
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 116
TRIBUTYLTIN COMPOUNDS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr. S. Dobson,
Institute of Terrestrial Ecology, United Kingdom,
and Dr. R. Cabridenc, Institut National de
Recherche Chimique Appliquée, France
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Tributyltin compounds.
(Environmental health criteria ; 116)
1.Trialkyltin compounds - adverse effects 2.Trialkyltin compounds
-toxicity I.Series
ISBN 92 4 157116 0 (NLM Classification: QV 290)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TRIBUTYLTIN COMPOUNDS
1. SUMMARY
1.1. Physical and chemical properties
1.2. Analytical methods
1.3. Sources of environmental pollution
1.4. Regulations on use
1.5. Environmental concentrations
1.6. Transport and transformation in the environment
1.7. Kinetics and metabolism
1.8. Effects on microorganisms
1.9. Effects on aquatic organisms
1.9.1. Effects on marine and estuarine organisms
1.9.2. Effects on freshwater organisms
1.9.3. Microcosm studies
1.10. Effects on terrestrial organisms
1.11. Effects on organisms in the field
1.12. Toxicity to laboratory mammals
1.12.1. Acute toxicity
1.12.2. Short-term toxicity
1.12.3. Long-term toxicity
1.12.4. Genotoxicity
1.12.5. Reproductive toxicity
1.12.6. Carcinogenicity
1.13. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of tributyltin compounds
2.2. Physical and chemical properties
2.3. Analytical methods
2.3.1. Measurement of organotin compounds
2.3.1.1 Extraction of tributyltin derivatives
2.3.1.2 Formation of volatile derivatives
2.3.1.3 Separation of organotin derivatives
2.3.1.4 Detection and measurement of different forms
of organotin
2.3.2. Interlaboratory calibrations
3. SOURCES OF ENVIRONMENTAL EXPOSURE
3.1. Uses
3.2. Production
3.3. Regulations
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
4.1. Adsorption onto and desorption from particles
4.2. Abiotic degradation
4.2.1. Hydrolytic cleavage of the tin-carbon bond
4.2.2. Photodegradation
4.3. Biodegradation
4.4. Bioaccumulation and elimination
5. ENVIRONMENTAL CONCENTRATIONS
5.1. Sea water and marine sediment
5.2. Fresh water and sediment
5.3. Sewage treatment
5.4. Biota
6 KINETICS AND METABOLISM
6.1. Metabolism of TBT in mammals
6.2. Metabolism of TBTO in other organisms
6.3. General mechanisms of toxicity of TBTO
6.3.1. General toxic mechanisms
6.3.2. Toxic mechanisms in bivalve molluscs
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: MICROORGANISMS
7.1. Bacteria and fungi
7.2. Freshwater algae
7.3. Estuarine and marine algae
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: AQUATIC ORGANISMS
8.1. Aquatic plants
8.2. Aquatic invertebrates
8.2.1. Trematode parasites of man
8.2.2. Freshwater molluscs
8.2.2.1 Acute toxicity
8.2.2.2 Short- and long-term toxicity
8.2.2.3 Factors affecting toxicity
8.2.3. Marine molluscs
8.2.3.1 Acute toxicity
8.2.3.2 Short- and long-term toxicity
8.2.3.3 Reproductive effects
8.2.3.4 Effects on growth
8.2.3.5 Shell thickening
8.2.3.6 Imposex
8.2.3.7 Genotoxicity
8.2.4. Crustaceans
8.2.4.1 Acute effects
8.2.4.2 Short- and long-term toxicity
8.2.4.3 Reproductive effects
8.2.4.4 Limb regeneration
8.2.4.5 Behavioural effects
8.2.5. Other aquatic invertebrates
8.2.5.1 Acute effects
8.2.5.2 Limb regeneration
8.3. Fish
8.3.1. Acute effects
8.3.2. Short- and long-term toxicity
8.3.3. Embryotoxicity
8.3.4. Behavioural effects
8.4. Amphibians
8.5. Multispecies studies
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: TERRESTRIAL ORGANISMS
9.1. Microcosm studies
9.2. Terrestrial insects
9.3. Terrestrial mammals
10. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: FIELD OBSERVATIONS
10.1. Effects on bivalves
10.2. Effects on gastropods: imposex
10.3. Effects on farmed fish
10.4. Effects of TBT-contaminated sediment
10.5. Effects of freshwater molluscicides
10.6. Effects from spills
10.7. The use of indicator species for monitoring the environment
11. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
11.1. Single exposure
11.1.1. Oral and parenteral administration
11.1.2. Dermal administration
11.1.3. Administration by inhalation
11.1.4. Irritation and sensitization
11.1.4.1 Skin irritation
11.1.4.2 Eye irritation
11.1.4.3 Skin sensitization
11.1.5. In vitro studies
11.2. Short-term toxicity
11.2.1. Oral dosing: general body effects
11.2.2. Inhalation studies
11.2.3. Histopathological effects
11.2.4. Haematological and biochemical effects
11.2.5. Effects on lymphoid organs and immune function
11.2.6. Mechanism of immunotoxicity
11.2.7. Effects on the endocrine system
11.3. Long-term toxicity
11.4. Genotoxicity
11.5. Reproductive toxicity
11.5.1. In vivo
11.5.2. in vitro
11.6. Carcinogenicity
12. EFFECTS ON HUMANS
12.1. Ingestion
12.2. Inhalation
12.3. Dermal exposure
12.4. Miscellaneous effects
13. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
13.1. Evaluation of human health risks
13.2. Evaluation of effects on the environment
14. RECOMMENDATIONS
14.1. Recommendations for protecting human and environmental health
14.2. Research needs
REFERENCES
RESUME
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET EFFETS SUR L'ENVIRONNEMENT
RECOMMANDATIONS
RESUMEN
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS SOBRE EL
MEDIO AMBIENTE
RECOMENDACIONES
WHO TASK GROUP ON TRIBUTYLTIN COMPOUNDS
Members
Dr C. Alzieu, French Institute for Research on Exploi-
tation of the Sea, Nantes, France
Dr I.J. Boyer, Division of Toxicological Review and Evalu-
ation, Food & Drug Administration, Washington, DC, USA
Dr A.H. El-Sabae, Faculty of Agriculture, Alexandria Uni-
versity, Alexandria, Egypt
Dr B. Gilbert, Company for the Development of Technology
Transfer (CODETEC), Cidade Universitaria, Campinas,
Brazil
Dr Y. Hayashi, Biological Safety Research Centre, National
Institute of Hygienic Sciences, Setagaya-ku, Tokyo,
Japan
Dr R. Koch, Institute for Geography & Geoecology, Academy
of Sciences, German Democratic Republic (Chairman)
Dr E.I. Krajnc, National Institute for Public Health and
Environmental Hygiene, Bilthoven, Netherlands
Dr H. Schweinfurth, Schering AG, Chemical Industry,
Bergkamen, Federal Republic of Germany
Mr D. Spatz, Office of Pesticide Programs, US Environmen-
tal Protection Agency, Washington, DC, USA
Dr A.R.D. Stebbing, Natural Environment Research Council,
Plymouth Marine Laboratory, Plymouth, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Agricultural
University, Wageningen, Netherlands
Dr J.E. Thain, Ministry of Agriculture, Fisheries and
Food, Fisheries Laboratory, Burnham-on-Crouch, United
Kingdom
Prof P.N. Viswanathan, Ecotoxicology Section, Industrial
Toxicology Research Centre, Lucknow, India
Observers
Mr J. Chadwick, Health and Safety Executive, Bootle,
United Kingdom
Dr R.J. Fielder, Department of Health, London, United
Kingdom
Dr R. Lange, Schering AG, Department of Experimental Toxi-
cology, Berlin, Federal Republic of Germany
Secretariat
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom (Rapporteur)
Dr M. Gilbert, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
(Secretary)
Mr P.D. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United
Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 or 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TRIBUTYLTIN COMPOUNDS
A WHO Task Group meeting on Environmental Health Cri-
teria for tributyltin compounds was held at the Institute
of Terrestrial Ecology (ITE), Monks Wood, United Kingdom,
from 11 to 15 September 1989. Dr M. Roberts, Director,
ITE, welcomed the participants on behalf of the host
institution and Dr M. Gilbert opened the meeting on behalf
of the three cooperating organizations of the IPCS (ILO,
UNEP, WHO). The Task Group reviewed and revised the draft
criteria document and made an evaluation of the risks for
human health and the environment from exposure to
tributyltin compounds.
The first draft of this document was prepared by Dr S.
Dobson (ITE) and Dr R. Cabridenc (Institut National de
Recherche Chimique Appliquée, France). Dr M. Gilbert and
Dr P.G. Jenkins, both members of the IPCS Central Unit,
were responsible for the technical development and
editing, respectively.
ABBREVIATIONS
AA atomic absorption
BCF bioconcentration factor
DBT dibutyltin
EC50 median effective concentration
EEC European Economic Community
EQT environmental quality target
FAA flameless atomic absorption
FMLP formyl methionyl leucyl phenylalanine
FPD flame photometric detector
GC gas chromatography
GLC gas-liquid chromatography
HPLC high-performance liquid chromatography
IC50 median inhibitory concentration
ip intraperitoneal
IU international unit
iv intravenous
LC50 median lethal concentration
LDH lactate dehydrogenase
LT50 median lethal time
MBT monobutyltin
MIC minimal inhibitory concentration
MS mass spectrometry
ND not detectable
NOEL no-observed-effect level
OECD Organization for Economic Cooperation and Development
PALS periarteriolar lymphocyte sheath
sc subcutaneous
T4 thyroxine
TBT tributyltin
TBTO tributyltin oxide
TLC thin-layer chromatography
TLV threshold limit value
1. SUMMARY
1.1. Physical and chemical properties
Tributyltin (TBT) compounds are organic derivatives of
tetravalent tin. They are characterized by the presence of
covalent bonds between carbon atoms and a tin atom and
have the general formula (n-C4H9)3 Sn-X (where X is
an anion). The purity of commercial tributyltin oxide
(TBTO) is generally above 96%; the principal impurities
are dibutyltin derivatives and, to a lesser extent, tetra-
butyltin and other trialkyltin compounds. TBTO is a
colourless liquid with a characteristic odour and a rela-
tive density of 1.17 to 1.18. The solubility in water is
low, varying between <1.0 and >100 mg/litre according to
the pH, temperature, and anions present in the water
(which determine speciation). In sea water and under nor-
mal conditions, TBT exists as three species (hydroxide,
chloride, and carbonate), which remain in equilibrium. At
pH values less than 7.0, the predominate forms are
Bu3SnOH2+ and Bu3SnCl, at pH 8, they are Bu3SnCl,
Bu3SnOH, and Bu3SnCO3-, and at pH values above 10,
Bu3SnOH and Bu3SnCO3- predominate.
The octanol/water partitioncoefficient (log Pow) lies
between 3.19 and 3.84 for distilled water and is 3.54 for
sea water. TBTO adsorbs strongly to particulate matter,
the reported adsorption coefficients ranging between 110
and 55 000. Vapour pressure is low but published values
show considerable variation. There was no loss of TBTO
from a solution of 1 mg/litre over 62 days, but 20% of the
water was lost by evaporation.
1.2. Analytical methods
Several methods are used for measuring tributyltin
derivatives in water, sediment, or biota. Atomic absorp-
tion spectrometry (AA) is the most common. AA spectrometry
with a flame allows a detection limit of 0.1 mg/litre.
Flameless AA, using atomization in an electric furnace
with graphite, is more sensitive and allows detection
limits of between 0.1 and 1.0 µg/litre water. There are
several different methods of extraction and for forming
volatile derivatives. Separation of these derivatives is
commonly done using "purge and trap" or gas chromato-
graphy. The detection limits are 0.5 and 5.0 µg/kg for
sediment and biota.
1.3. Sources of environmental pollution
Tributyltin compounds have been registered as mollus-
cicides, as antifoulants on boats, ships, quays, buoys,
crab pots, fish nets, and cages, as wood preservatives, as
slimicides on masonry, as disinfectants, and as biocides
for cooling systems, power station cooling towers, pulp
and paper mills, breweries, leather processing, and tex-
tile mills. TBT in antifouling paints was first marketed
in a form that allowed free release of the compound. More
recently, controlled-release paints, in which the TBT is
incorporated in a co-polymer matrix, have become avail-
able. Rubber matrices have also been developed to give
long-term slow release and lasting effectiveness for anti-
fouling paints and molluscicides. TBT is not used in agri-
culture because of high phytotoxicity.
1.4. Regulations on use
Many countries have restricted the use of TBT anti-
fouling paints as a result of effects on shellfish. The
regulations vary in detail from country to country, but
most ban the use of TBT paints on boats of 25 metres
length or less. Some countries have excluded boats with
aluminium hulls from this ban. In addition, some regu-
lations restrict the TBT content of paints or the leaching
rate of TBT from paints (to 4 or 5 µg/cm2 per day, long-
term).
1.5. Environmental concentrations
High levels of TBT in water, sediment, and biota have
been found close to pleasure boating activity, especially
in or near marinas, boat yards, and dry docks, fish nets
and cages treated with antifouling paints, and cooling
systems. The degree of tidal flushing and the turbidity
of the water influence TBT concentrations.
TBT levels have been found to reach 1.58 µg/litre in
sea water and estuaries, 7.1 µg/litre in fresh water,
26 300 µg/kg in coastal sediments, 3700 µg/kg in fresh-
water sediments, 6.39 mg/kg in bivalves, 1.92 mg/kg in
gastropods, and 11 mg/kg in fish. However, these maximum
concentrations of TBT should not be taken as representa-
tive, because a number of factors may give rise to anomal-
ously high values (e.g., paint particles in water and
sediment samples). It has been found that measured TBT
concentrations in the surface microlayer of both fresh
water and sea water are up to two orders of magnitude
above those measured just below the surface. However, it
should be noted that recorded levels of TBT in surface
microlayers may be highly affected by the method of
sampling.
Older data may not be comparable with newer data
because of improvements in the analytical methods avail-
able for measuring TBT in water, sediment, and tissue.
1.6. Transport and transformation in the environment
As a result of its low water solubility and lipophilic
character, TBT adsorbs readily onto particles. Between
10% and 95% of TBTO introduced into water is estimated to
undergo particulate adsorption. Progressive disappearance
of adsorbed TBT is not due to desorption but to degra-
dation. The degree of adsorption depends on the salinity,
nature and size of particles in suspension, amount of sus-
pended matter, temperature, and the presence of dissolved
organic matter.
The degradation of TBTO involves the splitting of the
carbon-tin-bond. This can result from various mechanisms
occurring simultaneously in the environment, including
physico-chemical mechanisms (hydrolysis and photodegra-
dation) and biological mechanisms (degradation by microor-
ganisms and metabolism by higher organisms). Whereas the
hydrolysis of organotin compounds occurs under conditions
of extreme pH, it is barely evident under normal environ-
mental conditions. Photodegradation occurs during labora-
tory exposure of solutions to UV light at 300 nm (and to a
lesser extent at 350 nm). Under natural conditions, pho-
tolysis is limited by the wavelength range of sunlight and
by the limited penetration of UV light into water. The
presence of photosensitizing substances can accelerate
photodegradation. Biodegradation depends on environmental
conditions such as temperature, oxygenation, pH, level of
mineral elements, the presence of easily biodegradable
organic substances for co-metabolism, and the nature of
the microflora and its capacity for adaptation. It also
depends on the TBTO concentration being lower than the
lethal or inhibitory threshold for the bacteria. As with
abiotic degradation, biotic breakdown of TBT is a pro-
gressive oxidative debutylization founded on the splitting
of the carbon-tin bond. Dibutyl derivatives are formed,
which are more readily degraded than tributyltin. Mono-
butyltins are mineralized slowly. Anaerobic degradation
does occur but there is a lack of agreement as to its
importance. Some workers consider that anaerobic degra-
dation is slow, others that it is more rapid than aerobic
degradation. Species of bacteria, algae, and wood-
degrading fungi have been identified that can degrade
TBTO. Estimates of the half-life of TBT in the environ-
ment vary widely.
TBT bioaccumulates in organisms because of its
solubility in fat. Bioconcentration factors of up to 7000
have been reported in laboratory investigations with
molluscs and fish, and higher values have been reported in
field studies. Uptake from food is more important than
uptake directly from the water. Higher concentration
factors in microorganisms (between 100 and 30 000) may
reflect adsorption rather than uptake into cells. There
is no indication that TBT is transferred to terrestrial
organisms via food chains.
1.7. Kinetics and metabolism
Tributyltin is absorbed from the gut (20-50% depending
on the vehicle) and via the skin of mammals (approximately
10%). It can be transferred across the blood-brain barrier
and from the placenta to the fetus. Absorbed material is
rapidly and widely distributed among tissues (principally
the liver and kidney).
TBT metabolism in mammals is rapid; metabolites are
detectable in blood within 3 h of TBT administration. In
in vitro studies, it has been shown that TBT is a
substrate for mixed-function oxidases, but these enzymes
are inhibited by very high concentrations of TBT.
The rate of TBT loss differs with different tissues,
and estimates for biological half-lives in mammals range
from 23 to about 30 days.
TBT metabolism also occurs in lower organisms, but it
is slower, particularly in molluscs, than in mammals. The
capacity for bioaccumulation is, therefore, much greater
than in mammals.
TBT compounds inhibit oxidative phosphorylation and
alter mitochondrial structure and function. TBT interferes
with calcification of the shell of oysters ( Crassostrea
species).
1.8. Effects on microorganisms
TBT is toxic to microorganisms and has been used
commercially as a bactericide and algicide. The concen-
trations that produce toxic effects vary considerably
according to the species. TBT is more toxic to gram-
positive bacteria (minimal inhibitory concentration (MIC)
between 0.2 and 0.8 mg/litre) than to gram-negative bac-
teria (MIC: 3 mg/litre). The TBT acetate MIC for fungi is
0.5-1 mg/litre and the TBTO MIC for the green alga
Chlorella pyrenoidosa is 0.5 mg/litre. The primary pro-
ductivity of a natural community of freshwater algae was
reduced by 50% at a TBTO concentration of 3 µg per litre.
Recently established no-observed-effect level (NOEL)
values for two species of algae are 18 and 32 µg per
litre. Toxicity to marine microorganisms is similarly
variable between species and between studies; NOEL values
are difficult to set but lie below 0.1 µg/litre for some
species. Algicidal concentrations range from <1.5 µg per
litre to >1000 µg/litre for different species.
1.9. Effects on aquatic organisms
1.9.1. Effects on marine and estuarine organisms
A summary diagram relating lethal and sublethal ef-
fects to measured marine and estuarine TBT concentrations
is presented in Fig. 1. Concentrations exceeding those
producing acute lethal effects have been found in many
different worldwide locations, particularly associated
with pleasure boating activity.
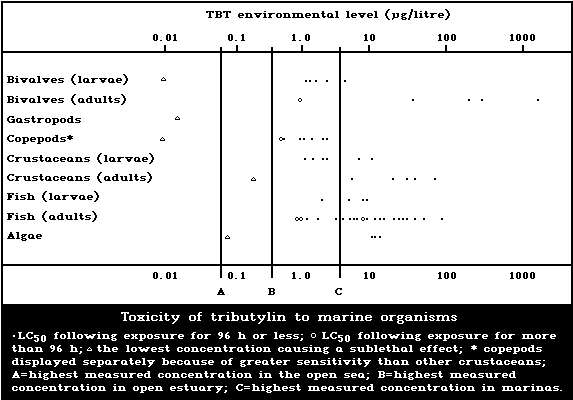 The development of the motile spores of a green
macroalga was the stage most sensitive to TBT (5-day
EC50: 0.001 µg/litre). There was reduced growth of a
marine angiosperm at TBT concentrations of 1 mg/kg sedi-
ment but no effect at 0.1 mg/kg.
Tributyltin is highly toxic to marine molluscs. It
has been shown experimentally to affect shell deposition
of growing oysters, gonadal development and gender of
adult oysters, settlement, growth, and mortality of larval
oysters and other bivalves, and to cause imposex (the
development of male characteristics) in female gastropods.
The NOEL for spat of the most sensitive oyster species
(Crassostrea gigas) has been reported to be about
20 ng/litre. TBT causes deformation of the shell of adult
oysters in a dose-related manner. No effect on shell mor-
phology was observed experimentally at TBT concentrations
of 2 ng/litre. The NOEL for the development of imposex in
female dogwhelks is below 1.5 ng/litre. Larval forms are
generally more sensitive than adults; in the case of oys-
ters this difference is particularly marked.
Copepods are more sensitive than other crustacean
groups to the acute lethal effects of TBT, LC50 values
for exposure periods up to 96 h ranging from 0.6 to
2.2 µg/litre. These values are comparable to those of
the more sensitive larvae of other crustacean groups. TBT
reduces reproductive performance, neonate survival, and
juvenile growth rate in crustaceans. The NOEL for repro-
duction in the mysid shrimp Acanthomysis sculpta has been
suggested to be 0.09 µg/litre. There was no avoidance of
TBT by the grass shrimp at concentrations up to 30 µg/litre.
The toxicity of tributyltin to marine fish is highly
variable, 96-h LC50 values ranging between 1.5 and
36 µg/litre. Larval stages are more sensitive than adults
(Fig. 1). There are indications that marine fish avoid
TBTO concentrations of 1 µg/litre or more.
1.9.2. Effects on freshwater organisms
A summary diagram relating lethal and sublethal
effects to measured TBT concentrations in fresh water is
presented in Fig. 2. Concentrations exceeding those pro-
ducing sublethal effects have been found, particularly
associated with pleasure boating activity.
The development of the motile spores of a green
macroalga was the stage most sensitive to TBT (5-day
EC50: 0.001 µg/litre). There was reduced growth of a
marine angiosperm at TBT concentrations of 1 mg/kg sedi-
ment but no effect at 0.1 mg/kg.
Tributyltin is highly toxic to marine molluscs. It
has been shown experimentally to affect shell deposition
of growing oysters, gonadal development and gender of
adult oysters, settlement, growth, and mortality of larval
oysters and other bivalves, and to cause imposex (the
development of male characteristics) in female gastropods.
The NOEL for spat of the most sensitive oyster species
(Crassostrea gigas) has been reported to be about
20 ng/litre. TBT causes deformation of the shell of adult
oysters in a dose-related manner. No effect on shell mor-
phology was observed experimentally at TBT concentrations
of 2 ng/litre. The NOEL for the development of imposex in
female dogwhelks is below 1.5 ng/litre. Larval forms are
generally more sensitive than adults; in the case of oys-
ters this difference is particularly marked.
Copepods are more sensitive than other crustacean
groups to the acute lethal effects of TBT, LC50 values
for exposure periods up to 96 h ranging from 0.6 to
2.2 µg/litre. These values are comparable to those of
the more sensitive larvae of other crustacean groups. TBT
reduces reproductive performance, neonate survival, and
juvenile growth rate in crustaceans. The NOEL for repro-
duction in the mysid shrimp Acanthomysis sculpta has been
suggested to be 0.09 µg/litre. There was no avoidance of
TBT by the grass shrimp at concentrations up to 30 µg/litre.
The toxicity of tributyltin to marine fish is highly
variable, 96-h LC50 values ranging between 1.5 and
36 µg/litre. Larval stages are more sensitive than adults
(Fig. 1). There are indications that marine fish avoid
TBTO concentrations of 1 µg/litre or more.
1.9.2. Effects on freshwater organisms
A summary diagram relating lethal and sublethal
effects to measured TBT concentrations in fresh water is
presented in Fig. 2. Concentrations exceeding those pro-
ducing sublethal effects have been found, particularly
associated with pleasure boating activity.
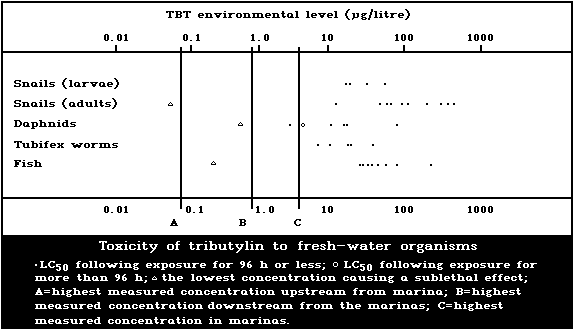 Fresh-water angiosperms were killed by a TBTO concen-
tration of 0.5 mg/litre, and growth was inhibited at
0.06 mg/litre or more.
Data on fresh-water invertebrate species are few, re-
lating to just three species other than target organisms.
Different salts of TBT yield 48-h LC50 values for Daphnia
of 2.3-70 µg/litre and for Tubifex of 5.5-33 µg/litre.
The NOEL for Daphnia has been estimated to be 0.5 µg per
litre, based on reversal of normal response to light. The
24-h LC50 for the Asiatic clam has been reported to be
2100 µg/litre, and for target snail adults in schistoso-
miasis control the corresponding values are 30-400 µg/litre.
Tributyltin has been shown to be toxic to schistosome
larvae in the aquatic stages; the LC50 (TBT fluoride)
was calculated to be 16.8 µg/litre for a 1-h exposure.
The TBT dose causing 99% to 100% suppression of cercarial
infectivity of mice was between 2 and 6 µg/litre.
The sensitivity of snails to TBT decreases with age,
but eggs are more resistant than both young and adults.
Egg laying is significantly effected at a TBTO concen-
tration of 0.001 µg/litre.
The acute toxicity of TBT to freshwater fish in LC50
tests up to 168 h ranges from 13 to 240 µg per litre.
The NOEL for the guppy was estimated to be 0.01 µg per
litre, based on histopathological effects.
No effect on survival was found when eggs and larvae
of the frog Rana temporaria were exposed to TBT concen-
trations of 3 µg/litre or less, but at 30 µg/litre sig-
nificant mortality was observed.
1.9.3. Microcosm studies
Microcosm studies modelling marine ecosystems have
been conducted with introduced organisms and in conditions
where inflowing sea water allowed colonization by other
organisms. Results showed decreases in both numbers of
individuals and in species diversity at TBTO concen-
trations in water between 0.06 and 3 µg/litre.
Results from freshwater model ecosystems suggest that
doses which kill freshwater snails also affect other
species, including fish.
1.10. Effects on terrestrial organisms
The exposure of terrestrial organisms to TBT results
primarily from its use as a wood preservative. TBTO is
toxic to bees housed in hives made from TBT-treated wood.
TBT was toxic to bats in a single study, but this result
was not statistically significant owing to high control
mortality. TBT compounds are toxic to insects exposed
topically or via feeding on treated wood. The acute tox-
icity of TBT to wild mice is moderate; estimated dietary
LC50 values, based on consumption of treated seeds used
in repellency tests, range from 37 to 240 mg/kg per day.
1.11. Effects on organisms in the field
Field observations have related high concentrations of
tributyltin to mortality and settlement failure of larval
bivalves, reduced growth, shell thickening and other mal-
formations in developing oysters, imposex in mud snails,
and imposex (concurrent with population decline) in the
dogwhelk. Complete failure of oyster fisheries was ident-
ified initially in France and afterwards in other
countries and related to water concentrations of TBT. The
effects were most marked in areas close to pleasure boat
marinas. Controlling the use of TBT antifouling paints on
small boats has resulted in recovery of oyster repro-
duction and growth. However, water concentrations of TBT
are still high enough in some areas to affect marine
gastropods.
Both shell growth and chambering in Pacific oysters
and imposex in dogwhelks have been used as biological
indicators of TBT contamination.
There have been few studies of the effects on organ-
isms of TBT in sediment, but there are indications that
the TBT is available to burrowing organisms and can cause
mortality in the field.
Gross toxic effects and histopathological changes have
been reported in farmed marine fish exposed to TBT by the
use of antifouling paints on retaining nets.
The use of TBT as a molluscicide against the fresh-
water snails that carry schistosomiasis (bilharzia) has
been proposed. Some field trials have been conducted which
show that it is difficult to apply TBT without damaging
non-target organisms.
1.12. Toxicity to laboratory mammals
1.12.1. Acute toxicity
Tributyltin is moderately to highly toxic to labora-
tory mammals, acute oral LD50 values ranging from 94 to
234 mg/kg body weight for the rat and from 44 to 230 mg/kg
body weight for the mouse. The acute toxicity to the
guinea-pig and the rabbit fall within the same range. The
variation comes from the "anion" component of the tri-
butyltin salt. These compounds exhibit greater lethal
potential when administered parenterally, as opposed to
orally, probably due to only partial absorption from the
gut.
Other effects of acute exposure may include alter-
ations in blood lipid levels, the endocrine system, liver,
and spleen, and transient deficits in brain development.
The toxicological significance of these effects, reported
after high single doses of the compound, is questionable
and the cause of death remains unknown.
The acute toxicity via the dermal route is low, the
LD50 being >9000 mg/kg body weight for the rabbit.
"Nose only" inhalation LD50 (4 h) for the rat is
77 mg/m3 (65 mg/m3 when only inhalable particles are
considered). TBT vapour/air mixtures produce no observ-
able toxic effects, even at saturation. However, TBT is
very hazardous as an inhaled aerosol, producing lung irri-
tation and oedema.
TBT is severely irritating to the skin and an extreme
irritant to the eye. TBTO is not a skin sensitizer.
1.12.2. Short-term toxicity
TBT compounds have been studied most extensively in
the rat (all the data in this section refer to the rat
unless otherwise indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg
body weight), high mortality rates were observed when the
exposure time exceeded 4 weeks. No deaths were noted at
100 mg/kg diet (10 mg/kg body weight) or after daily
administration of 12 mg/kg body weight by gavage. In rats
dosed during early post-natal life, 3 mg/kg body weight
resulted in increased deaths. The main symptoms at lethal
doses were loss of appetite, weakness, and emaciation.
Borderline effects on rat growth were observed at
50 mg/kg diet (6 mg/kg body weight) and 6 mg/kg body
weight (gavage studies). Mice are less sensitive, effects
being observed at 150 to 200 mg/kg diet (22 to 29 mg/kg
body weight).
Structural effects on endocrine organs, mainly the
pituitary and thyroid, have been noted in both short- and
long-term studies. Changes in circulating hormone concen-
trations and altered response to physiological stimuli
(pituitary trophic hormones) were observed in short-term
tests, but after long-term exposure most of these changes
appeared to be absent. The mechanism of action is not
known.
Exposure to TBTO aerosol at 2.8 mg/m3 produced high
mortality, respiratory distress, inflammatory reaction
within the respiratory tract and histopathological changes
of lymphatic organs. However, exposure to the highest
attainable vapour concentration (0.16 mg/m3) at room
temperature produced no effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular
necrosis and inflammatory changes in the bile duct were
observed in rats fed TBTO at a dietary level of 320 mg/kg
(approximately 25 mg/kg body weight) for 4 weeks and in
mice fed 80 mg/kg diet (approximately 12 mg/kg body
weight) for 90 days. Vacuolization of periportal hepato-
cytes was noted in dogs fed a dose of 10 mg/kg body weight
for 8 to 9 weeks. These changes were occasionally
accompanied by increased liver weight and increased serum
activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte
volume in rats, resulting from dosing with 80 mg/kg diet
(8 mg/kg body weight), indicate an effect on haemoglobin
synthesis, leading to microcytic hypochromic anaemia. The
decrease in splenic haemosiderin levels suggests alter-
ations in iron status. Anaemia has also been observed in
mice.
The formation of erythrocyte rosettes in mesenteric
lymph nodes has been observed in certain short-term inves-
tigations but not in long-term studies. The biological
significance of this finding (possibly transient) is
unclear.
The characteristic toxic effect of TBTO is on the
immune system; due to effects on the thymus, the cell-
mediated function is impaired. The mechanism of action is
unknown, but may involve the metabolic conversion to
dibutyltin compounds. Non-specific resistance is also
affected.
General effects on the immune system (e.g., on the
weight and morphology of lymphoid tissues, peripheral lym-
phocyte counts, and total serum immunoglobulin concen-
trations) have been reported in several different studies
with TBTO using rats and dogs, but not mice, at overtly
toxic dose levels (effects in mice have been seen with
tributyltin chloride at 150 mg/kg). Only the rat exhibits
general effects on the immune system without other overt
signs of toxicity and is clearly the most sensitive
species. The NOEL in short-term rat studies was 5 mg/kg
diet (0.6 mg/kg body weight). In studies with tributyltin
chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been
shown to compromise specific immune function in rat in
vivo host resistance studies. Decreased clearance of
Listeria monocytogenes was seen after exposure to a diet-
ary level of 50 mg/kg (the NOEL being 5 mg/kg per day),
and decreased resistance to Trichinella spiralis was seen
at 50 and 5 mg/kg diet, but not at 0.5 mg/kg diet (2.5,
0.25, and 0.025 mg/kg per day body weight, respectively).
Similar effects were seen in aged animals, but these were
less pronounced.
With present knowledge, the effects on host resistance
are probably of most relevance in assessing the potential
hazard to man, but there is insufficient experience in
these test systems to fully assess their significance.
However, some data on the significance of the T. spiralis
model are provided by findings in athymic nude rats after
the standard challenge. In these studies, the complete
absence of thymus-dependent immunity resulted in a 10- to
20-fold increase in muscle larvae counts; by contrast,
exposure to TBTO concentrations of 5 and 50 mg/kg diet
resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on
the effects of tributyltin compounds on the developing
immune system, there is no information on host resistance.
It would be prudent to base assessment of the poten-
tial hazard to humans on data from the most sensitive
species. Effects on host resistance to T. spiralis have
been seen at dietary levels as low as 5 mg/kg (equivalent
to 0.25 mg/kg per day body weight), the NOEL being
0.5 mg/kg (equivalent to 0.025 mg/kg per day). However,
the interpretation of the significance of these data for
human risk assessment is controversial. In all other
studies a concentration of 5 mg/kg per day in the diet
(equivalent to 0.5 mg/kg body weight, based on the short-
term studies) was the NOEL with respect to general, as
well as specific, effects on the immune system.
1.12.3. Long-term toxicity
A long-term study in rats indicates a marginal effect
of TBT on general toxicological parameters (of limited
toxicological significance) at a level of 5 mg/kg diet
(0.25 mg/kg body weight).
1.12.4. Genotoxicity
The genotoxicity of TBTO has been the subject of
extensive investigations. Negative results were obtained
in the vast majority of studies, and there is no convinc-
ing evidence that TBTO has any mutagenic potential.
1.12.5. Reproductive toxicity
The potential embryotoxicity of TBTO has been evalu-
ated in three mammalian species (mouse, rat, and rabbit)
after oral dosing of the mother. The main malformation
noted in rat and mouse fetuses was cleft palate, but this
occurred at dosages overtly toxic to the mothers. These
results are not considered to be indicative of teratogenic
effects of TBTO at doses below those producing maternal
toxicity. The lowest NOEL, with regard to embryotoxicity
and fetotoxicity for all three species, was 1.0 mg/kg body
weight.
1.12.6. Carcinogenicity
One carcinogenicity study has been carried out on
rats, in which neoplastic changes were observed in endo-
crine organs at 50 mg/kg diet. The pituitary tumours
reported at 0.5 mg/kg diet were considered as having no
biological significance since there was no dose-response
relationship. These tumour types usually appear in high
and variable background incidences, and their significance
is, therefore, questionable. A carcinogenicity study on
mice is in progress.
1.13. Effects on humans
Occupational exposure of workers to tributyltin has
been found to result in irritation of the upper respir-
atory tract. TBT as an aerosol poses a hazard to humans.
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The
potential problem is made worse by the lack of an
immediate response to the skin.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of tributyltin compounds
Tributyltins compounds are organic derivatives of tin
(SnIV) characterized by the presence of covalent bonds
between three carbon atoms and a tin atom. They conform to
the following general formula ( n-C4H9)3 Sn-X, where X is
an anion or a group linked covalently through a hetero-
atom.
The nature of X influences the physico-chemical
properties, notably the relative solubility in water and
non-polar solvents and the vapour pressure.
These compounds differ from inorganic tin both in
behaviour and effects. An important member of the group is
tributyltin oxide (TBTO; RTECS number, JN8750000). Commer-
cial TBTO has a purity generally above 96%. Principle
impurities are dibutyltin derivatives and, to a lesser
extent, tetrabutyl or dibutylalkyl tin compounds.
Other industrially important tributyltin derivatives
include tributyltin fluoride, tributyltin methacrylate (monomer
or copolymer), tributyltin benzoate, tributyltin linoleate,
tributyltin naphthenate, and tributyltin phosphate.
2.2. Physical and chemical properties
TBTO is flammable but does not form explosive mixtures
with air. It is a mild oxidizing agent. It reacts quanti-
tatively at room temperature with bromide or iodine with
cleavage of the Sn-O bond (a reaction that may be used for
quantitative analysis) (Bahr & Pawlenko, 1978).
In the presence of oxygen, light or heat, slow break-
down occurs with the formation of tetra-n-butyltin, di-
n-butyltin oxide, and eventually tin (IV) oxide by de-
alkylation (Evans & Karpel, 1985). This degradation may be
inhibited by the addition of 0.1-1.0% of stabilizers (such
as lactic or citric acids).
It has been suggested (Maguire et al., 1984; Laughlin
et al., 1986a) that TBTO in aqueous solution dissociates
with the formation of a hydrated tributyltin cation, which
can undergo reaction with anions present. Data are not
available on the equilibrium constants for these reac-
tions.
Laughlin et al. (1986a) showed that TBTO can react
with normal constituents of the sea water in the following
ways:
Bu3-Sn-O-Sn-Bu3+ HO -» 2Bu3-Sn-OH
Bu3-Sn-OH-H+ -» Bu3SnOH2+
Bu3-Sn-OH + CO32- -» Bu3SnCO3- + OH-
Bu3-Sn-OH2+ + Cl- -» Bu3-Sn-Cl + H2O
The predominant forms are Bu3SnOH2+ and Bu3SnCl
at pH < 7, Bu3SnCl, Bu3SnOH, and Bu3SnCO3- at pH 8,
and Bu3SnOH and Bu3SnCO3- at pH > 10.
Under normal conditions in sea water, it is considered
that the three species (hydroxide, chloride, and carbon-
ate) remain in equilibrium.
The physical and chemical properties of some commer-
cially available tributyltin compounds are listed in Table
1.
Varying data on the solubility of TBTO in water, which
ranges from < 1.0 to > 100 mg/litre at different tempera-
tures and pH values, may be related to the presence of
different anionic species as described above.
In the same way as described in the reaction between
TBTO and water, the TBT group can be transferred to other
oxygen-, nitrogen-, and sulfur-containing groups. Thus,
anaerobically in sediments, TBTO can be transformed to TBT
sulfide. With amino acids, or their derivatives such as
proteins, reaction can occur on the nitrogen and sulfur
atoms, and, with wood, it has been suggested that the TBT
group may react with hydroxylic groups (Blunden et al.,
1984) or form tributyltin carbonate (Smith et al., 1977).
Thus adsorption on to particulate matter could involve
chemical reaction as well as physical adsorption or sol-
ution. TBTO adsorbs strongly to particulate matter, the
reported adsorption coefficients ranging between 110 and
55 000.
Table 1. Identity and physical and chemical properties of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Oxide Benzoate Chloride Fluoride Linoleate Methacrylate Naphthenate
(TBTO) (TBTB) (TBTCl) (TBTF) (TBTL) (TBTM) (TBTN)
---------------------------------------------------------------------------------------------------------
IUPAC name distannoxane, stannane, stannane, stannane, stannane, stannane, stannane,
hexabutyl (benzyloxy) tributyl- tributyl- tributyl- tributyl- tributyl-
tributyl chloro fluoro (1-oxo-9,12- (2-methyl-1- mono (naph-
octadecadi- oxo-2-propyl) thenoyloxy)
enyl)oxy- oxy- derivatives
CAS name Bis(tributyl- Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin
tin) oxide benzoate chloride fluoride linoleate methacrylate naphthenate
CAS number 56-35-9 4342-36-3 1461-22-9 1983-10-4 24124-25-2 2155-70-6 85409-17-2
Molecular C24H54OSn2 C19H32O2Sn C12H27ClSn C12H27FSn C30H58O2Sn C16H32O2Sn
formula
Relative 596 411 325 309 568.7 374.7 ca.500
molecular
mass
Boiling 173 ca.135 140 > 350 ca.140 > 300 ca.125
point (°C) (130 Pa) (30 Pa) (1300 Pa) (extrapol) (50 Pa) (extrapol) (50 Pa)
Melting < -45 20 -16 240 < 0 16 < 0
point (°C)
Relative
density 1.17-1.18 ca.1.2 ca.1.2 1.25 1.05 1.14 ca.1.1
(20 °C)
Vapour
pressure (Pa 1 x 10-3 2 x 10-4 9 x 10-2 3 x 10-2 9 x 10-5
at 20 °C)
Refractive 1.4880-
index (20 °C) 1.4895
---------------------------------------------------------------------------------------------------------
TBTO is soluble in lipids and very soluble in a number
of organic solvents (ethanol, ether, halogenated hydro-
carbons, etc.).
The octanol/water partition coefficient (log Pow)
lies between 3.19 and 3.84 for distilled water and is 3.54
for sea water.
As shown in Table 1, the vapour pressures of TBT
compounds are low. The work of Maguire et al. (1983)
confirmed this directly by showing no loss of TBTO from a
1 mg/litre solution after 62 days; 20% of the water was
lost by evaporation.
2.3. Analytical methods
The control levels of contamination of different
environmental compartments (water, sediment, biota) and
the interpretation of laboratory experimental and field
study results regarding levels, fate, biodegradation, and
bioaccumulation of tributyltin compounds require sensitive
analytical techniques to allow identification and quanti-
fication.
2.3.1. Measurement of organotin compounds
These methods, which are summarized in Table 2, have
been applied initially to water and later to sediment and
biota. They must be sufficiently sensitive and specific to
allow monitoring of ng/litre levels, and they need to be
able to distinguish between different forms of organic tin
derivatives present in the environment, i.e. mono-, di-,
tri-, or tetra-butyltins and different species of alkyl
moieties (butyl, methyl). They have also to avoid all
interference from other metals and other organometallic
derivatives.
Generally there are four successive stages to analy-
sis, although some are optional:
* extraction;
* formation of volatile derivatives;
* separation of these derivatives;
* detection, identification, and quantification.
Table 2. Sampling, preparation, and analysis of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Medium Sampling method Sample volume Analytical method Detection limit Reference
---------------------------------------------------------------------------------------------------------
Air adsorption on 50-100 litres derivatization with Zimmerli &
Chromosorb, RMgX; GC/MS or Zimmermann
cation exchange GC/FPD (1980);
resin, or Tenax Muller (1987a)
Water 250 ml NaBH4 conversion to 0.1-2 ng/litre Hodge et al.
hydride; separation by (1979);
fractional distillation; Michel (1987);
AA Donard et al.
(1986); Braman &
Tompkins (1979);
Valkirs et al.
(1986); Weber
et al. (1986)
Water and extraction with 8 litres (water) derivatization with 1 ng/litre Maguire &
sediments dichloromethane or 1 g (sediment C5H11 MgBr; GC-FPD (water) Huneault (1981);
dry weight) or GC-FAA or 5 ng/mg Maguire &
(sediment Tkacz (1983,
dry weight) 1985); Maguire
et al. (1986)
Water and acidification, 1 litre derivatization with 10 ng/litre Meinema et al.
biota extraction with CH3 Mgl; GC-MS or AA (1978); Bjorklund
dichloromethane (1987a)
Water, 200 ml or NaBH4 conversion to 5 ng/litre or Matthias et
biota, or 16 litres hydride; extraction with 0.2 ng/litre al. (1986a,b);
sediments dichloromethane Humphrey &
Hope (1987)
Water and adsorption on 60 litres extraction with dichloro- 0.07 ng/litre Humphrey &
sediment silica (water) or 10 g methane/tropolone; deriva- (water) Hope (1987)
bonded C18 (sediment) tization with C5H7 MgBr; 0.2 mg/kg
GC-MS (sediment)
macroreticular 1 litre extraction with n-pentane < 1 ng/litre Muller
resin (water) diethylether (water) (1984)
adsorption (sediment); derivatization 0.5 mg/kg
with CH3MgCl; GC-MS (sediment)
---------------------------------------------------------------------------------------------------------
2.3.1.1 Extraction of tributyltin derivatives
Extraction may be independent of or coincident with
the formation of volatile derivatives. It is necessary for
sediments and biological tissues and can also be applied
in the analysis of water samples.
Following acidification, various organic solvents have
been used. The following are most often cited: methyliso-
butylketone, hexane, ethyl acetate, toluene, methanol,
chloroform, dichloromethane, and mixtures of tropolone
(2-hydroxy-2,4,6-cycloheptatrienone) with chloroform, ben-
zene, or dichloromethane.
In the case of water, liquid-liquid extraction may be
replaced by adsorption onto silica gel bonded with C18
aliphatic chains (Matthias et al., 1986a,b; Humphrey &
Hope, 1987).
2.3.1.2 Formation of volatile derivatives
Mono-, di-, and tri-butyltins are not sufficiently
volatile to assure their separation on gas-phase chromato-
graphy; it is, therefore, necessary to prepare more vol-
atile derivatives to allow better separation. Two pro-
cedures have been advocated:
* formation of alkyl derivatives (methyl or pentyl) by
the use of Grignard's reagent (reactive organomag-
nesium);
* formation of hydrides with the general structure
RnSnH4-n by reaction with sodium borohydride
(NaBH4) (Hodge et al., 1979).
These volatile derivatives can then be extracted using
organic solvents, such as dichloromethane, or purged by a
stream of hydrogen.
2.3.1.3 Separation of organotin derivatives
Less sensitive methods for direct separation of mono-,
di-, and tri-butyltins include high performance liquid
chromatography (Jewett & Brinckman, 1981) and thin-layer
chromatography. The latter method is only qualitative and
little used because of its low sensitivity.
2.3.1.4 Detection and measurement of different forms of organotin
Volatile derivatives prepared in the laboratory may be
separated by two procedures:
* separation as a function of boiling point with collec-
tion in a cold trap ("purge and trap" procedure);
* separation by gas chromatography.
After separation by GLC or by the "purge and trap"
procedure, it is possible to detect and quantify, at the
ng/litre level, different forms of organotin using the
following methods:
* a flame photometric detector selective for tin (FPD)
is considered satisfactory;
* a flame atomic absorption (AA) spectrometer or flame-
less atomic absorption (FAA) spectrometer using a
graphite furnace (tin is detected at 286.3 nm or
244.6 nm);
* a mass spectrometer (MS); this is useful for precise
identification of the substance but has limited sen-
sitivity.
There are several methods available for measuring TBT
down to detection limits of 0.2 to 5 ng/litre in water and
5 to 30 µg/kg (in tissues of biota and in sediments).
Some of them can be adapted for routine monitoring pur-
poses. It is necessary, however, to have sophisticated
equipment and the difficulty of the methods requires
experienced laboratories.
His & Robert (1980, 1985) developed a biological assay
based on toxic effects on larvae of the Pacific oyster,
Crassostrea gigas, sensitive only above 20 ng/litre and
nonspecific between organotin and other toxic compounds.
Colorimetric methods (Sherman & Carlson, 1980) have been
based on forming coloured derivatives with phenylfluorone
(nonspecific and with a sensitivity around 0.1 to 4 µg tin).
2.3.2. Interlaboratory calibrations
Interlaboratory comparison of assay methods have been
performed to compare the various proposed methods and to
validate their usefulness as standards.
Young et al. (1986) reported the conclusions of a
workshop held in the USA to examine the problems posed by
the analysis of organotins in water. Nine methods, based
on the principles outlined above, were considered as sat-
isfactory, since the range of results fell within + 15% of
the mean when the TBT concentration was in the order of
ng/litre.
Stephenson et al. (1987) reported the results of
interlaboratory calibrations conducted in 1986-1987 and
carried out on TBT derivatives in mussel tissues and in
sediments. The measurements were made in seven labora-
tories, each using its own technique and using different
extraction conditions, derivative formation, and detec-
tion. A first examination of results showed that they did
not vary by more than a factor of 3. The results were con-
sidered satisfactory.
Blair et al. (1986) took part in an interlaboratory
calibration exercise organised by the National Bureau of
Standards (NBS) in 1984 in the USA and carried out deter-
minations of TBT in water (at a concentration of 1 µg/litre).
Under the auspices of the OECD, it was decided
recently to organize a new worldwide intercalibration to
be carried out on:
* water samples containing 10 ng/litre each of mono-,
di-, and tri-butyltin;
* samples of dried sediment containing the above com-
pounds at a concentration of 100 µg/kg;
* samples of mussel tissue, frozen or freeze-dried, con-
taining the above compounds at 100 µg/kg.
It seems premature to impose a single analytical
method and preferable to allow a certain freedom of choice
between methods to allow sufficient sensitivity to be
attained. However, control of the competence of labora-
tories that carry out such difficult and complex analysis
is required through new calibration procedures.
3. SOURCES OF ENVIRONMENTAL EXPOSURE
3.1. Uses
Dutch scientists first recognized the biocidal proper-
ties of triorganotin compounds in the 1950s; major pro-
duction and use of these substances dates from this
period. It was found that the different triorganotin com-
pounds have different toxicities to different organisms.
Tributyltin compounds were found to be the most toxic of
the triorganotins to gram-positive bacteria and to fungi.
They were also found to have biocidal properties to a wide
spectrum of aquatic organisms.
In the early 1960s, both tributyltin oxide (TBTO) and
TBT fluoride were tested, mainly in Africa, as mollusci-
cides against several freshwater snail species that are
vectors of the disease schistosomiasis, the snails being
the intermediate hosts of the trematode parasite. This use
led to the introduction of TBT, during the mid 1960s, as
an antifouling paint on boats. At the same time TBT com-
pounds were being registered as wood preservatives (the
first registration was in 1958).
Tributyltin compounds have been registered as mollus-
cicides, as antifoulants on boats, ships, quays, buoys,
crabpots, fish nets, and cages, as wood preservatives, as
slimicides on masonry, as disinfectants, and as biocides
for cooling systems, power station cooling towers, pulp
and paper mills, breweries, leather processing, and tex-
tile mills.
When introduced as antifouling paints, TBT paints were
of the "free association" type, where the TBT is physi-
cally incorporated into the paint matrix. In this form it
has a high early release and very short life. Co-polymer
paints were introduced later; in these the TBT moiety is
chemically bonded to a polymer backbone, e.g., those
formed from TBT acrylate or methacrylate and the corre-
sponding acid. The biocide is released by chemical
hydrolysis of the organotin ester linkage. Dissolution is
slow from ships' hulls and a low level of released TBT can
be achieved over a prolonged period. TBT compounds have
also been impregnated into neoprene rubber to produce
elastomeric antifoulant coatings and slow-release mollus-
cicides. In this form, much of the TBT remains in the
matrix of the rubber, though the effectiveness lasts for
several years.
TBT compounds have not been suggested for use in agri-
culture because of their high phytotoxicity.
3.2. Production
The world consumption of tin in 1976 was estimated to
be 200 x 103 tonnes, of which 28 x 103 tonnes was
organotin. Approximately 40% of the total was consumed
in the USA (Zuckerman et al., 1978). The United Kingdom
Department of the Environment (1986) reported that the
worldwide use of organotin in 1980 was 30 x 103 tonnes.
This total was made up as follows:
* PVC stabilizers (dibutyl), approximately 20 x 103 tonnes;
* wood preservatives (tributyl), 3-4 x 103 tonnes;
* antifouling paints (tributyl), 2-3 x 103 tonnes;
* other uses of both di- and tri-butyltin, < 2 x 103 tonnes.
The annual world production of TBT compounds is esti-
mated to be 4000 to 5000 tonnes (Organotin Environmental
Programme Association (ORTEPA); personal communication to
IPCS, 1989).
The total annual use (production and imports) of
organotin compounds in Canada was reported by Thompson et
al. (1985) to be in excess of 1 x 103 tonnes. The total
annual production of TBTO in the Federal Republic of
Germany is reported to be 2 x 103 tonnes, of which 70%
is exported. National usage is as follows: 70% antifouling
paints; 20% timber protection; 10% textile and leather
protection; small amounts are also used as a preservative
in dispersion paints and as a disinfecting agent. Annual
tin emissions are reported to be less than 300 kg (TWG,
1988a). Annual TBT use in the Netherlands in 1985 was
reported to be 1.5 x 104 kg for wood preservation and
10 x 104 kg for antifouling paints (TWG, 1988c). Organo-
tin antifoulant use in Norway was 13.7 x 104 kg in 1986
for the treatment of nets and sea pens at approximately
600 fish farms (Linden, 1987). In Japan, usage was
estimated at 1300 tonnes in 1987, of which two-thirds was
used for antifouling paints on vessels and one-third for
antifouling of nets in fish culture.
A survey of total and retail sales of TBT-containing
paints and antifouling preparations for nets was carried
out in Finland in 1987. Of a total of 42 000 litres,
37 000 litres were sold retail. The concentration of TBT
in the antifouling paints was 4-18%. The previous use of
TBT as a slimicide or fungicide (estimated at 2.1 tonnes
per year during the period 1968-1970) has been discon-
tinued. The estimated sale of wood preservatives contain-
ing TBT was 130 tonnes in 1987; these contained between
0.9 and 1.8% of TBT. Champ & Pugh (1987) reported that
about 300 TBT antifouling paints were registered in the
USA in 1987, but only about 17 paints are now registered
for use (US EPA; personal communication to IPCS, 1989).
MAFF/HSE (1988) listed 345 different wood preservative
formulations, 24 surface biocides and 215 antifouling
paints containing TBT with registration approval for use
in the United Kingdom under the Control of Pesticides
Regulations. In 1989, the number of antifouling paints
containing TBT registered for use in the United Kingdom
had fallen to 148, with the number of wood preservatives
and surface biocides remaining about the same (337 and 26
registered products, respectively) (MAFF/HSE, 1989).
3.3. Regulations
In 1974, the USA set an occupational limit for organo-
tin compounds in air of 0.1 mg tin/m3 (time-weighted
average). In 1979, the American Conference of Governmental
Industrial Hygienists (ACGIH) recommended that the occu-
pational exposure standard for organotin compounds in air
should be set at a threshold limit value (time-weighted
average) of 0.1 mg tin/m3 and a short-term TLV at 0.2 mg
tin/m3. The Federal Republic of Germany was recommended,
in 1979, to adopt an occupational exposure standard for
organotin compounds in air of 0.1 mg tin/m3, specified
as a maximum worksite concentration (MAK). The United
Kingdom has also set a recommended occupational exposure
limit of 0.1 mg tin/m3.
A tentative acceptable daily intake (ADI) of 1.6 µg/kg
per day has been adopted in Japan.
In December 1979, the Japanese Government banned the
use of tributyltin compounds in certain products for
household use, e.g., paint, adhesive, wax, shoe polish,
and textile products.
Following the effects on the oyster industry in France
in the late 1970s, and the subsequent correlation of the
effects with TBT usage, the French government banned the
use of TBT antifouling paints for an initial trial period
of three months, which was later extended. In 1982, paints
containing more than 3% TBT by weight were banned on boats
of < 25 m in length, although boats with aluminium hulls
were excluded. Initially the regulation only covered the
Atlantic coast (January 1982) but was later extended
(September 1982) to the whole French coastline. All use of
organotin compounds in antifouling paints, at any concen-
tration, is now banned in France.
The exception in the regulations for TBT-based anti-
fouling paints that many countries have made for boats
with aluminium hulls is based on the fact that the copper-
based alternative paints react chemically with the alu-
minium.
In January 1986, the United Kingdom enforced regu-
lations that prohibited the retail sale and supply of
antifouling paints with a total tin concentration greater
than 7.5% by weight in co-polymer paints (reduced to 5.5%
in January 1987) or 2.5% in other paints. These regu-
lations were meant to control the use on small pleasure
craft, ban the sale of "free association" paints con-
taining high levels of organotin and set an upper limit on
organotin compounds in co-polymer paints. An ambient water
quality target of 20 ng/litre was set. The United Kingdom
Department of the Environment took steps to determine the
effectiveness of the legislation by setting up a monitor-
ing programme. Based on the results of this monitoring, a
total ban on the use of TBT paints on small boats (< 25 m)
and fish farming equipment was implemented in July 1987
(Abel et al., 1987). An environmental quality standard
(EQS) of 20 ng/litre for fresh water (covering both
potable water and protection of sensitive aquatic biota)
and 2 ng/litre for sea water has been set (United Kingdom
Department of the Environment, 1989).
The paint industry of the Federal Republic of Germany
(FRG) issued a renunciation in 1986 on the use of mono-
meric organotin compounds in antifouling paints and a
restriction to 3.8% TBT in co-polymeric paints. The FRG
has not, as yet, issued any national ban on TBT marine
antifouling paints and is awaiting the outcome of dis-
cussions on an EEC directive (TWG, 1988b). Champ & Pugh
(1987) reported that both Switzerland and the FRG have
banned all uses of TBT in antifouling paints in the fresh-
water environment.
In 1987, the US EPA reviewed TBT usage, weighing risks
to the environment against benefits to users. In the mean-
time, some individual States have passed their own regu-
lations. Both Virginia and Washington State have banned
the use of TBT antifouling paints on boats of < 25 m in
length, excepting those with aluminium hulls. Only paints
that conform to a leaching rate of 5 µg/cm2 per day
(steady state) can be used on boats longer than 25 m. Both
states continued to permit the use of TBT paints, with
acceptable leach rates, in 16 oz (0.45 kg) aerosol cans
for use on outboard motors and lower units. Maryland
instituted similar restrictions but set a lower permiss-
ible leaching rate of 1 µg/cm2 per day (steady state).
Since 1985, North Carolina, Oregon, and Michigan have
instituted restrictions on TBT use. California, Alaska,
New York, and New Jersey had TBT Bills pending in their
respective legislatures (Champ & Pugh, 1987). In April
1988, both the US House of Representatives and the Senate
passed bills to restrict the use of TBT in antifouling
paints. The legislation was signed by the President on
16th June 1988 and came into effect on 16th December 1988.
This Act established an interim release rate restriction
of 4.0 µg/cm2 per day (steady state) and a provision
prohibiting application of TBT antifouling paints to non-
aluminium vessels under 25 m length. Application to larger
vessels was restricted to certified applicators only. The
outboard motor or lower drive unit of a vessel less than
25 m in length was exempted. A limit on sales, delivery,
purchase, and receipt of TBT paints was set in December
1988 and a limit on use in June 1989 for existing stocks
of paint.
A voluntary ban on the use of TBT compounds for nets
in fish culture was imposed in 1987 by the National
Federation of Fisheries Cooperative Association of Japan.
In 1988, the Japanese Ministry of Health and Welfare and
the Japanese Ministry of International Trade and Industry
"designated" eight TBT compounds (and a further five TBT
compounds in 1989) on the basis of persistence, accumu-
lation, and toxicity. "Designated" indicates that no
final decision on regulation has yet been taken but that
the compounds have a recognized hazard. Following this
action, the Japan Paint Manufacturers Association volun-
tarily reduced the upper limit for TBT in paints to < 10%
wet weight for monomers and < 15% wet weight for polymers.
There is current action to monitor release rates from
paint products as the next step in limiting human exposure.
Maguire (1987) reported that tributyltin for the pres-
ervation of fish-farm nets is banned in Canada. In 1987,
the Canadian Department of Agriculture served notice that
antifouling uses of TBT compounds must conform to the
following: a maximum short-term (first 14 days) cumulative
release-rate from paint formulations of 168 µg/cm2; a
long-term average daily release of 4 µg/cm2; and a
minimum hull length of 19.5 m for the use of TBT antifoul-
ing paints on non-aluminium vessels.
In Australia, control measures on the use of TBT-based
paints were introduced in the States of New South Wales
and Victoria. TBT is prohibited for use on boats with a
hull length of less than 25 m, while a leaching rate of
5.0 µg/cm2 per day was set for hulls of 25 m or more.
Aluminium vessels are not exempt from the ban.
The Republic of Ireland instituted a by-law banning
the use of organotin compounds on boats and other aquatic
structures in April 1987 (Minchin et al., 1987).
Norway has also prohibited use of TBT in antifouling
paints except for boats longer than 25 m and those with
aluminium hulls; the regulation became effective from
January 1989. There is also prohibition on the sale, manu-
facture, and import of paints containing TBT without a
specific permit from the State Pollution Control auth-
ority. An agreement to prohibit use on nets of fish farms
has been concluded. Under the Helsinki Convention, the
Baltic States have formed an agreement on the banning of
TBT paints on small boats and have set up a joint monitor-
ing programme.
The Commission of the European Communities has made a
proposal to the Council of Ministers concerning restric-
tions on the use of antifouling paints that mirrors
national restrictions in member states (except that there
would be no derogation for boats with aluminium hulls).
This proposal is currently being considered by the
European Parliament and Council.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
Summary
Due to its physico-chemical properties, TBT introduced into
natural waters will partly adsorb onto particles. The quanti-
tative data show large variation due to differences in exper-
imental conditions such as salinity and concentration and
organic content of particulate matter. Once it is adsorbed,
decrease in TBT concentration takes place mainly by degra-
dation. It is known that TBT degradation rates in sediment are
slower than in the water column, particularly in anaerobic
conditions.
Although abiotic degradation occurs, the process remains
less important than biological action.
Biodegradation of TBTO in soil and water depends on the
environmental conditions and the toxic effect of the available
concentrations to the organisms involved. Hydroxylated inter-
mediates are formed during stepwise debutylation. Aerobic and
anaerobic organisms both cause biodegradation, but the relative
efficiency is not known conclusively. Illumination of the cul-
tures lowers the half-life, indicating the involvement of
photosynthetic organisms.
The lipophilic properties of TBTO contribute to bioaccumu-
lation in aquatic organisms, especially molluscs. Laboratory
and field studies corroborate this, although it is unclear how
adsorption processes complicate the results. Bioaccumulation in
all organisms studied is due, at least in part, to bioconcen-
tration from the water phase. Elimination takes place when
organisms are no longer exposed to tributyltin compounds.
Whether it is directly discharged into the environment or
diffuses progressively (at 1 to 10 µg/cm2 per day) from
coatings of the hulls of boats or nets, TBTO enters the aquatic
environment and is subject to transformation resulting from
physico-chemical and biochemical processes. Speciation is out-
lined in chapter 2.
4.1. Adsorption onto and desorption from particles
The effects of TBTO vary in relation to the state in
which the substance is present in the aquatic environment,
in particular whether it is available to organisms in
estuaries or sea shores. It is important to have infor-
mation on its distribution in natural waters likely to
have large amounts of suspended matter of various types.
Several workers (Valkirs et al., 1986; Maguire et al.,
1986; Randall et al., 1986; Harris & Cleary, 1987; Stang &
Seligman, 1987; Hinga et al., 1987) have conducted studies
on adsorption and desorption of TBTO in laboratory exper-
iments, observations in the field, studies conducted in
microcosms, and mathematical modelling.
Mathematical models have been developed to estimate
the distribution of TBT in enclosed or semi-enclosed har-
bours (Walton et al., 1986) and estuaries (Harris &
Cleary, 1987). Good agreement has been found between
measured and estimated concentrations of tin in San Diego
harbour, USA (Walton et al., 1986). The authors considered
the results useful in predicting levels in ecologically
sensitive areas of the bay. The Harris & Cleary (1987)
model was based on the estuary of the River Tamar in
south-west England. This model, still under development,
aimed to reduce inputs in order to allow the model to be
used by non-experts and to be applicable to all estuaries.
Output for the River Tamar suggested that sediment-bound
tin would be distributed up the estuary by tidal influence
leading to increased bound tin further from the open sea.
This effect would be most marked in the summer. Relative
to soluble TBT, this bound fraction does not currently
amount to a significant source of tin for organisms. The
authors point out, however, that this source may become
increasingly important as use of TBT declines and sedi-
ment-bound TBT represents the only available source of the
compound.
The chemical properties of TBT, particularly its lipo-
philic character and poor water solubility, are such that,
when TBTO is introduced into water, repartition will
occur, TBTO leaving the aqueous phase and preferentially
adsorbing onto particles (Hinga et al., 1987). Adsorption
and desorption are dependant on the nature of the sedi-
ment. Little data is available to indicate whether
adsorbed TBT is bioavailable.
If this phenomenon is generally evident, its intensity
varies considerably as a function of the method of study
used and the measurements made. Contradictory results are
apparent in the literature.
Reports from different authors using various con-
ditions have estimated that between 10% and 95% of TBTO
introduced into water is adsorbed onto particles. There
is, however, general agreement that the compound remains
strongly adsorbed. It has been stated that sediments
remain contaminated for at least 10 months; progressive
disappearance of TBTO is not due to desorption but to
degradation.
In an in situ study of Pearl Harbour sediment, the
rate of adsorption of tributyltin derivatives was found to
be 0.57 ng TBT/cm2 per day (Stang & Seligman, 1987).
There was, apparently, no desorption of TBTO itself but
dibutyltin derivatives formed by degradation desorbed with
rates varying between 0.16 and 0.55 ng DBT/cm2 per day.
Variability in results, more evident in field studies
than laboratory studies, is explained by the fact that
adsorption depends on many different factors, amongst
which are the following:
* salinity;
* nature and size of particles in suspension;
* amount of suspended particles;
* temperature;
* presence of dissolved organic matter.
Uncertainties are also evident in relation to the bio-
availability of TBT adsorbed onto sediment. Salazar et al.
(1987) considered that the effects of adsorbed TBTO were
partially masked, i.e. that the compound was unavailable
to organisms. This conclusion could not be verified
regarding effects on filtering or burrowing organisms
living in the sediment.
It is generally agreed that part of the TBTO accumu-
lates in the surface monolayer of natural waters. This
TBTO will also be adsorbed onto organic matter and lipid
material present on the surface.
4.2. Abiotic degradation
A number of studies have shown that a degradation
pathway for tributyltin compounds exists in the environ-
ment, which involves progressive debutylation. It is
theoretically completed with the liberation into water of
the tin oxide (SnO2).
R3SnX -> R2SnX2 -> RSnX3 -> SnX4
A number of studies have looked for evidence of such
degradation, the cause and mechanisms, and an understand-
ing of the kinetics in different environmental conditions
(Chapman & Price, 1972; Brinckman, 1981; Blunden et al.,
1984; Maguire & Tkacz, 1985; and Seligman et al., 1986a).
Degradation of TBTO proceeds via splitting of the
carbon-tin bond, which can result from various mechanisms
occurring simultaneously in the environment. These include
physico-chemical mechanisms (hydrolysis and photodegra-
dation) and biological mechanisms (degradation by microor-
ganisms and metabolism by higher organisms). While degra-
dation definitely occurs as a result of these different
mechanisms in laboratory studies, it is necessary to
assess the relative importance of these different pathways
to degradation of TBTO in the field.
4.2.1. Hydrolytic cleavage of the tin-carbon bond
Since hydrolysis of the tin-carbon bond of organotin
derivatives occurs only under conditions of extreme pH, it
is barely evident under normal environmental conditions.
Studies were carried out in darkness and a sterile
medium to assess the importance of hydrolysis in the
degradation of TBTO. According to the work of Maguire et
al. (1983) and of Maguire & Tkacz (1985), TBTO remains
stable for 11 months in distilled or natural water at
20 °C, in the dark, and in a sterile medium. Under various
conditions of pH, between 2.9 and 10.3, these authors
found no change in TBTO over 63 days. According to
Seligman et al. (1986a), slight degradation of TBTO was
apparent after 94 days in darkness in the presence of
formalin as a sterilizing agent.
It is, therefore, considered that degradation occurs
either not at all or only very slowly in normal environ-
mental conditions of pH and temperature, when monitored in
the dark and in a sterile medium.
4.2.2. Photodegradation
Photodegradation of TBTO by ultraviolet light is
theoretically possible. UV light with a wavelength longer
than 290 nm possesses an energy of 300 kJ/mol, whereas the
energy required to break the carbon-tin bond is 190-220
kJ/mol. At the same time, TBTO absorbs in the UV region at
300 nm and, less strongly, at 350 nm.
Field and laboratory measurements have shown that this
route of degradation can occur and that it forms deriva-
tives of dibutyltin. These seem to be resistant to pho-
tolysis, since very little monobutyltin is formed (Blunden
& Chapman, 1986). While the phenomenon clearly exists, its
importance varies considerably with different environmen-
tal conditions. Conditions of illumination, conditions of
transmission of light, and the presence of photosensi-
tizing substances (acetone, humic acids, etc.) can con-
siderably accelerate the process.
Results of laboratory studies vary considerably de-
pending on whether experiments are conducted under natural
sunlight or UV light of known wavelength. According to
Slesinger & Dresser (1978), the half-life of TBTO in sea
water subjected to ultraviolet light is 18.5 days. In the
presence of a photosensitizing substance, such as acetone,
the half-life is 3.5 days. Seligman et al. (1986a)
suggested that, under natural conditions, photodegradation
is less important than biological action, the development
of phytoplankton leading to a partial degradation of TBTO.
Their measurements were made at relatively high concen-
trations of TBTO (744 µg/litre). Under these conditions,
light caused no degradation over 144 days. According to
Lee et al. (1987), degradation of low concentrations of
TBTO (less than 5 ng/litre) in estuary water is increased
when the assay is conducted in light. The half-life is
between 6 and 12 days, and the presence of significant
concentrations of phytoplankton increases the speed of
degradation. According to Maguire et al. (1983), photoly-
sis under natural light conditions in distilled or natural
water is limited, leading to a TBTO half-life in excess of
89 days. Under experimental conditions of strong UV light,
degradation is apparent. At 300 nm the half-life of TBTO
is 1.1 days, whereas at 350 nm it is more than 18 days.
In these assays, it is possible to demonstrate the role of
humic acids, particularly fulvic acid, which considerably
augment the speed of photolysis. Under such conditions,
the half-life of TBTO falls to 0.6 days at 300 nm and to
6 days at 350 nm. Under natural conditions in the port of
Toronto, Canada, the degradation after 89 days, remained
less than 50%.
4.3. Biodegradation
A number of studies have been conducted to verify that
microorganisms, notably bacteria, are capable of degrading
TBTO. In practice, physico-chemical mechanisms and bio-
logical mechanisms of degradation overlap. Evidence for
biodegradation constitutes an important element in the
assessment of risk. Published studies of observations made
in the field or the laboratory have shown definite evi-
dence of biological degradation of TBTO. Biodegradation
kinetics depend on environmental conditions such as tem-
perature, oxygenation, pH, the level of mineral elements,
the presence of easily biodegradable organic substances,
and the nature of the microflora and the possibility of
their adaptation. Biodegradation also depends on the con-
centration of TBTO being lower than the lethal or inhibi-
tory threshold for the bacteria.
Biodegradation is based on the formation of intermedi-
ate hydroxylated derivatives, progressive oxidative
debutylization following the splitting of the carbon-tin
bond. Dibutyl derivatives are formed, which appear to be
degraded more rapidly than tributyl derivatives to give
monobutyl derivatives; these, conversely, are mineralized
slowly. The end product may be butene. The quantities of
carbon dioxide formed remain small. The biodegradation of
organotin compounds does not seem to involve the formation
of methyl derivatives of tin. Such methyl derivatives have
been measured in some studies (Braman & Tompkins, 1979;
Guard et al., 1981; Hallas et al., 1982; Brinckman et al.,
1983), but have been shown to be the result of the trans-
methylation of inorganic tin by certain marine bacteria
( Pseudomonas ) frequently found in estuaries.
Sheldon (1975) proposed the following scheme for
degradation involving microorganisms:
R3SnX -----> (R3Sn)2O -----> (R3Sn)2CO3
|
| UV or microorganisms
v
(R2SnO)n
|
| UV or microorganisms
v
(RSnO-)n
|
| UV or microorganisms
v
SnO2
A mechanism of biodegradation also exists under
anaerobic conditions (Maguire & Tkacz, 1985). Anaerobic
degradation is considered to be very slow by some workers
and more rapid than aerobic degradation by others.
Slesinger & Dresser (1978) conducted studies in a
Warburg respirometer under aerobic conditions and showed
that microflora derived from activated sludge and soil
were capable of partially degrading TBTO. The half-life
was 70 days, whereas under anaerobic conditions it was
200 days.
Henshaw et al. (1978) showed that pure cultures of
certain wood-degrading fungi, such as Coniophora puteana
and Coriolus polystictus, were capable of slowly biode-
grading TBTO and transforming it to dibutyl and monobutyl
derivatives.
Barug & Vonk (1980) studied the degradation of TBTO in
soil but could show no clear evidence for the action of
microorganisms. Under their experimental conditions, in
sterile or non-sterile medium, the half-life of TBTO
varied between 15 and 20 weeks depending on the soil type.
Barug (1981) was not able to isolate, from sediment or
soils, microorganisms capable of utilizing TBTO as a sole
carbon source. By contrast, in the presence of easily
biodegradable organic matter, biodegradation of TBTO is
apparent with the production of monobutyl derivatives and
smaller quantities of dibutyl derivatives. A number of
species were found to be capable of conducting such degra-
dation aerobically (bacteria: Pseudomonas aeruginosa and
Alcaligenes faecalis ; wood-degrading fungi: Coniophora
puteana, Trametes versicolor, and Chaetomium globosum ).
Under these conditions, they observed 70% degradation in
3 weeks. However, the breakdown of TBTO is not clearly
proved since the authors showed that TBTO accumulates in
the cell walls of bacteria and fungi.
Using water containing natural microflora, Olson &
Brinckman (1986) found no degradation of TBTO at a concen-
tration of 100 µg/litre and a temperature of 5 °C but did
record degradation at 28 °C. Their work also confirmed an
acceleration of degradation when the incubations were con-
ducted under light; the authors explained this acceler-
ation by invoking the role played by photosynthetic micro-
organisms.
Seligman et al. (1986a) also showed evidence for bio-
degradation; in medium polluted by TBTO at 0.5 µg/litre,
the TBTO half-life was 7 days in the dark and 6 days in
the light. In water containing 0.03 µg TBTO/litre, the
half-life was 19 days in the dark and 9 days in the light.
In all cases, dibutyl derivatives were formed and, to a
lesser extent, monobutyl derivatives. In studies with
14C-labelled TBTO, the measurement of 14CO2 production
suggested a half-life of between 50 and 75 days.
Stein & Kuster (1982) demonstrated that TBTO is elim-
inated from waste water passing through sewage treatment
plants by adsorption onto sludge and biodegradation by
sludge organisms, provided that concentrations of TBTO
remain less than 5 mg/litre (see also section 5.3).
According to Maguire et al. (1984), the green alga
Ankistrodesmus falcatus was capable of bioaccumulating
TBTO (with bioconcentration factors of 3 x 104) when it
was cultured in the presence of 20 µg TBTO/litre. When
the cultures were transferred to a non-contaminated
medium, 50% of the TBTO was transformed to dibutyl deriva-
tives or monobutyl derivatives and even to inorganic tin
over the course of 4 weeks. The assays were conducted on
axenic cultures of algae. It may be supposed that a bio-
logical effect was superimposed on physico-chemical degra-
dation mechanisms.
Maguire & Tkacz (1985) have shown that in sediments
there are oligochaetes that are also capable of metab-
olizing TBTO after it has been accumulated. However, the
simultaneous presence of bacteria in the test systems
means that a clear conclusion could not be reached.
According to Maguire et al. (1986), degradation can be
characterized as follows:
* Loss of TBTO by volatilization is very limited with a
half-life of more than 11 months.
* Hydrolysis of TBTO is equally slow with a half-life of
11 months.
* Photodegradation of TBTO plays a more important role
but the half-life of photodegradation is longer than
3 months. This route theoretically takes place but,
under natural conditions of illumination and the poor
penetration of UV light into turbid or coloured water,
it is inefficient.
* Aerobic biodegradation plays a role in water and sedi-
ment. The half-life varies considerably according to
conditions but is in the region of 4 to 5 months.
* Anaerobic degradation plays a role in water and sedi-
ment. The half-life varies considerably but is around
1.5 months.
The kinetics of degradation of dibutyl and monobutyl
tins are less well known. However, the degradation pro-
cesses of TBTO always results in the formation of metab-
olites less toxic than the parent compound.
Hinga et al. (1987) indicated a TBTO half-life of
between 5 and 19 days at 22-24 °C in model ecosystems.
Thain et al. (1987) suggested half-lives of 6 days in
fresh water and 60-90 days at 5 °C in sea water. In water
and sediment of the port of Toronto, the half-life varied
between 4 and 5 months (Maguire & Tkacz, 1985). In estuar-
ine waters of San Diego Bay, USA, the half-life varied
between 7 and 11 days at 12 °C, while in waters of the
Skidaway Estuary, it varied between 5 and 9 days at 28 °C
(Seligman et al., 1986a). Stang & Seligman (1986) using
contaminated sediment from San Diego Bay found that TBT
was degraded to monobutyltin. The degradation kinetic was
lower than in water, the half-life being approximately
162 days. In studies carried out by J.E. Thain & M.J.
Waldock (Personal communication to IPCS, 1989), naturally
contaminated sediments were maintained in the laboratory,
under flow-through conditions, at 12 °C. Degradation of
sediment-bound TBT was found to be a slow process. In
aerobic layers the half-life of TBT was between 4 and 5
months, but in deeper anaerobic layers a half-life value
was not obtained within 500 days.
4.4. Bioaccumulation and elimination
The lipophilic properties of TBTO and its moderately
high octanol-water partition coefficient (log Pow > 3)
contribute to bioaccumulation in living organisms.
Evidence for such mechanisms and an evaluation of
their importance is highly relevant for hazard assessment,
both for the environment and for humans, since some of the
organisms exposed to TBTO are human food items, e.g.,
bivalve molluscs, crustaceans, and fish. Alzieu et al.
(1980) showed that in contaminated areas tin levels in the
flesh of oysters were 100 times higher than concentrations
in the water.
Laboratory experiments have been conducted under dif-
ferent conditions to demonstrate such bioaccumulation, and
have shown that bioconcentration factors vary considerably
between species.
In estuarine bacteria, Blair et al. (1982) found bio-
concentration factors varying between 100 and 30 000 in
species resistant to concentrations of 20 mg TBTO/litre.
As was indicated earlier, such bioconcentration might
result either from adsorption to the surface of the organ-
isms or from true bioaccumulation into the cells. In
phytoplankton, Maguire et al. (1984) reported a bioconcen-
tration factor of 30 000 in the green alga Ankistrodesmus
falcatus exposed for 1 week to concentrations of 20 µg
TBTO/litre. In the diatom Isochrysis galbano, Laughlin et
al. (1986b) reported a bioconcentration factor of 5500.
Studies on the possibility of bioaccumulation and bio-
magnification in molluscs, particularly bivalve molluscs,
are prominent in the literature because of human consump-
tion of oysters and mussels. Alzieu et al. (1982) showed
that TBTO accumulated in oysters, maintained in tanks with
panels of antifouling paint based on TBTO, to levels of
25 mg/kg (dry weight) of tissue and that this resulted in
problems of cavitation of the shell. Waldock et al.
(1983), in studies of the Pacific oyster Crassostrea gigas
exposed for 22 days to TBTO concentrations of 0.15 µg/litre
and 1.25 µg/litre, reported bioconcentration factors of
6000 and 2000, respectively. In European oysters (Ostrea
edulis) exposed to the same concentrations, they found
concentration factors of 1500 and 1000, respectively. In
both cases, after transfer of the oysters to clean water
there was a 50% fall in TBTO levels due to loss or degra-
dation. Laughlin et al. (1986b) reported bioconcentration
factors between 1000 and 7000 for mussels (Mytilus edulis)
exposed for between 3 and 7 weeks to TBTO concentrations
of 23, 45, 63, 141, and 670 ng/litre. For the higher con-
centrations, a plateau in uptake was reached within
2 weeks, but for lower concentrations, no plateau was
reached within the 7-week experiment. The authors con-
sidered that the mussel would be a good indicator organism
for monitoring marine pollution. Cheng & Jensen (1989)
transferred mussels ( Mytilus edulis ) from an unpolluted
area into net bags suspended in a marina in Denmark. They
monitored tin uptake and water concentrations of tin over
a period of 51 days. Accumulation was found to increase
exponentially with time for both total tin and organic
tin. Bioconcentration factors of 5000 to 60 000, much
higher than those from laboratory experiments, were
reported. Transfer of the mussels to the laboratory after
exposure resulted in a half-time for loss of organic and
total tin of 40 and 25 days, respectively. Laughlin et al.
(1986b) showed that bioaccumulation of TBTO by mussels
was not significantly affected by the presence of humic
acids or kaolin but that the presence of mucins secreted
by bacteria did limit bioaccumulation. It was also shown
that bioaccumulation by mussels was greater if the phyto-
plankton used as a food organism (Isochrysis galbana) was
also contaminated with TBT. Contamination via food organ-
isms was more important than via the water.
When feeding crabs with the brine shrimp (Artemia
salina) containing concentrations of TBTO of 6200 µg/kg
wet weight, Evans & Laughlin (1984) found a concentration
factor of 4400. Allen et al. (1980) reported limited bio-
accumulation (< 50) in a 1-week study using freshwater
gastropods (Biomphalaria glabrata). In crustaceans, par-
ticularly the crab Rhithropanopeus harisii, accumulation
of TBTO from a water concentration of 0.28 µg/litre pro-
duced a moderate bioconcentration factor of 60 over 4 days.
Bioaccumulation of TBTO is equally evident in fish.
After exposure of the sheepshead minnow (Cyprinodon
variegatus) for 58 days to concentrations of TBTO varying
between 0.96 and 2.07 µg/litre, Ward et al. (1981)
reported a whole body concentration factor of 2600. After
returning the fish to clean water, loss of TBTO was rapid
over the first 7 days then slower. After 20 days, the
authors reported a loss of 74% from the muscle and 80%
from the viscera. Detection of dibutyltin, monobutyltin,
and inorganic tin suggested possible metabolism. Bressa et
al. (1984) exposed the mullet Liza aurata for 2 months to
concentrations of 5 µg TBTO/litre and reported biocon-
centration factors of 20 to 30 in the liver and kidneys
but no residues in the muscle. After transfer to clean
water, concentrations of tin fell in all organs. Short &
Thrower (1986) studied bioaccumulation in salmon
(Oncorhynchus tshawytscha) exposed for 96 h to concen-
trations of 1.49 µg/litre and obtained concentration
factors of 4300 in the liver, 1300 in the brain, and 200
in muscle. Tsuda et al. (1987) showed that TBTO was
accumulated by carp (Cyprinus carpio) exposed for 14 days
to concentrations varying between 1.8 and 2.4 ng/litre.
Over 10 days they found a plateau in uptake and a concen-
tration factor of 1000; metabolism was evident. Tsuda et
al. (1986) reported concentration factors ranging between
360 and 3400 for round crucian carp (Carassius carassius
grandoculis) tissues exposed to tributyltin chloride for
7 days.
5. ENVIRONMENTAL CONCENTRATIONS
Summary
Levels of TBT in water, sediment, and biota are elevated
within the proximity of marinas, commercial harbours, cooling
systems, and fish nets and cages treated with TBT-based anti-
foulant paints.
TBT levels have been found to reach 1.58 µg/litre in sea
water and estuaries, 7.1 µg/litre in fresh water, 26 300 µg/kg
in coastal sediments, 3700 µg/kg in fresh water sediments,
6.39 mg/kg in bivalves, 1.92 mg/kg in gastropods, and 11 mg/kg
in fish. However, these maximum concentrations of TBT should
not be taken as representative, because a number of factors may
give rise to anomalously high values (e.g., paint particles in
water and sediment samples).
It has been found that measured TBT concentrations in the
surface microlayer of both sea water and fresh water are up to
two orders of magnitude above those measured just below the
surface. However, it should be noted that the recorded levels
of TBT in surface microlayers may be highly affected by the
method of sampling.
Older data may not be comparable to newer data because of
improvements in the analytical methods available for measuring
TBT in water, sediment, and tissue.
5.1. Sea water and marine sediment
The concentrations of TBT in sea water and sediment
are shown in Tables 3 and 5, respectively. Many papers
have reported an association between increased levels of
TBT in water, sediment, and biota and proximity to
pleasure boating activity (especially marinas) and the use
of antifouling paints on fish nets and cages. The degree
of tidal flushing and turbidity of water also influence
TBT concentrations in particular locations.
Table 3. Concentrations of tributyltin in estuarine and sea water
---------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year deptha (µg/litre) Formb limit Reference
(metres) (µg/litre)
---------------------------------------------------------------------------------------
Denmark
Coastal waters 1986 0.1-0.2 <0.04 tin 0.04 Jensen & Cheng (1987)
Marinas 1986 <0.04-1.05 tin 0.04 Jensen & Cheng (1987)
Harbour areas 0.63-2.64 OTo ICES (1987)
Finland
Harbours 1988 0.2 0.02-0.2 TBT 0.01 Yla-Mononen (1988)
France
Bay of Arcachon 1982 0.1-0.3 OT Alzieu & Heral (1984)
1984 0.7-1.2 tin 0.15 Alzieu et al. (1986)
<0.15-0.5 OT 0.1 Alzieu et al. (1986)
1985 0.3-1.0 tin 0.15 Alzieu et al. (1986)
<0.15 OT 0.1 Alzieu et al. (1986)
Anse de Camaret,
Brest 1987 (1) <0.002-0.004 TBT 0.002 Alzieu et al. (1989)
Auray river 1986-
estuary 1987 (1) 0.009-0.069 TBT 0.002 Alzieu et al. (1989)
La Rochelle 1986-
1987 (1) 0.02-0.119 TBT 0.002 Alzieu et al. (1989)
Oleron Island 1986-
1987 (1) 0.039-1.5 TBT 0.002 Alzieu et al. (1989)
Arcachon Bay 1986-
1987 (1) <0.002-0.089 TBT 0.002 Alzieu et al. (1989)
Norway
Oslo fjord <0.01 TBTt 0.01 NIVA (1986)
Sweden
Coastal waters ND-0.04 TBTt Bjorklund (1987b)
United Kingdom
Essex coast 1982 0.1-0.2 <0.03-0.9 TBTt 0.03 Waldock & Miller (1983)
South-west coast 1984 <0.04-0.35 OT 0.04 Cleary & Stebbing (1985)
South-west coast 1986 surface 0.12-5.34 OT 0.04 Cleary & Stebbing (1987)
water
South-west coast 1986 0.5 <0.04-1.44 OT 0.04 Cleary & Stebbing (1987)
South-west coast 1986 bottom <0.04-2.6 OT 0.04 Cleary & Stebbing (1987)
South-west coast 1985 <0.02-0.68 TBT 0.02 Ebdon et al. (1988)
Poole harbour 1986 0.002-0.646 TBTt Langston et al. (1987)
Essex coast 1986 0.1 <0.001-0.831 TBT 0.001 Waldock et al. (1987b)
South coast 1986 0.1 <0.001-1.52 TBT 0.001 Waldock et al. (1987b)
South-west coast 1986 0.1 <0.001-1.27 TBT 0.001 Waldock et al. (1987b)
South Wales coast 1986 0.1 <0.001-0.29 TBT 0.001 Waldock et al. (1987b)
North Wales coast 1986 0.1 <0.001-0.012 TBT 0.001 Waldock et al. (1987b)
---------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year deptha (µg/litre) Formb limit Reference
(metres) (µg/litre)
---------------------------------------------------------------------------------------
USA
Chesapeake Bay 1985 surface ND-1.171 TBT 0.008- Hall et al. (1986)
microlayer 0.01
Chesapeake Bay
(South) 1986 0.15 ND-0.1 TBT 0.001 Huggett et al. (1986)
San Diego Bay 1986 >0.5 0.005-0.235 TBT 0.005 Seligman et al. (1986b)
Californian coast 1986 <0.002-0.6 TBT 0.001- Stallard et al. (1987)
0.002
San Diego Bay 1983-
1985 0.3-0.6 <0.01-0.93 TBT 0.01 Valkirs et al. (1986)
San Diego Bay 1983-
1985 (0.1) <0.01-0.55 TBT 0.01 Valkirs et al. (1986)
USA harbours &
estuaries (0.5) <0.005-0.35 TBT 0.005 Grovhoug et al. (1986)
Coos Bay, Oregon surface 0.007-0.014 TBT Wolniakowski et al.
water (1987)
---------------------------------------------------------------------------------------
a Figures in parentheses indicate distance from water bottom.
b TBT = sample analysed for TBT and expressed as TBT.
TBTt = sample analysed for TBT and expressed as tin.
tin = total tin expressed as tin.
OT = total organic tin expressed as tin.
OTo = total organic tin expressed as TBTO.
Alzieu et al. (1986) monitored tin and organotin con-
centrations in both water and oyster tissue from Arcachon
Bay, France, between 1982 and 1985. They found that levels
in oyster tissue decreased by 5 to 10 times over this
sampling period following French Government restrictions
on the use of TBT in antifouling paints. Alzieu et al.
(1989) monitored TBT water levels at various locations on
the French Atlantic coast in 1986 and 1987 (Table 3), and
found that concentrations generally ranged between < 0.002
and 0.1 µg TBT/litre with the exception of a marina on
Oleron Island, which had levels of up to 1.5 µg/litre.
Levels were highest both in marinas and in the autumn,
presumably when boats were being hosed off ready for the
winter. The authors concluded that levels of TBT had gen-
erally decreased since the restrictions on TBT antifouling
paints, but in certain marinas levels were significantly
higher, suggesting continued use of TBT paints in contra-
vention of restrictions.
Waldock & Miller (1983) measured TBT levels in water
samples collected monthly during 1982 at Burnham-on-Crouch
on the east coast of the United Kingdom. They found a rise
in TBT levels in May, at a time when boats were being
freshly painted with TBT antifouling paints. There was a
second rise in TBT water concentrations in August, at a
time when boats were repainted for the major sailing event
of the year. Analysis of water samples from several areas
on the Essex coast showed that the highest levels (up to
2.25 µg TBTO/litre) were associated with the highest
density of pleasure craft. The authors also reported that
a site used by a large number of boats (on the south
coast of the United Kingdom but situated on an open
coastal site and with less turbid water) had relatively
low TBT levels in the sea water (< 0.08 µg TBTO/litre in
early August).
Waldock et al. (1987b) analysed water samples from
nine sites around the United Kingdom coast during 1986
following restrictions placed on the tin content of anti-
fouling paints containing TBT in January 1986. They
sampled from an enclosed bay, an open coastal site, and
seven estuarine sites. Within these general areas,
locations were found which reflected the incoming water
from a river, an area fished for shellfish, and a harbour
or marina. Half of the 250 samples taken during 1986 were
found to equal or to be above the United Kingdom environ-
mental quality target level (EQT; 20 ng/litre). Levels
were barely above the detection limits at the sites
upstream of boats. Harbours and marinas showed the highest
levels with tidal flushing being an important factor in
determining amounts of TBT detected. A marina in Plymouth,
which has poor flushing, had TBT concentrations consist-
ently greater than 1 µg/litre from May to September,
whereas a marina in the estuary of the River Dart, with
good flushing, had levels of less than 0.2 µg/litre. Six
of the nine sites exceeded the EQT by 3 to 4 times; these
were all sites used regularly by yachts. The other three
sites not used by yachts all showed low but often detect-
able levels with just one sample exceeding the EQT. The
authors also found increased levels of TBT close to areas
where boats were hosed down. Other reports confirmed that
the distribution of TBT in water was associated with the
proximity to intense boating activity (Cleary & Stebbing,
1985; Ebdon et al., 1988). Langston et al. (1987) reported
that sediments, likewise, contained more TBT (up to
520 µg tin/kg) near marinas than at the harbour mouth
(20 µg tin/kg) in Poole harbour, United Kingdom. There
was poor flushing in the harbour and sediment was not dis-
tributed; this was reflected in the water levels, which
were 0.002 to 0.139 µg tin/litre in the general harbour
area and 0.234 to 0.646 µg/litre in the marina.
Cleary & Stebbing (1987) surveyed vertical water pro-
files in south-west England at sites already investigated
two years before. They did not find a systematic decline
in concentrations between the two surveys. The concen-
trations in the surface microlayer were 1.9 to 26.9 times
higher than those at 0.5 m below the surface (see Table 3).
Waldock et al. (1988) analysed water samples collected
in 1987 from commercial harbours and anchorages in the
United Kingdom. Significant concentrations were found;
several samples taken in the immediate vicinity of ships
had levels exceeding 0.05 µg TBT/litre. However, the
highest concentrations were found near to centres of
yachting activity, with over 0.6 µg/litre being found at
one site. The highest concentration found close to commer-
cial vessels in harbours was 0.078 µg/litre, but this was
within 2 m of an oil tanker. A concentration of 0.25 µg per
litre was recorded outside a shipyard where a 3000-tonne
vessel was being hosed down on the foreshore, and a con-
centration of 0.137 µg/litre was measured in surface
water close to a vessel at anchor in the River Fal. In
general, however, few samples taken in close proximity to
commercial ships exceeded 0.02 µg/litre.
Bacci & Gaggi (1989) monitored TBT and its degradation
products in harbours, marinas, and the open sea from the
northern Tyrrhenian Sea, Italy. Concentrations of up to
3.93 µg TBT/litre were measured in the various harbours
and marinas, but no organotin compounds were detected in
samples from the open sea. However, considering the detec-
tion limits of the analytical technique used (0.02 µg per
litre for both TBT and DBT), levels higher than the NOEL
(i.e. 0.01 µg/litre, UNEP, 1989) cannot be excluded.
From these preliminary results, it appears that, under
unfavourable meteorological conditions (e.g., moderate
southerly winds), significant quantities of TBT and
related compounds could contaminate open sea sites for a
few days per year.
The highest levels of TBT around the coasts of the USA
and Denmark were also associated with marinas or harbours
used by small pleasure craft, with TBT levels generally
showing a falling trend from the inner part to the
entrance (Grovhoug et al., 1986; Seligman et al., 1986b;
Jensen & Cheng, 1987). Stallard et al. (1987) analysed
both water and sediment from the Californian coast.
Highest TBT levels, up to 0.6 µg/litre water and 23 µg/kg
sediment, were found near marinas. Levels were lower in
other coastal areas and were lowest out in the open sea.
Valkirs et al. (1986) measured TBT in surface water (at a
depth of 0.3 to 0.6 m) and found that, over the period
1983-1985, TBT levels had increased in San Diego Bay, USA.
Seligman et al. (1989) measured TBT in the waters of
several harbours in the USA. Of the samples collected, 75%
contained TBT levels below the detection limit (< 5 ng per
litre). The highest concentrations were found in yacht
harbours and near to vessel repair facilities, with sig-
nificant levels being found near dry docks. The authors
also found a high degree of variability in TBT concen-
trations depending on the tidal movement, the season, and
intermittent point source discharges.
Hall et al. (1988a) measured TBT biweekly for a 4-
month period (June-September 1986) in the Port Annapolis
marina, Mears marina, Back Creek, and the Severn River
area of northern Chesapeake Bay, USA. Maximum concen-
trations of TBT were reported at both Port Annapolis
marina (1.8 µg tin/litre) and Mears marina (1.17 µg tin
per litre) during early June, followed by significant
reductions during late summer and early autumn. The day of
the week (Thursday-Monday) on which samples were taken
during the daily experiments was not found to signifi-
cantly affect TBT concentrations. Peak concentrations were
found to occur during a rising tide.
Balls (1987) reported that TBT levels in water were
initially (immediately after fish cages were treated with
antifoulants) 1 µg/litre (as tin) within fish cages,
falling to 0.1 µg/litre after 2 weeks and 0.005 µg per
litre after 5 months. Initial concentrations were 0.1 µg
tin/litre at a distance of 20 m from the cages, with
concentrations in the main body of the sea loch being
< 0.028 µg/litre.
5.2. Fresh water and sediment
Analysis for TBT compounds in the Great Lakes, N.
America, has revealed levels often comparable, and in many
cases higher (200 times higher in one sample), than those
measured in estuaries (Maguire et al., 1982; Maguire,
1984; Maguire et al., 1985; Maguire et al., 1986; Maguire
& Tkacz, 1987). Levels of TBT in water were found to be
greater in the surface microlayer than in the subsurface
samples. For example, water samples from Ontario lakes and
rivers showed surface levels of 0.15 to 60.7 µg tin/litre
compared to subsurface levels of between 0.01 and 2.91 µg
per litre (Maguire et al., 1982). TBT was found in the
Great Lakes and in rivers at levels up to those causing
effects on trout in the laboratory; Maguire & Tkacz (1987)
reported a level of 66.8 µg tin/litre in the surface
microlayer. In the United Kingdom, samples of fresh water
from near boatyards contained up to 3.2 µg TBT/litre
(Waldock 1989). In Lake Zurich and Swiss rivers, levels
were found to be much lower, i.e. up to 0.015 µg/litre
(Muller, 1987b). Kalbfus (1988) analysed water samples
from marinas on Lake Constance in 1987 and 1988 and found
that TBT levels rose to a peak in May which corresponded
to the boating activity on the lake. For example, at
Goren, TBT levels rose from 0.13 µg/litre in April to
0.58 µg/litre in May, but by July the levels had fallen
again to 0.028 µg/litre. At the same time TBT levels in
sediment rose from 830 µg/kg in May to 2700 µg/kg in
June and then to 3700 µg/kg in July. Similarly, when
samples were taken on Wannsee in Berlin, levels were found
to be 0.02 µg/litre when there were no boats on the
water, but at Tegel, Berlin, TBT levels were 0.25 µg per
litre when most of the boats were in the water. A coastal
marina at Kiel, on the Baltic, showed levels of 0.35 µg
per litre in April when only half of the moorings were
occupied.
Shiff et al. (1975) monitored water and mud samples
6.5 months after the application of controlled-release
BioMet SRM pellets (rubber formulation containing TBTO) in
Zimbabwe. The pellets were applied at a rate of 20 g/m2
for the control of freshwater snails, intermediate hosts
of the schistosomiasis parasite. Highest levels of organo-
tin were found in the mud immediately under the pellets
(up to 5 mg/kg). Levels in the mud dropped off rapidly
further away from the pellets; at 2 cm organotin levels
were < 0.6 mg/kg. The organotin level in surface water was
< 0.01 mg/litre and in background mud < 0.06 mg/kg.
Table 4. Concentrations of tributyltin in fresh water
------------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year depth (µg/litre) Forma limit Reference
(metres) (µg/litre)
------------------------------------------------------------------------------------------
Canada
Ontario lakes &
rivers 0.01-2.91 TBTt 0.01 Maguire et al. (1982)
Ontario lakes & surface
rivers microlayer 0.15-60.7 TBTt 0.01 Maguire et al. (1982)
St Clair River, surface
Ontario microlayer ND-0.03 TBTt 0.01 Maguire et al. (1985)
Canadian waterways 0.5 <0.01-2.34 TBTt 0.01 Maguire et al. (1986)
Ontario waterways surface 1.9-473 TBTt 1.0 Maguire & Tkacz (1987)
microlayer
Ontario waterways 0.5 <0.01-1.72 TBTt 0.01 Maguire & Tkacz (1987)
Quebec waterways surface 5.5 & 15.2 TBTt 1.0 Maguire & Tkacz (1987)
microlayer
Quebec waterways 0.5 <0.01-0.03 TBTt 0.01 Maguire & Tkacz (1987)
British Columbian
coast up to 0.078 TBT Humphrey & Hope (1987)
Federal Republic of Germany
Lake Constance 1987- up to 0.58 TBT Kalbfus (1988)
marinas 1988
Switzerland
Lake Zurich & 1985 surface 0.007-0.015 TBTc 0.001 Muller (1987b)
rivers water
Harbours 1983-
1984 0.005-1.636 TBT d
Rivers 1983-
1985 0.001-0.016 TBT d
United Kingdom
Wroxham Broad,
Norfolk 1987 up to 0.9b TBT Waldock et al. (1987a)
River Thames 1987 0.064c TBT Waldock et al. (1987a)
River Bure 1986-
1987 0.1 ND-1.54 TBT 0.001 Waldock (1989)
River Yare 1986-
1987 0.1 <0.001-3.26 TBT 0.001 Waldock (1989)
USA
New York State surface 2.0-23.8 TBTt 1.0 Maguire & Tkacz (1987)
waterways microlayer
------------------------------------------------------------------------------------------
a TBT = sample analysed for TBT and expressed as TBT. TBTt = sample analysed for TBT
and expressed as tin. TBTc = sample analysed for TBT and expressed as tributyltin chloride.
b Samples from local boatyards contained up to 1.5 µg/litre.
c Samples from marinas contained up to 1.3 µg/litre.
d Personal communication from M.D. Muller to IPCS.
Table 5. Concentrations of tributyltin in sediment
---------------------------------------------------------------------------------------
Sample Concentrationa Detection
Location Year depth (µg/kg) Formc limit Reference
(metres) (µg/kg)
---------------------------------------------------------------------------------------
Canada
Ontario lakes &
rivers 0.02 30.9-110 TBT 5 Maguire (1984)
Canadian waterways 0.02 <10-10 780 TBTt 10 Maguire et al. (1986)
British Columbian up to
coast 17 000 TBT Humphrey & Hope (1987)
Canada & USA
Detroit & St Clair
rivers 0.02 ND-70 TBTt 5 Maguire et al. (1985)
Netherlands
Eems-Dollard < 25b TBTt 25 TWG (1988c)
Various locations <50-8800 TBTt 50 TWG (1988c)
Switzerland
Lake Zurich 1880-d
1985 120 ND TBTc 0.01 Muller (1987b)
Lake Zurich 1980-
1984 120 280 TBTc 0.01 Muller (1987b)
Lake Zurich &
Boden 1984 2.0-3550 TBT e
United Kingdom
Poole Harbour,
Dorset 1986 20-520 TBTt Langston et al. (1987)
USA
Californian coast 1986 0.1 <2.0-23 TBT 1.0- Stallard et al. (1987)
2.0
San Diego Bay 1983 0.35 <2.0-300 TBT Stang & Seligman (1986)
USA harbours &
estuaries 1.4-178 OT Grovhoug et al. (1986)
Californian coast 15-527 TBT Stephenson et al.
(1987)
Virginian coast 0.02 23-290 TBT Rice et al. (1987)
Great Bay estuary 0.02 12-44 TBTt Weber et al. (1986)
---------------------------------------------------------------------------------------
a Concentrations given as µg/kg dry weight unless stated otherwise.
b Wet weight value.
c TBT = sample analysed for TBT and expressed as TBT.
TBTt = sample analysed for TBT and expressed as tin.
TBTc = sample analysed for TBT and expressed as tributyltin chloride.
OT = total organic tin expressed as tin.
d Museum core from the nineteenth century.
e Personal communication from M.D. Muller to IPCS.
5.3. Sewage treatment
The mono-, di-, and tri-butyltin content of waste
water entering a sewage treatment plant in Switzerland was
measured and its fate was monitored through the various
processes of settlement, digestion, and filtration of the
sewage (Fent, 1989a; Fent et al., 1989). Concentrations of
MBT, DBT, and TBT were 170, 152, and 155 ng/litre,
respectively, in the incoming raw waste water, averaged
over three days of monitoring. About 90% of the organotin
was associated with particulate matter, 10% being in sol-
ution (Table 6). A substantial amount of the incoming
butyltin compounds was lost from the effluent during pri-
mary settlement. The removal of particulate matter at this
stage took 74% of the incoming organotin. In the secondary
effluent, after activated sludge digestion, MBT and DBT
were found at levels similar to those in the primary
effluent; TBT concentrations were reduced to 6 ng/litre
and found only on the particulate matter. In the final
effluent from the plant, after filtration, concentrations
were 4, 3, and 4 ng/litre for MBT, DBT, and TBT, respect-
ively. Thus, 98% of the butyltin was removed from waste
water in the sewage plant. The authors point out that not
all treatment plants have filtration; in these cases only
87% would be removed and effluent concentrations of 9-70
ng/litre found. Levels of butyltin in the sewage sludge
(which is removed from the plant and used as fertiliser on
farm land) were 0.36, 0.38, and 0.34 mg/kg dry weight for
MBT, DBT, and TBT, respectively, in the raw sludge and
0.62, 1.23, and 1.12 mg/kg dry weight in the digested
sludge after 35 days of anaerobic conditions. The authors
point out that 900 kg/year of butyltin could be added to
Swiss soils via sewage sludge. The source of TBT detected
in the sludge was not identified or specified in the
report.
Table 6. Levels of organotin compounds in municipal waste watera
-----------------------------------------------------------------------------------------
MBT DBT TBT
Date water particles % water particles % water particles %
-----------------------------------------------------------------------------------------
23 February 1988 34 216 86 14 113 89 14 178 93
23 February 1988 25 181 88 10 163 94 14 158 92
28 February 1988 28 114 80 11 180 94 27 129 83
Mean 29 170 85 12 152 93 18 155 90
-----------------------------------------------------------------------------------------
a Levels in ng/litre are calculated as ions and corrected for recovery (55-70%); the
percentage of organotins associated with particles is also given. From Fent (1989a).
5.4. Biota
Concentrations of TBT in biota are given in Table 7.
Alzieu (1981) analysed the Pacific oyster (Crassostrea
gigas) for total tin levels following problems in the
French oyster industry in the late 1970s (see section
10.1). He reported that most of the tin accumulated was
in the digestive gland and in the gills. Highest residues
were found in oysters from the Bay of Arcachon (residues
in digestive gland and gill were up to 7.03 and 17.37
mg/kg, respectively), an area with large numbers of small
pleasure boats. Tin levels were stated to be influenced by
tidal flushing; both the Bay of Arcachon and Marennes
Oleron were used by a large number of boats, but residue
levels in oysters collected from the latter site had lower
tin levels (the Bay of Arcachon has poor tidal flushing
compared to Marennes Oleron). Alzieu & Heral (1984)
reported that the greatest accumulation of tin was in
close proximity to a marina. Oysters transferred to the
marina site accumulated a total tin level of 110 mg/kg
(dry weight) within 80 days, whereas oysters maintained as
controls in a local river or in the laboratory accumulated
< 1 mg/kg over the same period. Waldock & Miller (1983)
analysed oysters from the Essex coast, United Kingdom,
and, although both Pacific and European oysters (Ostrea
edulis) contained similar residues of total tin, the
Pacific oyster residues had a higher percentage of TBT.
There are seasonal differences in the levels of TBT
(and DBT) found in mussels (Mytilus edulis) in the field.
It has been suggested that, while these are predominantly
due to changes in boating activity affecting the avail-
ability of TBT to the organisms, physiological differences
in the animals at different times of year may also partly
explain the results. The relative amounts of TBT and DBT
in mussels are thought to reflect the rate of input to the
animal. A high ratio of DBT to TBT residues reflects low
input rates, and vice versa (Page, 1989).
Table 7. Concentrations of tributyltin in biota
---------------------------------------------------------------------------------------------------------
Detection
Concentrationc limit
Organism Year Locationa Organb (mg/kg) Formf (mg/kg) Reference
---------------------------------------------------------------------------------------------------------
Invertebrates
European oyster French coast DG 0.54-7.03 tin Alzieu
(Ostrea edulis) French coast gill <0.5-17.37 tin (1981)
1982 Essex coast, UK DG <0.23-2.05 TBTo 0.075 Waldock &
1982 Essex coast, UK rest <0.4-1.99 TBTo 0.075 Miller
(1983)
Pacific oyster French coast DG <0.5-2.5 tin Alzieu
(Crassostrea gigas) French coast gill <0.5-3.5 tin (1981)
1982 Essex coast, UK DG 4.05-8.64 TBTo 0.075 Waldock &
1982 Essex coast, UK rest 3.5-7.5 TBTo 0.075 Miller (1983)
Coos Bay, USA 0.05-0.189 TBT Wolniakowski
et al. (1987)
Eastern oyster Virginia, USA WB 0.59-1.57 TBT Rice et al. (1987)
(Crassostrea virginica) USA coast WB <0.12-3.9 TBT Wade et al. (1988)
Common mussel 1985- Japan ND-0.28g TBTo 0.05 EAJ (1988)
(Mytilus edulis) 1987 USA coast WB 0.25-3.85 TBT Wade et al.
(1988)
Asiatic mussel 1985- Japan 0.3-0.48g TBTo 0.05 EAJ (1988)
1987
Mussel Californian coast, 0.107-6.39 TBT Stephenson et
USA al. (1987)
Shellfish USA coast 0.23-7.35 OT Grovhoug et al. (1986)
B.C., Canada up to 1.8 TBT Humphrey & Hope (1987)
Netherlands <0.025-0.22 TBTt 0.025 TWG (1988c)
Dogwhelk Fal estuary, UK 0.023-0.786 TBTt Bryan et al. (1987)
(Nucella lapillus) South-west coast, 0.036-0.633 TBTt Gibbs et al.
UK (1987)
Various snail species 1988 Finnish harbours SP 0.04-0.1g TBT 0.01 Yla-Mononen
(1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Detection
Concentrationc limit
Organism Year Locationa Organb (mg/kg) Formf (mg/kg) Reference
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Fish
Herring 1984 Vancouver harbour, WB 0.24 TBTt 0.01 Maguire et
(Culpea harengus) Canada al. (1986)
Finfish B.C., Canada DM up to 11 TBT Humphrey &
Hope (1987)
Salmon species USA MT ND-0.2d TBT Short &
USA 0.28-0.9e TBT Thrower (1986)
Various fish species 1982- Jordan harbour, WB <0.01-0.02g TBTt 0.01 Maguire et
1983 Canada al. (1986)
Various fish species Japan MT ND-0.31d TBTc Hada (1986)
Various fish species 1985- Japan ND-1.7g TBTo 0.05 EAJ (1988)
1987
Various fish species Netherlands <0.025-0.26 tin 0.025 TWG (1988c)
Various fish species 1988 Finnish harbours WB <0.01-0.1g TBT 0.01 Yla-Mononen
(1988)
Birds
Oystercatcher 1986 Exe estuary, UK liver TR-0.08 TBTt 0.02 Osborn &
(Haematopus ostralegus) 1986 Exe estuary, UK MT 0.01-0.19 TBTt 0.02 Leach (1987)
Grey starling 1985- Japan ND (< 0.05)g TBTo 0.05 EAJ (1988)
1987
Black-tailed gull 1985- Japan ND (< 0.05)g TBTo 0.05 EAJ (1988)
1987
Mammals
Seals BL ND TBT 0.01 h
---------------------------------------------------------------------------------------------------------
a UK = United Kingdom; B.C. = British Columbia.
b DG = digestive gland; rest = tissues other than digestive gland; DM = dorsal muscle; MT = muscle
tissue; BL = blubber; WB = whole body; SP = soft parts.
c Concentrations measured as mg/kg dry weight unless stated otherwise; TR = trace; ND = not detectable.
d Fish collected from local fish markets.
e Salmon raised in TBT-treated sea pens.
f TBT = sample analysed for TBT and expressed as TBT; TBTt = sample analysed for TBT and expressed as
tin; TBTc = sample analysed for TBT and expressed as tributyltin chloride; OT = total organic tin
expressed as tin; tin = total tin expressed as tin; TBTo = sample analysed for TBT and expressed
as TBTO.
g Wet weight value.
h Personal communication from M.J. Waldock to IPCS.
Gibbs et al. (1987) found highest levels of TBT
(0.132-0.633 mg tin/kg dry weight) in dogwhelks from the
"enclosed" waters of Plymouth Sound and Torbay, United
Kingdom, whereas levels were less than 0.113 mg tin/kg on
the North Cornish coast. Bryan et al. (1987) reported
residues between 0.374 and 0.786 mg tin/kg (TBT fraction)
for dogwhelks from the Fal estuary, in the south-west of
England, whereas dogwhelks from around the Isle of Mull,
off the Scottish mainland, contained levels of less than
0.03 mg/kg. The Environment Agency of Japan monitored
various fish and shellfish species from different areas of
Japan between 1985 and 1987. The lowest levels of TBTO
(< 0.05 mg/kg wet weight) were found off the open coast
of Japan, higher levels being found in bays and estuaries.
The highest levels reported were in sea bass from the Seto
Inland Sea (up to 1.7 mg/kg). The level of TBTO in the
biota did not change significantly during the sampling
period (EAJ, 1988).
Since 1987 only vessels of > 25 m have been allowed to
use TBT antifouling paints in the United Kingdom (see
section 3.3). Bailey & Davies (1988a) analysed dogwhelk
and scallop from an area around an oil terminal frequented
by large ships at Sullom Voe, Shetland. Elevated tin
levels were found in both dogwhelk (up to 0.16 mg tin/kg
wet weight) and scallops (up to 0.23 mg/kg in gonadal
tissue) within Sullom Voe (especially in areas close to
the oil terminal) compared to those collected from the
surrounding area (< 0.03 mg/kg).
Increased levels of TBT in biota have been found
associated with fish nets and cages. Davies et al. (1987b)
found that residues of total tin in dogwhelks were higher
near fish cages in Loch Laxford (< 0.01-0.33 mg/kg), a sea
loch in Scotland, and in the harbour areas of Loch Crinan
(< 0.01-0.17 mg/kg) than outside the sea lochs (< 0.02 mg/kg).
Short & Thrower (1986) found TBT residues of between
0.28 and 0.9 mg tin/kg in salmon (Oncorhynchus
tshawytscha) maintained in TBT-treated sea pens for 3 to
19 months. The authors also monitored salmon for sale in
American fish markets and found TBT residues of up to 0.2
mg/kg. They also found that cooking does not effectively
destroy or remove TBT from salmon tissues.
6. KINETICS AND METABOLISM
Summary
Tributyltin is absorbed from the gut (20-50% depending on
the vehicle) and via the skin of mammals (about 10%), and can
be transferred across the blood-brain barrier and from the pla-
centa to the fetus. Absorbed material is rapidly and widely
distributed amongst tissues (principally liver and kidney).
Metabolism in mammals is rapid; metabolites are detectable
in blood within 3 h of TBT administration. TBT is a substrate
for mixed-function oxidases in vitro, but these enzymes are
inhibited by TBT in vitro at very high concentrations. Rate of
loss differs with different tissues and estimates for biologi-
cal half-lives in mammals range from 23 to about 30 days.
Metabolism occurs in lower organisms but is slower, par-
ticularly in molluscs. The capacity for bioaccumulation is,
therefore, much greater than in mammals.
TBT compounds inhibit oxidative phosphorylation and alter
mitochondrial structure and function. TBT interferes with the
calcification of the shell of oysters (Crassostrea species).
6.1. Metabolism of TBT in mammals
A number of workers have studied the absorption,
metabolism, and elimination of organotin derivatives in
various animals species, especially in mammals. Some
studies were conducted in vivo and others in vitro using
isolated liver microsomes.
The behaviour of organotin compounds depends partly on
their chemical structure and partly on speciation. How-
ever, the following statements generally apply:
* The distribution of TBT in organisms is usually rapid.
In a number of species (rat, mouse, rabbit, guinea-
pig), it is found preferentially in the liver and
kidney and, to a lesser extent, in the spleen, fat,
lungs, brain, and muscle.
* Excretion is via the bile rather than the urine.
* In tissues, particularly the liver, there is a process
of biotransformation characterized by progressive
de-alkylation leading to breakdown to inorganic tin
(Cremer, 1957; Bridges et al., 1967).
In an in vivo study, Brown et al. (1977) administered
113Sn-labelled TBTO to mice by ip injection. They re-
ported an initial rapid elimination, followed by a slower
phase, in the faeces. Part of the radiolabel was retained
in the tissues but turn-over occurred, with a biological
half-life for elimination of 23 to 29 days.
Evans et al. (1979), under similar conditions, admin-
istered 14C-labelled TBTO to mice in the drinking-water,
at low doses continuously for up to 30 days. There was
absorption from the intestine and accumulation in the
liver, spleen, kidney, and fat (and to a lesser degree in
muscle, lung, brain, and blood). In a second study, mice
were similarly dosed for 31 days. On cessation of dosing
with 14C-labelled TBTO, examination of the animals for a
further 15 days demonstrated loss of TBTO retained in
these tissues; the loss reached 97% in liver, 73% in
kidney, and 30% in fat, and the TBTO had disappeared
completely from the blood. Studies in metabolism cages
indicated that the principal route of loss was via the
faeces; limited amounts of labelled CO2 were exhaled.
Iwai et al. (1980) studied the distribution and
accumulation of tributyltin and its metabolites in areas
of the brain of rabbits. After a single oral dose of TBT
chloride, high concentrations of tributyltin were found in
the frontal and temporal lobes and in the cerebellum
initially. Thereafter, there was a rapid decrease in TBT
residues and an increase in levels of monobutyltin, which
persisted for much longer. Persistence occurred preferen-
tially in the grey matter rather than the white matter.
The authors' interpretation was that TBT, which passes
readily through the blood-brain barrier, is mainly de-
alkylated in the grey matter and that the metabolic
product remains there.
Humpel et al. (1986) administered 113Sn-labelled TBTO
orally to rats and found that the absorption varied
between 20% and 55% depending on the vehicle used. High
residues of tin were found in the liver and kidney (1 to 3
days after dosing) of which only approximately 5% was
unchanged TBT. Other tissues showed lower concentrations
of the label but the fraction of unchanged TBT was higher.
The exact nature of the metabolites could not be ident-
ified by the analytical method used (HPLC), but the
pattern was indicative of progressive debutylation. Daily
administration of TBTO for 14 days resulted in steadily
increasing concentrations of label in all tissues. Steady-
state levels were estimated to be reached after 3 to 4
weeks. When Snoeij et al. (1987) administered 14C-
labelled TBT acetate as a single oral dose to rats, about
20% absorption occurred. The presence of DBT and MBT in
plasma (after TLC separation) was demonstrated 3 h and
27 h after dosing.
TBT may cross the placenta to some extent, as was
shown by the presence of label in rat fetuses after a
single oral dose to the mother at day 18 of pregnancy. The
concentration in fetal tissue was comparable to that of
the mother's muscle tissue (Humpel et al., 1986).
After administration of neat 113Sn-labelled TBTO to
the intact skin of baboons for 7 h, 10 to 15% was esti-
mated to reach the systemic circulation (Humpel et al.,
1986).
Metabolism of tributyltin derivatives has been clearly
demonstrated in in vitro studies. Casida et al. (1971) and
Fish et al. (1975, 1976) studied the possible metabolism
of TBT acetate using rat hepatic microsomes in the pres-
ence of NADPH. They demonstrated hydroxylation by monooxy-
genases of the principal carbon-hydrogen bonds ( alpha and
beta to the tin atom) of 24% (at the alpha position) and
50% (at the beta position). The hydroxylated alpha metab-
olite is unstable and rapidly splits to form the dibutyl
derivative, followed by 1-butanol and then butane. Accord-
ing to Kimmel et al. (1977), the same type of reaction
occurs in microsome preparations from mice.
Uhl (1986) dissolved TBTO (9.88 or 5.54 mg) in a mix-
ture of 3 ml cherry brandy and 7 ml ethanol and gave it
orally to a volunteer. TBTO and its degradation products
were determined in urine by gas chromatography after reac-
tion with methyl magnesium bromide. Only 5.1% to 5.4% of
the dose was found in the urine, mainly as dibutyltin
metabolites. Butyltin levels in the urine decreased
rapidly during the first days after administration. After
dermal application of 20 µl (23.4 mg) of undiluted TBTO
on the arm of a volunteer, approximately 0.2% of the dose
was excreted in the urine, of which about 20% was found to
be tributyltin.
6.2. Metabolism of TBTO in other organisms
Lee (1985, 1986) examined the capacity of organisms
from various aquatic trophic levels to metabolize TBTO.
He used the blue crab (Callinectes sapidus), the brown
shrimp (Penaeus aztecus), a fish (the spot, Leiostomus
xanthurus), and the Eastern oyster (Crassostrea
virginica). The organisms were exposed to 14C-labelled
TBTO via the water (6 µg/litre for the crab and shrimp;
2 µg/litre for the fish and the oyster) and via food
(shrimp containing about 20 mg/kg) in the case of the crab
and fish. In all test species he reported a rapid uptake
of 14C-labelled TBTO into various organs. In crabs and
shrimps, he observed, after 3 days, the appearance of
various metabolites in the hepatopancreas (dibutyl, mono-
butyl, and polar derivatives). In the fish, the same was
seen in the liver. In oysters, the process was much slower
and metabolites appear only at low concentrations after 4
days. In vitro studies, conducted with liver microsomes
from fish and stomach microsomes from crabs, confirmed the
presence of a route of metabolism comparable to that in
mammals. Within microsomes, a cytochrome-P-450-dependent
oxygenase acts in the presence of NADPH and oxygen to
allow progressive degradation of TBTO. Such biochemical
mechanisms are apparent in a number of species. However,
their activity is limited in molluscs, particularly in
bivalves; thus the capacity of molluscs to metabolize
xenobiotics is generally weak. Tsuda et al. (1988) fol-
lowed the metabolism of tributyltin oxide in various
tissues of the carp Cyrpinus carpio over 14 days. In
muscle, there was little evidence of metabolites and
almost all of the tin present was in the form of tributyl-
tin. In the kidney, liver, and gall bladder, large amounts
of monobutyltin were evident. Little dibutyltin was
present in any of the tissues, suggesting that further
metabolism of the intermediate to the monobutyl form was
rapid. Ebdon et al. (1989) could not positively determine
whether dibutyltin and monobutyltin present in adult and
seed oysters in British estuaries derived from intake of
the metabolites or from metabolism within the oysters.
However, they observed that peak seasonal levels of the
metabolites occurred approximately 1 month after peaks of
tributyltin. They concluded that metabolism within the
oysters was responsible for the DBT and MBT present. This
also suggests that metabolism in oysters is slow.
6.3. General mechanisms of toxicity of TBTO
Different mechanisms of action have been advanced to
explain the biological effects and toxicity of TBTO. Some
of the mechanisms are present in all living organisms,
others only in certain species.
6.3.1. General toxic mechanisms
Several studies (Aldridge, 1958; Aldridge & Street,
1964, 1970) have demonstrated that the trialkyl deriva-
tives of tin, and notably tributyltin compounds, are
inhibitors of oxidative phosphorylation in mitochondria
and are, therefore, responsible for inhibiting energy
transfer. This inhibition results from various phenomena:
* disturbance of synthesis of ATP;
* action on mitochondrial membranes causing swelling and
rupture;
* alteration in ion transport across lipid membranes.
Rosenberg et al. (1980, 1981, 1984) and Rosenberg &
Drummond (1983) showed TBTO inhibition of cytochrome P-450
activity in cells from various tissues (liver, kidney,
small intestine mucosa) after dosing in vitro or in vivo.
Evans et al. (1979) demonstrated inhibition of oxidative
phosphorylation due to formation of complexes between tri-
alkyltin derivatives and proteins or certain alpha or beta
amino acids. They most notably form chemical links with
nitrogen and sulfur atoms in protein chains (see chapter 2).
6.3.2. Toxic mechanisms in bivalve molluscs
In bivalve molluscs, notably in oysters, one sublethal
effect of TBTO involves abnormal calcification. This is
shown particularly in Crassostrea gigas, the Pacific or
Eastern oyster, in areas contaminated with TBTO. The
effect is reproducible in experiments where healthy oys-
ters are transferred to contaminated areas, and also
reversible in transfers from contaminated to clean areas.
The abnormal calcification leads to distortion of the
shells; layers are formed successively of calcium carbon-
ate, flaking and open space (Alzieu et al., 1982), and
result partly from interference with synthesis of the
organic matter (gel) (which allows calcium deposition) and
partly from interference with crystallization of calcium
carbonate. Krampitz et al. (1976, 1983) showed that the
protein constituents of the interlamellar gel assisting
deposition of calcium were deficient in the amino acids
necessary for calcium fixation (serine, alanine, glycine,
glutamic acid, aspartic acid); these amino acids are com-
plexed by TBTO.
The work on mammals showing effects of TBTO on oxidat-
ive phosphorylation (Aldridge & Street, 1971) suggests
another possible effect, since ATP plays an important role
in the crystallization of calcium carbonate, as shown in
the following schematic diagram.
Fresh-water angiosperms were killed by a TBTO concen-
tration of 0.5 mg/litre, and growth was inhibited at
0.06 mg/litre or more.
Data on fresh-water invertebrate species are few, re-
lating to just three species other than target organisms.
Different salts of TBT yield 48-h LC50 values for Daphnia
of 2.3-70 µg/litre and for Tubifex of 5.5-33 µg/litre.
The NOEL for Daphnia has been estimated to be 0.5 µg per
litre, based on reversal of normal response to light. The
24-h LC50 for the Asiatic clam has been reported to be
2100 µg/litre, and for target snail adults in schistoso-
miasis control the corresponding values are 30-400 µg/litre.
Tributyltin has been shown to be toxic to schistosome
larvae in the aquatic stages; the LC50 (TBT fluoride)
was calculated to be 16.8 µg/litre for a 1-h exposure.
The TBT dose causing 99% to 100% suppression of cercarial
infectivity of mice was between 2 and 6 µg/litre.
The sensitivity of snails to TBT decreases with age,
but eggs are more resistant than both young and adults.
Egg laying is significantly effected at a TBTO concen-
tration of 0.001 µg/litre.
The acute toxicity of TBT to freshwater fish in LC50
tests up to 168 h ranges from 13 to 240 µg per litre.
The NOEL for the guppy was estimated to be 0.01 µg per
litre, based on histopathological effects.
No effect on survival was found when eggs and larvae
of the frog Rana temporaria were exposed to TBT concen-
trations of 3 µg/litre or less, but at 30 µg/litre sig-
nificant mortality was observed.
1.9.3. Microcosm studies
Microcosm studies modelling marine ecosystems have
been conducted with introduced organisms and in conditions
where inflowing sea water allowed colonization by other
organisms. Results showed decreases in both numbers of
individuals and in species diversity at TBTO concen-
trations in water between 0.06 and 3 µg/litre.
Results from freshwater model ecosystems suggest that
doses which kill freshwater snails also affect other
species, including fish.
1.10. Effects on terrestrial organisms
The exposure of terrestrial organisms to TBT results
primarily from its use as a wood preservative. TBTO is
toxic to bees housed in hives made from TBT-treated wood.
TBT was toxic to bats in a single study, but this result
was not statistically significant owing to high control
mortality. TBT compounds are toxic to insects exposed
topically or via feeding on treated wood. The acute tox-
icity of TBT to wild mice is moderate; estimated dietary
LC50 values, based on consumption of treated seeds used
in repellency tests, range from 37 to 240 mg/kg per day.
1.11. Effects on organisms in the field
Field observations have related high concentrations of
tributyltin to mortality and settlement failure of larval
bivalves, reduced growth, shell thickening and other mal-
formations in developing oysters, imposex in mud snails,
and imposex (concurrent with population decline) in the
dogwhelk. Complete failure of oyster fisheries was ident-
ified initially in France and afterwards in other
countries and related to water concentrations of TBT. The
effects were most marked in areas close to pleasure boat
marinas. Controlling the use of TBT antifouling paints on
small boats has resulted in recovery of oyster repro-
duction and growth. However, water concentrations of TBT
are still high enough in some areas to affect marine
gastropods.
Both shell growth and chambering in Pacific oysters
and imposex in dogwhelks have been used as biological
indicators of TBT contamination.
There have been few studies of the effects on organ-
isms of TBT in sediment, but there are indications that
the TBT is available to burrowing organisms and can cause
mortality in the field.
Gross toxic effects and histopathological changes have
been reported in farmed marine fish exposed to TBT by the
use of antifouling paints on retaining nets.
The use of TBT as a molluscicide against the fresh-
water snails that carry schistosomiasis (bilharzia) has
been proposed. Some field trials have been conducted which
show that it is difficult to apply TBT without damaging
non-target organisms.
1.12. Toxicity to laboratory mammals
1.12.1. Acute toxicity
Tributyltin is moderately to highly toxic to labora-
tory mammals, acute oral LD50 values ranging from 94 to
234 mg/kg body weight for the rat and from 44 to 230 mg/kg
body weight for the mouse. The acute toxicity to the
guinea-pig and the rabbit fall within the same range. The
variation comes from the "anion" component of the tri-
butyltin salt. These compounds exhibit greater lethal
potential when administered parenterally, as opposed to
orally, probably due to only partial absorption from the
gut.
Other effects of acute exposure may include alter-
ations in blood lipid levels, the endocrine system, liver,
and spleen, and transient deficits in brain development.
The toxicological significance of these effects, reported
after high single doses of the compound, is questionable
and the cause of death remains unknown.
The acute toxicity via the dermal route is low, the
LD50 being >9000 mg/kg body weight for the rabbit.
"Nose only" inhalation LD50 (4 h) for the rat is
77 mg/m3 (65 mg/m3 when only inhalable particles are
considered). TBT vapour/air mixtures produce no observ-
able toxic effects, even at saturation. However, TBT is
very hazardous as an inhaled aerosol, producing lung irri-
tation and oedema.
TBT is severely irritating to the skin and an extreme
irritant to the eye. TBTO is not a skin sensitizer.
1.12.2. Short-term toxicity
TBT compounds have been studied most extensively in
the rat (all the data in this section refer to the rat
unless otherwise indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg
body weight), high mortality rates were observed when the
exposure time exceeded 4 weeks. No deaths were noted at
100 mg/kg diet (10 mg/kg body weight) or after daily
administration of 12 mg/kg body weight by gavage. In rats
dosed during early post-natal life, 3 mg/kg body weight
resulted in increased deaths. The main symptoms at lethal
doses were loss of appetite, weakness, and emaciation.
Borderline effects on rat growth were observed at
50 mg/kg diet (6 mg/kg body weight) and 6 mg/kg body
weight (gavage studies). Mice are less sensitive, effects
being observed at 150 to 200 mg/kg diet (22 to 29 mg/kg
body weight).
Structural effects on endocrine organs, mainly the
pituitary and thyroid, have been noted in both short- and
long-term studies. Changes in circulating hormone concen-
trations and altered response to physiological stimuli
(pituitary trophic hormones) were observed in short-term
tests, but after long-term exposure most of these changes
appeared to be absent. The mechanism of action is not
known.
Exposure to TBTO aerosol at 2.8 mg/m3 produced high
mortality, respiratory distress, inflammatory reaction
within the respiratory tract and histopathological changes
of lymphatic organs. However, exposure to the highest
attainable vapour concentration (0.16 mg/m3) at room
temperature produced no effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular
necrosis and inflammatory changes in the bile duct were
observed in rats fed TBTO at a dietary level of 320 mg/kg
(approximately 25 mg/kg body weight) for 4 weeks and in
mice fed 80 mg/kg diet (approximately 12 mg/kg body
weight) for 90 days. Vacuolization of periportal hepato-
cytes was noted in dogs fed a dose of 10 mg/kg body weight
for 8 to 9 weeks. These changes were occasionally
accompanied by increased liver weight and increased serum
activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte
volume in rats, resulting from dosing with 80 mg/kg diet
(8 mg/kg body weight), indicate an effect on haemoglobin
synthesis, leading to microcytic hypochromic anaemia. The
decrease in splenic haemosiderin levels suggests alter-
ations in iron status. Anaemia has also been observed in
mice.
The formation of erythrocyte rosettes in mesenteric
lymph nodes has been observed in certain short-term inves-
tigations but not in long-term studies. The biological
significance of this finding (possibly transient) is
unclear.
The characteristic toxic effect of TBTO is on the
immune system; due to effects on the thymus, the cell-
mediated function is impaired. The mechanism of action is
unknown, but may involve the metabolic conversion to
dibutyltin compounds. Non-specific resistance is also
affected.
General effects on the immune system (e.g., on the
weight and morphology of lymphoid tissues, peripheral lym-
phocyte counts, and total serum immunoglobulin concen-
trations) have been reported in several different studies
with TBTO using rats and dogs, but not mice, at overtly
toxic dose levels (effects in mice have been seen with
tributyltin chloride at 150 mg/kg). Only the rat exhibits
general effects on the immune system without other overt
signs of toxicity and is clearly the most sensitive
species. The NOEL in short-term rat studies was 5 mg/kg
diet (0.6 mg/kg body weight). In studies with tributyltin
chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been
shown to compromise specific immune function in rat in
vivo host resistance studies. Decreased clearance of
Listeria monocytogenes was seen after exposure to a diet-
ary level of 50 mg/kg (the NOEL being 5 mg/kg per day),
and decreased resistance to Trichinella spiralis was seen
at 50 and 5 mg/kg diet, but not at 0.5 mg/kg diet (2.5,
0.25, and 0.025 mg/kg per day body weight, respectively).
Similar effects were seen in aged animals, but these were
less pronounced.
With present knowledge, the effects on host resistance
are probably of most relevance in assessing the potential
hazard to man, but there is insufficient experience in
these test systems to fully assess their significance.
However, some data on the significance of the T. spiralis
model are provided by findings in athymic nude rats after
the standard challenge. In these studies, the complete
absence of thymus-dependent immunity resulted in a 10- to
20-fold increase in muscle larvae counts; by contrast,
exposure to TBTO concentrations of 5 and 50 mg/kg diet
resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on
the effects of tributyltin compounds on the developing
immune system, there is no information on host resistance.
It would be prudent to base assessment of the poten-
tial hazard to humans on data from the most sensitive
species. Effects on host resistance to T. spiralis have
been seen at dietary levels as low as 5 mg/kg (equivalent
to 0.25 mg/kg per day body weight), the NOEL being
0.5 mg/kg (equivalent to 0.025 mg/kg per day). However,
the interpretation of the significance of these data for
human risk assessment is controversial. In all other
studies a concentration of 5 mg/kg per day in the diet
(equivalent to 0.5 mg/kg body weight, based on the short-
term studies) was the NOEL with respect to general, as
well as specific, effects on the immune system.
1.12.3. Long-term toxicity
A long-term study in rats indicates a marginal effect
of TBT on general toxicological parameters (of limited
toxicological significance) at a level of 5 mg/kg diet
(0.25 mg/kg body weight).
1.12.4. Genotoxicity
The genotoxicity of TBTO has been the subject of
extensive investigations. Negative results were obtained
in the vast majority of studies, and there is no convinc-
ing evidence that TBTO has any mutagenic potential.
1.12.5. Reproductive toxicity
The potential embryotoxicity of TBTO has been evalu-
ated in three mammalian species (mouse, rat, and rabbit)
after oral dosing of the mother. The main malformation
noted in rat and mouse fetuses was cleft palate, but this
occurred at dosages overtly toxic to the mothers. These
results are not considered to be indicative of teratogenic
effects of TBTO at doses below those producing maternal
toxicity. The lowest NOEL, with regard to embryotoxicity
and fetotoxicity for all three species, was 1.0 mg/kg body
weight.
1.12.6. Carcinogenicity
One carcinogenicity study has been carried out on
rats, in which neoplastic changes were observed in endo-
crine organs at 50 mg/kg diet. The pituitary tumours
reported at 0.5 mg/kg diet were considered as having no
biological significance since there was no dose-response
relationship. These tumour types usually appear in high
and variable background incidences, and their significance
is, therefore, questionable. A carcinogenicity study on
mice is in progress.
1.13. Effects on humans
Occupational exposure of workers to tributyltin has
been found to result in irritation of the upper respir-
atory tract. TBT as an aerosol poses a hazard to humans.
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The
potential problem is made worse by the lack of an
immediate response to the skin.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of tributyltin compounds
Tributyltins compounds are organic derivatives of tin
(SnIV) characterized by the presence of covalent bonds
between three carbon atoms and a tin atom. They conform to
the following general formula ( n-C4H9)3 Sn-X, where X is
an anion or a group linked covalently through a hetero-
atom.
The nature of X influences the physico-chemical
properties, notably the relative solubility in water and
non-polar solvents and the vapour pressure.
These compounds differ from inorganic tin both in
behaviour and effects. An important member of the group is
tributyltin oxide (TBTO; RTECS number, JN8750000). Commer-
cial TBTO has a purity generally above 96%. Principle
impurities are dibutyltin derivatives and, to a lesser
extent, tetrabutyl or dibutylalkyl tin compounds.
Other industrially important tributyltin derivatives
include tributyltin fluoride, tributyltin methacrylate (monomer
or copolymer), tributyltin benzoate, tributyltin linoleate,
tributyltin naphthenate, and tributyltin phosphate.
2.2. Physical and chemical properties
TBTO is flammable but does not form explosive mixtures
with air. It is a mild oxidizing agent. It reacts quanti-
tatively at room temperature with bromide or iodine with
cleavage of the Sn-O bond (a reaction that may be used for
quantitative analysis) (Bahr & Pawlenko, 1978).
In the presence of oxygen, light or heat, slow break-
down occurs with the formation of tetra-n-butyltin, di-
n-butyltin oxide, and eventually tin (IV) oxide by de-
alkylation (Evans & Karpel, 1985). This degradation may be
inhibited by the addition of 0.1-1.0% of stabilizers (such
as lactic or citric acids).
It has been suggested (Maguire et al., 1984; Laughlin
et al., 1986a) that TBTO in aqueous solution dissociates
with the formation of a hydrated tributyltin cation, which
can undergo reaction with anions present. Data are not
available on the equilibrium constants for these reac-
tions.
Laughlin et al. (1986a) showed that TBTO can react
with normal constituents of the sea water in the following
ways:
Bu3-Sn-O-Sn-Bu3+ HO -» 2Bu3-Sn-OH
Bu3-Sn-OH-H+ -» Bu3SnOH2+
Bu3-Sn-OH + CO32- -» Bu3SnCO3- + OH-
Bu3-Sn-OH2+ + Cl- -» Bu3-Sn-Cl + H2O
The predominant forms are Bu3SnOH2+ and Bu3SnCl
at pH < 7, Bu3SnCl, Bu3SnOH, and Bu3SnCO3- at pH 8,
and Bu3SnOH and Bu3SnCO3- at pH > 10.
Under normal conditions in sea water, it is considered
that the three species (hydroxide, chloride, and carbon-
ate) remain in equilibrium.
The physical and chemical properties of some commer-
cially available tributyltin compounds are listed in Table
1.
Varying data on the solubility of TBTO in water, which
ranges from < 1.0 to > 100 mg/litre at different tempera-
tures and pH values, may be related to the presence of
different anionic species as described above.
In the same way as described in the reaction between
TBTO and water, the TBT group can be transferred to other
oxygen-, nitrogen-, and sulfur-containing groups. Thus,
anaerobically in sediments, TBTO can be transformed to TBT
sulfide. With amino acids, or their derivatives such as
proteins, reaction can occur on the nitrogen and sulfur
atoms, and, with wood, it has been suggested that the TBT
group may react with hydroxylic groups (Blunden et al.,
1984) or form tributyltin carbonate (Smith et al., 1977).
Thus adsorption on to particulate matter could involve
chemical reaction as well as physical adsorption or sol-
ution. TBTO adsorbs strongly to particulate matter, the
reported adsorption coefficients ranging between 110 and
55 000.
Table 1. Identity and physical and chemical properties of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Oxide Benzoate Chloride Fluoride Linoleate Methacrylate Naphthenate
(TBTO) (TBTB) (TBTCl) (TBTF) (TBTL) (TBTM) (TBTN)
---------------------------------------------------------------------------------------------------------
IUPAC name distannoxane, stannane, stannane, stannane, stannane, stannane, stannane,
hexabutyl (benzyloxy) tributyl- tributyl- tributyl- tributyl- tributyl-
tributyl chloro fluoro (1-oxo-9,12- (2-methyl-1- mono (naph-
octadecadi- oxo-2-propyl) thenoyloxy)
enyl)oxy- oxy- derivatives
CAS name Bis(tributyl- Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin
tin) oxide benzoate chloride fluoride linoleate methacrylate naphthenate
CAS number 56-35-9 4342-36-3 1461-22-9 1983-10-4 24124-25-2 2155-70-6 85409-17-2
Molecular C24H54OSn2 C19H32O2Sn C12H27ClSn C12H27FSn C30H58O2Sn C16H32O2Sn
formula
Relative 596 411 325 309 568.7 374.7 ca.500
molecular
mass
Boiling 173 ca.135 140 > 350 ca.140 > 300 ca.125
point (°C) (130 Pa) (30 Pa) (1300 Pa) (extrapol) (50 Pa) (extrapol) (50 Pa)
Melting < -45 20 -16 240 < 0 16 < 0
point (°C)
Relative
density 1.17-1.18 ca.1.2 ca.1.2 1.25 1.05 1.14 ca.1.1
(20 °C)
Vapour
pressure (Pa 1 x 10-3 2 x 10-4 9 x 10-2 3 x 10-2 9 x 10-5
at 20 °C)
Refractive 1.4880-
index (20 °C) 1.4895
---------------------------------------------------------------------------------------------------------
TBTO is soluble in lipids and very soluble in a number
of organic solvents (ethanol, ether, halogenated hydro-
carbons, etc.).
The octanol/water partition coefficient (log Pow)
lies between 3.19 and 3.84 for distilled water and is 3.54
for sea water.
As shown in Table 1, the vapour pressures of TBT
compounds are low. The work of Maguire et al. (1983)
confirmed this directly by showing no loss of TBTO from a
1 mg/litre solution after 62 days; 20% of the water was
lost by evaporation.
2.3. Analytical methods
The control levels of contamination of different
environmental compartments (water, sediment, biota) and
the interpretation of laboratory experimental and field
study results regarding levels, fate, biodegradation, and
bioaccumulation of tributyltin compounds require sensitive
analytical techniques to allow identification and quanti-
fication.
2.3.1. Measurement of organotin compounds
These methods, which are summarized in Table 2, have
been applied initially to water and later to sediment and
biota. They must be sufficiently sensitive and specific to
allow monitoring of ng/litre levels, and they need to be
able to distinguish between different forms of organic tin
derivatives present in the environment, i.e. mono-, di-,
tri-, or tetra-butyltins and different species of alkyl
moieties (butyl, methyl). They have also to avoid all
interference from other metals and other organometallic
derivatives.
Generally there are four successive stages to analy-
sis, although some are optional:
* extraction;
* formation of volatile derivatives;
* separation of these derivatives;
* detection, identification, and quantification.
Table 2. Sampling, preparation, and analysis of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Medium Sampling method Sample volume Analytical method Detection limit Reference
---------------------------------------------------------------------------------------------------------
Air adsorption on 50-100 litres derivatization with Zimmerli &
Chromosorb, RMgX; GC/MS or Zimmermann
cation exchange GC/FPD (1980);
resin, or Tenax Muller (1987a)
Water 250 ml NaBH4 conversion to 0.1-2 ng/litre Hodge et al.
hydride; separation by (1979);
fractional distillation; Michel (1987);
AA Donard et al.
(1986); Braman &
Tompkins (1979);
Valkirs et al.
(1986); Weber
et al. (1986)
Water and extraction with 8 litres (water) derivatization with 1 ng/litre Maguire &
sediments dichloromethane or 1 g (sediment C5H11 MgBr; GC-FPD (water) Huneault (1981);
dry weight) or GC-FAA or 5 ng/mg Maguire &
(sediment Tkacz (1983,
dry weight) 1985); Maguire
et al. (1986)
Water and acidification, 1 litre derivatization with 10 ng/litre Meinema et al.
biota extraction with CH3 Mgl; GC-MS or AA (1978); Bjorklund
dichloromethane (1987a)
Water, 200 ml or NaBH4 conversion to 5 ng/litre or Matthias et
biota, or 16 litres hydride; extraction with 0.2 ng/litre al. (1986a,b);
sediments dichloromethane Humphrey &
Hope (1987)
Water and adsorption on 60 litres extraction with dichloro- 0.07 ng/litre Humphrey &
sediment silica (water) or 10 g methane/tropolone; deriva- (water) Hope (1987)
bonded C18 (sediment) tization with C5H7 MgBr; 0.2 mg/kg
GC-MS (sediment)
macroreticular 1 litre extraction with n-pentane < 1 ng/litre Muller
resin (water) diethylether (water) (1984)
adsorption (sediment); derivatization 0.5 mg/kg
with CH3MgCl; GC-MS (sediment)
---------------------------------------------------------------------------------------------------------
2.3.1.1 Extraction of tributyltin derivatives
Extraction may be independent of or coincident with
the formation of volatile derivatives. It is necessary for
sediments and biological tissues and can also be applied
in the analysis of water samples.
Following acidification, various organic solvents have
been used. The following are most often cited: methyliso-
butylketone, hexane, ethyl acetate, toluene, methanol,
chloroform, dichloromethane, and mixtures of tropolone
(2-hydroxy-2,4,6-cycloheptatrienone) with chloroform, ben-
zene, or dichloromethane.
In the case of water, liquid-liquid extraction may be
replaced by adsorption onto silica gel bonded with C18
aliphatic chains (Matthias et al., 1986a,b; Humphrey &
Hope, 1987).
2.3.1.2 Formation of volatile derivatives
Mono-, di-, and tri-butyltins are not sufficiently
volatile to assure their separation on gas-phase chromato-
graphy; it is, therefore, necessary to prepare more vol-
atile derivatives to allow better separation. Two pro-
cedures have been advocated:
* formation of alkyl derivatives (methyl or pentyl) by
the use of Grignard's reagent (reactive organomag-
nesium);
* formation of hydrides with the general structure
RnSnH4-n by reaction with sodium borohydride
(NaBH4) (Hodge et al., 1979).
These volatile derivatives can then be extracted using
organic solvents, such as dichloromethane, or purged by a
stream of hydrogen.
2.3.1.3 Separation of organotin derivatives
Less sensitive methods for direct separation of mono-,
di-, and tri-butyltins include high performance liquid
chromatography (Jewett & Brinckman, 1981) and thin-layer
chromatography. The latter method is only qualitative and
little used because of its low sensitivity.
2.3.1.4 Detection and measurement of different forms of organotin
Volatile derivatives prepared in the laboratory may be
separated by two procedures:
* separation as a function of boiling point with collec-
tion in a cold trap ("purge and trap" procedure);
* separation by gas chromatography.
After separation by GLC or by the "purge and trap"
procedure, it is possible to detect and quantify, at the
ng/litre level, different forms of organotin using the
following methods:
* a flame photometric detector selective for tin (FPD)
is considered satisfactory;
* a flame atomic absorption (AA) spectrometer or flame-
less atomic absorption (FAA) spectrometer using a
graphite furnace (tin is detected at 286.3 nm or
244.6 nm);
* a mass spectrometer (MS); this is useful for precise
identification of the substance but has limited sen-
sitivity.
There are several methods available for measuring TBT
down to detection limits of 0.2 to 5 ng/litre in water and
5 to 30 µg/kg (in tissues of biota and in sediments).
Some of them can be adapted for routine monitoring pur-
poses. It is necessary, however, to have sophisticated
equipment and the difficulty of the methods requires
experienced laboratories.
His & Robert (1980, 1985) developed a biological assay
based on toxic effects on larvae of the Pacific oyster,
Crassostrea gigas, sensitive only above 20 ng/litre and
nonspecific between organotin and other toxic compounds.
Colorimetric methods (Sherman & Carlson, 1980) have been
based on forming coloured derivatives with phenylfluorone
(nonspecific and with a sensitivity around 0.1 to 4 µg tin).
2.3.2. Interlaboratory calibrations
Interlaboratory comparison of assay methods have been
performed to compare the various proposed methods and to
validate their usefulness as standards.
Young et al. (1986) reported the conclusions of a
workshop held in the USA to examine the problems posed by
the analysis of organotins in water. Nine methods, based
on the principles outlined above, were considered as sat-
isfactory, since the range of results fell within + 15% of
the mean when the TBT concentration was in the order of
ng/litre.
Stephenson et al. (1987) reported the results of
interlaboratory calibrations conducted in 1986-1987 and
carried out on TBT derivatives in mussel tissues and in
sediments. The measurements were made in seven labora-
tories, each using its own technique and using different
extraction conditions, derivative formation, and detec-
tion. A first examination of results showed that they did
not vary by more than a factor of 3. The results were con-
sidered satisfactory.
Blair et al. (1986) took part in an interlaboratory
calibration exercise organised by the National Bureau of
Standards (NBS) in 1984 in the USA and carried out deter-
minations of TBT in water (at a concentration of 1 µg/litre).
Under the auspices of the OECD, it was decided
recently to organize a new worldwide intercalibration to
be carried out on:
* water samples containing 10 ng/litre each of mono-,
di-, and tri-butyltin;
* samples of dried sediment containing the above com-
pounds at a concentration of 100 µg/kg;
* samples of mussel tissue, frozen or freeze-dried, con-
taining the above compounds at 100 µg/kg.
It seems premature to impose a single analytical
method and preferable to allow a certain freedom of choice
between methods to allow sufficient sensitivity to be
attained. However, control of the competence of labora-
tories that carry out such difficult and complex analysis
is required through new calibration procedures.
3. SOURCES OF ENVIRONMENTAL EXPOSURE
3.1. Uses
Dutch scientists first recognized the biocidal proper-
ties of triorganotin compounds in the 1950s; major pro-
duction and use of these substances dates from this
period. It was found that the different triorganotin com-
pounds have different toxicities to different organisms.
Tributyltin compounds were found to be the most toxic of
the triorganotins to gram-positive bacteria and to fungi.
They were also found to have biocidal properties to a wide
spectrum of aquatic organisms.
In the early 1960s, both tributyltin oxide (TBTO) and
TBT fluoride were tested, mainly in Africa, as mollusci-
cides against several freshwater snail species that are
vectors of the disease schistosomiasis, the snails being
the intermediate hosts of the trematode parasite. This use
led to the introduction of TBT, during the mid 1960s, as
an antifouling paint on boats. At the same time TBT com-
pounds were being registered as wood preservatives (the
first registration was in 1958).
Tributyltin compounds have been registered as mollus-
cicides, as antifoulants on boats, ships, quays, buoys,
crabpots, fish nets, and cages, as wood preservatives, as
slimicides on masonry, as disinfectants, and as biocides
for cooling systems, power station cooling towers, pulp
and paper mills, breweries, leather processing, and tex-
tile mills.
When introduced as antifouling paints, TBT paints were
of the "free association" type, where the TBT is physi-
cally incorporated into the paint matrix. In this form it
has a high early release and very short life. Co-polymer
paints were introduced later; in these the TBT moiety is
chemically bonded to a polymer backbone, e.g., those
formed from TBT acrylate or methacrylate and the corre-
sponding acid. The biocide is released by chemical
hydrolysis of the organotin ester linkage. Dissolution is
slow from ships' hulls and a low level of released TBT can
be achieved over a prolonged period. TBT compounds have
also been impregnated into neoprene rubber to produce
elastomeric antifoulant coatings and slow-release mollus-
cicides. In this form, much of the TBT remains in the
matrix of the rubber, though the effectiveness lasts for
several years.
TBT compounds have not been suggested for use in agri-
culture because of their high phytotoxicity.
3.2. Production
The world consumption of tin in 1976 was estimated to
be 200 x 103 tonnes, of which 28 x 103 tonnes was
organotin. Approximately 40% of the total was consumed
in the USA (Zuckerman et al., 1978). The United Kingdom
Department of the Environment (1986) reported that the
worldwide use of organotin in 1980 was 30 x 103 tonnes.
This total was made up as follows:
* PVC stabilizers (dibutyl), approximately 20 x 103 tonnes;
* wood preservatives (tributyl), 3-4 x 103 tonnes;
* antifouling paints (tributyl), 2-3 x 103 tonnes;
* other uses of both di- and tri-butyltin, < 2 x 103 tonnes.
The annual world production of TBT compounds is esti-
mated to be 4000 to 5000 tonnes (Organotin Environmental
Programme Association (ORTEPA); personal communication to
IPCS, 1989).
The total annual use (production and imports) of
organotin compounds in Canada was reported by Thompson et
al. (1985) to be in excess of 1 x 103 tonnes. The total
annual production of TBTO in the Federal Republic of
Germany is reported to be 2 x 103 tonnes, of which 70%
is exported. National usage is as follows: 70% antifouling
paints; 20% timber protection; 10% textile and leather
protection; small amounts are also used as a preservative
in dispersion paints and as a disinfecting agent. Annual
tin emissions are reported to be less than 300 kg (TWG,
1988a). Annual TBT use in the Netherlands in 1985 was
reported to be 1.5 x 104 kg for wood preservation and
10 x 104 kg for antifouling paints (TWG, 1988c). Organo-
tin antifoulant use in Norway was 13.7 x 104 kg in 1986
for the treatment of nets and sea pens at approximately
600 fish farms (Linden, 1987). In Japan, usage was
estimated at 1300 tonnes in 1987, of which two-thirds was
used for antifouling paints on vessels and one-third for
antifouling of nets in fish culture.
A survey of total and retail sales of TBT-containing
paints and antifouling preparations for nets was carried
out in Finland in 1987. Of a total of 42 000 litres,
37 000 litres were sold retail. The concentration of TBT
in the antifouling paints was 4-18%. The previous use of
TBT as a slimicide or fungicide (estimated at 2.1 tonnes
per year during the period 1968-1970) has been discon-
tinued. The estimated sale of wood preservatives contain-
ing TBT was 130 tonnes in 1987; these contained between
0.9 and 1.8% of TBT. Champ & Pugh (1987) reported that
about 300 TBT antifouling paints were registered in the
USA in 1987, but only about 17 paints are now registered
for use (US EPA; personal communication to IPCS, 1989).
MAFF/HSE (1988) listed 345 different wood preservative
formulations, 24 surface biocides and 215 antifouling
paints containing TBT with registration approval for use
in the United Kingdom under the Control of Pesticides
Regulations. In 1989, the number of antifouling paints
containing TBT registered for use in the United Kingdom
had fallen to 148, with the number of wood preservatives
and surface biocides remaining about the same (337 and 26
registered products, respectively) (MAFF/HSE, 1989).
3.3. Regulations
In 1974, the USA set an occupational limit for organo-
tin compounds in air of 0.1 mg tin/m3 (time-weighted
average). In 1979, the American Conference of Governmental
Industrial Hygienists (ACGIH) recommended that the occu-
pational exposure standard for organotin compounds in air
should be set at a threshold limit value (time-weighted
average) of 0.1 mg tin/m3 and a short-term TLV at 0.2 mg
tin/m3. The Federal Republic of Germany was recommended,
in 1979, to adopt an occupational exposure standard for
organotin compounds in air of 0.1 mg tin/m3, specified
as a maximum worksite concentration (MAK). The United
Kingdom has also set a recommended occupational exposure
limit of 0.1 mg tin/m3.
A tentative acceptable daily intake (ADI) of 1.6 µg/kg
per day has been adopted in Japan.
In December 1979, the Japanese Government banned the
use of tributyltin compounds in certain products for
household use, e.g., paint, adhesive, wax, shoe polish,
and textile products.
Following the effects on the oyster industry in France
in the late 1970s, and the subsequent correlation of the
effects with TBT usage, the French government banned the
use of TBT antifouling paints for an initial trial period
of three months, which was later extended. In 1982, paints
containing more than 3% TBT by weight were banned on boats
of < 25 m in length, although boats with aluminium hulls
were excluded. Initially the regulation only covered the
Atlantic coast (January 1982) but was later extended
(September 1982) to the whole French coastline. All use of
organotin compounds in antifouling paints, at any concen-
tration, is now banned in France.
The exception in the regulations for TBT-based anti-
fouling paints that many countries have made for boats
with aluminium hulls is based on the fact that the copper-
based alternative paints react chemically with the alu-
minium.
In January 1986, the United Kingdom enforced regu-
lations that prohibited the retail sale and supply of
antifouling paints with a total tin concentration greater
than 7.5% by weight in co-polymer paints (reduced to 5.5%
in January 1987) or 2.5% in other paints. These regu-
lations were meant to control the use on small pleasure
craft, ban the sale of "free association" paints con-
taining high levels of organotin and set an upper limit on
organotin compounds in co-polymer paints. An ambient water
quality target of 20 ng/litre was set. The United Kingdom
Department of the Environment took steps to determine the
effectiveness of the legislation by setting up a monitor-
ing programme. Based on the results of this monitoring, a
total ban on the use of TBT paints on small boats (< 25 m)
and fish farming equipment was implemented in July 1987
(Abel et al., 1987). An environmental quality standard
(EQS) of 20 ng/litre for fresh water (covering both
potable water and protection of sensitive aquatic biota)
and 2 ng/litre for sea water has been set (United Kingdom
Department of the Environment, 1989).
The paint industry of the Federal Republic of Germany
(FRG) issued a renunciation in 1986 on the use of mono-
meric organotin compounds in antifouling paints and a
restriction to 3.8% TBT in co-polymeric paints. The FRG
has not, as yet, issued any national ban on TBT marine
antifouling paints and is awaiting the outcome of dis-
cussions on an EEC directive (TWG, 1988b). Champ & Pugh
(1987) reported that both Switzerland and the FRG have
banned all uses of TBT in antifouling paints in the fresh-
water environment.
In 1987, the US EPA reviewed TBT usage, weighing risks
to the environment against benefits to users. In the mean-
time, some individual States have passed their own regu-
lations. Both Virginia and Washington State have banned
the use of TBT antifouling paints on boats of < 25 m in
length, excepting those with aluminium hulls. Only paints
that conform to a leaching rate of 5 µg/cm2 per day
(steady state) can be used on boats longer than 25 m. Both
states continued to permit the use of TBT paints, with
acceptable leach rates, in 16 oz (0.45 kg) aerosol cans
for use on outboard motors and lower units. Maryland
instituted similar restrictions but set a lower permiss-
ible leaching rate of 1 µg/cm2 per day (steady state).
Since 1985, North Carolina, Oregon, and Michigan have
instituted restrictions on TBT use. California, Alaska,
New York, and New Jersey had TBT Bills pending in their
respective legislatures (Champ & Pugh, 1987). In April
1988, both the US House of Representatives and the Senate
passed bills to restrict the use of TBT in antifouling
paints. The legislation was signed by the President on
16th June 1988 and came into effect on 16th December 1988.
This Act established an interim release rate restriction
of 4.0 µg/cm2 per day (steady state) and a provision
prohibiting application of TBT antifouling paints to non-
aluminium vessels under 25 m length. Application to larger
vessels was restricted to certified applicators only. The
outboard motor or lower drive unit of a vessel less than
25 m in length was exempted. A limit on sales, delivery,
purchase, and receipt of TBT paints was set in December
1988 and a limit on use in June 1989 for existing stocks
of paint.
A voluntary ban on the use of TBT compounds for nets
in fish culture was imposed in 1987 by the National
Federation of Fisheries Cooperative Association of Japan.
In 1988, the Japanese Ministry of Health and Welfare and
the Japanese Ministry of International Trade and Industry
"designated" eight TBT compounds (and a further five TBT
compounds in 1989) on the basis of persistence, accumu-
lation, and toxicity. "Designated" indicates that no
final decision on regulation has yet been taken but that
the compounds have a recognized hazard. Following this
action, the Japan Paint Manufacturers Association volun-
tarily reduced the upper limit for TBT in paints to < 10%
wet weight for monomers and < 15% wet weight for polymers.
There is current action to monitor release rates from
paint products as the next step in limiting human exposure.
Maguire (1987) reported that tributyltin for the pres-
ervation of fish-farm nets is banned in Canada. In 1987,
the Canadian Department of Agriculture served notice that
antifouling uses of TBT compounds must conform to the
following: a maximum short-term (first 14 days) cumulative
release-rate from paint formulations of 168 µg/cm2; a
long-term average daily release of 4 µg/cm2; and a
minimum hull length of 19.5 m for the use of TBT antifoul-
ing paints on non-aluminium vessels.
In Australia, control measures on the use of TBT-based
paints were introduced in the States of New South Wales
and Victoria. TBT is prohibited for use on boats with a
hull length of less than 25 m, while a leaching rate of
5.0 µg/cm2 per day was set for hulls of 25 m or more.
Aluminium vessels are not exempt from the ban.
The Republic of Ireland instituted a by-law banning
the use of organotin compounds on boats and other aquatic
structures in April 1987 (Minchin et al., 1987).
Norway has also prohibited use of TBT in antifouling
paints except for boats longer than 25 m and those with
aluminium hulls; the regulation became effective from
January 1989. There is also prohibition on the sale, manu-
facture, and import of paints containing TBT without a
specific permit from the State Pollution Control auth-
ority. An agreement to prohibit use on nets of fish farms
has been concluded. Under the Helsinki Convention, the
Baltic States have formed an agreement on the banning of
TBT paints on small boats and have set up a joint monitor-
ing programme.
The Commission of the European Communities has made a
proposal to the Council of Ministers concerning restric-
tions on the use of antifouling paints that mirrors
national restrictions in member states (except that there
would be no derogation for boats with aluminium hulls).
This proposal is currently being considered by the
European Parliament and Council.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATION
Summary
Due to its physico-chemical properties, TBT introduced into
natural waters will partly adsorb onto particles. The quanti-
tative data show large variation due to differences in exper-
imental conditions such as salinity and concentration and
organic content of particulate matter. Once it is adsorbed,
decrease in TBT concentration takes place mainly by degra-
dation. It is known that TBT degradation rates in sediment are
slower than in the water column, particularly in anaerobic
conditions.
Although abiotic degradation occurs, the process remains
less important than biological action.
Biodegradation of TBTO in soil and water depends on the
environmental conditions and the toxic effect of the available
concentrations to the organisms involved. Hydroxylated inter-
mediates are formed during stepwise debutylation. Aerobic and
anaerobic organisms both cause biodegradation, but the relative
efficiency is not known conclusively. Illumination of the cul-
tures lowers the half-life, indicating the involvement of
photosynthetic organisms.
The lipophilic properties of TBTO contribute to bioaccumu-
lation in aquatic organisms, especially molluscs. Laboratory
and field studies corroborate this, although it is unclear how
adsorption processes complicate the results. Bioaccumulation in
all organisms studied is due, at least in part, to bioconcen-
tration from the water phase. Elimination takes place when
organisms are no longer exposed to tributyltin compounds.
Whether it is directly discharged into the environment or
diffuses progressively (at 1 to 10 µg/cm2 per day) from
coatings of the hulls of boats or nets, TBTO enters the aquatic
environment and is subject to transformation resulting from
physico-chemical and biochemical processes. Speciation is out-
lined in chapter 2.
4.1. Adsorption onto and desorption from particles
The effects of TBTO vary in relation to the state in
which the substance is present in the aquatic environment,
in particular whether it is available to organisms in
estuaries or sea shores. It is important to have infor-
mation on its distribution in natural waters likely to
have large amounts of suspended matter of various types.
Several workers (Valkirs et al., 1986; Maguire et al.,
1986; Randall et al., 1986; Harris & Cleary, 1987; Stang &
Seligman, 1987; Hinga et al., 1987) have conducted studies
on adsorption and desorption of TBTO in laboratory exper-
iments, observations in the field, studies conducted in
microcosms, and mathematical modelling.
Mathematical models have been developed to estimate
the distribution of TBT in enclosed or semi-enclosed har-
bours (Walton et al., 1986) and estuaries (Harris &
Cleary, 1987). Good agreement has been found between
measured and estimated concentrations of tin in San Diego
harbour, USA (Walton et al., 1986). The authors considered
the results useful in predicting levels in ecologically
sensitive areas of the bay. The Harris & Cleary (1987)
model was based on the estuary of the River Tamar in
south-west England. This model, still under development,
aimed to reduce inputs in order to allow the model to be
used by non-experts and to be applicable to all estuaries.
Output for the River Tamar suggested that sediment-bound
tin would be distributed up the estuary by tidal influence
leading to increased bound tin further from the open sea.
This effect would be most marked in the summer. Relative
to soluble TBT, this bound fraction does not currently
amount to a significant source of tin for organisms. The
authors point out, however, that this source may become
increasingly important as use of TBT declines and sedi-
ment-bound TBT represents the only available source of the
compound.
The chemical properties of TBT, particularly its lipo-
philic character and poor water solubility, are such that,
when TBTO is introduced into water, repartition will
occur, TBTO leaving the aqueous phase and preferentially
adsorbing onto particles (Hinga et al., 1987). Adsorption
and desorption are dependant on the nature of the sedi-
ment. Little data is available to indicate whether
adsorbed TBT is bioavailable.
If this phenomenon is generally evident, its intensity
varies considerably as a function of the method of study
used and the measurements made. Contradictory results are
apparent in the literature.
Reports from different authors using various con-
ditions have estimated that between 10% and 95% of TBTO
introduced into water is adsorbed onto particles. There
is, however, general agreement that the compound remains
strongly adsorbed. It has been stated that sediments
remain contaminated for at least 10 months; progressive
disappearance of TBTO is not due to desorption but to
degradation.
In an in situ study of Pearl Harbour sediment, the
rate of adsorption of tributyltin derivatives was found to
be 0.57 ng TBT/cm2 per day (Stang & Seligman, 1987).
There was, apparently, no desorption of TBTO itself but
dibutyltin derivatives formed by degradation desorbed with
rates varying between 0.16 and 0.55 ng DBT/cm2 per day.
Variability in results, more evident in field studies
than laboratory studies, is explained by the fact that
adsorption depends on many different factors, amongst
which are the following:
* salinity;
* nature and size of particles in suspension;
* amount of suspended particles;
* temperature;
* presence of dissolved organic matter.
Uncertainties are also evident in relation to the bio-
availability of TBT adsorbed onto sediment. Salazar et al.
(1987) considered that the effects of adsorbed TBTO were
partially masked, i.e. that the compound was unavailable
to organisms. This conclusion could not be verified
regarding effects on filtering or burrowing organisms
living in the sediment.
It is generally agreed that part of the TBTO accumu-
lates in the surface monolayer of natural waters. This
TBTO will also be adsorbed onto organic matter and lipid
material present on the surface.
4.2. Abiotic degradation
A number of studies have shown that a degradation
pathway for tributyltin compounds exists in the environ-
ment, which involves progressive debutylation. It is
theoretically completed with the liberation into water of
the tin oxide (SnO2).
R3SnX -> R2SnX2 -> RSnX3 -> SnX4
A number of studies have looked for evidence of such
degradation, the cause and mechanisms, and an understand-
ing of the kinetics in different environmental conditions
(Chapman & Price, 1972; Brinckman, 1981; Blunden et al.,
1984; Maguire & Tkacz, 1985; and Seligman et al., 1986a).
Degradation of TBTO proceeds via splitting of the
carbon-tin bond, which can result from various mechanisms
occurring simultaneously in the environment. These include
physico-chemical mechanisms (hydrolysis and photodegra-
dation) and biological mechanisms (degradation by microor-
ganisms and metabolism by higher organisms). While degra-
dation definitely occurs as a result of these different
mechanisms in laboratory studies, it is necessary to
assess the relative importance of these different pathways
to degradation of TBTO in the field.
4.2.1. Hydrolytic cleavage of the tin-carbon bond
Since hydrolysis of the tin-carbon bond of organotin
derivatives occurs only under conditions of extreme pH, it
is barely evident under normal environmental conditions.
Studies were carried out in darkness and a sterile
medium to assess the importance of hydrolysis in the
degradation of TBTO. According to the work of Maguire et
al. (1983) and of Maguire & Tkacz (1985), TBTO remains
stable for 11 months in distilled or natural water at
20 °C, in the dark, and in a sterile medium. Under various
conditions of pH, between 2.9 and 10.3, these authors
found no change in TBTO over 63 days. According to
Seligman et al. (1986a), slight degradation of TBTO was
apparent after 94 days in darkness in the presence of
formalin as a sterilizing agent.
It is, therefore, considered that degradation occurs
either not at all or only very slowly in normal environ-
mental conditions of pH and temperature, when monitored in
the dark and in a sterile medium.
4.2.2. Photodegradation
Photodegradation of TBTO by ultraviolet light is
theoretically possible. UV light with a wavelength longer
than 290 nm possesses an energy of 300 kJ/mol, whereas the
energy required to break the carbon-tin bond is 190-220
kJ/mol. At the same time, TBTO absorbs in the UV region at
300 nm and, less strongly, at 350 nm.
Field and laboratory measurements have shown that this
route of degradation can occur and that it forms deriva-
tives of dibutyltin. These seem to be resistant to pho-
tolysis, since very little monobutyltin is formed (Blunden
& Chapman, 1986). While the phenomenon clearly exists, its
importance varies considerably with different environmen-
tal conditions. Conditions of illumination, conditions of
transmission of light, and the presence of photosensi-
tizing substances (acetone, humic acids, etc.) can con-
siderably accelerate the process.
Results of laboratory studies vary considerably de-
pending on whether experiments are conducted under natural
sunlight or UV light of known wavelength. According to
Slesinger & Dresser (1978), the half-life of TBTO in sea
water subjected to ultraviolet light is 18.5 days. In the
presence of a photosensitizing substance, such as acetone,
the half-life is 3.5 days. Seligman et al. (1986a)
suggested that, under natural conditions, photodegradation
is less important than biological action, the development
of phytoplankton leading to a partial degradation of TBTO.
Their measurements were made at relatively high concen-
trations of TBTO (744 µg/litre). Under these conditions,
light caused no degradation over 144 days. According to
Lee et al. (1987), degradation of low concentrations of
TBTO (less than 5 ng/litre) in estuary water is increased
when the assay is conducted in light. The half-life is
between 6 and 12 days, and the presence of significant
concentrations of phytoplankton increases the speed of
degradation. According to Maguire et al. (1983), photoly-
sis under natural light conditions in distilled or natural
water is limited, leading to a TBTO half-life in excess of
89 days. Under experimental conditions of strong UV light,
degradation is apparent. At 300 nm the half-life of TBTO
is 1.1 days, whereas at 350 nm it is more than 18 days.
In these assays, it is possible to demonstrate the role of
humic acids, particularly fulvic acid, which considerably
augment the speed of photolysis. Under such conditions,
the half-life of TBTO falls to 0.6 days at 300 nm and to
6 days at 350 nm. Under natural conditions in the port of
Toronto, Canada, the degradation after 89 days, remained
less than 50%.
4.3. Biodegradation
A number of studies have been conducted to verify that
microorganisms, notably bacteria, are capable of degrading
TBTO. In practice, physico-chemical mechanisms and bio-
logical mechanisms of degradation overlap. Evidence for
biodegradation constitutes an important element in the
assessment of risk. Published studies of observations made
in the field or the laboratory have shown definite evi-
dence of biological degradation of TBTO. Biodegradation
kinetics depend on environmental conditions such as tem-
perature, oxygenation, pH, the level of mineral elements,
the presence of easily biodegradable organic substances,
and the nature of the microflora and the possibility of
their adaptation. Biodegradation also depends on the con-
centration of TBTO being lower than the lethal or inhibi-
tory threshold for the bacteria.
Biodegradation is based on the formation of intermedi-
ate hydroxylated derivatives, progressive oxidative
debutylization following the splitting of the carbon-tin
bond. Dibutyl derivatives are formed, which appear to be
degraded more rapidly than tributyl derivatives to give
monobutyl derivatives; these, conversely, are mineralized
slowly. The end product may be butene. The quantities of
carbon dioxide formed remain small. The biodegradation of
organotin compounds does not seem to involve the formation
of methyl derivatives of tin. Such methyl derivatives have
been measured in some studies (Braman & Tompkins, 1979;
Guard et al., 1981; Hallas et al., 1982; Brinckman et al.,
1983), but have been shown to be the result of the trans-
methylation of inorganic tin by certain marine bacteria
( Pseudomonas ) frequently found in estuaries.
Sheldon (1975) proposed the following scheme for
degradation involving microorganisms:
R3SnX -----> (R3Sn)2O -----> (R3Sn)2CO3
|
| UV or microorganisms
v
(R2SnO)n
|
| UV or microorganisms
v
(RSnO-)n
|
| UV or microorganisms
v
SnO2
A mechanism of biodegradation also exists under
anaerobic conditions (Maguire & Tkacz, 1985). Anaerobic
degradation is considered to be very slow by some workers
and more rapid than aerobic degradation by others.
Slesinger & Dresser (1978) conducted studies in a
Warburg respirometer under aerobic conditions and showed
that microflora derived from activated sludge and soil
were capable of partially degrading TBTO. The half-life
was 70 days, whereas under anaerobic conditions it was
200 days.
Henshaw et al. (1978) showed that pure cultures of
certain wood-degrading fungi, such as Coniophora puteana
and Coriolus polystictus, were capable of slowly biode-
grading TBTO and transforming it to dibutyl and monobutyl
derivatives.
Barug & Vonk (1980) studied the degradation of TBTO in
soil but could show no clear evidence for the action of
microorganisms. Under their experimental conditions, in
sterile or non-sterile medium, the half-life of TBTO
varied between 15 and 20 weeks depending on the soil type.
Barug (1981) was not able to isolate, from sediment or
soils, microorganisms capable of utilizing TBTO as a sole
carbon source. By contrast, in the presence of easily
biodegradable organic matter, biodegradation of TBTO is
apparent with the production of monobutyl derivatives and
smaller quantities of dibutyl derivatives. A number of
species were found to be capable of conducting such degra-
dation aerobically (bacteria: Pseudomonas aeruginosa and
Alcaligenes faecalis ; wood-degrading fungi: Coniophora
puteana, Trametes versicolor, and Chaetomium globosum ).
Under these conditions, they observed 70% degradation in
3 weeks. However, the breakdown of TBTO is not clearly
proved since the authors showed that TBTO accumulates in
the cell walls of bacteria and fungi.
Using water containing natural microflora, Olson &
Brinckman (1986) found no degradation of TBTO at a concen-
tration of 100 µg/litre and a temperature of 5 °C but did
record degradation at 28 °C. Their work also confirmed an
acceleration of degradation when the incubations were con-
ducted under light; the authors explained this acceler-
ation by invoking the role played by photosynthetic micro-
organisms.
Seligman et al. (1986a) also showed evidence for bio-
degradation; in medium polluted by TBTO at 0.5 µg/litre,
the TBTO half-life was 7 days in the dark and 6 days in
the light. In water containing 0.03 µg TBTO/litre, the
half-life was 19 days in the dark and 9 days in the light.
In all cases, dibutyl derivatives were formed and, to a
lesser extent, monobutyl derivatives. In studies with
14C-labelled TBTO, the measurement of 14CO2 production
suggested a half-life of between 50 and 75 days.
Stein & Kuster (1982) demonstrated that TBTO is elim-
inated from waste water passing through sewage treatment
plants by adsorption onto sludge and biodegradation by
sludge organisms, provided that concentrations of TBTO
remain less than 5 mg/litre (see also section 5.3).
According to Maguire et al. (1984), the green alga
Ankistrodesmus falcatus was capable of bioaccumulating
TBTO (with bioconcentration factors of 3 x 104) when it
was cultured in the presence of 20 µg TBTO/litre. When
the cultures were transferred to a non-contaminated
medium, 50% of the TBTO was transformed to dibutyl deriva-
tives or monobutyl derivatives and even to inorganic tin
over the course of 4 weeks. The assays were conducted on
axenic cultures of algae. It may be supposed that a bio-
logical effect was superimposed on physico-chemical degra-
dation mechanisms.
Maguire & Tkacz (1985) have shown that in sediments
there are oligochaetes that are also capable of metab-
olizing TBTO after it has been accumulated. However, the
simultaneous presence of bacteria in the test systems
means that a clear conclusion could not be reached.
According to Maguire et al. (1986), degradation can be
characterized as follows:
* Loss of TBTO by volatilization is very limited with a
half-life of more than 11 months.
* Hydrolysis of TBTO is equally slow with a half-life of
11 months.
* Photodegradation of TBTO plays a more important role
but the half-life of photodegradation is longer than
3 months. This route theoretically takes place but,
under natural conditions of illumination and the poor
penetration of UV light into turbid or coloured water,
it is inefficient.
* Aerobic biodegradation plays a role in water and sedi-
ment. The half-life varies considerably according to
conditions but is in the region of 4 to 5 months.
* Anaerobic degradation plays a role in water and sedi-
ment. The half-life varies considerably but is around
1.5 months.
The kinetics of degradation of dibutyl and monobutyl
tins are less well known. However, the degradation pro-
cesses of TBTO always results in the formation of metab-
olites less toxic than the parent compound.
Hinga et al. (1987) indicated a TBTO half-life of
between 5 and 19 days at 22-24 °C in model ecosystems.
Thain et al. (1987) suggested half-lives of 6 days in
fresh water and 60-90 days at 5 °C in sea water. In water
and sediment of the port of Toronto, the half-life varied
between 4 and 5 months (Maguire & Tkacz, 1985). In estuar-
ine waters of San Diego Bay, USA, the half-life varied
between 7 and 11 days at 12 °C, while in waters of the
Skidaway Estuary, it varied between 5 and 9 days at 28 °C
(Seligman et al., 1986a). Stang & Seligman (1986) using
contaminated sediment from San Diego Bay found that TBT
was degraded to monobutyltin. The degradation kinetic was
lower than in water, the half-life being approximately
162 days. In studies carried out by J.E. Thain & M.J.
Waldock (Personal communication to IPCS, 1989), naturally
contaminated sediments were maintained in the laboratory,
under flow-through conditions, at 12 °C. Degradation of
sediment-bound TBT was found to be a slow process. In
aerobic layers the half-life of TBT was between 4 and 5
months, but in deeper anaerobic layers a half-life value
was not obtained within 500 days.
4.4. Bioaccumulation and elimination
The lipophilic properties of TBTO and its moderately
high octanol-water partition coefficient (log Pow > 3)
contribute to bioaccumulation in living organisms.
Evidence for such mechanisms and an evaluation of
their importance is highly relevant for hazard assessment,
both for the environment and for humans, since some of the
organisms exposed to TBTO are human food items, e.g.,
bivalve molluscs, crustaceans, and fish. Alzieu et al.
(1980) showed that in contaminated areas tin levels in the
flesh of oysters were 100 times higher than concentrations
in the water.
Laboratory experiments have been conducted under dif-
ferent conditions to demonstrate such bioaccumulation, and
have shown that bioconcentration factors vary considerably
between species.
In estuarine bacteria, Blair et al. (1982) found bio-
concentration factors varying between 100 and 30 000 in
species resistant to concentrations of 20 mg TBTO/litre.
As was indicated earlier, such bioconcentration might
result either from adsorption to the surface of the organ-
isms or from true bioaccumulation into the cells. In
phytoplankton, Maguire et al. (1984) reported a bioconcen-
tration factor of 30 000 in the green alga Ankistrodesmus
falcatus exposed for 1 week to concentrations of 20 µg
TBTO/litre. In the diatom Isochrysis galbano, Laughlin et
al. (1986b) reported a bioconcentration factor of 5500.
Studies on the possibility of bioaccumulation and bio-
magnification in molluscs, particularly bivalve molluscs,
are prominent in the literature because of human consump-
tion of oysters and mussels. Alzieu et al. (1982) showed
that TBTO accumulated in oysters, maintained in tanks with
panels of antifouling paint based on TBTO, to levels of
25 mg/kg (dry weight) of tissue and that this resulted in
problems of cavitation of the shell. Waldock et al.
(1983), in studies of the Pacific oyster Crassostrea gigas
exposed for 22 days to TBTO concentrations of 0.15 µg/litre
and 1.25 µg/litre, reported bioconcentration factors of
6000 and 2000, respectively. In European oysters (Ostrea
edulis) exposed to the same concentrations, they found
concentration factors of 1500 and 1000, respectively. In
both cases, after transfer of the oysters to clean water
there was a 50% fall in TBTO levels due to loss or degra-
dation. Laughlin et al. (1986b) reported bioconcentration
factors between 1000 and 7000 for mussels (Mytilus edulis)
exposed for between 3 and 7 weeks to TBTO concentrations
of 23, 45, 63, 141, and 670 ng/litre. For the higher con-
centrations, a plateau in uptake was reached within
2 weeks, but for lower concentrations, no plateau was
reached within the 7-week experiment. The authors con-
sidered that the mussel would be a good indicator organism
for monitoring marine pollution. Cheng & Jensen (1989)
transferred mussels ( Mytilus edulis ) from an unpolluted
area into net bags suspended in a marina in Denmark. They
monitored tin uptake and water concentrations of tin over
a period of 51 days. Accumulation was found to increase
exponentially with time for both total tin and organic
tin. Bioconcentration factors of 5000 to 60 000, much
higher than those from laboratory experiments, were
reported. Transfer of the mussels to the laboratory after
exposure resulted in a half-time for loss of organic and
total tin of 40 and 25 days, respectively. Laughlin et al.
(1986b) showed that bioaccumulation of TBTO by mussels
was not significantly affected by the presence of humic
acids or kaolin but that the presence of mucins secreted
by bacteria did limit bioaccumulation. It was also shown
that bioaccumulation by mussels was greater if the phyto-
plankton used as a food organism (Isochrysis galbana) was
also contaminated with TBT. Contamination via food organ-
isms was more important than via the water.
When feeding crabs with the brine shrimp (Artemia
salina) containing concentrations of TBTO of 6200 µg/kg
wet weight, Evans & Laughlin (1984) found a concentration
factor of 4400. Allen et al. (1980) reported limited bio-
accumulation (< 50) in a 1-week study using freshwater
gastropods (Biomphalaria glabrata). In crustaceans, par-
ticularly the crab Rhithropanopeus harisii, accumulation
of TBTO from a water concentration of 0.28 µg/litre pro-
duced a moderate bioconcentration factor of 60 over 4 days.
Bioaccumulation of TBTO is equally evident in fish.
After exposure of the sheepshead minnow (Cyprinodon
variegatus) for 58 days to concentrations of TBTO varying
between 0.96 and 2.07 µg/litre, Ward et al. (1981)
reported a whole body concentration factor of 2600. After
returning the fish to clean water, loss of TBTO was rapid
over the first 7 days then slower. After 20 days, the
authors reported a loss of 74% from the muscle and 80%
from the viscera. Detection of dibutyltin, monobutyltin,
and inorganic tin suggested possible metabolism. Bressa et
al. (1984) exposed the mullet Liza aurata for 2 months to
concentrations of 5 µg TBTO/litre and reported biocon-
centration factors of 20 to 30 in the liver and kidneys
but no residues in the muscle. After transfer to clean
water, concentrations of tin fell in all organs. Short &
Thrower (1986) studied bioaccumulation in salmon
(Oncorhynchus tshawytscha) exposed for 96 h to concen-
trations of 1.49 µg/litre and obtained concentration
factors of 4300 in the liver, 1300 in the brain, and 200
in muscle. Tsuda et al. (1987) showed that TBTO was
accumulated by carp (Cyprinus carpio) exposed for 14 days
to concentrations varying between 1.8 and 2.4 ng/litre.
Over 10 days they found a plateau in uptake and a concen-
tration factor of 1000; metabolism was evident. Tsuda et
al. (1986) reported concentration factors ranging between
360 and 3400 for round crucian carp (Carassius carassius
grandoculis) tissues exposed to tributyltin chloride for
7 days.
5. ENVIRONMENTAL CONCENTRATIONS
Summary
Levels of TBT in water, sediment, and biota are elevated
within the proximity of marinas, commercial harbours, cooling
systems, and fish nets and cages treated with TBT-based anti-
foulant paints.
TBT levels have been found to reach 1.58 µg/litre in sea
water and estuaries, 7.1 µg/litre in fresh water, 26 300 µg/kg
in coastal sediments, 3700 µg/kg in fresh water sediments,
6.39 mg/kg in bivalves, 1.92 mg/kg in gastropods, and 11 mg/kg
in fish. However, these maximum concentrations of TBT should
not be taken as representative, because a number of factors may
give rise to anomalously high values (e.g., paint particles in
water and sediment samples).
It has been found that measured TBT concentrations in the
surface microlayer of both sea water and fresh water are up to
two orders of magnitude above those measured just below the
surface. However, it should be noted that the recorded levels
of TBT in surface microlayers may be highly affected by the
method of sampling.
Older data may not be comparable to newer data because of
improvements in the analytical methods available for measuring
TBT in water, sediment, and tissue.
5.1. Sea water and marine sediment
The concentrations of TBT in sea water and sediment
are shown in Tables 3 and 5, respectively. Many papers
have reported an association between increased levels of
TBT in water, sediment, and biota and proximity to
pleasure boating activity (especially marinas) and the use
of antifouling paints on fish nets and cages. The degree
of tidal flushing and turbidity of water also influence
TBT concentrations in particular locations.
Table 3. Concentrations of tributyltin in estuarine and sea water
---------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year deptha (µg/litre) Formb limit Reference
(metres) (µg/litre)
---------------------------------------------------------------------------------------
Denmark
Coastal waters 1986 0.1-0.2 <0.04 tin 0.04 Jensen & Cheng (1987)
Marinas 1986 <0.04-1.05 tin 0.04 Jensen & Cheng (1987)
Harbour areas 0.63-2.64 OTo ICES (1987)
Finland
Harbours 1988 0.2 0.02-0.2 TBT 0.01 Yla-Mononen (1988)
France
Bay of Arcachon 1982 0.1-0.3 OT Alzieu & Heral (1984)
1984 0.7-1.2 tin 0.15 Alzieu et al. (1986)
<0.15-0.5 OT 0.1 Alzieu et al. (1986)
1985 0.3-1.0 tin 0.15 Alzieu et al. (1986)
<0.15 OT 0.1 Alzieu et al. (1986)
Anse de Camaret,
Brest 1987 (1) <0.002-0.004 TBT 0.002 Alzieu et al. (1989)
Auray river 1986-
estuary 1987 (1) 0.009-0.069 TBT 0.002 Alzieu et al. (1989)
La Rochelle 1986-
1987 (1) 0.02-0.119 TBT 0.002 Alzieu et al. (1989)
Oleron Island 1986-
1987 (1) 0.039-1.5 TBT 0.002 Alzieu et al. (1989)
Arcachon Bay 1986-
1987 (1) <0.002-0.089 TBT 0.002 Alzieu et al. (1989)
Norway
Oslo fjord <0.01 TBTt 0.01 NIVA (1986)
Sweden
Coastal waters ND-0.04 TBTt Bjorklund (1987b)
United Kingdom
Essex coast 1982 0.1-0.2 <0.03-0.9 TBTt 0.03 Waldock & Miller (1983)
South-west coast 1984 <0.04-0.35 OT 0.04 Cleary & Stebbing (1985)
South-west coast 1986 surface 0.12-5.34 OT 0.04 Cleary & Stebbing (1987)
water
South-west coast 1986 0.5 <0.04-1.44 OT 0.04 Cleary & Stebbing (1987)
South-west coast 1986 bottom <0.04-2.6 OT 0.04 Cleary & Stebbing (1987)
South-west coast 1985 <0.02-0.68 TBT 0.02 Ebdon et al. (1988)
Poole harbour 1986 0.002-0.646 TBTt Langston et al. (1987)
Essex coast 1986 0.1 <0.001-0.831 TBT 0.001 Waldock et al. (1987b)
South coast 1986 0.1 <0.001-1.52 TBT 0.001 Waldock et al. (1987b)
South-west coast 1986 0.1 <0.001-1.27 TBT 0.001 Waldock et al. (1987b)
South Wales coast 1986 0.1 <0.001-0.29 TBT 0.001 Waldock et al. (1987b)
North Wales coast 1986 0.1 <0.001-0.012 TBT 0.001 Waldock et al. (1987b)
---------------------------------------------------------------------------------------
Table 3. (contd.)
---------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year deptha (µg/litre) Formb limit Reference
(metres) (µg/litre)
---------------------------------------------------------------------------------------
USA
Chesapeake Bay 1985 surface ND-1.171 TBT 0.008- Hall et al. (1986)
microlayer 0.01
Chesapeake Bay
(South) 1986 0.15 ND-0.1 TBT 0.001 Huggett et al. (1986)
San Diego Bay 1986 >0.5 0.005-0.235 TBT 0.005 Seligman et al. (1986b)
Californian coast 1986 <0.002-0.6 TBT 0.001- Stallard et al. (1987)
0.002
San Diego Bay 1983-
1985 0.3-0.6 <0.01-0.93 TBT 0.01 Valkirs et al. (1986)
San Diego Bay 1983-
1985 (0.1) <0.01-0.55 TBT 0.01 Valkirs et al. (1986)
USA harbours &
estuaries (0.5) <0.005-0.35 TBT 0.005 Grovhoug et al. (1986)
Coos Bay, Oregon surface 0.007-0.014 TBT Wolniakowski et al.
water (1987)
---------------------------------------------------------------------------------------
a Figures in parentheses indicate distance from water bottom.
b TBT = sample analysed for TBT and expressed as TBT.
TBTt = sample analysed for TBT and expressed as tin.
tin = total tin expressed as tin.
OT = total organic tin expressed as tin.
OTo = total organic tin expressed as TBTO.
Alzieu et al. (1986) monitored tin and organotin con-
centrations in both water and oyster tissue from Arcachon
Bay, France, between 1982 and 1985. They found that levels
in oyster tissue decreased by 5 to 10 times over this
sampling period following French Government restrictions
on the use of TBT in antifouling paints. Alzieu et al.
(1989) monitored TBT water levels at various locations on
the French Atlantic coast in 1986 and 1987 (Table 3), and
found that concentrations generally ranged between < 0.002
and 0.1 µg TBT/litre with the exception of a marina on
Oleron Island, which had levels of up to 1.5 µg/litre.
Levels were highest both in marinas and in the autumn,
presumably when boats were being hosed off ready for the
winter. The authors concluded that levels of TBT had gen-
erally decreased since the restrictions on TBT antifouling
paints, but in certain marinas levels were significantly
higher, suggesting continued use of TBT paints in contra-
vention of restrictions.
Waldock & Miller (1983) measured TBT levels in water
samples collected monthly during 1982 at Burnham-on-Crouch
on the east coast of the United Kingdom. They found a rise
in TBT levels in May, at a time when boats were being
freshly painted with TBT antifouling paints. There was a
second rise in TBT water concentrations in August, at a
time when boats were repainted for the major sailing event
of the year. Analysis of water samples from several areas
on the Essex coast showed that the highest levels (up to
2.25 µg TBTO/litre) were associated with the highest
density of pleasure craft. The authors also reported that
a site used by a large number of boats (on the south
coast of the United Kingdom but situated on an open
coastal site and with less turbid water) had relatively
low TBT levels in the sea water (< 0.08 µg TBTO/litre in
early August).
Waldock et al. (1987b) analysed water samples from
nine sites around the United Kingdom coast during 1986
following restrictions placed on the tin content of anti-
fouling paints containing TBT in January 1986. They
sampled from an enclosed bay, an open coastal site, and
seven estuarine sites. Within these general areas,
locations were found which reflected the incoming water
from a river, an area fished for shellfish, and a harbour
or marina. Half of the 250 samples taken during 1986 were
found to equal or to be above the United Kingdom environ-
mental quality target level (EQT; 20 ng/litre). Levels
were barely above the detection limits at the sites
upstream of boats. Harbours and marinas showed the highest
levels with tidal flushing being an important factor in
determining amounts of TBT detected. A marina in Plymouth,
which has poor flushing, had TBT concentrations consist-
ently greater than 1 µg/litre from May to September,
whereas a marina in the estuary of the River Dart, with
good flushing, had levels of less than 0.2 µg/litre. Six
of the nine sites exceeded the EQT by 3 to 4 times; these
were all sites used regularly by yachts. The other three
sites not used by yachts all showed low but often detect-
able levels with just one sample exceeding the EQT. The
authors also found increased levels of TBT close to areas
where boats were hosed down. Other reports confirmed that
the distribution of TBT in water was associated with the
proximity to intense boating activity (Cleary & Stebbing,
1985; Ebdon et al., 1988). Langston et al. (1987) reported
that sediments, likewise, contained more TBT (up to
520 µg tin/kg) near marinas than at the harbour mouth
(20 µg tin/kg) in Poole harbour, United Kingdom. There
was poor flushing in the harbour and sediment was not dis-
tributed; this was reflected in the water levels, which
were 0.002 to 0.139 µg tin/litre in the general harbour
area and 0.234 to 0.646 µg/litre in the marina.
Cleary & Stebbing (1987) surveyed vertical water pro-
files in south-west England at sites already investigated
two years before. They did not find a systematic decline
in concentrations between the two surveys. The concen-
trations in the surface microlayer were 1.9 to 26.9 times
higher than those at 0.5 m below the surface (see Table 3).
Waldock et al. (1988) analysed water samples collected
in 1987 from commercial harbours and anchorages in the
United Kingdom. Significant concentrations were found;
several samples taken in the immediate vicinity of ships
had levels exceeding 0.05 µg TBT/litre. However, the
highest concentrations were found near to centres of
yachting activity, with over 0.6 µg/litre being found at
one site. The highest concentration found close to commer-
cial vessels in harbours was 0.078 µg/litre, but this was
within 2 m of an oil tanker. A concentration of 0.25 µg per
litre was recorded outside a shipyard where a 3000-tonne
vessel was being hosed down on the foreshore, and a con-
centration of 0.137 µg/litre was measured in surface
water close to a vessel at anchor in the River Fal. In
general, however, few samples taken in close proximity to
commercial ships exceeded 0.02 µg/litre.
Bacci & Gaggi (1989) monitored TBT and its degradation
products in harbours, marinas, and the open sea from the
northern Tyrrhenian Sea, Italy. Concentrations of up to
3.93 µg TBT/litre were measured in the various harbours
and marinas, but no organotin compounds were detected in
samples from the open sea. However, considering the detec-
tion limits of the analytical technique used (0.02 µg per
litre for both TBT and DBT), levels higher than the NOEL
(i.e. 0.01 µg/litre, UNEP, 1989) cannot be excluded.
From these preliminary results, it appears that, under
unfavourable meteorological conditions (e.g., moderate
southerly winds), significant quantities of TBT and
related compounds could contaminate open sea sites for a
few days per year.
The highest levels of TBT around the coasts of the USA
and Denmark were also associated with marinas or harbours
used by small pleasure craft, with TBT levels generally
showing a falling trend from the inner part to the
entrance (Grovhoug et al., 1986; Seligman et al., 1986b;
Jensen & Cheng, 1987). Stallard et al. (1987) analysed
both water and sediment from the Californian coast.
Highest TBT levels, up to 0.6 µg/litre water and 23 µg/kg
sediment, were found near marinas. Levels were lower in
other coastal areas and were lowest out in the open sea.
Valkirs et al. (1986) measured TBT in surface water (at a
depth of 0.3 to 0.6 m) and found that, over the period
1983-1985, TBT levels had increased in San Diego Bay, USA.
Seligman et al. (1989) measured TBT in the waters of
several harbours in the USA. Of the samples collected, 75%
contained TBT levels below the detection limit (< 5 ng per
litre). The highest concentrations were found in yacht
harbours and near to vessel repair facilities, with sig-
nificant levels being found near dry docks. The authors
also found a high degree of variability in TBT concen-
trations depending on the tidal movement, the season, and
intermittent point source discharges.
Hall et al. (1988a) measured TBT biweekly for a 4-
month period (June-September 1986) in the Port Annapolis
marina, Mears marina, Back Creek, and the Severn River
area of northern Chesapeake Bay, USA. Maximum concen-
trations of TBT were reported at both Port Annapolis
marina (1.8 µg tin/litre) and Mears marina (1.17 µg tin
per litre) during early June, followed by significant
reductions during late summer and early autumn. The day of
the week (Thursday-Monday) on which samples were taken
during the daily experiments was not found to signifi-
cantly affect TBT concentrations. Peak concentrations were
found to occur during a rising tide.
Balls (1987) reported that TBT levels in water were
initially (immediately after fish cages were treated with
antifoulants) 1 µg/litre (as tin) within fish cages,
falling to 0.1 µg/litre after 2 weeks and 0.005 µg per
litre after 5 months. Initial concentrations were 0.1 µg
tin/litre at a distance of 20 m from the cages, with
concentrations in the main body of the sea loch being
< 0.028 µg/litre.
5.2. Fresh water and sediment
Analysis for TBT compounds in the Great Lakes, N.
America, has revealed levels often comparable, and in many
cases higher (200 times higher in one sample), than those
measured in estuaries (Maguire et al., 1982; Maguire,
1984; Maguire et al., 1985; Maguire et al., 1986; Maguire
& Tkacz, 1987). Levels of TBT in water were found to be
greater in the surface microlayer than in the subsurface
samples. For example, water samples from Ontario lakes and
rivers showed surface levels of 0.15 to 60.7 µg tin/litre
compared to subsurface levels of between 0.01 and 2.91 µg
per litre (Maguire et al., 1982). TBT was found in the
Great Lakes and in rivers at levels up to those causing
effects on trout in the laboratory; Maguire & Tkacz (1987)
reported a level of 66.8 µg tin/litre in the surface
microlayer. In the United Kingdom, samples of fresh water
from near boatyards contained up to 3.2 µg TBT/litre
(Waldock 1989). In Lake Zurich and Swiss rivers, levels
were found to be much lower, i.e. up to 0.015 µg/litre
(Muller, 1987b). Kalbfus (1988) analysed water samples
from marinas on Lake Constance in 1987 and 1988 and found
that TBT levels rose to a peak in May which corresponded
to the boating activity on the lake. For example, at
Goren, TBT levels rose from 0.13 µg/litre in April to
0.58 µg/litre in May, but by July the levels had fallen
again to 0.028 µg/litre. At the same time TBT levels in
sediment rose from 830 µg/kg in May to 2700 µg/kg in
June and then to 3700 µg/kg in July. Similarly, when
samples were taken on Wannsee in Berlin, levels were found
to be 0.02 µg/litre when there were no boats on the
water, but at Tegel, Berlin, TBT levels were 0.25 µg per
litre when most of the boats were in the water. A coastal
marina at Kiel, on the Baltic, showed levels of 0.35 µg
per litre in April when only half of the moorings were
occupied.
Shiff et al. (1975) monitored water and mud samples
6.5 months after the application of controlled-release
BioMet SRM pellets (rubber formulation containing TBTO) in
Zimbabwe. The pellets were applied at a rate of 20 g/m2
for the control of freshwater snails, intermediate hosts
of the schistosomiasis parasite. Highest levels of organo-
tin were found in the mud immediately under the pellets
(up to 5 mg/kg). Levels in the mud dropped off rapidly
further away from the pellets; at 2 cm organotin levels
were < 0.6 mg/kg. The organotin level in surface water was
< 0.01 mg/litre and in background mud < 0.06 mg/kg.
Table 4. Concentrations of tributyltin in fresh water
------------------------------------------------------------------------------------------
Sample Concentration Detection
Location Year depth (µg/litre) Forma limit Reference
(metres) (µg/litre)
------------------------------------------------------------------------------------------
Canada
Ontario lakes &
rivers 0.01-2.91 TBTt 0.01 Maguire et al. (1982)
Ontario lakes & surface
rivers microlayer 0.15-60.7 TBTt 0.01 Maguire et al. (1982)
St Clair River, surface
Ontario microlayer ND-0.03 TBTt 0.01 Maguire et al. (1985)
Canadian waterways 0.5 <0.01-2.34 TBTt 0.01 Maguire et al. (1986)
Ontario waterways surface 1.9-473 TBTt 1.0 Maguire & Tkacz (1987)
microlayer
Ontario waterways 0.5 <0.01-1.72 TBTt 0.01 Maguire & Tkacz (1987)
Quebec waterways surface 5.5 & 15.2 TBTt 1.0 Maguire & Tkacz (1987)
microlayer
Quebec waterways 0.5 <0.01-0.03 TBTt 0.01 Maguire & Tkacz (1987)
British Columbian
coast up to 0.078 TBT Humphrey & Hope (1987)
Federal Republic of Germany
Lake Constance 1987- up to 0.58 TBT Kalbfus (1988)
marinas 1988
Switzerland
Lake Zurich & 1985 surface 0.007-0.015 TBTc 0.001 Muller (1987b)
rivers water
Harbours 1983-
1984 0.005-1.636 TBT d
Rivers 1983-
1985 0.001-0.016 TBT d
United Kingdom
Wroxham Broad,
Norfolk 1987 up to 0.9b TBT Waldock et al. (1987a)
River Thames 1987 0.064c TBT Waldock et al. (1987a)
River Bure 1986-
1987 0.1 ND-1.54 TBT 0.001 Waldock (1989)
River Yare 1986-
1987 0.1 <0.001-3.26 TBT 0.001 Waldock (1989)
USA
New York State surface 2.0-23.8 TBTt 1.0 Maguire & Tkacz (1987)
waterways microlayer
------------------------------------------------------------------------------------------
a TBT = sample analysed for TBT and expressed as TBT. TBTt = sample analysed for TBT
and expressed as tin. TBTc = sample analysed for TBT and expressed as tributyltin chloride.
b Samples from local boatyards contained up to 1.5 µg/litre.
c Samples from marinas contained up to 1.3 µg/litre.
d Personal communication from M.D. Muller to IPCS.
Table 5. Concentrations of tributyltin in sediment
---------------------------------------------------------------------------------------
Sample Concentrationa Detection
Location Year depth (µg/kg) Formc limit Reference
(metres) (µg/kg)
---------------------------------------------------------------------------------------
Canada
Ontario lakes &
rivers 0.02 30.9-110 TBT 5 Maguire (1984)
Canadian waterways 0.02 <10-10 780 TBTt 10 Maguire et al. (1986)
British Columbian up to
coast 17 000 TBT Humphrey & Hope (1987)
Canada & USA
Detroit & St Clair
rivers 0.02 ND-70 TBTt 5 Maguire et al. (1985)
Netherlands
Eems-Dollard < 25b TBTt 25 TWG (1988c)
Various locations <50-8800 TBTt 50 TWG (1988c)
Switzerland
Lake Zurich 1880-d
1985 120 ND TBTc 0.01 Muller (1987b)
Lake Zurich 1980-
1984 120 280 TBTc 0.01 Muller (1987b)
Lake Zurich &
Boden 1984 2.0-3550 TBT e
United Kingdom
Poole Harbour,
Dorset 1986 20-520 TBTt Langston et al. (1987)
USA
Californian coast 1986 0.1 <2.0-23 TBT 1.0- Stallard et al. (1987)
2.0
San Diego Bay 1983 0.35 <2.0-300 TBT Stang & Seligman (1986)
USA harbours &
estuaries 1.4-178 OT Grovhoug et al. (1986)
Californian coast 15-527 TBT Stephenson et al.
(1987)
Virginian coast 0.02 23-290 TBT Rice et al. (1987)
Great Bay estuary 0.02 12-44 TBTt Weber et al. (1986)
---------------------------------------------------------------------------------------
a Concentrations given as µg/kg dry weight unless stated otherwise.
b Wet weight value.
c TBT = sample analysed for TBT and expressed as TBT.
TBTt = sample analysed for TBT and expressed as tin.
TBTc = sample analysed for TBT and expressed as tributyltin chloride.
OT = total organic tin expressed as tin.
d Museum core from the nineteenth century.
e Personal communication from M.D. Muller to IPCS.
5.3. Sewage treatment
The mono-, di-, and tri-butyltin content of waste
water entering a sewage treatment plant in Switzerland was
measured and its fate was monitored through the various
processes of settlement, digestion, and filtration of the
sewage (Fent, 1989a; Fent et al., 1989). Concentrations of
MBT, DBT, and TBT were 170, 152, and 155 ng/litre,
respectively, in the incoming raw waste water, averaged
over three days of monitoring. About 90% of the organotin
was associated with particulate matter, 10% being in sol-
ution (Table 6). A substantial amount of the incoming
butyltin compounds was lost from the effluent during pri-
mary settlement. The removal of particulate matter at this
stage took 74% of the incoming organotin. In the secondary
effluent, after activated sludge digestion, MBT and DBT
were found at levels similar to those in the primary
effluent; TBT concentrations were reduced to 6 ng/litre
and found only on the particulate matter. In the final
effluent from the plant, after filtration, concentrations
were 4, 3, and 4 ng/litre for MBT, DBT, and TBT, respect-
ively. Thus, 98% of the butyltin was removed from waste
water in the sewage plant. The authors point out that not
all treatment plants have filtration; in these cases only
87% would be removed and effluent concentrations of 9-70
ng/litre found. Levels of butyltin in the sewage sludge
(which is removed from the plant and used as fertiliser on
farm land) were 0.36, 0.38, and 0.34 mg/kg dry weight for
MBT, DBT, and TBT, respectively, in the raw sludge and
0.62, 1.23, and 1.12 mg/kg dry weight in the digested
sludge after 35 days of anaerobic conditions. The authors
point out that 900 kg/year of butyltin could be added to
Swiss soils via sewage sludge. The source of TBT detected
in the sludge was not identified or specified in the
report.
Table 6. Levels of organotin compounds in municipal waste watera
-----------------------------------------------------------------------------------------
MBT DBT TBT
Date water particles % water particles % water particles %
-----------------------------------------------------------------------------------------
23 February 1988 34 216 86 14 113 89 14 178 93
23 February 1988 25 181 88 10 163 94 14 158 92
28 February 1988 28 114 80 11 180 94 27 129 83
Mean 29 170 85 12 152 93 18 155 90
-----------------------------------------------------------------------------------------
a Levels in ng/litre are calculated as ions and corrected for recovery (55-70%); the
percentage of organotins associated with particles is also given. From Fent (1989a).
5.4. Biota
Concentrations of TBT in biota are given in Table 7.
Alzieu (1981) analysed the Pacific oyster (Crassostrea
gigas) for total tin levels following problems in the
French oyster industry in the late 1970s (see section
10.1). He reported that most of the tin accumulated was
in the digestive gland and in the gills. Highest residues
were found in oysters from the Bay of Arcachon (residues
in digestive gland and gill were up to 7.03 and 17.37
mg/kg, respectively), an area with large numbers of small
pleasure boats. Tin levels were stated to be influenced by
tidal flushing; both the Bay of Arcachon and Marennes
Oleron were used by a large number of boats, but residue
levels in oysters collected from the latter site had lower
tin levels (the Bay of Arcachon has poor tidal flushing
compared to Marennes Oleron). Alzieu & Heral (1984)
reported that the greatest accumulation of tin was in
close proximity to a marina. Oysters transferred to the
marina site accumulated a total tin level of 110 mg/kg
(dry weight) within 80 days, whereas oysters maintained as
controls in a local river or in the laboratory accumulated
< 1 mg/kg over the same period. Waldock & Miller (1983)
analysed oysters from the Essex coast, United Kingdom,
and, although both Pacific and European oysters (Ostrea
edulis) contained similar residues of total tin, the
Pacific oyster residues had a higher percentage of TBT.
There are seasonal differences in the levels of TBT
(and DBT) found in mussels (Mytilus edulis) in the field.
It has been suggested that, while these are predominantly
due to changes in boating activity affecting the avail-
ability of TBT to the organisms, physiological differences
in the animals at different times of year may also partly
explain the results. The relative amounts of TBT and DBT
in mussels are thought to reflect the rate of input to the
animal. A high ratio of DBT to TBT residues reflects low
input rates, and vice versa (Page, 1989).
Table 7. Concentrations of tributyltin in biota
---------------------------------------------------------------------------------------------------------
Detection
Concentrationc limit
Organism Year Locationa Organb (mg/kg) Formf (mg/kg) Reference
---------------------------------------------------------------------------------------------------------
Invertebrates
European oyster French coast DG 0.54-7.03 tin Alzieu
(Ostrea edulis) French coast gill <0.5-17.37 tin (1981)
1982 Essex coast, UK DG <0.23-2.05 TBTo 0.075 Waldock &
1982 Essex coast, UK rest <0.4-1.99 TBTo 0.075 Miller
(1983)
Pacific oyster French coast DG <0.5-2.5 tin Alzieu
(Crassostrea gigas) French coast gill <0.5-3.5 tin (1981)
1982 Essex coast, UK DG 4.05-8.64 TBTo 0.075 Waldock &
1982 Essex coast, UK rest 3.5-7.5 TBTo 0.075 Miller (1983)
Coos Bay, USA 0.05-0.189 TBT Wolniakowski
et al. (1987)
Eastern oyster Virginia, USA WB 0.59-1.57 TBT Rice et al. (1987)
(Crassostrea virginica) USA coast WB <0.12-3.9 TBT Wade et al. (1988)
Common mussel 1985- Japan ND-0.28g TBTo 0.05 EAJ (1988)
(Mytilus edulis) 1987 USA coast WB 0.25-3.85 TBT Wade et al.
(1988)
Asiatic mussel 1985- Japan 0.3-0.48g TBTo 0.05 EAJ (1988)
1987
Mussel Californian coast, 0.107-6.39 TBT Stephenson et
USA al. (1987)
Shellfish USA coast 0.23-7.35 OT Grovhoug et al. (1986)
B.C., Canada up to 1.8 TBT Humphrey & Hope (1987)
Netherlands <0.025-0.22 TBTt 0.025 TWG (1988c)
Dogwhelk Fal estuary, UK 0.023-0.786 TBTt Bryan et al. (1987)
(Nucella lapillus) South-west coast, 0.036-0.633 TBTt Gibbs et al.
UK (1987)
Various snail species 1988 Finnish harbours SP 0.04-0.1g TBT 0.01 Yla-Mononen
(1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Detection
Concentrationc limit
Organism Year Locationa Organb (mg/kg) Formf (mg/kg) Reference
----------------------------------------------------------------------------------------------------------------------------------------------------------------
Fish
Herring 1984 Vancouver harbour, WB 0.24 TBTt 0.01 Maguire et
(Culpea harengus) Canada al. (1986)
Finfish B.C., Canada DM up to 11 TBT Humphrey &
Hope (1987)
Salmon species USA MT ND-0.2d TBT Short &
USA 0.28-0.9e TBT Thrower (1986)
Various fish species 1982- Jordan harbour, WB <0.01-0.02g TBTt 0.01 Maguire et
1983 Canada al. (1986)
Various fish species Japan MT ND-0.31d TBTc Hada (1986)
Various fish species 1985- Japan ND-1.7g TBTo 0.05 EAJ (1988)
1987
Various fish species Netherlands <0.025-0.26 tin 0.025 TWG (1988c)
Various fish species 1988 Finnish harbours WB <0.01-0.1g TBT 0.01 Yla-Mononen
(1988)
Birds
Oystercatcher 1986 Exe estuary, UK liver TR-0.08 TBTt 0.02 Osborn &
(Haematopus ostralegus) 1986 Exe estuary, UK MT 0.01-0.19 TBTt 0.02 Leach (1987)
Grey starling 1985- Japan ND (< 0.05)g TBTo 0.05 EAJ (1988)
1987
Black-tailed gull 1985- Japan ND (< 0.05)g TBTo 0.05 EAJ (1988)
1987
Mammals
Seals BL ND TBT 0.01 h
---------------------------------------------------------------------------------------------------------
a UK = United Kingdom; B.C. = British Columbia.
b DG = digestive gland; rest = tissues other than digestive gland; DM = dorsal muscle; MT = muscle
tissue; BL = blubber; WB = whole body; SP = soft parts.
c Concentrations measured as mg/kg dry weight unless stated otherwise; TR = trace; ND = not detectable.
d Fish collected from local fish markets.
e Salmon raised in TBT-treated sea pens.
f TBT = sample analysed for TBT and expressed as TBT; TBTt = sample analysed for TBT and expressed as
tin; TBTc = sample analysed for TBT and expressed as tributyltin chloride; OT = total organic tin
expressed as tin; tin = total tin expressed as tin; TBTo = sample analysed for TBT and expressed
as TBTO.
g Wet weight value.
h Personal communication from M.J. Waldock to IPCS.
Gibbs et al. (1987) found highest levels of TBT
(0.132-0.633 mg tin/kg dry weight) in dogwhelks from the
"enclosed" waters of Plymouth Sound and Torbay, United
Kingdom, whereas levels were less than 0.113 mg tin/kg on
the North Cornish coast. Bryan et al. (1987) reported
residues between 0.374 and 0.786 mg tin/kg (TBT fraction)
for dogwhelks from the Fal estuary, in the south-west of
England, whereas dogwhelks from around the Isle of Mull,
off the Scottish mainland, contained levels of less than
0.03 mg/kg. The Environment Agency of Japan monitored
various fish and shellfish species from different areas of
Japan between 1985 and 1987. The lowest levels of TBTO
(< 0.05 mg/kg wet weight) were found off the open coast
of Japan, higher levels being found in bays and estuaries.
The highest levels reported were in sea bass from the Seto
Inland Sea (up to 1.7 mg/kg). The level of TBTO in the
biota did not change significantly during the sampling
period (EAJ, 1988).
Since 1987 only vessels of > 25 m have been allowed to
use TBT antifouling paints in the United Kingdom (see
section 3.3). Bailey & Davies (1988a) analysed dogwhelk
and scallop from an area around an oil terminal frequented
by large ships at Sullom Voe, Shetland. Elevated tin
levels were found in both dogwhelk (up to 0.16 mg tin/kg
wet weight) and scallops (up to 0.23 mg/kg in gonadal
tissue) within Sullom Voe (especially in areas close to
the oil terminal) compared to those collected from the
surrounding area (< 0.03 mg/kg).
Increased levels of TBT in biota have been found
associated with fish nets and cages. Davies et al. (1987b)
found that residues of total tin in dogwhelks were higher
near fish cages in Loch Laxford (< 0.01-0.33 mg/kg), a sea
loch in Scotland, and in the harbour areas of Loch Crinan
(< 0.01-0.17 mg/kg) than outside the sea lochs (< 0.02 mg/kg).
Short & Thrower (1986) found TBT residues of between
0.28 and 0.9 mg tin/kg in salmon (Oncorhynchus
tshawytscha) maintained in TBT-treated sea pens for 3 to
19 months. The authors also monitored salmon for sale in
American fish markets and found TBT residues of up to 0.2
mg/kg. They also found that cooking does not effectively
destroy or remove TBT from salmon tissues.
6. KINETICS AND METABOLISM
Summary
Tributyltin is absorbed from the gut (20-50% depending on
the vehicle) and via the skin of mammals (about 10%), and can
be transferred across the blood-brain barrier and from the pla-
centa to the fetus. Absorbed material is rapidly and widely
distributed amongst tissues (principally liver and kidney).
Metabolism in mammals is rapid; metabolites are detectable
in blood within 3 h of TBT administration. TBT is a substrate
for mixed-function oxidases in vitro, but these enzymes are
inhibited by TBT in vitro at very high concentrations. Rate of
loss differs with different tissues and estimates for biologi-
cal half-lives in mammals range from 23 to about 30 days.
Metabolism occurs in lower organisms but is slower, par-
ticularly in molluscs. The capacity for bioaccumulation is,
therefore, much greater than in mammals.
TBT compounds inhibit oxidative phosphorylation and alter
mitochondrial structure and function. TBT interferes with the
calcification of the shell of oysters (Crassostrea species).
6.1. Metabolism of TBT in mammals
A number of workers have studied the absorption,
metabolism, and elimination of organotin derivatives in
various animals species, especially in mammals. Some
studies were conducted in vivo and others in vitro using
isolated liver microsomes.
The behaviour of organotin compounds depends partly on
their chemical structure and partly on speciation. How-
ever, the following statements generally apply:
* The distribution of TBT in organisms is usually rapid.
In a number of species (rat, mouse, rabbit, guinea-
pig), it is found preferentially in the liver and
kidney and, to a lesser extent, in the spleen, fat,
lungs, brain, and muscle.
* Excretion is via the bile rather than the urine.
* In tissues, particularly the liver, there is a process
of biotransformation characterized by progressive
de-alkylation leading to breakdown to inorganic tin
(Cremer, 1957; Bridges et al., 1967).
In an in vivo study, Brown et al. (1977) administered
113Sn-labelled TBTO to mice by ip injection. They re-
ported an initial rapid elimination, followed by a slower
phase, in the faeces. Part of the radiolabel was retained
in the tissues but turn-over occurred, with a biological
half-life for elimination of 23 to 29 days.
Evans et al. (1979), under similar conditions, admin-
istered 14C-labelled TBTO to mice in the drinking-water,
at low doses continuously for up to 30 days. There was
absorption from the intestine and accumulation in the
liver, spleen, kidney, and fat (and to a lesser degree in
muscle, lung, brain, and blood). In a second study, mice
were similarly dosed for 31 days. On cessation of dosing
with 14C-labelled TBTO, examination of the animals for a
further 15 days demonstrated loss of TBTO retained in
these tissues; the loss reached 97% in liver, 73% in
kidney, and 30% in fat, and the TBTO had disappeared
completely from the blood. Studies in metabolism cages
indicated that the principal route of loss was via the
faeces; limited amounts of labelled CO2 were exhaled.
Iwai et al. (1980) studied the distribution and
accumulation of tributyltin and its metabolites in areas
of the brain of rabbits. After a single oral dose of TBT
chloride, high concentrations of tributyltin were found in
the frontal and temporal lobes and in the cerebellum
initially. Thereafter, there was a rapid decrease in TBT
residues and an increase in levels of monobutyltin, which
persisted for much longer. Persistence occurred preferen-
tially in the grey matter rather than the white matter.
The authors' interpretation was that TBT, which passes
readily through the blood-brain barrier, is mainly de-
alkylated in the grey matter and that the metabolic
product remains there.
Humpel et al. (1986) administered 113Sn-labelled TBTO
orally to rats and found that the absorption varied
between 20% and 55% depending on the vehicle used. High
residues of tin were found in the liver and kidney (1 to 3
days after dosing) of which only approximately 5% was
unchanged TBT. Other tissues showed lower concentrations
of the label but the fraction of unchanged TBT was higher.
The exact nature of the metabolites could not be ident-
ified by the analytical method used (HPLC), but the
pattern was indicative of progressive debutylation. Daily
administration of TBTO for 14 days resulted in steadily
increasing concentrations of label in all tissues. Steady-
state levels were estimated to be reached after 3 to 4
weeks. When Snoeij et al. (1987) administered 14C-
labelled TBT acetate as a single oral dose to rats, about
20% absorption occurred. The presence of DBT and MBT in
plasma (after TLC separation) was demonstrated 3 h and
27 h after dosing.
TBT may cross the placenta to some extent, as was
shown by the presence of label in rat fetuses after a
single oral dose to the mother at day 18 of pregnancy. The
concentration in fetal tissue was comparable to that of
the mother's muscle tissue (Humpel et al., 1986).
After administration of neat 113Sn-labelled TBTO to
the intact skin of baboons for 7 h, 10 to 15% was esti-
mated to reach the systemic circulation (Humpel et al.,
1986).
Metabolism of tributyltin derivatives has been clearly
demonstrated in in vitro studies. Casida et al. (1971) and
Fish et al. (1975, 1976) studied the possible metabolism
of TBT acetate using rat hepatic microsomes in the pres-
ence of NADPH. They demonstrated hydroxylation by monooxy-
genases of the principal carbon-hydrogen bonds ( alpha and
beta to the tin atom) of 24% (at the alpha position) and
50% (at the beta position). The hydroxylated alpha metab-
olite is unstable and rapidly splits to form the dibutyl
derivative, followed by 1-butanol and then butane. Accord-
ing to Kimmel et al. (1977), the same type of reaction
occurs in microsome preparations from mice.
Uhl (1986) dissolved TBTO (9.88 or 5.54 mg) in a mix-
ture of 3 ml cherry brandy and 7 ml ethanol and gave it
orally to a volunteer. TBTO and its degradation products
were determined in urine by gas chromatography after reac-
tion with methyl magnesium bromide. Only 5.1% to 5.4% of
the dose was found in the urine, mainly as dibutyltin
metabolites. Butyltin levels in the urine decreased
rapidly during the first days after administration. After
dermal application of 20 µl (23.4 mg) of undiluted TBTO
on the arm of a volunteer, approximately 0.2% of the dose
was excreted in the urine, of which about 20% was found to
be tributyltin.
6.2. Metabolism of TBTO in other organisms
Lee (1985, 1986) examined the capacity of organisms
from various aquatic trophic levels to metabolize TBTO.
He used the blue crab (Callinectes sapidus), the brown
shrimp (Penaeus aztecus), a fish (the spot, Leiostomus
xanthurus), and the Eastern oyster (Crassostrea
virginica). The organisms were exposed to 14C-labelled
TBTO via the water (6 µg/litre for the crab and shrimp;
2 µg/litre for the fish and the oyster) and via food
(shrimp containing about 20 mg/kg) in the case of the crab
and fish. In all test species he reported a rapid uptake
of 14C-labelled TBTO into various organs. In crabs and
shrimps, he observed, after 3 days, the appearance of
various metabolites in the hepatopancreas (dibutyl, mono-
butyl, and polar derivatives). In the fish, the same was
seen in the liver. In oysters, the process was much slower
and metabolites appear only at low concentrations after 4
days. In vitro studies, conducted with liver microsomes
from fish and stomach microsomes from crabs, confirmed the
presence of a route of metabolism comparable to that in
mammals. Within microsomes, a cytochrome-P-450-dependent
oxygenase acts in the presence of NADPH and oxygen to
allow progressive degradation of TBTO. Such biochemical
mechanisms are apparent in a number of species. However,
their activity is limited in molluscs, particularly in
bivalves; thus the capacity of molluscs to metabolize
xenobiotics is generally weak. Tsuda et al. (1988) fol-
lowed the metabolism of tributyltin oxide in various
tissues of the carp Cyrpinus carpio over 14 days. In
muscle, there was little evidence of metabolites and
almost all of the tin present was in the form of tributyl-
tin. In the kidney, liver, and gall bladder, large amounts
of monobutyltin were evident. Little dibutyltin was
present in any of the tissues, suggesting that further
metabolism of the intermediate to the monobutyl form was
rapid. Ebdon et al. (1989) could not positively determine
whether dibutyltin and monobutyltin present in adult and
seed oysters in British estuaries derived from intake of
the metabolites or from metabolism within the oysters.
However, they observed that peak seasonal levels of the
metabolites occurred approximately 1 month after peaks of
tributyltin. They concluded that metabolism within the
oysters was responsible for the DBT and MBT present. This
also suggests that metabolism in oysters is slow.
6.3. General mechanisms of toxicity of TBTO
Different mechanisms of action have been advanced to
explain the biological effects and toxicity of TBTO. Some
of the mechanisms are present in all living organisms,
others only in certain species.
6.3.1. General toxic mechanisms
Several studies (Aldridge, 1958; Aldridge & Street,
1964, 1970) have demonstrated that the trialkyl deriva-
tives of tin, and notably tributyltin compounds, are
inhibitors of oxidative phosphorylation in mitochondria
and are, therefore, responsible for inhibiting energy
transfer. This inhibition results from various phenomena:
* disturbance of synthesis of ATP;
* action on mitochondrial membranes causing swelling and
rupture;
* alteration in ion transport across lipid membranes.
Rosenberg et al. (1980, 1981, 1984) and Rosenberg &
Drummond (1983) showed TBTO inhibition of cytochrome P-450
activity in cells from various tissues (liver, kidney,
small intestine mucosa) after dosing in vitro or in vivo.
Evans et al. (1979) demonstrated inhibition of oxidative
phosphorylation due to formation of complexes between tri-
alkyltin derivatives and proteins or certain alpha or beta
amino acids. They most notably form chemical links with
nitrogen and sulfur atoms in protein chains (see chapter 2).
6.3.2. Toxic mechanisms in bivalve molluscs
In bivalve molluscs, notably in oysters, one sublethal
effect of TBTO involves abnormal calcification. This is
shown particularly in Crassostrea gigas, the Pacific or
Eastern oyster, in areas contaminated with TBTO. The
effect is reproducible in experiments where healthy oys-
ters are transferred to contaminated areas, and also
reversible in transfers from contaminated to clean areas.
The abnormal calcification leads to distortion of the
shells; layers are formed successively of calcium carbon-
ate, flaking and open space (Alzieu et al., 1982), and
result partly from interference with synthesis of the
organic matter (gel) (which allows calcium deposition) and
partly from interference with crystallization of calcium
carbonate. Krampitz et al. (1976, 1983) showed that the
protein constituents of the interlamellar gel assisting
deposition of calcium were deficient in the amino acids
necessary for calcium fixation (serine, alanine, glycine,
glutamic acid, aspartic acid); these amino acids are com-
plexed by TBTO.
The work on mammals showing effects of TBTO on oxidat-
ive phosphorylation (Aldridge & Street, 1971) suggests
another possible effect, since ATP plays an important role
in the crystallization of calcium carbonate, as shown in
the following schematic diagram.
 7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: MICROORGANISMS
Summary
Tributyltin is toxic to microorganisms and is used commer-
cially to control bacteria and fungi. Concentrations producing
toxic effects are very variable between species. The primary
productivity of a natural community of freshwater microalgae
was reduced by 50% at a TBTO concentration of 3 µg/litre. Re-
cently established no-observed-effect-levels for two species
are 18 and 32 µg/litre. Toxicity to sea-water microorganisms
is similarly variable between species and between studies; no-
observed-effect-levels are difficult to set but lie below 0.1 µg
per litre for some species. Most toxicity tests with microor-
ganisms use batch cultures, and in these systems concentrations
of TBT in solution may decline rapidly. Such tests may, there-
fore, underestimate true toxicity.
7.1. Bacteria and fungi
Bokranz & Plum (1975) presented data on the effective-
ness of TBT compounds against bacteria, algae, and fungi.
TBT is more toxic to gram-positive bacteria (such as
Staphylococcus aureus with a minimal inhibitory concen-
tration of between 0.2 and 0.8 mg/litre in culture) than
to gram-negative bacteria (such as Escherichia coli with
an MIC of 3.1 mg/litre) in serial dilution tests for vari-
ous TBT compounds. MIC values for four species of fungus
in culture (Botrytis, Penicillium, Aspergillus, and
Rhizopodium) varied from 0.5 to 1.0 mg/litre for TBT
acetate. Impregnation of textiles with TBTO, TBT sulfide,
or TBT fluoride at 0.01%, 0.05%, and 0.2% showed clear
inhibition of fungal growth on agar nutrient medium. The
TBT compounds were resistant to leaching from the textiles
when washed before culture at the two highest concen-
trations. Fungicidal action was also retained in textiles
treated with TBTO and subsequently buried in soil. In
tests with three species of fungus, limiting values for
complete inhibition of effects on wood were determined.
Values for TBTO ranged from 0.058 to 0.704 kg/m3 without
prior leaching of the compound and from 0.055 to 2.178 kg
per m3 after prior leaching. For TBT fluoride, values
ranged from 0.055 to 0.88 kg/m3 without prior leaching
and from 0.135 to 0.886 kg/m3 after leaching.
Soracco & Pope (1983) investigated the action of TBTO
on various physiological and biochemical activities of the
bacterium Legionella pneumophila, the organism causing
Legionnaires disease, which commonly lives in the water of
cooling systems. The minimal concentration of TBTO having
any effect on Legionella growth was about 0.02 mg/litre.
At concentrations between 0.5 and 1.1 mg/litre, TBTO re-
duced the growth rate initially and subsequently caused a
further reduction in growth rate. At 1.1 mg/litre, growth
was almost static, while at higher concentrations TBTO was
bactericidal, causing a reduction in the optical density
of the cultures. The effect of TBTO on the cultures was
dependent on cell density; its effectiveness was reduced
at high cell densities. Between 69% and 88% of the added
TBTO was found to be associated with the cells rather than
in free solution. There was a dose-response relationship
between TBTO concentration per unit biomass and the effect
on growth. The bacteriostatic concentration of 1.1
mg/litre did not kill the cells; transfer of cells from
this culture to fresh nutrient medium established that all
cells were still viable. A concentration of 2.24 mg/litre
was similarly lacking in bactericidal action and only a
dose of 11.2 mg/litre successfully killed cultures. The
most marked and immediate effect of TBTO was on intra-
cellular ATP levels and on "energy charge" (the ratio
between ATP and AMP in the cell). Three concentrations of
TBTO (0.112, 1.12, and 11.2 mg/litre) were tested and
produced reductions of intracellular ATP to 45%, 18%, and
15%, respectively, each within 1 min of addition of the
TBTO. The effect persisted at the lowest exposure level
for at least 3 h. Dramatic and immediate falls were also
seen in energy charge. The authors consider this to be the
major effect of the TBTO. Concomitant falls in nucleic
acid synthesis, synthesis of macromolecules, and CO2 pro-
duction were assumed to follow from the basic action. The
wide range of concentrations producing graded growth inhi-
bition, compared to the very small additional increase in
exposure required to cause cell death, suggested to the
authors that there were two separate mechanisms for the
growth inhibition and lethality of TBTO.
Argaman et al. (1984) investigated the toxic effect of
TBTO on activated sludge from municipal sewage treatment
plants. Sludge challenged with a single dose of TBTO was
inhibited (Warburg respirometer oxygen consumption
measurements) by concentrations of 25 µg/litre or more.
However, sludge pre-treated with TBTO at levels of 200 or
1000 µg/litre adapted to the TBTO and no effect was found
on the ability of sludge organisms to break down organic
materials.
7.2. Freshwater algae
The MIC for cultures of the green alga Chlorella
pyrenoidosa with TBTO was 0.5 mg/litre (Bokranz & Plum,
1975). Floch et al. (1964) reported a no-observed-effect-
level on the growth of a freshwater green alga (desmid) of
0.25 mg TBTO/litre or 0.15 mg TBT acetate/litre over an
exposure period of 10 days. No growth occurred at concen-
trations of 0.5 mg TBTO/litre or 0.3 mg TBT acetate/litre.
Deschiens & Floch (1968) reported an LC100 value for
Chlorella over 10 to 20 days of 0.5 mg TBTO/litre.
More recent studies have suggested that aquatic algae
are much more sensitive to TBT than earlier reports indi-
cated.
Wong et al. (1982) determined the IC50 (concen-
tration required to produce a 50% inhibition) for primary
productivity (uptake of 14C-labelled carbonate) and
reproduction of pure cultures of algae and for a natural
phytoplankton community from Lake Ontario, Canada. The
natural community was the most sensitive to TBTO, with an
IC50 for primary productivity of 3 µg/litre. Ankistro-
desmus falcatus showed similar patterns for the effect of
TBTO on primary productivity and on reproduction (growth),
though the latter was slightly more sensitive with an
IC50 of 5 µg/litre compared to 20 µg/litre for pro-
ductivity. The green alga Scenedesmus quadricaudata and
the cyanobacterium (blue-green alga) Anabaena flos-aquae
gave IC50 values for primary productivity of 16 and
13 µg/litre, respectively. RIVM (1989) reported 96-h
EC50 values for Chlorella and Scenedesmus pannonicus of
42 and 64 µg TBTO/litre, respectively, and no-observed-
effect-levels of 18 and 32 µg/litre, respectively.
7.3. Estuarine and marine algae
Many diatoms are highly resistant to the effects of
organometallic compounds. Thomas & Robinson (1987) studied
the tolerance of the diatom Amphora coffeaeformis to TBT
fluoride and found that its growth was unaffected at con-
centrations of less than 10-7mol/litre when the initial
culture cell density was 10 x 104 cells/ml. At the end of
the incubation, when the diatom had stopped growing, there
was no nitrate left in the medium. There was a significant
effect of TBT fluoride at 10-7mol/litre on growth when
the diatom was grown in nitrate-deficient medium. Growth
was also affected, to a lesser degree, by reducing the
silicate in the medium in the presence of TBT fluoride.
The authors concluded that TBTO tolerance in the diatom is
not due to the exclusion of the organotin but to detoxifi-
cation mechanisms requiring increased uptake of nitrate.
Recovery of the organisms after 24 h exposure supported
this theory. After short-term exposure to sublethal, but
inhibitory, concentrations of TBT fluoride, Amphora re-
covered within 24 h (Thomas & Robinson, 1986). Uptake of
nitrate was inhibited initially but recovered after 24 h.
Salazar (1985) exposed three species of marine phyto-
plankton (Gymnodinium splendens, Dunaliella sp., and
Phaeodactylum tricornutum) to TBTO concentrations of 1.5,
3, and 6 µg/litre for a period of 72 h. At the lowest
concentration, all of the G. splendens cells were killed
and growth of Dunliella sp. was inhibited. The growth of
Dunliella sp. was completely inhibited at both 3 and
6 µg/litre, whereas no effect on the growth of P.
tricornutum was observed at any of the test concen-
trations. Beaumont & Newman (1986) cultured the marine
algae Pavlova lutheri, Dunaliella tertiolecta, and
Skeletonema costatum with TBTO at 0.1, 1.0, and 5.0 µg
per litre. All algae exposed to 5.0 µg/litre died within
2 days. A comparison of the slope of the growth curve with
maximum increase in cell density in the culture showed
that all of the algae were significantly inhibited by TBTO
at the lowest concentration tested. This concentration was
not algicidal. Thain (1983) gave the algistatic concen-
tration for TBTO against Tetraselmis suecica as 560 to
1000 µg/litre and against Skeletonema costatum as 1.0 to
18 µg/litre, within 5 days of exposure. Corresponding
algicidal concentrations were > 1000 µg/litre for Tetra-
selmis and > 18 µg/litre for Skeletonema. Walsh et al.
(1985) calculated the EC50 values for growth inhibition
of the marine alga Skeletonema costatum by TBT acetate,
TBTO, TBT chloride, and TBT fluoride to be 0.36, 0.33,
0.36, and 0.25-0.50 µg/litre, respectively, for a 72-h
exposure period. The EC50 values for growth inhibition
of Thalassiosira pseudonanna, another marine species, by
TBT acetate and TBTO were 1.28 and 1.03 µg per litre,
respectively. The LC50 for Skeletonema was 14.7, 14.2,
11.5, and 11.9 µg/litre for TBT acetate, TBTO, TBT
chloride, and TBT fluoride, respectively. Algae did not
adapt to the presence of TBTO after exposure through
12 serial transfers over 12 weeks; EC50 values were the
same for previously exposed cells as for naive cells.
Dojmi Di Delupis et al. (1987) calculated the 8-day
EC50 for growth inhibition of the marine algal species
Dunaliella tertiolecta and Nitzschia sp., exposed to
TBTO, to be 4.53 µg/litre and 1.19 µg/litre, respect-
ively.
His et al. (1986) conducted bioassays to measure the
susceptibility of algae that are food organisms for oys-
ters to TBT-containing antifouling paints. Four algal
species were used in the studies: Isochrysis galbana
(Prymnesiophyceae); Chaetocerus calcitrans (Bacillario-
phyceae); Tetraselmis (Platymonas) suecica (Prasinophy-
ceae); and Phaeodactylum tricornutum (Bacillariophyceae).
Cultures were maintained in filtered sea water with a
salinity of 27o/oo and at a temperature of 20 °C. TBT
exposure was either to pure TBT acetate or as plates
painted with "International TBT antifouling" with a sur-
face area of between 0.01 and 1.0 cm2 in a culture of
2 litres. Culture density was estimated every 3 to 4 days
using a Coulter counter. Exposure to the pure TBT acetate
at 1 µg/litre had no effect on any of the algal cul-
tures. Isochrysis growth was totally inhibited by painted
panels of 1.0, 0.25, and 0.125 cm2 within 2 days of
culture. Smaller panels were then used to find the limit
of the effect. A 0.02-cm2 panel was also totally toxic to
growth of the alga; panels of 0.01 cm2 allowed growth
comparable to a control culture over the first week of
culture but then inhibited growth. By the 21st day of cul-
ture, the number of cells was reduced to 1.8 x 106 cells
per ml, compared to a control density of 3.2 x 106 cells
per ml. For Chaetocerus, panels of 0.02 cm2 were toxic
to the alga from the beginning of the culture period;
numbers of cells were reduced, indicating that not only
growth but also viability was affected. With panels of
0.01 cm2, there was complete inhibition of development
over the first 4 days, followed by a decrease in cell num-
bers from the original value. The two other algal species
were less sensitive. Phaeodactylum was inhibited by panels
of 1.0 and 0.5 cm2 from the outset of culture. Inhibition
also occurred with panels of 0.25 and 0.125 cm2, but
only after several days. Tetraselmis grew in the presence
of panels of all sizes up to 1.0 cm2, though at this
exposure, growth was reduced. Exposure to panels of 0.5
cm2 had little effect. The authors also tested the effect
on algal growth of fresh water from the river feeding the
area of interest and also the effect of river sediment. In
both cases, growth of the algae was greater than growth of
controls, suggesting a greater availability of nutrients.
However, it should be noted that no analysis was made of
actual water concentrations of TBT in this study; since
the precise exposure levels are unknown, the results are
difficult to interpret or evaluate.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: AQUATIC ORGANISMS
8.1. Aquatic plants
Summary
Few studies have been carried out on the effects of TBT on
aquatic plants. The lowest effect level was observed for
Enteromorpha intestinalis. Motile spores were inhibited from
settling by TBTO, the EC50 being 1 ng/litre; newly settled
spores increased in resistance with time. Results should be
interpreted with care because TBT concentrations were not
measured and the experimental protocols were incomplete.
Reduction in the growth of freshwater species was observed
at concentrations down to 0.06 mg/litre.
Davies et al. (1984) studied the effect of various TBT
compounds on spore development in the marine green macro-
alga, Enteromorpha intestinalis. The 5-day EC50 values
for newly-settled Enteromorpha spores ranged from
0.027 µg/litre (TBT benzoate) to 8.6 µg/litre (TBT acry-
late). The authors stated that the toxicity appeared to be
influenced by the type of anion. Using TBTO it was found
that sensitivity decreased with increasing settlement
time; the 5-day EC50 values for spore development ranged
from 0.22 µg/litre, when exposure began 30 min after
settlement, to 10 µg/litre, when exposure began 72 h
after settlement. Motile spores were the most sensitive
(5-day EC50 = 0.001 µg/litre).
The marine angiosperm Zostera marina showed reduced
growth at TBT concentrations in sediment of 1.0 mg/kg but
no effect at 0.1 mg/kg (Personal communication by M.J.
Waldock to IPCS, 1989).
Floch et al. (1964) exposed freshwater aquatic plants
to TBTO or TBT acetate, at water concentrations between
0.03 and 1.2 mg/litre, for 10 days. The duckweed Lemna
media and Canadian pondweed Elodea sp. both showed some
growth at TBTO concentrations of 0.03 mg/litre. Duckweed
maintained itself, without significant growth, at concen-
trations between 0.06 and 0.25 mg/litre, but died at 0.5
mg/litre. Elodea showed degeneration between 0.06 and 0.25
mg/litre and died at 0.5 mg/litre. Degeneration of Elodea
was evident at 0.15 mg TBT acetate/litre; growth occurred
at 0.03 mg/litre and death at 0.3 mg/litre. Lemna grew in
0.15 mg TBT acetate/litre and died at 1.2 mg/litre; there
was maintenance without growth at 0.6 mg/litre.
L.A. Boorman (personal communication to the IPCS,
1989) grew plants of two salt marsh species, Aster
tripolium and Limonium vulgare, in mud with added TBTO.
Plants of Aster were killed by sediment TBTO levels in
excess of 10 µg/kg (dry weight), while Limonium was not
significantly affected by levels of up to 150 µg/kg.
Chu (1976) found that the aquatic weed Ceratophyllum
died within 2 months exposure to a controlled release
rubber formulation containing 5% TBTO (5 mg/litre); the
exposure period being 24 h every 3 to 5 days.
8.2. Aquatic invertebrates
The acute toxicity of tributyltin to aquatic invert-
ebrates is summarized in Tables 8, 9, and 10. Larval
stages are considerably more sensitive to TBT than adults;
the LC50 for the larval Pacific oyster is 1.6 µg per
litre, over 48 h, whereas that for adults is 1800 µg per
litre (Thain, 1983). Other species show similar differ-
ences between life stages. The 96-h LC50 values for crus-
taceans range between 1.0 and 41 µg/litre. There are
fewer data on freshwater species; these relate to just
three species other than target organisms. Various TBT
salts give a range of 48-h LC50 values for Daphnia of 2.3
to 70 µg/litre and for Tubifex of 5.5 to 33 µg per
litre. The 24-h LC50 for the Asiatic clam is 2100 µg per
litre, and that for target snail adults in schistosomiasis
control is 30 to 400 µg/litre.
8.2.1. Trematode parasites of man
Some organotin molluscicides have been shown to be
toxic to schistosome larvae in the aquatic stages. Ritchie
et al. (1974) found that TBTO concentrations of 10 and
100 µg/litre rendered Schistosoma mansoni cercariae, the
infective stage released from the secondary host (water
snails), incapable of progressive movement, following a 5-
min exposure. Infectivity of the cercariae to mice was
completely suppressed. A 30-min exposure to concentrations
of 1 µg/litre or less had relatively little effect on
motility of cercariae and on subsequent infectivity of
mice. The authors also exposed S. mansoni miracidia to
TBTO and found that 10 µg/litre immobilized the miracidia
after a 40-min exposure and completely suppressed the
infectivity to snails (Biomphalaria glabrata). However, a
concentration of 1 µg/litre had no effect on motility
and infectivity after an exposure period of 120 min.
Table 8. Toxicity of tributyltin to marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Eastern oyster embryo statb 28 7.1 chloride 48 1.3 Roberts
(Crassostrea virginica) (0.78-1.38)d (1987)
larva 48 3.96
(2.42-4.21)d
European oyster adult statb oxide 48 >300 Thain
(Ostrea edulis) 96 210 (1983)
Pacific oyster larva statb oxide 48 1.6 Thain
(Crassostrea gigas) adult 48 1800 (1983)
adult 96 290
Mussel larva statb oxide 48 23 Thain
(Mytilus edulis) adult 48 300 (1983)
adult 96 38
Hard clam embryo statb 28 7.1 chloride 48 1.13 Roberts
(Mercenaria mercenaria) (0.72-1.31)d (1987)
larva 48 1.65d
Brown shrimp larva statb oxide 48 6.5 Thain
(Crangon crangon) larva 96 1.5 (1983)
adult 48 73
adult 96 41
Grass shrimp sub- flow 19.4- 9.8- 8.15- oxide 96 20d Walsh (1986)
(Palaemonetes pugio) adult 21.3 12.1 8.31 chloride 96 > 31d,f Bushong
et al. (1988)
Mysid shrimp < 1 day flow 24-26 19- 7.98- chloride 96 1.1 Goodman et
(Mysidopsis bahia) 22.3 8.01 (0.68-1.4)d al. (1988)
5 day 2
(1.4-2.6)d
10 day 2.2
(1.4-2.6)d
Shore crab larva statb oxide 48 110 Thain (1983)
(Carcinus maenus) 96 10
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Harpacticoid copepod adult stat 20-22 7 7.8 fluoride 96 2 Linden et
(Nitocra spinipes) (1-2)e al. (1979)
oxide 96 2
(1-3)e
Copepod sub- flow 20 10 chloride 72 0.6 Bushong et
(Eurytemora affinis) adult (0.1-2.0)d,f al. (1987)
sub- flow 20 10 chloride 48 1.4
adult (0.8-2.3)d,f
sub- stat 19.4- 10.1- 8.17- chloride 48 2.2 Hall et al.
adult 20.3 11.2 8.32 (0.2-7.3)d,f (1988b)
sub- stat 19.4- 10.1- 8.17- chloride 72 0.6
adult 20.3 11.2 8.32 (0-3.3)d,f
Copepod sub- flow 20 10 chloride 48 1.1 Bushong et
(Acartia tonsa) adult (0.7-2.2)d,f al. (1987)
adult statb 19.5- oxide 96 1.0 U'Ren (1983)
20.5 (0.8-1.2)d
Amphipod young flow 19.4- 9.8- 8.15- chloride 96 1.3d,f Bushong et
(Gammarus sp.) adult 21.3 12.1 8.31 chloride 96 5.3d,f al. (1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise); flow = flow-through conditions (TBT concentration in
water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
Table 9. Toxicity of tributyltin to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50d Reference
age flowa ature nessc tion (ug/litre)
(°C) (mg/litre) (h)
---------------------------------------------------------------------------------------------------------
Asiatic clam larva stat 20 oxide 24 2100e Foster
(Corbicula fluminea) (1981)
Water flea adult statb 21 chloride 96 5.9 (3.7-9.4)e Meador
(Daphnia magna) adult statb 21 chloride 120 3.4 (1.3-8.8)e (1986)
acetate 48 3.3 (1.5-6.0)e,f Polster &
oleate 48 8.5 (4.2-10.5)e,f Halacka
benzoate 48 4.3 (1.8-9.5)e,f (1971)
chloride 48 4.5 (1.2-9.3)e,f
laurate 48 4.7 (1.2-9.3)e,f
oxide 48 2.3 (1.2-5.2)e,f
juvenile stat 20 200 7.5 chloride 24 13e Vighi &
juvenile stat 20 200 7.5 oxide 24 14e Calamari
(1985)
juvenile stat 19 8.2 oxide 48 4.7 RIVM
(1989)
stat 20 oxide 48 70e Foster
(1981)
Tubifex worm acetate 48 8.0 (2.8-10.3)e,f Polster &
(Tubifex tubifex) oleate 48 17.0 (10.1-30.0)e,f Halacka
benzoate 48 16.0 (10.1-27.0)e,f (1971)
chloride 48 15.0 (10.1-23.0)e,f
laurate 48 33.0 (10.6-75.0)e,f
oxide 48 5.5 (1.6-10.3)e,f
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c Hardness expressed as mg/litre CaCO3).
d 95% confidence limits are given in brackets; concentrations expressed as the TBT salt used
unless otherwise stated.
e Nominal concentration.
f Concentration expressed as TBT.
Viyanant et al. (1982) exposed Schistosoma mansoni
cercariae to TBT fluoride and calculated an LC50 of
16.8 µg/litre and an LC90 of 21.7 µg/litre for a 1-h
exposure. Following a 1-h exposure of S. mansoni to TBT
fluoride, cercarial infectivity of mice was observed over
a period of 30 min. A 100% suppression of infectivity was
found at 6 µg/litre; effective doses (99%-100%) were
between 2 and 6 µg/litre.
8.2.2. Freshwater molluscs
Summary
The LC50 values for target fresh water snail adults in
schistosomiasis control range from 30 to 400 µg/litre. This
indicates very low selectivity of TBT as a Bilharzia mollusci-
cide, and a high risk to sensitive non-target aquatic species.
The lethal concentrations of TBT to adult Bilharzia snails
are also expected to inhibit motility and infectivity of both
cercariae and miracidiae in the contaminated water, as the sup-
pressive levels were found to be 10 µg/litre for both cer-
cariae and miracidiae of Schistosoma mansoni after 5 and 40
min, respectively. The 1-h LC 50 for cercariae is 16.8 µg per
litre.
Generally, the toxicity of TBT to freshwater snails depends
on the species, stage, age, temperature, pH, time of exposure,
time of observation, suspended matter, and type of structure
and formulation.
Pellets or matrices of natural rubber or synthetic polymers
impregnated with TBT produce a slow-release effective level of
the molluscicides, which results in low acute toxicity but
extended long-term toxicity.
Exposure of adult snails to TBT levels as low as 0.01 to
0.001 µg/litre reduced egg laying, inhibited hatchability of
the exposed eggs, and retarded the development of the surviving
offspring.
The no-observed-effect level for adult freshwater snails
(Limnaea stagnalis) was 0.32 µg/litre in long-term tests.
TBT compounds are strongly adsorbed on to suspended clay
and organic particles. These particulates are ingested by the
snails and provide one of the inputs for toxicity. Studies of
the impact of type and amount of suspended matter on TBT tox-
icity to snails vary in their conclusions.
Increasing the pH was found to enhance the molluscicidal
toxicity of slow-release TBT formulations.
The acute toxicity of TBT to adult freshwater snails
is summarized in Table 10. The data are based mainly on
species that are intermediate hosts in the life-cycle of
the parasite causing Bilharzia (schistosomiasis) in man,
TBT being used as a molluscicide to control the disease.
The toxic action of TBT is slow, the mortality at the end
of a 24-h exposure is often low, and for a more realistic
result a post-exposure observation period is required.
Rubber impregnated with TBT to produce a slow-release
molluscicide that maintains a low, but toxic, concen-
tration over a long period was developed by Cardarelli and
colleagues, and was shown to be very effective against
snail pest species such as Biomphalaria glabrata and B.
globosus (Berrios-Duran & Ritchie, 1968).
Molluscicidal activity has been demonstrated against
Bulinus spp., Biomphalaria spp., and certain operculate
freshwater molluscs, but organotin compounds have proved
to be not as toxic against the amphibious oncomelaniid
snails (McCullough et al., 1980).
8.2.2.1 Acute toxicity
Webbe (1963) found that young snails (Biomphalaria
sudanica and Bulinus nasutus) were more sensitive than
adults to TBT acetate, the 24-h LC50 values being 14 and
15 µg/litre for the two snail species, respectively.
When eggs from the same two species were exposed, the 24-h
LC50 values ranged from 100 to 1000 µg/litre. Paulini
(1964) found embryos of Biomphalaria glabrata to be more
susceptible than adults to TBT acetate. The 24-h LC50
values for embryos ranged from 26 µg/litre at 5-12 h of
age to 46 µg/litre at 77-87 h of age, whereas the value
for adults was 170 µg/litre.
Table 10. Acute toxicity of tributyltin to freshwater snails
---------------------------------------------------------------------------------------
Species TBT salt Exposure Post-exposure LC50 Reference
duration observation (µg/litre)
(h) (h)
---------------------------------------------------------------------------------------
Biomphalaria oxide 6 72 410 Seiffer & Schoof (1967)
glabrata acetate 6 72 290 Seiffer & Schoof (1967)
oxide 6 24 370 Ritchie et al. (1964)
oxide 24 24 40 Ritchie et al. (1964)
acetate 6 24 190 Ritchie et al. (1964)
acetate 24 24 85 Ritchie et al. (1964)
acetate 24 100-300 Hopf et al. (1967)
pentachloro-
phenate 24 100-400 Hopf et al. (1967)
oxide 24 50-100 Hopf et al. (1967)
oxide 24 30 Deschiens et al. (1966)
acetate 24 72 170 Paulini (1964)
Biomphalaria oxide 24 30 Deschiens et al. (1966)
contortus
Biomphalaria acetate 24 48 34 Webbe (1963)
sudanica
Bulinus nasutus acetate 24 48 32 Webbe (1963)
Bulinus oxide 17 24 10 de Villiers &
tropicus MacKenzie (1963)
Limnaea oxide 24 96 60 Temmink & Everts (1987)
stagnalis oxide 96 96 24 Temmink & Everts (1987)
oxide 96 42 RIVM (1989)
---------------------------------------------------------------------------------------
8.2.2.2 Short- and long-term toxicity
Ritchie et al. (1974) found that egg laying in
Biomphalaria glabrata was completely inhibited by 10 µg
TBTO/litre, all the snails being killed within 2 to 5
days. Egg laying was reduced, over a period of 2 to 3
weeks, by more than 90% at 1 µg/litre and by 50% at
0.1 µg/litre. At 0.01 µg/litre, egg laying was unaffec-
ted. Newly-laid eggs were exposed to TBTO for 34 days
followed by 50 days in clean water. Eggs exposed to 10 µg
per litre did not hatch, even after 50 days in clean
water. Of those exposed to 1 µg/litre, only 3% hatched
during the exposure and 35% of the rest hatched after
transfer to clean water (but with a delayed hatching
time). When newly-hatched snails were exposed to TBTO, 95%
of those exposed to 1 µg/litre from hatching died and
those that survived failed to lay eggs for 85 days. At
0.1 µg/litre, 60% of the snails died and egg laying in
the survivors was reduced by 80%. Egg laying was also
significantly reduced at both 0.01 and 0.001 µg/litre.
Upatham et al. (1980) studied the toxicity to Bulinus
abyssinicus of various controlled-release organotin mol-
luscicides, i.e. BioMet SRM rubber pellets (6% TBTO),
CBL-9B rubber pellets (20% TBT fluoride), and EC-13 float-
ing ethylene propylene co-polymer pellets (30% TBT fluor-
ide). The organotin compounds killed all of the snails
within 1 to 2 days at 100 mg/litre (active ingredient) and
within 5 to 7 days at 1 mg/litre, there being no signifi-
cant difference between the compounds. Changing the test
water daily had no significant effect. At lower concen-
trations the molluscicides required 36 to 40 days to kill
all the snails at an active ingredient concentration of
0.03 mg/litre, and at 0.3 mg/litre 9 to 10 days was re-
quired for BioMet SRM and CBL-9B, and 22 days for EC-13.
In long-term exposure tests, toxic effects on fresh-
water snails have been found at very low TBT concen-
trations. Cardarelli (1973) quoted a 120-day LC100 of
7 µg TBTO/litre for Biomphalaria glabrata. At 0.7 µg per
litre, 26% of the snails died within 120 days. RIVM (1989)
reported a NOEL for the freshwater snail Limnaea stagnalis
of 0.32 µg/litre in long-term tests.
8.2.2.3 Factors affecting toxicity
Paulini & de Souza (1970) studied the effect of
various factors on the molluscicidal activity of TBT to
freshly laid eggs of Biomphalaria glabrata. A concen-
tration of colloidal clay (the proportion of clay to
molluscicide was 1000:1) of 1000 mg/litre reduced the 24-h
LC50 (with a 7-day recovery period included in the mor-
tality count) by 30% for TBT acetate and by 7% for TBTO.
The addition of a yeast ( Saccharomyces sp.) suspension
(1 g/litre) reduced the 24-h LC50 (with a 24-h recovery
period) by 95% for TBTO and 72% for TBT acetate. The pro-
portion of yeast to molluscicide was 1000:1 for TBTO and
100:1 for TBT fluoride.
Cardarelli & Evans (1980) studied the effect of vari-
ous factors on the toxicity to snails of controlled-
release organotin molluscicides, i.e. BioMet SRM (6% TBTO
in natural rubber) and CBL-9B (20% TBT fluoride in natural
rubber). Using 100-mg/kg pellets, they found that increas-
ing the pH from 6 to 8 increased the toxicity (as measured
by both LT50 and LT100) of BioMet SRM but decreased
the toxicity of CBL-9B to both Biomphalaria glabrata and
Bulinus globosus. The authors found no effect of yeast or
humic acid (1 to 100 mg/litre) on the toxicity (LT100)
of these controlled-release molluscicides, neither did
they find an effect of suspended colloidal clays. They
concluded that, although the organotin compounds are
adsorbed, they are still toxic because the snails ingest
the added materials. Therefore, snails are still exposed
to TBT even after it is adsorbed to surfaces. In an exper-
iment to compare adsorbed uptake of TBT, soil-browsing
snails and isolated snails were compared during exposure
to slow-release organotin pellets. The molluscicides were
found to be more toxic when the snails were allowed to
browse on soil, and, at a distance of 60 cm from the pel-
lets, isolated snails were producing egg masses whereas
those browsing on soil were not. The authors also con-
cluded that the organotin molecules that come into contact
with soil particles are adsorbed and slowly form ligands
of a nontoxic nature, so that soil only remains toxic as
long as it is freshly exposed to organotin. Upatham et al.
(1980) found that the presence of mud or plant life,
compared to exposure in water alone, had no effect on the
toxicity (LT100) of either CBL-9B or EC-13 at 1 or 10
mg/litre (active ingredient) to Bulinus abyssinicus. When
the snails were exposed to BioMet SRM, no effect of mud or
plants was found at 10 mg/litre, but at 1 mg/litre a
slightly shorter time was required to kill all the snails
in water alone. Chu (1976) noted that organic materials
such as mud and weeds reduced the molluscicidal activity
of TBTO on Bulinus rohlfsi, when exposed to a rubber for-
mulation containing 5% TBTO (5 mg/litre TBTO), with a 24-h
exposure period every 3 to 5 days for 70 days and twice a
month for a further 7 months.
Macklad et al. (1983) found that the toxicity of
controlled-release molluscicides containing TBT fluoride
was dependent on the pre-exposure soaking time. The 48-h
LC50 for Biomphalaria alexandrina of 10 mg/litre total
available toxicant was achieved after 7, 3, and 1 days
soaking period for the formulations EC27 (10% TBT fluoride
in a plastic polymer), EC1320 (20% TBT fluoride in rub-
ber), and EC1330 (30% TBT fluoride in a polypropylene/
polyethylene mixture), respectively. EC1330 produced no
mortality following a 3-h soaking period and exposure to
concentrations ranging from 1 to 100 mg/litre for 48-h;
the authors suggested that the polypropylene/polyethylene
mixture (EC1330) needs to be sufficiently wet before it
begins to release TBT fluoride. A second experiment to
test the aging of slow-release molluscicides was carried
out. EC27 gave 89% snail mortality during a 48-h exposure
period to 50 mg/litre after a soaking time of 24-h. After
being left to age for 1 month the molluscicide was tested
again and had lost 80% of its original toxicity.
8.2.3. Marine molluscs
Summary
A large body of data exists on the effects of TBT on marine
molluscs and in particular on commercially important bivalves.
Sublethal effects occur at very low concentrations. It has been
shown experimentally that TBT affects shell deposition of grow-
ing oysters, gonad development and gender of adult oysters,
settlement, growth, and mortality of larval oysters and of
other bivalves, and causes imposex (the development of male
characteristics) in female gastropods. The NOEL for shell
thickening of the most sensitive oyster species (C. gigas) is
about 20 ng/litre. Embryo-larval stages are more susceptible
than adults; adverse effects on larval development have been
demonstrated at concentrations as low as 50 ng/litre. The NOEL
is 20 ng/litre.
The authors of recent work on imposex in Nucella have
determined threshold concentrations by extrapolation below the
limit of reliable detection. While the circumstantial evidence
in support is substantial (see chapter 10), the determination
of toxicological thresholds below the limits of chemical detec-
tion is not practicable. It is generally agreed that imposex
is not a specific index of TBT contamination, in that it can be
induced by other factors. The wide distribution of low concen-
trations of TBT and the incidence of imposex at levels similar
to or below the analytical detection limits make long-term
controlled experiments difficult. The establishment of NOELs
will have to await the development of better analytical tech-
niques.
8.2.3.1 Acute toxicity
Waldock & Thain (1985) calculated the 24-h EC50 (mor-
tality plus moribundity) of two organotin-containing fish-
net antifouling preparations for larval oysters to be
12 µg/litre for `Norimp 200' and 320 µg/litre for
`Flexgard'. These compare with a value for TBTO of 1.7 µg
per litre. Thain (1983) pointed out that adult bivalves
tend to appear more resistant to pollutants in standard
short-term tests since they can close their shell over the
test period and thus reduce exposure. Larval stages appear
to be much more sensitive in these tests.
8.2.3.2 Short- and long-term toxicity
Alzieu et al. (1982) kept Pacific oysters (Crassostrea
gigas) in 150-litre tanks that were successively filled
and drained according to the tidal period. To these tanks
were added panels coated on one side with TBT fluoride,
giving estimated concentrations of 0.2 and 2.0 µg TBT per
litre. All oysters died within 30 days in a tank contain-
ing the larger panel. In the tank with a panel surface of
50 cm2, 30% of oysters died after 110 days of exposure
and all oysters within 170 days.
His & Robert (1985) experimentally tested hypotheses
regarding the poor performance of Pacific oysters in the
Bay of Arcachon, France. Larvae were maintained in the
laboratory at TBT acetate concentrations ranging from 0.02
to 100 µg/litre. Growth was affected by all concen-
trations except 0.02 µg/litre. The next concentration
tested (0.05 µg/litre) reduced growth, led to mortality
within 10 days, and interrupted normal feeding by day 8.
No other effects were noted at 0.02 µg/litre and this was
regarded as the NOEL for larvae (see Table 11).
Table 11. Effects of TBT acetate on Crassostrea gigas larvae at
various water concentrationsa
--------------------------------------------------------------------------------
Water concentration Effect
(µg/litre)
--------------------------------------------------------------------------------
100 inhibition of fertilization
50 inhibition of segmentation
25 partial inhibition of segmentation (40%)
10 no formation of trochophores
3 to 5 no veligers; malformed trochophores
1 abnormal veligers; total mortality within 6 days
0.5 numbers of abnormal larvae; total mortality within 8 days;
perturbation of feeding regime, particularly from 4 to 8 days
after exposure; growth greatly reduced
0.2 percentage of D larvae showing abnormalities less elevated;
perturbation of feeding regime from day 4; progressive
mortality; total by day 12; weak growth
0.1 majority of D larvae normal; marked perturbation of feeding
regime from day 6; weak growth until day 6; some survivors
after 12 days
0.05 normal D larvae; perturbation of feeding regime, marked on day
8; significant mortality beginning at day 10; reduced growth
0.02 normal D larvae; little mortality; good growth; no effect of
TBT
--------------------------------------------------------------------------------
a From: His & Robert (1985).
Thain & Waldock (1985) exposed various bivalve spat
(common European oyster, Ostrea edulis; Pacific oyster,
Crassostrea gigas; common mussel, Mytilus edulis; and
carpet shells, Venerupis decussata and Venerupis semi-
decussata) to TBT leachate by maintaining them in flowing
sea-water tanks containing house slates painted with
`Micron 25R' (containing TBT methacrylate co-polymer). The
water concentrations of TBT were 0.24 or 2.6 µg/litre. At
2.6 µg/litre, growth was completely inhibited in all
groups and mortality was high (except for V. semidecussata)
within the 45-day exposure period; all mussels had died
within 14 days. At the lower exposure concentration,
growth was significantly inhibited in C. gigas, M. edulis,
and V. decussata but not in the other two species. In a
second study, the authors reported a severe reduction in
growth rate of recently metamorphosed oyster (Ostrea
edulis) spat after exposure to TBT leachate (0.06 µg per
litre) for 10 days under static conditions. Growth rate
was reduced between 0 and 10 days of exposure to 0.02 µg
per litre, but there was only slight reduction in growth,
relative to controls, between days 10 and 20.
Growth curves for oysters (Ostrea edulis), 2-3 mm in
size, exposed to different concentrations of TBTO are
given in Fig. 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: MICROORGANISMS
Summary
Tributyltin is toxic to microorganisms and is used commer-
cially to control bacteria and fungi. Concentrations producing
toxic effects are very variable between species. The primary
productivity of a natural community of freshwater microalgae
was reduced by 50% at a TBTO concentration of 3 µg/litre. Re-
cently established no-observed-effect-levels for two species
are 18 and 32 µg/litre. Toxicity to sea-water microorganisms
is similarly variable between species and between studies; no-
observed-effect-levels are difficult to set but lie below 0.1 µg
per litre for some species. Most toxicity tests with microor-
ganisms use batch cultures, and in these systems concentrations
of TBT in solution may decline rapidly. Such tests may, there-
fore, underestimate true toxicity.
7.1. Bacteria and fungi
Bokranz & Plum (1975) presented data on the effective-
ness of TBT compounds against bacteria, algae, and fungi.
TBT is more toxic to gram-positive bacteria (such as
Staphylococcus aureus with a minimal inhibitory concen-
tration of between 0.2 and 0.8 mg/litre in culture) than
to gram-negative bacteria (such as Escherichia coli with
an MIC of 3.1 mg/litre) in serial dilution tests for vari-
ous TBT compounds. MIC values for four species of fungus
in culture (Botrytis, Penicillium, Aspergillus, and
Rhizopodium) varied from 0.5 to 1.0 mg/litre for TBT
acetate. Impregnation of textiles with TBTO, TBT sulfide,
or TBT fluoride at 0.01%, 0.05%, and 0.2% showed clear
inhibition of fungal growth on agar nutrient medium. The
TBT compounds were resistant to leaching from the textiles
when washed before culture at the two highest concen-
trations. Fungicidal action was also retained in textiles
treated with TBTO and subsequently buried in soil. In
tests with three species of fungus, limiting values for
complete inhibition of effects on wood were determined.
Values for TBTO ranged from 0.058 to 0.704 kg/m3 without
prior leaching of the compound and from 0.055 to 2.178 kg
per m3 after prior leaching. For TBT fluoride, values
ranged from 0.055 to 0.88 kg/m3 without prior leaching
and from 0.135 to 0.886 kg/m3 after leaching.
Soracco & Pope (1983) investigated the action of TBTO
on various physiological and biochemical activities of the
bacterium Legionella pneumophila, the organism causing
Legionnaires disease, which commonly lives in the water of
cooling systems. The minimal concentration of TBTO having
any effect on Legionella growth was about 0.02 mg/litre.
At concentrations between 0.5 and 1.1 mg/litre, TBTO re-
duced the growth rate initially and subsequently caused a
further reduction in growth rate. At 1.1 mg/litre, growth
was almost static, while at higher concentrations TBTO was
bactericidal, causing a reduction in the optical density
of the cultures. The effect of TBTO on the cultures was
dependent on cell density; its effectiveness was reduced
at high cell densities. Between 69% and 88% of the added
TBTO was found to be associated with the cells rather than
in free solution. There was a dose-response relationship
between TBTO concentration per unit biomass and the effect
on growth. The bacteriostatic concentration of 1.1
mg/litre did not kill the cells; transfer of cells from
this culture to fresh nutrient medium established that all
cells were still viable. A concentration of 2.24 mg/litre
was similarly lacking in bactericidal action and only a
dose of 11.2 mg/litre successfully killed cultures. The
most marked and immediate effect of TBTO was on intra-
cellular ATP levels and on "energy charge" (the ratio
between ATP and AMP in the cell). Three concentrations of
TBTO (0.112, 1.12, and 11.2 mg/litre) were tested and
produced reductions of intracellular ATP to 45%, 18%, and
15%, respectively, each within 1 min of addition of the
TBTO. The effect persisted at the lowest exposure level
for at least 3 h. Dramatic and immediate falls were also
seen in energy charge. The authors consider this to be the
major effect of the TBTO. Concomitant falls in nucleic
acid synthesis, synthesis of macromolecules, and CO2 pro-
duction were assumed to follow from the basic action. The
wide range of concentrations producing graded growth inhi-
bition, compared to the very small additional increase in
exposure required to cause cell death, suggested to the
authors that there were two separate mechanisms for the
growth inhibition and lethality of TBTO.
Argaman et al. (1984) investigated the toxic effect of
TBTO on activated sludge from municipal sewage treatment
plants. Sludge challenged with a single dose of TBTO was
inhibited (Warburg respirometer oxygen consumption
measurements) by concentrations of 25 µg/litre or more.
However, sludge pre-treated with TBTO at levels of 200 or
1000 µg/litre adapted to the TBTO and no effect was found
on the ability of sludge organisms to break down organic
materials.
7.2. Freshwater algae
The MIC for cultures of the green alga Chlorella
pyrenoidosa with TBTO was 0.5 mg/litre (Bokranz & Plum,
1975). Floch et al. (1964) reported a no-observed-effect-
level on the growth of a freshwater green alga (desmid) of
0.25 mg TBTO/litre or 0.15 mg TBT acetate/litre over an
exposure period of 10 days. No growth occurred at concen-
trations of 0.5 mg TBTO/litre or 0.3 mg TBT acetate/litre.
Deschiens & Floch (1968) reported an LC100 value for
Chlorella over 10 to 20 days of 0.5 mg TBTO/litre.
More recent studies have suggested that aquatic algae
are much more sensitive to TBT than earlier reports indi-
cated.
Wong et al. (1982) determined the IC50 (concen-
tration required to produce a 50% inhibition) for primary
productivity (uptake of 14C-labelled carbonate) and
reproduction of pure cultures of algae and for a natural
phytoplankton community from Lake Ontario, Canada. The
natural community was the most sensitive to TBTO, with an
IC50 for primary productivity of 3 µg/litre. Ankistro-
desmus falcatus showed similar patterns for the effect of
TBTO on primary productivity and on reproduction (growth),
though the latter was slightly more sensitive with an
IC50 of 5 µg/litre compared to 20 µg/litre for pro-
ductivity. The green alga Scenedesmus quadricaudata and
the cyanobacterium (blue-green alga) Anabaena flos-aquae
gave IC50 values for primary productivity of 16 and
13 µg/litre, respectively. RIVM (1989) reported 96-h
EC50 values for Chlorella and Scenedesmus pannonicus of
42 and 64 µg TBTO/litre, respectively, and no-observed-
effect-levels of 18 and 32 µg/litre, respectively.
7.3. Estuarine and marine algae
Many diatoms are highly resistant to the effects of
organometallic compounds. Thomas & Robinson (1987) studied
the tolerance of the diatom Amphora coffeaeformis to TBT
fluoride and found that its growth was unaffected at con-
centrations of less than 10-7mol/litre when the initial
culture cell density was 10 x 104 cells/ml. At the end of
the incubation, when the diatom had stopped growing, there
was no nitrate left in the medium. There was a significant
effect of TBT fluoride at 10-7mol/litre on growth when
the diatom was grown in nitrate-deficient medium. Growth
was also affected, to a lesser degree, by reducing the
silicate in the medium in the presence of TBT fluoride.
The authors concluded that TBTO tolerance in the diatom is
not due to the exclusion of the organotin but to detoxifi-
cation mechanisms requiring increased uptake of nitrate.
Recovery of the organisms after 24 h exposure supported
this theory. After short-term exposure to sublethal, but
inhibitory, concentrations of TBT fluoride, Amphora re-
covered within 24 h (Thomas & Robinson, 1986). Uptake of
nitrate was inhibited initially but recovered after 24 h.
Salazar (1985) exposed three species of marine phyto-
plankton (Gymnodinium splendens, Dunaliella sp., and
Phaeodactylum tricornutum) to TBTO concentrations of 1.5,
3, and 6 µg/litre for a period of 72 h. At the lowest
concentration, all of the G. splendens cells were killed
and growth of Dunliella sp. was inhibited. The growth of
Dunliella sp. was completely inhibited at both 3 and
6 µg/litre, whereas no effect on the growth of P.
tricornutum was observed at any of the test concen-
trations. Beaumont & Newman (1986) cultured the marine
algae Pavlova lutheri, Dunaliella tertiolecta, and
Skeletonema costatum with TBTO at 0.1, 1.0, and 5.0 µg
per litre. All algae exposed to 5.0 µg/litre died within
2 days. A comparison of the slope of the growth curve with
maximum increase in cell density in the culture showed
that all of the algae were significantly inhibited by TBTO
at the lowest concentration tested. This concentration was
not algicidal. Thain (1983) gave the algistatic concen-
tration for TBTO against Tetraselmis suecica as 560 to
1000 µg/litre and against Skeletonema costatum as 1.0 to
18 µg/litre, within 5 days of exposure. Corresponding
algicidal concentrations were > 1000 µg/litre for Tetra-
selmis and > 18 µg/litre for Skeletonema. Walsh et al.
(1985) calculated the EC50 values for growth inhibition
of the marine alga Skeletonema costatum by TBT acetate,
TBTO, TBT chloride, and TBT fluoride to be 0.36, 0.33,
0.36, and 0.25-0.50 µg/litre, respectively, for a 72-h
exposure period. The EC50 values for growth inhibition
of Thalassiosira pseudonanna, another marine species, by
TBT acetate and TBTO were 1.28 and 1.03 µg per litre,
respectively. The LC50 for Skeletonema was 14.7, 14.2,
11.5, and 11.9 µg/litre for TBT acetate, TBTO, TBT
chloride, and TBT fluoride, respectively. Algae did not
adapt to the presence of TBTO after exposure through
12 serial transfers over 12 weeks; EC50 values were the
same for previously exposed cells as for naive cells.
Dojmi Di Delupis et al. (1987) calculated the 8-day
EC50 for growth inhibition of the marine algal species
Dunaliella tertiolecta and Nitzschia sp., exposed to
TBTO, to be 4.53 µg/litre and 1.19 µg/litre, respect-
ively.
His et al. (1986) conducted bioassays to measure the
susceptibility of algae that are food organisms for oys-
ters to TBT-containing antifouling paints. Four algal
species were used in the studies: Isochrysis galbana
(Prymnesiophyceae); Chaetocerus calcitrans (Bacillario-
phyceae); Tetraselmis (Platymonas) suecica (Prasinophy-
ceae); and Phaeodactylum tricornutum (Bacillariophyceae).
Cultures were maintained in filtered sea water with a
salinity of 27o/oo and at a temperature of 20 °C. TBT
exposure was either to pure TBT acetate or as plates
painted with "International TBT antifouling" with a sur-
face area of between 0.01 and 1.0 cm2 in a culture of
2 litres. Culture density was estimated every 3 to 4 days
using a Coulter counter. Exposure to the pure TBT acetate
at 1 µg/litre had no effect on any of the algal cul-
tures. Isochrysis growth was totally inhibited by painted
panels of 1.0, 0.25, and 0.125 cm2 within 2 days of
culture. Smaller panels were then used to find the limit
of the effect. A 0.02-cm2 panel was also totally toxic to
growth of the alga; panels of 0.01 cm2 allowed growth
comparable to a control culture over the first week of
culture but then inhibited growth. By the 21st day of cul-
ture, the number of cells was reduced to 1.8 x 106 cells
per ml, compared to a control density of 3.2 x 106 cells
per ml. For Chaetocerus, panels of 0.02 cm2 were toxic
to the alga from the beginning of the culture period;
numbers of cells were reduced, indicating that not only
growth but also viability was affected. With panels of
0.01 cm2, there was complete inhibition of development
over the first 4 days, followed by a decrease in cell num-
bers from the original value. The two other algal species
were less sensitive. Phaeodactylum was inhibited by panels
of 1.0 and 0.5 cm2 from the outset of culture. Inhibition
also occurred with panels of 0.25 and 0.125 cm2, but
only after several days. Tetraselmis grew in the presence
of panels of all sizes up to 1.0 cm2, though at this
exposure, growth was reduced. Exposure to panels of 0.5
cm2 had little effect. The authors also tested the effect
on algal growth of fresh water from the river feeding the
area of interest and also the effect of river sediment. In
both cases, growth of the algae was greater than growth of
controls, suggesting a greater availability of nutrients.
However, it should be noted that no analysis was made of
actual water concentrations of TBT in this study; since
the precise exposure levels are unknown, the results are
difficult to interpret or evaluate.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: AQUATIC ORGANISMS
8.1. Aquatic plants
Summary
Few studies have been carried out on the effects of TBT on
aquatic plants. The lowest effect level was observed for
Enteromorpha intestinalis. Motile spores were inhibited from
settling by TBTO, the EC50 being 1 ng/litre; newly settled
spores increased in resistance with time. Results should be
interpreted with care because TBT concentrations were not
measured and the experimental protocols were incomplete.
Reduction in the growth of freshwater species was observed
at concentrations down to 0.06 mg/litre.
Davies et al. (1984) studied the effect of various TBT
compounds on spore development in the marine green macro-
alga, Enteromorpha intestinalis. The 5-day EC50 values
for newly-settled Enteromorpha spores ranged from
0.027 µg/litre (TBT benzoate) to 8.6 µg/litre (TBT acry-
late). The authors stated that the toxicity appeared to be
influenced by the type of anion. Using TBTO it was found
that sensitivity decreased with increasing settlement
time; the 5-day EC50 values for spore development ranged
from 0.22 µg/litre, when exposure began 30 min after
settlement, to 10 µg/litre, when exposure began 72 h
after settlement. Motile spores were the most sensitive
(5-day EC50 = 0.001 µg/litre).
The marine angiosperm Zostera marina showed reduced
growth at TBT concentrations in sediment of 1.0 mg/kg but
no effect at 0.1 mg/kg (Personal communication by M.J.
Waldock to IPCS, 1989).
Floch et al. (1964) exposed freshwater aquatic plants
to TBTO or TBT acetate, at water concentrations between
0.03 and 1.2 mg/litre, for 10 days. The duckweed Lemna
media and Canadian pondweed Elodea sp. both showed some
growth at TBTO concentrations of 0.03 mg/litre. Duckweed
maintained itself, without significant growth, at concen-
trations between 0.06 and 0.25 mg/litre, but died at 0.5
mg/litre. Elodea showed degeneration between 0.06 and 0.25
mg/litre and died at 0.5 mg/litre. Degeneration of Elodea
was evident at 0.15 mg TBT acetate/litre; growth occurred
at 0.03 mg/litre and death at 0.3 mg/litre. Lemna grew in
0.15 mg TBT acetate/litre and died at 1.2 mg/litre; there
was maintenance without growth at 0.6 mg/litre.
L.A. Boorman (personal communication to the IPCS,
1989) grew plants of two salt marsh species, Aster
tripolium and Limonium vulgare, in mud with added TBTO.
Plants of Aster were killed by sediment TBTO levels in
excess of 10 µg/kg (dry weight), while Limonium was not
significantly affected by levels of up to 150 µg/kg.
Chu (1976) found that the aquatic weed Ceratophyllum
died within 2 months exposure to a controlled release
rubber formulation containing 5% TBTO (5 mg/litre); the
exposure period being 24 h every 3 to 5 days.
8.2. Aquatic invertebrates
The acute toxicity of tributyltin to aquatic invert-
ebrates is summarized in Tables 8, 9, and 10. Larval
stages are considerably more sensitive to TBT than adults;
the LC50 for the larval Pacific oyster is 1.6 µg per
litre, over 48 h, whereas that for adults is 1800 µg per
litre (Thain, 1983). Other species show similar differ-
ences between life stages. The 96-h LC50 values for crus-
taceans range between 1.0 and 41 µg/litre. There are
fewer data on freshwater species; these relate to just
three species other than target organisms. Various TBT
salts give a range of 48-h LC50 values for Daphnia of 2.3
to 70 µg/litre and for Tubifex of 5.5 to 33 µg per
litre. The 24-h LC50 for the Asiatic clam is 2100 µg per
litre, and that for target snail adults in schistosomiasis
control is 30 to 400 µg/litre.
8.2.1. Trematode parasites of man
Some organotin molluscicides have been shown to be
toxic to schistosome larvae in the aquatic stages. Ritchie
et al. (1974) found that TBTO concentrations of 10 and
100 µg/litre rendered Schistosoma mansoni cercariae, the
infective stage released from the secondary host (water
snails), incapable of progressive movement, following a 5-
min exposure. Infectivity of the cercariae to mice was
completely suppressed. A 30-min exposure to concentrations
of 1 µg/litre or less had relatively little effect on
motility of cercariae and on subsequent infectivity of
mice. The authors also exposed S. mansoni miracidia to
TBTO and found that 10 µg/litre immobilized the miracidia
after a 40-min exposure and completely suppressed the
infectivity to snails (Biomphalaria glabrata). However, a
concentration of 1 µg/litre had no effect on motility
and infectivity after an exposure period of 120 min.
Table 8. Toxicity of tributyltin to marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Eastern oyster embryo statb 28 7.1 chloride 48 1.3 Roberts
(Crassostrea virginica) (0.78-1.38)d (1987)
larva 48 3.96
(2.42-4.21)d
European oyster adult statb oxide 48 >300 Thain
(Ostrea edulis) 96 210 (1983)
Pacific oyster larva statb oxide 48 1.6 Thain
(Crassostrea gigas) adult 48 1800 (1983)
adult 96 290
Mussel larva statb oxide 48 23 Thain
(Mytilus edulis) adult 48 300 (1983)
adult 96 38
Hard clam embryo statb 28 7.1 chloride 48 1.13 Roberts
(Mercenaria mercenaria) (0.72-1.31)d (1987)
larva 48 1.65d
Brown shrimp larva statb oxide 48 6.5 Thain
(Crangon crangon) larva 96 1.5 (1983)
adult 48 73
adult 96 41
Grass shrimp sub- flow 19.4- 9.8- 8.15- oxide 96 20d Walsh (1986)
(Palaemonetes pugio) adult 21.3 12.1 8.31 chloride 96 > 31d,f Bushong
et al. (1988)
Mysid shrimp < 1 day flow 24-26 19- 7.98- chloride 96 1.1 Goodman et
(Mysidopsis bahia) 22.3 8.01 (0.68-1.4)d al. (1988)
5 day 2
(1.4-2.6)d
10 day 2.2
(1.4-2.6)d
Shore crab larva statb oxide 48 110 Thain (1983)
(Carcinus maenus) 96 10
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Harpacticoid copepod adult stat 20-22 7 7.8 fluoride 96 2 Linden et
(Nitocra spinipes) (1-2)e al. (1979)
oxide 96 2
(1-3)e
Copepod sub- flow 20 10 chloride 72 0.6 Bushong et
(Eurytemora affinis) adult (0.1-2.0)d,f al. (1987)
sub- flow 20 10 chloride 48 1.4
adult (0.8-2.3)d,f
sub- stat 19.4- 10.1- 8.17- chloride 48 2.2 Hall et al.
adult 20.3 11.2 8.32 (0.2-7.3)d,f (1988b)
sub- stat 19.4- 10.1- 8.17- chloride 72 0.6
adult 20.3 11.2 8.32 (0-3.3)d,f
Copepod sub- flow 20 10 chloride 48 1.1 Bushong et
(Acartia tonsa) adult (0.7-2.2)d,f al. (1987)
adult statb 19.5- oxide 96 1.0 U'Ren (1983)
20.5 (0.8-1.2)d
Amphipod young flow 19.4- 9.8- 8.15- chloride 96 1.3d,f Bushong et
(Gammarus sp.) adult 21.3 12.1 8.31 chloride 96 5.3d,f al. (1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise); flow = flow-through conditions (TBT concentration in
water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
Table 9. Toxicity of tributyltin to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50d Reference
age flowa ature nessc tion (ug/litre)
(°C) (mg/litre) (h)
---------------------------------------------------------------------------------------------------------
Asiatic clam larva stat 20 oxide 24 2100e Foster
(Corbicula fluminea) (1981)
Water flea adult statb 21 chloride 96 5.9 (3.7-9.4)e Meador
(Daphnia magna) adult statb 21 chloride 120 3.4 (1.3-8.8)e (1986)
acetate 48 3.3 (1.5-6.0)e,f Polster &
oleate 48 8.5 (4.2-10.5)e,f Halacka
benzoate 48 4.3 (1.8-9.5)e,f (1971)
chloride 48 4.5 (1.2-9.3)e,f
laurate 48 4.7 (1.2-9.3)e,f
oxide 48 2.3 (1.2-5.2)e,f
juvenile stat 20 200 7.5 chloride 24 13e Vighi &
juvenile stat 20 200 7.5 oxide 24 14e Calamari
(1985)
juvenile stat 19 8.2 oxide 48 4.7 RIVM
(1989)
stat 20 oxide 48 70e Foster
(1981)
Tubifex worm acetate 48 8.0 (2.8-10.3)e,f Polster &
(Tubifex tubifex) oleate 48 17.0 (10.1-30.0)e,f Halacka
benzoate 48 16.0 (10.1-27.0)e,f (1971)
chloride 48 15.0 (10.1-23.0)e,f
laurate 48 33.0 (10.6-75.0)e,f
oxide 48 5.5 (1.6-10.3)e,f
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c Hardness expressed as mg/litre CaCO3).
d 95% confidence limits are given in brackets; concentrations expressed as the TBT salt used
unless otherwise stated.
e Nominal concentration.
f Concentration expressed as TBT.
Viyanant et al. (1982) exposed Schistosoma mansoni
cercariae to TBT fluoride and calculated an LC50 of
16.8 µg/litre and an LC90 of 21.7 µg/litre for a 1-h
exposure. Following a 1-h exposure of S. mansoni to TBT
fluoride, cercarial infectivity of mice was observed over
a period of 30 min. A 100% suppression of infectivity was
found at 6 µg/litre; effective doses (99%-100%) were
between 2 and 6 µg/litre.
8.2.2. Freshwater molluscs
Summary
The LC50 values for target fresh water snail adults in
schistosomiasis control range from 30 to 400 µg/litre. This
indicates very low selectivity of TBT as a Bilharzia mollusci-
cide, and a high risk to sensitive non-target aquatic species.
The lethal concentrations of TBT to adult Bilharzia snails
are also expected to inhibit motility and infectivity of both
cercariae and miracidiae in the contaminated water, as the sup-
pressive levels were found to be 10 µg/litre for both cer-
cariae and miracidiae of Schistosoma mansoni after 5 and 40
min, respectively. The 1-h LC 50 for cercariae is 16.8 µg per
litre.
Generally, the toxicity of TBT to freshwater snails depends
on the species, stage, age, temperature, pH, time of exposure,
time of observation, suspended matter, and type of structure
and formulation.
Pellets or matrices of natural rubber or synthetic polymers
impregnated with TBT produce a slow-release effective level of
the molluscicides, which results in low acute toxicity but
extended long-term toxicity.
Exposure of adult snails to TBT levels as low as 0.01 to
0.001 µg/litre reduced egg laying, inhibited hatchability of
the exposed eggs, and retarded the development of the surviving
offspring.
The no-observed-effect level for adult freshwater snails
(Limnaea stagnalis) was 0.32 µg/litre in long-term tests.
TBT compounds are strongly adsorbed on to suspended clay
and organic particles. These particulates are ingested by the
snails and provide one of the inputs for toxicity. Studies of
the impact of type and amount of suspended matter on TBT tox-
icity to snails vary in their conclusions.
Increasing the pH was found to enhance the molluscicidal
toxicity of slow-release TBT formulations.
The acute toxicity of TBT to adult freshwater snails
is summarized in Table 10. The data are based mainly on
species that are intermediate hosts in the life-cycle of
the parasite causing Bilharzia (schistosomiasis) in man,
TBT being used as a molluscicide to control the disease.
The toxic action of TBT is slow, the mortality at the end
of a 24-h exposure is often low, and for a more realistic
result a post-exposure observation period is required.
Rubber impregnated with TBT to produce a slow-release
molluscicide that maintains a low, but toxic, concen-
tration over a long period was developed by Cardarelli and
colleagues, and was shown to be very effective against
snail pest species such as Biomphalaria glabrata and B.
globosus (Berrios-Duran & Ritchie, 1968).
Molluscicidal activity has been demonstrated against
Bulinus spp., Biomphalaria spp., and certain operculate
freshwater molluscs, but organotin compounds have proved
to be not as toxic against the amphibious oncomelaniid
snails (McCullough et al., 1980).
8.2.2.1 Acute toxicity
Webbe (1963) found that young snails (Biomphalaria
sudanica and Bulinus nasutus) were more sensitive than
adults to TBT acetate, the 24-h LC50 values being 14 and
15 µg/litre for the two snail species, respectively.
When eggs from the same two species were exposed, the 24-h
LC50 values ranged from 100 to 1000 µg/litre. Paulini
(1964) found embryos of Biomphalaria glabrata to be more
susceptible than adults to TBT acetate. The 24-h LC50
values for embryos ranged from 26 µg/litre at 5-12 h of
age to 46 µg/litre at 77-87 h of age, whereas the value
for adults was 170 µg/litre.
Table 10. Acute toxicity of tributyltin to freshwater snails
---------------------------------------------------------------------------------------
Species TBT salt Exposure Post-exposure LC50 Reference
duration observation (µg/litre)
(h) (h)
---------------------------------------------------------------------------------------
Biomphalaria oxide 6 72 410 Seiffer & Schoof (1967)
glabrata acetate 6 72 290 Seiffer & Schoof (1967)
oxide 6 24 370 Ritchie et al. (1964)
oxide 24 24 40 Ritchie et al. (1964)
acetate 6 24 190 Ritchie et al. (1964)
acetate 24 24 85 Ritchie et al. (1964)
acetate 24 100-300 Hopf et al. (1967)
pentachloro-
phenate 24 100-400 Hopf et al. (1967)
oxide 24 50-100 Hopf et al. (1967)
oxide 24 30 Deschiens et al. (1966)
acetate 24 72 170 Paulini (1964)
Biomphalaria oxide 24 30 Deschiens et al. (1966)
contortus
Biomphalaria acetate 24 48 34 Webbe (1963)
sudanica
Bulinus nasutus acetate 24 48 32 Webbe (1963)
Bulinus oxide 17 24 10 de Villiers &
tropicus MacKenzie (1963)
Limnaea oxide 24 96 60 Temmink & Everts (1987)
stagnalis oxide 96 96 24 Temmink & Everts (1987)
oxide 96 42 RIVM (1989)
---------------------------------------------------------------------------------------
8.2.2.2 Short- and long-term toxicity
Ritchie et al. (1974) found that egg laying in
Biomphalaria glabrata was completely inhibited by 10 µg
TBTO/litre, all the snails being killed within 2 to 5
days. Egg laying was reduced, over a period of 2 to 3
weeks, by more than 90% at 1 µg/litre and by 50% at
0.1 µg/litre. At 0.01 µg/litre, egg laying was unaffec-
ted. Newly-laid eggs were exposed to TBTO for 34 days
followed by 50 days in clean water. Eggs exposed to 10 µg
per litre did not hatch, even after 50 days in clean
water. Of those exposed to 1 µg/litre, only 3% hatched
during the exposure and 35% of the rest hatched after
transfer to clean water (but with a delayed hatching
time). When newly-hatched snails were exposed to TBTO, 95%
of those exposed to 1 µg/litre from hatching died and
those that survived failed to lay eggs for 85 days. At
0.1 µg/litre, 60% of the snails died and egg laying in
the survivors was reduced by 80%. Egg laying was also
significantly reduced at both 0.01 and 0.001 µg/litre.
Upatham et al. (1980) studied the toxicity to Bulinus
abyssinicus of various controlled-release organotin mol-
luscicides, i.e. BioMet SRM rubber pellets (6% TBTO),
CBL-9B rubber pellets (20% TBT fluoride), and EC-13 float-
ing ethylene propylene co-polymer pellets (30% TBT fluor-
ide). The organotin compounds killed all of the snails
within 1 to 2 days at 100 mg/litre (active ingredient) and
within 5 to 7 days at 1 mg/litre, there being no signifi-
cant difference between the compounds. Changing the test
water daily had no significant effect. At lower concen-
trations the molluscicides required 36 to 40 days to kill
all the snails at an active ingredient concentration of
0.03 mg/litre, and at 0.3 mg/litre 9 to 10 days was re-
quired for BioMet SRM and CBL-9B, and 22 days for EC-13.
In long-term exposure tests, toxic effects on fresh-
water snails have been found at very low TBT concen-
trations. Cardarelli (1973) quoted a 120-day LC100 of
7 µg TBTO/litre for Biomphalaria glabrata. At 0.7 µg per
litre, 26% of the snails died within 120 days. RIVM (1989)
reported a NOEL for the freshwater snail Limnaea stagnalis
of 0.32 µg/litre in long-term tests.
8.2.2.3 Factors affecting toxicity
Paulini & de Souza (1970) studied the effect of
various factors on the molluscicidal activity of TBT to
freshly laid eggs of Biomphalaria glabrata. A concen-
tration of colloidal clay (the proportion of clay to
molluscicide was 1000:1) of 1000 mg/litre reduced the 24-h
LC50 (with a 7-day recovery period included in the mor-
tality count) by 30% for TBT acetate and by 7% for TBTO.
The addition of a yeast ( Saccharomyces sp.) suspension
(1 g/litre) reduced the 24-h LC50 (with a 24-h recovery
period) by 95% for TBTO and 72% for TBT acetate. The pro-
portion of yeast to molluscicide was 1000:1 for TBTO and
100:1 for TBT fluoride.
Cardarelli & Evans (1980) studied the effect of vari-
ous factors on the toxicity to snails of controlled-
release organotin molluscicides, i.e. BioMet SRM (6% TBTO
in natural rubber) and CBL-9B (20% TBT fluoride in natural
rubber). Using 100-mg/kg pellets, they found that increas-
ing the pH from 6 to 8 increased the toxicity (as measured
by both LT50 and LT100) of BioMet SRM but decreased
the toxicity of CBL-9B to both Biomphalaria glabrata and
Bulinus globosus. The authors found no effect of yeast or
humic acid (1 to 100 mg/litre) on the toxicity (LT100)
of these controlled-release molluscicides, neither did
they find an effect of suspended colloidal clays. They
concluded that, although the organotin compounds are
adsorbed, they are still toxic because the snails ingest
the added materials. Therefore, snails are still exposed
to TBT even after it is adsorbed to surfaces. In an exper-
iment to compare adsorbed uptake of TBT, soil-browsing
snails and isolated snails were compared during exposure
to slow-release organotin pellets. The molluscicides were
found to be more toxic when the snails were allowed to
browse on soil, and, at a distance of 60 cm from the pel-
lets, isolated snails were producing egg masses whereas
those browsing on soil were not. The authors also con-
cluded that the organotin molecules that come into contact
with soil particles are adsorbed and slowly form ligands
of a nontoxic nature, so that soil only remains toxic as
long as it is freshly exposed to organotin. Upatham et al.
(1980) found that the presence of mud or plant life,
compared to exposure in water alone, had no effect on the
toxicity (LT100) of either CBL-9B or EC-13 at 1 or 10
mg/litre (active ingredient) to Bulinus abyssinicus. When
the snails were exposed to BioMet SRM, no effect of mud or
plants was found at 10 mg/litre, but at 1 mg/litre a
slightly shorter time was required to kill all the snails
in water alone. Chu (1976) noted that organic materials
such as mud and weeds reduced the molluscicidal activity
of TBTO on Bulinus rohlfsi, when exposed to a rubber for-
mulation containing 5% TBTO (5 mg/litre TBTO), with a 24-h
exposure period every 3 to 5 days for 70 days and twice a
month for a further 7 months.
Macklad et al. (1983) found that the toxicity of
controlled-release molluscicides containing TBT fluoride
was dependent on the pre-exposure soaking time. The 48-h
LC50 for Biomphalaria alexandrina of 10 mg/litre total
available toxicant was achieved after 7, 3, and 1 days
soaking period for the formulations EC27 (10% TBT fluoride
in a plastic polymer), EC1320 (20% TBT fluoride in rub-
ber), and EC1330 (30% TBT fluoride in a polypropylene/
polyethylene mixture), respectively. EC1330 produced no
mortality following a 3-h soaking period and exposure to
concentrations ranging from 1 to 100 mg/litre for 48-h;
the authors suggested that the polypropylene/polyethylene
mixture (EC1330) needs to be sufficiently wet before it
begins to release TBT fluoride. A second experiment to
test the aging of slow-release molluscicides was carried
out. EC27 gave 89% snail mortality during a 48-h exposure
period to 50 mg/litre after a soaking time of 24-h. After
being left to age for 1 month the molluscicide was tested
again and had lost 80% of its original toxicity.
8.2.3. Marine molluscs
Summary
A large body of data exists on the effects of TBT on marine
molluscs and in particular on commercially important bivalves.
Sublethal effects occur at very low concentrations. It has been
shown experimentally that TBT affects shell deposition of grow-
ing oysters, gonad development and gender of adult oysters,
settlement, growth, and mortality of larval oysters and of
other bivalves, and causes imposex (the development of male
characteristics) in female gastropods. The NOEL for shell
thickening of the most sensitive oyster species (C. gigas) is
about 20 ng/litre. Embryo-larval stages are more susceptible
than adults; adverse effects on larval development have been
demonstrated at concentrations as low as 50 ng/litre. The NOEL
is 20 ng/litre.
The authors of recent work on imposex in Nucella have
determined threshold concentrations by extrapolation below the
limit of reliable detection. While the circumstantial evidence
in support is substantial (see chapter 10), the determination
of toxicological thresholds below the limits of chemical detec-
tion is not practicable. It is generally agreed that imposex
is not a specific index of TBT contamination, in that it can be
induced by other factors. The wide distribution of low concen-
trations of TBT and the incidence of imposex at levels similar
to or below the analytical detection limits make long-term
controlled experiments difficult. The establishment of NOELs
will have to await the development of better analytical tech-
niques.
8.2.3.1 Acute toxicity
Waldock & Thain (1985) calculated the 24-h EC50 (mor-
tality plus moribundity) of two organotin-containing fish-
net antifouling preparations for larval oysters to be
12 µg/litre for `Norimp 200' and 320 µg/litre for
`Flexgard'. These compare with a value for TBTO of 1.7 µg
per litre. Thain (1983) pointed out that adult bivalves
tend to appear more resistant to pollutants in standard
short-term tests since they can close their shell over the
test period and thus reduce exposure. Larval stages appear
to be much more sensitive in these tests.
8.2.3.2 Short- and long-term toxicity
Alzieu et al. (1982) kept Pacific oysters (Crassostrea
gigas) in 150-litre tanks that were successively filled
and drained according to the tidal period. To these tanks
were added panels coated on one side with TBT fluoride,
giving estimated concentrations of 0.2 and 2.0 µg TBT per
litre. All oysters died within 30 days in a tank contain-
ing the larger panel. In the tank with a panel surface of
50 cm2, 30% of oysters died after 110 days of exposure
and all oysters within 170 days.
His & Robert (1985) experimentally tested hypotheses
regarding the poor performance of Pacific oysters in the
Bay of Arcachon, France. Larvae were maintained in the
laboratory at TBT acetate concentrations ranging from 0.02
to 100 µg/litre. Growth was affected by all concen-
trations except 0.02 µg/litre. The next concentration
tested (0.05 µg/litre) reduced growth, led to mortality
within 10 days, and interrupted normal feeding by day 8.
No other effects were noted at 0.02 µg/litre and this was
regarded as the NOEL for larvae (see Table 11).
Table 11. Effects of TBT acetate on Crassostrea gigas larvae at
various water concentrationsa
--------------------------------------------------------------------------------
Water concentration Effect
(µg/litre)
--------------------------------------------------------------------------------
100 inhibition of fertilization
50 inhibition of segmentation
25 partial inhibition of segmentation (40%)
10 no formation of trochophores
3 to 5 no veligers; malformed trochophores
1 abnormal veligers; total mortality within 6 days
0.5 numbers of abnormal larvae; total mortality within 8 days;
perturbation of feeding regime, particularly from 4 to 8 days
after exposure; growth greatly reduced
0.2 percentage of D larvae showing abnormalities less elevated;
perturbation of feeding regime from day 4; progressive
mortality; total by day 12; weak growth
0.1 majority of D larvae normal; marked perturbation of feeding
regime from day 6; weak growth until day 6; some survivors
after 12 days
0.05 normal D larvae; perturbation of feeding regime, marked on day
8; significant mortality beginning at day 10; reduced growth
0.02 normal D larvae; little mortality; good growth; no effect of
TBT
--------------------------------------------------------------------------------
a From: His & Robert (1985).
Thain & Waldock (1985) exposed various bivalve spat
(common European oyster, Ostrea edulis; Pacific oyster,
Crassostrea gigas; common mussel, Mytilus edulis; and
carpet shells, Venerupis decussata and Venerupis semi-
decussata) to TBT leachate by maintaining them in flowing
sea-water tanks containing house slates painted with
`Micron 25R' (containing TBT methacrylate co-polymer). The
water concentrations of TBT were 0.24 or 2.6 µg/litre. At
2.6 µg/litre, growth was completely inhibited in all
groups and mortality was high (except for V. semidecussata)
within the 45-day exposure period; all mussels had died
within 14 days. At the lower exposure concentration,
growth was significantly inhibited in C. gigas, M. edulis,
and V. decussata but not in the other two species. In a
second study, the authors reported a severe reduction in
growth rate of recently metamorphosed oyster (Ostrea
edulis) spat after exposure to TBT leachate (0.06 µg per
litre) for 10 days under static conditions. Growth rate
was reduced between 0 and 10 days of exposure to 0.02 µg
per litre, but there was only slight reduction in growth,
relative to controls, between days 10 and 20.
Growth curves for oysters (Ostrea edulis), 2-3 mm in
size, exposed to different concentrations of TBTO are
given in Fig. 3.
 Valkirs et al. (1987) calculated the 66-day LC50 of
TBT chloride for the mussel Mytilus edulis to be 0.97 µg
per litre under flow-through conditions. Beaumont & Budd
(1984) kept larvae of the common mussel, Mytilus edulis,
in filtered sea water containing TBTO concentrations of
0.1, 1.0, or 10.0 µg/litre. No larvae survived for longer
than 5 days at 10 µg/litre, or 10 days at 1.0 µg/litre.
Approximately half of the mussel larvae, at 0.1 µg per
litre, had died within 15 days. The survivors were mori-
bund, and their growth was significantly slower than that
of controls.
Laughlin et al. (1987) exposed the hard-shell clam
Mercenaria mercenaria to various concentrations of TBTO,
under static renewal conditions for larval (veliger)
stages and in flowing sea water for juveniles. They found
the post-larval settlement stages to be the least sensi-
tive. In a 25-day exposure, only juvenile clams exposed
at 10 µg/litre suffered 100% mortality, while those
exposed to 7.5 µg/litre or less showed mortality not
significantly different from that of controls. When
veligers were exposed, none survived TBTO levels of 1 µg
per litre or more for longer than 7 days, mortality being
100% within 2 days at 2.5, 5.0, and 7.5 µg/litre. At the
end of the 8-day experiment, all controls had become
pediveligers. Clams exposed to 0.6 µg/litre showed a sur-
vival level of approximately 40% of the control level, but
survivors achieved little growth and metamorphosis to
pediveligers did not occur. In another set of studies
(Laughlin et al., 1987, 1988), clams were exposed, from
fertilization to metamorphosis (approximately 14 days), to
TBTO concentrations of between 10 and 500 ng/litre. Clams
were also exposed for the first 5 days and then kept in
clean water for a further 9 days. Survival was found not
to be exposure dependent and a recovery period had no
effect. Growth was reduced at all concentrations, higher
exposure causing greater growth depression. At TBTO con-
centrations above 100 ng/litre, veligers failed to meta-
morphose to pediveligers within the 14-day exposure
period. Although the 9-day recovery period caused a slight
increase in growth, the animals were not significantly
larger than clams exposed continuously.
Pickwell & Steinert (1988) exposed adult mussels
(Mytilus edulis) and oysters (Crassostrea virginica) in a
flowing sea-water system contaminated with TBT from panels
painted with antifouling paint. The TBT concentration was
0.7 µg/litre and exposure lasted for 60 days. Haemolymph
was collected from the exposed animals and its protein
content measured. Mussel haemolymph protein content at the
end of the exposure period was 462 mg/litre, compared with
44 mg/litre in controls. Measurement of haemolymph lyso-
zyme activity and DNA content revealed no difference
between controls and treated mussels. This showed that
there had been no lysis of the haemocytes and no increase
in their numbers. Protein was not, therefore, derived from
blood cells. Oysters showed no similar effect of TBT
exposure, although haemolymph protein content was much
higher than in mussels. Over the course of the experiment,
there was approximately 50% mortality in mussels but no
deaths among the oysters.
8.2.3.3 Reproductive effects
Thain & Waldock (1986) reported the results of studies
on the reproduction of the European flat oyster (Ostrea
edulis) exposed to TBT antifouling paints. Three holding
tanks were set up each with 50 adult oysters weighing
between 50 and 70 g. One tank held controls and the other
two were exposed to TBT leaching from painted panels in a
mixing tank. A flow rate of 1 litre/min was maintained and
the two treatment tanks showed TBT concentrations of 0.24
and 2.6 µg/litre measured at the outflow. Only control
oysters released larvae during the course of the 75-day
experiment; about five million larvae were released
representing probably between four and six spawnings. At
the end of the experiment, the gonads of oysters from each
treatment were examined histologically. There were no
females in either of the treated groups of oysters (20%
females in the control group). At 2.6 µg/litre, 18 out
of 25 oysters examined were undifferentiated, 7 were male,
and none were female. There were 3 undifferentiated gonads
out of 27 at the lower exposure level. Gonadal thickness
was reduced in a dose-related manner. Mortality was low
in all groups (0 in controls and 3 and 5 in the two treat-
ment groups). Shell growth was reduced by TBT exposure;
25 oysters showed growth in the control group, 18 at
0.24 µg/litre, and 4 at 2.62 µg/litre). There were no
significant differences between the various groups of
oysters using two measures of condition (dry meat weight
compared with internal shell volume or compared with wet
meat weight). Final body burdens of TBT were 0.19, 0.40,
and 1.23 mg/kg wet meat weight for control, low-dose, and
high-dose groups, respectively.
Roberts et al. (1987) maintained adult oysters
(Crassostrea virginica) in TBT solutions containing 0.05,
0.1, 0.5, or 1.0 µg/litre for up to 8 weeks. The oysters
were brought into reproductive condition by increasing
water temperature. There were no deaths except at the
highest exposure concentration, where 20% to 30% mortality
occurred between the second and fourth weeks of exposure.
Gametes stripped from the exposed oysters were fertilized
by gametes from a reference population. There was no evi-
dence that TBT exposure had any effect on the ability of
gametes to be fertilized, and there was no statistically
significant effect of treatment on the gender of exposed
oysters over the 8-week exposure period.
8.2.3.4 Effects on growth
Waldock & Thain (1983) maintained spat of the Pacific
oyster (Crassostrea gigas) in experimental tanks, contain-
ing either TBTO, TBT and marine sediment, or just sedi-
ment, for 56 days. Weekly growth measurements (measured as
wet weight) showed enhanced weight gain in oysters exposed
to sediment alone (50 or 100 mg/litre). Low levels of TBTO
(0.15 µg/litre) inhibited growth and showed pronounced
thickening of the upper valve, and severe inhibition of
growth was noted at 1.6 µg/litre. Addition of sediment,
along with the TBT, slightly reduced the adverse effect on
oyster growth. This experiment was conducted to counter
arguments that sediment caused the effects observed in
oysters in the field.
Thain (1986) exposed Eastern oyster (Crassostrea
virginica) spat to TBTO concentrations of 0.02, 0.2, or
2.0 µg/litre under static renewal test conditions for 5
weeks. The percentage increase in growth was substantially
reduced at 2.0 µg/litre, whereas at the other two concen-
trations growth rate was similar to that of controls. No
deaths occurred and there was no evidence of shell thick-
ening or deformity in any of the treated animals.
Lawler & Aldrich (1987) exposed Pacific oyster spat to
TBTO concentrations of 0.01, 0.02, 0.05, 0.1, or 0.2 µg
per litre and monitored the average rate of oxygen con-
sumption before and after exposure. A significant negative
correlation was found between TBTO concentration and oxy-
gen consumption. There was also a significant relationship
between TBTO concentration in the water and feeding rates.
Feeding rates were measured by transmittance, which
increases as particulate food matter is removed from the
water. Change in transmittance was monitored after 1 h of
feeding. As the TBTO concentration in the water increased,
feeding rate decreased. Neither oxygen consumption nor
feeding was significantly affected below 0.05 µg/litre.
Increasing the TBTO level also progressively decreased the
ability of the oysters to compensate for hypoxia; this
effect was significant down to 0.01 µg/litre. Growth rate
(measured as average increase in valve length) was moni-
tored over a 48-day exposure period. There was a signifi-
cant negative correlation between increasing TBTO concen-
tration and growth. Growth was not significantly affected
at the lowest concentration of 0.01 µg TBTO/litre. At
levels of 0.05 µg/litre or more, there was an increased
incidence of shell thickening. However, reservations must
be expressed regarding the experimental design of this
work. The authors did not analyse TBT concentrations in
the test solutions. In addition, no analyses were carried
out on the dilution water, which may have been contami-
nated by ambient TBT, typically in excess of 10 ng/litre
(the lowest threshold determined). Furthermore, the ratio
of biomass to water was such that TBT would have been
rapidly removed from solution, so that nominal concen-
trations would have been maintained for only a few hours.
Although experimental solutions were replaced daily, the
rate of loss invalidates the threshold values cited. Simi-
lar criticisms can be made of other work using static or
static renewal systems.
Valkirs et al. (1987) exposed adults of both the
common mussel (Mytilus edulis) and Eastern oyster
(Crassostrea virginica) to TBT concentrations of 0.04,
0.13, 0.31, 0.73, or 1.89 µg/litre, for a 66-day period,
under flowing sea-water conditions. The TBT consisted of
leachate from plastic panels painted with antifouling
paint. Growth effects were measured by shell length, shell
width, and whole body wet weight (soft tissues and shell).
Since there was high mussel mortality at a TBT level of
1.89 µg/litre, growth effects were examined in animals
exposed to TBT concentrations of up to 0.73 µg/litre. No
significant effect on mussel shell width or whole body
weight was found, but a significant decrease in shell
length was observed at 0.31 and 0.73 µg/litre. More than
90% of all oysters tested within each concentration sur-
vived the test period. Statistical analysis of length,
width, and weight of oysters could not be carried out
since controls were significantly different from exposed
groups with respect to initial length of individuals. A
condition index (ratio of wet body weight to internal
shell volume) was calculated for both mussel and oyster.
No significant difference was found at any test concen-
tration for mussels. For oysters, the mean condition indi-
ces were significantly lower at concentrations of 0.73 and
1.89 µg/litre, compared both with the indices at lower
TBT concentrations and with controls.
Salazar & Salazar (1987) exposed juvenile common
mussels (Mytilus edulis), in flowing sea water, to TBT
concentrations of 70, 80, or 200 ng/litre in a 196-day
test and 40, 50, or 160 ng/litre in a 56-day test. Mussel
growth (measured as wet weight and length) was not sig-
nificantly affected up to 56 days in either test. From 63
days to the end of the experiment, there was a significant
reduction in growth at all exposure concentrations. No
significant mortality was reported in either experiment. A
group of controls kept under "field" conditions had
growth rates four times that of the laboratory controls
over a 56-day period, suggesting that bioassay conditions
were stressful for the mussels.
Stromgren & Bongard (1987) exposed juvenile mussels
(Mytilus edulis) to TBTO concentrations of 0.1 to
10 µg/litre in flowing sea water and measured shell
growth (length) at intervals of 24 to 48 h for 7 days. No
effect was observed at the lowest exposure concentration,
but at 0.4 µg/litre or more there was a significant
reduction in shell growth rate. The relationship between
TBTO concentration and growth response was approximately
hyperbolic. After 7 days of exposure, all groups treated
with 0.4 µg/litre or more showed growth rates of approxi-
mately 25% (or less) of the control value. The highest
exposure concentration reduced growth to approximately 5%
of the control value.
8.2.3.5 Shell thickening
Alzieu et al. (1982) reported that adult oysters
(Crassostrea gigas) developed gel centres in the shell
when they were exposed to TBT fluoride at a concentration
of 0.2 µg/litre.
Thain et al. (1987) exposed spat of the Pacific oyster
(Crassostrea gigas) to TBT concentrations of 2 to 200
ng/litre for 49 days. No effect on shell thickness was
observed in controls or at 2 ng TBT/litre. Between 20 and
200 ng/litre, there was a dose-related increase in shell
thickness. Severe "balling" occurred at both 100 and 200
ng/litre (where the overall appearance of the oyster shell
is spherical rather than having the flattened profile of
one valve of a normal oyster) (Fig. 4).
8.2.3.6 Imposex
The phenomenon of "imposex" was first observed in
the field (see section 10.2), the term being used to
describe the development of male characteristics by female
gastropods. The females develop a penis and ultimately
become infertile. Stages in the development of imposex are
illustrated in Fig. 5.
Smith (1981a) collected female mud snails (Nassarius
obsoletus) from three localities designated "dirty",
"intermediate", and "clean" on the basis of the degree
of imposex noted in the field (see section 10.2). A piece
of filter paper with 1.5 to 1.8 g of dried antifouling
paint containing TBT and lead arsenate (Alumacide) was
placed in 110-litre tanks, and the snails were exposed for
75 days. Halfway through the exposure period, the filter
paper was removed because the snails became lethargic. At
the end of the exposure period, all snails exposed to the
Alumacide had developed significantly more intense impo-
sex. Snails from the "clean" area showed an increase in
imposex incidence from 0% to 14.3%, whereas levels of
imposex remained constant in snails from "dirty" or
"intermediate" areas (> 95% incidence). Penis expression
in snails from "dirty" and "intermediate" areas
regressed significantly when they were transferred to
clean water. However, the extent to which the effects
observed in this study can be attributed to TBTO is
unclear, since the antifoulant contained two biocides and
no analytical measurements were made.
Valkirs et al. (1987) calculated the 66-day LC50 of
TBT chloride for the mussel Mytilus edulis to be 0.97 µg
per litre under flow-through conditions. Beaumont & Budd
(1984) kept larvae of the common mussel, Mytilus edulis,
in filtered sea water containing TBTO concentrations of
0.1, 1.0, or 10.0 µg/litre. No larvae survived for longer
than 5 days at 10 µg/litre, or 10 days at 1.0 µg/litre.
Approximately half of the mussel larvae, at 0.1 µg per
litre, had died within 15 days. The survivors were mori-
bund, and their growth was significantly slower than that
of controls.
Laughlin et al. (1987) exposed the hard-shell clam
Mercenaria mercenaria to various concentrations of TBTO,
under static renewal conditions for larval (veliger)
stages and in flowing sea water for juveniles. They found
the post-larval settlement stages to be the least sensi-
tive. In a 25-day exposure, only juvenile clams exposed
at 10 µg/litre suffered 100% mortality, while those
exposed to 7.5 µg/litre or less showed mortality not
significantly different from that of controls. When
veligers were exposed, none survived TBTO levels of 1 µg
per litre or more for longer than 7 days, mortality being
100% within 2 days at 2.5, 5.0, and 7.5 µg/litre. At the
end of the 8-day experiment, all controls had become
pediveligers. Clams exposed to 0.6 µg/litre showed a sur-
vival level of approximately 40% of the control level, but
survivors achieved little growth and metamorphosis to
pediveligers did not occur. In another set of studies
(Laughlin et al., 1987, 1988), clams were exposed, from
fertilization to metamorphosis (approximately 14 days), to
TBTO concentrations of between 10 and 500 ng/litre. Clams
were also exposed for the first 5 days and then kept in
clean water for a further 9 days. Survival was found not
to be exposure dependent and a recovery period had no
effect. Growth was reduced at all concentrations, higher
exposure causing greater growth depression. At TBTO con-
centrations above 100 ng/litre, veligers failed to meta-
morphose to pediveligers within the 14-day exposure
period. Although the 9-day recovery period caused a slight
increase in growth, the animals were not significantly
larger than clams exposed continuously.
Pickwell & Steinert (1988) exposed adult mussels
(Mytilus edulis) and oysters (Crassostrea virginica) in a
flowing sea-water system contaminated with TBT from panels
painted with antifouling paint. The TBT concentration was
0.7 µg/litre and exposure lasted for 60 days. Haemolymph
was collected from the exposed animals and its protein
content measured. Mussel haemolymph protein content at the
end of the exposure period was 462 mg/litre, compared with
44 mg/litre in controls. Measurement of haemolymph lyso-
zyme activity and DNA content revealed no difference
between controls and treated mussels. This showed that
there had been no lysis of the haemocytes and no increase
in their numbers. Protein was not, therefore, derived from
blood cells. Oysters showed no similar effect of TBT
exposure, although haemolymph protein content was much
higher than in mussels. Over the course of the experiment,
there was approximately 50% mortality in mussels but no
deaths among the oysters.
8.2.3.3 Reproductive effects
Thain & Waldock (1986) reported the results of studies
on the reproduction of the European flat oyster (Ostrea
edulis) exposed to TBT antifouling paints. Three holding
tanks were set up each with 50 adult oysters weighing
between 50 and 70 g. One tank held controls and the other
two were exposed to TBT leaching from painted panels in a
mixing tank. A flow rate of 1 litre/min was maintained and
the two treatment tanks showed TBT concentrations of 0.24
and 2.6 µg/litre measured at the outflow. Only control
oysters released larvae during the course of the 75-day
experiment; about five million larvae were released
representing probably between four and six spawnings. At
the end of the experiment, the gonads of oysters from each
treatment were examined histologically. There were no
females in either of the treated groups of oysters (20%
females in the control group). At 2.6 µg/litre, 18 out
of 25 oysters examined were undifferentiated, 7 were male,
and none were female. There were 3 undifferentiated gonads
out of 27 at the lower exposure level. Gonadal thickness
was reduced in a dose-related manner. Mortality was low
in all groups (0 in controls and 3 and 5 in the two treat-
ment groups). Shell growth was reduced by TBT exposure;
25 oysters showed growth in the control group, 18 at
0.24 µg/litre, and 4 at 2.62 µg/litre). There were no
significant differences between the various groups of
oysters using two measures of condition (dry meat weight
compared with internal shell volume or compared with wet
meat weight). Final body burdens of TBT were 0.19, 0.40,
and 1.23 mg/kg wet meat weight for control, low-dose, and
high-dose groups, respectively.
Roberts et al. (1987) maintained adult oysters
(Crassostrea virginica) in TBT solutions containing 0.05,
0.1, 0.5, or 1.0 µg/litre for up to 8 weeks. The oysters
were brought into reproductive condition by increasing
water temperature. There were no deaths except at the
highest exposure concentration, where 20% to 30% mortality
occurred between the second and fourth weeks of exposure.
Gametes stripped from the exposed oysters were fertilized
by gametes from a reference population. There was no evi-
dence that TBT exposure had any effect on the ability of
gametes to be fertilized, and there was no statistically
significant effect of treatment on the gender of exposed
oysters over the 8-week exposure period.
8.2.3.4 Effects on growth
Waldock & Thain (1983) maintained spat of the Pacific
oyster (Crassostrea gigas) in experimental tanks, contain-
ing either TBTO, TBT and marine sediment, or just sedi-
ment, for 56 days. Weekly growth measurements (measured as
wet weight) showed enhanced weight gain in oysters exposed
to sediment alone (50 or 100 mg/litre). Low levels of TBTO
(0.15 µg/litre) inhibited growth and showed pronounced
thickening of the upper valve, and severe inhibition of
growth was noted at 1.6 µg/litre. Addition of sediment,
along with the TBT, slightly reduced the adverse effect on
oyster growth. This experiment was conducted to counter
arguments that sediment caused the effects observed in
oysters in the field.
Thain (1986) exposed Eastern oyster (Crassostrea
virginica) spat to TBTO concentrations of 0.02, 0.2, or
2.0 µg/litre under static renewal test conditions for 5
weeks. The percentage increase in growth was substantially
reduced at 2.0 µg/litre, whereas at the other two concen-
trations growth rate was similar to that of controls. No
deaths occurred and there was no evidence of shell thick-
ening or deformity in any of the treated animals.
Lawler & Aldrich (1987) exposed Pacific oyster spat to
TBTO concentrations of 0.01, 0.02, 0.05, 0.1, or 0.2 µg
per litre and monitored the average rate of oxygen con-
sumption before and after exposure. A significant negative
correlation was found between TBTO concentration and oxy-
gen consumption. There was also a significant relationship
between TBTO concentration in the water and feeding rates.
Feeding rates were measured by transmittance, which
increases as particulate food matter is removed from the
water. Change in transmittance was monitored after 1 h of
feeding. As the TBTO concentration in the water increased,
feeding rate decreased. Neither oxygen consumption nor
feeding was significantly affected below 0.05 µg/litre.
Increasing the TBTO level also progressively decreased the
ability of the oysters to compensate for hypoxia; this
effect was significant down to 0.01 µg/litre. Growth rate
(measured as average increase in valve length) was moni-
tored over a 48-day exposure period. There was a signifi-
cant negative correlation between increasing TBTO concen-
tration and growth. Growth was not significantly affected
at the lowest concentration of 0.01 µg TBTO/litre. At
levels of 0.05 µg/litre or more, there was an increased
incidence of shell thickening. However, reservations must
be expressed regarding the experimental design of this
work. The authors did not analyse TBT concentrations in
the test solutions. In addition, no analyses were carried
out on the dilution water, which may have been contami-
nated by ambient TBT, typically in excess of 10 ng/litre
(the lowest threshold determined). Furthermore, the ratio
of biomass to water was such that TBT would have been
rapidly removed from solution, so that nominal concen-
trations would have been maintained for only a few hours.
Although experimental solutions were replaced daily, the
rate of loss invalidates the threshold values cited. Simi-
lar criticisms can be made of other work using static or
static renewal systems.
Valkirs et al. (1987) exposed adults of both the
common mussel (Mytilus edulis) and Eastern oyster
(Crassostrea virginica) to TBT concentrations of 0.04,
0.13, 0.31, 0.73, or 1.89 µg/litre, for a 66-day period,
under flowing sea-water conditions. The TBT consisted of
leachate from plastic panels painted with antifouling
paint. Growth effects were measured by shell length, shell
width, and whole body wet weight (soft tissues and shell).
Since there was high mussel mortality at a TBT level of
1.89 µg/litre, growth effects were examined in animals
exposed to TBT concentrations of up to 0.73 µg/litre. No
significant effect on mussel shell width or whole body
weight was found, but a significant decrease in shell
length was observed at 0.31 and 0.73 µg/litre. More than
90% of all oysters tested within each concentration sur-
vived the test period. Statistical analysis of length,
width, and weight of oysters could not be carried out
since controls were significantly different from exposed
groups with respect to initial length of individuals. A
condition index (ratio of wet body weight to internal
shell volume) was calculated for both mussel and oyster.
No significant difference was found at any test concen-
tration for mussels. For oysters, the mean condition indi-
ces were significantly lower at concentrations of 0.73 and
1.89 µg/litre, compared both with the indices at lower
TBT concentrations and with controls.
Salazar & Salazar (1987) exposed juvenile common
mussels (Mytilus edulis), in flowing sea water, to TBT
concentrations of 70, 80, or 200 ng/litre in a 196-day
test and 40, 50, or 160 ng/litre in a 56-day test. Mussel
growth (measured as wet weight and length) was not sig-
nificantly affected up to 56 days in either test. From 63
days to the end of the experiment, there was a significant
reduction in growth at all exposure concentrations. No
significant mortality was reported in either experiment. A
group of controls kept under "field" conditions had
growth rates four times that of the laboratory controls
over a 56-day period, suggesting that bioassay conditions
were stressful for the mussels.
Stromgren & Bongard (1987) exposed juvenile mussels
(Mytilus edulis) to TBTO concentrations of 0.1 to
10 µg/litre in flowing sea water and measured shell
growth (length) at intervals of 24 to 48 h for 7 days. No
effect was observed at the lowest exposure concentration,
but at 0.4 µg/litre or more there was a significant
reduction in shell growth rate. The relationship between
TBTO concentration and growth response was approximately
hyperbolic. After 7 days of exposure, all groups treated
with 0.4 µg/litre or more showed growth rates of approxi-
mately 25% (or less) of the control value. The highest
exposure concentration reduced growth to approximately 5%
of the control value.
8.2.3.5 Shell thickening
Alzieu et al. (1982) reported that adult oysters
(Crassostrea gigas) developed gel centres in the shell
when they were exposed to TBT fluoride at a concentration
of 0.2 µg/litre.
Thain et al. (1987) exposed spat of the Pacific oyster
(Crassostrea gigas) to TBT concentrations of 2 to 200
ng/litre for 49 days. No effect on shell thickness was
observed in controls or at 2 ng TBT/litre. Between 20 and
200 ng/litre, there was a dose-related increase in shell
thickness. Severe "balling" occurred at both 100 and 200
ng/litre (where the overall appearance of the oyster shell
is spherical rather than having the flattened profile of
one valve of a normal oyster) (Fig. 4).
8.2.3.6 Imposex
The phenomenon of "imposex" was first observed in
the field (see section 10.2), the term being used to
describe the development of male characteristics by female
gastropods. The females develop a penis and ultimately
become infertile. Stages in the development of imposex are
illustrated in Fig. 5.
Smith (1981a) collected female mud snails (Nassarius
obsoletus) from three localities designated "dirty",
"intermediate", and "clean" on the basis of the degree
of imposex noted in the field (see section 10.2). A piece
of filter paper with 1.5 to 1.8 g of dried antifouling
paint containing TBT and lead arsenate (Alumacide) was
placed in 110-litre tanks, and the snails were exposed for
75 days. Halfway through the exposure period, the filter
paper was removed because the snails became lethargic. At
the end of the exposure period, all snails exposed to the
Alumacide had developed significantly more intense impo-
sex. Snails from the "clean" area showed an increase in
imposex incidence from 0% to 14.3%, whereas levels of
imposex remained constant in snails from "dirty" or
"intermediate" areas (> 95% incidence). Penis expression
in snails from "dirty" and "intermediate" areas
regressed significantly when they were transferred to
clean water. However, the extent to which the effects
observed in this study can be attributed to TBTO is
unclear, since the antifoulant contained two biocides and
no analytical measurements were made.
 Feral & Le Gall (1983) attempted to identify which
part of the neuroendocrine system of a marine gastropod,
Ocenebra erinacea, was primarily affected in the
induction, by TBT, of the development of a penis in female
snails. A biological assay was established using isolated
female pedal ganglia or complete nervous system (intact
complex of pedal ganglia and cerebropleural ganglia) of
Ocenebra erinacea and isolated presumptive penis-forming
areas of a second species Crepidula fornicata. When pedal
ganglia were cultured with the presumptive penis-forming
area in a medium based on either clean or "polluted" sea
water (from areas known to have the imposex phenomenon in
the field) there was no penis development. Culturing the
whole nervous system in a medium based on clean sea water
also resulted in no penis growth. However, culturing the
complete nervous system with a medium based on "pol-
luted" sea water caused the growth of a penis. Culturing
in artificial sea water with added TBT (0.2 µg per litre)
also induced penis development. The authors concluded that
the primary effect of TBT is on the cerebropleural ganglia
of the snails (Fig. 6).
In studies by Bryan et al. (1986), the dogwhelk
Nucella lapillus was exposed to TBT concentrations of
0.02 µg tin/litre in tidal tanks, the TBT being leached
from a co-polymer antifouling paint. Within 4 months,
animals of both sexes had accumulated 1 mg tin/kg (as
TBT), and females showed a high degree of imposex, which
was still increasing. The authors stated that the exper-
iment tended to underestimate the exposure, since the diet
of barnacles was initially uncontaminated but would later
have contributed TBT to the dogwhelks via an extra route.
Gibbs et al. (1987) found that after 12 months of exposure
to 18.7 ng tin/litre, penis size in female dogwhelks was
increased, and that there was very little difference in
size between the sexes. The "control" dogwhelks in this
study were actually exposed to TBT at 1.5 ng/litre, which
was the background concentration in the sea water used.
These control females showed a penis bulk of 10-14% of
that of control males. Field observations on populations
living in < 0.5 ng/litre showed little penis development
(between 2% and 5% of male penis bulk). The NOEL for the
development of imposex is, therefore, less than 1.0 ng
TBT/litre.
Gibbs et al. (1988) reared dogwhelks in the laboratory
for 2 years from hatching in various concentrations of TBT
leached from antifouling paints. TBT concentrations in the
water were monitored at 1-2, 3-5, 20, and 100 ng tin/litre
and tissue concentrations in the whelks were also
measured. All exposed females were affected at all concen-
trations, developing a penis. Penis development (expressed
as a relative size index compared to males exposed to the
same TBT concentrations) in females was between 50 and 60%
of male penis bulk after exposure for 1 and 2 years to TBT
at a level of 1-2 ng tin/litre. The female penis reached
comparable size to that of the male on exposure to TBT
levels of 3-5 ng tin/litre or more. At increasing TBT
exposure concentrations, further male characteristics
developed and further female characteristics were
repressed. At TBT concentrations in the water of 1-2 ng
tin/litre, some females retained the capacity to breed
although others were sterilized by oviduct blockage. At
3-5 ng/litre, virtually all females were sterilized but
oogenesis was apparently normal. At 10 ng/litre, oogenesis
was suppressed, oocytes were resorbed, and spermatogenesis
was initiated. At 20 ng/litre, there was a functional
testis in the "females", with ripe sperm in the most-
affected animals.
Feral & Le Gall (1983) attempted to identify which
part of the neuroendocrine system of a marine gastropod,
Ocenebra erinacea, was primarily affected in the
induction, by TBT, of the development of a penis in female
snails. A biological assay was established using isolated
female pedal ganglia or complete nervous system (intact
complex of pedal ganglia and cerebropleural ganglia) of
Ocenebra erinacea and isolated presumptive penis-forming
areas of a second species Crepidula fornicata. When pedal
ganglia were cultured with the presumptive penis-forming
area in a medium based on either clean or "polluted" sea
water (from areas known to have the imposex phenomenon in
the field) there was no penis development. Culturing the
whole nervous system in a medium based on clean sea water
also resulted in no penis growth. However, culturing the
complete nervous system with a medium based on "pol-
luted" sea water caused the growth of a penis. Culturing
in artificial sea water with added TBT (0.2 µg per litre)
also induced penis development. The authors concluded that
the primary effect of TBT is on the cerebropleural ganglia
of the snails (Fig. 6).
In studies by Bryan et al. (1986), the dogwhelk
Nucella lapillus was exposed to TBT concentrations of
0.02 µg tin/litre in tidal tanks, the TBT being leached
from a co-polymer antifouling paint. Within 4 months,
animals of both sexes had accumulated 1 mg tin/kg (as
TBT), and females showed a high degree of imposex, which
was still increasing. The authors stated that the exper-
iment tended to underestimate the exposure, since the diet
of barnacles was initially uncontaminated but would later
have contributed TBT to the dogwhelks via an extra route.
Gibbs et al. (1987) found that after 12 months of exposure
to 18.7 ng tin/litre, penis size in female dogwhelks was
increased, and that there was very little difference in
size between the sexes. The "control" dogwhelks in this
study were actually exposed to TBT at 1.5 ng/litre, which
was the background concentration in the sea water used.
These control females showed a penis bulk of 10-14% of
that of control males. Field observations on populations
living in < 0.5 ng/litre showed little penis development
(between 2% and 5% of male penis bulk). The NOEL for the
development of imposex is, therefore, less than 1.0 ng
TBT/litre.
Gibbs et al. (1988) reared dogwhelks in the laboratory
for 2 years from hatching in various concentrations of TBT
leached from antifouling paints. TBT concentrations in the
water were monitored at 1-2, 3-5, 20, and 100 ng tin/litre
and tissue concentrations in the whelks were also
measured. All exposed females were affected at all concen-
trations, developing a penis. Penis development (expressed
as a relative size index compared to males exposed to the
same TBT concentrations) in females was between 50 and 60%
of male penis bulk after exposure for 1 and 2 years to TBT
at a level of 1-2 ng tin/litre. The female penis reached
comparable size to that of the male on exposure to TBT
levels of 3-5 ng tin/litre or more. At increasing TBT
exposure concentrations, further male characteristics
developed and further female characteristics were
repressed. At TBT concentrations in the water of 1-2 ng
tin/litre, some females retained the capacity to breed
although others were sterilized by oviduct blockage. At
3-5 ng/litre, virtually all females were sterilized but
oogenesis was apparently normal. At 10 ng/litre, oogenesis
was suppressed, oocytes were resorbed, and spermatogenesis
was initiated. At 20 ng/litre, there was a functional
testis in the "females", with ripe sperm in the most-
affected animals.
 Bryan et al. (1988) investigated the capacity of vari-
ous organotin compounds to induce imposex in the dogwhelk.
Whelks, already slightly affected by imposex, were taken
from the wild and exposed to 200 ng/litre tin, in the form
of TBT chloride, tri- n- propyl tin (TPrT), tetrabutyltin
(TTBT), dibutyltin (DBT), or triphenyltin (TPhT), for 14
days before being returned to the shore. Penis size, as a
percentage of male penis size, increased to 44% in females
exposed to TBT chloride, compared with 6% in controls.
TPrT increased relative penis size to only 14%, and no
other compound had any effect. In an attempt to eliminate
differences due to differential uptake of the compounds,
the organotin compounds were injected in a second exper-
iment. The females were maintained in the laboratory for
up to 105 days. TBT again induced increased penis size but
TTBT also showed an effect (19.5% compared to the 34%
shown after TBT injection). Other compounds were ineffec-
tive. The authors believed the effect of TTBT to be caused
by contamination by TBT and conversion to TBT in the tis-
sues. However, the increased imposex after treatment with
TPrT could not be explained in this way and the imposex
effect is considered to be not totally specific to tri-
butyltin.
8.2.3.7 Genotoxicity
Dixon & Prosser (1986) found that TBTO was not geno-
toxic, at concentrations of 0.05 to 5.0 µg tin/litre, to
the larvae of the mussel Mytilus edulis. Results were
based on chromosome analysis and sister chromatid
exchange (SCE). In 4-day acute toxicity studies, TBTO was
found to cause a dose-dependent reduction in both larval
survival and development of mussels. Survival ranged from
46% of controls at 0.05 µg/litre to 1.4% at 5 µg/litre.
The percentage of animals reaching the D-shell stage of
development was 8.7% of controls at the lowest dose, but
none reached this stage at either 1 or 5 µg/litre. How-
ever, when mussel larvae were exposed to a standard
mutagen (mitomycin C) or crude oil, in the presence of
TBTO, the SCE frequency increased to approximately twice
that found when larvae were exposed to either toxicant in
isolation (Dixon & McFadzen, 1987).
8.2.4. Crustaceans
Summary
Most of the available data for freshwater organisms are
derived from acute exposure tests with Daphnia. LC 50 data for
similar exposure times are variable (up to 2 orders of magni-
tude) probably due to age differences in the populations
studied. The NOEL for Daphnia has been estimated to be around
0.5 µg/litre, behaviour being the most sensitive parameter.
A range of marine species from copepods to crabs and lob-
sters has been studied, the lowest NOEL being 0.09 µg per
litre for reproductive effects in mysid shrimp.
The presence of sediment in test aquaria greatly reduces
the toxic effect of TBTO on estuarine crustaceans.
8.2.4.1 Acute effects
Using TBTO and TBT acetate, Floch et al. (1964) calcu-
lated LC100 and LC0 values for various species of fresh-
water invertebrates. The lethal concentrations of TBTO to
Daphnia magna over 24 and 72 h were 0.12 mg/litre and
0.06 mg/litre, respectively. Another aquatic crustacean,
Cypridopsis hartwigi, was less sensitive with lethal con-
centrations of 4 mg/litre for a 24-h exposure, 2 mg/litre
for a 48-h exposure, and 0.12 mg/litre for a 96-h
exposure. NOELs were 0.03 and 0.06 mg/litre for Daphnia
and Cypridopsis, respectively. For TBT acetate, the
LC100 was 0.15 mg/litre for a 72-h exposure of Daphnia
and 0.15 mg/litre for a 96-h exposure of Cypridopsis. The
LC0 was 0.075 mg/litre for both species.
However, more recent work has found Daphnia to be con-
siderably more sensitive to TBT. Meador (1986) reported a
96-h LC50 for Daphnia magna of 5.9 µg/litre and noted
that TBT is a slow-acting toxicant for Daphnia with
effects only being shown after 96 h or more of exposure.
Polster & Halacka (1971) quoted 48-h LC50 values for
Daphnia magna using various TBT salts, which ranged from
2.2 µg TBTO/litre to 8.5 µg TBT oleate/litre.
RIVM (1989) reported a 48-h EC50 for Daphnia of
4.7 µg/litre and a NOEL, over the same time period, of
0.56 µg/litre.
Davidson et al. (1986) calculated the 96-h LC50 to
be 0.42 µg/litre after exposing the mysid shrimp
Acanthomysis sculpta to a leachate of TBT.
When Walsh (1986) exposed the mole crab Emerita
talpoida to concentrations of 10 µg TBTO/litre of sea
water or 4500 µg/kg of sand, no effect on crab survival
was observed after 7 days of exposure. In continuous-flow
bioassays, 10 000 µg TBTO/kg of sediment did not kill
grass shrimp after a 96-h exposure.
8.2.4.2 Short- and long-term toxicity
U'Ren (1983) maintained the marine copepod Acartia
tonsa in solutions containing TBTO, under static con-
ditions, and calculated a 144-day LC50 of 0.55 µg per
litre; by combining moribundity and mortality as end-
points, the 144-day EC50 was found to be 0.4 µg/litre.
Laughlin et al. (1983) maintained mud crab larvae
(Rhithropanopeus harrisii), from hatching, in solutions
containing either TBTO at 0.5 to 25 µg/litre or TBT
sulfide at 0.5 to 50 µg/litre, under static renewal pro-
cedures. The survival of the zoeae, up to 15 days, was
unaffected by TBTO at levels up to 10 µg/litre. At 15 µg
per litre, 84% successfully moulted to the megalopa stage,
but at 5 µg TBTO/litre only 37% survived. Zoeal sur-
vival was unaffected by concentrations of TBT sulfide up
to 5 µg/litre. Survival at 20, 30, and 50 µg/litre was
78%, 26%, and 4%, respectively. The development rate, over
the same time period, decreased with increasing TBT con-
centration, although this was not statistically signifi-
cant below 10 µg TBTO/litre or 20 µg TBT sulfide/litre.
At the highest exposure concentrations (10 µg TBTO/litre
and 20 µg TBT sulfide/litre), metamorphosis was delayed
by approximately 2 days in the case of TBTO and 6 days for
TBT sulfide. Growth (measured as mean wet weight) was
significantly reduced at TBTO concentrations of 15 and
25 µg/litre or TBT sulfide concentrations of 20, 30, and
50 µg/litre, and showed dose dependency. Daily growth was
monitored for up to 12 days at concentrations (of both
TBTO and TBT sulfide) of 0.5, 1.0, and 5.0 µg per litre.
Although the final weights were not significantly differ-
ent, all TBT treatments caused an initial growth lag
during the first three days of exposure. Laughlin et al.
(1985) found the LC50 for exposure of zoeae of
Rhithropanopeus harrisii to TBTO during the 12 days of
zoeal development to be 55 nmol/litre.
Laughlin & French (1980) exposed shore crabs
(Hemigrapsus nudus), 2 to 3 days after hatching, to TBTO
(as "Biomet") for up to 14 days under static conditions.
At the highest concentrations (500 and 1000 µg/litre),
all the zoeae died within 2 days. Survival time increased
as the concentration of TBTO decreased from 100 to 25 µg
per litre; most larvae died within 8 days even at 25 µg
per litre. The estimated values of LT50 for 100, 75, 50,
and 25 µg/litre were 3.4, 4.8, 5.8, and 6.2 days,
respectively. Lobster larvae (Homarus americanus) were
much more sensitive; 100% being killed within 24 h and
2 days by 20 and 15 µg TBTO/litre, respectively. Concen-
trations of 10 and 5 µg/litre killed all larvae within
5 to 6 days. In the group exposed to 1 µg/litre, there
was a similar mortality pattern to the controls and high
mortality at the first ecdysis (3 to 5 days post-hatch).
However, in this group only a single larva metamorphosed
successfully, compared with 43% in the control group.
Davidson et al. (1986) kept juvenile mysid shrimps
(Acanthomysis sculpta), newly-released from the female,
in TBT concentrations of between 0.03 and 0.48 µg per
litre for a 63-day period under flow-through conditions.
The TBT source was leachate from panels coated with anti-
fouling paint. All animals died within 7 days at the
higher exposure level. There was no significant difference
in shrimp mortality between those exposed at levels up to
0.38 µg/litre and the controls, either at 22 or 41 days.
From day 41 to the end of the test (63 days), survival
decreased at 0.38 µg/litre and only 22.5% survived to the
end of the experiment, compared with 60% in the control
group. The authors stated that this indicated a lowering
of the NOEL for the entire life cycle of A. sculpta from
0.38 µg/litre to 0.25 µg/litre at 41 days. The increase
in mortality coincided with the release of juveniles by
the females, which indicated a sensitive time in the life
cycle. Both mean length and weight of females, at a TBT
level of 0.38 µg/litre, were significantly reduced after
63 days of exposure, but no effect of TBT concentrations
up to and including 0.38 µg/litre was observed in males.
A similar result was found in another study after 28 days
at 0.49 µg/litre; again no effect on length or weight was
found in males. There was a significant effect on the
length of developing juveniles and sub-adults. After 14
days of exposure to either 0.19 or 0.33 µg/litre, mysids
were significantly shorter; this was also the case at
0.2 µg/litre after 27 days. At TBT concentrations up to
and including 0.33 µg/litre, there was no effect on the
number of juveniles released per individual female, the
number of individuals in unhatched broods, or the number
of days from hatching of a female to the release of its
juveniles. However, there was a significant reduction in
the number of viable juveniles released at both 0.19 µg
per litre and 0.33 µg/litre. The authors suggest a NOEL
of 0.09 µg/litre for reproduction, the most sensitive
parameter found in the study.
Laughlin et al. (1984) exposed the Baltic amphipod
Gammarus oceanicus to TBTO or TBT fluoride concentrations
of 0.3 or 3.0 µg/litre for 8 weeks, under 48-h static
renewal conditions. They also exposed the amphipods to
leachates from TBT-containing antifouling paints, by
placing a 1 cm2 plexiglass plate, which was painted with
either "Micron 25" or "Interracing", in the tank for
5 weeks. Again the water was changed every 48 h. TBT
levels in the experiment with pure compounds, remained
constant, but the TBT concentration (measured as TBTO)
from the leachates gradually increased over each 48-h
exposure period to approximately 5.5 µg/litre for
"Interracing" and 0.5 µg/litre for "Micron 25",
reflecting the different leaching rates of the two com-
pounds. At the highest concentration of both TBTO and TBT
fluoride, 50% of the adults died within 10 to 12 days of
the exposure and all had died within 16 days (TBTO) or 33
days (TBT fluoride). The TBT paint "Interracing" caused
100% mortality within 1 week. The lower concentration of
the exposures to pure compounds and "Micron 25" resulted
in mortality patterns that were not dependent on TBT con-
centration but on senility. The exposures to 0.3 µg per
litre and "Micron 25" had significantly reduced the
number of surviving larvae by the end of the experiment.
Slight decreases in larval growth were observed after
exposure to TBTO and "Micron 25" but, generally, concen-
trations of less than 1 µg TBT/litre had little effect
on growth and no effect on whole animal oxygen consumption
rates.
Clark et al. (1987) monitored the survival of the
grass shrimp (Palaemonetes pugio) in water and water/sedi-
ment test aquaria after the addition of TBTO. When added
to water and tested in the absence of sediment, TBTO gave
96-h LC50 values comparable to other published results at
20 µg/litre. However, when the TBTO was admixed with
sediment rather than water, the LC50 could not be deter-
mined. TBT levels in sediment at 1 mg/kg in static and
10 mg/kg in flow-through tests showed no effects on shrimp
survival. Similar tests using Amphioxus gave an LC50 for
sediment containing TBTO of between 1 and 10 mg/kg over 4
and 10 days. The animals were killed by 10 µg TBT per
litre in water.
8.2.4.3 Reproductive effects
Hall et al. (1988b) maintained egg-carrying females of
the copepod Eurytemora affinis in TBT chloride at levels
of 0.1 or 0.5 µg/litre for up to 13 days. After 3 days
of exposure, the higher concentration had significantly
reduced the mean brood size to 0.2, compared with 15.2 in
controls. Neonate survival was significantly reduced at
0.1 µg/litre after 6 days (22% of control survival). No
offspring survived the higher exposure concentration. In a
second experiment, mean brood size, after 2 days of
exposure, was not significantly affected at concentrations
of between 12.5 and 200 ng/litre. Neonate survival, after
13 days, was unaffected by concentrations up to and
including 50 ng/litre; survival at 100 ng/litre was 76%
(compared to 22% survival at the same exposure level, in
the first experiment, over 6 days) and was further reduced
to 24% at 200 ng TBT/litre.
When Johansen & Mohlenberg (1987) exposed fertilized
mature female copepods (Acartia tonsa) to TBTO, egg pro-
duction was significantly reduced at the highest exposure
level of 0.1 µg/litre after 72 h. After 120 h, all
exposure concentrations had significantly reduced egg
production by 18%, 19%, and 37% relative to controls, at
TBTO concentrations of 0.01, 0.05, and 0.1 µg per litre,
respectively.
8.2.4.4 Limb regeneration
Weis et al. (1987a,b) exposed the fiddler crab (Uca
pugilator) to TBTO concentrations of 0.5, 5.0, or 50 µg
per litre under static renewal conditions. Regeneration
(autotomy) of 1 chela and 5 walking legs was induced by
pinching them off at the merus. Although some growth
retardation was observed, the most striking effect was the
development in regenerated limbs of deformities, e.g.,
backward curling or complete absence of the dactyl of the
claw, chelae or walking legs, stunted, unjointed, or bent
in the wrong direction. The percentage of males exhibiting
deformities was 17% at 0.5 µg/litre, 24% at 5 µg per
litre, and 67% at 50 µg/litre, the test solution being
changed twice weekly. In a second experiment, where the
test solution was changed three times weekly, the percent-
age deformities were as follows: males, 100% at both 5 and
50 µg/litre; females, 29% at 5 µg/litre and 100% at
50 µg/litre. There were no deformities in the control
groups during the experiments.
8.2.4.5 Behavioural effects
When Pinkney et al. (1985) gave the grass shrimp
(Palaemonetes pugio) a choice between TBTO-contaminated
water and clean water, it did not avoid total organic tin
concentrations of 2.3 to 30 µg/litre. The response data,
at both 2.3 and 30 µg/litre, were very similar.
Meador (1986) reported the effects of TBT chloride on
the photobehaviour of water fleas (Daphnia magna). The
normal response of Daphnia to a unidirectional light
source is to swim away from the light; this is an adaptive
response to avoid predators. The author reported that
Daphnia exposed to TBT chloride showed a reversal of this
behaviour and swam towards the light source (usually with
considerably increased swimming intensity). The threshold
concentration of TBT chloride causing this behavioural
reversal was 0.5 µg/litre (the LC50 over the same time
period was between 3.5 and 6 µg/litre).
8.2.5. Other aquatic invertebrates
Summary
Few studies have been carried out on other species. The
lowest effect level in annelids was observed for Nereis
diversicolor; mortality and behavioural effects were seen after
chronic exposure to 2 µg/litre. Limb regeneration was signifi-
cantly inhibited in a brittle star after exposure to 0.1 µg
per litre. Experiments have been carried out on four insect
species. The most sensitive was Notonectes, the LC0 being
0.03 mg/litre and the LC50 0.06 mg/litre.
8.2.5.1 Acute effects
Walsh et al. (1986a) exposed larvae of the lugworm
Arenicola cristata to either TBTO or TBT acetate, for 96
or 168 h, respectively. The concentrations that killed
100% of the animals were: 4 µg/litre (96 h) for TBTO;
10 µg/litre (96 h) and 5 µg/litre (168 h) for TBT acet-
ate. At 5 µg TBT acetate/litre, all larvae were abnormal
after 96 h of exposure. No deaths or abnormalities re-
sulted from exposure to 2 µg TBTO/litre for up to 168 h.
When Beaumont et al. (1987) exposed adult polychaetes
(Nereis diversicolor) to TBT in flowing sea water for up
to 22 days, there was 100% mortality by 22 days at 4 µg
per litre and 55% mortality at 2 µg/litre (20% control
deaths). Eversion of the proboscis was a more sensitive
indication of toxicity. No controls showed this effect,
whereas 75% of animals exposed to 2 µg/litre showed
everted proboscis after 22 days (50% after 20 days of
exposure).
Dragonfly larvae (Aeschna) sp. showed a 48-h LC100 of
0.25 mg TBTO/litre and an LC0 of 0.12 mg TBTO/litre.
Corresponding values for TBT acetate were 0.5 mg/litre
(72-h LC100) and 0.025 mg/litre (LC0). Notonectes sp.
yielded LC0 values of 0.03 mg/litre for both TBTO and TBT
acetate and 72-h LC100 values of 0.06 and 0.15 mg/litre
for TBTO and TBT acetate, respectively. Chironomid (midge)
larvae revealed a NOEL of 0.075 mg/litre for TBT acetate
and a 48-h LC100 of 0.15 mg/litre (Floch et al., 1964).
Cardarelli (1978) studied the efficacy of controlled-
release organotin compounds as mosquito larvicides. The
toxicity (LT100) of BioMet (6% TBTO in natural rubber),
CBL-9B (20% TBT fluoride in natural rubber), and
ECOPRO-1230 and ECOPRO-1330 (both are ethylene propylene
polymers containing 30% TBT fluoride) were tested against
larvae of the mosquito Culex quinquefasciatus. For BioMet
and CBL-9B, LT100 values ranged from 4 to 9 days for
toxicant concentrations of 0.1 to 10 mg/kg of pellet added
to the water, the toxicity increasing with increasing
toxicant concentration. A degradation product, TBT carbon-
ate, was found to be less toxic, the LT100 being 9 days
after exposure to 10 mg/litre. When the polymers were
tested at toxicant concentrations ranging from 0.9 to 32.6
mg/kg, toxicity was found to increase with increasing
toxicant concentration (LT100s range from 2 days to 16
days). Although initially toxicity tended to decrease as
the soaking time of the pellet increased, prior to
exposure, between days 100 and 500 of soaking toxicity
remained relatively unchanged. The authors observed that
the organotin compounds dramatically slowed or even
prevented morphogenesis.
8.2.5.2 Limb regeneration
In a study on limb regeneration, Walsh et al. (1986b)
maintained the brittle star Ophioderma brevispina in TBTO
concentrations of 0.01, 0.1, or 0.5 µg/litre under flow-
through conditions. On the first day of the experiment,
autotomy was induced in two arms, at opposite sides of the
disc, by pinching midway between disc and arm tip. The
animals were then exposed for four weeks to TBTO. There
were no deaths and no effect on disc diameter. Both 0.1
and 0.5 µg/litre significantly inhibited regeneration of
arms as measured by length; both groups showed average and
median lengths less than those in the lowest-dose group,
but variability precluded statistical significance. Aver-
age weights of limbs were also reduced in the groups
exposed to 0.1 and 0.5 µg/litre, but statistical analysis
was not carried out because pooled weights did not provide
enough data.
8.3. Fish
Summary
The acute toxicity of tributyltin to marine and freshwater
fish is highly variable, LC 50 values ranging from 1.5 to
240 µg/litre. It is unclear whether this is due to inherent
differences in sensitivity or to differences in route of
exposure. Many of the acute toxicity studies need to be inter-
preted with care, since the TBT concentrations cited are often
nominal and biologically available concentrations were not
measured.
In long-term toxicity tests, the NOEL for general toxico-
logical parameters (growth and behaviour) for trout yolk-sac
fry was greater than 0.2 µg/litre, and for medaka it was
3.2 µg/litre. Using histopathological parameters, the NOEL for
medaka was found to be 0.32 µg/litre, vacuolation of the reti-
nal epithelium being the most sensitive parameter.
Few embryotoxicity tests have been performed and it has not
been possible to establish a NOEL.
8.3.1. Acute effects
The 96-h LC50 of TBTO for marine fish ranges between
1.5 and 36 µg/litre (Table 12). Larvae seem to be more
sensitive than adults in those few studies examining dif-
ferent life stages in the same test. Fewer LC50 values
have been published for freshwater fish; they range from
13 to 240 µg/litre.
8.3.2. Short- and long-term toxicity
Matthiessen (1974) measured a lethal threshold concen-
tration of TBTO for Tilapia mossambica of between 8 and
16 µg/litre (24-h LC50, 28 µg/litre; LC5, 24 µg per
litre; LC90, 33 µg/litre) as a preliminary to studies
at sublethal concentrations. A temporary opacity of the
surface of the eyes developed at concentrations below this
threshold (> 8 µg/litre) but disappeared after a few
days. Other symptoms included sluggishness and difficult-
ies with balance. Melanophores in the skin were found to
be constricted, giving treated fish a paler appearance
than controls. The fish did not produce behaviourally
related display patterns, an activity important in the
species. As with the eye opacity, these other symptoms
disappeared after a few days or weeks, suggesting that the
fish develop some tolerance to the TBTO. The growth of
Tilapia exposed to 0, 5, or 8 µg TBTO/litre was moni-
tored over a 5-week period. These concentrations of TBTO
were estimated, on the basis of release rates, in water
after the TBTO had been used to control mollusc vectors
for schistosomiasis. There was no difference in growth
between fish exposed to 5 µg/litre and controls. Tilapia
exposed to 8 µg/litre showed negative growth and lost
about 6% of body weight over the experimental period. Eye
opacity was only found in the fish exposed to 8 µg per
litre, but this effect did not seem to affect feeding
behaviour. The only other change in the high-dose group
was an increase in aggressive encounters between males.
There was an increased reluctance amongst attacked males
to avoid conflict, which led to the death of several indi-
viduals. Fatal engagements between males are normally very
rare in this species.
When Seinen et al. (1981) exposed rainbow trout (Salmo
gairdneri) yolk-sac fry to TBT chloride concentrations of
0.2, 1, or 5 µg/litre for up to 110 days, all fry died
within 10 to 12 days (at the transition from yolk-sac fry
to the swimming fry stage) at 5 µg/litre, but there were
no deaths at lower doses. The major histopathological
change in fish that died after exposure to 5 µg per litre
was hydropic degeneration of tubule segments of the pro-
nephros. At 0.2 and 1 µg/litre, there was a dose-related
retardation of growth, resulting in a 44% decrease in body
weight (relative to controls) in the 1 µg/litre group
after 110 days. At the end of the experiment, both remain-
ing groups showed significant reductions in the haemo-
globin titre in the blood and in body weight. Only at the
higher concentration was there a significant decrease in
blood cell number. Relative liver weight was significantly
increased at both concentrations but relative numbers of
thymus cells were unaffected. The authors also measured
the relative area distribution of the various hepatic com-
partments in liver sections. The area occupied by nuclei
significantly increased at both concentrations, whereas
glycogen storage area decreased, but this was significant
only at the highest exposure level. Cytoplasmic area was
unaffected, as were all non-parenchymal compartments.
Table 12. Toxicity of tributyltin to fish
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salinity pH TBT salt Dura- LC50c Reference
age flowa ature (o/oo) tion (µg/
(°C) (h) litre)
---------------------------------------------------------------------------------------------------------
Marine and estuarine species
Sheepshead minnow sub-adult flow 19.4- 9.8- 8.15- chloride 48 >31d,f Bushong
(Cyprinodon variegatus) 21.3 12.1 8.31 72 28.1 et al. (1988)
(24-36.5)d,f
96 25.9
(22.8-30.1)d,f
Bleak 8 cm stat 10 7 7.8 fluoride 96 6-8e Linden et
(Alburnus alburnus) oxide 96 15 al. (1979)
(13-17)e
Inland silverside larva flow 20 10 chloride 48 7.7 Bushong et
(Menidia beryllina) (5.5-10.9)d,f al. (1987)
72 4.6
(3.3-6.2)d,f
Atlantic silverside sub-adult flow 20 10 chloride 48 12.7 Bushong et
(Menidia menidia) (7.8-15.2)d,f al. (1987)
72 9.3
(7.1-12.4)d,f
96 8.9
(6.7-11.6)d,f
Atlantic menhaden juvenile flow 20 10 chloride 48 6.8 Bushong et al. (1987)
(Brevoortia tyrannus) (4.1-infinite)d,f
72 5.2
(3.7-6.5)d,f
96 4.5
(3.6-6.4)d,f
Sole larva statb oxide 48 8.5 Thain (1983)
(Solea solea) larva 96 2.1
adult 48 88
Armed bullhead adult statb oxide 48 26 Thain (1983)
(Agonus cataphractus) 96 16
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Girella 2.4 g statb 19.9- 7.8- oxide 48 5.2d Kakuno &
(Girella punctata) 20.5 8.1 96 3.2d Kimura (1987)
Saltwater goby statb 20.2- 32.5- 8.1- oxide 24 12d Shimizu &
(Chasmichthys dolichognathus) 21.0 32.8 8.3 48 9d Kimura (1987)
96 4d
Chinook salmon juvenile stat 3-5 28 oxide 6 54d Short &
(Oncorhynchus tshawytscha) 12 20d Thrower (1987)
96 1.5d
Mummichog larvae flow 19.4- 9.8- 8.15- chloride 48 >32.2d,f Bushong et
(Fundulus heteroclitus) larvae 21.3 12.1 8.31 72 28.2 al. (1988)
(15.1-infinite)d,f
larvae 96 23.4
(15.2-30.9)d,f
sub-adult 48 >32.2d,f
sub-adult 72 28.3
(23.4-43.2)d,f
sub-adult 96 23.8
(20.8-28)d,f
Freshwater species
Rainbow trout yearling stat 18 250 oxide 24 28e Alabaster
(Salmo gairdneri) yearling statb 18 250 oxide 48 21e (1969)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Guppy 3-4 weeks statb 23 oxide 96 21 RIVM
(Lebistes reticulatus) chloride 168 21 (1989)
(16-29)e,f Polster &
oleate 168 33 Halacka
(19-60)e,f (1971)
benzoate 168 25
(19-32)e,f
laurate 168 30
(18-50)e,f
acetate 168 28
(21-33)e,f
oxide 168 39
(28-50)e,f
Medaka 4-5 weeks statb 23 oxide 96 17 RIVM
(Oryzias latipes) (1989)
Stickleback 4-5 weeks statb 19 oxide 96 13 RIVM
(Gasterosteus aculeatus) (1989)
Carp oxide 24 75 Temmink &
(Cyprinus carpio) oxide 96 32 Everts
(1987)
Golden orfe oxide 48 50e Plum
(Leuciscus idus melanotus) napht- 48 70e (1981)
henate
Bluegill sunfish stat 20 oxide 96 240e Foster
(Lepomis macrochirus) (1981)
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
g Hardness expressed as mg CaCO3/litre.
Pinkney et al. (1988) exposed 13- and 16-day-old
larvae of striped bass (Morone saxatilis) to varying con-
centrations of TBT from methacrylate-painted panels for 6
to 7 days. They found significant reductions in survival
at measured concentrations of 0.766 µg TBT/litre or more.
At lower exposure concentrations (0.067 µg/litre), growth
parameters changed in the 13-day-old larvae only. No
changes occurred in the growth parameters of 16-day-old
larvae exposed to 0.444 µg/litre or less. The authors did
not know whether this apparent difference in sensitivity
between larvae of different ages was a true effect or
simply the result of differences between the various
batches of larvae.
Wester et al. (1988) reported NOELs for the medaka
(Oryzias latipes) of 3.2 µg TBTO/litre for general toxi-
cological parameters (mortality, growth, general appear-
ance, abnormal behaviour) and 0.32 µg/litre for histo-
pathological effects during 1 month of exposure. The most
sensitive histopathological effect was the development of
vacuolation in the retinal epithelium of the eye. There
was a dose-related increase in hepatocellular vacuolation,
with swelling in pronounced cases. The vacuoles appeared
to be glycogen deposits, although, at higher doses, lipid
vacuoles were also noted. The NOEL for liver effects was
1 µg TBTO/litre. Kidney effects mostly involved the
tubule and included dilation, epithelial atrophy, degener-
ation and regeneration, and proteinaceous casts including
cellular debris (tubulonephrosis). Severe cases also
showed glomerular effects. Similar lesions were reported
after 3 months of exposure, with, in addition, effects on
the swim bladder, skin, oral cavity, and thyroid gland at
the highest concentration tested (10 µg/litre). Thymus
atrophy, reported by the same authors in the guppy, was
not found in the medaka, indicating some species speci-
ficity regarding the effects of TBT. Increased glycogen in
liver and muscle after TBTO treatment was demonstrated
analytically in the medaka, confirming histochemical
suggestions of increased glycogen in the guppy.
Shimizu & Kimura (1987) exposed the marine goby
Chasmichthys dolichognathus to TBTO in short (4 day) and
long (12 week) experiments, and reported a 96-h LC50 of
4 µg/litre. Long-term exposure to 2.1 µg/litre during
the season of gonadal recrudescence led to a significant
depression of the gonadosomatic index in male fish. There
was no effect on female fish in terms of gonadosomatic
index or ovarian histology.
8.3.3. Embryotoxicity
Newton et al. (1985) exposed eggs, embryos, and larvae
of the California grunion (Leuresthes tenuis) to plates
painted with 9.4% TBT methacrylate (0.5% TBTO; 44.7% cu-
prous oxide; 45.5% inert ingredient) and aged for 30 days
in flowing sea water. The authors claimed that the ex-
posure to concentrations of between 0.14 and 1.72 µg TBT
per litre had no adverse effect on hatching, growth, or
development. In fact the presence of TBT at such concen-
trations enhanced both hatching success and stimulated
growth. A concentration of 10 µg TBT/litre had no effect
on hatchability, but at 74 µg/litre hatching success was
reduced by approximately 50%. The authors observed no
effect on the survival of larvae, hatched from eggs
exposed to concentrations of 0.14 to 1.72 µg/litre, up to
7 days post-hatch. The presence of copper, however, should
be taken into consideration in the interpretation of these
results.
Weis et al. (1987a) reported considerable variation in
the response of embryos of the killifish (Fundulus
heteroclitus) to concentrations of TBTO ranging between 3
and 30 µg/litre. Two batches of embryos developed abnor-
malities at the highest concentration of TBTO tested
(30 µg/litre) and showed some mortality. Other batches of
embryos showed embryotoxicity for all tested concen-
trations (down to 3 µg/litre) but no abnormalities in the
embryos. Owing to this difference in sensitivity of dif-
ferent batches of eggs and the separation of embryotoxic
and teratogenic effects, it is difficult to establish a
NOEL for this species.
Fent (1989b) exposed fertilized eggs of the freshwater
minnow Phoxinus phoxinus to two TBT concentrations in
petri dishes in an environment chamber. The eggs were ex-
posed from the blastula stage (20 to 24 h after spawning),
and the water was renewed daily. Analytical determination
of TBT showed that the actual concentration had reduced
over the 24-h exposure period from 1.5 to 0.5 µg/litre in
the low-dose group and from 8.4 to 7.9 µg/litre in the
high-dose group. There were no differences in survival
between the control and low-dose groups. Hatching was nor-
mal but some fish showed vertebral malformations. At the
high dose, hatching was delayed or reduced and all hatched
larvae had severe vertebral malformations; many were
unable to uncurl. These larvae remained motionless at the
bottom of the dish. Some had oedema in the region of the
heart. Within 3 to 4 days of hatching, all of the larvae
in the high-dose group had died. A further series of
experiments similarly showed malformation at an exposure
level of 4.5 µg TBT/litre.
8.3.4. Behavioural effects
The avoidance response of the mummichog (Fundulus
heteroclitus) to TBTO has been studied by Pinkney et al.
(1985). When given a choice between TBTO-contaminated and
clean water, 4 out of 6 groups of fish avoided a total
organic tin level of 1 µg/litre. There were significant
avoidance responses for all test groups at total organic
tin levels of 3.7, 8, and 13.8 µg/litre. However, the
higher concentrations of tin did not result in an increase
in avoidance response. The authors found that, under the
same conditions, no avoidance response was shown by the
grass shrimp (see section 8.2.4.5). This shrimp is a major
food item of the mummichog in tidal marshes. Hall et al.
(1984) studied the avoidance response of two species of
juvenile estuarine fish, the striped bass (Morone
saxatilis) and Atlantic menhaden (Brevoortia tyrannus). An
avoidance response to TBTO was exhibited by bass at a
total organic tin concentration of 24.9 µg per litre.
Atlantic menhaden were more sensitive, showing a "mild"
avoidance at 5.5 µg/litre and a "strong" avoidance at
9.1 µg/litre.
Chliamovitch & Kuhn (1977) measured an EC50 for the
loss of positive rheotaxis of 30.8 µg/litre for the rain-
bow trout (Salmo gairdneri) and 53.2 µg/litre for a
tilapia (Tilapia rendalli).
8.4. Amphibians
Summary
There are few data on the effects of tributyltin on amphib-
ians. Survival of frog larvae was at least 80% of control
levels after exposure to 3 µg TBT/litre.
Floch et al. (1964) reported a NOEL for mortality of
tadpoles of two species of amphibians (Rana and Alytes sp.)
of 30 µg/litre. For both species, the 24-h LC100 for
TBTO was 75 µg/litre, the 48-h LC100 50 µg/litre, and
the 48-h LC100 for TBT acetate was 75 µg/litre.
Laughlin & Linden (1982) exposed eggs of the frog Rana
temporaria to concentrations of either TBTO or TBT fluor-
ide of 0.3, 3, or 30 µg/litre, for 5 days from the post-
gastrula stage of development, under static renewal con-
ditions. All surviving larvae hatched on either day 4 or
5 of exposure. Survival was at least 80% of control levels
after exposure to 0.3 or 3 µg/litre. Only at the highest
concentration (30 µg/litre) did TBT affect survival (40%
mortality with TBT fluoride and 50% mortality with TBTO).
The authors collected all surviving tadpoles and measured
wet and dry weight. Wet weights were significantly lower
in tadpoles exposed to 30 µg/litre but unaffected at
lower exposure concentrations. Tadpoles exposed to TBT
always showed higher mean dry weight than controls,
although increases were not consistently dose dependent.
The percentage of body water declined from 86-88.5% for
controls to 73.8% after exposure at the highest concen-
tration of TBT. The changes resulting from TBT treatment
were significant, but not the differences produced by the
different TBT compounds.
8.5. Multispecies studies
Summary
The few available microcosm studies indicate that the most
sensitive organism(s) within the microcosm are affected by TBT
at concentrations greater than 0.05 µg/litre. Recovery of
these organisms occurred a few months after exposure. Simul-
taneous exposure of snails and fish yields conflicting results,
snails appearing somewhat more sensitive than fish.
Beaumont et al. (1987) exposed sandy-substrate micro-
cosms of flowing sea water to TBT derived from slate
panels painted with "Micron 25" antifouling paint. Three
replicate microcosms were exposed for 4 months at high
(1-3 µg/litre) and at low (0.06-0.17 µg/litre) TBT con-
centrations in water, together with three control micro-
cosms. Flow rates were maintained by gravity from a header
tank at approximately 1 litre/min; the painted panels were
located in the header tank where water was passed over
them using a circulating pump. The substrate sediment was
sieved after collection (to 2 mm) to remove larger organ-
isms and allowed to settle in the microcosms for 3 weeks
before the addition of animals. The sediment was 10 cm
deep and the overlying water 15 cm deep in each microcosm.
At the beginning of the trial, 50 specimens of a bivalve
(Cerastoderma edule), a crustacean (Corophium volutator),
and two polychaetes (Nereis diversicolor and Cirratulus
cirratus) were introduced. After 4 weeks, 12 specimens of
the gastropod Littorina littorea were added and, after 6
weeks, 26 specimens of a second bivalve (Scrobicularia
plana) were added. Other species were found at the end of
the trial derived from small (< 2 mm) juveniles in the
sediment and inflowing sea water. Each day, 10 litres of a
suspension of the microalga Pavlova lutheri (5 x 106 cells/ml)
were added to the header tank as additional food supply.
The most sensitive of the introduced species was the
cockle (C. edule); 100% died within 2 weeks at the high
concentration of TBT and there was cumulative mortality at
the low TBT concentration over 17 weeks (14% of controls
died). Two-way analysis of variance indicated significant
differences between all three groups (i.e. control, and
low and high concentrations of TBT; p < 0.05). The other
bivalve (S. plana) showed high mortality at the highest
exposure level (100% after 10 weeks). At the low TBT con-
centration there were no time-related deaths in this
species and no significant difference from controls.
Among the polychaetes, high death rates were recorded
for N. diversicolor in all microcosms, including the
control, possibly because adults introduced were ripe and
died after spawning during the experiment. The other
polychaete (C. cirratus) survived well even after exposure
to the high concentration of TBT. Only one gastropod (L.
littorea) died in any of the microcosms. Juvenile bivalves
were the most common species found; their numbers and
diversity were lower in the low-dose microcosms (66 to
109) than in control microcosms (137 to 179), and they
were virtually absent from the high-dose microcosms (0, 1,
and 1 for the 3 replicates). The size of juvenile mussels
(Mytilus edulis) was also significantly reduced at the
low TBT concentration, relative to controls; other self-
introduced bivalve species were not affected by the low
TBT exposure. Measurements of chlorophyll a in sediment
cores, as a measure of algal biomass, indicated a signifi-
cant rise after exposure to high TBT levels. This is
explained by the relative insensitivity of algae to the
toxic effects of TBT and by reduced (or non-existent)
animal life available to consume the algae. The authors
emphasize the sensitivity of some species, for which TBT
is toxic at levels of 1 to 2 µg/litre under conditions
approximating natural exposure. They further emphasize
the great variation in sensitivity between species; for
example, while Mytilus edulis and Cerastoderma edule were
clearly affected at very low levels of TBT, other related
bivalves were largely unchecked. Differences in feeding
behaviour, leading to different effective exposure levels,
is offered as one possible explanation for differences in
response. Differential absorption or subsequent loss,
observed in laboratory studies with other molluscs, is
also proposed as a possible mechanism.
Henderson (1986) conducted long-term flow-through
microcosm studies on communities of marine organisms from
Pearl Harbour, Hawaii, where the organisms had been
exposed to leaching TBT from naval ships for some time.
Panels (20 x 25 cm) of roughened plexiglass were suspended
in the harbour for 14 weeks prior to the experiment to
allow settlement and growth of organisms, and 30 different
species were found on the panels. Prior to exposure, the
panels were kept for a further 5 weeks in the experimental
tanks. These tanks had a capacity of 155 litre and were
supplied with sea water pumped directly from the harbour
and through the tanks at a rate of 4 litres/min. The sea
water was passed through a 1 cm mesh with no further
filtration; organisms could, therefore, enter the tanks
easily and settle on the panels. The water was rich in
plankton consisting largely of diatoms, copepods, and
chaetognaths. Exposure to TBT derived from painted panels
treated with antifouling paint containing 9.4% TBT meth-
acrylate, 0.5% TBTO, 44.7% cuprous oxide, and 45.4% inert
ingredients. Nominal concentrations of TBT were 0, 0.05,
0.13, 0.31, 0.78, and 1.95 µg/litre, but actual mean
concentrations were 0.01, 0.04, 0.10, 0.54, 1.77, and
2.52 µg/litre, respectively. The measured copper levels
in the same samples were 1.0, 1.2, 1.2, 3.1, 4.4, and
5 µg/litre, respectively. Changes in coverage of the
panels was assessed using photographs taken with an under-
water camera at weekly or biweekly intervals throughout
the experimental period of approximately 60 days of
exposure and a further 60 days of recovery. One week after
the start of exposure, a further plastic panel was intro-
duced to monitor new colonization. There was a clear
decline in both total number of species and in species
diversity (reductions of 55%, 60%, and 80% at the three
highest exposure levels, respectively) on pre-fouled
panels exposed to TBT at 0.5, 1.8, and 2.5 µg/litre but
no effect at lower concentrations. The mortality of indi-
vidual species, in relation to TBT exposure, showed con-
siderable variation. The most sensitive of the six most
common species on the panels was Botrylloides spp, an
orange-coloured colonial tunicate, which showed 100% mor-
tality at TBT concentrations of 0.1 µg/litre or more. An
encrusting bryozoan, Schizoporella errata, showed 49% mor-
tality at 0.1 µg/litre and 80 to 100% mortality at higher
concentrations. Specimens of a second colonial tunicate,
Didemnus candidum, and the saddle oyster ( Anomis nobilis)
were all killed at concentrations of 0.5 µg/litre or
more. A tube worm (Hydroides elegans) and a solitary
tunicate (Ascidia spp.) survived even the highest
exposure level, though with considerable mortality at 1.8
and 2.5 µg/litre. Recovery of populations was complete
60 days after cessation of treatment. Settlement on the
panels not previously exposed was reduced by TBT concen-
trations of 0.1 µg/litre or more, but not by 0.04 µg per
litre. Algal settlement and growth on the walls of the
tanks was not affected by exposure to TBT at any concen-
tration; coralline algae increased their coverage of pre-
fouled panels. A total of 18 oysters (Crassostrea
virginica) placed in each tank prior to treatment was
monitored after 60 days. There was 50% mortality after
TBT exposure at 1.8 µg/litre but no significant reduction
in survival at lower concentrations. However, the con-
dition index of the oysters was affected by exposure to
0.1 µg/litre or more, but had returned to near normal
after 2 months recovery in clean water. The author
regarded 0.05 µg/litre as a reliable NOEL for the most
sensitive organisms exposed.
When Salazar & Salazar (1985) exposed copepods
(Acartia tonsa), mysids (Acanthomysis sculpta), and fish
(Citharichthys stigmaeus) to suspended sediment at a TBTO
concentration of 0.49 µg/litre water for 96 h, no sig-
nificant mortality was found. In a second experiment,
mysids (A. sculpta), worms (Neanthes arenaceodentata), and
clams (Macoma nasuta) were exposed to TBTO-contaminated
sediment with overlying water for 10 days in the case of
mysids and 20 days in the case of clams and worms. Levels
of TBTO in the sediment varied from 155 to 610 µg per kg,
falling during the exposure period, while measured levels
in the overlying water were about 0.2 µg/litre. No mor-
tality was observed during the exposure period.
Cardarelli (1973) exposed snails (Biomphalaria
glabrata) and fish (Lebistes reticulatus) to slow-
releasing molluscicides containing either TBTO or TBT
fluoride. At a daily release rate of 35 µg TBTO/litre,
100% mortality was achieved in snails within 60 days but
all of the fish also died within this period. At a TBTO
release rate of 7 µg/litre per day, snail mortality was
100% after 120 days exposure and fish mortality only 2%.
However, a second experiment gave a much higher fish mor-
tality of 46% after 120 days exposure to only 3.5 µg TBTO
per litre per day. Exposure to TBT fluoride at 7 µg per
litre per day killed all snails and fish within 60 days,
and 52% of fish and 100% of snails were killed after 90
days of exposure to 3.5 µg/litre per day.
In order to simulate the effect of molluscicides con-
taining 6% TBTO on biota, Jordan (1985) set up a model
ecosystem as follows: filtered river water was allowed to
flow into a tray containing washed mud (to simulate a
marsh), and overflow from this upper tank was allowed to
flow into a lower tank containing washed coarse sand (to
simulate a river). Snails (Biomphalaria glabrata and
Potamopyrgus coronatus) and guppies (Lebistes reticu-
latus) were added to the "marsh", while snails (B.
glabrata and Pomacea glauca), shrimps (Macrobrachium
faustinum), and L. reticulatus were added to the
"river". The molluscicide was added to the "marsh" as
pellets at levels of 2, 5, 10, or 20 g/m2 of marsh sur-
face. The percentage of both B. glagrata and L. reticu-
latus surviving 14 days of exposure was recorded (see
Table 13). The authors concluded that, in this model eco-
system, there was higher mortality of B. glabrata than of
L. reticulatus at low doses of slow-release TBTO, i.e. 2
and 5 g of pellet per m2 of "marsh".
Table 13. Percentage of B. glabrata and L. reticulatus surviving
14 days exposure to slow-release pellets containing 6% TBTO added
to the "marsh" in a "marsh" or "river" model ecosystema
----------------------------------------------------------------
Dose of B. glabrata L. reticulatus
molluscicide
"marsh" "river" "marsh" "river"
----------------------------------------------------------------
20 g/m2 00.0* 00.0* 00.0* 00.0*
(0/240) (0/240) (0/240) (0/240)
10 g/m2 00.0* 00.0* 00.0* 2.5*
(0/240) (0/240) (0/240) (6/240)
5 g/m2 00.0* 16.3** 23.3** 60.0+
(0/240) (39/240) (56/240) (144/240)
2 g/m2 52.9+ 75.4++ 75.8++ 78.8
(127/240) (181/240) (182/240) (189/240)
----------------------------------------------------------------
a From: Jordan (1985).
The percentages represent percent survival at various doses
for all weeks of exposure in replicate experiments. The
values marked with the same symbol are not statistically
different from each other at the 0.05 probability level. The
numbers in parentheses represent the number of survivors
compared to the number of animals exposed.
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: TERRESTRIAL ORGANISMS
Summary
Exposure of terrestrial organisms to TBT derives primarily
from its use as a wood preservative. However, little infor-
mation is available. TBTO has proved toxic to honey-bees coming
into close contact with TBTO-treated wood. TBT compounds are
toxic to insects exposed either topically or via feeding on
treated wood. The acute toxicity to wild small mammals is mod-
erate (between 37 and 240 mg/kg per day). The toxicity of TBTO
to bats is probable but not proven. There is no information on
other species.
9.1. Microcosm studies
Gile et al. (1982) introduced TBTO into a terrestrial
microcosm on pine posts, each microcosm containing four
posts (3.3 x 2.6 x 14 cm) treated with 14C-labelled TBTO
(167 mg/cm3). The microcosms consisted of soil and
endogenous soil organisms, ryegrass, earthworms, pillbugs
(woodlice), mealworms, crickets, garden snails, and a
gravid female gray-tailed vole. There were no effects on
any of the organisms over a period of 77 days. About 95%
of the TBTO remained in the posts for the whole of the
exposure period. A similar system set up to investigate
cricket mortality, with and without predation, showed no
effect of TBTO (Gillett et al., 1983).
9.2. Terrestrial insects
When Gardiner & Poller (1964) exposed larvae of the
common clothes moth (Tineola bisselliella) to wool treated
with a TBTO concentration of 1% by weight, all of the
exposed larvae were killed within the exposure period of
14 days. The action appeared to be that of a contact
insecticide because none of the cloth was eaten. Phenyltin
compounds were not as toxic as TBTO. Baker & Taylor (1967)
found that the action of TBTO more closely resembled the
slow toxicity of a stomach insecticide after exposing
wood-boring beetles (Lyctus brunneus) to impregnated wood.
Contact insecticides such as lindane ( gamma-hexachlorocyclo-
hexane) and dieldrin were 100 times more toxic than TBTO.
The LD50 of TBTO for another wood-boring species, Anobium
punctatum, was 0.254 kg/m3 (application rate to wood).
Saxena & Crowe (1988) applied TBTO, TBT chloride, and
TBT linoleate topically to the thorax of newly-emerged
insects of three species. The LD50 values ranged from
0.48% to 0.72% (dilutions with acetone) for the house fly
Musca domestica, 0.29% to 0.69% for the mosquito
Anophelese stephensi, and 0.52% to 0.87% for the cotton
stainer Dysdercus cingulatus. TBT compounds were more
toxic than the other organotin compounds tested (triphe-
nyltin, tricyclohexyltin, dimethyltin, phenyltin, and
diethyltin). The authors pointed out that TBT compounds
are considerably less toxic than trimethyltin compounds to
Musca sp.
In studies by Kalnins & Detroy (1984), wood was
treated with TBTO (1.9 kg/m3) after sawing and before
use in the construction of beehives. Five hives made from
the treated wood were stocked with bees. Tin residues of
3.24 mg/kg were found in bees during the first summer, and
residues of 8.67 mg/kg were found in the wax of the combs.
However, there were no detectable tin residues in honey.
There was high mortality in the bee colonies over winter,
only one colony surviving. No control colonies died over
winter. Residues in bees and wax in the second year (1.3
and 4.6 mg/kg, respectively) were lower than in the first
year in the one surviving colony.
9.3. Terrestrial mammals
Racey & Swift (1986) housed pipistrelle bats
(Pipistrellus pipistrellus) in roosting cages treated
with TBTO. The bats were pregnant females collected from
nursery roosts, and they were trained to feed on mealworms
before transfer to the experimental cages. The cages were
made of metal, lined with plywood, and were painted with
TBTO as a 1% solution in white spirit (the manufacturers
recommended rate for use of TBTO as a fungicide). The wood
was treated 2 months before the bats were introduced into
the cages. During the course of the 142-day experiment,
seven of the ten bats died. Median survival time was 100
days, with a range between 49 and 142 days. Two deaths in
the white spirit control group and three in the untreated
control group meant that results for TBTO were not stat-
istically significant.
Schafer & Bowles (1985) conducted toxicity and repel-
lency tests on deer mice (Peromyscus maniculatus) and
house mice (Mus musculus) using various TBT salts. Treat-
ment of feed seeds with 2% (by mass) produced clear repel-
lent effects of TBT in both species. The approximate
lethal dose (estimated by increasing the TBT dose until
the test animals died) for TBT acetate and fluoride was
320 mg/kg. The estimated dietary LC50, based on consump-
tion of treated seed used in the repellency tests, varied
between 37.5 mg/kg per day for TBT acetate and 238 mg/kg
per day for fluoride and sulfate. The LC50 for TBTO was
200 mg/kg per day.
10. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: FIELD OBSERVATIONS
Summary
Tributyltin compounds have had a wide range of uses. Most
concern has focussed on their use in the marine environment,
where TBT has been associated with mortality and failure of
settlement of bivalve larvae, reduced growth, shell thickening,
and other malformations in oysters, imposex (the development of
male reproductive appendages in female animals) in mud snails,
and imposex concurrent with population decline in dogwhelks.
Controls on the use of TBT in antifouling paints has led to
recovery of economically important shellfish growth and repro-
duction. Water concentrations of TBT are still high enough in
some areas to affect marine gastropods.
Field and laboratory results for marine molluscs are in
good agreement. Both imposex in dogwhelks and shell growth and
chambering in Pacific oysters are effective biological indi-
cators of TBT contamination.
There have been few studies into the effects on organisms
of TBT in sediment. There are indications that TBT is bioavail-
able to burrowing organisms and can cause mortality in the
field.
Gross toxic effects and histopathological changes have been
reported in farmed marine fish exposed to TBT through the use
of antifouling paints on retaining nets.
Although TBT has been detected in fresh water at high con-
centrations in some areas, there have been few studies on its
effects. Spills of large amounts of TBT from timber treatment
plants have caused ecological damage, but recovery occurred
within 9 months.
Field testing of tributyltin derivatives, mainly slow-
release formulations of TBTO, has shown that it is difficult to
apply TBT without damaging non-target organisms. Recovery
occurs through recolonization.
10.1. Effects on bivalves
In the 1970s, the French oyster industry was
undergoing a crisis. During the 1977, 1978, and 1979
seasons, very poor spatfalls were reported, together with
increasing reports of poor shell growth and shell malfor-
mations. An extensive survey of the occurrence and inten-
sity of malformations related to metal residues in oysters
suggested, for the first time, a connection with organotin
compounds (Alzieu, 1981). The shell thickening was found
to be due to the appearance of chambers in the oyster
shell and interlamellar gel formation in these cavities
(Alzieu, 1981; Alzieu et al., 1982). The authors of these
reports described in detail the shell abnormalities and
the process of calcification of the oyster shell, and
suggested mechanisms of TBT action. TBT is known to in-
hibit oxidative phosphorylation and it has been suggested
that this forms the basis of its action on the shell. It
is also known to complex amino acids. The effect on calci-
fication derives from inadequate calcium addition to the
organic matrix (a process dependent on ATP) and incorrect
deposition of this matrix. Alzieu et al. (1982) found good
correlation between the occurrence of shell thickening and
the proximity of ports where large numbers of boats were
usually moored. These field observations were corroborated
by the finding of similar shell abnormalities in the lab-
oratory when oysters were exposed to TBT fluoride, an
organotin compound present in antifouling paint used on
the boats. Oysters placed in flowing sea-water tanks con-
taining plates coated with TBT antifouling paints died
after a 30-day exposure to an estimated water concen-
tration of 2 µg/litre (organotin leachate) and shell
thickening was found to occur at water concentrations of
0.2 µg/litre.
Alzieu et al. (1982) assessed the shell quality of
18-month-old Pacific oysters ( Crassostrea gigas; also
known as the Japanese oyster) sampled along the eastern
coast of Oleron Island and in the vicinity of La Rochelle,
France. They concluded that the proximity of pleasure
craft ports or a commercial harbour could badly affect the
quality and growth of oyster shells. However, the presence
of other chemicals, along with TBT in the sea water made
direct assessment difficult. The authors, therefore,
carried out a set of experiments to confirm their
conclusions. Groups of oysters from areas with no shell
abnormalities were distributed to other locations, i.e. a
marina, a river, and the laboratory (three groups: a
control group; a group exposed to 50-cm2 panels coated
with TBT fluoride; and a group exposed to coated panels
500 cm2 in area). All oysters died within 30 days after
exposure to the larger panel in the laboratory and within
170 days after transfer to the marina. Oysters exposed to
the smaller panels showed a mortality rate of 30% after
110 days of exposure. Oysters transferred to the marina
site and those exposed to 50-cm2 TBT-coated panels devel-
oped gel-filled shell cavities after 100 and 110 days,
respectively, during the period of shell growth. Analysis
of the oysters for total tin content revealed levels of
< 1 mg/kg (dry weight) in "unpolluted" groups, 110 mg/kg
after 80 days at the marina site, and up to 25 mg/kg after
exposure to 50-cm2 panels in the laboratory.
Maurer et al. (1985) found TBT levels to be related to
inhibition of settlement of Pacific oyster in the Bay of
Arcachon and the Gironde estuary, France. The authors used
arrays of settling tubes mounted around a central tube
which was painted with "International TBT Antifouling"
at a rate of 6.4 g of paint on a surface of 8 dm2. The
settling tubes were mounted at varying distances (between
4.5 and 13 cm) from the central painted tube. In control
arrays, the central tube was unpainted. The arrays of
tubes were placed out in the two study areas in July 1982
and observations on settlement and growth were made in
September and November 1982. In the Gironde region, a
second method was also used; slates were painted with TBT
paint (21 g of "International TBT Antifouling" on a
surface of 26 dm2) and mounted 10 cm apart, alternating
unpainted with painted slates on a rod. The spacing of
tubes in the arrays was up to 25 cm from the central
painted tube. In the Gironde estuary, settlement was com-
parable with controls except on the painted tube; tubes
5 cm or more away from the TBT paint had similar numbers
of settling larvae as the control. However, deaths among
the settled larvae were high; 100% on tubes 15 cm away (or
less), 99.3% on the tube at 20 cm distant, and 78.3% on
the tube 25 cm distant from the paint. Slates showed high
settlement rates and a mortality of 82.5% at 10 cm away
from the paint. In the Bay of Arcachon, there was a much
lower settlement of larvae; in the Villa Algerienne area
there were 178 settlements/dm2 on the treated tubes
compared with 440 on controls, and in the Comprian area
81 or 83 settlements/dm2 on treated tubes compared with
323 on controls. Barnacles, which were less sensitive to
the paint, were also found to settle on the tubes. The
settlement period in this area was longer than that in the
Gironde estuary; some larvae settling later in the season
showed reduced growth compared with controls.
Thain & Waldock (1986) reported that the Pacific oys-
ter was introduced into the United Kingdom during the mid-
1970s. At this time, growth trials were performed at sev-
eral coastal locations. The oysters grew well at most
sites, but at some east coast sites, such as the estuary
of the River Crouch, they exhibited poor growth, reduced
meat yield, and shell thickening. At other sites, such as
the north Norfolk coast, there were good growth results
with none of the deformities found at the Crouch. The
cause of the poor growth results in some areas was inves-
tigated, and Key et al. (1976) found good correlation
between poor growth and high levels of fine suspended
particles. Areas of poor performance in the trials were
not used for the cultivation of the newly-introduced
oyster species. The types of shell abnormality exhibited
by oysters in France were very similar to those observed
in oysters from the east coast of England, which had been
attributed to sediment. The sediment in the marinas used
by Key et al. (1976) for resettlement studies was probably
contaminated with TBTO (Personal communication by M.J.
Waldock to IPCS). In 1982 it was decided to reassess the
causes of poor oyster shell growth in Britain. Levels of
TBT were measured in the estuaries of the Rivers Crouch
and Blackwater, on the east coast of England, and were
found regularly to exceed 0.2 µg/litre, a level shown by
the French studies to be harmful to the oyster (Thain &
Waldock, 1986). In a laboratory study, Waldock & Thain
(1983) investigated the effect of both suspended sediment
and TBT on growth and shell thickening in spat of the
Pacific oyster. They found that TBT levels of 0.16 µg per
litre inhibited and 1.6 µg/litre stopped growth. Shell
thickening was observed in the oysters exposed to TBT.
Exposure to sediment in "clean" water (i.e. containing
no TBT) actually enhanced growth. Thain & Waldock (1986)
reported that, during 1983, the laboratory finding that
TBT and not suspended sediment was affecting growth and
causing shell abnormalities in oyster was corroborated in
the field by studying oysters from different sites.
Alzieu & Portmann (1984) reported that although the
findings clearly implicated TBT as a major cause of growth
problems and abnormalities, they did not completely
exclude the possibility that other chemicals present might
have caused similar effects. They reviewed the effects of
a 1982 ban by French authorities on use of TBT paints on
boats shorter than 25 m. In the Bay of Arcachon area
(where, in 1980 and 1981, 95% to 100% of oysters had shown
deformities), the 1982 figures for deformities were 70% to
80% and by 1983 deformities had declined to 45% to 50%.
The number of oysters from the same area showing deform-
ities in both upper and lower shells was between 70% and
90%, in 1980 and 1981, and zero by 1983. The spatfall,
which in both 1980 and 1981 failed completely, sub-
sequently recovered; it was described as good in 1982 and
excellent by 1983. The authors also reported, however,
that these results were not reflected in another area
(La Rochelle Bay). This was attributed both to its close
proximity to a major commercial harbour and the fact that
the ban on the use of TBT antifouling paints on pleasure
craft in this area had not been as strictly observed as in
the Bay of Arcachon.
His et al. (1986) reported bioassays conducted on
oysters (Crassostrea gigas) using sea water collected from
the Bay of Arcachon both before and after the imposition
of a ban on the use of TBT paints on small boats. The sea
water collected in 1981 caused abnormalities in 40% of
larval oysters over 12 days of observation (compared with
4% in controls), whereas the sea water from 1982 produced
only 12% abnormalities over the same period. Growth of the
oyster larvae was also improved relative to the period
when TBT paints were still being used. In 1981, mean
growth of larval shells over 12 days was 133 µm, in Bay
of Arcachon sea water, compared with 142 µm in controls.
In 1982, mean growth was 162 µm, as opposed to 168 µm in
controls. Alzieu et al. (1986) stated that between 1980
and 1982 about 90% of oysters displayed anomalies in shell
calcification. Each year anomalies became apparent in
April and reached maximum intensity during June and July.
During the period 1983 to 1985, the percentage of oysters
displaying shell anomalies fell steadily. By 1985 none of
the oysters examined had malformations in both valves and
less than 40% had shell anomalies in one of the valves
(usually the upper valve). Alzieu et al. (1989) monitored
oysters in 1986 and 1987 and found that, although oysters
with deformities in both valves had stabilized at < 10%,
those with deformities in at least one valve had risen
again to a peak of 70% for both years.
Effects on oysters have also been reported near to
fish farms containing nets treated with TBT antifoulants.
Davies et al. (1987a) maintained caged Pacific oysters
(obtained from a nursery unit distant from any significant
sources of TBT) at varying distances from fish farms at
Loch Sween, Scotland. They found that significant accumu-
lation of tin was restricted to within 200 m of the fish
farms and that effects on oyster shell structure were
observed at a distance of 1000 m but not at 5000 m. A com-
parison of shell thickness index and tin accumulation
showed significant tin accumulation in those oysters with
the most severe shell thickening.
Studies in the USA and Japan have revealed similar
effects. Stephenson et al. (1986) transplanted culched and
culchless Pacific oysters (Crassostrea gigas) and two
species of mussel (Mytilus edulis and Mytilus
californianus) to areas of San Diego Bay, California, USA,
along a gradient of known sea-water TBT concentrations
(0.01-0.93 µg/litre). Reduced shell growth was observed
in all three species in areas where TBT levels were
highest. Oyster and Californian mussel samples (but not
M. edulis ) showed marked trends of reduced growth with
increasing TBT levels. Shell thickening in the oysters
also correlated with increasing TBT levels. Wolniakowski
et al. (1987) found Pacific oysters in Coos bay, Oregon,
USA, to have thickened and ball-shaped shells. When these
oysters were analysed, they were found to contain high
levels of TBT (49.7 to 189 µg/kg). The most marked
deformities occurred in animals collected in a marina and
near to where boats were painted. Okoshi et al. (1987)
transplanted spat of two different strains of Pacific
oysters (Crassostrea gigas) to two different experimental
field sites in northern Japan for 41 weeks. The number of
chambers in the oyster shells increased gradually in the
Miyagi strain at one site. In contrast, few chambers were
observed in the Hiroshima strain at either site. The
authors concluded that both genetic and environmental
factors were involved in the formation of shell chambers.
Crassostrea gigas is a non-indigenous species in the
United Kingdom and France. Stocks for breeding were intro-
duced into both countries in the late 1960s and originated
from the Miyagi region of Japan (Walne & Helm, 1979).
When Paul & Davies (1986) maintained scallops (Pecten
maximus) and Pacific oysters (Crassostrea gigas) in nets
coated with a TBTO-based paint, the mortality of scallop
spat was 24%, compared with a control mortality of less
than 6%, over the 31-week exposure period. Adult scallop
mortality was less than that of controls in the treated
group, and no effect was observed on growth. No oysters
died. Scallop spat in TBT-treated nets grew significantly
less rapidly than the control spat. Oyster growth, as
measured by mean shell length, was significantly reduced
and had largely ceased after 10 weeks of exposure. A sig-
nificant thickening of the oyster shell was observed
within 10 weeks of exposure and continued throughout the
31-week exposure. It was maintained even after transfer to
untreated nets for 10 weeks.
Minchin et al. (1987) monitored bivalve settlement in
Mulroy Bay, Ireland, between 1979 and 1986 using settling
panels placed in the sea. They found that, during this
period, settlement either failed or was reduced. Scallop
numbers fell from an average of > 1000 per panel in 1979
to zero in 1983. In 1984, no settlement of any bivalve
species was recorded on the panels. This reduced settle-
ment corresponded to the introduction of organotin fish-
net dips in local salmonid farms. The first use of TBT
paints appears to have been in 1981. This use of organotin
compounds ceased after 1985. In 1986, in all bivalves
monitored (except flame shells), there was again good
settlement. Scallops (Pecten maximus) and flame shells
(Lima hians and Chlamys varia) (all members of the
Pectinacea) were found to be particularly sensitive to
organotin compounds. It is not known whether the effect of
the TBT was on the reproduction of the bivalves or a toxic
effect on the larvae.
10.2. Effects on gastropods: imposex
Between the years 1972 and 1976, Smith (1981c)
sampled mud snails (Nassarius obsoletus) from four
locations bordering Long Island Sound in Westport and
Fairfield, Connecticut, USA. The snails were examined
for imposex (see section 8.2.3.6), and the results were
quantified by measuring the percentage of snails in a
sample showing any imposex and estimating the degree of
imposex within indi- vidual snails. In two of the
areas, adjacent to a yacht yard in Southport Harbour and
at the mouth of the harbour, 95% to 100% of snails had
some degree of imposex. Both showed significantly
more imposex than the other two sites, an area used
to moor a few old boats and an area protected from human
interference. The mud snails in this last area showed no
imposex. The area with a few old boats showed imposex
levels of 30% to 50%, much less than in the first two
areas. Smith (1981a) collected mud snails from three of
the locations, the yacht yard in the harbour (where
all female snails were abnormal and degree of imposex
greatest), the mouth of the harbour (where almost all
snails were abnormal but the degree of imposex changes
was intermediate), and the area protected from human
impact (at least 3.5 km from the nearest marina, where
all female snails were normal). The author labelled
these areas as "dirty", "intermediate", and "clean".
Snails transferred from the "clean" area to the
"dirty" area developed imposex. In those transferred
from the "dirty" area to the "clean" area the degree
of imposex was reduced. An analysis of chemicals
present in water from the "dirty" area was then carried
out and snails were exposed to some of the
contaminants individually, i.e. marina disinfectants,
detergents, copper antifouling paints, leaded
gasoline, combustion emissions, and two types of
TBT-based antifouling paints. Only the tin-containing
antifouling paints increased the level of penis
expression in female snails.
In a survey of the dogwhelk Nucella lapillus around
the south-west of England, Bryan et al. (1986) found
imposex to be widespread. The south coast of England was
the most severely affected. Populations showing the
highest incidence and highest intensity of imposex were
close to areas of boating or shipping activity. The degree
of imposex had increased markedly between 1969 and 1985 in
Plymouth Sound (an area on the south coast with large
numbers of small boats and ships), coinciding with the
introduction and increasing use of TBT-containing anti-
fouling paints in the area. Imposex correlated with
concentrations of sea-water tin (TBT fraction) and resi-
dues of tin in the dogwhelks (Fig. 6). Transferring whelks
from an area with little boating activity to Plymouth
marina resulted in a marked increase in the degree of
imposex. Bailey & Davies (1988b) found an increase in the
degree of imposex and also a higher incidence of penis
development in female dogwhelks collected in 1987 in the
Firth of Forth, Scotland, compared with those caught in
1975.
One of the effects of imposex on the female dogwhelk
is the blocking of the pallial oviduct, preventing the
release of egg capsules and rendering the female sterile.
A high incidence of females carrying aborted capsules was
found in declining populations close to sources of TBT.
The build-up of aborted capsules seemed, eventually, to be
lethal to the female; there were fewer females than
expected in affected areas (Gibbs & Bryan, 1986). The same
authors reported that the gross morphological changes
occurring in late imposex in the dogwhelk seem to be irre-
versible, since animals transferred from a moderately-
contaminated site to a "clean" site showed no resorption
of the penis. Gibbs & Bryan (1987) stated that imposex in
the dogwhelk was seen in sea water with TBT concentrations
of less than 1 ng tin/litre. They reported that the repro-
ductive failure, along with a lack of recruitment, had led
to population declines, almost to the point of extinction,
in areas of heavy TBT contamination. Gibbs et al. (1988)
experimentally transferred dogwhelks from an uncontami-
nated area to one showing water concentrations of 9-19 ng
tin/litre (TBT fraction) and demonstrated the development
of imposex within 18 months at the new location. These
transferred adults were able to spawn for much of this
period. The authors compared these results with results
for juveniles, which developed imposex earlier and were
sterile before reaching maturity. They discussed the
implications for the recolonization of areas where repro-
duction in the dogwhelk has been eliminated. Adults are
irreversibly affected by imposex. Recolonization is
unlikely until the TBT levels in sea water fall to around
2 ng tin/litre, a concentration at which the juveniles
are not sterilized before they reach sexual maturity. The
extent of recovery of populations would, therefore, depend
on the success of control measures and the water
concentrations resulting from bigger ships exempt from the
ban on TBT-containing antifouling paints.
Bryan et al. (1987) stated that while the evidence did
not show conclusively that TBT is solely responsible for
imposex in the dogwhelk, circumstantial evidence was con-
siderable. Imposex is related to the level of boating
activity. There is a significant relationship between
imposex and the body residue of organotin. Populations
that are in decline show the highest levels of TBT. Female
populations are declining faster than males and also have
the higher levels of TBT. The rise in the degree of
imposex coincided with the introduction and use of anti-
fouling paints containing TBT. Imposex has been shown to
be caused by TBT in other species of stenoglossan snails,
i.e. mud snails (Smith, 1981b). A significant decline in
imposex in monitored juvenile Nucella (Personal communi-
cation by P.E. Gibbs & G.W. Bryan to IPCS) followed the
introduction of TBT legislation in the United Kingdom.
Bryan et al. (1988) investigated the capacity of vari-
ous organotin compounds to induce imposex in the dogwhelk.
Whelks, already slightly affected by imposex, were taken
from the wild and exposed to 200 ng/litre tin, in the form
of TBT chloride, tri- n- propyl tin (TPrT), tetrabutyltin
(TTBT), dibutyltin (DBT), or triphenyltin (TPhT), for 14
days before being returned to the shore. Penis size, as a
percentage of male penis size, increased to 44% in females
exposed to TBT chloride, compared with 6% in controls.
TPrT increased relative penis size to only 14%, and no
other compound had any effect. In an attempt to eliminate
differences due to differential uptake of the compounds,
the organotin compounds were injected in a second exper-
iment. The females were maintained in the laboratory for
up to 105 days. TBT again induced increased penis size but
TTBT also showed an effect (19.5% compared to the 34%
shown after TBT injection). Other compounds were ineffec-
tive. The authors believed the effect of TTBT to be caused
by contamination by TBT and conversion to TBT in the tis-
sues. However, the increased imposex after treatment with
TPrT could not be explained in this way and the imposex
effect is considered to be not totally specific to tri-
butyltin.
8.2.3.7 Genotoxicity
Dixon & Prosser (1986) found that TBTO was not geno-
toxic, at concentrations of 0.05 to 5.0 µg tin/litre, to
the larvae of the mussel Mytilus edulis. Results were
based on chromosome analysis and sister chromatid
exchange (SCE). In 4-day acute toxicity studies, TBTO was
found to cause a dose-dependent reduction in both larval
survival and development of mussels. Survival ranged from
46% of controls at 0.05 µg/litre to 1.4% at 5 µg/litre.
The percentage of animals reaching the D-shell stage of
development was 8.7% of controls at the lowest dose, but
none reached this stage at either 1 or 5 µg/litre. How-
ever, when mussel larvae were exposed to a standard
mutagen (mitomycin C) or crude oil, in the presence of
TBTO, the SCE frequency increased to approximately twice
that found when larvae were exposed to either toxicant in
isolation (Dixon & McFadzen, 1987).
8.2.4. Crustaceans
Summary
Most of the available data for freshwater organisms are
derived from acute exposure tests with Daphnia. LC 50 data for
similar exposure times are variable (up to 2 orders of magni-
tude) probably due to age differences in the populations
studied. The NOEL for Daphnia has been estimated to be around
0.5 µg/litre, behaviour being the most sensitive parameter.
A range of marine species from copepods to crabs and lob-
sters has been studied, the lowest NOEL being 0.09 µg per
litre for reproductive effects in mysid shrimp.
The presence of sediment in test aquaria greatly reduces
the toxic effect of TBTO on estuarine crustaceans.
8.2.4.1 Acute effects
Using TBTO and TBT acetate, Floch et al. (1964) calcu-
lated LC100 and LC0 values for various species of fresh-
water invertebrates. The lethal concentrations of TBTO to
Daphnia magna over 24 and 72 h were 0.12 mg/litre and
0.06 mg/litre, respectively. Another aquatic crustacean,
Cypridopsis hartwigi, was less sensitive with lethal con-
centrations of 4 mg/litre for a 24-h exposure, 2 mg/litre
for a 48-h exposure, and 0.12 mg/litre for a 96-h
exposure. NOELs were 0.03 and 0.06 mg/litre for Daphnia
and Cypridopsis, respectively. For TBT acetate, the
LC100 was 0.15 mg/litre for a 72-h exposure of Daphnia
and 0.15 mg/litre for a 96-h exposure of Cypridopsis. The
LC0 was 0.075 mg/litre for both species.
However, more recent work has found Daphnia to be con-
siderably more sensitive to TBT. Meador (1986) reported a
96-h LC50 for Daphnia magna of 5.9 µg/litre and noted
that TBT is a slow-acting toxicant for Daphnia with
effects only being shown after 96 h or more of exposure.
Polster & Halacka (1971) quoted 48-h LC50 values for
Daphnia magna using various TBT salts, which ranged from
2.2 µg TBTO/litre to 8.5 µg TBT oleate/litre.
RIVM (1989) reported a 48-h EC50 for Daphnia of
4.7 µg/litre and a NOEL, over the same time period, of
0.56 µg/litre.
Davidson et al. (1986) calculated the 96-h LC50 to
be 0.42 µg/litre after exposing the mysid shrimp
Acanthomysis sculpta to a leachate of TBT.
When Walsh (1986) exposed the mole crab Emerita
talpoida to concentrations of 10 µg TBTO/litre of sea
water or 4500 µg/kg of sand, no effect on crab survival
was observed after 7 days of exposure. In continuous-flow
bioassays, 10 000 µg TBTO/kg of sediment did not kill
grass shrimp after a 96-h exposure.
8.2.4.2 Short- and long-term toxicity
U'Ren (1983) maintained the marine copepod Acartia
tonsa in solutions containing TBTO, under static con-
ditions, and calculated a 144-day LC50 of 0.55 µg per
litre; by combining moribundity and mortality as end-
points, the 144-day EC50 was found to be 0.4 µg/litre.
Laughlin et al. (1983) maintained mud crab larvae
(Rhithropanopeus harrisii), from hatching, in solutions
containing either TBTO at 0.5 to 25 µg/litre or TBT
sulfide at 0.5 to 50 µg/litre, under static renewal pro-
cedures. The survival of the zoeae, up to 15 days, was
unaffected by TBTO at levels up to 10 µg/litre. At 15 µg
per litre, 84% successfully moulted to the megalopa stage,
but at 5 µg TBTO/litre only 37% survived. Zoeal sur-
vival was unaffected by concentrations of TBT sulfide up
to 5 µg/litre. Survival at 20, 30, and 50 µg/litre was
78%, 26%, and 4%, respectively. The development rate, over
the same time period, decreased with increasing TBT con-
centration, although this was not statistically signifi-
cant below 10 µg TBTO/litre or 20 µg TBT sulfide/litre.
At the highest exposure concentrations (10 µg TBTO/litre
and 20 µg TBT sulfide/litre), metamorphosis was delayed
by approximately 2 days in the case of TBTO and 6 days for
TBT sulfide. Growth (measured as mean wet weight) was
significantly reduced at TBTO concentrations of 15 and
25 µg/litre or TBT sulfide concentrations of 20, 30, and
50 µg/litre, and showed dose dependency. Daily growth was
monitored for up to 12 days at concentrations (of both
TBTO and TBT sulfide) of 0.5, 1.0, and 5.0 µg per litre.
Although the final weights were not significantly differ-
ent, all TBT treatments caused an initial growth lag
during the first three days of exposure. Laughlin et al.
(1985) found the LC50 for exposure of zoeae of
Rhithropanopeus harrisii to TBTO during the 12 days of
zoeal development to be 55 nmol/litre.
Laughlin & French (1980) exposed shore crabs
(Hemigrapsus nudus), 2 to 3 days after hatching, to TBTO
(as "Biomet") for up to 14 days under static conditions.
At the highest concentrations (500 and 1000 µg/litre),
all the zoeae died within 2 days. Survival time increased
as the concentration of TBTO decreased from 100 to 25 µg
per litre; most larvae died within 8 days even at 25 µg
per litre. The estimated values of LT50 for 100, 75, 50,
and 25 µg/litre were 3.4, 4.8, 5.8, and 6.2 days,
respectively. Lobster larvae (Homarus americanus) were
much more sensitive; 100% being killed within 24 h and
2 days by 20 and 15 µg TBTO/litre, respectively. Concen-
trations of 10 and 5 µg/litre killed all larvae within
5 to 6 days. In the group exposed to 1 µg/litre, there
was a similar mortality pattern to the controls and high
mortality at the first ecdysis (3 to 5 days post-hatch).
However, in this group only a single larva metamorphosed
successfully, compared with 43% in the control group.
Davidson et al. (1986) kept juvenile mysid shrimps
(Acanthomysis sculpta), newly-released from the female,
in TBT concentrations of between 0.03 and 0.48 µg per
litre for a 63-day period under flow-through conditions.
The TBT source was leachate from panels coated with anti-
fouling paint. All animals died within 7 days at the
higher exposure level. There was no significant difference
in shrimp mortality between those exposed at levels up to
0.38 µg/litre and the controls, either at 22 or 41 days.
From day 41 to the end of the test (63 days), survival
decreased at 0.38 µg/litre and only 22.5% survived to the
end of the experiment, compared with 60% in the control
group. The authors stated that this indicated a lowering
of the NOEL for the entire life cycle of A. sculpta from
0.38 µg/litre to 0.25 µg/litre at 41 days. The increase
in mortality coincided with the release of juveniles by
the females, which indicated a sensitive time in the life
cycle. Both mean length and weight of females, at a TBT
level of 0.38 µg/litre, were significantly reduced after
63 days of exposure, but no effect of TBT concentrations
up to and including 0.38 µg/litre was observed in males.
A similar result was found in another study after 28 days
at 0.49 µg/litre; again no effect on length or weight was
found in males. There was a significant effect on the
length of developing juveniles and sub-adults. After 14
days of exposure to either 0.19 or 0.33 µg/litre, mysids
were significantly shorter; this was also the case at
0.2 µg/litre after 27 days. At TBT concentrations up to
and including 0.33 µg/litre, there was no effect on the
number of juveniles released per individual female, the
number of individuals in unhatched broods, or the number
of days from hatching of a female to the release of its
juveniles. However, there was a significant reduction in
the number of viable juveniles released at both 0.19 µg
per litre and 0.33 µg/litre. The authors suggest a NOEL
of 0.09 µg/litre for reproduction, the most sensitive
parameter found in the study.
Laughlin et al. (1984) exposed the Baltic amphipod
Gammarus oceanicus to TBTO or TBT fluoride concentrations
of 0.3 or 3.0 µg/litre for 8 weeks, under 48-h static
renewal conditions. They also exposed the amphipods to
leachates from TBT-containing antifouling paints, by
placing a 1 cm2 plexiglass plate, which was painted with
either "Micron 25" or "Interracing", in the tank for
5 weeks. Again the water was changed every 48 h. TBT
levels in the experiment with pure compounds, remained
constant, but the TBT concentration (measured as TBTO)
from the leachates gradually increased over each 48-h
exposure period to approximately 5.5 µg/litre for
"Interracing" and 0.5 µg/litre for "Micron 25",
reflecting the different leaching rates of the two com-
pounds. At the highest concentration of both TBTO and TBT
fluoride, 50% of the adults died within 10 to 12 days of
the exposure and all had died within 16 days (TBTO) or 33
days (TBT fluoride). The TBT paint "Interracing" caused
100% mortality within 1 week. The lower concentration of
the exposures to pure compounds and "Micron 25" resulted
in mortality patterns that were not dependent on TBT con-
centration but on senility. The exposures to 0.3 µg per
litre and "Micron 25" had significantly reduced the
number of surviving larvae by the end of the experiment.
Slight decreases in larval growth were observed after
exposure to TBTO and "Micron 25" but, generally, concen-
trations of less than 1 µg TBT/litre had little effect
on growth and no effect on whole animal oxygen consumption
rates.
Clark et al. (1987) monitored the survival of the
grass shrimp (Palaemonetes pugio) in water and water/sedi-
ment test aquaria after the addition of TBTO. When added
to water and tested in the absence of sediment, TBTO gave
96-h LC50 values comparable to other published results at
20 µg/litre. However, when the TBTO was admixed with
sediment rather than water, the LC50 could not be deter-
mined. TBT levels in sediment at 1 mg/kg in static and
10 mg/kg in flow-through tests showed no effects on shrimp
survival. Similar tests using Amphioxus gave an LC50 for
sediment containing TBTO of between 1 and 10 mg/kg over 4
and 10 days. The animals were killed by 10 µg TBT per
litre in water.
8.2.4.3 Reproductive effects
Hall et al. (1988b) maintained egg-carrying females of
the copepod Eurytemora affinis in TBT chloride at levels
of 0.1 or 0.5 µg/litre for up to 13 days. After 3 days
of exposure, the higher concentration had significantly
reduced the mean brood size to 0.2, compared with 15.2 in
controls. Neonate survival was significantly reduced at
0.1 µg/litre after 6 days (22% of control survival). No
offspring survived the higher exposure concentration. In a
second experiment, mean brood size, after 2 days of
exposure, was not significantly affected at concentrations
of between 12.5 and 200 ng/litre. Neonate survival, after
13 days, was unaffected by concentrations up to and
including 50 ng/litre; survival at 100 ng/litre was 76%
(compared to 22% survival at the same exposure level, in
the first experiment, over 6 days) and was further reduced
to 24% at 200 ng TBT/litre.
When Johansen & Mohlenberg (1987) exposed fertilized
mature female copepods (Acartia tonsa) to TBTO, egg pro-
duction was significantly reduced at the highest exposure
level of 0.1 µg/litre after 72 h. After 120 h, all
exposure concentrations had significantly reduced egg
production by 18%, 19%, and 37% relative to controls, at
TBTO concentrations of 0.01, 0.05, and 0.1 µg per litre,
respectively.
8.2.4.4 Limb regeneration
Weis et al. (1987a,b) exposed the fiddler crab (Uca
pugilator) to TBTO concentrations of 0.5, 5.0, or 50 µg
per litre under static renewal conditions. Regeneration
(autotomy) of 1 chela and 5 walking legs was induced by
pinching them off at the merus. Although some growth
retardation was observed, the most striking effect was the
development in regenerated limbs of deformities, e.g.,
backward curling or complete absence of the dactyl of the
claw, chelae or walking legs, stunted, unjointed, or bent
in the wrong direction. The percentage of males exhibiting
deformities was 17% at 0.5 µg/litre, 24% at 5 µg per
litre, and 67% at 50 µg/litre, the test solution being
changed twice weekly. In a second experiment, where the
test solution was changed three times weekly, the percent-
age deformities were as follows: males, 100% at both 5 and
50 µg/litre; females, 29% at 5 µg/litre and 100% at
50 µg/litre. There were no deformities in the control
groups during the experiments.
8.2.4.5 Behavioural effects
When Pinkney et al. (1985) gave the grass shrimp
(Palaemonetes pugio) a choice between TBTO-contaminated
water and clean water, it did not avoid total organic tin
concentrations of 2.3 to 30 µg/litre. The response data,
at both 2.3 and 30 µg/litre, were very similar.
Meador (1986) reported the effects of TBT chloride on
the photobehaviour of water fleas (Daphnia magna). The
normal response of Daphnia to a unidirectional light
source is to swim away from the light; this is an adaptive
response to avoid predators. The author reported that
Daphnia exposed to TBT chloride showed a reversal of this
behaviour and swam towards the light source (usually with
considerably increased swimming intensity). The threshold
concentration of TBT chloride causing this behavioural
reversal was 0.5 µg/litre (the LC50 over the same time
period was between 3.5 and 6 µg/litre).
8.2.5. Other aquatic invertebrates
Summary
Few studies have been carried out on other species. The
lowest effect level in annelids was observed for Nereis
diversicolor; mortality and behavioural effects were seen after
chronic exposure to 2 µg/litre. Limb regeneration was signifi-
cantly inhibited in a brittle star after exposure to 0.1 µg
per litre. Experiments have been carried out on four insect
species. The most sensitive was Notonectes, the LC0 being
0.03 mg/litre and the LC50 0.06 mg/litre.
8.2.5.1 Acute effects
Walsh et al. (1986a) exposed larvae of the lugworm
Arenicola cristata to either TBTO or TBT acetate, for 96
or 168 h, respectively. The concentrations that killed
100% of the animals were: 4 µg/litre (96 h) for TBTO;
10 µg/litre (96 h) and 5 µg/litre (168 h) for TBT acet-
ate. At 5 µg TBT acetate/litre, all larvae were abnormal
after 96 h of exposure. No deaths or abnormalities re-
sulted from exposure to 2 µg TBTO/litre for up to 168 h.
When Beaumont et al. (1987) exposed adult polychaetes
(Nereis diversicolor) to TBT in flowing sea water for up
to 22 days, there was 100% mortality by 22 days at 4 µg
per litre and 55% mortality at 2 µg/litre (20% control
deaths). Eversion of the proboscis was a more sensitive
indication of toxicity. No controls showed this effect,
whereas 75% of animals exposed to 2 µg/litre showed
everted proboscis after 22 days (50% after 20 days of
exposure).
Dragonfly larvae (Aeschna) sp. showed a 48-h LC100 of
0.25 mg TBTO/litre and an LC0 of 0.12 mg TBTO/litre.
Corresponding values for TBT acetate were 0.5 mg/litre
(72-h LC100) and 0.025 mg/litre (LC0). Notonectes sp.
yielded LC0 values of 0.03 mg/litre for both TBTO and TBT
acetate and 72-h LC100 values of 0.06 and 0.15 mg/litre
for TBTO and TBT acetate, respectively. Chironomid (midge)
larvae revealed a NOEL of 0.075 mg/litre for TBT acetate
and a 48-h LC100 of 0.15 mg/litre (Floch et al., 1964).
Cardarelli (1978) studied the efficacy of controlled-
release organotin compounds as mosquito larvicides. The
toxicity (LT100) of BioMet (6% TBTO in natural rubber),
CBL-9B (20% TBT fluoride in natural rubber), and
ECOPRO-1230 and ECOPRO-1330 (both are ethylene propylene
polymers containing 30% TBT fluoride) were tested against
larvae of the mosquito Culex quinquefasciatus. For BioMet
and CBL-9B, LT100 values ranged from 4 to 9 days for
toxicant concentrations of 0.1 to 10 mg/kg of pellet added
to the water, the toxicity increasing with increasing
toxicant concentration. A degradation product, TBT carbon-
ate, was found to be less toxic, the LT100 being 9 days
after exposure to 10 mg/litre. When the polymers were
tested at toxicant concentrations ranging from 0.9 to 32.6
mg/kg, toxicity was found to increase with increasing
toxicant concentration (LT100s range from 2 days to 16
days). Although initially toxicity tended to decrease as
the soaking time of the pellet increased, prior to
exposure, between days 100 and 500 of soaking toxicity
remained relatively unchanged. The authors observed that
the organotin compounds dramatically slowed or even
prevented morphogenesis.
8.2.5.2 Limb regeneration
In a study on limb regeneration, Walsh et al. (1986b)
maintained the brittle star Ophioderma brevispina in TBTO
concentrations of 0.01, 0.1, or 0.5 µg/litre under flow-
through conditions. On the first day of the experiment,
autotomy was induced in two arms, at opposite sides of the
disc, by pinching midway between disc and arm tip. The
animals were then exposed for four weeks to TBTO. There
were no deaths and no effect on disc diameter. Both 0.1
and 0.5 µg/litre significantly inhibited regeneration of
arms as measured by length; both groups showed average and
median lengths less than those in the lowest-dose group,
but variability precluded statistical significance. Aver-
age weights of limbs were also reduced in the groups
exposed to 0.1 and 0.5 µg/litre, but statistical analysis
was not carried out because pooled weights did not provide
enough data.
8.3. Fish
Summary
The acute toxicity of tributyltin to marine and freshwater
fish is highly variable, LC 50 values ranging from 1.5 to
240 µg/litre. It is unclear whether this is due to inherent
differences in sensitivity or to differences in route of
exposure. Many of the acute toxicity studies need to be inter-
preted with care, since the TBT concentrations cited are often
nominal and biologically available concentrations were not
measured.
In long-term toxicity tests, the NOEL for general toxico-
logical parameters (growth and behaviour) for trout yolk-sac
fry was greater than 0.2 µg/litre, and for medaka it was
3.2 µg/litre. Using histopathological parameters, the NOEL for
medaka was found to be 0.32 µg/litre, vacuolation of the reti-
nal epithelium being the most sensitive parameter.
Few embryotoxicity tests have been performed and it has not
been possible to establish a NOEL.
8.3.1. Acute effects
The 96-h LC50 of TBTO for marine fish ranges between
1.5 and 36 µg/litre (Table 12). Larvae seem to be more
sensitive than adults in those few studies examining dif-
ferent life stages in the same test. Fewer LC50 values
have been published for freshwater fish; they range from
13 to 240 µg/litre.
8.3.2. Short- and long-term toxicity
Matthiessen (1974) measured a lethal threshold concen-
tration of TBTO for Tilapia mossambica of between 8 and
16 µg/litre (24-h LC50, 28 µg/litre; LC5, 24 µg per
litre; LC90, 33 µg/litre) as a preliminary to studies
at sublethal concentrations. A temporary opacity of the
surface of the eyes developed at concentrations below this
threshold (> 8 µg/litre) but disappeared after a few
days. Other symptoms included sluggishness and difficult-
ies with balance. Melanophores in the skin were found to
be constricted, giving treated fish a paler appearance
than controls. The fish did not produce behaviourally
related display patterns, an activity important in the
species. As with the eye opacity, these other symptoms
disappeared after a few days or weeks, suggesting that the
fish develop some tolerance to the TBTO. The growth of
Tilapia exposed to 0, 5, or 8 µg TBTO/litre was moni-
tored over a 5-week period. These concentrations of TBTO
were estimated, on the basis of release rates, in water
after the TBTO had been used to control mollusc vectors
for schistosomiasis. There was no difference in growth
between fish exposed to 5 µg/litre and controls. Tilapia
exposed to 8 µg/litre showed negative growth and lost
about 6% of body weight over the experimental period. Eye
opacity was only found in the fish exposed to 8 µg per
litre, but this effect did not seem to affect feeding
behaviour. The only other change in the high-dose group
was an increase in aggressive encounters between males.
There was an increased reluctance amongst attacked males
to avoid conflict, which led to the death of several indi-
viduals. Fatal engagements between males are normally very
rare in this species.
When Seinen et al. (1981) exposed rainbow trout (Salmo
gairdneri) yolk-sac fry to TBT chloride concentrations of
0.2, 1, or 5 µg/litre for up to 110 days, all fry died
within 10 to 12 days (at the transition from yolk-sac fry
to the swimming fry stage) at 5 µg/litre, but there were
no deaths at lower doses. The major histopathological
change in fish that died after exposure to 5 µg per litre
was hydropic degeneration of tubule segments of the pro-
nephros. At 0.2 and 1 µg/litre, there was a dose-related
retardation of growth, resulting in a 44% decrease in body
weight (relative to controls) in the 1 µg/litre group
after 110 days. At the end of the experiment, both remain-
ing groups showed significant reductions in the haemo-
globin titre in the blood and in body weight. Only at the
higher concentration was there a significant decrease in
blood cell number. Relative liver weight was significantly
increased at both concentrations but relative numbers of
thymus cells were unaffected. The authors also measured
the relative area distribution of the various hepatic com-
partments in liver sections. The area occupied by nuclei
significantly increased at both concentrations, whereas
glycogen storage area decreased, but this was significant
only at the highest exposure level. Cytoplasmic area was
unaffected, as were all non-parenchymal compartments.
Table 12. Toxicity of tributyltin to fish
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salinity pH TBT salt Dura- LC50c Reference
age flowa ature (o/oo) tion (µg/
(°C) (h) litre)
---------------------------------------------------------------------------------------------------------
Marine and estuarine species
Sheepshead minnow sub-adult flow 19.4- 9.8- 8.15- chloride 48 >31d,f Bushong
(Cyprinodon variegatus) 21.3 12.1 8.31 72 28.1 et al. (1988)
(24-36.5)d,f
96 25.9
(22.8-30.1)d,f
Bleak 8 cm stat 10 7 7.8 fluoride 96 6-8e Linden et
(Alburnus alburnus) oxide 96 15 al. (1979)
(13-17)e
Inland silverside larva flow 20 10 chloride 48 7.7 Bushong et
(Menidia beryllina) (5.5-10.9)d,f al. (1987)
72 4.6
(3.3-6.2)d,f
Atlantic silverside sub-adult flow 20 10 chloride 48 12.7 Bushong et
(Menidia menidia) (7.8-15.2)d,f al. (1987)
72 9.3
(7.1-12.4)d,f
96 8.9
(6.7-11.6)d,f
Atlantic menhaden juvenile flow 20 10 chloride 48 6.8 Bushong et al. (1987)
(Brevoortia tyrannus) (4.1-infinite)d,f
72 5.2
(3.7-6.5)d,f
96 4.5
(3.6-6.4)d,f
Sole larva statb oxide 48 8.5 Thain (1983)
(Solea solea) larva 96 2.1
adult 48 88
Armed bullhead adult statb oxide 48 26 Thain (1983)
(Agonus cataphractus) 96 16
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Girella 2.4 g statb 19.9- 7.8- oxide 48 5.2d Kakuno &
(Girella punctata) 20.5 8.1 96 3.2d Kimura (1987)
Saltwater goby statb 20.2- 32.5- 8.1- oxide 24 12d Shimizu &
(Chasmichthys dolichognathus) 21.0 32.8 8.3 48 9d Kimura (1987)
96 4d
Chinook salmon juvenile stat 3-5 28 oxide 6 54d Short &
(Oncorhynchus tshawytscha) 12 20d Thrower (1987)
96 1.5d
Mummichog larvae flow 19.4- 9.8- 8.15- chloride 48 >32.2d,f Bushong et
(Fundulus heteroclitus) larvae 21.3 12.1 8.31 72 28.2 al. (1988)
(15.1-infinite)d,f
larvae 96 23.4
(15.2-30.9)d,f
sub-adult 48 >32.2d,f
sub-adult 72 28.3
(23.4-43.2)d,f
sub-adult 96 23.8
(20.8-28)d,f
Freshwater species
Rainbow trout yearling stat 18 250 oxide 24 28e Alabaster
(Salmo gairdneri) yearling statb 18 250 oxide 48 21e (1969)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Guppy 3-4 weeks statb 23 oxide 96 21 RIVM
(Lebistes reticulatus) chloride 168 21 (1989)
(16-29)e,f Polster &
oleate 168 33 Halacka
(19-60)e,f (1971)
benzoate 168 25
(19-32)e,f
laurate 168 30
(18-50)e,f
acetate 168 28
(21-33)e,f
oxide 168 39
(28-50)e,f
Medaka 4-5 weeks statb 23 oxide 96 17 RIVM
(Oryzias latipes) (1989)
Stickleback 4-5 weeks statb 19 oxide 96 13 RIVM
(Gasterosteus aculeatus) (1989)
Carp oxide 24 75 Temmink &
(Cyprinus carpio) oxide 96 32 Everts
(1987)
Golden orfe oxide 48 50e Plum
(Leuciscus idus melanotus) napht- 48 70e (1981)
henate
Bluegill sunfish stat 20 oxide 96 240e Foster
(Lepomis macrochirus) (1981)
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
g Hardness expressed as mg CaCO3/litre.
Pinkney et al. (1988) exposed 13- and 16-day-old
larvae of striped bass (Morone saxatilis) to varying con-
centrations of TBT from methacrylate-painted panels for 6
to 7 days. They found significant reductions in survival
at measured concentrations of 0.766 µg TBT/litre or more.
At lower exposure concentrations (0.067 µg/litre), growth
parameters changed in the 13-day-old larvae only. No
changes occurred in the growth parameters of 16-day-old
larvae exposed to 0.444 µg/litre or less. The authors did
not know whether this apparent difference in sensitivity
between larvae of different ages was a true effect or
simply the result of differences between the various
batches of larvae.
Wester et al. (1988) reported NOELs for the medaka
(Oryzias latipes) of 3.2 µg TBTO/litre for general toxi-
cological parameters (mortality, growth, general appear-
ance, abnormal behaviour) and 0.32 µg/litre for histo-
pathological effects during 1 month of exposure. The most
sensitive histopathological effect was the development of
vacuolation in the retinal epithelium of the eye. There
was a dose-related increase in hepatocellular vacuolation,
with swelling in pronounced cases. The vacuoles appeared
to be glycogen deposits, although, at higher doses, lipid
vacuoles were also noted. The NOEL for liver effects was
1 µg TBTO/litre. Kidney effects mostly involved the
tubule and included dilation, epithelial atrophy, degener-
ation and regeneration, and proteinaceous casts including
cellular debris (tubulonephrosis). Severe cases also
showed glomerular effects. Similar lesions were reported
after 3 months of exposure, with, in addition, effects on
the swim bladder, skin, oral cavity, and thyroid gland at
the highest concentration tested (10 µg/litre). Thymus
atrophy, reported by the same authors in the guppy, was
not found in the medaka, indicating some species speci-
ficity regarding the effects of TBT. Increased glycogen in
liver and muscle after TBTO treatment was demonstrated
analytically in the medaka, confirming histochemical
suggestions of increased glycogen in the guppy.
Shimizu & Kimura (1987) exposed the marine goby
Chasmichthys dolichognathus to TBTO in short (4 day) and
long (12 week) experiments, and reported a 96-h LC50 of
4 µg/litre. Long-term exposure to 2.1 µg/litre during
the season of gonadal recrudescence led to a significant
depression of the gonadosomatic index in male fish. There
was no effect on female fish in terms of gonadosomatic
index or ovarian histology.
8.3.3. Embryotoxicity
Newton et al. (1985) exposed eggs, embryos, and larvae
of the California grunion (Leuresthes tenuis) to plates
painted with 9.4% TBT methacrylate (0.5% TBTO; 44.7% cu-
prous oxide; 45.5% inert ingredient) and aged for 30 days
in flowing sea water. The authors claimed that the ex-
posure to concentrations of between 0.14 and 1.72 µg TBT
per litre had no adverse effect on hatching, growth, or
development. In fact the presence of TBT at such concen-
trations enhanced both hatching success and stimulated
growth. A concentration of 10 µg TBT/litre had no effect
on hatchability, but at 74 µg/litre hatching success was
reduced by approximately 50%. The authors observed no
effect on the survival of larvae, hatched from eggs
exposed to concentrations of 0.14 to 1.72 µg/litre, up to
7 days post-hatch. The presence of copper, however, should
be taken into consideration in the interpretation of these
results.
Weis et al. (1987a) reported considerable variation in
the response of embryos of the killifish (Fundulus
heteroclitus) to concentrations of TBTO ranging between 3
and 30 µg/litre. Two batches of embryos developed abnor-
malities at the highest concentration of TBTO tested
(30 µg/litre) and showed some mortality. Other batches of
embryos showed embryotoxicity for all tested concen-
trations (down to 3 µg/litre) but no abnormalities in the
embryos. Owing to this difference in sensitivity of dif-
ferent batches of eggs and the separation of embryotoxic
and teratogenic effects, it is difficult to establish a
NOEL for this species.
Fent (1989b) exposed fertilized eggs of the freshwater
minnow Phoxinus phoxinus to two TBT concentrations in
petri dishes in an environment chamber. The eggs were ex-
posed from the blastula stage (20 to 24 h after spawning),
and the water was renewed daily. Analytical determination
of TBT showed that the actual concentration had reduced
over the 24-h exposure period from 1.5 to 0.5 µg/litre in
the low-dose group and from 8.4 to 7.9 µg/litre in the
high-dose group. There were no differences in survival
between the control and low-dose groups. Hatching was nor-
mal but some fish showed vertebral malformations. At the
high dose, hatching was delayed or reduced and all hatched
larvae had severe vertebral malformations; many were
unable to uncurl. These larvae remained motionless at the
bottom of the dish. Some had oedema in the region of the
heart. Within 3 to 4 days of hatching, all of the larvae
in the high-dose group had died. A further series of
experiments similarly showed malformation at an exposure
level of 4.5 µg TBT/litre.
8.3.4. Behavioural effects
The avoidance response of the mummichog (Fundulus
heteroclitus) to TBTO has been studied by Pinkney et al.
(1985). When given a choice between TBTO-contaminated and
clean water, 4 out of 6 groups of fish avoided a total
organic tin level of 1 µg/litre. There were significant
avoidance responses for all test groups at total organic
tin levels of 3.7, 8, and 13.8 µg/litre. However, the
higher concentrations of tin did not result in an increase
in avoidance response. The authors found that, under the
same conditions, no avoidance response was shown by the
grass shrimp (see section 8.2.4.5). This shrimp is a major
food item of the mummichog in tidal marshes. Hall et al.
(1984) studied the avoidance response of two species of
juvenile estuarine fish, the striped bass (Morone
saxatilis) and Atlantic menhaden (Brevoortia tyrannus). An
avoidance response to TBTO was exhibited by bass at a
total organic tin concentration of 24.9 µg per litre.
Atlantic menhaden were more sensitive, showing a "mild"
avoidance at 5.5 µg/litre and a "strong" avoidance at
9.1 µg/litre.
Chliamovitch & Kuhn (1977) measured an EC50 for the
loss of positive rheotaxis of 30.8 µg/litre for the rain-
bow trout (Salmo gairdneri) and 53.2 µg/litre for a
tilapia (Tilapia rendalli).
8.4. Amphibians
Summary
There are few data on the effects of tributyltin on amphib-
ians. Survival of frog larvae was at least 80% of control
levels after exposure to 3 µg TBT/litre.
Floch et al. (1964) reported a NOEL for mortality of
tadpoles of two species of amphibians (Rana and Alytes sp.)
of 30 µg/litre. For both species, the 24-h LC100 for
TBTO was 75 µg/litre, the 48-h LC100 50 µg/litre, and
the 48-h LC100 for TBT acetate was 75 µg/litre.
Laughlin & Linden (1982) exposed eggs of the frog Rana
temporaria to concentrations of either TBTO or TBT fluor-
ide of 0.3, 3, or 30 µg/litre, for 5 days from the post-
gastrula stage of development, under static renewal con-
ditions. All surviving larvae hatched on either day 4 or
5 of exposure. Survival was at least 80% of control levels
after exposure to 0.3 or 3 µg/litre. Only at the highest
concentration (30 µg/litre) did TBT affect survival (40%
mortality with TBT fluoride and 50% mortality with TBTO).
The authors collected all surviving tadpoles and measured
wet and dry weight. Wet weights were significantly lower
in tadpoles exposed to 30 µg/litre but unaffected at
lower exposure concentrations. Tadpoles exposed to TBT
always showed higher mean dry weight than controls,
although increases were not consistently dose dependent.
The percentage of body water declined from 86-88.5% for
controls to 73.8% after exposure at the highest concen-
tration of TBT. The changes resulting from TBT treatment
were significant, but not the differences produced by the
different TBT compounds.
8.5. Multispecies studies
Summary
The few available microcosm studies indicate that the most
sensitive organism(s) within the microcosm are affected by TBT
at concentrations greater than 0.05 µg/litre. Recovery of
these organisms occurred a few months after exposure. Simul-
taneous exposure of snails and fish yields conflicting results,
snails appearing somewhat more sensitive than fish.
Beaumont et al. (1987) exposed sandy-substrate micro-
cosms of flowing sea water to TBT derived from slate
panels painted with "Micron 25" antifouling paint. Three
replicate microcosms were exposed for 4 months at high
(1-3 µg/litre) and at low (0.06-0.17 µg/litre) TBT con-
centrations in water, together with three control micro-
cosms. Flow rates were maintained by gravity from a header
tank at approximately 1 litre/min; the painted panels were
located in the header tank where water was passed over
them using a circulating pump. The substrate sediment was
sieved after collection (to 2 mm) to remove larger organ-
isms and allowed to settle in the microcosms for 3 weeks
before the addition of animals. The sediment was 10 cm
deep and the overlying water 15 cm deep in each microcosm.
At the beginning of the trial, 50 specimens of a bivalve
(Cerastoderma edule), a crustacean (Corophium volutator),
and two polychaetes (Nereis diversicolor and Cirratulus
cirratus) were introduced. After 4 weeks, 12 specimens of
the gastropod Littorina littorea were added and, after 6
weeks, 26 specimens of a second bivalve (Scrobicularia
plana) were added. Other species were found at the end of
the trial derived from small (< 2 mm) juveniles in the
sediment and inflowing sea water. Each day, 10 litres of a
suspension of the microalga Pavlova lutheri (5 x 106 cells/ml)
were added to the header tank as additional food supply.
The most sensitive of the introduced species was the
cockle (C. edule); 100% died within 2 weeks at the high
concentration of TBT and there was cumulative mortality at
the low TBT concentration over 17 weeks (14% of controls
died). Two-way analysis of variance indicated significant
differences between all three groups (i.e. control, and
low and high concentrations of TBT; p < 0.05). The other
bivalve (S. plana) showed high mortality at the highest
exposure level (100% after 10 weeks). At the low TBT con-
centration there were no time-related deaths in this
species and no significant difference from controls.
Among the polychaetes, high death rates were recorded
for N. diversicolor in all microcosms, including the
control, possibly because adults introduced were ripe and
died after spawning during the experiment. The other
polychaete (C. cirratus) survived well even after exposure
to the high concentration of TBT. Only one gastropod (L.
littorea) died in any of the microcosms. Juvenile bivalves
were the most common species found; their numbers and
diversity were lower in the low-dose microcosms (66 to
109) than in control microcosms (137 to 179), and they
were virtually absent from the high-dose microcosms (0, 1,
and 1 for the 3 replicates). The size of juvenile mussels
(Mytilus edulis) was also significantly reduced at the
low TBT concentration, relative to controls; other self-
introduced bivalve species were not affected by the low
TBT exposure. Measurements of chlorophyll a in sediment
cores, as a measure of algal biomass, indicated a signifi-
cant rise after exposure to high TBT levels. This is
explained by the relative insensitivity of algae to the
toxic effects of TBT and by reduced (or non-existent)
animal life available to consume the algae. The authors
emphasize the sensitivity of some species, for which TBT
is toxic at levels of 1 to 2 µg/litre under conditions
approximating natural exposure. They further emphasize
the great variation in sensitivity between species; for
example, while Mytilus edulis and Cerastoderma edule were
clearly affected at very low levels of TBT, other related
bivalves were largely unchecked. Differences in feeding
behaviour, leading to different effective exposure levels,
is offered as one possible explanation for differences in
response. Differential absorption or subsequent loss,
observed in laboratory studies with other molluscs, is
also proposed as a possible mechanism.
Henderson (1986) conducted long-term flow-through
microcosm studies on communities of marine organisms from
Pearl Harbour, Hawaii, where the organisms had been
exposed to leaching TBT from naval ships for some time.
Panels (20 x 25 cm) of roughened plexiglass were suspended
in the harbour for 14 weeks prior to the experiment to
allow settlement and growth of organisms, and 30 different
species were found on the panels. Prior to exposure, the
panels were kept for a further 5 weeks in the experimental
tanks. These tanks had a capacity of 155 litre and were
supplied with sea water pumped directly from the harbour
and through the tanks at a rate of 4 litres/min. The sea
water was passed through a 1 cm mesh with no further
filtration; organisms could, therefore, enter the tanks
easily and settle on the panels. The water was rich in
plankton consisting largely of diatoms, copepods, and
chaetognaths. Exposure to TBT derived from painted panels
treated with antifouling paint containing 9.4% TBT meth-
acrylate, 0.5% TBTO, 44.7% cuprous oxide, and 45.4% inert
ingredients. Nominal concentrations of TBT were 0, 0.05,
0.13, 0.31, 0.78, and 1.95 µg/litre, but actual mean
concentrations were 0.01, 0.04, 0.10, 0.54, 1.77, and
2.52 µg/litre, respectively. The measured copper levels
in the same samples were 1.0, 1.2, 1.2, 3.1, 4.4, and
5 µg/litre, respectively. Changes in coverage of the
panels was assessed using photographs taken with an under-
water camera at weekly or biweekly intervals throughout
the experimental period of approximately 60 days of
exposure and a further 60 days of recovery. One week after
the start of exposure, a further plastic panel was intro-
duced to monitor new colonization. There was a clear
decline in both total number of species and in species
diversity (reductions of 55%, 60%, and 80% at the three
highest exposure levels, respectively) on pre-fouled
panels exposed to TBT at 0.5, 1.8, and 2.5 µg/litre but
no effect at lower concentrations. The mortality of indi-
vidual species, in relation to TBT exposure, showed con-
siderable variation. The most sensitive of the six most
common species on the panels was Botrylloides spp, an
orange-coloured colonial tunicate, which showed 100% mor-
tality at TBT concentrations of 0.1 µg/litre or more. An
encrusting bryozoan, Schizoporella errata, showed 49% mor-
tality at 0.1 µg/litre and 80 to 100% mortality at higher
concentrations. Specimens of a second colonial tunicate,
Didemnus candidum, and the saddle oyster ( Anomis nobilis)
were all killed at concentrations of 0.5 µg/litre or
more. A tube worm (Hydroides elegans) and a solitary
tunicate (Ascidia spp.) survived even the highest
exposure level, though with considerable mortality at 1.8
and 2.5 µg/litre. Recovery of populations was complete
60 days after cessation of treatment. Settlement on the
panels not previously exposed was reduced by TBT concen-
trations of 0.1 µg/litre or more, but not by 0.04 µg per
litre. Algal settlement and growth on the walls of the
tanks was not affected by exposure to TBT at any concen-
tration; coralline algae increased their coverage of pre-
fouled panels. A total of 18 oysters (Crassostrea
virginica) placed in each tank prior to treatment was
monitored after 60 days. There was 50% mortality after
TBT exposure at 1.8 µg/litre but no significant reduction
in survival at lower concentrations. However, the con-
dition index of the oysters was affected by exposure to
0.1 µg/litre or more, but had returned to near normal
after 2 months recovery in clean water. The author
regarded 0.05 µg/litre as a reliable NOEL for the most
sensitive organisms exposed.
When Salazar & Salazar (1985) exposed copepods
(Acartia tonsa), mysids (Acanthomysis sculpta), and fish
(Citharichthys stigmaeus) to suspended sediment at a TBTO
concentration of 0.49 µg/litre water for 96 h, no sig-
nificant mortality was found. In a second experiment,
mysids (A. sculpta), worms (Neanthes arenaceodentata), and
clams (Macoma nasuta) were exposed to TBTO-contaminated
sediment with overlying water for 10 days in the case of
mysids and 20 days in the case of clams and worms. Levels
of TBTO in the sediment varied from 155 to 610 µg per kg,
falling during the exposure period, while measured levels
in the overlying water were about 0.2 µg/litre. No mor-
tality was observed during the exposure period.
Cardarelli (1973) exposed snails (Biomphalaria
glabrata) and fish (Lebistes reticulatus) to slow-
releasing molluscicides containing either TBTO or TBT
fluoride. At a daily release rate of 35 µg TBTO/litre,
100% mortality was achieved in snails within 60 days but
all of the fish also died within this period. At a TBTO
release rate of 7 µg/litre per day, snail mortality was
100% after 120 days exposure and fish mortality only 2%.
However, a second experiment gave a much higher fish mor-
tality of 46% after 120 days exposure to only 3.5 µg TBTO
per litre per day. Exposure to TBT fluoride at 7 µg per
litre per day killed all snails and fish within 60 days,
and 52% of fish and 100% of snails were killed after 90
days of exposure to 3.5 µg/litre per day.
In order to simulate the effect of molluscicides con-
taining 6% TBTO on biota, Jordan (1985) set up a model
ecosystem as follows: filtered river water was allowed to
flow into a tray containing washed mud (to simulate a
marsh), and overflow from this upper tank was allowed to
flow into a lower tank containing washed coarse sand (to
simulate a river). Snails (Biomphalaria glabrata and
Potamopyrgus coronatus) and guppies (Lebistes reticu-
latus) were added to the "marsh", while snails (B.
glabrata and Pomacea glauca), shrimps (Macrobrachium
faustinum), and L. reticulatus were added to the
"river". The molluscicide was added to the "marsh" as
pellets at levels of 2, 5, 10, or 20 g/m2 of marsh sur-
face. The percentage of both B. glagrata and L. reticu-
latus surviving 14 days of exposure was recorded (see
Table 13). The authors concluded that, in this model eco-
system, there was higher mortality of B. glabrata than of
L. reticulatus at low doses of slow-release TBTO, i.e. 2
and 5 g of pellet per m2 of "marsh".
Table 13. Percentage of B. glabrata and L. reticulatus surviving
14 days exposure to slow-release pellets containing 6% TBTO added
to the "marsh" in a "marsh" or "river" model ecosystema
----------------------------------------------------------------
Dose of B. glabrata L. reticulatus
molluscicide
"marsh" "river" "marsh" "river"
----------------------------------------------------------------
20 g/m2 00.0* 00.0* 00.0* 00.0*
(0/240) (0/240) (0/240) (0/240)
10 g/m2 00.0* 00.0* 00.0* 2.5*
(0/240) (0/240) (0/240) (6/240)
5 g/m2 00.0* 16.3** 23.3** 60.0+
(0/240) (39/240) (56/240) (144/240)
2 g/m2 52.9+ 75.4++ 75.8++ 78.8
(127/240) (181/240) (182/240) (189/240)
----------------------------------------------------------------
a From: Jordan (1985).
The percentages represent percent survival at various doses
for all weeks of exposure in replicate experiments. The
values marked with the same symbol are not statistically
different from each other at the 0.05 probability level. The
numbers in parentheses represent the number of survivors
compared to the number of animals exposed.
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: TERRESTRIAL ORGANISMS
Summary
Exposure of terrestrial organisms to TBT derives primarily
from its use as a wood preservative. However, little infor-
mation is available. TBTO has proved toxic to honey-bees coming
into close contact with TBTO-treated wood. TBT compounds are
toxic to insects exposed either topically or via feeding on
treated wood. The acute toxicity to wild small mammals is mod-
erate (between 37 and 240 mg/kg per day). The toxicity of TBTO
to bats is probable but not proven. There is no information on
other species.
9.1. Microcosm studies
Gile et al. (1982) introduced TBTO into a terrestrial
microcosm on pine posts, each microcosm containing four
posts (3.3 x 2.6 x 14 cm) treated with 14C-labelled TBTO
(167 mg/cm3). The microcosms consisted of soil and
endogenous soil organisms, ryegrass, earthworms, pillbugs
(woodlice), mealworms, crickets, garden snails, and a
gravid female gray-tailed vole. There were no effects on
any of the organisms over a period of 77 days. About 95%
of the TBTO remained in the posts for the whole of the
exposure period. A similar system set up to investigate
cricket mortality, with and without predation, showed no
effect of TBTO (Gillett et al., 1983).
9.2. Terrestrial insects
When Gardiner & Poller (1964) exposed larvae of the
common clothes moth (Tineola bisselliella) to wool treated
with a TBTO concentration of 1% by weight, all of the
exposed larvae were killed within the exposure period of
14 days. The action appeared to be that of a contact
insecticide because none of the cloth was eaten. Phenyltin
compounds were not as toxic as TBTO. Baker & Taylor (1967)
found that the action of TBTO more closely resembled the
slow toxicity of a stomach insecticide after exposing
wood-boring beetles (Lyctus brunneus) to impregnated wood.
Contact insecticides such as lindane ( gamma-hexachlorocyclo-
hexane) and dieldrin were 100 times more toxic than TBTO.
The LD50 of TBTO for another wood-boring species, Anobium
punctatum, was 0.254 kg/m3 (application rate to wood).
Saxena & Crowe (1988) applied TBTO, TBT chloride, and
TBT linoleate topically to the thorax of newly-emerged
insects of three species. The LD50 values ranged from
0.48% to 0.72% (dilutions with acetone) for the house fly
Musca domestica, 0.29% to 0.69% for the mosquito
Anophelese stephensi, and 0.52% to 0.87% for the cotton
stainer Dysdercus cingulatus. TBT compounds were more
toxic than the other organotin compounds tested (triphe-
nyltin, tricyclohexyltin, dimethyltin, phenyltin, and
diethyltin). The authors pointed out that TBT compounds
are considerably less toxic than trimethyltin compounds to
Musca sp.
In studies by Kalnins & Detroy (1984), wood was
treated with TBTO (1.9 kg/m3) after sawing and before
use in the construction of beehives. Five hives made from
the treated wood were stocked with bees. Tin residues of
3.24 mg/kg were found in bees during the first summer, and
residues of 8.67 mg/kg were found in the wax of the combs.
However, there were no detectable tin residues in honey.
There was high mortality in the bee colonies over winter,
only one colony surviving. No control colonies died over
winter. Residues in bees and wax in the second year (1.3
and 4.6 mg/kg, respectively) were lower than in the first
year in the one surviving colony.
9.3. Terrestrial mammals
Racey & Swift (1986) housed pipistrelle bats
(Pipistrellus pipistrellus) in roosting cages treated
with TBTO. The bats were pregnant females collected from
nursery roosts, and they were trained to feed on mealworms
before transfer to the experimental cages. The cages were
made of metal, lined with plywood, and were painted with
TBTO as a 1% solution in white spirit (the manufacturers
recommended rate for use of TBTO as a fungicide). The wood
was treated 2 months before the bats were introduced into
the cages. During the course of the 142-day experiment,
seven of the ten bats died. Median survival time was 100
days, with a range between 49 and 142 days. Two deaths in
the white spirit control group and three in the untreated
control group meant that results for TBTO were not stat-
istically significant.
Schafer & Bowles (1985) conducted toxicity and repel-
lency tests on deer mice (Peromyscus maniculatus) and
house mice (Mus musculus) using various TBT salts. Treat-
ment of feed seeds with 2% (by mass) produced clear repel-
lent effects of TBT in both species. The approximate
lethal dose (estimated by increasing the TBT dose until
the test animals died) for TBT acetate and fluoride was
320 mg/kg. The estimated dietary LC50, based on consump-
tion of treated seed used in the repellency tests, varied
between 37.5 mg/kg per day for TBT acetate and 238 mg/kg
per day for fluoride and sulfate. The LC50 for TBTO was
200 mg/kg per day.
10. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: FIELD OBSERVATIONS
Summary
Tributyltin compounds have had a wide range of uses. Most
concern has focussed on their use in the marine environment,
where TBT has been associated with mortality and failure of
settlement of bivalve larvae, reduced growth, shell thickening,
and other malformations in oysters, imposex (the development of
male reproductive appendages in female animals) in mud snails,
and imposex concurrent with population decline in dogwhelks.
Controls on the use of TBT in antifouling paints has led to
recovery of economically important shellfish growth and repro-
duction. Water concentrations of TBT are still high enough in
some areas to affect marine gastropods.
Field and laboratory results for marine molluscs are in
good agreement. Both imposex in dogwhelks and shell growth and
chambering in Pacific oysters are effective biological indi-
cators of TBT contamination.
There have been few studies into the effects on organisms
of TBT in sediment. There are indications that TBT is bioavail-
able to burrowing organisms and can cause mortality in the
field.
Gross toxic effects and histopathological changes have been
reported in farmed marine fish exposed to TBT through the use
of antifouling paints on retaining nets.
Although TBT has been detected in fresh water at high con-
centrations in some areas, there have been few studies on its
effects. Spills of large amounts of TBT from timber treatment
plants have caused ecological damage, but recovery occurred
within 9 months.
Field testing of tributyltin derivatives, mainly slow-
release formulations of TBTO, has shown that it is difficult to
apply TBT without damaging non-target organisms. Recovery
occurs through recolonization.
10.1. Effects on bivalves
In the 1970s, the French oyster industry was
undergoing a crisis. During the 1977, 1978, and 1979
seasons, very poor spatfalls were reported, together with
increasing reports of poor shell growth and shell malfor-
mations. An extensive survey of the occurrence and inten-
sity of malformations related to metal residues in oysters
suggested, for the first time, a connection with organotin
compounds (Alzieu, 1981). The shell thickening was found
to be due to the appearance of chambers in the oyster
shell and interlamellar gel formation in these cavities
(Alzieu, 1981; Alzieu et al., 1982). The authors of these
reports described in detail the shell abnormalities and
the process of calcification of the oyster shell, and
suggested mechanisms of TBT action. TBT is known to in-
hibit oxidative phosphorylation and it has been suggested
that this forms the basis of its action on the shell. It
is also known to complex amino acids. The effect on calci-
fication derives from inadequate calcium addition to the
organic matrix (a process dependent on ATP) and incorrect
deposition of this matrix. Alzieu et al. (1982) found good
correlation between the occurrence of shell thickening and
the proximity of ports where large numbers of boats were
usually moored. These field observations were corroborated
by the finding of similar shell abnormalities in the lab-
oratory when oysters were exposed to TBT fluoride, an
organotin compound present in antifouling paint used on
the boats. Oysters placed in flowing sea-water tanks con-
taining plates coated with TBT antifouling paints died
after a 30-day exposure to an estimated water concen-
tration of 2 µg/litre (organotin leachate) and shell
thickening was found to occur at water concentrations of
0.2 µg/litre.
Alzieu et al. (1982) assessed the shell quality of
18-month-old Pacific oysters ( Crassostrea gigas; also
known as the Japanese oyster) sampled along the eastern
coast of Oleron Island and in the vicinity of La Rochelle,
France. They concluded that the proximity of pleasure
craft ports or a commercial harbour could badly affect the
quality and growth of oyster shells. However, the presence
of other chemicals, along with TBT in the sea water made
direct assessment difficult. The authors, therefore,
carried out a set of experiments to confirm their
conclusions. Groups of oysters from areas with no shell
abnormalities were distributed to other locations, i.e. a
marina, a river, and the laboratory (three groups: a
control group; a group exposed to 50-cm2 panels coated
with TBT fluoride; and a group exposed to coated panels
500 cm2 in area). All oysters died within 30 days after
exposure to the larger panel in the laboratory and within
170 days after transfer to the marina. Oysters exposed to
the smaller panels showed a mortality rate of 30% after
110 days of exposure. Oysters transferred to the marina
site and those exposed to 50-cm2 TBT-coated panels devel-
oped gel-filled shell cavities after 100 and 110 days,
respectively, during the period of shell growth. Analysis
of the oysters for total tin content revealed levels of
< 1 mg/kg (dry weight) in "unpolluted" groups, 110 mg/kg
after 80 days at the marina site, and up to 25 mg/kg after
exposure to 50-cm2 panels in the laboratory.
Maurer et al. (1985) found TBT levels to be related to
inhibition of settlement of Pacific oyster in the Bay of
Arcachon and the Gironde estuary, France. The authors used
arrays of settling tubes mounted around a central tube
which was painted with "International TBT Antifouling"
at a rate of 6.4 g of paint on a surface of 8 dm2. The
settling tubes were mounted at varying distances (between
4.5 and 13 cm) from the central painted tube. In control
arrays, the central tube was unpainted. The arrays of
tubes were placed out in the two study areas in July 1982
and observations on settlement and growth were made in
September and November 1982. In the Gironde region, a
second method was also used; slates were painted with TBT
paint (21 g of "International TBT Antifouling" on a
surface of 26 dm2) and mounted 10 cm apart, alternating
unpainted with painted slates on a rod. The spacing of
tubes in the arrays was up to 25 cm from the central
painted tube. In the Gironde estuary, settlement was com-
parable with controls except on the painted tube; tubes
5 cm or more away from the TBT paint had similar numbers
of settling larvae as the control. However, deaths among
the settled larvae were high; 100% on tubes 15 cm away (or
less), 99.3% on the tube at 20 cm distant, and 78.3% on
the tube 25 cm distant from the paint. Slates showed high
settlement rates and a mortality of 82.5% at 10 cm away
from the paint. In the Bay of Arcachon, there was a much
lower settlement of larvae; in the Villa Algerienne area
there were 178 settlements/dm2 on the treated tubes
compared with 440 on controls, and in the Comprian area
81 or 83 settlements/dm2 on treated tubes compared with
323 on controls. Barnacles, which were less sensitive to
the paint, were also found to settle on the tubes. The
settlement period in this area was longer than that in the
Gironde estuary; some larvae settling later in the season
showed reduced growth compared with controls.
Thain & Waldock (1986) reported that the Pacific oys-
ter was introduced into the United Kingdom during the mid-
1970s. At this time, growth trials were performed at sev-
eral coastal locations. The oysters grew well at most
sites, but at some east coast sites, such as the estuary
of the River Crouch, they exhibited poor growth, reduced
meat yield, and shell thickening. At other sites, such as
the north Norfolk coast, there were good growth results
with none of the deformities found at the Crouch. The
cause of the poor growth results in some areas was inves-
tigated, and Key et al. (1976) found good correlation
between poor growth and high levels of fine suspended
particles. Areas of poor performance in the trials were
not used for the cultivation of the newly-introduced
oyster species. The types of shell abnormality exhibited
by oysters in France were very similar to those observed
in oysters from the east coast of England, which had been
attributed to sediment. The sediment in the marinas used
by Key et al. (1976) for resettlement studies was probably
contaminated with TBTO (Personal communication by M.J.
Waldock to IPCS). In 1982 it was decided to reassess the
causes of poor oyster shell growth in Britain. Levels of
TBT were measured in the estuaries of the Rivers Crouch
and Blackwater, on the east coast of England, and were
found regularly to exceed 0.2 µg/litre, a level shown by
the French studies to be harmful to the oyster (Thain &
Waldock, 1986). In a laboratory study, Waldock & Thain
(1983) investigated the effect of both suspended sediment
and TBT on growth and shell thickening in spat of the
Pacific oyster. They found that TBT levels of 0.16 µg per
litre inhibited and 1.6 µg/litre stopped growth. Shell
thickening was observed in the oysters exposed to TBT.
Exposure to sediment in "clean" water (i.e. containing
no TBT) actually enhanced growth. Thain & Waldock (1986)
reported that, during 1983, the laboratory finding that
TBT and not suspended sediment was affecting growth and
causing shell abnormalities in oyster was corroborated in
the field by studying oysters from different sites.
Alzieu & Portmann (1984) reported that although the
findings clearly implicated TBT as a major cause of growth
problems and abnormalities, they did not completely
exclude the possibility that other chemicals present might
have caused similar effects. They reviewed the effects of
a 1982 ban by French authorities on use of TBT paints on
boats shorter than 25 m. In the Bay of Arcachon area
(where, in 1980 and 1981, 95% to 100% of oysters had shown
deformities), the 1982 figures for deformities were 70% to
80% and by 1983 deformities had declined to 45% to 50%.
The number of oysters from the same area showing deform-
ities in both upper and lower shells was between 70% and
90%, in 1980 and 1981, and zero by 1983. The spatfall,
which in both 1980 and 1981 failed completely, sub-
sequently recovered; it was described as good in 1982 and
excellent by 1983. The authors also reported, however,
that these results were not reflected in another area
(La Rochelle Bay). This was attributed both to its close
proximity to a major commercial harbour and the fact that
the ban on the use of TBT antifouling paints on pleasure
craft in this area had not been as strictly observed as in
the Bay of Arcachon.
His et al. (1986) reported bioassays conducted on
oysters (Crassostrea gigas) using sea water collected from
the Bay of Arcachon both before and after the imposition
of a ban on the use of TBT paints on small boats. The sea
water collected in 1981 caused abnormalities in 40% of
larval oysters over 12 days of observation (compared with
4% in controls), whereas the sea water from 1982 produced
only 12% abnormalities over the same period. Growth of the
oyster larvae was also improved relative to the period
when TBT paints were still being used. In 1981, mean
growth of larval shells over 12 days was 133 µm, in Bay
of Arcachon sea water, compared with 142 µm in controls.
In 1982, mean growth was 162 µm, as opposed to 168 µm in
controls. Alzieu et al. (1986) stated that between 1980
and 1982 about 90% of oysters displayed anomalies in shell
calcification. Each year anomalies became apparent in
April and reached maximum intensity during June and July.
During the period 1983 to 1985, the percentage of oysters
displaying shell anomalies fell steadily. By 1985 none of
the oysters examined had malformations in both valves and
less than 40% had shell anomalies in one of the valves
(usually the upper valve). Alzieu et al. (1989) monitored
oysters in 1986 and 1987 and found that, although oysters
with deformities in both valves had stabilized at < 10%,
those with deformities in at least one valve had risen
again to a peak of 70% for both years.
Effects on oysters have also been reported near to
fish farms containing nets treated with TBT antifoulants.
Davies et al. (1987a) maintained caged Pacific oysters
(obtained from a nursery unit distant from any significant
sources of TBT) at varying distances from fish farms at
Loch Sween, Scotland. They found that significant accumu-
lation of tin was restricted to within 200 m of the fish
farms and that effects on oyster shell structure were
observed at a distance of 1000 m but not at 5000 m. A com-
parison of shell thickness index and tin accumulation
showed significant tin accumulation in those oysters with
the most severe shell thickening.
Studies in the USA and Japan have revealed similar
effects. Stephenson et al. (1986) transplanted culched and
culchless Pacific oysters (Crassostrea gigas) and two
species of mussel (Mytilus edulis and Mytilus
californianus) to areas of San Diego Bay, California, USA,
along a gradient of known sea-water TBT concentrations
(0.01-0.93 µg/litre). Reduced shell growth was observed
in all three species in areas where TBT levels were
highest. Oyster and Californian mussel samples (but not
M. edulis ) showed marked trends of reduced growth with
increasing TBT levels. Shell thickening in the oysters
also correlated with increasing TBT levels. Wolniakowski
et al. (1987) found Pacific oysters in Coos bay, Oregon,
USA, to have thickened and ball-shaped shells. When these
oysters were analysed, they were found to contain high
levels of TBT (49.7 to 189 µg/kg). The most marked
deformities occurred in animals collected in a marina and
near to where boats were painted. Okoshi et al. (1987)
transplanted spat of two different strains of Pacific
oysters (Crassostrea gigas) to two different experimental
field sites in northern Japan for 41 weeks. The number of
chambers in the oyster shells increased gradually in the
Miyagi strain at one site. In contrast, few chambers were
observed in the Hiroshima strain at either site. The
authors concluded that both genetic and environmental
factors were involved in the formation of shell chambers.
Crassostrea gigas is a non-indigenous species in the
United Kingdom and France. Stocks for breeding were intro-
duced into both countries in the late 1960s and originated
from the Miyagi region of Japan (Walne & Helm, 1979).
When Paul & Davies (1986) maintained scallops (Pecten
maximus) and Pacific oysters (Crassostrea gigas) in nets
coated with a TBTO-based paint, the mortality of scallop
spat was 24%, compared with a control mortality of less
than 6%, over the 31-week exposure period. Adult scallop
mortality was less than that of controls in the treated
group, and no effect was observed on growth. No oysters
died. Scallop spat in TBT-treated nets grew significantly
less rapidly than the control spat. Oyster growth, as
measured by mean shell length, was significantly reduced
and had largely ceased after 10 weeks of exposure. A sig-
nificant thickening of the oyster shell was observed
within 10 weeks of exposure and continued throughout the
31-week exposure. It was maintained even after transfer to
untreated nets for 10 weeks.
Minchin et al. (1987) monitored bivalve settlement in
Mulroy Bay, Ireland, between 1979 and 1986 using settling
panels placed in the sea. They found that, during this
period, settlement either failed or was reduced. Scallop
numbers fell from an average of > 1000 per panel in 1979
to zero in 1983. In 1984, no settlement of any bivalve
species was recorded on the panels. This reduced settle-
ment corresponded to the introduction of organotin fish-
net dips in local salmonid farms. The first use of TBT
paints appears to have been in 1981. This use of organotin
compounds ceased after 1985. In 1986, in all bivalves
monitored (except flame shells), there was again good
settlement. Scallops (Pecten maximus) and flame shells
(Lima hians and Chlamys varia) (all members of the
Pectinacea) were found to be particularly sensitive to
organotin compounds. It is not known whether the effect of
the TBT was on the reproduction of the bivalves or a toxic
effect on the larvae.
10.2. Effects on gastropods: imposex
Between the years 1972 and 1976, Smith (1981c)
sampled mud snails (Nassarius obsoletus) from four
locations bordering Long Island Sound in Westport and
Fairfield, Connecticut, USA. The snails were examined
for imposex (see section 8.2.3.6), and the results were
quantified by measuring the percentage of snails in a
sample showing any imposex and estimating the degree of
imposex within indi- vidual snails. In two of the
areas, adjacent to a yacht yard in Southport Harbour and
at the mouth of the harbour, 95% to 100% of snails had
some degree of imposex. Both showed significantly
more imposex than the other two sites, an area used
to moor a few old boats and an area protected from human
interference. The mud snails in this last area showed no
imposex. The area with a few old boats showed imposex
levels of 30% to 50%, much less than in the first two
areas. Smith (1981a) collected mud snails from three of
the locations, the yacht yard in the harbour (where
all female snails were abnormal and degree of imposex
greatest), the mouth of the harbour (where almost all
snails were abnormal but the degree of imposex changes
was intermediate), and the area protected from human
impact (at least 3.5 km from the nearest marina, where
all female snails were normal). The author labelled
these areas as "dirty", "intermediate", and "clean".
Snails transferred from the "clean" area to the
"dirty" area developed imposex. In those transferred
from the "dirty" area to the "clean" area the degree
of imposex was reduced. An analysis of chemicals
present in water from the "dirty" area was then carried
out and snails were exposed to some of the
contaminants individually, i.e. marina disinfectants,
detergents, copper antifouling paints, leaded
gasoline, combustion emissions, and two types of
TBT-based antifouling paints. Only the tin-containing
antifouling paints increased the level of penis
expression in female snails.
In a survey of the dogwhelk Nucella lapillus around
the south-west of England, Bryan et al. (1986) found
imposex to be widespread. The south coast of England was
the most severely affected. Populations showing the
highest incidence and highest intensity of imposex were
close to areas of boating or shipping activity. The degree
of imposex had increased markedly between 1969 and 1985 in
Plymouth Sound (an area on the south coast with large
numbers of small boats and ships), coinciding with the
introduction and increasing use of TBT-containing anti-
fouling paints in the area. Imposex correlated with
concentrations of sea-water tin (TBT fraction) and resi-
dues of tin in the dogwhelks (Fig. 6). Transferring whelks
from an area with little boating activity to Plymouth
marina resulted in a marked increase in the degree of
imposex. Bailey & Davies (1988b) found an increase in the
degree of imposex and also a higher incidence of penis
development in female dogwhelks collected in 1987 in the
Firth of Forth, Scotland, compared with those caught in
1975.
One of the effects of imposex on the female dogwhelk
is the blocking of the pallial oviduct, preventing the
release of egg capsules and rendering the female sterile.
A high incidence of females carrying aborted capsules was
found in declining populations close to sources of TBT.
The build-up of aborted capsules seemed, eventually, to be
lethal to the female; there were fewer females than
expected in affected areas (Gibbs & Bryan, 1986). The same
authors reported that the gross morphological changes
occurring in late imposex in the dogwhelk seem to be irre-
versible, since animals transferred from a moderately-
contaminated site to a "clean" site showed no resorption
of the penis. Gibbs & Bryan (1987) stated that imposex in
the dogwhelk was seen in sea water with TBT concentrations
of less than 1 ng tin/litre. They reported that the repro-
ductive failure, along with a lack of recruitment, had led
to population declines, almost to the point of extinction,
in areas of heavy TBT contamination. Gibbs et al. (1988)
experimentally transferred dogwhelks from an uncontami-
nated area to one showing water concentrations of 9-19 ng
tin/litre (TBT fraction) and demonstrated the development
of imposex within 18 months at the new location. These
transferred adults were able to spawn for much of this
period. The authors compared these results with results
for juveniles, which developed imposex earlier and were
sterile before reaching maturity. They discussed the
implications for the recolonization of areas where repro-
duction in the dogwhelk has been eliminated. Adults are
irreversibly affected by imposex. Recolonization is
unlikely until the TBT levels in sea water fall to around
2 ng tin/litre, a concentration at which the juveniles
are not sterilized before they reach sexual maturity. The
extent of recovery of populations would, therefore, depend
on the success of control measures and the water
concentrations resulting from bigger ships exempt from the
ban on TBT-containing antifouling paints.
Bryan et al. (1987) stated that while the evidence did
not show conclusively that TBT is solely responsible for
imposex in the dogwhelk, circumstantial evidence was con-
siderable. Imposex is related to the level of boating
activity. There is a significant relationship between
imposex and the body residue of organotin. Populations
that are in decline show the highest levels of TBT. Female
populations are declining faster than males and also have
the higher levels of TBT. The rise in the degree of
imposex coincided with the introduction and use of anti-
fouling paints containing TBT. Imposex has been shown to
be caused by TBT in other species of stenoglossan snails,
i.e. mud snails (Smith, 1981b). A significant decline in
imposex in monitored juvenile Nucella (Personal communi-
cation by P.E. Gibbs & G.W. Bryan to IPCS) followed the
introduction of TBT legislation in the United Kingdom.
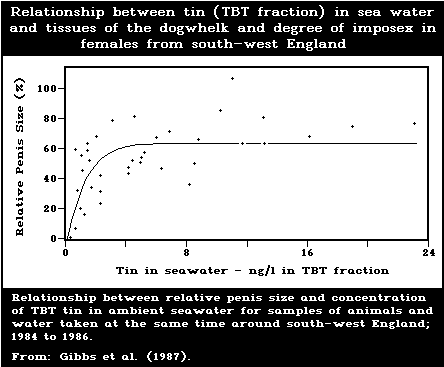
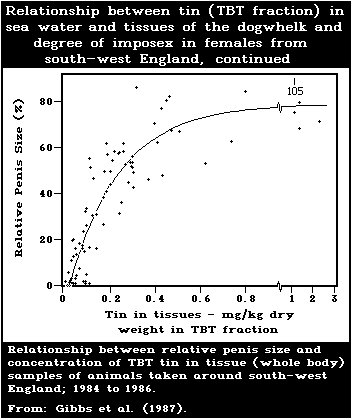 10.3. Effects on farmed fish
Wooten et al. (1986) reported the effects of exposure
to TBT antifouling paints applied to retaining nets for
farmed salmon. Fish exposed to newly-treated cage nets
were reported to be blind. The authors examined diseased
post-smolt salmon from cages treated with TBT at the time
of smolt transfer. Elevated tin levels were found in all
salmon exposed to the TBT paint; liver residues ranged
between 1.01 and 1.62 mg tin/kg wet weight. Residues in
the liver of blind fish ranged between 1.01 and 1.07 mg
tin/kg wet weight. Blindness had been caused by rupturing
of the eye; the eye lens was missing. Eye tissues appeared
normal histologically apart from the rupture; there were
no histological lesions. The kidney, liver, stomach, heart
and muscle of the blind fish appeared normal histologi-
cally. The intestine showed mucosal sloughing, vasodi-
lation, and pyknotic nuclei. The spleen had an "open
structure" with increased numbers of erythrocytes. There
was thickening of secondary gill filaments and some
necrosis and capillary separation in the epithelium. Other
fish in the affected population were reported to have
swellings along the lateral line. These were shown histo-
logically to be thick folds of epidermis under which there
was cellular infiltration of necrotic collagen forming an
abscess. Pancreas disease was also reported in affected
fish. In the liver there was a breakdown of lobular struc-
ture with lack of cohesion between cells. The effects were
thought to be consistent with poisoning by organotin.
10.4. Effects of TBT-contaminated sediment
Matthiessen & Thain (1989) studied the recolonization
by marine organisms in the field of sediment contaminated
with TBT-containing paint. Sediment was collected from
mudflats, and macroorganisms present were killed by
repeated freezing and thawing. TBT was added to the sedi-
ment in the form of abraded paint. The paint was abraded
with scourers used on yachts to produce material of the
kind likely to contaminate sediments normally. Sediment
samples containing 0.1, 1, 10, and 100 mg TBT/kg were
prepared. The contaminated sediment was returned to the
mudflats and placed in excavated trenches (3 m x 30 cm
wide x 20 cm deep) lined with polyethylene mesh (5-mm
apertures). Recolonization of the sediment could, there-
fore, take place both by settlement of organisms from
above and by lateral transfer of organisms from adjoining
mud. The sites were revisited five times during the next
160 days when samples were taken both to count recolon-
izing organisms and to measure TBT levels at various
depths. Surface sediment decontaminated rapidly; water
movement would have removed sediment and deposited new.
The subsurface TBT levels remained reasonably stable
throughout the experiment. Burrowing activity (estimated
by counting casts on the surface) of the polychaete
Arenicola marina was reduced at all concentrations of
TBT, though the effect of 0.1 mg TBT/kg disappeared after
3 months. A dose-related reduction in populations of the
burrowing polychaete Scoloplos armiger and burrowing
amphipod Urothoe poseidonis was observed over the whole
concentration range. There were no clear effects on other
species, including molluscs. The authors pointed out that
some unaffected groups were associated with the surface
sediment, where TBT was lost rapidly. Feeding behaviour
of these surface dwellers, such as the cockle (Cardium
edule), would lead to little TBT exposure, since they fil-
ter overlying sea water and ingest little or no sediment.
Species associated with deeper layers of sediment and
feeding on fine particles, such as Urothoe poseidonis,
showed greater effects of TBT, resulting, presumably,
from greater exposure rather than greater inherent sensi-
tivity. The authors also noted the wide range of sensi-
tivity of different species in laboratory tests without
sediment. The bioavailability of TBT associated with sedi-
ment would probably be different from that dissolved in
water.
10.5. Effects of freshwater molluscicides
Following earlier laboratory trials on the use of TBT
as a molluscicide for schistosomiasis control (Deschiens &
Floch, 1962), Deschiens et al. (1966) applied TBTO to a
pond used for fish culture, in Cameroon, of approximately
1 metre in depth and with an area of 50 m2 (approximately
50 tonnes of water) and a water temperature of 23 °C.
Caged pond snails (Biomphalaria [Australorbis] glabratus
and Bulinus contortus), 10 to a cage supplied with food,
were placed in the pond in different areas and at differ-
ent depths. Renewal of cages was performed to study the
persistence of the compound. The pond was treated with
TBTO, as "Biomet" (50% TBTO and 50% toxicologically
inert dispersant), at rates giving TBTO concentrations in
water of 0.015, 0.03, or 0.045 mg/litre, and the side
effects on fish and plankton were investigated. At 0.045
mg/litre, TBTO killed 100% of the snails and 70% of the
fish within 24 to 48 h. At 0.03 mg/litre, TBTO killed 100%
of snails within 2 to 3 days, but no effect on the fish
was observed within 15 days. At 0.015 mg/litre, the TBTO
killed all of the snails within 4 to 7 days and again had
no observable effect on the fish. The authors considered
this to be a practical concentration to achieve full
effect on snails without killing non-target organisms.
Various planktonic organisms (desmids, daphnids, and
aquatic insect larvae) were killed by TBTO at 0.5 mg/litre
experimentally. However, no effect was found on any of
these species in the pond treated at 0.015 mg/litre,
although there was some inhibition (but no deaths) of
these organisms at 0.03 and 0.045 mg/litre.
Gilbert et al. (1973) applied slow-release TBTO in
underseal (quick-drying asphalt-rubber-asbestos-clay paste)
to four sites, in Brazil, at concentrations of 15, 15, 50,
or 300 g TBTO/m2, to control Biomphalaria tenagophila
and B. straminea. Dead fish were observed at the sites
treated with 50 or 300 g/m2 and also at one of the two
sites treated with 15 g/m2, a static man-made pit. At
the other site, with a flow rate of 100 to 200 litre/min,
there was a 100% reduction in the snail population (B.
tenagophila) maintained for up to 12 months, while both
fish and aquatic insect life were apparently normal one
month after treatment. At this site abundant natural veg-
etation flourished in the immediate vicinity of the mol-
luscicide. Toledo et al. (1976) found that 15 g TBTO/m2
eliminated snails from 76% of treated sites for 1 year.
Guppies, which are often present in highly polluted sites,
normally suffered some mortality at the beginning of mol-
luscicidal treatment but in general they tolerated it
well. The authors found no lasting effect on plant or
insect life, and concluded that, because of the ability of
an unidentified fungus to grow profusely, microbiological
life continued in treated areas.
Shiff et al. (1975) applied BioMet SRM pellets (con-
taining TBTO) at concentrations of 20 and 30 g/m2 to a
night-storage dam in Zimbabwe. At the lower application
rate, pellets remained active against the snails (various
Biomphalaria spp.) for up to one year. At 30 g/m2, 100%
mortality of snails was achieved within 2 weeks of appli-
cation and was maintained up until the last sampling time
2 months after application. Prior to treatment, 80
specimens of Tilapia fish sp. and 1 of Clarias gariepinus
were caught. Four weeks after treatment 26 Tilapia were
caught and one dead Clarias was found. Eight weeks after
treatment 400 Tilapia were caught, of which 80 were
examined and all appeared normal.
Ayala et al. (1980) applied slow-release molluscicide
(rubber impregnated with 11% TBTO) at a rate of between 5
and 15 g/m2 to a site in Brazil and studied the effect on
non-target organisms. The TBTO molluscicide was initially
herbicidal towards floating vegetation but did not affect
either plants rooted in the mud or marginal plants. The
chemical was initially repellent to aquatic animals, some
of which were killed by the molluscicide. Within 3 months,
populations of non-target snails such as Pomacea sp.,
Drepanotrema sp., and a Physa sp. had returned to normal.
Although no fish had returned within 3 months, after 5
months numbers had returned to normal. In fact, 5 months
after application all but Spirogyra sp. were normal, and
the molluscicidal activity of the TBTO was retained for
more than one year. The authors concluded that after 3 to
5 months the action of the TBTO molluscicide is confined
to the bottom mud where Biomphalaria glabrata snails spend
much of their time.
10.6. Effects from spills
According to Waldock et al. (1987a), major inputs of
TBT into the environment may have arisen from spills of
timber-treatment products, often containing dieldrin and
pentachlorophenol as well as the organotin. They reported
that a detailed study was carried out after a spill con-
taminated a section of the Newmill Channel, Kent, United
Kingdom. There was a major kill of fish and all macroin-
vertebrates (except oligochaetes, chironomid larvae, and
elmid beetles) were also killed. Concentrations of TBT in
the water shortly after the spill were 540 µg per litre,
falling to 10 µg/litre after 3 weeks and 0.75 µg per
litre after 6 weeks. Nine months after the incident,
macroinvertebrate populations were reported to have
recovered. The authors stated that five such major spills
from timber treatment plants had been reported in the
United Kingdom within 2 years.
10.7. The use of indicator species for monitoring the environment
Both shell growth and shell chambering in Pacific
oysters and imposex in dogwhelks have been used as bio-
logical indicators of TBT contamination. Smith et al.
(1987) transferred juvenile Pacific oysters to major bays
and harbours of California, USA, known to contain elevated
levels of TBT. They observed a graded increase of stunted
growth and/or shell deformation, which was associated with
poor flushing and the proximity of large numbers of boats.
Gibbs et al. (1987) used the dogwhelk to monitor water
around the south-west peninsula of England. They found the
species to be a very sensitive indicator, especially if
the individuals were about 1 year old and in the early
stages of ovarian development. Davies et al. (1987a) found
imposex in dogwhelks to be a far more sensitive indicator
than either shell thickening in oysters or tin accumu-
lation. The dogwhelk has been used to identify areas of
contamination associated with seasonal small boat activity
and salmon farm cages in Scottish sea lochs (Davies et
al., 1987b), in areas of pleasure craft activity, fishing
harbours and a boat yard in the Firth of Forth (Scotland)
(Bailey & Davies, 1988b), and to investigate contamination
from an oil terminal in Sullom Voe, Shetland (Bailey &
Davies, 1988a).
11. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
11.1. Single exposure
Summary
The acute toxic effects of the various TBT compounds that
have been studied are comparable and are characteristically
delayed for several days. Oral LD 50 values in laboratory ani-
mals range from approximately 40 to 250 mg/kg body weight.
These compounds exhibit greater lethal potential when adminis-
tered parenterally, relative to the oral route, probably
because they are only partially absorbed from the gut. Acute
toxicity via the dermal route is low.
TBT compounds are potent skin irritants and extreme eye
irritants. Dermal exposure to TBTO appears to have little
sensitization potential.
Aerosols of TBT compounds are highly toxic. However, TBT
vapour/air mixtures at room temperature produce no effect, even
at saturation.
Other effects of acute exposure may include alterations in
blood lipid levels, the endocrine system, liver, and spleen,
and transient deficits in brain development. The toxicological
significance of these effects, reported after high single doses
of the compounds, is questionable and the cause of death
remains unknown.
11.1.1. Oral and parenteral administration
The acute toxicity of tributyltin to laboratory mam-
mals by various routes of administration is summarized in
Table 14. The acute oral LD50 for the rat ranges between
94 and 234 mg/kg body weight and for the mouse between 44
and 230 mg/kg body weight. Truhaut et al. (1976) pointed
to the delayed toxicity of tributyltin and, therefore, the
necessity to continue the observation of animals, after a
single acute dose, for several days. The period of post-
dosing observation is therefore indicated in Table 14.
LD50 values for ip and iv administrations of TBT are
very much lower than in the case of the oral route (10
mg/kg for rat ip and 6 mg/kg for mouse iv).
Table 14. Acute toxicity of tributyltin to laboratory mammals
------------------------------------------------------------------------------
Species TBT Route Observation LD50 (mg/kg Reference
perioda body weight)
(days)
------------------------------------------------------------------------------
Rat oxide oral 7 194 (165-227)b Elsea & Paynter (1958)
oxide oral 7 148 (113-195)c Elsea & Paynter (1958)
oxide oral 7 180 (132-228) Truhaut et al. (1976)
oxide oral 21 197 (137-273) Funahashi et al. (1980)
oxide oral 127 Schweinfurth (1985)
oxide ip 14 20 (18-21) Poitou et al. (1978)
oxide oral 234 Sheldon (1975)
fluoride oral 200 Sheldon (1975)
fluoride oral 14 94 Schweinfurth (1985)
chloride oral 14 122 Schweinfurth (1985)
acetate oral 113.5 Klimmer (1969)
benzoate oral 141 Klimmer (1969)
benzoate oral 14 99/203 Schweinfurth (1985)
oleate oral 225 Klimmer (1969)
linoleate oral 14 190 Schweinfurth (1985)
abietate oral 14 158 Schweinfurth (1985)
naphthenate oral 14 224 Schweinfurth (1985)
Mouse oxide oral 7 85 (52-130) Polster & Halacka (1971)
acetate oral 7 46 (25-85) Pelikan & Cerny (1968)
oleate oral 7 230 (175-301) Pelikan & Cerny (1968)
benzoate oral 7 108 (74-156) Pelikan & Cerny (1968)
chloride oral 7 117 (80-170) Pelikan & Cerny (1968)
laurate oral 7 180 (136-237) Pelikan & Cerny (1968)
oxide sc 7 200 (140-270) Polster & Halacka (1971)
oxide iv 7 6 (5.5-6.5) Truhaut et al. (1976)
oxide ip 14 16 (15-17) Poitou et al. (1978)
Rabbit fluoride dermal 680 Sheldon (1975)
------------------------------------------------------------------------------
a Following a single application of TBT, the observation period was as indicated.
b Application as aqueous suspension.
c Application in corn oil.
Pelikan & Cerny (1968) administered TBT (as the acet-
ate, benzoate, chloride, laurate, or oleate) to white mice
(body weight 25 g) in a single oral gavage dose of 500
mg/kg body weight, dissolved in sunflower oil. Ten animals
were used for each TBT compound. The mice were observed
for 8 h before being killed, and the viscera were examined
histologically. Four hours after treatment, all animals
showed signs of intoxication with the exception of those
given the laurate; after 8 h all showed toxic symptoms.
Gross damage was seen in the digestive tract, liver, and
spleen. Histological findings included a steatosis of the
liver cells in all animals (but to varying degrees),
traces of lipid in renal tubule cells (in those animals
receiving oleate or laurate) and many haemorrhages in the
digestive tract and kidneys. Effects on the liver and
spleen were also noted after dermal absorption of TBTO
(Pelikan & Cerny, 1969). However, no real conclusions can
be drawn from these studies as there was no clear reported
evidence that steatosis was caused either by the TBT or by
the fatty acids.
In studies by Funahashi et al. (1980), Sprague-Dawley
rats were given a single dose, in olive oil, of 100 mg
TBTO/kg body weight by intubation and were examined over
the following 21 days. Adrenal weight was slightly
increased 12 h after treatment and reached a maximum on
the second day. Histological changes in the adrenal had
returned to normal by day 14. The thyroid follicles showed
signs similar to those produced by hypophysectomy, i.e.
distension with colloid, flat epithelial cells. These
changes were severe after 72 h, but had returned to normal
within 14 days. Absolute pituitary weight was increased
slightly (and not significantly) 1 and 2 days after
dosing, while relative weight increased significantly.
There was atrophy of pituitary adrenocorticotrophin (ACTH)
cells 6 h after dosing. After 72 h, the staining of ACTH
cells became more intense. There were marked reductions
in the circulating levels of both thyroid stimulating
hormone (TSH) and thyroxine (T4) during the 72 h follow-
ing dosing, serum titres falling to one half and one sixth
of control values for the two hormones, respectively. The
authors concluded that TBTO had both a direct and an
indirect effect on the thyroid, since initially there was
a simultaneous rise in T4 and fall in TSH. Serum cortisol
levels increased to twice the control level 96 h after
treatment with TBTO at a level of 100 mg/kg body weight.
Intramuscular ACTH administration, 8 h after dosing, led
to increased cortisol in the blood of both treated and
control rats. After 16 h, controls showed release of
cortisol after ACTH stimulation, but treated rats showed a
decrease in circulating levels of cortisol after similar
stimulation.
Matsui et al. (1982) reported the effects of a single
dose of TBT fluoride (100 mg/kg body weight) given by
gastric intubation to Japanese white rabbits. A reversible
but pronounced hyperlipidaemia was observed, particularly
involving triglycerides and total cholesterol. Ultracen-
trifugation showed a marked increase in the chylomicron
plus VLDL (very low density lipoprotein) fraction. The
activity of lipoprotein lipase (LPL) in plasma was reduced
to about 50% of control levels. Fasting levels of blood
glucose were elevated, and the response to iv infusion of
glucose (given 3 days after the TBT fluoride) was
inhibited (insulin release reduced). The authors suggested
that the hyperlipidaemia was the result of reduced LPL
activity, which in turn was brought about by inhibition of
insulin release.
Calley et al. (1967) investigated the effects of TBT
acetate on liver function in rabbits. Following a single
oral dose of TBT acetate at 50 mg/kg body weight (half of
the measured LD50 in rabbits), only the serum glutamic-
pyruvate transaminase (SGPT) activity was affected. This
activity was not elevated after 48 h but was increased
within 144 h of dosing. Prothrombin time, alkaline phos-
phatase, and thymol turbidity showed no significant
changes from control values. SGPT is a highly specific
indicator of liver damage, directly reflecting liver cell
injury. Tributyltin acetate also significantly increased
the hexabarbitol-induced sleeping times of mice; a 50
mg/kg body weight oral dose increased the sleeping time
from 29 to 43 min following a standard dose of the
narcotic.
When Aldridge et al. (1977) administered TBTO (and its
gamma-keto, gamma-hydroxy, and delta-hydroxy metabolites)
intraperitoneally to mice on two consecutive days (at dose
levels of 12.5, 25, and 50 µmol/kg body weight), they
found no evidence of cerebral oedema after any of the
treatments.
Crofton et al. (1989) gave rat pups a single dose of
TBTO on day 5 following birth by gastric intubation with
0, 40, 50, or 60 mg/kg body weight. The monitoring of
behavioural parameters up to 62 days post-dosing showed no
persistent effect on motor activity or acoustic startle
response.
When Barnes & Stoner (1958) fed rats with tributyltin
acetate during the first 3 months after birth, they found
increased brain and spinal cord water content at the
highest dose tested (100 mg/kg diet). This increase was
not sufficiently great to be seen in histological sec-
tions. Bouldin et al. (1981) found no light or electron
microscopic evidence of neuronal damage in the hippocampus
or the pyriform cortex of neonate or adult rats exposed
daily to tributyltin acetate at 10 mg/kg body weight by
gavage for up to 30 days.
O'Callaghan & Miller (1988) reported the effects of a
single ip injection of TBTO on neonatal rat brain. The
rats were injected when 5 days old with 2, 3, or 4 mg/kg.
They were sacrificed at 13, 22, or 60 days of age, and
various proteins in homogenates of brain tissue were
measured by radioimmunoassay. Brain weight was reduced in
a dose-dependent manner, the cerebellum being most
affected. There was no evidence of altered brain histology
under the light microscope. Dose-dependent and region-
dependent decreases were found in P-38 (a synaptic-vesi-
cle-associated protein) and myelin basic protein (a
protein associated with oligodendroganglia and the myelin
sheath); there were decreases in both total (per tissue)
and concentration (per mg) levels of these proteins in the
cerebellum and forebrain but not the hippocampus. These
effects were seen at a dose level that did not affect body
weight. However, in contrast to the neurotoxic effects of
trimethyltin compounds, which are irreversible and persist
into adulthood, these neurotoxic effects of TBTO were
transitory at dose levels that did not affect body weight.
Robinson (1969) monitored tissue levels of catechol-
amines after ip administration of TBTO, in corn oil, to
rats at a level of 10 mg/kg body weight. This dose
exceeded the 6-day LD50, determined by the same authors
to be 7.21 mg/kg. Brain noradrenalin levels were signifi-
cantly reduced at 2, 24, and 48 h after dosing, whereas
5-hydroxytryptamine levels were only reduced (though
markedly) after 48 h. Noradrenalin levels in heart tissue
were also significantly reduced after 2, 24, and 48 h.
Adrenal adrenalin and noradrenalin levels were reduced
after 24 and 48 h, but only adrenalin was affected after
2 h.
11.1.2. Dermal administration
The acute LD50 of TBTO administered to rabbits via
the dermal route is very high, i.e. approximately 9000
mg/kg body weight (Table 14). Although there is dermal
absorption of TBT (see later), the degree of absorption is
not great enough to lead to acute toxic effects sys-
temically, except at very high exposure levels.
11.1.3. Administration by inhalation
In a "nose only" inhalation exposure (to minimize
dermal exposure and exposure through ingestion) lasting
4 h, the acute LC50 of TBTO for the rat was estimated to
be 77 mg/m3 by measurement of airborne droplets. This
value decreased to 65 mg/m3 when "inhalable" particles,
10 µm or less in diameter, were considered. There was
evidence of lung irritation and oedema in this study
(Schweinfurth, 1985). When groups of 10 male and 10 female
rats were each exposed to atmospheres containing almost
saturated vapours of TBTO, TBT benzoate, or TBT naphthen-
ate once for 7 h, no deaths occurred during exposure or in
a 14-day observation period following exposure. Only minor
clinical signs were noted occasionally during the exper-
iment, such as slight nasal discharge (Schweinfurth, 1985).
Truhaut et al. (1979) exposed mice to an aerosol of
TBTO in olive oil, for either a single 1-h period or seven
1-h periods on successive days, using TBTO concentrations
in air ranging between 0.05 and 0.4 mg/litre (50 to
400 mg/m3). Exploratory behaviour was scored over 5-min
periods 2 h after the single exposure was complete or 24 h
after the last of the seven exposure periods. The lower
two exposure doses caused significant increases in explo-
ratory behaviour (17% and 5% for 42 and 84 mg/kg, respect-
ively) while the higher exposure doses reduced exploratory
behaviour (-18% and -38% for 170 and 340 mg/kg, respect-
ively). Truhaut et al. (1981) found a median survival time
for mice of 22 min and for guinea-pigs of 9 min after
exposure to an aerosol of tributyltin (concentration not
stated). They reported that only tissues in the respirat-
ory system showed significant lesions. There was diffuse
congestion of the pulmonary blood vessels extending to the
septal capillary beds. There were also inflammatory
responses in the trachea and bronchii, secretion of mucus
in the bronchii and bronchioles, and distension and rup-
ture of the alveoli.
Anger et al. (1976) exposed guinea-pigs to aerosols of
TBTO ranging between 0.1 and 1 mg/litre air for 1 h. All
males exposed to 0.2 mg/litre died, and 12 out of 15
females exposed to 1 mg/litre died. No particular lesions
were observed in those animals that died, with the excep-
tion of a general congestion of the lungs. Exposure of
either males or females to 0.17 mg/litre caused no deaths,
though nasal irritation was observed; all these animals
survived a further 7 days of observation.
11.1.4. Irritation and sensitization
11.1.4.1 Skin irritation
In a study by Elsea & Paynter (1958), undiluted TBTO
was applied to the closely-clipped skin of the abdominal
area of rabbits, which was then covered with rubber dental
damming, gauze, and adhesive tape. After an exposure
period of 24 h, the covering was removed, and the TBTO was
washed off, as far as possible, by sponging with warm
water. This single application of doses up to 11.7 g/kg
body weight caused some of the rabbits to die from the
effects of TBTO absorbed into the body. There was, how-
ever, only moderate dermal irritation, characterized by
reddening, oedema, atonia, blanched areas, and areas of
brown discolouration. Examination of the skin at autopsy
showed some subcutaneous oedema. Repeated dermal appli-
cation of TBTO, impregnated into paper at 8 mg/kg, daily
for 5 days, only affected one animal out of four. This
rabbit showed slight erythema and oedema following the
first and second applications. Later in the experiment,
the skin appeared normal. No detailed histological examin-
ation of the skin was carried out.
Pelikan & Cerny (1969) applied to the shaved skin of
rats two preparations of TBTO, i.e. "Lastanax T" (con-
taining 20% TBTO plus a medium of water, alcohols of short
chain length, and n -alkyl-polyethylene oxide) and
"Lastanax P" (containing 15% TBTO plus a medium also con-
taining bis-(5-chloro-2-hydroxyphenyl)-methane). Actual
doses applied, corresponding to water dilutions of 100%,
33%, 10%, and 1% of the preparations, were 185, 61.5,
18.5, and 1.85 mg TBTO/kg body weight for Lastanax T and
145, 47, 14, and 1.4 mg TBTO for Lastanax P. Controls
received either water or the medium without TBTO. The
experiment was carried out in duplicate. In the first
series, clinical observations were made twice daily for
60 days, whereas in the second series rats were killed on
day 10 and the skin was examined histologically. Control
rats showed no clinical or histological effects on the
skin. However, clinical and histological changes were
found in all treated rats, even with the 1% dilution,
though severity increased with exposure dose. On the first
and second days after application, there was reddening of
the skin and oedema developed. From day 3, haemorrhagic
eschars developed with well defined borders; there was no
inflammation of the surrounding areas of skin. Between
these foci, numerous papules and pustules developed, some
with bleeding; the apices of some papules were necrosed.
Later the eschars joined to create larger areas of
ulceration. Healing of the areas began between the 9th
and 12th days; necroses disappeared by day 20, pustules 3
to 4 days before, and papules by the 30th to 33rd day. All
signs had disappeared after 35-38 days (exposure to 1% and
10% solutions) or after 45-50 days (exposure to 33% or
100% solutions). Changes with Lastanax P were similar, but
less severe, than with Lastanax T. In the histological
study, numerous large bullae, filled with leucocytes and
coagulated exudate (proteins), were found under the
stratum corneum of the epidermis after 10 days. Akanthosis
and vacuolation of epidermal cells occurred following
exposure to the 10% and 33% solutions and, to a lesser
extent, the 1% solution. Small haemorrhages were found.
Epidermal changes were dispersed and alternated with areas
of skin appearing normal. The authors suggested that the
TBTO NOEL for human skin should be set at 0.005% to 0.01%.
In a study by Middleton & Pratt (1977), TBT chloride
was applied to a shaved area of dorsal skin of male
Alderley Park (Wistar-derived) rats, four-weeks old (body
weight 50-80 g), in absolute ethanol as a solution of
10 mmol/litre. This dose was equivalent to 67 nmol/cm2.
The TBT had produced microscopic changes in the skin
within 2 h of application. Polymorphonuclear leucocytes
accumulated in capillaries and in dermal tissues. Epider-
mal cells showed progressive degenerative changes between
2 and 8 h after application until, by 8 h after appli-
cation, separation of the epidermis and dermis had
occurred and fluid collected in this separation. Many
inflammatory cells were present in both dermis and epider-
mis. There was widespread epidermal necrosis within 12 to
24 h, and separation of necrotic epidermis from the dermis
was almost complete by this time. The vesicles formed by
this separation were frequently packed with inflammatory
cells as well as exudative fluid. Regeneration of the
epidermal layer was observed after 18 to 24 h and dermal
inflammatory infiltration was regressing by this time.
Erythema, visually assessed and scored, reached a maximum
within 5 h of application and remained at this level for
48 h. The erythema had subsided by 72 h after application.
Vascular permeability of the skin was assessed by
injecting rats with a dye, tryphan blue, 45 min before
sacrifice. There was a biphasic response. At 2 h after
application of TBT chloride, there was an initial increase
in permeability of the skin to 135% of control levels. A
second peak began at 12 h and persisted for more than
25 h after application. The effect was still evident after
48 h and had occurred in both the treated and untreated
flanks of the animal, indicating absorption of the com-
pound and a systemic effect. The water content of the skin
was increased by TBT chloride, reaching a peak within 2 h
of application; by 10 h this effect had disappeared.
Middleton & Pratt (1978) reported that TBT produced focal
epidermal necroses and dermal inflammation at levels as
low as 33 nmol/cm2, fairly extensive epidermal necroses
and dermal inflammation at 67 nmol/cm2, and almost total
epidermal necrosis at 167 nmol/cm2.
11.1.4.2 Eye irritation
Pelikan (1969) applied TBTO, as "Lastanax T or P",
in a single dose of 0.03 ml to the left eye of rabbits.
Lastanax T is 20% TBTO in alcohols of short chain length,
non-ionic surface active substances ( n -alkyl-polyethyl-
ene oxide) and water. Lastanax P is 15% TBTO in the same
vehicle with the addition of bis-(5-chloro-2-hydroxyphe-
nyl) methane. A 10% and a 1% solution in water were used.
The 10% solution represented an actual TBTO dose of 6.1
and 4.6 mg/kg body weight for the two formulations,
respectively. With the 10% solution, both rabbits died,
11 and 12 days after application. Severe ulceration of the
eye preceded death. Histopathological examination of
internal organs after death revealed hyperaemia of the
brain and medulla oblongata and hyperplasia of reticulo-
endothelial cells of the spleen. Necrotic changes were
seen on the cornea of eyes treated with the 1% solutions
within 3 h of application, and symptoms worsened over the
next 2 to 5 days. Recovery was incomplete 100 days later.
11.1.4.3 Skin sensitization
Poitou et al. (1978) investigated the skin-sensitizing
potential of TBTO in guinea-pigs using the Magnussen-
Kligman method. The concentrations used for sensitization
were 1% (intradermal phase) and 5% (topical phase). Using
challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals.
11.1.5. In vitro studies
Johnson & Knowles (1983) demonstrated that incubation
of rat blood platelets with TBT chloride or TBTO, in
vitro, inhibited their ability to take up 14C-labelled 5-
hydroxytryptamine (5-HT). The organotin also caused
release of 14C-labelled 5-HT, taken up before exposure,
along with endogenous 5-HT. Treating rats ip with TBT
chloride at 5.0 mg/kg body weight also led to reduced up-
take of 5-HT (37% inhibition) 30 min after treatment. The
level of 5-HT in platelets, however, was unaffected by the
in vivo treatment with TBT chloride. knowles & Johnson
(1986) reported that exposure of rat platelets to TBT
chloride inhibited aggregation induced with ADP or colla-
gen. The inhibition of ADP-induced aggregation was depen-
dent on the dose of TBT and on the exposure time prior to
ADP addition. Exposure of the platelets to 5 µmol TBT per
litre for 5 min or 10 µmol/litre for 0.1 min produced
inhibition. However, exposure to 1 µmol/litre for 5 min
was ineffective. With an incubation time of 1 min, TBTO
was effective in lengthening the time taken for aggre-
gation after collagen induction at 0.625 µmol/litre, but
not at 0.5 µmol/litre.
The trialkyltins, including tributyltin, have been
shown to inhibit oxidative phosphorylation in rat mito-
chondria (Aldridge & Street, 1964). TBT stimulated
adenosinetriphosphatase (ATPase) activity and caused
limited swelling of rat liver mitochondria. These last two
effects occurred at similar concentrations of TBT in the
medium. The authors postulated that the two effects were
linked; the ATPase activity increased over a concentration
range of TBT and was always mirrored by a decrease in the
mitochondrial swelling. This relationship persisted
despite a complex response to TBT over the concentration
range; at higher concentrations there was a sudden, much
smaller mitochondrial swelling and a concomitant rise in
ATPase activity. TBT also inhibited the hydrolysis of ATP
by rat brain microsomes, although the concentrations
required were much higher than those affecting phosphory-
lation. The authors postulated that a combination of tri-
alkyltin compounds with negatively charged lipids is
involved in their biological activity.
Elferink et al. (1986) treated polymorphonuclear
leucocytes (PMNs), obtained from the peritoneal cavity of
rabbits, with an unspecified tributyltin (1 µmol/litre).
Uptake of opsonized zymosan was used as a measure of
phagocytosis, and enzyme (lysozyme) release to the super-
natant during incubation was also monitored. There was
almost complete inhibition of both parameters at
10-6 mol TBT/litre but no effect at 10-7 mol/litre. In-
hibition of phagocytosis was exactly paralleled by inhi-
bition of enzyme release. At concentrations of between
10-6 and 10-5 mol/litre, tributyltin caused lysis of the
cells with release of LDH, suggesting damage to the plasma
membrane. Exocytosis, induced by FMLP in the presence of
"cytocolasin B", was also inhibited by the TBT at con-
centrations of 10-6 mol/litre or more. There was little
effect of the compound on ATP levels in PMNs, and the
authors suggested that interference with ATP production
was not the basis for the effect of the TBT. Activation of
PMNs is accompanied by an increase in plasma membrane
permeability to Ca2+; this was strongly inhibited by the
TBT. The authors proposed two alternative explanations.
Either the Ca2+ permeability change is directly affected
or an earlier step is inhibited which, in turn, affects
calcium permeability. The observation that the exocytosis
effect could be counteracted by the addition of sulfydryl
compounds led the authors to conclude that the earliest
stages of activation of the PMNs were affected. Arakawa &
Wada (1984) reported a suppression of the chemotactic
response of rabbit leucocytes (neutrophils) towards for-
myl-methionyl-leucyl-phenylalanine by tributyltin chloride
at concentration between 0.1 and 10 µmol/litre in vitro.
Reinhardt et al. (1982) used cell detachment and
cloning efficiency of baby hamster kidney cells (BHK-21
C13) to quantify the cytotoxicity of organotin compounds.
The two parameters are independent, but each covers a
range of cellular damage. Cell detachment indicates irre-
versible damage to the cytoskeleton over a 4-h period,
while cloning efficiency covers influence on growth of
single cells over a 6-day period and includes cell re-
attachment followed by clone formation. The IC50 (concen-
tration at which there was a 50% reduction in cloning
efficiency) for TBTO was 5 x 10-7 mol/litre (0.3 mg/litre
medium) and for TBT chloride was 1.4 x 10-6 mol per litre
(0.5 mg/litre). The CD50 (50% effect on cell detachment)
was less sensitive to TBT, i.e. 3 x 10-5 mol/litre for
TBTO (18 mg/litre) and 4.3 x 10-5 mol/litre for TBT
chloride (14 mg/litre).
When Snoeij et al. (1986a) incubated isolated rat
thymocytes with TBT chloride, there was membrane damage
and disintegration of the cells at concentrations higher
than 1 µmol/litre. Various parameters of cell function
were investigated. The TBT chloride increased the consump-
tion of glucose in a dose-related manner at concentrations
in the culture medium higher than 0.1 µmol/litre; pro-
duction of lactate was also increased in parallel. The ef-
fect peaked at 1 µmol TBT/litre and thereafter declined;
damage to cell integrity occurred at these concentrations.
Over the same effective range of concentrations (0.1 to
1 µmol/litre), TBT decreased ATP levels. The ATP dimin-
ished very rapidly, within 2.5 min, and remained low for
at least 3 h. The incorporation of radiolabelled nucleo-
tide precursors into DNA and RNA and of amino acids into
protein was also affected. Thymidine incorporation into
DNA was reduced, relative to TBT dose, to a minimum of 20%
of control incorporation at 1 µmol TBT/litre. Uridine
incorporation was similarly reduced, but less effectively.
Inhibition of amino acid incorporation was substantial at
0.25 µmol TBT chloride/litre and protein synthesis was
virtually totally inhibited at 1 µmol/litre. The median
inhibitory concentrations (IC50) for thymidine, uridine,
proline, and leucine were 0.32 ± 0.04, 0.95 ± 0.06, 0.38
± 0.04, and 0.36 ± 0.03 µmol/litre, respectively. TBT
chloride concentrations of 0.1 µmol/litre or more mark-
edly reduced the production of cyclic AMP by the
thymocytes under stimulation from prostaglandin E1. TBT
chloride, therefore, has marked effects on the energy
metabolism of isolated thymocytes at concentrations well
below those affecting membrane integrity, and, as a
result, the authors stated that TBT chloride is best
characterized as an energy poison.
Snoeij et al. (1988a) similarly isolated rat thymo-
cytes, but separated them into fractions based on size.
The same parameters of cell function were investigated in
each of three fractions. Fraction 1 consisted of small,
non-proliferating thymocytes. Fractions 2 and 3 showed
some overlap in size, but fraction 2 was enriched in cells
actively synthesizing macromolecules, while fraction 3 was
enriched in dividing cells and showed the greatest pro-
liferative capacity. Resting ATP levels were highest in
the bigger cells, steady state levels increasing with
fraction number. TBT chloride reduced ATP levels in each
subfraction proportionally to between 62 and 64% of con-
trol values. Thus, TBT reduces intracellular ATP concen-
trations irrespective of cell volume or number of mito-
chondria. As with unfractionated thymocytes, TBT chloride
at both 0.25 and 1.0 µmol/litre inhibited thymidine
incorporation (to around 40% of control values at
0.25 µmol/litre and between 21 and 37% of control values
at 1 µmol/litre) and leucine incorporation (to between 13
and 29% of controls at 0.25 µmol/litre and 1 to 3% of
controls at 1 µmol/litre) in all fractions. In the case
of uridine incorporation, although there was inhibition in
most cases, 0.25 µmol TBT chloride/litre caused a stimu-
lation to 184% of control levels in fraction 1, the non-
proliferating cells.
11.2. Short-term toxicity
Summary
TBT compounds have been studied most extensively in the rat
(all the data in this section refer to the rat unless otherwise
indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg body
weight), high mortality rates were observed when the exposure
time exceeded 4 weeks. No deaths were noted at 100 mg/kg diet
(10 mg/kg body weight) or after daily administration of 12 mg
per kg body weight by gavage. In rats dosed during early post-
natal life, 3 mg/kg body weight resulted in increased deaths.
The main symptoms at lethal doses were loss of appetite, weak-
ness, and emaciation.
Borderline effects on rat growth were observed at 50 mg/kg
diet (6 mg/kg body weight) and 6 mg/kg body weight (gavage
studies). Mice are less sensitive, effects being observed at
150 to 200 mg/kg diet (22 to 29 mg/kg body weight).
Structural effects on endocrine organs, mainly the pitu-
itary and thyroid, have been noted in both short- and long-term
studies. Changes in circulating hormone concentrations and
altered response to physiological stimuli (pituitary trophic
hormones) were observed in short-term tests, but after long-
term exposure most of these changes appeared to be absent. The
mechanism of action is not known.
Exposure to TBTO aerosol at 2.8 mg/m 3 produced high mor-
tality, respiratory distress, inflammatory reaction within the
respiratory tract and histopathological changes of lymphatic
organs. However, exposure to the highest attainable vapour
concentration (0.16 mg/m 3 ) at room temperature produced no
effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular necrosis
and inflammatory changes in the bile duct were observed in rats
fed TBTO at a dietary level of 320 mg/kg (approximately 25
mg/kg body weight) for 4 weeks and in mice fed 80 mg/kg diet
(approximately 12 mg/kg body weight) for 90 days. Vacuolization
of periportal hepatocytes was noted in dogs fed a dose of 10
mg/kg body weight for 8 to 9 weeks. These changes were oc-
casionally accompanied by increased liver weight and increased
serum activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte vol-
ume in rats, resulting from dosing with 80 mg/kg diet (8 mg/kg
body weight), indicate an effect on haemoglobin synthesis,
leading to microcytic hypochromic anaemia. The decrease in
splenic haemosiderin levels suggests alterations in iron
status. Anaemia has also been observed in mice.
The formation of erythrocyte rosettes in mesenteric lymph
nodes has been observed in certain short-term investigations
but not in long-term studies. The biological significance of
this finding (possibly transient) is unclear.
The characteristic toxic effect of TBTO is on the immune
system; due to effects on the thymus, the cell-mediated func-
tion is impaired. The mechanism of action is unknown, but may
involve the metabolic conversion to dibutyltin compounds. Non-
specific resistance is also affected.
General effects on the immune system (e.g., on the weight
and morphology of lymphoid tissues, peripheral lymphocyte
counts, and total serum immunoglobulin concentrations) have
been reported in several different studies with TBTO using rats
and dogs, but not mice, at overtly toxic dose levels (effects
in mice have been seen with tributyltin chloride at 150 mg/kg).
Only the rat exhibits general effects on the immune system
without other overt signs of toxicity and is clearly the most
sensitive species. The NOEL in short-term rat studies was
5 mg/kg diet (0.6 mg/kg body weight). In studies with tributyl-
tin chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been shown
to compromise specific immune function in rat in vivo host re-
sistance studies. Decreased clearance of Listeria monocytogenes
was seen after exposure to a dietary level of 50 mg/kg (the
NOEL being 5 mg/kg per day), and decreased resistance to
Trichinella spiralis was seen at 50 and 5 mg/kg diet, but not
at 0.5 mg/kg diet (2.5, 0.25, and 0.025 mg/kg per day body
weight, respectively). Similar effects were seen in aged ani-
mals, but these were less pronounced.
With present knowledge, the effects on host resistance are
probably of most relevance in assessing the potential hazard to
man, but there is insufficient experience in these test systems
to fully assess their significance. However, some data on the
significance of the T. spiralis model are provided by findings
in athymic nude rats after the standard challenge. In these
studies, the complete absence of thymus-dependent immunity
resulted in a 10- to 20-fold increase in muscle larvae counts;
by contrast, exposure to TBTO concentrations of 5 and 50 mg/kg
diet resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on the
effects of tributyltin compounds on the developing immune sys-
tem, there is no information on host resistance.
It would be prudent to base assessment of the potential
hazard to humans on data from the most sensitive species.
Effects on host resistance to T. spiralis have been seen at
dietary levels as low as 5 mg/kg (equivalent to 0.25 mg/kg per
day body weight), the NOEL being 0.5 mg/kg (equivalent to
0.025 mg/kg per day). However, the interpretation of the sig-
nificance of these data for human risk assessment is contro-
versial. In all other studies a concentration of 5 mg/kg per
day in the diet (equivalent to 0.5 mg/kg body weight, based on
the short-term studies) was the NOEL with respect to general,
as well as specific, effects on the immune system.
11.2.1. Oral dosing: general body effects
Iwasaki et al. (1976) reported some oedema and
destruction of nerve axons following the administration of
0.01 ml TBTO/kg directly to the stomach of rats every day
for 28 days. No details of procedures or results were
presented in this report.
Schweinfurth (1985) found no evidence of brain oedema
after dosing rats with TBTO orally in arachis oil. Even
doses producing marked toxic effects on other organs (25
mg/kg body weight) failed to produce noticeable brain
oedema. However, triethyltin chloride produces brain
oedema at a dose of 1.5 mg/kg body weight.
Krajnc et al. (1984) investigated the short-term
effects of bis -tributyltin oxide in the rat. In exper-
iments lasting 4 weeks, Wistar rats were fed technical
TBTO at levels of 0, 5, 20, 80, or 320 mg/kg diet. All
animals survived the dosing period. Various symptoms of
poisoning were seen within 1 week of dosing in the group
fed 320 mg/kg diet, i.e. weakness, emaciation, roughened
fur, and blood-tinged discharge around the eyes and nose.
Some of these signs were seen at the lower doses after 4
weeks. Body weight gain was not affected by doses up to
20 mg/kg diet in males and 80 mg/kg in females. Males
showed reduced weight gain (96 g compared to control
weight gain of 117 g) over 4 weeks at 80 mg TBTO/kg. At
320 mg/kg diet, TBTO caused a reduction in body weight of
13 g in males and 21 g in females. Almost all of this
weight loss occurred in the first week of dosing, body
weight remaining constant after that. A 50% reduction in
food consumption was seen at this dose rate, compared to
control animals. Urine analysis, during the fourth week
of dosing, revealed no differences in urine volume, pro-
tein concentration, or creatinine clearance related to
treatment.
Funahashi et al. (1980) conducted histopathological
and biochemical studies on Sprague-Dawley rats given bis-
tributyltin oxide (TBTO) dissolved in olive oil. The rats
were dosed by intubation 5 times weekly for 13 or 26
weeks. Body weight was reduced after 13 weeks dosing at
6 mg/kg body weight, but not significantly so. After 26
weeks, body weight was significantly reduced relative to
controls (382 g compared to the control value of 439 g).
Dosing at 12 mg/kg reduced body weight significantly at
both 13 and 26 weeks (to 311 and 356 g, respectively).
There was a slight decrease, but unrelated to either dose
or time, in spleen weight. This decrease was just signifi-
cant at 6 mg/kg over 13 weeks and 12 mg/kg over 26 weeks,
but at no other time or dose.
When Mushak et al. (1982) dosed neonatal rats with TBT
acetate at levels of 1, 3, or 10 mg/kg per day from day 2
to day 29 of age, all rats given 1 mg/kg per day survived
with no apparent gross or histopathological effect. Totals
of 9 out of 24 and 17 out of 24 rats survived at dose
levels of 10 and 3 mg/kg per day, respectively, but all
showed reduced body weight. There were liver effects in
survivors.
11.2.2. Inhalation studies
A "nose only" inhalation study (lasting 4-5 weeks)
by Schweinfurth (1985) with rats exposed for 4 h/day (on
weekdays only; 21 to 24 exposure periods) to an aerosol of
TBTO (2.8 mg/m3) produced mortality (50% of males and
60% of females), apathy, and respiratory distress. Food
consumption and body weight gain were reduced. There were
inflammatory reactions within the respiratory tract and
lymphotoxic effects (depletion of lymphocytes in the
thymic cortex, atrophy of the thymus, and lymph nodes).
Inhalation of TBTO vapour/air mixtures produced no observ-
able effect. A concentration of 0.16 mg/m3 in the inha-
lation chamber, which corresponds to the equilibrium
vapour pressure of TBTO at room temperature, was con-
sidered to be the NOEL for rats.
When Gohlke et al. (1969) exposed rats to TBT chloride
in a 4-month inhalation study at nominal concentrations of
4 to 6 mg/m3, all animals survived the exposure. Towards
the end of the exposure, in the final month, the rats
showed minor irritation of the eye and nose. There was an
initial increase in relative liver weight but a signifi-
cant reduction over the whole experimental period. Fat
droplets were seen in the livers at autopsy, together with
a diffuse oedema of the brain. However, controls also
showed oedema. The oedema disappeared slowly as recovery
time after exposure increased. There were also inflamma-
tory changes in the respiratory tract of exposed animals.
Crofton et al. (1989) exposed pregnant female rats to
TBTO by intubation at 0 to 10 mg/kg per day on days 6 to
20 of gestation. There was no effect on the age at which
the testes of male offspring descended. However, females
showed a delay of approximately 2 days in vaginal opening,
compared to controls, after their mothers were exposed to
10 mg/kg per day.
11.2.3. Histopathological effects
Snoeij et al. (1985) reported that weanling rats fed
tributyltin chloride at a level of 150 mg/kg diet showed
marked reductions in body weight (treated rats, 87 g; con-
trol rats, 119 g) and brain weight (treated rats, 1.54 g;
control rats, 1.67 g) associated with a reduced food
intake of 25%. Thymus weight was reduced to 39% of the
control value over the 2-week feeding period.
Krajnc et al. (1984) found histopathological changes
in male and female Wistar rats exposed to 5, 20, 80, or
320 mg TBTO/kg diet for 4 weeks. No treatment-related
changes were found in the brain, heart, kidney, pancreas,
adrenals, popliteal lymph nodes, intestinal tract, or bone
marrow. Slight atrophy of hepatocytes (reduction in size)
in the centrilobular region was noted in some livers at
80 mg/kg and marked atrophy in 16 out of 20 livers at 320
mg/kg. The authors reported dystrophic calcification in
the liver at the highest dose level. Three animals showed
multiple-focus inflammation with necrosis of hepatic
parenchyma, which was associated with mononuclear and
polymorphonuclear infiltration, fibrosis, and hyperplasia
of the intrahepatic bile duct. In one animal there was
inflammation of the common bile duct. No bacteria or
viruses were found in the lesions. Similar necrotic
lesions were observed, in two animals, in a repeat study
at 320 mg TBTO/kg diet.
Mori et al. (1984) painted the shaved dorsal skin of
guinea-pigs daily for 50 days with an ethanolic solution
of TBTO at 10 or 40 mg/kg body weight. Monitoring of urine
and blood electrolyte levels during the course of the
treatment showed increased loss of sodium, chloride, phos-
phate, glucose, and amino acids in the urine. There was a
concomitant loss of electrolytes from serum. The effect
was most marked between 40 and 50 days of treatment. His-
tological lesions of the kidney tubules were observed when
the animals were killed after the 50th day.
11.2.4. Haematological and biochemical effects
Measurements of blood biochemical parameters of rats
fed TBTO for 4 weeks indicated few significant effects at
dietary dose levels below 320 mg/kg diet. Alanine amino
transferase (ALAT) activity was significantly increased,
in a dose-related manner, at 20 mg/kg or more, in both
males and females. The highest dose significantly de-
creased blood glucose in males, aspartate amino transfer-
ase (ASAT) in both males and females, and liver glycogen
in both sexes. Serum triglycerides, alkaline phosphatase,
and creatinine kinase activities were unaffected at any
dose level, as were blood lactate and pyruvate. At 80
mg/kg diet, TBTO significantly reduced blood haemoglobin
in both sexes and haematocrit in females. Mean erythro-
cytes volume was reduced in both sexes, as was mean cor-
puscular haemoglobin content (mass), but mean corpuscular
haemoglobin concentration and erythrocyte numbers were not
affected (Krajnc et al., 1984). A 6-week study at dietary
doses of 5, 20, and 80 mg/kg showed significant reductions
in haematocrit at 20 and 80 mg/kg and in blood haemoglobin
levels at 80 mg/kg. Iron concentration was reduced at 80
mg/kg, as was the isocitrate dehydrogenase (ICDH) activity
of erythrocytes. Numbers of erythrocytes, thrombocytes,
and reticulocytes were not significantly affected at any
dose level of TBTO, though there was a trend towards
increased numbers of reticulocytes. The authors suggested
an effect on haemoglobin synthesis and other indicators,
implying either reduced iron uptake or increased iron
loss. The enhanced ICDH activity and increasing reticulo-
cyte numbers indicated the presence of immature erythro-
cytes, and the authors could not exclude the possibility
of an in vivo haemolytic action of TBTO comparable to the
reported in vitro haemolysis (Krajnc et al., 1984).
Rosenberg et al. (1984) measured the activity of haem
oxygenase in mucosal cell fractions from control mice and
mice dosed by intubation with TBTO at 60 mg/kg body
weight. The activity of the enzyme, monitored by the
bilirubin absorbance spectrum, was substantially elevated,
compared to controls, 16 h after administration of the
TBTO. The activity of the same enzyme in liver and kidney
microsomes was not affected by this dose of TBTO given by
gavage, but was elevated when TBTO was given parenterally.
The activities of cytochrome P-450 and benzo(a)pyrene
hydroxylase were substantially reduced in the intestine of
TBTO-treated mice, this reduced activity being statisti-
cally significant for the latter but not for the former.
Similar results were obtained in liver fractions when TBTO
was applied parenterally.
11.2.5. Effects on lymphoid organs and immune function
Funahashi et al. (1980) reported the effects of feed-
ing TBTO in olive oil (0, 3, 6, or 12 mg/kg body weight
per day) for 13 or 26 weeks by gavage to groups of ten
male Sprague-Dawley rats, aged 5 weeks at the beginning of
the study. No analyses of haematology, immunoglobulin
levels, or specific aspects of immune function were per-
formed. A marked dose-related reduction in absolute and
relative thymus weight was seen following dosing for both
periods. Relative thymus weights were 682, 629, 340, and
313 mg/kg body weight after 13 weeks of dosing and 449,
313, 278, and 248 mg/kg body weight after 26 weeks of
dosing in the rats given 0, 3, 6, and 12 mg/kg per day,
respectively. All results, with the exception of those for
the group given 3 mg/kg body weight per day for 13 weeks,
were statistically significant (p < 0.003). Despite the
considerable reductions in thymus weight, the only histo-
logical observation was a slight reduction in the width of
the thymic cortex. A dose-related reduction in relative
spleen weight was seen after 26 weeks, which was statisti-
cally significant (p < 0.05) at the dose level of 12 mg/kg
body weight per day. Reduced body weight and increases in
relative pituitary and relative adrenal weights were stat-
istically significant (p < 0.01) at 12 mg/kg body weight
per day for 13 and 26 weeks and at 6 mg/kg body weight per
day for 26 weeks, and there was a significant (p < 0.05)
increase in relative adrenal weight at 6 mg/kg body weight
per day for 13 weeks. The relative pituitary weight was
also increased following 26 weeks of dosing at 3 mg/kg
body weight per day (p < 0.01). Based on thymus weight,
the NOEL was the most sensitive end-point in this study,
3 mg/kg body weight per day for 13 weeks. However, an
effect was seen at this dose when it was given for 26
weeks.
Funahashi et al. (1980) also reported that the
reduction in relative thymus weight, present 3 days after
a single dose of TBTO in olive oil of 100 mg/kg body
weight to 5-week-old male Sprague-Dawley rats, showed
signs of reversal at day 8. The reversibility of TBT-
induced thymus atrophy was also demonstrated by Snoeij et
al. (1988b). Groups of 4 or 5 young (4-5 weeks old) male
Wistar rats received a single gavage dose of TBT chloride
(0 or 16 mg/kg body weight) in corn oil. A 30% reduction
in relative thymus weight was evident in animals killed on
days 2, 3, or 4, but there was complete recovery by day 7.
A similar effect and recovery was seen in the thymus cell
counts. An equimolar dose of dibutyltin chloride produced
more pronounced effects, the recovery period being pro-
longed until day 9.
Krajnc et al. (1984) reported the effects of feeding
TBTO-containing diets (0, 5, 20, 80, or 320 mg/kg diet) to
groups (10 males and 10 females per group) of young SPF
Wistar rats for 4 weeks. These dose levels are equivalent
to approximately 0, 0.5, 2, 8, or 32 mg/kg body weight per
day, respectively, based on actual food consumption
measurements. Total leucocyte and circulating lymphocyte
counts were significantly reduced in males receiving 80
mg/kg diet (p < 0.01) and in both sexes receiving 320
mg/kg diet (p < 0.001). Eosinophil counts were signifi-
cantly reduced (p < 0.05) in males receiving 5, 80, or 320
mg/kg diet and in females given the highest dose. Monocyte
counts were increased significantly (p < 0.05) in males
receiving 20, 80, or 320 mg/kg diet but not in females.
Total immunoglobulin levels were significantly affected at
80 and 320 mg/kg diet. At 80 mg/kg diet, TBTO signifi-
cantly increased serum IgM to 132% (p < 0.01) and 145%
(p < 0.001) of control levels for males and females,
respectively. At the same dose, IgG in males was reduced
significantly (p < 0.05), but in females IgG was unaffec-
ted. A significant reduction (p < 0.001) in serum IgG was
found in both sexes fed TBTO at 320 mg/kg diet. Thymus and
relative thymus weights were significantly reduced in both
sexes (p < 0.001) at 80 and 320 mg/kg diet and in females
given 20 mg/kg diet (p < 0.05). Relative spleen weight
was significantly increased in males receiving 320 mg/kg
diet but this was probably related to the decreased body
weight of these animals. Histological examination showed
that all the animals given the highest dose exhibited
lymphocyte depletion from the thymic cortex, resulting in
an indistinct cortico-medullary junction and an increase
in ceroid/lipofuschin-loaded macrophages. Slight atrophy
of the thymic cortex was seen in two males fed 80 mg/kg
diet. Diffuse atrophy of the white pulp of the spleen was
seen in all animals at 320 mg/kg, the periarteriolar lym-
phocyte sheaths (PALS) being particularly affected. At 80
mg/kg diet, one male and two females showed slight splenic
atrophy. Depletion of T lymphocytes, determined by pan-T
immuno-staining, was seen in the PALS at 320 mg/kg diet.
There was an increase in the incidence of mesenteric lymph
nodes atrophy, observable in some animals fed 20 mg/kg and
increasing with dose to affect all animals given 320 mg/kg
diet. Both the paracortex and medulla of lymph nodes were
reduced in size and cellularity, and the numbers and size
of follicles were reduced by the highest TBTO dose level.
Again, the total number of T lymphocytes was strikingly
reduced in the paracortex by TBTO at 320 mg/kg diet, as
determined by immuno-staining. Although thymic involvement
was marked, it was not only thymus-dependent areas that
were affected. In rats exposed to the highest dose level,
which was overtly toxic, B lymphocyte areas also showed
low level activity, as indicated by fewer follicles and
inconspicuous germinal centres in lymph nodes and spleen.
An increase in the number of animals with rosettes in
sinuses in the mesenteric lymph nodes, composed of eryth-
rocytes surrounding mononuclear cells, was seen. This was
dose related: half of the animals dosed at 5 mg/kg and all
the animals treated with 80 or 320 mg/kg showed erythro-
cyte rosettes. The biological significance of these
rosettes is unclear. Specific aspects of immune function
were not tested. In further studies (personal communi-
cation by E.I. Krajnc to IPCS, 1989; Wester, in press), no
increase in rosette formation was observed at 5 mg/kg diet
or 50 mg/kg diet. Signs of general toxicity were observed
in animals treated with 320 mg/kg diet, i.e. reduced body
weight (p < 0.001) and increased serum ALAT activity. At
80 mg/kg, there was reduced body weight gain in males
(p < 0.05) and increased ALAT, and at 20 mg/kg increased
ALAT in males only. The liver was the only non-lymphoid
organ displaying histological changes: centrilobular hepa-
tocyte atrophy, reduced glycogen retention, parenchymal
necrosis, and hyperplasia of the intrahepatic bile duct
were seen in some animals treated at 320 mg/kg diet. Three
animals from the group given 80 mg/kg showed slight hepa-
tocyte atrophy. This study indicated a NOEL of 5 mg/kg
diet (approximately 0.5 mg/kg body weight per day).
Vos et al. (1984) investigated functional aspects of
the immunological effects reported by Krajnc et al. (1984)
in in vivo and ex vivo experiments using weanling (3 to 4
weeks old) male SPF Wistar rats fed diets containing TBTO.
Haematological parameters were not studied. Levels of
total circulating immunoglobulins (IgG and IgM) were
determined in animals fed diets containing 80 or 320 mg
TBTO/kg. At 320 mg/kg diet, IgM was significantly
increased (p < 0.05) and IgG was significantly decreased
(p < 0.001) on day 28 (no assays were performed on day 42
for this group). At 80 mg/kg diet, IgM was elevated by 30%
on day 42 (p < 0.01), while IgG was decreased by 30% on
days 28 and 42.
The effects of TBTO on lymphocyte counts and cell
viability in lymphoid organs was investigated, and several
specific tests of immune system function were also per-
formed. Numbers of B and T lymphocytes were significantly
reduced in the spleens (by 15% and 48%, respectively) of
animals fed diets containing 80 mg/kg diet. The ratio of
total T:B lymphocytes was decreased in a dose-related
manner following 9 weeks of exposure.
A significant reduction occurred in the numbers of
viable cells (trypan blue exclusion) obtained from the
thymus, spleen, and bone marrow of rats treated with TBTO
at 80 or 320 mg/kg diet. At 320 mg/kg diet, viability and
cell numbers were significantly (p < 0.05) reduced for
both thymus and spleen following 3, 8, and 20 days of ex-
posure, and bone marrow counts were significantly reduced
on days 8 and 20. Doses of 80 mg/kg diet produced signifi-
cant reductions in thymus cell count and viability on day
8 and thymus, spleen, and bone marrow counts on day 20.
The antibody response to sheep erythrocytes (ip),
tetanus toxoid (iv), ovalbumin (sc into the foot pad, in-
cluded H37Ra adjuvant), and the worm Trichinella spiralis
(oral) was assessed in animals receiving 20 or 80 mg TBTO
per kg diet for 6 weeks. The primary response to sheep
erythrocytes (0.5 ml of a 20% suspension, ip) was deter-
mined 10 days post inoculation (pi) and the secondary
response was determined on day 20 pi following an iv
booster inoculation on day 15 pi. The primary response was
unaffected, but there was a dose-related decrease in the
secondary response seen both in untreated and 2-mercapto-
ethanol-treated (IgM-inactivated) sera, reaching statisti-
cal significance (p < 0.05) in treated sera from the
highest-dose group. The response (IgG and IgM titres) to
tetanus toxoid inoculation measured only on day 21 pi was
equivocal, and that to ovalbumin measured on days 15, 21,
and 28 pi was unaffected by TBTO. IgG response to oral T.
spiralis infection was significantly (p < 0.05) increased
on day 21 pi at 20 mg/kg diet, but not on day 42 or at 80
mg/kg diet at either time. IgM response was unaffected.
IgE response, possibly the most relevant to resistance to
parasitic infection, was decreased in a dose-related man-
ner on days 21 and 42 following T. spiralis infection; log
titres were 3.8, 2.5 (p < 0.05), and 1.8 (p < 0.001) on
day 21 and 4.4, 3.6, and 3.4 (p < 0.05) on day 42 in the
control, 20-, and 80-mg/kg groups, respectively. Delayed-
type hypersensitivity was determined as change in skin
thickness following a challenge of ovalbumin to the skin
of the ear or tuberculin challenge to the skin of the
flank, made 3 and 4 weeks, respectively, following initial
immunization (sc into a footpad) 6 weeks after starting
dietary dosing of TBTO at 20 or 80 mg/kg. Compared to
controls similarly injected intradermally with medium,
significant reductions in response to ovalbumin challenge
were found 24, 48, and 72 h after dosing at 20 mg/kg (all
p < 0.05), and 24 h (p < 0.01) and 48 h (p < 0.05) after
dosing at 80 mg/kg. With tuberculin challenge, significant
effects were found in the group given 80 mg/kg diet after
24 h (p < 0.01), 48 h (p < 0.001), and 72 h (p < 0.001),
but only after 72 h (p < 0.05) at the lower dose level.
Reduced responses were seen at all three times after both
doses.
Thymus or spleen cells, obtained from rats fed TBTO at
20 or 80 mg/kg diet, were cultured with and without
mitogens (PHA = phytohaemagglutinin; Con A = concanavalin
A; PWM = pokeweed mitogen) for 24 h before the addition
of 3H-thymidine to monitor DNA synthesis. PHA and Con A
are both T cell mitogens and PWM is a mitogen for both T
and B cells. Due to a reduction in the number of viable
cells in thymic cultures from the high-dose group, respon-
siveness to mitogens was expressed per culture and per
thymus. Significant reductions in 3H-thymidine uptake
(expressed per thymus) were found in unstimulated (48%
reduction), PHA-treated (64%), Con A-treated (62%), and
PWM-treated (50%) cultures derived from the high-dose
group; PHA (48%) and PWM (28%) were the only responses
reduced at 20 mg/kg diet. Similar effects were seen on
splenic cultures, with a reduced number of viable cells
per spleen and significant reductions in response
(expressed per spleen) to PHA (50% reduction) and Con A
(45%) in cultures from the high-dose group; responses to
PWM and E. coli lipopolysaccharide (a B cell mitogen)
were significantly increased on a per culture basis but
not a per spleen basis. Cultures from the low-dose group
showed reduced response to PHA (25% reduction) and Con A
(20%), but these were not statistically significant.
TBTO, at both 20 and 80 mg/kg diet, reduced the
resistance of rats to infection by T. spiralis. The number
of worm larvae in muscle significantly increased in a
dose-dependent manner (74 000 in controls; 106 000 in rats
fed 20 mg TBTO/kg, p < 0.01; 198 000 in rats fed 80 mg/kg
p < 0.001) after standard infection with 1000 larvae by
mouth. The number of adult worms in the small intestine
also increased significantly after 10 (p < 0.05), 12
(p < 0.001), and 14 (p < 0.01) days in the high-dose
group, and after 12 (p < 0.05) and 14 (p < 0.001) days in
the low-dose groups. In a study of the inflammatory reac-
tion around larva-containing muscle cells in the tongues
of animals killed 14 days after infection, the response
(mononuclear cells and eosinophilic granulocytes) after
treatment with TBTO at 20 mg/kg diet was described as
"slightly reduced". However, there was a marked
reduction after treatment at 80 mg/kg diet.
The clearance of Listeria monocytogenes from the
spleen (a measure of host resistance) was monitored in
rats fed 20, 80, or 320 mg TBTO/kg diet for 6 or 7 weeks.
Statistically significant increases in the number of
viable bacteria were seen 2 days after an iv injection
into animals treated with 320 mg/kg diet for 6 (p < 0.01)
or 7 (p < 0.001) weeks and with 80 mg/kg diet for 6 weeks
(p < 0.001). A dose-related reduction, statistically sig-
nificant (p < 0.05) at 80 mg/kg diet, in viable bacteria
was seen 1 day post injection in the group receiving TBTO
for 7 weeks, but this was not seen in the 6-week study.
The ex vivo phagocytosis of L. monocytogenes by spleen-
and peritoneal-derived macrophages from TBTO treated (20
or 80 mg/kg diet) animals was not significantly affected,
although a dose-related reduction in the phagocytic
activity of splenic macrophages was seen.
Cells derived from the spleen and peritoneal cavity
were tested for spontaneous cell-mediated cytotoxicity
against murine YAC lymphoma target cells labelled
with 51Cr. "Specific release" of the chromium label
(release in experimental minus spontaneous release in the
controls) was used as the end-point of the assay. Stat-
istically significant reductions in specific release were
found with spleen cells from rats fed TBTO at 80 mg/kg
diet (p < 0.05), but not in spleen cells from those fed 20
mg/kg diet. Statistically significant effects (p < 0.05)
were found in peritoneal macrophages derived from rats
receiving 20 and 80 mg/kg diet, but there were no signifi-
cant effects on non-adherent cells ("natural killer
cells").
These studies were designed to investigate immune
function rather than to determine a no-observed-effect-
level, and the lowest dose used (20 mg/kg diet) produced
statistically significant effects (in particular, de-
pression of host resistance to T. spiralis and L. mono-
cytogenes ). No evidence of general toxicity was seen at
this dose level. The only sign of general toxicity
recorded was a significant reduction in body weight gain
seen after exposure to 320 mg/kg diet for 3, 8, and 20
days and to 80 mg/kg diet for 20 days.
A study of TBTO (0, 0.5, and 50 mg/kg diet) adminis-
tered to groups of weanling Wistar rats (five animals of
each sex per group) for 2 years was briefly reported by
Vos et al. (1985) and Wester (in press). It should be
noted that the intakes in this study were lower, on a body
weight basis, than in the shorter-term studies, being
equivalent to 0, 0.025, 0.25, and 2.5 mg/kg body weight
per day for the controls, and low, medium, and high doses,
respectively (based on actual intake measurements). A
significant decrease in peripheral lymphocyte count and a
statistically significant increase in platelets were seen
in the females fed 50 mg/kg diet for 1 year. Circulating
levels of total IgM and IgA were significantly increased
at 4-6 and 16-18 months, in rats given 50 mg/kg diet,
while significant reductions in IgG levels were recorded,
particularly in females.
General effects on the lymphoid organs were not
recorded, though several specific tests of immune function
were performed using the methods of Vos et al. (1984). A
dose-related decrease in resistance to T. spiralis infec-
tion was seen at 5 and 16 months, achieving statistical
significance (p < 0.05) at 5 and 50 mg/kg diet. IgE titres
were reduced, but IgA levels increased approximately 50-
fold at the highest dose level. No effects on delayed-type
hypersensitivity were seen, in contrast to results in the
short-term study by Vos et al. (1984). Host resistance to
L. monocytogenes (as measured by the number of viable
organisms in the spleen) was significantly reduced at 5
and 17 months in the 50-mg/kg group, but a significant
increase was seen at 17 months in the 5-mg/kg group.
Natural killer cell activity against YAC lymphoma cells
was reduced significantly at the highest dose level after
15-17 months. It will not be possible to fully assess this
study until the final report is published, although it
would appear that 0.5 mg/kg diet (0.025 mg/kg body weight
per day) was the NOEL.
A preliminary report on a study where diets containing
TBTO (0, 0.5, 5, or 50 mg/kg diet) were fed to Wistar rats
(12 months old) for 6 months, has been issued (personal
communication by E.I. Krajnc to IPCS, 1989). Specific
tests of immune function were measured after 5 months of
dosing. Significant (p < 0.05) reductions in host resist-
ance to T. spiralis and L. monocytogenes were seen at
50 mg/kg only. These results suggest that aged rats are
less sensitive to TBTO in the diet than weanlings animals,
although this may be due to a lower intake on a per kg
body weight basis. Full assessment of this study will need
to await the completion of the statistical analyses and
final report.
A series of studies have been performed to investigate
certain aspects of immune function (Schering, 1989a,b,c,d).
When groups (10 animals of each sex) of young (4 to 5
weeks old) Sprague-Dawley rats were fed diets containing
TBTO (0, 0.5, 2, 5, or 50 mg/kg diet) for 4 weeks, no sig-
nificant effects were seen on total or differential white
blood cell counts. Serum immunoglobulin levels were not
measured. A statistically significant decrease in absolute
and relative thymus weight (p < 0.01) was seen in males
fed 50 mg/kg. A decrease in absolute and relative spleen
weight was also seen in this group, though it was not sig-
nificant statistically. The viability of cultured spleen
cells (monitored with trypan blue exclusion) obtained from
females fed 5 mg/kg was reduced (p < 0.05), but other
groups were unaffected. A significant decrease in total
thymus cell count was seen in preparations from males fed
50 mg/kg only (p < 0.05). A slight but significant
(p < 0.05) reduction in the thickness of the thymic cortex
was also seen in these males, this being the only signifi-
cant histological finding. Specific measures of immune
function were not performed in this study but in parallel
studies. The NOEL in this study was 5 mg/kg diet
(approximately 0.6 mg/kg body weight per day, based on
measured intake) (Schering 1989a).
An assay of plaque-forming cells, a measure of humoral
immunity, revealed no effects due to TBTO (at levels of
0.5, 2, 5, or 50 mg/kg diet) fed to groups (10 animals of
each sex) of young (4 to 5 weeks old) Sprague-Dawley rats
for 5 weeks. The response was measured on day 36, follow-
ing an iv inoculation of sheep erythrocytes. The only sign
of toxicity was a reduction in body weight in males fed
50 mg/kg, but this was not statistically significant. No
investigation of general toxicity was performed. The NOEL
was 50 mg/kg diet (approximately 5.6 mg/kg body weight per
day) in this study (Schering, 1989b).
To assess the effects of TBTO on host resistance to
infection, the number of viable Listeria monocytogenes
cells in the spleen, 4 days after inoculation with
1.4 x 106 cells, was counted. No effects were produced in
groups (10 animals of each sex) of young Sprague-Dawley
rats fed diets containing TBTO at 0.5, 2, or 5 mg/kg diet
for 34 days. However, statistically significant increases
in numbers of viable bacteria were seen in the spleen of
males (p = 0.055) and females (p < 0.01) fed 50 mg/kg
diet. No investigations of general toxicity were per-
formed. The NOEL was 5 mg/kg diet (approximately 0.6 mg/kg
body weight per day) (Schering, 1989c).
The effects of TBTO on delayed-type hypersensitivity
reactions, a measure of cell-mediated immunity, were
assessed in groups (10 animals of each sex) of 4- to 5-
week-old Sprague-Dawley rats fed diets containing 0, 0.5,
2, 5, or 50 mg/kg diet for 37 days. A sensitizing dose of
100 µg bovine serum ablumin (BSA) mixed with Freund's
complete adjuvant was given on day 29, followed by a chal-
lenge dose of heat-inactivated BSA injected into a hind-
foot pad on day 37. No difference in response (increased
footpad thickness) was seen between test and control
groups. Body weight gain was unaffected, but no other
investigations of general toxicity were performed. The
NOEL in this study was 50 mg/kg diet (approximately 5.8
mg/kg body weight per day) (Schering 1989d).
The effects of inhaled TBTO were studied in groups (10
animals of each sex) of young (initial weights 86-131 g)
SPF Wistar rats exposed to 0, 0.03, 0.16, or 2.8 mg/m3
for 4 h/day, 5 days per week, for 21 to 24 exposures. The
two lower doses were provided by filtered vapour, and the
highest dose was provided by an aerosol with over 90% of
the particles < 5 µm in diameter (Schering 1983). Lympho-
cyte and total leucocyte counts, measured at 2 or 4 weeks,
were unaffected by TBTO. Some inconsistent changes in
reticulocyte counts were found, but these appeared to be
primarily related to use of the respiration chamber and
not to TBTO exposure. Immunoglobulins were not measured.
Thymolysis and lymphocyte depletion of the thymus-depen-
dent areas of the spleen and lymph nodes were reported in
the 11 animals (five males, six females) from the highest-
dose group that died during the study. No such lesions
were detected in the survivors, although in three sur-
vivors from the highest-dose group an increase in the
number of macrophages containing nuclear debris was seen
in the thymic cortex. No significant (p > 0.05) changes
were seen in absolute or relative weights of the thymus,
spleen, or iliac lymph node in animals surviving the
study, but no organ weights were recorded for those ani-
mals dying or sacrificed during the study. No specific
aspects of immune function were studied. Histological
signs of general toxicity were limited to those consistent
with inflammatory reactions within the respiratory tract
and, with one exception, were confined to animals exposed
to the aerosol. Food intake and body weight gain were
reduced in both sexes, the reduction being statistically
significant (p < 0.01) in male rats exposed to 2.8 mg/m3.
The cause of death in the 11 animals from the highest-dose
group was not ascertained (Schering, 1983).
When TBTO (0, 4, 20, 80, or 200 mg/kg diet) was fed to
groups (10 animals of each sex) of CD-1 mice, aged 6 to 7
weeks at commencement of the study, for 3 months, leuco-
cyte counts were increased at 80 and 200 mg/kg in both
sexes, although this increase did not reach statistical
significance (p > 0.05). Immunoglobulins were not
measured. Thymus weight was reduced in both sexes at 200
mg/kg, but this was not statistically significant
(p > 0.05). Spleen weights were increased in both sexes
at 80 and 200 mg/kg (reaching statistical significance
(p < 0.05) at 200 mg/kg), possibly secondary to effects on
erythrocytes. No specific immune function tests were per-
formed. Histological changes were seen in the livers of
both sexes at 80 and 200 mg/kg. Dose-related, statisti-
cally significant (p < 0.01) increases in absolute liver
weights were seen at 80 and 200 mg/kg, and adrenal weights
were significantly (p < 0.01) increased in the male rats
fed 200 mg/kg. The NOEL in this study was 20 mg/kg diet
(approximately 4 mg/kg body weight per day) (Biodynamics,
1989a).
In studies by Schering (1989e), groups (two animals of
each sex) of beagle dogs received variable doses of TBTO
in arachis oil by oral gavage:
Group 1: controls;
Group 2: 0.1 mg/kg body weight per day for 5 weeks,
0.2 mg/kg body weight per day for 4 to 5 weeks, then
10 mg/kg body weight per day for 8 to 9 weeks;
Group 3: 0.5 mg/kg body weight per day for 5 weeks
then 1 mg/kg body weight per day for 13 to 14 weeks;
Group 4: 2.5 mg/kg body weight per day for 5 weeks
then 5 mg/kg body weight per day for 13 to 14 weeks.
All males in groups 2 and 4 died as a result of mis-dosing
to the lungs. Increased leucocyte and neutrophil counts
were seen in group 4 at weeks 9 and 18 (p < 0.05), and
there was a statistically significant increase in leuco-
cyte count recorded at 13 weeks in group 2 (p < 0.05).
Non-statistically significant reductions in leucocyte,
neutrophil, and lymphocyte counts were found in group 2 at
18 weeks. Specific immunoglobulins were not measured.
Thymus weights were reduced in the two survivors of group
2 (0.9 ± 0.1 g compared with 4.0 ± 0.6 g in controls), and
slight increases in thymus weights were seen in groups 3
and 4. Iliac and mesenteric lymph node weights were
reduced, though spleen weights were increased, in the two
survivors from group 2. Histological changes in lymphoid
organs were confined to group 2 where a reduction of lym-
phocyte numbers in the thymus, spleen (particularly PALS),
and lymph nodes was seen. A dose-related increase in rela-
tive liver weight (33.1, 41.7, 49.9, and 57.6 g/kg body
weight in groups 1, 3, 4, and 2, respectively) was
accompanied by cytoplasmic vacuolation of hepatocytes in
group 2 (Schering, 1989e).
11.2.6. Mechanism of immunotoxicity
The precise mechanism of the immunotoxic effects of
TBTO is not yet clear. However, a hypothesis has been put
forward, based on the work of Snoeij (1987) and Pieters et
al. (1989).
a) Absorbed TBTO is present as the TBT+ cation or a salt
(chloride or carbonate). Studies using TBT chloride
are, therefore, relevant to TBTO immunotoxicity.
b) As dibutyltin chloride (DBT chloride), a metabolite of
TBT chloride, is, mole for mole, more potent that TBT
chloride at producing effects in the thymus and thymic
cells, it is probable that DBT chloride or another DBT
salt is the active species in TBTO toxicity.
c) The primary action of DBT is to suspend the maturation
of immature thymocytes by inhibiting their inter-
action/binding with thymic epithelial cells. The turn-
over period of thymocytes is 3 to 4 days. Therefore,
as cell proliferation/maturation is inhibited, rapid
depletion of thymocyte numbers without cytotoxicity is
expected, followed by a rapid proliferation (observed
by Snoeij et al. (1988b) and Funahashi et al. (1980))
on removal of DBT.
Other evidence indicating that TBT compounds have a par-
ticular effect on the thymus is provided by the in vitro
studies of Snoeij (1987), which show TBT chloride to be
cytotoxic to thymocytes.
The demonstration of reduced thymus weights in certain
fish species (e.g., the freshwater guppy) indicates that
TBT may have immunotoxic effects on a wide range of
species.
11.2.7. Effects on the endocrine system
The weights of both the adrenal and pituitary glands
of Sprague-Dawley rats were significantly increased after
exposure to 6 mg TBTO/kg body weight daily, by intubation,
for 26 weeks. Pituitary weight was also significantly
increased by 3 mg TBTO/kg body weight given daily for
26 weeks (Funahashi et al., 1980).
After 4 weeks, the serum insulin concentration of rats
was not significantly affected by dosing with TBTO at up
to 80 mg/kg diet, but was undetectable (< 2 milliIU/litre)
in rats fed 320 mg/kg (control levels of insulin in serum
were 111 and 74 milliIU/litre for males and females, re-
spectively) (Krajnc et al., 1984). In a further study, the
same authors monitored endocrine changes in male Wistar
rats exposed to TBTO in the diet (0, 20, or 80 mg/kg) for
6 weeks. Serum thyroxine, thyroid stimulating hormone
(TSH), and insulin levels were reduced significantly at
the higher dose level, but serum follicle stimulating
hormone (FSH) and corticosterone levels were unaffected.
Only insulin was significantly affected at 20 mg TBTO/kg.
The luteinizing hormone (LH) concentration in serum was
significantly increased by TBTO at 80 mg/kg, but was
unaffected by 20 mg/kg. The authors further examined endo-
crine function by monitoring hormone release following
physiological stimulus. Insulin release, following iv
administration of glucose, was unaffected by a 6-week
exposure to TBTO at either 20 or 80 mg/kg diet. The effect
of TBTO on insulin was attributed by the authors to a
decreased food intake. The release of TSH, after iv admin-
istration of thyrotrophin releasing hormone (TRH), showed
a tendency (p < 0.1) to be inhibited in rats fed at 80
mg/kg. The titre of circulating TSH, 20 min after TRH
administration, was significantly reduced compared with
controls. Release of both LH and FSH, in response to
luteinizing hormone releasing factor stimulation, was
enhanced in rats fed TBTO at both 20 and 80 mg/kg diet,
but only significantly so at the higher dose rate. Histo-
logical examination of endocrine organs after a 6-week
exposure to TBTO revealed some changes. No differences
were observed in either insulin- or glucagon-producing
cells in the pancreas. Some flattening of the epithelial
lining of the thyroid follicles was observed after
exposure of rats to 80 mg/kg but not to 20 mg/kg. Immuno-
cytochemical staining of the pituitary gland identified
each cell type producing the different pituitary hormones.
There was a dose-related decrease in both the intensity of
staining of TSH cells and the number of cells stained.
Conversely, there was a dose-related increase in the
staining intensity of LH cells. No effects were found on
FSH, growth hormone, or adrenocorticotrophin cells in the
pituitary (Krajnc et al., 1984).
11.3. Long-term toxicity
Wester (in press) carried out a 106-week toxicity
and carcinogenicity study with groups of 50 weanling
Wistar rats of each sex. An additional group of 10 rats
was used for an interim sacrifice after 1 year. TBTO was
fed at 0, 0.5, 5, or 50 mg/kg diet (equivalent to 0,
0.025, 0.25, or 2.5 mg/kg body weight). Increased food
consumption occurred in all treated males (not clearly
dose-related), and there was increased water consumption
in males at 5 and 50 mg/kg. During the second year, the
body weight of the highest-dose group was significantly
lower than that of controls. Excess mortality, compared
with that of controls, was confined to the 50 mg/kg group
towards the end of the experiment (see section 11.6).
Haematological changes (anaemia, lymphopenia, and thrombo-
cytosis) and increases in plasma enzyme activities (ALAT,
ASAT, and AP) were noted mainly at the high-dose level.
Serum IgM and IgA levels increased, while the IgG level
decreased (females). No effect was observed on circulating
concentrations of T4, free T4, TSH, LH, FSH, or insu-
lin; only the free T4:T4 ratio was decreased. Organ
weight changes consisted of increased liver, kidney,
adrenal, and pituitary weights and decreased thyroid
weight. Non-neoplastic histological alterations consisted
of a decrease in cell height of the thyroid follicles (at
50 mg/kg diet after 1 and 2 years), decrease in splenic
iron content (at 5 and 50 mg/kg after 1 year only), slight
bile duct proliferation (at 50 mg/kg after 1 year only),
and vacuolation of kidney proximal tubular epithelium and
nephrosis (at 50 mg/kg after 2 years only).
11.4. Genotoxicity
Summary
The genotoxicity of TBTO has been the subject of extensive
investigation. Negative results were obtained in the vast
majority of studies, and there is no convincing evidence that
TBTO has any mutagenic potential.
Davis et al. (1987) conducted a comprehensive study of
the genetic effects of TBTO using a wide range of tech-
niques in order to assess possible hazards in the use of
the compound as a molluscicide for the control of schisto-
somiasis. TBTO did not produce gene (point) mutations in
Salmonella typhimurium strains TA1530, TA1535, TA1538,
TA97, TA98, or TA100, either in the presence or absence of
an exogenous metabolic activation system (rat liver S9).
The compound did give some evidence of gene mutation in
Salmonella typhimurium TA100 using the fluctuation method
in the presence of S9, but no dose-response relationship
was seen; negative results were seen in the absence of S9.
TBTO did not induce point mutations in the yeast
Schizosaccharomyces pombe. Negative results were obtained
when TBTO was tested in the sex-linked recessive lethal
assay using Drosophila melanogaster, the compound being
given in food and by injection, indicating that TBTO did
not produce gene mutations in Drosophila. Negative results
were also obtained when the ability of TBTO to produce DNA
damage in Bacillus subtilis (recombination assay) or the
yeast Saccharomyces cerevisiae (mitotic gene conversion)
was tested.
The ability of TBTO to produce gene mutations in Sal-
monella has also been studied by Reimann & Lang (1987).
Negative results were obtained using Salmonella
typhimurium strains TA1535, TA1537, TA1538, TA98, and
TA100, both in the presence and absence of rat S9. Simi-
larly, negative results were obtained with six TBT esters
(abietate, borate, linoleate, naphthenate, phosphate, and
tallate). Further negative results were obtained when the
ability of TBTO to produce mitotic gene conversion in
Saccharomyces cerevisiae was tested.
The ability of TBTO to produce gene mutations in
mammalian cells in vitro was extensively investigated by
Davis et al. (1987), and negative results were consist-
ently obtained. TBTO did not induce gene mutations in V79
Chinese hamster cells (using resistance to 8-azaguanine,
ovabain, or 6-thioguanine as markers) in the presence of
rat liver S9. Negative results were also obtained in the
V79 cell assays when epidermal cells of mice and humans
(primary cultures) were used as the source of metabolic
activation in cell-mediated assays.
The in vitro clastogenic potential of TBTO has been
investigated in mammalian cells in two sets of studies.
When Davis et al. (1987) used Chinese hamster ovary (CHO)
cells, harvested at 8, 15, and 24 h, an increase in struc-
tural aberrations (mainly deletions), together with
endoreduplication, was seen, but only at the highest
concentration tested (5 µg/ml in the presence of S9 and
1.5 µg/ml in its absence). The increase in the presence
of S9 was seen only after 15 h, "toxicity" precluding
any analysis of results at this concentration after 8 h.
The increase in the absence of S9 was seen only at 8 h,
there being no increase at either 15 or 24 h. The results
of these studies are difficult to interpret, since the
effect was limited to concentrations associated with high
toxicity and no data on mitotic index was reported. No
increase in sister chromatid exchange was seen at any
concentration.
Reimann & Lang (1987) used human lymphocytes to test
the clastogenic potential of TBTO and obtained negative
results both in the presence and absence of S9. In the
latter case, TBTO was added 22 h after stimulation of the
cultures with phytohaemagglutinin, and cells were har-
vested 31 h later. In the former case, TBTO was added with
S9 after 26 h; 3 h later the S9 was removed, and the cells
were harvested 22 h later. Parallel studies on blood cul-
tures that were differentially stained with BUdR indicated
that almost all cells analysed for chromosome aberrations
were in the first mitotic stage. The highest TBTO concen-
trations used (0.1 µg/ml in the absence of S9 and
1 µg/ml in its presence) were associated with a marked
reduction in mitotic index (57% and 58%, respectively). No
increase in aberrations was seen at any dose level. This
study, therefore, failed to confirm the suggestion of some
clastogenic potential in the studies using CHO cells.
The ability of TBTO to produce chromosomal damage in
vivo has been investigated in two separate studies using
the micronucleus test. In one study, four doses of TBTO
(31.25, 62.5, 125, or 250 mg/kg body weight) were given by
gavage, in arachis oil, to NMRI mice, and bone marrow
cells were analysed for micronuclei in polychromatic
erythrocytes 24, 48, and 72 h after treatment (Reimann &
Lang, 1987). The highest dose level resulted in marked
lethality (16 out of 36 animals died), precluding any
analysis of the results. The 126-mg/kg dose level was also
associated with some deaths (4 mice died), but 5000 poly-
chromatic erythrocytes were analysed from five male and
five female mice at each harvest interval. Similar analy-
ses were carried out at the two lower dose levels. There
was no increase in micronuclei at any dose level or har-
vest time. This study provided no evidence to indicate
that TBTO produces chromosomal damage in bone marrow in
vivo. A second micronucleus study (Davis et al., 1987)
used Balb/c mice. Groups of 10 male and 10 female animals
were given 30 or 60 mg TBTO/kg as a solution in olive oil.
Bone marrow cells were harvested from five males and five
females after 30 h and 48 h, and 1000 polychromatic eryth-
rocytes were analysed from each for micronuclei. An
increase in micronuclei was seen only in the male mice
after 48 h and at the highest dose level. These results
conflict with those of Reinmann & Lang (1987). Further-
more, there was an unusually high spontaneous incidence of
micronuclei (up to 5.8 per 1000 polychromatic erythro-
cytes) in the study of Davis et al. (1987). These factors
prompted a re-analysis of the slides from this study by
the Institute of Occupational Health, Helsinki, Finland
(Schering 1986). This re-analysis, again using 1000 poly-
chromatic erythrocytes per animal, failed to confirm the
increase in micronuclei seen after 48 h in the male
animals given 60 mg TBTO/kg. A slight, but statistically
significant, increase in micronuclei was seen in the
female mice on re-analysis, but this was thought to be
biologically non-significant due to the high variability
in the control data. The re-analysis highlighted the
problem of interpreting studies in which only relatively
few polychromatic erythrocytes are analysed (1000) and
there is marked variability in the control data (with an
incidence outside the normal range at some time points).
Thus, no conclusions can be drawn from the micronucleus
study of Davis et al. (1987).
The ability of TBTO to inhibit metabolic cooperation
between V79 Chinese hamster 6-thioguanine-resistant and
sensitive cells has been investigated by Davis et al.
(1987). This assay has been suggested as a model for
tumour promoter activity. Negative results were obtained.
The significance of the assay is, however, unclear.
In summary, the genotoxicity of TBTO has been the
subject of extensive investigation. Negative results were
obtained in the vast majority of studies, and there is no
convincing evidence that TBTO has any mutagenic potential.
11.5. Reproductive toxicity
Summary
The potential embryotoxicity of TBTO has been evaluated in
three mammalian species (mouse, rat, and rabbit) after oral
dosing of the mother. The main malformation noted in rat and
mouse fetuses was cleft palate, but this occurred at dosages
overtly toxic to the mothers. These results are not considered
to be indicative of teratogenic effects of TBTO at doses below
those producing maternal toxicity. The lowest NOEL, with
regards to embryotoxicity and fetotoxicity for all three
species, was 1 mg/kg body weight.
11.5.1. In vivo
Reproductive toxicity has been studied in NMRI mice.
The highest dose used (35 mg/kg body weight) was chosen to
give minimal maternal mortality based on acute toxicity
tests. An increase in cleft palate was seen in the fetuses
of mice treated orally with 11.7 mg TBTO/kg body weight
(7% cleft palate), 23.4 mg/kg (24%), and 35 mg/kg (48%),
compared to the incidence in controls (0.7%). However, 11
out of a total of 15 affected mice were clustered in one
of the 18 litters and 15 litters contained none. The two
highest doses of TBTO also increased the frequency of
irregular ossification centres of sternabrae and of minor
abnormalities, such as fusion of the bases of os occipi-
talis. The strain of mice used in the study has a tendency
to produce these particular abnormalities as a result of
non-specific stress on the mother. The authors considered
that TBTO has a very low teratogenic potential; electron
microscopy 26 and 48 h after treatment showed no evidence
of damage to the embryos but considerable damage to the
maternal liver. At a level of 6 mg/kg body weight, TBTO
produced no increase in fetal abnormalities (Davis et al.,
1987).
Nemec (1987) investigated the maternal, embryotoxic,
and teratogenic effects of TBTO in New Zealand white
rabbits. The TBTO was administered in corn oil by gavage,
once a day from day 6 of gestation to day 18 inclusive, at
doses of 0.2, 1, and 2.5 mg/kg per day body weight in a
volume of 0.5 ml. Twenty female rabbits were dosed with
corn oil as controls and 20 rabbits were used at each dose
level. The rabbits were artificially inseminated and
injected iv with human chorionic gonadotrophin immediately
afterwards to ensure ovulation. With the exception of a
single female dosed at 1 mg/kg, all treated rabbits sur-
vived to day 29 of gestation when the experiment was
terminated. A total of 12 animals aborted during the
dosing period: 3, 1, 1, and 7 from the control, 0.2, 1,
and 2.5 mg/kg groups, respectively. Females aborting were
killed the same day and immediate postmortem examinations
carried out. The increased occurrence of abortions in the
highest-dose group was considered to be a secondary effect
of maternal toxicity. No clinical findings in the groups
given 0.2 or 1 mg/kg were considered to be the result of
TBTO treatment. There was a statistically significant mean
body weight loss in females dosed at 2.5 mg/kg per day
compared to controls between days 6 and 18 of gestation
(the period of actual dosing). Doses of 0.2 and 1.0 mg/kg
per day had no effect on growth or survival of fetuses.
There was a slight (statistically non-significant)
decrease in mean fetal weight in the group dosed with 2.5
mg/kg per day. This may represent minor fetotoxicity.
There were no differences in the types or frequency of
fetal malformations related to treatment and the con-
clusion was that TBTO was not teratogenic. Postmortem
examination of the mother rabbits indicated no changes
associated with treatment, and 1 mg/kg per day was con-
sidered to be the NOEL for maternal toxicity and toxicity
to the fetus.
When Crofton et al. (1989) treated pregnant Long-Evans
rats with TBTO by gastric intubation at 0 to 16 mg/kg per
day body weight from day 6 to day 20 of gestation, litter
size and pup weight were significantly reduced by doses of
10, 12, or 16 mg/kg per day but no effect was seen at
doses of 2.5 and 5 mg/kg per day. Maternal weight gain was
affected by the same dose levels that affected litter size
and pup weight. Pup survival was further reduced in the
first 3 days after birth. Litter size was reduced by 50%,
73%, and 96% at dose levels of 10, 12, and 16 mg/kg per
day, respectively, on day 1 post partum and by 63%, 88%,
and 100% on day 3 post partum. Pup weight on day 1 post
partum was reduced by 45%, 45%, and 68% at the three dose
levels. Two out of 71 pups born had cleft palate, but no
controls showed any abnormalities. These two pups were
both born dead. The five pups born to rats given 16 mg/kg
per day showed no malformations, but all died within 3
days. The authors concluded that it was impossible to
distinguish between possible fetotoxicity and maternal
toxicity, since all effects on offspring occurred at TBTO
dose rates that also affected the weight of the mother.
They also showed that pregnant female rats were more sen-
sitive to TBTO than non-pregnant females; the MTD (maximum
tolerated dose) for non-pregnant females was 16 mg/kg per
day, whereas pregnant females showed an MTD of between 5
and 10 mg/kg per day. Most effects on pups were transitory
in that survivors to adulthood showed only reductions in
body weight and brain weight compared to controls even at
the highest dose rates. Motor activity of offspring was
monitored in a maze fitted with photoelectric detectors.
During the pre-weaning period there was a significant
age/treatment interaction, but only on post-natal day 14
was there a significant response per individual day. All
doses of TBTO produced a significant decrease in activity
on that day. Post-weaning activity was reduced on post-
natal days 47 and 62 (p < 0.01) but only at a level of
10 mg/kg per day. There was no clear effect on acoustic
startle response.
In a two-generation reproduction study, TBTO was given
to rats at dietary concentrations of 0, 0.5, 5, and 50
mg/kg. The parental (F0) generation (30 males and 30
females per group) was exposed for 10 weeks before mating,
whereas the pre-mating treatment period for the F1 adults
(30 of each sex per group) was 15 weeks. Culling of
litters was performed on day 4 in the F1 and F2 gener-
ations. Preliminary data (Biodynamics, 1989b) on mor-
tality, body weight development, fertility indices, litter
data, and organ weights have been reported. There was no
evidence of compound-related mortality, and body weight
development was normal in the F0 generation. At 50 mg/kg
diet, the pup weights were decreased on days 14 and 21 in
the F1 generation and on days 7, 14, and 21 in the
F2 generation. Among the F1 parents, at 50 mg/kg, lower
body weights were noted in males throughout the pre-mating
period and in females during the first 3 weeks only. No
effects on mating, pregnancy, and fertility rates were
noted in either generation, and number of pups, litter
size, and pup survival were not affected by treatment in
either the F1 or the F2 generations. Relative and
absolute thymus weights were decreased in both sexes at
the dietary concentration of 50 mg/kg.
11.5.2. In vitro
Krowke et al. (1986) demonstrated an effect of TBTO on
the development of limb buds of mice in organ culture.
Clear-cut interference with differentiation was seen at
the lowest dose tested (0.03 mg/litre), while at 0.1 mg
per litre drastic impairment and abnormal development of
the paw skeleton were recorded. The effect was more pro-
nounced still at the highest dose tested (0.3 mg/litre).
In view of their failure to show effects on the embryo in
vivo, Davis et al. (1987) suggested that there is only
very limited movement of TBTO across the placenta of
mice.
11.6. Carcinogenicity
Summary
One carcinogenicity study on rats has been reported in
which neoplastic changes were observed in endocrine organs at
50 mg/kg diet. The pituitary tumours found at 0.5 mg/kg diet
are considered as having no biological significance since there
was no dose-response relationship. These tumour types appear
usually at high and variable background incidences. The signi-
ficance is, therefore, questionable. A second study on mice is
in progress.
Wester (in press) reported the results of a 106-week
study on carcinogenicty in Wistar rats at dietary TBTO
doses of 0, 0.5, 5, and 50 mg/kg (0, 0.025, 0.25, and
2.5 mg/kg body weight). At the highest dose level, general
toxicological effects were present (see section 11.3). The
incidence of benign tumours of the pituitary (mainly pro-
lactinomas) was elevated at 0.5 and 50 mg/kg, but not at
5 mg/kg diet, for both sexes. At 50 mg/kg, a significant
increase was noted in the incidence of adrenal medullary
tumours (pheochromocytomas) in both sexes and of parathy-
roid adenomas in male animals, while the incidence of
adrenal cortical tumours was significantly decreased at
0.5 and 50 mg/kg diet in males only. Isolated occurrence
of pancreatic carcinoma was found in treated female rats.
These were not considered to be compound related since
there was no dose dependency and the incidence rates were
low.
12. EFFECTS ON HUMANS
Summary
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The potential
problem is made worse by the lack of an immediate skin response.
12.1. Ingestion
There have been no reported cases of poisoning from
ingestion of TBTO or other TBT salts.
12.2. Inhalation
Seventy percent of the workers in a rubber factory
using TBTO in the vulcanizing process reported irritation
of the upper respiratory tract (and eyes). About 20% also
experienced lower chest symptoms (irritation, tightness,
and pain), but in all cases pulmonary function was unaf-
fected. The extent of the exposure was not recorded
(WHO/FAO, 1985).
12.3. Dermal exposure
Lyle (1958) described skin lesions in workers occu-
pationally exposed to dibutyl and tributyl tin compounds.
Skin burns were most commonly caused by small splashes of
liquid dibutyl or tributyl tin chlorides. Since the irri-
tancy of these compounds was not immediately apparent
(taking at least an hour to be perceived), small splashes
were frequently ignored by workers. More severe lesions on
the hands were caused by leaking gloves or failure to wear
hand protection. Some maintenance staff also suffered
burns after kneeling or rubbing against surfaces wet with
the chlorides. Clothes wet with these compounds produced
burns on the ventral skin and burns were seen immediately
above the level of protective boots on the calf of the
leg. Two kinds of lesion were seen in workers. The first
was an acute burn, which healed relatively quickly, and
the second a more diffuse dermatitis (seen in workers
wearing contaminated clothes in close contact with the
skin), which persisted. Treatment of the back of the hand
of volunteers with various undiluted TBT compounds estab-
lished that the chloride, acetate, laurate, and oxide all
produced acute burns. Burns were not caused by dibutyltin
esters or oxide or by tetrabutyltin, but were mostly due
to dibutyltin chloride or the tributyltin compounds. Red-
dening of the area treated was seen 2-3 h after treatment
with the compounds. Inflammation of the hair follicles
was the most obvious symptom, effects being confined to
the treated area. On the second day after treatment, min-
ute pustules appeared, which remained discrete and disap-
peared on the third or fourth day. After a week, all that
remained was a faint erythema. The burns were not reported
to be painful, either in the case of experimental or occu-
pational exposure. Sufferers complained of itching and
"stickiness" of the skin causing adherence of clothes.
Proper use of protective clothing, rapid washing of the
skin after exposure, and the use of aprons to prevent
wetting of overalls were found to be effective in pre-
venting burns, both acute and diffuse.
Baaijens (1987) described cases of accidental exposure
to TBTO during the manufacture of organotin compounds.
Severe dermatitis developed only where splashes of the
material had been retained on the skin for long periods.
In one case, a worker had been splashed over the face and
neck. He left the work area after the splash and showered.
An area behind one ear had not been washed and the derma-
titis had developed in this one area. There were no symp-
toms other than dermatitis. Another worker had been
splashed on the arm. He washed his skin but did not change
his overalls. Contact was extended and a large blister
developed on the arm. Monitoring of the urine tin content
showed no difference from normal in these two cases. A
third worker, who complained of an intense smell of TBTO,
suffered nausea and vomiting after 10 min of exposure.
Urine tin levels in this case were elevated for several
days. In all cases the symptoms disappeared within a few
days. The delayed irritancy of tributyltin was emphasized
by the author. It tends to lead to extended exposure since
the affected person does not perceive the effect for sev-
eral hours. The author found no relationship between
clinical or haematological parameters and normal occu-
pational exposure to organotin compounds.
Goh (1985) reported irritant effects and dermatitis in
painters applying TBTO formulations (0.6% TBTO in acrylic
resin-based paints) to buildings. The painters developed
rashes 8 to 10 h after exposure. When required to paint
ceilings, the men developed more severe symptoms on the
face, neck, and trunk, associated with dripping paint.
Severe itching, redness, swelling, and blistering were
recorded. Hospital examination showed extensive vesiculo-
bullous lesions, erythema, and oedema of the face, neck,
trunk, arms, and thighs. Although only two patients were
examined in detail, most of the workers on the site devel-
oped dermatitis. Patch testing of the two reported cases
showed erosion 48 and 96 h after patch tests using aqueous
solutions of TBTO (0.1 and 0.5 g/litre). Five other volun-
teer controls were patch-treated with solutions of 0.01
and 0.1 g/litre; these also showed a similar reaction to
the patients. No allergenic reaction was found in the
patch tests. Replacement of the paint with a preparation
having similar constituents other than TBTO prevented
further problems amongst the painters.
Lewis & Emmett (1987) describe contact dermatitis in a
shipwright resulting from exposure to TBTO-containing
antifouling paint. The man had been spray-painting blocks
of wood and his skin had been exposed to the spray. There
was no immediate sensation, but some irritation was evi-
dent within about an hour. Erythema and ulceration of the
exposed areas were noted on the second day. There were
also some mild pustular lesions on the mucous membrane of
the lips, presumed to be the result of wiping the mouth
with paint-contaminated arms. The authors conducted patch
testing with TBTO at aqueous concentrations of 0.1%,
0.01%, and 0.0015% using the A1 test tape method. At the
highest dose tested, there were large bullae after 48 h
and crusting after 96 h. The other two doses produced no
adverse effects. The delayed sensation and effect were
highlighted by the authors as being particular problems
with TBTO. Great care to prevent any exposure to products
containing TBTO at high concentrations was recommended.
Molin & Wahlberg (1975) investigated an outbreak of
dermatitis on the feet and ankles of trainee soldiers.
Seventy soldiers reported itching and sometimes painful
erythematous, vesiculous to bullous, and haemorrhagic
lesions after long marches on a hot day. Fifty other
soldiers reported less severe symptoms on another
occasion. The skin lesions disappeared within 1 or 2 weeks
in most cases after treatment with saline compresses and
topical steroid creams. In two cases, the skin was red and
slightly tender more than 6 months later. The outbreak was
traced to a single batch of socks that had been soaked in
7 times the recommended concentration of a disinfectant
solution containing TBTO. Recommended use of the disinfec-
tant was calculated to give about 0.001%, whereas the
concentration after the accidental over-use would have
been about 0.01%. Patch testing of eczema patients not
previously exposed to TBTO suggested that the primary
irritant concentration for the compound was between 0.1
and 0.01%. Soldiers patch-tested 2 months after their
dermatitis had fully healed gave negative results to both
0.001% and 0.01% TBTO. The authors suggested that the
effective concentration for TBTO as a disinfectant is too
close to the primary irritancy concentration to justify
the use of TBTO for disinfecting textiles.
Zedler (1961), on the basis of industrial experience,
stated that a concentration of 0.05% TBTO was not harmful
to the human skin.
12.4. Miscellaneous effects
Women using a latex spray paint containing TBTO as an
additive showed immediate irritant effects on the eye
(profuse lacrimation, eye inflammation) and the nasal
mucosa. The symptoms worsened over 14 days of spraying,
but subsided at the weekends and disappeared completely
when addition of TBTO to the paint was discontinued. The
extent of exposure in this case was not recorded (WHO/FAO,
1985).
Akatsuka et al. (1959) reported a case of occupational
poisoning with butyltin compounds where, along with symp-
toms of lassitude, slight occipital headaches, and stiff-
ness in the shoulders, there was a marked disturbance of
the sense of smell. The authors conducted studies on cats
to test whether this effect could be confirmed experimen-
tally. Exposure of the cats to a vapour mixture containing
mainly tributyltin bromide revealed a marked loss of the
sense of smell.
13. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
13.1. Evaluation of human health risks
Exposure of workers occurs principally during the
manufacture and formulation of tributyltin compounds, in
the application and removal of TBT paints, and from the
use of TBT in wood preservatives. Exposure of the general
public may come from the contamination of food, particu-
larly fish and shellfish, and from domestic application of
wood preservatives.
On the basis of both animal tests and direct obser-
vations on humans, it is clear that TBT compounds are
irritant to the skin and eyes and that inhalation of aero-
sols leads to respiratory irritation.
The handling of treated wood poses no dermal irritant
hazard once the wood has dried. However, aerosols of TBT
are very hazardous and re-entry to the treatment area
should be prohibited until the wood has thoroughly dried.
Acute systemic poisoning has never been reported and
clearance of TBT from the body is expected to occur within
a few days. Acute toxicity from handling TBT products is,
therefore, unlikely if proper precautions are taken.
Short- and long-term effects on experimental animals
have been reported in the liver and haematological and
endocrine systems. The effects of TBT compounds on the
immune system, and particularly on host resistance, have
proved the most sensitive parameter of toxicity in the
rat, the most sensitive species tested. The no-observed-
effect level (NOEL), using the Trichinella spiralis host-
resistance model, lies between 0.5 and 5.0 mg/kg diet
(0.025 and 0.25 mg/kg body weight), whereas using measures
of immune function it is 0.6 mg/kg body weight.
Owing to wide variation in the consumption of fish and
shellfish and local differences in residues of TBT in sea-
food, only illustrative estimates relating exposure and
NOEL values can be made. It needs to be emphasized that
local measurements of residues, local estimates of sea-
food consumption, and local decisions on acceptable safety
margins must be made to assess potential risk of these
compounds.
Using fish consumption figures of 15 and 150 g/day, a
value of 1 mg/kg for residues in fish, and an average
human body weight of 60 kg, the following safety margins
based on different immune endpoints are obtained.
---------------------------------------------------------
Fish Estimated Safety margin
consumption daily intake T. spiralis Other
(g/day) of TBT model immune
(µg/kg) parameters
---------------------------------------------------------
15 0.25 100-1000 2500
150 2.5 10-100 250
---------------------------------------------------------
Indiscriminate and irresponsible use of TBT compounds
and a failure to follow the recommendations, outlined in
this monograph, to reduce exposure of humans could lead to
intake of levels of TBT compounds hazardous to human
health.
Teratogenic effects have only occurred in experimental
animals at doses that caused overt maternal toxicity. The
teratogenic potential of TBT is, therefore, considered to
be very low.
Based on the results of comprehensive mutagenicity
studies, tributyltin compounds are not considered to have
mutagenic potential. In a carcinogenicity study on rats
with TBTO, an increased incidence was noted for endocrine
tumours that occur spontaneously at a high and variable
incidence. Therefore, the available evidence does not
clearly demonstrate a carcinogenic hazard of TBT compounds
for humans.
13.2. Evaluation of effects on the environment
Diffuse input of tributyltin (TBT) into the environ-
ment occurs predominantly from the use of TBT in antifoul-
ing paint. It could also occur if it were used as a mol-
luscicide. Point source contamination occurs if TBT is
used as a biocide in cooling systems, wood pulping,
leather processing, wood preservation processes, and tex-
tile treatment.
Due to their physico-chemical properties, TBT com-
pounds concentrate in the surface microlayer and in sedi-
ments. Abiotic degradation does not appear to be a major
mechanism of removal under environmental conditions.
Although TBTO is biodegradable in the water column, this
process is not rapid enough to prevent the occurrence of
elevated TBT levels in some areas. Bioaccumulation occurs
in most aquatic organisms, but in laboratory mammals,
metabolic degradation is a more efficient process.
TBT is extremely hazardous to some aquatic organisms
because it is toxic at very low concentrations in water.
Such concentrations have been found in some areas. Adverse
effects on non-target invertebrates, particularly mol-
luscs, have been reported in field studies, and these have
been sufficiently severe to lead to reproductive failure
and population decline. Adverse effects on the commercial
production of shellfish have been successfully reversed by
restrictions on the use of antifouling paints in some
areas, and these restrictions are also leading to the
reversal of imposex effects in gastropod populations. The
effects on farmed fish indicate that TBT-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is
likely to be low. TBT-treated wood could pose a hazard to
terrestrial organisms living in close contact with it.
The enhancement of TBT concentrations in the surface
microlayer may present a hazard to littoral organisms,
neustonic species (including benthic invertebrate and fish
larvae) and surface-feeding sea-birds and wildfowl.
Accumulation and low biodegradation of TBT in sediments
may present a hazard to aquatic organisms when these pol-
luted sediments are disturbed by natural processes or
dredging activities.
14. RECOMMENDATIONS
14.1. Recommendations for protecting human and environmental health
a) Member countries that have not yet regulated the use
of TBT compounds should be encouraged to do so.
b) There is a need for evaluation and, if necessary,
regulation of organotin input to the environment from
sources other than antifouling paints. For example,
this would include evaluation of the potential risk
from the application of TBT-contaminated sewage sludge
to soil.
c) Improved methods for the safe application, removal,
and disposal of organotin paints should be developed.
14.2. Research needs
a) Methods of detection and analysis need to be improved
to provide rapid and accurate measurements of butyltin
species in pg/litre concentrations. One reason for
this recommendation is that a biological effect, i.e.
imposex in gastropods, may occur at levels lower than
present detection limits.
b) There is a need for research into mechanisms which
concentrate rather than disperse TBT and which retard
degradation, with particular attention to the funda-
mental chemistry of TBT and its interaction with bio-
logical molecules. More study is needed on the uptake
of TBT at all trophic levels.
c) A study of the toxicity of TBT in aquatic organisms is
required. This work should investigate metabolism,
endocrine effects, and immunological toxicity, where
appropriate.
d) A search for other sensitive bioindicator species in
other groups, including freshwater species, is
required.
e) Models for the assessment of immunotoxicity in mammals
need to be validated and no-effect levels for relevant
parameters need to be defined more accurately.
f) A long-term toxicity study in a second mammalian
species should be undertaken.
g) A tumorigenicity study in a second mammalian species
should be undertaken.
h) Information on butyltin residue levels in fish and
shellfish for human consumption using speciating
methods is needed.
REFERENCES
ABEL, R., HATHAWAY, R.A., KING, N.J., VOSSER, J.L., & WILKINSON, T.G.
(1987) Assessment and regulatory actions for TBT in the UK. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1314-1319.
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A., KAWAME,
N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S., YANAGIBASHI, H.,
NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA, I., & IYO, T. (1959)
[Experimental studies on disturbance of sense of smell due to butyltin
compounds.] J. Tokyo med. Coll., 17: 1393-1402 (in Japanese).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int. Pest Control,
11: 29-35.
ALDRIDGE, W.N. (1958) The biochemistry of organotin compounds. Trialkyltins
and oxidation phosphorylation. Biochem. J., 69: 367-376.
ALDRIDGE, W.N. & STREET, B.W. (1964) Oxidative phosphorylation: Biochemical
effects and properties of trialkyltins. Biochem. J., 91: 287-297.
ALDRIDGE, W.N. & STREET, B.W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver mitochondria.
Biochem. J., 118: 171-179.
ALDRIDGE, W.N. & STREET, B.W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and triethyltin to
mitochondria and their effects on various mitochondrial functions. Biochem.
J., 124: 221-234.
ALDRIDGE, W.N., CASIDA, J.E., FISH, R.H., KIMMEL, E.C., & STREET, B.W.
(1977) Action on mitochondria and toxicity of metabolites of tri-n-butyltin
derivatives. Biochem. Pharmacol., 26: 1997-2000.
ALLEN, A.J., QUITTER, B.M., & RADICK, C.M. (1980) The biocidal mechanism of
controlled release bis (tri-n-butyltin) oxide in Biomphalaria glabrata. In:
Baker, R., ed. Controlled release of bioactive materials, New York, London,
Academic Press, p. 399.
ALZIEU, C.P. (1981) Evaluation des risques dus à l'emploi des peintures
anti-salissures dans les zones conchylicoles, Nantes, Institut scientifique
et technique des Pêches maritimes, 84 pp.
ALZIEU, C. & HERAL, M. (1984) Ecotoxicological effects of organotin
compounds on oyster culture. Ecotoxicol. Test. mar. Environ., 2: 187-196.
ALZIEU, C. & PORTMANN, J.E. (1984) The effect of tributyl tin on the
culture of C. gigas. Proceedings of the 15th Annual Shellfish Conference,
15-16 May, 1984, London, The Shellfish Association of Great Britain, 17 pp.
ALZIEU, C., HERAL, M., THIBAUD, Y., DARDIGNAC, M.-J., & FEUILLET, M. (1982)
Influence des peintures antisalissures à base d'organostanniques sur la
calcification de la coquille de l'huitre Crassostrea gigas. Rev. Trav.
Inst. Pêches Marit., 45: 101-116.
ALZIEU, C., SANJUAN, J., DELTREIL, J.P., & BOREL, M. (1986) Tin
contamination in Arcachon Bay: Effects on oyster shell anomalies. Mar.
Pollut. Bull., 17: 494-498.
ALZIEU, C., SANJUAN, J., MICHEL, P., BOREL, M., & DRENO, J.P. (1989)
Monitoring and assessment of butyltins in Atlantic coastal waters. Mar.
Pollut. Bull., 20: 22-26.
ANGER, J.P., ANGER, F., CANO, Y., CHAUVEL, Y., LOUVET, M., & VAN DEN
DRIESSCHE, J. (1976) Effets chez le cobaye, d'un aérosol à base d'oxyde de
tributylétain (OTBE). Eur. J. Toxicol., 9: 339-346.
ARAKAWA, Y. & WADA, O. (1984) Inhibition of neutrophil chemotaxis by
organotin compounds. Biochem. biophys. Res. Commun., 123: 543-548.
ARGAMAN, Y., HUCKS, C.E., & SHELBY, S.E. (1984) The effects of organotin on
the activated sludge process. Water Res., 18: 535-542.
AYALA, C.A.C., TOLEDO, J.V., DA SILVA NETTO, J.A., & GILBERT, B. (1980) The
effect of hexabutyldistannoxane (TBTO) and other molluscicides on non-
target species, Geneva, World Health Organization, Parasitic Diseases
Programme (Unpublished report).
BAAIJENS, P.A. (1987) Health effect screening and biological monitoring for
workers in organotin industries. In: Toxicology and analytics of the
tributyltins: The present status. Proceedings of an ORTEPA workshop,
Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands, ORTEP-
Association, pp. 191-208
BACCI, E. & GAGGI, C. (1989) Organotin compounds in harbour and marina
waters from the Northern Tyrrhenian Sea. Mar. Pollut. Bull., 20: 290-292.
BAHR, G. & PAWLENKO, S. (1978) Organic tin compounds. In: Bahr, G.,
Kalinowski, H.-O., & Pawlenko, S., ed. Organometallic compounds, germanium,
tin, Stuttgart, Georg Thieme Verlag, pp. 512-515 (Methods in Organic
Chemistry series).
BAILEY, S.K. & DAVIES, I.M. (1988a) Tributyltin contamination around an oil
terminal in Sullom Voe (Shetland). Environ. Pollut., 55: 161-172.
BAILEY, S.K. & DAVIES, I.M. (1988b) Tributyltin contamination in the Firth
of Forth (1975-87). Sci. total Environ., 76: 185-192.
BAKER, J.M. & TAYLOR, J.M. (1967) The toxicity of tributyltin oxide to the
wood-boring beetles Lyctus brunneus Steph. and Anobium punctatum (Deg.).
Ann. appl. Biol., 60: 181-190.
BALLS, P.W. (1987) Tributyltin (TBT) in the waters of a Scottish sea loch
arising from the use of antifoulant treated netting by salmon farms.
Aquaculture, 65: 227-237.
BARNES, J.M. & STONER, H.B. (1958) Toxic properties of some dialkyl and
trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARUG, D. (1981) Microbial degradation of bis (tributyltin) oxide.
Chemosphere, 10: 1145-1154.
BARUG, D. & VONK, J.W. (1980) Studies on the degradation of bis
(tributyltin) oxide in soil. Pestic. Sci., 11: 77-82.
BEAUMONT, A.R. & BUDD, M.D. (1984) High mortality of the larvae of the
common mussel at low concentrations of tributyltin. Mar. Pollut. Bull., 15:
402-405.
BEAUMONT, A.R. & NEWMAN, P.B. (1986) Low levels of tributyltin reduce
growth of marine micro-algae. Mar. Pollut. Bull., 17: 457-461.
BEAUMONT, A.R., NEWMAN, P.B., & WALDOCK, M.J. (1987) Sand substrate
microcosm studies on tributyl tin (TBT) toxicity to marine organisms, 24 pp
(Final report to the UK Department of the Environment, London. Contract No.
PECD 7/8/73).
BERRIOS-DURAN, L.A. & RITCHIE, L.S. (1968) Molluscicidal activity of
bis(tri-n-butyltin) oxide formulated in rubber. World Health Organ. Bull.,
39: 310-312.
BIODYNAMICS (1989a) A three month oral range-finding toxicity study in mice
with bis (tri-n-butyltin) oxide (TBTO), East Millstone, New Jersey,
Biodynamics (Final report to Aceto Chemical Corporation, M. + T. Chemicals
Inc., and Schering Berlin Inc.) (Unpublished).
BIODYNAMICS (1989b) A two-generation reproduction study in rats with TBTO,
East Millstone, New Jersey, Biodynamics (Interim report to Aceto Chemical
Corporation, M. + T. Chemicals Inc., and Schering Berlin Inc.)
(Unpublished).
BJORKLUND, I. (1987a) [Environmental aspects of ship's bottom paints],
Stockholm, Swedish Chemicals Inspectorate, Investigation Department, 16 pp
(in Swedish).
BJORKLUND, I. (1987b) [Environmental effect of antifouling paints],
Stockholm, Swedish National Environment Protection Board, 17 pp.
BLAIR, W.R., OLSON, G.J., BRINCKMAN, F.E., & IVERSON, W.P. (1982)
Accumulation and fate of tri-n-butyltin cation in estuarine bacteria.
Microbiol. Ecol., 8: 241-251.
BLAIR, W.R., OLSON, G.J., & BRINCKMAN, F.E. (1986) Speciation measurements
of butyltins: Application to controlled release rate determination and
production of reference standards. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1141-1145.
BLUNDEN, S.J. & CHAPMAN, A. (1986) Organotin compounds in the environment.
In: Craigh, P.J., ed. Organometallic compounds in the environment, Harlow,
Essex, Longman Group Ltd, pp. 111-159.
BLUNDEN, S.J., HOBBS, L.A., & SMITH, P.J. (1984) The environmental
chemistry of organotin compounds. In: Bowen, H.J.M, Blunden, S.J., Colbeck,
I., Harrison, R.M., Hobbs, L.A., Katz, S.A., Simkiss, K., Smith, P.J., &
Taylor, M.G., ed. Environmental chemistry, London, Royal Society of
Chemistry, Vol. 3, pp. 49-77.
BOKRANZ, A. & PLUM, H. (1975) Industrial manufacture and use of organotin
compounds, Bergkamen, Federal Republic of Germany, Schering AG, 33 pp.
BOORMAN, L.A. (1989) The effects of TBT on two salt marsh plant species,
Aster tripolium and Limonium vulgare. In Preparation.
BOULDIN, T.W., GOINES, N.D., BAGNELL, C.R., & KRIGMAN, M.R. (1981)
Pathogenesis of trimethyltin neuronal toxicity. Ultrastructural and
cytochemical observations. Am. J. Pathol., 104: 237-249.
BRAMAN, R.S. & TOMPKINS, M.A. (1979) Separation and determination of
nanogram amounts of inorganic and methyltin compounds in the environment.
Anal. Chem., 51: 12-19.
BRESSA, G., CIMA, L., CANOVA, F., & CARAVELLO, G.U. (1984) Bioaccumulation
of tin in the fish tissues (Liza aurata). In: Proceedings of the
International Conference on Environmental Contamination, London, July 1984
- Geneva, International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp. 812-815.
BRIDGES, J.W., DAVIES, D.S., & WILLIAMS, R.T. (1967) The fate of ethyltin
and diethyltin derivatives in the rat. Biochem. J., 105: 1261-1266.
BRINCKMAN, F.E. (1981) Environmental organotin chemistry today: Experiences
in the field and laboratory. J. organomet. Chem. Libr., 12: 343-376.
BRINCKMAN, F.E., JACKSON, J.A., BLAIR, W.R., OLSON, G.J., & IVERSON, W.P.
(1983) Ultratrace speciation and biogenesis of methyltin transport species
in estuarine waters. In: Trace metals in sea water, New York, Plenum Press,
pp. 39-72.
BROWN, R.A., NAZARIO, C.M., DE TIRADO, R.S., CASTRILLON, J., & AGARD, E.T.
(1977) A comparison of the half-life of inorganic and organic tin in the
mouse. Environ. Res., 13: 56-61.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1986) The
decline of the gastropod Nucella lapillus around south-west England:
Evidence for the effect of tributyltin from antifouling paints. J. Mar.
Biol. Assoc. UK, 66: 611-640.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1987) Copper,
zinc, and organotin as long-term factors governing the distribution of
organisms in the Fal estuary in southwest England. Estuaries, 10: 208-219.
BRYAN, G.W., GIBBS, P.E., & BURT, G.R. (1988) A comparison of the
effectiveness of tri-n-butyltin chloride and five other organotin compounds
in promoting the development of imposex in the dog-whelk Nucella lapillus.
J. Mar. Biol. Assoc. UK, 68: 733-744.
BUSHONG, S.J., HALL, W.S., JOHNSON, W.E., & HALL, L.W. (1987) Toxicity of
tributyltin to selected Chesapeake Bay biota. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1494-1503.
BUSHONG, S.J., HALL, L.W., HALL, W.S., JOHNSON, W.E., & HERMAN, R.L. (1988)
Acute toxicity of tributyltin to selected Chesapeake Bay fish and
invertebrates. Water Res., 22: 1027-1032.
CALLEY, D.J., GUESS, W.L., & AUTIAN, J. (1967) Hepatotoxicity of a series
of organotin esters. J. pharm. Sci., 56: 240-243.
CARDARELLI, N.F. (1973) Effects of ultralow molluscicide dosages on
Biomphalaria glabrata and Lebistes reticulatus in micro ecological systems:
Report I, Akron, Ohio, University of Akron, 15 pp.
CARDARELLI, N.F. (1978) Controlled release organotins as mosquito
larvicides. Mosq. News, 38: 328-333.
CARDARELLI, N.F. & EVANS, W. (1980) Chemodynamics and environmental
toxicology of controlled release organotin molluscicides. In: Baker, R.W.,
ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the Controlled Release Society, New York, London,
Academic Press, pp. 357-385.
CASIDA, J.E., KIMMEL, E.C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and trialkyleads by
liver microsomes. Acta chem. Scand., 25: 1497-1499.
CHAMP, M.A. & PUGH, W.L. (1987) Tributyltin antifouling paints:
Introduction and overview. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1296-1308.
CHAPMAN, A.H. & PRICE, J.W. (1972) Degradation of triphenyltin acetate by
ultraviolet light. Int. Pest Control, 14: 11-12.
CHENG, Z. & JENSEN, A. (1989) Accumulation of organic and inorganic tin in
blue mussel, Mytilus edulis, under natural conditions. Mar. Pollut. Bull.,
20: 281-286.
CHLIAMOVITCH, Y.-P. & KUHN, C. (1977) Behavioural, haematological and
histological studies on acute toxicity of bis(tri- n-butyltin) oxide on
Salmo gairdneri Richardson and Tilapia rendalli Boulenger. J. Fish Biol.,
10: 575-585.
CHU, K.Y. (1976) Effects of environmental factors on the molluscicidal
activities of slow-release hexabutyldistannoxane and copper sulfate. Bull.
World Health Organ., 54: 417-420.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & LORES, E.M. (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass shrimp
(Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Arch.
environ. Contam. Toxicol., 16: 401-407.
CLEARY, J.J. & STEBBING, A.R.D. (1985) Organotin and total tin in coastal
waters of southwest England. Mar. Pollut. Bull., 16: 350-355.
CLEARY, J.J. & STEBBING, A.R.D. (1987) Organotin in the surface microlayer
and subsurface waters of Southwest England. Mar. Pollut. Bull., 18: 238-
246.
CREMER, J.E. (1957) The metabolism in vitro of tissue slices from rats
given triethyltin compounds. Biochem. J., 67: 87-96.
CROFTON, K.M., DEAN, K.F., BONCEK, V.M., ROSEN, M.B., SHEETS, L.P.,
CHERNOFF, N., & REITER, L.W. (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: Postnatal evaluation of teratology and
behavior. Toxicol. appl. Pharmacol., 97: 113-123.
DAVIDSON, B.M., VALKIRS, A.O., & SELIGMAN, P.F. (1986) Acute and chronic
effects of tributyltin on the mysid Acanthomysis sculpta (Crustacea,
Mysidacea). In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., pp. 1219-1225.
DAVIES, R.J., FLETCHER, R.L., & FURTADO, S.E.J. (1984) The effects of
tributyltin compounds on spore development in the green alga Enteromorpha
intestinalis (L) Link. In: Proceedings of the 6th International Congress on
Marine Corrosion and Fouling, Athens, September 1984, pp. 557-565.
DAVIES, I.M., DRINKWATER, J., MCKIE, J.C., & BALLS, P. (1987a) Effects of
the use of tributyltin antifoulants in mariculture. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., pp. 1477-1481.
DAVIES, I.M., BAILEY, S.K., & MOORE, D.C. (1987b) Tributyltin in Scottish
sea Lochs, as indicated by degree of imposex in the dogwhelk, Nucella
lapillus (L.). Mar. Pollut. Bull., 18: 400-404.
DAVIS, A., BARALE, R., BRUN, G., FORSTER, R., GUNTHER, T., HAUTEFEUILLE,
H., VAN DER HEIJDEN, C.A., KNAAP, A.G.A.C., KROWKE, R., KUROKI, T.,
LOPRIENO, N., MALAVEILLE, C., MERKER, H.J., MONACO, M., MOSESSO, P.,
NEUBERT, D., NORPPA, H., SORSA, M., VOGEL, E., VOOGD, C.E., UMEDA, M., &
BARTSCH, H. (1987) Evaluation of the genetic and embryotoxic effects of
bis(tri- n-butyltin) oxide (TBTO), a broad-spectrum pesticide, in multiple
in vivo and in vitro short-term tests. Mutat. Res., 188: 65-95.
DESCHIENS, R. & FLOCH, H. (1962) Les propriétés molluscicides du chlorure
et de l'acétate de triphénylétain dans le cadre de la prophylaxie des
bilharzioses. C. R. Acad. Sci. Paris, 255: 1236-1237.
DESCHIENS, R. & FLOCH, H. (1968) Action biologique comparée de 6
molluscicides chimique dans le cadre de la prophylaxie des bilharzioses.
Conclusions pratiques. Bull. Soc. Pathol. Exot., 61: 640-650.
DESCHIENS, R., BROTTES, H., & MVOGO, L. (1966) Application sur le terrain,
au Cameroun, dans la prophylaxie des bilharzioses de l'action molluscicide
de l'oxyde de tributylétain. Bull. Soc. Pathol. Exot., 59: 968-973.
DE VILLIERS, J.P. & MACKENZIE, J.G. (1963) Organotin and organolead
molluscicides, Geneva, World Health Organization, Parasitic Diseases
Programme, p. 63 (Unpublished report Mol/Inf/13).
DIXON, D.R. & MCFADZEN, I. (1987) Bis(tributyltin) oxide (TBTO), an
antifouling compound, promotes SCE induction in the larvae of the common
mussel, Mytilus edulis. Mutagenesis, 2: 312.
DIXON, D.R. & PROSSER, H. (1986) An investigation of the genotoxic effects
of an organotin antifouling compound (bis(tributyltin) oxide) on the
chromosomes of the edible mussel, Mytilus edulis. Aquat. Toxicol., 8: 185-
195.
DOJMI DI DELUPIS, G., GUCCI, P.M.B., & VOLTERRA, L. (1987) Toxic effects of
bis-tributyltinoxide on phytoplancton. Main Group Metal Chem., 10: 77-82.
DONARD, O., RAPSOMANIKIS, S., & WEBER, J.H. (1986) Speciation of inorganic
tin and alkyltin compounds by atomic absorption spectrometry using
electrothermal quartz furnace after hydride generation. Anal. Chem., 58:
772-777.
EAJ (1988) Outline of TBT compounds monitored in Japan, Tokyo, Environment
Agency of Japan (OECD Clearing House Project on Organotins).
EBDON, L., EVANS, K., & HILL, S. (1988) The variation of tributyltin levels
with time in selected estuaries prior to the introduction of regulations
governing the use of tributyltin-based anti-fouling paints. Sci. total
Environ., 68: 207-223.
EBDON, L., EVANS, K., & HILL, S. (1989) The accumulation of organotins in
adult and seed oysters from selected estuaries prior to the introduction of
U.K. regulations governing the use of tributyltin-based antifouling paints.
Sci. total Environ., 83: 63-84.
ELFERINK, J.G.R., DEIERKAUF, M., & VAN STEVENINCK, J. (1986) Toxicity of
organotin compounds for polymorphonuclear leukocytes: the effect on
phagocyctosis and exocytosis. Biochem. Pharmacol., 35: 3727-3732.
ELSEA, J.R. & PAYNTER, O.E. (1958) Toxicological studies on bis(tri-n-
butyltin) oxide. Am. Med. Assoc. Arch. Ind. Health, 18: 214-217.
EVANS, C.J. & KARPEL, S. (1985) Organotin compounds in modern technology.
J. organomet. Chem. Libr., 16: 178-217.
EVANS, D.W & LAUGHLIN, R.B. (1984) Accumulation of bis (tributyltin) oxide
by the mud crab, Rhitropanopeus harrisii. Chemosphere, 13: 213-219.
EVANS, W.H., CARDARELLI, N.F., & SMITH, D.J. (1979) Accumulation and
excretion of [1-14C] bis (tri- n-butyltin) oxide in mice. J. Toxicol.
environ. Health, 5: 871-877.
FENT, K. (1989a) Organotin speciation in municipal wastewater and sewage
slude: Ecotoxicological consequences. Mar. environ. Res., 28: 477-483.
FENT, K. (1989b) Teratogenic effects of tributyltin on embryos of the
freshwater fish Phoxinus phoxinus. Presented at the OECD Workshop on
Tributyltin: Activities related to Field Studies at Sea, Centre IFREMER,
Nantes, 27-29 June, 1989 (Unpublished report).
FENT, K., FASSBIND, R., & SIEGRIST, H. (1989) Organotins in a municipal
wastewater treatment plant. Proceedings of the 1st European Conference on
Ecotoxicology, Copenhagen, Denmark, 17-19 October, 1988, Lyngby, Denmark,
The Technical University of Denmark, Laboratory of Environmental Sciences
and Ecology.
FERAL, C. & LE GALL, S. (1983) The influence of a pollutant factor
(tributyltin) on the neuroendocrine mechanism responsible for the
occurrence of a penis in the females of Ocenebra erinacea. In: Lever, J. &
Boer, H.H., ed. Molluscan neuroendocrinology. Proceedings of the
International Minisymposium on Molluscan Endocrinology, Amsterdam, North-
Holland Publishing Company, pp. 173-175.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1975) Bioorganotin chemistry:
Biological oxidation of tributyltin derivatives. J. organomet. Chem., 93:
C1-C4.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1976) Bioorganotin chemistry:
Biological oxidation of organotin compounds. In: Zuckerman, J.J., ed.
Organotin compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 197-203 (Advances in Chemistry Series No.
157).
FLOCH, H., DESCHIENS, R., & FLOCH, T. (1964) Sur les propriétés
molluscicides de l'oxyde et de l'acétate de tributylétain (prophylaxie des
bilharzioses). Bull. Soc. Pathol. Exot., 57: 454-465.
FOSTER, R.B. (1981) Use of Asiatic clam larvae in aquatic hazard
evaluations. In: Bates, J.M. & Weber, C.I., ed. Ecological assessments of
effluent impacts on communities of indigenous aquatic organisms,
Philadelphia, American Society for Testing and Materials, pp. 280-288 (ASTM
STP No. 730).
FUNAHASHI, N., IWASAKI, I., & IDE, G. (1980) Effects of bis(tri-n-butyltin)
oxide on endocrine and lymphoid organs of male rats. Acta pathol. Jpn., 30:
955-966.
GARDINER, B.G. & POLLER, R.C. (1964) Insecticidal activity and structure of
some organotin compounds. Bull. entomol. Res., 55: 17-21.
GIBBS, P.E. & BRYAN, G.W. (1986) Reproductive failure in populations of the
dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from
antifouling paints. J. Mar. Biol. Assoc. UK, 66: 767-777.
GIBBS, P.E. & BRYAN, G.W. (1987) TBT paints and the demise of the dog-
whelk, Nucella lapillus (Gastropoda). In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1482-1487.
GIBBS, P.E., BRYAN, G.W., PASCOE, P.L., & BURT, G.R. (1987) The use of the
dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT)
contamination. J. Mar. Biol. Assoc. UK, 67: 507-523.
GIBBS, P.E., PASCOE, P.L., & BURT, G.R. (1988) Sex change in the female
dogwhelk, Nucella lapillus, induced by tributyltin from antifouling paints.
J. Mar. Biol. Assoc. UK, 68: 715-731.
GILBERT, B., PAES LEME, L.A., FERREIRA, A.M., BULHOES, M.S., & CASTLETON,
C. (1973) Field tests of hexabutyldistannoxane (TBTO) in slow-release
formulations against Biomphalaria spp. Bull. World Health Organ., 49: 633-
636.
GILE, J.D., COLLINS, J.C., & GILLETT, J.W. (1982) Fate and impact of wood
preservatives in a terrestrial microcosm. J. agric. food Chem., 30: 295-
301.
GILLETT, J.W., GILE, J.D., & RUSSELL, L.K. (1983) Predator-prey (vole-
cricket) interactions: the effects of wood preservatives. Environ. Toxicol.
Chem., 2: 83-93.
GOH, C.L. (1985) Irritant dermatitis from tri-n-butyl tin oxide in paint.
Contact dermatitis, 12: 161-163.
GOHLKE, R., LEWA, W., STRACHOVSKY, A., & KOHLER, R. (1969) [Investi-gations
in experimental animals of the inhalatory effects of tributyltin chloride
in a subchronic experiment.] Z. gesamte Hyg. Grenzgeb., 15: 97-104 (in
German).
GOODMAN, L.R., CRIPE, G.M., MOODY, P.H., & HALSELL, D.G. (1988) Acute
toxicity of malathion, tetrabromobisphenol-A, and tributyltin chloride to
mysids (Mysidopsis bahia) of three ages. Bull. environ. Contam. Toxicol.,
41: 746-753.
GROVHOUG, J.G., SELIGMAN, P.F., VAFA, G., & FRANSHAM, R.L. (1986) Baseline
measurements of butyltin in U.S. harbors and estuaries. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1283-1288.
GUARD, H.E., COGBET, A.B., & COLEMAN, W.M. (1981) Methylation of
trimethyltin compounds by estuarine sediments. Science, 213: 770-771.
HADA, N. (1986) [Studies on the kinetics of tributyltin compounds in the
body: With special reference to their biological half times in goldfish and
rats as estimated by newly developed analytical method.] Nichidai Igaku
Zasshi, 45: 1005-1013 (in Japanese).
HALL, L.W., PINKNEY, A.E., ZEGER, S., BURTON, D.T., & LENKEVICH, M.J.
(1984) Behavioral responses to two estuarine fish species subjected to bis
(tri- n-butyltin) oxide. Water Resour. Bull., 20: 235-239.
HALL, L.W., LENKEVICH, M.J., HALL, W.S., PINKNEY, A.E., & BUSHONG, S.J.
(1986) Monitoring organotin concentrations in Maryland waters of Chesapeake
Bay. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1275-1279.
HALL, L.W., BUSHONG, S.J., JOHNSON, W.E., & HALL, W.S. (1988a) Spacial and
temporal distribution of butyltin compounds in a Northern Chesapeake Bay
marina and river system. Environ. Monit. Assess., 10: 229-244.
HALL, L.W., BUSHONG, S.J., HALL, W.S., & JOHNSON, W.E. (1988b) Acute and
chronic effects of tributyltin on a Chesapeake Bay copepod. Environ.
Toxicol. Chem., 7: 41-46.
HALLAS, L.E., MEANS, J.C., & COONEY, J.J. (1982) Methylation of tin by
estuarine microorganisms. Science, 215: 1505-1507.
HARRIS, J.R.W. & CLEARY, J.J. (1987) Particle-water partitioning and
organotin dispersal in an estuary. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1370-1374.
HENDERSON, R.S. (1986) Effects of organotin antifouling paint leachates on
Pearl Harbor organisms: a site specific flowthrough bioassay. In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1226-1233.
HENSHAW, B.G., LAIDLAW, R.A., ORSLER, R.J., CAREY, J.K., & SAVORY, J.G.
(1978) The permanence of tributyltin oxide in timber. In: Record of the
1978 Annual Convention of the British Wood Preserving Association,
Cambridge, 27-30 June, 1978, London, British Wood Preserving Association,
pp. 19-29.
HINGA, K.R., ADELMAN, D., & PILSON, M.E.Q. (1987) Radiolabeled butyl tin
studies in the Merl enclosed ecosystems. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1416-1420.
HIS, E. & ROBERT, R. (1980) Action d'un sel organo-métallique, l'acétate de
tributylétain sur les oeufs et les larves de Crassostrea gigas (Thunberg),
Copenhagen, International Council for the Exploration of the Sea (ICES)
Mariculture Commission, 27 pp (CM 1980/F).
HIS, E. & ROBERT, R. (1985) Développement des véligères de Crassostrea
gigas dans le Bassin d'Arcachon, études sur les mortalités larvaires. Rev.
Trav. Inst. Pêches Marit., 47: 63-88.
HIS, E., MAURER, D., & ROBERT, R. (1986) Observations complémentaires sur
les causes possibles des anomalies de la reproduction de Crassostrea gigas
(Thunberg) dans le Basin d'Arcachon. Rev. Trav. Inst. Pêches Marit., 48:
45-54.
HODGE, V.F., SEIDEL, S.L., & GOLDBERG, E.D. (1979) Determination of tin
(IV) and organotin compounds in natural waters, coastal sediments, and
macro algae by atomic absorption spectrometry. Anal. Chem., 51: 1256-1259.
HOPF, H.S., DUNCAN, J., BEESLEY, J.S.S., WEBLEY, D.J., & STURROCK, R.F.
(1967) Molluscicidal properties of organotin and organolead compounds.
World Health Organ. Bull., 36: 955-961.
HUGGETT, R.J., UNGER, M.A., & WESTBROOK, D.J. (1986) Organotin
concentrations in the southern Chesapeake Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1262-1265.
HUMPEL, M., KUHNE, G., TAUBER, U., & SCHULZE, P.E. (1986) Studies on the
kinetics of bis (tris- n -butyl-113tin) oxide (TBTO). In: Toxicology and
analytics of the tributyltins: The present status. Proceedings of an ORTEPA
workshop, Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands,
ORTEP-Association, pp. 122-142.
HUMPHREY, B. & HOPE, D. (1987) Analysis of water, sediments and biota for
organotin compounds. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1348-1351.
ICES (1987) Concentrations of organotin and total tin in open Danish sea
areas and in pleasure craft marinas along the sound, International Council
for the Exploration of the Sea (ICES) (TWG 14/9/3-E).
IWAI, H., MANABE, M., MATSUI, H., ONO, T., & WADA, O. (1980) Effects of
tributyltin and its metabolites on brain function. J. toxicol. Sci., 5:
257.
IWASAKI, I., FUNABASHI, N., TOIZUMI, S., & IDE, G. (1976) Histopathological
studies on rat's nervous system by oral administration of bis-(tri-n-
butyltin) oxide. (I). J. toxicol. Sci., 1: 99 (abstract).
JENSEN, A. & CHENG, Z. (1987) Total tin and organotin in seawater from
pleasure craft marinas along Danish coast of the Sound. In: Proceedings of
the 15th Conference of Baltic Oceanographers (CBO), Copenhagen, 1986,
Charlottenlung, Denmark, Marine Pollution Laboratory, Vol. 1, pp. 289-298.
JEWETT, K.L. & BRINCKMAN, F.E. (1981) Speciation of trace di- and
triorganotins in water by ion exchange HPLC-GFAA. J. chromatogr. Sci., 19:
583.
JOHANSEN, K. & MOHLENBERG, F. (1987) Impairment of egg production in
Acartia tonsa exposed to tributyltin oxide. Ophelia, 27: 137-141.
JOHNSON, T.L. & KNOWLES, C.O. (1983) Effects of organotins on rat
platelets. Toxicology, 29: 39-48.
JORDAN, P. (1985) Schistosomiasis - The St Lucia Project, Cambridge,
Cambridge University Press, 442 pp.
KAKUNO, A. & KIMURA, S. (1987) [Acute toxicity of bis (tributyltin) oxide
to girella (Girella punctata).] Bull. Tokai Reg. Fish. Res. Lab., 123: 41-
44 (in Japanese).
KALBFUS, W. (1988) TBT-burden from antifouling paints in different waters.
Presented at the OECD Workshop on Monitoring, Chemical Analysis and
Leaching Rates of TBT, Paris, 30 November-2 December, 1988, 11 pp
(Unpublished report).
KALNINS, M.A. & DETROY, B.F. (1984) Effect of wood preservative treatment
of beehives on honey bees and hive products. J. agric. food Chem., 32:
1176-1180.
KEY, D., NUNNY, R.S., DAVIDSON, P.E., & LEONARD, M.A. (1976) Abnormal shell
growth in the Pacific oyster Crassostrea gigas. Some preliminary results
from experiments undertaken in 1975, Copenhagen, International Council for
the Exploration of the Sea (ICES), 12 pp. (C. M. 1976/K/11).
KIMMEL, E.C., FISH, R.H., & CASIDA, J.E. (1977) Bioorganotin chemistry:
Metabolism of organotin compounds in microsomal monooxygenase system and in
mammals. J. agric. food Chem., 25: 1-8.
KLIMMER, O.R. (1969) [The use of organic tin compounds from the viewpoint
of experimental toxicology.] Arzneimittelforschung, 19: 934-939 (in
German).
KNOWLES, C.O. & JOHNSON, T.L. (1986) Influence of organotins on rat
platelet aggregation mechanism. Environ. Res., 39: 172-179.
KRAJNC, E.I. (1989) Presentation to OECD working group on TBT immune
effects. In Preparation.
KRAJNC, E.I., WESTER, P.W., LOEBER, J.G., VAN LEEUWEN, F.X.R., VOS, J.G.,
VAESSEN, H.A.M.G., & VAN DER HEIJDEN, C.A. (1984) Toxicity of bis(tri- n-
butyltin)oxide in the rat. 1. Short-term effects on general parameters and
on the endocrine and lymphoid systems. Toxicol. appl. Pharmacol., 75: 363-
386.
KRAMPITZ, G., ENGELS, J., & CAZAUX, C. (1976) Biochemical studies on water-
soluble proteins and related components of gastropods shells. In: Watabe,
N. & Wilbur, K.M., ed. The mechanisms of mineralization in the
invertebrates and plants, Columbia, University of South Carolina Press, pp.
155-173.
KRAMPITZ, G., DROLSHAGEN, H., & DELTREIL, J.P. (1983) Soluble matrix
components in malformed oyster shells. Experientia (Basel), 39: 1105-1106.
KROWKE, R., BLUTH, U., & NEUBERT, D. (1986) in vitro studies on the
embryotoxic potential of (bis[tri- n-butyltin])oxide in a limb bud culture
system. Arch. Toxicol., 58: 125-129.
LANGSTON, W.J., BURT, G.R., & ZHOU, M. (1987) Tin and organotin in water,
sediments, and benthic organisms of Poole Harbour. Mar. Pollut. Bull., 18:
634-639.
LAUGHLIN, R.B. & FRENCH, W.J. (1980) Comparative study of the acute
toxicity of a homologous series of trialkyltins to larval shore crabs,
Hemigrapsus nudus, and lobster, Homarus americanus. Bull. environ. Contam.
Toxicol., 25: 802-809.
LAUGHLIN, R. & LINDEN, O. (1982) Sublethal responses of the tadpoles of the
European frog Rana temporaria to two tributyltin compounds. Bull. environ.
Contam. Toxicol., 28: 494-499.
LAUGHLIN, R., FRENCH, W., & GUARD, H.E. (1983) Acute and sublethal toxicity
of tributyltin oxide (TBTO) and its putative environmental product,
tributyltin sulfide (TBTS) to zoeal mud crabs, Rhithropanopeus harrisii.
Water Air Soil Pollut., 20: 69-79.
LAUGHLIN, R., NORDLUND, K., & LINDEN, O. (1984) Long-term effects of
tributyltin compounds on the baltic amphipod, Gammarus oceanicus. Mar.
environ. Res., 12: 243-271.
LAUGHLIN, R.B., JOHANNESEN, R.B., FRENCH, W., GUARD, H., & BRINKMAN, F.E.
(1985) Structure-activity relationships for organotin compounds. Environ.
Toxicol. Chem., 4: 343-351.
LAUGHLIN, R.B., GUARD, H.E., & COLEMAN, W.M. (1986a) Tributyltin in
seawater: Speciation and octanol-water partition coefficient. Environ. Sci.
Technol., 20: 201-204.
LAUGHLIN, R.B., FRENCH, W., & GUARD, H.E. (1986b) Accumulation of bis
(tributyltin) oxide by the marine mussel Mytilus edulis. Environ. Sci.
Technol., 20: 884-890.
LAUGHLIN, R.B., PENDOLEY, P., & GUSTAFSON, R.G. (1987) Sublethal effects of
tributyltin on the hard shell clam, Mercenaria mercenaria. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1494-1498.
LAUGHLIN, R.B., GUSTAFSON, R., & PENDOLEY, P. (1988) Chronic embryo-larval
toxicity of tributyltin (TBT) to the hard shell clam Mercenaria mercenaria.
Mar. Ecol. Prog. Ser., 48: 29-36.
LAWLER, I.F. & ALDRICH, J.C. (1987) Sublethal effects of bis(tri- n-
butyltin)oxide on Crassostrea gigas spat. Mar. Pollut. Bull., 18: 274-278.
LEE, R.F. (1985) Metabolism of tributyltin oxide by crabs, oysters and
fish. Mar. environ. Res., 17: 145-148.
LEE, R.F. (1986) Metabolism of bis (tributyltin) oxide by estuarine
animals. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1182-1188.
LEE, R.F., VALKIRS, A.O., & SELIGMAN, P. (1987) Fate of tributyltin in
estuarine waters. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1411-1415.
LEWIS, P.G. & EMMETT, E.A. (1987) Irritant dermatitis from tributyl tin
oxide and contact allergy from chlorocresol. Contact dermatitis, 17: 129-
132.
LINDEN, O. (1987) The scope of the organotin issue in Scandinavia. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1320-1323.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LYLE, W.H. (1958) Lesions of the skin in process workers caused by contact
with butyl tin compounds. Br. J. ind. Med., 15: 193-196.
MCCULLOUGH, F.S., GAYRAL, P., DUNCAN, J., & CHRISTIE, J.D. (1980)
Molluscicides in schistosomiasis control. World Health Organ. Bull., 58:
681-689.
MACKLAD, F., TAMAN, F., & EL SEBAE, A.K. (1983) Toxicity of tributyltin
fluoride and copper sulfate in slow release formulation to Biomphalaria
alexandrina snails. Bull. High Inst. Public Health, 14: 1-12.
MAFF/HSE (1988) Pesticides 1988: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 399 pp (Reference
Book HMSO 500).
MAFF/HSE (1989) Pesticides 1989: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 407 pp (Reference
Book HMSO 500).
MAGUIRE, R.J. (1984) Butyltin compounds and inorganic tin in sediments in
Ontario. Environ. Sci. Technol., 18: 291-294.
MAGUIRE, R.J. (1987) Review: Environmental aspects of tributyltin. Appl.
organomet. Chem., 1: 475-498.
MAGUIRE, R.J. & HUNEAULT, H. (1981) Determination of butyltin species in
water by gas chromatography with flame photometric detection. J.
Chromatogr., 209: 458-462.
MAGUIRE, R.J. & TKACZ, R.J. (1983) Analysis of butyltin compounds by gas
chromatography. Comparison of flame photometric and atomic absorption
spectrophotometric detectors. J. Chromatogr., 268: 99-101.
MAGUIRE, R.J. & TKACZ, R.J. (1985) Degradation of the tri-n-butyltin
species in water and sediment from Toronto Harbor. J. agric. food Chem.,
33: 947-953.
MAGUIRE, R.J. & TKACZ, R.J. (1987) Concentration of tributyltin in the
microlayer of natural waters. Water Pollut. Res. J. Can., 22: 227-233.
MAGUIRE, R.J., CHAU, Y.K., BENGERT, G.A., HALE, E.J., WONG, P.T.S., &
KRAMAR, O. (1982) Occurrence of organotin compounds in Ontario lakes and
rivers. Environ. Sci. Technol., 16: 698-702.
MAGUIRE, R.J., CAREY, J.H., & HALE, E.J. (1983) Degradation of the tri-n-
butyltin species in water. J. agric. food Chem., 31: 1060-1065.
MAGUIRE, R.J., WONG, P.T.S., & RHAMEY, J.S. (1984) Accumulation and
metabolism of tri-n-butyltin cation by a green alga, Ankistrodesmus
falcatus. Can. J. Fish. aquat. Sci., 41: 537-540.
MAGUIRE, R.J., TKACZ, R.J., & SARTOR, D.L. (1985) Butyltin species and
inorganic tin in water and sediment of the Detroit and St. Clair rivers. J.
Great Lakes Res., 11: 320-327.
MAGUIRE, R.J., TKACZ, R.J., CHAU, Y.K., BENGERT, G.A., & WONG, P.T.S.
(1986) Occurrence of organotin compounds in water and sediment in Canada.
Chemosphere, 15: 253-274.
MATSUI, H., WADA, O., MANABE, S., ONO, T., IWAI, H., & FUJIKURA, T. (1982)
[Properties and mechanism of hyperlipidemia induced in rabbits by
tributyltin fluoride.] Jpn. J. ind. Health, 24: 163-171 (in Japanese).
MATTHIAS, C.L., BELLAMA, J.M., & BRINCKMAN, F.E. (1986a) Determination of
ultra-trace concentrations of butyltin compounds in water by simultaneous
hydridization/extraction with GC/FPD detection. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1146-1151.
MATTHIAS, C.L., OLSON, G.J., BELLAMA, J.M., & BRINCKMAN, F.E. (1986b)
Comprehensive method for determination of aquatic butyltin and
butylmethyltin species at ultratrace levels using simultaneous
hydridization/extraction with GC/FPD detection. Environ. Sci. Technol., 20:
609-615.
MATTHIESSEN, P. (1974) Some effects of slow-release bis (tri-n-butyl tin)
oxide on the tropical fish, Tilapia mossambica Peters. Controlled Release
Pesticide Symposium, Akron, Ohio, University of Akron, Community Technical
College, Engineering and Science Division, pp. 25.1-25.16.
MATTHIESSEN, P. & THAIN, J.E. (in press) A method for studying the impact
of polluted marine sediments on colonising organisms; tests with diesel-
based drilling mud and tributyltin antifouling paint. Hydrobiologia.
MAURER, D., HERAL, M., HIS, E., & RAZET, D. (1985) Influence d'une peinture
antisalissure à base de sels organométalliques de l'étain sur le captage en
milieu naturel de l'huitre Crassostrea gigas. Rev. Trav. Inst. Pêches
Marit., 47: 241-250.
MEADOR, J.P. (1986) An analysis of photobehavior of Daphnia magna exposed
to tributyltin. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1213-
1218.
MEINEMA, H.A., BURGER-WIERSMA, T., VERSLUIS DE HAAN, G., & GEVERS, E.C.
(1978) Determination of trace amounts of butyltin compounds in aqueous
systems by gas chromatography/mass spectrometry. Environ. Sci. Technol.,
12: 288-293.
MICHEL, P. (1987) Automatization of a hydride generation/A.A.S. system - an
improvement for organotin analysis. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1340-1343.
MIDDLETON, M.C. & PRATT, I. (1977) Skin water content as a quantitative
index of the vascular and histologic changes produced in rat skin by di- n-
butyltin and tri- n-butyltin. J. invest. Dermatol., 68: 379-384.
MIDDLETON, M.C. & PRATT, I. (1978) Changes in incorporation of
[3H]thymidine into DNA of rat skin following cutaneous application of
dibutyltin, tributyltin and 1-chloro-2:4-dinitrobenzene and the
relationship of these changes to a morphological assessment of the cellular
damage. J. invest. Dermatol., 71: 305-310.
MINCHIN, D., DUGGAN, C.B., & KING, W. (1987) Possible effects of organotins
on scallop recruitment. Mar. Pollut. Bull., 18: 604-608.
MOLIN, L. & WAHLBERG, J.E. (1975) Toxic skin reactions caused by
tributyltin oxide (TBTO) in socks. Berufs-dermatosen, 4: 138-142.
MORI, Y., IESATO, K., UEDA, S., MORI, T., IWASAKI, I., OHNISHI, K., SEINO,
Y., WAKASHIM, Y., WAKASHIN, M., & OKUDA, K. (1984) Renal tubular
disturbances induced by tributyl-tin oxide in guinea pigs: a secondary
Fanconi syndrome. Clin. Nephrol., 21: 118-125.
MULLER, H.A. (1987) Determination of tributyltin oxide in air. In:
Toxicology and analytics of the tributyltins: The present status.
Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986, Vlissingen-
Oost, The Netherlands, ORTEP Association, pp. 162-172.
MULLER, M.D. (1984) Tributyltin detection at trace levels in water and
sediments using GC with flame-photometric detection and GC-MS. Fresenius Z.
anal. Chem., 317: 32-36.
MULLER, M.D. (1987b) Comprehensive trace level determination of organotin
compounds in environmental samples using high-resolution gas chromatography
with flame photometric detection. Anal. Chem., 59: 617-623.
MUSHAK, P., KRIGMAN, M.R., & MAILMAN, R.B. (1982) Comparative organotin
toxicity in the developing rat: somatic and morphological changes and
relationship to accumulation of total tin. Neurobehav. Toxicol. Teratol.,
4: 209-215.
NEMEC, M.D. (1987) A teratology study in rabbits with TBTO: Final Report,
Wil Research Laboratories Inc., 210 pp (Report No. WIL-B0002) (Confidential
report to the Tributyl Tin Oxide Consortium).
NEWTON, F., THUM, A., DAVIDSON, B., VALKIRS, A., & SELIGMAN, P. (1985)
Effects on the growth and survival of eggs and embryos of the California
grunion (Leuresthes tenuis) exposed to trace levels of tributyltin, San
Diego, California, Naval Ocean Systems Center, 15 pp (Technical Report No.
1040).
NIVA (1986) [Organic tin compounds in fjord areas], Oslo, Norwegian
Institute for Water Research (NIVA) (in Norwegian).
O'CALLAGHAN, J.P. & MILLER, D.B. (1988) Acute exposure of the neonatal rat
to tributyltin results in decreases in biochemical indicators of
synaptogenesis and myelinogenesis. Pharmacol. exp. Ther., 246: 394-402.
OKOSHI, K., MORI, K., & NONURA, T. (1987) Characteristics of shell chamber
formation between the two races in Japanese oyster, Crassostrea gigas.
Aquaculture, 67: 313-320.
OLSON, G.J. & BRINCKMAN, F.E. (1986) Biodegradation of tributyltin by
Chesapeake Bay microorganisms. In: Proceedings of the Organotin Symposium,
Oceans '86 Conference, Washington, DC, USA, 23-25 September, 1986, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1196-1201.
OSBORN, D. & LEACH, D.V. (1987) Organotin in birds: Pilot study,
Huntingdon, Institute of Terrestrial Ecology, 15 pp (Final report to the UK
Department of the Environment. Contract No. F3CR/27/D4/01).
PAGE, D.S. (1989) An analytical method for butyltin species in shellfish.
Mar. Pollut. Bull., 20: 129-133.
PAUL, J.D. & DAVIES, I.M. (1986) Effects of copper- and tin-based anti-
fouling compounds on the growth of scallops (Pecten maximus) and oysters
(Crassostrea gigas). Aquaculture, 54: 191-203.
PAULINI, E. (1964) Laboratory experiments with some organotin compounds,
Geneva, World Health Organization, Parasitic Diseases Programme, pp. 1-3
(Unpublished report Mol/Inf/16).
PAULINI, E. & DE SOUZA, C.P. (1970) Influence of different suspended solids
in the water upon molluscicide activity, Geneva, World Health Organization,
Parasitic Diseases Programme, pp. 1-10 (Unpublished report PD/Mol/70.12).
PELIKAN, Z. (1969) Effects of bis(tri- n-butyltin) oxide on the eyes of
rabbits. Br. J. ind. Med., 26: 165-170.
PELIKAN, Z. & CERNY, E. (1968) [The toxic effects of tri- n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292 (in German).
PELIKAN, Z. & CERNY, E. (1969) Toxic effects of bis (tributyltin) oxide
(TBTO) on the skin of rats. Berufs-dermatosen, 17: 305-316.
PICKWELL, G.V. & STEINERT, S.A. (1988) Accumulation and effects of
organotin compounds in oysters and mussels: correlation with serum
biochemical and cytological factors and tissue burdens. Mar. environ. Res.,
24: 215-218.
PIETERS, R.H., KAMPINGA, J., BOL-SCHOENMAKERS, M., LAMB, B.W., PENNINKS,
A.H., & SEINEN, W. (1989) Organotin-induced thymus atrophy concerns the OX-
44+ immature thymocytes: Relation to the interaction between early
thymocytes and thymic epithelial cells? Thymus, 14(1-3): 79-88.
PINKNEY, A.E., HALL, L.W., LENKEVICH, M.J., BURTON, D.T., & ZEGER, S.
(1985) Comparison of avoidance responses of an estuarine fish, Fundulus
heteroclitus, and crustacean, Palaemonetes pugio, to bis (tri-n-butyltin)
oxide. Water Air Soil Pollut., 25: 33-40.
PINKNEY, A.E., MATTESON, L.L., & WRIGHT, D.A. (1988) Effects of tri-
butyltin on survival, growth, morphometry and RNA-DNA ratio of larval
striped bass, Morone saxatilis. In: Proceedings of the Organotin Symposium,
Oceans '88 Conference, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 987-991.
PLUM, H. (1981) Comportement des composés organostanniques vis-à-vis de
l'environnement. Inf. chim., 220: 135-139.
POITOU, P., MARIGNAC, B., CERTIN, C., & GRADISKI, D. (1978) Etude de
l'effet sur le système nerveux central et du pouvoir sensibilisant de
l'oxyde de tributylétain. Ann. Pharm. fr., 36: 569-572.
POLSTER, M. & HALACKA, K. (1971) [9. Contributions to the health-toxic
problems of some anti-microbially used organo-tin compounds.]
Ernährungsforschung, 16: 527-535 (in German).
RACEY, P.A. & SWIFT, S.M. (1986) The residual effects of remedial timber
treatments on bats. Biol. Conserv., 35: 205-214.
RANDALL, L., DONARD, O.F.X., & WEBER, J.H. (1986) Speciation of n-butyltin
compounds by atomic absorption spectrophotometry using electro-thermal
quartz furnace after hydride generation. Anal. Chim. Acta., 184: 197-203.
REIMANN, R. & LANG, R. (1987) Mutagenicity studies with tributyltin
compounds. In: Toxicology and analytics of the tributyltins: The present
status. Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986,
Vlissingen-Oost, The Netherlands, ORTEP Association, pp. 66-90.
REINHARDT, C.A., SCHAWALDER, H., & ZBINDEN, G. (1982) Cell detachment and
cloning efficiency as parameters for cytotoxicity. Toxicology, 25: 47-52.
RICE, C.D., ESPOURTEILLE, F.A., & HUGGETT, R.J. (1987) Analysis of
tributyltin in estuarine sediments and oyster tissue, Crassostrea
virginica. Appl. organomet. Chem., 1: 541-544.
RITCHIE, L.S., BERRIOS-DURAN, L.A., FRICK, L.P., & FOX, I. (1964)
Molluscicidal time-concentration relationships of organo-tin compounds.
World Health Organ. Bull., 31: 147-149.
RITCHIE, L.S., LOPEZ, V.A., & CORA, J.M. (1974) Prolonged applications of
an organotin against Biomphalaria glabrata and Schistosoma mansoni. In:
Molluscicides in schistosomiasis control, New York, London, Academic Press,
pp. 77-88.
RIVM (1989) In: Mathijssen-Spiekman, E.A.M., Canton, J.H., & Roghair, C.J.,
ed. [Investigation into the toxicity of TBTO for a number of freshwater
organisms], Bilthoven, National Institute of Public Health and
Environmental Hygiene, 48 pp (Report No. 668118.001) (in Dutch).
ROBERTS, M.H. (1987) Acute toxicity of tributyltin chloride to embryos and
larvae of two bivalve mollusks, Crassostrea virginica and Mercenaria
mercenaria. Bull. environ. Contam. Toxicol., 39: 1012-1019.
ROBERTS, M.H., BENDER, M.E., DE LISLE, P.F., SUTTON, H.C., & WILLIAMS, R.L.
(1987) Sex ratio and gamete production in American oysters exposed to
tributyltin in the laboratory. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1471-1476.
ROBINSON, I.N. (1969) Effects of some organotin compounds on tissue amine
levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROSENBERG, D.W. & DRUMMOND, G.S. (1983) Direct in vitro effects of bis
(tributyl) tin oxide on hepatic cytochrome P-450. Biochem. Pharmacol., 32:
3823-3829.
ROSENBERG, D.W., DRUMMOND, G.S., CORNISH, H.C., & KAPPAS, A. (1980)
Prolonged induction of hepatic haem oxygenase and decreases in cytochrome
P-450 content by organotin compounds. Biochem. J., 190: 465-468.
ROSENBERG, D.W., DRUMMOND, G.S., & KAPPAS, A. (1981) The influence of
organometals on heme metabolism. in vivo and in vitro studies with
organotins. Mol. Pharmacol., 21: 150-158.
ROSENBERG, D.W., ANDERSON, K.E., & KAPPAS, A. (1984) The potent induction
of intestinal heme oxygenase by the organotin compound, bis(tri- n-butyltin)
oxide. Biochem. biophys. Res. Commun., 119: 1022-1027.
SALAZAR, S.M. (1985) The effects of bis(tri-n-butyltin) oxide on three
species of marine phytoplankton, San Diego, California, Naval Ocean Systems
Center, 16 pp (Technical Report No. 1039).
SALAZAR, M.H. & SALAZAR, S.M. (1985) Ecological evaluation of
organotincontaminated sediment, San Diego, California, Naval Ocean Systems
Center, 21 pp (Technical Report No. 1050).
SALAZAR, M.H. & SALAZAR, S.M. (1987) Tributyltin effects on juvenile mussel
growth. In: Proceedings of the Organotin Symposium, Oceans '87 Conference,
Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1504-
1510.
SALAZAR, S.M., DAVIDSON, B.M., SALAZAR, M.H., STANG, P.M., & MEYERS-
SCHULTE, K.J. (1987) Effects of TBT on marine organisms: Field assessment
of a new site-specific bioassay system. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1461-1470.
SAXENA, P.N. & CROWE, A.J. (1988) An investigation of the efficacy of
organotin compounds for the control of the cotton stainer, Dysdercus
cingulatus, the mosquito, Anopheles stephensi, and the common house fly,
Musca domestica. Appl. organomet. Chem., 2: 185-187.
SCHAFER, E.W. & BOWLES, W.A. (1985) Acute oral toxicity and repellency of
933 chemicals to house and deer mice. Arch. environ. Contam. Toxicol., 14:
111-129.
SCHERING (1983) Repeated dose inhalation study of ZK21.995 in the rat for
29-32 days (21-24 exposures), Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. IC 1/83. Study No. TX81.177).
SCHERING (1986) Re-evaluation of a "mouse micronucleus test on TBTO",
Bergkamen, Federal Republic of Germany, Schering Inc. (Confidential report
No. IC 7186 prepared by the Institute of Occupational Health, Helsinki,
Finland, September 1984).
SCHERING (1989a) TBTO - 4 week oral (dietary administration) toxicity study
in the rat, Bergkamen, Federal Republic of Germany, Schering Inc. (Report
No. 280118 by Hazelton, France. Study No. 14/502).
SCHERING (1989b) TBTO - Plaque forming assay following a 5 week oral
toxicity study in the rat, Bergkamen, Federal Republic of Germany, Schering
Inc. (Report No. 283118 by Hazelton, France. Study No. 14/503).
SCHERING (1989c) TBTO - Resistance to Listeria monocytogene infection
following a 34 day oral toxicity study in the rat, Bergkamen, Federal
Republic of Germany, Schering Inc. (Report No. 282118 by Hazelton, France.
Study No. 14/505).
SCHERING (1989d) TBTO - Delayed type hypersensitivity test following a 37
day oral toxicity study in the rat, Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. 281118 by Hazelton, France).
SCHERING (1989e) TBTO - Systemic toxicity study in beagle dogs with daily
oral (intragastric) administration over a total of 18-19 weeks, Bergkamen,
Federal Republic of Germany, Schering Inc. (Report No. IC6/88).
SCHWEINFURTH, H. (1985) Toxicology of tributyltin compounds. Tin Uses, 143:
9-12.
SEIFFER, E.A. & SCHOOF, H.F. (1967) Tests of 15 experimental molluscicides
against Australorbis glabratus. Public Health Rep., 82: 833-839.
SEINEN, W., HELDER, T., VERNIJ, H., PENNINKS, A., & LEEUWANGH, P. (1981)
Short term toxicity of tri- n-butyltinchloride in rainbow trout (Salmo
gairdneri Richardson) yolk sac fry. Sci. total Environ., 19: 155-166.
SELIGMAN, P.F., VALKIRS, A.O., & LEE, R.F. (1986a) Degradation of
tributyltin in marine and estuarine waters. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1189-1195.
SELIGMAN, P.F., GROVHOUG, J.G. & RICHTER, K.E. (1986b) Measurement of
butyltins in San Diego Bay, CA: A monitoring strategy. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1289-1296.
SELIGMAN, P.F., GROVHOUG, J.G., VALKIRS, A.O., STANG, P.M., FRANSHAM, R.,
STALLARD, M.O., DAVIDSON, B., & LEE, R.F. (1989) Distribution and fate of
tributyltin in the United States marine environment. Appl. organomet.
Chem., (in press).
SHELDON, A.W. (1975) Effects of organotin anti-fouling coatings on man and
his environment. J. Paint Technol., 47: 54-58.
SHERMAN, L.R. & CARLSON, T.L. (1980) A modified phenylfluorone method for
determining organotin compounds in the ppb and sub-ppb range. J. anal.
Toxicol., 4: 31-33.
SHIFF, C.J., YIANNAKIS, C., & EVANS, A.C. (1975) Further trials with TBTO
and other slow release molluscicides in Rhodesia. In: Proceedings of the
Controlled Release Pesticide Symposium, Wright State University, Dayton,
Ohio, 8-10 September, 1975, pp. 177-188.
SHIMIZU, A. & KIMURA, S. (1987) [Effect of bis (tributyltin) oxide on
gonadal development of a salt-water goby, Chasmichthys dolichognathus:
Exposure during maturing period.] Bull. Tokai Reg. Fish. Res. Lab., 123:
45-49 (in Japanese).
SHORT, J.W. & THROWER, F.P. (1986) Accumulation of butyltins in muscle
tissue of chinook salmon reared in sea pens treated with tri- n-butyltin.
In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1177-1181.
SHORT, J.W. & THROWER, F.P. (1987) Toxicity of tri- n-butyl-tin to chinook
salmon, Oncorhynchus tshawytscha, adapted to seawater. Aquaculture, 61:
193-200.
SLESINGER, A.E. & DRESSER, I. (1978) The environmental chemistry of three
organotin chemicals. In: Good, M., ed. Report of the Organotin Workshop,
New Orleans, Louisiana, 17-19 February, 1978, pp. 115-162.
SMITH, B.S. (1981a) Male characteristics on female mud snails caused by
antifouling paints. J. appl. Toxicol., 1: 22-25.
SMITH, B.S. (1981b) Tributyltin compounds induce male characteristics on
female mud snails Nassarius obsoletus = Ilyanassa obsoleta. J. appl.
Toxicol., 1: 141-144.
SMITH, B.S. (1981c) Male characteristics in female Nassarius obsoletus:
Variations related to locality, season and year. Veliger, 23: 212-216.
SMITH, D.R., STEPHENSON, M.D., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1987) The use of transplanted juvenile oysters to monitor the toxic
effects of tributyltin in California waters. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1511-1516.
SMITH, P.J., CROWE, A.J., ALLEN, D.W., BROOKS, J.S., & FORMSTONE, R. (1977)
Study by 119m Sn Mossbauer spectroscopy of bis(tri- n-butyltin) oxide
adsorbed on cellulosic materials. Chem. Ind., 23: 874-875.
SNOEIJ, N.J. (1987) Triorganotin compounds in immunotoxicology and bio-
chemistry, Utrecht, The Netherlands, University of Utrecht, 170 pp (Ph.D.
Thesis).
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1985)
Toxicity of triorganotin compounds: Comparative in vivo studies with a
series of trialkyltin compounds and triphenyltin chloride in male rats.
Toxicol. appl. Pharmacol., 81: 274-286.
SNOEIJ, N.J., PUNT, P.M., PENNINKS, A.H., & SEINEN, W. (1986a) Effects of
tri- n-butyltin chloride on energy metabolism, macromolecular synthesis,
precursor uptake and cyclic AMP production in isolated rat thymocytes.
Biochim. Biophys. Acta, 852: 234-243.
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1986b)
Triorganotin-induced cytotoxicity to rat thymus, bone marrow and red blood
cells as determined by several in vitro assays. Toxicology, 39: 71-83.
SNOEIJ, N.J., PIETERS, R.H.H., PENNINKS, A.H., & SEINEN, W. (1987) Toxicity
of triorganotin compounds: Orally administered tri- n-butyltin compounds are
rapidly dealkylated in the rat. In: Triorganotincompounds in immunology and
biochemistry, Utrecht, The Netherlands, University of Utrecht, Chapter 4,
pp. 73-93 (Snoeij, N.J., Ph.D. Thesis).
SNOEIJ, N.J., BOL-SCHOENMAKERS, M., PENNINKS, A.H., & SEINEN, W. (1988a)
Differential effects of tri- n-butyltin chloride on macromolecular synthesis
and ATP levels of rat thymocyte subpopulations obtained by centrifugal
elutriation. Int. J. Immunopharmacol., 10: 29-37.
SNOEIJ, N.J., PENNINKS, A.H., & SEINEN, W. (1988b) Dibutyltin and
tributyltin compounds induce thymus atrophy in rats due to a selective
action on thymic lymphoblasts. Int. J. Immunopharmacol., 10: 891-899.
SORACCO, R.J. & POPE, D.H. (1983) Bacteriostatic and bactericidal modes of
action of bis (tributlytin) oxide on Legionella pneumophila. Appl. environ.
Microbiol., 45: 48-57.
STALLARD, M., HODGE, V., & GOLDBERG, E.D. (1987) TBT in California coastal
waters: Monitoring and assessment. Environ. Monit. Assess., 9: 195-220.
STANG, P.M. & SELIGMAN, P.F. (1986) Distribution and fate of butyltin
compounds in the sediment of San Diego Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1256-1261.
STANG, P.M. & SELIGMAN, P.F. (1987) In situ adsorption and desorption of
butyltin compounds from Pearl Harbor, Hawaii, sediment. In: Proceedings of
the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1386-1391.
STEIN, T. & KUSTER, K. (1982) Der biologische abbau von tributylzinnoxid
(TBTO) in einer belebtschlammanlage. Z. Wasser Abwasser Forsch., 15: 178-
180.
STEPHENSON, M.D., SMITH, D.R., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1986) Growth abnormalities in mussels and oysters from areas with high
levels of tributyltin in San Diego Bay. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1246-1251.
STEPHENSON, M.D., SMITH, D.R., HALL, L.W., JOHNSON, W.E., MICHEL, P.,
SHORT, J., WALDOCK, M., HUGGETT, R.J., SELIGMAN, P., & KOLA, S. (1987) An
international intercomparison of butyltin determinations in mussel tissue
and sediments. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1334-1338.
STROMGREN, T. & BONGARD, T. (1987) The effect of tributyltin oxide on
growth of Mytilus edulis. Mar. Pollut. Bull., 18: 30-31.
TEMMINK, J.H.M. & EVERTS, J.W. (1987) Comparative toxicity of tributyltin-
oxide (TBTO) for fish and snail. In: Proceedings of the Seventh World
Meeting of the ORTEP-Association, Amsterdam, 7-8 May, 1987, Vlissingen-
Oost, The Netherlands, ORTEP-Association, pp. 6-20.
THAIN, J.E. (1983) The acute toxicity of bis (tributyl tin) oxide to the
adults and larvae of some marine organisms, Copenhagen, International
Council for the Exploration of the Sea (ICES), 5 pp (Report No. C. M.
1983/E.13).
THAIN, J.E. (1986) Toxicity of TBT to bivalves: Effects on reproduction,
growth and survival. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1306-
1313.
THAIN, J.E. & WALDOCK, M.J. (1985) The growth of bivalve spat exposed to
organotin leachates from antifouling paints, Copenhagen, International
Council for the Exploration of the Sea (ICES), 10 pp (Report No. C. M.
1985/E.28).
THAIN, J.E. & WALDOCK, M.J. (1986) The impact of tributyl tin (TBT)
antifouling paints on molluscan fisheries. Water Sci. Technol., 18: 193-
202.
THAIN, J.E., WALDOCK, M.J., & WAITE, M.E. (1987) Toxicity and degradation
studies of tributyltin (TBT) and dibutyltin (DBT) in the aquatic
environment. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1398-1404.
THOMAS, T.E. & ROBINSON, M.G. (1986) The physiological effects of the
leachates from a self-polishing organotin antifouling paint on marine
diatoms. Mar. environ. Res., 18: 215-229.
THOMAS, T.E. & ROBINSON, M.G. (1987) Initial characterization of the
mechanisms responsible for the tolerance of Amphora coffeaeformis to copper
and tributyltin. Bot. mar., 30: 47-53.
THOMPSON, J.A.J., SHEFFER, M.G., PIERCE, R.C., CHAU, Y.K., COONEY, J.J.,
CULLEN, W.R., & MAGUIRE, R.J. (1985) Organotin compounds in the aquatic
environment: Scientific criteria for assessing their effects on
environmental quality, Ottawa, National Research Council Canada, 284 pp
(NRCC No. 22494).
TOLEDO, J.V., MONTEIRO DA SILVA, C.S., BULHOES, M.S., PAES LEME, L.A., DA
SILVA NETTO, J.A., & GILBERT, B. (1976) Snail control in urban sites in
Brazil with slow-release hexabutyldistannoxane and pentachlorophenol. World
Health Organ. Bull., 54: 421-425.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.-P., PHU LICH N., VAN DEN DRIESSCHE, J.,
GUESNIER, L.R., & MORIN, N. (1976) Contribution à l'étude toxicologique et
pharmacologique de l'oxyde de tributylétain (OTBE). Eur. J. Toxicol., 9:
31-40.
TRUHAUT, R., ANGER, J.P., REYMANN, J.M., CHAUVEL, Y., & VAN DEN DRIESSCHE,
J. (1979) Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicol. Eur. Res., 11: 181-186.
TRUHAUT, R., ANGER, J.P., ANGER, F., BRAULT, A., BRUNET, P., CANO, Y.,
LOUVET, M., SACCAVINI, J.C., VAN DEN DRIESSCHE, J., JANSON, C., & SUBLET,
Y. (1981) Dégradation thermique de l'oxyde de tributylétain (OTBE) et
toxicité pulmonaire des produits de combustion chez la souris et le cobaye.
Toxicol. Eur. Res., 111: 35-44.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1986)
Bioconcentration of butyltin compounds by round crucian carp. Toxicol.
environ. Chem., 12: 137-143.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1987)
Bioconcentration and metabolism of phenyl tin chlorides in carp. Water
Res., 21: 949-953.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1988)
Bioconcentration and metabolism of butyltin compounds in carp. Water Res.,
22: 647-651.
TWG (1988a) Uses and production of organotin compounds. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group), Brussels, 7-11 March, 1988, 6 pp (Report No. TWG 15/5/1-E).
TWG (1988b) Tributyl tin compounds in antifouling paints. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group, Brussels, 7-11 March, 1988, 2 pp, (Report No. TWG 15/5/2-E).
TWG (1988c) Organotins in the Netherlands. Presented by the Netherlands at
the Convention for the Prevention of Marine Pollution from Land-based
Sources (15th Meeting of the Technical Working Group, Brussels, 7-11 March,
1988, 11 pp (Report No. TWG 15/5/4-E).
UHL, S. (1986) [Population exposure to the environmental chemical
pentachloro-ophenol (PCP) and bis (tri-n- butyltin)oxide (TBTO)], Zurich,
Maus Offsetdruck Konstanz, 145 pp (ETH Thesis No. 8014, University of
Gottingen) (in German).
UNEP (1989) Mediterranean Action Plan. Assessment of organotin compounds as
marine pollutants in the Mediterranean, Athens, United Nations Environment
Programme (MAP Technical Report Series, No. 33).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1986) Organotin in
antifouling paints environmental considerations, London, UK Department of
the Environment, 82 pp (Pollution Paper No. 25).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1989) Water and the
environment, London, UK Department of the Environment, p. 26 (Circular No.
7/89).
UPATHAM, E.S., KOURA, M., AHMED, M.D., & AWAD, A.H. (1980) Laboratory
trials of controlled release molluscicides on Bulinus (Ph.) abyssinicus,
the intermediate host of Schistosoma haematobium in Somalia. In: Baker,
R.W., ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the controlled Release Society, New York, London,
Academic Press, pp. 461-469.
U'REN, S.C. (1983) Acute toxicity of bis(tributyltin) oxide to a marine
copepod. Mar. Pollut. Bull., 14: 303-306.
VALKIRS, A.O., SELIGMAN, P.F., STANG, P.M., HOMER, V., LIEBERMAN, S.H.,
VAFA, G., & DOOLEY, C.A. (1986) Measurement of butyltin compounds in San
Diego Bay. Mar. Pollut. Bull., 17: 319-324.
VALKIRS, A.O., DAVIDSON, B.M., & SELIGMAN, P.F. (1987) Sublethal growth
effects and mortality to marine bivalves from long-term exposure to
tributyltin. Chemosphere, 16: 201-220.
VIGHI, M. & CALAMARI, D. (1985) QSARs for organotin compounds on Daphnia
magna. Chemosphere, 14: 1925-1932.
VIYANANT, V., THIRACHANTRA, S., & SORNMANI, S. (1982) The effect of
controlled release copper sulphate and tributyltin fluoride on the
mortality and infectivity of Schistosoma mansoni cercariae. J. Helminthol.,
56: 85-92.
VOS, J.G., DE KLERC, A., KRAJNC, E.I., KRUIZINGA, W., VAN OMMEN, B., &
ROZING, J. (1984) Toxicity of bis(tri- n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of non-
specific resistance after short-term exposure. Toxicol. appl. Pharmacol.,
75: 387-408.
VOS, J.G., KRAJNC, E.I., & WESTER, P.W. (1985) Immunotoxicity of bis (tri-
n-butyltin) oxide. In: Dean, J., ed. Immunotoxicology and
immunopharmacology, New York, Raven Press, pp. 327-340.
WADE, T.L., GARCIA-ROMERO, B., & BROOKS, J.M. (1988) Tributyltin
contamination in bivalves from U.S. coastal estuaries. Environ. Sci.
Technol., 22: 1488-1493.
WALDOCK, M.J. (1989) Organotin concentrations in the Rivers Bure and Yare,
Norfolk Broads, England (Unpublished report of the Ministry of Agriculture,
Fisheries and Food, Fisheries Laboratory, Burnham-on-Crouch, Essex, UK).
WALDOCK, M.J. & MILLER, D. (1983) The determination of total and tributyl
tin in seawater and oysters in areas of high pleasure craft activity,
Copenhagen, International Council for the Exploration of the Sea (ICES), 18
pp (Report No. C. M. 1983/E.12).
WALDOCK, M.J. & THAIN, J.E. (1983) Shell thickening in Crassostrea gigas:
Organotin antifouling or sediment induced? Mar. Pollut. Bull., 14: 411-415.
WALDOCK, M.J. & THAIN, J.E. (1985) The comparative leach rates and toxicity
of two fish net antifouling preparations, Copenhagen, International Council
for the Exploration of the Sea (ICES), 5 pp (Report No. C. M. 1985/E.29).
WALDOCK, M.J., THAIN, J.E. & MILLER, D. (1983) The accumulation and
depuration of bis (tributyl tin) oxide in oysters: A comparison between the
Pacific oyster (Crassostrea gigas) and the European flat oyster (Ostrea
edulis), Copenhagen, International Council for the Exploration of the Sea
(ICES), 6 pp (Report No. C. M. 1983/E.52).
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1987a) Changes in concentrations
of organotins in U.K. rivers and estuaries following legislation in 1986.
In: Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1352-1356.
WALDOCK, M.J., THAIN, J.E., & WAITE, M.E. (1987b) The distribution and
potential toxic effects of TBT in UK estuaries during 1986. Appl.
organomet. Chem., 1: 287-301.
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1988) Inputs of TBT to the
marine environment from shipping activity in the U.K. Environ. Technol.
Lett., 9: 999-1010.
WALNE, P.R. & HELM, M.M. (1979) Introduction of Crassostrea gigas into the
United Kingdom. In: Mann, R., ed. Proceedings of a Symposium on Exotic
Species in Marinculture: Case histories of the Japanese Oyster Crassostrea
gigas (Thunberg), with implications for other fisheries, Woods Hole,
Massachusetts, USA, 18-20 September, 1978, Cambridge, London, MIT Press,
pp. 83-105.
WALSH, G.E. (1986) Organotin toxicity studies conducted with selected
marine organisms at EPA's environmental research laboratory, Gulf Breeze,
Florida. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1210-1212.
WALSH, G.E., MCLAUGHLAN, L.L., LORES, E.M., LOUIE, M.K., & DEANS, C.H.
(1985) Effects of organotins on growth and survival of two marine diatoms,
Skeletonema costatum and Thalassiosira pseudonana. Chemosphere, 14: 383-
392.
WALSH, G.E., LOUIE, M.K., MCLAUGHLIN, L.L., & LORES, E.M. (1986a) Lugworm
(Arenicola cristata) larvae in toxicity tests: Survival and development
when exposed to organotins. Environ. Toxicol. Chem., 5: 749-754.
WALSH, G.E., MCLAUGHLIN, L.L., LOUIE, M.K., DEANS, C.H., & LORES, E.M.
(1986b) Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin oxide and triphenyltin oxide.
Ecotoxicol. environ. Saf., 12: 95-100.
WALTON, R., ADEMA, C.M., & SELIGMAN, P.F. (1986) Mathematical modeling of
the transport and fate of organotin in harbors. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1297-1301.
WARD, G.S., CRAMM, G.C., PARRISH, P.R., TRACHMAN, H., & SLESINGER, A.
(1981) Bioaccumulation and chronic toxicity of bis(tributyltin) oxide
(TBTO): Tests with a saltwater fish. In: Branson, D.R. & Dickson, K.L., ed.
Proceedings of the Fourth Conference on Aquatic Toxicology and Hazard
Assessment, Philadelphia, American Society for Testing and Materials, pp.
183-200 (ASTM STP No. 737).
WEBBE, G. (1963) Laboratory tests of some new molluscicides (organotin
compounds), Geneva, World Health Organization, Parasitic Diseases
Programme, pp. 1-2 (Unpublished report Mol/Inf/14).
WEBER, J.H., DONARD, O.F.X., RANDALL, I., & HAN, J.S. (1986) Speciation of
methyl and butyltin compounds in the Great Bay estuary (N.H.). In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1280-1282.
WEIS, J.S., WEIS, P., & WANG, F. (1987a) Developmental effects of
tributyltin on the fiddler crab, Uca pugilator, and the killifish, Fundulus
heteroclitus. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1456-1460.
WEIS, J.S., GOTTLIEB, J., & KWIATKOWSKI, J. (1987b) Tributyltin retards
regeneration and produces deformities of limbs in the fiddler crab, Uca
pugilator. Arch. environ. Contam. Toxicol., 16: 321-326.
WESTER, P.W., CANTON, J.H., VAN IERSAL, A.A.J., KRANJC, E.I., & VAESSEN,
H.A.M.G. (1988) The toxicity of bis(tri-n- butyltin)oxide (TBTO) and di-n-
butyltindichloride (DBTC) in the small fish species Orysias latipes
(medaka) and Poecilia reticulata (guppy), Utrecht, The Netherlands,
University of Utrecht (Wester, P.W., Ph.D. Thesis).
WESTER, P.W. (in press) Chronic toxicity and carcinogenicity of bis (tri-n-
butyltin) oxide in the rat. Food Chem. Toxicol.
WHO/FAO (1984) Data sheet on pesticides No. 65: Bis (tributyltin)oxide,
Geneva, World Health Organization (VBC/PDS/DS/85.65).
WOLNIAKOWSKI, K.U., STEPHENSON, M.D., & ICHIKAWA, G.S. (1987) Tributyltin
concentrations and Pacific oyster deformations in Coos Bay, Oregon. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1438-1442.
WONG, P.T.S., CHAU, Y.K., KRAMAR, O., & BENGERT, G.A. (1982) Structure-
toxicity relationship of tin compounds on algae. Can. J. Fish. aquat. Sci.,
39: 483-488.
WOOTEN, R., DAVIES, I.M., MCKIE, J.C., & BRUNO, D.W. (1986) Chemical and
histopathological changes in salmon exposed to low concentrations of
tributyltin in seawater, Aberdeen, Department of Agriculture and Fisheries
for Scotland, 3 pp (Working Paper No. 15/86).
YLA-MONONEN, L. (1988) Finnish studies on the use, occurrence and effects
of organic tin compounds. Presented at the OECD Workshop on Monitoring,
Chemical Analysis and Leaching Rates of TBT, Paris, 30 November-2 December,
1988, 9 pp (Unpublished report).
YOUNG, D.R., SCHATZBERG, P., BRINCKMAN, F.E., CHAMP, M.A., HOLM, S.E., &
LANDY, R.B. (1986) Summary report - Interagency workshop on aquatic
sampling and analysis for organotin compounds. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1135-1140.
ZEDLER, R.J. (1961) Organotin as industrial biochemicals. Tin Uses,
53: 7-11.
ZIMMERLI, B. & ZIMMERMANN, H. (1980) Gas-chromatische bestimmung von spuren
von n-butylzinnverbindungen (tetra-, tri-, di-). Fresenius Z. anal. Chem.,
304: 23-27.
ZUCKERMAN, J.J., REISDORF, R.P., ELLIS, H.V., & WILKINSON, R.R. (1978)
Organotins in biology and the environment. In: Brinkman, F.E. & Bellama,
J.M., ed. Organometals and organometalloids, occurrence and fate in the
environment, Washington, DC, American Chemical Society, pp. 388-424 (ACS
Symposium Series No. 82).
RESUME
1. Identité, propriétés physiques et chimiques
Les dérivés du tributylétain (TBT) sont des dérivés
organiques de l'étain tétravalent. Ils se caractérisent
par la présence de liaisons covalentes entre des atomes de
carbone et un atome d'étain et leur formule générale est
la suivante: (n-C4H9)3 Sn-X (dans laquelle X désigne
un anion). La pureté de l'oxyde de tributylétain du com-
merce est généralement supérieure à 96%; les principales
impuretés sont le dibutylétain et dans une moindre mesure
le tétrabutylétain et d'autres trialkylétains. L'oxyde de
tributylétain est un liquide incolore d'odeur caractéris-
tique et de densité comprise entre 1,17 et 1,18. Il est
peu soluble dans l'eau (solubilité comprise entre moins
de 1,0 et plus de 100 mg/litre selon le pH, la température
et les anions présents dans l'eau qui déterminent l'espèce
chimique en cause). Dans l'eau de mer et dans les con-
ditions normales, on rencontre trois dérivés du tributylé-
tain: l'hydroxyde, le chlorure et le carbonate, qui sont
en équilibre. Aux pH inférieurs à 7,0 les formes prédomi-
nantes sont Bu3SnOH2+ et Bu3SnCl; à pH 8 ce sont
Bu3SnCl, Bu3SnOH et Bu3SnCO3-; au-delà de 10, ce
sont Bu3SnOH et Bu3SnCO3- qui prédominent.
Le coefficient de partage octanol/eau (log de Pow)
est compris entre 3,18 et 3,84 pour l'eau distillée et il
est égal à 3,54 pour l'eau de mer. L'oxyde de tributylé-
tain s'adsorbe fortement aux matières particulaires,
puisque les coefficients d'absorption indiqués dans la
littérature vont de 110 à 55 000. La tension de vapeur est
faible mais les valeurs publiées sont extrêmement varia-
bles. On n'a constaté aucune perte d'oxyde de tributylé-
tain à partir d'une solution de 1 mg/litre en 62 jours,
toutefois 20% de l'eau avait disparu par évaporation.
2. Méthodes d'analyse
On utilise plusieurs méthodes pour le dosage des
dérivés du tributylétain dans l'eau, les sédiments ou les
biotes. La plus communément utilisée est la spectrométrie
d'absorption atomique. La spectrométrie d'absorption
atomique avec flamme a une limite de détection de
0,1 mg/litre. Sans flamme, avec atomisation dans un four
électrique à graphite, elle est plus sensible et ses
limites de détection varient entre 0 et 1,0 µg/litre
d'eau. Il existe plusieurs méthodes d'extraction et de
préparation de dérivés volatils. La séparation de ces
dérivés s'effectue habituellement par piégeage ou par
chromatographie en phase gazeuse. Les limites de détection
se situent entre 0,5 et 5,0 µg/kg dans le cas des sédi-
ments et des biotes.
3. Sources de pollution de l'environnement
Les dérivés du tributylétain sont homologués comme
molluscicides, comme produits antisalissures pour la
préservation des coques de bateaux, des appontements, des
bouées, des casiers à crabes, des filets et des cages,
comme enduits de protection du bois, comme alguicides dans
le bâtiment, comme désinfectants et comme biocides dans
les systèmes de réfrigération, les tours de réfrigération
des centrales électriques, les usines de pâte à papier,
les brasseries, les tanneries et les usines textiles. Les
premières peintures antisalissures à base de TBT con-
tenaient ce produit sous une forme qui en permettait la
libération sans entrave. Plus récemment, sont apparues des
peintures dans lesquelles l'incorporation du TBT dans une
matrice en copolymère permet d'en limiter la libération.
On a également mis au point des matrices caoutchouteuses
qui permettent une libération lente et durable et assurent
aux peintures antisalissures et aux molluscicides une
efficacité prolongée. Le TBT n'est pas utilisé en agri-
culture en raison de sa forte phytotoxicité.
4. Réglementation
De nombreux pays ont restreint l'utilisation des
peintures antisalissures à base de TBT du fait de l'action
de cette substance sur les fruits de mer. Les détails de
la réglementation varient d'un pays à l'autre mais la
plupart interdisent l'emploi de peintures à base de TBT
sur les navires de moins de 25 mètres. Dans certains pays,
les navires à coque d'aluminium ne sont pas visés par
cette interdiction. En outre, certaines réglementations
limitent la teneur des peintures en TBT ou la lixiviation
de cette substance à partir des peintures qui en contien-
nent (4 à 5 µg/cm2 par jour sur une longue période).
5. Concentrations dans l'environnement
On a trouvé de fortes concentrations de TBT dans
l'eau, les sédiments et les biotes à proximité de zones de
plaisance, plus particulièrement de marinas, de chantiers
navals et de bassins de radoub, de filets et de cages
traités au moyen de peintures antisalissures et de sys-
tèmes de réfrigération. Ces concentrations de TBT dépen-
dent également de la submersion par la marée et de la
turbidité de l'eau.
On a observé que les concentrations de TBT pouvaient
atteindre 1,58 µg/litre dans l'eau de mer et les estuai-
res, 7,1 µg/litre dans l'eau douce, 26 300 µg/kg dans
les sédiments littoraux, 3700 µg/kg dans les sédiments
d'eau douce, 6,39 mg/kg dans les bivalves, 1,92 mg/kg dans
les gastéropodes et 11 mg/kg dans le poisson. Il ne faut
pas considérer cependant ces concentrations maximales
comme caractéristiques car un certain nombre de facteurs
peuvent donner lieu à des teneurs anormalement élevées
(par exemple la présence de particules de peinture dans
les échantillons d'eau et de sédiments). On a constaté que
les concentrations de TBT dans la micro-couche de surface
des eaux douces et des eaux de mer étaient jusqu'à 100
fois plus élevées que celles qu'on pouvait mesurer juste
en dessous de la surface. Toutefois, il convient de noter
que la concentration en TBT dans la micro-couche de
surface peut dépendre dans une très large mesure de la
technique d'échantillonnage.
Il se peut que les données anciennes ne soient pas
comparables aux données récentes en raison des amélio-
rations apportées aux méthodes de dosage du TBT dans
l'eau, les sédiments et les tissus.
6. Transport et transformation dans l'environnement
Du fait de sa faible solubilité dans l'eau et de son
caractère lipophile, le TBT s'adsorbe facilement aux par-
ticules. On estime que 10 à 95% de l'oxyde de tributylé-
tain qui pénètrent dans l'eau s'adsorbent ainsi sur les
particules. La disparition progressive du TBT absorbé
n'est pas due à sa désorption mais à sa dégradation. Le
degré d'adsorption dépend de la salinité, de la nature et
de la taille des particules en suspension, de la quantité
de matières en suspension, de la température et de la
présence de matières organiques dissoutes.
La dégradation de l'oxyde de tributylétain s'effectue
par rupture de la liaison carbone-étain. Celle-ci peut
résulter de divers mécanismes qui se produisent simultané-
ment dans l'environnement et notamment des mécanismes
physico-chimiques (hydrolyse et photodécomposition) ou
biologiques (dégradation par des micro-organismes et
métabolisation par des organismes supérieurs). L'hydrolyse
des dérivés organostanniques se produit à des valeurs
extrêmes du pH mais n'apparaît guère dans les conditions
qui règnent normalement dans l'environnement. La photo-
décomposition se produit par exposition en laboratoire de
solutions à un rayonnement ultra-violet de 300 nm (et à un
moindre degré, à un rayonnement de 350 nm). Dans le milieu
naturel, la photolyse est limitée par la longueur d'onde
du rayonnement solaire et par la pénétration du rayonne-
ment ultra-violet dans l'eau. La présence de substances
photosensibilisatrices peut accélérer la photodécomposi-
tion. La biodégradation dépend de l'état du milieu, et de
caractéristiques telles que sa température, son oxygéna-
tion, son pH, sa teneur en éléments minéraux, la présence
de substances organiques facilement biodégradables pouvant
subir une co-métabolisation ainsi que la nature de la
micro-flore et sa capacité à s'adapter. Elle ne peut
également avoir lieu que si la concentration en oxyde de
tributylétain est inférieure à la concentration létale ou
inhibitrice pour les bactéries. Comme dans le cas de la
décomposition abiotique, la dégradation biologique du TBT
comporte une débutylation oxydante progressive avec rup-
ture de la liaison carbone-étain. Il se forme des dérivés
dibutylés dont la dégradation est plus facile que celle du
tributylétain. Les monobutylétains sont lentement minéra-
lisés. Il se produit également une dégradation anaérobie
mais son importance reste discutée. Certains chercheurs
estiment que la dégradation en anaérobiose est lente alors
que d'autres la jugent plus rapide que la dégradation
aérobie. On a identifié des espèces de bactéries, d'algues
et de champignons attaquant le bois qui sont capables de
dégrader l'oxyde de tributylétain. Les estimations de la
demi-vie du TBT dans l'environnement varient dans
d'importantes proportions.
Le TBT s'accumule dans les organismes du fait de sa
solubilité dans les graisses. Des recherches en labora-
toire portant sur des mollusques et des poissons ont
donné, pour les facteurs de bioconcentration, des valeurs
allant jusqu'à 7000 mais des valeurs encore plus élevées
ont été observées lors d'études sur le terrain. L'absorp-
tion à partir de la nourriture est plus importante qu'à
partir de l'eau. Les facteurs de concentration plus élevés
observés chez les micro-organismes (entre 100 et 30 000)
peuvent s'expliquer par une adsorption plutôt que par une
absorption intracellulaire. Rien n'indique que le TBT
puisse passer dans les organismes terrestres par l'inter-
médiaire de la chaîne alimentaire.
7. Cinétique et métabolisme
Le tributylétain est absorbé au niveau intestinal (20
à 50% selon le véhicule) ainsi que par voie percutanée
chez les mammifères (dans la proportion d'environ 10%).
Il peut traverser la barrière hémo-méningée et passer du
placenta dans le foetus. Une fois absorbé, il est rapide-
ment et largement diffusé dans l'organisme (principalement
au niveau du foie et des reins).
Chez les mammifères, la métabolisation du TBT est
rapide; on peut déceler les métabolites dans le sang dans
les 3 heures suivant l'administration. Des études in vitro
ont montré que le TBT servait de substrat aux oxydases à
fonction mixte mais qu'il inhibait ces enzymes à très
forte concentration.
L'élimination du TBT s'effectue plus ou moins rapide-
ment selon la nature du tissu et les estimations de la
demi-vie biologique chez les mammifères varient de 23 à
environ 30 jours.
Les organismes inférieurs métabolisent également le
TBT mais le processus est plus lent - en particulier chez
les mollusques - que chez les mammifères. La capacité de
bioaccumulation est donc beaucoup plus importante que chez
les mammifères.
Les tributylétains inhibent la phosphorylation oxy-
dative et modifient la structure et la fonction des mito-
chondries. Le TBT empêche la calcification de la coquille
des huîtres (espèces du genre Crassostrea ).
8. Effets sur les micro-organismes
Le TBT est toxique pour les micro-organismes et on le
vend comme bactéricide et alguicide. Les concentrations
toxiques varient considérablement selon les espèces. Le
TBT est plus toxique pour les bactéries gram-positives
avec une concentration minimale inhibitrice (CMI) allant
de 0,2 à 0,8 mg/litre que pour les bactéries gram-néga-
tives (CMI des 3 mg/litre). La CMI de l'acétate de TBT
pour les champignons est de 0,5 à 1 mg/litre et celle de
l'oxyde de tributylétain est de 0,5 mg/litre pour l'algue
verte Chlorella pyrenoidosa. La productivité primaire
d'une communauté naturelle d'algues d'eau douce a été
réduite de 5% par une concentration d'oxyde de tributylé-
tain de 3 µg/litre. On a récemment établi la dose sans
effet observable pour 2 espèces d'algues; elle est
respectivement de 18 et 32 µg/litre. La toxicité pour
les microorganismes marins varie également selon les
espèces et selon les études; il est difficile d'établir
la valeur de la dose sans effet observable mais on pense
qu'elle est inférieure à 0,1 µg/litre pour certaines
espèces. Les concentrations alguicides vont de moins de
1,5 µg/litre à plus de 1000 µg/litre selon les espèces.
9. Effets sur les organismes aquatiques
9.1 Effets sur les organismes marins et estuariels
La Figure 1 donne un diagramme récapitulatif des
effets létaux et sublétaux que peuvent produire les con-
centrations de TBT relevées en mer et dans les estuaires.
Des concentrations supérieures à celles qui produisent les
effets létaux aigus ont été observées en différents points
du globe, notamment là où se déroulent des activités de
plaisance.
Ce sont les spores mobiles d'une algue verte géante
qui se sont révélés les plus sensibles au TBT (CE50 à 5
jours: 0,001 µg/litre). On a constaté une réduction de
la croissance d'un angiosperme marin à des concentrations
de TBT de 1 mg/kg de sédiments, aucun effet n'étant noté à
0,1 mg/kg.
10.3. Effects on farmed fish
Wooten et al. (1986) reported the effects of exposure
to TBT antifouling paints applied to retaining nets for
farmed salmon. Fish exposed to newly-treated cage nets
were reported to be blind. The authors examined diseased
post-smolt salmon from cages treated with TBT at the time
of smolt transfer. Elevated tin levels were found in all
salmon exposed to the TBT paint; liver residues ranged
between 1.01 and 1.62 mg tin/kg wet weight. Residues in
the liver of blind fish ranged between 1.01 and 1.07 mg
tin/kg wet weight. Blindness had been caused by rupturing
of the eye; the eye lens was missing. Eye tissues appeared
normal histologically apart from the rupture; there were
no histological lesions. The kidney, liver, stomach, heart
and muscle of the blind fish appeared normal histologi-
cally. The intestine showed mucosal sloughing, vasodi-
lation, and pyknotic nuclei. The spleen had an "open
structure" with increased numbers of erythrocytes. There
was thickening of secondary gill filaments and some
necrosis and capillary separation in the epithelium. Other
fish in the affected population were reported to have
swellings along the lateral line. These were shown histo-
logically to be thick folds of epidermis under which there
was cellular infiltration of necrotic collagen forming an
abscess. Pancreas disease was also reported in affected
fish. In the liver there was a breakdown of lobular struc-
ture with lack of cohesion between cells. The effects were
thought to be consistent with poisoning by organotin.
10.4. Effects of TBT-contaminated sediment
Matthiessen & Thain (1989) studied the recolonization
by marine organisms in the field of sediment contaminated
with TBT-containing paint. Sediment was collected from
mudflats, and macroorganisms present were killed by
repeated freezing and thawing. TBT was added to the sedi-
ment in the form of abraded paint. The paint was abraded
with scourers used on yachts to produce material of the
kind likely to contaminate sediments normally. Sediment
samples containing 0.1, 1, 10, and 100 mg TBT/kg were
prepared. The contaminated sediment was returned to the
mudflats and placed in excavated trenches (3 m x 30 cm
wide x 20 cm deep) lined with polyethylene mesh (5-mm
apertures). Recolonization of the sediment could, there-
fore, take place both by settlement of organisms from
above and by lateral transfer of organisms from adjoining
mud. The sites were revisited five times during the next
160 days when samples were taken both to count recolon-
izing organisms and to measure TBT levels at various
depths. Surface sediment decontaminated rapidly; water
movement would have removed sediment and deposited new.
The subsurface TBT levels remained reasonably stable
throughout the experiment. Burrowing activity (estimated
by counting casts on the surface) of the polychaete
Arenicola marina was reduced at all concentrations of
TBT, though the effect of 0.1 mg TBT/kg disappeared after
3 months. A dose-related reduction in populations of the
burrowing polychaete Scoloplos armiger and burrowing
amphipod Urothoe poseidonis was observed over the whole
concentration range. There were no clear effects on other
species, including molluscs. The authors pointed out that
some unaffected groups were associated with the surface
sediment, where TBT was lost rapidly. Feeding behaviour
of these surface dwellers, such as the cockle (Cardium
edule), would lead to little TBT exposure, since they fil-
ter overlying sea water and ingest little or no sediment.
Species associated with deeper layers of sediment and
feeding on fine particles, such as Urothoe poseidonis,
showed greater effects of TBT, resulting, presumably,
from greater exposure rather than greater inherent sensi-
tivity. The authors also noted the wide range of sensi-
tivity of different species in laboratory tests without
sediment. The bioavailability of TBT associated with sedi-
ment would probably be different from that dissolved in
water.
10.5. Effects of freshwater molluscicides
Following earlier laboratory trials on the use of TBT
as a molluscicide for schistosomiasis control (Deschiens &
Floch, 1962), Deschiens et al. (1966) applied TBTO to a
pond used for fish culture, in Cameroon, of approximately
1 metre in depth and with an area of 50 m2 (approximately
50 tonnes of water) and a water temperature of 23 °C.
Caged pond snails (Biomphalaria [Australorbis] glabratus
and Bulinus contortus), 10 to a cage supplied with food,
were placed in the pond in different areas and at differ-
ent depths. Renewal of cages was performed to study the
persistence of the compound. The pond was treated with
TBTO, as "Biomet" (50% TBTO and 50% toxicologically
inert dispersant), at rates giving TBTO concentrations in
water of 0.015, 0.03, or 0.045 mg/litre, and the side
effects on fish and plankton were investigated. At 0.045
mg/litre, TBTO killed 100% of the snails and 70% of the
fish within 24 to 48 h. At 0.03 mg/litre, TBTO killed 100%
of snails within 2 to 3 days, but no effect on the fish
was observed within 15 days. At 0.015 mg/litre, the TBTO
killed all of the snails within 4 to 7 days and again had
no observable effect on the fish. The authors considered
this to be a practical concentration to achieve full
effect on snails without killing non-target organisms.
Various planktonic organisms (desmids, daphnids, and
aquatic insect larvae) were killed by TBTO at 0.5 mg/litre
experimentally. However, no effect was found on any of
these species in the pond treated at 0.015 mg/litre,
although there was some inhibition (but no deaths) of
these organisms at 0.03 and 0.045 mg/litre.
Gilbert et al. (1973) applied slow-release TBTO in
underseal (quick-drying asphalt-rubber-asbestos-clay paste)
to four sites, in Brazil, at concentrations of 15, 15, 50,
or 300 g TBTO/m2, to control Biomphalaria tenagophila
and B. straminea. Dead fish were observed at the sites
treated with 50 or 300 g/m2 and also at one of the two
sites treated with 15 g/m2, a static man-made pit. At
the other site, with a flow rate of 100 to 200 litre/min,
there was a 100% reduction in the snail population (B.
tenagophila) maintained for up to 12 months, while both
fish and aquatic insect life were apparently normal one
month after treatment. At this site abundant natural veg-
etation flourished in the immediate vicinity of the mol-
luscicide. Toledo et al. (1976) found that 15 g TBTO/m2
eliminated snails from 76% of treated sites for 1 year.
Guppies, which are often present in highly polluted sites,
normally suffered some mortality at the beginning of mol-
luscicidal treatment but in general they tolerated it
well. The authors found no lasting effect on plant or
insect life, and concluded that, because of the ability of
an unidentified fungus to grow profusely, microbiological
life continued in treated areas.
Shiff et al. (1975) applied BioMet SRM pellets (con-
taining TBTO) at concentrations of 20 and 30 g/m2 to a
night-storage dam in Zimbabwe. At the lower application
rate, pellets remained active against the snails (various
Biomphalaria spp.) for up to one year. At 30 g/m2, 100%
mortality of snails was achieved within 2 weeks of appli-
cation and was maintained up until the last sampling time
2 months after application. Prior to treatment, 80
specimens of Tilapia fish sp. and 1 of Clarias gariepinus
were caught. Four weeks after treatment 26 Tilapia were
caught and one dead Clarias was found. Eight weeks after
treatment 400 Tilapia were caught, of which 80 were
examined and all appeared normal.
Ayala et al. (1980) applied slow-release molluscicide
(rubber impregnated with 11% TBTO) at a rate of between 5
and 15 g/m2 to a site in Brazil and studied the effect on
non-target organisms. The TBTO molluscicide was initially
herbicidal towards floating vegetation but did not affect
either plants rooted in the mud or marginal plants. The
chemical was initially repellent to aquatic animals, some
of which were killed by the molluscicide. Within 3 months,
populations of non-target snails such as Pomacea sp.,
Drepanotrema sp., and a Physa sp. had returned to normal.
Although no fish had returned within 3 months, after 5
months numbers had returned to normal. In fact, 5 months
after application all but Spirogyra sp. were normal, and
the molluscicidal activity of the TBTO was retained for
more than one year. The authors concluded that after 3 to
5 months the action of the TBTO molluscicide is confined
to the bottom mud where Biomphalaria glabrata snails spend
much of their time.
10.6. Effects from spills
According to Waldock et al. (1987a), major inputs of
TBT into the environment may have arisen from spills of
timber-treatment products, often containing dieldrin and
pentachlorophenol as well as the organotin. They reported
that a detailed study was carried out after a spill con-
taminated a section of the Newmill Channel, Kent, United
Kingdom. There was a major kill of fish and all macroin-
vertebrates (except oligochaetes, chironomid larvae, and
elmid beetles) were also killed. Concentrations of TBT in
the water shortly after the spill were 540 µg per litre,
falling to 10 µg/litre after 3 weeks and 0.75 µg per
litre after 6 weeks. Nine months after the incident,
macroinvertebrate populations were reported to have
recovered. The authors stated that five such major spills
from timber treatment plants had been reported in the
United Kingdom within 2 years.
10.7. The use of indicator species for monitoring the environment
Both shell growth and shell chambering in Pacific
oysters and imposex in dogwhelks have been used as bio-
logical indicators of TBT contamination. Smith et al.
(1987) transferred juvenile Pacific oysters to major bays
and harbours of California, USA, known to contain elevated
levels of TBT. They observed a graded increase of stunted
growth and/or shell deformation, which was associated with
poor flushing and the proximity of large numbers of boats.
Gibbs et al. (1987) used the dogwhelk to monitor water
around the south-west peninsula of England. They found the
species to be a very sensitive indicator, especially if
the individuals were about 1 year old and in the early
stages of ovarian development. Davies et al. (1987a) found
imposex in dogwhelks to be a far more sensitive indicator
than either shell thickening in oysters or tin accumu-
lation. The dogwhelk has been used to identify areas of
contamination associated with seasonal small boat activity
and salmon farm cages in Scottish sea lochs (Davies et
al., 1987b), in areas of pleasure craft activity, fishing
harbours and a boat yard in the Firth of Forth (Scotland)
(Bailey & Davies, 1988b), and to investigate contamination
from an oil terminal in Sullom Voe, Shetland (Bailey &
Davies, 1988a).
11. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
11.1. Single exposure
Summary
The acute toxic effects of the various TBT compounds that
have been studied are comparable and are characteristically
delayed for several days. Oral LD 50 values in laboratory ani-
mals range from approximately 40 to 250 mg/kg body weight.
These compounds exhibit greater lethal potential when adminis-
tered parenterally, relative to the oral route, probably
because they are only partially absorbed from the gut. Acute
toxicity via the dermal route is low.
TBT compounds are potent skin irritants and extreme eye
irritants. Dermal exposure to TBTO appears to have little
sensitization potential.
Aerosols of TBT compounds are highly toxic. However, TBT
vapour/air mixtures at room temperature produce no effect, even
at saturation.
Other effects of acute exposure may include alterations in
blood lipid levels, the endocrine system, liver, and spleen,
and transient deficits in brain development. The toxicological
significance of these effects, reported after high single doses
of the compounds, is questionable and the cause of death
remains unknown.
11.1.1. Oral and parenteral administration
The acute toxicity of tributyltin to laboratory mam-
mals by various routes of administration is summarized in
Table 14. The acute oral LD50 for the rat ranges between
94 and 234 mg/kg body weight and for the mouse between 44
and 230 mg/kg body weight. Truhaut et al. (1976) pointed
to the delayed toxicity of tributyltin and, therefore, the
necessity to continue the observation of animals, after a
single acute dose, for several days. The period of post-
dosing observation is therefore indicated in Table 14.
LD50 values for ip and iv administrations of TBT are
very much lower than in the case of the oral route (10
mg/kg for rat ip and 6 mg/kg for mouse iv).
Table 14. Acute toxicity of tributyltin to laboratory mammals
------------------------------------------------------------------------------
Species TBT Route Observation LD50 (mg/kg Reference
perioda body weight)
(days)
------------------------------------------------------------------------------
Rat oxide oral 7 194 (165-227)b Elsea & Paynter (1958)
oxide oral 7 148 (113-195)c Elsea & Paynter (1958)
oxide oral 7 180 (132-228) Truhaut et al. (1976)
oxide oral 21 197 (137-273) Funahashi et al. (1980)
oxide oral 127 Schweinfurth (1985)
oxide ip 14 20 (18-21) Poitou et al. (1978)
oxide oral 234 Sheldon (1975)
fluoride oral 200 Sheldon (1975)
fluoride oral 14 94 Schweinfurth (1985)
chloride oral 14 122 Schweinfurth (1985)
acetate oral 113.5 Klimmer (1969)
benzoate oral 141 Klimmer (1969)
benzoate oral 14 99/203 Schweinfurth (1985)
oleate oral 225 Klimmer (1969)
linoleate oral 14 190 Schweinfurth (1985)
abietate oral 14 158 Schweinfurth (1985)
naphthenate oral 14 224 Schweinfurth (1985)
Mouse oxide oral 7 85 (52-130) Polster & Halacka (1971)
acetate oral 7 46 (25-85) Pelikan & Cerny (1968)
oleate oral 7 230 (175-301) Pelikan & Cerny (1968)
benzoate oral 7 108 (74-156) Pelikan & Cerny (1968)
chloride oral 7 117 (80-170) Pelikan & Cerny (1968)
laurate oral 7 180 (136-237) Pelikan & Cerny (1968)
oxide sc 7 200 (140-270) Polster & Halacka (1971)
oxide iv 7 6 (5.5-6.5) Truhaut et al. (1976)
oxide ip 14 16 (15-17) Poitou et al. (1978)
Rabbit fluoride dermal 680 Sheldon (1975)
------------------------------------------------------------------------------
a Following a single application of TBT, the observation period was as indicated.
b Application as aqueous suspension.
c Application in corn oil.
Pelikan & Cerny (1968) administered TBT (as the acet-
ate, benzoate, chloride, laurate, or oleate) to white mice
(body weight 25 g) in a single oral gavage dose of 500
mg/kg body weight, dissolved in sunflower oil. Ten animals
were used for each TBT compound. The mice were observed
for 8 h before being killed, and the viscera were examined
histologically. Four hours after treatment, all animals
showed signs of intoxication with the exception of those
given the laurate; after 8 h all showed toxic symptoms.
Gross damage was seen in the digestive tract, liver, and
spleen. Histological findings included a steatosis of the
liver cells in all animals (but to varying degrees),
traces of lipid in renal tubule cells (in those animals
receiving oleate or laurate) and many haemorrhages in the
digestive tract and kidneys. Effects on the liver and
spleen were also noted after dermal absorption of TBTO
(Pelikan & Cerny, 1969). However, no real conclusions can
be drawn from these studies as there was no clear reported
evidence that steatosis was caused either by the TBT or by
the fatty acids.
In studies by Funahashi et al. (1980), Sprague-Dawley
rats were given a single dose, in olive oil, of 100 mg
TBTO/kg body weight by intubation and were examined over
the following 21 days. Adrenal weight was slightly
increased 12 h after treatment and reached a maximum on
the second day. Histological changes in the adrenal had
returned to normal by day 14. The thyroid follicles showed
signs similar to those produced by hypophysectomy, i.e.
distension with colloid, flat epithelial cells. These
changes were severe after 72 h, but had returned to normal
within 14 days. Absolute pituitary weight was increased
slightly (and not significantly) 1 and 2 days after
dosing, while relative weight increased significantly.
There was atrophy of pituitary adrenocorticotrophin (ACTH)
cells 6 h after dosing. After 72 h, the staining of ACTH
cells became more intense. There were marked reductions
in the circulating levels of both thyroid stimulating
hormone (TSH) and thyroxine (T4) during the 72 h follow-
ing dosing, serum titres falling to one half and one sixth
of control values for the two hormones, respectively. The
authors concluded that TBTO had both a direct and an
indirect effect on the thyroid, since initially there was
a simultaneous rise in T4 and fall in TSH. Serum cortisol
levels increased to twice the control level 96 h after
treatment with TBTO at a level of 100 mg/kg body weight.
Intramuscular ACTH administration, 8 h after dosing, led
to increased cortisol in the blood of both treated and
control rats. After 16 h, controls showed release of
cortisol after ACTH stimulation, but treated rats showed a
decrease in circulating levels of cortisol after similar
stimulation.
Matsui et al. (1982) reported the effects of a single
dose of TBT fluoride (100 mg/kg body weight) given by
gastric intubation to Japanese white rabbits. A reversible
but pronounced hyperlipidaemia was observed, particularly
involving triglycerides and total cholesterol. Ultracen-
trifugation showed a marked increase in the chylomicron
plus VLDL (very low density lipoprotein) fraction. The
activity of lipoprotein lipase (LPL) in plasma was reduced
to about 50% of control levels. Fasting levels of blood
glucose were elevated, and the response to iv infusion of
glucose (given 3 days after the TBT fluoride) was
inhibited (insulin release reduced). The authors suggested
that the hyperlipidaemia was the result of reduced LPL
activity, which in turn was brought about by inhibition of
insulin release.
Calley et al. (1967) investigated the effects of TBT
acetate on liver function in rabbits. Following a single
oral dose of TBT acetate at 50 mg/kg body weight (half of
the measured LD50 in rabbits), only the serum glutamic-
pyruvate transaminase (SGPT) activity was affected. This
activity was not elevated after 48 h but was increased
within 144 h of dosing. Prothrombin time, alkaline phos-
phatase, and thymol turbidity showed no significant
changes from control values. SGPT is a highly specific
indicator of liver damage, directly reflecting liver cell
injury. Tributyltin acetate also significantly increased
the hexabarbitol-induced sleeping times of mice; a 50
mg/kg body weight oral dose increased the sleeping time
from 29 to 43 min following a standard dose of the
narcotic.
When Aldridge et al. (1977) administered TBTO (and its
gamma-keto, gamma-hydroxy, and delta-hydroxy metabolites)
intraperitoneally to mice on two consecutive days (at dose
levels of 12.5, 25, and 50 µmol/kg body weight), they
found no evidence of cerebral oedema after any of the
treatments.
Crofton et al. (1989) gave rat pups a single dose of
TBTO on day 5 following birth by gastric intubation with
0, 40, 50, or 60 mg/kg body weight. The monitoring of
behavioural parameters up to 62 days post-dosing showed no
persistent effect on motor activity or acoustic startle
response.
When Barnes & Stoner (1958) fed rats with tributyltin
acetate during the first 3 months after birth, they found
increased brain and spinal cord water content at the
highest dose tested (100 mg/kg diet). This increase was
not sufficiently great to be seen in histological sec-
tions. Bouldin et al. (1981) found no light or electron
microscopic evidence of neuronal damage in the hippocampus
or the pyriform cortex of neonate or adult rats exposed
daily to tributyltin acetate at 10 mg/kg body weight by
gavage for up to 30 days.
O'Callaghan & Miller (1988) reported the effects of a
single ip injection of TBTO on neonatal rat brain. The
rats were injected when 5 days old with 2, 3, or 4 mg/kg.
They were sacrificed at 13, 22, or 60 days of age, and
various proteins in homogenates of brain tissue were
measured by radioimmunoassay. Brain weight was reduced in
a dose-dependent manner, the cerebellum being most
affected. There was no evidence of altered brain histology
under the light microscope. Dose-dependent and region-
dependent decreases were found in P-38 (a synaptic-vesi-
cle-associated protein) and myelin basic protein (a
protein associated with oligodendroganglia and the myelin
sheath); there were decreases in both total (per tissue)
and concentration (per mg) levels of these proteins in the
cerebellum and forebrain but not the hippocampus. These
effects were seen at a dose level that did not affect body
weight. However, in contrast to the neurotoxic effects of
trimethyltin compounds, which are irreversible and persist
into adulthood, these neurotoxic effects of TBTO were
transitory at dose levels that did not affect body weight.
Robinson (1969) monitored tissue levels of catechol-
amines after ip administration of TBTO, in corn oil, to
rats at a level of 10 mg/kg body weight. This dose
exceeded the 6-day LD50, determined by the same authors
to be 7.21 mg/kg. Brain noradrenalin levels were signifi-
cantly reduced at 2, 24, and 48 h after dosing, whereas
5-hydroxytryptamine levels were only reduced (though
markedly) after 48 h. Noradrenalin levels in heart tissue
were also significantly reduced after 2, 24, and 48 h.
Adrenal adrenalin and noradrenalin levels were reduced
after 24 and 48 h, but only adrenalin was affected after
2 h.
11.1.2. Dermal administration
The acute LD50 of TBTO administered to rabbits via
the dermal route is very high, i.e. approximately 9000
mg/kg body weight (Table 14). Although there is dermal
absorption of TBT (see later), the degree of absorption is
not great enough to lead to acute toxic effects sys-
temically, except at very high exposure levels.
11.1.3. Administration by inhalation
In a "nose only" inhalation exposure (to minimize
dermal exposure and exposure through ingestion) lasting
4 h, the acute LC50 of TBTO for the rat was estimated to
be 77 mg/m3 by measurement of airborne droplets. This
value decreased to 65 mg/m3 when "inhalable" particles,
10 µm or less in diameter, were considered. There was
evidence of lung irritation and oedema in this study
(Schweinfurth, 1985). When groups of 10 male and 10 female
rats were each exposed to atmospheres containing almost
saturated vapours of TBTO, TBT benzoate, or TBT naphthen-
ate once for 7 h, no deaths occurred during exposure or in
a 14-day observation period following exposure. Only minor
clinical signs were noted occasionally during the exper-
iment, such as slight nasal discharge (Schweinfurth, 1985).
Truhaut et al. (1979) exposed mice to an aerosol of
TBTO in olive oil, for either a single 1-h period or seven
1-h periods on successive days, using TBTO concentrations
in air ranging between 0.05 and 0.4 mg/litre (50 to
400 mg/m3). Exploratory behaviour was scored over 5-min
periods 2 h after the single exposure was complete or 24 h
after the last of the seven exposure periods. The lower
two exposure doses caused significant increases in explo-
ratory behaviour (17% and 5% for 42 and 84 mg/kg, respect-
ively) while the higher exposure doses reduced exploratory
behaviour (-18% and -38% for 170 and 340 mg/kg, respect-
ively). Truhaut et al. (1981) found a median survival time
for mice of 22 min and for guinea-pigs of 9 min after
exposure to an aerosol of tributyltin (concentration not
stated). They reported that only tissues in the respirat-
ory system showed significant lesions. There was diffuse
congestion of the pulmonary blood vessels extending to the
septal capillary beds. There were also inflammatory
responses in the trachea and bronchii, secretion of mucus
in the bronchii and bronchioles, and distension and rup-
ture of the alveoli.
Anger et al. (1976) exposed guinea-pigs to aerosols of
TBTO ranging between 0.1 and 1 mg/litre air for 1 h. All
males exposed to 0.2 mg/litre died, and 12 out of 15
females exposed to 1 mg/litre died. No particular lesions
were observed in those animals that died, with the excep-
tion of a general congestion of the lungs. Exposure of
either males or females to 0.17 mg/litre caused no deaths,
though nasal irritation was observed; all these animals
survived a further 7 days of observation.
11.1.4. Irritation and sensitization
11.1.4.1 Skin irritation
In a study by Elsea & Paynter (1958), undiluted TBTO
was applied to the closely-clipped skin of the abdominal
area of rabbits, which was then covered with rubber dental
damming, gauze, and adhesive tape. After an exposure
period of 24 h, the covering was removed, and the TBTO was
washed off, as far as possible, by sponging with warm
water. This single application of doses up to 11.7 g/kg
body weight caused some of the rabbits to die from the
effects of TBTO absorbed into the body. There was, how-
ever, only moderate dermal irritation, characterized by
reddening, oedema, atonia, blanched areas, and areas of
brown discolouration. Examination of the skin at autopsy
showed some subcutaneous oedema. Repeated dermal appli-
cation of TBTO, impregnated into paper at 8 mg/kg, daily
for 5 days, only affected one animal out of four. This
rabbit showed slight erythema and oedema following the
first and second applications. Later in the experiment,
the skin appeared normal. No detailed histological examin-
ation of the skin was carried out.
Pelikan & Cerny (1969) applied to the shaved skin of
rats two preparations of TBTO, i.e. "Lastanax T" (con-
taining 20% TBTO plus a medium of water, alcohols of short
chain length, and n -alkyl-polyethylene oxide) and
"Lastanax P" (containing 15% TBTO plus a medium also con-
taining bis-(5-chloro-2-hydroxyphenyl)-methane). Actual
doses applied, corresponding to water dilutions of 100%,
33%, 10%, and 1% of the preparations, were 185, 61.5,
18.5, and 1.85 mg TBTO/kg body weight for Lastanax T and
145, 47, 14, and 1.4 mg TBTO for Lastanax P. Controls
received either water or the medium without TBTO. The
experiment was carried out in duplicate. In the first
series, clinical observations were made twice daily for
60 days, whereas in the second series rats were killed on
day 10 and the skin was examined histologically. Control
rats showed no clinical or histological effects on the
skin. However, clinical and histological changes were
found in all treated rats, even with the 1% dilution,
though severity increased with exposure dose. On the first
and second days after application, there was reddening of
the skin and oedema developed. From day 3, haemorrhagic
eschars developed with well defined borders; there was no
inflammation of the surrounding areas of skin. Between
these foci, numerous papules and pustules developed, some
with bleeding; the apices of some papules were necrosed.
Later the eschars joined to create larger areas of
ulceration. Healing of the areas began between the 9th
and 12th days; necroses disappeared by day 20, pustules 3
to 4 days before, and papules by the 30th to 33rd day. All
signs had disappeared after 35-38 days (exposure to 1% and
10% solutions) or after 45-50 days (exposure to 33% or
100% solutions). Changes with Lastanax P were similar, but
less severe, than with Lastanax T. In the histological
study, numerous large bullae, filled with leucocytes and
coagulated exudate (proteins), were found under the
stratum corneum of the epidermis after 10 days. Akanthosis
and vacuolation of epidermal cells occurred following
exposure to the 10% and 33% solutions and, to a lesser
extent, the 1% solution. Small haemorrhages were found.
Epidermal changes were dispersed and alternated with areas
of skin appearing normal. The authors suggested that the
TBTO NOEL for human skin should be set at 0.005% to 0.01%.
In a study by Middleton & Pratt (1977), TBT chloride
was applied to a shaved area of dorsal skin of male
Alderley Park (Wistar-derived) rats, four-weeks old (body
weight 50-80 g), in absolute ethanol as a solution of
10 mmol/litre. This dose was equivalent to 67 nmol/cm2.
The TBT had produced microscopic changes in the skin
within 2 h of application. Polymorphonuclear leucocytes
accumulated in capillaries and in dermal tissues. Epider-
mal cells showed progressive degenerative changes between
2 and 8 h after application until, by 8 h after appli-
cation, separation of the epidermis and dermis had
occurred and fluid collected in this separation. Many
inflammatory cells were present in both dermis and epider-
mis. There was widespread epidermal necrosis within 12 to
24 h, and separation of necrotic epidermis from the dermis
was almost complete by this time. The vesicles formed by
this separation were frequently packed with inflammatory
cells as well as exudative fluid. Regeneration of the
epidermal layer was observed after 18 to 24 h and dermal
inflammatory infiltration was regressing by this time.
Erythema, visually assessed and scored, reached a maximum
within 5 h of application and remained at this level for
48 h. The erythema had subsided by 72 h after application.
Vascular permeability of the skin was assessed by
injecting rats with a dye, tryphan blue, 45 min before
sacrifice. There was a biphasic response. At 2 h after
application of TBT chloride, there was an initial increase
in permeability of the skin to 135% of control levels. A
second peak began at 12 h and persisted for more than
25 h after application. The effect was still evident after
48 h and had occurred in both the treated and untreated
flanks of the animal, indicating absorption of the com-
pound and a systemic effect. The water content of the skin
was increased by TBT chloride, reaching a peak within 2 h
of application; by 10 h this effect had disappeared.
Middleton & Pratt (1978) reported that TBT produced focal
epidermal necroses and dermal inflammation at levels as
low as 33 nmol/cm2, fairly extensive epidermal necroses
and dermal inflammation at 67 nmol/cm2, and almost total
epidermal necrosis at 167 nmol/cm2.
11.1.4.2 Eye irritation
Pelikan (1969) applied TBTO, as "Lastanax T or P",
in a single dose of 0.03 ml to the left eye of rabbits.
Lastanax T is 20% TBTO in alcohols of short chain length,
non-ionic surface active substances ( n -alkyl-polyethyl-
ene oxide) and water. Lastanax P is 15% TBTO in the same
vehicle with the addition of bis-(5-chloro-2-hydroxyphe-
nyl) methane. A 10% and a 1% solution in water were used.
The 10% solution represented an actual TBTO dose of 6.1
and 4.6 mg/kg body weight for the two formulations,
respectively. With the 10% solution, both rabbits died,
11 and 12 days after application. Severe ulceration of the
eye preceded death. Histopathological examination of
internal organs after death revealed hyperaemia of the
brain and medulla oblongata and hyperplasia of reticulo-
endothelial cells of the spleen. Necrotic changes were
seen on the cornea of eyes treated with the 1% solutions
within 3 h of application, and symptoms worsened over the
next 2 to 5 days. Recovery was incomplete 100 days later.
11.1.4.3 Skin sensitization
Poitou et al. (1978) investigated the skin-sensitizing
potential of TBTO in guinea-pigs using the Magnussen-
Kligman method. The concentrations used for sensitization
were 1% (intradermal phase) and 5% (topical phase). Using
challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals.
11.1.5. In vitro studies
Johnson & Knowles (1983) demonstrated that incubation
of rat blood platelets with TBT chloride or TBTO, in
vitro, inhibited their ability to take up 14C-labelled 5-
hydroxytryptamine (5-HT). The organotin also caused
release of 14C-labelled 5-HT, taken up before exposure,
along with endogenous 5-HT. Treating rats ip with TBT
chloride at 5.0 mg/kg body weight also led to reduced up-
take of 5-HT (37% inhibition) 30 min after treatment. The
level of 5-HT in platelets, however, was unaffected by the
in vivo treatment with TBT chloride. knowles & Johnson
(1986) reported that exposure of rat platelets to TBT
chloride inhibited aggregation induced with ADP or colla-
gen. The inhibition of ADP-induced aggregation was depen-
dent on the dose of TBT and on the exposure time prior to
ADP addition. Exposure of the platelets to 5 µmol TBT per
litre for 5 min or 10 µmol/litre for 0.1 min produced
inhibition. However, exposure to 1 µmol/litre for 5 min
was ineffective. With an incubation time of 1 min, TBTO
was effective in lengthening the time taken for aggre-
gation after collagen induction at 0.625 µmol/litre, but
not at 0.5 µmol/litre.
The trialkyltins, including tributyltin, have been
shown to inhibit oxidative phosphorylation in rat mito-
chondria (Aldridge & Street, 1964). TBT stimulated
adenosinetriphosphatase (ATPase) activity and caused
limited swelling of rat liver mitochondria. These last two
effects occurred at similar concentrations of TBT in the
medium. The authors postulated that the two effects were
linked; the ATPase activity increased over a concentration
range of TBT and was always mirrored by a decrease in the
mitochondrial swelling. This relationship persisted
despite a complex response to TBT over the concentration
range; at higher concentrations there was a sudden, much
smaller mitochondrial swelling and a concomitant rise in
ATPase activity. TBT also inhibited the hydrolysis of ATP
by rat brain microsomes, although the concentrations
required were much higher than those affecting phosphory-
lation. The authors postulated that a combination of tri-
alkyltin compounds with negatively charged lipids is
involved in their biological activity.
Elferink et al. (1986) treated polymorphonuclear
leucocytes (PMNs), obtained from the peritoneal cavity of
rabbits, with an unspecified tributyltin (1 µmol/litre).
Uptake of opsonized zymosan was used as a measure of
phagocytosis, and enzyme (lysozyme) release to the super-
natant during incubation was also monitored. There was
almost complete inhibition of both parameters at
10-6 mol TBT/litre but no effect at 10-7 mol/litre. In-
hibition of phagocytosis was exactly paralleled by inhi-
bition of enzyme release. At concentrations of between
10-6 and 10-5 mol/litre, tributyltin caused lysis of the
cells with release of LDH, suggesting damage to the plasma
membrane. Exocytosis, induced by FMLP in the presence of
"cytocolasin B", was also inhibited by the TBT at con-
centrations of 10-6 mol/litre or more. There was little
effect of the compound on ATP levels in PMNs, and the
authors suggested that interference with ATP production
was not the basis for the effect of the TBT. Activation of
PMNs is accompanied by an increase in plasma membrane
permeability to Ca2+; this was strongly inhibited by the
TBT. The authors proposed two alternative explanations.
Either the Ca2+ permeability change is directly affected
or an earlier step is inhibited which, in turn, affects
calcium permeability. The observation that the exocytosis
effect could be counteracted by the addition of sulfydryl
compounds led the authors to conclude that the earliest
stages of activation of the PMNs were affected. Arakawa &
Wada (1984) reported a suppression of the chemotactic
response of rabbit leucocytes (neutrophils) towards for-
myl-methionyl-leucyl-phenylalanine by tributyltin chloride
at concentration between 0.1 and 10 µmol/litre in vitro.
Reinhardt et al. (1982) used cell detachment and
cloning efficiency of baby hamster kidney cells (BHK-21
C13) to quantify the cytotoxicity of organotin compounds.
The two parameters are independent, but each covers a
range of cellular damage. Cell detachment indicates irre-
versible damage to the cytoskeleton over a 4-h period,
while cloning efficiency covers influence on growth of
single cells over a 6-day period and includes cell re-
attachment followed by clone formation. The IC50 (concen-
tration at which there was a 50% reduction in cloning
efficiency) for TBTO was 5 x 10-7 mol/litre (0.3 mg/litre
medium) and for TBT chloride was 1.4 x 10-6 mol per litre
(0.5 mg/litre). The CD50 (50% effect on cell detachment)
was less sensitive to TBT, i.e. 3 x 10-5 mol/litre for
TBTO (18 mg/litre) and 4.3 x 10-5 mol/litre for TBT
chloride (14 mg/litre).
When Snoeij et al. (1986a) incubated isolated rat
thymocytes with TBT chloride, there was membrane damage
and disintegration of the cells at concentrations higher
than 1 µmol/litre. Various parameters of cell function
were investigated. The TBT chloride increased the consump-
tion of glucose in a dose-related manner at concentrations
in the culture medium higher than 0.1 µmol/litre; pro-
duction of lactate was also increased in parallel. The ef-
fect peaked at 1 µmol TBT/litre and thereafter declined;
damage to cell integrity occurred at these concentrations.
Over the same effective range of concentrations (0.1 to
1 µmol/litre), TBT decreased ATP levels. The ATP dimin-
ished very rapidly, within 2.5 min, and remained low for
at least 3 h. The incorporation of radiolabelled nucleo-
tide precursors into DNA and RNA and of amino acids into
protein was also affected. Thymidine incorporation into
DNA was reduced, relative to TBT dose, to a minimum of 20%
of control incorporation at 1 µmol TBT/litre. Uridine
incorporation was similarly reduced, but less effectively.
Inhibition of amino acid incorporation was substantial at
0.25 µmol TBT chloride/litre and protein synthesis was
virtually totally inhibited at 1 µmol/litre. The median
inhibitory concentrations (IC50) for thymidine, uridine,
proline, and leucine were 0.32 ± 0.04, 0.95 ± 0.06, 0.38
± 0.04, and 0.36 ± 0.03 µmol/litre, respectively. TBT
chloride concentrations of 0.1 µmol/litre or more mark-
edly reduced the production of cyclic AMP by the
thymocytes under stimulation from prostaglandin E1. TBT
chloride, therefore, has marked effects on the energy
metabolism of isolated thymocytes at concentrations well
below those affecting membrane integrity, and, as a
result, the authors stated that TBT chloride is best
characterized as an energy poison.
Snoeij et al. (1988a) similarly isolated rat thymo-
cytes, but separated them into fractions based on size.
The same parameters of cell function were investigated in
each of three fractions. Fraction 1 consisted of small,
non-proliferating thymocytes. Fractions 2 and 3 showed
some overlap in size, but fraction 2 was enriched in cells
actively synthesizing macromolecules, while fraction 3 was
enriched in dividing cells and showed the greatest pro-
liferative capacity. Resting ATP levels were highest in
the bigger cells, steady state levels increasing with
fraction number. TBT chloride reduced ATP levels in each
subfraction proportionally to between 62 and 64% of con-
trol values. Thus, TBT reduces intracellular ATP concen-
trations irrespective of cell volume or number of mito-
chondria. As with unfractionated thymocytes, TBT chloride
at both 0.25 and 1.0 µmol/litre inhibited thymidine
incorporation (to around 40% of control values at
0.25 µmol/litre and between 21 and 37% of control values
at 1 µmol/litre) and leucine incorporation (to between 13
and 29% of controls at 0.25 µmol/litre and 1 to 3% of
controls at 1 µmol/litre) in all fractions. In the case
of uridine incorporation, although there was inhibition in
most cases, 0.25 µmol TBT chloride/litre caused a stimu-
lation to 184% of control levels in fraction 1, the non-
proliferating cells.
11.2. Short-term toxicity
Summary
TBT compounds have been studied most extensively in the rat
(all the data in this section refer to the rat unless otherwise
indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg body
weight), high mortality rates were observed when the exposure
time exceeded 4 weeks. No deaths were noted at 100 mg/kg diet
(10 mg/kg body weight) or after daily administration of 12 mg
per kg body weight by gavage. In rats dosed during early post-
natal life, 3 mg/kg body weight resulted in increased deaths.
The main symptoms at lethal doses were loss of appetite, weak-
ness, and emaciation.
Borderline effects on rat growth were observed at 50 mg/kg
diet (6 mg/kg body weight) and 6 mg/kg body weight (gavage
studies). Mice are less sensitive, effects being observed at
150 to 200 mg/kg diet (22 to 29 mg/kg body weight).
Structural effects on endocrine organs, mainly the pitu-
itary and thyroid, have been noted in both short- and long-term
studies. Changes in circulating hormone concentrations and
altered response to physiological stimuli (pituitary trophic
hormones) were observed in short-term tests, but after long-
term exposure most of these changes appeared to be absent. The
mechanism of action is not known.
Exposure to TBTO aerosol at 2.8 mg/m 3 produced high mor-
tality, respiratory distress, inflammatory reaction within the
respiratory tract and histopathological changes of lymphatic
organs. However, exposure to the highest attainable vapour
concentration (0.16 mg/m 3 ) at room temperature produced no
effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular necrosis
and inflammatory changes in the bile duct were observed in rats
fed TBTO at a dietary level of 320 mg/kg (approximately 25
mg/kg body weight) for 4 weeks and in mice fed 80 mg/kg diet
(approximately 12 mg/kg body weight) for 90 days. Vacuolization
of periportal hepatocytes was noted in dogs fed a dose of 10
mg/kg body weight for 8 to 9 weeks. These changes were oc-
casionally accompanied by increased liver weight and increased
serum activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte vol-
ume in rats, resulting from dosing with 80 mg/kg diet (8 mg/kg
body weight), indicate an effect on haemoglobin synthesis,
leading to microcytic hypochromic anaemia. The decrease in
splenic haemosiderin levels suggests alterations in iron
status. Anaemia has also been observed in mice.
The formation of erythrocyte rosettes in mesenteric lymph
nodes has been observed in certain short-term investigations
but not in long-term studies. The biological significance of
this finding (possibly transient) is unclear.
The characteristic toxic effect of TBTO is on the immune
system; due to effects on the thymus, the cell-mediated func-
tion is impaired. The mechanism of action is unknown, but may
involve the metabolic conversion to dibutyltin compounds. Non-
specific resistance is also affected.
General effects on the immune system (e.g., on the weight
and morphology of lymphoid tissues, peripheral lymphocyte
counts, and total serum immunoglobulin concentrations) have
been reported in several different studies with TBTO using rats
and dogs, but not mice, at overtly toxic dose levels (effects
in mice have been seen with tributyltin chloride at 150 mg/kg).
Only the rat exhibits general effects on the immune system
without other overt signs of toxicity and is clearly the most
sensitive species. The NOEL in short-term rat studies was
5 mg/kg diet (0.6 mg/kg body weight). In studies with tributyl-
tin chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been shown
to compromise specific immune function in rat in vivo host re-
sistance studies. Decreased clearance of Listeria monocytogenes
was seen after exposure to a dietary level of 50 mg/kg (the
NOEL being 5 mg/kg per day), and decreased resistance to
Trichinella spiralis was seen at 50 and 5 mg/kg diet, but not
at 0.5 mg/kg diet (2.5, 0.25, and 0.025 mg/kg per day body
weight, respectively). Similar effects were seen in aged ani-
mals, but these were less pronounced.
With present knowledge, the effects on host resistance are
probably of most relevance in assessing the potential hazard to
man, but there is insufficient experience in these test systems
to fully assess their significance. However, some data on the
significance of the T. spiralis model are provided by findings
in athymic nude rats after the standard challenge. In these
studies, the complete absence of thymus-dependent immunity
resulted in a 10- to 20-fold increase in muscle larvae counts;
by contrast, exposure to TBTO concentrations of 5 and 50 mg/kg
diet resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on the
effects of tributyltin compounds on the developing immune sys-
tem, there is no information on host resistance.
It would be prudent to base assessment of the potential
hazard to humans on data from the most sensitive species.
Effects on host resistance to T. spiralis have been seen at
dietary levels as low as 5 mg/kg (equivalent to 0.25 mg/kg per
day body weight), the NOEL being 0.5 mg/kg (equivalent to
0.025 mg/kg per day). However, the interpretation of the sig-
nificance of these data for human risk assessment is contro-
versial. In all other studies a concentration of 5 mg/kg per
day in the diet (equivalent to 0.5 mg/kg body weight, based on
the short-term studies) was the NOEL with respect to general,
as well as specific, effects on the immune system.
11.2.1. Oral dosing: general body effects
Iwasaki et al. (1976) reported some oedema and
destruction of nerve axons following the administration of
0.01 ml TBTO/kg directly to the stomach of rats every day
for 28 days. No details of procedures or results were
presented in this report.
Schweinfurth (1985) found no evidence of brain oedema
after dosing rats with TBTO orally in arachis oil. Even
doses producing marked toxic effects on other organs (25
mg/kg body weight) failed to produce noticeable brain
oedema. However, triethyltin chloride produces brain
oedema at a dose of 1.5 mg/kg body weight.
Krajnc et al. (1984) investigated the short-term
effects of bis -tributyltin oxide in the rat. In exper-
iments lasting 4 weeks, Wistar rats were fed technical
TBTO at levels of 0, 5, 20, 80, or 320 mg/kg diet. All
animals survived the dosing period. Various symptoms of
poisoning were seen within 1 week of dosing in the group
fed 320 mg/kg diet, i.e. weakness, emaciation, roughened
fur, and blood-tinged discharge around the eyes and nose.
Some of these signs were seen at the lower doses after 4
weeks. Body weight gain was not affected by doses up to
20 mg/kg diet in males and 80 mg/kg in females. Males
showed reduced weight gain (96 g compared to control
weight gain of 117 g) over 4 weeks at 80 mg TBTO/kg. At
320 mg/kg diet, TBTO caused a reduction in body weight of
13 g in males and 21 g in females. Almost all of this
weight loss occurred in the first week of dosing, body
weight remaining constant after that. A 50% reduction in
food consumption was seen at this dose rate, compared to
control animals. Urine analysis, during the fourth week
of dosing, revealed no differences in urine volume, pro-
tein concentration, or creatinine clearance related to
treatment.
Funahashi et al. (1980) conducted histopathological
and biochemical studies on Sprague-Dawley rats given bis-
tributyltin oxide (TBTO) dissolved in olive oil. The rats
were dosed by intubation 5 times weekly for 13 or 26
weeks. Body weight was reduced after 13 weeks dosing at
6 mg/kg body weight, but not significantly so. After 26
weeks, body weight was significantly reduced relative to
controls (382 g compared to the control value of 439 g).
Dosing at 12 mg/kg reduced body weight significantly at
both 13 and 26 weeks (to 311 and 356 g, respectively).
There was a slight decrease, but unrelated to either dose
or time, in spleen weight. This decrease was just signifi-
cant at 6 mg/kg over 13 weeks and 12 mg/kg over 26 weeks,
but at no other time or dose.
When Mushak et al. (1982) dosed neonatal rats with TBT
acetate at levels of 1, 3, or 10 mg/kg per day from day 2
to day 29 of age, all rats given 1 mg/kg per day survived
with no apparent gross or histopathological effect. Totals
of 9 out of 24 and 17 out of 24 rats survived at dose
levels of 10 and 3 mg/kg per day, respectively, but all
showed reduced body weight. There were liver effects in
survivors.
11.2.2. Inhalation studies
A "nose only" inhalation study (lasting 4-5 weeks)
by Schweinfurth (1985) with rats exposed for 4 h/day (on
weekdays only; 21 to 24 exposure periods) to an aerosol of
TBTO (2.8 mg/m3) produced mortality (50% of males and
60% of females), apathy, and respiratory distress. Food
consumption and body weight gain were reduced. There were
inflammatory reactions within the respiratory tract and
lymphotoxic effects (depletion of lymphocytes in the
thymic cortex, atrophy of the thymus, and lymph nodes).
Inhalation of TBTO vapour/air mixtures produced no observ-
able effect. A concentration of 0.16 mg/m3 in the inha-
lation chamber, which corresponds to the equilibrium
vapour pressure of TBTO at room temperature, was con-
sidered to be the NOEL for rats.
When Gohlke et al. (1969) exposed rats to TBT chloride
in a 4-month inhalation study at nominal concentrations of
4 to 6 mg/m3, all animals survived the exposure. Towards
the end of the exposure, in the final month, the rats
showed minor irritation of the eye and nose. There was an
initial increase in relative liver weight but a signifi-
cant reduction over the whole experimental period. Fat
droplets were seen in the livers at autopsy, together with
a diffuse oedema of the brain. However, controls also
showed oedema. The oedema disappeared slowly as recovery
time after exposure increased. There were also inflamma-
tory changes in the respiratory tract of exposed animals.
Crofton et al. (1989) exposed pregnant female rats to
TBTO by intubation at 0 to 10 mg/kg per day on days 6 to
20 of gestation. There was no effect on the age at which
the testes of male offspring descended. However, females
showed a delay of approximately 2 days in vaginal opening,
compared to controls, after their mothers were exposed to
10 mg/kg per day.
11.2.3. Histopathological effects
Snoeij et al. (1985) reported that weanling rats fed
tributyltin chloride at a level of 150 mg/kg diet showed
marked reductions in body weight (treated rats, 87 g; con-
trol rats, 119 g) and brain weight (treated rats, 1.54 g;
control rats, 1.67 g) associated with a reduced food
intake of 25%. Thymus weight was reduced to 39% of the
control value over the 2-week feeding period.
Krajnc et al. (1984) found histopathological changes
in male and female Wistar rats exposed to 5, 20, 80, or
320 mg TBTO/kg diet for 4 weeks. No treatment-related
changes were found in the brain, heart, kidney, pancreas,
adrenals, popliteal lymph nodes, intestinal tract, or bone
marrow. Slight atrophy of hepatocytes (reduction in size)
in the centrilobular region was noted in some livers at
80 mg/kg and marked atrophy in 16 out of 20 livers at 320
mg/kg. The authors reported dystrophic calcification in
the liver at the highest dose level. Three animals showed
multiple-focus inflammation with necrosis of hepatic
parenchyma, which was associated with mononuclear and
polymorphonuclear infiltration, fibrosis, and hyperplasia
of the intrahepatic bile duct. In one animal there was
inflammation of the common bile duct. No bacteria or
viruses were found in the lesions. Similar necrotic
lesions were observed, in two animals, in a repeat study
at 320 mg TBTO/kg diet.
Mori et al. (1984) painted the shaved dorsal skin of
guinea-pigs daily for 50 days with an ethanolic solution
of TBTO at 10 or 40 mg/kg body weight. Monitoring of urine
and blood electrolyte levels during the course of the
treatment showed increased loss of sodium, chloride, phos-
phate, glucose, and amino acids in the urine. There was a
concomitant loss of electrolytes from serum. The effect
was most marked between 40 and 50 days of treatment. His-
tological lesions of the kidney tubules were observed when
the animals were killed after the 50th day.
11.2.4. Haematological and biochemical effects
Measurements of blood biochemical parameters of rats
fed TBTO for 4 weeks indicated few significant effects at
dietary dose levels below 320 mg/kg diet. Alanine amino
transferase (ALAT) activity was significantly increased,
in a dose-related manner, at 20 mg/kg or more, in both
males and females. The highest dose significantly de-
creased blood glucose in males, aspartate amino transfer-
ase (ASAT) in both males and females, and liver glycogen
in both sexes. Serum triglycerides, alkaline phosphatase,
and creatinine kinase activities were unaffected at any
dose level, as were blood lactate and pyruvate. At 80
mg/kg diet, TBTO significantly reduced blood haemoglobin
in both sexes and haematocrit in females. Mean erythro-
cytes volume was reduced in both sexes, as was mean cor-
puscular haemoglobin content (mass), but mean corpuscular
haemoglobin concentration and erythrocyte numbers were not
affected (Krajnc et al., 1984). A 6-week study at dietary
doses of 5, 20, and 80 mg/kg showed significant reductions
in haematocrit at 20 and 80 mg/kg and in blood haemoglobin
levels at 80 mg/kg. Iron concentration was reduced at 80
mg/kg, as was the isocitrate dehydrogenase (ICDH) activity
of erythrocytes. Numbers of erythrocytes, thrombocytes,
and reticulocytes were not significantly affected at any
dose level of TBTO, though there was a trend towards
increased numbers of reticulocytes. The authors suggested
an effect on haemoglobin synthesis and other indicators,
implying either reduced iron uptake or increased iron
loss. The enhanced ICDH activity and increasing reticulo-
cyte numbers indicated the presence of immature erythro-
cytes, and the authors could not exclude the possibility
of an in vivo haemolytic action of TBTO comparable to the
reported in vitro haemolysis (Krajnc et al., 1984).
Rosenberg et al. (1984) measured the activity of haem
oxygenase in mucosal cell fractions from control mice and
mice dosed by intubation with TBTO at 60 mg/kg body
weight. The activity of the enzyme, monitored by the
bilirubin absorbance spectrum, was substantially elevated,
compared to controls, 16 h after administration of the
TBTO. The activity of the same enzyme in liver and kidney
microsomes was not affected by this dose of TBTO given by
gavage, but was elevated when TBTO was given parenterally.
The activities of cytochrome P-450 and benzo(a)pyrene
hydroxylase were substantially reduced in the intestine of
TBTO-treated mice, this reduced activity being statisti-
cally significant for the latter but not for the former.
Similar results were obtained in liver fractions when TBTO
was applied parenterally.
11.2.5. Effects on lymphoid organs and immune function
Funahashi et al. (1980) reported the effects of feed-
ing TBTO in olive oil (0, 3, 6, or 12 mg/kg body weight
per day) for 13 or 26 weeks by gavage to groups of ten
male Sprague-Dawley rats, aged 5 weeks at the beginning of
the study. No analyses of haematology, immunoglobulin
levels, or specific aspects of immune function were per-
formed. A marked dose-related reduction in absolute and
relative thymus weight was seen following dosing for both
periods. Relative thymus weights were 682, 629, 340, and
313 mg/kg body weight after 13 weeks of dosing and 449,
313, 278, and 248 mg/kg body weight after 26 weeks of
dosing in the rats given 0, 3, 6, and 12 mg/kg per day,
respectively. All results, with the exception of those for
the group given 3 mg/kg body weight per day for 13 weeks,
were statistically significant (p < 0.003). Despite the
considerable reductions in thymus weight, the only histo-
logical observation was a slight reduction in the width of
the thymic cortex. A dose-related reduction in relative
spleen weight was seen after 26 weeks, which was statisti-
cally significant (p < 0.05) at the dose level of 12 mg/kg
body weight per day. Reduced body weight and increases in
relative pituitary and relative adrenal weights were stat-
istically significant (p < 0.01) at 12 mg/kg body weight
per day for 13 and 26 weeks and at 6 mg/kg body weight per
day for 26 weeks, and there was a significant (p < 0.05)
increase in relative adrenal weight at 6 mg/kg body weight
per day for 13 weeks. The relative pituitary weight was
also increased following 26 weeks of dosing at 3 mg/kg
body weight per day (p < 0.01). Based on thymus weight,
the NOEL was the most sensitive end-point in this study,
3 mg/kg body weight per day for 13 weeks. However, an
effect was seen at this dose when it was given for 26
weeks.
Funahashi et al. (1980) also reported that the
reduction in relative thymus weight, present 3 days after
a single dose of TBTO in olive oil of 100 mg/kg body
weight to 5-week-old male Sprague-Dawley rats, showed
signs of reversal at day 8. The reversibility of TBT-
induced thymus atrophy was also demonstrated by Snoeij et
al. (1988b). Groups of 4 or 5 young (4-5 weeks old) male
Wistar rats received a single gavage dose of TBT chloride
(0 or 16 mg/kg body weight) in corn oil. A 30% reduction
in relative thymus weight was evident in animals killed on
days 2, 3, or 4, but there was complete recovery by day 7.
A similar effect and recovery was seen in the thymus cell
counts. An equimolar dose of dibutyltin chloride produced
more pronounced effects, the recovery period being pro-
longed until day 9.
Krajnc et al. (1984) reported the effects of feeding
TBTO-containing diets (0, 5, 20, 80, or 320 mg/kg diet) to
groups (10 males and 10 females per group) of young SPF
Wistar rats for 4 weeks. These dose levels are equivalent
to approximately 0, 0.5, 2, 8, or 32 mg/kg body weight per
day, respectively, based on actual food consumption
measurements. Total leucocyte and circulating lymphocyte
counts were significantly reduced in males receiving 80
mg/kg diet (p < 0.01) and in both sexes receiving 320
mg/kg diet (p < 0.001). Eosinophil counts were signifi-
cantly reduced (p < 0.05) in males receiving 5, 80, or 320
mg/kg diet and in females given the highest dose. Monocyte
counts were increased significantly (p < 0.05) in males
receiving 20, 80, or 320 mg/kg diet but not in females.
Total immunoglobulin levels were significantly affected at
80 and 320 mg/kg diet. At 80 mg/kg diet, TBTO signifi-
cantly increased serum IgM to 132% (p < 0.01) and 145%
(p < 0.001) of control levels for males and females,
respectively. At the same dose, IgG in males was reduced
significantly (p < 0.05), but in females IgG was unaffec-
ted. A significant reduction (p < 0.001) in serum IgG was
found in both sexes fed TBTO at 320 mg/kg diet. Thymus and
relative thymus weights were significantly reduced in both
sexes (p < 0.001) at 80 and 320 mg/kg diet and in females
given 20 mg/kg diet (p < 0.05). Relative spleen weight
was significantly increased in males receiving 320 mg/kg
diet but this was probably related to the decreased body
weight of these animals. Histological examination showed
that all the animals given the highest dose exhibited
lymphocyte depletion from the thymic cortex, resulting in
an indistinct cortico-medullary junction and an increase
in ceroid/lipofuschin-loaded macrophages. Slight atrophy
of the thymic cortex was seen in two males fed 80 mg/kg
diet. Diffuse atrophy of the white pulp of the spleen was
seen in all animals at 320 mg/kg, the periarteriolar lym-
phocyte sheaths (PALS) being particularly affected. At 80
mg/kg diet, one male and two females showed slight splenic
atrophy. Depletion of T lymphocytes, determined by pan-T
immuno-staining, was seen in the PALS at 320 mg/kg diet.
There was an increase in the incidence of mesenteric lymph
nodes atrophy, observable in some animals fed 20 mg/kg and
increasing with dose to affect all animals given 320 mg/kg
diet. Both the paracortex and medulla of lymph nodes were
reduced in size and cellularity, and the numbers and size
of follicles were reduced by the highest TBTO dose level.
Again, the total number of T lymphocytes was strikingly
reduced in the paracortex by TBTO at 320 mg/kg diet, as
determined by immuno-staining. Although thymic involvement
was marked, it was not only thymus-dependent areas that
were affected. In rats exposed to the highest dose level,
which was overtly toxic, B lymphocyte areas also showed
low level activity, as indicated by fewer follicles and
inconspicuous germinal centres in lymph nodes and spleen.
An increase in the number of animals with rosettes in
sinuses in the mesenteric lymph nodes, composed of eryth-
rocytes surrounding mononuclear cells, was seen. This was
dose related: half of the animals dosed at 5 mg/kg and all
the animals treated with 80 or 320 mg/kg showed erythro-
cyte rosettes. The biological significance of these
rosettes is unclear. Specific aspects of immune function
were not tested. In further studies (personal communi-
cation by E.I. Krajnc to IPCS, 1989; Wester, in press), no
increase in rosette formation was observed at 5 mg/kg diet
or 50 mg/kg diet. Signs of general toxicity were observed
in animals treated with 320 mg/kg diet, i.e. reduced body
weight (p < 0.001) and increased serum ALAT activity. At
80 mg/kg, there was reduced body weight gain in males
(p < 0.05) and increased ALAT, and at 20 mg/kg increased
ALAT in males only. The liver was the only non-lymphoid
organ displaying histological changes: centrilobular hepa-
tocyte atrophy, reduced glycogen retention, parenchymal
necrosis, and hyperplasia of the intrahepatic bile duct
were seen in some animals treated at 320 mg/kg diet. Three
animals from the group given 80 mg/kg showed slight hepa-
tocyte atrophy. This study indicated a NOEL of 5 mg/kg
diet (approximately 0.5 mg/kg body weight per day).
Vos et al. (1984) investigated functional aspects of
the immunological effects reported by Krajnc et al. (1984)
in in vivo and ex vivo experiments using weanling (3 to 4
weeks old) male SPF Wistar rats fed diets containing TBTO.
Haematological parameters were not studied. Levels of
total circulating immunoglobulins (IgG and IgM) were
determined in animals fed diets containing 80 or 320 mg
TBTO/kg. At 320 mg/kg diet, IgM was significantly
increased (p < 0.05) and IgG was significantly decreased
(p < 0.001) on day 28 (no assays were performed on day 42
for this group). At 80 mg/kg diet, IgM was elevated by 30%
on day 42 (p < 0.01), while IgG was decreased by 30% on
days 28 and 42.
The effects of TBTO on lymphocyte counts and cell
viability in lymphoid organs was investigated, and several
specific tests of immune system function were also per-
formed. Numbers of B and T lymphocytes were significantly
reduced in the spleens (by 15% and 48%, respectively) of
animals fed diets containing 80 mg/kg diet. The ratio of
total T:B lymphocytes was decreased in a dose-related
manner following 9 weeks of exposure.
A significant reduction occurred in the numbers of
viable cells (trypan blue exclusion) obtained from the
thymus, spleen, and bone marrow of rats treated with TBTO
at 80 or 320 mg/kg diet. At 320 mg/kg diet, viability and
cell numbers were significantly (p < 0.05) reduced for
both thymus and spleen following 3, 8, and 20 days of ex-
posure, and bone marrow counts were significantly reduced
on days 8 and 20. Doses of 80 mg/kg diet produced signifi-
cant reductions in thymus cell count and viability on day
8 and thymus, spleen, and bone marrow counts on day 20.
The antibody response to sheep erythrocytes (ip),
tetanus toxoid (iv), ovalbumin (sc into the foot pad, in-
cluded H37Ra adjuvant), and the worm Trichinella spiralis
(oral) was assessed in animals receiving 20 or 80 mg TBTO
per kg diet for 6 weeks. The primary response to sheep
erythrocytes (0.5 ml of a 20% suspension, ip) was deter-
mined 10 days post inoculation (pi) and the secondary
response was determined on day 20 pi following an iv
booster inoculation on day 15 pi. The primary response was
unaffected, but there was a dose-related decrease in the
secondary response seen both in untreated and 2-mercapto-
ethanol-treated (IgM-inactivated) sera, reaching statisti-
cal significance (p < 0.05) in treated sera from the
highest-dose group. The response (IgG and IgM titres) to
tetanus toxoid inoculation measured only on day 21 pi was
equivocal, and that to ovalbumin measured on days 15, 21,
and 28 pi was unaffected by TBTO. IgG response to oral T.
spiralis infection was significantly (p < 0.05) increased
on day 21 pi at 20 mg/kg diet, but not on day 42 or at 80
mg/kg diet at either time. IgM response was unaffected.
IgE response, possibly the most relevant to resistance to
parasitic infection, was decreased in a dose-related man-
ner on days 21 and 42 following T. spiralis infection; log
titres were 3.8, 2.5 (p < 0.05), and 1.8 (p < 0.001) on
day 21 and 4.4, 3.6, and 3.4 (p < 0.05) on day 42 in the
control, 20-, and 80-mg/kg groups, respectively. Delayed-
type hypersensitivity was determined as change in skin
thickness following a challenge of ovalbumin to the skin
of the ear or tuberculin challenge to the skin of the
flank, made 3 and 4 weeks, respectively, following initial
immunization (sc into a footpad) 6 weeks after starting
dietary dosing of TBTO at 20 or 80 mg/kg. Compared to
controls similarly injected intradermally with medium,
significant reductions in response to ovalbumin challenge
were found 24, 48, and 72 h after dosing at 20 mg/kg (all
p < 0.05), and 24 h (p < 0.01) and 48 h (p < 0.05) after
dosing at 80 mg/kg. With tuberculin challenge, significant
effects were found in the group given 80 mg/kg diet after
24 h (p < 0.01), 48 h (p < 0.001), and 72 h (p < 0.001),
but only after 72 h (p < 0.05) at the lower dose level.
Reduced responses were seen at all three times after both
doses.
Thymus or spleen cells, obtained from rats fed TBTO at
20 or 80 mg/kg diet, were cultured with and without
mitogens (PHA = phytohaemagglutinin; Con A = concanavalin
A; PWM = pokeweed mitogen) for 24 h before the addition
of 3H-thymidine to monitor DNA synthesis. PHA and Con A
are both T cell mitogens and PWM is a mitogen for both T
and B cells. Due to a reduction in the number of viable
cells in thymic cultures from the high-dose group, respon-
siveness to mitogens was expressed per culture and per
thymus. Significant reductions in 3H-thymidine uptake
(expressed per thymus) were found in unstimulated (48%
reduction), PHA-treated (64%), Con A-treated (62%), and
PWM-treated (50%) cultures derived from the high-dose
group; PHA (48%) and PWM (28%) were the only responses
reduced at 20 mg/kg diet. Similar effects were seen on
splenic cultures, with a reduced number of viable cells
per spleen and significant reductions in response
(expressed per spleen) to PHA (50% reduction) and Con A
(45%) in cultures from the high-dose group; responses to
PWM and E. coli lipopolysaccharide (a B cell mitogen)
were significantly increased on a per culture basis but
not a per spleen basis. Cultures from the low-dose group
showed reduced response to PHA (25% reduction) and Con A
(20%), but these were not statistically significant.
TBTO, at both 20 and 80 mg/kg diet, reduced the
resistance of rats to infection by T. spiralis. The number
of worm larvae in muscle significantly increased in a
dose-dependent manner (74 000 in controls; 106 000 in rats
fed 20 mg TBTO/kg, p < 0.01; 198 000 in rats fed 80 mg/kg
p < 0.001) after standard infection with 1000 larvae by
mouth. The number of adult worms in the small intestine
also increased significantly after 10 (p < 0.05), 12
(p < 0.001), and 14 (p < 0.01) days in the high-dose
group, and after 12 (p < 0.05) and 14 (p < 0.001) days in
the low-dose groups. In a study of the inflammatory reac-
tion around larva-containing muscle cells in the tongues
of animals killed 14 days after infection, the response
(mononuclear cells and eosinophilic granulocytes) after
treatment with TBTO at 20 mg/kg diet was described as
"slightly reduced". However, there was a marked
reduction after treatment at 80 mg/kg diet.
The clearance of Listeria monocytogenes from the
spleen (a measure of host resistance) was monitored in
rats fed 20, 80, or 320 mg TBTO/kg diet for 6 or 7 weeks.
Statistically significant increases in the number of
viable bacteria were seen 2 days after an iv injection
into animals treated with 320 mg/kg diet for 6 (p < 0.01)
or 7 (p < 0.001) weeks and with 80 mg/kg diet for 6 weeks
(p < 0.001). A dose-related reduction, statistically sig-
nificant (p < 0.05) at 80 mg/kg diet, in viable bacteria
was seen 1 day post injection in the group receiving TBTO
for 7 weeks, but this was not seen in the 6-week study.
The ex vivo phagocytosis of L. monocytogenes by spleen-
and peritoneal-derived macrophages from TBTO treated (20
or 80 mg/kg diet) animals was not significantly affected,
although a dose-related reduction in the phagocytic
activity of splenic macrophages was seen.
Cells derived from the spleen and peritoneal cavity
were tested for spontaneous cell-mediated cytotoxicity
against murine YAC lymphoma target cells labelled
with 51Cr. "Specific release" of the chromium label
(release in experimental minus spontaneous release in the
controls) was used as the end-point of the assay. Stat-
istically significant reductions in specific release were
found with spleen cells from rats fed TBTO at 80 mg/kg
diet (p < 0.05), but not in spleen cells from those fed 20
mg/kg diet. Statistically significant effects (p < 0.05)
were found in peritoneal macrophages derived from rats
receiving 20 and 80 mg/kg diet, but there were no signifi-
cant effects on non-adherent cells ("natural killer
cells").
These studies were designed to investigate immune
function rather than to determine a no-observed-effect-
level, and the lowest dose used (20 mg/kg diet) produced
statistically significant effects (in particular, de-
pression of host resistance to T. spiralis and L. mono-
cytogenes ). No evidence of general toxicity was seen at
this dose level. The only sign of general toxicity
recorded was a significant reduction in body weight gain
seen after exposure to 320 mg/kg diet for 3, 8, and 20
days and to 80 mg/kg diet for 20 days.
A study of TBTO (0, 0.5, and 50 mg/kg diet) adminis-
tered to groups of weanling Wistar rats (five animals of
each sex per group) for 2 years was briefly reported by
Vos et al. (1985) and Wester (in press). It should be
noted that the intakes in this study were lower, on a body
weight basis, than in the shorter-term studies, being
equivalent to 0, 0.025, 0.25, and 2.5 mg/kg body weight
per day for the controls, and low, medium, and high doses,
respectively (based on actual intake measurements). A
significant decrease in peripheral lymphocyte count and a
statistically significant increase in platelets were seen
in the females fed 50 mg/kg diet for 1 year. Circulating
levels of total IgM and IgA were significantly increased
at 4-6 and 16-18 months, in rats given 50 mg/kg diet,
while significant reductions in IgG levels were recorded,
particularly in females.
General effects on the lymphoid organs were not
recorded, though several specific tests of immune function
were performed using the methods of Vos et al. (1984). A
dose-related decrease in resistance to T. spiralis infec-
tion was seen at 5 and 16 months, achieving statistical
significance (p < 0.05) at 5 and 50 mg/kg diet. IgE titres
were reduced, but IgA levels increased approximately 50-
fold at the highest dose level. No effects on delayed-type
hypersensitivity were seen, in contrast to results in the
short-term study by Vos et al. (1984). Host resistance to
L. monocytogenes (as measured by the number of viable
organisms in the spleen) was significantly reduced at 5
and 17 months in the 50-mg/kg group, but a significant
increase was seen at 17 months in the 5-mg/kg group.
Natural killer cell activity against YAC lymphoma cells
was reduced significantly at the highest dose level after
15-17 months. It will not be possible to fully assess this
study until the final report is published, although it
would appear that 0.5 mg/kg diet (0.025 mg/kg body weight
per day) was the NOEL.
A preliminary report on a study where diets containing
TBTO (0, 0.5, 5, or 50 mg/kg diet) were fed to Wistar rats
(12 months old) for 6 months, has been issued (personal
communication by E.I. Krajnc to IPCS, 1989). Specific
tests of immune function were measured after 5 months of
dosing. Significant (p < 0.05) reductions in host resist-
ance to T. spiralis and L. monocytogenes were seen at
50 mg/kg only. These results suggest that aged rats are
less sensitive to TBTO in the diet than weanlings animals,
although this may be due to a lower intake on a per kg
body weight basis. Full assessment of this study will need
to await the completion of the statistical analyses and
final report.
A series of studies have been performed to investigate
certain aspects of immune function (Schering, 1989a,b,c,d).
When groups (10 animals of each sex) of young (4 to 5
weeks old) Sprague-Dawley rats were fed diets containing
TBTO (0, 0.5, 2, 5, or 50 mg/kg diet) for 4 weeks, no sig-
nificant effects were seen on total or differential white
blood cell counts. Serum immunoglobulin levels were not
measured. A statistically significant decrease in absolute
and relative thymus weight (p < 0.01) was seen in males
fed 50 mg/kg. A decrease in absolute and relative spleen
weight was also seen in this group, though it was not sig-
nificant statistically. The viability of cultured spleen
cells (monitored with trypan blue exclusion) obtained from
females fed 5 mg/kg was reduced (p < 0.05), but other
groups were unaffected. A significant decrease in total
thymus cell count was seen in preparations from males fed
50 mg/kg only (p < 0.05). A slight but significant
(p < 0.05) reduction in the thickness of the thymic cortex
was also seen in these males, this being the only signifi-
cant histological finding. Specific measures of immune
function were not performed in this study but in parallel
studies. The NOEL in this study was 5 mg/kg diet
(approximately 0.6 mg/kg body weight per day, based on
measured intake) (Schering 1989a).
An assay of plaque-forming cells, a measure of humoral
immunity, revealed no effects due to TBTO (at levels of
0.5, 2, 5, or 50 mg/kg diet) fed to groups (10 animals of
each sex) of young (4 to 5 weeks old) Sprague-Dawley rats
for 5 weeks. The response was measured on day 36, follow-
ing an iv inoculation of sheep erythrocytes. The only sign
of toxicity was a reduction in body weight in males fed
50 mg/kg, but this was not statistically significant. No
investigation of general toxicity was performed. The NOEL
was 50 mg/kg diet (approximately 5.6 mg/kg body weight per
day) in this study (Schering, 1989b).
To assess the effects of TBTO on host resistance to
infection, the number of viable Listeria monocytogenes
cells in the spleen, 4 days after inoculation with
1.4 x 106 cells, was counted. No effects were produced in
groups (10 animals of each sex) of young Sprague-Dawley
rats fed diets containing TBTO at 0.5, 2, or 5 mg/kg diet
for 34 days. However, statistically significant increases
in numbers of viable bacteria were seen in the spleen of
males (p = 0.055) and females (p < 0.01) fed 50 mg/kg
diet. No investigations of general toxicity were per-
formed. The NOEL was 5 mg/kg diet (approximately 0.6 mg/kg
body weight per day) (Schering, 1989c).
The effects of TBTO on delayed-type hypersensitivity
reactions, a measure of cell-mediated immunity, were
assessed in groups (10 animals of each sex) of 4- to 5-
week-old Sprague-Dawley rats fed diets containing 0, 0.5,
2, 5, or 50 mg/kg diet for 37 days. A sensitizing dose of
100 µg bovine serum ablumin (BSA) mixed with Freund's
complete adjuvant was given on day 29, followed by a chal-
lenge dose of heat-inactivated BSA injected into a hind-
foot pad on day 37. No difference in response (increased
footpad thickness) was seen between test and control
groups. Body weight gain was unaffected, but no other
investigations of general toxicity were performed. The
NOEL in this study was 50 mg/kg diet (approximately 5.8
mg/kg body weight per day) (Schering 1989d).
The effects of inhaled TBTO were studied in groups (10
animals of each sex) of young (initial weights 86-131 g)
SPF Wistar rats exposed to 0, 0.03, 0.16, or 2.8 mg/m3
for 4 h/day, 5 days per week, for 21 to 24 exposures. The
two lower doses were provided by filtered vapour, and the
highest dose was provided by an aerosol with over 90% of
the particles < 5 µm in diameter (Schering 1983). Lympho-
cyte and total leucocyte counts, measured at 2 or 4 weeks,
were unaffected by TBTO. Some inconsistent changes in
reticulocyte counts were found, but these appeared to be
primarily related to use of the respiration chamber and
not to TBTO exposure. Immunoglobulins were not measured.
Thymolysis and lymphocyte depletion of the thymus-depen-
dent areas of the spleen and lymph nodes were reported in
the 11 animals (five males, six females) from the highest-
dose group that died during the study. No such lesions
were detected in the survivors, although in three sur-
vivors from the highest-dose group an increase in the
number of macrophages containing nuclear debris was seen
in the thymic cortex. No significant (p > 0.05) changes
were seen in absolute or relative weights of the thymus,
spleen, or iliac lymph node in animals surviving the
study, but no organ weights were recorded for those ani-
mals dying or sacrificed during the study. No specific
aspects of immune function were studied. Histological
signs of general toxicity were limited to those consistent
with inflammatory reactions within the respiratory tract
and, with one exception, were confined to animals exposed
to the aerosol. Food intake and body weight gain were
reduced in both sexes, the reduction being statistically
significant (p < 0.01) in male rats exposed to 2.8 mg/m3.
The cause of death in the 11 animals from the highest-dose
group was not ascertained (Schering, 1983).
When TBTO (0, 4, 20, 80, or 200 mg/kg diet) was fed to
groups (10 animals of each sex) of CD-1 mice, aged 6 to 7
weeks at commencement of the study, for 3 months, leuco-
cyte counts were increased at 80 and 200 mg/kg in both
sexes, although this increase did not reach statistical
significance (p > 0.05). Immunoglobulins were not
measured. Thymus weight was reduced in both sexes at 200
mg/kg, but this was not statistically significant
(p > 0.05). Spleen weights were increased in both sexes
at 80 and 200 mg/kg (reaching statistical significance
(p < 0.05) at 200 mg/kg), possibly secondary to effects on
erythrocytes. No specific immune function tests were per-
formed. Histological changes were seen in the livers of
both sexes at 80 and 200 mg/kg. Dose-related, statisti-
cally significant (p < 0.01) increases in absolute liver
weights were seen at 80 and 200 mg/kg, and adrenal weights
were significantly (p < 0.01) increased in the male rats
fed 200 mg/kg. The NOEL in this study was 20 mg/kg diet
(approximately 4 mg/kg body weight per day) (Biodynamics,
1989a).
In studies by Schering (1989e), groups (two animals of
each sex) of beagle dogs received variable doses of TBTO
in arachis oil by oral gavage:
Group 1: controls;
Group 2: 0.1 mg/kg body weight per day for 5 weeks,
0.2 mg/kg body weight per day for 4 to 5 weeks, then
10 mg/kg body weight per day for 8 to 9 weeks;
Group 3: 0.5 mg/kg body weight per day for 5 weeks
then 1 mg/kg body weight per day for 13 to 14 weeks;
Group 4: 2.5 mg/kg body weight per day for 5 weeks
then 5 mg/kg body weight per day for 13 to 14 weeks.
All males in groups 2 and 4 died as a result of mis-dosing
to the lungs. Increased leucocyte and neutrophil counts
were seen in group 4 at weeks 9 and 18 (p < 0.05), and
there was a statistically significant increase in leuco-
cyte count recorded at 13 weeks in group 2 (p < 0.05).
Non-statistically significant reductions in leucocyte,
neutrophil, and lymphocyte counts were found in group 2 at
18 weeks. Specific immunoglobulins were not measured.
Thymus weights were reduced in the two survivors of group
2 (0.9 ± 0.1 g compared with 4.0 ± 0.6 g in controls), and
slight increases in thymus weights were seen in groups 3
and 4. Iliac and mesenteric lymph node weights were
reduced, though spleen weights were increased, in the two
survivors from group 2. Histological changes in lymphoid
organs were confined to group 2 where a reduction of lym-
phocyte numbers in the thymus, spleen (particularly PALS),
and lymph nodes was seen. A dose-related increase in rela-
tive liver weight (33.1, 41.7, 49.9, and 57.6 g/kg body
weight in groups 1, 3, 4, and 2, respectively) was
accompanied by cytoplasmic vacuolation of hepatocytes in
group 2 (Schering, 1989e).
11.2.6. Mechanism of immunotoxicity
The precise mechanism of the immunotoxic effects of
TBTO is not yet clear. However, a hypothesis has been put
forward, based on the work of Snoeij (1987) and Pieters et
al. (1989).
a) Absorbed TBTO is present as the TBT+ cation or a salt
(chloride or carbonate). Studies using TBT chloride
are, therefore, relevant to TBTO immunotoxicity.
b) As dibutyltin chloride (DBT chloride), a metabolite of
TBT chloride, is, mole for mole, more potent that TBT
chloride at producing effects in the thymus and thymic
cells, it is probable that DBT chloride or another DBT
salt is the active species in TBTO toxicity.
c) The primary action of DBT is to suspend the maturation
of immature thymocytes by inhibiting their inter-
action/binding with thymic epithelial cells. The turn-
over period of thymocytes is 3 to 4 days. Therefore,
as cell proliferation/maturation is inhibited, rapid
depletion of thymocyte numbers without cytotoxicity is
expected, followed by a rapid proliferation (observed
by Snoeij et al. (1988b) and Funahashi et al. (1980))
on removal of DBT.
Other evidence indicating that TBT compounds have a par-
ticular effect on the thymus is provided by the in vitro
studies of Snoeij (1987), which show TBT chloride to be
cytotoxic to thymocytes.
The demonstration of reduced thymus weights in certain
fish species (e.g., the freshwater guppy) indicates that
TBT may have immunotoxic effects on a wide range of
species.
11.2.7. Effects on the endocrine system
The weights of both the adrenal and pituitary glands
of Sprague-Dawley rats were significantly increased after
exposure to 6 mg TBTO/kg body weight daily, by intubation,
for 26 weeks. Pituitary weight was also significantly
increased by 3 mg TBTO/kg body weight given daily for
26 weeks (Funahashi et al., 1980).
After 4 weeks, the serum insulin concentration of rats
was not significantly affected by dosing with TBTO at up
to 80 mg/kg diet, but was undetectable (< 2 milliIU/litre)
in rats fed 320 mg/kg (control levels of insulin in serum
were 111 and 74 milliIU/litre for males and females, re-
spectively) (Krajnc et al., 1984). In a further study, the
same authors monitored endocrine changes in male Wistar
rats exposed to TBTO in the diet (0, 20, or 80 mg/kg) for
6 weeks. Serum thyroxine, thyroid stimulating hormone
(TSH), and insulin levels were reduced significantly at
the higher dose level, but serum follicle stimulating
hormone (FSH) and corticosterone levels were unaffected.
Only insulin was significantly affected at 20 mg TBTO/kg.
The luteinizing hormone (LH) concentration in serum was
significantly increased by TBTO at 80 mg/kg, but was
unaffected by 20 mg/kg. The authors further examined endo-
crine function by monitoring hormone release following
physiological stimulus. Insulin release, following iv
administration of glucose, was unaffected by a 6-week
exposure to TBTO at either 20 or 80 mg/kg diet. The effect
of TBTO on insulin was attributed by the authors to a
decreased food intake. The release of TSH, after iv admin-
istration of thyrotrophin releasing hormone (TRH), showed
a tendency (p < 0.1) to be inhibited in rats fed at 80
mg/kg. The titre of circulating TSH, 20 min after TRH
administration, was significantly reduced compared with
controls. Release of both LH and FSH, in response to
luteinizing hormone releasing factor stimulation, was
enhanced in rats fed TBTO at both 20 and 80 mg/kg diet,
but only significantly so at the higher dose rate. Histo-
logical examination of endocrine organs after a 6-week
exposure to TBTO revealed some changes. No differences
were observed in either insulin- or glucagon-producing
cells in the pancreas. Some flattening of the epithelial
lining of the thyroid follicles was observed after
exposure of rats to 80 mg/kg but not to 20 mg/kg. Immuno-
cytochemical staining of the pituitary gland identified
each cell type producing the different pituitary hormones.
There was a dose-related decrease in both the intensity of
staining of TSH cells and the number of cells stained.
Conversely, there was a dose-related increase in the
staining intensity of LH cells. No effects were found on
FSH, growth hormone, or adrenocorticotrophin cells in the
pituitary (Krajnc et al., 1984).
11.3. Long-term toxicity
Wester (in press) carried out a 106-week toxicity
and carcinogenicity study with groups of 50 weanling
Wistar rats of each sex. An additional group of 10 rats
was used for an interim sacrifice after 1 year. TBTO was
fed at 0, 0.5, 5, or 50 mg/kg diet (equivalent to 0,
0.025, 0.25, or 2.5 mg/kg body weight). Increased food
consumption occurred in all treated males (not clearly
dose-related), and there was increased water consumption
in males at 5 and 50 mg/kg. During the second year, the
body weight of the highest-dose group was significantly
lower than that of controls. Excess mortality, compared
with that of controls, was confined to the 50 mg/kg group
towards the end of the experiment (see section 11.6).
Haematological changes (anaemia, lymphopenia, and thrombo-
cytosis) and increases in plasma enzyme activities (ALAT,
ASAT, and AP) were noted mainly at the high-dose level.
Serum IgM and IgA levels increased, while the IgG level
decreased (females). No effect was observed on circulating
concentrations of T4, free T4, TSH, LH, FSH, or insu-
lin; only the free T4:T4 ratio was decreased. Organ
weight changes consisted of increased liver, kidney,
adrenal, and pituitary weights and decreased thyroid
weight. Non-neoplastic histological alterations consisted
of a decrease in cell height of the thyroid follicles (at
50 mg/kg diet after 1 and 2 years), decrease in splenic
iron content (at 5 and 50 mg/kg after 1 year only), slight
bile duct proliferation (at 50 mg/kg after 1 year only),
and vacuolation of kidney proximal tubular epithelium and
nephrosis (at 50 mg/kg after 2 years only).
11.4. Genotoxicity
Summary
The genotoxicity of TBTO has been the subject of extensive
investigation. Negative results were obtained in the vast
majority of studies, and there is no convincing evidence that
TBTO has any mutagenic potential.
Davis et al. (1987) conducted a comprehensive study of
the genetic effects of TBTO using a wide range of tech-
niques in order to assess possible hazards in the use of
the compound as a molluscicide for the control of schisto-
somiasis. TBTO did not produce gene (point) mutations in
Salmonella typhimurium strains TA1530, TA1535, TA1538,
TA97, TA98, or TA100, either in the presence or absence of
an exogenous metabolic activation system (rat liver S9).
The compound did give some evidence of gene mutation in
Salmonella typhimurium TA100 using the fluctuation method
in the presence of S9, but no dose-response relationship
was seen; negative results were seen in the absence of S9.
TBTO did not induce point mutations in the yeast
Schizosaccharomyces pombe. Negative results were obtained
when TBTO was tested in the sex-linked recessive lethal
assay using Drosophila melanogaster, the compound being
given in food and by injection, indicating that TBTO did
not produce gene mutations in Drosophila. Negative results
were also obtained when the ability of TBTO to produce DNA
damage in Bacillus subtilis (recombination assay) or the
yeast Saccharomyces cerevisiae (mitotic gene conversion)
was tested.
The ability of TBTO to produce gene mutations in Sal-
monella has also been studied by Reimann & Lang (1987).
Negative results were obtained using Salmonella
typhimurium strains TA1535, TA1537, TA1538, TA98, and
TA100, both in the presence and absence of rat S9. Simi-
larly, negative results were obtained with six TBT esters
(abietate, borate, linoleate, naphthenate, phosphate, and
tallate). Further negative results were obtained when the
ability of TBTO to produce mitotic gene conversion in
Saccharomyces cerevisiae was tested.
The ability of TBTO to produce gene mutations in
mammalian cells in vitro was extensively investigated by
Davis et al. (1987), and negative results were consist-
ently obtained. TBTO did not induce gene mutations in V79
Chinese hamster cells (using resistance to 8-azaguanine,
ovabain, or 6-thioguanine as markers) in the presence of
rat liver S9. Negative results were also obtained in the
V79 cell assays when epidermal cells of mice and humans
(primary cultures) were used as the source of metabolic
activation in cell-mediated assays.
The in vitro clastogenic potential of TBTO has been
investigated in mammalian cells in two sets of studies.
When Davis et al. (1987) used Chinese hamster ovary (CHO)
cells, harvested at 8, 15, and 24 h, an increase in struc-
tural aberrations (mainly deletions), together with
endoreduplication, was seen, but only at the highest
concentration tested (5 µg/ml in the presence of S9 and
1.5 µg/ml in its absence). The increase in the presence
of S9 was seen only after 15 h, "toxicity" precluding
any analysis of results at this concentration after 8 h.
The increase in the absence of S9 was seen only at 8 h,
there being no increase at either 15 or 24 h. The results
of these studies are difficult to interpret, since the
effect was limited to concentrations associated with high
toxicity and no data on mitotic index was reported. No
increase in sister chromatid exchange was seen at any
concentration.
Reimann & Lang (1987) used human lymphocytes to test
the clastogenic potential of TBTO and obtained negative
results both in the presence and absence of S9. In the
latter case, TBTO was added 22 h after stimulation of the
cultures with phytohaemagglutinin, and cells were har-
vested 31 h later. In the former case, TBTO was added with
S9 after 26 h; 3 h later the S9 was removed, and the cells
were harvested 22 h later. Parallel studies on blood cul-
tures that were differentially stained with BUdR indicated
that almost all cells analysed for chromosome aberrations
were in the first mitotic stage. The highest TBTO concen-
trations used (0.1 µg/ml in the absence of S9 and
1 µg/ml in its presence) were associated with a marked
reduction in mitotic index (57% and 58%, respectively). No
increase in aberrations was seen at any dose level. This
study, therefore, failed to confirm the suggestion of some
clastogenic potential in the studies using CHO cells.
The ability of TBTO to produce chromosomal damage in
vivo has been investigated in two separate studies using
the micronucleus test. In one study, four doses of TBTO
(31.25, 62.5, 125, or 250 mg/kg body weight) were given by
gavage, in arachis oil, to NMRI mice, and bone marrow
cells were analysed for micronuclei in polychromatic
erythrocytes 24, 48, and 72 h after treatment (Reimann &
Lang, 1987). The highest dose level resulted in marked
lethality (16 out of 36 animals died), precluding any
analysis of the results. The 126-mg/kg dose level was also
associated with some deaths (4 mice died), but 5000 poly-
chromatic erythrocytes were analysed from five male and
five female mice at each harvest interval. Similar analy-
ses were carried out at the two lower dose levels. There
was no increase in micronuclei at any dose level or har-
vest time. This study provided no evidence to indicate
that TBTO produces chromosomal damage in bone marrow in
vivo. A second micronucleus study (Davis et al., 1987)
used Balb/c mice. Groups of 10 male and 10 female animals
were given 30 or 60 mg TBTO/kg as a solution in olive oil.
Bone marrow cells were harvested from five males and five
females after 30 h and 48 h, and 1000 polychromatic eryth-
rocytes were analysed from each for micronuclei. An
increase in micronuclei was seen only in the male mice
after 48 h and at the highest dose level. These results
conflict with those of Reinmann & Lang (1987). Further-
more, there was an unusually high spontaneous incidence of
micronuclei (up to 5.8 per 1000 polychromatic erythro-
cytes) in the study of Davis et al. (1987). These factors
prompted a re-analysis of the slides from this study by
the Institute of Occupational Health, Helsinki, Finland
(Schering 1986). This re-analysis, again using 1000 poly-
chromatic erythrocytes per animal, failed to confirm the
increase in micronuclei seen after 48 h in the male
animals given 60 mg TBTO/kg. A slight, but statistically
significant, increase in micronuclei was seen in the
female mice on re-analysis, but this was thought to be
biologically non-significant due to the high variability
in the control data. The re-analysis highlighted the
problem of interpreting studies in which only relatively
few polychromatic erythrocytes are analysed (1000) and
there is marked variability in the control data (with an
incidence outside the normal range at some time points).
Thus, no conclusions can be drawn from the micronucleus
study of Davis et al. (1987).
The ability of TBTO to inhibit metabolic cooperation
between V79 Chinese hamster 6-thioguanine-resistant and
sensitive cells has been investigated by Davis et al.
(1987). This assay has been suggested as a model for
tumour promoter activity. Negative results were obtained.
The significance of the assay is, however, unclear.
In summary, the genotoxicity of TBTO has been the
subject of extensive investigation. Negative results were
obtained in the vast majority of studies, and there is no
convincing evidence that TBTO has any mutagenic potential.
11.5. Reproductive toxicity
Summary
The potential embryotoxicity of TBTO has been evaluated in
three mammalian species (mouse, rat, and rabbit) after oral
dosing of the mother. The main malformation noted in rat and
mouse fetuses was cleft palate, but this occurred at dosages
overtly toxic to the mothers. These results are not considered
to be indicative of teratogenic effects of TBTO at doses below
those producing maternal toxicity. The lowest NOEL, with
regards to embryotoxicity and fetotoxicity for all three
species, was 1 mg/kg body weight.
11.5.1. In vivo
Reproductive toxicity has been studied in NMRI mice.
The highest dose used (35 mg/kg body weight) was chosen to
give minimal maternal mortality based on acute toxicity
tests. An increase in cleft palate was seen in the fetuses
of mice treated orally with 11.7 mg TBTO/kg body weight
(7% cleft palate), 23.4 mg/kg (24%), and 35 mg/kg (48%),
compared to the incidence in controls (0.7%). However, 11
out of a total of 15 affected mice were clustered in one
of the 18 litters and 15 litters contained none. The two
highest doses of TBTO also increased the frequency of
irregular ossification centres of sternabrae and of minor
abnormalities, such as fusion of the bases of os occipi-
talis. The strain of mice used in the study has a tendency
to produce these particular abnormalities as a result of
non-specific stress on the mother. The authors considered
that TBTO has a very low teratogenic potential; electron
microscopy 26 and 48 h after treatment showed no evidence
of damage to the embryos but considerable damage to the
maternal liver. At a level of 6 mg/kg body weight, TBTO
produced no increase in fetal abnormalities (Davis et al.,
1987).
Nemec (1987) investigated the maternal, embryotoxic,
and teratogenic effects of TBTO in New Zealand white
rabbits. The TBTO was administered in corn oil by gavage,
once a day from day 6 of gestation to day 18 inclusive, at
doses of 0.2, 1, and 2.5 mg/kg per day body weight in a
volume of 0.5 ml. Twenty female rabbits were dosed with
corn oil as controls and 20 rabbits were used at each dose
level. The rabbits were artificially inseminated and
injected iv with human chorionic gonadotrophin immediately
afterwards to ensure ovulation. With the exception of a
single female dosed at 1 mg/kg, all treated rabbits sur-
vived to day 29 of gestation when the experiment was
terminated. A total of 12 animals aborted during the
dosing period: 3, 1, 1, and 7 from the control, 0.2, 1,
and 2.5 mg/kg groups, respectively. Females aborting were
killed the same day and immediate postmortem examinations
carried out. The increased occurrence of abortions in the
highest-dose group was considered to be a secondary effect
of maternal toxicity. No clinical findings in the groups
given 0.2 or 1 mg/kg were considered to be the result of
TBTO treatment. There was a statistically significant mean
body weight loss in females dosed at 2.5 mg/kg per day
compared to controls between days 6 and 18 of gestation
(the period of actual dosing). Doses of 0.2 and 1.0 mg/kg
per day had no effect on growth or survival of fetuses.
There was a slight (statistically non-significant)
decrease in mean fetal weight in the group dosed with 2.5
mg/kg per day. This may represent minor fetotoxicity.
There were no differences in the types or frequency of
fetal malformations related to treatment and the con-
clusion was that TBTO was not teratogenic. Postmortem
examination of the mother rabbits indicated no changes
associated with treatment, and 1 mg/kg per day was con-
sidered to be the NOEL for maternal toxicity and toxicity
to the fetus.
When Crofton et al. (1989) treated pregnant Long-Evans
rats with TBTO by gastric intubation at 0 to 16 mg/kg per
day body weight from day 6 to day 20 of gestation, litter
size and pup weight were significantly reduced by doses of
10, 12, or 16 mg/kg per day but no effect was seen at
doses of 2.5 and 5 mg/kg per day. Maternal weight gain was
affected by the same dose levels that affected litter size
and pup weight. Pup survival was further reduced in the
first 3 days after birth. Litter size was reduced by 50%,
73%, and 96% at dose levels of 10, 12, and 16 mg/kg per
day, respectively, on day 1 post partum and by 63%, 88%,
and 100% on day 3 post partum. Pup weight on day 1 post
partum was reduced by 45%, 45%, and 68% at the three dose
levels. Two out of 71 pups born had cleft palate, but no
controls showed any abnormalities. These two pups were
both born dead. The five pups born to rats given 16 mg/kg
per day showed no malformations, but all died within 3
days. The authors concluded that it was impossible to
distinguish between possible fetotoxicity and maternal
toxicity, since all effects on offspring occurred at TBTO
dose rates that also affected the weight of the mother.
They also showed that pregnant female rats were more sen-
sitive to TBTO than non-pregnant females; the MTD (maximum
tolerated dose) for non-pregnant females was 16 mg/kg per
day, whereas pregnant females showed an MTD of between 5
and 10 mg/kg per day. Most effects on pups were transitory
in that survivors to adulthood showed only reductions in
body weight and brain weight compared to controls even at
the highest dose rates. Motor activity of offspring was
monitored in a maze fitted with photoelectric detectors.
During the pre-weaning period there was a significant
age/treatment interaction, but only on post-natal day 14
was there a significant response per individual day. All
doses of TBTO produced a significant decrease in activity
on that day. Post-weaning activity was reduced on post-
natal days 47 and 62 (p < 0.01) but only at a level of
10 mg/kg per day. There was no clear effect on acoustic
startle response.
In a two-generation reproduction study, TBTO was given
to rats at dietary concentrations of 0, 0.5, 5, and 50
mg/kg. The parental (F0) generation (30 males and 30
females per group) was exposed for 10 weeks before mating,
whereas the pre-mating treatment period for the F1 adults
(30 of each sex per group) was 15 weeks. Culling of
litters was performed on day 4 in the F1 and F2 gener-
ations. Preliminary data (Biodynamics, 1989b) on mor-
tality, body weight development, fertility indices, litter
data, and organ weights have been reported. There was no
evidence of compound-related mortality, and body weight
development was normal in the F0 generation. At 50 mg/kg
diet, the pup weights were decreased on days 14 and 21 in
the F1 generation and on days 7, 14, and 21 in the
F2 generation. Among the F1 parents, at 50 mg/kg, lower
body weights were noted in males throughout the pre-mating
period and in females during the first 3 weeks only. No
effects on mating, pregnancy, and fertility rates were
noted in either generation, and number of pups, litter
size, and pup survival were not affected by treatment in
either the F1 or the F2 generations. Relative and
absolute thymus weights were decreased in both sexes at
the dietary concentration of 50 mg/kg.
11.5.2. In vitro
Krowke et al. (1986) demonstrated an effect of TBTO on
the development of limb buds of mice in organ culture.
Clear-cut interference with differentiation was seen at
the lowest dose tested (0.03 mg/litre), while at 0.1 mg
per litre drastic impairment and abnormal development of
the paw skeleton were recorded. The effect was more pro-
nounced still at the highest dose tested (0.3 mg/litre).
In view of their failure to show effects on the embryo in
vivo, Davis et al. (1987) suggested that there is only
very limited movement of TBTO across the placenta of
mice.
11.6. Carcinogenicity
Summary
One carcinogenicity study on rats has been reported in
which neoplastic changes were observed in endocrine organs at
50 mg/kg diet. The pituitary tumours found at 0.5 mg/kg diet
are considered as having no biological significance since there
was no dose-response relationship. These tumour types appear
usually at high and variable background incidences. The signi-
ficance is, therefore, questionable. A second study on mice is
in progress.
Wester (in press) reported the results of a 106-week
study on carcinogenicty in Wistar rats at dietary TBTO
doses of 0, 0.5, 5, and 50 mg/kg (0, 0.025, 0.25, and
2.5 mg/kg body weight). At the highest dose level, general
toxicological effects were present (see section 11.3). The
incidence of benign tumours of the pituitary (mainly pro-
lactinomas) was elevated at 0.5 and 50 mg/kg, but not at
5 mg/kg diet, for both sexes. At 50 mg/kg, a significant
increase was noted in the incidence of adrenal medullary
tumours (pheochromocytomas) in both sexes and of parathy-
roid adenomas in male animals, while the incidence of
adrenal cortical tumours was significantly decreased at
0.5 and 50 mg/kg diet in males only. Isolated occurrence
of pancreatic carcinoma was found in treated female rats.
These were not considered to be compound related since
there was no dose dependency and the incidence rates were
low.
12. EFFECTS ON HUMANS
Summary
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The potential
problem is made worse by the lack of an immediate skin response.
12.1. Ingestion
There have been no reported cases of poisoning from
ingestion of TBTO or other TBT salts.
12.2. Inhalation
Seventy percent of the workers in a rubber factory
using TBTO in the vulcanizing process reported irritation
of the upper respiratory tract (and eyes). About 20% also
experienced lower chest symptoms (irritation, tightness,
and pain), but in all cases pulmonary function was unaf-
fected. The extent of the exposure was not recorded
(WHO/FAO, 1985).
12.3. Dermal exposure
Lyle (1958) described skin lesions in workers occu-
pationally exposed to dibutyl and tributyl tin compounds.
Skin burns were most commonly caused by small splashes of
liquid dibutyl or tributyl tin chlorides. Since the irri-
tancy of these compounds was not immediately apparent
(taking at least an hour to be perceived), small splashes
were frequently ignored by workers. More severe lesions on
the hands were caused by leaking gloves or failure to wear
hand protection. Some maintenance staff also suffered
burns after kneeling or rubbing against surfaces wet with
the chlorides. Clothes wet with these compounds produced
burns on the ventral skin and burns were seen immediately
above the level of protective boots on the calf of the
leg. Two kinds of lesion were seen in workers. The first
was an acute burn, which healed relatively quickly, and
the second a more diffuse dermatitis (seen in workers
wearing contaminated clothes in close contact with the
skin), which persisted. Treatment of the back of the hand
of volunteers with various undiluted TBT compounds estab-
lished that the chloride, acetate, laurate, and oxide all
produced acute burns. Burns were not caused by dibutyltin
esters or oxide or by tetrabutyltin, but were mostly due
to dibutyltin chloride or the tributyltin compounds. Red-
dening of the area treated was seen 2-3 h after treatment
with the compounds. Inflammation of the hair follicles
was the most obvious symptom, effects being confined to
the treated area. On the second day after treatment, min-
ute pustules appeared, which remained discrete and disap-
peared on the third or fourth day. After a week, all that
remained was a faint erythema. The burns were not reported
to be painful, either in the case of experimental or occu-
pational exposure. Sufferers complained of itching and
"stickiness" of the skin causing adherence of clothes.
Proper use of protective clothing, rapid washing of the
skin after exposure, and the use of aprons to prevent
wetting of overalls were found to be effective in pre-
venting burns, both acute and diffuse.
Baaijens (1987) described cases of accidental exposure
to TBTO during the manufacture of organotin compounds.
Severe dermatitis developed only where splashes of the
material had been retained on the skin for long periods.
In one case, a worker had been splashed over the face and
neck. He left the work area after the splash and showered.
An area behind one ear had not been washed and the derma-
titis had developed in this one area. There were no symp-
toms other than dermatitis. Another worker had been
splashed on the arm. He washed his skin but did not change
his overalls. Contact was extended and a large blister
developed on the arm. Monitoring of the urine tin content
showed no difference from normal in these two cases. A
third worker, who complained of an intense smell of TBTO,
suffered nausea and vomiting after 10 min of exposure.
Urine tin levels in this case were elevated for several
days. In all cases the symptoms disappeared within a few
days. The delayed irritancy of tributyltin was emphasized
by the author. It tends to lead to extended exposure since
the affected person does not perceive the effect for sev-
eral hours. The author found no relationship between
clinical or haematological parameters and normal occu-
pational exposure to organotin compounds.
Goh (1985) reported irritant effects and dermatitis in
painters applying TBTO formulations (0.6% TBTO in acrylic
resin-based paints) to buildings. The painters developed
rashes 8 to 10 h after exposure. When required to paint
ceilings, the men developed more severe symptoms on the
face, neck, and trunk, associated with dripping paint.
Severe itching, redness, swelling, and blistering were
recorded. Hospital examination showed extensive vesiculo-
bullous lesions, erythema, and oedema of the face, neck,
trunk, arms, and thighs. Although only two patients were
examined in detail, most of the workers on the site devel-
oped dermatitis. Patch testing of the two reported cases
showed erosion 48 and 96 h after patch tests using aqueous
solutions of TBTO (0.1 and 0.5 g/litre). Five other volun-
teer controls were patch-treated with solutions of 0.01
and 0.1 g/litre; these also showed a similar reaction to
the patients. No allergenic reaction was found in the
patch tests. Replacement of the paint with a preparation
having similar constituents other than TBTO prevented
further problems amongst the painters.
Lewis & Emmett (1987) describe contact dermatitis in a
shipwright resulting from exposure to TBTO-containing
antifouling paint. The man had been spray-painting blocks
of wood and his skin had been exposed to the spray. There
was no immediate sensation, but some irritation was evi-
dent within about an hour. Erythema and ulceration of the
exposed areas were noted on the second day. There were
also some mild pustular lesions on the mucous membrane of
the lips, presumed to be the result of wiping the mouth
with paint-contaminated arms. The authors conducted patch
testing with TBTO at aqueous concentrations of 0.1%,
0.01%, and 0.0015% using the A1 test tape method. At the
highest dose tested, there were large bullae after 48 h
and crusting after 96 h. The other two doses produced no
adverse effects. The delayed sensation and effect were
highlighted by the authors as being particular problems
with TBTO. Great care to prevent any exposure to products
containing TBTO at high concentrations was recommended.
Molin & Wahlberg (1975) investigated an outbreak of
dermatitis on the feet and ankles of trainee soldiers.
Seventy soldiers reported itching and sometimes painful
erythematous, vesiculous to bullous, and haemorrhagic
lesions after long marches on a hot day. Fifty other
soldiers reported less severe symptoms on another
occasion. The skin lesions disappeared within 1 or 2 weeks
in most cases after treatment with saline compresses and
topical steroid creams. In two cases, the skin was red and
slightly tender more than 6 months later. The outbreak was
traced to a single batch of socks that had been soaked in
7 times the recommended concentration of a disinfectant
solution containing TBTO. Recommended use of the disinfec-
tant was calculated to give about 0.001%, whereas the
concentration after the accidental over-use would have
been about 0.01%. Patch testing of eczema patients not
previously exposed to TBTO suggested that the primary
irritant concentration for the compound was between 0.1
and 0.01%. Soldiers patch-tested 2 months after their
dermatitis had fully healed gave negative results to both
0.001% and 0.01% TBTO. The authors suggested that the
effective concentration for TBTO as a disinfectant is too
close to the primary irritancy concentration to justify
the use of TBTO for disinfecting textiles.
Zedler (1961), on the basis of industrial experience,
stated that a concentration of 0.05% TBTO was not harmful
to the human skin.
12.4. Miscellaneous effects
Women using a latex spray paint containing TBTO as an
additive showed immediate irritant effects on the eye
(profuse lacrimation, eye inflammation) and the nasal
mucosa. The symptoms worsened over 14 days of spraying,
but subsided at the weekends and disappeared completely
when addition of TBTO to the paint was discontinued. The
extent of exposure in this case was not recorded (WHO/FAO,
1985).
Akatsuka et al. (1959) reported a case of occupational
poisoning with butyltin compounds where, along with symp-
toms of lassitude, slight occipital headaches, and stiff-
ness in the shoulders, there was a marked disturbance of
the sense of smell. The authors conducted studies on cats
to test whether this effect could be confirmed experimen-
tally. Exposure of the cats to a vapour mixture containing
mainly tributyltin bromide revealed a marked loss of the
sense of smell.
13. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
13.1. Evaluation of human health risks
Exposure of workers occurs principally during the
manufacture and formulation of tributyltin compounds, in
the application and removal of TBT paints, and from the
use of TBT in wood preservatives. Exposure of the general
public may come from the contamination of food, particu-
larly fish and shellfish, and from domestic application of
wood preservatives.
On the basis of both animal tests and direct obser-
vations on humans, it is clear that TBT compounds are
irritant to the skin and eyes and that inhalation of aero-
sols leads to respiratory irritation.
The handling of treated wood poses no dermal irritant
hazard once the wood has dried. However, aerosols of TBT
are very hazardous and re-entry to the treatment area
should be prohibited until the wood has thoroughly dried.
Acute systemic poisoning has never been reported and
clearance of TBT from the body is expected to occur within
a few days. Acute toxicity from handling TBT products is,
therefore, unlikely if proper precautions are taken.
Short- and long-term effects on experimental animals
have been reported in the liver and haematological and
endocrine systems. The effects of TBT compounds on the
immune system, and particularly on host resistance, have
proved the most sensitive parameter of toxicity in the
rat, the most sensitive species tested. The no-observed-
effect level (NOEL), using the Trichinella spiralis host-
resistance model, lies between 0.5 and 5.0 mg/kg diet
(0.025 and 0.25 mg/kg body weight), whereas using measures
of immune function it is 0.6 mg/kg body weight.
Owing to wide variation in the consumption of fish and
shellfish and local differences in residues of TBT in sea-
food, only illustrative estimates relating exposure and
NOEL values can be made. It needs to be emphasized that
local measurements of residues, local estimates of sea-
food consumption, and local decisions on acceptable safety
margins must be made to assess potential risk of these
compounds.
Using fish consumption figures of 15 and 150 g/day, a
value of 1 mg/kg for residues in fish, and an average
human body weight of 60 kg, the following safety margins
based on different immune endpoints are obtained.
---------------------------------------------------------
Fish Estimated Safety margin
consumption daily intake T. spiralis Other
(g/day) of TBT model immune
(µg/kg) parameters
---------------------------------------------------------
15 0.25 100-1000 2500
150 2.5 10-100 250
---------------------------------------------------------
Indiscriminate and irresponsible use of TBT compounds
and a failure to follow the recommendations, outlined in
this monograph, to reduce exposure of humans could lead to
intake of levels of TBT compounds hazardous to human
health.
Teratogenic effects have only occurred in experimental
animals at doses that caused overt maternal toxicity. The
teratogenic potential of TBT is, therefore, considered to
be very low.
Based on the results of comprehensive mutagenicity
studies, tributyltin compounds are not considered to have
mutagenic potential. In a carcinogenicity study on rats
with TBTO, an increased incidence was noted for endocrine
tumours that occur spontaneously at a high and variable
incidence. Therefore, the available evidence does not
clearly demonstrate a carcinogenic hazard of TBT compounds
for humans.
13.2. Evaluation of effects on the environment
Diffuse input of tributyltin (TBT) into the environ-
ment occurs predominantly from the use of TBT in antifoul-
ing paint. It could also occur if it were used as a mol-
luscicide. Point source contamination occurs if TBT is
used as a biocide in cooling systems, wood pulping,
leather processing, wood preservation processes, and tex-
tile treatment.
Due to their physico-chemical properties, TBT com-
pounds concentrate in the surface microlayer and in sedi-
ments. Abiotic degradation does not appear to be a major
mechanism of removal under environmental conditions.
Although TBTO is biodegradable in the water column, this
process is not rapid enough to prevent the occurrence of
elevated TBT levels in some areas. Bioaccumulation occurs
in most aquatic organisms, but in laboratory mammals,
metabolic degradation is a more efficient process.
TBT is extremely hazardous to some aquatic organisms
because it is toxic at very low concentrations in water.
Such concentrations have been found in some areas. Adverse
effects on non-target invertebrates, particularly mol-
luscs, have been reported in field studies, and these have
been sufficiently severe to lead to reproductive failure
and population decline. Adverse effects on the commercial
production of shellfish have been successfully reversed by
restrictions on the use of antifouling paints in some
areas, and these restrictions are also leading to the
reversal of imposex effects in gastropod populations. The
effects on farmed fish indicate that TBT-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is
likely to be low. TBT-treated wood could pose a hazard to
terrestrial organisms living in close contact with it.
The enhancement of TBT concentrations in the surface
microlayer may present a hazard to littoral organisms,
neustonic species (including benthic invertebrate and fish
larvae) and surface-feeding sea-birds and wildfowl.
Accumulation and low biodegradation of TBT in sediments
may present a hazard to aquatic organisms when these pol-
luted sediments are disturbed by natural processes or
dredging activities.
14. RECOMMENDATIONS
14.1. Recommendations for protecting human and environmental health
a) Member countries that have not yet regulated the use
of TBT compounds should be encouraged to do so.
b) There is a need for evaluation and, if necessary,
regulation of organotin input to the environment from
sources other than antifouling paints. For example,
this would include evaluation of the potential risk
from the application of TBT-contaminated sewage sludge
to soil.
c) Improved methods for the safe application, removal,
and disposal of organotin paints should be developed.
14.2. Research needs
a) Methods of detection and analysis need to be improved
to provide rapid and accurate measurements of butyltin
species in pg/litre concentrations. One reason for
this recommendation is that a biological effect, i.e.
imposex in gastropods, may occur at levels lower than
present detection limits.
b) There is a need for research into mechanisms which
concentrate rather than disperse TBT and which retard
degradation, with particular attention to the funda-
mental chemistry of TBT and its interaction with bio-
logical molecules. More study is needed on the uptake
of TBT at all trophic levels.
c) A study of the toxicity of TBT in aquatic organisms is
required. This work should investigate metabolism,
endocrine effects, and immunological toxicity, where
appropriate.
d) A search for other sensitive bioindicator species in
other groups, including freshwater species, is
required.
e) Models for the assessment of immunotoxicity in mammals
need to be validated and no-effect levels for relevant
parameters need to be defined more accurately.
f) A long-term toxicity study in a second mammalian
species should be undertaken.
g) A tumorigenicity study in a second mammalian species
should be undertaken.
h) Information on butyltin residue levels in fish and
shellfish for human consumption using speciating
methods is needed.
REFERENCES
ABEL, R., HATHAWAY, R.A., KING, N.J., VOSSER, J.L., & WILKINSON, T.G.
(1987) Assessment and regulatory actions for TBT in the UK. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1314-1319.
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A., KAWAME,
N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S., YANAGIBASHI, H.,
NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA, I., & IYO, T. (1959)
[Experimental studies on disturbance of sense of smell due to butyltin
compounds.] J. Tokyo med. Coll., 17: 1393-1402 (in Japanese).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int. Pest Control,
11: 29-35.
ALDRIDGE, W.N. (1958) The biochemistry of organotin compounds. Trialkyltins
and oxidation phosphorylation. Biochem. J., 69: 367-376.
ALDRIDGE, W.N. & STREET, B.W. (1964) Oxidative phosphorylation: Biochemical
effects and properties of trialkyltins. Biochem. J., 91: 287-297.
ALDRIDGE, W.N. & STREET, B.W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver mitochondria.
Biochem. J., 118: 171-179.
ALDRIDGE, W.N. & STREET, B.W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and triethyltin to
mitochondria and their effects on various mitochondrial functions. Biochem.
J., 124: 221-234.
ALDRIDGE, W.N., CASIDA, J.E., FISH, R.H., KIMMEL, E.C., & STREET, B.W.
(1977) Action on mitochondria and toxicity of metabolites of tri-n-butyltin
derivatives. Biochem. Pharmacol., 26: 1997-2000.
ALLEN, A.J., QUITTER, B.M., & RADICK, C.M. (1980) The biocidal mechanism of
controlled release bis (tri-n-butyltin) oxide in Biomphalaria glabrata. In:
Baker, R., ed. Controlled release of bioactive materials, New York, London,
Academic Press, p. 399.
ALZIEU, C.P. (1981) Evaluation des risques dus à l'emploi des peintures
anti-salissures dans les zones conchylicoles, Nantes, Institut scientifique
et technique des Pêches maritimes, 84 pp.
ALZIEU, C. & HERAL, M. (1984) Ecotoxicological effects of organotin
compounds on oyster culture. Ecotoxicol. Test. mar. Environ., 2: 187-196.
ALZIEU, C. & PORTMANN, J.E. (1984) The effect of tributyl tin on the
culture of C. gigas. Proceedings of the 15th Annual Shellfish Conference,
15-16 May, 1984, London, The Shellfish Association of Great Britain, 17 pp.
ALZIEU, C., HERAL, M., THIBAUD, Y., DARDIGNAC, M.-J., & FEUILLET, M. (1982)
Influence des peintures antisalissures à base d'organostanniques sur la
calcification de la coquille de l'huitre Crassostrea gigas. Rev. Trav.
Inst. Pêches Marit., 45: 101-116.
ALZIEU, C., SANJUAN, J., DELTREIL, J.P., & BOREL, M. (1986) Tin
contamination in Arcachon Bay: Effects on oyster shell anomalies. Mar.
Pollut. Bull., 17: 494-498.
ALZIEU, C., SANJUAN, J., MICHEL, P., BOREL, M., & DRENO, J.P. (1989)
Monitoring and assessment of butyltins in Atlantic coastal waters. Mar.
Pollut. Bull., 20: 22-26.
ANGER, J.P., ANGER, F., CANO, Y., CHAUVEL, Y., LOUVET, M., & VAN DEN
DRIESSCHE, J. (1976) Effets chez le cobaye, d'un aérosol à base d'oxyde de
tributylétain (OTBE). Eur. J. Toxicol., 9: 339-346.
ARAKAWA, Y. & WADA, O. (1984) Inhibition of neutrophil chemotaxis by
organotin compounds. Biochem. biophys. Res. Commun., 123: 543-548.
ARGAMAN, Y., HUCKS, C.E., & SHELBY, S.E. (1984) The effects of organotin on
the activated sludge process. Water Res., 18: 535-542.
AYALA, C.A.C., TOLEDO, J.V., DA SILVA NETTO, J.A., & GILBERT, B. (1980) The
effect of hexabutyldistannoxane (TBTO) and other molluscicides on non-
target species, Geneva, World Health Organization, Parasitic Diseases
Programme (Unpublished report).
BAAIJENS, P.A. (1987) Health effect screening and biological monitoring for
workers in organotin industries. In: Toxicology and analytics of the
tributyltins: The present status. Proceedings of an ORTEPA workshop,
Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands, ORTEP-
Association, pp. 191-208
BACCI, E. & GAGGI, C. (1989) Organotin compounds in harbour and marina
waters from the Northern Tyrrhenian Sea. Mar. Pollut. Bull., 20: 290-292.
BAHR, G. & PAWLENKO, S. (1978) Organic tin compounds. In: Bahr, G.,
Kalinowski, H.-O., & Pawlenko, S., ed. Organometallic compounds, germanium,
tin, Stuttgart, Georg Thieme Verlag, pp. 512-515 (Methods in Organic
Chemistry series).
BAILEY, S.K. & DAVIES, I.M. (1988a) Tributyltin contamination around an oil
terminal in Sullom Voe (Shetland). Environ. Pollut., 55: 161-172.
BAILEY, S.K. & DAVIES, I.M. (1988b) Tributyltin contamination in the Firth
of Forth (1975-87). Sci. total Environ., 76: 185-192.
BAKER, J.M. & TAYLOR, J.M. (1967) The toxicity of tributyltin oxide to the
wood-boring beetles Lyctus brunneus Steph. and Anobium punctatum (Deg.).
Ann. appl. Biol., 60: 181-190.
BALLS, P.W. (1987) Tributyltin (TBT) in the waters of a Scottish sea loch
arising from the use of antifoulant treated netting by salmon farms.
Aquaculture, 65: 227-237.
BARNES, J.M. & STONER, H.B. (1958) Toxic properties of some dialkyl and
trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARUG, D. (1981) Microbial degradation of bis (tributyltin) oxide.
Chemosphere, 10: 1145-1154.
BARUG, D. & VONK, J.W. (1980) Studies on the degradation of bis
(tributyltin) oxide in soil. Pestic. Sci., 11: 77-82.
BEAUMONT, A.R. & BUDD, M.D. (1984) High mortality of the larvae of the
common mussel at low concentrations of tributyltin. Mar. Pollut. Bull., 15:
402-405.
BEAUMONT, A.R. & NEWMAN, P.B. (1986) Low levels of tributyltin reduce
growth of marine micro-algae. Mar. Pollut. Bull., 17: 457-461.
BEAUMONT, A.R., NEWMAN, P.B., & WALDOCK, M.J. (1987) Sand substrate
microcosm studies on tributyl tin (TBT) toxicity to marine organisms, 24 pp
(Final report to the UK Department of the Environment, London. Contract No.
PECD 7/8/73).
BERRIOS-DURAN, L.A. & RITCHIE, L.S. (1968) Molluscicidal activity of
bis(tri-n-butyltin) oxide formulated in rubber. World Health Organ. Bull.,
39: 310-312.
BIODYNAMICS (1989a) A three month oral range-finding toxicity study in mice
with bis (tri-n-butyltin) oxide (TBTO), East Millstone, New Jersey,
Biodynamics (Final report to Aceto Chemical Corporation, M. + T. Chemicals
Inc., and Schering Berlin Inc.) (Unpublished).
BIODYNAMICS (1989b) A two-generation reproduction study in rats with TBTO,
East Millstone, New Jersey, Biodynamics (Interim report to Aceto Chemical
Corporation, M. + T. Chemicals Inc., and Schering Berlin Inc.)
(Unpublished).
BJORKLUND, I. (1987a) [Environmental aspects of ship's bottom paints],
Stockholm, Swedish Chemicals Inspectorate, Investigation Department, 16 pp
(in Swedish).
BJORKLUND, I. (1987b) [Environmental effect of antifouling paints],
Stockholm, Swedish National Environment Protection Board, 17 pp.
BLAIR, W.R., OLSON, G.J., BRINCKMAN, F.E., & IVERSON, W.P. (1982)
Accumulation and fate of tri-n-butyltin cation in estuarine bacteria.
Microbiol. Ecol., 8: 241-251.
BLAIR, W.R., OLSON, G.J., & BRINCKMAN, F.E. (1986) Speciation measurements
of butyltins: Application to controlled release rate determination and
production of reference standards. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1141-1145.
BLUNDEN, S.J. & CHAPMAN, A. (1986) Organotin compounds in the environment.
In: Craigh, P.J., ed. Organometallic compounds in the environment, Harlow,
Essex, Longman Group Ltd, pp. 111-159.
BLUNDEN, S.J., HOBBS, L.A., & SMITH, P.J. (1984) The environmental
chemistry of organotin compounds. In: Bowen, H.J.M, Blunden, S.J., Colbeck,
I., Harrison, R.M., Hobbs, L.A., Katz, S.A., Simkiss, K., Smith, P.J., &
Taylor, M.G., ed. Environmental chemistry, London, Royal Society of
Chemistry, Vol. 3, pp. 49-77.
BOKRANZ, A. & PLUM, H. (1975) Industrial manufacture and use of organotin
compounds, Bergkamen, Federal Republic of Germany, Schering AG, 33 pp.
BOORMAN, L.A. (1989) The effects of TBT on two salt marsh plant species,
Aster tripolium and Limonium vulgare. In Preparation.
BOULDIN, T.W., GOINES, N.D., BAGNELL, C.R., & KRIGMAN, M.R. (1981)
Pathogenesis of trimethyltin neuronal toxicity. Ultrastructural and
cytochemical observations. Am. J. Pathol., 104: 237-249.
BRAMAN, R.S. & TOMPKINS, M.A. (1979) Separation and determination of
nanogram amounts of inorganic and methyltin compounds in the environment.
Anal. Chem., 51: 12-19.
BRESSA, G., CIMA, L., CANOVA, F., & CARAVELLO, G.U. (1984) Bioaccumulation
of tin in the fish tissues (Liza aurata). In: Proceedings of the
International Conference on Environmental Contamination, London, July 1984
- Geneva, International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp. 812-815.
BRIDGES, J.W., DAVIES, D.S., & WILLIAMS, R.T. (1967) The fate of ethyltin
and diethyltin derivatives in the rat. Biochem. J., 105: 1261-1266.
BRINCKMAN, F.E. (1981) Environmental organotin chemistry today: Experiences
in the field and laboratory. J. organomet. Chem. Libr., 12: 343-376.
BRINCKMAN, F.E., JACKSON, J.A., BLAIR, W.R., OLSON, G.J., & IVERSON, W.P.
(1983) Ultratrace speciation and biogenesis of methyltin transport species
in estuarine waters. In: Trace metals in sea water, New York, Plenum Press,
pp. 39-72.
BROWN, R.A., NAZARIO, C.M., DE TIRADO, R.S., CASTRILLON, J., & AGARD, E.T.
(1977) A comparison of the half-life of inorganic and organic tin in the
mouse. Environ. Res., 13: 56-61.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1986) The
decline of the gastropod Nucella lapillus around south-west England:
Evidence for the effect of tributyltin from antifouling paints. J. Mar.
Biol. Assoc. UK, 66: 611-640.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1987) Copper,
zinc, and organotin as long-term factors governing the distribution of
organisms in the Fal estuary in southwest England. Estuaries, 10: 208-219.
BRYAN, G.W., GIBBS, P.E., & BURT, G.R. (1988) A comparison of the
effectiveness of tri-n-butyltin chloride and five other organotin compounds
in promoting the development of imposex in the dog-whelk Nucella lapillus.
J. Mar. Biol. Assoc. UK, 68: 733-744.
BUSHONG, S.J., HALL, W.S., JOHNSON, W.E., & HALL, L.W. (1987) Toxicity of
tributyltin to selected Chesapeake Bay biota. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1494-1503.
BUSHONG, S.J., HALL, L.W., HALL, W.S., JOHNSON, W.E., & HERMAN, R.L. (1988)
Acute toxicity of tributyltin to selected Chesapeake Bay fish and
invertebrates. Water Res., 22: 1027-1032.
CALLEY, D.J., GUESS, W.L., & AUTIAN, J. (1967) Hepatotoxicity of a series
of organotin esters. J. pharm. Sci., 56: 240-243.
CARDARELLI, N.F. (1973) Effects of ultralow molluscicide dosages on
Biomphalaria glabrata and Lebistes reticulatus in micro ecological systems:
Report I, Akron, Ohio, University of Akron, 15 pp.
CARDARELLI, N.F. (1978) Controlled release organotins as mosquito
larvicides. Mosq. News, 38: 328-333.
CARDARELLI, N.F. & EVANS, W. (1980) Chemodynamics and environmental
toxicology of controlled release organotin molluscicides. In: Baker, R.W.,
ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the Controlled Release Society, New York, London,
Academic Press, pp. 357-385.
CASIDA, J.E., KIMMEL, E.C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and trialkyleads by
liver microsomes. Acta chem. Scand., 25: 1497-1499.
CHAMP, M.A. & PUGH, W.L. (1987) Tributyltin antifouling paints:
Introduction and overview. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1296-1308.
CHAPMAN, A.H. & PRICE, J.W. (1972) Degradation of triphenyltin acetate by
ultraviolet light. Int. Pest Control, 14: 11-12.
CHENG, Z. & JENSEN, A. (1989) Accumulation of organic and inorganic tin in
blue mussel, Mytilus edulis, under natural conditions. Mar. Pollut. Bull.,
20: 281-286.
CHLIAMOVITCH, Y.-P. & KUHN, C. (1977) Behavioural, haematological and
histological studies on acute toxicity of bis(tri- n-butyltin) oxide on
Salmo gairdneri Richardson and Tilapia rendalli Boulenger. J. Fish Biol.,
10: 575-585.
CHU, K.Y. (1976) Effects of environmental factors on the molluscicidal
activities of slow-release hexabutyldistannoxane and copper sulfate. Bull.
World Health Organ., 54: 417-420.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & LORES, E.M. (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass shrimp
(Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Arch.
environ. Contam. Toxicol., 16: 401-407.
CLEARY, J.J. & STEBBING, A.R.D. (1985) Organotin and total tin in coastal
waters of southwest England. Mar. Pollut. Bull., 16: 350-355.
CLEARY, J.J. & STEBBING, A.R.D. (1987) Organotin in the surface microlayer
and subsurface waters of Southwest England. Mar. Pollut. Bull., 18: 238-
246.
CREMER, J.E. (1957) The metabolism in vitro of tissue slices from rats
given triethyltin compounds. Biochem. J., 67: 87-96.
CROFTON, K.M., DEAN, K.F., BONCEK, V.M., ROSEN, M.B., SHEETS, L.P.,
CHERNOFF, N., & REITER, L.W. (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: Postnatal evaluation of teratology and
behavior. Toxicol. appl. Pharmacol., 97: 113-123.
DAVIDSON, B.M., VALKIRS, A.O., & SELIGMAN, P.F. (1986) Acute and chronic
effects of tributyltin on the mysid Acanthomysis sculpta (Crustacea,
Mysidacea). In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., pp. 1219-1225.
DAVIES, R.J., FLETCHER, R.L., & FURTADO, S.E.J. (1984) The effects of
tributyltin compounds on spore development in the green alga Enteromorpha
intestinalis (L) Link. In: Proceedings of the 6th International Congress on
Marine Corrosion and Fouling, Athens, September 1984, pp. 557-565.
DAVIES, I.M., DRINKWATER, J., MCKIE, J.C., & BALLS, P. (1987a) Effects of
the use of tributyltin antifoulants in mariculture. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., pp. 1477-1481.
DAVIES, I.M., BAILEY, S.K., & MOORE, D.C. (1987b) Tributyltin in Scottish
sea Lochs, as indicated by degree of imposex in the dogwhelk, Nucella
lapillus (L.). Mar. Pollut. Bull., 18: 400-404.
DAVIS, A., BARALE, R., BRUN, G., FORSTER, R., GUNTHER, T., HAUTEFEUILLE,
H., VAN DER HEIJDEN, C.A., KNAAP, A.G.A.C., KROWKE, R., KUROKI, T.,
LOPRIENO, N., MALAVEILLE, C., MERKER, H.J., MONACO, M., MOSESSO, P.,
NEUBERT, D., NORPPA, H., SORSA, M., VOGEL, E., VOOGD, C.E., UMEDA, M., &
BARTSCH, H. (1987) Evaluation of the genetic and embryotoxic effects of
bis(tri- n-butyltin) oxide (TBTO), a broad-spectrum pesticide, in multiple
in vivo and in vitro short-term tests. Mutat. Res., 188: 65-95.
DESCHIENS, R. & FLOCH, H. (1962) Les propriétés molluscicides du chlorure
et de l'acétate de triphénylétain dans le cadre de la prophylaxie des
bilharzioses. C. R. Acad. Sci. Paris, 255: 1236-1237.
DESCHIENS, R. & FLOCH, H. (1968) Action biologique comparée de 6
molluscicides chimique dans le cadre de la prophylaxie des bilharzioses.
Conclusions pratiques. Bull. Soc. Pathol. Exot., 61: 640-650.
DESCHIENS, R., BROTTES, H., & MVOGO, L. (1966) Application sur le terrain,
au Cameroun, dans la prophylaxie des bilharzioses de l'action molluscicide
de l'oxyde de tributylétain. Bull. Soc. Pathol. Exot., 59: 968-973.
DE VILLIERS, J.P. & MACKENZIE, J.G. (1963) Organotin and organolead
molluscicides, Geneva, World Health Organization, Parasitic Diseases
Programme, p. 63 (Unpublished report Mol/Inf/13).
DIXON, D.R. & MCFADZEN, I. (1987) Bis(tributyltin) oxide (TBTO), an
antifouling compound, promotes SCE induction in the larvae of the common
mussel, Mytilus edulis. Mutagenesis, 2: 312.
DIXON, D.R. & PROSSER, H. (1986) An investigation of the genotoxic effects
of an organotin antifouling compound (bis(tributyltin) oxide) on the
chromosomes of the edible mussel, Mytilus edulis. Aquat. Toxicol., 8: 185-
195.
DOJMI DI DELUPIS, G., GUCCI, P.M.B., & VOLTERRA, L. (1987) Toxic effects of
bis-tributyltinoxide on phytoplancton. Main Group Metal Chem., 10: 77-82.
DONARD, O., RAPSOMANIKIS, S., & WEBER, J.H. (1986) Speciation of inorganic
tin and alkyltin compounds by atomic absorption spectrometry using
electrothermal quartz furnace after hydride generation. Anal. Chem., 58:
772-777.
EAJ (1988) Outline of TBT compounds monitored in Japan, Tokyo, Environment
Agency of Japan (OECD Clearing House Project on Organotins).
EBDON, L., EVANS, K., & HILL, S. (1988) The variation of tributyltin levels
with time in selected estuaries prior to the introduction of regulations
governing the use of tributyltin-based anti-fouling paints. Sci. total
Environ., 68: 207-223.
EBDON, L., EVANS, K., & HILL, S. (1989) The accumulation of organotins in
adult and seed oysters from selected estuaries prior to the introduction of
U.K. regulations governing the use of tributyltin-based antifouling paints.
Sci. total Environ., 83: 63-84.
ELFERINK, J.G.R., DEIERKAUF, M., & VAN STEVENINCK, J. (1986) Toxicity of
organotin compounds for polymorphonuclear leukocytes: the effect on
phagocyctosis and exocytosis. Biochem. Pharmacol., 35: 3727-3732.
ELSEA, J.R. & PAYNTER, O.E. (1958) Toxicological studies on bis(tri-n-
butyltin) oxide. Am. Med. Assoc. Arch. Ind. Health, 18: 214-217.
EVANS, C.J. & KARPEL, S. (1985) Organotin compounds in modern technology.
J. organomet. Chem. Libr., 16: 178-217.
EVANS, D.W & LAUGHLIN, R.B. (1984) Accumulation of bis (tributyltin) oxide
by the mud crab, Rhitropanopeus harrisii. Chemosphere, 13: 213-219.
EVANS, W.H., CARDARELLI, N.F., & SMITH, D.J. (1979) Accumulation and
excretion of [1-14C] bis (tri- n-butyltin) oxide in mice. J. Toxicol.
environ. Health, 5: 871-877.
FENT, K. (1989a) Organotin speciation in municipal wastewater and sewage
slude: Ecotoxicological consequences. Mar. environ. Res., 28: 477-483.
FENT, K. (1989b) Teratogenic effects of tributyltin on embryos of the
freshwater fish Phoxinus phoxinus. Presented at the OECD Workshop on
Tributyltin: Activities related to Field Studies at Sea, Centre IFREMER,
Nantes, 27-29 June, 1989 (Unpublished report).
FENT, K., FASSBIND, R., & SIEGRIST, H. (1989) Organotins in a municipal
wastewater treatment plant. Proceedings of the 1st European Conference on
Ecotoxicology, Copenhagen, Denmark, 17-19 October, 1988, Lyngby, Denmark,
The Technical University of Denmark, Laboratory of Environmental Sciences
and Ecology.
FERAL, C. & LE GALL, S. (1983) The influence of a pollutant factor
(tributyltin) on the neuroendocrine mechanism responsible for the
occurrence of a penis in the females of Ocenebra erinacea. In: Lever, J. &
Boer, H.H., ed. Molluscan neuroendocrinology. Proceedings of the
International Minisymposium on Molluscan Endocrinology, Amsterdam, North-
Holland Publishing Company, pp. 173-175.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1975) Bioorganotin chemistry:
Biological oxidation of tributyltin derivatives. J. organomet. Chem., 93:
C1-C4.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1976) Bioorganotin chemistry:
Biological oxidation of organotin compounds. In: Zuckerman, J.J., ed.
Organotin compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 197-203 (Advances in Chemistry Series No.
157).
FLOCH, H., DESCHIENS, R., & FLOCH, T. (1964) Sur les propriétés
molluscicides de l'oxyde et de l'acétate de tributylétain (prophylaxie des
bilharzioses). Bull. Soc. Pathol. Exot., 57: 454-465.
FOSTER, R.B. (1981) Use of Asiatic clam larvae in aquatic hazard
evaluations. In: Bates, J.M. & Weber, C.I., ed. Ecological assessments of
effluent impacts on communities of indigenous aquatic organisms,
Philadelphia, American Society for Testing and Materials, pp. 280-288 (ASTM
STP No. 730).
FUNAHASHI, N., IWASAKI, I., & IDE, G. (1980) Effects of bis(tri-n-butyltin)
oxide on endocrine and lymphoid organs of male rats. Acta pathol. Jpn., 30:
955-966.
GARDINER, B.G. & POLLER, R.C. (1964) Insecticidal activity and structure of
some organotin compounds. Bull. entomol. Res., 55: 17-21.
GIBBS, P.E. & BRYAN, G.W. (1986) Reproductive failure in populations of the
dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from
antifouling paints. J. Mar. Biol. Assoc. UK, 66: 767-777.
GIBBS, P.E. & BRYAN, G.W. (1987) TBT paints and the demise of the dog-
whelk, Nucella lapillus (Gastropoda). In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1482-1487.
GIBBS, P.E., BRYAN, G.W., PASCOE, P.L., & BURT, G.R. (1987) The use of the
dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT)
contamination. J. Mar. Biol. Assoc. UK, 67: 507-523.
GIBBS, P.E., PASCOE, P.L., & BURT, G.R. (1988) Sex change in the female
dogwhelk, Nucella lapillus, induced by tributyltin from antifouling paints.
J. Mar. Biol. Assoc. UK, 68: 715-731.
GILBERT, B., PAES LEME, L.A., FERREIRA, A.M., BULHOES, M.S., & CASTLETON,
C. (1973) Field tests of hexabutyldistannoxane (TBTO) in slow-release
formulations against Biomphalaria spp. Bull. World Health Organ., 49: 633-
636.
GILE, J.D., COLLINS, J.C., & GILLETT, J.W. (1982) Fate and impact of wood
preservatives in a terrestrial microcosm. J. agric. food Chem., 30: 295-
301.
GILLETT, J.W., GILE, J.D., & RUSSELL, L.K. (1983) Predator-prey (vole-
cricket) interactions: the effects of wood preservatives. Environ. Toxicol.
Chem., 2: 83-93.
GOH, C.L. (1985) Irritant dermatitis from tri-n-butyl tin oxide in paint.
Contact dermatitis, 12: 161-163.
GOHLKE, R., LEWA, W., STRACHOVSKY, A., & KOHLER, R. (1969) [Investi-gations
in experimental animals of the inhalatory effects of tributyltin chloride
in a subchronic experiment.] Z. gesamte Hyg. Grenzgeb., 15: 97-104 (in
German).
GOODMAN, L.R., CRIPE, G.M., MOODY, P.H., & HALSELL, D.G. (1988) Acute
toxicity of malathion, tetrabromobisphenol-A, and tributyltin chloride to
mysids (Mysidopsis bahia) of three ages. Bull. environ. Contam. Toxicol.,
41: 746-753.
GROVHOUG, J.G., SELIGMAN, P.F., VAFA, G., & FRANSHAM, R.L. (1986) Baseline
measurements of butyltin in U.S. harbors and estuaries. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1283-1288.
GUARD, H.E., COGBET, A.B., & COLEMAN, W.M. (1981) Methylation of
trimethyltin compounds by estuarine sediments. Science, 213: 770-771.
HADA, N. (1986) [Studies on the kinetics of tributyltin compounds in the
body: With special reference to their biological half times in goldfish and
rats as estimated by newly developed analytical method.] Nichidai Igaku
Zasshi, 45: 1005-1013 (in Japanese).
HALL, L.W., PINKNEY, A.E., ZEGER, S., BURTON, D.T., & LENKEVICH, M.J.
(1984) Behavioral responses to two estuarine fish species subjected to bis
(tri- n-butyltin) oxide. Water Resour. Bull., 20: 235-239.
HALL, L.W., LENKEVICH, M.J., HALL, W.S., PINKNEY, A.E., & BUSHONG, S.J.
(1986) Monitoring organotin concentrations in Maryland waters of Chesapeake
Bay. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1275-1279.
HALL, L.W., BUSHONG, S.J., JOHNSON, W.E., & HALL, W.S. (1988a) Spacial and
temporal distribution of butyltin compounds in a Northern Chesapeake Bay
marina and river system. Environ. Monit. Assess., 10: 229-244.
HALL, L.W., BUSHONG, S.J., HALL, W.S., & JOHNSON, W.E. (1988b) Acute and
chronic effects of tributyltin on a Chesapeake Bay copepod. Environ.
Toxicol. Chem., 7: 41-46.
HALLAS, L.E., MEANS, J.C., & COONEY, J.J. (1982) Methylation of tin by
estuarine microorganisms. Science, 215: 1505-1507.
HARRIS, J.R.W. & CLEARY, J.J. (1987) Particle-water partitioning and
organotin dispersal in an estuary. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1370-1374.
HENDERSON, R.S. (1986) Effects of organotin antifouling paint leachates on
Pearl Harbor organisms: a site specific flowthrough bioassay. In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1226-1233.
HENSHAW, B.G., LAIDLAW, R.A., ORSLER, R.J., CAREY, J.K., & SAVORY, J.G.
(1978) The permanence of tributyltin oxide in timber. In: Record of the
1978 Annual Convention of the British Wood Preserving Association,
Cambridge, 27-30 June, 1978, London, British Wood Preserving Association,
pp. 19-29.
HINGA, K.R., ADELMAN, D., & PILSON, M.E.Q. (1987) Radiolabeled butyl tin
studies in the Merl enclosed ecosystems. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1416-1420.
HIS, E. & ROBERT, R. (1980) Action d'un sel organo-métallique, l'acétate de
tributylétain sur les oeufs et les larves de Crassostrea gigas (Thunberg),
Copenhagen, International Council for the Exploration of the Sea (ICES)
Mariculture Commission, 27 pp (CM 1980/F).
HIS, E. & ROBERT, R. (1985) Développement des véligères de Crassostrea
gigas dans le Bassin d'Arcachon, études sur les mortalités larvaires. Rev.
Trav. Inst. Pêches Marit., 47: 63-88.
HIS, E., MAURER, D., & ROBERT, R. (1986) Observations complémentaires sur
les causes possibles des anomalies de la reproduction de Crassostrea gigas
(Thunberg) dans le Basin d'Arcachon. Rev. Trav. Inst. Pêches Marit., 48:
45-54.
HODGE, V.F., SEIDEL, S.L., & GOLDBERG, E.D. (1979) Determination of tin
(IV) and organotin compounds in natural waters, coastal sediments, and
macro algae by atomic absorption spectrometry. Anal. Chem., 51: 1256-1259.
HOPF, H.S., DUNCAN, J., BEESLEY, J.S.S., WEBLEY, D.J., & STURROCK, R.F.
(1967) Molluscicidal properties of organotin and organolead compounds.
World Health Organ. Bull., 36: 955-961.
HUGGETT, R.J., UNGER, M.A., & WESTBROOK, D.J. (1986) Organotin
concentrations in the southern Chesapeake Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1262-1265.
HUMPEL, M., KUHNE, G., TAUBER, U., & SCHULZE, P.E. (1986) Studies on the
kinetics of bis (tris- n -butyl-113tin) oxide (TBTO). In: Toxicology and
analytics of the tributyltins: The present status. Proceedings of an ORTEPA
workshop, Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands,
ORTEP-Association, pp. 122-142.
HUMPHREY, B. & HOPE, D. (1987) Analysis of water, sediments and biota for
organotin compounds. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1348-1351.
ICES (1987) Concentrations of organotin and total tin in open Danish sea
areas and in pleasure craft marinas along the sound, International Council
for the Exploration of the Sea (ICES) (TWG 14/9/3-E).
IWAI, H., MANABE, M., MATSUI, H., ONO, T., & WADA, O. (1980) Effects of
tributyltin and its metabolites on brain function. J. toxicol. Sci., 5:
257.
IWASAKI, I., FUNABASHI, N., TOIZUMI, S., & IDE, G. (1976) Histopathological
studies on rat's nervous system by oral administration of bis-(tri-n-
butyltin) oxide. (I). J. toxicol. Sci., 1: 99 (abstract).
JENSEN, A. & CHENG, Z. (1987) Total tin and organotin in seawater from
pleasure craft marinas along Danish coast of the Sound. In: Proceedings of
the 15th Conference of Baltic Oceanographers (CBO), Copenhagen, 1986,
Charlottenlung, Denmark, Marine Pollution Laboratory, Vol. 1, pp. 289-298.
JEWETT, K.L. & BRINCKMAN, F.E. (1981) Speciation of trace di- and
triorganotins in water by ion exchange HPLC-GFAA. J. chromatogr. Sci., 19:
583.
JOHANSEN, K. & MOHLENBERG, F. (1987) Impairment of egg production in
Acartia tonsa exposed to tributyltin oxide. Ophelia, 27: 137-141.
JOHNSON, T.L. & KNOWLES, C.O. (1983) Effects of organotins on rat
platelets. Toxicology, 29: 39-48.
JORDAN, P. (1985) Schistosomiasis - The St Lucia Project, Cambridge,
Cambridge University Press, 442 pp.
KAKUNO, A. & KIMURA, S. (1987) [Acute toxicity of bis (tributyltin) oxide
to girella (Girella punctata).] Bull. Tokai Reg. Fish. Res. Lab., 123: 41-
44 (in Japanese).
KALBFUS, W. (1988) TBT-burden from antifouling paints in different waters.
Presented at the OECD Workshop on Monitoring, Chemical Analysis and
Leaching Rates of TBT, Paris, 30 November-2 December, 1988, 11 pp
(Unpublished report).
KALNINS, M.A. & DETROY, B.F. (1984) Effect of wood preservative treatment
of beehives on honey bees and hive products. J. agric. food Chem., 32:
1176-1180.
KEY, D., NUNNY, R.S., DAVIDSON, P.E., & LEONARD, M.A. (1976) Abnormal shell
growth in the Pacific oyster Crassostrea gigas. Some preliminary results
from experiments undertaken in 1975, Copenhagen, International Council for
the Exploration of the Sea (ICES), 12 pp. (C. M. 1976/K/11).
KIMMEL, E.C., FISH, R.H., & CASIDA, J.E. (1977) Bioorganotin chemistry:
Metabolism of organotin compounds in microsomal monooxygenase system and in
mammals. J. agric. food Chem., 25: 1-8.
KLIMMER, O.R. (1969) [The use of organic tin compounds from the viewpoint
of experimental toxicology.] Arzneimittelforschung, 19: 934-939 (in
German).
KNOWLES, C.O. & JOHNSON, T.L. (1986) Influence of organotins on rat
platelet aggregation mechanism. Environ. Res., 39: 172-179.
KRAJNC, E.I. (1989) Presentation to OECD working group on TBT immune
effects. In Preparation.
KRAJNC, E.I., WESTER, P.W., LOEBER, J.G., VAN LEEUWEN, F.X.R., VOS, J.G.,
VAESSEN, H.A.M.G., & VAN DER HEIJDEN, C.A. (1984) Toxicity of bis(tri- n-
butyltin)oxide in the rat. 1. Short-term effects on general parameters and
on the endocrine and lymphoid systems. Toxicol. appl. Pharmacol., 75: 363-
386.
KRAMPITZ, G., ENGELS, J., & CAZAUX, C. (1976) Biochemical studies on water-
soluble proteins and related components of gastropods shells. In: Watabe,
N. & Wilbur, K.M., ed. The mechanisms of mineralization in the
invertebrates and plants, Columbia, University of South Carolina Press, pp.
155-173.
KRAMPITZ, G., DROLSHAGEN, H., & DELTREIL, J.P. (1983) Soluble matrix
components in malformed oyster shells. Experientia (Basel), 39: 1105-1106.
KROWKE, R., BLUTH, U., & NEUBERT, D. (1986) in vitro studies on the
embryotoxic potential of (bis[tri- n-butyltin])oxide in a limb bud culture
system. Arch. Toxicol., 58: 125-129.
LANGSTON, W.J., BURT, G.R., & ZHOU, M. (1987) Tin and organotin in water,
sediments, and benthic organisms of Poole Harbour. Mar. Pollut. Bull., 18:
634-639.
LAUGHLIN, R.B. & FRENCH, W.J. (1980) Comparative study of the acute
toxicity of a homologous series of trialkyltins to larval shore crabs,
Hemigrapsus nudus, and lobster, Homarus americanus. Bull. environ. Contam.
Toxicol., 25: 802-809.
LAUGHLIN, R. & LINDEN, O. (1982) Sublethal responses of the tadpoles of the
European frog Rana temporaria to two tributyltin compounds. Bull. environ.
Contam. Toxicol., 28: 494-499.
LAUGHLIN, R., FRENCH, W., & GUARD, H.E. (1983) Acute and sublethal toxicity
of tributyltin oxide (TBTO) and its putative environmental product,
tributyltin sulfide (TBTS) to zoeal mud crabs, Rhithropanopeus harrisii.
Water Air Soil Pollut., 20: 69-79.
LAUGHLIN, R., NORDLUND, K., & LINDEN, O. (1984) Long-term effects of
tributyltin compounds on the baltic amphipod, Gammarus oceanicus. Mar.
environ. Res., 12: 243-271.
LAUGHLIN, R.B., JOHANNESEN, R.B., FRENCH, W., GUARD, H., & BRINKMAN, F.E.
(1985) Structure-activity relationships for organotin compounds. Environ.
Toxicol. Chem., 4: 343-351.
LAUGHLIN, R.B., GUARD, H.E., & COLEMAN, W.M. (1986a) Tributyltin in
seawater: Speciation and octanol-water partition coefficient. Environ. Sci.
Technol., 20: 201-204.
LAUGHLIN, R.B., FRENCH, W., & GUARD, H.E. (1986b) Accumulation of bis
(tributyltin) oxide by the marine mussel Mytilus edulis. Environ. Sci.
Technol., 20: 884-890.
LAUGHLIN, R.B., PENDOLEY, P., & GUSTAFSON, R.G. (1987) Sublethal effects of
tributyltin on the hard shell clam, Mercenaria mercenaria. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1494-1498.
LAUGHLIN, R.B., GUSTAFSON, R., & PENDOLEY, P. (1988) Chronic embryo-larval
toxicity of tributyltin (TBT) to the hard shell clam Mercenaria mercenaria.
Mar. Ecol. Prog. Ser., 48: 29-36.
LAWLER, I.F. & ALDRICH, J.C. (1987) Sublethal effects of bis(tri- n-
butyltin)oxide on Crassostrea gigas spat. Mar. Pollut. Bull., 18: 274-278.
LEE, R.F. (1985) Metabolism of tributyltin oxide by crabs, oysters and
fish. Mar. environ. Res., 17: 145-148.
LEE, R.F. (1986) Metabolism of bis (tributyltin) oxide by estuarine
animals. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1182-1188.
LEE, R.F., VALKIRS, A.O., & SELIGMAN, P. (1987) Fate of tributyltin in
estuarine waters. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1411-1415.
LEWIS, P.G. & EMMETT, E.A. (1987) Irritant dermatitis from tributyl tin
oxide and contact allergy from chlorocresol. Contact dermatitis, 17: 129-
132.
LINDEN, O. (1987) The scope of the organotin issue in Scandinavia. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1320-1323.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LYLE, W.H. (1958) Lesions of the skin in process workers caused by contact
with butyl tin compounds. Br. J. ind. Med., 15: 193-196.
MCCULLOUGH, F.S., GAYRAL, P., DUNCAN, J., & CHRISTIE, J.D. (1980)
Molluscicides in schistosomiasis control. World Health Organ. Bull., 58:
681-689.
MACKLAD, F., TAMAN, F., & EL SEBAE, A.K. (1983) Toxicity of tributyltin
fluoride and copper sulfate in slow release formulation to Biomphalaria
alexandrina snails. Bull. High Inst. Public Health, 14: 1-12.
MAFF/HSE (1988) Pesticides 1988: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 399 pp (Reference
Book HMSO 500).
MAFF/HSE (1989) Pesticides 1989: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 407 pp (Reference
Book HMSO 500).
MAGUIRE, R.J. (1984) Butyltin compounds and inorganic tin in sediments in
Ontario. Environ. Sci. Technol., 18: 291-294.
MAGUIRE, R.J. (1987) Review: Environmental aspects of tributyltin. Appl.
organomet. Chem., 1: 475-498.
MAGUIRE, R.J. & HUNEAULT, H. (1981) Determination of butyltin species in
water by gas chromatography with flame photometric detection. J.
Chromatogr., 209: 458-462.
MAGUIRE, R.J. & TKACZ, R.J. (1983) Analysis of butyltin compounds by gas
chromatography. Comparison of flame photometric and atomic absorption
spectrophotometric detectors. J. Chromatogr., 268: 99-101.
MAGUIRE, R.J. & TKACZ, R.J. (1985) Degradation of the tri-n-butyltin
species in water and sediment from Toronto Harbor. J. agric. food Chem.,
33: 947-953.
MAGUIRE, R.J. & TKACZ, R.J. (1987) Concentration of tributyltin in the
microlayer of natural waters. Water Pollut. Res. J. Can., 22: 227-233.
MAGUIRE, R.J., CHAU, Y.K., BENGERT, G.A., HALE, E.J., WONG, P.T.S., &
KRAMAR, O. (1982) Occurrence of organotin compounds in Ontario lakes and
rivers. Environ. Sci. Technol., 16: 698-702.
MAGUIRE, R.J., CAREY, J.H., & HALE, E.J. (1983) Degradation of the tri-n-
butyltin species in water. J. agric. food Chem., 31: 1060-1065.
MAGUIRE, R.J., WONG, P.T.S., & RHAMEY, J.S. (1984) Accumulation and
metabolism of tri-n-butyltin cation by a green alga, Ankistrodesmus
falcatus. Can. J. Fish. aquat. Sci., 41: 537-540.
MAGUIRE, R.J., TKACZ, R.J., & SARTOR, D.L. (1985) Butyltin species and
inorganic tin in water and sediment of the Detroit and St. Clair rivers. J.
Great Lakes Res., 11: 320-327.
MAGUIRE, R.J., TKACZ, R.J., CHAU, Y.K., BENGERT, G.A., & WONG, P.T.S.
(1986) Occurrence of organotin compounds in water and sediment in Canada.
Chemosphere, 15: 253-274.
MATSUI, H., WADA, O., MANABE, S., ONO, T., IWAI, H., & FUJIKURA, T. (1982)
[Properties and mechanism of hyperlipidemia induced in rabbits by
tributyltin fluoride.] Jpn. J. ind. Health, 24: 163-171 (in Japanese).
MATTHIAS, C.L., BELLAMA, J.M., & BRINCKMAN, F.E. (1986a) Determination of
ultra-trace concentrations of butyltin compounds in water by simultaneous
hydridization/extraction with GC/FPD detection. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1146-1151.
MATTHIAS, C.L., OLSON, G.J., BELLAMA, J.M., & BRINCKMAN, F.E. (1986b)
Comprehensive method for determination of aquatic butyltin and
butylmethyltin species at ultratrace levels using simultaneous
hydridization/extraction with GC/FPD detection. Environ. Sci. Technol., 20:
609-615.
MATTHIESSEN, P. (1974) Some effects of slow-release bis (tri-n-butyl tin)
oxide on the tropical fish, Tilapia mossambica Peters. Controlled Release
Pesticide Symposium, Akron, Ohio, University of Akron, Community Technical
College, Engineering and Science Division, pp. 25.1-25.16.
MATTHIESSEN, P. & THAIN, J.E. (in press) A method for studying the impact
of polluted marine sediments on colonising organisms; tests with diesel-
based drilling mud and tributyltin antifouling paint. Hydrobiologia.
MAURER, D., HERAL, M., HIS, E., & RAZET, D. (1985) Influence d'une peinture
antisalissure à base de sels organométalliques de l'étain sur le captage en
milieu naturel de l'huitre Crassostrea gigas. Rev. Trav. Inst. Pêches
Marit., 47: 241-250.
MEADOR, J.P. (1986) An analysis of photobehavior of Daphnia magna exposed
to tributyltin. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1213-
1218.
MEINEMA, H.A., BURGER-WIERSMA, T., VERSLUIS DE HAAN, G., & GEVERS, E.C.
(1978) Determination of trace amounts of butyltin compounds in aqueous
systems by gas chromatography/mass spectrometry. Environ. Sci. Technol.,
12: 288-293.
MICHEL, P. (1987) Automatization of a hydride generation/A.A.S. system - an
improvement for organotin analysis. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1340-1343.
MIDDLETON, M.C. & PRATT, I. (1977) Skin water content as a quantitative
index of the vascular and histologic changes produced in rat skin by di- n-
butyltin and tri- n-butyltin. J. invest. Dermatol., 68: 379-384.
MIDDLETON, M.C. & PRATT, I. (1978) Changes in incorporation of
[3H]thymidine into DNA of rat skin following cutaneous application of
dibutyltin, tributyltin and 1-chloro-2:4-dinitrobenzene and the
relationship of these changes to a morphological assessment of the cellular
damage. J. invest. Dermatol., 71: 305-310.
MINCHIN, D., DUGGAN, C.B., & KING, W. (1987) Possible effects of organotins
on scallop recruitment. Mar. Pollut. Bull., 18: 604-608.
MOLIN, L. & WAHLBERG, J.E. (1975) Toxic skin reactions caused by
tributyltin oxide (TBTO) in socks. Berufs-dermatosen, 4: 138-142.
MORI, Y., IESATO, K., UEDA, S., MORI, T., IWASAKI, I., OHNISHI, K., SEINO,
Y., WAKASHIM, Y., WAKASHIN, M., & OKUDA, K. (1984) Renal tubular
disturbances induced by tributyl-tin oxide in guinea pigs: a secondary
Fanconi syndrome. Clin. Nephrol., 21: 118-125.
MULLER, H.A. (1987) Determination of tributyltin oxide in air. In:
Toxicology and analytics of the tributyltins: The present status.
Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986, Vlissingen-
Oost, The Netherlands, ORTEP Association, pp. 162-172.
MULLER, M.D. (1984) Tributyltin detection at trace levels in water and
sediments using GC with flame-photometric detection and GC-MS. Fresenius Z.
anal. Chem., 317: 32-36.
MULLER, M.D. (1987b) Comprehensive trace level determination of organotin
compounds in environmental samples using high-resolution gas chromatography
with flame photometric detection. Anal. Chem., 59: 617-623.
MUSHAK, P., KRIGMAN, M.R., & MAILMAN, R.B. (1982) Comparative organotin
toxicity in the developing rat: somatic and morphological changes and
relationship to accumulation of total tin. Neurobehav. Toxicol. Teratol.,
4: 209-215.
NEMEC, M.D. (1987) A teratology study in rabbits with TBTO: Final Report,
Wil Research Laboratories Inc., 210 pp (Report No. WIL-B0002) (Confidential
report to the Tributyl Tin Oxide Consortium).
NEWTON, F., THUM, A., DAVIDSON, B., VALKIRS, A., & SELIGMAN, P. (1985)
Effects on the growth and survival of eggs and embryos of the California
grunion (Leuresthes tenuis) exposed to trace levels of tributyltin, San
Diego, California, Naval Ocean Systems Center, 15 pp (Technical Report No.
1040).
NIVA (1986) [Organic tin compounds in fjord areas], Oslo, Norwegian
Institute for Water Research (NIVA) (in Norwegian).
O'CALLAGHAN, J.P. & MILLER, D.B. (1988) Acute exposure of the neonatal rat
to tributyltin results in decreases in biochemical indicators of
synaptogenesis and myelinogenesis. Pharmacol. exp. Ther., 246: 394-402.
OKOSHI, K., MORI, K., & NONURA, T. (1987) Characteristics of shell chamber
formation between the two races in Japanese oyster, Crassostrea gigas.
Aquaculture, 67: 313-320.
OLSON, G.J. & BRINCKMAN, F.E. (1986) Biodegradation of tributyltin by
Chesapeake Bay microorganisms. In: Proceedings of the Organotin Symposium,
Oceans '86 Conference, Washington, DC, USA, 23-25 September, 1986, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1196-1201.
OSBORN, D. & LEACH, D.V. (1987) Organotin in birds: Pilot study,
Huntingdon, Institute of Terrestrial Ecology, 15 pp (Final report to the UK
Department of the Environment. Contract No. F3CR/27/D4/01).
PAGE, D.S. (1989) An analytical method for butyltin species in shellfish.
Mar. Pollut. Bull., 20: 129-133.
PAUL, J.D. & DAVIES, I.M. (1986) Effects of copper- and tin-based anti-
fouling compounds on the growth of scallops (Pecten maximus) and oysters
(Crassostrea gigas). Aquaculture, 54: 191-203.
PAULINI, E. (1964) Laboratory experiments with some organotin compounds,
Geneva, World Health Organization, Parasitic Diseases Programme, pp. 1-3
(Unpublished report Mol/Inf/16).
PAULINI, E. & DE SOUZA, C.P. (1970) Influence of different suspended solids
in the water upon molluscicide activity, Geneva, World Health Organization,
Parasitic Diseases Programme, pp. 1-10 (Unpublished report PD/Mol/70.12).
PELIKAN, Z. (1969) Effects of bis(tri- n-butyltin) oxide on the eyes of
rabbits. Br. J. ind. Med., 26: 165-170.
PELIKAN, Z. & CERNY, E. (1968) [The toxic effects of tri- n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292 (in German).
PELIKAN, Z. & CERNY, E. (1969) Toxic effects of bis (tributyltin) oxide
(TBTO) on the skin of rats. Berufs-dermatosen, 17: 305-316.
PICKWELL, G.V. & STEINERT, S.A. (1988) Accumulation and effects of
organotin compounds in oysters and mussels: correlation with serum
biochemical and cytological factors and tissue burdens. Mar. environ. Res.,
24: 215-218.
PIETERS, R.H., KAMPINGA, J., BOL-SCHOENMAKERS, M., LAMB, B.W., PENNINKS,
A.H., & SEINEN, W. (1989) Organotin-induced thymus atrophy concerns the OX-
44+ immature thymocytes: Relation to the interaction between early
thymocytes and thymic epithelial cells? Thymus, 14(1-3): 79-88.
PINKNEY, A.E., HALL, L.W., LENKEVICH, M.J., BURTON, D.T., & ZEGER, S.
(1985) Comparison of avoidance responses of an estuarine fish, Fundulus
heteroclitus, and crustacean, Palaemonetes pugio, to bis (tri-n-butyltin)
oxide. Water Air Soil Pollut., 25: 33-40.
PINKNEY, A.E., MATTESON, L.L., & WRIGHT, D.A. (1988) Effects of tri-
butyltin on survival, growth, morphometry and RNA-DNA ratio of larval
striped bass, Morone saxatilis. In: Proceedings of the Organotin Symposium,
Oceans '88 Conference, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 987-991.
PLUM, H. (1981) Comportement des composés organostanniques vis-à-vis de
l'environnement. Inf. chim., 220: 135-139.
POITOU, P., MARIGNAC, B., CERTIN, C., & GRADISKI, D. (1978) Etude de
l'effet sur le système nerveux central et du pouvoir sensibilisant de
l'oxyde de tributylétain. Ann. Pharm. fr., 36: 569-572.
POLSTER, M. & HALACKA, K. (1971) [9. Contributions to the health-toxic
problems of some anti-microbially used organo-tin compounds.]
Ernährungsforschung, 16: 527-535 (in German).
RACEY, P.A. & SWIFT, S.M. (1986) The residual effects of remedial timber
treatments on bats. Biol. Conserv., 35: 205-214.
RANDALL, L., DONARD, O.F.X., & WEBER, J.H. (1986) Speciation of n-butyltin
compounds by atomic absorption spectrophotometry using electro-thermal
quartz furnace after hydride generation. Anal. Chim. Acta., 184: 197-203.
REIMANN, R. & LANG, R. (1987) Mutagenicity studies with tributyltin
compounds. In: Toxicology and analytics of the tributyltins: The present
status. Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986,
Vlissingen-Oost, The Netherlands, ORTEP Association, pp. 66-90.
REINHARDT, C.A., SCHAWALDER, H., & ZBINDEN, G. (1982) Cell detachment and
cloning efficiency as parameters for cytotoxicity. Toxicology, 25: 47-52.
RICE, C.D., ESPOURTEILLE, F.A., & HUGGETT, R.J. (1987) Analysis of
tributyltin in estuarine sediments and oyster tissue, Crassostrea
virginica. Appl. organomet. Chem., 1: 541-544.
RITCHIE, L.S., BERRIOS-DURAN, L.A., FRICK, L.P., & FOX, I. (1964)
Molluscicidal time-concentration relationships of organo-tin compounds.
World Health Organ. Bull., 31: 147-149.
RITCHIE, L.S., LOPEZ, V.A., & CORA, J.M. (1974) Prolonged applications of
an organotin against Biomphalaria glabrata and Schistosoma mansoni. In:
Molluscicides in schistosomiasis control, New York, London, Academic Press,
pp. 77-88.
RIVM (1989) In: Mathijssen-Spiekman, E.A.M., Canton, J.H., & Roghair, C.J.,
ed. [Investigation into the toxicity of TBTO for a number of freshwater
organisms], Bilthoven, National Institute of Public Health and
Environmental Hygiene, 48 pp (Report No. 668118.001) (in Dutch).
ROBERTS, M.H. (1987) Acute toxicity of tributyltin chloride to embryos and
larvae of two bivalve mollusks, Crassostrea virginica and Mercenaria
mercenaria. Bull. environ. Contam. Toxicol., 39: 1012-1019.
ROBERTS, M.H., BENDER, M.E., DE LISLE, P.F., SUTTON, H.C., & WILLIAMS, R.L.
(1987) Sex ratio and gamete production in American oysters exposed to
tributyltin in the laboratory. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1471-1476.
ROBINSON, I.N. (1969) Effects of some organotin compounds on tissue amine
levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROSENBERG, D.W. & DRUMMOND, G.S. (1983) Direct in vitro effects of bis
(tributyl) tin oxide on hepatic cytochrome P-450. Biochem. Pharmacol., 32:
3823-3829.
ROSENBERG, D.W., DRUMMOND, G.S., CORNISH, H.C., & KAPPAS, A. (1980)
Prolonged induction of hepatic haem oxygenase and decreases in cytochrome
P-450 content by organotin compounds. Biochem. J., 190: 465-468.
ROSENBERG, D.W., DRUMMOND, G.S., & KAPPAS, A. (1981) The influence of
organometals on heme metabolism. in vivo and in vitro studies with
organotins. Mol. Pharmacol., 21: 150-158.
ROSENBERG, D.W., ANDERSON, K.E., & KAPPAS, A. (1984) The potent induction
of intestinal heme oxygenase by the organotin compound, bis(tri- n-butyltin)
oxide. Biochem. biophys. Res. Commun., 119: 1022-1027.
SALAZAR, S.M. (1985) The effects of bis(tri-n-butyltin) oxide on three
species of marine phytoplankton, San Diego, California, Naval Ocean Systems
Center, 16 pp (Technical Report No. 1039).
SALAZAR, M.H. & SALAZAR, S.M. (1985) Ecological evaluation of
organotincontaminated sediment, San Diego, California, Naval Ocean Systems
Center, 21 pp (Technical Report No. 1050).
SALAZAR, M.H. & SALAZAR, S.M. (1987) Tributyltin effects on juvenile mussel
growth. In: Proceedings of the Organotin Symposium, Oceans '87 Conference,
Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1504-
1510.
SALAZAR, S.M., DAVIDSON, B.M., SALAZAR, M.H., STANG, P.M., & MEYERS-
SCHULTE, K.J. (1987) Effects of TBT on marine organisms: Field assessment
of a new site-specific bioassay system. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1461-1470.
SAXENA, P.N. & CROWE, A.J. (1988) An investigation of the efficacy of
organotin compounds for the control of the cotton stainer, Dysdercus
cingulatus, the mosquito, Anopheles stephensi, and the common house fly,
Musca domestica. Appl. organomet. Chem., 2: 185-187.
SCHAFER, E.W. & BOWLES, W.A. (1985) Acute oral toxicity and repellency of
933 chemicals to house and deer mice. Arch. environ. Contam. Toxicol., 14:
111-129.
SCHERING (1983) Repeated dose inhalation study of ZK21.995 in the rat for
29-32 days (21-24 exposures), Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. IC 1/83. Study No. TX81.177).
SCHERING (1986) Re-evaluation of a "mouse micronucleus test on TBTO",
Bergkamen, Federal Republic of Germany, Schering Inc. (Confidential report
No. IC 7186 prepared by the Institute of Occupational Health, Helsinki,
Finland, September 1984).
SCHERING (1989a) TBTO - 4 week oral (dietary administration) toxicity study
in the rat, Bergkamen, Federal Republic of Germany, Schering Inc. (Report
No. 280118 by Hazelton, France. Study No. 14/502).
SCHERING (1989b) TBTO - Plaque forming assay following a 5 week oral
toxicity study in the rat, Bergkamen, Federal Republic of Germany, Schering
Inc. (Report No. 283118 by Hazelton, France. Study No. 14/503).
SCHERING (1989c) TBTO - Resistance to Listeria monocytogene infection
following a 34 day oral toxicity study in the rat, Bergkamen, Federal
Republic of Germany, Schering Inc. (Report No. 282118 by Hazelton, France.
Study No. 14/505).
SCHERING (1989d) TBTO - Delayed type hypersensitivity test following a 37
day oral toxicity study in the rat, Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. 281118 by Hazelton, France).
SCHERING (1989e) TBTO - Systemic toxicity study in beagle dogs with daily
oral (intragastric) administration over a total of 18-19 weeks, Bergkamen,
Federal Republic of Germany, Schering Inc. (Report No. IC6/88).
SCHWEINFURTH, H. (1985) Toxicology of tributyltin compounds. Tin Uses, 143:
9-12.
SEIFFER, E.A. & SCHOOF, H.F. (1967) Tests of 15 experimental molluscicides
against Australorbis glabratus. Public Health Rep., 82: 833-839.
SEINEN, W., HELDER, T., VERNIJ, H., PENNINKS, A., & LEEUWANGH, P. (1981)
Short term toxicity of tri- n-butyltinchloride in rainbow trout (Salmo
gairdneri Richardson) yolk sac fry. Sci. total Environ., 19: 155-166.
SELIGMAN, P.F., VALKIRS, A.O., & LEE, R.F. (1986a) Degradation of
tributyltin in marine and estuarine waters. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1189-1195.
SELIGMAN, P.F., GROVHOUG, J.G. & RICHTER, K.E. (1986b) Measurement of
butyltins in San Diego Bay, CA: A monitoring strategy. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1289-1296.
SELIGMAN, P.F., GROVHOUG, J.G., VALKIRS, A.O., STANG, P.M., FRANSHAM, R.,
STALLARD, M.O., DAVIDSON, B., & LEE, R.F. (1989) Distribution and fate of
tributyltin in the United States marine environment. Appl. organomet.
Chem., (in press).
SHELDON, A.W. (1975) Effects of organotin anti-fouling coatings on man and
his environment. J. Paint Technol., 47: 54-58.
SHERMAN, L.R. & CARLSON, T.L. (1980) A modified phenylfluorone method for
determining organotin compounds in the ppb and sub-ppb range. J. anal.
Toxicol., 4: 31-33.
SHIFF, C.J., YIANNAKIS, C., & EVANS, A.C. (1975) Further trials with TBTO
and other slow release molluscicides in Rhodesia. In: Proceedings of the
Controlled Release Pesticide Symposium, Wright State University, Dayton,
Ohio, 8-10 September, 1975, pp. 177-188.
SHIMIZU, A. & KIMURA, S. (1987) [Effect of bis (tributyltin) oxide on
gonadal development of a salt-water goby, Chasmichthys dolichognathus:
Exposure during maturing period.] Bull. Tokai Reg. Fish. Res. Lab., 123:
45-49 (in Japanese).
SHORT, J.W. & THROWER, F.P. (1986) Accumulation of butyltins in muscle
tissue of chinook salmon reared in sea pens treated with tri- n-butyltin.
In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1177-1181.
SHORT, J.W. & THROWER, F.P. (1987) Toxicity of tri- n-butyl-tin to chinook
salmon, Oncorhynchus tshawytscha, adapted to seawater. Aquaculture, 61:
193-200.
SLESINGER, A.E. & DRESSER, I. (1978) The environmental chemistry of three
organotin chemicals. In: Good, M., ed. Report of the Organotin Workshop,
New Orleans, Louisiana, 17-19 February, 1978, pp. 115-162.
SMITH, B.S. (1981a) Male characteristics on female mud snails caused by
antifouling paints. J. appl. Toxicol., 1: 22-25.
SMITH, B.S. (1981b) Tributyltin compounds induce male characteristics on
female mud snails Nassarius obsoletus = Ilyanassa obsoleta. J. appl.
Toxicol., 1: 141-144.
SMITH, B.S. (1981c) Male characteristics in female Nassarius obsoletus:
Variations related to locality, season and year. Veliger, 23: 212-216.
SMITH, D.R., STEPHENSON, M.D., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1987) The use of transplanted juvenile oysters to monitor the toxic
effects of tributyltin in California waters. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1511-1516.
SMITH, P.J., CROWE, A.J., ALLEN, D.W., BROOKS, J.S., & FORMSTONE, R. (1977)
Study by 119m Sn Mossbauer spectroscopy of bis(tri- n-butyltin) oxide
adsorbed on cellulosic materials. Chem. Ind., 23: 874-875.
SNOEIJ, N.J. (1987) Triorganotin compounds in immunotoxicology and bio-
chemistry, Utrecht, The Netherlands, University of Utrecht, 170 pp (Ph.D.
Thesis).
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1985)
Toxicity of triorganotin compounds: Comparative in vivo studies with a
series of trialkyltin compounds and triphenyltin chloride in male rats.
Toxicol. appl. Pharmacol., 81: 274-286.
SNOEIJ, N.J., PUNT, P.M., PENNINKS, A.H., & SEINEN, W. (1986a) Effects of
tri- n-butyltin chloride on energy metabolism, macromolecular synthesis,
precursor uptake and cyclic AMP production in isolated rat thymocytes.
Biochim. Biophys. Acta, 852: 234-243.
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1986b)
Triorganotin-induced cytotoxicity to rat thymus, bone marrow and red blood
cells as determined by several in vitro assays. Toxicology, 39: 71-83.
SNOEIJ, N.J., PIETERS, R.H.H., PENNINKS, A.H., & SEINEN, W. (1987) Toxicity
of triorganotin compounds: Orally administered tri- n-butyltin compounds are
rapidly dealkylated in the rat. In: Triorganotincompounds in immunology and
biochemistry, Utrecht, The Netherlands, University of Utrecht, Chapter 4,
pp. 73-93 (Snoeij, N.J., Ph.D. Thesis).
SNOEIJ, N.J., BOL-SCHOENMAKERS, M., PENNINKS, A.H., & SEINEN, W. (1988a)
Differential effects of tri- n-butyltin chloride on macromolecular synthesis
and ATP levels of rat thymocyte subpopulations obtained by centrifugal
elutriation. Int. J. Immunopharmacol., 10: 29-37.
SNOEIJ, N.J., PENNINKS, A.H., & SEINEN, W. (1988b) Dibutyltin and
tributyltin compounds induce thymus atrophy in rats due to a selective
action on thymic lymphoblasts. Int. J. Immunopharmacol., 10: 891-899.
SORACCO, R.J. & POPE, D.H. (1983) Bacteriostatic and bactericidal modes of
action of bis (tributlytin) oxide on Legionella pneumophila. Appl. environ.
Microbiol., 45: 48-57.
STALLARD, M., HODGE, V., & GOLDBERG, E.D. (1987) TBT in California coastal
waters: Monitoring and assessment. Environ. Monit. Assess., 9: 195-220.
STANG, P.M. & SELIGMAN, P.F. (1986) Distribution and fate of butyltin
compounds in the sediment of San Diego Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1256-1261.
STANG, P.M. & SELIGMAN, P.F. (1987) In situ adsorption and desorption of
butyltin compounds from Pearl Harbor, Hawaii, sediment. In: Proceedings of
the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1386-1391.
STEIN, T. & KUSTER, K. (1982) Der biologische abbau von tributylzinnoxid
(TBTO) in einer belebtschlammanlage. Z. Wasser Abwasser Forsch., 15: 178-
180.
STEPHENSON, M.D., SMITH, D.R., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1986) Growth abnormalities in mussels and oysters from areas with high
levels of tributyltin in San Diego Bay. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1246-1251.
STEPHENSON, M.D., SMITH, D.R., HALL, L.W., JOHNSON, W.E., MICHEL, P.,
SHORT, J., WALDOCK, M., HUGGETT, R.J., SELIGMAN, P., & KOLA, S. (1987) An
international intercomparison of butyltin determinations in mussel tissue
and sediments. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1334-1338.
STROMGREN, T. & BONGARD, T. (1987) The effect of tributyltin oxide on
growth of Mytilus edulis. Mar. Pollut. Bull., 18: 30-31.
TEMMINK, J.H.M. & EVERTS, J.W. (1987) Comparative toxicity of tributyltin-
oxide (TBTO) for fish and snail. In: Proceedings of the Seventh World
Meeting of the ORTEP-Association, Amsterdam, 7-8 May, 1987, Vlissingen-
Oost, The Netherlands, ORTEP-Association, pp. 6-20.
THAIN, J.E. (1983) The acute toxicity of bis (tributyl tin) oxide to the
adults and larvae of some marine organisms, Copenhagen, International
Council for the Exploration of the Sea (ICES), 5 pp (Report No. C. M.
1983/E.13).
THAIN, J.E. (1986) Toxicity of TBT to bivalves: Effects on reproduction,
growth and survival. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1306-
1313.
THAIN, J.E. & WALDOCK, M.J. (1985) The growth of bivalve spat exposed to
organotin leachates from antifouling paints, Copenhagen, International
Council for the Exploration of the Sea (ICES), 10 pp (Report No. C. M.
1985/E.28).
THAIN, J.E. & WALDOCK, M.J. (1986) The impact of tributyl tin (TBT)
antifouling paints on molluscan fisheries. Water Sci. Technol., 18: 193-
202.
THAIN, J.E., WALDOCK, M.J., & WAITE, M.E. (1987) Toxicity and degradation
studies of tributyltin (TBT) and dibutyltin (DBT) in the aquatic
environment. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1398-1404.
THOMAS, T.E. & ROBINSON, M.G. (1986) The physiological effects of the
leachates from a self-polishing organotin antifouling paint on marine
diatoms. Mar. environ. Res., 18: 215-229.
THOMAS, T.E. & ROBINSON, M.G. (1987) Initial characterization of the
mechanisms responsible for the tolerance of Amphora coffeaeformis to copper
and tributyltin. Bot. mar., 30: 47-53.
THOMPSON, J.A.J., SHEFFER, M.G., PIERCE, R.C., CHAU, Y.K., COONEY, J.J.,
CULLEN, W.R., & MAGUIRE, R.J. (1985) Organotin compounds in the aquatic
environment: Scientific criteria for assessing their effects on
environmental quality, Ottawa, National Research Council Canada, 284 pp
(NRCC No. 22494).
TOLEDO, J.V., MONTEIRO DA SILVA, C.S., BULHOES, M.S., PAES LEME, L.A., DA
SILVA NETTO, J.A., & GILBERT, B. (1976) Snail control in urban sites in
Brazil with slow-release hexabutyldistannoxane and pentachlorophenol. World
Health Organ. Bull., 54: 421-425.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.-P., PHU LICH N., VAN DEN DRIESSCHE, J.,
GUESNIER, L.R., & MORIN, N. (1976) Contribution à l'étude toxicologique et
pharmacologique de l'oxyde de tributylétain (OTBE). Eur. J. Toxicol., 9:
31-40.
TRUHAUT, R., ANGER, J.P., REYMANN, J.M., CHAUVEL, Y., & VAN DEN DRIESSCHE,
J. (1979) Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicol. Eur. Res., 11: 181-186.
TRUHAUT, R., ANGER, J.P., ANGER, F., BRAULT, A., BRUNET, P., CANO, Y.,
LOUVET, M., SACCAVINI, J.C., VAN DEN DRIESSCHE, J., JANSON, C., & SUBLET,
Y. (1981) Dégradation thermique de l'oxyde de tributylétain (OTBE) et
toxicité pulmonaire des produits de combustion chez la souris et le cobaye.
Toxicol. Eur. Res., 111: 35-44.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1986)
Bioconcentration of butyltin compounds by round crucian carp. Toxicol.
environ. Chem., 12: 137-143.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1987)
Bioconcentration and metabolism of phenyl tin chlorides in carp. Water
Res., 21: 949-953.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1988)
Bioconcentration and metabolism of butyltin compounds in carp. Water Res.,
22: 647-651.
TWG (1988a) Uses and production of organotin compounds. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group), Brussels, 7-11 March, 1988, 6 pp (Report No. TWG 15/5/1-E).
TWG (1988b) Tributyl tin compounds in antifouling paints. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group, Brussels, 7-11 March, 1988, 2 pp, (Report No. TWG 15/5/2-E).
TWG (1988c) Organotins in the Netherlands. Presented by the Netherlands at
the Convention for the Prevention of Marine Pollution from Land-based
Sources (15th Meeting of the Technical Working Group, Brussels, 7-11 March,
1988, 11 pp (Report No. TWG 15/5/4-E).
UHL, S. (1986) [Population exposure to the environmental chemical
pentachloro-ophenol (PCP) and bis (tri-n- butyltin)oxide (TBTO)], Zurich,
Maus Offsetdruck Konstanz, 145 pp (ETH Thesis No. 8014, University of
Gottingen) (in German).
UNEP (1989) Mediterranean Action Plan. Assessment of organotin compounds as
marine pollutants in the Mediterranean, Athens, United Nations Environment
Programme (MAP Technical Report Series, No. 33).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1986) Organotin in
antifouling paints environmental considerations, London, UK Department of
the Environment, 82 pp (Pollution Paper No. 25).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1989) Water and the
environment, London, UK Department of the Environment, p. 26 (Circular No.
7/89).
UPATHAM, E.S., KOURA, M., AHMED, M.D., & AWAD, A.H. (1980) Laboratory
trials of controlled release molluscicides on Bulinus (Ph.) abyssinicus,
the intermediate host of Schistosoma haematobium in Somalia. In: Baker,
R.W., ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the controlled Release Society, New York, London,
Academic Press, pp. 461-469.
U'REN, S.C. (1983) Acute toxicity of bis(tributyltin) oxide to a marine
copepod. Mar. Pollut. Bull., 14: 303-306.
VALKIRS, A.O., SELIGMAN, P.F., STANG, P.M., HOMER, V., LIEBERMAN, S.H.,
VAFA, G., & DOOLEY, C.A. (1986) Measurement of butyltin compounds in San
Diego Bay. Mar. Pollut. Bull., 17: 319-324.
VALKIRS, A.O., DAVIDSON, B.M., & SELIGMAN, P.F. (1987) Sublethal growth
effects and mortality to marine bivalves from long-term exposure to
tributyltin. Chemosphere, 16: 201-220.
VIGHI, M. & CALAMARI, D. (1985) QSARs for organotin compounds on Daphnia
magna. Chemosphere, 14: 1925-1932.
VIYANANT, V., THIRACHANTRA, S., & SORNMANI, S. (1982) The effect of
controlled release copper sulphate and tributyltin fluoride on the
mortality and infectivity of Schistosoma mansoni cercariae. J. Helminthol.,
56: 85-92.
VOS, J.G., DE KLERC, A., KRAJNC, E.I., KRUIZINGA, W., VAN OMMEN, B., &
ROZING, J. (1984) Toxicity of bis(tri- n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of non-
specific resistance after short-term exposure. Toxicol. appl. Pharmacol.,
75: 387-408.
VOS, J.G., KRAJNC, E.I., & WESTER, P.W. (1985) Immunotoxicity of bis (tri-
n-butyltin) oxide. In: Dean, J., ed. Immunotoxicology and
immunopharmacology, New York, Raven Press, pp. 327-340.
WADE, T.L., GARCIA-ROMERO, B., & BROOKS, J.M. (1988) Tributyltin
contamination in bivalves from U.S. coastal estuaries. Environ. Sci.
Technol., 22: 1488-1493.
WALDOCK, M.J. (1989) Organotin concentrations in the Rivers Bure and Yare,
Norfolk Broads, England (Unpublished report of the Ministry of Agriculture,
Fisheries and Food, Fisheries Laboratory, Burnham-on-Crouch, Essex, UK).
WALDOCK, M.J. & MILLER, D. (1983) The determination of total and tributyl
tin in seawater and oysters in areas of high pleasure craft activity,
Copenhagen, International Council for the Exploration of the Sea (ICES), 18
pp (Report No. C. M. 1983/E.12).
WALDOCK, M.J. & THAIN, J.E. (1983) Shell thickening in Crassostrea gigas:
Organotin antifouling or sediment induced? Mar. Pollut. Bull., 14: 411-415.
WALDOCK, M.J. & THAIN, J.E. (1985) The comparative leach rates and toxicity
of two fish net antifouling preparations, Copenhagen, International Council
for the Exploration of the Sea (ICES), 5 pp (Report No. C. M. 1985/E.29).
WALDOCK, M.J., THAIN, J.E. & MILLER, D. (1983) The accumulation and
depuration of bis (tributyl tin) oxide in oysters: A comparison between the
Pacific oyster (Crassostrea gigas) and the European flat oyster (Ostrea
edulis), Copenhagen, International Council for the Exploration of the Sea
(ICES), 6 pp (Report No. C. M. 1983/E.52).
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1987a) Changes in concentrations
of organotins in U.K. rivers and estuaries following legislation in 1986.
In: Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1352-1356.
WALDOCK, M.J., THAIN, J.E., & WAITE, M.E. (1987b) The distribution and
potential toxic effects of TBT in UK estuaries during 1986. Appl.
organomet. Chem., 1: 287-301.
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1988) Inputs of TBT to the
marine environment from shipping activity in the U.K. Environ. Technol.
Lett., 9: 999-1010.
WALNE, P.R. & HELM, M.M. (1979) Introduction of Crassostrea gigas into the
United Kingdom. In: Mann, R., ed. Proceedings of a Symposium on Exotic
Species in Marinculture: Case histories of the Japanese Oyster Crassostrea
gigas (Thunberg), with implications for other fisheries, Woods Hole,
Massachusetts, USA, 18-20 September, 1978, Cambridge, London, MIT Press,
pp. 83-105.
WALSH, G.E. (1986) Organotin toxicity studies conducted with selected
marine organisms at EPA's environmental research laboratory, Gulf Breeze,
Florida. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1210-1212.
WALSH, G.E., MCLAUGHLAN, L.L., LORES, E.M., LOUIE, M.K., & DEANS, C.H.
(1985) Effects of organotins on growth and survival of two marine diatoms,
Skeletonema costatum and Thalassiosira pseudonana. Chemosphere, 14: 383-
392.
WALSH, G.E., LOUIE, M.K., MCLAUGHLIN, L.L., & LORES, E.M. (1986a) Lugworm
(Arenicola cristata) larvae in toxicity tests: Survival and development
when exposed to organotins. Environ. Toxicol. Chem., 5: 749-754.
WALSH, G.E., MCLAUGHLIN, L.L., LOUIE, M.K., DEANS, C.H., & LORES, E.M.
(1986b) Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin oxide and triphenyltin oxide.
Ecotoxicol. environ. Saf., 12: 95-100.
WALTON, R., ADEMA, C.M., & SELIGMAN, P.F. (1986) Mathematical modeling of
the transport and fate of organotin in harbors. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1297-1301.
WARD, G.S., CRAMM, G.C., PARRISH, P.R., TRACHMAN, H., & SLESINGER, A.
(1981) Bioaccumulation and chronic toxicity of bis(tributyltin) oxide
(TBTO): Tests with a saltwater fish. In: Branson, D.R. & Dickson, K.L., ed.
Proceedings of the Fourth Conference on Aquatic Toxicology and Hazard
Assessment, Philadelphia, American Society for Testing and Materials, pp.
183-200 (ASTM STP No. 737).
WEBBE, G. (1963) Laboratory tests of some new molluscicides (organotin
compounds), Geneva, World Health Organization, Parasitic Diseases
Programme, pp. 1-2 (Unpublished report Mol/Inf/14).
WEBER, J.H., DONARD, O.F.X., RANDALL, I., & HAN, J.S. (1986) Speciation of
methyl and butyltin compounds in the Great Bay estuary (N.H.). In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1280-1282.
WEIS, J.S., WEIS, P., & WANG, F. (1987a) Developmental effects of
tributyltin on the fiddler crab, Uca pugilator, and the killifish, Fundulus
heteroclitus. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1456-1460.
WEIS, J.S., GOTTLIEB, J., & KWIATKOWSKI, J. (1987b) Tributyltin retards
regeneration and produces deformities of limbs in the fiddler crab, Uca
pugilator. Arch. environ. Contam. Toxicol., 16: 321-326.
WESTER, P.W., CANTON, J.H., VAN IERSAL, A.A.J., KRANJC, E.I., & VAESSEN,
H.A.M.G. (1988) The toxicity of bis(tri-n- butyltin)oxide (TBTO) and di-n-
butyltindichloride (DBTC) in the small fish species Orysias latipes
(medaka) and Poecilia reticulata (guppy), Utrecht, The Netherlands,
University of Utrecht (Wester, P.W., Ph.D. Thesis).
WESTER, P.W. (in press) Chronic toxicity and carcinogenicity of bis (tri-n-
butyltin) oxide in the rat. Food Chem. Toxicol.
WHO/FAO (1984) Data sheet on pesticides No. 65: Bis (tributyltin)oxide,
Geneva, World Health Organization (VBC/PDS/DS/85.65).
WOLNIAKOWSKI, K.U., STEPHENSON, M.D., & ICHIKAWA, G.S. (1987) Tributyltin
concentrations and Pacific oyster deformations in Coos Bay, Oregon. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1438-1442.
WONG, P.T.S., CHAU, Y.K., KRAMAR, O., & BENGERT, G.A. (1982) Structure-
toxicity relationship of tin compounds on algae. Can. J. Fish. aquat. Sci.,
39: 483-488.
WOOTEN, R., DAVIES, I.M., MCKIE, J.C., & BRUNO, D.W. (1986) Chemical and
histopathological changes in salmon exposed to low concentrations of
tributyltin in seawater, Aberdeen, Department of Agriculture and Fisheries
for Scotland, 3 pp (Working Paper No. 15/86).
YLA-MONONEN, L. (1988) Finnish studies on the use, occurrence and effects
of organic tin compounds. Presented at the OECD Workshop on Monitoring,
Chemical Analysis and Leaching Rates of TBT, Paris, 30 November-2 December,
1988, 9 pp (Unpublished report).
YOUNG, D.R., SCHATZBERG, P., BRINCKMAN, F.E., CHAMP, M.A., HOLM, S.E., &
LANDY, R.B. (1986) Summary report - Interagency workshop on aquatic
sampling and analysis for organotin compounds. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1135-1140.
ZEDLER, R.J. (1961) Organotin as industrial biochemicals. Tin Uses,
53: 7-11.
ZIMMERLI, B. & ZIMMERMANN, H. (1980) Gas-chromatische bestimmung von spuren
von n-butylzinnverbindungen (tetra-, tri-, di-). Fresenius Z. anal. Chem.,
304: 23-27.
ZUCKERMAN, J.J., REISDORF, R.P., ELLIS, H.V., & WILKINSON, R.R. (1978)
Organotins in biology and the environment. In: Brinkman, F.E. & Bellama,
J.M., ed. Organometals and organometalloids, occurrence and fate in the
environment, Washington, DC, American Chemical Society, pp. 388-424 (ACS
Symposium Series No. 82).
RESUME
1. Identité, propriétés physiques et chimiques
Les dérivés du tributylétain (TBT) sont des dérivés
organiques de l'étain tétravalent. Ils se caractérisent
par la présence de liaisons covalentes entre des atomes de
carbone et un atome d'étain et leur formule générale est
la suivante: (n-C4H9)3 Sn-X (dans laquelle X désigne
un anion). La pureté de l'oxyde de tributylétain du com-
merce est généralement supérieure à 96%; les principales
impuretés sont le dibutylétain et dans une moindre mesure
le tétrabutylétain et d'autres trialkylétains. L'oxyde de
tributylétain est un liquide incolore d'odeur caractéris-
tique et de densité comprise entre 1,17 et 1,18. Il est
peu soluble dans l'eau (solubilité comprise entre moins
de 1,0 et plus de 100 mg/litre selon le pH, la température
et les anions présents dans l'eau qui déterminent l'espèce
chimique en cause). Dans l'eau de mer et dans les con-
ditions normales, on rencontre trois dérivés du tributylé-
tain: l'hydroxyde, le chlorure et le carbonate, qui sont
en équilibre. Aux pH inférieurs à 7,0 les formes prédomi-
nantes sont Bu3SnOH2+ et Bu3SnCl; à pH 8 ce sont
Bu3SnCl, Bu3SnOH et Bu3SnCO3-; au-delà de 10, ce
sont Bu3SnOH et Bu3SnCO3- qui prédominent.
Le coefficient de partage octanol/eau (log de Pow)
est compris entre 3,18 et 3,84 pour l'eau distillée et il
est égal à 3,54 pour l'eau de mer. L'oxyde de tributylé-
tain s'adsorbe fortement aux matières particulaires,
puisque les coefficients d'absorption indiqués dans la
littérature vont de 110 à 55 000. La tension de vapeur est
faible mais les valeurs publiées sont extrêmement varia-
bles. On n'a constaté aucune perte d'oxyde de tributylé-
tain à partir d'une solution de 1 mg/litre en 62 jours,
toutefois 20% de l'eau avait disparu par évaporation.
2. Méthodes d'analyse
On utilise plusieurs méthodes pour le dosage des
dérivés du tributylétain dans l'eau, les sédiments ou les
biotes. La plus communément utilisée est la spectrométrie
d'absorption atomique. La spectrométrie d'absorption
atomique avec flamme a une limite de détection de
0,1 mg/litre. Sans flamme, avec atomisation dans un four
électrique à graphite, elle est plus sensible et ses
limites de détection varient entre 0 et 1,0 µg/litre
d'eau. Il existe plusieurs méthodes d'extraction et de
préparation de dérivés volatils. La séparation de ces
dérivés s'effectue habituellement par piégeage ou par
chromatographie en phase gazeuse. Les limites de détection
se situent entre 0,5 et 5,0 µg/kg dans le cas des sédi-
ments et des biotes.
3. Sources de pollution de l'environnement
Les dérivés du tributylétain sont homologués comme
molluscicides, comme produits antisalissures pour la
préservation des coques de bateaux, des appontements, des
bouées, des casiers à crabes, des filets et des cages,
comme enduits de protection du bois, comme alguicides dans
le bâtiment, comme désinfectants et comme biocides dans
les systèmes de réfrigération, les tours de réfrigération
des centrales électriques, les usines de pâte à papier,
les brasseries, les tanneries et les usines textiles. Les
premières peintures antisalissures à base de TBT con-
tenaient ce produit sous une forme qui en permettait la
libération sans entrave. Plus récemment, sont apparues des
peintures dans lesquelles l'incorporation du TBT dans une
matrice en copolymère permet d'en limiter la libération.
On a également mis au point des matrices caoutchouteuses
qui permettent une libération lente et durable et assurent
aux peintures antisalissures et aux molluscicides une
efficacité prolongée. Le TBT n'est pas utilisé en agri-
culture en raison de sa forte phytotoxicité.
4. Réglementation
De nombreux pays ont restreint l'utilisation des
peintures antisalissures à base de TBT du fait de l'action
de cette substance sur les fruits de mer. Les détails de
la réglementation varient d'un pays à l'autre mais la
plupart interdisent l'emploi de peintures à base de TBT
sur les navires de moins de 25 mètres. Dans certains pays,
les navires à coque d'aluminium ne sont pas visés par
cette interdiction. En outre, certaines réglementations
limitent la teneur des peintures en TBT ou la lixiviation
de cette substance à partir des peintures qui en contien-
nent (4 à 5 µg/cm2 par jour sur une longue période).
5. Concentrations dans l'environnement
On a trouvé de fortes concentrations de TBT dans
l'eau, les sédiments et les biotes à proximité de zones de
plaisance, plus particulièrement de marinas, de chantiers
navals et de bassins de radoub, de filets et de cages
traités au moyen de peintures antisalissures et de sys-
tèmes de réfrigération. Ces concentrations de TBT dépen-
dent également de la submersion par la marée et de la
turbidité de l'eau.
On a observé que les concentrations de TBT pouvaient
atteindre 1,58 µg/litre dans l'eau de mer et les estuai-
res, 7,1 µg/litre dans l'eau douce, 26 300 µg/kg dans
les sédiments littoraux, 3700 µg/kg dans les sédiments
d'eau douce, 6,39 mg/kg dans les bivalves, 1,92 mg/kg dans
les gastéropodes et 11 mg/kg dans le poisson. Il ne faut
pas considérer cependant ces concentrations maximales
comme caractéristiques car un certain nombre de facteurs
peuvent donner lieu à des teneurs anormalement élevées
(par exemple la présence de particules de peinture dans
les échantillons d'eau et de sédiments). On a constaté que
les concentrations de TBT dans la micro-couche de surface
des eaux douces et des eaux de mer étaient jusqu'à 100
fois plus élevées que celles qu'on pouvait mesurer juste
en dessous de la surface. Toutefois, il convient de noter
que la concentration en TBT dans la micro-couche de
surface peut dépendre dans une très large mesure de la
technique d'échantillonnage.
Il se peut que les données anciennes ne soient pas
comparables aux données récentes en raison des amélio-
rations apportées aux méthodes de dosage du TBT dans
l'eau, les sédiments et les tissus.
6. Transport et transformation dans l'environnement
Du fait de sa faible solubilité dans l'eau et de son
caractère lipophile, le TBT s'adsorbe facilement aux par-
ticules. On estime que 10 à 95% de l'oxyde de tributylé-
tain qui pénètrent dans l'eau s'adsorbent ainsi sur les
particules. La disparition progressive du TBT absorbé
n'est pas due à sa désorption mais à sa dégradation. Le
degré d'adsorption dépend de la salinité, de la nature et
de la taille des particules en suspension, de la quantité
de matières en suspension, de la température et de la
présence de matières organiques dissoutes.
La dégradation de l'oxyde de tributylétain s'effectue
par rupture de la liaison carbone-étain. Celle-ci peut
résulter de divers mécanismes qui se produisent simultané-
ment dans l'environnement et notamment des mécanismes
physico-chimiques (hydrolyse et photodécomposition) ou
biologiques (dégradation par des micro-organismes et
métabolisation par des organismes supérieurs). L'hydrolyse
des dérivés organostanniques se produit à des valeurs
extrêmes du pH mais n'apparaît guère dans les conditions
qui règnent normalement dans l'environnement. La photo-
décomposition se produit par exposition en laboratoire de
solutions à un rayonnement ultra-violet de 300 nm (et à un
moindre degré, à un rayonnement de 350 nm). Dans le milieu
naturel, la photolyse est limitée par la longueur d'onde
du rayonnement solaire et par la pénétration du rayonne-
ment ultra-violet dans l'eau. La présence de substances
photosensibilisatrices peut accélérer la photodécomposi-
tion. La biodégradation dépend de l'état du milieu, et de
caractéristiques telles que sa température, son oxygéna-
tion, son pH, sa teneur en éléments minéraux, la présence
de substances organiques facilement biodégradables pouvant
subir une co-métabolisation ainsi que la nature de la
micro-flore et sa capacité à s'adapter. Elle ne peut
également avoir lieu que si la concentration en oxyde de
tributylétain est inférieure à la concentration létale ou
inhibitrice pour les bactéries. Comme dans le cas de la
décomposition abiotique, la dégradation biologique du TBT
comporte une débutylation oxydante progressive avec rup-
ture de la liaison carbone-étain. Il se forme des dérivés
dibutylés dont la dégradation est plus facile que celle du
tributylétain. Les monobutylétains sont lentement minéra-
lisés. Il se produit également une dégradation anaérobie
mais son importance reste discutée. Certains chercheurs
estiment que la dégradation en anaérobiose est lente alors
que d'autres la jugent plus rapide que la dégradation
aérobie. On a identifié des espèces de bactéries, d'algues
et de champignons attaquant le bois qui sont capables de
dégrader l'oxyde de tributylétain. Les estimations de la
demi-vie du TBT dans l'environnement varient dans
d'importantes proportions.
Le TBT s'accumule dans les organismes du fait de sa
solubilité dans les graisses. Des recherches en labora-
toire portant sur des mollusques et des poissons ont
donné, pour les facteurs de bioconcentration, des valeurs
allant jusqu'à 7000 mais des valeurs encore plus élevées
ont été observées lors d'études sur le terrain. L'absorp-
tion à partir de la nourriture est plus importante qu'à
partir de l'eau. Les facteurs de concentration plus élevés
observés chez les micro-organismes (entre 100 et 30 000)
peuvent s'expliquer par une adsorption plutôt que par une
absorption intracellulaire. Rien n'indique que le TBT
puisse passer dans les organismes terrestres par l'inter-
médiaire de la chaîne alimentaire.
7. Cinétique et métabolisme
Le tributylétain est absorbé au niveau intestinal (20
à 50% selon le véhicule) ainsi que par voie percutanée
chez les mammifères (dans la proportion d'environ 10%).
Il peut traverser la barrière hémo-méningée et passer du
placenta dans le foetus. Une fois absorbé, il est rapide-
ment et largement diffusé dans l'organisme (principalement
au niveau du foie et des reins).
Chez les mammifères, la métabolisation du TBT est
rapide; on peut déceler les métabolites dans le sang dans
les 3 heures suivant l'administration. Des études in vitro
ont montré que le TBT servait de substrat aux oxydases à
fonction mixte mais qu'il inhibait ces enzymes à très
forte concentration.
L'élimination du TBT s'effectue plus ou moins rapide-
ment selon la nature du tissu et les estimations de la
demi-vie biologique chez les mammifères varient de 23 à
environ 30 jours.
Les organismes inférieurs métabolisent également le
TBT mais le processus est plus lent - en particulier chez
les mollusques - que chez les mammifères. La capacité de
bioaccumulation est donc beaucoup plus importante que chez
les mammifères.
Les tributylétains inhibent la phosphorylation oxy-
dative et modifient la structure et la fonction des mito-
chondries. Le TBT empêche la calcification de la coquille
des huîtres (espèces du genre Crassostrea ).
8. Effets sur les micro-organismes
Le TBT est toxique pour les micro-organismes et on le
vend comme bactéricide et alguicide. Les concentrations
toxiques varient considérablement selon les espèces. Le
TBT est plus toxique pour les bactéries gram-positives
avec une concentration minimale inhibitrice (CMI) allant
de 0,2 à 0,8 mg/litre que pour les bactéries gram-néga-
tives (CMI des 3 mg/litre). La CMI de l'acétate de TBT
pour les champignons est de 0,5 à 1 mg/litre et celle de
l'oxyde de tributylétain est de 0,5 mg/litre pour l'algue
verte Chlorella pyrenoidosa. La productivité primaire
d'une communauté naturelle d'algues d'eau douce a été
réduite de 5% par une concentration d'oxyde de tributylé-
tain de 3 µg/litre. On a récemment établi la dose sans
effet observable pour 2 espèces d'algues; elle est
respectivement de 18 et 32 µg/litre. La toxicité pour
les microorganismes marins varie également selon les
espèces et selon les études; il est difficile d'établir
la valeur de la dose sans effet observable mais on pense
qu'elle est inférieure à 0,1 µg/litre pour certaines
espèces. Les concentrations alguicides vont de moins de
1,5 µg/litre à plus de 1000 µg/litre selon les espèces.
9. Effets sur les organismes aquatiques
9.1 Effets sur les organismes marins et estuariels
La Figure 1 donne un diagramme récapitulatif des
effets létaux et sublétaux que peuvent produire les con-
centrations de TBT relevées en mer et dans les estuaires.
Des concentrations supérieures à celles qui produisent les
effets létaux aigus ont été observées en différents points
du globe, notamment là où se déroulent des activités de
plaisance.
Ce sont les spores mobiles d'une algue verte géante
qui se sont révélés les plus sensibles au TBT (CE50 à 5
jours: 0,001 µg/litre). On a constaté une réduction de
la croissance d'un angiosperme marin à des concentrations
de TBT de 1 mg/kg de sédiments, aucun effet n'étant noté à
0,1 mg/kg.
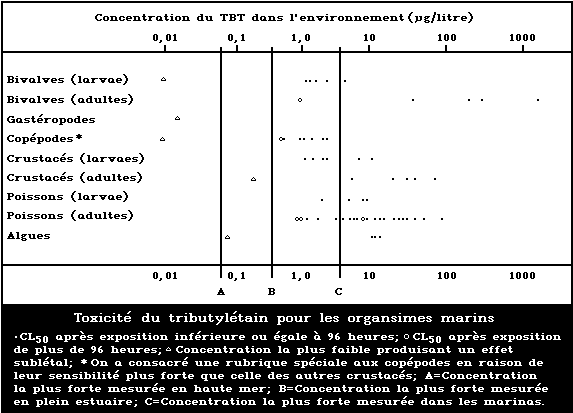 Le tributylétain est très toxique pour les mollusques
marins. On a montré expérimentalement qu'il perturbait la
formation de la coquille, le développement des gonades et
la différenciation sexuelle des huîtres adultes, leur
fixation et leur croissance; en outre on a noté une morta-
lité des larves d'huîtres et d'autres bivalves et
l'apparition de caractères mâles chez les gastéropodes
femelles. La dose sans effet observable serait de
20 ng/litre pour le naissain de l'espèce d'huître la plus
sensible, l'huître japonaise (Crassostrea gigas). Chez les
adultes, il se produit également une déformation de la
coquille qui est liée à la dose. Expérimentalement, on n'a
pas observé d'effets sur la morphologie coquillière à des
concentrations de TBT de 2 ng/litre. La dose sans effet
observable correspondant à l'apparition de caractères
mâles chez les mollusques femelles du genre Thais est
inférieure à 1,5 ng/litre. Les formes larvaires sont
généralement plus sensibles que les adultes; la différence
est particulièrement marquée dans le cas des huîtres.
Les copépodes sont plus sensibles que les autres
crustacés aux effets létaux aigus du TBT, avec des valeurs
de la CL50 pour des périodes allant jusqu'à 96 heures
comprises entre 0,6 et 2,2 µg/litre. Ces valeurs sont
comparables à celles qui s'appliquent aux larves les plus
sensibles des autres groupes de crustacés. Le TBT réduit
la capacité de reproduction, la survie néonatale et la
vitesse de croissance des crustacés. Dans le cas de la
crevette Acanthomysis sculpta, un mysidé, la dose sans
effet observable sur la reproduction serait de
0,09 µg/litre. La crevette ne cherche pas à éviter le TBT
au-dessous de 30 µg/litre.
La toxicité du tributylétain pour les poissons de mer
est très variable, les valeurs de la CL50 à 96 heures
allant de 1,5 à 36 µg/litre. Les stades larvaires sont
plus sensibles que les adultes (Figure 1). Il semblerait
que les poissons de mer évitent l'oxyde de tributylétain à
partir de 1 µg/litre.
9.2 Effets sur les organismes d'eau douce
Un diagramme récapitulatif concernant les effets
létaux et sublétaux des concentrations de TBT mesurées
dans l'eau douce est donné à la Figure 2. On a observé la
présence de concentrations supérieures à celles qui
produisent des effets sublétaux, notamment dans les zones
de navigation de plaisance.
Une concentration d'oxyde de tributylétain de
0,5 mg/litre a été mortelle pour des angiospermes d'eau
douce et leur croissance était inhibée dès 0,06 mg/litre.
On ne dispose que de peu de données sur les inverté-
brés d'eau douce, tout au plus sur trois espèces autres
que les organismes cibles. Pour divers sels de tributylé-
tain on a obtenu des valeurs de la CL50 à 48 heures de
2,3-70 µg/litre pour la daphnie et de 5,5-33 µg par
litre pour les vers de vase. La dose sans effet observable
pour la daphnie est évaluée à 0,5 µg/litre, le critère
choisi étant la réapparition d'une réaction normale à la
lumière. En ce qui concerne la palourde d'Asie, on indique
une CL50 à 24 heures de 2100 µg/litre, les valeurs
corrrespondantes allant de 30 à 400 µg/litre pour les
mollusques adultes que l'on cherche à détruire dans les
opérations de lutte contre la schistosomiase.
On a montré que le tributylétain était toxique pour
les larves de schistosome à leur stade aquatique; la
CL50 du fluorure de tributylétain est de 16,8 µg par
litre pour une exposition d'une heure. Une dose de TBT
comprise en 2 et 6 µg/litre supprime à hauteur de 99 à
100% l'infectiosité des cercaires pour la souris.
La sensibilité des mollusques au TBT diminue avec
l'âge mais les oeufs sont plus résistants que les jeunes
ou les adultes. La ponte est notablement affectée à une
concentration en oxyde de tributylétainde 0,001 µg/litre.
La toxicité aiguë du TBT pour les poissons d'eau douce
s'est située, pour des périodes allant jusqu'à 168 heures,
dans les limites de 13 à 240 µg/litre, valeurs correspon-
dant à la CL50. Dans le cas du guppy, la dose sans effet
histopathologique observable a été estimée à 0,01 µg/litre.
Après exposition de grenouilles Rana temporaria à des
concentrations inférieures ou égales 3 µg/litre, on n'a
observé aucun effet sur la survie des oeufs ni des larves;
en revanche, à la concentration de 30 µg/litre on a noté
une mortalité sensible.
Le tributylétain est très toxique pour les mollusques
marins. On a montré expérimentalement qu'il perturbait la
formation de la coquille, le développement des gonades et
la différenciation sexuelle des huîtres adultes, leur
fixation et leur croissance; en outre on a noté une morta-
lité des larves d'huîtres et d'autres bivalves et
l'apparition de caractères mâles chez les gastéropodes
femelles. La dose sans effet observable serait de
20 ng/litre pour le naissain de l'espèce d'huître la plus
sensible, l'huître japonaise (Crassostrea gigas). Chez les
adultes, il se produit également une déformation de la
coquille qui est liée à la dose. Expérimentalement, on n'a
pas observé d'effets sur la morphologie coquillière à des
concentrations de TBT de 2 ng/litre. La dose sans effet
observable correspondant à l'apparition de caractères
mâles chez les mollusques femelles du genre Thais est
inférieure à 1,5 ng/litre. Les formes larvaires sont
généralement plus sensibles que les adultes; la différence
est particulièrement marquée dans le cas des huîtres.
Les copépodes sont plus sensibles que les autres
crustacés aux effets létaux aigus du TBT, avec des valeurs
de la CL50 pour des périodes allant jusqu'à 96 heures
comprises entre 0,6 et 2,2 µg/litre. Ces valeurs sont
comparables à celles qui s'appliquent aux larves les plus
sensibles des autres groupes de crustacés. Le TBT réduit
la capacité de reproduction, la survie néonatale et la
vitesse de croissance des crustacés. Dans le cas de la
crevette Acanthomysis sculpta, un mysidé, la dose sans
effet observable sur la reproduction serait de
0,09 µg/litre. La crevette ne cherche pas à éviter le TBT
au-dessous de 30 µg/litre.
La toxicité du tributylétain pour les poissons de mer
est très variable, les valeurs de la CL50 à 96 heures
allant de 1,5 à 36 µg/litre. Les stades larvaires sont
plus sensibles que les adultes (Figure 1). Il semblerait
que les poissons de mer évitent l'oxyde de tributylétain à
partir de 1 µg/litre.
9.2 Effets sur les organismes d'eau douce
Un diagramme récapitulatif concernant les effets
létaux et sublétaux des concentrations de TBT mesurées
dans l'eau douce est donné à la Figure 2. On a observé la
présence de concentrations supérieures à celles qui
produisent des effets sublétaux, notamment dans les zones
de navigation de plaisance.
Une concentration d'oxyde de tributylétain de
0,5 mg/litre a été mortelle pour des angiospermes d'eau
douce et leur croissance était inhibée dès 0,06 mg/litre.
On ne dispose que de peu de données sur les inverté-
brés d'eau douce, tout au plus sur trois espèces autres
que les organismes cibles. Pour divers sels de tributylé-
tain on a obtenu des valeurs de la CL50 à 48 heures de
2,3-70 µg/litre pour la daphnie et de 5,5-33 µg par
litre pour les vers de vase. La dose sans effet observable
pour la daphnie est évaluée à 0,5 µg/litre, le critère
choisi étant la réapparition d'une réaction normale à la
lumière. En ce qui concerne la palourde d'Asie, on indique
une CL50 à 24 heures de 2100 µg/litre, les valeurs
corrrespondantes allant de 30 à 400 µg/litre pour les
mollusques adultes que l'on cherche à détruire dans les
opérations de lutte contre la schistosomiase.
On a montré que le tributylétain était toxique pour
les larves de schistosome à leur stade aquatique; la
CL50 du fluorure de tributylétain est de 16,8 µg par
litre pour une exposition d'une heure. Une dose de TBT
comprise en 2 et 6 µg/litre supprime à hauteur de 99 à
100% l'infectiosité des cercaires pour la souris.
La sensibilité des mollusques au TBT diminue avec
l'âge mais les oeufs sont plus résistants que les jeunes
ou les adultes. La ponte est notablement affectée à une
concentration en oxyde de tributylétainde 0,001 µg/litre.
La toxicité aiguë du TBT pour les poissons d'eau douce
s'est située, pour des périodes allant jusqu'à 168 heures,
dans les limites de 13 à 240 µg/litre, valeurs correspon-
dant à la CL50. Dans le cas du guppy, la dose sans effet
histopathologique observable a été estimée à 0,01 µg/litre.
Après exposition de grenouilles Rana temporaria à des
concentrations inférieures ou égales 3 µg/litre, on n'a
observé aucun effet sur la survie des oeufs ni des larves;
en revanche, à la concentration de 30 µg/litre on a noté
une mortalité sensible.
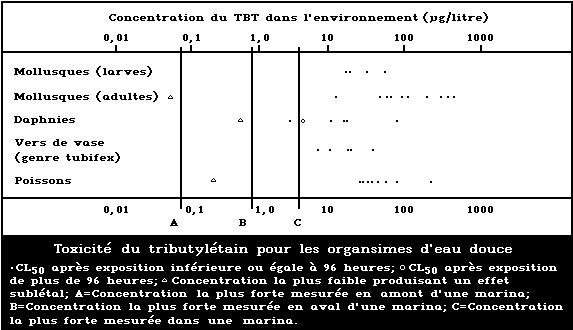 9.3 Etudes de microcosme
En vue de la modélisation des écosystèmes marins on a
effectué des études de microcosme consistant à introduire
dans ces milieux certains organismes et en se plaçant dans
des conditions où un apport d'eau de mer permettait la
colonisation du milieu par d'autres biotes. On a constaté
qu'à des concentrations d'oxyde de tributylétain comprises
entre 0,06 et 3 µg/litre, il y avait réduction du nombre
d'individus et une moindre diversité des espèces.
Les résultats obtenus par modélisation d'écosystèmes
d'eau douce montrent que les doses qui tuent les mol-
lusques sont également nocives pour d'autres espèces,
notamment les poissons.
10. Effets sur les organismes terrestres
L'exposition des organismes terrestres au TBT
découlent essentiellement de l'utilisation de ces subs-
tances pour la protection du bois. L'oxyde de tributylé-
tain est toxique pour la population des ruches dont le
bois de construction a été traité au TBT. Une étude,
d'ailleurs unique, a montré que le TBT était toxique pour
les chauves-souris mais le résultat ne peut pas être con-
sidéré comme statistiquement significatif en raison de la
forte mortalité des témoins. Les dérivés du tributylétain
sont toxiques pour les insectes exposés, soit topiquement
soit par suite de xylophagie. Pour les souris de type
sauvage, la toxicité aiguë du TBT est moyenne; les valeurs
de la CL50 par voie alimentaire, calculées d'après la
consommation de semences traitées et soumises à des
épreuves de répulsivité, vont de 37 à 240 mg/kg par jour.
11. Effets sur l'aquaculture
Les observations effectuées dans des zones d'aquacul-
ture ont permis d'attribuer à la présence de fortes con-
centrations de tributylétain, un certain nombre d'effets
nocifs observés sur des bivalves tels que: mortalité,
incapacité à se fixer, moindre croissance, épaississement
de la coquille et autres malformations notamment chez les
huîtres, apparition de caractères mâles chez les gastéro-
podes (avec diminution simultanée des populations) ainsi
que chez les mollusques du genre Thais. C'est en France
que pour la première fois on a attribué à la présence de
TBT dans l'eau, l'anéantissement total de parcs à huîtres,
observations qui ont été faites ensuite dans d'autres
pays. Les effets étaient particulièrement marqués dans les
secteurs proches de marinas destinées aux navires de
plaisance. La réglementation de l'usage des peintures
antisalissures à base TBT sur les petits navires a permis
aux huîtres de retrouver leur capacité de reproduction et
de croissance. Toutefois la concentration du TBT dans
l'eau reste suffisamment forte dans certains secteurs pour
nuire aux gastéropodes marins. On utilise la croissance
de la coquille et son gaufrage chez les huîtres japonaises
ainsi que l'apparition de caractères mâles chez les
mollusques du genre Thais comme indicateurs biologiques
d'une contamination par du tributylétain.
Peu d'études ont été consacrées aux effets du TBT
sédimentaire sur la faune marine mais on pense qu'il peut
être absorbé par les organismes fouisseurs et provoquer
une certaine mortalité.
Des effets toxiques macroscopiques et des altérations
histopathologiques ont été observés dans des élevages de
poissons de mer, les filets délimitant les bassins ayant
été traités au moyen de peintures aintisalissures à base
de TBT.
On a proposé d'utiliser du TBT comme molluscicides
pour détruire les mollusques d'eau douce qui transmettent
la bilharziose (bilharzies). Un certain nombre d'essais
ont été effectués sur le terrain, dont il ressort que le
TBT est difficile à utiliser sans préjudice pour les
organismes non visés.
12. Toxicité pour les mammifères de laboratoire
12.1 Toxicité aiguë
Le tributylétain est moyennement à fortement toxique
pour les mammifères de laboratoire, avec des valeurs de la
DL50 par voie orale allant de 94 à 234 mg/kg de poids
corporel chez le rat et de 44 à 230 mg/kg de poids
corporel chez la souris. Pour le cobaye et le lapin, les
valeurs sont du même ordre de grandeur. Les variations
enregistrées sont dues aux différents anions entrant dans
la composition du sel de tributylétain. Ces composés
entraînent une mortalité plus forte lorsqu'ils sont
administrés par voie parentérale plutôt que par voie
orale, probablement du fait qu'ils ne sont que partielle-
ment absorbés au niveau intestinal.
Parmi les autres effets toxiques aigus on peut citer
des anomalies concernant les taux de lipide sanguins, le
système endocrinien, le foie, la rate et un déficit
passager dans le développement cérébral. La portée toxi-
cologique réelle de ces effets, qui n'ont été observés
qu'après administration de doses uniques élevées de ces
composés, reste discutable et la cause effective de la
mort n'est pas véritablement connue.
Par voie percutanée, la toxicité aiguë est faible, la
DL50 étant supérieure à 9000 mg/kg de poids corporel chez
le lapin. Chez le rat, après inhalation purement nasale,
la DL50 à 4 heures se situait à 77 mg/m3 (65 mg/m3 si
l'on ne tient compte que des particules respirables). Des
mélanges d'air et de vapeurs de tributylétain ne produi-
sent pas d'effets toxiques observables, même à saturation.
Toutefois le TBT est très dangereux sous forme d'aérosol
lorsqu'il est inhalé et il produit alors une irritation et
un oedème des poumons.
Le TBT est très irritant pour la peau et extrêmement
irritant pour l'oeil. L'oxyde de tributylétain n'a pas
d'effet sensibilisateur cutané.
12.2 Toxicité à court terme
Les composés du tributylétain ont été très étudiés
chez le rat (toutes les données présentées dans ce qui
suit concernent cet animal, sauf indication contraire).
On a observé un fort taux de mortalité après une durée
d'exposition de plus de quatre semaines à des doses dans
l'alimentation de 320 mg/kg (environ 25 mg/kg de poids
corporel). Acune mortalité n'a été observée à la dose de
100 mg/kg de nourriture (10 mg/kg de poids corporel) ni
après l'administration par gavage d'une dose correspondant
à 12 mg de TBT/kg de poids corporel. Administrée à des
ratons peu après leur naissance, une dose de 3 mg/kg de
poids corporel a augmenté la mortalité. Les principaux
symptômes observés après administration de doses mortelles
consistaient en perte d'appétit, faiblesse et émaciation.
On a observé des effets marginaux sur la croissance
aux doses de 50 mg/kg de nourriture (6 mg/kg de poids
corporel) et de 6 mg/kg de poids corporel (administrées
par gavage). Les souris sont moins sensibles, ces effets
n'étant observés qu'à partir de 150 à 200 mg/kg de nour-
riture (22 à 29 mg/kg de poids corporel).
Des effets structuraux ont été observés sur les
organes endocrines, essentiellement l'hypophyse et la
thyroïde, lors d'études à court et à long terme. Lors des
études à court terme, on a observé des anomalies dans la
concentration des hormones circulantes ainsi que dans la
réponse aux stimuli physiologiques (trophines hypophy-
saires); toutefois après une exposition de longue durée,
la plupart de ces anomalies avaient disparu. Le mécanisme
sous-jacent n'est pas connu.
L'exposition à un aérosol d'oxyde de tributylétain à
la dose de 2,8 mg/m3 a déterminé une forte mortalité,
une détresse respiratoire, une réaction inflammatoire au
niveau des voies respiratoires et des anomalies histo-
pathologiques des organes lymphatiques. En revanche,
l'exposition à des vapeurs à la concentration la plus
forte possible (0,16 mg/m3) à la température ambiante,
n'a produit aucun effet.
On a signalé des effets toxiques au niveau du foie et
des canaux biliaires chez ces trois espèces de mammifères.
Ainsi une nécrose des cellules hépatiques et des altéra-
tions inflammatoires du canal cholédoque ont été obser-
vées chez des rats qui avaient reçu de l'oxyde de
tributylétain à raison de 320 mg/kg de nourriture (soit
approximativement 25 mg/kg de poids corporel) pendant
quatre semaines et des souris qui en avaient reçu 80 mg/kg
de nourriture (soit approximativement 12 mg/kg de poids
corporel) pendant trois mois. Chez des chiens soumis à une
dose de 10 mg/kg de poids corporel pendant huit à neuf
semaines, on a observé une vacuolisation des hépatocytes
de la région périportale. Ces altérations s'accompagnaient
occasionnellement d'un accroissement du poids du foie et
d'une augmentation de l'activité sérique des enzymes
hépatiques.
La réduction de la concentration d'hémoglobine et de
l'hématocrite chez le rat, à la suite de l'administration
d'une dose correspondant à 80 mg/kg de nourriture (soit
8 mg/kg de poids corporel), montre qu'il y a un effet sur
la synthèse de l'hémoglobine qui conduit à une anémie
hypochrome microcytaire. La réduction des taux d'hémosi-
dérine splénique donne à penser qu'il y a action au niveau
des réserves martiales. Une anémie a également été
observée chez la souris.
La formation de rosettes érythrocytaires au niveau des
ganglions lymphatiques mésentériques a été observée lors
de certaines études à court terme mais pas lors d'études à
long terme. La portée biologique de cette observation
(vraisemblablement passagère) reste obscure.
L'oxyde de tributylétain exerce un effet toxique
caractéristique sur le système immunitaire; du fait de son
action sur le thymus, il perturbe les fonctions immuni-
taires à médiation cellulaire. Le mode d'action demeure
inconnu mais il pourrait y avoir conversion métabolique en
dibutylétain. La résistance non spécifique est également
amoindrie.
Un certain nombre d'études sur rats et chiens mais à
l'exclusion des souris, ont été effectuées avec de l'oxyde
de tributylétain et ont révélé l'existence d'effets
généraux sur le système immunitaire (par exemple poids et
morphologie des tissus lymphoïdes, numération des lympho-
cytes périphériques, concentration totale des immunoglo-
bulines sériques), à des doses largement toxiques (des
effets ont été observés chez la souris à des doses de
150 mg/kg de chlorure de tributylétain). Seul le rat
manifeste des effets généraux sur le système immunitaire
sans autres signes patents de toxicité et il se révèle
indiscutablement être l'espèce la plus sensible. Des
études à court terme sur le rat ont permis d'établir que
la dose sans effet observable était de 5 mg/kg de nour-
riture (soit 0,6 mg/kg de poids corporel). Lors des études
portant sur le chlorure de tributylétain, on a observé
des effets analogues au niveau du thymus. Ces effets
disparaissaient rapidement lorsqu'on cessait d'administrer
la substance. On a montré que l'oxyde de tributylétain,
étudié dans le cadre de travaux sur la résistance de
l'hôte, perturbait les fonctions immunitaires spécifiques
du rat. L'organisme de cet animal présentait une moindre
aptitude à éliminer les Listeria monocytogenes après
exposition à une dose de 50 mg/kg de nourriture (dose sans
effet observable: 5 mg/kg et par jour), et une moindre
résistance à Trichinella spiralis a été observée aux doses
respectives de 50 et 5 mg/kg de nourriture, cet effet
disparaissant à la dose de 0,5 mg/kg de nourriture (ce qui
correspond à des doses quotidiennes respectives de 2,5,
0,25 et 0,025 mg/kg de poids corporel). Des effets
analogues ont été observés chez des animaux âgés mais ils
étaient moins marqués.
Dans l'état actuel des connaissances, les effets sur
la résistance de l'hôte sont probablement très utiles pour
évaluer les risques pour la santé humaine, mais l'on ne
possède pas une expérience suffisante de ces systèmes
d'épreuve pour tirer des conclusions définitives des
résultats obtenus. Il reste que l'observation de rats
glabres athymiques soumis à une inoculation de T. spiralis
virulentes a permis l'interprétation des résultats
obtenus sur le modèle T. spiralis. En effet, l'absence
totale d'immunité thymo-dépendante a multiplié par 10 à 20
le nombre de larves présentes dans les muscles; par contre
l'exposition à des concentrations d'oxyde de tributylétain
respectivement égales à 5 et 50 mg/kg de nourriture a
multiplié par 2 et 4 respectivement le nombre de ces
larves.
Il existe quelques données concernant les effets du
tributylétain sur le système immunitaire en développement
mais sans aucune information sur la résistance de l'hôte.
Pour évaluer les dangers potentiels pour l'homme, il
serait plus prudent de prendre en considération les effets
produits sur l'espèce la plus sensible. On a observé des
effets sur la résistance de l'hôte à T. spiralis à des
concentrations dans l'alimentation ne dépassant pas
5 mg/kg (soit l'équivalent de 0,25 mg/kg de poids corporel
par jour), la dose sans effet observable étant égale à
0,25 mg/kg (ce qui correspond à 0,025 mg/kg par jour).
Toutefois, l'interprétation de ces résultats en vue d'une
évaluation du risque pour l'homme reste controversée.
Toutes les études qui ont été effectuées font ressortir
que la dose quotidienne sans effet observable pour ce qui
est des effets généraux ou spécifiques sur le système
immunitaire se situe à 5 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel selon les
études à court terme).
12.3 Toxicité à long terme
Une étude de longue durée chez le rat a montré que le
tributylétain a une effet marginal sur les paramètres
toxicologiques généraux (sans grande signification toxi-
cologique) à la dose de 5 mg/kg de nourriture (soit
0,25 mg/kg de poids corporel).
12.4 Génotoxicité
La génotoxicité de l'oxyde de tributylétain a fait
l'objet d'études approfondies. La plupart de ces études
ont donné des résultats négatifs et rien n'indique de
façon convaincante que ce produit puisse présenter le
moindre risque mutagène.
12.5 Toxicité vis-à-vis de la fonction de reproduction
On a évalué l'embryotoxicité potentielle de l'oxyde de
tributylétain sur trois espèces de mammifères (souris, rat
et lapin), après administration de la substance par voie
orale à la mère. La principale malformation observée chez
les foetus de rat et de souris était une fissure congéni-
tale du palais osseux mais il est vrai qu'elle ne se
produisait qu'à des doses manifestement toxiques pour la
mère. On ne peut pas considérer que ces résultats
témoignent d'une activité tératogène aux doses inférieures
à celles qui sont toxiques pour la mère. La dose minimale
sans effet observable pour ce qui est de l'embryotoxicité
et de la foetotoxicité chez ces trois espèces se situait à
1,0 mg/kg de poids corporel.
12.6 Cancérogénicité
Une étude de cancérogénicité a été effectuée sur des
rats et on a observé à cette occasion des modifications
néoplasiques au niveau des organes endocrines à la dose de
50 mg/kg de poids corporel. Les tumeurs hypophysaires
observées à la dose de 0,5 mg/kg de nourriture ne semblent
pas avoir d'importance biologique car il n'y a pas de
relation précise dose-réponse. Ces types de tumeurs
apparaissent en général chez les témoins à des fréquences
élevées et variables et leur signification reste donc
discutable. Une autre étude de cancérogénicité est en
cours sur des souris.
13. Effets sur l'homme
On a constaté que l'exposition professionnelle
d'ouvriers à des dérivés du tributylétain produisait une
irritation des voies respiratoires supérieures. Ces
substances sont dangereuses pour l'homme lorsqu'elles sont
sous la forme d'aérosols. L'oxyde de tributylétain est
irritant pour la peau et les muqueuses oculaires et il a
été fait état de dermatites graves à la suite d'un contact
direct avec la peau. Le risque est d'autant plus grave que
la réaction cutanée n'est pas immédiate.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour l'homme
Les travailleurs sont principalement exposés lors de
la préparation et de la formulation des dérivés du tri-
butylétain, de l'application ou de l'élimination de
peintures à base de TBT et de l'utilisation de produits de
protection du bois à base de ces substances. Quant à la
population dans son ensemble, elle peut être exposée par
contamination de la nourriture, en particulier des
poissons et des coquillages, et lors de l'utilisation de
produits de protection du bois à usage ménager.
Si l'on s'appuie sur l'expérimentation animale et
l'observation directe sur l'homme, il est clair que les
dérivés du tributylétain sont irritants pour la peau et
les yeux et que l'inhalation d'aérosols conduit à une
irritation des voies respiratoires.
La manipulation de bois traités n'entraîne aucune
irritation de l'épiderme une fois que le produit a séché.
Cependant les aérosols de TBT sont extrêmement dangereux
et il ne faut pas revenir dans le local où le bois a été
traité avant séchage complet.
On n'a jamais fait état d'intoxications aiguës généra-
lisées par le TBT qui en principe doit s'éliminer de
l'organisme en l'espace de quelques jours. Il est donc peu
probable que l'on puisse s'exposer à des intoxications
aiguës par la manipulation de produits à base de tri-
butylétain si l'on prend les précautions voulues.
On a fait état d'effets à court et à long terme chez
des animaux de laboratoire, sur le foie, le sang et les
glandes endocrines. Chez le rat, qui est l'espèce la plus
sensible, c'est l'effet au niveau du système immunitaire
et en particulier sur la résistance de l'hôte, qui
constitue le paramètre le plus sensible de la toxicité de
ces produits. En utilisant comme modèle la résistance de
l'hôte à l'infestation par Trichinella spiralis, on
obtient une dose sans effet observable qui se situe entre
0,5 et 5 mg/kg de nourriture (c'est-à-dire 0,025 et 0,25
mg/kg de poids corporel) alors que si l'on se rapporte à
l'action sur la fonction immunitaire, la dose est égale à
0,6 mg/kg de poids corporel.
En raison des grandes variations dans la consommation
de poissons et de coquillages ainsi que dans les teneurs
locales des fruits de mer en résidus de TBT, on ne peut
donner que quelques exemples pour illustrer l'exposition
résultante et les valeurs des doses sans effet observable.
Il importe de souligner que pour déterminer le risque
résultant de la présence de ces composés, il faut procéder
sur place à des mesures de résidus dans les denrées,
évaluer la consommation locale de fruits de mer et définir
une marge de sécurité acceptable.
En retenant pour la consommation de poisson les
chiffres de 15 et 150 grammes par jour, de 1 mg/kg pour
les résidus de TBT dans le poisson et un poids corporel
moyen de 60 kg, on obtient pour l'homme les marges
suivantes de sécurité selon les divers paramètres immuni-
taires.
---------------------------------------------------------------------
Consommation Apport estimatif Marge de sécurité
de poisson journalier de Modèle Autres paramètres
(g/jour) TBT (µg/kg) T. Spiralis immunitaires
---------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
---------------------------------------------------------------------
Utiliser des dérivés du TBT à tort et à travers et de
manière irresponsable sans suivre les recommandations qui
figurent dans la présente monographie pour réduire l'expo-
sition humaine peut conduire à l'ingestion de quantités de
TBT dangereuses pour la santé humaine.
On a noté jusqu'ici d'effets tératogènes, chez l'ani-
mal de laboratoire, qu'à des doses manifestement toxiques
pour la mère. On peut donc considérer que l'activité
tératogène du TBT est très faible.
En s'appuyant sur les résultats d'études très
complètes de mutagénicité, on estime que les dérivés du
tributylétain n'ont aucun pouvoir mutagène. Une étude de
cancérogénicité chez le rat, portant sur l'oxyde de
tributylétain, a fait apparaître une incidence accrue de
tumeurs endocriniennes; mais il s'agissait de tumeurs
spontanées dont l'incidence, généralement élevée, est très
variable. Ces résultats ne constituent donc pas un
argument bien net en faveur d'un risque cancérogène pour
l'homme.
2. Evaluation du risque écologique
La pénétration diffuse du tributylétain (TBT) dans
l'environnement a principalement pour origine l'utili-
sation de peintures antisalissures qui en contiennent.
Une contamination ponctuelle peut se produire lorsqu'on
utilise du TBT comme biocide dans les systèmes de réfri-
gération, lors du traitement de la pulpe de bois, le
tannage du cuir, la préservation du bois et le traitement
des textiles.
Du fait de leurs priopriétés physico-chimiques, les
dérivés du TBT se concentrent dans la micro-couche de
surface ainsi que dans les sédiments. Il ne semble pas
que le principal mécanisme d'élimination de ces substances
dans les conditions naturelles soit une dégradation abio-
tique. L'oxyde de tributylétain est biodégradable dans
l'eau mais le processus n'est pas suffisamment rapide pour
empêcher la présence dans certaines zones de concen-
trations élevées en TBT. Une bioaccumulation se produit
dans la plupart des organismes aquatiques mais chez les
mammifères de laboratoire, la dégradation métabolique est
plus efficace.
Le TBT est extrêment dangereux pour certains orga-
nismes aquatiques, du fait de sa toxicité à très faibles
concentrations dans l'eau. On le rencontre à ces concen-
trations dans certaines zones. On a signalé la présence
d'effets nocifs sur des invertébrés non visés, en parti-
culier des mollusques et ces effets sont suffisamment
graves pour bloquer la reproduction et entraîner un déclin
des populations de mollusques. Ces effets néfastes pour
la conchyliculture ont pu être combattus avec succès grâce
à des restrictions imposées à l'utilisation des peintures
antisalissures dans certains secteurs, restrictions qui
ont également permis d'éviter l'apparition de caractères
mâles chez les gastéropodes femelles. En ce qui concerne
le pisciculture, il convient de ne pas utiliser de pein-
tures à base de TBT sur les filets qui limitent les
bassins.
D'une manière générale, le risque pour l'environnement
terrestre est vraisemblablement faible. Cependant le
traitement du bois par ces substances pourrait se révéler
dangereux pour les organismes qui vivent à son contact.
L'augmentation de la teneur en TBT de la micro-couche
superficielle pourrait se révéler dangereuse pour la faune
côtière, pour les neustons (y compris les invertébrés
benthiques et les larves de poissons) ainsi que pour les
oiseaux de mer et le gibier d'eau qui se nourrissent en
surface. L'accumulation et le faible taux de biodégra-
dation du TBT dans les sédiments peut présenter un risque
pour les organismes aquatiques lorsque des sédiments
pollués sont soulevés par des processus naturels ou des
activités de dragage.
RECOMMANDATIONS
1. Recommandations pour la protection de la santé humaine
et de l'environnement
a) Les pays membres qui jusqu'ici n'ont pas réglementé
l'utilisation des dérivés du TBT devraient être
invités à le faire.
b) Il est nécessaire d'évaluer la pénétration des dérivés
organo-stanniques dans l'environnement à partir de
sources autres que les peintures antisalissures et, si
besoin est, d'édicter une réglementation à ce sujet.
Il faut en particulier évaluer le risque résultant du
déversement sur le sol de boues d'égouts contaminés
par du tributylétain.
c) Il faudrait améliorer, du point de vue de la sécurité,
les techniques d'application, d'élimination et d'éva-
cuation des peintures à base d'organo-stanniques.
2. Recherches à effectuer
a) Il faut améliorer les méthodes de recherche et de
dosage du butylétain afin d'avoir une estimation
rapide et exacte des concentrations de l'ordre de
pg/litre. Une des raisons de cette recommandation
tient à un effet biologique, à savoir l'apparition de
caractères mâles chez les gastéropodes femelles, qui
est susceptible de se produire à des concentrations
plus basses que les limites actuelles de détection.
b) Il faut effectuer des recherches sur les mécanimes par
lesquels le TBT se concentre au lieu de se disperser
et qui retardent sa décomposition; à cet effet on
étudiera avec une attention particulière la chimie
fondamentale du tributylétain et ses interactions avec
les molécules biologiques. Il faut étudier davantage
la fixation du TBT à tous les niveaux de la chaîne
alimentaire.
c) Il est nécessaire d'entreprendre des études sur la
toxicité du TBT pour les organismes aquatiques. On
étudiera en particulier le métabolisme, les effets
endocriniens et immunologiques, selon le cas.
d) Il est nécessaire de rechercher d'autres espèces sen-
sibles capables de servir d'indicateurs biologiques,
notamment parmi les espèces d'eau douce.
e) Il faut valider les modèles permettant l'évaluation de
l'immunotoxicité chez les mammifères et définir de
façon plus précise les doses sans effet toxique
correspondant aux paramètres pertinents.
f) Etude de toxicité chronique à entreprendre sur une
deuxième espèce de mammifères.
g) Etude de tumorigénicité à entreprendre sur une
deuxième espèce de mammifères.
h) Données sur les résidus de butylétain dans le poisson
et les coquillages destinés à la consommation humaine
en distinguant les différentes espèces.
RESUMEN
1. Propiedades físicas y químicas
Los compuestos de tributilestaño (TBE) son derivados
orgánicos del estaño tetravalente. Caracterizados por la
presencia de enlaces covalentes entre átomos de carbono y
un átomo de estaño, tienen la siguiente fórmula general:
(n-C4H9)3 Sn-X, en la que X es un anión. En general,
la pureza del óxido de tributilestaño (OTBE) comercial
pasa del 96%; las principales impurezas están constituidas
por derivados del dibutilestaño y, en menor grado, por
compuestos de tetrabutilestaño y otros compuestos tri-
alquílicos de ese elemento. El OTBE es un líquido incoloro
con olor característico y una densidad relativa de 1,17 a
1,18. La solubilidad en el agua es baja, variando entre
< 1,0 y > 100 mg/litro según el pH, la temperatura, y los
aniones presentes en el agua (que determinan la especifi-
cidad). En el agua del mar y en condiciones normales, el
TBE aparece en tres formas o especies (hidróxido, cloruro
y carbonato), que se mantienen en equilibrio. En valores
de pH inferiores a 7,0, las formas predominantes son
Bu3SnOH2+ y Bu3SnCl, a pH 8 son Bu3SnCl, Bu3SnOH
y Bu3SnCO3-, mientras que cuando el pH pasa de 10
predominan Bu3SnOH y Bu3SnCO3-.
El coeficiente de partición octanol/agua (log Poa)
varía entre 3,19 y 3,84 para el agua destilada y es de
3,54 para el agua del mar. El OTBE adsorbe intensamente
las partículas, con coeficientes de adsorción comprendidos
entre 110 y 55 000 según los informes publicados. La
presión de vapor es baja, pero los valores publicados
acusan variaciones considerables. En una solución de 1
mg/litro no se observó disminución alguna del OTBE durante
62 días, pero el 20% del agua se perdió por evaporación.
2. Métodos analíticos
Se utilizan diversos métodos para medir los derivados
tributilestánnicos en el agua, en el sedimento o en la
flora y la fauna (biota). El más usado es la espectro-
metría de absorción atómica (AA). La espectrometría de AA
con llama permite alcanzar un límite de detección de 0,1
mg/litro. Resulta más sensible la AA sin llama, basada en
la atomización en un horno eléctrico con grafito, cuyos
límites de detección están comprendidos entre 0,1 y
1,0 µg/litro de agua. Existen diferentes métodos de
extracción y para formar derivados volátiles. La sepa-
ración de estos derivados suele hacerse por "purga y
captura" o cromatografía de gases. Los límites de detec-
ción son de 0,5 y 5,0 µg/kg para el sedimento y la
biota.
3. Fuentes de contaminación ambiental
Se han registrado diversos compuestos de tributile-
staño como molusquicidas, productos antiincrustantes en
botes y otras embarcaciones, muelles, boyas, jaulas de
langostas, redes de pesca y nasas, como conservadores de
la madera, como "productos anti-cieno" en los trabajos
de albañilería, como desinfectantes y como biocidas en los
sistemas de refrigeración, las torres de refrigeración de
las centrales electrógenas, las fábricas de pulpa y de
papel, las cervecerías, las industrias del cuero y los
telares. En las pinturas antiincrustantes, el TBE se
comercializó al principio bajo una forma que permitía la
liberación sin trabas del compuesto. En fecha más reciente
se han puesto a la venta pinturas de liberación controlada
en las que el TBE se incorpora a una matriz copolimérica.
También se han ideado matrices de caucho para hacer más
lenta y retrasar la liberación del compuesto, con la
consiguiente prolongación de la eficacia de las pinturas
antiincrustantes y de los molusquicidas. El TBE no se
utiliza en la agricultura por su elevada fitotoxicidad.
4. Reglamentación del empleo
Muchos países han restringido el empleo de pinturas
antiincrustantes con TBE por los efectos de éste sobre los
mariscos. Los reglamentos varían en detalle de unos países
a otros, pero casi siempre prohíben el uso de pinturas de
TBE en las embarcaciones de 24 metros de longitud o menos.
Algunos países han excluido de esta prohibición a las
embarcaciones con casco de aluminio. Además, en algunos
reglamentos se restringe el contenido de TBE en las
pinturas o el ritmo con que se libera este compuesto de
las mismas (a 4 o 5 µg/cm2 por día, a largo plazo).
5. Concentraciones en el medio ambiente
Se han encontrado concentraciones elevadas de TBE en
el agua, los sedimentos y la biota próximos a las zonas de
navegación de recreo, especialmente en "marinas" o
fondeaderos de yates, embarcaderos, diques secos, redes de
pesca y nasas tratadas con pinturas antiincrustantes, así
como en los sistemas de refrigeración. La intensidad de
las mareas y la turbiedad del agua influyen en las con-
centraciones de TBE.
Se ha visto que las concentraciones de TBE pueden
llegar a 1,58 µg/litro en el agua del mar y en los estu-
arios, a 7,1 µ/litro en el agua dulce, a 26 300 µg/kg en
los sedimentos costeros, a 3700 µg/kg en los sedimentos
de agua dulce, a 6,39 mg/kg en los bivalvos, a 1,92 mg/kg
en los gasterópodos y a 11 mg/kg en los peces. Ahora bien,
no hay que considerar como representativas esas concentra-
ciones máximas de TBE, toda vez que diversos factores
pueden dar lugar a valores anormalmente elevados (por
ejemplo, las partículas de pintura en las muestras de agua
y de sedimentos). Se ha comprobado que en las concentra-
ciones de TBE en la microcapa superficial del agua, tanto
dulce como salada, pueden ser dos veces más altas que las
medidas inmediatamente por debajo de la superficie. Sin
embargo, conviene tener en cuenta que los valores regis-
trados de TBE en las microcapas superficiales pueden verse
muy afectados por el método de muestreo.
Es posible que los datos antiguos no sean comparables
con los más recientes a causa del perfeccionamiento de los
métodos analíticos disponibles para determinar el TBE en
el agua, los sedimentos y los tejidos.
6. Transporte y transformación en el medio ambiente
A consecuencia de su baja hidrosolubilidad y de su
carácter lipofílico, el TBE se adsorbe fácilmente en las
partículas. Se calcula que entre el 10% y el 95% del OTBE
introducido en el agua se adsorbe de ese modo. La
desaparición progresiva del TBE adsorbido no se debe a la
desorción sino a la degradación. El grado de adsorción
depende de la salinidad, de la naturaleza y el tamaño de
las partículas en suspensión, de la cantidad de material
suspendido, de la temperatura y de la presencia de materia
orgánica disuelta.
La degradación del OTBE entraña la escisión del enlace
carbono-estaño. Este fenómeno puede deberse a diversos
mecanismos que intervienen simultáneamente en el medio
ambiente, unos fisicoquímicos (hidrólisis y fotodegrada-
ción) y otros biológicos (degradación por microorganismos
y metabolización por organismos superiores). Mientras que
en condiciones extremas de pH se produce una hidrólisis de
los compuestos de estaño orgánico, ésta es apenas evidente
en condiciones ambientales normales. En el laboratorio se
observa fotodegradación cuando se exponen soluciones a una
irradiación ultravioleta de 300 nm (y, en menor grado, de
350 nm). En condiciones naturales, la fotólisis está
limitada por la gama de longitudes de onda de la luz solar
y por la escasa penetración de la luz ultravioleta en el
agua. La presencia de sustancias fotosensibilizadoras
puede acelerar la fotodegradación. La biodegradación
depende de condiciones ambientales tales como la tempera-
tura, la oxigenación, el pH, el nivel de elementos miner-
ales, la presencia de sustancias orgánicas fácilmente
biodegradables a efectos de cometabolismo y la naturaleza
de la microflora y su capacidad de adaptación. También
depende de que la concentración de OTBE sea más baja que
el umbral letal o inhibitorio para las bacterias. Como en
la degradación abiótica, la ruptura biótica del TBE es un
proceso progresivo de debutilización oxidativa fundado en
la escisión del enlace carbono-estaño, en el que se forman
derivados dibutílicos que se degradan más fácilmente que
el tributilestaño. Los monobutilestaños se mineralizan
lentamente. Aunque existe degradación anaerobia, no se ha
llegado a un acuerdo sobre su importancia. Algunos autores
estiman que es lenta, mientras que otros piensan que es
más rápida que la degradación aerobia. Se han identificado
varias especies de bacterias, algas y hongos nocivos para
la madera que pueden degradar el OTBE. Las estimaciones
de la semivida del TBE en el medio ambiente acusan grandes
variaciones.
El TBE se bioacumula en los organismos a causa de su
solubilidad en las grasas. En investigaciones de labora-
torio con moluscos y peces se han visto que los factores
de bioconcentración pueden llegar a un valor de 7000, y
aun se han obtenido valores más altos en los estudios
sobre el terreno. La absorción a partir de los alimentos
es más importante que la que se efectúa directamente a
partir del agua. La presencia de factores de concentración
más altos en los microorganismos (entre 100 y 30 000)
puede deberse más a adsorción que a absorción intracelu-
lar. No hay ninguna indicación de que el TBE se transfiera
a los microorganismos terrestres a través de las cadenas
alimentarias.
7. Cinética y metabolismo
El tributilestaño se absorbe a través del intestino
(20-50%, según el vehículo) y de la piel en los mamíferos
(10% aproximadamente), pudiendo atravesar la barrera
hematoencefálica y pasar de la placenta al feto. El
material absorbido se distribuye rápida y ampliamente por
los tejidos (principalmente el hígado y el riñón).
En los mamíferos, el metabolismo del TBE es rápido: a
las 3 h de administrar TBE pueden ya descubrirse metabo-
litos en la sangre. Los estudios in vitro han revelado
que el TBE es un sustrato para ciertas oxidasas de función
mixta, pero las concentraciones muy altas de TBE inhiben
esas enzimas.
La velocidad de desaparición del TBE difiere de unos
tejidos a otros; las estimaciones de la semivida biológica
en los mamíferos van desde 23 hasta unos 30 días.
La metabolización del TBE se observa también en los
organismos inferiores, pero es más lenta (particularmente
en los moluscos) que en los mamíferos. La capacidad de
bioacumulación, por consiguiente, es mucho mayor que en
éstos.
Los compuestos de TBE inhiben la fosforilización
oxidativa y alteran la estructura y las funciones de las
mitocondrias. El TBE interfiere en la calcificación de la
concha de las ostras ( Crassostrea spp).
8. Efectos en los microorganismos
El TBE es tóxico para los microorganismos y se ha
utilizado comercialmente como bactericida y alguicida.
Las concentraciones que provocan efectos tóxicos varían
mucho de unas especies a otras. El TBE es más tóxico para
las bacterias gram-positivas (concentración inhibitoria
mínima (CIM): entre 0,2 y 0,8 mg/litro) que para las gram-
negativas (CIM: 3 mg/litro). La CIM del acetato de TBE
para los hongos es de 0,5-1 mg/litro y la CIM del OTBE
para el alga verde Chlorella pyrenoidosa es de 0,5
mg/litro. La productividad primaria de una comunidad
natural de algas de agua dulce se redujo en un 50% con una
concentración de OTBE de 3 µg/litro. En fecha reciente
se han determinado en dos especies de algas niveles de
efecto no observado (NENO) de 18 y 32 µg/litro. De igual
modo, la toxicidad para los microorganismos marinos varía
de unas especies a otras y de unos estudios a otros; los
NENO son difíciles de establecer pero no llegan a
0,1 µg/litro en algunas especies. Las concentraciones
alguicidas varían entre < 1,5 µg/litro y > 1000 µg por
litro según las especies.
9. Efectos en los organismos acuáticos
9.1 Efectos en los organismos de agua salada (mar y estuarios)
En la figura 1 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las con-
centraciones de TBE medidas en diversos organismos del mar
y de los estuarios. En todo el mundo, especialmente en los
lugares relacionados con actividades náuticas recreativas,
se han encontrado concentraciones superiores a las que
producen efectos letales.
9.3 Etudes de microcosme
En vue de la modélisation des écosystèmes marins on a
effectué des études de microcosme consistant à introduire
dans ces milieux certains organismes et en se plaçant dans
des conditions où un apport d'eau de mer permettait la
colonisation du milieu par d'autres biotes. On a constaté
qu'à des concentrations d'oxyde de tributylétain comprises
entre 0,06 et 3 µg/litre, il y avait réduction du nombre
d'individus et une moindre diversité des espèces.
Les résultats obtenus par modélisation d'écosystèmes
d'eau douce montrent que les doses qui tuent les mol-
lusques sont également nocives pour d'autres espèces,
notamment les poissons.
10. Effets sur les organismes terrestres
L'exposition des organismes terrestres au TBT
découlent essentiellement de l'utilisation de ces subs-
tances pour la protection du bois. L'oxyde de tributylé-
tain est toxique pour la population des ruches dont le
bois de construction a été traité au TBT. Une étude,
d'ailleurs unique, a montré que le TBT était toxique pour
les chauves-souris mais le résultat ne peut pas être con-
sidéré comme statistiquement significatif en raison de la
forte mortalité des témoins. Les dérivés du tributylétain
sont toxiques pour les insectes exposés, soit topiquement
soit par suite de xylophagie. Pour les souris de type
sauvage, la toxicité aiguë du TBT est moyenne; les valeurs
de la CL50 par voie alimentaire, calculées d'après la
consommation de semences traitées et soumises à des
épreuves de répulsivité, vont de 37 à 240 mg/kg par jour.
11. Effets sur l'aquaculture
Les observations effectuées dans des zones d'aquacul-
ture ont permis d'attribuer à la présence de fortes con-
centrations de tributylétain, un certain nombre d'effets
nocifs observés sur des bivalves tels que: mortalité,
incapacité à se fixer, moindre croissance, épaississement
de la coquille et autres malformations notamment chez les
huîtres, apparition de caractères mâles chez les gastéro-
podes (avec diminution simultanée des populations) ainsi
que chez les mollusques du genre Thais. C'est en France
que pour la première fois on a attribué à la présence de
TBT dans l'eau, l'anéantissement total de parcs à huîtres,
observations qui ont été faites ensuite dans d'autres
pays. Les effets étaient particulièrement marqués dans les
secteurs proches de marinas destinées aux navires de
plaisance. La réglementation de l'usage des peintures
antisalissures à base TBT sur les petits navires a permis
aux huîtres de retrouver leur capacité de reproduction et
de croissance. Toutefois la concentration du TBT dans
l'eau reste suffisamment forte dans certains secteurs pour
nuire aux gastéropodes marins. On utilise la croissance
de la coquille et son gaufrage chez les huîtres japonaises
ainsi que l'apparition de caractères mâles chez les
mollusques du genre Thais comme indicateurs biologiques
d'une contamination par du tributylétain.
Peu d'études ont été consacrées aux effets du TBT
sédimentaire sur la faune marine mais on pense qu'il peut
être absorbé par les organismes fouisseurs et provoquer
une certaine mortalité.
Des effets toxiques macroscopiques et des altérations
histopathologiques ont été observés dans des élevages de
poissons de mer, les filets délimitant les bassins ayant
été traités au moyen de peintures aintisalissures à base
de TBT.
On a proposé d'utiliser du TBT comme molluscicides
pour détruire les mollusques d'eau douce qui transmettent
la bilharziose (bilharzies). Un certain nombre d'essais
ont été effectués sur le terrain, dont il ressort que le
TBT est difficile à utiliser sans préjudice pour les
organismes non visés.
12. Toxicité pour les mammifères de laboratoire
12.1 Toxicité aiguë
Le tributylétain est moyennement à fortement toxique
pour les mammifères de laboratoire, avec des valeurs de la
DL50 par voie orale allant de 94 à 234 mg/kg de poids
corporel chez le rat et de 44 à 230 mg/kg de poids
corporel chez la souris. Pour le cobaye et le lapin, les
valeurs sont du même ordre de grandeur. Les variations
enregistrées sont dues aux différents anions entrant dans
la composition du sel de tributylétain. Ces composés
entraînent une mortalité plus forte lorsqu'ils sont
administrés par voie parentérale plutôt que par voie
orale, probablement du fait qu'ils ne sont que partielle-
ment absorbés au niveau intestinal.
Parmi les autres effets toxiques aigus on peut citer
des anomalies concernant les taux de lipide sanguins, le
système endocrinien, le foie, la rate et un déficit
passager dans le développement cérébral. La portée toxi-
cologique réelle de ces effets, qui n'ont été observés
qu'après administration de doses uniques élevées de ces
composés, reste discutable et la cause effective de la
mort n'est pas véritablement connue.
Par voie percutanée, la toxicité aiguë est faible, la
DL50 étant supérieure à 9000 mg/kg de poids corporel chez
le lapin. Chez le rat, après inhalation purement nasale,
la DL50 à 4 heures se situait à 77 mg/m3 (65 mg/m3 si
l'on ne tient compte que des particules respirables). Des
mélanges d'air et de vapeurs de tributylétain ne produi-
sent pas d'effets toxiques observables, même à saturation.
Toutefois le TBT est très dangereux sous forme d'aérosol
lorsqu'il est inhalé et il produit alors une irritation et
un oedème des poumons.
Le TBT est très irritant pour la peau et extrêmement
irritant pour l'oeil. L'oxyde de tributylétain n'a pas
d'effet sensibilisateur cutané.
12.2 Toxicité à court terme
Les composés du tributylétain ont été très étudiés
chez le rat (toutes les données présentées dans ce qui
suit concernent cet animal, sauf indication contraire).
On a observé un fort taux de mortalité après une durée
d'exposition de plus de quatre semaines à des doses dans
l'alimentation de 320 mg/kg (environ 25 mg/kg de poids
corporel). Acune mortalité n'a été observée à la dose de
100 mg/kg de nourriture (10 mg/kg de poids corporel) ni
après l'administration par gavage d'une dose correspondant
à 12 mg de TBT/kg de poids corporel. Administrée à des
ratons peu après leur naissance, une dose de 3 mg/kg de
poids corporel a augmenté la mortalité. Les principaux
symptômes observés après administration de doses mortelles
consistaient en perte d'appétit, faiblesse et émaciation.
On a observé des effets marginaux sur la croissance
aux doses de 50 mg/kg de nourriture (6 mg/kg de poids
corporel) et de 6 mg/kg de poids corporel (administrées
par gavage). Les souris sont moins sensibles, ces effets
n'étant observés qu'à partir de 150 à 200 mg/kg de nour-
riture (22 à 29 mg/kg de poids corporel).
Des effets structuraux ont été observés sur les
organes endocrines, essentiellement l'hypophyse et la
thyroïde, lors d'études à court et à long terme. Lors des
études à court terme, on a observé des anomalies dans la
concentration des hormones circulantes ainsi que dans la
réponse aux stimuli physiologiques (trophines hypophy-
saires); toutefois après une exposition de longue durée,
la plupart de ces anomalies avaient disparu. Le mécanisme
sous-jacent n'est pas connu.
L'exposition à un aérosol d'oxyde de tributylétain à
la dose de 2,8 mg/m3 a déterminé une forte mortalité,
une détresse respiratoire, une réaction inflammatoire au
niveau des voies respiratoires et des anomalies histo-
pathologiques des organes lymphatiques. En revanche,
l'exposition à des vapeurs à la concentration la plus
forte possible (0,16 mg/m3) à la température ambiante,
n'a produit aucun effet.
On a signalé des effets toxiques au niveau du foie et
des canaux biliaires chez ces trois espèces de mammifères.
Ainsi une nécrose des cellules hépatiques et des altéra-
tions inflammatoires du canal cholédoque ont été obser-
vées chez des rats qui avaient reçu de l'oxyde de
tributylétain à raison de 320 mg/kg de nourriture (soit
approximativement 25 mg/kg de poids corporel) pendant
quatre semaines et des souris qui en avaient reçu 80 mg/kg
de nourriture (soit approximativement 12 mg/kg de poids
corporel) pendant trois mois. Chez des chiens soumis à une
dose de 10 mg/kg de poids corporel pendant huit à neuf
semaines, on a observé une vacuolisation des hépatocytes
de la région périportale. Ces altérations s'accompagnaient
occasionnellement d'un accroissement du poids du foie et
d'une augmentation de l'activité sérique des enzymes
hépatiques.
La réduction de la concentration d'hémoglobine et de
l'hématocrite chez le rat, à la suite de l'administration
d'une dose correspondant à 80 mg/kg de nourriture (soit
8 mg/kg de poids corporel), montre qu'il y a un effet sur
la synthèse de l'hémoglobine qui conduit à une anémie
hypochrome microcytaire. La réduction des taux d'hémosi-
dérine splénique donne à penser qu'il y a action au niveau
des réserves martiales. Une anémie a également été
observée chez la souris.
La formation de rosettes érythrocytaires au niveau des
ganglions lymphatiques mésentériques a été observée lors
de certaines études à court terme mais pas lors d'études à
long terme. La portée biologique de cette observation
(vraisemblablement passagère) reste obscure.
L'oxyde de tributylétain exerce un effet toxique
caractéristique sur le système immunitaire; du fait de son
action sur le thymus, il perturbe les fonctions immuni-
taires à médiation cellulaire. Le mode d'action demeure
inconnu mais il pourrait y avoir conversion métabolique en
dibutylétain. La résistance non spécifique est également
amoindrie.
Un certain nombre d'études sur rats et chiens mais à
l'exclusion des souris, ont été effectuées avec de l'oxyde
de tributylétain et ont révélé l'existence d'effets
généraux sur le système immunitaire (par exemple poids et
morphologie des tissus lymphoïdes, numération des lympho-
cytes périphériques, concentration totale des immunoglo-
bulines sériques), à des doses largement toxiques (des
effets ont été observés chez la souris à des doses de
150 mg/kg de chlorure de tributylétain). Seul le rat
manifeste des effets généraux sur le système immunitaire
sans autres signes patents de toxicité et il se révèle
indiscutablement être l'espèce la plus sensible. Des
études à court terme sur le rat ont permis d'établir que
la dose sans effet observable était de 5 mg/kg de nour-
riture (soit 0,6 mg/kg de poids corporel). Lors des études
portant sur le chlorure de tributylétain, on a observé
des effets analogues au niveau du thymus. Ces effets
disparaissaient rapidement lorsqu'on cessait d'administrer
la substance. On a montré que l'oxyde de tributylétain,
étudié dans le cadre de travaux sur la résistance de
l'hôte, perturbait les fonctions immunitaires spécifiques
du rat. L'organisme de cet animal présentait une moindre
aptitude à éliminer les Listeria monocytogenes après
exposition à une dose de 50 mg/kg de nourriture (dose sans
effet observable: 5 mg/kg et par jour), et une moindre
résistance à Trichinella spiralis a été observée aux doses
respectives de 50 et 5 mg/kg de nourriture, cet effet
disparaissant à la dose de 0,5 mg/kg de nourriture (ce qui
correspond à des doses quotidiennes respectives de 2,5,
0,25 et 0,025 mg/kg de poids corporel). Des effets
analogues ont été observés chez des animaux âgés mais ils
étaient moins marqués.
Dans l'état actuel des connaissances, les effets sur
la résistance de l'hôte sont probablement très utiles pour
évaluer les risques pour la santé humaine, mais l'on ne
possède pas une expérience suffisante de ces systèmes
d'épreuve pour tirer des conclusions définitives des
résultats obtenus. Il reste que l'observation de rats
glabres athymiques soumis à une inoculation de T. spiralis
virulentes a permis l'interprétation des résultats
obtenus sur le modèle T. spiralis. En effet, l'absence
totale d'immunité thymo-dépendante a multiplié par 10 à 20
le nombre de larves présentes dans les muscles; par contre
l'exposition à des concentrations d'oxyde de tributylétain
respectivement égales à 5 et 50 mg/kg de nourriture a
multiplié par 2 et 4 respectivement le nombre de ces
larves.
Il existe quelques données concernant les effets du
tributylétain sur le système immunitaire en développement
mais sans aucune information sur la résistance de l'hôte.
Pour évaluer les dangers potentiels pour l'homme, il
serait plus prudent de prendre en considération les effets
produits sur l'espèce la plus sensible. On a observé des
effets sur la résistance de l'hôte à T. spiralis à des
concentrations dans l'alimentation ne dépassant pas
5 mg/kg (soit l'équivalent de 0,25 mg/kg de poids corporel
par jour), la dose sans effet observable étant égale à
0,25 mg/kg (ce qui correspond à 0,025 mg/kg par jour).
Toutefois, l'interprétation de ces résultats en vue d'une
évaluation du risque pour l'homme reste controversée.
Toutes les études qui ont été effectuées font ressortir
que la dose quotidienne sans effet observable pour ce qui
est des effets généraux ou spécifiques sur le système
immunitaire se situe à 5 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel selon les
études à court terme).
12.3 Toxicité à long terme
Une étude de longue durée chez le rat a montré que le
tributylétain a une effet marginal sur les paramètres
toxicologiques généraux (sans grande signification toxi-
cologique) à la dose de 5 mg/kg de nourriture (soit
0,25 mg/kg de poids corporel).
12.4 Génotoxicité
La génotoxicité de l'oxyde de tributylétain a fait
l'objet d'études approfondies. La plupart de ces études
ont donné des résultats négatifs et rien n'indique de
façon convaincante que ce produit puisse présenter le
moindre risque mutagène.
12.5 Toxicité vis-à-vis de la fonction de reproduction
On a évalué l'embryotoxicité potentielle de l'oxyde de
tributylétain sur trois espèces de mammifères (souris, rat
et lapin), après administration de la substance par voie
orale à la mère. La principale malformation observée chez
les foetus de rat et de souris était une fissure congéni-
tale du palais osseux mais il est vrai qu'elle ne se
produisait qu'à des doses manifestement toxiques pour la
mère. On ne peut pas considérer que ces résultats
témoignent d'une activité tératogène aux doses inférieures
à celles qui sont toxiques pour la mère. La dose minimale
sans effet observable pour ce qui est de l'embryotoxicité
et de la foetotoxicité chez ces trois espèces se situait à
1,0 mg/kg de poids corporel.
12.6 Cancérogénicité
Une étude de cancérogénicité a été effectuée sur des
rats et on a observé à cette occasion des modifications
néoplasiques au niveau des organes endocrines à la dose de
50 mg/kg de poids corporel. Les tumeurs hypophysaires
observées à la dose de 0,5 mg/kg de nourriture ne semblent
pas avoir d'importance biologique car il n'y a pas de
relation précise dose-réponse. Ces types de tumeurs
apparaissent en général chez les témoins à des fréquences
élevées et variables et leur signification reste donc
discutable. Une autre étude de cancérogénicité est en
cours sur des souris.
13. Effets sur l'homme
On a constaté que l'exposition professionnelle
d'ouvriers à des dérivés du tributylétain produisait une
irritation des voies respiratoires supérieures. Ces
substances sont dangereuses pour l'homme lorsqu'elles sont
sous la forme d'aérosols. L'oxyde de tributylétain est
irritant pour la peau et les muqueuses oculaires et il a
été fait état de dermatites graves à la suite d'un contact
direct avec la peau. Le risque est d'autant plus grave que
la réaction cutanée n'est pas immédiate.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour l'homme
Les travailleurs sont principalement exposés lors de
la préparation et de la formulation des dérivés du tri-
butylétain, de l'application ou de l'élimination de
peintures à base de TBT et de l'utilisation de produits de
protection du bois à base de ces substances. Quant à la
population dans son ensemble, elle peut être exposée par
contamination de la nourriture, en particulier des
poissons et des coquillages, et lors de l'utilisation de
produits de protection du bois à usage ménager.
Si l'on s'appuie sur l'expérimentation animale et
l'observation directe sur l'homme, il est clair que les
dérivés du tributylétain sont irritants pour la peau et
les yeux et que l'inhalation d'aérosols conduit à une
irritation des voies respiratoires.
La manipulation de bois traités n'entraîne aucune
irritation de l'épiderme une fois que le produit a séché.
Cependant les aérosols de TBT sont extrêmement dangereux
et il ne faut pas revenir dans le local où le bois a été
traité avant séchage complet.
On n'a jamais fait état d'intoxications aiguës généra-
lisées par le TBT qui en principe doit s'éliminer de
l'organisme en l'espace de quelques jours. Il est donc peu
probable que l'on puisse s'exposer à des intoxications
aiguës par la manipulation de produits à base de tri-
butylétain si l'on prend les précautions voulues.
On a fait état d'effets à court et à long terme chez
des animaux de laboratoire, sur le foie, le sang et les
glandes endocrines. Chez le rat, qui est l'espèce la plus
sensible, c'est l'effet au niveau du système immunitaire
et en particulier sur la résistance de l'hôte, qui
constitue le paramètre le plus sensible de la toxicité de
ces produits. En utilisant comme modèle la résistance de
l'hôte à l'infestation par Trichinella spiralis, on
obtient une dose sans effet observable qui se situe entre
0,5 et 5 mg/kg de nourriture (c'est-à-dire 0,025 et 0,25
mg/kg de poids corporel) alors que si l'on se rapporte à
l'action sur la fonction immunitaire, la dose est égale à
0,6 mg/kg de poids corporel.
En raison des grandes variations dans la consommation
de poissons et de coquillages ainsi que dans les teneurs
locales des fruits de mer en résidus de TBT, on ne peut
donner que quelques exemples pour illustrer l'exposition
résultante et les valeurs des doses sans effet observable.
Il importe de souligner que pour déterminer le risque
résultant de la présence de ces composés, il faut procéder
sur place à des mesures de résidus dans les denrées,
évaluer la consommation locale de fruits de mer et définir
une marge de sécurité acceptable.
En retenant pour la consommation de poisson les
chiffres de 15 et 150 grammes par jour, de 1 mg/kg pour
les résidus de TBT dans le poisson et un poids corporel
moyen de 60 kg, on obtient pour l'homme les marges
suivantes de sécurité selon les divers paramètres immuni-
taires.
---------------------------------------------------------------------
Consommation Apport estimatif Marge de sécurité
de poisson journalier de Modèle Autres paramètres
(g/jour) TBT (µg/kg) T. Spiralis immunitaires
---------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
---------------------------------------------------------------------
Utiliser des dérivés du TBT à tort et à travers et de
manière irresponsable sans suivre les recommandations qui
figurent dans la présente monographie pour réduire l'expo-
sition humaine peut conduire à l'ingestion de quantités de
TBT dangereuses pour la santé humaine.
On a noté jusqu'ici d'effets tératogènes, chez l'ani-
mal de laboratoire, qu'à des doses manifestement toxiques
pour la mère. On peut donc considérer que l'activité
tératogène du TBT est très faible.
En s'appuyant sur les résultats d'études très
complètes de mutagénicité, on estime que les dérivés du
tributylétain n'ont aucun pouvoir mutagène. Une étude de
cancérogénicité chez le rat, portant sur l'oxyde de
tributylétain, a fait apparaître une incidence accrue de
tumeurs endocriniennes; mais il s'agissait de tumeurs
spontanées dont l'incidence, généralement élevée, est très
variable. Ces résultats ne constituent donc pas un
argument bien net en faveur d'un risque cancérogène pour
l'homme.
2. Evaluation du risque écologique
La pénétration diffuse du tributylétain (TBT) dans
l'environnement a principalement pour origine l'utili-
sation de peintures antisalissures qui en contiennent.
Une contamination ponctuelle peut se produire lorsqu'on
utilise du TBT comme biocide dans les systèmes de réfri-
gération, lors du traitement de la pulpe de bois, le
tannage du cuir, la préservation du bois et le traitement
des textiles.
Du fait de leurs priopriétés physico-chimiques, les
dérivés du TBT se concentrent dans la micro-couche de
surface ainsi que dans les sédiments. Il ne semble pas
que le principal mécanisme d'élimination de ces substances
dans les conditions naturelles soit une dégradation abio-
tique. L'oxyde de tributylétain est biodégradable dans
l'eau mais le processus n'est pas suffisamment rapide pour
empêcher la présence dans certaines zones de concen-
trations élevées en TBT. Une bioaccumulation se produit
dans la plupart des organismes aquatiques mais chez les
mammifères de laboratoire, la dégradation métabolique est
plus efficace.
Le TBT est extrêment dangereux pour certains orga-
nismes aquatiques, du fait de sa toxicité à très faibles
concentrations dans l'eau. On le rencontre à ces concen-
trations dans certaines zones. On a signalé la présence
d'effets nocifs sur des invertébrés non visés, en parti-
culier des mollusques et ces effets sont suffisamment
graves pour bloquer la reproduction et entraîner un déclin
des populations de mollusques. Ces effets néfastes pour
la conchyliculture ont pu être combattus avec succès grâce
à des restrictions imposées à l'utilisation des peintures
antisalissures dans certains secteurs, restrictions qui
ont également permis d'éviter l'apparition de caractères
mâles chez les gastéropodes femelles. En ce qui concerne
le pisciculture, il convient de ne pas utiliser de pein-
tures à base de TBT sur les filets qui limitent les
bassins.
D'une manière générale, le risque pour l'environnement
terrestre est vraisemblablement faible. Cependant le
traitement du bois par ces substances pourrait se révéler
dangereux pour les organismes qui vivent à son contact.
L'augmentation de la teneur en TBT de la micro-couche
superficielle pourrait se révéler dangereuse pour la faune
côtière, pour les neustons (y compris les invertébrés
benthiques et les larves de poissons) ainsi que pour les
oiseaux de mer et le gibier d'eau qui se nourrissent en
surface. L'accumulation et le faible taux de biodégra-
dation du TBT dans les sédiments peut présenter un risque
pour les organismes aquatiques lorsque des sédiments
pollués sont soulevés par des processus naturels ou des
activités de dragage.
RECOMMANDATIONS
1. Recommandations pour la protection de la santé humaine
et de l'environnement
a) Les pays membres qui jusqu'ici n'ont pas réglementé
l'utilisation des dérivés du TBT devraient être
invités à le faire.
b) Il est nécessaire d'évaluer la pénétration des dérivés
organo-stanniques dans l'environnement à partir de
sources autres que les peintures antisalissures et, si
besoin est, d'édicter une réglementation à ce sujet.
Il faut en particulier évaluer le risque résultant du
déversement sur le sol de boues d'égouts contaminés
par du tributylétain.
c) Il faudrait améliorer, du point de vue de la sécurité,
les techniques d'application, d'élimination et d'éva-
cuation des peintures à base d'organo-stanniques.
2. Recherches à effectuer
a) Il faut améliorer les méthodes de recherche et de
dosage du butylétain afin d'avoir une estimation
rapide et exacte des concentrations de l'ordre de
pg/litre. Une des raisons de cette recommandation
tient à un effet biologique, à savoir l'apparition de
caractères mâles chez les gastéropodes femelles, qui
est susceptible de se produire à des concentrations
plus basses que les limites actuelles de détection.
b) Il faut effectuer des recherches sur les mécanimes par
lesquels le TBT se concentre au lieu de se disperser
et qui retardent sa décomposition; à cet effet on
étudiera avec une attention particulière la chimie
fondamentale du tributylétain et ses interactions avec
les molécules biologiques. Il faut étudier davantage
la fixation du TBT à tous les niveaux de la chaîne
alimentaire.
c) Il est nécessaire d'entreprendre des études sur la
toxicité du TBT pour les organismes aquatiques. On
étudiera en particulier le métabolisme, les effets
endocriniens et immunologiques, selon le cas.
d) Il est nécessaire de rechercher d'autres espèces sen-
sibles capables de servir d'indicateurs biologiques,
notamment parmi les espèces d'eau douce.
e) Il faut valider les modèles permettant l'évaluation de
l'immunotoxicité chez les mammifères et définir de
façon plus précise les doses sans effet toxique
correspondant aux paramètres pertinents.
f) Etude de toxicité chronique à entreprendre sur une
deuxième espèce de mammifères.
g) Etude de tumorigénicité à entreprendre sur une
deuxième espèce de mammifères.
h) Données sur les résidus de butylétain dans le poisson
et les coquillages destinés à la consommation humaine
en distinguant les différentes espèces.
RESUMEN
1. Propiedades físicas y químicas
Los compuestos de tributilestaño (TBE) son derivados
orgánicos del estaño tetravalente. Caracterizados por la
presencia de enlaces covalentes entre átomos de carbono y
un átomo de estaño, tienen la siguiente fórmula general:
(n-C4H9)3 Sn-X, en la que X es un anión. En general,
la pureza del óxido de tributilestaño (OTBE) comercial
pasa del 96%; las principales impurezas están constituidas
por derivados del dibutilestaño y, en menor grado, por
compuestos de tetrabutilestaño y otros compuestos tri-
alquílicos de ese elemento. El OTBE es un líquido incoloro
con olor característico y una densidad relativa de 1,17 a
1,18. La solubilidad en el agua es baja, variando entre
< 1,0 y > 100 mg/litro según el pH, la temperatura, y los
aniones presentes en el agua (que determinan la especifi-
cidad). En el agua del mar y en condiciones normales, el
TBE aparece en tres formas o especies (hidróxido, cloruro
y carbonato), que se mantienen en equilibrio. En valores
de pH inferiores a 7,0, las formas predominantes son
Bu3SnOH2+ y Bu3SnCl, a pH 8 son Bu3SnCl, Bu3SnOH
y Bu3SnCO3-, mientras que cuando el pH pasa de 10
predominan Bu3SnOH y Bu3SnCO3-.
El coeficiente de partición octanol/agua (log Poa)
varía entre 3,19 y 3,84 para el agua destilada y es de
3,54 para el agua del mar. El OTBE adsorbe intensamente
las partículas, con coeficientes de adsorción comprendidos
entre 110 y 55 000 según los informes publicados. La
presión de vapor es baja, pero los valores publicados
acusan variaciones considerables. En una solución de 1
mg/litro no se observó disminución alguna del OTBE durante
62 días, pero el 20% del agua se perdió por evaporación.
2. Métodos analíticos
Se utilizan diversos métodos para medir los derivados
tributilestánnicos en el agua, en el sedimento o en la
flora y la fauna (biota). El más usado es la espectro-
metría de absorción atómica (AA). La espectrometría de AA
con llama permite alcanzar un límite de detección de 0,1
mg/litro. Resulta más sensible la AA sin llama, basada en
la atomización en un horno eléctrico con grafito, cuyos
límites de detección están comprendidos entre 0,1 y
1,0 µg/litro de agua. Existen diferentes métodos de
extracción y para formar derivados volátiles. La sepa-
ración de estos derivados suele hacerse por "purga y
captura" o cromatografía de gases. Los límites de detec-
ción son de 0,5 y 5,0 µg/kg para el sedimento y la
biota.
3. Fuentes de contaminación ambiental
Se han registrado diversos compuestos de tributile-
staño como molusquicidas, productos antiincrustantes en
botes y otras embarcaciones, muelles, boyas, jaulas de
langostas, redes de pesca y nasas, como conservadores de
la madera, como "productos anti-cieno" en los trabajos
de albañilería, como desinfectantes y como biocidas en los
sistemas de refrigeración, las torres de refrigeración de
las centrales electrógenas, las fábricas de pulpa y de
papel, las cervecerías, las industrias del cuero y los
telares. En las pinturas antiincrustantes, el TBE se
comercializó al principio bajo una forma que permitía la
liberación sin trabas del compuesto. En fecha más reciente
se han puesto a la venta pinturas de liberación controlada
en las que el TBE se incorpora a una matriz copolimérica.
También se han ideado matrices de caucho para hacer más
lenta y retrasar la liberación del compuesto, con la
consiguiente prolongación de la eficacia de las pinturas
antiincrustantes y de los molusquicidas. El TBE no se
utiliza en la agricultura por su elevada fitotoxicidad.
4. Reglamentación del empleo
Muchos países han restringido el empleo de pinturas
antiincrustantes con TBE por los efectos de éste sobre los
mariscos. Los reglamentos varían en detalle de unos países
a otros, pero casi siempre prohíben el uso de pinturas de
TBE en las embarcaciones de 24 metros de longitud o menos.
Algunos países han excluido de esta prohibición a las
embarcaciones con casco de aluminio. Además, en algunos
reglamentos se restringe el contenido de TBE en las
pinturas o el ritmo con que se libera este compuesto de
las mismas (a 4 o 5 µg/cm2 por día, a largo plazo).
5. Concentraciones en el medio ambiente
Se han encontrado concentraciones elevadas de TBE en
el agua, los sedimentos y la biota próximos a las zonas de
navegación de recreo, especialmente en "marinas" o
fondeaderos de yates, embarcaderos, diques secos, redes de
pesca y nasas tratadas con pinturas antiincrustantes, así
como en los sistemas de refrigeración. La intensidad de
las mareas y la turbiedad del agua influyen en las con-
centraciones de TBE.
Se ha visto que las concentraciones de TBE pueden
llegar a 1,58 µg/litro en el agua del mar y en los estu-
arios, a 7,1 µ/litro en el agua dulce, a 26 300 µg/kg en
los sedimentos costeros, a 3700 µg/kg en los sedimentos
de agua dulce, a 6,39 mg/kg en los bivalvos, a 1,92 mg/kg
en los gasterópodos y a 11 mg/kg en los peces. Ahora bien,
no hay que considerar como representativas esas concentra-
ciones máximas de TBE, toda vez que diversos factores
pueden dar lugar a valores anormalmente elevados (por
ejemplo, las partículas de pintura en las muestras de agua
y de sedimentos). Se ha comprobado que en las concentra-
ciones de TBE en la microcapa superficial del agua, tanto
dulce como salada, pueden ser dos veces más altas que las
medidas inmediatamente por debajo de la superficie. Sin
embargo, conviene tener en cuenta que los valores regis-
trados de TBE en las microcapas superficiales pueden verse
muy afectados por el método de muestreo.
Es posible que los datos antiguos no sean comparables
con los más recientes a causa del perfeccionamiento de los
métodos analíticos disponibles para determinar el TBE en
el agua, los sedimentos y los tejidos.
6. Transporte y transformación en el medio ambiente
A consecuencia de su baja hidrosolubilidad y de su
carácter lipofílico, el TBE se adsorbe fácilmente en las
partículas. Se calcula que entre el 10% y el 95% del OTBE
introducido en el agua se adsorbe de ese modo. La
desaparición progresiva del TBE adsorbido no se debe a la
desorción sino a la degradación. El grado de adsorción
depende de la salinidad, de la naturaleza y el tamaño de
las partículas en suspensión, de la cantidad de material
suspendido, de la temperatura y de la presencia de materia
orgánica disuelta.
La degradación del OTBE entraña la escisión del enlace
carbono-estaño. Este fenómeno puede deberse a diversos
mecanismos que intervienen simultáneamente en el medio
ambiente, unos fisicoquímicos (hidrólisis y fotodegrada-
ción) y otros biológicos (degradación por microorganismos
y metabolización por organismos superiores). Mientras que
en condiciones extremas de pH se produce una hidrólisis de
los compuestos de estaño orgánico, ésta es apenas evidente
en condiciones ambientales normales. En el laboratorio se
observa fotodegradación cuando se exponen soluciones a una
irradiación ultravioleta de 300 nm (y, en menor grado, de
350 nm). En condiciones naturales, la fotólisis está
limitada por la gama de longitudes de onda de la luz solar
y por la escasa penetración de la luz ultravioleta en el
agua. La presencia de sustancias fotosensibilizadoras
puede acelerar la fotodegradación. La biodegradación
depende de condiciones ambientales tales como la tempera-
tura, la oxigenación, el pH, el nivel de elementos miner-
ales, la presencia de sustancias orgánicas fácilmente
biodegradables a efectos de cometabolismo y la naturaleza
de la microflora y su capacidad de adaptación. También
depende de que la concentración de OTBE sea más baja que
el umbral letal o inhibitorio para las bacterias. Como en
la degradación abiótica, la ruptura biótica del TBE es un
proceso progresivo de debutilización oxidativa fundado en
la escisión del enlace carbono-estaño, en el que se forman
derivados dibutílicos que se degradan más fácilmente que
el tributilestaño. Los monobutilestaños se mineralizan
lentamente. Aunque existe degradación anaerobia, no se ha
llegado a un acuerdo sobre su importancia. Algunos autores
estiman que es lenta, mientras que otros piensan que es
más rápida que la degradación aerobia. Se han identificado
varias especies de bacterias, algas y hongos nocivos para
la madera que pueden degradar el OTBE. Las estimaciones
de la semivida del TBE en el medio ambiente acusan grandes
variaciones.
El TBE se bioacumula en los organismos a causa de su
solubilidad en las grasas. En investigaciones de labora-
torio con moluscos y peces se han visto que los factores
de bioconcentración pueden llegar a un valor de 7000, y
aun se han obtenido valores más altos en los estudios
sobre el terreno. La absorción a partir de los alimentos
es más importante que la que se efectúa directamente a
partir del agua. La presencia de factores de concentración
más altos en los microorganismos (entre 100 y 30 000)
puede deberse más a adsorción que a absorción intracelu-
lar. No hay ninguna indicación de que el TBE se transfiera
a los microorganismos terrestres a través de las cadenas
alimentarias.
7. Cinética y metabolismo
El tributilestaño se absorbe a través del intestino
(20-50%, según el vehículo) y de la piel en los mamíferos
(10% aproximadamente), pudiendo atravesar la barrera
hematoencefálica y pasar de la placenta al feto. El
material absorbido se distribuye rápida y ampliamente por
los tejidos (principalmente el hígado y el riñón).
En los mamíferos, el metabolismo del TBE es rápido: a
las 3 h de administrar TBE pueden ya descubrirse metabo-
litos en la sangre. Los estudios in vitro han revelado
que el TBE es un sustrato para ciertas oxidasas de función
mixta, pero las concentraciones muy altas de TBE inhiben
esas enzimas.
La velocidad de desaparición del TBE difiere de unos
tejidos a otros; las estimaciones de la semivida biológica
en los mamíferos van desde 23 hasta unos 30 días.
La metabolización del TBE se observa también en los
organismos inferiores, pero es más lenta (particularmente
en los moluscos) que en los mamíferos. La capacidad de
bioacumulación, por consiguiente, es mucho mayor que en
éstos.
Los compuestos de TBE inhiben la fosforilización
oxidativa y alteran la estructura y las funciones de las
mitocondrias. El TBE interfiere en la calcificación de la
concha de las ostras ( Crassostrea spp).
8. Efectos en los microorganismos
El TBE es tóxico para los microorganismos y se ha
utilizado comercialmente como bactericida y alguicida.
Las concentraciones que provocan efectos tóxicos varían
mucho de unas especies a otras. El TBE es más tóxico para
las bacterias gram-positivas (concentración inhibitoria
mínima (CIM): entre 0,2 y 0,8 mg/litro) que para las gram-
negativas (CIM: 3 mg/litro). La CIM del acetato de TBE
para los hongos es de 0,5-1 mg/litro y la CIM del OTBE
para el alga verde Chlorella pyrenoidosa es de 0,5
mg/litro. La productividad primaria de una comunidad
natural de algas de agua dulce se redujo en un 50% con una
concentración de OTBE de 3 µg/litro. En fecha reciente
se han determinado en dos especies de algas niveles de
efecto no observado (NENO) de 18 y 32 µg/litro. De igual
modo, la toxicidad para los microorganismos marinos varía
de unas especies a otras y de unos estudios a otros; los
NENO son difíciles de establecer pero no llegan a
0,1 µg/litro en algunas especies. Las concentraciones
alguicidas varían entre < 1,5 µg/litro y > 1000 µg por
litro según las especies.
9. Efectos en los organismos acuáticos
9.1 Efectos en los organismos de agua salada (mar y estuarios)
En la figura 1 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las con-
centraciones de TBE medidas en diversos organismos del mar
y de los estuarios. En todo el mundo, especialmente en los
lugares relacionados con actividades náuticas recreativas,
se han encontrado concentraciones superiores a las que
producen efectos letales.
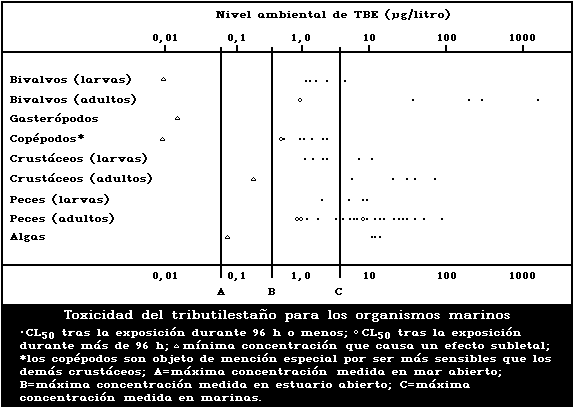 El desarrollo de las esporas móviles de una macroalga
verde se ha revelado como el índice evolutivo más sensible
al TBE (CE50 de 5 días: 0,001 µg/litro). El crecimiento
de una angiosperma marina se redujo en concentraciones de
TBE de un mg/kg de sedimento, pero no se observó ningún
efecto a 0,1 mg/kg.
El tributilestaño es sumamente tóxico para los
moluscos marinos. Experimentalmente se ha demostrado que
altera la formación de la concha en las ostras en creci-
miento, así como el desarrollo gonadal y el sexo de las
ostras adultas, la formación de colonias, el crecimiento y
la mortalidad de las ostras y otros bivalvos en la fase
larvaria, y que causa "imposex" (desarrollo de caracter-
ísticas masculinas) en los gasterópodos hembras. Se ha
obtenido un valor NENO de 20 ng/litro para la freza de la
especie de ostra más sensible (Crassostrea gigas). El TBE
provoca una deformación de la concha de las ostras adultas
más o menos acentuada según la dosis. No se ha observado
experimentalmente ningún efecto sobre la morfología de la
concha con concentraciones de TBE de 2 ng/litro. En las
hembras de buccino, el valor NENO para el imposex no llega
a 1,5 ng/litro. En general, las formas larvarias son más
sensibles que los adultos, siendo esta diferencia
especialmente marcada en el caso de las ostras.
Los copépodos son más sensibles que otros grupos de
crustáceos al efecto letal agudo del TBE; los valores de
CL50 para periodos de exposición de hasta 96 h van de
0,6 a 2,2 µg/litro. Estas cifras son comparables a las
obtenidas en las larvas más sensibles de otros grupos de
crustáceos. El TBE reduce el rendimiento reproductor, la
supervivencia de los recién nacidos y la tasa de creci-
miento juvenil en los crustáceos. En el camarón mísido
Acanthomysis sculpta el valor NENO para la reproducción
parece ser de 0,09 µg/litro. Otro camarón, el llamado
por los anglosajones "grass shrimp", no evita el TBE a
concentraciones que pueden llegar hasta 30 µg/litro.
La toxicidad del tributilestaño para los peces marinos
es sumamente variable; los valores de la CL50 a 96 h van
desde 1,5 hasta 36 µg/litro. Las fases larvarias son más
sensibles que los adultos (fig. 1). Hay indicios de que
los peces marinos evitan las concentraciones de OTBE de
1 µg/litro o más.
9.2 Efectos en los organismos de agua dulce
En la figura 2 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las
concentraciones de TBE medidas en agua dulce. Se han
observado concentraciones mayores que las que provocan
efectos subletales en diversos sitios, especialmente en
relación con actividades náuticas recreativas.
Una concentración OTBE de 0,5 mg/litro produjo la
muerte de las angioespermas de agua dulce, mientras que el
crecimiento se inhibió a 0,06 mg/litro o más.
El desarrollo de las esporas móviles de una macroalga
verde se ha revelado como el índice evolutivo más sensible
al TBE (CE50 de 5 días: 0,001 µg/litro). El crecimiento
de una angiosperma marina se redujo en concentraciones de
TBE de un mg/kg de sedimento, pero no se observó ningún
efecto a 0,1 mg/kg.
El tributilestaño es sumamente tóxico para los
moluscos marinos. Experimentalmente se ha demostrado que
altera la formación de la concha en las ostras en creci-
miento, así como el desarrollo gonadal y el sexo de las
ostras adultas, la formación de colonias, el crecimiento y
la mortalidad de las ostras y otros bivalvos en la fase
larvaria, y que causa "imposex" (desarrollo de caracter-
ísticas masculinas) en los gasterópodos hembras. Se ha
obtenido un valor NENO de 20 ng/litro para la freza de la
especie de ostra más sensible (Crassostrea gigas). El TBE
provoca una deformación de la concha de las ostras adultas
más o menos acentuada según la dosis. No se ha observado
experimentalmente ningún efecto sobre la morfología de la
concha con concentraciones de TBE de 2 ng/litro. En las
hembras de buccino, el valor NENO para el imposex no llega
a 1,5 ng/litro. En general, las formas larvarias son más
sensibles que los adultos, siendo esta diferencia
especialmente marcada en el caso de las ostras.
Los copépodos son más sensibles que otros grupos de
crustáceos al efecto letal agudo del TBE; los valores de
CL50 para periodos de exposición de hasta 96 h van de
0,6 a 2,2 µg/litro. Estas cifras son comparables a las
obtenidas en las larvas más sensibles de otros grupos de
crustáceos. El TBE reduce el rendimiento reproductor, la
supervivencia de los recién nacidos y la tasa de creci-
miento juvenil en los crustáceos. En el camarón mísido
Acanthomysis sculpta el valor NENO para la reproducción
parece ser de 0,09 µg/litro. Otro camarón, el llamado
por los anglosajones "grass shrimp", no evita el TBE a
concentraciones que pueden llegar hasta 30 µg/litro.
La toxicidad del tributilestaño para los peces marinos
es sumamente variable; los valores de la CL50 a 96 h van
desde 1,5 hasta 36 µg/litro. Las fases larvarias son más
sensibles que los adultos (fig. 1). Hay indicios de que
los peces marinos evitan las concentraciones de OTBE de
1 µg/litro o más.
9.2 Efectos en los organismos de agua dulce
En la figura 2 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las
concentraciones de TBE medidas en agua dulce. Se han
observado concentraciones mayores que las que provocan
efectos subletales en diversos sitios, especialmente en
relación con actividades náuticas recreativas.
Una concentración OTBE de 0,5 mg/litro produjo la
muerte de las angioespermas de agua dulce, mientras que el
crecimiento se inhibió a 0,06 mg/litro o más.
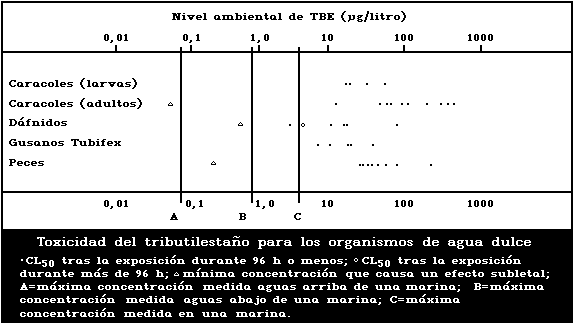 Los datos sobre los invertebrados de agua dulce son
escasos y no comprenden más que tres especies, además de
los organismos tomados como objetivo. Con diferentes sales
de TBE se han obtenido valores de CL50 en 48 h para
Daphnia de 2,3-70 µg/litro y para Tubifex de 5,5-
33 µg/litro. Basándose en la reversión de la respuesta
normal a la luz, se ha calculado que el NENO para Daphnia
es de 0,5 µg/litro. La CL50 de 24 h para la almeja
asiática parece ser, según se ha señalado, de 2100 µg por
litro, mientras que para los moluscos adultos contra los
que se dirige la lucha antiesquistosomiásica los valores
correspondientes son de 30-400 µg/litro.
Se ha demostrado que el tributilestaño es tóxico para
las larvas de esquistosoma en las fases acuáticas; la
CL50 (fluoruro de TBE) parece ser, según los cálculos
realizados, de 16,8 µg/litro en el caso de una exposi-
ción de 1 h. La dosis de TBE que suprime el 99-100% de la
infectividad de las cercarias para el ratón está compren-
dida entre 2 y 6 µg/litro.
La sensibilidad de los moluscos al TBE disminuye con
la edad, pero los huevos son más resistentes que los
individuos jóvenes y adultos. La puesta de huevos se ve
considerablemente afectada a una concentración de OTBE de
0,001 µg/litro.
En las pruebas de CL50 con exposiciones de hasta
168 h, la toxicidad aguda del TBE para los peces de agua
dulce varía entre 13 y 240 µg/litro. Basándose en los
efectos histopatológicos, se ha calculado que el valor
NENO para el "guppy" es de 0,01 µg/litro.
No se ha observado ningún efecto sobre la super-
vivencia tras la exposición de huevos y larvas de la rana
Rana temporaria a concentraciones de TBE de 3 µg por
litro o menos; en cambio, la mortalidad fue significativa
a 30 µg/litro.
9.3 Estudios microcósmicos
Se han realizado algunos "estudios microcósmicos"
utilizando como modelo ecosistemas marinos en los que se
habían introducido organismos y en los que la entrada de
agua de mar facilitaba la colonización por otros organ-
ismos. Los resultados obtenidos muestran que tanto el
número de individuos como la diversidad de las especies
disminuyen cuando la concentración de OTBE en el agua se
sitúa entre 0,06 y 3 µg/litro.
Los resultados obtenidos con modelos de ecosistemas de
agua dulce hacen pensar que las dosis que matan los cara-
coles afectan también a otras especies, en particular los
peces.
10. Efectos en los organismos terrestres
La exposición de organismos terrestres al TBE proviene
sobre todo del uso de este compuesto como conservador de
la madera. El OTBE es tóxico para las abejas que viven en
colmenas de madera tratadas con TBE. Este producto se
mostró tóxico para los murciélagos en un estudio aislado,
pero la elevada mortalidad de los testigos resta signifi-
cación estadística a este resultado. Los compuestos de
TBE son tóxicos para los insectos que están en contacto o
se alimentan con madera tratada. El TBE tiene una toxi-
cidad aguda moderada para los ratones en libertad y,
basándose en el consumo de semillas tratadas en las
pruebas de repelentes, se calcula que los valores de
CL50 en la dieta se sitúan entre 37 y 240 mg/kg al día.
11. Efectos en organismos estudiados sobre el terreno
Las observaciones sobre el terreno han permitido
establecer una relación entre las concentraciones elevadas
de tributilestaño y la mortalidad de las larvas de
bivalvos, la incapacidad de las mismas para constituir
colonias, el retraso del crecimiento, el engrosamiento de
la concha y otras malformaciones de las ostras en
desarrollo, el imposex en los caracoles de suelos
cenagosos y el imposex (con disminución concurrente de la
población) en el buccino. En Francia inicialmente, y más
tarde en otros países, se ha identificado y atribuido la
destrucción completa de los criaderos de ostras a la
presencia de TBE en el agua. Los efectos son más acusados
en las zonas próximas a los centros de deportes náuticos.
Al dejar de emplear pinturas antiincrustantes a base de
TBE en las pequeñas embarcaciones se restablecieron la
reproducción y el crecimiento de las ostras. Sin embargo,
en algunas zonas las concentraciones de TBE en el agua
siguen siendo bastante elevadas para afectar a los
gasterópodos marinos.
Tanto el desarrollo y formación de la concha en las
ostras del Pacífico como el imposex en el buccino se han
utilizado como indicadores biológicos de contaminación por
TBE.
Aunque se han hecho pocos estudios sobre los efectos
en los organismos del TBE presente en los sedimentos, hay
indicios de que este compuesto puede llegar a los animales
que viven en madrigueras y producir mortalidad en condi-
ciones prácticas.
Se han observado efectos tóxicos macroscópicos y
alteraciones histopatológicas en los criaderos de pesca
marítima expuestos al TBE por el uso de pinturas antiin-
crustantes en las redes de contención.
Se ha propuesto la utilización de TBE como molusqui-
cida contra los caracoles de agua dulce que transmiten la
esquistosomiasis (bilharziasis). Varios ensayos prácticos
han demostrado que es difícil aplicar el TBE sin que
resulten perjudicados otros organismos distintos de los
tomados como objetivo.
12. Toxicidad para los mamíferos de laboratorio
12.1 Toxicidad aguda
El tributilestaño tiene una toxicidad entre moderada y
alta para los mamíferos de laboratorio; los valores de la
DL50 oral aguda varían desde 94 a 234 mg/kg de peso
corporal para la rata y desde 44 hasta 230 mg/kg de peso
corporal para el ratón. La toxicidad aguda para el cobayo
y el conejo es del mismo orden. La variación se debe al
componente aniónico de la sal de tributilestaño. Estos
compuestos tienen un potencial letal mayor por vía paren-
teral que por vía oral, probablemente porque la absorción
intestinal es incompleta.
Entre otros efectos de la exposición aguda cabe citar
alteraciones de las cifras de lípidos sanguíneos, el sis-
tema endocrino, el hígado y el bazo, así como un déficit
transitorio del desarrollo cerebral. La importancia toxi-
cológica de estos efectos, observados tras la administra-
ción de una fuerte dosis única del compuesto, es discuti-
ble y sigue ignorándose cuál es la causa de la muerte.
La toxicidad aguda por vía dérmica es baja; la
DL50 es > 9000 mg/kg de peso corporal para el conejo.
La DL50 por inhalación exclusivamente nasal (4 h) es de
77 mg/m3 (65 mg/m3 cuando sólo se tienen en cuenta las
partículas inhalables) para la rata. Las mezclas de aire
y vapores de TBE no producen efectos tóxicos observables,
ni siquiera en el punto de saturación. Sin embargo, el TBE
es muy peligroso cuando se inhala en forma de aerosol,
produciendo irritación y edema de los pulmones.
El TBE es muy irritante para la piel y sumamente irri-
tante para los ojos. El OTBE no produce sensibilización
cutánea.
12.2 Toxicidad a corto plazo
Los compuestos de TBE han sido muy estudiados en la
rata, hasta el punto de que todos los datos expuestos en
esta sección se refieren a ese animal a menos que se
indique otra cosa.
Con dosis de 320 mg/kg (unos 25 mg/kg de peso
corporal) en la dieta se han obtenido altas tasas de
mortalidad cuando la exposición se prolonga más de 4
semanas. No se observaron muertes con 100 mg/kg en la
dieta (10 mg/kg de peso corporal) ni tras la administra-
ción forzada de 12 mg/kg de peso corporal al día. En las
ratas tratadas en sus primeros días de vida, la mortalidad
aumentó con una dosis de 3 mg/kg de peso corporal. Los
principales síntomas causados por las dosis letales fueron
inapetencia, debilidad y emaciación.
Se han observado efectos "limítrofes" en el creci-
miento de la rata con dosis de 50 mg/kg (6 mg/kg de peso
corporal) en la dieta y de 6 mg/kg de peso corporal en
estudios de administración forzada. Los ratones se
muestran menos sensibles, observándose los efectos del
compuesto con dosis de 150 a 200 mg/kg (22 a 29 mg/kg de
peso corporal) en la dieta.
Tanto los estudios a corto plazo como en los pro-
longados se han observado efectos estructurales sobre los
órganos endocrinos, principalmente la hipófisis y el
tiroides. Las pruebas a corto plazo pusieron de manifiesto
alteraciones en la concentración de las hormonas circu-
lantes y en la respuesta a los estímulos fisiológicos
(hormonas tróficas hipofisarias), pero tras la exposición
prolongada desaparecieron casi todas esas alteraciones.
El mecanismo de acción es desconocido.
La exposición a un aerosol de OTBE a razón de 2,8
mg/m3 produjo un aumento de la mortalidad, así como
dificultades respiratorias, inflamación del tracto
respiratorio y alteraciones histopatológicas de los
órganos linfáticos. En cambio, la exposición a la máxima
concentración de vapor accesible (0,16 mg/m3) a la
temperatura del local no produjo ningún efecto.
En tres especies de mamíferos se han señalado efectos
tóxicos en el hígado y los conductos biliares. En ratas a
las que se administró OTBE en la dieta a razón de 320
mg/kg (unos 25 mg/kg de peso corporal) durante 4 semanas y
en ratones tratados con 80 mg/kg (unos 12 mg/kg de peso
corporal) en la dieta durante 90 días se produjo una
necrosis hepatocelular con alteraciones inflamatorias del
árbol biliar. En perros que recibieron una dosis de 10
mg/kg de peso corporal durante 8 o 9 semanas se observó
una vacuolización de los hepatocitos periportales. Estos
cambios se acompañaban a veces de un aumento del peso del
hígado y de la actividad sérica de las enzimas hepáticas.
El descenso de la concentración de hemoglobina y del
volumen eritrocítico en la rata, causado por la adminis-
tración de 80 mg/kg (8 mg/kg de peso corporal) en la
dieta, traduce un efecto en la síntesis de la hemoglobina
que da lugar a una anemia hipocrómica microcítica. El
descenso de las cifras de hemosiderina esplénica sugiere
una alteración del equilibrio del hierro. En los ratones
se ha observado también anemia.
En ciertas investigaciones a corto plazo, pero no en
los estudios prolongados, se ha observado formación de
rosetas de hematíes en los ganglios linfáticos del
mesenterio. No está clara la significación biológica de
este fenómeno (posiblemente transitorio).
El efecto tóxico característico del OTBE tiene lugar
en el sistema inmunitario: a causa de los efectos en el
timo, la inmunidad celular se menoscaba. Aunque se
desconoce el mecanismo de acción, es posible que esté
relacionado con la conversión metabólica en compuestos de
dibutilestaño. También resulta afectada la resistencia
inespecífica.
Asimismo se han observado efectos generales en el
sistema inmunitario (por ejemplo, en el peso y la
morfología de los tejidos linfoides, los recuentos de
linfocitos periféricos y las concentraciones totales de
inmunoglobulinas séricas) en diferentes estudios con OTBE
realizados en ratas y perros, pero no en ratones, mediante
dosis claramente tóxicas (en el ratón se han registrado
efectos con dosis de cloruro de tributilestaño de 150
mg/kg). Solamente la rata presenta signos de toxicidad y,
evidentemente, es la especie más sensible. En los estudios
a corto plazo, el NENO para esta especie fue de 5 mg/kg
(0,6 mg/kg de peso corporal) en la dieta. En los estudios
con cloruro de tributilestaño se han observado efectos
análogos en el timo, rápidamente reversibles tan pronto
como se interrumpía la administración. Según se ha
observado en estudios in vivo de resistencia del huésped,
el OTBE pone en peligro la función inmunitaria específica
en la rata. Tras la exposición a un nivel de 50 mg/kg en
la dieta (siendo el valor NENO de 5 mg/kg al día), se
observó una disminución del "aclaramiento" de Listeria
monocytogenes, mientras que con 50 y 5 mg/kg en la dieta,
pero no con 0,5 mg/kg (2,5, 0,25 y 0,025 mg/kg de peso
corporal al día, respectivamente) se produjo un descenso
de la resistencia a Trichinella spiralis. Los mismos
efectos, aunque menos pronunciados, se observaron en
animales de más edad.
Según los conocimientos actuales, los efectos en la
resistencia del huésped se deben probablemente a una
evaluación más adecuada de los posibles riesgos para el
hombre; sin embargo, no se tiene suficiente experiencia
con los sistemas de prueba para apreciar bien su
importancia. En cualquier caso, las observaciones en
ratas atímicas "desnudas", estimuladas en las condi-
ciones ordinarias, proporcionan algunos datos para
interpretar el modelo de T. spiralis. En estos estudios,
la ausencia completa de inmunidad timodependiente aumentó
de 10 a 20 veces los recuentos de larvas en el músculo; en
cambio, la exposición a concentraciones de OTBE de 5 y 50
mg/kg en la dieta duplicó y cuadruplicó, respectivamente,
la cifra inicial.
Aunque ahora se dispone de algunos datos sobre los
efectos de los compuestos de tributilestaño en el
desarrollo del sistema inmunitario, carecemos de informa-
ción sobre la resistencia del huésped.
Lo prudente sería evaluar los posibles riesgos para el
hombre en función de los datos obtenidos en la especie más
sensible. Los efectos sobre la resistencia del huésped a
T. spiralis se han observado ya con niveles en la dieta
de 5 mg/kg (equivalentes a 0,25 mg/kg de peso corporal al
día), por lo que el valor NENO es de 0,5 mg/kg (equiva-
lente a 0,025 mg/kg al día). Sin embargo, no existe
acuerdo sobre el significado de estos datos para evaluar
los riesgos en el hombre. En todos los demás estudios,
una concentración de 5 mg/kg al día en la dieta (equiva-
lente a 0,5 mg/kg de peso corporal, sobre la base de los
estudios a corto plazo) correspondía al valor NENO con
respecto a los efectos en el sistema inmunitario, tanto
generales como específicos.
12.3 Toxicidad a largo plazo
Un estudio a largo plazo en las ratas sugiere un
efecto marginal del TBE en los parámetros toxicológicos
generales (cuya significación toxicológica es limitada) a
una concentración de 5 mg/kg (0,25 mg/kg de peso corporal)
en la dieta.
12.4 Genotoxicidad
La genotoxicidad del OTBE ha sido objeto de detenidas
investigaciones. En la gran mayoría de los estudios se
han obtenido resultados negativos, y no hay ninguna prueba
convincente de que el OTBE tenga propiedades mutagénicas.
12.5 Toxicidad en el sistema reproductor
En tres especies de mamíferos (ratón, rata y conejo)
se ha evaluado la posible embriotoxicidad del OTBE tras
administrarlo por vía oral a la madre. La principal
malformación observada en los fetos de rata y de ratón fue
la fisura palatina, pero este efecto sólo se registró con
dosis claramente tóxicas para las madres. Tal resultado
no se ha considerado como indicio de efectos teratogénicos
del OTBE en dosis inferiores a las que producen toxicidad
materna. El NENO más bajo en lo relativo a la embriotoxi-
cidad y la fetotoxicidad para todos las especies fue de
1,0 mg/kg de peso corporal.
12.6 Carcinogenicidad
En las ratas se ha realizado un estudio de carcino-
genicidad en el que se obtuvieron alteraciones neoplásicas
de los órganos endocrinos con 50 mg/kg en la dieta. En
cuanto a los tumores hipofisarios registrados con 0,5
mg/kg en la dieta, se consideró que no tenían signifi-
cación biológica por no existir relación dosis-respuesta.
Estos tipos de tumores suelen aparecer con una incidencia
general elevada y variable, por lo que su significación
parece discutible. Está en curso un estudio de carcino-
genicidad en el ratón.
13. Efectos en el ser humano
Se ha observado que la exposición profesional de los
trabajadores al tributilestaño provoca irritación de las
vías respiratorias superiores. En forma de aerosol, el
TBE entraña un riesgo para las personas. El OTBE ejerce
un efecto irritante en la piel y en los ojos, habiéndose
observado casos de dermatitis grave a consecuencia del
contacto directo con la piel. El problema se agrava por
la falta de una respuesta cutánea inmediata.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS
SOBRE EL MEDIO AMBIENTE
1. Evaluación del riesgo para las personas
La exposición de los trabajadores se produce sobre
todo en las actividades de fabricación y formulación de
compuestos de tributilestaño, durante la aplicación y la
eliminación de pinturas a base de TBE y a consecuencia del
empleo de TBE como conservador de la madera. La exposición
del público en general puede deberse a la contaminación de
los alimentos, particularmente el pescado y los mariscos,
así como a la aplicación doméstica de productos de protec-
ción de la madera.
A juzgar por las pruebas realizadas en animales y por
la observación directa de las personas, parece evidente
que los compuestos de TBE ejercen efectos irritantes en la
piel y en los ojos y que la inhalación de los aerosoles
provoca irritación respiratoria.
La manipulación de madera tratada no entraña riesgos
de irritación dérmica si se ha secado el material. En
cambio, los aerosoles de TBE son muy peligrosos y no debe
permitirse que la madera vuelva a entrar en la zona de
tratamiento hasta que esté perfectamente seca.
No se ha señalado ningún caso de intoxicación sis-
témica aguda y, probablemente, el TBE se elimina del
organismo al cabo de pocos días. Por consiguiente, no es
de temer que el manejo de productos de TBE entrañe peligro
de toxicidad aguda si se toman las precauciones
adecuadas.
En los animales de laboratorio se han observado
efectos a corto y a largo plazo en el hígado y en los
sistemas hematológico y endocrino. Los efectos de los
compuestos de TBE en el sistema inmunitario, y especial-
mente en la resistencia del huésped, han resultado ser el
parámetro más sensible de toxicidad en la rata, que es la
especie más sensible de todas las estudiadas. Cuando se
utiliza Trichinella spiralis como modelo de resistencia
del huésped, el nivel de efecto no observado (NENO) se
sitúa entre 0,5 y 5,0 mg/kg (0,025 y 0,25 mg/kg de peso
corporal) en la dieta, mientras que si se utilizan índices
de función inmunitaria es de 0,6 mg/kg de peso corporal.
Debido a las grandes variaciones en el consumo de
pescado y mariscos y a las diferencias locales de la
cantidad de residuos de TBE presentes en la fauna marina,
sólo a título de orientación pueden hacerse estimaciones
de los valores de exposición y del NENO. Conviene tener
en cuenta que para evaluar el riesgo potencial de estos
compuestos hay que proceder en el plano local a determinar
los residuos, calcular el consumo de pescado y mariscos y
fijar los límites aceptables de seguridad.
Partiendo de cifras de consumo de pescado de 15 y 150
g/día, de un valor de residuos en el pescado de 1 mg/kg y
de un peso medio de las personas de 60 kilos, se han
obtenido los siguientes márgenes de seguridad basados en
diferentes puntos finales inmunológicos.
----------------------------------------------------------------------
Consumo de Ingestión diaria Margen de seguridad
pescado estimada de TBE Modelo de Otros parámetros
(g/día) (µg/kg) T. spiralis inmunológicos
----------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
----------------------------------------------------------------------
El empleo indiscriminado e irresponsable de compuesto
de TBE y la observancia de las recomendaciones esbozadas
en la presente monografía para reducir la exposición de
las personas puede dar lugar a la ingestión de concentra-
ciones de compuesto de TBE peligrosas para la salud
humana.
En los animales de experimentación sólo de han
observado efectos teratogénicos con dosis que causan
signos patentes de toxicidad materna. Por consiguiente,
se considera que el potencial teratogénico de TBE es muy
bajo.
Basándose en los resultados de detallados estudios de
mutagenicidad, se considera que los compuestos de
tributilestaño no tienen potencial mutagénico. En un
estudio de carcinogenicidad realizada en la rata con OTBE,
se observó un aumento de la incidencia de ciertos tumores
endocrinos que aparecen espontáneamente con una incidencia
elevada y variable. Por consiguiente, los datos
disponibles no indican claramente que los compuestos de
TBE entrañen riesgos carcinogénicos para las personas.
2. Evaluación de los riesgos ambientales
La difusión del tributilestaño (TBE) en el medio
ambiente se debe sobre todo al empleo de ese compuesto en
las pinturas antiincrustantes. También puede deberse al
empleo de TBE como molusquicida. La contaminación en la
fuente se produce a consecuencia de utilizar TBE como
biocida en los sistemas de refrigeración, la fabricación
de pulpa de madera, el tratamiento de los cueros, los
procesos de conservación de la madera y el tratamiento de
los productos textiles.
Debido a sus propiedades fisicoquímicas, los compues-
tos de TBE se concentran en la microcapa superficial y en
los sedimentos. La degradación abiótica no parece ser un
mecanismo importante de eliminación del TBE en condiciones
ambientales. Aunque el OTBE es biodegradable en la columna
de agua, este proceso no es suficientemente rápido para
impedir la aparición de concentraciones elevadas de TBE en
algunos sitios. En la mayor parte de los organismos
acuáticos se produce bioacumulación pero la degradación
metabólica es un proceso más eficaz en los mamíferos de
laboratorio.
El TBE es sumamente peligroso para algunos organismos
acuáticos por ser tóxico incluso a concentraciones muy
bajas en el agua. Tales concentraciones se han señalado
en diversos lugares. En los estudios sobre el terreno se
han observado efectos adversos sobre invertebrados,
especialmente moluscos, a los que no se pretendía atacar,
y esos efectos resultan suficientemente graves para inter-
ferir en la reproducción y provocar un descenso de la
población. En algunos sitios ha sido posible anular los
efectos adversos en la producción comercial de mariscos
restringuiendo el empleo de pinturas antiincrustantes, con
lo que también se ha logrado suprimir el efecto de imposex
en las poblaciones de gasterópodos. Los efectos en las
piscifactorías hacen pensar que no conviene utilizar
pinturas que contengan TBE en las redes de contención.
Para el medio terrestre, el riesgo general parece ser
bajo. La madera tratada con TBE podría ser peligrosa para
los organismos terrestres que viven en estrecho contacto
con ella.
El aumento de las concentraciones de TBE en la micro-
capa superficial puede ser peligroso para los organismos
del litoral, las especies neustónicas (inclusive inverte-
brados bénticos y larvas de peces) y las aves salvajes y
marinas que se alimentan en la superficie del agua. La
acumulación y la baja biodegradación del TBE en los sedi-
mentos puede representar un peligro para los organismos
acuáticos cuando esos sedimentos contaminados se movilizan
por procesos naturales u operaciones de dragado.
RECOMENDACIONES
1. Recomendaciones para proteger la salud humana y la
higiene del medio
a) Hay que instar a los países Miembros que todavía no
hayan reglamentado el uso de compuestos de TBE a que
lo hagan.
b) Es necesario evaluar y, si procede reglamentar, el
ingreso en el medio ambiente de estaño orgánico pro-
cedente de fuentes distintas de las pinturas antiin-
crustantes. Por ejemplo, convendría evaluar el riesgo
potencial que entraña la aplicación al suelo de lodos
de alcantarillado contaminados con TBE.
c) Habrá que mejorar los métodos utilizables para apli-
car, eliminar y evacuar en condiciones de seguridad
las pinturas de estaño orgánico.
2. Investigaciones necesarias
a) Habrá que mejorar los métodos de detección y análisis
a fin de poder determinar con rapidez y precisión los
compuestos de butilestaño presentes en concentraciones
de pg/litro. Una de las razones que justifican esa
recomendación es que ciertos efectos biológicos (por
ejemplo, el imposex en los gasterópodos) pueden pro-
ducirse con concentraciones inferiores a los actuales
límites de detección.
b) Esnecesario estudiar los mecanismos que concentran en
vez de dispersar el TBE y que retrasan la degradación,
prestando especial atención a los fundamentos químicos
de ese compuesto y a su interaccción con las moléculas
biológicas. Se necesitan más estudios sobre la absor-
ción del TBE en todos los niveles tróficos.
c) Habrá que estudiar la toxicidad del TBE en los organ-
ismos acuáticos. Estos estudios deberán versar, si
procede, sobre el metabolismo, los efectos endocrinos
y la toxicidad inmunológica.
d) Habrá que buscar otras especies sensibles (en parti-
cular de agua dulce) que sirvan de bioindicador en
otros grupos.
e) Habrá que confirmar la validez de los modelos utili-
zados para evaluar la inmunotoxicidad en los mamíferos
y definir con más precisión los niveles sin efecto de
los parámetros pertinentes.
f) Convendría emprender un estudio de toxicidad crónica
en una segunda especie de mamífero.
g) Convendría emprender un estudio de tumorigenicidad en
una segunda especie de mamífero.
h) Hay que obtener datos mediante métodos de especiación
sobre los niveles de residuos de butilestaño en los
peces y mariscos destinados al consumo humano.
Los datos sobre los invertebrados de agua dulce son
escasos y no comprenden más que tres especies, además de
los organismos tomados como objetivo. Con diferentes sales
de TBE se han obtenido valores de CL50 en 48 h para
Daphnia de 2,3-70 µg/litro y para Tubifex de 5,5-
33 µg/litro. Basándose en la reversión de la respuesta
normal a la luz, se ha calculado que el NENO para Daphnia
es de 0,5 µg/litro. La CL50 de 24 h para la almeja
asiática parece ser, según se ha señalado, de 2100 µg por
litro, mientras que para los moluscos adultos contra los
que se dirige la lucha antiesquistosomiásica los valores
correspondientes son de 30-400 µg/litro.
Se ha demostrado que el tributilestaño es tóxico para
las larvas de esquistosoma en las fases acuáticas; la
CL50 (fluoruro de TBE) parece ser, según los cálculos
realizados, de 16,8 µg/litro en el caso de una exposi-
ción de 1 h. La dosis de TBE que suprime el 99-100% de la
infectividad de las cercarias para el ratón está compren-
dida entre 2 y 6 µg/litro.
La sensibilidad de los moluscos al TBE disminuye con
la edad, pero los huevos son más resistentes que los
individuos jóvenes y adultos. La puesta de huevos se ve
considerablemente afectada a una concentración de OTBE de
0,001 µg/litro.
En las pruebas de CL50 con exposiciones de hasta
168 h, la toxicidad aguda del TBE para los peces de agua
dulce varía entre 13 y 240 µg/litro. Basándose en los
efectos histopatológicos, se ha calculado que el valor
NENO para el "guppy" es de 0,01 µg/litro.
No se ha observado ningún efecto sobre la super-
vivencia tras la exposición de huevos y larvas de la rana
Rana temporaria a concentraciones de TBE de 3 µg por
litro o menos; en cambio, la mortalidad fue significativa
a 30 µg/litro.
9.3 Estudios microcósmicos
Se han realizado algunos "estudios microcósmicos"
utilizando como modelo ecosistemas marinos en los que se
habían introducido organismos y en los que la entrada de
agua de mar facilitaba la colonización por otros organ-
ismos. Los resultados obtenidos muestran que tanto el
número de individuos como la diversidad de las especies
disminuyen cuando la concentración de OTBE en el agua se
sitúa entre 0,06 y 3 µg/litro.
Los resultados obtenidos con modelos de ecosistemas de
agua dulce hacen pensar que las dosis que matan los cara-
coles afectan también a otras especies, en particular los
peces.
10. Efectos en los organismos terrestres
La exposición de organismos terrestres al TBE proviene
sobre todo del uso de este compuesto como conservador de
la madera. El OTBE es tóxico para las abejas que viven en
colmenas de madera tratadas con TBE. Este producto se
mostró tóxico para los murciélagos en un estudio aislado,
pero la elevada mortalidad de los testigos resta signifi-
cación estadística a este resultado. Los compuestos de
TBE son tóxicos para los insectos que están en contacto o
se alimentan con madera tratada. El TBE tiene una toxi-
cidad aguda moderada para los ratones en libertad y,
basándose en el consumo de semillas tratadas en las
pruebas de repelentes, se calcula que los valores de
CL50 en la dieta se sitúan entre 37 y 240 mg/kg al día.
11. Efectos en organismos estudiados sobre el terreno
Las observaciones sobre el terreno han permitido
establecer una relación entre las concentraciones elevadas
de tributilestaño y la mortalidad de las larvas de
bivalvos, la incapacidad de las mismas para constituir
colonias, el retraso del crecimiento, el engrosamiento de
la concha y otras malformaciones de las ostras en
desarrollo, el imposex en los caracoles de suelos
cenagosos y el imposex (con disminución concurrente de la
población) en el buccino. En Francia inicialmente, y más
tarde en otros países, se ha identificado y atribuido la
destrucción completa de los criaderos de ostras a la
presencia de TBE en el agua. Los efectos son más acusados
en las zonas próximas a los centros de deportes náuticos.
Al dejar de emplear pinturas antiincrustantes a base de
TBE en las pequeñas embarcaciones se restablecieron la
reproducción y el crecimiento de las ostras. Sin embargo,
en algunas zonas las concentraciones de TBE en el agua
siguen siendo bastante elevadas para afectar a los
gasterópodos marinos.
Tanto el desarrollo y formación de la concha en las
ostras del Pacífico como el imposex en el buccino se han
utilizado como indicadores biológicos de contaminación por
TBE.
Aunque se han hecho pocos estudios sobre los efectos
en los organismos del TBE presente en los sedimentos, hay
indicios de que este compuesto puede llegar a los animales
que viven en madrigueras y producir mortalidad en condi-
ciones prácticas.
Se han observado efectos tóxicos macroscópicos y
alteraciones histopatológicas en los criaderos de pesca
marítima expuestos al TBE por el uso de pinturas antiin-
crustantes en las redes de contención.
Se ha propuesto la utilización de TBE como molusqui-
cida contra los caracoles de agua dulce que transmiten la
esquistosomiasis (bilharziasis). Varios ensayos prácticos
han demostrado que es difícil aplicar el TBE sin que
resulten perjudicados otros organismos distintos de los
tomados como objetivo.
12. Toxicidad para los mamíferos de laboratorio
12.1 Toxicidad aguda
El tributilestaño tiene una toxicidad entre moderada y
alta para los mamíferos de laboratorio; los valores de la
DL50 oral aguda varían desde 94 a 234 mg/kg de peso
corporal para la rata y desde 44 hasta 230 mg/kg de peso
corporal para el ratón. La toxicidad aguda para el cobayo
y el conejo es del mismo orden. La variación se debe al
componente aniónico de la sal de tributilestaño. Estos
compuestos tienen un potencial letal mayor por vía paren-
teral que por vía oral, probablemente porque la absorción
intestinal es incompleta.
Entre otros efectos de la exposición aguda cabe citar
alteraciones de las cifras de lípidos sanguíneos, el sis-
tema endocrino, el hígado y el bazo, así como un déficit
transitorio del desarrollo cerebral. La importancia toxi-
cológica de estos efectos, observados tras la administra-
ción de una fuerte dosis única del compuesto, es discuti-
ble y sigue ignorándose cuál es la causa de la muerte.
La toxicidad aguda por vía dérmica es baja; la
DL50 es > 9000 mg/kg de peso corporal para el conejo.
La DL50 por inhalación exclusivamente nasal (4 h) es de
77 mg/m3 (65 mg/m3 cuando sólo se tienen en cuenta las
partículas inhalables) para la rata. Las mezclas de aire
y vapores de TBE no producen efectos tóxicos observables,
ni siquiera en el punto de saturación. Sin embargo, el TBE
es muy peligroso cuando se inhala en forma de aerosol,
produciendo irritación y edema de los pulmones.
El TBE es muy irritante para la piel y sumamente irri-
tante para los ojos. El OTBE no produce sensibilización
cutánea.
12.2 Toxicidad a corto plazo
Los compuestos de TBE han sido muy estudiados en la
rata, hasta el punto de que todos los datos expuestos en
esta sección se refieren a ese animal a menos que se
indique otra cosa.
Con dosis de 320 mg/kg (unos 25 mg/kg de peso
corporal) en la dieta se han obtenido altas tasas de
mortalidad cuando la exposición se prolonga más de 4
semanas. No se observaron muertes con 100 mg/kg en la
dieta (10 mg/kg de peso corporal) ni tras la administra-
ción forzada de 12 mg/kg de peso corporal al día. En las
ratas tratadas en sus primeros días de vida, la mortalidad
aumentó con una dosis de 3 mg/kg de peso corporal. Los
principales síntomas causados por las dosis letales fueron
inapetencia, debilidad y emaciación.
Se han observado efectos "limítrofes" en el creci-
miento de la rata con dosis de 50 mg/kg (6 mg/kg de peso
corporal) en la dieta y de 6 mg/kg de peso corporal en
estudios de administración forzada. Los ratones se
muestran menos sensibles, observándose los efectos del
compuesto con dosis de 150 a 200 mg/kg (22 a 29 mg/kg de
peso corporal) en la dieta.
Tanto los estudios a corto plazo como en los pro-
longados se han observado efectos estructurales sobre los
órganos endocrinos, principalmente la hipófisis y el
tiroides. Las pruebas a corto plazo pusieron de manifiesto
alteraciones en la concentración de las hormonas circu-
lantes y en la respuesta a los estímulos fisiológicos
(hormonas tróficas hipofisarias), pero tras la exposición
prolongada desaparecieron casi todas esas alteraciones.
El mecanismo de acción es desconocido.
La exposición a un aerosol de OTBE a razón de 2,8
mg/m3 produjo un aumento de la mortalidad, así como
dificultades respiratorias, inflamación del tracto
respiratorio y alteraciones histopatológicas de los
órganos linfáticos. En cambio, la exposición a la máxima
concentración de vapor accesible (0,16 mg/m3) a la
temperatura del local no produjo ningún efecto.
En tres especies de mamíferos se han señalado efectos
tóxicos en el hígado y los conductos biliares. En ratas a
las que se administró OTBE en la dieta a razón de 320
mg/kg (unos 25 mg/kg de peso corporal) durante 4 semanas y
en ratones tratados con 80 mg/kg (unos 12 mg/kg de peso
corporal) en la dieta durante 90 días se produjo una
necrosis hepatocelular con alteraciones inflamatorias del
árbol biliar. En perros que recibieron una dosis de 10
mg/kg de peso corporal durante 8 o 9 semanas se observó
una vacuolización de los hepatocitos periportales. Estos
cambios se acompañaban a veces de un aumento del peso del
hígado y de la actividad sérica de las enzimas hepáticas.
El descenso de la concentración de hemoglobina y del
volumen eritrocítico en la rata, causado por la adminis-
tración de 80 mg/kg (8 mg/kg de peso corporal) en la
dieta, traduce un efecto en la síntesis de la hemoglobina
que da lugar a una anemia hipocrómica microcítica. El
descenso de las cifras de hemosiderina esplénica sugiere
una alteración del equilibrio del hierro. En los ratones
se ha observado también anemia.
En ciertas investigaciones a corto plazo, pero no en
los estudios prolongados, se ha observado formación de
rosetas de hematíes en los ganglios linfáticos del
mesenterio. No está clara la significación biológica de
este fenómeno (posiblemente transitorio).
El efecto tóxico característico del OTBE tiene lugar
en el sistema inmunitario: a causa de los efectos en el
timo, la inmunidad celular se menoscaba. Aunque se
desconoce el mecanismo de acción, es posible que esté
relacionado con la conversión metabólica en compuestos de
dibutilestaño. También resulta afectada la resistencia
inespecífica.
Asimismo se han observado efectos generales en el
sistema inmunitario (por ejemplo, en el peso y la
morfología de los tejidos linfoides, los recuentos de
linfocitos periféricos y las concentraciones totales de
inmunoglobulinas séricas) en diferentes estudios con OTBE
realizados en ratas y perros, pero no en ratones, mediante
dosis claramente tóxicas (en el ratón se han registrado
efectos con dosis de cloruro de tributilestaño de 150
mg/kg). Solamente la rata presenta signos de toxicidad y,
evidentemente, es la especie más sensible. En los estudios
a corto plazo, el NENO para esta especie fue de 5 mg/kg
(0,6 mg/kg de peso corporal) en la dieta. En los estudios
con cloruro de tributilestaño se han observado efectos
análogos en el timo, rápidamente reversibles tan pronto
como se interrumpía la administración. Según se ha
observado en estudios in vivo de resistencia del huésped,
el OTBE pone en peligro la función inmunitaria específica
en la rata. Tras la exposición a un nivel de 50 mg/kg en
la dieta (siendo el valor NENO de 5 mg/kg al día), se
observó una disminución del "aclaramiento" de Listeria
monocytogenes, mientras que con 50 y 5 mg/kg en la dieta,
pero no con 0,5 mg/kg (2,5, 0,25 y 0,025 mg/kg de peso
corporal al día, respectivamente) se produjo un descenso
de la resistencia a Trichinella spiralis. Los mismos
efectos, aunque menos pronunciados, se observaron en
animales de más edad.
Según los conocimientos actuales, los efectos en la
resistencia del huésped se deben probablemente a una
evaluación más adecuada de los posibles riesgos para el
hombre; sin embargo, no se tiene suficiente experiencia
con los sistemas de prueba para apreciar bien su
importancia. En cualquier caso, las observaciones en
ratas atímicas "desnudas", estimuladas en las condi-
ciones ordinarias, proporcionan algunos datos para
interpretar el modelo de T. spiralis. En estos estudios,
la ausencia completa de inmunidad timodependiente aumentó
de 10 a 20 veces los recuentos de larvas en el músculo; en
cambio, la exposición a concentraciones de OTBE de 5 y 50
mg/kg en la dieta duplicó y cuadruplicó, respectivamente,
la cifra inicial.
Aunque ahora se dispone de algunos datos sobre los
efectos de los compuestos de tributilestaño en el
desarrollo del sistema inmunitario, carecemos de informa-
ción sobre la resistencia del huésped.
Lo prudente sería evaluar los posibles riesgos para el
hombre en función de los datos obtenidos en la especie más
sensible. Los efectos sobre la resistencia del huésped a
T. spiralis se han observado ya con niveles en la dieta
de 5 mg/kg (equivalentes a 0,25 mg/kg de peso corporal al
día), por lo que el valor NENO es de 0,5 mg/kg (equiva-
lente a 0,025 mg/kg al día). Sin embargo, no existe
acuerdo sobre el significado de estos datos para evaluar
los riesgos en el hombre. En todos los demás estudios,
una concentración de 5 mg/kg al día en la dieta (equiva-
lente a 0,5 mg/kg de peso corporal, sobre la base de los
estudios a corto plazo) correspondía al valor NENO con
respecto a los efectos en el sistema inmunitario, tanto
generales como específicos.
12.3 Toxicidad a largo plazo
Un estudio a largo plazo en las ratas sugiere un
efecto marginal del TBE en los parámetros toxicológicos
generales (cuya significación toxicológica es limitada) a
una concentración de 5 mg/kg (0,25 mg/kg de peso corporal)
en la dieta.
12.4 Genotoxicidad
La genotoxicidad del OTBE ha sido objeto de detenidas
investigaciones. En la gran mayoría de los estudios se
han obtenido resultados negativos, y no hay ninguna prueba
convincente de que el OTBE tenga propiedades mutagénicas.
12.5 Toxicidad en el sistema reproductor
En tres especies de mamíferos (ratón, rata y conejo)
se ha evaluado la posible embriotoxicidad del OTBE tras
administrarlo por vía oral a la madre. La principal
malformación observada en los fetos de rata y de ratón fue
la fisura palatina, pero este efecto sólo se registró con
dosis claramente tóxicas para las madres. Tal resultado
no se ha considerado como indicio de efectos teratogénicos
del OTBE en dosis inferiores a las que producen toxicidad
materna. El NENO más bajo en lo relativo a la embriotoxi-
cidad y la fetotoxicidad para todos las especies fue de
1,0 mg/kg de peso corporal.
12.6 Carcinogenicidad
En las ratas se ha realizado un estudio de carcino-
genicidad en el que se obtuvieron alteraciones neoplásicas
de los órganos endocrinos con 50 mg/kg en la dieta. En
cuanto a los tumores hipofisarios registrados con 0,5
mg/kg en la dieta, se consideró que no tenían signifi-
cación biológica por no existir relación dosis-respuesta.
Estos tipos de tumores suelen aparecer con una incidencia
general elevada y variable, por lo que su significación
parece discutible. Está en curso un estudio de carcino-
genicidad en el ratón.
13. Efectos en el ser humano
Se ha observado que la exposición profesional de los
trabajadores al tributilestaño provoca irritación de las
vías respiratorias superiores. En forma de aerosol, el
TBE entraña un riesgo para las personas. El OTBE ejerce
un efecto irritante en la piel y en los ojos, habiéndose
observado casos de dermatitis grave a consecuencia del
contacto directo con la piel. El problema se agrava por
la falta de una respuesta cutánea inmediata.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS
SOBRE EL MEDIO AMBIENTE
1. Evaluación del riesgo para las personas
La exposición de los trabajadores se produce sobre
todo en las actividades de fabricación y formulación de
compuestos de tributilestaño, durante la aplicación y la
eliminación de pinturas a base de TBE y a consecuencia del
empleo de TBE como conservador de la madera. La exposición
del público en general puede deberse a la contaminación de
los alimentos, particularmente el pescado y los mariscos,
así como a la aplicación doméstica de productos de protec-
ción de la madera.
A juzgar por las pruebas realizadas en animales y por
la observación directa de las personas, parece evidente
que los compuestos de TBE ejercen efectos irritantes en la
piel y en los ojos y que la inhalación de los aerosoles
provoca irritación respiratoria.
La manipulación de madera tratada no entraña riesgos
de irritación dérmica si se ha secado el material. En
cambio, los aerosoles de TBE son muy peligrosos y no debe
permitirse que la madera vuelva a entrar en la zona de
tratamiento hasta que esté perfectamente seca.
No se ha señalado ningún caso de intoxicación sis-
témica aguda y, probablemente, el TBE se elimina del
organismo al cabo de pocos días. Por consiguiente, no es
de temer que el manejo de productos de TBE entrañe peligro
de toxicidad aguda si se toman las precauciones
adecuadas.
En los animales de laboratorio se han observado
efectos a corto y a largo plazo en el hígado y en los
sistemas hematológico y endocrino. Los efectos de los
compuestos de TBE en el sistema inmunitario, y especial-
mente en la resistencia del huésped, han resultado ser el
parámetro más sensible de toxicidad en la rata, que es la
especie más sensible de todas las estudiadas. Cuando se
utiliza Trichinella spiralis como modelo de resistencia
del huésped, el nivel de efecto no observado (NENO) se
sitúa entre 0,5 y 5,0 mg/kg (0,025 y 0,25 mg/kg de peso
corporal) en la dieta, mientras que si se utilizan índices
de función inmunitaria es de 0,6 mg/kg de peso corporal.
Debido a las grandes variaciones en el consumo de
pescado y mariscos y a las diferencias locales de la
cantidad de residuos de TBE presentes en la fauna marina,
sólo a título de orientación pueden hacerse estimaciones
de los valores de exposición y del NENO. Conviene tener
en cuenta que para evaluar el riesgo potencial de estos
compuestos hay que proceder en el plano local a determinar
los residuos, calcular el consumo de pescado y mariscos y
fijar los límites aceptables de seguridad.
Partiendo de cifras de consumo de pescado de 15 y 150
g/día, de un valor de residuos en el pescado de 1 mg/kg y
de un peso medio de las personas de 60 kilos, se han
obtenido los siguientes márgenes de seguridad basados en
diferentes puntos finales inmunológicos.
----------------------------------------------------------------------
Consumo de Ingestión diaria Margen de seguridad
pescado estimada de TBE Modelo de Otros parámetros
(g/día) (µg/kg) T. spiralis inmunológicos
----------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
----------------------------------------------------------------------
El empleo indiscriminado e irresponsable de compuesto
de TBE y la observancia de las recomendaciones esbozadas
en la presente monografía para reducir la exposición de
las personas puede dar lugar a la ingestión de concentra-
ciones de compuesto de TBE peligrosas para la salud
humana.
En los animales de experimentación sólo de han
observado efectos teratogénicos con dosis que causan
signos patentes de toxicidad materna. Por consiguiente,
se considera que el potencial teratogénico de TBE es muy
bajo.
Basándose en los resultados de detallados estudios de
mutagenicidad, se considera que los compuestos de
tributilestaño no tienen potencial mutagénico. En un
estudio de carcinogenicidad realizada en la rata con OTBE,
se observó un aumento de la incidencia de ciertos tumores
endocrinos que aparecen espontáneamente con una incidencia
elevada y variable. Por consiguiente, los datos
disponibles no indican claramente que los compuestos de
TBE entrañen riesgos carcinogénicos para las personas.
2. Evaluación de los riesgos ambientales
La difusión del tributilestaño (TBE) en el medio
ambiente se debe sobre todo al empleo de ese compuesto en
las pinturas antiincrustantes. También puede deberse al
empleo de TBE como molusquicida. La contaminación en la
fuente se produce a consecuencia de utilizar TBE como
biocida en los sistemas de refrigeración, la fabricación
de pulpa de madera, el tratamiento de los cueros, los
procesos de conservación de la madera y el tratamiento de
los productos textiles.
Debido a sus propiedades fisicoquímicas, los compues-
tos de TBE se concentran en la microcapa superficial y en
los sedimentos. La degradación abiótica no parece ser un
mecanismo importante de eliminación del TBE en condiciones
ambientales. Aunque el OTBE es biodegradable en la columna
de agua, este proceso no es suficientemente rápido para
impedir la aparición de concentraciones elevadas de TBE en
algunos sitios. En la mayor parte de los organismos
acuáticos se produce bioacumulación pero la degradación
metabólica es un proceso más eficaz en los mamíferos de
laboratorio.
El TBE es sumamente peligroso para algunos organismos
acuáticos por ser tóxico incluso a concentraciones muy
bajas en el agua. Tales concentraciones se han señalado
en diversos lugares. En los estudios sobre el terreno se
han observado efectos adversos sobre invertebrados,
especialmente moluscos, a los que no se pretendía atacar,
y esos efectos resultan suficientemente graves para inter-
ferir en la reproducción y provocar un descenso de la
población. En algunos sitios ha sido posible anular los
efectos adversos en la producción comercial de mariscos
restringuiendo el empleo de pinturas antiincrustantes, con
lo que también se ha logrado suprimir el efecto de imposex
en las poblaciones de gasterópodos. Los efectos en las
piscifactorías hacen pensar que no conviene utilizar
pinturas que contengan TBE en las redes de contención.
Para el medio terrestre, el riesgo general parece ser
bajo. La madera tratada con TBE podría ser peligrosa para
los organismos terrestres que viven en estrecho contacto
con ella.
El aumento de las concentraciones de TBE en la micro-
capa superficial puede ser peligroso para los organismos
del litoral, las especies neustónicas (inclusive inverte-
brados bénticos y larvas de peces) y las aves salvajes y
marinas que se alimentan en la superficie del agua. La
acumulación y la baja biodegradación del TBE en los sedi-
mentos puede representar un peligro para los organismos
acuáticos cuando esos sedimentos contaminados se movilizan
por procesos naturales u operaciones de dragado.
RECOMENDACIONES
1. Recomendaciones para proteger la salud humana y la
higiene del medio
a) Hay que instar a los países Miembros que todavía no
hayan reglamentado el uso de compuestos de TBE a que
lo hagan.
b) Es necesario evaluar y, si procede reglamentar, el
ingreso en el medio ambiente de estaño orgánico pro-
cedente de fuentes distintas de las pinturas antiin-
crustantes. Por ejemplo, convendría evaluar el riesgo
potencial que entraña la aplicación al suelo de lodos
de alcantarillado contaminados con TBE.
c) Habrá que mejorar los métodos utilizables para apli-
car, eliminar y evacuar en condiciones de seguridad
las pinturas de estaño orgánico.
2. Investigaciones necesarias
a) Habrá que mejorar los métodos de detección y análisis
a fin de poder determinar con rapidez y precisión los
compuestos de butilestaño presentes en concentraciones
de pg/litro. Una de las razones que justifican esa
recomendación es que ciertos efectos biológicos (por
ejemplo, el imposex en los gasterópodos) pueden pro-
ducirse con concentraciones inferiores a los actuales
límites de detección.
b) Esnecesario estudiar los mecanismos que concentran en
vez de dispersar el TBE y que retrasan la degradación,
prestando especial atención a los fundamentos químicos
de ese compuesto y a su interaccción con las moléculas
biológicas. Se necesitan más estudios sobre la absor-
ción del TBE en todos los niveles tróficos.
c) Habrá que estudiar la toxicidad del TBE en los organ-
ismos acuáticos. Estos estudios deberán versar, si
procede, sobre el metabolismo, los efectos endocrinos
y la toxicidad inmunológica.
d) Habrá que buscar otras especies sensibles (en parti-
cular de agua dulce) que sirvan de bioindicador en
otros grupos.
e) Habrá que confirmar la validez de los modelos utili-
zados para evaluar la inmunotoxicidad en los mamíferos
y definir con más precisión los niveles sin efecto de
los parámetros pertinentes.
f) Convendría emprender un estudio de toxicidad crónica
en una segunda especie de mamífero.
g) Convendría emprender un estudio de tumorigenicidad en
una segunda especie de mamífero.
h) Hay que obtener datos mediante métodos de especiación
sobre los niveles de residuos de butilestaño en los
peces y mariscos destinados al consumo humano.
 The development of the motile spores of a green
macroalga was the stage most sensitive to TBT (5-day
EC50: 0.001 µg/litre). There was reduced growth of a
marine angiosperm at TBT concentrations of 1 mg/kg sedi-
ment but no effect at 0.1 mg/kg.
Tributyltin is highly toxic to marine molluscs. It
has been shown experimentally to affect shell deposition
of growing oysters, gonadal development and gender of
adult oysters, settlement, growth, and mortality of larval
oysters and other bivalves, and to cause imposex (the
development of male characteristics) in female gastropods.
The NOEL for spat of the most sensitive oyster species
(Crassostrea gigas) has been reported to be about
20 ng/litre. TBT causes deformation of the shell of adult
oysters in a dose-related manner. No effect on shell mor-
phology was observed experimentally at TBT concentrations
of 2 ng/litre. The NOEL for the development of imposex in
female dogwhelks is below 1.5 ng/litre. Larval forms are
generally more sensitive than adults; in the case of oys-
ters this difference is particularly marked.
Copepods are more sensitive than other crustacean
groups to the acute lethal effects of TBT, LC50 values
for exposure periods up to 96 h ranging from 0.6 to
2.2 µg/litre. These values are comparable to those of
the more sensitive larvae of other crustacean groups. TBT
reduces reproductive performance, neonate survival, and
juvenile growth rate in crustaceans. The NOEL for repro-
duction in the mysid shrimp Acanthomysis sculpta has been
suggested to be 0.09 µg/litre. There was no avoidance of
TBT by the grass shrimp at concentrations up to 30 µg/litre.
The toxicity of tributyltin to marine fish is highly
variable, 96-h LC50 values ranging between 1.5 and
36 µg/litre. Larval stages are more sensitive than adults
(Fig. 1). There are indications that marine fish avoid
TBTO concentrations of 1 µg/litre or more.
1.9.2. Effects on freshwater organisms
A summary diagram relating lethal and sublethal
effects to measured TBT concentrations in fresh water is
presented in Fig. 2. Concentrations exceeding those pro-
ducing sublethal effects have been found, particularly
associated with pleasure boating activity.
The development of the motile spores of a green
macroalga was the stage most sensitive to TBT (5-day
EC50: 0.001 µg/litre). There was reduced growth of a
marine angiosperm at TBT concentrations of 1 mg/kg sedi-
ment but no effect at 0.1 mg/kg.
Tributyltin is highly toxic to marine molluscs. It
has been shown experimentally to affect shell deposition
of growing oysters, gonadal development and gender of
adult oysters, settlement, growth, and mortality of larval
oysters and other bivalves, and to cause imposex (the
development of male characteristics) in female gastropods.
The NOEL for spat of the most sensitive oyster species
(Crassostrea gigas) has been reported to be about
20 ng/litre. TBT causes deformation of the shell of adult
oysters in a dose-related manner. No effect on shell mor-
phology was observed experimentally at TBT concentrations
of 2 ng/litre. The NOEL for the development of imposex in
female dogwhelks is below 1.5 ng/litre. Larval forms are
generally more sensitive than adults; in the case of oys-
ters this difference is particularly marked.
Copepods are more sensitive than other crustacean
groups to the acute lethal effects of TBT, LC50 values
for exposure periods up to 96 h ranging from 0.6 to
2.2 µg/litre. These values are comparable to those of
the more sensitive larvae of other crustacean groups. TBT
reduces reproductive performance, neonate survival, and
juvenile growth rate in crustaceans. The NOEL for repro-
duction in the mysid shrimp Acanthomysis sculpta has been
suggested to be 0.09 µg/litre. There was no avoidance of
TBT by the grass shrimp at concentrations up to 30 µg/litre.
The toxicity of tributyltin to marine fish is highly
variable, 96-h LC50 values ranging between 1.5 and
36 µg/litre. Larval stages are more sensitive than adults
(Fig. 1). There are indications that marine fish avoid
TBTO concentrations of 1 µg/litre or more.
1.9.2. Effects on freshwater organisms
A summary diagram relating lethal and sublethal
effects to measured TBT concentrations in fresh water is
presented in Fig. 2. Concentrations exceeding those pro-
ducing sublethal effects have been found, particularly
associated with pleasure boating activity.
 Fresh-water angiosperms were killed by a TBTO concen-
tration of 0.5 mg/litre, and growth was inhibited at
0.06 mg/litre or more.
Data on fresh-water invertebrate species are few, re-
lating to just three species other than target organisms.
Different salts of TBT yield 48-h LC50 values for Daphnia
of 2.3-70 µg/litre and for Tubifex of 5.5-33 µg/litre.
The NOEL for Daphnia has been estimated to be 0.5 µg per
litre, based on reversal of normal response to light. The
24-h LC50 for the Asiatic clam has been reported to be
2100 µg/litre, and for target snail adults in schistoso-
miasis control the corresponding values are 30-400 µg/litre.
Tributyltin has been shown to be toxic to schistosome
larvae in the aquatic stages; the LC50 (TBT fluoride)
was calculated to be 16.8 µg/litre for a 1-h exposure.
The TBT dose causing 99% to 100% suppression of cercarial
infectivity of mice was between 2 and 6 µg/litre.
The sensitivity of snails to TBT decreases with age,
but eggs are more resistant than both young and adults.
Egg laying is significantly effected at a TBTO concen-
tration of 0.001 µg/litre.
The acute toxicity of TBT to freshwater fish in LC50
tests up to 168 h ranges from 13 to 240 µg per litre.
The NOEL for the guppy was estimated to be 0.01 µg per
litre, based on histopathological effects.
No effect on survival was found when eggs and larvae
of the frog Rana temporaria were exposed to TBT concen-
trations of 3 µg/litre or less, but at 30 µg/litre sig-
nificant mortality was observed.
1.9.3. Microcosm studies
Microcosm studies modelling marine ecosystems have
been conducted with introduced organisms and in conditions
where inflowing sea water allowed colonization by other
organisms. Results showed decreases in both numbers of
individuals and in species diversity at TBTO concen-
trations in water between 0.06 and 3 µg/litre.
Results from freshwater model ecosystems suggest that
doses which kill freshwater snails also affect other
species, including fish.
1.10. Effects on terrestrial organisms
The exposure of terrestrial organisms to TBT results
primarily from its use as a wood preservative. TBTO is
toxic to bees housed in hives made from TBT-treated wood.
TBT was toxic to bats in a single study, but this result
was not statistically significant owing to high control
mortality. TBT compounds are toxic to insects exposed
topically or via feeding on treated wood. The acute tox-
icity of TBT to wild mice is moderate; estimated dietary
LC50 values, based on consumption of treated seeds used
in repellency tests, range from 37 to 240 mg/kg per day.
1.11. Effects on organisms in the field
Field observations have related high concentrations of
tributyltin to mortality and settlement failure of larval
bivalves, reduced growth, shell thickening and other mal-
formations in developing oysters, imposex in mud snails,
and imposex (concurrent with population decline) in the
dogwhelk. Complete failure of oyster fisheries was ident-
ified initially in France and afterwards in other
countries and related to water concentrations of TBT. The
effects were most marked in areas close to pleasure boat
marinas. Controlling the use of TBT antifouling paints on
small boats has resulted in recovery of oyster repro-
duction and growth. However, water concentrations of TBT
are still high enough in some areas to affect marine
gastropods.
Both shell growth and chambering in Pacific oysters
and imposex in dogwhelks have been used as biological
indicators of TBT contamination.
There have been few studies of the effects on organ-
isms of TBT in sediment, but there are indications that
the TBT is available to burrowing organisms and can cause
mortality in the field.
Gross toxic effects and histopathological changes have
been reported in farmed marine fish exposed to TBT by the
use of antifouling paints on retaining nets.
The use of TBT as a molluscicide against the fresh-
water snails that carry schistosomiasis (bilharzia) has
been proposed. Some field trials have been conducted which
show that it is difficult to apply TBT without damaging
non-target organisms.
1.12. Toxicity to laboratory mammals
1.12.1. Acute toxicity
Tributyltin is moderately to highly toxic to labora-
tory mammals, acute oral LD50 values ranging from 94 to
234 mg/kg body weight for the rat and from 44 to 230 mg/kg
body weight for the mouse. The acute toxicity to the
guinea-pig and the rabbit fall within the same range. The
variation comes from the "anion" component of the tri-
butyltin salt. These compounds exhibit greater lethal
potential when administered parenterally, as opposed to
orally, probably due to only partial absorption from the
gut.
Other effects of acute exposure may include alter-
ations in blood lipid levels, the endocrine system, liver,
and spleen, and transient deficits in brain development.
The toxicological significance of these effects, reported
after high single doses of the compound, is questionable
and the cause of death remains unknown.
The acute toxicity via the dermal route is low, the
LD50 being >9000 mg/kg body weight for the rabbit.
"Nose only" inhalation LD50 (4 h) for the rat is
77 mg/m3 (65 mg/m3 when only inhalable particles are
considered). TBT vapour/air mixtures produce no observ-
able toxic effects, even at saturation. However, TBT is
very hazardous as an inhaled aerosol, producing lung irri-
tation and oedema.
TBT is severely irritating to the skin and an extreme
irritant to the eye. TBTO is not a skin sensitizer.
1.12.2. Short-term toxicity
TBT compounds have been studied most extensively in
the rat (all the data in this section refer to the rat
unless otherwise indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg
body weight), high mortality rates were observed when the
exposure time exceeded 4 weeks. No deaths were noted at
100 mg/kg diet (10 mg/kg body weight) or after daily
administration of 12 mg/kg body weight by gavage. In rats
dosed during early post-natal life, 3 mg/kg body weight
resulted in increased deaths. The main symptoms at lethal
doses were loss of appetite, weakness, and emaciation.
Borderline effects on rat growth were observed at
50 mg/kg diet (6 mg/kg body weight) and 6 mg/kg body
weight (gavage studies). Mice are less sensitive, effects
being observed at 150 to 200 mg/kg diet (22 to 29 mg/kg
body weight).
Structural effects on endocrine organs, mainly the
pituitary and thyroid, have been noted in both short- and
long-term studies. Changes in circulating hormone concen-
trations and altered response to physiological stimuli
(pituitary trophic hormones) were observed in short-term
tests, but after long-term exposure most of these changes
appeared to be absent. The mechanism of action is not
known.
Exposure to TBTO aerosol at 2.8 mg/m3 produced high
mortality, respiratory distress, inflammatory reaction
within the respiratory tract and histopathological changes
of lymphatic organs. However, exposure to the highest
attainable vapour concentration (0.16 mg/m3) at room
temperature produced no effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular
necrosis and inflammatory changes in the bile duct were
observed in rats fed TBTO at a dietary level of 320 mg/kg
(approximately 25 mg/kg body weight) for 4 weeks and in
mice fed 80 mg/kg diet (approximately 12 mg/kg body
weight) for 90 days. Vacuolization of periportal hepato-
cytes was noted in dogs fed a dose of 10 mg/kg body weight
for 8 to 9 weeks. These changes were occasionally
accompanied by increased liver weight and increased serum
activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte
volume in rats, resulting from dosing with 80 mg/kg diet
(8 mg/kg body weight), indicate an effect on haemoglobin
synthesis, leading to microcytic hypochromic anaemia. The
decrease in splenic haemosiderin levels suggests alter-
ations in iron status. Anaemia has also been observed in
mice.
The formation of erythrocyte rosettes in mesenteric
lymph nodes has been observed in certain short-term inves-
tigations but not in long-term studies. The biological
significance of this finding (possibly transient) is
unclear.
The characteristic toxic effect of TBTO is on the
immune system; due to effects on the thymus, the cell-
mediated function is impaired. The mechanism of action is
unknown, but may involve the metabolic conversion to
dibutyltin compounds. Non-specific resistance is also
affected.
General effects on the immune system (e.g., on the
weight and morphology of lymphoid tissues, peripheral lym-
phocyte counts, and total serum immunoglobulin concen-
trations) have been reported in several different studies
with TBTO using rats and dogs, but not mice, at overtly
toxic dose levels (effects in mice have been seen with
tributyltin chloride at 150 mg/kg). Only the rat exhibits
general effects on the immune system without other overt
signs of toxicity and is clearly the most sensitive
species. The NOEL in short-term rat studies was 5 mg/kg
diet (0.6 mg/kg body weight). In studies with tributyltin
chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been
shown to compromise specific immune function in rat in
vivo host resistance studies. Decreased clearance of
Listeria monocytogenes was seen after exposure to a diet-
ary level of 50 mg/kg (the NOEL being 5 mg/kg per day),
and decreased resistance to Trichinella spiralis was seen
at 50 and 5 mg/kg diet, but not at 0.5 mg/kg diet (2.5,
0.25, and 0.025 mg/kg per day body weight, respectively).
Similar effects were seen in aged animals, but these were
less pronounced.
With present knowledge, the effects on host resistance
are probably of most relevance in assessing the potential
hazard to man, but there is insufficient experience in
these test systems to fully assess their significance.
However, some data on the significance of the T. spiralis
model are provided by findings in athymic nude rats after
the standard challenge. In these studies, the complete
absence of thymus-dependent immunity resulted in a 10- to
20-fold increase in muscle larvae counts; by contrast,
exposure to TBTO concentrations of 5 and 50 mg/kg diet
resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on
the effects of tributyltin compounds on the developing
immune system, there is no information on host resistance.
It would be prudent to base assessment of the poten-
tial hazard to humans on data from the most sensitive
species. Effects on host resistance to T. spiralis have
been seen at dietary levels as low as 5 mg/kg (equivalent
to 0.25 mg/kg per day body weight), the NOEL being
0.5 mg/kg (equivalent to 0.025 mg/kg per day). However,
the interpretation of the significance of these data for
human risk assessment is controversial. In all other
studies a concentration of 5 mg/kg per day in the diet
(equivalent to 0.5 mg/kg body weight, based on the short-
term studies) was the NOEL with respect to general, as
well as specific, effects on the immune system.
1.12.3. Long-term toxicity
A long-term study in rats indicates a marginal effect
of TBT on general toxicological parameters (of limited
toxicological significance) at a level of 5 mg/kg diet
(0.25 mg/kg body weight).
1.12.4. Genotoxicity
The genotoxicity of TBTO has been the subject of
extensive investigations. Negative results were obtained
in the vast majority of studies, and there is no convinc-
ing evidence that TBTO has any mutagenic potential.
1.12.5. Reproductive toxicity
The potential embryotoxicity of TBTO has been evalu-
ated in three mammalian species (mouse, rat, and rabbit)
after oral dosing of the mother. The main malformation
noted in rat and mouse fetuses was cleft palate, but this
occurred at dosages overtly toxic to the mothers. These
results are not considered to be indicative of teratogenic
effects of TBTO at doses below those producing maternal
toxicity. The lowest NOEL, with regard to embryotoxicity
and fetotoxicity for all three species, was 1.0 mg/kg body
weight.
1.12.6. Carcinogenicity
One carcinogenicity study has been carried out on
rats, in which neoplastic changes were observed in endo-
crine organs at 50 mg/kg diet. The pituitary tumours
reported at 0.5 mg/kg diet were considered as having no
biological significance since there was no dose-response
relationship. These tumour types usually appear in high
and variable background incidences, and their significance
is, therefore, questionable. A carcinogenicity study on
mice is in progress.
1.13. Effects on humans
Occupational exposure of workers to tributyltin has
been found to result in irritation of the upper respir-
atory tract. TBT as an aerosol poses a hazard to humans.
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The
potential problem is made worse by the lack of an
immediate response to the skin.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of tributyltin compounds
Tributyltins compounds are organic derivatives of tin
(SnIV) characterized by the presence of covalent bonds
between three carbon atoms and a tin atom. They conform to
the following general formula ( n-C4H9)3 Sn-X, where X is
an anion or a group linked covalently through a hetero-
atom.
The nature of X influences the physico-chemical
properties, notably the relative solubility in water and
non-polar solvents and the vapour pressure.
These compounds differ from inorganic tin both in
behaviour and effects. An important member of the group is
tributyltin oxide (TBTO; RTECS number, JN8750000). Commer-
cial TBTO has a purity generally above 96%. Principle
impurities are dibutyltin derivatives and, to a lesser
extent, tetrabutyl or dibutylalkyl tin compounds.
Other industrially important tributyltin derivatives
include tributyltin fluoride, tributyltin methacrylate (monomer
or copolymer), tributyltin benzoate, tributyltin linoleate,
tributyltin naphthenate, and tributyltin phosphate.
2.2. Physical and chemical properties
TBTO is flammable but does not form explosive mixtures
with air. It is a mild oxidizing agent. It reacts quanti-
tatively at room temperature with bromide or iodine with
cleavage of the Sn-O bond (a reaction that may be used for
quantitative analysis) (Bahr & Pawlenko, 1978).
In the presence of oxygen, light or heat, slow break-
down occurs with the formation of tetra-n-butyltin, di-
n-butyltin oxide, and eventually tin (IV) oxide by de-
alkylation (Evans & Karpel, 1985). This degradation may be
inhibited by the addition of 0.1-1.0% of stabilizers (such
as lactic or citric acids).
It has been suggested (Maguire et al., 1984; Laughlin
et al., 1986a) that TBTO in aqueous solution dissociates
with the formation of a hydrated tributyltin cation, which
can undergo reaction with anions present. Data are not
available on the equilibrium constants for these reac-
tions.
Laughlin et al. (1986a) showed that TBTO can react
with normal constituents of the sea water in the following
ways:
Bu3-Sn-O-Sn-Bu3+ HO -» 2Bu3-Sn-OH
Bu3-Sn-OH-H+ -» Bu3SnOH2+
Bu3-Sn-OH + CO32- -» Bu3SnCO3- + OH-
Bu3-Sn-OH2+ + Cl- -» Bu3-Sn-Cl + H2O
The predominant forms are Bu3SnOH2+ and Bu3SnCl
at pH < 7, Bu3SnCl, Bu3SnOH, and Bu3SnCO3- at pH 8,
and Bu3SnOH and Bu3SnCO3- at pH > 10.
Under normal conditions in sea water, it is considered
that the three species (hydroxide, chloride, and carbon-
ate) remain in equilibrium.
The physical and chemical properties of some commer-
cially available tributyltin compounds are listed in Table
1.
Varying data on the solubility of TBTO in water, which
ranges from < 1.0 to > 100 mg/litre at different tempera-
tures and pH values, may be related to the presence of
different anionic species as described above.
In the same way as described in the reaction between
TBTO and water, the TBT group can be transferred to other
oxygen-, nitrogen-, and sulfur-containing groups. Thus,
anaerobically in sediments, TBTO can be transformed to TBT
sulfide. With amino acids, or their derivatives such as
proteins, reaction can occur on the nitrogen and sulfur
atoms, and, with wood, it has been suggested that the TBT
group may react with hydroxylic groups (Blunden et al.,
1984) or form tributyltin carbonate (Smith et al., 1977).
Thus adsorption on to particulate matter could involve
chemical reaction as well as physical adsorption or sol-
ution. TBTO adsorbs strongly to particulate matter, the
reported adsorption coefficients ranging between 110 and
55 000.
Table 1. Identity and physical and chemical properties of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Oxide Benzoate Chloride Fluoride Linoleate Methacrylate Naphthenate
(TBTO) (TBTB) (TBTCl) (TBTF) (TBTL) (TBTM) (TBTN)
---------------------------------------------------------------------------------------------------------
IUPAC name distannoxane, stannane, stannane, stannane, stannane, stannane, stannane,
hexabutyl (benzyloxy) tributyl- tributyl- tributyl- tributyl- tributyl-
tributyl chloro fluoro (1-oxo-9,12- (2-methyl-1- mono (naph-
octadecadi- oxo-2-propyl) thenoyloxy)
enyl)oxy- oxy- derivatives
CAS name Bis(tributyl- Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin
tin) oxide benzoate chloride fluoride linoleate methacrylate naphthenate
CAS number
Fresh-water angiosperms were killed by a TBTO concen-
tration of 0.5 mg/litre, and growth was inhibited at
0.06 mg/litre or more.
Data on fresh-water invertebrate species are few, re-
lating to just three species other than target organisms.
Different salts of TBT yield 48-h LC50 values for Daphnia
of 2.3-70 µg/litre and for Tubifex of 5.5-33 µg/litre.
The NOEL for Daphnia has been estimated to be 0.5 µg per
litre, based on reversal of normal response to light. The
24-h LC50 for the Asiatic clam has been reported to be
2100 µg/litre, and for target snail adults in schistoso-
miasis control the corresponding values are 30-400 µg/litre.
Tributyltin has been shown to be toxic to schistosome
larvae in the aquatic stages; the LC50 (TBT fluoride)
was calculated to be 16.8 µg/litre for a 1-h exposure.
The TBT dose causing 99% to 100% suppression of cercarial
infectivity of mice was between 2 and 6 µg/litre.
The sensitivity of snails to TBT decreases with age,
but eggs are more resistant than both young and adults.
Egg laying is significantly effected at a TBTO concen-
tration of 0.001 µg/litre.
The acute toxicity of TBT to freshwater fish in LC50
tests up to 168 h ranges from 13 to 240 µg per litre.
The NOEL for the guppy was estimated to be 0.01 µg per
litre, based on histopathological effects.
No effect on survival was found when eggs and larvae
of the frog Rana temporaria were exposed to TBT concen-
trations of 3 µg/litre or less, but at 30 µg/litre sig-
nificant mortality was observed.
1.9.3. Microcosm studies
Microcosm studies modelling marine ecosystems have
been conducted with introduced organisms and in conditions
where inflowing sea water allowed colonization by other
organisms. Results showed decreases in both numbers of
individuals and in species diversity at TBTO concen-
trations in water between 0.06 and 3 µg/litre.
Results from freshwater model ecosystems suggest that
doses which kill freshwater snails also affect other
species, including fish.
1.10. Effects on terrestrial organisms
The exposure of terrestrial organisms to TBT results
primarily from its use as a wood preservative. TBTO is
toxic to bees housed in hives made from TBT-treated wood.
TBT was toxic to bats in a single study, but this result
was not statistically significant owing to high control
mortality. TBT compounds are toxic to insects exposed
topically or via feeding on treated wood. The acute tox-
icity of TBT to wild mice is moderate; estimated dietary
LC50 values, based on consumption of treated seeds used
in repellency tests, range from 37 to 240 mg/kg per day.
1.11. Effects on organisms in the field
Field observations have related high concentrations of
tributyltin to mortality and settlement failure of larval
bivalves, reduced growth, shell thickening and other mal-
formations in developing oysters, imposex in mud snails,
and imposex (concurrent with population decline) in the
dogwhelk. Complete failure of oyster fisheries was ident-
ified initially in France and afterwards in other
countries and related to water concentrations of TBT. The
effects were most marked in areas close to pleasure boat
marinas. Controlling the use of TBT antifouling paints on
small boats has resulted in recovery of oyster repro-
duction and growth. However, water concentrations of TBT
are still high enough in some areas to affect marine
gastropods.
Both shell growth and chambering in Pacific oysters
and imposex in dogwhelks have been used as biological
indicators of TBT contamination.
There have been few studies of the effects on organ-
isms of TBT in sediment, but there are indications that
the TBT is available to burrowing organisms and can cause
mortality in the field.
Gross toxic effects and histopathological changes have
been reported in farmed marine fish exposed to TBT by the
use of antifouling paints on retaining nets.
The use of TBT as a molluscicide against the fresh-
water snails that carry schistosomiasis (bilharzia) has
been proposed. Some field trials have been conducted which
show that it is difficult to apply TBT without damaging
non-target organisms.
1.12. Toxicity to laboratory mammals
1.12.1. Acute toxicity
Tributyltin is moderately to highly toxic to labora-
tory mammals, acute oral LD50 values ranging from 94 to
234 mg/kg body weight for the rat and from 44 to 230 mg/kg
body weight for the mouse. The acute toxicity to the
guinea-pig and the rabbit fall within the same range. The
variation comes from the "anion" component of the tri-
butyltin salt. These compounds exhibit greater lethal
potential when administered parenterally, as opposed to
orally, probably due to only partial absorption from the
gut.
Other effects of acute exposure may include alter-
ations in blood lipid levels, the endocrine system, liver,
and spleen, and transient deficits in brain development.
The toxicological significance of these effects, reported
after high single doses of the compound, is questionable
and the cause of death remains unknown.
The acute toxicity via the dermal route is low, the
LD50 being >9000 mg/kg body weight for the rabbit.
"Nose only" inhalation LD50 (4 h) for the rat is
77 mg/m3 (65 mg/m3 when only inhalable particles are
considered). TBT vapour/air mixtures produce no observ-
able toxic effects, even at saturation. However, TBT is
very hazardous as an inhaled aerosol, producing lung irri-
tation and oedema.
TBT is severely irritating to the skin and an extreme
irritant to the eye. TBTO is not a skin sensitizer.
1.12.2. Short-term toxicity
TBT compounds have been studied most extensively in
the rat (all the data in this section refer to the rat
unless otherwise indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg
body weight), high mortality rates were observed when the
exposure time exceeded 4 weeks. No deaths were noted at
100 mg/kg diet (10 mg/kg body weight) or after daily
administration of 12 mg/kg body weight by gavage. In rats
dosed during early post-natal life, 3 mg/kg body weight
resulted in increased deaths. The main symptoms at lethal
doses were loss of appetite, weakness, and emaciation.
Borderline effects on rat growth were observed at
50 mg/kg diet (6 mg/kg body weight) and 6 mg/kg body
weight (gavage studies). Mice are less sensitive, effects
being observed at 150 to 200 mg/kg diet (22 to 29 mg/kg
body weight).
Structural effects on endocrine organs, mainly the
pituitary and thyroid, have been noted in both short- and
long-term studies. Changes in circulating hormone concen-
trations and altered response to physiological stimuli
(pituitary trophic hormones) were observed in short-term
tests, but after long-term exposure most of these changes
appeared to be absent. The mechanism of action is not
known.
Exposure to TBTO aerosol at 2.8 mg/m3 produced high
mortality, respiratory distress, inflammatory reaction
within the respiratory tract and histopathological changes
of lymphatic organs. However, exposure to the highest
attainable vapour concentration (0.16 mg/m3) at room
temperature produced no effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular
necrosis and inflammatory changes in the bile duct were
observed in rats fed TBTO at a dietary level of 320 mg/kg
(approximately 25 mg/kg body weight) for 4 weeks and in
mice fed 80 mg/kg diet (approximately 12 mg/kg body
weight) for 90 days. Vacuolization of periportal hepato-
cytes was noted in dogs fed a dose of 10 mg/kg body weight
for 8 to 9 weeks. These changes were occasionally
accompanied by increased liver weight and increased serum
activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte
volume in rats, resulting from dosing with 80 mg/kg diet
(8 mg/kg body weight), indicate an effect on haemoglobin
synthesis, leading to microcytic hypochromic anaemia. The
decrease in splenic haemosiderin levels suggests alter-
ations in iron status. Anaemia has also been observed in
mice.
The formation of erythrocyte rosettes in mesenteric
lymph nodes has been observed in certain short-term inves-
tigations but not in long-term studies. The biological
significance of this finding (possibly transient) is
unclear.
The characteristic toxic effect of TBTO is on the
immune system; due to effects on the thymus, the cell-
mediated function is impaired. The mechanism of action is
unknown, but may involve the metabolic conversion to
dibutyltin compounds. Non-specific resistance is also
affected.
General effects on the immune system (e.g., on the
weight and morphology of lymphoid tissues, peripheral lym-
phocyte counts, and total serum immunoglobulin concen-
trations) have been reported in several different studies
with TBTO using rats and dogs, but not mice, at overtly
toxic dose levels (effects in mice have been seen with
tributyltin chloride at 150 mg/kg). Only the rat exhibits
general effects on the immune system without other overt
signs of toxicity and is clearly the most sensitive
species. The NOEL in short-term rat studies was 5 mg/kg
diet (0.6 mg/kg body weight). In studies with tributyltin
chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been
shown to compromise specific immune function in rat in
vivo host resistance studies. Decreased clearance of
Listeria monocytogenes was seen after exposure to a diet-
ary level of 50 mg/kg (the NOEL being 5 mg/kg per day),
and decreased resistance to Trichinella spiralis was seen
at 50 and 5 mg/kg diet, but not at 0.5 mg/kg diet (2.5,
0.25, and 0.025 mg/kg per day body weight, respectively).
Similar effects were seen in aged animals, but these were
less pronounced.
With present knowledge, the effects on host resistance
are probably of most relevance in assessing the potential
hazard to man, but there is insufficient experience in
these test systems to fully assess their significance.
However, some data on the significance of the T. spiralis
model are provided by findings in athymic nude rats after
the standard challenge. In these studies, the complete
absence of thymus-dependent immunity resulted in a 10- to
20-fold increase in muscle larvae counts; by contrast,
exposure to TBTO concentrations of 5 and 50 mg/kg diet
resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on
the effects of tributyltin compounds on the developing
immune system, there is no information on host resistance.
It would be prudent to base assessment of the poten-
tial hazard to humans on data from the most sensitive
species. Effects on host resistance to T. spiralis have
been seen at dietary levels as low as 5 mg/kg (equivalent
to 0.25 mg/kg per day body weight), the NOEL being
0.5 mg/kg (equivalent to 0.025 mg/kg per day). However,
the interpretation of the significance of these data for
human risk assessment is controversial. In all other
studies a concentration of 5 mg/kg per day in the diet
(equivalent to 0.5 mg/kg body weight, based on the short-
term studies) was the NOEL with respect to general, as
well as specific, effects on the immune system.
1.12.3. Long-term toxicity
A long-term study in rats indicates a marginal effect
of TBT on general toxicological parameters (of limited
toxicological significance) at a level of 5 mg/kg diet
(0.25 mg/kg body weight).
1.12.4. Genotoxicity
The genotoxicity of TBTO has been the subject of
extensive investigations. Negative results were obtained
in the vast majority of studies, and there is no convinc-
ing evidence that TBTO has any mutagenic potential.
1.12.5. Reproductive toxicity
The potential embryotoxicity of TBTO has been evalu-
ated in three mammalian species (mouse, rat, and rabbit)
after oral dosing of the mother. The main malformation
noted in rat and mouse fetuses was cleft palate, but this
occurred at dosages overtly toxic to the mothers. These
results are not considered to be indicative of teratogenic
effects of TBTO at doses below those producing maternal
toxicity. The lowest NOEL, with regard to embryotoxicity
and fetotoxicity for all three species, was 1.0 mg/kg body
weight.
1.12.6. Carcinogenicity
One carcinogenicity study has been carried out on
rats, in which neoplastic changes were observed in endo-
crine organs at 50 mg/kg diet. The pituitary tumours
reported at 0.5 mg/kg diet were considered as having no
biological significance since there was no dose-response
relationship. These tumour types usually appear in high
and variable background incidences, and their significance
is, therefore, questionable. A carcinogenicity study on
mice is in progress.
1.13. Effects on humans
Occupational exposure of workers to tributyltin has
been found to result in irritation of the upper respir-
atory tract. TBT as an aerosol poses a hazard to humans.
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The
potential problem is made worse by the lack of an
immediate response to the skin.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity of tributyltin compounds
Tributyltins compounds are organic derivatives of tin
(SnIV) characterized by the presence of covalent bonds
between three carbon atoms and a tin atom. They conform to
the following general formula ( n-C4H9)3 Sn-X, where X is
an anion or a group linked covalently through a hetero-
atom.
The nature of X influences the physico-chemical
properties, notably the relative solubility in water and
non-polar solvents and the vapour pressure.
These compounds differ from inorganic tin both in
behaviour and effects. An important member of the group is
tributyltin oxide (TBTO; RTECS number, JN8750000). Commer-
cial TBTO has a purity generally above 96%. Principle
impurities are dibutyltin derivatives and, to a lesser
extent, tetrabutyl or dibutylalkyl tin compounds.
Other industrially important tributyltin derivatives
include tributyltin fluoride, tributyltin methacrylate (monomer
or copolymer), tributyltin benzoate, tributyltin linoleate,
tributyltin naphthenate, and tributyltin phosphate.
2.2. Physical and chemical properties
TBTO is flammable but does not form explosive mixtures
with air. It is a mild oxidizing agent. It reacts quanti-
tatively at room temperature with bromide or iodine with
cleavage of the Sn-O bond (a reaction that may be used for
quantitative analysis) (Bahr & Pawlenko, 1978).
In the presence of oxygen, light or heat, slow break-
down occurs with the formation of tetra-n-butyltin, di-
n-butyltin oxide, and eventually tin (IV) oxide by de-
alkylation (Evans & Karpel, 1985). This degradation may be
inhibited by the addition of 0.1-1.0% of stabilizers (such
as lactic or citric acids).
It has been suggested (Maguire et al., 1984; Laughlin
et al., 1986a) that TBTO in aqueous solution dissociates
with the formation of a hydrated tributyltin cation, which
can undergo reaction with anions present. Data are not
available on the equilibrium constants for these reac-
tions.
Laughlin et al. (1986a) showed that TBTO can react
with normal constituents of the sea water in the following
ways:
Bu3-Sn-O-Sn-Bu3+ HO -» 2Bu3-Sn-OH
Bu3-Sn-OH-H+ -» Bu3SnOH2+
Bu3-Sn-OH + CO32- -» Bu3SnCO3- + OH-
Bu3-Sn-OH2+ + Cl- -» Bu3-Sn-Cl + H2O
The predominant forms are Bu3SnOH2+ and Bu3SnCl
at pH < 7, Bu3SnCl, Bu3SnOH, and Bu3SnCO3- at pH 8,
and Bu3SnOH and Bu3SnCO3- at pH > 10.
Under normal conditions in sea water, it is considered
that the three species (hydroxide, chloride, and carbon-
ate) remain in equilibrium.
The physical and chemical properties of some commer-
cially available tributyltin compounds are listed in Table
1.
Varying data on the solubility of TBTO in water, which
ranges from < 1.0 to > 100 mg/litre at different tempera-
tures and pH values, may be related to the presence of
different anionic species as described above.
In the same way as described in the reaction between
TBTO and water, the TBT group can be transferred to other
oxygen-, nitrogen-, and sulfur-containing groups. Thus,
anaerobically in sediments, TBTO can be transformed to TBT
sulfide. With amino acids, or their derivatives such as
proteins, reaction can occur on the nitrogen and sulfur
atoms, and, with wood, it has been suggested that the TBT
group may react with hydroxylic groups (Blunden et al.,
1984) or form tributyltin carbonate (Smith et al., 1977).
Thus adsorption on to particulate matter could involve
chemical reaction as well as physical adsorption or sol-
ution. TBTO adsorbs strongly to particulate matter, the
reported adsorption coefficients ranging between 110 and
55 000.
Table 1. Identity and physical and chemical properties of tributyltin compounds
---------------------------------------------------------------------------------------------------------
Oxide Benzoate Chloride Fluoride Linoleate Methacrylate Naphthenate
(TBTO) (TBTB) (TBTCl) (TBTF) (TBTL) (TBTM) (TBTN)
---------------------------------------------------------------------------------------------------------
IUPAC name distannoxane, stannane, stannane, stannane, stannane, stannane, stannane,
hexabutyl (benzyloxy) tributyl- tributyl- tributyl- tributyl- tributyl-
tributyl chloro fluoro (1-oxo-9,12- (2-methyl-1- mono (naph-
octadecadi- oxo-2-propyl) thenoyloxy)
enyl)oxy- oxy- derivatives
CAS name Bis(tributyl- Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin Tributyltin
tin) oxide benzoate chloride fluoride linoleate methacrylate naphthenate
CAS number  7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: MICROORGANISMS
Summary
Tributyltin is toxic to microorganisms and is used commer-
cially to control bacteria and fungi. Concentrations producing
toxic effects are very variable between species. The primary
productivity of a natural community of freshwater microalgae
was reduced by 50% at a TBTO concentration of 3 µg/litre. Re-
cently established no-observed-effect-levels for two species
are 18 and 32 µg/litre. Toxicity to sea-water microorganisms
is similarly variable between species and between studies; no-
observed-effect-levels are difficult to set but lie below 0.1 µg
per litre for some species. Most toxicity tests with microor-
ganisms use batch cultures, and in these systems concentrations
of TBT in solution may decline rapidly. Such tests may, there-
fore, underestimate true toxicity.
7.1. Bacteria and fungi
Bokranz & Plum (1975) presented data on the effective-
ness of TBT compounds against bacteria, algae, and fungi.
TBT is more toxic to gram-positive bacteria (such as
Staphylococcus aureus with a minimal inhibitory concen-
tration of between 0.2 and 0.8 mg/litre in culture) than
to gram-negative bacteria (such as Escherichia coli with
an MIC of 3.1 mg/litre) in serial dilution tests for vari-
ous TBT compounds. MIC values for four species of fungus
in culture (Botrytis, Penicillium, Aspergillus, and
Rhizopodium) varied from 0.5 to 1.0 mg/litre for TBT
acetate. Impregnation of textiles with TBTO, TBT sulfide,
or TBT fluoride at 0.01%, 0.05%, and 0.2% showed clear
inhibition of fungal growth on agar nutrient medium. The
TBT compounds were resistant to leaching from the textiles
when washed before culture at the two highest concen-
trations. Fungicidal action was also retained in textiles
treated with TBTO and subsequently buried in soil. In
tests with three species of fungus, limiting values for
complete inhibition of effects on wood were determined.
Values for TBTO ranged from 0.058 to 0.704 kg/m3 without
prior leaching of the compound and from 0.055 to 2.178 kg
per m3 after prior leaching. For TBT fluoride, values
ranged from 0.055 to 0.88 kg/m3 without prior leaching
and from 0.135 to 0.886 kg/m3 after leaching.
Soracco & Pope (1983) investigated the action of TBTO
on various physiological and biochemical activities of the
bacterium Legionella pneumophila, the organism causing
Legionnaires disease, which commonly lives in the water of
cooling systems. The minimal concentration of TBTO having
any effect on Legionella growth was about 0.02 mg/litre.
At concentrations between 0.5 and 1.1 mg/litre, TBTO re-
duced the growth rate initially and subsequently caused a
further reduction in growth rate. At 1.1 mg/litre, growth
was almost static, while at higher concentrations TBTO was
bactericidal, causing a reduction in the optical density
of the cultures. The effect of TBTO on the cultures was
dependent on cell density; its effectiveness was reduced
at high cell densities. Between 69% and 88% of the added
TBTO was found to be associated with the cells rather than
in free solution. There was a dose-response relationship
between TBTO concentration per unit biomass and the effect
on growth. The bacteriostatic concentration of 1.1
mg/litre did not kill the cells; transfer of cells from
this culture to fresh nutrient medium established that all
cells were still viable. A concentration of 2.24 mg/litre
was similarly lacking in bactericidal action and only a
dose of 11.2 mg/litre successfully killed cultures. The
most marked and immediate effect of TBTO was on intra-
cellular ATP levels and on "energy charge" (the ratio
between ATP and AMP in the cell). Three concentrations of
TBTO (0.112, 1.12, and 11.2 mg/litre) were tested and
produced reductions of intracellular ATP to 45%, 18%, and
15%, respectively, each within 1 min of addition of the
TBTO. The effect persisted at the lowest exposure level
for at least 3 h. Dramatic and immediate falls were also
seen in energy charge. The authors consider this to be the
major effect of the TBTO. Concomitant falls in nucleic
acid synthesis, synthesis of macromolecules, and CO2 pro-
duction were assumed to follow from the basic action. The
wide range of concentrations producing graded growth inhi-
bition, compared to the very small additional increase in
exposure required to cause cell death, suggested to the
authors that there were two separate mechanisms for the
growth inhibition and lethality of TBTO.
Argaman et al. (1984) investigated the toxic effect of
TBTO on activated sludge from municipal sewage treatment
plants. Sludge challenged with a single dose of TBTO was
inhibited (Warburg respirometer oxygen consumption
measurements) by concentrations of 25 µg/litre or more.
However, sludge pre-treated with TBTO at levels of 200 or
1000 µg/litre adapted to the TBTO and no effect was found
on the ability of sludge organisms to break down organic
materials.
7.2. Freshwater algae
The MIC for cultures of the green alga Chlorella
pyrenoidosa with TBTO was 0.5 mg/litre (Bokranz & Plum,
1975). Floch et al. (1964) reported a no-observed-effect-
level on the growth of a freshwater green alga (desmid) of
0.25 mg TBTO/litre or 0.15 mg TBT acetate/litre over an
exposure period of 10 days. No growth occurred at concen-
trations of 0.5 mg TBTO/litre or 0.3 mg TBT acetate/litre.
Deschiens & Floch (1968) reported an LC100 value for
Chlorella over 10 to 20 days of 0.5 mg TBTO/litre.
More recent studies have suggested that aquatic algae
are much more sensitive to TBT than earlier reports indi-
cated.
Wong et al. (1982) determined the IC50 (concen-
tration required to produce a 50% inhibition) for primary
productivity (uptake of 14C-labelled carbonate) and
reproduction of pure cultures of algae and for a natural
phytoplankton community from Lake Ontario, Canada. The
natural community was the most sensitive to TBTO, with an
IC50 for primary productivity of 3 µg/litre. Ankistro-
desmus falcatus showed similar patterns for the effect of
TBTO on primary productivity and on reproduction (growth),
though the latter was slightly more sensitive with an
IC50 of 5 µg/litre compared to 20 µg/litre for pro-
ductivity. The green alga Scenedesmus quadricaudata and
the cyanobacterium (blue-green alga) Anabaena flos-aquae
gave IC50 values for primary productivity of 16 and
13 µg/litre, respectively. RIVM (1989) reported 96-h
EC50 values for Chlorella and Scenedesmus pannonicus of
42 and 64 µg TBTO/litre, respectively, and no-observed-
effect-levels of 18 and 32 µg/litre, respectively.
7.3. Estuarine and marine algae
Many diatoms are highly resistant to the effects of
organometallic compounds. Thomas & Robinson (1987) studied
the tolerance of the diatom Amphora coffeaeformis to TBT
fluoride and found that its growth was unaffected at con-
centrations of less than 10-7mol/litre when the initial
culture cell density was 10 x 104 cells/ml. At the end of
the incubation, when the diatom had stopped growing, there
was no nitrate left in the medium. There was a significant
effect of TBT fluoride at 10-7mol/litre on growth when
the diatom was grown in nitrate-deficient medium. Growth
was also affected, to a lesser degree, by reducing the
silicate in the medium in the presence of TBT fluoride.
The authors concluded that TBTO tolerance in the diatom is
not due to the exclusion of the organotin but to detoxifi-
cation mechanisms requiring increased uptake of nitrate.
Recovery of the organisms after 24 h exposure supported
this theory. After short-term exposure to sublethal, but
inhibitory, concentrations of TBT fluoride, Amphora re-
covered within 24 h (Thomas & Robinson, 1986). Uptake of
nitrate was inhibited initially but recovered after 24 h.
Salazar (1985) exposed three species of marine phyto-
plankton (Gymnodinium splendens, Dunaliella sp., and
Phaeodactylum tricornutum) to TBTO concentrations of 1.5,
3, and 6 µg/litre for a period of 72 h. At the lowest
concentration, all of the G. splendens cells were killed
and growth of Dunliella sp. was inhibited. The growth of
Dunliella sp. was completely inhibited at both 3 and
6 µg/litre, whereas no effect on the growth of P.
tricornutum was observed at any of the test concen-
trations. Beaumont & Newman (1986) cultured the marine
algae Pavlova lutheri, Dunaliella tertiolecta, and
Skeletonema costatum with TBTO at 0.1, 1.0, and 5.0 µg
per litre. All algae exposed to 5.0 µg/litre died within
2 days. A comparison of the slope of the growth curve with
maximum increase in cell density in the culture showed
that all of the algae were significantly inhibited by TBTO
at the lowest concentration tested. This concentration was
not algicidal. Thain (1983) gave the algistatic concen-
tration for TBTO against Tetraselmis suecica as 560 to
1000 µg/litre and against Skeletonema costatum as 1.0 to
18 µg/litre, within 5 days of exposure. Corresponding
algicidal concentrations were > 1000 µg/litre for Tetra-
selmis and > 18 µg/litre for Skeletonema. Walsh et al.
(1985) calculated the EC50 values for growth inhibition
of the marine alga Skeletonema costatum by TBT acetate,
TBTO, TBT chloride, and TBT fluoride to be 0.36, 0.33,
0.36, and 0.25-0.50 µg/litre, respectively, for a 72-h
exposure period. The EC50 values for growth inhibition
of Thalassiosira pseudonanna, another marine species, by
TBT acetate and TBTO were 1.28 and 1.03 µg per litre,
respectively. The LC50 for Skeletonema was 14.7, 14.2,
11.5, and 11.9 µg/litre for TBT acetate, TBTO, TBT
chloride, and TBT fluoride, respectively. Algae did not
adapt to the presence of TBTO after exposure through
12 serial transfers over 12 weeks; EC50 values were the
same for previously exposed cells as for naive cells.
Dojmi Di Delupis et al. (1987) calculated the 8-day
EC50 for growth inhibition of the marine algal species
Dunaliella tertiolecta and Nitzschia sp., exposed to
TBTO, to be 4.53 µg/litre and 1.19 µg/litre, respect-
ively.
His et al. (1986) conducted bioassays to measure the
susceptibility of algae that are food organisms for oys-
ters to TBT-containing antifouling paints. Four algal
species were used in the studies: Isochrysis galbana
(Prymnesiophyceae); Chaetocerus calcitrans (Bacillario-
phyceae); Tetraselmis (Platymonas) suecica (Prasinophy-
ceae); and Phaeodactylum tricornutum (Bacillariophyceae).
Cultures were maintained in filtered sea water with a
salinity of 27o/oo and at a temperature of 20 °C. TBT
exposure was either to pure TBT acetate or as plates
painted with "International TBT antifouling" with a sur-
face area of between 0.01 and 1.0 cm2 in a culture of
2 litres. Culture density was estimated every 3 to 4 days
using a Coulter counter. Exposure to the pure TBT acetate
at 1 µg/litre had no effect on any of the algal cul-
tures. Isochrysis growth was totally inhibited by painted
panels of 1.0, 0.25, and 0.125 cm2 within 2 days of
culture. Smaller panels were then used to find the limit
of the effect. A 0.02-cm2 panel was also totally toxic to
growth of the alga; panels of 0.01 cm2 allowed growth
comparable to a control culture over the first week of
culture but then inhibited growth. By the 21st day of cul-
ture, the number of cells was reduced to 1.8 x 106 cells
per ml, compared to a control density of 3.2 x 106 cells
per ml. For Chaetocerus, panels of 0.02 cm2 were toxic
to the alga from the beginning of the culture period;
numbers of cells were reduced, indicating that not only
growth but also viability was affected. With panels of
0.01 cm2, there was complete inhibition of development
over the first 4 days, followed by a decrease in cell num-
bers from the original value. The two other algal species
were less sensitive. Phaeodactylum was inhibited by panels
of 1.0 and 0.5 cm2 from the outset of culture. Inhibition
also occurred with panels of 0.25 and 0.125 cm2, but
only after several days. Tetraselmis grew in the presence
of panels of all sizes up to 1.0 cm2, though at this
exposure, growth was reduced. Exposure to panels of 0.5
cm2 had little effect. The authors also tested the effect
on algal growth of fresh water from the river feeding the
area of interest and also the effect of river sediment. In
both cases, growth of the algae was greater than growth of
controls, suggesting a greater availability of nutrients.
However, it should be noted that no analysis was made of
actual water concentrations of TBT in this study; since
the precise exposure levels are unknown, the results are
difficult to interpret or evaluate.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: AQUATIC ORGANISMS
8.1. Aquatic plants
Summary
Few studies have been carried out on the effects of TBT on
aquatic plants. The lowest effect level was observed for
Enteromorpha intestinalis. Motile spores were inhibited from
settling by TBTO, the EC50 being 1 ng/litre; newly settled
spores increased in resistance with time. Results should be
interpreted with care because TBT concentrations were not
measured and the experimental protocols were incomplete.
Reduction in the growth of freshwater species was observed
at concentrations down to 0.06 mg/litre.
Davies et al. (1984) studied the effect of various TBT
compounds on spore development in the marine green macro-
alga, Enteromorpha intestinalis. The 5-day EC50 values
for newly-settled Enteromorpha spores ranged from
0.027 µg/litre (TBT benzoate) to 8.6 µg/litre (TBT acry-
late). The authors stated that the toxicity appeared to be
influenced by the type of anion. Using TBTO it was found
that sensitivity decreased with increasing settlement
time; the 5-day EC50 values for spore development ranged
from 0.22 µg/litre, when exposure began 30 min after
settlement, to 10 µg/litre, when exposure began 72 h
after settlement. Motile spores were the most sensitive
(5-day EC50 = 0.001 µg/litre).
The marine angiosperm Zostera marina showed reduced
growth at TBT concentrations in sediment of 1.0 mg/kg but
no effect at 0.1 mg/kg (Personal communication by M.J.
Waldock to IPCS, 1989).
Floch et al. (1964) exposed freshwater aquatic plants
to TBTO or TBT acetate, at water concentrations between
0.03 and 1.2 mg/litre, for 10 days. The duckweed Lemna
media and Canadian pondweed Elodea sp. both showed some
growth at TBTO concentrations of 0.03 mg/litre. Duckweed
maintained itself, without significant growth, at concen-
trations between 0.06 and 0.25 mg/litre, but died at 0.5
mg/litre. Elodea showed degeneration between 0.06 and 0.25
mg/litre and died at 0.5 mg/litre. Degeneration of Elodea
was evident at 0.15 mg TBT acetate/litre; growth occurred
at 0.03 mg/litre and death at 0.3 mg/litre. Lemna grew in
0.15 mg TBT acetate/litre and died at 1.2 mg/litre; there
was maintenance without growth at 0.6 mg/litre.
L.A. Boorman (personal communication to the IPCS,
1989) grew plants of two salt marsh species, Aster
tripolium and Limonium vulgare, in mud with added TBTO.
Plants of Aster were killed by sediment TBTO levels in
excess of 10 µg/kg (dry weight), while Limonium was not
significantly affected by levels of up to 150 µg/kg.
Chu (1976) found that the aquatic weed Ceratophyllum
died within 2 months exposure to a controlled release
rubber formulation containing 5% TBTO (5 mg/litre); the
exposure period being 24 h every 3 to 5 days.
8.2. Aquatic invertebrates
The acute toxicity of tributyltin to aquatic invert-
ebrates is summarized in Tables 8, 9, and 10. Larval
stages are considerably more sensitive to TBT than adults;
the LC50 for the larval Pacific oyster is 1.6 µg per
litre, over 48 h, whereas that for adults is 1800 µg per
litre (Thain, 1983). Other species show similar differ-
ences between life stages. The 96-h LC50 values for crus-
taceans range between 1.0 and 41 µg/litre. There are
fewer data on freshwater species; these relate to just
three species other than target organisms. Various TBT
salts give a range of 48-h LC50 values for Daphnia of 2.3
to 70 µg/litre and for Tubifex of 5.5 to 33 µg per
litre. The 24-h LC50 for the Asiatic clam is 2100 µg per
litre, and that for target snail adults in schistosomiasis
control is 30 to 400 µg/litre.
8.2.1. Trematode parasites of man
Some organotin molluscicides have been shown to be
toxic to schistosome larvae in the aquatic stages. Ritchie
et al. (1974) found that TBTO concentrations of 10 and
100 µg/litre rendered Schistosoma mansoni cercariae, the
infective stage released from the secondary host (water
snails), incapable of progressive movement, following a 5-
min exposure. Infectivity of the cercariae to mice was
completely suppressed. A 30-min exposure to concentrations
of 1 µg/litre or less had relatively little effect on
motility of cercariae and on subsequent infectivity of
mice. The authors also exposed S. mansoni miracidia to
TBTO and found that 10 µg/litre immobilized the miracidia
after a 40-min exposure and completely suppressed the
infectivity to snails (Biomphalaria glabrata). However, a
concentration of 1 µg/litre had no effect on motility
and infectivity after an exposure period of 120 min.
Table 8. Toxicity of tributyltin to marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Eastern oyster embryo statb 28 7.1 chloride 48 1.3 Roberts
(Crassostrea virginica) (0.78-1.38)d (1987)
larva 48 3.96
(2.42-4.21)d
European oyster adult statb oxide 48 >300 Thain
(Ostrea edulis) 96 210 (1983)
Pacific oyster larva statb oxide 48 1.6 Thain
(Crassostrea gigas) adult 48 1800 (1983)
adult 96 290
Mussel larva statb oxide 48 23 Thain
(Mytilus edulis) adult 48 300 (1983)
adult 96 38
Hard clam embryo statb 28 7.1 chloride 48 1.13 Roberts
(Mercenaria mercenaria) (0.72-1.31)d (1987)
larva 48 1.65d
Brown shrimp larva statb oxide 48 6.5 Thain
(Crangon crangon) larva 96 1.5 (1983)
adult 48 73
adult 96 41
Grass shrimp sub- flow 19.4- 9.8- 8.15- oxide 96 20d Walsh (1986)
(Palaemonetes pugio) adult 21.3 12.1 8.31 chloride 96 > 31d,f Bushong
et al. (1988)
Mysid shrimp < 1 day flow 24-26 19- 7.98- chloride 96 1.1 Goodman et
(Mysidopsis bahia) 22.3 8.01 (0.68-1.4)d al. (1988)
5 day 2
(1.4-2.6)d
10 day 2.2
(1.4-2.6)d
Shore crab larva statb oxide 48 110 Thain (1983)
(Carcinus maenus) 96 10
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Harpacticoid copepod adult stat 20-22 7 7.8 fluoride 96 2 Linden et
(Nitocra spinipes) (1-2)e al. (1979)
oxide 96 2
(1-3)e
Copepod sub- flow 20 10 chloride 72 0.6 Bushong et
(Eurytemora affinis) adult (0.1-2.0)d,f al. (1987)
sub- flow 20 10 chloride 48 1.4
adult (0.8-2.3)d,f
sub- stat 19.4- 10.1- 8.17- chloride 48 2.2 Hall et al.
adult 20.3 11.2 8.32 (0.2-7.3)d,f (1988b)
sub- stat 19.4- 10.1- 8.17- chloride 72 0.6
adult 20.3 11.2 8.32 (0-3.3)d,f
Copepod sub- flow 20 10 chloride 48 1.1 Bushong et
(Acartia tonsa) adult (0.7-2.2)d,f al. (1987)
adult statb 19.5- oxide 96 1.0 U'Ren (1983)
20.5 (0.8-1.2)d
Amphipod young flow 19.4- 9.8- 8.15- chloride 96 1.3d,f Bushong et
(Gammarus sp.) adult 21.3 12.1 8.31 chloride 96 5.3d,f al. (1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise); flow = flow-through conditions (TBT concentration in
water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
Table 9. Toxicity of tributyltin to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50d Reference
age flowa ature nessc tion (ug/litre)
(°C) (mg/litre) (h)
---------------------------------------------------------------------------------------------------------
Asiatic clam larva stat 20 oxide 24 2100e Foster
(Corbicula fluminea) (1981)
Water flea adult statb 21 chloride 96 5.9 (3.7-9.4)e Meador
(Daphnia magna) adult statb 21 chloride 120 3.4 (1.3-8.8)e (1986)
acetate 48 3.3 (1.5-6.0)e,f Polster &
oleate 48 8.5 (4.2-10.5)e,f Halacka
benzoate 48 4.3 (1.8-9.5)e,f (1971)
chloride 48 4.5 (1.2-9.3)e,f
laurate 48 4.7 (1.2-9.3)e,f
oxide 48 2.3 (1.2-5.2)e,f
juvenile stat 20 200 7.5 chloride 24 13e Vighi &
juvenile stat 20 200 7.5 oxide 24 14e Calamari
(1985)
juvenile stat 19 8.2 oxide 48 4.7 RIVM
(1989)
stat 20 oxide 48 70e Foster
(1981)
Tubifex worm acetate 48 8.0 (2.8-10.3)e,f Polster &
(Tubifex tubifex) oleate 48 17.0 (10.1-30.0)e,f Halacka
benzoate 48 16.0 (10.1-27.0)e,f (1971)
chloride 48 15.0 (10.1-23.0)e,f
laurate 48 33.0 (10.6-75.0)e,f
oxide 48 5.5 (1.6-10.3)e,f
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c Hardness expressed as mg/litre CaCO3).
d 95% confidence limits are given in brackets; concentrations expressed as the TBT salt used
unless otherwise stated.
e Nominal concentration.
f Concentration expressed as TBT.
Viyanant et al. (1982) exposed Schistosoma mansoni
cercariae to TBT fluoride and calculated an LC50 of
16.8 µg/litre and an LC90 of 21.7 µg/litre for a 1-h
exposure. Following a 1-h exposure of S. mansoni to TBT
fluoride, cercarial infectivity of mice was observed over
a period of 30 min. A 100% suppression of infectivity was
found at 6 µg/litre; effective doses (99%-100%) were
between 2 and 6 µg/litre.
8.2.2. Freshwater molluscs
Summary
The LC50 values for target fresh water snail adults in
schistosomiasis control range from 30 to 400 µg/litre. This
indicates very low selectivity of TBT as a Bilharzia mollusci-
cide, and a high risk to sensitive non-target aquatic species.
The lethal concentrations of TBT to adult Bilharzia snails
are also expected to inhibit motility and infectivity of both
cercariae and miracidiae in the contaminated water, as the sup-
pressive levels were found to be 10 µg/litre for both cer-
cariae and miracidiae of Schistosoma mansoni after 5 and 40
min, respectively. The 1-h LC 50 for cercariae is 16.8 µg per
litre.
Generally, the toxicity of TBT to freshwater snails depends
on the species, stage, age, temperature, pH, time of exposure,
time of observation, suspended matter, and type of structure
and formulation.
Pellets or matrices of natural rubber or synthetic polymers
impregnated with TBT produce a slow-release effective level of
the molluscicides, which results in low acute toxicity but
extended long-term toxicity.
Exposure of adult snails to TBT levels as low as 0.01 to
0.001 µg/litre reduced egg laying, inhibited hatchability of
the exposed eggs, and retarded the development of the surviving
offspring.
The no-observed-effect level for adult freshwater snails
(Limnaea stagnalis) was 0.32 µg/litre in long-term tests.
TBT compounds are strongly adsorbed on to suspended clay
and organic particles. These particulates are ingested by the
snails and provide one of the inputs for toxicity. Studies of
the impact of type and amount of suspended matter on TBT tox-
icity to snails vary in their conclusions.
Increasing the pH was found to enhance the molluscicidal
toxicity of slow-release TBT formulations.
The acute toxicity of TBT to adult freshwater snails
is summarized in Table 10. The data are based mainly on
species that are intermediate hosts in the life-cycle of
the parasite causing Bilharzia (schistosomiasis) in man,
TBT being used as a molluscicide to control the disease.
The toxic action of TBT is slow, the mortality at the end
of a 24-h exposure is often low, and for a more realistic
result a post-exposure observation period is required.
Rubber impregnated with TBT to produce a slow-release
molluscicide that maintains a low, but toxic, concen-
tration over a long period was developed by Cardarelli and
colleagues, and was shown to be very effective against
snail pest species such as Biomphalaria glabrata and B.
globosus (Berrios-Duran & Ritchie, 1968).
Molluscicidal activity has been demonstrated against
Bulinus spp., Biomphalaria spp., and certain operculate
freshwater molluscs, but organotin compounds have proved
to be not as toxic against the amphibious oncomelaniid
snails (McCullough et al., 1980).
8.2.2.1 Acute toxicity
Webbe (1963) found that young snails (Biomphalaria
sudanica and Bulinus nasutus) were more sensitive than
adults to TBT acetate, the 24-h LC50 values being 14 and
15 µg/litre for the two snail species, respectively.
When eggs from the same two species were exposed, the 24-h
LC50 values ranged from 100 to 1000 µg/litre. Paulini
(1964) found embryos of Biomphalaria glabrata to be more
susceptible than adults to TBT acetate. The 24-h LC50
values for embryos ranged from 26 µg/litre at 5-12 h of
age to 46 µg/litre at 77-87 h of age, whereas the value
for adults was 170 µg/litre.
Table 10. Acute toxicity of tributyltin to freshwater snails
---------------------------------------------------------------------------------------
Species TBT salt Exposure Post-exposure LC50 Reference
duration observation (µg/litre)
(h) (h)
---------------------------------------------------------------------------------------
Biomphalaria oxide 6 72 410 Seiffer & Schoof (1967)
glabrata acetate 6 72 290 Seiffer & Schoof (1967)
oxide 6 24 370 Ritchie et al. (1964)
oxide 24 24 40 Ritchie et al. (1964)
acetate 6 24 190 Ritchie et al. (1964)
acetate 24 24 85 Ritchie et al. (1964)
acetate 24 100-300 Hopf et al. (1967)
pentachloro-
phenate 24 100-400 Hopf et al. (1967)
oxide 24 50-100 Hopf et al. (1967)
oxide 24 30 Deschiens et al. (1966)
acetate 24 72 170 Paulini (1964)
Biomphalaria oxide 24 30 Deschiens et al. (1966)
contortus
Biomphalaria acetate 24 48 34 Webbe (1963)
sudanica
Bulinus nasutus acetate 24 48 32 Webbe (1963)
Bulinus oxide 17 24 10 de Villiers &
tropicus MacKenzie (1963)
Limnaea oxide 24 96 60 Temmink & Everts (1987)
stagnalis oxide 96 96 24 Temmink & Everts (1987)
oxide 96 42 RIVM (1989)
---------------------------------------------------------------------------------------
8.2.2.2 Short- and long-term toxicity
Ritchie et al. (1974) found that egg laying in
Biomphalaria glabrata was completely inhibited by 10 µg
TBTO/litre, all the snails being killed within 2 to 5
days. Egg laying was reduced, over a period of 2 to 3
weeks, by more than 90% at 1 µg/litre and by 50% at
0.1 µg/litre. At 0.01 µg/litre, egg laying was unaffec-
ted. Newly-laid eggs were exposed to TBTO for 34 days
followed by 50 days in clean water. Eggs exposed to 10 µg
per litre did not hatch, even after 50 days in clean
water. Of those exposed to 1 µg/litre, only 3% hatched
during the exposure and 35% of the rest hatched after
transfer to clean water (but with a delayed hatching
time). When newly-hatched snails were exposed to TBTO, 95%
of those exposed to 1 µg/litre from hatching died and
those that survived failed to lay eggs for 85 days. At
0.1 µg/litre, 60% of the snails died and egg laying in
the survivors was reduced by 80%. Egg laying was also
significantly reduced at both 0.01 and 0.001 µg/litre.
Upatham et al. (1980) studied the toxicity to Bulinus
abyssinicus of various controlled-release organotin mol-
luscicides, i.e. BioMet SRM rubber pellets (6% TBTO),
CBL-9B rubber pellets (20% TBT fluoride), and EC-13 float-
ing ethylene propylene co-polymer pellets (30% TBT fluor-
ide). The organotin compounds killed all of the snails
within 1 to 2 days at 100 mg/litre (active ingredient) and
within 5 to 7 days at 1 mg/litre, there being no signifi-
cant difference between the compounds. Changing the test
water daily had no significant effect. At lower concen-
trations the molluscicides required 36 to 40 days to kill
all the snails at an active ingredient concentration of
0.03 mg/litre, and at 0.3 mg/litre 9 to 10 days was re-
quired for BioMet SRM and CBL-9B, and 22 days for EC-13.
In long-term exposure tests, toxic effects on fresh-
water snails have been found at very low TBT concen-
trations. Cardarelli (1973) quoted a 120-day LC100 of
7 µg TBTO/litre for Biomphalaria glabrata. At 0.7 µg per
litre, 26% of the snails died within 120 days. RIVM (1989)
reported a NOEL for the freshwater snail Limnaea stagnalis
of 0.32 µg/litre in long-term tests.
8.2.2.3 Factors affecting toxicity
Paulini & de Souza (1970) studied the effect of
various factors on the molluscicidal activity of TBT to
freshly laid eggs of Biomphalaria glabrata. A concen-
tration of colloidal clay (the proportion of clay to
molluscicide was 1000:1) of 1000 mg/litre reduced the 24-h
LC50 (with a 7-day recovery period included in the mor-
tality count) by 30% for TBT acetate and by 7% for TBTO.
The addition of a yeast ( Saccharomyces sp.) suspension
(1 g/litre) reduced the 24-h LC50 (with a 24-h recovery
period) by 95% for TBTO and 72% for TBT acetate. The pro-
portion of yeast to molluscicide was 1000:1 for TBTO and
100:1 for TBT fluoride.
Cardarelli & Evans (1980) studied the effect of vari-
ous factors on the toxicity to snails of controlled-
release organotin molluscicides, i.e. BioMet SRM (6% TBTO
in natural rubber) and CBL-9B (20% TBT fluoride in natural
rubber). Using 100-mg/kg pellets, they found that increas-
ing the pH from 6 to 8 increased the toxicity (as measured
by both LT50 and LT100) of BioMet SRM but decreased
the toxicity of CBL-9B to both Biomphalaria glabrata and
Bulinus globosus. The authors found no effect of yeast or
humic acid (1 to 100 mg/litre) on the toxicity (LT100)
of these controlled-release molluscicides, neither did
they find an effect of suspended colloidal clays. They
concluded that, although the organotin compounds are
adsorbed, they are still toxic because the snails ingest
the added materials. Therefore, snails are still exposed
to TBT even after it is adsorbed to surfaces. In an exper-
iment to compare adsorbed uptake of TBT, soil-browsing
snails and isolated snails were compared during exposure
to slow-release organotin pellets. The molluscicides were
found to be more toxic when the snails were allowed to
browse on soil, and, at a distance of 60 cm from the pel-
lets, isolated snails were producing egg masses whereas
those browsing on soil were not. The authors also con-
cluded that the organotin molecules that come into contact
with soil particles are adsorbed and slowly form ligands
of a nontoxic nature, so that soil only remains toxic as
long as it is freshly exposed to organotin. Upatham et al.
(1980) found that the presence of mud or plant life,
compared to exposure in water alone, had no effect on the
toxicity (LT100) of either CBL-9B or EC-13 at 1 or 10
mg/litre (active ingredient) to Bulinus abyssinicus. When
the snails were exposed to BioMet SRM, no effect of mud or
plants was found at 10 mg/litre, but at 1 mg/litre a
slightly shorter time was required to kill all the snails
in water alone. Chu (1976) noted that organic materials
such as mud and weeds reduced the molluscicidal activity
of TBTO on Bulinus rohlfsi, when exposed to a rubber for-
mulation containing 5% TBTO (5 mg/litre TBTO), with a 24-h
exposure period every 3 to 5 days for 70 days and twice a
month for a further 7 months.
Macklad et al. (1983) found that the toxicity of
controlled-release molluscicides containing TBT fluoride
was dependent on the pre-exposure soaking time. The 48-h
LC50 for Biomphalaria alexandrina of 10 mg/litre total
available toxicant was achieved after 7, 3, and 1 days
soaking period for the formulations EC27 (10% TBT fluoride
in a plastic polymer), EC1320 (20% TBT fluoride in rub-
ber), and EC1330 (30% TBT fluoride in a polypropylene/
polyethylene mixture), respectively. EC1330 produced no
mortality following a 3-h soaking period and exposure to
concentrations ranging from 1 to 100 mg/litre for 48-h;
the authors suggested that the polypropylene/polyethylene
mixture (EC1330) needs to be sufficiently wet before it
begins to release TBT fluoride. A second experiment to
test the aging of slow-release molluscicides was carried
out. EC27 gave 89% snail mortality during a 48-h exposure
period to 50 mg/litre after a soaking time of 24-h. After
being left to age for 1 month the molluscicide was tested
again and had lost 80% of its original toxicity.
8.2.3. Marine molluscs
Summary
A large body of data exists on the effects of TBT on marine
molluscs and in particular on commercially important bivalves.
Sublethal effects occur at very low concentrations. It has been
shown experimentally that TBT affects shell deposition of grow-
ing oysters, gonad development and gender of adult oysters,
settlement, growth, and mortality of larval oysters and of
other bivalves, and causes imposex (the development of male
characteristics) in female gastropods. The NOEL for shell
thickening of the most sensitive oyster species (C. gigas) is
about 20 ng/litre. Embryo-larval stages are more susceptible
than adults; adverse effects on larval development have been
demonstrated at concentrations as low as 50 ng/litre. The NOEL
is 20 ng/litre.
The authors of recent work on imposex in Nucella have
determined threshold concentrations by extrapolation below the
limit of reliable detection. While the circumstantial evidence
in support is substantial (see chapter 10), the determination
of toxicological thresholds below the limits of chemical detec-
tion is not practicable. It is generally agreed that imposex
is not a specific index of TBT contamination, in that it can be
induced by other factors. The wide distribution of low concen-
trations of TBT and the incidence of imposex at levels similar
to or below the analytical detection limits make long-term
controlled experiments difficult. The establishment of NOELs
will have to await the development of better analytical tech-
niques.
8.2.3.1 Acute toxicity
Waldock & Thain (1985) calculated the 24-h EC50 (mor-
tality plus moribundity) of two organotin-containing fish-
net antifouling preparations for larval oysters to be
12 µg/litre for `Norimp 200' and 320 µg/litre for
`Flexgard'. These compare with a value for TBTO of 1.7 µg
per litre. Thain (1983) pointed out that adult bivalves
tend to appear more resistant to pollutants in standard
short-term tests since they can close their shell over the
test period and thus reduce exposure. Larval stages appear
to be much more sensitive in these tests.
8.2.3.2 Short- and long-term toxicity
Alzieu et al. (1982) kept Pacific oysters (Crassostrea
gigas) in 150-litre tanks that were successively filled
and drained according to the tidal period. To these tanks
were added panels coated on one side with TBT fluoride,
giving estimated concentrations of 0.2 and 2.0 µg TBT per
litre. All oysters died within 30 days in a tank contain-
ing the larger panel. In the tank with a panel surface of
50 cm2, 30% of oysters died after 110 days of exposure
and all oysters within 170 days.
His & Robert (1985) experimentally tested hypotheses
regarding the poor performance of Pacific oysters in the
Bay of Arcachon, France. Larvae were maintained in the
laboratory at TBT acetate concentrations ranging from 0.02
to 100 µg/litre. Growth was affected by all concen-
trations except 0.02 µg/litre. The next concentration
tested (0.05 µg/litre) reduced growth, led to mortality
within 10 days, and interrupted normal feeding by day 8.
No other effects were noted at 0.02 µg/litre and this was
regarded as the NOEL for larvae (see Table 11).
Table 11. Effects of TBT acetate on Crassostrea gigas larvae at
various water concentrationsa
--------------------------------------------------------------------------------
Water concentration Effect
(µg/litre)
--------------------------------------------------------------------------------
100 inhibition of fertilization
50 inhibition of segmentation
25 partial inhibition of segmentation (40%)
10 no formation of trochophores
3 to 5 no veligers; malformed trochophores
1 abnormal veligers; total mortality within 6 days
0.5 numbers of abnormal larvae; total mortality within 8 days;
perturbation of feeding regime, particularly from 4 to 8 days
after exposure; growth greatly reduced
0.2 percentage of D larvae showing abnormalities less elevated;
perturbation of feeding regime from day 4; progressive
mortality; total by day 12; weak growth
0.1 majority of D larvae normal; marked perturbation of feeding
regime from day 6; weak growth until day 6; some survivors
after 12 days
0.05 normal D larvae; perturbation of feeding regime, marked on day
8; significant mortality beginning at day 10; reduced growth
0.02 normal D larvae; little mortality; good growth; no effect of
TBT
--------------------------------------------------------------------------------
a From: His & Robert (1985).
Thain & Waldock (1985) exposed various bivalve spat
(common European oyster, Ostrea edulis; Pacific oyster,
Crassostrea gigas; common mussel, Mytilus edulis; and
carpet shells, Venerupis decussata and Venerupis semi-
decussata) to TBT leachate by maintaining them in flowing
sea-water tanks containing house slates painted with
`Micron 25R' (containing TBT methacrylate co-polymer). The
water concentrations of TBT were 0.24 or 2.6 µg/litre. At
2.6 µg/litre, growth was completely inhibited in all
groups and mortality was high (except for V. semidecussata)
within the 45-day exposure period; all mussels had died
within 14 days. At the lower exposure concentration,
growth was significantly inhibited in C. gigas, M. edulis,
and V. decussata but not in the other two species. In a
second study, the authors reported a severe reduction in
growth rate of recently metamorphosed oyster (Ostrea
edulis) spat after exposure to TBT leachate (0.06 µg per
litre) for 10 days under static conditions. Growth rate
was reduced between 0 and 10 days of exposure to 0.02 µg
per litre, but there was only slight reduction in growth,
relative to controls, between days 10 and 20.
Growth curves for oysters (Ostrea edulis), 2-3 mm in
size, exposed to different concentrations of TBTO are
given in Fig. 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: MICROORGANISMS
Summary
Tributyltin is toxic to microorganisms and is used commer-
cially to control bacteria and fungi. Concentrations producing
toxic effects are very variable between species. The primary
productivity of a natural community of freshwater microalgae
was reduced by 50% at a TBTO concentration of 3 µg/litre. Re-
cently established no-observed-effect-levels for two species
are 18 and 32 µg/litre. Toxicity to sea-water microorganisms
is similarly variable between species and between studies; no-
observed-effect-levels are difficult to set but lie below 0.1 µg
per litre for some species. Most toxicity tests with microor-
ganisms use batch cultures, and in these systems concentrations
of TBT in solution may decline rapidly. Such tests may, there-
fore, underestimate true toxicity.
7.1. Bacteria and fungi
Bokranz & Plum (1975) presented data on the effective-
ness of TBT compounds against bacteria, algae, and fungi.
TBT is more toxic to gram-positive bacteria (such as
Staphylococcus aureus with a minimal inhibitory concen-
tration of between 0.2 and 0.8 mg/litre in culture) than
to gram-negative bacteria (such as Escherichia coli with
an MIC of 3.1 mg/litre) in serial dilution tests for vari-
ous TBT compounds. MIC values for four species of fungus
in culture (Botrytis, Penicillium, Aspergillus, and
Rhizopodium) varied from 0.5 to 1.0 mg/litre for TBT
acetate. Impregnation of textiles with TBTO, TBT sulfide,
or TBT fluoride at 0.01%, 0.05%, and 0.2% showed clear
inhibition of fungal growth on agar nutrient medium. The
TBT compounds were resistant to leaching from the textiles
when washed before culture at the two highest concen-
trations. Fungicidal action was also retained in textiles
treated with TBTO and subsequently buried in soil. In
tests with three species of fungus, limiting values for
complete inhibition of effects on wood were determined.
Values for TBTO ranged from 0.058 to 0.704 kg/m3 without
prior leaching of the compound and from 0.055 to 2.178 kg
per m3 after prior leaching. For TBT fluoride, values
ranged from 0.055 to 0.88 kg/m3 without prior leaching
and from 0.135 to 0.886 kg/m3 after leaching.
Soracco & Pope (1983) investigated the action of TBTO
on various physiological and biochemical activities of the
bacterium Legionella pneumophila, the organism causing
Legionnaires disease, which commonly lives in the water of
cooling systems. The minimal concentration of TBTO having
any effect on Legionella growth was about 0.02 mg/litre.
At concentrations between 0.5 and 1.1 mg/litre, TBTO re-
duced the growth rate initially and subsequently caused a
further reduction in growth rate. At 1.1 mg/litre, growth
was almost static, while at higher concentrations TBTO was
bactericidal, causing a reduction in the optical density
of the cultures. The effect of TBTO on the cultures was
dependent on cell density; its effectiveness was reduced
at high cell densities. Between 69% and 88% of the added
TBTO was found to be associated with the cells rather than
in free solution. There was a dose-response relationship
between TBTO concentration per unit biomass and the effect
on growth. The bacteriostatic concentration of 1.1
mg/litre did not kill the cells; transfer of cells from
this culture to fresh nutrient medium established that all
cells were still viable. A concentration of 2.24 mg/litre
was similarly lacking in bactericidal action and only a
dose of 11.2 mg/litre successfully killed cultures. The
most marked and immediate effect of TBTO was on intra-
cellular ATP levels and on "energy charge" (the ratio
between ATP and AMP in the cell). Three concentrations of
TBTO (0.112, 1.12, and 11.2 mg/litre) were tested and
produced reductions of intracellular ATP to 45%, 18%, and
15%, respectively, each within 1 min of addition of the
TBTO. The effect persisted at the lowest exposure level
for at least 3 h. Dramatic and immediate falls were also
seen in energy charge. The authors consider this to be the
major effect of the TBTO. Concomitant falls in nucleic
acid synthesis, synthesis of macromolecules, and CO2 pro-
duction were assumed to follow from the basic action. The
wide range of concentrations producing graded growth inhi-
bition, compared to the very small additional increase in
exposure required to cause cell death, suggested to the
authors that there were two separate mechanisms for the
growth inhibition and lethality of TBTO.
Argaman et al. (1984) investigated the toxic effect of
TBTO on activated sludge from municipal sewage treatment
plants. Sludge challenged with a single dose of TBTO was
inhibited (Warburg respirometer oxygen consumption
measurements) by concentrations of 25 µg/litre or more.
However, sludge pre-treated with TBTO at levels of 200 or
1000 µg/litre adapted to the TBTO and no effect was found
on the ability of sludge organisms to break down organic
materials.
7.2. Freshwater algae
The MIC for cultures of the green alga Chlorella
pyrenoidosa with TBTO was 0.5 mg/litre (Bokranz & Plum,
1975). Floch et al. (1964) reported a no-observed-effect-
level on the growth of a freshwater green alga (desmid) of
0.25 mg TBTO/litre or 0.15 mg TBT acetate/litre over an
exposure period of 10 days. No growth occurred at concen-
trations of 0.5 mg TBTO/litre or 0.3 mg TBT acetate/litre.
Deschiens & Floch (1968) reported an LC100 value for
Chlorella over 10 to 20 days of 0.5 mg TBTO/litre.
More recent studies have suggested that aquatic algae
are much more sensitive to TBT than earlier reports indi-
cated.
Wong et al. (1982) determined the IC50 (concen-
tration required to produce a 50% inhibition) for primary
productivity (uptake of 14C-labelled carbonate) and
reproduction of pure cultures of algae and for a natural
phytoplankton community from Lake Ontario, Canada. The
natural community was the most sensitive to TBTO, with an
IC50 for primary productivity of 3 µg/litre. Ankistro-
desmus falcatus showed similar patterns for the effect of
TBTO on primary productivity and on reproduction (growth),
though the latter was slightly more sensitive with an
IC50 of 5 µg/litre compared to 20 µg/litre for pro-
ductivity. The green alga Scenedesmus quadricaudata and
the cyanobacterium (blue-green alga) Anabaena flos-aquae
gave IC50 values for primary productivity of 16 and
13 µg/litre, respectively. RIVM (1989) reported 96-h
EC50 values for Chlorella and Scenedesmus pannonicus of
42 and 64 µg TBTO/litre, respectively, and no-observed-
effect-levels of 18 and 32 µg/litre, respectively.
7.3. Estuarine and marine algae
Many diatoms are highly resistant to the effects of
organometallic compounds. Thomas & Robinson (1987) studied
the tolerance of the diatom Amphora coffeaeformis to TBT
fluoride and found that its growth was unaffected at con-
centrations of less than 10-7mol/litre when the initial
culture cell density was 10 x 104 cells/ml. At the end of
the incubation, when the diatom had stopped growing, there
was no nitrate left in the medium. There was a significant
effect of TBT fluoride at 10-7mol/litre on growth when
the diatom was grown in nitrate-deficient medium. Growth
was also affected, to a lesser degree, by reducing the
silicate in the medium in the presence of TBT fluoride.
The authors concluded that TBTO tolerance in the diatom is
not due to the exclusion of the organotin but to detoxifi-
cation mechanisms requiring increased uptake of nitrate.
Recovery of the organisms after 24 h exposure supported
this theory. After short-term exposure to sublethal, but
inhibitory, concentrations of TBT fluoride, Amphora re-
covered within 24 h (Thomas & Robinson, 1986). Uptake of
nitrate was inhibited initially but recovered after 24 h.
Salazar (1985) exposed three species of marine phyto-
plankton (Gymnodinium splendens, Dunaliella sp., and
Phaeodactylum tricornutum) to TBTO concentrations of 1.5,
3, and 6 µg/litre for a period of 72 h. At the lowest
concentration, all of the G. splendens cells were killed
and growth of Dunliella sp. was inhibited. The growth of
Dunliella sp. was completely inhibited at both 3 and
6 µg/litre, whereas no effect on the growth of P.
tricornutum was observed at any of the test concen-
trations. Beaumont & Newman (1986) cultured the marine
algae Pavlova lutheri, Dunaliella tertiolecta, and
Skeletonema costatum with TBTO at 0.1, 1.0, and 5.0 µg
per litre. All algae exposed to 5.0 µg/litre died within
2 days. A comparison of the slope of the growth curve with
maximum increase in cell density in the culture showed
that all of the algae were significantly inhibited by TBTO
at the lowest concentration tested. This concentration was
not algicidal. Thain (1983) gave the algistatic concen-
tration for TBTO against Tetraselmis suecica as 560 to
1000 µg/litre and against Skeletonema costatum as 1.0 to
18 µg/litre, within 5 days of exposure. Corresponding
algicidal concentrations were > 1000 µg/litre for Tetra-
selmis and > 18 µg/litre for Skeletonema. Walsh et al.
(1985) calculated the EC50 values for growth inhibition
of the marine alga Skeletonema costatum by TBT acetate,
TBTO, TBT chloride, and TBT fluoride to be 0.36, 0.33,
0.36, and 0.25-0.50 µg/litre, respectively, for a 72-h
exposure period. The EC50 values for growth inhibition
of Thalassiosira pseudonanna, another marine species, by
TBT acetate and TBTO were 1.28 and 1.03 µg per litre,
respectively. The LC50 for Skeletonema was 14.7, 14.2,
11.5, and 11.9 µg/litre for TBT acetate, TBTO, TBT
chloride, and TBT fluoride, respectively. Algae did not
adapt to the presence of TBTO after exposure through
12 serial transfers over 12 weeks; EC50 values were the
same for previously exposed cells as for naive cells.
Dojmi Di Delupis et al. (1987) calculated the 8-day
EC50 for growth inhibition of the marine algal species
Dunaliella tertiolecta and Nitzschia sp., exposed to
TBTO, to be 4.53 µg/litre and 1.19 µg/litre, respect-
ively.
His et al. (1986) conducted bioassays to measure the
susceptibility of algae that are food organisms for oys-
ters to TBT-containing antifouling paints. Four algal
species were used in the studies: Isochrysis galbana
(Prymnesiophyceae); Chaetocerus calcitrans (Bacillario-
phyceae); Tetraselmis (Platymonas) suecica (Prasinophy-
ceae); and Phaeodactylum tricornutum (Bacillariophyceae).
Cultures were maintained in filtered sea water with a
salinity of 27o/oo and at a temperature of 20 °C. TBT
exposure was either to pure TBT acetate or as plates
painted with "International TBT antifouling" with a sur-
face area of between 0.01 and 1.0 cm2 in a culture of
2 litres. Culture density was estimated every 3 to 4 days
using a Coulter counter. Exposure to the pure TBT acetate
at 1 µg/litre had no effect on any of the algal cul-
tures. Isochrysis growth was totally inhibited by painted
panels of 1.0, 0.25, and 0.125 cm2 within 2 days of
culture. Smaller panels were then used to find the limit
of the effect. A 0.02-cm2 panel was also totally toxic to
growth of the alga; panels of 0.01 cm2 allowed growth
comparable to a control culture over the first week of
culture but then inhibited growth. By the 21st day of cul-
ture, the number of cells was reduced to 1.8 x 106 cells
per ml, compared to a control density of 3.2 x 106 cells
per ml. For Chaetocerus, panels of 0.02 cm2 were toxic
to the alga from the beginning of the culture period;
numbers of cells were reduced, indicating that not only
growth but also viability was affected. With panels of
0.01 cm2, there was complete inhibition of development
over the first 4 days, followed by a decrease in cell num-
bers from the original value. The two other algal species
were less sensitive. Phaeodactylum was inhibited by panels
of 1.0 and 0.5 cm2 from the outset of culture. Inhibition
also occurred with panels of 0.25 and 0.125 cm2, but
only after several days. Tetraselmis grew in the presence
of panels of all sizes up to 1.0 cm2, though at this
exposure, growth was reduced. Exposure to panels of 0.5
cm2 had little effect. The authors also tested the effect
on algal growth of fresh water from the river feeding the
area of interest and also the effect of river sediment. In
both cases, growth of the algae was greater than growth of
controls, suggesting a greater availability of nutrients.
However, it should be noted that no analysis was made of
actual water concentrations of TBT in this study; since
the precise exposure levels are unknown, the results are
difficult to interpret or evaluate.
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: AQUATIC ORGANISMS
8.1. Aquatic plants
Summary
Few studies have been carried out on the effects of TBT on
aquatic plants. The lowest effect level was observed for
Enteromorpha intestinalis. Motile spores were inhibited from
settling by TBTO, the EC50 being 1 ng/litre; newly settled
spores increased in resistance with time. Results should be
interpreted with care because TBT concentrations were not
measured and the experimental protocols were incomplete.
Reduction in the growth of freshwater species was observed
at concentrations down to 0.06 mg/litre.
Davies et al. (1984) studied the effect of various TBT
compounds on spore development in the marine green macro-
alga, Enteromorpha intestinalis. The 5-day EC50 values
for newly-settled Enteromorpha spores ranged from
0.027 µg/litre (TBT benzoate) to 8.6 µg/litre (TBT acry-
late). The authors stated that the toxicity appeared to be
influenced by the type of anion. Using TBTO it was found
that sensitivity decreased with increasing settlement
time; the 5-day EC50 values for spore development ranged
from 0.22 µg/litre, when exposure began 30 min after
settlement, to 10 µg/litre, when exposure began 72 h
after settlement. Motile spores were the most sensitive
(5-day EC50 = 0.001 µg/litre).
The marine angiosperm Zostera marina showed reduced
growth at TBT concentrations in sediment of 1.0 mg/kg but
no effect at 0.1 mg/kg (Personal communication by M.J.
Waldock to IPCS, 1989).
Floch et al. (1964) exposed freshwater aquatic plants
to TBTO or TBT acetate, at water concentrations between
0.03 and 1.2 mg/litre, for 10 days. The duckweed Lemna
media and Canadian pondweed Elodea sp. both showed some
growth at TBTO concentrations of 0.03 mg/litre. Duckweed
maintained itself, without significant growth, at concen-
trations between 0.06 and 0.25 mg/litre, but died at 0.5
mg/litre. Elodea showed degeneration between 0.06 and 0.25
mg/litre and died at 0.5 mg/litre. Degeneration of Elodea
was evident at 0.15 mg TBT acetate/litre; growth occurred
at 0.03 mg/litre and death at 0.3 mg/litre. Lemna grew in
0.15 mg TBT acetate/litre and died at 1.2 mg/litre; there
was maintenance without growth at 0.6 mg/litre.
L.A. Boorman (personal communication to the IPCS,
1989) grew plants of two salt marsh species, Aster
tripolium and Limonium vulgare, in mud with added TBTO.
Plants of Aster were killed by sediment TBTO levels in
excess of 10 µg/kg (dry weight), while Limonium was not
significantly affected by levels of up to 150 µg/kg.
Chu (1976) found that the aquatic weed Ceratophyllum
died within 2 months exposure to a controlled release
rubber formulation containing 5% TBTO (5 mg/litre); the
exposure period being 24 h every 3 to 5 days.
8.2. Aquatic invertebrates
The acute toxicity of tributyltin to aquatic invert-
ebrates is summarized in Tables 8, 9, and 10. Larval
stages are considerably more sensitive to TBT than adults;
the LC50 for the larval Pacific oyster is 1.6 µg per
litre, over 48 h, whereas that for adults is 1800 µg per
litre (Thain, 1983). Other species show similar differ-
ences between life stages. The 96-h LC50 values for crus-
taceans range between 1.0 and 41 µg/litre. There are
fewer data on freshwater species; these relate to just
three species other than target organisms. Various TBT
salts give a range of 48-h LC50 values for Daphnia of 2.3
to 70 µg/litre and for Tubifex of 5.5 to 33 µg per
litre. The 24-h LC50 for the Asiatic clam is 2100 µg per
litre, and that for target snail adults in schistosomiasis
control is 30 to 400 µg/litre.
8.2.1. Trematode parasites of man
Some organotin molluscicides have been shown to be
toxic to schistosome larvae in the aquatic stages. Ritchie
et al. (1974) found that TBTO concentrations of 10 and
100 µg/litre rendered Schistosoma mansoni cercariae, the
infective stage released from the secondary host (water
snails), incapable of progressive movement, following a 5-
min exposure. Infectivity of the cercariae to mice was
completely suppressed. A 30-min exposure to concentrations
of 1 µg/litre or less had relatively little effect on
motility of cercariae and on subsequent infectivity of
mice. The authors also exposed S. mansoni miracidia to
TBTO and found that 10 µg/litre immobilized the miracidia
after a 40-min exposure and completely suppressed the
infectivity to snails (Biomphalaria glabrata). However, a
concentration of 1 µg/litre had no effect on motility
and infectivity after an exposure period of 120 min.
Table 8. Toxicity of tributyltin to marine invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Eastern oyster embryo statb 28 7.1 chloride 48 1.3 Roberts
(Crassostrea virginica) (0.78-1.38)d (1987)
larva 48 3.96
(2.42-4.21)d
European oyster adult statb oxide 48 >300 Thain
(Ostrea edulis) 96 210 (1983)
Pacific oyster larva statb oxide 48 1.6 Thain
(Crassostrea gigas) adult 48 1800 (1983)
adult 96 290
Mussel larva statb oxide 48 23 Thain
(Mytilus edulis) adult 48 300 (1983)
adult 96 38
Hard clam embryo statb 28 7.1 chloride 48 1.13 Roberts
(Mercenaria mercenaria) (0.72-1.31)d (1987)
larva 48 1.65d
Brown shrimp larva statb oxide 48 6.5 Thain
(Crangon crangon) larva 96 1.5 (1983)
adult 48 73
adult 96 41
Grass shrimp sub- flow 19.4- 9.8- 8.15- oxide 96 20d Walsh (1986)
(Palaemonetes pugio) adult 21.3 12.1 8.31 chloride 96 > 31d,f Bushong
et al. (1988)
Mysid shrimp < 1 day flow 24-26 19- 7.98- chloride 96 1.1 Goodman et
(Mysidopsis bahia) 22.3 8.01 (0.68-1.4)d al. (1988)
5 day 2
(1.4-2.6)d
10 day 2.2
(1.4-2.6)d
Shore crab larva statb oxide 48 110 Thain (1983)
(Carcinus maenus) 96 10
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salin- pH TBT salt Dura- LC50c Reference
age flowa ature ity tion (ug/litre)
(°C) (o/oo) (h)
---------------------------------------------------------------------------------------------------------
Harpacticoid copepod adult stat 20-22 7 7.8 fluoride 96 2 Linden et
(Nitocra spinipes) (1-2)e al. (1979)
oxide 96 2
(1-3)e
Copepod sub- flow 20 10 chloride 72 0.6 Bushong et
(Eurytemora affinis) adult (0.1-2.0)d,f al. (1987)
sub- flow 20 10 chloride 48 1.4
adult (0.8-2.3)d,f
sub- stat 19.4- 10.1- 8.17- chloride 48 2.2 Hall et al.
adult 20.3 11.2 8.32 (0.2-7.3)d,f (1988b)
sub- stat 19.4- 10.1- 8.17- chloride 72 0.6
adult 20.3 11.2 8.32 (0-3.3)d,f
Copepod sub- flow 20 10 chloride 48 1.1 Bushong et
(Acartia tonsa) adult (0.7-2.2)d,f al. (1987)
adult statb 19.5- oxide 96 1.0 U'Ren (1983)
20.5 (0.8-1.2)d
Amphipod young flow 19.4- 9.8- 8.15- chloride 96 1.3d,f Bushong et
(Gammarus sp.) adult 21.3 12.1 8.31 chloride 96 5.3d,f al. (1988)
----------------------------------------------------------------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise); flow = flow-through conditions (TBT concentration in
water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
Table 9. Toxicity of tributyltin to freshwater invertebrates
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50d Reference
age flowa ature nessc tion (ug/litre)
(°C) (mg/litre) (h)
---------------------------------------------------------------------------------------------------------
Asiatic clam larva stat 20 oxide 24 2100e Foster
(Corbicula fluminea) (1981)
Water flea adult statb 21 chloride 96 5.9 (3.7-9.4)e Meador
(Daphnia magna) adult statb 21 chloride 120 3.4 (1.3-8.8)e (1986)
acetate 48 3.3 (1.5-6.0)e,f Polster &
oleate 48 8.5 (4.2-10.5)e,f Halacka
benzoate 48 4.3 (1.8-9.5)e,f (1971)
chloride 48 4.5 (1.2-9.3)e,f
laurate 48 4.7 (1.2-9.3)e,f
oxide 48 2.3 (1.2-5.2)e,f
juvenile stat 20 200 7.5 chloride 24 13e Vighi &
juvenile stat 20 200 7.5 oxide 24 14e Calamari
(1985)
juvenile stat 19 8.2 oxide 48 4.7 RIVM
(1989)
stat 20 oxide 48 70e Foster
(1981)
Tubifex worm acetate 48 8.0 (2.8-10.3)e,f Polster &
(Tubifex tubifex) oleate 48 17.0 (10.1-30.0)e,f Halacka
benzoate 48 16.0 (10.1-27.0)e,f (1971)
chloride 48 15.0 (10.1-23.0)e,f
laurate 48 33.0 (10.6-75.0)e,f
oxide 48 5.5 (1.6-10.3)e,f
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c Hardness expressed as mg/litre CaCO3).
d 95% confidence limits are given in brackets; concentrations expressed as the TBT salt used
unless otherwise stated.
e Nominal concentration.
f Concentration expressed as TBT.
Viyanant et al. (1982) exposed Schistosoma mansoni
cercariae to TBT fluoride and calculated an LC50 of
16.8 µg/litre and an LC90 of 21.7 µg/litre for a 1-h
exposure. Following a 1-h exposure of S. mansoni to TBT
fluoride, cercarial infectivity of mice was observed over
a period of 30 min. A 100% suppression of infectivity was
found at 6 µg/litre; effective doses (99%-100%) were
between 2 and 6 µg/litre.
8.2.2. Freshwater molluscs
Summary
The LC50 values for target fresh water snail adults in
schistosomiasis control range from 30 to 400 µg/litre. This
indicates very low selectivity of TBT as a Bilharzia mollusci-
cide, and a high risk to sensitive non-target aquatic species.
The lethal concentrations of TBT to adult Bilharzia snails
are also expected to inhibit motility and infectivity of both
cercariae and miracidiae in the contaminated water, as the sup-
pressive levels were found to be 10 µg/litre for both cer-
cariae and miracidiae of Schistosoma mansoni after 5 and 40
min, respectively. The 1-h LC 50 for cercariae is 16.8 µg per
litre.
Generally, the toxicity of TBT to freshwater snails depends
on the species, stage, age, temperature, pH, time of exposure,
time of observation, suspended matter, and type of structure
and formulation.
Pellets or matrices of natural rubber or synthetic polymers
impregnated with TBT produce a slow-release effective level of
the molluscicides, which results in low acute toxicity but
extended long-term toxicity.
Exposure of adult snails to TBT levels as low as 0.01 to
0.001 µg/litre reduced egg laying, inhibited hatchability of
the exposed eggs, and retarded the development of the surviving
offspring.
The no-observed-effect level for adult freshwater snails
(Limnaea stagnalis) was 0.32 µg/litre in long-term tests.
TBT compounds are strongly adsorbed on to suspended clay
and organic particles. These particulates are ingested by the
snails and provide one of the inputs for toxicity. Studies of
the impact of type and amount of suspended matter on TBT tox-
icity to snails vary in their conclusions.
Increasing the pH was found to enhance the molluscicidal
toxicity of slow-release TBT formulations.
The acute toxicity of TBT to adult freshwater snails
is summarized in Table 10. The data are based mainly on
species that are intermediate hosts in the life-cycle of
the parasite causing Bilharzia (schistosomiasis) in man,
TBT being used as a molluscicide to control the disease.
The toxic action of TBT is slow, the mortality at the end
of a 24-h exposure is often low, and for a more realistic
result a post-exposure observation period is required.
Rubber impregnated with TBT to produce a slow-release
molluscicide that maintains a low, but toxic, concen-
tration over a long period was developed by Cardarelli and
colleagues, and was shown to be very effective against
snail pest species such as Biomphalaria glabrata and B.
globosus (Berrios-Duran & Ritchie, 1968).
Molluscicidal activity has been demonstrated against
Bulinus spp., Biomphalaria spp., and certain operculate
freshwater molluscs, but organotin compounds have proved
to be not as toxic against the amphibious oncomelaniid
snails (McCullough et al., 1980).
8.2.2.1 Acute toxicity
Webbe (1963) found that young snails (Biomphalaria
sudanica and Bulinus nasutus) were more sensitive than
adults to TBT acetate, the 24-h LC50 values being 14 and
15 µg/litre for the two snail species, respectively.
When eggs from the same two species were exposed, the 24-h
LC50 values ranged from 100 to 1000 µg/litre. Paulini
(1964) found embryos of Biomphalaria glabrata to be more
susceptible than adults to TBT acetate. The 24-h LC50
values for embryos ranged from 26 µg/litre at 5-12 h of
age to 46 µg/litre at 77-87 h of age, whereas the value
for adults was 170 µg/litre.
Table 10. Acute toxicity of tributyltin to freshwater snails
---------------------------------------------------------------------------------------
Species TBT salt Exposure Post-exposure LC50 Reference
duration observation (µg/litre)
(h) (h)
---------------------------------------------------------------------------------------
Biomphalaria oxide 6 72 410 Seiffer & Schoof (1967)
glabrata acetate 6 72 290 Seiffer & Schoof (1967)
oxide 6 24 370 Ritchie et al. (1964)
oxide 24 24 40 Ritchie et al. (1964)
acetate 6 24 190 Ritchie et al. (1964)
acetate 24 24 85 Ritchie et al. (1964)
acetate 24 100-300 Hopf et al. (1967)
pentachloro-
phenate 24 100-400 Hopf et al. (1967)
oxide 24 50-100 Hopf et al. (1967)
oxide 24 30 Deschiens et al. (1966)
acetate 24 72 170 Paulini (1964)
Biomphalaria oxide 24 30 Deschiens et al. (1966)
contortus
Biomphalaria acetate 24 48 34 Webbe (1963)
sudanica
Bulinus nasutus acetate 24 48 32 Webbe (1963)
Bulinus oxide 17 24 10 de Villiers &
tropicus MacKenzie (1963)
Limnaea oxide 24 96 60 Temmink & Everts (1987)
stagnalis oxide 96 96 24 Temmink & Everts (1987)
oxide 96 42 RIVM (1989)
---------------------------------------------------------------------------------------
8.2.2.2 Short- and long-term toxicity
Ritchie et al. (1974) found that egg laying in
Biomphalaria glabrata was completely inhibited by 10 µg
TBTO/litre, all the snails being killed within 2 to 5
days. Egg laying was reduced, over a period of 2 to 3
weeks, by more than 90% at 1 µg/litre and by 50% at
0.1 µg/litre. At 0.01 µg/litre, egg laying was unaffec-
ted. Newly-laid eggs were exposed to TBTO for 34 days
followed by 50 days in clean water. Eggs exposed to 10 µg
per litre did not hatch, even after 50 days in clean
water. Of those exposed to 1 µg/litre, only 3% hatched
during the exposure and 35% of the rest hatched after
transfer to clean water (but with a delayed hatching
time). When newly-hatched snails were exposed to TBTO, 95%
of those exposed to 1 µg/litre from hatching died and
those that survived failed to lay eggs for 85 days. At
0.1 µg/litre, 60% of the snails died and egg laying in
the survivors was reduced by 80%. Egg laying was also
significantly reduced at both 0.01 and 0.001 µg/litre.
Upatham et al. (1980) studied the toxicity to Bulinus
abyssinicus of various controlled-release organotin mol-
luscicides, i.e. BioMet SRM rubber pellets (6% TBTO),
CBL-9B rubber pellets (20% TBT fluoride), and EC-13 float-
ing ethylene propylene co-polymer pellets (30% TBT fluor-
ide). The organotin compounds killed all of the snails
within 1 to 2 days at 100 mg/litre (active ingredient) and
within 5 to 7 days at 1 mg/litre, there being no signifi-
cant difference between the compounds. Changing the test
water daily had no significant effect. At lower concen-
trations the molluscicides required 36 to 40 days to kill
all the snails at an active ingredient concentration of
0.03 mg/litre, and at 0.3 mg/litre 9 to 10 days was re-
quired for BioMet SRM and CBL-9B, and 22 days for EC-13.
In long-term exposure tests, toxic effects on fresh-
water snails have been found at very low TBT concen-
trations. Cardarelli (1973) quoted a 120-day LC100 of
7 µg TBTO/litre for Biomphalaria glabrata. At 0.7 µg per
litre, 26% of the snails died within 120 days. RIVM (1989)
reported a NOEL for the freshwater snail Limnaea stagnalis
of 0.32 µg/litre in long-term tests.
8.2.2.3 Factors affecting toxicity
Paulini & de Souza (1970) studied the effect of
various factors on the molluscicidal activity of TBT to
freshly laid eggs of Biomphalaria glabrata. A concen-
tration of colloidal clay (the proportion of clay to
molluscicide was 1000:1) of 1000 mg/litre reduced the 24-h
LC50 (with a 7-day recovery period included in the mor-
tality count) by 30% for TBT acetate and by 7% for TBTO.
The addition of a yeast ( Saccharomyces sp.) suspension
(1 g/litre) reduced the 24-h LC50 (with a 24-h recovery
period) by 95% for TBTO and 72% for TBT acetate. The pro-
portion of yeast to molluscicide was 1000:1 for TBTO and
100:1 for TBT fluoride.
Cardarelli & Evans (1980) studied the effect of vari-
ous factors on the toxicity to snails of controlled-
release organotin molluscicides, i.e. BioMet SRM (6% TBTO
in natural rubber) and CBL-9B (20% TBT fluoride in natural
rubber). Using 100-mg/kg pellets, they found that increas-
ing the pH from 6 to 8 increased the toxicity (as measured
by both LT50 and LT100) of BioMet SRM but decreased
the toxicity of CBL-9B to both Biomphalaria glabrata and
Bulinus globosus. The authors found no effect of yeast or
humic acid (1 to 100 mg/litre) on the toxicity (LT100)
of these controlled-release molluscicides, neither did
they find an effect of suspended colloidal clays. They
concluded that, although the organotin compounds are
adsorbed, they are still toxic because the snails ingest
the added materials. Therefore, snails are still exposed
to TBT even after it is adsorbed to surfaces. In an exper-
iment to compare adsorbed uptake of TBT, soil-browsing
snails and isolated snails were compared during exposure
to slow-release organotin pellets. The molluscicides were
found to be more toxic when the snails were allowed to
browse on soil, and, at a distance of 60 cm from the pel-
lets, isolated snails were producing egg masses whereas
those browsing on soil were not. The authors also con-
cluded that the organotin molecules that come into contact
with soil particles are adsorbed and slowly form ligands
of a nontoxic nature, so that soil only remains toxic as
long as it is freshly exposed to organotin. Upatham et al.
(1980) found that the presence of mud or plant life,
compared to exposure in water alone, had no effect on the
toxicity (LT100) of either CBL-9B or EC-13 at 1 or 10
mg/litre (active ingredient) to Bulinus abyssinicus. When
the snails were exposed to BioMet SRM, no effect of mud or
plants was found at 10 mg/litre, but at 1 mg/litre a
slightly shorter time was required to kill all the snails
in water alone. Chu (1976) noted that organic materials
such as mud and weeds reduced the molluscicidal activity
of TBTO on Bulinus rohlfsi, when exposed to a rubber for-
mulation containing 5% TBTO (5 mg/litre TBTO), with a 24-h
exposure period every 3 to 5 days for 70 days and twice a
month for a further 7 months.
Macklad et al. (1983) found that the toxicity of
controlled-release molluscicides containing TBT fluoride
was dependent on the pre-exposure soaking time. The 48-h
LC50 for Biomphalaria alexandrina of 10 mg/litre total
available toxicant was achieved after 7, 3, and 1 days
soaking period for the formulations EC27 (10% TBT fluoride
in a plastic polymer), EC1320 (20% TBT fluoride in rub-
ber), and EC1330 (30% TBT fluoride in a polypropylene/
polyethylene mixture), respectively. EC1330 produced no
mortality following a 3-h soaking period and exposure to
concentrations ranging from 1 to 100 mg/litre for 48-h;
the authors suggested that the polypropylene/polyethylene
mixture (EC1330) needs to be sufficiently wet before it
begins to release TBT fluoride. A second experiment to
test the aging of slow-release molluscicides was carried
out. EC27 gave 89% snail mortality during a 48-h exposure
period to 50 mg/litre after a soaking time of 24-h. After
being left to age for 1 month the molluscicide was tested
again and had lost 80% of its original toxicity.
8.2.3. Marine molluscs
Summary
A large body of data exists on the effects of TBT on marine
molluscs and in particular on commercially important bivalves.
Sublethal effects occur at very low concentrations. It has been
shown experimentally that TBT affects shell deposition of grow-
ing oysters, gonad development and gender of adult oysters,
settlement, growth, and mortality of larval oysters and of
other bivalves, and causes imposex (the development of male
characteristics) in female gastropods. The NOEL for shell
thickening of the most sensitive oyster species (C. gigas) is
about 20 ng/litre. Embryo-larval stages are more susceptible
than adults; adverse effects on larval development have been
demonstrated at concentrations as low as 50 ng/litre. The NOEL
is 20 ng/litre.
The authors of recent work on imposex in Nucella have
determined threshold concentrations by extrapolation below the
limit of reliable detection. While the circumstantial evidence
in support is substantial (see chapter 10), the determination
of toxicological thresholds below the limits of chemical detec-
tion is not practicable. It is generally agreed that imposex
is not a specific index of TBT contamination, in that it can be
induced by other factors. The wide distribution of low concen-
trations of TBT and the incidence of imposex at levels similar
to or below the analytical detection limits make long-term
controlled experiments difficult. The establishment of NOELs
will have to await the development of better analytical tech-
niques.
8.2.3.1 Acute toxicity
Waldock & Thain (1985) calculated the 24-h EC50 (mor-
tality plus moribundity) of two organotin-containing fish-
net antifouling preparations for larval oysters to be
12 µg/litre for `Norimp 200' and 320 µg/litre for
`Flexgard'. These compare with a value for TBTO of 1.7 µg
per litre. Thain (1983) pointed out that adult bivalves
tend to appear more resistant to pollutants in standard
short-term tests since they can close their shell over the
test period and thus reduce exposure. Larval stages appear
to be much more sensitive in these tests.
8.2.3.2 Short- and long-term toxicity
Alzieu et al. (1982) kept Pacific oysters (Crassostrea
gigas) in 150-litre tanks that were successively filled
and drained according to the tidal period. To these tanks
were added panels coated on one side with TBT fluoride,
giving estimated concentrations of 0.2 and 2.0 µg TBT per
litre. All oysters died within 30 days in a tank contain-
ing the larger panel. In the tank with a panel surface of
50 cm2, 30% of oysters died after 110 days of exposure
and all oysters within 170 days.
His & Robert (1985) experimentally tested hypotheses
regarding the poor performance of Pacific oysters in the
Bay of Arcachon, France. Larvae were maintained in the
laboratory at TBT acetate concentrations ranging from 0.02
to 100 µg/litre. Growth was affected by all concen-
trations except 0.02 µg/litre. The next concentration
tested (0.05 µg/litre) reduced growth, led to mortality
within 10 days, and interrupted normal feeding by day 8.
No other effects were noted at 0.02 µg/litre and this was
regarded as the NOEL for larvae (see Table 11).
Table 11. Effects of TBT acetate on Crassostrea gigas larvae at
various water concentrationsa
--------------------------------------------------------------------------------
Water concentration Effect
(µg/litre)
--------------------------------------------------------------------------------
100 inhibition of fertilization
50 inhibition of segmentation
25 partial inhibition of segmentation (40%)
10 no formation of trochophores
3 to 5 no veligers; malformed trochophores
1 abnormal veligers; total mortality within 6 days
0.5 numbers of abnormal larvae; total mortality within 8 days;
perturbation of feeding regime, particularly from 4 to 8 days
after exposure; growth greatly reduced
0.2 percentage of D larvae showing abnormalities less elevated;
perturbation of feeding regime from day 4; progressive
mortality; total by day 12; weak growth
0.1 majority of D larvae normal; marked perturbation of feeding
regime from day 6; weak growth until day 6; some survivors
after 12 days
0.05 normal D larvae; perturbation of feeding regime, marked on day
8; significant mortality beginning at day 10; reduced growth
0.02 normal D larvae; little mortality; good growth; no effect of
TBT
--------------------------------------------------------------------------------
a From: His & Robert (1985).
Thain & Waldock (1985) exposed various bivalve spat
(common European oyster, Ostrea edulis; Pacific oyster,
Crassostrea gigas; common mussel, Mytilus edulis; and
carpet shells, Venerupis decussata and Venerupis semi-
decussata) to TBT leachate by maintaining them in flowing
sea-water tanks containing house slates painted with
`Micron 25R' (containing TBT methacrylate co-polymer). The
water concentrations of TBT were 0.24 or 2.6 µg/litre. At
2.6 µg/litre, growth was completely inhibited in all
groups and mortality was high (except for V. semidecussata)
within the 45-day exposure period; all mussels had died
within 14 days. At the lower exposure concentration,
growth was significantly inhibited in C. gigas, M. edulis,
and V. decussata but not in the other two species. In a
second study, the authors reported a severe reduction in
growth rate of recently metamorphosed oyster (Ostrea
edulis) spat after exposure to TBT leachate (0.06 µg per
litre) for 10 days under static conditions. Growth rate
was reduced between 0 and 10 days of exposure to 0.02 µg
per litre, but there was only slight reduction in growth,
relative to controls, between days 10 and 20.
Growth curves for oysters (Ostrea edulis), 2-3 mm in
size, exposed to different concentrations of TBTO are
given in Fig. 3.
 Valkirs et al. (1987) calculated the 66-day LC50 of
TBT chloride for the mussel Mytilus edulis to be 0.97 µg
per litre under flow-through conditions. Beaumont & Budd
(1984) kept larvae of the common mussel, Mytilus edulis,
in filtered sea water containing TBTO concentrations of
0.1, 1.0, or 10.0 µg/litre. No larvae survived for longer
than 5 days at 10 µg/litre, or 10 days at 1.0 µg/litre.
Approximately half of the mussel larvae, at 0.1 µg per
litre, had died within 15 days. The survivors were mori-
bund, and their growth was significantly slower than that
of controls.
Laughlin et al. (1987) exposed the hard-shell clam
Mercenaria mercenaria to various concentrations of TBTO,
under static renewal conditions for larval (veliger)
stages and in flowing sea water for juveniles. They found
the post-larval settlement stages to be the least sensi-
tive. In a 25-day exposure, only juvenile clams exposed
at 10 µg/litre suffered 100% mortality, while those
exposed to 7.5 µg/litre or less showed mortality not
significantly different from that of controls. When
veligers were exposed, none survived TBTO levels of 1 µg
per litre or more for longer than 7 days, mortality being
100% within 2 days at 2.5, 5.0, and 7.5 µg/litre. At the
end of the 8-day experiment, all controls had become
pediveligers. Clams exposed to 0.6 µg/litre showed a sur-
vival level of approximately 40% of the control level, but
survivors achieved little growth and metamorphosis to
pediveligers did not occur. In another set of studies
(Laughlin et al., 1987, 1988), clams were exposed, from
fertilization to metamorphosis (approximately 14 days), to
TBTO concentrations of between 10 and 500 ng/litre. Clams
were also exposed for the first 5 days and then kept in
clean water for a further 9 days. Survival was found not
to be exposure dependent and a recovery period had no
effect. Growth was reduced at all concentrations, higher
exposure causing greater growth depression. At TBTO con-
centrations above 100 ng/litre, veligers failed to meta-
morphose to pediveligers within the 14-day exposure
period. Although the 9-day recovery period caused a slight
increase in growth, the animals were not significantly
larger than clams exposed continuously.
Pickwell & Steinert (1988) exposed adult mussels
(Mytilus edulis) and oysters (Crassostrea virginica) in a
flowing sea-water system contaminated with TBT from panels
painted with antifouling paint. The TBT concentration was
0.7 µg/litre and exposure lasted for 60 days. Haemolymph
was collected from the exposed animals and its protein
content measured. Mussel haemolymph protein content at the
end of the exposure period was 462 mg/litre, compared with
44 mg/litre in controls. Measurement of haemolymph lyso-
zyme activity and DNA content revealed no difference
between controls and treated mussels. This showed that
there had been no lysis of the haemocytes and no increase
in their numbers. Protein was not, therefore, derived from
blood cells. Oysters showed no similar effect of TBT
exposure, although haemolymph protein content was much
higher than in mussels. Over the course of the experiment,
there was approximately 50% mortality in mussels but no
deaths among the oysters.
8.2.3.3 Reproductive effects
Thain & Waldock (1986) reported the results of studies
on the reproduction of the European flat oyster (Ostrea
edulis) exposed to TBT antifouling paints. Three holding
tanks were set up each with 50 adult oysters weighing
between 50 and 70 g. One tank held controls and the other
two were exposed to TBT leaching from painted panels in a
mixing tank. A flow rate of 1 litre/min was maintained and
the two treatment tanks showed TBT concentrations of 0.24
and 2.6 µg/litre measured at the outflow. Only control
oysters released larvae during the course of the 75-day
experiment; about five million larvae were released
representing probably between four and six spawnings. At
the end of the experiment, the gonads of oysters from each
treatment were examined histologically. There were no
females in either of the treated groups of oysters (20%
females in the control group). At 2.6 µg/litre, 18 out
of 25 oysters examined were undifferentiated, 7 were male,
and none were female. There were 3 undifferentiated gonads
out of 27 at the lower exposure level. Gonadal thickness
was reduced in a dose-related manner. Mortality was low
in all groups (0 in controls and 3 and 5 in the two treat-
ment groups). Shell growth was reduced by TBT exposure;
25 oysters showed growth in the control group, 18 at
0.24 µg/litre, and 4 at 2.62 µg/litre). There were no
significant differences between the various groups of
oysters using two measures of condition (dry meat weight
compared with internal shell volume or compared with wet
meat weight). Final body burdens of TBT were 0.19, 0.40,
and 1.23 mg/kg wet meat weight for control, low-dose, and
high-dose groups, respectively.
Roberts et al. (1987) maintained adult oysters
(Crassostrea virginica) in TBT solutions containing 0.05,
0.1, 0.5, or 1.0 µg/litre for up to 8 weeks. The oysters
were brought into reproductive condition by increasing
water temperature. There were no deaths except at the
highest exposure concentration, where 20% to 30% mortality
occurred between the second and fourth weeks of exposure.
Gametes stripped from the exposed oysters were fertilized
by gametes from a reference population. There was no evi-
dence that TBT exposure had any effect on the ability of
gametes to be fertilized, and there was no statistically
significant effect of treatment on the gender of exposed
oysters over the 8-week exposure period.
8.2.3.4 Effects on growth
Waldock & Thain (1983) maintained spat of the Pacific
oyster (Crassostrea gigas) in experimental tanks, contain-
ing either TBTO, TBT and marine sediment, or just sedi-
ment, for 56 days. Weekly growth measurements (measured as
wet weight) showed enhanced weight gain in oysters exposed
to sediment alone (50 or 100 mg/litre). Low levels of TBTO
(0.15 µg/litre) inhibited growth and showed pronounced
thickening of the upper valve, and severe inhibition of
growth was noted at 1.6 µg/litre. Addition of sediment,
along with the TBT, slightly reduced the adverse effect on
oyster growth. This experiment was conducted to counter
arguments that sediment caused the effects observed in
oysters in the field.
Thain (1986) exposed Eastern oyster (Crassostrea
virginica) spat to TBTO concentrations of 0.02, 0.2, or
2.0 µg/litre under static renewal test conditions for 5
weeks. The percentage increase in growth was substantially
reduced at 2.0 µg/litre, whereas at the other two concen-
trations growth rate was similar to that of controls. No
deaths occurred and there was no evidence of shell thick-
ening or deformity in any of the treated animals.
Lawler & Aldrich (1987) exposed Pacific oyster spat to
TBTO concentrations of 0.01, 0.02, 0.05, 0.1, or 0.2 µg
per litre and monitored the average rate of oxygen con-
sumption before and after exposure. A significant negative
correlation was found between TBTO concentration and oxy-
gen consumption. There was also a significant relationship
between TBTO concentration in the water and feeding rates.
Feeding rates were measured by transmittance, which
increases as particulate food matter is removed from the
water. Change in transmittance was monitored after 1 h of
feeding. As the TBTO concentration in the water increased,
feeding rate decreased. Neither oxygen consumption nor
feeding was significantly affected below 0.05 µg/litre.
Increasing the TBTO level also progressively decreased the
ability of the oysters to compensate for hypoxia; this
effect was significant down to 0.01 µg/litre. Growth rate
(measured as average increase in valve length) was moni-
tored over a 48-day exposure period. There was a signifi-
cant negative correlation between increasing TBTO concen-
tration and growth. Growth was not significantly affected
at the lowest concentration of 0.01 µg TBTO/litre. At
levels of 0.05 µg/litre or more, there was an increased
incidence of shell thickening. However, reservations must
be expressed regarding the experimental design of this
work. The authors did not analyse TBT concentrations in
the test solutions. In addition, no analyses were carried
out on the dilution water, which may have been contami-
nated by ambient TBT, typically in excess of 10 ng/litre
(the lowest threshold determined). Furthermore, the ratio
of biomass to water was such that TBT would have been
rapidly removed from solution, so that nominal concen-
trations would have been maintained for only a few hours.
Although experimental solutions were replaced daily, the
rate of loss invalidates the threshold values cited. Simi-
lar criticisms can be made of other work using static or
static renewal systems.
Valkirs et al. (1987) exposed adults of both the
common mussel (Mytilus edulis) and Eastern oyster
(Crassostrea virginica) to TBT concentrations of 0.04,
0.13, 0.31, 0.73, or 1.89 µg/litre, for a 66-day period,
under flowing sea-water conditions. The TBT consisted of
leachate from plastic panels painted with antifouling
paint. Growth effects were measured by shell length, shell
width, and whole body wet weight (soft tissues and shell).
Since there was high mussel mortality at a TBT level of
1.89 µg/litre, growth effects were examined in animals
exposed to TBT concentrations of up to 0.73 µg/litre. No
significant effect on mussel shell width or whole body
weight was found, but a significant decrease in shell
length was observed at 0.31 and 0.73 µg/litre. More than
90% of all oysters tested within each concentration sur-
vived the test period. Statistical analysis of length,
width, and weight of oysters could not be carried out
since controls were significantly different from exposed
groups with respect to initial length of individuals. A
condition index (ratio of wet body weight to internal
shell volume) was calculated for both mussel and oyster.
No significant difference was found at any test concen-
tration for mussels. For oysters, the mean condition indi-
ces were significantly lower at concentrations of 0.73 and
1.89 µg/litre, compared both with the indices at lower
TBT concentrations and with controls.
Salazar & Salazar (1987) exposed juvenile common
mussels (Mytilus edulis), in flowing sea water, to TBT
concentrations of 70, 80, or 200 ng/litre in a 196-day
test and 40, 50, or 160 ng/litre in a 56-day test. Mussel
growth (measured as wet weight and length) was not sig-
nificantly affected up to 56 days in either test. From 63
days to the end of the experiment, there was a significant
reduction in growth at all exposure concentrations. No
significant mortality was reported in either experiment. A
group of controls kept under "field" conditions had
growth rates four times that of the laboratory controls
over a 56-day period, suggesting that bioassay conditions
were stressful for the mussels.
Stromgren & Bongard (1987) exposed juvenile mussels
(Mytilus edulis) to TBTO concentrations of 0.1 to
10 µg/litre in flowing sea water and measured shell
growth (length) at intervals of 24 to 48 h for 7 days. No
effect was observed at the lowest exposure concentration,
but at 0.4 µg/litre or more there was a significant
reduction in shell growth rate. The relationship between
TBTO concentration and growth response was approximately
hyperbolic. After 7 days of exposure, all groups treated
with 0.4 µg/litre or more showed growth rates of approxi-
mately 25% (or less) of the control value. The highest
exposure concentration reduced growth to approximately 5%
of the control value.
8.2.3.5 Shell thickening
Alzieu et al. (1982) reported that adult oysters
(Crassostrea gigas) developed gel centres in the shell
when they were exposed to TBT fluoride at a concentration
of 0.2 µg/litre.
Thain et al. (1987) exposed spat of the Pacific oyster
(Crassostrea gigas) to TBT concentrations of 2 to 200
ng/litre for 49 days. No effect on shell thickness was
observed in controls or at 2 ng TBT/litre. Between 20 and
200 ng/litre, there was a dose-related increase in shell
thickness. Severe "balling" occurred at both 100 and 200
ng/litre (where the overall appearance of the oyster shell
is spherical rather than having the flattened profile of
one valve of a normal oyster) (Fig. 4).
8.2.3.6 Imposex
The phenomenon of "imposex" was first observed in
the field (see section 10.2), the term being used to
describe the development of male characteristics by female
gastropods. The females develop a penis and ultimately
become infertile. Stages in the development of imposex are
illustrated in Fig. 5.
Smith (1981a) collected female mud snails (Nassarius
obsoletus) from three localities designated "dirty",
"intermediate", and "clean" on the basis of the degree
of imposex noted in the field (see section 10.2). A piece
of filter paper with 1.5 to 1.8 g of dried antifouling
paint containing TBT and lead arsenate (Alumacide) was
placed in 110-litre tanks, and the snails were exposed for
75 days. Halfway through the exposure period, the filter
paper was removed because the snails became lethargic. At
the end of the exposure period, all snails exposed to the
Alumacide had developed significantly more intense impo-
sex. Snails from the "clean" area showed an increase in
imposex incidence from 0% to 14.3%, whereas levels of
imposex remained constant in snails from "dirty" or
"intermediate" areas (> 95% incidence). Penis expression
in snails from "dirty" and "intermediate" areas
regressed significantly when they were transferred to
clean water. However, the extent to which the effects
observed in this study can be attributed to TBTO is
unclear, since the antifoulant contained two biocides and
no analytical measurements were made.
Valkirs et al. (1987) calculated the 66-day LC50 of
TBT chloride for the mussel Mytilus edulis to be 0.97 µg
per litre under flow-through conditions. Beaumont & Budd
(1984) kept larvae of the common mussel, Mytilus edulis,
in filtered sea water containing TBTO concentrations of
0.1, 1.0, or 10.0 µg/litre. No larvae survived for longer
than 5 days at 10 µg/litre, or 10 days at 1.0 µg/litre.
Approximately half of the mussel larvae, at 0.1 µg per
litre, had died within 15 days. The survivors were mori-
bund, and their growth was significantly slower than that
of controls.
Laughlin et al. (1987) exposed the hard-shell clam
Mercenaria mercenaria to various concentrations of TBTO,
under static renewal conditions for larval (veliger)
stages and in flowing sea water for juveniles. They found
the post-larval settlement stages to be the least sensi-
tive. In a 25-day exposure, only juvenile clams exposed
at 10 µg/litre suffered 100% mortality, while those
exposed to 7.5 µg/litre or less showed mortality not
significantly different from that of controls. When
veligers were exposed, none survived TBTO levels of 1 µg
per litre or more for longer than 7 days, mortality being
100% within 2 days at 2.5, 5.0, and 7.5 µg/litre. At the
end of the 8-day experiment, all controls had become
pediveligers. Clams exposed to 0.6 µg/litre showed a sur-
vival level of approximately 40% of the control level, but
survivors achieved little growth and metamorphosis to
pediveligers did not occur. In another set of studies
(Laughlin et al., 1987, 1988), clams were exposed, from
fertilization to metamorphosis (approximately 14 days), to
TBTO concentrations of between 10 and 500 ng/litre. Clams
were also exposed for the first 5 days and then kept in
clean water for a further 9 days. Survival was found not
to be exposure dependent and a recovery period had no
effect. Growth was reduced at all concentrations, higher
exposure causing greater growth depression. At TBTO con-
centrations above 100 ng/litre, veligers failed to meta-
morphose to pediveligers within the 14-day exposure
period. Although the 9-day recovery period caused a slight
increase in growth, the animals were not significantly
larger than clams exposed continuously.
Pickwell & Steinert (1988) exposed adult mussels
(Mytilus edulis) and oysters (Crassostrea virginica) in a
flowing sea-water system contaminated with TBT from panels
painted with antifouling paint. The TBT concentration was
0.7 µg/litre and exposure lasted for 60 days. Haemolymph
was collected from the exposed animals and its protein
content measured. Mussel haemolymph protein content at the
end of the exposure period was 462 mg/litre, compared with
44 mg/litre in controls. Measurement of haemolymph lyso-
zyme activity and DNA content revealed no difference
between controls and treated mussels. This showed that
there had been no lysis of the haemocytes and no increase
in their numbers. Protein was not, therefore, derived from
blood cells. Oysters showed no similar effect of TBT
exposure, although haemolymph protein content was much
higher than in mussels. Over the course of the experiment,
there was approximately 50% mortality in mussels but no
deaths among the oysters.
8.2.3.3 Reproductive effects
Thain & Waldock (1986) reported the results of studies
on the reproduction of the European flat oyster (Ostrea
edulis) exposed to TBT antifouling paints. Three holding
tanks were set up each with 50 adult oysters weighing
between 50 and 70 g. One tank held controls and the other
two were exposed to TBT leaching from painted panels in a
mixing tank. A flow rate of 1 litre/min was maintained and
the two treatment tanks showed TBT concentrations of 0.24
and 2.6 µg/litre measured at the outflow. Only control
oysters released larvae during the course of the 75-day
experiment; about five million larvae were released
representing probably between four and six spawnings. At
the end of the experiment, the gonads of oysters from each
treatment were examined histologically. There were no
females in either of the treated groups of oysters (20%
females in the control group). At 2.6 µg/litre, 18 out
of 25 oysters examined were undifferentiated, 7 were male,
and none were female. There were 3 undifferentiated gonads
out of 27 at the lower exposure level. Gonadal thickness
was reduced in a dose-related manner. Mortality was low
in all groups (0 in controls and 3 and 5 in the two treat-
ment groups). Shell growth was reduced by TBT exposure;
25 oysters showed growth in the control group, 18 at
0.24 µg/litre, and 4 at 2.62 µg/litre). There were no
significant differences between the various groups of
oysters using two measures of condition (dry meat weight
compared with internal shell volume or compared with wet
meat weight). Final body burdens of TBT were 0.19, 0.40,
and 1.23 mg/kg wet meat weight for control, low-dose, and
high-dose groups, respectively.
Roberts et al. (1987) maintained adult oysters
(Crassostrea virginica) in TBT solutions containing 0.05,
0.1, 0.5, or 1.0 µg/litre for up to 8 weeks. The oysters
were brought into reproductive condition by increasing
water temperature. There were no deaths except at the
highest exposure concentration, where 20% to 30% mortality
occurred between the second and fourth weeks of exposure.
Gametes stripped from the exposed oysters were fertilized
by gametes from a reference population. There was no evi-
dence that TBT exposure had any effect on the ability of
gametes to be fertilized, and there was no statistically
significant effect of treatment on the gender of exposed
oysters over the 8-week exposure period.
8.2.3.4 Effects on growth
Waldock & Thain (1983) maintained spat of the Pacific
oyster (Crassostrea gigas) in experimental tanks, contain-
ing either TBTO, TBT and marine sediment, or just sedi-
ment, for 56 days. Weekly growth measurements (measured as
wet weight) showed enhanced weight gain in oysters exposed
to sediment alone (50 or 100 mg/litre). Low levels of TBTO
(0.15 µg/litre) inhibited growth and showed pronounced
thickening of the upper valve, and severe inhibition of
growth was noted at 1.6 µg/litre. Addition of sediment,
along with the TBT, slightly reduced the adverse effect on
oyster growth. This experiment was conducted to counter
arguments that sediment caused the effects observed in
oysters in the field.
Thain (1986) exposed Eastern oyster (Crassostrea
virginica) spat to TBTO concentrations of 0.02, 0.2, or
2.0 µg/litre under static renewal test conditions for 5
weeks. The percentage increase in growth was substantially
reduced at 2.0 µg/litre, whereas at the other two concen-
trations growth rate was similar to that of controls. No
deaths occurred and there was no evidence of shell thick-
ening or deformity in any of the treated animals.
Lawler & Aldrich (1987) exposed Pacific oyster spat to
TBTO concentrations of 0.01, 0.02, 0.05, 0.1, or 0.2 µg
per litre and monitored the average rate of oxygen con-
sumption before and after exposure. A significant negative
correlation was found between TBTO concentration and oxy-
gen consumption. There was also a significant relationship
between TBTO concentration in the water and feeding rates.
Feeding rates were measured by transmittance, which
increases as particulate food matter is removed from the
water. Change in transmittance was monitored after 1 h of
feeding. As the TBTO concentration in the water increased,
feeding rate decreased. Neither oxygen consumption nor
feeding was significantly affected below 0.05 µg/litre.
Increasing the TBTO level also progressively decreased the
ability of the oysters to compensate for hypoxia; this
effect was significant down to 0.01 µg/litre. Growth rate
(measured as average increase in valve length) was moni-
tored over a 48-day exposure period. There was a signifi-
cant negative correlation between increasing TBTO concen-
tration and growth. Growth was not significantly affected
at the lowest concentration of 0.01 µg TBTO/litre. At
levels of 0.05 µg/litre or more, there was an increased
incidence of shell thickening. However, reservations must
be expressed regarding the experimental design of this
work. The authors did not analyse TBT concentrations in
the test solutions. In addition, no analyses were carried
out on the dilution water, which may have been contami-
nated by ambient TBT, typically in excess of 10 ng/litre
(the lowest threshold determined). Furthermore, the ratio
of biomass to water was such that TBT would have been
rapidly removed from solution, so that nominal concen-
trations would have been maintained for only a few hours.
Although experimental solutions were replaced daily, the
rate of loss invalidates the threshold values cited. Simi-
lar criticisms can be made of other work using static or
static renewal systems.
Valkirs et al. (1987) exposed adults of both the
common mussel (Mytilus edulis) and Eastern oyster
(Crassostrea virginica) to TBT concentrations of 0.04,
0.13, 0.31, 0.73, or 1.89 µg/litre, for a 66-day period,
under flowing sea-water conditions. The TBT consisted of
leachate from plastic panels painted with antifouling
paint. Growth effects were measured by shell length, shell
width, and whole body wet weight (soft tissues and shell).
Since there was high mussel mortality at a TBT level of
1.89 µg/litre, growth effects were examined in animals
exposed to TBT concentrations of up to 0.73 µg/litre. No
significant effect on mussel shell width or whole body
weight was found, but a significant decrease in shell
length was observed at 0.31 and 0.73 µg/litre. More than
90% of all oysters tested within each concentration sur-
vived the test period. Statistical analysis of length,
width, and weight of oysters could not be carried out
since controls were significantly different from exposed
groups with respect to initial length of individuals. A
condition index (ratio of wet body weight to internal
shell volume) was calculated for both mussel and oyster.
No significant difference was found at any test concen-
tration for mussels. For oysters, the mean condition indi-
ces were significantly lower at concentrations of 0.73 and
1.89 µg/litre, compared both with the indices at lower
TBT concentrations and with controls.
Salazar & Salazar (1987) exposed juvenile common
mussels (Mytilus edulis), in flowing sea water, to TBT
concentrations of 70, 80, or 200 ng/litre in a 196-day
test and 40, 50, or 160 ng/litre in a 56-day test. Mussel
growth (measured as wet weight and length) was not sig-
nificantly affected up to 56 days in either test. From 63
days to the end of the experiment, there was a significant
reduction in growth at all exposure concentrations. No
significant mortality was reported in either experiment. A
group of controls kept under "field" conditions had
growth rates four times that of the laboratory controls
over a 56-day period, suggesting that bioassay conditions
were stressful for the mussels.
Stromgren & Bongard (1987) exposed juvenile mussels
(Mytilus edulis) to TBTO concentrations of 0.1 to
10 µg/litre in flowing sea water and measured shell
growth (length) at intervals of 24 to 48 h for 7 days. No
effect was observed at the lowest exposure concentration,
but at 0.4 µg/litre or more there was a significant
reduction in shell growth rate. The relationship between
TBTO concentration and growth response was approximately
hyperbolic. After 7 days of exposure, all groups treated
with 0.4 µg/litre or more showed growth rates of approxi-
mately 25% (or less) of the control value. The highest
exposure concentration reduced growth to approximately 5%
of the control value.
8.2.3.5 Shell thickening
Alzieu et al. (1982) reported that adult oysters
(Crassostrea gigas) developed gel centres in the shell
when they were exposed to TBT fluoride at a concentration
of 0.2 µg/litre.
Thain et al. (1987) exposed spat of the Pacific oyster
(Crassostrea gigas) to TBT concentrations of 2 to 200
ng/litre for 49 days. No effect on shell thickness was
observed in controls or at 2 ng TBT/litre. Between 20 and
200 ng/litre, there was a dose-related increase in shell
thickness. Severe "balling" occurred at both 100 and 200
ng/litre (where the overall appearance of the oyster shell
is spherical rather than having the flattened profile of
one valve of a normal oyster) (Fig. 4).
8.2.3.6 Imposex
The phenomenon of "imposex" was first observed in
the field (see section 10.2), the term being used to
describe the development of male characteristics by female
gastropods. The females develop a penis and ultimately
become infertile. Stages in the development of imposex are
illustrated in Fig. 5.
Smith (1981a) collected female mud snails (Nassarius
obsoletus) from three localities designated "dirty",
"intermediate", and "clean" on the basis of the degree
of imposex noted in the field (see section 10.2). A piece
of filter paper with 1.5 to 1.8 g of dried antifouling
paint containing TBT and lead arsenate (Alumacide) was
placed in 110-litre tanks, and the snails were exposed for
75 days. Halfway through the exposure period, the filter
paper was removed because the snails became lethargic. At
the end of the exposure period, all snails exposed to the
Alumacide had developed significantly more intense impo-
sex. Snails from the "clean" area showed an increase in
imposex incidence from 0% to 14.3%, whereas levels of
imposex remained constant in snails from "dirty" or
"intermediate" areas (> 95% incidence). Penis expression
in snails from "dirty" and "intermediate" areas
regressed significantly when they were transferred to
clean water. However, the extent to which the effects
observed in this study can be attributed to TBTO is
unclear, since the antifoulant contained two biocides and
no analytical measurements were made.
 Feral & Le Gall (1983) attempted to identify which
part of the neuroendocrine system of a marine gastropod,
Ocenebra erinacea, was primarily affected in the
induction, by TBT, of the development of a penis in female
snails. A biological assay was established using isolated
female pedal ganglia or complete nervous system (intact
complex of pedal ganglia and cerebropleural ganglia) of
Ocenebra erinacea and isolated presumptive penis-forming
areas of a second species Crepidula fornicata. When pedal
ganglia were cultured with the presumptive penis-forming
area in a medium based on either clean or "polluted" sea
water (from areas known to have the imposex phenomenon in
the field) there was no penis development. Culturing the
whole nervous system in a medium based on clean sea water
also resulted in no penis growth. However, culturing the
complete nervous system with a medium based on "pol-
luted" sea water caused the growth of a penis. Culturing
in artificial sea water with added TBT (0.2 µg per litre)
also induced penis development. The authors concluded that
the primary effect of TBT is on the cerebropleural ganglia
of the snails (Fig. 6).
In studies by Bryan et al. (1986), the dogwhelk
Nucella lapillus was exposed to TBT concentrations of
0.02 µg tin/litre in tidal tanks, the TBT being leached
from a co-polymer antifouling paint. Within 4 months,
animals of both sexes had accumulated 1 mg tin/kg (as
TBT), and females showed a high degree of imposex, which
was still increasing. The authors stated that the exper-
iment tended to underestimate the exposure, since the diet
of barnacles was initially uncontaminated but would later
have contributed TBT to the dogwhelks via an extra route.
Gibbs et al. (1987) found that after 12 months of exposure
to 18.7 ng tin/litre, penis size in female dogwhelks was
increased, and that there was very little difference in
size between the sexes. The "control" dogwhelks in this
study were actually exposed to TBT at 1.5 ng/litre, which
was the background concentration in the sea water used.
These control females showed a penis bulk of 10-14% of
that of control males. Field observations on populations
living in < 0.5 ng/litre showed little penis development
(between 2% and 5% of male penis bulk). The NOEL for the
development of imposex is, therefore, less than 1.0 ng
TBT/litre.
Gibbs et al. (1988) reared dogwhelks in the laboratory
for 2 years from hatching in various concentrations of TBT
leached from antifouling paints. TBT concentrations in the
water were monitored at 1-2, 3-5, 20, and 100 ng tin/litre
and tissue concentrations in the whelks were also
measured. All exposed females were affected at all concen-
trations, developing a penis. Penis development (expressed
as a relative size index compared to males exposed to the
same TBT concentrations) in females was between 50 and 60%
of male penis bulk after exposure for 1 and 2 years to TBT
at a level of 1-2 ng tin/litre. The female penis reached
comparable size to that of the male on exposure to TBT
levels of 3-5 ng tin/litre or more. At increasing TBT
exposure concentrations, further male characteristics
developed and further female characteristics were
repressed. At TBT concentrations in the water of 1-2 ng
tin/litre, some females retained the capacity to breed
although others were sterilized by oviduct blockage. At
3-5 ng/litre, virtually all females were sterilized but
oogenesis was apparently normal. At 10 ng/litre, oogenesis
was suppressed, oocytes were resorbed, and spermatogenesis
was initiated. At 20 ng/litre, there was a functional
testis in the "females", with ripe sperm in the most-
affected animals.
Feral & Le Gall (1983) attempted to identify which
part of the neuroendocrine system of a marine gastropod,
Ocenebra erinacea, was primarily affected in the
induction, by TBT, of the development of a penis in female
snails. A biological assay was established using isolated
female pedal ganglia or complete nervous system (intact
complex of pedal ganglia and cerebropleural ganglia) of
Ocenebra erinacea and isolated presumptive penis-forming
areas of a second species Crepidula fornicata. When pedal
ganglia were cultured with the presumptive penis-forming
area in a medium based on either clean or "polluted" sea
water (from areas known to have the imposex phenomenon in
the field) there was no penis development. Culturing the
whole nervous system in a medium based on clean sea water
also resulted in no penis growth. However, culturing the
complete nervous system with a medium based on "pol-
luted" sea water caused the growth of a penis. Culturing
in artificial sea water with added TBT (0.2 µg per litre)
also induced penis development. The authors concluded that
the primary effect of TBT is on the cerebropleural ganglia
of the snails (Fig. 6).
In studies by Bryan et al. (1986), the dogwhelk
Nucella lapillus was exposed to TBT concentrations of
0.02 µg tin/litre in tidal tanks, the TBT being leached
from a co-polymer antifouling paint. Within 4 months,
animals of both sexes had accumulated 1 mg tin/kg (as
TBT), and females showed a high degree of imposex, which
was still increasing. The authors stated that the exper-
iment tended to underestimate the exposure, since the diet
of barnacles was initially uncontaminated but would later
have contributed TBT to the dogwhelks via an extra route.
Gibbs et al. (1987) found that after 12 months of exposure
to 18.7 ng tin/litre, penis size in female dogwhelks was
increased, and that there was very little difference in
size between the sexes. The "control" dogwhelks in this
study were actually exposed to TBT at 1.5 ng/litre, which
was the background concentration in the sea water used.
These control females showed a penis bulk of 10-14% of
that of control males. Field observations on populations
living in < 0.5 ng/litre showed little penis development
(between 2% and 5% of male penis bulk). The NOEL for the
development of imposex is, therefore, less than 1.0 ng
TBT/litre.
Gibbs et al. (1988) reared dogwhelks in the laboratory
for 2 years from hatching in various concentrations of TBT
leached from antifouling paints. TBT concentrations in the
water were monitored at 1-2, 3-5, 20, and 100 ng tin/litre
and tissue concentrations in the whelks were also
measured. All exposed females were affected at all concen-
trations, developing a penis. Penis development (expressed
as a relative size index compared to males exposed to the
same TBT concentrations) in females was between 50 and 60%
of male penis bulk after exposure for 1 and 2 years to TBT
at a level of 1-2 ng tin/litre. The female penis reached
comparable size to that of the male on exposure to TBT
levels of 3-5 ng tin/litre or more. At increasing TBT
exposure concentrations, further male characteristics
developed and further female characteristics were
repressed. At TBT concentrations in the water of 1-2 ng
tin/litre, some females retained the capacity to breed
although others were sterilized by oviduct blockage. At
3-5 ng/litre, virtually all females were sterilized but
oogenesis was apparently normal. At 10 ng/litre, oogenesis
was suppressed, oocytes were resorbed, and spermatogenesis
was initiated. At 20 ng/litre, there was a functional
testis in the "females", with ripe sperm in the most-
affected animals.
 Bryan et al. (1988) investigated the capacity of vari-
ous organotin compounds to induce imposex in the dogwhelk.
Whelks, already slightly affected by imposex, were taken
from the wild and exposed to 200 ng/litre tin, in the form
of TBT chloride, tri- n- propyl tin (TPrT), tetrabutyltin
(TTBT), dibutyltin (DBT), or triphenyltin (TPhT), for 14
days before being returned to the shore. Penis size, as a
percentage of male penis size, increased to 44% in females
exposed to TBT chloride, compared with 6% in controls.
TPrT increased relative penis size to only 14%, and no
other compound had any effect. In an attempt to eliminate
differences due to differential uptake of the compounds,
the organotin compounds were injected in a second exper-
iment. The females were maintained in the laboratory for
up to 105 days. TBT again induced increased penis size but
TTBT also showed an effect (19.5% compared to the 34%
shown after TBT injection). Other compounds were ineffec-
tive. The authors believed the effect of TTBT to be caused
by contamination by TBT and conversion to TBT in the tis-
sues. However, the increased imposex after treatment with
TPrT could not be explained in this way and the imposex
effect is considered to be not totally specific to tri-
butyltin.
8.2.3.7 Genotoxicity
Dixon & Prosser (1986) found that TBTO was not geno-
toxic, at concentrations of 0.05 to 5.0 µg tin/litre, to
the larvae of the mussel Mytilus edulis. Results were
based on chromosome analysis and sister chromatid
exchange (SCE). In 4-day acute toxicity studies, TBTO was
found to cause a dose-dependent reduction in both larval
survival and development of mussels. Survival ranged from
46% of controls at 0.05 µg/litre to 1.4% at 5 µg/litre.
The percentage of animals reaching the D-shell stage of
development was 8.7% of controls at the lowest dose, but
none reached this stage at either 1 or 5 µg/litre. How-
ever, when mussel larvae were exposed to a standard
mutagen (mitomycin C) or crude oil, in the presence of
TBTO, the SCE frequency increased to approximately twice
that found when larvae were exposed to either toxicant in
isolation (Dixon & McFadzen, 1987).
8.2.4. Crustaceans
Summary
Most of the available data for freshwater organisms are
derived from acute exposure tests with Daphnia. LC 50 data for
similar exposure times are variable (up to 2 orders of magni-
tude) probably due to age differences in the populations
studied. The NOEL for Daphnia has been estimated to be around
0.5 µg/litre, behaviour being the most sensitive parameter.
A range of marine species from copepods to crabs and lob-
sters has been studied, the lowest NOEL being 0.09 µg per
litre for reproductive effects in mysid shrimp.
The presence of sediment in test aquaria greatly reduces
the toxic effect of TBTO on estuarine crustaceans.
8.2.4.1 Acute effects
Using TBTO and TBT acetate, Floch et al. (1964) calcu-
lated LC100 and LC0 values for various species of fresh-
water invertebrates. The lethal concentrations of TBTO to
Daphnia magna over 24 and 72 h were 0.12 mg/litre and
0.06 mg/litre, respectively. Another aquatic crustacean,
Cypridopsis hartwigi, was less sensitive with lethal con-
centrations of 4 mg/litre for a 24-h exposure, 2 mg/litre
for a 48-h exposure, and 0.12 mg/litre for a 96-h
exposure. NOELs were 0.03 and 0.06 mg/litre for Daphnia
and Cypridopsis, respectively. For TBT acetate, the
LC100 was 0.15 mg/litre for a 72-h exposure of Daphnia
and 0.15 mg/litre for a 96-h exposure of Cypridopsis. The
LC0 was 0.075 mg/litre for both species.
However, more recent work has found Daphnia to be con-
siderably more sensitive to TBT. Meador (1986) reported a
96-h LC50 for Daphnia magna of 5.9 µg/litre and noted
that TBT is a slow-acting toxicant for Daphnia with
effects only being shown after 96 h or more of exposure.
Polster & Halacka (1971) quoted 48-h LC50 values for
Daphnia magna using various TBT salts, which ranged from
2.2 µg TBTO/litre to 8.5 µg TBT oleate/litre.
RIVM (1989) reported a 48-h EC50 for Daphnia of
4.7 µg/litre and a NOEL, over the same time period, of
0.56 µg/litre.
Davidson et al. (1986) calculated the 96-h LC50 to
be 0.42 µg/litre after exposing the mysid shrimp
Acanthomysis sculpta to a leachate of TBT.
When Walsh (1986) exposed the mole crab Emerita
talpoida to concentrations of 10 µg TBTO/litre of sea
water or 4500 µg/kg of sand, no effect on crab survival
was observed after 7 days of exposure. In continuous-flow
bioassays, 10 000 µg TBTO/kg of sediment did not kill
grass shrimp after a 96-h exposure.
8.2.4.2 Short- and long-term toxicity
U'Ren (1983) maintained the marine copepod Acartia
tonsa in solutions containing TBTO, under static con-
ditions, and calculated a 144-day LC50 of 0.55 µg per
litre; by combining moribundity and mortality as end-
points, the 144-day EC50 was found to be 0.4 µg/litre.
Laughlin et al. (1983) maintained mud crab larvae
(Rhithropanopeus harrisii), from hatching, in solutions
containing either TBTO at 0.5 to 25 µg/litre or TBT
sulfide at 0.5 to 50 µg/litre, under static renewal pro-
cedures. The survival of the zoeae, up to 15 days, was
unaffected by TBTO at levels up to 10 µg/litre. At 15 µg
per litre, 84% successfully moulted to the megalopa stage,
but at 5 µg TBTO/litre only 37% survived. Zoeal sur-
vival was unaffected by concentrations of TBT sulfide up
to 5 µg/litre. Survival at 20, 30, and 50 µg/litre was
78%, 26%, and 4%, respectively. The development rate, over
the same time period, decreased with increasing TBT con-
centration, although this was not statistically signifi-
cant below 10 µg TBTO/litre or 20 µg TBT sulfide/litre.
At the highest exposure concentrations (10 µg TBTO/litre
and 20 µg TBT sulfide/litre), metamorphosis was delayed
by approximately 2 days in the case of TBTO and 6 days for
TBT sulfide. Growth (measured as mean wet weight) was
significantly reduced at TBTO concentrations of 15 and
25 µg/litre or TBT sulfide concentrations of 20, 30, and
50 µg/litre, and showed dose dependency. Daily growth was
monitored for up to 12 days at concentrations (of both
TBTO and TBT sulfide) of 0.5, 1.0, and 5.0 µg per litre.
Although the final weights were not significantly differ-
ent, all TBT treatments caused an initial growth lag
during the first three days of exposure. Laughlin et al.
(1985) found the LC50 for exposure of zoeae of
Rhithropanopeus harrisii to TBTO during the 12 days of
zoeal development to be 55 nmol/litre.
Laughlin & French (1980) exposed shore crabs
(Hemigrapsus nudus), 2 to 3 days after hatching, to TBTO
(as "Biomet") for up to 14 days under static conditions.
At the highest concentrations (500 and 1000 µg/litre),
all the zoeae died within 2 days. Survival time increased
as the concentration of TBTO decreased from 100 to 25 µg
per litre; most larvae died within 8 days even at 25 µg
per litre. The estimated values of LT50 for 100, 75, 50,
and 25 µg/litre were 3.4, 4.8, 5.8, and 6.2 days,
respectively. Lobster larvae (Homarus americanus) were
much more sensitive; 100% being killed within 24 h and
2 days by 20 and 15 µg TBTO/litre, respectively. Concen-
trations of 10 and 5 µg/litre killed all larvae within
5 to 6 days. In the group exposed to 1 µg/litre, there
was a similar mortality pattern to the controls and high
mortality at the first ecdysis (3 to 5 days post-hatch).
However, in this group only a single larva metamorphosed
successfully, compared with 43% in the control group.
Davidson et al. (1986) kept juvenile mysid shrimps
(Acanthomysis sculpta), newly-released from the female,
in TBT concentrations of between 0.03 and 0.48 µg per
litre for a 63-day period under flow-through conditions.
The TBT source was leachate from panels coated with anti-
fouling paint. All animals died within 7 days at the
higher exposure level. There was no significant difference
in shrimp mortality between those exposed at levels up to
0.38 µg/litre and the controls, either at 22 or 41 days.
From day 41 to the end of the test (63 days), survival
decreased at 0.38 µg/litre and only 22.5% survived to the
end of the experiment, compared with 60% in the control
group. The authors stated that this indicated a lowering
of the NOEL for the entire life cycle of A. sculpta from
0.38 µg/litre to 0.25 µg/litre at 41 days. The increase
in mortality coincided with the release of juveniles by
the females, which indicated a sensitive time in the life
cycle. Both mean length and weight of females, at a TBT
level of 0.38 µg/litre, were significantly reduced after
63 days of exposure, but no effect of TBT concentrations
up to and including 0.38 µg/litre was observed in males.
A similar result was found in another study after 28 days
at 0.49 µg/litre; again no effect on length or weight was
found in males. There was a significant effect on the
length of developing juveniles and sub-adults. After 14
days of exposure to either 0.19 or 0.33 µg/litre, mysids
were significantly shorter; this was also the case at
0.2 µg/litre after 27 days. At TBT concentrations up to
and including 0.33 µg/litre, there was no effect on the
number of juveniles released per individual female, the
number of individuals in unhatched broods, or the number
of days from hatching of a female to the release of its
juveniles. However, there was a significant reduction in
the number of viable juveniles released at both 0.19 µg
per litre and 0.33 µg/litre. The authors suggest a NOEL
of 0.09 µg/litre for reproduction, the most sensitive
parameter found in the study.
Laughlin et al. (1984) exposed the Baltic amphipod
Gammarus oceanicus to TBTO or TBT fluoride concentrations
of 0.3 or 3.0 µg/litre for 8 weeks, under 48-h static
renewal conditions. They also exposed the amphipods to
leachates from TBT-containing antifouling paints, by
placing a 1 cm2 plexiglass plate, which was painted with
either "Micron 25" or "Interracing", in the tank for
5 weeks. Again the water was changed every 48 h. TBT
levels in the experiment with pure compounds, remained
constant, but the TBT concentration (measured as TBTO)
from the leachates gradually increased over each 48-h
exposure period to approximately 5.5 µg/litre for
"Interracing" and 0.5 µg/litre for "Micron 25",
reflecting the different leaching rates of the two com-
pounds. At the highest concentration of both TBTO and TBT
fluoride, 50% of the adults died within 10 to 12 days of
the exposure and all had died within 16 days (TBTO) or 33
days (TBT fluoride). The TBT paint "Interracing" caused
100% mortality within 1 week. The lower concentration of
the exposures to pure compounds and "Micron 25" resulted
in mortality patterns that were not dependent on TBT con-
centration but on senility. The exposures to 0.3 µg per
litre and "Micron 25" had significantly reduced the
number of surviving larvae by the end of the experiment.
Slight decreases in larval growth were observed after
exposure to TBTO and "Micron 25" but, generally, concen-
trations of less than 1 µg TBT/litre had little effect
on growth and no effect on whole animal oxygen consumption
rates.
Clark et al. (1987) monitored the survival of the
grass shrimp (Palaemonetes pugio) in water and water/sedi-
ment test aquaria after the addition of TBTO. When added
to water and tested in the absence of sediment, TBTO gave
96-h LC50 values comparable to other published results at
20 µg/litre. However, when the TBTO was admixed with
sediment rather than water, the LC50 could not be deter-
mined. TBT levels in sediment at 1 mg/kg in static and
10 mg/kg in flow-through tests showed no effects on shrimp
survival. Similar tests using Amphioxus gave an LC50 for
sediment containing TBTO of between 1 and 10 mg/kg over 4
and 10 days. The animals were killed by 10 µg TBT per
litre in water.
8.2.4.3 Reproductive effects
Hall et al. (1988b) maintained egg-carrying females of
the copepod Eurytemora affinis in TBT chloride at levels
of 0.1 or 0.5 µg/litre for up to 13 days. After 3 days
of exposure, the higher concentration had significantly
reduced the mean brood size to 0.2, compared with 15.2 in
controls. Neonate survival was significantly reduced at
0.1 µg/litre after 6 days (22% of control survival). No
offspring survived the higher exposure concentration. In a
second experiment, mean brood size, after 2 days of
exposure, was not significantly affected at concentrations
of between 12.5 and 200 ng/litre. Neonate survival, after
13 days, was unaffected by concentrations up to and
including 50 ng/litre; survival at 100 ng/litre was 76%
(compared to 22% survival at the same exposure level, in
the first experiment, over 6 days) and was further reduced
to 24% at 200 ng TBT/litre.
When Johansen & Mohlenberg (1987) exposed fertilized
mature female copepods (Acartia tonsa) to TBTO, egg pro-
duction was significantly reduced at the highest exposure
level of 0.1 µg/litre after 72 h. After 120 h, all
exposure concentrations had significantly reduced egg
production by 18%, 19%, and 37% relative to controls, at
TBTO concentrations of 0.01, 0.05, and 0.1 µg per litre,
respectively.
8.2.4.4 Limb regeneration
Weis et al. (1987a,b) exposed the fiddler crab (Uca
pugilator) to TBTO concentrations of 0.5, 5.0, or 50 µg
per litre under static renewal conditions. Regeneration
(autotomy) of 1 chela and 5 walking legs was induced by
pinching them off at the merus. Although some growth
retardation was observed, the most striking effect was the
development in regenerated limbs of deformities, e.g.,
backward curling or complete absence of the dactyl of the
claw, chelae or walking legs, stunted, unjointed, or bent
in the wrong direction. The percentage of males exhibiting
deformities was 17% at 0.5 µg/litre, 24% at 5 µg per
litre, and 67% at 50 µg/litre, the test solution being
changed twice weekly. In a second experiment, where the
test solution was changed three times weekly, the percent-
age deformities were as follows: males, 100% at both 5 and
50 µg/litre; females, 29% at 5 µg/litre and 100% at
50 µg/litre. There were no deformities in the control
groups during the experiments.
8.2.4.5 Behavioural effects
When Pinkney et al. (1985) gave the grass shrimp
(Palaemonetes pugio) a choice between TBTO-contaminated
water and clean water, it did not avoid total organic tin
concentrations of 2.3 to 30 µg/litre. The response data,
at both 2.3 and 30 µg/litre, were very similar.
Meador (1986) reported the effects of TBT chloride on
the photobehaviour of water fleas (Daphnia magna). The
normal response of Daphnia to a unidirectional light
source is to swim away from the light; this is an adaptive
response to avoid predators. The author reported that
Daphnia exposed to TBT chloride showed a reversal of this
behaviour and swam towards the light source (usually with
considerably increased swimming intensity). The threshold
concentration of TBT chloride causing this behavioural
reversal was 0.5 µg/litre (the LC50 over the same time
period was between 3.5 and 6 µg/litre).
8.2.5. Other aquatic invertebrates
Summary
Few studies have been carried out on other species. The
lowest effect level in annelids was observed for Nereis
diversicolor; mortality and behavioural effects were seen after
chronic exposure to 2 µg/litre. Limb regeneration was signifi-
cantly inhibited in a brittle star after exposure to 0.1 µg
per litre. Experiments have been carried out on four insect
species. The most sensitive was Notonectes, the LC0 being
0.03 mg/litre and the LC50 0.06 mg/litre.
8.2.5.1 Acute effects
Walsh et al. (1986a) exposed larvae of the lugworm
Arenicola cristata to either TBTO or TBT acetate, for 96
or 168 h, respectively. The concentrations that killed
100% of the animals were: 4 µg/litre (96 h) for TBTO;
10 µg/litre (96 h) and 5 µg/litre (168 h) for TBT acet-
ate. At 5 µg TBT acetate/litre, all larvae were abnormal
after 96 h of exposure. No deaths or abnormalities re-
sulted from exposure to 2 µg TBTO/litre for up to 168 h.
When Beaumont et al. (1987) exposed adult polychaetes
(Nereis diversicolor) to TBT in flowing sea water for up
to 22 days, there was 100% mortality by 22 days at 4 µg
per litre and 55% mortality at 2 µg/litre (20% control
deaths). Eversion of the proboscis was a more sensitive
indication of toxicity. No controls showed this effect,
whereas 75% of animals exposed to 2 µg/litre showed
everted proboscis after 22 days (50% after 20 days of
exposure).
Dragonfly larvae (Aeschna) sp. showed a 48-h LC100 of
0.25 mg TBTO/litre and an LC0 of 0.12 mg TBTO/litre.
Corresponding values for TBT acetate were 0.5 mg/litre
(72-h LC100) and 0.025 mg/litre (LC0). Notonectes sp.
yielded LC0 values of 0.03 mg/litre for both TBTO and TBT
acetate and 72-h LC100 values of 0.06 and 0.15 mg/litre
for TBTO and TBT acetate, respectively. Chironomid (midge)
larvae revealed a NOEL of 0.075 mg/litre for TBT acetate
and a 48-h LC100 of 0.15 mg/litre (Floch et al., 1964).
Cardarelli (1978) studied the efficacy of controlled-
release organotin compounds as mosquito larvicides. The
toxicity (LT100) of BioMet (6% TBTO in natural rubber),
CBL-9B (20% TBT fluoride in natural rubber), and
ECOPRO-1230 and ECOPRO-1330 (both are ethylene propylene
polymers containing 30% TBT fluoride) were tested against
larvae of the mosquito Culex quinquefasciatus. For BioMet
and CBL-9B, LT100 values ranged from 4 to 9 days for
toxicant concentrations of 0.1 to 10 mg/kg of pellet added
to the water, the toxicity increasing with increasing
toxicant concentration. A degradation product, TBT carbon-
ate, was found to be less toxic, the LT100 being 9 days
after exposure to 10 mg/litre. When the polymers were
tested at toxicant concentrations ranging from 0.9 to 32.6
mg/kg, toxicity was found to increase with increasing
toxicant concentration (LT100s range from 2 days to 16
days). Although initially toxicity tended to decrease as
the soaking time of the pellet increased, prior to
exposure, between days 100 and 500 of soaking toxicity
remained relatively unchanged. The authors observed that
the organotin compounds dramatically slowed or even
prevented morphogenesis.
8.2.5.2 Limb regeneration
In a study on limb regeneration, Walsh et al. (1986b)
maintained the brittle star Ophioderma brevispina in TBTO
concentrations of 0.01, 0.1, or 0.5 µg/litre under flow-
through conditions. On the first day of the experiment,
autotomy was induced in two arms, at opposite sides of the
disc, by pinching midway between disc and arm tip. The
animals were then exposed for four weeks to TBTO. There
were no deaths and no effect on disc diameter. Both 0.1
and 0.5 µg/litre significantly inhibited regeneration of
arms as measured by length; both groups showed average and
median lengths less than those in the lowest-dose group,
but variability precluded statistical significance. Aver-
age weights of limbs were also reduced in the groups
exposed to 0.1 and 0.5 µg/litre, but statistical analysis
was not carried out because pooled weights did not provide
enough data.
8.3. Fish
Summary
The acute toxicity of tributyltin to marine and freshwater
fish is highly variable, LC 50 values ranging from 1.5 to
240 µg/litre. It is unclear whether this is due to inherent
differences in sensitivity or to differences in route of
exposure. Many of the acute toxicity studies need to be inter-
preted with care, since the TBT concentrations cited are often
nominal and biologically available concentrations were not
measured.
In long-term toxicity tests, the NOEL for general toxico-
logical parameters (growth and behaviour) for trout yolk-sac
fry was greater than 0.2 µg/litre, and for medaka it was
3.2 µg/litre. Using histopathological parameters, the NOEL for
medaka was found to be 0.32 µg/litre, vacuolation of the reti-
nal epithelium being the most sensitive parameter.
Few embryotoxicity tests have been performed and it has not
been possible to establish a NOEL.
8.3.1. Acute effects
The 96-h LC50 of TBTO for marine fish ranges between
1.5 and 36 µg/litre (Table 12). Larvae seem to be more
sensitive than adults in those few studies examining dif-
ferent life stages in the same test. Fewer LC50 values
have been published for freshwater fish; they range from
13 to 240 µg/litre.
8.3.2. Short- and long-term toxicity
Matthiessen (1974) measured a lethal threshold concen-
tration of TBTO for Tilapia mossambica of between 8 and
16 µg/litre (24-h LC50, 28 µg/litre; LC5, 24 µg per
litre; LC90, 33 µg/litre) as a preliminary to studies
at sublethal concentrations. A temporary opacity of the
surface of the eyes developed at concentrations below this
threshold (> 8 µg/litre) but disappeared after a few
days. Other symptoms included sluggishness and difficult-
ies with balance. Melanophores in the skin were found to
be constricted, giving treated fish a paler appearance
than controls. The fish did not produce behaviourally
related display patterns, an activity important in the
species. As with the eye opacity, these other symptoms
disappeared after a few days or weeks, suggesting that the
fish develop some tolerance to the TBTO. The growth of
Tilapia exposed to 0, 5, or 8 µg TBTO/litre was moni-
tored over a 5-week period. These concentrations of TBTO
were estimated, on the basis of release rates, in water
after the TBTO had been used to control mollusc vectors
for schistosomiasis. There was no difference in growth
between fish exposed to 5 µg/litre and controls. Tilapia
exposed to 8 µg/litre showed negative growth and lost
about 6% of body weight over the experimental period. Eye
opacity was only found in the fish exposed to 8 µg per
litre, but this effect did not seem to affect feeding
behaviour. The only other change in the high-dose group
was an increase in aggressive encounters between males.
There was an increased reluctance amongst attacked males
to avoid conflict, which led to the death of several indi-
viduals. Fatal engagements between males are normally very
rare in this species.
When Seinen et al. (1981) exposed rainbow trout (Salmo
gairdneri) yolk-sac fry to TBT chloride concentrations of
0.2, 1, or 5 µg/litre for up to 110 days, all fry died
within 10 to 12 days (at the transition from yolk-sac fry
to the swimming fry stage) at 5 µg/litre, but there were
no deaths at lower doses. The major histopathological
change in fish that died after exposure to 5 µg per litre
was hydropic degeneration of tubule segments of the pro-
nephros. At 0.2 and 1 µg/litre, there was a dose-related
retardation of growth, resulting in a 44% decrease in body
weight (relative to controls) in the 1 µg/litre group
after 110 days. At the end of the experiment, both remain-
ing groups showed significant reductions in the haemo-
globin titre in the blood and in body weight. Only at the
higher concentration was there a significant decrease in
blood cell number. Relative liver weight was significantly
increased at both concentrations but relative numbers of
thymus cells were unaffected. The authors also measured
the relative area distribution of the various hepatic com-
partments in liver sections. The area occupied by nuclei
significantly increased at both concentrations, whereas
glycogen storage area decreased, but this was significant
only at the highest exposure level. Cytoplasmic area was
unaffected, as were all non-parenchymal compartments.
Table 12. Toxicity of tributyltin to fish
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salinity pH TBT salt Dura- LC50c Reference
age flowa ature (o/oo) tion (µg/
(°C) (h) litre)
---------------------------------------------------------------------------------------------------------
Marine and estuarine species
Sheepshead minnow sub-adult flow 19.4- 9.8- 8.15- chloride 48 >31d,f Bushong
(Cyprinodon variegatus) 21.3 12.1 8.31 72 28.1 et al. (1988)
(24-36.5)d,f
96 25.9
(22.8-30.1)d,f
Bleak 8 cm stat 10 7 7.8 fluoride 96 6-8e Linden et
(Alburnus alburnus) oxide 96 15 al. (1979)
(13-17)e
Inland silverside larva flow 20 10 chloride 48 7.7 Bushong et
(Menidia beryllina) (5.5-10.9)d,f al. (1987)
72 4.6
(3.3-6.2)d,f
Atlantic silverside sub-adult flow 20 10 chloride 48 12.7 Bushong et
(Menidia menidia) (7.8-15.2)d,f al. (1987)
72 9.3
(7.1-12.4)d,f
96 8.9
(6.7-11.6)d,f
Atlantic menhaden juvenile flow 20 10 chloride 48 6.8 Bushong et al. (1987)
(Brevoortia tyrannus) (4.1-infinite)d,f
72 5.2
(3.7-6.5)d,f
96 4.5
(3.6-6.4)d,f
Sole larva statb oxide 48 8.5 Thain (1983)
(Solea solea) larva 96 2.1
adult 48 88
Armed bullhead adult statb oxide 48 26 Thain (1983)
(Agonus cataphractus) 96 16
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Girella 2.4 g statb 19.9- 7.8- oxide 48 5.2d Kakuno &
(Girella punctata) 20.5 8.1 96 3.2d Kimura (1987)
Saltwater goby statb 20.2- 32.5- 8.1- oxide 24 12d Shimizu &
(Chasmichthys dolichognathus) 21.0 32.8 8.3 48 9d Kimura (1987)
96 4d
Chinook salmon juvenile stat 3-5 28 oxide 6 54d Short &
(Oncorhynchus tshawytscha) 12 20d Thrower (1987)
96 1.5d
Mummichog larvae flow 19.4- 9.8- 8.15- chloride 48 >32.2d,f Bushong et
(Fundulus heteroclitus) larvae 21.3 12.1 8.31 72 28.2 al. (1988)
(15.1-infinite)d,f
larvae 96 23.4
(15.2-30.9)d,f
sub-adult 48 >32.2d,f
sub-adult 72 28.3
(23.4-43.2)d,f
sub-adult 96 23.8
(20.8-28)d,f
Freshwater species
Rainbow trout yearling stat 18 250 oxide 24 28e Alabaster
(Salmo gairdneri) yearling statb 18 250 oxide 48 21e (1969)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Guppy 3-4 weeks statb 23 oxide 96 21 RIVM
(Lebistes reticulatus) chloride 168 21 (1989)
(16-29)e,f Polster &
oleate 168 33 Halacka
(19-60)e,f (1971)
benzoate 168 25
(19-32)e,f
laurate 168 30
(18-50)e,f
acetate 168 28
(21-33)e,f
oxide 168 39
(28-50)e,f
Medaka 4-5 weeks statb 23 oxide 96 17 RIVM
(Oryzias latipes) (1989)
Stickleback 4-5 weeks statb 19 oxide 96 13 RIVM
(Gasterosteus aculeatus) (1989)
Carp oxide 24 75 Temmink &
(Cyprinus carpio) oxide 96 32 Everts
(1987)
Golden orfe oxide 48 50e Plum
(Leuciscus idus melanotus) napht- 48 70e (1981)
henate
Bluegill sunfish stat 20 oxide 96 240e Foster
(Lepomis macrochirus) (1981)
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
g Hardness expressed as mg CaCO3/litre.
Pinkney et al. (1988) exposed 13- and 16-day-old
larvae of striped bass (Morone saxatilis) to varying con-
centrations of TBT from methacrylate-painted panels for 6
to 7 days. They found significant reductions in survival
at measured concentrations of 0.766 µg TBT/litre or more.
At lower exposure concentrations (0.067 µg/litre), growth
parameters changed in the 13-day-old larvae only. No
changes occurred in the growth parameters of 16-day-old
larvae exposed to 0.444 µg/litre or less. The authors did
not know whether this apparent difference in sensitivity
between larvae of different ages was a true effect or
simply the result of differences between the various
batches of larvae.
Wester et al. (1988) reported NOELs for the medaka
(Oryzias latipes) of 3.2 µg TBTO/litre for general toxi-
cological parameters (mortality, growth, general appear-
ance, abnormal behaviour) and 0.32 µg/litre for histo-
pathological effects during 1 month of exposure. The most
sensitive histopathological effect was the development of
vacuolation in the retinal epithelium of the eye. There
was a dose-related increase in hepatocellular vacuolation,
with swelling in pronounced cases. The vacuoles appeared
to be glycogen deposits, although, at higher doses, lipid
vacuoles were also noted. The NOEL for liver effects was
1 µg TBTO/litre. Kidney effects mostly involved the
tubule and included dilation, epithelial atrophy, degener-
ation and regeneration, and proteinaceous casts including
cellular debris (tubulonephrosis). Severe cases also
showed glomerular effects. Similar lesions were reported
after 3 months of exposure, with, in addition, effects on
the swim bladder, skin, oral cavity, and thyroid gland at
the highest concentration tested (10 µg/litre). Thymus
atrophy, reported by the same authors in the guppy, was
not found in the medaka, indicating some species speci-
ficity regarding the effects of TBT. Increased glycogen in
liver and muscle after TBTO treatment was demonstrated
analytically in the medaka, confirming histochemical
suggestions of increased glycogen in the guppy.
Shimizu & Kimura (1987) exposed the marine goby
Chasmichthys dolichognathus to TBTO in short (4 day) and
long (12 week) experiments, and reported a 96-h LC50 of
4 µg/litre. Long-term exposure to 2.1 µg/litre during
the season of gonadal recrudescence led to a significant
depression of the gonadosomatic index in male fish. There
was no effect on female fish in terms of gonadosomatic
index or ovarian histology.
8.3.3. Embryotoxicity
Newton et al. (1985) exposed eggs, embryos, and larvae
of the California grunion (Leuresthes tenuis) to plates
painted with 9.4% TBT methacrylate (0.5% TBTO; 44.7% cu-
prous oxide; 45.5% inert ingredient) and aged for 30 days
in flowing sea water. The authors claimed that the ex-
posure to concentrations of between 0.14 and 1.72 µg TBT
per litre had no adverse effect on hatching, growth, or
development. In fact the presence of TBT at such concen-
trations enhanced both hatching success and stimulated
growth. A concentration of 10 µg TBT/litre had no effect
on hatchability, but at 74 µg/litre hatching success was
reduced by approximately 50%. The authors observed no
effect on the survival of larvae, hatched from eggs
exposed to concentrations of 0.14 to 1.72 µg/litre, up to
7 days post-hatch. The presence of copper, however, should
be taken into consideration in the interpretation of these
results.
Weis et al. (1987a) reported considerable variation in
the response of embryos of the killifish (Fundulus
heteroclitus) to concentrations of TBTO ranging between 3
and 30 µg/litre. Two batches of embryos developed abnor-
malities at the highest concentration of TBTO tested
(30 µg/litre) and showed some mortality. Other batches of
embryos showed embryotoxicity for all tested concen-
trations (down to 3 µg/litre) but no abnormalities in the
embryos. Owing to this difference in sensitivity of dif-
ferent batches of eggs and the separation of embryotoxic
and teratogenic effects, it is difficult to establish a
NOEL for this species.
Fent (1989b) exposed fertilized eggs of the freshwater
minnow Phoxinus phoxinus to two TBT concentrations in
petri dishes in an environment chamber. The eggs were ex-
posed from the blastula stage (20 to 24 h after spawning),
and the water was renewed daily. Analytical determination
of TBT showed that the actual concentration had reduced
over the 24-h exposure period from 1.5 to 0.5 µg/litre in
the low-dose group and from 8.4 to 7.9 µg/litre in the
high-dose group. There were no differences in survival
between the control and low-dose groups. Hatching was nor-
mal but some fish showed vertebral malformations. At the
high dose, hatching was delayed or reduced and all hatched
larvae had severe vertebral malformations; many were
unable to uncurl. These larvae remained motionless at the
bottom of the dish. Some had oedema in the region of the
heart. Within 3 to 4 days of hatching, all of the larvae
in the high-dose group had died. A further series of
experiments similarly showed malformation at an exposure
level of 4.5 µg TBT/litre.
8.3.4. Behavioural effects
The avoidance response of the mummichog (Fundulus
heteroclitus) to TBTO has been studied by Pinkney et al.
(1985). When given a choice between TBTO-contaminated and
clean water, 4 out of 6 groups of fish avoided a total
organic tin level of 1 µg/litre. There were significant
avoidance responses for all test groups at total organic
tin levels of 3.7, 8, and 13.8 µg/litre. However, the
higher concentrations of tin did not result in an increase
in avoidance response. The authors found that, under the
same conditions, no avoidance response was shown by the
grass shrimp (see section 8.2.4.5). This shrimp is a major
food item of the mummichog in tidal marshes. Hall et al.
(1984) studied the avoidance response of two species of
juvenile estuarine fish, the striped bass (Morone
saxatilis) and Atlantic menhaden (Brevoortia tyrannus). An
avoidance response to TBTO was exhibited by bass at a
total organic tin concentration of 24.9 µg per litre.
Atlantic menhaden were more sensitive, showing a "mild"
avoidance at 5.5 µg/litre and a "strong" avoidance at
9.1 µg/litre.
Chliamovitch & Kuhn (1977) measured an EC50 for the
loss of positive rheotaxis of 30.8 µg/litre for the rain-
bow trout (Salmo gairdneri) and 53.2 µg/litre for a
tilapia (Tilapia rendalli).
8.4. Amphibians
Summary
There are few data on the effects of tributyltin on amphib-
ians. Survival of frog larvae was at least 80% of control
levels after exposure to 3 µg TBT/litre.
Floch et al. (1964) reported a NOEL for mortality of
tadpoles of two species of amphibians (Rana and Alytes sp.)
of 30 µg/litre. For both species, the 24-h LC100 for
TBTO was 75 µg/litre, the 48-h LC100 50 µg/litre, and
the 48-h LC100 for TBT acetate was 75 µg/litre.
Laughlin & Linden (1982) exposed eggs of the frog Rana
temporaria to concentrations of either TBTO or TBT fluor-
ide of 0.3, 3, or 30 µg/litre, for 5 days from the post-
gastrula stage of development, under static renewal con-
ditions. All surviving larvae hatched on either day 4 or
5 of exposure. Survival was at least 80% of control levels
after exposure to 0.3 or 3 µg/litre. Only at the highest
concentration (30 µg/litre) did TBT affect survival (40%
mortality with TBT fluoride and 50% mortality with TBTO).
The authors collected all surviving tadpoles and measured
wet and dry weight. Wet weights were significantly lower
in tadpoles exposed to 30 µg/litre but unaffected at
lower exposure concentrations. Tadpoles exposed to TBT
always showed higher mean dry weight than controls,
although increases were not consistently dose dependent.
The percentage of body water declined from 86-88.5% for
controls to 73.8% after exposure at the highest concen-
tration of TBT. The changes resulting from TBT treatment
were significant, but not the differences produced by the
different TBT compounds.
8.5. Multispecies studies
Summary
The few available microcosm studies indicate that the most
sensitive organism(s) within the microcosm are affected by TBT
at concentrations greater than 0.05 µg/litre. Recovery of
these organisms occurred a few months after exposure. Simul-
taneous exposure of snails and fish yields conflicting results,
snails appearing somewhat more sensitive than fish.
Beaumont et al. (1987) exposed sandy-substrate micro-
cosms of flowing sea water to TBT derived from slate
panels painted with "Micron 25" antifouling paint. Three
replicate microcosms were exposed for 4 months at high
(1-3 µg/litre) and at low (0.06-0.17 µg/litre) TBT con-
centrations in water, together with three control micro-
cosms. Flow rates were maintained by gravity from a header
tank at approximately 1 litre/min; the painted panels were
located in the header tank where water was passed over
them using a circulating pump. The substrate sediment was
sieved after collection (to 2 mm) to remove larger organ-
isms and allowed to settle in the microcosms for 3 weeks
before the addition of animals. The sediment was 10 cm
deep and the overlying water 15 cm deep in each microcosm.
At the beginning of the trial, 50 specimens of a bivalve
(Cerastoderma edule), a crustacean (Corophium volutator),
and two polychaetes (Nereis diversicolor and Cirratulus
cirratus) were introduced. After 4 weeks, 12 specimens of
the gastropod Littorina littorea were added and, after 6
weeks, 26 specimens of a second bivalve (Scrobicularia
plana) were added. Other species were found at the end of
the trial derived from small (< 2 mm) juveniles in the
sediment and inflowing sea water. Each day, 10 litres of a
suspension of the microalga Pavlova lutheri (5 x 106 cells/ml)
were added to the header tank as additional food supply.
The most sensitive of the introduced species was the
cockle (C. edule); 100% died within 2 weeks at the high
concentration of TBT and there was cumulative mortality at
the low TBT concentration over 17 weeks (14% of controls
died). Two-way analysis of variance indicated significant
differences between all three groups (i.e. control, and
low and high concentrations of TBT; p < 0.05). The other
bivalve (S. plana) showed high mortality at the highest
exposure level (100% after 10 weeks). At the low TBT con-
centration there were no time-related deaths in this
species and no significant difference from controls.
Among the polychaetes, high death rates were recorded
for N. diversicolor in all microcosms, including the
control, possibly because adults introduced were ripe and
died after spawning during the experiment. The other
polychaete (C. cirratus) survived well even after exposure
to the high concentration of TBT. Only one gastropod (L.
littorea) died in any of the microcosms. Juvenile bivalves
were the most common species found; their numbers and
diversity were lower in the low-dose microcosms (66 to
109) than in control microcosms (137 to 179), and they
were virtually absent from the high-dose microcosms (0, 1,
and 1 for the 3 replicates). The size of juvenile mussels
(Mytilus edulis) was also significantly reduced at the
low TBT concentration, relative to controls; other self-
introduced bivalve species were not affected by the low
TBT exposure. Measurements of chlorophyll a in sediment
cores, as a measure of algal biomass, indicated a signifi-
cant rise after exposure to high TBT levels. This is
explained by the relative insensitivity of algae to the
toxic effects of TBT and by reduced (or non-existent)
animal life available to consume the algae. The authors
emphasize the sensitivity of some species, for which TBT
is toxic at levels of 1 to 2 µg/litre under conditions
approximating natural exposure. They further emphasize
the great variation in sensitivity between species; for
example, while Mytilus edulis and Cerastoderma edule were
clearly affected at very low levels of TBT, other related
bivalves were largely unchecked. Differences in feeding
behaviour, leading to different effective exposure levels,
is offered as one possible explanation for differences in
response. Differential absorption or subsequent loss,
observed in laboratory studies with other molluscs, is
also proposed as a possible mechanism.
Henderson (1986) conducted long-term flow-through
microcosm studies on communities of marine organisms from
Pearl Harbour, Hawaii, where the organisms had been
exposed to leaching TBT from naval ships for some time.
Panels (20 x 25 cm) of roughened plexiglass were suspended
in the harbour for 14 weeks prior to the experiment to
allow settlement and growth of organisms, and 30 different
species were found on the panels. Prior to exposure, the
panels were kept for a further 5 weeks in the experimental
tanks. These tanks had a capacity of 155 litre and were
supplied with sea water pumped directly from the harbour
and through the tanks at a rate of 4 litres/min. The sea
water was passed through a 1 cm mesh with no further
filtration; organisms could, therefore, enter the tanks
easily and settle on the panels. The water was rich in
plankton consisting largely of diatoms, copepods, and
chaetognaths. Exposure to TBT derived from painted panels
treated with antifouling paint containing 9.4% TBT meth-
acrylate, 0.5% TBTO, 44.7% cuprous oxide, and 45.4% inert
ingredients. Nominal concentrations of TBT were 0, 0.05,
0.13, 0.31, 0.78, and 1.95 µg/litre, but actual mean
concentrations were 0.01, 0.04, 0.10, 0.54, 1.77, and
2.52 µg/litre, respectively. The measured copper levels
in the same samples were 1.0, 1.2, 1.2, 3.1, 4.4, and
5 µg/litre, respectively. Changes in coverage of the
panels was assessed using photographs taken with an under-
water camera at weekly or biweekly intervals throughout
the experimental period of approximately 60 days of
exposure and a further 60 days of recovery. One week after
the start of exposure, a further plastic panel was intro-
duced to monitor new colonization. There was a clear
decline in both total number of species and in species
diversity (reductions of 55%, 60%, and 80% at the three
highest exposure levels, respectively) on pre-fouled
panels exposed to TBT at 0.5, 1.8, and 2.5 µg/litre but
no effect at lower concentrations. The mortality of indi-
vidual species, in relation to TBT exposure, showed con-
siderable variation. The most sensitive of the six most
common species on the panels was Botrylloides spp, an
orange-coloured colonial tunicate, which showed 100% mor-
tality at TBT concentrations of 0.1 µg/litre or more. An
encrusting bryozoan, Schizoporella errata, showed 49% mor-
tality at 0.1 µg/litre and 80 to 100% mortality at higher
concentrations. Specimens of a second colonial tunicate,
Didemnus candidum, and the saddle oyster ( Anomis nobilis)
were all killed at concentrations of 0.5 µg/litre or
more. A tube worm (Hydroides elegans) and a solitary
tunicate (Ascidia spp.) survived even the highest
exposure level, though with considerable mortality at 1.8
and 2.5 µg/litre. Recovery of populations was complete
60 days after cessation of treatment. Settlement on the
panels not previously exposed was reduced by TBT concen-
trations of 0.1 µg/litre or more, but not by 0.04 µg per
litre. Algal settlement and growth on the walls of the
tanks was not affected by exposure to TBT at any concen-
tration; coralline algae increased their coverage of pre-
fouled panels. A total of 18 oysters (Crassostrea
virginica) placed in each tank prior to treatment was
monitored after 60 days. There was 50% mortality after
TBT exposure at 1.8 µg/litre but no significant reduction
in survival at lower concentrations. However, the con-
dition index of the oysters was affected by exposure to
0.1 µg/litre or more, but had returned to near normal
after 2 months recovery in clean water. The author
regarded 0.05 µg/litre as a reliable NOEL for the most
sensitive organisms exposed.
When Salazar & Salazar (1985) exposed copepods
(Acartia tonsa), mysids (Acanthomysis sculpta), and fish
(Citharichthys stigmaeus) to suspended sediment at a TBTO
concentration of 0.49 µg/litre water for 96 h, no sig-
nificant mortality was found. In a second experiment,
mysids (A. sculpta), worms (Neanthes arenaceodentata), and
clams (Macoma nasuta) were exposed to TBTO-contaminated
sediment with overlying water for 10 days in the case of
mysids and 20 days in the case of clams and worms. Levels
of TBTO in the sediment varied from 155 to 610 µg per kg,
falling during the exposure period, while measured levels
in the overlying water were about 0.2 µg/litre. No mor-
tality was observed during the exposure period.
Cardarelli (1973) exposed snails (Biomphalaria
glabrata) and fish (Lebistes reticulatus) to slow-
releasing molluscicides containing either TBTO or TBT
fluoride. At a daily release rate of 35 µg TBTO/litre,
100% mortality was achieved in snails within 60 days but
all of the fish also died within this period. At a TBTO
release rate of 7 µg/litre per day, snail mortality was
100% after 120 days exposure and fish mortality only 2%.
However, a second experiment gave a much higher fish mor-
tality of 46% after 120 days exposure to only 3.5 µg TBTO
per litre per day. Exposure to TBT fluoride at 7 µg per
litre per day killed all snails and fish within 60 days,
and 52% of fish and 100% of snails were killed after 90
days of exposure to 3.5 µg/litre per day.
In order to simulate the effect of molluscicides con-
taining 6% TBTO on biota, Jordan (1985) set up a model
ecosystem as follows: filtered river water was allowed to
flow into a tray containing washed mud (to simulate a
marsh), and overflow from this upper tank was allowed to
flow into a lower tank containing washed coarse sand (to
simulate a river). Snails (Biomphalaria glabrata and
Potamopyrgus coronatus) and guppies (Lebistes reticu-
latus) were added to the "marsh", while snails (B.
glabrata and Pomacea glauca), shrimps (Macrobrachium
faustinum), and L. reticulatus were added to the
"river". The molluscicide was added to the "marsh" as
pellets at levels of 2, 5, 10, or 20 g/m2 of marsh sur-
face. The percentage of both B. glagrata and L. reticu-
latus surviving 14 days of exposure was recorded (see
Table 13). The authors concluded that, in this model eco-
system, there was higher mortality of B. glabrata than of
L. reticulatus at low doses of slow-release TBTO, i.e. 2
and 5 g of pellet per m2 of "marsh".
Table 13. Percentage of B. glabrata and L. reticulatus surviving
14 days exposure to slow-release pellets containing 6% TBTO added
to the "marsh" in a "marsh" or "river" model ecosystema
----------------------------------------------------------------
Dose of B. glabrata L. reticulatus
molluscicide
"marsh" "river" "marsh" "river"
----------------------------------------------------------------
20 g/m2 00.0* 00.0* 00.0* 00.0*
(0/240) (0/240) (0/240) (0/240)
10 g/m2 00.0* 00.0* 00.0* 2.5*
(0/240) (0/240) (0/240) (6/240)
5 g/m2 00.0* 16.3** 23.3** 60.0+
(0/240) (39/240) (56/240) (144/240)
2 g/m2 52.9+ 75.4++ 75.8++ 78.8
(127/240) (181/240) (182/240) (189/240)
----------------------------------------------------------------
a From: Jordan (1985).
The percentages represent percent survival at various doses
for all weeks of exposure in replicate experiments. The
values marked with the same symbol are not statistically
different from each other at the 0.05 probability level. The
numbers in parentheses represent the number of survivors
compared to the number of animals exposed.
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: TERRESTRIAL ORGANISMS
Summary
Exposure of terrestrial organisms to TBT derives primarily
from its use as a wood preservative. However, little infor-
mation is available. TBTO has proved toxic to honey-bees coming
into close contact with TBTO-treated wood. TBT compounds are
toxic to insects exposed either topically or via feeding on
treated wood. The acute toxicity to wild small mammals is mod-
erate (between 37 and 240 mg/kg per day). The toxicity of TBTO
to bats is probable but not proven. There is no information on
other species.
9.1. Microcosm studies
Gile et al. (1982) introduced TBTO into a terrestrial
microcosm on pine posts, each microcosm containing four
posts (3.3 x 2.6 x 14 cm) treated with 14C-labelled TBTO
(167 mg/cm3). The microcosms consisted of soil and
endogenous soil organisms, ryegrass, earthworms, pillbugs
(woodlice), mealworms, crickets, garden snails, and a
gravid female gray-tailed vole. There were no effects on
any of the organisms over a period of 77 days. About 95%
of the TBTO remained in the posts for the whole of the
exposure period. A similar system set up to investigate
cricket mortality, with and without predation, showed no
effect of TBTO (Gillett et al., 1983).
9.2. Terrestrial insects
When Gardiner & Poller (1964) exposed larvae of the
common clothes moth (Tineola bisselliella) to wool treated
with a TBTO concentration of 1% by weight, all of the
exposed larvae were killed within the exposure period of
14 days. The action appeared to be that of a contact
insecticide because none of the cloth was eaten. Phenyltin
compounds were not as toxic as TBTO. Baker & Taylor (1967)
found that the action of TBTO more closely resembled the
slow toxicity of a stomach insecticide after exposing
wood-boring beetles (Lyctus brunneus) to impregnated wood.
Contact insecticides such as lindane ( gamma-hexachlorocyclo-
hexane) and dieldrin were 100 times more toxic than TBTO.
The LD50 of TBTO for another wood-boring species, Anobium
punctatum, was 0.254 kg/m3 (application rate to wood).
Saxena & Crowe (1988) applied TBTO, TBT chloride, and
TBT linoleate topically to the thorax of newly-emerged
insects of three species. The LD50 values ranged from
0.48% to 0.72% (dilutions with acetone) for the house fly
Musca domestica, 0.29% to 0.69% for the mosquito
Anophelese stephensi, and 0.52% to 0.87% for the cotton
stainer Dysdercus cingulatus. TBT compounds were more
toxic than the other organotin compounds tested (triphe-
nyltin, tricyclohexyltin, dimethyltin, phenyltin, and
diethyltin). The authors pointed out that TBT compounds
are considerably less toxic than trimethyltin compounds to
Musca sp.
In studies by Kalnins & Detroy (1984), wood was
treated with TBTO (1.9 kg/m3) after sawing and before
use in the construction of beehives. Five hives made from
the treated wood were stocked with bees. Tin residues of
3.24 mg/kg were found in bees during the first summer, and
residues of 8.67 mg/kg were found in the wax of the combs.
However, there were no detectable tin residues in honey.
There was high mortality in the bee colonies over winter,
only one colony surviving. No control colonies died over
winter. Residues in bees and wax in the second year (1.3
and 4.6 mg/kg, respectively) were lower than in the first
year in the one surviving colony.
9.3. Terrestrial mammals
Racey & Swift (1986) housed pipistrelle bats
(Pipistrellus pipistrellus) in roosting cages treated
with TBTO. The bats were pregnant females collected from
nursery roosts, and they were trained to feed on mealworms
before transfer to the experimental cages. The cages were
made of metal, lined with plywood, and were painted with
TBTO as a 1% solution in white spirit (the manufacturers
recommended rate for use of TBTO as a fungicide). The wood
was treated 2 months before the bats were introduced into
the cages. During the course of the 142-day experiment,
seven of the ten bats died. Median survival time was 100
days, with a range between 49 and 142 days. Two deaths in
the white spirit control group and three in the untreated
control group meant that results for TBTO were not stat-
istically significant.
Schafer & Bowles (1985) conducted toxicity and repel-
lency tests on deer mice (Peromyscus maniculatus) and
house mice (Mus musculus) using various TBT salts. Treat-
ment of feed seeds with 2% (by mass) produced clear repel-
lent effects of TBT in both species. The approximate
lethal dose (estimated by increasing the TBT dose until
the test animals died) for TBT acetate and fluoride was
320 mg/kg. The estimated dietary LC50, based on consump-
tion of treated seed used in the repellency tests, varied
between 37.5 mg/kg per day for TBT acetate and 238 mg/kg
per day for fluoride and sulfate. The LC50 for TBTO was
200 mg/kg per day.
10. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: FIELD OBSERVATIONS
Summary
Tributyltin compounds have had a wide range of uses. Most
concern has focussed on their use in the marine environment,
where TBT has been associated with mortality and failure of
settlement of bivalve larvae, reduced growth, shell thickening,
and other malformations in oysters, imposex (the development of
male reproductive appendages in female animals) in mud snails,
and imposex concurrent with population decline in dogwhelks.
Controls on the use of TBT in antifouling paints has led to
recovery of economically important shellfish growth and repro-
duction. Water concentrations of TBT are still high enough in
some areas to affect marine gastropods.
Field and laboratory results for marine molluscs are in
good agreement. Both imposex in dogwhelks and shell growth and
chambering in Pacific oysters are effective biological indi-
cators of TBT contamination.
There have been few studies into the effects on organisms
of TBT in sediment. There are indications that TBT is bioavail-
able to burrowing organisms and can cause mortality in the
field.
Gross toxic effects and histopathological changes have been
reported in farmed marine fish exposed to TBT through the use
of antifouling paints on retaining nets.
Although TBT has been detected in fresh water at high con-
centrations in some areas, there have been few studies on its
effects. Spills of large amounts of TBT from timber treatment
plants have caused ecological damage, but recovery occurred
within 9 months.
Field testing of tributyltin derivatives, mainly slow-
release formulations of TBTO, has shown that it is difficult to
apply TBT without damaging non-target organisms. Recovery
occurs through recolonization.
10.1. Effects on bivalves
In the 1970s, the French oyster industry was
undergoing a crisis. During the 1977, 1978, and 1979
seasons, very poor spatfalls were reported, together with
increasing reports of poor shell growth and shell malfor-
mations. An extensive survey of the occurrence and inten-
sity of malformations related to metal residues in oysters
suggested, for the first time, a connection with organotin
compounds (Alzieu, 1981). The shell thickening was found
to be due to the appearance of chambers in the oyster
shell and interlamellar gel formation in these cavities
(Alzieu, 1981; Alzieu et al., 1982). The authors of these
reports described in detail the shell abnormalities and
the process of calcification of the oyster shell, and
suggested mechanisms of TBT action. TBT is known to in-
hibit oxidative phosphorylation and it has been suggested
that this forms the basis of its action on the shell. It
is also known to complex amino acids. The effect on calci-
fication derives from inadequate calcium addition to the
organic matrix (a process dependent on ATP) and incorrect
deposition of this matrix. Alzieu et al. (1982) found good
correlation between the occurrence of shell thickening and
the proximity of ports where large numbers of boats were
usually moored. These field observations were corroborated
by the finding of similar shell abnormalities in the lab-
oratory when oysters were exposed to TBT fluoride, an
organotin compound present in antifouling paint used on
the boats. Oysters placed in flowing sea-water tanks con-
taining plates coated with TBT antifouling paints died
after a 30-day exposure to an estimated water concen-
tration of 2 µg/litre (organotin leachate) and shell
thickening was found to occur at water concentrations of
0.2 µg/litre.
Alzieu et al. (1982) assessed the shell quality of
18-month-old Pacific oysters ( Crassostrea gigas; also
known as the Japanese oyster) sampled along the eastern
coast of Oleron Island and in the vicinity of La Rochelle,
France. They concluded that the proximity of pleasure
craft ports or a commercial harbour could badly affect the
quality and growth of oyster shells. However, the presence
of other chemicals, along with TBT in the sea water made
direct assessment difficult. The authors, therefore,
carried out a set of experiments to confirm their
conclusions. Groups of oysters from areas with no shell
abnormalities were distributed to other locations, i.e. a
marina, a river, and the laboratory (three groups: a
control group; a group exposed to 50-cm2 panels coated
with TBT fluoride; and a group exposed to coated panels
500 cm2 in area). All oysters died within 30 days after
exposure to the larger panel in the laboratory and within
170 days after transfer to the marina. Oysters exposed to
the smaller panels showed a mortality rate of 30% after
110 days of exposure. Oysters transferred to the marina
site and those exposed to 50-cm2 TBT-coated panels devel-
oped gel-filled shell cavities after 100 and 110 days,
respectively, during the period of shell growth. Analysis
of the oysters for total tin content revealed levels of
< 1 mg/kg (dry weight) in "unpolluted" groups, 110 mg/kg
after 80 days at the marina site, and up to 25 mg/kg after
exposure to 50-cm2 panels in the laboratory.
Maurer et al. (1985) found TBT levels to be related to
inhibition of settlement of Pacific oyster in the Bay of
Arcachon and the Gironde estuary, France. The authors used
arrays of settling tubes mounted around a central tube
which was painted with "International TBT Antifouling"
at a rate of 6.4 g of paint on a surface of 8 dm2. The
settling tubes were mounted at varying distances (between
4.5 and 13 cm) from the central painted tube. In control
arrays, the central tube was unpainted. The arrays of
tubes were placed out in the two study areas in July 1982
and observations on settlement and growth were made in
September and November 1982. In the Gironde region, a
second method was also used; slates were painted with TBT
paint (21 g of "International TBT Antifouling" on a
surface of 26 dm2) and mounted 10 cm apart, alternating
unpainted with painted slates on a rod. The spacing of
tubes in the arrays was up to 25 cm from the central
painted tube. In the Gironde estuary, settlement was com-
parable with controls except on the painted tube; tubes
5 cm or more away from the TBT paint had similar numbers
of settling larvae as the control. However, deaths among
the settled larvae were high; 100% on tubes 15 cm away (or
less), 99.3% on the tube at 20 cm distant, and 78.3% on
the tube 25 cm distant from the paint. Slates showed high
settlement rates and a mortality of 82.5% at 10 cm away
from the paint. In the Bay of Arcachon, there was a much
lower settlement of larvae; in the Villa Algerienne area
there were 178 settlements/dm2 on the treated tubes
compared with 440 on controls, and in the Comprian area
81 or 83 settlements/dm2 on treated tubes compared with
323 on controls. Barnacles, which were less sensitive to
the paint, were also found to settle on the tubes. The
settlement period in this area was longer than that in the
Gironde estuary; some larvae settling later in the season
showed reduced growth compared with controls.
Thain & Waldock (1986) reported that the Pacific oys-
ter was introduced into the United Kingdom during the mid-
1970s. At this time, growth trials were performed at sev-
eral coastal locations. The oysters grew well at most
sites, but at some east coast sites, such as the estuary
of the River Crouch, they exhibited poor growth, reduced
meat yield, and shell thickening. At other sites, such as
the north Norfolk coast, there were good growth results
with none of the deformities found at the Crouch. The
cause of the poor growth results in some areas was inves-
tigated, and Key et al. (1976) found good correlation
between poor growth and high levels of fine suspended
particles. Areas of poor performance in the trials were
not used for the cultivation of the newly-introduced
oyster species. The types of shell abnormality exhibited
by oysters in France were very similar to those observed
in oysters from the east coast of England, which had been
attributed to sediment. The sediment in the marinas used
by Key et al. (1976) for resettlement studies was probably
contaminated with TBTO (Personal communication by M.J.
Waldock to IPCS). In 1982 it was decided to reassess the
causes of poor oyster shell growth in Britain. Levels of
TBT were measured in the estuaries of the Rivers Crouch
and Blackwater, on the east coast of England, and were
found regularly to exceed 0.2 µg/litre, a level shown by
the French studies to be harmful to the oyster (Thain &
Waldock, 1986). In a laboratory study, Waldock & Thain
(1983) investigated the effect of both suspended sediment
and TBT on growth and shell thickening in spat of the
Pacific oyster. They found that TBT levels of 0.16 µg per
litre inhibited and 1.6 µg/litre stopped growth. Shell
thickening was observed in the oysters exposed to TBT.
Exposure to sediment in "clean" water (i.e. containing
no TBT) actually enhanced growth. Thain & Waldock (1986)
reported that, during 1983, the laboratory finding that
TBT and not suspended sediment was affecting growth and
causing shell abnormalities in oyster was corroborated in
the field by studying oysters from different sites.
Alzieu & Portmann (1984) reported that although the
findings clearly implicated TBT as a major cause of growth
problems and abnormalities, they did not completely
exclude the possibility that other chemicals present might
have caused similar effects. They reviewed the effects of
a 1982 ban by French authorities on use of TBT paints on
boats shorter than 25 m. In the Bay of Arcachon area
(where, in 1980 and 1981, 95% to 100% of oysters had shown
deformities), the 1982 figures for deformities were 70% to
80% and by 1983 deformities had declined to 45% to 50%.
The number of oysters from the same area showing deform-
ities in both upper and lower shells was between 70% and
90%, in 1980 and 1981, and zero by 1983. The spatfall,
which in both 1980 and 1981 failed completely, sub-
sequently recovered; it was described as good in 1982 and
excellent by 1983. The authors also reported, however,
that these results were not reflected in another area
(La Rochelle Bay). This was attributed both to its close
proximity to a major commercial harbour and the fact that
the ban on the use of TBT antifouling paints on pleasure
craft in this area had not been as strictly observed as in
the Bay of Arcachon.
His et al. (1986) reported bioassays conducted on
oysters (Crassostrea gigas) using sea water collected from
the Bay of Arcachon both before and after the imposition
of a ban on the use of TBT paints on small boats. The sea
water collected in 1981 caused abnormalities in 40% of
larval oysters over 12 days of observation (compared with
4% in controls), whereas the sea water from 1982 produced
only 12% abnormalities over the same period. Growth of the
oyster larvae was also improved relative to the period
when TBT paints were still being used. In 1981, mean
growth of larval shells over 12 days was 133 µm, in Bay
of Arcachon sea water, compared with 142 µm in controls.
In 1982, mean growth was 162 µm, as opposed to 168 µm in
controls. Alzieu et al. (1986) stated that between 1980
and 1982 about 90% of oysters displayed anomalies in shell
calcification. Each year anomalies became apparent in
April and reached maximum intensity during June and July.
During the period 1983 to 1985, the percentage of oysters
displaying shell anomalies fell steadily. By 1985 none of
the oysters examined had malformations in both valves and
less than 40% had shell anomalies in one of the valves
(usually the upper valve). Alzieu et al. (1989) monitored
oysters in 1986 and 1987 and found that, although oysters
with deformities in both valves had stabilized at < 10%,
those with deformities in at least one valve had risen
again to a peak of 70% for both years.
Effects on oysters have also been reported near to
fish farms containing nets treated with TBT antifoulants.
Davies et al. (1987a) maintained caged Pacific oysters
(obtained from a nursery unit distant from any significant
sources of TBT) at varying distances from fish farms at
Loch Sween, Scotland. They found that significant accumu-
lation of tin was restricted to within 200 m of the fish
farms and that effects on oyster shell structure were
observed at a distance of 1000 m but not at 5000 m. A com-
parison of shell thickness index and tin accumulation
showed significant tin accumulation in those oysters with
the most severe shell thickening.
Studies in the USA and Japan have revealed similar
effects. Stephenson et al. (1986) transplanted culched and
culchless Pacific oysters (Crassostrea gigas) and two
species of mussel (Mytilus edulis and Mytilus
californianus) to areas of San Diego Bay, California, USA,
along a gradient of known sea-water TBT concentrations
(0.01-0.93 µg/litre). Reduced shell growth was observed
in all three species in areas where TBT levels were
highest. Oyster and Californian mussel samples (but not
M. edulis ) showed marked trends of reduced growth with
increasing TBT levels. Shell thickening in the oysters
also correlated with increasing TBT levels. Wolniakowski
et al. (1987) found Pacific oysters in Coos bay, Oregon,
USA, to have thickened and ball-shaped shells. When these
oysters were analysed, they were found to contain high
levels of TBT (49.7 to 189 µg/kg). The most marked
deformities occurred in animals collected in a marina and
near to where boats were painted. Okoshi et al. (1987)
transplanted spat of two different strains of Pacific
oysters (Crassostrea gigas) to two different experimental
field sites in northern Japan for 41 weeks. The number of
chambers in the oyster shells increased gradually in the
Miyagi strain at one site. In contrast, few chambers were
observed in the Hiroshima strain at either site. The
authors concluded that both genetic and environmental
factors were involved in the formation of shell chambers.
Crassostrea gigas is a non-indigenous species in the
United Kingdom and France. Stocks for breeding were intro-
duced into both countries in the late 1960s and originated
from the Miyagi region of Japan (Walne & Helm, 1979).
When Paul & Davies (1986) maintained scallops (Pecten
maximus) and Pacific oysters (Crassostrea gigas) in nets
coated with a TBTO-based paint, the mortality of scallop
spat was 24%, compared with a control mortality of less
than 6%, over the 31-week exposure period. Adult scallop
mortality was less than that of controls in the treated
group, and no effect was observed on growth. No oysters
died. Scallop spat in TBT-treated nets grew significantly
less rapidly than the control spat. Oyster growth, as
measured by mean shell length, was significantly reduced
and had largely ceased after 10 weeks of exposure. A sig-
nificant thickening of the oyster shell was observed
within 10 weeks of exposure and continued throughout the
31-week exposure. It was maintained even after transfer to
untreated nets for 10 weeks.
Minchin et al. (1987) monitored bivalve settlement in
Mulroy Bay, Ireland, between 1979 and 1986 using settling
panels placed in the sea. They found that, during this
period, settlement either failed or was reduced. Scallop
numbers fell from an average of > 1000 per panel in 1979
to zero in 1983. In 1984, no settlement of any bivalve
species was recorded on the panels. This reduced settle-
ment corresponded to the introduction of organotin fish-
net dips in local salmonid farms. The first use of TBT
paints appears to have been in 1981. This use of organotin
compounds ceased after 1985. In 1986, in all bivalves
monitored (except flame shells), there was again good
settlement. Scallops (Pecten maximus) and flame shells
(Lima hians and Chlamys varia) (all members of the
Pectinacea) were found to be particularly sensitive to
organotin compounds. It is not known whether the effect of
the TBT was on the reproduction of the bivalves or a toxic
effect on the larvae.
10.2. Effects on gastropods: imposex
Between the years 1972 and 1976, Smith (1981c)
sampled mud snails (Nassarius obsoletus) from four
locations bordering Long Island Sound in Westport and
Fairfield, Connecticut, USA. The snails were examined
for imposex (see section 8.2.3.6), and the results were
quantified by measuring the percentage of snails in a
sample showing any imposex and estimating the degree of
imposex within indi- vidual snails. In two of the
areas, adjacent to a yacht yard in Southport Harbour and
at the mouth of the harbour, 95% to 100% of snails had
some degree of imposex. Both showed significantly
more imposex than the other two sites, an area used
to moor a few old boats and an area protected from human
interference. The mud snails in this last area showed no
imposex. The area with a few old boats showed imposex
levels of 30% to 50%, much less than in the first two
areas. Smith (1981a) collected mud snails from three of
the locations, the yacht yard in the harbour (where
all female snails were abnormal and degree of imposex
greatest), the mouth of the harbour (where almost all
snails were abnormal but the degree of imposex changes
was intermediate), and the area protected from human
impact (at least 3.5 km from the nearest marina, where
all female snails were normal). The author labelled
these areas as "dirty", "intermediate", and "clean".
Snails transferred from the "clean" area to the
"dirty" area developed imposex. In those transferred
from the "dirty" area to the "clean" area the degree
of imposex was reduced. An analysis of chemicals
present in water from the "dirty" area was then carried
out and snails were exposed to some of the
contaminants individually, i.e. marina disinfectants,
detergents, copper antifouling paints, leaded
gasoline, combustion emissions, and two types of
TBT-based antifouling paints. Only the tin-containing
antifouling paints increased the level of penis
expression in female snails.
In a survey of the dogwhelk Nucella lapillus around
the south-west of England, Bryan et al. (1986) found
imposex to be widespread. The south coast of England was
the most severely affected. Populations showing the
highest incidence and highest intensity of imposex were
close to areas of boating or shipping activity. The degree
of imposex had increased markedly between 1969 and 1985 in
Plymouth Sound (an area on the south coast with large
numbers of small boats and ships), coinciding with the
introduction and increasing use of TBT-containing anti-
fouling paints in the area. Imposex correlated with
concentrations of sea-water tin (TBT fraction) and resi-
dues of tin in the dogwhelks (Fig. 6). Transferring whelks
from an area with little boating activity to Plymouth
marina resulted in a marked increase in the degree of
imposex. Bailey & Davies (1988b) found an increase in the
degree of imposex and also a higher incidence of penis
development in female dogwhelks collected in 1987 in the
Firth of Forth, Scotland, compared with those caught in
1975.
One of the effects of imposex on the female dogwhelk
is the blocking of the pallial oviduct, preventing the
release of egg capsules and rendering the female sterile.
A high incidence of females carrying aborted capsules was
found in declining populations close to sources of TBT.
The build-up of aborted capsules seemed, eventually, to be
lethal to the female; there were fewer females than
expected in affected areas (Gibbs & Bryan, 1986). The same
authors reported that the gross morphological changes
occurring in late imposex in the dogwhelk seem to be irre-
versible, since animals transferred from a moderately-
contaminated site to a "clean" site showed no resorption
of the penis. Gibbs & Bryan (1987) stated that imposex in
the dogwhelk was seen in sea water with TBT concentrations
of less than 1 ng tin/litre. They reported that the repro-
ductive failure, along with a lack of recruitment, had led
to population declines, almost to the point of extinction,
in areas of heavy TBT contamination. Gibbs et al. (1988)
experimentally transferred dogwhelks from an uncontami-
nated area to one showing water concentrations of 9-19 ng
tin/litre (TBT fraction) and demonstrated the development
of imposex within 18 months at the new location. These
transferred adults were able to spawn for much of this
period. The authors compared these results with results
for juveniles, which developed imposex earlier and were
sterile before reaching maturity. They discussed the
implications for the recolonization of areas where repro-
duction in the dogwhelk has been eliminated. Adults are
irreversibly affected by imposex. Recolonization is
unlikely until the TBT levels in sea water fall to around
2 ng tin/litre, a concentration at which the juveniles
are not sterilized before they reach sexual maturity. The
extent of recovery of populations would, therefore, depend
on the success of control measures and the water
concentrations resulting from bigger ships exempt from the
ban on TBT-containing antifouling paints.
Bryan et al. (1987) stated that while the evidence did
not show conclusively that TBT is solely responsible for
imposex in the dogwhelk, circumstantial evidence was con-
siderable. Imposex is related to the level of boating
activity. There is a significant relationship between
imposex and the body residue of organotin. Populations
that are in decline show the highest levels of TBT. Female
populations are declining faster than males and also have
the higher levels of TBT. The rise in the degree of
imposex coincided with the introduction and use of anti-
fouling paints containing TBT. Imposex has been shown to
be caused by TBT in other species of stenoglossan snails,
i.e. mud snails (Smith, 1981b). A significant decline in
imposex in monitored juvenile Nucella (Personal communi-
cation by P.E. Gibbs & G.W. Bryan to IPCS) followed the
introduction of TBT legislation in the United Kingdom.
Bryan et al. (1988) investigated the capacity of vari-
ous organotin compounds to induce imposex in the dogwhelk.
Whelks, already slightly affected by imposex, were taken
from the wild and exposed to 200 ng/litre tin, in the form
of TBT chloride, tri- n- propyl tin (TPrT), tetrabutyltin
(TTBT), dibutyltin (DBT), or triphenyltin (TPhT), for 14
days before being returned to the shore. Penis size, as a
percentage of male penis size, increased to 44% in females
exposed to TBT chloride, compared with 6% in controls.
TPrT increased relative penis size to only 14%, and no
other compound had any effect. In an attempt to eliminate
differences due to differential uptake of the compounds,
the organotin compounds were injected in a second exper-
iment. The females were maintained in the laboratory for
up to 105 days. TBT again induced increased penis size but
TTBT also showed an effect (19.5% compared to the 34%
shown after TBT injection). Other compounds were ineffec-
tive. The authors believed the effect of TTBT to be caused
by contamination by TBT and conversion to TBT in the tis-
sues. However, the increased imposex after treatment with
TPrT could not be explained in this way and the imposex
effect is considered to be not totally specific to tri-
butyltin.
8.2.3.7 Genotoxicity
Dixon & Prosser (1986) found that TBTO was not geno-
toxic, at concentrations of 0.05 to 5.0 µg tin/litre, to
the larvae of the mussel Mytilus edulis. Results were
based on chromosome analysis and sister chromatid
exchange (SCE). In 4-day acute toxicity studies, TBTO was
found to cause a dose-dependent reduction in both larval
survival and development of mussels. Survival ranged from
46% of controls at 0.05 µg/litre to 1.4% at 5 µg/litre.
The percentage of animals reaching the D-shell stage of
development was 8.7% of controls at the lowest dose, but
none reached this stage at either 1 or 5 µg/litre. How-
ever, when mussel larvae were exposed to a standard
mutagen (mitomycin C) or crude oil, in the presence of
TBTO, the SCE frequency increased to approximately twice
that found when larvae were exposed to either toxicant in
isolation (Dixon & McFadzen, 1987).
8.2.4. Crustaceans
Summary
Most of the available data for freshwater organisms are
derived from acute exposure tests with Daphnia. LC 50 data for
similar exposure times are variable (up to 2 orders of magni-
tude) probably due to age differences in the populations
studied. The NOEL for Daphnia has been estimated to be around
0.5 µg/litre, behaviour being the most sensitive parameter.
A range of marine species from copepods to crabs and lob-
sters has been studied, the lowest NOEL being 0.09 µg per
litre for reproductive effects in mysid shrimp.
The presence of sediment in test aquaria greatly reduces
the toxic effect of TBTO on estuarine crustaceans.
8.2.4.1 Acute effects
Using TBTO and TBT acetate, Floch et al. (1964) calcu-
lated LC100 and LC0 values for various species of fresh-
water invertebrates. The lethal concentrations of TBTO to
Daphnia magna over 24 and 72 h were 0.12 mg/litre and
0.06 mg/litre, respectively. Another aquatic crustacean,
Cypridopsis hartwigi, was less sensitive with lethal con-
centrations of 4 mg/litre for a 24-h exposure, 2 mg/litre
for a 48-h exposure, and 0.12 mg/litre for a 96-h
exposure. NOELs were 0.03 and 0.06 mg/litre for Daphnia
and Cypridopsis, respectively. For TBT acetate, the
LC100 was 0.15 mg/litre for a 72-h exposure of Daphnia
and 0.15 mg/litre for a 96-h exposure of Cypridopsis. The
LC0 was 0.075 mg/litre for both species.
However, more recent work has found Daphnia to be con-
siderably more sensitive to TBT. Meador (1986) reported a
96-h LC50 for Daphnia magna of 5.9 µg/litre and noted
that TBT is a slow-acting toxicant for Daphnia with
effects only being shown after 96 h or more of exposure.
Polster & Halacka (1971) quoted 48-h LC50 values for
Daphnia magna using various TBT salts, which ranged from
2.2 µg TBTO/litre to 8.5 µg TBT oleate/litre.
RIVM (1989) reported a 48-h EC50 for Daphnia of
4.7 µg/litre and a NOEL, over the same time period, of
0.56 µg/litre.
Davidson et al. (1986) calculated the 96-h LC50 to
be 0.42 µg/litre after exposing the mysid shrimp
Acanthomysis sculpta to a leachate of TBT.
When Walsh (1986) exposed the mole crab Emerita
talpoida to concentrations of 10 µg TBTO/litre of sea
water or 4500 µg/kg of sand, no effect on crab survival
was observed after 7 days of exposure. In continuous-flow
bioassays, 10 000 µg TBTO/kg of sediment did not kill
grass shrimp after a 96-h exposure.
8.2.4.2 Short- and long-term toxicity
U'Ren (1983) maintained the marine copepod Acartia
tonsa in solutions containing TBTO, under static con-
ditions, and calculated a 144-day LC50 of 0.55 µg per
litre; by combining moribundity and mortality as end-
points, the 144-day EC50 was found to be 0.4 µg/litre.
Laughlin et al. (1983) maintained mud crab larvae
(Rhithropanopeus harrisii), from hatching, in solutions
containing either TBTO at 0.5 to 25 µg/litre or TBT
sulfide at 0.5 to 50 µg/litre, under static renewal pro-
cedures. The survival of the zoeae, up to 15 days, was
unaffected by TBTO at levels up to 10 µg/litre. At 15 µg
per litre, 84% successfully moulted to the megalopa stage,
but at 5 µg TBTO/litre only 37% survived. Zoeal sur-
vival was unaffected by concentrations of TBT sulfide up
to 5 µg/litre. Survival at 20, 30, and 50 µg/litre was
78%, 26%, and 4%, respectively. The development rate, over
the same time period, decreased with increasing TBT con-
centration, although this was not statistically signifi-
cant below 10 µg TBTO/litre or 20 µg TBT sulfide/litre.
At the highest exposure concentrations (10 µg TBTO/litre
and 20 µg TBT sulfide/litre), metamorphosis was delayed
by approximately 2 days in the case of TBTO and 6 days for
TBT sulfide. Growth (measured as mean wet weight) was
significantly reduced at TBTO concentrations of 15 and
25 µg/litre or TBT sulfide concentrations of 20, 30, and
50 µg/litre, and showed dose dependency. Daily growth was
monitored for up to 12 days at concentrations (of both
TBTO and TBT sulfide) of 0.5, 1.0, and 5.0 µg per litre.
Although the final weights were not significantly differ-
ent, all TBT treatments caused an initial growth lag
during the first three days of exposure. Laughlin et al.
(1985) found the LC50 for exposure of zoeae of
Rhithropanopeus harrisii to TBTO during the 12 days of
zoeal development to be 55 nmol/litre.
Laughlin & French (1980) exposed shore crabs
(Hemigrapsus nudus), 2 to 3 days after hatching, to TBTO
(as "Biomet") for up to 14 days under static conditions.
At the highest concentrations (500 and 1000 µg/litre),
all the zoeae died within 2 days. Survival time increased
as the concentration of TBTO decreased from 100 to 25 µg
per litre; most larvae died within 8 days even at 25 µg
per litre. The estimated values of LT50 for 100, 75, 50,
and 25 µg/litre were 3.4, 4.8, 5.8, and 6.2 days,
respectively. Lobster larvae (Homarus americanus) were
much more sensitive; 100% being killed within 24 h and
2 days by 20 and 15 µg TBTO/litre, respectively. Concen-
trations of 10 and 5 µg/litre killed all larvae within
5 to 6 days. In the group exposed to 1 µg/litre, there
was a similar mortality pattern to the controls and high
mortality at the first ecdysis (3 to 5 days post-hatch).
However, in this group only a single larva metamorphosed
successfully, compared with 43% in the control group.
Davidson et al. (1986) kept juvenile mysid shrimps
(Acanthomysis sculpta), newly-released from the female,
in TBT concentrations of between 0.03 and 0.48 µg per
litre for a 63-day period under flow-through conditions.
The TBT source was leachate from panels coated with anti-
fouling paint. All animals died within 7 days at the
higher exposure level. There was no significant difference
in shrimp mortality between those exposed at levels up to
0.38 µg/litre and the controls, either at 22 or 41 days.
From day 41 to the end of the test (63 days), survival
decreased at 0.38 µg/litre and only 22.5% survived to the
end of the experiment, compared with 60% in the control
group. The authors stated that this indicated a lowering
of the NOEL for the entire life cycle of A. sculpta from
0.38 µg/litre to 0.25 µg/litre at 41 days. The increase
in mortality coincided with the release of juveniles by
the females, which indicated a sensitive time in the life
cycle. Both mean length and weight of females, at a TBT
level of 0.38 µg/litre, were significantly reduced after
63 days of exposure, but no effect of TBT concentrations
up to and including 0.38 µg/litre was observed in males.
A similar result was found in another study after 28 days
at 0.49 µg/litre; again no effect on length or weight was
found in males. There was a significant effect on the
length of developing juveniles and sub-adults. After 14
days of exposure to either 0.19 or 0.33 µg/litre, mysids
were significantly shorter; this was also the case at
0.2 µg/litre after 27 days. At TBT concentrations up to
and including 0.33 µg/litre, there was no effect on the
number of juveniles released per individual female, the
number of individuals in unhatched broods, or the number
of days from hatching of a female to the release of its
juveniles. However, there was a significant reduction in
the number of viable juveniles released at both 0.19 µg
per litre and 0.33 µg/litre. The authors suggest a NOEL
of 0.09 µg/litre for reproduction, the most sensitive
parameter found in the study.
Laughlin et al. (1984) exposed the Baltic amphipod
Gammarus oceanicus to TBTO or TBT fluoride concentrations
of 0.3 or 3.0 µg/litre for 8 weeks, under 48-h static
renewal conditions. They also exposed the amphipods to
leachates from TBT-containing antifouling paints, by
placing a 1 cm2 plexiglass plate, which was painted with
either "Micron 25" or "Interracing", in the tank for
5 weeks. Again the water was changed every 48 h. TBT
levels in the experiment with pure compounds, remained
constant, but the TBT concentration (measured as TBTO)
from the leachates gradually increased over each 48-h
exposure period to approximately 5.5 µg/litre for
"Interracing" and 0.5 µg/litre for "Micron 25",
reflecting the different leaching rates of the two com-
pounds. At the highest concentration of both TBTO and TBT
fluoride, 50% of the adults died within 10 to 12 days of
the exposure and all had died within 16 days (TBTO) or 33
days (TBT fluoride). The TBT paint "Interracing" caused
100% mortality within 1 week. The lower concentration of
the exposures to pure compounds and "Micron 25" resulted
in mortality patterns that were not dependent on TBT con-
centration but on senility. The exposures to 0.3 µg per
litre and "Micron 25" had significantly reduced the
number of surviving larvae by the end of the experiment.
Slight decreases in larval growth were observed after
exposure to TBTO and "Micron 25" but, generally, concen-
trations of less than 1 µg TBT/litre had little effect
on growth and no effect on whole animal oxygen consumption
rates.
Clark et al. (1987) monitored the survival of the
grass shrimp (Palaemonetes pugio) in water and water/sedi-
ment test aquaria after the addition of TBTO. When added
to water and tested in the absence of sediment, TBTO gave
96-h LC50 values comparable to other published results at
20 µg/litre. However, when the TBTO was admixed with
sediment rather than water, the LC50 could not be deter-
mined. TBT levels in sediment at 1 mg/kg in static and
10 mg/kg in flow-through tests showed no effects on shrimp
survival. Similar tests using Amphioxus gave an LC50 for
sediment containing TBTO of between 1 and 10 mg/kg over 4
and 10 days. The animals were killed by 10 µg TBT per
litre in water.
8.2.4.3 Reproductive effects
Hall et al. (1988b) maintained egg-carrying females of
the copepod Eurytemora affinis in TBT chloride at levels
of 0.1 or 0.5 µg/litre for up to 13 days. After 3 days
of exposure, the higher concentration had significantly
reduced the mean brood size to 0.2, compared with 15.2 in
controls. Neonate survival was significantly reduced at
0.1 µg/litre after 6 days (22% of control survival). No
offspring survived the higher exposure concentration. In a
second experiment, mean brood size, after 2 days of
exposure, was not significantly affected at concentrations
of between 12.5 and 200 ng/litre. Neonate survival, after
13 days, was unaffected by concentrations up to and
including 50 ng/litre; survival at 100 ng/litre was 76%
(compared to 22% survival at the same exposure level, in
the first experiment, over 6 days) and was further reduced
to 24% at 200 ng TBT/litre.
When Johansen & Mohlenberg (1987) exposed fertilized
mature female copepods (Acartia tonsa) to TBTO, egg pro-
duction was significantly reduced at the highest exposure
level of 0.1 µg/litre after 72 h. After 120 h, all
exposure concentrations had significantly reduced egg
production by 18%, 19%, and 37% relative to controls, at
TBTO concentrations of 0.01, 0.05, and 0.1 µg per litre,
respectively.
8.2.4.4 Limb regeneration
Weis et al. (1987a,b) exposed the fiddler crab (Uca
pugilator) to TBTO concentrations of 0.5, 5.0, or 50 µg
per litre under static renewal conditions. Regeneration
(autotomy) of 1 chela and 5 walking legs was induced by
pinching them off at the merus. Although some growth
retardation was observed, the most striking effect was the
development in regenerated limbs of deformities, e.g.,
backward curling or complete absence of the dactyl of the
claw, chelae or walking legs, stunted, unjointed, or bent
in the wrong direction. The percentage of males exhibiting
deformities was 17% at 0.5 µg/litre, 24% at 5 µg per
litre, and 67% at 50 µg/litre, the test solution being
changed twice weekly. In a second experiment, where the
test solution was changed three times weekly, the percent-
age deformities were as follows: males, 100% at both 5 and
50 µg/litre; females, 29% at 5 µg/litre and 100% at
50 µg/litre. There were no deformities in the control
groups during the experiments.
8.2.4.5 Behavioural effects
When Pinkney et al. (1985) gave the grass shrimp
(Palaemonetes pugio) a choice between TBTO-contaminated
water and clean water, it did not avoid total organic tin
concentrations of 2.3 to 30 µg/litre. The response data,
at both 2.3 and 30 µg/litre, were very similar.
Meador (1986) reported the effects of TBT chloride on
the photobehaviour of water fleas (Daphnia magna). The
normal response of Daphnia to a unidirectional light
source is to swim away from the light; this is an adaptive
response to avoid predators. The author reported that
Daphnia exposed to TBT chloride showed a reversal of this
behaviour and swam towards the light source (usually with
considerably increased swimming intensity). The threshold
concentration of TBT chloride causing this behavioural
reversal was 0.5 µg/litre (the LC50 over the same time
period was between 3.5 and 6 µg/litre).
8.2.5. Other aquatic invertebrates
Summary
Few studies have been carried out on other species. The
lowest effect level in annelids was observed for Nereis
diversicolor; mortality and behavioural effects were seen after
chronic exposure to 2 µg/litre. Limb regeneration was signifi-
cantly inhibited in a brittle star after exposure to 0.1 µg
per litre. Experiments have been carried out on four insect
species. The most sensitive was Notonectes, the LC0 being
0.03 mg/litre and the LC50 0.06 mg/litre.
8.2.5.1 Acute effects
Walsh et al. (1986a) exposed larvae of the lugworm
Arenicola cristata to either TBTO or TBT acetate, for 96
or 168 h, respectively. The concentrations that killed
100% of the animals were: 4 µg/litre (96 h) for TBTO;
10 µg/litre (96 h) and 5 µg/litre (168 h) for TBT acet-
ate. At 5 µg TBT acetate/litre, all larvae were abnormal
after 96 h of exposure. No deaths or abnormalities re-
sulted from exposure to 2 µg TBTO/litre for up to 168 h.
When Beaumont et al. (1987) exposed adult polychaetes
(Nereis diversicolor) to TBT in flowing sea water for up
to 22 days, there was 100% mortality by 22 days at 4 µg
per litre and 55% mortality at 2 µg/litre (20% control
deaths). Eversion of the proboscis was a more sensitive
indication of toxicity. No controls showed this effect,
whereas 75% of animals exposed to 2 µg/litre showed
everted proboscis after 22 days (50% after 20 days of
exposure).
Dragonfly larvae (Aeschna) sp. showed a 48-h LC100 of
0.25 mg TBTO/litre and an LC0 of 0.12 mg TBTO/litre.
Corresponding values for TBT acetate were 0.5 mg/litre
(72-h LC100) and 0.025 mg/litre (LC0). Notonectes sp.
yielded LC0 values of 0.03 mg/litre for both TBTO and TBT
acetate and 72-h LC100 values of 0.06 and 0.15 mg/litre
for TBTO and TBT acetate, respectively. Chironomid (midge)
larvae revealed a NOEL of 0.075 mg/litre for TBT acetate
and a 48-h LC100 of 0.15 mg/litre (Floch et al., 1964).
Cardarelli (1978) studied the efficacy of controlled-
release organotin compounds as mosquito larvicides. The
toxicity (LT100) of BioMet (6% TBTO in natural rubber),
CBL-9B (20% TBT fluoride in natural rubber), and
ECOPRO-1230 and ECOPRO-1330 (both are ethylene propylene
polymers containing 30% TBT fluoride) were tested against
larvae of the mosquito Culex quinquefasciatus. For BioMet
and CBL-9B, LT100 values ranged from 4 to 9 days for
toxicant concentrations of 0.1 to 10 mg/kg of pellet added
to the water, the toxicity increasing with increasing
toxicant concentration. A degradation product, TBT carbon-
ate, was found to be less toxic, the LT100 being 9 days
after exposure to 10 mg/litre. When the polymers were
tested at toxicant concentrations ranging from 0.9 to 32.6
mg/kg, toxicity was found to increase with increasing
toxicant concentration (LT100s range from 2 days to 16
days). Although initially toxicity tended to decrease as
the soaking time of the pellet increased, prior to
exposure, between days 100 and 500 of soaking toxicity
remained relatively unchanged. The authors observed that
the organotin compounds dramatically slowed or even
prevented morphogenesis.
8.2.5.2 Limb regeneration
In a study on limb regeneration, Walsh et al. (1986b)
maintained the brittle star Ophioderma brevispina in TBTO
concentrations of 0.01, 0.1, or 0.5 µg/litre under flow-
through conditions. On the first day of the experiment,
autotomy was induced in two arms, at opposite sides of the
disc, by pinching midway between disc and arm tip. The
animals were then exposed for four weeks to TBTO. There
were no deaths and no effect on disc diameter. Both 0.1
and 0.5 µg/litre significantly inhibited regeneration of
arms as measured by length; both groups showed average and
median lengths less than those in the lowest-dose group,
but variability precluded statistical significance. Aver-
age weights of limbs were also reduced in the groups
exposed to 0.1 and 0.5 µg/litre, but statistical analysis
was not carried out because pooled weights did not provide
enough data.
8.3. Fish
Summary
The acute toxicity of tributyltin to marine and freshwater
fish is highly variable, LC 50 values ranging from 1.5 to
240 µg/litre. It is unclear whether this is due to inherent
differences in sensitivity or to differences in route of
exposure. Many of the acute toxicity studies need to be inter-
preted with care, since the TBT concentrations cited are often
nominal and biologically available concentrations were not
measured.
In long-term toxicity tests, the NOEL for general toxico-
logical parameters (growth and behaviour) for trout yolk-sac
fry was greater than 0.2 µg/litre, and for medaka it was
3.2 µg/litre. Using histopathological parameters, the NOEL for
medaka was found to be 0.32 µg/litre, vacuolation of the reti-
nal epithelium being the most sensitive parameter.
Few embryotoxicity tests have been performed and it has not
been possible to establish a NOEL.
8.3.1. Acute effects
The 96-h LC50 of TBTO for marine fish ranges between
1.5 and 36 µg/litre (Table 12). Larvae seem to be more
sensitive than adults in those few studies examining dif-
ferent life stages in the same test. Fewer LC50 values
have been published for freshwater fish; they range from
13 to 240 µg/litre.
8.3.2. Short- and long-term toxicity
Matthiessen (1974) measured a lethal threshold concen-
tration of TBTO for Tilapia mossambica of between 8 and
16 µg/litre (24-h LC50, 28 µg/litre; LC5, 24 µg per
litre; LC90, 33 µg/litre) as a preliminary to studies
at sublethal concentrations. A temporary opacity of the
surface of the eyes developed at concentrations below this
threshold (> 8 µg/litre) but disappeared after a few
days. Other symptoms included sluggishness and difficult-
ies with balance. Melanophores in the skin were found to
be constricted, giving treated fish a paler appearance
than controls. The fish did not produce behaviourally
related display patterns, an activity important in the
species. As with the eye opacity, these other symptoms
disappeared after a few days or weeks, suggesting that the
fish develop some tolerance to the TBTO. The growth of
Tilapia exposed to 0, 5, or 8 µg TBTO/litre was moni-
tored over a 5-week period. These concentrations of TBTO
were estimated, on the basis of release rates, in water
after the TBTO had been used to control mollusc vectors
for schistosomiasis. There was no difference in growth
between fish exposed to 5 µg/litre and controls. Tilapia
exposed to 8 µg/litre showed negative growth and lost
about 6% of body weight over the experimental period. Eye
opacity was only found in the fish exposed to 8 µg per
litre, but this effect did not seem to affect feeding
behaviour. The only other change in the high-dose group
was an increase in aggressive encounters between males.
There was an increased reluctance amongst attacked males
to avoid conflict, which led to the death of several indi-
viduals. Fatal engagements between males are normally very
rare in this species.
When Seinen et al. (1981) exposed rainbow trout (Salmo
gairdneri) yolk-sac fry to TBT chloride concentrations of
0.2, 1, or 5 µg/litre for up to 110 days, all fry died
within 10 to 12 days (at the transition from yolk-sac fry
to the swimming fry stage) at 5 µg/litre, but there were
no deaths at lower doses. The major histopathological
change in fish that died after exposure to 5 µg per litre
was hydropic degeneration of tubule segments of the pro-
nephros. At 0.2 and 1 µg/litre, there was a dose-related
retardation of growth, resulting in a 44% decrease in body
weight (relative to controls) in the 1 µg/litre group
after 110 days. At the end of the experiment, both remain-
ing groups showed significant reductions in the haemo-
globin titre in the blood and in body weight. Only at the
higher concentration was there a significant decrease in
blood cell number. Relative liver weight was significantly
increased at both concentrations but relative numbers of
thymus cells were unaffected. The authors also measured
the relative area distribution of the various hepatic com-
partments in liver sections. The area occupied by nuclei
significantly increased at both concentrations, whereas
glycogen storage area decreased, but this was significant
only at the highest exposure level. Cytoplasmic area was
unaffected, as were all non-parenchymal compartments.
Table 12. Toxicity of tributyltin to fish
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Salinity pH TBT salt Dura- LC50c Reference
age flowa ature (o/oo) tion (µg/
(°C) (h) litre)
---------------------------------------------------------------------------------------------------------
Marine and estuarine species
Sheepshead minnow sub-adult flow 19.4- 9.8- 8.15- chloride 48 >31d,f Bushong
(Cyprinodon variegatus) 21.3 12.1 8.31 72 28.1 et al. (1988)
(24-36.5)d,f
96 25.9
(22.8-30.1)d,f
Bleak 8 cm stat 10 7 7.8 fluoride 96 6-8e Linden et
(Alburnus alburnus) oxide 96 15 al. (1979)
(13-17)e
Inland silverside larva flow 20 10 chloride 48 7.7 Bushong et
(Menidia beryllina) (5.5-10.9)d,f al. (1987)
72 4.6
(3.3-6.2)d,f
Atlantic silverside sub-adult flow 20 10 chloride 48 12.7 Bushong et
(Menidia menidia) (7.8-15.2)d,f al. (1987)
72 9.3
(7.1-12.4)d,f
96 8.9
(6.7-11.6)d,f
Atlantic menhaden juvenile flow 20 10 chloride 48 6.8 Bushong et al. (1987)
(Brevoortia tyrannus) (4.1-infinite)d,f
72 5.2
(3.7-6.5)d,f
96 4.5
(3.6-6.4)d,f
Sole larva statb oxide 48 8.5 Thain (1983)
(Solea solea) larva 96 2.1
adult 48 88
Armed bullhead adult statb oxide 48 26 Thain (1983)
(Agonus cataphractus) 96 16
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Girella 2.4 g statb 19.9- 7.8- oxide 48 5.2d Kakuno &
(Girella punctata) 20.5 8.1 96 3.2d Kimura (1987)
Saltwater goby statb 20.2- 32.5- 8.1- oxide 24 12d Shimizu &
(Chasmichthys dolichognathus) 21.0 32.8 8.3 48 9d Kimura (1987)
96 4d
Chinook salmon juvenile stat 3-5 28 oxide 6 54d Short &
(Oncorhynchus tshawytscha) 12 20d Thrower (1987)
96 1.5d
Mummichog larvae flow 19.4- 9.8- 8.15- chloride 48 >32.2d,f Bushong et
(Fundulus heteroclitus) larvae 21.3 12.1 8.31 72 28.2 al. (1988)
(15.1-infinite)d,f
larvae 96 23.4
(15.2-30.9)d,f
sub-adult 48 >32.2d,f
sub-adult 72 28.3
(23.4-43.2)d,f
sub-adult 96 23.8
(20.8-28)d,f
Freshwater species
Rainbow trout yearling stat 18 250 oxide 24 28e Alabaster
(Salmo gairdneri) yearling statb 18 250 oxide 48 21e (1969)
---------------------------------------------------------------------------------------------------------
Table 12. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Size/ Stat/ Temper- Hard- pH TBT salt Dura- LC50c Reference
age flowa ature nessg tion (µg/
(°C) (mg/litre) (h) litre)
---------------------------------------------------------------------------------------------------------
Guppy 3-4 weeks statb 23 oxide 96 21 RIVM
(Lebistes reticulatus) chloride 168 21 (1989)
(16-29)e,f Polster &
oleate 168 33 Halacka
(19-60)e,f (1971)
benzoate 168 25
(19-32)e,f
laurate 168 30
(18-50)e,f
acetate 168 28
(21-33)e,f
oxide 168 39
(28-50)e,f
Medaka 4-5 weeks statb 23 oxide 96 17 RIVM
(Oryzias latipes) (1989)
Stickleback 4-5 weeks statb 19 oxide 96 13 RIVM
(Gasterosteus aculeatus) (1989)
Carp oxide 24 75 Temmink &
(Cyprinus carpio) oxide 96 32 Everts
(1987)
Golden orfe oxide 48 50e Plum
(Leuciscus idus melanotus) napht- 48 70e (1981)
henate
Bluegill sunfish stat 20 oxide 96 240e Foster
(Lepomis macrochirus) (1981)
---------------------------------------------------------------------------------------------------------
a stat = static conditions (water unchanged for the duration of the test unless stated otherwise);
flow = flow-through conditions (TBT concentration in water continuously maintained).
b Static renewal conditions (water changed periodically).
c 95% confidence limits are given in brackets.
d Measured concentration.
e Nominal concentration.
f Concentration expressed as TBT.
g Hardness expressed as mg CaCO3/litre.
Pinkney et al. (1988) exposed 13- and 16-day-old
larvae of striped bass (Morone saxatilis) to varying con-
centrations of TBT from methacrylate-painted panels for 6
to 7 days. They found significant reductions in survival
at measured concentrations of 0.766 µg TBT/litre or more.
At lower exposure concentrations (0.067 µg/litre), growth
parameters changed in the 13-day-old larvae only. No
changes occurred in the growth parameters of 16-day-old
larvae exposed to 0.444 µg/litre or less. The authors did
not know whether this apparent difference in sensitivity
between larvae of different ages was a true effect or
simply the result of differences between the various
batches of larvae.
Wester et al. (1988) reported NOELs for the medaka
(Oryzias latipes) of 3.2 µg TBTO/litre for general toxi-
cological parameters (mortality, growth, general appear-
ance, abnormal behaviour) and 0.32 µg/litre for histo-
pathological effects during 1 month of exposure. The most
sensitive histopathological effect was the development of
vacuolation in the retinal epithelium of the eye. There
was a dose-related increase in hepatocellular vacuolation,
with swelling in pronounced cases. The vacuoles appeared
to be glycogen deposits, although, at higher doses, lipid
vacuoles were also noted. The NOEL for liver effects was
1 µg TBTO/litre. Kidney effects mostly involved the
tubule and included dilation, epithelial atrophy, degener-
ation and regeneration, and proteinaceous casts including
cellular debris (tubulonephrosis). Severe cases also
showed glomerular effects. Similar lesions were reported
after 3 months of exposure, with, in addition, effects on
the swim bladder, skin, oral cavity, and thyroid gland at
the highest concentration tested (10 µg/litre). Thymus
atrophy, reported by the same authors in the guppy, was
not found in the medaka, indicating some species speci-
ficity regarding the effects of TBT. Increased glycogen in
liver and muscle after TBTO treatment was demonstrated
analytically in the medaka, confirming histochemical
suggestions of increased glycogen in the guppy.
Shimizu & Kimura (1987) exposed the marine goby
Chasmichthys dolichognathus to TBTO in short (4 day) and
long (12 week) experiments, and reported a 96-h LC50 of
4 µg/litre. Long-term exposure to 2.1 µg/litre during
the season of gonadal recrudescence led to a significant
depression of the gonadosomatic index in male fish. There
was no effect on female fish in terms of gonadosomatic
index or ovarian histology.
8.3.3. Embryotoxicity
Newton et al. (1985) exposed eggs, embryos, and larvae
of the California grunion (Leuresthes tenuis) to plates
painted with 9.4% TBT methacrylate (0.5% TBTO; 44.7% cu-
prous oxide; 45.5% inert ingredient) and aged for 30 days
in flowing sea water. The authors claimed that the ex-
posure to concentrations of between 0.14 and 1.72 µg TBT
per litre had no adverse effect on hatching, growth, or
development. In fact the presence of TBT at such concen-
trations enhanced both hatching success and stimulated
growth. A concentration of 10 µg TBT/litre had no effect
on hatchability, but at 74 µg/litre hatching success was
reduced by approximately 50%. The authors observed no
effect on the survival of larvae, hatched from eggs
exposed to concentrations of 0.14 to 1.72 µg/litre, up to
7 days post-hatch. The presence of copper, however, should
be taken into consideration in the interpretation of these
results.
Weis et al. (1987a) reported considerable variation in
the response of embryos of the killifish (Fundulus
heteroclitus) to concentrations of TBTO ranging between 3
and 30 µg/litre. Two batches of embryos developed abnor-
malities at the highest concentration of TBTO tested
(30 µg/litre) and showed some mortality. Other batches of
embryos showed embryotoxicity for all tested concen-
trations (down to 3 µg/litre) but no abnormalities in the
embryos. Owing to this difference in sensitivity of dif-
ferent batches of eggs and the separation of embryotoxic
and teratogenic effects, it is difficult to establish a
NOEL for this species.
Fent (1989b) exposed fertilized eggs of the freshwater
minnow Phoxinus phoxinus to two TBT concentrations in
petri dishes in an environment chamber. The eggs were ex-
posed from the blastula stage (20 to 24 h after spawning),
and the water was renewed daily. Analytical determination
of TBT showed that the actual concentration had reduced
over the 24-h exposure period from 1.5 to 0.5 µg/litre in
the low-dose group and from 8.4 to 7.9 µg/litre in the
high-dose group. There were no differences in survival
between the control and low-dose groups. Hatching was nor-
mal but some fish showed vertebral malformations. At the
high dose, hatching was delayed or reduced and all hatched
larvae had severe vertebral malformations; many were
unable to uncurl. These larvae remained motionless at the
bottom of the dish. Some had oedema in the region of the
heart. Within 3 to 4 days of hatching, all of the larvae
in the high-dose group had died. A further series of
experiments similarly showed malformation at an exposure
level of 4.5 µg TBT/litre.
8.3.4. Behavioural effects
The avoidance response of the mummichog (Fundulus
heteroclitus) to TBTO has been studied by Pinkney et al.
(1985). When given a choice between TBTO-contaminated and
clean water, 4 out of 6 groups of fish avoided a total
organic tin level of 1 µg/litre. There were significant
avoidance responses for all test groups at total organic
tin levels of 3.7, 8, and 13.8 µg/litre. However, the
higher concentrations of tin did not result in an increase
in avoidance response. The authors found that, under the
same conditions, no avoidance response was shown by the
grass shrimp (see section 8.2.4.5). This shrimp is a major
food item of the mummichog in tidal marshes. Hall et al.
(1984) studied the avoidance response of two species of
juvenile estuarine fish, the striped bass (Morone
saxatilis) and Atlantic menhaden (Brevoortia tyrannus). An
avoidance response to TBTO was exhibited by bass at a
total organic tin concentration of 24.9 µg per litre.
Atlantic menhaden were more sensitive, showing a "mild"
avoidance at 5.5 µg/litre and a "strong" avoidance at
9.1 µg/litre.
Chliamovitch & Kuhn (1977) measured an EC50 for the
loss of positive rheotaxis of 30.8 µg/litre for the rain-
bow trout (Salmo gairdneri) and 53.2 µg/litre for a
tilapia (Tilapia rendalli).
8.4. Amphibians
Summary
There are few data on the effects of tributyltin on amphib-
ians. Survival of frog larvae was at least 80% of control
levels after exposure to 3 µg TBT/litre.
Floch et al. (1964) reported a NOEL for mortality of
tadpoles of two species of amphibians (Rana and Alytes sp.)
of 30 µg/litre. For both species, the 24-h LC100 for
TBTO was 75 µg/litre, the 48-h LC100 50 µg/litre, and
the 48-h LC100 for TBT acetate was 75 µg/litre.
Laughlin & Linden (1982) exposed eggs of the frog Rana
temporaria to concentrations of either TBTO or TBT fluor-
ide of 0.3, 3, or 30 µg/litre, for 5 days from the post-
gastrula stage of development, under static renewal con-
ditions. All surviving larvae hatched on either day 4 or
5 of exposure. Survival was at least 80% of control levels
after exposure to 0.3 or 3 µg/litre. Only at the highest
concentration (30 µg/litre) did TBT affect survival (40%
mortality with TBT fluoride and 50% mortality with TBTO).
The authors collected all surviving tadpoles and measured
wet and dry weight. Wet weights were significantly lower
in tadpoles exposed to 30 µg/litre but unaffected at
lower exposure concentrations. Tadpoles exposed to TBT
always showed higher mean dry weight than controls,
although increases were not consistently dose dependent.
The percentage of body water declined from 86-88.5% for
controls to 73.8% after exposure at the highest concen-
tration of TBT. The changes resulting from TBT treatment
were significant, but not the differences produced by the
different TBT compounds.
8.5. Multispecies studies
Summary
The few available microcosm studies indicate that the most
sensitive organism(s) within the microcosm are affected by TBT
at concentrations greater than 0.05 µg/litre. Recovery of
these organisms occurred a few months after exposure. Simul-
taneous exposure of snails and fish yields conflicting results,
snails appearing somewhat more sensitive than fish.
Beaumont et al. (1987) exposed sandy-substrate micro-
cosms of flowing sea water to TBT derived from slate
panels painted with "Micron 25" antifouling paint. Three
replicate microcosms were exposed for 4 months at high
(1-3 µg/litre) and at low (0.06-0.17 µg/litre) TBT con-
centrations in water, together with three control micro-
cosms. Flow rates were maintained by gravity from a header
tank at approximately 1 litre/min; the painted panels were
located in the header tank where water was passed over
them using a circulating pump. The substrate sediment was
sieved after collection (to 2 mm) to remove larger organ-
isms and allowed to settle in the microcosms for 3 weeks
before the addition of animals. The sediment was 10 cm
deep and the overlying water 15 cm deep in each microcosm.
At the beginning of the trial, 50 specimens of a bivalve
(Cerastoderma edule), a crustacean (Corophium volutator),
and two polychaetes (Nereis diversicolor and Cirratulus
cirratus) were introduced. After 4 weeks, 12 specimens of
the gastropod Littorina littorea were added and, after 6
weeks, 26 specimens of a second bivalve (Scrobicularia
plana) were added. Other species were found at the end of
the trial derived from small (< 2 mm) juveniles in the
sediment and inflowing sea water. Each day, 10 litres of a
suspension of the microalga Pavlova lutheri (5 x 106 cells/ml)
were added to the header tank as additional food supply.
The most sensitive of the introduced species was the
cockle (C. edule); 100% died within 2 weeks at the high
concentration of TBT and there was cumulative mortality at
the low TBT concentration over 17 weeks (14% of controls
died). Two-way analysis of variance indicated significant
differences between all three groups (i.e. control, and
low and high concentrations of TBT; p < 0.05). The other
bivalve (S. plana) showed high mortality at the highest
exposure level (100% after 10 weeks). At the low TBT con-
centration there were no time-related deaths in this
species and no significant difference from controls.
Among the polychaetes, high death rates were recorded
for N. diversicolor in all microcosms, including the
control, possibly because adults introduced were ripe and
died after spawning during the experiment. The other
polychaete (C. cirratus) survived well even after exposure
to the high concentration of TBT. Only one gastropod (L.
littorea) died in any of the microcosms. Juvenile bivalves
were the most common species found; their numbers and
diversity were lower in the low-dose microcosms (66 to
109) than in control microcosms (137 to 179), and they
were virtually absent from the high-dose microcosms (0, 1,
and 1 for the 3 replicates). The size of juvenile mussels
(Mytilus edulis) was also significantly reduced at the
low TBT concentration, relative to controls; other self-
introduced bivalve species were not affected by the low
TBT exposure. Measurements of chlorophyll a in sediment
cores, as a measure of algal biomass, indicated a signifi-
cant rise after exposure to high TBT levels. This is
explained by the relative insensitivity of algae to the
toxic effects of TBT and by reduced (or non-existent)
animal life available to consume the algae. The authors
emphasize the sensitivity of some species, for which TBT
is toxic at levels of 1 to 2 µg/litre under conditions
approximating natural exposure. They further emphasize
the great variation in sensitivity between species; for
example, while Mytilus edulis and Cerastoderma edule were
clearly affected at very low levels of TBT, other related
bivalves were largely unchecked. Differences in feeding
behaviour, leading to different effective exposure levels,
is offered as one possible explanation for differences in
response. Differential absorption or subsequent loss,
observed in laboratory studies with other molluscs, is
also proposed as a possible mechanism.
Henderson (1986) conducted long-term flow-through
microcosm studies on communities of marine organisms from
Pearl Harbour, Hawaii, where the organisms had been
exposed to leaching TBT from naval ships for some time.
Panels (20 x 25 cm) of roughened plexiglass were suspended
in the harbour for 14 weeks prior to the experiment to
allow settlement and growth of organisms, and 30 different
species were found on the panels. Prior to exposure, the
panels were kept for a further 5 weeks in the experimental
tanks. These tanks had a capacity of 155 litre and were
supplied with sea water pumped directly from the harbour
and through the tanks at a rate of 4 litres/min. The sea
water was passed through a 1 cm mesh with no further
filtration; organisms could, therefore, enter the tanks
easily and settle on the panels. The water was rich in
plankton consisting largely of diatoms, copepods, and
chaetognaths. Exposure to TBT derived from painted panels
treated with antifouling paint containing 9.4% TBT meth-
acrylate, 0.5% TBTO, 44.7% cuprous oxide, and 45.4% inert
ingredients. Nominal concentrations of TBT were 0, 0.05,
0.13, 0.31, 0.78, and 1.95 µg/litre, but actual mean
concentrations were 0.01, 0.04, 0.10, 0.54, 1.77, and
2.52 µg/litre, respectively. The measured copper levels
in the same samples were 1.0, 1.2, 1.2, 3.1, 4.4, and
5 µg/litre, respectively. Changes in coverage of the
panels was assessed using photographs taken with an under-
water camera at weekly or biweekly intervals throughout
the experimental period of approximately 60 days of
exposure and a further 60 days of recovery. One week after
the start of exposure, a further plastic panel was intro-
duced to monitor new colonization. There was a clear
decline in both total number of species and in species
diversity (reductions of 55%, 60%, and 80% at the three
highest exposure levels, respectively) on pre-fouled
panels exposed to TBT at 0.5, 1.8, and 2.5 µg/litre but
no effect at lower concentrations. The mortality of indi-
vidual species, in relation to TBT exposure, showed con-
siderable variation. The most sensitive of the six most
common species on the panels was Botrylloides spp, an
orange-coloured colonial tunicate, which showed 100% mor-
tality at TBT concentrations of 0.1 µg/litre or more. An
encrusting bryozoan, Schizoporella errata, showed 49% mor-
tality at 0.1 µg/litre and 80 to 100% mortality at higher
concentrations. Specimens of a second colonial tunicate,
Didemnus candidum, and the saddle oyster ( Anomis nobilis)
were all killed at concentrations of 0.5 µg/litre or
more. A tube worm (Hydroides elegans) and a solitary
tunicate (Ascidia spp.) survived even the highest
exposure level, though with considerable mortality at 1.8
and 2.5 µg/litre. Recovery of populations was complete
60 days after cessation of treatment. Settlement on the
panels not previously exposed was reduced by TBT concen-
trations of 0.1 µg/litre or more, but not by 0.04 µg per
litre. Algal settlement and growth on the walls of the
tanks was not affected by exposure to TBT at any concen-
tration; coralline algae increased their coverage of pre-
fouled panels. A total of 18 oysters (Crassostrea
virginica) placed in each tank prior to treatment was
monitored after 60 days. There was 50% mortality after
TBT exposure at 1.8 µg/litre but no significant reduction
in survival at lower concentrations. However, the con-
dition index of the oysters was affected by exposure to
0.1 µg/litre or more, but had returned to near normal
after 2 months recovery in clean water. The author
regarded 0.05 µg/litre as a reliable NOEL for the most
sensitive organisms exposed.
When Salazar & Salazar (1985) exposed copepods
(Acartia tonsa), mysids (Acanthomysis sculpta), and fish
(Citharichthys stigmaeus) to suspended sediment at a TBTO
concentration of 0.49 µg/litre water for 96 h, no sig-
nificant mortality was found. In a second experiment,
mysids (A. sculpta), worms (Neanthes arenaceodentata), and
clams (Macoma nasuta) were exposed to TBTO-contaminated
sediment with overlying water for 10 days in the case of
mysids and 20 days in the case of clams and worms. Levels
of TBTO in the sediment varied from 155 to 610 µg per kg,
falling during the exposure period, while measured levels
in the overlying water were about 0.2 µg/litre. No mor-
tality was observed during the exposure period.
Cardarelli (1973) exposed snails (Biomphalaria
glabrata) and fish (Lebistes reticulatus) to slow-
releasing molluscicides containing either TBTO or TBT
fluoride. At a daily release rate of 35 µg TBTO/litre,
100% mortality was achieved in snails within 60 days but
all of the fish also died within this period. At a TBTO
release rate of 7 µg/litre per day, snail mortality was
100% after 120 days exposure and fish mortality only 2%.
However, a second experiment gave a much higher fish mor-
tality of 46% after 120 days exposure to only 3.5 µg TBTO
per litre per day. Exposure to TBT fluoride at 7 µg per
litre per day killed all snails and fish within 60 days,
and 52% of fish and 100% of snails were killed after 90
days of exposure to 3.5 µg/litre per day.
In order to simulate the effect of molluscicides con-
taining 6% TBTO on biota, Jordan (1985) set up a model
ecosystem as follows: filtered river water was allowed to
flow into a tray containing washed mud (to simulate a
marsh), and overflow from this upper tank was allowed to
flow into a lower tank containing washed coarse sand (to
simulate a river). Snails (Biomphalaria glabrata and
Potamopyrgus coronatus) and guppies (Lebistes reticu-
latus) were added to the "marsh", while snails (B.
glabrata and Pomacea glauca), shrimps (Macrobrachium
faustinum), and L. reticulatus were added to the
"river". The molluscicide was added to the "marsh" as
pellets at levels of 2, 5, 10, or 20 g/m2 of marsh sur-
face. The percentage of both B. glagrata and L. reticu-
latus surviving 14 days of exposure was recorded (see
Table 13). The authors concluded that, in this model eco-
system, there was higher mortality of B. glabrata than of
L. reticulatus at low doses of slow-release TBTO, i.e. 2
and 5 g of pellet per m2 of "marsh".
Table 13. Percentage of B. glabrata and L. reticulatus surviving
14 days exposure to slow-release pellets containing 6% TBTO added
to the "marsh" in a "marsh" or "river" model ecosystema
----------------------------------------------------------------
Dose of B. glabrata L. reticulatus
molluscicide
"marsh" "river" "marsh" "river"
----------------------------------------------------------------
20 g/m2 00.0* 00.0* 00.0* 00.0*
(0/240) (0/240) (0/240) (0/240)
10 g/m2 00.0* 00.0* 00.0* 2.5*
(0/240) (0/240) (0/240) (6/240)
5 g/m2 00.0* 16.3** 23.3** 60.0+
(0/240) (39/240) (56/240) (144/240)
2 g/m2 52.9+ 75.4++ 75.8++ 78.8
(127/240) (181/240) (182/240) (189/240)
----------------------------------------------------------------
a From: Jordan (1985).
The percentages represent percent survival at various doses
for all weeks of exposure in replicate experiments. The
values marked with the same symbol are not statistically
different from each other at the 0.05 probability level. The
numbers in parentheses represent the number of survivors
compared to the number of animals exposed.
9. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: TERRESTRIAL ORGANISMS
Summary
Exposure of terrestrial organisms to TBT derives primarily
from its use as a wood preservative. However, little infor-
mation is available. TBTO has proved toxic to honey-bees coming
into close contact with TBTO-treated wood. TBT compounds are
toxic to insects exposed either topically or via feeding on
treated wood. The acute toxicity to wild small mammals is mod-
erate (between 37 and 240 mg/kg per day). The toxicity of TBTO
to bats is probable but not proven. There is no information on
other species.
9.1. Microcosm studies
Gile et al. (1982) introduced TBTO into a terrestrial
microcosm on pine posts, each microcosm containing four
posts (3.3 x 2.6 x 14 cm) treated with 14C-labelled TBTO
(167 mg/cm3). The microcosms consisted of soil and
endogenous soil organisms, ryegrass, earthworms, pillbugs
(woodlice), mealworms, crickets, garden snails, and a
gravid female gray-tailed vole. There were no effects on
any of the organisms over a period of 77 days. About 95%
of the TBTO remained in the posts for the whole of the
exposure period. A similar system set up to investigate
cricket mortality, with and without predation, showed no
effect of TBTO (Gillett et al., 1983).
9.2. Terrestrial insects
When Gardiner & Poller (1964) exposed larvae of the
common clothes moth (Tineola bisselliella) to wool treated
with a TBTO concentration of 1% by weight, all of the
exposed larvae were killed within the exposure period of
14 days. The action appeared to be that of a contact
insecticide because none of the cloth was eaten. Phenyltin
compounds were not as toxic as TBTO. Baker & Taylor (1967)
found that the action of TBTO more closely resembled the
slow toxicity of a stomach insecticide after exposing
wood-boring beetles (Lyctus brunneus) to impregnated wood.
Contact insecticides such as lindane ( gamma-hexachlorocyclo-
hexane) and dieldrin were 100 times more toxic than TBTO.
The LD50 of TBTO for another wood-boring species, Anobium
punctatum, was 0.254 kg/m3 (application rate to wood).
Saxena & Crowe (1988) applied TBTO, TBT chloride, and
TBT linoleate topically to the thorax of newly-emerged
insects of three species. The LD50 values ranged from
0.48% to 0.72% (dilutions with acetone) for the house fly
Musca domestica, 0.29% to 0.69% for the mosquito
Anophelese stephensi, and 0.52% to 0.87% for the cotton
stainer Dysdercus cingulatus. TBT compounds were more
toxic than the other organotin compounds tested (triphe-
nyltin, tricyclohexyltin, dimethyltin, phenyltin, and
diethyltin). The authors pointed out that TBT compounds
are considerably less toxic than trimethyltin compounds to
Musca sp.
In studies by Kalnins & Detroy (1984), wood was
treated with TBTO (1.9 kg/m3) after sawing and before
use in the construction of beehives. Five hives made from
the treated wood were stocked with bees. Tin residues of
3.24 mg/kg were found in bees during the first summer, and
residues of 8.67 mg/kg were found in the wax of the combs.
However, there were no detectable tin residues in honey.
There was high mortality in the bee colonies over winter,
only one colony surviving. No control colonies died over
winter. Residues in bees and wax in the second year (1.3
and 4.6 mg/kg, respectively) were lower than in the first
year in the one surviving colony.
9.3. Terrestrial mammals
Racey & Swift (1986) housed pipistrelle bats
(Pipistrellus pipistrellus) in roosting cages treated
with TBTO. The bats were pregnant females collected from
nursery roosts, and they were trained to feed on mealworms
before transfer to the experimental cages. The cages were
made of metal, lined with plywood, and were painted with
TBTO as a 1% solution in white spirit (the manufacturers
recommended rate for use of TBTO as a fungicide). The wood
was treated 2 months before the bats were introduced into
the cages. During the course of the 142-day experiment,
seven of the ten bats died. Median survival time was 100
days, with a range between 49 and 142 days. Two deaths in
the white spirit control group and three in the untreated
control group meant that results for TBTO were not stat-
istically significant.
Schafer & Bowles (1985) conducted toxicity and repel-
lency tests on deer mice (Peromyscus maniculatus) and
house mice (Mus musculus) using various TBT salts. Treat-
ment of feed seeds with 2% (by mass) produced clear repel-
lent effects of TBT in both species. The approximate
lethal dose (estimated by increasing the TBT dose until
the test animals died) for TBT acetate and fluoride was
320 mg/kg. The estimated dietary LC50, based on consump-
tion of treated seed used in the repellency tests, varied
between 37.5 mg/kg per day for TBT acetate and 238 mg/kg
per day for fluoride and sulfate. The LC50 for TBTO was
200 mg/kg per day.
10. EFFECTS ON ORGANISMS IN THE ENVIRONMENT: FIELD OBSERVATIONS
Summary
Tributyltin compounds have had a wide range of uses. Most
concern has focussed on their use in the marine environment,
where TBT has been associated with mortality and failure of
settlement of bivalve larvae, reduced growth, shell thickening,
and other malformations in oysters, imposex (the development of
male reproductive appendages in female animals) in mud snails,
and imposex concurrent with population decline in dogwhelks.
Controls on the use of TBT in antifouling paints has led to
recovery of economically important shellfish growth and repro-
duction. Water concentrations of TBT are still high enough in
some areas to affect marine gastropods.
Field and laboratory results for marine molluscs are in
good agreement. Both imposex in dogwhelks and shell growth and
chambering in Pacific oysters are effective biological indi-
cators of TBT contamination.
There have been few studies into the effects on organisms
of TBT in sediment. There are indications that TBT is bioavail-
able to burrowing organisms and can cause mortality in the
field.
Gross toxic effects and histopathological changes have been
reported in farmed marine fish exposed to TBT through the use
of antifouling paints on retaining nets.
Although TBT has been detected in fresh water at high con-
centrations in some areas, there have been few studies on its
effects. Spills of large amounts of TBT from timber treatment
plants have caused ecological damage, but recovery occurred
within 9 months.
Field testing of tributyltin derivatives, mainly slow-
release formulations of TBTO, has shown that it is difficult to
apply TBT without damaging non-target organisms. Recovery
occurs through recolonization.
10.1. Effects on bivalves
In the 1970s, the French oyster industry was
undergoing a crisis. During the 1977, 1978, and 1979
seasons, very poor spatfalls were reported, together with
increasing reports of poor shell growth and shell malfor-
mations. An extensive survey of the occurrence and inten-
sity of malformations related to metal residues in oysters
suggested, for the first time, a connection with organotin
compounds (Alzieu, 1981). The shell thickening was found
to be due to the appearance of chambers in the oyster
shell and interlamellar gel formation in these cavities
(Alzieu, 1981; Alzieu et al., 1982). The authors of these
reports described in detail the shell abnormalities and
the process of calcification of the oyster shell, and
suggested mechanisms of TBT action. TBT is known to in-
hibit oxidative phosphorylation and it has been suggested
that this forms the basis of its action on the shell. It
is also known to complex amino acids. The effect on calci-
fication derives from inadequate calcium addition to the
organic matrix (a process dependent on ATP) and incorrect
deposition of this matrix. Alzieu et al. (1982) found good
correlation between the occurrence of shell thickening and
the proximity of ports where large numbers of boats were
usually moored. These field observations were corroborated
by the finding of similar shell abnormalities in the lab-
oratory when oysters were exposed to TBT fluoride, an
organotin compound present in antifouling paint used on
the boats. Oysters placed in flowing sea-water tanks con-
taining plates coated with TBT antifouling paints died
after a 30-day exposure to an estimated water concen-
tration of 2 µg/litre (organotin leachate) and shell
thickening was found to occur at water concentrations of
0.2 µg/litre.
Alzieu et al. (1982) assessed the shell quality of
18-month-old Pacific oysters ( Crassostrea gigas; also
known as the Japanese oyster) sampled along the eastern
coast of Oleron Island and in the vicinity of La Rochelle,
France. They concluded that the proximity of pleasure
craft ports or a commercial harbour could badly affect the
quality and growth of oyster shells. However, the presence
of other chemicals, along with TBT in the sea water made
direct assessment difficult. The authors, therefore,
carried out a set of experiments to confirm their
conclusions. Groups of oysters from areas with no shell
abnormalities were distributed to other locations, i.e. a
marina, a river, and the laboratory (three groups: a
control group; a group exposed to 50-cm2 panels coated
with TBT fluoride; and a group exposed to coated panels
500 cm2 in area). All oysters died within 30 days after
exposure to the larger panel in the laboratory and within
170 days after transfer to the marina. Oysters exposed to
the smaller panels showed a mortality rate of 30% after
110 days of exposure. Oysters transferred to the marina
site and those exposed to 50-cm2 TBT-coated panels devel-
oped gel-filled shell cavities after 100 and 110 days,
respectively, during the period of shell growth. Analysis
of the oysters for total tin content revealed levels of
< 1 mg/kg (dry weight) in "unpolluted" groups, 110 mg/kg
after 80 days at the marina site, and up to 25 mg/kg after
exposure to 50-cm2 panels in the laboratory.
Maurer et al. (1985) found TBT levels to be related to
inhibition of settlement of Pacific oyster in the Bay of
Arcachon and the Gironde estuary, France. The authors used
arrays of settling tubes mounted around a central tube
which was painted with "International TBT Antifouling"
at a rate of 6.4 g of paint on a surface of 8 dm2. The
settling tubes were mounted at varying distances (between
4.5 and 13 cm) from the central painted tube. In control
arrays, the central tube was unpainted. The arrays of
tubes were placed out in the two study areas in July 1982
and observations on settlement and growth were made in
September and November 1982. In the Gironde region, a
second method was also used; slates were painted with TBT
paint (21 g of "International TBT Antifouling" on a
surface of 26 dm2) and mounted 10 cm apart, alternating
unpainted with painted slates on a rod. The spacing of
tubes in the arrays was up to 25 cm from the central
painted tube. In the Gironde estuary, settlement was com-
parable with controls except on the painted tube; tubes
5 cm or more away from the TBT paint had similar numbers
of settling larvae as the control. However, deaths among
the settled larvae were high; 100% on tubes 15 cm away (or
less), 99.3% on the tube at 20 cm distant, and 78.3% on
the tube 25 cm distant from the paint. Slates showed high
settlement rates and a mortality of 82.5% at 10 cm away
from the paint. In the Bay of Arcachon, there was a much
lower settlement of larvae; in the Villa Algerienne area
there were 178 settlements/dm2 on the treated tubes
compared with 440 on controls, and in the Comprian area
81 or 83 settlements/dm2 on treated tubes compared with
323 on controls. Barnacles, which were less sensitive to
the paint, were also found to settle on the tubes. The
settlement period in this area was longer than that in the
Gironde estuary; some larvae settling later in the season
showed reduced growth compared with controls.
Thain & Waldock (1986) reported that the Pacific oys-
ter was introduced into the United Kingdom during the mid-
1970s. At this time, growth trials were performed at sev-
eral coastal locations. The oysters grew well at most
sites, but at some east coast sites, such as the estuary
of the River Crouch, they exhibited poor growth, reduced
meat yield, and shell thickening. At other sites, such as
the north Norfolk coast, there were good growth results
with none of the deformities found at the Crouch. The
cause of the poor growth results in some areas was inves-
tigated, and Key et al. (1976) found good correlation
between poor growth and high levels of fine suspended
particles. Areas of poor performance in the trials were
not used for the cultivation of the newly-introduced
oyster species. The types of shell abnormality exhibited
by oysters in France were very similar to those observed
in oysters from the east coast of England, which had been
attributed to sediment. The sediment in the marinas used
by Key et al. (1976) for resettlement studies was probably
contaminated with TBTO (Personal communication by M.J.
Waldock to IPCS). In 1982 it was decided to reassess the
causes of poor oyster shell growth in Britain. Levels of
TBT were measured in the estuaries of the Rivers Crouch
and Blackwater, on the east coast of England, and were
found regularly to exceed 0.2 µg/litre, a level shown by
the French studies to be harmful to the oyster (Thain &
Waldock, 1986). In a laboratory study, Waldock & Thain
(1983) investigated the effect of both suspended sediment
and TBT on growth and shell thickening in spat of the
Pacific oyster. They found that TBT levels of 0.16 µg per
litre inhibited and 1.6 µg/litre stopped growth. Shell
thickening was observed in the oysters exposed to TBT.
Exposure to sediment in "clean" water (i.e. containing
no TBT) actually enhanced growth. Thain & Waldock (1986)
reported that, during 1983, the laboratory finding that
TBT and not suspended sediment was affecting growth and
causing shell abnormalities in oyster was corroborated in
the field by studying oysters from different sites.
Alzieu & Portmann (1984) reported that although the
findings clearly implicated TBT as a major cause of growth
problems and abnormalities, they did not completely
exclude the possibility that other chemicals present might
have caused similar effects. They reviewed the effects of
a 1982 ban by French authorities on use of TBT paints on
boats shorter than 25 m. In the Bay of Arcachon area
(where, in 1980 and 1981, 95% to 100% of oysters had shown
deformities), the 1982 figures for deformities were 70% to
80% and by 1983 deformities had declined to 45% to 50%.
The number of oysters from the same area showing deform-
ities in both upper and lower shells was between 70% and
90%, in 1980 and 1981, and zero by 1983. The spatfall,
which in both 1980 and 1981 failed completely, sub-
sequently recovered; it was described as good in 1982 and
excellent by 1983. The authors also reported, however,
that these results were not reflected in another area
(La Rochelle Bay). This was attributed both to its close
proximity to a major commercial harbour and the fact that
the ban on the use of TBT antifouling paints on pleasure
craft in this area had not been as strictly observed as in
the Bay of Arcachon.
His et al. (1986) reported bioassays conducted on
oysters (Crassostrea gigas) using sea water collected from
the Bay of Arcachon both before and after the imposition
of a ban on the use of TBT paints on small boats. The sea
water collected in 1981 caused abnormalities in 40% of
larval oysters over 12 days of observation (compared with
4% in controls), whereas the sea water from 1982 produced
only 12% abnormalities over the same period. Growth of the
oyster larvae was also improved relative to the period
when TBT paints were still being used. In 1981, mean
growth of larval shells over 12 days was 133 µm, in Bay
of Arcachon sea water, compared with 142 µm in controls.
In 1982, mean growth was 162 µm, as opposed to 168 µm in
controls. Alzieu et al. (1986) stated that between 1980
and 1982 about 90% of oysters displayed anomalies in shell
calcification. Each year anomalies became apparent in
April and reached maximum intensity during June and July.
During the period 1983 to 1985, the percentage of oysters
displaying shell anomalies fell steadily. By 1985 none of
the oysters examined had malformations in both valves and
less than 40% had shell anomalies in one of the valves
(usually the upper valve). Alzieu et al. (1989) monitored
oysters in 1986 and 1987 and found that, although oysters
with deformities in both valves had stabilized at < 10%,
those with deformities in at least one valve had risen
again to a peak of 70% for both years.
Effects on oysters have also been reported near to
fish farms containing nets treated with TBT antifoulants.
Davies et al. (1987a) maintained caged Pacific oysters
(obtained from a nursery unit distant from any significant
sources of TBT) at varying distances from fish farms at
Loch Sween, Scotland. They found that significant accumu-
lation of tin was restricted to within 200 m of the fish
farms and that effects on oyster shell structure were
observed at a distance of 1000 m but not at 5000 m. A com-
parison of shell thickness index and tin accumulation
showed significant tin accumulation in those oysters with
the most severe shell thickening.
Studies in the USA and Japan have revealed similar
effects. Stephenson et al. (1986) transplanted culched and
culchless Pacific oysters (Crassostrea gigas) and two
species of mussel (Mytilus edulis and Mytilus
californianus) to areas of San Diego Bay, California, USA,
along a gradient of known sea-water TBT concentrations
(0.01-0.93 µg/litre). Reduced shell growth was observed
in all three species in areas where TBT levels were
highest. Oyster and Californian mussel samples (but not
M. edulis ) showed marked trends of reduced growth with
increasing TBT levels. Shell thickening in the oysters
also correlated with increasing TBT levels. Wolniakowski
et al. (1987) found Pacific oysters in Coos bay, Oregon,
USA, to have thickened and ball-shaped shells. When these
oysters were analysed, they were found to contain high
levels of TBT (49.7 to 189 µg/kg). The most marked
deformities occurred in animals collected in a marina and
near to where boats were painted. Okoshi et al. (1987)
transplanted spat of two different strains of Pacific
oysters (Crassostrea gigas) to two different experimental
field sites in northern Japan for 41 weeks. The number of
chambers in the oyster shells increased gradually in the
Miyagi strain at one site. In contrast, few chambers were
observed in the Hiroshima strain at either site. The
authors concluded that both genetic and environmental
factors were involved in the formation of shell chambers.
Crassostrea gigas is a non-indigenous species in the
United Kingdom and France. Stocks for breeding were intro-
duced into both countries in the late 1960s and originated
from the Miyagi region of Japan (Walne & Helm, 1979).
When Paul & Davies (1986) maintained scallops (Pecten
maximus) and Pacific oysters (Crassostrea gigas) in nets
coated with a TBTO-based paint, the mortality of scallop
spat was 24%, compared with a control mortality of less
than 6%, over the 31-week exposure period. Adult scallop
mortality was less than that of controls in the treated
group, and no effect was observed on growth. No oysters
died. Scallop spat in TBT-treated nets grew significantly
less rapidly than the control spat. Oyster growth, as
measured by mean shell length, was significantly reduced
and had largely ceased after 10 weeks of exposure. A sig-
nificant thickening of the oyster shell was observed
within 10 weeks of exposure and continued throughout the
31-week exposure. It was maintained even after transfer to
untreated nets for 10 weeks.
Minchin et al. (1987) monitored bivalve settlement in
Mulroy Bay, Ireland, between 1979 and 1986 using settling
panels placed in the sea. They found that, during this
period, settlement either failed or was reduced. Scallop
numbers fell from an average of > 1000 per panel in 1979
to zero in 1983. In 1984, no settlement of any bivalve
species was recorded on the panels. This reduced settle-
ment corresponded to the introduction of organotin fish-
net dips in local salmonid farms. The first use of TBT
paints appears to have been in 1981. This use of organotin
compounds ceased after 1985. In 1986, in all bivalves
monitored (except flame shells), there was again good
settlement. Scallops (Pecten maximus) and flame shells
(Lima hians and Chlamys varia) (all members of the
Pectinacea) were found to be particularly sensitive to
organotin compounds. It is not known whether the effect of
the TBT was on the reproduction of the bivalves or a toxic
effect on the larvae.
10.2. Effects on gastropods: imposex
Between the years 1972 and 1976, Smith (1981c)
sampled mud snails (Nassarius obsoletus) from four
locations bordering Long Island Sound in Westport and
Fairfield, Connecticut, USA. The snails were examined
for imposex (see section 8.2.3.6), and the results were
quantified by measuring the percentage of snails in a
sample showing any imposex and estimating the degree of
imposex within indi- vidual snails. In two of the
areas, adjacent to a yacht yard in Southport Harbour and
at the mouth of the harbour, 95% to 100% of snails had
some degree of imposex. Both showed significantly
more imposex than the other two sites, an area used
to moor a few old boats and an area protected from human
interference. The mud snails in this last area showed no
imposex. The area with a few old boats showed imposex
levels of 30% to 50%, much less than in the first two
areas. Smith (1981a) collected mud snails from three of
the locations, the yacht yard in the harbour (where
all female snails were abnormal and degree of imposex
greatest), the mouth of the harbour (where almost all
snails were abnormal but the degree of imposex changes
was intermediate), and the area protected from human
impact (at least 3.5 km from the nearest marina, where
all female snails were normal). The author labelled
these areas as "dirty", "intermediate", and "clean".
Snails transferred from the "clean" area to the
"dirty" area developed imposex. In those transferred
from the "dirty" area to the "clean" area the degree
of imposex was reduced. An analysis of chemicals
present in water from the "dirty" area was then carried
out and snails were exposed to some of the
contaminants individually, i.e. marina disinfectants,
detergents, copper antifouling paints, leaded
gasoline, combustion emissions, and two types of
TBT-based antifouling paints. Only the tin-containing
antifouling paints increased the level of penis
expression in female snails.
In a survey of the dogwhelk Nucella lapillus around
the south-west of England, Bryan et al. (1986) found
imposex to be widespread. The south coast of England was
the most severely affected. Populations showing the
highest incidence and highest intensity of imposex were
close to areas of boating or shipping activity. The degree
of imposex had increased markedly between 1969 and 1985 in
Plymouth Sound (an area on the south coast with large
numbers of small boats and ships), coinciding with the
introduction and increasing use of TBT-containing anti-
fouling paints in the area. Imposex correlated with
concentrations of sea-water tin (TBT fraction) and resi-
dues of tin in the dogwhelks (Fig. 6). Transferring whelks
from an area with little boating activity to Plymouth
marina resulted in a marked increase in the degree of
imposex. Bailey & Davies (1988b) found an increase in the
degree of imposex and also a higher incidence of penis
development in female dogwhelks collected in 1987 in the
Firth of Forth, Scotland, compared with those caught in
1975.
One of the effects of imposex on the female dogwhelk
is the blocking of the pallial oviduct, preventing the
release of egg capsules and rendering the female sterile.
A high incidence of females carrying aborted capsules was
found in declining populations close to sources of TBT.
The build-up of aborted capsules seemed, eventually, to be
lethal to the female; there were fewer females than
expected in affected areas (Gibbs & Bryan, 1986). The same
authors reported that the gross morphological changes
occurring in late imposex in the dogwhelk seem to be irre-
versible, since animals transferred from a moderately-
contaminated site to a "clean" site showed no resorption
of the penis. Gibbs & Bryan (1987) stated that imposex in
the dogwhelk was seen in sea water with TBT concentrations
of less than 1 ng tin/litre. They reported that the repro-
ductive failure, along with a lack of recruitment, had led
to population declines, almost to the point of extinction,
in areas of heavy TBT contamination. Gibbs et al. (1988)
experimentally transferred dogwhelks from an uncontami-
nated area to one showing water concentrations of 9-19 ng
tin/litre (TBT fraction) and demonstrated the development
of imposex within 18 months at the new location. These
transferred adults were able to spawn for much of this
period. The authors compared these results with results
for juveniles, which developed imposex earlier and were
sterile before reaching maturity. They discussed the
implications for the recolonization of areas where repro-
duction in the dogwhelk has been eliminated. Adults are
irreversibly affected by imposex. Recolonization is
unlikely until the TBT levels in sea water fall to around
2 ng tin/litre, a concentration at which the juveniles
are not sterilized before they reach sexual maturity. The
extent of recovery of populations would, therefore, depend
on the success of control measures and the water
concentrations resulting from bigger ships exempt from the
ban on TBT-containing antifouling paints.
Bryan et al. (1987) stated that while the evidence did
not show conclusively that TBT is solely responsible for
imposex in the dogwhelk, circumstantial evidence was con-
siderable. Imposex is related to the level of boating
activity. There is a significant relationship between
imposex and the body residue of organotin. Populations
that are in decline show the highest levels of TBT. Female
populations are declining faster than males and also have
the higher levels of TBT. The rise in the degree of
imposex coincided with the introduction and use of anti-
fouling paints containing TBT. Imposex has been shown to
be caused by TBT in other species of stenoglossan snails,
i.e. mud snails (Smith, 1981b). A significant decline in
imposex in monitored juvenile Nucella (Personal communi-
cation by P.E. Gibbs & G.W. Bryan to IPCS) followed the
introduction of TBT legislation in the United Kingdom.

 10.3. Effects on farmed fish
Wooten et al. (1986) reported the effects of exposure
to TBT antifouling paints applied to retaining nets for
farmed salmon. Fish exposed to newly-treated cage nets
were reported to be blind. The authors examined diseased
post-smolt salmon from cages treated with TBT at the time
of smolt transfer. Elevated tin levels were found in all
salmon exposed to the TBT paint; liver residues ranged
between 1.01 and 1.62 mg tin/kg wet weight. Residues in
the liver of blind fish ranged between 1.01 and 1.07 mg
tin/kg wet weight. Blindness had been caused by rupturing
of the eye; the eye lens was missing. Eye tissues appeared
normal histologically apart from the rupture; there were
no histological lesions. The kidney, liver, stomach, heart
and muscle of the blind fish appeared normal histologi-
cally. The intestine showed mucosal sloughing, vasodi-
lation, and pyknotic nuclei. The spleen had an "open
structure" with increased numbers of erythrocytes. There
was thickening of secondary gill filaments and some
necrosis and capillary separation in the epithelium. Other
fish in the affected population were reported to have
swellings along the lateral line. These were shown histo-
logically to be thick folds of epidermis under which there
was cellular infiltration of necrotic collagen forming an
abscess. Pancreas disease was also reported in affected
fish. In the liver there was a breakdown of lobular struc-
ture with lack of cohesion between cells. The effects were
thought to be consistent with poisoning by organotin.
10.4. Effects of TBT-contaminated sediment
Matthiessen & Thain (1989) studied the recolonization
by marine organisms in the field of sediment contaminated
with TBT-containing paint. Sediment was collected from
mudflats, and macroorganisms present were killed by
repeated freezing and thawing. TBT was added to the sedi-
ment in the form of abraded paint. The paint was abraded
with scourers used on yachts to produce material of the
kind likely to contaminate sediments normally. Sediment
samples containing 0.1, 1, 10, and 100 mg TBT/kg were
prepared. The contaminated sediment was returned to the
mudflats and placed in excavated trenches (3 m x 30 cm
wide x 20 cm deep) lined with polyethylene mesh (5-mm
apertures). Recolonization of the sediment could, there-
fore, take place both by settlement of organisms from
above and by lateral transfer of organisms from adjoining
mud. The sites were revisited five times during the next
160 days when samples were taken both to count recolon-
izing organisms and to measure TBT levels at various
depths. Surface sediment decontaminated rapidly; water
movement would have removed sediment and deposited new.
The subsurface TBT levels remained reasonably stable
throughout the experiment. Burrowing activity (estimated
by counting casts on the surface) of the polychaete
Arenicola marina was reduced at all concentrations of
TBT, though the effect of 0.1 mg TBT/kg disappeared after
3 months. A dose-related reduction in populations of the
burrowing polychaete Scoloplos armiger and burrowing
amphipod Urothoe poseidonis was observed over the whole
concentration range. There were no clear effects on other
species, including molluscs. The authors pointed out that
some unaffected groups were associated with the surface
sediment, where TBT was lost rapidly. Feeding behaviour
of these surface dwellers, such as the cockle (Cardium
edule), would lead to little TBT exposure, since they fil-
ter overlying sea water and ingest little or no sediment.
Species associated with deeper layers of sediment and
feeding on fine particles, such as Urothoe poseidonis,
showed greater effects of TBT, resulting, presumably,
from greater exposure rather than greater inherent sensi-
tivity. The authors also noted the wide range of sensi-
tivity of different species in laboratory tests without
sediment. The bioavailability of TBT associated with sedi-
ment would probably be different from that dissolved in
water.
10.5. Effects of freshwater molluscicides
Following earlier laboratory trials on the use of TBT
as a molluscicide for schistosomiasis control (Deschiens &
Floch, 1962), Deschiens et al. (1966) applied TBTO to a
pond used for fish culture, in Cameroon, of approximately
1 metre in depth and with an area of 50 m2 (approximately
50 tonnes of water) and a water temperature of 23 °C.
Caged pond snails (Biomphalaria [Australorbis] glabratus
and Bulinus contortus), 10 to a cage supplied with food,
were placed in the pond in different areas and at differ-
ent depths. Renewal of cages was performed to study the
persistence of the compound. The pond was treated with
TBTO, as "Biomet" (50% TBTO and 50% toxicologically
inert dispersant), at rates giving TBTO concentrations in
water of 0.015, 0.03, or 0.045 mg/litre, and the side
effects on fish and plankton were investigated. At 0.045
mg/litre, TBTO killed 100% of the snails and 70% of the
fish within 24 to 48 h. At 0.03 mg/litre, TBTO killed 100%
of snails within 2 to 3 days, but no effect on the fish
was observed within 15 days. At 0.015 mg/litre, the TBTO
killed all of the snails within 4 to 7 days and again had
no observable effect on the fish. The authors considered
this to be a practical concentration to achieve full
effect on snails without killing non-target organisms.
Various planktonic organisms (desmids, daphnids, and
aquatic insect larvae) were killed by TBTO at 0.5 mg/litre
experimentally. However, no effect was found on any of
these species in the pond treated at 0.015 mg/litre,
although there was some inhibition (but no deaths) of
these organisms at 0.03 and 0.045 mg/litre.
Gilbert et al. (1973) applied slow-release TBTO in
underseal (quick-drying asphalt-rubber-asbestos-clay paste)
to four sites, in Brazil, at concentrations of 15, 15, 50,
or 300 g TBTO/m2, to control Biomphalaria tenagophila
and B. straminea. Dead fish were observed at the sites
treated with 50 or 300 g/m2 and also at one of the two
sites treated with 15 g/m2, a static man-made pit. At
the other site, with a flow rate of 100 to 200 litre/min,
there was a 100% reduction in the snail population (B.
tenagophila) maintained for up to 12 months, while both
fish and aquatic insect life were apparently normal one
month after treatment. At this site abundant natural veg-
etation flourished in the immediate vicinity of the mol-
luscicide. Toledo et al. (1976) found that 15 g TBTO/m2
eliminated snails from 76% of treated sites for 1 year.
Guppies, which are often present in highly polluted sites,
normally suffered some mortality at the beginning of mol-
luscicidal treatment but in general they tolerated it
well. The authors found no lasting effect on plant or
insect life, and concluded that, because of the ability of
an unidentified fungus to grow profusely, microbiological
life continued in treated areas.
Shiff et al. (1975) applied BioMet SRM pellets (con-
taining TBTO) at concentrations of 20 and 30 g/m2 to a
night-storage dam in Zimbabwe. At the lower application
rate, pellets remained active against the snails (various
Biomphalaria spp.) for up to one year. At 30 g/m2, 100%
mortality of snails was achieved within 2 weeks of appli-
cation and was maintained up until the last sampling time
2 months after application. Prior to treatment, 80
specimens of Tilapia fish sp. and 1 of Clarias gariepinus
were caught. Four weeks after treatment 26 Tilapia were
caught and one dead Clarias was found. Eight weeks after
treatment 400 Tilapia were caught, of which 80 were
examined and all appeared normal.
Ayala et al. (1980) applied slow-release molluscicide
(rubber impregnated with 11% TBTO) at a rate of between 5
and 15 g/m2 to a site in Brazil and studied the effect on
non-target organisms. The TBTO molluscicide was initially
herbicidal towards floating vegetation but did not affect
either plants rooted in the mud or marginal plants. The
chemical was initially repellent to aquatic animals, some
of which were killed by the molluscicide. Within 3 months,
populations of non-target snails such as Pomacea sp.,
Drepanotrema sp., and a Physa sp. had returned to normal.
Although no fish had returned within 3 months, after 5
months numbers had returned to normal. In fact, 5 months
after application all but Spirogyra sp. were normal, and
the molluscicidal activity of the TBTO was retained for
more than one year. The authors concluded that after 3 to
5 months the action of the TBTO molluscicide is confined
to the bottom mud where Biomphalaria glabrata snails spend
much of their time.
10.6. Effects from spills
According to Waldock et al. (1987a), major inputs of
TBT into the environment may have arisen from spills of
timber-treatment products, often containing dieldrin and
pentachlorophenol as well as the organotin. They reported
that a detailed study was carried out after a spill con-
taminated a section of the Newmill Channel, Kent, United
Kingdom. There was a major kill of fish and all macroin-
vertebrates (except oligochaetes, chironomid larvae, and
elmid beetles) were also killed. Concentrations of TBT in
the water shortly after the spill were 540 µg per litre,
falling to 10 µg/litre after 3 weeks and 0.75 µg per
litre after 6 weeks. Nine months after the incident,
macroinvertebrate populations were reported to have
recovered. The authors stated that five such major spills
from timber treatment plants had been reported in the
United Kingdom within 2 years.
10.7. The use of indicator species for monitoring the environment
Both shell growth and shell chambering in Pacific
oysters and imposex in dogwhelks have been used as bio-
logical indicators of TBT contamination. Smith et al.
(1987) transferred juvenile Pacific oysters to major bays
and harbours of California, USA, known to contain elevated
levels of TBT. They observed a graded increase of stunted
growth and/or shell deformation, which was associated with
poor flushing and the proximity of large numbers of boats.
Gibbs et al. (1987) used the dogwhelk to monitor water
around the south-west peninsula of England. They found the
species to be a very sensitive indicator, especially if
the individuals were about 1 year old and in the early
stages of ovarian development. Davies et al. (1987a) found
imposex in dogwhelks to be a far more sensitive indicator
than either shell thickening in oysters or tin accumu-
lation. The dogwhelk has been used to identify areas of
contamination associated with seasonal small boat activity
and salmon farm cages in Scottish sea lochs (Davies et
al., 1987b), in areas of pleasure craft activity, fishing
harbours and a boat yard in the Firth of Forth (Scotland)
(Bailey & Davies, 1988b), and to investigate contamination
from an oil terminal in Sullom Voe, Shetland (Bailey &
Davies, 1988a).
11. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
11.1. Single exposure
Summary
The acute toxic effects of the various TBT compounds that
have been studied are comparable and are characteristically
delayed for several days. Oral LD 50 values in laboratory ani-
mals range from approximately 40 to 250 mg/kg body weight.
These compounds exhibit greater lethal potential when adminis-
tered parenterally, relative to the oral route, probably
because they are only partially absorbed from the gut. Acute
toxicity via the dermal route is low.
TBT compounds are potent skin irritants and extreme eye
irritants. Dermal exposure to TBTO appears to have little
sensitization potential.
Aerosols of TBT compounds are highly toxic. However, TBT
vapour/air mixtures at room temperature produce no effect, even
at saturation.
Other effects of acute exposure may include alterations in
blood lipid levels, the endocrine system, liver, and spleen,
and transient deficits in brain development. The toxicological
significance of these effects, reported after high single doses
of the compounds, is questionable and the cause of death
remains unknown.
11.1.1. Oral and parenteral administration
The acute toxicity of tributyltin to laboratory mam-
mals by various routes of administration is summarized in
Table 14. The acute oral LD50 for the rat ranges between
94 and 234 mg/kg body weight and for the mouse between 44
and 230 mg/kg body weight. Truhaut et al. (1976) pointed
to the delayed toxicity of tributyltin and, therefore, the
necessity to continue the observation of animals, after a
single acute dose, for several days. The period of post-
dosing observation is therefore indicated in Table 14.
LD50 values for ip and iv administrations of TBT are
very much lower than in the case of the oral route (10
mg/kg for rat ip and 6 mg/kg for mouse iv).
Table 14. Acute toxicity of tributyltin to laboratory mammals
------------------------------------------------------------------------------
Species TBT Route Observation LD50 (mg/kg Reference
perioda body weight)
(days)
------------------------------------------------------------------------------
Rat oxide oral 7 194 (165-227)b Elsea & Paynter (1958)
oxide oral 7 148 (113-195)c Elsea & Paynter (1958)
oxide oral 7 180 (132-228) Truhaut et al. (1976)
oxide oral 21 197 (137-273) Funahashi et al. (1980)
oxide oral 127 Schweinfurth (1985)
oxide ip 14 20 (18-21) Poitou et al. (1978)
oxide oral 234 Sheldon (1975)
fluoride oral 200 Sheldon (1975)
fluoride oral 14 94 Schweinfurth (1985)
chloride oral 14 122 Schweinfurth (1985)
acetate oral 113.5 Klimmer (1969)
benzoate oral 141 Klimmer (1969)
benzoate oral 14 99/203 Schweinfurth (1985)
oleate oral 225 Klimmer (1969)
linoleate oral 14 190 Schweinfurth (1985)
abietate oral 14 158 Schweinfurth (1985)
naphthenate oral 14 224 Schweinfurth (1985)
Mouse oxide oral 7 85 (52-130) Polster & Halacka (1971)
acetate oral 7 46 (25-85) Pelikan & Cerny (1968)
oleate oral 7 230 (175-301) Pelikan & Cerny (1968)
benzoate oral 7 108 (74-156) Pelikan & Cerny (1968)
chloride oral 7 117 (80-170) Pelikan & Cerny (1968)
laurate oral 7 180 (136-237) Pelikan & Cerny (1968)
oxide sc 7 200 (140-270) Polster & Halacka (1971)
oxide iv 7 6 (5.5-6.5) Truhaut et al. (1976)
oxide ip 14 16 (15-17) Poitou et al. (1978)
Rabbit fluoride dermal 680 Sheldon (1975)
------------------------------------------------------------------------------
a Following a single application of TBT, the observation period was as indicated.
b Application as aqueous suspension.
c Application in corn oil.
Pelikan & Cerny (1968) administered TBT (as the acet-
ate, benzoate, chloride, laurate, or oleate) to white mice
(body weight 25 g) in a single oral gavage dose of 500
mg/kg body weight, dissolved in sunflower oil. Ten animals
were used for each TBT compound. The mice were observed
for 8 h before being killed, and the viscera were examined
histologically. Four hours after treatment, all animals
showed signs of intoxication with the exception of those
given the laurate; after 8 h all showed toxic symptoms.
Gross damage was seen in the digestive tract, liver, and
spleen. Histological findings included a steatosis of the
liver cells in all animals (but to varying degrees),
traces of lipid in renal tubule cells (in those animals
receiving oleate or laurate) and many haemorrhages in the
digestive tract and kidneys. Effects on the liver and
spleen were also noted after dermal absorption of TBTO
(Pelikan & Cerny, 1969). However, no real conclusions can
be drawn from these studies as there was no clear reported
evidence that steatosis was caused either by the TBT or by
the fatty acids.
In studies by Funahashi et al. (1980), Sprague-Dawley
rats were given a single dose, in olive oil, of 100 mg
TBTO/kg body weight by intubation and were examined over
the following 21 days. Adrenal weight was slightly
increased 12 h after treatment and reached a maximum on
the second day. Histological changes in the adrenal had
returned to normal by day 14. The thyroid follicles showed
signs similar to those produced by hypophysectomy, i.e.
distension with colloid, flat epithelial cells. These
changes were severe after 72 h, but had returned to normal
within 14 days. Absolute pituitary weight was increased
slightly (and not significantly) 1 and 2 days after
dosing, while relative weight increased significantly.
There was atrophy of pituitary adrenocorticotrophin (ACTH)
cells 6 h after dosing. After 72 h, the staining of ACTH
cells became more intense. There were marked reductions
in the circulating levels of both thyroid stimulating
hormone (TSH) and thyroxine (T4) during the 72 h follow-
ing dosing, serum titres falling to one half and one sixth
of control values for the two hormones, respectively. The
authors concluded that TBTO had both a direct and an
indirect effect on the thyroid, since initially there was
a simultaneous rise in T4 and fall in TSH. Serum cortisol
levels increased to twice the control level 96 h after
treatment with TBTO at a level of 100 mg/kg body weight.
Intramuscular ACTH administration, 8 h after dosing, led
to increased cortisol in the blood of both treated and
control rats. After 16 h, controls showed release of
cortisol after ACTH stimulation, but treated rats showed a
decrease in circulating levels of cortisol after similar
stimulation.
Matsui et al. (1982) reported the effects of a single
dose of TBT fluoride (100 mg/kg body weight) given by
gastric intubation to Japanese white rabbits. A reversible
but pronounced hyperlipidaemia was observed, particularly
involving triglycerides and total cholesterol. Ultracen-
trifugation showed a marked increase in the chylomicron
plus VLDL (very low density lipoprotein) fraction. The
activity of lipoprotein lipase (LPL) in plasma was reduced
to about 50% of control levels. Fasting levels of blood
glucose were elevated, and the response to iv infusion of
glucose (given 3 days after the TBT fluoride) was
inhibited (insulin release reduced). The authors suggested
that the hyperlipidaemia was the result of reduced LPL
activity, which in turn was brought about by inhibition of
insulin release.
Calley et al. (1967) investigated the effects of TBT
acetate on liver function in rabbits. Following a single
oral dose of TBT acetate at 50 mg/kg body weight (half of
the measured LD50 in rabbits), only the serum glutamic-
pyruvate transaminase (SGPT) activity was affected. This
activity was not elevated after 48 h but was increased
within 144 h of dosing. Prothrombin time, alkaline phos-
phatase, and thymol turbidity showed no significant
changes from control values. SGPT is a highly specific
indicator of liver damage, directly reflecting liver cell
injury. Tributyltin acetate also significantly increased
the hexabarbitol-induced sleeping times of mice; a 50
mg/kg body weight oral dose increased the sleeping time
from 29 to 43 min following a standard dose of the
narcotic.
When Aldridge et al. (1977) administered TBTO (and its
gamma-keto, gamma-hydroxy, and delta-hydroxy metabolites)
intraperitoneally to mice on two consecutive days (at dose
levels of 12.5, 25, and 50 µmol/kg body weight), they
found no evidence of cerebral oedema after any of the
treatments.
Crofton et al. (1989) gave rat pups a single dose of
TBTO on day 5 following birth by gastric intubation with
0, 40, 50, or 60 mg/kg body weight. The monitoring of
behavioural parameters up to 62 days post-dosing showed no
persistent effect on motor activity or acoustic startle
response.
When Barnes & Stoner (1958) fed rats with tributyltin
acetate during the first 3 months after birth, they found
increased brain and spinal cord water content at the
highest dose tested (100 mg/kg diet). This increase was
not sufficiently great to be seen in histological sec-
tions. Bouldin et al. (1981) found no light or electron
microscopic evidence of neuronal damage in the hippocampus
or the pyriform cortex of neonate or adult rats exposed
daily to tributyltin acetate at 10 mg/kg body weight by
gavage for up to 30 days.
O'Callaghan & Miller (1988) reported the effects of a
single ip injection of TBTO on neonatal rat brain. The
rats were injected when 5 days old with 2, 3, or 4 mg/kg.
They were sacrificed at 13, 22, or 60 days of age, and
various proteins in homogenates of brain tissue were
measured by radioimmunoassay. Brain weight was reduced in
a dose-dependent manner, the cerebellum being most
affected. There was no evidence of altered brain histology
under the light microscope. Dose-dependent and region-
dependent decreases were found in P-38 (a synaptic-vesi-
cle-associated protein) and myelin basic protein (a
protein associated with oligodendroganglia and the myelin
sheath); there were decreases in both total (per tissue)
and concentration (per mg) levels of these proteins in the
cerebellum and forebrain but not the hippocampus. These
effects were seen at a dose level that did not affect body
weight. However, in contrast to the neurotoxic effects of
trimethyltin compounds, which are irreversible and persist
into adulthood, these neurotoxic effects of TBTO were
transitory at dose levels that did not affect body weight.
Robinson (1969) monitored tissue levels of catechol-
amines after ip administration of TBTO, in corn oil, to
rats at a level of 10 mg/kg body weight. This dose
exceeded the 6-day LD50, determined by the same authors
to be 7.21 mg/kg. Brain noradrenalin levels were signifi-
cantly reduced at 2, 24, and 48 h after dosing, whereas
5-hydroxytryptamine levels were only reduced (though
markedly) after 48 h. Noradrenalin levels in heart tissue
were also significantly reduced after 2, 24, and 48 h.
Adrenal adrenalin and noradrenalin levels were reduced
after 24 and 48 h, but only adrenalin was affected after
2 h.
11.1.2. Dermal administration
The acute LD50 of TBTO administered to rabbits via
the dermal route is very high, i.e. approximately 9000
mg/kg body weight (Table 14). Although there is dermal
absorption of TBT (see later), the degree of absorption is
not great enough to lead to acute toxic effects sys-
temically, except at very high exposure levels.
11.1.3. Administration by inhalation
In a "nose only" inhalation exposure (to minimize
dermal exposure and exposure through ingestion) lasting
4 h, the acute LC50 of TBTO for the rat was estimated to
be 77 mg/m3 by measurement of airborne droplets. This
value decreased to 65 mg/m3 when "inhalable" particles,
10 µm or less in diameter, were considered. There was
evidence of lung irritation and oedema in this study
(Schweinfurth, 1985). When groups of 10 male and 10 female
rats were each exposed to atmospheres containing almost
saturated vapours of TBTO, TBT benzoate, or TBT naphthen-
ate once for 7 h, no deaths occurred during exposure or in
a 14-day observation period following exposure. Only minor
clinical signs were noted occasionally during the exper-
iment, such as slight nasal discharge (Schweinfurth, 1985).
Truhaut et al. (1979) exposed mice to an aerosol of
TBTO in olive oil, for either a single 1-h period or seven
1-h periods on successive days, using TBTO concentrations
in air ranging between 0.05 and 0.4 mg/litre (50 to
400 mg/m3). Exploratory behaviour was scored over 5-min
periods 2 h after the single exposure was complete or 24 h
after the last of the seven exposure periods. The lower
two exposure doses caused significant increases in explo-
ratory behaviour (17% and 5% for 42 and 84 mg/kg, respect-
ively) while the higher exposure doses reduced exploratory
behaviour (-18% and -38% for 170 and 340 mg/kg, respect-
ively). Truhaut et al. (1981) found a median survival time
for mice of 22 min and for guinea-pigs of 9 min after
exposure to an aerosol of tributyltin (concentration not
stated). They reported that only tissues in the respirat-
ory system showed significant lesions. There was diffuse
congestion of the pulmonary blood vessels extending to the
septal capillary beds. There were also inflammatory
responses in the trachea and bronchii, secretion of mucus
in the bronchii and bronchioles, and distension and rup-
ture of the alveoli.
Anger et al. (1976) exposed guinea-pigs to aerosols of
TBTO ranging between 0.1 and 1 mg/litre air for 1 h. All
males exposed to 0.2 mg/litre died, and 12 out of 15
females exposed to 1 mg/litre died. No particular lesions
were observed in those animals that died, with the excep-
tion of a general congestion of the lungs. Exposure of
either males or females to 0.17 mg/litre caused no deaths,
though nasal irritation was observed; all these animals
survived a further 7 days of observation.
11.1.4. Irritation and sensitization
11.1.4.1 Skin irritation
In a study by Elsea & Paynter (1958), undiluted TBTO
was applied to the closely-clipped skin of the abdominal
area of rabbits, which was then covered with rubber dental
damming, gauze, and adhesive tape. After an exposure
period of 24 h, the covering was removed, and the TBTO was
washed off, as far as possible, by sponging with warm
water. This single application of doses up to 11.7 g/kg
body weight caused some of the rabbits to die from the
effects of TBTO absorbed into the body. There was, how-
ever, only moderate dermal irritation, characterized by
reddening, oedema, atonia, blanched areas, and areas of
brown discolouration. Examination of the skin at autopsy
showed some subcutaneous oedema. Repeated dermal appli-
cation of TBTO, impregnated into paper at 8 mg/kg, daily
for 5 days, only affected one animal out of four. This
rabbit showed slight erythema and oedema following the
first and second applications. Later in the experiment,
the skin appeared normal. No detailed histological examin-
ation of the skin was carried out.
Pelikan & Cerny (1969) applied to the shaved skin of
rats two preparations of TBTO, i.e. "Lastanax T" (con-
taining 20% TBTO plus a medium of water, alcohols of short
chain length, and n -alkyl-polyethylene oxide) and
"Lastanax P" (containing 15% TBTO plus a medium also con-
taining bis-(5-chloro-2-hydroxyphenyl)-methane). Actual
doses applied, corresponding to water dilutions of 100%,
33%, 10%, and 1% of the preparations, were 185, 61.5,
18.5, and 1.85 mg TBTO/kg body weight for Lastanax T and
145, 47, 14, and 1.4 mg TBTO for Lastanax P. Controls
received either water or the medium without TBTO. The
experiment was carried out in duplicate. In the first
series, clinical observations were made twice daily for
60 days, whereas in the second series rats were killed on
day 10 and the skin was examined histologically. Control
rats showed no clinical or histological effects on the
skin. However, clinical and histological changes were
found in all treated rats, even with the 1% dilution,
though severity increased with exposure dose. On the first
and second days after application, there was reddening of
the skin and oedema developed. From day 3, haemorrhagic
eschars developed with well defined borders; there was no
inflammation of the surrounding areas of skin. Between
these foci, numerous papules and pustules developed, some
with bleeding; the apices of some papules were necrosed.
Later the eschars joined to create larger areas of
ulceration. Healing of the areas began between the 9th
and 12th days; necroses disappeared by day 20, pustules 3
to 4 days before, and papules by the 30th to 33rd day. All
signs had disappeared after 35-38 days (exposure to 1% and
10% solutions) or after 45-50 days (exposure to 33% or
100% solutions). Changes with Lastanax P were similar, but
less severe, than with Lastanax T. In the histological
study, numerous large bullae, filled with leucocytes and
coagulated exudate (proteins), were found under the
stratum corneum of the epidermis after 10 days. Akanthosis
and vacuolation of epidermal cells occurred following
exposure to the 10% and 33% solutions and, to a lesser
extent, the 1% solution. Small haemorrhages were found.
Epidermal changes were dispersed and alternated with areas
of skin appearing normal. The authors suggested that the
TBTO NOEL for human skin should be set at 0.005% to 0.01%.
In a study by Middleton & Pratt (1977), TBT chloride
was applied to a shaved area of dorsal skin of male
Alderley Park (Wistar-derived) rats, four-weeks old (body
weight 50-80 g), in absolute ethanol as a solution of
10 mmol/litre. This dose was equivalent to 67 nmol/cm2.
The TBT had produced microscopic changes in the skin
within 2 h of application. Polymorphonuclear leucocytes
accumulated in capillaries and in dermal tissues. Epider-
mal cells showed progressive degenerative changes between
2 and 8 h after application until, by 8 h after appli-
cation, separation of the epidermis and dermis had
occurred and fluid collected in this separation. Many
inflammatory cells were present in both dermis and epider-
mis. There was widespread epidermal necrosis within 12 to
24 h, and separation of necrotic epidermis from the dermis
was almost complete by this time. The vesicles formed by
this separation were frequently packed with inflammatory
cells as well as exudative fluid. Regeneration of the
epidermal layer was observed after 18 to 24 h and dermal
inflammatory infiltration was regressing by this time.
Erythema, visually assessed and scored, reached a maximum
within 5 h of application and remained at this level for
48 h. The erythema had subsided by 72 h after application.
Vascular permeability of the skin was assessed by
injecting rats with a dye, tryphan blue, 45 min before
sacrifice. There was a biphasic response. At 2 h after
application of TBT chloride, there was an initial increase
in permeability of the skin to 135% of control levels. A
second peak began at 12 h and persisted for more than
25 h after application. The effect was still evident after
48 h and had occurred in both the treated and untreated
flanks of the animal, indicating absorption of the com-
pound and a systemic effect. The water content of the skin
was increased by TBT chloride, reaching a peak within 2 h
of application; by 10 h this effect had disappeared.
Middleton & Pratt (1978) reported that TBT produced focal
epidermal necroses and dermal inflammation at levels as
low as 33 nmol/cm2, fairly extensive epidermal necroses
and dermal inflammation at 67 nmol/cm2, and almost total
epidermal necrosis at 167 nmol/cm2.
11.1.4.2 Eye irritation
Pelikan (1969) applied TBTO, as "Lastanax T or P",
in a single dose of 0.03 ml to the left eye of rabbits.
Lastanax T is 20% TBTO in alcohols of short chain length,
non-ionic surface active substances ( n -alkyl-polyethyl-
ene oxide) and water. Lastanax P is 15% TBTO in the same
vehicle with the addition of bis-(5-chloro-2-hydroxyphe-
nyl) methane. A 10% and a 1% solution in water were used.
The 10% solution represented an actual TBTO dose of 6.1
and 4.6 mg/kg body weight for the two formulations,
respectively. With the 10% solution, both rabbits died,
11 and 12 days after application. Severe ulceration of the
eye preceded death. Histopathological examination of
internal organs after death revealed hyperaemia of the
brain and medulla oblongata and hyperplasia of reticulo-
endothelial cells of the spleen. Necrotic changes were
seen on the cornea of eyes treated with the 1% solutions
within 3 h of application, and symptoms worsened over the
next 2 to 5 days. Recovery was incomplete 100 days later.
11.1.4.3 Skin sensitization
Poitou et al. (1978) investigated the skin-sensitizing
potential of TBTO in guinea-pigs using the Magnussen-
Kligman method. The concentrations used for sensitization
were 1% (intradermal phase) and 5% (topical phase). Using
challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals.
11.1.5. In vitro studies
Johnson & Knowles (1983) demonstrated that incubation
of rat blood platelets with TBT chloride or TBTO, in
vitro, inhibited their ability to take up 14C-labelled 5-
hydroxytryptamine (5-HT). The organotin also caused
release of 14C-labelled 5-HT, taken up before exposure,
along with endogenous 5-HT. Treating rats ip with TBT
chloride at 5.0 mg/kg body weight also led to reduced up-
take of 5-HT (37% inhibition) 30 min after treatment. The
level of 5-HT in platelets, however, was unaffected by the
in vivo treatment with TBT chloride. knowles & Johnson
(1986) reported that exposure of rat platelets to TBT
chloride inhibited aggregation induced with ADP or colla-
gen. The inhibition of ADP-induced aggregation was depen-
dent on the dose of TBT and on the exposure time prior to
ADP addition. Exposure of the platelets to 5 µmol TBT per
litre for 5 min or 10 µmol/litre for 0.1 min produced
inhibition. However, exposure to 1 µmol/litre for 5 min
was ineffective. With an incubation time of 1 min, TBTO
was effective in lengthening the time taken for aggre-
gation after collagen induction at 0.625 µmol/litre, but
not at 0.5 µmol/litre.
The trialkyltins, including tributyltin, have been
shown to inhibit oxidative phosphorylation in rat mito-
chondria (Aldridge & Street, 1964). TBT stimulated
adenosinetriphosphatase (ATPase) activity and caused
limited swelling of rat liver mitochondria. These last two
effects occurred at similar concentrations of TBT in the
medium. The authors postulated that the two effects were
linked; the ATPase activity increased over a concentration
range of TBT and was always mirrored by a decrease in the
mitochondrial swelling. This relationship persisted
despite a complex response to TBT over the concentration
range; at higher concentrations there was a sudden, much
smaller mitochondrial swelling and a concomitant rise in
ATPase activity. TBT also inhibited the hydrolysis of ATP
by rat brain microsomes, although the concentrations
required were much higher than those affecting phosphory-
lation. The authors postulated that a combination of tri-
alkyltin compounds with negatively charged lipids is
involved in their biological activity.
Elferink et al. (1986) treated polymorphonuclear
leucocytes (PMNs), obtained from the peritoneal cavity of
rabbits, with an unspecified tributyltin (1 µmol/litre).
Uptake of opsonized zymosan was used as a measure of
phagocytosis, and enzyme (lysozyme) release to the super-
natant during incubation was also monitored. There was
almost complete inhibition of both parameters at
10-6 mol TBT/litre but no effect at 10-7 mol/litre. In-
hibition of phagocytosis was exactly paralleled by inhi-
bition of enzyme release. At concentrations of between
10-6 and 10-5 mol/litre, tributyltin caused lysis of the
cells with release of LDH, suggesting damage to the plasma
membrane. Exocytosis, induced by FMLP in the presence of
"cytocolasin B", was also inhibited by the TBT at con-
centrations of 10-6 mol/litre or more. There was little
effect of the compound on ATP levels in PMNs, and the
authors suggested that interference with ATP production
was not the basis for the effect of the TBT. Activation of
PMNs is accompanied by an increase in plasma membrane
permeability to Ca2+; this was strongly inhibited by the
TBT. The authors proposed two alternative explanations.
Either the Ca2+ permeability change is directly affected
or an earlier step is inhibited which, in turn, affects
calcium permeability. The observation that the exocytosis
effect could be counteracted by the addition of sulfydryl
compounds led the authors to conclude that the earliest
stages of activation of the PMNs were affected. Arakawa &
Wada (1984) reported a suppression of the chemotactic
response of rabbit leucocytes (neutrophils) towards for-
myl-methionyl-leucyl-phenylalanine by tributyltin chloride
at concentration between 0.1 and 10 µmol/litre in vitro.
Reinhardt et al. (1982) used cell detachment and
cloning efficiency of baby hamster kidney cells (BHK-21
C13) to quantify the cytotoxicity of organotin compounds.
The two parameters are independent, but each covers a
range of cellular damage. Cell detachment indicates irre-
versible damage to the cytoskeleton over a 4-h period,
while cloning efficiency covers influence on growth of
single cells over a 6-day period and includes cell re-
attachment followed by clone formation. The IC50 (concen-
tration at which there was a 50% reduction in cloning
efficiency) for TBTO was 5 x 10-7 mol/litre (0.3 mg/litre
medium) and for TBT chloride was 1.4 x 10-6 mol per litre
(0.5 mg/litre). The CD50 (50% effect on cell detachment)
was less sensitive to TBT, i.e. 3 x 10-5 mol/litre for
TBTO (18 mg/litre) and 4.3 x 10-5 mol/litre for TBT
chloride (14 mg/litre).
When Snoeij et al. (1986a) incubated isolated rat
thymocytes with TBT chloride, there was membrane damage
and disintegration of the cells at concentrations higher
than 1 µmol/litre. Various parameters of cell function
were investigated. The TBT chloride increased the consump-
tion of glucose in a dose-related manner at concentrations
in the culture medium higher than 0.1 µmol/litre; pro-
duction of lactate was also increased in parallel. The ef-
fect peaked at 1 µmol TBT/litre and thereafter declined;
damage to cell integrity occurred at these concentrations.
Over the same effective range of concentrations (0.1 to
1 µmol/litre), TBT decreased ATP levels. The ATP dimin-
ished very rapidly, within 2.5 min, and remained low for
at least 3 h. The incorporation of radiolabelled nucleo-
tide precursors into DNA and RNA and of amino acids into
protein was also affected. Thymidine incorporation into
DNA was reduced, relative to TBT dose, to a minimum of 20%
of control incorporation at 1 µmol TBT/litre. Uridine
incorporation was similarly reduced, but less effectively.
Inhibition of amino acid incorporation was substantial at
0.25 µmol TBT chloride/litre and protein synthesis was
virtually totally inhibited at 1 µmol/litre. The median
inhibitory concentrations (IC50) for thymidine, uridine,
proline, and leucine were 0.32 ± 0.04, 0.95 ± 0.06, 0.38
± 0.04, and 0.36 ± 0.03 µmol/litre, respectively. TBT
chloride concentrations of 0.1 µmol/litre or more mark-
edly reduced the production of cyclic AMP by the
thymocytes under stimulation from prostaglandin E1. TBT
chloride, therefore, has marked effects on the energy
metabolism of isolated thymocytes at concentrations well
below those affecting membrane integrity, and, as a
result, the authors stated that TBT chloride is best
characterized as an energy poison.
Snoeij et al. (1988a) similarly isolated rat thymo-
cytes, but separated them into fractions based on size.
The same parameters of cell function were investigated in
each of three fractions. Fraction 1 consisted of small,
non-proliferating thymocytes. Fractions 2 and 3 showed
some overlap in size, but fraction 2 was enriched in cells
actively synthesizing macromolecules, while fraction 3 was
enriched in dividing cells and showed the greatest pro-
liferative capacity. Resting ATP levels were highest in
the bigger cells, steady state levels increasing with
fraction number. TBT chloride reduced ATP levels in each
subfraction proportionally to between 62 and 64% of con-
trol values. Thus, TBT reduces intracellular ATP concen-
trations irrespective of cell volume or number of mito-
chondria. As with unfractionated thymocytes, TBT chloride
at both 0.25 and 1.0 µmol/litre inhibited thymidine
incorporation (to around 40% of control values at
0.25 µmol/litre and between 21 and 37% of control values
at 1 µmol/litre) and leucine incorporation (to between 13
and 29% of controls at 0.25 µmol/litre and 1 to 3% of
controls at 1 µmol/litre) in all fractions. In the case
of uridine incorporation, although there was inhibition in
most cases, 0.25 µmol TBT chloride/litre caused a stimu-
lation to 184% of control levels in fraction 1, the non-
proliferating cells.
11.2. Short-term toxicity
Summary
TBT compounds have been studied most extensively in the rat
(all the data in this section refer to the rat unless otherwise
indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg body
weight), high mortality rates were observed when the exposure
time exceeded 4 weeks. No deaths were noted at 100 mg/kg diet
(10 mg/kg body weight) or after daily administration of 12 mg
per kg body weight by gavage. In rats dosed during early post-
natal life, 3 mg/kg body weight resulted in increased deaths.
The main symptoms at lethal doses were loss of appetite, weak-
ness, and emaciation.
Borderline effects on rat growth were observed at 50 mg/kg
diet (6 mg/kg body weight) and 6 mg/kg body weight (gavage
studies). Mice are less sensitive, effects being observed at
150 to 200 mg/kg diet (22 to 29 mg/kg body weight).
Structural effects on endocrine organs, mainly the pitu-
itary and thyroid, have been noted in both short- and long-term
studies. Changes in circulating hormone concentrations and
altered response to physiological stimuli (pituitary trophic
hormones) were observed in short-term tests, but after long-
term exposure most of these changes appeared to be absent. The
mechanism of action is not known.
Exposure to TBTO aerosol at 2.8 mg/m 3 produced high mor-
tality, respiratory distress, inflammatory reaction within the
respiratory tract and histopathological changes of lymphatic
organs. However, exposure to the highest attainable vapour
concentration (0.16 mg/m 3 ) at room temperature produced no
effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular necrosis
and inflammatory changes in the bile duct were observed in rats
fed TBTO at a dietary level of 320 mg/kg (approximately 25
mg/kg body weight) for 4 weeks and in mice fed 80 mg/kg diet
(approximately 12 mg/kg body weight) for 90 days. Vacuolization
of periportal hepatocytes was noted in dogs fed a dose of 10
mg/kg body weight for 8 to 9 weeks. These changes were oc-
casionally accompanied by increased liver weight and increased
serum activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte vol-
ume in rats, resulting from dosing with 80 mg/kg diet (8 mg/kg
body weight), indicate an effect on haemoglobin synthesis,
leading to microcytic hypochromic anaemia. The decrease in
splenic haemosiderin levels suggests alterations in iron
status. Anaemia has also been observed in mice.
The formation of erythrocyte rosettes in mesenteric lymph
nodes has been observed in certain short-term investigations
but not in long-term studies. The biological significance of
this finding (possibly transient) is unclear.
The characteristic toxic effect of TBTO is on the immune
system; due to effects on the thymus, the cell-mediated func-
tion is impaired. The mechanism of action is unknown, but may
involve the metabolic conversion to dibutyltin compounds. Non-
specific resistance is also affected.
General effects on the immune system (e.g., on the weight
and morphology of lymphoid tissues, peripheral lymphocyte
counts, and total serum immunoglobulin concentrations) have
been reported in several different studies with TBTO using rats
and dogs, but not mice, at overtly toxic dose levels (effects
in mice have been seen with tributyltin chloride at 150 mg/kg).
Only the rat exhibits general effects on the immune system
without other overt signs of toxicity and is clearly the most
sensitive species. The NOEL in short-term rat studies was
5 mg/kg diet (0.6 mg/kg body weight). In studies with tributyl-
tin chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been shown
to compromise specific immune function in rat in vivo host re-
sistance studies. Decreased clearance of Listeria monocytogenes
was seen after exposure to a dietary level of 50 mg/kg (the
NOEL being 5 mg/kg per day), and decreased resistance to
Trichinella spiralis was seen at 50 and 5 mg/kg diet, but not
at 0.5 mg/kg diet (2.5, 0.25, and 0.025 mg/kg per day body
weight, respectively). Similar effects were seen in aged ani-
mals, but these were less pronounced.
With present knowledge, the effects on host resistance are
probably of most relevance in assessing the potential hazard to
man, but there is insufficient experience in these test systems
to fully assess their significance. However, some data on the
significance of the T. spiralis model are provided by findings
in athymic nude rats after the standard challenge. In these
studies, the complete absence of thymus-dependent immunity
resulted in a 10- to 20-fold increase in muscle larvae counts;
by contrast, exposure to TBTO concentrations of 5 and 50 mg/kg
diet resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on the
effects of tributyltin compounds on the developing immune sys-
tem, there is no information on host resistance.
It would be prudent to base assessment of the potential
hazard to humans on data from the most sensitive species.
Effects on host resistance to T. spiralis have been seen at
dietary levels as low as 5 mg/kg (equivalent to 0.25 mg/kg per
day body weight), the NOEL being 0.5 mg/kg (equivalent to
0.025 mg/kg per day). However, the interpretation of the sig-
nificance of these data for human risk assessment is contro-
versial. In all other studies a concentration of 5 mg/kg per
day in the diet (equivalent to 0.5 mg/kg body weight, based on
the short-term studies) was the NOEL with respect to general,
as well as specific, effects on the immune system.
11.2.1. Oral dosing: general body effects
Iwasaki et al. (1976) reported some oedema and
destruction of nerve axons following the administration of
0.01 ml TBTO/kg directly to the stomach of rats every day
for 28 days. No details of procedures or results were
presented in this report.
Schweinfurth (1985) found no evidence of brain oedema
after dosing rats with TBTO orally in arachis oil. Even
doses producing marked toxic effects on other organs (25
mg/kg body weight) failed to produce noticeable brain
oedema. However, triethyltin chloride produces brain
oedema at a dose of 1.5 mg/kg body weight.
Krajnc et al. (1984) investigated the short-term
effects of bis -tributyltin oxide in the rat. In exper-
iments lasting 4 weeks, Wistar rats were fed technical
TBTO at levels of 0, 5, 20, 80, or 320 mg/kg diet. All
animals survived the dosing period. Various symptoms of
poisoning were seen within 1 week of dosing in the group
fed 320 mg/kg diet, i.e. weakness, emaciation, roughened
fur, and blood-tinged discharge around the eyes and nose.
Some of these signs were seen at the lower doses after 4
weeks. Body weight gain was not affected by doses up to
20 mg/kg diet in males and 80 mg/kg in females. Males
showed reduced weight gain (96 g compared to control
weight gain of 117 g) over 4 weeks at 80 mg TBTO/kg. At
320 mg/kg diet, TBTO caused a reduction in body weight of
13 g in males and 21 g in females. Almost all of this
weight loss occurred in the first week of dosing, body
weight remaining constant after that. A 50% reduction in
food consumption was seen at this dose rate, compared to
control animals. Urine analysis, during the fourth week
of dosing, revealed no differences in urine volume, pro-
tein concentration, or creatinine clearance related to
treatment.
Funahashi et al. (1980) conducted histopathological
and biochemical studies on Sprague-Dawley rats given bis-
tributyltin oxide (TBTO) dissolved in olive oil. The rats
were dosed by intubation 5 times weekly for 13 or 26
weeks. Body weight was reduced after 13 weeks dosing at
6 mg/kg body weight, but not significantly so. After 26
weeks, body weight was significantly reduced relative to
controls (382 g compared to the control value of 439 g).
Dosing at 12 mg/kg reduced body weight significantly at
both 13 and 26 weeks (to 311 and 356 g, respectively).
There was a slight decrease, but unrelated to either dose
or time, in spleen weight. This decrease was just signifi-
cant at 6 mg/kg over 13 weeks and 12 mg/kg over 26 weeks,
but at no other time or dose.
When Mushak et al. (1982) dosed neonatal rats with TBT
acetate at levels of 1, 3, or 10 mg/kg per day from day 2
to day 29 of age, all rats given 1 mg/kg per day survived
with no apparent gross or histopathological effect. Totals
of 9 out of 24 and 17 out of 24 rats survived at dose
levels of 10 and 3 mg/kg per day, respectively, but all
showed reduced body weight. There were liver effects in
survivors.
11.2.2. Inhalation studies
A "nose only" inhalation study (lasting 4-5 weeks)
by Schweinfurth (1985) with rats exposed for 4 h/day (on
weekdays only; 21 to 24 exposure periods) to an aerosol of
TBTO (2.8 mg/m3) produced mortality (50% of males and
60% of females), apathy, and respiratory distress. Food
consumption and body weight gain were reduced. There were
inflammatory reactions within the respiratory tract and
lymphotoxic effects (depletion of lymphocytes in the
thymic cortex, atrophy of the thymus, and lymph nodes).
Inhalation of TBTO vapour/air mixtures produced no observ-
able effect. A concentration of 0.16 mg/m3 in the inha-
lation chamber, which corresponds to the equilibrium
vapour pressure of TBTO at room temperature, was con-
sidered to be the NOEL for rats.
When Gohlke et al. (1969) exposed rats to TBT chloride
in a 4-month inhalation study at nominal concentrations of
4 to 6 mg/m3, all animals survived the exposure. Towards
the end of the exposure, in the final month, the rats
showed minor irritation of the eye and nose. There was an
initial increase in relative liver weight but a signifi-
cant reduction over the whole experimental period. Fat
droplets were seen in the livers at autopsy, together with
a diffuse oedema of the brain. However, controls also
showed oedema. The oedema disappeared slowly as recovery
time after exposure increased. There were also inflamma-
tory changes in the respiratory tract of exposed animals.
Crofton et al. (1989) exposed pregnant female rats to
TBTO by intubation at 0 to 10 mg/kg per day on days 6 to
20 of gestation. There was no effect on the age at which
the testes of male offspring descended. However, females
showed a delay of approximately 2 days in vaginal opening,
compared to controls, after their mothers were exposed to
10 mg/kg per day.
11.2.3. Histopathological effects
Snoeij et al. (1985) reported that weanling rats fed
tributyltin chloride at a level of 150 mg/kg diet showed
marked reductions in body weight (treated rats, 87 g; con-
trol rats, 119 g) and brain weight (treated rats, 1.54 g;
control rats, 1.67 g) associated with a reduced food
intake of 25%. Thymus weight was reduced to 39% of the
control value over the 2-week feeding period.
Krajnc et al. (1984) found histopathological changes
in male and female Wistar rats exposed to 5, 20, 80, or
320 mg TBTO/kg diet for 4 weeks. No treatment-related
changes were found in the brain, heart, kidney, pancreas,
adrenals, popliteal lymph nodes, intestinal tract, or bone
marrow. Slight atrophy of hepatocytes (reduction in size)
in the centrilobular region was noted in some livers at
80 mg/kg and marked atrophy in 16 out of 20 livers at 320
mg/kg. The authors reported dystrophic calcification in
the liver at the highest dose level. Three animals showed
multiple-focus inflammation with necrosis of hepatic
parenchyma, which was associated with mononuclear and
polymorphonuclear infiltration, fibrosis, and hyperplasia
of the intrahepatic bile duct. In one animal there was
inflammation of the common bile duct. No bacteria or
viruses were found in the lesions. Similar necrotic
lesions were observed, in two animals, in a repeat study
at 320 mg TBTO/kg diet.
Mori et al. (1984) painted the shaved dorsal skin of
guinea-pigs daily for 50 days with an ethanolic solution
of TBTO at 10 or 40 mg/kg body weight. Monitoring of urine
and blood electrolyte levels during the course of the
treatment showed increased loss of sodium, chloride, phos-
phate, glucose, and amino acids in the urine. There was a
concomitant loss of electrolytes from serum. The effect
was most marked between 40 and 50 days of treatment. His-
tological lesions of the kidney tubules were observed when
the animals were killed after the 50th day.
11.2.4. Haematological and biochemical effects
Measurements of blood biochemical parameters of rats
fed TBTO for 4 weeks indicated few significant effects at
dietary dose levels below 320 mg/kg diet. Alanine amino
transferase (ALAT) activity was significantly increased,
in a dose-related manner, at 20 mg/kg or more, in both
males and females. The highest dose significantly de-
creased blood glucose in males, aspartate amino transfer-
ase (ASAT) in both males and females, and liver glycogen
in both sexes. Serum triglycerides, alkaline phosphatase,
and creatinine kinase activities were unaffected at any
dose level, as were blood lactate and pyruvate. At 80
mg/kg diet, TBTO significantly reduced blood haemoglobin
in both sexes and haematocrit in females. Mean erythro-
cytes volume was reduced in both sexes, as was mean cor-
puscular haemoglobin content (mass), but mean corpuscular
haemoglobin concentration and erythrocyte numbers were not
affected (Krajnc et al., 1984). A 6-week study at dietary
doses of 5, 20, and 80 mg/kg showed significant reductions
in haematocrit at 20 and 80 mg/kg and in blood haemoglobin
levels at 80 mg/kg. Iron concentration was reduced at 80
mg/kg, as was the isocitrate dehydrogenase (ICDH) activity
of erythrocytes. Numbers of erythrocytes, thrombocytes,
and reticulocytes were not significantly affected at any
dose level of TBTO, though there was a trend towards
increased numbers of reticulocytes. The authors suggested
an effect on haemoglobin synthesis and other indicators,
implying either reduced iron uptake or increased iron
loss. The enhanced ICDH activity and increasing reticulo-
cyte numbers indicated the presence of immature erythro-
cytes, and the authors could not exclude the possibility
of an in vivo haemolytic action of TBTO comparable to the
reported in vitro haemolysis (Krajnc et al., 1984).
Rosenberg et al. (1984) measured the activity of haem
oxygenase in mucosal cell fractions from control mice and
mice dosed by intubation with TBTO at 60 mg/kg body
weight. The activity of the enzyme, monitored by the
bilirubin absorbance spectrum, was substantially elevated,
compared to controls, 16 h after administration of the
TBTO. The activity of the same enzyme in liver and kidney
microsomes was not affected by this dose of TBTO given by
gavage, but was elevated when TBTO was given parenterally.
The activities of cytochrome P-450 and benzo(a)pyrene
hydroxylase were substantially reduced in the intestine of
TBTO-treated mice, this reduced activity being statisti-
cally significant for the latter but not for the former.
Similar results were obtained in liver fractions when TBTO
was applied parenterally.
11.2.5. Effects on lymphoid organs and immune function
Funahashi et al. (1980) reported the effects of feed-
ing TBTO in olive oil (0, 3, 6, or 12 mg/kg body weight
per day) for 13 or 26 weeks by gavage to groups of ten
male Sprague-Dawley rats, aged 5 weeks at the beginning of
the study. No analyses of haematology, immunoglobulin
levels, or specific aspects of immune function were per-
formed. A marked dose-related reduction in absolute and
relative thymus weight was seen following dosing for both
periods. Relative thymus weights were 682, 629, 340, and
313 mg/kg body weight after 13 weeks of dosing and 449,
313, 278, and 248 mg/kg body weight after 26 weeks of
dosing in the rats given 0, 3, 6, and 12 mg/kg per day,
respectively. All results, with the exception of those for
the group given 3 mg/kg body weight per day for 13 weeks,
were statistically significant (p < 0.003). Despite the
considerable reductions in thymus weight, the only histo-
logical observation was a slight reduction in the width of
the thymic cortex. A dose-related reduction in relative
spleen weight was seen after 26 weeks, which was statisti-
cally significant (p < 0.05) at the dose level of 12 mg/kg
body weight per day. Reduced body weight and increases in
relative pituitary and relative adrenal weights were stat-
istically significant (p < 0.01) at 12 mg/kg body weight
per day for 13 and 26 weeks and at 6 mg/kg body weight per
day for 26 weeks, and there was a significant (p < 0.05)
increase in relative adrenal weight at 6 mg/kg body weight
per day for 13 weeks. The relative pituitary weight was
also increased following 26 weeks of dosing at 3 mg/kg
body weight per day (p < 0.01). Based on thymus weight,
the NOEL was the most sensitive end-point in this study,
3 mg/kg body weight per day for 13 weeks. However, an
effect was seen at this dose when it was given for 26
weeks.
Funahashi et al. (1980) also reported that the
reduction in relative thymus weight, present 3 days after
a single dose of TBTO in olive oil of 100 mg/kg body
weight to 5-week-old male Sprague-Dawley rats, showed
signs of reversal at day 8. The reversibility of TBT-
induced thymus atrophy was also demonstrated by Snoeij et
al. (1988b). Groups of 4 or 5 young (4-5 weeks old) male
Wistar rats received a single gavage dose of TBT chloride
(0 or 16 mg/kg body weight) in corn oil. A 30% reduction
in relative thymus weight was evident in animals killed on
days 2, 3, or 4, but there was complete recovery by day 7.
A similar effect and recovery was seen in the thymus cell
counts. An equimolar dose of dibutyltin chloride produced
more pronounced effects, the recovery period being pro-
longed until day 9.
Krajnc et al. (1984) reported the effects of feeding
TBTO-containing diets (0, 5, 20, 80, or 320 mg/kg diet) to
groups (10 males and 10 females per group) of young SPF
Wistar rats for 4 weeks. These dose levels are equivalent
to approximately 0, 0.5, 2, 8, or 32 mg/kg body weight per
day, respectively, based on actual food consumption
measurements. Total leucocyte and circulating lymphocyte
counts were significantly reduced in males receiving 80
mg/kg diet (p < 0.01) and in both sexes receiving 320
mg/kg diet (p < 0.001). Eosinophil counts were signifi-
cantly reduced (p < 0.05) in males receiving 5, 80, or 320
mg/kg diet and in females given the highest dose. Monocyte
counts were increased significantly (p < 0.05) in males
receiving 20, 80, or 320 mg/kg diet but not in females.
Total immunoglobulin levels were significantly affected at
80 and 320 mg/kg diet. At 80 mg/kg diet, TBTO signifi-
cantly increased serum IgM to 132% (p < 0.01) and 145%
(p < 0.001) of control levels for males and females,
respectively. At the same dose, IgG in males was reduced
significantly (p < 0.05), but in females IgG was unaffec-
ted. A significant reduction (p < 0.001) in serum IgG was
found in both sexes fed TBTO at 320 mg/kg diet. Thymus and
relative thymus weights were significantly reduced in both
sexes (p < 0.001) at 80 and 320 mg/kg diet and in females
given 20 mg/kg diet (p < 0.05). Relative spleen weight
was significantly increased in males receiving 320 mg/kg
diet but this was probably related to the decreased body
weight of these animals. Histological examination showed
that all the animals given the highest dose exhibited
lymphocyte depletion from the thymic cortex, resulting in
an indistinct cortico-medullary junction and an increase
in ceroid/lipofuschin-loaded macrophages. Slight atrophy
of the thymic cortex was seen in two males fed 80 mg/kg
diet. Diffuse atrophy of the white pulp of the spleen was
seen in all animals at 320 mg/kg, the periarteriolar lym-
phocyte sheaths (PALS) being particularly affected. At 80
mg/kg diet, one male and two females showed slight splenic
atrophy. Depletion of T lymphocytes, determined by pan-T
immuno-staining, was seen in the PALS at 320 mg/kg diet.
There was an increase in the incidence of mesenteric lymph
nodes atrophy, observable in some animals fed 20 mg/kg and
increasing with dose to affect all animals given 320 mg/kg
diet. Both the paracortex and medulla of lymph nodes were
reduced in size and cellularity, and the numbers and size
of follicles were reduced by the highest TBTO dose level.
Again, the total number of T lymphocytes was strikingly
reduced in the paracortex by TBTO at 320 mg/kg diet, as
determined by immuno-staining. Although thymic involvement
was marked, it was not only thymus-dependent areas that
were affected. In rats exposed to the highest dose level,
which was overtly toxic, B lymphocyte areas also showed
low level activity, as indicated by fewer follicles and
inconspicuous germinal centres in lymph nodes and spleen.
An increase in the number of animals with rosettes in
sinuses in the mesenteric lymph nodes, composed of eryth-
rocytes surrounding mononuclear cells, was seen. This was
dose related: half of the animals dosed at 5 mg/kg and all
the animals treated with 80 or 320 mg/kg showed erythro-
cyte rosettes. The biological significance of these
rosettes is unclear. Specific aspects of immune function
were not tested. In further studies (personal communi-
cation by E.I. Krajnc to IPCS, 1989; Wester, in press), no
increase in rosette formation was observed at 5 mg/kg diet
or 50 mg/kg diet. Signs of general toxicity were observed
in animals treated with 320 mg/kg diet, i.e. reduced body
weight (p < 0.001) and increased serum ALAT activity. At
80 mg/kg, there was reduced body weight gain in males
(p < 0.05) and increased ALAT, and at 20 mg/kg increased
ALAT in males only. The liver was the only non-lymphoid
organ displaying histological changes: centrilobular hepa-
tocyte atrophy, reduced glycogen retention, parenchymal
necrosis, and hyperplasia of the intrahepatic bile duct
were seen in some animals treated at 320 mg/kg diet. Three
animals from the group given 80 mg/kg showed slight hepa-
tocyte atrophy. This study indicated a NOEL of 5 mg/kg
diet (approximately 0.5 mg/kg body weight per day).
Vos et al. (1984) investigated functional aspects of
the immunological effects reported by Krajnc et al. (1984)
in in vivo and ex vivo experiments using weanling (3 to 4
weeks old) male SPF Wistar rats fed diets containing TBTO.
Haematological parameters were not studied. Levels of
total circulating immunoglobulins (IgG and IgM) were
determined in animals fed diets containing 80 or 320 mg
TBTO/kg. At 320 mg/kg diet, IgM was significantly
increased (p < 0.05) and IgG was significantly decreased
(p < 0.001) on day 28 (no assays were performed on day 42
for this group). At 80 mg/kg diet, IgM was elevated by 30%
on day 42 (p < 0.01), while IgG was decreased by 30% on
days 28 and 42.
The effects of TBTO on lymphocyte counts and cell
viability in lymphoid organs was investigated, and several
specific tests of immune system function were also per-
formed. Numbers of B and T lymphocytes were significantly
reduced in the spleens (by 15% and 48%, respectively) of
animals fed diets containing 80 mg/kg diet. The ratio of
total T:B lymphocytes was decreased in a dose-related
manner following 9 weeks of exposure.
A significant reduction occurred in the numbers of
viable cells (trypan blue exclusion) obtained from the
thymus, spleen, and bone marrow of rats treated with TBTO
at 80 or 320 mg/kg diet. At 320 mg/kg diet, viability and
cell numbers were significantly (p < 0.05) reduced for
both thymus and spleen following 3, 8, and 20 days of ex-
posure, and bone marrow counts were significantly reduced
on days 8 and 20. Doses of 80 mg/kg diet produced signifi-
cant reductions in thymus cell count and viability on day
8 and thymus, spleen, and bone marrow counts on day 20.
The antibody response to sheep erythrocytes (ip),
tetanus toxoid (iv), ovalbumin (sc into the foot pad, in-
cluded H37Ra adjuvant), and the worm Trichinella spiralis
(oral) was assessed in animals receiving 20 or 80 mg TBTO
per kg diet for 6 weeks. The primary response to sheep
erythrocytes (0.5 ml of a 20% suspension, ip) was deter-
mined 10 days post inoculation (pi) and the secondary
response was determined on day 20 pi following an iv
booster inoculation on day 15 pi. The primary response was
unaffected, but there was a dose-related decrease in the
secondary response seen both in untreated and 2-mercapto-
ethanol-treated (IgM-inactivated) sera, reaching statisti-
cal significance (p < 0.05) in treated sera from the
highest-dose group. The response (IgG and IgM titres) to
tetanus toxoid inoculation measured only on day 21 pi was
equivocal, and that to ovalbumin measured on days 15, 21,
and 28 pi was unaffected by TBTO. IgG response to oral T.
spiralis infection was significantly (p < 0.05) increased
on day 21 pi at 20 mg/kg diet, but not on day 42 or at 80
mg/kg diet at either time. IgM response was unaffected.
IgE response, possibly the most relevant to resistance to
parasitic infection, was decreased in a dose-related man-
ner on days 21 and 42 following T. spiralis infection; log
titres were 3.8, 2.5 (p < 0.05), and 1.8 (p < 0.001) on
day 21 and 4.4, 3.6, and 3.4 (p < 0.05) on day 42 in the
control, 20-, and 80-mg/kg groups, respectively. Delayed-
type hypersensitivity was determined as change in skin
thickness following a challenge of ovalbumin to the skin
of the ear or tuberculin challenge to the skin of the
flank, made 3 and 4 weeks, respectively, following initial
immunization (sc into a footpad) 6 weeks after starting
dietary dosing of TBTO at 20 or 80 mg/kg. Compared to
controls similarly injected intradermally with medium,
significant reductions in response to ovalbumin challenge
were found 24, 48, and 72 h after dosing at 20 mg/kg (all
p < 0.05), and 24 h (p < 0.01) and 48 h (p < 0.05) after
dosing at 80 mg/kg. With tuberculin challenge, significant
effects were found in the group given 80 mg/kg diet after
24 h (p < 0.01), 48 h (p < 0.001), and 72 h (p < 0.001),
but only after 72 h (p < 0.05) at the lower dose level.
Reduced responses were seen at all three times after both
doses.
Thymus or spleen cells, obtained from rats fed TBTO at
20 or 80 mg/kg diet, were cultured with and without
mitogens (PHA = phytohaemagglutinin; Con A = concanavalin
A; PWM = pokeweed mitogen) for 24 h before the addition
of 3H-thymidine to monitor DNA synthesis. PHA and Con A
are both T cell mitogens and PWM is a mitogen for both T
and B cells. Due to a reduction in the number of viable
cells in thymic cultures from the high-dose group, respon-
siveness to mitogens was expressed per culture and per
thymus. Significant reductions in 3H-thymidine uptake
(expressed per thymus) were found in unstimulated (48%
reduction), PHA-treated (64%), Con A-treated (62%), and
PWM-treated (50%) cultures derived from the high-dose
group; PHA (48%) and PWM (28%) were the only responses
reduced at 20 mg/kg diet. Similar effects were seen on
splenic cultures, with a reduced number of viable cells
per spleen and significant reductions in response
(expressed per spleen) to PHA (50% reduction) and Con A
(45%) in cultures from the high-dose group; responses to
PWM and E. coli lipopolysaccharide (a B cell mitogen)
were significantly increased on a per culture basis but
not a per spleen basis. Cultures from the low-dose group
showed reduced response to PHA (25% reduction) and Con A
(20%), but these were not statistically significant.
TBTO, at both 20 and 80 mg/kg diet, reduced the
resistance of rats to infection by T. spiralis. The number
of worm larvae in muscle significantly increased in a
dose-dependent manner (74 000 in controls; 106 000 in rats
fed 20 mg TBTO/kg, p < 0.01; 198 000 in rats fed 80 mg/kg
p < 0.001) after standard infection with 1000 larvae by
mouth. The number of adult worms in the small intestine
also increased significantly after 10 (p < 0.05), 12
(p < 0.001), and 14 (p < 0.01) days in the high-dose
group, and after 12 (p < 0.05) and 14 (p < 0.001) days in
the low-dose groups. In a study of the inflammatory reac-
tion around larva-containing muscle cells in the tongues
of animals killed 14 days after infection, the response
(mononuclear cells and eosinophilic granulocytes) after
treatment with TBTO at 20 mg/kg diet was described as
"slightly reduced". However, there was a marked
reduction after treatment at 80 mg/kg diet.
The clearance of Listeria monocytogenes from the
spleen (a measure of host resistance) was monitored in
rats fed 20, 80, or 320 mg TBTO/kg diet for 6 or 7 weeks.
Statistically significant increases in the number of
viable bacteria were seen 2 days after an iv injection
into animals treated with 320 mg/kg diet for 6 (p < 0.01)
or 7 (p < 0.001) weeks and with 80 mg/kg diet for 6 weeks
(p < 0.001). A dose-related reduction, statistically sig-
nificant (p < 0.05) at 80 mg/kg diet, in viable bacteria
was seen 1 day post injection in the group receiving TBTO
for 7 weeks, but this was not seen in the 6-week study.
The ex vivo phagocytosis of L. monocytogenes by spleen-
and peritoneal-derived macrophages from TBTO treated (20
or 80 mg/kg diet) animals was not significantly affected,
although a dose-related reduction in the phagocytic
activity of splenic macrophages was seen.
Cells derived from the spleen and peritoneal cavity
were tested for spontaneous cell-mediated cytotoxicity
against murine YAC lymphoma target cells labelled
with 51Cr. "Specific release" of the chromium label
(release in experimental minus spontaneous release in the
controls) was used as the end-point of the assay. Stat-
istically significant reductions in specific release were
found with spleen cells from rats fed TBTO at 80 mg/kg
diet (p < 0.05), but not in spleen cells from those fed 20
mg/kg diet. Statistically significant effects (p < 0.05)
were found in peritoneal macrophages derived from rats
receiving 20 and 80 mg/kg diet, but there were no signifi-
cant effects on non-adherent cells ("natural killer
cells").
These studies were designed to investigate immune
function rather than to determine a no-observed-effect-
level, and the lowest dose used (20 mg/kg diet) produced
statistically significant effects (in particular, de-
pression of host resistance to T. spiralis and L. mono-
cytogenes ). No evidence of general toxicity was seen at
this dose level. The only sign of general toxicity
recorded was a significant reduction in body weight gain
seen after exposure to 320 mg/kg diet for 3, 8, and 20
days and to 80 mg/kg diet for 20 days.
A study of TBTO (0, 0.5, and 50 mg/kg diet) adminis-
tered to groups of weanling Wistar rats (five animals of
each sex per group) for 2 years was briefly reported by
Vos et al. (1985) and Wester (in press). It should be
noted that the intakes in this study were lower, on a body
weight basis, than in the shorter-term studies, being
equivalent to 0, 0.025, 0.25, and 2.5 mg/kg body weight
per day for the controls, and low, medium, and high doses,
respectively (based on actual intake measurements). A
significant decrease in peripheral lymphocyte count and a
statistically significant increase in platelets were seen
in the females fed 50 mg/kg diet for 1 year. Circulating
levels of total IgM and IgA were significantly increased
at 4-6 and 16-18 months, in rats given 50 mg/kg diet,
while significant reductions in IgG levels were recorded,
particularly in females.
General effects on the lymphoid organs were not
recorded, though several specific tests of immune function
were performed using the methods of Vos et al. (1984). A
dose-related decrease in resistance to T. spiralis infec-
tion was seen at 5 and 16 months, achieving statistical
significance (p < 0.05) at 5 and 50 mg/kg diet. IgE titres
were reduced, but IgA levels increased approximately 50-
fold at the highest dose level. No effects on delayed-type
hypersensitivity were seen, in contrast to results in the
short-term study by Vos et al. (1984). Host resistance to
L. monocytogenes (as measured by the number of viable
organisms in the spleen) was significantly reduced at 5
and 17 months in the 50-mg/kg group, but a significant
increase was seen at 17 months in the 5-mg/kg group.
Natural killer cell activity against YAC lymphoma cells
was reduced significantly at the highest dose level after
15-17 months. It will not be possible to fully assess this
study until the final report is published, although it
would appear that 0.5 mg/kg diet (0.025 mg/kg body weight
per day) was the NOEL.
A preliminary report on a study where diets containing
TBTO (0, 0.5, 5, or 50 mg/kg diet) were fed to Wistar rats
(12 months old) for 6 months, has been issued (personal
communication by E.I. Krajnc to IPCS, 1989). Specific
tests of immune function were measured after 5 months of
dosing. Significant (p < 0.05) reductions in host resist-
ance to T. spiralis and L. monocytogenes were seen at
50 mg/kg only. These results suggest that aged rats are
less sensitive to TBTO in the diet than weanlings animals,
although this may be due to a lower intake on a per kg
body weight basis. Full assessment of this study will need
to await the completion of the statistical analyses and
final report.
A series of studies have been performed to investigate
certain aspects of immune function (Schering, 1989a,b,c,d).
When groups (10 animals of each sex) of young (4 to 5
weeks old) Sprague-Dawley rats were fed diets containing
TBTO (0, 0.5, 2, 5, or 50 mg/kg diet) for 4 weeks, no sig-
nificant effects were seen on total or differential white
blood cell counts. Serum immunoglobulin levels were not
measured. A statistically significant decrease in absolute
and relative thymus weight (p < 0.01) was seen in males
fed 50 mg/kg. A decrease in absolute and relative spleen
weight was also seen in this group, though it was not sig-
nificant statistically. The viability of cultured spleen
cells (monitored with trypan blue exclusion) obtained from
females fed 5 mg/kg was reduced (p < 0.05), but other
groups were unaffected. A significant decrease in total
thymus cell count was seen in preparations from males fed
50 mg/kg only (p < 0.05). A slight but significant
(p < 0.05) reduction in the thickness of the thymic cortex
was also seen in these males, this being the only signifi-
cant histological finding. Specific measures of immune
function were not performed in this study but in parallel
studies. The NOEL in this study was 5 mg/kg diet
(approximately 0.6 mg/kg body weight per day, based on
measured intake) (Schering 1989a).
An assay of plaque-forming cells, a measure of humoral
immunity, revealed no effects due to TBTO (at levels of
0.5, 2, 5, or 50 mg/kg diet) fed to groups (10 animals of
each sex) of young (4 to 5 weeks old) Sprague-Dawley rats
for 5 weeks. The response was measured on day 36, follow-
ing an iv inoculation of sheep erythrocytes. The only sign
of toxicity was a reduction in body weight in males fed
50 mg/kg, but this was not statistically significant. No
investigation of general toxicity was performed. The NOEL
was 50 mg/kg diet (approximately 5.6 mg/kg body weight per
day) in this study (Schering, 1989b).
To assess the effects of TBTO on host resistance to
infection, the number of viable Listeria monocytogenes
cells in the spleen, 4 days after inoculation with
1.4 x 106 cells, was counted. No effects were produced in
groups (10 animals of each sex) of young Sprague-Dawley
rats fed diets containing TBTO at 0.5, 2, or 5 mg/kg diet
for 34 days. However, statistically significant increases
in numbers of viable bacteria were seen in the spleen of
males (p = 0.055) and females (p < 0.01) fed 50 mg/kg
diet. No investigations of general toxicity were per-
formed. The NOEL was 5 mg/kg diet (approximately 0.6 mg/kg
body weight per day) (Schering, 1989c).
The effects of TBTO on delayed-type hypersensitivity
reactions, a measure of cell-mediated immunity, were
assessed in groups (10 animals of each sex) of 4- to 5-
week-old Sprague-Dawley rats fed diets containing 0, 0.5,
2, 5, or 50 mg/kg diet for 37 days. A sensitizing dose of
100 µg bovine serum ablumin (BSA) mixed with Freund's
complete adjuvant was given on day 29, followed by a chal-
lenge dose of heat-inactivated BSA injected into a hind-
foot pad on day 37. No difference in response (increased
footpad thickness) was seen between test and control
groups. Body weight gain was unaffected, but no other
investigations of general toxicity were performed. The
NOEL in this study was 50 mg/kg diet (approximately 5.8
mg/kg body weight per day) (Schering 1989d).
The effects of inhaled TBTO were studied in groups (10
animals of each sex) of young (initial weights 86-131 g)
SPF Wistar rats exposed to 0, 0.03, 0.16, or 2.8 mg/m3
for 4 h/day, 5 days per week, for 21 to 24 exposures. The
two lower doses were provided by filtered vapour, and the
highest dose was provided by an aerosol with over 90% of
the particles < 5 µm in diameter (Schering 1983). Lympho-
cyte and total leucocyte counts, measured at 2 or 4 weeks,
were unaffected by TBTO. Some inconsistent changes in
reticulocyte counts were found, but these appeared to be
primarily related to use of the respiration chamber and
not to TBTO exposure. Immunoglobulins were not measured.
Thymolysis and lymphocyte depletion of the thymus-depen-
dent areas of the spleen and lymph nodes were reported in
the 11 animals (five males, six females) from the highest-
dose group that died during the study. No such lesions
were detected in the survivors, although in three sur-
vivors from the highest-dose group an increase in the
number of macrophages containing nuclear debris was seen
in the thymic cortex. No significant (p > 0.05) changes
were seen in absolute or relative weights of the thymus,
spleen, or iliac lymph node in animals surviving the
study, but no organ weights were recorded for those ani-
mals dying or sacrificed during the study. No specific
aspects of immune function were studied. Histological
signs of general toxicity were limited to those consistent
with inflammatory reactions within the respiratory tract
and, with one exception, were confined to animals exposed
to the aerosol. Food intake and body weight gain were
reduced in both sexes, the reduction being statistically
significant (p < 0.01) in male rats exposed to 2.8 mg/m3.
The cause of death in the 11 animals from the highest-dose
group was not ascertained (Schering, 1983).
When TBTO (0, 4, 20, 80, or 200 mg/kg diet) was fed to
groups (10 animals of each sex) of CD-1 mice, aged 6 to 7
weeks at commencement of the study, for 3 months, leuco-
cyte counts were increased at 80 and 200 mg/kg in both
sexes, although this increase did not reach statistical
significance (p > 0.05). Immunoglobulins were not
measured. Thymus weight was reduced in both sexes at 200
mg/kg, but this was not statistically significant
(p > 0.05). Spleen weights were increased in both sexes
at 80 and 200 mg/kg (reaching statistical significance
(p < 0.05) at 200 mg/kg), possibly secondary to effects on
erythrocytes. No specific immune function tests were per-
formed. Histological changes were seen in the livers of
both sexes at 80 and 200 mg/kg. Dose-related, statisti-
cally significant (p < 0.01) increases in absolute liver
weights were seen at 80 and 200 mg/kg, and adrenal weights
were significantly (p < 0.01) increased in the male rats
fed 200 mg/kg. The NOEL in this study was 20 mg/kg diet
(approximately 4 mg/kg body weight per day) (Biodynamics,
1989a).
In studies by Schering (1989e), groups (two animals of
each sex) of beagle dogs received variable doses of TBTO
in arachis oil by oral gavage:
Group 1: controls;
Group 2: 0.1 mg/kg body weight per day for 5 weeks,
0.2 mg/kg body weight per day for 4 to 5 weeks, then
10 mg/kg body weight per day for 8 to 9 weeks;
Group 3: 0.5 mg/kg body weight per day for 5 weeks
then 1 mg/kg body weight per day for 13 to 14 weeks;
Group 4: 2.5 mg/kg body weight per day for 5 weeks
then 5 mg/kg body weight per day for 13 to 14 weeks.
All males in groups 2 and 4 died as a result of mis-dosing
to the lungs. Increased leucocyte and neutrophil counts
were seen in group 4 at weeks 9 and 18 (p < 0.05), and
there was a statistically significant increase in leuco-
cyte count recorded at 13 weeks in group 2 (p < 0.05).
Non-statistically significant reductions in leucocyte,
neutrophil, and lymphocyte counts were found in group 2 at
18 weeks. Specific immunoglobulins were not measured.
Thymus weights were reduced in the two survivors of group
2 (0.9 ± 0.1 g compared with 4.0 ± 0.6 g in controls), and
slight increases in thymus weights were seen in groups 3
and 4. Iliac and mesenteric lymph node weights were
reduced, though spleen weights were increased, in the two
survivors from group 2. Histological changes in lymphoid
organs were confined to group 2 where a reduction of lym-
phocyte numbers in the thymus, spleen (particularly PALS),
and lymph nodes was seen. A dose-related increase in rela-
tive liver weight (33.1, 41.7, 49.9, and 57.6 g/kg body
weight in groups 1, 3, 4, and 2, respectively) was
accompanied by cytoplasmic vacuolation of hepatocytes in
group 2 (Schering, 1989e).
11.2.6. Mechanism of immunotoxicity
The precise mechanism of the immunotoxic effects of
TBTO is not yet clear. However, a hypothesis has been put
forward, based on the work of Snoeij (1987) and Pieters et
al. (1989).
a) Absorbed TBTO is present as the TBT+ cation or a salt
(chloride or carbonate). Studies using TBT chloride
are, therefore, relevant to TBTO immunotoxicity.
b) As dibutyltin chloride (DBT chloride), a metabolite of
TBT chloride, is, mole for mole, more potent that TBT
chloride at producing effects in the thymus and thymic
cells, it is probable that DBT chloride or another DBT
salt is the active species in TBTO toxicity.
c) The primary action of DBT is to suspend the maturation
of immature thymocytes by inhibiting their inter-
action/binding with thymic epithelial cells. The turn-
over period of thymocytes is 3 to 4 days. Therefore,
as cell proliferation/maturation is inhibited, rapid
depletion of thymocyte numbers without cytotoxicity is
expected, followed by a rapid proliferation (observed
by Snoeij et al. (1988b) and Funahashi et al. (1980))
on removal of DBT.
Other evidence indicating that TBT compounds have a par-
ticular effect on the thymus is provided by the in vitro
studies of Snoeij (1987), which show TBT chloride to be
cytotoxic to thymocytes.
The demonstration of reduced thymus weights in certain
fish species (e.g., the freshwater guppy) indicates that
TBT may have immunotoxic effects on a wide range of
species.
11.2.7. Effects on the endocrine system
The weights of both the adrenal and pituitary glands
of Sprague-Dawley rats were significantly increased after
exposure to 6 mg TBTO/kg body weight daily, by intubation,
for 26 weeks. Pituitary weight was also significantly
increased by 3 mg TBTO/kg body weight given daily for
26 weeks (Funahashi et al., 1980).
After 4 weeks, the serum insulin concentration of rats
was not significantly affected by dosing with TBTO at up
to 80 mg/kg diet, but was undetectable (< 2 milliIU/litre)
in rats fed 320 mg/kg (control levels of insulin in serum
were 111 and 74 milliIU/litre for males and females, re-
spectively) (Krajnc et al., 1984). In a further study, the
same authors monitored endocrine changes in male Wistar
rats exposed to TBTO in the diet (0, 20, or 80 mg/kg) for
6 weeks. Serum thyroxine, thyroid stimulating hormone
(TSH), and insulin levels were reduced significantly at
the higher dose level, but serum follicle stimulating
hormone (FSH) and corticosterone levels were unaffected.
Only insulin was significantly affected at 20 mg TBTO/kg.
The luteinizing hormone (LH) concentration in serum was
significantly increased by TBTO at 80 mg/kg, but was
unaffected by 20 mg/kg. The authors further examined endo-
crine function by monitoring hormone release following
physiological stimulus. Insulin release, following iv
administration of glucose, was unaffected by a 6-week
exposure to TBTO at either 20 or 80 mg/kg diet. The effect
of TBTO on insulin was attributed by the authors to a
decreased food intake. The release of TSH, after iv admin-
istration of thyrotrophin releasing hormone (TRH), showed
a tendency (p < 0.1) to be inhibited in rats fed at 80
mg/kg. The titre of circulating TSH, 20 min after TRH
administration, was significantly reduced compared with
controls. Release of both LH and FSH, in response to
luteinizing hormone releasing factor stimulation, was
enhanced in rats fed TBTO at both 20 and 80 mg/kg diet,
but only significantly so at the higher dose rate. Histo-
logical examination of endocrine organs after a 6-week
exposure to TBTO revealed some changes. No differences
were observed in either insulin- or glucagon-producing
cells in the pancreas. Some flattening of the epithelial
lining of the thyroid follicles was observed after
exposure of rats to 80 mg/kg but not to 20 mg/kg. Immuno-
cytochemical staining of the pituitary gland identified
each cell type producing the different pituitary hormones.
There was a dose-related decrease in both the intensity of
staining of TSH cells and the number of cells stained.
Conversely, there was a dose-related increase in the
staining intensity of LH cells. No effects were found on
FSH, growth hormone, or adrenocorticotrophin cells in the
pituitary (Krajnc et al., 1984).
11.3. Long-term toxicity
Wester (in press) carried out a 106-week toxicity
and carcinogenicity study with groups of 50 weanling
Wistar rats of each sex. An additional group of 10 rats
was used for an interim sacrifice after 1 year. TBTO was
fed at 0, 0.5, 5, or 50 mg/kg diet (equivalent to 0,
0.025, 0.25, or 2.5 mg/kg body weight). Increased food
consumption occurred in all treated males (not clearly
dose-related), and there was increased water consumption
in males at 5 and 50 mg/kg. During the second year, the
body weight of the highest-dose group was significantly
lower than that of controls. Excess mortality, compared
with that of controls, was confined to the 50 mg/kg group
towards the end of the experiment (see section 11.6).
Haematological changes (anaemia, lymphopenia, and thrombo-
cytosis) and increases in plasma enzyme activities (ALAT,
ASAT, and AP) were noted mainly at the high-dose level.
Serum IgM and IgA levels increased, while the IgG level
decreased (females). No effect was observed on circulating
concentrations of T4, free T4, TSH, LH, FSH, or insu-
lin; only the free T4:T4 ratio was decreased. Organ
weight changes consisted of increased liver, kidney,
adrenal, and pituitary weights and decreased thyroid
weight. Non-neoplastic histological alterations consisted
of a decrease in cell height of the thyroid follicles (at
50 mg/kg diet after 1 and 2 years), decrease in splenic
iron content (at 5 and 50 mg/kg after 1 year only), slight
bile duct proliferation (at 50 mg/kg after 1 year only),
and vacuolation of kidney proximal tubular epithelium and
nephrosis (at 50 mg/kg after 2 years only).
11.4. Genotoxicity
Summary
The genotoxicity of TBTO has been the subject of extensive
investigation. Negative results were obtained in the vast
majority of studies, and there is no convincing evidence that
TBTO has any mutagenic potential.
Davis et al. (1987) conducted a comprehensive study of
the genetic effects of TBTO using a wide range of tech-
niques in order to assess possible hazards in the use of
the compound as a molluscicide for the control of schisto-
somiasis. TBTO did not produce gene (point) mutations in
Salmonella typhimurium strains TA1530, TA1535, TA1538,
TA97, TA98, or TA100, either in the presence or absence of
an exogenous metabolic activation system (rat liver S9).
The compound did give some evidence of gene mutation in
Salmonella typhimurium TA100 using the fluctuation method
in the presence of S9, but no dose-response relationship
was seen; negative results were seen in the absence of S9.
TBTO did not induce point mutations in the yeast
Schizosaccharomyces pombe. Negative results were obtained
when TBTO was tested in the sex-linked recessive lethal
assay using Drosophila melanogaster, the compound being
given in food and by injection, indicating that TBTO did
not produce gene mutations in Drosophila. Negative results
were also obtained when the ability of TBTO to produce DNA
damage in Bacillus subtilis (recombination assay) or the
yeast Saccharomyces cerevisiae (mitotic gene conversion)
was tested.
The ability of TBTO to produce gene mutations in Sal-
monella has also been studied by Reimann & Lang (1987).
Negative results were obtained using Salmonella
typhimurium strains TA1535, TA1537, TA1538, TA98, and
TA100, both in the presence and absence of rat S9. Simi-
larly, negative results were obtained with six TBT esters
(abietate, borate, linoleate, naphthenate, phosphate, and
tallate). Further negative results were obtained when the
ability of TBTO to produce mitotic gene conversion in
Saccharomyces cerevisiae was tested.
The ability of TBTO to produce gene mutations in
mammalian cells in vitro was extensively investigated by
Davis et al. (1987), and negative results were consist-
ently obtained. TBTO did not induce gene mutations in V79
Chinese hamster cells (using resistance to 8-azaguanine,
ovabain, or 6-thioguanine as markers) in the presence of
rat liver S9. Negative results were also obtained in the
V79 cell assays when epidermal cells of mice and humans
(primary cultures) were used as the source of metabolic
activation in cell-mediated assays.
The in vitro clastogenic potential of TBTO has been
investigated in mammalian cells in two sets of studies.
When Davis et al. (1987) used Chinese hamster ovary (CHO)
cells, harvested at 8, 15, and 24 h, an increase in struc-
tural aberrations (mainly deletions), together with
endoreduplication, was seen, but only at the highest
concentration tested (5 µg/ml in the presence of S9 and
1.5 µg/ml in its absence). The increase in the presence
of S9 was seen only after 15 h, "toxicity" precluding
any analysis of results at this concentration after 8 h.
The increase in the absence of S9 was seen only at 8 h,
there being no increase at either 15 or 24 h. The results
of these studies are difficult to interpret, since the
effect was limited to concentrations associated with high
toxicity and no data on mitotic index was reported. No
increase in sister chromatid exchange was seen at any
concentration.
Reimann & Lang (1987) used human lymphocytes to test
the clastogenic potential of TBTO and obtained negative
results both in the presence and absence of S9. In the
latter case, TBTO was added 22 h after stimulation of the
cultures with phytohaemagglutinin, and cells were har-
vested 31 h later. In the former case, TBTO was added with
S9 after 26 h; 3 h later the S9 was removed, and the cells
were harvested 22 h later. Parallel studies on blood cul-
tures that were differentially stained with BUdR indicated
that almost all cells analysed for chromosome aberrations
were in the first mitotic stage. The highest TBTO concen-
trations used (0.1 µg/ml in the absence of S9 and
1 µg/ml in its presence) were associated with a marked
reduction in mitotic index (57% and 58%, respectively). No
increase in aberrations was seen at any dose level. This
study, therefore, failed to confirm the suggestion of some
clastogenic potential in the studies using CHO cells.
The ability of TBTO to produce chromosomal damage in
vivo has been investigated in two separate studies using
the micronucleus test. In one study, four doses of TBTO
(31.25, 62.5, 125, or 250 mg/kg body weight) were given by
gavage, in arachis oil, to NMRI mice, and bone marrow
cells were analysed for micronuclei in polychromatic
erythrocytes 24, 48, and 72 h after treatment (Reimann &
Lang, 1987). The highest dose level resulted in marked
lethality (16 out of 36 animals died), precluding any
analysis of the results. The 126-mg/kg dose level was also
associated with some deaths (4 mice died), but 5000 poly-
chromatic erythrocytes were analysed from five male and
five female mice at each harvest interval. Similar analy-
ses were carried out at the two lower dose levels. There
was no increase in micronuclei at any dose level or har-
vest time. This study provided no evidence to indicate
that TBTO produces chromosomal damage in bone marrow in
vivo. A second micronucleus study (Davis et al., 1987)
used Balb/c mice. Groups of 10 male and 10 female animals
were given 30 or 60 mg TBTO/kg as a solution in olive oil.
Bone marrow cells were harvested from five males and five
females after 30 h and 48 h, and 1000 polychromatic eryth-
rocytes were analysed from each for micronuclei. An
increase in micronuclei was seen only in the male mice
after 48 h and at the highest dose level. These results
conflict with those of Reinmann & Lang (1987). Further-
more, there was an unusually high spontaneous incidence of
micronuclei (up to 5.8 per 1000 polychromatic erythro-
cytes) in the study of Davis et al. (1987). These factors
prompted a re-analysis of the slides from this study by
the Institute of Occupational Health, Helsinki, Finland
(Schering 1986). This re-analysis, again using 1000 poly-
chromatic erythrocytes per animal, failed to confirm the
increase in micronuclei seen after 48 h in the male
animals given 60 mg TBTO/kg. A slight, but statistically
significant, increase in micronuclei was seen in the
female mice on re-analysis, but this was thought to be
biologically non-significant due to the high variability
in the control data. The re-analysis highlighted the
problem of interpreting studies in which only relatively
few polychromatic erythrocytes are analysed (1000) and
there is marked variability in the control data (with an
incidence outside the normal range at some time points).
Thus, no conclusions can be drawn from the micronucleus
study of Davis et al. (1987).
The ability of TBTO to inhibit metabolic cooperation
between V79 Chinese hamster 6-thioguanine-resistant and
sensitive cells has been investigated by Davis et al.
(1987). This assay has been suggested as a model for
tumour promoter activity. Negative results were obtained.
The significance of the assay is, however, unclear.
In summary, the genotoxicity of TBTO has been the
subject of extensive investigation. Negative results were
obtained in the vast majority of studies, and there is no
convincing evidence that TBTO has any mutagenic potential.
11.5. Reproductive toxicity
Summary
The potential embryotoxicity of TBTO has been evaluated in
three mammalian species (mouse, rat, and rabbit) after oral
dosing of the mother. The main malformation noted in rat and
mouse fetuses was cleft palate, but this occurred at dosages
overtly toxic to the mothers. These results are not considered
to be indicative of teratogenic effects of TBTO at doses below
those producing maternal toxicity. The lowest NOEL, with
regards to embryotoxicity and fetotoxicity for all three
species, was 1 mg/kg body weight.
11.5.1. In vivo
Reproductive toxicity has been studied in NMRI mice.
The highest dose used (35 mg/kg body weight) was chosen to
give minimal maternal mortality based on acute toxicity
tests. An increase in cleft palate was seen in the fetuses
of mice treated orally with 11.7 mg TBTO/kg body weight
(7% cleft palate), 23.4 mg/kg (24%), and 35 mg/kg (48%),
compared to the incidence in controls (0.7%). However, 11
out of a total of 15 affected mice were clustered in one
of the 18 litters and 15 litters contained none. The two
highest doses of TBTO also increased the frequency of
irregular ossification centres of sternabrae and of minor
abnormalities, such as fusion of the bases of os occipi-
talis. The strain of mice used in the study has a tendency
to produce these particular abnormalities as a result of
non-specific stress on the mother. The authors considered
that TBTO has a very low teratogenic potential; electron
microscopy 26 and 48 h after treatment showed no evidence
of damage to the embryos but considerable damage to the
maternal liver. At a level of 6 mg/kg body weight, TBTO
produced no increase in fetal abnormalities (Davis et al.,
1987).
Nemec (1987) investigated the maternal, embryotoxic,
and teratogenic effects of TBTO in New Zealand white
rabbits. The TBTO was administered in corn oil by gavage,
once a day from day 6 of gestation to day 18 inclusive, at
doses of 0.2, 1, and 2.5 mg/kg per day body weight in a
volume of 0.5 ml. Twenty female rabbits were dosed with
corn oil as controls and 20 rabbits were used at each dose
level. The rabbits were artificially inseminated and
injected iv with human chorionic gonadotrophin immediately
afterwards to ensure ovulation. With the exception of a
single female dosed at 1 mg/kg, all treated rabbits sur-
vived to day 29 of gestation when the experiment was
terminated. A total of 12 animals aborted during the
dosing period: 3, 1, 1, and 7 from the control, 0.2, 1,
and 2.5 mg/kg groups, respectively. Females aborting were
killed the same day and immediate postmortem examinations
carried out. The increased occurrence of abortions in the
highest-dose group was considered to be a secondary effect
of maternal toxicity. No clinical findings in the groups
given 0.2 or 1 mg/kg were considered to be the result of
TBTO treatment. There was a statistically significant mean
body weight loss in females dosed at 2.5 mg/kg per day
compared to controls between days 6 and 18 of gestation
(the period of actual dosing). Doses of 0.2 and 1.0 mg/kg
per day had no effect on growth or survival of fetuses.
There was a slight (statistically non-significant)
decrease in mean fetal weight in the group dosed with 2.5
mg/kg per day. This may represent minor fetotoxicity.
There were no differences in the types or frequency of
fetal malformations related to treatment and the con-
clusion was that TBTO was not teratogenic. Postmortem
examination of the mother rabbits indicated no changes
associated with treatment, and 1 mg/kg per day was con-
sidered to be the NOEL for maternal toxicity and toxicity
to the fetus.
When Crofton et al. (1989) treated pregnant Long-Evans
rats with TBTO by gastric intubation at 0 to 16 mg/kg per
day body weight from day 6 to day 20 of gestation, litter
size and pup weight were significantly reduced by doses of
10, 12, or 16 mg/kg per day but no effect was seen at
doses of 2.5 and 5 mg/kg per day. Maternal weight gain was
affected by the same dose levels that affected litter size
and pup weight. Pup survival was further reduced in the
first 3 days after birth. Litter size was reduced by 50%,
73%, and 96% at dose levels of 10, 12, and 16 mg/kg per
day, respectively, on day 1 post partum and by 63%, 88%,
and 100% on day 3 post partum. Pup weight on day 1 post
partum was reduced by 45%, 45%, and 68% at the three dose
levels. Two out of 71 pups born had cleft palate, but no
controls showed any abnormalities. These two pups were
both born dead. The five pups born to rats given 16 mg/kg
per day showed no malformations, but all died within 3
days. The authors concluded that it was impossible to
distinguish between possible fetotoxicity and maternal
toxicity, since all effects on offspring occurred at TBTO
dose rates that also affected the weight of the mother.
They also showed that pregnant female rats were more sen-
sitive to TBTO than non-pregnant females; the MTD (maximum
tolerated dose) for non-pregnant females was 16 mg/kg per
day, whereas pregnant females showed an MTD of between 5
and 10 mg/kg per day. Most effects on pups were transitory
in that survivors to adulthood showed only reductions in
body weight and brain weight compared to controls even at
the highest dose rates. Motor activity of offspring was
monitored in a maze fitted with photoelectric detectors.
During the pre-weaning period there was a significant
age/treatment interaction, but only on post-natal day 14
was there a significant response per individual day. All
doses of TBTO produced a significant decrease in activity
on that day. Post-weaning activity was reduced on post-
natal days 47 and 62 (p < 0.01) but only at a level of
10 mg/kg per day. There was no clear effect on acoustic
startle response.
In a two-generation reproduction study, TBTO was given
to rats at dietary concentrations of 0, 0.5, 5, and 50
mg/kg. The parental (F0) generation (30 males and 30
females per group) was exposed for 10 weeks before mating,
whereas the pre-mating treatment period for the F1 adults
(30 of each sex per group) was 15 weeks. Culling of
litters was performed on day 4 in the F1 and F2 gener-
ations. Preliminary data (Biodynamics, 1989b) on mor-
tality, body weight development, fertility indices, litter
data, and organ weights have been reported. There was no
evidence of compound-related mortality, and body weight
development was normal in the F0 generation. At 50 mg/kg
diet, the pup weights were decreased on days 14 and 21 in
the F1 generation and on days 7, 14, and 21 in the
F2 generation. Among the F1 parents, at 50 mg/kg, lower
body weights were noted in males throughout the pre-mating
period and in females during the first 3 weeks only. No
effects on mating, pregnancy, and fertility rates were
noted in either generation, and number of pups, litter
size, and pup survival were not affected by treatment in
either the F1 or the F2 generations. Relative and
absolute thymus weights were decreased in both sexes at
the dietary concentration of 50 mg/kg.
11.5.2. In vitro
Krowke et al. (1986) demonstrated an effect of TBTO on
the development of limb buds of mice in organ culture.
Clear-cut interference with differentiation was seen at
the lowest dose tested (0.03 mg/litre), while at 0.1 mg
per litre drastic impairment and abnormal development of
the paw skeleton were recorded. The effect was more pro-
nounced still at the highest dose tested (0.3 mg/litre).
In view of their failure to show effects on the embryo in
vivo, Davis et al. (1987) suggested that there is only
very limited movement of TBTO across the placenta of
mice.
11.6. Carcinogenicity
Summary
One carcinogenicity study on rats has been reported in
which neoplastic changes were observed in endocrine organs at
50 mg/kg diet. The pituitary tumours found at 0.5 mg/kg diet
are considered as having no biological significance since there
was no dose-response relationship. These tumour types appear
usually at high and variable background incidences. The signi-
ficance is, therefore, questionable. A second study on mice is
in progress.
Wester (in press) reported the results of a 106-week
study on carcinogenicty in Wistar rats at dietary TBTO
doses of 0, 0.5, 5, and 50 mg/kg (0, 0.025, 0.25, and
2.5 mg/kg body weight). At the highest dose level, general
toxicological effects were present (see section 11.3). The
incidence of benign tumours of the pituitary (mainly pro-
lactinomas) was elevated at 0.5 and 50 mg/kg, but not at
5 mg/kg diet, for both sexes. At 50 mg/kg, a significant
increase was noted in the incidence of adrenal medullary
tumours (pheochromocytomas) in both sexes and of parathy-
roid adenomas in male animals, while the incidence of
adrenal cortical tumours was significantly decreased at
0.5 and 50 mg/kg diet in males only. Isolated occurrence
of pancreatic carcinoma was found in treated female rats.
These were not considered to be compound related since
there was no dose dependency and the incidence rates were
low.
12. EFFECTS ON HUMANS
Summary
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The potential
problem is made worse by the lack of an immediate skin response.
12.1. Ingestion
There have been no reported cases of poisoning from
ingestion of TBTO or other TBT salts.
12.2. Inhalation
Seventy percent of the workers in a rubber factory
using TBTO in the vulcanizing process reported irritation
of the upper respiratory tract (and eyes). About 20% also
experienced lower chest symptoms (irritation, tightness,
and pain), but in all cases pulmonary function was unaf-
fected. The extent of the exposure was not recorded
(WHO/FAO, 1985).
12.3. Dermal exposure
Lyle (1958) described skin lesions in workers occu-
pationally exposed to dibutyl and tributyl tin compounds.
Skin burns were most commonly caused by small splashes of
liquid dibutyl or tributyl tin chlorides. Since the irri-
tancy of these compounds was not immediately apparent
(taking at least an hour to be perceived), small splashes
were frequently ignored by workers. More severe lesions on
the hands were caused by leaking gloves or failure to wear
hand protection. Some maintenance staff also suffered
burns after kneeling or rubbing against surfaces wet with
the chlorides. Clothes wet with these compounds produced
burns on the ventral skin and burns were seen immediately
above the level of protective boots on the calf of the
leg. Two kinds of lesion were seen in workers. The first
was an acute burn, which healed relatively quickly, and
the second a more diffuse dermatitis (seen in workers
wearing contaminated clothes in close contact with the
skin), which persisted. Treatment of the back of the hand
of volunteers with various undiluted TBT compounds estab-
lished that the chloride, acetate, laurate, and oxide all
produced acute burns. Burns were not caused by dibutyltin
esters or oxide or by tetrabutyltin, but were mostly due
to dibutyltin chloride or the tributyltin compounds. Red-
dening of the area treated was seen 2-3 h after treatment
with the compounds. Inflammation of the hair follicles
was the most obvious symptom, effects being confined to
the treated area. On the second day after treatment, min-
ute pustules appeared, which remained discrete and disap-
peared on the third or fourth day. After a week, all that
remained was a faint erythema. The burns were not reported
to be painful, either in the case of experimental or occu-
pational exposure. Sufferers complained of itching and
"stickiness" of the skin causing adherence of clothes.
Proper use of protective clothing, rapid washing of the
skin after exposure, and the use of aprons to prevent
wetting of overalls were found to be effective in pre-
venting burns, both acute and diffuse.
Baaijens (1987) described cases of accidental exposure
to TBTO during the manufacture of organotin compounds.
Severe dermatitis developed only where splashes of the
material had been retained on the skin for long periods.
In one case, a worker had been splashed over the face and
neck. He left the work area after the splash and showered.
An area behind one ear had not been washed and the derma-
titis had developed in this one area. There were no symp-
toms other than dermatitis. Another worker had been
splashed on the arm. He washed his skin but did not change
his overalls. Contact was extended and a large blister
developed on the arm. Monitoring of the urine tin content
showed no difference from normal in these two cases. A
third worker, who complained of an intense smell of TBTO,
suffered nausea and vomiting after 10 min of exposure.
Urine tin levels in this case were elevated for several
days. In all cases the symptoms disappeared within a few
days. The delayed irritancy of tributyltin was emphasized
by the author. It tends to lead to extended exposure since
the affected person does not perceive the effect for sev-
eral hours. The author found no relationship between
clinical or haematological parameters and normal occu-
pational exposure to organotin compounds.
Goh (1985) reported irritant effects and dermatitis in
painters applying TBTO formulations (0.6% TBTO in acrylic
resin-based paints) to buildings. The painters developed
rashes 8 to 10 h after exposure. When required to paint
ceilings, the men developed more severe symptoms on the
face, neck, and trunk, associated with dripping paint.
Severe itching, redness, swelling, and blistering were
recorded. Hospital examination showed extensive vesiculo-
bullous lesions, erythema, and oedema of the face, neck,
trunk, arms, and thighs. Although only two patients were
examined in detail, most of the workers on the site devel-
oped dermatitis. Patch testing of the two reported cases
showed erosion 48 and 96 h after patch tests using aqueous
solutions of TBTO (0.1 and 0.5 g/litre). Five other volun-
teer controls were patch-treated with solutions of 0.01
and 0.1 g/litre; these also showed a similar reaction to
the patients. No allergenic reaction was found in the
patch tests. Replacement of the paint with a preparation
having similar constituents other than TBTO prevented
further problems amongst the painters.
Lewis & Emmett (1987) describe contact dermatitis in a
shipwright resulting from exposure to TBTO-containing
antifouling paint. The man had been spray-painting blocks
of wood and his skin had been exposed to the spray. There
was no immediate sensation, but some irritation was evi-
dent within about an hour. Erythema and ulceration of the
exposed areas were noted on the second day. There were
also some mild pustular lesions on the mucous membrane of
the lips, presumed to be the result of wiping the mouth
with paint-contaminated arms. The authors conducted patch
testing with TBTO at aqueous concentrations of 0.1%,
0.01%, and 0.0015% using the A1 test tape method. At the
highest dose tested, there were large bullae after 48 h
and crusting after 96 h. The other two doses produced no
adverse effects. The delayed sensation and effect were
highlighted by the authors as being particular problems
with TBTO. Great care to prevent any exposure to products
containing TBTO at high concentrations was recommended.
Molin & Wahlberg (1975) investigated an outbreak of
dermatitis on the feet and ankles of trainee soldiers.
Seventy soldiers reported itching and sometimes painful
erythematous, vesiculous to bullous, and haemorrhagic
lesions after long marches on a hot day. Fifty other
soldiers reported less severe symptoms on another
occasion. The skin lesions disappeared within 1 or 2 weeks
in most cases after treatment with saline compresses and
topical steroid creams. In two cases, the skin was red and
slightly tender more than 6 months later. The outbreak was
traced to a single batch of socks that had been soaked in
7 times the recommended concentration of a disinfectant
solution containing TBTO. Recommended use of the disinfec-
tant was calculated to give about 0.001%, whereas the
concentration after the accidental over-use would have
been about 0.01%. Patch testing of eczema patients not
previously exposed to TBTO suggested that the primary
irritant concentration for the compound was between 0.1
and 0.01%. Soldiers patch-tested 2 months after their
dermatitis had fully healed gave negative results to both
0.001% and 0.01% TBTO. The authors suggested that the
effective concentration for TBTO as a disinfectant is too
close to the primary irritancy concentration to justify
the use of TBTO for disinfecting textiles.
Zedler (1961), on the basis of industrial experience,
stated that a concentration of 0.05% TBTO was not harmful
to the human skin.
12.4. Miscellaneous effects
Women using a latex spray paint containing TBTO as an
additive showed immediate irritant effects on the eye
(profuse lacrimation, eye inflammation) and the nasal
mucosa. The symptoms worsened over 14 days of spraying,
but subsided at the weekends and disappeared completely
when addition of TBTO to the paint was discontinued. The
extent of exposure in this case was not recorded (WHO/FAO,
1985).
Akatsuka et al. (1959) reported a case of occupational
poisoning with butyltin compounds where, along with symp-
toms of lassitude, slight occipital headaches, and stiff-
ness in the shoulders, there was a marked disturbance of
the sense of smell. The authors conducted studies on cats
to test whether this effect could be confirmed experimen-
tally. Exposure of the cats to a vapour mixture containing
mainly tributyltin bromide revealed a marked loss of the
sense of smell.
13. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
13.1. Evaluation of human health risks
Exposure of workers occurs principally during the
manufacture and formulation of tributyltin compounds, in
the application and removal of TBT paints, and from the
use of TBT in wood preservatives. Exposure of the general
public may come from the contamination of food, particu-
larly fish and shellfish, and from domestic application of
wood preservatives.
On the basis of both animal tests and direct obser-
vations on humans, it is clear that TBT compounds are
irritant to the skin and eyes and that inhalation of aero-
sols leads to respiratory irritation.
The handling of treated wood poses no dermal irritant
hazard once the wood has dried. However, aerosols of TBT
are very hazardous and re-entry to the treatment area
should be prohibited until the wood has thoroughly dried.
Acute systemic poisoning has never been reported and
clearance of TBT from the body is expected to occur within
a few days. Acute toxicity from handling TBT products is,
therefore, unlikely if proper precautions are taken.
Short- and long-term effects on experimental animals
have been reported in the liver and haematological and
endocrine systems. The effects of TBT compounds on the
immune system, and particularly on host resistance, have
proved the most sensitive parameter of toxicity in the
rat, the most sensitive species tested. The no-observed-
effect level (NOEL), using the Trichinella spiralis host-
resistance model, lies between 0.5 and 5.0 mg/kg diet
(0.025 and 0.25 mg/kg body weight), whereas using measures
of immune function it is 0.6 mg/kg body weight.
Owing to wide variation in the consumption of fish and
shellfish and local differences in residues of TBT in sea-
food, only illustrative estimates relating exposure and
NOEL values can be made. It needs to be emphasized that
local measurements of residues, local estimates of sea-
food consumption, and local decisions on acceptable safety
margins must be made to assess potential risk of these
compounds.
Using fish consumption figures of 15 and 150 g/day, a
value of 1 mg/kg for residues in fish, and an average
human body weight of 60 kg, the following safety margins
based on different immune endpoints are obtained.
---------------------------------------------------------
Fish Estimated Safety margin
consumption daily intake T. spiralis Other
(g/day) of TBT model immune
(µg/kg) parameters
---------------------------------------------------------
15 0.25 100-1000 2500
150 2.5 10-100 250
---------------------------------------------------------
Indiscriminate and irresponsible use of TBT compounds
and a failure to follow the recommendations, outlined in
this monograph, to reduce exposure of humans could lead to
intake of levels of TBT compounds hazardous to human
health.
Teratogenic effects have only occurred in experimental
animals at doses that caused overt maternal toxicity. The
teratogenic potential of TBT is, therefore, considered to
be very low.
Based on the results of comprehensive mutagenicity
studies, tributyltin compounds are not considered to have
mutagenic potential. In a carcinogenicity study on rats
with TBTO, an increased incidence was noted for endocrine
tumours that occur spontaneously at a high and variable
incidence. Therefore, the available evidence does not
clearly demonstrate a carcinogenic hazard of TBT compounds
for humans.
13.2. Evaluation of effects on the environment
Diffuse input of tributyltin (TBT) into the environ-
ment occurs predominantly from the use of TBT in antifoul-
ing paint. It could also occur if it were used as a mol-
luscicide. Point source contamination occurs if TBT is
used as a biocide in cooling systems, wood pulping,
leather processing, wood preservation processes, and tex-
tile treatment.
Due to their physico-chemical properties, TBT com-
pounds concentrate in the surface microlayer and in sedi-
ments. Abiotic degradation does not appear to be a major
mechanism of removal under environmental conditions.
Although TBTO is biodegradable in the water column, this
process is not rapid enough to prevent the occurrence of
elevated TBT levels in some areas. Bioaccumulation occurs
in most aquatic organisms, but in laboratory mammals,
metabolic degradation is a more efficient process.
TBT is extremely hazardous to some aquatic organisms
because it is toxic at very low concentrations in water.
Such concentrations have been found in some areas. Adverse
effects on non-target invertebrates, particularly mol-
luscs, have been reported in field studies, and these have
been sufficiently severe to lead to reproductive failure
and population decline. Adverse effects on the commercial
production of shellfish have been successfully reversed by
restrictions on the use of antifouling paints in some
areas, and these restrictions are also leading to the
reversal of imposex effects in gastropod populations. The
effects on farmed fish indicate that TBT-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is
likely to be low. TBT-treated wood could pose a hazard to
terrestrial organisms living in close contact with it.
The enhancement of TBT concentrations in the surface
microlayer may present a hazard to littoral organisms,
neustonic species (including benthic invertebrate and fish
larvae) and surface-feeding sea-birds and wildfowl.
Accumulation and low biodegradation of TBT in sediments
may present a hazard to aquatic organisms when these pol-
luted sediments are disturbed by natural processes or
dredging activities.
14. RECOMMENDATIONS
14.1. Recommendations for protecting human and environmental health
a) Member countries that have not yet regulated the use
of TBT compounds should be encouraged to do so.
b) There is a need for evaluation and, if necessary,
regulation of organotin input to the environment from
sources other than antifouling paints. For example,
this would include evaluation of the potential risk
from the application of TBT-contaminated sewage sludge
to soil.
c) Improved methods for the safe application, removal,
and disposal of organotin paints should be developed.
14.2. Research needs
a) Methods of detection and analysis need to be improved
to provide rapid and accurate measurements of butyltin
species in pg/litre concentrations. One reason for
this recommendation is that a biological effect, i.e.
imposex in gastropods, may occur at levels lower than
present detection limits.
b) There is a need for research into mechanisms which
concentrate rather than disperse TBT and which retard
degradation, with particular attention to the funda-
mental chemistry of TBT and its interaction with bio-
logical molecules. More study is needed on the uptake
of TBT at all trophic levels.
c) A study of the toxicity of TBT in aquatic organisms is
required. This work should investigate metabolism,
endocrine effects, and immunological toxicity, where
appropriate.
d) A search for other sensitive bioindicator species in
other groups, including freshwater species, is
required.
e) Models for the assessment of immunotoxicity in mammals
need to be validated and no-effect levels for relevant
parameters need to be defined more accurately.
f) A long-term toxicity study in a second mammalian
species should be undertaken.
g) A tumorigenicity study in a second mammalian species
should be undertaken.
h) Information on butyltin residue levels in fish and
shellfish for human consumption using speciating
methods is needed.
REFERENCES
ABEL, R., HATHAWAY, R.A., KING, N.J., VOSSER, J.L., & WILKINSON, T.G.
(1987) Assessment and regulatory actions for TBT in the UK. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1314-1319.
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A., KAWAME,
N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S., YANAGIBASHI, H.,
NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA, I., & IYO, T. (1959)
[Experimental studies on disturbance of sense of smell due to butyltin
compounds.] J. Tokyo med. Coll., 17: 1393-1402 (in Japanese).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int. Pest Control,
11: 29-35.
ALDRIDGE, W.N. (1958) The biochemistry of organotin compounds. Trialkyltins
and oxidation phosphorylation. Biochem. J., 69: 367-376.
ALDRIDGE, W.N. & STREET, B.W. (1964) Oxidative phosphorylation: Biochemical
effects and properties of trialkyltins. Biochem. J., 91: 287-297.
ALDRIDGE, W.N. & STREET, B.W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver mitochondria.
Biochem. J., 118: 171-179.
ALDRIDGE, W.N. & STREET, B.W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and triethyltin to
mitochondria and their effects on various mitochondrial functions. Biochem.
J., 124: 221-234.
ALDRIDGE, W.N., CASIDA, J.E., FISH, R.H., KIMMEL, E.C., & STREET, B.W.
(1977) Action on mitochondria and toxicity of metabolites of tri-n-butyltin
derivatives. Biochem. Pharmacol., 26: 1997-2000.
ALLEN, A.J., QUITTER, B.M., & RADICK, C.M. (1980) The biocidal mechanism of
controlled release bis (tri-n-butyltin) oxide in Biomphalaria glabrata. In:
Baker, R., ed. Controlled release of bioactive materials, New York, London,
Academic Press, p. 399.
ALZIEU, C.P. (1981) Evaluation des risques dus à l'emploi des peintures
anti-salissures dans les zones conchylicoles, Nantes, Institut scientifique
et technique des Pêches maritimes, 84 pp.
ALZIEU, C. & HERAL, M. (1984) Ecotoxicological effects of organotin
compounds on oyster culture. Ecotoxicol. Test. mar. Environ., 2: 187-196.
ALZIEU, C. & PORTMANN, J.E. (1984) The effect of tributyl tin on the
culture of C. gigas. Proceedings of the 15th Annual Shellfish Conference,
15-16 May, 1984, London, The Shellfish Association of Great Britain, 17 pp.
ALZIEU, C., HERAL, M., THIBAUD, Y., DARDIGNAC, M.-J., & FEUILLET, M. (1982)
Influence des peintures antisalissures à base d'organostanniques sur la
calcification de la coquille de l'huitre Crassostrea gigas. Rev. Trav.
Inst. Pêches Marit., 45: 101-116.
ALZIEU, C., SANJUAN, J., DELTREIL, J.P., & BOREL, M. (1986) Tin
contamination in Arcachon Bay: Effects on oyster shell anomalies. Mar.
Pollut. Bull., 17: 494-498.
ALZIEU, C., SANJUAN, J., MICHEL, P., BOREL, M., & DRENO, J.P. (1989)
Monitoring and assessment of butyltins in Atlantic coastal waters. Mar.
Pollut. Bull., 20: 22-26.
ANGER, J.P., ANGER, F., CANO, Y., CHAUVEL, Y., LOUVET, M., & VAN DEN
DRIESSCHE, J. (1976) Effets chez le cobaye, d'un aérosol à base d'oxyde de
tributylétain (OTBE). Eur. J. Toxicol., 9: 339-346.
ARAKAWA, Y. & WADA, O. (1984) Inhibition of neutrophil chemotaxis by
organotin compounds. Biochem. biophys. Res. Commun., 123: 543-548.
ARGAMAN, Y., HUCKS, C.E., & SHELBY, S.E. (1984) The effects of organotin on
the activated sludge process. Water Res., 18: 535-542.
AYALA, C.A.C., TOLEDO, J.V., DA SILVA NETTO, J.A., & GILBERT, B. (1980) The
effect of hexabutyldistannoxane (TBTO) and other molluscicides on non-
target species, Geneva, World Health Organization, Parasitic Diseases
Programme (Unpublished report).
BAAIJENS, P.A. (1987) Health effect screening and biological monitoring for
workers in organotin industries. In: Toxicology and analytics of the
tributyltins: The present status. Proceedings of an ORTEPA workshop,
Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands, ORTEP-
Association, pp. 191-208
BACCI, E. & GAGGI, C. (1989) Organotin compounds in harbour and marina
waters from the Northern Tyrrhenian Sea. Mar. Pollut. Bull., 20: 290-292.
BAHR, G. & PAWLENKO, S. (1978) Organic tin compounds. In: Bahr, G.,
Kalinowski, H.-O., & Pawlenko, S., ed. Organometallic compounds, germanium,
tin, Stuttgart, Georg Thieme Verlag, pp. 512-515 (Methods in Organic
Chemistry series).
BAILEY, S.K. & DAVIES, I.M. (1988a) Tributyltin contamination around an oil
terminal in Sullom Voe (Shetland). Environ. Pollut., 55: 161-172.
BAILEY, S.K. & DAVIES, I.M. (1988b) Tributyltin contamination in the Firth
of Forth (1975-87). Sci. total Environ., 76: 185-192.
BAKER, J.M. & TAYLOR, J.M. (1967) The toxicity of tributyltin oxide to the
wood-boring beetles Lyctus brunneus Steph. and Anobium punctatum (Deg.).
Ann. appl. Biol., 60: 181-190.
BALLS, P.W. (1987) Tributyltin (TBT) in the waters of a Scottish sea loch
arising from the use of antifoulant treated netting by salmon farms.
Aquaculture, 65: 227-237.
BARNES, J.M. & STONER, H.B. (1958) Toxic properties of some dialkyl and
trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARUG, D. (1981) Microbial degradation of bis (tributyltin) oxide.
Chemosphere, 10: 1145-1154.
BARUG, D. & VONK, J.W. (1980) Studies on the degradation of bis
(tributyltin) oxide in soil. Pestic. Sci., 11: 77-82.
BEAUMONT, A.R. & BUDD, M.D. (1984) High mortality of the larvae of the
common mussel at low concentrations of tributyltin. Mar. Pollut. Bull., 15:
402-405.
BEAUMONT, A.R. & NEWMAN, P.B. (1986) Low levels of tributyltin reduce
growth of marine micro-algae. Mar. Pollut. Bull., 17: 457-461.
BEAUMONT, A.R., NEWMAN, P.B., & WALDOCK, M.J. (1987) Sand substrate
microcosm studies on tributyl tin (TBT) toxicity to marine organisms, 24 pp
(Final report to the UK Department of the Environment, London. Contract No.
PECD 7/8/73).
BERRIOS-DURAN, L.A. & RITCHIE, L.S. (1968) Molluscicidal activity of
bis(tri-n-butyltin) oxide formulated in rubber. World Health Organ. Bull.,
39: 310-312.
BIODYNAMICS (1989a) A three month oral range-finding toxicity study in mice
with bis (tri-n-butyltin) oxide (TBTO), East Millstone, New Jersey,
Biodynamics (Final report to Aceto Chemical Corporation, M. + T. Chemicals
Inc., and Schering Berlin Inc.) (Unpublished).
BIODYNAMICS (1989b) A two-generation reproduction study in rats with TBTO,
East Millstone, New Jersey, Biodynamics (Interim report to Aceto Chemical
Corporation, M. + T. Chemicals Inc., and Schering Berlin Inc.)
(Unpublished).
BJORKLUND, I. (1987a) [Environmental aspects of ship's bottom paints],
Stockholm, Swedish Chemicals Inspectorate, Investigation Department, 16 pp
(in Swedish).
BJORKLUND, I. (1987b) [Environmental effect of antifouling paints],
Stockholm, Swedish National Environment Protection Board, 17 pp.
BLAIR, W.R., OLSON, G.J., BRINCKMAN, F.E., & IVERSON, W.P. (1982)
Accumulation and fate of tri-n-butyltin cation in estuarine bacteria.
Microbiol. Ecol., 8: 241-251.
BLAIR, W.R., OLSON, G.J., & BRINCKMAN, F.E. (1986) Speciation measurements
of butyltins: Application to controlled release rate determination and
production of reference standards. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1141-1145.
BLUNDEN, S.J. & CHAPMAN, A. (1986) Organotin compounds in the environment.
In: Craigh, P.J., ed. Organometallic compounds in the environment, Harlow,
Essex, Longman Group Ltd, pp. 111-159.
BLUNDEN, S.J., HOBBS, L.A., & SMITH, P.J. (1984) The environmental
chemistry of organotin compounds. In: Bowen, H.J.M, Blunden, S.J., Colbeck,
I., Harrison, R.M., Hobbs, L.A., Katz, S.A., Simkiss, K., Smith, P.J., &
Taylor, M.G., ed. Environmental chemistry, London, Royal Society of
Chemistry, Vol. 3, pp. 49-77.
BOKRANZ, A. & PLUM, H. (1975) Industrial manufacture and use of organotin
compounds, Bergkamen, Federal Republic of Germany, Schering AG, 33 pp.
BOORMAN, L.A. (1989) The effects of TBT on two salt marsh plant species,
Aster tripolium and Limonium vulgare. In Preparation.
BOULDIN, T.W., GOINES, N.D., BAGNELL, C.R., & KRIGMAN, M.R. (1981)
Pathogenesis of trimethyltin neuronal toxicity. Ultrastructural and
cytochemical observations. Am. J. Pathol., 104: 237-249.
BRAMAN, R.S. & TOMPKINS, M.A. (1979) Separation and determination of
nanogram amounts of inorganic and methyltin compounds in the environment.
Anal. Chem., 51: 12-19.
BRESSA, G., CIMA, L., CANOVA, F., & CARAVELLO, G.U. (1984) Bioaccumulation
of tin in the fish tissues (Liza aurata). In: Proceedings of the
International Conference on Environmental Contamination, London, July 1984
- Geneva, International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp. 812-815.
BRIDGES, J.W., DAVIES, D.S., & WILLIAMS, R.T. (1967) The fate of ethyltin
and diethyltin derivatives in the rat. Biochem. J., 105: 1261-1266.
BRINCKMAN, F.E. (1981) Environmental organotin chemistry today: Experiences
in the field and laboratory. J. organomet. Chem. Libr., 12: 343-376.
BRINCKMAN, F.E., JACKSON, J.A., BLAIR, W.R., OLSON, G.J., & IVERSON, W.P.
(1983) Ultratrace speciation and biogenesis of methyltin transport species
in estuarine waters. In: Trace metals in sea water, New York, Plenum Press,
pp. 39-72.
BROWN, R.A., NAZARIO, C.M., DE TIRADO, R.S., CASTRILLON, J., & AGARD, E.T.
(1977) A comparison of the half-life of inorganic and organic tin in the
mouse. Environ. Res., 13: 56-61.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1986) The
decline of the gastropod Nucella lapillus around south-west England:
Evidence for the effect of tributyltin from antifouling paints. J. Mar.
Biol. Assoc. UK, 66: 611-640.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1987) Copper,
zinc, and organotin as long-term factors governing the distribution of
organisms in the Fal estuary in southwest England. Estuaries, 10: 208-219.
BRYAN, G.W., GIBBS, P.E., & BURT, G.R. (1988) A comparison of the
effectiveness of tri-n-butyltin chloride and five other organotin compounds
in promoting the development of imposex in the dog-whelk Nucella lapillus.
J. Mar. Biol. Assoc. UK, 68: 733-744.
BUSHONG, S.J., HALL, W.S., JOHNSON, W.E., & HALL, L.W. (1987) Toxicity of
tributyltin to selected Chesapeake Bay biota. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1494-1503.
BUSHONG, S.J., HALL, L.W., HALL, W.S., JOHNSON, W.E., & HERMAN, R.L. (1988)
Acute toxicity of tributyltin to selected Chesapeake Bay fish and
invertebrates. Water Res., 22: 1027-1032.
CALLEY, D.J., GUESS, W.L., & AUTIAN, J. (1967) Hepatotoxicity of a series
of organotin esters. J. pharm. Sci., 56: 240-243.
CARDARELLI, N.F. (1973) Effects of ultralow molluscicide dosages on
Biomphalaria glabrata and Lebistes reticulatus in micro ecological systems:
Report I, Akron, Ohio, University of Akron, 15 pp.
CARDARELLI, N.F. (1978) Controlled release organotins as mosquito
larvicides. Mosq. News, 38: 328-333.
CARDARELLI, N.F. & EVANS, W. (1980) Chemodynamics and environmental
toxicology of controlled release organotin molluscicides. In: Baker, R.W.,
ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the Controlled Release Society, New York, London,
Academic Press, pp. 357-385.
CASIDA, J.E., KIMMEL, E.C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and trialkyleads by
liver microsomes. Acta chem. Scand., 25: 1497-1499.
CHAMP, M.A. & PUGH, W.L. (1987) Tributyltin antifouling paints:
Introduction and overview. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1296-1308.
CHAPMAN, A.H. & PRICE, J.W. (1972) Degradation of triphenyltin acetate by
ultraviolet light. Int. Pest Control, 14: 11-12.
CHENG, Z. & JENSEN, A. (1989) Accumulation of organic and inorganic tin in
blue mussel, Mytilus edulis, under natural conditions. Mar. Pollut. Bull.,
20: 281-286.
CHLIAMOVITCH, Y.-P. & KUHN, C. (1977) Behavioural, haematological and
histological studies on acute toxicity of bis(tri- n-butyltin) oxide on
Salmo gairdneri Richardson and Tilapia rendalli Boulenger. J. Fish Biol.,
10: 575-585.
CHU, K.Y. (1976) Effects of environmental factors on the molluscicidal
activities of slow-release hexabutyldistannoxane and copper sulfate. Bull.
World Health Organ., 54: 417-420.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & LORES, E.M. (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass shrimp
(Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Arch.
environ. Contam. Toxicol., 16: 401-407.
CLEARY, J.J. & STEBBING, A.R.D. (1985) Organotin and total tin in coastal
waters of southwest England. Mar. Pollut. Bull., 16: 350-355.
CLEARY, J.J. & STEBBING, A.R.D. (1987) Organotin in the surface microlayer
and subsurface waters of Southwest England. Mar. Pollut. Bull., 18: 238-
246.
CREMER, J.E. (1957) The metabolism in vitro of tissue slices from rats
given triethyltin compounds. Biochem. J., 67: 87-96.
CROFTON, K.M., DEAN, K.F., BONCEK, V.M., ROSEN, M.B., SHEETS, L.P.,
CHERNOFF, N., & REITER, L.W. (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: Postnatal evaluation of teratology and
behavior. Toxicol. appl. Pharmacol., 97: 113-123.
DAVIDSON, B.M., VALKIRS, A.O., & SELIGMAN, P.F. (1986) Acute and chronic
effects of tributyltin on the mysid Acanthomysis sculpta (Crustacea,
Mysidacea). In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., pp. 1219-1225.
DAVIES, R.J., FLETCHER, R.L., & FURTADO, S.E.J. (1984) The effects of
tributyltin compounds on spore development in the green alga Enteromorpha
intestinalis (L) Link. In: Proceedings of the 6th International Congress on
Marine Corrosion and Fouling, Athens, September 1984, pp. 557-565.
DAVIES, I.M., DRINKWATER, J., MCKIE, J.C., & BALLS, P. (1987a) Effects of
the use of tributyltin antifoulants in mariculture. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., pp. 1477-1481.
DAVIES, I.M., BAILEY, S.K., & MOORE, D.C. (1987b) Tributyltin in Scottish
sea Lochs, as indicated by degree of imposex in the dogwhelk, Nucella
lapillus (L.). Mar. Pollut. Bull., 18: 400-404.
DAVIS, A., BARALE, R., BRUN, G., FORSTER, R., GUNTHER, T., HAUTEFEUILLE,
H., VAN DER HEIJDEN, C.A., KNAAP, A.G.A.C., KROWKE, R., KUROKI, T.,
LOPRIENO, N., MALAVEILLE, C., MERKER, H.J., MONACO, M., MOSESSO, P.,
NEUBERT, D., NORPPA, H., SORSA, M., VOGEL, E., VOOGD, C.E., UMEDA, M., &
BARTSCH, H. (1987) Evaluation of the genetic and embryotoxic effects of
bis(tri- n-butyltin) oxide (TBTO), a broad-spectrum pesticide, in multiple
in vivo and in vitro short-term tests. Mutat. Res., 188: 65-95.
DESCHIENS, R. & FLOCH, H. (1962) Les propriétés molluscicides du chlorure
et de l'acétate de triphénylétain dans le cadre de la prophylaxie des
bilharzioses. C. R. Acad. Sci. Paris, 255: 1236-1237.
DESCHIENS, R. & FLOCH, H. (1968) Action biologique comparée de 6
molluscicides chimique dans le cadre de la prophylaxie des bilharzioses.
Conclusions pratiques. Bull. Soc. Pathol. Exot., 61: 640-650.
DESCHIENS, R., BROTTES, H., & MVOGO, L. (1966) Application sur le terrain,
au Cameroun, dans la prophylaxie des bilharzioses de l'action molluscicide
de l'oxyde de tributylétain. Bull. Soc. Pathol. Exot., 59: 968-973.
DE VILLIERS, J.P. & MACKENZIE, J.G. (1963) Organotin and organolead
molluscicides, Geneva, World Health Organization, Parasitic Diseases
Programme, p. 63 (Unpublished report Mol/Inf/13).
DIXON, D.R. & MCFADZEN, I. (1987) Bis(tributyltin) oxide (TBTO), an
antifouling compound, promotes SCE induction in the larvae of the common
mussel, Mytilus edulis. Mutagenesis, 2: 312.
DIXON, D.R. & PROSSER, H. (1986) An investigation of the genotoxic effects
of an organotin antifouling compound (bis(tributyltin) oxide) on the
chromosomes of the edible mussel, Mytilus edulis. Aquat. Toxicol., 8: 185-
195.
DOJMI DI DELUPIS, G., GUCCI, P.M.B., & VOLTERRA, L. (1987) Toxic effects of
bis-tributyltinoxide on phytoplancton. Main Group Metal Chem., 10: 77-82.
DONARD, O., RAPSOMANIKIS, S., & WEBER, J.H. (1986) Speciation of inorganic
tin and alkyltin compounds by atomic absorption spectrometry using
electrothermal quartz furnace after hydride generation. Anal. Chem., 58:
772-777.
EAJ (1988) Outline of TBT compounds monitored in Japan, Tokyo, Environment
Agency of Japan (OECD Clearing House Project on Organotins).
EBDON, L., EVANS, K., & HILL, S. (1988) The variation of tributyltin levels
with time in selected estuaries prior to the introduction of regulations
governing the use of tributyltin-based anti-fouling paints. Sci. total
Environ., 68: 207-223.
EBDON, L., EVANS, K., & HILL, S. (1989) The accumulation of organotins in
adult and seed oysters from selected estuaries prior to the introduction of
U.K. regulations governing the use of tributyltin-based antifouling paints.
Sci. total Environ., 83: 63-84.
ELFERINK, J.G.R., DEIERKAUF, M., & VAN STEVENINCK, J. (1986) Toxicity of
organotin compounds for polymorphonuclear leukocytes: the effect on
phagocyctosis and exocytosis. Biochem. Pharmacol., 35: 3727-3732.
ELSEA, J.R. & PAYNTER, O.E. (1958) Toxicological studies on bis(tri-n-
butyltin) oxide. Am. Med. Assoc. Arch. Ind. Health, 18: 214-217.
EVANS, C.J. & KARPEL, S. (1985) Organotin compounds in modern technology.
J. organomet. Chem. Libr., 16: 178-217.
EVANS, D.W & LAUGHLIN, R.B. (1984) Accumulation of bis (tributyltin) oxide
by the mud crab, Rhitropanopeus harrisii. Chemosphere, 13: 213-219.
EVANS, W.H., CARDARELLI, N.F., & SMITH, D.J. (1979) Accumulation and
excretion of [1-14C] bis (tri- n-butyltin) oxide in mice. J. Toxicol.
environ. Health, 5: 871-877.
FENT, K. (1989a) Organotin speciation in municipal wastewater and sewage
slude: Ecotoxicological consequences. Mar. environ. Res., 28: 477-483.
FENT, K. (1989b) Teratogenic effects of tributyltin on embryos of the
freshwater fish Phoxinus phoxinus. Presented at the OECD Workshop on
Tributyltin: Activities related to Field Studies at Sea, Centre IFREMER,
Nantes, 27-29 June, 1989 (Unpublished report).
FENT, K., FASSBIND, R., & SIEGRIST, H. (1989) Organotins in a municipal
wastewater treatment plant. Proceedings of the 1st European Conference on
Ecotoxicology, Copenhagen, Denmark, 17-19 October, 1988, Lyngby, Denmark,
The Technical University of Denmark, Laboratory of Environmental Sciences
and Ecology.
FERAL, C. & LE GALL, S. (1983) The influence of a pollutant factor
(tributyltin) on the neuroendocrine mechanism responsible for the
occurrence of a penis in the females of Ocenebra erinacea. In: Lever, J. &
Boer, H.H., ed. Molluscan neuroendocrinology. Proceedings of the
International Minisymposium on Molluscan Endocrinology, Amsterdam, North-
Holland Publishing Company, pp. 173-175.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1975) Bioorganotin chemistry:
Biological oxidation of tributyltin derivatives. J. organomet. Chem., 93:
C1-C4.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1976) Bioorganotin chemistry:
Biological oxidation of organotin compounds. In: Zuckerman, J.J., ed.
Organotin compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 197-203 (Advances in Chemistry Series No.
157).
FLOCH, H., DESCHIENS, R., & FLOCH, T. (1964) Sur les propriétés
molluscicides de l'oxyde et de l'acétate de tributylétain (prophylaxie des
bilharzioses). Bull. Soc. Pathol. Exot., 57: 454-465.
FOSTER, R.B. (1981) Use of Asiatic clam larvae in aquatic hazard
evaluations. In: Bates, J.M. & Weber, C.I., ed. Ecological assessments of
effluent impacts on communities of indigenous aquatic organisms,
Philadelphia, American Society for Testing and Materials, pp. 280-288 (ASTM
STP No. 730).
FUNAHASHI, N., IWASAKI, I., & IDE, G. (1980) Effects of bis(tri-n-butyltin)
oxide on endocrine and lymphoid organs of male rats. Acta pathol. Jpn., 30:
955-966.
GARDINER, B.G. & POLLER, R.C. (1964) Insecticidal activity and structure of
some organotin compounds. Bull. entomol. Res., 55: 17-21.
GIBBS, P.E. & BRYAN, G.W. (1986) Reproductive failure in populations of the
dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from
antifouling paints. J. Mar. Biol. Assoc. UK, 66: 767-777.
GIBBS, P.E. & BRYAN, G.W. (1987) TBT paints and the demise of the dog-
whelk, Nucella lapillus (Gastropoda). In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1482-1487.
GIBBS, P.E., BRYAN, G.W., PASCOE, P.L., & BURT, G.R. (1987) The use of the
dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT)
contamination. J. Mar. Biol. Assoc. UK, 67: 507-523.
GIBBS, P.E., PASCOE, P.L., & BURT, G.R. (1988) Sex change in the female
dogwhelk, Nucella lapillus, induced by tributyltin from antifouling paints.
J. Mar. Biol. Assoc. UK, 68: 715-731.
GILBERT, B., PAES LEME, L.A., FERREIRA, A.M., BULHOES, M.S., & CASTLETON,
C. (1973) Field tests of hexabutyldistannoxane (TBTO) in slow-release
formulations against Biomphalaria spp. Bull. World Health Organ., 49: 633-
636.
GILE, J.D., COLLINS, J.C., & GILLETT, J.W. (1982) Fate and impact of wood
preservatives in a terrestrial microcosm. J. agric. food Chem., 30: 295-
301.
GILLETT, J.W., GILE, J.D., & RUSSELL, L.K. (1983) Predator-prey (vole-
cricket) interactions: the effects of wood preservatives. Environ. Toxicol.
Chem., 2: 83-93.
GOH, C.L. (1985) Irritant dermatitis from tri-n-butyl tin oxide in paint.
Contact dermatitis, 12: 161-163.
GOHLKE, R., LEWA, W., STRACHOVSKY, A., & KOHLER, R. (1969) [Investi-gations
in experimental animals of the inhalatory effects of tributyltin chloride
in a subchronic experiment.] Z. gesamte Hyg. Grenzgeb., 15: 97-104 (in
German).
GOODMAN, L.R., CRIPE, G.M., MOODY, P.H., & HALSELL, D.G. (1988) Acute
toxicity of malathion, tetrabromobisphenol-A, and tributyltin chloride to
mysids (Mysidopsis bahia) of three ages. Bull. environ. Contam. Toxicol.,
41: 746-753.
GROVHOUG, J.G., SELIGMAN, P.F., VAFA, G., & FRANSHAM, R.L. (1986) Baseline
measurements of butyltin in U.S. harbors and estuaries. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1283-1288.
GUARD, H.E., COGBET, A.B., & COLEMAN, W.M. (1981) Methylation of
trimethyltin compounds by estuarine sediments. Science, 213: 770-771.
HADA, N. (1986) [Studies on the kinetics of tributyltin compounds in the
body: With special reference to their biological half times in goldfish and
rats as estimated by newly developed analytical method.] Nichidai Igaku
Zasshi, 45: 1005-1013 (in Japanese).
HALL, L.W., PINKNEY, A.E., ZEGER, S., BURTON, D.T., & LENKEVICH, M.J.
(1984) Behavioral responses to two estuarine fish species subjected to bis
(tri- n-butyltin) oxide. Water Resour. Bull., 20: 235-239.
HALL, L.W., LENKEVICH, M.J., HALL, W.S., PINKNEY, A.E., & BUSHONG, S.J.
(1986) Monitoring organotin concentrations in Maryland waters of Chesapeake
Bay. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1275-1279.
HALL, L.W., BUSHONG, S.J., JOHNSON, W.E., & HALL, W.S. (1988a) Spacial and
temporal distribution of butyltin compounds in a Northern Chesapeake Bay
marina and river system. Environ. Monit. Assess., 10: 229-244.
HALL, L.W., BUSHONG, S.J., HALL, W.S., & JOHNSON, W.E. (1988b) Acute and
chronic effects of tributyltin on a Chesapeake Bay copepod. Environ.
Toxicol. Chem., 7: 41-46.
HALLAS, L.E., MEANS, J.C., & COONEY, J.J. (1982) Methylation of tin by
estuarine microorganisms. Science, 215: 1505-1507.
HARRIS, J.R.W. & CLEARY, J.J. (1987) Particle-water partitioning and
organotin dispersal in an estuary. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1370-1374.
HENDERSON, R.S. (1986) Effects of organotin antifouling paint leachates on
Pearl Harbor organisms: a site specific flowthrough bioassay. In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1226-1233.
HENSHAW, B.G., LAIDLAW, R.A., ORSLER, R.J., CAREY, J.K., & SAVORY, J.G.
(1978) The permanence of tributyltin oxide in timber. In: Record of the
1978 Annual Convention of the British Wood Preserving Association,
Cambridge, 27-30 June, 1978, London, British Wood Preserving Association,
pp. 19-29.
HINGA, K.R., ADELMAN, D., & PILSON, M.E.Q. (1987) Radiolabeled butyl tin
studies in the Merl enclosed ecosystems. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1416-1420.
HIS, E. & ROBERT, R. (1980) Action d'un sel organo-métallique, l'acétate de
tributylétain sur les oeufs et les larves de Crassostrea gigas (Thunberg),
Copenhagen, International Council for the Exploration of the Sea (ICES)
Mariculture Commission, 27 pp (CM 1980/F).
HIS, E. & ROBERT, R. (1985) Développement des véligères de Crassostrea
gigas dans le Bassin d'Arcachon, études sur les mortalités larvaires. Rev.
Trav. Inst. Pêches Marit., 47: 63-88.
HIS, E., MAURER, D., & ROBERT, R. (1986) Observations complémentaires sur
les causes possibles des anomalies de la reproduction de Crassostrea gigas
(Thunberg) dans le Basin d'Arcachon. Rev. Trav. Inst. Pêches Marit., 48:
45-54.
HODGE, V.F., SEIDEL, S.L., & GOLDBERG, E.D. (1979) Determination of tin
(IV) and organotin compounds in natural waters, coastal sediments, and
macro algae by atomic absorption spectrometry. Anal. Chem., 51: 1256-1259.
HOPF, H.S., DUNCAN, J., BEESLEY, J.S.S., WEBLEY, D.J., & STURROCK, R.F.
(1967) Molluscicidal properties of organotin and organolead compounds.
World Health Organ. Bull., 36: 955-961.
HUGGETT, R.J., UNGER, M.A., & WESTBROOK, D.J. (1986) Organotin
concentrations in the southern Chesapeake Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1262-1265.
HUMPEL, M., KUHNE, G., TAUBER, U., & SCHULZE, P.E. (1986) Studies on the
kinetics of bis (tris- n -butyl-113tin) oxide (TBTO). In: Toxicology and
analytics of the tributyltins: The present status. Proceedings of an ORTEPA
workshop, Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands,
ORTEP-Association, pp. 122-142.
HUMPHREY, B. & HOPE, D. (1987) Analysis of water, sediments and biota for
organotin compounds. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1348-1351.
ICES (1987) Concentrations of organotin and total tin in open Danish sea
areas and in pleasure craft marinas along the sound, International Council
for the Exploration of the Sea (ICES) (TWG 14/9/3-E).
IWAI, H., MANABE, M., MATSUI, H., ONO, T., & WADA, O. (1980) Effects of
tributyltin and its metabolites on brain function. J. toxicol. Sci., 5:
257.
IWASAKI, I., FUNABASHI, N., TOIZUMI, S., & IDE, G. (1976) Histopathological
studies on rat's nervous system by oral administration of bis-(tri-n-
butyltin) oxide. (I). J. toxicol. Sci., 1: 99 (abstract).
JENSEN, A. & CHENG, Z. (1987) Total tin and organotin in seawater from
pleasure craft marinas along Danish coast of the Sound. In: Proceedings of
the 15th Conference of Baltic Oceanographers (CBO), Copenhagen, 1986,
Charlottenlung, Denmark, Marine Pollution Laboratory, Vol. 1, pp. 289-298.
JEWETT, K.L. & BRINCKMAN, F.E. (1981) Speciation of trace di- and
triorganotins in water by ion exchange HPLC-GFAA. J. chromatogr. Sci., 19:
583.
JOHANSEN, K. & MOHLENBERG, F. (1987) Impairment of egg production in
Acartia tonsa exposed to tributyltin oxide. Ophelia, 27: 137-141.
JOHNSON, T.L. & KNOWLES, C.O. (1983) Effects of organotins on rat
platelets. Toxicology, 29: 39-48.
JORDAN, P. (1985) Schistosomiasis - The St Lucia Project, Cambridge,
Cambridge University Press, 442 pp.
KAKUNO, A. & KIMURA, S. (1987) [Acute toxicity of bis (tributyltin) oxide
to girella (Girella punctata).] Bull. Tokai Reg. Fish. Res. Lab., 123: 41-
44 (in Japanese).
KALBFUS, W. (1988) TBT-burden from antifouling paints in different waters.
Presented at the OECD Workshop on Monitoring, Chemical Analysis and
Leaching Rates of TBT, Paris, 30 November-2 December, 1988, 11 pp
(Unpublished report).
KALNINS, M.A. & DETROY, B.F. (1984) Effect of wood preservative treatment
of beehives on honey bees and hive products. J. agric. food Chem., 32:
1176-1180.
KEY, D., NUNNY, R.S., DAVIDSON, P.E., & LEONARD, M.A. (1976) Abnormal shell
growth in the Pacific oyster Crassostrea gigas. Some preliminary results
from experiments undertaken in 1975, Copenhagen, International Council for
the Exploration of the Sea (ICES), 12 pp. (C. M. 1976/K/11).
KIMMEL, E.C., FISH, R.H., & CASIDA, J.E. (1977) Bioorganotin chemistry:
Metabolism of organotin compounds in microsomal monooxygenase system and in
mammals. J. agric. food Chem., 25: 1-8.
KLIMMER, O.R. (1969) [The use of organic tin compounds from the viewpoint
of experimental toxicology.] Arzneimittelforschung, 19: 934-939 (in
German).
KNOWLES, C.O. & JOHNSON, T.L. (1986) Influence of organotins on rat
platelet aggregation mechanism. Environ. Res., 39: 172-179.
KRAJNC, E.I. (1989) Presentation to OECD working group on TBT immune
effects. In Preparation.
KRAJNC, E.I., WESTER, P.W., LOEBER, J.G., VAN LEEUWEN, F.X.R., VOS, J.G.,
VAESSEN, H.A.M.G., & VAN DER HEIJDEN, C.A. (1984) Toxicity of bis(tri- n-
butyltin)oxide in the rat. 1. Short-term effects on general parameters and
on the endocrine and lymphoid systems. Toxicol. appl. Pharmacol., 75: 363-
386.
KRAMPITZ, G., ENGELS, J., & CAZAUX, C. (1976) Biochemical studies on water-
soluble proteins and related components of gastropods shells. In: Watabe,
N. & Wilbur, K.M., ed. The mechanisms of mineralization in the
invertebrates and plants, Columbia, University of South Carolina Press, pp.
155-173.
KRAMPITZ, G., DROLSHAGEN, H., & DELTREIL, J.P. (1983) Soluble matrix
components in malformed oyster shells. Experientia (Basel), 39: 1105-1106.
KROWKE, R., BLUTH, U., & NEUBERT, D. (1986) in vitro studies on the
embryotoxic potential of (bis[tri- n-butyltin])oxide in a limb bud culture
system. Arch. Toxicol., 58: 125-129.
LANGSTON, W.J., BURT, G.R., & ZHOU, M. (1987) Tin and organotin in water,
sediments, and benthic organisms of Poole Harbour. Mar. Pollut. Bull., 18:
634-639.
LAUGHLIN, R.B. & FRENCH, W.J. (1980) Comparative study of the acute
toxicity of a homologous series of trialkyltins to larval shore crabs,
Hemigrapsus nudus, and lobster, Homarus americanus. Bull. environ. Contam.
Toxicol., 25: 802-809.
LAUGHLIN, R. & LINDEN, O. (1982) Sublethal responses of the tadpoles of the
European frog Rana temporaria to two tributyltin compounds. Bull. environ.
Contam. Toxicol., 28: 494-499.
LAUGHLIN, R., FRENCH, W., & GUARD, H.E. (1983) Acute and sublethal toxicity
of tributyltin oxide (TBTO) and its putative environmental product,
tributyltin sulfide (TBTS) to zoeal mud crabs, Rhithropanopeus harrisii.
Water Air Soil Pollut., 20: 69-79.
LAUGHLIN, R., NORDLUND, K., & LINDEN, O. (1984) Long-term effects of
tributyltin compounds on the baltic amphipod, Gammarus oceanicus. Mar.
environ. Res., 12: 243-271.
LAUGHLIN, R.B., JOHANNESEN, R.B., FRENCH, W., GUARD, H., & BRINKMAN, F.E.
(1985) Structure-activity relationships for organotin compounds. Environ.
Toxicol. Chem., 4: 343-351.
LAUGHLIN, R.B., GUARD, H.E., & COLEMAN, W.M. (1986a) Tributyltin in
seawater: Speciation and octanol-water partition coefficient. Environ. Sci.
Technol., 20: 201-204.
LAUGHLIN, R.B., FRENCH, W., & GUARD, H.E. (1986b) Accumulation of bis
(tributyltin) oxide by the marine mussel Mytilus edulis. Environ. Sci.
Technol., 20: 884-890.
LAUGHLIN, R.B., PENDOLEY, P., & GUSTAFSON, R.G. (1987) Sublethal effects of
tributyltin on the hard shell clam, Mercenaria mercenaria. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1494-1498.
LAUGHLIN, R.B., GUSTAFSON, R., & PENDOLEY, P. (1988) Chronic embryo-larval
toxicity of tributyltin (TBT) to the hard shell clam Mercenaria mercenaria.
Mar. Ecol. Prog. Ser., 48: 29-36.
LAWLER, I.F. & ALDRICH, J.C. (1987) Sublethal effects of bis(tri- n-
butyltin)oxide on Crassostrea gigas spat. Mar. Pollut. Bull., 18: 274-278.
LEE, R.F. (1985) Metabolism of tributyltin oxide by crabs, oysters and
fish. Mar. environ. Res., 17: 145-148.
LEE, R.F. (1986) Metabolism of bis (tributyltin) oxide by estuarine
animals. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1182-1188.
LEE, R.F., VALKIRS, A.O., & SELIGMAN, P. (1987) Fate of tributyltin in
estuarine waters. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1411-1415.
LEWIS, P.G. & EMMETT, E.A. (1987) Irritant dermatitis from tributyl tin
oxide and contact allergy from chlorocresol. Contact dermatitis, 17: 129-
132.
LINDEN, O. (1987) The scope of the organotin issue in Scandinavia. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1320-1323.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LYLE, W.H. (1958) Lesions of the skin in process workers caused by contact
with butyl tin compounds. Br. J. ind. Med., 15: 193-196.
MCCULLOUGH, F.S., GAYRAL, P., DUNCAN, J., & CHRISTIE, J.D. (1980)
Molluscicides in schistosomiasis control. World Health Organ. Bull., 58:
681-689.
MACKLAD, F., TAMAN, F., & EL SEBAE, A.K. (1983) Toxicity of tributyltin
fluoride and copper sulfate in slow release formulation to Biomphalaria
alexandrina snails. Bull. High Inst. Public Health, 14: 1-12.
MAFF/HSE (1988) Pesticides 1988: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 399 pp (Reference
Book HMSO 500).
MAFF/HSE (1989) Pesticides 1989: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 407 pp (Reference
Book HMSO 500).
MAGUIRE, R.J. (1984) Butyltin compounds and inorganic tin in sediments in
Ontario. Environ. Sci. Technol., 18: 291-294.
MAGUIRE, R.J. (1987) Review: Environmental aspects of tributyltin. Appl.
organomet. Chem., 1: 475-498.
MAGUIRE, R.J. & HUNEAULT, H. (1981) Determination of butyltin species in
water by gas chromatography with flame photometric detection. J.
Chromatogr., 209: 458-462.
MAGUIRE, R.J. & TKACZ, R.J. (1983) Analysis of butyltin compounds by gas
chromatography. Comparison of flame photometric and atomic absorption
spectrophotometric detectors. J. Chromatogr., 268: 99-101.
MAGUIRE, R.J. & TKACZ, R.J. (1985) Degradation of the tri-n-butyltin
species in water and sediment from Toronto Harbor. J. agric. food Chem.,
33: 947-953.
MAGUIRE, R.J. & TKACZ, R.J. (1987) Concentration of tributyltin in the
microlayer of natural waters. Water Pollut. Res. J. Can., 22: 227-233.
MAGUIRE, R.J., CHAU, Y.K., BENGERT, G.A., HALE, E.J., WONG, P.T.S., &
KRAMAR, O. (1982) Occurrence of organotin compounds in Ontario lakes and
rivers. Environ. Sci. Technol., 16: 698-702.
MAGUIRE, R.J., CAREY, J.H., & HALE, E.J. (1983) Degradation of the tri-n-
butyltin species in water. J. agric. food Chem., 31: 1060-1065.
MAGUIRE, R.J., WONG, P.T.S., & RHAMEY, J.S. (1984) Accumulation and
metabolism of tri-n-butyltin cation by a green alga, Ankistrodesmus
falcatus. Can. J. Fish. aquat. Sci., 41: 537-540.
MAGUIRE, R.J., TKACZ, R.J., & SARTOR, D.L. (1985) Butyltin species and
inorganic tin in water and sediment of the Detroit and St. Clair rivers. J.
Great Lakes Res., 11: 320-327.
MAGUIRE, R.J., TKACZ, R.J., CHAU, Y.K., BENGERT, G.A., & WONG, P.T.S.
(1986) Occurrence of organotin compounds in water and sediment in Canada.
Chemosphere, 15: 253-274.
MATSUI, H., WADA, O., MANABE, S., ONO, T., IWAI, H., & FUJIKURA, T. (1982)
[Properties and mechanism of hyperlipidemia induced in rabbits by
tributyltin fluoride.] Jpn. J. ind. Health, 24: 163-171 (in Japanese).
MATTHIAS, C.L., BELLAMA, J.M., & BRINCKMAN, F.E. (1986a) Determination of
ultra-trace concentrations of butyltin compounds in water by simultaneous
hydridization/extraction with GC/FPD detection. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1146-1151.
MATTHIAS, C.L., OLSON, G.J., BELLAMA, J.M., & BRINCKMAN, F.E. (1986b)
Comprehensive method for determination of aquatic butyltin and
butylmethyltin species at ultratrace levels using simultaneous
hydridization/extraction with GC/FPD detection. Environ. Sci. Technol., 20:
609-615.
MATTHIESSEN, P. (1974) Some effects of slow-release bis (tri-n-butyl tin)
oxide on the tropical fish, Tilapia mossambica Peters. Controlled Release
Pesticide Symposium, Akron, Ohio, University of Akron, Community Technical
College, Engineering and Science Division, pp. 25.1-25.16.
MATTHIESSEN, P. & THAIN, J.E. (in press) A method for studying the impact
of polluted marine sediments on colonising organisms; tests with diesel-
based drilling mud and tributyltin antifouling paint. Hydrobiologia.
MAURER, D., HERAL, M., HIS, E., & RAZET, D. (1985) Influence d'une peinture
antisalissure à base de sels organométalliques de l'étain sur le captage en
milieu naturel de l'huitre Crassostrea gigas. Rev. Trav. Inst. Pêches
Marit., 47: 241-250.
MEADOR, J.P. (1986) An analysis of photobehavior of Daphnia magna exposed
to tributyltin. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1213-
1218.
MEINEMA, H.A., BURGER-WIERSMA, T., VERSLUIS DE HAAN, G., & GEVERS, E.C.
(1978) Determination of trace amounts of butyltin compounds in aqueous
systems by gas chromatography/mass spectrometry. Environ. Sci. Technol.,
12: 288-293.
MICHEL, P. (1987) Automatization of a hydride generation/A.A.S. system - an
improvement for organotin analysis. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1340-1343.
MIDDLETON, M.C. & PRATT, I. (1977) Skin water content as a quantitative
index of the vascular and histologic changes produced in rat skin by di- n-
butyltin and tri- n-butyltin. J. invest. Dermatol., 68: 379-384.
MIDDLETON, M.C. & PRATT, I. (1978) Changes in incorporation of
[3H]thymidine into DNA of rat skin following cutaneous application of
dibutyltin, tributyltin and 1-chloro-2:4-dinitrobenzene and the
relationship of these changes to a morphological assessment of the cellular
damage. J. invest. Dermatol., 71: 305-310.
MINCHIN, D., DUGGAN, C.B., & KING, W. (1987) Possible effects of organotins
on scallop recruitment. Mar. Pollut. Bull., 18: 604-608.
MOLIN, L. & WAHLBERG, J.E. (1975) Toxic skin reactions caused by
tributyltin oxide (TBTO) in socks. Berufs-dermatosen, 4: 138-142.
MORI, Y., IESATO, K., UEDA, S., MORI, T., IWASAKI, I., OHNISHI, K., SEINO,
Y., WAKASHIM, Y., WAKASHIN, M., & OKUDA, K. (1984) Renal tubular
disturbances induced by tributyl-tin oxide in guinea pigs: a secondary
Fanconi syndrome. Clin. Nephrol., 21: 118-125.
MULLER, H.A. (1987) Determination of tributyltin oxide in air. In:
Toxicology and analytics of the tributyltins: The present status.
Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986, Vlissingen-
Oost, The Netherlands, ORTEP Association, pp. 162-172.
MULLER, M.D. (1984) Tributyltin detection at trace levels in water and
sediments using GC with flame-photometric detection and GC-MS. Fresenius Z.
anal. Chem., 317: 32-36.
MULLER, M.D. (1987b) Comprehensive trace level determination of organotin
compounds in environmental samples using high-resolution gas chromatography
with flame photometric detection. Anal. Chem., 59: 617-623.
MUSHAK, P., KRIGMAN, M.R., & MAILMAN, R.B. (1982) Comparative organotin
toxicity in the developing rat: somatic and morphological changes and
relationship to accumulation of total tin. Neurobehav. Toxicol. Teratol.,
4: 209-215.
NEMEC, M.D. (1987) A teratology study in rabbits with TBTO: Final Report,
Wil Research Laboratories Inc., 210 pp (Report No. WIL-B0002) (Confidential
report to the Tributyl Tin Oxide Consortium).
NEWTON, F., THUM, A., DAVIDSON, B., VALKIRS, A., & SELIGMAN, P. (1985)
Effects on the growth and survival of eggs and embryos of the California
grunion (Leuresthes tenuis) exposed to trace levels of tributyltin, San
Diego, California, Naval Ocean Systems Center, 15 pp (Technical Report No.
1040).
NIVA (1986) [Organic tin compounds in fjord areas], Oslo, Norwegian
Institute for Water Research (NIVA) (in Norwegian).
O'CALLAGHAN, J.P. & MILLER, D.B. (1988) Acute exposure of the neonatal rat
to tributyltin results in decreases in biochemical indicators of
synaptogenesis and myelinogenesis. Pharmacol. exp. Ther., 246: 394-402.
OKOSHI, K., MORI, K., & NONURA, T. (1987) Characteristics of shell chamber
formation between the two races in Japanese oyster, Crassostrea gigas.
Aquaculture, 67: 313-320.
OLSON, G.J. & BRINCKMAN, F.E. (1986) Biodegradation of tributyltin by
Chesapeake Bay microorganisms. In: Proceedings of the Organotin Symposium,
Oceans '86 Conference, Washington, DC, USA, 23-25 September, 1986, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1196-1201.
OSBORN, D. & LEACH, D.V. (1987) Organotin in birds: Pilot study,
Huntingdon, Institute of Terrestrial Ecology, 15 pp (Final report to the UK
Department of the Environment. Contract No. F3CR/27/D4/01).
PAGE, D.S. (1989) An analytical method for butyltin species in shellfish.
Mar. Pollut. Bull., 20: 129-133.
PAUL, J.D. & DAVIES, I.M. (1986) Effects of copper- and tin-based anti-
fouling compounds on the growth of scallops (Pecten maximus) and oysters
(Crassostrea gigas). Aquaculture, 54: 191-203.
PAULINI, E. (1964) Laboratory experiments with some organotin compounds,
Geneva, World Health Organization, Parasitic Diseases Programme, pp. 1-3
(Unpublished report Mol/Inf/16).
PAULINI, E. & DE SOUZA, C.P. (1970) Influence of different suspended solids
in the water upon molluscicide activity, Geneva, World Health Organization,
Parasitic Diseases Programme, pp. 1-10 (Unpublished report PD/Mol/70.12).
PELIKAN, Z. (1969) Effects of bis(tri- n-butyltin) oxide on the eyes of
rabbits. Br. J. ind. Med., 26: 165-170.
PELIKAN, Z. & CERNY, E. (1968) [The toxic effects of tri- n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292 (in German).
PELIKAN, Z. & CERNY, E. (1969) Toxic effects of bis (tributyltin) oxide
(TBTO) on the skin of rats. Berufs-dermatosen, 17: 305-316.
PICKWELL, G.V. & STEINERT, S.A. (1988) Accumulation and effects of
organotin compounds in oysters and mussels: correlation with serum
biochemical and cytological factors and tissue burdens. Mar. environ. Res.,
24: 215-218.
PIETERS, R.H., KAMPINGA, J., BOL-SCHOENMAKERS, M., LAMB, B.W., PENNINKS,
A.H., & SEINEN, W. (1989) Organotin-induced thymus atrophy concerns the OX-
44+ immature thymocytes: Relation to the interaction between early
thymocytes and thymic epithelial cells? Thymus, 14(1-3): 79-88.
PINKNEY, A.E., HALL, L.W., LENKEVICH, M.J., BURTON, D.T., & ZEGER, S.
(1985) Comparison of avoidance responses of an estuarine fish, Fundulus
heteroclitus, and crustacean, Palaemonetes pugio, to bis (tri-n-butyltin)
oxide. Water Air Soil Pollut., 25: 33-40.
PINKNEY, A.E., MATTESON, L.L., & WRIGHT, D.A. (1988) Effects of tri-
butyltin on survival, growth, morphometry and RNA-DNA ratio of larval
striped bass, Morone saxatilis. In: Proceedings of the Organotin Symposium,
Oceans '88 Conference, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 987-991.
PLUM, H. (1981) Comportement des composés organostanniques vis-à-vis de
l'environnement. Inf. chim., 220: 135-139.
POITOU, P., MARIGNAC, B., CERTIN, C., & GRADISKI, D. (1978) Etude de
l'effet sur le système nerveux central et du pouvoir sensibilisant de
l'oxyde de tributylétain. Ann. Pharm. fr., 36: 569-572.
POLSTER, M. & HALACKA, K. (1971) [9. Contributions to the health-toxic
problems of some anti-microbially used organo-tin compounds.]
Ernährungsforschung, 16: 527-535 (in German).
RACEY, P.A. & SWIFT, S.M. (1986) The residual effects of remedial timber
treatments on bats. Biol. Conserv., 35: 205-214.
RANDALL, L., DONARD, O.F.X., & WEBER, J.H. (1986) Speciation of n-butyltin
compounds by atomic absorption spectrophotometry using electro-thermal
quartz furnace after hydride generation. Anal. Chim. Acta., 184: 197-203.
REIMANN, R. & LANG, R. (1987) Mutagenicity studies with tributyltin
compounds. In: Toxicology and analytics of the tributyltins: The present
status. Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986,
Vlissingen-Oost, The Netherlands, ORTEP Association, pp. 66-90.
REINHARDT, C.A., SCHAWALDER, H., & ZBINDEN, G. (1982) Cell detachment and
cloning efficiency as parameters for cytotoxicity. Toxicology, 25: 47-52.
RICE, C.D., ESPOURTEILLE, F.A., & HUGGETT, R.J. (1987) Analysis of
tributyltin in estuarine sediments and oyster tissue, Crassostrea
virginica. Appl. organomet. Chem., 1: 541-544.
RITCHIE, L.S., BERRIOS-DURAN, L.A., FRICK, L.P., & FOX, I. (1964)
Molluscicidal time-concentration relationships of organo-tin compounds.
World Health Organ. Bull., 31: 147-149.
RITCHIE, L.S., LOPEZ, V.A., & CORA, J.M. (1974) Prolonged applications of
an organotin against Biomphalaria glabrata and Schistosoma mansoni. In:
Molluscicides in schistosomiasis control, New York, London, Academic Press,
pp. 77-88.
RIVM (1989) In: Mathijssen-Spiekman, E.A.M., Canton, J.H., & Roghair, C.J.,
ed. [Investigation into the toxicity of TBTO for a number of freshwater
organisms], Bilthoven, National Institute of Public Health and
Environmental Hygiene, 48 pp (Report No. 668118.001) (in Dutch).
ROBERTS, M.H. (1987) Acute toxicity of tributyltin chloride to embryos and
larvae of two bivalve mollusks, Crassostrea virginica and Mercenaria
mercenaria. Bull. environ. Contam. Toxicol., 39: 1012-1019.
ROBERTS, M.H., BENDER, M.E., DE LISLE, P.F., SUTTON, H.C., & WILLIAMS, R.L.
(1987) Sex ratio and gamete production in American oysters exposed to
tributyltin in the laboratory. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1471-1476.
ROBINSON, I.N. (1969) Effects of some organotin compounds on tissue amine
levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROSENBERG, D.W. & DRUMMOND, G.S. (1983) Direct in vitro effects of bis
(tributyl) tin oxide on hepatic cytochrome P-450. Biochem. Pharmacol., 32:
3823-3829.
ROSENBERG, D.W., DRUMMOND, G.S., CORNISH, H.C., & KAPPAS, A. (1980)
Prolonged induction of hepatic haem oxygenase and decreases in cytochrome
P-450 content by organotin compounds. Biochem. J., 190: 465-468.
ROSENBERG, D.W., DRUMMOND, G.S., & KAPPAS, A. (1981) The influence of
organometals on heme metabolism. in vivo and in vitro studies with
organotins. Mol. Pharmacol., 21: 150-158.
ROSENBERG, D.W., ANDERSON, K.E., & KAPPAS, A. (1984) The potent induction
of intestinal heme oxygenase by the organotin compound, bis(tri- n-butyltin)
oxide. Biochem. biophys. Res. Commun., 119: 1022-1027.
SALAZAR, S.M. (1985) The effects of bis(tri-n-butyltin) oxide on three
species of marine phytoplankton, San Diego, California, Naval Ocean Systems
Center, 16 pp (Technical Report No. 1039).
SALAZAR, M.H. & SALAZAR, S.M. (1985) Ecological evaluation of
organotincontaminated sediment, San Diego, California, Naval Ocean Systems
Center, 21 pp (Technical Report No. 1050).
SALAZAR, M.H. & SALAZAR, S.M. (1987) Tributyltin effects on juvenile mussel
growth. In: Proceedings of the Organotin Symposium, Oceans '87 Conference,
Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1504-
1510.
SALAZAR, S.M., DAVIDSON, B.M., SALAZAR, M.H., STANG, P.M., & MEYERS-
SCHULTE, K.J. (1987) Effects of TBT on marine organisms: Field assessment
of a new site-specific bioassay system. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1461-1470.
SAXENA, P.N. & CROWE, A.J. (1988) An investigation of the efficacy of
organotin compounds for the control of the cotton stainer, Dysdercus
cingulatus, the mosquito, Anopheles stephensi, and the common house fly,
Musca domestica. Appl. organomet. Chem., 2: 185-187.
SCHAFER, E.W. & BOWLES, W.A. (1985) Acute oral toxicity and repellency of
933 chemicals to house and deer mice. Arch. environ. Contam. Toxicol., 14:
111-129.
SCHERING (1983) Repeated dose inhalation study of ZK21.995 in the rat for
29-32 days (21-24 exposures), Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. IC 1/83. Study No. TX81.177).
SCHERING (1986) Re-evaluation of a "mouse micronucleus test on TBTO",
Bergkamen, Federal Republic of Germany, Schering Inc. (Confidential report
No. IC 7186 prepared by the Institute of Occupational Health, Helsinki,
Finland, September 1984).
SCHERING (1989a) TBTO - 4 week oral (dietary administration) toxicity study
in the rat, Bergkamen, Federal Republic of Germany, Schering Inc. (Report
No. 280118 by Hazelton, France. Study No. 14/502).
SCHERING (1989b) TBTO - Plaque forming assay following a 5 week oral
toxicity study in the rat, Bergkamen, Federal Republic of Germany, Schering
Inc. (Report No. 283118 by Hazelton, France. Study No. 14/503).
SCHERING (1989c) TBTO - Resistance to Listeria monocytogene infection
following a 34 day oral toxicity study in the rat, Bergkamen, Federal
Republic of Germany, Schering Inc. (Report No. 282118 by Hazelton, France.
Study No. 14/505).
SCHERING (1989d) TBTO - Delayed type hypersensitivity test following a 37
day oral toxicity study in the rat, Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. 281118 by Hazelton, France).
SCHERING (1989e) TBTO - Systemic toxicity study in beagle dogs with daily
oral (intragastric) administration over a total of 18-19 weeks, Bergkamen,
Federal Republic of Germany, Schering Inc. (Report No. IC6/88).
SCHWEINFURTH, H. (1985) Toxicology of tributyltin compounds. Tin Uses, 143:
9-12.
SEIFFER, E.A. & SCHOOF, H.F. (1967) Tests of 15 experimental molluscicides
against Australorbis glabratus. Public Health Rep., 82: 833-839.
SEINEN, W., HELDER, T., VERNIJ, H., PENNINKS, A., & LEEUWANGH, P. (1981)
Short term toxicity of tri- n-butyltinchloride in rainbow trout (Salmo
gairdneri Richardson) yolk sac fry. Sci. total Environ., 19: 155-166.
SELIGMAN, P.F., VALKIRS, A.O., & LEE, R.F. (1986a) Degradation of
tributyltin in marine and estuarine waters. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1189-1195.
SELIGMAN, P.F., GROVHOUG, J.G. & RICHTER, K.E. (1986b) Measurement of
butyltins in San Diego Bay, CA: A monitoring strategy. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1289-1296.
SELIGMAN, P.F., GROVHOUG, J.G., VALKIRS, A.O., STANG, P.M., FRANSHAM, R.,
STALLARD, M.O., DAVIDSON, B., & LEE, R.F. (1989) Distribution and fate of
tributyltin in the United States marine environment. Appl. organomet.
Chem., (in press).
SHELDON, A.W. (1975) Effects of organotin anti-fouling coatings on man and
his environment. J. Paint Technol., 47: 54-58.
SHERMAN, L.R. & CARLSON, T.L. (1980) A modified phenylfluorone method for
determining organotin compounds in the ppb and sub-ppb range. J. anal.
Toxicol., 4: 31-33.
SHIFF, C.J., YIANNAKIS, C., & EVANS, A.C. (1975) Further trials with TBTO
and other slow release molluscicides in Rhodesia. In: Proceedings of the
Controlled Release Pesticide Symposium, Wright State University, Dayton,
Ohio, 8-10 September, 1975, pp. 177-188.
SHIMIZU, A. & KIMURA, S. (1987) [Effect of bis (tributyltin) oxide on
gonadal development of a salt-water goby, Chasmichthys dolichognathus:
Exposure during maturing period.] Bull. Tokai Reg. Fish. Res. Lab., 123:
45-49 (in Japanese).
SHORT, J.W. & THROWER, F.P. (1986) Accumulation of butyltins in muscle
tissue of chinook salmon reared in sea pens treated with tri- n-butyltin.
In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1177-1181.
SHORT, J.W. & THROWER, F.P. (1987) Toxicity of tri- n-butyl-tin to chinook
salmon, Oncorhynchus tshawytscha, adapted to seawater. Aquaculture, 61:
193-200.
SLESINGER, A.E. & DRESSER, I. (1978) The environmental chemistry of three
organotin chemicals. In: Good, M., ed. Report of the Organotin Workshop,
New Orleans, Louisiana, 17-19 February, 1978, pp. 115-162.
SMITH, B.S. (1981a) Male characteristics on female mud snails caused by
antifouling paints. J. appl. Toxicol., 1: 22-25.
SMITH, B.S. (1981b) Tributyltin compounds induce male characteristics on
female mud snails Nassarius obsoletus = Ilyanassa obsoleta. J. appl.
Toxicol., 1: 141-144.
SMITH, B.S. (1981c) Male characteristics in female Nassarius obsoletus:
Variations related to locality, season and year. Veliger, 23: 212-216.
SMITH, D.R., STEPHENSON, M.D., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1987) The use of transplanted juvenile oysters to monitor the toxic
effects of tributyltin in California waters. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1511-1516.
SMITH, P.J., CROWE, A.J., ALLEN, D.W., BROOKS, J.S., & FORMSTONE, R. (1977)
Study by 119m Sn Mossbauer spectroscopy of bis(tri- n-butyltin) oxide
adsorbed on cellulosic materials. Chem. Ind., 23: 874-875.
SNOEIJ, N.J. (1987) Triorganotin compounds in immunotoxicology and bio-
chemistry, Utrecht, The Netherlands, University of Utrecht, 170 pp (Ph.D.
Thesis).
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1985)
Toxicity of triorganotin compounds: Comparative in vivo studies with a
series of trialkyltin compounds and triphenyltin chloride in male rats.
Toxicol. appl. Pharmacol., 81: 274-286.
SNOEIJ, N.J., PUNT, P.M., PENNINKS, A.H., & SEINEN, W. (1986a) Effects of
tri- n-butyltin chloride on energy metabolism, macromolecular synthesis,
precursor uptake and cyclic AMP production in isolated rat thymocytes.
Biochim. Biophys. Acta, 852: 234-243.
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1986b)
Triorganotin-induced cytotoxicity to rat thymus, bone marrow and red blood
cells as determined by several in vitro assays. Toxicology, 39: 71-83.
SNOEIJ, N.J., PIETERS, R.H.H., PENNINKS, A.H., & SEINEN, W. (1987) Toxicity
of triorganotin compounds: Orally administered tri- n-butyltin compounds are
rapidly dealkylated in the rat. In: Triorganotincompounds in immunology and
biochemistry, Utrecht, The Netherlands, University of Utrecht, Chapter 4,
pp. 73-93 (Snoeij, N.J., Ph.D. Thesis).
SNOEIJ, N.J., BOL-SCHOENMAKERS, M., PENNINKS, A.H., & SEINEN, W. (1988a)
Differential effects of tri- n-butyltin chloride on macromolecular synthesis
and ATP levels of rat thymocyte subpopulations obtained by centrifugal
elutriation. Int. J. Immunopharmacol., 10: 29-37.
SNOEIJ, N.J., PENNINKS, A.H., & SEINEN, W. (1988b) Dibutyltin and
tributyltin compounds induce thymus atrophy in rats due to a selective
action on thymic lymphoblasts. Int. J. Immunopharmacol., 10: 891-899.
SORACCO, R.J. & POPE, D.H. (1983) Bacteriostatic and bactericidal modes of
action of bis (tributlytin) oxide on Legionella pneumophila. Appl. environ.
Microbiol., 45: 48-57.
STALLARD, M., HODGE, V., & GOLDBERG, E.D. (1987) TBT in California coastal
waters: Monitoring and assessment. Environ. Monit. Assess., 9: 195-220.
STANG, P.M. & SELIGMAN, P.F. (1986) Distribution and fate of butyltin
compounds in the sediment of San Diego Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1256-1261.
STANG, P.M. & SELIGMAN, P.F. (1987) In situ adsorption and desorption of
butyltin compounds from Pearl Harbor, Hawaii, sediment. In: Proceedings of
the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1386-1391.
STEIN, T. & KUSTER, K. (1982) Der biologische abbau von tributylzinnoxid
(TBTO) in einer belebtschlammanlage. Z. Wasser Abwasser Forsch., 15: 178-
180.
STEPHENSON, M.D., SMITH, D.R., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1986) Growth abnormalities in mussels and oysters from areas with high
levels of tributyltin in San Diego Bay. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1246-1251.
STEPHENSON, M.D., SMITH, D.R., HALL, L.W., JOHNSON, W.E., MICHEL, P.,
SHORT, J., WALDOCK, M., HUGGETT, R.J., SELIGMAN, P., & KOLA, S. (1987) An
international intercomparison of butyltin determinations in mussel tissue
and sediments. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1334-1338.
STROMGREN, T. & BONGARD, T. (1987) The effect of tributyltin oxide on
growth of Mytilus edulis. Mar. Pollut. Bull., 18: 30-31.
TEMMINK, J.H.M. & EVERTS, J.W. (1987) Comparative toxicity of tributyltin-
oxide (TBTO) for fish and snail. In: Proceedings of the Seventh World
Meeting of the ORTEP-Association, Amsterdam, 7-8 May, 1987, Vlissingen-
Oost, The Netherlands, ORTEP-Association, pp. 6-20.
THAIN, J.E. (1983) The acute toxicity of bis (tributyl tin) oxide to the
adults and larvae of some marine organisms, Copenhagen, International
Council for the Exploration of the Sea (ICES), 5 pp (Report No. C. M.
1983/E.13).
THAIN, J.E. (1986) Toxicity of TBT to bivalves: Effects on reproduction,
growth and survival. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1306-
1313.
THAIN, J.E. & WALDOCK, M.J. (1985) The growth of bivalve spat exposed to
organotin leachates from antifouling paints, Copenhagen, International
Council for the Exploration of the Sea (ICES), 10 pp (Report No. C. M.
1985/E.28).
THAIN, J.E. & WALDOCK, M.J. (1986) The impact of tributyl tin (TBT)
antifouling paints on molluscan fisheries. Water Sci. Technol., 18: 193-
202.
THAIN, J.E., WALDOCK, M.J., & WAITE, M.E. (1987) Toxicity and degradation
studies of tributyltin (TBT) and dibutyltin (DBT) in the aquatic
environment. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1398-1404.
THOMAS, T.E. & ROBINSON, M.G. (1986) The physiological effects of the
leachates from a self-polishing organotin antifouling paint on marine
diatoms. Mar. environ. Res., 18: 215-229.
THOMAS, T.E. & ROBINSON, M.G. (1987) Initial characterization of the
mechanisms responsible for the tolerance of Amphora coffeaeformis to copper
and tributyltin. Bot. mar., 30: 47-53.
THOMPSON, J.A.J., SHEFFER, M.G., PIERCE, R.C., CHAU, Y.K., COONEY, J.J.,
CULLEN, W.R., & MAGUIRE, R.J. (1985) Organotin compounds in the aquatic
environment: Scientific criteria for assessing their effects on
environmental quality, Ottawa, National Research Council Canada, 284 pp
(NRCC No. 22494).
TOLEDO, J.V., MONTEIRO DA SILVA, C.S., BULHOES, M.S., PAES LEME, L.A., DA
SILVA NETTO, J.A., & GILBERT, B. (1976) Snail control in urban sites in
Brazil with slow-release hexabutyldistannoxane and pentachlorophenol. World
Health Organ. Bull., 54: 421-425.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.-P., PHU LICH N., VAN DEN DRIESSCHE, J.,
GUESNIER, L.R., & MORIN, N. (1976) Contribution à l'étude toxicologique et
pharmacologique de l'oxyde de tributylétain (OTBE). Eur. J. Toxicol., 9:
31-40.
TRUHAUT, R., ANGER, J.P., REYMANN, J.M., CHAUVEL, Y., & VAN DEN DRIESSCHE,
J. (1979) Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicol. Eur. Res., 11: 181-186.
TRUHAUT, R., ANGER, J.P., ANGER, F., BRAULT, A., BRUNET, P., CANO, Y.,
LOUVET, M., SACCAVINI, J.C., VAN DEN DRIESSCHE, J., JANSON, C., & SUBLET,
Y. (1981) Dégradation thermique de l'oxyde de tributylétain (OTBE) et
toxicité pulmonaire des produits de combustion chez la souris et le cobaye.
Toxicol. Eur. Res., 111: 35-44.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1986)
Bioconcentration of butyltin compounds by round crucian carp. Toxicol.
environ. Chem., 12: 137-143.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1987)
Bioconcentration and metabolism of phenyl tin chlorides in carp. Water
Res., 21: 949-953.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1988)
Bioconcentration and metabolism of butyltin compounds in carp. Water Res.,
22: 647-651.
TWG (1988a) Uses and production of organotin compounds. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group), Brussels, 7-11 March, 1988, 6 pp (Report No. TWG 15/5/1-E).
TWG (1988b) Tributyl tin compounds in antifouling paints. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group, Brussels, 7-11 March, 1988, 2 pp, (Report No. TWG 15/5/2-E).
TWG (1988c) Organotins in the Netherlands. Presented by the Netherlands at
the Convention for the Prevention of Marine Pollution from Land-based
Sources (15th Meeting of the Technical Working Group, Brussels, 7-11 March,
1988, 11 pp (Report No. TWG 15/5/4-E).
UHL, S. (1986) [Population exposure to the environmental chemical
pentachloro-ophenol (PCP) and bis (tri-n- butyltin)oxide (TBTO)], Zurich,
Maus Offsetdruck Konstanz, 145 pp (ETH Thesis No. 8014, University of
Gottingen) (in German).
UNEP (1989) Mediterranean Action Plan. Assessment of organotin compounds as
marine pollutants in the Mediterranean, Athens, United Nations Environment
Programme (MAP Technical Report Series, No. 33).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1986) Organotin in
antifouling paints environmental considerations, London, UK Department of
the Environment, 82 pp (Pollution Paper No. 25).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1989) Water and the
environment, London, UK Department of the Environment, p. 26 (Circular No.
7/89).
UPATHAM, E.S., KOURA, M., AHMED, M.D., & AWAD, A.H. (1980) Laboratory
trials of controlled release molluscicides on Bulinus (Ph.) abyssinicus,
the intermediate host of Schistosoma haematobium in Somalia. In: Baker,
R.W., ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the controlled Release Society, New York, London,
Academic Press, pp. 461-469.
U'REN, S.C. (1983) Acute toxicity of bis(tributyltin) oxide to a marine
copepod. Mar. Pollut. Bull., 14: 303-306.
VALKIRS, A.O., SELIGMAN, P.F., STANG, P.M., HOMER, V., LIEBERMAN, S.H.,
VAFA, G., & DOOLEY, C.A. (1986) Measurement of butyltin compounds in San
Diego Bay. Mar. Pollut. Bull., 17: 319-324.
VALKIRS, A.O., DAVIDSON, B.M., & SELIGMAN, P.F. (1987) Sublethal growth
effects and mortality to marine bivalves from long-term exposure to
tributyltin. Chemosphere, 16: 201-220.
VIGHI, M. & CALAMARI, D. (1985) QSARs for organotin compounds on Daphnia
magna. Chemosphere, 14: 1925-1932.
VIYANANT, V., THIRACHANTRA, S., & SORNMANI, S. (1982) The effect of
controlled release copper sulphate and tributyltin fluoride on the
mortality and infectivity of Schistosoma mansoni cercariae. J. Helminthol.,
56: 85-92.
VOS, J.G., DE KLERC, A., KRAJNC, E.I., KRUIZINGA, W., VAN OMMEN, B., &
ROZING, J. (1984) Toxicity of bis(tri- n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of non-
specific resistance after short-term exposure. Toxicol. appl. Pharmacol.,
75: 387-408.
VOS, J.G., KRAJNC, E.I., & WESTER, P.W. (1985) Immunotoxicity of bis (tri-
n-butyltin) oxide. In: Dean, J., ed. Immunotoxicology and
immunopharmacology, New York, Raven Press, pp. 327-340.
WADE, T.L., GARCIA-ROMERO, B., & BROOKS, J.M. (1988) Tributyltin
contamination in bivalves from U.S. coastal estuaries. Environ. Sci.
Technol., 22: 1488-1493.
WALDOCK, M.J. (1989) Organotin concentrations in the Rivers Bure and Yare,
Norfolk Broads, England (Unpublished report of the Ministry of Agriculture,
Fisheries and Food, Fisheries Laboratory, Burnham-on-Crouch, Essex, UK).
WALDOCK, M.J. & MILLER, D. (1983) The determination of total and tributyl
tin in seawater and oysters in areas of high pleasure craft activity,
Copenhagen, International Council for the Exploration of the Sea (ICES), 18
pp (Report No. C. M. 1983/E.12).
WALDOCK, M.J. & THAIN, J.E. (1983) Shell thickening in Crassostrea gigas:
Organotin antifouling or sediment induced? Mar. Pollut. Bull., 14: 411-415.
WALDOCK, M.J. & THAIN, J.E. (1985) The comparative leach rates and toxicity
of two fish net antifouling preparations, Copenhagen, International Council
for the Exploration of the Sea (ICES), 5 pp (Report No. C. M. 1985/E.29).
WALDOCK, M.J., THAIN, J.E. & MILLER, D. (1983) The accumulation and
depuration of bis (tributyl tin) oxide in oysters: A comparison between the
Pacific oyster (Crassostrea gigas) and the European flat oyster (Ostrea
edulis), Copenhagen, International Council for the Exploration of the Sea
(ICES), 6 pp (Report No. C. M. 1983/E.52).
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1987a) Changes in concentrations
of organotins in U.K. rivers and estuaries following legislation in 1986.
In: Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1352-1356.
WALDOCK, M.J., THAIN, J.E., & WAITE, M.E. (1987b) The distribution and
potential toxic effects of TBT in UK estuaries during 1986. Appl.
organomet. Chem., 1: 287-301.
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1988) Inputs of TBT to the
marine environment from shipping activity in the U.K. Environ. Technol.
Lett., 9: 999-1010.
WALNE, P.R. & HELM, M.M. (1979) Introduction of Crassostrea gigas into the
United Kingdom. In: Mann, R., ed. Proceedings of a Symposium on Exotic
Species in Marinculture: Case histories of the Japanese Oyster Crassostrea
gigas (Thunberg), with implications for other fisheries, Woods Hole,
Massachusetts, USA, 18-20 September, 1978, Cambridge, London, MIT Press,
pp. 83-105.
WALSH, G.E. (1986) Organotin toxicity studies conducted with selected
marine organisms at EPA's environmental research laboratory, Gulf Breeze,
Florida. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1210-1212.
WALSH, G.E., MCLAUGHLAN, L.L., LORES, E.M., LOUIE, M.K., & DEANS, C.H.
(1985) Effects of organotins on growth and survival of two marine diatoms,
Skeletonema costatum and Thalassiosira pseudonana. Chemosphere, 14: 383-
392.
WALSH, G.E., LOUIE, M.K., MCLAUGHLIN, L.L., & LORES, E.M. (1986a) Lugworm
(Arenicola cristata) larvae in toxicity tests: Survival and development
when exposed to organotins. Environ. Toxicol. Chem., 5: 749-754.
WALSH, G.E., MCLAUGHLIN, L.L., LOUIE, M.K., DEANS, C.H., & LORES, E.M.
(1986b) Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin oxide and triphenyltin oxide.
Ecotoxicol. environ. Saf., 12: 95-100.
WALTON, R., ADEMA, C.M., & SELIGMAN, P.F. (1986) Mathematical modeling of
the transport and fate of organotin in harbors. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1297-1301.
WARD, G.S., CRAMM, G.C., PARRISH, P.R., TRACHMAN, H., & SLESINGER, A.
(1981) Bioaccumulation and chronic toxicity of bis(tributyltin) oxide
(TBTO): Tests with a saltwater fish. In: Branson, D.R. & Dickson, K.L., ed.
Proceedings of the Fourth Conference on Aquatic Toxicology and Hazard
Assessment, Philadelphia, American Society for Testing and Materials, pp.
183-200 (ASTM STP No. 737).
WEBBE, G. (1963) Laboratory tests of some new molluscicides (organotin
compounds), Geneva, World Health Organization, Parasitic Diseases
Programme, pp. 1-2 (Unpublished report Mol/Inf/14).
WEBER, J.H., DONARD, O.F.X., RANDALL, I., & HAN, J.S. (1986) Speciation of
methyl and butyltin compounds in the Great Bay estuary (N.H.). In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1280-1282.
WEIS, J.S., WEIS, P., & WANG, F. (1987a) Developmental effects of
tributyltin on the fiddler crab, Uca pugilator, and the killifish, Fundulus
heteroclitus. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1456-1460.
WEIS, J.S., GOTTLIEB, J., & KWIATKOWSKI, J. (1987b) Tributyltin retards
regeneration and produces deformities of limbs in the fiddler crab, Uca
pugilator. Arch. environ. Contam. Toxicol., 16: 321-326.
WESTER, P.W., CANTON, J.H., VAN IERSAL, A.A.J., KRANJC, E.I., & VAESSEN,
H.A.M.G. (1988) The toxicity of bis(tri-n- butyltin)oxide (TBTO) and di-n-
butyltindichloride (DBTC) in the small fish species Orysias latipes
(medaka) and Poecilia reticulata (guppy), Utrecht, The Netherlands,
University of Utrecht (Wester, P.W., Ph.D. Thesis).
WESTER, P.W. (in press) Chronic toxicity and carcinogenicity of bis (tri-n-
butyltin) oxide in the rat. Food Chem. Toxicol.
WHO/FAO (1984) Data sheet on pesticides No. 65: Bis (tributyltin)oxide,
Geneva, World Health Organization (VBC/PDS/DS/85.65).
WOLNIAKOWSKI, K.U., STEPHENSON, M.D., & ICHIKAWA, G.S. (1987) Tributyltin
concentrations and Pacific oyster deformations in Coos Bay, Oregon. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1438-1442.
WONG, P.T.S., CHAU, Y.K., KRAMAR, O., & BENGERT, G.A. (1982) Structure-
toxicity relationship of tin compounds on algae. Can. J. Fish. aquat. Sci.,
39: 483-488.
WOOTEN, R., DAVIES, I.M., MCKIE, J.C., & BRUNO, D.W. (1986) Chemical and
histopathological changes in salmon exposed to low concentrations of
tributyltin in seawater, Aberdeen, Department of Agriculture and Fisheries
for Scotland, 3 pp (Working Paper No. 15/86).
YLA-MONONEN, L. (1988) Finnish studies on the use, occurrence and effects
of organic tin compounds. Presented at the OECD Workshop on Monitoring,
Chemical Analysis and Leaching Rates of TBT, Paris, 30 November-2 December,
1988, 9 pp (Unpublished report).
YOUNG, D.R., SCHATZBERG, P., BRINCKMAN, F.E., CHAMP, M.A., HOLM, S.E., &
LANDY, R.B. (1986) Summary report - Interagency workshop on aquatic
sampling and analysis for organotin compounds. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1135-1140.
ZEDLER, R.J. (1961) Organotin as industrial biochemicals. Tin Uses,
53: 7-11.
ZIMMERLI, B. & ZIMMERMANN, H. (1980) Gas-chromatische bestimmung von spuren
von n-butylzinnverbindungen (tetra-, tri-, di-). Fresenius Z. anal. Chem.,
304: 23-27.
ZUCKERMAN, J.J., REISDORF, R.P., ELLIS, H.V., & WILKINSON, R.R. (1978)
Organotins in biology and the environment. In: Brinkman, F.E. & Bellama,
J.M., ed. Organometals and organometalloids, occurrence and fate in the
environment, Washington, DC, American Chemical Society, pp. 388-424 (ACS
Symposium Series No. 82).
RESUME
1. Identité, propriétés physiques et chimiques
Les dérivés du tributylétain (TBT) sont des dérivés
organiques de l'étain tétravalent. Ils se caractérisent
par la présence de liaisons covalentes entre des atomes de
carbone et un atome d'étain et leur formule générale est
la suivante: (n-C4H9)3 Sn-X (dans laquelle X désigne
un anion). La pureté de l'oxyde de tributylétain du com-
merce est généralement supérieure à 96%; les principales
impuretés sont le dibutylétain et dans une moindre mesure
le tétrabutylétain et d'autres trialkylétains. L'oxyde de
tributylétain est un liquide incolore d'odeur caractéris-
tique et de densité comprise entre 1,17 et 1,18. Il est
peu soluble dans l'eau (solubilité comprise entre moins
de 1,0 et plus de 100 mg/litre selon le pH, la température
et les anions présents dans l'eau qui déterminent l'espèce
chimique en cause). Dans l'eau de mer et dans les con-
ditions normales, on rencontre trois dérivés du tributylé-
tain: l'hydroxyde, le chlorure et le carbonate, qui sont
en équilibre. Aux pH inférieurs à 7,0 les formes prédomi-
nantes sont Bu3SnOH2+ et Bu3SnCl; à pH 8 ce sont
Bu3SnCl, Bu3SnOH et Bu3SnCO3-; au-delà de 10, ce
sont Bu3SnOH et Bu3SnCO3- qui prédominent.
Le coefficient de partage octanol/eau (log de Pow)
est compris entre 3,18 et 3,84 pour l'eau distillée et il
est égal à 3,54 pour l'eau de mer. L'oxyde de tributylé-
tain s'adsorbe fortement aux matières particulaires,
puisque les coefficients d'absorption indiqués dans la
littérature vont de 110 à 55 000. La tension de vapeur est
faible mais les valeurs publiées sont extrêmement varia-
bles. On n'a constaté aucune perte d'oxyde de tributylé-
tain à partir d'une solution de 1 mg/litre en 62 jours,
toutefois 20% de l'eau avait disparu par évaporation.
2. Méthodes d'analyse
On utilise plusieurs méthodes pour le dosage des
dérivés du tributylétain dans l'eau, les sédiments ou les
biotes. La plus communément utilisée est la spectrométrie
d'absorption atomique. La spectrométrie d'absorption
atomique avec flamme a une limite de détection de
0,1 mg/litre. Sans flamme, avec atomisation dans un four
électrique à graphite, elle est plus sensible et ses
limites de détection varient entre 0 et 1,0 µg/litre
d'eau. Il existe plusieurs méthodes d'extraction et de
préparation de dérivés volatils. La séparation de ces
dérivés s'effectue habituellement par piégeage ou par
chromatographie en phase gazeuse. Les limites de détection
se situent entre 0,5 et 5,0 µg/kg dans le cas des sédi-
ments et des biotes.
3. Sources de pollution de l'environnement
Les dérivés du tributylétain sont homologués comme
molluscicides, comme produits antisalissures pour la
préservation des coques de bateaux, des appontements, des
bouées, des casiers à crabes, des filets et des cages,
comme enduits de protection du bois, comme alguicides dans
le bâtiment, comme désinfectants et comme biocides dans
les systèmes de réfrigération, les tours de réfrigération
des centrales électriques, les usines de pâte à papier,
les brasseries, les tanneries et les usines textiles. Les
premières peintures antisalissures à base de TBT con-
tenaient ce produit sous une forme qui en permettait la
libération sans entrave. Plus récemment, sont apparues des
peintures dans lesquelles l'incorporation du TBT dans une
matrice en copolymère permet d'en limiter la libération.
On a également mis au point des matrices caoutchouteuses
qui permettent une libération lente et durable et assurent
aux peintures antisalissures et aux molluscicides une
efficacité prolongée. Le TBT n'est pas utilisé en agri-
culture en raison de sa forte phytotoxicité.
4. Réglementation
De nombreux pays ont restreint l'utilisation des
peintures antisalissures à base de TBT du fait de l'action
de cette substance sur les fruits de mer. Les détails de
la réglementation varient d'un pays à l'autre mais la
plupart interdisent l'emploi de peintures à base de TBT
sur les navires de moins de 25 mètres. Dans certains pays,
les navires à coque d'aluminium ne sont pas visés par
cette interdiction. En outre, certaines réglementations
limitent la teneur des peintures en TBT ou la lixiviation
de cette substance à partir des peintures qui en contien-
nent (4 à 5 µg/cm2 par jour sur une longue période).
5. Concentrations dans l'environnement
On a trouvé de fortes concentrations de TBT dans
l'eau, les sédiments et les biotes à proximité de zones de
plaisance, plus particulièrement de marinas, de chantiers
navals et de bassins de radoub, de filets et de cages
traités au moyen de peintures antisalissures et de sys-
tèmes de réfrigération. Ces concentrations de TBT dépen-
dent également de la submersion par la marée et de la
turbidité de l'eau.
On a observé que les concentrations de TBT pouvaient
atteindre 1,58 µg/litre dans l'eau de mer et les estuai-
res, 7,1 µg/litre dans l'eau douce, 26 300 µg/kg dans
les sédiments littoraux, 3700 µg/kg dans les sédiments
d'eau douce, 6,39 mg/kg dans les bivalves, 1,92 mg/kg dans
les gastéropodes et 11 mg/kg dans le poisson. Il ne faut
pas considérer cependant ces concentrations maximales
comme caractéristiques car un certain nombre de facteurs
peuvent donner lieu à des teneurs anormalement élevées
(par exemple la présence de particules de peinture dans
les échantillons d'eau et de sédiments). On a constaté que
les concentrations de TBT dans la micro-couche de surface
des eaux douces et des eaux de mer étaient jusqu'à 100
fois plus élevées que celles qu'on pouvait mesurer juste
en dessous de la surface. Toutefois, il convient de noter
que la concentration en TBT dans la micro-couche de
surface peut dépendre dans une très large mesure de la
technique d'échantillonnage.
Il se peut que les données anciennes ne soient pas
comparables aux données récentes en raison des amélio-
rations apportées aux méthodes de dosage du TBT dans
l'eau, les sédiments et les tissus.
6. Transport et transformation dans l'environnement
Du fait de sa faible solubilité dans l'eau et de son
caractère lipophile, le TBT s'adsorbe facilement aux par-
ticules. On estime que 10 à 95% de l'oxyde de tributylé-
tain qui pénètrent dans l'eau s'adsorbent ainsi sur les
particules. La disparition progressive du TBT absorbé
n'est pas due à sa désorption mais à sa dégradation. Le
degré d'adsorption dépend de la salinité, de la nature et
de la taille des particules en suspension, de la quantité
de matières en suspension, de la température et de la
présence de matières organiques dissoutes.
La dégradation de l'oxyde de tributylétain s'effectue
par rupture de la liaison carbone-étain. Celle-ci peut
résulter de divers mécanismes qui se produisent simultané-
ment dans l'environnement et notamment des mécanismes
physico-chimiques (hydrolyse et photodécomposition) ou
biologiques (dégradation par des micro-organismes et
métabolisation par des organismes supérieurs). L'hydrolyse
des dérivés organostanniques se produit à des valeurs
extrêmes du pH mais n'apparaît guère dans les conditions
qui règnent normalement dans l'environnement. La photo-
décomposition se produit par exposition en laboratoire de
solutions à un rayonnement ultra-violet de 300 nm (et à un
moindre degré, à un rayonnement de 350 nm). Dans le milieu
naturel, la photolyse est limitée par la longueur d'onde
du rayonnement solaire et par la pénétration du rayonne-
ment ultra-violet dans l'eau. La présence de substances
photosensibilisatrices peut accélérer la photodécomposi-
tion. La biodégradation dépend de l'état du milieu, et de
caractéristiques telles que sa température, son oxygéna-
tion, son pH, sa teneur en éléments minéraux, la présence
de substances organiques facilement biodégradables pouvant
subir une co-métabolisation ainsi que la nature de la
micro-flore et sa capacité à s'adapter. Elle ne peut
également avoir lieu que si la concentration en oxyde de
tributylétain est inférieure à la concentration létale ou
inhibitrice pour les bactéries. Comme dans le cas de la
décomposition abiotique, la dégradation biologique du TBT
comporte une débutylation oxydante progressive avec rup-
ture de la liaison carbone-étain. Il se forme des dérivés
dibutylés dont la dégradation est plus facile que celle du
tributylétain. Les monobutylétains sont lentement minéra-
lisés. Il se produit également une dégradation anaérobie
mais son importance reste discutée. Certains chercheurs
estiment que la dégradation en anaérobiose est lente alors
que d'autres la jugent plus rapide que la dégradation
aérobie. On a identifié des espèces de bactéries, d'algues
et de champignons attaquant le bois qui sont capables de
dégrader l'oxyde de tributylétain. Les estimations de la
demi-vie du TBT dans l'environnement varient dans
d'importantes proportions.
Le TBT s'accumule dans les organismes du fait de sa
solubilité dans les graisses. Des recherches en labora-
toire portant sur des mollusques et des poissons ont
donné, pour les facteurs de bioconcentration, des valeurs
allant jusqu'à 7000 mais des valeurs encore plus élevées
ont été observées lors d'études sur le terrain. L'absorp-
tion à partir de la nourriture est plus importante qu'à
partir de l'eau. Les facteurs de concentration plus élevés
observés chez les micro-organismes (entre 100 et 30 000)
peuvent s'expliquer par une adsorption plutôt que par une
absorption intracellulaire. Rien n'indique que le TBT
puisse passer dans les organismes terrestres par l'inter-
médiaire de la chaîne alimentaire.
7. Cinétique et métabolisme
Le tributylétain est absorbé au niveau intestinal (20
à 50% selon le véhicule) ainsi que par voie percutanée
chez les mammifères (dans la proportion d'environ 10%).
Il peut traverser la barrière hémo-méningée et passer du
placenta dans le foetus. Une fois absorbé, il est rapide-
ment et largement diffusé dans l'organisme (principalement
au niveau du foie et des reins).
Chez les mammifères, la métabolisation du TBT est
rapide; on peut déceler les métabolites dans le sang dans
les 3 heures suivant l'administration. Des études in vitro
ont montré que le TBT servait de substrat aux oxydases à
fonction mixte mais qu'il inhibait ces enzymes à très
forte concentration.
L'élimination du TBT s'effectue plus ou moins rapide-
ment selon la nature du tissu et les estimations de la
demi-vie biologique chez les mammifères varient de 23 à
environ 30 jours.
Les organismes inférieurs métabolisent également le
TBT mais le processus est plus lent - en particulier chez
les mollusques - que chez les mammifères. La capacité de
bioaccumulation est donc beaucoup plus importante que chez
les mammifères.
Les tributylétains inhibent la phosphorylation oxy-
dative et modifient la structure et la fonction des mito-
chondries. Le TBT empêche la calcification de la coquille
des huîtres (espèces du genre Crassostrea ).
8. Effets sur les micro-organismes
Le TBT est toxique pour les micro-organismes et on le
vend comme bactéricide et alguicide. Les concentrations
toxiques varient considérablement selon les espèces. Le
TBT est plus toxique pour les bactéries gram-positives
avec une concentration minimale inhibitrice (CMI) allant
de 0,2 à 0,8 mg/litre que pour les bactéries gram-néga-
tives (CMI des 3 mg/litre). La CMI de l'acétate de TBT
pour les champignons est de 0,5 à 1 mg/litre et celle de
l'oxyde de tributylétain est de 0,5 mg/litre pour l'algue
verte Chlorella pyrenoidosa. La productivité primaire
d'une communauté naturelle d'algues d'eau douce a été
réduite de 5% par une concentration d'oxyde de tributylé-
tain de 3 µg/litre. On a récemment établi la dose sans
effet observable pour 2 espèces d'algues; elle est
respectivement de 18 et 32 µg/litre. La toxicité pour
les microorganismes marins varie également selon les
espèces et selon les études; il est difficile d'établir
la valeur de la dose sans effet observable mais on pense
qu'elle est inférieure à 0,1 µg/litre pour certaines
espèces. Les concentrations alguicides vont de moins de
1,5 µg/litre à plus de 1000 µg/litre selon les espèces.
9. Effets sur les organismes aquatiques
9.1 Effets sur les organismes marins et estuariels
La Figure 1 donne un diagramme récapitulatif des
effets létaux et sublétaux que peuvent produire les con-
centrations de TBT relevées en mer et dans les estuaires.
Des concentrations supérieures à celles qui produisent les
effets létaux aigus ont été observées en différents points
du globe, notamment là où se déroulent des activités de
plaisance.
Ce sont les spores mobiles d'une algue verte géante
qui se sont révélés les plus sensibles au TBT (CE50 à 5
jours: 0,001 µg/litre). On a constaté une réduction de
la croissance d'un angiosperme marin à des concentrations
de TBT de 1 mg/kg de sédiments, aucun effet n'étant noté à
0,1 mg/kg.
10.3. Effects on farmed fish
Wooten et al. (1986) reported the effects of exposure
to TBT antifouling paints applied to retaining nets for
farmed salmon. Fish exposed to newly-treated cage nets
were reported to be blind. The authors examined diseased
post-smolt salmon from cages treated with TBT at the time
of smolt transfer. Elevated tin levels were found in all
salmon exposed to the TBT paint; liver residues ranged
between 1.01 and 1.62 mg tin/kg wet weight. Residues in
the liver of blind fish ranged between 1.01 and 1.07 mg
tin/kg wet weight. Blindness had been caused by rupturing
of the eye; the eye lens was missing. Eye tissues appeared
normal histologically apart from the rupture; there were
no histological lesions. The kidney, liver, stomach, heart
and muscle of the blind fish appeared normal histologi-
cally. The intestine showed mucosal sloughing, vasodi-
lation, and pyknotic nuclei. The spleen had an "open
structure" with increased numbers of erythrocytes. There
was thickening of secondary gill filaments and some
necrosis and capillary separation in the epithelium. Other
fish in the affected population were reported to have
swellings along the lateral line. These were shown histo-
logically to be thick folds of epidermis under which there
was cellular infiltration of necrotic collagen forming an
abscess. Pancreas disease was also reported in affected
fish. In the liver there was a breakdown of lobular struc-
ture with lack of cohesion between cells. The effects were
thought to be consistent with poisoning by organotin.
10.4. Effects of TBT-contaminated sediment
Matthiessen & Thain (1989) studied the recolonization
by marine organisms in the field of sediment contaminated
with TBT-containing paint. Sediment was collected from
mudflats, and macroorganisms present were killed by
repeated freezing and thawing. TBT was added to the sedi-
ment in the form of abraded paint. The paint was abraded
with scourers used on yachts to produce material of the
kind likely to contaminate sediments normally. Sediment
samples containing 0.1, 1, 10, and 100 mg TBT/kg were
prepared. The contaminated sediment was returned to the
mudflats and placed in excavated trenches (3 m x 30 cm
wide x 20 cm deep) lined with polyethylene mesh (5-mm
apertures). Recolonization of the sediment could, there-
fore, take place both by settlement of organisms from
above and by lateral transfer of organisms from adjoining
mud. The sites were revisited five times during the next
160 days when samples were taken both to count recolon-
izing organisms and to measure TBT levels at various
depths. Surface sediment decontaminated rapidly; water
movement would have removed sediment and deposited new.
The subsurface TBT levels remained reasonably stable
throughout the experiment. Burrowing activity (estimated
by counting casts on the surface) of the polychaete
Arenicola marina was reduced at all concentrations of
TBT, though the effect of 0.1 mg TBT/kg disappeared after
3 months. A dose-related reduction in populations of the
burrowing polychaete Scoloplos armiger and burrowing
amphipod Urothoe poseidonis was observed over the whole
concentration range. There were no clear effects on other
species, including molluscs. The authors pointed out that
some unaffected groups were associated with the surface
sediment, where TBT was lost rapidly. Feeding behaviour
of these surface dwellers, such as the cockle (Cardium
edule), would lead to little TBT exposure, since they fil-
ter overlying sea water and ingest little or no sediment.
Species associated with deeper layers of sediment and
feeding on fine particles, such as Urothoe poseidonis,
showed greater effects of TBT, resulting, presumably,
from greater exposure rather than greater inherent sensi-
tivity. The authors also noted the wide range of sensi-
tivity of different species in laboratory tests without
sediment. The bioavailability of TBT associated with sedi-
ment would probably be different from that dissolved in
water.
10.5. Effects of freshwater molluscicides
Following earlier laboratory trials on the use of TBT
as a molluscicide for schistosomiasis control (Deschiens &
Floch, 1962), Deschiens et al. (1966) applied TBTO to a
pond used for fish culture, in Cameroon, of approximately
1 metre in depth and with an area of 50 m2 (approximately
50 tonnes of water) and a water temperature of 23 °C.
Caged pond snails (Biomphalaria [Australorbis] glabratus
and Bulinus contortus), 10 to a cage supplied with food,
were placed in the pond in different areas and at differ-
ent depths. Renewal of cages was performed to study the
persistence of the compound. The pond was treated with
TBTO, as "Biomet" (50% TBTO and 50% toxicologically
inert dispersant), at rates giving TBTO concentrations in
water of 0.015, 0.03, or 0.045 mg/litre, and the side
effects on fish and plankton were investigated. At 0.045
mg/litre, TBTO killed 100% of the snails and 70% of the
fish within 24 to 48 h. At 0.03 mg/litre, TBTO killed 100%
of snails within 2 to 3 days, but no effect on the fish
was observed within 15 days. At 0.015 mg/litre, the TBTO
killed all of the snails within 4 to 7 days and again had
no observable effect on the fish. The authors considered
this to be a practical concentration to achieve full
effect on snails without killing non-target organisms.
Various planktonic organisms (desmids, daphnids, and
aquatic insect larvae) were killed by TBTO at 0.5 mg/litre
experimentally. However, no effect was found on any of
these species in the pond treated at 0.015 mg/litre,
although there was some inhibition (but no deaths) of
these organisms at 0.03 and 0.045 mg/litre.
Gilbert et al. (1973) applied slow-release TBTO in
underseal (quick-drying asphalt-rubber-asbestos-clay paste)
to four sites, in Brazil, at concentrations of 15, 15, 50,
or 300 g TBTO/m2, to control Biomphalaria tenagophila
and B. straminea. Dead fish were observed at the sites
treated with 50 or 300 g/m2 and also at one of the two
sites treated with 15 g/m2, a static man-made pit. At
the other site, with a flow rate of 100 to 200 litre/min,
there was a 100% reduction in the snail population (B.
tenagophila) maintained for up to 12 months, while both
fish and aquatic insect life were apparently normal one
month after treatment. At this site abundant natural veg-
etation flourished in the immediate vicinity of the mol-
luscicide. Toledo et al. (1976) found that 15 g TBTO/m2
eliminated snails from 76% of treated sites for 1 year.
Guppies, which are often present in highly polluted sites,
normally suffered some mortality at the beginning of mol-
luscicidal treatment but in general they tolerated it
well. The authors found no lasting effect on plant or
insect life, and concluded that, because of the ability of
an unidentified fungus to grow profusely, microbiological
life continued in treated areas.
Shiff et al. (1975) applied BioMet SRM pellets (con-
taining TBTO) at concentrations of 20 and 30 g/m2 to a
night-storage dam in Zimbabwe. At the lower application
rate, pellets remained active against the snails (various
Biomphalaria spp.) for up to one year. At 30 g/m2, 100%
mortality of snails was achieved within 2 weeks of appli-
cation and was maintained up until the last sampling time
2 months after application. Prior to treatment, 80
specimens of Tilapia fish sp. and 1 of Clarias gariepinus
were caught. Four weeks after treatment 26 Tilapia were
caught and one dead Clarias was found. Eight weeks after
treatment 400 Tilapia were caught, of which 80 were
examined and all appeared normal.
Ayala et al. (1980) applied slow-release molluscicide
(rubber impregnated with 11% TBTO) at a rate of between 5
and 15 g/m2 to a site in Brazil and studied the effect on
non-target organisms. The TBTO molluscicide was initially
herbicidal towards floating vegetation but did not affect
either plants rooted in the mud or marginal plants. The
chemical was initially repellent to aquatic animals, some
of which were killed by the molluscicide. Within 3 months,
populations of non-target snails such as Pomacea sp.,
Drepanotrema sp., and a Physa sp. had returned to normal.
Although no fish had returned within 3 months, after 5
months numbers had returned to normal. In fact, 5 months
after application all but Spirogyra sp. were normal, and
the molluscicidal activity of the TBTO was retained for
more than one year. The authors concluded that after 3 to
5 months the action of the TBTO molluscicide is confined
to the bottom mud where Biomphalaria glabrata snails spend
much of their time.
10.6. Effects from spills
According to Waldock et al. (1987a), major inputs of
TBT into the environment may have arisen from spills of
timber-treatment products, often containing dieldrin and
pentachlorophenol as well as the organotin. They reported
that a detailed study was carried out after a spill con-
taminated a section of the Newmill Channel, Kent, United
Kingdom. There was a major kill of fish and all macroin-
vertebrates (except oligochaetes, chironomid larvae, and
elmid beetles) were also killed. Concentrations of TBT in
the water shortly after the spill were 540 µg per litre,
falling to 10 µg/litre after 3 weeks and 0.75 µg per
litre after 6 weeks. Nine months after the incident,
macroinvertebrate populations were reported to have
recovered. The authors stated that five such major spills
from timber treatment plants had been reported in the
United Kingdom within 2 years.
10.7. The use of indicator species for monitoring the environment
Both shell growth and shell chambering in Pacific
oysters and imposex in dogwhelks have been used as bio-
logical indicators of TBT contamination. Smith et al.
(1987) transferred juvenile Pacific oysters to major bays
and harbours of California, USA, known to contain elevated
levels of TBT. They observed a graded increase of stunted
growth and/or shell deformation, which was associated with
poor flushing and the proximity of large numbers of boats.
Gibbs et al. (1987) used the dogwhelk to monitor water
around the south-west peninsula of England. They found the
species to be a very sensitive indicator, especially if
the individuals were about 1 year old and in the early
stages of ovarian development. Davies et al. (1987a) found
imposex in dogwhelks to be a far more sensitive indicator
than either shell thickening in oysters or tin accumu-
lation. The dogwhelk has been used to identify areas of
contamination associated with seasonal small boat activity
and salmon farm cages in Scottish sea lochs (Davies et
al., 1987b), in areas of pleasure craft activity, fishing
harbours and a boat yard in the Firth of Forth (Scotland)
(Bailey & Davies, 1988b), and to investigate contamination
from an oil terminal in Sullom Voe, Shetland (Bailey &
Davies, 1988a).
11. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
11.1. Single exposure
Summary
The acute toxic effects of the various TBT compounds that
have been studied are comparable and are characteristically
delayed for several days. Oral LD 50 values in laboratory ani-
mals range from approximately 40 to 250 mg/kg body weight.
These compounds exhibit greater lethal potential when adminis-
tered parenterally, relative to the oral route, probably
because they are only partially absorbed from the gut. Acute
toxicity via the dermal route is low.
TBT compounds are potent skin irritants and extreme eye
irritants. Dermal exposure to TBTO appears to have little
sensitization potential.
Aerosols of TBT compounds are highly toxic. However, TBT
vapour/air mixtures at room temperature produce no effect, even
at saturation.
Other effects of acute exposure may include alterations in
blood lipid levels, the endocrine system, liver, and spleen,
and transient deficits in brain development. The toxicological
significance of these effects, reported after high single doses
of the compounds, is questionable and the cause of death
remains unknown.
11.1.1. Oral and parenteral administration
The acute toxicity of tributyltin to laboratory mam-
mals by various routes of administration is summarized in
Table 14. The acute oral LD50 for the rat ranges between
94 and 234 mg/kg body weight and for the mouse between 44
and 230 mg/kg body weight. Truhaut et al. (1976) pointed
to the delayed toxicity of tributyltin and, therefore, the
necessity to continue the observation of animals, after a
single acute dose, for several days. The period of post-
dosing observation is therefore indicated in Table 14.
LD50 values for ip and iv administrations of TBT are
very much lower than in the case of the oral route (10
mg/kg for rat ip and 6 mg/kg for mouse iv).
Table 14. Acute toxicity of tributyltin to laboratory mammals
------------------------------------------------------------------------------
Species TBT Route Observation LD50 (mg/kg Reference
perioda body weight)
(days)
------------------------------------------------------------------------------
Rat oxide oral 7 194 (165-227)b Elsea & Paynter (1958)
oxide oral 7 148 (113-195)c Elsea & Paynter (1958)
oxide oral 7 180 (132-228) Truhaut et al. (1976)
oxide oral 21 197 (137-273) Funahashi et al. (1980)
oxide oral 127 Schweinfurth (1985)
oxide ip 14 20 (18-21) Poitou et al. (1978)
oxide oral 234 Sheldon (1975)
fluoride oral 200 Sheldon (1975)
fluoride oral 14 94 Schweinfurth (1985)
chloride oral 14 122 Schweinfurth (1985)
acetate oral 113.5 Klimmer (1969)
benzoate oral 141 Klimmer (1969)
benzoate oral 14 99/203 Schweinfurth (1985)
oleate oral 225 Klimmer (1969)
linoleate oral 14 190 Schweinfurth (1985)
abietate oral 14 158 Schweinfurth (1985)
naphthenate oral 14 224 Schweinfurth (1985)
Mouse oxide oral 7 85 (52-130) Polster & Halacka (1971)
acetate oral 7 46 (25-85) Pelikan & Cerny (1968)
oleate oral 7 230 (175-301) Pelikan & Cerny (1968)
benzoate oral 7 108 (74-156) Pelikan & Cerny (1968)
chloride oral 7 117 (80-170) Pelikan & Cerny (1968)
laurate oral 7 180 (136-237) Pelikan & Cerny (1968)
oxide sc 7 200 (140-270) Polster & Halacka (1971)
oxide iv 7 6 (5.5-6.5) Truhaut et al. (1976)
oxide ip 14 16 (15-17) Poitou et al. (1978)
Rabbit fluoride dermal 680 Sheldon (1975)
------------------------------------------------------------------------------
a Following a single application of TBT, the observation period was as indicated.
b Application as aqueous suspension.
c Application in corn oil.
Pelikan & Cerny (1968) administered TBT (as the acet-
ate, benzoate, chloride, laurate, or oleate) to white mice
(body weight 25 g) in a single oral gavage dose of 500
mg/kg body weight, dissolved in sunflower oil. Ten animals
were used for each TBT compound. The mice were observed
for 8 h before being killed, and the viscera were examined
histologically. Four hours after treatment, all animals
showed signs of intoxication with the exception of those
given the laurate; after 8 h all showed toxic symptoms.
Gross damage was seen in the digestive tract, liver, and
spleen. Histological findings included a steatosis of the
liver cells in all animals (but to varying degrees),
traces of lipid in renal tubule cells (in those animals
receiving oleate or laurate) and many haemorrhages in the
digestive tract and kidneys. Effects on the liver and
spleen were also noted after dermal absorption of TBTO
(Pelikan & Cerny, 1969). However, no real conclusions can
be drawn from these studies as there was no clear reported
evidence that steatosis was caused either by the TBT or by
the fatty acids.
In studies by Funahashi et al. (1980), Sprague-Dawley
rats were given a single dose, in olive oil, of 100 mg
TBTO/kg body weight by intubation and were examined over
the following 21 days. Adrenal weight was slightly
increased 12 h after treatment and reached a maximum on
the second day. Histological changes in the adrenal had
returned to normal by day 14. The thyroid follicles showed
signs similar to those produced by hypophysectomy, i.e.
distension with colloid, flat epithelial cells. These
changes were severe after 72 h, but had returned to normal
within 14 days. Absolute pituitary weight was increased
slightly (and not significantly) 1 and 2 days after
dosing, while relative weight increased significantly.
There was atrophy of pituitary adrenocorticotrophin (ACTH)
cells 6 h after dosing. After 72 h, the staining of ACTH
cells became more intense. There were marked reductions
in the circulating levels of both thyroid stimulating
hormone (TSH) and thyroxine (T4) during the 72 h follow-
ing dosing, serum titres falling to one half and one sixth
of control values for the two hormones, respectively. The
authors concluded that TBTO had both a direct and an
indirect effect on the thyroid, since initially there was
a simultaneous rise in T4 and fall in TSH. Serum cortisol
levels increased to twice the control level 96 h after
treatment with TBTO at a level of 100 mg/kg body weight.
Intramuscular ACTH administration, 8 h after dosing, led
to increased cortisol in the blood of both treated and
control rats. After 16 h, controls showed release of
cortisol after ACTH stimulation, but treated rats showed a
decrease in circulating levels of cortisol after similar
stimulation.
Matsui et al. (1982) reported the effects of a single
dose of TBT fluoride (100 mg/kg body weight) given by
gastric intubation to Japanese white rabbits. A reversible
but pronounced hyperlipidaemia was observed, particularly
involving triglycerides and total cholesterol. Ultracen-
trifugation showed a marked increase in the chylomicron
plus VLDL (very low density lipoprotein) fraction. The
activity of lipoprotein lipase (LPL) in plasma was reduced
to about 50% of control levels. Fasting levels of blood
glucose were elevated, and the response to iv infusion of
glucose (given 3 days after the TBT fluoride) was
inhibited (insulin release reduced). The authors suggested
that the hyperlipidaemia was the result of reduced LPL
activity, which in turn was brought about by inhibition of
insulin release.
Calley et al. (1967) investigated the effects of TBT
acetate on liver function in rabbits. Following a single
oral dose of TBT acetate at 50 mg/kg body weight (half of
the measured LD50 in rabbits), only the serum glutamic-
pyruvate transaminase (SGPT) activity was affected. This
activity was not elevated after 48 h but was increased
within 144 h of dosing. Prothrombin time, alkaline phos-
phatase, and thymol turbidity showed no significant
changes from control values. SGPT is a highly specific
indicator of liver damage, directly reflecting liver cell
injury. Tributyltin acetate also significantly increased
the hexabarbitol-induced sleeping times of mice; a 50
mg/kg body weight oral dose increased the sleeping time
from 29 to 43 min following a standard dose of the
narcotic.
When Aldridge et al. (1977) administered TBTO (and its
gamma-keto, gamma-hydroxy, and delta-hydroxy metabolites)
intraperitoneally to mice on two consecutive days (at dose
levels of 12.5, 25, and 50 µmol/kg body weight), they
found no evidence of cerebral oedema after any of the
treatments.
Crofton et al. (1989) gave rat pups a single dose of
TBTO on day 5 following birth by gastric intubation with
0, 40, 50, or 60 mg/kg body weight. The monitoring of
behavioural parameters up to 62 days post-dosing showed no
persistent effect on motor activity or acoustic startle
response.
When Barnes & Stoner (1958) fed rats with tributyltin
acetate during the first 3 months after birth, they found
increased brain and spinal cord water content at the
highest dose tested (100 mg/kg diet). This increase was
not sufficiently great to be seen in histological sec-
tions. Bouldin et al. (1981) found no light or electron
microscopic evidence of neuronal damage in the hippocampus
or the pyriform cortex of neonate or adult rats exposed
daily to tributyltin acetate at 10 mg/kg body weight by
gavage for up to 30 days.
O'Callaghan & Miller (1988) reported the effects of a
single ip injection of TBTO on neonatal rat brain. The
rats were injected when 5 days old with 2, 3, or 4 mg/kg.
They were sacrificed at 13, 22, or 60 days of age, and
various proteins in homogenates of brain tissue were
measured by radioimmunoassay. Brain weight was reduced in
a dose-dependent manner, the cerebellum being most
affected. There was no evidence of altered brain histology
under the light microscope. Dose-dependent and region-
dependent decreases were found in P-38 (a synaptic-vesi-
cle-associated protein) and myelin basic protein (a
protein associated with oligodendroganglia and the myelin
sheath); there were decreases in both total (per tissue)
and concentration (per mg) levels of these proteins in the
cerebellum and forebrain but not the hippocampus. These
effects were seen at a dose level that did not affect body
weight. However, in contrast to the neurotoxic effects of
trimethyltin compounds, which are irreversible and persist
into adulthood, these neurotoxic effects of TBTO were
transitory at dose levels that did not affect body weight.
Robinson (1969) monitored tissue levels of catechol-
amines after ip administration of TBTO, in corn oil, to
rats at a level of 10 mg/kg body weight. This dose
exceeded the 6-day LD50, determined by the same authors
to be 7.21 mg/kg. Brain noradrenalin levels were signifi-
cantly reduced at 2, 24, and 48 h after dosing, whereas
5-hydroxytryptamine levels were only reduced (though
markedly) after 48 h. Noradrenalin levels in heart tissue
were also significantly reduced after 2, 24, and 48 h.
Adrenal adrenalin and noradrenalin levels were reduced
after 24 and 48 h, but only adrenalin was affected after
2 h.
11.1.2. Dermal administration
The acute LD50 of TBTO administered to rabbits via
the dermal route is very high, i.e. approximately 9000
mg/kg body weight (Table 14). Although there is dermal
absorption of TBT (see later), the degree of absorption is
not great enough to lead to acute toxic effects sys-
temically, except at very high exposure levels.
11.1.3. Administration by inhalation
In a "nose only" inhalation exposure (to minimize
dermal exposure and exposure through ingestion) lasting
4 h, the acute LC50 of TBTO for the rat was estimated to
be 77 mg/m3 by measurement of airborne droplets. This
value decreased to 65 mg/m3 when "inhalable" particles,
10 µm or less in diameter, were considered. There was
evidence of lung irritation and oedema in this study
(Schweinfurth, 1985). When groups of 10 male and 10 female
rats were each exposed to atmospheres containing almost
saturated vapours of TBTO, TBT benzoate, or TBT naphthen-
ate once for 7 h, no deaths occurred during exposure or in
a 14-day observation period following exposure. Only minor
clinical signs were noted occasionally during the exper-
iment, such as slight nasal discharge (Schweinfurth, 1985).
Truhaut et al. (1979) exposed mice to an aerosol of
TBTO in olive oil, for either a single 1-h period or seven
1-h periods on successive days, using TBTO concentrations
in air ranging between 0.05 and 0.4 mg/litre (50 to
400 mg/m3). Exploratory behaviour was scored over 5-min
periods 2 h after the single exposure was complete or 24 h
after the last of the seven exposure periods. The lower
two exposure doses caused significant increases in explo-
ratory behaviour (17% and 5% for 42 and 84 mg/kg, respect-
ively) while the higher exposure doses reduced exploratory
behaviour (-18% and -38% for 170 and 340 mg/kg, respect-
ively). Truhaut et al. (1981) found a median survival time
for mice of 22 min and for guinea-pigs of 9 min after
exposure to an aerosol of tributyltin (concentration not
stated). They reported that only tissues in the respirat-
ory system showed significant lesions. There was diffuse
congestion of the pulmonary blood vessels extending to the
septal capillary beds. There were also inflammatory
responses in the trachea and bronchii, secretion of mucus
in the bronchii and bronchioles, and distension and rup-
ture of the alveoli.
Anger et al. (1976) exposed guinea-pigs to aerosols of
TBTO ranging between 0.1 and 1 mg/litre air for 1 h. All
males exposed to 0.2 mg/litre died, and 12 out of 15
females exposed to 1 mg/litre died. No particular lesions
were observed in those animals that died, with the excep-
tion of a general congestion of the lungs. Exposure of
either males or females to 0.17 mg/litre caused no deaths,
though nasal irritation was observed; all these animals
survived a further 7 days of observation.
11.1.4. Irritation and sensitization
11.1.4.1 Skin irritation
In a study by Elsea & Paynter (1958), undiluted TBTO
was applied to the closely-clipped skin of the abdominal
area of rabbits, which was then covered with rubber dental
damming, gauze, and adhesive tape. After an exposure
period of 24 h, the covering was removed, and the TBTO was
washed off, as far as possible, by sponging with warm
water. This single application of doses up to 11.7 g/kg
body weight caused some of the rabbits to die from the
effects of TBTO absorbed into the body. There was, how-
ever, only moderate dermal irritation, characterized by
reddening, oedema, atonia, blanched areas, and areas of
brown discolouration. Examination of the skin at autopsy
showed some subcutaneous oedema. Repeated dermal appli-
cation of TBTO, impregnated into paper at 8 mg/kg, daily
for 5 days, only affected one animal out of four. This
rabbit showed slight erythema and oedema following the
first and second applications. Later in the experiment,
the skin appeared normal. No detailed histological examin-
ation of the skin was carried out.
Pelikan & Cerny (1969) applied to the shaved skin of
rats two preparations of TBTO, i.e. "Lastanax T" (con-
taining 20% TBTO plus a medium of water, alcohols of short
chain length, and n -alkyl-polyethylene oxide) and
"Lastanax P" (containing 15% TBTO plus a medium also con-
taining bis-(5-chloro-2-hydroxyphenyl)-methane). Actual
doses applied, corresponding to water dilutions of 100%,
33%, 10%, and 1% of the preparations, were 185, 61.5,
18.5, and 1.85 mg TBTO/kg body weight for Lastanax T and
145, 47, 14, and 1.4 mg TBTO for Lastanax P. Controls
received either water or the medium without TBTO. The
experiment was carried out in duplicate. In the first
series, clinical observations were made twice daily for
60 days, whereas in the second series rats were killed on
day 10 and the skin was examined histologically. Control
rats showed no clinical or histological effects on the
skin. However, clinical and histological changes were
found in all treated rats, even with the 1% dilution,
though severity increased with exposure dose. On the first
and second days after application, there was reddening of
the skin and oedema developed. From day 3, haemorrhagic
eschars developed with well defined borders; there was no
inflammation of the surrounding areas of skin. Between
these foci, numerous papules and pustules developed, some
with bleeding; the apices of some papules were necrosed.
Later the eschars joined to create larger areas of
ulceration. Healing of the areas began between the 9th
and 12th days; necroses disappeared by day 20, pustules 3
to 4 days before, and papules by the 30th to 33rd day. All
signs had disappeared after 35-38 days (exposure to 1% and
10% solutions) or after 45-50 days (exposure to 33% or
100% solutions). Changes with Lastanax P were similar, but
less severe, than with Lastanax T. In the histological
study, numerous large bullae, filled with leucocytes and
coagulated exudate (proteins), were found under the
stratum corneum of the epidermis after 10 days. Akanthosis
and vacuolation of epidermal cells occurred following
exposure to the 10% and 33% solutions and, to a lesser
extent, the 1% solution. Small haemorrhages were found.
Epidermal changes were dispersed and alternated with areas
of skin appearing normal. The authors suggested that the
TBTO NOEL for human skin should be set at 0.005% to 0.01%.
In a study by Middleton & Pratt (1977), TBT chloride
was applied to a shaved area of dorsal skin of male
Alderley Park (Wistar-derived) rats, four-weeks old (body
weight 50-80 g), in absolute ethanol as a solution of
10 mmol/litre. This dose was equivalent to 67 nmol/cm2.
The TBT had produced microscopic changes in the skin
within 2 h of application. Polymorphonuclear leucocytes
accumulated in capillaries and in dermal tissues. Epider-
mal cells showed progressive degenerative changes between
2 and 8 h after application until, by 8 h after appli-
cation, separation of the epidermis and dermis had
occurred and fluid collected in this separation. Many
inflammatory cells were present in both dermis and epider-
mis. There was widespread epidermal necrosis within 12 to
24 h, and separation of necrotic epidermis from the dermis
was almost complete by this time. The vesicles formed by
this separation were frequently packed with inflammatory
cells as well as exudative fluid. Regeneration of the
epidermal layer was observed after 18 to 24 h and dermal
inflammatory infiltration was regressing by this time.
Erythema, visually assessed and scored, reached a maximum
within 5 h of application and remained at this level for
48 h. The erythema had subsided by 72 h after application.
Vascular permeability of the skin was assessed by
injecting rats with a dye, tryphan blue, 45 min before
sacrifice. There was a biphasic response. At 2 h after
application of TBT chloride, there was an initial increase
in permeability of the skin to 135% of control levels. A
second peak began at 12 h and persisted for more than
25 h after application. The effect was still evident after
48 h and had occurred in both the treated and untreated
flanks of the animal, indicating absorption of the com-
pound and a systemic effect. The water content of the skin
was increased by TBT chloride, reaching a peak within 2 h
of application; by 10 h this effect had disappeared.
Middleton & Pratt (1978) reported that TBT produced focal
epidermal necroses and dermal inflammation at levels as
low as 33 nmol/cm2, fairly extensive epidermal necroses
and dermal inflammation at 67 nmol/cm2, and almost total
epidermal necrosis at 167 nmol/cm2.
11.1.4.2 Eye irritation
Pelikan (1969) applied TBTO, as "Lastanax T or P",
in a single dose of 0.03 ml to the left eye of rabbits.
Lastanax T is 20% TBTO in alcohols of short chain length,
non-ionic surface active substances ( n -alkyl-polyethyl-
ene oxide) and water. Lastanax P is 15% TBTO in the same
vehicle with the addition of bis-(5-chloro-2-hydroxyphe-
nyl) methane. A 10% and a 1% solution in water were used.
The 10% solution represented an actual TBTO dose of 6.1
and 4.6 mg/kg body weight for the two formulations,
respectively. With the 10% solution, both rabbits died,
11 and 12 days after application. Severe ulceration of the
eye preceded death. Histopathological examination of
internal organs after death revealed hyperaemia of the
brain and medulla oblongata and hyperplasia of reticulo-
endothelial cells of the spleen. Necrotic changes were
seen on the cornea of eyes treated with the 1% solutions
within 3 h of application, and symptoms worsened over the
next 2 to 5 days. Recovery was incomplete 100 days later.
11.1.4.3 Skin sensitization
Poitou et al. (1978) investigated the skin-sensitizing
potential of TBTO in guinea-pigs using the Magnussen-
Kligman method. The concentrations used for sensitization
were 1% (intradermal phase) and 5% (topical phase). Using
challenge concentrations of 0.25% and 0.1%, no sensitizing
action was demonstrated in the 20 test animals.
11.1.5. In vitro studies
Johnson & Knowles (1983) demonstrated that incubation
of rat blood platelets with TBT chloride or TBTO, in
vitro, inhibited their ability to take up 14C-labelled 5-
hydroxytryptamine (5-HT). The organotin also caused
release of 14C-labelled 5-HT, taken up before exposure,
along with endogenous 5-HT. Treating rats ip with TBT
chloride at 5.0 mg/kg body weight also led to reduced up-
take of 5-HT (37% inhibition) 30 min after treatment. The
level of 5-HT in platelets, however, was unaffected by the
in vivo treatment with TBT chloride. knowles & Johnson
(1986) reported that exposure of rat platelets to TBT
chloride inhibited aggregation induced with ADP or colla-
gen. The inhibition of ADP-induced aggregation was depen-
dent on the dose of TBT and on the exposure time prior to
ADP addition. Exposure of the platelets to 5 µmol TBT per
litre for 5 min or 10 µmol/litre for 0.1 min produced
inhibition. However, exposure to 1 µmol/litre for 5 min
was ineffective. With an incubation time of 1 min, TBTO
was effective in lengthening the time taken for aggre-
gation after collagen induction at 0.625 µmol/litre, but
not at 0.5 µmol/litre.
The trialkyltins, including tributyltin, have been
shown to inhibit oxidative phosphorylation in rat mito-
chondria (Aldridge & Street, 1964). TBT stimulated
adenosinetriphosphatase (ATPase) activity and caused
limited swelling of rat liver mitochondria. These last two
effects occurred at similar concentrations of TBT in the
medium. The authors postulated that the two effects were
linked; the ATPase activity increased over a concentration
range of TBT and was always mirrored by a decrease in the
mitochondrial swelling. This relationship persisted
despite a complex response to TBT over the concentration
range; at higher concentrations there was a sudden, much
smaller mitochondrial swelling and a concomitant rise in
ATPase activity. TBT also inhibited the hydrolysis of ATP
by rat brain microsomes, although the concentrations
required were much higher than those affecting phosphory-
lation. The authors postulated that a combination of tri-
alkyltin compounds with negatively charged lipids is
involved in their biological activity.
Elferink et al. (1986) treated polymorphonuclear
leucocytes (PMNs), obtained from the peritoneal cavity of
rabbits, with an unspecified tributyltin (1 µmol/litre).
Uptake of opsonized zymosan was used as a measure of
phagocytosis, and enzyme (lysozyme) release to the super-
natant during incubation was also monitored. There was
almost complete inhibition of both parameters at
10-6 mol TBT/litre but no effect at 10-7 mol/litre. In-
hibition of phagocytosis was exactly paralleled by inhi-
bition of enzyme release. At concentrations of between
10-6 and 10-5 mol/litre, tributyltin caused lysis of the
cells with release of LDH, suggesting damage to the plasma
membrane. Exocytosis, induced by FMLP in the presence of
"cytocolasin B", was also inhibited by the TBT at con-
centrations of 10-6 mol/litre or more. There was little
effect of the compound on ATP levels in PMNs, and the
authors suggested that interference with ATP production
was not the basis for the effect of the TBT. Activation of
PMNs is accompanied by an increase in plasma membrane
permeability to Ca2+; this was strongly inhibited by the
TBT. The authors proposed two alternative explanations.
Either the Ca2+ permeability change is directly affected
or an earlier step is inhibited which, in turn, affects
calcium permeability. The observation that the exocytosis
effect could be counteracted by the addition of sulfydryl
compounds led the authors to conclude that the earliest
stages of activation of the PMNs were affected. Arakawa &
Wada (1984) reported a suppression of the chemotactic
response of rabbit leucocytes (neutrophils) towards for-
myl-methionyl-leucyl-phenylalanine by tributyltin chloride
at concentration between 0.1 and 10 µmol/litre in vitro.
Reinhardt et al. (1982) used cell detachment and
cloning efficiency of baby hamster kidney cells (BHK-21
C13) to quantify the cytotoxicity of organotin compounds.
The two parameters are independent, but each covers a
range of cellular damage. Cell detachment indicates irre-
versible damage to the cytoskeleton over a 4-h period,
while cloning efficiency covers influence on growth of
single cells over a 6-day period and includes cell re-
attachment followed by clone formation. The IC50 (concen-
tration at which there was a 50% reduction in cloning
efficiency) for TBTO was 5 x 10-7 mol/litre (0.3 mg/litre
medium) and for TBT chloride was 1.4 x 10-6 mol per litre
(0.5 mg/litre). The CD50 (50% effect on cell detachment)
was less sensitive to TBT, i.e. 3 x 10-5 mol/litre for
TBTO (18 mg/litre) and 4.3 x 10-5 mol/litre for TBT
chloride (14 mg/litre).
When Snoeij et al. (1986a) incubated isolated rat
thymocytes with TBT chloride, there was membrane damage
and disintegration of the cells at concentrations higher
than 1 µmol/litre. Various parameters of cell function
were investigated. The TBT chloride increased the consump-
tion of glucose in a dose-related manner at concentrations
in the culture medium higher than 0.1 µmol/litre; pro-
duction of lactate was also increased in parallel. The ef-
fect peaked at 1 µmol TBT/litre and thereafter declined;
damage to cell integrity occurred at these concentrations.
Over the same effective range of concentrations (0.1 to
1 µmol/litre), TBT decreased ATP levels. The ATP dimin-
ished very rapidly, within 2.5 min, and remained low for
at least 3 h. The incorporation of radiolabelled nucleo-
tide precursors into DNA and RNA and of amino acids into
protein was also affected. Thymidine incorporation into
DNA was reduced, relative to TBT dose, to a minimum of 20%
of control incorporation at 1 µmol TBT/litre. Uridine
incorporation was similarly reduced, but less effectively.
Inhibition of amino acid incorporation was substantial at
0.25 µmol TBT chloride/litre and protein synthesis was
virtually totally inhibited at 1 µmol/litre. The median
inhibitory concentrations (IC50) for thymidine, uridine,
proline, and leucine were 0.32 ± 0.04, 0.95 ± 0.06, 0.38
± 0.04, and 0.36 ± 0.03 µmol/litre, respectively. TBT
chloride concentrations of 0.1 µmol/litre or more mark-
edly reduced the production of cyclic AMP by the
thymocytes under stimulation from prostaglandin E1. TBT
chloride, therefore, has marked effects on the energy
metabolism of isolated thymocytes at concentrations well
below those affecting membrane integrity, and, as a
result, the authors stated that TBT chloride is best
characterized as an energy poison.
Snoeij et al. (1988a) similarly isolated rat thymo-
cytes, but separated them into fractions based on size.
The same parameters of cell function were investigated in
each of three fractions. Fraction 1 consisted of small,
non-proliferating thymocytes. Fractions 2 and 3 showed
some overlap in size, but fraction 2 was enriched in cells
actively synthesizing macromolecules, while fraction 3 was
enriched in dividing cells and showed the greatest pro-
liferative capacity. Resting ATP levels were highest in
the bigger cells, steady state levels increasing with
fraction number. TBT chloride reduced ATP levels in each
subfraction proportionally to between 62 and 64% of con-
trol values. Thus, TBT reduces intracellular ATP concen-
trations irrespective of cell volume or number of mito-
chondria. As with unfractionated thymocytes, TBT chloride
at both 0.25 and 1.0 µmol/litre inhibited thymidine
incorporation (to around 40% of control values at
0.25 µmol/litre and between 21 and 37% of control values
at 1 µmol/litre) and leucine incorporation (to between 13
and 29% of controls at 0.25 µmol/litre and 1 to 3% of
controls at 1 µmol/litre) in all fractions. In the case
of uridine incorporation, although there was inhibition in
most cases, 0.25 µmol TBT chloride/litre caused a stimu-
lation to 184% of control levels in fraction 1, the non-
proliferating cells.
11.2. Short-term toxicity
Summary
TBT compounds have been studied most extensively in the rat
(all the data in this section refer to the rat unless otherwise
indicated).
At dietary doses of 320 mg/kg (approximately 25 mg/kg body
weight), high mortality rates were observed when the exposure
time exceeded 4 weeks. No deaths were noted at 100 mg/kg diet
(10 mg/kg body weight) or after daily administration of 12 mg
per kg body weight by gavage. In rats dosed during early post-
natal life, 3 mg/kg body weight resulted in increased deaths.
The main symptoms at lethal doses were loss of appetite, weak-
ness, and emaciation.
Borderline effects on rat growth were observed at 50 mg/kg
diet (6 mg/kg body weight) and 6 mg/kg body weight (gavage
studies). Mice are less sensitive, effects being observed at
150 to 200 mg/kg diet (22 to 29 mg/kg body weight).
Structural effects on endocrine organs, mainly the pitu-
itary and thyroid, have been noted in both short- and long-term
studies. Changes in circulating hormone concentrations and
altered response to physiological stimuli (pituitary trophic
hormones) were observed in short-term tests, but after long-
term exposure most of these changes appeared to be absent. The
mechanism of action is not known.
Exposure to TBTO aerosol at 2.8 mg/m 3 produced high mor-
tality, respiratory distress, inflammatory reaction within the
respiratory tract and histopathological changes of lymphatic
organs. However, exposure to the highest attainable vapour
concentration (0.16 mg/m 3 ) at room temperature produced no
effects.
Toxic effects on the liver and bile ducts have been
reported in three mammalian species. Hepatocellular necrosis
and inflammatory changes in the bile duct were observed in rats
fed TBTO at a dietary level of 320 mg/kg (approximately 25
mg/kg body weight) for 4 weeks and in mice fed 80 mg/kg diet
(approximately 12 mg/kg body weight) for 90 days. Vacuolization
of periportal hepatocytes was noted in dogs fed a dose of 10
mg/kg body weight for 8 to 9 weeks. These changes were oc-
casionally accompanied by increased liver weight and increased
serum activities of liver enzymes.
Decreases in haemoglobin concentration and erythrocyte vol-
ume in rats, resulting from dosing with 80 mg/kg diet (8 mg/kg
body weight), indicate an effect on haemoglobin synthesis,
leading to microcytic hypochromic anaemia. The decrease in
splenic haemosiderin levels suggests alterations in iron
status. Anaemia has also been observed in mice.
The formation of erythrocyte rosettes in mesenteric lymph
nodes has been observed in certain short-term investigations
but not in long-term studies. The biological significance of
this finding (possibly transient) is unclear.
The characteristic toxic effect of TBTO is on the immune
system; due to effects on the thymus, the cell-mediated func-
tion is impaired. The mechanism of action is unknown, but may
involve the metabolic conversion to dibutyltin compounds. Non-
specific resistance is also affected.
General effects on the immune system (e.g., on the weight
and morphology of lymphoid tissues, peripheral lymphocyte
counts, and total serum immunoglobulin concentrations) have
been reported in several different studies with TBTO using rats
and dogs, but not mice, at overtly toxic dose levels (effects
in mice have been seen with tributyltin chloride at 150 mg/kg).
Only the rat exhibits general effects on the immune system
without other overt signs of toxicity and is clearly the most
sensitive species. The NOEL in short-term rat studies was
5 mg/kg diet (0.6 mg/kg body weight). In studies with tributyl-
tin chloride, analogous effects on the thymus were seen. These
were readily reversible when dosing ceased. TBTO has been shown
to compromise specific immune function in rat in vivo host re-
sistance studies. Decreased clearance of Listeria monocytogenes
was seen after exposure to a dietary level of 50 mg/kg (the
NOEL being 5 mg/kg per day), and decreased resistance to
Trichinella spiralis was seen at 50 and 5 mg/kg diet, but not
at 0.5 mg/kg diet (2.5, 0.25, and 0.025 mg/kg per day body
weight, respectively). Similar effects were seen in aged ani-
mals, but these were less pronounced.
With present knowledge, the effects on host resistance are
probably of most relevance in assessing the potential hazard to
man, but there is insufficient experience in these test systems
to fully assess their significance. However, some data on the
significance of the T. spiralis model are provided by findings
in athymic nude rats after the standard challenge. In these
studies, the complete absence of thymus-dependent immunity
resulted in a 10- to 20-fold increase in muscle larvae counts;
by contrast, exposure to TBTO concentrations of 5 and 50 mg/kg
diet resulted in a 2-fold and a 4-fold increase, respectively.
Although some data are now available from studies on the
effects of tributyltin compounds on the developing immune sys-
tem, there is no information on host resistance.
It would be prudent to base assessment of the potential
hazard to humans on data from the most sensitive species.
Effects on host resistance to T. spiralis have been seen at
dietary levels as low as 5 mg/kg (equivalent to 0.25 mg/kg per
day body weight), the NOEL being 0.5 mg/kg (equivalent to
0.025 mg/kg per day). However, the interpretation of the sig-
nificance of these data for human risk assessment is contro-
versial. In all other studies a concentration of 5 mg/kg per
day in the diet (equivalent to 0.5 mg/kg body weight, based on
the short-term studies) was the NOEL with respect to general,
as well as specific, effects on the immune system.
11.2.1. Oral dosing: general body effects
Iwasaki et al. (1976) reported some oedema and
destruction of nerve axons following the administration of
0.01 ml TBTO/kg directly to the stomach of rats every day
for 28 days. No details of procedures or results were
presented in this report.
Schweinfurth (1985) found no evidence of brain oedema
after dosing rats with TBTO orally in arachis oil. Even
doses producing marked toxic effects on other organs (25
mg/kg body weight) failed to produce noticeable brain
oedema. However, triethyltin chloride produces brain
oedema at a dose of 1.5 mg/kg body weight.
Krajnc et al. (1984) investigated the short-term
effects of bis -tributyltin oxide in the rat. In exper-
iments lasting 4 weeks, Wistar rats were fed technical
TBTO at levels of 0, 5, 20, 80, or 320 mg/kg diet. All
animals survived the dosing period. Various symptoms of
poisoning were seen within 1 week of dosing in the group
fed 320 mg/kg diet, i.e. weakness, emaciation, roughened
fur, and blood-tinged discharge around the eyes and nose.
Some of these signs were seen at the lower doses after 4
weeks. Body weight gain was not affected by doses up to
20 mg/kg diet in males and 80 mg/kg in females. Males
showed reduced weight gain (96 g compared to control
weight gain of 117 g) over 4 weeks at 80 mg TBTO/kg. At
320 mg/kg diet, TBTO caused a reduction in body weight of
13 g in males and 21 g in females. Almost all of this
weight loss occurred in the first week of dosing, body
weight remaining constant after that. A 50% reduction in
food consumption was seen at this dose rate, compared to
control animals. Urine analysis, during the fourth week
of dosing, revealed no differences in urine volume, pro-
tein concentration, or creatinine clearance related to
treatment.
Funahashi et al. (1980) conducted histopathological
and biochemical studies on Sprague-Dawley rats given bis-
tributyltin oxide (TBTO) dissolved in olive oil. The rats
were dosed by intubation 5 times weekly for 13 or 26
weeks. Body weight was reduced after 13 weeks dosing at
6 mg/kg body weight, but not significantly so. After 26
weeks, body weight was significantly reduced relative to
controls (382 g compared to the control value of 439 g).
Dosing at 12 mg/kg reduced body weight significantly at
both 13 and 26 weeks (to 311 and 356 g, respectively).
There was a slight decrease, but unrelated to either dose
or time, in spleen weight. This decrease was just signifi-
cant at 6 mg/kg over 13 weeks and 12 mg/kg over 26 weeks,
but at no other time or dose.
When Mushak et al. (1982) dosed neonatal rats with TBT
acetate at levels of 1, 3, or 10 mg/kg per day from day 2
to day 29 of age, all rats given 1 mg/kg per day survived
with no apparent gross or histopathological effect. Totals
of 9 out of 24 and 17 out of 24 rats survived at dose
levels of 10 and 3 mg/kg per day, respectively, but all
showed reduced body weight. There were liver effects in
survivors.
11.2.2. Inhalation studies
A "nose only" inhalation study (lasting 4-5 weeks)
by Schweinfurth (1985) with rats exposed for 4 h/day (on
weekdays only; 21 to 24 exposure periods) to an aerosol of
TBTO (2.8 mg/m3) produced mortality (50% of males and
60% of females), apathy, and respiratory distress. Food
consumption and body weight gain were reduced. There were
inflammatory reactions within the respiratory tract and
lymphotoxic effects (depletion of lymphocytes in the
thymic cortex, atrophy of the thymus, and lymph nodes).
Inhalation of TBTO vapour/air mixtures produced no observ-
able effect. A concentration of 0.16 mg/m3 in the inha-
lation chamber, which corresponds to the equilibrium
vapour pressure of TBTO at room temperature, was con-
sidered to be the NOEL for rats.
When Gohlke et al. (1969) exposed rats to TBT chloride
in a 4-month inhalation study at nominal concentrations of
4 to 6 mg/m3, all animals survived the exposure. Towards
the end of the exposure, in the final month, the rats
showed minor irritation of the eye and nose. There was an
initial increase in relative liver weight but a signifi-
cant reduction over the whole experimental period. Fat
droplets were seen in the livers at autopsy, together with
a diffuse oedema of the brain. However, controls also
showed oedema. The oedema disappeared slowly as recovery
time after exposure increased. There were also inflamma-
tory changes in the respiratory tract of exposed animals.
Crofton et al. (1989) exposed pregnant female rats to
TBTO by intubation at 0 to 10 mg/kg per day on days 6 to
20 of gestation. There was no effect on the age at which
the testes of male offspring descended. However, females
showed a delay of approximately 2 days in vaginal opening,
compared to controls, after their mothers were exposed to
10 mg/kg per day.
11.2.3. Histopathological effects
Snoeij et al. (1985) reported that weanling rats fed
tributyltin chloride at a level of 150 mg/kg diet showed
marked reductions in body weight (treated rats, 87 g; con-
trol rats, 119 g) and brain weight (treated rats, 1.54 g;
control rats, 1.67 g) associated with a reduced food
intake of 25%. Thymus weight was reduced to 39% of the
control value over the 2-week feeding period.
Krajnc et al. (1984) found histopathological changes
in male and female Wistar rats exposed to 5, 20, 80, or
320 mg TBTO/kg diet for 4 weeks. No treatment-related
changes were found in the brain, heart, kidney, pancreas,
adrenals, popliteal lymph nodes, intestinal tract, or bone
marrow. Slight atrophy of hepatocytes (reduction in size)
in the centrilobular region was noted in some livers at
80 mg/kg and marked atrophy in 16 out of 20 livers at 320
mg/kg. The authors reported dystrophic calcification in
the liver at the highest dose level. Three animals showed
multiple-focus inflammation with necrosis of hepatic
parenchyma, which was associated with mononuclear and
polymorphonuclear infiltration, fibrosis, and hyperplasia
of the intrahepatic bile duct. In one animal there was
inflammation of the common bile duct. No bacteria or
viruses were found in the lesions. Similar necrotic
lesions were observed, in two animals, in a repeat study
at 320 mg TBTO/kg diet.
Mori et al. (1984) painted the shaved dorsal skin of
guinea-pigs daily for 50 days with an ethanolic solution
of TBTO at 10 or 40 mg/kg body weight. Monitoring of urine
and blood electrolyte levels during the course of the
treatment showed increased loss of sodium, chloride, phos-
phate, glucose, and amino acids in the urine. There was a
concomitant loss of electrolytes from serum. The effect
was most marked between 40 and 50 days of treatment. His-
tological lesions of the kidney tubules were observed when
the animals were killed after the 50th day.
11.2.4. Haematological and biochemical effects
Measurements of blood biochemical parameters of rats
fed TBTO for 4 weeks indicated few significant effects at
dietary dose levels below 320 mg/kg diet. Alanine amino
transferase (ALAT) activity was significantly increased,
in a dose-related manner, at 20 mg/kg or more, in both
males and females. The highest dose significantly de-
creased blood glucose in males, aspartate amino transfer-
ase (ASAT) in both males and females, and liver glycogen
in both sexes. Serum triglycerides, alkaline phosphatase,
and creatinine kinase activities were unaffected at any
dose level, as were blood lactate and pyruvate. At 80
mg/kg diet, TBTO significantly reduced blood haemoglobin
in both sexes and haematocrit in females. Mean erythro-
cytes volume was reduced in both sexes, as was mean cor-
puscular haemoglobin content (mass), but mean corpuscular
haemoglobin concentration and erythrocyte numbers were not
affected (Krajnc et al., 1984). A 6-week study at dietary
doses of 5, 20, and 80 mg/kg showed significant reductions
in haematocrit at 20 and 80 mg/kg and in blood haemoglobin
levels at 80 mg/kg. Iron concentration was reduced at 80
mg/kg, as was the isocitrate dehydrogenase (ICDH) activity
of erythrocytes. Numbers of erythrocytes, thrombocytes,
and reticulocytes were not significantly affected at any
dose level of TBTO, though there was a trend towards
increased numbers of reticulocytes. The authors suggested
an effect on haemoglobin synthesis and other indicators,
implying either reduced iron uptake or increased iron
loss. The enhanced ICDH activity and increasing reticulo-
cyte numbers indicated the presence of immature erythro-
cytes, and the authors could not exclude the possibility
of an in vivo haemolytic action of TBTO comparable to the
reported in vitro haemolysis (Krajnc et al., 1984).
Rosenberg et al. (1984) measured the activity of haem
oxygenase in mucosal cell fractions from control mice and
mice dosed by intubation with TBTO at 60 mg/kg body
weight. The activity of the enzyme, monitored by the
bilirubin absorbance spectrum, was substantially elevated,
compared to controls, 16 h after administration of the
TBTO. The activity of the same enzyme in liver and kidney
microsomes was not affected by this dose of TBTO given by
gavage, but was elevated when TBTO was given parenterally.
The activities of cytochrome P-450 and benzo(a)pyrene
hydroxylase were substantially reduced in the intestine of
TBTO-treated mice, this reduced activity being statisti-
cally significant for the latter but not for the former.
Similar results were obtained in liver fractions when TBTO
was applied parenterally.
11.2.5. Effects on lymphoid organs and immune function
Funahashi et al. (1980) reported the effects of feed-
ing TBTO in olive oil (0, 3, 6, or 12 mg/kg body weight
per day) for 13 or 26 weeks by gavage to groups of ten
male Sprague-Dawley rats, aged 5 weeks at the beginning of
the study. No analyses of haematology, immunoglobulin
levels, or specific aspects of immune function were per-
formed. A marked dose-related reduction in absolute and
relative thymus weight was seen following dosing for both
periods. Relative thymus weights were 682, 629, 340, and
313 mg/kg body weight after 13 weeks of dosing and 449,
313, 278, and 248 mg/kg body weight after 26 weeks of
dosing in the rats given 0, 3, 6, and 12 mg/kg per day,
respectively. All results, with the exception of those for
the group given 3 mg/kg body weight per day for 13 weeks,
were statistically significant (p < 0.003). Despite the
considerable reductions in thymus weight, the only histo-
logical observation was a slight reduction in the width of
the thymic cortex. A dose-related reduction in relative
spleen weight was seen after 26 weeks, which was statisti-
cally significant (p < 0.05) at the dose level of 12 mg/kg
body weight per day. Reduced body weight and increases in
relative pituitary and relative adrenal weights were stat-
istically significant (p < 0.01) at 12 mg/kg body weight
per day for 13 and 26 weeks and at 6 mg/kg body weight per
day for 26 weeks, and there was a significant (p < 0.05)
increase in relative adrenal weight at 6 mg/kg body weight
per day for 13 weeks. The relative pituitary weight was
also increased following 26 weeks of dosing at 3 mg/kg
body weight per day (p < 0.01). Based on thymus weight,
the NOEL was the most sensitive end-point in this study,
3 mg/kg body weight per day for 13 weeks. However, an
effect was seen at this dose when it was given for 26
weeks.
Funahashi et al. (1980) also reported that the
reduction in relative thymus weight, present 3 days after
a single dose of TBTO in olive oil of 100 mg/kg body
weight to 5-week-old male Sprague-Dawley rats, showed
signs of reversal at day 8. The reversibility of TBT-
induced thymus atrophy was also demonstrated by Snoeij et
al. (1988b). Groups of 4 or 5 young (4-5 weeks old) male
Wistar rats received a single gavage dose of TBT chloride
(0 or 16 mg/kg body weight) in corn oil. A 30% reduction
in relative thymus weight was evident in animals killed on
days 2, 3, or 4, but there was complete recovery by day 7.
A similar effect and recovery was seen in the thymus cell
counts. An equimolar dose of dibutyltin chloride produced
more pronounced effects, the recovery period being pro-
longed until day 9.
Krajnc et al. (1984) reported the effects of feeding
TBTO-containing diets (0, 5, 20, 80, or 320 mg/kg diet) to
groups (10 males and 10 females per group) of young SPF
Wistar rats for 4 weeks. These dose levels are equivalent
to approximately 0, 0.5, 2, 8, or 32 mg/kg body weight per
day, respectively, based on actual food consumption
measurements. Total leucocyte and circulating lymphocyte
counts were significantly reduced in males receiving 80
mg/kg diet (p < 0.01) and in both sexes receiving 320
mg/kg diet (p < 0.001). Eosinophil counts were signifi-
cantly reduced (p < 0.05) in males receiving 5, 80, or 320
mg/kg diet and in females given the highest dose. Monocyte
counts were increased significantly (p < 0.05) in males
receiving 20, 80, or 320 mg/kg diet but not in females.
Total immunoglobulin levels were significantly affected at
80 and 320 mg/kg diet. At 80 mg/kg diet, TBTO signifi-
cantly increased serum IgM to 132% (p < 0.01) and 145%
(p < 0.001) of control levels for males and females,
respectively. At the same dose, IgG in males was reduced
significantly (p < 0.05), but in females IgG was unaffec-
ted. A significant reduction (p < 0.001) in serum IgG was
found in both sexes fed TBTO at 320 mg/kg diet. Thymus and
relative thymus weights were significantly reduced in both
sexes (p < 0.001) at 80 and 320 mg/kg diet and in females
given 20 mg/kg diet (p < 0.05). Relative spleen weight
was significantly increased in males receiving 320 mg/kg
diet but this was probably related to the decreased body
weight of these animals. Histological examination showed
that all the animals given the highest dose exhibited
lymphocyte depletion from the thymic cortex, resulting in
an indistinct cortico-medullary junction and an increase
in ceroid/lipofuschin-loaded macrophages. Slight atrophy
of the thymic cortex was seen in two males fed 80 mg/kg
diet. Diffuse atrophy of the white pulp of the spleen was
seen in all animals at 320 mg/kg, the periarteriolar lym-
phocyte sheaths (PALS) being particularly affected. At 80
mg/kg diet, one male and two females showed slight splenic
atrophy. Depletion of T lymphocytes, determined by pan-T
immuno-staining, was seen in the PALS at 320 mg/kg diet.
There was an increase in the incidence of mesenteric lymph
nodes atrophy, observable in some animals fed 20 mg/kg and
increasing with dose to affect all animals given 320 mg/kg
diet. Both the paracortex and medulla of lymph nodes were
reduced in size and cellularity, and the numbers and size
of follicles were reduced by the highest TBTO dose level.
Again, the total number of T lymphocytes was strikingly
reduced in the paracortex by TBTO at 320 mg/kg diet, as
determined by immuno-staining. Although thymic involvement
was marked, it was not only thymus-dependent areas that
were affected. In rats exposed to the highest dose level,
which was overtly toxic, B lymphocyte areas also showed
low level activity, as indicated by fewer follicles and
inconspicuous germinal centres in lymph nodes and spleen.
An increase in the number of animals with rosettes in
sinuses in the mesenteric lymph nodes, composed of eryth-
rocytes surrounding mononuclear cells, was seen. This was
dose related: half of the animals dosed at 5 mg/kg and all
the animals treated with 80 or 320 mg/kg showed erythro-
cyte rosettes. The biological significance of these
rosettes is unclear. Specific aspects of immune function
were not tested. In further studies (personal communi-
cation by E.I. Krajnc to IPCS, 1989; Wester, in press), no
increase in rosette formation was observed at 5 mg/kg diet
or 50 mg/kg diet. Signs of general toxicity were observed
in animals treated with 320 mg/kg diet, i.e. reduced body
weight (p < 0.001) and increased serum ALAT activity. At
80 mg/kg, there was reduced body weight gain in males
(p < 0.05) and increased ALAT, and at 20 mg/kg increased
ALAT in males only. The liver was the only non-lymphoid
organ displaying histological changes: centrilobular hepa-
tocyte atrophy, reduced glycogen retention, parenchymal
necrosis, and hyperplasia of the intrahepatic bile duct
were seen in some animals treated at 320 mg/kg diet. Three
animals from the group given 80 mg/kg showed slight hepa-
tocyte atrophy. This study indicated a NOEL of 5 mg/kg
diet (approximately 0.5 mg/kg body weight per day).
Vos et al. (1984) investigated functional aspects of
the immunological effects reported by Krajnc et al. (1984)
in in vivo and ex vivo experiments using weanling (3 to 4
weeks old) male SPF Wistar rats fed diets containing TBTO.
Haematological parameters were not studied. Levels of
total circulating immunoglobulins (IgG and IgM) were
determined in animals fed diets containing 80 or 320 mg
TBTO/kg. At 320 mg/kg diet, IgM was significantly
increased (p < 0.05) and IgG was significantly decreased
(p < 0.001) on day 28 (no assays were performed on day 42
for this group). At 80 mg/kg diet, IgM was elevated by 30%
on day 42 (p < 0.01), while IgG was decreased by 30% on
days 28 and 42.
The effects of TBTO on lymphocyte counts and cell
viability in lymphoid organs was investigated, and several
specific tests of immune system function were also per-
formed. Numbers of B and T lymphocytes were significantly
reduced in the spleens (by 15% and 48%, respectively) of
animals fed diets containing 80 mg/kg diet. The ratio of
total T:B lymphocytes was decreased in a dose-related
manner following 9 weeks of exposure.
A significant reduction occurred in the numbers of
viable cells (trypan blue exclusion) obtained from the
thymus, spleen, and bone marrow of rats treated with TBTO
at 80 or 320 mg/kg diet. At 320 mg/kg diet, viability and
cell numbers were significantly (p < 0.05) reduced for
both thymus and spleen following 3, 8, and 20 days of ex-
posure, and bone marrow counts were significantly reduced
on days 8 and 20. Doses of 80 mg/kg diet produced signifi-
cant reductions in thymus cell count and viability on day
8 and thymus, spleen, and bone marrow counts on day 20.
The antibody response to sheep erythrocytes (ip),
tetanus toxoid (iv), ovalbumin (sc into the foot pad, in-
cluded H37Ra adjuvant), and the worm Trichinella spiralis
(oral) was assessed in animals receiving 20 or 80 mg TBTO
per kg diet for 6 weeks. The primary response to sheep
erythrocytes (0.5 ml of a 20% suspension, ip) was deter-
mined 10 days post inoculation (pi) and the secondary
response was determined on day 20 pi following an iv
booster inoculation on day 15 pi. The primary response was
unaffected, but there was a dose-related decrease in the
secondary response seen both in untreated and 2-mercapto-
ethanol-treated (IgM-inactivated) sera, reaching statisti-
cal significance (p < 0.05) in treated sera from the
highest-dose group. The response (IgG and IgM titres) to
tetanus toxoid inoculation measured only on day 21 pi was
equivocal, and that to ovalbumin measured on days 15, 21,
and 28 pi was unaffected by TBTO. IgG response to oral T.
spiralis infection was significantly (p < 0.05) increased
on day 21 pi at 20 mg/kg diet, but not on day 42 or at 80
mg/kg diet at either time. IgM response was unaffected.
IgE response, possibly the most relevant to resistance to
parasitic infection, was decreased in a dose-related man-
ner on days 21 and 42 following T. spiralis infection; log
titres were 3.8, 2.5 (p < 0.05), and 1.8 (p < 0.001) on
day 21 and 4.4, 3.6, and 3.4 (p < 0.05) on day 42 in the
control, 20-, and 80-mg/kg groups, respectively. Delayed-
type hypersensitivity was determined as change in skin
thickness following a challenge of ovalbumin to the skin
of the ear or tuberculin challenge to the skin of the
flank, made 3 and 4 weeks, respectively, following initial
immunization (sc into a footpad) 6 weeks after starting
dietary dosing of TBTO at 20 or 80 mg/kg. Compared to
controls similarly injected intradermally with medium,
significant reductions in response to ovalbumin challenge
were found 24, 48, and 72 h after dosing at 20 mg/kg (all
p < 0.05), and 24 h (p < 0.01) and 48 h (p < 0.05) after
dosing at 80 mg/kg. With tuberculin challenge, significant
effects were found in the group given 80 mg/kg diet after
24 h (p < 0.01), 48 h (p < 0.001), and 72 h (p < 0.001),
but only after 72 h (p < 0.05) at the lower dose level.
Reduced responses were seen at all three times after both
doses.
Thymus or spleen cells, obtained from rats fed TBTO at
20 or 80 mg/kg diet, were cultured with and without
mitogens (PHA = phytohaemagglutinin; Con A = concanavalin
A; PWM = pokeweed mitogen) for 24 h before the addition
of 3H-thymidine to monitor DNA synthesis. PHA and Con A
are both T cell mitogens and PWM is a mitogen for both T
and B cells. Due to a reduction in the number of viable
cells in thymic cultures from the high-dose group, respon-
siveness to mitogens was expressed per culture and per
thymus. Significant reductions in 3H-thymidine uptake
(expressed per thymus) were found in unstimulated (48%
reduction), PHA-treated (64%), Con A-treated (62%), and
PWM-treated (50%) cultures derived from the high-dose
group; PHA (48%) and PWM (28%) were the only responses
reduced at 20 mg/kg diet. Similar effects were seen on
splenic cultures, with a reduced number of viable cells
per spleen and significant reductions in response
(expressed per spleen) to PHA (50% reduction) and Con A
(45%) in cultures from the high-dose group; responses to
PWM and E. coli lipopolysaccharide (a B cell mitogen)
were significantly increased on a per culture basis but
not a per spleen basis. Cultures from the low-dose group
showed reduced response to PHA (25% reduction) and Con A
(20%), but these were not statistically significant.
TBTO, at both 20 and 80 mg/kg diet, reduced the
resistance of rats to infection by T. spiralis. The number
of worm larvae in muscle significantly increased in a
dose-dependent manner (74 000 in controls; 106 000 in rats
fed 20 mg TBTO/kg, p < 0.01; 198 000 in rats fed 80 mg/kg
p < 0.001) after standard infection with 1000 larvae by
mouth. The number of adult worms in the small intestine
also increased significantly after 10 (p < 0.05), 12
(p < 0.001), and 14 (p < 0.01) days in the high-dose
group, and after 12 (p < 0.05) and 14 (p < 0.001) days in
the low-dose groups. In a study of the inflammatory reac-
tion around larva-containing muscle cells in the tongues
of animals killed 14 days after infection, the response
(mononuclear cells and eosinophilic granulocytes) after
treatment with TBTO at 20 mg/kg diet was described as
"slightly reduced". However, there was a marked
reduction after treatment at 80 mg/kg diet.
The clearance of Listeria monocytogenes from the
spleen (a measure of host resistance) was monitored in
rats fed 20, 80, or 320 mg TBTO/kg diet for 6 or 7 weeks.
Statistically significant increases in the number of
viable bacteria were seen 2 days after an iv injection
into animals treated with 320 mg/kg diet for 6 (p < 0.01)
or 7 (p < 0.001) weeks and with 80 mg/kg diet for 6 weeks
(p < 0.001). A dose-related reduction, statistically sig-
nificant (p < 0.05) at 80 mg/kg diet, in viable bacteria
was seen 1 day post injection in the group receiving TBTO
for 7 weeks, but this was not seen in the 6-week study.
The ex vivo phagocytosis of L. monocytogenes by spleen-
and peritoneal-derived macrophages from TBTO treated (20
or 80 mg/kg diet) animals was not significantly affected,
although a dose-related reduction in the phagocytic
activity of splenic macrophages was seen.
Cells derived from the spleen and peritoneal cavity
were tested for spontaneous cell-mediated cytotoxicity
against murine YAC lymphoma target cells labelled
with 51Cr. "Specific release" of the chromium label
(release in experimental minus spontaneous release in the
controls) was used as the end-point of the assay. Stat-
istically significant reductions in specific release were
found with spleen cells from rats fed TBTO at 80 mg/kg
diet (p < 0.05), but not in spleen cells from those fed 20
mg/kg diet. Statistically significant effects (p < 0.05)
were found in peritoneal macrophages derived from rats
receiving 20 and 80 mg/kg diet, but there were no signifi-
cant effects on non-adherent cells ("natural killer
cells").
These studies were designed to investigate immune
function rather than to determine a no-observed-effect-
level, and the lowest dose used (20 mg/kg diet) produced
statistically significant effects (in particular, de-
pression of host resistance to T. spiralis and L. mono-
cytogenes ). No evidence of general toxicity was seen at
this dose level. The only sign of general toxicity
recorded was a significant reduction in body weight gain
seen after exposure to 320 mg/kg diet for 3, 8, and 20
days and to 80 mg/kg diet for 20 days.
A study of TBTO (0, 0.5, and 50 mg/kg diet) adminis-
tered to groups of weanling Wistar rats (five animals of
each sex per group) for 2 years was briefly reported by
Vos et al. (1985) and Wester (in press). It should be
noted that the intakes in this study were lower, on a body
weight basis, than in the shorter-term studies, being
equivalent to 0, 0.025, 0.25, and 2.5 mg/kg body weight
per day for the controls, and low, medium, and high doses,
respectively (based on actual intake measurements). A
significant decrease in peripheral lymphocyte count and a
statistically significant increase in platelets were seen
in the females fed 50 mg/kg diet for 1 year. Circulating
levels of total IgM and IgA were significantly increased
at 4-6 and 16-18 months, in rats given 50 mg/kg diet,
while significant reductions in IgG levels were recorded,
particularly in females.
General effects on the lymphoid organs were not
recorded, though several specific tests of immune function
were performed using the methods of Vos et al. (1984). A
dose-related decrease in resistance to T. spiralis infec-
tion was seen at 5 and 16 months, achieving statistical
significance (p < 0.05) at 5 and 50 mg/kg diet. IgE titres
were reduced, but IgA levels increased approximately 50-
fold at the highest dose level. No effects on delayed-type
hypersensitivity were seen, in contrast to results in the
short-term study by Vos et al. (1984). Host resistance to
L. monocytogenes (as measured by the number of viable
organisms in the spleen) was significantly reduced at 5
and 17 months in the 50-mg/kg group, but a significant
increase was seen at 17 months in the 5-mg/kg group.
Natural killer cell activity against YAC lymphoma cells
was reduced significantly at the highest dose level after
15-17 months. It will not be possible to fully assess this
study until the final report is published, although it
would appear that 0.5 mg/kg diet (0.025 mg/kg body weight
per day) was the NOEL.
A preliminary report on a study where diets containing
TBTO (0, 0.5, 5, or 50 mg/kg diet) were fed to Wistar rats
(12 months old) for 6 months, has been issued (personal
communication by E.I. Krajnc to IPCS, 1989). Specific
tests of immune function were measured after 5 months of
dosing. Significant (p < 0.05) reductions in host resist-
ance to T. spiralis and L. monocytogenes were seen at
50 mg/kg only. These results suggest that aged rats are
less sensitive to TBTO in the diet than weanlings animals,
although this may be due to a lower intake on a per kg
body weight basis. Full assessment of this study will need
to await the completion of the statistical analyses and
final report.
A series of studies have been performed to investigate
certain aspects of immune function (Schering, 1989a,b,c,d).
When groups (10 animals of each sex) of young (4 to 5
weeks old) Sprague-Dawley rats were fed diets containing
TBTO (0, 0.5, 2, 5, or 50 mg/kg diet) for 4 weeks, no sig-
nificant effects were seen on total or differential white
blood cell counts. Serum immunoglobulin levels were not
measured. A statistically significant decrease in absolute
and relative thymus weight (p < 0.01) was seen in males
fed 50 mg/kg. A decrease in absolute and relative spleen
weight was also seen in this group, though it was not sig-
nificant statistically. The viability of cultured spleen
cells (monitored with trypan blue exclusion) obtained from
females fed 5 mg/kg was reduced (p < 0.05), but other
groups were unaffected. A significant decrease in total
thymus cell count was seen in preparations from males fed
50 mg/kg only (p < 0.05). A slight but significant
(p < 0.05) reduction in the thickness of the thymic cortex
was also seen in these males, this being the only signifi-
cant histological finding. Specific measures of immune
function were not performed in this study but in parallel
studies. The NOEL in this study was 5 mg/kg diet
(approximately 0.6 mg/kg body weight per day, based on
measured intake) (Schering 1989a).
An assay of plaque-forming cells, a measure of humoral
immunity, revealed no effects due to TBTO (at levels of
0.5, 2, 5, or 50 mg/kg diet) fed to groups (10 animals of
each sex) of young (4 to 5 weeks old) Sprague-Dawley rats
for 5 weeks. The response was measured on day 36, follow-
ing an iv inoculation of sheep erythrocytes. The only sign
of toxicity was a reduction in body weight in males fed
50 mg/kg, but this was not statistically significant. No
investigation of general toxicity was performed. The NOEL
was 50 mg/kg diet (approximately 5.6 mg/kg body weight per
day) in this study (Schering, 1989b).
To assess the effects of TBTO on host resistance to
infection, the number of viable Listeria monocytogenes
cells in the spleen, 4 days after inoculation with
1.4 x 106 cells, was counted. No effects were produced in
groups (10 animals of each sex) of young Sprague-Dawley
rats fed diets containing TBTO at 0.5, 2, or 5 mg/kg diet
for 34 days. However, statistically significant increases
in numbers of viable bacteria were seen in the spleen of
males (p = 0.055) and females (p < 0.01) fed 50 mg/kg
diet. No investigations of general toxicity were per-
formed. The NOEL was 5 mg/kg diet (approximately 0.6 mg/kg
body weight per day) (Schering, 1989c).
The effects of TBTO on delayed-type hypersensitivity
reactions, a measure of cell-mediated immunity, were
assessed in groups (10 animals of each sex) of 4- to 5-
week-old Sprague-Dawley rats fed diets containing 0, 0.5,
2, 5, or 50 mg/kg diet for 37 days. A sensitizing dose of
100 µg bovine serum ablumin (BSA) mixed with Freund's
complete adjuvant was given on day 29, followed by a chal-
lenge dose of heat-inactivated BSA injected into a hind-
foot pad on day 37. No difference in response (increased
footpad thickness) was seen between test and control
groups. Body weight gain was unaffected, but no other
investigations of general toxicity were performed. The
NOEL in this study was 50 mg/kg diet (approximately 5.8
mg/kg body weight per day) (Schering 1989d).
The effects of inhaled TBTO were studied in groups (10
animals of each sex) of young (initial weights 86-131 g)
SPF Wistar rats exposed to 0, 0.03, 0.16, or 2.8 mg/m3
for 4 h/day, 5 days per week, for 21 to 24 exposures. The
two lower doses were provided by filtered vapour, and the
highest dose was provided by an aerosol with over 90% of
the particles < 5 µm in diameter (Schering 1983). Lympho-
cyte and total leucocyte counts, measured at 2 or 4 weeks,
were unaffected by TBTO. Some inconsistent changes in
reticulocyte counts were found, but these appeared to be
primarily related to use of the respiration chamber and
not to TBTO exposure. Immunoglobulins were not measured.
Thymolysis and lymphocyte depletion of the thymus-depen-
dent areas of the spleen and lymph nodes were reported in
the 11 animals (five males, six females) from the highest-
dose group that died during the study. No such lesions
were detected in the survivors, although in three sur-
vivors from the highest-dose group an increase in the
number of macrophages containing nuclear debris was seen
in the thymic cortex. No significant (p > 0.05) changes
were seen in absolute or relative weights of the thymus,
spleen, or iliac lymph node in animals surviving the
study, but no organ weights were recorded for those ani-
mals dying or sacrificed during the study. No specific
aspects of immune function were studied. Histological
signs of general toxicity were limited to those consistent
with inflammatory reactions within the respiratory tract
and, with one exception, were confined to animals exposed
to the aerosol. Food intake and body weight gain were
reduced in both sexes, the reduction being statistically
significant (p < 0.01) in male rats exposed to 2.8 mg/m3.
The cause of death in the 11 animals from the highest-dose
group was not ascertained (Schering, 1983).
When TBTO (0, 4, 20, 80, or 200 mg/kg diet) was fed to
groups (10 animals of each sex) of CD-1 mice, aged 6 to 7
weeks at commencement of the study, for 3 months, leuco-
cyte counts were increased at 80 and 200 mg/kg in both
sexes, although this increase did not reach statistical
significance (p > 0.05). Immunoglobulins were not
measured. Thymus weight was reduced in both sexes at 200
mg/kg, but this was not statistically significant
(p > 0.05). Spleen weights were increased in both sexes
at 80 and 200 mg/kg (reaching statistical significance
(p < 0.05) at 200 mg/kg), possibly secondary to effects on
erythrocytes. No specific immune function tests were per-
formed. Histological changes were seen in the livers of
both sexes at 80 and 200 mg/kg. Dose-related, statisti-
cally significant (p < 0.01) increases in absolute liver
weights were seen at 80 and 200 mg/kg, and adrenal weights
were significantly (p < 0.01) increased in the male rats
fed 200 mg/kg. The NOEL in this study was 20 mg/kg diet
(approximately 4 mg/kg body weight per day) (Biodynamics,
1989a).
In studies by Schering (1989e), groups (two animals of
each sex) of beagle dogs received variable doses of TBTO
in arachis oil by oral gavage:
Group 1: controls;
Group 2: 0.1 mg/kg body weight per day for 5 weeks,
0.2 mg/kg body weight per day for 4 to 5 weeks, then
10 mg/kg body weight per day for 8 to 9 weeks;
Group 3: 0.5 mg/kg body weight per day for 5 weeks
then 1 mg/kg body weight per day for 13 to 14 weeks;
Group 4: 2.5 mg/kg body weight per day for 5 weeks
then 5 mg/kg body weight per day for 13 to 14 weeks.
All males in groups 2 and 4 died as a result of mis-dosing
to the lungs. Increased leucocyte and neutrophil counts
were seen in group 4 at weeks 9 and 18 (p < 0.05), and
there was a statistically significant increase in leuco-
cyte count recorded at 13 weeks in group 2 (p < 0.05).
Non-statistically significant reductions in leucocyte,
neutrophil, and lymphocyte counts were found in group 2 at
18 weeks. Specific immunoglobulins were not measured.
Thymus weights were reduced in the two survivors of group
2 (0.9 ± 0.1 g compared with 4.0 ± 0.6 g in controls), and
slight increases in thymus weights were seen in groups 3
and 4. Iliac and mesenteric lymph node weights were
reduced, though spleen weights were increased, in the two
survivors from group 2. Histological changes in lymphoid
organs were confined to group 2 where a reduction of lym-
phocyte numbers in the thymus, spleen (particularly PALS),
and lymph nodes was seen. A dose-related increase in rela-
tive liver weight (33.1, 41.7, 49.9, and 57.6 g/kg body
weight in groups 1, 3, 4, and 2, respectively) was
accompanied by cytoplasmic vacuolation of hepatocytes in
group 2 (Schering, 1989e).
11.2.6. Mechanism of immunotoxicity
The precise mechanism of the immunotoxic effects of
TBTO is not yet clear. However, a hypothesis has been put
forward, based on the work of Snoeij (1987) and Pieters et
al. (1989).
a) Absorbed TBTO is present as the TBT+ cation or a salt
(chloride or carbonate). Studies using TBT chloride
are, therefore, relevant to TBTO immunotoxicity.
b) As dibutyltin chloride (DBT chloride), a metabolite of
TBT chloride, is, mole for mole, more potent that TBT
chloride at producing effects in the thymus and thymic
cells, it is probable that DBT chloride or another DBT
salt is the active species in TBTO toxicity.
c) The primary action of DBT is to suspend the maturation
of immature thymocytes by inhibiting their inter-
action/binding with thymic epithelial cells. The turn-
over period of thymocytes is 3 to 4 days. Therefore,
as cell proliferation/maturation is inhibited, rapid
depletion of thymocyte numbers without cytotoxicity is
expected, followed by a rapid proliferation (observed
by Snoeij et al. (1988b) and Funahashi et al. (1980))
on removal of DBT.
Other evidence indicating that TBT compounds have a par-
ticular effect on the thymus is provided by the in vitro
studies of Snoeij (1987), which show TBT chloride to be
cytotoxic to thymocytes.
The demonstration of reduced thymus weights in certain
fish species (e.g., the freshwater guppy) indicates that
TBT may have immunotoxic effects on a wide range of
species.
11.2.7. Effects on the endocrine system
The weights of both the adrenal and pituitary glands
of Sprague-Dawley rats were significantly increased after
exposure to 6 mg TBTO/kg body weight daily, by intubation,
for 26 weeks. Pituitary weight was also significantly
increased by 3 mg TBTO/kg body weight given daily for
26 weeks (Funahashi et al., 1980).
After 4 weeks, the serum insulin concentration of rats
was not significantly affected by dosing with TBTO at up
to 80 mg/kg diet, but was undetectable (< 2 milliIU/litre)
in rats fed 320 mg/kg (control levels of insulin in serum
were 111 and 74 milliIU/litre for males and females, re-
spectively) (Krajnc et al., 1984). In a further study, the
same authors monitored endocrine changes in male Wistar
rats exposed to TBTO in the diet (0, 20, or 80 mg/kg) for
6 weeks. Serum thyroxine, thyroid stimulating hormone
(TSH), and insulin levels were reduced significantly at
the higher dose level, but serum follicle stimulating
hormone (FSH) and corticosterone levels were unaffected.
Only insulin was significantly affected at 20 mg TBTO/kg.
The luteinizing hormone (LH) concentration in serum was
significantly increased by TBTO at 80 mg/kg, but was
unaffected by 20 mg/kg. The authors further examined endo-
crine function by monitoring hormone release following
physiological stimulus. Insulin release, following iv
administration of glucose, was unaffected by a 6-week
exposure to TBTO at either 20 or 80 mg/kg diet. The effect
of TBTO on insulin was attributed by the authors to a
decreased food intake. The release of TSH, after iv admin-
istration of thyrotrophin releasing hormone (TRH), showed
a tendency (p < 0.1) to be inhibited in rats fed at 80
mg/kg. The titre of circulating TSH, 20 min after TRH
administration, was significantly reduced compared with
controls. Release of both LH and FSH, in response to
luteinizing hormone releasing factor stimulation, was
enhanced in rats fed TBTO at both 20 and 80 mg/kg diet,
but only significantly so at the higher dose rate. Histo-
logical examination of endocrine organs after a 6-week
exposure to TBTO revealed some changes. No differences
were observed in either insulin- or glucagon-producing
cells in the pancreas. Some flattening of the epithelial
lining of the thyroid follicles was observed after
exposure of rats to 80 mg/kg but not to 20 mg/kg. Immuno-
cytochemical staining of the pituitary gland identified
each cell type producing the different pituitary hormones.
There was a dose-related decrease in both the intensity of
staining of TSH cells and the number of cells stained.
Conversely, there was a dose-related increase in the
staining intensity of LH cells. No effects were found on
FSH, growth hormone, or adrenocorticotrophin cells in the
pituitary (Krajnc et al., 1984).
11.3. Long-term toxicity
Wester (in press) carried out a 106-week toxicity
and carcinogenicity study with groups of 50 weanling
Wistar rats of each sex. An additional group of 10 rats
was used for an interim sacrifice after 1 year. TBTO was
fed at 0, 0.5, 5, or 50 mg/kg diet (equivalent to 0,
0.025, 0.25, or 2.5 mg/kg body weight). Increased food
consumption occurred in all treated males (not clearly
dose-related), and there was increased water consumption
in males at 5 and 50 mg/kg. During the second year, the
body weight of the highest-dose group was significantly
lower than that of controls. Excess mortality, compared
with that of controls, was confined to the 50 mg/kg group
towards the end of the experiment (see section 11.6).
Haematological changes (anaemia, lymphopenia, and thrombo-
cytosis) and increases in plasma enzyme activities (ALAT,
ASAT, and AP) were noted mainly at the high-dose level.
Serum IgM and IgA levels increased, while the IgG level
decreased (females). No effect was observed on circulating
concentrations of T4, free T4, TSH, LH, FSH, or insu-
lin; only the free T4:T4 ratio was decreased. Organ
weight changes consisted of increased liver, kidney,
adrenal, and pituitary weights and decreased thyroid
weight. Non-neoplastic histological alterations consisted
of a decrease in cell height of the thyroid follicles (at
50 mg/kg diet after 1 and 2 years), decrease in splenic
iron content (at 5 and 50 mg/kg after 1 year only), slight
bile duct proliferation (at 50 mg/kg after 1 year only),
and vacuolation of kidney proximal tubular epithelium and
nephrosis (at 50 mg/kg after 2 years only).
11.4. Genotoxicity
Summary
The genotoxicity of TBTO has been the subject of extensive
investigation. Negative results were obtained in the vast
majority of studies, and there is no convincing evidence that
TBTO has any mutagenic potential.
Davis et al. (1987) conducted a comprehensive study of
the genetic effects of TBTO using a wide range of tech-
niques in order to assess possible hazards in the use of
the compound as a molluscicide for the control of schisto-
somiasis. TBTO did not produce gene (point) mutations in
Salmonella typhimurium strains TA1530, TA1535, TA1538,
TA97, TA98, or TA100, either in the presence or absence of
an exogenous metabolic activation system (rat liver S9).
The compound did give some evidence of gene mutation in
Salmonella typhimurium TA100 using the fluctuation method
in the presence of S9, but no dose-response relationship
was seen; negative results were seen in the absence of S9.
TBTO did not induce point mutations in the yeast
Schizosaccharomyces pombe. Negative results were obtained
when TBTO was tested in the sex-linked recessive lethal
assay using Drosophila melanogaster, the compound being
given in food and by injection, indicating that TBTO did
not produce gene mutations in Drosophila. Negative results
were also obtained when the ability of TBTO to produce DNA
damage in Bacillus subtilis (recombination assay) or the
yeast Saccharomyces cerevisiae (mitotic gene conversion)
was tested.
The ability of TBTO to produce gene mutations in Sal-
monella has also been studied by Reimann & Lang (1987).
Negative results were obtained using Salmonella
typhimurium strains TA1535, TA1537, TA1538, TA98, and
TA100, both in the presence and absence of rat S9. Simi-
larly, negative results were obtained with six TBT esters
(abietate, borate, linoleate, naphthenate, phosphate, and
tallate). Further negative results were obtained when the
ability of TBTO to produce mitotic gene conversion in
Saccharomyces cerevisiae was tested.
The ability of TBTO to produce gene mutations in
mammalian cells in vitro was extensively investigated by
Davis et al. (1987), and negative results were consist-
ently obtained. TBTO did not induce gene mutations in V79
Chinese hamster cells (using resistance to 8-azaguanine,
ovabain, or 6-thioguanine as markers) in the presence of
rat liver S9. Negative results were also obtained in the
V79 cell assays when epidermal cells of mice and humans
(primary cultures) were used as the source of metabolic
activation in cell-mediated assays.
The in vitro clastogenic potential of TBTO has been
investigated in mammalian cells in two sets of studies.
When Davis et al. (1987) used Chinese hamster ovary (CHO)
cells, harvested at 8, 15, and 24 h, an increase in struc-
tural aberrations (mainly deletions), together with
endoreduplication, was seen, but only at the highest
concentration tested (5 µg/ml in the presence of S9 and
1.5 µg/ml in its absence). The increase in the presence
of S9 was seen only after 15 h, "toxicity" precluding
any analysis of results at this concentration after 8 h.
The increase in the absence of S9 was seen only at 8 h,
there being no increase at either 15 or 24 h. The results
of these studies are difficult to interpret, since the
effect was limited to concentrations associated with high
toxicity and no data on mitotic index was reported. No
increase in sister chromatid exchange was seen at any
concentration.
Reimann & Lang (1987) used human lymphocytes to test
the clastogenic potential of TBTO and obtained negative
results both in the presence and absence of S9. In the
latter case, TBTO was added 22 h after stimulation of the
cultures with phytohaemagglutinin, and cells were har-
vested 31 h later. In the former case, TBTO was added with
S9 after 26 h; 3 h later the S9 was removed, and the cells
were harvested 22 h later. Parallel studies on blood cul-
tures that were differentially stained with BUdR indicated
that almost all cells analysed for chromosome aberrations
were in the first mitotic stage. The highest TBTO concen-
trations used (0.1 µg/ml in the absence of S9 and
1 µg/ml in its presence) were associated with a marked
reduction in mitotic index (57% and 58%, respectively). No
increase in aberrations was seen at any dose level. This
study, therefore, failed to confirm the suggestion of some
clastogenic potential in the studies using CHO cells.
The ability of TBTO to produce chromosomal damage in
vivo has been investigated in two separate studies using
the micronucleus test. In one study, four doses of TBTO
(31.25, 62.5, 125, or 250 mg/kg body weight) were given by
gavage, in arachis oil, to NMRI mice, and bone marrow
cells were analysed for micronuclei in polychromatic
erythrocytes 24, 48, and 72 h after treatment (Reimann &
Lang, 1987). The highest dose level resulted in marked
lethality (16 out of 36 animals died), precluding any
analysis of the results. The 126-mg/kg dose level was also
associated with some deaths (4 mice died), but 5000 poly-
chromatic erythrocytes were analysed from five male and
five female mice at each harvest interval. Similar analy-
ses were carried out at the two lower dose levels. There
was no increase in micronuclei at any dose level or har-
vest time. This study provided no evidence to indicate
that TBTO produces chromosomal damage in bone marrow in
vivo. A second micronucleus study (Davis et al., 1987)
used Balb/c mice. Groups of 10 male and 10 female animals
were given 30 or 60 mg TBTO/kg as a solution in olive oil.
Bone marrow cells were harvested from five males and five
females after 30 h and 48 h, and 1000 polychromatic eryth-
rocytes were analysed from each for micronuclei. An
increase in micronuclei was seen only in the male mice
after 48 h and at the highest dose level. These results
conflict with those of Reinmann & Lang (1987). Further-
more, there was an unusually high spontaneous incidence of
micronuclei (up to 5.8 per 1000 polychromatic erythro-
cytes) in the study of Davis et al. (1987). These factors
prompted a re-analysis of the slides from this study by
the Institute of Occupational Health, Helsinki, Finland
(Schering 1986). This re-analysis, again using 1000 poly-
chromatic erythrocytes per animal, failed to confirm the
increase in micronuclei seen after 48 h in the male
animals given 60 mg TBTO/kg. A slight, but statistically
significant, increase in micronuclei was seen in the
female mice on re-analysis, but this was thought to be
biologically non-significant due to the high variability
in the control data. The re-analysis highlighted the
problem of interpreting studies in which only relatively
few polychromatic erythrocytes are analysed (1000) and
there is marked variability in the control data (with an
incidence outside the normal range at some time points).
Thus, no conclusions can be drawn from the micronucleus
study of Davis et al. (1987).
The ability of TBTO to inhibit metabolic cooperation
between V79 Chinese hamster 6-thioguanine-resistant and
sensitive cells has been investigated by Davis et al.
(1987). This assay has been suggested as a model for
tumour promoter activity. Negative results were obtained.
The significance of the assay is, however, unclear.
In summary, the genotoxicity of TBTO has been the
subject of extensive investigation. Negative results were
obtained in the vast majority of studies, and there is no
convincing evidence that TBTO has any mutagenic potential.
11.5. Reproductive toxicity
Summary
The potential embryotoxicity of TBTO has been evaluated in
three mammalian species (mouse, rat, and rabbit) after oral
dosing of the mother. The main malformation noted in rat and
mouse fetuses was cleft palate, but this occurred at dosages
overtly toxic to the mothers. These results are not considered
to be indicative of teratogenic effects of TBTO at doses below
those producing maternal toxicity. The lowest NOEL, with
regards to embryotoxicity and fetotoxicity for all three
species, was 1 mg/kg body weight.
11.5.1. In vivo
Reproductive toxicity has been studied in NMRI mice.
The highest dose used (35 mg/kg body weight) was chosen to
give minimal maternal mortality based on acute toxicity
tests. An increase in cleft palate was seen in the fetuses
of mice treated orally with 11.7 mg TBTO/kg body weight
(7% cleft palate), 23.4 mg/kg (24%), and 35 mg/kg (48%),
compared to the incidence in controls (0.7%). However, 11
out of a total of 15 affected mice were clustered in one
of the 18 litters and 15 litters contained none. The two
highest doses of TBTO also increased the frequency of
irregular ossification centres of sternabrae and of minor
abnormalities, such as fusion of the bases of os occipi-
talis. The strain of mice used in the study has a tendency
to produce these particular abnormalities as a result of
non-specific stress on the mother. The authors considered
that TBTO has a very low teratogenic potential; electron
microscopy 26 and 48 h after treatment showed no evidence
of damage to the embryos but considerable damage to the
maternal liver. At a level of 6 mg/kg body weight, TBTO
produced no increase in fetal abnormalities (Davis et al.,
1987).
Nemec (1987) investigated the maternal, embryotoxic,
and teratogenic effects of TBTO in New Zealand white
rabbits. The TBTO was administered in corn oil by gavage,
once a day from day 6 of gestation to day 18 inclusive, at
doses of 0.2, 1, and 2.5 mg/kg per day body weight in a
volume of 0.5 ml. Twenty female rabbits were dosed with
corn oil as controls and 20 rabbits were used at each dose
level. The rabbits were artificially inseminated and
injected iv with human chorionic gonadotrophin immediately
afterwards to ensure ovulation. With the exception of a
single female dosed at 1 mg/kg, all treated rabbits sur-
vived to day 29 of gestation when the experiment was
terminated. A total of 12 animals aborted during the
dosing period: 3, 1, 1, and 7 from the control, 0.2, 1,
and 2.5 mg/kg groups, respectively. Females aborting were
killed the same day and immediate postmortem examinations
carried out. The increased occurrence of abortions in the
highest-dose group was considered to be a secondary effect
of maternal toxicity. No clinical findings in the groups
given 0.2 or 1 mg/kg were considered to be the result of
TBTO treatment. There was a statistically significant mean
body weight loss in females dosed at 2.5 mg/kg per day
compared to controls between days 6 and 18 of gestation
(the period of actual dosing). Doses of 0.2 and 1.0 mg/kg
per day had no effect on growth or survival of fetuses.
There was a slight (statistically non-significant)
decrease in mean fetal weight in the group dosed with 2.5
mg/kg per day. This may represent minor fetotoxicity.
There were no differences in the types or frequency of
fetal malformations related to treatment and the con-
clusion was that TBTO was not teratogenic. Postmortem
examination of the mother rabbits indicated no changes
associated with treatment, and 1 mg/kg per day was con-
sidered to be the NOEL for maternal toxicity and toxicity
to the fetus.
When Crofton et al. (1989) treated pregnant Long-Evans
rats with TBTO by gastric intubation at 0 to 16 mg/kg per
day body weight from day 6 to day 20 of gestation, litter
size and pup weight were significantly reduced by doses of
10, 12, or 16 mg/kg per day but no effect was seen at
doses of 2.5 and 5 mg/kg per day. Maternal weight gain was
affected by the same dose levels that affected litter size
and pup weight. Pup survival was further reduced in the
first 3 days after birth. Litter size was reduced by 50%,
73%, and 96% at dose levels of 10, 12, and 16 mg/kg per
day, respectively, on day 1 post partum and by 63%, 88%,
and 100% on day 3 post partum. Pup weight on day 1 post
partum was reduced by 45%, 45%, and 68% at the three dose
levels. Two out of 71 pups born had cleft palate, but no
controls showed any abnormalities. These two pups were
both born dead. The five pups born to rats given 16 mg/kg
per day showed no malformations, but all died within 3
days. The authors concluded that it was impossible to
distinguish between possible fetotoxicity and maternal
toxicity, since all effects on offspring occurred at TBTO
dose rates that also affected the weight of the mother.
They also showed that pregnant female rats were more sen-
sitive to TBTO than non-pregnant females; the MTD (maximum
tolerated dose) for non-pregnant females was 16 mg/kg per
day, whereas pregnant females showed an MTD of between 5
and 10 mg/kg per day. Most effects on pups were transitory
in that survivors to adulthood showed only reductions in
body weight and brain weight compared to controls even at
the highest dose rates. Motor activity of offspring was
monitored in a maze fitted with photoelectric detectors.
During the pre-weaning period there was a significant
age/treatment interaction, but only on post-natal day 14
was there a significant response per individual day. All
doses of TBTO produced a significant decrease in activity
on that day. Post-weaning activity was reduced on post-
natal days 47 and 62 (p < 0.01) but only at a level of
10 mg/kg per day. There was no clear effect on acoustic
startle response.
In a two-generation reproduction study, TBTO was given
to rats at dietary concentrations of 0, 0.5, 5, and 50
mg/kg. The parental (F0) generation (30 males and 30
females per group) was exposed for 10 weeks before mating,
whereas the pre-mating treatment period for the F1 adults
(30 of each sex per group) was 15 weeks. Culling of
litters was performed on day 4 in the F1 and F2 gener-
ations. Preliminary data (Biodynamics, 1989b) on mor-
tality, body weight development, fertility indices, litter
data, and organ weights have been reported. There was no
evidence of compound-related mortality, and body weight
development was normal in the F0 generation. At 50 mg/kg
diet, the pup weights were decreased on days 14 and 21 in
the F1 generation and on days 7, 14, and 21 in the
F2 generation. Among the F1 parents, at 50 mg/kg, lower
body weights were noted in males throughout the pre-mating
period and in females during the first 3 weeks only. No
effects on mating, pregnancy, and fertility rates were
noted in either generation, and number of pups, litter
size, and pup survival were not affected by treatment in
either the F1 or the F2 generations. Relative and
absolute thymus weights were decreased in both sexes at
the dietary concentration of 50 mg/kg.
11.5.2. In vitro
Krowke et al. (1986) demonstrated an effect of TBTO on
the development of limb buds of mice in organ culture.
Clear-cut interference with differentiation was seen at
the lowest dose tested (0.03 mg/litre), while at 0.1 mg
per litre drastic impairment and abnormal development of
the paw skeleton were recorded. The effect was more pro-
nounced still at the highest dose tested (0.3 mg/litre).
In view of their failure to show effects on the embryo in
vivo, Davis et al. (1987) suggested that there is only
very limited movement of TBTO across the placenta of
mice.
11.6. Carcinogenicity
Summary
One carcinogenicity study on rats has been reported in
which neoplastic changes were observed in endocrine organs at
50 mg/kg diet. The pituitary tumours found at 0.5 mg/kg diet
are considered as having no biological significance since there
was no dose-response relationship. These tumour types appear
usually at high and variable background incidences. The signi-
ficance is, therefore, questionable. A second study on mice is
in progress.
Wester (in press) reported the results of a 106-week
study on carcinogenicty in Wistar rats at dietary TBTO
doses of 0, 0.5, 5, and 50 mg/kg (0, 0.025, 0.25, and
2.5 mg/kg body weight). At the highest dose level, general
toxicological effects were present (see section 11.3). The
incidence of benign tumours of the pituitary (mainly pro-
lactinomas) was elevated at 0.5 and 50 mg/kg, but not at
5 mg/kg diet, for both sexes. At 50 mg/kg, a significant
increase was noted in the incidence of adrenal medullary
tumours (pheochromocytomas) in both sexes and of parathy-
roid adenomas in male animals, while the incidence of
adrenal cortical tumours was significantly decreased at
0.5 and 50 mg/kg diet in males only. Isolated occurrence
of pancreatic carcinoma was found in treated female rats.
These were not considered to be compound related since
there was no dose dependency and the incidence rates were
low.
12. EFFECTS ON HUMANS
Summary
TBTO is a skin and eye irritant and severe dermatitis has
been reported after direct contact with the skin. The potential
problem is made worse by the lack of an immediate skin response.
12.1. Ingestion
There have been no reported cases of poisoning from
ingestion of TBTO or other TBT salts.
12.2. Inhalation
Seventy percent of the workers in a rubber factory
using TBTO in the vulcanizing process reported irritation
of the upper respiratory tract (and eyes). About 20% also
experienced lower chest symptoms (irritation, tightness,
and pain), but in all cases pulmonary function was unaf-
fected. The extent of the exposure was not recorded
(WHO/FAO, 1985).
12.3. Dermal exposure
Lyle (1958) described skin lesions in workers occu-
pationally exposed to dibutyl and tributyl tin compounds.
Skin burns were most commonly caused by small splashes of
liquid dibutyl or tributyl tin chlorides. Since the irri-
tancy of these compounds was not immediately apparent
(taking at least an hour to be perceived), small splashes
were frequently ignored by workers. More severe lesions on
the hands were caused by leaking gloves or failure to wear
hand protection. Some maintenance staff also suffered
burns after kneeling or rubbing against surfaces wet with
the chlorides. Clothes wet with these compounds produced
burns on the ventral skin and burns were seen immediately
above the level of protective boots on the calf of the
leg. Two kinds of lesion were seen in workers. The first
was an acute burn, which healed relatively quickly, and
the second a more diffuse dermatitis (seen in workers
wearing contaminated clothes in close contact with the
skin), which persisted. Treatment of the back of the hand
of volunteers with various undiluted TBT compounds estab-
lished that the chloride, acetate, laurate, and oxide all
produced acute burns. Burns were not caused by dibutyltin
esters or oxide or by tetrabutyltin, but were mostly due
to dibutyltin chloride or the tributyltin compounds. Red-
dening of the area treated was seen 2-3 h after treatment
with the compounds. Inflammation of the hair follicles
was the most obvious symptom, effects being confined to
the treated area. On the second day after treatment, min-
ute pustules appeared, which remained discrete and disap-
peared on the third or fourth day. After a week, all that
remained was a faint erythema. The burns were not reported
to be painful, either in the case of experimental or occu-
pational exposure. Sufferers complained of itching and
"stickiness" of the skin causing adherence of clothes.
Proper use of protective clothing, rapid washing of the
skin after exposure, and the use of aprons to prevent
wetting of overalls were found to be effective in pre-
venting burns, both acute and diffuse.
Baaijens (1987) described cases of accidental exposure
to TBTO during the manufacture of organotin compounds.
Severe dermatitis developed only where splashes of the
material had been retained on the skin for long periods.
In one case, a worker had been splashed over the face and
neck. He left the work area after the splash and showered.
An area behind one ear had not been washed and the derma-
titis had developed in this one area. There were no symp-
toms other than dermatitis. Another worker had been
splashed on the arm. He washed his skin but did not change
his overalls. Contact was extended and a large blister
developed on the arm. Monitoring of the urine tin content
showed no difference from normal in these two cases. A
third worker, who complained of an intense smell of TBTO,
suffered nausea and vomiting after 10 min of exposure.
Urine tin levels in this case were elevated for several
days. In all cases the symptoms disappeared within a few
days. The delayed irritancy of tributyltin was emphasized
by the author. It tends to lead to extended exposure since
the affected person does not perceive the effect for sev-
eral hours. The author found no relationship between
clinical or haematological parameters and normal occu-
pational exposure to organotin compounds.
Goh (1985) reported irritant effects and dermatitis in
painters applying TBTO formulations (0.6% TBTO in acrylic
resin-based paints) to buildings. The painters developed
rashes 8 to 10 h after exposure. When required to paint
ceilings, the men developed more severe symptoms on the
face, neck, and trunk, associated with dripping paint.
Severe itching, redness, swelling, and blistering were
recorded. Hospital examination showed extensive vesiculo-
bullous lesions, erythema, and oedema of the face, neck,
trunk, arms, and thighs. Although only two patients were
examined in detail, most of the workers on the site devel-
oped dermatitis. Patch testing of the two reported cases
showed erosion 48 and 96 h after patch tests using aqueous
solutions of TBTO (0.1 and 0.5 g/litre). Five other volun-
teer controls were patch-treated with solutions of 0.01
and 0.1 g/litre; these also showed a similar reaction to
the patients. No allergenic reaction was found in the
patch tests. Replacement of the paint with a preparation
having similar constituents other than TBTO prevented
further problems amongst the painters.
Lewis & Emmett (1987) describe contact dermatitis in a
shipwright resulting from exposure to TBTO-containing
antifouling paint. The man had been spray-painting blocks
of wood and his skin had been exposed to the spray. There
was no immediate sensation, but some irritation was evi-
dent within about an hour. Erythema and ulceration of the
exposed areas were noted on the second day. There were
also some mild pustular lesions on the mucous membrane of
the lips, presumed to be the result of wiping the mouth
with paint-contaminated arms. The authors conducted patch
testing with TBTO at aqueous concentrations of 0.1%,
0.01%, and 0.0015% using the A1 test tape method. At the
highest dose tested, there were large bullae after 48 h
and crusting after 96 h. The other two doses produced no
adverse effects. The delayed sensation and effect were
highlighted by the authors as being particular problems
with TBTO. Great care to prevent any exposure to products
containing TBTO at high concentrations was recommended.
Molin & Wahlberg (1975) investigated an outbreak of
dermatitis on the feet and ankles of trainee soldiers.
Seventy soldiers reported itching and sometimes painful
erythematous, vesiculous to bullous, and haemorrhagic
lesions after long marches on a hot day. Fifty other
soldiers reported less severe symptoms on another
occasion. The skin lesions disappeared within 1 or 2 weeks
in most cases after treatment with saline compresses and
topical steroid creams. In two cases, the skin was red and
slightly tender more than 6 months later. The outbreak was
traced to a single batch of socks that had been soaked in
7 times the recommended concentration of a disinfectant
solution containing TBTO. Recommended use of the disinfec-
tant was calculated to give about 0.001%, whereas the
concentration after the accidental over-use would have
been about 0.01%. Patch testing of eczema patients not
previously exposed to TBTO suggested that the primary
irritant concentration for the compound was between 0.1
and 0.01%. Soldiers patch-tested 2 months after their
dermatitis had fully healed gave negative results to both
0.001% and 0.01% TBTO. The authors suggested that the
effective concentration for TBTO as a disinfectant is too
close to the primary irritancy concentration to justify
the use of TBTO for disinfecting textiles.
Zedler (1961), on the basis of industrial experience,
stated that a concentration of 0.05% TBTO was not harmful
to the human skin.
12.4. Miscellaneous effects
Women using a latex spray paint containing TBTO as an
additive showed immediate irritant effects on the eye
(profuse lacrimation, eye inflammation) and the nasal
mucosa. The symptoms worsened over 14 days of spraying,
but subsided at the weekends and disappeared completely
when addition of TBTO to the paint was discontinued. The
extent of exposure in this case was not recorded (WHO/FAO,
1985).
Akatsuka et al. (1959) reported a case of occupational
poisoning with butyltin compounds where, along with symp-
toms of lassitude, slight occipital headaches, and stiff-
ness in the shoulders, there was a marked disturbance of
the sense of smell. The authors conducted studies on cats
to test whether this effect could be confirmed experimen-
tally. Exposure of the cats to a vapour mixture containing
mainly tributyltin bromide revealed a marked loss of the
sense of smell.
13. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
13.1. Evaluation of human health risks
Exposure of workers occurs principally during the
manufacture and formulation of tributyltin compounds, in
the application and removal of TBT paints, and from the
use of TBT in wood preservatives. Exposure of the general
public may come from the contamination of food, particu-
larly fish and shellfish, and from domestic application of
wood preservatives.
On the basis of both animal tests and direct obser-
vations on humans, it is clear that TBT compounds are
irritant to the skin and eyes and that inhalation of aero-
sols leads to respiratory irritation.
The handling of treated wood poses no dermal irritant
hazard once the wood has dried. However, aerosols of TBT
are very hazardous and re-entry to the treatment area
should be prohibited until the wood has thoroughly dried.
Acute systemic poisoning has never been reported and
clearance of TBT from the body is expected to occur within
a few days. Acute toxicity from handling TBT products is,
therefore, unlikely if proper precautions are taken.
Short- and long-term effects on experimental animals
have been reported in the liver and haematological and
endocrine systems. The effects of TBT compounds on the
immune system, and particularly on host resistance, have
proved the most sensitive parameter of toxicity in the
rat, the most sensitive species tested. The no-observed-
effect level (NOEL), using the Trichinella spiralis host-
resistance model, lies between 0.5 and 5.0 mg/kg diet
(0.025 and 0.25 mg/kg body weight), whereas using measures
of immune function it is 0.6 mg/kg body weight.
Owing to wide variation in the consumption of fish and
shellfish and local differences in residues of TBT in sea-
food, only illustrative estimates relating exposure and
NOEL values can be made. It needs to be emphasized that
local measurements of residues, local estimates of sea-
food consumption, and local decisions on acceptable safety
margins must be made to assess potential risk of these
compounds.
Using fish consumption figures of 15 and 150 g/day, a
value of 1 mg/kg for residues in fish, and an average
human body weight of 60 kg, the following safety margins
based on different immune endpoints are obtained.
---------------------------------------------------------
Fish Estimated Safety margin
consumption daily intake T. spiralis Other
(g/day) of TBT model immune
(µg/kg) parameters
---------------------------------------------------------
15 0.25 100-1000 2500
150 2.5 10-100 250
---------------------------------------------------------
Indiscriminate and irresponsible use of TBT compounds
and a failure to follow the recommendations, outlined in
this monograph, to reduce exposure of humans could lead to
intake of levels of TBT compounds hazardous to human
health.
Teratogenic effects have only occurred in experimental
animals at doses that caused overt maternal toxicity. The
teratogenic potential of TBT is, therefore, considered to
be very low.
Based on the results of comprehensive mutagenicity
studies, tributyltin compounds are not considered to have
mutagenic potential. In a carcinogenicity study on rats
with TBTO, an increased incidence was noted for endocrine
tumours that occur spontaneously at a high and variable
incidence. Therefore, the available evidence does not
clearly demonstrate a carcinogenic hazard of TBT compounds
for humans.
13.2. Evaluation of effects on the environment
Diffuse input of tributyltin (TBT) into the environ-
ment occurs predominantly from the use of TBT in antifoul-
ing paint. It could also occur if it were used as a mol-
luscicide. Point source contamination occurs if TBT is
used as a biocide in cooling systems, wood pulping,
leather processing, wood preservation processes, and tex-
tile treatment.
Due to their physico-chemical properties, TBT com-
pounds concentrate in the surface microlayer and in sedi-
ments. Abiotic degradation does not appear to be a major
mechanism of removal under environmental conditions.
Although TBTO is biodegradable in the water column, this
process is not rapid enough to prevent the occurrence of
elevated TBT levels in some areas. Bioaccumulation occurs
in most aquatic organisms, but in laboratory mammals,
metabolic degradation is a more efficient process.
TBT is extremely hazardous to some aquatic organisms
because it is toxic at very low concentrations in water.
Such concentrations have been found in some areas. Adverse
effects on non-target invertebrates, particularly mol-
luscs, have been reported in field studies, and these have
been sufficiently severe to lead to reproductive failure
and population decline. Adverse effects on the commercial
production of shellfish have been successfully reversed by
restrictions on the use of antifouling paints in some
areas, and these restrictions are also leading to the
reversal of imposex effects in gastropod populations. The
effects on farmed fish indicate that TBT-containing paints
should not be used on restraining nets.
The general hazard to the terrestrial environment is
likely to be low. TBT-treated wood could pose a hazard to
terrestrial organisms living in close contact with it.
The enhancement of TBT concentrations in the surface
microlayer may present a hazard to littoral organisms,
neustonic species (including benthic invertebrate and fish
larvae) and surface-feeding sea-birds and wildfowl.
Accumulation and low biodegradation of TBT in sediments
may present a hazard to aquatic organisms when these pol-
luted sediments are disturbed by natural processes or
dredging activities.
14. RECOMMENDATIONS
14.1. Recommendations for protecting human and environmental health
a) Member countries that have not yet regulated the use
of TBT compounds should be encouraged to do so.
b) There is a need for evaluation and, if necessary,
regulation of organotin input to the environment from
sources other than antifouling paints. For example,
this would include evaluation of the potential risk
from the application of TBT-contaminated sewage sludge
to soil.
c) Improved methods for the safe application, removal,
and disposal of organotin paints should be developed.
14.2. Research needs
a) Methods of detection and analysis need to be improved
to provide rapid and accurate measurements of butyltin
species in pg/litre concentrations. One reason for
this recommendation is that a biological effect, i.e.
imposex in gastropods, may occur at levels lower than
present detection limits.
b) There is a need for research into mechanisms which
concentrate rather than disperse TBT and which retard
degradation, with particular attention to the funda-
mental chemistry of TBT and its interaction with bio-
logical molecules. More study is needed on the uptake
of TBT at all trophic levels.
c) A study of the toxicity of TBT in aquatic organisms is
required. This work should investigate metabolism,
endocrine effects, and immunological toxicity, where
appropriate.
d) A search for other sensitive bioindicator species in
other groups, including freshwater species, is
required.
e) Models for the assessment of immunotoxicity in mammals
need to be validated and no-effect levels for relevant
parameters need to be defined more accurately.
f) A long-term toxicity study in a second mammalian
species should be undertaken.
g) A tumorigenicity study in a second mammalian species
should be undertaken.
h) Information on butyltin residue levels in fish and
shellfish for human consumption using speciating
methods is needed.
REFERENCES
ABEL, R., HATHAWAY, R.A., KING, N.J., VOSSER, J.L., & WILKINSON, T.G.
(1987) Assessment and regulatory actions for TBT in the UK. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1314-1319.
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A., KAWAME,
N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S., YANAGIBASHI, H.,
NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA, I., & IYO, T. (1959)
[Experimental studies on disturbance of sense of smell due to butyltin
compounds.] J. Tokyo med. Coll., 17: 1393-1402 (in Japanese).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides, insecticides,
fungicides, wetting agents and miscellaneous substances. Int. Pest Control,
11: 29-35.
ALDRIDGE, W.N. (1958) The biochemistry of organotin compounds. Trialkyltins
and oxidation phosphorylation. Biochem. J., 69: 367-376.
ALDRIDGE, W.N. & STREET, B.W. (1964) Oxidative phosphorylation: Biochemical
effects and properties of trialkyltins. Biochem. J., 91: 287-297.
ALDRIDGE, W.N. & STREET, B.W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver mitochondria.
Biochem. J., 118: 171-179.
ALDRIDGE, W.N. & STREET, B.W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and triethyltin to
mitochondria and their effects on various mitochondrial functions. Biochem.
J., 124: 221-234.
ALDRIDGE, W.N., CASIDA, J.E., FISH, R.H., KIMMEL, E.C., & STREET, B.W.
(1977) Action on mitochondria and toxicity of metabolites of tri-n-butyltin
derivatives. Biochem. Pharmacol., 26: 1997-2000.
ALLEN, A.J., QUITTER, B.M., & RADICK, C.M. (1980) The biocidal mechanism of
controlled release bis (tri-n-butyltin) oxide in Biomphalaria glabrata. In:
Baker, R., ed. Controlled release of bioactive materials, New York, London,
Academic Press, p. 399.
ALZIEU, C.P. (1981) Evaluation des risques dus à l'emploi des peintures
anti-salissures dans les zones conchylicoles, Nantes, Institut scientifique
et technique des Pêches maritimes, 84 pp.
ALZIEU, C. & HERAL, M. (1984) Ecotoxicological effects of organotin
compounds on oyster culture. Ecotoxicol. Test. mar. Environ., 2: 187-196.
ALZIEU, C. & PORTMANN, J.E. (1984) The effect of tributyl tin on the
culture of C. gigas. Proceedings of the 15th Annual Shellfish Conference,
15-16 May, 1984, London, The Shellfish Association of Great Britain, 17 pp.
ALZIEU, C., HERAL, M., THIBAUD, Y., DARDIGNAC, M.-J., & FEUILLET, M. (1982)
Influence des peintures antisalissures à base d'organostanniques sur la
calcification de la coquille de l'huitre Crassostrea gigas. Rev. Trav.
Inst. Pêches Marit., 45: 101-116.
ALZIEU, C., SANJUAN, J., DELTREIL, J.P., & BOREL, M. (1986) Tin
contamination in Arcachon Bay: Effects on oyster shell anomalies. Mar.
Pollut. Bull., 17: 494-498.
ALZIEU, C., SANJUAN, J., MICHEL, P., BOREL, M., & DRENO, J.P. (1989)
Monitoring and assessment of butyltins in Atlantic coastal waters. Mar.
Pollut. Bull., 20: 22-26.
ANGER, J.P., ANGER, F., CANO, Y., CHAUVEL, Y., LOUVET, M., & VAN DEN
DRIESSCHE, J. (1976) Effets chez le cobaye, d'un aérosol à base d'oxyde de
tributylétain (OTBE). Eur. J. Toxicol., 9: 339-346.
ARAKAWA, Y. & WADA, O. (1984) Inhibition of neutrophil chemotaxis by
organotin compounds. Biochem. biophys. Res. Commun., 123: 543-548.
ARGAMAN, Y., HUCKS, C.E., & SHELBY, S.E. (1984) The effects of organotin on
the activated sludge process. Water Res., 18: 535-542.
AYALA, C.A.C., TOLEDO, J.V., DA SILVA NETTO, J.A., & GILBERT, B. (1980) The
effect of hexabutyldistannoxane (TBTO) and other molluscicides on non-
target species, Geneva, World Health Organization, Parasitic Diseases
Programme (Unpublished report).
BAAIJENS, P.A. (1987) Health effect screening and biological monitoring for
workers in organotin industries. In: Toxicology and analytics of the
tributyltins: The present status. Proceedings of an ORTEPA workshop,
Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands, ORTEP-
Association, pp. 191-208
BACCI, E. & GAGGI, C. (1989) Organotin compounds in harbour and marina
waters from the Northern Tyrrhenian Sea. Mar. Pollut. Bull., 20: 290-292.
BAHR, G. & PAWLENKO, S. (1978) Organic tin compounds. In: Bahr, G.,
Kalinowski, H.-O., & Pawlenko, S., ed. Organometallic compounds, germanium,
tin, Stuttgart, Georg Thieme Verlag, pp. 512-515 (Methods in Organic
Chemistry series).
BAILEY, S.K. & DAVIES, I.M. (1988a) Tributyltin contamination around an oil
terminal in Sullom Voe (Shetland). Environ. Pollut., 55: 161-172.
BAILEY, S.K. & DAVIES, I.M. (1988b) Tributyltin contamination in the Firth
of Forth (1975-87). Sci. total Environ., 76: 185-192.
BAKER, J.M. & TAYLOR, J.M. (1967) The toxicity of tributyltin oxide to the
wood-boring beetles Lyctus brunneus Steph. and Anobium punctatum (Deg.).
Ann. appl. Biol., 60: 181-190.
BALLS, P.W. (1987) Tributyltin (TBT) in the waters of a Scottish sea loch
arising from the use of antifoulant treated netting by salmon farms.
Aquaculture, 65: 227-237.
BARNES, J.M. & STONER, H.B. (1958) Toxic properties of some dialkyl and
trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARUG, D. (1981) Microbial degradation of bis (tributyltin) oxide.
Chemosphere, 10: 1145-1154.
BARUG, D. & VONK, J.W. (1980) Studies on the degradation of bis
(tributyltin) oxide in soil. Pestic. Sci., 11: 77-82.
BEAUMONT, A.R. & BUDD, M.D. (1984) High mortality of the larvae of the
common mussel at low concentrations of tributyltin. Mar. Pollut. Bull., 15:
402-405.
BEAUMONT, A.R. & NEWMAN, P.B. (1986) Low levels of tributyltin reduce
growth of marine micro-algae. Mar. Pollut. Bull., 17: 457-461.
BEAUMONT, A.R., NEWMAN, P.B., & WALDOCK, M.J. (1987) Sand substrate
microcosm studies on tributyl tin (TBT) toxicity to marine organisms, 24 pp
(Final report to the UK Department of the Environment, London. Contract No.
PECD 7/8/73).
BERRIOS-DURAN, L.A. & RITCHIE, L.S. (1968) Molluscicidal activity of
bis(tri-n-butyltin) oxide formulated in rubber. World Health Organ. Bull.,
39: 310-312.
BIODYNAMICS (1989a) A three month oral range-finding toxicity study in mice
with bis (tri-n-butyltin) oxide (TBTO), East Millstone, New Jersey,
Biodynamics (Final report to Aceto Chemical Corporation, M. + T. Chemicals
Inc., and Schering Berlin Inc.) (Unpublished).
BIODYNAMICS (1989b) A two-generation reproduction study in rats with TBTO,
East Millstone, New Jersey, Biodynamics (Interim report to Aceto Chemical
Corporation, M. + T. Chemicals Inc., and Schering Berlin Inc.)
(Unpublished).
BJORKLUND, I. (1987a) [Environmental aspects of ship's bottom paints],
Stockholm, Swedish Chemicals Inspectorate, Investigation Department, 16 pp
(in Swedish).
BJORKLUND, I. (1987b) [Environmental effect of antifouling paints],
Stockholm, Swedish National Environment Protection Board, 17 pp.
BLAIR, W.R., OLSON, G.J., BRINCKMAN, F.E., & IVERSON, W.P. (1982)
Accumulation and fate of tri-n-butyltin cation in estuarine bacteria.
Microbiol. Ecol., 8: 241-251.
BLAIR, W.R., OLSON, G.J., & BRINCKMAN, F.E. (1986) Speciation measurements
of butyltins: Application to controlled release rate determination and
production of reference standards. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1141-1145.
BLUNDEN, S.J. & CHAPMAN, A. (1986) Organotin compounds in the environment.
In: Craigh, P.J., ed. Organometallic compounds in the environment, Harlow,
Essex, Longman Group Ltd, pp. 111-159.
BLUNDEN, S.J., HOBBS, L.A., & SMITH, P.J. (1984) The environmental
chemistry of organotin compounds. In: Bowen, H.J.M, Blunden, S.J., Colbeck,
I., Harrison, R.M., Hobbs, L.A., Katz, S.A., Simkiss, K., Smith, P.J., &
Taylor, M.G., ed. Environmental chemistry, London, Royal Society of
Chemistry, Vol. 3, pp. 49-77.
BOKRANZ, A. & PLUM, H. (1975) Industrial manufacture and use of organotin
compounds, Bergkamen, Federal Republic of Germany, Schering AG, 33 pp.
BOORMAN, L.A. (1989) The effects of TBT on two salt marsh plant species,
Aster tripolium and Limonium vulgare. In Preparation.
BOULDIN, T.W., GOINES, N.D., BAGNELL, C.R., & KRIGMAN, M.R. (1981)
Pathogenesis of trimethyltin neuronal toxicity. Ultrastructural and
cytochemical observations. Am. J. Pathol., 104: 237-249.
BRAMAN, R.S. & TOMPKINS, M.A. (1979) Separation and determination of
nanogram amounts of inorganic and methyltin compounds in the environment.
Anal. Chem., 51: 12-19.
BRESSA, G., CIMA, L., CANOVA, F., & CARAVELLO, G.U. (1984) Bioaccumulation
of tin in the fish tissues (Liza aurata). In: Proceedings of the
International Conference on Environmental Contamination, London, July 1984
- Geneva, International Register of Potentially Toxic Chemicals, United
Nations Environment Programme, pp. 812-815.
BRIDGES, J.W., DAVIES, D.S., & WILLIAMS, R.T. (1967) The fate of ethyltin
and diethyltin derivatives in the rat. Biochem. J., 105: 1261-1266.
BRINCKMAN, F.E. (1981) Environmental organotin chemistry today: Experiences
in the field and laboratory. J. organomet. Chem. Libr., 12: 343-376.
BRINCKMAN, F.E., JACKSON, J.A., BLAIR, W.R., OLSON, G.J., & IVERSON, W.P.
(1983) Ultratrace speciation and biogenesis of methyltin transport species
in estuarine waters. In: Trace metals in sea water, New York, Plenum Press,
pp. 39-72.
BROWN, R.A., NAZARIO, C.M., DE TIRADO, R.S., CASTRILLON, J., & AGARD, E.T.
(1977) A comparison of the half-life of inorganic and organic tin in the
mouse. Environ. Res., 13: 56-61.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1986) The
decline of the gastropod Nucella lapillus around south-west England:
Evidence for the effect of tributyltin from antifouling paints. J. Mar.
Biol. Assoc. UK, 66: 611-640.
BRYAN, G.W., GIBBS, P.E., HUMMERSTONE, L.G., & BURT, G.R. (1987) Copper,
zinc, and organotin as long-term factors governing the distribution of
organisms in the Fal estuary in southwest England. Estuaries, 10: 208-219.
BRYAN, G.W., GIBBS, P.E., & BURT, G.R. (1988) A comparison of the
effectiveness of tri-n-butyltin chloride and five other organotin compounds
in promoting the development of imposex in the dog-whelk Nucella lapillus.
J. Mar. Biol. Assoc. UK, 68: 733-744.
BUSHONG, S.J., HALL, W.S., JOHNSON, W.E., & HALL, L.W. (1987) Toxicity of
tributyltin to selected Chesapeake Bay biota. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1494-1503.
BUSHONG, S.J., HALL, L.W., HALL, W.S., JOHNSON, W.E., & HERMAN, R.L. (1988)
Acute toxicity of tributyltin to selected Chesapeake Bay fish and
invertebrates. Water Res., 22: 1027-1032.
CALLEY, D.J., GUESS, W.L., & AUTIAN, J. (1967) Hepatotoxicity of a series
of organotin esters. J. pharm. Sci., 56: 240-243.
CARDARELLI, N.F. (1973) Effects of ultralow molluscicide dosages on
Biomphalaria glabrata and Lebistes reticulatus in micro ecological systems:
Report I, Akron, Ohio, University of Akron, 15 pp.
CARDARELLI, N.F. (1978) Controlled release organotins as mosquito
larvicides. Mosq. News, 38: 328-333.
CARDARELLI, N.F. & EVANS, W. (1980) Chemodynamics and environmental
toxicology of controlled release organotin molluscicides. In: Baker, R.W.,
ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the Controlled Release Society, New York, London,
Academic Press, pp. 357-385.
CASIDA, J.E., KIMMEL, E.C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and trialkyleads by
liver microsomes. Acta chem. Scand., 25: 1497-1499.
CHAMP, M.A. & PUGH, W.L. (1987) Tributyltin antifouling paints:
Introduction and overview. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1296-1308.
CHAPMAN, A.H. & PRICE, J.W. (1972) Degradation of triphenyltin acetate by
ultraviolet light. Int. Pest Control, 14: 11-12.
CHENG, Z. & JENSEN, A. (1989) Accumulation of organic and inorganic tin in
blue mussel, Mytilus edulis, under natural conditions. Mar. Pollut. Bull.,
20: 281-286.
CHLIAMOVITCH, Y.-P. & KUHN, C. (1977) Behavioural, haematological and
histological studies on acute toxicity of bis(tri- n-butyltin) oxide on
Salmo gairdneri Richardson and Tilapia rendalli Boulenger. J. Fish Biol.,
10: 575-585.
CHU, K.Y. (1976) Effects of environmental factors on the molluscicidal
activities of slow-release hexabutyldistannoxane and copper sulfate. Bull.
World Health Organ., 54: 417-420.
CLARK, J.R., PATRICK, J.M., MOORE, J.C., & LORES, E.M. (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass shrimp
(Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Arch.
environ. Contam. Toxicol., 16: 401-407.
CLEARY, J.J. & STEBBING, A.R.D. (1985) Organotin and total tin in coastal
waters of southwest England. Mar. Pollut. Bull., 16: 350-355.
CLEARY, J.J. & STEBBING, A.R.D. (1987) Organotin in the surface microlayer
and subsurface waters of Southwest England. Mar. Pollut. Bull., 18: 238-
246.
CREMER, J.E. (1957) The metabolism in vitro of tissue slices from rats
given triethyltin compounds. Biochem. J., 67: 87-96.
CROFTON, K.M., DEAN, K.F., BONCEK, V.M., ROSEN, M.B., SHEETS, L.P.,
CHERNOFF, N., & REITER, L.W. (1989) Prenatal or postnatal exposure to
bis(tri- n-butyltin)oxide in the rat: Postnatal evaluation of teratology and
behavior. Toxicol. appl. Pharmacol., 97: 113-123.
DAVIDSON, B.M., VALKIRS, A.O., & SELIGMAN, P.F. (1986) Acute and chronic
effects of tributyltin on the mysid Acanthomysis sculpta (Crustacea,
Mysidacea). In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., pp. 1219-1225.
DAVIES, R.J., FLETCHER, R.L., & FURTADO, S.E.J. (1984) The effects of
tributyltin compounds on spore development in the green alga Enteromorpha
intestinalis (L) Link. In: Proceedings of the 6th International Congress on
Marine Corrosion and Fouling, Athens, September 1984, pp. 557-565.
DAVIES, I.M., DRINKWATER, J., MCKIE, J.C., & BALLS, P. (1987a) Effects of
the use of tributyltin antifoulants in mariculture. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., pp. 1477-1481.
DAVIES, I.M., BAILEY, S.K., & MOORE, D.C. (1987b) Tributyltin in Scottish
sea Lochs, as indicated by degree of imposex in the dogwhelk, Nucella
lapillus (L.). Mar. Pollut. Bull., 18: 400-404.
DAVIS, A., BARALE, R., BRUN, G., FORSTER, R., GUNTHER, T., HAUTEFEUILLE,
H., VAN DER HEIJDEN, C.A., KNAAP, A.G.A.C., KROWKE, R., KUROKI, T.,
LOPRIENO, N., MALAVEILLE, C., MERKER, H.J., MONACO, M., MOSESSO, P.,
NEUBERT, D., NORPPA, H., SORSA, M., VOGEL, E., VOOGD, C.E., UMEDA, M., &
BARTSCH, H. (1987) Evaluation of the genetic and embryotoxic effects of
bis(tri- n-butyltin) oxide (TBTO), a broad-spectrum pesticide, in multiple
in vivo and in vitro short-term tests. Mutat. Res., 188: 65-95.
DESCHIENS, R. & FLOCH, H. (1962) Les propriétés molluscicides du chlorure
et de l'acétate de triphénylétain dans le cadre de la prophylaxie des
bilharzioses. C. R. Acad. Sci. Paris, 255: 1236-1237.
DESCHIENS, R. & FLOCH, H. (1968) Action biologique comparée de 6
molluscicides chimique dans le cadre de la prophylaxie des bilharzioses.
Conclusions pratiques. Bull. Soc. Pathol. Exot., 61: 640-650.
DESCHIENS, R., BROTTES, H., & MVOGO, L. (1966) Application sur le terrain,
au Cameroun, dans la prophylaxie des bilharzioses de l'action molluscicide
de l'oxyde de tributylétain. Bull. Soc. Pathol. Exot., 59: 968-973.
DE VILLIERS, J.P. & MACKENZIE, J.G. (1963) Organotin and organolead
molluscicides, Geneva, World Health Organization, Parasitic Diseases
Programme, p. 63 (Unpublished report Mol/Inf/13).
DIXON, D.R. & MCFADZEN, I. (1987) Bis(tributyltin) oxide (TBTO), an
antifouling compound, promotes SCE induction in the larvae of the common
mussel, Mytilus edulis. Mutagenesis, 2: 312.
DIXON, D.R. & PROSSER, H. (1986) An investigation of the genotoxic effects
of an organotin antifouling compound (bis(tributyltin) oxide) on the
chromosomes of the edible mussel, Mytilus edulis. Aquat. Toxicol., 8: 185-
195.
DOJMI DI DELUPIS, G., GUCCI, P.M.B., & VOLTERRA, L. (1987) Toxic effects of
bis-tributyltinoxide on phytoplancton. Main Group Metal Chem., 10: 77-82.
DONARD, O., RAPSOMANIKIS, S., & WEBER, J.H. (1986) Speciation of inorganic
tin and alkyltin compounds by atomic absorption spectrometry using
electrothermal quartz furnace after hydride generation. Anal. Chem., 58:
772-777.
EAJ (1988) Outline of TBT compounds monitored in Japan, Tokyo, Environment
Agency of Japan (OECD Clearing House Project on Organotins).
EBDON, L., EVANS, K., & HILL, S. (1988) The variation of tributyltin levels
with time in selected estuaries prior to the introduction of regulations
governing the use of tributyltin-based anti-fouling paints. Sci. total
Environ., 68: 207-223.
EBDON, L., EVANS, K., & HILL, S. (1989) The accumulation of organotins in
adult and seed oysters from selected estuaries prior to the introduction of
U.K. regulations governing the use of tributyltin-based antifouling paints.
Sci. total Environ., 83: 63-84.
ELFERINK, J.G.R., DEIERKAUF, M., & VAN STEVENINCK, J. (1986) Toxicity of
organotin compounds for polymorphonuclear leukocytes: the effect on
phagocyctosis and exocytosis. Biochem. Pharmacol., 35: 3727-3732.
ELSEA, J.R. & PAYNTER, O.E. (1958) Toxicological studies on bis(tri-n-
butyltin) oxide. Am. Med. Assoc. Arch. Ind. Health, 18: 214-217.
EVANS, C.J. & KARPEL, S. (1985) Organotin compounds in modern technology.
J. organomet. Chem. Libr., 16: 178-217.
EVANS, D.W & LAUGHLIN, R.B. (1984) Accumulation of bis (tributyltin) oxide
by the mud crab, Rhitropanopeus harrisii. Chemosphere, 13: 213-219.
EVANS, W.H., CARDARELLI, N.F., & SMITH, D.J. (1979) Accumulation and
excretion of [1-14C] bis (tri- n-butyltin) oxide in mice. J. Toxicol.
environ. Health, 5: 871-877.
FENT, K. (1989a) Organotin speciation in municipal wastewater and sewage
slude: Ecotoxicological consequences. Mar. environ. Res., 28: 477-483.
FENT, K. (1989b) Teratogenic effects of tributyltin on embryos of the
freshwater fish Phoxinus phoxinus. Presented at the OECD Workshop on
Tributyltin: Activities related to Field Studies at Sea, Centre IFREMER,
Nantes, 27-29 June, 1989 (Unpublished report).
FENT, K., FASSBIND, R., & SIEGRIST, H. (1989) Organotins in a municipal
wastewater treatment plant. Proceedings of the 1st European Conference on
Ecotoxicology, Copenhagen, Denmark, 17-19 October, 1988, Lyngby, Denmark,
The Technical University of Denmark, Laboratory of Environmental Sciences
and Ecology.
FERAL, C. & LE GALL, S. (1983) The influence of a pollutant factor
(tributyltin) on the neuroendocrine mechanism responsible for the
occurrence of a penis in the females of Ocenebra erinacea. In: Lever, J. &
Boer, H.H., ed. Molluscan neuroendocrinology. Proceedings of the
International Minisymposium on Molluscan Endocrinology, Amsterdam, North-
Holland Publishing Company, pp. 173-175.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1975) Bioorganotin chemistry:
Biological oxidation of tributyltin derivatives. J. organomet. Chem., 93:
C1-C4.
FISH, R.H., KIMMEL, E.C., & CASIDA, J.E. (1976) Bioorganotin chemistry:
Biological oxidation of organotin compounds. In: Zuckerman, J.J., ed.
Organotin compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 197-203 (Advances in Chemistry Series No.
157).
FLOCH, H., DESCHIENS, R., & FLOCH, T. (1964) Sur les propriétés
molluscicides de l'oxyde et de l'acétate de tributylétain (prophylaxie des
bilharzioses). Bull. Soc. Pathol. Exot., 57: 454-465.
FOSTER, R.B. (1981) Use of Asiatic clam larvae in aquatic hazard
evaluations. In: Bates, J.M. & Weber, C.I., ed. Ecological assessments of
effluent impacts on communities of indigenous aquatic organisms,
Philadelphia, American Society for Testing and Materials, pp. 280-288 (ASTM
STP No. 730).
FUNAHASHI, N., IWASAKI, I., & IDE, G. (1980) Effects of bis(tri-n-butyltin)
oxide on endocrine and lymphoid organs of male rats. Acta pathol. Jpn., 30:
955-966.
GARDINER, B.G. & POLLER, R.C. (1964) Insecticidal activity and structure of
some organotin compounds. Bull. entomol. Res., 55: 17-21.
GIBBS, P.E. & BRYAN, G.W. (1986) Reproductive failure in populations of the
dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from
antifouling paints. J. Mar. Biol. Assoc. UK, 66: 767-777.
GIBBS, P.E. & BRYAN, G.W. (1987) TBT paints and the demise of the dog-
whelk, Nucella lapillus (Gastropoda). In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1482-1487.
GIBBS, P.E., BRYAN, G.W., PASCOE, P.L., & BURT, G.R. (1987) The use of the
dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT)
contamination. J. Mar. Biol. Assoc. UK, 67: 507-523.
GIBBS, P.E., PASCOE, P.L., & BURT, G.R. (1988) Sex change in the female
dogwhelk, Nucella lapillus, induced by tributyltin from antifouling paints.
J. Mar. Biol. Assoc. UK, 68: 715-731.
GILBERT, B., PAES LEME, L.A., FERREIRA, A.M., BULHOES, M.S., & CASTLETON,
C. (1973) Field tests of hexabutyldistannoxane (TBTO) in slow-release
formulations against Biomphalaria spp. Bull. World Health Organ., 49: 633-
636.
GILE, J.D., COLLINS, J.C., & GILLETT, J.W. (1982) Fate and impact of wood
preservatives in a terrestrial microcosm. J. agric. food Chem., 30: 295-
301.
GILLETT, J.W., GILE, J.D., & RUSSELL, L.K. (1983) Predator-prey (vole-
cricket) interactions: the effects of wood preservatives. Environ. Toxicol.
Chem., 2: 83-93.
GOH, C.L. (1985) Irritant dermatitis from tri-n-butyl tin oxide in paint.
Contact dermatitis, 12: 161-163.
GOHLKE, R., LEWA, W., STRACHOVSKY, A., & KOHLER, R. (1969) [Investi-gations
in experimental animals of the inhalatory effects of tributyltin chloride
in a subchronic experiment.] Z. gesamte Hyg. Grenzgeb., 15: 97-104 (in
German).
GOODMAN, L.R., CRIPE, G.M., MOODY, P.H., & HALSELL, D.G. (1988) Acute
toxicity of malathion, tetrabromobisphenol-A, and tributyltin chloride to
mysids (Mysidopsis bahia) of three ages. Bull. environ. Contam. Toxicol.,
41: 746-753.
GROVHOUG, J.G., SELIGMAN, P.F., VAFA, G., & FRANSHAM, R.L. (1986) Baseline
measurements of butyltin in U.S. harbors and estuaries. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1283-1288.
GUARD, H.E., COGBET, A.B., & COLEMAN, W.M. (1981) Methylation of
trimethyltin compounds by estuarine sediments. Science, 213: 770-771.
HADA, N. (1986) [Studies on the kinetics of tributyltin compounds in the
body: With special reference to their biological half times in goldfish and
rats as estimated by newly developed analytical method.] Nichidai Igaku
Zasshi, 45: 1005-1013 (in Japanese).
HALL, L.W., PINKNEY, A.E., ZEGER, S., BURTON, D.T., & LENKEVICH, M.J.
(1984) Behavioral responses to two estuarine fish species subjected to bis
(tri- n-butyltin) oxide. Water Resour. Bull., 20: 235-239.
HALL, L.W., LENKEVICH, M.J., HALL, W.S., PINKNEY, A.E., & BUSHONG, S.J.
(1986) Monitoring organotin concentrations in Maryland waters of Chesapeake
Bay. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1275-1279.
HALL, L.W., BUSHONG, S.J., JOHNSON, W.E., & HALL, W.S. (1988a) Spacial and
temporal distribution of butyltin compounds in a Northern Chesapeake Bay
marina and river system. Environ. Monit. Assess., 10: 229-244.
HALL, L.W., BUSHONG, S.J., HALL, W.S., & JOHNSON, W.E. (1988b) Acute and
chronic effects of tributyltin on a Chesapeake Bay copepod. Environ.
Toxicol. Chem., 7: 41-46.
HALLAS, L.E., MEANS, J.C., & COONEY, J.J. (1982) Methylation of tin by
estuarine microorganisms. Science, 215: 1505-1507.
HARRIS, J.R.W. & CLEARY, J.J. (1987) Particle-water partitioning and
organotin dispersal in an estuary. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1370-1374.
HENDERSON, R.S. (1986) Effects of organotin antifouling paint leachates on
Pearl Harbor organisms: a site specific flowthrough bioassay. In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1226-1233.
HENSHAW, B.G., LAIDLAW, R.A., ORSLER, R.J., CAREY, J.K., & SAVORY, J.G.
(1978) The permanence of tributyltin oxide in timber. In: Record of the
1978 Annual Convention of the British Wood Preserving Association,
Cambridge, 27-30 June, 1978, London, British Wood Preserving Association,
pp. 19-29.
HINGA, K.R., ADELMAN, D., & PILSON, M.E.Q. (1987) Radiolabeled butyl tin
studies in the Merl enclosed ecosystems. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1416-1420.
HIS, E. & ROBERT, R. (1980) Action d'un sel organo-métallique, l'acétate de
tributylétain sur les oeufs et les larves de Crassostrea gigas (Thunberg),
Copenhagen, International Council for the Exploration of the Sea (ICES)
Mariculture Commission, 27 pp (CM 1980/F).
HIS, E. & ROBERT, R. (1985) Développement des véligères de Crassostrea
gigas dans le Bassin d'Arcachon, études sur les mortalités larvaires. Rev.
Trav. Inst. Pêches Marit., 47: 63-88.
HIS, E., MAURER, D., & ROBERT, R. (1986) Observations complémentaires sur
les causes possibles des anomalies de la reproduction de Crassostrea gigas
(Thunberg) dans le Basin d'Arcachon. Rev. Trav. Inst. Pêches Marit., 48:
45-54.
HODGE, V.F., SEIDEL, S.L., & GOLDBERG, E.D. (1979) Determination of tin
(IV) and organotin compounds in natural waters, coastal sediments, and
macro algae by atomic absorption spectrometry. Anal. Chem., 51: 1256-1259.
HOPF, H.S., DUNCAN, J., BEESLEY, J.S.S., WEBLEY, D.J., & STURROCK, R.F.
(1967) Molluscicidal properties of organotin and organolead compounds.
World Health Organ. Bull., 36: 955-961.
HUGGETT, R.J., UNGER, M.A., & WESTBROOK, D.J. (1986) Organotin
concentrations in the southern Chesapeake Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1262-1265.
HUMPEL, M., KUHNE, G., TAUBER, U., & SCHULZE, P.E. (1986) Studies on the
kinetics of bis (tris- n -butyl-113tin) oxide (TBTO). In: Toxicology and
analytics of the tributyltins: The present status. Proceedings of an ORTEPA
workshop, Berlin, 15-16 May, 1986, Vlissingen-Oost, The Netherlands,
ORTEP-Association, pp. 122-142.
HUMPHREY, B. & HOPE, D. (1987) Analysis of water, sediments and biota for
organotin compounds. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1348-1351.
ICES (1987) Concentrations of organotin and total tin in open Danish sea
areas and in pleasure craft marinas along the sound, International Council
for the Exploration of the Sea (ICES) (TWG 14/9/3-E).
IWAI, H., MANABE, M., MATSUI, H., ONO, T., & WADA, O. (1980) Effects of
tributyltin and its metabolites on brain function. J. toxicol. Sci., 5:
257.
IWASAKI, I., FUNABASHI, N., TOIZUMI, S., & IDE, G. (1976) Histopathological
studies on rat's nervous system by oral administration of bis-(tri-n-
butyltin) oxide. (I). J. toxicol. Sci., 1: 99 (abstract).
JENSEN, A. & CHENG, Z. (1987) Total tin and organotin in seawater from
pleasure craft marinas along Danish coast of the Sound. In: Proceedings of
the 15th Conference of Baltic Oceanographers (CBO), Copenhagen, 1986,
Charlottenlung, Denmark, Marine Pollution Laboratory, Vol. 1, pp. 289-298.
JEWETT, K.L. & BRINCKMAN, F.E. (1981) Speciation of trace di- and
triorganotins in water by ion exchange HPLC-GFAA. J. chromatogr. Sci., 19:
583.
JOHANSEN, K. & MOHLENBERG, F. (1987) Impairment of egg production in
Acartia tonsa exposed to tributyltin oxide. Ophelia, 27: 137-141.
JOHNSON, T.L. & KNOWLES, C.O. (1983) Effects of organotins on rat
platelets. Toxicology, 29: 39-48.
JORDAN, P. (1985) Schistosomiasis - The St Lucia Project, Cambridge,
Cambridge University Press, 442 pp.
KAKUNO, A. & KIMURA, S. (1987) [Acute toxicity of bis (tributyltin) oxide
to girella (Girella punctata).] Bull. Tokai Reg. Fish. Res. Lab., 123: 41-
44 (in Japanese).
KALBFUS, W. (1988) TBT-burden from antifouling paints in different waters.
Presented at the OECD Workshop on Monitoring, Chemical Analysis and
Leaching Rates of TBT, Paris, 30 November-2 December, 1988, 11 pp
(Unpublished report).
KALNINS, M.A. & DETROY, B.F. (1984) Effect of wood preservative treatment
of beehives on honey bees and hive products. J. agric. food Chem., 32:
1176-1180.
KEY, D., NUNNY, R.S., DAVIDSON, P.E., & LEONARD, M.A. (1976) Abnormal shell
growth in the Pacific oyster Crassostrea gigas. Some preliminary results
from experiments undertaken in 1975, Copenhagen, International Council for
the Exploration of the Sea (ICES), 12 pp. (C. M. 1976/K/11).
KIMMEL, E.C., FISH, R.H., & CASIDA, J.E. (1977) Bioorganotin chemistry:
Metabolism of organotin compounds in microsomal monooxygenase system and in
mammals. J. agric. food Chem., 25: 1-8.
KLIMMER, O.R. (1969) [The use of organic tin compounds from the viewpoint
of experimental toxicology.] Arzneimittelforschung, 19: 934-939 (in
German).
KNOWLES, C.O. & JOHNSON, T.L. (1986) Influence of organotins on rat
platelet aggregation mechanism. Environ. Res., 39: 172-179.
KRAJNC, E.I. (1989) Presentation to OECD working group on TBT immune
effects. In Preparation.
KRAJNC, E.I., WESTER, P.W., LOEBER, J.G., VAN LEEUWEN, F.X.R., VOS, J.G.,
VAESSEN, H.A.M.G., & VAN DER HEIJDEN, C.A. (1984) Toxicity of bis(tri- n-
butyltin)oxide in the rat. 1. Short-term effects on general parameters and
on the endocrine and lymphoid systems. Toxicol. appl. Pharmacol., 75: 363-
386.
KRAMPITZ, G., ENGELS, J., & CAZAUX, C. (1976) Biochemical studies on water-
soluble proteins and related components of gastropods shells. In: Watabe,
N. & Wilbur, K.M., ed. The mechanisms of mineralization in the
invertebrates and plants, Columbia, University of South Carolina Press, pp.
155-173.
KRAMPITZ, G., DROLSHAGEN, H., & DELTREIL, J.P. (1983) Soluble matrix
components in malformed oyster shells. Experientia (Basel), 39: 1105-1106.
KROWKE, R., BLUTH, U., & NEUBERT, D. (1986) in vitro studies on the
embryotoxic potential of (bis[tri- n-butyltin])oxide in a limb bud culture
system. Arch. Toxicol., 58: 125-129.
LANGSTON, W.J., BURT, G.R., & ZHOU, M. (1987) Tin and organotin in water,
sediments, and benthic organisms of Poole Harbour. Mar. Pollut. Bull., 18:
634-639.
LAUGHLIN, R.B. & FRENCH, W.J. (1980) Comparative study of the acute
toxicity of a homologous series of trialkyltins to larval shore crabs,
Hemigrapsus nudus, and lobster, Homarus americanus. Bull. environ. Contam.
Toxicol., 25: 802-809.
LAUGHLIN, R. & LINDEN, O. (1982) Sublethal responses of the tadpoles of the
European frog Rana temporaria to two tributyltin compounds. Bull. environ.
Contam. Toxicol., 28: 494-499.
LAUGHLIN, R., FRENCH, W., & GUARD, H.E. (1983) Acute and sublethal toxicity
of tributyltin oxide (TBTO) and its putative environmental product,
tributyltin sulfide (TBTS) to zoeal mud crabs, Rhithropanopeus harrisii.
Water Air Soil Pollut., 20: 69-79.
LAUGHLIN, R., NORDLUND, K., & LINDEN, O. (1984) Long-term effects of
tributyltin compounds on the baltic amphipod, Gammarus oceanicus. Mar.
environ. Res., 12: 243-271.
LAUGHLIN, R.B., JOHANNESEN, R.B., FRENCH, W., GUARD, H., & BRINKMAN, F.E.
(1985) Structure-activity relationships for organotin compounds. Environ.
Toxicol. Chem., 4: 343-351.
LAUGHLIN, R.B., GUARD, H.E., & COLEMAN, W.M. (1986a) Tributyltin in
seawater: Speciation and octanol-water partition coefficient. Environ. Sci.
Technol., 20: 201-204.
LAUGHLIN, R.B., FRENCH, W., & GUARD, H.E. (1986b) Accumulation of bis
(tributyltin) oxide by the marine mussel Mytilus edulis. Environ. Sci.
Technol., 20: 884-890.
LAUGHLIN, R.B., PENDOLEY, P., & GUSTAFSON, R.G. (1987) Sublethal effects of
tributyltin on the hard shell clam, Mercenaria mercenaria. In: Proceedings
of the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1494-1498.
LAUGHLIN, R.B., GUSTAFSON, R., & PENDOLEY, P. (1988) Chronic embryo-larval
toxicity of tributyltin (TBT) to the hard shell clam Mercenaria mercenaria.
Mar. Ecol. Prog. Ser., 48: 29-36.
LAWLER, I.F. & ALDRICH, J.C. (1987) Sublethal effects of bis(tri- n-
butyltin)oxide on Crassostrea gigas spat. Mar. Pollut. Bull., 18: 274-278.
LEE, R.F. (1985) Metabolism of tributyltin oxide by crabs, oysters and
fish. Mar. environ. Res., 17: 145-148.
LEE, R.F. (1986) Metabolism of bis (tributyltin) oxide by estuarine
animals. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1182-1188.
LEE, R.F., VALKIRS, A.O., & SELIGMAN, P. (1987) Fate of tributyltin in
estuarine waters. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1411-1415.
LEWIS, P.G. & EMMETT, E.A. (1987) Irritant dermatitis from tributyl tin
oxide and contact allergy from chlorocresol. Contact dermatitis, 17: 129-
132.
LINDEN, O. (1987) The scope of the organotin issue in Scandinavia. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1320-1323.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G. (1979) The
acute toxicity of 78 chemicals and pesticide formulations against two
brackish water organisms, the bleak (Alburnus alburnus) and the
harpacticoid Nitocra spinipes. Chemosphere, 8: 843-851.
LYLE, W.H. (1958) Lesions of the skin in process workers caused by contact
with butyl tin compounds. Br. J. ind. Med., 15: 193-196.
MCCULLOUGH, F.S., GAYRAL, P., DUNCAN, J., & CHRISTIE, J.D. (1980)
Molluscicides in schistosomiasis control. World Health Organ. Bull., 58:
681-689.
MACKLAD, F., TAMAN, F., & EL SEBAE, A.K. (1983) Toxicity of tributyltin
fluoride and copper sulfate in slow release formulation to Biomphalaria
alexandrina snails. Bull. High Inst. Public Health, 14: 1-12.
MAFF/HSE (1988) Pesticides 1988: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 399 pp (Reference
Book HMSO 500).
MAFF/HSE (1989) Pesticides 1989: Pesticides approved under the control of
pesticides regulations 1986, London, UK Ministry of Agriculture, Fisheries
and Food (MAFF) and UK Health and Safety Executive (HSE), 407 pp (Reference
Book HMSO 500).
MAGUIRE, R.J. (1984) Butyltin compounds and inorganic tin in sediments in
Ontario. Environ. Sci. Technol., 18: 291-294.
MAGUIRE, R.J. (1987) Review: Environmental aspects of tributyltin. Appl.
organomet. Chem., 1: 475-498.
MAGUIRE, R.J. & HUNEAULT, H. (1981) Determination of butyltin species in
water by gas chromatography with flame photometric detection. J.
Chromatogr., 209: 458-462.
MAGUIRE, R.J. & TKACZ, R.J. (1983) Analysis of butyltin compounds by gas
chromatography. Comparison of flame photometric and atomic absorption
spectrophotometric detectors. J. Chromatogr., 268: 99-101.
MAGUIRE, R.J. & TKACZ, R.J. (1985) Degradation of the tri-n-butyltin
species in water and sediment from Toronto Harbor. J. agric. food Chem.,
33: 947-953.
MAGUIRE, R.J. & TKACZ, R.J. (1987) Concentration of tributyltin in the
microlayer of natural waters. Water Pollut. Res. J. Can., 22: 227-233.
MAGUIRE, R.J., CHAU, Y.K., BENGERT, G.A., HALE, E.J., WONG, P.T.S., &
KRAMAR, O. (1982) Occurrence of organotin compounds in Ontario lakes and
rivers. Environ. Sci. Technol., 16: 698-702.
MAGUIRE, R.J., CAREY, J.H., & HALE, E.J. (1983) Degradation of the tri-n-
butyltin species in water. J. agric. food Chem., 31: 1060-1065.
MAGUIRE, R.J., WONG, P.T.S., & RHAMEY, J.S. (1984) Accumulation and
metabolism of tri-n-butyltin cation by a green alga, Ankistrodesmus
falcatus. Can. J. Fish. aquat. Sci., 41: 537-540.
MAGUIRE, R.J., TKACZ, R.J., & SARTOR, D.L. (1985) Butyltin species and
inorganic tin in water and sediment of the Detroit and St. Clair rivers. J.
Great Lakes Res., 11: 320-327.
MAGUIRE, R.J., TKACZ, R.J., CHAU, Y.K., BENGERT, G.A., & WONG, P.T.S.
(1986) Occurrence of organotin compounds in water and sediment in Canada.
Chemosphere, 15: 253-274.
MATSUI, H., WADA, O., MANABE, S., ONO, T., IWAI, H., & FUJIKURA, T. (1982)
[Properties and mechanism of hyperlipidemia induced in rabbits by
tributyltin fluoride.] Jpn. J. ind. Health, 24: 163-171 (in Japanese).
MATTHIAS, C.L., BELLAMA, J.M., & BRINCKMAN, F.E. (1986a) Determination of
ultra-trace concentrations of butyltin compounds in water by simultaneous
hydridization/extraction with GC/FPD detection. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1146-1151.
MATTHIAS, C.L., OLSON, G.J., BELLAMA, J.M., & BRINCKMAN, F.E. (1986b)
Comprehensive method for determination of aquatic butyltin and
butylmethyltin species at ultratrace levels using simultaneous
hydridization/extraction with GC/FPD detection. Environ. Sci. Technol., 20:
609-615.
MATTHIESSEN, P. (1974) Some effects of slow-release bis (tri-n-butyl tin)
oxide on the tropical fish, Tilapia mossambica Peters. Controlled Release
Pesticide Symposium, Akron, Ohio, University of Akron, Community Technical
College, Engineering and Science Division, pp. 25.1-25.16.
MATTHIESSEN, P. & THAIN, J.E. (in press) A method for studying the impact
of polluted marine sediments on colonising organisms; tests with diesel-
based drilling mud and tributyltin antifouling paint. Hydrobiologia.
MAURER, D., HERAL, M., HIS, E., & RAZET, D. (1985) Influence d'une peinture
antisalissure à base de sels organométalliques de l'étain sur le captage en
milieu naturel de l'huitre Crassostrea gigas. Rev. Trav. Inst. Pêches
Marit., 47: 241-250.
MEADOR, J.P. (1986) An analysis of photobehavior of Daphnia magna exposed
to tributyltin. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1213-
1218.
MEINEMA, H.A., BURGER-WIERSMA, T., VERSLUIS DE HAAN, G., & GEVERS, E.C.
(1978) Determination of trace amounts of butyltin compounds in aqueous
systems by gas chromatography/mass spectrometry. Environ. Sci. Technol.,
12: 288-293.
MICHEL, P. (1987) Automatization of a hydride generation/A.A.S. system - an
improvement for organotin analysis. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1340-1343.
MIDDLETON, M.C. & PRATT, I. (1977) Skin water content as a quantitative
index of the vascular and histologic changes produced in rat skin by di- n-
butyltin and tri- n-butyltin. J. invest. Dermatol., 68: 379-384.
MIDDLETON, M.C. & PRATT, I. (1978) Changes in incorporation of
[3H]thymidine into DNA of rat skin following cutaneous application of
dibutyltin, tributyltin and 1-chloro-2:4-dinitrobenzene and the
relationship of these changes to a morphological assessment of the cellular
damage. J. invest. Dermatol., 71: 305-310.
MINCHIN, D., DUGGAN, C.B., & KING, W. (1987) Possible effects of organotins
on scallop recruitment. Mar. Pollut. Bull., 18: 604-608.
MOLIN, L. & WAHLBERG, J.E. (1975) Toxic skin reactions caused by
tributyltin oxide (TBTO) in socks. Berufs-dermatosen, 4: 138-142.
MORI, Y., IESATO, K., UEDA, S., MORI, T., IWASAKI, I., OHNISHI, K., SEINO,
Y., WAKASHIM, Y., WAKASHIN, M., & OKUDA, K. (1984) Renal tubular
disturbances induced by tributyl-tin oxide in guinea pigs: a secondary
Fanconi syndrome. Clin. Nephrol., 21: 118-125.
MULLER, H.A. (1987) Determination of tributyltin oxide in air. In:
Toxicology and analytics of the tributyltins: The present status.
Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986, Vlissingen-
Oost, The Netherlands, ORTEP Association, pp. 162-172.
MULLER, M.D. (1984) Tributyltin detection at trace levels in water and
sediments using GC with flame-photometric detection and GC-MS. Fresenius Z.
anal. Chem., 317: 32-36.
MULLER, M.D. (1987b) Comprehensive trace level determination of organotin
compounds in environmental samples using high-resolution gas chromatography
with flame photometric detection. Anal. Chem., 59: 617-623.
MUSHAK, P., KRIGMAN, M.R., & MAILMAN, R.B. (1982) Comparative organotin
toxicity in the developing rat: somatic and morphological changes and
relationship to accumulation of total tin. Neurobehav. Toxicol. Teratol.,
4: 209-215.
NEMEC, M.D. (1987) A teratology study in rabbits with TBTO: Final Report,
Wil Research Laboratories Inc., 210 pp (Report No. WIL-B0002) (Confidential
report to the Tributyl Tin Oxide Consortium).
NEWTON, F., THUM, A., DAVIDSON, B., VALKIRS, A., & SELIGMAN, P. (1985)
Effects on the growth and survival of eggs and embryos of the California
grunion (Leuresthes tenuis) exposed to trace levels of tributyltin, San
Diego, California, Naval Ocean Systems Center, 15 pp (Technical Report No.
1040).
NIVA (1986) [Organic tin compounds in fjord areas], Oslo, Norwegian
Institute for Water Research (NIVA) (in Norwegian).
O'CALLAGHAN, J.P. & MILLER, D.B. (1988) Acute exposure of the neonatal rat
to tributyltin results in decreases in biochemical indicators of
synaptogenesis and myelinogenesis. Pharmacol. exp. Ther., 246: 394-402.
OKOSHI, K., MORI, K., & NONURA, T. (1987) Characteristics of shell chamber
formation between the two races in Japanese oyster, Crassostrea gigas.
Aquaculture, 67: 313-320.
OLSON, G.J. & BRINCKMAN, F.E. (1986) Biodegradation of tributyltin by
Chesapeake Bay microorganisms. In: Proceedings of the Organotin Symposium,
Oceans '86 Conference, Washington, DC, USA, 23-25 September, 1986, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1196-1201.
OSBORN, D. & LEACH, D.V. (1987) Organotin in birds: Pilot study,
Huntingdon, Institute of Terrestrial Ecology, 15 pp (Final report to the UK
Department of the Environment. Contract No. F3CR/27/D4/01).
PAGE, D.S. (1989) An analytical method for butyltin species in shellfish.
Mar. Pollut. Bull., 20: 129-133.
PAUL, J.D. & DAVIES, I.M. (1986) Effects of copper- and tin-based anti-
fouling compounds on the growth of scallops (Pecten maximus) and oysters
(Crassostrea gigas). Aquaculture, 54: 191-203.
PAULINI, E. (1964) Laboratory experiments with some organotin compounds,
Geneva, World Health Organization, Parasitic Diseases Programme, pp. 1-3
(Unpublished report Mol/Inf/16).
PAULINI, E. & DE SOUZA, C.P. (1970) Influence of different suspended solids
in the water upon molluscicide activity, Geneva, World Health Organization,
Parasitic Diseases Programme, pp. 1-10 (Unpublished report PD/Mol/70.12).
PELIKAN, Z. (1969) Effects of bis(tri- n-butyltin) oxide on the eyes of
rabbits. Br. J. ind. Med., 26: 165-170.
PELIKAN, Z. & CERNY, E. (1968) [The toxic effects of tri- n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292 (in German).
PELIKAN, Z. & CERNY, E. (1969) Toxic effects of bis (tributyltin) oxide
(TBTO) on the skin of rats. Berufs-dermatosen, 17: 305-316.
PICKWELL, G.V. & STEINERT, S.A. (1988) Accumulation and effects of
organotin compounds in oysters and mussels: correlation with serum
biochemical and cytological factors and tissue burdens. Mar. environ. Res.,
24: 215-218.
PIETERS, R.H., KAMPINGA, J., BOL-SCHOENMAKERS, M., LAMB, B.W., PENNINKS,
A.H., & SEINEN, W. (1989) Organotin-induced thymus atrophy concerns the OX-
44+ immature thymocytes: Relation to the interaction between early
thymocytes and thymic epithelial cells? Thymus, 14(1-3): 79-88.
PINKNEY, A.E., HALL, L.W., LENKEVICH, M.J., BURTON, D.T., & ZEGER, S.
(1985) Comparison of avoidance responses of an estuarine fish, Fundulus
heteroclitus, and crustacean, Palaemonetes pugio, to bis (tri-n-butyltin)
oxide. Water Air Soil Pollut., 25: 33-40.
PINKNEY, A.E., MATTESON, L.L., & WRIGHT, D.A. (1988) Effects of tri-
butyltin on survival, growth, morphometry and RNA-DNA ratio of larval
striped bass, Morone saxatilis. In: Proceedings of the Organotin Symposium,
Oceans '88 Conference, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 987-991.
PLUM, H. (1981) Comportement des composés organostanniques vis-à-vis de
l'environnement. Inf. chim., 220: 135-139.
POITOU, P., MARIGNAC, B., CERTIN, C., & GRADISKI, D. (1978) Etude de
l'effet sur le système nerveux central et du pouvoir sensibilisant de
l'oxyde de tributylétain. Ann. Pharm. fr., 36: 569-572.
POLSTER, M. & HALACKA, K. (1971) [9. Contributions to the health-toxic
problems of some anti-microbially used organo-tin compounds.]
Ernährungsforschung, 16: 527-535 (in German).
RACEY, P.A. & SWIFT, S.M. (1986) The residual effects of remedial timber
treatments on bats. Biol. Conserv., 35: 205-214.
RANDALL, L., DONARD, O.F.X., & WEBER, J.H. (1986) Speciation of n-butyltin
compounds by atomic absorption spectrophotometry using electro-thermal
quartz furnace after hydride generation. Anal. Chim. Acta., 184: 197-203.
REIMANN, R. & LANG, R. (1987) Mutagenicity studies with tributyltin
compounds. In: Toxicology and analytics of the tributyltins: The present
status. Proceedings of an ORTEPA workshop, Berlin, 15-16 May, 1986,
Vlissingen-Oost, The Netherlands, ORTEP Association, pp. 66-90.
REINHARDT, C.A., SCHAWALDER, H., & ZBINDEN, G. (1982) Cell detachment and
cloning efficiency as parameters for cytotoxicity. Toxicology, 25: 47-52.
RICE, C.D., ESPOURTEILLE, F.A., & HUGGETT, R.J. (1987) Analysis of
tributyltin in estuarine sediments and oyster tissue, Crassostrea
virginica. Appl. organomet. Chem., 1: 541-544.
RITCHIE, L.S., BERRIOS-DURAN, L.A., FRICK, L.P., & FOX, I. (1964)
Molluscicidal time-concentration relationships of organo-tin compounds.
World Health Organ. Bull., 31: 147-149.
RITCHIE, L.S., LOPEZ, V.A., & CORA, J.M. (1974) Prolonged applications of
an organotin against Biomphalaria glabrata and Schistosoma mansoni. In:
Molluscicides in schistosomiasis control, New York, London, Academic Press,
pp. 77-88.
RIVM (1989) In: Mathijssen-Spiekman, E.A.M., Canton, J.H., & Roghair, C.J.,
ed. [Investigation into the toxicity of TBTO for a number of freshwater
organisms], Bilthoven, National Institute of Public Health and
Environmental Hygiene, 48 pp (Report No. 668118.001) (in Dutch).
ROBERTS, M.H. (1987) Acute toxicity of tributyltin chloride to embryos and
larvae of two bivalve mollusks, Crassostrea virginica and Mercenaria
mercenaria. Bull. environ. Contam. Toxicol., 39: 1012-1019.
ROBERTS, M.H., BENDER, M.E., DE LISLE, P.F., SUTTON, H.C., & WILLIAMS, R.L.
(1987) Sex ratio and gamete production in American oysters exposed to
tributyltin in the laboratory. In: Proceedings of the Organotin Symposium,
Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28 September-1
October, 1987, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1471-1476.
ROBINSON, I.N. (1969) Effects of some organotin compounds on tissue amine
levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROSENBERG, D.W. & DRUMMOND, G.S. (1983) Direct in vitro effects of bis
(tributyl) tin oxide on hepatic cytochrome P-450. Biochem. Pharmacol., 32:
3823-3829.
ROSENBERG, D.W., DRUMMOND, G.S., CORNISH, H.C., & KAPPAS, A. (1980)
Prolonged induction of hepatic haem oxygenase and decreases in cytochrome
P-450 content by organotin compounds. Biochem. J., 190: 465-468.
ROSENBERG, D.W., DRUMMOND, G.S., & KAPPAS, A. (1981) The influence of
organometals on heme metabolism. in vivo and in vitro studies with
organotins. Mol. Pharmacol., 21: 150-158.
ROSENBERG, D.W., ANDERSON, K.E., & KAPPAS, A. (1984) The potent induction
of intestinal heme oxygenase by the organotin compound, bis(tri- n-butyltin)
oxide. Biochem. biophys. Res. Commun., 119: 1022-1027.
SALAZAR, S.M. (1985) The effects of bis(tri-n-butyltin) oxide on three
species of marine phytoplankton, San Diego, California, Naval Ocean Systems
Center, 16 pp (Technical Report No. 1039).
SALAZAR, M.H. & SALAZAR, S.M. (1985) Ecological evaluation of
organotincontaminated sediment, San Diego, California, Naval Ocean Systems
Center, 21 pp (Technical Report No. 1050).
SALAZAR, M.H. & SALAZAR, S.M. (1987) Tributyltin effects on juvenile mussel
growth. In: Proceedings of the Organotin Symposium, Oceans '87 Conference,
Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1504-
1510.
SALAZAR, S.M., DAVIDSON, B.M., SALAZAR, M.H., STANG, P.M., & MEYERS-
SCHULTE, K.J. (1987) Effects of TBT on marine organisms: Field assessment
of a new site-specific bioassay system. In: Proceedings of the Organotin
Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada, 28
September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1461-1470.
SAXENA, P.N. & CROWE, A.J. (1988) An investigation of the efficacy of
organotin compounds for the control of the cotton stainer, Dysdercus
cingulatus, the mosquito, Anopheles stephensi, and the common house fly,
Musca domestica. Appl. organomet. Chem., 2: 185-187.
SCHAFER, E.W. & BOWLES, W.A. (1985) Acute oral toxicity and repellency of
933 chemicals to house and deer mice. Arch. environ. Contam. Toxicol., 14:
111-129.
SCHERING (1983) Repeated dose inhalation study of ZK21.995 in the rat for
29-32 days (21-24 exposures), Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. IC 1/83. Study No. TX81.177).
SCHERING (1986) Re-evaluation of a "mouse micronucleus test on TBTO",
Bergkamen, Federal Republic of Germany, Schering Inc. (Confidential report
No. IC 7186 prepared by the Institute of Occupational Health, Helsinki,
Finland, September 1984).
SCHERING (1989a) TBTO - 4 week oral (dietary administration) toxicity study
in the rat, Bergkamen, Federal Republic of Germany, Schering Inc. (Report
No. 280118 by Hazelton, France. Study No. 14/502).
SCHERING (1989b) TBTO - Plaque forming assay following a 5 week oral
toxicity study in the rat, Bergkamen, Federal Republic of Germany, Schering
Inc. (Report No. 283118 by Hazelton, France. Study No. 14/503).
SCHERING (1989c) TBTO - Resistance to Listeria monocytogene infection
following a 34 day oral toxicity study in the rat, Bergkamen, Federal
Republic of Germany, Schering Inc. (Report No. 282118 by Hazelton, France.
Study No. 14/505).
SCHERING (1989d) TBTO - Delayed type hypersensitivity test following a 37
day oral toxicity study in the rat, Bergkamen, Federal Republic of Germany,
Schering Inc. (Report No. 281118 by Hazelton, France).
SCHERING (1989e) TBTO - Systemic toxicity study in beagle dogs with daily
oral (intragastric) administration over a total of 18-19 weeks, Bergkamen,
Federal Republic of Germany, Schering Inc. (Report No. IC6/88).
SCHWEINFURTH, H. (1985) Toxicology of tributyltin compounds. Tin Uses, 143:
9-12.
SEIFFER, E.A. & SCHOOF, H.F. (1967) Tests of 15 experimental molluscicides
against Australorbis glabratus. Public Health Rep., 82: 833-839.
SEINEN, W., HELDER, T., VERNIJ, H., PENNINKS, A., & LEEUWANGH, P. (1981)
Short term toxicity of tri- n-butyltinchloride in rainbow trout (Salmo
gairdneri Richardson) yolk sac fry. Sci. total Environ., 19: 155-166.
SELIGMAN, P.F., VALKIRS, A.O., & LEE, R.F. (1986a) Degradation of
tributyltin in marine and estuarine waters. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1189-1195.
SELIGMAN, P.F., GROVHOUG, J.G. & RICHTER, K.E. (1986b) Measurement of
butyltins in San Diego Bay, CA: A monitoring strategy. In: Proceedings of
the Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1289-1296.
SELIGMAN, P.F., GROVHOUG, J.G., VALKIRS, A.O., STANG, P.M., FRANSHAM, R.,
STALLARD, M.O., DAVIDSON, B., & LEE, R.F. (1989) Distribution and fate of
tributyltin in the United States marine environment. Appl. organomet.
Chem., (in press).
SHELDON, A.W. (1975) Effects of organotin anti-fouling coatings on man and
his environment. J. Paint Technol., 47: 54-58.
SHERMAN, L.R. & CARLSON, T.L. (1980) A modified phenylfluorone method for
determining organotin compounds in the ppb and sub-ppb range. J. anal.
Toxicol., 4: 31-33.
SHIFF, C.J., YIANNAKIS, C., & EVANS, A.C. (1975) Further trials with TBTO
and other slow release molluscicides in Rhodesia. In: Proceedings of the
Controlled Release Pesticide Symposium, Wright State University, Dayton,
Ohio, 8-10 September, 1975, pp. 177-188.
SHIMIZU, A. & KIMURA, S. (1987) [Effect of bis (tributyltin) oxide on
gonadal development of a salt-water goby, Chasmichthys dolichognathus:
Exposure during maturing period.] Bull. Tokai Reg. Fish. Res. Lab., 123:
45-49 (in Japanese).
SHORT, J.W. & THROWER, F.P. (1986) Accumulation of butyltins in muscle
tissue of chinook salmon reared in sea pens treated with tri- n-butyltin.
In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1177-1181.
SHORT, J.W. & THROWER, F.P. (1987) Toxicity of tri- n-butyl-tin to chinook
salmon, Oncorhynchus tshawytscha, adapted to seawater. Aquaculture, 61:
193-200.
SLESINGER, A.E. & DRESSER, I. (1978) The environmental chemistry of three
organotin chemicals. In: Good, M., ed. Report of the Organotin Workshop,
New Orleans, Louisiana, 17-19 February, 1978, pp. 115-162.
SMITH, B.S. (1981a) Male characteristics on female mud snails caused by
antifouling paints. J. appl. Toxicol., 1: 22-25.
SMITH, B.S. (1981b) Tributyltin compounds induce male characteristics on
female mud snails Nassarius obsoletus = Ilyanassa obsoleta. J. appl.
Toxicol., 1: 141-144.
SMITH, B.S. (1981c) Male characteristics in female Nassarius obsoletus:
Variations related to locality, season and year. Veliger, 23: 212-216.
SMITH, D.R., STEPHENSON, M.D., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1987) The use of transplanted juvenile oysters to monitor the toxic
effects of tributyltin in California waters. In: Proceedings of the
Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia, Canada,
28 September-1 October, 1987, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1511-1516.
SMITH, P.J., CROWE, A.J., ALLEN, D.W., BROOKS, J.S., & FORMSTONE, R. (1977)
Study by 119m Sn Mossbauer spectroscopy of bis(tri- n-butyltin) oxide
adsorbed on cellulosic materials. Chem. Ind., 23: 874-875.
SNOEIJ, N.J. (1987) Triorganotin compounds in immunotoxicology and bio-
chemistry, Utrecht, The Netherlands, University of Utrecht, 170 pp (Ph.D.
Thesis).
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1985)
Toxicity of triorganotin compounds: Comparative in vivo studies with a
series of trialkyltin compounds and triphenyltin chloride in male rats.
Toxicol. appl. Pharmacol., 81: 274-286.
SNOEIJ, N.J., PUNT, P.M., PENNINKS, A.H., & SEINEN, W. (1986a) Effects of
tri- n-butyltin chloride on energy metabolism, macromolecular synthesis,
precursor uptake and cyclic AMP production in isolated rat thymocytes.
Biochim. Biophys. Acta, 852: 234-243.
SNOEIJ, N.J., VAN IERSEL, A.A.J., PENNINKS, A.H., & SEINEN, W. (1986b)
Triorganotin-induced cytotoxicity to rat thymus, bone marrow and red blood
cells as determined by several in vitro assays. Toxicology, 39: 71-83.
SNOEIJ, N.J., PIETERS, R.H.H., PENNINKS, A.H., & SEINEN, W. (1987) Toxicity
of triorganotin compounds: Orally administered tri- n-butyltin compounds are
rapidly dealkylated in the rat. In: Triorganotincompounds in immunology and
biochemistry, Utrecht, The Netherlands, University of Utrecht, Chapter 4,
pp. 73-93 (Snoeij, N.J., Ph.D. Thesis).
SNOEIJ, N.J., BOL-SCHOENMAKERS, M., PENNINKS, A.H., & SEINEN, W. (1988a)
Differential effects of tri- n-butyltin chloride on macromolecular synthesis
and ATP levels of rat thymocyte subpopulations obtained by centrifugal
elutriation. Int. J. Immunopharmacol., 10: 29-37.
SNOEIJ, N.J., PENNINKS, A.H., & SEINEN, W. (1988b) Dibutyltin and
tributyltin compounds induce thymus atrophy in rats due to a selective
action on thymic lymphoblasts. Int. J. Immunopharmacol., 10: 891-899.
SORACCO, R.J. & POPE, D.H. (1983) Bacteriostatic and bactericidal modes of
action of bis (tributlytin) oxide on Legionella pneumophila. Appl. environ.
Microbiol., 45: 48-57.
STALLARD, M., HODGE, V., & GOLDBERG, E.D. (1987) TBT in California coastal
waters: Monitoring and assessment. Environ. Monit. Assess., 9: 195-220.
STANG, P.M. & SELIGMAN, P.F. (1986) Distribution and fate of butyltin
compounds in the sediment of San Diego Bay. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1256-1261.
STANG, P.M. & SELIGMAN, P.F. (1987) In situ adsorption and desorption of
butyltin compounds from Pearl Harbor, Hawaii, sediment. In: Proceedings of
the Organotin Symposium, Oceans '87 Conference, Halifax, Nova Scotia,
Canada, 28 September-1 October, 1987, New York, The Institute of Electrical
and Electronics Engineers, Inc., Vol. 4, pp. 1386-1391.
STEIN, T. & KUSTER, K. (1982) Der biologische abbau von tributylzinnoxid
(TBTO) in einer belebtschlammanlage. Z. Wasser Abwasser Forsch., 15: 178-
180.
STEPHENSON, M.D., SMITH, D.R., GOETZL, J., ICHIKAWA, G., & MARTIN, M.
(1986) Growth abnormalities in mussels and oysters from areas with high
levels of tributyltin in San Diego Bay. In: Proceedings of the Organotin
Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25 September,
1986, New York, The Institute of Electrical and Electronics Engineers,
Inc., Vol. 4, pp. 1246-1251.
STEPHENSON, M.D., SMITH, D.R., HALL, L.W., JOHNSON, W.E., MICHEL, P.,
SHORT, J., WALDOCK, M., HUGGETT, R.J., SELIGMAN, P., & KOLA, S. (1987) An
international intercomparison of butyltin determinations in mussel tissue
and sediments. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1334-1338.
STROMGREN, T. & BONGARD, T. (1987) The effect of tributyltin oxide on
growth of Mytilus edulis. Mar. Pollut. Bull., 18: 30-31.
TEMMINK, J.H.M. & EVERTS, J.W. (1987) Comparative toxicity of tributyltin-
oxide (TBTO) for fish and snail. In: Proceedings of the Seventh World
Meeting of the ORTEP-Association, Amsterdam, 7-8 May, 1987, Vlissingen-
Oost, The Netherlands, ORTEP-Association, pp. 6-20.
THAIN, J.E. (1983) The acute toxicity of bis (tributyl tin) oxide to the
adults and larvae of some marine organisms, Copenhagen, International
Council for the Exploration of the Sea (ICES), 5 pp (Report No. C. M.
1983/E.13).
THAIN, J.E. (1986) Toxicity of TBT to bivalves: Effects on reproduction,
growth and survival. In: Proceedings of the Organotin Symposium, Oceans '86
Conference, Washington, DC, USA, 23-25 September, 1986, New York, The
Institute of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1306-
1313.
THAIN, J.E. & WALDOCK, M.J. (1985) The growth of bivalve spat exposed to
organotin leachates from antifouling paints, Copenhagen, International
Council for the Exploration of the Sea (ICES), 10 pp (Report No. C. M.
1985/E.28).
THAIN, J.E. & WALDOCK, M.J. (1986) The impact of tributyl tin (TBT)
antifouling paints on molluscan fisheries. Water Sci. Technol., 18: 193-
202.
THAIN, J.E., WALDOCK, M.J., & WAITE, M.E. (1987) Toxicity and degradation
studies of tributyltin (TBT) and dibutyltin (DBT) in the aquatic
environment. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1398-1404.
THOMAS, T.E. & ROBINSON, M.G. (1986) The physiological effects of the
leachates from a self-polishing organotin antifouling paint on marine
diatoms. Mar. environ. Res., 18: 215-229.
THOMAS, T.E. & ROBINSON, M.G. (1987) Initial characterization of the
mechanisms responsible for the tolerance of Amphora coffeaeformis to copper
and tributyltin. Bot. mar., 30: 47-53.
THOMPSON, J.A.J., SHEFFER, M.G., PIERCE, R.C., CHAU, Y.K., COONEY, J.J.,
CULLEN, W.R., & MAGUIRE, R.J. (1985) Organotin compounds in the aquatic
environment: Scientific criteria for assessing their effects on
environmental quality, Ottawa, National Research Council Canada, 284 pp
(NRCC No. 22494).
TOLEDO, J.V., MONTEIRO DA SILVA, C.S., BULHOES, M.S., PAES LEME, L.A., DA
SILVA NETTO, J.A., & GILBERT, B. (1976) Snail control in urban sites in
Brazil with slow-release hexabutyldistannoxane and pentachlorophenol. World
Health Organ. Bull., 54: 421-425.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.-P., PHU LICH N., VAN DEN DRIESSCHE, J.,
GUESNIER, L.R., & MORIN, N. (1976) Contribution à l'étude toxicologique et
pharmacologique de l'oxyde de tributylétain (OTBE). Eur. J. Toxicol., 9:
31-40.
TRUHAUT, R., ANGER, J.P., REYMANN, J.M., CHAUVEL, Y., & VAN DEN DRIESSCHE,
J. (1979) Influence de l'oxyde de tributylétain (OTBE) en aérosol sur le
comportement exploratoire chez la souris. Toxicol. Eur. Res., 11: 181-186.
TRUHAUT, R., ANGER, J.P., ANGER, F., BRAULT, A., BRUNET, P., CANO, Y.,
LOUVET, M., SACCAVINI, J.C., VAN DEN DRIESSCHE, J., JANSON, C., & SUBLET,
Y. (1981) Dégradation thermique de l'oxyde de tributylétain (OTBE) et
toxicité pulmonaire des produits de combustion chez la souris et le cobaye.
Toxicol. Eur. Res., 111: 35-44.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1986)
Bioconcentration of butyltin compounds by round crucian carp. Toxicol.
environ. Chem., 12: 137-143.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1987)
Bioconcentration and metabolism of phenyl tin chlorides in carp. Water
Res., 21: 949-953.
TSUDA, T., NAKANISHI, H., AOKI, S., & TAKEBAYASHI, J. (1988)
Bioconcentration and metabolism of butyltin compounds in carp. Water Res.,
22: 647-651.
TWG (1988a) Uses and production of organotin compounds. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group), Brussels, 7-11 March, 1988, 6 pp (Report No. TWG 15/5/1-E).
TWG (1988b) Tributyl tin compounds in antifouling paints. Presented by the
Federal Republic of Germany at the Convention for the Prevention of Marine
Pollution from Land-based Sources (15th Meeting of the Technical Working
Group, Brussels, 7-11 March, 1988, 2 pp, (Report No. TWG 15/5/2-E).
TWG (1988c) Organotins in the Netherlands. Presented by the Netherlands at
the Convention for the Prevention of Marine Pollution from Land-based
Sources (15th Meeting of the Technical Working Group, Brussels, 7-11 March,
1988, 11 pp (Report No. TWG 15/5/4-E).
UHL, S. (1986) [Population exposure to the environmental chemical
pentachloro-ophenol (PCP) and bis (tri-n- butyltin)oxide (TBTO)], Zurich,
Maus Offsetdruck Konstanz, 145 pp (ETH Thesis No. 8014, University of
Gottingen) (in German).
UNEP (1989) Mediterranean Action Plan. Assessment of organotin compounds as
marine pollutants in the Mediterranean, Athens, United Nations Environment
Programme (MAP Technical Report Series, No. 33).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1986) Organotin in
antifouling paints environmental considerations, London, UK Department of
the Environment, 82 pp (Pollution Paper No. 25).
UNITED KINGDOM DEPARTMENT OF THE ENVIRONMENT (1989) Water and the
environment, London, UK Department of the Environment, p. 26 (Circular No.
7/89).
UPATHAM, E.S., KOURA, M., AHMED, M.D., & AWAD, A.H. (1980) Laboratory
trials of controlled release molluscicides on Bulinus (Ph.) abyssinicus,
the intermediate host of Schistosoma haematobium in Somalia. In: Baker,
R.W., ed. Controlled release of bioactive materials. Proceedings of the 6th
International Meeting of the controlled Release Society, New York, London,
Academic Press, pp. 461-469.
U'REN, S.C. (1983) Acute toxicity of bis(tributyltin) oxide to a marine
copepod. Mar. Pollut. Bull., 14: 303-306.
VALKIRS, A.O., SELIGMAN, P.F., STANG, P.M., HOMER, V., LIEBERMAN, S.H.,
VAFA, G., & DOOLEY, C.A. (1986) Measurement of butyltin compounds in San
Diego Bay. Mar. Pollut. Bull., 17: 319-324.
VALKIRS, A.O., DAVIDSON, B.M., & SELIGMAN, P.F. (1987) Sublethal growth
effects and mortality to marine bivalves from long-term exposure to
tributyltin. Chemosphere, 16: 201-220.
VIGHI, M. & CALAMARI, D. (1985) QSARs for organotin compounds on Daphnia
magna. Chemosphere, 14: 1925-1932.
VIYANANT, V., THIRACHANTRA, S., & SORNMANI, S. (1982) The effect of
controlled release copper sulphate and tributyltin fluoride on the
mortality and infectivity of Schistosoma mansoni cercariae. J. Helminthol.,
56: 85-92.
VOS, J.G., DE KLERC, A., KRAJNC, E.I., KRUIZINGA, W., VAN OMMEN, B., &
ROZING, J. (1984) Toxicity of bis(tri- n-butyltin)oxide in the rat. II.
Suppression of thymus-dependent immune responses and of parameters of non-
specific resistance after short-term exposure. Toxicol. appl. Pharmacol.,
75: 387-408.
VOS, J.G., KRAJNC, E.I., & WESTER, P.W. (1985) Immunotoxicity of bis (tri-
n-butyltin) oxide. In: Dean, J., ed. Immunotoxicology and
immunopharmacology, New York, Raven Press, pp. 327-340.
WADE, T.L., GARCIA-ROMERO, B., & BROOKS, J.M. (1988) Tributyltin
contamination in bivalves from U.S. coastal estuaries. Environ. Sci.
Technol., 22: 1488-1493.
WALDOCK, M.J. (1989) Organotin concentrations in the Rivers Bure and Yare,
Norfolk Broads, England (Unpublished report of the Ministry of Agriculture,
Fisheries and Food, Fisheries Laboratory, Burnham-on-Crouch, Essex, UK).
WALDOCK, M.J. & MILLER, D. (1983) The determination of total and tributyl
tin in seawater and oysters in areas of high pleasure craft activity,
Copenhagen, International Council for the Exploration of the Sea (ICES), 18
pp (Report No. C. M. 1983/E.12).
WALDOCK, M.J. & THAIN, J.E. (1983) Shell thickening in Crassostrea gigas:
Organotin antifouling or sediment induced? Mar. Pollut. Bull., 14: 411-415.
WALDOCK, M.J. & THAIN, J.E. (1985) The comparative leach rates and toxicity
of two fish net antifouling preparations, Copenhagen, International Council
for the Exploration of the Sea (ICES), 5 pp (Report No. C. M. 1985/E.29).
WALDOCK, M.J., THAIN, J.E. & MILLER, D. (1983) The accumulation and
depuration of bis (tributyl tin) oxide in oysters: A comparison between the
Pacific oyster (Crassostrea gigas) and the European flat oyster (Ostrea
edulis), Copenhagen, International Council for the Exploration of the Sea
(ICES), 6 pp (Report No. C. M. 1983/E.52).
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1987a) Changes in concentrations
of organotins in U.K. rivers and estuaries following legislation in 1986.
In: Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1352-1356.
WALDOCK, M.J., THAIN, J.E., & WAITE, M.E. (1987b) The distribution and
potential toxic effects of TBT in UK estuaries during 1986. Appl.
organomet. Chem., 1: 287-301.
WALDOCK, M.J., WAITE, M.E., & THAIN, J.E. (1988) Inputs of TBT to the
marine environment from shipping activity in the U.K. Environ. Technol.
Lett., 9: 999-1010.
WALNE, P.R. & HELM, M.M. (1979) Introduction of Crassostrea gigas into the
United Kingdom. In: Mann, R., ed. Proceedings of a Symposium on Exotic
Species in Marinculture: Case histories of the Japanese Oyster Crassostrea
gigas (Thunberg), with implications for other fisheries, Woods Hole,
Massachusetts, USA, 18-20 September, 1978, Cambridge, London, MIT Press,
pp. 83-105.
WALSH, G.E. (1986) Organotin toxicity studies conducted with selected
marine organisms at EPA's environmental research laboratory, Gulf Breeze,
Florida. In: Proceedings of the Organotin Symposium, Oceans '86 Conference,
Washington, DC, USA, 23-25 September, 1986, New York, The Institute of
Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1210-1212.
WALSH, G.E., MCLAUGHLAN, L.L., LORES, E.M., LOUIE, M.K., & DEANS, C.H.
(1985) Effects of organotins on growth and survival of two marine diatoms,
Skeletonema costatum and Thalassiosira pseudonana. Chemosphere, 14: 383-
392.
WALSH, G.E., LOUIE, M.K., MCLAUGHLIN, L.L., & LORES, E.M. (1986a) Lugworm
(Arenicola cristata) larvae in toxicity tests: Survival and development
when exposed to organotins. Environ. Toxicol. Chem., 5: 749-754.
WALSH, G.E., MCLAUGHLIN, L.L., LOUIE, M.K., DEANS, C.H., & LORES, E.M.
(1986b) Inhibition of arm regeneration by Ophioderma brevispina
(Echinodermata, Ophiuroidea) by tributyltin oxide and triphenyltin oxide.
Ecotoxicol. environ. Saf., 12: 95-100.
WALTON, R., ADEMA, C.M., & SELIGMAN, P.F. (1986) Mathematical modeling of
the transport and fate of organotin in harbors. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1297-1301.
WARD, G.S., CRAMM, G.C., PARRISH, P.R., TRACHMAN, H., & SLESINGER, A.
(1981) Bioaccumulation and chronic toxicity of bis(tributyltin) oxide
(TBTO): Tests with a saltwater fish. In: Branson, D.R. & Dickson, K.L., ed.
Proceedings of the Fourth Conference on Aquatic Toxicology and Hazard
Assessment, Philadelphia, American Society for Testing and Materials, pp.
183-200 (ASTM STP No. 737).
WEBBE, G. (1963) Laboratory tests of some new molluscicides (organotin
compounds), Geneva, World Health Organization, Parasitic Diseases
Programme, pp. 1-2 (Unpublished report Mol/Inf/14).
WEBER, J.H., DONARD, O.F.X., RANDALL, I., & HAN, J.S. (1986) Speciation of
methyl and butyltin compounds in the Great Bay estuary (N.H.). In:
Proceedings of the Organotin Symposium, Oceans '86 Conference, Washington,
DC, USA, 23-25 September, 1986, New York, The Institute of Electrical and
Electronics Engineers, Inc., Vol. 4, pp. 1280-1282.
WEIS, J.S., WEIS, P., & WANG, F. (1987a) Developmental effects of
tributyltin on the fiddler crab, Uca pugilator, and the killifish, Fundulus
heteroclitus. In: Proceedings of the Organotin Symposium, Oceans '87
Conference, Halifax, Nova Scotia, Canada, 28 September-1 October, 1987, New
York, The Institute of Electrical and Electronics Engineers, Inc., Vol. 4,
pp. 1456-1460.
WEIS, J.S., GOTTLIEB, J., & KWIATKOWSKI, J. (1987b) Tributyltin retards
regeneration and produces deformities of limbs in the fiddler crab, Uca
pugilator. Arch. environ. Contam. Toxicol., 16: 321-326.
WESTER, P.W., CANTON, J.H., VAN IERSAL, A.A.J., KRANJC, E.I., & VAESSEN,
H.A.M.G. (1988) The toxicity of bis(tri-n- butyltin)oxide (TBTO) and di-n-
butyltindichloride (DBTC) in the small fish species Orysias latipes
(medaka) and Poecilia reticulata (guppy), Utrecht, The Netherlands,
University of Utrecht (Wester, P.W., Ph.D. Thesis).
WESTER, P.W. (in press) Chronic toxicity and carcinogenicity of bis (tri-n-
butyltin) oxide in the rat. Food Chem. Toxicol.
WHO/FAO (1984) Data sheet on pesticides No. 65: Bis (tributyltin)oxide,
Geneva, World Health Organization (VBC/PDS/DS/85.65).
WOLNIAKOWSKI, K.U., STEPHENSON, M.D., & ICHIKAWA, G.S. (1987) Tributyltin
concentrations and Pacific oyster deformations in Coos Bay, Oregon. In:
Proceedings of the Organotin Symposium, Oceans '87 Conference, Halifax,
Nova Scotia, Canada, 28 September-1 October, 1987, New York, The Institute
of Electrical and Electronics Engineers, Inc., Vol. 4, pp. 1438-1442.
WONG, P.T.S., CHAU, Y.K., KRAMAR, O., & BENGERT, G.A. (1982) Structure-
toxicity relationship of tin compounds on algae. Can. J. Fish. aquat. Sci.,
39: 483-488.
WOOTEN, R., DAVIES, I.M., MCKIE, J.C., & BRUNO, D.W. (1986) Chemical and
histopathological changes in salmon exposed to low concentrations of
tributyltin in seawater, Aberdeen, Department of Agriculture and Fisheries
for Scotland, 3 pp (Working Paper No. 15/86).
YLA-MONONEN, L. (1988) Finnish studies on the use, occurrence and effects
of organic tin compounds. Presented at the OECD Workshop on Monitoring,
Chemical Analysis and Leaching Rates of TBT, Paris, 30 November-2 December,
1988, 9 pp (Unpublished report).
YOUNG, D.R., SCHATZBERG, P., BRINCKMAN, F.E., CHAMP, M.A., HOLM, S.E., &
LANDY, R.B. (1986) Summary report - Interagency workshop on aquatic
sampling and analysis for organotin compounds. In: Proceedings of the
Organotin Symposium, Oceans '86 Conference, Washington, DC, USA, 23-25
September, 1986, New York, The Institute of Electrical and Electronics
Engineers, Inc., Vol. 4, pp. 1135-1140.
ZEDLER, R.J. (1961) Organotin as industrial biochemicals. Tin Uses,
53: 7-11.
ZIMMERLI, B. & ZIMMERMANN, H. (1980) Gas-chromatische bestimmung von spuren
von n-butylzinnverbindungen (tetra-, tri-, di-). Fresenius Z. anal. Chem.,
304: 23-27.
ZUCKERMAN, J.J., REISDORF, R.P., ELLIS, H.V., & WILKINSON, R.R. (1978)
Organotins in biology and the environment. In: Brinkman, F.E. & Bellama,
J.M., ed. Organometals and organometalloids, occurrence and fate in the
environment, Washington, DC, American Chemical Society, pp. 388-424 (ACS
Symposium Series No. 82).
RESUME
1. Identité, propriétés physiques et chimiques
Les dérivés du tributylétain (TBT) sont des dérivés
organiques de l'étain tétravalent. Ils se caractérisent
par la présence de liaisons covalentes entre des atomes de
carbone et un atome d'étain et leur formule générale est
la suivante: (n-C4H9)3 Sn-X (dans laquelle X désigne
un anion). La pureté de l'oxyde de tributylétain du com-
merce est généralement supérieure à 96%; les principales
impuretés sont le dibutylétain et dans une moindre mesure
le tétrabutylétain et d'autres trialkylétains. L'oxyde de
tributylétain est un liquide incolore d'odeur caractéris-
tique et de densité comprise entre 1,17 et 1,18. Il est
peu soluble dans l'eau (solubilité comprise entre moins
de 1,0 et plus de 100 mg/litre selon le pH, la température
et les anions présents dans l'eau qui déterminent l'espèce
chimique en cause). Dans l'eau de mer et dans les con-
ditions normales, on rencontre trois dérivés du tributylé-
tain: l'hydroxyde, le chlorure et le carbonate, qui sont
en équilibre. Aux pH inférieurs à 7,0 les formes prédomi-
nantes sont Bu3SnOH2+ et Bu3SnCl; à pH 8 ce sont
Bu3SnCl, Bu3SnOH et Bu3SnCO3-; au-delà de 10, ce
sont Bu3SnOH et Bu3SnCO3- qui prédominent.
Le coefficient de partage octanol/eau (log de Pow)
est compris entre 3,18 et 3,84 pour l'eau distillée et il
est égal à 3,54 pour l'eau de mer. L'oxyde de tributylé-
tain s'adsorbe fortement aux matières particulaires,
puisque les coefficients d'absorption indiqués dans la
littérature vont de 110 à 55 000. La tension de vapeur est
faible mais les valeurs publiées sont extrêmement varia-
bles. On n'a constaté aucune perte d'oxyde de tributylé-
tain à partir d'une solution de 1 mg/litre en 62 jours,
toutefois 20% de l'eau avait disparu par évaporation.
2. Méthodes d'analyse
On utilise plusieurs méthodes pour le dosage des
dérivés du tributylétain dans l'eau, les sédiments ou les
biotes. La plus communément utilisée est la spectrométrie
d'absorption atomique. La spectrométrie d'absorption
atomique avec flamme a une limite de détection de
0,1 mg/litre. Sans flamme, avec atomisation dans un four
électrique à graphite, elle est plus sensible et ses
limites de détection varient entre 0 et 1,0 µg/litre
d'eau. Il existe plusieurs méthodes d'extraction et de
préparation de dérivés volatils. La séparation de ces
dérivés s'effectue habituellement par piégeage ou par
chromatographie en phase gazeuse. Les limites de détection
se situent entre 0,5 et 5,0 µg/kg dans le cas des sédi-
ments et des biotes.
3. Sources de pollution de l'environnement
Les dérivés du tributylétain sont homologués comme
molluscicides, comme produits antisalissures pour la
préservation des coques de bateaux, des appontements, des
bouées, des casiers à crabes, des filets et des cages,
comme enduits de protection du bois, comme alguicides dans
le bâtiment, comme désinfectants et comme biocides dans
les systèmes de réfrigération, les tours de réfrigération
des centrales électriques, les usines de pâte à papier,
les brasseries, les tanneries et les usines textiles. Les
premières peintures antisalissures à base de TBT con-
tenaient ce produit sous une forme qui en permettait la
libération sans entrave. Plus récemment, sont apparues des
peintures dans lesquelles l'incorporation du TBT dans une
matrice en copolymère permet d'en limiter la libération.
On a également mis au point des matrices caoutchouteuses
qui permettent une libération lente et durable et assurent
aux peintures antisalissures et aux molluscicides une
efficacité prolongée. Le TBT n'est pas utilisé en agri-
culture en raison de sa forte phytotoxicité.
4. Réglementation
De nombreux pays ont restreint l'utilisation des
peintures antisalissures à base de TBT du fait de l'action
de cette substance sur les fruits de mer. Les détails de
la réglementation varient d'un pays à l'autre mais la
plupart interdisent l'emploi de peintures à base de TBT
sur les navires de moins de 25 mètres. Dans certains pays,
les navires à coque d'aluminium ne sont pas visés par
cette interdiction. En outre, certaines réglementations
limitent la teneur des peintures en TBT ou la lixiviation
de cette substance à partir des peintures qui en contien-
nent (4 à 5 µg/cm2 par jour sur une longue période).
5. Concentrations dans l'environnement
On a trouvé de fortes concentrations de TBT dans
l'eau, les sédiments et les biotes à proximité de zones de
plaisance, plus particulièrement de marinas, de chantiers
navals et de bassins de radoub, de filets et de cages
traités au moyen de peintures antisalissures et de sys-
tèmes de réfrigération. Ces concentrations de TBT dépen-
dent également de la submersion par la marée et de la
turbidité de l'eau.
On a observé que les concentrations de TBT pouvaient
atteindre 1,58 µg/litre dans l'eau de mer et les estuai-
res, 7,1 µg/litre dans l'eau douce, 26 300 µg/kg dans
les sédiments littoraux, 3700 µg/kg dans les sédiments
d'eau douce, 6,39 mg/kg dans les bivalves, 1,92 mg/kg dans
les gastéropodes et 11 mg/kg dans le poisson. Il ne faut
pas considérer cependant ces concentrations maximales
comme caractéristiques car un certain nombre de facteurs
peuvent donner lieu à des teneurs anormalement élevées
(par exemple la présence de particules de peinture dans
les échantillons d'eau et de sédiments). On a constaté que
les concentrations de TBT dans la micro-couche de surface
des eaux douces et des eaux de mer étaient jusqu'à 100
fois plus élevées que celles qu'on pouvait mesurer juste
en dessous de la surface. Toutefois, il convient de noter
que la concentration en TBT dans la micro-couche de
surface peut dépendre dans une très large mesure de la
technique d'échantillonnage.
Il se peut que les données anciennes ne soient pas
comparables aux données récentes en raison des amélio-
rations apportées aux méthodes de dosage du TBT dans
l'eau, les sédiments et les tissus.
6. Transport et transformation dans l'environnement
Du fait de sa faible solubilité dans l'eau et de son
caractère lipophile, le TBT s'adsorbe facilement aux par-
ticules. On estime que 10 à 95% de l'oxyde de tributylé-
tain qui pénètrent dans l'eau s'adsorbent ainsi sur les
particules. La disparition progressive du TBT absorbé
n'est pas due à sa désorption mais à sa dégradation. Le
degré d'adsorption dépend de la salinité, de la nature et
de la taille des particules en suspension, de la quantité
de matières en suspension, de la température et de la
présence de matières organiques dissoutes.
La dégradation de l'oxyde de tributylétain s'effectue
par rupture de la liaison carbone-étain. Celle-ci peut
résulter de divers mécanismes qui se produisent simultané-
ment dans l'environnement et notamment des mécanismes
physico-chimiques (hydrolyse et photodécomposition) ou
biologiques (dégradation par des micro-organismes et
métabolisation par des organismes supérieurs). L'hydrolyse
des dérivés organostanniques se produit à des valeurs
extrêmes du pH mais n'apparaît guère dans les conditions
qui règnent normalement dans l'environnement. La photo-
décomposition se produit par exposition en laboratoire de
solutions à un rayonnement ultra-violet de 300 nm (et à un
moindre degré, à un rayonnement de 350 nm). Dans le milieu
naturel, la photolyse est limitée par la longueur d'onde
du rayonnement solaire et par la pénétration du rayonne-
ment ultra-violet dans l'eau. La présence de substances
photosensibilisatrices peut accélérer la photodécomposi-
tion. La biodégradation dépend de l'état du milieu, et de
caractéristiques telles que sa température, son oxygéna-
tion, son pH, sa teneur en éléments minéraux, la présence
de substances organiques facilement biodégradables pouvant
subir une co-métabolisation ainsi que la nature de la
micro-flore et sa capacité à s'adapter. Elle ne peut
également avoir lieu que si la concentration en oxyde de
tributylétain est inférieure à la concentration létale ou
inhibitrice pour les bactéries. Comme dans le cas de la
décomposition abiotique, la dégradation biologique du TBT
comporte une débutylation oxydante progressive avec rup-
ture de la liaison carbone-étain. Il se forme des dérivés
dibutylés dont la dégradation est plus facile que celle du
tributylétain. Les monobutylétains sont lentement minéra-
lisés. Il se produit également une dégradation anaérobie
mais son importance reste discutée. Certains chercheurs
estiment que la dégradation en anaérobiose est lente alors
que d'autres la jugent plus rapide que la dégradation
aérobie. On a identifié des espèces de bactéries, d'algues
et de champignons attaquant le bois qui sont capables de
dégrader l'oxyde de tributylétain. Les estimations de la
demi-vie du TBT dans l'environnement varient dans
d'importantes proportions.
Le TBT s'accumule dans les organismes du fait de sa
solubilité dans les graisses. Des recherches en labora-
toire portant sur des mollusques et des poissons ont
donné, pour les facteurs de bioconcentration, des valeurs
allant jusqu'à 7000 mais des valeurs encore plus élevées
ont été observées lors d'études sur le terrain. L'absorp-
tion à partir de la nourriture est plus importante qu'à
partir de l'eau. Les facteurs de concentration plus élevés
observés chez les micro-organismes (entre 100 et 30 000)
peuvent s'expliquer par une adsorption plutôt que par une
absorption intracellulaire. Rien n'indique que le TBT
puisse passer dans les organismes terrestres par l'inter-
médiaire de la chaîne alimentaire.
7. Cinétique et métabolisme
Le tributylétain est absorbé au niveau intestinal (20
à 50% selon le véhicule) ainsi que par voie percutanée
chez les mammifères (dans la proportion d'environ 10%).
Il peut traverser la barrière hémo-méningée et passer du
placenta dans le foetus. Une fois absorbé, il est rapide-
ment et largement diffusé dans l'organisme (principalement
au niveau du foie et des reins).
Chez les mammifères, la métabolisation du TBT est
rapide; on peut déceler les métabolites dans le sang dans
les 3 heures suivant l'administration. Des études in vitro
ont montré que le TBT servait de substrat aux oxydases à
fonction mixte mais qu'il inhibait ces enzymes à très
forte concentration.
L'élimination du TBT s'effectue plus ou moins rapide-
ment selon la nature du tissu et les estimations de la
demi-vie biologique chez les mammifères varient de 23 à
environ 30 jours.
Les organismes inférieurs métabolisent également le
TBT mais le processus est plus lent - en particulier chez
les mollusques - que chez les mammifères. La capacité de
bioaccumulation est donc beaucoup plus importante que chez
les mammifères.
Les tributylétains inhibent la phosphorylation oxy-
dative et modifient la structure et la fonction des mito-
chondries. Le TBT empêche la calcification de la coquille
des huîtres (espèces du genre Crassostrea ).
8. Effets sur les micro-organismes
Le TBT est toxique pour les micro-organismes et on le
vend comme bactéricide et alguicide. Les concentrations
toxiques varient considérablement selon les espèces. Le
TBT est plus toxique pour les bactéries gram-positives
avec une concentration minimale inhibitrice (CMI) allant
de 0,2 à 0,8 mg/litre que pour les bactéries gram-néga-
tives (CMI des 3 mg/litre). La CMI de l'acétate de TBT
pour les champignons est de 0,5 à 1 mg/litre et celle de
l'oxyde de tributylétain est de 0,5 mg/litre pour l'algue
verte Chlorella pyrenoidosa. La productivité primaire
d'une communauté naturelle d'algues d'eau douce a été
réduite de 5% par une concentration d'oxyde de tributylé-
tain de 3 µg/litre. On a récemment établi la dose sans
effet observable pour 2 espèces d'algues; elle est
respectivement de 18 et 32 µg/litre. La toxicité pour
les microorganismes marins varie également selon les
espèces et selon les études; il est difficile d'établir
la valeur de la dose sans effet observable mais on pense
qu'elle est inférieure à 0,1 µg/litre pour certaines
espèces. Les concentrations alguicides vont de moins de
1,5 µg/litre à plus de 1000 µg/litre selon les espèces.
9. Effets sur les organismes aquatiques
9.1 Effets sur les organismes marins et estuariels
La Figure 1 donne un diagramme récapitulatif des
effets létaux et sublétaux que peuvent produire les con-
centrations de TBT relevées en mer et dans les estuaires.
Des concentrations supérieures à celles qui produisent les
effets létaux aigus ont été observées en différents points
du globe, notamment là où se déroulent des activités de
plaisance.
Ce sont les spores mobiles d'une algue verte géante
qui se sont révélés les plus sensibles au TBT (CE50 à 5
jours: 0,001 µg/litre). On a constaté une réduction de
la croissance d'un angiosperme marin à des concentrations
de TBT de 1 mg/kg de sédiments, aucun effet n'étant noté à
0,1 mg/kg.
 Le tributylétain est très toxique pour les mollusques
marins. On a montré expérimentalement qu'il perturbait la
formation de la coquille, le développement des gonades et
la différenciation sexuelle des huîtres adultes, leur
fixation et leur croissance; en outre on a noté une morta-
lité des larves d'huîtres et d'autres bivalves et
l'apparition de caractères mâles chez les gastéropodes
femelles. La dose sans effet observable serait de
20 ng/litre pour le naissain de l'espèce d'huître la plus
sensible, l'huître japonaise (Crassostrea gigas). Chez les
adultes, il se produit également une déformation de la
coquille qui est liée à la dose. Expérimentalement, on n'a
pas observé d'effets sur la morphologie coquillière à des
concentrations de TBT de 2 ng/litre. La dose sans effet
observable correspondant à l'apparition de caractères
mâles chez les mollusques femelles du genre Thais est
inférieure à 1,5 ng/litre. Les formes larvaires sont
généralement plus sensibles que les adultes; la différence
est particulièrement marquée dans le cas des huîtres.
Les copépodes sont plus sensibles que les autres
crustacés aux effets létaux aigus du TBT, avec des valeurs
de la CL50 pour des périodes allant jusqu'à 96 heures
comprises entre 0,6 et 2,2 µg/litre. Ces valeurs sont
comparables à celles qui s'appliquent aux larves les plus
sensibles des autres groupes de crustacés. Le TBT réduit
la capacité de reproduction, la survie néonatale et la
vitesse de croissance des crustacés. Dans le cas de la
crevette Acanthomysis sculpta, un mysidé, la dose sans
effet observable sur la reproduction serait de
0,09 µg/litre. La crevette ne cherche pas à éviter le TBT
au-dessous de 30 µg/litre.
La toxicité du tributylétain pour les poissons de mer
est très variable, les valeurs de la CL50 à 96 heures
allant de 1,5 à 36 µg/litre. Les stades larvaires sont
plus sensibles que les adultes (Figure 1). Il semblerait
que les poissons de mer évitent l'oxyde de tributylétain à
partir de 1 µg/litre.
9.2 Effets sur les organismes d'eau douce
Un diagramme récapitulatif concernant les effets
létaux et sublétaux des concentrations de TBT mesurées
dans l'eau douce est donné à la Figure 2. On a observé la
présence de concentrations supérieures à celles qui
produisent des effets sublétaux, notamment dans les zones
de navigation de plaisance.
Une concentration d'oxyde de tributylétain de
0,5 mg/litre a été mortelle pour des angiospermes d'eau
douce et leur croissance était inhibée dès 0,06 mg/litre.
On ne dispose que de peu de données sur les inverté-
brés d'eau douce, tout au plus sur trois espèces autres
que les organismes cibles. Pour divers sels de tributylé-
tain on a obtenu des valeurs de la CL50 à 48 heures de
2,3-70 µg/litre pour la daphnie et de 5,5-33 µg par
litre pour les vers de vase. La dose sans effet observable
pour la daphnie est évaluée à 0,5 µg/litre, le critère
choisi étant la réapparition d'une réaction normale à la
lumière. En ce qui concerne la palourde d'Asie, on indique
une CL50 à 24 heures de 2100 µg/litre, les valeurs
corrrespondantes allant de 30 à 400 µg/litre pour les
mollusques adultes que l'on cherche à détruire dans les
opérations de lutte contre la schistosomiase.
On a montré que le tributylétain était toxique pour
les larves de schistosome à leur stade aquatique; la
CL50 du fluorure de tributylétain est de 16,8 µg par
litre pour une exposition d'une heure. Une dose de TBT
comprise en 2 et 6 µg/litre supprime à hauteur de 99 à
100% l'infectiosité des cercaires pour la souris.
La sensibilité des mollusques au TBT diminue avec
l'âge mais les oeufs sont plus résistants que les jeunes
ou les adultes. La ponte est notablement affectée à une
concentration en oxyde de tributylétainde 0,001 µg/litre.
La toxicité aiguë du TBT pour les poissons d'eau douce
s'est située, pour des périodes allant jusqu'à 168 heures,
dans les limites de 13 à 240 µg/litre, valeurs correspon-
dant à la CL50. Dans le cas du guppy, la dose sans effet
histopathologique observable a été estimée à 0,01 µg/litre.
Après exposition de grenouilles Rana temporaria à des
concentrations inférieures ou égales 3 µg/litre, on n'a
observé aucun effet sur la survie des oeufs ni des larves;
en revanche, à la concentration de 30 µg/litre on a noté
une mortalité sensible.
Le tributylétain est très toxique pour les mollusques
marins. On a montré expérimentalement qu'il perturbait la
formation de la coquille, le développement des gonades et
la différenciation sexuelle des huîtres adultes, leur
fixation et leur croissance; en outre on a noté une morta-
lité des larves d'huîtres et d'autres bivalves et
l'apparition de caractères mâles chez les gastéropodes
femelles. La dose sans effet observable serait de
20 ng/litre pour le naissain de l'espèce d'huître la plus
sensible, l'huître japonaise (Crassostrea gigas). Chez les
adultes, il se produit également une déformation de la
coquille qui est liée à la dose. Expérimentalement, on n'a
pas observé d'effets sur la morphologie coquillière à des
concentrations de TBT de 2 ng/litre. La dose sans effet
observable correspondant à l'apparition de caractères
mâles chez les mollusques femelles du genre Thais est
inférieure à 1,5 ng/litre. Les formes larvaires sont
généralement plus sensibles que les adultes; la différence
est particulièrement marquée dans le cas des huîtres.
Les copépodes sont plus sensibles que les autres
crustacés aux effets létaux aigus du TBT, avec des valeurs
de la CL50 pour des périodes allant jusqu'à 96 heures
comprises entre 0,6 et 2,2 µg/litre. Ces valeurs sont
comparables à celles qui s'appliquent aux larves les plus
sensibles des autres groupes de crustacés. Le TBT réduit
la capacité de reproduction, la survie néonatale et la
vitesse de croissance des crustacés. Dans le cas de la
crevette Acanthomysis sculpta, un mysidé, la dose sans
effet observable sur la reproduction serait de
0,09 µg/litre. La crevette ne cherche pas à éviter le TBT
au-dessous de 30 µg/litre.
La toxicité du tributylétain pour les poissons de mer
est très variable, les valeurs de la CL50 à 96 heures
allant de 1,5 à 36 µg/litre. Les stades larvaires sont
plus sensibles que les adultes (Figure 1). Il semblerait
que les poissons de mer évitent l'oxyde de tributylétain à
partir de 1 µg/litre.
9.2 Effets sur les organismes d'eau douce
Un diagramme récapitulatif concernant les effets
létaux et sublétaux des concentrations de TBT mesurées
dans l'eau douce est donné à la Figure 2. On a observé la
présence de concentrations supérieures à celles qui
produisent des effets sublétaux, notamment dans les zones
de navigation de plaisance.
Une concentration d'oxyde de tributylétain de
0,5 mg/litre a été mortelle pour des angiospermes d'eau
douce et leur croissance était inhibée dès 0,06 mg/litre.
On ne dispose que de peu de données sur les inverté-
brés d'eau douce, tout au plus sur trois espèces autres
que les organismes cibles. Pour divers sels de tributylé-
tain on a obtenu des valeurs de la CL50 à 48 heures de
2,3-70 µg/litre pour la daphnie et de 5,5-33 µg par
litre pour les vers de vase. La dose sans effet observable
pour la daphnie est évaluée à 0,5 µg/litre, le critère
choisi étant la réapparition d'une réaction normale à la
lumière. En ce qui concerne la palourde d'Asie, on indique
une CL50 à 24 heures de 2100 µg/litre, les valeurs
corrrespondantes allant de 30 à 400 µg/litre pour les
mollusques adultes que l'on cherche à détruire dans les
opérations de lutte contre la schistosomiase.
On a montré que le tributylétain était toxique pour
les larves de schistosome à leur stade aquatique; la
CL50 du fluorure de tributylétain est de 16,8 µg par
litre pour une exposition d'une heure. Une dose de TBT
comprise en 2 et 6 µg/litre supprime à hauteur de 99 à
100% l'infectiosité des cercaires pour la souris.
La sensibilité des mollusques au TBT diminue avec
l'âge mais les oeufs sont plus résistants que les jeunes
ou les adultes. La ponte est notablement affectée à une
concentration en oxyde de tributylétainde 0,001 µg/litre.
La toxicité aiguë du TBT pour les poissons d'eau douce
s'est située, pour des périodes allant jusqu'à 168 heures,
dans les limites de 13 à 240 µg/litre, valeurs correspon-
dant à la CL50. Dans le cas du guppy, la dose sans effet
histopathologique observable a été estimée à 0,01 µg/litre.
Après exposition de grenouilles Rana temporaria à des
concentrations inférieures ou égales 3 µg/litre, on n'a
observé aucun effet sur la survie des oeufs ni des larves;
en revanche, à la concentration de 30 µg/litre on a noté
une mortalité sensible.
 9.3 Etudes de microcosme
En vue de la modélisation des écosystèmes marins on a
effectué des études de microcosme consistant à introduire
dans ces milieux certains organismes et en se plaçant dans
des conditions où un apport d'eau de mer permettait la
colonisation du milieu par d'autres biotes. On a constaté
qu'à des concentrations d'oxyde de tributylétain comprises
entre 0,06 et 3 µg/litre, il y avait réduction du nombre
d'individus et une moindre diversité des espèces.
Les résultats obtenus par modélisation d'écosystèmes
d'eau douce montrent que les doses qui tuent les mol-
lusques sont également nocives pour d'autres espèces,
notamment les poissons.
10. Effets sur les organismes terrestres
L'exposition des organismes terrestres au TBT
découlent essentiellement de l'utilisation de ces subs-
tances pour la protection du bois. L'oxyde de tributylé-
tain est toxique pour la population des ruches dont le
bois de construction a été traité au TBT. Une étude,
d'ailleurs unique, a montré que le TBT était toxique pour
les chauves-souris mais le résultat ne peut pas être con-
sidéré comme statistiquement significatif en raison de la
forte mortalité des témoins. Les dérivés du tributylétain
sont toxiques pour les insectes exposés, soit topiquement
soit par suite de xylophagie. Pour les souris de type
sauvage, la toxicité aiguë du TBT est moyenne; les valeurs
de la CL50 par voie alimentaire, calculées d'après la
consommation de semences traitées et soumises à des
épreuves de répulsivité, vont de 37 à 240 mg/kg par jour.
11. Effets sur l'aquaculture
Les observations effectuées dans des zones d'aquacul-
ture ont permis d'attribuer à la présence de fortes con-
centrations de tributylétain, un certain nombre d'effets
nocifs observés sur des bivalves tels que: mortalité,
incapacité à se fixer, moindre croissance, épaississement
de la coquille et autres malformations notamment chez les
huîtres, apparition de caractères mâles chez les gastéro-
podes (avec diminution simultanée des populations) ainsi
que chez les mollusques du genre Thais. C'est en France
que pour la première fois on a attribué à la présence de
TBT dans l'eau, l'anéantissement total de parcs à huîtres,
observations qui ont été faites ensuite dans d'autres
pays. Les effets étaient particulièrement marqués dans les
secteurs proches de marinas destinées aux navires de
plaisance. La réglementation de l'usage des peintures
antisalissures à base TBT sur les petits navires a permis
aux huîtres de retrouver leur capacité de reproduction et
de croissance. Toutefois la concentration du TBT dans
l'eau reste suffisamment forte dans certains secteurs pour
nuire aux gastéropodes marins. On utilise la croissance
de la coquille et son gaufrage chez les huîtres japonaises
ainsi que l'apparition de caractères mâles chez les
mollusques du genre Thais comme indicateurs biologiques
d'une contamination par du tributylétain.
Peu d'études ont été consacrées aux effets du TBT
sédimentaire sur la faune marine mais on pense qu'il peut
être absorbé par les organismes fouisseurs et provoquer
une certaine mortalité.
Des effets toxiques macroscopiques et des altérations
histopathologiques ont été observés dans des élevages de
poissons de mer, les filets délimitant les bassins ayant
été traités au moyen de peintures aintisalissures à base
de TBT.
On a proposé d'utiliser du TBT comme molluscicides
pour détruire les mollusques d'eau douce qui transmettent
la bilharziose (bilharzies). Un certain nombre d'essais
ont été effectués sur le terrain, dont il ressort que le
TBT est difficile à utiliser sans préjudice pour les
organismes non visés.
12. Toxicité pour les mammifères de laboratoire
12.1 Toxicité aiguë
Le tributylétain est moyennement à fortement toxique
pour les mammifères de laboratoire, avec des valeurs de la
DL50 par voie orale allant de 94 à 234 mg/kg de poids
corporel chez le rat et de 44 à 230 mg/kg de poids
corporel chez la souris. Pour le cobaye et le lapin, les
valeurs sont du même ordre de grandeur. Les variations
enregistrées sont dues aux différents anions entrant dans
la composition du sel de tributylétain. Ces composés
entraînent une mortalité plus forte lorsqu'ils sont
administrés par voie parentérale plutôt que par voie
orale, probablement du fait qu'ils ne sont que partielle-
ment absorbés au niveau intestinal.
Parmi les autres effets toxiques aigus on peut citer
des anomalies concernant les taux de lipide sanguins, le
système endocrinien, le foie, la rate et un déficit
passager dans le développement cérébral. La portée toxi-
cologique réelle de ces effets, qui n'ont été observés
qu'après administration de doses uniques élevées de ces
composés, reste discutable et la cause effective de la
mort n'est pas véritablement connue.
Par voie percutanée, la toxicité aiguë est faible, la
DL50 étant supérieure à 9000 mg/kg de poids corporel chez
le lapin. Chez le rat, après inhalation purement nasale,
la DL50 à 4 heures se situait à 77 mg/m3 (65 mg/m3 si
l'on ne tient compte que des particules respirables). Des
mélanges d'air et de vapeurs de tributylétain ne produi-
sent pas d'effets toxiques observables, même à saturation.
Toutefois le TBT est très dangereux sous forme d'aérosol
lorsqu'il est inhalé et il produit alors une irritation et
un oedème des poumons.
Le TBT est très irritant pour la peau et extrêmement
irritant pour l'oeil. L'oxyde de tributylétain n'a pas
d'effet sensibilisateur cutané.
12.2 Toxicité à court terme
Les composés du tributylétain ont été très étudiés
chez le rat (toutes les données présentées dans ce qui
suit concernent cet animal, sauf indication contraire).
On a observé un fort taux de mortalité après une durée
d'exposition de plus de quatre semaines à des doses dans
l'alimentation de 320 mg/kg (environ 25 mg/kg de poids
corporel). Acune mortalité n'a été observée à la dose de
100 mg/kg de nourriture (10 mg/kg de poids corporel) ni
après l'administration par gavage d'une dose correspondant
à 12 mg de TBT/kg de poids corporel. Administrée à des
ratons peu après leur naissance, une dose de 3 mg/kg de
poids corporel a augmenté la mortalité. Les principaux
symptômes observés après administration de doses mortelles
consistaient en perte d'appétit, faiblesse et émaciation.
On a observé des effets marginaux sur la croissance
aux doses de 50 mg/kg de nourriture (6 mg/kg de poids
corporel) et de 6 mg/kg de poids corporel (administrées
par gavage). Les souris sont moins sensibles, ces effets
n'étant observés qu'à partir de 150 à 200 mg/kg de nour-
riture (22 à 29 mg/kg de poids corporel).
Des effets structuraux ont été observés sur les
organes endocrines, essentiellement l'hypophyse et la
thyroïde, lors d'études à court et à long terme. Lors des
études à court terme, on a observé des anomalies dans la
concentration des hormones circulantes ainsi que dans la
réponse aux stimuli physiologiques (trophines hypophy-
saires); toutefois après une exposition de longue durée,
la plupart de ces anomalies avaient disparu. Le mécanisme
sous-jacent n'est pas connu.
L'exposition à un aérosol d'oxyde de tributylétain à
la dose de 2,8 mg/m3 a déterminé une forte mortalité,
une détresse respiratoire, une réaction inflammatoire au
niveau des voies respiratoires et des anomalies histo-
pathologiques des organes lymphatiques. En revanche,
l'exposition à des vapeurs à la concentration la plus
forte possible (0,16 mg/m3) à la température ambiante,
n'a produit aucun effet.
On a signalé des effets toxiques au niveau du foie et
des canaux biliaires chez ces trois espèces de mammifères.
Ainsi une nécrose des cellules hépatiques et des altéra-
tions inflammatoires du canal cholédoque ont été obser-
vées chez des rats qui avaient reçu de l'oxyde de
tributylétain à raison de 320 mg/kg de nourriture (soit
approximativement 25 mg/kg de poids corporel) pendant
quatre semaines et des souris qui en avaient reçu 80 mg/kg
de nourriture (soit approximativement 12 mg/kg de poids
corporel) pendant trois mois. Chez des chiens soumis à une
dose de 10 mg/kg de poids corporel pendant huit à neuf
semaines, on a observé une vacuolisation des hépatocytes
de la région périportale. Ces altérations s'accompagnaient
occasionnellement d'un accroissement du poids du foie et
d'une augmentation de l'activité sérique des enzymes
hépatiques.
La réduction de la concentration d'hémoglobine et de
l'hématocrite chez le rat, à la suite de l'administration
d'une dose correspondant à 80 mg/kg de nourriture (soit
8 mg/kg de poids corporel), montre qu'il y a un effet sur
la synthèse de l'hémoglobine qui conduit à une anémie
hypochrome microcytaire. La réduction des taux d'hémosi-
dérine splénique donne à penser qu'il y a action au niveau
des réserves martiales. Une anémie a également été
observée chez la souris.
La formation de rosettes érythrocytaires au niveau des
ganglions lymphatiques mésentériques a été observée lors
de certaines études à court terme mais pas lors d'études à
long terme. La portée biologique de cette observation
(vraisemblablement passagère) reste obscure.
L'oxyde de tributylétain exerce un effet toxique
caractéristique sur le système immunitaire; du fait de son
action sur le thymus, il perturbe les fonctions immuni-
taires à médiation cellulaire. Le mode d'action demeure
inconnu mais il pourrait y avoir conversion métabolique en
dibutylétain. La résistance non spécifique est également
amoindrie.
Un certain nombre d'études sur rats et chiens mais à
l'exclusion des souris, ont été effectuées avec de l'oxyde
de tributylétain et ont révélé l'existence d'effets
généraux sur le système immunitaire (par exemple poids et
morphologie des tissus lymphoïdes, numération des lympho-
cytes périphériques, concentration totale des immunoglo-
bulines sériques), à des doses largement toxiques (des
effets ont été observés chez la souris à des doses de
150 mg/kg de chlorure de tributylétain). Seul le rat
manifeste des effets généraux sur le système immunitaire
sans autres signes patents de toxicité et il se révèle
indiscutablement être l'espèce la plus sensible. Des
études à court terme sur le rat ont permis d'établir que
la dose sans effet observable était de 5 mg/kg de nour-
riture (soit 0,6 mg/kg de poids corporel). Lors des études
portant sur le chlorure de tributylétain, on a observé
des effets analogues au niveau du thymus. Ces effets
disparaissaient rapidement lorsqu'on cessait d'administrer
la substance. On a montré que l'oxyde de tributylétain,
étudié dans le cadre de travaux sur la résistance de
l'hôte, perturbait les fonctions immunitaires spécifiques
du rat. L'organisme de cet animal présentait une moindre
aptitude à éliminer les Listeria monocytogenes après
exposition à une dose de 50 mg/kg de nourriture (dose sans
effet observable: 5 mg/kg et par jour), et une moindre
résistance à Trichinella spiralis a été observée aux doses
respectives de 50 et 5 mg/kg de nourriture, cet effet
disparaissant à la dose de 0,5 mg/kg de nourriture (ce qui
correspond à des doses quotidiennes respectives de 2,5,
0,25 et 0,025 mg/kg de poids corporel). Des effets
analogues ont été observés chez des animaux âgés mais ils
étaient moins marqués.
Dans l'état actuel des connaissances, les effets sur
la résistance de l'hôte sont probablement très utiles pour
évaluer les risques pour la santé humaine, mais l'on ne
possède pas une expérience suffisante de ces systèmes
d'épreuve pour tirer des conclusions définitives des
résultats obtenus. Il reste que l'observation de rats
glabres athymiques soumis à une inoculation de T. spiralis
virulentes a permis l'interprétation des résultats
obtenus sur le modèle T. spiralis. En effet, l'absence
totale d'immunité thymo-dépendante a multiplié par 10 à 20
le nombre de larves présentes dans les muscles; par contre
l'exposition à des concentrations d'oxyde de tributylétain
respectivement égales à 5 et 50 mg/kg de nourriture a
multiplié par 2 et 4 respectivement le nombre de ces
larves.
Il existe quelques données concernant les effets du
tributylétain sur le système immunitaire en développement
mais sans aucune information sur la résistance de l'hôte.
Pour évaluer les dangers potentiels pour l'homme, il
serait plus prudent de prendre en considération les effets
produits sur l'espèce la plus sensible. On a observé des
effets sur la résistance de l'hôte à T. spiralis à des
concentrations dans l'alimentation ne dépassant pas
5 mg/kg (soit l'équivalent de 0,25 mg/kg de poids corporel
par jour), la dose sans effet observable étant égale à
0,25 mg/kg (ce qui correspond à 0,025 mg/kg par jour).
Toutefois, l'interprétation de ces résultats en vue d'une
évaluation du risque pour l'homme reste controversée.
Toutes les études qui ont été effectuées font ressortir
que la dose quotidienne sans effet observable pour ce qui
est des effets généraux ou spécifiques sur le système
immunitaire se situe à 5 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel selon les
études à court terme).
12.3 Toxicité à long terme
Une étude de longue durée chez le rat a montré que le
tributylétain a une effet marginal sur les paramètres
toxicologiques généraux (sans grande signification toxi-
cologique) à la dose de 5 mg/kg de nourriture (soit
0,25 mg/kg de poids corporel).
12.4 Génotoxicité
La génotoxicité de l'oxyde de tributylétain a fait
l'objet d'études approfondies. La plupart de ces études
ont donné des résultats négatifs et rien n'indique de
façon convaincante que ce produit puisse présenter le
moindre risque mutagène.
12.5 Toxicité vis-à-vis de la fonction de reproduction
On a évalué l'embryotoxicité potentielle de l'oxyde de
tributylétain sur trois espèces de mammifères (souris, rat
et lapin), après administration de la substance par voie
orale à la mère. La principale malformation observée chez
les foetus de rat et de souris était une fissure congéni-
tale du palais osseux mais il est vrai qu'elle ne se
produisait qu'à des doses manifestement toxiques pour la
mère. On ne peut pas considérer que ces résultats
témoignent d'une activité tératogène aux doses inférieures
à celles qui sont toxiques pour la mère. La dose minimale
sans effet observable pour ce qui est de l'embryotoxicité
et de la foetotoxicité chez ces trois espèces se situait à
1,0 mg/kg de poids corporel.
12.6 Cancérogénicité
Une étude de cancérogénicité a été effectuée sur des
rats et on a observé à cette occasion des modifications
néoplasiques au niveau des organes endocrines à la dose de
50 mg/kg de poids corporel. Les tumeurs hypophysaires
observées à la dose de 0,5 mg/kg de nourriture ne semblent
pas avoir d'importance biologique car il n'y a pas de
relation précise dose-réponse. Ces types de tumeurs
apparaissent en général chez les témoins à des fréquences
élevées et variables et leur signification reste donc
discutable. Une autre étude de cancérogénicité est en
cours sur des souris.
13. Effets sur l'homme
On a constaté que l'exposition professionnelle
d'ouvriers à des dérivés du tributylétain produisait une
irritation des voies respiratoires supérieures. Ces
substances sont dangereuses pour l'homme lorsqu'elles sont
sous la forme d'aérosols. L'oxyde de tributylétain est
irritant pour la peau et les muqueuses oculaires et il a
été fait état de dermatites graves à la suite d'un contact
direct avec la peau. Le risque est d'autant plus grave que
la réaction cutanée n'est pas immédiate.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour l'homme
Les travailleurs sont principalement exposés lors de
la préparation et de la formulation des dérivés du tri-
butylétain, de l'application ou de l'élimination de
peintures à base de TBT et de l'utilisation de produits de
protection du bois à base de ces substances. Quant à la
population dans son ensemble, elle peut être exposée par
contamination de la nourriture, en particulier des
poissons et des coquillages, et lors de l'utilisation de
produits de protection du bois à usage ménager.
Si l'on s'appuie sur l'expérimentation animale et
l'observation directe sur l'homme, il est clair que les
dérivés du tributylétain sont irritants pour la peau et
les yeux et que l'inhalation d'aérosols conduit à une
irritation des voies respiratoires.
La manipulation de bois traités n'entraîne aucune
irritation de l'épiderme une fois que le produit a séché.
Cependant les aérosols de TBT sont extrêmement dangereux
et il ne faut pas revenir dans le local où le bois a été
traité avant séchage complet.
On n'a jamais fait état d'intoxications aiguës généra-
lisées par le TBT qui en principe doit s'éliminer de
l'organisme en l'espace de quelques jours. Il est donc peu
probable que l'on puisse s'exposer à des intoxications
aiguës par la manipulation de produits à base de tri-
butylétain si l'on prend les précautions voulues.
On a fait état d'effets à court et à long terme chez
des animaux de laboratoire, sur le foie, le sang et les
glandes endocrines. Chez le rat, qui est l'espèce la plus
sensible, c'est l'effet au niveau du système immunitaire
et en particulier sur la résistance de l'hôte, qui
constitue le paramètre le plus sensible de la toxicité de
ces produits. En utilisant comme modèle la résistance de
l'hôte à l'infestation par Trichinella spiralis, on
obtient une dose sans effet observable qui se situe entre
0,5 et 5 mg/kg de nourriture (c'est-à-dire 0,025 et 0,25
mg/kg de poids corporel) alors que si l'on se rapporte à
l'action sur la fonction immunitaire, la dose est égale à
0,6 mg/kg de poids corporel.
En raison des grandes variations dans la consommation
de poissons et de coquillages ainsi que dans les teneurs
locales des fruits de mer en résidus de TBT, on ne peut
donner que quelques exemples pour illustrer l'exposition
résultante et les valeurs des doses sans effet observable.
Il importe de souligner que pour déterminer le risque
résultant de la présence de ces composés, il faut procéder
sur place à des mesures de résidus dans les denrées,
évaluer la consommation locale de fruits de mer et définir
une marge de sécurité acceptable.
En retenant pour la consommation de poisson les
chiffres de 15 et 150 grammes par jour, de 1 mg/kg pour
les résidus de TBT dans le poisson et un poids corporel
moyen de 60 kg, on obtient pour l'homme les marges
suivantes de sécurité selon les divers paramètres immuni-
taires.
---------------------------------------------------------------------
Consommation Apport estimatif Marge de sécurité
de poisson journalier de Modèle Autres paramètres
(g/jour) TBT (µg/kg) T. Spiralis immunitaires
---------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
---------------------------------------------------------------------
Utiliser des dérivés du TBT à tort et à travers et de
manière irresponsable sans suivre les recommandations qui
figurent dans la présente monographie pour réduire l'expo-
sition humaine peut conduire à l'ingestion de quantités de
TBT dangereuses pour la santé humaine.
On a noté jusqu'ici d'effets tératogènes, chez l'ani-
mal de laboratoire, qu'à des doses manifestement toxiques
pour la mère. On peut donc considérer que l'activité
tératogène du TBT est très faible.
En s'appuyant sur les résultats d'études très
complètes de mutagénicité, on estime que les dérivés du
tributylétain n'ont aucun pouvoir mutagène. Une étude de
cancérogénicité chez le rat, portant sur l'oxyde de
tributylétain, a fait apparaître une incidence accrue de
tumeurs endocriniennes; mais il s'agissait de tumeurs
spontanées dont l'incidence, généralement élevée, est très
variable. Ces résultats ne constituent donc pas un
argument bien net en faveur d'un risque cancérogène pour
l'homme.
2. Evaluation du risque écologique
La pénétration diffuse du tributylétain (TBT) dans
l'environnement a principalement pour origine l'utili-
sation de peintures antisalissures qui en contiennent.
Une contamination ponctuelle peut se produire lorsqu'on
utilise du TBT comme biocide dans les systèmes de réfri-
gération, lors du traitement de la pulpe de bois, le
tannage du cuir, la préservation du bois et le traitement
des textiles.
Du fait de leurs priopriétés physico-chimiques, les
dérivés du TBT se concentrent dans la micro-couche de
surface ainsi que dans les sédiments. Il ne semble pas
que le principal mécanisme d'élimination de ces substances
dans les conditions naturelles soit une dégradation abio-
tique. L'oxyde de tributylétain est biodégradable dans
l'eau mais le processus n'est pas suffisamment rapide pour
empêcher la présence dans certaines zones de concen-
trations élevées en TBT. Une bioaccumulation se produit
dans la plupart des organismes aquatiques mais chez les
mammifères de laboratoire, la dégradation métabolique est
plus efficace.
Le TBT est extrêment dangereux pour certains orga-
nismes aquatiques, du fait de sa toxicité à très faibles
concentrations dans l'eau. On le rencontre à ces concen-
trations dans certaines zones. On a signalé la présence
d'effets nocifs sur des invertébrés non visés, en parti-
culier des mollusques et ces effets sont suffisamment
graves pour bloquer la reproduction et entraîner un déclin
des populations de mollusques. Ces effets néfastes pour
la conchyliculture ont pu être combattus avec succès grâce
à des restrictions imposées à l'utilisation des peintures
antisalissures dans certains secteurs, restrictions qui
ont également permis d'éviter l'apparition de caractères
mâles chez les gastéropodes femelles. En ce qui concerne
le pisciculture, il convient de ne pas utiliser de pein-
tures à base de TBT sur les filets qui limitent les
bassins.
D'une manière générale, le risque pour l'environnement
terrestre est vraisemblablement faible. Cependant le
traitement du bois par ces substances pourrait se révéler
dangereux pour les organismes qui vivent à son contact.
L'augmentation de la teneur en TBT de la micro-couche
superficielle pourrait se révéler dangereuse pour la faune
côtière, pour les neustons (y compris les invertébrés
benthiques et les larves de poissons) ainsi que pour les
oiseaux de mer et le gibier d'eau qui se nourrissent en
surface. L'accumulation et le faible taux de biodégra-
dation du TBT dans les sédiments peut présenter un risque
pour les organismes aquatiques lorsque des sédiments
pollués sont soulevés par des processus naturels ou des
activités de dragage.
RECOMMANDATIONS
1. Recommandations pour la protection de la santé humaine
et de l'environnement
a) Les pays membres qui jusqu'ici n'ont pas réglementé
l'utilisation des dérivés du TBT devraient être
invités à le faire.
b) Il est nécessaire d'évaluer la pénétration des dérivés
organo-stanniques dans l'environnement à partir de
sources autres que les peintures antisalissures et, si
besoin est, d'édicter une réglementation à ce sujet.
Il faut en particulier évaluer le risque résultant du
déversement sur le sol de boues d'égouts contaminés
par du tributylétain.
c) Il faudrait améliorer, du point de vue de la sécurité,
les techniques d'application, d'élimination et d'éva-
cuation des peintures à base d'organo-stanniques.
2. Recherches à effectuer
a) Il faut améliorer les méthodes de recherche et de
dosage du butylétain afin d'avoir une estimation
rapide et exacte des concentrations de l'ordre de
pg/litre. Une des raisons de cette recommandation
tient à un effet biologique, à savoir l'apparition de
caractères mâles chez les gastéropodes femelles, qui
est susceptible de se produire à des concentrations
plus basses que les limites actuelles de détection.
b) Il faut effectuer des recherches sur les mécanimes par
lesquels le TBT se concentre au lieu de se disperser
et qui retardent sa décomposition; à cet effet on
étudiera avec une attention particulière la chimie
fondamentale du tributylétain et ses interactions avec
les molécules biologiques. Il faut étudier davantage
la fixation du TBT à tous les niveaux de la chaîne
alimentaire.
c) Il est nécessaire d'entreprendre des études sur la
toxicité du TBT pour les organismes aquatiques. On
étudiera en particulier le métabolisme, les effets
endocriniens et immunologiques, selon le cas.
d) Il est nécessaire de rechercher d'autres espèces sen-
sibles capables de servir d'indicateurs biologiques,
notamment parmi les espèces d'eau douce.
e) Il faut valider les modèles permettant l'évaluation de
l'immunotoxicité chez les mammifères et définir de
façon plus précise les doses sans effet toxique
correspondant aux paramètres pertinents.
f) Etude de toxicité chronique à entreprendre sur une
deuxième espèce de mammifères.
g) Etude de tumorigénicité à entreprendre sur une
deuxième espèce de mammifères.
h) Données sur les résidus de butylétain dans le poisson
et les coquillages destinés à la consommation humaine
en distinguant les différentes espèces.
RESUMEN
1. Propiedades físicas y químicas
Los compuestos de tributilestaño (TBE) son derivados
orgánicos del estaño tetravalente. Caracterizados por la
presencia de enlaces covalentes entre átomos de carbono y
un átomo de estaño, tienen la siguiente fórmula general:
(n-C4H9)3 Sn-X, en la que X es un anión. En general,
la pureza del óxido de tributilestaño (OTBE) comercial
pasa del 96%; las principales impurezas están constituidas
por derivados del dibutilestaño y, en menor grado, por
compuestos de tetrabutilestaño y otros compuestos tri-
alquílicos de ese elemento. El OTBE es un líquido incoloro
con olor característico y una densidad relativa de 1,17 a
1,18. La solubilidad en el agua es baja, variando entre
< 1,0 y > 100 mg/litro según el pH, la temperatura, y los
aniones presentes en el agua (que determinan la especifi-
cidad). En el agua del mar y en condiciones normales, el
TBE aparece en tres formas o especies (hidróxido, cloruro
y carbonato), que se mantienen en equilibrio. En valores
de pH inferiores a 7,0, las formas predominantes son
Bu3SnOH2+ y Bu3SnCl, a pH 8 son Bu3SnCl, Bu3SnOH
y Bu3SnCO3-, mientras que cuando el pH pasa de 10
predominan Bu3SnOH y Bu3SnCO3-.
El coeficiente de partición octanol/agua (log Poa)
varía entre 3,19 y 3,84 para el agua destilada y es de
3,54 para el agua del mar. El OTBE adsorbe intensamente
las partículas, con coeficientes de adsorción comprendidos
entre 110 y 55 000 según los informes publicados. La
presión de vapor es baja, pero los valores publicados
acusan variaciones considerables. En una solución de 1
mg/litro no se observó disminución alguna del OTBE durante
62 días, pero el 20% del agua se perdió por evaporación.
2. Métodos analíticos
Se utilizan diversos métodos para medir los derivados
tributilestánnicos en el agua, en el sedimento o en la
flora y la fauna (biota). El más usado es la espectro-
metría de absorción atómica (AA). La espectrometría de AA
con llama permite alcanzar un límite de detección de 0,1
mg/litro. Resulta más sensible la AA sin llama, basada en
la atomización en un horno eléctrico con grafito, cuyos
límites de detección están comprendidos entre 0,1 y
1,0 µg/litro de agua. Existen diferentes métodos de
extracción y para formar derivados volátiles. La sepa-
ración de estos derivados suele hacerse por "purga y
captura" o cromatografía de gases. Los límites de detec-
ción son de 0,5 y 5,0 µg/kg para el sedimento y la
biota.
3. Fuentes de contaminación ambiental
Se han registrado diversos compuestos de tributile-
staño como molusquicidas, productos antiincrustantes en
botes y otras embarcaciones, muelles, boyas, jaulas de
langostas, redes de pesca y nasas, como conservadores de
la madera, como "productos anti-cieno" en los trabajos
de albañilería, como desinfectantes y como biocidas en los
sistemas de refrigeración, las torres de refrigeración de
las centrales electrógenas, las fábricas de pulpa y de
papel, las cervecerías, las industrias del cuero y los
telares. En las pinturas antiincrustantes, el TBE se
comercializó al principio bajo una forma que permitía la
liberación sin trabas del compuesto. En fecha más reciente
se han puesto a la venta pinturas de liberación controlada
en las que el TBE se incorpora a una matriz copolimérica.
También se han ideado matrices de caucho para hacer más
lenta y retrasar la liberación del compuesto, con la
consiguiente prolongación de la eficacia de las pinturas
antiincrustantes y de los molusquicidas. El TBE no se
utiliza en la agricultura por su elevada fitotoxicidad.
4. Reglamentación del empleo
Muchos países han restringido el empleo de pinturas
antiincrustantes con TBE por los efectos de éste sobre los
mariscos. Los reglamentos varían en detalle de unos países
a otros, pero casi siempre prohíben el uso de pinturas de
TBE en las embarcaciones de 24 metros de longitud o menos.
Algunos países han excluido de esta prohibición a las
embarcaciones con casco de aluminio. Además, en algunos
reglamentos se restringe el contenido de TBE en las
pinturas o el ritmo con que se libera este compuesto de
las mismas (a 4 o 5 µg/cm2 por día, a largo plazo).
5. Concentraciones en el medio ambiente
Se han encontrado concentraciones elevadas de TBE en
el agua, los sedimentos y la biota próximos a las zonas de
navegación de recreo, especialmente en "marinas" o
fondeaderos de yates, embarcaderos, diques secos, redes de
pesca y nasas tratadas con pinturas antiincrustantes, así
como en los sistemas de refrigeración. La intensidad de
las mareas y la turbiedad del agua influyen en las con-
centraciones de TBE.
Se ha visto que las concentraciones de TBE pueden
llegar a 1,58 µg/litro en el agua del mar y en los estu-
arios, a 7,1 µ/litro en el agua dulce, a 26 300 µg/kg en
los sedimentos costeros, a 3700 µg/kg en los sedimentos
de agua dulce, a 6,39 mg/kg en los bivalvos, a 1,92 mg/kg
en los gasterópodos y a 11 mg/kg en los peces. Ahora bien,
no hay que considerar como representativas esas concentra-
ciones máximas de TBE, toda vez que diversos factores
pueden dar lugar a valores anormalmente elevados (por
ejemplo, las partículas de pintura en las muestras de agua
y de sedimentos). Se ha comprobado que en las concentra-
ciones de TBE en la microcapa superficial del agua, tanto
dulce como salada, pueden ser dos veces más altas que las
medidas inmediatamente por debajo de la superficie. Sin
embargo, conviene tener en cuenta que los valores regis-
trados de TBE en las microcapas superficiales pueden verse
muy afectados por el método de muestreo.
Es posible que los datos antiguos no sean comparables
con los más recientes a causa del perfeccionamiento de los
métodos analíticos disponibles para determinar el TBE en
el agua, los sedimentos y los tejidos.
6. Transporte y transformación en el medio ambiente
A consecuencia de su baja hidrosolubilidad y de su
carácter lipofílico, el TBE se adsorbe fácilmente en las
partículas. Se calcula que entre el 10% y el 95% del OTBE
introducido en el agua se adsorbe de ese modo. La
desaparición progresiva del TBE adsorbido no se debe a la
desorción sino a la degradación. El grado de adsorción
depende de la salinidad, de la naturaleza y el tamaño de
las partículas en suspensión, de la cantidad de material
suspendido, de la temperatura y de la presencia de materia
orgánica disuelta.
La degradación del OTBE entraña la escisión del enlace
carbono-estaño. Este fenómeno puede deberse a diversos
mecanismos que intervienen simultáneamente en el medio
ambiente, unos fisicoquímicos (hidrólisis y fotodegrada-
ción) y otros biológicos (degradación por microorganismos
y metabolización por organismos superiores). Mientras que
en condiciones extremas de pH se produce una hidrólisis de
los compuestos de estaño orgánico, ésta es apenas evidente
en condiciones ambientales normales. En el laboratorio se
observa fotodegradación cuando se exponen soluciones a una
irradiación ultravioleta de 300 nm (y, en menor grado, de
350 nm). En condiciones naturales, la fotólisis está
limitada por la gama de longitudes de onda de la luz solar
y por la escasa penetración de la luz ultravioleta en el
agua. La presencia de sustancias fotosensibilizadoras
puede acelerar la fotodegradación. La biodegradación
depende de condiciones ambientales tales como la tempera-
tura, la oxigenación, el pH, el nivel de elementos miner-
ales, la presencia de sustancias orgánicas fácilmente
biodegradables a efectos de cometabolismo y la naturaleza
de la microflora y su capacidad de adaptación. También
depende de que la concentración de OTBE sea más baja que
el umbral letal o inhibitorio para las bacterias. Como en
la degradación abiótica, la ruptura biótica del TBE es un
proceso progresivo de debutilización oxidativa fundado en
la escisión del enlace carbono-estaño, en el que se forman
derivados dibutílicos que se degradan más fácilmente que
el tributilestaño. Los monobutilestaños se mineralizan
lentamente. Aunque existe degradación anaerobia, no se ha
llegado a un acuerdo sobre su importancia. Algunos autores
estiman que es lenta, mientras que otros piensan que es
más rápida que la degradación aerobia. Se han identificado
varias especies de bacterias, algas y hongos nocivos para
la madera que pueden degradar el OTBE. Las estimaciones
de la semivida del TBE en el medio ambiente acusan grandes
variaciones.
El TBE se bioacumula en los organismos a causa de su
solubilidad en las grasas. En investigaciones de labora-
torio con moluscos y peces se han visto que los factores
de bioconcentración pueden llegar a un valor de 7000, y
aun se han obtenido valores más altos en los estudios
sobre el terreno. La absorción a partir de los alimentos
es más importante que la que se efectúa directamente a
partir del agua. La presencia de factores de concentración
más altos en los microorganismos (entre 100 y 30 000)
puede deberse más a adsorción que a absorción intracelu-
lar. No hay ninguna indicación de que el TBE se transfiera
a los microorganismos terrestres a través de las cadenas
alimentarias.
7. Cinética y metabolismo
El tributilestaño se absorbe a través del intestino
(20-50%, según el vehículo) y de la piel en los mamíferos
(10% aproximadamente), pudiendo atravesar la barrera
hematoencefálica y pasar de la placenta al feto. El
material absorbido se distribuye rápida y ampliamente por
los tejidos (principalmente el hígado y el riñón).
En los mamíferos, el metabolismo del TBE es rápido: a
las 3 h de administrar TBE pueden ya descubrirse metabo-
litos en la sangre. Los estudios in vitro han revelado
que el TBE es un sustrato para ciertas oxidasas de función
mixta, pero las concentraciones muy altas de TBE inhiben
esas enzimas.
La velocidad de desaparición del TBE difiere de unos
tejidos a otros; las estimaciones de la semivida biológica
en los mamíferos van desde 23 hasta unos 30 días.
La metabolización del TBE se observa también en los
organismos inferiores, pero es más lenta (particularmente
en los moluscos) que en los mamíferos. La capacidad de
bioacumulación, por consiguiente, es mucho mayor que en
éstos.
Los compuestos de TBE inhiben la fosforilización
oxidativa y alteran la estructura y las funciones de las
mitocondrias. El TBE interfiere en la calcificación de la
concha de las ostras ( Crassostrea spp).
8. Efectos en los microorganismos
El TBE es tóxico para los microorganismos y se ha
utilizado comercialmente como bactericida y alguicida.
Las concentraciones que provocan efectos tóxicos varían
mucho de unas especies a otras. El TBE es más tóxico para
las bacterias gram-positivas (concentración inhibitoria
mínima (CIM): entre 0,2 y 0,8 mg/litro) que para las gram-
negativas (CIM: 3 mg/litro). La CIM del acetato de TBE
para los hongos es de 0,5-1 mg/litro y la CIM del OTBE
para el alga verde Chlorella pyrenoidosa es de 0,5
mg/litro. La productividad primaria de una comunidad
natural de algas de agua dulce se redujo en un 50% con una
concentración de OTBE de 3 µg/litro. En fecha reciente
se han determinado en dos especies de algas niveles de
efecto no observado (NENO) de 18 y 32 µg/litro. De igual
modo, la toxicidad para los microorganismos marinos varía
de unas especies a otras y de unos estudios a otros; los
NENO son difíciles de establecer pero no llegan a
0,1 µg/litro en algunas especies. Las concentraciones
alguicidas varían entre < 1,5 µg/litro y > 1000 µg por
litro según las especies.
9. Efectos en los organismos acuáticos
9.1 Efectos en los organismos de agua salada (mar y estuarios)
En la figura 1 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las con-
centraciones de TBE medidas en diversos organismos del mar
y de los estuarios. En todo el mundo, especialmente en los
lugares relacionados con actividades náuticas recreativas,
se han encontrado concentraciones superiores a las que
producen efectos letales.
9.3 Etudes de microcosme
En vue de la modélisation des écosystèmes marins on a
effectué des études de microcosme consistant à introduire
dans ces milieux certains organismes et en se plaçant dans
des conditions où un apport d'eau de mer permettait la
colonisation du milieu par d'autres biotes. On a constaté
qu'à des concentrations d'oxyde de tributylétain comprises
entre 0,06 et 3 µg/litre, il y avait réduction du nombre
d'individus et une moindre diversité des espèces.
Les résultats obtenus par modélisation d'écosystèmes
d'eau douce montrent que les doses qui tuent les mol-
lusques sont également nocives pour d'autres espèces,
notamment les poissons.
10. Effets sur les organismes terrestres
L'exposition des organismes terrestres au TBT
découlent essentiellement de l'utilisation de ces subs-
tances pour la protection du bois. L'oxyde de tributylé-
tain est toxique pour la population des ruches dont le
bois de construction a été traité au TBT. Une étude,
d'ailleurs unique, a montré que le TBT était toxique pour
les chauves-souris mais le résultat ne peut pas être con-
sidéré comme statistiquement significatif en raison de la
forte mortalité des témoins. Les dérivés du tributylétain
sont toxiques pour les insectes exposés, soit topiquement
soit par suite de xylophagie. Pour les souris de type
sauvage, la toxicité aiguë du TBT est moyenne; les valeurs
de la CL50 par voie alimentaire, calculées d'après la
consommation de semences traitées et soumises à des
épreuves de répulsivité, vont de 37 à 240 mg/kg par jour.
11. Effets sur l'aquaculture
Les observations effectuées dans des zones d'aquacul-
ture ont permis d'attribuer à la présence de fortes con-
centrations de tributylétain, un certain nombre d'effets
nocifs observés sur des bivalves tels que: mortalité,
incapacité à se fixer, moindre croissance, épaississement
de la coquille et autres malformations notamment chez les
huîtres, apparition de caractères mâles chez les gastéro-
podes (avec diminution simultanée des populations) ainsi
que chez les mollusques du genre Thais. C'est en France
que pour la première fois on a attribué à la présence de
TBT dans l'eau, l'anéantissement total de parcs à huîtres,
observations qui ont été faites ensuite dans d'autres
pays. Les effets étaient particulièrement marqués dans les
secteurs proches de marinas destinées aux navires de
plaisance. La réglementation de l'usage des peintures
antisalissures à base TBT sur les petits navires a permis
aux huîtres de retrouver leur capacité de reproduction et
de croissance. Toutefois la concentration du TBT dans
l'eau reste suffisamment forte dans certains secteurs pour
nuire aux gastéropodes marins. On utilise la croissance
de la coquille et son gaufrage chez les huîtres japonaises
ainsi que l'apparition de caractères mâles chez les
mollusques du genre Thais comme indicateurs biologiques
d'une contamination par du tributylétain.
Peu d'études ont été consacrées aux effets du TBT
sédimentaire sur la faune marine mais on pense qu'il peut
être absorbé par les organismes fouisseurs et provoquer
une certaine mortalité.
Des effets toxiques macroscopiques et des altérations
histopathologiques ont été observés dans des élevages de
poissons de mer, les filets délimitant les bassins ayant
été traités au moyen de peintures aintisalissures à base
de TBT.
On a proposé d'utiliser du TBT comme molluscicides
pour détruire les mollusques d'eau douce qui transmettent
la bilharziose (bilharzies). Un certain nombre d'essais
ont été effectués sur le terrain, dont il ressort que le
TBT est difficile à utiliser sans préjudice pour les
organismes non visés.
12. Toxicité pour les mammifères de laboratoire
12.1 Toxicité aiguë
Le tributylétain est moyennement à fortement toxique
pour les mammifères de laboratoire, avec des valeurs de la
DL50 par voie orale allant de 94 à 234 mg/kg de poids
corporel chez le rat et de 44 à 230 mg/kg de poids
corporel chez la souris. Pour le cobaye et le lapin, les
valeurs sont du même ordre de grandeur. Les variations
enregistrées sont dues aux différents anions entrant dans
la composition du sel de tributylétain. Ces composés
entraînent une mortalité plus forte lorsqu'ils sont
administrés par voie parentérale plutôt que par voie
orale, probablement du fait qu'ils ne sont que partielle-
ment absorbés au niveau intestinal.
Parmi les autres effets toxiques aigus on peut citer
des anomalies concernant les taux de lipide sanguins, le
système endocrinien, le foie, la rate et un déficit
passager dans le développement cérébral. La portée toxi-
cologique réelle de ces effets, qui n'ont été observés
qu'après administration de doses uniques élevées de ces
composés, reste discutable et la cause effective de la
mort n'est pas véritablement connue.
Par voie percutanée, la toxicité aiguë est faible, la
DL50 étant supérieure à 9000 mg/kg de poids corporel chez
le lapin. Chez le rat, après inhalation purement nasale,
la DL50 à 4 heures se situait à 77 mg/m3 (65 mg/m3 si
l'on ne tient compte que des particules respirables). Des
mélanges d'air et de vapeurs de tributylétain ne produi-
sent pas d'effets toxiques observables, même à saturation.
Toutefois le TBT est très dangereux sous forme d'aérosol
lorsqu'il est inhalé et il produit alors une irritation et
un oedème des poumons.
Le TBT est très irritant pour la peau et extrêmement
irritant pour l'oeil. L'oxyde de tributylétain n'a pas
d'effet sensibilisateur cutané.
12.2 Toxicité à court terme
Les composés du tributylétain ont été très étudiés
chez le rat (toutes les données présentées dans ce qui
suit concernent cet animal, sauf indication contraire).
On a observé un fort taux de mortalité après une durée
d'exposition de plus de quatre semaines à des doses dans
l'alimentation de 320 mg/kg (environ 25 mg/kg de poids
corporel). Acune mortalité n'a été observée à la dose de
100 mg/kg de nourriture (10 mg/kg de poids corporel) ni
après l'administration par gavage d'une dose correspondant
à 12 mg de TBT/kg de poids corporel. Administrée à des
ratons peu après leur naissance, une dose de 3 mg/kg de
poids corporel a augmenté la mortalité. Les principaux
symptômes observés après administration de doses mortelles
consistaient en perte d'appétit, faiblesse et émaciation.
On a observé des effets marginaux sur la croissance
aux doses de 50 mg/kg de nourriture (6 mg/kg de poids
corporel) et de 6 mg/kg de poids corporel (administrées
par gavage). Les souris sont moins sensibles, ces effets
n'étant observés qu'à partir de 150 à 200 mg/kg de nour-
riture (22 à 29 mg/kg de poids corporel).
Des effets structuraux ont été observés sur les
organes endocrines, essentiellement l'hypophyse et la
thyroïde, lors d'études à court et à long terme. Lors des
études à court terme, on a observé des anomalies dans la
concentration des hormones circulantes ainsi que dans la
réponse aux stimuli physiologiques (trophines hypophy-
saires); toutefois après une exposition de longue durée,
la plupart de ces anomalies avaient disparu. Le mécanisme
sous-jacent n'est pas connu.
L'exposition à un aérosol d'oxyde de tributylétain à
la dose de 2,8 mg/m3 a déterminé une forte mortalité,
une détresse respiratoire, une réaction inflammatoire au
niveau des voies respiratoires et des anomalies histo-
pathologiques des organes lymphatiques. En revanche,
l'exposition à des vapeurs à la concentration la plus
forte possible (0,16 mg/m3) à la température ambiante,
n'a produit aucun effet.
On a signalé des effets toxiques au niveau du foie et
des canaux biliaires chez ces trois espèces de mammifères.
Ainsi une nécrose des cellules hépatiques et des altéra-
tions inflammatoires du canal cholédoque ont été obser-
vées chez des rats qui avaient reçu de l'oxyde de
tributylétain à raison de 320 mg/kg de nourriture (soit
approximativement 25 mg/kg de poids corporel) pendant
quatre semaines et des souris qui en avaient reçu 80 mg/kg
de nourriture (soit approximativement 12 mg/kg de poids
corporel) pendant trois mois. Chez des chiens soumis à une
dose de 10 mg/kg de poids corporel pendant huit à neuf
semaines, on a observé une vacuolisation des hépatocytes
de la région périportale. Ces altérations s'accompagnaient
occasionnellement d'un accroissement du poids du foie et
d'une augmentation de l'activité sérique des enzymes
hépatiques.
La réduction de la concentration d'hémoglobine et de
l'hématocrite chez le rat, à la suite de l'administration
d'une dose correspondant à 80 mg/kg de nourriture (soit
8 mg/kg de poids corporel), montre qu'il y a un effet sur
la synthèse de l'hémoglobine qui conduit à une anémie
hypochrome microcytaire. La réduction des taux d'hémosi-
dérine splénique donne à penser qu'il y a action au niveau
des réserves martiales. Une anémie a également été
observée chez la souris.
La formation de rosettes érythrocytaires au niveau des
ganglions lymphatiques mésentériques a été observée lors
de certaines études à court terme mais pas lors d'études à
long terme. La portée biologique de cette observation
(vraisemblablement passagère) reste obscure.
L'oxyde de tributylétain exerce un effet toxique
caractéristique sur le système immunitaire; du fait de son
action sur le thymus, il perturbe les fonctions immuni-
taires à médiation cellulaire. Le mode d'action demeure
inconnu mais il pourrait y avoir conversion métabolique en
dibutylétain. La résistance non spécifique est également
amoindrie.
Un certain nombre d'études sur rats et chiens mais à
l'exclusion des souris, ont été effectuées avec de l'oxyde
de tributylétain et ont révélé l'existence d'effets
généraux sur le système immunitaire (par exemple poids et
morphologie des tissus lymphoïdes, numération des lympho-
cytes périphériques, concentration totale des immunoglo-
bulines sériques), à des doses largement toxiques (des
effets ont été observés chez la souris à des doses de
150 mg/kg de chlorure de tributylétain). Seul le rat
manifeste des effets généraux sur le système immunitaire
sans autres signes patents de toxicité et il se révèle
indiscutablement être l'espèce la plus sensible. Des
études à court terme sur le rat ont permis d'établir que
la dose sans effet observable était de 5 mg/kg de nour-
riture (soit 0,6 mg/kg de poids corporel). Lors des études
portant sur le chlorure de tributylétain, on a observé
des effets analogues au niveau du thymus. Ces effets
disparaissaient rapidement lorsqu'on cessait d'administrer
la substance. On a montré que l'oxyde de tributylétain,
étudié dans le cadre de travaux sur la résistance de
l'hôte, perturbait les fonctions immunitaires spécifiques
du rat. L'organisme de cet animal présentait une moindre
aptitude à éliminer les Listeria monocytogenes après
exposition à une dose de 50 mg/kg de nourriture (dose sans
effet observable: 5 mg/kg et par jour), et une moindre
résistance à Trichinella spiralis a été observée aux doses
respectives de 50 et 5 mg/kg de nourriture, cet effet
disparaissant à la dose de 0,5 mg/kg de nourriture (ce qui
correspond à des doses quotidiennes respectives de 2,5,
0,25 et 0,025 mg/kg de poids corporel). Des effets
analogues ont été observés chez des animaux âgés mais ils
étaient moins marqués.
Dans l'état actuel des connaissances, les effets sur
la résistance de l'hôte sont probablement très utiles pour
évaluer les risques pour la santé humaine, mais l'on ne
possède pas une expérience suffisante de ces systèmes
d'épreuve pour tirer des conclusions définitives des
résultats obtenus. Il reste que l'observation de rats
glabres athymiques soumis à une inoculation de T. spiralis
virulentes a permis l'interprétation des résultats
obtenus sur le modèle T. spiralis. En effet, l'absence
totale d'immunité thymo-dépendante a multiplié par 10 à 20
le nombre de larves présentes dans les muscles; par contre
l'exposition à des concentrations d'oxyde de tributylétain
respectivement égales à 5 et 50 mg/kg de nourriture a
multiplié par 2 et 4 respectivement le nombre de ces
larves.
Il existe quelques données concernant les effets du
tributylétain sur le système immunitaire en développement
mais sans aucune information sur la résistance de l'hôte.
Pour évaluer les dangers potentiels pour l'homme, il
serait plus prudent de prendre en considération les effets
produits sur l'espèce la plus sensible. On a observé des
effets sur la résistance de l'hôte à T. spiralis à des
concentrations dans l'alimentation ne dépassant pas
5 mg/kg (soit l'équivalent de 0,25 mg/kg de poids corporel
par jour), la dose sans effet observable étant égale à
0,25 mg/kg (ce qui correspond à 0,025 mg/kg par jour).
Toutefois, l'interprétation de ces résultats en vue d'une
évaluation du risque pour l'homme reste controversée.
Toutes les études qui ont été effectuées font ressortir
que la dose quotidienne sans effet observable pour ce qui
est des effets généraux ou spécifiques sur le système
immunitaire se situe à 5 mg/kg de nourriture (soit
l'équivalent de 0,5 mg/kg de poids corporel selon les
études à court terme).
12.3 Toxicité à long terme
Une étude de longue durée chez le rat a montré que le
tributylétain a une effet marginal sur les paramètres
toxicologiques généraux (sans grande signification toxi-
cologique) à la dose de 5 mg/kg de nourriture (soit
0,25 mg/kg de poids corporel).
12.4 Génotoxicité
La génotoxicité de l'oxyde de tributylétain a fait
l'objet d'études approfondies. La plupart de ces études
ont donné des résultats négatifs et rien n'indique de
façon convaincante que ce produit puisse présenter le
moindre risque mutagène.
12.5 Toxicité vis-à-vis de la fonction de reproduction
On a évalué l'embryotoxicité potentielle de l'oxyde de
tributylétain sur trois espèces de mammifères (souris, rat
et lapin), après administration de la substance par voie
orale à la mère. La principale malformation observée chez
les foetus de rat et de souris était une fissure congéni-
tale du palais osseux mais il est vrai qu'elle ne se
produisait qu'à des doses manifestement toxiques pour la
mère. On ne peut pas considérer que ces résultats
témoignent d'une activité tératogène aux doses inférieures
à celles qui sont toxiques pour la mère. La dose minimale
sans effet observable pour ce qui est de l'embryotoxicité
et de la foetotoxicité chez ces trois espèces se situait à
1,0 mg/kg de poids corporel.
12.6 Cancérogénicité
Une étude de cancérogénicité a été effectuée sur des
rats et on a observé à cette occasion des modifications
néoplasiques au niveau des organes endocrines à la dose de
50 mg/kg de poids corporel. Les tumeurs hypophysaires
observées à la dose de 0,5 mg/kg de nourriture ne semblent
pas avoir d'importance biologique car il n'y a pas de
relation précise dose-réponse. Ces types de tumeurs
apparaissent en général chez les témoins à des fréquences
élevées et variables et leur signification reste donc
discutable. Une autre étude de cancérogénicité est en
cours sur des souris.
13. Effets sur l'homme
On a constaté que l'exposition professionnelle
d'ouvriers à des dérivés du tributylétain produisait une
irritation des voies respiratoires supérieures. Ces
substances sont dangereuses pour l'homme lorsqu'elles sont
sous la forme d'aérosols. L'oxyde de tributylétain est
irritant pour la peau et les muqueuses oculaires et il a
été fait état de dermatites graves à la suite d'un contact
direct avec la peau. Le risque est d'autant plus grave que
la réaction cutanée n'est pas immédiate.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour l'homme
Les travailleurs sont principalement exposés lors de
la préparation et de la formulation des dérivés du tri-
butylétain, de l'application ou de l'élimination de
peintures à base de TBT et de l'utilisation de produits de
protection du bois à base de ces substances. Quant à la
population dans son ensemble, elle peut être exposée par
contamination de la nourriture, en particulier des
poissons et des coquillages, et lors de l'utilisation de
produits de protection du bois à usage ménager.
Si l'on s'appuie sur l'expérimentation animale et
l'observation directe sur l'homme, il est clair que les
dérivés du tributylétain sont irritants pour la peau et
les yeux et que l'inhalation d'aérosols conduit à une
irritation des voies respiratoires.
La manipulation de bois traités n'entraîne aucune
irritation de l'épiderme une fois que le produit a séché.
Cependant les aérosols de TBT sont extrêmement dangereux
et il ne faut pas revenir dans le local où le bois a été
traité avant séchage complet.
On n'a jamais fait état d'intoxications aiguës généra-
lisées par le TBT qui en principe doit s'éliminer de
l'organisme en l'espace de quelques jours. Il est donc peu
probable que l'on puisse s'exposer à des intoxications
aiguës par la manipulation de produits à base de tri-
butylétain si l'on prend les précautions voulues.
On a fait état d'effets à court et à long terme chez
des animaux de laboratoire, sur le foie, le sang et les
glandes endocrines. Chez le rat, qui est l'espèce la plus
sensible, c'est l'effet au niveau du système immunitaire
et en particulier sur la résistance de l'hôte, qui
constitue le paramètre le plus sensible de la toxicité de
ces produits. En utilisant comme modèle la résistance de
l'hôte à l'infestation par Trichinella spiralis, on
obtient une dose sans effet observable qui se situe entre
0,5 et 5 mg/kg de nourriture (c'est-à-dire 0,025 et 0,25
mg/kg de poids corporel) alors que si l'on se rapporte à
l'action sur la fonction immunitaire, la dose est égale à
0,6 mg/kg de poids corporel.
En raison des grandes variations dans la consommation
de poissons et de coquillages ainsi que dans les teneurs
locales des fruits de mer en résidus de TBT, on ne peut
donner que quelques exemples pour illustrer l'exposition
résultante et les valeurs des doses sans effet observable.
Il importe de souligner que pour déterminer le risque
résultant de la présence de ces composés, il faut procéder
sur place à des mesures de résidus dans les denrées,
évaluer la consommation locale de fruits de mer et définir
une marge de sécurité acceptable.
En retenant pour la consommation de poisson les
chiffres de 15 et 150 grammes par jour, de 1 mg/kg pour
les résidus de TBT dans le poisson et un poids corporel
moyen de 60 kg, on obtient pour l'homme les marges
suivantes de sécurité selon les divers paramètres immuni-
taires.
---------------------------------------------------------------------
Consommation Apport estimatif Marge de sécurité
de poisson journalier de Modèle Autres paramètres
(g/jour) TBT (µg/kg) T. Spiralis immunitaires
---------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
---------------------------------------------------------------------
Utiliser des dérivés du TBT à tort et à travers et de
manière irresponsable sans suivre les recommandations qui
figurent dans la présente monographie pour réduire l'expo-
sition humaine peut conduire à l'ingestion de quantités de
TBT dangereuses pour la santé humaine.
On a noté jusqu'ici d'effets tératogènes, chez l'ani-
mal de laboratoire, qu'à des doses manifestement toxiques
pour la mère. On peut donc considérer que l'activité
tératogène du TBT est très faible.
En s'appuyant sur les résultats d'études très
complètes de mutagénicité, on estime que les dérivés du
tributylétain n'ont aucun pouvoir mutagène. Une étude de
cancérogénicité chez le rat, portant sur l'oxyde de
tributylétain, a fait apparaître une incidence accrue de
tumeurs endocriniennes; mais il s'agissait de tumeurs
spontanées dont l'incidence, généralement élevée, est très
variable. Ces résultats ne constituent donc pas un
argument bien net en faveur d'un risque cancérogène pour
l'homme.
2. Evaluation du risque écologique
La pénétration diffuse du tributylétain (TBT) dans
l'environnement a principalement pour origine l'utili-
sation de peintures antisalissures qui en contiennent.
Une contamination ponctuelle peut se produire lorsqu'on
utilise du TBT comme biocide dans les systèmes de réfri-
gération, lors du traitement de la pulpe de bois, le
tannage du cuir, la préservation du bois et le traitement
des textiles.
Du fait de leurs priopriétés physico-chimiques, les
dérivés du TBT se concentrent dans la micro-couche de
surface ainsi que dans les sédiments. Il ne semble pas
que le principal mécanisme d'élimination de ces substances
dans les conditions naturelles soit une dégradation abio-
tique. L'oxyde de tributylétain est biodégradable dans
l'eau mais le processus n'est pas suffisamment rapide pour
empêcher la présence dans certaines zones de concen-
trations élevées en TBT. Une bioaccumulation se produit
dans la plupart des organismes aquatiques mais chez les
mammifères de laboratoire, la dégradation métabolique est
plus efficace.
Le TBT est extrêment dangereux pour certains orga-
nismes aquatiques, du fait de sa toxicité à très faibles
concentrations dans l'eau. On le rencontre à ces concen-
trations dans certaines zones. On a signalé la présence
d'effets nocifs sur des invertébrés non visés, en parti-
culier des mollusques et ces effets sont suffisamment
graves pour bloquer la reproduction et entraîner un déclin
des populations de mollusques. Ces effets néfastes pour
la conchyliculture ont pu être combattus avec succès grâce
à des restrictions imposées à l'utilisation des peintures
antisalissures dans certains secteurs, restrictions qui
ont également permis d'éviter l'apparition de caractères
mâles chez les gastéropodes femelles. En ce qui concerne
le pisciculture, il convient de ne pas utiliser de pein-
tures à base de TBT sur les filets qui limitent les
bassins.
D'une manière générale, le risque pour l'environnement
terrestre est vraisemblablement faible. Cependant le
traitement du bois par ces substances pourrait se révéler
dangereux pour les organismes qui vivent à son contact.
L'augmentation de la teneur en TBT de la micro-couche
superficielle pourrait se révéler dangereuse pour la faune
côtière, pour les neustons (y compris les invertébrés
benthiques et les larves de poissons) ainsi que pour les
oiseaux de mer et le gibier d'eau qui se nourrissent en
surface. L'accumulation et le faible taux de biodégra-
dation du TBT dans les sédiments peut présenter un risque
pour les organismes aquatiques lorsque des sédiments
pollués sont soulevés par des processus naturels ou des
activités de dragage.
RECOMMANDATIONS
1. Recommandations pour la protection de la santé humaine
et de l'environnement
a) Les pays membres qui jusqu'ici n'ont pas réglementé
l'utilisation des dérivés du TBT devraient être
invités à le faire.
b) Il est nécessaire d'évaluer la pénétration des dérivés
organo-stanniques dans l'environnement à partir de
sources autres que les peintures antisalissures et, si
besoin est, d'édicter une réglementation à ce sujet.
Il faut en particulier évaluer le risque résultant du
déversement sur le sol de boues d'égouts contaminés
par du tributylétain.
c) Il faudrait améliorer, du point de vue de la sécurité,
les techniques d'application, d'élimination et d'éva-
cuation des peintures à base d'organo-stanniques.
2. Recherches à effectuer
a) Il faut améliorer les méthodes de recherche et de
dosage du butylétain afin d'avoir une estimation
rapide et exacte des concentrations de l'ordre de
pg/litre. Une des raisons de cette recommandation
tient à un effet biologique, à savoir l'apparition de
caractères mâles chez les gastéropodes femelles, qui
est susceptible de se produire à des concentrations
plus basses que les limites actuelles de détection.
b) Il faut effectuer des recherches sur les mécanimes par
lesquels le TBT se concentre au lieu de se disperser
et qui retardent sa décomposition; à cet effet on
étudiera avec une attention particulière la chimie
fondamentale du tributylétain et ses interactions avec
les molécules biologiques. Il faut étudier davantage
la fixation du TBT à tous les niveaux de la chaîne
alimentaire.
c) Il est nécessaire d'entreprendre des études sur la
toxicité du TBT pour les organismes aquatiques. On
étudiera en particulier le métabolisme, les effets
endocriniens et immunologiques, selon le cas.
d) Il est nécessaire de rechercher d'autres espèces sen-
sibles capables de servir d'indicateurs biologiques,
notamment parmi les espèces d'eau douce.
e) Il faut valider les modèles permettant l'évaluation de
l'immunotoxicité chez les mammifères et définir de
façon plus précise les doses sans effet toxique
correspondant aux paramètres pertinents.
f) Etude de toxicité chronique à entreprendre sur une
deuxième espèce de mammifères.
g) Etude de tumorigénicité à entreprendre sur une
deuxième espèce de mammifères.
h) Données sur les résidus de butylétain dans le poisson
et les coquillages destinés à la consommation humaine
en distinguant les différentes espèces.
RESUMEN
1. Propiedades físicas y químicas
Los compuestos de tributilestaño (TBE) son derivados
orgánicos del estaño tetravalente. Caracterizados por la
presencia de enlaces covalentes entre átomos de carbono y
un átomo de estaño, tienen la siguiente fórmula general:
(n-C4H9)3 Sn-X, en la que X es un anión. En general,
la pureza del óxido de tributilestaño (OTBE) comercial
pasa del 96%; las principales impurezas están constituidas
por derivados del dibutilestaño y, en menor grado, por
compuestos de tetrabutilestaño y otros compuestos tri-
alquílicos de ese elemento. El OTBE es un líquido incoloro
con olor característico y una densidad relativa de 1,17 a
1,18. La solubilidad en el agua es baja, variando entre
< 1,0 y > 100 mg/litro según el pH, la temperatura, y los
aniones presentes en el agua (que determinan la especifi-
cidad). En el agua del mar y en condiciones normales, el
TBE aparece en tres formas o especies (hidróxido, cloruro
y carbonato), que se mantienen en equilibrio. En valores
de pH inferiores a 7,0, las formas predominantes son
Bu3SnOH2+ y Bu3SnCl, a pH 8 son Bu3SnCl, Bu3SnOH
y Bu3SnCO3-, mientras que cuando el pH pasa de 10
predominan Bu3SnOH y Bu3SnCO3-.
El coeficiente de partición octanol/agua (log Poa)
varía entre 3,19 y 3,84 para el agua destilada y es de
3,54 para el agua del mar. El OTBE adsorbe intensamente
las partículas, con coeficientes de adsorción comprendidos
entre 110 y 55 000 según los informes publicados. La
presión de vapor es baja, pero los valores publicados
acusan variaciones considerables. En una solución de 1
mg/litro no se observó disminución alguna del OTBE durante
62 días, pero el 20% del agua se perdió por evaporación.
2. Métodos analíticos
Se utilizan diversos métodos para medir los derivados
tributilestánnicos en el agua, en el sedimento o en la
flora y la fauna (biota). El más usado es la espectro-
metría de absorción atómica (AA). La espectrometría de AA
con llama permite alcanzar un límite de detección de 0,1
mg/litro. Resulta más sensible la AA sin llama, basada en
la atomización en un horno eléctrico con grafito, cuyos
límites de detección están comprendidos entre 0,1 y
1,0 µg/litro de agua. Existen diferentes métodos de
extracción y para formar derivados volátiles. La sepa-
ración de estos derivados suele hacerse por "purga y
captura" o cromatografía de gases. Los límites de detec-
ción son de 0,5 y 5,0 µg/kg para el sedimento y la
biota.
3. Fuentes de contaminación ambiental
Se han registrado diversos compuestos de tributile-
staño como molusquicidas, productos antiincrustantes en
botes y otras embarcaciones, muelles, boyas, jaulas de
langostas, redes de pesca y nasas, como conservadores de
la madera, como "productos anti-cieno" en los trabajos
de albañilería, como desinfectantes y como biocidas en los
sistemas de refrigeración, las torres de refrigeración de
las centrales electrógenas, las fábricas de pulpa y de
papel, las cervecerías, las industrias del cuero y los
telares. En las pinturas antiincrustantes, el TBE se
comercializó al principio bajo una forma que permitía la
liberación sin trabas del compuesto. En fecha más reciente
se han puesto a la venta pinturas de liberación controlada
en las que el TBE se incorpora a una matriz copolimérica.
También se han ideado matrices de caucho para hacer más
lenta y retrasar la liberación del compuesto, con la
consiguiente prolongación de la eficacia de las pinturas
antiincrustantes y de los molusquicidas. El TBE no se
utiliza en la agricultura por su elevada fitotoxicidad.
4. Reglamentación del empleo
Muchos países han restringido el empleo de pinturas
antiincrustantes con TBE por los efectos de éste sobre los
mariscos. Los reglamentos varían en detalle de unos países
a otros, pero casi siempre prohíben el uso de pinturas de
TBE en las embarcaciones de 24 metros de longitud o menos.
Algunos países han excluido de esta prohibición a las
embarcaciones con casco de aluminio. Además, en algunos
reglamentos se restringe el contenido de TBE en las
pinturas o el ritmo con que se libera este compuesto de
las mismas (a 4 o 5 µg/cm2 por día, a largo plazo).
5. Concentraciones en el medio ambiente
Se han encontrado concentraciones elevadas de TBE en
el agua, los sedimentos y la biota próximos a las zonas de
navegación de recreo, especialmente en "marinas" o
fondeaderos de yates, embarcaderos, diques secos, redes de
pesca y nasas tratadas con pinturas antiincrustantes, así
como en los sistemas de refrigeración. La intensidad de
las mareas y la turbiedad del agua influyen en las con-
centraciones de TBE.
Se ha visto que las concentraciones de TBE pueden
llegar a 1,58 µg/litro en el agua del mar y en los estu-
arios, a 7,1 µ/litro en el agua dulce, a 26 300 µg/kg en
los sedimentos costeros, a 3700 µg/kg en los sedimentos
de agua dulce, a 6,39 mg/kg en los bivalvos, a 1,92 mg/kg
en los gasterópodos y a 11 mg/kg en los peces. Ahora bien,
no hay que considerar como representativas esas concentra-
ciones máximas de TBE, toda vez que diversos factores
pueden dar lugar a valores anormalmente elevados (por
ejemplo, las partículas de pintura en las muestras de agua
y de sedimentos). Se ha comprobado que en las concentra-
ciones de TBE en la microcapa superficial del agua, tanto
dulce como salada, pueden ser dos veces más altas que las
medidas inmediatamente por debajo de la superficie. Sin
embargo, conviene tener en cuenta que los valores regis-
trados de TBE en las microcapas superficiales pueden verse
muy afectados por el método de muestreo.
Es posible que los datos antiguos no sean comparables
con los más recientes a causa del perfeccionamiento de los
métodos analíticos disponibles para determinar el TBE en
el agua, los sedimentos y los tejidos.
6. Transporte y transformación en el medio ambiente
A consecuencia de su baja hidrosolubilidad y de su
carácter lipofílico, el TBE se adsorbe fácilmente en las
partículas. Se calcula que entre el 10% y el 95% del OTBE
introducido en el agua se adsorbe de ese modo. La
desaparición progresiva del TBE adsorbido no se debe a la
desorción sino a la degradación. El grado de adsorción
depende de la salinidad, de la naturaleza y el tamaño de
las partículas en suspensión, de la cantidad de material
suspendido, de la temperatura y de la presencia de materia
orgánica disuelta.
La degradación del OTBE entraña la escisión del enlace
carbono-estaño. Este fenómeno puede deberse a diversos
mecanismos que intervienen simultáneamente en el medio
ambiente, unos fisicoquímicos (hidrólisis y fotodegrada-
ción) y otros biológicos (degradación por microorganismos
y metabolización por organismos superiores). Mientras que
en condiciones extremas de pH se produce una hidrólisis de
los compuestos de estaño orgánico, ésta es apenas evidente
en condiciones ambientales normales. En el laboratorio se
observa fotodegradación cuando se exponen soluciones a una
irradiación ultravioleta de 300 nm (y, en menor grado, de
350 nm). En condiciones naturales, la fotólisis está
limitada por la gama de longitudes de onda de la luz solar
y por la escasa penetración de la luz ultravioleta en el
agua. La presencia de sustancias fotosensibilizadoras
puede acelerar la fotodegradación. La biodegradación
depende de condiciones ambientales tales como la tempera-
tura, la oxigenación, el pH, el nivel de elementos miner-
ales, la presencia de sustancias orgánicas fácilmente
biodegradables a efectos de cometabolismo y la naturaleza
de la microflora y su capacidad de adaptación. También
depende de que la concentración de OTBE sea más baja que
el umbral letal o inhibitorio para las bacterias. Como en
la degradación abiótica, la ruptura biótica del TBE es un
proceso progresivo de debutilización oxidativa fundado en
la escisión del enlace carbono-estaño, en el que se forman
derivados dibutílicos que se degradan más fácilmente que
el tributilestaño. Los monobutilestaños se mineralizan
lentamente. Aunque existe degradación anaerobia, no se ha
llegado a un acuerdo sobre su importancia. Algunos autores
estiman que es lenta, mientras que otros piensan que es
más rápida que la degradación aerobia. Se han identificado
varias especies de bacterias, algas y hongos nocivos para
la madera que pueden degradar el OTBE. Las estimaciones
de la semivida del TBE en el medio ambiente acusan grandes
variaciones.
El TBE se bioacumula en los organismos a causa de su
solubilidad en las grasas. En investigaciones de labora-
torio con moluscos y peces se han visto que los factores
de bioconcentración pueden llegar a un valor de 7000, y
aun se han obtenido valores más altos en los estudios
sobre el terreno. La absorción a partir de los alimentos
es más importante que la que se efectúa directamente a
partir del agua. La presencia de factores de concentración
más altos en los microorganismos (entre 100 y 30 000)
puede deberse más a adsorción que a absorción intracelu-
lar. No hay ninguna indicación de que el TBE se transfiera
a los microorganismos terrestres a través de las cadenas
alimentarias.
7. Cinética y metabolismo
El tributilestaño se absorbe a través del intestino
(20-50%, según el vehículo) y de la piel en los mamíferos
(10% aproximadamente), pudiendo atravesar la barrera
hematoencefálica y pasar de la placenta al feto. El
material absorbido se distribuye rápida y ampliamente por
los tejidos (principalmente el hígado y el riñón).
En los mamíferos, el metabolismo del TBE es rápido: a
las 3 h de administrar TBE pueden ya descubrirse metabo-
litos en la sangre. Los estudios in vitro han revelado
que el TBE es un sustrato para ciertas oxidasas de función
mixta, pero las concentraciones muy altas de TBE inhiben
esas enzimas.
La velocidad de desaparición del TBE difiere de unos
tejidos a otros; las estimaciones de la semivida biológica
en los mamíferos van desde 23 hasta unos 30 días.
La metabolización del TBE se observa también en los
organismos inferiores, pero es más lenta (particularmente
en los moluscos) que en los mamíferos. La capacidad de
bioacumulación, por consiguiente, es mucho mayor que en
éstos.
Los compuestos de TBE inhiben la fosforilización
oxidativa y alteran la estructura y las funciones de las
mitocondrias. El TBE interfiere en la calcificación de la
concha de las ostras ( Crassostrea spp).
8. Efectos en los microorganismos
El TBE es tóxico para los microorganismos y se ha
utilizado comercialmente como bactericida y alguicida.
Las concentraciones que provocan efectos tóxicos varían
mucho de unas especies a otras. El TBE es más tóxico para
las bacterias gram-positivas (concentración inhibitoria
mínima (CIM): entre 0,2 y 0,8 mg/litro) que para las gram-
negativas (CIM: 3 mg/litro). La CIM del acetato de TBE
para los hongos es de 0,5-1 mg/litro y la CIM del OTBE
para el alga verde Chlorella pyrenoidosa es de 0,5
mg/litro. La productividad primaria de una comunidad
natural de algas de agua dulce se redujo en un 50% con una
concentración de OTBE de 3 µg/litro. En fecha reciente
se han determinado en dos especies de algas niveles de
efecto no observado (NENO) de 18 y 32 µg/litro. De igual
modo, la toxicidad para los microorganismos marinos varía
de unas especies a otras y de unos estudios a otros; los
NENO son difíciles de establecer pero no llegan a
0,1 µg/litro en algunas especies. Las concentraciones
alguicidas varían entre < 1,5 µg/litro y > 1000 µg por
litro según las especies.
9. Efectos en los organismos acuáticos
9.1 Efectos en los organismos de agua salada (mar y estuarios)
En la figura 1 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las con-
centraciones de TBE medidas en diversos organismos del mar
y de los estuarios. En todo el mundo, especialmente en los
lugares relacionados con actividades náuticas recreativas,
se han encontrado concentraciones superiores a las que
producen efectos letales.
 El desarrollo de las esporas móviles de una macroalga
verde se ha revelado como el índice evolutivo más sensible
al TBE (CE50 de 5 días: 0,001 µg/litro). El crecimiento
de una angiosperma marina se redujo en concentraciones de
TBE de un mg/kg de sedimento, pero no se observó ningún
efecto a 0,1 mg/kg.
El tributilestaño es sumamente tóxico para los
moluscos marinos. Experimentalmente se ha demostrado que
altera la formación de la concha en las ostras en creci-
miento, así como el desarrollo gonadal y el sexo de las
ostras adultas, la formación de colonias, el crecimiento y
la mortalidad de las ostras y otros bivalvos en la fase
larvaria, y que causa "imposex" (desarrollo de caracter-
ísticas masculinas) en los gasterópodos hembras. Se ha
obtenido un valor NENO de 20 ng/litro para la freza de la
especie de ostra más sensible (Crassostrea gigas). El TBE
provoca una deformación de la concha de las ostras adultas
más o menos acentuada según la dosis. No se ha observado
experimentalmente ningún efecto sobre la morfología de la
concha con concentraciones de TBE de 2 ng/litro. En las
hembras de buccino, el valor NENO para el imposex no llega
a 1,5 ng/litro. En general, las formas larvarias son más
sensibles que los adultos, siendo esta diferencia
especialmente marcada en el caso de las ostras.
Los copépodos son más sensibles que otros grupos de
crustáceos al efecto letal agudo del TBE; los valores de
CL50 para periodos de exposición de hasta 96 h van de
0,6 a 2,2 µg/litro. Estas cifras son comparables a las
obtenidas en las larvas más sensibles de otros grupos de
crustáceos. El TBE reduce el rendimiento reproductor, la
supervivencia de los recién nacidos y la tasa de creci-
miento juvenil en los crustáceos. En el camarón mísido
Acanthomysis sculpta el valor NENO para la reproducción
parece ser de 0,09 µg/litro. Otro camarón, el llamado
por los anglosajones "grass shrimp", no evita el TBE a
concentraciones que pueden llegar hasta 30 µg/litro.
La toxicidad del tributilestaño para los peces marinos
es sumamente variable; los valores de la CL50 a 96 h van
desde 1,5 hasta 36 µg/litro. Las fases larvarias son más
sensibles que los adultos (fig. 1). Hay indicios de que
los peces marinos evitan las concentraciones de OTBE de
1 µg/litro o más.
9.2 Efectos en los organismos de agua dulce
En la figura 2 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las
concentraciones de TBE medidas en agua dulce. Se han
observado concentraciones mayores que las que provocan
efectos subletales en diversos sitios, especialmente en
relación con actividades náuticas recreativas.
Una concentración OTBE de 0,5 mg/litro produjo la
muerte de las angioespermas de agua dulce, mientras que el
crecimiento se inhibió a 0,06 mg/litro o más.
El desarrollo de las esporas móviles de una macroalga
verde se ha revelado como el índice evolutivo más sensible
al TBE (CE50 de 5 días: 0,001 µg/litro). El crecimiento
de una angiosperma marina se redujo en concentraciones de
TBE de un mg/kg de sedimento, pero no se observó ningún
efecto a 0,1 mg/kg.
El tributilestaño es sumamente tóxico para los
moluscos marinos. Experimentalmente se ha demostrado que
altera la formación de la concha en las ostras en creci-
miento, así como el desarrollo gonadal y el sexo de las
ostras adultas, la formación de colonias, el crecimiento y
la mortalidad de las ostras y otros bivalvos en la fase
larvaria, y que causa "imposex" (desarrollo de caracter-
ísticas masculinas) en los gasterópodos hembras. Se ha
obtenido un valor NENO de 20 ng/litro para la freza de la
especie de ostra más sensible (Crassostrea gigas). El TBE
provoca una deformación de la concha de las ostras adultas
más o menos acentuada según la dosis. No se ha observado
experimentalmente ningún efecto sobre la morfología de la
concha con concentraciones de TBE de 2 ng/litro. En las
hembras de buccino, el valor NENO para el imposex no llega
a 1,5 ng/litro. En general, las formas larvarias son más
sensibles que los adultos, siendo esta diferencia
especialmente marcada en el caso de las ostras.
Los copépodos son más sensibles que otros grupos de
crustáceos al efecto letal agudo del TBE; los valores de
CL50 para periodos de exposición de hasta 96 h van de
0,6 a 2,2 µg/litro. Estas cifras son comparables a las
obtenidas en las larvas más sensibles de otros grupos de
crustáceos. El TBE reduce el rendimiento reproductor, la
supervivencia de los recién nacidos y la tasa de creci-
miento juvenil en los crustáceos. En el camarón mísido
Acanthomysis sculpta el valor NENO para la reproducción
parece ser de 0,09 µg/litro. Otro camarón, el llamado
por los anglosajones "grass shrimp", no evita el TBE a
concentraciones que pueden llegar hasta 30 µg/litro.
La toxicidad del tributilestaño para los peces marinos
es sumamente variable; los valores de la CL50 a 96 h van
desde 1,5 hasta 36 µg/litro. Las fases larvarias son más
sensibles que los adultos (fig. 1). Hay indicios de que
los peces marinos evitan las concentraciones de OTBE de
1 µg/litro o más.
9.2 Efectos en los organismos de agua dulce
En la figura 2 se resumen en un diagrama las rela-
ciones entre los efectos letales y subletales y las
concentraciones de TBE medidas en agua dulce. Se han
observado concentraciones mayores que las que provocan
efectos subletales en diversos sitios, especialmente en
relación con actividades náuticas recreativas.
Una concentración OTBE de 0,5 mg/litro produjo la
muerte de las angioespermas de agua dulce, mientras que el
crecimiento se inhibió a 0,06 mg/litro o más.
 Los datos sobre los invertebrados de agua dulce son
escasos y no comprenden más que tres especies, además de
los organismos tomados como objetivo. Con diferentes sales
de TBE se han obtenido valores de CL50 en 48 h para
Daphnia de 2,3-70 µg/litro y para Tubifex de 5,5-
33 µg/litro. Basándose en la reversión de la respuesta
normal a la luz, se ha calculado que el NENO para Daphnia
es de 0,5 µg/litro. La CL50 de 24 h para la almeja
asiática parece ser, según se ha señalado, de 2100 µg por
litro, mientras que para los moluscos adultos contra los
que se dirige la lucha antiesquistosomiásica los valores
correspondientes son de 30-400 µg/litro.
Se ha demostrado que el tributilestaño es tóxico para
las larvas de esquistosoma en las fases acuáticas; la
CL50 (fluoruro de TBE) parece ser, según los cálculos
realizados, de 16,8 µg/litro en el caso de una exposi-
ción de 1 h. La dosis de TBE que suprime el 99-100% de la
infectividad de las cercarias para el ratón está compren-
dida entre 2 y 6 µg/litro.
La sensibilidad de los moluscos al TBE disminuye con
la edad, pero los huevos son más resistentes que los
individuos jóvenes y adultos. La puesta de huevos se ve
considerablemente afectada a una concentración de OTBE de
0,001 µg/litro.
En las pruebas de CL50 con exposiciones de hasta
168 h, la toxicidad aguda del TBE para los peces de agua
dulce varía entre 13 y 240 µg/litro. Basándose en los
efectos histopatológicos, se ha calculado que el valor
NENO para el "guppy" es de 0,01 µg/litro.
No se ha observado ningún efecto sobre la super-
vivencia tras la exposición de huevos y larvas de la rana
Rana temporaria a concentraciones de TBE de 3 µg por
litro o menos; en cambio, la mortalidad fue significativa
a 30 µg/litro.
9.3 Estudios microcósmicos
Se han realizado algunos "estudios microcósmicos"
utilizando como modelo ecosistemas marinos en los que se
habían introducido organismos y en los que la entrada de
agua de mar facilitaba la colonización por otros organ-
ismos. Los resultados obtenidos muestran que tanto el
número de individuos como la diversidad de las especies
disminuyen cuando la concentración de OTBE en el agua se
sitúa entre 0,06 y 3 µg/litro.
Los resultados obtenidos con modelos de ecosistemas de
agua dulce hacen pensar que las dosis que matan los cara-
coles afectan también a otras especies, en particular los
peces.
10. Efectos en los organismos terrestres
La exposición de organismos terrestres al TBE proviene
sobre todo del uso de este compuesto como conservador de
la madera. El OTBE es tóxico para las abejas que viven en
colmenas de madera tratadas con TBE. Este producto se
mostró tóxico para los murciélagos en un estudio aislado,
pero la elevada mortalidad de los testigos resta signifi-
cación estadística a este resultado. Los compuestos de
TBE son tóxicos para los insectos que están en contacto o
se alimentan con madera tratada. El TBE tiene una toxi-
cidad aguda moderada para los ratones en libertad y,
basándose en el consumo de semillas tratadas en las
pruebas de repelentes, se calcula que los valores de
CL50 en la dieta se sitúan entre 37 y 240 mg/kg al día.
11. Efectos en organismos estudiados sobre el terreno
Las observaciones sobre el terreno han permitido
establecer una relación entre las concentraciones elevadas
de tributilestaño y la mortalidad de las larvas de
bivalvos, la incapacidad de las mismas para constituir
colonias, el retraso del crecimiento, el engrosamiento de
la concha y otras malformaciones de las ostras en
desarrollo, el imposex en los caracoles de suelos
cenagosos y el imposex (con disminución concurrente de la
población) en el buccino. En Francia inicialmente, y más
tarde en otros países, se ha identificado y atribuido la
destrucción completa de los criaderos de ostras a la
presencia de TBE en el agua. Los efectos son más acusados
en las zonas próximas a los centros de deportes náuticos.
Al dejar de emplear pinturas antiincrustantes a base de
TBE en las pequeñas embarcaciones se restablecieron la
reproducción y el crecimiento de las ostras. Sin embargo,
en algunas zonas las concentraciones de TBE en el agua
siguen siendo bastante elevadas para afectar a los
gasterópodos marinos.
Tanto el desarrollo y formación de la concha en las
ostras del Pacífico como el imposex en el buccino se han
utilizado como indicadores biológicos de contaminación por
TBE.
Aunque se han hecho pocos estudios sobre los efectos
en los organismos del TBE presente en los sedimentos, hay
indicios de que este compuesto puede llegar a los animales
que viven en madrigueras y producir mortalidad en condi-
ciones prácticas.
Se han observado efectos tóxicos macroscópicos y
alteraciones histopatológicas en los criaderos de pesca
marítima expuestos al TBE por el uso de pinturas antiin-
crustantes en las redes de contención.
Se ha propuesto la utilización de TBE como molusqui-
cida contra los caracoles de agua dulce que transmiten la
esquistosomiasis (bilharziasis). Varios ensayos prácticos
han demostrado que es difícil aplicar el TBE sin que
resulten perjudicados otros organismos distintos de los
tomados como objetivo.
12. Toxicidad para los mamíferos de laboratorio
12.1 Toxicidad aguda
El tributilestaño tiene una toxicidad entre moderada y
alta para los mamíferos de laboratorio; los valores de la
DL50 oral aguda varían desde 94 a 234 mg/kg de peso
corporal para la rata y desde 44 hasta 230 mg/kg de peso
corporal para el ratón. La toxicidad aguda para el cobayo
y el conejo es del mismo orden. La variación se debe al
componente aniónico de la sal de tributilestaño. Estos
compuestos tienen un potencial letal mayor por vía paren-
teral que por vía oral, probablemente porque la absorción
intestinal es incompleta.
Entre otros efectos de la exposición aguda cabe citar
alteraciones de las cifras de lípidos sanguíneos, el sis-
tema endocrino, el hígado y el bazo, así como un déficit
transitorio del desarrollo cerebral. La importancia toxi-
cológica de estos efectos, observados tras la administra-
ción de una fuerte dosis única del compuesto, es discuti-
ble y sigue ignorándose cuál es la causa de la muerte.
La toxicidad aguda por vía dérmica es baja; la
DL50 es > 9000 mg/kg de peso corporal para el conejo.
La DL50 por inhalación exclusivamente nasal (4 h) es de
77 mg/m3 (65 mg/m3 cuando sólo se tienen en cuenta las
partículas inhalables) para la rata. Las mezclas de aire
y vapores de TBE no producen efectos tóxicos observables,
ni siquiera en el punto de saturación. Sin embargo, el TBE
es muy peligroso cuando se inhala en forma de aerosol,
produciendo irritación y edema de los pulmones.
El TBE es muy irritante para la piel y sumamente irri-
tante para los ojos. El OTBE no produce sensibilización
cutánea.
12.2 Toxicidad a corto plazo
Los compuestos de TBE han sido muy estudiados en la
rata, hasta el punto de que todos los datos expuestos en
esta sección se refieren a ese animal a menos que se
indique otra cosa.
Con dosis de 320 mg/kg (unos 25 mg/kg de peso
corporal) en la dieta se han obtenido altas tasas de
mortalidad cuando la exposición se prolonga más de 4
semanas. No se observaron muertes con 100 mg/kg en la
dieta (10 mg/kg de peso corporal) ni tras la administra-
ción forzada de 12 mg/kg de peso corporal al día. En las
ratas tratadas en sus primeros días de vida, la mortalidad
aumentó con una dosis de 3 mg/kg de peso corporal. Los
principales síntomas causados por las dosis letales fueron
inapetencia, debilidad y emaciación.
Se han observado efectos "limítrofes" en el creci-
miento de la rata con dosis de 50 mg/kg (6 mg/kg de peso
corporal) en la dieta y de 6 mg/kg de peso corporal en
estudios de administración forzada. Los ratones se
muestran menos sensibles, observándose los efectos del
compuesto con dosis de 150 a 200 mg/kg (22 a 29 mg/kg de
peso corporal) en la dieta.
Tanto los estudios a corto plazo como en los pro-
longados se han observado efectos estructurales sobre los
órganos endocrinos, principalmente la hipófisis y el
tiroides. Las pruebas a corto plazo pusieron de manifiesto
alteraciones en la concentración de las hormonas circu-
lantes y en la respuesta a los estímulos fisiológicos
(hormonas tróficas hipofisarias), pero tras la exposición
prolongada desaparecieron casi todas esas alteraciones.
El mecanismo de acción es desconocido.
La exposición a un aerosol de OTBE a razón de 2,8
mg/m3 produjo un aumento de la mortalidad, así como
dificultades respiratorias, inflamación del tracto
respiratorio y alteraciones histopatológicas de los
órganos linfáticos. En cambio, la exposición a la máxima
concentración de vapor accesible (0,16 mg/m3) a la
temperatura del local no produjo ningún efecto.
En tres especies de mamíferos se han señalado efectos
tóxicos en el hígado y los conductos biliares. En ratas a
las que se administró OTBE en la dieta a razón de 320
mg/kg (unos 25 mg/kg de peso corporal) durante 4 semanas y
en ratones tratados con 80 mg/kg (unos 12 mg/kg de peso
corporal) en la dieta durante 90 días se produjo una
necrosis hepatocelular con alteraciones inflamatorias del
árbol biliar. En perros que recibieron una dosis de 10
mg/kg de peso corporal durante 8 o 9 semanas se observó
una vacuolización de los hepatocitos periportales. Estos
cambios se acompañaban a veces de un aumento del peso del
hígado y de la actividad sérica de las enzimas hepáticas.
El descenso de la concentración de hemoglobina y del
volumen eritrocítico en la rata, causado por la adminis-
tración de 80 mg/kg (8 mg/kg de peso corporal) en la
dieta, traduce un efecto en la síntesis de la hemoglobina
que da lugar a una anemia hipocrómica microcítica. El
descenso de las cifras de hemosiderina esplénica sugiere
una alteración del equilibrio del hierro. En los ratones
se ha observado también anemia.
En ciertas investigaciones a corto plazo, pero no en
los estudios prolongados, se ha observado formación de
rosetas de hematíes en los ganglios linfáticos del
mesenterio. No está clara la significación biológica de
este fenómeno (posiblemente transitorio).
El efecto tóxico característico del OTBE tiene lugar
en el sistema inmunitario: a causa de los efectos en el
timo, la inmunidad celular se menoscaba. Aunque se
desconoce el mecanismo de acción, es posible que esté
relacionado con la conversión metabólica en compuestos de
dibutilestaño. También resulta afectada la resistencia
inespecífica.
Asimismo se han observado efectos generales en el
sistema inmunitario (por ejemplo, en el peso y la
morfología de los tejidos linfoides, los recuentos de
linfocitos periféricos y las concentraciones totales de
inmunoglobulinas séricas) en diferentes estudios con OTBE
realizados en ratas y perros, pero no en ratones, mediante
dosis claramente tóxicas (en el ratón se han registrado
efectos con dosis de cloruro de tributilestaño de 150
mg/kg). Solamente la rata presenta signos de toxicidad y,
evidentemente, es la especie más sensible. En los estudios
a corto plazo, el NENO para esta especie fue de 5 mg/kg
(0,6 mg/kg de peso corporal) en la dieta. En los estudios
con cloruro de tributilestaño se han observado efectos
análogos en el timo, rápidamente reversibles tan pronto
como se interrumpía la administración. Según se ha
observado en estudios in vivo de resistencia del huésped,
el OTBE pone en peligro la función inmunitaria específica
en la rata. Tras la exposición a un nivel de 50 mg/kg en
la dieta (siendo el valor NENO de 5 mg/kg al día), se
observó una disminución del "aclaramiento" de Listeria
monocytogenes, mientras que con 50 y 5 mg/kg en la dieta,
pero no con 0,5 mg/kg (2,5, 0,25 y 0,025 mg/kg de peso
corporal al día, respectivamente) se produjo un descenso
de la resistencia a Trichinella spiralis. Los mismos
efectos, aunque menos pronunciados, se observaron en
animales de más edad.
Según los conocimientos actuales, los efectos en la
resistencia del huésped se deben probablemente a una
evaluación más adecuada de los posibles riesgos para el
hombre; sin embargo, no se tiene suficiente experiencia
con los sistemas de prueba para apreciar bien su
importancia. En cualquier caso, las observaciones en
ratas atímicas "desnudas", estimuladas en las condi-
ciones ordinarias, proporcionan algunos datos para
interpretar el modelo de T. spiralis. En estos estudios,
la ausencia completa de inmunidad timodependiente aumentó
de 10 a 20 veces los recuentos de larvas en el músculo; en
cambio, la exposición a concentraciones de OTBE de 5 y 50
mg/kg en la dieta duplicó y cuadruplicó, respectivamente,
la cifra inicial.
Aunque ahora se dispone de algunos datos sobre los
efectos de los compuestos de tributilestaño en el
desarrollo del sistema inmunitario, carecemos de informa-
ción sobre la resistencia del huésped.
Lo prudente sería evaluar los posibles riesgos para el
hombre en función de los datos obtenidos en la especie más
sensible. Los efectos sobre la resistencia del huésped a
T. spiralis se han observado ya con niveles en la dieta
de 5 mg/kg (equivalentes a 0,25 mg/kg de peso corporal al
día), por lo que el valor NENO es de 0,5 mg/kg (equiva-
lente a 0,025 mg/kg al día). Sin embargo, no existe
acuerdo sobre el significado de estos datos para evaluar
los riesgos en el hombre. En todos los demás estudios,
una concentración de 5 mg/kg al día en la dieta (equiva-
lente a 0,5 mg/kg de peso corporal, sobre la base de los
estudios a corto plazo) correspondía al valor NENO con
respecto a los efectos en el sistema inmunitario, tanto
generales como específicos.
12.3 Toxicidad a largo plazo
Un estudio a largo plazo en las ratas sugiere un
efecto marginal del TBE en los parámetros toxicológicos
generales (cuya significación toxicológica es limitada) a
una concentración de 5 mg/kg (0,25 mg/kg de peso corporal)
en la dieta.
12.4 Genotoxicidad
La genotoxicidad del OTBE ha sido objeto de detenidas
investigaciones. En la gran mayoría de los estudios se
han obtenido resultados negativos, y no hay ninguna prueba
convincente de que el OTBE tenga propiedades mutagénicas.
12.5 Toxicidad en el sistema reproductor
En tres especies de mamíferos (ratón, rata y conejo)
se ha evaluado la posible embriotoxicidad del OTBE tras
administrarlo por vía oral a la madre. La principal
malformación observada en los fetos de rata y de ratón fue
la fisura palatina, pero este efecto sólo se registró con
dosis claramente tóxicas para las madres. Tal resultado
no se ha considerado como indicio de efectos teratogénicos
del OTBE en dosis inferiores a las que producen toxicidad
materna. El NENO más bajo en lo relativo a la embriotoxi-
cidad y la fetotoxicidad para todos las especies fue de
1,0 mg/kg de peso corporal.
12.6 Carcinogenicidad
En las ratas se ha realizado un estudio de carcino-
genicidad en el que se obtuvieron alteraciones neoplásicas
de los órganos endocrinos con 50 mg/kg en la dieta. En
cuanto a los tumores hipofisarios registrados con 0,5
mg/kg en la dieta, se consideró que no tenían signifi-
cación biológica por no existir relación dosis-respuesta.
Estos tipos de tumores suelen aparecer con una incidencia
general elevada y variable, por lo que su significación
parece discutible. Está en curso un estudio de carcino-
genicidad en el ratón.
13. Efectos en el ser humano
Se ha observado que la exposición profesional de los
trabajadores al tributilestaño provoca irritación de las
vías respiratorias superiores. En forma de aerosol, el
TBE entraña un riesgo para las personas. El OTBE ejerce
un efecto irritante en la piel y en los ojos, habiéndose
observado casos de dermatitis grave a consecuencia del
contacto directo con la piel. El problema se agrava por
la falta de una respuesta cutánea inmediata.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS
SOBRE EL MEDIO AMBIENTE
1. Evaluación del riesgo para las personas
La exposición de los trabajadores se produce sobre
todo en las actividades de fabricación y formulación de
compuestos de tributilestaño, durante la aplicación y la
eliminación de pinturas a base de TBE y a consecuencia del
empleo de TBE como conservador de la madera. La exposición
del público en general puede deberse a la contaminación de
los alimentos, particularmente el pescado y los mariscos,
así como a la aplicación doméstica de productos de protec-
ción de la madera.
A juzgar por las pruebas realizadas en animales y por
la observación directa de las personas, parece evidente
que los compuestos de TBE ejercen efectos irritantes en la
piel y en los ojos y que la inhalación de los aerosoles
provoca irritación respiratoria.
La manipulación de madera tratada no entraña riesgos
de irritación dérmica si se ha secado el material. En
cambio, los aerosoles de TBE son muy peligrosos y no debe
permitirse que la madera vuelva a entrar en la zona de
tratamiento hasta que esté perfectamente seca.
No se ha señalado ningún caso de intoxicación sis-
témica aguda y, probablemente, el TBE se elimina del
organismo al cabo de pocos días. Por consiguiente, no es
de temer que el manejo de productos de TBE entrañe peligro
de toxicidad aguda si se toman las precauciones
adecuadas.
En los animales de laboratorio se han observado
efectos a corto y a largo plazo en el hígado y en los
sistemas hematológico y endocrino. Los efectos de los
compuestos de TBE en el sistema inmunitario, y especial-
mente en la resistencia del huésped, han resultado ser el
parámetro más sensible de toxicidad en la rata, que es la
especie más sensible de todas las estudiadas. Cuando se
utiliza Trichinella spiralis como modelo de resistencia
del huésped, el nivel de efecto no observado (NENO) se
sitúa entre 0,5 y 5,0 mg/kg (0,025 y 0,25 mg/kg de peso
corporal) en la dieta, mientras que si se utilizan índices
de función inmunitaria es de 0,6 mg/kg de peso corporal.
Debido a las grandes variaciones en el consumo de
pescado y mariscos y a las diferencias locales de la
cantidad de residuos de TBE presentes en la fauna marina,
sólo a título de orientación pueden hacerse estimaciones
de los valores de exposición y del NENO. Conviene tener
en cuenta que para evaluar el riesgo potencial de estos
compuestos hay que proceder en el plano local a determinar
los residuos, calcular el consumo de pescado y mariscos y
fijar los límites aceptables de seguridad.
Partiendo de cifras de consumo de pescado de 15 y 150
g/día, de un valor de residuos en el pescado de 1 mg/kg y
de un peso medio de las personas de 60 kilos, se han
obtenido los siguientes márgenes de seguridad basados en
diferentes puntos finales inmunológicos.
----------------------------------------------------------------------
Consumo de Ingestión diaria Margen de seguridad
pescado estimada de TBE Modelo de Otros parámetros
(g/día) (µg/kg) T. spiralis inmunológicos
----------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
----------------------------------------------------------------------
El empleo indiscriminado e irresponsable de compuesto
de TBE y la observancia de las recomendaciones esbozadas
en la presente monografía para reducir la exposición de
las personas puede dar lugar a la ingestión de concentra-
ciones de compuesto de TBE peligrosas para la salud
humana.
En los animales de experimentación sólo de han
observado efectos teratogénicos con dosis que causan
signos patentes de toxicidad materna. Por consiguiente,
se considera que el potencial teratogénico de TBE es muy
bajo.
Basándose en los resultados de detallados estudios de
mutagenicidad, se considera que los compuestos de
tributilestaño no tienen potencial mutagénico. En un
estudio de carcinogenicidad realizada en la rata con OTBE,
se observó un aumento de la incidencia de ciertos tumores
endocrinos que aparecen espontáneamente con una incidencia
elevada y variable. Por consiguiente, los datos
disponibles no indican claramente que los compuestos de
TBE entrañen riesgos carcinogénicos para las personas.
2. Evaluación de los riesgos ambientales
La difusión del tributilestaño (TBE) en el medio
ambiente se debe sobre todo al empleo de ese compuesto en
las pinturas antiincrustantes. También puede deberse al
empleo de TBE como molusquicida. La contaminación en la
fuente se produce a consecuencia de utilizar TBE como
biocida en los sistemas de refrigeración, la fabricación
de pulpa de madera, el tratamiento de los cueros, los
procesos de conservación de la madera y el tratamiento de
los productos textiles.
Debido a sus propiedades fisicoquímicas, los compues-
tos de TBE se concentran en la microcapa superficial y en
los sedimentos. La degradación abiótica no parece ser un
mecanismo importante de eliminación del TBE en condiciones
ambientales. Aunque el OTBE es biodegradable en la columna
de agua, este proceso no es suficientemente rápido para
impedir la aparición de concentraciones elevadas de TBE en
algunos sitios. En la mayor parte de los organismos
acuáticos se produce bioacumulación pero la degradación
metabólica es un proceso más eficaz en los mamíferos de
laboratorio.
El TBE es sumamente peligroso para algunos organismos
acuáticos por ser tóxico incluso a concentraciones muy
bajas en el agua. Tales concentraciones se han señalado
en diversos lugares. En los estudios sobre el terreno se
han observado efectos adversos sobre invertebrados,
especialmente moluscos, a los que no se pretendía atacar,
y esos efectos resultan suficientemente graves para inter-
ferir en la reproducción y provocar un descenso de la
población. En algunos sitios ha sido posible anular los
efectos adversos en la producción comercial de mariscos
restringuiendo el empleo de pinturas antiincrustantes, con
lo que también se ha logrado suprimir el efecto de imposex
en las poblaciones de gasterópodos. Los efectos en las
piscifactorías hacen pensar que no conviene utilizar
pinturas que contengan TBE en las redes de contención.
Para el medio terrestre, el riesgo general parece ser
bajo. La madera tratada con TBE podría ser peligrosa para
los organismos terrestres que viven en estrecho contacto
con ella.
El aumento de las concentraciones de TBE en la micro-
capa superficial puede ser peligroso para los organismos
del litoral, las especies neustónicas (inclusive inverte-
brados bénticos y larvas de peces) y las aves salvajes y
marinas que se alimentan en la superficie del agua. La
acumulación y la baja biodegradación del TBE en los sedi-
mentos puede representar un peligro para los organismos
acuáticos cuando esos sedimentos contaminados se movilizan
por procesos naturales u operaciones de dragado.
RECOMENDACIONES
1. Recomendaciones para proteger la salud humana y la
higiene del medio
a) Hay que instar a los países Miembros que todavía no
hayan reglamentado el uso de compuestos de TBE a que
lo hagan.
b) Es necesario evaluar y, si procede reglamentar, el
ingreso en el medio ambiente de estaño orgánico pro-
cedente de fuentes distintas de las pinturas antiin-
crustantes. Por ejemplo, convendría evaluar el riesgo
potencial que entraña la aplicación al suelo de lodos
de alcantarillado contaminados con TBE.
c) Habrá que mejorar los métodos utilizables para apli-
car, eliminar y evacuar en condiciones de seguridad
las pinturas de estaño orgánico.
2. Investigaciones necesarias
a) Habrá que mejorar los métodos de detección y análisis
a fin de poder determinar con rapidez y precisión los
compuestos de butilestaño presentes en concentraciones
de pg/litro. Una de las razones que justifican esa
recomendación es que ciertos efectos biológicos (por
ejemplo, el imposex en los gasterópodos) pueden pro-
ducirse con concentraciones inferiores a los actuales
límites de detección.
b) Esnecesario estudiar los mecanismos que concentran en
vez de dispersar el TBE y que retrasan la degradación,
prestando especial atención a los fundamentos químicos
de ese compuesto y a su interaccción con las moléculas
biológicas. Se necesitan más estudios sobre la absor-
ción del TBE en todos los niveles tróficos.
c) Habrá que estudiar la toxicidad del TBE en los organ-
ismos acuáticos. Estos estudios deberán versar, si
procede, sobre el metabolismo, los efectos endocrinos
y la toxicidad inmunológica.
d) Habrá que buscar otras especies sensibles (en parti-
cular de agua dulce) que sirvan de bioindicador en
otros grupos.
e) Habrá que confirmar la validez de los modelos utili-
zados para evaluar la inmunotoxicidad en los mamíferos
y definir con más precisión los niveles sin efecto de
los parámetros pertinentes.
f) Convendría emprender un estudio de toxicidad crónica
en una segunda especie de mamífero.
g) Convendría emprender un estudio de tumorigenicidad en
una segunda especie de mamífero.
h) Hay que obtener datos mediante métodos de especiación
sobre los niveles de residuos de butilestaño en los
peces y mariscos destinados al consumo humano.
Los datos sobre los invertebrados de agua dulce son
escasos y no comprenden más que tres especies, además de
los organismos tomados como objetivo. Con diferentes sales
de TBE se han obtenido valores de CL50 en 48 h para
Daphnia de 2,3-70 µg/litro y para Tubifex de 5,5-
33 µg/litro. Basándose en la reversión de la respuesta
normal a la luz, se ha calculado que el NENO para Daphnia
es de 0,5 µg/litro. La CL50 de 24 h para la almeja
asiática parece ser, según se ha señalado, de 2100 µg por
litro, mientras que para los moluscos adultos contra los
que se dirige la lucha antiesquistosomiásica los valores
correspondientes son de 30-400 µg/litro.
Se ha demostrado que el tributilestaño es tóxico para
las larvas de esquistosoma en las fases acuáticas; la
CL50 (fluoruro de TBE) parece ser, según los cálculos
realizados, de 16,8 µg/litro en el caso de una exposi-
ción de 1 h. La dosis de TBE que suprime el 99-100% de la
infectividad de las cercarias para el ratón está compren-
dida entre 2 y 6 µg/litro.
La sensibilidad de los moluscos al TBE disminuye con
la edad, pero los huevos son más resistentes que los
individuos jóvenes y adultos. La puesta de huevos se ve
considerablemente afectada a una concentración de OTBE de
0,001 µg/litro.
En las pruebas de CL50 con exposiciones de hasta
168 h, la toxicidad aguda del TBE para los peces de agua
dulce varía entre 13 y 240 µg/litro. Basándose en los
efectos histopatológicos, se ha calculado que el valor
NENO para el "guppy" es de 0,01 µg/litro.
No se ha observado ningún efecto sobre la super-
vivencia tras la exposición de huevos y larvas de la rana
Rana temporaria a concentraciones de TBE de 3 µg por
litro o menos; en cambio, la mortalidad fue significativa
a 30 µg/litro.
9.3 Estudios microcósmicos
Se han realizado algunos "estudios microcósmicos"
utilizando como modelo ecosistemas marinos en los que se
habían introducido organismos y en los que la entrada de
agua de mar facilitaba la colonización por otros organ-
ismos. Los resultados obtenidos muestran que tanto el
número de individuos como la diversidad de las especies
disminuyen cuando la concentración de OTBE en el agua se
sitúa entre 0,06 y 3 µg/litro.
Los resultados obtenidos con modelos de ecosistemas de
agua dulce hacen pensar que las dosis que matan los cara-
coles afectan también a otras especies, en particular los
peces.
10. Efectos en los organismos terrestres
La exposición de organismos terrestres al TBE proviene
sobre todo del uso de este compuesto como conservador de
la madera. El OTBE es tóxico para las abejas que viven en
colmenas de madera tratadas con TBE. Este producto se
mostró tóxico para los murciélagos en un estudio aislado,
pero la elevada mortalidad de los testigos resta signifi-
cación estadística a este resultado. Los compuestos de
TBE son tóxicos para los insectos que están en contacto o
se alimentan con madera tratada. El TBE tiene una toxi-
cidad aguda moderada para los ratones en libertad y,
basándose en el consumo de semillas tratadas en las
pruebas de repelentes, se calcula que los valores de
CL50 en la dieta se sitúan entre 37 y 240 mg/kg al día.
11. Efectos en organismos estudiados sobre el terreno
Las observaciones sobre el terreno han permitido
establecer una relación entre las concentraciones elevadas
de tributilestaño y la mortalidad de las larvas de
bivalvos, la incapacidad de las mismas para constituir
colonias, el retraso del crecimiento, el engrosamiento de
la concha y otras malformaciones de las ostras en
desarrollo, el imposex en los caracoles de suelos
cenagosos y el imposex (con disminución concurrente de la
población) en el buccino. En Francia inicialmente, y más
tarde en otros países, se ha identificado y atribuido la
destrucción completa de los criaderos de ostras a la
presencia de TBE en el agua. Los efectos son más acusados
en las zonas próximas a los centros de deportes náuticos.
Al dejar de emplear pinturas antiincrustantes a base de
TBE en las pequeñas embarcaciones se restablecieron la
reproducción y el crecimiento de las ostras. Sin embargo,
en algunas zonas las concentraciones de TBE en el agua
siguen siendo bastante elevadas para afectar a los
gasterópodos marinos.
Tanto el desarrollo y formación de la concha en las
ostras del Pacífico como el imposex en el buccino se han
utilizado como indicadores biológicos de contaminación por
TBE.
Aunque se han hecho pocos estudios sobre los efectos
en los organismos del TBE presente en los sedimentos, hay
indicios de que este compuesto puede llegar a los animales
que viven en madrigueras y producir mortalidad en condi-
ciones prácticas.
Se han observado efectos tóxicos macroscópicos y
alteraciones histopatológicas en los criaderos de pesca
marítima expuestos al TBE por el uso de pinturas antiin-
crustantes en las redes de contención.
Se ha propuesto la utilización de TBE como molusqui-
cida contra los caracoles de agua dulce que transmiten la
esquistosomiasis (bilharziasis). Varios ensayos prácticos
han demostrado que es difícil aplicar el TBE sin que
resulten perjudicados otros organismos distintos de los
tomados como objetivo.
12. Toxicidad para los mamíferos de laboratorio
12.1 Toxicidad aguda
El tributilestaño tiene una toxicidad entre moderada y
alta para los mamíferos de laboratorio; los valores de la
DL50 oral aguda varían desde 94 a 234 mg/kg de peso
corporal para la rata y desde 44 hasta 230 mg/kg de peso
corporal para el ratón. La toxicidad aguda para el cobayo
y el conejo es del mismo orden. La variación se debe al
componente aniónico de la sal de tributilestaño. Estos
compuestos tienen un potencial letal mayor por vía paren-
teral que por vía oral, probablemente porque la absorción
intestinal es incompleta.
Entre otros efectos de la exposición aguda cabe citar
alteraciones de las cifras de lípidos sanguíneos, el sis-
tema endocrino, el hígado y el bazo, así como un déficit
transitorio del desarrollo cerebral. La importancia toxi-
cológica de estos efectos, observados tras la administra-
ción de una fuerte dosis única del compuesto, es discuti-
ble y sigue ignorándose cuál es la causa de la muerte.
La toxicidad aguda por vía dérmica es baja; la
DL50 es > 9000 mg/kg de peso corporal para el conejo.
La DL50 por inhalación exclusivamente nasal (4 h) es de
77 mg/m3 (65 mg/m3 cuando sólo se tienen en cuenta las
partículas inhalables) para la rata. Las mezclas de aire
y vapores de TBE no producen efectos tóxicos observables,
ni siquiera en el punto de saturación. Sin embargo, el TBE
es muy peligroso cuando se inhala en forma de aerosol,
produciendo irritación y edema de los pulmones.
El TBE es muy irritante para la piel y sumamente irri-
tante para los ojos. El OTBE no produce sensibilización
cutánea.
12.2 Toxicidad a corto plazo
Los compuestos de TBE han sido muy estudiados en la
rata, hasta el punto de que todos los datos expuestos en
esta sección se refieren a ese animal a menos que se
indique otra cosa.
Con dosis de 320 mg/kg (unos 25 mg/kg de peso
corporal) en la dieta se han obtenido altas tasas de
mortalidad cuando la exposición se prolonga más de 4
semanas. No se observaron muertes con 100 mg/kg en la
dieta (10 mg/kg de peso corporal) ni tras la administra-
ción forzada de 12 mg/kg de peso corporal al día. En las
ratas tratadas en sus primeros días de vida, la mortalidad
aumentó con una dosis de 3 mg/kg de peso corporal. Los
principales síntomas causados por las dosis letales fueron
inapetencia, debilidad y emaciación.
Se han observado efectos "limítrofes" en el creci-
miento de la rata con dosis de 50 mg/kg (6 mg/kg de peso
corporal) en la dieta y de 6 mg/kg de peso corporal en
estudios de administración forzada. Los ratones se
muestran menos sensibles, observándose los efectos del
compuesto con dosis de 150 a 200 mg/kg (22 a 29 mg/kg de
peso corporal) en la dieta.
Tanto los estudios a corto plazo como en los pro-
longados se han observado efectos estructurales sobre los
órganos endocrinos, principalmente la hipófisis y el
tiroides. Las pruebas a corto plazo pusieron de manifiesto
alteraciones en la concentración de las hormonas circu-
lantes y en la respuesta a los estímulos fisiológicos
(hormonas tróficas hipofisarias), pero tras la exposición
prolongada desaparecieron casi todas esas alteraciones.
El mecanismo de acción es desconocido.
La exposición a un aerosol de OTBE a razón de 2,8
mg/m3 produjo un aumento de la mortalidad, así como
dificultades respiratorias, inflamación del tracto
respiratorio y alteraciones histopatológicas de los
órganos linfáticos. En cambio, la exposición a la máxima
concentración de vapor accesible (0,16 mg/m3) a la
temperatura del local no produjo ningún efecto.
En tres especies de mamíferos se han señalado efectos
tóxicos en el hígado y los conductos biliares. En ratas a
las que se administró OTBE en la dieta a razón de 320
mg/kg (unos 25 mg/kg de peso corporal) durante 4 semanas y
en ratones tratados con 80 mg/kg (unos 12 mg/kg de peso
corporal) en la dieta durante 90 días se produjo una
necrosis hepatocelular con alteraciones inflamatorias del
árbol biliar. En perros que recibieron una dosis de 10
mg/kg de peso corporal durante 8 o 9 semanas se observó
una vacuolización de los hepatocitos periportales. Estos
cambios se acompañaban a veces de un aumento del peso del
hígado y de la actividad sérica de las enzimas hepáticas.
El descenso de la concentración de hemoglobina y del
volumen eritrocítico en la rata, causado por la adminis-
tración de 80 mg/kg (8 mg/kg de peso corporal) en la
dieta, traduce un efecto en la síntesis de la hemoglobina
que da lugar a una anemia hipocrómica microcítica. El
descenso de las cifras de hemosiderina esplénica sugiere
una alteración del equilibrio del hierro. En los ratones
se ha observado también anemia.
En ciertas investigaciones a corto plazo, pero no en
los estudios prolongados, se ha observado formación de
rosetas de hematíes en los ganglios linfáticos del
mesenterio. No está clara la significación biológica de
este fenómeno (posiblemente transitorio).
El efecto tóxico característico del OTBE tiene lugar
en el sistema inmunitario: a causa de los efectos en el
timo, la inmunidad celular se menoscaba. Aunque se
desconoce el mecanismo de acción, es posible que esté
relacionado con la conversión metabólica en compuestos de
dibutilestaño. También resulta afectada la resistencia
inespecífica.
Asimismo se han observado efectos generales en el
sistema inmunitario (por ejemplo, en el peso y la
morfología de los tejidos linfoides, los recuentos de
linfocitos periféricos y las concentraciones totales de
inmunoglobulinas séricas) en diferentes estudios con OTBE
realizados en ratas y perros, pero no en ratones, mediante
dosis claramente tóxicas (en el ratón se han registrado
efectos con dosis de cloruro de tributilestaño de 150
mg/kg). Solamente la rata presenta signos de toxicidad y,
evidentemente, es la especie más sensible. En los estudios
a corto plazo, el NENO para esta especie fue de 5 mg/kg
(0,6 mg/kg de peso corporal) en la dieta. En los estudios
con cloruro de tributilestaño se han observado efectos
análogos en el timo, rápidamente reversibles tan pronto
como se interrumpía la administración. Según se ha
observado en estudios in vivo de resistencia del huésped,
el OTBE pone en peligro la función inmunitaria específica
en la rata. Tras la exposición a un nivel de 50 mg/kg en
la dieta (siendo el valor NENO de 5 mg/kg al día), se
observó una disminución del "aclaramiento" de Listeria
monocytogenes, mientras que con 50 y 5 mg/kg en la dieta,
pero no con 0,5 mg/kg (2,5, 0,25 y 0,025 mg/kg de peso
corporal al día, respectivamente) se produjo un descenso
de la resistencia a Trichinella spiralis. Los mismos
efectos, aunque menos pronunciados, se observaron en
animales de más edad.
Según los conocimientos actuales, los efectos en la
resistencia del huésped se deben probablemente a una
evaluación más adecuada de los posibles riesgos para el
hombre; sin embargo, no se tiene suficiente experiencia
con los sistemas de prueba para apreciar bien su
importancia. En cualquier caso, las observaciones en
ratas atímicas "desnudas", estimuladas en las condi-
ciones ordinarias, proporcionan algunos datos para
interpretar el modelo de T. spiralis. En estos estudios,
la ausencia completa de inmunidad timodependiente aumentó
de 10 a 20 veces los recuentos de larvas en el músculo; en
cambio, la exposición a concentraciones de OTBE de 5 y 50
mg/kg en la dieta duplicó y cuadruplicó, respectivamente,
la cifra inicial.
Aunque ahora se dispone de algunos datos sobre los
efectos de los compuestos de tributilestaño en el
desarrollo del sistema inmunitario, carecemos de informa-
ción sobre la resistencia del huésped.
Lo prudente sería evaluar los posibles riesgos para el
hombre en función de los datos obtenidos en la especie más
sensible. Los efectos sobre la resistencia del huésped a
T. spiralis se han observado ya con niveles en la dieta
de 5 mg/kg (equivalentes a 0,25 mg/kg de peso corporal al
día), por lo que el valor NENO es de 0,5 mg/kg (equiva-
lente a 0,025 mg/kg al día). Sin embargo, no existe
acuerdo sobre el significado de estos datos para evaluar
los riesgos en el hombre. En todos los demás estudios,
una concentración de 5 mg/kg al día en la dieta (equiva-
lente a 0,5 mg/kg de peso corporal, sobre la base de los
estudios a corto plazo) correspondía al valor NENO con
respecto a los efectos en el sistema inmunitario, tanto
generales como específicos.
12.3 Toxicidad a largo plazo
Un estudio a largo plazo en las ratas sugiere un
efecto marginal del TBE en los parámetros toxicológicos
generales (cuya significación toxicológica es limitada) a
una concentración de 5 mg/kg (0,25 mg/kg de peso corporal)
en la dieta.
12.4 Genotoxicidad
La genotoxicidad del OTBE ha sido objeto de detenidas
investigaciones. En la gran mayoría de los estudios se
han obtenido resultados negativos, y no hay ninguna prueba
convincente de que el OTBE tenga propiedades mutagénicas.
12.5 Toxicidad en el sistema reproductor
En tres especies de mamíferos (ratón, rata y conejo)
se ha evaluado la posible embriotoxicidad del OTBE tras
administrarlo por vía oral a la madre. La principal
malformación observada en los fetos de rata y de ratón fue
la fisura palatina, pero este efecto sólo se registró con
dosis claramente tóxicas para las madres. Tal resultado
no se ha considerado como indicio de efectos teratogénicos
del OTBE en dosis inferiores a las que producen toxicidad
materna. El NENO más bajo en lo relativo a la embriotoxi-
cidad y la fetotoxicidad para todos las especies fue de
1,0 mg/kg de peso corporal.
12.6 Carcinogenicidad
En las ratas se ha realizado un estudio de carcino-
genicidad en el que se obtuvieron alteraciones neoplásicas
de los órganos endocrinos con 50 mg/kg en la dieta. En
cuanto a los tumores hipofisarios registrados con 0,5
mg/kg en la dieta, se consideró que no tenían signifi-
cación biológica por no existir relación dosis-respuesta.
Estos tipos de tumores suelen aparecer con una incidencia
general elevada y variable, por lo que su significación
parece discutible. Está en curso un estudio de carcino-
genicidad en el ratón.
13. Efectos en el ser humano
Se ha observado que la exposición profesional de los
trabajadores al tributilestaño provoca irritación de las
vías respiratorias superiores. En forma de aerosol, el
TBE entraña un riesgo para las personas. El OTBE ejerce
un efecto irritante en la piel y en los ojos, habiéndose
observado casos de dermatitis grave a consecuencia del
contacto directo con la piel. El problema se agrava por
la falta de una respuesta cutánea inmediata.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS
SOBRE EL MEDIO AMBIENTE
1. Evaluación del riesgo para las personas
La exposición de los trabajadores se produce sobre
todo en las actividades de fabricación y formulación de
compuestos de tributilestaño, durante la aplicación y la
eliminación de pinturas a base de TBE y a consecuencia del
empleo de TBE como conservador de la madera. La exposición
del público en general puede deberse a la contaminación de
los alimentos, particularmente el pescado y los mariscos,
así como a la aplicación doméstica de productos de protec-
ción de la madera.
A juzgar por las pruebas realizadas en animales y por
la observación directa de las personas, parece evidente
que los compuestos de TBE ejercen efectos irritantes en la
piel y en los ojos y que la inhalación de los aerosoles
provoca irritación respiratoria.
La manipulación de madera tratada no entraña riesgos
de irritación dérmica si se ha secado el material. En
cambio, los aerosoles de TBE son muy peligrosos y no debe
permitirse que la madera vuelva a entrar en la zona de
tratamiento hasta que esté perfectamente seca.
No se ha señalado ningún caso de intoxicación sis-
témica aguda y, probablemente, el TBE se elimina del
organismo al cabo de pocos días. Por consiguiente, no es
de temer que el manejo de productos de TBE entrañe peligro
de toxicidad aguda si se toman las precauciones
adecuadas.
En los animales de laboratorio se han observado
efectos a corto y a largo plazo en el hígado y en los
sistemas hematológico y endocrino. Los efectos de los
compuestos de TBE en el sistema inmunitario, y especial-
mente en la resistencia del huésped, han resultado ser el
parámetro más sensible de toxicidad en la rata, que es la
especie más sensible de todas las estudiadas. Cuando se
utiliza Trichinella spiralis como modelo de resistencia
del huésped, el nivel de efecto no observado (NENO) se
sitúa entre 0,5 y 5,0 mg/kg (0,025 y 0,25 mg/kg de peso
corporal) en la dieta, mientras que si se utilizan índices
de función inmunitaria es de 0,6 mg/kg de peso corporal.
Debido a las grandes variaciones en el consumo de
pescado y mariscos y a las diferencias locales de la
cantidad de residuos de TBE presentes en la fauna marina,
sólo a título de orientación pueden hacerse estimaciones
de los valores de exposición y del NENO. Conviene tener
en cuenta que para evaluar el riesgo potencial de estos
compuestos hay que proceder en el plano local a determinar
los residuos, calcular el consumo de pescado y mariscos y
fijar los límites aceptables de seguridad.
Partiendo de cifras de consumo de pescado de 15 y 150
g/día, de un valor de residuos en el pescado de 1 mg/kg y
de un peso medio de las personas de 60 kilos, se han
obtenido los siguientes márgenes de seguridad basados en
diferentes puntos finales inmunológicos.
----------------------------------------------------------------------
Consumo de Ingestión diaria Margen de seguridad
pescado estimada de TBE Modelo de Otros parámetros
(g/día) (µg/kg) T. spiralis inmunológicos
----------------------------------------------------------------------
15 0,25 100-1000 2500
150 2,5 10-100 250
----------------------------------------------------------------------
El empleo indiscriminado e irresponsable de compuesto
de TBE y la observancia de las recomendaciones esbozadas
en la presente monografía para reducir la exposición de
las personas puede dar lugar a la ingestión de concentra-
ciones de compuesto de TBE peligrosas para la salud
humana.
En los animales de experimentación sólo de han
observado efectos teratogénicos con dosis que causan
signos patentes de toxicidad materna. Por consiguiente,
se considera que el potencial teratogénico de TBE es muy
bajo.
Basándose en los resultados de detallados estudios de
mutagenicidad, se considera que los compuestos de
tributilestaño no tienen potencial mutagénico. En un
estudio de carcinogenicidad realizada en la rata con OTBE,
se observó un aumento de la incidencia de ciertos tumores
endocrinos que aparecen espontáneamente con una incidencia
elevada y variable. Por consiguiente, los datos
disponibles no indican claramente que los compuestos de
TBE entrañen riesgos carcinogénicos para las personas.
2. Evaluación de los riesgos ambientales
La difusión del tributilestaño (TBE) en el medio
ambiente se debe sobre todo al empleo de ese compuesto en
las pinturas antiincrustantes. También puede deberse al
empleo de TBE como molusquicida. La contaminación en la
fuente se produce a consecuencia de utilizar TBE como
biocida en los sistemas de refrigeración, la fabricación
de pulpa de madera, el tratamiento de los cueros, los
procesos de conservación de la madera y el tratamiento de
los productos textiles.
Debido a sus propiedades fisicoquímicas, los compues-
tos de TBE se concentran en la microcapa superficial y en
los sedimentos. La degradación abiótica no parece ser un
mecanismo importante de eliminación del TBE en condiciones
ambientales. Aunque el OTBE es biodegradable en la columna
de agua, este proceso no es suficientemente rápido para
impedir la aparición de concentraciones elevadas de TBE en
algunos sitios. En la mayor parte de los organismos
acuáticos se produce bioacumulación pero la degradación
metabólica es un proceso más eficaz en los mamíferos de
laboratorio.
El TBE es sumamente peligroso para algunos organismos
acuáticos por ser tóxico incluso a concentraciones muy
bajas en el agua. Tales concentraciones se han señalado
en diversos lugares. En los estudios sobre el terreno se
han observado efectos adversos sobre invertebrados,
especialmente moluscos, a los que no se pretendía atacar,
y esos efectos resultan suficientemente graves para inter-
ferir en la reproducción y provocar un descenso de la
población. En algunos sitios ha sido posible anular los
efectos adversos en la producción comercial de mariscos
restringuiendo el empleo de pinturas antiincrustantes, con
lo que también se ha logrado suprimir el efecto de imposex
en las poblaciones de gasterópodos. Los efectos en las
piscifactorías hacen pensar que no conviene utilizar
pinturas que contengan TBE en las redes de contención.
Para el medio terrestre, el riesgo general parece ser
bajo. La madera tratada con TBE podría ser peligrosa para
los organismos terrestres que viven en estrecho contacto
con ella.
El aumento de las concentraciones de TBE en la micro-
capa superficial puede ser peligroso para los organismos
del litoral, las especies neustónicas (inclusive inverte-
brados bénticos y larvas de peces) y las aves salvajes y
marinas que se alimentan en la superficie del agua. La
acumulación y la baja biodegradación del TBE en los sedi-
mentos puede representar un peligro para los organismos
acuáticos cuando esos sedimentos contaminados se movilizan
por procesos naturales u operaciones de dragado.
RECOMENDACIONES
1. Recomendaciones para proteger la salud humana y la
higiene del medio
a) Hay que instar a los países Miembros que todavía no
hayan reglamentado el uso de compuestos de TBE a que
lo hagan.
b) Es necesario evaluar y, si procede reglamentar, el
ingreso en el medio ambiente de estaño orgánico pro-
cedente de fuentes distintas de las pinturas antiin-
crustantes. Por ejemplo, convendría evaluar el riesgo
potencial que entraña la aplicación al suelo de lodos
de alcantarillado contaminados con TBE.
c) Habrá que mejorar los métodos utilizables para apli-
car, eliminar y evacuar en condiciones de seguridad
las pinturas de estaño orgánico.
2. Investigaciones necesarias
a) Habrá que mejorar los métodos de detección y análisis
a fin de poder determinar con rapidez y precisión los
compuestos de butilestaño presentes en concentraciones
de pg/litro. Una de las razones que justifican esa
recomendación es que ciertos efectos biológicos (por
ejemplo, el imposex en los gasterópodos) pueden pro-
ducirse con concentraciones inferiores a los actuales
límites de detección.
b) Esnecesario estudiar los mecanismos que concentran en
vez de dispersar el TBE y que retrasan la degradación,
prestando especial atención a los fundamentos químicos
de ese compuesto y a su interaccción con las moléculas
biológicas. Se necesitan más estudios sobre la absor-
ción del TBE en todos los niveles tróficos.
c) Habrá que estudiar la toxicidad del TBE en los organ-
ismos acuáticos. Estos estudios deberán versar, si
procede, sobre el metabolismo, los efectos endocrinos
y la toxicidad inmunológica.
d) Habrá que buscar otras especies sensibles (en parti-
cular de agua dulce) que sirvan de bioindicador en
otros grupos.
e) Habrá que confirmar la validez de los modelos utili-
zados para evaluar la inmunotoxicidad en los mamíferos
y definir con más precisión los niveles sin efecto de
los parámetros pertinentes.
f) Convendría emprender un estudio de toxicidad crónica
en una segunda especie de mamífero.
g) Convendría emprender un estudio de tumorigenicidad en
una segunda especie de mamífero.
h) Hay que obtener datos mediante métodos de especiación
sobre los niveles de residuos de butilestaño en los
peces y mariscos destinados al consumo humano.
