
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 115
2-METHOXYETHANOL, 2-ETHOXYETHANOL,
AND THEIR ACETATES
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
2-Methoxyethanol, 2-ethoxyethanol, and their acetates.
(Environmental health criteria ; 115)
1.Ethylene glycols - adverse effects 2.Ethylene glycols - toxicity
I.Series
ISBN 92 4 157115 2 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 2-METHOXYETHANOL, 2-ETHOXYETHANOL,
AND THEIR ACETATES
1. SUMMARY AND CONCLUSIONS
1.1. Identity, physical and chemical properties, analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution, and transformation
1.4. Environmental levels and human exposures
1.5. Kinetics and metabolism
1.6. Effects of organisms in the environment
1.7. Effects on experimental animals and in vitro test systems
1.7.1. Systemic toxicity
1.7.2. Carcinogenicity and mutagenicity
1.7.3. Male reproductive system
1.7.4. Developmental toxicity
1.8. Effects on man
1.9. Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Industrial production
3.2.1.1 Manufacturing processes
3.2.1.2 World production figures
3.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Short-term exposures
8.2.1. Haematological and immunological effects
8.2.2. Effects on liver and kidney
8.2.3. Behavioural and neurological effects
8.3. Skin and eye irritation; sensitization
8.4. Long-term exposures
8.5. Effects on reproduction and fetal development
8.5.1. Effects on the male reproductive system
8.5.1.1 Oral exposure
8.5.1.2 Inhalation studies
8.5.2. Embryotoxicity and developmental effects
8.5.2.1 2-Methoxyethanol
8.5.2.2 2-Ethoxyethanol
8.5.3. Teratogenicity
8.5.3.1 2-Methoxyethanol
8.5.3.2 2-Ethoxyethanol and 2-ethoxyethanol acetate
8.6. Mutagenicity and related end-points
8.7. Carcinogenicity
8.8. Mechanism of toxicity - mode of action
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Poisoning reports
9.2. Occupational exposure
9.2.1. Repeated exposure
9.2.2. Epidemiological studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS
11.1. Health protection
11.2. Further research
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET CONCLUSIONS
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
RECOMMANDATIONS
RESUMEN Y CONCLUSIONES
EVALUACION DE LOS RIESGOS Y EFECTOS EN EL MEDIO AMBIENTE
RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR 2-METHOXYETHANOL,
2-ETHOXYETHANOL, AND THEIR ACETATES
Members
Dr W. Denkhaus, Institute for Occupational and Social Medi-
cine, University of Mainz, Mainz, Federal Republic of
Germany
Dr R.J. Fielder, Medical TEH Division, Department of Health,
Hannibal House, Elephant and Castle, London, United King-
dom
Dr B. Gilbert, Company for the Development of Technology
Transfer (CODETEC), Cidade Universitaria, Campinas,
Brazil (Vice-Chairman)
Dr B. Hardin, Division of Standards Development and Tech-
nology Transfer, National Institute for Occupational
Safety and Health, Cincinnati, Ohio, USA
Dr M. Ikeda, Department of Public Health, Kyoto University
Faculty of Medicine, Kyoto, Japan (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr L. Rosenstein, Office of Toxic Substances, US Environ-
mental Protection Agency, Washington DC, USA
Dr J. Sokal, Institute of Occupational Medicine, Division of
Industrial Toxicology, Lodz, Poland
Dr H. Veulemans, Laboratory for Occupational Hygiene, De-
partment of Occupational Medicine, University of Leuven,
Leuven, Belgium
Representatives of other Organizations
Dr K. Miller, International Commission on Occupational
Health, British Industrial Biological Research Associ-
ation, Carshalton, Surrey, United Kingdom
Observers
Dr A. Cicolella, Institut National de Recherche et de Sécur-
ité, Vandoeuvre, France
Secretariat
Dr G. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization,
Research Triangle Park, North Carolina, USA (Secretary)
Dr H. Teitelbaum, US Environmental Protection Agency,
Office of Toxic Substances, Washington DC, USA
(Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 or 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 2-METHOXYETHANOL, 2-ETHOXYETHANOL,
AND THEIR ACETATES
A WHO Task Group on Environmental Health Criteria for
2-Methoxyethanol, 2-Ethoxyethanol, and their Acetates met
at the British Industrial Biological Research Association
(BIBRA), Surrey, United Kingdom, from 4 to 7 April 1989.
The meeting was sponsored by the United Kingdom Department
of Health and Social Services. Dr S.D. Gangoli, Director,
BIBRA, welcomed the participants on behalf of the host
institution, and Dr G.C. Becking opened the meeting on
behalf of the three co-operating organizations of the IPCS
(ILO/UNEP/WHO). The Task Group reviewed and revised the
draft document and made an evaluation of the risks for
humans and the environment from exposure to these four
glycol ethers.
The first and second drafts of this document were
prepared by Dr H. TEITELBAUM, US Environmental Protection
Agency, Washington DC, USA.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
Dr G. Becking and Dr P.G. Jenkins, both members of the
IPCS Central Unit, were responsible for the overall
scientific content and technical editing, respectively.
ABBREVIATIONS
ADH alcohol dehydrogenase
EAA ethoxyacetic acid
ECG electrocardiogram
2-EE 2-ethoxyethanol
2-EEA 2-ethoxyethyl acetate
GC-FID gas chromatography with flame ionization detector
HPLC high performance liquid chromatography
LOEL lowest-observed-effect level
MAA methoxyacetic acid
2-ME 2-methoxyethanol
2-MEA 2-methoxyethyl acetate
NIOSH National Institute for Occupational Safety and Health (USA)
NOEL no-observed-effect level
ODC ornithine decarboxylase
OSHA Occupational Safety and Health Administration (USA)
PCB polychlorinated biphenyl
SCE sister chromatid exchange
TWA time-weighted average
UDS unscheduled DNA synthesis
1. SUMMARY AND CONCLUSIONS
1.1. Identity, Physical and Chemical Properties, Analytical Methods
This monograph considers only the methyl and ethyl
ethers of ethylene glycol, i.e. 2-methoxyethanol (2-ME)
and 2-ethoxyethanol (2-EE), and their respective acetate
esters, 2-methoxyethyl acetate (2-MEA) and 2-ethoxyethyl
acetate (2-EEA). These four compounds are all stable,
colourless, flammable liquids with a mild ethereal odour
and are all miscible with (or in the case of 2-EEA very
soluble in) water and miscible with a large number of
organic solvents.
Analytical methods are available for the detection of
these glycol ethers or their metabolites in various media
(air, water, blood, and urine). They often employ adsorp-
tion or extraction procedures to concentrate the sample,
followed by gas chromatographic analysis. Using gas or
high performance liquid chromatography, 2-methoxyacetic
acid (MAA) and 2-ethoxyacetic acid (EAA), (metabolites of
2-ME and 2-EE) can be measured in urine, usually after
derivatization, at concentrations between 5 and 100 µg/ml.
1.2. Sources of Human and Environmental Exposure
The four glycol ethers reviewed are all produced by
the reaction of ethylene oxide with the appropriate
alcohol, followed, when required, by esterification with
ethanoic acid.
Data for world production of these glycol ethers are
not available. However, the combined annual production
in Western Europe, USA, and Japan is approximately 79 x
103 tonnes of 2-ME and 205 x 103 tonnes of 2-EE. A
large proportion is used in the coatings industry (paints,
stains, and lacquers) and as solvents for printing inks,
resins and dyes, and home and industrial cleaners. They
are also used as anti-icing additives in hydraulic fluids
and jet fuel.
1.3. Environmental Transport, Distribution, and Transformation
The solubility of these glycol ethers in water and
their relatively low vapour pressure could result in their
build-up in water in the absence of degradation. However,
degradation by microorganisms in soil, sewage sludge, and
water appears to prevent this possibility.
Atmospheric emissions resulting from the use of glycol
ethers as evaporative solvents result in the greatest
environmental exposure. In the general environment, photo-
lytic degradation appears to be rapid, and levels below
0.0007 mg/m3 (2 x 10-4 ppm) would be expected.
Under aerobic conditions glycol ethers are degraded
rapidly by microorganisms to carbon dioxide and water,
whereas under anaerobic conditions methane and carbon
dioxide are the major end-products.
1.4. Environmental Levels and Human Exposures
The use of glycol ethers can result in significant
widespread emissions to the environment. There is particu-
lar concern for direct human exposure in industry, in
small work-shops, and during home use of products con-
taining glycol ethers. Occupational exposure values of
< 0.1 mg/m3 to > 150 mg/m3 have been reported. Signifi-
cant exposure could occur to users of consumer products
but no data are available.
In addition to exposure from airborne glycol ethers,
humans may be exposed dermally. Blood analyses confirm
rapid absorption by this route, which may contribute more
than airborne exposure to the total body burden.
1.5. Kinetics and Metabolism
All four glycol ethers have been shown to be readily
absorbed through the skin, lungs, and gastrointestinal
tract. The highest levels detected in distribution studies
on 2-ME in pregnant mice were in the maternal liver,
blood, and gastrointestinal tract, and in the placenta,
yolk sac, and numerous embryonic structures.
The metabolic transformation of 2-ME gives two primary
metabolites: MAA and 2-methoxyacetyl glycine. Metabolism
to carbon dioxide represents a secondary, minor route.
The conversion in plasma of 2-ME to MAA is rapid, with a
half-life of 0.6 h in rats, but the excretion of MAA is
slow, with a half-life of about 20 h in the rat and 77 h
in man.
In laboratory animals, administration of 2-EE led to
the production of EAA and 2-ethoxyacetyl glycine, EAA
being the major metabolite appearing in the presumptive
target organ, the testes. In a human study using 2-EEA, a
similar metabolic pathway was seen, the acetate being
hydrolyzed first to 2-EE and subsequently oxidized to EAA.
The resultant EAA was excreted with an estimated half-life
of 21-42 h. Experimental work suggests that the retention
or accumulation of metabolites could be toxicologically
significant assuming that these metabolites are respon-
sible for the observed target-organ toxicity.
1.6. Effects on Organisms in the Environment
The toxicity of 2-ME and 2-EE to microorganisms and
aquatic animals appears to be low. For microorganisms,
the lethal concentration in the medium is greater than 2%.
Growth inhibition of green algae by 2-ME was noted at 104
mg/litre and of cyanobacteria (blue-green algae) at 100 mg
per litre. The acute toxicity of 2-EE is very low for arthro-
pods (LC50 > 4 g/litre) and freshwater fish (LC50 > 10 g per
litre). The glycol ether acetates (2-MEA and 2-EEA) are
far more toxic to fish. The LC50 of 2-EEA for fathead
minnows is 46 mg/litre and that of 2-MEA for tidewater
silverfish and bluegills is 45 mg/litre. There have been
no long-term studies.
1.7. Effects on Experimental Animals and In Vitro Test Systems
1.7.1. Systemic toxicity
The toxicity of 2-ME and 2-EE to experimental animals
has been much more widely studied than that of 2-MEA and
2-EEA.
2-ME and 2-EE and their acetates have similar
lethalities after single exposures and they show low acute
lethality whether exposure is via the dermal, oral, or
inhalation route. Oral LD50 values for a variety of
species range between 900 and 3400 mg/kg body weight for
2-ME, 1400 and 5500 mg/kg for 2-EE, 1250 and 3930 mg/kg
for 2-MEA, and 1300 and 5100 mg/kg for 2-EEA. Inhalation
LC50 values of 4603 mg/m3 (2-ME) and 6698 mg/m3 (2-EE)
have been reported in mice.
Only limited data on skin and eye irritation or on the
sensitization potential of these glycol ethers in animals
is available. It would appear that they are not irritating
to the skin, but that they can cause eye irritation. No
skin irritation or skin sensitization has been reported in
humans in spite of extensive exposures.
Short-term inhalation exposure (up to 90 days) of
experimental animals to high concentrations (> 9313 mg
2-ME/m3 and > 1450 mg 2-EE/m3) has been shown to lead
to adverse effects on blood parameters, the nervous
system, and testes, thymus, kidney, liver, and lung. At
lower exposure levels, effects are observed on the
haemopoietic system and testes. For example, rats exposed
by inhalation to 2-ME for 13 weeks at levels between 93
and 930 mg/m3 exhibited reduced packed cell volume and
white blood cell, haemoglobin, platelet, and serum protein
concen-trations at the highest dose only, while similarly
exposed rabbits had decreased thymus size, in addition to
the decreased blood parameters, at 930 mg/m3. 2-EE
exhibited similar but less severe effects in rats and
rabbits when animals were exposed for 13 weeks at a level
of 1450 mg/m3. No data are available from long-term
studies.
1.7.2. Carcinogenicity and mutagenicity
The mutagenicity of 2-ME has been investigated in a
range of in vitro systems using bacteria and mammalian
cells. Although most studies yielded negative results,
there were reports of positive mutagenicity results at
very high 2-ME concentrations in CHO cells when investi-
gated for chromosome aberration (at 6830 µg/ml or more)
and sister chromatid exchange (3170 µg/ml or more).
However, in vivo studies for chromosome aberrations and
micronuclei were negative. Only very limited information
on the mutagenic potential of 2-EE is available, and there
are no carcinogenicity data for these glycol ethers.
1.7.3. Male reproductive system
The effect of 2-ME on the male reproductive system has
been intensively investigated following both oral and
inhalation exposure in rodents. Degenerative changes in
the germinal epithelium of the seminiferous tubules were
consistently noted. Similar effects were seen with 2-EE
but at somewhat higher dose levels.
Oral dosing of rats with 2-ME for 1-11 days resulted
in a dose-related decrease in sperm count and changes in
sperm motility and morphology at dose levels of 100 mg/kg
body weight or more. Marked histological damage was seen
in the testes at autopsy. The no-observed-effect level
(NOEL) was 50 mg/kg. Reduced fertility was still evident
8 weeks after exposure to 200 mg/kg. Similar effects were
seen at dose levels of 500 mg 2-EE/kg or more, given for
up to 11 days, the NOEL for 11-day treatment being
250 mg/kg. However, when sperm reserves were depleted by
repeated mating, some reduction in sperm counts was seen
at the lowest dose investigated (150 mg/kg). Fertility
studies following a single oral dose of 250 mg 2-ME/kg or
more revealed complete sterility in both rats and mice at
5 weeks post dosing, some decrease in fertility being seen
at 125 mg/kg.
When the inhalation route was investigated, similar
degenerative changes in the testes were seen with 2-ME.
Effects were observed following a single exposure (4 h) to
1944 mg/m3 or more but not to 933 mg/m3. NOEL values
were 311 mg/m3 in rats following exposure for 13 weeks (6
h/day, 5 day/week) and 933 mg/m3 (6 h/day) in mice fol-
lowing exposure on 9 occasions over 11 days. Exposure of
rabbits to 2-ME for 13 weeks (6 h/day, 5 days/week) re-
sulted in marked effects on the testes at 311 mg/m3 or
more; marginal effects were seen at 93 mg/m3, and a
NOEL was not identified.
1.7.4. Developmental toxicity
Developmental toxicity has been observed in several
species of laboratory animals following exposure by all
routes of administration, i.e. oral, inhalation, and
dermal. 2-ME produced teratogenic effects in mice, rats,
rabbits, and monkeys. 2-EE and 2-EEA were teratogenic in
rats and rabbits. Although 2-MEA has not been tested for
developmental toxicity, metabolic profiles (see section 6)
suggest that it is reasonable to expect that 2-MEA would
have a toxicity similar to that of 2-ME.
The widest range of dose/response data (doses of 31.25
to 1000 mg/kg per day) is available for 2-ME. In this
gavage study using mice, (2-ME was administered on days 7
to 14 of gestation), the NOEL for maternal toxicity was
125 mg/kg per day. However, malformations were observed
at 62.5 mg/kg per day and skeletal variations at 31.25
mg/kg per day. A NOEL for developmental toxicity was not
reported. In single-dose studies, mice were treated with
2-ME by gavage on gestation day 11; 100 mg/kg was not
fetotoxic, while 175 mg/kg produced digit defects without
other signs of maternal or fetal toxicity. Cardiovascular
defects and ECG abnormalities were observed in neonatal
rats following treatment on days 7 to 13 of gestation with
25 mg/kg per day. Since that was the lowest dose tested,
this study yielded no developmental NOEL (maternal
toxicity was not observed at that dose). Similarly, no
NOEL for developmental toxicity could be determined when
monkeys were treated by gavage with 2-ME at 0.16, 0.32, or
0.47 mmol/kg per day on days 20 to 45 of gestation.
Fetotoxicity in mice and rats and malformations in
rabbits were observed following exposure by inhalation to
2-ME at 156 mg/m3. For all three species, the NOEL for
developmental effects was 31 mg/m3. However, behavioural
and neurochemical alterations were seen in the offspring
of rats exposed to 78 mg 2-ME/m3 on days 7-13 or 14-20 of
gestation.
Following inhalation exposure of rats (743 mg/m3) and
rabbits (589 mg/m3), 2-EE was found to be teratogenic
(in the presence of slight maternal toxicity). Another
study reported fetotoxicity but no malformations in rats
exposed to 184 or 920 mg 2-EE/m3, and in rabbits exposed
to 644 mg 2-EE/m3. NOEL values for developmental effects
were 37 mg/m3 for rats and 184 mg/m3 for rabbits. Behav-
ioural and neurochemical alterations were seen in the off-
spring of rats exposed to 368 mg 2-EE/m3 on days 7-13 or
14-20 of gestation.
Rats treated by dermal application of 0.25 ml undi-
luted 2-EE (four times daily on gestation days 7-16)
exhibited marked fetotoxicity and a high incidence of
malformation in the absence of maternal toxicity. Similar
effects were noted following 2-EEA treatment of rats,
using the same protocol, at an equimolar dose (0.35 ml,
four times daily).
Inhalation exposure of rabbits to 2-EEA on gestation
days 6-18 produced teratogenic responses at 2176 mg/m3 and
544 mg/m3 in two different studies, the developmental
NOEL values in these two studies being 135 mg/m3 and 270
mg/m3. Exposure of rats to 2-EEA on days 6-15 of ges-
tation produced fetotoxicity at 540 mg/m3 and malfor-
mation at 1080 mg/m3. The developmental NOEL was 170
mg/m3.
1.8. Effects on man
Information on the toxic effects of these four glycol
ethers on humans is limited. The results from the few
case reports and workplace epidemiological studies are
consistent with the adverse effects seen in experimental
animals. No reports quantifying general population
exposure and health effects have been found.
In two non-fatal cases of poisoning by ingestion of
100 ml 2-ME, the predominant signs and symptoms were
nausea, vertigo, cyanosis, tachycardia, hyperventilation,
and metabolic acidosis, with some evidence of renal
failure. Similar but less severe symptoms were found in a
person ingesting 40 ml 2-EE. In a fatal poisoning
resulting from the ingestion of 400 ml 2-ME, postmortem
findings showed acute haemorrhagic gastritis, fatty
degeneration of the liver, and degenerative changes in
renal tubules.
Repeated exposure of workers to 2-ME and 2-EE, in
addition to other solvents, resulted in anaemia,
leucopenia, general weakness, and ataxia. No reliable
estimation of exposure was made in many of these reported
studies. Haematological effects of glycol ethers on humans
have been documented and the development of macrocytic
anaemia in a worker exposed to 2-ME (average 105 mg/m3),
along with other solvents, has been described.
Bone marrow toxicity has been reported in workers
exposed dermally to 2-ME, and immunological effects have
been noted in workers following prolonged exposure (8-35
years) to 2-ME and 2-EE (mean exposures being 6.1 mg/m3
and 4.8 mg/m3, respectively).
Epidemiological studies of workers exposed to 2-ME and
2-EE have shown some evidence of adverse effect on the
male reproductive system, with an increased frequency of
reduced sperm counts. Exposure to 2-EE (37 workers) at
levels up to 88.5 mg/m3 led to change in semen indices.
Among 73 workers exposed to 2-ME (up to 17.7 mg/m3) and
2-EE (up to 80.5 mg/m3), there was an increased fre-
quency of reduced sperm counts and also evidence of haema-
tological effects when exposures (TWA) were 2.6 mg/m3 for
2-ME and 9.9 mg/m3 for 2-EE.
The adverse effects noted in humans exposed occu-
pationally are consistent with those noted in experimental
animals. However, due to deficiencies in exposure
assessments and to mixed exposures, no dose-response
relationships can be determined.
1.9. Conclusions
Many people may be exposed to these four glycol ethers
at levels comparable to industrial levels through the use
of consumer and trade products. Significant occupational
exposure may occur both through inhalation and skin
absorption. Limited measurements of air levels in the
workplace range from < 0.1 mg/m3 to > 150 mg/m3.
Both 2-ME and 2-EE demonstrate low toxicity to micro-
organisms and aquatic species. No data exist to ascertain
the potential for adverse effects on environmental
species from long-term exposure.
In rats, the NOEL in acute studies for testicular
effects was 933 mg 2-ME/m3, and the NOEL for repeated
exposure was 311 mg/m3. In repeated exposure studies using
the most sensitive species, the rabbit, a clear effect was
detected at 311 mg/m3, whereas at 93 mg/m3 there was a
marginal effect (1 in 5 animals). Evidence from studies
on men exposed occupationally to 2-ME and 2-EE indicates
that these glycol ethers can produce testicular toxicity
in humans.
Developmental toxicity has been observed in all
species (mice, rats, and rabbits exposed to 2-ME at 156
mg/m3 or more. The NOEL for all three species was 31
mg/m3. Behavioural and neurochemical alterations in
rats followed in utero exposure to 78 mg/m3, no NOEL
being identified. 2-EE and 2-EEA were slightly less
potent. Developmental effects were observed in rats and
rabbits following exposure to 2-EE at 368 mg/m3 or
more. These effects were slight in rats exposed to 184 mg
2-EE/m3, but 37 mg/m3 was a clear NOEL for both rats
and rabbits.
Haematological effects are produced by these glycol
ethers in mice, rat, rabbits, dogs, hamsters, and guinea-
pigs. This agrees with haematological effects reported in
some of the limited number of studies of industrial
workers exposed repeatedly to 2-EE and/or 2-ME. In
repeated-exposure animal studies, the NOEL was 93 mg
2-ME/m3 in rabbits and 368 mg 2-EE/m3 in rats and
rabbits. No data have been found to evaluate quantitat-
ively the haematological effects that follow acute
exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
The four glycol ethers discussed in this monograph,
i.e. 2-methoxyethanol (2-ME), 2-methoxyethyl acetate
(2-MEA), 2-ethoxyethanol (2-EE), and 2-ethoxyethyl acetate
(2-EEA), are stable flammable liquids with a slight odour
at normal room temperature and pressure. Their structural
formulae are:
H H H
| | |
H - C - O - C - C - OH 2-methoxyethanol
| | |
H H H
H H H H
| | | |
H - C - C - O - C - C - OH 2-ethoxyethanol
| | | |
H H H H
H H H O H
| | | || |
H - C - O - C - C - O - C - C - H 2-methoxyethyl acetate
| | | |
H H H H
H H H H O H
| | | | || |
H - C - C - O - C - C - O - C - C - H 2-ethoxyethyl acetate
| | | | |
H H H H H
Information on the identity of the four selected
glycol ethers is presented in Table 1.
2.2. Physical and Chemical Properties
A summary of the physical and chemical properties of
these four glycol ethers (2-ME; 2-MEA; 2-EE; 2-EEA) is
given in Table 2.
2.3. Conversion Factors
2-Methoxyethanol (2-ME) 1 ppm = 3.11 mg/m3
2-Methoxyethyl Acetate (2-MEA) 1 ppm = 4.83 mg/m3
2-Ethoxyethanol (2-EE) 1 ppm = 3.68 mg/m3
2-Ethoxyethyl Acetate (2-EEA) 1 ppm = 5.40 mg/m3
2.4. Analytical Methods
Several analytical procedures used for the determi-
nation of 2-ME, 2-MEA, 2-EE, and 2-EEA in various environ-
mental media are summarized in Table 3. In some reports,
the useful range was indicated but not the limit of
detection.
In reporting the methods validated by NIOSH (1987a,
1987b), only the range that has been confirmed as accurate
is shown. However, these methods may be capable of
measuring much lower levels of glycol ethers in air pro-
viding adequate sampling times are employed and desorption
efficiencies ascertained.
Variations on the basic NIOSH sampling and gas chroma-
tographic methods have been reported by Denkhaus et al.
(1986), and the measurement of glycol ethers in the
workplace using diffusive monitors has been described by
Hamlin et al. (1982).
Metabolites of 2-EE and 2-ME in urine have been
measured using either gas chromatography (Groeseneken et
al., 1986a, 1989b; Smallwood et al., 1984, 1988), or HPLC
analysis (Cheever et al., 1984).
Using methylene chloride extractions of acidified
urine, followed by derivatization with pentafluorobenzyl
bromide, average recoveries of 78 and 91% were obtained
for methoxyacetic acid (MAA) and ethoxyacetic acid (EAA),
respectively. Detection limits for GC-FID analysis were
11.4 µg/ml for MAA and 5.0 µg/ml for EAA (Smallwood et
al., 1984). Smallwood et al. (1988) have reported that a
range of 5 to 100 µg EAA/ml in urine can be analysed.
Preliminary results indicate that this procedure can be
used to detect exposure to 2-EE in shipyard workers using
2-EE-containing paints. Groeseneken et al. (1986a)
utilized similar extraction procedures and GC-FID analysis
after diazomethane derivatization. Although recoveries
were low (50-60%), the method could quantify 0.15 mg MAA
per litre and 0.07 mg EAA/litre. Groeseneken et al.
(1989b) have recently described an improved method for
detecting MAA and EAA in urine. Recoveries were in excess
of 90%, linear standard curves were obtained over a broad
range (0.1-200 mg/litre), and the possible interference by
gly-colic acid in the assay previously described
(Groeseneken et al. 1986a) was eliminated. Cheever et al.
(1984) ana-lysed urine samples, directly at pH 3 by HPLC,
for EAA after animals were dosed with 230 mg 2-EE/kg body
weight, but no limit of detection or appropriate range for
use was reported. However, this method may be useful for
biological monitoring of exposed populations.
Table 1. The identity of selected glycol ethers
----------------------------------------------------------------------------------------------------
Chemical CAS Number Molecular Common Some common
formula synonyms trade names
----------------------------------------------------------------------------------------------------
2-Methoxyethanol 109-86-4 C3H8O2 Ethylene glycol Methyl Cellosolve;
(2-ME) monomethyl ether; Jeffersol EM;
ethanol, 2-methoxy; Dowanol EM; Poly-solv
EGM ether EM; Methyl oxitol.
2-Methoxyethyl 110-49-6 C5H10O3 Ethylene Methyl Cellosolve
acetate glycol monomethyl acetate
(2-MEA) ether acetate;
ethanol, 2-methoxy-
acetate
2-Ethoxyethanol 110-80-5 C4H10O2 Ethylene glycol Cellosolve, Dowanol
(2-EE) monoethyl ether EE; Oxitol; Ethoxol
2-Ethoxyethyl 111-15-9 C6H12O3 Ethylene glycol Cellosolve acetate;
acetate monoethyl ether Ethyl Cellosolve
(2-EEA) acetate; acetic acetate; Oxitol
acid, 2-ethoxyethyl acetate; Poly-Solv EE
ester.
----------------------------------------------------------------------------------------------------
Table 2. Physical and chemical properties of selected glycol ethersa
---------------------------------------------------------------------------------------------------------
Chemical Relative Density Boiling Vapour Relative Flash Water
molecular (g/ml at point pressure vapour point solubility
mass 20 °C) (°C) (mmHg) density (°C)
(air=1 )
---------------------------------------------------------------------------------------------------------
2-Methoxyethanol 76.09 0.960 124 6.2 at 20 °C 2.6 46.1 open cup infinite
(2-ME) 9.7 at 25 °C 41.7 closed cup
2-Methoxyethyl 118.13 1.005 145 2.0 at 20 °C 4.07 55.6 closed cup infinite
Acetate 5.3 at 25 °C
(2-MEA)
2-Ethoxyethanol 90.12 0.93 135 3.8 at 20 °C 3.0 49 open cup infinite
(2-EE) 5.3 at 25 °C 44 closed cup
2-Ethoxyethyl 132.16 0.975 156 1.2 at 20 °C 4.72 51.1 closed cup 23 g/100g
Acetate 1.1 at 25 °C at 20 °C
(2-EEA)
---------------------------------------------------------------------------------------------------------
a Data from: Rowe & Wolf (1982) and Mellan (1977).
Table 3. Analytical methods for selected glycol ethers and their metabolites
---------------------------------------------------------------------------------------------------------
Matrix Sampling method Analytical Limit of detection Reference
extraction/cleanup methoda or useful range
---------------------------------------------------------------------------------------------------------
Air Adsorption on charcoal, GC-FID range (mg/m3): NIOSH (1987a, 1987b)
elution with methylene 2-ME 44-160;
chloride, carbon 2-MEA 51-214;
disulfide or methylene 2-EE 340-1460
chloride; methanol
Air Inhaled or expired air GC-FID NR Groeseneken et al.
(2-EE) pumped through silica (1986b)
gel, desorbed with
methanol (88% efficient)
Air Diffusive sampling, GC-FID range: 5-20 mg/m3 Hamlin et al. (1982)
(2-ME, 2-EE) adsorption on Tenax
thermal desorption
Air Personal monitors with GC-FID range: 0.5-250 mg/m3 Health and Safety
(2-ME, 2-EE) pump adsorption on Executive (1988)
Tenex thermal desorption
Water Direct analysis of HPLC-UV 5 mg/litre; Bailey et al. (1985)
(2-EEA) aqueous solutions range: 5-1000 mg/litre
Blood Methylene chloride GC-FID 2-ME 8.8 mg/kg; range Smallwood et al.
(2-ME, 2-EE) extraction in presence 8-946 mg/kg (1984)
of anhydrous sodium 2-EE 5.0 mg/kg; range
sulfate. Average 6-895 mg/kg
recoveries 2-ME (78%),
2-EE (84%)
Blood Head-space elution GC-FID NR Denkhaus et al.
(2-ME, 2-EE) (1986)
Urine Methylene GC-FID MAA 11.4 mg/litre; range Smallwood et al.
(MAA, EAA) extraction followed 11.4-1140 mg/litre (1984)
by derivatization with EAA 5.0 mg/litre; range
pentafluorobenzyl bromide 10-1000 mg/litre
Table 3 (contd)
---------------------------------------------------------------------------------------------------------
Matrix Sampling method Analytical Limit of detection Reference
extraction/cleanup methoda or useful range
---------------------------------------------------------------------------------------------------------
Urine Lyophilization followed GC-FID MAA 0.03 mg/litre; range Groeseneken et al.
(MAA, EAA) by derivatization 0.1-200 mg/litre (1989b)
with pentafluorobenzyl EAA 0.03 mg/litre; range
0.1-200 mg/litre
-----------------------------------------------------------------------------------------------------------
a GC-FID = gas chromatography-flame ionization detector;
HPLC-UV = high performance liquid chromatography with ultraviolet light detection;
NR = not reported.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
The two glycol ethers and their acetates (2-ME, 2-MEA,
2-EE, 2-EEA) have not been reported to occur as natural
products.
3.2. Man-Made Sources
3.2.1. Industrial production
3.2.1.1 Manufacturing processes
The production process for 2-ME and 2-EE involves the
reaction of the relevant alcohol with ethylene oxide to
produce the required glycol ether (Kirk-Othmer, 1980).
The acetates, 2-MEA and 2-EEA, are produced by standard
esterification techniques using 2-ME or 2-EE, the acid
anhydride or chloride, and an acid catalyst (Kirk-Othmer,
1980).
3.2.1.2 World production figures
The use of 2-ME and 2-EE has declined over the past
few years because they have been partially replaced in
some countries by less toxic substances. Estimates of
production levels for three major industrialized areas are
shown in Table 4. Production figures for other regions of
the world have not been found.
Table 4. Estimated production (in tonnes) of 2-EE and 2-ME in 1981a
-----------------------------------------------------------
Region or Country 2-ME 2-EE
-----------------------------------------------------------
Western Europe 37 000 116 000
Japan 3100 9800
United States 39 000 79 000
-----------------------------------------------------------
a These are estimates taken from US EPA (1987);
production figures for the rest of the world have not
been found.
3.3. Uses
2-ME, 2-EE, 2-MEA, and 2-EEA have a wide range of uses
as solvents with particular applications in paints,
stains, inks, lacquers, and the production of food-contact
plastics. The major function of these agents is to
dissolve various components of mixtures, in both aqueous
and non-aqueous systems, and to keep them in solution
until the last stages of evaporation. It is these
dispersive applications that cause the greatest concern
for widespread human and environmental exposure.
In addition, these four glycol ethers are used as
resin solvents, in surface coatings and inks for silk-
screen printing and in photographic and photolithographic
processes, as solvents for dyes in textile and leather
finishing, and as general solvents in a wide variety of
home and industrial cleaners. 2-ME is used extensively as
an anti-icing additive in hydraulic fluids and jet fuel
for military and small civilian jet aircraft, as well as
in hydraulic brake fluids (Mellan, 1977).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
The greatest environmental exposure to glycol ethers
results from their direct release into the atmosphere
when they are used as evaporative solvents. Given the
amounts synthesized and transported, there is also a great
potential for environmental exposure from accidental
releases and the disposal of cleaning products and con-
tainers. Discharges of this type result in the transport
of these chemicals to land and water. Because of their
water solubility and low vapour pressure, they could build
up in water in the absence of degradation. However, their
levels in soil and water would be expected to decrease
fairly quickly because of rapid hydrolysis and/or oxi-
dation. Also, adapted sludge has been reported to digest
these compounds (Verschueren, 1977), giving 90% degra-
dation of 2-EE after 5.5 days.
Since all of the major degradation processes in soil
and water are oxidative, the potential exists for persist-
ent contamination of anaerobic soils, such as landfills,
and their underlying anaerobic aquifers. Contamination of
ground water by 2-EE and 2-EEA from leaking storage tanks
has been observed (Botta et al., 1984). However, 2-ME can
apparently serve as a substrate for anaerobic methane fer-
mentation and is digested by anaerobic sludge (Tanaka et
al., 1986). Under such conditions, contamination of soil
and ground water would be transitory.
4.2. Biotransformation
Under normal aerobic conditions, 2-ME, 2-EE, and their
acetates would be expected to be degraded readily to car-
bon dioxide and water by microorganisms. Under anaerobic
conditions, 2-ME is degraded by mesophilic sludge through
at least two pathways, depending on temperature and pH,
with methane and carbon dioxide being the end products in
both cases (Tanaka et al., 1986). Optimal conditions for
degradation are pH 7.5 and 30-35°C.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
The patterns of use of these four glycol ethers can
result in significant, widespread emissions to the environment.
Therefore, there is a great potential for exposure to
people in the workplace, as well as to the general pop-
ulation and to the environment. However, no data on the
levels of 2-ME, 2-EE, and their acetates in the general
environment have been found. As a result of the rates of
degradation and the physical and chemical properties of
these compounds, it is highly unlikely that food chain
accumulation would occur.
5.2. General Population Exposure
No data have been found that would allow an estimate
to be made of the exposure to the general population using
these evaporative solvents. However, there is particular
concern for direct human exposure in small workshops and
by individual users, where the products are being used in
environments with either poor or non-existent ventilation,
or where skin exposure may not be controlled adequately.
5.3. Occupational Exposure
Workers, other than those in large industrial estab-
lishments, constitute the largest population group subject
to high exposure. In the USA, airborne exposures have been
measured for some of the trades, many of the industrial
uses, and for workers involved in the manufacture of these
compounds (Table 5). In a survey of European manufacturing
sites, the time-weighted averages (TWAs) for workers ex-
posed to 2-ME, 2-MEA, 2-EE, and 2-EEA were reported as
28.9, 4.3, 15.8, and 14.6 mg/m3 (9.3, 0.9, 4.3, and 2.7
ppm), respectively (ECETOC, 1985). As estimates of ex-
posure, these measurements do not take into account dermal
or aerosol exposures, which may be very significant (see
section 9.2). A summary of the exposure of workers in the
semiconductor industry to 2-ME, 2-MEA, 2-EE, or 2-EEA
(Table 6) (Paustenbach, 1988) reports exposures lower than
those in other industries within the USA (Table 5). These
measurements do not accurately reflect exposure during
uses such as maintenance painting (as opposed to
industrial production), because here there is a wide vari-
ation in exposure conditions. Modelling of the possible
range of exposures in trade and consumer uses might pro-
vide some useful data.
The exposure data available from large industries
suggest that the majority of exposures are "low", i.e.
exposures for 2-ME are below 0.1 mg/m3 (0.03 ppm) and for
2-EE are below 1.8 mg/m3 (0.5 ppm). However, in almost
all industries studied there are some workers exposed to
much higher levels (see section 9). For example, monitor-
ing carried out in a number of industries using glycol
ethers (Hamlin et al., 1982) involved both personal and
area monitoring and covered a range of applications in
flexographic and gravure printing, car refinishing, film
coating, and printing ink manufacture. Although atmos-
pheric concentrations were generally low, levels of up to
74 mg 2-EE/m3 (20 ppm) and 146 mg 2-ME/m3 (47 ppm) were
reported in some poorly ventilated areas. There was wide
variation in the exposure between different plants, even
when these plants used the same process.
Air samples (2654 total) from 336 plants in Belgium
have been analysed for glycol ethers (including 2-ME,
2-EE, and their acetates) (Veulemans et al., 1987b). One
or more glycol ethers were detected in 262 air samples
covering 78 plants, 2-EE and its acetate being detected
most often. Detectable levels were of the order of 9.2 mg
per m3, 25% being above 18.4 mg/m3.
Engineering models have been used to estimate ex-
posure resulting from the use of paint, coatings, stains,
etc., containing 2-ME, 2-EE, and their acetates. Such
models indicate that peak exposure values of > 30 ppm and
1-h average exposures of > 5 ppm will occur when paints
and similar products containing more than 2% of these sol-
vents are used (US EPA, 1987). Much higher exposure levels
are possible when the concentration of 2-ME or 2-EE in the
paint is higher. These estimates apply to situations where
protective equipment or special engineering controls were
not available. Under industrial conditions, exposure may
be lower than these models predict if ventilation, exhaust
hoods, or other protective equipment are used.
Table 5. Summary of occupational exposures (mg/m3) to glycol ethers in the USAa
------------------------------------------------------------------------------------------------------------
Chemical and Arithmetic Arithmetic Standard Geometric Geometric
job category rangeb mean deviation mean deviation
------------------------------------------------------------------------------------------------------------
2-MEc
Operator 0.31-188.5 (0.1 -60.6) 59.28 (19.06) 8.40 (2.70) 23.10 (7.43) 56.98 (18.32)
Miscellaneous 9.33 (3.00) 3.11 (1.00) 9.33 (3.00)
Painter 0.31- 10.3 (0.1 - 3.3) 6.87 (2.21) 7.43 (2.39) 2.08 (0.67) 7.43 (2.39)
Painter/screener 0.31- 12.1 (0.1 - 3.9) 6.22 (2.00) 7.15 (2.30) 1.95 (0.63) 5.91 (1.90)
2-MEd
Painter 3.76- 18.7 (1.21- 6.01) 11.23 (3.61) 5.69 (1.83) 8.40 (2.70) 7.46 (2.40)
Operator 5.19- 7.68 (1.67- 2.47) 6.44 (2.07) 3.76 (1.21) 6.31 (2.03) 1.24 (0.40)
2-MEe
Operator 0.31- 35.14 (0.1 -11.3) 7.28 (2.34) 9.39 (3.02) 1.37 (0.44) 11.26 (3.62)
2-MEAc
Miscellaneous 0.48- 40.57 (0.1 - 8.4) 12.94 (2.68) 13.09 (2.71) 1.69 (0.35) 16.81 (3.48)
Operator 0.48- 26.56 (0.1 - 5.5) 7.87 (1.63) 13.04 (2.70) 1.98 (0.41) 10.19 (2.11)
Printer/screener 0.48- 5.07 (0.1 - 1.05) 2.13 (0.44) 16.23 (3.36) 0.87 (0.18) 3.86 (0.80)
Painter 0.48- 14.49 (0.1 - 3.0) 1.88 (0.39) 15.94 (3.30) 0.77 (0.16) 3.31 (0.70)
2-EEc
Painting 0.37-313.9 (0.1 -85.3) 71.21 (19.35) 13.69 (3.72) 2.72 (0.74) 74.30 (20.19)
Printer 0.37- 87.95 (0.1 -23.9) 17.77 (4.83) 9.09 (2.47) 4.49 (1.22) 19.95 (5.42)
Coating/adhesive 0.37- 36.80 (0.1 -10.0) 5.89 (1.60) 13.14 (3.57) 0.99 (0.27) 11.85 (3.22)
Mechanical industry 0.37 (0.10) 3.68 (1.00) 0.04 (0.01)
Leather 0.37 (0.10) 3.68 (1.00) 0.04 (0.01)
Operation/prod. 0.37 (0.10) 3.68 (1.00) 0.04 (0.01)
--------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------
Chemical and Arithmetic Arithmetic Standard Geometric Geometric
job category rangeb mean deviation mean deviation
--------------------------------------------------------------------------------------------------------------
2-EEd
Printer/screener 6.92-161.9 (1.88-44.0) 84.90 (23.07) 6.92 (1.88) 59.80 (16.25) 59.28 (16.11)
2-EEe
Printer/screener 0.37- 54.10 (0.1-14.79) 16.74 (4.55) 9.02 (2.45) 3.35 (0.91) 18.58 (5.05)
2-EEAc
Leather 6.75- 51.30 (1.25-9.5) 30.89 (5.72) 9.34 (1.73) 22.90 (4.24) 18.36 (3.40)
& coating 0.54-272.38 (0.1-50.44) 20.79 (3.85) 21.98 (4.07) 2.70 (0.50) 51.68 (9.57)
Prod./maint. 0.54- 27.0 (0.1- 5.0) 9.50 (1.76) 13.77 (2.55) 2.59 (0.48) 11.29 (2.09)
2-EEAd
Painter 1.03- 5.08 (0.19-0.94) 3.08 (0.57) 9.88 (1.83) 2.27 (0.42) 2.05 (0.38)
2-EAe
Miscellaneous 15.12-230.04 (2.8-42.6) 67.61 (12.52) 14.04 (2.60) 36.72 (6.80) 82.57 (15.29)
--------------------------------------------------------------------------------------------------------------
a From: US EPA (1987). Values were reported as ppm in the original report and are given in parentheses.
b Values of 0.1 ppm or less are reported as 0.1 ppm.
c Federal (USA) OSHA data.
d California OSHA data.
e NIOSH data.
Table 6. Exposure to glycol ethers within the semiconductor industry in the USA (mg/m3)a
------------------------------------------------------------------------------------------------------------
2-EEA 2-EEA 2-ME 2-ME 2-MEA 2-MEA
Sampling data No. Range Mean + SD No. Range Mean + SD No. Range Mean + SD
------------------------------------------------------------------------------------------------------------
Personal (TWA) 96 0.0054-2.7 0.27±0.43 6 0.12 -3.11 0.68±1.18 16 ND 0.048±0.00
Personal
(Short-term) 21 0.0054-97.2 15.23±29.2 1 NA 80.0 1 NA 82.0
Area (TWA) 128 0.0054-9.72 0.27±0.86 4 0.093-2.49 0.72±1.18 20 ND 0.048±0.00
Area
(Short-term) 10 0.027-81.0 8.42±25.49 1 NA 80.9 1 NA 87.0
------------------------------------------------------------------------------------------------------------
a From: Paustenbach (1988).
No. = number of samples.
Analytical limit of detection: 2-EEA, 0.0054 mg/m3; 2-ME, 0.093 mg/3; and 2-MEA, 0.048 mg/m3.
TWA = time-weighted average.
NA = not applicable.
ND = not detectable.
SD = standard deviation.
6. KINETICS AND METABOLISM
6.1. Absorption
As would be expected from their chemical structures
and solubilities, all four glycol ethers are readily
absorbed through the skin, lungs, and gastrointestinal
tract. Utilizing in vitro techniques, a rate of absorption
of 2-EEA through beagle skin of 2.3 mg/cm2 per h has been
measured (Guest et al., 1984). For isolated human epider-
mis, the following absorption rates have been determined:
2-ME, 2.8 mg/cm2 per h; 2-EE, 0.8 mg/cm2 per h; and
2-EEA, 0.8 mg per cm2 per h (Dugard et al., 1984).
In vivo studies in humans showed rapid absorption of
2-ME after dermal application of 15 ml of solvent (Nakaaki
et al., 1980). Two hours after application, blood levels
reached 200-300 µg/ml. This rate of absorption was ap-
proximately 10 times greater than that of methanol,
acetone, or methyl acetate.
Indirect evidence exists to show that 2-EE is well
absorbed from the gastrointestinal tract of rats. After a
single oral dose of 14C-2EE (230 mg/kg body weight), 76-
80% of the dose was excreted in the urine within 96 h
(Cheever et al., 1984).
6.2. Distribution
Glycol ethers are rapidly metabolized and eliminated
in the mammalian species that have been studied (see sec-
tions 6.3 and 6.4). Very few studies have therefore been
conducted to examine tissue distribution.
Using radioactive 2-ME, Sleet et al. (1986) noted that
radioactivity was present throughout the maternal and con-
ceptus compartments only 5 min after oral administration
of a tracer dose to pregnant mice. The highest levels
were noted in maternal liver, blood, and gastrointestinal
tract, and in the placenta, yolk sac, and embryonic struc-
tures such as limb buds, somites, and neuroepithelium.
Maternal blood levels declined to between 2 and 10% of
peak levels after 24 h. At 6 h post-administration, 69% of
the radioactivity in maternal liver and 33% of that in the
embryo were acid soluble.
6.3. Metabolic Transformation
The glycol ether acetates, 2-EEA and 2-MEA, are rap-
idly hydrolysed in vivo to the free glycol ether (2-EE and
2-ME, respectively) and acetate in rats (Romer et al.,
1985). The metabolism of 2-ME has been studied by Miller
et al. (1983a) and Moss et al. (1985), who found that
methoxyacetic acid (MAA) and methoxyacetyl glycine are the
primary metabolites. MAA accounted for 50 to 60% of the
urinary radioactivity and methoxyacetyl glycine for 18 to
25% during the 48-h observation period following a single
intraperitoneal dose of 2-[methoxy-14C]ethanol (250 mg per
kg body weight) (Moss et al., 1985). The conversion in
plasma of 2-ME to 2-MAA was rapid, the half-life for the
disappearance of 2-ME being 36 min. In the study reported
by Miller et al. (1983a) using 14C labelled 2-ME, 12% of
the dose was eliminated as 14CO2 after 48 h, suggesting
that either 2-ME or its metabolite 2-MAA underwent further
oxidative metabolism.
2-Ethoxyacetic acid (EAA) and 2-ethoxyacetyl glycine
have been found in the urine of rats that had been admin-
istered a single oral dose of 230 mg 2-EE/kg body weight
(Cheever et al., 1984).
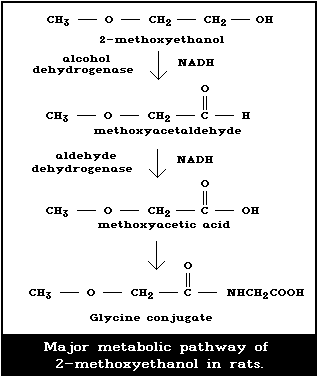
Fig. 1 shows the proposed pathway for the metabolism
of 2-ME in the rat (Miller et al., 1983a; Moss et al.,
1985; Foster et al., 1986). This route of metabolism
involves the enzyme alcohol dehydrogenase (ADH), as shown
by the blocking of 2-ME metabolism by the known ADH in-
hibitor 4-methylpyrazole (Moss et al., 1985). In addition,
the administration of ethanol before exposure of rats to
2-ME or 2-EE has been found to prolong the retention of
these glycol ethers in the blood (Romer et al., 1985).
This effect was noted at ethanol blood levels above
3 mmole/litre. The retention of 2-ME or 2-EE in the body
was attributed to the competitive inhibition by ethanol of
the common metabolizing enzyme, alcohol dehydrogenase.
 When rats were administered 2-EE at doses from
0.5 mg/kg to 100 mg/kg, Groeseneken et al. (1988) found
increasing relative amounts of EAA in urine (from 13.4% to
36.8% of the total dose). This could be the result of
competition by other metabolic pathways, which would
become more easily saturated at the higher dosage levels.
At 2-EE doses equivalent to the lowest doses in these
animal studies, it was estimated that humans excrete 30-
35% as EAA. Furthermore, 27% (on average) of the EAA was
excreted as the glycine conjugate in the rat, whereas no
glycine conjugation was observed in humans.
The in vitro nasal mucosal carboxylesterase activity
of mice was compared to the activity of other mice tissues
and to the nasal mucosal carboxylesterase activity of
rats, rabbits, or dogs when exposed to 2-MEA or 2-EEA
(Stott & McKenna, 1985). The specific activity in nasal
carboxylase was found to be similar to that of the liver
in mice, but it was greater than the activity found in the
kidney, lung, or blood of mice. Nasal mucosal carboxyl-
esterase activity of mice was comparable to that of dogs,
slightly higher than the activity in rats, and nearly six-
fold higher than the activity in rabbits. These in vitro
studies suggest that considerable hydrolysis may occur in
the intact animal, resulting in the formation of acetic
acid at the initial route of entry.
6.4. Elimination and Excretion
Although 2-ME is rapidly metabolized to 2-MAA after an
intraperitoneal dose of 250 mg/kg body weight in the rat,
the excretion of 2-MAA is fairly slow (half-life of ap-
proximately 20 h) (Moss et al., 1985). In humans, an elim-
ination half-life for 2-MAA of 77.1 h has been reported
(Groeseneken et al., 1989a). The elimination of radioac-
tive 2-EAA (ethyl 1,214C) has been reported in the rat
(half-life of approximately 8 h) (Guest et al., 1984) and
in humans (half-life of approximately 21-42 h)
(Groeseneken, 1986b,c, 1988; Veulemans, 1987a). In rats,
the administration of an oral dose of 230 mg 2-EE/kg body
weight led to the production of EAA and N -ethoxyacety gly-
cine (> 76% of the dose), EAA being the major metabolite
found in testes after 2 h (Cheever et al., 1984).
The urinary excretion of EAA during and after single
4-h exposures to 14, 28, or 50 mg 2-EEA/m3 in human
volunteers has been reported by Groeseneken et al. (1987).
The distribution/excretion time course during and after
2-EEA exposure was similar to that observed for 2-EE.
This indicates that humans, like rodents, hydrolyse the
acetate to 2-EE, which is then converted to the EAA metab-
olite and excreted. The excretion of the EAA metabolite
was observed to be biphasic. A second peak of excretion
occurred approximately 3 h after the first, suggesting
some type of redistribution of the glycol ethers, or of a
metabolite, from a peripheral compartment.
The urinary excretion of EAA was evaluated under field
conditions in which women volunteers were exposed daily to
2-EE or 2-EEA in the process of silk-screen printing
(Veulemans et al., 1987a). Urinary EAA was measured during
5 days of normal production and was also detectable after
a 12-day stop in production. The excretion of EAA in-
creased during the work week, yet it was still detectable
after 12 days without exposure. These data suggest that
the retention of EAA, or of other 2-EE or 2-EEA metab-
olite, may be toxicologically significant if EAA is the
active metabolite responsible for the observed toxicity.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Given the physical and chemical properties of these
four glycol ethers and the known rates of degradation in
the environment (section 4), there is minimal concern that
hazardous levels of these substances will occur. Although
only a few studies have been reported, the available data
support this conclusion. For example, both 2-ME and 2-EE
have been tested for effects on microorganisms and aquatic
animals. The lethal concentration of 2-ME and 2-EE to
microorganisms (Cladosporium resinae, Pseudomonas aeru-
ginosa, Gliomastix sp, and Candida sp is > 2% in the
medium (Neihof & Bailey, 1978; Lee & Wong, 1979). The
exposure of C. resinae lasted 30 to 42 days, whereas the
other organisms were exposed for 4 months. Very low
toxicity was shown by 2-ME to the green alga Scenedesmus
quadricauda (growth inhibition only at > 10 g/litre) and
the cyano-bacterium (blue-green alga) Microcystis aerugin-
osa (growth inhibition only at > 100 mg/litre) (Bringmann
& Kuhn, 1978). 2-EE has very low toxicity to the brine
shrimp (Artemia salina) (LC50 > 4 g/litre) (Price et
al., 1974) and to another arthropod Daphnia magna
(Hermens et al., 1984). The toxicity of 2-EE to fresh-
water fish is also very low, the LC50 (96 h) for the
bluegill (Lepomis macrochirus) being > 10 g/litre. However,
2-EEA is far more toxic to fish in this assay, LC50 (96 h)
values of 60 mg/litre (Bailey et al., 1985) and 46 mg per
litre in fathead minnows (Pimephales promelas) (Purdy,
1987) having been reported. These results confirm those of
Juhnke & Ludemann (1978), who reported an LC50 (48 h)
value of 107-141 mg per litre when using the Golden Orfe
(Leuciscus idus melanotus) test. The reason for this low
LC50 has not been studied, but Dawson et al. (1977) re-
ported similarly low LC50 values for 2-MEA in fish (45 mg
per litre in tidewater silverfish (Menidia beryllina)
and bluegill (Lepomis chirus).
Effects on other organisms have not been reported.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data indicating the acute oral, dermal, intraperito-
neal, and inhalation toxicities of 2-ME and 2-EE are given
in Table 7. As shown, these two glycol ethers have similar
toxicities, and are of low acute toxicity whether animals
are exposed via the dermal, oral, or respiratory routes.
Even after intraperitoneal injection, the reported levels
of acute toxicity are still low (1.7-2.46 g per kg body
weight).
Dyspnoea, somnolence, ataxia, and prostration were re-
ported after near lethal doses of 2-EE were given to rats,
mice, guinea-pigs, or rabbits (Stenger et al., 1971).
Haemoglobinuria and/or haematuria were reported after a
single oral administration of 2-EE with a dose approaching
the LD50 (Laug et al., 1939). Microscopic examination of
the kidneys of rats, mice, guinea-pigs, or rabbits given
2-EE orally revealed severe tubular degeneration, conges-
tion, and cast formation; in some animals most of the
cortical tubules were necrotic (Laug et al., 1939). No
data on the purity of the 2-EE used in these early studies
were reported.
Single 4-h inhalation exposures of rats to 1866 mg 2-ME
per m3 or more led to testicular atrophy as early as 24 h
after exposure (Doe, 1984b).
8.2. Short-term Exposures
Repeated exposure of experimental animals to 2-ME and
2-EE by various routes of administration (7-90 days) have
revealed adverse effects on the haematological and nervous
systems and on the testes, thymus, kidney, liver, and
lung. From data on the metabolic transformation and
elimination of 2-MEA and 2-EEA in animals and man (see
section 6), it is probable that the toxicities of these
glycol ether acetates are similar to those of the parent
chemicals 2-ME and 2-EE.
8.2.1. Haematological and immunological effects
Following repeated exposures to 2-ME and 2-EE using
various routes of administration, haematological effects
have been observed in several species. A summary of the
most relevant studies is given in Tables 8 (2-ME) and 9
(2-EE).
Table 7. Acute toxicity data for 2-methoxyethanol and 2-ethoxyethanol and their acetatesa
-------------------------------------------------------------------------------------------------------------
Compound Species Intra- Oral LD50 Dermal Inhalation References
peritoneal LD50 LC50
LD50
-------------------------------------------------------------------------------------------------------------
2-Methoxyethanol Rat 2460 2460-3400 Smyth et al. (1941); Goldberg et al.
(1962); Pisko & Verbilov (1988)
Mouse 2200 2167-2560 4603 Werner et al. (1943); Saparmamedov
(1480) (1974); Pisko & Verbilov (1988)
Guinea-pig 950 Karel et al. (1947)
Rabbit 890-1450 1300 Carpenter et al. (1956); Pisko &
Verbilov (1988)
2-Ethoxyethanol Rat 2000 2125-5500 Smyth et al. (1941); Laug et al.(1939)
Stenger et al. (1971); Pisko & Verbilov
(1988)
Mouse 1700 4000-4800 6698 Werner et al. (1943); Laug et al.(1939)
(1820) Stenger et al. (1971); Saparmemedov
(1974); Pisko & Verbilov (1988)
Guinea-pig 1400-2600 Karel et al. (1947); Laug et al.(1939)
Rabbit 3300- Stenger et al. (1971); Smyth et al.
15 200 (1941)
2-Methoxyethanol Rat 3930 Smyth et al. (1941)
acetate Guinea-pig 1250 Kirk-Othmer (1980)
Rabbit 5557
2-Ethoxyethanol Rat 5100 Smyth et al. (1941)
acetate Guinea-pig 1910 Kirk-Othmer (1980)
Rabbit 10 333
-------------------------------------------------------------------------------------------------------------
a Doses are given as mg/kg body weight except for inhalation doses,
which are in mg/m3 (values in parentheses are ppm)
Concerning changes in blood parameters, a comparison
of Tables 7, 8, and 9 indicates clearly that 2-ME is more
toxic than 2-EE in several species, although 2-EE has been
less widely studied. For example, Nagano et al. (1979)
showed that 2-EE administered orally for 5 weeks at 2000
mg/kg body weight per day did not result in changes in
haematological parameters other than a reduced leucocyte
count. No effects were observed at 1000 mg/kg. Under a
similar dosing regimen, 2-ME caused a dose-related re-
duction in leucocytes at 500 mg/kg per day, other haemato-
logical parameters being unaffected at 250 mg/kg per day
(Nagano et al., 1979).
Some of the doses used in the studies summarized in
Tables 7 and 8 resulted in histological and functional
changes in the bone marrow and thymus. In an inhalation
study, Miller et al. (1983b) noted thymic atrophy and de-
creased thymus weights in male and female rats exposed to
933 mg 2-ME/m3 for 13 weeks. Miller et al. (1981) also
noted decreased weights of thymus, spleen, and mesenteric
lymph nodes in rats exposed to 933 or 3110 mg 2-ME/m3 for
9 days, and reduced bone marrow cellularity was observed
at 3110 mg/m3. In one study there was at least partial
reversal of these effects 22 days after an exposure last-
ing 4 days (Grant et al., 1985).
When MAA, the metabolite of 2-ME, was administered to
rats by gavage at 300 mg/kg body weight per day for 8
days, it resulted in reduced erythrocyte counts, haemo-
globin concentrations, and packed cell volumes, and a
marked reduction in leucocyte counts. Thymus and spleen
weights were also decreased and a severe lymphoid
depletion was seen in the thymus. Similar, but less
marked, effects on blood and thymus were seen in some
animals administered 100 mg/kg per day. No treatment-
related effects were reported at 30 mg/kg per day (Miller
et al., 1982).
Studies on immunological function show that 2-ME and
2-EE yield different results. De Delbarre et al. (1980)
studied the effect of 2-ME and 2-EE on the humoral respon-
siveness to antigenic stimuli and on adjuvant arthritis in
the rat. 2-ME had an inhibitory effect on adjuvant ar-
thritis at doses greater than 18.6 mg/kg body weight per
day administered subcutaneously, whereas 2-EE was not
effective at doses up to 150 mg/kg. A dose of 150 mg 2-ME
per kg per day delayed rejection of skin grafts and 75 mg
2-ME/kg per day (for 28 days) significantly depressed
antibody production. Again, 2-EE had no effect on these
parameters. House et al. (1985) administered 2-ME to mice
by gavage (10 doses of 250, 500, or 1000 mg 2-ME/kg body
weight per day for 2 weeks) and noted a 48% reduction in
thymus weight at 500 and 1000 mg/kg. However, there was no
significant alteration in immune function or host resist-
ance. Similar findings were reported when the same dose
levels of MAA, the major metabolite of 2-ME (section 6),
were administered.
Table 8. Haematological effects of 2-methoxyethanol in animalsa
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat inhalation 10 males 6 h/day; 9 days; RBC fragility, no effect at Miller et al.
10 females 311, 933, 3110 mg/m3 3110 mg/m3 but reduced RBC, (1981)
Hb, and PCV levels and bone
marrow cellularity seen in M
and F; similar findings in F
at 933 mg/m3; reduced WBC
at 3110 and 933 mg/m3;
reduced thymus weight
Rat inhalation 10 males 6 h/day; 5 days/week only effect at 2 lowest Miller et al.
10 females for 13 weeks; doses was reduced body (1983b)
93.3, 311, 933 mg/m3 weight gain in F; at 933
mg/m3 reduced WBC, Hb,
PCV, and platelets in both
sexes after 4 and 12 weeks;
reduced serum proteins at 13
weeks, thymic atrophy
Rabbit inhalation 5 males 6 h/day; 5 days/week decreased thymus size and Miller et al.
5 females for 13 weeks; body weight, PCV, WBC, (1983b)
93.3, 311, 933 mg/m3 platelets and Hb at 933
mg/m3; slight to moderate
decrease in lymphoid tissue
at 311 mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week decreased RBC, Hb and Hct; Werner et al.
2 controls for 12 weeks; increased immature (1943)
750 mg/m3 granulocytes
Mouse oral 5 males 5 times/week for 4/5 mice at 2000 mg/kg died Nagano et al.
5 weeks; 62.5, 125, before completion; doses at (1979)
250, 500, 1000, 2000 or above 500 mg/kg resulted
mg/kg body weight/day in reduced WBC, RBC, and PCV
Table 8 (contd.)
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat oral 24 males once per day for reduced thymus and spleen Grant et al.
4 days; 100 and 500 weight at 500 mg/kg on days (1985)
mg/kg body weight/day; 1-8, recovery by day 22;
sacrificed reduced WBC at both 100 and
500 mg/kg, largely
reversible by day 22
Hamster oral 4 males once daily, 5 days/week decrease in WBC at 500 mg/kg Nagano et al.
for 5 weeks; 62.5, 125, the only effect on blood (1984)
500 mg/kg body weight/day
Guinea oral 3 males once daily, 5 days/week about 50% decrease in WBC at Nagano et al.
-pig for 5 weeks; 250 and 500 both doses (1984)
mg/kg body weight/day
--------------------------------------------------------------------------------------------------------
a M = male; F = female; PCV = packed cell volume; Hct = haematocrit; RBC = red blood cell count;
WBC = white blood cell count; Hb = haemoglobin; No. = number of animals per group
Table 9. Haematological effects of 2-ethoxyethanol in animalsa
-------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Response Reference
time; level
-------------------------------------------------------------------------------------------------------
Rat inhalation 15 males 6 h/day; 5 days/week decreased leucocyte count Barbee et al.
15 females for 13 weeks; 92, 368, in females at 1472 mg/m3, (1984)
1472 mg/m3 no other significant
biological effect reported
Rabbit inhalation 10 males 6 h/day; 5 days/week for decreased Hb, Hct, and Barbee et al.
10 females 13 weeks; 92, 368, 1472 RBC in both males and (1984)
mg/m3 females only at 1472
mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week for slight reduction in RBC, Werner et al.
2 control 12 weeks; 3091 mg/m3 Hb, and PCV, microcytosis, (1943)
hypochromia, and poly-
chromatophilia were seen,
marked increase in
immature granulocytes
Mouse oral 5 males 5 times/week for 5 lower WBC at 2000 mg/kg, Nagano et al.
weeks; 62.5, 125, 250, but no effect on (1979)
500, 3110, 2000 mg/kg erythrocyte parameters
body weight/day
-------------------------------------------------------------------------------------------------------
a PCV = packed cell volume; Hct = haematocrit;
RBC = red blood cell count; WBC = white blood cell count; Hb = haemoglobin
No. = number of animals per group
8.2.2 Effects on liver and kidney
Effects on the liver, such as reduced cytoplasmic den-
sity, disruption of lobular structure, elevated plasma
fibrinogen, reduced serum proteins, and elevated liver
weights, have been reported in certain studies in which
rats, mice, or rabbits were exposed to 2-ME or 2-EE at
levels in excess of 300 ppm (933 and 1104 mg/m3, respect-
ively), for periods of up to 13 weeks. Many of the effects
observed were reversible, and no consistent pattern was
noted among the various studies (Miller et al., 1981;
1983b; Stenger et al., 1971). Hepatic changes have been
observed at inhalation exposures to 2-ME and 2-EE in ex-
cess of 300 ppm (933 and 1104 mg/m3, respectively).
No pathological changes could be detected in the kid-
neys of rats exposed to approximately 933 mg 2-ME/m3 for
6 or 7 h per day, 5 days a week, for 13 weeks (Miller et
al., 1981, 1983b). In studies with 2-EE, no treatment-
related pathology was reported after inhalation exposure
of rats to 1362 mg/m3 (370 ppm) for 5 weeks or dogs to
3091 mg/m3 (840 ppm) for 12 weeks (Werner et al., 1943).
8.2.3 Behavioural and neurological effects
There have been few reports on the effects of 2-ME
and 2-EE on the function of the nervous system in animals.
Ataxia was reported after inhalation exposure of rats to
18.96 g 2-EE/m3 (5152 ppm) for 8h. After exposure of rats
to levels of 2-ME between 1555 and 12 440 mg/m3, 4 h per
day for up to 7 days, inhibition of an avoidance-escape
conditioned response was observed without any alteration
of motor function (Goldberg et al., 1962). The same
authors reported also a significant inhibition of this
response after 14 days exposure to 1210-4836 mg 2-ME per
m3 (389-1555 ppm). After 3 weeks recovery, rats whose
avoidance response had been inhibited on the 14th day
showed a return to normal. Savolainen (1980) reported a
partial loss of motor function in the hind limbs of rats
after exposure to 1244 mg 2-ME/m3 (400 ppm) for 6 h/day,
5 days per week, for 2 weeks. This hindlimb paralysis
coincided with the glial cell toxicity noted during the
second week of exposure. Recovery was incomplete after 2
weeks post-exposure, the animals receiving the highest
dose showing minor paresis.
8.3 Skin and Eye Irritation; Sensitization
No satisfactory data on the sensitization potential of
2-ME and 2-EE in animals, and only limited data on their
irritant properties to eyes, have been reported. Weil &
Scala (1971) found 2-ME to be an eye irritant. Lailler et
al. (1975) reported that 0.1 ml of undiluted 2-ME led to
corneal and conjunctival oedema and increased vascular
leakage in the conjunctiva and aqueous humour. A 25% aque-
ous solution was much less active.
8.4 Long-term Exposures
No adequate long-term animal studies on 2-ME and 2-EE
or their acetates have been reported to date.
8.5 Effects on Reproduction and Fetal Development
The effects of glycol ethers, particularly 2-ME, on
animal reproduction, fertility, and teratogenicity have
been extensively studied. Some of the early studies on
reproduction have been reviewed by Hardin (1983). The many
studies conducted in several countries and in several
animal species are in general agreement on the nature of
developmental and reproductive effects.
8.5.1 Effects on the male reproductive system
8.5.1.1 Oral exposure
The pathological changes observed in the testes of
rats after the administration of 2-ME have been well
characterized by Foster et al. (1983). The severe degener-
ative changes noted in the germinal epithelium of the
seminiferous tubules of different laboratory animals were
similar, irrespective of species or route of adminis-
tration. Daily doses of 2-ME were administered orally to
rats (50, 100, 250, or 500 mg/kg) for periods between 1
and 11 days, and 250, 500, or 1000 mg 2-EE/kg body weight
per day was administered in a similar regimen (Foster et
al., 1983; Creasy & Foster, 1984; Creasy et al., 1985).
After 2-ME administration, testicular damage was observed
1 day post dosing with 100 mg/kg or more, the lesion being
localized in the late primary spermatocytes. Continuous
dosing with 2-ME resulted not only in progressive deletion
of the primary spermatocytes but also in degenerative
changes in secondary spermatocytes and dividing sper-
matids. Changes in sperm motility, morphology, and concen-
tration were also reported. The cessation in maturation
of early primary spermatocytes led to a depletion of the
spermatid population, resulting in tubules containing only
Sertoli cells, spermatogonia, and early primary spermato-
cytes. Decreased testicular weight was reported at 250 mg
per kg or more. Although most of the effects seen after a
4-day treatment with 2-ME were reversible within 8 weeks,
a small proportion of tubules showed incomplete recovery
indicating a non-reversible, long-term effect. Similar
findings were reported after 2-EE administration, but only
after 11 days of dosing with 500 and 1000 mg/kg. No
effects on the testes were observed following oral admin-
istration of 250 mg 2-EE/kg per day or 50 mg 2-ME/kg per
day.
Subsequent work by Chapin et al. (1985a,b) supports
the hypothesis that 2-ME administered to male rats at
doses of 100 or 200 mg/kg daily for 5 days affects the
spermatogonia. In these studies male rats were given doses
of 0, 50, 100, or 200 mg/kg per day for 5 days, and recov-
ery was investigated by evaluating the testicular damage
in sacrificed animals at 8 subsequent weekly intervals or
alternatively by investigating fertility through mating
with two females per week for 8 weeks. Treatment with 100
or 200 mg/kg resulted in widespread testicular damage and
cell death immediately after treatment, with only very
mild effects being noted at 50 mg/kg (this was thus a
marginal-effect level rather than a no-effect level).
There was some evidence of recovery from testicular damage
towards the end of the study period, but reduced fertility
was still apparent weeks after the cessation of treatment
with 200 mg/kg. Thus, the recovery was neither as
complete nor as rapid as that noted by Foster et al.
(1983).
The in vivo and in vitro effects of 2-ME and MAA ex-
posures, respectively, were compared by evaluating effects
using electron microscopy (Creasy et al., 1986). 2-ME
caused cell death in the pachytene spermatocytes in vivo ,
as well as focal breakdown of the plasma membranes be-
tween spermatocytes and Sertoli cells. Similar, but less
frequent, breaks were seen in vitro when mixed cultures of
Sertoli and germ cells were treated with MAA.
Subsequent studies by Foster et al. (1987), in which
the alkoxyacetic acids of 2-ME and 2-EE (MAA and EAA,
respectively) were given orally in doses equimolar to the
studies described earlier (Foster et al., 1983), yielded
similar patterns of testicular degeneration and effects on
spermatocyte development. The similarity of effects at
equimolar concentrations between the two acetic acid de-
rivatives and their respective glycol ethers suggests that
these metabolites may either be the causative factors or
at least play a role in the production of the observed
degenerative effects.
The assessment of the effects of low doses of poten-
tial toxicants on sperm fertility and production is diffi-
cult because of the large amount of sperm usually produced
by most test species and the large sperm reserves. Using
an experimental design that required bi-daily matings of
Long-Evans male rats, the effects of orally administered
2-EE (0, 150, or 300 mg/kg body weight per day; 5 days per
week for 6 weeks) on sperm reserves and on pachytene sper-
matocytes was evaluated (Hurtt & Zenick, 1986). Further
groups of rats received the same doses of 2-EE, but were
not subjected to repeated mating. After 6 weeks of treat-
ment, the animals were sacrificed and the effect of 2-EE
administration on organ weight, testicular spermatid
count, cauda epididymal sperm count, and sperm morphology
was determined. Exposures to 2-EE resulted in significant
decreases in testicular weight, spermatid count, and epi-
didymal sperm count for both mated and unmated animals at
the highest dose level. However, the effects were also
seen at 150 mg/kg per day in repeatedly mated animals.
Adult male rats treated orally with 2-EE (936 mg/kg
body weight, 5 days/week for 6 weeks) were found to have
decreased sperm counts and altered sperm morphologies
after 5 and 6 weeks of exposure when compared with
vehicle-control animals, and sperm motility was decreased
at week 6 (Oudiz & Zenick, 1986). These data indicate that
the pachytene spermatocyte is the target cell most sensi-
tive to the effects of 2-EE.
The reproductive toxicity of 2-EE in CD-1 mice has
been evaluated in a continuous breeding protocol (Lamb et
al., 1984). Male and female mice (20 males and 20 females
per dose group) were given access to drinking-water con-
taining 0.5, 1.0, or 2.0% 2-EE and housed as breeding
pairs continuously for 14 weeks. Treated animals were then
paired with controls for breeding. At 1 and 2% 2-EE, sig-
nificant adverse effects on fertility were seen, the re-
productive capacity of both males and females being af-
fected. Testicular atrophy, decreased sperm motility, and
increased abnormal sperm levels were seen in treated
males, but no treatment-related pathological effects were
seen in females even though reduced fertility was noted in
the cross-mating phase.
The reproductive toxicity of a single oral dose of
2-ME (0, 500, 750, 1000, or 1500 mg/kg) was assessed in
adult male CD rats and CD-1 mice by Anderson et al.
(1987). Animals from each group were sacrificed at weekly
intervals during weeks 3-8 post exposure and evaluated for
sperm counts, sperm morphology, and testicular histology.
A dose-related toxicological response in spermatocytes was
observed in both species. In a companion study, male rats
and mice were exposed to 2-ME (0, 125, 250, or 500 mg/kg)
and permitted to mate during weeks 1-10 post exposure.
Following mating, pregnant females were sacrificed on day
17 of pregnancy and each uterus was evaluated for dominant
lethal effects. The only effect noted was a decrease in
total number of implants at week 6 following treatment
with 500 mg/kg. In a study in which both rats and mice
were given single doses at 0, 500, 750, 1000, or 1500 mg
per kg and mated 5 and 6 weeks later, dominant lethal
studies showed a dose-related decrease in fertility in
rats, with complete sterility in all but the lowest dose
group after 6 weeks. No effects on the reproductive
capacity of mice were noted. There was no statistically
significant evidence for the induction of dominant lethal
mutations or abnormalities in the F1 generation of either
species.
8.5.1.2 Inhalation studies
Testicular damage has been reported in rats exposed to
2-ME by inhalation for a single 4-h period (Doe, 1984b).
Exposure to 1944 mg/m3 resulted in histological evidence
of damage to the maturing spermatids, testicular atrophy
occurring at 3887 mg/m3 or more. The NOEL was 933 mg/m3.
The effect of repeated exposure to 2-ME has also been
investigated using the inhalation route. Exposure of rats
to 3110 mg/m3 (6 h/day) for 9 out of 11 days resulted in
degenerative changes and necrosis in the germinal epi-
thelium of the seminiferous tubules, but no effects on the
testes were observed with a dose of 933 mg/m3. However,
Doe et al. (1983) reported pronounced atrophy of the sem-
iniferous tubules in rats exposed to 933 mg 2-ME/m3 or
more (6 h/day, 5 days/week) for 13 weeks. The NOEL was
311 mg/m3 (Miller et al., 1983b). Studies were also car-
ried out in rabbits, using exposure levels of 93.3, 311,
and 933 mg/m3 (6 h/day, 5 days/week), and most animals
exposed to 311 mg/m3 showed reduced testis size and
severe degenerative changes in the tubules. Reduced testis
weight was also seen in two out of five animals exposed to
93.3 mg/m3, and one animal showed histological changes
in the germinal epithelium. A NOEL could not be ident-
ified, but 93.3 mg/m3 was near the minimal effective dose
in rabbits.
In addition, an increased incidence of sperm abnor-
malities (principally sperm with banana shaped or amorph-
orous heads) has been noted in mice exposed to 1555 mg
2-ME/m3 (7 h per day for 5 days) but not to 78 mg per
m3 (McGregor et al., 1983).
8.5.2 Embryotoxicity and developmental effects
8.5.2.1 2-Methoxyethanol
2-ME has been shown to lead to embryotoxic and devel-
opmental effects in laboratory animals following inha-
lation, oral, or dermal exposure. The effects of 2-ME
exposure over the entire organ-forming period of gestation
have been studied in rabbits, rats, and mice, the rabbit
being the most sensitive species.
The embryotoxicity of 2-ME after gastric intubation
was evaluated in mice (31.25, 62.5, 125, 250, 500, or 1000
mg/kg body weight) on days 7-14 of gestation (Nagano et
al., 1981). No maternal deaths were observed, but maternal
weight gain was reduced in the mice that received doses of
250 mg/kg per day or more. Maternal toxicity was not seen
at 123 mg/kg or less. On day 18 of gestation, fetuses were
examined and an increase in fetal death rate was observed
at doses of 250 mg/kg or more. At doses of 500 and 1000 mg
per kg, all fetuses were dead at caesarean section, except
for one in the 500-mg/kg group. Approximately 44% (57/130)
of the live fetuses in the 250-mg/kg group were found to
have gross anomalies, including exencephaly (24), umbili-
cal hernia (3), and abnormal digits (29). One fetus had
both exencephaly and abnormal digits. The lone surviving
fetus in the 500-mg/kg group had exencephaly and abnormal
digits. Minimal skeletal malformations were also observed
at the lowest dose of 31.25 mg/kg body weight (a matern-
ally non-toxic dose). Thus, a NOEL could not be ascer-
tained.
Dose-dependent electrocardiographic changes were
detected on gestation day 20 in the rat fetuses of mothers
exposed orally to 2-ME (0, 25, or 50 mg/kg body weight) on
days 7 to 13 of gestation (Toraason et al., 1985). Cardio-
vascular malformations were observed, including right
ductus arteriosus and ventricular septal defects. QRS in-
tervals were significantly prolonged, particularly in the
highest dose group, suggesting intra-ventricular conduc-
tion delays.
Ornithine decarboxylase (ODC) activity is highest
during rapid growth and development in fetuses and is
sensitive to maternal exposure to chemicals and drugs.
Toraason et al. (1986a,b) evaluated the effect on ODC ac-
tivity in the fetuses of pregnant rats that received 25 mg
2-ME/kg by gavage during gestation days 7-13 or 13-19. The
activity was most affected in fetuses exposed during ges-
tation days 7 to 13. It was highest in 3-day old rats and
declined sharply thereafter. No functional or morphologi-
cal effects were observed in fetuses exposed at maternal
dose levels of 25 mg/kg during gestation days 7-13 or in
fetuses exposed at that level during days 13-19.
Nelson et al. (1984a) treated male rats by inhalation
(78 mg 2-ME/m3 for 7 h/day, 7 days/week for 6 weeks) and
subsequently bred these to untreated females. In addition,
groups of 15 pregnant rats were treated with the same dose
(7 h/day on gestation days 7-13 or 14-20) and allowed to
deliver and rear their young. Neuromotor function activity
and simple learning ability were assessed on days 10-90
after birth. Offspring from dams treated between gestation
days 7-13 showed significant changes in avoidance con-
ditioning, and changes in neurochemical levels were ob-
served in the brains of 21-day-old offspring from the
paternally exposed group as well as from both maternally
exposed groups.
8.5.2.2 2-Ethoxyethanol
A dose-finding study in pregnant rats revealed that no
offspring survived inhalation exposures of 3312 mg 2-EE
per m3, 7 h/day, during gestation days 7-13 or 14-20
(Nelson et al., 1981). The authors reported 34% neonatal
deaths at 736 mg/m3 under similar exposure conditions.
Offspring from dams exposed to 368 mg/m3 on gestation
days 7-13 or 14-20 showed impaired performance in behav-
ioural tests and neurochemical alterations in brain samples.
Using inhalation exposures of 478, 1435, and 2208 mg
2-EE/m3 on gestation days 7-15 (7 h/day), Nelson et al.
(1984b) reported complete resorption of all rat fetuses at
2208 mg/m3, reduced fetal weight at 1435 mg/m3, and a
NOEL of 478 mg/m3. Andrew & Hardin (1984), exposed preg-
nant rats to 733 and 2823 mg/m3 for 7 h/day throughout
gestation and observed increased fetal resorptions at 733
mg/m3 as well as skeletal and cardiovascular abnormali-
ties. At 2823 mg/m3 the resorption frequency reached 100%.
Developmental toxicity has been noted in rats after
dermal application of 2-EE (0.25 or 0.5 ml, four times per
day) (Hardin et al., 1982) or 2-EEA (0.35 ml, four times
per day) (Hardin et al., 1984) on gestation days 7-16.
Increased resorption rates and fetal deaths, decreased
viable fetus weights, and increased cardiovascular defects
and skeletal malformations were seen, even at the lowest
dose tested.
The effect of simultaneous administration of ethanol
(10% in drinking water) and 2-EE (368 mg/m3 by inha-
lation) has been investigated, in view of their related
metabolism (via the enzyme alcohol dehydrogenase) (Nelson
et al., 1982, Nelson et al., 1984c). Although the results
obtained are difficult to interpret, ethanol adminis-
tration early in gestation tended to reduce the behav-
ioural effects induced by 2-EE, whereas ethanol given late
in gestation enhanced these effects.
8.5.3 Teratogenicity
8.5.3.1 2-Methoxyethanol
The teratogenic potential of dermally administered
2-ME was estimated in pregnant rats with the Chernoff-
Kavlock screening test (Wickramaratne, 1986). Various con-
centrations (0, 3, 10, 30, or 100%) of 2-ME in physiologi-
cal saline were applied to shaved skin and occluded for
6 h of exposure on gestation days 6-17. Animals were per-
mitted to have their litters and rear the pups until 5
days postpartum, when the study was terminated. No adverse
effects were noted at the 3% dose level. Small litter
sizes and decreased fetal survival were observed at the
10% level. At 30%, lethality was observed in all fetuses,
and all pregnant females died when exposed dermally to
100% 2-ME.
The teratogenicity of 2-ME in mice, rats, and rabbits
has been evaluated by Hanley et al., (1984). Pregnant rats
and rabbits were exposed by inhalation to 0, 9, 31, or 156
mg/m3 for 6 h/day on gestation days 6-18 (rabbits) or 6-
15 (rats), and mice were exposed to 0, 31, or 156 mg per
m3 for 6 h/day on days 6-15 of gestation. No teratogenic
effects were found in CF-1 mice and Fischer-344 rats under
the conditions of this study, although slight fetotoxicity
was noted in both species. In New Zealand white rabbits
exposed to 156 mg/m3 there was a significant increase in
resorption rate and incidence of malformations involving
all organ systems, as well as a significant decrease in
mean fetal body weight when compared to controls. Of the
fetuses from dams exposed to 156 mg per m3, 63% exhibi-
ted at least one malformation and 91% of the litters had
at least one fetus with a malformation. There were no
teratogenic or fetotoxic effects in any of the three
species evaluated at 31 mg/m3.
Developmental phase-specific and dose-related terato-
genic anomalies in CD-1 mice resulting from 2-ME exposure
have been evaluated (Horton et al., 1985). 2-ME was admin-
istered by gavage to pregnant females at doses of 250 mg
per kg (during gestation days 7 to 9, 8 to 10, or 9 to 11;
during days 7 to 8, 9 to 10, or 10 to 11; or once a day on
gestation days 9, 10, 11, 12, or 13) in order to identify
the most sensitive developmental phases for the anomalies
under study. Resulting malformations were specifically re-
lated to the developmental stage at the time of exposure,
with exencephaly being observed between days 7 to 10 and
paw anomalies (syndactyly, oligodactyly, and stunted digit
number 1) during the later stages of development (days 9-
12). The dose dependency of digit malformation was studied
by administering single doses by gavage (100, 175, 250,
300, 350, 400, or 450 mg 2-ME/kg) on gestation day 11.
Dose-related paw malformations were noted in all litters,
with forepaws being more susceptible than hindpaws in
terms of the number and severity of malformations. At 175
mg/kg digit anomalies were induced without concurrent
reductions in fetal weight, while at 250 mg/kg or more
digit anomalies occurred concurrently with reduced fetal
body weight. In this study, the NOEL for the single ex-
posure (day 11) was 100 mg/kg. Although in this same
strain of mice digital malformations were not detected in
near-term fetuses given 100 mg 2-ME/kg body weight by
gavage on gestation day 11 (Greene et al., 1987), there
was a slight increase in the amount of cell death in
approximately 50% of the limb buds from embryos collected
24 h after dosing.
In a preliminary study, the teratogenic potential of
2-ME was determined in Cynomolgus monkeys using doses of
0.16, 0.32, and 0.47 mmol/kg administered by gavage
throughout the period of organogenesis (Scott et al.,
1987). Fetal death was dose-related (2/13 at 0.16 mmol
per kg; 3/10 at 0.32 mmol/kg; and 8/8 at 0.47 mmol/kg).
At the highest dose level, four fetuses were lost through
abortion and one of the remaining four, which was re-
covered by hysterotomy, demonstrated malformation (ectro-
dactyly) of the forelimbs. The MAA content of maternal
sera was followed throughout the treatment period. By the
25th day of treatment, MAA levels had more than doubled in
all treatment groups, compared to the values determined on
day one, indicating that multiple exposure to 2-ME can
lead to accumulation of the potentially embryotoxic metab-
olite MAA, the possible causative factor in the embryonic
deaths and teratogenicity observed (see section 8.8).
8.5.3.2 2-Ethoxyethanol and 2-Ethoxyethanol acetate
Andrew & Hardin (1984) have studied the teratogenic
potential of 2-EE in rats and rabbits. Rats (29-38 per
group) were exposed by inhalation to 0, 552, or 2388 mg
per m3 5 days per week for 3 weeks immediately prior to
mating and then to 0, 743, or 2823 mg/m3 for 7 h/day from
gestation day 1 to 19. Pregnant rabbits received 589 or
2271 mg/m3 for 7 h/day from gestation day 1 to 18. In
the New Zealand white rabbits exposed to 2271 mg/m3, the
rate of early resorptions was 100%. Even at 689 mg/m3, the
number of early resorptions per litter was 6 times that in
the control group. There was no evidence of severe intra-
uterine growth retardation in surviving fetuses, but a
significant increase in the incidence of major malfor-
mations (ventral wall defects and fusion of the aorta with
the pulmonary artery), minor anomalies, and skeletal vari-
ants. Teratogenic effects of 2-EE and also 2-EEA were re-
ported in Dutch Belted Rabbits after inhalation exposures
of 644 mg 2-EE/m3 and 2160 mg 2-EEA/m3 (Doe, 1984a).
In this study the author considered the NOEL in rabbits to
be 184 mg/m3 for 2-EE and 135 mg/m3 for 2-EEA.
In the study by Andrew & Hardin (1984), the highest
2-EE dose (2823 mg/m3) in rats led to 100% resorption,
as in rabbits. The resorption rate per litter in the
group gestationally exposed to 743 mg/m3 was about twice
the control value. Fetal body size was significantly
decreased, indicating retardation of intrauterine growth.
The significant increase in the incidence of cardiovascu-
lar defects (transposed and retrotracheal pulmonary
artery) and the increased incidence of common skeletal
variants and anomalies over control values were indicative
of a teratogenic effect of 2-EE in rats. Doe (1984a)
reported fetotoxicity without teratogenicity in rats after
inhalation exposure to 184 and 920 mg/m3, but no adverse
effects were reported after exposure to 37 mg/m3.
The teratogenic potential of 2-EE was evaluated in
pregnant Sprague-Dawley rats using dermal application
(0.25 or 0.50 ml, four times/day during gestation days 7
to 16) (Hardin et al., 1982). No signs of maternal
toxicity were noted except for ataxia on the last day of
dosing in the high-dose group, as well as a significant
decrease in body weight gain in the last half of ges-
tation. All fetuses in the high-dose group suffered intra-
uterine death. In the low-dose group, there was a signifi-
cant increase in the number of females with 100% dead
implants (p < 0.001), and in the incidence of skeletal
variations (p < 0.05). Also, reductions in fetal body
weight (p < 0.001) and cardiovascular malformations
(p < 0.05) were observed, as were significant decreases in
the number of live fetuses per litter (p < 0.001).
Hardin et al. (1984) studied the developmental tox-
icity of 2-EEA in rats after dermal application of 0.35 ml
2-EEA four times per day on days 7 to 16 of gestation
(daily application 1.37 g). 2-EEA was strongly embryo-
toxic, as reflected by significantly increased frequencies
of completely resorbed litters and dead implants per
litter. Also, the body weight of live fetuses was reduced
relative to water-treated controls. The spectrum and fre-
quency of malformations and variations noted in 2-EEA-
treated animals was similar to that described by Hardin et
al. (1982) in a previous study.
The developmental toxicity (including teratogenicity)
of 2-EEA in rats and rabbits following inhalation ex-
posure has been evaluated by Tyl et al. (1988). Fischer
344 rats (30 animals/group) and New Zealand white rabbits
(24 animals/group) were exposed to 2-EEA concentrations of
0, 270, 540, 1080, or 1620 mg/m3, 6 h/day on gestational
days 6-15 (rats) and 6-18 (rabbits). Fetuses were examined
for external, visceral, and skeletal malformations and
variations on gestational day 21 (rats) and 29 (rabbits).
In both rats and rabbits, maternal toxicity and develop-
mental toxicity were observed at exposures of 540 mg/m3 or
more. Teratogenic responses were increased at 1080 and
1620 mg/m3 with a 100% incidence of total malformations
being observed at the highest dose. An exposure of 270 mg
per m3 was considered a NOEL in both species, no evi-
dence of maternal or developmental toxicity being reported
at this dose.
8.6 Mutagenicity and Related End-Points
The only published data on 2-EE concerns a point mu-
tation test using Escherichia coli, which was reported to
be negative (Szybalski, 1958). However, 2-ME has been
tested in several in vitro and in vivo systems for its
genotoxic potential. The mutagenicity of 2-ME has been
evaluated in the following test systems: Salmonella typhi-
murium, unscheduled DNA synthesis (UDS) in human embryo
fibroblasts, bone marrow metaphase analysis in male and
female rats, dominant lethality in male rats, and a sex-
linked recessive lethal test in Drosophila melanogaster.
No evidence of mutagenicity in S. typhimurium was found
using five strains, with and without metabolic activation,
and dose levels up to 33 mg 2-ME per plate. When 2-ME was
tested in the presence of alcohol dehydrogenase no evi-
dence was found that metabolites of 2-ME were mutagenic in
S. typhimurium. Similarly, the addition of 2-ME at con-
centrations up to 10 mg/ml of medium did not lead to
changes in UDS in human embryo fibroblasts (McGregor et
al., 1983).
The production in CHO cells of sister chromatid ex-
changes (SCEs) and chromosome aberrations by 2-EE was
studied by Galloway et al. (1987). Both assays were
carried out with and without activation by an exogenous
enzyme system from rat liver chemically induced with the
PCB mixture Aroclor 1254. Aberrations were found only in
the absence of metabolic activation when using 2-EE con-
centrations between 4780 and 9510 µg/ml of medium. The
lowest effective concentration was 6830 µg/ml. SCEs were
found with and without activation, the lowest effective
concentration being 3170 µg/ml when the range studied
was 951-9510 µg/ml.
Aneuploidy was induced in the diploid yeast strain
D61.M ( Saccharomyces cerevisiae ) at 2-MEA levels of 3-
5.7% in the medium (Zimmermann et al., 1985). However,
there were no chemically related effects in this yeast
strain on point mutation or recombination after exposure
to 2-MEA. The relevance to mammals of these findings in
yeast is not clear.
No statistically significant increase in chromosome
aberrations was seen in male or female rats after exposure
by inhalation to 2-ME (78 or 1555 mg/m3), 7 h/day, for 1
or 5 days. Where possible 50 metaphases per rat were
scored from groups of 10 animals (McGregor et al., 1983).
Basler (1986) dosed Chinese hamsters intraperitoneally (10
animals) with two thirds of the LD50 of 2-MEA (approxi-
mately 1333 mg/kg body weight in corn oil) and the animals
were sacrificed 12, 24, 48, and 72 h after the single
dose. No statistically significantly increase in micro-
nucleated erythrocytes was noted.
Two dominant lethal studies in rats have been carried
out using 2-ME. McGregor et al. (1983) exposed groups of
10 male CD rats by inhalation to 78 or 1555 mg/m3, 7 h
per day, for 5 days. Control and low-dose groups gave
similar results, but the results at 1555 mg/m3 were
difficult to interpret. A high proportion of early deaths
was noted, which could be partly explained in terms of the
low implantation frequency reported. Therefore, a dominant
lethal effect at 1555 mg/m3 could not be demonstrated
conclusively. Rao et al. (1983), using male Sprague-
Dawley rats exposed to 93, 311, or 933 mg 2-ME/m3 by in-
halation 6 h/day, 5 days/week for 13 weeks, found no domi-
nant lethal effect at 93 or 311 mg/m3. An assessment of
dominant lethality was impossible at 933 mg/m3 due to
almost complete infertility. No positive control was in-
cluded.
In a sex-linked recessive lethal test in Drosophila
melanogaster reported by McGregor et al. (1983), the
results were inconclusive. Given the low absolute number
of mutants (low sample size), the inconsistency with which
various broods were affected, and the lack of a dose-
response relationship, a firm conclusion on the mutagen-
icity of 2-ME in D. melanogaster cannot be made.
8.7 Carcinogenicity
No carcinogenicity data are available on these glycol
ethers.
8.8 Mechanism of Toxicity - Mode of Action
The metabolic fate of 2-ME, 2-EE, and their acetates
has been well studied in both animals (Miller et al., 1983a;
Moss et al., 1985) and man (NIOSH, 1986, Groeseneken et
al., 1987, Veulemans, 1987a). Due to rapid hydrolysis of
the acetates to the monoalkyl glycol ether (see section
6), the putative toxic metabolite are the same for 2-ME or
2-MEA and for 2-EE or 2-EEA.
Both in vitro and in vivo studies have supported the
hypothesis that the toxic effects of 2-ME and 2-EE are
elicited by the toxicity of 2-methoxyacetaldehyde and
methoxyacetic acid (MAA) from 2-ME and ethoxyacetaldehyde
and ethoxyacetic acid (EAA) from 2-EE.
Gray et al. (1985) studied the effects in vitro of
2-ME, 2-EE, MAA, and EAA on mixed cultures of Sertoli and
germ cells from rat testes. At concentrations up to 50
mmol/litre medium, no morphological damage was noted for
2-ME or 2-EE, whereas administration of MAA or EAA at 2-10
mmol/litre led to degeneration of the pachytene and div-
iding spermatocytes, the probable target cells of the
parent ethers in vivo (Chapin et al., 1985b, Creasy et
al., 1985; Foster et al., 1986, Oudiz and Zenick, 1986).
Foster et al. (1986) reported that 2-methoxyacetaldehyde
(2-MALD), a possible metabolite of 2-ME, can produce
specific testicular effects in vitro and in vivo at doses
much lower than those required for MAA (0.2 and 0.5 mmol
per litre in vitro ). In more recent studies, Foster et
al. (1987) compared the in vivo and in vitro testicular
effects of MAA and EAA in rats. Single oral doses of MAA
and EAA (equimolar with 100, 250, or 500 mg 2-ME/kg body
weight) were administered and cell morphology was moni-
tored for 14 days. MAA produced damage to spermatocytes
undergoing meiotic maturation and division within 24 h of
treatment, whereas EAA produced these changes only at the
highest dose. Similar results were seen in vitro at a dose
(5 mmol/litre medium) equivalent to the steady state
plasma level of MAA after administration of 500 mg/kg.
Evidence that MAA is important in causing 2-ME terato-
genicity was reported by Yonemoto et al. (1984). MAA, but
not 2-ME, was found to interfere with organogenesis when
the two compounds were investigated using rat embryos in
culture.
Results from in vivo studies in mice (Welsch et al.,
1987; Sleet et al., 1988) and rats (Ritter et al., 1985)
further substantiate the hypothesis that monoalkyl glycol
ethers are metabolized to acetic acid metabolites via
alcohol dehydrogenase (ADH) and aldehyde dehydrogenases,
and that the ultimate toxin is actually the alkoxy acid
metabolite of the glycol ether or a further metabolite.
In mice (Sleet et al., 1987), 2-ME and MAA were equipotent
in producing teratogenic lesions leading to malformations
of the digits of all paws. Similar effects were noted by
Brown et al. (1984) and Ritter et al. (1985) in rats. Sub-
sequent work showed that the embryotoxic and teratogenic
effects of 2-ME in mice could be prevented by the concomi-
tant administration of 0.12 or 1.2 mmol/kg of 4-methyl-
pyrazole, an inhibitor of ADH, but that 4-methylpyrazole
was without effect after the administration of MAA (Sleet
et al., 1988).
9. EFFECTS ON MAN
Only limited information on the toxic effects of gly-
col ethers in humans is available. This information comes
largely from case reports in the early literature dealing
with accidental poisoning and/or workplace exposure. Such
reports provide only minimal information relating specific
effects to exposure levels. Only a few epidemiological
studies have been reported.
9.1 General Population Exposure
The widespread use of consumer products such as
paints, stains, inks, lacquers, surface coatings, and
home/industrial cleaners that contain one or more glycol
ethers means that broad segments of the general population
could be exposed. However, no reports quantifying such
exposures have been found nor any that describe skin irri-
tation or sensitization from exposures to glycol ethers in
humans, despite widespread exposure.
9.1.1 Poisoning reports
A 44-year-old man consumed 400 ml of 2-ME mixed with
brandy and died without regaining consciousness 5 h after
he was admitted to hospital in a comatose state (Young &
Woolner, 1946). Postmortem findings included acute
haemorrhagic gastritis, fatty degeneration of the liver,
and black coloured kidneys with marked degenerative
changes in the renal tubules.
Two non-fatal cases of poisoning by 2-ME in men aged
41 and 23 were reported by Nitter-Hauge (1970). Both
patients consumed 100 ml 2-ME. In addition to nervous
system disorders (agitation, confusion), the predominant
clinical features were nausea, cyanosis, hyperventilation,
slight tachycardia, and metabolic acidosis. In one patient
there were suggestions of moderate kidney failure. No
evidence of liver damage was found and both patients re-
covered within 4 weeks.
Ingestion of approximately 40 ml 2-EE by a 44-year-old
women led to vertigo, unconsciousness, effects on the
central nervous system, and metabolic acidosis (Fucik,
1969). She recovered within 44 days from all symptoms,
including kidney insufficiency and signs of liver damage,
although neurasthenia was still evident at that time.
9.2 Occupational Exposure
9.2.1 Repeated exposure
Reports on the effects of glycol ethers on humans fol-
lowing repeated exposure are available from occupational
studies. Early reports provided evidence that the repeated
exposure of humans to solvents containing 2-ME resulted in
such effects as anaemia, leucopenia, lethargy, general
weakness, dizziness, ataxia, and unequal or exaggerated
reflexes. A diagnosis of toxic encephalopathy was made by
Donley (1936) and Parson & Parsons (1938), but no reliable
estimation of exposure to 2-ME was given. Greenburg et al.
(1938) reported levels of 2-ME between 78 and 236 mg per
m3 in one manufacturing plant where 19 male subjects
(ages 16-26) were examined. The median duration of ex-
posure was 5 weeks and the maximum was 2 years. Signs of
nervous system dysfunction (fatigue, hand tremor, and
lethargy) were found, but no clear relationship between
the incidence of symptoms and the duration of exposure
could be ascertained in this small population. No control
group was included in this study.
The development of macrocytic anaemia has been de-
scribed in a case report of a worker exposed to 2-ME and
other solvents during microfilm manufacturing. Exposure
to 2-ME averaged 109 mg/m3 (35 ppm) (56-180 mg/m3, 18-
58 ppm), to methyl ethyl ketone 1-5 ppm, and to propylene
glycol monomethyl ether 4-13 ppm for a duration of 20
months. Follow-up analyses conducted 1 month after termin-
ating exposure indicated a return to normal limits for all
haematological parameters studied (Cohen, 1984).
The haematological effects of glycol ethers on humans
have also been documented in workplace surveys. Obvious
symptoms are not always manifested in peripheral blood.
The evaluation in a printing plant of seven workers with
dermal and respiratory exposure to dipropylene glycol
monomethyl ether, ethylene glycol monoethyl ether, and a
range of aliphatic, aromatic, and halogenated hydrocarbons
used for offset and multicolour printing revealed no
alteration in the peripheral blood (Cullen et al., 1983).
Although there was evidence of bone marrow injury in three
of the seven workers, no direct relationship could be
drawn to glycol ether exposure. The exposure to other sol-
vents by this relatively small group of workers makes more
definitive conclusions impossible. Bone marrow toxicity
had earlier been reported in two case studies of workers
exposed dermally to 2-ME (Ohi & Wegmann, 1978).
Denkhaus et al. (1986) investigated the cellular im-
mune response of nine workers aged 25-58 years of age,
heavily exposed to mixtures of organic solvents (including
2-ME and 2-EE) for 8-35 (mean 18.9) years, by the analysis
of subpopulations of peripheral blood lymphocytes. A con-
trol group of matched healthy controls was included. The
mean exposures to 2-ME and 2-EE were 6.1 mg/m3 (peak
150 mg/m3) and 4.8 mg/m3 (peak 53 mg/m3), respect-
ively. Based on samples taken during working shifts, mean
blood levels were 40.1 µg 2-ME/100 ml (peak: 965 µg/100
ml) and 2.0 µg 2-EE/100 ml (peak: 92.7 µg/100 ml). At
these levels (and in the presence of other solvents such
as 1-butanol, isobutanol, 2-butoxybutanol, toluene, m-xylene,
2-butanone, and 2-hexanone), the exposed workers had de-
creased levels of helper T-cells, and increased levels of
natural killer cells and human B-lymphocytes. However,
the levels of suppressor cells were normal. The authors
noted that similar changes in lymphocyte subpopulations
are found in states of general immunodeficiency and in
immunogenetic forms of aplastic anaemia.
9.2.2 Epidemiological studies
In a cross-sectional study, male employees in a plant
manufacturing 2-ME in the USA were examined for haema-
tological effects and possible sterility (Cook et al.,
1982). Personal air sampling indicated levels of exposure
of 1.3 mg/m3 or less in one working location and 19-26 mg
per m3 in another. Forty workers, engaged in production
and distribution of 2-ME (75% of the possible total popu-
lation) and 25 controls (57% of total eligible population)
were examined for haematological effects, and six exposed
and nine control workers participated in an examination of
testis size, semen abnormalities, and levels of serum
follicle-stimulating hormone (FSH). No increase in the
incidence of leucopenia or anaemia was noted, but de-
creases in white cell count and testicular size were noted
in the most highly exposed workers. These results were not
statistically significant but correlate with known effects
in experimental animals.
A cross-sectional evaluation of semen quality (sperm
concentration, pH, volume, viability, motility, velocity,
and morphology) was carried out in 37 workers exposed to
2-EE used as a resin solvent in a metal casting plant
(NIOSH, 1986). The study population represented 50% of
the exposed workforce. A control group of 38 workers from
other locations in the plant was included. Full-shift
breathing zone airborne exposure to 2-EE ranged from non-
detectable to 88 mg/m3. Dermal exposure was indicated by
urinary excretion of EAA at levels from non-detectable to
163 mg/g creatinine. Workers exposed to 2-EE had signifi-
cantly lower average sperm counts than controls (113
versus 154 million per ejaculate), although both exposed
and control groups had lower sperm counts than those found
in other occupational groups. The two groups studied did
not differ significantly with respect to other semen
characteristics or testicular size.
The effect of 2-ME and 2-EE exposure on male repro-
ductive factors and blood has been studied in shipyard
painters by Sparer et al. (1988a,b), Welch et al. (1988),
and Welch & Cullen (1988). Airborne levels (time-weighted
average: TWA) were determined in 102 samples over six
workshifts and were 0-80.5 mg 2-EE/m3 (mean 9.9 and
median level 4.4 mg/m3), and 0-17.7 mg 2-ME/m3 (mean
2.6 and median 1.4 mg/m3). Given the methods used by
Sparer et al. (1988a,b) the authors concluded these were
probably underestimates of exposure, particularly in the
mixing room and inside large tanks. Urinary excretion of
EAA indicated dermal exposure as well as airborne ex-
posure in some workers. In 73 painters, from a total
population of 153, an increased prevalence of oligospermia
and azoospermia was noted and there was an increased odds
ratio for a lower sperm count per ejaculate in exposed
workers as compared to the 40 controls studied (Welch et
al., 1988). In addition, when 94 painters from the same
population were examined for haematological effects of
chemical exposures, 10% were found to be anaemic and 5%
exhibited granulocytopenia (Welch & Cullen, 1988). None of
these effects were noted in the 55 control subjects exam-
ined. The reproductive effects observed were in agreement
with those reported previously by NIOSH (1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure
Many people may be exposed to 2-methoxyethanol (2-ME),
2-ethoxyethanol (2-EE), and their acetates (2-MEA and 2-
EEA), at levels comparable to industrial levels, through
the use of consumer and trade products. On the other
hand, exposure through food, water, or the ambient air is
probably negligible. This inference is based only on the
physical and chemical properties of these compounds and
evidence of rapid environmental degradation.
Significant occupational exposure may occur both
through inhalation and skin absorbtion. Limited measure-
ments of air levels in the workplace range from less than
0.1 mg/m3 to more than 150 mg/m3. However, the avail-
able monitoring is quite limited and large variations may
occur both within and among industries. Because of the
potential for skin absorption, air monitoring alone may
underestimate total exposure. Total uptake may be best
estimated from biological monitoring. Occupations where
extensive exposure is possible include, for example,
painting, printing, and cleaning, but it should be borne
in mind that there are many other occupations where these
compounds are also used and where exposure is of concern.
10.1.2. Health Effects
The major effects of concern for humans are develop-
mental, testicular, and haematological toxicity. These
are demonstrated by extensive and consistent data in ani-
mals and some human data. All these effects can be caused
by both short-term and longer-term exposures. In experi-
mental animals, very high repeated exposure to 2-ME and
2-EE (over 930 and 1450 mg/m3, respectively) produces
neurobehavioural, hepatic, and renal toxic effects. These
are also observed in human poisoning situations.
These four glycol ethers exhibit very similar testicu-
lar and developmental toxicities, in all species evalu-
ated, and by all routes of exposure that have been em-
ployed (inhalation, dermal, and oral). Mechanistic studies
indicate that for both testicular and developmental ef-
fects, metabolism to the alkoxyacetic acid derivative is a
necessary activation step. Metabolism takes place via the
alcohol dehydrogenase system that is common to humans and
laboratory animals. The toxic metabolites, methoxyacetic
acid (MAA) and ethoxyacetic acid (EAA), have been detected
in the urine of humans exposed to these solvents. The con-
sistency of response across laboratory animal species
studied, combined with the similarity of metabolism in
humans, makes it clear that humans should be presumed to
be subject to the testicular and developmental effects of
these glycol ethers. Available data on the excretion of
alkoxyacetic acids by humans indicate prolonged retention
compared with that in laboratory animals, suggesting that
humans may be more sensitive than the most sensitive ex-
perimental animal species. The rapid skin absorbtion of
these compounds is of particular concern. Teratogenicity
and other developmental effects have been observed follow-
ing the application of 2-ME, 2-EE, and 2-EEA to the intact
skin of rats.
Testicular damage has been observed in the rat, mouse,
and rabbit following exposure to these glycol ethers
through both the inhalation and the oral route. Single in-
halation exposures of rats to 1944 mg 2-ME/m3 or more for
4 h and repeated exposure to 933 mg 2-ME/m3 or more for
13 weeks resulted in histological evidence of testicular
damage. The NOEL for acute exposure was 933 mg/m3 and for
repeated exposure was 311 mg/m3. The mouse appeared
somewhat less sensitive, the NOEL for repeated exposure
being 933 mg/m3. However, the rabbit was more sensitive,
with marked testicular change being seen after repeated
exposure to 311 mg/m3 and a marginal effect (one in five
rabbits affected) being seen at 93 mg/m3. Similar
effects have been seen following oral exposure of rats to
2-ME, with short-term (including single dose) exposure to
100 mg/kg producing testicular damage. The NOEL in a sub-
acute (11 days) study was 50 mg/kg. 2-EE is somewhat less
potent with regard to its testicular toxicity than 2-ME;
effects were only seen at dose levels of 500 mg/kg or
more, the NOEL being 250 mg/kg.
Evidence from studies in men exposed occupationally
to 2-ME and 2-EE is consistent with the animal data and
indicates that these glycol ethers can produce testicular
toxicity in humans. Epidemiological studies on small
groups of workers exposed to 2-EE in a metal casting
plant and in shipyard painters exposed to both 2-ME and
2-EE consistently revealed an increased incidence of re-
duced sperm counts. Data on exposure levels were limited,
but there was evidence, in each case, of dermal exposure
as well as exposure via inhalation.
Developmental toxicity has been observed in the rat,
mouse, rabbit, and monkey following exposure to these
glycol ethers using dermal, oral, and inhalation routes.
Twelve daily applications of undiluted 2-ME to the shaved
skin of pregnant rats (with 6-h occlusion) was lethal,
while ten open applications of 2-EE (1.0 ml/day) or 2-EEA
(1.4 ml/day) were teratogenic but not maternally toxic.
Twelve occluded applications of 10% 2-ME in saline proved
developmentally toxic, the NOEL in this study being 3%
2-ME. No-observed-effect levels have not been demonstrated
following repeated oral dosing of pregnant animals with
2-ME. The lowest-observed-effect level (LOEL) for oral
administration of 2-ME was 31.25 mg/kg per day for mice,
25 mg/kg per day for rats, and 0.16 mmol/kg per day for
monkeys. Single-dose studies have been reported only for
2-ME in mice, the results being that on gestation day 11
(the most sensitive day) the NOEL was 100 mg/kg and the
LOEL was 175 mg/kg. Both 2-EE and 2-EEA have been evalu-
ated by inhalation exposure in rats and rabbits. Rats were
exposed to 2-EE in two studies, resulting in teratogenic
effects (743 mg/m3 for 7 h/day on gestation days 1-19)
or fetotoxic effects (184 and 920 mg/m3 for 6 h/day on
gestation days 6-15). In the latter study, the NOEL was
37 mg/m3. Rabbits exposed to 2-EE also exhibited
teratogenic effects (589 mg/m3 for 7 h/day on gestation
days 1-18) or fetotoxic effects (644 mg/m3 for 6 h/day
on gestation days 6-18). In the latter study, the NOEL was
184 mg/m3. When rabbits were exposed to 2-EEA for
6 h/day on gestation days 6-18, teratogenic effects were
seen at 2160 mg/m3 in one study and 1620 mg/m3 in
another. Fetotoxicity appeared in both studies at 540 mg
per m3 and the lowest exposure level in each study (135
and 270 mg/m3, respectively) was the NOEL. Rats exposed
to 2-EEA by inhalation for 6 h/day on gestation days 6-15
showed the same pattern of response: teratogenic effects
at 1620 mg/m3, fetotoxicity at 1080 mg/m3, and no ef-
fect at 270 mg/m3.
Thus developmental toxicity has been observed in all
species (mice, rats, and rabbits) exposed to 2-ME at 156
mg/m3 or more. The NOEL for all three species was 31 mg
per m3. Behavioural and neurochemical alterations in
rats followed in utero exposure at 78 mg/m3, with no
NOEL being identified.
2-EE and 2-EEA were slightly less potent. Develop-
mental effects in rats and rabbits followed all 2-EE ex-
posures at 368 mg/m3 or more. Slight developmental ef-
fects were seen in rats exposed at 184 mg 2-EE/m3, but
37 mg/m3 was a clear NOEL. For 2-EEA, the NOEL was 170 mg
per m3 for both rats and rabbits.
Haematological effects from single acute dose ex-
posures have been observed in animals and in human poison-
ings. Repeated inhalation exposure of the most sensitive
species, the rabbit, to 2-ME for 13 weeks, 5 times per
week, yielded a NOEL of 93 mg/m3. Repeat doses of 2-ME
also cause haematological toxicity in mice, rabbits, dogs,
hamsters, and guinea-pigs. 2-EE is less potent in causing
haematological effects than 2-ME. The NOEL for haemato-
logical effects in rats and rabbits exposed to 2-EE for 13
weeks, 5 times per week for 6 h/day, is 368 mg/m3. Dogs
and mice also show haematological effects from repeated
2-EE exposure at higher levels. Exposure to the acetate
esters of 2-EE and 2-ME would be expected to cause similar
effects at similar exposure levels, but there are too few
data on exposure and haematological effects of these com-
pounds to determine under what conditions single human ex-
posures will lead to haematological effects.
Industrial exposure levels have been reported at or
near the NOEL for haematological effects in animals after
repeated doses of both 2-ME and 2-EE. This fact, together
with the probable greater sensitivity of humans and the
expected accumulation of metabolites in human blood, indi-
cates that haematological effects may well occur from in-
dustrial and consumer exposure. This has been confirmed by
the haematological effects reported in some of the limited
number of studies of industrial workers with repeated 2-EE
and/or 2-ME exposure.
10.2. Evaluation of Effects on the Environment
Environmental exposure to these glycol ethers can
arise as a consequence of their direct release into the
atmosphere from their use as evaporative solvents. Dis-
charges to the land and water from accidental release may
also result in environmental exposure. Accumulation in
soil and surface water could only occur in the absence of
degradation. However, these glycol ethers are rapidly
degraded by chemical and biological processes and accumu-
lation is not expected. 2-MEA and 2-EEA are also expected
to hydrolyse readily and subsequently biodegrade under
aerobic conditions. However, contamination of anaerobic
soils and aquifers remains a potential problem, but this
condition is expected to be transitory resulting in negli-
gible risk.
Both 2-ME and 2-EE demonstrate low toxicity to micro-
oganisms and aquatic species. The glycol ether acetates,
however, are far more acutely toxic. No data exists to
ascertain the potential for adverse effects from long-term
exposure to environmental species.
11. RECOMMENDATIONS
11.1. Health Protection
1. Alternative less toxic solvents should be identified
to replace 2-methoxyethanol, 2-ethoxyethanol, and their
esters, particularly in consumer products. Assessment of
the effects of other ethylene glycol ethers is also of
particular importance, because some may cause effects
similar to the four glycol ethers evaluated here.
2. In view of the known toxic effects of these glycol
ethers, authorities should seriously consider appropriate
strategies to alert users of these chemicals to their
hazards, particularly those arising from dermal ex-
posures.
3. In view of recent toxicological data and the potential
for considerable dermal absorption of these glycol ethers,
national occupational exposure limits should be recon-
sidered to insure that the total daily dose to workers by
all routes of administration does not pose an undue risk
to health.
4. Single dose effects occur in animals at fairly high
exposure levels. Prudent use of these compounds (attention
to personal hygiene, suitable protective devices, and
adequate ventilation) is recommended to reduce the health
risks. The data indicate that more extensive protection
may be required to prevent developmental effects, as well
as effects on blood and testis, from repeated exposure.
11.2. Further Research
1. In view of the implication of methoxyacetic acid (MAA)
and ethoxyacetic acid (EAA) (the principal identified
metabolites of 2-ME, 2-EE, and of their esters) in the
toxicity to the male reproductive system, their mechanism
of action should be investigated. If it transpires that
MAA and EAA are not the primary agents concerned, these
should be identified and their mechanism of action
elucidated.
2. These four glycol ethers are known to have both
haematological effects and male reproductive effects
(sperm count reduction). The available data, although
limited, appear to suggest that the two effects become
evident at similar dose levels. The mechanism of action
should be investigated for both organ systems, and
haematological effects and sperm counts should be examined
in parallel to determine if haematological changes offer
warning signs for other effects of these compounds.
3. Air monitoring alone is not sufficient to assure low
exposure. Biological monitoring can aid in detecting
failures in protective measures. At present the
relationship between biological indicators of exposure,
total body uptake, and observed health effects have not
been sufficiently established. Further work is necessary
to provide the basis for using biological monitoring in
determining safe exposures.
4. Epidemiological studies and/or targeted health sur-
veillance in populations subject to high exposure to these
glycol ethers should be designed in order to estimate
exposure-effect relationships for the purpose of determin-
ing safe exposures, providing that overall exposure can be
properly and adequately evaluated by appropriate environ-
mental and biological monitoring.
5. The possibility that these compounds may cause effects
on the female gonads should be investigated through
multigeneration reproductive studies in animals.
6. Available research indicates that humans may metab-
olize these glycol ethers to the corresponding alkoxy-
acetic acids to a greater extent than rats, and that the
half-life for urinary excretion of these toxic metabolites
is about four times longer in humans than in rats.
Furthermore, rats conjugate a large portion of the acid
metabolites, whereas humans do not. These differences
might contribute to the relatively higher sensitivity of
humans to these glycol ethers. Detailed knowledge of the
metabolism and excretion kinetics would improve the
ability to predict safe exposure levels.
7. The results obtained from short-term (13-week) studies
indicate effects on various organ systems. However, there
have been no studies of sufficient duration that would
allow the reversibility of such effects to be assessed.
Therefore, it is suggested that stop-studies be undertaken
in which experimental animals are exposed to these glycol
ethers for at least 13 weeks, followed by a suitable
recovery period. Important physiological parameters
should then be evaluated in order to determine whether or
not these effects are transient.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Regulatory standards for 2-ME, 2-MEA, and 2-EE, estab-
lished by national bodies in different countries and the
European Economic Community, are summarized in the data
profile of the International Register of Potentially Toxic
Chemicals (IRPTC, 1987).
REFERENCES
ANDERSON, D., BRINKWORTH, M.H., JENKINSON, P.C., CLODE, S.A., CREASY,
D.M., & GANGOLLI, S.D. (1987) Effect of ethylene glycol monomethyl ether
on spermatogenesis, dominant lethality, and F1 abnormalities in the rat
and the mouse after treatment of F0 males. Teratog. Carcinog. Mutagen.,
7:141-158.
ANDREW, F.D. & HARDIN, B.D. (1984) Developmental effects after inhalation
exposure of gravid rabbits and rats to ethylene glycol monoethyl ether.
Environ. Health Perspect., 57: 13-23.
BAILEY, H.C., LIU, D.H.W., & JAVITZ, H.A. (1985) Time/toxicity
relationships in short-term static, dynamic, and plug-flow bioassays. In:
Bahner, R.C. & Hansen, D.J., ed. Aquatic toxicology and hazard assessment:
Eighth Symposium, Philadelphia, American Society for Testing and
Materials, pp. 193-212 (ASTM STP 891).
BARBEE, S.V., TERRILL, J.B., DESOUSA, D.J., & CONAWAY, C.C. (1984)
Subchronic inhalation toxicology of ethylene glycol monoethyl ether in the
rat and rabbit. Environ. Health Perspect., 57: 157-163.
BASLER, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by the
micronucleus test. Mutat. Res., 174: 11-13.
BOTTA, D., CASTELLANI PIRRI, L., & MANTICA, E. (1984) Ground water
pollution by organic solvents and their microbial degradation products,
Luxembourg, Commission of the European Communities, pp. 261-275 (EUR-
8518).
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) Aeruginosa and
Scenedesmus quadricauda. Mitt. intern. Ver. theor. angew. Limnol., 21:
275-284.
BROWN, N.A., HOLT, D., & WEBB, M. (1984) The teratogenicity of
methoxyacetic acid in the rat. Toxicol. Lett., 22: 93-100.
CARPENTER, C.P., POZZANI, V.C., WEIL, C.S., NAIR, J.H., KECK, G.A., &
SMITH, H.F. (1956) The toxicity of butyl cellosolve solvent. Arch. ind.
Health, 14: 114-131.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., & LAMB, J.C. (1985a) Effects of
ethylene glycol monomethyl ether (EGME) on mating performance and
epididymal sperm parameters in F344 rats. Fundam. appl. Toxicol., 5: 182-
189.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., SWAISGOOD, R.R., & LAMB, J.S.
(1985b) The recovery of the testis over 8 weeks after short-term dosing
with ethylene glycol monomethyl ether: histology, cell specific enzymes,
and rete testis fluid protein. Fundam. appl. Toxicol., 5: 515-525.
CHEEVER, K.L., PLOTNICK, H.B., RICHARDS, D.E., & WEIGEL, W.W. (1984)
Metabolism and excretion of 2-ethoxyethanol in the adult male rat.
Environ. Health Perspect., 57: 241-248.
COHEN, R. (1984) Reversible subacute ethylene glycol monomethyl ether
toxicity associated with microfilm production: a case report. Am. J. ind.
Med., 6: 441-446.
COOK, R.R., BODNER, K.M., KOLESAR, R.C., VAN PEENEN, P.F.D., DICKSON,
G.S., & FLANAGAN, K. (1982) A cross-sectional study of ethylene glycol
monomethyl ether process employees. Arch. environ. Health, 37: 346-351.
CREASY, D.M. & FOSTER, P.M.D. (1984) The morphological development of
glycol ether-induced testicular atrophy in the rat. Exp. mol. Pathol., 40:
169-176.
CREASY, D.M., FLYNN, J.C., GRAY, T.J.B., & BUTLER, W.H. (1985) A
quantitative study of stage-specific spermatocyte damage following
administration of ethylene glycol monomethyl ether in the rat. Exp. mol.
Pathol., 43: 321-336.
CREASY, D.M., JONES, H.B., BEECH, L.M., & GRAY, T.J.B. (1986) The effects
of two testicular toxins on the ultrastructural morphology of mixed
cultures of Sertoli and germ cells: a comparison with in vivo effects.
Food chem. Toxicol., 24: 655-656.
CULLEN, M.R., RADO, T., WALDRON, J.A., SPARER, J., & WELCH, L.S. (1983)
Bone marrow injury in lithographers exposed to glycol ethers and organic
solvents used in multicolor offset and ultraviolet curing printing
processes. Arch. environ. Health, 38(6): 347-354.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J.
hazard. Mater., 1: 303-318.
DE DELBARRE, F., KAHAN, A., DE GERY, A., & KONRAD, K. (1980) Action
immunomodulatrice du méthoxy-2 éthanol et de dérivés homologues chez le
rat. C.R. Acad. Sci. Paris, 291: 215-218.
DENKHAUS, W., STELDERN, D., BOTZENHARDT, U., & KONIETZKO, H. (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int. Arch. occup.
environ. Health, 57: 109-115.
DOE, J.E. (1984a) Ethylene glycol monoethyl ether and ethylene glycol
monoethyl ether acetate teratology studies. Environ. Health Perspect., 57:
33-41.
DOE, J.E. (1984b) Further studies on the toxicology of the glycol ethers
with emphasis on rapid screening and hazard assessment. Environ. Health
Perspect., 57: 199-206.
DOE, J.E., SAMUELS, D.M., TINSTON, D.J., & WICKRAMARATNE, G.A.D. (1983)
Comparative aspects of the reproductive toxicology by inhalation in rats
of ethylene glycol monomethyl ether and propylene glycol monomethyl ether.
Toxicol. appl. Pharmacol., 69: 43-47.
DONLEY, D.E. (1936) Toxic encephalopathy and volatile solvents in
industry. J. ind. Hyg. Toxicol., 18: 571-577.
DUGARD, P.H., WALKER, M., MAWDSLEY, S.J., & SCOTT, R.C. (1984) Absorption
of some glycol ethers through human skin in vitro . Env. Health Perspect.,
57: 193-197.
ECETOC (1985) The toxicology of glycol ethers and its relevance to man,
Brussels, European Chemical Industry Ecology and Toxicology Centre
(Technical Report No. 17).
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., THOMAS, L.V., COOK, M.W., &
GANGOLLI, S.D. (1983) Testicular toxicity of ethylene glycol monomethyl
and monoethyl ethers in the rat. Toxicol. appl. Pharmacol., 69: 385-399.
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., & GRAY, T.J.B. (1984)
Testicular toxicity produced by ethylene glycol monomethyl and monoethyl
ethers in the rat. Environ. Health Perspect., 57: 207.
FOSTER, P.M.D., BLACKBURN, D.M., MOORE, R.B., & LLOYD, S.C. (1986)
Testicular toxicity of 2-methoxyacetaldehyde, a possible metabolite of
ethylene glycol monomethyl ether, in the rat. Toxicol. Lett., 32: 73-80.
FOSTER, P.M.D., LLOYD, S.C., & BLACKBURN, D.M. (1987) Comparison of the in
vivo and in vitro testicular effects produced by methoxy-, ethoxy-, and n-
butoxy acetic acids in the rat. Toxicology, 43: 17-30.
FUCIK, J. (1969) Poisoning by ethylene glycol monoethyl ether. Prac. Lek.,
21: 116-118.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO, J.,
MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese hamster
ovary cells: evaluations of 108 chemicals. Environ. mol. Mutagen., 10(10):
1-175.
GOLDBERG, M.E., HARN, C., & SMYTH, H.F. (1962) Toxicological implication
of altered behaviour induced by an industrial vapour. Toxicol. appl.
Pharmacol., 4: 148-164.
GRANT, D., SULSH, S., JONES, H.B., GANGOLLI, S.D., & BUTLER, W.H. (1985)
Acute toxicity and recovery in the hemopoietic system of rats after
treatment with ethylene glycol monomethyl and monobutyl ethers. Toxicol.
appl. Pharmacol., 77: 187-200.
GRAY, T.J.B., MOSS, E.J., CREASY, D.M., & GANGOLLI, S.D. (1985) Studies on
the toxicity of some glycol ethers and alkoxyacetic acids in primary
testicular cell cultures. Toxicol. appl. Pharmacol., 79: 490-501.
GREENBURG, L., MAYERS, M.R., GOLDWATER, L.J., BURKE, W.J., & MOSCOWITZ, S.
(1938) Health hazards in the manufacture of "fused collars". 1. Exposure
to ethylene glycol monomethyl ether. J. ind. Hyg. Toxicol., 20: 134-147.
GREENE, J.A., SLEET, R.B., MORGAN, K.T., & WELSCH, F. (1987) Cytotoxic
effects of ethylene glycol monomethyl ether in the forelimb bud of the
mouse embryo. Teratology, 36: 23-34.
GROESENEKEN, D., VAN VLEM, E., VEULEMANS, H., & MASSCHELEIN, R. (1986a)
Gas chromatographic determination of methoxyacetic and ethoxyacetic acid
in urine. Br. J. ind. Med., 43: 62-65.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986b) Respiratory
uptake and elimination of ethylene glycol monoethyl ether after
experimental human exposure. Br. J. ind. Med., 43: 544-549.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986c) Urinary
excretion of ethoxyacetic acid after experimental human exposure to
ethylene glycol monoethyl ether. Br. J. ind. Med., 43: 615-619.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1987)
Ethoxyacetic acid: a metabolite of ethylene glycol monoethyl ether acetate
in man. Br. J. ind. Med., 44: 488-493.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1988)
Comparative urinary excretion of ethoxyacetic acid in man and rat after
single low doses of ethylene glycol monoethyl ether. Toxicol. Lett., 41:
57-68.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989a)
Experimental human exposure to ethylene glycol monomethyl ether. Int.
Arch. occup. environ. Health, 61: 243-247.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989b) An
improved method for the determination in urine of alkoxyacetic acids. Int.
Arch. occup. environ. Health, 61: 249-254.
GUEST, D., HAMILTON, M.L., DEISINGER, P.J., & DIVINCENZO, G.D. (1984)
Pulmonary and percutaneous absorption of 2-propoxyethyl acetate and 2-
ethoxyethyl acetate in beagle dogs. Environ. Health Perspect., 57: 177-
183.
HAMLIN, J.W., HUDSON, B., SHEEN, A.D., & SAUNDERS, K.J. (1982) The
measurement of glycol ether levels in the workplace. Polym. Paint Colour
J., October 13: 61-63.
HANLEY, T.R., Jr, YANO, B.L., NITSCHKE, K.D., & JOHN, J.A. (1984)
Comparison of the teratogenic potential of inhaled ethylene glycol
monomethyl ether in rats, mice, and rabbits. Toxicol. appl. Pharmacol.,
75: 409-422.
HARDIN, B.D. (1983) Reproductive toxicity of the glycol ethers.
Toxicology, 27: 91-102.
HARDIN, B.D., NIEMEIER, R.W., SMITH, R.J., KUCZUK, M.H., MATHINOS, P.R., &
WEAVER, T.F. (1982) Teratogenicity of 2-ethoxyethanol by dermal
application. Drug chem. Toxicol., 5(3): 277-294.
HARDIN, B.D., GOAD, P.T., & BURG, J.R. (1984) Developmental toxicity of
four glycol ethers applied cutaneously to rats. Environ. Health Perspect.,
57: 69-74.
HEALTH AND SAFETY EXECUTIVE (1988) Methods for the determination of
hazardous substances: Glycol ethers and glycol acetate vapours in air,
London, UK Health and Safety Executive, pp. 1-7 (MDHS-23).
HERMENS, J., CANTON, H., JANSSEN, P., & DEJONG, R. (1984) Quantitative
structure-activity relationships and toxicity of mixtures of chemicals
with anaesthetic potency: Acute lethal and sublethal toxicity to Daphnia
magna. Aquat. Toxicol., 5: 143-154.
HORTON, V.L., SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1985)
Developmental phase-specific and dose-related teratogenic effects of
ethylene glycol monomethyl ether in CD-1 mice. Toxicol. appl. Pharmacol.,
80: 108-118.
HOUSE, R.V., LAUER, L.D., MURRAY, M.J., WARD, E.C., & DEAN, J.H. (1985)
Immunological studies in B6C3F1 mice following exposure to ethylene glycol
monomethyl ether and its principal metabolite methoxyacetic acid. Toxicol.
appl. Pharmacol., 77: 358-362.
HURTT, M.E. & ZENICK, H. (1986) Decreasing epididymal sperm reserves
enhances the detection of ethoxyethanol-induced spermatotoxicity. Fundam.
appl. Toxicol., 7: 348-353.
IRPTC (1987) IRPTC legal file 1986 - Volume 1, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
JUHNKE, I. & LUDEMANN, D. (1978) The results obtained with the Golden Orfe
test during the examination of 200 chemical compounds for acute fish
toxicity. Wasser Abwasser Forsch., 11: 161-164.
KAREL, L., LANDING, B.H., & HARVEY, T.S. (1947) The intraperitoneal
toxicity of some glycols, glycol ethers, glycol esters and phthalates in
mice. J. Pharmacol. exp. Ther., 90: 338-347.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 9 - Ethanol,
3rd ed., New York, Chichester, Brisbane, Toronto, John Wiley & Sons.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 11 - Glycols
(Ethylene and Propylene), 3rd ed., New York, Chichester, Brisbane,
Toronto, John Wiley & Sons.
LAILLER, J., PLAZONNET, B., LE DOUAREC, J.C., & GONIN, M.J. (1975)
Evaluation of ocular irriation in the rabbit: Development of an objective
method of studying eye irritation. Proc. Eur. Soc. Toxicol., 17: 336-350.
LAMB, J.C., DUSHYANT, K.G., RUSSELL, V.S., HAMMEL, L., & SABHARNAL, P.S.
(1984) Reproductive toxicity of ethylene glycol monoethyl ether tested by
continuous breeding of CD-1 mice. Environ. Health Perspect., 57: 85-90.
LAUG, E.P., CALVERY, H.O., MORRIS, H.J., & WOODARD, G. (1939) The
toxicology of some glycols and derivatives. J. ind. Hyg. Toxicol., 21:
173-201.
LEE, K.H. & WONG, H.A. (1979) Toxic effects of some alcohol and ethylene
glycol derivatives on Cladosporium resinae. Appl. environ. Microbiol., 38:
24-28.
MCGREGOR, D.B., WILLINS, M.J., MCDONALD, P., HOLMSTROM, M., MCDONALD, D.,
& NIEMEIER, R.W. (1983) Genetic effects of 2-methoxyethanol and bis(2-
methoxyethyl)ether. Toxicol. appl. Pharmacol., 70: 303-316.
MELLAN, I. (1977) Glycol ethers and esters. In: Industrial Solvents
Handbook, 2nd ed., Park Ridge, New Jersey, Noyes Data Corporation, pp.
346-399, 513-551.
MILLER, R.R., AYERS, J.A., CALHOUN, L.L., YOUNG, J.T., & MCKENNA, M.J.
(1981) Comparative short-term inhalation toxicity of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in rats and mice.
Toxicol. appl. Pharmacol., 61: 368-377.
MILLER, R.R., CARREON, R.E., YOUNG, J.T., & MCKENNA, M.J. (1982) Toxicity
of methoxyacetate acid in rats. Fundam. appl. Toxicol., 2: 155-160.
MILLER, R.R., HERMANN, E.A., LANGVARDT, P.W., MCKENNA, M.J., & SCHWETZ,
B.A. (1983a) Comparative metabolism and disposition of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in male rats.
Toxicol. appl. Pharmacol., 67: 229-237.
MILLER, R.R., AYRES, J.A., YOUNG, J.T., & MCKENNA, M.J. (1983b) Ethylene
glycol monomethyl ether. I. Subchronic vapor inhalation study with rats
and rabbits. Fundam. appl. Toxicol., 3: 49-54.
MOSS, E.J., THOMAS, L.V., COOK, M.W., WALTERS, D.C., FOSTER, P.M.D.,
CREASY, D.M., & GRAY, T.J.B. (1985) The role of metabolism in 2-
methoxyethanol-induced testicular toxicity. Toxicol. appl. Pharmacol., 79:
480-489.
NAGANO, K., NAKAYAMA, E., KOGANA, M., DOBAYASKI, H., ADACHI, H., & YAMADA,
T. (1979) Testicular atrophy of mice induced by ethylene glycol monoalkyl
ethers. Jpn. J. ind. Health, 21: 29-35.
NAGANO, K., NAKAYAMA, E., DOBAYASKI, H., YAMADA, T., ADACHI, H.,
NISHIZAWA, T., OZAWA, H., NAKAICHI, M., OKUKDA, H., MINAMI, K., &
YAMAZAKI, K. (1981) Embryotoxic effects of ethylene glycol monomethyl
ether in mice. Toxicology, 20: 335-343.
NAGANO, K., NAKAYAMA, E., OOBAYASHI, H., NISHIZAWA, T., OKUDA, H., &
YAMAZAKI, K. (1984) Experimental studies on toxicity of ethylene glycol
alkyl ethers in Japan. Environ. Health Perspect., 57: 75-84.
NAKAAKI, K., FUKABORI, S., & TADA, O. (1980) An experimental study on
percutaneous absorption of some organic solvents. J. Sci. Labour, 56(12):
1-9.
NEIHOF, R.A. & BAILEY, C.A. (1978) Biocidal properties of anti-icing
additives for aircraft fuels. Appl. environ. Microbiol., 35: 698-703.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., TAYLOR, B.J., & HORNUNG,
R.W. (1981) Ethoxyethanol behavioral teratology in rats. Neuro-
toxicology., 2(2): 231-249.
NELSON, B.K., BRIGHTWELL, W.S., & SETZER, J.V. (1982) Prenatal
interactions between ethanol and the industrial solvent 2-ethoxyethanol in
rats: maternal and behavioral teratogenic effects. Neurobehav. Toxicol.
Teratol., 4: 387-394.
NELSON, B.K., BRIGHTWELL, W.S., BURG, J.R., & MASSARI, V.J. (1984a)
Behavioral and neurochemical alterations in the offspring of rats after
maternal or paternal inhalation exposure to the industrial solvent 2-
methoxyethanol. Pharmacol. Biochem. Behav., 20: 269-279.
NELSON, B.K., SETZER, J.V., BRIGHTWELL, W.S., MATHINOS, P.R., KUCZUR,
M.H., WEAVER, T.E., & GOAD, P.T. (1984b) Comparative inhalation
teratogenicity of four glycol ether solvents and amino derivative in rats.
Environ. Health Perspect., 57: 261-271.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., & O'DONOHUE, T.L. (1984c)
Reproductive toxicity of the industrial solvent 2-ethoxyethanol in rats
and interactive effects of ethanol. Environ. Health Perspect., 57: 255-
259.
NIOSH (1986) Health hazard evaluation report: Precision Castparts
Corporation, Portland, Oregon, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (Report No. HETA-84-415-1688).
NIOSH (1987a) Alcohols IV. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1403.
NIOSH (1987b) Esters I. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1450.
NITTER-HAUGE, S. (1970) Poisoning with ethylene glycol monomethyl ether:
report of two cases. Acta med. Scand., 188: 277-280.
OHI, G. & WEGMANN, D.H. (1978) Transcutaneous ethylene glycol monomethyl
ether poisoning in the work setting. J. occup. Med., 20: 675-676.
OUDIZ, D. & ZENICK, H. (1986) In vivo and in vitro evaluations of
spermatotoxicity induced by 2-ethoxyethanol treatment. Toxicol. appl.
Pharmacol., 84: 576-583.
PARSONS, C.E. & PARSONS, M.E.M. (1938) Toxic encephalopathy and
"granulopenic anaemia" due to volatile solvents in industry: report of
two cases. J. ind. Hyg. Toxicol., 20: 124-135.
PAUSTENBACH, D.J. (1988) Assessment of the developmental risks resulting
from occupational exposure to selected glycol ethers within the
semiconductor industry. J. Toxicol. environ. Health, 23: 29-75.
PISKO, G.T. & VERBILOV, A.A. (1988) Toxicity of monomethyl, monoethyl and
monobutyl ethers of ethylene glycol. Gig. Tr. prof. Zabol., 3: 48-49.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J. Water Pollut. Control Fed., 46: 63-77.
RAO, K.S., COBEL-GEARD, S.R., YOUNG, J.T., HANLEY, T.R., Jr, HAYES, W.C.,
JOHN, J.A., & MILLER, R.R. (1983) Ethylene glycol monomethyl ether II.
Reproductive and dominant lethal studies in rats. Fundam. appl. Toxicol.,
3: 80-85.
RITTER, E.J., SCOTT, W.J., RANDALL, J.L., & RITTER, J.M. (1985)
Teratogenicity of dimethoxyethyl phthalate and its metabolites
methoxyethanol and methoxyacetic acid in the rat. Teratology, 32: 25-31.
ROMER, K.G., BALGE, F., & FREUNDT, K.J. (1985) Ethanol-induced
accumulation of ethylene glycol monoalkyl ethers in rats. Drug chem.
Toxicol., 8(4): 255-264.
ROWE, V.K. & WOLF, M.A. (1982) Derivatives of glycols. In: Clayton, G.D. &
Clayton, F.E., ed. Patty's industrial hygiene toxicology, Vol. 2, pp.
3909-4052.
SAPARMAMEDOV, E. (1974) [Toxicity of some simple ethylene glycol ethers
(single experiments).] Zdravookhr Turkm., 18(9): 26-31 (in Russian)
(English translation from US NIOSH.).
SAVOLAINEN, H. (1980) Glial cell toxicity of ethyleneglycol monomethyl
ether vapour. Environ. Res., 22: 423-430.
SCOTT, W.J., FRADKIN, R., NAU, H., & WITTFOHT, W. (1987) Teratologic
potential of 2-methoxyethanol (2-ME) in non-human primates. Teratology,
35(2): 66 (abstract).
SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1986) Localization of
radioactivity from 2-methoxy[1,2-14C]ethanol in maternal and conceptus
compartments of CD-1 mice. Toxicol. appl. Pharmacol., 84: 25-35.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1987) The teratogenicity and
disposition of the glycol ether 2-methoxyethanol and their relationship in
CD-1 mice. In: Welsch, F., ed. Approaches to elucidate mechanisms in
teratogenesis, New York, Hemisphere Publishing Co., pp. 33-57.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1988) The relationship of
embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicol. appl.
Pharmacol., 93: 195-207.
SMALLWOOD, A.W., DEBORD, K.E., & LOWRY, L.K. (1984) Analyses of ethylene
glycol monoalkyl ethers and their proposed metabolites in blood and urine.
Environ. Health Perspect., 57: 249-253.
SMALLWOOD, A.W., DEBORD, K., BURG, J., MOSELEY, C., & LOWRY, L. (1988)
Determination of urinary 2-ethoxyacetic acid as an indicator of
occupational exposure to 2-ethoxyethanol. Appl. ind. Hyg., 3(2): 47-50.
SMYTH, H.F., SEATON, J., & FISCHER, L. (1941) The single dose toxicity of
some glycols and derivatives. J. ind. Hyg. Toxicol., 23: 259-268.
SPARER, J., WELCH, L.S., MCMANUS, K., & CULLEN, M.R. (1988a) Effects of
exposure to glycol ethers in shipyard painters. I. Evaluation of exposure.
Am. J. ind. Med., 14: 497-507.
SPARER, J., WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R.
(1988b) Effects of exposure to glycol ethers in shipyard painters. II.
Male reproduction. Am. J. ind. Med., 14: 509-526.
STENGER, E.G., AEPPLI, L., MULLER, D., PEHEIM, E., & THOMANN, P. (1971)
Toxicology of ethylene glycol monoethyl ether. Arzneim Forsch., 21: 880-
885.
STOTT, W.T. & MCKENNA, M.J. (1985) Hydrolysis of several glycol ether
acetates and acrylate esters by nasal mucosal carboxylesterase in vitro .
Fundam. appl. Toxicol., 5: 399-404.
SZYBALSKI, W. (1958) Special microbiological systems II. Observations on
chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76: 475-488.
TANAKA, K., MIKAMI, E., & SUZUKI, T. (1986) Methane fermentation of 2-
methoxyethanol by mesophilic digesting sludge. J. Ferment. Technol.,
64(4): 305-309.
TORAASON, M., STRINGER, B., STOBER, P., & HARDIN, B.D. (1985)
Electrocardiographic study of rat fetuses exposed to ethylene glycol
monomethyl ether (EGME). Teratology, 32: 33-39.
TORAASON, M., BREITENSTEIN, M.J., & SMITH, R.J. (1986a) Ethylene glycol
monomethyl ether (EGME) inhibits rat embryo ornithine decarboxylase (ODC)
activity. Drug chem. Toxicol., 9: 191-203.
TORAASON, M., STRINGER, B., & SMITH, R. (1986b) Ornithine decarboxylase
activity in the neonatal rat heart following prenatal exposure to ethylene
glycol monomethyl ether. Drug chem. Toxicol., 9(1): 1-14.
TYL, R.W., PRITTS, I.M., FRANCE, K.A., FISHER, L.C., & TYLER, T.R. (1988)
Developmental toxicity evaluation of inhaled 2-ethoxyethanol acetate in
Fischer 344 rats and New Zealand white rabbits. Fundam. appl. Toxicol.,
10: 20-39.
US EPA (1987) Environmental health criteria: 2-methoxyethanol, 2-
ethoxyethanol, and their acetates, Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances.
VERSCHUEREN, K. (1977) Ethylene glycol monomethyl ether. In: Handbook of
experimental data on organic chemicals, New York, Van Nostrand-Reinhold
Company, 327 pp.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987a)
Field study of the urinary excretion of ethoxyacetic acid during repeated
daily exposure to the ethyl ether of ethylene glycol and the ethyl ether
of ethylene glycol acetate. Scand. J. Work Environ. Health, 13: 239-242.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987b)
Survey of ethylene glycol ether exposures in Belgian industries and
workshops. Am. Ind. Hyg. Assoc. J., 48(8): 671-676.
WEIL, C.S. & SCALA, R.A. (1971) Study of intra- and inter-laboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol. appl. Pharmacol., 19: 276-360.
WELCH, L.S. & CULLEN, M.R. (1988) Effects of exposure to glycol ethers in
shipyard painters. III. Hematologic effects. Am. J. ind. Med., 14: 527-
536.
WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R. (1988) Effects
of exposure to ethylene glycol ethers on shipyard painters: II. Male
reproduction. Am. J. ind. Med., 14: 509-526.
WELSCH, R., SLEET, R.B., & GREENE, J.A. (1987) Attenuation of 2-
methoxyethanol and methoxyacetic acid-induced digit malformations in mice
by simple physiological compounds: implications for the role of further
metabolism of methoxyacetic acid in developmental toxicity. J. biochem.
Toxicol., 2: 225-240.
WERNER, H.W., MITCHELL, J.L., MILLER, J.W., & VON OETTINGEN, W.F. (1943)
Effects of repeated exposure of dogs to monoalkyl ethylene glycol ether
vapors. J. ind. Hyg. Toxicol., 25(9): 409-414.
WICKRAMARATNE, G.A., de S. (1986) The teratogenic potential and dose-
response of dermally administered ethylene glycol monomethyl ether (EGME)
estimated in rats with the Chernoff-Kavlock assay. J. appl. Toxicol.,
6(3): 165-166.
YONEMOTO, J., BROWN, N.A., & WEBB, M. (1984) Effects of dimethoxyethyl
phthalate, monomethoxyacetic acid on post implantation rat embryos in
culture. Toxicol. Lett., 21: 97-102.
YOUNG, E.G. & WOOLNER, L.B. (1946) A case of fatal poisoning from 2-
methoxyethanol. J. ind. Hyg. Toxicol., 28: 267-268.
ZIMMERMANN, F.K., MAYER, V.W., SCHEEL, I., & RESNICK, M.A. (1985) Acetone,
methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae.
Mutat. Res., 149: 339-351.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
La présente monographie ne traite que des éthers
méthyliques et éthyliques de l'éthylène-glycol, c'est-à-
dire le méthoxy-2 éthanol (2-ME), l'éthoxy-2 éthanol
(2-EE) et leurs esters acétiques respectifs, à savoir
l'acétate de méthoxy-2 éthyle (2-MEA) et l'acétate
d'éthoxy-2 éthyle (2-EEA). Ces composés se présentent tous
les quatre sous la forme de liquides stables incolores et
inflammables, dotés d'une légère odeur éthérée; ils sont
tous miscibles à l'eau (ou tout au moins dans le cas du
2-EAA très soluble dans celle-ci) et miscibles à un grand
nombre de solvants organiques.
Il existe des méthodes d'analyse permettant la mise en
évidence de ces éthers du glycol et de leurs métabolites
dans divers milieux (air, eau, sang et urine). Ces
méthodes font souvent appel à des techniques d'adsorption
ou d'extraction afin de concentrer l'échantillon, suivies
d'une analyse par chromatographie en phase gazeuse. La
chromatographie en phase gazeuse ou la chromatographie
liquide à haute performance permettent de doser l'acide
méthoxy-2 acétique (MAA) ainsi que l'acide éthoxy-2
acétique (EAA) (qui sont des métabolites du du 2-ME et du
2-EE) dans les urines, généralement après obtention de
dérivés convenables, à des concentrations entre 5 et
100 µg/ml.
2. Sources d'exposition humaine et environnementale
Les quatre éthers du glycol étudiés s'obtiennent tous
par réaction de l'oxyde d'éthylène sur l'alcool convenable
puis, si nécessaire, par estérification à l'aide d'acide
éthanoïque.
On ne dispose pas de données concernant la production
mondiale de ces éthers du glycol. Toutefois on peut
avancer que la production annuelle globale de l'Europe
occidentale, des Etats-Unis et du Japon se situe aux envi-
rons de 79 x 103 tonnes de 2-ME et de 205 x 103 tonnes
de 2-EE. Ils sont en grande partie utilisés pour la
production industrielle de divers revêtements (peintures,
teintures, laques, vernis, etc.) et comme solvants pour la
préparation d'encres d'impression, de résines et de
colorants, ainsi que pour la fabrication de détachants
domestiques et industriels. On les utilise également comme
additifs de dégivrage dans les liquides hydrauliques et
les carburéacteurs.
3. Transport, distribution et transformation dans l'environnement
Du fait de leur solubilité dans l'eau et de leur
tension de vapeur relativement basse, ces éthers
pourraient, en l'absence de décomposition, s'accumuler
dans l'eau. Toutefois, il semble que cette éventualité
soit exclue du fait de leur dégradation par des micro-
organismes présents dans le sol, les boues d'effluents et
l'eau.
C'est l'utilisation de ces éthers comme solvants vola-
tils qui, du fait des émissions atmosphériques auxquelles
elle donne lieu, entraîne l'exposition environnementale la
plus importante. Dans l'environnement général, ils
subissent une photolyse rapide et l'on pourrait s'attendre
à des concentrations inférieures à 0,0007 mg/m3 (2 x
10-4 ppm).
En aérobiose, les éthers du glycol subissent une
dégradation microbienne rapide en dioxyde de carbone et en
eau, alors qu'en anaérobiose, les principaux produits
finals sont le méthane et le dioxyde de carbone.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation d'éthers du glycol peut entraîner des
nombreuses émissions dans l'environnement. C'est en parti-
culier l'exposition humaine directe dans l'industrie, dans
les petits ateliers et au cours de l'utilisation domes-
tique de produits à base d'éthers du glycol qui est spéci-
alement préoccupante. En ce qui concerne l'exposition pro-
fessionnelle, les valeurs signalées vont de concentrations
< 0,1 mg/m3 à des concentrations > 150 mg/m3. L'utili-
sation de certains produits de consommation à base
d'éthers du glycol pourrait provoquer une exposition
notable des usagers mais on ne dispose pas de données à ce
sujet.
Outre l'exposition par la voie atmosphérique, l'homme
peut également être exposé par la voie dermique. L'analyse
du sang confirme que ces produits sont rapidement absorbés
par cette voie, qui contribue probablement davantage à la
charge totale de l'organisme que l'exposition par voie
aérienne.
5. Cinétique et métabolisme
Ces quatre éthers sont rapidement absorbés au niveau
de la peau, des poumons et des voies digestives. Des
études de répartition portant sur le 2-ME chez des souris
gravides ont montré que c'est dans le foie maternel, les
voies digestives, le placenta, le sac vitellin et de
nombreuses structures embryonnaires que se rencontraient
les concentrations les plus fortes.
La métabolisation du 2-ME donne naissance à deux
métabolites primaires : le MAA et la méthoxy-2 acétyl-
glycine. La transformation en dioxyde de carbone corres-
pond à une voie métabolique secondaire de moindre
importance. La conversion plasmatique du 2-ME en MAA
s'effectue rapidement, avec une demi-vie de 0,6 heure chez
le rat; en revanche l'excrétion de la MAA est lente, sa
demi-vie étant d'environ 20 heures chez le rat et de 77
heures chez l'homme.
L'administration à des animaux de laboratoire de 2-EE
a conduit à la production d'EAA et d'éthoxy-2 acétyl-
glycine, l'EAA étant le principal métabolite qui se mani-
feste dans l'organe supposé être l'organe cible, à savoir
les testicules. Chez l'homme, une étude sur le 2-EAA a
permis d'observer une voie métabolique analogue, l'acétate
étant d'abord hydrolysé en 2-EE puis oxydé en EAA. Cet
EAA a été ensuite excrété avec une demi-vie estimative de
21 à 42 heures. L'expérience semble indiquer que la
rétention ou l'accumulation des métabolites pourrait être
toxicologiquement importante dans la mesure où ces méta-
bolites seraient responsables de la toxicité observée au
niveau de l'organe cible.
6. Effets sur les êtres vivants dans leur milieu naturel
Il semble que le 2-ME et le 2-EE présentent une faible
toxicité pour les micro-organismes et les animaux aqua-
tiques. En ce qui concerne les micro-organismes, la con-
centration létale dans le milieu est supérieure à 2 %. On
a constaté une inhibition de la croissance des algues
vertes par le 2-ME à la concentration de 104 mg/litre et
de celle des cyanobactéries (algues bleu/vert) à la con-
centration de 100 mg/litre. La toxicité aiguë du 2-EE est
très faible pour les arthropodes (CL50 > à 4 g/litre) et
les poissons d'eau douce (CL50 > à 10 g/litre). Les acé-
tates des éthers du glycol (2-MEA et 2-EAA) sont beaucoup
plus toxiques pour les poissons. Ainsi la CL50 du 2-EEA
pour le vairon Pimephales promelas est de 46 mg par litre
tandis que celle du 2-MAA est de 45 mg/litre pour le
tarpon et pour Lepomis machrochirus. Il n'y a pas eu
d'études à long terme.
7. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
7.1 Toxicité générale
La toxicité du 2-ME et du 2-EE chez l'animal d'expéri-
ence a été beaucoup plus étudiée que celle du 2-MEA et du
2-EAA.
En ce qui concerne le 2-ME et le 2-EE ainsi que leurs
acétates, la dose létale après exposition unique est du
même ordre et ces composés présentent une faible toxicité
aiguë, que l'exposition ait lieu par voie dermique, orale
ou par inhalation. Pour diverses espèces, les valeurs de
la DL50 vont de 900 à 3400 mg/kg de poids corporel pour
le 2-ME, de 1400 à 5500 mg/kg pour le 2-EE, de 1250 à
3900 mg/kg pour le 2-MEA et de 1300 à 5100 mg/kg pour le
2-EAA. Des valeurs de 4603 mg/m3 (2-ME) et de 6698 mg
par m3 (2-EE) ont été signalées pour la CL50 par inhala-
tion chez la souris.
On ne possède que peu de données concernant les effets
irritants au niveau des yeux et de la peau ou le pouvoir
de sensibilisation de ces éthers du glycol chez l'animal.
Il semblerait qu'ils ne soient pas irritants pour la peau,
mais qu'ils puissent l'être pour l'oeil. Chez l'homme,
malgré de fortes expositions, on n'a jamais signalé
d'irritation cutanée ni de sensibilisation à ce niveau.
On a montré qu'en exposant par voie respiratoire des
animaux d'expérience pendant des périodes allant jusqu'à
90 jours, à de fortes concentrations (> 9313 mg de 2-ME
par m3 et > 1450 mg de 2-EE/m3) on déterminait des
effets nocifs sur les paramètres hématologiques, le
système nerveux, les testicules, le thymus, les reins, le
foie et les poumons. A des concentrations plus faibles,
les effets ne s'observaient qu'au niveau du système
hématopoïétique et des testicules. Par exemple, des rats
exposés par inhalation à du 2-ME pendant 13 semaines à des
doses comprises entre 93 et 930 mg/m3, présentaient une
réduction de l'hématocrite, du nombre de leucocytes, de
l'hémoglobine, des plaquettes et des protéines sériques,
mais seulement à la dose la plus forte. Chez des lapins
exposés de la même manière, on notait une réduction de la
taille du thymus et une altération des paramètres hémato-
logiques, à la dose de 903 mg/m3. Le 2-EE a produit des
effets analogues, mais moins graves, chez le rat et le
lapin lors d'une exposition de 13 semaines à la concen-
tration de 1450 mg/m3. On ne dispose d'aucune donnée
résultant d'études à long terme.
7.2 Cancérogénicité et mutagénicité
On a étudié la mutagénicité du 2-ME sur toute une
série de systèmes in vitro constitués de bactéries ou de
cellules mammaliennes. La plupart des études ont fourni
des résultats négatifs, toutefois on a tout de même
signalé des résultats positifs à de très fortes concen-
trations de 2-ME sur des cellules CHO. Il s'agissait
d'aberrations chromosomiques (à des concentrations supéri-
eures à 6830 µg/ml) et d'échanges entre chromatides
soeurs (à des concentrations supérieures à 3170 µg par
ml). La recherche d'aberrations chromosomiques et de
micronoyaux n'a rien donné in vivo . On ne dispose que de
données limitées sur le pouvoir mutagène du 2-EE; en
outre, il n'existe pas de données sur la cancérogénicité
de ces éthers du glycol.
7.3 Organes mâles de la reproduction
On a étudié de manière approfondie l'effet du 2-ME sur
l'appareil reproducteur mâle après administration par voie
orale ou respiratoire de ces substances à des rongeurs.
La présence de modifications dégénératives au niveau de
l'épithélium germinal des tubes séminifères à été systé-
matiquement observée. Des effets analogues ont été con-
statés avec le 2-EE, mais à des doses un peu inférieures.
L'administration par voie orale à des rats de 2-ME
pendant 1 à 11 jours a provoqué une réduction du nombre
des spermatozoïdes et des modifications de leur mobilité
et de leur morphologie, liées à la dose, à partir de
100 mg/kg de poids corporel. L'autopsie a révélé une
atteinte histologique marquée des testicules. La dose
sans effet observable (NOEL) était de 50 mg/kg. La
réduction de la fertilité était encore manifeste huit
semaines après une exposition à 200 mg/kg. Des effets
analogues ont été observés dans le cas du 2-EE à des doses
supérieures ou égales à 500 mg/kg, administrées pendant
des périodes allant jusqu'à 11 jours, la dose sans effet
observable sur 11 jours étant de 250 mg/kg. Toutefois
l'épuisement des réserves de spermatozoïdes, par suite
d'accouplements répétés, s'est accompagné à la dose la
plus faible étudiée (150 mg/kg), d'une réduction de leur
nombre. Après avoir administré par voie orale à des rats
et à des souris une dose unique de 250 mg ou davantage de
2-ME/kg de poids corporel, on a observé chez les animaux
une stérilité complète cinq semaines après l'admini-
stration, une certaine réduction de la fécondité étant
observée dès 125 mg/kg.
L'administration du 2-ME par la voie respiratoire a
donné lieu à des modifications dégénératives analogues au
niveau des testicules. Les effets en question ont été
observés après exposition unique de 4 heures à des doses
supérieures ou égales à 1944 mg/m3, aucun effet n'étant
observé à 933 mg/m3. Les valeurs de la dose sans effet
observable étaient de 311 mg/m3 chez les rats après
exposition de 13 semaines (6 heures par jour, 5 jours par
semaine) et de 933 mg/m3 (6 heures par jour) chez les
souris après exposition à neuf reprises sur une durée
totale de 11 jours. L'exposition de lapins à du 2-ME
pendant 13 semaines (6 heures par jour, 5 jours par
semaine) a entraîné des effets marqués au niveau des
testicules à la dose de 311 mg/m3 ou davantage; à la
dose de 93 mg/m3, on a observé des effets limites; il
n'a pas été possible de déterminer la dose sans effet
observable.
7.4 Toxicité foetale
On a observé des effets toxiques sur le développement
de plusieurs espèces d'animaux de laboratoire après
exposition par toutes les voies possibles, c'est-à-dire
orale, respiratoire et dermique. Le 2-ME a produit des
effets tératogènes chez la souris, le rat, le lapin et le
singe. Le 2-EE et le 2-EEA se sont révélés tératogènes
chez le rat et le lapin. Bien que le 2-MEA n'ait pas
encore été étudié de ce point de vue, son profil méta-
bolique (voir section 6) incite à penser qu'il a
vraisembleblement une toxicité analogue à celle du 2-ME.
C'est dans le cas du 2-ME que l'on possède l'ensemble
le plus complet de données dose-réponse (doses de 31,25 à
1000 mg/kg/j). Lors de cette étude portant sur des souris
auxquelles le 2-ME avait été administré par gavage
(administration les jours 7 et 14 de la gestation), on a
obtenu une dose sans effet observable de 125 mg/kg par
jour en ce qui concerne la toxicité maternelle. Toutefois
des malformations ont été observées à partir de doses
quotidiennes de 62,5 mg/kg et des modifications au niveau
du squelette à partir de 31,25 mg/kg par jour. La dose
sans effet observable relative à la toxicité foetale n'a
pas été indiquée. Lors d'études portant sur des doses
uniques, des souris ont reçu par gavage du 2-ME au onzième
jour de la gestation; la dose de 100 mg/kg n'était pas
foetotoxique tandis que celle de 175 mg/kg a produit des
anomalies digitales, mais sans autres signes de toxicité
maternelle ou foetale. Des anomalis cardio-vasculaires et
électrocardiographiques ont été observés chez les rats
nouveau-nés après administration les 7ème et 13ème jours
de la gestation d'une dose quotidienne de 25 mg/kg. Etant
donné qu'il s'agissait de la dose la plus faible expéri-
mentée, l'étude n'a pas permis d'établir une dose sans
effet observable sur le foetus (on n'a pas observé de
toxicité maternelle à cette dose). De même, aucune dose
de ce type n'a pu être déterminée après administration par
gavage à des guenons de 2-ME aux doses quotidiennes
respectives de 0,16, 0,32, ou 0,47 mmol/kg, du 20ème au
45ème jour de la gestation.
Après exposition par voie respiratoire à du 2-ME à la
dose de 156 mg/m3, on a observé une toxicité foetale
chez des rats et des souris et des malformations chez des
lapins. Pour l'ensemble de ces trois espèces, la dose sans
effet observable sur le développement foetal était de 31
mg/m3. Toutefois des anomalies comportementales et
neurochimiques ont été observées dans la descendance de
rattes exposées du 7ème au 13ème jour ou du 14ème au 20ème
jour de leur gestation à une dose de 78 mg de 2-ME par m3.
Après avoir exposé des rats à des doses de 743 mg par
m3 de 2-EE et des lapins à des doses 589 mg/m3 de la
même substance, on a constaté que ce produit était térato-
gène (avec en outre une certaine toxicité maternelle).
Dans une autre étude, on a constaté une toxicité foetale,
mais sans malformations, chez des rats exposés à des doses
de 184 ou 920 mg de 2-EE par m3 et chez des lapins ex-
posés à la dose de 644 mg/m3 de la même substance. Pour
ce qui est des effets sur le développement foetal, la
valeur de la dose sans effet observable était de 37 mg par
m3 pour le rat et de 184 mg/m3 pour le lapin. On a ob-
servé des anomalies comportementales et neurochimiques
dans la descendance de rattes exposées du 7ème au 13ème
jour et du 14ème au 20ème jour de leur gestation à la dose
de 360 mg de 2-EE par m3.
Des rattes soumises à une application dermique de 0,25
ml de 2-EE non dilué quatre fois par jour du 7ème et 16ème
jour de la gestation, ont eu une descendance où l'on
notait une foetotoxicité marquée et une forte incidence de
malformations malgré l'absence de toxicité maternelle. Des
effets analogues ont été observés à la suite d'un traite-
ment indentique par le 2-EEA selon le même protocole au
moyen d'une dose équimolaire (0,35 ml, quatre fois par
jour).
En exposant par la voie respiratoire des lapines à du
2-EEA du 6ème au 18ème jour de la gestation, on a obtenu
au cours de deux études différentes, des réponses térato-
gènes aux doses de 2176 mg/m3 et 544 mg/m3, la valeur
de la dose sans effet observable sur le développement
foetal étant respectivement de 135 mg/m3 et 270 mg par
m3. L'exposition de rattes du 6ème au 15ème jour de leur
gestation à du 2-EEA a entraîné dans leur descendance une
toxicité foetale à la dose de 540 mg/m3 et des malfor-
mations à la dose de 1080 mg/m3. La dose sans effet ob-
servable sur le développement foetal était de 170 mg par
m3.
8. Effets sur l'homme
On ne dispose que de renseignements limités sur les
effets toxiques chez l'homme de ces quatre éthers du
glycol. Les résultats fournis par quelques études de cas
ou études épidémiologiques sur les lieux de travail sont
dans la ligne des effets observés chez les animaux de
laboratoire. On n'a pas eu connaissance de rapports qui
chiffrent l'exposition de la population en général ni les
effets sur la santé.
Lors de deux cas non mortels d'empoisonnement par
ingestion d'un volume de 100 ml de 2-ME, on a noté les
principaux symptômes suivants : nausée, vertiges, cyanose,
tachycardie, hyperventilation et acidose métabolique avec
quelques signes d'insuffisance rénale. Des sympômes
analogues mais moins graves ont été observés chez une
personne qui avait ingéré 40 ml de 2-EE. Lors d'un
empoisonnement mortel par ingestion de 400 ml de 2-ME,
l'autopsie a révélé une gastrite hémorragique aiguë, une
dégénérescence graisseuse du foie et une altération
dégénérative des tubules rénaux.
L'exposition réitérée de travailleurs à du 2-ME et à
du 2-EE, en plus d'autres solvants, a entraîné chez eux de
l'anémie, un leucopénie, une faiblesse générale et une
ataxie. Dans nombre de ces études, il n'a pas été possible
de trouver une estimation fiable de l'exposition des
sujets. On a rapporté des effets hématologiques dus aux
éthers du glycol chez l'homme et on a notamment décrit
l'apparition d'une anémie macrocytaire chez un travailleur
exposé à du 2-ME (dose moyenne 105 ml/m3), ainsi qu'à
d'autres solvants.
Il a été fait état d'une toxicité médullaire chez des
ouvriers dont l'épiderme était exposé à du 2-ME, et des
effets immunologiques ont été également notés à la suite
d'une exposition prolongée (8 à 35 années) au 2-ME et au
2-EE (doses moyennes d'exposition 6,1 mg/m3 et 4,8 mg par
m3 respectivement).
Des études épidémiologiques effectuées sur des
ouvriers exposés à du 2-ME et à du 2-EE ont révélé des
anomalies au niveau de la fonction de reproduction, avec
une fréquenc accrue des cas d'oligospermie. L'exposition
à du 2-EE (37 ouvriers) à des concentrations pouvant
atteindre 88,5 mg/m3 a entraîné une modification du sper-
mogramme. Parmi 73 ouvriers exposés à du 2-ME (jusqu'à
17,7 mg/m3) et à du 2-EE (jusqu'à 80,5 mg/m3), on a
constaté une fréquence accrue de cas d'oligospermie et
observé certains effets hématologiques, pour des doses
d'exposition (TWA) de 2,6 mg/m3 dans le cas du 2-ME et de
9,9 mg/m3 dans celui du 2-EE.
Les effets indésirables constatés chez l'homme par
suite d'une exposition professionnelle correspondent à
ceux qui ont été observés chez les animaux de laboratoire.
Cependant, l'évaluation de l'exposition présentant un
certain nombre d'insuffisances et du fait qu'il s'agissait
d'expositions simultanées à plusieurs substances, il n'a
pas été possible d'en déduire une relation dose-réponse.
9. Conclusions
De nombreuses personnes peuvent être exposées à ces
quatre éthers du glycol à des concentrations comparables à
celles que l'on rencontre dans l'industrie, par suite de
l'utilisation de certains produits de consommation ou de
produits commerciaux. Une exposition professionnelle non
négligeable peut se produire par inhalation ou par résorp-
tion cutanée. Des mesures faites en petit nombre dans
l'air des lieux de travail indiquent des teneurs allant <
0,1 mg/m3 à > 150 mg/m3.
Le 2-ME et le 2-EE sont tous deux d'une faible toxi-
cité pour les micro-organismes et les espèces aquatiques.
Il n'existe aucune donnée qui permettrait d'évaluer le
risque d'effets indésirables sur les êtres vivants dans
leur milieu naturel par suite d'une exposition de longue
durée.
Chez le rat, la dose de 2-ME sans effets aigus observ-
ables au niveau testiculaire, est de 933 mg/m3; en cas
d'exposition répétée, la dose sans effet observable est de
311 mg/m3. En exposant de manière répétée l'espèce la
plus sensible, à savoir le lapin, on a observé un effet
net dès 311 mg/m3; cet effet était limite à la dose de
93 mg/m3 (un animal sur cinq). Les données fournies par
des études effectuées sur des sujets humains exposés de
par leur profession à du 2-ME et à du 2-EE montrent que
ces éthers du glycol exercent une certaine toxicité
testiculaire.
Chez toutes les espèces étudiées (souris, rats et
lapins) on a observé après exposition au 2-ME à des doses
égales ou supérieures à 156 mg/m3, une toxicité vis-à-
vis du développement foetal. Des anomalies comportement-
ales et neurochimiques ont été observées chez le rat après
exposition in utero à 78 mg/m3 de cette substance sans
qu'on puisse déterminer de dose sans effet observable. Le
2-EE et le 2-EEA se sont révélés légèrement moins actifs.
Des effets ont été également observés sur le développement
de foetus de rats et de lapins après exposition à du 2-EE
à des doses égales ou supérieures 368 mg/m3. Ces effets
étaient légers chez les rats exposés à 184 mg de 2-EE par
m3 mais on a pu néanmoins fixer nettement la dose sans
effet observable chez le rat et le lapin à la valeur de
38 mg/m3.
Ces éthers du glycol produisent des effets hématolo-
giques chez la souris, le rat, le lapin, le chien, le
hamster et le cobaye. Ces résultats sont en accord avec
les anomalies hématologiques observées lors des quelques
études consacrées à des travailleurs de l'industrie qui
avaient subi des expositions répétées à du 2-EE et/ou du
2-ME. Lors d'études sur l'animal comportant une expo-
sition répétée à ces deux substances, on a fixé à 93 mg
par m3 la dose de 2-ME sans effet observable chez le
lapin et à 368 mg/m3 la dose de 2-EE sans effet obser-
vable chez le rat et le lapin. On n'a pas pu obtenir de
données qui permettent une évaluation quatitative des
effets hématologiques aigus consécutifs à une exposition.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
1.1 Exposition
Nombreuses sont les personnes qui peuvent être
exposées au méthoxy-2 éthanol (2-ME), à l'éthoxy-2 éthanol
(2-EE) et à leurs acétates (2-MEA et 2-EEA) à des concen-
trations comparables à celles que l'on rencontre dans
l'industrie, lors de l'utilisation de produits de consom-
mation et de produits commerciaux. En revanche l'expo-
sition par l'intermédiaire des denrées alimentaires, de
l'eau ou de l'air ambiant est probablement négligeable.
Cette hypothèse ne repose que sur les propriétés
physiques et chimiques de ces composés et sur le fait
qu'ils se dégradent rapidement dans l'environnement.
Une exposition professionnelle non négligeable peut se
produire par inhalation ou résorption cutanée. Les
quelques mesures de concentrations dans l'air des lieux de
travail ont donné des valeurs qui vont de moins de 0,1 mg
par m3 à plus de 150 mg/m3. Toutefois les données de
surveillance existantes sont très limitées et il peut y
avoir d'importantes variations entre les différentes
industries et dans une même industrie. En raison du risque
de résorption cutanée, une simple surveillance de l'air
des lieux de travail risque de sous estimer l'exposition
totale. Pour évaluer la charge totale de l'organisme, la
meilleure méthode consiste à faire un contrôle biologique.
Une forte exposition peut se produire lors de travaux tels
que la peinture, l'impression ou le nettoyage mais il faut
se souvenir que ces composés sont utilisés à l'occasion
d'un grand nombre d'autres activités au cours desquelles
on pourrait craindre une exposition.
1.2 Effets sur la santé
Les principaux effets qu'on peut craindre chez l'homme
tiennent à l'action toxique de ces composés sur le
développement foetal, les testicules et les paramètres
hématologiques. La réalité de ces effets est attestée par
des données nombreuses et cohérentes obtenues chez l'ani-
mal ainsi que par quelques données concernant l'homme.
Tous ces effets peuvent appraître par suite d'expositions
à court ou à long terme. Chez l'animal de laboratoire,
une exposition répétée à des très fortes doses de 2-ME et
de 2-EE (plus de 930 ou 1450 mg/m3, respectivement)
entraîne des effets toxiques qui se traduisent par des
anomalies neuro-comportementales, hépatiques et rénales.
On les observe également dans les cas d'intoxication
humaine.
Ces quatre éthers du glycol exercent des effets
toxiques très voisins tant sur les testicules que sur le
développement du foetus, et ce, chez toutes les espèces
étudiées et par toutes les voies d'exposition qui ont été
utilisées (voie respiratoire, voie percutanée, voie
orale). L'étude du mécanisme de ces effets montre que
dans les deux cas, une phase d'activation est nécessaire,
à savoir la métabolisation en un dérivé de l'acide alkoxy-
acétique correspondant. La métabolisation s'effectue en
présence du système de l'alcool déshydrogénase qui est
commun à l'homme et aux animaux de laboratoire. Des méta-
bolites toxiques, l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA) ont été décelés dans l'urine de
sujets exposés à ces solvants. La régularité de cette
réponse toxique d'une espèce animale à l'autre, jointe à
l'analogie du métabolisme chez l'homme et l'animal,
montrent à l'évidence que l'homme pourrait être la cible
des mêmes effets toxiques sur les testicules et le
développement foetal. Les données dont on dispose au sujet
de l'excrétion des acides alkoxyacétiques chez l'homme
indiquent que leur durée de rétention est plus longue chez
celui-ci que chez l'animal, ce qui incite à penser que
l'homme pourrait être plus sensible à ces effets que
l'animal de laboratoire le plus sensible. C'est la
résorption cutanée rapide de ces composés qui est parti-
culièrement préocccupante. On a observé des effets térato-
gènes et autres anomalies du developpement à la suite de
l'application de 2-ME, de 2-EE et de 2-EAA sur la peau
intacte du rat.
Des lésions testiculaires ont été observées chez le
rat, la souris et le lapin à la suite d'une exposition à
ces éthers du glycol, soit par la voie respiratoire soit
par la voie orale. Chez le rat, une seule exposition par
voie respiratoire à des doses de 2-ME supérieures ou
égales à 1944 mg/m3 pendant 4 heures et une exposition
répétée à des doses supérieures ou égales à 933 mg par
m3 pendant 13 semaines ont déterminé des anomalies histo-
logiques au niveau testiculaire. Dans le cas de l'expo-
sition unique, la dose sans effet observable était de 933
mg/m3; elle était 311 mg/m3 dans le cas d'expositions
répétées. La souris a semblé un peu moins sensible, la
dose sans effet observable dans son cas étant, pour des
expositions répétées, de 933 mg/m3. Toutefois le lapin
l'était davantage, avec des altérations testiculaires
marquées qu'on pouvait observer après des expositions
répétées à 311 mg/m3 et la présence d'effets limites (un
lapin sur cinq) dès la dose de 93 mg/m3. Des effets ana-
logues ont été observés à la suite de l'exposition par
voie orale de rats à du 2-ME, une exposition de courte
durée (et notamment à une dose unique) ayant déterminé des
lésions testiculaires à partir de 100 mg/m3. Dans le
cas d'une étude portant sur les effets subaigus (11 jours)
la dose sans effet observable a été de 50 mg/kg. Le 2-EE
présente une toxicité testiculaire un peu moindre que
celle du 2-ME; ces effets n'apparaissent qu'à des doses
de 500 mg/kg ou davantage et la dose sans effet observable
se situe à 250 mg/kg.
Les données fournies par les études menées sur des
sujets humains exposés de par leur profession à du 2-ME et
à du 2-EE cadrent avec les données obtenues sur l'animal
et montrent que ces éthers du glycol peuvent produire des
effets toxiques au niveau testiculaire chez l'homme. Des
études épidémiologiques portant sur de petits groupes de
travailleurs exposés au 2-EE dans un atelier de fonte de
métaux et chez des peintres d'un chantier naval exposés à
ces deux composés, ont révélé une oligospermie systéma-
tique. Les données relatives aux niveaux d'exposition sont
limitées mais dans chaque cas, il y a lieu de penser qu'il
y a eu exposition par voie cutanée et respiratoire.
On a observé chez le rat, la souris, le lapin et le
singe des effets toxiques sur le développement embryon-
naire après exposition à ces éthers du glycol par voie
percutanée, orale ou respiratoire. Du 2-ME non dilué
appliqué à 12 reprises au cours d'une journée sur la peau
rasée de rattes gravides (zone d'application couverte
pendant 6 heures) s'est révélé mortel pour les animaux
alors qu'appliqué à 10 reprises sans pansement, du 2-EE
(1,0 ml/jour) ou du 2-EEA (1,4 ml/jour) ont produit des
effets tératogènes mais n'ont pas été toxiques pour les
femelles gravides. Au cours d'une autre étude, 12 appli-
cations de 2-ME à 10 % dans du soluté physiologique, avec
pansement sur le site d'application, ont entraîné des
effets toxiques sur le développement embryonnaire, la dose
sans effet observable se situant à la concentration de
3 %. Après administration répétée par voie orale de 2-ME
à des animaux gravides, on n'a pas observé de dose sans
effet toxique. La dose la plus faible produisant un effet
observable dans le cas de l'administration par voie orale
était de 31,5 mg/kg par jour pour la souris, de 25 mg/kg
pour le rat et de 0,16 mmol/kg pour le singe. En ce qui
concerne l'administration d'une dose unique, on ne possède
des résultats que pour le 2-ME chez la souris, avec une
dose sans effet observable pour le 11ème jour de la
gestation (le jour le plus sensible) de 100 mg/kg et une
valeur de 175 mg/kg pour la dose la plus faible avec effet
observable. Le 2-EE et le 2-EEA ont fait l'objet d'études
au cours desquelles des rats et des lapins ont été exposés
à ces deux composés par la voie respiratoire. L'expo-
sition des rats au 2-EE a fait l'objet de deux études qui
ont révélé des effets tératogènes (743 mg/m3, 7 heures
par jour, du premier au 19ème jour de la gestation) ou des
effets foetotoxiques (184 et 920 mg/m3, 6 heures par
jour, du 6ème au 15ème jour de la gestation). Dans cette
dernière étude, on a trouvé une dose sans effet observable
de 37 mg/m3. Chez des lapins exposés à du 2-EE on a
également observé des effets tératogènes (589 mg/m3, 7
heures par jour du premier au 18ème jour de la gestation)
et des effets foetotoxiques (644 mg/m3, 6 heures par
jour, du 6ème au 18ème jour de la gestation). Dans cette
dernière étude, la dose sans effet observable était de
184 mg/m3. Chez des lapines exposées à du 2-EEA 6 heures
par jour du 6ème au 18ème jour de la gestation, on a
observé des effets tératogènes à la dose de 2160 mg/m3
dans une étude et à la dose de 1620 mg/m3 dans une
autre. Les deux études ont montré l'apparition d'une
foetotoxicité à 540 mg/m3; la dose sans effet observable
correspondant, dans chaque étude, à la dose la plus faible
étudiée (respectivement 135 et 270 mg/m3). Des rats
exposés par voie respiratoire à du 2-EEA 6 heures par jour
du 6ème au 15ème jour de la gestation ont présenté le même
de type de réactions : effets tératogènes à 1620 mg par
m3, foetotoxicité à 1080 mg/m3 et aucun effet à 260 mg
par m3.
Des effets toxiques sur le développement embryonnaire
ont donc été observés chez toutes les espèces (souris,
rats et lapins), exposées à des doses supérieures ou
égales à 156 mg de 2-ME. Pour ces trois espèces, la dose
sans effet observable était de 31 mg/m3. Après expo-
sition in utero à 78 mg/m3, on a observé chez la descen-
dance des altérations comportementales et neurochimiques
sans qu'il soit possible de déterminer la dose sans effet
observable.
Le 2-EE et le 2-EEA se sont révélés légèrement moins
actifs. Des effets toxiques sur le développement embryon-
naire ont été observés chez des rats et des lapins après
exposition à des doses supérieures ou égales à 368 mg par
m3 de 2-EE. De légers effets de ce type ont été également
observés chez des rats exposés à 184 mg de 2-EE/m3, la
dose sans effet observable étant clairement établie à
37 mg/m3. En ce qui concerne le 2-EEA, la dose sans
effet observable était de 170 mg/m3 pour les rats et les
lapins.
Des effets hématologiques consécutifs à une exposition
à une dose unique on été observés chez l'animal ainsi qu'à
la suite d'intoxications chez l'homme. L'exposition
répétée par voie respiratoire, de lapins, l'espèce la plus
sensible, à du 2-ME 5 fois par semaine pendant 13
semaines, a permis de fixer la dose sans effet observable
à 93 mg/m3. Administré à répétition, le 2-ME produit
également des anomalies hématologiques chez la souris, le
lapin, le chien, le hamster et le cobaye. Le 2-EE est
moins actif à cet égard que le 2-ME. En ce qui concerne
les effets hématologiques chez le rat et le lapin, après
exposition à du 2-EE pendant 13 semaines, 5 fois par
semaine et 6 heures par jour, on peut fixer la dose sans
effet observable à 368 mg/m3. Chez le chien et la
souris, on observe également après, exposition répétée à
des doses plus élevées, un certain nombre d'effets hémato-
logiques. L'exposition aux acétates de 2-EE et de 2-ME
devrait provoquer des effets analogues, toutes choses
égales d'ailleurs, mais les données dont on dispose sur
l'exposition et les effets hématologiques de ces composés
sont trop limitées pour qu'on puisse établir les con-
ditions dans lesquelles une seule exposition conduirait à
des effets hématologiques chez l'homme.
On a observé dans l'industrie des niveaux d'exposition
voisins de la dose sans effet hématologique observable que
l'on a pu déterminer chez l'animal après expositions
répétées à du 2-ME et à du 2-EE. On peut en conclure,
compte tenu de la sensibilité probablement plus grande de
l'homme et de l'accumulation vraisemblable de métabolites
dans le sang, que l'exposition résultant de l'utilisation
de produits de consommation ou d'activités dans
l'industrie pourrait entraîner des effets hématologiques
de ce genre. A l'appui de cette hypothèse, on peut citer
les effets hématologiques effectivement signalés lors de
quelques études effectuées chez des travailleurs de
l'industrie ayant subi des expositions répétées au 2-EE
et/ou au 2-ME.
2. Evaluation des effets sur l'environnement
La libération directe dans l'atmosphère ou l'utili-
sation de ces produits comme solvants volatils peut con-
duire à une exposition dans le milieu ambiant. Ce peut
être également le cas par suite d'une libération acciden-
telle sur le sol ou dans l'eau. L'accumulation dans le
sol et les eaux superficielles ne peut se produire qu'en
l'absence de dégradation de ces composés. Or, on sait que
ces éthers du glycol se décomposent rapidement sous
l'effet de processus chimiques et biologiques; ils ne
devraient donce pas s'accumuler. Le 2-MEA et le 2-EAA
devraient également s'hydrolyser rapidement et subir
ensuite une biodégradation aérobie. Toutefois dans des
conditions d'anaérobiose, on pourrait craindre une
contamination des sols et des nappes phréatiques encore
que le phénomène soit vraisemblablement transitoire et le
risque correspondant négligeable.
Le 2-ME et le 2-EE sont peu toxiques pour les micro-
organismes et les espèces aquatiques. Leurs acétates, en
revanche, présentent une toxicité aiguë beaucoup plus
forte. On ne dispose pas de données à partir desquelles
on puisse déterminer leur potentiel nocif pour les espèces
vivantes en cas d'exposition prolongée.
RECOMMANDATIONS
1. Protection de la santé
1. Il conviendrait de recercher des solvants moins
toxiques que le méthoxy-2 éthanol, l'éthoxy-2 éthanol et
leurs esters, en particulier pour les remplacer dans les
produits de consommation. Il est également important
d'évaluer les effets des autres éthers de l'éthylène-
glycol car certains d'entre eux pourraient excercer des
effets analogues aux quatre éthers qui ont fait l'objet de
la présente évaluation.
2. Du fait des effets toxiques reconnus de ces éthers du
glycol, les autorités responsables devraient se préoccuper
sérieusement de trouver les moyens d'avertir les consom-
mateurs des dangers que présente l'utilisation de ces
produits, en particulier en cas d'exposition par voie
cutanée.
3. Compte tenu des données toxicologiques récentes et de
la possibilité d'une très forte absorption percutanée des
ces éthers du glycol, il importe de revoir les limites
nationales d'exposition professionnelle pour faire en
sorte que la dose quotidienne totale à laquelle sont
exposés les travailleurs par n'importe quelle voie, ne
menace pas leur santé.
4. Lorsqu'elle est assez importante, une dose unique peut
produire des effets sur l'animal. Pour réduire les
risques, il est recommandé d'utiliser ces produits avec
prudence (veiller à l'hygiène personnelle, utiliser des
dispositifs protecteurs appropriés et assurer une venti-
lation suffisante). Les données montrent que les effets
toxiques sur le développement embryonnaires ainsi que sur
le sang et les testicules, consécutifs à une exposition
répétée, pourraient exiger des mesures de protection plus
importantes.
2. Recherches à affectuer
1. Du fait que l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA), qui sont les principaux métabolites
du 2-ME, du 2-EE et de leurs esters, exercent des effets
toxiques sur les organes reproducteurs mâles, il faudrait
en étudier le mode d'action. S'ils apparaît que le MAA et
EAA ne sont pas les véritables responsables de ces effets,
ceux-ci devront être identifiés et leur mode d'action
élucidé.
2. Ces quatre éthers du glycol ont des effets sur le sang
et sur la fonction de reproduction masculine (oligo-
spermie). Les données disponibles, bien qu'en nombre
limité, indiquent que ces deux effets se manifestent à des
doses analogues. Il faudrait étudier le mode d'action de
ces composés sur ces deux types d'organes et étudier
parallèlement le nombre de spermatozoïdes et les effets
hématologiques afin de voir si ces derniers sont suscept-
ibles de jouer le rôle de signal d'alarme pour d'autres
effets toxiques de ces composés.
3. La surveillance de l'air ne suffit pas à assurer une
faible exposition. Un contrôle biologique peut permettre
de déceler les insuffisances des mesures de protection. A
l'heure actuelle on n'a pas établi avec une certitude
suffisante la relation qui existe entre les indicateurs
biologiques de l'exposition, la charge totale de l'organ-
isme et les effets physiologiques observés. Des travaux
sont encore nécessaires pour pouvoir établir les limites
de sécurité en fonction des résultats de la surveillance
biologique.
4. Il faudrait envisager des études épidémiologiques ou
une surveillance sanitaire spécifique au sein de popu-
lations qui sont fortement exposées à ces éthers du glycol
afin d'obtenir des relations exposition-effets; on
pourrait alors déterminer les limites de sécurité, à
condition toutefois que l'exposition globale puisse être
correctement et suffisamment évaluée par la surveillance
de l'environnement et des contrôles biologiques.
5. Il conviendrait d'étudier l'éventualité d'effets sur
l'appareil reproducteur féminin en procédant à des études
de reproduction sur plusieurs générations d'animaux de
laboratoire.
6. Les résultats disponibles indiquent que l'homme
pourrait métaboliser dans une plus grande proportion que
le rat ces éthers du glycol en acides alkoxyacétiques
correspondants, la demi-vie des ces métabolites toxiques
s'étant environ quatre fois plus longue chez l'homme. Par
ailleurs l'organisme du rat est capable de conjuguer une
fraction plus importante des métabolites acides que ne le
fait celui de l'homme. Ces différences pourraient
entraîner une sensibilité relativement plus importante de
l'homme aux éthers du glycol. Une connaissance plus
précise du métabolisme et de la cinétique d'excrétion de
ces composés permettrait de mieux prévoir les limites de
sécurité.
7. Les résultats fournis par des études à court terme
(13 semaines) montrent que des effets s'exercent sur
divers organes. Toutefois, il n'a pas été procédé à des
études suffisamment longues pour qu'on puisse déterminer
si ces effets sont réversibles ou non. Il est donc
recommandé de procéder à des études au cours desquelles
des animaux de laboratoire subiront une exposition d'au
moins 13 semaines à ces composés, suivie d'une période de
récupération. A l'issue de cette période, on devra
déterminer les paramètres physiologiques importants afin
de voir si les effets sont passagers ou non.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, métodos analíticos
En esta monografía sólo se tienen en cuenta los éteres
metílico y etílico del etilenglicol, es decir el 2-metoxi-
etanol (2-ME) y el 2-etoxietanol (2-EE), así como sus
ésteres acéticos respectivos, el acetato de 2-metoxietilo
(2-MEA) y el acetato de 2-etoxietilo (2-EEA). Estos cuatro
compuestos son todos ellos líquidos estables, incoloros e
inflamables con un olor levemente etéreo y todos ellos son
miscibles con (o, en el caso del 2-EEA, muy solubles en)
agua y miscibles con numerosos solventes orgánicos.
Se dispone de métodos analíticos para detectar estos
éteres glicólicos o sus metabolitos en diversos medios
(aire, agua, sangre y orina). En muchos casos se basan en
el empleo de métodos de adsorción o extracción para con-
centrar la muestra, seguidos de un análisis por cromato-
grafía de gases. Mediante la cromatografía de gases o la
cromatografía de líquidos de alto rendimiento, cabe la
posibilidad de determinar cuantitativamente el ácido
2-metoxiacético (MAA) y el ácido 2-etoxiacético (EAA) -
metabolitos del 2-ME y del 2-EE - en la orina, por lo gen-
eral tras derivación, a concentraciones de 5-100 µg por
ml.
2. Fuentes de exposición humana y ambiental
Los cuatro éteres glicólicos examinados están todos
ellos producidos por la reacción del óxido etilénico con
el alcohol apropiado, seguida, si procede, de esterifica-
ción con ácido etanoico.
No se dispone de datos sobre la producción mundial de
estos éteres glicólicos. Sin embargo, la producción anual
combinada de Europa occidental, los Estados Unidos de Amé-
rica y el Japón es aproximadamente del 79 x 103 toneladas de
2-ME y 205 x 103 toneladas de 2-EE. Una proporción con-
siderable se utiliza en la fabricación de pinturas, color-
antes y lacas así como en forma de solventes para tinta de
imprenta, resinas y tintes, y como productos de limpieza
domésticos e industriales. También se utilizan estos com-
puestos como aditivos anticongelantes en los líquidos hi-
dráulicos y el combustible de los reactores.
3. Transporte, distribución y transformación en el medio ambiente
La hidrosolubilidad de estos éteres glicólicos y su
presión de vapor relativamente baja podrían dar lugar a su
acumulación en el agua en ausencia de degradación. Sin
embargo, esta posibilidad queda aparentemente excluida
debido a la degradación por los microorganismos presentes
en el suelo, los cienos de alcantarilla y el agua.
Las emisiones atmosféricas causadas por el uso de
éteres glicólicos como solventes volátiles originan la
máxima exposición ambiental. En el medio ambiente general,
la degradación fotolítica parece ser rápida y cabe prever
niveles inferiores a 0,0007 mg/m3 (2 x 10-4 ppm).
En condiciones aeróbicas los microorganismos degradan
rápidamente los éteres glicólicos en forma de dióxido de
carbono y agua, mientras que en condiciones de anaerobi-
osis los principales productos finales son el metano y el
dióxido de carbono.
4. Niveles en el medio ambiente y exposición humana
El uso de éteres glicólicos puede dar lugar a emisi-
ones considerables y muy extendidas en el medio ambiente.
Suscita especial preocupación la exposición humana directa
en la industria, en los talleres de dimensiones modestas y
como consecuencia del empleo doméstico de productos que
contienen dichos éteres. Se han señalado valores de expo-
sición profesional comprendidos entre < 0,1 mg/m3 y > 150
mg/m3. Los usuarios de productos de consumo pueden
sufrir una exposición considerable, aunque no se dispone
de datos al respecto.
Además de la exposición a los éteres glicólicos pres-
entes en la atmósfera, las personas pueden estar expuestas
por vía cutánea. Los análisis de sangre confirman que la
absorción es rápida por esta vía, que puede contribuir más
que la exposición atmosférica a la carga total que recibe
el organismo.
5. Cinética y metabolismo
Se ha demostrado que los cuatro éteres glicólicos
pueden absorberse rápidamente a través de la piel, los
pulmones y el tracto gastrointestinal. Los valores más
elevados que se han obtenido en los estudios sobre la
distribución del 2-ME en ratonas gestantes se sitúan en el
hígado, la sangre y el tracto gastrointestinal de la
madre, así como en la placenta, el saco vitelino y en
numerosas estructuras del embrión.
La transformación metabólica del 2-ME produce dos
metabolitos primarios: MAA y glicina de 2-metoxiacetilo.
La metabolización a dióxido de carbono representa una vía
secundaria de menor importancia. La conversión del 2-ME
en MAA en el plasma se produce rápidamente, con una vida
media de 0,6 h en las ratas, pero la secreción del MAA es
lenta, con una vida media de 20 h aproximadamente en la
rata y de 77 h en el hombre.
En los animales de laboratorio, la administración de
2-EE da lugar a la producción de EAA y de glicina de
2-etoxiacetilo; el EAA es el principal metabolito que
aparece en los testículos, que son el "órgano diana"
presunto. En un estudio humano en el que se utilizó 2-EEA
se observó una vía metabólica análoga: el acetato se hi-
drolizó primero en 2-EE y luego se transformó en EAA por
oxidación. El EAA resultante se excretó con una vida media
estimada en 21-42 h. Los estudios experimentales hacen
pensar que la retención o acumulación de los metabolitos
podrían ser importantes desde el punto de vista toxico-
lógico en el supuesto de que dichos metabolitos sean la
causa de la toxicidad observada en el "órgano diana".
6. Efectos en los organismos presentes en el medio ambiente
La toxicidad del 2-ME y del 2-EE para los microorga-
nismos y animales acuáticos parece ser baja. En el caso
de los microorganismos, la concentración letal en el medio
es superior al 2%. Con el 2-ME se ha observado inhibición
del crecimiento de las algas verdes a 104 mg/litro y de
las cianobacterias (algas verde azuladas) a 100 mg/litro.
La toxicidad aguda del 2-EE es muy baja para los artrópo-
dos (CL50 > 4 g/litro) y para los peces de agua dulce
(CL50 > 10 g/litro). Los acetatos de éteres glicólicos
(2-MEA y 2-EEA) son mucho más tóxicos para los peces. La
CL50 del 2-EEA para el Phoxinus cabezudo es 46 mg/litro y
la del 2-MEA para el pez plateado de la pleamar y los
Lepomis de agallas azules es de 45 mg/litro. No se han
hecho estudios a largo plazo.
7. Efectos en los animales de experimentación y en los
sistemas de experimentación in vitro
7.1 Toxicidad sistémica
La toxicidad del 2-ME y del 2-EE en los animales de
experimentación se ha estudiado en medida mucho mayor que
la del 2-MA y del 2-EEA.
El 2-ME y el 2-EE y sus acetatos dan tasas análogas de
letalidad tras una exposición única y producen una letali-
dad aguda baja cuando la exposición tiene lugar por vía
dérmica u oral o por inhalación. Los valores de la DL50 oral
en las diversas especies estudiadas oscilan entre 900 y
3400 mg/kg de peso corporal en el caso del 2-ME, entre
1400 y 5500 mg/kg en el del 2-EE, entre 1250 y 3930 mg/kg
en el del 2-MEA, y entre 1300 y 5100 mg/kg en el de 2-EEA.
En los ratones se han obtenido valores de la CL50 por
inhalación de 4603 mg/m3 (2-ME) y 6698 mg/m3 (2-EE).
Sólo se dispone de datos limitados acerca de la irri-
tación cutánea u ocular o del potencial de sensibilización
de estos éteres glicólicos en los animales. Al parecer,
no son irritantes para la piel, pero pueden causar irri-
tación en los ojos. En el hombre no se ha observado irri-
tación ni sensibilización cutáneas ni siquiera en caso de
gran exposición.
La exposición por inhalación a corto plazo (hasta 90
días) de los animales de experimentación a concentraciones
elevadas (> 9313 mg de 2-ME/m3 y > 1450 mg de 2-EE/m3)
ejerce, según se ha demostrado, efectos adversos en los
parámetros sanguíneos, el sistema nervioso y los testícu-
los, el timo, el riñón, el hígado y los pulmones. Utiliz-
ando niveles más bajos de exposición, se han observado
efectos en el sistema hematopoyético y en los testículos.
Así, por ejemplo, las ratas expuestas durante 13 semanas a
la inhalación de 2-ME a concentraciones comprendidas entre
93 y 930 mg/m3 presentaron una reducción del volumen
hematocrito y de los glóbulos blancos, la hemoglobina, las
plaquetas y las concentraciones de proteínas séricas sola-
mente cuando se aplicaba la dosis máxima, mientras que los
ratones expuestos del mismo modo presentaron disminución
del tamaño del timo además de la disminución de los pará-
metros sanguíneos con concentraciones de 930 mg/m3. Las
ratas y conejos expuestos al 2-EE presentaron efectos
análogos pero menos intensos cuando soportaron durante 13
semanas una concentración de 1450 mg/m3. No se dispone
de datos sobre estudios a largo plazo.
7.2 Carcinogenicidad y mutagenicidad
La mutagenicidad del 2-ME se ha estudiado en una gama
de sistemas in vitro utilizando bacterias y células de
mamífero. Aunque la mayor parte de los estudios han dado
resultados negativos, algunos informes acusan resultados
positivos en cuanto a la mutagenicidad de las concentraci-
ones muy altas de 2-ME en células CHO estudiadas desde el
punto de vista de las aberraciones cromosómicas (a 6830 µg
por ml o más) o del intercambio de cromátides equiparables
(3170 µg/ml o más). En cambio, la investigación in vivo
de aberraciones cromosómicas y micronúcleos ha dado
siempre resultados negativos. Sólo se dispone de una
información muy limitada sobre el potencial mutagénico del
2-EE y no se dispone de ningún dato sobre la carcinogen-
icidad de estos éteres glicólicos.
7.3 Sistema reproductor masculino
Los efectos del 2-ME en el sistema reproductor mascu-
lino se han estudiado detenidamente en roedores expuestos
por vía oral o por inhalación. En el epitelio germinal de
los tubos seminíferos se han observado constantemente
alteraciones degenerativas. Análogos efectos se han obten-
ido con el 2-EE, si bien con niveles de dosificación algo
más elevados.
En la rata, la administración oral de 2-ME durante 1-
11 días ha dado lugar a un descenso del recuento de esper-
matozoides con cambios de la motilidad y la morfología de
éstos en relación con la dosis utilizada; como niveles de
dosificación se utilizaron 100 mg/kg de peso corporal o
más. En la autopsia se encontraron acusadas alteraciones
histológicas en los testículos. El nivel de efecto no
observado (NENO) fue de 50 mg/kg. La reducción de la fer-
tilidad seguía siendo patente a las 8 semanas de la expo-
sición a 200 mg/kg. Análogos efectos se observaron con
dosificaciones de 500 mg de 2-EE/kg o más, administrados
durante 11 días como máximo; en el tratamiento de 11 días
el NENO fue de 250 mg/kg. En cambio, cuando las reservas
de espermatozoides están reducidas por la frecuencia de
los acoplamientos, se observó cierta reducción de los
recuentos con la dosis más baja estudiada. Los estudios
de fertilidad consecutivos a la administración de una sola
dosis oral de 250 mg de 2-ME/kg o más mostraron una ester-
ilidad completa, tanto en las ratas como en los ratones, a
partir de las 5 semanas de administración; con 125 mg/kg
se observó ya cierto descenso de la fertilidad.
En los experimentos de inhalación se observaron alter-
aciones degenerativas análogas en los testículos con el
2-ME. Los efectos se observaron tras una sola exposición
(4 h) a 1944 mg/m3 o más pero no con 933 mg/m3. El NENO
fue de 311 mg/m3 en la rata tras la exposición durante 13
semanas (6 h/día, 5 días/semana) y de 933 mg/m3 (6 h/día)
en los ratones tras la exposición en 9 ocasiones en el
curso de 11 días. En los conejos expuestos al 2-ME durante
13 semanas (6 h/día, 5 días/semana) se observaron efectos
marcados en los testículos con concentraciones de 311
mg/m3 o más y efectos marginales con 93 mg/m3; no se
determinó el NENO.
7.4 Toxicidad para el desarrollo
En varias especies de animales de laboratorio se ha
observado toxicidad para el desarrollo tras la exposición
a los compuestos utilizando todas las vías de administra-
ción: oral, inhalatoria y dérmica. El 2-ME produjo efectos
teratógenos en ratones, ratas, conejos y monos. El 2-EE y
el 2-EEA resultaron teratógenos en las ratas y los
ratones. Aunque no se ha estudiado la toxicidad sobre el
desarrollo del 2-MEA, los perfiles metabólicos (véase la
sección 6) hacen pensar que es posible que el 2-MEA tenga
una toxicidad análoga a la del 2-ME.
En relación con el 2-ME se dispone de la gama más
amplia de datos dosis/respuesta (dosis de 31,25 por 1000
mg/kg por día). En este estudio de administración inten-
siva en el que se utilizaron ratones (2-ME administrado
entre el 7° y el 14° día de gestación) el NENO correspon-
diente a la toxicidad materna fue de 125 mg/kg por día.
No obstante, se observaron malformaciones con 62,5 mg/kg
por día y variaciones esqueléticas con 31,25 mg/kg por
día. No se señaló ningún NENO de toxicidad para el desar-
rollo. En el marco de estudios de dosis única, se trató a
ratones con 2-ME administrado por alimentación forzada el
11° día de la gestación; la dosis de 100 mg/kg no era
fetotóxica mientras que la de 175 mg/kg produjo anomalías
digitales sin otros signos de toxicidad materna o fetal.
En la ratas recién nacidas se observaron defectos cardio-
vasculares y anomalías del ECG tras el tratamiento de las
madres con 25 mg/kg por día durante los días 7° a 13° de
la gestación. Como ésta fue la dosis más baja que se
ensayó, este estudio no ha permitido establecer un NENO
para el desarrollo (con esa dosis no se observó toxicidad
materna). De igual modo, no pudo determinarse ningún NENO
de toxicidad para el desarrollo en un estudio de trata-
miento de monos por alimentación forzada con 2-ME a 0,16,
0,32 o 0,47 mmol/kg por día durante los días 20° a 45° de
la gestación.
Tras la exposición a la inhalación de 2-ME a 156 mg
por m3 se ha observado fetotoxicidad en los ratones y
ratas y malformaciones en los conejos. En las tres espe-
cies, el NENO correspondiente a los efectos sobre el
desarrollo fue de 31 mg/m3. Sin embargo, en la descen-
dencia de las ratas expuestas a 78 mg 2-ME/m3 durante los
días 7-13 o 14-20 de la gestación se observaron altera-
ciones conductuales y neuroquímicas.
Tras la exposición por inhalación de ratas (743 mg por
m3) y conejos (589 mg/m3), el 2-EE se reveló terató-
geno (en presencia de ligera toxicidad materna). En otro
estudio se observó fetotoxicidad pero no malformaciones en
las ratas expuestas a 184 ó 920 mg de 2-EE/m3, así como
en los conejos expuestos a 644 mg de 2-EE/m3. Los val-
ores del NENO para los efectos en el desarrollo fueron de
37 mg/m3 en las ratas y de 184 mg/m3 en los conejos. En
la descendencia de las ratas expuestas a 368 mg de 2-EE
por m3 durante los días 7-13 ó 14-20 de la gestación se
observaron alteraciones conductuales y neuroquímicas.
Las ratas tratadas por aplicación dérmica de 0,25 ml
de 2-EE sin diluir (cuatro veces al día en los días 7-16
de la gestación) acusaron una considerable fetotoxicidad y
una elevada incidencia de malformaciones en ausencia de
toxicidad materna. Análogos efectos se observaron tras el
tratamiento de las ratas con 2-EEA, utilizando el mismo
protocolo, a una dosis equimolar (0,35 ml, cuatro veces al
día).
La exposición por inhalación de 2-EEA de conejas
durante los días 6-18 de la gestación provocó respuestas
teratógenas con 2176 mg/m3 y 544 mg/m3 en dos estudios
diferentes, en los cuales los valores del NENO para el
desarrollo fueron de 135 mg/m3 y 270 mg/m3. Las ratas
expuestas al 2-EEA durante los días 6-15 de la gestación
acusaron fetotoxicidad a 540 mg/m3 y malformaciones a
1080 mg/m3. El NENO para el desarrollo fue de 170 mg por
m3.
8. Efectos en el hombre
La información disponible sobre los efectos tóxicos de
estos cuatro éteres glicólicos en el ser humano es limi-
tada. Los resultados de los escasos informes sobre casos
individuales y estudios epidemiológicos en el lugar de
trabajo corroboran los efectos adversos observados en los
animales de experimentación. No se ha encontrado ningún
informe en el que se cuantifique la exposición y los
efectos adversos en la población general.
En dos casos no mortales de envenenamiento por inges-
tión de 100 ml de 2-ME, los signos y síntomas predomi-
nantes fueron náuseas, vértigo, cianosis, taquicardia,
hiperventilación y acidosis metabólica, con algunos indi-
cios de insuficiencia renal. Síntomas análogos, aunque
menos graves, se observaron en un sujeto que ingirió 40 ml
de 2-EE. En un caso de intoxicación mortal causada por la
ingestión de 400 ml de 2-ME, la autopsia reveló una gas-
tritis hemorrágica aguda con degeneración grasa del hígado
y alteraciones degenerativas de los túbulos renales.
La exposición repetida de los trabajadores al 2-ME y
al 2-EE, así como a otros solventes, ha dado lugar a
anemia, leucopenia, debilidad general y ataxia. En muchos
de estos estudios no se ha hecho ninguna estimación fide-
digna de la exposición. Los efectos hematológicos de los
éteres glicólicos en el ser humano están bien documentados
y se ha descrito la aparición de anemia macrocítica en un
trabajador expuesto al 2-ME (promedio: 105 mg/m3), así
como a otros solventes.
En los trabajadores expuestos por vía dérmica al 2-ME
se han observado efectos tóxicos en la médula ósea, y tam-
bién se han observado efectos inmunológicos en trabaja-
dores sometidos a una exposición prolongada (8-35 años) al
2-ME y al 2-EE (los promedios de exposición fueron de 6,1
mg/m3 y 4,8 mg/m3, respectivamente).
Los estudios epidemiológicos realizados en trabaja-
dores expuestos al 2-ME y al 2-EE han revelado algunos
indicios de efectos adversos en el sistema reproductor
masculino, con un aumento de la frecuencia de recuentos
reducidos de espermatozoides. La exposición al 2-EE (37
trabajadores) a concentraciones de hasta 88,5 mg/m3 pro-
vocaron una alteración de los índices seminales. En un
grupo de 73 trabajadores expuestos al 2-ME (hasta 17,7 mg
por m3) y al 2-EE (hasta 80,5 mg/m3) se observó una
mayor frecuencia de recuentos reducidos de espermatozoides
y también signos de efectos hematológicos con exposiciones
de 2,6 mg/m3 para el 2-ME y de 9,9 mg/m3 para el 2-EE
(TWA).
Los efectos adversos observados en las personas pro-
fesionalmente expuestas coinciden con los señalados en los
animales de experimentación. Sin embargo, debido a defici-
encias en las evaluaciones de la exposición y a las expo-
siciones mixtas, no se han podido determinar relaciones
dosis-respuesta.
9. Conclusiones
Muchas personas pueden estar expuestas a concentra-
ciones de estos cuatro éteres glicólicos comparables a las
industriales a consecuencia del empleo de productos comer-
ciales y de consumo. Tanto por inhalación como por absor-
ción cutánea pueden producirse exposiciones profesionales
importantes. En un número limitado de determinaciones de
la concentración atmosférica en los lugares de trabajo se
han obtenido valores comprendidos entre < 0,1 mg/m3 y >
150 mg/m3.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
para los microorganismos y las especies acuáticas. No se
dispone de datos que permitan precisar la capacidad poten-
cial de las exposiciones prolongadas para ejercer efectos
adversos sobre las especies presentes en el medio ambiente.
En las ratas se ha obtenido un NENO para los efectos
testiculares de 933 mg de 2-ME/m3, así como un NENO para
la exposición repetida de 311 mg/m3. En los experimentos
de exposición repetida con la especie más sensible, el
conejo, se ha detectado un efecto neto con 311 mg/m3, mien-
tras que a 93 mg/m3 se observaba un efecto marginal (1 de
5 animales). En las personas expuestas profesionalmente al
2-ME y al 2-EE se han encontrado indicios de que estos
éteres glicólicos pueden producir toxicidad testicular en
el ser humano.
En todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más se ha observado toxi-
cidad para el desarrollo. Para las tres especies se tuvo
un NENO de 31 mg/m3. En las ratas expuestas in utero a
78 mg/m3 se produjeron alteraciones conductuales y neuro-
químicas, pero no se estableció ningún valor de NENO. El
2-EE y el 2-EEA eran algo menos potentes. En la rata y en
el conejo se han observado efectos sobre el desarrollo
tras la exposición a 2-EE a concentraciones de 368 mg por
m3 o más. Estos efectos eran ligeros en las ratas ex-
puestas a 184 mg de 2-EE/m3, pero tanto en las ratas como
en los conejos se pudo establecer un NENO bien definido a
37 mg/m3.
Estos éteres glicólicos producen efectos hematológicos
en los ratones, las ratas, los conejos, los perros, los
hamsters, y los cobayos. Esta observación concuerda con
los efectos hematológicos señalados en algunos de los
escasos estudios efectuados en trabajadores industriales
expuestos repetidamente al 2-EE y/o al 2-ME. En los estu-
dios de exposición repetida de animales se obtuvo un NENO
de 93 mg de 2-ME/m3 en los conejos y de 368 mg de 2-EE
por m3 en las ratas y los conejos. No se han obtenido
datos que permitan evaluar cuantitativamente los efectos
hematológicos que siguen a la exposición aguda.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y EFECTOS
EN EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
1.1 Exposición
Muchas personas pueden estar expuestas al 2-metoxi-
etanol (2-ME), al 2-etoxietanol (2-EE) y a sus acetatos
(2-MEA y 2-EEA) en concentraciones comparables a las
industriales como consecuencia del empleo de productos
comerciales y de consumo. En cambio, la exposición por
los alimentos, el agua o el aire ambiente es probablemente
insignificante. Esta impresión se basa únicamente en las
propiedades físicas y químicas de estos compuestos y en
los indicios de que experimentan una rápida degradación en
el medio ambiente.
Tanto por inhalación como por absorción cutánea puede
producirse una exposición profesional importante. Las
escasas determinaciones de las concentraciones en la
atmósfera de los lugares de trabajo han dado valores
comprendidos entre menos de 0,1 mg/m3 y más de 150 mg por
m3. Sin embargo, las posibilidades de vigilancia son muy
limitadas y cabe la posibilidad de que haya grandes vari-
aciones entre diferentes industrias e incluso dentro de
una misma industria. Habida cuenta de las posibilidades de
absorción cutánea, la vigilancia del aire por sí sola
puede dar una subestimación de la exposición total. La
vigilancia biológica es el mejor método para calcular la
absorción total. Entre los trabajos que entrañan una expo-
sición considerable figuran, por ejemplo, los de pintura,
imprenta y limpieza; ahora bien, no hay que olvidar que
estos compuestos se utilizan también en otras muchas acti-
vidades profesionales en las que la exposición debe ser
motivo de inquietud.
1.2 Efectos en la salud
Los principales motivos de inquietud en el ser humano
son los efectos en el desarrollo, los efectos testiculares
y los vinculados a la toxicidad hematológica. Estos
efectos han sido demostrados por una multitud de datos
sólidos obtenidos en el animal y por algunos datos de
origen humano. Todos ellos pueden estar causados por una
exposición a corto o a largo plazo. En los animales de
experimentación, la exposición muy repetida al 2-ME y al
2-EE (más de 939 y 1450 mg/m3, respectivamente) produce
efectos tóxicos neuroconductuales, hepáticos y renales,
los cuales se observan también en casos de intoxicación
humana.
Estos cuatro éteres glicólicos dan valores muy simi-
lares de toxicidad testicular y de toxicidad para el
desarrollo en todas las especies estudiadas y por todas
las vías de exposición que se han ensayado (inhalatoria,
dérmica y oral). En los estudios sobre el mecanismo de
acción se ha visto que la metabolización al derivado del
ácido alcoxiacético constituye una etapa indispensable de
activación, tanto en los efectos sobre el desarrollo como
en los testiculares. Dicho metabolismo se efectúa mediante
el sistema de deshidrogenasa alcohólica que es común al
hombre y a los animales de laboratorio. Los metabolitos
tóxicos, el ácido metoxiacético (MAA) y el ácido etoxi-
acético (EAA), aparecen en la orina de las personas ex-
puestas a estos solventes. La coherencia de las respuestas
en las distintas especies de animales de laboratorio estu-
diadas, junto con la semejanza del metabolismo en el ser
humano, permiten concluir que el hombre está probablemente
expuesto a los efectos testiculares y sobre el desarrollo
de estos éteres glicólicos. Los datos disponibles sobre
la excreción de ácidos alcoxiacéticos por el hombre
sugieren la existencia de una retención prolongada en com-
paración con la que se observa en los animales de labora-
torio, lo cual hace pensar que las personas quizás sean
más sensibles que las especies experimentales de mayor
sensibilidad. Un motivo de especial inquietud es la rápida
absorción cutánea de estos compuestos. Se han observado
efectos teratógenos y otros efectos sobre el desarrollo
tras la aplicación de 2-ME, 2-EE y 2-EEA en la piel
intacta de la rata.
En la rata, el ratón y el conejo se han observado
alteraciones testiculares tras la exposición a estos
éteres glicólicos, tanto por inhalación como por vía oral.
Una exposición aislada de la rata a la inhalación de 1944
mg de 2-ME/m3 o más durante 4 h y la exposición repetida
a 933 mg de 2-ME/m3 o más durante 13 semanas han provo-
cado signos histológicos evidentes de alteración testi-
cular. El NENO para la exposición aguda fue de 933 mg/m3
y para la exposición repetida de 311 mg/m3. El ratón
parece ser menos sensible, siendo el NENO para la exposi-
ción repetida de 933 mg/m3. En cambio, el conejo resulta
más sensible, observándose en él alteraciones testiculares
marcadas tras la exposición repetida a 311 mg/m3 y un
efecto marginal (uno en cinco conejos afectados) a 93 mg
por m3. Efectos análogos se han observado tras la exposi-
ción oral de la rata al 2-ME, con aparición de lesiones
testiculares tras la exposición breve (inclusive de dosis
única) a 100 mg/kg. En un estu-dio subagudo (11 días) el
NENO fue de 50 mg/kg. El 2-EE es algo menos potente
respecto a la toxicidad testicular que el 2-ME; sólo se
observaron efectos con dosificaciones de 500 mg/kg o más,
siendo el NENO de 250 mg/kg.
Los datos obtenidos en las personas profesionalmente
expuestas al 2-ME y al 2-EE coinciden con los de los estu-
dios en animales e indican que estos éteres glicólicos
pueden producir toxicidad testicular en el ser humano.
Los estudios epidemiológicos de pequeños grupos de traba-
jadores expuestos al 2-EE en una empresa de fundición de
metales y de pintores de embarcaciones expuestos tanto al
2-ME como al 2-EE muestran indefectiblemente una mayor
incidencia de recuentos reducidos de espermatozoides. Los
datos sobre los niveles de exposición, aunque limitados,
aportan en cada caso pruebas de la exposición dérmica así
como de la exposición por inhalación.
En la rata, el ratón, el conejo y el mono se han
observado efectos tóxicos sobre el desarrollo tras la
exposición a estos éteres glicólicos por vía dérmica, oral
o inhalatoria. Con 12 aplicaciones diarias de 2-ME sin
diluir en la piel rasurada de ratas gestantes (oclusión:
6 h) se obtuvo un efecto letal, mientras que 10 aplica-
ciones abiertas de 2-EE (1,0 ml/día) o 2-EEA (1,4 ml/día)
se mostraron teratógenas pero no tóxicas para la madre.
Doce aplicaciones cerradas de 2-ME al 10% en suero salino
resultaron tóxicas para el desarrollo (en este estudio el
NENO fue del 3% para el 2-ME. No se han encontrado niveles
sin efecto aparente tras la administración oral repetida
de 2-ME a las hembras preñadas. El nivel de efecto obser-
vado mínimo (NEOM) en la administración oral de 2-ME fue
de 31,25 mg/kg por día en el caso de los ratones, de 25 mg
por kg por día en el de las ratas y de 0,16 mmol/kg por
día en el de los monos. Sólo se han hecho experimentos de
dosis única con el 2-ME en los ratones, con el resultado
de que el 11° día de la gestación (que es el día más sen-
sible) el NENO era de 100 mg/kg y el NEOM de 175 mg/kg.
Tanto el 2-EE como el 2-EEA se han evaluado en ratas y
conejos por inhalación. A las ratas se las expuso al 2-EE
en dos estudios, en los que se obtuvieron efectos terató-
genos (743 mg/m3 durante 7 h/día los días 1-19 de la ges-
tación) o efectos fetotóxicos (184 y 920 mg/m3 durante 6 h
por día los días 6-15 de la gestación). En este último
estudio, el NENO fue de 37 mg/m3. También los conejos
expuestos al 2-EE presentaron efectos teratógenos (589 mg
por m3 durante 7 h/día los días 1-18 de la gestación) o
efectos fetotóxicos (644 mg/m3 durante 6 h/día los días
6-18 de la gestación). En el último estudio, el NENO fue
de 184 mg/m3. En los conejos expuestos al 2-EEA durante
6 h/día los días 6-18 de la gestación se obtuvieron efec-
tos teratógenos a 2160 mg/m3 en un estudio y a 1620 mg
por m3 en otro. En ambos estudios se observó fetotoxici-
dad a 540 mg/m3 y en uno y otro el nivel de exposición
mínimo (135 y 270 mg/m3, respectivamente) coincidía con
el NENO. Las ratas expuestas al 2-EEA por inhalación dur-
ante 6 h/día los días 6-15 de la gestación presentaron el
mismo tipo de expuesta: efectos teratógenos a 1620 mg por
m3, fetotoxicidad a 1080 mg/m3 y ausencia de todo ef-
ecto a 270 mg/m3.
Así, pues, se ha observado toxicidad para el desar-
rollo en todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más. Para las tres espe-
cies, el NENO fue de 31 mg/m3. En ratas expuestas in
utero a 78 mg/m3 se han observado alteraciones conduc-
tuales y neuroquímicas, no habiéndose señalado ningún
NENO.
El 2-EE y el 2-EEA han resultado algo menos potentes.
En la rata y el conejo, todas las exposiciones al 2-EE a
368 mg/m3 o más fueron seguidas de efectos sobre el des-
arrollo. En las ratas expuestas a 184 mg de 2-EE/m3 estos ef-
ectos eran leves, pero 37 mg/m3 constituía un NENO patente.
En el caso del 2-EEA, el NENO era de 170 mg/m3 tanto en
la rata como en el conejo.
Tanto en los animales como en los casos de envenena-
miento humano se han observado efectos hematológicos tras
la exposición a una dosis aguda única. La exposición por
inhalación repetida de la especie más sensible, el conejo,
al 2-ME durante 13 semanas y a razón de cinco veces por
semana dio un NEI de 93 mg/m3. También las dosis repeti-
das de 2-ME provocan toxicidad hematológica en los
ratones, conejos, perros, hámsters y cobayos. El 2-EE es
menos potente que el 2-ME como causa de efectos hemato-
lógicos. El NENO correspondiente a estos efectos fue de
368 mg/m3 en las ratas y los conejos expuestos a 2-EE
durante 13 semanas a razón de cinco veces por semana
durante 6 h/día. También en los perros y los ratones se
han registrado efectos hematológicos tras la exposición
repetida a concentraciones más elevadas de 2-EE. La
exposición a los ésteres acéticos del 2-EE y del 2-ME
quizá provoque efectos análogos a los mismos niveles de
exposición, pero los datos disponibles sobre exposición y
efectos hematológicos de esos compuestos son demasiado
escasos para determinar en qué condiciones una exposición
humana aislada tendrá efectos hematológicos.
Se han observado niveles de exposición industrial
próximos o idénticos al NENO de efectos hematológicos en
animales expuestos a dosis repetidas de 2-ME o de 2-EE.
Este hecho, junto con la mayor sensibilidad que probable-
mente tienen las personas y la acumulación previsible de
metabolitos en la sangre humana, hace pensar que tanto la
exposición industrial como la de los consumidores pueden
tener efectos hematológicos. Esto ha sido confirmado por
la observación de efectos de este tipo en algunos de los
escasos estudios realizados sobre trabajadores industri-
ales expuestos repetidamente al 2-EE, al 2-ME o a ambos
compuestos a la vez.
2. Evaluación de los efectos sobre el medio ambiente
La exposición ambiental a estos éteres glicólicos
puede producirse como consecuencia de su paso directo a la
atmósfera cuando se utilizan como solventes volátiles.
También pueden ser causa de exposición ambiental los ver-
tidos en el suelo y el agua a consecuencia de escapes ac-
cidentales. La acumulación en el suelo y en las aguas sup-
erficiales sólo podría producirse en ausencia de degrada-
ción. Sin embargo, estos éteres glicólicos se degradan
rápidamente a causa de procesos químicos y biológicos, por
lo que no es probable que se acumulen. Tanto el 2-MEA como
el 2-EEA pueden hidrolizarse fácilmente y, por consigui-
ente, biodegradarse en condiciones aerobias. En cambio,
la contaminación de acuíferos y suelos anaerobios sigue
planteando un problema potencial, aunque es de esperar que
no pase de ser una situación transitoria y, por ende, poco
peligrosa.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
con los microorganismos y las especies acuáticas. Los
acetatos de éteres glicólicos, en cambio, tienen una toxi-
cidad aguda mucho mayor. No se dispone de datos para cal-
cular las posibilidades de efectos adversos en especies
del medio ambiente a causa de la exposición prolongada.
RECOMENDACIONES
1. Protección de la salud
1. Hay que identificar otros solventes menos tóxicos que
puedan sustituir al 2-metoxietanol, al 2-etoxietanol y a
sus ésteres, especialmente en los productos destinados al
consumo. También es particularme importante evaluar los
efectos de otros éteres etilenglicólicos, ya que algunos
pueden tener efectos análogos a los de los cuatro éteres
glicólicos que aquí se evalúan.
2. Habida cuenta de los conocidos efectos tóxicos de
estos éteres glicólicos, las autoridades deben ocuparse
seriamente de establecer estrategias apropiadas para
informar a los usuarios de esos productos acerca de los
riesgos que entrañan, especialmente los resultantes de la
exposición dérmica.
3. En vista de los nuevos datos toxicológicos y de las
posibilidades de absorción dérmica importante de estos
éteres glicólicos, habrá que reconsiderar los límites de
exposición profesional fijados por los países a fin de que
la dosis diaria total que reciben los trabajadores por
todas las vías de administración no plantee un riesgo
indebido para la salud.
4. En los animales se observan efectos de dosis única en
niveles de exposición bastante altos. Con objeto de
reducir los riesgos para la salud, se recomienda utilizar
con prudencia estos compuestos (prestando atención a la
higiene personal, los dispositivos apropiados de protec-
ción y la ventilación adecuada). Los datos disponibles
indican que puede ser necesario aumentar la protección a
fin de evitar efectos en el desarrollo, así como en la
sangre y los testículos como resultando de la exposición
repetida.
2. Investigaciones necesarias
1. En vista de la intervención del ácido metoxiacético
(MAA) y etoxiacético (EAA) (que son los principales meta-
bolitos identificados del 2-ME, del 2-EE y de sus ésteres)
en la toxicidad para el sistema reproductor masculino,
habrá que investigar su mecanismo de acción. Si de ello
se deduce que el MAA y el EAA no son los principales agen-
tes responsables, habrá que tratar de identificar éstos y
de aclarar su mecanismo de acción.
2. Estos cuatro éteres glicólicos tienen a la vez efectos
hematológicos y efectos sobre el sitema reproductor mascu-
lino (reducción del recuento de espermatozoides). Los
datos disponibles, aunque limitados, parecen indicar que
ambos efectos se hacen evidentes a niveles de dosificación
análogos. Habrá que investigar el mecanismo de acción en
ambos sistemas orgánicos y examinar paralelamente los
efectos hematológicos y los recuentos de espermatozoides
con el fin de determinar si las alteraciones de la sangre
proporcionan signos de alarma con repecto a otros efectos
de estos compuestos.
3. La vigilancia del aire no basta por sí sola para
garantizar una exposición de poca intensidad. La vigilan-
cia biológica puede contribuir a detectar defectos de las
medidas de protección. De momento no se ha establecido
claramente la relación existente entre los indicadores
biológicos de exposición, la absorción corporal total y
los efectos observados en la salud. Habrá que proseguir
las investigaciones a fin de adquirir una base para apli-
car la vigilancia biológica a la determinación de la
seguridad en las exposiciones.
4. Hay que proyectar estudios epidemiológicos y/o traba-
jos específicos de vigilancia sanitaria en poblaciones muy
expuestas a estos éteres glicólicos a fin de estimar las
relaciones exposición-efecto con miras a determinar expo-
siciones seguras, siempre que se pueda evaluar adecuada y
suficientemente la exposición total mediante la vigilancia
ambiental y biológica.
5. Habrá que investigar la posibilidad de que estos com-
puestos ejerzan efectos sobre las gónadas femeninas medi-
ante estudios de reproducción multigeneracional en ani-
males.
6. Los datos disponibles indican que el ser humano puede
metabolizar estos éteres glicólicos hasta los correspon-
dientes ácidos alcoxiacéticos en mayor medida que la rata,
y que la vida media de la excreción urinaria de estos
metabolitos tóxicos es aproximadamente cuatro veces más
prolongada en las personas que en las ratas. Por otra
parte, éstas conjugan una mayor cantidad de los metaboli-
tos ácidos, cosa que no hacen las personas. Estas diferen-
cias podrían explicar la sensibilidad relativamente elev-
ada del ser humano a estos éteres glicólicos. Un conocimi-
ento detallado del metabolismo y de la cinética de excre-
ción mejoraría nuestra capacidad para predecir cuáles son
los niveles de exposición seguros.
7. Los resultados obtenidos en estudios a corto plazo (13
semanas) indican efectos en diversos sistemas orgánicos.
En cambio, no se han hecho estudios de suficiente duración
que permitan evaluar la reversibilidad de tales efectos.
Por consiguiente, convendría que se emprendieran estudios
de interrupción en los que los animales de experimentación
estuvieran expuestos a estos éteres glicólicos al menos
durante 13 semanas, seguidas de un periodo apropiado de
recuperación. Cabría evaluar así importantes parámetros
fisiológicos con miras a determinar si esos efectos son o
no transitorios.
When rats were administered 2-EE at doses from
0.5 mg/kg to 100 mg/kg, Groeseneken et al. (1988) found
increasing relative amounts of EAA in urine (from 13.4% to
36.8% of the total dose). This could be the result of
competition by other metabolic pathways, which would
become more easily saturated at the higher dosage levels.
At 2-EE doses equivalent to the lowest doses in these
animal studies, it was estimated that humans excrete 30-
35% as EAA. Furthermore, 27% (on average) of the EAA was
excreted as the glycine conjugate in the rat, whereas no
glycine conjugation was observed in humans.
The in vitro nasal mucosal carboxylesterase activity
of mice was compared to the activity of other mice tissues
and to the nasal mucosal carboxylesterase activity of
rats, rabbits, or dogs when exposed to 2-MEA or 2-EEA
(Stott & McKenna, 1985). The specific activity in nasal
carboxylase was found to be similar to that of the liver
in mice, but it was greater than the activity found in the
kidney, lung, or blood of mice. Nasal mucosal carboxyl-
esterase activity of mice was comparable to that of dogs,
slightly higher than the activity in rats, and nearly six-
fold higher than the activity in rabbits. These in vitro
studies suggest that considerable hydrolysis may occur in
the intact animal, resulting in the formation of acetic
acid at the initial route of entry.
6.4. Elimination and Excretion
Although 2-ME is rapidly metabolized to 2-MAA after an
intraperitoneal dose of 250 mg/kg body weight in the rat,
the excretion of 2-MAA is fairly slow (half-life of ap-
proximately 20 h) (Moss et al., 1985). In humans, an elim-
ination half-life for 2-MAA of 77.1 h has been reported
(Groeseneken et al., 1989a). The elimination of radioac-
tive 2-EAA (ethyl 1,214C) has been reported in the rat
(half-life of approximately 8 h) (Guest et al., 1984) and
in humans (half-life of approximately 21-42 h)
(Groeseneken, 1986b,c, 1988; Veulemans, 1987a). In rats,
the administration of an oral dose of 230 mg 2-EE/kg body
weight led to the production of EAA and N -ethoxyacety gly-
cine (> 76% of the dose), EAA being the major metabolite
found in testes after 2 h (Cheever et al., 1984).
The urinary excretion of EAA during and after single
4-h exposures to 14, 28, or 50 mg 2-EEA/m3 in human
volunteers has been reported by Groeseneken et al. (1987).
The distribution/excretion time course during and after
2-EEA exposure was similar to that observed for 2-EE.
This indicates that humans, like rodents, hydrolyse the
acetate to 2-EE, which is then converted to the EAA metab-
olite and excreted. The excretion of the EAA metabolite
was observed to be biphasic. A second peak of excretion
occurred approximately 3 h after the first, suggesting
some type of redistribution of the glycol ethers, or of a
metabolite, from a peripheral compartment.
The urinary excretion of EAA was evaluated under field
conditions in which women volunteers were exposed daily to
2-EE or 2-EEA in the process of silk-screen printing
(Veulemans et al., 1987a). Urinary EAA was measured during
5 days of normal production and was also detectable after
a 12-day stop in production. The excretion of EAA in-
creased during the work week, yet it was still detectable
after 12 days without exposure. These data suggest that
the retention of EAA, or of other 2-EE or 2-EEA metab-
olite, may be toxicologically significant if EAA is the
active metabolite responsible for the observed toxicity.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Given the physical and chemical properties of these
four glycol ethers and the known rates of degradation in
the environment (section 4), there is minimal concern that
hazardous levels of these substances will occur. Although
only a few studies have been reported, the available data
support this conclusion. For example, both 2-ME and 2-EE
have been tested for effects on microorganisms and aquatic
animals. The lethal concentration of 2-ME and 2-EE to
microorganisms (Cladosporium resinae, Pseudomonas aeru-
ginosa, Gliomastix sp, and Candida sp is > 2% in the
medium (Neihof & Bailey, 1978; Lee & Wong, 1979). The
exposure of C. resinae lasted 30 to 42 days, whereas the
other organisms were exposed for 4 months. Very low
toxicity was shown by 2-ME to the green alga Scenedesmus
quadricauda (growth inhibition only at > 10 g/litre) and
the cyano-bacterium (blue-green alga) Microcystis aerugin-
osa (growth inhibition only at > 100 mg/litre) (Bringmann
& Kuhn, 1978). 2-EE has very low toxicity to the brine
shrimp (Artemia salina) (LC50 > 4 g/litre) (Price et
al., 1974) and to another arthropod Daphnia magna
(Hermens et al., 1984). The toxicity of 2-EE to fresh-
water fish is also very low, the LC50 (96 h) for the
bluegill (Lepomis macrochirus) being > 10 g/litre. However,
2-EEA is far more toxic to fish in this assay, LC50 (96 h)
values of 60 mg/litre (Bailey et al., 1985) and 46 mg per
litre in fathead minnows (Pimephales promelas) (Purdy,
1987) having been reported. These results confirm those of
Juhnke & Ludemann (1978), who reported an LC50 (48 h)
value of 107-141 mg per litre when using the Golden Orfe
(Leuciscus idus melanotus) test. The reason for this low
LC50 has not been studied, but Dawson et al. (1977) re-
ported similarly low LC50 values for 2-MEA in fish (45 mg
per litre in tidewater silverfish (Menidia beryllina)
and bluegill (Lepomis chirus).
Effects on other organisms have not been reported.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data indicating the acute oral, dermal, intraperito-
neal, and inhalation toxicities of 2-ME and 2-EE are given
in Table 7. As shown, these two glycol ethers have similar
toxicities, and are of low acute toxicity whether animals
are exposed via the dermal, oral, or respiratory routes.
Even after intraperitoneal injection, the reported levels
of acute toxicity are still low (1.7-2.46 g per kg body
weight).
Dyspnoea, somnolence, ataxia, and prostration were re-
ported after near lethal doses of 2-EE were given to rats,
mice, guinea-pigs, or rabbits (Stenger et al., 1971).
Haemoglobinuria and/or haematuria were reported after a
single oral administration of 2-EE with a dose approaching
the LD50 (Laug et al., 1939). Microscopic examination of
the kidneys of rats, mice, guinea-pigs, or rabbits given
2-EE orally revealed severe tubular degeneration, conges-
tion, and cast formation; in some animals most of the
cortical tubules were necrotic (Laug et al., 1939). No
data on the purity of the 2-EE used in these early studies
were reported.
Single 4-h inhalation exposures of rats to 1866 mg 2-ME
per m3 or more led to testicular atrophy as early as 24 h
after exposure (Doe, 1984b).
8.2. Short-term Exposures
Repeated exposure of experimental animals to 2-ME and
2-EE by various routes of administration (7-90 days) have
revealed adverse effects on the haematological and nervous
systems and on the testes, thymus, kidney, liver, and
lung. From data on the metabolic transformation and
elimination of 2-MEA and 2-EEA in animals and man (see
section 6), it is probable that the toxicities of these
glycol ether acetates are similar to those of the parent
chemicals 2-ME and 2-EE.
8.2.1. Haematological and immunological effects
Following repeated exposures to 2-ME and 2-EE using
various routes of administration, haematological effects
have been observed in several species. A summary of the
most relevant studies is given in Tables 8 (2-ME) and 9
(2-EE).
Table 7. Acute toxicity data for 2-methoxyethanol and 2-ethoxyethanol and their acetatesa
-------------------------------------------------------------------------------------------------------------
Compound Species Intra- Oral LD50 Dermal Inhalation References
peritoneal LD50 LC50
LD50
-------------------------------------------------------------------------------------------------------------
2-Methoxyethanol Rat 2460 2460-3400 Smyth et al. (1941); Goldberg et al.
(1962); Pisko & Verbilov (1988)
Mouse 2200 2167-2560 4603 Werner et al. (1943); Saparmamedov
(1480) (1974); Pisko & Verbilov (1988)
Guinea-pig 950 Karel et al. (1947)
Rabbit 890-1450 1300 Carpenter et al. (1956); Pisko &
Verbilov (1988)
2-Ethoxyethanol Rat 2000 2125-5500 Smyth et al. (1941); Laug et al.(1939)
Stenger et al. (1971); Pisko & Verbilov
(1988)
Mouse 1700 4000-4800 6698 Werner et al. (1943); Laug et al.(1939)
(1820) Stenger et al. (1971); Saparmemedov
(1974); Pisko & Verbilov (1988)
Guinea-pig 1400-2600 Karel et al. (1947); Laug et al.(1939)
Rabbit 3300- Stenger et al. (1971); Smyth et al.
15 200 (1941)
2-Methoxyethanol Rat 3930 Smyth et al. (1941)
acetate Guinea-pig 1250 Kirk-Othmer (1980)
Rabbit 5557
2-Ethoxyethanol Rat 5100 Smyth et al. (1941)
acetate Guinea-pig 1910 Kirk-Othmer (1980)
Rabbit 10 333
-------------------------------------------------------------------------------------------------------------
a Doses are given as mg/kg body weight except for inhalation doses,
which are in mg/m3 (values in parentheses are ppm)
Concerning changes in blood parameters, a comparison
of Tables 7, 8, and 9 indicates clearly that 2-ME is more
toxic than 2-EE in several species, although 2-EE has been
less widely studied. For example, Nagano et al. (1979)
showed that 2-EE administered orally for 5 weeks at 2000
mg/kg body weight per day did not result in changes in
haematological parameters other than a reduced leucocyte
count. No effects were observed at 1000 mg/kg. Under a
similar dosing regimen, 2-ME caused a dose-related re-
duction in leucocytes at 500 mg/kg per day, other haemato-
logical parameters being unaffected at 250 mg/kg per day
(Nagano et al., 1979).
Some of the doses used in the studies summarized in
Tables 7 and 8 resulted in histological and functional
changes in the bone marrow and thymus. In an inhalation
study, Miller et al. (1983b) noted thymic atrophy and de-
creased thymus weights in male and female rats exposed to
933 mg 2-ME/m3 for 13 weeks. Miller et al. (1981) also
noted decreased weights of thymus, spleen, and mesenteric
lymph nodes in rats exposed to 933 or 3110 mg 2-ME/m3 for
9 days, and reduced bone marrow cellularity was observed
at 3110 mg/m3. In one study there was at least partial
reversal of these effects 22 days after an exposure last-
ing 4 days (Grant et al., 1985).
When MAA, the metabolite of 2-ME, was administered to
rats by gavage at 300 mg/kg body weight per day for 8
days, it resulted in reduced erythrocyte counts, haemo-
globin concentrations, and packed cell volumes, and a
marked reduction in leucocyte counts. Thymus and spleen
weights were also decreased and a severe lymphoid
depletion was seen in the thymus. Similar, but less
marked, effects on blood and thymus were seen in some
animals administered 100 mg/kg per day. No treatment-
related effects were reported at 30 mg/kg per day (Miller
et al., 1982).
Studies on immunological function show that 2-ME and
2-EE yield different results. De Delbarre et al. (1980)
studied the effect of 2-ME and 2-EE on the humoral respon-
siveness to antigenic stimuli and on adjuvant arthritis in
the rat. 2-ME had an inhibitory effect on adjuvant ar-
thritis at doses greater than 18.6 mg/kg body weight per
day administered subcutaneously, whereas 2-EE was not
effective at doses up to 150 mg/kg. A dose of 150 mg 2-ME
per kg per day delayed rejection of skin grafts and 75 mg
2-ME/kg per day (for 28 days) significantly depressed
antibody production. Again, 2-EE had no effect on these
parameters. House et al. (1985) administered 2-ME to mice
by gavage (10 doses of 250, 500, or 1000 mg 2-ME/kg body
weight per day for 2 weeks) and noted a 48% reduction in
thymus weight at 500 and 1000 mg/kg. However, there was no
significant alteration in immune function or host resist-
ance. Similar findings were reported when the same dose
levels of MAA, the major metabolite of 2-ME (section 6),
were administered.
Table 8. Haematological effects of 2-methoxyethanol in animalsa
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat inhalation 10 males 6 h/day; 9 days; RBC fragility, no effect at Miller et al.
10 females 311, 933, 3110 mg/m3 3110 mg/m3 but reduced RBC, (1981)
Hb, and PCV levels and bone
marrow cellularity seen in M
and F; similar findings in F
at 933 mg/m3; reduced WBC
at 3110 and 933 mg/m3;
reduced thymus weight
Rat inhalation 10 males 6 h/day; 5 days/week only effect at 2 lowest Miller et al.
10 females for 13 weeks; doses was reduced body (1983b)
93.3, 311, 933 mg/m3 weight gain in F; at 933
mg/m3 reduced WBC, Hb,
PCV, and platelets in both
sexes after 4 and 12 weeks;
reduced serum proteins at 13
weeks, thymic atrophy
Rabbit inhalation 5 males 6 h/day; 5 days/week decreased thymus size and Miller et al.
5 females for 13 weeks; body weight, PCV, WBC, (1983b)
93.3, 311, 933 mg/m3 platelets and Hb at 933
mg/m3; slight to moderate
decrease in lymphoid tissue
at 311 mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week decreased RBC, Hb and Hct; Werner et al.
2 controls for 12 weeks; increased immature (1943)
750 mg/m3 granulocytes
Mouse oral 5 males 5 times/week for 4/5 mice at 2000 mg/kg died Nagano et al.
5 weeks; 62.5, 125, before completion; doses at (1979)
250, 500, 1000, 2000 or above 500 mg/kg resulted
mg/kg body weight/day in reduced WBC, RBC, and PCV
Table 8 (contd.)
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat oral 24 males once per day for reduced thymus and spleen Grant et al.
4 days; 100 and 500 weight at 500 mg/kg on days (1985)
mg/kg body weight/day; 1-8, recovery by day 22;
sacrificed reduced WBC at both 100 and
500 mg/kg, largely
reversible by day 22
Hamster oral 4 males once daily, 5 days/week decrease in WBC at 500 mg/kg Nagano et al.
for 5 weeks; 62.5, 125, the only effect on blood (1984)
500 mg/kg body weight/day
Guinea oral 3 males once daily, 5 days/week about 50% decrease in WBC at Nagano et al.
-pig for 5 weeks; 250 and 500 both doses (1984)
mg/kg body weight/day
--------------------------------------------------------------------------------------------------------
a M = male; F = female; PCV = packed cell volume; Hct = haematocrit; RBC = red blood cell count;
WBC = white blood cell count; Hb = haemoglobin; No. = number of animals per group
Table 9. Haematological effects of 2-ethoxyethanol in animalsa
-------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Response Reference
time; level
-------------------------------------------------------------------------------------------------------
Rat inhalation 15 males 6 h/day; 5 days/week decreased leucocyte count Barbee et al.
15 females for 13 weeks; 92, 368, in females at 1472 mg/m3, (1984)
1472 mg/m3 no other significant
biological effect reported
Rabbit inhalation 10 males 6 h/day; 5 days/week for decreased Hb, Hct, and Barbee et al.
10 females 13 weeks; 92, 368, 1472 RBC in both males and (1984)
mg/m3 females only at 1472
mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week for slight reduction in RBC, Werner et al.
2 control 12 weeks; 3091 mg/m3 Hb, and PCV, microcytosis, (1943)
hypochromia, and poly-
chromatophilia were seen,
marked increase in
immature granulocytes
Mouse oral 5 males 5 times/week for 5 lower WBC at 2000 mg/kg, Nagano et al.
weeks; 62.5, 125, 250, but no effect on (1979)
500, 3110, 2000 mg/kg erythrocyte parameters
body weight/day
-------------------------------------------------------------------------------------------------------
a PCV = packed cell volume; Hct = haematocrit;
RBC = red blood cell count; WBC = white blood cell count; Hb = haemoglobin
No. = number of animals per group
8.2.2 Effects on liver and kidney
Effects on the liver, such as reduced cytoplasmic den-
sity, disruption of lobular structure, elevated plasma
fibrinogen, reduced serum proteins, and elevated liver
weights, have been reported in certain studies in which
rats, mice, or rabbits were exposed to 2-ME or 2-EE at
levels in excess of 300 ppm (933 and 1104 mg/m3, respect-
ively), for periods of up to 13 weeks. Many of the effects
observed were reversible, and no consistent pattern was
noted among the various studies (Miller et al., 1981;
1983b; Stenger et al., 1971). Hepatic changes have been
observed at inhalation exposures to 2-ME and 2-EE in ex-
cess of 300 ppm (933 and 1104 mg/m3, respectively).
No pathological changes could be detected in the kid-
neys of rats exposed to approximately 933 mg 2-ME/m3 for
6 or 7 h per day, 5 days a week, for 13 weeks (Miller et
al., 1981, 1983b). In studies with 2-EE, no treatment-
related pathology was reported after inhalation exposure
of rats to 1362 mg/m3 (370 ppm) for 5 weeks or dogs to
3091 mg/m3 (840 ppm) for 12 weeks (Werner et al., 1943).
8.2.3 Behavioural and neurological effects
There have been few reports on the effects of 2-ME
and 2-EE on the function of the nervous system in animals.
Ataxia was reported after inhalation exposure of rats to
18.96 g 2-EE/m3 (5152 ppm) for 8h. After exposure of rats
to levels of 2-ME between 1555 and 12 440 mg/m3, 4 h per
day for up to 7 days, inhibition of an avoidance-escape
conditioned response was observed without any alteration
of motor function (Goldberg et al., 1962). The same
authors reported also a significant inhibition of this
response after 14 days exposure to 1210-4836 mg 2-ME per
m3 (389-1555 ppm). After 3 weeks recovery, rats whose
avoidance response had been inhibited on the 14th day
showed a return to normal. Savolainen (1980) reported a
partial loss of motor function in the hind limbs of rats
after exposure to 1244 mg 2-ME/m3 (400 ppm) for 6 h/day,
5 days per week, for 2 weeks. This hindlimb paralysis
coincided with the glial cell toxicity noted during the
second week of exposure. Recovery was incomplete after 2
weeks post-exposure, the animals receiving the highest
dose showing minor paresis.
8.3 Skin and Eye Irritation; Sensitization
No satisfactory data on the sensitization potential of
2-ME and 2-EE in animals, and only limited data on their
irritant properties to eyes, have been reported. Weil &
Scala (1971) found 2-ME to be an eye irritant. Lailler et
al. (1975) reported that 0.1 ml of undiluted 2-ME led to
corneal and conjunctival oedema and increased vascular
leakage in the conjunctiva and aqueous humour. A 25% aque-
ous solution was much less active.
8.4 Long-term Exposures
No adequate long-term animal studies on 2-ME and 2-EE
or their acetates have been reported to date.
8.5 Effects on Reproduction and Fetal Development
The effects of glycol ethers, particularly 2-ME, on
animal reproduction, fertility, and teratogenicity have
been extensively studied. Some of the early studies on
reproduction have been reviewed by Hardin (1983). The many
studies conducted in several countries and in several
animal species are in general agreement on the nature of
developmental and reproductive effects.
8.5.1 Effects on the male reproductive system
8.5.1.1 Oral exposure
The pathological changes observed in the testes of
rats after the administration of 2-ME have been well
characterized by Foster et al. (1983). The severe degener-
ative changes noted in the germinal epithelium of the
seminiferous tubules of different laboratory animals were
similar, irrespective of species or route of adminis-
tration. Daily doses of 2-ME were administered orally to
rats (50, 100, 250, or 500 mg/kg) for periods between 1
and 11 days, and 250, 500, or 1000 mg 2-EE/kg body weight
per day was administered in a similar regimen (Foster et
al., 1983; Creasy & Foster, 1984; Creasy et al., 1985).
After 2-ME administration, testicular damage was observed
1 day post dosing with 100 mg/kg or more, the lesion being
localized in the late primary spermatocytes. Continuous
dosing with 2-ME resulted not only in progressive deletion
of the primary spermatocytes but also in degenerative
changes in secondary spermatocytes and dividing sper-
matids. Changes in sperm motility, morphology, and concen-
tration were also reported. The cessation in maturation
of early primary spermatocytes led to a depletion of the
spermatid population, resulting in tubules containing only
Sertoli cells, spermatogonia, and early primary spermato-
cytes. Decreased testicular weight was reported at 250 mg
per kg or more. Although most of the effects seen after a
4-day treatment with 2-ME were reversible within 8 weeks,
a small proportion of tubules showed incomplete recovery
indicating a non-reversible, long-term effect. Similar
findings were reported after 2-EE administration, but only
after 11 days of dosing with 500 and 1000 mg/kg. No
effects on the testes were observed following oral admin-
istration of 250 mg 2-EE/kg per day or 50 mg 2-ME/kg per
day.
Subsequent work by Chapin et al. (1985a,b) supports
the hypothesis that 2-ME administered to male rats at
doses of 100 or 200 mg/kg daily for 5 days affects the
spermatogonia. In these studies male rats were given doses
of 0, 50, 100, or 200 mg/kg per day for 5 days, and recov-
ery was investigated by evaluating the testicular damage
in sacrificed animals at 8 subsequent weekly intervals or
alternatively by investigating fertility through mating
with two females per week for 8 weeks. Treatment with 100
or 200 mg/kg resulted in widespread testicular damage and
cell death immediately after treatment, with only very
mild effects being noted at 50 mg/kg (this was thus a
marginal-effect level rather than a no-effect level).
There was some evidence of recovery from testicular damage
towards the end of the study period, but reduced fertility
was still apparent weeks after the cessation of treatment
with 200 mg/kg. Thus, the recovery was neither as
complete nor as rapid as that noted by Foster et al.
(1983).
The in vivo and in vitro effects of 2-ME and MAA ex-
posures, respectively, were compared by evaluating effects
using electron microscopy (Creasy et al., 1986). 2-ME
caused cell death in the pachytene spermatocytes in vivo ,
as well as focal breakdown of the plasma membranes be-
tween spermatocytes and Sertoli cells. Similar, but less
frequent, breaks were seen in vitro when mixed cultures of
Sertoli and germ cells were treated with MAA.
Subsequent studies by Foster et al. (1987), in which
the alkoxyacetic acids of 2-ME and 2-EE (MAA and EAA,
respectively) were given orally in doses equimolar to the
studies described earlier (Foster et al., 1983), yielded
similar patterns of testicular degeneration and effects on
spermatocyte development. The similarity of effects at
equimolar concentrations between the two acetic acid de-
rivatives and their respective glycol ethers suggests that
these metabolites may either be the causative factors or
at least play a role in the production of the observed
degenerative effects.
The assessment of the effects of low doses of poten-
tial toxicants on sperm fertility and production is diffi-
cult because of the large amount of sperm usually produced
by most test species and the large sperm reserves. Using
an experimental design that required bi-daily matings of
Long-Evans male rats, the effects of orally administered
2-EE (0, 150, or 300 mg/kg body weight per day; 5 days per
week for 6 weeks) on sperm reserves and on pachytene sper-
matocytes was evaluated (Hurtt & Zenick, 1986). Further
groups of rats received the same doses of 2-EE, but were
not subjected to repeated mating. After 6 weeks of treat-
ment, the animals were sacrificed and the effect of 2-EE
administration on organ weight, testicular spermatid
count, cauda epididymal sperm count, and sperm morphology
was determined. Exposures to 2-EE resulted in significant
decreases in testicular weight, spermatid count, and epi-
didymal sperm count for both mated and unmated animals at
the highest dose level. However, the effects were also
seen at 150 mg/kg per day in repeatedly mated animals.
Adult male rats treated orally with 2-EE (936 mg/kg
body weight, 5 days/week for 6 weeks) were found to have
decreased sperm counts and altered sperm morphologies
after 5 and 6 weeks of exposure when compared with
vehicle-control animals, and sperm motility was decreased
at week 6 (Oudiz & Zenick, 1986). These data indicate that
the pachytene spermatocyte is the target cell most sensi-
tive to the effects of 2-EE.
The reproductive toxicity of 2-EE in CD-1 mice has
been evaluated in a continuous breeding protocol (Lamb et
al., 1984). Male and female mice (20 males and 20 females
per dose group) were given access to drinking-water con-
taining 0.5, 1.0, or 2.0% 2-EE and housed as breeding
pairs continuously for 14 weeks. Treated animals were then
paired with controls for breeding. At 1 and 2% 2-EE, sig-
nificant adverse effects on fertility were seen, the re-
productive capacity of both males and females being af-
fected. Testicular atrophy, decreased sperm motility, and
increased abnormal sperm levels were seen in treated
males, but no treatment-related pathological effects were
seen in females even though reduced fertility was noted in
the cross-mating phase.
The reproductive toxicity of a single oral dose of
2-ME (0, 500, 750, 1000, or 1500 mg/kg) was assessed in
adult male CD rats and CD-1 mice by Anderson et al.
(1987). Animals from each group were sacrificed at weekly
intervals during weeks 3-8 post exposure and evaluated for
sperm counts, sperm morphology, and testicular histology.
A dose-related toxicological response in spermatocytes was
observed in both species. In a companion study, male rats
and mice were exposed to 2-ME (0, 125, 250, or 500 mg/kg)
and permitted to mate during weeks 1-10 post exposure.
Following mating, pregnant females were sacrificed on day
17 of pregnancy and each uterus was evaluated for dominant
lethal effects. The only effect noted was a decrease in
total number of implants at week 6 following treatment
with 500 mg/kg. In a study in which both rats and mice
were given single doses at 0, 500, 750, 1000, or 1500 mg
per kg and mated 5 and 6 weeks later, dominant lethal
studies showed a dose-related decrease in fertility in
rats, with complete sterility in all but the lowest dose
group after 6 weeks. No effects on the reproductive
capacity of mice were noted. There was no statistically
significant evidence for the induction of dominant lethal
mutations or abnormalities in the F1 generation of either
species.
8.5.1.2 Inhalation studies
Testicular damage has been reported in rats exposed to
2-ME by inhalation for a single 4-h period (Doe, 1984b).
Exposure to 1944 mg/m3 resulted in histological evidence
of damage to the maturing spermatids, testicular atrophy
occurring at 3887 mg/m3 or more. The NOEL was 933 mg/m3.
The effect of repeated exposure to 2-ME has also been
investigated using the inhalation route. Exposure of rats
to 3110 mg/m3 (6 h/day) for 9 out of 11 days resulted in
degenerative changes and necrosis in the germinal epi-
thelium of the seminiferous tubules, but no effects on the
testes were observed with a dose of 933 mg/m3. However,
Doe et al. (1983) reported pronounced atrophy of the sem-
iniferous tubules in rats exposed to 933 mg 2-ME/m3 or
more (6 h/day, 5 days/week) for 13 weeks. The NOEL was
311 mg/m3 (Miller et al., 1983b). Studies were also car-
ried out in rabbits, using exposure levels of 93.3, 311,
and 933 mg/m3 (6 h/day, 5 days/week), and most animals
exposed to 311 mg/m3 showed reduced testis size and
severe degenerative changes in the tubules. Reduced testis
weight was also seen in two out of five animals exposed to
93.3 mg/m3, and one animal showed histological changes
in the germinal epithelium. A NOEL could not be ident-
ified, but 93.3 mg/m3 was near the minimal effective dose
in rabbits.
In addition, an increased incidence of sperm abnor-
malities (principally sperm with banana shaped or amorph-
orous heads) has been noted in mice exposed to 1555 mg
2-ME/m3 (7 h per day for 5 days) but not to 78 mg per
m3 (McGregor et al., 1983).
8.5.2 Embryotoxicity and developmental effects
8.5.2.1 2-Methoxyethanol
2-ME has been shown to lead to embryotoxic and devel-
opmental effects in laboratory animals following inha-
lation, oral, or dermal exposure. The effects of 2-ME
exposure over the entire organ-forming period of gestation
have been studied in rabbits, rats, and mice, the rabbit
being the most sensitive species.
The embryotoxicity of 2-ME after gastric intubation
was evaluated in mice (31.25, 62.5, 125, 250, 500, or 1000
mg/kg body weight) on days 7-14 of gestation (Nagano et
al., 1981). No maternal deaths were observed, but maternal
weight gain was reduced in the mice that received doses of
250 mg/kg per day or more. Maternal toxicity was not seen
at 123 mg/kg or less. On day 18 of gestation, fetuses were
examined and an increase in fetal death rate was observed
at doses of 250 mg/kg or more. At doses of 500 and 1000 mg
per kg, all fetuses were dead at caesarean section, except
for one in the 500-mg/kg group. Approximately 44% (57/130)
of the live fetuses in the 250-mg/kg group were found to
have gross anomalies, including exencephaly (24), umbili-
cal hernia (3), and abnormal digits (29). One fetus had
both exencephaly and abnormal digits. The lone surviving
fetus in the 500-mg/kg group had exencephaly and abnormal
digits. Minimal skeletal malformations were also observed
at the lowest dose of 31.25 mg/kg body weight (a matern-
ally non-toxic dose). Thus, a NOEL could not be ascer-
tained.
Dose-dependent electrocardiographic changes were
detected on gestation day 20 in the rat fetuses of mothers
exposed orally to 2-ME (0, 25, or 50 mg/kg body weight) on
days 7 to 13 of gestation (Toraason et al., 1985). Cardio-
vascular malformations were observed, including right
ductus arteriosus and ventricular septal defects. QRS in-
tervals were significantly prolonged, particularly in the
highest dose group, suggesting intra-ventricular conduc-
tion delays.
Ornithine decarboxylase (ODC) activity is highest
during rapid growth and development in fetuses and is
sensitive to maternal exposure to chemicals and drugs.
Toraason et al. (1986a,b) evaluated the effect on ODC ac-
tivity in the fetuses of pregnant rats that received 25 mg
2-ME/kg by gavage during gestation days 7-13 or 13-19. The
activity was most affected in fetuses exposed during ges-
tation days 7 to 13. It was highest in 3-day old rats and
declined sharply thereafter. No functional or morphologi-
cal effects were observed in fetuses exposed at maternal
dose levels of 25 mg/kg during gestation days 7-13 or in
fetuses exposed at that level during days 13-19.
Nelson et al. (1984a) treated male rats by inhalation
(78 mg 2-ME/m3 for 7 h/day, 7 days/week for 6 weeks) and
subsequently bred these to untreated females. In addition,
groups of 15 pregnant rats were treated with the same dose
(7 h/day on gestation days 7-13 or 14-20) and allowed to
deliver and rear their young. Neuromotor function activity
and simple learning ability were assessed on days 10-90
after birth. Offspring from dams treated between gestation
days 7-13 showed significant changes in avoidance con-
ditioning, and changes in neurochemical levels were ob-
served in the brains of 21-day-old offspring from the
paternally exposed group as well as from both maternally
exposed groups.
8.5.2.2 2-Ethoxyethanol
A dose-finding study in pregnant rats revealed that no
offspring survived inhalation exposures of 3312 mg 2-EE
per m3, 7 h/day, during gestation days 7-13 or 14-20
(Nelson et al., 1981). The authors reported 34% neonatal
deaths at 736 mg/m3 under similar exposure conditions.
Offspring from dams exposed to 368 mg/m3 on gestation
days 7-13 or 14-20 showed impaired performance in behav-
ioural tests and neurochemical alterations in brain samples.
Using inhalation exposures of 478, 1435, and 2208 mg
2-EE/m3 on gestation days 7-15 (7 h/day), Nelson et al.
(1984b) reported complete resorption of all rat fetuses at
2208 mg/m3, reduced fetal weight at 1435 mg/m3, and a
NOEL of 478 mg/m3. Andrew & Hardin (1984), exposed preg-
nant rats to 733 and 2823 mg/m3 for 7 h/day throughout
gestation and observed increased fetal resorptions at 733
mg/m3 as well as skeletal and cardiovascular abnormali-
ties. At 2823 mg/m3 the resorption frequency reached 100%.
Developmental toxicity has been noted in rats after
dermal application of 2-EE (0.25 or 0.5 ml, four times per
day) (Hardin et al., 1982) or 2-EEA (0.35 ml, four times
per day) (Hardin et al., 1984) on gestation days 7-16.
Increased resorption rates and fetal deaths, decreased
viable fetus weights, and increased cardiovascular defects
and skeletal malformations were seen, even at the lowest
dose tested.
The effect of simultaneous administration of ethanol
(10% in drinking water) and 2-EE (368 mg/m3 by inha-
lation) has been investigated, in view of their related
metabolism (via the enzyme alcohol dehydrogenase) (Nelson
et al., 1982, Nelson et al., 1984c). Although the results
obtained are difficult to interpret, ethanol adminis-
tration early in gestation tended to reduce the behav-
ioural effects induced by 2-EE, whereas ethanol given late
in gestation enhanced these effects.
8.5.3 Teratogenicity
8.5.3.1 2-Methoxyethanol
The teratogenic potential of dermally administered
2-ME was estimated in pregnant rats with the Chernoff-
Kavlock screening test (Wickramaratne, 1986). Various con-
centrations (0, 3, 10, 30, or 100%) of 2-ME in physiologi-
cal saline were applied to shaved skin and occluded for
6 h of exposure on gestation days 6-17. Animals were per-
mitted to have their litters and rear the pups until 5
days postpartum, when the study was terminated. No adverse
effects were noted at the 3% dose level. Small litter
sizes and decreased fetal survival were observed at the
10% level. At 30%, lethality was observed in all fetuses,
and all pregnant females died when exposed dermally to
100% 2-ME.
The teratogenicity of 2-ME in mice, rats, and rabbits
has been evaluated by Hanley et al., (1984). Pregnant rats
and rabbits were exposed by inhalation to 0, 9, 31, or 156
mg/m3 for 6 h/day on gestation days 6-18 (rabbits) or 6-
15 (rats), and mice were exposed to 0, 31, or 156 mg per
m3 for 6 h/day on days 6-15 of gestation. No teratogenic
effects were found in CF-1 mice and Fischer-344 rats under
the conditions of this study, although slight fetotoxicity
was noted in both species. In New Zealand white rabbits
exposed to 156 mg/m3 there was a significant increase in
resorption rate and incidence of malformations involving
all organ systems, as well as a significant decrease in
mean fetal body weight when compared to controls. Of the
fetuses from dams exposed to 156 mg per m3, 63% exhibi-
ted at least one malformation and 91% of the litters had
at least one fetus with a malformation. There were no
teratogenic or fetotoxic effects in any of the three
species evaluated at 31 mg/m3.
Developmental phase-specific and dose-related terato-
genic anomalies in CD-1 mice resulting from 2-ME exposure
have been evaluated (Horton et al., 1985). 2-ME was admin-
istered by gavage to pregnant females at doses of 250 mg
per kg (during gestation days 7 to 9, 8 to 10, or 9 to 11;
during days 7 to 8, 9 to 10, or 10 to 11; or once a day on
gestation days 9, 10, 11, 12, or 13) in order to identify
the most sensitive developmental phases for the anomalies
under study. Resulting malformations were specifically re-
lated to the developmental stage at the time of exposure,
with exencephaly being observed between days 7 to 10 and
paw anomalies (syndactyly, oligodactyly, and stunted digit
number 1) during the later stages of development (days 9-
12). The dose dependency of digit malformation was studied
by administering single doses by gavage (100, 175, 250,
300, 350, 400, or 450 mg 2-ME/kg) on gestation day 11.
Dose-related paw malformations were noted in all litters,
with forepaws being more susceptible than hindpaws in
terms of the number and severity of malformations. At 175
mg/kg digit anomalies were induced without concurrent
reductions in fetal weight, while at 250 mg/kg or more
digit anomalies occurred concurrently with reduced fetal
body weight. In this study, the NOEL for the single ex-
posure (day 11) was 100 mg/kg. Although in this same
strain of mice digital malformations were not detected in
near-term fetuses given 100 mg 2-ME/kg body weight by
gavage on gestation day 11 (Greene et al., 1987), there
was a slight increase in the amount of cell death in
approximately 50% of the limb buds from embryos collected
24 h after dosing.
In a preliminary study, the teratogenic potential of
2-ME was determined in Cynomolgus monkeys using doses of
0.16, 0.32, and 0.47 mmol/kg administered by gavage
throughout the period of organogenesis (Scott et al.,
1987). Fetal death was dose-related (2/13 at 0.16 mmol
per kg; 3/10 at 0.32 mmol/kg; and 8/8 at 0.47 mmol/kg).
At the highest dose level, four fetuses were lost through
abortion and one of the remaining four, which was re-
covered by hysterotomy, demonstrated malformation (ectro-
dactyly) of the forelimbs. The MAA content of maternal
sera was followed throughout the treatment period. By the
25th day of treatment, MAA levels had more than doubled in
all treatment groups, compared to the values determined on
day one, indicating that multiple exposure to 2-ME can
lead to accumulation of the potentially embryotoxic metab-
olite MAA, the possible causative factor in the embryonic
deaths and teratogenicity observed (see section 8.8).
8.5.3.2 2-Ethoxyethanol and 2-Ethoxyethanol acetate
Andrew & Hardin (1984) have studied the teratogenic
potential of 2-EE in rats and rabbits. Rats (29-38 per
group) were exposed by inhalation to 0, 552, or 2388 mg
per m3 5 days per week for 3 weeks immediately prior to
mating and then to 0, 743, or 2823 mg/m3 for 7 h/day from
gestation day 1 to 19. Pregnant rabbits received 589 or
2271 mg/m3 for 7 h/day from gestation day 1 to 18. In
the New Zealand white rabbits exposed to 2271 mg/m3, the
rate of early resorptions was 100%. Even at 689 mg/m3, the
number of early resorptions per litter was 6 times that in
the control group. There was no evidence of severe intra-
uterine growth retardation in surviving fetuses, but a
significant increase in the incidence of major malfor-
mations (ventral wall defects and fusion of the aorta with
the pulmonary artery), minor anomalies, and skeletal vari-
ants. Teratogenic effects of 2-EE and also 2-EEA were re-
ported in Dutch Belted Rabbits after inhalation exposures
of 644 mg 2-EE/m3 and 2160 mg 2-EEA/m3 (Doe, 1984a).
In this study the author considered the NOEL in rabbits to
be 184 mg/m3 for 2-EE and 135 mg/m3 for 2-EEA.
In the study by Andrew & Hardin (1984), the highest
2-EE dose (2823 mg/m3) in rats led to 100% resorption,
as in rabbits. The resorption rate per litter in the
group gestationally exposed to 743 mg/m3 was about twice
the control value. Fetal body size was significantly
decreased, indicating retardation of intrauterine growth.
The significant increase in the incidence of cardiovascu-
lar defects (transposed and retrotracheal pulmonary
artery) and the increased incidence of common skeletal
variants and anomalies over control values were indicative
of a teratogenic effect of 2-EE in rats. Doe (1984a)
reported fetotoxicity without teratogenicity in rats after
inhalation exposure to 184 and 920 mg/m3, but no adverse
effects were reported after exposure to 37 mg/m3.
The teratogenic potential of 2-EE was evaluated in
pregnant Sprague-Dawley rats using dermal application
(0.25 or 0.50 ml, four times/day during gestation days 7
to 16) (Hardin et al., 1982). No signs of maternal
toxicity were noted except for ataxia on the last day of
dosing in the high-dose group, as well as a significant
decrease in body weight gain in the last half of ges-
tation. All fetuses in the high-dose group suffered intra-
uterine death. In the low-dose group, there was a signifi-
cant increase in the number of females with 100% dead
implants (p < 0.001), and in the incidence of skeletal
variations (p < 0.05). Also, reductions in fetal body
weight (p < 0.001) and cardiovascular malformations
(p < 0.05) were observed, as were significant decreases in
the number of live fetuses per litter (p < 0.001).
Hardin et al. (1984) studied the developmental tox-
icity of 2-EEA in rats after dermal application of 0.35 ml
2-EEA four times per day on days 7 to 16 of gestation
(daily application 1.37 g). 2-EEA was strongly embryo-
toxic, as reflected by significantly increased frequencies
of completely resorbed litters and dead implants per
litter. Also, the body weight of live fetuses was reduced
relative to water-treated controls. The spectrum and fre-
quency of malformations and variations noted in 2-EEA-
treated animals was similar to that described by Hardin et
al. (1982) in a previous study.
The developmental toxicity (including teratogenicity)
of 2-EEA in rats and rabbits following inhalation ex-
posure has been evaluated by Tyl et al. (1988). Fischer
344 rats (30 animals/group) and New Zealand white rabbits
(24 animals/group) were exposed to 2-EEA concentrations of
0, 270, 540, 1080, or 1620 mg/m3, 6 h/day on gestational
days 6-15 (rats) and 6-18 (rabbits). Fetuses were examined
for external, visceral, and skeletal malformations and
variations on gestational day 21 (rats) and 29 (rabbits).
In both rats and rabbits, maternal toxicity and develop-
mental toxicity were observed at exposures of 540 mg/m3 or
more. Teratogenic responses were increased at 1080 and
1620 mg/m3 with a 100% incidence of total malformations
being observed at the highest dose. An exposure of 270 mg
per m3 was considered a NOEL in both species, no evi-
dence of maternal or developmental toxicity being reported
at this dose.
8.6 Mutagenicity and Related End-Points
The only published data on 2-EE concerns a point mu-
tation test using Escherichia coli, which was reported to
be negative (Szybalski, 1958). However, 2-ME has been
tested in several in vitro and in vivo systems for its
genotoxic potential. The mutagenicity of 2-ME has been
evaluated in the following test systems: Salmonella typhi-
murium, unscheduled DNA synthesis (UDS) in human embryo
fibroblasts, bone marrow metaphase analysis in male and
female rats, dominant lethality in male rats, and a sex-
linked recessive lethal test in Drosophila melanogaster.
No evidence of mutagenicity in S. typhimurium was found
using five strains, with and without metabolic activation,
and dose levels up to 33 mg 2-ME per plate. When 2-ME was
tested in the presence of alcohol dehydrogenase no evi-
dence was found that metabolites of 2-ME were mutagenic in
S. typhimurium. Similarly, the addition of 2-ME at con-
centrations up to 10 mg/ml of medium did not lead to
changes in UDS in human embryo fibroblasts (McGregor et
al., 1983).
The production in CHO cells of sister chromatid ex-
changes (SCEs) and chromosome aberrations by 2-EE was
studied by Galloway et al. (1987). Both assays were
carried out with and without activation by an exogenous
enzyme system from rat liver chemically induced with the
PCB mixture Aroclor 1254. Aberrations were found only in
the absence of metabolic activation when using 2-EE con-
centrations between 4780 and 9510 µg/ml of medium. The
lowest effective concentration was 6830 µg/ml. SCEs were
found with and without activation, the lowest effective
concentration being 3170 µg/ml when the range studied
was 951-9510 µg/ml.
Aneuploidy was induced in the diploid yeast strain
D61.M ( Saccharomyces cerevisiae ) at 2-MEA levels of 3-
5.7% in the medium (Zimmermann et al., 1985). However,
there were no chemically related effects in this yeast
strain on point mutation or recombination after exposure
to 2-MEA. The relevance to mammals of these findings in
yeast is not clear.
No statistically significant increase in chromosome
aberrations was seen in male or female rats after exposure
by inhalation to 2-ME (78 or 1555 mg/m3), 7 h/day, for 1
or 5 days. Where possible 50 metaphases per rat were
scored from groups of 10 animals (McGregor et al., 1983).
Basler (1986) dosed Chinese hamsters intraperitoneally (10
animals) with two thirds of the LD50 of 2-MEA (approxi-
mately 1333 mg/kg body weight in corn oil) and the animals
were sacrificed 12, 24, 48, and 72 h after the single
dose. No statistically significantly increase in micro-
nucleated erythrocytes was noted.
Two dominant lethal studies in rats have been carried
out using 2-ME. McGregor et al. (1983) exposed groups of
10 male CD rats by inhalation to 78 or 1555 mg/m3, 7 h
per day, for 5 days. Control and low-dose groups gave
similar results, but the results at 1555 mg/m3 were
difficult to interpret. A high proportion of early deaths
was noted, which could be partly explained in terms of the
low implantation frequency reported. Therefore, a dominant
lethal effect at 1555 mg/m3 could not be demonstrated
conclusively. Rao et al. (1983), using male Sprague-
Dawley rats exposed to 93, 311, or 933 mg 2-ME/m3 by in-
halation 6 h/day, 5 days/week for 13 weeks, found no domi-
nant lethal effect at 93 or 311 mg/m3. An assessment of
dominant lethality was impossible at 933 mg/m3 due to
almost complete infertility. No positive control was in-
cluded.
In a sex-linked recessive lethal test in Drosophila
melanogaster reported by McGregor et al. (1983), the
results were inconclusive. Given the low absolute number
of mutants (low sample size), the inconsistency with which
various broods were affected, and the lack of a dose-
response relationship, a firm conclusion on the mutagen-
icity of 2-ME in D. melanogaster cannot be made.
8.7 Carcinogenicity
No carcinogenicity data are available on these glycol
ethers.
8.8 Mechanism of Toxicity - Mode of Action
The metabolic fate of 2-ME, 2-EE, and their acetates
has been well studied in both animals (Miller et al., 1983a;
Moss et al., 1985) and man (NIOSH, 1986, Groeseneken et
al., 1987, Veulemans, 1987a). Due to rapid hydrolysis of
the acetates to the monoalkyl glycol ether (see section
6), the putative toxic metabolite are the same for 2-ME or
2-MEA and for 2-EE or 2-EEA.
Both in vitro and in vivo studies have supported the
hypothesis that the toxic effects of 2-ME and 2-EE are
elicited by the toxicity of 2-methoxyacetaldehyde and
methoxyacetic acid (MAA) from 2-ME and ethoxyacetaldehyde
and ethoxyacetic acid (EAA) from 2-EE.
Gray et al. (1985) studied the effects in vitro of
2-ME, 2-EE, MAA, and EAA on mixed cultures of Sertoli and
germ cells from rat testes. At concentrations up to 50
mmol/litre medium, no morphological damage was noted for
2-ME or 2-EE, whereas administration of MAA or EAA at 2-10
mmol/litre led to degeneration of the pachytene and div-
iding spermatocytes, the probable target cells of the
parent ethers in vivo (Chapin et al., 1985b, Creasy et
al., 1985; Foster et al., 1986, Oudiz and Zenick, 1986).
Foster et al. (1986) reported that 2-methoxyacetaldehyde
(2-MALD), a possible metabolite of 2-ME, can produce
specific testicular effects in vitro and in vivo at doses
much lower than those required for MAA (0.2 and 0.5 mmol
per litre in vitro ). In more recent studies, Foster et
al. (1987) compared the in vivo and in vitro testicular
effects of MAA and EAA in rats. Single oral doses of MAA
and EAA (equimolar with 100, 250, or 500 mg 2-ME/kg body
weight) were administered and cell morphology was moni-
tored for 14 days. MAA produced damage to spermatocytes
undergoing meiotic maturation and division within 24 h of
treatment, whereas EAA produced these changes only at the
highest dose. Similar results were seen in vitro at a dose
(5 mmol/litre medium) equivalent to the steady state
plasma level of MAA after administration of 500 mg/kg.
Evidence that MAA is important in causing 2-ME terato-
genicity was reported by Yonemoto et al. (1984). MAA, but
not 2-ME, was found to interfere with organogenesis when
the two compounds were investigated using rat embryos in
culture.
Results from in vivo studies in mice (Welsch et al.,
1987; Sleet et al., 1988) and rats (Ritter et al., 1985)
further substantiate the hypothesis that monoalkyl glycol
ethers are metabolized to acetic acid metabolites via
alcohol dehydrogenase (ADH) and aldehyde dehydrogenases,
and that the ultimate toxin is actually the alkoxy acid
metabolite of the glycol ether or a further metabolite.
In mice (Sleet et al., 1987), 2-ME and MAA were equipotent
in producing teratogenic lesions leading to malformations
of the digits of all paws. Similar effects were noted by
Brown et al. (1984) and Ritter et al. (1985) in rats. Sub-
sequent work showed that the embryotoxic and teratogenic
effects of 2-ME in mice could be prevented by the concomi-
tant administration of 0.12 or 1.2 mmol/kg of 4-methyl-
pyrazole, an inhibitor of ADH, but that 4-methylpyrazole
was without effect after the administration of MAA (Sleet
et al., 1988).
9. EFFECTS ON MAN
Only limited information on the toxic effects of gly-
col ethers in humans is available. This information comes
largely from case reports in the early literature dealing
with accidental poisoning and/or workplace exposure. Such
reports provide only minimal information relating specific
effects to exposure levels. Only a few epidemiological
studies have been reported.
9.1 General Population Exposure
The widespread use of consumer products such as
paints, stains, inks, lacquers, surface coatings, and
home/industrial cleaners that contain one or more glycol
ethers means that broad segments of the general population
could be exposed. However, no reports quantifying such
exposures have been found nor any that describe skin irri-
tation or sensitization from exposures to glycol ethers in
humans, despite widespread exposure.
9.1.1 Poisoning reports
A 44-year-old man consumed 400 ml of 2-ME mixed with
brandy and died without regaining consciousness 5 h after
he was admitted to hospital in a comatose state (Young &
Woolner, 1946). Postmortem findings included acute
haemorrhagic gastritis, fatty degeneration of the liver,
and black coloured kidneys with marked degenerative
changes in the renal tubules.
Two non-fatal cases of poisoning by 2-ME in men aged
41 and 23 were reported by Nitter-Hauge (1970). Both
patients consumed 100 ml 2-ME. In addition to nervous
system disorders (agitation, confusion), the predominant
clinical features were nausea, cyanosis, hyperventilation,
slight tachycardia, and metabolic acidosis. In one patient
there were suggestions of moderate kidney failure. No
evidence of liver damage was found and both patients re-
covered within 4 weeks.
Ingestion of approximately 40 ml 2-EE by a 44-year-old
women led to vertigo, unconsciousness, effects on the
central nervous system, and metabolic acidosis (Fucik,
1969). She recovered within 44 days from all symptoms,
including kidney insufficiency and signs of liver damage,
although neurasthenia was still evident at that time.
9.2 Occupational Exposure
9.2.1 Repeated exposure
Reports on the effects of glycol ethers on humans fol-
lowing repeated exposure are available from occupational
studies. Early reports provided evidence that the repeated
exposure of humans to solvents containing 2-ME resulted in
such effects as anaemia, leucopenia, lethargy, general
weakness, dizziness, ataxia, and unequal or exaggerated
reflexes. A diagnosis of toxic encephalopathy was made by
Donley (1936) and Parson & Parsons (1938), but no reliable
estimation of exposure to 2-ME was given. Greenburg et al.
(1938) reported levels of 2-ME between 78 and 236 mg per
m3 in one manufacturing plant where 19 male subjects
(ages 16-26) were examined. The median duration of ex-
posure was 5 weeks and the maximum was 2 years. Signs of
nervous system dysfunction (fatigue, hand tremor, and
lethargy) were found, but no clear relationship between
the incidence of symptoms and the duration of exposure
could be ascertained in this small population. No control
group was included in this study.
The development of macrocytic anaemia has been de-
scribed in a case report of a worker exposed to 2-ME and
other solvents during microfilm manufacturing. Exposure
to 2-ME averaged 109 mg/m3 (35 ppm) (56-180 mg/m3, 18-
58 ppm), to methyl ethyl ketone 1-5 ppm, and to propylene
glycol monomethyl ether 4-13 ppm for a duration of 20
months. Follow-up analyses conducted 1 month after termin-
ating exposure indicated a return to normal limits for all
haematological parameters studied (Cohen, 1984).
The haematological effects of glycol ethers on humans
have also been documented in workplace surveys. Obvious
symptoms are not always manifested in peripheral blood.
The evaluation in a printing plant of seven workers with
dermal and respiratory exposure to dipropylene glycol
monomethyl ether, ethylene glycol monoethyl ether, and a
range of aliphatic, aromatic, and halogenated hydrocarbons
used for offset and multicolour printing revealed no
alteration in the peripheral blood (Cullen et al., 1983).
Although there was evidence of bone marrow injury in three
of the seven workers, no direct relationship could be
drawn to glycol ether exposure. The exposure to other sol-
vents by this relatively small group of workers makes more
definitive conclusions impossible. Bone marrow toxicity
had earlier been reported in two case studies of workers
exposed dermally to 2-ME (Ohi & Wegmann, 1978).
Denkhaus et al. (1986) investigated the cellular im-
mune response of nine workers aged 25-58 years of age,
heavily exposed to mixtures of organic solvents (including
2-ME and 2-EE) for 8-35 (mean 18.9) years, by the analysis
of subpopulations of peripheral blood lymphocytes. A con-
trol group of matched healthy controls was included. The
mean exposures to 2-ME and 2-EE were 6.1 mg/m3 (peak
150 mg/m3) and 4.8 mg/m3 (peak 53 mg/m3), respect-
ively. Based on samples taken during working shifts, mean
blood levels were 40.1 µg 2-ME/100 ml (peak: 965 µg/100
ml) and 2.0 µg 2-EE/100 ml (peak: 92.7 µg/100 ml). At
these levels (and in the presence of other solvents such
as 1-butanol, isobutanol, 2-butoxybutanol, toluene, m-xylene,
2-butanone, and 2-hexanone), the exposed workers had de-
creased levels of helper T-cells, and increased levels of
natural killer cells and human B-lymphocytes. However,
the levels of suppressor cells were normal. The authors
noted that similar changes in lymphocyte subpopulations
are found in states of general immunodeficiency and in
immunogenetic forms of aplastic anaemia.
9.2.2 Epidemiological studies
In a cross-sectional study, male employees in a plant
manufacturing 2-ME in the USA were examined for haema-
tological effects and possible sterility (Cook et al.,
1982). Personal air sampling indicated levels of exposure
of 1.3 mg/m3 or less in one working location and 19-26 mg
per m3 in another. Forty workers, engaged in production
and distribution of 2-ME (75% of the possible total popu-
lation) and 25 controls (57% of total eligible population)
were examined for haematological effects, and six exposed
and nine control workers participated in an examination of
testis size, semen abnormalities, and levels of serum
follicle-stimulating hormone (FSH). No increase in the
incidence of leucopenia or anaemia was noted, but de-
creases in white cell count and testicular size were noted
in the most highly exposed workers. These results were not
statistically significant but correlate with known effects
in experimental animals.
A cross-sectional evaluation of semen quality (sperm
concentration, pH, volume, viability, motility, velocity,
and morphology) was carried out in 37 workers exposed to
2-EE used as a resin solvent in a metal casting plant
(NIOSH, 1986). The study population represented 50% of
the exposed workforce. A control group of 38 workers from
other locations in the plant was included. Full-shift
breathing zone airborne exposure to 2-EE ranged from non-
detectable to 88 mg/m3. Dermal exposure was indicated by
urinary excretion of EAA at levels from non-detectable to
163 mg/g creatinine. Workers exposed to 2-EE had signifi-
cantly lower average sperm counts than controls (113
versus 154 million per ejaculate), although both exposed
and control groups had lower sperm counts than those found
in other occupational groups. The two groups studied did
not differ significantly with respect to other semen
characteristics or testicular size.
The effect of 2-ME and 2-EE exposure on male repro-
ductive factors and blood has been studied in shipyard
painters by Sparer et al. (1988a,b), Welch et al. (1988),
and Welch & Cullen (1988). Airborne levels (time-weighted
average: TWA) were determined in 102 samples over six
workshifts and were 0-80.5 mg 2-EE/m3 (mean 9.9 and
median level 4.4 mg/m3), and 0-17.7 mg 2-ME/m3 (mean
2.6 and median 1.4 mg/m3). Given the methods used by
Sparer et al. (1988a,b) the authors concluded these were
probably underestimates of exposure, particularly in the
mixing room and inside large tanks. Urinary excretion of
EAA indicated dermal exposure as well as airborne ex-
posure in some workers. In 73 painters, from a total
population of 153, an increased prevalence of oligospermia
and azoospermia was noted and there was an increased odds
ratio for a lower sperm count per ejaculate in exposed
workers as compared to the 40 controls studied (Welch et
al., 1988). In addition, when 94 painters from the same
population were examined for haematological effects of
chemical exposures, 10% were found to be anaemic and 5%
exhibited granulocytopenia (Welch & Cullen, 1988). None of
these effects were noted in the 55 control subjects exam-
ined. The reproductive effects observed were in agreement
with those reported previously by NIOSH (1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure
Many people may be exposed to 2-methoxyethanol (2-ME),
2-ethoxyethanol (2-EE), and their acetates (2-MEA and 2-
EEA), at levels comparable to industrial levels, through
the use of consumer and trade products. On the other
hand, exposure through food, water, or the ambient air is
probably negligible. This inference is based only on the
physical and chemical properties of these compounds and
evidence of rapid environmental degradation.
Significant occupational exposure may occur both
through inhalation and skin absorbtion. Limited measure-
ments of air levels in the workplace range from less than
0.1 mg/m3 to more than 150 mg/m3. However, the avail-
able monitoring is quite limited and large variations may
occur both within and among industries. Because of the
potential for skin absorption, air monitoring alone may
underestimate total exposure. Total uptake may be best
estimated from biological monitoring. Occupations where
extensive exposure is possible include, for example,
painting, printing, and cleaning, but it should be borne
in mind that there are many other occupations where these
compounds are also used and where exposure is of concern.
10.1.2. Health Effects
The major effects of concern for humans are develop-
mental, testicular, and haematological toxicity. These
are demonstrated by extensive and consistent data in ani-
mals and some human data. All these effects can be caused
by both short-term and longer-term exposures. In experi-
mental animals, very high repeated exposure to 2-ME and
2-EE (over 930 and 1450 mg/m3, respectively) produces
neurobehavioural, hepatic, and renal toxic effects. These
are also observed in human poisoning situations.
These four glycol ethers exhibit very similar testicu-
lar and developmental toxicities, in all species evalu-
ated, and by all routes of exposure that have been em-
ployed (inhalation, dermal, and oral). Mechanistic studies
indicate that for both testicular and developmental ef-
fects, metabolism to the alkoxyacetic acid derivative is a
necessary activation step. Metabolism takes place via the
alcohol dehydrogenase system that is common to humans and
laboratory animals. The toxic metabolites, methoxyacetic
acid (MAA) and ethoxyacetic acid (EAA), have been detected
in the urine of humans exposed to these solvents. The con-
sistency of response across laboratory animal species
studied, combined with the similarity of metabolism in
humans, makes it clear that humans should be presumed to
be subject to the testicular and developmental effects of
these glycol ethers. Available data on the excretion of
alkoxyacetic acids by humans indicate prolonged retention
compared with that in laboratory animals, suggesting that
humans may be more sensitive than the most sensitive ex-
perimental animal species. The rapid skin absorbtion of
these compounds is of particular concern. Teratogenicity
and other developmental effects have been observed follow-
ing the application of 2-ME, 2-EE, and 2-EEA to the intact
skin of rats.
Testicular damage has been observed in the rat, mouse,
and rabbit following exposure to these glycol ethers
through both the inhalation and the oral route. Single in-
halation exposures of rats to 1944 mg 2-ME/m3 or more for
4 h and repeated exposure to 933 mg 2-ME/m3 or more for
13 weeks resulted in histological evidence of testicular
damage. The NOEL for acute exposure was 933 mg/m3 and for
repeated exposure was 311 mg/m3. The mouse appeared
somewhat less sensitive, the NOEL for repeated exposure
being 933 mg/m3. However, the rabbit was more sensitive,
with marked testicular change being seen after repeated
exposure to 311 mg/m3 and a marginal effect (one in five
rabbits affected) being seen at 93 mg/m3. Similar
effects have been seen following oral exposure of rats to
2-ME, with short-term (including single dose) exposure to
100 mg/kg producing testicular damage. The NOEL in a sub-
acute (11 days) study was 50 mg/kg. 2-EE is somewhat less
potent with regard to its testicular toxicity than 2-ME;
effects were only seen at dose levels of 500 mg/kg or
more, the NOEL being 250 mg/kg.
Evidence from studies in men exposed occupationally
to 2-ME and 2-EE is consistent with the animal data and
indicates that these glycol ethers can produce testicular
toxicity in humans. Epidemiological studies on small
groups of workers exposed to 2-EE in a metal casting
plant and in shipyard painters exposed to both 2-ME and
2-EE consistently revealed an increased incidence of re-
duced sperm counts. Data on exposure levels were limited,
but there was evidence, in each case, of dermal exposure
as well as exposure via inhalation.
Developmental toxicity has been observed in the rat,
mouse, rabbit, and monkey following exposure to these
glycol ethers using dermal, oral, and inhalation routes.
Twelve daily applications of undiluted 2-ME to the shaved
skin of pregnant rats (with 6-h occlusion) was lethal,
while ten open applications of 2-EE (1.0 ml/day) or 2-EEA
(1.4 ml/day) were teratogenic but not maternally toxic.
Twelve occluded applications of 10% 2-ME in saline proved
developmentally toxic, the NOEL in this study being 3%
2-ME. No-observed-effect levels have not been demonstrated
following repeated oral dosing of pregnant animals with
2-ME. The lowest-observed-effect level (LOEL) for oral
administration of 2-ME was 31.25 mg/kg per day for mice,
25 mg/kg per day for rats, and 0.16 mmol/kg per day for
monkeys. Single-dose studies have been reported only for
2-ME in mice, the results being that on gestation day 11
(the most sensitive day) the NOEL was 100 mg/kg and the
LOEL was 175 mg/kg. Both 2-EE and 2-EEA have been evalu-
ated by inhalation exposure in rats and rabbits. Rats were
exposed to 2-EE in two studies, resulting in teratogenic
effects (743 mg/m3 for 7 h/day on gestation days 1-19)
or fetotoxic effects (184 and 920 mg/m3 for 6 h/day on
gestation days 6-15). In the latter study, the NOEL was
37 mg/m3. Rabbits exposed to 2-EE also exhibited
teratogenic effects (589 mg/m3 for 7 h/day on gestation
days 1-18) or fetotoxic effects (644 mg/m3 for 6 h/day
on gestation days 6-18). In the latter study, the NOEL was
184 mg/m3. When rabbits were exposed to 2-EEA for
6 h/day on gestation days 6-18, teratogenic effects were
seen at 2160 mg/m3 in one study and 1620 mg/m3 in
another. Fetotoxicity appeared in both studies at 540 mg
per m3 and the lowest exposure level in each study (135
and 270 mg/m3, respectively) was the NOEL. Rats exposed
to 2-EEA by inhalation for 6 h/day on gestation days 6-15
showed the same pattern of response: teratogenic effects
at 1620 mg/m3, fetotoxicity at 1080 mg/m3, and no ef-
fect at 270 mg/m3.
Thus developmental toxicity has been observed in all
species (mice, rats, and rabbits) exposed to 2-ME at 156
mg/m3 or more. The NOEL for all three species was 31 mg
per m3. Behavioural and neurochemical alterations in
rats followed in utero exposure at 78 mg/m3, with no
NOEL being identified.
2-EE and 2-EEA were slightly less potent. Develop-
mental effects in rats and rabbits followed all 2-EE ex-
posures at 368 mg/m3 or more. Slight developmental ef-
fects were seen in rats exposed at 184 mg 2-EE/m3, but
37 mg/m3 was a clear NOEL. For 2-EEA, the NOEL was 170 mg
per m3 for both rats and rabbits.
Haematological effects from single acute dose ex-
posures have been observed in animals and in human poison-
ings. Repeated inhalation exposure of the most sensitive
species, the rabbit, to 2-ME for 13 weeks, 5 times per
week, yielded a NOEL of 93 mg/m3. Repeat doses of 2-ME
also cause haematological toxicity in mice, rabbits, dogs,
hamsters, and guinea-pigs. 2-EE is less potent in causing
haematological effects than 2-ME. The NOEL for haemato-
logical effects in rats and rabbits exposed to 2-EE for 13
weeks, 5 times per week for 6 h/day, is 368 mg/m3. Dogs
and mice also show haematological effects from repeated
2-EE exposure at higher levels. Exposure to the acetate
esters of 2-EE and 2-ME would be expected to cause similar
effects at similar exposure levels, but there are too few
data on exposure and haematological effects of these com-
pounds to determine under what conditions single human ex-
posures will lead to haematological effects.
Industrial exposure levels have been reported at or
near the NOEL for haematological effects in animals after
repeated doses of both 2-ME and 2-EE. This fact, together
with the probable greater sensitivity of humans and the
expected accumulation of metabolites in human blood, indi-
cates that haematological effects may well occur from in-
dustrial and consumer exposure. This has been confirmed by
the haematological effects reported in some of the limited
number of studies of industrial workers with repeated 2-EE
and/or 2-ME exposure.
10.2. Evaluation of Effects on the Environment
Environmental exposure to these glycol ethers can
arise as a consequence of their direct release into the
atmosphere from their use as evaporative solvents. Dis-
charges to the land and water from accidental release may
also result in environmental exposure. Accumulation in
soil and surface water could only occur in the absence of
degradation. However, these glycol ethers are rapidly
degraded by chemical and biological processes and accumu-
lation is not expected. 2-MEA and 2-EEA are also expected
to hydrolyse readily and subsequently biodegrade under
aerobic conditions. However, contamination of anaerobic
soils and aquifers remains a potential problem, but this
condition is expected to be transitory resulting in negli-
gible risk.
Both 2-ME and 2-EE demonstrate low toxicity to micro-
oganisms and aquatic species. The glycol ether acetates,
however, are far more acutely toxic. No data exists to
ascertain the potential for adverse effects from long-term
exposure to environmental species.
11. RECOMMENDATIONS
11.1. Health Protection
1. Alternative less toxic solvents should be identified
to replace 2-methoxyethanol, 2-ethoxyethanol, and their
esters, particularly in consumer products. Assessment of
the effects of other ethylene glycol ethers is also of
particular importance, because some may cause effects
similar to the four glycol ethers evaluated here.
2. In view of the known toxic effects of these glycol
ethers, authorities should seriously consider appropriate
strategies to alert users of these chemicals to their
hazards, particularly those arising from dermal ex-
posures.
3. In view of recent toxicological data and the potential
for considerable dermal absorption of these glycol ethers,
national occupational exposure limits should be recon-
sidered to insure that the total daily dose to workers by
all routes of administration does not pose an undue risk
to health.
4. Single dose effects occur in animals at fairly high
exposure levels. Prudent use of these compounds (attention
to personal hygiene, suitable protective devices, and
adequate ventilation) is recommended to reduce the health
risks. The data indicate that more extensive protection
may be required to prevent developmental effects, as well
as effects on blood and testis, from repeated exposure.
11.2. Further Research
1. In view of the implication of methoxyacetic acid (MAA)
and ethoxyacetic acid (EAA) (the principal identified
metabolites of 2-ME, 2-EE, and of their esters) in the
toxicity to the male reproductive system, their mechanism
of action should be investigated. If it transpires that
MAA and EAA are not the primary agents concerned, these
should be identified and their mechanism of action
elucidated.
2. These four glycol ethers are known to have both
haematological effects and male reproductive effects
(sperm count reduction). The available data, although
limited, appear to suggest that the two effects become
evident at similar dose levels. The mechanism of action
should be investigated for both organ systems, and
haematological effects and sperm counts should be examined
in parallel to determine if haematological changes offer
warning signs for other effects of these compounds.
3. Air monitoring alone is not sufficient to assure low
exposure. Biological monitoring can aid in detecting
failures in protective measures. At present the
relationship between biological indicators of exposure,
total body uptake, and observed health effects have not
been sufficiently established. Further work is necessary
to provide the basis for using biological monitoring in
determining safe exposures.
4. Epidemiological studies and/or targeted health sur-
veillance in populations subject to high exposure to these
glycol ethers should be designed in order to estimate
exposure-effect relationships for the purpose of determin-
ing safe exposures, providing that overall exposure can be
properly and adequately evaluated by appropriate environ-
mental and biological monitoring.
5. The possibility that these compounds may cause effects
on the female gonads should be investigated through
multigeneration reproductive studies in animals.
6. Available research indicates that humans may metab-
olize these glycol ethers to the corresponding alkoxy-
acetic acids to a greater extent than rats, and that the
half-life for urinary excretion of these toxic metabolites
is about four times longer in humans than in rats.
Furthermore, rats conjugate a large portion of the acid
metabolites, whereas humans do not. These differences
might contribute to the relatively higher sensitivity of
humans to these glycol ethers. Detailed knowledge of the
metabolism and excretion kinetics would improve the
ability to predict safe exposure levels.
7. The results obtained from short-term (13-week) studies
indicate effects on various organ systems. However, there
have been no studies of sufficient duration that would
allow the reversibility of such effects to be assessed.
Therefore, it is suggested that stop-studies be undertaken
in which experimental animals are exposed to these glycol
ethers for at least 13 weeks, followed by a suitable
recovery period. Important physiological parameters
should then be evaluated in order to determine whether or
not these effects are transient.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Regulatory standards for 2-ME, 2-MEA, and 2-EE, estab-
lished by national bodies in different countries and the
European Economic Community, are summarized in the data
profile of the International Register of Potentially Toxic
Chemicals (IRPTC, 1987).
REFERENCES
ANDERSON, D., BRINKWORTH, M.H., JENKINSON, P.C., CLODE, S.A., CREASY,
D.M., & GANGOLLI, S.D. (1987) Effect of ethylene glycol monomethyl ether
on spermatogenesis, dominant lethality, and F1 abnormalities in the rat
and the mouse after treatment of F0 males. Teratog. Carcinog. Mutagen.,
7:141-158.
ANDREW, F.D. & HARDIN, B.D. (1984) Developmental effects after inhalation
exposure of gravid rabbits and rats to ethylene glycol monoethyl ether.
Environ. Health Perspect., 57: 13-23.
BAILEY, H.C., LIU, D.H.W., & JAVITZ, H.A. (1985) Time/toxicity
relationships in short-term static, dynamic, and plug-flow bioassays. In:
Bahner, R.C. & Hansen, D.J., ed. Aquatic toxicology and hazard assessment:
Eighth Symposium, Philadelphia, American Society for Testing and
Materials, pp. 193-212 (ASTM STP 891).
BARBEE, S.V., TERRILL, J.B., DESOUSA, D.J., & CONAWAY, C.C. (1984)
Subchronic inhalation toxicology of ethylene glycol monoethyl ether in the
rat and rabbit. Environ. Health Perspect., 57: 157-163.
BASLER, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by the
micronucleus test. Mutat. Res., 174: 11-13.
BOTTA, D., CASTELLANI PIRRI, L., & MANTICA, E. (1984) Ground water
pollution by organic solvents and their microbial degradation products,
Luxembourg, Commission of the European Communities, pp. 261-275 (EUR-
8518).
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) Aeruginosa and
Scenedesmus quadricauda. Mitt. intern. Ver. theor. angew. Limnol., 21:
275-284.
BROWN, N.A., HOLT, D., & WEBB, M. (1984) The teratogenicity of
methoxyacetic acid in the rat. Toxicol. Lett., 22: 93-100.
CARPENTER, C.P., POZZANI, V.C., WEIL, C.S., NAIR, J.H., KECK, G.A., &
SMITH, H.F. (1956) The toxicity of butyl cellosolve solvent. Arch. ind.
Health, 14: 114-131.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., & LAMB, J.C. (1985a) Effects of
ethylene glycol monomethyl ether (EGME) on mating performance and
epididymal sperm parameters in F344 rats. Fundam. appl. Toxicol., 5: 182-
189.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., SWAISGOOD, R.R., & LAMB, J.S.
(1985b) The recovery of the testis over 8 weeks after short-term dosing
with ethylene glycol monomethyl ether: histology, cell specific enzymes,
and rete testis fluid protein. Fundam. appl. Toxicol., 5: 515-525.
CHEEVER, K.L., PLOTNICK, H.B., RICHARDS, D.E., & WEIGEL, W.W. (1984)
Metabolism and excretion of 2-ethoxyethanol in the adult male rat.
Environ. Health Perspect., 57: 241-248.
COHEN, R. (1984) Reversible subacute ethylene glycol monomethyl ether
toxicity associated with microfilm production: a case report. Am. J. ind.
Med., 6: 441-446.
COOK, R.R., BODNER, K.M., KOLESAR, R.C., VAN PEENEN, P.F.D., DICKSON,
G.S., & FLANAGAN, K. (1982) A cross-sectional study of ethylene glycol
monomethyl ether process employees. Arch. environ. Health, 37: 346-351.
CREASY, D.M. & FOSTER, P.M.D. (1984) The morphological development of
glycol ether-induced testicular atrophy in the rat. Exp. mol. Pathol., 40:
169-176.
CREASY, D.M., FLYNN, J.C., GRAY, T.J.B., & BUTLER, W.H. (1985) A
quantitative study of stage-specific spermatocyte damage following
administration of ethylene glycol monomethyl ether in the rat. Exp. mol.
Pathol., 43: 321-336.
CREASY, D.M., JONES, H.B., BEECH, L.M., & GRAY, T.J.B. (1986) The effects
of two testicular toxins on the ultrastructural morphology of mixed
cultures of Sertoli and germ cells: a comparison with in vivo effects.
Food chem. Toxicol., 24: 655-656.
CULLEN, M.R., RADO, T., WALDRON, J.A., SPARER, J., & WELCH, L.S. (1983)
Bone marrow injury in lithographers exposed to glycol ethers and organic
solvents used in multicolor offset and ultraviolet curing printing
processes. Arch. environ. Health, 38(6): 347-354.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J.
hazard. Mater., 1: 303-318.
DE DELBARRE, F., KAHAN, A., DE GERY, A., & KONRAD, K. (1980) Action
immunomodulatrice du méthoxy-2 éthanol et de dérivés homologues chez le
rat. C.R. Acad. Sci. Paris, 291: 215-218.
DENKHAUS, W., STELDERN, D., BOTZENHARDT, U., & KONIETZKO, H. (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int. Arch. occup.
environ. Health, 57: 109-115.
DOE, J.E. (1984a) Ethylene glycol monoethyl ether and ethylene glycol
monoethyl ether acetate teratology studies. Environ. Health Perspect., 57:
33-41.
DOE, J.E. (1984b) Further studies on the toxicology of the glycol ethers
with emphasis on rapid screening and hazard assessment. Environ. Health
Perspect., 57: 199-206.
DOE, J.E., SAMUELS, D.M., TINSTON, D.J., & WICKRAMARATNE, G.A.D. (1983)
Comparative aspects of the reproductive toxicology by inhalation in rats
of ethylene glycol monomethyl ether and propylene glycol monomethyl ether.
Toxicol. appl. Pharmacol., 69: 43-47.
DONLEY, D.E. (1936) Toxic encephalopathy and volatile solvents in
industry. J. ind. Hyg. Toxicol., 18: 571-577.
DUGARD, P.H., WALKER, M., MAWDSLEY, S.J., & SCOTT, R.C. (1984) Absorption
of some glycol ethers through human skin in vitro . Env. Health Perspect.,
57: 193-197.
ECETOC (1985) The toxicology of glycol ethers and its relevance to man,
Brussels, European Chemical Industry Ecology and Toxicology Centre
(Technical Report No. 17).
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., THOMAS, L.V., COOK, M.W., &
GANGOLLI, S.D. (1983) Testicular toxicity of ethylene glycol monomethyl
and monoethyl ethers in the rat. Toxicol. appl. Pharmacol., 69: 385-399.
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., & GRAY, T.J.B. (1984)
Testicular toxicity produced by ethylene glycol monomethyl and monoethyl
ethers in the rat. Environ. Health Perspect., 57: 207.
FOSTER, P.M.D., BLACKBURN, D.M., MOORE, R.B., & LLOYD, S.C. (1986)
Testicular toxicity of 2-methoxyacetaldehyde, a possible metabolite of
ethylene glycol monomethyl ether, in the rat. Toxicol. Lett., 32: 73-80.
FOSTER, P.M.D., LLOYD, S.C., & BLACKBURN, D.M. (1987) Comparison of the in
vivo and in vitro testicular effects produced by methoxy-, ethoxy-, and n-
butoxy acetic acids in the rat. Toxicology, 43: 17-30.
FUCIK, J. (1969) Poisoning by ethylene glycol monoethyl ether. Prac. Lek.,
21: 116-118.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO, J.,
MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese hamster
ovary cells: evaluations of 108 chemicals. Environ. mol. Mutagen., 10(10):
1-175.
GOLDBERG, M.E., HARN, C., & SMYTH, H.F. (1962) Toxicological implication
of altered behaviour induced by an industrial vapour. Toxicol. appl.
Pharmacol., 4: 148-164.
GRANT, D., SULSH, S., JONES, H.B., GANGOLLI, S.D., & BUTLER, W.H. (1985)
Acute toxicity and recovery in the hemopoietic system of rats after
treatment with ethylene glycol monomethyl and monobutyl ethers. Toxicol.
appl. Pharmacol., 77: 187-200.
GRAY, T.J.B., MOSS, E.J., CREASY, D.M., & GANGOLLI, S.D. (1985) Studies on
the toxicity of some glycol ethers and alkoxyacetic acids in primary
testicular cell cultures. Toxicol. appl. Pharmacol., 79: 490-501.
GREENBURG, L., MAYERS, M.R., GOLDWATER, L.J., BURKE, W.J., & MOSCOWITZ, S.
(1938) Health hazards in the manufacture of "fused collars". 1. Exposure
to ethylene glycol monomethyl ether. J. ind. Hyg. Toxicol., 20: 134-147.
GREENE, J.A., SLEET, R.B., MORGAN, K.T., & WELSCH, F. (1987) Cytotoxic
effects of ethylene glycol monomethyl ether in the forelimb bud of the
mouse embryo. Teratology, 36: 23-34.
GROESENEKEN, D., VAN VLEM, E., VEULEMANS, H., & MASSCHELEIN, R. (1986a)
Gas chromatographic determination of methoxyacetic and ethoxyacetic acid
in urine. Br. J. ind. Med., 43: 62-65.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986b) Respiratory
uptake and elimination of ethylene glycol monoethyl ether after
experimental human exposure. Br. J. ind. Med., 43: 544-549.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986c) Urinary
excretion of ethoxyacetic acid after experimental human exposure to
ethylene glycol monoethyl ether. Br. J. ind. Med., 43: 615-619.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1987)
Ethoxyacetic acid: a metabolite of ethylene glycol monoethyl ether acetate
in man. Br. J. ind. Med., 44: 488-493.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1988)
Comparative urinary excretion of ethoxyacetic acid in man and rat after
single low doses of ethylene glycol monoethyl ether. Toxicol. Lett., 41:
57-68.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989a)
Experimental human exposure to ethylene glycol monomethyl ether. Int.
Arch. occup. environ. Health, 61: 243-247.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989b) An
improved method for the determination in urine of alkoxyacetic acids. Int.
Arch. occup. environ. Health, 61: 249-254.
GUEST, D., HAMILTON, M.L., DEISINGER, P.J., & DIVINCENZO, G.D. (1984)
Pulmonary and percutaneous absorption of 2-propoxyethyl acetate and 2-
ethoxyethyl acetate in beagle dogs. Environ. Health Perspect., 57: 177-
183.
HAMLIN, J.W., HUDSON, B., SHEEN, A.D., & SAUNDERS, K.J. (1982) The
measurement of glycol ether levels in the workplace. Polym. Paint Colour
J., October 13: 61-63.
HANLEY, T.R., Jr, YANO, B.L., NITSCHKE, K.D., & JOHN, J.A. (1984)
Comparison of the teratogenic potential of inhaled ethylene glycol
monomethyl ether in rats, mice, and rabbits. Toxicol. appl. Pharmacol.,
75: 409-422.
HARDIN, B.D. (1983) Reproductive toxicity of the glycol ethers.
Toxicology, 27: 91-102.
HARDIN, B.D., NIEMEIER, R.W., SMITH, R.J., KUCZUK, M.H., MATHINOS, P.R., &
WEAVER, T.F. (1982) Teratogenicity of 2-ethoxyethanol by dermal
application. Drug chem. Toxicol., 5(3): 277-294.
HARDIN, B.D., GOAD, P.T., & BURG, J.R. (1984) Developmental toxicity of
four glycol ethers applied cutaneously to rats. Environ. Health Perspect.,
57: 69-74.
HEALTH AND SAFETY EXECUTIVE (1988) Methods for the determination of
hazardous substances: Glycol ethers and glycol acetate vapours in air,
London, UK Health and Safety Executive, pp. 1-7 (MDHS-23).
HERMENS, J., CANTON, H., JANSSEN, P., & DEJONG, R. (1984) Quantitative
structure-activity relationships and toxicity of mixtures of chemicals
with anaesthetic potency: Acute lethal and sublethal toxicity to Daphnia
magna. Aquat. Toxicol., 5: 143-154.
HORTON, V.L., SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1985)
Developmental phase-specific and dose-related teratogenic effects of
ethylene glycol monomethyl ether in CD-1 mice. Toxicol. appl. Pharmacol.,
80: 108-118.
HOUSE, R.V., LAUER, L.D., MURRAY, M.J., WARD, E.C., & DEAN, J.H. (1985)
Immunological studies in B6C3F1 mice following exposure to ethylene glycol
monomethyl ether and its principal metabolite methoxyacetic acid. Toxicol.
appl. Pharmacol., 77: 358-362.
HURTT, M.E. & ZENICK, H. (1986) Decreasing epididymal sperm reserves
enhances the detection of ethoxyethanol-induced spermatotoxicity. Fundam.
appl. Toxicol., 7: 348-353.
IRPTC (1987) IRPTC legal file 1986 - Volume 1, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
JUHNKE, I. & LUDEMANN, D. (1978) The results obtained with the Golden Orfe
test during the examination of 200 chemical compounds for acute fish
toxicity. Wasser Abwasser Forsch., 11: 161-164.
KAREL, L., LANDING, B.H., & HARVEY, T.S. (1947) The intraperitoneal
toxicity of some glycols, glycol ethers, glycol esters and phthalates in
mice. J. Pharmacol. exp. Ther., 90: 338-347.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 9 - Ethanol,
3rd ed., New York, Chichester, Brisbane, Toronto, John Wiley & Sons.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 11 - Glycols
(Ethylene and Propylene), 3rd ed., New York, Chichester, Brisbane,
Toronto, John Wiley & Sons.
LAILLER, J., PLAZONNET, B., LE DOUAREC, J.C., & GONIN, M.J. (1975)
Evaluation of ocular irriation in the rabbit: Development of an objective
method of studying eye irritation. Proc. Eur. Soc. Toxicol., 17: 336-350.
LAMB, J.C., DUSHYANT, K.G., RUSSELL, V.S., HAMMEL, L., & SABHARNAL, P.S.
(1984) Reproductive toxicity of ethylene glycol monoethyl ether tested by
continuous breeding of CD-1 mice. Environ. Health Perspect., 57: 85-90.
LAUG, E.P., CALVERY, H.O., MORRIS, H.J., & WOODARD, G. (1939) The
toxicology of some glycols and derivatives. J. ind. Hyg. Toxicol., 21:
173-201.
LEE, K.H. & WONG, H.A. (1979) Toxic effects of some alcohol and ethylene
glycol derivatives on Cladosporium resinae. Appl. environ. Microbiol., 38:
24-28.
MCGREGOR, D.B., WILLINS, M.J., MCDONALD, P., HOLMSTROM, M., MCDONALD, D.,
& NIEMEIER, R.W. (1983) Genetic effects of 2-methoxyethanol and bis(2-
methoxyethyl)ether. Toxicol. appl. Pharmacol., 70: 303-316.
MELLAN, I. (1977) Glycol ethers and esters. In: Industrial Solvents
Handbook, 2nd ed., Park Ridge, New Jersey, Noyes Data Corporation, pp.
346-399, 513-551.
MILLER, R.R., AYERS, J.A., CALHOUN, L.L., YOUNG, J.T., & MCKENNA, M.J.
(1981) Comparative short-term inhalation toxicity of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in rats and mice.
Toxicol. appl. Pharmacol., 61: 368-377.
MILLER, R.R., CARREON, R.E., YOUNG, J.T., & MCKENNA, M.J. (1982) Toxicity
of methoxyacetate acid in rats. Fundam. appl. Toxicol., 2: 155-160.
MILLER, R.R., HERMANN, E.A., LANGVARDT, P.W., MCKENNA, M.J., & SCHWETZ,
B.A. (1983a) Comparative metabolism and disposition of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in male rats.
Toxicol. appl. Pharmacol., 67: 229-237.
MILLER, R.R., AYRES, J.A., YOUNG, J.T., & MCKENNA, M.J. (1983b) Ethylene
glycol monomethyl ether. I. Subchronic vapor inhalation study with rats
and rabbits. Fundam. appl. Toxicol., 3: 49-54.
MOSS, E.J., THOMAS, L.V., COOK, M.W., WALTERS, D.C., FOSTER, P.M.D.,
CREASY, D.M., & GRAY, T.J.B. (1985) The role of metabolism in 2-
methoxyethanol-induced testicular toxicity. Toxicol. appl. Pharmacol., 79:
480-489.
NAGANO, K., NAKAYAMA, E., KOGANA, M., DOBAYASKI, H., ADACHI, H., & YAMADA,
T. (1979) Testicular atrophy of mice induced by ethylene glycol monoalkyl
ethers. Jpn. J. ind. Health, 21: 29-35.
NAGANO, K., NAKAYAMA, E., DOBAYASKI, H., YAMADA, T., ADACHI, H.,
NISHIZAWA, T., OZAWA, H., NAKAICHI, M., OKUKDA, H., MINAMI, K., &
YAMAZAKI, K. (1981) Embryotoxic effects of ethylene glycol monomethyl
ether in mice. Toxicology, 20: 335-343.
NAGANO, K., NAKAYAMA, E., OOBAYASHI, H., NISHIZAWA, T., OKUDA, H., &
YAMAZAKI, K. (1984) Experimental studies on toxicity of ethylene glycol
alkyl ethers in Japan. Environ. Health Perspect., 57: 75-84.
NAKAAKI, K., FUKABORI, S., & TADA, O. (1980) An experimental study on
percutaneous absorption of some organic solvents. J. Sci. Labour, 56(12):
1-9.
NEIHOF, R.A. & BAILEY, C.A. (1978) Biocidal properties of anti-icing
additives for aircraft fuels. Appl. environ. Microbiol., 35: 698-703.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., TAYLOR, B.J., & HORNUNG,
R.W. (1981) Ethoxyethanol behavioral teratology in rats. Neuro-
toxicology., 2(2): 231-249.
NELSON, B.K., BRIGHTWELL, W.S., & SETZER, J.V. (1982) Prenatal
interactions between ethanol and the industrial solvent 2-ethoxyethanol in
rats: maternal and behavioral teratogenic effects. Neurobehav. Toxicol.
Teratol., 4: 387-394.
NELSON, B.K., BRIGHTWELL, W.S., BURG, J.R., & MASSARI, V.J. (1984a)
Behavioral and neurochemical alterations in the offspring of rats after
maternal or paternal inhalation exposure to the industrial solvent 2-
methoxyethanol. Pharmacol. Biochem. Behav., 20: 269-279.
NELSON, B.K., SETZER, J.V., BRIGHTWELL, W.S., MATHINOS, P.R., KUCZUR,
M.H., WEAVER, T.E., & GOAD, P.T. (1984b) Comparative inhalation
teratogenicity of four glycol ether solvents and amino derivative in rats.
Environ. Health Perspect., 57: 261-271.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., & O'DONOHUE, T.L. (1984c)
Reproductive toxicity of the industrial solvent 2-ethoxyethanol in rats
and interactive effects of ethanol. Environ. Health Perspect., 57: 255-
259.
NIOSH (1986) Health hazard evaluation report: Precision Castparts
Corporation, Portland, Oregon, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (Report No. HETA-84-415-1688).
NIOSH (1987a) Alcohols IV. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1403.
NIOSH (1987b) Esters I. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1450.
NITTER-HAUGE, S. (1970) Poisoning with ethylene glycol monomethyl ether:
report of two cases. Acta med. Scand., 188: 277-280.
OHI, G. & WEGMANN, D.H. (1978) Transcutaneous ethylene glycol monomethyl
ether poisoning in the work setting. J. occup. Med., 20: 675-676.
OUDIZ, D. & ZENICK, H. (1986) In vivo and in vitro evaluations of
spermatotoxicity induced by 2-ethoxyethanol treatment. Toxicol. appl.
Pharmacol., 84: 576-583.
PARSONS, C.E. & PARSONS, M.E.M. (1938) Toxic encephalopathy and
"granulopenic anaemia" due to volatile solvents in industry: report of
two cases. J. ind. Hyg. Toxicol., 20: 124-135.
PAUSTENBACH, D.J. (1988) Assessment of the developmental risks resulting
from occupational exposure to selected glycol ethers within the
semiconductor industry. J. Toxicol. environ. Health, 23: 29-75.
PISKO, G.T. & VERBILOV, A.A. (1988) Toxicity of monomethyl, monoethyl and
monobutyl ethers of ethylene glycol. Gig. Tr. prof. Zabol., 3: 48-49.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J. Water Pollut. Control Fed., 46: 63-77.
RAO, K.S., COBEL-GEARD, S.R., YOUNG, J.T., HANLEY, T.R., Jr, HAYES, W.C.,
JOHN, J.A., & MILLER, R.R. (1983) Ethylene glycol monomethyl ether II.
Reproductive and dominant lethal studies in rats. Fundam. appl. Toxicol.,
3: 80-85.
RITTER, E.J., SCOTT, W.J., RANDALL, J.L., & RITTER, J.M. (1985)
Teratogenicity of dimethoxyethyl phthalate and its metabolites
methoxyethanol and methoxyacetic acid in the rat. Teratology, 32: 25-31.
ROMER, K.G., BALGE, F., & FREUNDT, K.J. (1985) Ethanol-induced
accumulation of ethylene glycol monoalkyl ethers in rats. Drug chem.
Toxicol., 8(4): 255-264.
ROWE, V.K. & WOLF, M.A. (1982) Derivatives of glycols. In: Clayton, G.D. &
Clayton, F.E., ed. Patty's industrial hygiene toxicology, Vol. 2, pp.
3909-4052.
SAPARMAMEDOV, E. (1974) [Toxicity of some simple ethylene glycol ethers
(single experiments).] Zdravookhr Turkm., 18(9): 26-31 (in Russian)
(English translation from US NIOSH.).
SAVOLAINEN, H. (1980) Glial cell toxicity of ethyleneglycol monomethyl
ether vapour. Environ. Res., 22: 423-430.
SCOTT, W.J., FRADKIN, R., NAU, H., & WITTFOHT, W. (1987) Teratologic
potential of 2-methoxyethanol (2-ME) in non-human primates. Teratology,
35(2): 66 (abstract).
SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1986) Localization of
radioactivity from 2-methoxy[1,2-14C]ethanol in maternal and conceptus
compartments of CD-1 mice. Toxicol. appl. Pharmacol., 84: 25-35.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1987) The teratogenicity and
disposition of the glycol ether 2-methoxyethanol and their relationship in
CD-1 mice. In: Welsch, F., ed. Approaches to elucidate mechanisms in
teratogenesis, New York, Hemisphere Publishing Co., pp. 33-57.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1988) The relationship of
embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicol. appl.
Pharmacol., 93: 195-207.
SMALLWOOD, A.W., DEBORD, K.E., & LOWRY, L.K. (1984) Analyses of ethylene
glycol monoalkyl ethers and their proposed metabolites in blood and urine.
Environ. Health Perspect., 57: 249-253.
SMALLWOOD, A.W., DEBORD, K., BURG, J., MOSELEY, C., & LOWRY, L. (1988)
Determination of urinary 2-ethoxyacetic acid as an indicator of
occupational exposure to 2-ethoxyethanol. Appl. ind. Hyg., 3(2): 47-50.
SMYTH, H.F., SEATON, J., & FISCHER, L. (1941) The single dose toxicity of
some glycols and derivatives. J. ind. Hyg. Toxicol., 23: 259-268.
SPARER, J., WELCH, L.S., MCMANUS, K., & CULLEN, M.R. (1988a) Effects of
exposure to glycol ethers in shipyard painters. I. Evaluation of exposure.
Am. J. ind. Med., 14: 497-507.
SPARER, J., WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R.
(1988b) Effects of exposure to glycol ethers in shipyard painters. II.
Male reproduction. Am. J. ind. Med., 14: 509-526.
STENGER, E.G., AEPPLI, L., MULLER, D., PEHEIM, E., & THOMANN, P. (1971)
Toxicology of ethylene glycol monoethyl ether. Arzneim Forsch., 21: 880-
885.
STOTT, W.T. & MCKENNA, M.J. (1985) Hydrolysis of several glycol ether
acetates and acrylate esters by nasal mucosal carboxylesterase in vitro .
Fundam. appl. Toxicol., 5: 399-404.
SZYBALSKI, W. (1958) Special microbiological systems II. Observations on
chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76: 475-488.
TANAKA, K., MIKAMI, E., & SUZUKI, T. (1986) Methane fermentation of 2-
methoxyethanol by mesophilic digesting sludge. J. Ferment. Technol.,
64(4): 305-309.
TORAASON, M., STRINGER, B., STOBER, P., & HARDIN, B.D. (1985)
Electrocardiographic study of rat fetuses exposed to ethylene glycol
monomethyl ether (EGME). Teratology, 32: 33-39.
TORAASON, M., BREITENSTEIN, M.J., & SMITH, R.J. (1986a) Ethylene glycol
monomethyl ether (EGME) inhibits rat embryo ornithine decarboxylase (ODC)
activity. Drug chem. Toxicol., 9: 191-203.
TORAASON, M., STRINGER, B., & SMITH, R. (1986b) Ornithine decarboxylase
activity in the neonatal rat heart following prenatal exposure to ethylene
glycol monomethyl ether. Drug chem. Toxicol., 9(1): 1-14.
TYL, R.W., PRITTS, I.M., FRANCE, K.A., FISHER, L.C., & TYLER, T.R. (1988)
Developmental toxicity evaluation of inhaled 2-ethoxyethanol acetate in
Fischer 344 rats and New Zealand white rabbits. Fundam. appl. Toxicol.,
10: 20-39.
US EPA (1987) Environmental health criteria: 2-methoxyethanol, 2-
ethoxyethanol, and their acetates, Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances.
VERSCHUEREN, K. (1977) Ethylene glycol monomethyl ether. In: Handbook of
experimental data on organic chemicals, New York, Van Nostrand-Reinhold
Company, 327 pp.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987a)
Field study of the urinary excretion of ethoxyacetic acid during repeated
daily exposure to the ethyl ether of ethylene glycol and the ethyl ether
of ethylene glycol acetate. Scand. J. Work Environ. Health, 13: 239-242.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987b)
Survey of ethylene glycol ether exposures in Belgian industries and
workshops. Am. Ind. Hyg. Assoc. J., 48(8): 671-676.
WEIL, C.S. & SCALA, R.A. (1971) Study of intra- and inter-laboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol. appl. Pharmacol., 19: 276-360.
WELCH, L.S. & CULLEN, M.R. (1988) Effects of exposure to glycol ethers in
shipyard painters. III. Hematologic effects. Am. J. ind. Med., 14: 527-
536.
WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R. (1988) Effects
of exposure to ethylene glycol ethers on shipyard painters: II. Male
reproduction. Am. J. ind. Med., 14: 509-526.
WELSCH, R., SLEET, R.B., & GREENE, J.A. (1987) Attenuation of 2-
methoxyethanol and methoxyacetic acid-induced digit malformations in mice
by simple physiological compounds: implications for the role of further
metabolism of methoxyacetic acid in developmental toxicity. J. biochem.
Toxicol., 2: 225-240.
WERNER, H.W., MITCHELL, J.L., MILLER, J.W., & VON OETTINGEN, W.F. (1943)
Effects of repeated exposure of dogs to monoalkyl ethylene glycol ether
vapors. J. ind. Hyg. Toxicol., 25(9): 409-414.
WICKRAMARATNE, G.A., de S. (1986) The teratogenic potential and dose-
response of dermally administered ethylene glycol monomethyl ether (EGME)
estimated in rats with the Chernoff-Kavlock assay. J. appl. Toxicol.,
6(3): 165-166.
YONEMOTO, J., BROWN, N.A., & WEBB, M. (1984) Effects of dimethoxyethyl
phthalate, monomethoxyacetic acid on post implantation rat embryos in
culture. Toxicol. Lett., 21: 97-102.
YOUNG, E.G. & WOOLNER, L.B. (1946) A case of fatal poisoning from 2-
methoxyethanol. J. ind. Hyg. Toxicol., 28: 267-268.
ZIMMERMANN, F.K., MAYER, V.W., SCHEEL, I., & RESNICK, M.A. (1985) Acetone,
methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae.
Mutat. Res., 149: 339-351.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
La présente monographie ne traite que des éthers
méthyliques et éthyliques de l'éthylène-glycol, c'est-à-
dire le méthoxy-2 éthanol (2-ME), l'éthoxy-2 éthanol
(2-EE) et leurs esters acétiques respectifs, à savoir
l'acétate de méthoxy-2 éthyle (2-MEA) et l'acétate
d'éthoxy-2 éthyle (2-EEA). Ces composés se présentent tous
les quatre sous la forme de liquides stables incolores et
inflammables, dotés d'une légère odeur éthérée; ils sont
tous miscibles à l'eau (ou tout au moins dans le cas du
2-EAA très soluble dans celle-ci) et miscibles à un grand
nombre de solvants organiques.
Il existe des méthodes d'analyse permettant la mise en
évidence de ces éthers du glycol et de leurs métabolites
dans divers milieux (air, eau, sang et urine). Ces
méthodes font souvent appel à des techniques d'adsorption
ou d'extraction afin de concentrer l'échantillon, suivies
d'une analyse par chromatographie en phase gazeuse. La
chromatographie en phase gazeuse ou la chromatographie
liquide à haute performance permettent de doser l'acide
méthoxy-2 acétique (MAA) ainsi que l'acide éthoxy-2
acétique (EAA) (qui sont des métabolites du du 2-ME et du
2-EE) dans les urines, généralement après obtention de
dérivés convenables, à des concentrations entre 5 et
100 µg/ml.
2. Sources d'exposition humaine et environnementale
Les quatre éthers du glycol étudiés s'obtiennent tous
par réaction de l'oxyde d'éthylène sur l'alcool convenable
puis, si nécessaire, par estérification à l'aide d'acide
éthanoïque.
On ne dispose pas de données concernant la production
mondiale de ces éthers du glycol. Toutefois on peut
avancer que la production annuelle globale de l'Europe
occidentale, des Etats-Unis et du Japon se situe aux envi-
rons de 79 x 103 tonnes de 2-ME et de 205 x 103 tonnes
de 2-EE. Ils sont en grande partie utilisés pour la
production industrielle de divers revêtements (peintures,
teintures, laques, vernis, etc.) et comme solvants pour la
préparation d'encres d'impression, de résines et de
colorants, ainsi que pour la fabrication de détachants
domestiques et industriels. On les utilise également comme
additifs de dégivrage dans les liquides hydrauliques et
les carburéacteurs.
3. Transport, distribution et transformation dans l'environnement
Du fait de leur solubilité dans l'eau et de leur
tension de vapeur relativement basse, ces éthers
pourraient, en l'absence de décomposition, s'accumuler
dans l'eau. Toutefois, il semble que cette éventualité
soit exclue du fait de leur dégradation par des micro-
organismes présents dans le sol, les boues d'effluents et
l'eau.
C'est l'utilisation de ces éthers comme solvants vola-
tils qui, du fait des émissions atmosphériques auxquelles
elle donne lieu, entraîne l'exposition environnementale la
plus importante. Dans l'environnement général, ils
subissent une photolyse rapide et l'on pourrait s'attendre
à des concentrations inférieures à 0,0007 mg/m3 (2 x
10-4 ppm).
En aérobiose, les éthers du glycol subissent une
dégradation microbienne rapide en dioxyde de carbone et en
eau, alors qu'en anaérobiose, les principaux produits
finals sont le méthane et le dioxyde de carbone.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation d'éthers du glycol peut entraîner des
nombreuses émissions dans l'environnement. C'est en parti-
culier l'exposition humaine directe dans l'industrie, dans
les petits ateliers et au cours de l'utilisation domes-
tique de produits à base d'éthers du glycol qui est spéci-
alement préoccupante. En ce qui concerne l'exposition pro-
fessionnelle, les valeurs signalées vont de concentrations
< 0,1 mg/m3 à des concentrations > 150 mg/m3. L'utili-
sation de certains produits de consommation à base
d'éthers du glycol pourrait provoquer une exposition
notable des usagers mais on ne dispose pas de données à ce
sujet.
Outre l'exposition par la voie atmosphérique, l'homme
peut également être exposé par la voie dermique. L'analyse
du sang confirme que ces produits sont rapidement absorbés
par cette voie, qui contribue probablement davantage à la
charge totale de l'organisme que l'exposition par voie
aérienne.
5. Cinétique et métabolisme
Ces quatre éthers sont rapidement absorbés au niveau
de la peau, des poumons et des voies digestives. Des
études de répartition portant sur le 2-ME chez des souris
gravides ont montré que c'est dans le foie maternel, les
voies digestives, le placenta, le sac vitellin et de
nombreuses structures embryonnaires que se rencontraient
les concentrations les plus fortes.
La métabolisation du 2-ME donne naissance à deux
métabolites primaires : le MAA et la méthoxy-2 acétyl-
glycine. La transformation en dioxyde de carbone corres-
pond à une voie métabolique secondaire de moindre
importance. La conversion plasmatique du 2-ME en MAA
s'effectue rapidement, avec une demi-vie de 0,6 heure chez
le rat; en revanche l'excrétion de la MAA est lente, sa
demi-vie étant d'environ 20 heures chez le rat et de 77
heures chez l'homme.
L'administration à des animaux de laboratoire de 2-EE
a conduit à la production d'EAA et d'éthoxy-2 acétyl-
glycine, l'EAA étant le principal métabolite qui se mani-
feste dans l'organe supposé être l'organe cible, à savoir
les testicules. Chez l'homme, une étude sur le 2-EAA a
permis d'observer une voie métabolique analogue, l'acétate
étant d'abord hydrolysé en 2-EE puis oxydé en EAA. Cet
EAA a été ensuite excrété avec une demi-vie estimative de
21 à 42 heures. L'expérience semble indiquer que la
rétention ou l'accumulation des métabolites pourrait être
toxicologiquement importante dans la mesure où ces méta-
bolites seraient responsables de la toxicité observée au
niveau de l'organe cible.
6. Effets sur les êtres vivants dans leur milieu naturel
Il semble que le 2-ME et le 2-EE présentent une faible
toxicité pour les micro-organismes et les animaux aqua-
tiques. En ce qui concerne les micro-organismes, la con-
centration létale dans le milieu est supérieure à 2 %. On
a constaté une inhibition de la croissance des algues
vertes par le 2-ME à la concentration de 104 mg/litre et
de celle des cyanobactéries (algues bleu/vert) à la con-
centration de 100 mg/litre. La toxicité aiguë du 2-EE est
très faible pour les arthropodes (CL50 > à 4 g/litre) et
les poissons d'eau douce (CL50 > à 10 g/litre). Les acé-
tates des éthers du glycol (2-MEA et 2-EAA) sont beaucoup
plus toxiques pour les poissons. Ainsi la CL50 du 2-EEA
pour le vairon Pimephales promelas est de 46 mg par litre
tandis que celle du 2-MAA est de 45 mg/litre pour le
tarpon et pour Lepomis machrochirus. Il n'y a pas eu
d'études à long terme.
7. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
7.1 Toxicité générale
La toxicité du 2-ME et du 2-EE chez l'animal d'expéri-
ence a été beaucoup plus étudiée que celle du 2-MEA et du
2-EAA.
En ce qui concerne le 2-ME et le 2-EE ainsi que leurs
acétates, la dose létale après exposition unique est du
même ordre et ces composés présentent une faible toxicité
aiguë, que l'exposition ait lieu par voie dermique, orale
ou par inhalation. Pour diverses espèces, les valeurs de
la DL50 vont de 900 à 3400 mg/kg de poids corporel pour
le 2-ME, de 1400 à 5500 mg/kg pour le 2-EE, de 1250 à
3900 mg/kg pour le 2-MEA et de 1300 à 5100 mg/kg pour le
2-EAA. Des valeurs de 4603 mg/m3 (2-ME) et de 6698 mg
par m3 (2-EE) ont été signalées pour la CL50 par inhala-
tion chez la souris.
On ne possède que peu de données concernant les effets
irritants au niveau des yeux et de la peau ou le pouvoir
de sensibilisation de ces éthers du glycol chez l'animal.
Il semblerait qu'ils ne soient pas irritants pour la peau,
mais qu'ils puissent l'être pour l'oeil. Chez l'homme,
malgré de fortes expositions, on n'a jamais signalé
d'irritation cutanée ni de sensibilisation à ce niveau.
On a montré qu'en exposant par voie respiratoire des
animaux d'expérience pendant des périodes allant jusqu'à
90 jours, à de fortes concentrations (> 9313 mg de 2-ME
par m3 et > 1450 mg de 2-EE/m3) on déterminait des
effets nocifs sur les paramètres hématologiques, le
système nerveux, les testicules, le thymus, les reins, le
foie et les poumons. A des concentrations plus faibles,
les effets ne s'observaient qu'au niveau du système
hématopoïétique et des testicules. Par exemple, des rats
exposés par inhalation à du 2-ME pendant 13 semaines à des
doses comprises entre 93 et 930 mg/m3, présentaient une
réduction de l'hématocrite, du nombre de leucocytes, de
l'hémoglobine, des plaquettes et des protéines sériques,
mais seulement à la dose la plus forte. Chez des lapins
exposés de la même manière, on notait une réduction de la
taille du thymus et une altération des paramètres hémato-
logiques, à la dose de 903 mg/m3. Le 2-EE a produit des
effets analogues, mais moins graves, chez le rat et le
lapin lors d'une exposition de 13 semaines à la concen-
tration de 1450 mg/m3. On ne dispose d'aucune donnée
résultant d'études à long terme.
7.2 Cancérogénicité et mutagénicité
On a étudié la mutagénicité du 2-ME sur toute une
série de systèmes in vitro constitués de bactéries ou de
cellules mammaliennes. La plupart des études ont fourni
des résultats négatifs, toutefois on a tout de même
signalé des résultats positifs à de très fortes concen-
trations de 2-ME sur des cellules CHO. Il s'agissait
d'aberrations chromosomiques (à des concentrations supéri-
eures à 6830 µg/ml) et d'échanges entre chromatides
soeurs (à des concentrations supérieures à 3170 µg par
ml). La recherche d'aberrations chromosomiques et de
micronoyaux n'a rien donné in vivo . On ne dispose que de
données limitées sur le pouvoir mutagène du 2-EE; en
outre, il n'existe pas de données sur la cancérogénicité
de ces éthers du glycol.
7.3 Organes mâles de la reproduction
On a étudié de manière approfondie l'effet du 2-ME sur
l'appareil reproducteur mâle après administration par voie
orale ou respiratoire de ces substances à des rongeurs.
La présence de modifications dégénératives au niveau de
l'épithélium germinal des tubes séminifères à été systé-
matiquement observée. Des effets analogues ont été con-
statés avec le 2-EE, mais à des doses un peu inférieures.
L'administration par voie orale à des rats de 2-ME
pendant 1 à 11 jours a provoqué une réduction du nombre
des spermatozoïdes et des modifications de leur mobilité
et de leur morphologie, liées à la dose, à partir de
100 mg/kg de poids corporel. L'autopsie a révélé une
atteinte histologique marquée des testicules. La dose
sans effet observable (NOEL) était de 50 mg/kg. La
réduction de la fertilité était encore manifeste huit
semaines après une exposition à 200 mg/kg. Des effets
analogues ont été observés dans le cas du 2-EE à des doses
supérieures ou égales à 500 mg/kg, administrées pendant
des périodes allant jusqu'à 11 jours, la dose sans effet
observable sur 11 jours étant de 250 mg/kg. Toutefois
l'épuisement des réserves de spermatozoïdes, par suite
d'accouplements répétés, s'est accompagné à la dose la
plus faible étudiée (150 mg/kg), d'une réduction de leur
nombre. Après avoir administré par voie orale à des rats
et à des souris une dose unique de 250 mg ou davantage de
2-ME/kg de poids corporel, on a observé chez les animaux
une stérilité complète cinq semaines après l'admini-
stration, une certaine réduction de la fécondité étant
observée dès 125 mg/kg.
L'administration du 2-ME par la voie respiratoire a
donné lieu à des modifications dégénératives analogues au
niveau des testicules. Les effets en question ont été
observés après exposition unique de 4 heures à des doses
supérieures ou égales à 1944 mg/m3, aucun effet n'étant
observé à 933 mg/m3. Les valeurs de la dose sans effet
observable étaient de 311 mg/m3 chez les rats après
exposition de 13 semaines (6 heures par jour, 5 jours par
semaine) et de 933 mg/m3 (6 heures par jour) chez les
souris après exposition à neuf reprises sur une durée
totale de 11 jours. L'exposition de lapins à du 2-ME
pendant 13 semaines (6 heures par jour, 5 jours par
semaine) a entraîné des effets marqués au niveau des
testicules à la dose de 311 mg/m3 ou davantage; à la
dose de 93 mg/m3, on a observé des effets limites; il
n'a pas été possible de déterminer la dose sans effet
observable.
7.4 Toxicité foetale
On a observé des effets toxiques sur le développement
de plusieurs espèces d'animaux de laboratoire après
exposition par toutes les voies possibles, c'est-à-dire
orale, respiratoire et dermique. Le 2-ME a produit des
effets tératogènes chez la souris, le rat, le lapin et le
singe. Le 2-EE et le 2-EEA se sont révélés tératogènes
chez le rat et le lapin. Bien que le 2-MEA n'ait pas
encore été étudié de ce point de vue, son profil méta-
bolique (voir section 6) incite à penser qu'il a
vraisembleblement une toxicité analogue à celle du 2-ME.
C'est dans le cas du 2-ME que l'on possède l'ensemble
le plus complet de données dose-réponse (doses de 31,25 à
1000 mg/kg/j). Lors de cette étude portant sur des souris
auxquelles le 2-ME avait été administré par gavage
(administration les jours 7 et 14 de la gestation), on a
obtenu une dose sans effet observable de 125 mg/kg par
jour en ce qui concerne la toxicité maternelle. Toutefois
des malformations ont été observées à partir de doses
quotidiennes de 62,5 mg/kg et des modifications au niveau
du squelette à partir de 31,25 mg/kg par jour. La dose
sans effet observable relative à la toxicité foetale n'a
pas été indiquée. Lors d'études portant sur des doses
uniques, des souris ont reçu par gavage du 2-ME au onzième
jour de la gestation; la dose de 100 mg/kg n'était pas
foetotoxique tandis que celle de 175 mg/kg a produit des
anomalies digitales, mais sans autres signes de toxicité
maternelle ou foetale. Des anomalis cardio-vasculaires et
électrocardiographiques ont été observés chez les rats
nouveau-nés après administration les 7ème et 13ème jours
de la gestation d'une dose quotidienne de 25 mg/kg. Etant
donné qu'il s'agissait de la dose la plus faible expéri-
mentée, l'étude n'a pas permis d'établir une dose sans
effet observable sur le foetus (on n'a pas observé de
toxicité maternelle à cette dose). De même, aucune dose
de ce type n'a pu être déterminée après administration par
gavage à des guenons de 2-ME aux doses quotidiennes
respectives de 0,16, 0,32, ou 0,47 mmol/kg, du 20ème au
45ème jour de la gestation.
Après exposition par voie respiratoire à du 2-ME à la
dose de 156 mg/m3, on a observé une toxicité foetale
chez des rats et des souris et des malformations chez des
lapins. Pour l'ensemble de ces trois espèces, la dose sans
effet observable sur le développement foetal était de 31
mg/m3. Toutefois des anomalies comportementales et
neurochimiques ont été observées dans la descendance de
rattes exposées du 7ème au 13ème jour ou du 14ème au 20ème
jour de leur gestation à une dose de 78 mg de 2-ME par m3.
Après avoir exposé des rats à des doses de 743 mg par
m3 de 2-EE et des lapins à des doses 589 mg/m3 de la
même substance, on a constaté que ce produit était térato-
gène (avec en outre une certaine toxicité maternelle).
Dans une autre étude, on a constaté une toxicité foetale,
mais sans malformations, chez des rats exposés à des doses
de 184 ou 920 mg de 2-EE par m3 et chez des lapins ex-
posés à la dose de 644 mg/m3 de la même substance. Pour
ce qui est des effets sur le développement foetal, la
valeur de la dose sans effet observable était de 37 mg par
m3 pour le rat et de 184 mg/m3 pour le lapin. On a ob-
servé des anomalies comportementales et neurochimiques
dans la descendance de rattes exposées du 7ème au 13ème
jour et du 14ème au 20ème jour de leur gestation à la dose
de 360 mg de 2-EE par m3.
Des rattes soumises à une application dermique de 0,25
ml de 2-EE non dilué quatre fois par jour du 7ème et 16ème
jour de la gestation, ont eu une descendance où l'on
notait une foetotoxicité marquée et une forte incidence de
malformations malgré l'absence de toxicité maternelle. Des
effets analogues ont été observés à la suite d'un traite-
ment indentique par le 2-EEA selon le même protocole au
moyen d'une dose équimolaire (0,35 ml, quatre fois par
jour).
En exposant par la voie respiratoire des lapines à du
2-EEA du 6ème au 18ème jour de la gestation, on a obtenu
au cours de deux études différentes, des réponses térato-
gènes aux doses de 2176 mg/m3 et 544 mg/m3, la valeur
de la dose sans effet observable sur le développement
foetal étant respectivement de 135 mg/m3 et 270 mg par
m3. L'exposition de rattes du 6ème au 15ème jour de leur
gestation à du 2-EEA a entraîné dans leur descendance une
toxicité foetale à la dose de 540 mg/m3 et des malfor-
mations à la dose de 1080 mg/m3. La dose sans effet ob-
servable sur le développement foetal était de 170 mg par
m3.
8. Effets sur l'homme
On ne dispose que de renseignements limités sur les
effets toxiques chez l'homme de ces quatre éthers du
glycol. Les résultats fournis par quelques études de cas
ou études épidémiologiques sur les lieux de travail sont
dans la ligne des effets observés chez les animaux de
laboratoire. On n'a pas eu connaissance de rapports qui
chiffrent l'exposition de la population en général ni les
effets sur la santé.
Lors de deux cas non mortels d'empoisonnement par
ingestion d'un volume de 100 ml de 2-ME, on a noté les
principaux symptômes suivants : nausée, vertiges, cyanose,
tachycardie, hyperventilation et acidose métabolique avec
quelques signes d'insuffisance rénale. Des sympômes
analogues mais moins graves ont été observés chez une
personne qui avait ingéré 40 ml de 2-EE. Lors d'un
empoisonnement mortel par ingestion de 400 ml de 2-ME,
l'autopsie a révélé une gastrite hémorragique aiguë, une
dégénérescence graisseuse du foie et une altération
dégénérative des tubules rénaux.
L'exposition réitérée de travailleurs à du 2-ME et à
du 2-EE, en plus d'autres solvants, a entraîné chez eux de
l'anémie, un leucopénie, une faiblesse générale et une
ataxie. Dans nombre de ces études, il n'a pas été possible
de trouver une estimation fiable de l'exposition des
sujets. On a rapporté des effets hématologiques dus aux
éthers du glycol chez l'homme et on a notamment décrit
l'apparition d'une anémie macrocytaire chez un travailleur
exposé à du 2-ME (dose moyenne 105 ml/m3), ainsi qu'à
d'autres solvants.
Il a été fait état d'une toxicité médullaire chez des
ouvriers dont l'épiderme était exposé à du 2-ME, et des
effets immunologiques ont été également notés à la suite
d'une exposition prolongée (8 à 35 années) au 2-ME et au
2-EE (doses moyennes d'exposition 6,1 mg/m3 et 4,8 mg par
m3 respectivement).
Des études épidémiologiques effectuées sur des
ouvriers exposés à du 2-ME et à du 2-EE ont révélé des
anomalies au niveau de la fonction de reproduction, avec
une fréquenc accrue des cas d'oligospermie. L'exposition
à du 2-EE (37 ouvriers) à des concentrations pouvant
atteindre 88,5 mg/m3 a entraîné une modification du sper-
mogramme. Parmi 73 ouvriers exposés à du 2-ME (jusqu'à
17,7 mg/m3) et à du 2-EE (jusqu'à 80,5 mg/m3), on a
constaté une fréquence accrue de cas d'oligospermie et
observé certains effets hématologiques, pour des doses
d'exposition (TWA) de 2,6 mg/m3 dans le cas du 2-ME et de
9,9 mg/m3 dans celui du 2-EE.
Les effets indésirables constatés chez l'homme par
suite d'une exposition professionnelle correspondent à
ceux qui ont été observés chez les animaux de laboratoire.
Cependant, l'évaluation de l'exposition présentant un
certain nombre d'insuffisances et du fait qu'il s'agissait
d'expositions simultanées à plusieurs substances, il n'a
pas été possible d'en déduire une relation dose-réponse.
9. Conclusions
De nombreuses personnes peuvent être exposées à ces
quatre éthers du glycol à des concentrations comparables à
celles que l'on rencontre dans l'industrie, par suite de
l'utilisation de certains produits de consommation ou de
produits commerciaux. Une exposition professionnelle non
négligeable peut se produire par inhalation ou par résorp-
tion cutanée. Des mesures faites en petit nombre dans
l'air des lieux de travail indiquent des teneurs allant <
0,1 mg/m3 à > 150 mg/m3.
Le 2-ME et le 2-EE sont tous deux d'une faible toxi-
cité pour les micro-organismes et les espèces aquatiques.
Il n'existe aucune donnée qui permettrait d'évaluer le
risque d'effets indésirables sur les êtres vivants dans
leur milieu naturel par suite d'une exposition de longue
durée.
Chez le rat, la dose de 2-ME sans effets aigus observ-
ables au niveau testiculaire, est de 933 mg/m3; en cas
d'exposition répétée, la dose sans effet observable est de
311 mg/m3. En exposant de manière répétée l'espèce la
plus sensible, à savoir le lapin, on a observé un effet
net dès 311 mg/m3; cet effet était limite à la dose de
93 mg/m3 (un animal sur cinq). Les données fournies par
des études effectuées sur des sujets humains exposés de
par leur profession à du 2-ME et à du 2-EE montrent que
ces éthers du glycol exercent une certaine toxicité
testiculaire.
Chez toutes les espèces étudiées (souris, rats et
lapins) on a observé après exposition au 2-ME à des doses
égales ou supérieures à 156 mg/m3, une toxicité vis-à-
vis du développement foetal. Des anomalies comportement-
ales et neurochimiques ont été observées chez le rat après
exposition in utero à 78 mg/m3 de cette substance sans
qu'on puisse déterminer de dose sans effet observable. Le
2-EE et le 2-EEA se sont révélés légèrement moins actifs.
Des effets ont été également observés sur le développement
de foetus de rats et de lapins après exposition à du 2-EE
à des doses égales ou supérieures 368 mg/m3. Ces effets
étaient légers chez les rats exposés à 184 mg de 2-EE par
m3 mais on a pu néanmoins fixer nettement la dose sans
effet observable chez le rat et le lapin à la valeur de
38 mg/m3.
Ces éthers du glycol produisent des effets hématolo-
giques chez la souris, le rat, le lapin, le chien, le
hamster et le cobaye. Ces résultats sont en accord avec
les anomalies hématologiques observées lors des quelques
études consacrées à des travailleurs de l'industrie qui
avaient subi des expositions répétées à du 2-EE et/ou du
2-ME. Lors d'études sur l'animal comportant une expo-
sition répétée à ces deux substances, on a fixé à 93 mg
par m3 la dose de 2-ME sans effet observable chez le
lapin et à 368 mg/m3 la dose de 2-EE sans effet obser-
vable chez le rat et le lapin. On n'a pas pu obtenir de
données qui permettent une évaluation quatitative des
effets hématologiques aigus consécutifs à une exposition.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
1.1 Exposition
Nombreuses sont les personnes qui peuvent être
exposées au méthoxy-2 éthanol (2-ME), à l'éthoxy-2 éthanol
(2-EE) et à leurs acétates (2-MEA et 2-EEA) à des concen-
trations comparables à celles que l'on rencontre dans
l'industrie, lors de l'utilisation de produits de consom-
mation et de produits commerciaux. En revanche l'expo-
sition par l'intermédiaire des denrées alimentaires, de
l'eau ou de l'air ambiant est probablement négligeable.
Cette hypothèse ne repose que sur les propriétés
physiques et chimiques de ces composés et sur le fait
qu'ils se dégradent rapidement dans l'environnement.
Une exposition professionnelle non négligeable peut se
produire par inhalation ou résorption cutanée. Les
quelques mesures de concentrations dans l'air des lieux de
travail ont donné des valeurs qui vont de moins de 0,1 mg
par m3 à plus de 150 mg/m3. Toutefois les données de
surveillance existantes sont très limitées et il peut y
avoir d'importantes variations entre les différentes
industries et dans une même industrie. En raison du risque
de résorption cutanée, une simple surveillance de l'air
des lieux de travail risque de sous estimer l'exposition
totale. Pour évaluer la charge totale de l'organisme, la
meilleure méthode consiste à faire un contrôle biologique.
Une forte exposition peut se produire lors de travaux tels
que la peinture, l'impression ou le nettoyage mais il faut
se souvenir que ces composés sont utilisés à l'occasion
d'un grand nombre d'autres activités au cours desquelles
on pourrait craindre une exposition.
1.2 Effets sur la santé
Les principaux effets qu'on peut craindre chez l'homme
tiennent à l'action toxique de ces composés sur le
développement foetal, les testicules et les paramètres
hématologiques. La réalité de ces effets est attestée par
des données nombreuses et cohérentes obtenues chez l'ani-
mal ainsi que par quelques données concernant l'homme.
Tous ces effets peuvent appraître par suite d'expositions
à court ou à long terme. Chez l'animal de laboratoire,
une exposition répétée à des très fortes doses de 2-ME et
de 2-EE (plus de 930 ou 1450 mg/m3, respectivement)
entraîne des effets toxiques qui se traduisent par des
anomalies neuro-comportementales, hépatiques et rénales.
On les observe également dans les cas d'intoxication
humaine.
Ces quatre éthers du glycol exercent des effets
toxiques très voisins tant sur les testicules que sur le
développement du foetus, et ce, chez toutes les espèces
étudiées et par toutes les voies d'exposition qui ont été
utilisées (voie respiratoire, voie percutanée, voie
orale). L'étude du mécanisme de ces effets montre que
dans les deux cas, une phase d'activation est nécessaire,
à savoir la métabolisation en un dérivé de l'acide alkoxy-
acétique correspondant. La métabolisation s'effectue en
présence du système de l'alcool déshydrogénase qui est
commun à l'homme et aux animaux de laboratoire. Des méta-
bolites toxiques, l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA) ont été décelés dans l'urine de
sujets exposés à ces solvants. La régularité de cette
réponse toxique d'une espèce animale à l'autre, jointe à
l'analogie du métabolisme chez l'homme et l'animal,
montrent à l'évidence que l'homme pourrait être la cible
des mêmes effets toxiques sur les testicules et le
développement foetal. Les données dont on dispose au sujet
de l'excrétion des acides alkoxyacétiques chez l'homme
indiquent que leur durée de rétention est plus longue chez
celui-ci que chez l'animal, ce qui incite à penser que
l'homme pourrait être plus sensible à ces effets que
l'animal de laboratoire le plus sensible. C'est la
résorption cutanée rapide de ces composés qui est parti-
culièrement préocccupante. On a observé des effets térato-
gènes et autres anomalies du developpement à la suite de
l'application de 2-ME, de 2-EE et de 2-EAA sur la peau
intacte du rat.
Des lésions testiculaires ont été observées chez le
rat, la souris et le lapin à la suite d'une exposition à
ces éthers du glycol, soit par la voie respiratoire soit
par la voie orale. Chez le rat, une seule exposition par
voie respiratoire à des doses de 2-ME supérieures ou
égales à 1944 mg/m3 pendant 4 heures et une exposition
répétée à des doses supérieures ou égales à 933 mg par
m3 pendant 13 semaines ont déterminé des anomalies histo-
logiques au niveau testiculaire. Dans le cas de l'expo-
sition unique, la dose sans effet observable était de 933
mg/m3; elle était 311 mg/m3 dans le cas d'expositions
répétées. La souris a semblé un peu moins sensible, la
dose sans effet observable dans son cas étant, pour des
expositions répétées, de 933 mg/m3. Toutefois le lapin
l'était davantage, avec des altérations testiculaires
marquées qu'on pouvait observer après des expositions
répétées à 311 mg/m3 et la présence d'effets limites (un
lapin sur cinq) dès la dose de 93 mg/m3. Des effets ana-
logues ont été observés à la suite de l'exposition par
voie orale de rats à du 2-ME, une exposition de courte
durée (et notamment à une dose unique) ayant déterminé des
lésions testiculaires à partir de 100 mg/m3. Dans le
cas d'une étude portant sur les effets subaigus (11 jours)
la dose sans effet observable a été de 50 mg/kg. Le 2-EE
présente une toxicité testiculaire un peu moindre que
celle du 2-ME; ces effets n'apparaissent qu'à des doses
de 500 mg/kg ou davantage et la dose sans effet observable
se situe à 250 mg/kg.
Les données fournies par les études menées sur des
sujets humains exposés de par leur profession à du 2-ME et
à du 2-EE cadrent avec les données obtenues sur l'animal
et montrent que ces éthers du glycol peuvent produire des
effets toxiques au niveau testiculaire chez l'homme. Des
études épidémiologiques portant sur de petits groupes de
travailleurs exposés au 2-EE dans un atelier de fonte de
métaux et chez des peintres d'un chantier naval exposés à
ces deux composés, ont révélé une oligospermie systéma-
tique. Les données relatives aux niveaux d'exposition sont
limitées mais dans chaque cas, il y a lieu de penser qu'il
y a eu exposition par voie cutanée et respiratoire.
On a observé chez le rat, la souris, le lapin et le
singe des effets toxiques sur le développement embryon-
naire après exposition à ces éthers du glycol par voie
percutanée, orale ou respiratoire. Du 2-ME non dilué
appliqué à 12 reprises au cours d'une journée sur la peau
rasée de rattes gravides (zone d'application couverte
pendant 6 heures) s'est révélé mortel pour les animaux
alors qu'appliqué à 10 reprises sans pansement, du 2-EE
(1,0 ml/jour) ou du 2-EEA (1,4 ml/jour) ont produit des
effets tératogènes mais n'ont pas été toxiques pour les
femelles gravides. Au cours d'une autre étude, 12 appli-
cations de 2-ME à 10 % dans du soluté physiologique, avec
pansement sur le site d'application, ont entraîné des
effets toxiques sur le développement embryonnaire, la dose
sans effet observable se situant à la concentration de
3 %. Après administration répétée par voie orale de 2-ME
à des animaux gravides, on n'a pas observé de dose sans
effet toxique. La dose la plus faible produisant un effet
observable dans le cas de l'administration par voie orale
était de 31,5 mg/kg par jour pour la souris, de 25 mg/kg
pour le rat et de 0,16 mmol/kg pour le singe. En ce qui
concerne l'administration d'une dose unique, on ne possède
des résultats que pour le 2-ME chez la souris, avec une
dose sans effet observable pour le 11ème jour de la
gestation (le jour le plus sensible) de 100 mg/kg et une
valeur de 175 mg/kg pour la dose la plus faible avec effet
observable. Le 2-EE et le 2-EEA ont fait l'objet d'études
au cours desquelles des rats et des lapins ont été exposés
à ces deux composés par la voie respiratoire. L'expo-
sition des rats au 2-EE a fait l'objet de deux études qui
ont révélé des effets tératogènes (743 mg/m3, 7 heures
par jour, du premier au 19ème jour de la gestation) ou des
effets foetotoxiques (184 et 920 mg/m3, 6 heures par
jour, du 6ème au 15ème jour de la gestation). Dans cette
dernière étude, on a trouvé une dose sans effet observable
de 37 mg/m3. Chez des lapins exposés à du 2-EE on a
également observé des effets tératogènes (589 mg/m3, 7
heures par jour du premier au 18ème jour de la gestation)
et des effets foetotoxiques (644 mg/m3, 6 heures par
jour, du 6ème au 18ème jour de la gestation). Dans cette
dernière étude, la dose sans effet observable était de
184 mg/m3. Chez des lapines exposées à du 2-EEA 6 heures
par jour du 6ème au 18ème jour de la gestation, on a
observé des effets tératogènes à la dose de 2160 mg/m3
dans une étude et à la dose de 1620 mg/m3 dans une
autre. Les deux études ont montré l'apparition d'une
foetotoxicité à 540 mg/m3; la dose sans effet observable
correspondant, dans chaque étude, à la dose la plus faible
étudiée (respectivement 135 et 270 mg/m3). Des rats
exposés par voie respiratoire à du 2-EEA 6 heures par jour
du 6ème au 15ème jour de la gestation ont présenté le même
de type de réactions : effets tératogènes à 1620 mg par
m3, foetotoxicité à 1080 mg/m3 et aucun effet à 260 mg
par m3.
Des effets toxiques sur le développement embryonnaire
ont donc été observés chez toutes les espèces (souris,
rats et lapins), exposées à des doses supérieures ou
égales à 156 mg de 2-ME. Pour ces trois espèces, la dose
sans effet observable était de 31 mg/m3. Après expo-
sition in utero à 78 mg/m3, on a observé chez la descen-
dance des altérations comportementales et neurochimiques
sans qu'il soit possible de déterminer la dose sans effet
observable.
Le 2-EE et le 2-EEA se sont révélés légèrement moins
actifs. Des effets toxiques sur le développement embryon-
naire ont été observés chez des rats et des lapins après
exposition à des doses supérieures ou égales à 368 mg par
m3 de 2-EE. De légers effets de ce type ont été également
observés chez des rats exposés à 184 mg de 2-EE/m3, la
dose sans effet observable étant clairement établie à
37 mg/m3. En ce qui concerne le 2-EEA, la dose sans
effet observable était de 170 mg/m3 pour les rats et les
lapins.
Des effets hématologiques consécutifs à une exposition
à une dose unique on été observés chez l'animal ainsi qu'à
la suite d'intoxications chez l'homme. L'exposition
répétée par voie respiratoire, de lapins, l'espèce la plus
sensible, à du 2-ME 5 fois par semaine pendant 13
semaines, a permis de fixer la dose sans effet observable
à 93 mg/m3. Administré à répétition, le 2-ME produit
également des anomalies hématologiques chez la souris, le
lapin, le chien, le hamster et le cobaye. Le 2-EE est
moins actif à cet égard que le 2-ME. En ce qui concerne
les effets hématologiques chez le rat et le lapin, après
exposition à du 2-EE pendant 13 semaines, 5 fois par
semaine et 6 heures par jour, on peut fixer la dose sans
effet observable à 368 mg/m3. Chez le chien et la
souris, on observe également après, exposition répétée à
des doses plus élevées, un certain nombre d'effets hémato-
logiques. L'exposition aux acétates de 2-EE et de 2-ME
devrait provoquer des effets analogues, toutes choses
égales d'ailleurs, mais les données dont on dispose sur
l'exposition et les effets hématologiques de ces composés
sont trop limitées pour qu'on puisse établir les con-
ditions dans lesquelles une seule exposition conduirait à
des effets hématologiques chez l'homme.
On a observé dans l'industrie des niveaux d'exposition
voisins de la dose sans effet hématologique observable que
l'on a pu déterminer chez l'animal après expositions
répétées à du 2-ME et à du 2-EE. On peut en conclure,
compte tenu de la sensibilité probablement plus grande de
l'homme et de l'accumulation vraisemblable de métabolites
dans le sang, que l'exposition résultant de l'utilisation
de produits de consommation ou d'activités dans
l'industrie pourrait entraîner des effets hématologiques
de ce genre. A l'appui de cette hypothèse, on peut citer
les effets hématologiques effectivement signalés lors de
quelques études effectuées chez des travailleurs de
l'industrie ayant subi des expositions répétées au 2-EE
et/ou au 2-ME.
2. Evaluation des effets sur l'environnement
La libération directe dans l'atmosphère ou l'utili-
sation de ces produits comme solvants volatils peut con-
duire à une exposition dans le milieu ambiant. Ce peut
être également le cas par suite d'une libération acciden-
telle sur le sol ou dans l'eau. L'accumulation dans le
sol et les eaux superficielles ne peut se produire qu'en
l'absence de dégradation de ces composés. Or, on sait que
ces éthers du glycol se décomposent rapidement sous
l'effet de processus chimiques et biologiques; ils ne
devraient donce pas s'accumuler. Le 2-MEA et le 2-EAA
devraient également s'hydrolyser rapidement et subir
ensuite une biodégradation aérobie. Toutefois dans des
conditions d'anaérobiose, on pourrait craindre une
contamination des sols et des nappes phréatiques encore
que le phénomène soit vraisemblablement transitoire et le
risque correspondant négligeable.
Le 2-ME et le 2-EE sont peu toxiques pour les micro-
organismes et les espèces aquatiques. Leurs acétates, en
revanche, présentent une toxicité aiguë beaucoup plus
forte. On ne dispose pas de données à partir desquelles
on puisse déterminer leur potentiel nocif pour les espèces
vivantes en cas d'exposition prolongée.
RECOMMANDATIONS
1. Protection de la santé
1. Il conviendrait de recercher des solvants moins
toxiques que le méthoxy-2 éthanol, l'éthoxy-2 éthanol et
leurs esters, en particulier pour les remplacer dans les
produits de consommation. Il est également important
d'évaluer les effets des autres éthers de l'éthylène-
glycol car certains d'entre eux pourraient excercer des
effets analogues aux quatre éthers qui ont fait l'objet de
la présente évaluation.
2. Du fait des effets toxiques reconnus de ces éthers du
glycol, les autorités responsables devraient se préoccuper
sérieusement de trouver les moyens d'avertir les consom-
mateurs des dangers que présente l'utilisation de ces
produits, en particulier en cas d'exposition par voie
cutanée.
3. Compte tenu des données toxicologiques récentes et de
la possibilité d'une très forte absorption percutanée des
ces éthers du glycol, il importe de revoir les limites
nationales d'exposition professionnelle pour faire en
sorte que la dose quotidienne totale à laquelle sont
exposés les travailleurs par n'importe quelle voie, ne
menace pas leur santé.
4. Lorsqu'elle est assez importante, une dose unique peut
produire des effets sur l'animal. Pour réduire les
risques, il est recommandé d'utiliser ces produits avec
prudence (veiller à l'hygiène personnelle, utiliser des
dispositifs protecteurs appropriés et assurer une venti-
lation suffisante). Les données montrent que les effets
toxiques sur le développement embryonnaires ainsi que sur
le sang et les testicules, consécutifs à une exposition
répétée, pourraient exiger des mesures de protection plus
importantes.
2. Recherches à affectuer
1. Du fait que l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA), qui sont les principaux métabolites
du 2-ME, du 2-EE et de leurs esters, exercent des effets
toxiques sur les organes reproducteurs mâles, il faudrait
en étudier le mode d'action. S'ils apparaît que le MAA et
EAA ne sont pas les véritables responsables de ces effets,
ceux-ci devront être identifiés et leur mode d'action
élucidé.
2. Ces quatre éthers du glycol ont des effets sur le sang
et sur la fonction de reproduction masculine (oligo-
spermie). Les données disponibles, bien qu'en nombre
limité, indiquent que ces deux effets se manifestent à des
doses analogues. Il faudrait étudier le mode d'action de
ces composés sur ces deux types d'organes et étudier
parallèlement le nombre de spermatozoïdes et les effets
hématologiques afin de voir si ces derniers sont suscept-
ibles de jouer le rôle de signal d'alarme pour d'autres
effets toxiques de ces composés.
3. La surveillance de l'air ne suffit pas à assurer une
faible exposition. Un contrôle biologique peut permettre
de déceler les insuffisances des mesures de protection. A
l'heure actuelle on n'a pas établi avec une certitude
suffisante la relation qui existe entre les indicateurs
biologiques de l'exposition, la charge totale de l'organ-
isme et les effets physiologiques observés. Des travaux
sont encore nécessaires pour pouvoir établir les limites
de sécurité en fonction des résultats de la surveillance
biologique.
4. Il faudrait envisager des études épidémiologiques ou
une surveillance sanitaire spécifique au sein de popu-
lations qui sont fortement exposées à ces éthers du glycol
afin d'obtenir des relations exposition-effets; on
pourrait alors déterminer les limites de sécurité, à
condition toutefois que l'exposition globale puisse être
correctement et suffisamment évaluée par la surveillance
de l'environnement et des contrôles biologiques.
5. Il conviendrait d'étudier l'éventualité d'effets sur
l'appareil reproducteur féminin en procédant à des études
de reproduction sur plusieurs générations d'animaux de
laboratoire.
6. Les résultats disponibles indiquent que l'homme
pourrait métaboliser dans une plus grande proportion que
le rat ces éthers du glycol en acides alkoxyacétiques
correspondants, la demi-vie des ces métabolites toxiques
s'étant environ quatre fois plus longue chez l'homme. Par
ailleurs l'organisme du rat est capable de conjuguer une
fraction plus importante des métabolites acides que ne le
fait celui de l'homme. Ces différences pourraient
entraîner une sensibilité relativement plus importante de
l'homme aux éthers du glycol. Une connaissance plus
précise du métabolisme et de la cinétique d'excrétion de
ces composés permettrait de mieux prévoir les limites de
sécurité.
7. Les résultats fournis par des études à court terme
(13 semaines) montrent que des effets s'exercent sur
divers organes. Toutefois, il n'a pas été procédé à des
études suffisamment longues pour qu'on puisse déterminer
si ces effets sont réversibles ou non. Il est donc
recommandé de procéder à des études au cours desquelles
des animaux de laboratoire subiront une exposition d'au
moins 13 semaines à ces composés, suivie d'une période de
récupération. A l'issue de cette période, on devra
déterminer les paramètres physiologiques importants afin
de voir si les effets sont passagers ou non.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, métodos analíticos
En esta monografía sólo se tienen en cuenta los éteres
metílico y etílico del etilenglicol, es decir el 2-metoxi-
etanol (2-ME) y el 2-etoxietanol (2-EE), así como sus
ésteres acéticos respectivos, el acetato de 2-metoxietilo
(2-MEA) y el acetato de 2-etoxietilo (2-EEA). Estos cuatro
compuestos son todos ellos líquidos estables, incoloros e
inflamables con un olor levemente etéreo y todos ellos son
miscibles con (o, en el caso del 2-EEA, muy solubles en)
agua y miscibles con numerosos solventes orgánicos.
Se dispone de métodos analíticos para detectar estos
éteres glicólicos o sus metabolitos en diversos medios
(aire, agua, sangre y orina). En muchos casos se basan en
el empleo de métodos de adsorción o extracción para con-
centrar la muestra, seguidos de un análisis por cromato-
grafía de gases. Mediante la cromatografía de gases o la
cromatografía de líquidos de alto rendimiento, cabe la
posibilidad de determinar cuantitativamente el ácido
2-metoxiacético (MAA) y el ácido 2-etoxiacético (EAA) -
metabolitos del 2-ME y del 2-EE - en la orina, por lo gen-
eral tras derivación, a concentraciones de 5-100 µg por
ml.
2. Fuentes de exposición humana y ambiental
Los cuatro éteres glicólicos examinados están todos
ellos producidos por la reacción del óxido etilénico con
el alcohol apropiado, seguida, si procede, de esterifica-
ción con ácido etanoico.
No se dispone de datos sobre la producción mundial de
estos éteres glicólicos. Sin embargo, la producción anual
combinada de Europa occidental, los Estados Unidos de Amé-
rica y el Japón es aproximadamente del 79 x 103 toneladas de
2-ME y 205 x 103 toneladas de 2-EE. Una proporción con-
siderable se utiliza en la fabricación de pinturas, color-
antes y lacas así como en forma de solventes para tinta de
imprenta, resinas y tintes, y como productos de limpieza
domésticos e industriales. También se utilizan estos com-
puestos como aditivos anticongelantes en los líquidos hi-
dráulicos y el combustible de los reactores.
3. Transporte, distribución y transformación en el medio ambiente
La hidrosolubilidad de estos éteres glicólicos y su
presión de vapor relativamente baja podrían dar lugar a su
acumulación en el agua en ausencia de degradación. Sin
embargo, esta posibilidad queda aparentemente excluida
debido a la degradación por los microorganismos presentes
en el suelo, los cienos de alcantarilla y el agua.
Las emisiones atmosféricas causadas por el uso de
éteres glicólicos como solventes volátiles originan la
máxima exposición ambiental. En el medio ambiente general,
la degradación fotolítica parece ser rápida y cabe prever
niveles inferiores a 0,0007 mg/m3 (2 x 10-4 ppm).
En condiciones aeróbicas los microorganismos degradan
rápidamente los éteres glicólicos en forma de dióxido de
carbono y agua, mientras que en condiciones de anaerobi-
osis los principales productos finales son el metano y el
dióxido de carbono.
4. Niveles en el medio ambiente y exposición humana
El uso de éteres glicólicos puede dar lugar a emisi-
ones considerables y muy extendidas en el medio ambiente.
Suscita especial preocupación la exposición humana directa
en la industria, en los talleres de dimensiones modestas y
como consecuencia del empleo doméstico de productos que
contienen dichos éteres. Se han señalado valores de expo-
sición profesional comprendidos entre < 0,1 mg/m3 y > 150
mg/m3. Los usuarios de productos de consumo pueden
sufrir una exposición considerable, aunque no se dispone
de datos al respecto.
Además de la exposición a los éteres glicólicos pres-
entes en la atmósfera, las personas pueden estar expuestas
por vía cutánea. Los análisis de sangre confirman que la
absorción es rápida por esta vía, que puede contribuir más
que la exposición atmosférica a la carga total que recibe
el organismo.
5. Cinética y metabolismo
Se ha demostrado que los cuatro éteres glicólicos
pueden absorberse rápidamente a través de la piel, los
pulmones y el tracto gastrointestinal. Los valores más
elevados que se han obtenido en los estudios sobre la
distribución del 2-ME en ratonas gestantes se sitúan en el
hígado, la sangre y el tracto gastrointestinal de la
madre, así como en la placenta, el saco vitelino y en
numerosas estructuras del embrión.
La transformación metabólica del 2-ME produce dos
metabolitos primarios: MAA y glicina de 2-metoxiacetilo.
La metabolización a dióxido de carbono representa una vía
secundaria de menor importancia. La conversión del 2-ME
en MAA en el plasma se produce rápidamente, con una vida
media de 0,6 h en las ratas, pero la secreción del MAA es
lenta, con una vida media de 20 h aproximadamente en la
rata y de 77 h en el hombre.
En los animales de laboratorio, la administración de
2-EE da lugar a la producción de EAA y de glicina de
2-etoxiacetilo; el EAA es el principal metabolito que
aparece en los testículos, que son el "órgano diana"
presunto. En un estudio humano en el que se utilizó 2-EEA
se observó una vía metabólica análoga: el acetato se hi-
drolizó primero en 2-EE y luego se transformó en EAA por
oxidación. El EAA resultante se excretó con una vida media
estimada en 21-42 h. Los estudios experimentales hacen
pensar que la retención o acumulación de los metabolitos
podrían ser importantes desde el punto de vista toxico-
lógico en el supuesto de que dichos metabolitos sean la
causa de la toxicidad observada en el "órgano diana".
6. Efectos en los organismos presentes en el medio ambiente
La toxicidad del 2-ME y del 2-EE para los microorga-
nismos y animales acuáticos parece ser baja. En el caso
de los microorganismos, la concentración letal en el medio
es superior al 2%. Con el 2-ME se ha observado inhibición
del crecimiento de las algas verdes a 104 mg/litro y de
las cianobacterias (algas verde azuladas) a 100 mg/litro.
La toxicidad aguda del 2-EE es muy baja para los artrópo-
dos (CL50 > 4 g/litro) y para los peces de agua dulce
(CL50 > 10 g/litro). Los acetatos de éteres glicólicos
(2-MEA y 2-EEA) son mucho más tóxicos para los peces. La
CL50 del 2-EEA para el Phoxinus cabezudo es 46 mg/litro y
la del 2-MEA para el pez plateado de la pleamar y los
Lepomis de agallas azules es de 45 mg/litro. No se han
hecho estudios a largo plazo.
7. Efectos en los animales de experimentación y en los
sistemas de experimentación in vitro
7.1 Toxicidad sistémica
La toxicidad del 2-ME y del 2-EE en los animales de
experimentación se ha estudiado en medida mucho mayor que
la del 2-MA y del 2-EEA.
El 2-ME y el 2-EE y sus acetatos dan tasas análogas de
letalidad tras una exposición única y producen una letali-
dad aguda baja cuando la exposición tiene lugar por vía
dérmica u oral o por inhalación. Los valores de la DL50 oral
en las diversas especies estudiadas oscilan entre 900 y
3400 mg/kg de peso corporal en el caso del 2-ME, entre
1400 y 5500 mg/kg en el del 2-EE, entre 1250 y 3930 mg/kg
en el del 2-MEA, y entre 1300 y 5100 mg/kg en el de 2-EEA.
En los ratones se han obtenido valores de la CL50 por
inhalación de 4603 mg/m3 (2-ME) y 6698 mg/m3 (2-EE).
Sólo se dispone de datos limitados acerca de la irri-
tación cutánea u ocular o del potencial de sensibilización
de estos éteres glicólicos en los animales. Al parecer,
no son irritantes para la piel, pero pueden causar irri-
tación en los ojos. En el hombre no se ha observado irri-
tación ni sensibilización cutáneas ni siquiera en caso de
gran exposición.
La exposición por inhalación a corto plazo (hasta 90
días) de los animales de experimentación a concentraciones
elevadas (> 9313 mg de 2-ME/m3 y > 1450 mg de 2-EE/m3)
ejerce, según se ha demostrado, efectos adversos en los
parámetros sanguíneos, el sistema nervioso y los testícu-
los, el timo, el riñón, el hígado y los pulmones. Utiliz-
ando niveles más bajos de exposición, se han observado
efectos en el sistema hematopoyético y en los testículos.
Así, por ejemplo, las ratas expuestas durante 13 semanas a
la inhalación de 2-ME a concentraciones comprendidas entre
93 y 930 mg/m3 presentaron una reducción del volumen
hematocrito y de los glóbulos blancos, la hemoglobina, las
plaquetas y las concentraciones de proteínas séricas sola-
mente cuando se aplicaba la dosis máxima, mientras que los
ratones expuestos del mismo modo presentaron disminución
del tamaño del timo además de la disminución de los pará-
metros sanguíneos con concentraciones de 930 mg/m3. Las
ratas y conejos expuestos al 2-EE presentaron efectos
análogos pero menos intensos cuando soportaron durante 13
semanas una concentración de 1450 mg/m3. No se dispone
de datos sobre estudios a largo plazo.
7.2 Carcinogenicidad y mutagenicidad
La mutagenicidad del 2-ME se ha estudiado en una gama
de sistemas in vitro utilizando bacterias y células de
mamífero. Aunque la mayor parte de los estudios han dado
resultados negativos, algunos informes acusan resultados
positivos en cuanto a la mutagenicidad de las concentraci-
ones muy altas de 2-ME en células CHO estudiadas desde el
punto de vista de las aberraciones cromosómicas (a 6830 µg
por ml o más) o del intercambio de cromátides equiparables
(3170 µg/ml o más). En cambio, la investigación in vivo
de aberraciones cromosómicas y micronúcleos ha dado
siempre resultados negativos. Sólo se dispone de una
información muy limitada sobre el potencial mutagénico del
2-EE y no se dispone de ningún dato sobre la carcinogen-
icidad de estos éteres glicólicos.
7.3 Sistema reproductor masculino
Los efectos del 2-ME en el sistema reproductor mascu-
lino se han estudiado detenidamente en roedores expuestos
por vía oral o por inhalación. En el epitelio germinal de
los tubos seminíferos se han observado constantemente
alteraciones degenerativas. Análogos efectos se han obten-
ido con el 2-EE, si bien con niveles de dosificación algo
más elevados.
En la rata, la administración oral de 2-ME durante 1-
11 días ha dado lugar a un descenso del recuento de esper-
matozoides con cambios de la motilidad y la morfología de
éstos en relación con la dosis utilizada; como niveles de
dosificación se utilizaron 100 mg/kg de peso corporal o
más. En la autopsia se encontraron acusadas alteraciones
histológicas en los testículos. El nivel de efecto no
observado (NENO) fue de 50 mg/kg. La reducción de la fer-
tilidad seguía siendo patente a las 8 semanas de la expo-
sición a 200 mg/kg. Análogos efectos se observaron con
dosificaciones de 500 mg de 2-EE/kg o más, administrados
durante 11 días como máximo; en el tratamiento de 11 días
el NENO fue de 250 mg/kg. En cambio, cuando las reservas
de espermatozoides están reducidas por la frecuencia de
los acoplamientos, se observó cierta reducción de los
recuentos con la dosis más baja estudiada. Los estudios
de fertilidad consecutivos a la administración de una sola
dosis oral de 250 mg de 2-ME/kg o más mostraron una ester-
ilidad completa, tanto en las ratas como en los ratones, a
partir de las 5 semanas de administración; con 125 mg/kg
se observó ya cierto descenso de la fertilidad.
En los experimentos de inhalación se observaron alter-
aciones degenerativas análogas en los testículos con el
2-ME. Los efectos se observaron tras una sola exposición
(4 h) a 1944 mg/m3 o más pero no con 933 mg/m3. El NENO
fue de 311 mg/m3 en la rata tras la exposición durante 13
semanas (6 h/día, 5 días/semana) y de 933 mg/m3 (6 h/día)
en los ratones tras la exposición en 9 ocasiones en el
curso de 11 días. En los conejos expuestos al 2-ME durante
13 semanas (6 h/día, 5 días/semana) se observaron efectos
marcados en los testículos con concentraciones de 311
mg/m3 o más y efectos marginales con 93 mg/m3; no se
determinó el NENO.
7.4 Toxicidad para el desarrollo
En varias especies de animales de laboratorio se ha
observado toxicidad para el desarrollo tras la exposición
a los compuestos utilizando todas las vías de administra-
ción: oral, inhalatoria y dérmica. El 2-ME produjo efectos
teratógenos en ratones, ratas, conejos y monos. El 2-EE y
el 2-EEA resultaron teratógenos en las ratas y los
ratones. Aunque no se ha estudiado la toxicidad sobre el
desarrollo del 2-MEA, los perfiles metabólicos (véase la
sección 6) hacen pensar que es posible que el 2-MEA tenga
una toxicidad análoga a la del 2-ME.
En relación con el 2-ME se dispone de la gama más
amplia de datos dosis/respuesta (dosis de 31,25 por 1000
mg/kg por día). En este estudio de administración inten-
siva en el que se utilizaron ratones (2-ME administrado
entre el 7° y el 14° día de gestación) el NENO correspon-
diente a la toxicidad materna fue de 125 mg/kg por día.
No obstante, se observaron malformaciones con 62,5 mg/kg
por día y variaciones esqueléticas con 31,25 mg/kg por
día. No se señaló ningún NENO de toxicidad para el desar-
rollo. En el marco de estudios de dosis única, se trató a
ratones con 2-ME administrado por alimentación forzada el
11° día de la gestación; la dosis de 100 mg/kg no era
fetotóxica mientras que la de 175 mg/kg produjo anomalías
digitales sin otros signos de toxicidad materna o fetal.
En la ratas recién nacidas se observaron defectos cardio-
vasculares y anomalías del ECG tras el tratamiento de las
madres con 25 mg/kg por día durante los días 7° a 13° de
la gestación. Como ésta fue la dosis más baja que se
ensayó, este estudio no ha permitido establecer un NENO
para el desarrollo (con esa dosis no se observó toxicidad
materna). De igual modo, no pudo determinarse ningún NENO
de toxicidad para el desarrollo en un estudio de trata-
miento de monos por alimentación forzada con 2-ME a 0,16,
0,32 o 0,47 mmol/kg por día durante los días 20° a 45° de
la gestación.
Tras la exposición a la inhalación de 2-ME a 156 mg
por m3 se ha observado fetotoxicidad en los ratones y
ratas y malformaciones en los conejos. En las tres espe-
cies, el NENO correspondiente a los efectos sobre el
desarrollo fue de 31 mg/m3. Sin embargo, en la descen-
dencia de las ratas expuestas a 78 mg 2-ME/m3 durante los
días 7-13 o 14-20 de la gestación se observaron altera-
ciones conductuales y neuroquímicas.
Tras la exposición por inhalación de ratas (743 mg por
m3) y conejos (589 mg/m3), el 2-EE se reveló terató-
geno (en presencia de ligera toxicidad materna). En otro
estudio se observó fetotoxicidad pero no malformaciones en
las ratas expuestas a 184 ó 920 mg de 2-EE/m3, así como
en los conejos expuestos a 644 mg de 2-EE/m3. Los val-
ores del NENO para los efectos en el desarrollo fueron de
37 mg/m3 en las ratas y de 184 mg/m3 en los conejos. En
la descendencia de las ratas expuestas a 368 mg de 2-EE
por m3 durante los días 7-13 ó 14-20 de la gestación se
observaron alteraciones conductuales y neuroquímicas.
Las ratas tratadas por aplicación dérmica de 0,25 ml
de 2-EE sin diluir (cuatro veces al día en los días 7-16
de la gestación) acusaron una considerable fetotoxicidad y
una elevada incidencia de malformaciones en ausencia de
toxicidad materna. Análogos efectos se observaron tras el
tratamiento de las ratas con 2-EEA, utilizando el mismo
protocolo, a una dosis equimolar (0,35 ml, cuatro veces al
día).
La exposición por inhalación de 2-EEA de conejas
durante los días 6-18 de la gestación provocó respuestas
teratógenas con 2176 mg/m3 y 544 mg/m3 en dos estudios
diferentes, en los cuales los valores del NENO para el
desarrollo fueron de 135 mg/m3 y 270 mg/m3. Las ratas
expuestas al 2-EEA durante los días 6-15 de la gestación
acusaron fetotoxicidad a 540 mg/m3 y malformaciones a
1080 mg/m3. El NENO para el desarrollo fue de 170 mg por
m3.
8. Efectos en el hombre
La información disponible sobre los efectos tóxicos de
estos cuatro éteres glicólicos en el ser humano es limi-
tada. Los resultados de los escasos informes sobre casos
individuales y estudios epidemiológicos en el lugar de
trabajo corroboran los efectos adversos observados en los
animales de experimentación. No se ha encontrado ningún
informe en el que se cuantifique la exposición y los
efectos adversos en la población general.
En dos casos no mortales de envenenamiento por inges-
tión de 100 ml de 2-ME, los signos y síntomas predomi-
nantes fueron náuseas, vértigo, cianosis, taquicardia,
hiperventilación y acidosis metabólica, con algunos indi-
cios de insuficiencia renal. Síntomas análogos, aunque
menos graves, se observaron en un sujeto que ingirió 40 ml
de 2-EE. En un caso de intoxicación mortal causada por la
ingestión de 400 ml de 2-ME, la autopsia reveló una gas-
tritis hemorrágica aguda con degeneración grasa del hígado
y alteraciones degenerativas de los túbulos renales.
La exposición repetida de los trabajadores al 2-ME y
al 2-EE, así como a otros solventes, ha dado lugar a
anemia, leucopenia, debilidad general y ataxia. En muchos
de estos estudios no se ha hecho ninguna estimación fide-
digna de la exposición. Los efectos hematológicos de los
éteres glicólicos en el ser humano están bien documentados
y se ha descrito la aparición de anemia macrocítica en un
trabajador expuesto al 2-ME (promedio: 105 mg/m3), así
como a otros solventes.
En los trabajadores expuestos por vía dérmica al 2-ME
se han observado efectos tóxicos en la médula ósea, y tam-
bién se han observado efectos inmunológicos en trabaja-
dores sometidos a una exposición prolongada (8-35 años) al
2-ME y al 2-EE (los promedios de exposición fueron de 6,1
mg/m3 y 4,8 mg/m3, respectivamente).
Los estudios epidemiológicos realizados en trabaja-
dores expuestos al 2-ME y al 2-EE han revelado algunos
indicios de efectos adversos en el sistema reproductor
masculino, con un aumento de la frecuencia de recuentos
reducidos de espermatozoides. La exposición al 2-EE (37
trabajadores) a concentraciones de hasta 88,5 mg/m3 pro-
vocaron una alteración de los índices seminales. En un
grupo de 73 trabajadores expuestos al 2-ME (hasta 17,7 mg
por m3) y al 2-EE (hasta 80,5 mg/m3) se observó una
mayor frecuencia de recuentos reducidos de espermatozoides
y también signos de efectos hematológicos con exposiciones
de 2,6 mg/m3 para el 2-ME y de 9,9 mg/m3 para el 2-EE
(TWA).
Los efectos adversos observados en las personas pro-
fesionalmente expuestas coinciden con los señalados en los
animales de experimentación. Sin embargo, debido a defici-
encias en las evaluaciones de la exposición y a las expo-
siciones mixtas, no se han podido determinar relaciones
dosis-respuesta.
9. Conclusiones
Muchas personas pueden estar expuestas a concentra-
ciones de estos cuatro éteres glicólicos comparables a las
industriales a consecuencia del empleo de productos comer-
ciales y de consumo. Tanto por inhalación como por absor-
ción cutánea pueden producirse exposiciones profesionales
importantes. En un número limitado de determinaciones de
la concentración atmosférica en los lugares de trabajo se
han obtenido valores comprendidos entre < 0,1 mg/m3 y >
150 mg/m3.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
para los microorganismos y las especies acuáticas. No se
dispone de datos que permitan precisar la capacidad poten-
cial de las exposiciones prolongadas para ejercer efectos
adversos sobre las especies presentes en el medio ambiente.
En las ratas se ha obtenido un NENO para los efectos
testiculares de 933 mg de 2-ME/m3, así como un NENO para
la exposición repetida de 311 mg/m3. En los experimentos
de exposición repetida con la especie más sensible, el
conejo, se ha detectado un efecto neto con 311 mg/m3, mien-
tras que a 93 mg/m3 se observaba un efecto marginal (1 de
5 animales). En las personas expuestas profesionalmente al
2-ME y al 2-EE se han encontrado indicios de que estos
éteres glicólicos pueden producir toxicidad testicular en
el ser humano.
En todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más se ha observado toxi-
cidad para el desarrollo. Para las tres especies se tuvo
un NENO de 31 mg/m3. En las ratas expuestas in utero a
78 mg/m3 se produjeron alteraciones conductuales y neuro-
químicas, pero no se estableció ningún valor de NENO. El
2-EE y el 2-EEA eran algo menos potentes. En la rata y en
el conejo se han observado efectos sobre el desarrollo
tras la exposición a 2-EE a concentraciones de 368 mg por
m3 o más. Estos efectos eran ligeros en las ratas ex-
puestas a 184 mg de 2-EE/m3, pero tanto en las ratas como
en los conejos se pudo establecer un NENO bien definido a
37 mg/m3.
Estos éteres glicólicos producen efectos hematológicos
en los ratones, las ratas, los conejos, los perros, los
hamsters, y los cobayos. Esta observación concuerda con
los efectos hematológicos señalados en algunos de los
escasos estudios efectuados en trabajadores industriales
expuestos repetidamente al 2-EE y/o al 2-ME. En los estu-
dios de exposición repetida de animales se obtuvo un NENO
de 93 mg de 2-ME/m3 en los conejos y de 368 mg de 2-EE
por m3 en las ratas y los conejos. No se han obtenido
datos que permitan evaluar cuantitativamente los efectos
hematológicos que siguen a la exposición aguda.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y EFECTOS
EN EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
1.1 Exposición
Muchas personas pueden estar expuestas al 2-metoxi-
etanol (2-ME), al 2-etoxietanol (2-EE) y a sus acetatos
(2-MEA y 2-EEA) en concentraciones comparables a las
industriales como consecuencia del empleo de productos
comerciales y de consumo. En cambio, la exposición por
los alimentos, el agua o el aire ambiente es probablemente
insignificante. Esta impresión se basa únicamente en las
propiedades físicas y químicas de estos compuestos y en
los indicios de que experimentan una rápida degradación en
el medio ambiente.
Tanto por inhalación como por absorción cutánea puede
producirse una exposición profesional importante. Las
escasas determinaciones de las concentraciones en la
atmósfera de los lugares de trabajo han dado valores
comprendidos entre menos de 0,1 mg/m3 y más de 150 mg por
m3. Sin embargo, las posibilidades de vigilancia son muy
limitadas y cabe la posibilidad de que haya grandes vari-
aciones entre diferentes industrias e incluso dentro de
una misma industria. Habida cuenta de las posibilidades de
absorción cutánea, la vigilancia del aire por sí sola
puede dar una subestimación de la exposición total. La
vigilancia biológica es el mejor método para calcular la
absorción total. Entre los trabajos que entrañan una expo-
sición considerable figuran, por ejemplo, los de pintura,
imprenta y limpieza; ahora bien, no hay que olvidar que
estos compuestos se utilizan también en otras muchas acti-
vidades profesionales en las que la exposición debe ser
motivo de inquietud.
1.2 Efectos en la salud
Los principales motivos de inquietud en el ser humano
son los efectos en el desarrollo, los efectos testiculares
y los vinculados a la toxicidad hematológica. Estos
efectos han sido demostrados por una multitud de datos
sólidos obtenidos en el animal y por algunos datos de
origen humano. Todos ellos pueden estar causados por una
exposición a corto o a largo plazo. En los animales de
experimentación, la exposición muy repetida al 2-ME y al
2-EE (más de 939 y 1450 mg/m3, respectivamente) produce
efectos tóxicos neuroconductuales, hepáticos y renales,
los cuales se observan también en casos de intoxicación
humana.
Estos cuatro éteres glicólicos dan valores muy simi-
lares de toxicidad testicular y de toxicidad para el
desarrollo en todas las especies estudiadas y por todas
las vías de exposición que se han ensayado (inhalatoria,
dérmica y oral). En los estudios sobre el mecanismo de
acción se ha visto que la metabolización al derivado del
ácido alcoxiacético constituye una etapa indispensable de
activación, tanto en los efectos sobre el desarrollo como
en los testiculares. Dicho metabolismo se efectúa mediante
el sistema de deshidrogenasa alcohólica que es común al
hombre y a los animales de laboratorio. Los metabolitos
tóxicos, el ácido metoxiacético (MAA) y el ácido etoxi-
acético (EAA), aparecen en la orina de las personas ex-
puestas a estos solventes. La coherencia de las respuestas
en las distintas especies de animales de laboratorio estu-
diadas, junto con la semejanza del metabolismo en el ser
humano, permiten concluir que el hombre está probablemente
expuesto a los efectos testiculares y sobre el desarrollo
de estos éteres glicólicos. Los datos disponibles sobre
la excreción de ácidos alcoxiacéticos por el hombre
sugieren la existencia de una retención prolongada en com-
paración con la que se observa en los animales de labora-
torio, lo cual hace pensar que las personas quizás sean
más sensibles que las especies experimentales de mayor
sensibilidad. Un motivo de especial inquietud es la rápida
absorción cutánea de estos compuestos. Se han observado
efectos teratógenos y otros efectos sobre el desarrollo
tras la aplicación de 2-ME, 2-EE y 2-EEA en la piel
intacta de la rata.
En la rata, el ratón y el conejo se han observado
alteraciones testiculares tras la exposición a estos
éteres glicólicos, tanto por inhalación como por vía oral.
Una exposición aislada de la rata a la inhalación de 1944
mg de 2-ME/m3 o más durante 4 h y la exposición repetida
a 933 mg de 2-ME/m3 o más durante 13 semanas han provo-
cado signos histológicos evidentes de alteración testi-
cular. El NENO para la exposición aguda fue de 933 mg/m3
y para la exposición repetida de 311 mg/m3. El ratón
parece ser menos sensible, siendo el NENO para la exposi-
ción repetida de 933 mg/m3. En cambio, el conejo resulta
más sensible, observándose en él alteraciones testiculares
marcadas tras la exposición repetida a 311 mg/m3 y un
efecto marginal (uno en cinco conejos afectados) a 93 mg
por m3. Efectos análogos se han observado tras la exposi-
ción oral de la rata al 2-ME, con aparición de lesiones
testiculares tras la exposición breve (inclusive de dosis
única) a 100 mg/kg. En un estu-dio subagudo (11 días) el
NENO fue de 50 mg/kg. El 2-EE es algo menos potente
respecto a la toxicidad testicular que el 2-ME; sólo se
observaron efectos con dosificaciones de 500 mg/kg o más,
siendo el NENO de 250 mg/kg.
Los datos obtenidos en las personas profesionalmente
expuestas al 2-ME y al 2-EE coinciden con los de los estu-
dios en animales e indican que estos éteres glicólicos
pueden producir toxicidad testicular en el ser humano.
Los estudios epidemiológicos de pequeños grupos de traba-
jadores expuestos al 2-EE en una empresa de fundición de
metales y de pintores de embarcaciones expuestos tanto al
2-ME como al 2-EE muestran indefectiblemente una mayor
incidencia de recuentos reducidos de espermatozoides. Los
datos sobre los niveles de exposición, aunque limitados,
aportan en cada caso pruebas de la exposición dérmica así
como de la exposición por inhalación.
En la rata, el ratón, el conejo y el mono se han
observado efectos tóxicos sobre el desarrollo tras la
exposición a estos éteres glicólicos por vía dérmica, oral
o inhalatoria. Con 12 aplicaciones diarias de 2-ME sin
diluir en la piel rasurada de ratas gestantes (oclusión:
6 h) se obtuvo un efecto letal, mientras que 10 aplica-
ciones abiertas de 2-EE (1,0 ml/día) o 2-EEA (1,4 ml/día)
se mostraron teratógenas pero no tóxicas para la madre.
Doce aplicaciones cerradas de 2-ME al 10% en suero salino
resultaron tóxicas para el desarrollo (en este estudio el
NENO fue del 3% para el 2-ME. No se han encontrado niveles
sin efecto aparente tras la administración oral repetida
de 2-ME a las hembras preñadas. El nivel de efecto obser-
vado mínimo (NEOM) en la administración oral de 2-ME fue
de 31,25 mg/kg por día en el caso de los ratones, de 25 mg
por kg por día en el de las ratas y de 0,16 mmol/kg por
día en el de los monos. Sólo se han hecho experimentos de
dosis única con el 2-ME en los ratones, con el resultado
de que el 11° día de la gestación (que es el día más sen-
sible) el NENO era de 100 mg/kg y el NEOM de 175 mg/kg.
Tanto el 2-EE como el 2-EEA se han evaluado en ratas y
conejos por inhalación. A las ratas se las expuso al 2-EE
en dos estudios, en los que se obtuvieron efectos terató-
genos (743 mg/m3 durante 7 h/día los días 1-19 de la ges-
tación) o efectos fetotóxicos (184 y 920 mg/m3 durante 6 h
por día los días 6-15 de la gestación). En este último
estudio, el NENO fue de 37 mg/m3. También los conejos
expuestos al 2-EE presentaron efectos teratógenos (589 mg
por m3 durante 7 h/día los días 1-18 de la gestación) o
efectos fetotóxicos (644 mg/m3 durante 6 h/día los días
6-18 de la gestación). En el último estudio, el NENO fue
de 184 mg/m3. En los conejos expuestos al 2-EEA durante
6 h/día los días 6-18 de la gestación se obtuvieron efec-
tos teratógenos a 2160 mg/m3 en un estudio y a 1620 mg
por m3 en otro. En ambos estudios se observó fetotoxici-
dad a 540 mg/m3 y en uno y otro el nivel de exposición
mínimo (135 y 270 mg/m3, respectivamente) coincidía con
el NENO. Las ratas expuestas al 2-EEA por inhalación dur-
ante 6 h/día los días 6-15 de la gestación presentaron el
mismo tipo de expuesta: efectos teratógenos a 1620 mg por
m3, fetotoxicidad a 1080 mg/m3 y ausencia de todo ef-
ecto a 270 mg/m3.
Así, pues, se ha observado toxicidad para el desar-
rollo en todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más. Para las tres espe-
cies, el NENO fue de 31 mg/m3. En ratas expuestas in
utero a 78 mg/m3 se han observado alteraciones conduc-
tuales y neuroquímicas, no habiéndose señalado ningún
NENO.
El 2-EE y el 2-EEA han resultado algo menos potentes.
En la rata y el conejo, todas las exposiciones al 2-EE a
368 mg/m3 o más fueron seguidas de efectos sobre el des-
arrollo. En las ratas expuestas a 184 mg de 2-EE/m3 estos ef-
ectos eran leves, pero 37 mg/m3 constituía un NENO patente.
En el caso del 2-EEA, el NENO era de 170 mg/m3 tanto en
la rata como en el conejo.
Tanto en los animales como en los casos de envenena-
miento humano se han observado efectos hematológicos tras
la exposición a una dosis aguda única. La exposición por
inhalación repetida de la especie más sensible, el conejo,
al 2-ME durante 13 semanas y a razón de cinco veces por
semana dio un NEI de 93 mg/m3. También las dosis repeti-
das de 2-ME provocan toxicidad hematológica en los
ratones, conejos, perros, hámsters y cobayos. El 2-EE es
menos potente que el 2-ME como causa de efectos hemato-
lógicos. El NENO correspondiente a estos efectos fue de
368 mg/m3 en las ratas y los conejos expuestos a 2-EE
durante 13 semanas a razón de cinco veces por semana
durante 6 h/día. También en los perros y los ratones se
han registrado efectos hematológicos tras la exposición
repetida a concentraciones más elevadas de 2-EE. La
exposición a los ésteres acéticos del 2-EE y del 2-ME
quizá provoque efectos análogos a los mismos niveles de
exposición, pero los datos disponibles sobre exposición y
efectos hematológicos de esos compuestos son demasiado
escasos para determinar en qué condiciones una exposición
humana aislada tendrá efectos hematológicos.
Se han observado niveles de exposición industrial
próximos o idénticos al NENO de efectos hematológicos en
animales expuestos a dosis repetidas de 2-ME o de 2-EE.
Este hecho, junto con la mayor sensibilidad que probable-
mente tienen las personas y la acumulación previsible de
metabolitos en la sangre humana, hace pensar que tanto la
exposición industrial como la de los consumidores pueden
tener efectos hematológicos. Esto ha sido confirmado por
la observación de efectos de este tipo en algunos de los
escasos estudios realizados sobre trabajadores industri-
ales expuestos repetidamente al 2-EE, al 2-ME o a ambos
compuestos a la vez.
2. Evaluación de los efectos sobre el medio ambiente
La exposición ambiental a estos éteres glicólicos
puede producirse como consecuencia de su paso directo a la
atmósfera cuando se utilizan como solventes volátiles.
También pueden ser causa de exposición ambiental los ver-
tidos en el suelo y el agua a consecuencia de escapes ac-
cidentales. La acumulación en el suelo y en las aguas sup-
erficiales sólo podría producirse en ausencia de degrada-
ción. Sin embargo, estos éteres glicólicos se degradan
rápidamente a causa de procesos químicos y biológicos, por
lo que no es probable que se acumulen. Tanto el 2-MEA como
el 2-EEA pueden hidrolizarse fácilmente y, por consigui-
ente, biodegradarse en condiciones aerobias. En cambio,
la contaminación de acuíferos y suelos anaerobios sigue
planteando un problema potencial, aunque es de esperar que
no pase de ser una situación transitoria y, por ende, poco
peligrosa.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
con los microorganismos y las especies acuáticas. Los
acetatos de éteres glicólicos, en cambio, tienen una toxi-
cidad aguda mucho mayor. No se dispone de datos para cal-
cular las posibilidades de efectos adversos en especies
del medio ambiente a causa de la exposición prolongada.
RECOMENDACIONES
1. Protección de la salud
1. Hay que identificar otros solventes menos tóxicos que
puedan sustituir al 2-metoxietanol, al 2-etoxietanol y a
sus ésteres, especialmente en los productos destinados al
consumo. También es particularme importante evaluar los
efectos de otros éteres etilenglicólicos, ya que algunos
pueden tener efectos análogos a los de los cuatro éteres
glicólicos que aquí se evalúan.
2. Habida cuenta de los conocidos efectos tóxicos de
estos éteres glicólicos, las autoridades deben ocuparse
seriamente de establecer estrategias apropiadas para
informar a los usuarios de esos productos acerca de los
riesgos que entrañan, especialmente los resultantes de la
exposición dérmica.
3. En vista de los nuevos datos toxicológicos y de las
posibilidades de absorción dérmica importante de estos
éteres glicólicos, habrá que reconsiderar los límites de
exposición profesional fijados por los países a fin de que
la dosis diaria total que reciben los trabajadores por
todas las vías de administración no plantee un riesgo
indebido para la salud.
4. En los animales se observan efectos de dosis única en
niveles de exposición bastante altos. Con objeto de
reducir los riesgos para la salud, se recomienda utilizar
con prudencia estos compuestos (prestando atención a la
higiene personal, los dispositivos apropiados de protec-
ción y la ventilación adecuada). Los datos disponibles
indican que puede ser necesario aumentar la protección a
fin de evitar efectos en el desarrollo, así como en la
sangre y los testículos como resultando de la exposición
repetida.
2. Investigaciones necesarias
1. En vista de la intervención del ácido metoxiacético
(MAA) y etoxiacético (EAA) (que son los principales meta-
bolitos identificados del 2-ME, del 2-EE y de sus ésteres)
en la toxicidad para el sistema reproductor masculino,
habrá que investigar su mecanismo de acción. Si de ello
se deduce que el MAA y el EAA no son los principales agen-
tes responsables, habrá que tratar de identificar éstos y
de aclarar su mecanismo de acción.
2. Estos cuatro éteres glicólicos tienen a la vez efectos
hematológicos y efectos sobre el sitema reproductor mascu-
lino (reducción del recuento de espermatozoides). Los
datos disponibles, aunque limitados, parecen indicar que
ambos efectos se hacen evidentes a niveles de dosificación
análogos. Habrá que investigar el mecanismo de acción en
ambos sistemas orgánicos y examinar paralelamente los
efectos hematológicos y los recuentos de espermatozoides
con el fin de determinar si las alteraciones de la sangre
proporcionan signos de alarma con repecto a otros efectos
de estos compuestos.
3. La vigilancia del aire no basta por sí sola para
garantizar una exposición de poca intensidad. La vigilan-
cia biológica puede contribuir a detectar defectos de las
medidas de protección. De momento no se ha establecido
claramente la relación existente entre los indicadores
biológicos de exposición, la absorción corporal total y
los efectos observados en la salud. Habrá que proseguir
las investigaciones a fin de adquirir una base para apli-
car la vigilancia biológica a la determinación de la
seguridad en las exposiciones.
4. Hay que proyectar estudios epidemiológicos y/o traba-
jos específicos de vigilancia sanitaria en poblaciones muy
expuestas a estos éteres glicólicos a fin de estimar las
relaciones exposición-efecto con miras a determinar expo-
siciones seguras, siempre que se pueda evaluar adecuada y
suficientemente la exposición total mediante la vigilancia
ambiental y biológica.
5. Habrá que investigar la posibilidad de que estos com-
puestos ejerzan efectos sobre las gónadas femeninas medi-
ante estudios de reproducción multigeneracional en ani-
males.
6. Los datos disponibles indican que el ser humano puede
metabolizar estos éteres glicólicos hasta los correspon-
dientes ácidos alcoxiacéticos en mayor medida que la rata,
y que la vida media de la excreción urinaria de estos
metabolitos tóxicos es aproximadamente cuatro veces más
prolongada en las personas que en las ratas. Por otra
parte, éstas conjugan una mayor cantidad de los metaboli-
tos ácidos, cosa que no hacen las personas. Estas diferen-
cias podrían explicar la sensibilidad relativamente elev-
ada del ser humano a estos éteres glicólicos. Un conocimi-
ento detallado del metabolismo y de la cinética de excre-
ción mejoraría nuestra capacidad para predecir cuáles son
los niveles de exposición seguros.
7. Los resultados obtenidos en estudios a corto plazo (13
semanas) indican efectos en diversos sistemas orgánicos.
En cambio, no se han hecho estudios de suficiente duración
que permitan evaluar la reversibilidad de tales efectos.
Por consiguiente, convendría que se emprendieran estudios
de interrupción en los que los animales de experimentación
estuvieran expuestos a estos éteres glicólicos al menos
durante 13 semanas, seguidas de un periodo apropiado de
recuperación. Cabría evaluar así importantes parámetros
fisiológicos con miras a determinar si esos efectos son o
no transitorios.
 When rats were administered 2-EE at doses from
0.5 mg/kg to 100 mg/kg, Groeseneken et al. (1988) found
increasing relative amounts of EAA in urine (from 13.4% to
36.8% of the total dose). This could be the result of
competition by other metabolic pathways, which would
become more easily saturated at the higher dosage levels.
At 2-EE doses equivalent to the lowest doses in these
animal studies, it was estimated that humans excrete 30-
35% as EAA. Furthermore, 27% (on average) of the EAA was
excreted as the glycine conjugate in the rat, whereas no
glycine conjugation was observed in humans.
The in vitro nasal mucosal carboxylesterase activity
of mice was compared to the activity of other mice tissues
and to the nasal mucosal carboxylesterase activity of
rats, rabbits, or dogs when exposed to 2-MEA or 2-EEA
(Stott & McKenna, 1985). The specific activity in nasal
carboxylase was found to be similar to that of the liver
in mice, but it was greater than the activity found in the
kidney, lung, or blood of mice. Nasal mucosal carboxyl-
esterase activity of mice was comparable to that of dogs,
slightly higher than the activity in rats, and nearly six-
fold higher than the activity in rabbits. These in vitro
studies suggest that considerable hydrolysis may occur in
the intact animal, resulting in the formation of acetic
acid at the initial route of entry.
6.4. Elimination and Excretion
Although 2-ME is rapidly metabolized to 2-MAA after an
intraperitoneal dose of 250 mg/kg body weight in the rat,
the excretion of 2-MAA is fairly slow (half-life of ap-
proximately 20 h) (Moss et al., 1985). In humans, an elim-
ination half-life for 2-MAA of 77.1 h has been reported
(Groeseneken et al., 1989a). The elimination of radioac-
tive 2-EAA (ethyl 1,214C) has been reported in the rat
(half-life of approximately 8 h) (Guest et al., 1984) and
in humans (half-life of approximately 21-42 h)
(Groeseneken, 1986b,c, 1988; Veulemans, 1987a). In rats,
the administration of an oral dose of 230 mg 2-EE/kg body
weight led to the production of EAA and N -ethoxyacety gly-
cine (> 76% of the dose), EAA being the major metabolite
found in testes after 2 h (Cheever et al., 1984).
The urinary excretion of EAA during and after single
4-h exposures to 14, 28, or 50 mg 2-EEA/m3 in human
volunteers has been reported by Groeseneken et al. (1987).
The distribution/excretion time course during and after
2-EEA exposure was similar to that observed for 2-EE.
This indicates that humans, like rodents, hydrolyse the
acetate to 2-EE, which is then converted to the EAA metab-
olite and excreted. The excretion of the EAA metabolite
was observed to be biphasic. A second peak of excretion
occurred approximately 3 h after the first, suggesting
some type of redistribution of the glycol ethers, or of a
metabolite, from a peripheral compartment.
The urinary excretion of EAA was evaluated under field
conditions in which women volunteers were exposed daily to
2-EE or 2-EEA in the process of silk-screen printing
(Veulemans et al., 1987a). Urinary EAA was measured during
5 days of normal production and was also detectable after
a 12-day stop in production. The excretion of EAA in-
creased during the work week, yet it was still detectable
after 12 days without exposure. These data suggest that
the retention of EAA, or of other 2-EE or 2-EEA metab-
olite, may be toxicologically significant if EAA is the
active metabolite responsible for the observed toxicity.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Given the physical and chemical properties of these
four glycol ethers and the known rates of degradation in
the environment (section 4), there is minimal concern that
hazardous levels of these substances will occur. Although
only a few studies have been reported, the available data
support this conclusion. For example, both 2-ME and 2-EE
have been tested for effects on microorganisms and aquatic
animals. The lethal concentration of 2-ME and 2-EE to
microorganisms (Cladosporium resinae, Pseudomonas aeru-
ginosa, Gliomastix sp, and Candida sp is > 2% in the
medium (Neihof & Bailey, 1978; Lee & Wong, 1979). The
exposure of C. resinae lasted 30 to 42 days, whereas the
other organisms were exposed for 4 months. Very low
toxicity was shown by 2-ME to the green alga Scenedesmus
quadricauda (growth inhibition only at > 10 g/litre) and
the cyano-bacterium (blue-green alga) Microcystis aerugin-
osa (growth inhibition only at > 100 mg/litre) (Bringmann
& Kuhn, 1978). 2-EE has very low toxicity to the brine
shrimp (Artemia salina) (LC50 > 4 g/litre) (Price et
al., 1974) and to another arthropod Daphnia magna
(Hermens et al., 1984). The toxicity of 2-EE to fresh-
water fish is also very low, the LC50 (96 h) for the
bluegill (Lepomis macrochirus) being > 10 g/litre. However,
2-EEA is far more toxic to fish in this assay, LC50 (96 h)
values of 60 mg/litre (Bailey et al., 1985) and 46 mg per
litre in fathead minnows (Pimephales promelas) (Purdy,
1987) having been reported. These results confirm those of
Juhnke & Ludemann (1978), who reported an LC50 (48 h)
value of 107-141 mg per litre when using the Golden Orfe
(Leuciscus idus melanotus) test. The reason for this low
LC50 has not been studied, but Dawson et al. (1977) re-
ported similarly low LC50 values for 2-MEA in fish (45 mg
per litre in tidewater silverfish (Menidia beryllina)
and bluegill (Lepomis chirus).
Effects on other organisms have not been reported.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data indicating the acute oral, dermal, intraperito-
neal, and inhalation toxicities of 2-ME and 2-EE are given
in Table 7. As shown, these two glycol ethers have similar
toxicities, and are of low acute toxicity whether animals
are exposed via the dermal, oral, or respiratory routes.
Even after intraperitoneal injection, the reported levels
of acute toxicity are still low (1.7-2.46 g per kg body
weight).
Dyspnoea, somnolence, ataxia, and prostration were re-
ported after near lethal doses of 2-EE were given to rats,
mice, guinea-pigs, or rabbits (Stenger et al., 1971).
Haemoglobinuria and/or haematuria were reported after a
single oral administration of 2-EE with a dose approaching
the LD50 (Laug et al., 1939). Microscopic examination of
the kidneys of rats, mice, guinea-pigs, or rabbits given
2-EE orally revealed severe tubular degeneration, conges-
tion, and cast formation; in some animals most of the
cortical tubules were necrotic (Laug et al., 1939). No
data on the purity of the 2-EE used in these early studies
were reported.
Single 4-h inhalation exposures of rats to 1866 mg 2-ME
per m3 or more led to testicular atrophy as early as 24 h
after exposure (Doe, 1984b).
8.2. Short-term Exposures
Repeated exposure of experimental animals to 2-ME and
2-EE by various routes of administration (7-90 days) have
revealed adverse effects on the haematological and nervous
systems and on the testes, thymus, kidney, liver, and
lung. From data on the metabolic transformation and
elimination of 2-MEA and 2-EEA in animals and man (see
section 6), it is probable that the toxicities of these
glycol ether acetates are similar to those of the parent
chemicals 2-ME and 2-EE.
8.2.1. Haematological and immunological effects
Following repeated exposures to 2-ME and 2-EE using
various routes of administration, haematological effects
have been observed in several species. A summary of the
most relevant studies is given in Tables 8 (2-ME) and 9
(2-EE).
Table 7. Acute toxicity data for 2-methoxyethanol and 2-ethoxyethanol and their acetatesa
-------------------------------------------------------------------------------------------------------------
Compound Species Intra- Oral LD50 Dermal Inhalation References
peritoneal LD50 LC50
LD50
-------------------------------------------------------------------------------------------------------------
2-Methoxyethanol Rat 2460 2460-3400 Smyth et al. (1941); Goldberg et al.
(1962); Pisko & Verbilov (1988)
Mouse 2200 2167-2560 4603 Werner et al. (1943); Saparmamedov
(1480) (1974); Pisko & Verbilov (1988)
Guinea-pig 950 Karel et al. (1947)
Rabbit 890-1450 1300 Carpenter et al. (1956); Pisko &
Verbilov (1988)
2-Ethoxyethanol Rat 2000 2125-5500 Smyth et al. (1941); Laug et al.(1939)
Stenger et al. (1971); Pisko & Verbilov
(1988)
Mouse 1700 4000-4800 6698 Werner et al. (1943); Laug et al.(1939)
(1820) Stenger et al. (1971); Saparmemedov
(1974); Pisko & Verbilov (1988)
Guinea-pig 1400-2600 Karel et al. (1947); Laug et al.(1939)
Rabbit 3300- Stenger et al. (1971); Smyth et al.
15 200 (1941)
2-Methoxyethanol Rat 3930 Smyth et al. (1941)
acetate Guinea-pig 1250 Kirk-Othmer (1980)
Rabbit 5557
2-Ethoxyethanol Rat 5100 Smyth et al. (1941)
acetate Guinea-pig 1910 Kirk-Othmer (1980)
Rabbit 10 333
-------------------------------------------------------------------------------------------------------------
a Doses are given as mg/kg body weight except for inhalation doses,
which are in mg/m3 (values in parentheses are ppm)
Concerning changes in blood parameters, a comparison
of Tables 7, 8, and 9 indicates clearly that 2-ME is more
toxic than 2-EE in several species, although 2-EE has been
less widely studied. For example, Nagano et al. (1979)
showed that 2-EE administered orally for 5 weeks at 2000
mg/kg body weight per day did not result in changes in
haematological parameters other than a reduced leucocyte
count. No effects were observed at 1000 mg/kg. Under a
similar dosing regimen, 2-ME caused a dose-related re-
duction in leucocytes at 500 mg/kg per day, other haemato-
logical parameters being unaffected at 250 mg/kg per day
(Nagano et al., 1979).
Some of the doses used in the studies summarized in
Tables 7 and 8 resulted in histological and functional
changes in the bone marrow and thymus. In an inhalation
study, Miller et al. (1983b) noted thymic atrophy and de-
creased thymus weights in male and female rats exposed to
933 mg 2-ME/m3 for 13 weeks. Miller et al. (1981) also
noted decreased weights of thymus, spleen, and mesenteric
lymph nodes in rats exposed to 933 or 3110 mg 2-ME/m3 for
9 days, and reduced bone marrow cellularity was observed
at 3110 mg/m3. In one study there was at least partial
reversal of these effects 22 days after an exposure last-
ing 4 days (Grant et al., 1985).
When MAA, the metabolite of 2-ME, was administered to
rats by gavage at 300 mg/kg body weight per day for 8
days, it resulted in reduced erythrocyte counts, haemo-
globin concentrations, and packed cell volumes, and a
marked reduction in leucocyte counts. Thymus and spleen
weights were also decreased and a severe lymphoid
depletion was seen in the thymus. Similar, but less
marked, effects on blood and thymus were seen in some
animals administered 100 mg/kg per day. No treatment-
related effects were reported at 30 mg/kg per day (Miller
et al., 1982).
Studies on immunological function show that 2-ME and
2-EE yield different results. De Delbarre et al. (1980)
studied the effect of 2-ME and 2-EE on the humoral respon-
siveness to antigenic stimuli and on adjuvant arthritis in
the rat. 2-ME had an inhibitory effect on adjuvant ar-
thritis at doses greater than 18.6 mg/kg body weight per
day administered subcutaneously, whereas 2-EE was not
effective at doses up to 150 mg/kg. A dose of 150 mg 2-ME
per kg per day delayed rejection of skin grafts and 75 mg
2-ME/kg per day (for 28 days) significantly depressed
antibody production. Again, 2-EE had no effect on these
parameters. House et al. (1985) administered 2-ME to mice
by gavage (10 doses of 250, 500, or 1000 mg 2-ME/kg body
weight per day for 2 weeks) and noted a 48% reduction in
thymus weight at 500 and 1000 mg/kg. However, there was no
significant alteration in immune function or host resist-
ance. Similar findings were reported when the same dose
levels of MAA, the major metabolite of 2-ME (section 6),
were administered.
Table 8. Haematological effects of 2-methoxyethanol in animalsa
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat inhalation 10 males 6 h/day; 9 days; RBC fragility, no effect at Miller et al.
10 females 311, 933, 3110 mg/m3 3110 mg/m3 but reduced RBC, (1981)
Hb, and PCV levels and bone
marrow cellularity seen in M
and F; similar findings in F
at 933 mg/m3; reduced WBC
at 3110 and 933 mg/m3;
reduced thymus weight
Rat inhalation 10 males 6 h/day; 5 days/week only effect at 2 lowest Miller et al.
10 females for 13 weeks; doses was reduced body (1983b)
93.3, 311, 933 mg/m3 weight gain in F; at 933
mg/m3 reduced WBC, Hb,
PCV, and platelets in both
sexes after 4 and 12 weeks;
reduced serum proteins at 13
weeks, thymic atrophy
Rabbit inhalation 5 males 6 h/day; 5 days/week decreased thymus size and Miller et al.
5 females for 13 weeks; body weight, PCV, WBC, (1983b)
93.3, 311, 933 mg/m3 platelets and Hb at 933
mg/m3; slight to moderate
decrease in lymphoid tissue
at 311 mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week decreased RBC, Hb and Hct; Werner et al.
2 controls for 12 weeks; increased immature (1943)
750 mg/m3 granulocytes
Mouse oral 5 males 5 times/week for 4/5 mice at 2000 mg/kg died Nagano et al.
5 weeks; 62.5, 125, before completion; doses at (1979)
250, 500, 1000, 2000 or above 500 mg/kg resulted
mg/kg body weight/day in reduced WBC, RBC, and PCV
Table 8 (contd.)
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat oral 24 males once per day for reduced thymus and spleen Grant et al.
4 days; 100 and 500 weight at 500 mg/kg on days (1985)
mg/kg body weight/day; 1-8, recovery by day 22;
sacrificed reduced WBC at both 100 and
500 mg/kg, largely
reversible by day 22
Hamster oral 4 males once daily, 5 days/week decrease in WBC at 500 mg/kg Nagano et al.
for 5 weeks; 62.5, 125, the only effect on blood (1984)
500 mg/kg body weight/day
Guinea oral 3 males once daily, 5 days/week about 50% decrease in WBC at Nagano et al.
-pig for 5 weeks; 250 and 500 both doses (1984)
mg/kg body weight/day
--------------------------------------------------------------------------------------------------------
a M = male; F = female; PCV = packed cell volume; Hct = haematocrit; RBC = red blood cell count;
WBC = white blood cell count; Hb = haemoglobin; No. = number of animals per group
Table 9. Haematological effects of 2-ethoxyethanol in animalsa
-------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Response Reference
time; level
-------------------------------------------------------------------------------------------------------
Rat inhalation 15 males 6 h/day; 5 days/week decreased leucocyte count Barbee et al.
15 females for 13 weeks; 92, 368, in females at 1472 mg/m3, (1984)
1472 mg/m3 no other significant
biological effect reported
Rabbit inhalation 10 males 6 h/day; 5 days/week for decreased Hb, Hct, and Barbee et al.
10 females 13 weeks; 92, 368, 1472 RBC in both males and (1984)
mg/m3 females only at 1472
mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week for slight reduction in RBC, Werner et al.
2 control 12 weeks; 3091 mg/m3 Hb, and PCV, microcytosis, (1943)
hypochromia, and poly-
chromatophilia were seen,
marked increase in
immature granulocytes
Mouse oral 5 males 5 times/week for 5 lower WBC at 2000 mg/kg, Nagano et al.
weeks; 62.5, 125, 250, but no effect on (1979)
500, 3110, 2000 mg/kg erythrocyte parameters
body weight/day
-------------------------------------------------------------------------------------------------------
a PCV = packed cell volume; Hct = haematocrit;
RBC = red blood cell count; WBC = white blood cell count; Hb = haemoglobin
No. = number of animals per group
8.2.2 Effects on liver and kidney
Effects on the liver, such as reduced cytoplasmic den-
sity, disruption of lobular structure, elevated plasma
fibrinogen, reduced serum proteins, and elevated liver
weights, have been reported in certain studies in which
rats, mice, or rabbits were exposed to 2-ME or 2-EE at
levels in excess of 300 ppm (933 and 1104 mg/m3, respect-
ively), for periods of up to 13 weeks. Many of the effects
observed were reversible, and no consistent pattern was
noted among the various studies (Miller et al., 1981;
1983b; Stenger et al., 1971). Hepatic changes have been
observed at inhalation exposures to 2-ME and 2-EE in ex-
cess of 300 ppm (933 and 1104 mg/m3, respectively).
No pathological changes could be detected in the kid-
neys of rats exposed to approximately 933 mg 2-ME/m3 for
6 or 7 h per day, 5 days a week, for 13 weeks (Miller et
al., 1981, 1983b). In studies with 2-EE, no treatment-
related pathology was reported after inhalation exposure
of rats to 1362 mg/m3 (370 ppm) for 5 weeks or dogs to
3091 mg/m3 (840 ppm) for 12 weeks (Werner et al., 1943).
8.2.3 Behavioural and neurological effects
There have been few reports on the effects of 2-ME
and 2-EE on the function of the nervous system in animals.
Ataxia was reported after inhalation exposure of rats to
18.96 g 2-EE/m3 (5152 ppm) for 8h. After exposure of rats
to levels of 2-ME between 1555 and 12 440 mg/m3, 4 h per
day for up to 7 days, inhibition of an avoidance-escape
conditioned response was observed without any alteration
of motor function (Goldberg et al., 1962). The same
authors reported also a significant inhibition of this
response after 14 days exposure to 1210-4836 mg 2-ME per
m3 (389-1555 ppm). After 3 weeks recovery, rats whose
avoidance response had been inhibited on the 14th day
showed a return to normal. Savolainen (1980) reported a
partial loss of motor function in the hind limbs of rats
after exposure to 1244 mg 2-ME/m3 (400 ppm) for 6 h/day,
5 days per week, for 2 weeks. This hindlimb paralysis
coincided with the glial cell toxicity noted during the
second week of exposure. Recovery was incomplete after 2
weeks post-exposure, the animals receiving the highest
dose showing minor paresis.
8.3 Skin and Eye Irritation; Sensitization
No satisfactory data on the sensitization potential of
2-ME and 2-EE in animals, and only limited data on their
irritant properties to eyes, have been reported. Weil &
Scala (1971) found 2-ME to be an eye irritant. Lailler et
al. (1975) reported that 0.1 ml of undiluted 2-ME led to
corneal and conjunctival oedema and increased vascular
leakage in the conjunctiva and aqueous humour. A 25% aque-
ous solution was much less active.
8.4 Long-term Exposures
No adequate long-term animal studies on 2-ME and 2-EE
or their acetates have been reported to date.
8.5 Effects on Reproduction and Fetal Development
The effects of glycol ethers, particularly 2-ME, on
animal reproduction, fertility, and teratogenicity have
been extensively studied. Some of the early studies on
reproduction have been reviewed by Hardin (1983). The many
studies conducted in several countries and in several
animal species are in general agreement on the nature of
developmental and reproductive effects.
8.5.1 Effects on the male reproductive system
8.5.1.1 Oral exposure
The pathological changes observed in the testes of
rats after the administration of 2-ME have been well
characterized by Foster et al. (1983). The severe degener-
ative changes noted in the germinal epithelium of the
seminiferous tubules of different laboratory animals were
similar, irrespective of species or route of adminis-
tration. Daily doses of 2-ME were administered orally to
rats (50, 100, 250, or 500 mg/kg) for periods between 1
and 11 days, and 250, 500, or 1000 mg 2-EE/kg body weight
per day was administered in a similar regimen (Foster et
al., 1983; Creasy & Foster, 1984; Creasy et al., 1985).
After 2-ME administration, testicular damage was observed
1 day post dosing with 100 mg/kg or more, the lesion being
localized in the late primary spermatocytes. Continuous
dosing with 2-ME resulted not only in progressive deletion
of the primary spermatocytes but also in degenerative
changes in secondary spermatocytes and dividing sper-
matids. Changes in sperm motility, morphology, and concen-
tration were also reported. The cessation in maturation
of early primary spermatocytes led to a depletion of the
spermatid population, resulting in tubules containing only
Sertoli cells, spermatogonia, and early primary spermato-
cytes. Decreased testicular weight was reported at 250 mg
per kg or more. Although most of the effects seen after a
4-day treatment with 2-ME were reversible within 8 weeks,
a small proportion of tubules showed incomplete recovery
indicating a non-reversible, long-term effect. Similar
findings were reported after 2-EE administration, but only
after 11 days of dosing with 500 and 1000 mg/kg. No
effects on the testes were observed following oral admin-
istration of 250 mg 2-EE/kg per day or 50 mg 2-ME/kg per
day.
Subsequent work by Chapin et al. (1985a,b) supports
the hypothesis that 2-ME administered to male rats at
doses of 100 or 200 mg/kg daily for 5 days affects the
spermatogonia. In these studies male rats were given doses
of 0, 50, 100, or 200 mg/kg per day for 5 days, and recov-
ery was investigated by evaluating the testicular damage
in sacrificed animals at 8 subsequent weekly intervals or
alternatively by investigating fertility through mating
with two females per week for 8 weeks. Treatment with 100
or 200 mg/kg resulted in widespread testicular damage and
cell death immediately after treatment, with only very
mild effects being noted at 50 mg/kg (this was thus a
marginal-effect level rather than a no-effect level).
There was some evidence of recovery from testicular damage
towards the end of the study period, but reduced fertility
was still apparent weeks after the cessation of treatment
with 200 mg/kg. Thus, the recovery was neither as
complete nor as rapid as that noted by Foster et al.
(1983).
The in vivo and in vitro effects of 2-ME and MAA ex-
posures, respectively, were compared by evaluating effects
using electron microscopy (Creasy et al., 1986). 2-ME
caused cell death in the pachytene spermatocytes in vivo ,
as well as focal breakdown of the plasma membranes be-
tween spermatocytes and Sertoli cells. Similar, but less
frequent, breaks were seen in vitro when mixed cultures of
Sertoli and germ cells were treated with MAA.
Subsequent studies by Foster et al. (1987), in which
the alkoxyacetic acids of 2-ME and 2-EE (MAA and EAA,
respectively) were given orally in doses equimolar to the
studies described earlier (Foster et al., 1983), yielded
similar patterns of testicular degeneration and effects on
spermatocyte development. The similarity of effects at
equimolar concentrations between the two acetic acid de-
rivatives and their respective glycol ethers suggests that
these metabolites may either be the causative factors or
at least play a role in the production of the observed
degenerative effects.
The assessment of the effects of low doses of poten-
tial toxicants on sperm fertility and production is diffi-
cult because of the large amount of sperm usually produced
by most test species and the large sperm reserves. Using
an experimental design that required bi-daily matings of
Long-Evans male rats, the effects of orally administered
2-EE (0, 150, or 300 mg/kg body weight per day; 5 days per
week for 6 weeks) on sperm reserves and on pachytene sper-
matocytes was evaluated (Hurtt & Zenick, 1986). Further
groups of rats received the same doses of 2-EE, but were
not subjected to repeated mating. After 6 weeks of treat-
ment, the animals were sacrificed and the effect of 2-EE
administration on organ weight, testicular spermatid
count, cauda epididymal sperm count, and sperm morphology
was determined. Exposures to 2-EE resulted in significant
decreases in testicular weight, spermatid count, and epi-
didymal sperm count for both mated and unmated animals at
the highest dose level. However, the effects were also
seen at 150 mg/kg per day in repeatedly mated animals.
Adult male rats treated orally with 2-EE (936 mg/kg
body weight, 5 days/week for 6 weeks) were found to have
decreased sperm counts and altered sperm morphologies
after 5 and 6 weeks of exposure when compared with
vehicle-control animals, and sperm motility was decreased
at week 6 (Oudiz & Zenick, 1986). These data indicate that
the pachytene spermatocyte is the target cell most sensi-
tive to the effects of 2-EE.
The reproductive toxicity of 2-EE in CD-1 mice has
been evaluated in a continuous breeding protocol (Lamb et
al., 1984). Male and female mice (20 males and 20 females
per dose group) were given access to drinking-water con-
taining 0.5, 1.0, or 2.0% 2-EE and housed as breeding
pairs continuously for 14 weeks. Treated animals were then
paired with controls for breeding. At 1 and 2% 2-EE, sig-
nificant adverse effects on fertility were seen, the re-
productive capacity of both males and females being af-
fected. Testicular atrophy, decreased sperm motility, and
increased abnormal sperm levels were seen in treated
males, but no treatment-related pathological effects were
seen in females even though reduced fertility was noted in
the cross-mating phase.
The reproductive toxicity of a single oral dose of
2-ME (0, 500, 750, 1000, or 1500 mg/kg) was assessed in
adult male CD rats and CD-1 mice by Anderson et al.
(1987). Animals from each group were sacrificed at weekly
intervals during weeks 3-8 post exposure and evaluated for
sperm counts, sperm morphology, and testicular histology.
A dose-related toxicological response in spermatocytes was
observed in both species. In a companion study, male rats
and mice were exposed to 2-ME (0, 125, 250, or 500 mg/kg)
and permitted to mate during weeks 1-10 post exposure.
Following mating, pregnant females were sacrificed on day
17 of pregnancy and each uterus was evaluated for dominant
lethal effects. The only effect noted was a decrease in
total number of implants at week 6 following treatment
with 500 mg/kg. In a study in which both rats and mice
were given single doses at 0, 500, 750, 1000, or 1500 mg
per kg and mated 5 and 6 weeks later, dominant lethal
studies showed a dose-related decrease in fertility in
rats, with complete sterility in all but the lowest dose
group after 6 weeks. No effects on the reproductive
capacity of mice were noted. There was no statistically
significant evidence for the induction of dominant lethal
mutations or abnormalities in the F1 generation of either
species.
8.5.1.2 Inhalation studies
Testicular damage has been reported in rats exposed to
2-ME by inhalation for a single 4-h period (Doe, 1984b).
Exposure to 1944 mg/m3 resulted in histological evidence
of damage to the maturing spermatids, testicular atrophy
occurring at 3887 mg/m3 or more. The NOEL was 933 mg/m3.
The effect of repeated exposure to 2-ME has also been
investigated using the inhalation route. Exposure of rats
to 3110 mg/m3 (6 h/day) for 9 out of 11 days resulted in
degenerative changes and necrosis in the germinal epi-
thelium of the seminiferous tubules, but no effects on the
testes were observed with a dose of 933 mg/m3. However,
Doe et al. (1983) reported pronounced atrophy of the sem-
iniferous tubules in rats exposed to 933 mg 2-ME/m3 or
more (6 h/day, 5 days/week) for 13 weeks. The NOEL was
311 mg/m3 (Miller et al., 1983b). Studies were also car-
ried out in rabbits, using exposure levels of 93.3, 311,
and 933 mg/m3 (6 h/day, 5 days/week), and most animals
exposed to 311 mg/m3 showed reduced testis size and
severe degenerative changes in the tubules. Reduced testis
weight was also seen in two out of five animals exposed to
93.3 mg/m3, and one animal showed histological changes
in the germinal epithelium. A NOEL could not be ident-
ified, but 93.3 mg/m3 was near the minimal effective dose
in rabbits.
In addition, an increased incidence of sperm abnor-
malities (principally sperm with banana shaped or amorph-
orous heads) has been noted in mice exposed to 1555 mg
2-ME/m3 (7 h per day for 5 days) but not to 78 mg per
m3 (McGregor et al., 1983).
8.5.2 Embryotoxicity and developmental effects
8.5.2.1 2-Methoxyethanol
2-ME has been shown to lead to embryotoxic and devel-
opmental effects in laboratory animals following inha-
lation, oral, or dermal exposure. The effects of 2-ME
exposure over the entire organ-forming period of gestation
have been studied in rabbits, rats, and mice, the rabbit
being the most sensitive species.
The embryotoxicity of 2-ME after gastric intubation
was evaluated in mice (31.25, 62.5, 125, 250, 500, or 1000
mg/kg body weight) on days 7-14 of gestation (Nagano et
al., 1981). No maternal deaths were observed, but maternal
weight gain was reduced in the mice that received doses of
250 mg/kg per day or more. Maternal toxicity was not seen
at 123 mg/kg or less. On day 18 of gestation, fetuses were
examined and an increase in fetal death rate was observed
at doses of 250 mg/kg or more. At doses of 500 and 1000 mg
per kg, all fetuses were dead at caesarean section, except
for one in the 500-mg/kg group. Approximately 44% (57/130)
of the live fetuses in the 250-mg/kg group were found to
have gross anomalies, including exencephaly (24), umbili-
cal hernia (3), and abnormal digits (29). One fetus had
both exencephaly and abnormal digits. The lone surviving
fetus in the 500-mg/kg group had exencephaly and abnormal
digits. Minimal skeletal malformations were also observed
at the lowest dose of 31.25 mg/kg body weight (a matern-
ally non-toxic dose). Thus, a NOEL could not be ascer-
tained.
Dose-dependent electrocardiographic changes were
detected on gestation day 20 in the rat fetuses of mothers
exposed orally to 2-ME (0, 25, or 50 mg/kg body weight) on
days 7 to 13 of gestation (Toraason et al., 1985). Cardio-
vascular malformations were observed, including right
ductus arteriosus and ventricular septal defects. QRS in-
tervals were significantly prolonged, particularly in the
highest dose group, suggesting intra-ventricular conduc-
tion delays.
Ornithine decarboxylase (ODC) activity is highest
during rapid growth and development in fetuses and is
sensitive to maternal exposure to chemicals and drugs.
Toraason et al. (1986a,b) evaluated the effect on ODC ac-
tivity in the fetuses of pregnant rats that received 25 mg
2-ME/kg by gavage during gestation days 7-13 or 13-19. The
activity was most affected in fetuses exposed during ges-
tation days 7 to 13. It was highest in 3-day old rats and
declined sharply thereafter. No functional or morphologi-
cal effects were observed in fetuses exposed at maternal
dose levels of 25 mg/kg during gestation days 7-13 or in
fetuses exposed at that level during days 13-19.
Nelson et al. (1984a) treated male rats by inhalation
(78 mg 2-ME/m3 for 7 h/day, 7 days/week for 6 weeks) and
subsequently bred these to untreated females. In addition,
groups of 15 pregnant rats were treated with the same dose
(7 h/day on gestation days 7-13 or 14-20) and allowed to
deliver and rear their young. Neuromotor function activity
and simple learning ability were assessed on days 10-90
after birth. Offspring from dams treated between gestation
days 7-13 showed significant changes in avoidance con-
ditioning, and changes in neurochemical levels were ob-
served in the brains of 21-day-old offspring from the
paternally exposed group as well as from both maternally
exposed groups.
8.5.2.2 2-Ethoxyethanol
A dose-finding study in pregnant rats revealed that no
offspring survived inhalation exposures of 3312 mg 2-EE
per m3, 7 h/day, during gestation days 7-13 or 14-20
(Nelson et al., 1981). The authors reported 34% neonatal
deaths at 736 mg/m3 under similar exposure conditions.
Offspring from dams exposed to 368 mg/m3 on gestation
days 7-13 or 14-20 showed impaired performance in behav-
ioural tests and neurochemical alterations in brain samples.
Using inhalation exposures of 478, 1435, and 2208 mg
2-EE/m3 on gestation days 7-15 (7 h/day), Nelson et al.
(1984b) reported complete resorption of all rat fetuses at
2208 mg/m3, reduced fetal weight at 1435 mg/m3, and a
NOEL of 478 mg/m3. Andrew & Hardin (1984), exposed preg-
nant rats to 733 and 2823 mg/m3 for 7 h/day throughout
gestation and observed increased fetal resorptions at 733
mg/m3 as well as skeletal and cardiovascular abnormali-
ties. At 2823 mg/m3 the resorption frequency reached 100%.
Developmental toxicity has been noted in rats after
dermal application of 2-EE (0.25 or 0.5 ml, four times per
day) (Hardin et al., 1982) or 2-EEA (0.35 ml, four times
per day) (Hardin et al., 1984) on gestation days 7-16.
Increased resorption rates and fetal deaths, decreased
viable fetus weights, and increased cardiovascular defects
and skeletal malformations were seen, even at the lowest
dose tested.
The effect of simultaneous administration of ethanol
(10% in drinking water) and 2-EE (368 mg/m3 by inha-
lation) has been investigated, in view of their related
metabolism (via the enzyme alcohol dehydrogenase) (Nelson
et al., 1982, Nelson et al., 1984c). Although the results
obtained are difficult to interpret, ethanol adminis-
tration early in gestation tended to reduce the behav-
ioural effects induced by 2-EE, whereas ethanol given late
in gestation enhanced these effects.
8.5.3 Teratogenicity
8.5.3.1 2-Methoxyethanol
The teratogenic potential of dermally administered
2-ME was estimated in pregnant rats with the Chernoff-
Kavlock screening test (Wickramaratne, 1986). Various con-
centrations (0, 3, 10, 30, or 100%) of 2-ME in physiologi-
cal saline were applied to shaved skin and occluded for
6 h of exposure on gestation days 6-17. Animals were per-
mitted to have their litters and rear the pups until 5
days postpartum, when the study was terminated. No adverse
effects were noted at the 3% dose level. Small litter
sizes and decreased fetal survival were observed at the
10% level. At 30%, lethality was observed in all fetuses,
and all pregnant females died when exposed dermally to
100% 2-ME.
The teratogenicity of 2-ME in mice, rats, and rabbits
has been evaluated by Hanley et al., (1984). Pregnant rats
and rabbits were exposed by inhalation to 0, 9, 31, or 156
mg/m3 for 6 h/day on gestation days 6-18 (rabbits) or 6-
15 (rats), and mice were exposed to 0, 31, or 156 mg per
m3 for 6 h/day on days 6-15 of gestation. No teratogenic
effects were found in CF-1 mice and Fischer-344 rats under
the conditions of this study, although slight fetotoxicity
was noted in both species. In New Zealand white rabbits
exposed to 156 mg/m3 there was a significant increase in
resorption rate and incidence of malformations involving
all organ systems, as well as a significant decrease in
mean fetal body weight when compared to controls. Of the
fetuses from dams exposed to 156 mg per m3, 63% exhibi-
ted at least one malformation and 91% of the litters had
at least one fetus with a malformation. There were no
teratogenic or fetotoxic effects in any of the three
species evaluated at 31 mg/m3.
Developmental phase-specific and dose-related terato-
genic anomalies in CD-1 mice resulting from 2-ME exposure
have been evaluated (Horton et al., 1985). 2-ME was admin-
istered by gavage to pregnant females at doses of 250 mg
per kg (during gestation days 7 to 9, 8 to 10, or 9 to 11;
during days 7 to 8, 9 to 10, or 10 to 11; or once a day on
gestation days 9, 10, 11, 12, or 13) in order to identify
the most sensitive developmental phases for the anomalies
under study. Resulting malformations were specifically re-
lated to the developmental stage at the time of exposure,
with exencephaly being observed between days 7 to 10 and
paw anomalies (syndactyly, oligodactyly, and stunted digit
number 1) during the later stages of development (days 9-
12). The dose dependency of digit malformation was studied
by administering single doses by gavage (100, 175, 250,
300, 350, 400, or 450 mg 2-ME/kg) on gestation day 11.
Dose-related paw malformations were noted in all litters,
with forepaws being more susceptible than hindpaws in
terms of the number and severity of malformations. At 175
mg/kg digit anomalies were induced without concurrent
reductions in fetal weight, while at 250 mg/kg or more
digit anomalies occurred concurrently with reduced fetal
body weight. In this study, the NOEL for the single ex-
posure (day 11) was 100 mg/kg. Although in this same
strain of mice digital malformations were not detected in
near-term fetuses given 100 mg 2-ME/kg body weight by
gavage on gestation day 11 (Greene et al., 1987), there
was a slight increase in the amount of cell death in
approximately 50% of the limb buds from embryos collected
24 h after dosing.
In a preliminary study, the teratogenic potential of
2-ME was determined in Cynomolgus monkeys using doses of
0.16, 0.32, and 0.47 mmol/kg administered by gavage
throughout the period of organogenesis (Scott et al.,
1987). Fetal death was dose-related (2/13 at 0.16 mmol
per kg; 3/10 at 0.32 mmol/kg; and 8/8 at 0.47 mmol/kg).
At the highest dose level, four fetuses were lost through
abortion and one of the remaining four, which was re-
covered by hysterotomy, demonstrated malformation (ectro-
dactyly) of the forelimbs. The MAA content of maternal
sera was followed throughout the treatment period. By the
25th day of treatment, MAA levels had more than doubled in
all treatment groups, compared to the values determined on
day one, indicating that multiple exposure to 2-ME can
lead to accumulation of the potentially embryotoxic metab-
olite MAA, the possible causative factor in the embryonic
deaths and teratogenicity observed (see section 8.8).
8.5.3.2 2-Ethoxyethanol and 2-Ethoxyethanol acetate
Andrew & Hardin (1984) have studied the teratogenic
potential of 2-EE in rats and rabbits. Rats (29-38 per
group) were exposed by inhalation to 0, 552, or 2388 mg
per m3 5 days per week for 3 weeks immediately prior to
mating and then to 0, 743, or 2823 mg/m3 for 7 h/day from
gestation day 1 to 19. Pregnant rabbits received 589 or
2271 mg/m3 for 7 h/day from gestation day 1 to 18. In
the New Zealand white rabbits exposed to 2271 mg/m3, the
rate of early resorptions was 100%. Even at 689 mg/m3, the
number of early resorptions per litter was 6 times that in
the control group. There was no evidence of severe intra-
uterine growth retardation in surviving fetuses, but a
significant increase in the incidence of major malfor-
mations (ventral wall defects and fusion of the aorta with
the pulmonary artery), minor anomalies, and skeletal vari-
ants. Teratogenic effects of 2-EE and also 2-EEA were re-
ported in Dutch Belted Rabbits after inhalation exposures
of 644 mg 2-EE/m3 and 2160 mg 2-EEA/m3 (Doe, 1984a).
In this study the author considered the NOEL in rabbits to
be 184 mg/m3 for 2-EE and 135 mg/m3 for 2-EEA.
In the study by Andrew & Hardin (1984), the highest
2-EE dose (2823 mg/m3) in rats led to 100% resorption,
as in rabbits. The resorption rate per litter in the
group gestationally exposed to 743 mg/m3 was about twice
the control value. Fetal body size was significantly
decreased, indicating retardation of intrauterine growth.
The significant increase in the incidence of cardiovascu-
lar defects (transposed and retrotracheal pulmonary
artery) and the increased incidence of common skeletal
variants and anomalies over control values were indicative
of a teratogenic effect of 2-EE in rats. Doe (1984a)
reported fetotoxicity without teratogenicity in rats after
inhalation exposure to 184 and 920 mg/m3, but no adverse
effects were reported after exposure to 37 mg/m3.
The teratogenic potential of 2-EE was evaluated in
pregnant Sprague-Dawley rats using dermal application
(0.25 or 0.50 ml, four times/day during gestation days 7
to 16) (Hardin et al., 1982). No signs of maternal
toxicity were noted except for ataxia on the last day of
dosing in the high-dose group, as well as a significant
decrease in body weight gain in the last half of ges-
tation. All fetuses in the high-dose group suffered intra-
uterine death. In the low-dose group, there was a signifi-
cant increase in the number of females with 100% dead
implants (p < 0.001), and in the incidence of skeletal
variations (p < 0.05). Also, reductions in fetal body
weight (p < 0.001) and cardiovascular malformations
(p < 0.05) were observed, as were significant decreases in
the number of live fetuses per litter (p < 0.001).
Hardin et al. (1984) studied the developmental tox-
icity of 2-EEA in rats after dermal application of 0.35 ml
2-EEA four times per day on days 7 to 16 of gestation
(daily application 1.37 g). 2-EEA was strongly embryo-
toxic, as reflected by significantly increased frequencies
of completely resorbed litters and dead implants per
litter. Also, the body weight of live fetuses was reduced
relative to water-treated controls. The spectrum and fre-
quency of malformations and variations noted in 2-EEA-
treated animals was similar to that described by Hardin et
al. (1982) in a previous study.
The developmental toxicity (including teratogenicity)
of 2-EEA in rats and rabbits following inhalation ex-
posure has been evaluated by Tyl et al. (1988). Fischer
344 rats (30 animals/group) and New Zealand white rabbits
(24 animals/group) were exposed to 2-EEA concentrations of
0, 270, 540, 1080, or 1620 mg/m3, 6 h/day on gestational
days 6-15 (rats) and 6-18 (rabbits). Fetuses were examined
for external, visceral, and skeletal malformations and
variations on gestational day 21 (rats) and 29 (rabbits).
In both rats and rabbits, maternal toxicity and develop-
mental toxicity were observed at exposures of 540 mg/m3 or
more. Teratogenic responses were increased at 1080 and
1620 mg/m3 with a 100% incidence of total malformations
being observed at the highest dose. An exposure of 270 mg
per m3 was considered a NOEL in both species, no evi-
dence of maternal or developmental toxicity being reported
at this dose.
8.6 Mutagenicity and Related End-Points
The only published data on 2-EE concerns a point mu-
tation test using Escherichia coli, which was reported to
be negative (Szybalski, 1958). However, 2-ME has been
tested in several in vitro and in vivo systems for its
genotoxic potential. The mutagenicity of 2-ME has been
evaluated in the following test systems: Salmonella typhi-
murium, unscheduled DNA synthesis (UDS) in human embryo
fibroblasts, bone marrow metaphase analysis in male and
female rats, dominant lethality in male rats, and a sex-
linked recessive lethal test in Drosophila melanogaster.
No evidence of mutagenicity in S. typhimurium was found
using five strains, with and without metabolic activation,
and dose levels up to 33 mg 2-ME per plate. When 2-ME was
tested in the presence of alcohol dehydrogenase no evi-
dence was found that metabolites of 2-ME were mutagenic in
S. typhimurium. Similarly, the addition of 2-ME at con-
centrations up to 10 mg/ml of medium did not lead to
changes in UDS in human embryo fibroblasts (McGregor et
al., 1983).
The production in CHO cells of sister chromatid ex-
changes (SCEs) and chromosome aberrations by 2-EE was
studied by Galloway et al. (1987). Both assays were
carried out with and without activation by an exogenous
enzyme system from rat liver chemically induced with the
PCB mixture Aroclor 1254. Aberrations were found only in
the absence of metabolic activation when using 2-EE con-
centrations between 4780 and 9510 µg/ml of medium. The
lowest effective concentration was 6830 µg/ml. SCEs were
found with and without activation, the lowest effective
concentration being 3170 µg/ml when the range studied
was 951-9510 µg/ml.
Aneuploidy was induced in the diploid yeast strain
D61.M ( Saccharomyces cerevisiae ) at 2-MEA levels of 3-
5.7% in the medium (Zimmermann et al., 1985). However,
there were no chemically related effects in this yeast
strain on point mutation or recombination after exposure
to 2-MEA. The relevance to mammals of these findings in
yeast is not clear.
No statistically significant increase in chromosome
aberrations was seen in male or female rats after exposure
by inhalation to 2-ME (78 or 1555 mg/m3), 7 h/day, for 1
or 5 days. Where possible 50 metaphases per rat were
scored from groups of 10 animals (McGregor et al., 1983).
Basler (1986) dosed Chinese hamsters intraperitoneally (10
animals) with two thirds of the LD50 of 2-MEA (approxi-
mately 1333 mg/kg body weight in corn oil) and the animals
were sacrificed 12, 24, 48, and 72 h after the single
dose. No statistically significantly increase in micro-
nucleated erythrocytes was noted.
Two dominant lethal studies in rats have been carried
out using 2-ME. McGregor et al. (1983) exposed groups of
10 male CD rats by inhalation to 78 or 1555 mg/m3, 7 h
per day, for 5 days. Control and low-dose groups gave
similar results, but the results at 1555 mg/m3 were
difficult to interpret. A high proportion of early deaths
was noted, which could be partly explained in terms of the
low implantation frequency reported. Therefore, a dominant
lethal effect at 1555 mg/m3 could not be demonstrated
conclusively. Rao et al. (1983), using male Sprague-
Dawley rats exposed to 93, 311, or 933 mg 2-ME/m3 by in-
halation 6 h/day, 5 days/week for 13 weeks, found no domi-
nant lethal effect at 93 or 311 mg/m3. An assessment of
dominant lethality was impossible at 933 mg/m3 due to
almost complete infertility. No positive control was in-
cluded.
In a sex-linked recessive lethal test in Drosophila
melanogaster reported by McGregor et al. (1983), the
results were inconclusive. Given the low absolute number
of mutants (low sample size), the inconsistency with which
various broods were affected, and the lack of a dose-
response relationship, a firm conclusion on the mutagen-
icity of 2-ME in D. melanogaster cannot be made.
8.7 Carcinogenicity
No carcinogenicity data are available on these glycol
ethers.
8.8 Mechanism of Toxicity - Mode of Action
The metabolic fate of 2-ME, 2-EE, and their acetates
has been well studied in both animals (Miller et al., 1983a;
Moss et al., 1985) and man (NIOSH, 1986, Groeseneken et
al., 1987, Veulemans, 1987a). Due to rapid hydrolysis of
the acetates to the monoalkyl glycol ether (see section
6), the putative toxic metabolite are the same for 2-ME or
2-MEA and for 2-EE or 2-EEA.
Both in vitro and in vivo studies have supported the
hypothesis that the toxic effects of 2-ME and 2-EE are
elicited by the toxicity of 2-methoxyacetaldehyde and
methoxyacetic acid (MAA) from 2-ME and ethoxyacetaldehyde
and ethoxyacetic acid (EAA) from 2-EE.
Gray et al. (1985) studied the effects in vitro of
2-ME, 2-EE, MAA, and EAA on mixed cultures of Sertoli and
germ cells from rat testes. At concentrations up to 50
mmol/litre medium, no morphological damage was noted for
2-ME or 2-EE, whereas administration of MAA or EAA at 2-10
mmol/litre led to degeneration of the pachytene and div-
iding spermatocytes, the probable target cells of the
parent ethers in vivo (Chapin et al., 1985b, Creasy et
al., 1985; Foster et al., 1986, Oudiz and Zenick, 1986).
Foster et al. (1986) reported that 2-methoxyacetaldehyde
(2-MALD), a possible metabolite of 2-ME, can produce
specific testicular effects in vitro and in vivo at doses
much lower than those required for MAA (0.2 and 0.5 mmol
per litre in vitro ). In more recent studies, Foster et
al. (1987) compared the in vivo and in vitro testicular
effects of MAA and EAA in rats. Single oral doses of MAA
and EAA (equimolar with 100, 250, or 500 mg 2-ME/kg body
weight) were administered and cell morphology was moni-
tored for 14 days. MAA produced damage to spermatocytes
undergoing meiotic maturation and division within 24 h of
treatment, whereas EAA produced these changes only at the
highest dose. Similar results were seen in vitro at a dose
(5 mmol/litre medium) equivalent to the steady state
plasma level of MAA after administration of 500 mg/kg.
Evidence that MAA is important in causing 2-ME terato-
genicity was reported by Yonemoto et al. (1984). MAA, but
not 2-ME, was found to interfere with organogenesis when
the two compounds were investigated using rat embryos in
culture.
Results from in vivo studies in mice (Welsch et al.,
1987; Sleet et al., 1988) and rats (Ritter et al., 1985)
further substantiate the hypothesis that monoalkyl glycol
ethers are metabolized to acetic acid metabolites via
alcohol dehydrogenase (ADH) and aldehyde dehydrogenases,
and that the ultimate toxin is actually the alkoxy acid
metabolite of the glycol ether or a further metabolite.
In mice (Sleet et al., 1987), 2-ME and MAA were equipotent
in producing teratogenic lesions leading to malformations
of the digits of all paws. Similar effects were noted by
Brown et al. (1984) and Ritter et al. (1985) in rats. Sub-
sequent work showed that the embryotoxic and teratogenic
effects of 2-ME in mice could be prevented by the concomi-
tant administration of 0.12 or 1.2 mmol/kg of 4-methyl-
pyrazole, an inhibitor of ADH, but that 4-methylpyrazole
was without effect after the administration of MAA (Sleet
et al., 1988).
9. EFFECTS ON MAN
Only limited information on the toxic effects of gly-
col ethers in humans is available. This information comes
largely from case reports in the early literature dealing
with accidental poisoning and/or workplace exposure. Such
reports provide only minimal information relating specific
effects to exposure levels. Only a few epidemiological
studies have been reported.
9.1 General Population Exposure
The widespread use of consumer products such as
paints, stains, inks, lacquers, surface coatings, and
home/industrial cleaners that contain one or more glycol
ethers means that broad segments of the general population
could be exposed. However, no reports quantifying such
exposures have been found nor any that describe skin irri-
tation or sensitization from exposures to glycol ethers in
humans, despite widespread exposure.
9.1.1 Poisoning reports
A 44-year-old man consumed 400 ml of 2-ME mixed with
brandy and died without regaining consciousness 5 h after
he was admitted to hospital in a comatose state (Young &
Woolner, 1946). Postmortem findings included acute
haemorrhagic gastritis, fatty degeneration of the liver,
and black coloured kidneys with marked degenerative
changes in the renal tubules.
Two non-fatal cases of poisoning by 2-ME in men aged
41 and 23 were reported by Nitter-Hauge (1970). Both
patients consumed 100 ml 2-ME. In addition to nervous
system disorders (agitation, confusion), the predominant
clinical features were nausea, cyanosis, hyperventilation,
slight tachycardia, and metabolic acidosis. In one patient
there were suggestions of moderate kidney failure. No
evidence of liver damage was found and both patients re-
covered within 4 weeks.
Ingestion of approximately 40 ml 2-EE by a 44-year-old
women led to vertigo, unconsciousness, effects on the
central nervous system, and metabolic acidosis (Fucik,
1969). She recovered within 44 days from all symptoms,
including kidney insufficiency and signs of liver damage,
although neurasthenia was still evident at that time.
9.2 Occupational Exposure
9.2.1 Repeated exposure
Reports on the effects of glycol ethers on humans fol-
lowing repeated exposure are available from occupational
studies. Early reports provided evidence that the repeated
exposure of humans to solvents containing 2-ME resulted in
such effects as anaemia, leucopenia, lethargy, general
weakness, dizziness, ataxia, and unequal or exaggerated
reflexes. A diagnosis of toxic encephalopathy was made by
Donley (1936) and Parson & Parsons (1938), but no reliable
estimation of exposure to 2-ME was given. Greenburg et al.
(1938) reported levels of 2-ME between 78 and 236 mg per
m3 in one manufacturing plant where 19 male subjects
(ages 16-26) were examined. The median duration of ex-
posure was 5 weeks and the maximum was 2 years. Signs of
nervous system dysfunction (fatigue, hand tremor, and
lethargy) were found, but no clear relationship between
the incidence of symptoms and the duration of exposure
could be ascertained in this small population. No control
group was included in this study.
The development of macrocytic anaemia has been de-
scribed in a case report of a worker exposed to 2-ME and
other solvents during microfilm manufacturing. Exposure
to 2-ME averaged 109 mg/m3 (35 ppm) (56-180 mg/m3, 18-
58 ppm), to methyl ethyl ketone 1-5 ppm, and to propylene
glycol monomethyl ether 4-13 ppm for a duration of 20
months. Follow-up analyses conducted 1 month after termin-
ating exposure indicated a return to normal limits for all
haematological parameters studied (Cohen, 1984).
The haematological effects of glycol ethers on humans
have also been documented in workplace surveys. Obvious
symptoms are not always manifested in peripheral blood.
The evaluation in a printing plant of seven workers with
dermal and respiratory exposure to dipropylene glycol
monomethyl ether, ethylene glycol monoethyl ether, and a
range of aliphatic, aromatic, and halogenated hydrocarbons
used for offset and multicolour printing revealed no
alteration in the peripheral blood (Cullen et al., 1983).
Although there was evidence of bone marrow injury in three
of the seven workers, no direct relationship could be
drawn to glycol ether exposure. The exposure to other sol-
vents by this relatively small group of workers makes more
definitive conclusions impossible. Bone marrow toxicity
had earlier been reported in two case studies of workers
exposed dermally to 2-ME (Ohi & Wegmann, 1978).
Denkhaus et al. (1986) investigated the cellular im-
mune response of nine workers aged 25-58 years of age,
heavily exposed to mixtures of organic solvents (including
2-ME and 2-EE) for 8-35 (mean 18.9) years, by the analysis
of subpopulations of peripheral blood lymphocytes. A con-
trol group of matched healthy controls was included. The
mean exposures to 2-ME and 2-EE were 6.1 mg/m3 (peak
150 mg/m3) and 4.8 mg/m3 (peak 53 mg/m3), respect-
ively. Based on samples taken during working shifts, mean
blood levels were 40.1 µg 2-ME/100 ml (peak: 965 µg/100
ml) and 2.0 µg 2-EE/100 ml (peak: 92.7 µg/100 ml). At
these levels (and in the presence of other solvents such
as 1-butanol, isobutanol, 2-butoxybutanol, toluene, m-xylene,
2-butanone, and 2-hexanone), the exposed workers had de-
creased levels of helper T-cells, and increased levels of
natural killer cells and human B-lymphocytes. However,
the levels of suppressor cells were normal. The authors
noted that similar changes in lymphocyte subpopulations
are found in states of general immunodeficiency and in
immunogenetic forms of aplastic anaemia.
9.2.2 Epidemiological studies
In a cross-sectional study, male employees in a plant
manufacturing 2-ME in the USA were examined for haema-
tological effects and possible sterility (Cook et al.,
1982). Personal air sampling indicated levels of exposure
of 1.3 mg/m3 or less in one working location and 19-26 mg
per m3 in another. Forty workers, engaged in production
and distribution of 2-ME (75% of the possible total popu-
lation) and 25 controls (57% of total eligible population)
were examined for haematological effects, and six exposed
and nine control workers participated in an examination of
testis size, semen abnormalities, and levels of serum
follicle-stimulating hormone (FSH). No increase in the
incidence of leucopenia or anaemia was noted, but de-
creases in white cell count and testicular size were noted
in the most highly exposed workers. These results were not
statistically significant but correlate with known effects
in experimental animals.
A cross-sectional evaluation of semen quality (sperm
concentration, pH, volume, viability, motility, velocity,
and morphology) was carried out in 37 workers exposed to
2-EE used as a resin solvent in a metal casting plant
(NIOSH, 1986). The study population represented 50% of
the exposed workforce. A control group of 38 workers from
other locations in the plant was included. Full-shift
breathing zone airborne exposure to 2-EE ranged from non-
detectable to 88 mg/m3. Dermal exposure was indicated by
urinary excretion of EAA at levels from non-detectable to
163 mg/g creatinine. Workers exposed to 2-EE had signifi-
cantly lower average sperm counts than controls (113
versus 154 million per ejaculate), although both exposed
and control groups had lower sperm counts than those found
in other occupational groups. The two groups studied did
not differ significantly with respect to other semen
characteristics or testicular size.
The effect of 2-ME and 2-EE exposure on male repro-
ductive factors and blood has been studied in shipyard
painters by Sparer et al. (1988a,b), Welch et al. (1988),
and Welch & Cullen (1988). Airborne levels (time-weighted
average: TWA) were determined in 102 samples over six
workshifts and were 0-80.5 mg 2-EE/m3 (mean 9.9 and
median level 4.4 mg/m3), and 0-17.7 mg 2-ME/m3 (mean
2.6 and median 1.4 mg/m3). Given the methods used by
Sparer et al. (1988a,b) the authors concluded these were
probably underestimates of exposure, particularly in the
mixing room and inside large tanks. Urinary excretion of
EAA indicated dermal exposure as well as airborne ex-
posure in some workers. In 73 painters, from a total
population of 153, an increased prevalence of oligospermia
and azoospermia was noted and there was an increased odds
ratio for a lower sperm count per ejaculate in exposed
workers as compared to the 40 controls studied (Welch et
al., 1988). In addition, when 94 painters from the same
population were examined for haematological effects of
chemical exposures, 10% were found to be anaemic and 5%
exhibited granulocytopenia (Welch & Cullen, 1988). None of
these effects were noted in the 55 control subjects exam-
ined. The reproductive effects observed were in agreement
with those reported previously by NIOSH (1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure
Many people may be exposed to 2-methoxyethanol (2-ME),
2-ethoxyethanol (2-EE), and their acetates (2-MEA and 2-
EEA), at levels comparable to industrial levels, through
the use of consumer and trade products. On the other
hand, exposure through food, water, or the ambient air is
probably negligible. This inference is based only on the
physical and chemical properties of these compounds and
evidence of rapid environmental degradation.
Significant occupational exposure may occur both
through inhalation and skin absorbtion. Limited measure-
ments of air levels in the workplace range from less than
0.1 mg/m3 to more than 150 mg/m3. However, the avail-
able monitoring is quite limited and large variations may
occur both within and among industries. Because of the
potential for skin absorption, air monitoring alone may
underestimate total exposure. Total uptake may be best
estimated from biological monitoring. Occupations where
extensive exposure is possible include, for example,
painting, printing, and cleaning, but it should be borne
in mind that there are many other occupations where these
compounds are also used and where exposure is of concern.
10.1.2. Health Effects
The major effects of concern for humans are develop-
mental, testicular, and haematological toxicity. These
are demonstrated by extensive and consistent data in ani-
mals and some human data. All these effects can be caused
by both short-term and longer-term exposures. In experi-
mental animals, very high repeated exposure to 2-ME and
2-EE (over 930 and 1450 mg/m3, respectively) produces
neurobehavioural, hepatic, and renal toxic effects. These
are also observed in human poisoning situations.
These four glycol ethers exhibit very similar testicu-
lar and developmental toxicities, in all species evalu-
ated, and by all routes of exposure that have been em-
ployed (inhalation, dermal, and oral). Mechanistic studies
indicate that for both testicular and developmental ef-
fects, metabolism to the alkoxyacetic acid derivative is a
necessary activation step. Metabolism takes place via the
alcohol dehydrogenase system that is common to humans and
laboratory animals. The toxic metabolites, methoxyacetic
acid (MAA) and ethoxyacetic acid (EAA), have been detected
in the urine of humans exposed to these solvents. The con-
sistency of response across laboratory animal species
studied, combined with the similarity of metabolism in
humans, makes it clear that humans should be presumed to
be subject to the testicular and developmental effects of
these glycol ethers. Available data on the excretion of
alkoxyacetic acids by humans indicate prolonged retention
compared with that in laboratory animals, suggesting that
humans may be more sensitive than the most sensitive ex-
perimental animal species. The rapid skin absorbtion of
these compounds is of particular concern. Teratogenicity
and other developmental effects have been observed follow-
ing the application of 2-ME, 2-EE, and 2-EEA to the intact
skin of rats.
Testicular damage has been observed in the rat, mouse,
and rabbit following exposure to these glycol ethers
through both the inhalation and the oral route. Single in-
halation exposures of rats to 1944 mg 2-ME/m3 or more for
4 h and repeated exposure to 933 mg 2-ME/m3 or more for
13 weeks resulted in histological evidence of testicular
damage. The NOEL for acute exposure was 933 mg/m3 and for
repeated exposure was 311 mg/m3. The mouse appeared
somewhat less sensitive, the NOEL for repeated exposure
being 933 mg/m3. However, the rabbit was more sensitive,
with marked testicular change being seen after repeated
exposure to 311 mg/m3 and a marginal effect (one in five
rabbits affected) being seen at 93 mg/m3. Similar
effects have been seen following oral exposure of rats to
2-ME, with short-term (including single dose) exposure to
100 mg/kg producing testicular damage. The NOEL in a sub-
acute (11 days) study was 50 mg/kg. 2-EE is somewhat less
potent with regard to its testicular toxicity than 2-ME;
effects were only seen at dose levels of 500 mg/kg or
more, the NOEL being 250 mg/kg.
Evidence from studies in men exposed occupationally
to 2-ME and 2-EE is consistent with the animal data and
indicates that these glycol ethers can produce testicular
toxicity in humans. Epidemiological studies on small
groups of workers exposed to 2-EE in a metal casting
plant and in shipyard painters exposed to both 2-ME and
2-EE consistently revealed an increased incidence of re-
duced sperm counts. Data on exposure levels were limited,
but there was evidence, in each case, of dermal exposure
as well as exposure via inhalation.
Developmental toxicity has been observed in the rat,
mouse, rabbit, and monkey following exposure to these
glycol ethers using dermal, oral, and inhalation routes.
Twelve daily applications of undiluted 2-ME to the shaved
skin of pregnant rats (with 6-h occlusion) was lethal,
while ten open applications of 2-EE (1.0 ml/day) or 2-EEA
(1.4 ml/day) were teratogenic but not maternally toxic.
Twelve occluded applications of 10% 2-ME in saline proved
developmentally toxic, the NOEL in this study being 3%
2-ME. No-observed-effect levels have not been demonstrated
following repeated oral dosing of pregnant animals with
2-ME. The lowest-observed-effect level (LOEL) for oral
administration of 2-ME was 31.25 mg/kg per day for mice,
25 mg/kg per day for rats, and 0.16 mmol/kg per day for
monkeys. Single-dose studies have been reported only for
2-ME in mice, the results being that on gestation day 11
(the most sensitive day) the NOEL was 100 mg/kg and the
LOEL was 175 mg/kg. Both 2-EE and 2-EEA have been evalu-
ated by inhalation exposure in rats and rabbits. Rats were
exposed to 2-EE in two studies, resulting in teratogenic
effects (743 mg/m3 for 7 h/day on gestation days 1-19)
or fetotoxic effects (184 and 920 mg/m3 for 6 h/day on
gestation days 6-15). In the latter study, the NOEL was
37 mg/m3. Rabbits exposed to 2-EE also exhibited
teratogenic effects (589 mg/m3 for 7 h/day on gestation
days 1-18) or fetotoxic effects (644 mg/m3 for 6 h/day
on gestation days 6-18). In the latter study, the NOEL was
184 mg/m3. When rabbits were exposed to 2-EEA for
6 h/day on gestation days 6-18, teratogenic effects were
seen at 2160 mg/m3 in one study and 1620 mg/m3 in
another. Fetotoxicity appeared in both studies at 540 mg
per m3 and the lowest exposure level in each study (135
and 270 mg/m3, respectively) was the NOEL. Rats exposed
to 2-EEA by inhalation for 6 h/day on gestation days 6-15
showed the same pattern of response: teratogenic effects
at 1620 mg/m3, fetotoxicity at 1080 mg/m3, and no ef-
fect at 270 mg/m3.
Thus developmental toxicity has been observed in all
species (mice, rats, and rabbits) exposed to 2-ME at 156
mg/m3 or more. The NOEL for all three species was 31 mg
per m3. Behavioural and neurochemical alterations in
rats followed in utero exposure at 78 mg/m3, with no
NOEL being identified.
2-EE and 2-EEA were slightly less potent. Develop-
mental effects in rats and rabbits followed all 2-EE ex-
posures at 368 mg/m3 or more. Slight developmental ef-
fects were seen in rats exposed at 184 mg 2-EE/m3, but
37 mg/m3 was a clear NOEL. For 2-EEA, the NOEL was 170 mg
per m3 for both rats and rabbits.
Haematological effects from single acute dose ex-
posures have been observed in animals and in human poison-
ings. Repeated inhalation exposure of the most sensitive
species, the rabbit, to 2-ME for 13 weeks, 5 times per
week, yielded a NOEL of 93 mg/m3. Repeat doses of 2-ME
also cause haematological toxicity in mice, rabbits, dogs,
hamsters, and guinea-pigs. 2-EE is less potent in causing
haematological effects than 2-ME. The NOEL for haemato-
logical effects in rats and rabbits exposed to 2-EE for 13
weeks, 5 times per week for 6 h/day, is 368 mg/m3. Dogs
and mice also show haematological effects from repeated
2-EE exposure at higher levels. Exposure to the acetate
esters of 2-EE and 2-ME would be expected to cause similar
effects at similar exposure levels, but there are too few
data on exposure and haematological effects of these com-
pounds to determine under what conditions single human ex-
posures will lead to haematological effects.
Industrial exposure levels have been reported at or
near the NOEL for haematological effects in animals after
repeated doses of both 2-ME and 2-EE. This fact, together
with the probable greater sensitivity of humans and the
expected accumulation of metabolites in human blood, indi-
cates that haematological effects may well occur from in-
dustrial and consumer exposure. This has been confirmed by
the haematological effects reported in some of the limited
number of studies of industrial workers with repeated 2-EE
and/or 2-ME exposure.
10.2. Evaluation of Effects on the Environment
Environmental exposure to these glycol ethers can
arise as a consequence of their direct release into the
atmosphere from their use as evaporative solvents. Dis-
charges to the land and water from accidental release may
also result in environmental exposure. Accumulation in
soil and surface water could only occur in the absence of
degradation. However, these glycol ethers are rapidly
degraded by chemical and biological processes and accumu-
lation is not expected. 2-MEA and 2-EEA are also expected
to hydrolyse readily and subsequently biodegrade under
aerobic conditions. However, contamination of anaerobic
soils and aquifers remains a potential problem, but this
condition is expected to be transitory resulting in negli-
gible risk.
Both 2-ME and 2-EE demonstrate low toxicity to micro-
oganisms and aquatic species. The glycol ether acetates,
however, are far more acutely toxic. No data exists to
ascertain the potential for adverse effects from long-term
exposure to environmental species.
11. RECOMMENDATIONS
11.1. Health Protection
1. Alternative less toxic solvents should be identified
to replace 2-methoxyethanol, 2-ethoxyethanol, and their
esters, particularly in consumer products. Assessment of
the effects of other ethylene glycol ethers is also of
particular importance, because some may cause effects
similar to the four glycol ethers evaluated here.
2. In view of the known toxic effects of these glycol
ethers, authorities should seriously consider appropriate
strategies to alert users of these chemicals to their
hazards, particularly those arising from dermal ex-
posures.
3. In view of recent toxicological data and the potential
for considerable dermal absorption of these glycol ethers,
national occupational exposure limits should be recon-
sidered to insure that the total daily dose to workers by
all routes of administration does not pose an undue risk
to health.
4. Single dose effects occur in animals at fairly high
exposure levels. Prudent use of these compounds (attention
to personal hygiene, suitable protective devices, and
adequate ventilation) is recommended to reduce the health
risks. The data indicate that more extensive protection
may be required to prevent developmental effects, as well
as effects on blood and testis, from repeated exposure.
11.2. Further Research
1. In view of the implication of methoxyacetic acid (MAA)
and ethoxyacetic acid (EAA) (the principal identified
metabolites of 2-ME, 2-EE, and of their esters) in the
toxicity to the male reproductive system, their mechanism
of action should be investigated. If it transpires that
MAA and EAA are not the primary agents concerned, these
should be identified and their mechanism of action
elucidated.
2. These four glycol ethers are known to have both
haematological effects and male reproductive effects
(sperm count reduction). The available data, although
limited, appear to suggest that the two effects become
evident at similar dose levels. The mechanism of action
should be investigated for both organ systems, and
haematological effects and sperm counts should be examined
in parallel to determine if haematological changes offer
warning signs for other effects of these compounds.
3. Air monitoring alone is not sufficient to assure low
exposure. Biological monitoring can aid in detecting
failures in protective measures. At present the
relationship between biological indicators of exposure,
total body uptake, and observed health effects have not
been sufficiently established. Further work is necessary
to provide the basis for using biological monitoring in
determining safe exposures.
4. Epidemiological studies and/or targeted health sur-
veillance in populations subject to high exposure to these
glycol ethers should be designed in order to estimate
exposure-effect relationships for the purpose of determin-
ing safe exposures, providing that overall exposure can be
properly and adequately evaluated by appropriate environ-
mental and biological monitoring.
5. The possibility that these compounds may cause effects
on the female gonads should be investigated through
multigeneration reproductive studies in animals.
6. Available research indicates that humans may metab-
olize these glycol ethers to the corresponding alkoxy-
acetic acids to a greater extent than rats, and that the
half-life for urinary excretion of these toxic metabolites
is about four times longer in humans than in rats.
Furthermore, rats conjugate a large portion of the acid
metabolites, whereas humans do not. These differences
might contribute to the relatively higher sensitivity of
humans to these glycol ethers. Detailed knowledge of the
metabolism and excretion kinetics would improve the
ability to predict safe exposure levels.
7. The results obtained from short-term (13-week) studies
indicate effects on various organ systems. However, there
have been no studies of sufficient duration that would
allow the reversibility of such effects to be assessed.
Therefore, it is suggested that stop-studies be undertaken
in which experimental animals are exposed to these glycol
ethers for at least 13 weeks, followed by a suitable
recovery period. Important physiological parameters
should then be evaluated in order to determine whether or
not these effects are transient.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Regulatory standards for 2-ME, 2-MEA, and 2-EE, estab-
lished by national bodies in different countries and the
European Economic Community, are summarized in the data
profile of the International Register of Potentially Toxic
Chemicals (IRPTC, 1987).
REFERENCES
ANDERSON, D., BRINKWORTH, M.H., JENKINSON, P.C., CLODE, S.A., CREASY,
D.M., & GANGOLLI, S.D. (1987) Effect of ethylene glycol monomethyl ether
on spermatogenesis, dominant lethality, and F1 abnormalities in the rat
and the mouse after treatment of F0 males. Teratog. Carcinog. Mutagen.,
7:141-158.
ANDREW, F.D. & HARDIN, B.D. (1984) Developmental effects after inhalation
exposure of gravid rabbits and rats to ethylene glycol monoethyl ether.
Environ. Health Perspect., 57: 13-23.
BAILEY, H.C., LIU, D.H.W., & JAVITZ, H.A. (1985) Time/toxicity
relationships in short-term static, dynamic, and plug-flow bioassays. In:
Bahner, R.C. & Hansen, D.J., ed. Aquatic toxicology and hazard assessment:
Eighth Symposium, Philadelphia, American Society for Testing and
Materials, pp. 193-212 (ASTM STP 891).
BARBEE, S.V., TERRILL, J.B., DESOUSA, D.J., & CONAWAY, C.C. (1984)
Subchronic inhalation toxicology of ethylene glycol monoethyl ether in the
rat and rabbit. Environ. Health Perspect., 57: 157-163.
BASLER, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by the
micronucleus test. Mutat. Res., 174: 11-13.
BOTTA, D., CASTELLANI PIRRI, L., & MANTICA, E. (1984) Ground water
pollution by organic solvents and their microbial degradation products,
Luxembourg, Commission of the European Communities, pp. 261-275 (EUR-
8518).
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) Aeruginosa and
Scenedesmus quadricauda. Mitt. intern. Ver. theor. angew. Limnol., 21:
275-284.
BROWN, N.A., HOLT, D., & WEBB, M. (1984) The teratogenicity of
methoxyacetic acid in the rat. Toxicol. Lett., 22: 93-100.
CARPENTER, C.P., POZZANI, V.C., WEIL, C.S., NAIR, J.H., KECK, G.A., &
SMITH, H.F. (1956) The toxicity of butyl cellosolve solvent. Arch. ind.
Health, 14: 114-131.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., & LAMB, J.C. (1985a) Effects of
ethylene glycol monomethyl ether (EGME) on mating performance and
epididymal sperm parameters in F344 rats. Fundam. appl. Toxicol., 5: 182-
189.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., SWAISGOOD, R.R., & LAMB, J.S.
(1985b) The recovery of the testis over 8 weeks after short-term dosing
with ethylene glycol monomethyl ether: histology, cell specific enzymes,
and rete testis fluid protein. Fundam. appl. Toxicol., 5: 515-525.
CHEEVER, K.L., PLOTNICK, H.B., RICHARDS, D.E., & WEIGEL, W.W. (1984)
Metabolism and excretion of 2-ethoxyethanol in the adult male rat.
Environ. Health Perspect., 57: 241-248.
COHEN, R. (1984) Reversible subacute ethylene glycol monomethyl ether
toxicity associated with microfilm production: a case report. Am. J. ind.
Med., 6: 441-446.
COOK, R.R., BODNER, K.M., KOLESAR, R.C., VAN PEENEN, P.F.D., DICKSON,
G.S., & FLANAGAN, K. (1982) A cross-sectional study of ethylene glycol
monomethyl ether process employees. Arch. environ. Health, 37: 346-351.
CREASY, D.M. & FOSTER, P.M.D. (1984) The morphological development of
glycol ether-induced testicular atrophy in the rat. Exp. mol. Pathol., 40:
169-176.
CREASY, D.M., FLYNN, J.C., GRAY, T.J.B., & BUTLER, W.H. (1985) A
quantitative study of stage-specific spermatocyte damage following
administration of ethylene glycol monomethyl ether in the rat. Exp. mol.
Pathol., 43: 321-336.
CREASY, D.M., JONES, H.B., BEECH, L.M., & GRAY, T.J.B. (1986) The effects
of two testicular toxins on the ultrastructural morphology of mixed
cultures of Sertoli and germ cells: a comparison with in vivo effects.
Food chem. Toxicol., 24: 655-656.
CULLEN, M.R., RADO, T., WALDRON, J.A., SPARER, J., & WELCH, L.S. (1983)
Bone marrow injury in lithographers exposed to glycol ethers and organic
solvents used in multicolor offset and ultraviolet curing printing
processes. Arch. environ. Health, 38(6): 347-354.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J.
hazard. Mater., 1: 303-318.
DE DELBARRE, F., KAHAN, A., DE GERY, A., & KONRAD, K. (1980) Action
immunomodulatrice du méthoxy-2 éthanol et de dérivés homologues chez le
rat. C.R. Acad. Sci. Paris, 291: 215-218.
DENKHAUS, W., STELDERN, D., BOTZENHARDT, U., & KONIETZKO, H. (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int. Arch. occup.
environ. Health, 57: 109-115.
DOE, J.E. (1984a) Ethylene glycol monoethyl ether and ethylene glycol
monoethyl ether acetate teratology studies. Environ. Health Perspect., 57:
33-41.
DOE, J.E. (1984b) Further studies on the toxicology of the glycol ethers
with emphasis on rapid screening and hazard assessment. Environ. Health
Perspect., 57: 199-206.
DOE, J.E., SAMUELS, D.M., TINSTON, D.J., & WICKRAMARATNE, G.A.D. (1983)
Comparative aspects of the reproductive toxicology by inhalation in rats
of ethylene glycol monomethyl ether and propylene glycol monomethyl ether.
Toxicol. appl. Pharmacol., 69: 43-47.
DONLEY, D.E. (1936) Toxic encephalopathy and volatile solvents in
industry. J. ind. Hyg. Toxicol., 18: 571-577.
DUGARD, P.H., WALKER, M., MAWDSLEY, S.J., & SCOTT, R.C. (1984) Absorption
of some glycol ethers through human skin in vitro . Env. Health Perspect.,
57: 193-197.
ECETOC (1985) The toxicology of glycol ethers and its relevance to man,
Brussels, European Chemical Industry Ecology and Toxicology Centre
(Technical Report No. 17).
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., THOMAS, L.V., COOK, M.W., &
GANGOLLI, S.D. (1983) Testicular toxicity of ethylene glycol monomethyl
and monoethyl ethers in the rat. Toxicol. appl. Pharmacol., 69: 385-399.
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., & GRAY, T.J.B. (1984)
Testicular toxicity produced by ethylene glycol monomethyl and monoethyl
ethers in the rat. Environ. Health Perspect., 57: 207.
FOSTER, P.M.D., BLACKBURN, D.M., MOORE, R.B., & LLOYD, S.C. (1986)
Testicular toxicity of 2-methoxyacetaldehyde, a possible metabolite of
ethylene glycol monomethyl ether, in the rat. Toxicol. Lett., 32: 73-80.
FOSTER, P.M.D., LLOYD, S.C., & BLACKBURN, D.M. (1987) Comparison of the in
vivo and in vitro testicular effects produced by methoxy-, ethoxy-, and n-
butoxy acetic acids in the rat. Toxicology, 43: 17-30.
FUCIK, J. (1969) Poisoning by ethylene glycol monoethyl ether. Prac. Lek.,
21: 116-118.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO, J.,
MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese hamster
ovary cells: evaluations of 108 chemicals. Environ. mol. Mutagen., 10(10):
1-175.
GOLDBERG, M.E., HARN, C., & SMYTH, H.F. (1962) Toxicological implication
of altered behaviour induced by an industrial vapour. Toxicol. appl.
Pharmacol., 4: 148-164.
GRANT, D., SULSH, S., JONES, H.B., GANGOLLI, S.D., & BUTLER, W.H. (1985)
Acute toxicity and recovery in the hemopoietic system of rats after
treatment with ethylene glycol monomethyl and monobutyl ethers. Toxicol.
appl. Pharmacol., 77: 187-200.
GRAY, T.J.B., MOSS, E.J., CREASY, D.M., & GANGOLLI, S.D. (1985) Studies on
the toxicity of some glycol ethers and alkoxyacetic acids in primary
testicular cell cultures. Toxicol. appl. Pharmacol., 79: 490-501.
GREENBURG, L., MAYERS, M.R., GOLDWATER, L.J., BURKE, W.J., & MOSCOWITZ, S.
(1938) Health hazards in the manufacture of "fused collars". 1. Exposure
to ethylene glycol monomethyl ether. J. ind. Hyg. Toxicol., 20: 134-147.
GREENE, J.A., SLEET, R.B., MORGAN, K.T., & WELSCH, F. (1987) Cytotoxic
effects of ethylene glycol monomethyl ether in the forelimb bud of the
mouse embryo. Teratology, 36: 23-34.
GROESENEKEN, D., VAN VLEM, E., VEULEMANS, H., & MASSCHELEIN, R. (1986a)
Gas chromatographic determination of methoxyacetic and ethoxyacetic acid
in urine. Br. J. ind. Med., 43: 62-65.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986b) Respiratory
uptake and elimination of ethylene glycol monoethyl ether after
experimental human exposure. Br. J. ind. Med., 43: 544-549.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986c) Urinary
excretion of ethoxyacetic acid after experimental human exposure to
ethylene glycol monoethyl ether. Br. J. ind. Med., 43: 615-619.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1987)
Ethoxyacetic acid: a metabolite of ethylene glycol monoethyl ether acetate
in man. Br. J. ind. Med., 44: 488-493.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1988)
Comparative urinary excretion of ethoxyacetic acid in man and rat after
single low doses of ethylene glycol monoethyl ether. Toxicol. Lett., 41:
57-68.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989a)
Experimental human exposure to ethylene glycol monomethyl ether. Int.
Arch. occup. environ. Health, 61: 243-247.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989b) An
improved method for the determination in urine of alkoxyacetic acids. Int.
Arch. occup. environ. Health, 61: 249-254.
GUEST, D., HAMILTON, M.L., DEISINGER, P.J., & DIVINCENZO, G.D. (1984)
Pulmonary and percutaneous absorption of 2-propoxyethyl acetate and 2-
ethoxyethyl acetate in beagle dogs. Environ. Health Perspect., 57: 177-
183.
HAMLIN, J.W., HUDSON, B., SHEEN, A.D., & SAUNDERS, K.J. (1982) The
measurement of glycol ether levels in the workplace. Polym. Paint Colour
J., October 13: 61-63.
HANLEY, T.R., Jr, YANO, B.L., NITSCHKE, K.D., & JOHN, J.A. (1984)
Comparison of the teratogenic potential of inhaled ethylene glycol
monomethyl ether in rats, mice, and rabbits. Toxicol. appl. Pharmacol.,
75: 409-422.
HARDIN, B.D. (1983) Reproductive toxicity of the glycol ethers.
Toxicology, 27: 91-102.
HARDIN, B.D., NIEMEIER, R.W., SMITH, R.J., KUCZUK, M.H., MATHINOS, P.R., &
WEAVER, T.F. (1982) Teratogenicity of 2-ethoxyethanol by dermal
application. Drug chem. Toxicol., 5(3): 277-294.
HARDIN, B.D., GOAD, P.T., & BURG, J.R. (1984) Developmental toxicity of
four glycol ethers applied cutaneously to rats. Environ. Health Perspect.,
57: 69-74.
HEALTH AND SAFETY EXECUTIVE (1988) Methods for the determination of
hazardous substances: Glycol ethers and glycol acetate vapours in air,
London, UK Health and Safety Executive, pp. 1-7 (MDHS-23).
HERMENS, J., CANTON, H., JANSSEN, P., & DEJONG, R. (1984) Quantitative
structure-activity relationships and toxicity of mixtures of chemicals
with anaesthetic potency: Acute lethal and sublethal toxicity to Daphnia
magna. Aquat. Toxicol., 5: 143-154.
HORTON, V.L., SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1985)
Developmental phase-specific and dose-related teratogenic effects of
ethylene glycol monomethyl ether in CD-1 mice. Toxicol. appl. Pharmacol.,
80: 108-118.
HOUSE, R.V., LAUER, L.D., MURRAY, M.J., WARD, E.C., & DEAN, J.H. (1985)
Immunological studies in B6C3F1 mice following exposure to ethylene glycol
monomethyl ether and its principal metabolite methoxyacetic acid. Toxicol.
appl. Pharmacol., 77: 358-362.
HURTT, M.E. & ZENICK, H. (1986) Decreasing epididymal sperm reserves
enhances the detection of ethoxyethanol-induced spermatotoxicity. Fundam.
appl. Toxicol., 7: 348-353.
IRPTC (1987) IRPTC legal file 1986 - Volume 1, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
JUHNKE, I. & LUDEMANN, D. (1978) The results obtained with the Golden Orfe
test during the examination of 200 chemical compounds for acute fish
toxicity. Wasser Abwasser Forsch., 11: 161-164.
KAREL, L., LANDING, B.H., & HARVEY, T.S. (1947) The intraperitoneal
toxicity of some glycols, glycol ethers, glycol esters and phthalates in
mice. J. Pharmacol. exp. Ther., 90: 338-347.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 9 - Ethanol,
3rd ed., New York, Chichester, Brisbane, Toronto, John Wiley & Sons.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 11 - Glycols
(Ethylene and Propylene), 3rd ed., New York, Chichester, Brisbane,
Toronto, John Wiley & Sons.
LAILLER, J., PLAZONNET, B., LE DOUAREC, J.C., & GONIN, M.J. (1975)
Evaluation of ocular irriation in the rabbit: Development of an objective
method of studying eye irritation. Proc. Eur. Soc. Toxicol., 17: 336-350.
LAMB, J.C., DUSHYANT, K.G., RUSSELL, V.S., HAMMEL, L., & SABHARNAL, P.S.
(1984) Reproductive toxicity of ethylene glycol monoethyl ether tested by
continuous breeding of CD-1 mice. Environ. Health Perspect., 57: 85-90.
LAUG, E.P., CALVERY, H.O., MORRIS, H.J., & WOODARD, G. (1939) The
toxicology of some glycols and derivatives. J. ind. Hyg. Toxicol., 21:
173-201.
LEE, K.H. & WONG, H.A. (1979) Toxic effects of some alcohol and ethylene
glycol derivatives on Cladosporium resinae. Appl. environ. Microbiol., 38:
24-28.
MCGREGOR, D.B., WILLINS, M.J., MCDONALD, P., HOLMSTROM, M., MCDONALD, D.,
& NIEMEIER, R.W. (1983) Genetic effects of 2-methoxyethanol and bis(2-
methoxyethyl)ether. Toxicol. appl. Pharmacol., 70: 303-316.
MELLAN, I. (1977) Glycol ethers and esters. In: Industrial Solvents
Handbook, 2nd ed., Park Ridge, New Jersey, Noyes Data Corporation, pp.
346-399, 513-551.
MILLER, R.R., AYERS, J.A., CALHOUN, L.L., YOUNG, J.T., & MCKENNA, M.J.
(1981) Comparative short-term inhalation toxicity of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in rats and mice.
Toxicol. appl. Pharmacol., 61: 368-377.
MILLER, R.R., CARREON, R.E., YOUNG, J.T., & MCKENNA, M.J. (1982) Toxicity
of methoxyacetate acid in rats. Fundam. appl. Toxicol., 2: 155-160.
MILLER, R.R., HERMANN, E.A., LANGVARDT, P.W., MCKENNA, M.J., & SCHWETZ,
B.A. (1983a) Comparative metabolism and disposition of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in male rats.
Toxicol. appl. Pharmacol., 67: 229-237.
MILLER, R.R., AYRES, J.A., YOUNG, J.T., & MCKENNA, M.J. (1983b) Ethylene
glycol monomethyl ether. I. Subchronic vapor inhalation study with rats
and rabbits. Fundam. appl. Toxicol., 3: 49-54.
MOSS, E.J., THOMAS, L.V., COOK, M.W., WALTERS, D.C., FOSTER, P.M.D.,
CREASY, D.M., & GRAY, T.J.B. (1985) The role of metabolism in 2-
methoxyethanol-induced testicular toxicity. Toxicol. appl. Pharmacol., 79:
480-489.
NAGANO, K., NAKAYAMA, E., KOGANA, M., DOBAYASKI, H., ADACHI, H., & YAMADA,
T. (1979) Testicular atrophy of mice induced by ethylene glycol monoalkyl
ethers. Jpn. J. ind. Health, 21: 29-35.
NAGANO, K., NAKAYAMA, E., DOBAYASKI, H., YAMADA, T., ADACHI, H.,
NISHIZAWA, T., OZAWA, H., NAKAICHI, M., OKUKDA, H., MINAMI, K., &
YAMAZAKI, K. (1981) Embryotoxic effects of ethylene glycol monomethyl
ether in mice. Toxicology, 20: 335-343.
NAGANO, K., NAKAYAMA, E., OOBAYASHI, H., NISHIZAWA, T., OKUDA, H., &
YAMAZAKI, K. (1984) Experimental studies on toxicity of ethylene glycol
alkyl ethers in Japan. Environ. Health Perspect., 57: 75-84.
NAKAAKI, K., FUKABORI, S., & TADA, O. (1980) An experimental study on
percutaneous absorption of some organic solvents. J. Sci. Labour, 56(12):
1-9.
NEIHOF, R.A. & BAILEY, C.A. (1978) Biocidal properties of anti-icing
additives for aircraft fuels. Appl. environ. Microbiol., 35: 698-703.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., TAYLOR, B.J., & HORNUNG,
R.W. (1981) Ethoxyethanol behavioral teratology in rats. Neuro-
toxicology., 2(2): 231-249.
NELSON, B.K., BRIGHTWELL, W.S., & SETZER, J.V. (1982) Prenatal
interactions between ethanol and the industrial solvent 2-ethoxyethanol in
rats: maternal and behavioral teratogenic effects. Neurobehav. Toxicol.
Teratol., 4: 387-394.
NELSON, B.K., BRIGHTWELL, W.S., BURG, J.R., & MASSARI, V.J. (1984a)
Behavioral and neurochemical alterations in the offspring of rats after
maternal or paternal inhalation exposure to the industrial solvent 2-
methoxyethanol. Pharmacol. Biochem. Behav., 20: 269-279.
NELSON, B.K., SETZER, J.V., BRIGHTWELL, W.S., MATHINOS, P.R., KUCZUR,
M.H., WEAVER, T.E., & GOAD, P.T. (1984b) Comparative inhalation
teratogenicity of four glycol ether solvents and amino derivative in rats.
Environ. Health Perspect., 57: 261-271.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., & O'DONOHUE, T.L. (1984c)
Reproductive toxicity of the industrial solvent 2-ethoxyethanol in rats
and interactive effects of ethanol. Environ. Health Perspect., 57: 255-
259.
NIOSH (1986) Health hazard evaluation report: Precision Castparts
Corporation, Portland, Oregon, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (Report No. HETA-84-415-1688).
NIOSH (1987a) Alcohols IV. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1403.
NIOSH (1987b) Esters I. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1450.
NITTER-HAUGE, S. (1970) Poisoning with ethylene glycol monomethyl ether:
report of two cases. Acta med. Scand., 188: 277-280.
OHI, G. & WEGMANN, D.H. (1978) Transcutaneous ethylene glycol monomethyl
ether poisoning in the work setting. J. occup. Med., 20: 675-676.
OUDIZ, D. & ZENICK, H. (1986) In vivo and in vitro evaluations of
spermatotoxicity induced by 2-ethoxyethanol treatment. Toxicol. appl.
Pharmacol., 84: 576-583.
PARSONS, C.E. & PARSONS, M.E.M. (1938) Toxic encephalopathy and
"granulopenic anaemia" due to volatile solvents in industry: report of
two cases. J. ind. Hyg. Toxicol., 20: 124-135.
PAUSTENBACH, D.J. (1988) Assessment of the developmental risks resulting
from occupational exposure to selected glycol ethers within the
semiconductor industry. J. Toxicol. environ. Health, 23: 29-75.
PISKO, G.T. & VERBILOV, A.A. (1988) Toxicity of monomethyl, monoethyl and
monobutyl ethers of ethylene glycol. Gig. Tr. prof. Zabol., 3: 48-49.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J. Water Pollut. Control Fed., 46: 63-77.
RAO, K.S., COBEL-GEARD, S.R., YOUNG, J.T., HANLEY, T.R., Jr, HAYES, W.C.,
JOHN, J.A., & MILLER, R.R. (1983) Ethylene glycol monomethyl ether II.
Reproductive and dominant lethal studies in rats. Fundam. appl. Toxicol.,
3: 80-85.
RITTER, E.J., SCOTT, W.J., RANDALL, J.L., & RITTER, J.M. (1985)
Teratogenicity of dimethoxyethyl phthalate and its metabolites
methoxyethanol and methoxyacetic acid in the rat. Teratology, 32: 25-31.
ROMER, K.G., BALGE, F., & FREUNDT, K.J. (1985) Ethanol-induced
accumulation of ethylene glycol monoalkyl ethers in rats. Drug chem.
Toxicol., 8(4): 255-264.
ROWE, V.K. & WOLF, M.A. (1982) Derivatives of glycols. In: Clayton, G.D. &
Clayton, F.E., ed. Patty's industrial hygiene toxicology, Vol. 2, pp.
3909-4052.
SAPARMAMEDOV, E. (1974) [Toxicity of some simple ethylene glycol ethers
(single experiments).] Zdravookhr Turkm., 18(9): 26-31 (in Russian)
(English translation from US NIOSH.).
SAVOLAINEN, H. (1980) Glial cell toxicity of ethyleneglycol monomethyl
ether vapour. Environ. Res., 22: 423-430.
SCOTT, W.J., FRADKIN, R., NAU, H., & WITTFOHT, W. (1987) Teratologic
potential of 2-methoxyethanol (2-ME) in non-human primates. Teratology,
35(2): 66 (abstract).
SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1986) Localization of
radioactivity from 2-methoxy[1,2-14C]ethanol in maternal and conceptus
compartments of CD-1 mice. Toxicol. appl. Pharmacol., 84: 25-35.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1987) The teratogenicity and
disposition of the glycol ether 2-methoxyethanol and their relationship in
CD-1 mice. In: Welsch, F., ed. Approaches to elucidate mechanisms in
teratogenesis, New York, Hemisphere Publishing Co., pp. 33-57.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1988) The relationship of
embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicol. appl.
Pharmacol., 93: 195-207.
SMALLWOOD, A.W., DEBORD, K.E., & LOWRY, L.K. (1984) Analyses of ethylene
glycol monoalkyl ethers and their proposed metabolites in blood and urine.
Environ. Health Perspect., 57: 249-253.
SMALLWOOD, A.W., DEBORD, K., BURG, J., MOSELEY, C., & LOWRY, L. (1988)
Determination of urinary 2-ethoxyacetic acid as an indicator of
occupational exposure to 2-ethoxyethanol. Appl. ind. Hyg., 3(2): 47-50.
SMYTH, H.F., SEATON, J., & FISCHER, L. (1941) The single dose toxicity of
some glycols and derivatives. J. ind. Hyg. Toxicol., 23: 259-268.
SPARER, J., WELCH, L.S., MCMANUS, K., & CULLEN, M.R. (1988a) Effects of
exposure to glycol ethers in shipyard painters. I. Evaluation of exposure.
Am. J. ind. Med., 14: 497-507.
SPARER, J., WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R.
(1988b) Effects of exposure to glycol ethers in shipyard painters. II.
Male reproduction. Am. J. ind. Med., 14: 509-526.
STENGER, E.G., AEPPLI, L., MULLER, D., PEHEIM, E., & THOMANN, P. (1971)
Toxicology of ethylene glycol monoethyl ether. Arzneim Forsch., 21: 880-
885.
STOTT, W.T. & MCKENNA, M.J. (1985) Hydrolysis of several glycol ether
acetates and acrylate esters by nasal mucosal carboxylesterase in vitro .
Fundam. appl. Toxicol., 5: 399-404.
SZYBALSKI, W. (1958) Special microbiological systems II. Observations on
chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76: 475-488.
TANAKA, K., MIKAMI, E., & SUZUKI, T. (1986) Methane fermentation of 2-
methoxyethanol by mesophilic digesting sludge. J. Ferment. Technol.,
64(4): 305-309.
TORAASON, M., STRINGER, B., STOBER, P., & HARDIN, B.D. (1985)
Electrocardiographic study of rat fetuses exposed to ethylene glycol
monomethyl ether (EGME). Teratology, 32: 33-39.
TORAASON, M., BREITENSTEIN, M.J., & SMITH, R.J. (1986a) Ethylene glycol
monomethyl ether (EGME) inhibits rat embryo ornithine decarboxylase (ODC)
activity. Drug chem. Toxicol., 9: 191-203.
TORAASON, M., STRINGER, B., & SMITH, R. (1986b) Ornithine decarboxylase
activity in the neonatal rat heart following prenatal exposure to ethylene
glycol monomethyl ether. Drug chem. Toxicol., 9(1): 1-14.
TYL, R.W., PRITTS, I.M., FRANCE, K.A., FISHER, L.C., & TYLER, T.R. (1988)
Developmental toxicity evaluation of inhaled 2-ethoxyethanol acetate in
Fischer 344 rats and New Zealand white rabbits. Fundam. appl. Toxicol.,
10: 20-39.
US EPA (1987) Environmental health criteria: 2-methoxyethanol, 2-
ethoxyethanol, and their acetates, Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances.
VERSCHUEREN, K. (1977) Ethylene glycol monomethyl ether. In: Handbook of
experimental data on organic chemicals, New York, Van Nostrand-Reinhold
Company, 327 pp.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987a)
Field study of the urinary excretion of ethoxyacetic acid during repeated
daily exposure to the ethyl ether of ethylene glycol and the ethyl ether
of ethylene glycol acetate. Scand. J. Work Environ. Health, 13: 239-242.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987b)
Survey of ethylene glycol ether exposures in Belgian industries and
workshops. Am. Ind. Hyg. Assoc. J., 48(8): 671-676.
WEIL, C.S. & SCALA, R.A. (1971) Study of intra- and inter-laboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol. appl. Pharmacol., 19: 276-360.
WELCH, L.S. & CULLEN, M.R. (1988) Effects of exposure to glycol ethers in
shipyard painters. III. Hematologic effects. Am. J. ind. Med., 14: 527-
536.
WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R. (1988) Effects
of exposure to ethylene glycol ethers on shipyard painters: II. Male
reproduction. Am. J. ind. Med., 14: 509-526.
WELSCH, R., SLEET, R.B., & GREENE, J.A. (1987) Attenuation of 2-
methoxyethanol and methoxyacetic acid-induced digit malformations in mice
by simple physiological compounds: implications for the role of further
metabolism of methoxyacetic acid in developmental toxicity. J. biochem.
Toxicol., 2: 225-240.
WERNER, H.W., MITCHELL, J.L., MILLER, J.W., & VON OETTINGEN, W.F. (1943)
Effects of repeated exposure of dogs to monoalkyl ethylene glycol ether
vapors. J. ind. Hyg. Toxicol., 25(9): 409-414.
WICKRAMARATNE, G.A., de S. (1986) The teratogenic potential and dose-
response of dermally administered ethylene glycol monomethyl ether (EGME)
estimated in rats with the Chernoff-Kavlock assay. J. appl. Toxicol.,
6(3): 165-166.
YONEMOTO, J., BROWN, N.A., & WEBB, M. (1984) Effects of dimethoxyethyl
phthalate, monomethoxyacetic acid on post implantation rat embryos in
culture. Toxicol. Lett., 21: 97-102.
YOUNG, E.G. & WOOLNER, L.B. (1946) A case of fatal poisoning from 2-
methoxyethanol. J. ind. Hyg. Toxicol., 28: 267-268.
ZIMMERMANN, F.K., MAYER, V.W., SCHEEL, I., & RESNICK, M.A. (1985) Acetone,
methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae.
Mutat. Res., 149: 339-351.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
La présente monographie ne traite que des éthers
méthyliques et éthyliques de l'éthylène-glycol, c'est-à-
dire le méthoxy-2 éthanol (2-ME), l'éthoxy-2 éthanol
(2-EE) et leurs esters acétiques respectifs, à savoir
l'acétate de méthoxy-2 éthyle (2-MEA) et l'acétate
d'éthoxy-2 éthyle (2-EEA). Ces composés se présentent tous
les quatre sous la forme de liquides stables incolores et
inflammables, dotés d'une légère odeur éthérée; ils sont
tous miscibles à l'eau (ou tout au moins dans le cas du
2-EAA très soluble dans celle-ci) et miscibles à un grand
nombre de solvants organiques.
Il existe des méthodes d'analyse permettant la mise en
évidence de ces éthers du glycol et de leurs métabolites
dans divers milieux (air, eau, sang et urine). Ces
méthodes font souvent appel à des techniques d'adsorption
ou d'extraction afin de concentrer l'échantillon, suivies
d'une analyse par chromatographie en phase gazeuse. La
chromatographie en phase gazeuse ou la chromatographie
liquide à haute performance permettent de doser l'acide
méthoxy-2 acétique (MAA) ainsi que l'acide éthoxy-2
acétique (EAA) (qui sont des métabolites du du 2-ME et du
2-EE) dans les urines, généralement après obtention de
dérivés convenables, à des concentrations entre 5 et
100 µg/ml.
2. Sources d'exposition humaine et environnementale
Les quatre éthers du glycol étudiés s'obtiennent tous
par réaction de l'oxyde d'éthylène sur l'alcool convenable
puis, si nécessaire, par estérification à l'aide d'acide
éthanoïque.
On ne dispose pas de données concernant la production
mondiale de ces éthers du glycol. Toutefois on peut
avancer que la production annuelle globale de l'Europe
occidentale, des Etats-Unis et du Japon se situe aux envi-
rons de 79 x 103 tonnes de 2-ME et de 205 x 103 tonnes
de 2-EE. Ils sont en grande partie utilisés pour la
production industrielle de divers revêtements (peintures,
teintures, laques, vernis, etc.) et comme solvants pour la
préparation d'encres d'impression, de résines et de
colorants, ainsi que pour la fabrication de détachants
domestiques et industriels. On les utilise également comme
additifs de dégivrage dans les liquides hydrauliques et
les carburéacteurs.
3. Transport, distribution et transformation dans l'environnement
Du fait de leur solubilité dans l'eau et de leur
tension de vapeur relativement basse, ces éthers
pourraient, en l'absence de décomposition, s'accumuler
dans l'eau. Toutefois, il semble que cette éventualité
soit exclue du fait de leur dégradation par des micro-
organismes présents dans le sol, les boues d'effluents et
l'eau.
C'est l'utilisation de ces éthers comme solvants vola-
tils qui, du fait des émissions atmosphériques auxquelles
elle donne lieu, entraîne l'exposition environnementale la
plus importante. Dans l'environnement général, ils
subissent une photolyse rapide et l'on pourrait s'attendre
à des concentrations inférieures à 0,0007 mg/m3 (2 x
10-4 ppm).
En aérobiose, les éthers du glycol subissent une
dégradation microbienne rapide en dioxyde de carbone et en
eau, alors qu'en anaérobiose, les principaux produits
finals sont le méthane et le dioxyde de carbone.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation d'éthers du glycol peut entraîner des
nombreuses émissions dans l'environnement. C'est en parti-
culier l'exposition humaine directe dans l'industrie, dans
les petits ateliers et au cours de l'utilisation domes-
tique de produits à base d'éthers du glycol qui est spéci-
alement préoccupante. En ce qui concerne l'exposition pro-
fessionnelle, les valeurs signalées vont de concentrations
< 0,1 mg/m3 à des concentrations > 150 mg/m3. L'utili-
sation de certains produits de consommation à base
d'éthers du glycol pourrait provoquer une exposition
notable des usagers mais on ne dispose pas de données à ce
sujet.
Outre l'exposition par la voie atmosphérique, l'homme
peut également être exposé par la voie dermique. L'analyse
du sang confirme que ces produits sont rapidement absorbés
par cette voie, qui contribue probablement davantage à la
charge totale de l'organisme que l'exposition par voie
aérienne.
5. Cinétique et métabolisme
Ces quatre éthers sont rapidement absorbés au niveau
de la peau, des poumons et des voies digestives. Des
études de répartition portant sur le 2-ME chez des souris
gravides ont montré que c'est dans le foie maternel, les
voies digestives, le placenta, le sac vitellin et de
nombreuses structures embryonnaires que se rencontraient
les concentrations les plus fortes.
La métabolisation du 2-ME donne naissance à deux
métabolites primaires : le MAA et la méthoxy-2 acétyl-
glycine. La transformation en dioxyde de carbone corres-
pond à une voie métabolique secondaire de moindre
importance. La conversion plasmatique du 2-ME en MAA
s'effectue rapidement, avec une demi-vie de 0,6 heure chez
le rat; en revanche l'excrétion de la MAA est lente, sa
demi-vie étant d'environ 20 heures chez le rat et de 77
heures chez l'homme.
L'administration à des animaux de laboratoire de 2-EE
a conduit à la production d'EAA et d'éthoxy-2 acétyl-
glycine, l'EAA étant le principal métabolite qui se mani-
feste dans l'organe supposé être l'organe cible, à savoir
les testicules. Chez l'homme, une étude sur le 2-EAA a
permis d'observer une voie métabolique analogue, l'acétate
étant d'abord hydrolysé en 2-EE puis oxydé en EAA. Cet
EAA a été ensuite excrété avec une demi-vie estimative de
21 à 42 heures. L'expérience semble indiquer que la
rétention ou l'accumulation des métabolites pourrait être
toxicologiquement importante dans la mesure où ces méta-
bolites seraient responsables de la toxicité observée au
niveau de l'organe cible.
6. Effets sur les êtres vivants dans leur milieu naturel
Il semble que le 2-ME et le 2-EE présentent une faible
toxicité pour les micro-organismes et les animaux aqua-
tiques. En ce qui concerne les micro-organismes, la con-
centration létale dans le milieu est supérieure à 2 %. On
a constaté une inhibition de la croissance des algues
vertes par le 2-ME à la concentration de 104 mg/litre et
de celle des cyanobactéries (algues bleu/vert) à la con-
centration de 100 mg/litre. La toxicité aiguë du 2-EE est
très faible pour les arthropodes (CL50 > à 4 g/litre) et
les poissons d'eau douce (CL50 > à 10 g/litre). Les acé-
tates des éthers du glycol (2-MEA et 2-EAA) sont beaucoup
plus toxiques pour les poissons. Ainsi la CL50 du 2-EEA
pour le vairon Pimephales promelas est de 46 mg par litre
tandis que celle du 2-MAA est de 45 mg/litre pour le
tarpon et pour Lepomis machrochirus. Il n'y a pas eu
d'études à long terme.
7. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
7.1 Toxicité générale
La toxicité du 2-ME et du 2-EE chez l'animal d'expéri-
ence a été beaucoup plus étudiée que celle du 2-MEA et du
2-EAA.
En ce qui concerne le 2-ME et le 2-EE ainsi que leurs
acétates, la dose létale après exposition unique est du
même ordre et ces composés présentent une faible toxicité
aiguë, que l'exposition ait lieu par voie dermique, orale
ou par inhalation. Pour diverses espèces, les valeurs de
la DL50 vont de 900 à 3400 mg/kg de poids corporel pour
le 2-ME, de 1400 à 5500 mg/kg pour le 2-EE, de 1250 à
3900 mg/kg pour le 2-MEA et de 1300 à 5100 mg/kg pour le
2-EAA. Des valeurs de 4603 mg/m3 (2-ME) et de 6698 mg
par m3 (2-EE) ont été signalées pour la CL50 par inhala-
tion chez la souris.
On ne possède que peu de données concernant les effets
irritants au niveau des yeux et de la peau ou le pouvoir
de sensibilisation de ces éthers du glycol chez l'animal.
Il semblerait qu'ils ne soient pas irritants pour la peau,
mais qu'ils puissent l'être pour l'oeil. Chez l'homme,
malgré de fortes expositions, on n'a jamais signalé
d'irritation cutanée ni de sensibilisation à ce niveau.
On a montré qu'en exposant par voie respiratoire des
animaux d'expérience pendant des périodes allant jusqu'à
90 jours, à de fortes concentrations (> 9313 mg de 2-ME
par m3 et > 1450 mg de 2-EE/m3) on déterminait des
effets nocifs sur les paramètres hématologiques, le
système nerveux, les testicules, le thymus, les reins, le
foie et les poumons. A des concentrations plus faibles,
les effets ne s'observaient qu'au niveau du système
hématopoïétique et des testicules. Par exemple, des rats
exposés par inhalation à du 2-ME pendant 13 semaines à des
doses comprises entre 93 et 930 mg/m3, présentaient une
réduction de l'hématocrite, du nombre de leucocytes, de
l'hémoglobine, des plaquettes et des protéines sériques,
mais seulement à la dose la plus forte. Chez des lapins
exposés de la même manière, on notait une réduction de la
taille du thymus et une altération des paramètres hémato-
logiques, à la dose de 903 mg/m3. Le 2-EE a produit des
effets analogues, mais moins graves, chez le rat et le
lapin lors d'une exposition de 13 semaines à la concen-
tration de 1450 mg/m3. On ne dispose d'aucune donnée
résultant d'études à long terme.
7.2 Cancérogénicité et mutagénicité
On a étudié la mutagénicité du 2-ME sur toute une
série de systèmes in vitro constitués de bactéries ou de
cellules mammaliennes. La plupart des études ont fourni
des résultats négatifs, toutefois on a tout de même
signalé des résultats positifs à de très fortes concen-
trations de 2-ME sur des cellules CHO. Il s'agissait
d'aberrations chromosomiques (à des concentrations supéri-
eures à 6830 µg/ml) et d'échanges entre chromatides
soeurs (à des concentrations supérieures à 3170 µg par
ml). La recherche d'aberrations chromosomiques et de
micronoyaux n'a rien donné in vivo . On ne dispose que de
données limitées sur le pouvoir mutagène du 2-EE; en
outre, il n'existe pas de données sur la cancérogénicité
de ces éthers du glycol.
7.3 Organes mâles de la reproduction
On a étudié de manière approfondie l'effet du 2-ME sur
l'appareil reproducteur mâle après administration par voie
orale ou respiratoire de ces substances à des rongeurs.
La présence de modifications dégénératives au niveau de
l'épithélium germinal des tubes séminifères à été systé-
matiquement observée. Des effets analogues ont été con-
statés avec le 2-EE, mais à des doses un peu inférieures.
L'administration par voie orale à des rats de 2-ME
pendant 1 à 11 jours a provoqué une réduction du nombre
des spermatozoïdes et des modifications de leur mobilité
et de leur morphologie, liées à la dose, à partir de
100 mg/kg de poids corporel. L'autopsie a révélé une
atteinte histologique marquée des testicules. La dose
sans effet observable (NOEL) était de 50 mg/kg. La
réduction de la fertilité était encore manifeste huit
semaines après une exposition à 200 mg/kg. Des effets
analogues ont été observés dans le cas du 2-EE à des doses
supérieures ou égales à 500 mg/kg, administrées pendant
des périodes allant jusqu'à 11 jours, la dose sans effet
observable sur 11 jours étant de 250 mg/kg. Toutefois
l'épuisement des réserves de spermatozoïdes, par suite
d'accouplements répétés, s'est accompagné à la dose la
plus faible étudiée (150 mg/kg), d'une réduction de leur
nombre. Après avoir administré par voie orale à des rats
et à des souris une dose unique de 250 mg ou davantage de
2-ME/kg de poids corporel, on a observé chez les animaux
une stérilité complète cinq semaines après l'admini-
stration, une certaine réduction de la fécondité étant
observée dès 125 mg/kg.
L'administration du 2-ME par la voie respiratoire a
donné lieu à des modifications dégénératives analogues au
niveau des testicules. Les effets en question ont été
observés après exposition unique de 4 heures à des doses
supérieures ou égales à 1944 mg/m3, aucun effet n'étant
observé à 933 mg/m3. Les valeurs de la dose sans effet
observable étaient de 311 mg/m3 chez les rats après
exposition de 13 semaines (6 heures par jour, 5 jours par
semaine) et de 933 mg/m3 (6 heures par jour) chez les
souris après exposition à neuf reprises sur une durée
totale de 11 jours. L'exposition de lapins à du 2-ME
pendant 13 semaines (6 heures par jour, 5 jours par
semaine) a entraîné des effets marqués au niveau des
testicules à la dose de 311 mg/m3 ou davantage; à la
dose de 93 mg/m3, on a observé des effets limites; il
n'a pas été possible de déterminer la dose sans effet
observable.
7.4 Toxicité foetale
On a observé des effets toxiques sur le développement
de plusieurs espèces d'animaux de laboratoire après
exposition par toutes les voies possibles, c'est-à-dire
orale, respiratoire et dermique. Le 2-ME a produit des
effets tératogènes chez la souris, le rat, le lapin et le
singe. Le 2-EE et le 2-EEA se sont révélés tératogènes
chez le rat et le lapin. Bien que le 2-MEA n'ait pas
encore été étudié de ce point de vue, son profil méta-
bolique (voir section 6) incite à penser qu'il a
vraisembleblement une toxicité analogue à celle du 2-ME.
C'est dans le cas du 2-ME que l'on possède l'ensemble
le plus complet de données dose-réponse (doses de 31,25 à
1000 mg/kg/j). Lors de cette étude portant sur des souris
auxquelles le 2-ME avait été administré par gavage
(administration les jours 7 et 14 de la gestation), on a
obtenu une dose sans effet observable de 125 mg/kg par
jour en ce qui concerne la toxicité maternelle. Toutefois
des malformations ont été observées à partir de doses
quotidiennes de 62,5 mg/kg et des modifications au niveau
du squelette à partir de 31,25 mg/kg par jour. La dose
sans effet observable relative à la toxicité foetale n'a
pas été indiquée. Lors d'études portant sur des doses
uniques, des souris ont reçu par gavage du 2-ME au onzième
jour de la gestation; la dose de 100 mg/kg n'était pas
foetotoxique tandis que celle de 175 mg/kg a produit des
anomalies digitales, mais sans autres signes de toxicité
maternelle ou foetale. Des anomalis cardio-vasculaires et
électrocardiographiques ont été observés chez les rats
nouveau-nés après administration les 7ème et 13ème jours
de la gestation d'une dose quotidienne de 25 mg/kg. Etant
donné qu'il s'agissait de la dose la plus faible expéri-
mentée, l'étude n'a pas permis d'établir une dose sans
effet observable sur le foetus (on n'a pas observé de
toxicité maternelle à cette dose). De même, aucune dose
de ce type n'a pu être déterminée après administration par
gavage à des guenons de 2-ME aux doses quotidiennes
respectives de 0,16, 0,32, ou 0,47 mmol/kg, du 20ème au
45ème jour de la gestation.
Après exposition par voie respiratoire à du 2-ME à la
dose de 156 mg/m3, on a observé une toxicité foetale
chez des rats et des souris et des malformations chez des
lapins. Pour l'ensemble de ces trois espèces, la dose sans
effet observable sur le développement foetal était de 31
mg/m3. Toutefois des anomalies comportementales et
neurochimiques ont été observées dans la descendance de
rattes exposées du 7ème au 13ème jour ou du 14ème au 20ème
jour de leur gestation à une dose de 78 mg de 2-ME par m3.
Après avoir exposé des rats à des doses de 743 mg par
m3 de 2-EE et des lapins à des doses 589 mg/m3 de la
même substance, on a constaté que ce produit était térato-
gène (avec en outre une certaine toxicité maternelle).
Dans une autre étude, on a constaté une toxicité foetale,
mais sans malformations, chez des rats exposés à des doses
de 184 ou 920 mg de 2-EE par m3 et chez des lapins ex-
posés à la dose de 644 mg/m3 de la même substance. Pour
ce qui est des effets sur le développement foetal, la
valeur de la dose sans effet observable était de 37 mg par
m3 pour le rat et de 184 mg/m3 pour le lapin. On a ob-
servé des anomalies comportementales et neurochimiques
dans la descendance de rattes exposées du 7ème au 13ème
jour et du 14ème au 20ème jour de leur gestation à la dose
de 360 mg de 2-EE par m3.
Des rattes soumises à une application dermique de 0,25
ml de 2-EE non dilué quatre fois par jour du 7ème et 16ème
jour de la gestation, ont eu une descendance où l'on
notait une foetotoxicité marquée et une forte incidence de
malformations malgré l'absence de toxicité maternelle. Des
effets analogues ont été observés à la suite d'un traite-
ment indentique par le 2-EEA selon le même protocole au
moyen d'une dose équimolaire (0,35 ml, quatre fois par
jour).
En exposant par la voie respiratoire des lapines à du
2-EEA du 6ème au 18ème jour de la gestation, on a obtenu
au cours de deux études différentes, des réponses térato-
gènes aux doses de 2176 mg/m3 et 544 mg/m3, la valeur
de la dose sans effet observable sur le développement
foetal étant respectivement de 135 mg/m3 et 270 mg par
m3. L'exposition de rattes du 6ème au 15ème jour de leur
gestation à du 2-EEA a entraîné dans leur descendance une
toxicité foetale à la dose de 540 mg/m3 et des malfor-
mations à la dose de 1080 mg/m3. La dose sans effet ob-
servable sur le développement foetal était de 170 mg par
m3.
8. Effets sur l'homme
On ne dispose que de renseignements limités sur les
effets toxiques chez l'homme de ces quatre éthers du
glycol. Les résultats fournis par quelques études de cas
ou études épidémiologiques sur les lieux de travail sont
dans la ligne des effets observés chez les animaux de
laboratoire. On n'a pas eu connaissance de rapports qui
chiffrent l'exposition de la population en général ni les
effets sur la santé.
Lors de deux cas non mortels d'empoisonnement par
ingestion d'un volume de 100 ml de 2-ME, on a noté les
principaux symptômes suivants : nausée, vertiges, cyanose,
tachycardie, hyperventilation et acidose métabolique avec
quelques signes d'insuffisance rénale. Des sympômes
analogues mais moins graves ont été observés chez une
personne qui avait ingéré 40 ml de 2-EE. Lors d'un
empoisonnement mortel par ingestion de 400 ml de 2-ME,
l'autopsie a révélé une gastrite hémorragique aiguë, une
dégénérescence graisseuse du foie et une altération
dégénérative des tubules rénaux.
L'exposition réitérée de travailleurs à du 2-ME et à
du 2-EE, en plus d'autres solvants, a entraîné chez eux de
l'anémie, un leucopénie, une faiblesse générale et une
ataxie. Dans nombre de ces études, il n'a pas été possible
de trouver une estimation fiable de l'exposition des
sujets. On a rapporté des effets hématologiques dus aux
éthers du glycol chez l'homme et on a notamment décrit
l'apparition d'une anémie macrocytaire chez un travailleur
exposé à du 2-ME (dose moyenne 105 ml/m3), ainsi qu'à
d'autres solvants.
Il a été fait état d'une toxicité médullaire chez des
ouvriers dont l'épiderme était exposé à du 2-ME, et des
effets immunologiques ont été également notés à la suite
d'une exposition prolongée (8 à 35 années) au 2-ME et au
2-EE (doses moyennes d'exposition 6,1 mg/m3 et 4,8 mg par
m3 respectivement).
Des études épidémiologiques effectuées sur des
ouvriers exposés à du 2-ME et à du 2-EE ont révélé des
anomalies au niveau de la fonction de reproduction, avec
une fréquenc accrue des cas d'oligospermie. L'exposition
à du 2-EE (37 ouvriers) à des concentrations pouvant
atteindre 88,5 mg/m3 a entraîné une modification du sper-
mogramme. Parmi 73 ouvriers exposés à du 2-ME (jusqu'à
17,7 mg/m3) et à du 2-EE (jusqu'à 80,5 mg/m3), on a
constaté une fréquence accrue de cas d'oligospermie et
observé certains effets hématologiques, pour des doses
d'exposition (TWA) de 2,6 mg/m3 dans le cas du 2-ME et de
9,9 mg/m3 dans celui du 2-EE.
Les effets indésirables constatés chez l'homme par
suite d'une exposition professionnelle correspondent à
ceux qui ont été observés chez les animaux de laboratoire.
Cependant, l'évaluation de l'exposition présentant un
certain nombre d'insuffisances et du fait qu'il s'agissait
d'expositions simultanées à plusieurs substances, il n'a
pas été possible d'en déduire une relation dose-réponse.
9. Conclusions
De nombreuses personnes peuvent être exposées à ces
quatre éthers du glycol à des concentrations comparables à
celles que l'on rencontre dans l'industrie, par suite de
l'utilisation de certains produits de consommation ou de
produits commerciaux. Une exposition professionnelle non
négligeable peut se produire par inhalation ou par résorp-
tion cutanée. Des mesures faites en petit nombre dans
l'air des lieux de travail indiquent des teneurs allant <
0,1 mg/m3 à > 150 mg/m3.
Le 2-ME et le 2-EE sont tous deux d'une faible toxi-
cité pour les micro-organismes et les espèces aquatiques.
Il n'existe aucune donnée qui permettrait d'évaluer le
risque d'effets indésirables sur les êtres vivants dans
leur milieu naturel par suite d'une exposition de longue
durée.
Chez le rat, la dose de 2-ME sans effets aigus observ-
ables au niveau testiculaire, est de 933 mg/m3; en cas
d'exposition répétée, la dose sans effet observable est de
311 mg/m3. En exposant de manière répétée l'espèce la
plus sensible, à savoir le lapin, on a observé un effet
net dès 311 mg/m3; cet effet était limite à la dose de
93 mg/m3 (un animal sur cinq). Les données fournies par
des études effectuées sur des sujets humains exposés de
par leur profession à du 2-ME et à du 2-EE montrent que
ces éthers du glycol exercent une certaine toxicité
testiculaire.
Chez toutes les espèces étudiées (souris, rats et
lapins) on a observé après exposition au 2-ME à des doses
égales ou supérieures à 156 mg/m3, une toxicité vis-à-
vis du développement foetal. Des anomalies comportement-
ales et neurochimiques ont été observées chez le rat après
exposition in utero à 78 mg/m3 de cette substance sans
qu'on puisse déterminer de dose sans effet observable. Le
2-EE et le 2-EEA se sont révélés légèrement moins actifs.
Des effets ont été également observés sur le développement
de foetus de rats et de lapins après exposition à du 2-EE
à des doses égales ou supérieures 368 mg/m3. Ces effets
étaient légers chez les rats exposés à 184 mg de 2-EE par
m3 mais on a pu néanmoins fixer nettement la dose sans
effet observable chez le rat et le lapin à la valeur de
38 mg/m3.
Ces éthers du glycol produisent des effets hématolo-
giques chez la souris, le rat, le lapin, le chien, le
hamster et le cobaye. Ces résultats sont en accord avec
les anomalies hématologiques observées lors des quelques
études consacrées à des travailleurs de l'industrie qui
avaient subi des expositions répétées à du 2-EE et/ou du
2-ME. Lors d'études sur l'animal comportant une expo-
sition répétée à ces deux substances, on a fixé à 93 mg
par m3 la dose de 2-ME sans effet observable chez le
lapin et à 368 mg/m3 la dose de 2-EE sans effet obser-
vable chez le rat et le lapin. On n'a pas pu obtenir de
données qui permettent une évaluation quatitative des
effets hématologiques aigus consécutifs à une exposition.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
1.1 Exposition
Nombreuses sont les personnes qui peuvent être
exposées au méthoxy-2 éthanol (2-ME), à l'éthoxy-2 éthanol
(2-EE) et à leurs acétates (2-MEA et 2-EEA) à des concen-
trations comparables à celles que l'on rencontre dans
l'industrie, lors de l'utilisation de produits de consom-
mation et de produits commerciaux. En revanche l'expo-
sition par l'intermédiaire des denrées alimentaires, de
l'eau ou de l'air ambiant est probablement négligeable.
Cette hypothèse ne repose que sur les propriétés
physiques et chimiques de ces composés et sur le fait
qu'ils se dégradent rapidement dans l'environnement.
Une exposition professionnelle non négligeable peut se
produire par inhalation ou résorption cutanée. Les
quelques mesures de concentrations dans l'air des lieux de
travail ont donné des valeurs qui vont de moins de 0,1 mg
par m3 à plus de 150 mg/m3. Toutefois les données de
surveillance existantes sont très limitées et il peut y
avoir d'importantes variations entre les différentes
industries et dans une même industrie. En raison du risque
de résorption cutanée, une simple surveillance de l'air
des lieux de travail risque de sous estimer l'exposition
totale. Pour évaluer la charge totale de l'organisme, la
meilleure méthode consiste à faire un contrôle biologique.
Une forte exposition peut se produire lors de travaux tels
que la peinture, l'impression ou le nettoyage mais il faut
se souvenir que ces composés sont utilisés à l'occasion
d'un grand nombre d'autres activités au cours desquelles
on pourrait craindre une exposition.
1.2 Effets sur la santé
Les principaux effets qu'on peut craindre chez l'homme
tiennent à l'action toxique de ces composés sur le
développement foetal, les testicules et les paramètres
hématologiques. La réalité de ces effets est attestée par
des données nombreuses et cohérentes obtenues chez l'ani-
mal ainsi que par quelques données concernant l'homme.
Tous ces effets peuvent appraître par suite d'expositions
à court ou à long terme. Chez l'animal de laboratoire,
une exposition répétée à des très fortes doses de 2-ME et
de 2-EE (plus de 930 ou 1450 mg/m3, respectivement)
entraîne des effets toxiques qui se traduisent par des
anomalies neuro-comportementales, hépatiques et rénales.
On les observe également dans les cas d'intoxication
humaine.
Ces quatre éthers du glycol exercent des effets
toxiques très voisins tant sur les testicules que sur le
développement du foetus, et ce, chez toutes les espèces
étudiées et par toutes les voies d'exposition qui ont été
utilisées (voie respiratoire, voie percutanée, voie
orale). L'étude du mécanisme de ces effets montre que
dans les deux cas, une phase d'activation est nécessaire,
à savoir la métabolisation en un dérivé de l'acide alkoxy-
acétique correspondant. La métabolisation s'effectue en
présence du système de l'alcool déshydrogénase qui est
commun à l'homme et aux animaux de laboratoire. Des méta-
bolites toxiques, l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA) ont été décelés dans l'urine de
sujets exposés à ces solvants. La régularité de cette
réponse toxique d'une espèce animale à l'autre, jointe à
l'analogie du métabolisme chez l'homme et l'animal,
montrent à l'évidence que l'homme pourrait être la cible
des mêmes effets toxiques sur les testicules et le
développement foetal. Les données dont on dispose au sujet
de l'excrétion des acides alkoxyacétiques chez l'homme
indiquent que leur durée de rétention est plus longue chez
celui-ci que chez l'animal, ce qui incite à penser que
l'homme pourrait être plus sensible à ces effets que
l'animal de laboratoire le plus sensible. C'est la
résorption cutanée rapide de ces composés qui est parti-
culièrement préocccupante. On a observé des effets térato-
gènes et autres anomalies du developpement à la suite de
l'application de 2-ME, de 2-EE et de 2-EAA sur la peau
intacte du rat.
Des lésions testiculaires ont été observées chez le
rat, la souris et le lapin à la suite d'une exposition à
ces éthers du glycol, soit par la voie respiratoire soit
par la voie orale. Chez le rat, une seule exposition par
voie respiratoire à des doses de 2-ME supérieures ou
égales à 1944 mg/m3 pendant 4 heures et une exposition
répétée à des doses supérieures ou égales à 933 mg par
m3 pendant 13 semaines ont déterminé des anomalies histo-
logiques au niveau testiculaire. Dans le cas de l'expo-
sition unique, la dose sans effet observable était de 933
mg/m3; elle était 311 mg/m3 dans le cas d'expositions
répétées. La souris a semblé un peu moins sensible, la
dose sans effet observable dans son cas étant, pour des
expositions répétées, de 933 mg/m3. Toutefois le lapin
l'était davantage, avec des altérations testiculaires
marquées qu'on pouvait observer après des expositions
répétées à 311 mg/m3 et la présence d'effets limites (un
lapin sur cinq) dès la dose de 93 mg/m3. Des effets ana-
logues ont été observés à la suite de l'exposition par
voie orale de rats à du 2-ME, une exposition de courte
durée (et notamment à une dose unique) ayant déterminé des
lésions testiculaires à partir de 100 mg/m3. Dans le
cas d'une étude portant sur les effets subaigus (11 jours)
la dose sans effet observable a été de 50 mg/kg. Le 2-EE
présente une toxicité testiculaire un peu moindre que
celle du 2-ME; ces effets n'apparaissent qu'à des doses
de 500 mg/kg ou davantage et la dose sans effet observable
se situe à 250 mg/kg.
Les données fournies par les études menées sur des
sujets humains exposés de par leur profession à du 2-ME et
à du 2-EE cadrent avec les données obtenues sur l'animal
et montrent que ces éthers du glycol peuvent produire des
effets toxiques au niveau testiculaire chez l'homme. Des
études épidémiologiques portant sur de petits groupes de
travailleurs exposés au 2-EE dans un atelier de fonte de
métaux et chez des peintres d'un chantier naval exposés à
ces deux composés, ont révélé une oligospermie systéma-
tique. Les données relatives aux niveaux d'exposition sont
limitées mais dans chaque cas, il y a lieu de penser qu'il
y a eu exposition par voie cutanée et respiratoire.
On a observé chez le rat, la souris, le lapin et le
singe des effets toxiques sur le développement embryon-
naire après exposition à ces éthers du glycol par voie
percutanée, orale ou respiratoire. Du 2-ME non dilué
appliqué à 12 reprises au cours d'une journée sur la peau
rasée de rattes gravides (zone d'application couverte
pendant 6 heures) s'est révélé mortel pour les animaux
alors qu'appliqué à 10 reprises sans pansement, du 2-EE
(1,0 ml/jour) ou du 2-EEA (1,4 ml/jour) ont produit des
effets tératogènes mais n'ont pas été toxiques pour les
femelles gravides. Au cours d'une autre étude, 12 appli-
cations de 2-ME à 10 % dans du soluté physiologique, avec
pansement sur le site d'application, ont entraîné des
effets toxiques sur le développement embryonnaire, la dose
sans effet observable se situant à la concentration de
3 %. Après administration répétée par voie orale de 2-ME
à des animaux gravides, on n'a pas observé de dose sans
effet toxique. La dose la plus faible produisant un effet
observable dans le cas de l'administration par voie orale
était de 31,5 mg/kg par jour pour la souris, de 25 mg/kg
pour le rat et de 0,16 mmol/kg pour le singe. En ce qui
concerne l'administration d'une dose unique, on ne possède
des résultats que pour le 2-ME chez la souris, avec une
dose sans effet observable pour le 11ème jour de la
gestation (le jour le plus sensible) de 100 mg/kg et une
valeur de 175 mg/kg pour la dose la plus faible avec effet
observable. Le 2-EE et le 2-EEA ont fait l'objet d'études
au cours desquelles des rats et des lapins ont été exposés
à ces deux composés par la voie respiratoire. L'expo-
sition des rats au 2-EE a fait l'objet de deux études qui
ont révélé des effets tératogènes (743 mg/m3, 7 heures
par jour, du premier au 19ème jour de la gestation) ou des
effets foetotoxiques (184 et 920 mg/m3, 6 heures par
jour, du 6ème au 15ème jour de la gestation). Dans cette
dernière étude, on a trouvé une dose sans effet observable
de 37 mg/m3. Chez des lapins exposés à du 2-EE on a
également observé des effets tératogènes (589 mg/m3, 7
heures par jour du premier au 18ème jour de la gestation)
et des effets foetotoxiques (644 mg/m3, 6 heures par
jour, du 6ème au 18ème jour de la gestation). Dans cette
dernière étude, la dose sans effet observable était de
184 mg/m3. Chez des lapines exposées à du 2-EEA 6 heures
par jour du 6ème au 18ème jour de la gestation, on a
observé des effets tératogènes à la dose de 2160 mg/m3
dans une étude et à la dose de 1620 mg/m3 dans une
autre. Les deux études ont montré l'apparition d'une
foetotoxicité à 540 mg/m3; la dose sans effet observable
correspondant, dans chaque étude, à la dose la plus faible
étudiée (respectivement 135 et 270 mg/m3). Des rats
exposés par voie respiratoire à du 2-EEA 6 heures par jour
du 6ème au 15ème jour de la gestation ont présenté le même
de type de réactions : effets tératogènes à 1620 mg par
m3, foetotoxicité à 1080 mg/m3 et aucun effet à 260 mg
par m3.
Des effets toxiques sur le développement embryonnaire
ont donc été observés chez toutes les espèces (souris,
rats et lapins), exposées à des doses supérieures ou
égales à 156 mg de 2-ME. Pour ces trois espèces, la dose
sans effet observable était de 31 mg/m3. Après expo-
sition in utero à 78 mg/m3, on a observé chez la descen-
dance des altérations comportementales et neurochimiques
sans qu'il soit possible de déterminer la dose sans effet
observable.
Le 2-EE et le 2-EEA se sont révélés légèrement moins
actifs. Des effets toxiques sur le développement embryon-
naire ont été observés chez des rats et des lapins après
exposition à des doses supérieures ou égales à 368 mg par
m3 de 2-EE. De légers effets de ce type ont été également
observés chez des rats exposés à 184 mg de 2-EE/m3, la
dose sans effet observable étant clairement établie à
37 mg/m3. En ce qui concerne le 2-EEA, la dose sans
effet observable était de 170 mg/m3 pour les rats et les
lapins.
Des effets hématologiques consécutifs à une exposition
à une dose unique on été observés chez l'animal ainsi qu'à
la suite d'intoxications chez l'homme. L'exposition
répétée par voie respiratoire, de lapins, l'espèce la plus
sensible, à du 2-ME 5 fois par semaine pendant 13
semaines, a permis de fixer la dose sans effet observable
à 93 mg/m3. Administré à répétition, le 2-ME produit
également des anomalies hématologiques chez la souris, le
lapin, le chien, le hamster et le cobaye. Le 2-EE est
moins actif à cet égard que le 2-ME. En ce qui concerne
les effets hématologiques chez le rat et le lapin, après
exposition à du 2-EE pendant 13 semaines, 5 fois par
semaine et 6 heures par jour, on peut fixer la dose sans
effet observable à 368 mg/m3. Chez le chien et la
souris, on observe également après, exposition répétée à
des doses plus élevées, un certain nombre d'effets hémato-
logiques. L'exposition aux acétates de 2-EE et de 2-ME
devrait provoquer des effets analogues, toutes choses
égales d'ailleurs, mais les données dont on dispose sur
l'exposition et les effets hématologiques de ces composés
sont trop limitées pour qu'on puisse établir les con-
ditions dans lesquelles une seule exposition conduirait à
des effets hématologiques chez l'homme.
On a observé dans l'industrie des niveaux d'exposition
voisins de la dose sans effet hématologique observable que
l'on a pu déterminer chez l'animal après expositions
répétées à du 2-ME et à du 2-EE. On peut en conclure,
compte tenu de la sensibilité probablement plus grande de
l'homme et de l'accumulation vraisemblable de métabolites
dans le sang, que l'exposition résultant de l'utilisation
de produits de consommation ou d'activités dans
l'industrie pourrait entraîner des effets hématologiques
de ce genre. A l'appui de cette hypothèse, on peut citer
les effets hématologiques effectivement signalés lors de
quelques études effectuées chez des travailleurs de
l'industrie ayant subi des expositions répétées au 2-EE
et/ou au 2-ME.
2. Evaluation des effets sur l'environnement
La libération directe dans l'atmosphère ou l'utili-
sation de ces produits comme solvants volatils peut con-
duire à une exposition dans le milieu ambiant. Ce peut
être également le cas par suite d'une libération acciden-
telle sur le sol ou dans l'eau. L'accumulation dans le
sol et les eaux superficielles ne peut se produire qu'en
l'absence de dégradation de ces composés. Or, on sait que
ces éthers du glycol se décomposent rapidement sous
l'effet de processus chimiques et biologiques; ils ne
devraient donce pas s'accumuler. Le 2-MEA et le 2-EAA
devraient également s'hydrolyser rapidement et subir
ensuite une biodégradation aérobie. Toutefois dans des
conditions d'anaérobiose, on pourrait craindre une
contamination des sols et des nappes phréatiques encore
que le phénomène soit vraisemblablement transitoire et le
risque correspondant négligeable.
Le 2-ME et le 2-EE sont peu toxiques pour les micro-
organismes et les espèces aquatiques. Leurs acétates, en
revanche, présentent une toxicité aiguë beaucoup plus
forte. On ne dispose pas de données à partir desquelles
on puisse déterminer leur potentiel nocif pour les espèces
vivantes en cas d'exposition prolongée.
RECOMMANDATIONS
1. Protection de la santé
1. Il conviendrait de recercher des solvants moins
toxiques que le méthoxy-2 éthanol, l'éthoxy-2 éthanol et
leurs esters, en particulier pour les remplacer dans les
produits de consommation. Il est également important
d'évaluer les effets des autres éthers de l'éthylène-
glycol car certains d'entre eux pourraient excercer des
effets analogues aux quatre éthers qui ont fait l'objet de
la présente évaluation.
2. Du fait des effets toxiques reconnus de ces éthers du
glycol, les autorités responsables devraient se préoccuper
sérieusement de trouver les moyens d'avertir les consom-
mateurs des dangers que présente l'utilisation de ces
produits, en particulier en cas d'exposition par voie
cutanée.
3. Compte tenu des données toxicologiques récentes et de
la possibilité d'une très forte absorption percutanée des
ces éthers du glycol, il importe de revoir les limites
nationales d'exposition professionnelle pour faire en
sorte que la dose quotidienne totale à laquelle sont
exposés les travailleurs par n'importe quelle voie, ne
menace pas leur santé.
4. Lorsqu'elle est assez importante, une dose unique peut
produire des effets sur l'animal. Pour réduire les
risques, il est recommandé d'utiliser ces produits avec
prudence (veiller à l'hygiène personnelle, utiliser des
dispositifs protecteurs appropriés et assurer une venti-
lation suffisante). Les données montrent que les effets
toxiques sur le développement embryonnaires ainsi que sur
le sang et les testicules, consécutifs à une exposition
répétée, pourraient exiger des mesures de protection plus
importantes.
2. Recherches à affectuer
1. Du fait que l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA), qui sont les principaux métabolites
du 2-ME, du 2-EE et de leurs esters, exercent des effets
toxiques sur les organes reproducteurs mâles, il faudrait
en étudier le mode d'action. S'ils apparaît que le MAA et
EAA ne sont pas les véritables responsables de ces effets,
ceux-ci devront être identifiés et leur mode d'action
élucidé.
2. Ces quatre éthers du glycol ont des effets sur le sang
et sur la fonction de reproduction masculine (oligo-
spermie). Les données disponibles, bien qu'en nombre
limité, indiquent que ces deux effets se manifestent à des
doses analogues. Il faudrait étudier le mode d'action de
ces composés sur ces deux types d'organes et étudier
parallèlement le nombre de spermatozoïdes et les effets
hématologiques afin de voir si ces derniers sont suscept-
ibles de jouer le rôle de signal d'alarme pour d'autres
effets toxiques de ces composés.
3. La surveillance de l'air ne suffit pas à assurer une
faible exposition. Un contrôle biologique peut permettre
de déceler les insuffisances des mesures de protection. A
l'heure actuelle on n'a pas établi avec une certitude
suffisante la relation qui existe entre les indicateurs
biologiques de l'exposition, la charge totale de l'organ-
isme et les effets physiologiques observés. Des travaux
sont encore nécessaires pour pouvoir établir les limites
de sécurité en fonction des résultats de la surveillance
biologique.
4. Il faudrait envisager des études épidémiologiques ou
une surveillance sanitaire spécifique au sein de popu-
lations qui sont fortement exposées à ces éthers du glycol
afin d'obtenir des relations exposition-effets; on
pourrait alors déterminer les limites de sécurité, à
condition toutefois que l'exposition globale puisse être
correctement et suffisamment évaluée par la surveillance
de l'environnement et des contrôles biologiques.
5. Il conviendrait d'étudier l'éventualité d'effets sur
l'appareil reproducteur féminin en procédant à des études
de reproduction sur plusieurs générations d'animaux de
laboratoire.
6. Les résultats disponibles indiquent que l'homme
pourrait métaboliser dans une plus grande proportion que
le rat ces éthers du glycol en acides alkoxyacétiques
correspondants, la demi-vie des ces métabolites toxiques
s'étant environ quatre fois plus longue chez l'homme. Par
ailleurs l'organisme du rat est capable de conjuguer une
fraction plus importante des métabolites acides que ne le
fait celui de l'homme. Ces différences pourraient
entraîner une sensibilité relativement plus importante de
l'homme aux éthers du glycol. Une connaissance plus
précise du métabolisme et de la cinétique d'excrétion de
ces composés permettrait de mieux prévoir les limites de
sécurité.
7. Les résultats fournis par des études à court terme
(13 semaines) montrent que des effets s'exercent sur
divers organes. Toutefois, il n'a pas été procédé à des
études suffisamment longues pour qu'on puisse déterminer
si ces effets sont réversibles ou non. Il est donc
recommandé de procéder à des études au cours desquelles
des animaux de laboratoire subiront une exposition d'au
moins 13 semaines à ces composés, suivie d'une période de
récupération. A l'issue de cette période, on devra
déterminer les paramètres physiologiques importants afin
de voir si les effets sont passagers ou non.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, métodos analíticos
En esta monografía sólo se tienen en cuenta los éteres
metílico y etílico del etilenglicol, es decir el 2-metoxi-
etanol (2-ME) y el 2-etoxietanol (2-EE), así como sus
ésteres acéticos respectivos, el acetato de 2-metoxietilo
(2-MEA) y el acetato de 2-etoxietilo (2-EEA). Estos cuatro
compuestos son todos ellos líquidos estables, incoloros e
inflamables con un olor levemente etéreo y todos ellos son
miscibles con (o, en el caso del 2-EEA, muy solubles en)
agua y miscibles con numerosos solventes orgánicos.
Se dispone de métodos analíticos para detectar estos
éteres glicólicos o sus metabolitos en diversos medios
(aire, agua, sangre y orina). En muchos casos se basan en
el empleo de métodos de adsorción o extracción para con-
centrar la muestra, seguidos de un análisis por cromato-
grafía de gases. Mediante la cromatografía de gases o la
cromatografía de líquidos de alto rendimiento, cabe la
posibilidad de determinar cuantitativamente el ácido
2-metoxiacético (MAA) y el ácido 2-etoxiacético (EAA) -
metabolitos del 2-ME y del 2-EE - en la orina, por lo gen-
eral tras derivación, a concentraciones de 5-100 µg por
ml.
2. Fuentes de exposición humana y ambiental
Los cuatro éteres glicólicos examinados están todos
ellos producidos por la reacción del óxido etilénico con
el alcohol apropiado, seguida, si procede, de esterifica-
ción con ácido etanoico.
No se dispone de datos sobre la producción mundial de
estos éteres glicólicos. Sin embargo, la producción anual
combinada de Europa occidental, los Estados Unidos de Amé-
rica y el Japón es aproximadamente del 79 x 103 toneladas de
2-ME y 205 x 103 toneladas de 2-EE. Una proporción con-
siderable se utiliza en la fabricación de pinturas, color-
antes y lacas así como en forma de solventes para tinta de
imprenta, resinas y tintes, y como productos de limpieza
domésticos e industriales. También se utilizan estos com-
puestos como aditivos anticongelantes en los líquidos hi-
dráulicos y el combustible de los reactores.
3. Transporte, distribución y transformación en el medio ambiente
La hidrosolubilidad de estos éteres glicólicos y su
presión de vapor relativamente baja podrían dar lugar a su
acumulación en el agua en ausencia de degradación. Sin
embargo, esta posibilidad queda aparentemente excluida
debido a la degradación por los microorganismos presentes
en el suelo, los cienos de alcantarilla y el agua.
Las emisiones atmosféricas causadas por el uso de
éteres glicólicos como solventes volátiles originan la
máxima exposición ambiental. En el medio ambiente general,
la degradación fotolítica parece ser rápida y cabe prever
niveles inferiores a 0,0007 mg/m3 (2 x 10-4 ppm).
En condiciones aeróbicas los microorganismos degradan
rápidamente los éteres glicólicos en forma de dióxido de
carbono y agua, mientras que en condiciones de anaerobi-
osis los principales productos finales son el metano y el
dióxido de carbono.
4. Niveles en el medio ambiente y exposición humana
El uso de éteres glicólicos puede dar lugar a emisi-
ones considerables y muy extendidas en el medio ambiente.
Suscita especial preocupación la exposición humana directa
en la industria, en los talleres de dimensiones modestas y
como consecuencia del empleo doméstico de productos que
contienen dichos éteres. Se han señalado valores de expo-
sición profesional comprendidos entre < 0,1 mg/m3 y > 150
mg/m3. Los usuarios de productos de consumo pueden
sufrir una exposición considerable, aunque no se dispone
de datos al respecto.
Además de la exposición a los éteres glicólicos pres-
entes en la atmósfera, las personas pueden estar expuestas
por vía cutánea. Los análisis de sangre confirman que la
absorción es rápida por esta vía, que puede contribuir más
que la exposición atmosférica a la carga total que recibe
el organismo.
5. Cinética y metabolismo
Se ha demostrado que los cuatro éteres glicólicos
pueden absorberse rápidamente a través de la piel, los
pulmones y el tracto gastrointestinal. Los valores más
elevados que se han obtenido en los estudios sobre la
distribución del 2-ME en ratonas gestantes se sitúan en el
hígado, la sangre y el tracto gastrointestinal de la
madre, así como en la placenta, el saco vitelino y en
numerosas estructuras del embrión.
La transformación metabólica del 2-ME produce dos
metabolitos primarios: MAA y glicina de 2-metoxiacetilo.
La metabolización a dióxido de carbono representa una vía
secundaria de menor importancia. La conversión del 2-ME
en MAA en el plasma se produce rápidamente, con una vida
media de 0,6 h en las ratas, pero la secreción del MAA es
lenta, con una vida media de 20 h aproximadamente en la
rata y de 77 h en el hombre.
En los animales de laboratorio, la administración de
2-EE da lugar a la producción de EAA y de glicina de
2-etoxiacetilo; el EAA es el principal metabolito que
aparece en los testículos, que son el "órgano diana"
presunto. En un estudio humano en el que se utilizó 2-EEA
se observó una vía metabólica análoga: el acetato se hi-
drolizó primero en 2-EE y luego se transformó en EAA por
oxidación. El EAA resultante se excretó con una vida media
estimada en 21-42 h. Los estudios experimentales hacen
pensar que la retención o acumulación de los metabolitos
podrían ser importantes desde el punto de vista toxico-
lógico en el supuesto de que dichos metabolitos sean la
causa de la toxicidad observada en el "órgano diana".
6. Efectos en los organismos presentes en el medio ambiente
La toxicidad del 2-ME y del 2-EE para los microorga-
nismos y animales acuáticos parece ser baja. En el caso
de los microorganismos, la concentración letal en el medio
es superior al 2%. Con el 2-ME se ha observado inhibición
del crecimiento de las algas verdes a 104 mg/litro y de
las cianobacterias (algas verde azuladas) a 100 mg/litro.
La toxicidad aguda del 2-EE es muy baja para los artrópo-
dos (CL50 > 4 g/litro) y para los peces de agua dulce
(CL50 > 10 g/litro). Los acetatos de éteres glicólicos
(2-MEA y 2-EEA) son mucho más tóxicos para los peces. La
CL50 del 2-EEA para el Phoxinus cabezudo es 46 mg/litro y
la del 2-MEA para el pez plateado de la pleamar y los
Lepomis de agallas azules es de 45 mg/litro. No se han
hecho estudios a largo plazo.
7. Efectos en los animales de experimentación y en los
sistemas de experimentación in vitro
7.1 Toxicidad sistémica
La toxicidad del 2-ME y del 2-EE en los animales de
experimentación se ha estudiado en medida mucho mayor que
la del 2-MA y del 2-EEA.
El 2-ME y el 2-EE y sus acetatos dan tasas análogas de
letalidad tras una exposición única y producen una letali-
dad aguda baja cuando la exposición tiene lugar por vía
dérmica u oral o por inhalación. Los valores de la DL50 oral
en las diversas especies estudiadas oscilan entre 900 y
3400 mg/kg de peso corporal en el caso del 2-ME, entre
1400 y 5500 mg/kg en el del 2-EE, entre 1250 y 3930 mg/kg
en el del 2-MEA, y entre 1300 y 5100 mg/kg en el de 2-EEA.
En los ratones se han obtenido valores de la CL50 por
inhalación de 4603 mg/m3 (2-ME) y 6698 mg/m3 (2-EE).
Sólo se dispone de datos limitados acerca de la irri-
tación cutánea u ocular o del potencial de sensibilización
de estos éteres glicólicos en los animales. Al parecer,
no son irritantes para la piel, pero pueden causar irri-
tación en los ojos. En el hombre no se ha observado irri-
tación ni sensibilización cutáneas ni siquiera en caso de
gran exposición.
La exposición por inhalación a corto plazo (hasta 90
días) de los animales de experimentación a concentraciones
elevadas (> 9313 mg de 2-ME/m3 y > 1450 mg de 2-EE/m3)
ejerce, según se ha demostrado, efectos adversos en los
parámetros sanguíneos, el sistema nervioso y los testícu-
los, el timo, el riñón, el hígado y los pulmones. Utiliz-
ando niveles más bajos de exposición, se han observado
efectos en el sistema hematopoyético y en los testículos.
Así, por ejemplo, las ratas expuestas durante 13 semanas a
la inhalación de 2-ME a concentraciones comprendidas entre
93 y 930 mg/m3 presentaron una reducción del volumen
hematocrito y de los glóbulos blancos, la hemoglobina, las
plaquetas y las concentraciones de proteínas séricas sola-
mente cuando se aplicaba la dosis máxima, mientras que los
ratones expuestos del mismo modo presentaron disminución
del tamaño del timo además de la disminución de los pará-
metros sanguíneos con concentraciones de 930 mg/m3. Las
ratas y conejos expuestos al 2-EE presentaron efectos
análogos pero menos intensos cuando soportaron durante 13
semanas una concentración de 1450 mg/m3. No se dispone
de datos sobre estudios a largo plazo.
7.2 Carcinogenicidad y mutagenicidad
La mutagenicidad del 2-ME se ha estudiado en una gama
de sistemas in vitro utilizando bacterias y células de
mamífero. Aunque la mayor parte de los estudios han dado
resultados negativos, algunos informes acusan resultados
positivos en cuanto a la mutagenicidad de las concentraci-
ones muy altas de 2-ME en células CHO estudiadas desde el
punto de vista de las aberraciones cromosómicas (a 6830 µg
por ml o más) o del intercambio de cromátides equiparables
(3170 µg/ml o más). En cambio, la investigación in vivo
de aberraciones cromosómicas y micronúcleos ha dado
siempre resultados negativos. Sólo se dispone de una
información muy limitada sobre el potencial mutagénico del
2-EE y no se dispone de ningún dato sobre la carcinogen-
icidad de estos éteres glicólicos.
7.3 Sistema reproductor masculino
Los efectos del 2-ME en el sistema reproductor mascu-
lino se han estudiado detenidamente en roedores expuestos
por vía oral o por inhalación. En el epitelio germinal de
los tubos seminíferos se han observado constantemente
alteraciones degenerativas. Análogos efectos se han obten-
ido con el 2-EE, si bien con niveles de dosificación algo
más elevados.
En la rata, la administración oral de 2-ME durante 1-
11 días ha dado lugar a un descenso del recuento de esper-
matozoides con cambios de la motilidad y la morfología de
éstos en relación con la dosis utilizada; como niveles de
dosificación se utilizaron 100 mg/kg de peso corporal o
más. En la autopsia se encontraron acusadas alteraciones
histológicas en los testículos. El nivel de efecto no
observado (NENO) fue de 50 mg/kg. La reducción de la fer-
tilidad seguía siendo patente a las 8 semanas de la expo-
sición a 200 mg/kg. Análogos efectos se observaron con
dosificaciones de 500 mg de 2-EE/kg o más, administrados
durante 11 días como máximo; en el tratamiento de 11 días
el NENO fue de 250 mg/kg. En cambio, cuando las reservas
de espermatozoides están reducidas por la frecuencia de
los acoplamientos, se observó cierta reducción de los
recuentos con la dosis más baja estudiada. Los estudios
de fertilidad consecutivos a la administración de una sola
dosis oral de 250 mg de 2-ME/kg o más mostraron una ester-
ilidad completa, tanto en las ratas como en los ratones, a
partir de las 5 semanas de administración; con 125 mg/kg
se observó ya cierto descenso de la fertilidad.
En los experimentos de inhalación se observaron alter-
aciones degenerativas análogas en los testículos con el
2-ME. Los efectos se observaron tras una sola exposición
(4 h) a 1944 mg/m3 o más pero no con 933 mg/m3. El NENO
fue de 311 mg/m3 en la rata tras la exposición durante 13
semanas (6 h/día, 5 días/semana) y de 933 mg/m3 (6 h/día)
en los ratones tras la exposición en 9 ocasiones en el
curso de 11 días. En los conejos expuestos al 2-ME durante
13 semanas (6 h/día, 5 días/semana) se observaron efectos
marcados en los testículos con concentraciones de 311
mg/m3 o más y efectos marginales con 93 mg/m3; no se
determinó el NENO.
7.4 Toxicidad para el desarrollo
En varias especies de animales de laboratorio se ha
observado toxicidad para el desarrollo tras la exposición
a los compuestos utilizando todas las vías de administra-
ción: oral, inhalatoria y dérmica. El 2-ME produjo efectos
teratógenos en ratones, ratas, conejos y monos. El 2-EE y
el 2-EEA resultaron teratógenos en las ratas y los
ratones. Aunque no se ha estudiado la toxicidad sobre el
desarrollo del 2-MEA, los perfiles metabólicos (véase la
sección 6) hacen pensar que es posible que el 2-MEA tenga
una toxicidad análoga a la del 2-ME.
En relación con el 2-ME se dispone de la gama más
amplia de datos dosis/respuesta (dosis de 31,25 por 1000
mg/kg por día). En este estudio de administración inten-
siva en el que se utilizaron ratones (2-ME administrado
entre el 7° y el 14° día de gestación) el NENO correspon-
diente a la toxicidad materna fue de 125 mg/kg por día.
No obstante, se observaron malformaciones con 62,5 mg/kg
por día y variaciones esqueléticas con 31,25 mg/kg por
día. No se señaló ningún NENO de toxicidad para el desar-
rollo. En el marco de estudios de dosis única, se trató a
ratones con 2-ME administrado por alimentación forzada el
11° día de la gestación; la dosis de 100 mg/kg no era
fetotóxica mientras que la de 175 mg/kg produjo anomalías
digitales sin otros signos de toxicidad materna o fetal.
En la ratas recién nacidas se observaron defectos cardio-
vasculares y anomalías del ECG tras el tratamiento de las
madres con 25 mg/kg por día durante los días 7° a 13° de
la gestación. Como ésta fue la dosis más baja que se
ensayó, este estudio no ha permitido establecer un NENO
para el desarrollo (con esa dosis no se observó toxicidad
materna). De igual modo, no pudo determinarse ningún NENO
de toxicidad para el desarrollo en un estudio de trata-
miento de monos por alimentación forzada con 2-ME a 0,16,
0,32 o 0,47 mmol/kg por día durante los días 20° a 45° de
la gestación.
Tras la exposición a la inhalación de 2-ME a 156 mg
por m3 se ha observado fetotoxicidad en los ratones y
ratas y malformaciones en los conejos. En las tres espe-
cies, el NENO correspondiente a los efectos sobre el
desarrollo fue de 31 mg/m3. Sin embargo, en la descen-
dencia de las ratas expuestas a 78 mg 2-ME/m3 durante los
días 7-13 o 14-20 de la gestación se observaron altera-
ciones conductuales y neuroquímicas.
Tras la exposición por inhalación de ratas (743 mg por
m3) y conejos (589 mg/m3), el 2-EE se reveló terató-
geno (en presencia de ligera toxicidad materna). En otro
estudio se observó fetotoxicidad pero no malformaciones en
las ratas expuestas a 184 ó 920 mg de 2-EE/m3, así como
en los conejos expuestos a 644 mg de 2-EE/m3. Los val-
ores del NENO para los efectos en el desarrollo fueron de
37 mg/m3 en las ratas y de 184 mg/m3 en los conejos. En
la descendencia de las ratas expuestas a 368 mg de 2-EE
por m3 durante los días 7-13 ó 14-20 de la gestación se
observaron alteraciones conductuales y neuroquímicas.
Las ratas tratadas por aplicación dérmica de 0,25 ml
de 2-EE sin diluir (cuatro veces al día en los días 7-16
de la gestación) acusaron una considerable fetotoxicidad y
una elevada incidencia de malformaciones en ausencia de
toxicidad materna. Análogos efectos se observaron tras el
tratamiento de las ratas con 2-EEA, utilizando el mismo
protocolo, a una dosis equimolar (0,35 ml, cuatro veces al
día).
La exposición por inhalación de 2-EEA de conejas
durante los días 6-18 de la gestación provocó respuestas
teratógenas con 2176 mg/m3 y 544 mg/m3 en dos estudios
diferentes, en los cuales los valores del NENO para el
desarrollo fueron de 135 mg/m3 y 270 mg/m3. Las ratas
expuestas al 2-EEA durante los días 6-15 de la gestación
acusaron fetotoxicidad a 540 mg/m3 y malformaciones a
1080 mg/m3. El NENO para el desarrollo fue de 170 mg por
m3.
8. Efectos en el hombre
La información disponible sobre los efectos tóxicos de
estos cuatro éteres glicólicos en el ser humano es limi-
tada. Los resultados de los escasos informes sobre casos
individuales y estudios epidemiológicos en el lugar de
trabajo corroboran los efectos adversos observados en los
animales de experimentación. No se ha encontrado ningún
informe en el que se cuantifique la exposición y los
efectos adversos en la población general.
En dos casos no mortales de envenenamiento por inges-
tión de 100 ml de 2-ME, los signos y síntomas predomi-
nantes fueron náuseas, vértigo, cianosis, taquicardia,
hiperventilación y acidosis metabólica, con algunos indi-
cios de insuficiencia renal. Síntomas análogos, aunque
menos graves, se observaron en un sujeto que ingirió 40 ml
de 2-EE. En un caso de intoxicación mortal causada por la
ingestión de 400 ml de 2-ME, la autopsia reveló una gas-
tritis hemorrágica aguda con degeneración grasa del hígado
y alteraciones degenerativas de los túbulos renales.
La exposición repetida de los trabajadores al 2-ME y
al 2-EE, así como a otros solventes, ha dado lugar a
anemia, leucopenia, debilidad general y ataxia. En muchos
de estos estudios no se ha hecho ninguna estimación fide-
digna de la exposición. Los efectos hematológicos de los
éteres glicólicos en el ser humano están bien documentados
y se ha descrito la aparición de anemia macrocítica en un
trabajador expuesto al 2-ME (promedio: 105 mg/m3), así
como a otros solventes.
En los trabajadores expuestos por vía dérmica al 2-ME
se han observado efectos tóxicos en la médula ósea, y tam-
bién se han observado efectos inmunológicos en trabaja-
dores sometidos a una exposición prolongada (8-35 años) al
2-ME y al 2-EE (los promedios de exposición fueron de 6,1
mg/m3 y 4,8 mg/m3, respectivamente).
Los estudios epidemiológicos realizados en trabaja-
dores expuestos al 2-ME y al 2-EE han revelado algunos
indicios de efectos adversos en el sistema reproductor
masculino, con un aumento de la frecuencia de recuentos
reducidos de espermatozoides. La exposición al 2-EE (37
trabajadores) a concentraciones de hasta 88,5 mg/m3 pro-
vocaron una alteración de los índices seminales. En un
grupo de 73 trabajadores expuestos al 2-ME (hasta 17,7 mg
por m3) y al 2-EE (hasta 80,5 mg/m3) se observó una
mayor frecuencia de recuentos reducidos de espermatozoides
y también signos de efectos hematológicos con exposiciones
de 2,6 mg/m3 para el 2-ME y de 9,9 mg/m3 para el 2-EE
(TWA).
Los efectos adversos observados en las personas pro-
fesionalmente expuestas coinciden con los señalados en los
animales de experimentación. Sin embargo, debido a defici-
encias en las evaluaciones de la exposición y a las expo-
siciones mixtas, no se han podido determinar relaciones
dosis-respuesta.
9. Conclusiones
Muchas personas pueden estar expuestas a concentra-
ciones de estos cuatro éteres glicólicos comparables a las
industriales a consecuencia del empleo de productos comer-
ciales y de consumo. Tanto por inhalación como por absor-
ción cutánea pueden producirse exposiciones profesionales
importantes. En un número limitado de determinaciones de
la concentración atmosférica en los lugares de trabajo se
han obtenido valores comprendidos entre < 0,1 mg/m3 y >
150 mg/m3.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
para los microorganismos y las especies acuáticas. No se
dispone de datos que permitan precisar la capacidad poten-
cial de las exposiciones prolongadas para ejercer efectos
adversos sobre las especies presentes en el medio ambiente.
En las ratas se ha obtenido un NENO para los efectos
testiculares de 933 mg de 2-ME/m3, así como un NENO para
la exposición repetida de 311 mg/m3. En los experimentos
de exposición repetida con la especie más sensible, el
conejo, se ha detectado un efecto neto con 311 mg/m3, mien-
tras que a 93 mg/m3 se observaba un efecto marginal (1 de
5 animales). En las personas expuestas profesionalmente al
2-ME y al 2-EE se han encontrado indicios de que estos
éteres glicólicos pueden producir toxicidad testicular en
el ser humano.
En todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más se ha observado toxi-
cidad para el desarrollo. Para las tres especies se tuvo
un NENO de 31 mg/m3. En las ratas expuestas in utero a
78 mg/m3 se produjeron alteraciones conductuales y neuro-
químicas, pero no se estableció ningún valor de NENO. El
2-EE y el 2-EEA eran algo menos potentes. En la rata y en
el conejo se han observado efectos sobre el desarrollo
tras la exposición a 2-EE a concentraciones de 368 mg por
m3 o más. Estos efectos eran ligeros en las ratas ex-
puestas a 184 mg de 2-EE/m3, pero tanto en las ratas como
en los conejos se pudo establecer un NENO bien definido a
37 mg/m3.
Estos éteres glicólicos producen efectos hematológicos
en los ratones, las ratas, los conejos, los perros, los
hamsters, y los cobayos. Esta observación concuerda con
los efectos hematológicos señalados en algunos de los
escasos estudios efectuados en trabajadores industriales
expuestos repetidamente al 2-EE y/o al 2-ME. En los estu-
dios de exposición repetida de animales se obtuvo un NENO
de 93 mg de 2-ME/m3 en los conejos y de 368 mg de 2-EE
por m3 en las ratas y los conejos. No se han obtenido
datos que permitan evaluar cuantitativamente los efectos
hematológicos que siguen a la exposición aguda.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y EFECTOS
EN EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
1.1 Exposición
Muchas personas pueden estar expuestas al 2-metoxi-
etanol (2-ME), al 2-etoxietanol (2-EE) y a sus acetatos
(2-MEA y 2-EEA) en concentraciones comparables a las
industriales como consecuencia del empleo de productos
comerciales y de consumo. En cambio, la exposición por
los alimentos, el agua o el aire ambiente es probablemente
insignificante. Esta impresión se basa únicamente en las
propiedades físicas y químicas de estos compuestos y en
los indicios de que experimentan una rápida degradación en
el medio ambiente.
Tanto por inhalación como por absorción cutánea puede
producirse una exposición profesional importante. Las
escasas determinaciones de las concentraciones en la
atmósfera de los lugares de trabajo han dado valores
comprendidos entre menos de 0,1 mg/m3 y más de 150 mg por
m3. Sin embargo, las posibilidades de vigilancia son muy
limitadas y cabe la posibilidad de que haya grandes vari-
aciones entre diferentes industrias e incluso dentro de
una misma industria. Habida cuenta de las posibilidades de
absorción cutánea, la vigilancia del aire por sí sola
puede dar una subestimación de la exposición total. La
vigilancia biológica es el mejor método para calcular la
absorción total. Entre los trabajos que entrañan una expo-
sición considerable figuran, por ejemplo, los de pintura,
imprenta y limpieza; ahora bien, no hay que olvidar que
estos compuestos se utilizan también en otras muchas acti-
vidades profesionales en las que la exposición debe ser
motivo de inquietud.
1.2 Efectos en la salud
Los principales motivos de inquietud en el ser humano
son los efectos en el desarrollo, los efectos testiculares
y los vinculados a la toxicidad hematológica. Estos
efectos han sido demostrados por una multitud de datos
sólidos obtenidos en el animal y por algunos datos de
origen humano. Todos ellos pueden estar causados por una
exposición a corto o a largo plazo. En los animales de
experimentación, la exposición muy repetida al 2-ME y al
2-EE (más de 939 y 1450 mg/m3, respectivamente) produce
efectos tóxicos neuroconductuales, hepáticos y renales,
los cuales se observan también en casos de intoxicación
humana.
Estos cuatro éteres glicólicos dan valores muy simi-
lares de toxicidad testicular y de toxicidad para el
desarrollo en todas las especies estudiadas y por todas
las vías de exposición que se han ensayado (inhalatoria,
dérmica y oral). En los estudios sobre el mecanismo de
acción se ha visto que la metabolización al derivado del
ácido alcoxiacético constituye una etapa indispensable de
activación, tanto en los efectos sobre el desarrollo como
en los testiculares. Dicho metabolismo se efectúa mediante
el sistema de deshidrogenasa alcohólica que es común al
hombre y a los animales de laboratorio. Los metabolitos
tóxicos, el ácido metoxiacético (MAA) y el ácido etoxi-
acético (EAA), aparecen en la orina de las personas ex-
puestas a estos solventes. La coherencia de las respuestas
en las distintas especies de animales de laboratorio estu-
diadas, junto con la semejanza del metabolismo en el ser
humano, permiten concluir que el hombre está probablemente
expuesto a los efectos testiculares y sobre el desarrollo
de estos éteres glicólicos. Los datos disponibles sobre
la excreción de ácidos alcoxiacéticos por el hombre
sugieren la existencia de una retención prolongada en com-
paración con la que se observa en los animales de labora-
torio, lo cual hace pensar que las personas quizás sean
más sensibles que las especies experimentales de mayor
sensibilidad. Un motivo de especial inquietud es la rápida
absorción cutánea de estos compuestos. Se han observado
efectos teratógenos y otros efectos sobre el desarrollo
tras la aplicación de 2-ME, 2-EE y 2-EEA en la piel
intacta de la rata.
En la rata, el ratón y el conejo se han observado
alteraciones testiculares tras la exposición a estos
éteres glicólicos, tanto por inhalación como por vía oral.
Una exposición aislada de la rata a la inhalación de 1944
mg de 2-ME/m3 o más durante 4 h y la exposición repetida
a 933 mg de 2-ME/m3 o más durante 13 semanas han provo-
cado signos histológicos evidentes de alteración testi-
cular. El NENO para la exposición aguda fue de 933 mg/m3
y para la exposición repetida de 311 mg/m3. El ratón
parece ser menos sensible, siendo el NENO para la exposi-
ción repetida de 933 mg/m3. En cambio, el conejo resulta
más sensible, observándose en él alteraciones testiculares
marcadas tras la exposición repetida a 311 mg/m3 y un
efecto marginal (uno en cinco conejos afectados) a 93 mg
por m3. Efectos análogos se han observado tras la exposi-
ción oral de la rata al 2-ME, con aparición de lesiones
testiculares tras la exposición breve (inclusive de dosis
única) a 100 mg/kg. En un estu-dio subagudo (11 días) el
NENO fue de 50 mg/kg. El 2-EE es algo menos potente
respecto a la toxicidad testicular que el 2-ME; sólo se
observaron efectos con dosificaciones de 500 mg/kg o más,
siendo el NENO de 250 mg/kg.
Los datos obtenidos en las personas profesionalmente
expuestas al 2-ME y al 2-EE coinciden con los de los estu-
dios en animales e indican que estos éteres glicólicos
pueden producir toxicidad testicular en el ser humano.
Los estudios epidemiológicos de pequeños grupos de traba-
jadores expuestos al 2-EE en una empresa de fundición de
metales y de pintores de embarcaciones expuestos tanto al
2-ME como al 2-EE muestran indefectiblemente una mayor
incidencia de recuentos reducidos de espermatozoides. Los
datos sobre los niveles de exposición, aunque limitados,
aportan en cada caso pruebas de la exposición dérmica así
como de la exposición por inhalación.
En la rata, el ratón, el conejo y el mono se han
observado efectos tóxicos sobre el desarrollo tras la
exposición a estos éteres glicólicos por vía dérmica, oral
o inhalatoria. Con 12 aplicaciones diarias de 2-ME sin
diluir en la piel rasurada de ratas gestantes (oclusión:
6 h) se obtuvo un efecto letal, mientras que 10 aplica-
ciones abiertas de 2-EE (1,0 ml/día) o 2-EEA (1,4 ml/día)
se mostraron teratógenas pero no tóxicas para la madre.
Doce aplicaciones cerradas de 2-ME al 10% en suero salino
resultaron tóxicas para el desarrollo (en este estudio el
NENO fue del 3% para el 2-ME. No se han encontrado niveles
sin efecto aparente tras la administración oral repetida
de 2-ME a las hembras preñadas. El nivel de efecto obser-
vado mínimo (NEOM) en la administración oral de 2-ME fue
de 31,25 mg/kg por día en el caso de los ratones, de 25 mg
por kg por día en el de las ratas y de 0,16 mmol/kg por
día en el de los monos. Sólo se han hecho experimentos de
dosis única con el 2-ME en los ratones, con el resultado
de que el 11° día de la gestación (que es el día más sen-
sible) el NENO era de 100 mg/kg y el NEOM de 175 mg/kg.
Tanto el 2-EE como el 2-EEA se han evaluado en ratas y
conejos por inhalación. A las ratas se las expuso al 2-EE
en dos estudios, en los que se obtuvieron efectos terató-
genos (743 mg/m3 durante 7 h/día los días 1-19 de la ges-
tación) o efectos fetotóxicos (184 y 920 mg/m3 durante 6 h
por día los días 6-15 de la gestación). En este último
estudio, el NENO fue de 37 mg/m3. También los conejos
expuestos al 2-EE presentaron efectos teratógenos (589 mg
por m3 durante 7 h/día los días 1-18 de la gestación) o
efectos fetotóxicos (644 mg/m3 durante 6 h/día los días
6-18 de la gestación). En el último estudio, el NENO fue
de 184 mg/m3. En los conejos expuestos al 2-EEA durante
6 h/día los días 6-18 de la gestación se obtuvieron efec-
tos teratógenos a 2160 mg/m3 en un estudio y a 1620 mg
por m3 en otro. En ambos estudios se observó fetotoxici-
dad a 540 mg/m3 y en uno y otro el nivel de exposición
mínimo (135 y 270 mg/m3, respectivamente) coincidía con
el NENO. Las ratas expuestas al 2-EEA por inhalación dur-
ante 6 h/día los días 6-15 de la gestación presentaron el
mismo tipo de expuesta: efectos teratógenos a 1620 mg por
m3, fetotoxicidad a 1080 mg/m3 y ausencia de todo ef-
ecto a 270 mg/m3.
Así, pues, se ha observado toxicidad para el desar-
rollo en todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más. Para las tres espe-
cies, el NENO fue de 31 mg/m3. En ratas expuestas in
utero a 78 mg/m3 se han observado alteraciones conduc-
tuales y neuroquímicas, no habiéndose señalado ningún
NENO.
El 2-EE y el 2-EEA han resultado algo menos potentes.
En la rata y el conejo, todas las exposiciones al 2-EE a
368 mg/m3 o más fueron seguidas de efectos sobre el des-
arrollo. En las ratas expuestas a 184 mg de 2-EE/m3 estos ef-
ectos eran leves, pero 37 mg/m3 constituía un NENO patente.
En el caso del 2-EEA, el NENO era de 170 mg/m3 tanto en
la rata como en el conejo.
Tanto en los animales como en los casos de envenena-
miento humano se han observado efectos hematológicos tras
la exposición a una dosis aguda única. La exposición por
inhalación repetida de la especie más sensible, el conejo,
al 2-ME durante 13 semanas y a razón de cinco veces por
semana dio un NEI de 93 mg/m3. También las dosis repeti-
das de 2-ME provocan toxicidad hematológica en los
ratones, conejos, perros, hámsters y cobayos. El 2-EE es
menos potente que el 2-ME como causa de efectos hemato-
lógicos. El NENO correspondiente a estos efectos fue de
368 mg/m3 en las ratas y los conejos expuestos a 2-EE
durante 13 semanas a razón de cinco veces por semana
durante 6 h/día. También en los perros y los ratones se
han registrado efectos hematológicos tras la exposición
repetida a concentraciones más elevadas de 2-EE. La
exposición a los ésteres acéticos del 2-EE y del 2-ME
quizá provoque efectos análogos a los mismos niveles de
exposición, pero los datos disponibles sobre exposición y
efectos hematológicos de esos compuestos son demasiado
escasos para determinar en qué condiciones una exposición
humana aislada tendrá efectos hematológicos.
Se han observado niveles de exposición industrial
próximos o idénticos al NENO de efectos hematológicos en
animales expuestos a dosis repetidas de 2-ME o de 2-EE.
Este hecho, junto con la mayor sensibilidad que probable-
mente tienen las personas y la acumulación previsible de
metabolitos en la sangre humana, hace pensar que tanto la
exposición industrial como la de los consumidores pueden
tener efectos hematológicos. Esto ha sido confirmado por
la observación de efectos de este tipo en algunos de los
escasos estudios realizados sobre trabajadores industri-
ales expuestos repetidamente al 2-EE, al 2-ME o a ambos
compuestos a la vez.
2. Evaluación de los efectos sobre el medio ambiente
La exposición ambiental a estos éteres glicólicos
puede producirse como consecuencia de su paso directo a la
atmósfera cuando se utilizan como solventes volátiles.
También pueden ser causa de exposición ambiental los ver-
tidos en el suelo y el agua a consecuencia de escapes ac-
cidentales. La acumulación en el suelo y en las aguas sup-
erficiales sólo podría producirse en ausencia de degrada-
ción. Sin embargo, estos éteres glicólicos se degradan
rápidamente a causa de procesos químicos y biológicos, por
lo que no es probable que se acumulen. Tanto el 2-MEA como
el 2-EEA pueden hidrolizarse fácilmente y, por consigui-
ente, biodegradarse en condiciones aerobias. En cambio,
la contaminación de acuíferos y suelos anaerobios sigue
planteando un problema potencial, aunque es de esperar que
no pase de ser una situación transitoria y, por ende, poco
peligrosa.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
con los microorganismos y las especies acuáticas. Los
acetatos de éteres glicólicos, en cambio, tienen una toxi-
cidad aguda mucho mayor. No se dispone de datos para cal-
cular las posibilidades de efectos adversos en especies
del medio ambiente a causa de la exposición prolongada.
RECOMENDACIONES
1. Protección de la salud
1. Hay que identificar otros solventes menos tóxicos que
puedan sustituir al 2-metoxietanol, al 2-etoxietanol y a
sus ésteres, especialmente en los productos destinados al
consumo. También es particularme importante evaluar los
efectos de otros éteres etilenglicólicos, ya que algunos
pueden tener efectos análogos a los de los cuatro éteres
glicólicos que aquí se evalúan.
2. Habida cuenta de los conocidos efectos tóxicos de
estos éteres glicólicos, las autoridades deben ocuparse
seriamente de establecer estrategias apropiadas para
informar a los usuarios de esos productos acerca de los
riesgos que entrañan, especialmente los resultantes de la
exposición dérmica.
3. En vista de los nuevos datos toxicológicos y de las
posibilidades de absorción dérmica importante de estos
éteres glicólicos, habrá que reconsiderar los límites de
exposición profesional fijados por los países a fin de que
la dosis diaria total que reciben los trabajadores por
todas las vías de administración no plantee un riesgo
indebido para la salud.
4. En los animales se observan efectos de dosis única en
niveles de exposición bastante altos. Con objeto de
reducir los riesgos para la salud, se recomienda utilizar
con prudencia estos compuestos (prestando atención a la
higiene personal, los dispositivos apropiados de protec-
ción y la ventilación adecuada). Los datos disponibles
indican que puede ser necesario aumentar la protección a
fin de evitar efectos en el desarrollo, así como en la
sangre y los testículos como resultando de la exposición
repetida.
2. Investigaciones necesarias
1. En vista de la intervención del ácido metoxiacético
(MAA) y etoxiacético (EAA) (que son los principales meta-
bolitos identificados del 2-ME, del 2-EE y de sus ésteres)
en la toxicidad para el sistema reproductor masculino,
habrá que investigar su mecanismo de acción. Si de ello
se deduce que el MAA y el EAA no son los principales agen-
tes responsables, habrá que tratar de identificar éstos y
de aclarar su mecanismo de acción.
2. Estos cuatro éteres glicólicos tienen a la vez efectos
hematológicos y efectos sobre el sitema reproductor mascu-
lino (reducción del recuento de espermatozoides). Los
datos disponibles, aunque limitados, parecen indicar que
ambos efectos se hacen evidentes a niveles de dosificación
análogos. Habrá que investigar el mecanismo de acción en
ambos sistemas orgánicos y examinar paralelamente los
efectos hematológicos y los recuentos de espermatozoides
con el fin de determinar si las alteraciones de la sangre
proporcionan signos de alarma con repecto a otros efectos
de estos compuestos.
3. La vigilancia del aire no basta por sí sola para
garantizar una exposición de poca intensidad. La vigilan-
cia biológica puede contribuir a detectar defectos de las
medidas de protección. De momento no se ha establecido
claramente la relación existente entre los indicadores
biológicos de exposición, la absorción corporal total y
los efectos observados en la salud. Habrá que proseguir
las investigaciones a fin de adquirir una base para apli-
car la vigilancia biológica a la determinación de la
seguridad en las exposiciones.
4. Hay que proyectar estudios epidemiológicos y/o traba-
jos específicos de vigilancia sanitaria en poblaciones muy
expuestas a estos éteres glicólicos a fin de estimar las
relaciones exposición-efecto con miras a determinar expo-
siciones seguras, siempre que se pueda evaluar adecuada y
suficientemente la exposición total mediante la vigilancia
ambiental y biológica.
5. Habrá que investigar la posibilidad de que estos com-
puestos ejerzan efectos sobre las gónadas femeninas medi-
ante estudios de reproducción multigeneracional en ani-
males.
6. Los datos disponibles indican que el ser humano puede
metabolizar estos éteres glicólicos hasta los correspon-
dientes ácidos alcoxiacéticos en mayor medida que la rata,
y que la vida media de la excreción urinaria de estos
metabolitos tóxicos es aproximadamente cuatro veces más
prolongada en las personas que en las ratas. Por otra
parte, éstas conjugan una mayor cantidad de los metaboli-
tos ácidos, cosa que no hacen las personas. Estas diferen-
cias podrían explicar la sensibilidad relativamente elev-
ada del ser humano a estos éteres glicólicos. Un conocimi-
ento detallado del metabolismo y de la cinética de excre-
ción mejoraría nuestra capacidad para predecir cuáles son
los niveles de exposición seguros.
7. Los resultados obtenidos en estudios a corto plazo (13
semanas) indican efectos en diversos sistemas orgánicos.
En cambio, no se han hecho estudios de suficiente duración
que permitan evaluar la reversibilidad de tales efectos.
Por consiguiente, convendría que se emprendieran estudios
de interrupción en los que los animales de experimentación
estuvieran expuestos a estos éteres glicólicos al menos
durante 13 semanas, seguidas de un periodo apropiado de
recuperación. Cabría evaluar así importantes parámetros
fisiológicos con miras a determinar si esos efectos son o
no transitorios.
When rats were administered 2-EE at doses from
0.5 mg/kg to 100 mg/kg, Groeseneken et al. (1988) found
increasing relative amounts of EAA in urine (from 13.4% to
36.8% of the total dose). This could be the result of
competition by other metabolic pathways, which would
become more easily saturated at the higher dosage levels.
At 2-EE doses equivalent to the lowest doses in these
animal studies, it was estimated that humans excrete 30-
35% as EAA. Furthermore, 27% (on average) of the EAA was
excreted as the glycine conjugate in the rat, whereas no
glycine conjugation was observed in humans.
The in vitro nasal mucosal carboxylesterase activity
of mice was compared to the activity of other mice tissues
and to the nasal mucosal carboxylesterase activity of
rats, rabbits, or dogs when exposed to 2-MEA or 2-EEA
(Stott & McKenna, 1985). The specific activity in nasal
carboxylase was found to be similar to that of the liver
in mice, but it was greater than the activity found in the
kidney, lung, or blood of mice. Nasal mucosal carboxyl-
esterase activity of mice was comparable to that of dogs,
slightly higher than the activity in rats, and nearly six-
fold higher than the activity in rabbits. These in vitro
studies suggest that considerable hydrolysis may occur in
the intact animal, resulting in the formation of acetic
acid at the initial route of entry.
6.4. Elimination and Excretion
Although 2-ME is rapidly metabolized to 2-MAA after an
intraperitoneal dose of 250 mg/kg body weight in the rat,
the excretion of 2-MAA is fairly slow (half-life of ap-
proximately 20 h) (Moss et al., 1985). In humans, an elim-
ination half-life for 2-MAA of 77.1 h has been reported
(Groeseneken et al., 1989a). The elimination of radioac-
tive 2-EAA (ethyl 1,214C) has been reported in the rat
(half-life of approximately 8 h) (Guest et al., 1984) and
in humans (half-life of approximately 21-42 h)
(Groeseneken, 1986b,c, 1988; Veulemans, 1987a). In rats,
the administration of an oral dose of 230 mg 2-EE/kg body
weight led to the production of EAA and N -ethoxyacety gly-
cine (> 76% of the dose), EAA being the major metabolite
found in testes after 2 h (Cheever et al., 1984).
The urinary excretion of EAA during and after single
4-h exposures to 14, 28, or 50 mg 2-EEA/m3 in human
volunteers has been reported by Groeseneken et al. (1987).
The distribution/excretion time course during and after
2-EEA exposure was similar to that observed for 2-EE.
This indicates that humans, like rodents, hydrolyse the
acetate to 2-EE, which is then converted to the EAA metab-
olite and excreted. The excretion of the EAA metabolite
was observed to be biphasic. A second peak of excretion
occurred approximately 3 h after the first, suggesting
some type of redistribution of the glycol ethers, or of a
metabolite, from a peripheral compartment.
The urinary excretion of EAA was evaluated under field
conditions in which women volunteers were exposed daily to
2-EE or 2-EEA in the process of silk-screen printing
(Veulemans et al., 1987a). Urinary EAA was measured during
5 days of normal production and was also detectable after
a 12-day stop in production. The excretion of EAA in-
creased during the work week, yet it was still detectable
after 12 days without exposure. These data suggest that
the retention of EAA, or of other 2-EE or 2-EEA metab-
olite, may be toxicologically significant if EAA is the
active metabolite responsible for the observed toxicity.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Given the physical and chemical properties of these
four glycol ethers and the known rates of degradation in
the environment (section 4), there is minimal concern that
hazardous levels of these substances will occur. Although
only a few studies have been reported, the available data
support this conclusion. For example, both 2-ME and 2-EE
have been tested for effects on microorganisms and aquatic
animals. The lethal concentration of 2-ME and 2-EE to
microorganisms (Cladosporium resinae, Pseudomonas aeru-
ginosa, Gliomastix sp, and Candida sp is > 2% in the
medium (Neihof & Bailey, 1978; Lee & Wong, 1979). The
exposure of C. resinae lasted 30 to 42 days, whereas the
other organisms were exposed for 4 months. Very low
toxicity was shown by 2-ME to the green alga Scenedesmus
quadricauda (growth inhibition only at > 10 g/litre) and
the cyano-bacterium (blue-green alga) Microcystis aerugin-
osa (growth inhibition only at > 100 mg/litre) (Bringmann
& Kuhn, 1978). 2-EE has very low toxicity to the brine
shrimp (Artemia salina) (LC50 > 4 g/litre) (Price et
al., 1974) and to another arthropod Daphnia magna
(Hermens et al., 1984). The toxicity of 2-EE to fresh-
water fish is also very low, the LC50 (96 h) for the
bluegill (Lepomis macrochirus) being > 10 g/litre. However,
2-EEA is far more toxic to fish in this assay, LC50 (96 h)
values of 60 mg/litre (Bailey et al., 1985) and 46 mg per
litre in fathead minnows (Pimephales promelas) (Purdy,
1987) having been reported. These results confirm those of
Juhnke & Ludemann (1978), who reported an LC50 (48 h)
value of 107-141 mg per litre when using the Golden Orfe
(Leuciscus idus melanotus) test. The reason for this low
LC50 has not been studied, but Dawson et al. (1977) re-
ported similarly low LC50 values for 2-MEA in fish (45 mg
per litre in tidewater silverfish (Menidia beryllina)
and bluegill (Lepomis chirus).
Effects on other organisms have not been reported.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data indicating the acute oral, dermal, intraperito-
neal, and inhalation toxicities of 2-ME and 2-EE are given
in Table 7. As shown, these two glycol ethers have similar
toxicities, and are of low acute toxicity whether animals
are exposed via the dermal, oral, or respiratory routes.
Even after intraperitoneal injection, the reported levels
of acute toxicity are still low (1.7-2.46 g per kg body
weight).
Dyspnoea, somnolence, ataxia, and prostration were re-
ported after near lethal doses of 2-EE were given to rats,
mice, guinea-pigs, or rabbits (Stenger et al., 1971).
Haemoglobinuria and/or haematuria were reported after a
single oral administration of 2-EE with a dose approaching
the LD50 (Laug et al., 1939). Microscopic examination of
the kidneys of rats, mice, guinea-pigs, or rabbits given
2-EE orally revealed severe tubular degeneration, conges-
tion, and cast formation; in some animals most of the
cortical tubules were necrotic (Laug et al., 1939). No
data on the purity of the 2-EE used in these early studies
were reported.
Single 4-h inhalation exposures of rats to 1866 mg 2-ME
per m3 or more led to testicular atrophy as early as 24 h
after exposure (Doe, 1984b).
8.2. Short-term Exposures
Repeated exposure of experimental animals to 2-ME and
2-EE by various routes of administration (7-90 days) have
revealed adverse effects on the haematological and nervous
systems and on the testes, thymus, kidney, liver, and
lung. From data on the metabolic transformation and
elimination of 2-MEA and 2-EEA in animals and man (see
section 6), it is probable that the toxicities of these
glycol ether acetates are similar to those of the parent
chemicals 2-ME and 2-EE.
8.2.1. Haematological and immunological effects
Following repeated exposures to 2-ME and 2-EE using
various routes of administration, haematological effects
have been observed in several species. A summary of the
most relevant studies is given in Tables 8 (2-ME) and 9
(2-EE).
Table 7. Acute toxicity data for 2-methoxyethanol and 2-ethoxyethanol and their acetatesa
-------------------------------------------------------------------------------------------------------------
Compound Species Intra- Oral LD50 Dermal Inhalation References
peritoneal LD50 LC50
LD50
-------------------------------------------------------------------------------------------------------------
2-Methoxyethanol Rat 2460 2460-3400 Smyth et al. (1941); Goldberg et al.
(1962); Pisko & Verbilov (1988)
Mouse 2200 2167-2560 4603 Werner et al. (1943); Saparmamedov
(1480) (1974); Pisko & Verbilov (1988)
Guinea-pig 950 Karel et al. (1947)
Rabbit 890-1450 1300 Carpenter et al. (1956); Pisko &
Verbilov (1988)
2-Ethoxyethanol Rat 2000 2125-5500 Smyth et al. (1941); Laug et al.(1939)
Stenger et al. (1971); Pisko & Verbilov
(1988)
Mouse 1700 4000-4800 6698 Werner et al. (1943); Laug et al.(1939)
(1820) Stenger et al. (1971); Saparmemedov
(1974); Pisko & Verbilov (1988)
Guinea-pig 1400-2600 Karel et al. (1947); Laug et al.(1939)
Rabbit 3300- Stenger et al. (1971); Smyth et al.
15 200 (1941)
2-Methoxyethanol Rat 3930 Smyth et al. (1941)
acetate Guinea-pig 1250 Kirk-Othmer (1980)
Rabbit 5557
2-Ethoxyethanol Rat 5100 Smyth et al. (1941)
acetate Guinea-pig 1910 Kirk-Othmer (1980)
Rabbit 10 333
-------------------------------------------------------------------------------------------------------------
a Doses are given as mg/kg body weight except for inhalation doses,
which are in mg/m3 (values in parentheses are ppm)
Concerning changes in blood parameters, a comparison
of Tables 7, 8, and 9 indicates clearly that 2-ME is more
toxic than 2-EE in several species, although 2-EE has been
less widely studied. For example, Nagano et al. (1979)
showed that 2-EE administered orally for 5 weeks at 2000
mg/kg body weight per day did not result in changes in
haematological parameters other than a reduced leucocyte
count. No effects were observed at 1000 mg/kg. Under a
similar dosing regimen, 2-ME caused a dose-related re-
duction in leucocytes at 500 mg/kg per day, other haemato-
logical parameters being unaffected at 250 mg/kg per day
(Nagano et al., 1979).
Some of the doses used in the studies summarized in
Tables 7 and 8 resulted in histological and functional
changes in the bone marrow and thymus. In an inhalation
study, Miller et al. (1983b) noted thymic atrophy and de-
creased thymus weights in male and female rats exposed to
933 mg 2-ME/m3 for 13 weeks. Miller et al. (1981) also
noted decreased weights of thymus, spleen, and mesenteric
lymph nodes in rats exposed to 933 or 3110 mg 2-ME/m3 for
9 days, and reduced bone marrow cellularity was observed
at 3110 mg/m3. In one study there was at least partial
reversal of these effects 22 days after an exposure last-
ing 4 days (Grant et al., 1985).
When MAA, the metabolite of 2-ME, was administered to
rats by gavage at 300 mg/kg body weight per day for 8
days, it resulted in reduced erythrocyte counts, haemo-
globin concentrations, and packed cell volumes, and a
marked reduction in leucocyte counts. Thymus and spleen
weights were also decreased and a severe lymphoid
depletion was seen in the thymus. Similar, but less
marked, effects on blood and thymus were seen in some
animals administered 100 mg/kg per day. No treatment-
related effects were reported at 30 mg/kg per day (Miller
et al., 1982).
Studies on immunological function show that 2-ME and
2-EE yield different results. De Delbarre et al. (1980)
studied the effect of 2-ME and 2-EE on the humoral respon-
siveness to antigenic stimuli and on adjuvant arthritis in
the rat. 2-ME had an inhibitory effect on adjuvant ar-
thritis at doses greater than 18.6 mg/kg body weight per
day administered subcutaneously, whereas 2-EE was not
effective at doses up to 150 mg/kg. A dose of 150 mg 2-ME
per kg per day delayed rejection of skin grafts and 75 mg
2-ME/kg per day (for 28 days) significantly depressed
antibody production. Again, 2-EE had no effect on these
parameters. House et al. (1985) administered 2-ME to mice
by gavage (10 doses of 250, 500, or 1000 mg 2-ME/kg body
weight per day for 2 weeks) and noted a 48% reduction in
thymus weight at 500 and 1000 mg/kg. However, there was no
significant alteration in immune function or host resist-
ance. Similar findings were reported when the same dose
levels of MAA, the major metabolite of 2-ME (section 6),
were administered.
Table 8. Haematological effects of 2-methoxyethanol in animalsa
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat inhalation 10 males 6 h/day; 9 days; RBC fragility, no effect at Miller et al.
10 females 311, 933, 3110 mg/m3 3110 mg/m3 but reduced RBC, (1981)
Hb, and PCV levels and bone
marrow cellularity seen in M
and F; similar findings in F
at 933 mg/m3; reduced WBC
at 3110 and 933 mg/m3;
reduced thymus weight
Rat inhalation 10 males 6 h/day; 5 days/week only effect at 2 lowest Miller et al.
10 females for 13 weeks; doses was reduced body (1983b)
93.3, 311, 933 mg/m3 weight gain in F; at 933
mg/m3 reduced WBC, Hb,
PCV, and platelets in both
sexes after 4 and 12 weeks;
reduced serum proteins at 13
weeks, thymic atrophy
Rabbit inhalation 5 males 6 h/day; 5 days/week decreased thymus size and Miller et al.
5 females for 13 weeks; body weight, PCV, WBC, (1983b)
93.3, 311, 933 mg/m3 platelets and Hb at 933
mg/m3; slight to moderate
decrease in lymphoid tissue
at 311 mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week decreased RBC, Hb and Hct; Werner et al.
2 controls for 12 weeks; increased immature (1943)
750 mg/m3 granulocytes
Mouse oral 5 males 5 times/week for 4/5 mice at 2000 mg/kg died Nagano et al.
5 weeks; 62.5, 125, before completion; doses at (1979)
250, 500, 1000, 2000 or above 500 mg/kg resulted
mg/kg body weight/day in reduced WBC, RBC, and PCV
Table 8 (contd.)
--------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Effect References
time; level
--------------------------------------------------------------------------------------------------------
Rat oral 24 males once per day for reduced thymus and spleen Grant et al.
4 days; 100 and 500 weight at 500 mg/kg on days (1985)
mg/kg body weight/day; 1-8, recovery by day 22;
sacrificed reduced WBC at both 100 and
500 mg/kg, largely
reversible by day 22
Hamster oral 4 males once daily, 5 days/week decrease in WBC at 500 mg/kg Nagano et al.
for 5 weeks; 62.5, 125, the only effect on blood (1984)
500 mg/kg body weight/day
Guinea oral 3 males once daily, 5 days/week about 50% decrease in WBC at Nagano et al.
-pig for 5 weeks; 250 and 500 both doses (1984)
mg/kg body weight/day
--------------------------------------------------------------------------------------------------------
a M = male; F = female; PCV = packed cell volume; Hct = haematocrit; RBC = red blood cell count;
WBC = white blood cell count; Hb = haemoglobin; No. = number of animals per group
Table 9. Haematological effects of 2-ethoxyethanol in animalsa
-------------------------------------------------------------------------------------------------------
Species Route No. Dose frequency; Response Reference
time; level
-------------------------------------------------------------------------------------------------------
Rat inhalation 15 males 6 h/day; 5 days/week decreased leucocyte count Barbee et al.
15 females for 13 weeks; 92, 368, in females at 1472 mg/m3, (1984)
1472 mg/m3 no other significant
biological effect reported
Rabbit inhalation 10 males 6 h/day; 5 days/week for decreased Hb, Hct, and Barbee et al.
10 females 13 weeks; 92, 368, 1472 RBC in both males and (1984)
mg/m3 females only at 1472
mg/m3
Dog inhalation 2 treated 7 h/day; 5 days/week for slight reduction in RBC, Werner et al.
2 control 12 weeks; 3091 mg/m3 Hb, and PCV, microcytosis, (1943)
hypochromia, and poly-
chromatophilia were seen,
marked increase in
immature granulocytes
Mouse oral 5 males 5 times/week for 5 lower WBC at 2000 mg/kg, Nagano et al.
weeks; 62.5, 125, 250, but no effect on (1979)
500, 3110, 2000 mg/kg erythrocyte parameters
body weight/day
-------------------------------------------------------------------------------------------------------
a PCV = packed cell volume; Hct = haematocrit;
RBC = red blood cell count; WBC = white blood cell count; Hb = haemoglobin
No. = number of animals per group
8.2.2 Effects on liver and kidney
Effects on the liver, such as reduced cytoplasmic den-
sity, disruption of lobular structure, elevated plasma
fibrinogen, reduced serum proteins, and elevated liver
weights, have been reported in certain studies in which
rats, mice, or rabbits were exposed to 2-ME or 2-EE at
levels in excess of 300 ppm (933 and 1104 mg/m3, respect-
ively), for periods of up to 13 weeks. Many of the effects
observed were reversible, and no consistent pattern was
noted among the various studies (Miller et al., 1981;
1983b; Stenger et al., 1971). Hepatic changes have been
observed at inhalation exposures to 2-ME and 2-EE in ex-
cess of 300 ppm (933 and 1104 mg/m3, respectively).
No pathological changes could be detected in the kid-
neys of rats exposed to approximately 933 mg 2-ME/m3 for
6 or 7 h per day, 5 days a week, for 13 weeks (Miller et
al., 1981, 1983b). In studies with 2-EE, no treatment-
related pathology was reported after inhalation exposure
of rats to 1362 mg/m3 (370 ppm) for 5 weeks or dogs to
3091 mg/m3 (840 ppm) for 12 weeks (Werner et al., 1943).
8.2.3 Behavioural and neurological effects
There have been few reports on the effects of 2-ME
and 2-EE on the function of the nervous system in animals.
Ataxia was reported after inhalation exposure of rats to
18.96 g 2-EE/m3 (5152 ppm) for 8h. After exposure of rats
to levels of 2-ME between 1555 and 12 440 mg/m3, 4 h per
day for up to 7 days, inhibition of an avoidance-escape
conditioned response was observed without any alteration
of motor function (Goldberg et al., 1962). The same
authors reported also a significant inhibition of this
response after 14 days exposure to 1210-4836 mg 2-ME per
m3 (389-1555 ppm). After 3 weeks recovery, rats whose
avoidance response had been inhibited on the 14th day
showed a return to normal. Savolainen (1980) reported a
partial loss of motor function in the hind limbs of rats
after exposure to 1244 mg 2-ME/m3 (400 ppm) for 6 h/day,
5 days per week, for 2 weeks. This hindlimb paralysis
coincided with the glial cell toxicity noted during the
second week of exposure. Recovery was incomplete after 2
weeks post-exposure, the animals receiving the highest
dose showing minor paresis.
8.3 Skin and Eye Irritation; Sensitization
No satisfactory data on the sensitization potential of
2-ME and 2-EE in animals, and only limited data on their
irritant properties to eyes, have been reported. Weil &
Scala (1971) found 2-ME to be an eye irritant. Lailler et
al. (1975) reported that 0.1 ml of undiluted 2-ME led to
corneal and conjunctival oedema and increased vascular
leakage in the conjunctiva and aqueous humour. A 25% aque-
ous solution was much less active.
8.4 Long-term Exposures
No adequate long-term animal studies on 2-ME and 2-EE
or their acetates have been reported to date.
8.5 Effects on Reproduction and Fetal Development
The effects of glycol ethers, particularly 2-ME, on
animal reproduction, fertility, and teratogenicity have
been extensively studied. Some of the early studies on
reproduction have been reviewed by Hardin (1983). The many
studies conducted in several countries and in several
animal species are in general agreement on the nature of
developmental and reproductive effects.
8.5.1 Effects on the male reproductive system
8.5.1.1 Oral exposure
The pathological changes observed in the testes of
rats after the administration of 2-ME have been well
characterized by Foster et al. (1983). The severe degener-
ative changes noted in the germinal epithelium of the
seminiferous tubules of different laboratory animals were
similar, irrespective of species or route of adminis-
tration. Daily doses of 2-ME were administered orally to
rats (50, 100, 250, or 500 mg/kg) for periods between 1
and 11 days, and 250, 500, or 1000 mg 2-EE/kg body weight
per day was administered in a similar regimen (Foster et
al., 1983; Creasy & Foster, 1984; Creasy et al., 1985).
After 2-ME administration, testicular damage was observed
1 day post dosing with 100 mg/kg or more, the lesion being
localized in the late primary spermatocytes. Continuous
dosing with 2-ME resulted not only in progressive deletion
of the primary spermatocytes but also in degenerative
changes in secondary spermatocytes and dividing sper-
matids. Changes in sperm motility, morphology, and concen-
tration were also reported. The cessation in maturation
of early primary spermatocytes led to a depletion of the
spermatid population, resulting in tubules containing only
Sertoli cells, spermatogonia, and early primary spermato-
cytes. Decreased testicular weight was reported at 250 mg
per kg or more. Although most of the effects seen after a
4-day treatment with 2-ME were reversible within 8 weeks,
a small proportion of tubules showed incomplete recovery
indicating a non-reversible, long-term effect. Similar
findings were reported after 2-EE administration, but only
after 11 days of dosing with 500 and 1000 mg/kg. No
effects on the testes were observed following oral admin-
istration of 250 mg 2-EE/kg per day or 50 mg 2-ME/kg per
day.
Subsequent work by Chapin et al. (1985a,b) supports
the hypothesis that 2-ME administered to male rats at
doses of 100 or 200 mg/kg daily for 5 days affects the
spermatogonia. In these studies male rats were given doses
of 0, 50, 100, or 200 mg/kg per day for 5 days, and recov-
ery was investigated by evaluating the testicular damage
in sacrificed animals at 8 subsequent weekly intervals or
alternatively by investigating fertility through mating
with two females per week for 8 weeks. Treatment with 100
or 200 mg/kg resulted in widespread testicular damage and
cell death immediately after treatment, with only very
mild effects being noted at 50 mg/kg (this was thus a
marginal-effect level rather than a no-effect level).
There was some evidence of recovery from testicular damage
towards the end of the study period, but reduced fertility
was still apparent weeks after the cessation of treatment
with 200 mg/kg. Thus, the recovery was neither as
complete nor as rapid as that noted by Foster et al.
(1983).
The in vivo and in vitro effects of 2-ME and MAA ex-
posures, respectively, were compared by evaluating effects
using electron microscopy (Creasy et al., 1986). 2-ME
caused cell death in the pachytene spermatocytes in vivo ,
as well as focal breakdown of the plasma membranes be-
tween spermatocytes and Sertoli cells. Similar, but less
frequent, breaks were seen in vitro when mixed cultures of
Sertoli and germ cells were treated with MAA.
Subsequent studies by Foster et al. (1987), in which
the alkoxyacetic acids of 2-ME and 2-EE (MAA and EAA,
respectively) were given orally in doses equimolar to the
studies described earlier (Foster et al., 1983), yielded
similar patterns of testicular degeneration and effects on
spermatocyte development. The similarity of effects at
equimolar concentrations between the two acetic acid de-
rivatives and their respective glycol ethers suggests that
these metabolites may either be the causative factors or
at least play a role in the production of the observed
degenerative effects.
The assessment of the effects of low doses of poten-
tial toxicants on sperm fertility and production is diffi-
cult because of the large amount of sperm usually produced
by most test species and the large sperm reserves. Using
an experimental design that required bi-daily matings of
Long-Evans male rats, the effects of orally administered
2-EE (0, 150, or 300 mg/kg body weight per day; 5 days per
week for 6 weeks) on sperm reserves and on pachytene sper-
matocytes was evaluated (Hurtt & Zenick, 1986). Further
groups of rats received the same doses of 2-EE, but were
not subjected to repeated mating. After 6 weeks of treat-
ment, the animals were sacrificed and the effect of 2-EE
administration on organ weight, testicular spermatid
count, cauda epididymal sperm count, and sperm morphology
was determined. Exposures to 2-EE resulted in significant
decreases in testicular weight, spermatid count, and epi-
didymal sperm count for both mated and unmated animals at
the highest dose level. However, the effects were also
seen at 150 mg/kg per day in repeatedly mated animals.
Adult male rats treated orally with 2-EE (936 mg/kg
body weight, 5 days/week for 6 weeks) were found to have
decreased sperm counts and altered sperm morphologies
after 5 and 6 weeks of exposure when compared with
vehicle-control animals, and sperm motility was decreased
at week 6 (Oudiz & Zenick, 1986). These data indicate that
the pachytene spermatocyte is the target cell most sensi-
tive to the effects of 2-EE.
The reproductive toxicity of 2-EE in CD-1 mice has
been evaluated in a continuous breeding protocol (Lamb et
al., 1984). Male and female mice (20 males and 20 females
per dose group) were given access to drinking-water con-
taining 0.5, 1.0, or 2.0% 2-EE and housed as breeding
pairs continuously for 14 weeks. Treated animals were then
paired with controls for breeding. At 1 and 2% 2-EE, sig-
nificant adverse effects on fertility were seen, the re-
productive capacity of both males and females being af-
fected. Testicular atrophy, decreased sperm motility, and
increased abnormal sperm levels were seen in treated
males, but no treatment-related pathological effects were
seen in females even though reduced fertility was noted in
the cross-mating phase.
The reproductive toxicity of a single oral dose of
2-ME (0, 500, 750, 1000, or 1500 mg/kg) was assessed in
adult male CD rats and CD-1 mice by Anderson et al.
(1987). Animals from each group were sacrificed at weekly
intervals during weeks 3-8 post exposure and evaluated for
sperm counts, sperm morphology, and testicular histology.
A dose-related toxicological response in spermatocytes was
observed in both species. In a companion study, male rats
and mice were exposed to 2-ME (0, 125, 250, or 500 mg/kg)
and permitted to mate during weeks 1-10 post exposure.
Following mating, pregnant females were sacrificed on day
17 of pregnancy and each uterus was evaluated for dominant
lethal effects. The only effect noted was a decrease in
total number of implants at week 6 following treatment
with 500 mg/kg. In a study in which both rats and mice
were given single doses at 0, 500, 750, 1000, or 1500 mg
per kg and mated 5 and 6 weeks later, dominant lethal
studies showed a dose-related decrease in fertility in
rats, with complete sterility in all but the lowest dose
group after 6 weeks. No effects on the reproductive
capacity of mice were noted. There was no statistically
significant evidence for the induction of dominant lethal
mutations or abnormalities in the F1 generation of either
species.
8.5.1.2 Inhalation studies
Testicular damage has been reported in rats exposed to
2-ME by inhalation for a single 4-h period (Doe, 1984b).
Exposure to 1944 mg/m3 resulted in histological evidence
of damage to the maturing spermatids, testicular atrophy
occurring at 3887 mg/m3 or more. The NOEL was 933 mg/m3.
The effect of repeated exposure to 2-ME has also been
investigated using the inhalation route. Exposure of rats
to 3110 mg/m3 (6 h/day) for 9 out of 11 days resulted in
degenerative changes and necrosis in the germinal epi-
thelium of the seminiferous tubules, but no effects on the
testes were observed with a dose of 933 mg/m3. However,
Doe et al. (1983) reported pronounced atrophy of the sem-
iniferous tubules in rats exposed to 933 mg 2-ME/m3 or
more (6 h/day, 5 days/week) for 13 weeks. The NOEL was
311 mg/m3 (Miller et al., 1983b). Studies were also car-
ried out in rabbits, using exposure levels of 93.3, 311,
and 933 mg/m3 (6 h/day, 5 days/week), and most animals
exposed to 311 mg/m3 showed reduced testis size and
severe degenerative changes in the tubules. Reduced testis
weight was also seen in two out of five animals exposed to
93.3 mg/m3, and one animal showed histological changes
in the germinal epithelium. A NOEL could not be ident-
ified, but 93.3 mg/m3 was near the minimal effective dose
in rabbits.
In addition, an increased incidence of sperm abnor-
malities (principally sperm with banana shaped or amorph-
orous heads) has been noted in mice exposed to 1555 mg
2-ME/m3 (7 h per day for 5 days) but not to 78 mg per
m3 (McGregor et al., 1983).
8.5.2 Embryotoxicity and developmental effects
8.5.2.1 2-Methoxyethanol
2-ME has been shown to lead to embryotoxic and devel-
opmental effects in laboratory animals following inha-
lation, oral, or dermal exposure. The effects of 2-ME
exposure over the entire organ-forming period of gestation
have been studied in rabbits, rats, and mice, the rabbit
being the most sensitive species.
The embryotoxicity of 2-ME after gastric intubation
was evaluated in mice (31.25, 62.5, 125, 250, 500, or 1000
mg/kg body weight) on days 7-14 of gestation (Nagano et
al., 1981). No maternal deaths were observed, but maternal
weight gain was reduced in the mice that received doses of
250 mg/kg per day or more. Maternal toxicity was not seen
at 123 mg/kg or less. On day 18 of gestation, fetuses were
examined and an increase in fetal death rate was observed
at doses of 250 mg/kg or more. At doses of 500 and 1000 mg
per kg, all fetuses were dead at caesarean section, except
for one in the 500-mg/kg group. Approximately 44% (57/130)
of the live fetuses in the 250-mg/kg group were found to
have gross anomalies, including exencephaly (24), umbili-
cal hernia (3), and abnormal digits (29). One fetus had
both exencephaly and abnormal digits. The lone surviving
fetus in the 500-mg/kg group had exencephaly and abnormal
digits. Minimal skeletal malformations were also observed
at the lowest dose of 31.25 mg/kg body weight (a matern-
ally non-toxic dose). Thus, a NOEL could not be ascer-
tained.
Dose-dependent electrocardiographic changes were
detected on gestation day 20 in the rat fetuses of mothers
exposed orally to 2-ME (0, 25, or 50 mg/kg body weight) on
days 7 to 13 of gestation (Toraason et al., 1985). Cardio-
vascular malformations were observed, including right
ductus arteriosus and ventricular septal defects. QRS in-
tervals were significantly prolonged, particularly in the
highest dose group, suggesting intra-ventricular conduc-
tion delays.
Ornithine decarboxylase (ODC) activity is highest
during rapid growth and development in fetuses and is
sensitive to maternal exposure to chemicals and drugs.
Toraason et al. (1986a,b) evaluated the effect on ODC ac-
tivity in the fetuses of pregnant rats that received 25 mg
2-ME/kg by gavage during gestation days 7-13 or 13-19. The
activity was most affected in fetuses exposed during ges-
tation days 7 to 13. It was highest in 3-day old rats and
declined sharply thereafter. No functional or morphologi-
cal effects were observed in fetuses exposed at maternal
dose levels of 25 mg/kg during gestation days 7-13 or in
fetuses exposed at that level during days 13-19.
Nelson et al. (1984a) treated male rats by inhalation
(78 mg 2-ME/m3 for 7 h/day, 7 days/week for 6 weeks) and
subsequently bred these to untreated females. In addition,
groups of 15 pregnant rats were treated with the same dose
(7 h/day on gestation days 7-13 or 14-20) and allowed to
deliver and rear their young. Neuromotor function activity
and simple learning ability were assessed on days 10-90
after birth. Offspring from dams treated between gestation
days 7-13 showed significant changes in avoidance con-
ditioning, and changes in neurochemical levels were ob-
served in the brains of 21-day-old offspring from the
paternally exposed group as well as from both maternally
exposed groups.
8.5.2.2 2-Ethoxyethanol
A dose-finding study in pregnant rats revealed that no
offspring survived inhalation exposures of 3312 mg 2-EE
per m3, 7 h/day, during gestation days 7-13 or 14-20
(Nelson et al., 1981). The authors reported 34% neonatal
deaths at 736 mg/m3 under similar exposure conditions.
Offspring from dams exposed to 368 mg/m3 on gestation
days 7-13 or 14-20 showed impaired performance in behav-
ioural tests and neurochemical alterations in brain samples.
Using inhalation exposures of 478, 1435, and 2208 mg
2-EE/m3 on gestation days 7-15 (7 h/day), Nelson et al.
(1984b) reported complete resorption of all rat fetuses at
2208 mg/m3, reduced fetal weight at 1435 mg/m3, and a
NOEL of 478 mg/m3. Andrew & Hardin (1984), exposed preg-
nant rats to 733 and 2823 mg/m3 for 7 h/day throughout
gestation and observed increased fetal resorptions at 733
mg/m3 as well as skeletal and cardiovascular abnormali-
ties. At 2823 mg/m3 the resorption frequency reached 100%.
Developmental toxicity has been noted in rats after
dermal application of 2-EE (0.25 or 0.5 ml, four times per
day) (Hardin et al., 1982) or 2-EEA (0.35 ml, four times
per day) (Hardin et al., 1984) on gestation days 7-16.
Increased resorption rates and fetal deaths, decreased
viable fetus weights, and increased cardiovascular defects
and skeletal malformations were seen, even at the lowest
dose tested.
The effect of simultaneous administration of ethanol
(10% in drinking water) and 2-EE (368 mg/m3 by inha-
lation) has been investigated, in view of their related
metabolism (via the enzyme alcohol dehydrogenase) (Nelson
et al., 1982, Nelson et al., 1984c). Although the results
obtained are difficult to interpret, ethanol adminis-
tration early in gestation tended to reduce the behav-
ioural effects induced by 2-EE, whereas ethanol given late
in gestation enhanced these effects.
8.5.3 Teratogenicity
8.5.3.1 2-Methoxyethanol
The teratogenic potential of dermally administered
2-ME was estimated in pregnant rats with the Chernoff-
Kavlock screening test (Wickramaratne, 1986). Various con-
centrations (0, 3, 10, 30, or 100%) of 2-ME in physiologi-
cal saline were applied to shaved skin and occluded for
6 h of exposure on gestation days 6-17. Animals were per-
mitted to have their litters and rear the pups until 5
days postpartum, when the study was terminated. No adverse
effects were noted at the 3% dose level. Small litter
sizes and decreased fetal survival were observed at the
10% level. At 30%, lethality was observed in all fetuses,
and all pregnant females died when exposed dermally to
100% 2-ME.
The teratogenicity of 2-ME in mice, rats, and rabbits
has been evaluated by Hanley et al., (1984). Pregnant rats
and rabbits were exposed by inhalation to 0, 9, 31, or 156
mg/m3 for 6 h/day on gestation days 6-18 (rabbits) or 6-
15 (rats), and mice were exposed to 0, 31, or 156 mg per
m3 for 6 h/day on days 6-15 of gestation. No teratogenic
effects were found in CF-1 mice and Fischer-344 rats under
the conditions of this study, although slight fetotoxicity
was noted in both species. In New Zealand white rabbits
exposed to 156 mg/m3 there was a significant increase in
resorption rate and incidence of malformations involving
all organ systems, as well as a significant decrease in
mean fetal body weight when compared to controls. Of the
fetuses from dams exposed to 156 mg per m3, 63% exhibi-
ted at least one malformation and 91% of the litters had
at least one fetus with a malformation. There were no
teratogenic or fetotoxic effects in any of the three
species evaluated at 31 mg/m3.
Developmental phase-specific and dose-related terato-
genic anomalies in CD-1 mice resulting from 2-ME exposure
have been evaluated (Horton et al., 1985). 2-ME was admin-
istered by gavage to pregnant females at doses of 250 mg
per kg (during gestation days 7 to 9, 8 to 10, or 9 to 11;
during days 7 to 8, 9 to 10, or 10 to 11; or once a day on
gestation days 9, 10, 11, 12, or 13) in order to identify
the most sensitive developmental phases for the anomalies
under study. Resulting malformations were specifically re-
lated to the developmental stage at the time of exposure,
with exencephaly being observed between days 7 to 10 and
paw anomalies (syndactyly, oligodactyly, and stunted digit
number 1) during the later stages of development (days 9-
12). The dose dependency of digit malformation was studied
by administering single doses by gavage (100, 175, 250,
300, 350, 400, or 450 mg 2-ME/kg) on gestation day 11.
Dose-related paw malformations were noted in all litters,
with forepaws being more susceptible than hindpaws in
terms of the number and severity of malformations. At 175
mg/kg digit anomalies were induced without concurrent
reductions in fetal weight, while at 250 mg/kg or more
digit anomalies occurred concurrently with reduced fetal
body weight. In this study, the NOEL for the single ex-
posure (day 11) was 100 mg/kg. Although in this same
strain of mice digital malformations were not detected in
near-term fetuses given 100 mg 2-ME/kg body weight by
gavage on gestation day 11 (Greene et al., 1987), there
was a slight increase in the amount of cell death in
approximately 50% of the limb buds from embryos collected
24 h after dosing.
In a preliminary study, the teratogenic potential of
2-ME was determined in Cynomolgus monkeys using doses of
0.16, 0.32, and 0.47 mmol/kg administered by gavage
throughout the period of organogenesis (Scott et al.,
1987). Fetal death was dose-related (2/13 at 0.16 mmol
per kg; 3/10 at 0.32 mmol/kg; and 8/8 at 0.47 mmol/kg).
At the highest dose level, four fetuses were lost through
abortion and one of the remaining four, which was re-
covered by hysterotomy, demonstrated malformation (ectro-
dactyly) of the forelimbs. The MAA content of maternal
sera was followed throughout the treatment period. By the
25th day of treatment, MAA levels had more than doubled in
all treatment groups, compared to the values determined on
day one, indicating that multiple exposure to 2-ME can
lead to accumulation of the potentially embryotoxic metab-
olite MAA, the possible causative factor in the embryonic
deaths and teratogenicity observed (see section 8.8).
8.5.3.2 2-Ethoxyethanol and 2-Ethoxyethanol acetate
Andrew & Hardin (1984) have studied the teratogenic
potential of 2-EE in rats and rabbits. Rats (29-38 per
group) were exposed by inhalation to 0, 552, or 2388 mg
per m3 5 days per week for 3 weeks immediately prior to
mating and then to 0, 743, or 2823 mg/m3 for 7 h/day from
gestation day 1 to 19. Pregnant rabbits received 589 or
2271 mg/m3 for 7 h/day from gestation day 1 to 18. In
the New Zealand white rabbits exposed to 2271 mg/m3, the
rate of early resorptions was 100%. Even at 689 mg/m3, the
number of early resorptions per litter was 6 times that in
the control group. There was no evidence of severe intra-
uterine growth retardation in surviving fetuses, but a
significant increase in the incidence of major malfor-
mations (ventral wall defects and fusion of the aorta with
the pulmonary artery), minor anomalies, and skeletal vari-
ants. Teratogenic effects of 2-EE and also 2-EEA were re-
ported in Dutch Belted Rabbits after inhalation exposures
of 644 mg 2-EE/m3 and 2160 mg 2-EEA/m3 (Doe, 1984a).
In this study the author considered the NOEL in rabbits to
be 184 mg/m3 for 2-EE and 135 mg/m3 for 2-EEA.
In the study by Andrew & Hardin (1984), the highest
2-EE dose (2823 mg/m3) in rats led to 100% resorption,
as in rabbits. The resorption rate per litter in the
group gestationally exposed to 743 mg/m3 was about twice
the control value. Fetal body size was significantly
decreased, indicating retardation of intrauterine growth.
The significant increase in the incidence of cardiovascu-
lar defects (transposed and retrotracheal pulmonary
artery) and the increased incidence of common skeletal
variants and anomalies over control values were indicative
of a teratogenic effect of 2-EE in rats. Doe (1984a)
reported fetotoxicity without teratogenicity in rats after
inhalation exposure to 184 and 920 mg/m3, but no adverse
effects were reported after exposure to 37 mg/m3.
The teratogenic potential of 2-EE was evaluated in
pregnant Sprague-Dawley rats using dermal application
(0.25 or 0.50 ml, four times/day during gestation days 7
to 16) (Hardin et al., 1982). No signs of maternal
toxicity were noted except for ataxia on the last day of
dosing in the high-dose group, as well as a significant
decrease in body weight gain in the last half of ges-
tation. All fetuses in the high-dose group suffered intra-
uterine death. In the low-dose group, there was a signifi-
cant increase in the number of females with 100% dead
implants (p < 0.001), and in the incidence of skeletal
variations (p < 0.05). Also, reductions in fetal body
weight (p < 0.001) and cardiovascular malformations
(p < 0.05) were observed, as were significant decreases in
the number of live fetuses per litter (p < 0.001).
Hardin et al. (1984) studied the developmental tox-
icity of 2-EEA in rats after dermal application of 0.35 ml
2-EEA four times per day on days 7 to 16 of gestation
(daily application 1.37 g). 2-EEA was strongly embryo-
toxic, as reflected by significantly increased frequencies
of completely resorbed litters and dead implants per
litter. Also, the body weight of live fetuses was reduced
relative to water-treated controls. The spectrum and fre-
quency of malformations and variations noted in 2-EEA-
treated animals was similar to that described by Hardin et
al. (1982) in a previous study.
The developmental toxicity (including teratogenicity)
of 2-EEA in rats and rabbits following inhalation ex-
posure has been evaluated by Tyl et al. (1988). Fischer
344 rats (30 animals/group) and New Zealand white rabbits
(24 animals/group) were exposed to 2-EEA concentrations of
0, 270, 540, 1080, or 1620 mg/m3, 6 h/day on gestational
days 6-15 (rats) and 6-18 (rabbits). Fetuses were examined
for external, visceral, and skeletal malformations and
variations on gestational day 21 (rats) and 29 (rabbits).
In both rats and rabbits, maternal toxicity and develop-
mental toxicity were observed at exposures of 540 mg/m3 or
more. Teratogenic responses were increased at 1080 and
1620 mg/m3 with a 100% incidence of total malformations
being observed at the highest dose. An exposure of 270 mg
per m3 was considered a NOEL in both species, no evi-
dence of maternal or developmental toxicity being reported
at this dose.
8.6 Mutagenicity and Related End-Points
The only published data on 2-EE concerns a point mu-
tation test using Escherichia coli, which was reported to
be negative (Szybalski, 1958). However, 2-ME has been
tested in several in vitro and in vivo systems for its
genotoxic potential. The mutagenicity of 2-ME has been
evaluated in the following test systems: Salmonella typhi-
murium, unscheduled DNA synthesis (UDS) in human embryo
fibroblasts, bone marrow metaphase analysis in male and
female rats, dominant lethality in male rats, and a sex-
linked recessive lethal test in Drosophila melanogaster.
No evidence of mutagenicity in S. typhimurium was found
using five strains, with and without metabolic activation,
and dose levels up to 33 mg 2-ME per plate. When 2-ME was
tested in the presence of alcohol dehydrogenase no evi-
dence was found that metabolites of 2-ME were mutagenic in
S. typhimurium. Similarly, the addition of 2-ME at con-
centrations up to 10 mg/ml of medium did not lead to
changes in UDS in human embryo fibroblasts (McGregor et
al., 1983).
The production in CHO cells of sister chromatid ex-
changes (SCEs) and chromosome aberrations by 2-EE was
studied by Galloway et al. (1987). Both assays were
carried out with and without activation by an exogenous
enzyme system from rat liver chemically induced with the
PCB mixture Aroclor 1254. Aberrations were found only in
the absence of metabolic activation when using 2-EE con-
centrations between 4780 and 9510 µg/ml of medium. The
lowest effective concentration was 6830 µg/ml. SCEs were
found with and without activation, the lowest effective
concentration being 3170 µg/ml when the range studied
was 951-9510 µg/ml.
Aneuploidy was induced in the diploid yeast strain
D61.M ( Saccharomyces cerevisiae ) at 2-MEA levels of 3-
5.7% in the medium (Zimmermann et al., 1985). However,
there were no chemically related effects in this yeast
strain on point mutation or recombination after exposure
to 2-MEA. The relevance to mammals of these findings in
yeast is not clear.
No statistically significant increase in chromosome
aberrations was seen in male or female rats after exposure
by inhalation to 2-ME (78 or 1555 mg/m3), 7 h/day, for 1
or 5 days. Where possible 50 metaphases per rat were
scored from groups of 10 animals (McGregor et al., 1983).
Basler (1986) dosed Chinese hamsters intraperitoneally (10
animals) with two thirds of the LD50 of 2-MEA (approxi-
mately 1333 mg/kg body weight in corn oil) and the animals
were sacrificed 12, 24, 48, and 72 h after the single
dose. No statistically significantly increase in micro-
nucleated erythrocytes was noted.
Two dominant lethal studies in rats have been carried
out using 2-ME. McGregor et al. (1983) exposed groups of
10 male CD rats by inhalation to 78 or 1555 mg/m3, 7 h
per day, for 5 days. Control and low-dose groups gave
similar results, but the results at 1555 mg/m3 were
difficult to interpret. A high proportion of early deaths
was noted, which could be partly explained in terms of the
low implantation frequency reported. Therefore, a dominant
lethal effect at 1555 mg/m3 could not be demonstrated
conclusively. Rao et al. (1983), using male Sprague-
Dawley rats exposed to 93, 311, or 933 mg 2-ME/m3 by in-
halation 6 h/day, 5 days/week for 13 weeks, found no domi-
nant lethal effect at 93 or 311 mg/m3. An assessment of
dominant lethality was impossible at 933 mg/m3 due to
almost complete infertility. No positive control was in-
cluded.
In a sex-linked recessive lethal test in Drosophila
melanogaster reported by McGregor et al. (1983), the
results were inconclusive. Given the low absolute number
of mutants (low sample size), the inconsistency with which
various broods were affected, and the lack of a dose-
response relationship, a firm conclusion on the mutagen-
icity of 2-ME in D. melanogaster cannot be made.
8.7 Carcinogenicity
No carcinogenicity data are available on these glycol
ethers.
8.8 Mechanism of Toxicity - Mode of Action
The metabolic fate of 2-ME, 2-EE, and their acetates
has been well studied in both animals (Miller et al., 1983a;
Moss et al., 1985) and man (NIOSH, 1986, Groeseneken et
al., 1987, Veulemans, 1987a). Due to rapid hydrolysis of
the acetates to the monoalkyl glycol ether (see section
6), the putative toxic metabolite are the same for 2-ME or
2-MEA and for 2-EE or 2-EEA.
Both in vitro and in vivo studies have supported the
hypothesis that the toxic effects of 2-ME and 2-EE are
elicited by the toxicity of 2-methoxyacetaldehyde and
methoxyacetic acid (MAA) from 2-ME and ethoxyacetaldehyde
and ethoxyacetic acid (EAA) from 2-EE.
Gray et al. (1985) studied the effects in vitro of
2-ME, 2-EE, MAA, and EAA on mixed cultures of Sertoli and
germ cells from rat testes. At concentrations up to 50
mmol/litre medium, no morphological damage was noted for
2-ME or 2-EE, whereas administration of MAA or EAA at 2-10
mmol/litre led to degeneration of the pachytene and div-
iding spermatocytes, the probable target cells of the
parent ethers in vivo (Chapin et al., 1985b, Creasy et
al., 1985; Foster et al., 1986, Oudiz and Zenick, 1986).
Foster et al. (1986) reported that 2-methoxyacetaldehyde
(2-MALD), a possible metabolite of 2-ME, can produce
specific testicular effects in vitro and in vivo at doses
much lower than those required for MAA (0.2 and 0.5 mmol
per litre in vitro ). In more recent studies, Foster et
al. (1987) compared the in vivo and in vitro testicular
effects of MAA and EAA in rats. Single oral doses of MAA
and EAA (equimolar with 100, 250, or 500 mg 2-ME/kg body
weight) were administered and cell morphology was moni-
tored for 14 days. MAA produced damage to spermatocytes
undergoing meiotic maturation and division within 24 h of
treatment, whereas EAA produced these changes only at the
highest dose. Similar results were seen in vitro at a dose
(5 mmol/litre medium) equivalent to the steady state
plasma level of MAA after administration of 500 mg/kg.
Evidence that MAA is important in causing 2-ME terato-
genicity was reported by Yonemoto et al. (1984). MAA, but
not 2-ME, was found to interfere with organogenesis when
the two compounds were investigated using rat embryos in
culture.
Results from in vivo studies in mice (Welsch et al.,
1987; Sleet et al., 1988) and rats (Ritter et al., 1985)
further substantiate the hypothesis that monoalkyl glycol
ethers are metabolized to acetic acid metabolites via
alcohol dehydrogenase (ADH) and aldehyde dehydrogenases,
and that the ultimate toxin is actually the alkoxy acid
metabolite of the glycol ether or a further metabolite.
In mice (Sleet et al., 1987), 2-ME and MAA were equipotent
in producing teratogenic lesions leading to malformations
of the digits of all paws. Similar effects were noted by
Brown et al. (1984) and Ritter et al. (1985) in rats. Sub-
sequent work showed that the embryotoxic and teratogenic
effects of 2-ME in mice could be prevented by the concomi-
tant administration of 0.12 or 1.2 mmol/kg of 4-methyl-
pyrazole, an inhibitor of ADH, but that 4-methylpyrazole
was without effect after the administration of MAA (Sleet
et al., 1988).
9. EFFECTS ON MAN
Only limited information on the toxic effects of gly-
col ethers in humans is available. This information comes
largely from case reports in the early literature dealing
with accidental poisoning and/or workplace exposure. Such
reports provide only minimal information relating specific
effects to exposure levels. Only a few epidemiological
studies have been reported.
9.1 General Population Exposure
The widespread use of consumer products such as
paints, stains, inks, lacquers, surface coatings, and
home/industrial cleaners that contain one or more glycol
ethers means that broad segments of the general population
could be exposed. However, no reports quantifying such
exposures have been found nor any that describe skin irri-
tation or sensitization from exposures to glycol ethers in
humans, despite widespread exposure.
9.1.1 Poisoning reports
A 44-year-old man consumed 400 ml of 2-ME mixed with
brandy and died without regaining consciousness 5 h after
he was admitted to hospital in a comatose state (Young &
Woolner, 1946). Postmortem findings included acute
haemorrhagic gastritis, fatty degeneration of the liver,
and black coloured kidneys with marked degenerative
changes in the renal tubules.
Two non-fatal cases of poisoning by 2-ME in men aged
41 and 23 were reported by Nitter-Hauge (1970). Both
patients consumed 100 ml 2-ME. In addition to nervous
system disorders (agitation, confusion), the predominant
clinical features were nausea, cyanosis, hyperventilation,
slight tachycardia, and metabolic acidosis. In one patient
there were suggestions of moderate kidney failure. No
evidence of liver damage was found and both patients re-
covered within 4 weeks.
Ingestion of approximately 40 ml 2-EE by a 44-year-old
women led to vertigo, unconsciousness, effects on the
central nervous system, and metabolic acidosis (Fucik,
1969). She recovered within 44 days from all symptoms,
including kidney insufficiency and signs of liver damage,
although neurasthenia was still evident at that time.
9.2 Occupational Exposure
9.2.1 Repeated exposure
Reports on the effects of glycol ethers on humans fol-
lowing repeated exposure are available from occupational
studies. Early reports provided evidence that the repeated
exposure of humans to solvents containing 2-ME resulted in
such effects as anaemia, leucopenia, lethargy, general
weakness, dizziness, ataxia, and unequal or exaggerated
reflexes. A diagnosis of toxic encephalopathy was made by
Donley (1936) and Parson & Parsons (1938), but no reliable
estimation of exposure to 2-ME was given. Greenburg et al.
(1938) reported levels of 2-ME between 78 and 236 mg per
m3 in one manufacturing plant where 19 male subjects
(ages 16-26) were examined. The median duration of ex-
posure was 5 weeks and the maximum was 2 years. Signs of
nervous system dysfunction (fatigue, hand tremor, and
lethargy) were found, but no clear relationship between
the incidence of symptoms and the duration of exposure
could be ascertained in this small population. No control
group was included in this study.
The development of macrocytic anaemia has been de-
scribed in a case report of a worker exposed to 2-ME and
other solvents during microfilm manufacturing. Exposure
to 2-ME averaged 109 mg/m3 (35 ppm) (56-180 mg/m3, 18-
58 ppm), to methyl ethyl ketone 1-5 ppm, and to propylene
glycol monomethyl ether 4-13 ppm for a duration of 20
months. Follow-up analyses conducted 1 month after termin-
ating exposure indicated a return to normal limits for all
haematological parameters studied (Cohen, 1984).
The haematological effects of glycol ethers on humans
have also been documented in workplace surveys. Obvious
symptoms are not always manifested in peripheral blood.
The evaluation in a printing plant of seven workers with
dermal and respiratory exposure to dipropylene glycol
monomethyl ether, ethylene glycol monoethyl ether, and a
range of aliphatic, aromatic, and halogenated hydrocarbons
used for offset and multicolour printing revealed no
alteration in the peripheral blood (Cullen et al., 1983).
Although there was evidence of bone marrow injury in three
of the seven workers, no direct relationship could be
drawn to glycol ether exposure. The exposure to other sol-
vents by this relatively small group of workers makes more
definitive conclusions impossible. Bone marrow toxicity
had earlier been reported in two case studies of workers
exposed dermally to 2-ME (Ohi & Wegmann, 1978).
Denkhaus et al. (1986) investigated the cellular im-
mune response of nine workers aged 25-58 years of age,
heavily exposed to mixtures of organic solvents (including
2-ME and 2-EE) for 8-35 (mean 18.9) years, by the analysis
of subpopulations of peripheral blood lymphocytes. A con-
trol group of matched healthy controls was included. The
mean exposures to 2-ME and 2-EE were 6.1 mg/m3 (peak
150 mg/m3) and 4.8 mg/m3 (peak 53 mg/m3), respect-
ively. Based on samples taken during working shifts, mean
blood levels were 40.1 µg 2-ME/100 ml (peak: 965 µg/100
ml) and 2.0 µg 2-EE/100 ml (peak: 92.7 µg/100 ml). At
these levels (and in the presence of other solvents such
as 1-butanol, isobutanol, 2-butoxybutanol, toluene, m-xylene,
2-butanone, and 2-hexanone), the exposed workers had de-
creased levels of helper T-cells, and increased levels of
natural killer cells and human B-lymphocytes. However,
the levels of suppressor cells were normal. The authors
noted that similar changes in lymphocyte subpopulations
are found in states of general immunodeficiency and in
immunogenetic forms of aplastic anaemia.
9.2.2 Epidemiological studies
In a cross-sectional study, male employees in a plant
manufacturing 2-ME in the USA were examined for haema-
tological effects and possible sterility (Cook et al.,
1982). Personal air sampling indicated levels of exposure
of 1.3 mg/m3 or less in one working location and 19-26 mg
per m3 in another. Forty workers, engaged in production
and distribution of 2-ME (75% of the possible total popu-
lation) and 25 controls (57% of total eligible population)
were examined for haematological effects, and six exposed
and nine control workers participated in an examination of
testis size, semen abnormalities, and levels of serum
follicle-stimulating hormone (FSH). No increase in the
incidence of leucopenia or anaemia was noted, but de-
creases in white cell count and testicular size were noted
in the most highly exposed workers. These results were not
statistically significant but correlate with known effects
in experimental animals.
A cross-sectional evaluation of semen quality (sperm
concentration, pH, volume, viability, motility, velocity,
and morphology) was carried out in 37 workers exposed to
2-EE used as a resin solvent in a metal casting plant
(NIOSH, 1986). The study population represented 50% of
the exposed workforce. A control group of 38 workers from
other locations in the plant was included. Full-shift
breathing zone airborne exposure to 2-EE ranged from non-
detectable to 88 mg/m3. Dermal exposure was indicated by
urinary excretion of EAA at levels from non-detectable to
163 mg/g creatinine. Workers exposed to 2-EE had signifi-
cantly lower average sperm counts than controls (113
versus 154 million per ejaculate), although both exposed
and control groups had lower sperm counts than those found
in other occupational groups. The two groups studied did
not differ significantly with respect to other semen
characteristics or testicular size.
The effect of 2-ME and 2-EE exposure on male repro-
ductive factors and blood has been studied in shipyard
painters by Sparer et al. (1988a,b), Welch et al. (1988),
and Welch & Cullen (1988). Airborne levels (time-weighted
average: TWA) were determined in 102 samples over six
workshifts and were 0-80.5 mg 2-EE/m3 (mean 9.9 and
median level 4.4 mg/m3), and 0-17.7 mg 2-ME/m3 (mean
2.6 and median 1.4 mg/m3). Given the methods used by
Sparer et al. (1988a,b) the authors concluded these were
probably underestimates of exposure, particularly in the
mixing room and inside large tanks. Urinary excretion of
EAA indicated dermal exposure as well as airborne ex-
posure in some workers. In 73 painters, from a total
population of 153, an increased prevalence of oligospermia
and azoospermia was noted and there was an increased odds
ratio for a lower sperm count per ejaculate in exposed
workers as compared to the 40 controls studied (Welch et
al., 1988). In addition, when 94 painters from the same
population were examined for haematological effects of
chemical exposures, 10% were found to be anaemic and 5%
exhibited granulocytopenia (Welch & Cullen, 1988). None of
these effects were noted in the 55 control subjects exam-
ined. The reproductive effects observed were in agreement
with those reported previously by NIOSH (1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
10.1.1. Exposure
Many people may be exposed to 2-methoxyethanol (2-ME),
2-ethoxyethanol (2-EE), and their acetates (2-MEA and 2-
EEA), at levels comparable to industrial levels, through
the use of consumer and trade products. On the other
hand, exposure through food, water, or the ambient air is
probably negligible. This inference is based only on the
physical and chemical properties of these compounds and
evidence of rapid environmental degradation.
Significant occupational exposure may occur both
through inhalation and skin absorbtion. Limited measure-
ments of air levels in the workplace range from less than
0.1 mg/m3 to more than 150 mg/m3. However, the avail-
able monitoring is quite limited and large variations may
occur both within and among industries. Because of the
potential for skin absorption, air monitoring alone may
underestimate total exposure. Total uptake may be best
estimated from biological monitoring. Occupations where
extensive exposure is possible include, for example,
painting, printing, and cleaning, but it should be borne
in mind that there are many other occupations where these
compounds are also used and where exposure is of concern.
10.1.2. Health Effects
The major effects of concern for humans are develop-
mental, testicular, and haematological toxicity. These
are demonstrated by extensive and consistent data in ani-
mals and some human data. All these effects can be caused
by both short-term and longer-term exposures. In experi-
mental animals, very high repeated exposure to 2-ME and
2-EE (over 930 and 1450 mg/m3, respectively) produces
neurobehavioural, hepatic, and renal toxic effects. These
are also observed in human poisoning situations.
These four glycol ethers exhibit very similar testicu-
lar and developmental toxicities, in all species evalu-
ated, and by all routes of exposure that have been em-
ployed (inhalation, dermal, and oral). Mechanistic studies
indicate that for both testicular and developmental ef-
fects, metabolism to the alkoxyacetic acid derivative is a
necessary activation step. Metabolism takes place via the
alcohol dehydrogenase system that is common to humans and
laboratory animals. The toxic metabolites, methoxyacetic
acid (MAA) and ethoxyacetic acid (EAA), have been detected
in the urine of humans exposed to these solvents. The con-
sistency of response across laboratory animal species
studied, combined with the similarity of metabolism in
humans, makes it clear that humans should be presumed to
be subject to the testicular and developmental effects of
these glycol ethers. Available data on the excretion of
alkoxyacetic acids by humans indicate prolonged retention
compared with that in laboratory animals, suggesting that
humans may be more sensitive than the most sensitive ex-
perimental animal species. The rapid skin absorbtion of
these compounds is of particular concern. Teratogenicity
and other developmental effects have been observed follow-
ing the application of 2-ME, 2-EE, and 2-EEA to the intact
skin of rats.
Testicular damage has been observed in the rat, mouse,
and rabbit following exposure to these glycol ethers
through both the inhalation and the oral route. Single in-
halation exposures of rats to 1944 mg 2-ME/m3 or more for
4 h and repeated exposure to 933 mg 2-ME/m3 or more for
13 weeks resulted in histological evidence of testicular
damage. The NOEL for acute exposure was 933 mg/m3 and for
repeated exposure was 311 mg/m3. The mouse appeared
somewhat less sensitive, the NOEL for repeated exposure
being 933 mg/m3. However, the rabbit was more sensitive,
with marked testicular change being seen after repeated
exposure to 311 mg/m3 and a marginal effect (one in five
rabbits affected) being seen at 93 mg/m3. Similar
effects have been seen following oral exposure of rats to
2-ME, with short-term (including single dose) exposure to
100 mg/kg producing testicular damage. The NOEL in a sub-
acute (11 days) study was 50 mg/kg. 2-EE is somewhat less
potent with regard to its testicular toxicity than 2-ME;
effects were only seen at dose levels of 500 mg/kg or
more, the NOEL being 250 mg/kg.
Evidence from studies in men exposed occupationally
to 2-ME and 2-EE is consistent with the animal data and
indicates that these glycol ethers can produce testicular
toxicity in humans. Epidemiological studies on small
groups of workers exposed to 2-EE in a metal casting
plant and in shipyard painters exposed to both 2-ME and
2-EE consistently revealed an increased incidence of re-
duced sperm counts. Data on exposure levels were limited,
but there was evidence, in each case, of dermal exposure
as well as exposure via inhalation.
Developmental toxicity has been observed in the rat,
mouse, rabbit, and monkey following exposure to these
glycol ethers using dermal, oral, and inhalation routes.
Twelve daily applications of undiluted 2-ME to the shaved
skin of pregnant rats (with 6-h occlusion) was lethal,
while ten open applications of 2-EE (1.0 ml/day) or 2-EEA
(1.4 ml/day) were teratogenic but not maternally toxic.
Twelve occluded applications of 10% 2-ME in saline proved
developmentally toxic, the NOEL in this study being 3%
2-ME. No-observed-effect levels have not been demonstrated
following repeated oral dosing of pregnant animals with
2-ME. The lowest-observed-effect level (LOEL) for oral
administration of 2-ME was 31.25 mg/kg per day for mice,
25 mg/kg per day for rats, and 0.16 mmol/kg per day for
monkeys. Single-dose studies have been reported only for
2-ME in mice, the results being that on gestation day 11
(the most sensitive day) the NOEL was 100 mg/kg and the
LOEL was 175 mg/kg. Both 2-EE and 2-EEA have been evalu-
ated by inhalation exposure in rats and rabbits. Rats were
exposed to 2-EE in two studies, resulting in teratogenic
effects (743 mg/m3 for 7 h/day on gestation days 1-19)
or fetotoxic effects (184 and 920 mg/m3 for 6 h/day on
gestation days 6-15). In the latter study, the NOEL was
37 mg/m3. Rabbits exposed to 2-EE also exhibited
teratogenic effects (589 mg/m3 for 7 h/day on gestation
days 1-18) or fetotoxic effects (644 mg/m3 for 6 h/day
on gestation days 6-18). In the latter study, the NOEL was
184 mg/m3. When rabbits were exposed to 2-EEA for
6 h/day on gestation days 6-18, teratogenic effects were
seen at 2160 mg/m3 in one study and 1620 mg/m3 in
another. Fetotoxicity appeared in both studies at 540 mg
per m3 and the lowest exposure level in each study (135
and 270 mg/m3, respectively) was the NOEL. Rats exposed
to 2-EEA by inhalation for 6 h/day on gestation days 6-15
showed the same pattern of response: teratogenic effects
at 1620 mg/m3, fetotoxicity at 1080 mg/m3, and no ef-
fect at 270 mg/m3.
Thus developmental toxicity has been observed in all
species (mice, rats, and rabbits) exposed to 2-ME at 156
mg/m3 or more. The NOEL for all three species was 31 mg
per m3. Behavioural and neurochemical alterations in
rats followed in utero exposure at 78 mg/m3, with no
NOEL being identified.
2-EE and 2-EEA were slightly less potent. Develop-
mental effects in rats and rabbits followed all 2-EE ex-
posures at 368 mg/m3 or more. Slight developmental ef-
fects were seen in rats exposed at 184 mg 2-EE/m3, but
37 mg/m3 was a clear NOEL. For 2-EEA, the NOEL was 170 mg
per m3 for both rats and rabbits.
Haematological effects from single acute dose ex-
posures have been observed in animals and in human poison-
ings. Repeated inhalation exposure of the most sensitive
species, the rabbit, to 2-ME for 13 weeks, 5 times per
week, yielded a NOEL of 93 mg/m3. Repeat doses of 2-ME
also cause haematological toxicity in mice, rabbits, dogs,
hamsters, and guinea-pigs. 2-EE is less potent in causing
haematological effects than 2-ME. The NOEL for haemato-
logical effects in rats and rabbits exposed to 2-EE for 13
weeks, 5 times per week for 6 h/day, is 368 mg/m3. Dogs
and mice also show haematological effects from repeated
2-EE exposure at higher levels. Exposure to the acetate
esters of 2-EE and 2-ME would be expected to cause similar
effects at similar exposure levels, but there are too few
data on exposure and haematological effects of these com-
pounds to determine under what conditions single human ex-
posures will lead to haematological effects.
Industrial exposure levels have been reported at or
near the NOEL for haematological effects in animals after
repeated doses of both 2-ME and 2-EE. This fact, together
with the probable greater sensitivity of humans and the
expected accumulation of metabolites in human blood, indi-
cates that haematological effects may well occur from in-
dustrial and consumer exposure. This has been confirmed by
the haematological effects reported in some of the limited
number of studies of industrial workers with repeated 2-EE
and/or 2-ME exposure.
10.2. Evaluation of Effects on the Environment
Environmental exposure to these glycol ethers can
arise as a consequence of their direct release into the
atmosphere from their use as evaporative solvents. Dis-
charges to the land and water from accidental release may
also result in environmental exposure. Accumulation in
soil and surface water could only occur in the absence of
degradation. However, these glycol ethers are rapidly
degraded by chemical and biological processes and accumu-
lation is not expected. 2-MEA and 2-EEA are also expected
to hydrolyse readily and subsequently biodegrade under
aerobic conditions. However, contamination of anaerobic
soils and aquifers remains a potential problem, but this
condition is expected to be transitory resulting in negli-
gible risk.
Both 2-ME and 2-EE demonstrate low toxicity to micro-
oganisms and aquatic species. The glycol ether acetates,
however, are far more acutely toxic. No data exists to
ascertain the potential for adverse effects from long-term
exposure to environmental species.
11. RECOMMENDATIONS
11.1. Health Protection
1. Alternative less toxic solvents should be identified
to replace 2-methoxyethanol, 2-ethoxyethanol, and their
esters, particularly in consumer products. Assessment of
the effects of other ethylene glycol ethers is also of
particular importance, because some may cause effects
similar to the four glycol ethers evaluated here.
2. In view of the known toxic effects of these glycol
ethers, authorities should seriously consider appropriate
strategies to alert users of these chemicals to their
hazards, particularly those arising from dermal ex-
posures.
3. In view of recent toxicological data and the potential
for considerable dermal absorption of these glycol ethers,
national occupational exposure limits should be recon-
sidered to insure that the total daily dose to workers by
all routes of administration does not pose an undue risk
to health.
4. Single dose effects occur in animals at fairly high
exposure levels. Prudent use of these compounds (attention
to personal hygiene, suitable protective devices, and
adequate ventilation) is recommended to reduce the health
risks. The data indicate that more extensive protection
may be required to prevent developmental effects, as well
as effects on blood and testis, from repeated exposure.
11.2. Further Research
1. In view of the implication of methoxyacetic acid (MAA)
and ethoxyacetic acid (EAA) (the principal identified
metabolites of 2-ME, 2-EE, and of their esters) in the
toxicity to the male reproductive system, their mechanism
of action should be investigated. If it transpires that
MAA and EAA are not the primary agents concerned, these
should be identified and their mechanism of action
elucidated.
2. These four glycol ethers are known to have both
haematological effects and male reproductive effects
(sperm count reduction). The available data, although
limited, appear to suggest that the two effects become
evident at similar dose levels. The mechanism of action
should be investigated for both organ systems, and
haematological effects and sperm counts should be examined
in parallel to determine if haematological changes offer
warning signs for other effects of these compounds.
3. Air monitoring alone is not sufficient to assure low
exposure. Biological monitoring can aid in detecting
failures in protective measures. At present the
relationship between biological indicators of exposure,
total body uptake, and observed health effects have not
been sufficiently established. Further work is necessary
to provide the basis for using biological monitoring in
determining safe exposures.
4. Epidemiological studies and/or targeted health sur-
veillance in populations subject to high exposure to these
glycol ethers should be designed in order to estimate
exposure-effect relationships for the purpose of determin-
ing safe exposures, providing that overall exposure can be
properly and adequately evaluated by appropriate environ-
mental and biological monitoring.
5. The possibility that these compounds may cause effects
on the female gonads should be investigated through
multigeneration reproductive studies in animals.
6. Available research indicates that humans may metab-
olize these glycol ethers to the corresponding alkoxy-
acetic acids to a greater extent than rats, and that the
half-life for urinary excretion of these toxic metabolites
is about four times longer in humans than in rats.
Furthermore, rats conjugate a large portion of the acid
metabolites, whereas humans do not. These differences
might contribute to the relatively higher sensitivity of
humans to these glycol ethers. Detailed knowledge of the
metabolism and excretion kinetics would improve the
ability to predict safe exposure levels.
7. The results obtained from short-term (13-week) studies
indicate effects on various organ systems. However, there
have been no studies of sufficient duration that would
allow the reversibility of such effects to be assessed.
Therefore, it is suggested that stop-studies be undertaken
in which experimental animals are exposed to these glycol
ethers for at least 13 weeks, followed by a suitable
recovery period. Important physiological parameters
should then be evaluated in order to determine whether or
not these effects are transient.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Regulatory standards for 2-ME, 2-MEA, and 2-EE, estab-
lished by national bodies in different countries and the
European Economic Community, are summarized in the data
profile of the International Register of Potentially Toxic
Chemicals (IRPTC, 1987).
REFERENCES
ANDERSON, D., BRINKWORTH, M.H., JENKINSON, P.C., CLODE, S.A., CREASY,
D.M., & GANGOLLI, S.D. (1987) Effect of ethylene glycol monomethyl ether
on spermatogenesis, dominant lethality, and F1 abnormalities in the rat
and the mouse after treatment of F0 males. Teratog. Carcinog. Mutagen.,
7:141-158.
ANDREW, F.D. & HARDIN, B.D. (1984) Developmental effects after inhalation
exposure of gravid rabbits and rats to ethylene glycol monoethyl ether.
Environ. Health Perspect., 57: 13-23.
BAILEY, H.C., LIU, D.H.W., & JAVITZ, H.A. (1985) Time/toxicity
relationships in short-term static, dynamic, and plug-flow bioassays. In:
Bahner, R.C. & Hansen, D.J., ed. Aquatic toxicology and hazard assessment:
Eighth Symposium, Philadelphia, American Society for Testing and
Materials, pp. 193-212 (ASTM STP 891).
BARBEE, S.V., TERRILL, J.B., DESOUSA, D.J., & CONAWAY, C.C. (1984)
Subchronic inhalation toxicology of ethylene glycol monoethyl ether in the
rat and rabbit. Environ. Health Perspect., 57: 157-163.
BASLER, A. (1986) Aneuploidy-inducing chemicals in yeast evaluated by the
micronucleus test. Mutat. Res., 174: 11-13.
BOTTA, D., CASTELLANI PIRRI, L., & MANTICA, E. (1984) Ground water
pollution by organic solvents and their microbial degradation products,
Luxembourg, Commission of the European Communities, pp. 261-275 (EUR-
8518).
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for their toxicity
threshold: Model organisms Microcystis (Diplocystis) Aeruginosa and
Scenedesmus quadricauda. Mitt. intern. Ver. theor. angew. Limnol., 21:
275-284.
BROWN, N.A., HOLT, D., & WEBB, M. (1984) The teratogenicity of
methoxyacetic acid in the rat. Toxicol. Lett., 22: 93-100.
CARPENTER, C.P., POZZANI, V.C., WEIL, C.S., NAIR, J.H., KECK, G.A., &
SMITH, H.F. (1956) The toxicity of butyl cellosolve solvent. Arch. ind.
Health, 14: 114-131.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., & LAMB, J.C. (1985a) Effects of
ethylene glycol monomethyl ether (EGME) on mating performance and
epididymal sperm parameters in F344 rats. Fundam. appl. Toxicol., 5: 182-
189.
CHAPIN, R.E., DUTTON, S.L., ROSS, M.D., SWAISGOOD, R.R., & LAMB, J.S.
(1985b) The recovery of the testis over 8 weeks after short-term dosing
with ethylene glycol monomethyl ether: histology, cell specific enzymes,
and rete testis fluid protein. Fundam. appl. Toxicol., 5: 515-525.
CHEEVER, K.L., PLOTNICK, H.B., RICHARDS, D.E., & WEIGEL, W.W. (1984)
Metabolism and excretion of 2-ethoxyethanol in the adult male rat.
Environ. Health Perspect., 57: 241-248.
COHEN, R. (1984) Reversible subacute ethylene glycol monomethyl ether
toxicity associated with microfilm production: a case report. Am. J. ind.
Med., 6: 441-446.
COOK, R.R., BODNER, K.M., KOLESAR, R.C., VAN PEENEN, P.F.D., DICKSON,
G.S., & FLANAGAN, K. (1982) A cross-sectional study of ethylene glycol
monomethyl ether process employees. Arch. environ. Health, 37: 346-351.
CREASY, D.M. & FOSTER, P.M.D. (1984) The morphological development of
glycol ether-induced testicular atrophy in the rat. Exp. mol. Pathol., 40:
169-176.
CREASY, D.M., FLYNN, J.C., GRAY, T.J.B., & BUTLER, W.H. (1985) A
quantitative study of stage-specific spermatocyte damage following
administration of ethylene glycol monomethyl ether in the rat. Exp. mol.
Pathol., 43: 321-336.
CREASY, D.M., JONES, H.B., BEECH, L.M., & GRAY, T.J.B. (1986) The effects
of two testicular toxins on the ultrastructural morphology of mixed
cultures of Sertoli and germ cells: a comparison with in vivo effects.
Food chem. Toxicol., 24: 655-656.
CULLEN, M.R., RADO, T., WALDRON, J.A., SPARER, J., & WELCH, L.S. (1983)
Bone marrow injury in lithographers exposed to glycol ethers and organic
solvents used in multicolor offset and ultraviolet curing printing
processes. Arch. environ. Health, 38(6): 347-354.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J.
hazard. Mater., 1: 303-318.
DE DELBARRE, F., KAHAN, A., DE GERY, A., & KONRAD, K. (1980) Action
immunomodulatrice du méthoxy-2 éthanol et de dérivés homologues chez le
rat. C.R. Acad. Sci. Paris, 291: 215-218.
DENKHAUS, W., STELDERN, D., BOTZENHARDT, U., & KONIETZKO, H. (1986)
Lymphocyte subpopulations in solvent-exposed workers. Int. Arch. occup.
environ. Health, 57: 109-115.
DOE, J.E. (1984a) Ethylene glycol monoethyl ether and ethylene glycol
monoethyl ether acetate teratology studies. Environ. Health Perspect., 57:
33-41.
DOE, J.E. (1984b) Further studies on the toxicology of the glycol ethers
with emphasis on rapid screening and hazard assessment. Environ. Health
Perspect., 57: 199-206.
DOE, J.E., SAMUELS, D.M., TINSTON, D.J., & WICKRAMARATNE, G.A.D. (1983)
Comparative aspects of the reproductive toxicology by inhalation in rats
of ethylene glycol monomethyl ether and propylene glycol monomethyl ether.
Toxicol. appl. Pharmacol., 69: 43-47.
DONLEY, D.E. (1936) Toxic encephalopathy and volatile solvents in
industry. J. ind. Hyg. Toxicol., 18: 571-577.
DUGARD, P.H., WALKER, M., MAWDSLEY, S.J., & SCOTT, R.C. (1984) Absorption
of some glycol ethers through human skin in vitro . Env. Health Perspect.,
57: 193-197.
ECETOC (1985) The toxicology of glycol ethers and its relevance to man,
Brussels, European Chemical Industry Ecology and Toxicology Centre
(Technical Report No. 17).
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., THOMAS, L.V., COOK, M.W., &
GANGOLLI, S.D. (1983) Testicular toxicity of ethylene glycol monomethyl
and monoethyl ethers in the rat. Toxicol. appl. Pharmacol., 69: 385-399.
FOSTER, P.M.D., CREASY, D.M., FOSTER, J.R., & GRAY, T.J.B. (1984)
Testicular toxicity produced by ethylene glycol monomethyl and monoethyl
ethers in the rat. Environ. Health Perspect., 57: 207.
FOSTER, P.M.D., BLACKBURN, D.M., MOORE, R.B., & LLOYD, S.C. (1986)
Testicular toxicity of 2-methoxyacetaldehyde, a possible metabolite of
ethylene glycol monomethyl ether, in the rat. Toxicol. Lett., 32: 73-80.
FOSTER, P.M.D., LLOYD, S.C., & BLACKBURN, D.M. (1987) Comparison of the in
vivo and in vitro testicular effects produced by methoxy-, ethoxy-, and n-
butoxy acetic acids in the rat. Toxicology, 43: 17-30.
FUCIK, J. (1969) Poisoning by ethylene glycol monoethyl ether. Prac. Lek.,
21: 116-118.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO, J.,
MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese hamster
ovary cells: evaluations of 108 chemicals. Environ. mol. Mutagen., 10(10):
1-175.
GOLDBERG, M.E., HARN, C., & SMYTH, H.F. (1962) Toxicological implication
of altered behaviour induced by an industrial vapour. Toxicol. appl.
Pharmacol., 4: 148-164.
GRANT, D., SULSH, S., JONES, H.B., GANGOLLI, S.D., & BUTLER, W.H. (1985)
Acute toxicity and recovery in the hemopoietic system of rats after
treatment with ethylene glycol monomethyl and monobutyl ethers. Toxicol.
appl. Pharmacol., 77: 187-200.
GRAY, T.J.B., MOSS, E.J., CREASY, D.M., & GANGOLLI, S.D. (1985) Studies on
the toxicity of some glycol ethers and alkoxyacetic acids in primary
testicular cell cultures. Toxicol. appl. Pharmacol., 79: 490-501.
GREENBURG, L., MAYERS, M.R., GOLDWATER, L.J., BURKE, W.J., & MOSCOWITZ, S.
(1938) Health hazards in the manufacture of "fused collars". 1. Exposure
to ethylene glycol monomethyl ether. J. ind. Hyg. Toxicol., 20: 134-147.
GREENE, J.A., SLEET, R.B., MORGAN, K.T., & WELSCH, F. (1987) Cytotoxic
effects of ethylene glycol monomethyl ether in the forelimb bud of the
mouse embryo. Teratology, 36: 23-34.
GROESENEKEN, D., VAN VLEM, E., VEULEMANS, H., & MASSCHELEIN, R. (1986a)
Gas chromatographic determination of methoxyacetic and ethoxyacetic acid
in urine. Br. J. ind. Med., 43: 62-65.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986b) Respiratory
uptake and elimination of ethylene glycol monoethyl ether after
experimental human exposure. Br. J. ind. Med., 43: 544-549.
GROESENEKEN, D., VEULEMANS, H., & MASSCHELEIN, R. (1986c) Urinary
excretion of ethoxyacetic acid after experimental human exposure to
ethylene glycol monoethyl ether. Br. J. ind. Med., 43: 615-619.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1987)
Ethoxyacetic acid: a metabolite of ethylene glycol monoethyl ether acetate
in man. Br. J. ind. Med., 44: 488-493.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1988)
Comparative urinary excretion of ethoxyacetic acid in man and rat after
single low doses of ethylene glycol monoethyl ether. Toxicol. Lett., 41:
57-68.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989a)
Experimental human exposure to ethylene glycol monomethyl ether. Int.
Arch. occup. environ. Health, 61: 243-247.
GROESENEKEN, D., VEULEMANS, H., MASSCHELEIN, R., & VAN VLEM, E. (1989b) An
improved method for the determination in urine of alkoxyacetic acids. Int.
Arch. occup. environ. Health, 61: 249-254.
GUEST, D., HAMILTON, M.L., DEISINGER, P.J., & DIVINCENZO, G.D. (1984)
Pulmonary and percutaneous absorption of 2-propoxyethyl acetate and 2-
ethoxyethyl acetate in beagle dogs. Environ. Health Perspect., 57: 177-
183.
HAMLIN, J.W., HUDSON, B., SHEEN, A.D., & SAUNDERS, K.J. (1982) The
measurement of glycol ether levels in the workplace. Polym. Paint Colour
J., October 13: 61-63.
HANLEY, T.R., Jr, YANO, B.L., NITSCHKE, K.D., & JOHN, J.A. (1984)
Comparison of the teratogenic potential of inhaled ethylene glycol
monomethyl ether in rats, mice, and rabbits. Toxicol. appl. Pharmacol.,
75: 409-422.
HARDIN, B.D. (1983) Reproductive toxicity of the glycol ethers.
Toxicology, 27: 91-102.
HARDIN, B.D., NIEMEIER, R.W., SMITH, R.J., KUCZUK, M.H., MATHINOS, P.R., &
WEAVER, T.F. (1982) Teratogenicity of 2-ethoxyethanol by dermal
application. Drug chem. Toxicol., 5(3): 277-294.
HARDIN, B.D., GOAD, P.T., & BURG, J.R. (1984) Developmental toxicity of
four glycol ethers applied cutaneously to rats. Environ. Health Perspect.,
57: 69-74.
HEALTH AND SAFETY EXECUTIVE (1988) Methods for the determination of
hazardous substances: Glycol ethers and glycol acetate vapours in air,
London, UK Health and Safety Executive, pp. 1-7 (MDHS-23).
HERMENS, J., CANTON, H., JANSSEN, P., & DEJONG, R. (1984) Quantitative
structure-activity relationships and toxicity of mixtures of chemicals
with anaesthetic potency: Acute lethal and sublethal toxicity to Daphnia
magna. Aquat. Toxicol., 5: 143-154.
HORTON, V.L., SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1985)
Developmental phase-specific and dose-related teratogenic effects of
ethylene glycol monomethyl ether in CD-1 mice. Toxicol. appl. Pharmacol.,
80: 108-118.
HOUSE, R.V., LAUER, L.D., MURRAY, M.J., WARD, E.C., & DEAN, J.H. (1985)
Immunological studies in B6C3F1 mice following exposure to ethylene glycol
monomethyl ether and its principal metabolite methoxyacetic acid. Toxicol.
appl. Pharmacol., 77: 358-362.
HURTT, M.E. & ZENICK, H. (1986) Decreasing epididymal sperm reserves
enhances the detection of ethoxyethanol-induced spermatotoxicity. Fundam.
appl. Toxicol., 7: 348-353.
IRPTC (1987) IRPTC legal file 1986 - Volume 1, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
JUHNKE, I. & LUDEMANN, D. (1978) The results obtained with the Golden Orfe
test during the examination of 200 chemical compounds for acute fish
toxicity. Wasser Abwasser Forsch., 11: 161-164.
KAREL, L., LANDING, B.H., & HARVEY, T.S. (1947) The intraperitoneal
toxicity of some glycols, glycol ethers, glycol esters and phthalates in
mice. J. Pharmacol. exp. Ther., 90: 338-347.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 9 - Ethanol,
3rd ed., New York, Chichester, Brisbane, Toronto, John Wiley & Sons.
KIRK-OTHMER (1980) Encyclopedia of chemical technology: Vol. 11 - Glycols
(Ethylene and Propylene), 3rd ed., New York, Chichester, Brisbane,
Toronto, John Wiley & Sons.
LAILLER, J., PLAZONNET, B., LE DOUAREC, J.C., & GONIN, M.J. (1975)
Evaluation of ocular irriation in the rabbit: Development of an objective
method of studying eye irritation. Proc. Eur. Soc. Toxicol., 17: 336-350.
LAMB, J.C., DUSHYANT, K.G., RUSSELL, V.S., HAMMEL, L., & SABHARNAL, P.S.
(1984) Reproductive toxicity of ethylene glycol monoethyl ether tested by
continuous breeding of CD-1 mice. Environ. Health Perspect., 57: 85-90.
LAUG, E.P., CALVERY, H.O., MORRIS, H.J., & WOODARD, G. (1939) The
toxicology of some glycols and derivatives. J. ind. Hyg. Toxicol., 21:
173-201.
LEE, K.H. & WONG, H.A. (1979) Toxic effects of some alcohol and ethylene
glycol derivatives on Cladosporium resinae. Appl. environ. Microbiol., 38:
24-28.
MCGREGOR, D.B., WILLINS, M.J., MCDONALD, P., HOLMSTROM, M., MCDONALD, D.,
& NIEMEIER, R.W. (1983) Genetic effects of 2-methoxyethanol and bis(2-
methoxyethyl)ether. Toxicol. appl. Pharmacol., 70: 303-316.
MELLAN, I. (1977) Glycol ethers and esters. In: Industrial Solvents
Handbook, 2nd ed., Park Ridge, New Jersey, Noyes Data Corporation, pp.
346-399, 513-551.
MILLER, R.R., AYERS, J.A., CALHOUN, L.L., YOUNG, J.T., & MCKENNA, M.J.
(1981) Comparative short-term inhalation toxicity of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in rats and mice.
Toxicol. appl. Pharmacol., 61: 368-377.
MILLER, R.R., CARREON, R.E., YOUNG, J.T., & MCKENNA, M.J. (1982) Toxicity
of methoxyacetate acid in rats. Fundam. appl. Toxicol., 2: 155-160.
MILLER, R.R., HERMANN, E.A., LANGVARDT, P.W., MCKENNA, M.J., & SCHWETZ,
B.A. (1983a) Comparative metabolism and disposition of ethylene glycol
monomethyl ether and propylene glycol monomethyl ether in male rats.
Toxicol. appl. Pharmacol., 67: 229-237.
MILLER, R.R., AYRES, J.A., YOUNG, J.T., & MCKENNA, M.J. (1983b) Ethylene
glycol monomethyl ether. I. Subchronic vapor inhalation study with rats
and rabbits. Fundam. appl. Toxicol., 3: 49-54.
MOSS, E.J., THOMAS, L.V., COOK, M.W., WALTERS, D.C., FOSTER, P.M.D.,
CREASY, D.M., & GRAY, T.J.B. (1985) The role of metabolism in 2-
methoxyethanol-induced testicular toxicity. Toxicol. appl. Pharmacol., 79:
480-489.
NAGANO, K., NAKAYAMA, E., KOGANA, M., DOBAYASKI, H., ADACHI, H., & YAMADA,
T. (1979) Testicular atrophy of mice induced by ethylene glycol monoalkyl
ethers. Jpn. J. ind. Health, 21: 29-35.
NAGANO, K., NAKAYAMA, E., DOBAYASKI, H., YAMADA, T., ADACHI, H.,
NISHIZAWA, T., OZAWA, H., NAKAICHI, M., OKUKDA, H., MINAMI, K., &
YAMAZAKI, K. (1981) Embryotoxic effects of ethylene glycol monomethyl
ether in mice. Toxicology, 20: 335-343.
NAGANO, K., NAKAYAMA, E., OOBAYASHI, H., NISHIZAWA, T., OKUDA, H., &
YAMAZAKI, K. (1984) Experimental studies on toxicity of ethylene glycol
alkyl ethers in Japan. Environ. Health Perspect., 57: 75-84.
NAKAAKI, K., FUKABORI, S., & TADA, O. (1980) An experimental study on
percutaneous absorption of some organic solvents. J. Sci. Labour, 56(12):
1-9.
NEIHOF, R.A. & BAILEY, C.A. (1978) Biocidal properties of anti-icing
additives for aircraft fuels. Appl. environ. Microbiol., 35: 698-703.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., TAYLOR, B.J., & HORNUNG,
R.W. (1981) Ethoxyethanol behavioral teratology in rats. Neuro-
toxicology., 2(2): 231-249.
NELSON, B.K., BRIGHTWELL, W.S., & SETZER, J.V. (1982) Prenatal
interactions between ethanol and the industrial solvent 2-ethoxyethanol in
rats: maternal and behavioral teratogenic effects. Neurobehav. Toxicol.
Teratol., 4: 387-394.
NELSON, B.K., BRIGHTWELL, W.S., BURG, J.R., & MASSARI, V.J. (1984a)
Behavioral and neurochemical alterations in the offspring of rats after
maternal or paternal inhalation exposure to the industrial solvent 2-
methoxyethanol. Pharmacol. Biochem. Behav., 20: 269-279.
NELSON, B.K., SETZER, J.V., BRIGHTWELL, W.S., MATHINOS, P.R., KUCZUR,
M.H., WEAVER, T.E., & GOAD, P.T. (1984b) Comparative inhalation
teratogenicity of four glycol ether solvents and amino derivative in rats.
Environ. Health Perspect., 57: 261-271.
NELSON, B.K., BRIGHTWELL, W.S., SETZER, J.V., & O'DONOHUE, T.L. (1984c)
Reproductive toxicity of the industrial solvent 2-ethoxyethanol in rats
and interactive effects of ethanol. Environ. Health Perspect., 57: 255-
259.
NIOSH (1986) Health hazard evaluation report: Precision Castparts
Corporation, Portland, Oregon, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (Report No. HETA-84-415-1688).
NIOSH (1987a) Alcohols IV. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1403.
NIOSH (1987b) Esters I. In: NIOSH manual of analytical methods,
Cincinnati, Ohio, National Institute for Occupational Safety and Health,
p. 1450.
NITTER-HAUGE, S. (1970) Poisoning with ethylene glycol monomethyl ether:
report of two cases. Acta med. Scand., 188: 277-280.
OHI, G. & WEGMANN, D.H. (1978) Transcutaneous ethylene glycol monomethyl
ether poisoning in the work setting. J. occup. Med., 20: 675-676.
OUDIZ, D. & ZENICK, H. (1986) In vivo and in vitro evaluations of
spermatotoxicity induced by 2-ethoxyethanol treatment. Toxicol. appl.
Pharmacol., 84: 576-583.
PARSONS, C.E. & PARSONS, M.E.M. (1938) Toxic encephalopathy and
"granulopenic anaemia" due to volatile solvents in industry: report of
two cases. J. ind. Hyg. Toxicol., 20: 124-135.
PAUSTENBACH, D.J. (1988) Assessment of the developmental risks resulting
from occupational exposure to selected glycol ethers within the
semiconductor industry. J. Toxicol. environ. Health, 23: 29-75.
PISKO, G.T. & VERBILOV, A.A. (1988) Toxicity of monomethyl, monoethyl and
monobutyl ethers of ethylene glycol. Gig. Tr. prof. Zabol., 3: 48-49.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp bioassay and
seawater BOD of petrochemicals. J. Water Pollut. Control Fed., 46: 63-77.
RAO, K.S., COBEL-GEARD, S.R., YOUNG, J.T., HANLEY, T.R., Jr, HAYES, W.C.,
JOHN, J.A., & MILLER, R.R. (1983) Ethylene glycol monomethyl ether II.
Reproductive and dominant lethal studies in rats. Fundam. appl. Toxicol.,
3: 80-85.
RITTER, E.J., SCOTT, W.J., RANDALL, J.L., & RITTER, J.M. (1985)
Teratogenicity of dimethoxyethyl phthalate and its metabolites
methoxyethanol and methoxyacetic acid in the rat. Teratology, 32: 25-31.
ROMER, K.G., BALGE, F., & FREUNDT, K.J. (1985) Ethanol-induced
accumulation of ethylene glycol monoalkyl ethers in rats. Drug chem.
Toxicol., 8(4): 255-264.
ROWE, V.K. & WOLF, M.A. (1982) Derivatives of glycols. In: Clayton, G.D. &
Clayton, F.E., ed. Patty's industrial hygiene toxicology, Vol. 2, pp.
3909-4052.
SAPARMAMEDOV, E. (1974) [Toxicity of some simple ethylene glycol ethers
(single experiments).] Zdravookhr Turkm., 18(9): 26-31 (in Russian)
(English translation from US NIOSH.).
SAVOLAINEN, H. (1980) Glial cell toxicity of ethyleneglycol monomethyl
ether vapour. Environ. Res., 22: 423-430.
SCOTT, W.J., FRADKIN, R., NAU, H., & WITTFOHT, W. (1987) Teratologic
potential of 2-methoxyethanol (2-ME) in non-human primates. Teratology,
35(2): 66 (abstract).
SLEET, R.B., JOHN-GREENE, J.A., & WELSCH, F. (1986) Localization of
radioactivity from 2-methoxy[1,2-14C]ethanol in maternal and conceptus
compartments of CD-1 mice. Toxicol. appl. Pharmacol., 84: 25-35.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1987) The teratogenicity and
disposition of the glycol ether 2-methoxyethanol and their relationship in
CD-1 mice. In: Welsch, F., ed. Approaches to elucidate mechanisms in
teratogenesis, New York, Hemisphere Publishing Co., pp. 33-57.
SLEET, R.B., GREENE, J.A., & WELSCH, F. (1988) The relationship of
embryotoxicity to disposition of 2-methoxyethanol in mice. Toxicol. appl.
Pharmacol., 93: 195-207.
SMALLWOOD, A.W., DEBORD, K.E., & LOWRY, L.K. (1984) Analyses of ethylene
glycol monoalkyl ethers and their proposed metabolites in blood and urine.
Environ. Health Perspect., 57: 249-253.
SMALLWOOD, A.W., DEBORD, K., BURG, J., MOSELEY, C., & LOWRY, L. (1988)
Determination of urinary 2-ethoxyacetic acid as an indicator of
occupational exposure to 2-ethoxyethanol. Appl. ind. Hyg., 3(2): 47-50.
SMYTH, H.F., SEATON, J., & FISCHER, L. (1941) The single dose toxicity of
some glycols and derivatives. J. ind. Hyg. Toxicol., 23: 259-268.
SPARER, J., WELCH, L.S., MCMANUS, K., & CULLEN, M.R. (1988a) Effects of
exposure to glycol ethers in shipyard painters. I. Evaluation of exposure.
Am. J. ind. Med., 14: 497-507.
SPARER, J., WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R.
(1988b) Effects of exposure to glycol ethers in shipyard painters. II.
Male reproduction. Am. J. ind. Med., 14: 509-526.
STENGER, E.G., AEPPLI, L., MULLER, D., PEHEIM, E., & THOMANN, P. (1971)
Toxicology of ethylene glycol monoethyl ether. Arzneim Forsch., 21: 880-
885.
STOTT, W.T. & MCKENNA, M.J. (1985) Hydrolysis of several glycol ether
acetates and acrylate esters by nasal mucosal carboxylesterase in vitro .
Fundam. appl. Toxicol., 5: 399-404.
SZYBALSKI, W. (1958) Special microbiological systems II. Observations on
chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76: 475-488.
TANAKA, K., MIKAMI, E., & SUZUKI, T. (1986) Methane fermentation of 2-
methoxyethanol by mesophilic digesting sludge. J. Ferment. Technol.,
64(4): 305-309.
TORAASON, M., STRINGER, B., STOBER, P., & HARDIN, B.D. (1985)
Electrocardiographic study of rat fetuses exposed to ethylene glycol
monomethyl ether (EGME). Teratology, 32: 33-39.
TORAASON, M., BREITENSTEIN, M.J., & SMITH, R.J. (1986a) Ethylene glycol
monomethyl ether (EGME) inhibits rat embryo ornithine decarboxylase (ODC)
activity. Drug chem. Toxicol., 9: 191-203.
TORAASON, M., STRINGER, B., & SMITH, R. (1986b) Ornithine decarboxylase
activity in the neonatal rat heart following prenatal exposure to ethylene
glycol monomethyl ether. Drug chem. Toxicol., 9(1): 1-14.
TYL, R.W., PRITTS, I.M., FRANCE, K.A., FISHER, L.C., & TYLER, T.R. (1988)
Developmental toxicity evaluation of inhaled 2-ethoxyethanol acetate in
Fischer 344 rats and New Zealand white rabbits. Fundam. appl. Toxicol.,
10: 20-39.
US EPA (1987) Environmental health criteria: 2-methoxyethanol, 2-
ethoxyethanol, and their acetates, Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances.
VERSCHUEREN, K. (1977) Ethylene glycol monomethyl ether. In: Handbook of
experimental data on organic chemicals, New York, Van Nostrand-Reinhold
Company, 327 pp.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987a)
Field study of the urinary excretion of ethoxyacetic acid during repeated
daily exposure to the ethyl ether of ethylene glycol and the ethyl ether
of ethylene glycol acetate. Scand. J. Work Environ. Health, 13: 239-242.
VEULEMANS, H., GROESENEKEN, D., MASSCHELEIN, R., & VAN VLEM, E. (1987b)
Survey of ethylene glycol ether exposures in Belgian industries and
workshops. Am. Ind. Hyg. Assoc. J., 48(8): 671-676.
WEIL, C.S. & SCALA, R.A. (1971) Study of intra- and inter-laboratory
variability in the results of rabbit eye and skin irritation tests.
Toxicol. appl. Pharmacol., 19: 276-360.
WELCH, L.S. & CULLEN, M.R. (1988) Effects of exposure to glycol ethers in
shipyard painters. III. Hematologic effects. Am. J. ind. Med., 14: 527-
536.
WELCH, L.S., SCHRADER, S.M., TURNER, T.W., & CULLEN, M.R. (1988) Effects
of exposure to ethylene glycol ethers on shipyard painters: II. Male
reproduction. Am. J. ind. Med., 14: 509-526.
WELSCH, R., SLEET, R.B., & GREENE, J.A. (1987) Attenuation of 2-
methoxyethanol and methoxyacetic acid-induced digit malformations in mice
by simple physiological compounds: implications for the role of further
metabolism of methoxyacetic acid in developmental toxicity. J. biochem.
Toxicol., 2: 225-240.
WERNER, H.W., MITCHELL, J.L., MILLER, J.W., & VON OETTINGEN, W.F. (1943)
Effects of repeated exposure of dogs to monoalkyl ethylene glycol ether
vapors. J. ind. Hyg. Toxicol., 25(9): 409-414.
WICKRAMARATNE, G.A., de S. (1986) The teratogenic potential and dose-
response of dermally administered ethylene glycol monomethyl ether (EGME)
estimated in rats with the Chernoff-Kavlock assay. J. appl. Toxicol.,
6(3): 165-166.
YONEMOTO, J., BROWN, N.A., & WEBB, M. (1984) Effects of dimethoxyethyl
phthalate, monomethoxyacetic acid on post implantation rat embryos in
culture. Toxicol. Lett., 21: 97-102.
YOUNG, E.G. & WOOLNER, L.B. (1946) A case of fatal poisoning from 2-
methoxyethanol. J. ind. Hyg. Toxicol., 28: 267-268.
ZIMMERMANN, F.K., MAYER, V.W., SCHEEL, I., & RESNICK, M.A. (1985) Acetone,
methyl ethyl ketone, ethyl acetate, acetonitrile and other polar aprotic
solvents are strong inducers of aneuploidy in Saccharomyces cerevisiae.
Mutat. Res., 149: 339-351.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
La présente monographie ne traite que des éthers
méthyliques et éthyliques de l'éthylène-glycol, c'est-à-
dire le méthoxy-2 éthanol (2-ME), l'éthoxy-2 éthanol
(2-EE) et leurs esters acétiques respectifs, à savoir
l'acétate de méthoxy-2 éthyle (2-MEA) et l'acétate
d'éthoxy-2 éthyle (2-EEA). Ces composés se présentent tous
les quatre sous la forme de liquides stables incolores et
inflammables, dotés d'une légère odeur éthérée; ils sont
tous miscibles à l'eau (ou tout au moins dans le cas du
2-EAA très soluble dans celle-ci) et miscibles à un grand
nombre de solvants organiques.
Il existe des méthodes d'analyse permettant la mise en
évidence de ces éthers du glycol et de leurs métabolites
dans divers milieux (air, eau, sang et urine). Ces
méthodes font souvent appel à des techniques d'adsorption
ou d'extraction afin de concentrer l'échantillon, suivies
d'une analyse par chromatographie en phase gazeuse. La
chromatographie en phase gazeuse ou la chromatographie
liquide à haute performance permettent de doser l'acide
méthoxy-2 acétique (MAA) ainsi que l'acide éthoxy-2
acétique (EAA) (qui sont des métabolites du du 2-ME et du
2-EE) dans les urines, généralement après obtention de
dérivés convenables, à des concentrations entre 5 et
100 µg/ml.
2. Sources d'exposition humaine et environnementale
Les quatre éthers du glycol étudiés s'obtiennent tous
par réaction de l'oxyde d'éthylène sur l'alcool convenable
puis, si nécessaire, par estérification à l'aide d'acide
éthanoïque.
On ne dispose pas de données concernant la production
mondiale de ces éthers du glycol. Toutefois on peut
avancer que la production annuelle globale de l'Europe
occidentale, des Etats-Unis et du Japon se situe aux envi-
rons de 79 x 103 tonnes de 2-ME et de 205 x 103 tonnes
de 2-EE. Ils sont en grande partie utilisés pour la
production industrielle de divers revêtements (peintures,
teintures, laques, vernis, etc.) et comme solvants pour la
préparation d'encres d'impression, de résines et de
colorants, ainsi que pour la fabrication de détachants
domestiques et industriels. On les utilise également comme
additifs de dégivrage dans les liquides hydrauliques et
les carburéacteurs.
3. Transport, distribution et transformation dans l'environnement
Du fait de leur solubilité dans l'eau et de leur
tension de vapeur relativement basse, ces éthers
pourraient, en l'absence de décomposition, s'accumuler
dans l'eau. Toutefois, il semble que cette éventualité
soit exclue du fait de leur dégradation par des micro-
organismes présents dans le sol, les boues d'effluents et
l'eau.
C'est l'utilisation de ces éthers comme solvants vola-
tils qui, du fait des émissions atmosphériques auxquelles
elle donne lieu, entraîne l'exposition environnementale la
plus importante. Dans l'environnement général, ils
subissent une photolyse rapide et l'on pourrait s'attendre
à des concentrations inférieures à 0,0007 mg/m3 (2 x
10-4 ppm).
En aérobiose, les éthers du glycol subissent une
dégradation microbienne rapide en dioxyde de carbone et en
eau, alors qu'en anaérobiose, les principaux produits
finals sont le méthane et le dioxyde de carbone.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation d'éthers du glycol peut entraîner des
nombreuses émissions dans l'environnement. C'est en parti-
culier l'exposition humaine directe dans l'industrie, dans
les petits ateliers et au cours de l'utilisation domes-
tique de produits à base d'éthers du glycol qui est spéci-
alement préoccupante. En ce qui concerne l'exposition pro-
fessionnelle, les valeurs signalées vont de concentrations
< 0,1 mg/m3 à des concentrations > 150 mg/m3. L'utili-
sation de certains produits de consommation à base
d'éthers du glycol pourrait provoquer une exposition
notable des usagers mais on ne dispose pas de données à ce
sujet.
Outre l'exposition par la voie atmosphérique, l'homme
peut également être exposé par la voie dermique. L'analyse
du sang confirme que ces produits sont rapidement absorbés
par cette voie, qui contribue probablement davantage à la
charge totale de l'organisme que l'exposition par voie
aérienne.
5. Cinétique et métabolisme
Ces quatre éthers sont rapidement absorbés au niveau
de la peau, des poumons et des voies digestives. Des
études de répartition portant sur le 2-ME chez des souris
gravides ont montré que c'est dans le foie maternel, les
voies digestives, le placenta, le sac vitellin et de
nombreuses structures embryonnaires que se rencontraient
les concentrations les plus fortes.
La métabolisation du 2-ME donne naissance à deux
métabolites primaires : le MAA et la méthoxy-2 acétyl-
glycine. La transformation en dioxyde de carbone corres-
pond à une voie métabolique secondaire de moindre
importance. La conversion plasmatique du 2-ME en MAA
s'effectue rapidement, avec une demi-vie de 0,6 heure chez
le rat; en revanche l'excrétion de la MAA est lente, sa
demi-vie étant d'environ 20 heures chez le rat et de 77
heures chez l'homme.
L'administration à des animaux de laboratoire de 2-EE
a conduit à la production d'EAA et d'éthoxy-2 acétyl-
glycine, l'EAA étant le principal métabolite qui se mani-
feste dans l'organe supposé être l'organe cible, à savoir
les testicules. Chez l'homme, une étude sur le 2-EAA a
permis d'observer une voie métabolique analogue, l'acétate
étant d'abord hydrolysé en 2-EE puis oxydé en EAA. Cet
EAA a été ensuite excrété avec une demi-vie estimative de
21 à 42 heures. L'expérience semble indiquer que la
rétention ou l'accumulation des métabolites pourrait être
toxicologiquement importante dans la mesure où ces méta-
bolites seraient responsables de la toxicité observée au
niveau de l'organe cible.
6. Effets sur les êtres vivants dans leur milieu naturel
Il semble que le 2-ME et le 2-EE présentent une faible
toxicité pour les micro-organismes et les animaux aqua-
tiques. En ce qui concerne les micro-organismes, la con-
centration létale dans le milieu est supérieure à 2 %. On
a constaté une inhibition de la croissance des algues
vertes par le 2-ME à la concentration de 104 mg/litre et
de celle des cyanobactéries (algues bleu/vert) à la con-
centration de 100 mg/litre. La toxicité aiguë du 2-EE est
très faible pour les arthropodes (CL50 > à 4 g/litre) et
les poissons d'eau douce (CL50 > à 10 g/litre). Les acé-
tates des éthers du glycol (2-MEA et 2-EAA) sont beaucoup
plus toxiques pour les poissons. Ainsi la CL50 du 2-EEA
pour le vairon Pimephales promelas est de 46 mg par litre
tandis que celle du 2-MAA est de 45 mg/litre pour le
tarpon et pour Lepomis machrochirus. Il n'y a pas eu
d'études à long terme.
7. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
7.1 Toxicité générale
La toxicité du 2-ME et du 2-EE chez l'animal d'expéri-
ence a été beaucoup plus étudiée que celle du 2-MEA et du
2-EAA.
En ce qui concerne le 2-ME et le 2-EE ainsi que leurs
acétates, la dose létale après exposition unique est du
même ordre et ces composés présentent une faible toxicité
aiguë, que l'exposition ait lieu par voie dermique, orale
ou par inhalation. Pour diverses espèces, les valeurs de
la DL50 vont de 900 à 3400 mg/kg de poids corporel pour
le 2-ME, de 1400 à 5500 mg/kg pour le 2-EE, de 1250 à
3900 mg/kg pour le 2-MEA et de 1300 à 5100 mg/kg pour le
2-EAA. Des valeurs de 4603 mg/m3 (2-ME) et de 6698 mg
par m3 (2-EE) ont été signalées pour la CL50 par inhala-
tion chez la souris.
On ne possède que peu de données concernant les effets
irritants au niveau des yeux et de la peau ou le pouvoir
de sensibilisation de ces éthers du glycol chez l'animal.
Il semblerait qu'ils ne soient pas irritants pour la peau,
mais qu'ils puissent l'être pour l'oeil. Chez l'homme,
malgré de fortes expositions, on n'a jamais signalé
d'irritation cutanée ni de sensibilisation à ce niveau.
On a montré qu'en exposant par voie respiratoire des
animaux d'expérience pendant des périodes allant jusqu'à
90 jours, à de fortes concentrations (> 9313 mg de 2-ME
par m3 et > 1450 mg de 2-EE/m3) on déterminait des
effets nocifs sur les paramètres hématologiques, le
système nerveux, les testicules, le thymus, les reins, le
foie et les poumons. A des concentrations plus faibles,
les effets ne s'observaient qu'au niveau du système
hématopoïétique et des testicules. Par exemple, des rats
exposés par inhalation à du 2-ME pendant 13 semaines à des
doses comprises entre 93 et 930 mg/m3, présentaient une
réduction de l'hématocrite, du nombre de leucocytes, de
l'hémoglobine, des plaquettes et des protéines sériques,
mais seulement à la dose la plus forte. Chez des lapins
exposés de la même manière, on notait une réduction de la
taille du thymus et une altération des paramètres hémato-
logiques, à la dose de 903 mg/m3. Le 2-EE a produit des
effets analogues, mais moins graves, chez le rat et le
lapin lors d'une exposition de 13 semaines à la concen-
tration de 1450 mg/m3. On ne dispose d'aucune donnée
résultant d'études à long terme.
7.2 Cancérogénicité et mutagénicité
On a étudié la mutagénicité du 2-ME sur toute une
série de systèmes in vitro constitués de bactéries ou de
cellules mammaliennes. La plupart des études ont fourni
des résultats négatifs, toutefois on a tout de même
signalé des résultats positifs à de très fortes concen-
trations de 2-ME sur des cellules CHO. Il s'agissait
d'aberrations chromosomiques (à des concentrations supéri-
eures à 6830 µg/ml) et d'échanges entre chromatides
soeurs (à des concentrations supérieures à 3170 µg par
ml). La recherche d'aberrations chromosomiques et de
micronoyaux n'a rien donné in vivo . On ne dispose que de
données limitées sur le pouvoir mutagène du 2-EE; en
outre, il n'existe pas de données sur la cancérogénicité
de ces éthers du glycol.
7.3 Organes mâles de la reproduction
On a étudié de manière approfondie l'effet du 2-ME sur
l'appareil reproducteur mâle après administration par voie
orale ou respiratoire de ces substances à des rongeurs.
La présence de modifications dégénératives au niveau de
l'épithélium germinal des tubes séminifères à été systé-
matiquement observée. Des effets analogues ont été con-
statés avec le 2-EE, mais à des doses un peu inférieures.
L'administration par voie orale à des rats de 2-ME
pendant 1 à 11 jours a provoqué une réduction du nombre
des spermatozoïdes et des modifications de leur mobilité
et de leur morphologie, liées à la dose, à partir de
100 mg/kg de poids corporel. L'autopsie a révélé une
atteinte histologique marquée des testicules. La dose
sans effet observable (NOEL) était de 50 mg/kg. La
réduction de la fertilité était encore manifeste huit
semaines après une exposition à 200 mg/kg. Des effets
analogues ont été observés dans le cas du 2-EE à des doses
supérieures ou égales à 500 mg/kg, administrées pendant
des périodes allant jusqu'à 11 jours, la dose sans effet
observable sur 11 jours étant de 250 mg/kg. Toutefois
l'épuisement des réserves de spermatozoïdes, par suite
d'accouplements répétés, s'est accompagné à la dose la
plus faible étudiée (150 mg/kg), d'une réduction de leur
nombre. Après avoir administré par voie orale à des rats
et à des souris une dose unique de 250 mg ou davantage de
2-ME/kg de poids corporel, on a observé chez les animaux
une stérilité complète cinq semaines après l'admini-
stration, une certaine réduction de la fécondité étant
observée dès 125 mg/kg.
L'administration du 2-ME par la voie respiratoire a
donné lieu à des modifications dégénératives analogues au
niveau des testicules. Les effets en question ont été
observés après exposition unique de 4 heures à des doses
supérieures ou égales à 1944 mg/m3, aucun effet n'étant
observé à 933 mg/m3. Les valeurs de la dose sans effet
observable étaient de 311 mg/m3 chez les rats après
exposition de 13 semaines (6 heures par jour, 5 jours par
semaine) et de 933 mg/m3 (6 heures par jour) chez les
souris après exposition à neuf reprises sur une durée
totale de 11 jours. L'exposition de lapins à du 2-ME
pendant 13 semaines (6 heures par jour, 5 jours par
semaine) a entraîné des effets marqués au niveau des
testicules à la dose de 311 mg/m3 ou davantage; à la
dose de 93 mg/m3, on a observé des effets limites; il
n'a pas été possible de déterminer la dose sans effet
observable.
7.4 Toxicité foetale
On a observé des effets toxiques sur le développement
de plusieurs espèces d'animaux de laboratoire après
exposition par toutes les voies possibles, c'est-à-dire
orale, respiratoire et dermique. Le 2-ME a produit des
effets tératogènes chez la souris, le rat, le lapin et le
singe. Le 2-EE et le 2-EEA se sont révélés tératogènes
chez le rat et le lapin. Bien que le 2-MEA n'ait pas
encore été étudié de ce point de vue, son profil méta-
bolique (voir section 6) incite à penser qu'il a
vraisembleblement une toxicité analogue à celle du 2-ME.
C'est dans le cas du 2-ME que l'on possède l'ensemble
le plus complet de données dose-réponse (doses de 31,25 à
1000 mg/kg/j). Lors de cette étude portant sur des souris
auxquelles le 2-ME avait été administré par gavage
(administration les jours 7 et 14 de la gestation), on a
obtenu une dose sans effet observable de 125 mg/kg par
jour en ce qui concerne la toxicité maternelle. Toutefois
des malformations ont été observées à partir de doses
quotidiennes de 62,5 mg/kg et des modifications au niveau
du squelette à partir de 31,25 mg/kg par jour. La dose
sans effet observable relative à la toxicité foetale n'a
pas été indiquée. Lors d'études portant sur des doses
uniques, des souris ont reçu par gavage du 2-ME au onzième
jour de la gestation; la dose de 100 mg/kg n'était pas
foetotoxique tandis que celle de 175 mg/kg a produit des
anomalies digitales, mais sans autres signes de toxicité
maternelle ou foetale. Des anomalis cardio-vasculaires et
électrocardiographiques ont été observés chez les rats
nouveau-nés après administration les 7ème et 13ème jours
de la gestation d'une dose quotidienne de 25 mg/kg. Etant
donné qu'il s'agissait de la dose la plus faible expéri-
mentée, l'étude n'a pas permis d'établir une dose sans
effet observable sur le foetus (on n'a pas observé de
toxicité maternelle à cette dose). De même, aucune dose
de ce type n'a pu être déterminée après administration par
gavage à des guenons de 2-ME aux doses quotidiennes
respectives de 0,16, 0,32, ou 0,47 mmol/kg, du 20ème au
45ème jour de la gestation.
Après exposition par voie respiratoire à du 2-ME à la
dose de 156 mg/m3, on a observé une toxicité foetale
chez des rats et des souris et des malformations chez des
lapins. Pour l'ensemble de ces trois espèces, la dose sans
effet observable sur le développement foetal était de 31
mg/m3. Toutefois des anomalies comportementales et
neurochimiques ont été observées dans la descendance de
rattes exposées du 7ème au 13ème jour ou du 14ème au 20ème
jour de leur gestation à une dose de 78 mg de 2-ME par m3.
Après avoir exposé des rats à des doses de 743 mg par
m3 de 2-EE et des lapins à des doses 589 mg/m3 de la
même substance, on a constaté que ce produit était térato-
gène (avec en outre une certaine toxicité maternelle).
Dans une autre étude, on a constaté une toxicité foetale,
mais sans malformations, chez des rats exposés à des doses
de 184 ou 920 mg de 2-EE par m3 et chez des lapins ex-
posés à la dose de 644 mg/m3 de la même substance. Pour
ce qui est des effets sur le développement foetal, la
valeur de la dose sans effet observable était de 37 mg par
m3 pour le rat et de 184 mg/m3 pour le lapin. On a ob-
servé des anomalies comportementales et neurochimiques
dans la descendance de rattes exposées du 7ème au 13ème
jour et du 14ème au 20ème jour de leur gestation à la dose
de 360 mg de 2-EE par m3.
Des rattes soumises à une application dermique de 0,25
ml de 2-EE non dilué quatre fois par jour du 7ème et 16ème
jour de la gestation, ont eu une descendance où l'on
notait une foetotoxicité marquée et une forte incidence de
malformations malgré l'absence de toxicité maternelle. Des
effets analogues ont été observés à la suite d'un traite-
ment indentique par le 2-EEA selon le même protocole au
moyen d'une dose équimolaire (0,35 ml, quatre fois par
jour).
En exposant par la voie respiratoire des lapines à du
2-EEA du 6ème au 18ème jour de la gestation, on a obtenu
au cours de deux études différentes, des réponses térato-
gènes aux doses de 2176 mg/m3 et 544 mg/m3, la valeur
de la dose sans effet observable sur le développement
foetal étant respectivement de 135 mg/m3 et 270 mg par
m3. L'exposition de rattes du 6ème au 15ème jour de leur
gestation à du 2-EEA a entraîné dans leur descendance une
toxicité foetale à la dose de 540 mg/m3 et des malfor-
mations à la dose de 1080 mg/m3. La dose sans effet ob-
servable sur le développement foetal était de 170 mg par
m3.
8. Effets sur l'homme
On ne dispose que de renseignements limités sur les
effets toxiques chez l'homme de ces quatre éthers du
glycol. Les résultats fournis par quelques études de cas
ou études épidémiologiques sur les lieux de travail sont
dans la ligne des effets observés chez les animaux de
laboratoire. On n'a pas eu connaissance de rapports qui
chiffrent l'exposition de la population en général ni les
effets sur la santé.
Lors de deux cas non mortels d'empoisonnement par
ingestion d'un volume de 100 ml de 2-ME, on a noté les
principaux symptômes suivants : nausée, vertiges, cyanose,
tachycardie, hyperventilation et acidose métabolique avec
quelques signes d'insuffisance rénale. Des sympômes
analogues mais moins graves ont été observés chez une
personne qui avait ingéré 40 ml de 2-EE. Lors d'un
empoisonnement mortel par ingestion de 400 ml de 2-ME,
l'autopsie a révélé une gastrite hémorragique aiguë, une
dégénérescence graisseuse du foie et une altération
dégénérative des tubules rénaux.
L'exposition réitérée de travailleurs à du 2-ME et à
du 2-EE, en plus d'autres solvants, a entraîné chez eux de
l'anémie, un leucopénie, une faiblesse générale et une
ataxie. Dans nombre de ces études, il n'a pas été possible
de trouver une estimation fiable de l'exposition des
sujets. On a rapporté des effets hématologiques dus aux
éthers du glycol chez l'homme et on a notamment décrit
l'apparition d'une anémie macrocytaire chez un travailleur
exposé à du 2-ME (dose moyenne 105 ml/m3), ainsi qu'à
d'autres solvants.
Il a été fait état d'une toxicité médullaire chez des
ouvriers dont l'épiderme était exposé à du 2-ME, et des
effets immunologiques ont été également notés à la suite
d'une exposition prolongée (8 à 35 années) au 2-ME et au
2-EE (doses moyennes d'exposition 6,1 mg/m3 et 4,8 mg par
m3 respectivement).
Des études épidémiologiques effectuées sur des
ouvriers exposés à du 2-ME et à du 2-EE ont révélé des
anomalies au niveau de la fonction de reproduction, avec
une fréquenc accrue des cas d'oligospermie. L'exposition
à du 2-EE (37 ouvriers) à des concentrations pouvant
atteindre 88,5 mg/m3 a entraîné une modification du sper-
mogramme. Parmi 73 ouvriers exposés à du 2-ME (jusqu'à
17,7 mg/m3) et à du 2-EE (jusqu'à 80,5 mg/m3), on a
constaté une fréquence accrue de cas d'oligospermie et
observé certains effets hématologiques, pour des doses
d'exposition (TWA) de 2,6 mg/m3 dans le cas du 2-ME et de
9,9 mg/m3 dans celui du 2-EE.
Les effets indésirables constatés chez l'homme par
suite d'une exposition professionnelle correspondent à
ceux qui ont été observés chez les animaux de laboratoire.
Cependant, l'évaluation de l'exposition présentant un
certain nombre d'insuffisances et du fait qu'il s'agissait
d'expositions simultanées à plusieurs substances, il n'a
pas été possible d'en déduire une relation dose-réponse.
9. Conclusions
De nombreuses personnes peuvent être exposées à ces
quatre éthers du glycol à des concentrations comparables à
celles que l'on rencontre dans l'industrie, par suite de
l'utilisation de certains produits de consommation ou de
produits commerciaux. Une exposition professionnelle non
négligeable peut se produire par inhalation ou par résorp-
tion cutanée. Des mesures faites en petit nombre dans
l'air des lieux de travail indiquent des teneurs allant <
0,1 mg/m3 à > 150 mg/m3.
Le 2-ME et le 2-EE sont tous deux d'une faible toxi-
cité pour les micro-organismes et les espèces aquatiques.
Il n'existe aucune donnée qui permettrait d'évaluer le
risque d'effets indésirables sur les êtres vivants dans
leur milieu naturel par suite d'une exposition de longue
durée.
Chez le rat, la dose de 2-ME sans effets aigus observ-
ables au niveau testiculaire, est de 933 mg/m3; en cas
d'exposition répétée, la dose sans effet observable est de
311 mg/m3. En exposant de manière répétée l'espèce la
plus sensible, à savoir le lapin, on a observé un effet
net dès 311 mg/m3; cet effet était limite à la dose de
93 mg/m3 (un animal sur cinq). Les données fournies par
des études effectuées sur des sujets humains exposés de
par leur profession à du 2-ME et à du 2-EE montrent que
ces éthers du glycol exercent une certaine toxicité
testiculaire.
Chez toutes les espèces étudiées (souris, rats et
lapins) on a observé après exposition au 2-ME à des doses
égales ou supérieures à 156 mg/m3, une toxicité vis-à-
vis du développement foetal. Des anomalies comportement-
ales et neurochimiques ont été observées chez le rat après
exposition in utero à 78 mg/m3 de cette substance sans
qu'on puisse déterminer de dose sans effet observable. Le
2-EE et le 2-EEA se sont révélés légèrement moins actifs.
Des effets ont été également observés sur le développement
de foetus de rats et de lapins après exposition à du 2-EE
à des doses égales ou supérieures 368 mg/m3. Ces effets
étaient légers chez les rats exposés à 184 mg de 2-EE par
m3 mais on a pu néanmoins fixer nettement la dose sans
effet observable chez le rat et le lapin à la valeur de
38 mg/m3.
Ces éthers du glycol produisent des effets hématolo-
giques chez la souris, le rat, le lapin, le chien, le
hamster et le cobaye. Ces résultats sont en accord avec
les anomalies hématologiques observées lors des quelques
études consacrées à des travailleurs de l'industrie qui
avaient subi des expositions répétées à du 2-EE et/ou du
2-ME. Lors d'études sur l'animal comportant une expo-
sition répétée à ces deux substances, on a fixé à 93 mg
par m3 la dose de 2-ME sans effet observable chez le
lapin et à 368 mg/m3 la dose de 2-EE sans effet obser-
vable chez le rat et le lapin. On n'a pas pu obtenir de
données qui permettent une évaluation quatitative des
effets hématologiques aigus consécutifs à une exposition.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
1.1 Exposition
Nombreuses sont les personnes qui peuvent être
exposées au méthoxy-2 éthanol (2-ME), à l'éthoxy-2 éthanol
(2-EE) et à leurs acétates (2-MEA et 2-EEA) à des concen-
trations comparables à celles que l'on rencontre dans
l'industrie, lors de l'utilisation de produits de consom-
mation et de produits commerciaux. En revanche l'expo-
sition par l'intermédiaire des denrées alimentaires, de
l'eau ou de l'air ambiant est probablement négligeable.
Cette hypothèse ne repose que sur les propriétés
physiques et chimiques de ces composés et sur le fait
qu'ils se dégradent rapidement dans l'environnement.
Une exposition professionnelle non négligeable peut se
produire par inhalation ou résorption cutanée. Les
quelques mesures de concentrations dans l'air des lieux de
travail ont donné des valeurs qui vont de moins de 0,1 mg
par m3 à plus de 150 mg/m3. Toutefois les données de
surveillance existantes sont très limitées et il peut y
avoir d'importantes variations entre les différentes
industries et dans une même industrie. En raison du risque
de résorption cutanée, une simple surveillance de l'air
des lieux de travail risque de sous estimer l'exposition
totale. Pour évaluer la charge totale de l'organisme, la
meilleure méthode consiste à faire un contrôle biologique.
Une forte exposition peut se produire lors de travaux tels
que la peinture, l'impression ou le nettoyage mais il faut
se souvenir que ces composés sont utilisés à l'occasion
d'un grand nombre d'autres activités au cours desquelles
on pourrait craindre une exposition.
1.2 Effets sur la santé
Les principaux effets qu'on peut craindre chez l'homme
tiennent à l'action toxique de ces composés sur le
développement foetal, les testicules et les paramètres
hématologiques. La réalité de ces effets est attestée par
des données nombreuses et cohérentes obtenues chez l'ani-
mal ainsi que par quelques données concernant l'homme.
Tous ces effets peuvent appraître par suite d'expositions
à court ou à long terme. Chez l'animal de laboratoire,
une exposition répétée à des très fortes doses de 2-ME et
de 2-EE (plus de 930 ou 1450 mg/m3, respectivement)
entraîne des effets toxiques qui se traduisent par des
anomalies neuro-comportementales, hépatiques et rénales.
On les observe également dans les cas d'intoxication
humaine.
Ces quatre éthers du glycol exercent des effets
toxiques très voisins tant sur les testicules que sur le
développement du foetus, et ce, chez toutes les espèces
étudiées et par toutes les voies d'exposition qui ont été
utilisées (voie respiratoire, voie percutanée, voie
orale). L'étude du mécanisme de ces effets montre que
dans les deux cas, une phase d'activation est nécessaire,
à savoir la métabolisation en un dérivé de l'acide alkoxy-
acétique correspondant. La métabolisation s'effectue en
présence du système de l'alcool déshydrogénase qui est
commun à l'homme et aux animaux de laboratoire. Des méta-
bolites toxiques, l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA) ont été décelés dans l'urine de
sujets exposés à ces solvants. La régularité de cette
réponse toxique d'une espèce animale à l'autre, jointe à
l'analogie du métabolisme chez l'homme et l'animal,
montrent à l'évidence que l'homme pourrait être la cible
des mêmes effets toxiques sur les testicules et le
développement foetal. Les données dont on dispose au sujet
de l'excrétion des acides alkoxyacétiques chez l'homme
indiquent que leur durée de rétention est plus longue chez
celui-ci que chez l'animal, ce qui incite à penser que
l'homme pourrait être plus sensible à ces effets que
l'animal de laboratoire le plus sensible. C'est la
résorption cutanée rapide de ces composés qui est parti-
culièrement préocccupante. On a observé des effets térato-
gènes et autres anomalies du developpement à la suite de
l'application de 2-ME, de 2-EE et de 2-EAA sur la peau
intacte du rat.
Des lésions testiculaires ont été observées chez le
rat, la souris et le lapin à la suite d'une exposition à
ces éthers du glycol, soit par la voie respiratoire soit
par la voie orale. Chez le rat, une seule exposition par
voie respiratoire à des doses de 2-ME supérieures ou
égales à 1944 mg/m3 pendant 4 heures et une exposition
répétée à des doses supérieures ou égales à 933 mg par
m3 pendant 13 semaines ont déterminé des anomalies histo-
logiques au niveau testiculaire. Dans le cas de l'expo-
sition unique, la dose sans effet observable était de 933
mg/m3; elle était 311 mg/m3 dans le cas d'expositions
répétées. La souris a semblé un peu moins sensible, la
dose sans effet observable dans son cas étant, pour des
expositions répétées, de 933 mg/m3. Toutefois le lapin
l'était davantage, avec des altérations testiculaires
marquées qu'on pouvait observer après des expositions
répétées à 311 mg/m3 et la présence d'effets limites (un
lapin sur cinq) dès la dose de 93 mg/m3. Des effets ana-
logues ont été observés à la suite de l'exposition par
voie orale de rats à du 2-ME, une exposition de courte
durée (et notamment à une dose unique) ayant déterminé des
lésions testiculaires à partir de 100 mg/m3. Dans le
cas d'une étude portant sur les effets subaigus (11 jours)
la dose sans effet observable a été de 50 mg/kg. Le 2-EE
présente une toxicité testiculaire un peu moindre que
celle du 2-ME; ces effets n'apparaissent qu'à des doses
de 500 mg/kg ou davantage et la dose sans effet observable
se situe à 250 mg/kg.
Les données fournies par les études menées sur des
sujets humains exposés de par leur profession à du 2-ME et
à du 2-EE cadrent avec les données obtenues sur l'animal
et montrent que ces éthers du glycol peuvent produire des
effets toxiques au niveau testiculaire chez l'homme. Des
études épidémiologiques portant sur de petits groupes de
travailleurs exposés au 2-EE dans un atelier de fonte de
métaux et chez des peintres d'un chantier naval exposés à
ces deux composés, ont révélé une oligospermie systéma-
tique. Les données relatives aux niveaux d'exposition sont
limitées mais dans chaque cas, il y a lieu de penser qu'il
y a eu exposition par voie cutanée et respiratoire.
On a observé chez le rat, la souris, le lapin et le
singe des effets toxiques sur le développement embryon-
naire après exposition à ces éthers du glycol par voie
percutanée, orale ou respiratoire. Du 2-ME non dilué
appliqué à 12 reprises au cours d'une journée sur la peau
rasée de rattes gravides (zone d'application couverte
pendant 6 heures) s'est révélé mortel pour les animaux
alors qu'appliqué à 10 reprises sans pansement, du 2-EE
(1,0 ml/jour) ou du 2-EEA (1,4 ml/jour) ont produit des
effets tératogènes mais n'ont pas été toxiques pour les
femelles gravides. Au cours d'une autre étude, 12 appli-
cations de 2-ME à 10 % dans du soluté physiologique, avec
pansement sur le site d'application, ont entraîné des
effets toxiques sur le développement embryonnaire, la dose
sans effet observable se situant à la concentration de
3 %. Après administration répétée par voie orale de 2-ME
à des animaux gravides, on n'a pas observé de dose sans
effet toxique. La dose la plus faible produisant un effet
observable dans le cas de l'administration par voie orale
était de 31,5 mg/kg par jour pour la souris, de 25 mg/kg
pour le rat et de 0,16 mmol/kg pour le singe. En ce qui
concerne l'administration d'une dose unique, on ne possède
des résultats que pour le 2-ME chez la souris, avec une
dose sans effet observable pour le 11ème jour de la
gestation (le jour le plus sensible) de 100 mg/kg et une
valeur de 175 mg/kg pour la dose la plus faible avec effet
observable. Le 2-EE et le 2-EEA ont fait l'objet d'études
au cours desquelles des rats et des lapins ont été exposés
à ces deux composés par la voie respiratoire. L'expo-
sition des rats au 2-EE a fait l'objet de deux études qui
ont révélé des effets tératogènes (743 mg/m3, 7 heures
par jour, du premier au 19ème jour de la gestation) ou des
effets foetotoxiques (184 et 920 mg/m3, 6 heures par
jour, du 6ème au 15ème jour de la gestation). Dans cette
dernière étude, on a trouvé une dose sans effet observable
de 37 mg/m3. Chez des lapins exposés à du 2-EE on a
également observé des effets tératogènes (589 mg/m3, 7
heures par jour du premier au 18ème jour de la gestation)
et des effets foetotoxiques (644 mg/m3, 6 heures par
jour, du 6ème au 18ème jour de la gestation). Dans cette
dernière étude, la dose sans effet observable était de
184 mg/m3. Chez des lapines exposées à du 2-EEA 6 heures
par jour du 6ème au 18ème jour de la gestation, on a
observé des effets tératogènes à la dose de 2160 mg/m3
dans une étude et à la dose de 1620 mg/m3 dans une
autre. Les deux études ont montré l'apparition d'une
foetotoxicité à 540 mg/m3; la dose sans effet observable
correspondant, dans chaque étude, à la dose la plus faible
étudiée (respectivement 135 et 270 mg/m3). Des rats
exposés par voie respiratoire à du 2-EEA 6 heures par jour
du 6ème au 15ème jour de la gestation ont présenté le même
de type de réactions : effets tératogènes à 1620 mg par
m3, foetotoxicité à 1080 mg/m3 et aucun effet à 260 mg
par m3.
Des effets toxiques sur le développement embryonnaire
ont donc été observés chez toutes les espèces (souris,
rats et lapins), exposées à des doses supérieures ou
égales à 156 mg de 2-ME. Pour ces trois espèces, la dose
sans effet observable était de 31 mg/m3. Après expo-
sition in utero à 78 mg/m3, on a observé chez la descen-
dance des altérations comportementales et neurochimiques
sans qu'il soit possible de déterminer la dose sans effet
observable.
Le 2-EE et le 2-EEA se sont révélés légèrement moins
actifs. Des effets toxiques sur le développement embryon-
naire ont été observés chez des rats et des lapins après
exposition à des doses supérieures ou égales à 368 mg par
m3 de 2-EE. De légers effets de ce type ont été également
observés chez des rats exposés à 184 mg de 2-EE/m3, la
dose sans effet observable étant clairement établie à
37 mg/m3. En ce qui concerne le 2-EEA, la dose sans
effet observable était de 170 mg/m3 pour les rats et les
lapins.
Des effets hématologiques consécutifs à une exposition
à une dose unique on été observés chez l'animal ainsi qu'à
la suite d'intoxications chez l'homme. L'exposition
répétée par voie respiratoire, de lapins, l'espèce la plus
sensible, à du 2-ME 5 fois par semaine pendant 13
semaines, a permis de fixer la dose sans effet observable
à 93 mg/m3. Administré à répétition, le 2-ME produit
également des anomalies hématologiques chez la souris, le
lapin, le chien, le hamster et le cobaye. Le 2-EE est
moins actif à cet égard que le 2-ME. En ce qui concerne
les effets hématologiques chez le rat et le lapin, après
exposition à du 2-EE pendant 13 semaines, 5 fois par
semaine et 6 heures par jour, on peut fixer la dose sans
effet observable à 368 mg/m3. Chez le chien et la
souris, on observe également après, exposition répétée à
des doses plus élevées, un certain nombre d'effets hémato-
logiques. L'exposition aux acétates de 2-EE et de 2-ME
devrait provoquer des effets analogues, toutes choses
égales d'ailleurs, mais les données dont on dispose sur
l'exposition et les effets hématologiques de ces composés
sont trop limitées pour qu'on puisse établir les con-
ditions dans lesquelles une seule exposition conduirait à
des effets hématologiques chez l'homme.
On a observé dans l'industrie des niveaux d'exposition
voisins de la dose sans effet hématologique observable que
l'on a pu déterminer chez l'animal après expositions
répétées à du 2-ME et à du 2-EE. On peut en conclure,
compte tenu de la sensibilité probablement plus grande de
l'homme et de l'accumulation vraisemblable de métabolites
dans le sang, que l'exposition résultant de l'utilisation
de produits de consommation ou d'activités dans
l'industrie pourrait entraîner des effets hématologiques
de ce genre. A l'appui de cette hypothèse, on peut citer
les effets hématologiques effectivement signalés lors de
quelques études effectuées chez des travailleurs de
l'industrie ayant subi des expositions répétées au 2-EE
et/ou au 2-ME.
2. Evaluation des effets sur l'environnement
La libération directe dans l'atmosphère ou l'utili-
sation de ces produits comme solvants volatils peut con-
duire à une exposition dans le milieu ambiant. Ce peut
être également le cas par suite d'une libération acciden-
telle sur le sol ou dans l'eau. L'accumulation dans le
sol et les eaux superficielles ne peut se produire qu'en
l'absence de dégradation de ces composés. Or, on sait que
ces éthers du glycol se décomposent rapidement sous
l'effet de processus chimiques et biologiques; ils ne
devraient donce pas s'accumuler. Le 2-MEA et le 2-EAA
devraient également s'hydrolyser rapidement et subir
ensuite une biodégradation aérobie. Toutefois dans des
conditions d'anaérobiose, on pourrait craindre une
contamination des sols et des nappes phréatiques encore
que le phénomène soit vraisemblablement transitoire et le
risque correspondant négligeable.
Le 2-ME et le 2-EE sont peu toxiques pour les micro-
organismes et les espèces aquatiques. Leurs acétates, en
revanche, présentent une toxicité aiguë beaucoup plus
forte. On ne dispose pas de données à partir desquelles
on puisse déterminer leur potentiel nocif pour les espèces
vivantes en cas d'exposition prolongée.
RECOMMANDATIONS
1. Protection de la santé
1. Il conviendrait de recercher des solvants moins
toxiques que le méthoxy-2 éthanol, l'éthoxy-2 éthanol et
leurs esters, en particulier pour les remplacer dans les
produits de consommation. Il est également important
d'évaluer les effets des autres éthers de l'éthylène-
glycol car certains d'entre eux pourraient excercer des
effets analogues aux quatre éthers qui ont fait l'objet de
la présente évaluation.
2. Du fait des effets toxiques reconnus de ces éthers du
glycol, les autorités responsables devraient se préoccuper
sérieusement de trouver les moyens d'avertir les consom-
mateurs des dangers que présente l'utilisation de ces
produits, en particulier en cas d'exposition par voie
cutanée.
3. Compte tenu des données toxicologiques récentes et de
la possibilité d'une très forte absorption percutanée des
ces éthers du glycol, il importe de revoir les limites
nationales d'exposition professionnelle pour faire en
sorte que la dose quotidienne totale à laquelle sont
exposés les travailleurs par n'importe quelle voie, ne
menace pas leur santé.
4. Lorsqu'elle est assez importante, une dose unique peut
produire des effets sur l'animal. Pour réduire les
risques, il est recommandé d'utiliser ces produits avec
prudence (veiller à l'hygiène personnelle, utiliser des
dispositifs protecteurs appropriés et assurer une venti-
lation suffisante). Les données montrent que les effets
toxiques sur le développement embryonnaires ainsi que sur
le sang et les testicules, consécutifs à une exposition
répétée, pourraient exiger des mesures de protection plus
importantes.
2. Recherches à affectuer
1. Du fait que l'acide méthoxyacétique (MAA) et l'acide
éthoxyacétique (EAA), qui sont les principaux métabolites
du 2-ME, du 2-EE et de leurs esters, exercent des effets
toxiques sur les organes reproducteurs mâles, il faudrait
en étudier le mode d'action. S'ils apparaît que le MAA et
EAA ne sont pas les véritables responsables de ces effets,
ceux-ci devront être identifiés et leur mode d'action
élucidé.
2. Ces quatre éthers du glycol ont des effets sur le sang
et sur la fonction de reproduction masculine (oligo-
spermie). Les données disponibles, bien qu'en nombre
limité, indiquent que ces deux effets se manifestent à des
doses analogues. Il faudrait étudier le mode d'action de
ces composés sur ces deux types d'organes et étudier
parallèlement le nombre de spermatozoïdes et les effets
hématologiques afin de voir si ces derniers sont suscept-
ibles de jouer le rôle de signal d'alarme pour d'autres
effets toxiques de ces composés.
3. La surveillance de l'air ne suffit pas à assurer une
faible exposition. Un contrôle biologique peut permettre
de déceler les insuffisances des mesures de protection. A
l'heure actuelle on n'a pas établi avec une certitude
suffisante la relation qui existe entre les indicateurs
biologiques de l'exposition, la charge totale de l'organ-
isme et les effets physiologiques observés. Des travaux
sont encore nécessaires pour pouvoir établir les limites
de sécurité en fonction des résultats de la surveillance
biologique.
4. Il faudrait envisager des études épidémiologiques ou
une surveillance sanitaire spécifique au sein de popu-
lations qui sont fortement exposées à ces éthers du glycol
afin d'obtenir des relations exposition-effets; on
pourrait alors déterminer les limites de sécurité, à
condition toutefois que l'exposition globale puisse être
correctement et suffisamment évaluée par la surveillance
de l'environnement et des contrôles biologiques.
5. Il conviendrait d'étudier l'éventualité d'effets sur
l'appareil reproducteur féminin en procédant à des études
de reproduction sur plusieurs générations d'animaux de
laboratoire.
6. Les résultats disponibles indiquent que l'homme
pourrait métaboliser dans une plus grande proportion que
le rat ces éthers du glycol en acides alkoxyacétiques
correspondants, la demi-vie des ces métabolites toxiques
s'étant environ quatre fois plus longue chez l'homme. Par
ailleurs l'organisme du rat est capable de conjuguer une
fraction plus importante des métabolites acides que ne le
fait celui de l'homme. Ces différences pourraient
entraîner une sensibilité relativement plus importante de
l'homme aux éthers du glycol. Une connaissance plus
précise du métabolisme et de la cinétique d'excrétion de
ces composés permettrait de mieux prévoir les limites de
sécurité.
7. Les résultats fournis par des études à court terme
(13 semaines) montrent que des effets s'exercent sur
divers organes. Toutefois, il n'a pas été procédé à des
études suffisamment longues pour qu'on puisse déterminer
si ces effets sont réversibles ou non. Il est donc
recommandé de procéder à des études au cours desquelles
des animaux de laboratoire subiront une exposition d'au
moins 13 semaines à ces composés, suivie d'une période de
récupération. A l'issue de cette période, on devra
déterminer les paramètres physiologiques importants afin
de voir si les effets sont passagers ou non.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas, métodos analíticos
En esta monografía sólo se tienen en cuenta los éteres
metílico y etílico del etilenglicol, es decir el 2-metoxi-
etanol (2-ME) y el 2-etoxietanol (2-EE), así como sus
ésteres acéticos respectivos, el acetato de 2-metoxietilo
(2-MEA) y el acetato de 2-etoxietilo (2-EEA). Estos cuatro
compuestos son todos ellos líquidos estables, incoloros e
inflamables con un olor levemente etéreo y todos ellos son
miscibles con (o, en el caso del 2-EEA, muy solubles en)
agua y miscibles con numerosos solventes orgánicos.
Se dispone de métodos analíticos para detectar estos
éteres glicólicos o sus metabolitos en diversos medios
(aire, agua, sangre y orina). En muchos casos se basan en
el empleo de métodos de adsorción o extracción para con-
centrar la muestra, seguidos de un análisis por cromato-
grafía de gases. Mediante la cromatografía de gases o la
cromatografía de líquidos de alto rendimiento, cabe la
posibilidad de determinar cuantitativamente el ácido
2-metoxiacético (MAA) y el ácido 2-etoxiacético (EAA) -
metabolitos del 2-ME y del 2-EE - en la orina, por lo gen-
eral tras derivación, a concentraciones de 5-100 µg por
ml.
2. Fuentes de exposición humana y ambiental
Los cuatro éteres glicólicos examinados están todos
ellos producidos por la reacción del óxido etilénico con
el alcohol apropiado, seguida, si procede, de esterifica-
ción con ácido etanoico.
No se dispone de datos sobre la producción mundial de
estos éteres glicólicos. Sin embargo, la producción anual
combinada de Europa occidental, los Estados Unidos de Amé-
rica y el Japón es aproximadamente del 79 x 103 toneladas de
2-ME y 205 x 103 toneladas de 2-EE. Una proporción con-
siderable se utiliza en la fabricación de pinturas, color-
antes y lacas así como en forma de solventes para tinta de
imprenta, resinas y tintes, y como productos de limpieza
domésticos e industriales. También se utilizan estos com-
puestos como aditivos anticongelantes en los líquidos hi-
dráulicos y el combustible de los reactores.
3. Transporte, distribución y transformación en el medio ambiente
La hidrosolubilidad de estos éteres glicólicos y su
presión de vapor relativamente baja podrían dar lugar a su
acumulación en el agua en ausencia de degradación. Sin
embargo, esta posibilidad queda aparentemente excluida
debido a la degradación por los microorganismos presentes
en el suelo, los cienos de alcantarilla y el agua.
Las emisiones atmosféricas causadas por el uso de
éteres glicólicos como solventes volátiles originan la
máxima exposición ambiental. En el medio ambiente general,
la degradación fotolítica parece ser rápida y cabe prever
niveles inferiores a 0,0007 mg/m3 (2 x 10-4 ppm).
En condiciones aeróbicas los microorganismos degradan
rápidamente los éteres glicólicos en forma de dióxido de
carbono y agua, mientras que en condiciones de anaerobi-
osis los principales productos finales son el metano y el
dióxido de carbono.
4. Niveles en el medio ambiente y exposición humana
El uso de éteres glicólicos puede dar lugar a emisi-
ones considerables y muy extendidas en el medio ambiente.
Suscita especial preocupación la exposición humana directa
en la industria, en los talleres de dimensiones modestas y
como consecuencia del empleo doméstico de productos que
contienen dichos éteres. Se han señalado valores de expo-
sición profesional comprendidos entre < 0,1 mg/m3 y > 150
mg/m3. Los usuarios de productos de consumo pueden
sufrir una exposición considerable, aunque no se dispone
de datos al respecto.
Además de la exposición a los éteres glicólicos pres-
entes en la atmósfera, las personas pueden estar expuestas
por vía cutánea. Los análisis de sangre confirman que la
absorción es rápida por esta vía, que puede contribuir más
que la exposición atmosférica a la carga total que recibe
el organismo.
5. Cinética y metabolismo
Se ha demostrado que los cuatro éteres glicólicos
pueden absorberse rápidamente a través de la piel, los
pulmones y el tracto gastrointestinal. Los valores más
elevados que se han obtenido en los estudios sobre la
distribución del 2-ME en ratonas gestantes se sitúan en el
hígado, la sangre y el tracto gastrointestinal de la
madre, así como en la placenta, el saco vitelino y en
numerosas estructuras del embrión.
La transformación metabólica del 2-ME produce dos
metabolitos primarios: MAA y glicina de 2-metoxiacetilo.
La metabolización a dióxido de carbono representa una vía
secundaria de menor importancia. La conversión del 2-ME
en MAA en el plasma se produce rápidamente, con una vida
media de 0,6 h en las ratas, pero la secreción del MAA es
lenta, con una vida media de 20 h aproximadamente en la
rata y de 77 h en el hombre.
En los animales de laboratorio, la administración de
2-EE da lugar a la producción de EAA y de glicina de
2-etoxiacetilo; el EAA es el principal metabolito que
aparece en los testículos, que son el "órgano diana"
presunto. En un estudio humano en el que se utilizó 2-EEA
se observó una vía metabólica análoga: el acetato se hi-
drolizó primero en 2-EE y luego se transformó en EAA por
oxidación. El EAA resultante se excretó con una vida media
estimada en 21-42 h. Los estudios experimentales hacen
pensar que la retención o acumulación de los metabolitos
podrían ser importantes desde el punto de vista toxico-
lógico en el supuesto de que dichos metabolitos sean la
causa de la toxicidad observada en el "órgano diana".
6. Efectos en los organismos presentes en el medio ambiente
La toxicidad del 2-ME y del 2-EE para los microorga-
nismos y animales acuáticos parece ser baja. En el caso
de los microorganismos, la concentración letal en el medio
es superior al 2%. Con el 2-ME se ha observado inhibición
del crecimiento de las algas verdes a 104 mg/litro y de
las cianobacterias (algas verde azuladas) a 100 mg/litro.
La toxicidad aguda del 2-EE es muy baja para los artrópo-
dos (CL50 > 4 g/litro) y para los peces de agua dulce
(CL50 > 10 g/litro). Los acetatos de éteres glicólicos
(2-MEA y 2-EEA) son mucho más tóxicos para los peces. La
CL50 del 2-EEA para el Phoxinus cabezudo es 46 mg/litro y
la del 2-MEA para el pez plateado de la pleamar y los
Lepomis de agallas azules es de 45 mg/litro. No se han
hecho estudios a largo plazo.
7. Efectos en los animales de experimentación y en los
sistemas de experimentación in vitro
7.1 Toxicidad sistémica
La toxicidad del 2-ME y del 2-EE en los animales de
experimentación se ha estudiado en medida mucho mayor que
la del 2-MA y del 2-EEA.
El 2-ME y el 2-EE y sus acetatos dan tasas análogas de
letalidad tras una exposición única y producen una letali-
dad aguda baja cuando la exposición tiene lugar por vía
dérmica u oral o por inhalación. Los valores de la DL50 oral
en las diversas especies estudiadas oscilan entre 900 y
3400 mg/kg de peso corporal en el caso del 2-ME, entre
1400 y 5500 mg/kg en el del 2-EE, entre 1250 y 3930 mg/kg
en el del 2-MEA, y entre 1300 y 5100 mg/kg en el de 2-EEA.
En los ratones se han obtenido valores de la CL50 por
inhalación de 4603 mg/m3 (2-ME) y 6698 mg/m3 (2-EE).
Sólo se dispone de datos limitados acerca de la irri-
tación cutánea u ocular o del potencial de sensibilización
de estos éteres glicólicos en los animales. Al parecer,
no son irritantes para la piel, pero pueden causar irri-
tación en los ojos. En el hombre no se ha observado irri-
tación ni sensibilización cutáneas ni siquiera en caso de
gran exposición.
La exposición por inhalación a corto plazo (hasta 90
días) de los animales de experimentación a concentraciones
elevadas (> 9313 mg de 2-ME/m3 y > 1450 mg de 2-EE/m3)
ejerce, según se ha demostrado, efectos adversos en los
parámetros sanguíneos, el sistema nervioso y los testícu-
los, el timo, el riñón, el hígado y los pulmones. Utiliz-
ando niveles más bajos de exposición, se han observado
efectos en el sistema hematopoyético y en los testículos.
Así, por ejemplo, las ratas expuestas durante 13 semanas a
la inhalación de 2-ME a concentraciones comprendidas entre
93 y 930 mg/m3 presentaron una reducción del volumen
hematocrito y de los glóbulos blancos, la hemoglobina, las
plaquetas y las concentraciones de proteínas séricas sola-
mente cuando se aplicaba la dosis máxima, mientras que los
ratones expuestos del mismo modo presentaron disminución
del tamaño del timo además de la disminución de los pará-
metros sanguíneos con concentraciones de 930 mg/m3. Las
ratas y conejos expuestos al 2-EE presentaron efectos
análogos pero menos intensos cuando soportaron durante 13
semanas una concentración de 1450 mg/m3. No se dispone
de datos sobre estudios a largo plazo.
7.2 Carcinogenicidad y mutagenicidad
La mutagenicidad del 2-ME se ha estudiado en una gama
de sistemas in vitro utilizando bacterias y células de
mamífero. Aunque la mayor parte de los estudios han dado
resultados negativos, algunos informes acusan resultados
positivos en cuanto a la mutagenicidad de las concentraci-
ones muy altas de 2-ME en células CHO estudiadas desde el
punto de vista de las aberraciones cromosómicas (a 6830 µg
por ml o más) o del intercambio de cromátides equiparables
(3170 µg/ml o más). En cambio, la investigación in vivo
de aberraciones cromosómicas y micronúcleos ha dado
siempre resultados negativos. Sólo se dispone de una
información muy limitada sobre el potencial mutagénico del
2-EE y no se dispone de ningún dato sobre la carcinogen-
icidad de estos éteres glicólicos.
7.3 Sistema reproductor masculino
Los efectos del 2-ME en el sistema reproductor mascu-
lino se han estudiado detenidamente en roedores expuestos
por vía oral o por inhalación. En el epitelio germinal de
los tubos seminíferos se han observado constantemente
alteraciones degenerativas. Análogos efectos se han obten-
ido con el 2-EE, si bien con niveles de dosificación algo
más elevados.
En la rata, la administración oral de 2-ME durante 1-
11 días ha dado lugar a un descenso del recuento de esper-
matozoides con cambios de la motilidad y la morfología de
éstos en relación con la dosis utilizada; como niveles de
dosificación se utilizaron 100 mg/kg de peso corporal o
más. En la autopsia se encontraron acusadas alteraciones
histológicas en los testículos. El nivel de efecto no
observado (NENO) fue de 50 mg/kg. La reducción de la fer-
tilidad seguía siendo patente a las 8 semanas de la expo-
sición a 200 mg/kg. Análogos efectos se observaron con
dosificaciones de 500 mg de 2-EE/kg o más, administrados
durante 11 días como máximo; en el tratamiento de 11 días
el NENO fue de 250 mg/kg. En cambio, cuando las reservas
de espermatozoides están reducidas por la frecuencia de
los acoplamientos, se observó cierta reducción de los
recuentos con la dosis más baja estudiada. Los estudios
de fertilidad consecutivos a la administración de una sola
dosis oral de 250 mg de 2-ME/kg o más mostraron una ester-
ilidad completa, tanto en las ratas como en los ratones, a
partir de las 5 semanas de administración; con 125 mg/kg
se observó ya cierto descenso de la fertilidad.
En los experimentos de inhalación se observaron alter-
aciones degenerativas análogas en los testículos con el
2-ME. Los efectos se observaron tras una sola exposición
(4 h) a 1944 mg/m3 o más pero no con 933 mg/m3. El NENO
fue de 311 mg/m3 en la rata tras la exposición durante 13
semanas (6 h/día, 5 días/semana) y de 933 mg/m3 (6 h/día)
en los ratones tras la exposición en 9 ocasiones en el
curso de 11 días. En los conejos expuestos al 2-ME durante
13 semanas (6 h/día, 5 días/semana) se observaron efectos
marcados en los testículos con concentraciones de 311
mg/m3 o más y efectos marginales con 93 mg/m3; no se
determinó el NENO.
7.4 Toxicidad para el desarrollo
En varias especies de animales de laboratorio se ha
observado toxicidad para el desarrollo tras la exposición
a los compuestos utilizando todas las vías de administra-
ción: oral, inhalatoria y dérmica. El 2-ME produjo efectos
teratógenos en ratones, ratas, conejos y monos. El 2-EE y
el 2-EEA resultaron teratógenos en las ratas y los
ratones. Aunque no se ha estudiado la toxicidad sobre el
desarrollo del 2-MEA, los perfiles metabólicos (véase la
sección 6) hacen pensar que es posible que el 2-MEA tenga
una toxicidad análoga a la del 2-ME.
En relación con el 2-ME se dispone de la gama más
amplia de datos dosis/respuesta (dosis de 31,25 por 1000
mg/kg por día). En este estudio de administración inten-
siva en el que se utilizaron ratones (2-ME administrado
entre el 7° y el 14° día de gestación) el NENO correspon-
diente a la toxicidad materna fue de 125 mg/kg por día.
No obstante, se observaron malformaciones con 62,5 mg/kg
por día y variaciones esqueléticas con 31,25 mg/kg por
día. No se señaló ningún NENO de toxicidad para el desar-
rollo. En el marco de estudios de dosis única, se trató a
ratones con 2-ME administrado por alimentación forzada el
11° día de la gestación; la dosis de 100 mg/kg no era
fetotóxica mientras que la de 175 mg/kg produjo anomalías
digitales sin otros signos de toxicidad materna o fetal.
En la ratas recién nacidas se observaron defectos cardio-
vasculares y anomalías del ECG tras el tratamiento de las
madres con 25 mg/kg por día durante los días 7° a 13° de
la gestación. Como ésta fue la dosis más baja que se
ensayó, este estudio no ha permitido establecer un NENO
para el desarrollo (con esa dosis no se observó toxicidad
materna). De igual modo, no pudo determinarse ningún NENO
de toxicidad para el desarrollo en un estudio de trata-
miento de monos por alimentación forzada con 2-ME a 0,16,
0,32 o 0,47 mmol/kg por día durante los días 20° a 45° de
la gestación.
Tras la exposición a la inhalación de 2-ME a 156 mg
por m3 se ha observado fetotoxicidad en los ratones y
ratas y malformaciones en los conejos. En las tres espe-
cies, el NENO correspondiente a los efectos sobre el
desarrollo fue de 31 mg/m3. Sin embargo, en la descen-
dencia de las ratas expuestas a 78 mg 2-ME/m3 durante los
días 7-13 o 14-20 de la gestación se observaron altera-
ciones conductuales y neuroquímicas.
Tras la exposición por inhalación de ratas (743 mg por
m3) y conejos (589 mg/m3), el 2-EE se reveló terató-
geno (en presencia de ligera toxicidad materna). En otro
estudio se observó fetotoxicidad pero no malformaciones en
las ratas expuestas a 184 ó 920 mg de 2-EE/m3, así como
en los conejos expuestos a 644 mg de 2-EE/m3. Los val-
ores del NENO para los efectos en el desarrollo fueron de
37 mg/m3 en las ratas y de 184 mg/m3 en los conejos. En
la descendencia de las ratas expuestas a 368 mg de 2-EE
por m3 durante los días 7-13 ó 14-20 de la gestación se
observaron alteraciones conductuales y neuroquímicas.
Las ratas tratadas por aplicación dérmica de 0,25 ml
de 2-EE sin diluir (cuatro veces al día en los días 7-16
de la gestación) acusaron una considerable fetotoxicidad y
una elevada incidencia de malformaciones en ausencia de
toxicidad materna. Análogos efectos se observaron tras el
tratamiento de las ratas con 2-EEA, utilizando el mismo
protocolo, a una dosis equimolar (0,35 ml, cuatro veces al
día).
La exposición por inhalación de 2-EEA de conejas
durante los días 6-18 de la gestación provocó respuestas
teratógenas con 2176 mg/m3 y 544 mg/m3 en dos estudios
diferentes, en los cuales los valores del NENO para el
desarrollo fueron de 135 mg/m3 y 270 mg/m3. Las ratas
expuestas al 2-EEA durante los días 6-15 de la gestación
acusaron fetotoxicidad a 540 mg/m3 y malformaciones a
1080 mg/m3. El NENO para el desarrollo fue de 170 mg por
m3.
8. Efectos en el hombre
La información disponible sobre los efectos tóxicos de
estos cuatro éteres glicólicos en el ser humano es limi-
tada. Los resultados de los escasos informes sobre casos
individuales y estudios epidemiológicos en el lugar de
trabajo corroboran los efectos adversos observados en los
animales de experimentación. No se ha encontrado ningún
informe en el que se cuantifique la exposición y los
efectos adversos en la población general.
En dos casos no mortales de envenenamiento por inges-
tión de 100 ml de 2-ME, los signos y síntomas predomi-
nantes fueron náuseas, vértigo, cianosis, taquicardia,
hiperventilación y acidosis metabólica, con algunos indi-
cios de insuficiencia renal. Síntomas análogos, aunque
menos graves, se observaron en un sujeto que ingirió 40 ml
de 2-EE. En un caso de intoxicación mortal causada por la
ingestión de 400 ml de 2-ME, la autopsia reveló una gas-
tritis hemorrágica aguda con degeneración grasa del hígado
y alteraciones degenerativas de los túbulos renales.
La exposición repetida de los trabajadores al 2-ME y
al 2-EE, así como a otros solventes, ha dado lugar a
anemia, leucopenia, debilidad general y ataxia. En muchos
de estos estudios no se ha hecho ninguna estimación fide-
digna de la exposición. Los efectos hematológicos de los
éteres glicólicos en el ser humano están bien documentados
y se ha descrito la aparición de anemia macrocítica en un
trabajador expuesto al 2-ME (promedio: 105 mg/m3), así
como a otros solventes.
En los trabajadores expuestos por vía dérmica al 2-ME
se han observado efectos tóxicos en la médula ósea, y tam-
bién se han observado efectos inmunológicos en trabaja-
dores sometidos a una exposición prolongada (8-35 años) al
2-ME y al 2-EE (los promedios de exposición fueron de 6,1
mg/m3 y 4,8 mg/m3, respectivamente).
Los estudios epidemiológicos realizados en trabaja-
dores expuestos al 2-ME y al 2-EE han revelado algunos
indicios de efectos adversos en el sistema reproductor
masculino, con un aumento de la frecuencia de recuentos
reducidos de espermatozoides. La exposición al 2-EE (37
trabajadores) a concentraciones de hasta 88,5 mg/m3 pro-
vocaron una alteración de los índices seminales. En un
grupo de 73 trabajadores expuestos al 2-ME (hasta 17,7 mg
por m3) y al 2-EE (hasta 80,5 mg/m3) se observó una
mayor frecuencia de recuentos reducidos de espermatozoides
y también signos de efectos hematológicos con exposiciones
de 2,6 mg/m3 para el 2-ME y de 9,9 mg/m3 para el 2-EE
(TWA).
Los efectos adversos observados en las personas pro-
fesionalmente expuestas coinciden con los señalados en los
animales de experimentación. Sin embargo, debido a defici-
encias en las evaluaciones de la exposición y a las expo-
siciones mixtas, no se han podido determinar relaciones
dosis-respuesta.
9. Conclusiones
Muchas personas pueden estar expuestas a concentra-
ciones de estos cuatro éteres glicólicos comparables a las
industriales a consecuencia del empleo de productos comer-
ciales y de consumo. Tanto por inhalación como por absor-
ción cutánea pueden producirse exposiciones profesionales
importantes. En un número limitado de determinaciones de
la concentración atmosférica en los lugares de trabajo se
han obtenido valores comprendidos entre < 0,1 mg/m3 y >
150 mg/m3.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
para los microorganismos y las especies acuáticas. No se
dispone de datos que permitan precisar la capacidad poten-
cial de las exposiciones prolongadas para ejercer efectos
adversos sobre las especies presentes en el medio ambiente.
En las ratas se ha obtenido un NENO para los efectos
testiculares de 933 mg de 2-ME/m3, así como un NENO para
la exposición repetida de 311 mg/m3. En los experimentos
de exposición repetida con la especie más sensible, el
conejo, se ha detectado un efecto neto con 311 mg/m3, mien-
tras que a 93 mg/m3 se observaba un efecto marginal (1 de
5 animales). En las personas expuestas profesionalmente al
2-ME y al 2-EE se han encontrado indicios de que estos
éteres glicólicos pueden producir toxicidad testicular en
el ser humano.
En todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más se ha observado toxi-
cidad para el desarrollo. Para las tres especies se tuvo
un NENO de 31 mg/m3. En las ratas expuestas in utero a
78 mg/m3 se produjeron alteraciones conductuales y neuro-
químicas, pero no se estableció ningún valor de NENO. El
2-EE y el 2-EEA eran algo menos potentes. En la rata y en
el conejo se han observado efectos sobre el desarrollo
tras la exposición a 2-EE a concentraciones de 368 mg por
m3 o más. Estos efectos eran ligeros en las ratas ex-
puestas a 184 mg de 2-EE/m3, pero tanto en las ratas como
en los conejos se pudo establecer un NENO bien definido a
37 mg/m3.
Estos éteres glicólicos producen efectos hematológicos
en los ratones, las ratas, los conejos, los perros, los
hamsters, y los cobayos. Esta observación concuerda con
los efectos hematológicos señalados en algunos de los
escasos estudios efectuados en trabajadores industriales
expuestos repetidamente al 2-EE y/o al 2-ME. En los estu-
dios de exposición repetida de animales se obtuvo un NENO
de 93 mg de 2-ME/m3 en los conejos y de 368 mg de 2-EE
por m3 en las ratas y los conejos. No se han obtenido
datos que permitan evaluar cuantitativamente los efectos
hematológicos que siguen a la exposición aguda.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y EFECTOS
EN EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
1.1 Exposición
Muchas personas pueden estar expuestas al 2-metoxi-
etanol (2-ME), al 2-etoxietanol (2-EE) y a sus acetatos
(2-MEA y 2-EEA) en concentraciones comparables a las
industriales como consecuencia del empleo de productos
comerciales y de consumo. En cambio, la exposición por
los alimentos, el agua o el aire ambiente es probablemente
insignificante. Esta impresión se basa únicamente en las
propiedades físicas y químicas de estos compuestos y en
los indicios de que experimentan una rápida degradación en
el medio ambiente.
Tanto por inhalación como por absorción cutánea puede
producirse una exposición profesional importante. Las
escasas determinaciones de las concentraciones en la
atmósfera de los lugares de trabajo han dado valores
comprendidos entre menos de 0,1 mg/m3 y más de 150 mg por
m3. Sin embargo, las posibilidades de vigilancia son muy
limitadas y cabe la posibilidad de que haya grandes vari-
aciones entre diferentes industrias e incluso dentro de
una misma industria. Habida cuenta de las posibilidades de
absorción cutánea, la vigilancia del aire por sí sola
puede dar una subestimación de la exposición total. La
vigilancia biológica es el mejor método para calcular la
absorción total. Entre los trabajos que entrañan una expo-
sición considerable figuran, por ejemplo, los de pintura,
imprenta y limpieza; ahora bien, no hay que olvidar que
estos compuestos se utilizan también en otras muchas acti-
vidades profesionales en las que la exposición debe ser
motivo de inquietud.
1.2 Efectos en la salud
Los principales motivos de inquietud en el ser humano
son los efectos en el desarrollo, los efectos testiculares
y los vinculados a la toxicidad hematológica. Estos
efectos han sido demostrados por una multitud de datos
sólidos obtenidos en el animal y por algunos datos de
origen humano. Todos ellos pueden estar causados por una
exposición a corto o a largo plazo. En los animales de
experimentación, la exposición muy repetida al 2-ME y al
2-EE (más de 939 y 1450 mg/m3, respectivamente) produce
efectos tóxicos neuroconductuales, hepáticos y renales,
los cuales se observan también en casos de intoxicación
humana.
Estos cuatro éteres glicólicos dan valores muy simi-
lares de toxicidad testicular y de toxicidad para el
desarrollo en todas las especies estudiadas y por todas
las vías de exposición que se han ensayado (inhalatoria,
dérmica y oral). En los estudios sobre el mecanismo de
acción se ha visto que la metabolización al derivado del
ácido alcoxiacético constituye una etapa indispensable de
activación, tanto en los efectos sobre el desarrollo como
en los testiculares. Dicho metabolismo se efectúa mediante
el sistema de deshidrogenasa alcohólica que es común al
hombre y a los animales de laboratorio. Los metabolitos
tóxicos, el ácido metoxiacético (MAA) y el ácido etoxi-
acético (EAA), aparecen en la orina de las personas ex-
puestas a estos solventes. La coherencia de las respuestas
en las distintas especies de animales de laboratorio estu-
diadas, junto con la semejanza del metabolismo en el ser
humano, permiten concluir que el hombre está probablemente
expuesto a los efectos testiculares y sobre el desarrollo
de estos éteres glicólicos. Los datos disponibles sobre
la excreción de ácidos alcoxiacéticos por el hombre
sugieren la existencia de una retención prolongada en com-
paración con la que se observa en los animales de labora-
torio, lo cual hace pensar que las personas quizás sean
más sensibles que las especies experimentales de mayor
sensibilidad. Un motivo de especial inquietud es la rápida
absorción cutánea de estos compuestos. Se han observado
efectos teratógenos y otros efectos sobre el desarrollo
tras la aplicación de 2-ME, 2-EE y 2-EEA en la piel
intacta de la rata.
En la rata, el ratón y el conejo se han observado
alteraciones testiculares tras la exposición a estos
éteres glicólicos, tanto por inhalación como por vía oral.
Una exposición aislada de la rata a la inhalación de 1944
mg de 2-ME/m3 o más durante 4 h y la exposición repetida
a 933 mg de 2-ME/m3 o más durante 13 semanas han provo-
cado signos histológicos evidentes de alteración testi-
cular. El NENO para la exposición aguda fue de 933 mg/m3
y para la exposición repetida de 311 mg/m3. El ratón
parece ser menos sensible, siendo el NENO para la exposi-
ción repetida de 933 mg/m3. En cambio, el conejo resulta
más sensible, observándose en él alteraciones testiculares
marcadas tras la exposición repetida a 311 mg/m3 y un
efecto marginal (uno en cinco conejos afectados) a 93 mg
por m3. Efectos análogos se han observado tras la exposi-
ción oral de la rata al 2-ME, con aparición de lesiones
testiculares tras la exposición breve (inclusive de dosis
única) a 100 mg/kg. En un estu-dio subagudo (11 días) el
NENO fue de 50 mg/kg. El 2-EE es algo menos potente
respecto a la toxicidad testicular que el 2-ME; sólo se
observaron efectos con dosificaciones de 500 mg/kg o más,
siendo el NENO de 250 mg/kg.
Los datos obtenidos en las personas profesionalmente
expuestas al 2-ME y al 2-EE coinciden con los de los estu-
dios en animales e indican que estos éteres glicólicos
pueden producir toxicidad testicular en el ser humano.
Los estudios epidemiológicos de pequeños grupos de traba-
jadores expuestos al 2-EE en una empresa de fundición de
metales y de pintores de embarcaciones expuestos tanto al
2-ME como al 2-EE muestran indefectiblemente una mayor
incidencia de recuentos reducidos de espermatozoides. Los
datos sobre los niveles de exposición, aunque limitados,
aportan en cada caso pruebas de la exposición dérmica así
como de la exposición por inhalación.
En la rata, el ratón, el conejo y el mono se han
observado efectos tóxicos sobre el desarrollo tras la
exposición a estos éteres glicólicos por vía dérmica, oral
o inhalatoria. Con 12 aplicaciones diarias de 2-ME sin
diluir en la piel rasurada de ratas gestantes (oclusión:
6 h) se obtuvo un efecto letal, mientras que 10 aplica-
ciones abiertas de 2-EE (1,0 ml/día) o 2-EEA (1,4 ml/día)
se mostraron teratógenas pero no tóxicas para la madre.
Doce aplicaciones cerradas de 2-ME al 10% en suero salino
resultaron tóxicas para el desarrollo (en este estudio el
NENO fue del 3% para el 2-ME. No se han encontrado niveles
sin efecto aparente tras la administración oral repetida
de 2-ME a las hembras preñadas. El nivel de efecto obser-
vado mínimo (NEOM) en la administración oral de 2-ME fue
de 31,25 mg/kg por día en el caso de los ratones, de 25 mg
por kg por día en el de las ratas y de 0,16 mmol/kg por
día en el de los monos. Sólo se han hecho experimentos de
dosis única con el 2-ME en los ratones, con el resultado
de que el 11° día de la gestación (que es el día más sen-
sible) el NENO era de 100 mg/kg y el NEOM de 175 mg/kg.
Tanto el 2-EE como el 2-EEA se han evaluado en ratas y
conejos por inhalación. A las ratas se las expuso al 2-EE
en dos estudios, en los que se obtuvieron efectos terató-
genos (743 mg/m3 durante 7 h/día los días 1-19 de la ges-
tación) o efectos fetotóxicos (184 y 920 mg/m3 durante 6 h
por día los días 6-15 de la gestación). En este último
estudio, el NENO fue de 37 mg/m3. También los conejos
expuestos al 2-EE presentaron efectos teratógenos (589 mg
por m3 durante 7 h/día los días 1-18 de la gestación) o
efectos fetotóxicos (644 mg/m3 durante 6 h/día los días
6-18 de la gestación). En el último estudio, el NENO fue
de 184 mg/m3. En los conejos expuestos al 2-EEA durante
6 h/día los días 6-18 de la gestación se obtuvieron efec-
tos teratógenos a 2160 mg/m3 en un estudio y a 1620 mg
por m3 en otro. En ambos estudios se observó fetotoxici-
dad a 540 mg/m3 y en uno y otro el nivel de exposición
mínimo (135 y 270 mg/m3, respectivamente) coincidía con
el NENO. Las ratas expuestas al 2-EEA por inhalación dur-
ante 6 h/día los días 6-15 de la gestación presentaron el
mismo tipo de expuesta: efectos teratógenos a 1620 mg por
m3, fetotoxicidad a 1080 mg/m3 y ausencia de todo ef-
ecto a 270 mg/m3.
Así, pues, se ha observado toxicidad para el desar-
rollo en todas las especies (ratones, ratas y conejos) ex-
puestas al 2-ME a 156 mg/m3 o más. Para las tres espe-
cies, el NENO fue de 31 mg/m3. En ratas expuestas in
utero a 78 mg/m3 se han observado alteraciones conduc-
tuales y neuroquímicas, no habiéndose señalado ningún
NENO.
El 2-EE y el 2-EEA han resultado algo menos potentes.
En la rata y el conejo, todas las exposiciones al 2-EE a
368 mg/m3 o más fueron seguidas de efectos sobre el des-
arrollo. En las ratas expuestas a 184 mg de 2-EE/m3 estos ef-
ectos eran leves, pero 37 mg/m3 constituía un NENO patente.
En el caso del 2-EEA, el NENO era de 170 mg/m3 tanto en
la rata como en el conejo.
Tanto en los animales como en los casos de envenena-
miento humano se han observado efectos hematológicos tras
la exposición a una dosis aguda única. La exposición por
inhalación repetida de la especie más sensible, el conejo,
al 2-ME durante 13 semanas y a razón de cinco veces por
semana dio un NEI de 93 mg/m3. También las dosis repeti-
das de 2-ME provocan toxicidad hematológica en los
ratones, conejos, perros, hámsters y cobayos. El 2-EE es
menos potente que el 2-ME como causa de efectos hemato-
lógicos. El NENO correspondiente a estos efectos fue de
368 mg/m3 en las ratas y los conejos expuestos a 2-EE
durante 13 semanas a razón de cinco veces por semana
durante 6 h/día. También en los perros y los ratones se
han registrado efectos hematológicos tras la exposición
repetida a concentraciones más elevadas de 2-EE. La
exposición a los ésteres acéticos del 2-EE y del 2-ME
quizá provoque efectos análogos a los mismos niveles de
exposición, pero los datos disponibles sobre exposición y
efectos hematológicos de esos compuestos son demasiado
escasos para determinar en qué condiciones una exposición
humana aislada tendrá efectos hematológicos.
Se han observado niveles de exposición industrial
próximos o idénticos al NENO de efectos hematológicos en
animales expuestos a dosis repetidas de 2-ME o de 2-EE.
Este hecho, junto con la mayor sensibilidad que probable-
mente tienen las personas y la acumulación previsible de
metabolitos en la sangre humana, hace pensar que tanto la
exposición industrial como la de los consumidores pueden
tener efectos hematológicos. Esto ha sido confirmado por
la observación de efectos de este tipo en algunos de los
escasos estudios realizados sobre trabajadores industri-
ales expuestos repetidamente al 2-EE, al 2-ME o a ambos
compuestos a la vez.
2. Evaluación de los efectos sobre el medio ambiente
La exposición ambiental a estos éteres glicólicos
puede producirse como consecuencia de su paso directo a la
atmósfera cuando se utilizan como solventes volátiles.
También pueden ser causa de exposición ambiental los ver-
tidos en el suelo y el agua a consecuencia de escapes ac-
cidentales. La acumulación en el suelo y en las aguas sup-
erficiales sólo podría producirse en ausencia de degrada-
ción. Sin embargo, estos éteres glicólicos se degradan
rápidamente a causa de procesos químicos y biológicos, por
lo que no es probable que se acumulen. Tanto el 2-MEA como
el 2-EEA pueden hidrolizarse fácilmente y, por consigui-
ente, biodegradarse en condiciones aerobias. En cambio,
la contaminación de acuíferos y suelos anaerobios sigue
planteando un problema potencial, aunque es de esperar que
no pase de ser una situación transitoria y, por ende, poco
peligrosa.
Tanto el 2-ME como el 2-EE se muestran poco tóxicos
con los microorganismos y las especies acuáticas. Los
acetatos de éteres glicólicos, en cambio, tienen una toxi-
cidad aguda mucho mayor. No se dispone de datos para cal-
cular las posibilidades de efectos adversos en especies
del medio ambiente a causa de la exposición prolongada.
RECOMENDACIONES
1. Protección de la salud
1. Hay que identificar otros solventes menos tóxicos que
puedan sustituir al 2-metoxietanol, al 2-etoxietanol y a
sus ésteres, especialmente en los productos destinados al
consumo. También es particularme importante evaluar los
efectos de otros éteres etilenglicólicos, ya que algunos
pueden tener efectos análogos a los de los cuatro éteres
glicólicos que aquí se evalúan.
2. Habida cuenta de los conocidos efectos tóxicos de
estos éteres glicólicos, las autoridades deben ocuparse
seriamente de establecer estrategias apropiadas para
informar a los usuarios de esos productos acerca de los
riesgos que entrañan, especialmente los resultantes de la
exposición dérmica.
3. En vista de los nuevos datos toxicológicos y de las
posibilidades de absorción dérmica importante de estos
éteres glicólicos, habrá que reconsiderar los límites de
exposición profesional fijados por los países a fin de que
la dosis diaria total que reciben los trabajadores por
todas las vías de administración no plantee un riesgo
indebido para la salud.
4. En los animales se observan efectos de dosis única en
niveles de exposición bastante altos. Con objeto de
reducir los riesgos para la salud, se recomienda utilizar
con prudencia estos compuestos (prestando atención a la
higiene personal, los dispositivos apropiados de protec-
ción y la ventilación adecuada). Los datos disponibles
indican que puede ser necesario aumentar la protección a
fin de evitar efectos en el desarrollo, así como en la
sangre y los testículos como resultando de la exposición
repetida.
2. Investigaciones necesarias
1. En vista de la intervención del ácido metoxiacético
(MAA) y etoxiacético (EAA) (que son los principales meta-
bolitos identificados del 2-ME, del 2-EE y de sus ésteres)
en la toxicidad para el sistema reproductor masculino,
habrá que investigar su mecanismo de acción. Si de ello
se deduce que el MAA y el EAA no son los principales agen-
tes responsables, habrá que tratar de identificar éstos y
de aclarar su mecanismo de acción.
2. Estos cuatro éteres glicólicos tienen a la vez efectos
hematológicos y efectos sobre el sitema reproductor mascu-
lino (reducción del recuento de espermatozoides). Los
datos disponibles, aunque limitados, parecen indicar que
ambos efectos se hacen evidentes a niveles de dosificación
análogos. Habrá que investigar el mecanismo de acción en
ambos sistemas orgánicos y examinar paralelamente los
efectos hematológicos y los recuentos de espermatozoides
con el fin de determinar si las alteraciones de la sangre
proporcionan signos de alarma con repecto a otros efectos
de estos compuestos.
3. La vigilancia del aire no basta por sí sola para
garantizar una exposición de poca intensidad. La vigilan-
cia biológica puede contribuir a detectar defectos de las
medidas de protección. De momento no se ha establecido
claramente la relación existente entre los indicadores
biológicos de exposición, la absorción corporal total y
los efectos observados en la salud. Habrá que proseguir
las investigaciones a fin de adquirir una base para apli-
car la vigilancia biológica a la determinación de la
seguridad en las exposiciones.
4. Hay que proyectar estudios epidemiológicos y/o traba-
jos específicos de vigilancia sanitaria en poblaciones muy
expuestas a estos éteres glicólicos a fin de estimar las
relaciones exposición-efecto con miras a determinar expo-
siciones seguras, siempre que se pueda evaluar adecuada y
suficientemente la exposición total mediante la vigilancia
ambiental y biológica.
5. Habrá que investigar la posibilidad de que estos com-
puestos ejerzan efectos sobre las gónadas femeninas medi-
ante estudios de reproducción multigeneracional en ani-
males.
6. Los datos disponibles indican que el ser humano puede
metabolizar estos éteres glicólicos hasta los correspon-
dientes ácidos alcoxiacéticos en mayor medida que la rata,
y que la vida media de la excreción urinaria de estos
metabolitos tóxicos es aproximadamente cuatro veces más
prolongada en las personas que en las ratas. Por otra
parte, éstas conjugan una mayor cantidad de los metaboli-
tos ácidos, cosa que no hacen las personas. Estas diferen-
cias podrían explicar la sensibilidad relativamente elev-
ada del ser humano a estos éteres glicólicos. Un conocimi-
ento detallado del metabolismo y de la cinética de excre-
ción mejoraría nuestra capacidad para predecir cuáles son
los niveles de exposición seguros.
7. Los resultados obtenidos en estudios a corto plazo (13
semanas) indican efectos en diversos sistemas orgánicos.
En cambio, no se han hecho estudios de suficiente duración
que permitan evaluar la reversibilidad de tales efectos.
Por consiguiente, convendría que se emprendieran estudios
de interrupción en los que los animales de experimentación
estuvieran expuestos a estos éteres glicólicos al menos
durante 13 semanas, seguidas de un periodo apropiado de
recuperación. Cabría evaluar así importantes parámetros
fisiológicos con miras a determinar si esos efectos son o
no transitorios.
