose sur une induction enzymatique. La
méthylisobutylcétone potentialise également l'effet cholestatique
du manganèse administré avec ou sans bilirubine.
Des babouins exposés pendant sept jours à une dose de 205
mg/m3 (50 ppm) ont présenté des troubles neuro-comportementaux.
La méthylisobutylcétone est foetotoxique à une concentration
manifestement toxique pour la mère (12 300 mg par m3, 3000 ppm)
mais elle n'est pas embryotoxique ni tératogène à cette
concentration. A la concentration de 4100 mg/m3 (1000 ppm), on
n'a pas observé d'embryotoxicité, de foetotoxicité ni de
tératogénicité chez les rats et les souris.
On a recherché la génotoxicité éventuelle de la
méthylisobutylcétone en pratiquant un certain nombre d'épreuves à
court terme, consistant notamment en tests sur des cellules
mammaliennes, des bactéries et des levures ainsi que dans la
recherche de micro-noyaux chez la souris. Les résultats indiquent
que la méthyliso-butylcétone n'est pas génotoxique. En ce qui
concerne la génotoxicité à long terme ou la cancérogénicité, on ne
dispose d'aucune donnée.
A la concentration de 410 mg/m3 (100 ppm), la
méthylisobutylcétone peut produire des symptômes chez l'homme
consistant en irritation occulaire, migraines, nausées, vertiges et
fatigue et qui correspondent à un effet dépresseur réversible sur
le système nerveux central. Toutefois, rien n'indique l'existence
de lésions permanentes.
La toxicité pour les organismes et micro-organismes aquatiques
est faible.
Compte tenu de la volatilité relativement forte de la
méthylisobutylcétone, de sa photoconversion rapide dans
l'atmosphère, de sa biodégradabilité et de sa faible toxicité pour
les mammifères et la faune aquatique, il est vraisemblable que
cette substance ne peut exercer d'effets néfastes sur
l'environnement qu'à la suite de déversements accidentels ou de la
décharge incontrôlée d'effluents industriels.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des effets sur l'environnement
La méthylisobutylcétone ne devrait pas persister dans
l'environnement. Elle se volatilise lentement à partir du sol et de
l'eau et subit une biodégradation rapide dans l'eau douce et l'eau
salée. Dans l'atmosphère, on pense qu'elle est décomposée par les
radicaux libres OH avec une demi-vie d'environ 14 heures. Elle ne
s'accumule probablement pas et présente une faible toxicité pour
les micro-organismes, les poissons, les algues et les invertébrés
aquatiques. Ce n'est qu'en cas de déversement accidentel ou de
rejet incontrôlé de déchets que cette substance est susceptible
d'atteindre des concentrations toxiques pour les êtres vivants.
2. Evaluation des risques pour la santé humaine
La population générale n'est exposée qu'à de faibles
concentrations de méthylisobutylcétone. On en a décelé de faibles
quantités dans les denrées alimentaires et dans l'eau de
consommation ou autres boissons (produits panifiés, 10,9 mg/kg;
produits laitiers, 11,5 mg/kg; gélatines, puddings, 10,9 mg/kg;
boissons diverses, 10,2 mg/kg). En ce qui concerne la population
générale, deux pays ont fixé des concentrations maximales dans
l'air ambiant qui se situent dans les limites de 0,1 à 0,2 mg/m3.
L'exposition professionnelle se produit notamment lors de la
production et de l'utilisation de vernis, de peintures et de
solvants d'extraction. La principale voie de pénétration est
l'inhalation. Le faible seuil olfactif (1,64 mg/m3) et les effets
irritants de cette substance peuvent avertir de la présence de
fortes concentrations. L'exposition à ces concentrations de 10 à
410 mg/m3 (2,4 à 100 ppm) a produit une irritation perceptible au
niveau des yeux, du nez et de la gorge; à 820 mg/m3 (200 ppm), on
éprouve une sensation de malaise. Entre 10 et 410 mg par m3 (2,4
à 100 ppm), on a également observé des céphalées, des nausées et
des vertiges. Une exposition de deux heures à des concentrations
allant jusqu'à 200 mg/m3 (50 ppm) n'a pas produit d'effets
sensibles à en juger par un simple test de temps de réaction et de
calcul mental.
On ne dispose que d'un seul rapport faisant état d'une
exposition professionnelle de longue durée à une dose de 2050
mg/m3 (500 ppm), 20 à 30 minutes par jour et à 328 mg/m3 (80
ppm) pour la majeure partie du reste de la journée. Plus de la
moitié des 19 ouvriers exposés se sont plaints de faiblesse, de
perte d'appétit, de maux de tête, d'irritation oculaire, de maux
d'estomac, de nausées, de vomissements et de maux de gorge.
Quelques ouvriers ont éprouvé de l'insomnie, de la somnolence et
une perte d'équilibre. Chez quatre d'entre eux on a constaté une
légère hypertrophie du foie et chez six autres une colite non
spécifique. Cinq années plus tard, les méthodes de travail
s'étaient considérablement améliorées et les concentrations
maximales réduites au cinquième des teneurs précédentes. Quelques
ouvriers se sont encore plaints d'irritation au niveau des yeux et
des voies respiratoires supérieures ainsi que de symptômes
digestifs et neurologiques. La contact prolongé avec la peau a
provoqué une irritation cutanée et des crevasses.
Il ressort de l'expérimentation animale que la toxicité
générale aiguë de la méthylisobutylcétone est faible, par voie
orale ou par inhalation. Lors d'une étude de 90 jours, des rats
Sprague-Dawley ont reçu par gavage de la méthylisobutylcétone à des
doses quotidiennes de 50, de 250 et 1000 mg/kg de poids corporel.
On a noté une léthargie chez des animaux du groupe soumis à la dose
la plus élevée et, chez les mâles, une réduction du gain de poids.
Les animaux de ce groupe présentaient une néphropathie généralisée,
avec augmentation du poids relatif des reins et une hypertrophie du
foie. Cette augmentation du poids relatif des reins était également
notable chez les animaux soumis à la dose de 250 mg/kg, mais à
cette dose, on ne constatait qu'une légère hypertrophie du foie
chez les mâles. Quelle que soit la dose, on n'a pas constaté de
lésion histopathologique au niveau du foie ou des autres tissus. La
dose sans effet observable a été évaluée à 60 mg/kg et par jour.
Lors d'une étude de même durée au cours de laquelle on a fait
inhaler à des rats et des souris des concentrations allant jusqu'à
4100 mg/m3 (1000 ppm) on n'a pas relevé de signes de toxicité
engageant le pronostic vital. Toute fois des altérations
morphologiques réversibles liées à l'administration de cette
substance ont été observées au niveau du foie et des reins. A la
concentration de 4100 mg par m3, on observait des signes de
dépression du système nerveux central. La méthylisobutylcétone a
provoqué une augmentation du poids du foie (à une dose supérieure
à 1025 mg/m3, soit 250 ppm) et provoqué l'induction des enzymes
microsomiques du foie. C'est ce dernier mécanisme qui serait à la
base de l'exacerbation de la toxicité des alcanes halogénés et de
la potentialisation de la neurotoxicité dunw-hexane. Lors d'études
de 90 jours sur des souris, des rats, des chiens et des singes, on
a observé, chez les rats seulement, l'apparition d'inclusions
hyalines dans les tubules proximaux des reins (néphrose tubulaire
à inclusions hyalines d'origine toxique). Cet effet observé chez
les rats mâles était réversible et il est douteux qu'il ait une
signification quelconque en toxicologie humaine. La
méthylisobutylcétone réduit l'activité de l'alcool-déshydrogénase
hépatique chez la souris in vitro . On a également constaté
qu'elle potentialisait les effets cholestatiques du manganèse en
présence ou en l'absence de bilirubine.
Des rats et des souris exposés par inhalation à des
concentrations de 1230, 4100 ou 12 300 mg/m3 (300, 1000 ou 3000
ppm) du sixième au quinzième jours de la gestation puis sacrifiés
le vingt-et-unième jour (rats) ou le dixhuitième jour (souris), ont
présenté des signes marqués d'intoxication à la concentration la
plus forte. Cette concentration était foetotoxique (réduction du
poids foetal et ossification retardée) mais n'était ni
embryotoxique ni tératogène. Aux concentrations de 4100 et 1230
mg/m3, on n'a constaté aucune toxicité pour les mères ni signe
d'embryotoxicité, de foetotoxicité ou de tératogénicité.
La méthylisobutylcétone n'a pas produit de mutation génique
dans des systèmes d'épreuve bactériens (Salmonella typhimurium et
Escherichia coli ), qu'il y ait ou non activation métabolique. On
a également obtenu des résultats négatifs dans différentes épreuves
(avec ou sans activation métabolique) à la recherche de conversions
géniques mitotiques dans des levures ( Saccharomyces cerevisiae )
ou lors d'épreuves de mutation génique sur des cellules
mammaliennes en culture (lymphome murin). La recherche in vitro
d'une synthèse anarchique de l'ADN dans des hépatocytes primaires
de rat et de lésions chromosomiques structurales dans des cellules
de foie de rat en culture (RL4) s'est révélée négative. Chez la
souris, la recherche in vivo de micro-noyaux s'est également
révélée négative. Toutes ces données montrent que la
méthyl-isobutylcétone n'est pas génotoxique.
RECOMMANDATIONS
Les concentrations de méthylisobutylcétone auxquelles la
population, dans son ensemble, est susceptible d'être exposée, ne
présentent vraisemblablement aucun danger. La principale voie
d'exposition professionnelle est la voie respiratoire, aussi les
concentrations atmosphériques devront être maintenues en dessous
des limites recommandées d'exposition professionnelle, grâce à un
aménagement convenable des procédés de production et à des moyens
mécaniques tels que la ventilation. Il convient d'éviter toute
contamination de la peau et des yeux. Des vêtements protecteurs
appropriés ainsi que des masques respiratoires doivent être placés
dans les ateliers confinés; ils seront utilisés en cas d'urgence ou
pour effectuer certaines opérations d'entretien. La
méthylisobutylcétone est inflammable et doit donc être manipulée
avec les précautions d'usage.
La méthylisobutylcétone présente une faible toxicité pour les
micro-organismes et pour les poissons et sa demivie dans
l'environnement est courte. Il s'ensuit qu'elle ne présente aucun
risque pour l'environnement, dans la mesure où des mesures
appropriées sont prises pour réduire les émissions au minimum. La
décharge de quantités importantes dans l'environnement pourrait
avoir localement des effets indésirables.
RECHERCHES A EFFECTUER
1. La méthylisobutylcétone affecte un certain nombre de systèmes
enzymatiques. Elle peut donc avoir une influence sensible sur
la biotransformation des produits xénobiotiques métabolisés
par ces enzymes. Etant donné que l'homme est généralement
exposé à plusieurs composés différents, il faudrait
entre-prendre des études sur les effets combinés de mélanges
contenant de la méthylisobutylcétone.
2. On ne dispose que de très peu d'informations sur la relation
dose-réponse relative aux effets toxiques de la
méthylisobutylcétone sur le système nerveux central (par
exemple temps de réaction, effets comportementaux), sur les
voies respiratoires supérieures, sur les muqueuses et sur la
fonction rénale. Il faudrait obtenir davantage de
renseignements sur la toxico-cinétique de cette cétone, soit
seule soit associée à d'autres solvants. Il faudra également
étudier la pénétration percutanée de la méthylisobutylcétone.
3. Il faudrait entreprendre des études épidémiologiques pour
élucider les effets exercés à long terme sur le système
nerveux central par des concentrations moyennes de
méthylisobutylcétone soit seule soit associée à d'autres
solvants.
RESUMEN
La metil isobutil acetona (MIBA) es un líquido transparente de
buen olor que se produce a escala comercial y tiene un uso muy
extendido como disolvente. Puede medirse mediante cromatografía de
gases con detección de ionización de llama. Se evapora rápidamente
a la atmósfera, donde se fototransforma en poco tiempo. La MIBA es
fácilmente biodegradable, lo que, junto con su moderada solubilidad
en el agua y su reducido coeficiente de partición octanol/agua,
sugiere que tiene un bajo potencial de bioacumulación. Los límites
de exposición profesional varían entre 100-410 mg/m3 (promedio
ponderado en el tiempo) y 5-300 mg/m3 (valor máximo) en distintos
países.
La MIBA se metaboliza fácilmente para dar productos de
excreción hidrosolubles y su toxicidad sistémica aguda en animales
es baja por las vías de exposición oral y de inhalación. No se ha
observado axonopatía periférica en estudios realizados en animales.
No se dispone de datos exactos sobre la CL50. La exposición a 16
400 mg/m3 (4000 ppm) durante 4 horas resultó letal para la rata.
Las concentraciones de MIBA líquida y en vapor comprendidas entre
10 y 410 mg/m3 (2,4-100 ppm) son irritantes para los ojos y las
vías respiratorias superiores. Con concentraciones de hasta 200
mg/m3 (50 ppm) no se observaron en el hombre efectos
significativos en una prueba sencilla de tiempo de reacción ni en
una prueba de aritmética mental. El contacto cutáneo prolongado o
repetido puede desecar y descamar la piel. La aspiración accidental
de MIBA líquida puede provocar pneumonitis química.
En un estudio de ceba de ratas durante 90 días, se determinó
un nivel sin efecto observado de 50 mg/kg. En estudios de
inhalación durante 90 días en ratas y ratones, concentraciones de
hasta 4100 mg/m3 (1000 ppm) no produjeron ningún signo de
toxicidad con peligro para la vida. No obstante, se notificaron
cambios morfológicos reversibles relacionados con el compuesto en
el hígado y el riñón. En varios estudios se observó que
concentraciones de MIBA tan bajas como 1025 mg/m3 (250 ppm) eran
capaces de aumentar el tamaño del hígado. Mediante la exposición a
4100 mg/m3 (1000 ppm) durante 50 días, se indujo actividad
metabólica en las enzimas microsómicas del hígado del pollo. A
dosis más elevadas (hasta 8180 mg/m3, 1996 ppm) los efectos se
limitaron a un aumento del peso del hígado sin lesiones
histológicas. En estudios durante 90 días con ratones, ratas,
perros y monos, sólo las ratas macho presentaron corpúsculos
hialinos en los túbulos proximales del riñón (nefrosis tubulotóxica
de corpúsculos hialinos). Este efecto en la rata macho resultó ser
reversible y de dudosa importancia para el hombre. La inducción
enzimática puede ser la base de la potenciación de la toxicidad de
los haloalcanos por la MIBA. Se observó también que la MIBA era
capaz de potenciar el efecto colestático del manganeso administrado
con o sin bilirrubina.
En papiones expuestos durante 7 días a 205 mg/m3 (50 ppm),
se observaron efectos en el neurocomportamiento.
La MIBA resulta fetotóxica a una concentración que produce sin
lugar a dudas toxicidad materna (12 300 mg/m3, 3000 ppm) pero no
es embriotóxica ni teratogénica en esa concentración. En una
concentración de 4100 mg/m3 (1000 ppm), no resultó ni
embriotóxica, ni fetotóxica ni teratogénica en la rata ni en el
ratón.
Se ha estudiado la genotoxicidad de la MIBA en varios ensayos
a corto plazo, inclusive pruebas in vitro con bacterias, levaduras
y células de mamíferos y un ensayo de micronúcleos en el ratón.
Esos estudios indican que la MIBA no es genotóxica. No se dispone
de informes sobre estudios a largo plazo ni estudios de
carcinogenicidad.
Aunque con una concentración de 410 mg/m3 (100 ppm) la MIBA
puede inducir en el hombre síntomas como irritación ocular, dolores
de cabeza, náuseas, mareos y fatiga, que corresponden a un efecto
reversible de depresión del sistema nervioso central, no existen
pruebas de que produzca lesiones permanentes en el sistema
nervioso.
La toxicidad de la MIBA para organismos y micro-organismos
acuáticos es baja.
La volatilidad relativamente alta de la MIBA, su rápida
fototransformación en la atmósfera, su fácil biodegradación y su
baja toxicidad para mamíferos y organismos acuáticos indican que
los efectos medioambientales adversos de esta sustancia
probablemente sólo se producirán como consecuencia de vertidos
accidentales o de efluentes industriales no controlados.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS
EFECTOS EN EL MEDIO AMBIENTE
1. Evaluación de los efectos en el medio ambiente
La MIBA tiene pocas probabilidades de persistir en el medio
ambiente. Se volatiliza poco a poco desde el suelo y el agua y se
biodegrada fácilmente en agua dulce y salada. En la atmósfera, se
ha calculado que la MIBA es degradada por los radicales OHÊ una
semivida de aproximadamente 14 horas. En principio, la MIBA no se
bioacumula y tiene una toxicidad baja para micro-organismos, peces,
algas e invertebrados acuáticos. Sólo en los casos de vertido
accidental o de evacuación inadecuada de desechos en el medio
ambiente es probable que los niveles de MIBA provoquen toxicidad en
los organismos del entorno.
2. Evaluación de los riesgos para la salud humana
La población general está expuesta a niveles reducidos de MIBA.
Se han detectado sólo pequeñas cantidades en los alimentos, el agua
potable y otras bebidas (alimentos horneados, 10,9 mg/kg; productos
lácteos congelados, 11,5 mg/kg; gelatinas y budines, 10,9 mg/kg;
bebidas, 10,2 mg/kg). En cuanto a la exposición de la población
general, dos países han definido concentraciones máximas en el aire
entre 0,1 y 0,2 mg/m3.
La exposición profesional tiene lugar especialmente en la
producción y utilización de lacas, pinturas y disolventes de
extracción. La principal vía de entrada es por inhalación. El bajo
umbral olfativo (1,64 mg/m3) y los efectos irritantes pueden
servir como indicadores de las concentraciones elevadas. La
exposición a niveles de 10-410 mg/m3 (2,4-100 ppm) produjo
irritación perceptible de los ojos, la nariz, o la garganta y 820
mg/m3 (200 ppm) produjeron molestias. Con un nivel de 10-410
mg/m3 (2,4-100 ppm) también se produjeron síntomas como dolor de
cabeza, náuseas y vértigos. No se observaron efectos significativos
debidos a una exposición durante 2 horas a hasta 200 mg/m3 (50
ppm) al realizar una prueba sencilla de tiempo de reacción ni una
prueba de aritmética mental.
En el único informe sobre un estudio de la exposición
profesional a largo plazo, en el que se expuso a trabajadores a
2050 mg MIBA/m3 (500 ppm) durante 20-30 minutos al día y a 328
mg/m3 (80 ppm) durante la mayor parte del resto de la jornada
laboral, más de la mitad de los 19 trabajadores se quejaron de
debilidad, pérdida del apetito, dolores de cabeza, irritación
ocular, dolor de estómago, náuseas, vómitos y dolor de garganta.
Algunos trabajadores sufrieron insomio, somnolencia y cierta
inestabilidad. En cuatro se observó un ligero agrandamiento del
hígado y en seis se observó colitis no específica. Al cabo de 5
años, las prácticas laborales habían mejorado en gran medida y las
concentraciones más elevadas se redujeron a aproximadamente la
quinta parte del nivel anterior. Algunos trabajadores siguieron
quejándose de irritación en los ojos y de las vías respiratorias
superiores así como de síntomas gastrointestinales y del sistema
nervioso central. El contacto cutáneo prolongado con la MIBA
provocó irritación y descamación de la piel.
En estudios en animales, la toxicidad sistémica aguda de la
MIBA es baja por las vías oral y respiratoria. En un estudio de 90
días de duración, se cebó con MIBA a ratas Sprague-Dawley en dosis
diarias de 50, 250 ó 1000 mg/kg de peso corporal. Se observó
letargo en el grupo que recibió la dosis más alta y en los machos
se observó una reducción del ritmo de aumento del peso corporal. En
este grupo se observó nefropatía generalizada, con aumento del peso
relativo del riñón y hepatomegalia. El peso relativo del riñón
también aumentó en los animales alimentados con 250 mg/kg al día,
y se observó ligera hepatomegalia sólo en los machos. No
aparecieron lesiones histopatológicas en el hígado ni en otros
tejidos con ninguna de las dosis administradas. Se concluyó que el
nivel de efecto no observado era de 50 mg/kg al día. En estudios de
inhalación durante 90 días realizados en ratas y ratones, las
concentraciones de hasta 4100 mg/m3 (1000 ppm) no originaron
ningún signo de toxicidad que pusiera en peligro la vida. No
obstante, se comunicó la observación de cambios morfológicos
reversibles relacionados con el compuesto en el hígado y el riñón.
Con niveles de 4100 mg/m3 se observaron signos de depresión del
sistema nervioso central. La MIBA fue capaz de aumentar el peso del
hígado (concentración > 1025 mg/m3 (250 ppm)) y de inducir el
metabolismo microsómico hepático. Esto puede explicar la
exacerbación de la toxicidad de los haloalcanos y la potenciación
de la neurotoxicidad del n‹hexano. En estudios de 90 días con
ratones, ratas, perros y monos, sólo las ratas macho desarrollaron
corpúsculos hialinos en los túbulos proximales del riñón (nefrosis
tubulotóxica de corpúsculos hialinos). Este efecto en ratas macho
resultó ser reversible y de dudosa importancia para el hombre. In
vitro, la MIBA reduce la actividad de la deshidrogenasa alcohólica
en el hígado del ratón. También se ha observado que potencia los
efectos colestáticos del manganeso administrado con o sin
bilirrubina.
En ratas y ratones expuestos a la inhalación de MIBA en
concentraciones de 1230, 4100 ó 12 300 mg/m3 (300, 1000 ó 3000
ppm) en los días 6 a 15 de la gestación y sacrificados el día 21
(ratas) o el día 18 (ratones), se observó una notable toxicidad
materna a la concentración más elevada en ambas especies. Esta
concentración produjo fetotoxicidad (peso del cuerpo fetal reducido
y retraso en la osificación) pero no resultó embriotóxico ni
teratogénico. A 4100 y 1230 mg/m3 no se observó ni toxicidad
materna ni síntomas de embriotoxicidad, fetotoxicidad o
teratogenicidad.
La MIBA no indujo mutación genética en sistemas de ensayo
bacterianos (Salmonella typhimurium y Escherichia coli), con o sin
activación metabólica. También se obtuvieron resultados negativos
en los ensayos (tanto con y sin activación metabólica) para la
conversión mitótica de genes en levaduras (Saccharomyces
cerevisiae) y en pruebas de mutación génica con cultivos de células
de mamíferos (linfoma de ratón). Los ensayos in vitro sobre
síntesis no controlada de ADN en hepatocitos primarios de rata y
sobre lesiones cromosómicas estructurales en cultivos de células
hepáticas de rata (RL4) resultaron negativos. En el ratón, un
ensayo de micronúcleo in vivo resultó negativo. Estos datos indican
que la MIBA no es genotóxica.
RECOMENDACIONES
En los niveles de MIBA a que está expuesta la población humana
general, es poco probable que se plantee riesgo alguno. En el medio
laboral, donde la principal vía de exposición es por inhalación,
los niveles atmosféricos deben mantenerse por debajo de los límites
de exposición profesional recomendados mediante procesos de trabajo
y controles de ingeniería, inclusive la ventilación, de diseño
adecuado. Debe evitarse la contaminación de la piel y de los ojos.
Debe facilitarse el uso de prendas protectoras adecuadas y de
protección respiratoria en los espacios cerrados, en casos de
emergencia y para ciertas operaciones de mantenimiento. La MIBA es
inflamable y debe manipularse teniendo esta característica en
cuenta.
La MIBA tiene baja toxicidad para los microorganismos y los
peces, y su semivida en el medio ambiente es corta. Por
consiguiente, no presenta riesgos para el medio ambiente siempre
que se apliquen las medidas de control adecuadas para reducir al
mínimo las emisiones. El vertido en gran escala podría ejercer
efectos adversos en el medio ambiente a escala local.
OTRAS INVESTIGACIONES
1. LA MIBA afecta a varios sistemas enzimáticos. Por lo tanto,
puede influir de modo significativo en la bio-transformación
de sustancias biológicas externas que son metabolizadas por
estas enzimas. Como las personas suelen estar expuestas a más
de un compuesto, deben llevarse a cabo estudios sobre los
efectos combinados de muestras que contengan MIBA.
2. Se dispone de muy poca información sobre las relaciones
dosis‹respuesta en cuanto a los efectos de la MIBA en el
sistema nervioso central humano (por ejemplo, tiempo de
reacción, efectos conductuales) en las vías respiratorias
superiores y las mucosas, o en la función renal. Se necesita
más información sobre la toxicocinética de la MIBA por sí sola
y mezclada con otros disolventes. Debe evaluarse la
penetración cutánea de la MIBA.
3. Deben emprenderse estudios epidemiológicos para dilucidar los
efectos que tiene en el sistema nervioso la exposición a largo
plazo a concentraciones moderadas de MIBA, por sí sola o
mezclada con otros disolventes.
 HMP and 4-methyl-2-pentanone have also been identified in rats
(Pilon, 1987). MIBK increased aniline hydroxylase activity and
cytochrome P-450 concentration in chicken liver microsomes after
inhalation (4100 mg/m3, 1000 ppm, for 50 days) (Abou-Donia et
al., 1985b). The induction by MIBK was apparently of similar
capacity to that of methyl n -butyl ketone (Abou-Donia et al.,
1985a). It is likely that MIBK can induce its own oxidative
metabolism as well as that of other substances. The study of
Malyscheva (1988) (section 8.2.4) suggested that MIBK is absorbed
through the skin as well as via the oral and inhalation routes
(section 8.1). A comparison of intraperitoneal and oral LD50
values (Zakhari et al., 1977) suggests an oral absorption of 30% or
more. Malyscheva (1988) showed dermal absorption followed by
extensive distribution (toxic signs in many organs). Likewise,
inhalation studies producing liver and kidney changes are
suggestive of extensive distribution (MacEwen et al., 1971; Vernot
et al., 1971).
The structure of MIBK precludes the metabolic production of
2,5-hexane-dione, the neurotoxic agent formed from both hexane and
methyl n -butyl ketone.
6.1.1 Effect on liver alcohol dehydrogenase in vitro
MIBK in N,N -dimethylacetamide has been shown to reduce the
activity of mouse liver alcohol dehydrogenase in vitro
(Cunningham et al., 1989).
6.2 Humans
MIBK and other substituted 2-pentanones have been reported in
urine samples from unexposed humans (Zlatkis & Liebich, 1971). MIBK
was detected in various tissues and body fluids (section 2.4.1.3)
of two individuals who suffered fatal exposure to a mixture of
organic solvents. The concentration range of MIBK in the tissues
and body fluids of the two decedents was 1.4-2.5 and 0.2-0.8 mg/kg,
respectively. The tissue distribution differed markedly between the
two individuals (Bellanca et al., 1982). The inhalation study
carried out by Dick et al. (1990) with human volunteers suggested
that exposures to 410 mg MIBK/m3 (100 ppm) for 4 h causes steady
state blood levels to be attained. Blood and breath samples
collected 90 min after exposure indicated essentially complete
clearance of the absorbed MIBK. Hjelm et al. (1990) exposed human
volunteers for 2 h during light physical excercise to MIBK (10,
100, and 200 mg/m3; 2.4, 24.4, and 48.8 ppm). The concentration
of MIBK in blood rose rapidly after the onset of exposure, during
which no plateau level was reached. No tendency towards saturation
kinetics was observed over the dose range, the apparent blood
clearance being 1.6 litres/h per kg throughout. Only 0.04% of the
total MIBK dose was eliminated unchanged via the kidneys within 3
h after exposure.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Toxicity data reported in this chapter should be interpreted
with caution, since the tests were conducted under static
conditions using nominal rather than measured concentrations.
Actual concentrations experienced by the test organisms cannot be
determined for these tests.
7.1 Microorganisms
MIBK has low toxicity for microorganisms as indicated by the
threshold concentrations required for inhibition of growth (Table
3).
Table 3. Toxicity of MIBK for microorganisms
Species Threshold Duration of Reference
concentration for study
growth inhibition
(mg/litre)
Protozoa
Saprozoic > 800 48 h Bringmann & Kühn (1981)
flagellate
(Chilomonas
paramaecium)
Bacteriovorous 450 72 h Bringmann & Kühn (1981)
flagellate
(Entosiphon
sulcatum)
Bacteriovorous 950 20 h Bringmann & Kühn (1981)
ciliate ( Uronema
parduczi)
Bacterium
Pseudomonas putida 275 16 h Bringmann & Kühn (1977b)
Table 4. Acute toxicity of MIBK for aquatic organisms
Species LC50 Duration Reference
(mg/litre) of study
Freshwater fish
Golden orfe 672-744 48 h Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 460 24 h Bridié et al. (1979b)
(Carassius auratus)
Fathead minnow approx. 525 96 h Call et al. (1985)
(Pimephales promelas)
Invertebrates
Freshwater
Water flea 4280 24 h Bringmann & Kühn (1977a)
(Daphnia magna) 1550 24 h Bringmann & Kühn (1982)
Marine
Brine shrimp 1230 24 h Price et al. (1974)
(Artemia salina)
Freshwater algae
Green algae 725 a 8 days Bringmann & Kühn (1977b)
(Scenedesmus
quadricauda)
Bluegreen algae 136 a 8 days Bringmann & Kühn (1978)
(Microcystis
aeruginosa)
a Threshold concentration for reduction of total biomass
7.2 Aquatic organisms
MIBK appears to have low toxicity for aquatic organisms (Table
4). The maximum zero lethality concentration (LC0) is in the
range of 480-720 mg/litre (Juhnke & Lüdemann, 1978). Using a QSAR
model, Lipnick et al. (1987) showed that the 24-h LC50 of 460
mg/litre reported by Bridié et al. (1979a) in goldfish (Carassius
auratus) fitted a narcotic mechanism of action.
Aquatic invertebrates are less sensitive than fish to the
toxicity of MIBK, 24-h LC50 values of 4280 mg/litre (Bringmann &
Kuhn, 1977a) and 1550 mg/litre (Bringmann & Kuhn, 1982) having been
reported for the water flea Daphnia magna and 1230 mg/litre for
the brine shrimp Artemia salina (Price et al., 1974). The
LC100 and LC0 values for Daphnia magna were 5000 and 2280
mg/litre, respectively (Bringmann & Kuhn, 1977a).
The toxicity of MIBK was also measured in the green alga
Scenedesmus quadricauda , in which the 8-day threshold for
toxicity was 725 mg/litre (Bringmann & Kuhn, 1977b), and in the
relatively more sensitive cyanobacterium (blue-green alga)
Microcystis aeruginosa , in which the toxicity threshold was 136
mg/litre (Bringmann & Kuhn, 1978).
7.3 Terrestrial organisms
Experimental data are not available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
MIBK is of low acute toxicity by the oral and inhalation routes
of exposure (Table 5).
The maximum time for which rats could be exposed to a saturated
atmosphere of MIBK without dying was 15 min (Smyth et al., 1951).
In one study, six rats survived a 4-h exposure to 8200 mg MIBK/m3
(2000 ppm), but, following a 4-h exposure to 16 400 mg/m3 (4000
ppm), all six animals died within 14 days.
In studies by Specht (1938) and Specht et al. (1940), female
guinea-pigs were exposed to MIBK concentrations of 4100, 69 000,
and 115 000 mg/m3 (1000, 16 800, and 28 000 ppm, respectively)
for up to 24 h. In view of the method used for generating the
atmosphere (allowing measured amounts of MIBK to evaporate freely
to one cubic meter volume of air at 25-26 °C), the two higher
levels must be greatly exaggerated because the saturation
concentration in air for MIBK at 25 °C is 40 000 mg/m3. At 4100
mg/m3 there was minimal eye or nasal irritation. However, there
was a decreased respiratory rate during the first 6 h of exposure,
which was attributed to a narcotic effect. The higher levels
produced obvious signs of eye and nose irritation, followed by
salivation, lacrimation, ataxia, progressive narcosis, and death.
The highest level killed 50% of the animals within 45 min. Autopsy
and histopathological investigations in some animals showed fatty
livers and congestion of the brain, lungs, and spleen, but no
damage to the heart and kidneys was observed.
A single ip injection of 500 mg MIBK/kg body weight in
guinea-pigs did not induce changes in the serum ornithine-carbamyl
transferase level, and there were no histopathological changes in
the liver. However, an injection of 1000 mg/kg body weight killed
one out of four animals, and a slight increase in the serum
ornithine-carbamyl transferase level was seen in the survivors 24
h after dosing. Histopathologically, there was possible lipid
accumulation in liver cells but no evidence of liver damage
(Divincenzo & Krasavage, 1974). It should be noted that an ip
injection of 560 mg/kg produced minimal effects in rats (Vezina et
al., 1985), suggesting that mice are more sensitive than rats (the
ip LD50 is 590 mg/kg in mice, see Table 5).
Table 5. LD50 and LC50 values for MIBK in rats and mice
Route of Species Duration LD50 or LC50 Reference
exposure of exposure
Oral (LD50) rat 4570 mg/kg body weight Smyth et al. (1951)
rat 4600 mg/kg body weight Batyrova (1973)
rat 2080 mg/kg body weight RTECS (1987)
mouse 2850 mg/kg body weight Batyrova (1973)
mouse 1900 mg/kg body weight Zakhari et al. (1977)
Intraperitoneal (LD50) mouse 590 mg/kg body weight Zakhari et al. (1977)
Inhalation (LC50) rat 4 h 8.2-16.4 g/m3 Smyth et al. (1951); Smyth (1956)
mouse 2 h 20.5 g/m3 Batyrova (1973)
mouse 45 mins 74.2 g/m3 Zakhari et al. (1977)
(18 105 ppm)
In male Sprague-Dawley rats, a single oral dose of MIBK
enhanced the hepatotoxicity of a single ip dose of chloroform given
24 h later. The no-observed-effect and minimal-effect levels of
MIBK were 375 and 560 mg/kg body weight, respectively (Vezina et
al., 1985). The ketone potentiation of haloalkane-induced
hepatonecrosis has been attributed to enhanced bioactivation of the
haloalkane, which is mediated by the increased cytochrome P-450
activity induced by the ketone (Branchflower et al., 1983). The
extent of potentiation of carbon tetrachloride liver toxicity (as
shown by an increase in plasma alanine transaminase activity and
bilirubin concentration) was found to depend on the concentration
of both MIBK and carbon tetrachloride in male rats. The minimum
effective MIBK dose decreased 10-fold when the carbon tetrachloride
dose was increased from 0.01 ml/kg to 0.1 ml/kg. These findings
suggest that liver injury is determined by the product of MIBK and
carbon tetrachloride doses (Pilon et al., 1988). Attention should
be paid to this when working in environments containing mixtures of
solvents.
8.2 Short-term exposure
8.2.1 Inhalation
In rats exposed to 410 mg/m3 (100 ppm) for 2 weeks, there was
an increase in kidney weight. An increase in both liver and kidney
weights was observed after exposure to 820 mg/m3 (200 ppm) for 2
weeks or 410 mg/m3 (100 ppm) for 90 days. Histopathological
investigations showed hyaline droplets in the proximal tubules of
the kidney and this finding was named hyaline droplet toxic tubular
nephrosis (MacEwen et al., 1971; Vernot et al., 1971). In rats,
dogs, and monkeys exposed continuously for 90 days to MIBK at 410
mg/m3 (100 ppm) under reduced oxygen tension and reduced
atmospheric pressure (65% oxygen at 34.7 k Pa), liver and kidney
weights were increased in rats after 90 days. Hyaline droplets were
observed in rat kidney epithelium after 15 days, but this effect
was reversible after a 3- to 4-week recovery period. No
histopathological changes were reported in monkeys or dogs and no
sex differences were reported for any parameter (MacEwen et al.,
1971).
Four groups of six male and six female F-344 rats and six male
and six female B6C3F1 mice were exposed to 0, 44, 2050, or 8180
mg MIBK/m3 (0, 10.1, 501, or 1996 ppm, respectively) for 6 h/day
(for 5 days with 2 days off followed by 4 more consecutive days of
exposure) (Dodd et al., 1982; Phillips et al., 1987). Lacrimation
was observed in the highest dose group, but no ophthalmological
lesions or alterations in body weight gain were found. Liver
weight, expressed as a percentage of body weight, was increased in
male and female rats and in female mice exposed to 8180 mg/m3. A
statistically significant increase in liver weight was also
observed in male rats exposed to 2050 mg/m3. Male and female rats
and female mice showed a significant increase in both absolute and
relative kidney weights when exposed to 8180 mg/m3. However, male
mice at this exposure level exhibited a significant decrease in
relative kidney weight. Of the animals exposed to 2050 mg/m3,
only male rats exhibited an increase in kidney weight, but this was
not statistically significantly different from the control value.
Hyaline droplet formation was seen in the kidneys of male rats
exposed to 2050 and 8180 mg/m3. Epithelial regeneration of the
proximal convoluted tubules was also seen at 8180 mg/m3. There
were no histopathological abnormalities in rats and mice exposed to
414 mg/m3, and this concentration was considered a clear
no-observed-adverse-effect level (Dodd et al., 1982). In a
subsequent study, four groups of 14 male and 14 female F-344 rats
and 14 male and 14 female B6C3F1 mice were exposed to 0, 205,
1025, or 4100 mg MIBK/m3 (0, 50, 250, or 1000 ppm, respectively)
for 6 h/day (5 days/week for 90 days). No growth retardation or
clinical effects were observed in either rats or mice. Clinical
observations were made in addition to measurements of body and
organ weights (heart, kidneys, liver, lungs, and testes).
Haematology, ophthalmology, gross pathology, and histology were
also investigated and, in rats, water consumption and urine and
serum chemistry analyses were made. Male rats and mice exposed to
4100 mg/m3 (1000 ppm) showed a slight increase in liver weight
(approximately 11%) and in liver weight per body weight ratio, and
liver weight was also slightly increased in male mice exposed to
1025 mg/m3 (250 ppm). However, neither gross nor microscopic
hepatic lesions were observed, and urinalysis and serum chemistry
values were normal. In male rats exposed to 1025-4100 mg/m3
(250-1000 ppm), there was an increase in the number of hyaline
droplets within the proximal tubular cells of the kidney. No other
gross or microscopic renal changes were observed (Dodd & Eisler,
1983; Phillips et al., 1987). It was considered that the hyaline
droplet effects produced by MIBK may be specific to the male rat
due to the presence of alpha-2-µglobulin (Phillips et al., 1987).
In studies by Brondeau et al. (1989), groups of five hens were
continuously exposed for 50 days by inhalation to either 4100 mg
MIBK/m3 (1000 ppm) or 3520 mg n -hexane/m3 (1000 ppm) or for
30 days to a mixture of 4100 mg MIBK/m3 and 3520 mg n
-hexane/m3. Inhalation of n -hexane alone had no effect on
hepatic microsomal enzymes, but inhalation of MIBK or the MIBK/ n
-hexane mixture increased significantly the aniline hydroxylase
activity and cytochrome P-450 content of the liver (Abou-Donia et
al., 1985b). Inhalation of 2440 to 12 400 mg MIBK/m3 (595 to 3020
ppm) in rats produced enhancement of the liver cytochrome P-450
content and glutathione- S -transferase activity and also enhanced
the ability of 1,2-dichlorobenzene to increase serum glutamate
dehydrogenase activity.
Experiments in open-chest cats demonstrated that significant
pulmonary hypertension and vasoconstriction, with reduced pulmonary
arterial flow, occurred as a result of MIBK inhalation for 5 min at
all concentrations tested (410 to 41 000 mg/m3 (100 to 10 000
ppm)). Systemic arterial pressure and vascular resistance were not
significantly affected (Zakhari et al., 1977). Broncho-constriction
was also produced by MIBK inhalation for 5 min, the effect being
statistically significant at concentrations at or above 2050
mg/m3 (500 ppm) or 4100 mg/m3 (1000 ppm) for pulmonary
resistance or transpulmonary pressure, respectively. Adult mongrel
dogs with open-chest surgery showed pulmonary hypertension at an
inhalation concentration of 20.5 mg MIBK/m3 (5 ppm) for 5 min. At
41 mg/m3 (10 ppm), myocardial contractility occurred (Zakhari et
al., 1977).
8.2.2 Oral
Three daily doses of 375 or 1500 mg MIBK/kg body weight given
by gavage to rats reduced the bile flow produced by an intravenous
injection of taurocholate (20 mg per kg body weight) (Plaa &
Ayotte, 1985). The effect of MIBK on the cholestatic activity of
manganese, with or without bilirubin, has also been investigated in
male Sprague-Dawley rats. MIBK was administered by gavage in corn
oil at doses ranging from 188 to 1502 mg/kg body weight once a day
for 1, 3, or 7 days (Vezina et al., 1985; Vezina and Plaa, 1987).
MIBK was not cholestatic but, at doses of 375 mg/kg or more, was
found to potentiate the cholestasis induced by a
manganese-bilirubin combination when this was given 18 h after the
1-day treatment with MIBK. When given for 3 or 7 days, MIBK
produced a dose-related enhancement of the cholestasis induced by
a manganese-bilirubin combination. A 3-day treatment with MIBK (750
mg/kg) was also shown to potentiate the cholestasis induced by
manganese alone. Two known metabolites of MIBK (HMP and
4-methyl-2-pentanol) were also able to potentiate the cholestatic
effect of the manganese-bilirubin combination or of manganese alone
in male Sprague-Dawley rats. When the metabolite was given by
gavage 18 h prior to the administration of the manganese-bilirubin
combination, cholestasis was potentiated by 375 mg/kg or 1502 mg/kg
(expressed as equivalents of MIBK) of 4-methyl-2-pentanol or HMP,
respectively. Lower doses did not decrease bile flow rate.
4-Methyl-2-pentanol was also more effective than HMP as a
potentiator following daily treatment for 3 days prior to
manganese-bilirubin administration. However, with manganese alone,
HMP was more effective. It was suggested that the potentiation of
cholestasis may be associated with metabolic induction or might
reflect a separate mechanism of action involving a membrane
interaction (Vezina & Plaa, 1988).
The toxic effects of MIBK in Sprague-Dawley rats (groups of 30
animals of each sex) were examined following 13 weeks of oral
gavage administration at levels of 0, 59, 250, or 1000 mg/kg daily.
Body weight, food consumption, organ weight, morbidity, clinical
chemistry, haematology, and histopathology evaluations were
performed. All surviving animals were killed after 90 days (13
weeks) and 10 animals of each sex per group were examined.
Nephrotoxicity was seen as a general nephropathy for both male and
female rats administered 1000 mg/kg per day. Although increased
liver and kidney weights were observed for males and females at
1000 mg/kg per day, there were no corresponding histopathological
lesions present in the liver. The effects seen at 1000 mg/kg per
day were present to a significantly lesser extent in the females
and males fed 250 mg/kg per day. No effects were observed at 50
mg/kg per day, identifying a no-observed-effect level
(Microbiological Associates, 1986).
8.2.3 Parenteral
In studies by Krasavage et al. (1982), rats (strain and number
not specified) were given intraperitoneal injections of MIBK or a
mixture of methyl ethyl ketone (MEK) and MIBK (9:1 by volume), 5
times/week, for 35 weeks. The dose levels for the first 2 weeks
were 10, 30, and 100 mg per kg body weight, and these were then
doubled for the remainder of the treatment period. Body weight gain
suppression was seen after 3-4 weeks of treatment. The only other
effect noted was transient narcosis during the first 4 weeks at the
highest dose. Pulmonary vascular effects were observed following an
intraperitoneal administration of MIBK to cats (threshold dose 8
mg/kg), but bronchoconstriction was not seen following an
intraperitoneal administration of 4-32 mg/kg (Zakhari et al.,
1977).
8.2.4 Skin application
In rats exposed dermally to 300-600 mg MIBK/kg per day for 4
months, dose- and time-dependent morphological changes were
observed in the skin, brain, liver, adrenals, spleen, and testis.
Body temperature decreased and oxygen consumption increased
(Malyscheva, 1988).
8.3 Skin, eye, and respiratory irritation; sensitization
8.3.1 Skin irritation
A single 10-h occluded application of MIBK to the shaved skin
of rabbits produced erythema, which occurred immediately after the
application and persisted for up to 24 h. Daily applications of 10
ml on 10 cm2 skin for 7 days caused drying and flaking of the
surface (Krasavage et al., 1982).
8.3.2 Eye irritation
Undiluted MIBK (0.1 ml) produced some irritation within 10 min
when instilled in the rabbit eye. Inflammation and conjunctival
swelling occurred within 8 h; the inflammation, swelling, and
exudate present at 24 h had disappeared by 60 h (Krasavage et al.,
1982).
8.3.3 Respiratory irritation
De Ceaurriz et al. (1981) measured the reflex decrease in
respiratory rate in male Swiss OF1 mice as an index of sensory
irritation. MIBK caused a concentration-dependent decrease in
respiratory rate during a 5-min exposure, and a 50% decrease in
respiratory rate (RD50) was seen at 13 100 mg/m3 (3195 ppm).
Specht et al. (1940) attributed the decreased respiratory rate to
a narcotic effect. It should be recognized that the reduction may
not be due to sensory irritation.
8.3.4 Skin sensitization
There are no reports of skin sensitization studies.
8.4 Long-term exposure
No long-term toxicity studies have been reported.
8.5 Reproduction, embryotoxicity, and teratogenicity
In studies by Tyl (1984) and Tyl et al. (1987), groups of 35
pregnant Fischer-344 rats and 30 pregnant CD-1 mice were exposed to
1230, 4100, or 12 300 mg MIBK/m3 (300, 1000, or 3000 ppm) on days
6-15 (inclusive) of gestation. The animals were sacrificed on days
21 (rats) or 18 (mice) and fetuses examined for external, visceral,
and skeletal alterations. In rats, exposure to 12 300 mg/m3
resulted in maternal toxicity with decreased body weight gain,
increased relative kidney weight, decreased food consumption, and
fetotoxicity (reduced fetal body weight per litter and delays in
skeletal ossification). Clinical signs in dams included loss of
coordination, negative toe pinch, paresis, muscular weakness,
piloerection, lacrimation, and perioral encrustation. No increase
in fetal malformation was observed in any group. At 1230 and 4100
mg/m3, there was no maternal, embryo, or fetal toxicity, or
malformations. However, reduced fetal body weight and delay in some
ossification parameters were observed at the lowest dose but not at
the intermediate dose level. These effects were attributed to the
larger litter size (average 10.8) in this group compared to that in
controls (9.5).
In mice, exposure to 12 300 mg/m3 produced maternal toxicity
with increased mortality (3/25), increased absolute and relative
liver weights, and fetotoxicity (increased incidence of dead
fetuses, reduced fetal body weight per litter, and delayed or
reduced ossification). Clinical signs in dams included irregular
gait, paresis, hypoactivity, ataxia, negative toe pinch, and
lacrimation. There was no treatment-related increase in
embryotoxicity or fetal malformations at any exposure concentration
tested. No significant treatment-related maternal, embryo, or fetal
toxicity (including malformations) was observed at 1230 or 4100
mg/m3.
Table 6. Mutagenicity and related end-points
System Dose Response Reference
Bacterial Assays
Salmonella typhimurium a 0.04-4 µg/plate negative Chemical
strains TA98, TA100, Manufacturers
TA1537, TA1538 Association
(1984);
O'Donoghue et
al. (1988)
Salmonella typhimurium a
strains TA1535, TA1537 Up to 8000 µg/ml negative Brooks et al.
TA1538, TA98, TA100 (1988)
Eschericia coli
strains WP2 and Up to 8000 µg/ml negative Brooks et al.
WP2 uvr A (1988)
Yeast Assays
Saccharomyces cerevisiae JDI Up to 5 mg/ml negative Brooks et al.
mitotic gene conversion (1988)
assay ± rat liver S9
Mammalian cell assays in vitro :
L51784 TK +/- mouse 0.001-100 µl/ml negative Chemical
lymphoma mutation assay (preliminary assay) Manufacturers
(± rat liver S9) 0.4-6 µl/ml negative Association
(1984);
O'Donoghue et
al. (1988)
Primary rat hepatocytes; 0.01-100 µl/ml negative Chemical
unscheduled DNA synthesis Manufacturers
(DNA repair) Association
(1984);
O'Donoghue et
al. (1988)
Cultured rat liver cells Up to 1000 µl/ml negative Brooks et al.
chromosomal damage assay (half the dose for (1988)
RL4 cells 50% inhibition of
cell growth)
Table 6 (contd).
System Dose Response Reference
Balb/3T3; cell transformation 2-5 µl/ml (-S9) inconclusive Chemical
assay ± rat liver S9 1-7 µl/ml (+S9) Manufacturers
Association
(1984);
O'Donoghue et
al. (1988)
Mammalian in vivo assay
Mouse (male and female) 0.73 ml/kg ip negative Chemical
micronucleus assay (maximum tolerated Manufacturers
(polychromatic dose level) Association
erythrocytes) (1984);
O'Donoghue et
al. (1988).
a Preincubation mutation assay incorporating Arochlor-induced rat liver S9
8.6 Mutagenicity and related end-points
A summary of the reported data on MIBK mutagenicity is given
in Table 6.
8.6.1 Bacterial assays
A pre-incubation assay with Salmonella typhimurium (strains
TA98, TA100, TA1537, TAl538) was conducted at dose levels of 0.04
to 4 µg/plate both in the presence and absence of a metabolic
activation system prepared from Aroclor-induced rat liver
homogenate (S9 fraction). Precautions were taken to prevent the
escape of MIBK vapour and assure prolonged exposure of the bacteria
to the test substance. MIBK did not cause an increase in reverse gene
mutation (Chemical Manufacturers Association 1984; O'Donoghue et
al., 1988).
Both MIBK and the oxidative metabolite 4-hydroxy-4-methyl-2-
pentanone (HMP) (see section 6.1) were tested for mutagenicity in
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538, and in Eschericia coli strains WP2 and WP2 uvr A
(Brooks et al., 1988). Aroclor-induced rat liver S9 fraction was
included. No induction of reverse gene mutation was observed up to
a maximum concentration of 8000 µg MIBK/ml (pre-incubation assay in
a sealed container) or 4000 µg HMP/plate (plate incorporation
assay).
8.6.2 Yeast assay for mitotic gene conversions
MIBK and the metabolite HMP were assayed for mitotic gene
conversion using log-phase cultures of the yeast Saccharomyces
cerevisiae JD1 (Brooks et al., 1988). Compounds were tested up to
a concentration of 5 mg/ml in the presence and absence of rat liver
S9 fraction in a sealed container for 18 h. Neither compound
induced mitotic gene conversion.
8.6.3 L5178Y TK+/- mouse lymphoma assay
A preliminary assay was carried out in the presence and absence
of a metabolic activation system at doses of 0.001-100 µl/ml. The
non-activated cultures showed 3 to 157% total relative growth,
while the cultures containing the rat liver S9 fraction had a
relative growth of 23-95% compared with untreated control cultures.
No increase in mutation frequencies was observed in cultures
containing the metabolic activation system, but in the
non-activated cultures, a 2-fold increase above controls was seen
at two non-consecutive doses. An increase in mutation frequency of
approximately 5 times the concurrent control occurred at one test
concentration, but this concentration also caused 97% cell death.
In the absence of a dose-related effect, this result was considered
equivocal. A repeat assay was performed using duplicate cultures
and a narrower range of doses (0.4-6 µl/ml). The total relative
growth ranged from 1 to 80% in non-metabolically activated cells
and from 28 to 63% in cultures that contained the S9 fraction. None
of the activated cultures revealed increased mutation frequencies.
A borderline positive result was found at 6 µl/ml, but different
mutation frequencies occurred in the duplicate cultures and 96-99%
of the cells were killed (Chemical Manufacturers Association, 1984;
O'Donoghue et al., 1988).
8.6.4 Unscheduled DNA synthesis in primary rat hepatocytes in vitro
When MIBK was tested at five dose levels ranging from 0.01
µl/ml to 100 µl/ml in a single assay, there was an increase of less
than 5 fold in labelled nuclear grains in cells treated with MIBK
compared with cells of the solvent control plates (Chemical
Manufacturers Association, 1984; O'Donoghue et al., 1988). Since
the value did not exceed that of the negative control by two
standard deviations of the control value, it was considered that,
under the conditions tested, MIBK did not cause a significant
increase in the nuclear grain count.
8.6.5 Mouse micronucleus assay
Male and female mice were administered MIBK by ip injection at
the maximum tolerated dose level of 0.73 ml/kg body weight, and
bone marrow polychromatic erythrocytes were estimated 12, 24, and
48 h later. There were no significant differences between the
treated and control animals in the ratio of polychromatic to
normochromatic erythrocytes. The number of micronucleated
polychromatic erythrocytes per 1000 cells was not significantly
increased in the MIBK-treated animals (Chemical Manufacturers
Association, 1984; O'Donoghue et al., 1988).
8.6.6 Assay for structural chromosome damage
MIBK (purity > 98.5%) and the metabolite HMP were tested (24-h
exposure) in cultured rat liver cells (RL4) for the ability to
induce chromosomal damage. Metabolic activation with S9 mix was not
used because RL4 cells are metabolically competent. The
concentrations of MIBK employed were 0.125, 0.25, and 0.5 times the
concentration required for 50% inhibition of cell growth. Maximum
concentrations tested were thus 1000 µg MIBK/ml and 4000µg HMP/ml
(this HMP level was equivalent to the concentration required for
>60% growth inhibition). Incubations with MIBK were sealed to
prevent loss by evaporation. MIBK did not produce chromosomal
damage, but HMP gave a small increase (which was not dose related)
in chromatid damage within the concentration range 2000-4000 µg/ml.
It should be noted that HMP did not induce reverse gene mutation in
bacteria or mitotic gene conversion in yeast (Brooks et al., 1988).
8.6.7 Cell transformation assay
In studies reported by the Chemical Manufacturers Association
(1984) and O'Donoghue et al. (1988), MIBK was tested in the
Balb/3T3 (clone A31-1) morphological transformation assay. Doses of
2.4, 3.6, and 4.8 µl MIBK/ml were added to the culture medium in
the absence of a metabolic activation system, and 1, 2, and 4 µl
MIBK/ml were added in the presence of such a system (Aroclorinduced
rat liver S9 fraction). MIBK produced a positive response in the
non-activated cultures only (4.8 µl MIBK per ml gave 3 type III
foci in 15 dishes). A confirmatory study was conducted with doses
of 2, 3, 4, and 5 µl/ml and 4, 5, 6, and 7 µl/ml, respectively, in
the presence and absence of S9 fraction. No significant increase in
the number of transformed foci was found in this study, either in
the presence or absence of the metabolic activation system. Thus,
the effect of MIBK on cell transformation was not reproducible in
the two assays and the ambiguity of the results makes them
unreliable.
8.7 Carcinogenicity
No carcinogenicity studies have been reported.
8.8 Neurotoxicity
In a study by Krasavage et al. (1982), rats were given ip
injections of MIBK, or a mixture of methyl ethyl ketone and MIBK
(9:1 by volume), 5 times/week, for 35 weeks. The dose levels of 10,
30, and 100 mg/kg body weight were doubled after 2 weeks of
treatment. Transient anaesthesia was noted during the first 4 weeks
in the highest dose group, but there was no evidence of peripheral
neuropathy. In dogs administered 300 mg MIBK/kg body weight per day
subcutaneously (sc) for 11 months, electromyographic examination
showed no evidence of neurotoxicity.
Cats treated subcutaneously with 150 mg MIBK/kg body weight per
day or a mixture of methyl ethyl ketone/MIBK (9:1) twice daily, 5
times/week, for up to 8.5 months showed no evidence of nervous
system damage (Spencer & Schaumburg, 1976). In beagle dogs
receiving similar treatment, there were no neurotoxic changes
(Krasavage et al., 1982).
Groups of male rats were exposed to 5330 mg/m3 (1300 ppm)
methyl n -butyl ketone for 4 months or 6150 mg/m3 (1500 ppm)
MIBK for 5 months (Spencer et al., 1975). Methyl n -butyl ketone
produced a toxic distal axonopathy. MIBK produced minimal distal
axonal changes, but 3% methyl n -butyl ketone was present as a
contaminant in the MIBK, and the design of the cages used may have
caused compression neuropathy (Spencer et al., 1975; Spencer &
Schaumburg, 1976). Animals exposed to MIBK showed slight signs of
narcosis, but body weight gain was normal, and, at 5 months, there
were no clinical signs of neurological dysfunction. Rats exposed
for 3 h to 102 mg MIBK/m3 (25 ppm) showed a 58% increase in
pressor lever response, which had not returned to control levels 7
days after the end of exposure (Geller et al., 1978). The maximum
motor-fibre conduction velocity in the tail nerve decreased
markedly when male rats were treated with methyl n -butyl ketone
(401 mg/kg, 5 times/week for 55 weeks) but not when they were
treated with MIBK (601 mg/kg, 5 times/week for 55 weeks). However,
treatment with MIBK (201 mg/kg) facilitated the neurotoxic effect
of methyl n -butyl ketone (401 mg/kg) possibly due to the
demonstrated ability of MIBK to increase the metabolic activity of
10 000 g liver supernatants towards both MIBK and methyl n -butyl
ketone (Nagano et al., 1988).
Discriminatory behaviour and memory in baboons was not affected
by exposures of 82-164 mg/m3 (20-40 ppm) (Geller et al., 1978).
Geller et al. (1979) reported an effect on accuracy of performance
of tasks in a ``delayed match-to-sample discrimination-test'' in
baboons exposed for 7 days to 205 mg/m3 (50 ppm) MIBK, but there
was no change in response when MIBK was combined with methyl ethyl
ketone at 295 mg/m3 (100 ppm).
De Ceaurriz et al. (1984) exposed male Swiss OF1 mice to an
atmosphere containing MIBK and measured the total duration of
immobility during a 3-min ``behavioural despair'' swimming test. At
concentrations of 2714, 3104, 3309, and 3657 mg/m3 (662, 757,
807, and 892 ppm), a dose-dependent decrease of mobility was
observed (25, 38, 46, and 70% respectively). The authors noted that
the mean active level of MIBK was lower for this neurobehavioural
effect (3292 mg/m3 (893 ppm) for 50% inhibition of immobility)
than for an effect on sensory irritation (13 100 mg/m3 (3195 ppm)
for 50% inhibition of respiratory rate) (De Ceaurriz et al., 1981,
section 8.3.3).
In inhalation studies on hens (five per group) into the effect
of MIBK on n -hexane-induced neurotoxicity, a continuous exposure
period of 90 days was followed by a 30-day observation period
(Abou-Donia et al., 1985b). One group of hens was exposed to 3520
mgnw-hexane/m3 (1000 ppm) and another group to 4100 mg MIBK/m3
(1000 ppm). Four additional groups were exposed simultaneously to
3520 mg n -hexane/m3 (1000 ppm) and 410, 1025, 2050, or 4100 mg
MIBK/m3 (100, 250, 500, or 1000 ppm, respectively). A control
group was exposed to ambient air in an exposure chamber. Hens
continuously exposed to 4100 mg MIBK/m3 developed weakness of the
legs with subsequent recovery. Inhalation of 3520 mg n
-hexane/m3 produced mild ataxia. Exposure to 3520 mg n
-hexane/m3 together with 1025, 2050, or 4100 mg MIBK/m3
resulted in signs of neurotoxicity including paralysis, the
severity of which depended on the MIBK concentration. Hens
continuously exposed to the 3520/410 mg/m3 n -hexane/MIBK
mixture exhibited severe ataxia throughout the observation period.
Histopathological examination of hens exposed to the n
-hexane/MIBK mixture showed large swollen axons and degeneration
of the axon and myelin of the spinal cord and peripheral nerves.
There were no histopathological abnormalities in the central
nervous system of hens exposed only to MIBK. This demonstrates that
MIBK potentiates the neurotoxic action of n -hexane. In a
subsequent study, in which hens were exposed for 50 days to MIBK at
4100 mg/m3 (1000 ppm) or to n -hexane, it was suggested that
the potentiation by MIBK of n -hexane neurotoxicity is related to
the induction by MIBK of liver microsomal cytochrome P-450,
resulting in increased metabolism of n -hexane to its neurotoxic
metabolites (sections 6.1, 8.2.1). The findings also suggest that
the neurotoxicity of technical methyl butyl-ketone (methyl n
-butyl ketone/MIBK (7/3)) was correctly attributed to the methyl
n -butyl ketone component (Abdo et al., 1982). Simultaneous
treatment of hens with 41, 205, or 410 mg/m3 (10, 50, or 100 ppm)
technical methyl butyl ketone, 5 days/week for 90 days, with a
dermal application of technical grade 0 -ethyl- 0 -4-nitrophenyl
phenylphosphonothionate (EPN, 1.0 mg/kg, 85%) greatly enhanced the
neurotoxic effects. It was proposed that MIBK partially contributed
to this potentiation by inducing cytochrome P-450 and thus
enhancing the formation of neurotoxic products from methyl- n
-butyl ketone and EPN (Abou-Donia et al., 1985a).
The effect of MIBK on the duration of ethanol-induced loss of
righting reflex and on ethanol elimination has been studied in
mice. MIBK was dissolved in corn oil and injected ip 30 min before
ethanol (4 g/kg ip). At a dose of 501 mg/kg, MIBK significantly
prolonged the duration of ethanol-induced loss of righting reflex.
The concentrations of ethanol in blood and brain on return of the
righting reflex were similar in MIBK-treated and control animals
(Cunningham et al., 1989).
8.9 In vitro toxicity assays
In contrast to methyl n -butyl ketone and n -hexane, MIBK
caused little or no cytopathological or growth-inhibiting effects
in cultured mouse neuroblastoma cells (Selkoe et al., 1978). MIBK
in N,N -dimethylacetamide reduced the activity of mouse liver
alcohol dehydrogenase in vitro (Cunningham et al., 1989). MIBK
was found to inhibit the sulfhydryl-dependent creatine kinase and
adenylate kinase enzymes in vitro but not to the same extent as
did the neurotoxic agent, methyl- n -butyl ketone (Lapin et al.,
1982).
9. EFFECTS ON MAN
9.1 Acute toxicity
In a study on the sensory threshold, Silverman et al. (1946)
exposed 12 volunteers of both sexes to various concentrations of
MIBK for a 15-min period. This period permitted an accurate
observation of olfactory fatigue and increasing or decreasing
irritation of mucous membranes. The sensory response limit was 410
mg/m3 (100 ppm). The majority of the subjects found the odour
objectionable at 820 mg/m3 (200 ppm), and the vapour irritated
the eyes. The low odour threshold (1.64 mg/m3) (Ruth, 1986) and
the irritant effects can provide warning of high concentrations.
Because of its low viscosity, MIBK may, when swallowed, also be
aspirated into the lungs causing a chemical pneumonitis (Panson &
Winek, 1980).
9.2 Short-term exposure
Workers exposed to 410 mg MIBK/m3 (100 ppm) complained either
of headache and nausea or of respiratory irritation (Elkins, 1959).
Tolerance was said to be acquired during the working week but was
lost over the weekend. Reduction of the exposure to 82 mg/m3 (20
ppm) largely eliminated the complaints. In the study of Hjelm et
al. (1990) (see section 6.3) on human volunteers, CNS symptoms
(headache and/or vertigo and/or nausea) were reported at 2-h
exposure levels of 10-200 mg MIBK/m3 (2.4-48.8 ppm). There were
no significant effects from exposure on the performance of a
reaction time task or a test of mental arithmetic.
9.3 Eye and respiratory irritation
Exposure to a concentration of 820 mg MIBK/m3 (200 ppm) for
a 15-min period caused eye irritation in 12 human volunteers
(Silverman et al., 1946). Undiluted MIBK splashed in the eyes may
cause painful irritation (Shell, 1957). A group of workers exposed
to 410 mg MIBK/m3 (100 ppm) complained of respiratory tract
irritation, but there were no complaints at 82 mg/m3 (20 ppm)
(Elkins, 1959). In the study of Hjelm et al. (1990) (see section
6.3) on human volunteers, irritation particularly of the nose and
throat was reported at 2-h exposure levels of 10, 100, and 200
mg/m3 (2.4, 24.4, and 48.8 ppm).
9.4 Long-term exposure
In workers exposed to up to 2050 mg MIBK/m3 (500 ppm) for
20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, over half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomiting, and sore throat. A few of the
workers experienced insomnia, somnolence, heartburn, intestinal
pain, and some unsteadiness. Four workers had slightly enlarged
livers and six had a nonspecific colitis. Clinical chemistry
examination revealed no abnormalities in any of the workers. Five
years later, work practices had greatly improved, the highest MIBK
concentration was 410-430 mg/m3 (100-105 ppm), and the general
concentration was 205 mg/m3 (50 ppm). A few workers still
complained of gastrointestinal and central nervous system effects,
and slight liver enlargement had persisted in two workers, but
other symptoms had disappeared (Armeli et al., 1968).
9.5 Placental transfer
MIBK was detected in maternal and umbilical cord blood samples
from 11 patients (Dowty et al., 1976).
9.6 Neurotoxicity
A few isolated cases of peripheral neuropathy have been
reported after exposure to spray paint or lacquer thinner that
apparently contained MIBK and other hydrocarbon solvents, including
neurotoxic agents (Oh & Kim, 1976; Aubuchon et al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of effects on the environment
MIBK is not likely to persist in the environment. It will
slowly volatilize from soil and water and is readily biodegraded in
fresh and salt water. In the atmosphere, MIBK is estimated to be
degraded by OHÊ radicals with a half-life of approximately 14 h.
MIBK is not expected to bioaccumulate and has a low toxicity for
microorganisms, fish, algae, and aquatic invertebrates. Only in
cases of accidental spillage or inappropriate disposal of wastes
into the environment are levels of MIBK likely to cause toxicity to
organisms in the environment.
10.2 Evaluation of health risks for humans
The general population is exposed to low levels of MIBK. Only
small quantities have been detected in food, drinking-water, and
other beverages (baked goods, 10.9 mg/kg; frozen dairy products,
11.5 mg/kg; gelatins, puddings, 10.9 mg/kg; beverages, 10.2 mg/kg).
For general population exposure, maximum ambient air concentrations
in the range of 0.1 to 0.2 mg/m3 have been defined by two
countries.
Occupational exposure occurs particularly in the production and
use of lacquers, paints, and extraction solvents. The major route
of entry is by inhalation. The low odour threshold (1.64 mg/m3)
and the irritant effects can provide warning of high
concentrations. Exposure to levels of 10-410 mg/m3 (2.4-100 ppm)
produced perceptible irritation of either the eyes, nose, or
throat, and 820 mg/m3 (200 ppm) produced discomfort. Symptoms
such as headache, nausea, and vertigo also occurred at a level of
10-410 mg/m3 (2.4-100 ppm). There were no significant effects
from a 2-h exposure of up to 200 mg/m3 (50 ppm) on a simple
reaction time task or test of mental arithmetic.
In the single report concerning long-term occupational
exposure, where workers were exposed to 2050 mg MIBK/m3 (500 ppm)
for 20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, more than half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomitting, and sore throat. A few workers
experienced insomnia, somnolence, and some unsteadiness. Four had
slightly enlarged livers and six had nonspecific colitis. Five
years later, work practices had greatly improved and the highest
concentrations were reduced to about one fifth of the previous
level. A few workers still complained of irritation of the eyes and
upper respiratory tract as well as gastrointestinal and central
nervous system symptoms. Prolonged skin contact with MIBK caused
irritation and flaking of the skin.
In animal studies, acute systemic MIBK toxicity is low by the
oral and inhalation routes. In a 90-day study, Sprague-Dawley rats
were given MIBK by gavage at doses of 50, 250, or 1000 mg/kg body
weight per day. Lethargy was noted in the highest-dose group and
males showed reduced body weight gain. In this group there was
generalized nephropathy, with an increase in relative kidney weight
and hepatomegaly. Relative kidney weight was also increased in the
animals fed 250 mg/kg per day, and slight hepatomegaly was reported
in male rats only. There were no histopathological lesions in the
liver or other tissues at any dose level. It was concluded that the
NOEL was 50 mg/kg per day. In 90-day inhalation studies on rats and
mice, concentrations of up to 4100 mg/m3 (1000 ppm) did not
result in any life-threatening signs of toxicity. However,
compound-related reversible morphological changes in the liver and
kidney were reported. Levels of 4100 mg/m3 produced evidence of
central nervous system depression. MIBK was capable of increasing
liver weight (at > 1025 mg/m3 (250 ppm)) and inducing hepatic
microsomal metabolism. This may be the explanation for the
exacerbation of haloalkane toxicity and the potentiation of the
neurotoxicity of n -hexane. In 90-day studies with mice, rats,
dogs, and monkeys, only male rats developed hyaline droplets in the
proximal tubules of the kidney (hyaline droplet toxic tubular
nephrosis). This effect in male rats was reversible and of doubtful
significance for humans. MIBK reduces the activity of mouse liver
alcohol dehydrogenase in vitro . It has also been found to
potentiate the cholestatic effects of manganese given with or
without bilirubin.
In rats and mice exposed to MIBK by inhalation at
concentrations of 1230, 4100, or 12 300 mg/m3 (300, 1000, or 3000
ppm) on days 6 to 15 of gestation and sacrificed on day 21 (rats)
or day 18 (mice), marked maternal toxicity was observed at the
highest concentration in both species. This concentration produced
fetotoxicity (reduced fetal body weight and delayed ossification)
but was not embryotoxic or teratogenic. At 4100 and 1230 mg/m3
there was no maternal toxicity and no evidence of embryotoxicity,
fetotoxicity, or teratogenicity.
MIBK did not induce gene mutation in bacterial test systems
(Salmonella typhimurium and Escherichia coli ) either with or
without metabolic activation. Negative results were also obtained
in tests (both with and without metabolic activation) for mitotic
gene conversion in yeast ( Saccharomyces cerevisiae ) and in gene
mutation tests using cultured mammalian cells (mouse lymphoma). In
vitro assays for unscheduled DNA synthesis in primary rat
hepatocytes and for structural chromosome damage in cultured rat
liver cells (RL4) were negative. An in vivo micronucleus test in
mice was negative. These data indicate that MIBK is not genotoxic.
11. RECOMMENDATIONS
At the levels of MIBK to which the general human population is
exposed, there is unlikely to be any hazard. In the occupational
health context, where the major route of exposure is by inhalation,
atmospheric levels should be kept below the recommended
occupational exposure limits by suitably designed work processes
and engineering controls, including ventilation. Skin and eye
contamination should be avoided. Suitable protective clothing and
respiratory protection should be readily available for use in
enclosed spaces, in emergencies, and for certain maintenance
operations. MIBK is inflammable and should be handled accordingly.
MIBK has low toxicity for microorganisms and fish, and its
half-life in the environment is short. Consequently, there is no
risk to the environment provided there are adequate controls to
minimize emissions. Large-scale release could have local adverse
effects on the environment.
12. FURTHER RESEARCH
1. MIBK affects a number of enzyme systems. Therefore, it can
significantly influence the biotransformation of xenobiotics that
are metabolized by these enzymes. Since humans are usually exposed
to more than one compound, studies on the combined effects of
mixtures containing MIBK should be undertaken.
2. There is very little information available on the dose-response
relationships for the effects of MIBK on the human central nervous
system (e.g., reaction time, behavioural effects), on the upper
airways and mucous membranes, or on kidney function. More
information on toxico-kinetics is needed for MIBK alone and in
mixture with other solvents. The skin penetration of MIBK should be
assessed.
3. Epidemiological studies should be undertaken to elucidate the
effects on the nervous system of long-term exposure to moderate
concentrations of MIBK alone or in mixture with other solvents.
REFERENCES
ABDO, K.M., GRAHAM, D.G., TIMMINS, P.R., & ABOU-DONIA, M.B. (1982)
Neurotoxicity of continuous (90 days) inhalation of technical grade
methyl butyl ketone in hens. J. Toxicol. environ. Health, 9:
199-215.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & ABDO, K.M.
(1985a) The joint neurotoxic action of inhaled methyl butyl ketone
vapour and dermally applied 0-ethyl 0-4-nitrophenyl
phenylphosphonothioate in hens: potentiating effect. Toxicol. appl.
Pharmacol., 79: 69-82.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & TIMMONS, P.R.
(1985b) The synergism of n -hexane-induced neurotoxicity by
methyl isobutyl ketone following subchronic (90 days) inhalation in
hens: induction of hepatic microsomal cytochrome P-450. Toxicol.
appl. Pharmacol., 81: 1-16.
ANALYTICAL QUALITY CONTROL (1972) Handbook for analytical quality
control in water and waste water laboratories, Cincinnati, Ohio,
National Environmental Research Centre.
ARMELI, G., LINARI, F., & MARTORANO, G. (1968) [Clinical and
haematochemical examinations in workers exposed to the action of a
higher ketone (MIBK) repeated after 5 years.] Lav. Um., 20:
418-424 (in Italian).
ATKINSON, R., ASCHMANN, S.M., CARTER, W.P.L., & PITTS, J.N., Jr
(1982) Rate constants for the gas-phase reaction of OH radicals
with a series of ketones at 299 ± 2 ° K. Int. J. chem. Kinet.,
14: 839-847.
AUBUCHON, J., ROBINS, H.I., & VISESKUL, C. (1979) Peripheral
neuropathy after exposure to methyl isobutyl ketone in spray paint.
Lancet, August 18: 363-364.
AUSTERN, B.M., DOBBS, R.A., & COHEN, J.M. (1975)
Gas-chromatographic determination of selected organic compounds
added to wastewater. Environ. Sci. Technol., 9: 588-590.
BAMBERGER, R.L., ESPOSITO, G.G., JACOBS, B.W., PODOLAK, G.E., &
MAZUR, J.F. (1978) A new personal sampler for organic vapors. Am.
Ind. Hyg. Assoc. J., 39: 701-708.
BASU, P., CARPENTER, J., CHEN, C., NELSON, H., PERRY, W., SHOCLET,
A., TAYLOR, D., & ZAFRAN, F. (1968) Human exposure assessment:
Methyl isobutyl ketone, Washington, DC, US Environmental Protection
Agency (EPA contract 68-01.4839).
BATYROVA, T.F. (1973) Substantiation of the maximum permissible
concentration of methylisobutyl ketone in air or workrooms. Gig.
Tr. prof. Zabol., 17(11): 52-53.
BELLANCA, J.A., DAVIS, P.L., DONNELLY, B., DAL CORTIVO, L.A., &
WEINBERG, S.B. (1982) Detection and quantitation of multiple
volatile compounds in tissues by GC and GC/MS. J. anal. Toxicol.,
6: 238-240.
BRANCHFLOWER, R.V., SCHULICK, R.D., GEORGE, J.W., & POHL, L.R.
(1983) Comparison of the effects of methyl n -butyl ketone and
phenobarbital on rat liver cytochromes P-450 and the metabolism of
chloroform to phosgene. Toxicol. appl. Pharmacol., 71: 414-421.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER M. (1979a) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUHN, R. (1977a) [Findings concerning the harmful
effect of water-endangering substances on, Daphnia magna .] Z.
Wasser Abwasser Forsch., 10: 161-166 (in German).
BRINGMANN, G. & KUHN, R. (1977b) [Limit values for the harmful
effect of water-endangering substances on bacteria ( Pseudomonas
putida ) and green algae ( Scenedesmus quadricauda ).] Z. Wasser
Abwasser Forsch., 10: 87-98 (in German).
BRINGMANN, G. & KUHN, R. (1978) [Limit values for the harmful
effect of water-endangering substances on blue-green algae
( Microcystis aeruginosa ) and green algae ( Scenedesmus
quadricauda ) in the cell multiplication inhibitor test.] Vom
Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effects of
pollutants on flagellates and ciliates, and/or on holozoic
bacteria-eating and saprozoic protozoa.] GWF-Wasser Abwasser,
122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna straus tested by an approved
standardized procedure.] Z. Wasser Abwasser Forsch., 15: 1-6 (in
German).
BRONDEAU, M.T., BAN, M., BONNET, P., GUENIER, J.P., & DE CEAURRIZ,
J. (1989) Acetone compared to other ketones in modifying the
hepatotoxicity of inhaled 1,2-dichlorobenzene in rats and mice.
Toxicol. Lett. 49: 69-78.
BROOKS, T.M., MEYER, A.L., & HUTSON, D.H. (1988) The genetic
toxicology of some hydrocarbon and oxygenated solvents.
Mutagenesis, 3: 227-232.
BROWN, K.W. & DONNELLY, K.C. (1988) An estimation of the risk
associated with the organic constituents of hazardous and municipal
waste landfill leachates. Hazardous Waste Hazardous Mater., 5(1):
1-30.
BROWN, R.H. & PURNELL, C.J. (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. J. Chromatogr.,
178: 79-90.
BURNHAM, A.K., CALDER, G.V., FRITZ, J.S., JUNK, G.A., SVEC, H.J.,
& WILLIS, R. (1972) Identification and estimation of neutral
organic contaminants in potable water. Anal. Chem., 44: 139-142.
CALL, D.J., BROOKE, L.T., KNUTH, M.L., POIRIER, S.H., & HOGLUND,
M.D. (1985) Fish subchronic toxicity prediction model for
industrial organic chemicals that produce narcosis. Environ.
Toxicol. Chem., 4: 335-341.
CEC (1976) Analysis of organic micropollutants in water,
Luxembourg, Commission of the European Communities (Cost 64b bis).
CFR (1987) Code of Federal Regulations. Methods of analysis for
organic chemicals in groundwater at hazardous waste sites,
Washington, DC, US Government Printing Office (Appendix IX, 40 CFR,
Part 264).
CHEMICAL MANUFACTURERS ASSOCIATION (1984) Ketones Program Panel,
Vol. 1 - Methyl isobutyl ketone: Mutagenicity and teratology
studies, Washington, DC, Chemical Manufacturers Association.
CORWIN, J.F. (1969) Volatile oxygen-containing organic compounds in
sea water: determination. Bull. mar. Sci., 19: 504-509.
COX, R.A., DERWENT, R.G., & WILLIAMS, M.R. (1980) Atmospheric
photooxidation reactions. Rates, reactivity and mechanism for
reaction of organic compounds with hydroxyl radicals. Environ. Sci.
Technol., 14: 57-61.
CUNNINGHAM, J., SHARKAWI, M., & PLAA, G.L. (1989) Pharmacological
and metabolic interactions between ethanol and methyl n -butyl
ketone, methyl isobutyl ketone, methyl ethyl ketone, or acetone in
mice. Fundam. appl. Toxicol., 13: 102-9.
DE CEAURRIZ, J., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J., MICILLINO, J.C., MARIGNAC, B., BONNET, P., MULLER,
J., & GUENIER, J.P. (1984) Quantitative evolution of sensory
irritating and neurobehavioural properties of aliphatic ketones in
mice. Food Chem. Toxicol., 22, 545-549.
DICK, R., DANKOVIC, D., SETZER, J., PHIPPS, F., & LOWRY, L. (1990)
Body burden profiles of methyl ethyl ketone and methyl isobutyl
ketone exposure in human subjects. Toxicologist, 10: 122.
DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic
solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
DIVINCENZO, G.D., KAPLAN, C.J., & DEDINAS, J. (1976)
Characterization of the metabolites of methyl n -butyl ketone,
methyl iso-butyl ketone and methyl ethyl ketone in guinea pig serum
and their clearance. Toxicol. appl. Pharmacol., 36: 511-522.
DODD, D.E. & EISLER, D.L. (1983) Methyl isobutyl ketone ninety-day
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 46-504
submitted to US EPA).
DODD, D.E., LONGO, L.C., & EISLER, D.L. (1982) Nine-day vapour
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 45-501
submitted to US EPA).
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic
constituents. Pediatr. Res., 10: 696-701.
ECDIN (1990) Data bank on environmental chemicals, Ispra (Varese),
Establishment, Joint Research Centre of the Commission of the
European Communities.
ELKINS, H.B. (1959) Chemistry of industrial toxicology, New York,
John Wiley and Sons, p. 121.
ELLISON, W.K. & WALLBANK, T.E. (1974) Solvents in sewage and
industrial waste waters. Identification and determination. Water
Pollut. Control, 73: 656-672.
ELOFSSON, S.A., GAMBERALE, F., HINDMARSH, T., IREGREN, A.,
ISAKSSON, A., JOHNSSON, I., KNAVE, B., LYDAHL, E., MINDUS, P.,
PERSSON, H.E., PHILIPSON, B., STEBY, M., STRUWE, G., SODERMAN, E.,
WENNBERG, A., & WIDEN, L. (1980) Exposure to organic solvents: a
cross-sectional epidemiologic investigation on occupationally
exposed car and industrial spray painters with special reference to
the nervous system. Scand. J. Work Environ. Health, 6: 239-273.
FAWELL, J.K. & HUNT, S. (1981) Organic micropollutants in drinking
water, Medmenham, Water Research Centre (Technical Report No. 159).
FERNANDES, M. (1985) Methodology for the analysis of volatile
compounds in food packaging materials. Coletanea Inst. Technol.
Aliment., 15: 49-59.
FIRE PREVENTION (1981) Information sheets on hazardous materials,
London, London Fire Protection Association, p. 47 (H97 No. 140).
FRANCIS, A.J., IDEN, G.T., NINE, B.J., & CHANG, C.K. (1980)
Characterization of organics in leachates from low level
radioactive waste disposal sites. Nucl. Technol., 50: 158-163.
FROSTLING, H., HOFF, A., JACOBSSON, S., PFAFFLI, P., VAINIOTALO,
S., ZITTING, A., & TECHN, D. (1984) Analytical, occupational and
toxicologic aspects of the degradation products of polypropylene
plastics. Scand. J. Work Environ. Health., 10: 163-169.
GARMAN, J.R., FREUND, T., & LAWLESS, E.W. (1987) Testing for
groundwater contamination at hazardous waste sites. Chromatogr.
Sci., 25: 328-344.
GELLER, I., ROWLANDS, J.R., & KAPLAN, H.L. (1978) Effects of
ketones on operant behaviour of laboratory animals. In: Voluntary
inhalation of industrial solvents, Washington, DC, US Department of
Health, Education and Welfare, p. 363 (DHEW Publication No.
79-779).
GELLER I., GAUSE, E., KAPLAN, H., & HARTMANN, R.J. (1979) Effects
of acetone, methyl ethyl ketone, and methyl isobutyl ketone on a
match-to-sample task in the baboon. Pharmacol. Biochem. Behav.,
11: 401-406.
HAMPTON, C.V., PIERSON, W.R., HARVEY, T.M., UPDEGROVE, W.S., &
MARANO, R.S. (1982) Hydrocarbon gases emitted from vehicles on the
road. 1. A qualitative gas chromatography/mass spectroscopy survey.
Environ. Sci. Technol., 16: 287-298.
HANNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioural effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HJELM, E.W., HAGBERG, M., IREGREN, A., & LÖF, A. (1990) Exposure to
methyl isobutyl ketone: toxicokinetics and occurrence of irritative
and CNS symptoms in man. Int. Arch. occup. environ. Health, 62:
19-26.
IRPTC (1990) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JUHNKE, I. & LÜDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute fish toxicity in the orfe test.] Z.
Wasser Abwasser Forsch., 11(5): 161-164 (in German).
KEITH, L.H. (1974) Chemical characterization of industrial waste
waters by gas chromatography-mass spectrometry. Sci. total
Environ., 3: 87-102.
KRASAVAGE, W.J., O'DONOGHUE, J.L., & DIVINCENZO, G.D. (1982) Methyl
isobutyl ketone. In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, New York, John Wiley and Sons,
Vol. 2e, pp. 4747-4751.
KRISTENSSON, J. & BEVING, H. (1987) A study of painters
occupationally exposed to water and solvent based paints,
Luxembourg, Commission of the European Communities, pp. 71-72 (EUR.
10555).
LANDE, S.S., DURKIN, P.R., CHRISTOPHER, D.H., HOWARD, P.H., &
SAXENA, J. (1976) Investigation of selected potential environmental
contamination: ketonic solvents, Syracuse, New York, Center for
Chemical Hazard Assessment Research Corporation, p. 252.
LAPIN, E.P., WEISSBARTH, S., MAKER, H.S., & LEHRER, G.M. (1982) The
sensitivities of creatine and adenylate kinases to the neurotoxins
acrylamide and metyl n-butyl ketone. Environ. Res., 28: 21-31.
LEO, A. & WEININGER, D. (1984) Medicinal chemistry report,
Claremont, California, Pomona College.
LEVIN, J.-O. & CARLEBORG, L. (1987) Evaluation of solid sorbents
for sampling ketones in work-room air. Ann. occup. Hyg., 31:
31-38.
LIPNICK, R.L., WATSON, K.R., & STRAUSZ, A.K. (1987) A QSAR study of
the acute toxicity of some industrial organic chemicals to
goldfish. Narcosis, electrophile and proelectrophile mechanisms.
Xenobiotica, 17, 1011-1025.
MACEWEN, J.D., VERNOT, E.H., & HAUN, C.C. (1971) Effects of 90-day
continous exposure to methyl isobutyl ketone on dogs, monkeys, and
rats, Ohio, Wright-Patterson AFB, Aerospace Medical Research
Laboratory (Report No. AMRL TR-71-65).
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 7: 611- 614.
MALYSCHEVA, M.V. (1988) [The effect of the skin route of
administration of methyl isobutyl ketone on its toxicity.] Gig. i
Sanit., 10: 79-80 (in Russian).
MICROBIOLOGICAL ASSOCIATES (1986) Subchronic toxicity of methyl
isobutyl ketone in Sprague-Dawley rats. Preliminary report,
Research Triangle Park, North Carolina, Research Triangle Park
Institute, (Study No. 5221.04).
MITI (1978) The biodegradability and bioaccumulation of new and
existing chemical substances, Tokyo, Ministry of International
Trade and Industry, Chemical Products Safety Division, Basic
Industries Bureau.
MOSHLAKOVA, L.A., & INDINA, T.V. (1986) [Gas chromatographic
determination of ketones present simultaneously in the air of the
work area and in the washings from workers' skin. Gig. i Sanit.,
2: 90-91 (in Russian).
NAGANO, M., HARADA, K., MISUMI, J., & NOMURA, S. (1988) [Effect of
methyl isobutyl ketone on methyl n-butyl ketone neurotoxicity in
rats.] Sangyo Igaku, 30: 50-51 (in Japanese).
NIOSH (1984) NIOSH manual of analytical methods: ketones I, 3rd
ed., Cincinnati, Ohio, US National Institute for Occupational
Safety and Health,Vol. 2 (No. 1300).
NTIS (1985) Scientific literature review of aliphatic ketones,
secondary alcohols and related esters in flavour usage. Volume 1 -
Introduction and summary, tables of data bibliography: Al,
Washington, DC, National Technical Information Service, Part 2
(PB85-141059).
O'DONOGHUE, J.L., HAWORTH, S.R., CURREN, R.D., KIRBY, P.E., LAWLOR,
T., MORAN, E.J., PHILLIPS, R.D., PUTNAM, D.L., ROGERS-BACK, A.M.,
SLESINSKI, R.S., & THILAGAR, A. (1988) Mutagenicity studies on
ketone solvents: methyl ethyl ketone, methyl isobutyl ketone and
isophorone. Mutat. Res., 206: 149-161.
OECD (1977) The asessment of environmental chemicals: production
figures and use patterns for some high volume chemicals, Paris,
Organization for Economic Cooperation and Development
(ENV/Chem./77.6).
OECD (1984) Data interpretation guides for initial hazard
assessment of chemicals (provisional), Paris, Organisation for
Economic Cooperation and Development, p. 31.
OH, S.J. & KIM, J.M. (1976) Giant axonal swelling in "Huffer's"
neuropathy. Arch. Neurol., 33: 583-586.
PANSON, R.D. & WINEK, C.L. (1980) Aspiration toxicity of ketones.
Clin. Toxicol., 17: 271-317.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERICKSON, M.D. (1982) Purgeable organic compounds
in mothers' milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PHILLIPS, R.D., MORAN, E.J., DODD, D.E., FOWLER, E.H., KARY, C.D.,
&O'DONOGHUE, J. (1987) A 14-week vapor inhalation toxicity study of
methyl isobutyl ketone. Fundam. appl. Toxicol., 9: 380-388.
PILON, D. (1987) Interaction cétones/hydrocarbures halogènes:
Utilization des métabolites cétoniques comme indices d'exposition
aux cétones, Montreal, University of Montreal (Ph.D. Thesis).
PILON, D., BRODEUR, J., & PLAA, G.L. (1988) Potentiation of carbon
tetrachloride-induced liver injury by ketonic and ketogenic
compounds: role of the CCl4 dose. Toxicol. appl. Pharmacol.,
94: 183-190.
PLAA, G.L. & AYOTTE, D. (1985) Taurolithocholate-induced
intrahepatic cholestasis: potentiation by methyl isobutyl ketone
and methyl n-butylketone in rats. Toxicol. appl. Pharmacol., 80:
228-234.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimps
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77
RACCIO, J.M. & WIDOMSKI, J.R. (1981) Quality control of flavors in
soft drinks and the analysis of residual solvents in food packaging
films utilizing headspace sampling with open tubular columns.
Chromatogr. Newsl., 9(2): 42-45.
RIPPSTEIN, W.J. & COLEMAN, M.E. (1984) [Toxicological evaluation on
the Columbian spacecraft.] Kosmet. Biol. Aviakosm. Med., 18:
87-96 (in Russian).
RTECS (1987) Registry of toxic effects of chemical substances,
1985-86 ed., Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Vol. 1-6 (DHSS (NIOSH) Publication No. 87-114).
RUTH, J.H. (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am. Ind. Hyg. Assoc. J., 47:
A142-A151.
SABROE, S. & OLSEN, J. (1979) Health complaints and work conditions
among lacquerers in the Danish furniture industry. Scand. J. soc.
Med., 7: 97-104
SATO, A. & NAKAJIMA, T. (1979) Partition coefficients of some
aromatic hydrocarbons and ketones in water, blood and oil. Brit. J.
ind. Med., 36, 231-234.
SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13: 349-352.
SAX, N.I. (1979) Dangerous properties of industrial materials, 6th
ed, New York, Van Norstrand Reinhold Company, p. 750.
SELKOE, D.J., LUCKENBILL-EDDS, L., & SHELANSKI, M.L. (1978) Effects
of neurotoxic industrial solvents on cultured neuroblastoma cells:
methyl n -butyl ketone, n -hexane, and derivatives. J.
Neuropathol. exp. Neurol., 37: 768-789.
SHELL (1957) Methyl isobutyl ketone: industrial hygiene bulletin,
New York, Shell Chemical Corporation, pp. 57-112.
SILVERMAN, L., SCHULTE, H.F., & FIRST, M.W. (1946) Further studies
on sensory response to certain industrial solvent vapors. J. ind.
Hyg. Toxicol., 28: 262-266.
SMYTH, H.F. (1956) Hygienic standards for daily inhalation. Am.
Ind. Hyg. Assoc. J., 17: 129-266.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SPECHT, H. (1938) Acute response of guinea pigs to inhalation of
methyl isobutyl ketone, Washington, DC, US Public Health Service,
pp. 292-300 (US Public Health Report No. 53).
SPECHT, H., MILLER, J.W., VALAER, P.J., & SAYERS, R.R. (1940) Acute
response of guinea pigs to the inhalation of ketone vapours,
Washington, DC, US Public Health Service, Division of Industrial
Hygiene (NIH Bulletin No. 176).
SPENCER, P.S. & SCHAUMBURG, H.H. (1976) Feline nervous system
response to chronic intoxication with commercial grades of methyl
n -butyl ketone, methyl isobutyl ketone and methyl ethyl ketone.
Toxicol. appl. Pharmacol., 37: 301-311.
SPENCER, P.S., SCHAUMBURG, H.H., RALEIGH, R.L., & TERHAAR, C.J.
(1975) Nervous system degeneration produced by the industrial
solvent methyl n -butyl ketone. Arch. Neurol., 32: 219-222.
TNO (1983a) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 2.
TNO (1983b) Volatile compounds in food: Qualitative data, Zeist,
Netherlands, Organization for Applied Scientific Research.
TNO (1986) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 5.
TNO (1987) Volatile compounds in food: Supplement 4, Zeist,
Netherlands, Organization for Applied Scientific Research.
TOMCZYK, H. & ROGACZEWSKA, T. (1979) [Gas chromatographic
determination of airborne methyl isobutyl ketone, methyl isobutyl
carbinol, acetone, toluene and o-xylene.] Med. Pr., XXX(6):
417-423 (in Polish).
TYL, R.W. (1984) A teratologic evaluation of methyl isobutyl ketone
in Fischer 344 rats and CD-1 mice following inhalation exposure,
Washington, DC, Chemical Manufacturers Association (Bushy Run
Research Center Report No. 47.505).
TYL, R.W., FRANCE, K.A., FISHER, L.C., PRITTS, I.M., TYLER, T.R.,
PHILLIPS, R.D., & MORAN, E.J. (1987) Developmental toxicity
evaluation of inhaled methyl isobutyl ketone in Fischer 344 rats
and CD-l mice. Fundam. appl. Toxicol., 8: 319-327.
VERNOT, E.H., MACEWEN, J.D., & HARRIS, E.S. (1971) Continuous
exposure of animals to methyl isobutyl ketone, Ohio, Wright
Patterson AFB, Aerospace Medical Research Laboratory (US NTIS AD
Report No. 751443).
VERSCHUEREN, K. (1983) Handbook of environmental data on organic
chemicals, 2nd ed., New York, Van Nostrand Reinhold Company, pp.
459-461.
VEZINA, M. & PLAA, G.L. (1987) Potentiation by methyl isobutyl
ketone of the cholestasis induced in rats by a manganese-bilirubin
combination or manganese alone. Toxicol. appl. Pharmacol. 91:
477-483.
VEZINA, M. & PLAA, G.L. (1988) Methyl isobutyl ketone metabolites
and potentiation of the cholestasis induced in rats by a
manganese-bilirubin combination or manganese alone. Toxicol. appl.
Pharmacol. 92: 419-427.
VEZINA, M., AYOTTE, P., & PLAA, G.L. (1985) Potentiation of
necrogenic and cholestatic liver injury by 4-methyl-2-pentanone.
Can. Fed. Biol. Soc., 28: 221.
WEBB, R.G., GARRISON, A.W., KEITH, L.H., & MCGUIRE, J.H. (1973)
Current practice in GC-MS analysis of organics in water,
Washington, DC, US Environmental Protection Agency (EPA Report No.
R2-73-277) (NTIS PB 224 947/2).
WELLER, J.P. & WOLF, M. (1989) Mass spectroscopy and headspace gas
chromatography. Beitr. gerichtl. Med., 47: 525-532.
ZAKHARI, S., LEVY, P., LIEBOWITZ, M., & AVIADO, D.M. (1977) Acute
oral, intraperitoneal, and inhalation toxicity of methyl isobutyl
ketone in the mouse. In: Goldberg, L., ed. Isopropanol and ketones
in the environment, Cleveland, Ohio, CRC Press, Part 3, Chapter
10-14, pp. 93-133.
ZLATKIS, A. & LIEBICH, H.M. (1971) Profile of volatile metabolites
in human urine. Clin. Chem., 17: 592-594.
RESUME
La méthylisobutylcétone est un liquide limpide d'odeur
douceâtre produite en vue d'une vaste utilisation commerciale comme
solvant. On peut la doser par chromatographie en phase gazeuse avec
détection par ionisation de flamme. Elle s'évapore rapidement dans
l'atmosphère où elle subit une photoconversion à brève échéance. La
méthylisobutylcétone est facilement biodégradable et, compte tenu
de sa solubilité moyenne dans l'eau et de son faible coefficient de
partage entre l'octanol et l'eau, elle devrait présenter un faible
potentiel de bioaccumulation. Les limites d'exposition
professionnelle sont de 100-400 mg/m3 (moyenne pondérée par
rapport au temps: TWA) et de 5-300 mg/m3 (valeur plafond: CLV)
selon les pays.
La méthylisobutylcétone est rapidement métabolisée en produits
d'excrétion solubles dans l'eau et sa toxicité aiguë générale est
faible chez l'animal après exposition par voie orale ou
respiratoire. L'expérimentation animale n'a pas révélé
d'axonopathie périphérique. On ne dispose pas de données précises
sur la CL50. Une exposition de 4 heures à une concentration de 16
400 mg/m3 (4000 ppm) a été mortelle pour des rats. La
méthylisobutylcétone liquide ou sous forme de vapeurs à la
concentration de 10 à 410 mg/m3 (2,4 à 100 ppm) est irritante
pour les yeux et les voies respiratoires supérieures. Des
concentrations allant jusqu'à 200 mg/m3 (50 ppm) n'ont produit
aucun effet sensible sur l'homme lors d'épreuves portant sur le
temps de réaction et le calcul mental. Un contact prolongé ou
répété avec la peau peut produire un dessèchement et des crevasses.
L'aspiration accidentelle de méthylisobutylcétone liquide peut
provoquer une pneumonie chimique.
Lors d'une étude de 90 jours effectuée en gavant des rats, on
a obtenu une dose sans effet observable de 50 mg/kg par jour. Des
études d'inhalation de 90 jours portant sur des rats et des souris
à des concentrations allant jusqu'à 4100 mg/m3 (1000 ppm) n'ont
pas révélé de signes de toxicité engageant le pronostic vital.
Toute fois des altérations morphologiques réversibles liées à
l'administration de ce composé ont été observées au niveau du foie
et des reins. Dans un certain nombre d'études, on a observé une
hypertrophie du foie dès la dose de 1025 mg/m3 (250 ppm). Exposés
à 4100 mg/m3 (1000 ppm) pendant 50 jours, des poulets ont
présenté une induction des enzymes microsomiques. A doses plus
élevées (jusqu'à 8180 mg/m3, 1996 ppm) les effets se limitaient
à un accroissement du poids du foie sans lésion histologique. Lors
d'études de 90 jours effectuées sur des souris, des rats, des
chiens et des singes, seuls les rats mâles ont présenté des
altérations histologiques: présence de gouttelettes hyalines dans
les tubules proximaux des reins (néphrose tubulaire toxique à
inclusions hyalines). Cet effet s'est révélé réversible et sa
portée en toxicologie humaine demeure incertaine. Il est possible
que la potentialisation de la toxicité des alcanes halogénés par la
méthylisobutylcétone repose sur une induction enzymatique. La
méthylisobutylcétone potentialise également l'effet cholestatique
du manganèse administré avec ou sans bilirubine.
Des babouins exposés pendant sept jours à une dose de 205
mg/m3 (50 ppm) ont présenté des troubles neuro-comportementaux.
La méthylisobutylcétone est foetotoxique à une concentration
manifestement toxique pour la mère (12 300 mg par m3, 3000 ppm)
mais elle n'est pas embryotoxique ni tératogène à cette
concentration. A la concentration de 4100 mg/m3 (1000 ppm), on
n'a pas observé d'embryotoxicité, de foetotoxicité ni de
tératogénicité chez les rats et les souris.
On a recherché la génotoxicité éventuelle de la
méthylisobutylcétone en pratiquant un certain nombre d'épreuves à
court terme, consistant notamment en tests sur des cellules
mammaliennes, des bactéries et des levures ainsi que dans la
recherche de micro-noyaux chez la souris. Les résultats indiquent
que la méthyliso-butylcétone n'est pas génotoxique. En ce qui
concerne la génotoxicité à long terme ou la cancérogénicité, on ne
dispose d'aucune donnée.
A la concentration de 410 mg/m3 (100 ppm), la
méthylisobutylcétone peut produire des symptômes chez l'homme
consistant en irritation occulaire, migraines, nausées, vertiges et
fatigue et qui correspondent à un effet dépresseur réversible sur
le système nerveux central. Toutefois, rien n'indique l'existence
de lésions permanentes.
La toxicité pour les organismes et micro-organismes aquatiques
est faible.
Compte tenu de la volatilité relativement forte de la
méthylisobutylcétone, de sa photoconversion rapide dans
l'atmosphère, de sa biodégradabilité et de sa faible toxicité pour
les mammifères et la faune aquatique, il est vraisemblable que
cette substance ne peut exercer d'effets néfastes sur
l'environnement qu'à la suite de déversements accidentels ou de la
décharge incontrôlée d'effluents industriels.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des effets sur l'environnement
La méthylisobutylcétone ne devrait pas persister dans
l'environnement. Elle se volatilise lentement à partir du sol et de
l'eau et subit une biodégradation rapide dans l'eau douce et l'eau
salée. Dans l'atmosphère, on pense qu'elle est décomposée par les
radicaux libres OH avec une demi-vie d'environ 14 heures. Elle ne
s'accumule probablement pas et présente une faible toxicité pour
les micro-organismes, les poissons, les algues et les invertébrés
aquatiques. Ce n'est qu'en cas de déversement accidentel ou de
rejet incontrôlé de déchets que cette substance est susceptible
d'atteindre des concentrations toxiques pour les êtres vivants.
2. Evaluation des risques pour la santé humaine
La population générale n'est exposée qu'à de faibles
concentrations de méthylisobutylcétone. On en a décelé de faibles
quantités dans les denrées alimentaires et dans l'eau de
consommation ou autres boissons (produits panifiés, 10,9 mg/kg;
produits laitiers, 11,5 mg/kg; gélatines, puddings, 10,9 mg/kg;
boissons diverses, 10,2 mg/kg). En ce qui concerne la population
générale, deux pays ont fixé des concentrations maximales dans
l'air ambiant qui se situent dans les limites de 0,1 à 0,2 mg/m3.
L'exposition professionnelle se produit notamment lors de la
production et de l'utilisation de vernis, de peintures et de
solvants d'extraction. La principale voie de pénétration est
l'inhalation. Le faible seuil olfactif (1,64 mg/m3) et les effets
irritants de cette substance peuvent avertir de la présence de
fortes concentrations. L'exposition à ces concentrations de 10 à
410 mg/m3 (2,4 à 100 ppm) a produit une irritation perceptible au
niveau des yeux, du nez et de la gorge; à 820 mg/m3 (200 ppm), on
éprouve une sensation de malaise. Entre 10 et 410 mg par m3 (2,4
à 100 ppm), on a également observé des céphalées, des nausées et
des vertiges. Une exposition de deux heures à des concentrations
allant jusqu'à 200 mg/m3 (50 ppm) n'a pas produit d'effets
sensibles à en juger par un simple test de temps de réaction et de
calcul mental.
On ne dispose que d'un seul rapport faisant état d'une
exposition professionnelle de longue durée à une dose de 2050
mg/m3 (500 ppm), 20 à 30 minutes par jour et à 328 mg/m3 (80
ppm) pour la majeure partie du reste de la journée. Plus de la
moitié des 19 ouvriers exposés se sont plaints de faiblesse, de
perte d'appétit, de maux de tête, d'irritation oculaire, de maux
d'estomac, de nausées, de vomissements et de maux de gorge.
Quelques ouvriers ont éprouvé de l'insomnie, de la somnolence et
une perte d'équilibre. Chez quatre d'entre eux on a constaté une
légère hypertrophie du foie et chez six autres une colite non
spécifique. Cinq années plus tard, les méthodes de travail
s'étaient considérablement améliorées et les concentrations
maximales réduites au cinquième des teneurs précédentes. Quelques
ouvriers se sont encore plaints d'irritation au niveau des yeux et
des voies respiratoires supérieures ainsi que de symptômes
digestifs et neurologiques. La contact prolongé avec la peau a
provoqué une irritation cutanée et des crevasses.
Il ressort de l'expérimentation animale que la toxicité
générale aiguë de la méthylisobutylcétone est faible, par voie
orale ou par inhalation. Lors d'une étude de 90 jours, des rats
Sprague-Dawley ont reçu par gavage de la méthylisobutylcétone à des
doses quotidiennes de 50, de 250 et 1000 mg/kg de poids corporel.
On a noté une léthargie chez des animaux du groupe soumis à la dose
la plus élevée et, chez les mâles, une réduction du gain de poids.
Les animaux de ce groupe présentaient une néphropathie généralisée,
avec augmentation du poids relatif des reins et une hypertrophie du
foie. Cette augmentation du poids relatif des reins était également
notable chez les animaux soumis à la dose de 250 mg/kg, mais à
cette dose, on ne constatait qu'une légère hypertrophie du foie
chez les mâles. Quelle que soit la dose, on n'a pas constaté de
lésion histopathologique au niveau du foie ou des autres tissus. La
dose sans effet observable a été évaluée à 60 mg/kg et par jour.
Lors d'une étude de même durée au cours de laquelle on a fait
inhaler à des rats et des souris des concentrations allant jusqu'à
4100 mg/m3 (1000 ppm) on n'a pas relevé de signes de toxicité
engageant le pronostic vital. Toute fois des altérations
morphologiques réversibles liées à l'administration de cette
substance ont été observées au niveau du foie et des reins. A la
concentration de 4100 mg par m3, on observait des signes de
dépression du système nerveux central. La méthylisobutylcétone a
provoqué une augmentation du poids du foie (à une dose supérieure
à 1025 mg/m3, soit 250 ppm) et provoqué l'induction des enzymes
microsomiques du foie. C'est ce dernier mécanisme qui serait à la
base de l'exacerbation de la toxicité des alcanes halogénés et de
la potentialisation de la neurotoxicité dunw-hexane. Lors d'études
de 90 jours sur des souris, des rats, des chiens et des singes, on
a observé, chez les rats seulement, l'apparition d'inclusions
hyalines dans les tubules proximaux des reins (néphrose tubulaire
à inclusions hyalines d'origine toxique). Cet effet observé chez
les rats mâles était réversible et il est douteux qu'il ait une
signification quelconque en toxicologie humaine. La
méthylisobutylcétone réduit l'activité de l'alcool-déshydrogénase
hépatique chez la souris in vitro . On a également constaté
qu'elle potentialisait les effets cholestatiques du manganèse en
présence ou en l'absence de bilirubine.
Des rats et des souris exposés par inhalation à des
concentrations de 1230, 4100 ou 12 300 mg/m3 (300, 1000 ou 3000
ppm) du sixième au quinzième jours de la gestation puis sacrifiés
le vingt-et-unième jour (rats) ou le dixhuitième jour (souris), ont
présenté des signes marqués d'intoxication à la concentration la
plus forte. Cette concentration était foetotoxique (réduction du
poids foetal et ossification retardée) mais n'était ni
embryotoxique ni tératogène. Aux concentrations de 4100 et 1230
mg/m3, on n'a constaté aucune toxicité pour les mères ni signe
d'embryotoxicité, de foetotoxicité ou de tératogénicité.
La méthylisobutylcétone n'a pas produit de mutation génique
dans des systèmes d'épreuve bactériens (Salmonella typhimurium et
Escherichia coli ), qu'il y ait ou non activation métabolique. On
a également obtenu des résultats négatifs dans différentes épreuves
(avec ou sans activation métabolique) à la recherche de conversions
géniques mitotiques dans des levures ( Saccharomyces cerevisiae )
ou lors d'épreuves de mutation génique sur des cellules
mammaliennes en culture (lymphome murin). La recherche in vitro
d'une synthèse anarchique de l'ADN dans des hépatocytes primaires
de rat et de lésions chromosomiques structurales dans des cellules
de foie de rat en culture (RL4) s'est révélée négative. Chez la
souris, la recherche in vivo de micro-noyaux s'est également
révélée négative. Toutes ces données montrent que la
méthyl-isobutylcétone n'est pas génotoxique.
RECOMMANDATIONS
Les concentrations de méthylisobutylcétone auxquelles la
population, dans son ensemble, est susceptible d'être exposée, ne
présentent vraisemblablement aucun danger. La principale voie
d'exposition professionnelle est la voie respiratoire, aussi les
concentrations atmosphériques devront être maintenues en dessous
des limites recommandées d'exposition professionnelle, grâce à un
aménagement convenable des procédés de production et à des moyens
mécaniques tels que la ventilation. Il convient d'éviter toute
contamination de la peau et des yeux. Des vêtements protecteurs
appropriés ainsi que des masques respiratoires doivent être placés
dans les ateliers confinés; ils seront utilisés en cas d'urgence ou
pour effectuer certaines opérations d'entretien. La
méthylisobutylcétone est inflammable et doit donc être manipulée
avec les précautions d'usage.
La méthylisobutylcétone présente une faible toxicité pour les
micro-organismes et pour les poissons et sa demivie dans
l'environnement est courte. Il s'ensuit qu'elle ne présente aucun
risque pour l'environnement, dans la mesure où des mesures
appropriées sont prises pour réduire les émissions au minimum. La
décharge de quantités importantes dans l'environnement pourrait
avoir localement des effets indésirables.
RECHERCHES A EFFECTUER
1. La méthylisobutylcétone affecte un certain nombre de systèmes
enzymatiques. Elle peut donc avoir une influence sensible sur
la biotransformation des produits xénobiotiques métabolisés
par ces enzymes. Etant donné que l'homme est généralement
exposé à plusieurs composés différents, il faudrait
entre-prendre des études sur les effets combinés de mélanges
contenant de la méthylisobutylcétone.
2. On ne dispose que de très peu d'informations sur la relation
dose-réponse relative aux effets toxiques de la
méthylisobutylcétone sur le système nerveux central (par
exemple temps de réaction, effets comportementaux), sur les
voies respiratoires supérieures, sur les muqueuses et sur la
fonction rénale. Il faudrait obtenir davantage de
renseignements sur la toxico-cinétique de cette cétone, soit
seule soit associée à d'autres solvants. Il faudra également
étudier la pénétration percutanée de la méthylisobutylcétone.
3. Il faudrait entreprendre des études épidémiologiques pour
élucider les effets exercés à long terme sur le système
nerveux central par des concentrations moyennes de
méthylisobutylcétone soit seule soit associée à d'autres
solvants.
RESUMEN
La metil isobutil acetona (MIBA) es un líquido transparente de
buen olor que se produce a escala comercial y tiene un uso muy
extendido como disolvente. Puede medirse mediante cromatografía de
gases con detección de ionización de llama. Se evapora rápidamente
a la atmósfera, donde se fototransforma en poco tiempo. La MIBA es
fácilmente biodegradable, lo que, junto con su moderada solubilidad
en el agua y su reducido coeficiente de partición octanol/agua,
sugiere que tiene un bajo potencial de bioacumulación. Los límites
de exposición profesional varían entre 100-410 mg/m3 (promedio
ponderado en el tiempo) y 5-300 mg/m3 (valor máximo) en distintos
países.
La MIBA se metaboliza fácilmente para dar productos de
excreción hidrosolubles y su toxicidad sistémica aguda en animales
es baja por las vías de exposición oral y de inhalación. No se ha
observado axonopatía periférica en estudios realizados en animales.
No se dispone de datos exactos sobre la CL50. La exposición a 16
400 mg/m3 (4000 ppm) durante 4 horas resultó letal para la rata.
Las concentraciones de MIBA líquida y en vapor comprendidas entre
10 y 410 mg/m3 (2,4-100 ppm) son irritantes para los ojos y las
vías respiratorias superiores. Con concentraciones de hasta 200
mg/m3 (50 ppm) no se observaron en el hombre efectos
significativos en una prueba sencilla de tiempo de reacción ni en
una prueba de aritmética mental. El contacto cutáneo prolongado o
repetido puede desecar y descamar la piel. La aspiración accidental
de MIBA líquida puede provocar pneumonitis química.
En un estudio de ceba de ratas durante 90 días, se determinó
un nivel sin efecto observado de 50 mg/kg. En estudios de
inhalación durante 90 días en ratas y ratones, concentraciones de
hasta 4100 mg/m3 (1000 ppm) no produjeron ningún signo de
toxicidad con peligro para la vida. No obstante, se notificaron
cambios morfológicos reversibles relacionados con el compuesto en
el hígado y el riñón. En varios estudios se observó que
concentraciones de MIBA tan bajas como 1025 mg/m3 (250 ppm) eran
capaces de aumentar el tamaño del hígado. Mediante la exposición a
4100 mg/m3 (1000 ppm) durante 50 días, se indujo actividad
metabólica en las enzimas microsómicas del hígado del pollo. A
dosis más elevadas (hasta 8180 mg/m3, 1996 ppm) los efectos se
limitaron a un aumento del peso del hígado sin lesiones
histológicas. En estudios durante 90 días con ratones, ratas,
perros y monos, sólo las ratas macho presentaron corpúsculos
hialinos en los túbulos proximales del riñón (nefrosis tubulotóxica
de corpúsculos hialinos). Este efecto en la rata macho resultó ser
reversible y de dudosa importancia para el hombre. La inducción
enzimática puede ser la base de la potenciación de la toxicidad de
los haloalcanos por la MIBA. Se observó también que la MIBA era
capaz de potenciar el efecto colestático del manganeso administrado
con o sin bilirrubina.
En papiones expuestos durante 7 días a 205 mg/m3 (50 ppm),
se observaron efectos en el neurocomportamiento.
La MIBA resulta fetotóxica a una concentración que produce sin
lugar a dudas toxicidad materna (12 300 mg/m3, 3000 ppm) pero no
es embriotóxica ni teratogénica en esa concentración. En una
concentración de 4100 mg/m3 (1000 ppm), no resultó ni
embriotóxica, ni fetotóxica ni teratogénica en la rata ni en el
ratón.
Se ha estudiado la genotoxicidad de la MIBA en varios ensayos
a corto plazo, inclusive pruebas in vitro con bacterias, levaduras
y células de mamíferos y un ensayo de micronúcleos en el ratón.
Esos estudios indican que la MIBA no es genotóxica. No se dispone
de informes sobre estudios a largo plazo ni estudios de
carcinogenicidad.
Aunque con una concentración de 410 mg/m3 (100 ppm) la MIBA
puede inducir en el hombre síntomas como irritación ocular, dolores
de cabeza, náuseas, mareos y fatiga, que corresponden a un efecto
reversible de depresión del sistema nervioso central, no existen
pruebas de que produzca lesiones permanentes en el sistema
nervioso.
La toxicidad de la MIBA para organismos y micro-organismos
acuáticos es baja.
La volatilidad relativamente alta de la MIBA, su rápida
fototransformación en la atmósfera, su fácil biodegradación y su
baja toxicidad para mamíferos y organismos acuáticos indican que
los efectos medioambientales adversos de esta sustancia
probablemente sólo se producirán como consecuencia de vertidos
accidentales o de efluentes industriales no controlados.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS
EFECTOS EN EL MEDIO AMBIENTE
1. Evaluación de los efectos en el medio ambiente
La MIBA tiene pocas probabilidades de persistir en el medio
ambiente. Se volatiliza poco a poco desde el suelo y el agua y se
biodegrada fácilmente en agua dulce y salada. En la atmósfera, se
ha calculado que la MIBA es degradada por los radicales OHÊ una
semivida de aproximadamente 14 horas. En principio, la MIBA no se
bioacumula y tiene una toxicidad baja para micro-organismos, peces,
algas e invertebrados acuáticos. Sólo en los casos de vertido
accidental o de evacuación inadecuada de desechos en el medio
ambiente es probable que los niveles de MIBA provoquen toxicidad en
los organismos del entorno.
2. Evaluación de los riesgos para la salud humana
La población general está expuesta a niveles reducidos de MIBA.
Se han detectado sólo pequeñas cantidades en los alimentos, el agua
potable y otras bebidas (alimentos horneados, 10,9 mg/kg; productos
lácteos congelados, 11,5 mg/kg; gelatinas y budines, 10,9 mg/kg;
bebidas, 10,2 mg/kg). En cuanto a la exposición de la población
general, dos países han definido concentraciones máximas en el aire
entre 0,1 y 0,2 mg/m3.
La exposición profesional tiene lugar especialmente en la
producción y utilización de lacas, pinturas y disolventes de
extracción. La principal vía de entrada es por inhalación. El bajo
umbral olfativo (1,64 mg/m3) y los efectos irritantes pueden
servir como indicadores de las concentraciones elevadas. La
exposición a niveles de 10-410 mg/m3 (2,4-100 ppm) produjo
irritación perceptible de los ojos, la nariz, o la garganta y 820
mg/m3 (200 ppm) produjeron molestias. Con un nivel de 10-410
mg/m3 (2,4-100 ppm) también se produjeron síntomas como dolor de
cabeza, náuseas y vértigos. No se observaron efectos significativos
debidos a una exposición durante 2 horas a hasta 200 mg/m3 (50
ppm) al realizar una prueba sencilla de tiempo de reacción ni una
prueba de aritmética mental.
En el único informe sobre un estudio de la exposición
profesional a largo plazo, en el que se expuso a trabajadores a
2050 mg MIBA/m3 (500 ppm) durante 20-30 minutos al día y a 328
mg/m3 (80 ppm) durante la mayor parte del resto de la jornada
laboral, más de la mitad de los 19 trabajadores se quejaron de
debilidad, pérdida del apetito, dolores de cabeza, irritación
ocular, dolor de estómago, náuseas, vómitos y dolor de garganta.
Algunos trabajadores sufrieron insomio, somnolencia y cierta
inestabilidad. En cuatro se observó un ligero agrandamiento del
hígado y en seis se observó colitis no específica. Al cabo de 5
años, las prácticas laborales habían mejorado en gran medida y las
concentraciones más elevadas se redujeron a aproximadamente la
quinta parte del nivel anterior. Algunos trabajadores siguieron
quejándose de irritación en los ojos y de las vías respiratorias
superiores así como de síntomas gastrointestinales y del sistema
nervioso central. El contacto cutáneo prolongado con la MIBA
provocó irritación y descamación de la piel.
En estudios en animales, la toxicidad sistémica aguda de la
MIBA es baja por las vías oral y respiratoria. En un estudio de 90
días de duración, se cebó con MIBA a ratas Sprague-Dawley en dosis
diarias de 50, 250 ó 1000 mg/kg de peso corporal. Se observó
letargo en el grupo que recibió la dosis más alta y en los machos
se observó una reducción del ritmo de aumento del peso corporal. En
este grupo se observó nefropatía generalizada, con aumento del peso
relativo del riñón y hepatomegalia. El peso relativo del riñón
también aumentó en los animales alimentados con 250 mg/kg al día,
y se observó ligera hepatomegalia sólo en los machos. No
aparecieron lesiones histopatológicas en el hígado ni en otros
tejidos con ninguna de las dosis administradas. Se concluyó que el
nivel de efecto no observado era de 50 mg/kg al día. En estudios de
inhalación durante 90 días realizados en ratas y ratones, las
concentraciones de hasta 4100 mg/m3 (1000 ppm) no originaron
ningún signo de toxicidad que pusiera en peligro la vida. No
obstante, se comunicó la observación de cambios morfológicos
reversibles relacionados con el compuesto en el hígado y el riñón.
Con niveles de 4100 mg/m3 se observaron signos de depresión del
sistema nervioso central. La MIBA fue capaz de aumentar el peso del
hígado (concentración > 1025 mg/m3 (250 ppm)) y de inducir el
metabolismo microsómico hepático. Esto puede explicar la
exacerbación de la toxicidad de los haloalcanos y la potenciación
de la neurotoxicidad del n‹hexano. En estudios de 90 días con
ratones, ratas, perros y monos, sólo las ratas macho desarrollaron
corpúsculos hialinos en los túbulos proximales del riñón (nefrosis
tubulotóxica de corpúsculos hialinos). Este efecto en ratas macho
resultó ser reversible y de dudosa importancia para el hombre. In
vitro, la MIBA reduce la actividad de la deshidrogenasa alcohólica
en el hígado del ratón. También se ha observado que potencia los
efectos colestáticos del manganeso administrado con o sin
bilirrubina.
En ratas y ratones expuestos a la inhalación de MIBA en
concentraciones de 1230, 4100 ó 12 300 mg/m3 (300, 1000 ó 3000
ppm) en los días 6 a 15 de la gestación y sacrificados el día 21
(ratas) o el día 18 (ratones), se observó una notable toxicidad
materna a la concentración más elevada en ambas especies. Esta
concentración produjo fetotoxicidad (peso del cuerpo fetal reducido
y retraso en la osificación) pero no resultó embriotóxico ni
teratogénico. A 4100 y 1230 mg/m3 no se observó ni toxicidad
materna ni síntomas de embriotoxicidad, fetotoxicidad o
teratogenicidad.
La MIBA no indujo mutación genética en sistemas de ensayo
bacterianos (Salmonella typhimurium y Escherichia coli), con o sin
activación metabólica. También se obtuvieron resultados negativos
en los ensayos (tanto con y sin activación metabólica) para la
conversión mitótica de genes en levaduras (Saccharomyces
cerevisiae) y en pruebas de mutación génica con cultivos de células
de mamíferos (linfoma de ratón). Los ensayos in vitro sobre
síntesis no controlada de ADN en hepatocitos primarios de rata y
sobre lesiones cromosómicas estructurales en cultivos de células
hepáticas de rata (RL4) resultaron negativos. En el ratón, un
ensayo de micronúcleo in vivo resultó negativo. Estos datos indican
que la MIBA no es genotóxica.
RECOMENDACIONES
En los niveles de MIBA a que está expuesta la población humana
general, es poco probable que se plantee riesgo alguno. En el medio
laboral, donde la principal vía de exposición es por inhalación,
los niveles atmosféricos deben mantenerse por debajo de los límites
de exposición profesional recomendados mediante procesos de trabajo
y controles de ingeniería, inclusive la ventilación, de diseño
adecuado. Debe evitarse la contaminación de la piel y de los ojos.
Debe facilitarse el uso de prendas protectoras adecuadas y de
protección respiratoria en los espacios cerrados, en casos de
emergencia y para ciertas operaciones de mantenimiento. La MIBA es
inflamable y debe manipularse teniendo esta característica en
cuenta.
La MIBA tiene baja toxicidad para los microorganismos y los
peces, y su semivida en el medio ambiente es corta. Por
consiguiente, no presenta riesgos para el medio ambiente siempre
que se apliquen las medidas de control adecuadas para reducir al
mínimo las emisiones. El vertido en gran escala podría ejercer
efectos adversos en el medio ambiente a escala local.
OTRAS INVESTIGACIONES
1. LA MIBA afecta a varios sistemas enzimáticos. Por lo tanto,
puede influir de modo significativo en la bio-transformación
de sustancias biológicas externas que son metabolizadas por
estas enzimas. Como las personas suelen estar expuestas a más
de un compuesto, deben llevarse a cabo estudios sobre los
efectos combinados de muestras que contengan MIBA.
2. Se dispone de muy poca información sobre las relaciones
dosis‹respuesta en cuanto a los efectos de la MIBA en el
sistema nervioso central humano (por ejemplo, tiempo de
reacción, efectos conductuales) en las vías respiratorias
superiores y las mucosas, o en la función renal. Se necesita
más información sobre la toxicocinética de la MIBA por sí sola
y mezclada con otros disolventes. Debe evaluarse la
penetración cutánea de la MIBA.
3. Deben emprenderse estudios epidemiológicos para dilucidar los
efectos que tiene en el sistema nervioso la exposición a largo
plazo a concentraciones moderadas de MIBA, por sí sola o
mezclada con otros disolventes.
HMP and 4-methyl-2-pentanone have also been identified in rats
(Pilon, 1987). MIBK increased aniline hydroxylase activity and
cytochrome P-450 concentration in chicken liver microsomes after
inhalation (4100 mg/m3, 1000 ppm, for 50 days) (Abou-Donia et
al., 1985b). The induction by MIBK was apparently of similar
capacity to that of methyl n -butyl ketone (Abou-Donia et al.,
1985a). It is likely that MIBK can induce its own oxidative
metabolism as well as that of other substances. The study of
Malyscheva (1988) (section 8.2.4) suggested that MIBK is absorbed
through the skin as well as via the oral and inhalation routes
(section 8.1). A comparison of intraperitoneal and oral LD50
values (Zakhari et al., 1977) suggests an oral absorption of 30% or
more. Malyscheva (1988) showed dermal absorption followed by
extensive distribution (toxic signs in many organs). Likewise,
inhalation studies producing liver and kidney changes are
suggestive of extensive distribution (MacEwen et al., 1971; Vernot
et al., 1971).
The structure of MIBK precludes the metabolic production of
2,5-hexane-dione, the neurotoxic agent formed from both hexane and
methyl n -butyl ketone.
6.1.1 Effect on liver alcohol dehydrogenase in vitro
MIBK in N,N -dimethylacetamide has been shown to reduce the
activity of mouse liver alcohol dehydrogenase in vitro
(Cunningham et al., 1989).
6.2 Humans
MIBK and other substituted 2-pentanones have been reported in
urine samples from unexposed humans (Zlatkis & Liebich, 1971). MIBK
was detected in various tissues and body fluids (section 2.4.1.3)
of two individuals who suffered fatal exposure to a mixture of
organic solvents. The concentration range of MIBK in the tissues
and body fluids of the two decedents was 1.4-2.5 and 0.2-0.8 mg/kg,
respectively. The tissue distribution differed markedly between the
two individuals (Bellanca et al., 1982). The inhalation study
carried out by Dick et al. (1990) with human volunteers suggested
that exposures to 410 mg MIBK/m3 (100 ppm) for 4 h causes steady
state blood levels to be attained. Blood and breath samples
collected 90 min after exposure indicated essentially complete
clearance of the absorbed MIBK. Hjelm et al. (1990) exposed human
volunteers for 2 h during light physical excercise to MIBK (10,
100, and 200 mg/m3; 2.4, 24.4, and 48.8 ppm). The concentration
of MIBK in blood rose rapidly after the onset of exposure, during
which no plateau level was reached. No tendency towards saturation
kinetics was observed over the dose range, the apparent blood
clearance being 1.6 litres/h per kg throughout. Only 0.04% of the
total MIBK dose was eliminated unchanged via the kidneys within 3
h after exposure.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Toxicity data reported in this chapter should be interpreted
with caution, since the tests were conducted under static
conditions using nominal rather than measured concentrations.
Actual concentrations experienced by the test organisms cannot be
determined for these tests.
7.1 Microorganisms
MIBK has low toxicity for microorganisms as indicated by the
threshold concentrations required for inhibition of growth (Table
3).
Table 3. Toxicity of MIBK for microorganisms
Species Threshold Duration of Reference
concentration for study
growth inhibition
(mg/litre)
Protozoa
Saprozoic > 800 48 h Bringmann & Kühn (1981)
flagellate
(Chilomonas
paramaecium)
Bacteriovorous 450 72 h Bringmann & Kühn (1981)
flagellate
(Entosiphon
sulcatum)
Bacteriovorous 950 20 h Bringmann & Kühn (1981)
ciliate ( Uronema
parduczi)
Bacterium
Pseudomonas putida 275 16 h Bringmann & Kühn (1977b)
Table 4. Acute toxicity of MIBK for aquatic organisms
Species LC50 Duration Reference
(mg/litre) of study
Freshwater fish
Golden orfe 672-744 48 h Juhnke & Lüdemann (1978)
(Leuciscus idus
melanotus)
Goldfish 460 24 h Bridié et al. (1979b)
(Carassius auratus)
Fathead minnow approx. 525 96 h Call et al. (1985)
(Pimephales promelas)
Invertebrates
Freshwater
Water flea 4280 24 h Bringmann & Kühn (1977a)
(Daphnia magna) 1550 24 h Bringmann & Kühn (1982)
Marine
Brine shrimp 1230 24 h Price et al. (1974)
(Artemia salina)
Freshwater algae
Green algae 725 a 8 days Bringmann & Kühn (1977b)
(Scenedesmus
quadricauda)
Bluegreen algae 136 a 8 days Bringmann & Kühn (1978)
(Microcystis
aeruginosa)
a Threshold concentration for reduction of total biomass
7.2 Aquatic organisms
MIBK appears to have low toxicity for aquatic organisms (Table
4). The maximum zero lethality concentration (LC0) is in the
range of 480-720 mg/litre (Juhnke & Lüdemann, 1978). Using a QSAR
model, Lipnick et al. (1987) showed that the 24-h LC50 of 460
mg/litre reported by Bridié et al. (1979a) in goldfish (Carassius
auratus) fitted a narcotic mechanism of action.
Aquatic invertebrates are less sensitive than fish to the
toxicity of MIBK, 24-h LC50 values of 4280 mg/litre (Bringmann &
Kuhn, 1977a) and 1550 mg/litre (Bringmann & Kuhn, 1982) having been
reported for the water flea Daphnia magna and 1230 mg/litre for
the brine shrimp Artemia salina (Price et al., 1974). The
LC100 and LC0 values for Daphnia magna were 5000 and 2280
mg/litre, respectively (Bringmann & Kuhn, 1977a).
The toxicity of MIBK was also measured in the green alga
Scenedesmus quadricauda , in which the 8-day threshold for
toxicity was 725 mg/litre (Bringmann & Kuhn, 1977b), and in the
relatively more sensitive cyanobacterium (blue-green alga)
Microcystis aeruginosa , in which the toxicity threshold was 136
mg/litre (Bringmann & Kuhn, 1978).
7.3 Terrestrial organisms
Experimental data are not available.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
MIBK is of low acute toxicity by the oral and inhalation routes
of exposure (Table 5).
The maximum time for which rats could be exposed to a saturated
atmosphere of MIBK without dying was 15 min (Smyth et al., 1951).
In one study, six rats survived a 4-h exposure to 8200 mg MIBK/m3
(2000 ppm), but, following a 4-h exposure to 16 400 mg/m3 (4000
ppm), all six animals died within 14 days.
In studies by Specht (1938) and Specht et al. (1940), female
guinea-pigs were exposed to MIBK concentrations of 4100, 69 000,
and 115 000 mg/m3 (1000, 16 800, and 28 000 ppm, respectively)
for up to 24 h. In view of the method used for generating the
atmosphere (allowing measured amounts of MIBK to evaporate freely
to one cubic meter volume of air at 25-26 °C), the two higher
levels must be greatly exaggerated because the saturation
concentration in air for MIBK at 25 °C is 40 000 mg/m3. At 4100
mg/m3 there was minimal eye or nasal irritation. However, there
was a decreased respiratory rate during the first 6 h of exposure,
which was attributed to a narcotic effect. The higher levels
produced obvious signs of eye and nose irritation, followed by
salivation, lacrimation, ataxia, progressive narcosis, and death.
The highest level killed 50% of the animals within 45 min. Autopsy
and histopathological investigations in some animals showed fatty
livers and congestion of the brain, lungs, and spleen, but no
damage to the heart and kidneys was observed.
A single ip injection of 500 mg MIBK/kg body weight in
guinea-pigs did not induce changes in the serum ornithine-carbamyl
transferase level, and there were no histopathological changes in
the liver. However, an injection of 1000 mg/kg body weight killed
one out of four animals, and a slight increase in the serum
ornithine-carbamyl transferase level was seen in the survivors 24
h after dosing. Histopathologically, there was possible lipid
accumulation in liver cells but no evidence of liver damage
(Divincenzo & Krasavage, 1974). It should be noted that an ip
injection of 560 mg/kg produced minimal effects in rats (Vezina et
al., 1985), suggesting that mice are more sensitive than rats (the
ip LD50 is 590 mg/kg in mice, see Table 5).
Table 5. LD50 and LC50 values for MIBK in rats and mice
Route of Species Duration LD50 or LC50 Reference
exposure of exposure
Oral (LD50) rat 4570 mg/kg body weight Smyth et al. (1951)
rat 4600 mg/kg body weight Batyrova (1973)
rat 2080 mg/kg body weight RTECS (1987)
mouse 2850 mg/kg body weight Batyrova (1973)
mouse 1900 mg/kg body weight Zakhari et al. (1977)
Intraperitoneal (LD50) mouse 590 mg/kg body weight Zakhari et al. (1977)
Inhalation (LC50) rat 4 h 8.2-16.4 g/m3 Smyth et al. (1951); Smyth (1956)
mouse 2 h 20.5 g/m3 Batyrova (1973)
mouse 45 mins 74.2 g/m3 Zakhari et al. (1977)
(18 105 ppm)
In male Sprague-Dawley rats, a single oral dose of MIBK
enhanced the hepatotoxicity of a single ip dose of chloroform given
24 h later. The no-observed-effect and minimal-effect levels of
MIBK were 375 and 560 mg/kg body weight, respectively (Vezina et
al., 1985). The ketone potentiation of haloalkane-induced
hepatonecrosis has been attributed to enhanced bioactivation of the
haloalkane, which is mediated by the increased cytochrome P-450
activity induced by the ketone (Branchflower et al., 1983). The
extent of potentiation of carbon tetrachloride liver toxicity (as
shown by an increase in plasma alanine transaminase activity and
bilirubin concentration) was found to depend on the concentration
of both MIBK and carbon tetrachloride in male rats. The minimum
effective MIBK dose decreased 10-fold when the carbon tetrachloride
dose was increased from 0.01 ml/kg to 0.1 ml/kg. These findings
suggest that liver injury is determined by the product of MIBK and
carbon tetrachloride doses (Pilon et al., 1988). Attention should
be paid to this when working in environments containing mixtures of
solvents.
8.2 Short-term exposure
8.2.1 Inhalation
In rats exposed to 410 mg/m3 (100 ppm) for 2 weeks, there was
an increase in kidney weight. An increase in both liver and kidney
weights was observed after exposure to 820 mg/m3 (200 ppm) for 2
weeks or 410 mg/m3 (100 ppm) for 90 days. Histopathological
investigations showed hyaline droplets in the proximal tubules of
the kidney and this finding was named hyaline droplet toxic tubular
nephrosis (MacEwen et al., 1971; Vernot et al., 1971). In rats,
dogs, and monkeys exposed continuously for 90 days to MIBK at 410
mg/m3 (100 ppm) under reduced oxygen tension and reduced
atmospheric pressure (65% oxygen at 34.7 k Pa), liver and kidney
weights were increased in rats after 90 days. Hyaline droplets were
observed in rat kidney epithelium after 15 days, but this effect
was reversible after a 3- to 4-week recovery period. No
histopathological changes were reported in monkeys or dogs and no
sex differences were reported for any parameter (MacEwen et al.,
1971).
Four groups of six male and six female F-344 rats and six male
and six female B6C3F1 mice were exposed to 0, 44, 2050, or 8180
mg MIBK/m3 (0, 10.1, 501, or 1996 ppm, respectively) for 6 h/day
(for 5 days with 2 days off followed by 4 more consecutive days of
exposure) (Dodd et al., 1982; Phillips et al., 1987). Lacrimation
was observed in the highest dose group, but no ophthalmological
lesions or alterations in body weight gain were found. Liver
weight, expressed as a percentage of body weight, was increased in
male and female rats and in female mice exposed to 8180 mg/m3. A
statistically significant increase in liver weight was also
observed in male rats exposed to 2050 mg/m3. Male and female rats
and female mice showed a significant increase in both absolute and
relative kidney weights when exposed to 8180 mg/m3. However, male
mice at this exposure level exhibited a significant decrease in
relative kidney weight. Of the animals exposed to 2050 mg/m3,
only male rats exhibited an increase in kidney weight, but this was
not statistically significantly different from the control value.
Hyaline droplet formation was seen in the kidneys of male rats
exposed to 2050 and 8180 mg/m3. Epithelial regeneration of the
proximal convoluted tubules was also seen at 8180 mg/m3. There
were no histopathological abnormalities in rats and mice exposed to
414 mg/m3, and this concentration was considered a clear
no-observed-adverse-effect level (Dodd et al., 1982). In a
subsequent study, four groups of 14 male and 14 female F-344 rats
and 14 male and 14 female B6C3F1 mice were exposed to 0, 205,
1025, or 4100 mg MIBK/m3 (0, 50, 250, or 1000 ppm, respectively)
for 6 h/day (5 days/week for 90 days). No growth retardation or
clinical effects were observed in either rats or mice. Clinical
observations were made in addition to measurements of body and
organ weights (heart, kidneys, liver, lungs, and testes).
Haematology, ophthalmology, gross pathology, and histology were
also investigated and, in rats, water consumption and urine and
serum chemistry analyses were made. Male rats and mice exposed to
4100 mg/m3 (1000 ppm) showed a slight increase in liver weight
(approximately 11%) and in liver weight per body weight ratio, and
liver weight was also slightly increased in male mice exposed to
1025 mg/m3 (250 ppm). However, neither gross nor microscopic
hepatic lesions were observed, and urinalysis and serum chemistry
values were normal. In male rats exposed to 1025-4100 mg/m3
(250-1000 ppm), there was an increase in the number of hyaline
droplets within the proximal tubular cells of the kidney. No other
gross or microscopic renal changes were observed (Dodd & Eisler,
1983; Phillips et al., 1987). It was considered that the hyaline
droplet effects produced by MIBK may be specific to the male rat
due to the presence of alpha-2-µglobulin (Phillips et al., 1987).
In studies by Brondeau et al. (1989), groups of five hens were
continuously exposed for 50 days by inhalation to either 4100 mg
MIBK/m3 (1000 ppm) or 3520 mg n -hexane/m3 (1000 ppm) or for
30 days to a mixture of 4100 mg MIBK/m3 and 3520 mg n
-hexane/m3. Inhalation of n -hexane alone had no effect on
hepatic microsomal enzymes, but inhalation of MIBK or the MIBK/ n
-hexane mixture increased significantly the aniline hydroxylase
activity and cytochrome P-450 content of the liver (Abou-Donia et
al., 1985b). Inhalation of 2440 to 12 400 mg MIBK/m3 (595 to 3020
ppm) in rats produced enhancement of the liver cytochrome P-450
content and glutathione- S -transferase activity and also enhanced
the ability of 1,2-dichlorobenzene to increase serum glutamate
dehydrogenase activity.
Experiments in open-chest cats demonstrated that significant
pulmonary hypertension and vasoconstriction, with reduced pulmonary
arterial flow, occurred as a result of MIBK inhalation for 5 min at
all concentrations tested (410 to 41 000 mg/m3 (100 to 10 000
ppm)). Systemic arterial pressure and vascular resistance were not
significantly affected (Zakhari et al., 1977). Broncho-constriction
was also produced by MIBK inhalation for 5 min, the effect being
statistically significant at concentrations at or above 2050
mg/m3 (500 ppm) or 4100 mg/m3 (1000 ppm) for pulmonary
resistance or transpulmonary pressure, respectively. Adult mongrel
dogs with open-chest surgery showed pulmonary hypertension at an
inhalation concentration of 20.5 mg MIBK/m3 (5 ppm) for 5 min. At
41 mg/m3 (10 ppm), myocardial contractility occurred (Zakhari et
al., 1977).
8.2.2 Oral
Three daily doses of 375 or 1500 mg MIBK/kg body weight given
by gavage to rats reduced the bile flow produced by an intravenous
injection of taurocholate (20 mg per kg body weight) (Plaa &
Ayotte, 1985). The effect of MIBK on the cholestatic activity of
manganese, with or without bilirubin, has also been investigated in
male Sprague-Dawley rats. MIBK was administered by gavage in corn
oil at doses ranging from 188 to 1502 mg/kg body weight once a day
for 1, 3, or 7 days (Vezina et al., 1985; Vezina and Plaa, 1987).
MIBK was not cholestatic but, at doses of 375 mg/kg or more, was
found to potentiate the cholestasis induced by a
manganese-bilirubin combination when this was given 18 h after the
1-day treatment with MIBK. When given for 3 or 7 days, MIBK
produced a dose-related enhancement of the cholestasis induced by
a manganese-bilirubin combination. A 3-day treatment with MIBK (750
mg/kg) was also shown to potentiate the cholestasis induced by
manganese alone. Two known metabolites of MIBK (HMP and
4-methyl-2-pentanol) were also able to potentiate the cholestatic
effect of the manganese-bilirubin combination or of manganese alone
in male Sprague-Dawley rats. When the metabolite was given by
gavage 18 h prior to the administration of the manganese-bilirubin
combination, cholestasis was potentiated by 375 mg/kg or 1502 mg/kg
(expressed as equivalents of MIBK) of 4-methyl-2-pentanol or HMP,
respectively. Lower doses did not decrease bile flow rate.
4-Methyl-2-pentanol was also more effective than HMP as a
potentiator following daily treatment for 3 days prior to
manganese-bilirubin administration. However, with manganese alone,
HMP was more effective. It was suggested that the potentiation of
cholestasis may be associated with metabolic induction or might
reflect a separate mechanism of action involving a membrane
interaction (Vezina & Plaa, 1988).
The toxic effects of MIBK in Sprague-Dawley rats (groups of 30
animals of each sex) were examined following 13 weeks of oral
gavage administration at levels of 0, 59, 250, or 1000 mg/kg daily.
Body weight, food consumption, organ weight, morbidity, clinical
chemistry, haematology, and histopathology evaluations were
performed. All surviving animals were killed after 90 days (13
weeks) and 10 animals of each sex per group were examined.
Nephrotoxicity was seen as a general nephropathy for both male and
female rats administered 1000 mg/kg per day. Although increased
liver and kidney weights were observed for males and females at
1000 mg/kg per day, there were no corresponding histopathological
lesions present in the liver. The effects seen at 1000 mg/kg per
day were present to a significantly lesser extent in the females
and males fed 250 mg/kg per day. No effects were observed at 50
mg/kg per day, identifying a no-observed-effect level
(Microbiological Associates, 1986).
8.2.3 Parenteral
In studies by Krasavage et al. (1982), rats (strain and number
not specified) were given intraperitoneal injections of MIBK or a
mixture of methyl ethyl ketone (MEK) and MIBK (9:1 by volume), 5
times/week, for 35 weeks. The dose levels for the first 2 weeks
were 10, 30, and 100 mg per kg body weight, and these were then
doubled for the remainder of the treatment period. Body weight gain
suppression was seen after 3-4 weeks of treatment. The only other
effect noted was transient narcosis during the first 4 weeks at the
highest dose. Pulmonary vascular effects were observed following an
intraperitoneal administration of MIBK to cats (threshold dose 8
mg/kg), but bronchoconstriction was not seen following an
intraperitoneal administration of 4-32 mg/kg (Zakhari et al.,
1977).
8.2.4 Skin application
In rats exposed dermally to 300-600 mg MIBK/kg per day for 4
months, dose- and time-dependent morphological changes were
observed in the skin, brain, liver, adrenals, spleen, and testis.
Body temperature decreased and oxygen consumption increased
(Malyscheva, 1988).
8.3 Skin, eye, and respiratory irritation; sensitization
8.3.1 Skin irritation
A single 10-h occluded application of MIBK to the shaved skin
of rabbits produced erythema, which occurred immediately after the
application and persisted for up to 24 h. Daily applications of 10
ml on 10 cm2 skin for 7 days caused drying and flaking of the
surface (Krasavage et al., 1982).
8.3.2 Eye irritation
Undiluted MIBK (0.1 ml) produced some irritation within 10 min
when instilled in the rabbit eye. Inflammation and conjunctival
swelling occurred within 8 h; the inflammation, swelling, and
exudate present at 24 h had disappeared by 60 h (Krasavage et al.,
1982).
8.3.3 Respiratory irritation
De Ceaurriz et al. (1981) measured the reflex decrease in
respiratory rate in male Swiss OF1 mice as an index of sensory
irritation. MIBK caused a concentration-dependent decrease in
respiratory rate during a 5-min exposure, and a 50% decrease in
respiratory rate (RD50) was seen at 13 100 mg/m3 (3195 ppm).
Specht et al. (1940) attributed the decreased respiratory rate to
a narcotic effect. It should be recognized that the reduction may
not be due to sensory irritation.
8.3.4 Skin sensitization
There are no reports of skin sensitization studies.
8.4 Long-term exposure
No long-term toxicity studies have been reported.
8.5 Reproduction, embryotoxicity, and teratogenicity
In studies by Tyl (1984) and Tyl et al. (1987), groups of 35
pregnant Fischer-344 rats and 30 pregnant CD-1 mice were exposed to
1230, 4100, or 12 300 mg MIBK/m3 (300, 1000, or 3000 ppm) on days
6-15 (inclusive) of gestation. The animals were sacrificed on days
21 (rats) or 18 (mice) and fetuses examined for external, visceral,
and skeletal alterations. In rats, exposure to 12 300 mg/m3
resulted in maternal toxicity with decreased body weight gain,
increased relative kidney weight, decreased food consumption, and
fetotoxicity (reduced fetal body weight per litter and delays in
skeletal ossification). Clinical signs in dams included loss of
coordination, negative toe pinch, paresis, muscular weakness,
piloerection, lacrimation, and perioral encrustation. No increase
in fetal malformation was observed in any group. At 1230 and 4100
mg/m3, there was no maternal, embryo, or fetal toxicity, or
malformations. However, reduced fetal body weight and delay in some
ossification parameters were observed at the lowest dose but not at
the intermediate dose level. These effects were attributed to the
larger litter size (average 10.8) in this group compared to that in
controls (9.5).
In mice, exposure to 12 300 mg/m3 produced maternal toxicity
with increased mortality (3/25), increased absolute and relative
liver weights, and fetotoxicity (increased incidence of dead
fetuses, reduced fetal body weight per litter, and delayed or
reduced ossification). Clinical signs in dams included irregular
gait, paresis, hypoactivity, ataxia, negative toe pinch, and
lacrimation. There was no treatment-related increase in
embryotoxicity or fetal malformations at any exposure concentration
tested. No significant treatment-related maternal, embryo, or fetal
toxicity (including malformations) was observed at 1230 or 4100
mg/m3.
Table 6. Mutagenicity and related end-points
System Dose Response Reference
Bacterial Assays
Salmonella typhimurium a 0.04-4 µg/plate negative Chemical
strains TA98, TA100, Manufacturers
TA1537, TA1538 Association
(1984);
O'Donoghue et
al. (1988)
Salmonella typhimurium a
strains TA1535, TA1537 Up to 8000 µg/ml negative Brooks et al.
TA1538, TA98, TA100 (1988)
Eschericia coli
strains WP2 and Up to 8000 µg/ml negative Brooks et al.
WP2 uvr A (1988)
Yeast Assays
Saccharomyces cerevisiae JDI Up to 5 mg/ml negative Brooks et al.
mitotic gene conversion (1988)
assay ± rat liver S9
Mammalian cell assays in vitro :
L51784 TK +/- mouse 0.001-100 µl/ml negative Chemical
lymphoma mutation assay (preliminary assay) Manufacturers
(± rat liver S9) 0.4-6 µl/ml negative Association
(1984);
O'Donoghue et
al. (1988)
Primary rat hepatocytes; 0.01-100 µl/ml negative Chemical
unscheduled DNA synthesis Manufacturers
(DNA repair) Association
(1984);
O'Donoghue et
al. (1988)
Cultured rat liver cells Up to 1000 µl/ml negative Brooks et al.
chromosomal damage assay (half the dose for (1988)
RL4 cells 50% inhibition of
cell growth)
Table 6 (contd).
System Dose Response Reference
Balb/3T3; cell transformation 2-5 µl/ml (-S9) inconclusive Chemical
assay ± rat liver S9 1-7 µl/ml (+S9) Manufacturers
Association
(1984);
O'Donoghue et
al. (1988)
Mammalian in vivo assay
Mouse (male and female) 0.73 ml/kg ip negative Chemical
micronucleus assay (maximum tolerated Manufacturers
(polychromatic dose level) Association
erythrocytes) (1984);
O'Donoghue et
al. (1988).
a Preincubation mutation assay incorporating Arochlor-induced rat liver S9
8.6 Mutagenicity and related end-points
A summary of the reported data on MIBK mutagenicity is given
in Table 6.
8.6.1 Bacterial assays
A pre-incubation assay with Salmonella typhimurium (strains
TA98, TA100, TA1537, TAl538) was conducted at dose levels of 0.04
to 4 µg/plate both in the presence and absence of a metabolic
activation system prepared from Aroclor-induced rat liver
homogenate (S9 fraction). Precautions were taken to prevent the
escape of MIBK vapour and assure prolonged exposure of the bacteria
to the test substance. MIBK did not cause an increase in reverse gene
mutation (Chemical Manufacturers Association 1984; O'Donoghue et
al., 1988).
Both MIBK and the oxidative metabolite 4-hydroxy-4-methyl-2-
pentanone (HMP) (see section 6.1) were tested for mutagenicity in
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538, and in Eschericia coli strains WP2 and WP2 uvr A
(Brooks et al., 1988). Aroclor-induced rat liver S9 fraction was
included. No induction of reverse gene mutation was observed up to
a maximum concentration of 8000 µg MIBK/ml (pre-incubation assay in
a sealed container) or 4000 µg HMP/plate (plate incorporation
assay).
8.6.2 Yeast assay for mitotic gene conversions
MIBK and the metabolite HMP were assayed for mitotic gene
conversion using log-phase cultures of the yeast Saccharomyces
cerevisiae JD1 (Brooks et al., 1988). Compounds were tested up to
a concentration of 5 mg/ml in the presence and absence of rat liver
S9 fraction in a sealed container for 18 h. Neither compound
induced mitotic gene conversion.
8.6.3 L5178Y TK+/- mouse lymphoma assay
A preliminary assay was carried out in the presence and absence
of a metabolic activation system at doses of 0.001-100 µl/ml. The
non-activated cultures showed 3 to 157% total relative growth,
while the cultures containing the rat liver S9 fraction had a
relative growth of 23-95% compared with untreated control cultures.
No increase in mutation frequencies was observed in cultures
containing the metabolic activation system, but in the
non-activated cultures, a 2-fold increase above controls was seen
at two non-consecutive doses. An increase in mutation frequency of
approximately 5 times the concurrent control occurred at one test
concentration, but this concentration also caused 97% cell death.
In the absence of a dose-related effect, this result was considered
equivocal. A repeat assay was performed using duplicate cultures
and a narrower range of doses (0.4-6 µl/ml). The total relative
growth ranged from 1 to 80% in non-metabolically activated cells
and from 28 to 63% in cultures that contained the S9 fraction. None
of the activated cultures revealed increased mutation frequencies.
A borderline positive result was found at 6 µl/ml, but different
mutation frequencies occurred in the duplicate cultures and 96-99%
of the cells were killed (Chemical Manufacturers Association, 1984;
O'Donoghue et al., 1988).
8.6.4 Unscheduled DNA synthesis in primary rat hepatocytes in vitro
When MIBK was tested at five dose levels ranging from 0.01
µl/ml to 100 µl/ml in a single assay, there was an increase of less
than 5 fold in labelled nuclear grains in cells treated with MIBK
compared with cells of the solvent control plates (Chemical
Manufacturers Association, 1984; O'Donoghue et al., 1988). Since
the value did not exceed that of the negative control by two
standard deviations of the control value, it was considered that,
under the conditions tested, MIBK did not cause a significant
increase in the nuclear grain count.
8.6.5 Mouse micronucleus assay
Male and female mice were administered MIBK by ip injection at
the maximum tolerated dose level of 0.73 ml/kg body weight, and
bone marrow polychromatic erythrocytes were estimated 12, 24, and
48 h later. There were no significant differences between the
treated and control animals in the ratio of polychromatic to
normochromatic erythrocytes. The number of micronucleated
polychromatic erythrocytes per 1000 cells was not significantly
increased in the MIBK-treated animals (Chemical Manufacturers
Association, 1984; O'Donoghue et al., 1988).
8.6.6 Assay for structural chromosome damage
MIBK (purity > 98.5%) and the metabolite HMP were tested (24-h
exposure) in cultured rat liver cells (RL4) for the ability to
induce chromosomal damage. Metabolic activation with S9 mix was not
used because RL4 cells are metabolically competent. The
concentrations of MIBK employed were 0.125, 0.25, and 0.5 times the
concentration required for 50% inhibition of cell growth. Maximum
concentrations tested were thus 1000 µg MIBK/ml and 4000µg HMP/ml
(this HMP level was equivalent to the concentration required for
>60% growth inhibition). Incubations with MIBK were sealed to
prevent loss by evaporation. MIBK did not produce chromosomal
damage, but HMP gave a small increase (which was not dose related)
in chromatid damage within the concentration range 2000-4000 µg/ml.
It should be noted that HMP did not induce reverse gene mutation in
bacteria or mitotic gene conversion in yeast (Brooks et al., 1988).
8.6.7 Cell transformation assay
In studies reported by the Chemical Manufacturers Association
(1984) and O'Donoghue et al. (1988), MIBK was tested in the
Balb/3T3 (clone A31-1) morphological transformation assay. Doses of
2.4, 3.6, and 4.8 µl MIBK/ml were added to the culture medium in
the absence of a metabolic activation system, and 1, 2, and 4 µl
MIBK/ml were added in the presence of such a system (Aroclorinduced
rat liver S9 fraction). MIBK produced a positive response in the
non-activated cultures only (4.8 µl MIBK per ml gave 3 type III
foci in 15 dishes). A confirmatory study was conducted with doses
of 2, 3, 4, and 5 µl/ml and 4, 5, 6, and 7 µl/ml, respectively, in
the presence and absence of S9 fraction. No significant increase in
the number of transformed foci was found in this study, either in
the presence or absence of the metabolic activation system. Thus,
the effect of MIBK on cell transformation was not reproducible in
the two assays and the ambiguity of the results makes them
unreliable.
8.7 Carcinogenicity
No carcinogenicity studies have been reported.
8.8 Neurotoxicity
In a study by Krasavage et al. (1982), rats were given ip
injections of MIBK, or a mixture of methyl ethyl ketone and MIBK
(9:1 by volume), 5 times/week, for 35 weeks. The dose levels of 10,
30, and 100 mg/kg body weight were doubled after 2 weeks of
treatment. Transient anaesthesia was noted during the first 4 weeks
in the highest dose group, but there was no evidence of peripheral
neuropathy. In dogs administered 300 mg MIBK/kg body weight per day
subcutaneously (sc) for 11 months, electromyographic examination
showed no evidence of neurotoxicity.
Cats treated subcutaneously with 150 mg MIBK/kg body weight per
day or a mixture of methyl ethyl ketone/MIBK (9:1) twice daily, 5
times/week, for up to 8.5 months showed no evidence of nervous
system damage (Spencer & Schaumburg, 1976). In beagle dogs
receiving similar treatment, there were no neurotoxic changes
(Krasavage et al., 1982).
Groups of male rats were exposed to 5330 mg/m3 (1300 ppm)
methyl n -butyl ketone for 4 months or 6150 mg/m3 (1500 ppm)
MIBK for 5 months (Spencer et al., 1975). Methyl n -butyl ketone
produced a toxic distal axonopathy. MIBK produced minimal distal
axonal changes, but 3% methyl n -butyl ketone was present as a
contaminant in the MIBK, and the design of the cages used may have
caused compression neuropathy (Spencer et al., 1975; Spencer &
Schaumburg, 1976). Animals exposed to MIBK showed slight signs of
narcosis, but body weight gain was normal, and, at 5 months, there
were no clinical signs of neurological dysfunction. Rats exposed
for 3 h to 102 mg MIBK/m3 (25 ppm) showed a 58% increase in
pressor lever response, which had not returned to control levels 7
days after the end of exposure (Geller et al., 1978). The maximum
motor-fibre conduction velocity in the tail nerve decreased
markedly when male rats were treated with methyl n -butyl ketone
(401 mg/kg, 5 times/week for 55 weeks) but not when they were
treated with MIBK (601 mg/kg, 5 times/week for 55 weeks). However,
treatment with MIBK (201 mg/kg) facilitated the neurotoxic effect
of methyl n -butyl ketone (401 mg/kg) possibly due to the
demonstrated ability of MIBK to increase the metabolic activity of
10 000 g liver supernatants towards both MIBK and methyl n -butyl
ketone (Nagano et al., 1988).
Discriminatory behaviour and memory in baboons was not affected
by exposures of 82-164 mg/m3 (20-40 ppm) (Geller et al., 1978).
Geller et al. (1979) reported an effect on accuracy of performance
of tasks in a ``delayed match-to-sample discrimination-test'' in
baboons exposed for 7 days to 205 mg/m3 (50 ppm) MIBK, but there
was no change in response when MIBK was combined with methyl ethyl
ketone at 295 mg/m3 (100 ppm).
De Ceaurriz et al. (1984) exposed male Swiss OF1 mice to an
atmosphere containing MIBK and measured the total duration of
immobility during a 3-min ``behavioural despair'' swimming test. At
concentrations of 2714, 3104, 3309, and 3657 mg/m3 (662, 757,
807, and 892 ppm), a dose-dependent decrease of mobility was
observed (25, 38, 46, and 70% respectively). The authors noted that
the mean active level of MIBK was lower for this neurobehavioural
effect (3292 mg/m3 (893 ppm) for 50% inhibition of immobility)
than for an effect on sensory irritation (13 100 mg/m3 (3195 ppm)
for 50% inhibition of respiratory rate) (De Ceaurriz et al., 1981,
section 8.3.3).
In inhalation studies on hens (five per group) into the effect
of MIBK on n -hexane-induced neurotoxicity, a continuous exposure
period of 90 days was followed by a 30-day observation period
(Abou-Donia et al., 1985b). One group of hens was exposed to 3520
mgnw-hexane/m3 (1000 ppm) and another group to 4100 mg MIBK/m3
(1000 ppm). Four additional groups were exposed simultaneously to
3520 mg n -hexane/m3 (1000 ppm) and 410, 1025, 2050, or 4100 mg
MIBK/m3 (100, 250, 500, or 1000 ppm, respectively). A control
group was exposed to ambient air in an exposure chamber. Hens
continuously exposed to 4100 mg MIBK/m3 developed weakness of the
legs with subsequent recovery. Inhalation of 3520 mg n
-hexane/m3 produced mild ataxia. Exposure to 3520 mg n
-hexane/m3 together with 1025, 2050, or 4100 mg MIBK/m3
resulted in signs of neurotoxicity including paralysis, the
severity of which depended on the MIBK concentration. Hens
continuously exposed to the 3520/410 mg/m3 n -hexane/MIBK
mixture exhibited severe ataxia throughout the observation period.
Histopathological examination of hens exposed to the n
-hexane/MIBK mixture showed large swollen axons and degeneration
of the axon and myelin of the spinal cord and peripheral nerves.
There were no histopathological abnormalities in the central
nervous system of hens exposed only to MIBK. This demonstrates that
MIBK potentiates the neurotoxic action of n -hexane. In a
subsequent study, in which hens were exposed for 50 days to MIBK at
4100 mg/m3 (1000 ppm) or to n -hexane, it was suggested that
the potentiation by MIBK of n -hexane neurotoxicity is related to
the induction by MIBK of liver microsomal cytochrome P-450,
resulting in increased metabolism of n -hexane to its neurotoxic
metabolites (sections 6.1, 8.2.1). The findings also suggest that
the neurotoxicity of technical methyl butyl-ketone (methyl n
-butyl ketone/MIBK (7/3)) was correctly attributed to the methyl
n -butyl ketone component (Abdo et al., 1982). Simultaneous
treatment of hens with 41, 205, or 410 mg/m3 (10, 50, or 100 ppm)
technical methyl butyl ketone, 5 days/week for 90 days, with a
dermal application of technical grade 0 -ethyl- 0 -4-nitrophenyl
phenylphosphonothionate (EPN, 1.0 mg/kg, 85%) greatly enhanced the
neurotoxic effects. It was proposed that MIBK partially contributed
to this potentiation by inducing cytochrome P-450 and thus
enhancing the formation of neurotoxic products from methyl- n
-butyl ketone and EPN (Abou-Donia et al., 1985a).
The effect of MIBK on the duration of ethanol-induced loss of
righting reflex and on ethanol elimination has been studied in
mice. MIBK was dissolved in corn oil and injected ip 30 min before
ethanol (4 g/kg ip). At a dose of 501 mg/kg, MIBK significantly
prolonged the duration of ethanol-induced loss of righting reflex.
The concentrations of ethanol in blood and brain on return of the
righting reflex were similar in MIBK-treated and control animals
(Cunningham et al., 1989).
8.9 In vitro toxicity assays
In contrast to methyl n -butyl ketone and n -hexane, MIBK
caused little or no cytopathological or growth-inhibiting effects
in cultured mouse neuroblastoma cells (Selkoe et al., 1978). MIBK
in N,N -dimethylacetamide reduced the activity of mouse liver
alcohol dehydrogenase in vitro (Cunningham et al., 1989). MIBK
was found to inhibit the sulfhydryl-dependent creatine kinase and
adenylate kinase enzymes in vitro but not to the same extent as
did the neurotoxic agent, methyl- n -butyl ketone (Lapin et al.,
1982).
9. EFFECTS ON MAN
9.1 Acute toxicity
In a study on the sensory threshold, Silverman et al. (1946)
exposed 12 volunteers of both sexes to various concentrations of
MIBK for a 15-min period. This period permitted an accurate
observation of olfactory fatigue and increasing or decreasing
irritation of mucous membranes. The sensory response limit was 410
mg/m3 (100 ppm). The majority of the subjects found the odour
objectionable at 820 mg/m3 (200 ppm), and the vapour irritated
the eyes. The low odour threshold (1.64 mg/m3) (Ruth, 1986) and
the irritant effects can provide warning of high concentrations.
Because of its low viscosity, MIBK may, when swallowed, also be
aspirated into the lungs causing a chemical pneumonitis (Panson &
Winek, 1980).
9.2 Short-term exposure
Workers exposed to 410 mg MIBK/m3 (100 ppm) complained either
of headache and nausea or of respiratory irritation (Elkins, 1959).
Tolerance was said to be acquired during the working week but was
lost over the weekend. Reduction of the exposure to 82 mg/m3 (20
ppm) largely eliminated the complaints. In the study of Hjelm et
al. (1990) (see section 6.3) on human volunteers, CNS symptoms
(headache and/or vertigo and/or nausea) were reported at 2-h
exposure levels of 10-200 mg MIBK/m3 (2.4-48.8 ppm). There were
no significant effects from exposure on the performance of a
reaction time task or a test of mental arithmetic.
9.3 Eye and respiratory irritation
Exposure to a concentration of 820 mg MIBK/m3 (200 ppm) for
a 15-min period caused eye irritation in 12 human volunteers
(Silverman et al., 1946). Undiluted MIBK splashed in the eyes may
cause painful irritation (Shell, 1957). A group of workers exposed
to 410 mg MIBK/m3 (100 ppm) complained of respiratory tract
irritation, but there were no complaints at 82 mg/m3 (20 ppm)
(Elkins, 1959). In the study of Hjelm et al. (1990) (see section
6.3) on human volunteers, irritation particularly of the nose and
throat was reported at 2-h exposure levels of 10, 100, and 200
mg/m3 (2.4, 24.4, and 48.8 ppm).
9.4 Long-term exposure
In workers exposed to up to 2050 mg MIBK/m3 (500 ppm) for
20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, over half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomiting, and sore throat. A few of the
workers experienced insomnia, somnolence, heartburn, intestinal
pain, and some unsteadiness. Four workers had slightly enlarged
livers and six had a nonspecific colitis. Clinical chemistry
examination revealed no abnormalities in any of the workers. Five
years later, work practices had greatly improved, the highest MIBK
concentration was 410-430 mg/m3 (100-105 ppm), and the general
concentration was 205 mg/m3 (50 ppm). A few workers still
complained of gastrointestinal and central nervous system effects,
and slight liver enlargement had persisted in two workers, but
other symptoms had disappeared (Armeli et al., 1968).
9.5 Placental transfer
MIBK was detected in maternal and umbilical cord blood samples
from 11 patients (Dowty et al., 1976).
9.6 Neurotoxicity
A few isolated cases of peripheral neuropathy have been
reported after exposure to spray paint or lacquer thinner that
apparently contained MIBK and other hydrocarbon solvents, including
neurotoxic agents (Oh & Kim, 1976; Aubuchon et al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of effects on the environment
MIBK is not likely to persist in the environment. It will
slowly volatilize from soil and water and is readily biodegraded in
fresh and salt water. In the atmosphere, MIBK is estimated to be
degraded by OHÊ radicals with a half-life of approximately 14 h.
MIBK is not expected to bioaccumulate and has a low toxicity for
microorganisms, fish, algae, and aquatic invertebrates. Only in
cases of accidental spillage or inappropriate disposal of wastes
into the environment are levels of MIBK likely to cause toxicity to
organisms in the environment.
10.2 Evaluation of health risks for humans
The general population is exposed to low levels of MIBK. Only
small quantities have been detected in food, drinking-water, and
other beverages (baked goods, 10.9 mg/kg; frozen dairy products,
11.5 mg/kg; gelatins, puddings, 10.9 mg/kg; beverages, 10.2 mg/kg).
For general population exposure, maximum ambient air concentrations
in the range of 0.1 to 0.2 mg/m3 have been defined by two
countries.
Occupational exposure occurs particularly in the production and
use of lacquers, paints, and extraction solvents. The major route
of entry is by inhalation. The low odour threshold (1.64 mg/m3)
and the irritant effects can provide warning of high
concentrations. Exposure to levels of 10-410 mg/m3 (2.4-100 ppm)
produced perceptible irritation of either the eyes, nose, or
throat, and 820 mg/m3 (200 ppm) produced discomfort. Symptoms
such as headache, nausea, and vertigo also occurred at a level of
10-410 mg/m3 (2.4-100 ppm). There were no significant effects
from a 2-h exposure of up to 200 mg/m3 (50 ppm) on a simple
reaction time task or test of mental arithmetic.
In the single report concerning long-term occupational
exposure, where workers were exposed to 2050 mg MIBK/m3 (500 ppm)
for 20-30 min per day and to 328 mg/m3 (80 ppm) for much of the
remainder of the working day, more than half of the 19 workers
complained of weakness, loss of appetite, headache, eye irritation,
stomach ache, nausea, vomitting, and sore throat. A few workers
experienced insomnia, somnolence, and some unsteadiness. Four had
slightly enlarged livers and six had nonspecific colitis. Five
years later, work practices had greatly improved and the highest
concentrations were reduced to about one fifth of the previous
level. A few workers still complained of irritation of the eyes and
upper respiratory tract as well as gastrointestinal and central
nervous system symptoms. Prolonged skin contact with MIBK caused
irritation and flaking of the skin.
In animal studies, acute systemic MIBK toxicity is low by the
oral and inhalation routes. In a 90-day study, Sprague-Dawley rats
were given MIBK by gavage at doses of 50, 250, or 1000 mg/kg body
weight per day. Lethargy was noted in the highest-dose group and
males showed reduced body weight gain. In this group there was
generalized nephropathy, with an increase in relative kidney weight
and hepatomegaly. Relative kidney weight was also increased in the
animals fed 250 mg/kg per day, and slight hepatomegaly was reported
in male rats only. There were no histopathological lesions in the
liver or other tissues at any dose level. It was concluded that the
NOEL was 50 mg/kg per day. In 90-day inhalation studies on rats and
mice, concentrations of up to 4100 mg/m3 (1000 ppm) did not
result in any life-threatening signs of toxicity. However,
compound-related reversible morphological changes in the liver and
kidney were reported. Levels of 4100 mg/m3 produced evidence of
central nervous system depression. MIBK was capable of increasing
liver weight (at > 1025 mg/m3 (250 ppm)) and inducing hepatic
microsomal metabolism. This may be the explanation for the
exacerbation of haloalkane toxicity and the potentiation of the
neurotoxicity of n -hexane. In 90-day studies with mice, rats,
dogs, and monkeys, only male rats developed hyaline droplets in the
proximal tubules of the kidney (hyaline droplet toxic tubular
nephrosis). This effect in male rats was reversible and of doubtful
significance for humans. MIBK reduces the activity of mouse liver
alcohol dehydrogenase in vitro . It has also been found to
potentiate the cholestatic effects of manganese given with or
without bilirubin.
In rats and mice exposed to MIBK by inhalation at
concentrations of 1230, 4100, or 12 300 mg/m3 (300, 1000, or 3000
ppm) on days 6 to 15 of gestation and sacrificed on day 21 (rats)
or day 18 (mice), marked maternal toxicity was observed at the
highest concentration in both species. This concentration produced
fetotoxicity (reduced fetal body weight and delayed ossification)
but was not embryotoxic or teratogenic. At 4100 and 1230 mg/m3
there was no maternal toxicity and no evidence of embryotoxicity,
fetotoxicity, or teratogenicity.
MIBK did not induce gene mutation in bacterial test systems
(Salmonella typhimurium and Escherichia coli ) either with or
without metabolic activation. Negative results were also obtained
in tests (both with and without metabolic activation) for mitotic
gene conversion in yeast ( Saccharomyces cerevisiae ) and in gene
mutation tests using cultured mammalian cells (mouse lymphoma). In
vitro assays for unscheduled DNA synthesis in primary rat
hepatocytes and for structural chromosome damage in cultured rat
liver cells (RL4) were negative. An in vivo micronucleus test in
mice was negative. These data indicate that MIBK is not genotoxic.
11. RECOMMENDATIONS
At the levels of MIBK to which the general human population is
exposed, there is unlikely to be any hazard. In the occupational
health context, where the major route of exposure is by inhalation,
atmospheric levels should be kept below the recommended
occupational exposure limits by suitably designed work processes
and engineering controls, including ventilation. Skin and eye
contamination should be avoided. Suitable protective clothing and
respiratory protection should be readily available for use in
enclosed spaces, in emergencies, and for certain maintenance
operations. MIBK is inflammable and should be handled accordingly.
MIBK has low toxicity for microorganisms and fish, and its
half-life in the environment is short. Consequently, there is no
risk to the environment provided there are adequate controls to
minimize emissions. Large-scale release could have local adverse
effects on the environment.
12. FURTHER RESEARCH
1. MIBK affects a number of enzyme systems. Therefore, it can
significantly influence the biotransformation of xenobiotics that
are metabolized by these enzymes. Since humans are usually exposed
to more than one compound, studies on the combined effects of
mixtures containing MIBK should be undertaken.
2. There is very little information available on the dose-response
relationships for the effects of MIBK on the human central nervous
system (e.g., reaction time, behavioural effects), on the upper
airways and mucous membranes, or on kidney function. More
information on toxico-kinetics is needed for MIBK alone and in
mixture with other solvents. The skin penetration of MIBK should be
assessed.
3. Epidemiological studies should be undertaken to elucidate the
effects on the nervous system of long-term exposure to moderate
concentrations of MIBK alone or in mixture with other solvents.
REFERENCES
ABDO, K.M., GRAHAM, D.G., TIMMINS, P.R., & ABOU-DONIA, M.B. (1982)
Neurotoxicity of continuous (90 days) inhalation of technical grade
methyl butyl ketone in hens. J. Toxicol. environ. Health, 9:
199-215.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & ABDO, K.M.
(1985a) The joint neurotoxic action of inhaled methyl butyl ketone
vapour and dermally applied 0-ethyl 0-4-nitrophenyl
phenylphosphonothioate in hens: potentiating effect. Toxicol. appl.
Pharmacol., 79: 69-82.
ABOU-DONIA, M.B., LAPADULA, D.M., CAMPBELL, G., & TIMMONS, P.R.
(1985b) The synergism of n -hexane-induced neurotoxicity by
methyl isobutyl ketone following subchronic (90 days) inhalation in
hens: induction of hepatic microsomal cytochrome P-450. Toxicol.
appl. Pharmacol., 81: 1-16.
ANALYTICAL QUALITY CONTROL (1972) Handbook for analytical quality
control in water and waste water laboratories, Cincinnati, Ohio,
National Environmental Research Centre.
ARMELI, G., LINARI, F., & MARTORANO, G. (1968) [Clinical and
haematochemical examinations in workers exposed to the action of a
higher ketone (MIBK) repeated after 5 years.] Lav. Um., 20:
418-424 (in Italian).
ATKINSON, R., ASCHMANN, S.M., CARTER, W.P.L., & PITTS, J.N., Jr
(1982) Rate constants for the gas-phase reaction of OH radicals
with a series of ketones at 299 ± 2 ° K. Int. J. chem. Kinet.,
14: 839-847.
AUBUCHON, J., ROBINS, H.I., & VISESKUL, C. (1979) Peripheral
neuropathy after exposure to methyl isobutyl ketone in spray paint.
Lancet, August 18: 363-364.
AUSTERN, B.M., DOBBS, R.A., & COHEN, J.M. (1975)
Gas-chromatographic determination of selected organic compounds
added to wastewater. Environ. Sci. Technol., 9: 588-590.
BAMBERGER, R.L., ESPOSITO, G.G., JACOBS, B.W., PODOLAK, G.E., &
MAZUR, J.F. (1978) A new personal sampler for organic vapors. Am.
Ind. Hyg. Assoc. J., 39: 701-708.
BASU, P., CARPENTER, J., CHEN, C., NELSON, H., PERRY, W., SHOCLET,
A., TAYLOR, D., & ZAFRAN, F. (1968) Human exposure assessment:
Methyl isobutyl ketone, Washington, DC, US Environmental Protection
Agency (EPA contract 68-01.4839).
BATYROVA, T.F. (1973) Substantiation of the maximum permissible
concentration of methylisobutyl ketone in air or workrooms. Gig.
Tr. prof. Zabol., 17(11): 52-53.
BELLANCA, J.A., DAVIS, P.L., DONNELLY, B., DAL CORTIVO, L.A., &
WEINBERG, S.B. (1982) Detection and quantitation of multiple
volatile compounds in tissues by GC and GC/MS. J. anal. Toxicol.,
6: 238-240.
BRANCHFLOWER, R.V., SCHULICK, R.D., GEORGE, J.W., & POHL, L.R.
(1983) Comparison of the effects of methyl n -butyl ketone and
phenobarbital on rat liver cytochromes P-450 and the metabolism of
chloroform to phosgene. Toxicol. appl. Pharmacol., 71: 414-421.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER M. (1979a) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRINGMANN, G. & KUHN, R. (1977a) [Findings concerning the harmful
effect of water-endangering substances on, Daphnia magna .] Z.
Wasser Abwasser Forsch., 10: 161-166 (in German).
BRINGMANN, G. & KUHN, R. (1977b) [Limit values for the harmful
effect of water-endangering substances on bacteria ( Pseudomonas
putida ) and green algae ( Scenedesmus quadricauda ).] Z. Wasser
Abwasser Forsch., 10: 87-98 (in German).
BRINGMANN, G. & KUHN, R. (1978) [Limit values for the harmful
effect of water-endangering substances on blue-green algae
( Microcystis aeruginosa ) and green algae ( Scenedesmus
quadricauda ) in the cell multiplication inhibitor test.] Vom
Wasser, 50: 45-60 (in German).
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effects of
pollutants on flagellates and ciliates, and/or on holozoic
bacteria-eating and saprozoic protozoa.] GWF-Wasser Abwasser,
122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Results of toxic action of water
pollutants on Daphnia magna straus tested by an approved
standardized procedure.] Z. Wasser Abwasser Forsch., 15: 1-6 (in
German).
BRONDEAU, M.T., BAN, M., BONNET, P., GUENIER, J.P., & DE CEAURRIZ,
J. (1989) Acetone compared to other ketones in modifying the
hepatotoxicity of inhaled 1,2-dichlorobenzene in rats and mice.
Toxicol. Lett. 49: 69-78.
BROOKS, T.M., MEYER, A.L., & HUTSON, D.H. (1988) The genetic
toxicology of some hydrocarbon and oxygenated solvents.
Mutagenesis, 3: 227-232.
BROWN, K.W. & DONNELLY, K.C. (1988) An estimation of the risk
associated with the organic constituents of hazardous and municipal
waste landfill leachates. Hazardous Waste Hazardous Mater., 5(1):
1-30.
BROWN, R.H. & PURNELL, C.J. (1979) Collection and analysis of trace
organic vapour pollutants in ambient atmospheres. J. Chromatogr.,
178: 79-90.
BURNHAM, A.K., CALDER, G.V., FRITZ, J.S., JUNK, G.A., SVEC, H.J.,
& WILLIS, R. (1972) Identification and estimation of neutral
organic contaminants in potable water. Anal. Chem., 44: 139-142.
CALL, D.J., BROOKE, L.T., KNUTH, M.L., POIRIER, S.H., & HOGLUND,
M.D. (1985) Fish subchronic toxicity prediction model for
industrial organic chemicals that produce narcosis. Environ.
Toxicol. Chem., 4: 335-341.
CEC (1976) Analysis of organic micropollutants in water,
Luxembourg, Commission of the European Communities (Cost 64b bis).
CFR (1987) Code of Federal Regulations. Methods of analysis for
organic chemicals in groundwater at hazardous waste sites,
Washington, DC, US Government Printing Office (Appendix IX, 40 CFR,
Part 264).
CHEMICAL MANUFACTURERS ASSOCIATION (1984) Ketones Program Panel,
Vol. 1 - Methyl isobutyl ketone: Mutagenicity and teratology
studies, Washington, DC, Chemical Manufacturers Association.
CORWIN, J.F. (1969) Volatile oxygen-containing organic compounds in
sea water: determination. Bull. mar. Sci., 19: 504-509.
COX, R.A., DERWENT, R.G., & WILLIAMS, M.R. (1980) Atmospheric
photooxidation reactions. Rates, reactivity and mechanism for
reaction of organic compounds with hydroxyl radicals. Environ. Sci.
Technol., 14: 57-61.
CUNNINGHAM, J., SHARKAWI, M., & PLAA, G.L. (1989) Pharmacological
and metabolic interactions between ethanol and methyl n -butyl
ketone, methyl isobutyl ketone, methyl ethyl ketone, or acetone in
mice. Fundam. appl. Toxicol., 13: 102-9.
DE CEAURRIZ, J., MICILLINO, J.C., BONNET, P., & GUENIER, J.P.
(1981) Sensory irritation caused by various industrial airborne
chemicals. Toxicol. Lett., 9: 137-143.
DE CEAURRIZ, J., MICILLINO, J.C., MARIGNAC, B., BONNET, P., MULLER,
J., & GUENIER, J.P. (1984) Quantitative evolution of sensory
irritating and neurobehavioural properties of aliphatic ketones in
mice. Food Chem. Toxicol., 22, 545-549.
DICK, R., DANKOVIC, D., SETZER, J., PHIPPS, F., & LOWRY, L. (1990)
Body burden profiles of methyl ethyl ketone and methyl isobutyl
ketone exposure in human subjects. Toxicologist, 10: 122.
DIVINCENZO, G.D. & KRASAVAGE, W.J. (1974) Serum ornithine carbamyl
transferase as a liver response test for exposure to organic
solvents. Am. Ind. Hyg. Assoc. J., 35: 21-29.
DIVINCENZO, G.D., KAPLAN, C.J., & DEDINAS, J. (1976)
Characterization of the metabolites of methyl n -butyl ketone,
methyl iso-butyl ketone and methyl ethyl ketone in guinea pig serum
and their clearance. Toxicol. appl. Pharmacol., 36: 511-522.
DODD, D.E. & EISLER, D.L. (1983) Methyl isobutyl ketone ninety-day
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 46-504
submitted to US EPA).
DODD, D.E., LONGO, L.C., & EISLER, D.L. (1982) Nine-day vapour
inhalation study on rats and mice, Washington, DC, Chemical
Manufacturers Association (Bushy Run Research Center, Report 45-501
submitted to US EPA).
DOWTY, B.J., LASETER, J.L., & STORER, J. (1976) The transplacental
migration and accumulation in blood of volatile organic
constituents. Pediatr. Res., 10: 696-701.
ECDIN (1990) Data bank on environmental chemicals, Ispra (Varese),
Establishment, Joint Research Centre of the Commission of the
European Communities.
ELKINS, H.B. (1959) Chemistry of industrial toxicology, New York,
John Wiley and Sons, p. 121.
ELLISON, W.K. & WALLBANK, T.E. (1974) Solvents in sewage and
industrial waste waters. Identification and determination. Water
Pollut. Control, 73: 656-672.
ELOFSSON, S.A., GAMBERALE, F., HINDMARSH, T., IREGREN, A.,
ISAKSSON, A., JOHNSSON, I., KNAVE, B., LYDAHL, E., MINDUS, P.,
PERSSON, H.E., PHILIPSON, B., STEBY, M., STRUWE, G., SODERMAN, E.,
WENNBERG, A., & WIDEN, L. (1980) Exposure to organic solvents: a
cross-sectional epidemiologic investigation on occupationally
exposed car and industrial spray painters with special reference to
the nervous system. Scand. J. Work Environ. Health, 6: 239-273.
FAWELL, J.K. & HUNT, S. (1981) Organic micropollutants in drinking
water, Medmenham, Water Research Centre (Technical Report No. 159).
FERNANDES, M. (1985) Methodology for the analysis of volatile
compounds in food packaging materials. Coletanea Inst. Technol.
Aliment., 15: 49-59.
FIRE PREVENTION (1981) Information sheets on hazardous materials,
London, London Fire Protection Association, p. 47 (H97 No. 140).
FRANCIS, A.J., IDEN, G.T., NINE, B.J., & CHANG, C.K. (1980)
Characterization of organics in leachates from low level
radioactive waste disposal sites. Nucl. Technol., 50: 158-163.
FROSTLING, H., HOFF, A., JACOBSSON, S., PFAFFLI, P., VAINIOTALO,
S., ZITTING, A., & TECHN, D. (1984) Analytical, occupational and
toxicologic aspects of the degradation products of polypropylene
plastics. Scand. J. Work Environ. Health., 10: 163-169.
GARMAN, J.R., FREUND, T., & LAWLESS, E.W. (1987) Testing for
groundwater contamination at hazardous waste sites. Chromatogr.
Sci., 25: 328-344.
GELLER, I., ROWLANDS, J.R., & KAPLAN, H.L. (1978) Effects of
ketones on operant behaviour of laboratory animals. In: Voluntary
inhalation of industrial solvents, Washington, DC, US Department of
Health, Education and Welfare, p. 363 (DHEW Publication No.
79-779).
GELLER I., GAUSE, E., KAPLAN, H., & HARTMANN, R.J. (1979) Effects
of acetone, methyl ethyl ketone, and methyl isobutyl ketone on a
match-to-sample task in the baboon. Pharmacol. Biochem. Behav.,
11: 401-406.
HAMPTON, C.V., PIERSON, W.R., HARVEY, T.M., UPDEGROVE, W.S., &
MARANO, R.S. (1982) Hydrocarbon gases emitted from vehicles on the
road. 1. A qualitative gas chromatography/mass spectroscopy survey.
Environ. Sci. Technol., 16: 287-298.
HANNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioural effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HJELM, E.W., HAGBERG, M., IREGREN, A., & LÖF, A. (1990) Exposure to
methyl isobutyl ketone: toxicokinetics and occurrence of irritative
and CNS symptoms in man. Int. Arch. occup. environ. Health, 62:
19-26.
IRPTC (1990) IRPTC legal file, Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
JUHNKE, I. & LÜDEMANN, D. (1978) [Results of the testing of 200
chemical compounds for acute fish toxicity in the orfe test.] Z.
Wasser Abwasser Forsch., 11(5): 161-164 (in German).
KEITH, L.H. (1974) Chemical characterization of industrial waste
waters by gas chromatography-mass spectrometry. Sci. total
Environ., 3: 87-102.
KRASAVAGE, W.J., O'DONOGHUE, J.L., & DIVINCENZO, G.D. (1982) Methyl
isobutyl ketone. In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, New York, John Wiley and Sons,
Vol. 2e, pp. 4747-4751.
KRISTENSSON, J. & BEVING, H. (1987) A study of painters
occupationally exposed to water and solvent based paints,
Luxembourg, Commission of the European Communities, pp. 71-72 (EUR.
10555).
LANDE, S.S., DURKIN, P.R., CHRISTOPHER, D.H., HOWARD, P.H., &
SAXENA, J. (1976) Investigation of selected potential environmental
contamination: ketonic solvents, Syracuse, New York, Center for
Chemical Hazard Assessment Research Corporation, p. 252.
LAPIN, E.P., WEISSBARTH, S., MAKER, H.S., & LEHRER, G.M. (1982) The
sensitivities of creatine and adenylate kinases to the neurotoxins
acrylamide and metyl n-butyl ketone. Environ. Res., 28: 21-31.
LEO, A. & WEININGER, D. (1984) Medicinal chemistry report,
Claremont, California, Pomona College.
LEVIN, J.-O. & CARLEBORG, L. (1987) Evaluation of solid sorbents
for sampling ketones in work-room air. Ann. occup. Hyg., 31:
31-38.
LIPNICK, R.L., WATSON, K.R., & STRAUSZ, A.K. (1987) A QSAR study of
the acute toxicity of some industrial organic chemicals to
goldfish. Narcosis, electrophile and proelectrophile mechanisms.
Xenobiotica, 17, 1011-1025.
MACEWEN, J.D., VERNOT, E.H., & HAUN, C.C. (1971) Effects of 90-day
continous exposure to methyl isobutyl ketone on dogs, monkeys, and
rats, Ohio, Wright-Patterson AFB, Aerospace Medical Research
Laboratory (Report No. AMRL TR-71-65).
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 7: 611- 614.
MALYSCHEVA, M.V. (1988) [The effect of the skin route of
administration of methyl isobutyl ketone on its toxicity.] Gig. i
Sanit., 10: 79-80 (in Russian).
MICROBIOLOGICAL ASSOCIATES (1986) Subchronic toxicity of methyl
isobutyl ketone in Sprague-Dawley rats. Preliminary report,
Research Triangle Park, North Carolina, Research Triangle Park
Institute, (Study No. 5221.04).
MITI (1978) The biodegradability and bioaccumulation of new and
existing chemical substances, Tokyo, Ministry of International
Trade and Industry, Chemical Products Safety Division, Basic
Industries Bureau.
MOSHLAKOVA, L.A., & INDINA, T.V. (1986) [Gas chromatographic
determination of ketones present simultaneously in the air of the
work area and in the washings from workers' skin. Gig. i Sanit.,
2: 90-91 (in Russian).
NAGANO, M., HARADA, K., MISUMI, J., & NOMURA, S. (1988) [Effect of
methyl isobutyl ketone on methyl n-butyl ketone neurotoxicity in
rats.] Sangyo Igaku, 30: 50-51 (in Japanese).
NIOSH (1984) NIOSH manual of analytical methods: ketones I, 3rd
ed., Cincinnati, Ohio, US National Institute for Occupational
Safety and Health,Vol. 2 (No. 1300).
NTIS (1985) Scientific literature review of aliphatic ketones,
secondary alcohols and related esters in flavour usage. Volume 1 -
Introduction and summary, tables of data bibliography: Al,
Washington, DC, National Technical Information Service, Part 2
(PB85-141059).
O'DONOGHUE, J.L., HAWORTH, S.R., CURREN, R.D., KIRBY, P.E., LAWLOR,
T., MORAN, E.J., PHILLIPS, R.D., PUTNAM, D.L., ROGERS-BACK, A.M.,
SLESINSKI, R.S., & THILAGAR, A. (1988) Mutagenicity studies on
ketone solvents: methyl ethyl ketone, methyl isobutyl ketone and
isophorone. Mutat. Res., 206: 149-161.
OECD (1977) The asessment of environmental chemicals: production
figures and use patterns for some high volume chemicals, Paris,
Organization for Economic Cooperation and Development
(ENV/Chem./77.6).
OECD (1984) Data interpretation guides for initial hazard
assessment of chemicals (provisional), Paris, Organisation for
Economic Cooperation and Development, p. 31.
OH, S.J. & KIM, J.M. (1976) Giant axonal swelling in "Huffer's"
neuropathy. Arch. Neurol., 33: 583-586.
PANSON, R.D. & WINEK, C.L. (1980) Aspiration toxicity of ketones.
Clin. Toxicol., 17: 271-317.
PELLIZZARI, E.D., HARTWELL, T.D., HARRIS, B.S.H., WADDELL, R.D.,
WHITAKER, D.A., & ERICKSON, M.D. (1982) Purgeable organic compounds
in mothers' milk. Bull. environ. Contam. Toxicol., 28: 322-328.
PHILLIPS, R.D., MORAN, E.J., DODD, D.E., FOWLER, E.H., KARY, C.D.,
&O'DONOGHUE, J. (1987) A 14-week vapor inhalation toxicity study of
methyl isobutyl ketone. Fundam. appl. Toxicol., 9: 380-388.
PILON, D. (1987) Interaction cétones/hydrocarbures halogènes:
Utilization des métabolites cétoniques comme indices d'exposition
aux cétones, Montreal, University of Montreal (Ph.D. Thesis).
PILON, D., BRODEUR, J., & PLAA, G.L. (1988) Potentiation of carbon
tetrachloride-induced liver injury by ketonic and ketogenic
compounds: role of the CCl4 dose. Toxicol. appl. Pharmacol.,
94: 183-190.
PLAA, G.L. & AYOTTE, D. (1985) Taurolithocholate-induced
intrahepatic cholestasis: potentiation by methyl isobutyl ketone
and methyl n-butylketone in rats. Toxicol. appl. Pharmacol., 80:
228-234.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimps
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77
RACCIO, J.M. & WIDOMSKI, J.R. (1981) Quality control of flavors in
soft drinks and the analysis of residual solvents in food packaging
films utilizing headspace sampling with open tubular columns.
Chromatogr. Newsl., 9(2): 42-45.
RIPPSTEIN, W.J. & COLEMAN, M.E. (1984) [Toxicological evaluation on
the Columbian spacecraft.] Kosmet. Biol. Aviakosm. Med., 18:
87-96 (in Russian).
RTECS (1987) Registry of toxic effects of chemical substances,
1985-86 ed., Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Vol. 1-6 (DHSS (NIOSH) Publication No. 87-114).
RUTH, J.H. (1986) Odor threshold and irritation levels of several
chemical substances: a review. Am. Ind. Hyg. Assoc. J., 47:
A142-A151.
SABROE, S. & OLSEN, J. (1979) Health complaints and work conditions
among lacquerers in the Danish furniture industry. Scand. J. soc.
Med., 7: 97-104
SATO, A. & NAKAJIMA, T. (1979) Partition coefficients of some
aromatic hydrocarbons and ketones in water, blood and oil. Brit. J.
ind. Med., 36, 231-234.
SAWHNEY, B.L. & KOZLOSKI, R.P. (1984) Organic pollutants in
leachates from landfill sites. J. environ. Qual., 13: 349-352.
SAX, N.I. (1979) Dangerous properties of industrial materials, 6th
ed, New York, Van Norstrand Reinhold Company, p. 750.
SELKOE, D.J., LUCKENBILL-EDDS, L., & SHELANSKI, M.L. (1978) Effects
of neurotoxic industrial solvents on cultured neuroblastoma cells:
methyl n -butyl ketone, n -hexane, and derivatives. J.
Neuropathol. exp. Neurol., 37: 768-789.
SHELL (1957) Methyl isobutyl ketone: industrial hygiene bulletin,
New York, Shell Chemical Corporation, pp. 57-112.
SILVERMAN, L., SCHULTE, H.F., & FIRST, M.W. (1946) Further studies
on sensory response to certain industrial solvent vapors. J. ind.
Hyg. Toxicol., 28: 262-266.
SMYTH, H.F. (1956) Hygienic standards for daily inhalation. Am.
Ind. Hyg. Assoc. J., 17: 129-266.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SPECHT, H. (1938) Acute response of guinea pigs to inhalation of
methyl isobutyl ketone, Washington, DC, US Public Health Service,
pp. 292-300 (US Public Health Report No. 53).
SPECHT, H., MILLER, J.W., VALAER, P.J., & SAYERS, R.R. (1940) Acute
response of guinea pigs to the inhalation of ketone vapours,
Washington, DC, US Public Health Service, Division of Industrial
Hygiene (NIH Bulletin No. 176).
SPENCER, P.S. & SCHAUMBURG, H.H. (1976) Feline nervous system
response to chronic intoxication with commercial grades of methyl
n -butyl ketone, methyl isobutyl ketone and methyl ethyl ketone.
Toxicol. appl. Pharmacol., 37: 301-311.
SPENCER, P.S., SCHAUMBURG, H.H., RALEIGH, R.L., & TERHAAR, C.J.
(1975) Nervous system degeneration produced by the industrial
solvent methyl n -butyl ketone. Arch. Neurol., 32: 219-222.
TNO (1983a) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 2.
TNO (1983b) Volatile compounds in food: Qualitative data, Zeist,
Netherlands, Organization for Applied Scientific Research.
TNO (1986) Volatile compounds in food: Quantitative data, Zeist,
Netherlands, Organization for Applied Scientific Research, Vol. 5.
TNO (1987) Volatile compounds in food: Supplement 4, Zeist,
Netherlands, Organization for Applied Scientific Research.
TOMCZYK, H. & ROGACZEWSKA, T. (1979) [Gas chromatographic
determination of airborne methyl isobutyl ketone, methyl isobutyl
carbinol, acetone, toluene and o-xylene.] Med. Pr., XXX(6):
417-423 (in Polish).
TYL, R.W. (1984) A teratologic evaluation of methyl isobutyl ketone
in Fischer 344 rats and CD-1 mice following inhalation exposure,
Washington, DC, Chemical Manufacturers Association (Bushy Run
Research Center Report No. 47.505).
TYL, R.W., FRANCE, K.A., FISHER, L.C., PRITTS, I.M., TYLER, T.R.,
PHILLIPS, R.D., & MORAN, E.J. (1987) Developmental toxicity
evaluation of inhaled methyl isobutyl ketone in Fischer 344 rats
and CD-l mice. Fundam. appl. Toxicol., 8: 319-327.
VERNOT, E.H., MACEWEN, J.D., & HARRIS, E.S. (1971) Continuous
exposure of animals to methyl isobutyl ketone, Ohio, Wright
Patterson AFB, Aerospace Medical Research Laboratory (US NTIS AD
Report No. 751443).
VERSCHUEREN, K. (1983) Handbook of environmental data on organic
chemicals, 2nd ed., New York, Van Nostrand Reinhold Company, pp.
459-461.
VEZINA, M. & PLAA, G.L. (1987) Potentiation by methyl isobutyl
ketone of the cholestasis induced in rats by a manganese-bilirubin
combination or manganese alone. Toxicol. appl. Pharmacol. 91:
477-483.
VEZINA, M. & PLAA, G.L. (1988) Methyl isobutyl ketone metabolites
and potentiation of the cholestasis induced in rats by a
manganese-bilirubin combination or manganese alone. Toxicol. appl.
Pharmacol. 92: 419-427.
VEZINA, M., AYOTTE, P., & PLAA, G.L. (1985) Potentiation of
necrogenic and cholestatic liver injury by 4-methyl-2-pentanone.
Can. Fed. Biol. Soc., 28: 221.
WEBB, R.G., GARRISON, A.W., KEITH, L.H., & MCGUIRE, J.H. (1973)
Current practice in GC-MS analysis of organics in water,
Washington, DC, US Environmental Protection Agency (EPA Report No.
R2-73-277) (NTIS PB 224 947/2).
WELLER, J.P. & WOLF, M. (1989) Mass spectroscopy and headspace gas
chromatography. Beitr. gerichtl. Med., 47: 525-532.
ZAKHARI, S., LEVY, P., LIEBOWITZ, M., & AVIADO, D.M. (1977) Acute
oral, intraperitoneal, and inhalation toxicity of methyl isobutyl
ketone in the mouse. In: Goldberg, L., ed. Isopropanol and ketones
in the environment, Cleveland, Ohio, CRC Press, Part 3, Chapter
10-14, pp. 93-133.
ZLATKIS, A. & LIEBICH, H.M. (1971) Profile of volatile metabolites
in human urine. Clin. Chem., 17: 592-594.
RESUME
La méthylisobutylcétone est un liquide limpide d'odeur
douceâtre produite en vue d'une vaste utilisation commerciale comme
solvant. On peut la doser par chromatographie en phase gazeuse avec
détection par ionisation de flamme. Elle s'évapore rapidement dans
l'atmosphère où elle subit une photoconversion à brève échéance. La
méthylisobutylcétone est facilement biodégradable et, compte tenu
de sa solubilité moyenne dans l'eau et de son faible coefficient de
partage entre l'octanol et l'eau, elle devrait présenter un faible
potentiel de bioaccumulation. Les limites d'exposition
professionnelle sont de 100-400 mg/m3 (moyenne pondérée par
rapport au temps: TWA) et de 5-300 mg/m3 (valeur plafond: CLV)
selon les pays.
La méthylisobutylcétone est rapidement métabolisée en produits
d'excrétion solubles dans l'eau et sa toxicité aiguë générale est
faible chez l'animal après exposition par voie orale ou
respiratoire. L'expérimentation animale n'a pas révélé
d'axonopathie périphérique. On ne dispose pas de données précises
sur la CL50. Une exposition de 4 heures à une concentration de 16
400 mg/m3 (4000 ppm) a été mortelle pour des rats. La
méthylisobutylcétone liquide ou sous forme de vapeurs à la
concentration de 10 à 410 mg/m3 (2,4 à 100 ppm) est irritante
pour les yeux et les voies respiratoires supérieures. Des
concentrations allant jusqu'à 200 mg/m3 (50 ppm) n'ont produit
aucun effet sensible sur l'homme lors d'épreuves portant sur le
temps de réaction et le calcul mental. Un contact prolongé ou
répété avec la peau peut produire un dessèchement et des crevasses.
L'aspiration accidentelle de méthylisobutylcétone liquide peut
provoquer une pneumonie chimique.
Lors d'une étude de 90 jours effectuée en gavant des rats, on
a obtenu une dose sans effet observable de 50 mg/kg par jour. Des
études d'inhalation de 90 jours portant sur des rats et des souris
à des concentrations allant jusqu'à 4100 mg/m3 (1000 ppm) n'ont
pas révélé de signes de toxicité engageant le pronostic vital.
Toute fois des altérations morphologiques réversibles liées à
l'administration de ce composé ont été observées au niveau du foie
et des reins. Dans un certain nombre d'études, on a observé une
hypertrophie du foie dès la dose de 1025 mg/m3 (250 ppm). Exposés
à 4100 mg/m3 (1000 ppm) pendant 50 jours, des poulets ont
présenté une induction des enzymes microsomiques. A doses plus
élevées (jusqu'à 8180 mg/m3, 1996 ppm) les effets se limitaient
à un accroissement du poids du foie sans lésion histologique. Lors
d'études de 90 jours effectuées sur des souris, des rats, des
chiens et des singes, seuls les rats mâles ont présenté des
altérations histologiques: présence de gouttelettes hyalines dans
les tubules proximaux des reins (néphrose tubulaire toxique à
inclusions hyalines). Cet effet s'est révélé réversible et sa
portée en toxicologie humaine demeure incertaine. Il est possible
que la potentialisation de la toxicité des alcanes halogénés par la
méthylisobutylcétone repose sur une induction enzymatique. La
méthylisobutylcétone potentialise également l'effet cholestatique
du manganèse administré avec ou sans bilirubine.
Des babouins exposés pendant sept jours à une dose de 205
mg/m3 (50 ppm) ont présenté des troubles neuro-comportementaux.
La méthylisobutylcétone est foetotoxique à une concentration
manifestement toxique pour la mère (12 300 mg par m3, 3000 ppm)
mais elle n'est pas embryotoxique ni tératogène à cette
concentration. A la concentration de 4100 mg/m3 (1000 ppm), on
n'a pas observé d'embryotoxicité, de foetotoxicité ni de
tératogénicité chez les rats et les souris.
On a recherché la génotoxicité éventuelle de la
méthylisobutylcétone en pratiquant un certain nombre d'épreuves à
court terme, consistant notamment en tests sur des cellules
mammaliennes, des bactéries et des levures ainsi que dans la
recherche de micro-noyaux chez la souris. Les résultats indiquent
que la méthyliso-butylcétone n'est pas génotoxique. En ce qui
concerne la génotoxicité à long terme ou la cancérogénicité, on ne
dispose d'aucune donnée.
A la concentration de 410 mg/m3 (100 ppm), la
méthylisobutylcétone peut produire des symptômes chez l'homme
consistant en irritation occulaire, migraines, nausées, vertiges et
fatigue et qui correspondent à un effet dépresseur réversible sur
le système nerveux central. Toutefois, rien n'indique l'existence
de lésions permanentes.
La toxicité pour les organismes et micro-organismes aquatiques
est faible.
Compte tenu de la volatilité relativement forte de la
méthylisobutylcétone, de sa photoconversion rapide dans
l'atmosphère, de sa biodégradabilité et de sa faible toxicité pour
les mammifères et la faune aquatique, il est vraisemblable que
cette substance ne peut exercer d'effets néfastes sur
l'environnement qu'à la suite de déversements accidentels ou de la
décharge incontrôlée d'effluents industriels.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des effets sur l'environnement
La méthylisobutylcétone ne devrait pas persister dans
l'environnement. Elle se volatilise lentement à partir du sol et de
l'eau et subit une biodégradation rapide dans l'eau douce et l'eau
salée. Dans l'atmosphère, on pense qu'elle est décomposée par les
radicaux libres OH avec une demi-vie d'environ 14 heures. Elle ne
s'accumule probablement pas et présente une faible toxicité pour
les micro-organismes, les poissons, les algues et les invertébrés
aquatiques. Ce n'est qu'en cas de déversement accidentel ou de
rejet incontrôlé de déchets que cette substance est susceptible
d'atteindre des concentrations toxiques pour les êtres vivants.
2. Evaluation des risques pour la santé humaine
La population générale n'est exposée qu'à de faibles
concentrations de méthylisobutylcétone. On en a décelé de faibles
quantités dans les denrées alimentaires et dans l'eau de
consommation ou autres boissons (produits panifiés, 10,9 mg/kg;
produits laitiers, 11,5 mg/kg; gélatines, puddings, 10,9 mg/kg;
boissons diverses, 10,2 mg/kg). En ce qui concerne la population
générale, deux pays ont fixé des concentrations maximales dans
l'air ambiant qui se situent dans les limites de 0,1 à 0,2 mg/m3.
L'exposition professionnelle se produit notamment lors de la
production et de l'utilisation de vernis, de peintures et de
solvants d'extraction. La principale voie de pénétration est
l'inhalation. Le faible seuil olfactif (1,64 mg/m3) et les effets
irritants de cette substance peuvent avertir de la présence de
fortes concentrations. L'exposition à ces concentrations de 10 à
410 mg/m3 (2,4 à 100 ppm) a produit une irritation perceptible au
niveau des yeux, du nez et de la gorge; à 820 mg/m3 (200 ppm), on
éprouve une sensation de malaise. Entre 10 et 410 mg par m3 (2,4
à 100 ppm), on a également observé des céphalées, des nausées et
des vertiges. Une exposition de deux heures à des concentrations
allant jusqu'à 200 mg/m3 (50 ppm) n'a pas produit d'effets
sensibles à en juger par un simple test de temps de réaction et de
calcul mental.
On ne dispose que d'un seul rapport faisant état d'une
exposition professionnelle de longue durée à une dose de 2050
mg/m3 (500 ppm), 20 à 30 minutes par jour et à 328 mg/m3 (80
ppm) pour la majeure partie du reste de la journée. Plus de la
moitié des 19 ouvriers exposés se sont plaints de faiblesse, de
perte d'appétit, de maux de tête, d'irritation oculaire, de maux
d'estomac, de nausées, de vomissements et de maux de gorge.
Quelques ouvriers ont éprouvé de l'insomnie, de la somnolence et
une perte d'équilibre. Chez quatre d'entre eux on a constaté une
légère hypertrophie du foie et chez six autres une colite non
spécifique. Cinq années plus tard, les méthodes de travail
s'étaient considérablement améliorées et les concentrations
maximales réduites au cinquième des teneurs précédentes. Quelques
ouvriers se sont encore plaints d'irritation au niveau des yeux et
des voies respiratoires supérieures ainsi que de symptômes
digestifs et neurologiques. La contact prolongé avec la peau a
provoqué une irritation cutanée et des crevasses.
Il ressort de l'expérimentation animale que la toxicité
générale aiguë de la méthylisobutylcétone est faible, par voie
orale ou par inhalation. Lors d'une étude de 90 jours, des rats
Sprague-Dawley ont reçu par gavage de la méthylisobutylcétone à des
doses quotidiennes de 50, de 250 et 1000 mg/kg de poids corporel.
On a noté une léthargie chez des animaux du groupe soumis à la dose
la plus élevée et, chez les mâles, une réduction du gain de poids.
Les animaux de ce groupe présentaient une néphropathie généralisée,
avec augmentation du poids relatif des reins et une hypertrophie du
foie. Cette augmentation du poids relatif des reins était également
notable chez les animaux soumis à la dose de 250 mg/kg, mais à
cette dose, on ne constatait qu'une légère hypertrophie du foie
chez les mâles. Quelle que soit la dose, on n'a pas constaté de
lésion histopathologique au niveau du foie ou des autres tissus. La
dose sans effet observable a été évaluée à 60 mg/kg et par jour.
Lors d'une étude de même durée au cours de laquelle on a fait
inhaler à des rats et des souris des concentrations allant jusqu'à
4100 mg/m3 (1000 ppm) on n'a pas relevé de signes de toxicité
engageant le pronostic vital. Toute fois des altérations
morphologiques réversibles liées à l'administration de cette
substance ont été observées au niveau du foie et des reins. A la
concentration de 4100 mg par m3, on observait des signes de
dépression du système nerveux central. La méthylisobutylcétone a
provoqué une augmentation du poids du foie (à une dose supérieure
à 1025 mg/m3, soit 250 ppm) et provoqué l'induction des enzymes
microsomiques du foie. C'est ce dernier mécanisme qui serait à la
base de l'exacerbation de la toxicité des alcanes halogénés et de
la potentialisation de la neurotoxicité dunw-hexane. Lors d'études
de 90 jours sur des souris, des rats, des chiens et des singes, on
a observé, chez les rats seulement, l'apparition d'inclusions
hyalines dans les tubules proximaux des reins (néphrose tubulaire
à inclusions hyalines d'origine toxique). Cet effet observé chez
les rats mâles était réversible et il est douteux qu'il ait une
signification quelconque en toxicologie humaine. La
méthylisobutylcétone réduit l'activité de l'alcool-déshydrogénase
hépatique chez la souris in vitro . On a également constaté
qu'elle potentialisait les effets cholestatiques du manganèse en
présence ou en l'absence de bilirubine.
Des rats et des souris exposés par inhalation à des
concentrations de 1230, 4100 ou 12 300 mg/m3 (300, 1000 ou 3000
ppm) du sixième au quinzième jours de la gestation puis sacrifiés
le vingt-et-unième jour (rats) ou le dixhuitième jour (souris), ont
présenté des signes marqués d'intoxication à la concentration la
plus forte. Cette concentration était foetotoxique (réduction du
poids foetal et ossification retardée) mais n'était ni
embryotoxique ni tératogène. Aux concentrations de 4100 et 1230
mg/m3, on n'a constaté aucune toxicité pour les mères ni signe
d'embryotoxicité, de foetotoxicité ou de tératogénicité.
La méthylisobutylcétone n'a pas produit de mutation génique
dans des systèmes d'épreuve bactériens (Salmonella typhimurium et
Escherichia coli ), qu'il y ait ou non activation métabolique. On
a également obtenu des résultats négatifs dans différentes épreuves
(avec ou sans activation métabolique) à la recherche de conversions
géniques mitotiques dans des levures ( Saccharomyces cerevisiae )
ou lors d'épreuves de mutation génique sur des cellules
mammaliennes en culture (lymphome murin). La recherche in vitro
d'une synthèse anarchique de l'ADN dans des hépatocytes primaires
de rat et de lésions chromosomiques structurales dans des cellules
de foie de rat en culture (RL4) s'est révélée négative. Chez la
souris, la recherche in vivo de micro-noyaux s'est également
révélée négative. Toutes ces données montrent que la
méthyl-isobutylcétone n'est pas génotoxique.
RECOMMANDATIONS
Les concentrations de méthylisobutylcétone auxquelles la
population, dans son ensemble, est susceptible d'être exposée, ne
présentent vraisemblablement aucun danger. La principale voie
d'exposition professionnelle est la voie respiratoire, aussi les
concentrations atmosphériques devront être maintenues en dessous
des limites recommandées d'exposition professionnelle, grâce à un
aménagement convenable des procédés de production et à des moyens
mécaniques tels que la ventilation. Il convient d'éviter toute
contamination de la peau et des yeux. Des vêtements protecteurs
appropriés ainsi que des masques respiratoires doivent être placés
dans les ateliers confinés; ils seront utilisés en cas d'urgence ou
pour effectuer certaines opérations d'entretien. La
méthylisobutylcétone est inflammable et doit donc être manipulée
avec les précautions d'usage.
La méthylisobutylcétone présente une faible toxicité pour les
micro-organismes et pour les poissons et sa demivie dans
l'environnement est courte. Il s'ensuit qu'elle ne présente aucun
risque pour l'environnement, dans la mesure où des mesures
appropriées sont prises pour réduire les émissions au minimum. La
décharge de quantités importantes dans l'environnement pourrait
avoir localement des effets indésirables.
RECHERCHES A EFFECTUER
1. La méthylisobutylcétone affecte un certain nombre de systèmes
enzymatiques. Elle peut donc avoir une influence sensible sur
la biotransformation des produits xénobiotiques métabolisés
par ces enzymes. Etant donné que l'homme est généralement
exposé à plusieurs composés différents, il faudrait
entre-prendre des études sur les effets combinés de mélanges
contenant de la méthylisobutylcétone.
2. On ne dispose que de très peu d'informations sur la relation
dose-réponse relative aux effets toxiques de la
méthylisobutylcétone sur le système nerveux central (par
exemple temps de réaction, effets comportementaux), sur les
voies respiratoires supérieures, sur les muqueuses et sur la
fonction rénale. Il faudrait obtenir davantage de
renseignements sur la toxico-cinétique de cette cétone, soit
seule soit associée à d'autres solvants. Il faudra également
étudier la pénétration percutanée de la méthylisobutylcétone.
3. Il faudrait entreprendre des études épidémiologiques pour
élucider les effets exercés à long terme sur le système
nerveux central par des concentrations moyennes de
méthylisobutylcétone soit seule soit associée à d'autres
solvants.
RESUMEN
La metil isobutil acetona (MIBA) es un líquido transparente de
buen olor que se produce a escala comercial y tiene un uso muy
extendido como disolvente. Puede medirse mediante cromatografía de
gases con detección de ionización de llama. Se evapora rápidamente
a la atmósfera, donde se fototransforma en poco tiempo. La MIBA es
fácilmente biodegradable, lo que, junto con su moderada solubilidad
en el agua y su reducido coeficiente de partición octanol/agua,
sugiere que tiene un bajo potencial de bioacumulación. Los límites
de exposición profesional varían entre 100-410 mg/m3 (promedio
ponderado en el tiempo) y 5-300 mg/m3 (valor máximo) en distintos
países.
La MIBA se metaboliza fácilmente para dar productos de
excreción hidrosolubles y su toxicidad sistémica aguda en animales
es baja por las vías de exposición oral y de inhalación. No se ha
observado axonopatía periférica en estudios realizados en animales.
No se dispone de datos exactos sobre la CL50. La exposición a 16
400 mg/m3 (4000 ppm) durante 4 horas resultó letal para la rata.
Las concentraciones de MIBA líquida y en vapor comprendidas entre
10 y 410 mg/m3 (2,4-100 ppm) son irritantes para los ojos y las
vías respiratorias superiores. Con concentraciones de hasta 200
mg/m3 (50 ppm) no se observaron en el hombre efectos
significativos en una prueba sencilla de tiempo de reacción ni en
una prueba de aritmética mental. El contacto cutáneo prolongado o
repetido puede desecar y descamar la piel. La aspiración accidental
de MIBA líquida puede provocar pneumonitis química.
En un estudio de ceba de ratas durante 90 días, se determinó
un nivel sin efecto observado de 50 mg/kg. En estudios de
inhalación durante 90 días en ratas y ratones, concentraciones de
hasta 4100 mg/m3 (1000 ppm) no produjeron ningún signo de
toxicidad con peligro para la vida. No obstante, se notificaron
cambios morfológicos reversibles relacionados con el compuesto en
el hígado y el riñón. En varios estudios se observó que
concentraciones de MIBA tan bajas como 1025 mg/m3 (250 ppm) eran
capaces de aumentar el tamaño del hígado. Mediante la exposición a
4100 mg/m3 (1000 ppm) durante 50 días, se indujo actividad
metabólica en las enzimas microsómicas del hígado del pollo. A
dosis más elevadas (hasta 8180 mg/m3, 1996 ppm) los efectos se
limitaron a un aumento del peso del hígado sin lesiones
histológicas. En estudios durante 90 días con ratones, ratas,
perros y monos, sólo las ratas macho presentaron corpúsculos
hialinos en los túbulos proximales del riñón (nefrosis tubulotóxica
de corpúsculos hialinos). Este efecto en la rata macho resultó ser
reversible y de dudosa importancia para el hombre. La inducción
enzimática puede ser la base de la potenciación de la toxicidad de
los haloalcanos por la MIBA. Se observó también que la MIBA era
capaz de potenciar el efecto colestático del manganeso administrado
con o sin bilirrubina.
En papiones expuestos durante 7 días a 205 mg/m3 (50 ppm),
se observaron efectos en el neurocomportamiento.
La MIBA resulta fetotóxica a una concentración que produce sin
lugar a dudas toxicidad materna (12 300 mg/m3, 3000 ppm) pero no
es embriotóxica ni teratogénica en esa concentración. En una
concentración de 4100 mg/m3 (1000 ppm), no resultó ni
embriotóxica, ni fetotóxica ni teratogénica en la rata ni en el
ratón.
Se ha estudiado la genotoxicidad de la MIBA en varios ensayos
a corto plazo, inclusive pruebas in vitro con bacterias, levaduras
y células de mamíferos y un ensayo de micronúcleos en el ratón.
Esos estudios indican que la MIBA no es genotóxica. No se dispone
de informes sobre estudios a largo plazo ni estudios de
carcinogenicidad.
Aunque con una concentración de 410 mg/m3 (100 ppm) la MIBA
puede inducir en el hombre síntomas como irritación ocular, dolores
de cabeza, náuseas, mareos y fatiga, que corresponden a un efecto
reversible de depresión del sistema nervioso central, no existen
pruebas de que produzca lesiones permanentes en el sistema
nervioso.
La toxicidad de la MIBA para organismos y micro-organismos
acuáticos es baja.
La volatilidad relativamente alta de la MIBA, su rápida
fototransformación en la atmósfera, su fácil biodegradación y su
baja toxicidad para mamíferos y organismos acuáticos indican que
los efectos medioambientales adversos de esta sustancia
probablemente sólo se producirán como consecuencia de vertidos
accidentales o de efluentes industriales no controlados.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS
EFECTOS EN EL MEDIO AMBIENTE
1. Evaluación de los efectos en el medio ambiente
La MIBA tiene pocas probabilidades de persistir en el medio
ambiente. Se volatiliza poco a poco desde el suelo y el agua y se
biodegrada fácilmente en agua dulce y salada. En la atmósfera, se
ha calculado que la MIBA es degradada por los radicales OHÊ una
semivida de aproximadamente 14 horas. En principio, la MIBA no se
bioacumula y tiene una toxicidad baja para micro-organismos, peces,
algas e invertebrados acuáticos. Sólo en los casos de vertido
accidental o de evacuación inadecuada de desechos en el medio
ambiente es probable que los niveles de MIBA provoquen toxicidad en
los organismos del entorno.
2. Evaluación de los riesgos para la salud humana
La población general está expuesta a niveles reducidos de MIBA.
Se han detectado sólo pequeñas cantidades en los alimentos, el agua
potable y otras bebidas (alimentos horneados, 10,9 mg/kg; productos
lácteos congelados, 11,5 mg/kg; gelatinas y budines, 10,9 mg/kg;
bebidas, 10,2 mg/kg). En cuanto a la exposición de la población
general, dos países han definido concentraciones máximas en el aire
entre 0,1 y 0,2 mg/m3.
La exposición profesional tiene lugar especialmente en la
producción y utilización de lacas, pinturas y disolventes de
extracción. La principal vía de entrada es por inhalación. El bajo
umbral olfativo (1,64 mg/m3) y los efectos irritantes pueden
servir como indicadores de las concentraciones elevadas. La
exposición a niveles de 10-410 mg/m3 (2,4-100 ppm) produjo
irritación perceptible de los ojos, la nariz, o la garganta y 820
mg/m3 (200 ppm) produjeron molestias. Con un nivel de 10-410
mg/m3 (2,4-100 ppm) también se produjeron síntomas como dolor de
cabeza, náuseas y vértigos. No se observaron efectos significativos
debidos a una exposición durante 2 horas a hasta 200 mg/m3 (50
ppm) al realizar una prueba sencilla de tiempo de reacción ni una
prueba de aritmética mental.
En el único informe sobre un estudio de la exposición
profesional a largo plazo, en el que se expuso a trabajadores a
2050 mg MIBA/m3 (500 ppm) durante 20-30 minutos al día y a 328
mg/m3 (80 ppm) durante la mayor parte del resto de la jornada
laboral, más de la mitad de los 19 trabajadores se quejaron de
debilidad, pérdida del apetito, dolores de cabeza, irritación
ocular, dolor de estómago, náuseas, vómitos y dolor de garganta.
Algunos trabajadores sufrieron insomio, somnolencia y cierta
inestabilidad. En cuatro se observó un ligero agrandamiento del
hígado y en seis se observó colitis no específica. Al cabo de 5
años, las prácticas laborales habían mejorado en gran medida y las
concentraciones más elevadas se redujeron a aproximadamente la
quinta parte del nivel anterior. Algunos trabajadores siguieron
quejándose de irritación en los ojos y de las vías respiratorias
superiores así como de síntomas gastrointestinales y del sistema
nervioso central. El contacto cutáneo prolongado con la MIBA
provocó irritación y descamación de la piel.
En estudios en animales, la toxicidad sistémica aguda de la
MIBA es baja por las vías oral y respiratoria. En un estudio de 90
días de duración, se cebó con MIBA a ratas Sprague-Dawley en dosis
diarias de 50, 250 ó 1000 mg/kg de peso corporal. Se observó
letargo en el grupo que recibió la dosis más alta y en los machos
se observó una reducción del ritmo de aumento del peso corporal. En
este grupo se observó nefropatía generalizada, con aumento del peso
relativo del riñón y hepatomegalia. El peso relativo del riñón
también aumentó en los animales alimentados con 250 mg/kg al día,
y se observó ligera hepatomegalia sólo en los machos. No
aparecieron lesiones histopatológicas en el hígado ni en otros
tejidos con ninguna de las dosis administradas. Se concluyó que el
nivel de efecto no observado era de 50 mg/kg al día. En estudios de
inhalación durante 90 días realizados en ratas y ratones, las
concentraciones de hasta 4100 mg/m3 (1000 ppm) no originaron
ningún signo de toxicidad que pusiera en peligro la vida. No
obstante, se comunicó la observación de cambios morfológicos
reversibles relacionados con el compuesto en el hígado y el riñón.
Con niveles de 4100 mg/m3 se observaron signos de depresión del
sistema nervioso central. La MIBA fue capaz de aumentar el peso del
hígado (concentración > 1025 mg/m3 (250 ppm)) y de inducir el
metabolismo microsómico hepático. Esto puede explicar la
exacerbación de la toxicidad de los haloalcanos y la potenciación
de la neurotoxicidad del n‹hexano. En estudios de 90 días con
ratones, ratas, perros y monos, sólo las ratas macho desarrollaron
corpúsculos hialinos en los túbulos proximales del riñón (nefrosis
tubulotóxica de corpúsculos hialinos). Este efecto en ratas macho
resultó ser reversible y de dudosa importancia para el hombre. In
vitro, la MIBA reduce la actividad de la deshidrogenasa alcohólica
en el hígado del ratón. También se ha observado que potencia los
efectos colestáticos del manganeso administrado con o sin
bilirrubina.
En ratas y ratones expuestos a la inhalación de MIBA en
concentraciones de 1230, 4100 ó 12 300 mg/m3 (300, 1000 ó 3000
ppm) en los días 6 a 15 de la gestación y sacrificados el día 21
(ratas) o el día 18 (ratones), se observó una notable toxicidad
materna a la concentración más elevada en ambas especies. Esta
concentración produjo fetotoxicidad (peso del cuerpo fetal reducido
y retraso en la osificación) pero no resultó embriotóxico ni
teratogénico. A 4100 y 1230 mg/m3 no se observó ni toxicidad
materna ni síntomas de embriotoxicidad, fetotoxicidad o
teratogenicidad.
La MIBA no indujo mutación genética en sistemas de ensayo
bacterianos (Salmonella typhimurium y Escherichia coli), con o sin
activación metabólica. También se obtuvieron resultados negativos
en los ensayos (tanto con y sin activación metabólica) para la
conversión mitótica de genes en levaduras (Saccharomyces
cerevisiae) y en pruebas de mutación génica con cultivos de células
de mamíferos (linfoma de ratón). Los ensayos in vitro sobre
síntesis no controlada de ADN en hepatocitos primarios de rata y
sobre lesiones cromosómicas estructurales en cultivos de células
hepáticas de rata (RL4) resultaron negativos. En el ratón, un
ensayo de micronúcleo in vivo resultó negativo. Estos datos indican
que la MIBA no es genotóxica.
RECOMENDACIONES
En los niveles de MIBA a que está expuesta la población humana
general, es poco probable que se plantee riesgo alguno. En el medio
laboral, donde la principal vía de exposición es por inhalación,
los niveles atmosféricos deben mantenerse por debajo de los límites
de exposición profesional recomendados mediante procesos de trabajo
y controles de ingeniería, inclusive la ventilación, de diseño
adecuado. Debe evitarse la contaminación de la piel y de los ojos.
Debe facilitarse el uso de prendas protectoras adecuadas y de
protección respiratoria en los espacios cerrados, en casos de
emergencia y para ciertas operaciones de mantenimiento. La MIBA es
inflamable y debe manipularse teniendo esta característica en
cuenta.
La MIBA tiene baja toxicidad para los microorganismos y los
peces, y su semivida en el medio ambiente es corta. Por
consiguiente, no presenta riesgos para el medio ambiente siempre
que se apliquen las medidas de control adecuadas para reducir al
mínimo las emisiones. El vertido en gran escala podría ejercer
efectos adversos en el medio ambiente a escala local.
OTRAS INVESTIGACIONES
1. LA MIBA afecta a varios sistemas enzimáticos. Por lo tanto,
puede influir de modo significativo en la bio-transformación
de sustancias biológicas externas que son metabolizadas por
estas enzimas. Como las personas suelen estar expuestas a más
de un compuesto, deben llevarse a cabo estudios sobre los
efectos combinados de muestras que contengan MIBA.
2. Se dispone de muy poca información sobre las relaciones
dosis‹respuesta en cuanto a los efectos de la MIBA en el
sistema nervioso central humano (por ejemplo, tiempo de
reacción, efectos conductuales) en las vías respiratorias
superiores y las mucosas, o en la función renal. Se necesita
más información sobre la toxicocinética de la MIBA por sí sola
y mezclada con otros disolventes. Debe evaluarse la
penetración cutánea de la MIBA.
3. Deben emprenderse estudios epidemiológicos para dilucidar los
efectos que tiene en el sistema nervioso la exposición a largo
plazo a concentraciones moderadas de MIBA, por sí sola o
mezclada con otros disolventes.
