
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 111
TRIPHENYL PHOSPHATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr. A. Nakamura,
National Institute for Hygienic Sciences, Japan
World Health Orgnization
Geneva, 1991
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Triphenyl phosphate.
(Environmental health criteria ; 111)
1.Organophosphorus compounds - adverse effects 2.Organophosphorus
compounds - toxicity I.Series
ISBN 92 4 157111 X (NLM Classification: QV 627)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TRIPHENYL PHOSPHATE
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution, and transformation
1.4. Environmental levels and human exposure
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and in vitro test systems
1.7. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factor
2.4. Analytical methods
2.4.1. Sample extraction
2.4.2. Clean-up procedures
2.4.3. Gas chromatography and mass spectrometry
2.4.4. Contamination of analytical reagents
2.4.5. Other analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
3.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and transformation in the environment
4.1.1. Release to the environment
4.1.2. Fate in water and sediment
4.1.3. Biodegradation
4.1.4. Water treatment
4.2. Bioaccumulation
4.2.1. Fish
4.2.2. Chironomid larvae
4.2.3. Environmental fate in artificial pond water
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Sediment and soil
5.1.4. Fish
5.2. General population exposure
5.2.1. Food
5.2.2. Drinking-water
5.2.3. Human tissues
5.3. Occupational exposure
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Unicellular algae and fungi
6.2. Aquatic organisms
6.3. Insects
7. KINETICS AND METABOLISM
8. EFFECTS ON ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Short-term exposure
8.3. Skin irritation
8.4. Reproduction
8.5. Mutagenicity
8.6. Carcinogenicity
8.7. Neurotoxicity
8.8. In vitro studies
9. EFFECTS ON HUMANS
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure levels
10.1.2. Toxic effects
10.2. Evaluation of effects on the environment
10.2.1. Exposure levels
10.2.2. Toxic effects
11. RECOMMENDATIONS
11.1. Recommendations for further research
REFERENCES
RESUME
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
RECOMMANDATIONS
RESUMEN
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN EL
MEDIO AMBIENTE
RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRIPHENYL PHOSPHATE
Members
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Abbots Ripton, Huntingdon, Cambridgeshire, England (Chairman)
Dr S. Fairhurst, Medical Division, Health and Safety Executive,
Bootle, Merseyside, England (Joint Rapporteur)
Ms N. Kanoh, Division of Information on Chemical Safety, National
Institute of Hygienic Sciences, Setagaya-ku, Tokyo, Japan
Dr A. Nakamura, Division of Medical Devices, National Institute of
Hygienic Sciences, Setagaya-ku, Tokyo, Japan
Dr M. Tasheva, Department of Toxicology, Institute of Hygiene and
Occupational Health, Sofia, Bulgaria
Dr B. Veronesi, Neurotoxicology Division, US Environmental Protection
Agency, Research Triangle Park, North Carolina, USA
Mr W.D. Wagner, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr R. Wallentowicz, Exposure Assessment Application Branch, US
Environmental Protection Agency, Washington, DC, USA (Joint Rapporteur)
Dr Shen-Zhi Zhang, Beijing Municipal Centre for Hygiene and Epidemic
Control, Beijing, China
Observers
Dr M. Beth, Berufsgenossenschaft der Chemischen Industrie (BG Chemie),
Heidelberg, Federal Republic of Germany
Dr R. Kleinstück, Bayer AG, Leverkusen, Federal Republic of Germany
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, Division of
Environmental Health, World Health Organization, Switzerland (Secretary)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TRIPHENYL PHOSPHATE
A WHO Task Group meeting on Environmental Health Criteria for
Triphenyl Phosphate was held at the British Industrial Biological
Research Association (BIBRA), Carshalton, United Kingdom, from 9 to 13
October 1989. Dr S.D. Gangolli, Director, BIBRA, welcomed the
participants on behalf of the host institution and Dr M. Gilbert opened
the meeting on behalf of the three cooperating organizations of the IPCS
(ILO, UNEP, WHO). The Task Group reviewed and revised the draft criteria
document and made an evaluation of the risks for human health and the
environment from exposure to triphenyl phosphate.
The first draft of this document was prepared by Dr A. Nakamura,
National Institute for Hygienic Sciences, Japan. Dr M. Gilbert and Dr
P.G. Jenkins, both members of the IPCS Central Unit, were responsible
for the overall scientific content and editing, respectively.
ABBREVIATIONS
BCF bioconcentration factor
EC effective concentration
HPLC high performance liquid chromatography
LC lethal concentration
LD lethal dose
ND not detected
OPIDN organophosphate-induced delayed neuropathy
TAP triaryl phosphate
TCP tricresyl phosphate
TLC thin-layer chromatography
TPP triphenyl phosphate
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical methods
Triphenyl phosphate (TPP) is a non-flammable, non-explosive,
colourless, crystalline substance. Its partition coefficient between
octanol and water (log Pow) is 4.61-4.76. At normal ambient
temperatures, it hydrolyses rapidly in alkaline solution, producing
diphenyl phosphate and phenol, but very slowly in acidic or neutral
solutions.
The analytical method of choice is gas-liquid chromatography with
nitrogen-phosphorus sensitive or flame photometric detection. The
detection limit in water is about 20 ng/litre.
1.2. Sources of human and environmental exposure
TPP is manufactured from phosphorus oxychloride and phenol. It is
used as a flame retardant in phenolic and phenylene-oxide-based resins
for the manufacture of electrical and automobile components and as a
non-flammable plasticizer in cellulose acetate for photographic films.
It is also a component of hydraulic fluids or lubricant oils and has a
number of other minor uses.
Exposure of the general population through normal use can be
regarded as minimal.
1.3. Environmental transport, distribution, and transformation
Triaryl phosphates enter into the aquatic environment mainly via
hydraulic fluid leakage as well as by leaching from plastics and, to a
minor extent, from manufacturing processes. Because of its low water
solubility and relatively high soil adsorption coefficient, TPP is
rapidly adsorbed on river (or pond) sediments. Its biodegradation in
aqueous environments is rapid.
The degradation of TPP involves a stepwise enzymatic hydrolysis to
orthophosphate and phenolic moieties.
The bioconcentration factors (BCF) measured for several species of
fish range from 6 to 18 900 and the depuration half-life ranges from 1.2
to 49.6 h.
The release of TPP from production sites to the air represents a
source of human exposure in the occupational environment. The combustion
of plastics and volatilization from plastics or water surfaces may also
be a major pathway to the atmosphere.
1.4. Environmental levels and human exposure
TPP has been widely found in air, water, sediment, and aquatic
organisms, but levels in environmental samples are low. The maximum
levels reported are 23.2 ng/m3 in air, 7900 ng/litre in river water,
4000 ng/g in sediment, and 600 ng/g in fish.
1.5. Effects on organisms in the environment
The growth of algae is completely inhibited at TPP concentrations of
1 mg/litre or more but is stimulated at lower concentrations (0.1 and
0.05 mg/litre). The nitrogenase activity of Anabaena flos-aquae
decreases in a dose-dependent manner from 84% at 0.1 mg/litre to 68% at
5.0 mg/litre.
TPP is the most acutely toxic of the various triaryl phosphates to
fish, shrimps, and daphnids. The acute toxicity index of TPP for fish
(96-h LC50) ranges from 290 mg/litre in bluegills to 0.36 mg/litre in
rainbow trout. The large difference in EC0 values between trout and
fathead minnows may be due to the difference in their ability to
metabolize TPP. Sublethal effects on fish include morphological
abnormalities such as congestion, degeneration, and haemorrhage from the
smaller blood vessels (mainly in the gills) and behavioural
abnormalities. The immobility of fish exposed to 0.21-0.29 mg per litre
completely disappeared within 7 days when the fish were transferred to
clean water.
1.6. Effects on experimental animals and in vitro test systems
The oral LD50 of TPP has been estimated to be >6.4 g/kg in rats and
>2.0 g/kg in chickens.
TPP doses ranging from 0.5 to 2 g/kg were well tolerated by rabbits
after intramuscular injection and by chickens after oral administration.
In a 35-day feeding study, depression of body weight gain and increase
in liver weight were observed at a dose of TPP in male Holtzman rats.
TPP was not teratogenic in Sprague-Dawley rats at dose levels up to
690 mg/kg body weight. No reproduction studies have been reported.
There are no data on the mutagenicity of TPP from well-validated
tests, and there has been no adequate carcinogenicity study.
TPP did not cause delayed neurotoxicity following single
subcutaneous exposures in cats (up to 1 g/kg) or in a 4-month study in
Sprague-Dawley rats at dose levels up to 1% in the feed.
No immunotoxic effects were reported from a 120-day study in rats
fed dose levels up to 1% in the feed.
1.7. Effects on humans
While a statistically significant reduction in red blood cell
cholinesterase has been reported in some workers, there has been no
evidence of neurological disease in workers in a TPP-manufacturing
plant. There have been no reports of delayed neurotoxicity in cases of
TPP poisoning. Contact dermatitis due to TPP has been described.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical structure:
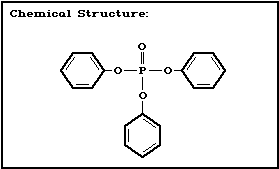 Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number: 115-86-6
RTECS registry number: TC8400000
Synonyms: Triphenyl phosphate; Triphenyl-phosphate; TPP
Trade name: Phosflex TPP(R),; Disflamoll TP(R),; Celluflex TPP(R),
Manufacturers and suppliers (Modern Plastics Encyclopedia, 1975):
Ashland Chemical Co.; Celanese Co.; Daihachi Chemical Industry Co.,
Ltd.; East Coast Chemicals Co.; B.F. Goodrich Chemical Co.; Mobay
Chemical Co.; Monsanto Chemical Co.; Rhone-Poulenc Co.; Showa Ether Co.,
Ltd.; Stauffer Chemical Co.
2.2. Physical and chemical properties
The physical properties of TPP are listed in Table 1.
Table 1. Physical properties of TPP
-------------------------------------------------------------------------------
Physical state crystalline solid
Colour colourless
Odour very slightly aromatic
Melting point (°C) 49-50a; 49b; 49.2c
Boiling point (°C) 245 (11 mmHg)a,b; 220 (5 mmHg)c;
234 (5 mmHg)d; 370e
Relative density 1.185-1.202 (25 °C)c; 1.185 (25 °C)d;
1.2055b
Refractive index (at 25 °C) 1.552-1.563c
Flash point (°C) 220b; 225c
Viscosity (cSt) 11 (50 °C); 9.9 (55 °C)c
Vapour pressure (mmHg) 0.15 (150 °C); 1.90 (200 °C)c;
1.0 (193.5 °C)e
Henry's Law constant 1.8-3.6 x 10-7 atm-m3/mol
Solubility in organic solvents soluble in benzene, chloroform, ether,
acetone; moderately soluble in ethanola
Solubility in water (mg/litre) 1.9f; 0.73g; 2.1 (±0.1)h
Octanol-water partition coefficient 4.63f; 4.61i; 4.76j
(log Pow)
-------------------------------------------------------------------------------
a Windholz (1983)
b Hine et al. (1981)
c Modern Plastics Encyclopedia (1975)
d Lefaux (1972)
e Sutton et al. (1960)
f Saeger et al. (1979)
g Hollifield (1979)
h Ofstad & Sletten (1985)
i Kenmotsu et al. (1980b)
j Sasaki et al. (1981)
TPP is non-flammable and non-explosive. It begins to decompose at
about 600 °C but is not completely degraded even at 1000 °C in inert
gas. Under these conditions, TPP yields aromatic hydrocarbons
(naphthalene, biphenyl, phenanthrene, anthracene, etc.), oxygenated
aromatic compounds (phenol, dibenzofuran, diphenyl ether) and phosphoric
oxides (ortho- , pyro-, meta-, and poly-phosphoric acids). With a large
excess of air, complete combustion to carbon dioxide is accomplished
within the temperature range 800-900 °C (Lhomme et al., 1984).
At ordinary temperature, TPP is hydrolysed very slowly in acidic and
neutral solutions but rapidly in alkaline solutions. The hydrolysis
rate constants and half-lives are summarized in Table 2. In studies by
Barnard et al. (1961), alkaline hydrolysis of TPP yielded diphenyl
phosphate, but further hydrolysis to monophenyl phosphate and phosphoric
acid was not observed under the experimental conditions used. Under
strong acidic conditions and at high temperature (100 °C), TPP readily
hydrolyses to give phosphoric acid (Barnard et al., 1966).
In studies by Finnegan & Matson (1972), the photolysis of TPP
yielded biphenyl (2%), the recovered ester amounting to 48%. The quantum
yield for biphenyl formation was 6 x 10-4.
2.3. Conversion factor
Triphenyl phosphate 1 ppm = 13.35 mg/m3 air
2.4. Analytical methods
Analytical methods for determining TPP in air, water, sediment,
fish, and biological tissues are summarized in Table 3. General
procedures for TPP analysis are similar to those for tricresyl phosphate
(TCP) (WHO, 1990). The detection limit of TPP in water is approximately
20 ng/litre.
Diphenyl phosphate, a hydrolysis product of TPP, has been determined
in sediment by extraction with aqueous methanol, clean-up with XAD-2
resin and C-18 bonded silica cartridge, butylation, and gas
chromatographic determination (Muir et al., 1983b).
TPP is present in several commercial triaryl phosphates, e.g.,
Santicizer-140,(R) Pydraul 50E,(R) (Monsanto Co.), Fyrquel GT,(R) and
Phosflex 41-P,(R) (Stauffer Chemical Co.) (Deo & Howard, 1978). When TPP
is identified, other triaryl phosphates are often detected at the same
time.
2.4.1. Sample extraction
TPP is extracted from water, sediment, fish, and air along with TCP.
WHO (1990) gives details of methods.
Table 2. Hydrolysis rate constants and half-lives of TPP in aqueous solution
---------------------------------------------------------------------------------------------------------
Rate constants
Solution Temper- pH K1 K2 K1' Half- Reference
ature first second pseudo- life
(°C) order order first
(sec-1) (M-1.sec-1) order
---------------------------------------------------------------------------------------------------------
Water 27 alkaline 2.7 x 10-1 Wolfe
(1980)
60% dioxane-water 0 alkaline 2.35 x 10-3 Barnard
10.1 alkaline 4.77 x 10-3 et al.
24.7 alkaline 1.06 x 10-2 (1961)
35 alkaline 2.32 x 10-2 Barnard
et al.
(1961)
NaOH (0.1 mol/ 22 13.0 0.49 h Muir et
litre)/acetone (1:1) al.
(1983a)
H3BO4/NaOH buffer 25 9 3 days Mayer et
al. (1981)
Buffered water 21 ± 2 8.2 9.3 x 10-2/day 7.5 Howard &
days Deo (1979)
9.5 1.3 Howard &
days Deo (1979)
Dioxane-water (3:1) 100 neutral 6.0 x 10-8/day 130 Barnard et
days al. (1961)
KH2PO4/Na2HPO4 25 7 19 Mayer et
days al. (1981)
KHC8H4O4/NaOH buffer 25 5 28 Mayer et
days al. (1981)
Dioxane-water (3:2) 100 neutral 6 x 10-8 Barnard
0.122M HClO4 1.43 x 10-5 et al.
1.21M HClO4 10.8 x 10-5 (1966)
3.02M HClO4 6.45 x 10-5 Barnard
et al.
(1966)
---------------------------------------------------------------------------------------------------------
Table 3. Methods for the determination of TPP
---------------------------------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
---------------------------------------------------------------------------------------------------------
Workplace collect with Millipore filter, extract GC/FPD 1 µg per TCP & TPP US NIOSH
air with ethanol sample (1982)
Environment trap with glycerol-Florisil column, eluate GC/FPD 1 ng/m3 simultaneous Yasuda
air with methanol, add water, and extact with method for (1980)
hexane trialkyl/aryl
phosphates
Air collect by aspiration through ethanol, TLC 5 ng/plate TCP & TPP Druyan
hydrolyse with NaOH; the resultant phenols (1975)
are reacted with p -O2NC6H4N2+ and
separated with silica gel plate
Drinking- adsorb with XAD-2 resin, eluate with GC/NPD 1 ng/litre method for Lebel et
water acetone-hexane or acetone GC/MS low level al. (1979,
trialkyl/aryl 1981)
phosphates
River or extract with methylene chloride or benzene GC/NPD 0.02 µg/ simultaneous Kenmotsu et
sea water GC/FPD litre method for al. (1980a,
(TPP) trialkyl/aryl 1981b,
phosphates 1982b)
GC/MS 0.05 µg/ Muir et al.
litre (1981)
(TCP) Ishikawa et
al. (1985)
Farm pond reflux with methanol-water (9+1) or meth- GC/NPD 1 ng/g simultaneous Muir et al.
sediment ylene chloride-methanol (1+1), clean-up by method for (1980, 1981)
acid alumina column chromatography triaryl
phosphates
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
---------------------------------------------------------------------------------------------------------
River extract with acetonitrile or acetone, GC/FPD 5 ng/g simultaneous Kenmotsu et
or sea clean-up by charcoal or Florisil column GC/MS method for al.(1980a,
sediment chromatography trialkyl/aryl 1981b, 1982a,
phosphates 1982b, 1983)
Ishikawa et
al. (1985)
Fish extract with hexane or methanol, clean-up GC/NPD 1 ng/g simultaneous Muir et
by gel permeation column chromatography GC/MS method for al. (1980,
and acid alumina column chromatograpy triaryl 1981, 1983)
phosphates
Fish extract with acetonitrile and methylene GC/FPD 5 ng/g simultaneous Kenmotsu et
chloride, clean-up by acetonitrile-hexane GC/MS method for al. (1980a)
graphy, concentrated sulfuric acid extrac- trialkyl/aryl
tion and Florisil column chromatography phosphates
Human extract with benzene or acetone-hexane GC/NPD 1 ng/g simultaneous Lebel &
adipose (15 + 85), clean-up by gel permeation GC/FPD method for Williams
tissues chromatography trialkyl/aryl (1983)
phosphates
---------------------------------------------------------------------------------------------------------
2.4.2. Clean-up procedures
Clean-up procedures for TPP are similar to those for TCP (WHO,
1990). It is difficult to separate TPP from other triaryl phosphates by
Florisil or gel permeation chromatography.
2.4.3. Gas chromatography and mass spectrometry
TPP is analysed simultaneously with TCP. GC and mass spectrometry
procedures are described in WHO (1990).
2.4.4. Contamination of analytical reagents
Triaryl phosphates (TAPs) are widely used as flame retardants in
plastics and hydraulic fluids. Their wide-spread use and release into
the environment produces trace contamination of reagents used for
analysis. Trace amounts of TPP have been found in Super Q water
(Williams & Lebel, 1981), Corning water (Lebel et al., 1981), hexane,
acetonitrile, and methylene chloride (Daft, 1982). TAPs have also been
found in cyclohexane (Bowers et al., 1981), hexane (Hudec et al., 1981),
and analytical grade filters (Daft, 1982). Care must be taken to avoid
contamination of reagents in order to obtain reliable data in trace
analysis of TPP.
2.4.5. Other analytical methods
A colorimetric method has been developed for determination of TPP in
air (Druyan, 1975), but the interference by other TAPs was not
investigated. Thin-layer chromatography (TLC) has been used for the
determination of TPP in air (Druyan, 1975) and in plastics (Peereboom,
1960; Braun, 1965). The octanol/water partition coefficient of TPP has
been determined by reversed phase TLC (Renberg et al., 1980). It is
difficult to separate the various TAPs by TLC (Bloom, 1973). Tittarelli
& Mascherpa (1981) described a highly specific HPLC detector for TAPs
using a graphite furnace atomic absorption spectrometer. In general,
TLC and HPLC have not been used as widely as GLC for the analysis of
TPP.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
TPP does not occur naturally in the environment. Figures concerning
total world production are not available, but 7250 tonnes was produced
in the USA in 1977 (Boethling & Cooper, 1985) and 3750 tonnes in Japan
in 1984.
TPP is produced from phosphorus oxychloride and phenol.
3.2. Uses
TPP was used, in Japan in 1984, as a flame-retardant in phenolics
and phenylene-oxide-based resin for the manufacture of electrical and
automobile components (3200 tonnes), as a non-flammable plasticizer in
cellulose acetate for photographic films (500 tonnes), and for other
miscellaneous purposes (50 tonnes)a. Other uses of TPP are as a non-
combustible substitute for camphor in celluloid (which renders
acetylcellulose, nitrocellulose, airplane "dope", etc. stable and
fireproof), for impregnating roofing paper, and as a plasticizer in
lacquers and varnishes (Windholz, 1983). It is also used as a
plasticizer in vinyl automotive upholstery (Ahrens et al., 1978) and in
cellulose acetate articles (Pegum, 1966).
TPP is also found as a component of hydraulic fluids and lubricant
oils (WHO, 1990; Table 6), and of other triaryl phosphate esters: methyl
diphenyl phosphate (triphenyl phosphate content, ca. 5%); 2-ethylhexyl
diphenyl phosphate (ca. 5%); trixylenyl phosphate (ca. 5%); iso-decyl
diphenyl phosphate (ca. 45%); cresyl diphenyl phosphate (ca. 45%);
isopropylphenyl diphenyl phosphate (ca. 45%) (Daft, 1982).
a Personal communication to IPCS from the Association of the Plasticizer
Industry of Japan (1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Summary
TPP has been found in various environmental media, but usually at
low levels. It may be released by leakage at sites of production and use
and by the combustion of plastics. No figures are available on the
amounts released into the environment.
The solubility of TPP in water is low, and it is readily adsorbed
onto sediment.
The rate of biodegradation in water is dependent on water quality (1
mg/litre was degraded in 4 days in River Mississippi water). Little or
no degradation occurs in heat-sterilized river water. The degradation
pathway is reported to involve stepwise enzymatic hydrolysis.
Water treatment techniques, both for waste water and drinking-water,
reduce TPP levels by at least an order of magnitude.
Bioaccumulation data are available from laboratory studies, but
should be considered to represent a bioaccumulation potential.
Depuration, as measured by clearance rate constant, is higher for
rainbow trout than for fathead minnows by about 50%.
4.1. Transport and transformation in the environment
4.1.1. Release to the environment
The release of TPP into the air at production sites represents a
potential source of human contamination. It has been suggested that,
since TPP in the reactor and purification vessels is hot, mist and
vapour coming from leaks in the reactor and from the open receiving tank
are the main source of TPP in the air (Sutton et al., 1960) (see also
section 5.2). Recent figures however are not available. A low
concentration (0.057 mg/m3) of TPP was detected near a zinc die cast
machine where hydraulic fluids were used (US NIOSH, 1980).
Combustion of plastics or volatilization from plastics or water
surface may also be a major pathway to the atmosphere. Vick et al.
(1978) found TPP emitted in the vapour phase and on particulate matter
from a utility plant. The concentrations were not reported.
The entry of TPP into the aquatic environment is thought to occur
principally via hydraulic fluid leakage, as well as by leaching from
vinyl plastics and, to a minor extent, from manufacturing processes
(Ahrens et al., 1978; Mayer et al., 1981; WHO, 1990).
4.1.2. Fate in water and sediment
The solubility of TPP in water is low (Table 1).
Monitoring studies have shown trialkyl and triaryl phosphates to be
present in water and sediment sampled near major industrialized sites
(Konasewich et al., 1978; Sheldon & Hites, 1978, 1979; Mayer et al.,
1981; Williams & Lebel, 1981; Aldous, 1982; Williams et al., 1982;
Ishikawa et al., 1985). The adsorption coefficient of TPP on marine
sediment was found to be 59 (Kenmotsu et al., 1980b). Muir et al.
(1982) showed rapid equilibrium of TPP with the bottom sediment in a
shallow pond (depth 0.5 m) within 10 h.
4.1.3. Biodegradation
TPP (200 µg) was completely degraded within 4 days in 200 ml of
River Mississippi (USA) water at room temperature (Saeger et al., 1979).
Howard & Deo (1979) measured the degradation rate constants for TPP in
non-sterilized natural water (Seneca River and Lake Ontario, USA).
Little degradation occurred for the first two days, followed by a loss
more rapid than in distilled water at comparable pH. After two days,
the pseudo-first-order rate constants at pH 8.2 were 0.64 and 0.34 days-1
for the two natural water samples, and 0.093 days-1 for distilled
water. The rapid degradation (99.2% in 7 days) of TPP (1 mg/litre) was
also found in a river die-away study using Neya and Oh River water
(Osaka, Japan), whereas no degradation was observed during 15 days in
heat-sterilized river water (Hattori et al., 1981). In clear non-
sterilized sea water, the degradation was very slow (35.1% in 14 days)
(Hattori et al., 1981).
Primary biodegradation rates from semicontinuous activated sludge
studies generally show the same trend in degradation rates as river die-
away studies; TPP (3-13 mg per litre, 24-h feed) revealed 96% (± 2%)
degradation (Saeger et al., 1979). The ultimate biodegradability was
measured using the apparatus and procedure developed by Thompson &
Duthie and modified by Sturm; the theoretical carbon dioxide evolution
from TPP (18.3 mg/litre) was 81.8% (Saeger et al., 1979).
The degradation pathway for TPP is reported to involve stepwise
enzymatic hydrolysis to orthophosphate and phenolic moieties. The
phenol would be expected to undergo further degradation (Barrett et al.,
1969; Pickard et al., 1975).
4.1.4. Water treatment
Data from FMC Corporation (USA) show that TPP (0.74 mg per litre) in
waste water was reduced to 0.07 mg/litre in the effluent water by
biological treatment (Boethling & Cooper, 1985). TPP was reduced from
16 µg/litre to 2 µg/litre by classical secondary treatment methods, and
from 0.2 µg/litre to 0.03 µg/litre by standard techniques for drinking-
water treatment (Sheldon & Hites, 1979). Fukushima & Kawai (1986) also
reported that TPP (0.054-2.12 µg/litre) in untreated water was reduced
to 0.005-0.082 µg/litre by conventional waste water treatment.
4.2. Bioaccumulation
4.2.1. Fish
Data on the bioconcentration and depuration of TPP are given in
Table 4. None of the exposures were considered to be representative of
realistic environmental levels. Moreover the bioconcentration factor
(BCF) measured in the laboratory must be considered to represent a
bioaccumulation potential rather than an absolute bioaccumulation factor
(Veith et al., 1979).
Several equations have been presented to predict the
bioconcentration factors of organic chemicals in various fish strains
using octanol-water partition coefficient (Pow) or water solubility
(Neely et al., 1974; Lu & Metcalf, 1975; Kanazawa, 1978; Veith et al.,
1979; Sasaki et al., 1982).
Of six tissues of fish exposed with 14C-triphenyl phosphate, liver
had the highest concentration (10 µg/g at 4 h post-treatment) and also
showed the highest rate of 14C-triphenyl phosphate depuration. The
rapid clearance from liver suggests extensive TPP metabolism. Rates of
uptake of radioactivity (µg/g tissue per h) by the six tissues were as
follows: liver, 2.75; kidney, 2.01; caeca, 0.62; intestine, 0.53;
muscle, 0.45; blood, 0.56 (Muir et al., 1980b).
Clearance of TPP was biphasic with more rapid rates of clearance in
the first 6 days after transfer to clean water, especially in the case
of rainbow trout (Muir et al., 1983a). The clearance rate constant was
higher for rainbow trout than for fathead minnows by about 50% (Muir et
al., 1983a).
Table 4. Bioaccumulation and clearance of TPP by fish
---------------------------------------------------------------------------------------------------------
Species Temp Flow/ Analy- BCF Exposure Uptake Clearance Depur- Reference
(°C) stat tical (k1/k2) concentration rate rate ation
methoda (mg/litre) (k1, (k2 x 103, half life
h-1) h-1) (h)
---------------------------------------------------------------------------------------------------------
Killfish 25 Stat GC-FPD 157-390 1 1.2 Sasaki et al.
(Oryzeas (1982)
latpes) GC-FPD 250-500 0.25 Sasaki et al.
(1981)
Flow GC-FPD 84-193 1 Sasaki et al.
(1982)
Rainbow 10 Stat TR 1368 ± 329b 0.005-0.05 11.6-17.4 42.5 Muir et al.
trout (1983a)
(Salmo TR 573 ± 97c 9.7c
gairdneri) TR 931 ± 122d 17.7d
HER 324 ± 99c 20.7
10 Stat TR 2590b 0.05 43.36 17.9 (fast) Muir et al.
(1980b)
TR 18 900b 2.45 (slow)
12 Stat GC-FPD 271 12.96 Sitthichaikasem
(1978)
Fathead 10 Stat TR 1743 ± 282b 0.005-0.05 7.6-14.0 49.6 Muir et al.
minnows (1983a)
(Pimephales TR 561 ± 115c 7.2c
promelas) TR 218 ± 55d 15.4d
HER 420 ± 25c 30.0
Goldfish 25 Stat GC-FPD 6-11 Sasaki et al.
(Carassius (1981)
auratus)
---------------------------------------------------------------------------------------------------------
a GC-FPD = gas chromatography (flame photometric detector) after suitable extraction;
TR = total radioactivity; HER = hexane-extractable radioactivity.
b BCF was calculated by the "initial rate method".
c The static test method was used (Zitko, 1980).
d K1 and k2 were derived by non-linear regression calculation.
4.2.2. Chironomid larvae
Muir et al. (1983b) studied the accumulation of TPP by Chironomus
tentans larvae exposed to water and sediment spiked with 14C-triphenyl
phosphate. The overall accumulation has been described by the following
equation:
dCc/dt = k1(Cw) - k2(Cc) + k3(Cs)
where Cc is the concentration in the larvae, Cw the concentration in
water, Cs the concentration in sediment, k1, k3 are uptake rate
constants, and k2 is the elimination rate constant.
The rate constant k3 has been described by the following equation:
k3 = k1(Cw/Cs) x (CFs - CFw)/(CFw)
where CFs is the equilibrium concentration factor for larvae in sediment
and CFw is the equilibrium concentration factor for larvae in water.
The results are summarized in Table 5.
Table 5. Uptake rate constants (k1) calculated by use of a first-order
kinetic model and concentration factors for uptake of TPP by Chironomid larvae
------------------------------------------------------------------------------------
High concentration Low concentration
(500 µg/kg sediment) (50 µg/kg sediment)
System k1(h-1) CFw CFs k1(h-1) CFw CFs
------------------------------------------------------------------------------------
Pond sediment 0.4 ± 0.1 6 ± 0 12 ± 10 0.6 ± 0.2 12 ± 4 18 ± 8
River sediment 2.1 ± 0.8 45 ± 17a 78 ± 34a 4.2 ± 1.6 88 ± 34 90 ± 41
Sand sediment 3.3 ± 1.0 64 ± 33b 173 ± 69b 10.4 ± 3.1 208 ± 62b 138 ± 17b
------------------------------------------------------------------------------------
a Significant difference (P = 0.05) between mean larval concentrations in water
and in sediment using the t-test.
b Significant difference (P = 0.01) between mean larval concentrations in water
and in sediment using the t-test.
The relative contribution of sediment and water to the body burdens
observed in larvae (24-h exposure) was estimated by calculating uptake
rates for water (k1)(Cw) and for sediment (k3)(Cs). The results
indicate that contributions from water and sediment were roughly
equivalent for most sediments for a sediment-to-water ratio of 1:5. The
authors noted however:
"A greater ratio of water to sediment would tend to increase
the contribution of uptake from water proportionally. A
water-to-sediment ratio of 100:1 would reduce the contribution
of sediment uptake to 10%, and would make it difficult to show
significant differences between water and sediment
experiments".
Initial elimination rate constants and half-lives of TPP for larvae
exposed to different sediment-water systems are shown in Table 6.
Table 6. Elimination rate constants and half-lives of TPP for
Chironamid larvae
-----------------------------------------------------
Elimination rate
System k2 (h-1) Half-life (h)
-----------------------------------------------------
Pond sediment 0.023 ± 0.012 30.4 ± 16.1
River sediment 0.011 ± 0.004 62.7 ± 24.5
Sand sediment 0.016 ± 0.010 44.4 ± 28.0
River water 0.039 ± 0.013 17.6 ± 6.0
-----------------------------------------------------
4.2.3. Environmental fate in artificial pond water
The environmental fate of radiolabelled TPP (60 µg per litre) in
artificial pond water was studied by Muir et al. (1982). The
radioactivity observed in each pond compartment is shown in Table 7.
Small losses of TPP by volatilization were thought to occur, but this
was not confirmed by direct measurement above the water surface.
Despite differences in fish species and water temperatures between
laboratory and field experiments, the observed body burdens of TPP were
similar, for the first 24 h of the experiment, to those predicted on the
basis of laboratory data. However, at 72 and 240 h, the predicted values
were higher than those observed. These results were considered to
reflect more rapid clearance of TPP by fathead minnows than by rainbow
trout.
Table 7. Percentage of TPP radioactivity in the
various compartments of a pond
----------------------------------------------------
Time(h) Water Sediment Duckweed Fish Total
----------------------------------------------------
10 74 29 1.4 2.7 107.1
24 52 34 1.4 3.4 90.8
32 31 - - - -
48 34 43 1.2 1.3 79.5
72 28 33 0.9 0.9 62.8
120 23 40 0.5 0.6 64.1
240 13 36 0.5 0.5 50.0
----------------------------------------------------
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Summary
TPP has been widely found in air, water, sediment, and aquatic
organisms, but only at low levels. Higher levels have been found only
in sediments near industrialized areas.
Ambient air concentrations of TPP in rural areas range from 0.5 to
1.4 ng/m3 and in urban areas from 0.9 to 14.1 ng/m3.
TPP levels in surface water from 3 to 700 ng/litre have been
measured, values up to 7900 ng/litre occurring near facilities producing
either aryl phosphates or hydraulic fluids containing TPP. These high
values probably result from TPP bound to suspended sediment. Reported
drinking-water levels are several orders of magnitude lower (0.3-30
ng/litre), suggesting that TPP is removed by adsorption to filtration
media in water-treatment plants. No TPP has been detected in potable
water from wells. There is no information available on levels in
groundwater.
Levels of TPP in river and marine sediment range from 0.2 to 200
ng/g, but values of up to 4000 ng/g have been reported in sediments near
manufacturing sites for automobile parts. There has been one report of
TPP being detected in agricultural soils, but no values were given.
In fish and shellfish, TPP tissue levels from 2 to 150 ng per g have
been reported. No TPP has been detected in human adipose tissue and
there are no data on TPP for any other species.
Exposure to humans can occur by several routes, including the
ingestion of contaminated drinking-water, fish, shellfish, and other
foodstuffs. US FDA total-diet studies have found average daily intake
levels of 0.3-4.4, 1.2-1.6, and 0.5-1.6 ng per kg body weight per day
for infants, toddlers, and adults, respectively. It should be noted that
TPP occurred in less than 1% of the foods in these diets.
Occupational exposure can occur in manufacturing industries and other
areas such as automobile or aircraft facilities handling hydraulic fluids.
Levels of 0.008 to 29.6 mg/m3 have been detected in air, the highest
values occurring at TPP-manufacturing sites.
5.1. Environmental levels
TPP has been found widely in air, water, sediment, and aquatic
organisms. The levels of TPP in environmental samples are low (Table 8),
although moderately high levels have often been found in sediment
collected near heavily industrialized areas (Table 9 and 10).
5.1.1. Air
Yasuda (1980) measured the distribution of various organic
phosphorus compounds in the atmosphere above the eastern Seto Inland
Sea, Japan, and found TPP at levels of 0.5-1.4 ng/m3 in 3 out of 4
samples. He also measured concentrations of phosphate esters in the
atmosphere above the Dogo Plain and Ozu Basin of Western Shikoku, Japan,
these being mainly agricultural areas. TPP was detected only in the
urban air of a middle-size city (Matsuyama) at levels of 0.9-14.1 ng/m3.
5.1.2. Water
Although there have been many studies of TAPs in water, TPP has not
often been detected in natural waters. According to the annual reports
of the Environment Agency of Japan, TPP has not been detected in river
or sea water at any sampling points in Japan. Detection limits varied
from 20 to 200 ng/litre at the various laboratories (EAJ, 1977, 1981).
Kawai et al. (1978) detected TPP in river water sampled in Osaka, Japan,
at levels of 50-700 ng per litre, and Ishikawa et al. (1985) detected
levels of 13-31 ng/litre in 5 out of 16 samples of river water in
Kitakyushu City, Japan, but none in sea water. Both cities are located
in the most heavily industrialized area of Japan. In Tokyo, Japan, TPP
was not found in river or sea water (detection limit: 20 ng/litre) by
Wakabayashi (1980), whereas a level of 3 ng/litre was detected in sea
water by Sugiyama & Tanaka (1982).
High concentrations of TAPs have frequently been detected in river
water sampled near producer and user sites. Sheldon & Hites (1978)
found 100-300 ng TPP/litre in 2 out of 5 samples of Delaware River (USA)
water collected in winter, and 100-400 ng/litre in 11 out of 12 samples
collected in summer. The highest level (16 000 ng/litre) of TPP was
found in a waste stream entering Philadelphia's NorthEast Sewage
Treatment plant for industrial effluents (Sheldon & Hites, 1979).
Concentrations of TPP in four samples of Kanawha River (USA) water
collected 13 km downstream from the outfall of an aryl phosphate
manufacturing plant ranged from 300 to 1200 ng/litre (Boethling &
Cooper, 1985). Mayer et al. (1981) also detected TPP (100-7900
ng/litre) in Mississippi River (USA) water sampled at St. Louis
(Missouri), where two phosphate ester hydraulic fluids were being
produced by Monsanto Co.
Table 8. Concentrations of TPP in environmental air, water, sediment, and fish at various locations
---------------------------------------------------------------------------------------------------------
Year Location Sample Concentrationa Number of Reference
samples
(detected/
analysed)
---------------------------------------------------------------------------------------------------------
1975 Japan (various river and sea water ND (20-200 ng/litre) (0/100) EAJ (1977)
locations) river and sea sediment ND (2-50 ng/g) (0/100)
fish ND (5-50 ng/g) (0/100)
1976 Osaka (Japan) river water 50-700 ng/litre (11/13) Kawai et al.
(1978)
1976 Shikoku (Japan) atmosphere 0.9-14.1 ng/m3 (4/19) Yasuda (1980)
1977 Eastern Seto atmosphere 0.5-1.4 ng/m3 (3/4)
Inland Sea (Japan)
1978 Eastern Ontario drinking-water 0.3-2.6 ng/litre (12/12) Lebel et al.
water treatment (1981)
plant (Canada)
1978 Tokyo (Japan) river water ND (20 ng/litre) (0/12) Wakabayashi
sea water ND (20 ng/litre) (0/3) (1980)
river sediment 0.7-3.3 ng/g (10/15)
sea sediment 0.2-0.3 ng/g (2/3)
1979 Canada (various drinking-water 0.3-8.6 ng/litre (20/60) Williams &
locations) Lebel (1981)
1980 Great Lake (Canada) drinking-water 0.2-4.8 ng/litre (11/12) Williams et
al. (1982)
1980 Kitakyushu river water 13-31 ng/litre (3/16) Ishikawa et
City (Japan) sea water ND (10 ng/litre) (0/9) al. (1985)
sea sediment ND (5 ng/g) (0/6)
1980 Seto Inland fish and shellfish 2-6 ng/g (12/41) Kenmotsu et
Sea (Japan) al. (1981a)
1981 Tokyo Bay sea water 3 ng/litre Sugiyama &
(Japan) Tanaka (1982)
NR USA drinking-water 10-120 ng/litre Muir (1984)
---------------------------------------------------------------------------------------------------------
a Figures in parentheses are detection limits
ND = not detected; NR = not reported
Table 9. Concentration of TPP in water, sediment, and fish muscle at industrialized and non-industrialized sites in the USAa
-------------------------------------------------------------------------------------------------
Location Water (ng/litre) Sediment (ng/g) Fish (ng/g)
-------------------------------------------------------------------------------------------------
Waukegan Harbor, Illinois ND (0/5) 10 (2/3) ND (0/13)
Waukegan Bay, Illinois ND (0/4) ND (0/3) NR
Upper Saginaw River, Michigan 100 (3/3) 10 (3/3) ND (0/12)
Saginaw River at Lake Huron 600-700 (4/4) 1000-4000 (3/3) 100 (1/10)
Illinois River, Grafton Illinois 100 (4/4) ND (0/3) ND (0/4)
Missouri River, Fenton, Missouri ND (0/4) ND (0/3) ND (0/9)
Missouri River at Chesterfield, Missouri 100 (1/4) ND (0/4) NR
Missouri River, Halls Ferry, Missouri 100-200 (3/3) ND (0/3) NR
Mississippi River above St. Louise, Missouri ND (0/5) NR 500 (1/3)
Mississippi River at St. Louise, Missouri 100-7900 (9/15) 100 (2/6) NR
Mississippi River below St. Louise, Missouri 100-400 (3/3) ND (0/3) 100 (1/4)
Kanawha River, Winfield, W. Virginia 100-800 (3/3) 20-200 (3/6) 100-600 (13/27)
San Francisco Bay, California 100 (2/5) NR NR
-------------------------------------------------------------------------------------------------
a From: Mayer et al. (1981); measurements were made from November 1977 to May 1978; figures in
parentheses indicate number of samples (detected/analysed); detection limits were 100 ng/litre
(water), 10 ng/g (sediment), and 100 ng/g (fish); ND = not detected; NR = not reported
Table 10. Concentrations of TPP near sites producing or using trialkyl/aryl phosphates
---------------------------------------------------------------------------------------------------------
Year Location Sample Concentration Number of Reference
sample
(detected/
analysed)
---------------------------------------------------------------------------------------------------------
1975 New Orleans (USA) finished water 120 ng/litre NR Boethling
& Cooper
(1985)
1976 Delaware River (USA) river water (winter) 100-300 ng/litre (2/5) Sheldon &
river water (summer) 100-400 ng/litre (11/11) Hites
(1978)
1977 Delaware River (USA) influent of sewage 16 000 ng/litre NR Sheldon &
treatment plant Hites
effluent of sewage 2000 ng/litre NR (1979)
treatment plant
river water 200-300 ng/litre (3/3)
effluent of water 30 ng/litre NR
treatment plant
1978 Kanawha River (USA) river water 300-1200 ng/litre NR Boethling
& Cooper
(1985)
1980 FMC Corp. Plant (USA) waste water 740 000 ng/litre NR Boethling
effluent water 7000 ng/litre NR & Cooper
(1985)
1980 Automobile manufacture (USA) workplace air 0.008-0.057 mg/m3 (6/6) US NIOSH
(1980)
1983 Saginaw River (USA) river water 700 ng/g (1/4) Boethling
& Cooper
(1985)
NR TPP manufacturing plant (USA) workplace air 0.5-29.6 mg/m3 (78/78) Sutton et
al. (1960)
NR Waukegan Harbor, Illinois (USA) fish (carp, goldfish) 60-150 ng/g (3/3) Lombardo &
Egry (1979)
---------------------------------------------------------------------------------------------------------
5.1.3. Sediment and soil
Relatively high concentrations of TPP have occasionally been found
in sediments collected near heavily industrialized areas. Mayer et al.
(1981) found TPP levels of 1000-4000 ng/g in sediment from the Saginaw
River (Lake Huron) sampled at 1.6-3.2 km downstream from several plants
manufacturing automobile spare parts (Boethling & Cooper, 1985). They
also detected TPP levels of 10-200 ng/g at Waukegan Harbor (Illinois),
Upper Saginaw River (Michigan), the Mississippi River at St. Louis
(Missouri), and the Kanawha River at Winfield (West Virginia) (Mayer et
al., 1981). According to the annual reports of the Environment Agency
of Japan, TPP has not been found at any sampling points in Japan. The
detection limits varied from 2 to 50 ng/g at the various laboratories
(EAJ, 1977). Wakabayashi (1980) detected TPP levels of 0.7-3.3 ng/g in
10 out of 15 river sediment samples, and 0.2-0.3 ng/g in 2 out of 3 sea
sediment samples analysed in Tokyo.
Caines & Holden (1976) identified TPP in agricultural soils
collected from vineyards in Scotland, but the concentration was not
reported.
5.1.4. Fish
Lombardo & Egry (1979) found TPP levels of 60-150 ng/g in carp and
goldfish caught near a site at Waukegan Harbor (USA) where aryl
phosphate hydraulic fluids were used. Mayer et al. (1981) detected
concentrations of 100-600 ng per g in 16 out of 82 samples collected in
several rivers in the USA. According to the annual reports of the
Environment Agency of Japan, TPP has not been detected in fish caught at
any sampling points in Japan. The detection limits ranged from 5 to 50
ng/g at the various laboratories (EAJ, 1977, 1981). Kenmotsu et al.
(1981a) found TPP levels of 2-6 ng/g in 12 out of 41 samples collected
from Seto Inland Sea, Japan.
5.2. General population exposure
5.2.1. Food
Gilbert et al. (1986) analysed composite total-diet samples
(representative of 15 different commodity food types encompassing an
average adult diet for each of eight regions in the United Kingdom) for
the presence of trialkyl and triaryl phosphates. Of the food groups,
offal, other animal products, and nuts consistently contained the
highest levels, but the proportion of individual compounds in the
different food groups varied. Trioctyl phosphate was the major
component in the carcass meat, offal, and poultry groups, and there were
significant amounts of TPP and TBP. Total phosphate intake was
estimated to be between 0.07 and 0.1 mg per person per day.
Gunderson (1988) reported the presence of TPP in samples collected
between April 1982 and April 1984 during FDA total-diet studies. The
mean daily intakes of TPP were 0.3-4.4, 1.2-1.6, and 0.5-1.6 ng/kg body
weight per day for infants, toddlers, and adults, respectively.
5.2.2. Drinking-water
Lebel et al. (1981) analysed TAPs in drinking-water sampled from
eastern Ontario water treatment plants and found TPP levels of 0.3-2.6
ng/litre in all of the 12 samples collected. An extended survey of
drinking-water was conducted in Canada (Williams & Lebel, 1981). TPP
was detected at levels of 0.3-8.6 ng/litre in 7 out of 60 samples of
treated potable water obtained at the treatment plants of 29
municipalities. Higher levels of TPP were present in treated water
obtained from river sources, compared with samples from lake sources,
and TPP was not found in potable water from wells. TPP was also
detected in 11 out of 12 samples of drinking-water obtained from 12
water treatment plants located around the Great Lakes (USA and Canada)
at concentrations from 0.2 to 4.8 ng/litre (Williams et al., 1982).
Sheldon & Hites (1979) reported a relatively high level of TPP (30
ng/litre) in finished drinking-water sampled from a water treatment
plant located near a sewage treatment plant handling industrial
effluents.
In general, the concentration of TPP in drinking-water is 100- to
1000-fold lower than that in river or lake water. Due to its adsorption
to sediment, TPP can efficiently be removed by filtration at water
treatment plants.
5.2.3. Human tissues
There has been only one report of TAPs being present in human
adipose tissues (Lebel & Williams, 1983). TPP was not detected.
5.3. Occupational exposure
Sutton et al. (1960) investigated the concentration of TPP in the
air of a TPP-manufacturing plant. The levels found at various locations
are indicated in Table 11.
Table 11. Air concentrations of TPP at various
locations in a manufacturing plant
-------------------------------------------------------
Location Number of Range Mean
samples (mg/m3) (mg/m3)
-------------------------------------------------------
Reactor room 6 1.4-2.2 1.8
Purification area
General room air 6 1.0-3.9 2.4
Receiving tank 10 5.0-29.6 12.0
Flaker room
General room air 6 1.8-3.7 2.6
Flaker 11 2.6-6.8 4.5
Bagging area
General room air 5 2.2-7.8 4.1
Bagger 23 0.5-20.8 8.2
Stacking bags 11 0.7-7.4 5.4
-------------------------------------------------------
TPP has been detected at concentrations of 0.008-0.057 mg/m3 in the
air at automobile manufacturing plants where hydraulic fluids are used
(US NIOSH, 1980).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Summary
The primary productivity of green algal cultures was inhibited (to
50%) by exposure to TPP (0.26 to 0.5 mg/litre) for 7 days, and the
nitrogenase activity of cyanobacteria (blue-green algae) was inhibited
at 5 mg/litre. Fungal spore germination was unaffected at 5 x 10-3
mol/litre.
The 48-h LC50 for Daphnia is 1.0 mg/litre and 96-h LC50
values for fish range from 0.36 to 290 mg/litre. The no-observed-effect
level for growth and survival of rainbow trout fry is 1.4 µg/litre.
There is no information on the toxicity of TPP to organisms living
in or ingesting sediment and none on terrestrial species other than
fungi.
6.1. Unicellular algae and fungi
TPP was the most toxic compound among six TAPs tested for effects on
the primary productivity of algae (Wong & Chau, 1984). The growth of
green algae was completely inhibited at concentrations of 1 mg/litre or
more but stimulated at lower concentrations (0.1 and 0.05 mg/litre)
(Wong & Chau, 1984). The nitrogenase activity, measured by the acetylene
reduction technique, of a cyanobacterium (blue-green alga: Anabaena
flos-aquae) was affected by TPP. Additions of 0.1, 1.0, and 5.0
mg/litre reduced the nitrogenase activity to 84, 77, and 68% of the
control value, respectively (Wong & Chau, 1984). These data are
summarized in Table 12.
TPP did not show any toxicity, as measured by spore germination, to
the fungus Aspergillus niger at a concentration of 5 x 10-3
mol/litre. However, it inhibited fungal respiration by 8-9% (Eto et
al., 1975).
6.2. Aquatic organisms
Data on the toxicity of TPP to aquatic organisms are given in Table 13.
Table 12. Toxicity of TPP and its products for freshwater unicellular algae
---------------------------------------------------------------------------------------------------------
Organism Chemical Temper- Species Effect Concen- Reference
ature (mg/litre) tration
(°C) (mg/litre)
---------------------------------------------------------------------------------------------------------
Alga TPP 20 Ankistrodesmus falcatus 7-day IC50 for 0.26 Wong &
acicularis primary productivity Chau
(1984)
Green alga TPP 20 Scenedesmus quadricaudata 7-day IC50 for 0.50 Wong &
primary productivity Chau
(1984)
Green alga TPP Selenastrum capricornutum 96-h EC50 2 Mayer
P50E Selenastrum capricornutum 96-h EC50 5 et al.
P115E Selenastrum capricornutum 96-h EC50 > 1000 (1981)
Cyanobacterium TPP 20 Anabaena flos-aquae 28-h Inhibition of 5 Wong &
(blue-green alga) 61% of control acetylene reduction Chau
(1984)
Lake Ontario TPP 20 IC50 for primary 0.2 Wong &
phytoplankton productivity Chau
(1984)
---------------------------------------------------------------------------------------------------------
Table 13. Toxicity of TPP for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organisms Age/size Temper- pH Flow/ Hard- End-point Parameter Concent- Reference
ature stat ness or ration
(°C) (mg/ criteria (mg/litre)
litre) used
---------------------------------------------------------------------------------------------------------
Rainbow trout Fry: 0.11 g, stat 96-h LC50 0.36 Palawski
(Salmo 24 mm, 12 days et al.
gairdneri) past swim-up (1983)
stage
Fry: 0.11 g, stat immobility, 96-h LC50 0.30 Palawski
24 mm, 12 days mortality, et al.
past swim-up loss of (1983)
stage equilibrium
Fry 12(±1) 7.2 stat 272 mortality 96-h LC50 0.40 Mayer et
flow 272 and growth 90-d EC0 >0.0014 al. (1981)
inhibition
0.60 g 12 7.4 stat 40 96-h LC50 0.37 Mayer &
Ellersieck
(1986)
Fathead minnow stat 96-h LC50 0.66 Mayer
(Pimephales Egg and fry flow mortality 90-d EC0 0.087-0.27 et al.
promelas) Egg and fry flow and growth 90-d EC0 >0.23 (1981)
inhibition
1.00 g 22 7.3 stat 44 96-h LC50 1.0 Mayer &
Ellersieck
(1986)
Sheepshead minnow stat 96-h LC50 0.32-0.56 Mayer et
(Cyprinodon al. (1981)
viriegatus)
Bluegill 33-75 mm 23 7.6- stat 55 96-h LC50 290 Dawson et
(Leptomis 7.9 al. (1977)
macrochirus)
Killifish 0.1-0.2 g 25 stat 96-h LC50 1.2 Sasaki et
(Oryzias al. (1981)
latipes)
Table 13. Toxicity of TPP for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organisms Age/size Temper- pH Flow/ Hard- End-point Parameter Concent- Reference
ature stat ness or ration
(°C) (mg/ criteria (mg/litre)
litre) used
---------------------------------------------------------------------------------------------------------
Goldfish 0.8-2.8 g stat 96-h LC50 0.70 Sasaki et
(Carassius al. (1981)
auratus)
Channel catfish 0.23 g 22 7.5 stat 38 96-h LC50 0.42 Mayer &
(Ictalurus Ellersieck
punctatus) (1986)
Tidewater 40-100 mm 20 stat 96-h LC50 95 Dawson
silverside et al.
(Menidia (1977)
beryllina)
Mysid shrimp stat 96-h LC50 0.18-0.32 Mayer
(Mysidopsis et al.
bahia) (1981)
Water flea stat 48-h EC50 1.0 Mayer
et al.
(1981)
---------------------------------------------------------------------------------------------------------
The 96-h LC50 values for pure TPP to fish range from 0.36 mg/litre
for the rainbow trout (Palawski et al., 1983) to 290 mg/litre for the
bluegill (Dawson et al., 1977).
The growth and survival of rainbow trout fry were not affected when
they were exposed to TPP at a concentration of 0.0014 mg/litre (Mayer et
al., 1981). At 0.23 mg/litre, the survival of fathead minnow fry was
significantly reduced, but neither the growth of the survivors nor
hatchability was affected (Mayer et al., 1981; Palawski et al., 1983).
Sublethal effects of TPP on fish include morphological and
behavioural abnormalities (Wagemann et al., 1974; Lockhart et al.,
1975). Spinal curvature was observed in surviving rainbow trout exposed
for 24-72 h at concentrations near the LC50 (Sasaki et al., 1981;
Palawski et al., 1983).
Death of goldfish occurred in a 20-litre water tank in which a piece
(18 x 38 cm) of car seat upholstery containing TPP had been immersed
(Ahrens et al., 1978). Goldfish exposed to TPP (concentration not
stated) showed histopathological lesions characterized by congestion,
degeneration, and haemorrhage of the smaller blood vessels, principally
venules and capillaries. Such vascular pathology was most pronounced in
the gills. Similar but less pronounced congestion of the smaller blood
vessels was noted in the brain, spinal cord, pseudobranch and kidneys
(Ahrens et al., 1978). Immobility of fish exposed to 0.21-0.29 mg
TPP/litre disappeared within 7 days after exposure had stopped
(Palawski, et al., 1983).
Exposure of aquatic organisms to TPP would normally arise from
spillage of hydraulic fluids containing this compound. Studies have been
made of the effects of various products, especially Pydraul 50E and
115E, Houghtosafe 1120, and Santicizer 154, on fish and aquatic
invertebrates. Where comparisons have been made between different
components of the fluids, TPP has been shown to be responsible for most
of the acute toxicity observed. However, certain characteristic
sublethal symptoms seen with these hydraulic fluids (such as cataracts
of the eye lens, effects on bone development and collagen content,
haemorrhagic lesions of the dorsal and gill regions, and vertebral
deformity) do not occur after exposure to TPP. They are therefore caused
by other fluid components (Wagemann et al., 1974; Dawson et al., 1977;
Nevins & Johnson, 1978; Mayer et al., 1981; Adams et al., 1983).
6.3. Insects
In studies on 5th-instar small brown planthopper larvae (Laodelphax
striatellus), the chemical being applied by contact, the 21-h LD50 was
570.2 µg TPP per tube, but TPP-OH was without effect (Eto et al., 1975).
The 24-h LD50 in similar studies on adult female (2-5 days old) house
flies (Musca domestica) was >1000 µg TPP per jar (Plapp & Tong, 1966).
When adult female (4-5 days old) green rice leafhoppers were treated
topically, the 24-h LD50 for TPP was 4.6 mg/g and for TPP-OH was 11.53
mg/g (Eto et al., 1975).
7. KINETICS AND METABOLISM
No data on the kinetics and metabolism of TPP in experimental
animals are available. Eto et al. (1975) reported that treated
houseflies transform TPP into diphenyl p -hydroxyphenyl phosphate
(TPP-OH) in vivo.
8. EFFECTS ON ANIMALS AND IN VITRO SYSTEMS
Summary
Acute toxicity data exist for several species of animals and
indicate low toxicity via the oral and dermal routes (1320 to 10 800
mg/kg and >7900 mg/kg, respectively). No inhalation data are
available. TPP also exhibits low toxicity in short-term studies and is
not irritant to mouse skin. In rats, no effects were seen in mothers or
offspring following repeated dietary exposure of 166-690 mg/kg per day
for a period of 91 days, including mating and gestation periods.
The neurotoxicity of TPP has been debated since the early studies of
Smith et al. (1930, 1932), which reported delayed neuropathy in cats and
monkeys exposed to TPP in acute and short-term studies. However, Wills
et al. (1979) could demonstrate no ataxia or neuropathic damage in cats
exposed to 99.9%-pure TPP. Consequently, the validity of the Smith
studies has been questioned. Other toxicity studies using behavioural
and morphological end-points have demonstrated that TPP administered
short-term to cats and chickens fails to produce neuro-toxic changes. A
mixture of triaryl (including cresyl and phenyl) phosphates produced
neurochemical changes and minor peripheral nerve pathology in the caudal
nerve of rats; acute intraperitoneal injection of 150 mg or less
produced neither biochemical nor morphological change.
Negative results have been reported for several in vitro
mutagenicity studies. No satisfactory studies are available on
carcinogenicity.
8.1. Single exposure
Acute toxicity data resulting from single exposure to TPP are
summarized in Table 14. Little information is available on the acute
signs of toxicity.
8.2. Short-term exposure
Sutton et al. (1960) reported a 35-day feeding study in male
Holtzman rats with TPP at doses of 1 and 5 g/kg. Depression of body
weight gain and an increase of liver weight were observed in the high-
dose group. No haematological changes were found.
Table 14. Acute toxicity of triphenyl phosphate
-------------------------------------------------------------
Species Route of LD50 Reference
adminis- (mg/kg)
tration
-------------------------------------------------------------
Rat oral 3500 Hierholzer et al. (1957)
Rat oral 3800 Antonyuk (1974)
Rat oral 10 800 Johannsen et al. (1977)
Rat oral > 5000 US EPA (1986)
Mouse oral > 3000 Sutton et al. (1960)
Mouse oral 1320 Antonyuk (1974)
Mouse oral > 5000 US EPA (1986)
Guinea-pig oral > 4000 Sutton et al. (1960)
Chicken oral > 2000 Smith et al. (1932)
Chicken oral > 5000 Johannsen et al. (1977)
Rabbit dermal > 7900 Johannsen et al. (1977)
-------------------------------------------------------------
In studies by Hinton et al. (1987), TPP was fed to weanling Sprague-
Dawley rats (10 of each sex per group) at dose levels of 0, 2.5, 5, 7.5,
or 10 g/kg for 120 days. The immunotoxicity evaluation included total
protein analysis, electrophoretic analysis of serum proteins, lymphoid
organ weights in relation to growth, and histopathology, together with
expanded immunohistochemical evaluation of B- and T-lymphocyte regions
in the spleen, thymus, and lymph nodes using immunoperoxidase staining.
Assessment was made of the humoral response to a T-lymphocyte-dependent
antigen, sheep red blood cells; it began at mid-term of the feeding
period for the primary response and was followed by secondary and
tertiary booster immunizations at intervals of 3 weeks. The kinetics of
the response were measured by haemolysin assay of relative antibody
titres at days 3, 4, 5, and 6 post injection. No significant effects on
the response were noted for either sex at any of the dose levels tested.
The only effects noted were a decreased rate of growth at high levels of
TPP and increased levels of alpha- and ß-globulins (Hinton et al.,
1987).
When Antonyuk (1974) administered TPP orally for 3 months to rats at
doses of 380 or 1900 mg/kg, there were no deaths, no abnormal growths,
and no inhibition of cholinesterase activity. In another study,
Antonyuk (1974) administered 650 to 1900 mg/kg orally to rats for 3
months with no significant toxic effects.
8.3. Skin irritation
No significant skin irritation was observed when a gauze pad soaked
with approximately 0.5 ml of a 70% solution of TPP in alcohol was
applied to the skin of mice for 72 h (Sutton et al., 1960).
8.4. Reproduction
In studies by Welsh et al. (1987), male and female Sprague-Dawley
(Spartan) rats (40 of each sex per group) were fed dietary levels of 0,
2.5, 5, 7.5, or 10 mg TPP/kg (from 4 weeks post weaning for 91 days,
through mating and gestation). At these dietary levels, the daily
intake of TPP during pregnancy was 0, 166, 341, 516, and 690 mg/kg body
weight, respectively. TPP exposure had no toxic effects on mothers or
offspring at these dosages. The types of developmental anomalies were
similar in both treated and control animals, and no significant increase
in the incidence of anomalies was seen in the treated groups as compared
to control values. TPP was not teratogenic in Sprague-Dawley rats at the
levels tested.
8.5. Mutagenicity
Szybalski (1958) reported negative results with TPP in a paper disk
method using streptomycin-dependent mutants of E.coli.
TPP did not demonstrate mutagenic activity in microbial assays
employing Salmonella typhimurium (TA 1535, TA 1537, TA 1538, TA 98, and
TA 100 strains) and Saccharomyces cerevisiae (D4 strain) indicator
organisms. All studies were carried out both in the presence and
absence of metabolic activation (Monsanto, 1979).
Negative results were also reported in Ames tests conducted with
Salmonella typhimurium strains TA 98, TA 100, TA 1535, and TA 1537, in
the absence or presence of rat liver S9 (Zeiger et al., 1987).
TPP was tested for its ability to induce mutations at the thymidine
kinase (TK) locus in cultured L5178Y mouse lymphoma cells. When tested
with or without metabolic activation, TPP did not induce significant
mutations at the TK locus (Monsanto, 1979).
8.6. Carcinogenicity
Theiss et al. (1977) studied the occurrence of lung adenomas in
strain A/St male mice, 6 to 8 weeks old, using doses of 80, 40, or 20 mg
TPP/kg injected intraperitoneally 1, 3, and 18 times, respectively, into
groups of 20 mice. Twenty-four weeks after the first injection, the
animals were sacrificed, and the frequency of lung tumours was compared
with that in the control group of 50 animals treated with tricarpylin
(vehicle). The pulmonary adenoma response to TPP was not significantly
greater than the response of the control mice. This study was
considered inadequate due to the low survival of animals in two of the
three experimental groups and the short duration of the study.
8.7. Neurotoxicity
In 1930, Smith and his associates found that single and multiple
doses of technical grade TPP produced generalized delayed paralysis in
cats and monkeys but not in chickens or rabbits (Smith et al., 1930).
Smith et al. (1932) attempted to ascertain the minimum lethal dose
of TPP. Rabbits survived after an intramuscular injection of 1 g/kg;
chickens also were unaffected after oral administrations of 0.5 to 2
g/kg. In cats, the minimum toxic dose by subcutaneous injection was
about 0.2 g/kg and the reaction was of the delayed type; a neurotoxic
action and flaccid paralysis were followed by death.
Johannsen et al. (1977) dosed chickens with cumulative doses of 60
g/kg but failed to produce ataxia or neuropathology suggestive of
organophosphate-induced delayed neuropathy (OPIDN).
In an attempt to re-evaluate the delayed neurotoxicity, Wills et al.
(1979) reported that 99.9%-pure TPP did not produce any evidence of
axonal degeneration, demyelination, or any other pathological changes at
11 levels of the nervous system (from the cerebral cortex to peripheral
nerves) when subcutaneously injected into cats at doses of 0.4, 0.7, or
1.0 g/kg. Prostration occurred at the higher doses. Wills et al. (1979)
suggested that the samples of TPP used by Smith et al. (1930) may have
contained impurities that were capable of producing axonal degeneration
and demyelination.
Sobotka et al. (1986) fed young male Sprague-Dawley rats (10 per
group) diets containing TPP at levels of 0, 2.5, 5, 7.5, or 10 g/kg, for
4 months. Treatment-related decreases in growth rate, in the absence of
changes in food consumption, were found at all dietary levels above 2.5
g/kg. There was no evidence of neuromotor toxicity following subchronic
dietary exposure to TPP.
In a study by Vainiotalo et al. (1987), a commercial cresyl diphenyl
phosphate preparation was analysed and found to contain approximately
35% triphenyl phosphate, 45% cresyl diphenyl phosphates, 18% dicresyl
phenyl phosphates, and 2% tricresyl phosphates. The product was almost
free of the o-cresyl isomers, as revealed by the analysis of its
alkaline hydrolysis products. A single intraperitoneal injection (150 or
300 mg/kg) of this mixture caused the induction of microsomal cytochrome
P-450 in the liver of Wistar rats, a concomitant increase in the
activities of mixed function monooxygenases, and proliferation of smooth
endoplasmic reticulum 24 h after the treatment. The activity of
pseudocholinesterase in blood was inhibited 4 h and 24 h after the
injection but the effect leveled off. Treatment with 300 mg/kg
inhibited brain 2',3'-cyclic-nucleotide 3'-phosphohydrolase through the
2-week observation period associated with demyelination in peripheral
nerves.
8.8. In Vitro studies
In vitro TPP was found to cause significant direct inhibition of
monocyte antigen presentation at non-cytotoxic concentrations as low as
1 µmol/litre (Esa et al., 1988).
9. EFFECTS ON HUMANS
Sutton et al. (1960) found no evidence of neurological disease or
other abnormalities in 32 workers exposed to TPP vapour, mist, or dust
(at a time-weighted air concentration of 3.5 mg/m3) for an average of
7.4 years. In six of these workers, who were exposed more regularly to
TPP, there was a statistically significant asymptomatic reduction in
erythrocyte cholinesterase values, but no plasma cholinesterase
depression.
In 39 workers exposed to an organophosphate ester mixture with about
30% TPP and 70% different isopropyl TPPs, a significantly lower level of
serum IgM and a lower activity (of borderline significance; p = 0.05) of
erythrocyte cholinesterase, compared to controls, were reported.
However, plasma cholinesterase activity and the other observed
parameters were not significantly affected (Emmett et al., 1984).
A few individuals have been reported to show positive reactions in
patch tests using cellulose acetate film containing both TCP and TPP.
However, the causative agent could not be identified and may have been
TCP (Hjorth, 1964). A single case of allergy to spectacle frames could
also have been due to TCP (Carlsen et al., 1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
Animal data indicate that TPP has low toxicity. It produces no
irritant effect on animal skin. Despite an early report to the contrary,
TPP is not considered neurotoxic in animals or man. The no-observed-
adverse-effect level on mothers and offspring from a 90-day rat study
was at 690 mg/kg per day. Both exposure of the general population and
occupational exposure to TPP are low.
TPP is not mutagenic.
The available data indicate no hazard to humans.
10.1.1. Exposure levels
Exposure of the general population to TPP through various
environmental media, including drinking-water, is likely. The
concentrations of TPP measured in drinking-water in Canada and the USA
are extremely low. TPP has often been detected in urban air, although
the levels are low. Vaporization of TPP from heated vinyl automotive
upholstery under hot weather has been suggested, but no data on
concentrations in cars are available. In a survey of TAPs in human
adipose tissues, TPP was not detected. There are insufficient data to
evaluate the significance of the general population exposure to TPP.
Significant air concentrations (0.5-29.6 mg/m3) have been reported
in a TPP manufacturing plant, but recent figures are not available.
More data on occupational exposure to TPP in manufacturing plants are
required.
10.1.2. Toxic effects
The toxicity profile of TPP is quite inadequate for a full
evaluation of its hazard.
There is no evidence that TPP has mutagenic activity in bacteria or
that it has carcinogenic activity, based on a study in one animal
species. No evidence that TPP causes delayed neurotoxicity has so far
been obtained in animal experiments. In a 35-day feeding study in rats,
depression of body weight gain and increase in liver weight were
observed at a dose of 5 g/kg. No adequate data are available on the
effects of TPP on reproduction, i.e. function of gonads, fertility,
parturition, and growth and development of offspring.
Contact dermatitis due to TPP has been described.
10.2. Evaluation of effects on the environment
Water concentrations of TPP in the environment are low and toxic
effects on aquatic organisms are unlikely. Spills of hydraulic fluids
containing TPP would be expected to cause local kills. Since TPP is
removed rapidly from the tissues of fish when exposure ends and
bioconcentration factors are moderate, bioaccumulation is not considered
to be a hazard.
High concentrations of TPP in sediment near production plants have
been reported. TPP bound to sediment has been shown to be bioavailable
to one organism living in sediment, but no toxicity data on sediment-
living or sediment-ingesting species exist. There is, therefore, the
possibility of effects on aquatic communities.
10.2.1. Exposure levels
TPP is found in air, surface water, soil, sediment, and aquatic
organisms sampled in heavily industrialized areas. The highest reported
concentration of TPP in industrial water effluent is 16 µg/litre, while
that in river water is 7.9 µg/litre. Taking into account the rapid
biodegradation of TPP in aqueous environments, normal concentrations of
TPP in aqueous environments are unlikely to adversely affect aquatic
organisms. However, disposal of TPP-treated vinyl fabric upholstery
into a pond would result in a sufficiently high concentration of TPP to
kill fish.
10.2.2. Toxic effects
Among the various triaryl phosphates, TPP is the most acutely toxic
compound to fish, shrimps, and daphnids. The 96-h LC50 of TPP for fish
ranges from 0.36 mg/litre in rainbow trout to 290 mg/litre in bluegill.
Salmonids are generally sensitive to TPP, but the growth and survival of
rainbow trout fry were not affected when they were exposed to TPP at a
concentration of 0.0014 mg/litre. Histopathological lesions in goldfish
exposed to TPP consist of congestion, degeneration, and haemorrhage of
the small blood vessels, principally venules and capillaries. Such
vascular pathology is most pronounced in the gills.
The growth of algae was completely inhibited at TPP concentrations
of 1 mg/litre or more but was stimulated at lower concentrations (0.1
and 0.05 mg/litre). The nitrogenase activity of Anabaena flos-aquae was
significantly reduced, even at 0.1 mg/litre.
11. RECOMMENDATIONS
11.1. Recommendations for further research
There is a need for skin sensitization, in vitro cytogenicity, and
pharmacokinetic studies on triphenyl phosphate.
REFERENCES
ADAMS, W.J., KIMERLE, R.A., HEIDOLPH, B.B., & MICHAEL, P.R. (1983) Field
comparison of laboratory-derived acute and chronic toxicity data,
Philadelphia, American Society of Testing and Materials, pp. 367-385
(ASTM STP 802).
AHRENS, V.D., HENION, J.D., MAYLIN, G.A., LEIBOVITZ, L., ST. JOHN, L.E.,
Jr, & LISK, D.J. (1978) A water-extractable toxic compound in vinyl
upholstery fabric. Bull. environ. Contam. Toxicol., 20: 418-422.
ALDOUS, C.N. (1982) Alterations in rat brain norepinephrine and dopamine
levels and synthesis rates in response to five neurotoxic chemicals:
acrylamide, 2,5-hexanedione, tri-o-tolyl phosphate, leptophos, and
methyl mercuric chloride. Diss. Abstr. Int., 43(5): 1441B.
ANTONYUK, O.K. (1974) Hygienic evaluation of the plasticizer triphenyl
phosphate added to polymer composition. Gig. i Sanit., 8: 98-99.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., & WELCH,
V.A. (1961) The reactions of organic phosphates. Part V. The hydrolysis
of triphenyl and trimethyl phosphates. J. Chem. Soc., 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER, B.,
VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic phosphates. Part
VI. The hydrolysis of aryl phosphates. J. Chem. Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphorylenzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY, S.K.
(1974) Detection and estimation of tricresyl phosphate in mustard oil.
Forensic Sci., 3: 263-270.
BLOOM, P.J. (1973) Application des chromatographies sur couch mince et
gazliquide à l'analyse qualitative et quantitative des esters des acides
phosphorique et phosphoreux. J. Chromatogr., 75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and effects of
triaryl and trialkyl/aryl phosphate esters. Residue Rev., 94: 49-99.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., & KARASEK,
F.W. (1981) Trace impurities in solvents commonly used for gas
chromatographic analysis of environmental samples. J. Chromatogr., 206:
279-288.
BRAUN, D. (1965) [Qualitative analysis of plasticizers by using thin-
layer chromatography.] Chimia, 19(2): 77-82 (in German).
CAINES, L.A. & HOLDEN, A.V. (1976) Stream pollution by an organomercury
compound. Bull. environ. Contam. Toxicol., 16(4): 483-485.
CARLSEN, L., ANDERSEN, K.E., & EGSGAARD, H. (1986) Triphenyl phosphate
allergy from spectacle frames. Contact dermatitis, 15: 274-277.
Ciba-Geigy Corporation: Acute oral toxicity to rats and mice, EPA-OTS
document 86-870000069.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues in
foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The
acute toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J. hazard. Mater., 1(4): 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic mass
spectrometric analysis of some commercial aryl phosphate oils. J. Assoc.
Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by thin-
layer chromatography.] Gig. i Sanit., 10: 62-65 (in Russian).
EAJ (1977) [Environmental monitoring of chemicals.] Tokyo, Environment
Agency Japan, pp. 212-214 (Environmental Survey Report Series No. 3) (in
Japanese).
EAJ (1981) Environmental monitoring of chemicals: Environmental survey
report of 1978, 1979 F.Y., Tokyo, Environment Agency Japan, pp. 171-183.
EMMETT, E.A., DANNENBERG, A.M., LEWIS, P.G., FOX, R., BLEECKER, M.,
TANAKA, F., SYNKOWSKI, D.R., & LEVINE, M.S. (1984) A clinical study of
industrial workers exposed to organophosphate esters and a comparison
group, Pittsfield, Massachusetts, General Electric Company (Prepared for
the US Environmental Protection Agency, Washington).
EMMETT, E.A., LEWIS, P.G., TANAKA, F., BLEECKER, M., FOX, R.,
DARLINGTON, A.C., SYNKOWSKI, D.R., DANNENBERG, A.M., Jr, TAYLOR, W.J., &
LEVINE, M.S. (1985) Industrial exposure to organophosphorus compounds.
Studies of a group of workers with a decrease in esterase-staining
monocytes. J. occup. Med., 27(12): 905-914.
ESA, A.H., WARR, G.A., & NEWCOMBE, D.S. (1988) Immunotoxicity of
organophosphorous compounds. Clin. Immunol. Immunopathol., 49: 41-52.
ETO, M., HASHIMOTO, Y., OZAKI, K., & SASAKI, Y. (1975) [Fungitoxicity
and insecticide synergism of monothioquinol phosphate esters and related
compounds.] Botyu Kagaku, 40: 110-117 (in Japanese).
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl phosphate
esters. A new photochemical coupling reaction. J. Am. Chem. Soc., 94:
4780-4782.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GILBERT, J., SHEPHERD, M.J., WALLWORK, M.A., & SHARMAN, M. (1986) A
survey of trialkyl and triaryl phosphates in the United Kingdom total
diet samples. Food Addit. Contam., 3(2): 113-122.
GUNDERSON, E.L. (1988) FDA total diet study, April 1982 - April 1984,
dietary intakes of pesticides, selected elements and other chemicals. J.
Assoc. Off. Anal. Chem., 71(6): 1200-1209.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Suishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HIERHOLZER, K., NOETZEL, H., & SCHMIDT, L. (1957) [Comparative
toxicological study on triphenyl phosphate and tricresyl phosphate.]
Arzneimittelforschung, 7: 585-588.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD, G.T.
(1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's industrial hygiene
and toxicology, 3rd revised ed., New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
HINTON, D.M., JESSOP, J.J., ARNOLD, A., ALBERT, R.H., & HINES, F.A.
(1987) Evaluation of immunotoxicity in a subchronic feeding study of
triphenyl phosphate. Toxicol. ind. Health, 3(1): 71-89.
HJORTH, N. (1964) Contact dermatitis from cellulose acetate film. Cross-
sensitization between tricresyl phosphate (TCP) and triphenyl phosphate
(TPP). Contact dermatitis, 12: 86-100.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water solubility
of highly insoluble organic chemicals of environmental interest. Bull.
environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. B ull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(dichloro-
propyl)phosphate, a mutagenic flame retardant: Frequent occurrence in
human seminal plasma. Science, 211: 951-952.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water Res.,
19: 119-125.
JOHANNSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J., RADUE,
R.W., & GRAHAM, P.R. (1977) Evaluation of delayed neurotoxicity and
dose-response relationships of phosphate esters in the adult hen.
Toxicol. appl. Pharmacol., 41: 291-304.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. Pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water pollution
caused by organophosphoric compounds.] Kankyo Gijyutsu, 7: 668-675
(in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment and sea
water.] J. Food Hyg, Soc. Jpn, 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental pollutants.
V: Environmental fate of organic phosphoric acid triesters.] Okayama-ken
Kankyo Hoken Senta Nemnpo, 4: 103-110 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination of
organic phosphate esters in environment samples.] Okayama-ken Kankyo
Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T. (1982a)
[An environmental survey of chemicals. XIX. Determination of
organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken Senta
Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T. (1982b)
[Studies on the biological toxicity of several pollutants in
environments. VII. GC/MS Spectrometric determination of organophosphoric
acid triesters in sediment.] Okayama-ken Kankyo Hoken Senta Nempo, 6:
142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemical. XXII. GC/MS spectrometric
determination of organophosphoric acid triesters in sediment (2).]
Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in Japanese).
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on organic
and heavy metal contaminants in the Lakes Erie, Michigan, Huron and
Superior basins. Great Lakes Water Qual. Bd.
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic phosphate
triesters in human adipose tissue. J. Assoc. Off. Anal. Chem., 66:
691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from drinking
water at the ng/L level, using macroreticular resin. J. Assoc. Off.
Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas chromatographic
determination of trialkyl/aryl phosphates in drinking water, following
isolation using macroreticular resin. J. Assoc. Off. Anal. Chem.,
64: 991-998.
LHOMME, V., BRUNEAU, C., SOYER, N., & BRAULT, A. (1984) Thermal behavior
of some organic phosphates. Ind. Eng. Chem. Prod. Res. Dev., 23:
98-102.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY, D.
(1975) Chronic toxicity of a synthetic triaryl phosphate oil to fish.
Environ. Physiol. Biochem., 5: 361-369.
LOCKHART, W.L., METNER, D.A., BLOUW, A.P., & MUIR, D.C.G. (1982)
Prediction of biological availability of organic chemical pollutants to
aquatic animals and plants. In: Pearson, J.G., Foster, R.B., & Bishop,
W.E., ed. Aquatic toxicology and hazard assessment: Fifth conference,
Philadelphia, American Society for Testing and Materials, pp. 259-272
(ASTM STP 766).
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62(1): 47-51.
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem. Environ.
Health Perspect., 10: 269-284.
MAYER, F.L., Jr, MAYER, K.S., & ELLERSIECK, M.R. (1986) Relation of
survival to other endpoints in chronic toxicity tests with fish.
Environ. Toxicol. Chem., 5(8): 737-748.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE, P.M., &
SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An aquatic
environmental assessment of Pydrauls 50E and 115E, In: Branson, D.R. &
Dickson, K.L., ed. Aquatic Toxicology and Hazard Assessment: Fourth
Conference, Philadelphia, American Society for Testing and Materials,
pp. 103-123 (ASTM STP 737).
MOCHIDA, K., GOMYODA, M., FUJITA, T., & YAMAGATA, K. (1988) Tricresyl
phosphate and triphenyl phosphate are toxic to cultured human, monkey,
and dog cells. Zentralbl. Bakterial. Mikrobiol. Hyg., B185(4-5):
427-429.
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising Supplement
52: 10A, p. 697, New York McGraw-Hill Inc.
MONSANTO INDUSTRIAL CHEMICALS CO. (1979) Summary of the mutagenicity
study, neurotoxicity study, teratology study, long-term feeding study
and 90-day inhalation study which Monsanto has on the aryl phosphate,
Saint-Louis, Missouri, Monsanto Industrial Chemicals Co. (Prepared for
the US Environmental Protection Agency) (EPA-OTS document No. 40-
7942057).
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980a) Determination of
several triaryl phosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., BLOUW, A.P., & LOCKHART, W.L. (1980b)
Environmental dynamics of phosphate esters. I. Uptake and
bioaccumulation of triphenyl phosphate by rainbow trout. Chemosphere,
9: 525-532.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and cleanup
of fish, sediment, and water for determination of triaryl phosphates by
gas-liquid chromatography. J. Assoc. Off. Anal. Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Toxicol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983a) Environmental
dynamics of phosphate esters. III. Comparison of the bioconcentration of
four triaryl phosphates by fish. Chemosphere, 12: 155-166.
MUIR, D.C.G., TOWNSEND, B.E., & LOCKHART, W.L. (1983b) Bioavailability
of six organic chemicals to Chironous tentans larvae in sediment and
water. Environ. Toxicol. Chem., 2: 269-281.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition coefficient to
measure bioconcentration potential of organic chemicals in fish.
Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate ester
mixtures to invertebrates and fish. Bull. environ. Contam. Toxicol.,
19: 250-256.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performanace liquid
chromatographic analysis on radial compression column of the neurotoxic
tri-o-cresyl phosphate and metabolites. Anal. Biochem., 135: 296-303.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresyl phosphate. Sci. total Environ.,
43: 233-241.
PALAWSKI, D., BUCKLER, D.R., & MAYER, F.L. (1983) Survival and condition
of Rainbow trout (Salmo gairdneri) after acute exposures to methyl
parathion, triphenyl phosphate, and DEF. Bull. environ. Contam.
Toxicol., 30: 614-620.
PEEREBOOM, J.W.C. (1960) The analysis of plasticizers by micro-
adsorption chromatography. J. Chromatogr., 4: 323-328.
PEGUM, J.S. (1966) Contact dermatitis from plastics containing triaryl
phosphtes. Br. J. Dermatol., 78: 626-631.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975) Utilization of
triaryl phosphates by a mixed bacterial population. Can. J. Microbiol.,
21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with synergistic
properties. J. econ. Entomol., 59(1): 11-15.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin layer
chromatography. Evaluation of methods and application on phosphate
esters, polychlorinated paraffins and some PCB-substitutes. Chemosphere,
9: 683-691.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P., &
TUCKER, E.S. (1979) Environmental fate of selected phosphate esters.
Environ. Sci. Technol., 13: 840-844.
SASAKI, K., TAKEDA, M., & UCHIYAMA, M. (1981) Toxicity, absorption and
elimination of phosphoric acid triesters by Killifish and Goldfish.
Bull. environ. Contam. Toxicol., 27: 775-782.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by Killifish
(Oryzeas laptipes). Bull. environ. Contam. Toxicol., 28: 752-759.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the Delaware
River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, M.I., EVOLVE, E., & FRAZIER, W.H. (1930) Pharmacological action
of certain phenol esters with special reference to the etiology of so-
called ginger paralysis. Public Health Rep., 45: 2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies on the
pharmacology of certain phenol esters with special reference to the
relation of chemical constitution and physiologic action. Natl. Inst.
Health Bull., 160: 1-53.
SOBOTKA, T.J., BRODIE, R.E., ARNOLD, A., WEST, G.L., & O'DONNELL, M.W.
(1986) Neuromotor function in rats during subchronic dietary exposure to
triphenyl phosphate. Neurobehav. Toxicol. Teratol., 8: 7-10.
SUGIYAMA, H. & TANAKA, K. (1982) [Investigation of trace organic
chemicals in the sea water of the Tokyo Bay by gas chromatography mass-
spectrometry.] Bull. Kanagawa Prefect. environ. Center, 4: 33-38 (in
Japanese).
SUTTON, W.L., TERHAAR, C.J., MILLER, F.A., SCHERBERGER, R.F., RILEY,
E.C., ROUDABUSH, R.L., & FASSETT, D.W. (1960) Studies on the industrial
hygiene and toxicology of triphenyl phosphate. Arch. environ. Health,
1: 45-58.
SZYBALSKI, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76:
475-489.
THEISS, J.C., STONER, G.D., SHIMKIN, M.B., & WEISBURGER, E.K. (1977)
Test for carcinogenicity of organic contaminants of United States
drinking waters by pulmonary tumor response in strain A mice. Cancer
Res., 37: 2717-2720.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorous compounds. Anal. Chem., 53: 1466-1499.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division, General
Motors Corporation, Rochester, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (PB82-104530).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chasse, Louisiana,
Cincinnati, Ohio, National Institute for Occupational Safety and Health
(Report No. IWA-89-10).
VAINIOTALO, S., VERKKALA, E., SAVOLAINEN, H., NICKELS, J., & ZITTING, A.
(1987) Acute biological effects of commercial cresyl diphenyl phosphate
in rats. Toxicology, 44(1): 31-44.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A simple
colorimetric method for estimation of tricresyl phosphate in edible
oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish
Res. Board Can., 36: 1040-1048.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J., & SVEC, H.J. (1978)
Organic emissions from combustion of combination coal/refuse to produce
electricity. Chemosphere, 7: 893-902.
WAGEMANN, R., GRAHAM, B., & LOCKHART, W.L. (1974) Studies on chemical
degradation and fish toxicity of a synthetic triaryl phosphate
lubrication oil, IMOL S-140, Ottawa, Environment Canada, Fisheries and
Marine Service (Technical Report No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution and toxicological
aspects of a triaryl phosphate synthetic oil]. Annual Report of the
Tokyo Metropolitan Research Institute for Environmental Protection, 11:
110-113 (in Japanese).
WELSH, J.J., COLLINS, T.F.X., WHITBY, K.E., BLACK, T.N., & ARNOLD, A.
(1987) Teratogenic potential of triphenyl phosphate in Sprague-Dawley
(Spartan) rats. Toxicol. ind. Health, 3(3): 357-369.
WHO (1990) Environmental Health Criteria 110: Tricresyl phosphate,
Geneva, World Health Organization.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of tri(haloalkyl)-
, trialkyl-, and triaryl phosphates in Canadian drinking water. Bull.
environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON, R.
(1982) Determination of mutagenic potential and organic contaminants of
Great Lakes drinking water. Chemosphere, 11: 263-276.
WILLS, J.H., BARRON, K., GROBLEWSKI, G.E., BENITZ, K.F., & JOHNSON, M.K.
(1979) Does triphenyl phosphate produce delayed neurotoxic effects?
Toxicol. Lett., 4: 21-24.
WINDHOLZ, M., ed. (1983) The Merck Index, 10th ed., Rahway, New Jersey,
Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate esters:
Application of linear free energy relationships to estimate hydrolysis
rate constants for use in environmental fate assessment. Chemosphere,
9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-65.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides in the
atmosphere above the Dogo plain and Ozu basin.] J. Chem. Soc. Jpn, 1980:
645-653 (in Japanese).
ZEIGER, E., ANDERSON, B., HAWORTH, S., LAWLOR, T., MORTELMANS, K., &
SPECK, W. (1987) Salmonella mutagenicity tests: III. Results from the
testing of 255 chemicals. Environ. Mutagen., 9(Suppl. 9): 1-110.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop, Can. Tech. Rep. Fish. aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de triphényle (TPP) est une substance cristalline,
ininflammable, inexplosible et incolore. Son coefficient de partage
entre l'octanol et l'eau (log de Pow) est de 4,61-4,76. A la température
ambiante ordinaire, il s'hydrolyse rapidement en milieu alcalin pour
donner du phosphate de diphényle et du phénol, mais l'hydrolyse est très
lente en milieu acide ou neutre.
Pour l'analyse, la méthode choix est la chromatographie gaz-liquide,
avec détection au moyen d'un dispositif sensible à l'azote/phosphore ou
par photométrie de flamme. La limite de détection dans l'eau est
d'environ 20 ng/litre.
2. Sources d'exposition humaine et environnementale
Le phosphate de triphényle est produit à partir de l'oxychlorure de
phosphore et du phénol. Il est utilisé comme retardateur de flamme dans
les résines phénoliques et les résines à base d'oxydes de phénylène que
l'on utilise pour la fabrication de pièces d'automobiles et de
l'appareillage électrique; on l'emploie également comme plastifiant
ininflammable dans l'acétate de cellulose servant à la confection des
pellicules photographiques. Il entre également dans la compositision des
liquides hydrauliques et des huiles lubrifiantes à côté d'un certain
nombre d'autres usages de moindre importance.
On peut considérer qu'en utilisation normale, la population dans son
ensemble n'encourt qu'une exposition minime.
3. Transport, distribution et transformation dans l'environnement
Les phosphates de triaryle pénètrent dans le milieu aquatique par
suite de fuites de liquides hydrauliques, de la lixiviation de certains
plastiques et en faibles quantités, lors des divers processus de
fabrication. En raison de sa faible solubilité dans l'eau et de son
coefficient d'adsorption au sol relativement élevé, le phosphate de
triphényle se fixe rapidement sur les sédiments de rivières ou des
étangs. En milieu aquatique, il subit une biodégradation rapide.
La dégradation du phosphate de triphényle comporte une hydrolyse
enzymatique par étapes en orthophosphate et phénol.
Les facteurs de bioconcentration mesurés chez plusieurs espèces de
poissons vont de 6 à 18 900 et la demi-vie d'élimination varie de 1,2 à
49,6 heures.
La libération de cette substance dans l'air des unités de production
constitue une source d'exposition humaine sur les lieux de travail. La
combustion des matières plastiques et la volatilisation du phosphate de
triphényle à partir de ces substances ou de la surface de l'eau peut
également constituer une voie importante de pénétration dans
l'atmosphère.
4. Niveaux dans l'environnement et exposition humaine
On trouve un peu partout du phosphate de triphényle dans l'air,
l'eau, les sédiments et les organismes aquatiques, mais les prélèvements
effectués n'en contiennent que de faibles quantités. Les teneurs les
plus fortes qui aient été signalées sont de 23,2 ng/m3 dans l'air, 7900
ng/litre dans des cours d'eau, 4000 ng/g dans des sédiments et de 600
ng/g dans le poisson.
5. Effets sur les êtres vivants dans leur milieu naturel
La croissance des algues est complètement inhibée à des
concentrations de 1 mg/litre ou davantage mais elle est en revanche
stimulée à des concentrations plus faibles (0,1 et 0,05 mg/litre).
L'activité de la nitrogénase d' Anabaena flos-aquae diminue à mesure que
la dose augmente, passant de 84% à 0,1 mg/litre à 68% à 5,0 mg/litre.
Le phosphate de triphényle est, parmi les phosphates de triaryle,
celui qui présente la plus forte toxicité aiguë vis-à-vis des poissons,
des crevettes et des daphnies. L'indice de toxicité aiguë de ce
phosphate pour le poisson (CL50 à 96 h) va de 290 mg/litre pour Lepomis
macrochirus, à 0,36 mg/litre pour la truite arc-en-ciel. La grande
différence entre la truite et les vairons du genre Pimephales en ce qui
concerne les valeurs de la CL50, pourraient être due à des différences
dans leur aptitude à métaboliser le phosphate de triphényle. Parmi les
effets sublétaux observés chez les poissons, on peut citer des anomalies
morphologiques telles que congestion, dégénérescence et hémorragie au
niveau des petits vaisseaux sanguins (essentiellement branchiaux) ainsi
que des anomalies de comportement. L'immobilité des poissons exposés à
0,21-0,29 mg/litre de phosphate de triphényle a complètement disparu
dans les sept jours qui ont suivi le changement d'eau.
6. Effets sur les animaux d'expérience et les systèmes d'épreuves in vitro
On estime que la DL50 par voie orale est supérieure à 6,4 g/kg chez
le rat et à 2,0 g/kg chez le poulet.
Des doses de phosphate de triphényle allant de 0,5 à 2 g/kg ont été
bien tolérées par des lapins après injection intramusculaire et par des
poulets après administration orale. Lors d'une étude d'alimentation de
35 jours, on a observé après administration de cette substance à des
rats Holtzman mâles, une réduction du gain de poids corporel et une
augmentation du poids du foie.
On n'a pas observé d'effets tératogènes chez des rats Sprague-Dawley
à des doses allant jusqu'à 690 mg/kg de poids corporel. On n'a pas
publié d'études concernant la reproduction.
On ne dispose pas de données sur la mutagénicité du phosphate de
triphényle qui résultent d'épreuves correctement validées et il n'y a
pas eu non plus d'études de cancérogénicité convenables.
Après des injections sous-cutanées de phosphate de triphényle à des
chats (jusqu'à 1 g/kg) on n'a pas observé de neurotoxicité retardée; on
n'en a pas observé non plus après une étude de 4 mois sur des rats
Sprague-Dawley qui en recevaient dans leur alimentation des doses allant
jusqu'à 1% de la ration.
Aucun effet immunotoxique n'a été signalé après une étude de 120
jours pendant laquelle des rats ont reçu du phosphate de triphényle dans
leur nourriture à des doses allant jusqu'à 1%.
7. Effets sur l'homme
On a signalé une réduction statistiquement significative de la
cholinestérase érythrocytaire chez certains travailleurs, mais aucun
signe d'affection neurologique n'a été relevé chez des ouvriers qui
travaillaient dans une unité de production de phosphate de triphényle.
On n'a pas signalé non plus de neurotoxicité retardée parmi les cas
d'intoxication par le phosphate de triphényle. On a décrit des cas de
dermatite de contact dus au phosphate de triphényle.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
Les données tirées des études sur l'animal montrent que le phosphate
de triphényle est peu toxique. Appliqué sur la peau d'animaux de
laboratoire, il ne produit pas d'irritation. On estime que le phosphate
de triphényle n'est pas neurotoxique pour l'homme ni l'animal, bien
qu'un premier rapport ait pu affirmer le contraire. Lors d'une étude de
90 jours sur des rats, on a évalué à 690 mg/kg par jour la dose sans
effet nocif observable pour les mères et leur descendance. L'exposition
professionnelle et l'exposition de la population dans son ensemble
demeurent à un faible niveau.
Le phosphate de triphényle n'est pas mutagène.
Selon des données disponibles, il ne présente aucun danger pour
l'homme.
1.1 Niveaux d'exposition
Il y a probablement un risque d'exposition de la population générale
au phosphate de triphényle par l'intermédiaire des divers compartiments
de l'environnement et notamment par l'eau de consommation. Toutefois les
concentrations de phosphate de triphényle mesurées dans de l'eau de
boisson au Canada et aux Etats-Unis se sont révélées extrêmement
faibles. On en a souvent décelé la présence dans l'air des villes mais à
faibles concentrations. On a pu craindre que l'échauffement des sièges
d'automobiles en vinyle, lorsque la température extérieure est très
élevée, puisse conduire à la vaporisation du phosphate de triphényle
utilisé comme plastifiant, mais on ne dispose d'aucune donnée sur les
concentrations présentes à l'intérieur des véhicules. Lors d'une enquête
portant sur la teneur des tissus adipeux humains en phosphates de
triaryle, on n'a pas décelé la présence de phosphate de triphényle. On
ne dispose pas de données suffisantes pour se faire une idée de
l'importance de l'exposition de la population générale au phosphate de
triphényle. On a signalé des concentrations importantes de ce produit
dans l'air d'une unité de production (0,5 à 29,6 mg/m3) mais on ne
dispose pas de chiffres récents. Il serait bon d'avoir davantage de
données sur l'exposition professionnelle au phosphate de triphényle dans
les unités de production.
1.2 Effets toxiques
Le profil de toxicité du phosphate de triphényle ne permet guère
d'évaluer de façon complète le danger qu'il représente.
On a noté aucun signe d'activité mutagène chez les bactéries ni
d'ailleurs d'activité cancérogène, en se basant pour cela sur une étude
relative à une seule espèce animale. L'expérimentation animale n'a pas
pu jusqu'ici, mettre en évidence une neurotoxicité retardée attribuable
à cette substance. Lors d'une étude d'alimentation de 35 jours sur des
rats, on a relevé à la dose de 5 g/kg, une réduction du gain de poids
corporel et une augmentation du poids du foie. On ne possède pas de
données suffisantes sur les effets qu'il pourrait exercer sur la
fonction de reproduction (gonades, fécondité, parturitions, croissance
et développement de la progéniture).
On a décrit des cas de dermatite de contact attribuable au phosphate
de triphényle.
2. Evaluation des effets sur l'environnement
Dans l'eau, la concentration du phosphate de triphényle est faible et
il est peu probable qu'il exerce des effets toxiques sur les organismes
aquatiques. Il peut y avoir mortalité locale par suite du déversement
accidentel de liquides hydrauliques contenant du phosphate de
triphényle. Cependant, comme ce phosphate s'élimine rapidement des
tissus pisciaires lorsque cesse l'exposition et que les facteurs de
bioconcentration sont moyens, on ne pense pas qu'il y ait véritablement
risque de bioaccumulation.
On a fait état de fortes concentrations de phosphate de triphényle
dans les sédiments proches des unités de production. Il a été montré en
outre que le phosphate de triphényle lié aux sédiments pouvait être fixé
par un organisme qui y était présent mais on ne possède aucune donnée de
toxicité sur les espèces qui vivent dans les sédiments ou qui s'en
nourrissent. Reste que des effets sur les populations aquatiques sont
possibles.
2.1 Niveaux d'exposition
Dans les régions très industrialisées, les prélèvements effectués
dans l'air, dans les eaux superficielles, dans le sol, les sédiments et
parmi les organismes aquatiques indiquent la présence de phosphate de
triphényle. La concentration la plus élevée qui ait été signalée dans
des effluents industriels était de 16 µg/litre; dans un cours d'eau,
elle était de 7,9 µg/litre. Si l'on prend en considération la
biodégradation rapide du phosphate de triphényle dans le milieu
aquatique, il est peu probable que les concentrations que l'on rencontre
normalement puissent se révéler nocives pour les organismes qui y
vivent. Toutefois la décharge dans des mares de déchets de garnitures de
sièges en vinyle traité par du phosphate de triphényle, pourrait donner
lieu à des concentrations mortelles pour les poissons.
2.2 Effets toxiques
Parmi les divers phosphates de triaryle, le phosphate de triphényle
est celui dont la toxicité aiguë est la plus forte pour les poissons,
les crevettes et les daphnies. La CL50 à 96 h varie de 0,36 mg/litre
pour la truite arc-en-ciel à 290 mg/litre pour Lepomis macrochirus. Les
salmonidés sont en général sensibles au phosphate de triphényle mais on
a constaté qui ni la croissance ni la survie des alevins de truite arc-
en-ciel ne souffraient d'une exposition à cette substance à la
concentration de 0,0014 mg/litre. Chez des poissons rouges exposés à du
phosphate de triphényle, on a constaté un certain nombre d'anomalies
histologiques: congestion, dégénérescence et hémorragie au niveau des
petits vaisseaux sanguins, principalement les veinules et les
capillaires. Cette pathologie vasculaire est plus prononcée au niveau
des branchies.
La présence de concentrations de phosphate de triphényle de l'ordre
de 1 mg/litre ou davantage a complètement inhibé la croissance de
certaines algues alors que des concentrations plus faibles (0,1 et 0,05
mg/litre) avaient l'effet contraire. L'activité de la nitrogénase
d' Anabaena flos-aquae a été sensiblement réduite, même à la
concentration de 0,1 mg/litre.
RECOMMANDATIONS
1. Recommandations relatives aux recherches à effectuer
a) Etudes à entreprendre sur la sensibilisation cutanée.
b) Nécessité d'une étude de cytogénicité in vitro.
c) Nécessité d'études pharmacocinétiques selon les différentes voies
d'absorption.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El trifenilfosfato (TFF) es una sustancia no inflamable, no
explosiva, incolora y cristalina. Su coeficiente de reparto en octanol y
agua (log Poa) es de 4,61-4,76. A temperatura ambiente normal se
hidroliza rápidamente en solución alcalina, dando difenilfosfato y
fenol, y muy lentamente en soluciones ácidas o neutras.
El método analítico más apropiado es la cromatografía gas-líquido con
un detector sensible al nitrógeno-fósforo o uno fotométrico de llama. El
límite de detección en el agua es de unos 20 ng/litro.
2. Fuentes de exposición humana y ambiental
El TFF se fabrica a partir de oxicloruro de fósforo y fenol. Se
utiliza como pirorretardante en resinas fenólicas y de óxido de fenileno
en la producción de componentes eléctricos y del automóvil y como
plastificante no inflamable en acetato de celulosa para películas
fotográficas. También es un componente de fluidos hidráulicos o aceites
lubricantes y tiene otros usos de menor importancia.
La exposición de la población general por el uso normal puede
considerarse mínima.
3. Transporte, distribución y transformación en el medio ambiente
Los triarilfosfatos entran en el medio acuático principalmente por
escapes de fluidos hidráulicos, así como por lixiviación a partir de los
plásticos y, en menor medida, a partir de los procesos de fabricación. A
causa de su baja solubilidad en agua y su coeficiente de adsorción en el
suelo relativamente alto, el TFF se adsorbe con rapidez en los
sedimentos de los ríos (o de las charcas). Su biodegradación en el medio
acuoso es rápida.
El TFF se degrada mediante una hidrólisis enzimática escalonada que
lo divide en ortofosfato y componentes fenólicos.
Los factores de bioconcentración (FBC) medidos en varias especies de
peces oscilan entre 6 y 18 900, y la semivida de depuración va de 1,2 a
49,6 h.
La liberación de TFF desde los lugares de producción al aire
representa una fuente de exposición humana en el ambiente de trabajo. La
combustión de plásticos y la volatilización a partir de ellos o de las
superficies acuáticas también pueden ser importantes vías de ingreso en
la atmósfera.
4. Niveles medioambientales y exposición humana
El TFF se ha detectado con frecuencia en el aire, el agua, los
sedimentos y los organismos acuáticos, pero los niveles en muestras
medioambientales son bajos. Los niveles máximos detectados son de 23,2
ng/m3 en el aire, 7900 ng/litro en agua de río, 4000 ng/g en sedimentos
y 600 ng/g en peces.
5. Efectos sobre los seres vivos del medio ambiente
Las concentraciones de TFF iguales o superiores a 1 mg/litro inhiben
completamente el crecimiento de las algas, pero la concentraciones más
bajas (0,1 y 0,05 mg/litro) lo estimulan. La actividad de la nitrogenasa
de Anabaena flos-aquae decrece en función de la dosis desde 84% a 0,1
mg/litro hasta 68% a 5,0 mg/litro.
De los distintos triarilfosfatos, el TFF es el más tóxico para los
peces, los camarones y los dáfnidos. El índice de toxicidad aguda del
TFF para los peces (CL50 96 h) oscila entre 290 mg/litro en Lepomis
macrochirus y 0,36 mg/litro en la trucha arco iris. La gran diferencia
entre los valores de CE0 de la trucha y de Pimephales promelas puede
deberse a su distinta capacidad para metabolizar el TFF. Entre los
efectos subletales que produce en los peces figuran anomalías
morfológicas como congestión, degeneración y hemorragias de los vasos
sanguíneos más pequeños (principalmente de las branquias) y anomalías en
el comportamiento. La inmovilidad de los peces expuestos a
concentraciones de 0,21-0,29 mg/litro desapareció totalmente al cabo de
siete días cuando se los puso en agua limpia.
6. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
Se ha calculado que por vía oral la DL50 del TFF en ratas es > 6,4
g/kg y en pollos es > 2,0 g/kg.
Los conejos y los pollos toleraron bien dosis de TFF de 0,5 a 2,0
g/kg por vía intramuscular y oral respectivamente. En un estudio de
alimentación de 35 días en machos de rata Holtzman, con una dosis de TFF
se observó una disminución en la ganancia de peso corporal y un aumento
del peso del hígado.
Con dosis de TFF de hasta 690 mg/kg de peso corporal no se produjeron
efectos teratogénicos en ratas Sprague-Dawley. No se conocen estudios
sobre la reproducción.
No hay datos acerca de la capacidad mutagénica del TFF obtenidos en
ensayos bien contrastados y no se han hecho estudios adecuados de
carcinogenicidad.
La aplicación a gatos de una única dosis subcutánea de TFF (de hasta
1 g/kg) no causó neurotoxicidad diferida; tampoco se observó en un
estudio de cuatro meses en ratas Sprague-Dawley con dosis de hasta el 1%
en el alimento.
No se comunicaron efectos inmunotóxicos tras un estudio de 120 días
en ratas a las que se administraron dosis de hasta el 1% en el alimento.
7. Efectos en la especie humana
Se ha comunicado que, si bien algunos trabajadores de instalaciones
de producción de TFF han mostrado una reducción estadísticamente
significativa de la colinesterasa eritrocítica, no hay manifestaciones
de enfermedades neurológicas. No hay informes de neurotoxicidad diferida
en casos de intoxicación por TFF. Se han descrito casos de dermatitis de
contacto debida al TFF.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Los datos obtenidos en animales indican que el TFF tiene una
toxicidad baja. No produce efectos irritantes en la piel de los
animales. A pesar de un primer informe en sentido contrario, no se
considera que el TFF sea neurotóxico para los animales o el hombre. El
nivel sin efecto adverso observado fue, en las madres y en las crías, de
690 mg/kg al día en un estudio de 90 días realizado en ratas. La
exposición al TFF es baja tanto en la población general como en los
trabajadores.
El TFF no tiene efectos mutagénicos.
Los datos disponibles indican que no entraña peligro para los seres
humanos.
1.1 Niveles de exposición
Puede considerarse probable la exposición de la población general al
TFF por conducto de distintos medios ambientales, incluida el agua de
bebida. Se han medido concentraciones extraordinariamente bajas de TFF
en el agua de bebida del Canadá y los EE.UU. Con frecuencia se ha
detectado TFF en el aire urbano, aunque los niveles son bajos. Se ha
hablado de vaporización de TFF al calentarse el vinilo de la tapicería
de los automóviles, pero no se dispone de datos sobre la concentración
en los coches. En un estudio de los triarilfosfatos en el tejido adiposo
humano no se detectó TFF. Estos datos no bastan para evaluar la
importancia de la exposición de la población general al TFF.
Se ha comunicado la existencia de concentraciones importantes (0,5-
29,6 mg/m3) en el aire de unas instalaciones de producción de TFF, pero
no se dispone de cifras recientes. Se necesitan más datos sobre la
exposición profesional al TFF en los lugares de fabricación.
1.2 Efectos tóxicos
Los datos de que se dispone sobre la toxicidad del TFF son totalmente
insuficientes para una valoración completa del riesgo que representa.
No hay pruebas de que el TFF tenga actividad mutagénica en bacterias
ni de que tenga actividad carcinogénica, de acuerdo con un estudio sobre
una especie animal. De momento no se han obtenido pruebas de que el TFF
cause neurotoxicidad diferida en animales de experimentación. En un
estudio de alimentación de 35 días en ratas se observó una disminución
en la ganancia de peso corporal y un aumento de peso del hígado con una
dosis de 5 g/kg. No se dispone de datos adecuados en cuanto a los
efectos del TFF en la reproducción, es decir, en la función de las
gónadas, la fertilidad, el parto y el crecimiento y desarrollo de la
descendencia.
Se han descrito casos de dermatitis de contacto causada por el TFF.
2. Evaluación de los efectos en el medio ambiente
Las concentraciones de TFF en el agua ambiental son bajas y los
efectos tóxicos en los organismos acuáticos son poco probables. Los
vertidos de fluidos hidráulicos con TFF podrían tener efectos letales a
nivel local. Puesto que el TFF se elimina rápidamente de los tejidos de
los peces al terminar la exposición y los factores de bioconcentración
son moderados, no se considera que la bioacumulación sea un peligro.
Se ha informado de la presencia de altas concentraciones de TFF en
sedimentos cercanos a instalaciones de producción. Se ha demostrado que
cierto organismo que vive en los sedimentos puede utilizar el TFF fijado
por éstos, pero no hay datos de toxicidad sobre las especies que viven
en los sedimentos o se alimentan de ellos. Existe, por consiguiente, la
posibilidad de efectos en las comunidades acuáticas.
2.1 Niveles de exposición
El TFF se halla en el aire, el agua superficial, el suelo, los
sedimentos y los organismos acuáticos recogidos en zonas muy
industrializadas. La concentración más alta de TFF en efluentes de aguas
industriales que se ha comunicado es de 16 µg/litro, mientras que en
aguas fluviales es de 7,9 µg/litro. Teniendo en cuenta la rápida bio-
degradación del TFF en el medio acuoso, es poco probable que las
concentraciones normales de TFF en él afecten de manera adversa a los
organismos acuáticos. Sin embargo, si se arrojase a una charca tejido de
tapicería de vinilo tratado con TFF se produciría una concentración
suficientemente alta para matar a los peces.
2.2 Efectos tóxicos
Entre los diferentes triarilfosfatos, el TFF es el compuesto más
tóxico para peces, camarones y dáfnidos. Los valores de la CL50 en 96
horas de TFF para los peces varían entre 0,36 mg/litro en la trucha arco
iris y 290 mg/litro en Lepomis macrochirus. Aunque los salmónidos en
general son sensibles al TFF, el crecimiento y la supervivencia de los
alevines de trucha arco iris no se vieron afectados cuando éstos se
expusieron a una concentración de TFF de 0,0014 mg/litro. Los ejemplares
de Carassius auratus expuestos al TFF presentaron lesiones
histopatológicas consistentes en congestión, degeneración y hemorragia
de los vasos sanguíneos pequeños, principalmente vénulas y capilares.
Esta patología vascular es más pronunciada en las branquias.
Las concentraciones de TFF iguales o superiores a 1 mg/litro
inhibieron completamente el crecimento de las algas, pero a
concentraciones más bajas (0,1 y 0,05 mg/litro) lo estimularonn. La
actividad de la nitrogenasa de Anabaena flos-aquae se redujo de forma
significativa, incluso a una concentración de 0,1 mg/litro.
RECOMENDACIONES
1. Recomendaciones para futuras investigaciones
a) Se deberían realizar estudios de sensibilización cutánea.
b) Es necesario realizar un estudio de citogenicidad in vitro.
c) Se precisan estudios farmacocinéticos de las diferentes vías.
Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number: 115-86-6
RTECS registry number: TC8400000
Synonyms: Triphenyl phosphate; Triphenyl-phosphate; TPP
Trade name: Phosflex TPP(R),; Disflamoll TP(R),; Celluflex TPP(R),
Manufacturers and suppliers (Modern Plastics Encyclopedia, 1975):
Ashland Chemical Co.; Celanese Co.; Daihachi Chemical Industry Co.,
Ltd.; East Coast Chemicals Co.; B.F. Goodrich Chemical Co.; Mobay
Chemical Co.; Monsanto Chemical Co.; Rhone-Poulenc Co.; Showa Ether Co.,
Ltd.; Stauffer Chemical Co.
2.2. Physical and chemical properties
The physical properties of TPP are listed in Table 1.
Table 1. Physical properties of TPP
-------------------------------------------------------------------------------
Physical state crystalline solid
Colour colourless
Odour very slightly aromatic
Melting point (°C) 49-50a; 49b; 49.2c
Boiling point (°C) 245 (11 mmHg)a,b; 220 (5 mmHg)c;
234 (5 mmHg)d; 370e
Relative density 1.185-1.202 (25 °C)c; 1.185 (25 °C)d;
1.2055b
Refractive index (at 25 °C) 1.552-1.563c
Flash point (°C) 220b; 225c
Viscosity (cSt) 11 (50 °C); 9.9 (55 °C)c
Vapour pressure (mmHg) 0.15 (150 °C); 1.90 (200 °C)c;
1.0 (193.5 °C)e
Henry's Law constant 1.8-3.6 x 10-7 atm-m3/mol
Solubility in organic solvents soluble in benzene, chloroform, ether,
acetone; moderately soluble in ethanola
Solubility in water (mg/litre) 1.9f; 0.73g; 2.1 (±0.1)h
Octanol-water partition coefficient 4.63f; 4.61i; 4.76j
(log Pow)
-------------------------------------------------------------------------------
a Windholz (1983)
b Hine et al. (1981)
c Modern Plastics Encyclopedia (1975)
d Lefaux (1972)
e Sutton et al. (1960)
f Saeger et al. (1979)
g Hollifield (1979)
h Ofstad & Sletten (1985)
i Kenmotsu et al. (1980b)
j Sasaki et al. (1981)
TPP is non-flammable and non-explosive. It begins to decompose at
about 600 °C but is not completely degraded even at 1000 °C in inert
gas. Under these conditions, TPP yields aromatic hydrocarbons
(naphthalene, biphenyl, phenanthrene, anthracene, etc.), oxygenated
aromatic compounds (phenol, dibenzofuran, diphenyl ether) and phosphoric
oxides (ortho- , pyro-, meta-, and poly-phosphoric acids). With a large
excess of air, complete combustion to carbon dioxide is accomplished
within the temperature range 800-900 °C (Lhomme et al., 1984).
At ordinary temperature, TPP is hydrolysed very slowly in acidic and
neutral solutions but rapidly in alkaline solutions. The hydrolysis
rate constants and half-lives are summarized in Table 2. In studies by
Barnard et al. (1961), alkaline hydrolysis of TPP yielded diphenyl
phosphate, but further hydrolysis to monophenyl phosphate and phosphoric
acid was not observed under the experimental conditions used. Under
strong acidic conditions and at high temperature (100 °C), TPP readily
hydrolyses to give phosphoric acid (Barnard et al., 1966).
In studies by Finnegan & Matson (1972), the photolysis of TPP
yielded biphenyl (2%), the recovered ester amounting to 48%. The quantum
yield for biphenyl formation was 6 x 10-4.
2.3. Conversion factor
Triphenyl phosphate 1 ppm = 13.35 mg/m3 air
2.4. Analytical methods
Analytical methods for determining TPP in air, water, sediment,
fish, and biological tissues are summarized in Table 3. General
procedures for TPP analysis are similar to those for tricresyl phosphate
(TCP) (WHO, 1990). The detection limit of TPP in water is approximately
20 ng/litre.
Diphenyl phosphate, a hydrolysis product of TPP, has been determined
in sediment by extraction with aqueous methanol, clean-up with XAD-2
resin and C-18 bonded silica cartridge, butylation, and gas
chromatographic determination (Muir et al., 1983b).
TPP is present in several commercial triaryl phosphates, e.g.,
Santicizer-140,(R) Pydraul 50E,(R) (Monsanto Co.), Fyrquel GT,(R) and
Phosflex 41-P,(R) (Stauffer Chemical Co.) (Deo & Howard, 1978). When TPP
is identified, other triaryl phosphates are often detected at the same
time.
2.4.1. Sample extraction
TPP is extracted from water, sediment, fish, and air along with TCP.
WHO (1990) gives details of methods.
Table 2. Hydrolysis rate constants and half-lives of TPP in aqueous solution
---------------------------------------------------------------------------------------------------------
Rate constants
Solution Temper- pH K1 K2 K1' Half- Reference
ature first second pseudo- life
(°C) order order first
(sec-1) (M-1.sec-1) order
---------------------------------------------------------------------------------------------------------
Water 27 alkaline 2.7 x 10-1 Wolfe
(1980)
60% dioxane-water 0 alkaline 2.35 x 10-3 Barnard
10.1 alkaline 4.77 x 10-3 et al.
24.7 alkaline 1.06 x 10-2 (1961)
35 alkaline 2.32 x 10-2 Barnard
et al.
(1961)
NaOH (0.1 mol/ 22 13.0 0.49 h Muir et
litre)/acetone (1:1) al.
(1983a)
H3BO4/NaOH buffer 25 9 3 days Mayer et
al. (1981)
Buffered water 21 ± 2 8.2 9.3 x 10-2/day 7.5 Howard &
days Deo (1979)
9.5 1.3 Howard &
days Deo (1979)
Dioxane-water (3:1) 100 neutral 6.0 x 10-8/day 130 Barnard et
days al. (1961)
KH2PO4/Na2HPO4 25 7 19 Mayer et
days al. (1981)
KHC8H4O4/NaOH buffer 25 5 28 Mayer et
days al. (1981)
Dioxane-water (3:2) 100 neutral 6 x 10-8 Barnard
0.122M HClO4 1.43 x 10-5 et al.
1.21M HClO4 10.8 x 10-5 (1966)
3.02M HClO4 6.45 x 10-5 Barnard
et al.
(1966)
---------------------------------------------------------------------------------------------------------
Table 3. Methods for the determination of TPP
---------------------------------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
---------------------------------------------------------------------------------------------------------
Workplace collect with Millipore filter, extract GC/FPD 1 µg per TCP & TPP US NIOSH
air with ethanol sample (1982)
Environment trap with glycerol-Florisil column, eluate GC/FPD 1 ng/m3 simultaneous Yasuda
air with methanol, add water, and extact with method for (1980)
hexane trialkyl/aryl
phosphates
Air collect by aspiration through ethanol, TLC 5 ng/plate TCP & TPP Druyan
hydrolyse with NaOH; the resultant phenols (1975)
are reacted with p -O2NC6H4N2+ and
separated with silica gel plate
Drinking- adsorb with XAD-2 resin, eluate with GC/NPD 1 ng/litre method for Lebel et
water acetone-hexane or acetone GC/MS low level al. (1979,
trialkyl/aryl 1981)
phosphates
River or extract with methylene chloride or benzene GC/NPD 0.02 µg/ simultaneous Kenmotsu et
sea water GC/FPD litre method for al. (1980a,
(TPP) trialkyl/aryl 1981b,
phosphates 1982b)
GC/MS 0.05 µg/ Muir et al.
litre (1981)
(TCP) Ishikawa et
al. (1985)
Farm pond reflux with methanol-water (9+1) or meth- GC/NPD 1 ng/g simultaneous Muir et al.
sediment ylene chloride-methanol (1+1), clean-up by method for (1980, 1981)
acid alumina column chromatography triaryl
phosphates
Table 3. (contd.)
---------------------------------------------------------------------------------------------------------
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
---------------------------------------------------------------------------------------------------------
River extract with acetonitrile or acetone, GC/FPD 5 ng/g simultaneous Kenmotsu et
or sea clean-up by charcoal or Florisil column GC/MS method for al.(1980a,
sediment chromatography trialkyl/aryl 1981b, 1982a,
phosphates 1982b, 1983)
Ishikawa et
al. (1985)
Fish extract with hexane or methanol, clean-up GC/NPD 1 ng/g simultaneous Muir et
by gel permeation column chromatography GC/MS method for al. (1980,
and acid alumina column chromatograpy triaryl 1981, 1983)
phosphates
Fish extract with acetonitrile and methylene GC/FPD 5 ng/g simultaneous Kenmotsu et
chloride, clean-up by acetonitrile-hexane GC/MS method for al. (1980a)
graphy, concentrated sulfuric acid extrac- trialkyl/aryl
tion and Florisil column chromatography phosphates
Human extract with benzene or acetone-hexane GC/NPD 1 ng/g simultaneous Lebel &
adipose (15 + 85), clean-up by gel permeation GC/FPD method for Williams
tissues chromatography trialkyl/aryl (1983)
phosphates
---------------------------------------------------------------------------------------------------------
2.4.2. Clean-up procedures
Clean-up procedures for TPP are similar to those for TCP (WHO,
1990). It is difficult to separate TPP from other triaryl phosphates by
Florisil or gel permeation chromatography.
2.4.3. Gas chromatography and mass spectrometry
TPP is analysed simultaneously with TCP. GC and mass spectrometry
procedures are described in WHO (1990).
2.4.4. Contamination of analytical reagents
Triaryl phosphates (TAPs) are widely used as flame retardants in
plastics and hydraulic fluids. Their wide-spread use and release into
the environment produces trace contamination of reagents used for
analysis. Trace amounts of TPP have been found in Super Q water
(Williams & Lebel, 1981), Corning water (Lebel et al., 1981), hexane,
acetonitrile, and methylene chloride (Daft, 1982). TAPs have also been
found in cyclohexane (Bowers et al., 1981), hexane (Hudec et al., 1981),
and analytical grade filters (Daft, 1982). Care must be taken to avoid
contamination of reagents in order to obtain reliable data in trace
analysis of TPP.
2.4.5. Other analytical methods
A colorimetric method has been developed for determination of TPP in
air (Druyan, 1975), but the interference by other TAPs was not
investigated. Thin-layer chromatography (TLC) has been used for the
determination of TPP in air (Druyan, 1975) and in plastics (Peereboom,
1960; Braun, 1965). The octanol/water partition coefficient of TPP has
been determined by reversed phase TLC (Renberg et al., 1980). It is
difficult to separate the various TAPs by TLC (Bloom, 1973). Tittarelli
& Mascherpa (1981) described a highly specific HPLC detector for TAPs
using a graphite furnace atomic absorption spectrometer. In general,
TLC and HPLC have not been used as widely as GLC for the analysis of
TPP.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
TPP does not occur naturally in the environment. Figures concerning
total world production are not available, but 7250 tonnes was produced
in the USA in 1977 (Boethling & Cooper, 1985) and 3750 tonnes in Japan
in 1984.
TPP is produced from phosphorus oxychloride and phenol.
3.2. Uses
TPP was used, in Japan in 1984, as a flame-retardant in phenolics
and phenylene-oxide-based resin for the manufacture of electrical and
automobile components (3200 tonnes), as a non-flammable plasticizer in
cellulose acetate for photographic films (500 tonnes), and for other
miscellaneous purposes (50 tonnes)a. Other uses of TPP are as a non-
combustible substitute for camphor in celluloid (which renders
acetylcellulose, nitrocellulose, airplane "dope", etc. stable and
fireproof), for impregnating roofing paper, and as a plasticizer in
lacquers and varnishes (Windholz, 1983). It is also used as a
plasticizer in vinyl automotive upholstery (Ahrens et al., 1978) and in
cellulose acetate articles (Pegum, 1966).
TPP is also found as a component of hydraulic fluids and lubricant
oils (WHO, 1990; Table 6), and of other triaryl phosphate esters: methyl
diphenyl phosphate (triphenyl phosphate content, ca. 5%); 2-ethylhexyl
diphenyl phosphate (ca. 5%); trixylenyl phosphate (ca. 5%); iso-decyl
diphenyl phosphate (ca. 45%); cresyl diphenyl phosphate (ca. 45%);
isopropylphenyl diphenyl phosphate (ca. 45%) (Daft, 1982).
a Personal communication to IPCS from the Association of the Plasticizer
Industry of Japan (1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Summary
TPP has been found in various environmental media, but usually at
low levels. It may be released by leakage at sites of production and use
and by the combustion of plastics. No figures are available on the
amounts released into the environment.
The solubility of TPP in water is low, and it is readily adsorbed
onto sediment.
The rate of biodegradation in water is dependent on water quality (1
mg/litre was degraded in 4 days in River Mississippi water). Little or
no degradation occurs in heat-sterilized river water. The degradation
pathway is reported to involve stepwise enzymatic hydrolysis.
Water treatment techniques, both for waste water and drinking-water,
reduce TPP levels by at least an order of magnitude.
Bioaccumulation data are available from laboratory studies, but
should be considered to represent a bioaccumulation potential.
Depuration, as measured by clearance rate constant, is higher for
rainbow trout than for fathead minnows by about 50%.
4.1. Transport and transformation in the environment
4.1.1. Release to the environment
The release of TPP into the air at production sites represents a
potential source of human contamination. It has been suggested that,
since TPP in the reactor and purification vessels is hot, mist and
vapour coming from leaks in the reactor and from the open receiving tank
are the main source of TPP in the air (Sutton et al., 1960) (see also
section 5.2). Recent figures however are not available. A low
concentration (0.057 mg/m3) of TPP was detected near a zinc die cast
machine where hydraulic fluids were used (US NIOSH, 1980).
Combustion of plastics or volatilization from plastics or water
surface may also be a major pathway to the atmosphere. Vick et al.
(1978) found TPP emitted in the vapour phase and on particulate matter
from a utility plant. The concentrations were not reported.
The entry of TPP into the aquatic environment is thought to occur
principally via hydraulic fluid leakage, as well as by leaching from
vinyl plastics and, to a minor extent, from manufacturing processes
(Ahrens et al., 1978; Mayer et al., 1981; WHO, 1990).
4.1.2. Fate in water and sediment
The solubility of TPP in water is low (Table 1).
Monitoring studies have shown trialkyl and triaryl phosphates to be
present in water and sediment sampled near major industrialized sites
(Konasewich et al., 1978; Sheldon & Hites, 1978, 1979; Mayer et al.,
1981; Williams & Lebel, 1981; Aldous, 1982; Williams et al., 1982;
Ishikawa et al., 1985). The adsorption coefficient of TPP on marine
sediment was found to be 59 (Kenmotsu et al., 1980b). Muir et al.
(1982) showed rapid equilibrium of TPP with the bottom sediment in a
shallow pond (depth 0.5 m) within 10 h.
4.1.3. Biodegradation
TPP (200 µg) was completely degraded within 4 days in 200 ml of
River Mississippi (USA) water at room temperature (Saeger et al., 1979).
Howard & Deo (1979) measured the degradation rate constants for TPP in
non-sterilized natural water (Seneca River and Lake Ontario, USA).
Little degradation occurred for the first two days, followed by a loss
more rapid than in distilled water at comparable pH. After two days,
the pseudo-first-order rate constants at pH 8.2 were 0.64 and 0.34 days-1
for the two natural water samples, and 0.093 days-1 for distilled
water. The rapid degradation (99.2% in 7 days) of TPP (1 mg/litre) was
also found in a river die-away study using Neya and Oh River water
(Osaka, Japan), whereas no degradation was observed during 15 days in
heat-sterilized river water (Hattori et al., 1981). In clear non-
sterilized sea water, the degradation was very slow (35.1% in 14 days)
(Hattori et al., 1981).
Primary biodegradation rates from semicontinuous activated sludge
studies generally show the same trend in degradation rates as river die-
away studies; TPP (3-13 mg per litre, 24-h feed) revealed 96% (± 2%)
degradation (Saeger et al., 1979). The ultimate biodegradability was
measured using the apparatus and procedure developed by Thompson &
Duthie and modified by Sturm; the theoretical carbon dioxide evolution
from TPP (18.3 mg/litre) was 81.8% (Saeger et al., 1979).
The degradation pathway for TPP is reported to involve stepwise
enzymatic hydrolysis to orthophosphate and phenolic moieties. The
phenol would be expected to undergo further degradation (Barrett et al.,
1969; Pickard et al., 1975).
4.1.4. Water treatment
Data from FMC Corporation (USA) show that TPP (0.74 mg per litre) in
waste water was reduced to 0.07 mg/litre in the effluent water by
biological treatment (Boethling & Cooper, 1985). TPP was reduced from
16 µg/litre to 2 µg/litre by classical secondary treatment methods, and
from 0.2 µg/litre to 0.03 µg/litre by standard techniques for drinking-
water treatment (Sheldon & Hites, 1979). Fukushima & Kawai (1986) also
reported that TPP (0.054-2.12 µg/litre) in untreated water was reduced
to 0.005-0.082 µg/litre by conventional waste water treatment.
4.2. Bioaccumulation
4.2.1. Fish
Data on the bioconcentration and depuration of TPP are given in
Table 4. None of the exposures were considered to be representative of
realistic environmental levels. Moreover the bioconcentration factor
(BCF) measured in the laboratory must be considered to represent a
bioaccumulation potential rather than an absolute bioaccumulation factor
(Veith et al., 1979).
Several equations have been presented to predict the
bioconcentration factors of organic chemicals in various fish strains
using octanol-water partition coefficient (Pow) or water solubility
(Neely et al., 1974; Lu & Metcalf, 1975; Kanazawa, 1978; Veith et al.,
1979; Sasaki et al., 1982).
Of six tissues of fish exposed with 14C-triphenyl phosphate, liver
had the highest concentration (10 µg/g at 4 h post-treatment) and also
showed the highest rate of 14C-triphenyl phosphate depuration. The
rapid clearance from liver suggests extensive TPP metabolism. Rates of
uptake of radioactivity (µg/g tissue per h) by the six tissues were as
follows: liver, 2.75; kidney, 2.01; caeca, 0.62; intestine, 0.53;
muscle, 0.45; blood, 0.56 (Muir et al., 1980b).
Clearance of TPP was biphasic with more rapid rates of clearance in
the first 6 days after transfer to clean water, especially in the case
of rainbow trout (Muir et al., 1983a). The clearance rate constant was
higher for rainbow trout than for fathead minnows by about 50% (Muir et
al., 1983a).
Table 4. Bioaccumulation and clearance of TPP by fish
---------------------------------------------------------------------------------------------------------
Species Temp Flow/ Analy- BCF Exposure Uptake Clearance Depur- Reference
(°C) stat tical (k1/k2) concentration rate rate ation
methoda (mg/litre) (k1, (k2 x 103, half life
h-1) h-1) (h)
---------------------------------------------------------------------------------------------------------
Killfish 25 Stat GC-FPD 157-390 1 1.2 Sasaki et al.
(Oryzeas (1982)
latpes) GC-FPD 250-500 0.25 Sasaki et al.
(1981)
Flow GC-FPD 84-193 1 Sasaki et al.
(1982)
Rainbow 10 Stat TR 1368 ± 329b 0.005-0.05 11.6-17.4 42.5 Muir et al.
trout (1983a)
(Salmo TR 573 ± 97c 9.7c
gairdneri) TR 931 ± 122d 17.7d
HER 324 ± 99c 20.7
10 Stat TR 2590b 0.05 43.36 17.9 (fast) Muir et al.
(1980b)
TR 18 900b 2.45 (slow)
12 Stat GC-FPD 271 12.96 Sitthichaikasem
(1978)
Fathead 10 Stat TR 1743 ± 282b 0.005-0.05 7.6-14.0 49.6 Muir et al.
minnows (1983a)
(Pimephales TR 561 ± 115c 7.2c
promelas) TR 218 ± 55d 15.4d
HER 420 ± 25c 30.0
Goldfish 25 Stat GC-FPD 6-11 Sasaki et al.
(Carassius (1981)
auratus)
---------------------------------------------------------------------------------------------------------
a GC-FPD = gas chromatography (flame photometric detector) after suitable extraction;
TR = total radioactivity; HER = hexane-extractable radioactivity.
b BCF was calculated by the "initial rate method".
c The static test method was used (Zitko, 1980).
d K1 and k2 were derived by non-linear regression calculation.
4.2.2. Chironomid larvae
Muir et al. (1983b) studied the accumulation of TPP by Chironomus
tentans larvae exposed to water and sediment spiked with 14C-triphenyl
phosphate. The overall accumulation has been described by the following
equation:
dCc/dt = k1(Cw) - k2(Cc) + k3(Cs)
where Cc is the concentration in the larvae, Cw the concentration in
water, Cs the concentration in sediment, k1, k3 are uptake rate
constants, and k2 is the elimination rate constant.
The rate constant k3 has been described by the following equation:
k3 = k1(Cw/Cs) x (CFs - CFw)/(CFw)
where CFs is the equilibrium concentration factor for larvae in sediment
and CFw is the equilibrium concentration factor for larvae in water.
The results are summarized in Table 5.
Table 5. Uptake rate constants (k1) calculated by use of a first-order
kinetic model and concentration factors for uptake of TPP by Chironomid larvae
------------------------------------------------------------------------------------
High concentration Low concentration
(500 µg/kg sediment) (50 µg/kg sediment)
System k1(h-1) CFw CFs k1(h-1) CFw CFs
------------------------------------------------------------------------------------
Pond sediment 0.4 ± 0.1 6 ± 0 12 ± 10 0.6 ± 0.2 12 ± 4 18 ± 8
River sediment 2.1 ± 0.8 45 ± 17a 78 ± 34a 4.2 ± 1.6 88 ± 34 90 ± 41
Sand sediment 3.3 ± 1.0 64 ± 33b 173 ± 69b 10.4 ± 3.1 208 ± 62b 138 ± 17b
------------------------------------------------------------------------------------
a Significant difference (P = 0.05) between mean larval concentrations in water
and in sediment using the t-test.
b Significant difference (P = 0.01) between mean larval concentrations in water
and in sediment using the t-test.
The relative contribution of sediment and water to the body burdens
observed in larvae (24-h exposure) was estimated by calculating uptake
rates for water (k1)(Cw) and for sediment (k3)(Cs). The results
indicate that contributions from water and sediment were roughly
equivalent for most sediments for a sediment-to-water ratio of 1:5. The
authors noted however:
"A greater ratio of water to sediment would tend to increase
the contribution of uptake from water proportionally. A
water-to-sediment ratio of 100:1 would reduce the contribution
of sediment uptake to 10%, and would make it difficult to show
significant differences between water and sediment
experiments".
Initial elimination rate constants and half-lives of TPP for larvae
exposed to different sediment-water systems are shown in Table 6.
Table 6. Elimination rate constants and half-lives of TPP for
Chironamid larvae
-----------------------------------------------------
Elimination rate
System k2 (h-1) Half-life (h)
-----------------------------------------------------
Pond sediment 0.023 ± 0.012 30.4 ± 16.1
River sediment 0.011 ± 0.004 62.7 ± 24.5
Sand sediment 0.016 ± 0.010 44.4 ± 28.0
River water 0.039 ± 0.013 17.6 ± 6.0
-----------------------------------------------------
4.2.3. Environmental fate in artificial pond water
The environmental fate of radiolabelled TPP (60 µg per litre) in
artificial pond water was studied by Muir et al. (1982). The
radioactivity observed in each pond compartment is shown in Table 7.
Small losses of TPP by volatilization were thought to occur, but this
was not confirmed by direct measurement above the water surface.
Despite differences in fish species and water temperatures between
laboratory and field experiments, the observed body burdens of TPP were
similar, for the first 24 h of the experiment, to those predicted on the
basis of laboratory data. However, at 72 and 240 h, the predicted values
were higher than those observed. These results were considered to
reflect more rapid clearance of TPP by fathead minnows than by rainbow
trout.
Table 7. Percentage of TPP radioactivity in the
various compartments of a pond
----------------------------------------------------
Time(h) Water Sediment Duckweed Fish Total
----------------------------------------------------
10 74 29 1.4 2.7 107.1
24 52 34 1.4 3.4 90.8
32 31 - - - -
48 34 43 1.2 1.3 79.5
72 28 33 0.9 0.9 62.8
120 23 40 0.5 0.6 64.1
240 13 36 0.5 0.5 50.0
----------------------------------------------------
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Summary
TPP has been widely found in air, water, sediment, and aquatic
organisms, but only at low levels. Higher levels have been found only
in sediments near industrialized areas.
Ambient air concentrations of TPP in rural areas range from 0.5 to
1.4 ng/m3 and in urban areas from 0.9 to 14.1 ng/m3.
TPP levels in surface water from 3 to 700 ng/litre have been
measured, values up to 7900 ng/litre occurring near facilities producing
either aryl phosphates or hydraulic fluids containing TPP. These high
values probably result from TPP bound to suspended sediment. Reported
drinking-water levels are several orders of magnitude lower (0.3-30
ng/litre), suggesting that TPP is removed by adsorption to filtration
media in water-treatment plants. No TPP has been detected in potable
water from wells. There is no information available on levels in
groundwater.
Levels of TPP in river and marine sediment range from 0.2 to 200
ng/g, but values of up to 4000 ng/g have been reported in sediments near
manufacturing sites for automobile parts. There has been one report of
TPP being detected in agricultural soils, but no values were given.
In fish and shellfish, TPP tissue levels from 2 to 150 ng per g have
been reported. No TPP has been detected in human adipose tissue and
there are no data on TPP for any other species.
Exposure to humans can occur by several routes, including the
ingestion of contaminated drinking-water, fish, shellfish, and other
foodstuffs. US FDA total-diet studies have found average daily intake
levels of 0.3-4.4, 1.2-1.6, and 0.5-1.6 ng per kg body weight per day
for infants, toddlers, and adults, respectively. It should be noted that
TPP occurred in less than 1% of the foods in these diets.
Occupational exposure can occur in manufacturing industries and other
areas such as automobile or aircraft facilities handling hydraulic fluids.
Levels of 0.008 to 29.6 mg/m3 have been detected in air, the highest
values occurring at TPP-manufacturing sites.
5.1. Environmental levels
TPP has been found widely in air, water, sediment, and aquatic
organisms. The levels of TPP in environmental samples are low (Table 8),
although moderately high levels have often been found in sediment
collected near heavily industrialized areas (Table 9 and 10).
5.1.1. Air
Yasuda (1980) measured the distribution of various organic
phosphorus compounds in the atmosphere above the eastern Seto Inland
Sea, Japan, and found TPP at levels of 0.5-1.4 ng/m3 in 3 out of 4
samples. He also measured concentrations of phosphate esters in the
atmosphere above the Dogo Plain and Ozu Basin of Western Shikoku, Japan,
these being mainly agricultural areas. TPP was detected only in the
urban air of a middle-size city (Matsuyama) at levels of 0.9-14.1 ng/m3.
5.1.2. Water
Although there have been many studies of TAPs in water, TPP has not
often been detected in natural waters. According to the annual reports
of the Environment Agency of Japan, TPP has not been detected in river
or sea water at any sampling points in Japan. Detection limits varied
from 20 to 200 ng/litre at the various laboratories (EAJ, 1977, 1981).
Kawai et al. (1978) detected TPP in river water sampled in Osaka, Japan,
at levels of 50-700 ng per litre, and Ishikawa et al. (1985) detected
levels of 13-31 ng/litre in 5 out of 16 samples of river water in
Kitakyushu City, Japan, but none in sea water. Both cities are located
in the most heavily industrialized area of Japan. In Tokyo, Japan, TPP
was not found in river or sea water (detection limit: 20 ng/litre) by
Wakabayashi (1980), whereas a level of 3 ng/litre was detected in sea
water by Sugiyama & Tanaka (1982).
High concentrations of TAPs have frequently been detected in river
water sampled near producer and user sites. Sheldon & Hites (1978)
found 100-300 ng TPP/litre in 2 out of 5 samples of Delaware River (USA)
water collected in winter, and 100-400 ng/litre in 11 out of 12 samples
collected in summer. The highest level (16 000 ng/litre) of TPP was
found in a waste stream entering Philadelphia's NorthEast Sewage
Treatment plant for industrial effluents (Sheldon & Hites, 1979).
Concentrations of TPP in four samples of Kanawha River (USA) water
collected 13 km downstream from the outfall of an aryl phosphate
manufacturing plant ranged from 300 to 1200 ng/litre (Boethling &
Cooper, 1985). Mayer et al. (1981) also detected TPP (100-7900
ng/litre) in Mississippi River (USA) water sampled at St. Louis
(Missouri), where two phosphate ester hydraulic fluids were being
produced by Monsanto Co.
Table 8. Concentrations of TPP in environmental air, water, sediment, and fish at various locations
---------------------------------------------------------------------------------------------------------
Year Location Sample Concentrationa Number of Reference
samples
(detected/
analysed)
---------------------------------------------------------------------------------------------------------
1975 Japan (various river and sea water ND (20-200 ng/litre) (0/100) EAJ (1977)
locations) river and sea sediment ND (2-50 ng/g) (0/100)
fish ND (5-50 ng/g) (0/100)
1976 Osaka (Japan) river water 50-700 ng/litre (11/13) Kawai et al.
(1978)
1976 Shikoku (Japan) atmosphere 0.9-14.1 ng/m3 (4/19) Yasuda (1980)
1977 Eastern Seto atmosphere 0.5-1.4 ng/m3 (3/4)
Inland Sea (Japan)
1978 Eastern Ontario drinking-water 0.3-2.6 ng/litre (12/12) Lebel et al.
water treatment (1981)
plant (Canada)
1978 Tokyo (Japan) river water ND (20 ng/litre) (0/12) Wakabayashi
sea water ND (20 ng/litre) (0/3) (1980)
river sediment 0.7-3.3 ng/g (10/15)
sea sediment 0.2-0.3 ng/g (2/3)
1979 Canada (various drinking-water 0.3-8.6 ng/litre (20/60) Williams &
locations) Lebel (1981)
1980 Great Lake (Canada) drinking-water 0.2-4.8 ng/litre (11/12) Williams et
al. (1982)
1980 Kitakyushu river water 13-31 ng/litre (3/16) Ishikawa et
City (Japan) sea water ND (10 ng/litre) (0/9) al. (1985)
sea sediment ND (5 ng/g) (0/6)
1980 Seto Inland fish and shellfish 2-6 ng/g (12/41) Kenmotsu et
Sea (Japan) al. (1981a)
1981 Tokyo Bay sea water 3 ng/litre Sugiyama &
(Japan) Tanaka (1982)
NR USA drinking-water 10-120 ng/litre Muir (1984)
---------------------------------------------------------------------------------------------------------
a Figures in parentheses are detection limits
ND = not detected; NR = not reported
Table 9. Concentration of TPP in water, sediment, and fish muscle at industrialized and non-industrialized sites in the USAa
-------------------------------------------------------------------------------------------------
Location Water (ng/litre) Sediment (ng/g) Fish (ng/g)
-------------------------------------------------------------------------------------------------
Waukegan Harbor, Illinois ND (0/5) 10 (2/3) ND (0/13)
Waukegan Bay, Illinois ND (0/4) ND (0/3) NR
Upper Saginaw River, Michigan 100 (3/3) 10 (3/3) ND (0/12)
Saginaw River at Lake Huron 600-700 (4/4) 1000-4000 (3/3) 100 (1/10)
Illinois River, Grafton Illinois 100 (4/4) ND (0/3) ND (0/4)
Missouri River, Fenton, Missouri ND (0/4) ND (0/3) ND (0/9)
Missouri River at Chesterfield, Missouri 100 (1/4) ND (0/4) NR
Missouri River, Halls Ferry, Missouri 100-200 (3/3) ND (0/3) NR
Mississippi River above St. Louise, Missouri ND (0/5) NR 500 (1/3)
Mississippi River at St. Louise, Missouri 100-7900 (9/15) 100 (2/6) NR
Mississippi River below St. Louise, Missouri 100-400 (3/3) ND (0/3) 100 (1/4)
Kanawha River, Winfield, W. Virginia 100-800 (3/3) 20-200 (3/6) 100-600 (13/27)
San Francisco Bay, California 100 (2/5) NR NR
-------------------------------------------------------------------------------------------------
a From: Mayer et al. (1981); measurements were made from November 1977 to May 1978; figures in
parentheses indicate number of samples (detected/analysed); detection limits were 100 ng/litre
(water), 10 ng/g (sediment), and 100 ng/g (fish); ND = not detected; NR = not reported
Table 10. Concentrations of TPP near sites producing or using trialkyl/aryl phosphates
---------------------------------------------------------------------------------------------------------
Year Location Sample Concentration Number of Reference
sample
(detected/
analysed)
---------------------------------------------------------------------------------------------------------
1975 New Orleans (USA) finished water 120 ng/litre NR Boethling
& Cooper
(1985)
1976 Delaware River (USA) river water (winter) 100-300 ng/litre (2/5) Sheldon &
river water (summer) 100-400 ng/litre (11/11) Hites
(1978)
1977 Delaware River (USA) influent of sewage 16 000 ng/litre NR Sheldon &
treatment plant Hites
effluent of sewage 2000 ng/litre NR (1979)
treatment plant
river water 200-300 ng/litre (3/3)
effluent of water 30 ng/litre NR
treatment plant
1978 Kanawha River (USA) river water 300-1200 ng/litre NR Boethling
& Cooper
(1985)
1980 FMC Corp. Plant (USA) waste water 740 000 ng/litre NR Boethling
effluent water 7000 ng/litre NR & Cooper
(1985)
1980 Automobile manufacture (USA) workplace air 0.008-0.057 mg/m3 (6/6) US NIOSH
(1980)
1983 Saginaw River (USA) river water 700 ng/g (1/4) Boethling
& Cooper
(1985)
NR TPP manufacturing plant (USA) workplace air 0.5-29.6 mg/m3 (78/78) Sutton et
al. (1960)
NR Waukegan Harbor, Illinois (USA) fish (carp, goldfish) 60-150 ng/g (3/3) Lombardo &
Egry (1979)
---------------------------------------------------------------------------------------------------------
5.1.3. Sediment and soil
Relatively high concentrations of TPP have occasionally been found
in sediments collected near heavily industrialized areas. Mayer et al.
(1981) found TPP levels of 1000-4000 ng/g in sediment from the Saginaw
River (Lake Huron) sampled at 1.6-3.2 km downstream from several plants
manufacturing automobile spare parts (Boethling & Cooper, 1985). They
also detected TPP levels of 10-200 ng/g at Waukegan Harbor (Illinois),
Upper Saginaw River (Michigan), the Mississippi River at St. Louis
(Missouri), and the Kanawha River at Winfield (West Virginia) (Mayer et
al., 1981). According to the annual reports of the Environment Agency
of Japan, TPP has not been found at any sampling points in Japan. The
detection limits varied from 2 to 50 ng/g at the various laboratories
(EAJ, 1977). Wakabayashi (1980) detected TPP levels of 0.7-3.3 ng/g in
10 out of 15 river sediment samples, and 0.2-0.3 ng/g in 2 out of 3 sea
sediment samples analysed in Tokyo.
Caines & Holden (1976) identified TPP in agricultural soils
collected from vineyards in Scotland, but the concentration was not
reported.
5.1.4. Fish
Lombardo & Egry (1979) found TPP levels of 60-150 ng/g in carp and
goldfish caught near a site at Waukegan Harbor (USA) where aryl
phosphate hydraulic fluids were used. Mayer et al. (1981) detected
concentrations of 100-600 ng per g in 16 out of 82 samples collected in
several rivers in the USA. According to the annual reports of the
Environment Agency of Japan, TPP has not been detected in fish caught at
any sampling points in Japan. The detection limits ranged from 5 to 50
ng/g at the various laboratories (EAJ, 1977, 1981). Kenmotsu et al.
(1981a) found TPP levels of 2-6 ng/g in 12 out of 41 samples collected
from Seto Inland Sea, Japan.
5.2. General population exposure
5.2.1. Food
Gilbert et al. (1986) analysed composite total-diet samples
(representative of 15 different commodity food types encompassing an
average adult diet for each of eight regions in the United Kingdom) for
the presence of trialkyl and triaryl phosphates. Of the food groups,
offal, other animal products, and nuts consistently contained the
highest levels, but the proportion of individual compounds in the
different food groups varied. Trioctyl phosphate was the major
component in the carcass meat, offal, and poultry groups, and there were
significant amounts of TPP and TBP. Total phosphate intake was
estimated to be between 0.07 and 0.1 mg per person per day.
Gunderson (1988) reported the presence of TPP in samples collected
between April 1982 and April 1984 during FDA total-diet studies. The
mean daily intakes of TPP were 0.3-4.4, 1.2-1.6, and 0.5-1.6 ng/kg body
weight per day for infants, toddlers, and adults, respectively.
5.2.2. Drinking-water
Lebel et al. (1981) analysed TAPs in drinking-water sampled from
eastern Ontario water treatment plants and found TPP levels of 0.3-2.6
ng/litre in all of the 12 samples collected. An extended survey of
drinking-water was conducted in Canada (Williams & Lebel, 1981). TPP
was detected at levels of 0.3-8.6 ng/litre in 7 out of 60 samples of
treated potable water obtained at the treatment plants of 29
municipalities. Higher levels of TPP were present in treated water
obtained from river sources, compared with samples from lake sources,
and TPP was not found in potable water from wells. TPP was also
detected in 11 out of 12 samples of drinking-water obtained from 12
water treatment plants located around the Great Lakes (USA and Canada)
at concentrations from 0.2 to 4.8 ng/litre (Williams et al., 1982).
Sheldon & Hites (1979) reported a relatively high level of TPP (30
ng/litre) in finished drinking-water sampled from a water treatment
plant located near a sewage treatment plant handling industrial
effluents.
In general, the concentration of TPP in drinking-water is 100- to
1000-fold lower than that in river or lake water. Due to its adsorption
to sediment, TPP can efficiently be removed by filtration at water
treatment plants.
5.2.3. Human tissues
There has been only one report of TAPs being present in human
adipose tissues (Lebel & Williams, 1983). TPP was not detected.
5.3. Occupational exposure
Sutton et al. (1960) investigated the concentration of TPP in the
air of a TPP-manufacturing plant. The levels found at various locations
are indicated in Table 11.
Table 11. Air concentrations of TPP at various
locations in a manufacturing plant
-------------------------------------------------------
Location Number of Range Mean
samples (mg/m3) (mg/m3)
-------------------------------------------------------
Reactor room 6 1.4-2.2 1.8
Purification area
General room air 6 1.0-3.9 2.4
Receiving tank 10 5.0-29.6 12.0
Flaker room
General room air 6 1.8-3.7 2.6
Flaker 11 2.6-6.8 4.5
Bagging area
General room air 5 2.2-7.8 4.1
Bagger 23 0.5-20.8 8.2
Stacking bags 11 0.7-7.4 5.4
-------------------------------------------------------
TPP has been detected at concentrations of 0.008-0.057 mg/m3 in the
air at automobile manufacturing plants where hydraulic fluids are used
(US NIOSH, 1980).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Summary
The primary productivity of green algal cultures was inhibited (to
50%) by exposure to TPP (0.26 to 0.5 mg/litre) for 7 days, and the
nitrogenase activity of cyanobacteria (blue-green algae) was inhibited
at 5 mg/litre. Fungal spore germination was unaffected at 5 x 10-3
mol/litre.
The 48-h LC50 for Daphnia is 1.0 mg/litre and 96-h LC50
values for fish range from 0.36 to 290 mg/litre. The no-observed-effect
level for growth and survival of rainbow trout fry is 1.4 µg/litre.
There is no information on the toxicity of TPP to organisms living
in or ingesting sediment and none on terrestrial species other than
fungi.
6.1. Unicellular algae and fungi
TPP was the most toxic compound among six TAPs tested for effects on
the primary productivity of algae (Wong & Chau, 1984). The growth of
green algae was completely inhibited at concentrations of 1 mg/litre or
more but stimulated at lower concentrations (0.1 and 0.05 mg/litre)
(Wong & Chau, 1984). The nitrogenase activity, measured by the acetylene
reduction technique, of a cyanobacterium (blue-green alga: Anabaena
flos-aquae) was affected by TPP. Additions of 0.1, 1.0, and 5.0
mg/litre reduced the nitrogenase activity to 84, 77, and 68% of the
control value, respectively (Wong & Chau, 1984). These data are
summarized in Table 12.
TPP did not show any toxicity, as measured by spore germination, to
the fungus Aspergillus niger at a concentration of 5 x 10-3
mol/litre. However, it inhibited fungal respiration by 8-9% (Eto et
al., 1975).
6.2. Aquatic organisms
Data on the toxicity of TPP to aquatic organisms are given in Table 13.
Table 12. Toxicity of TPP and its products for freshwater unicellular algae
---------------------------------------------------------------------------------------------------------
Organism Chemical Temper- Species Effect Concen- Reference
ature (mg/litre) tration
(°C) (mg/litre)
---------------------------------------------------------------------------------------------------------
Alga TPP 20 Ankistrodesmus falcatus 7-day IC50 for 0.26 Wong &
acicularis primary productivity Chau
(1984)
Green alga TPP 20 Scenedesmus quadricaudata 7-day IC50 for 0.50 Wong &
primary productivity Chau
(1984)
Green alga TPP Selenastrum capricornutum 96-h EC50 2 Mayer
P50E Selenastrum capricornutum 96-h EC50 5 et al.
P115E Selenastrum capricornutum 96-h EC50 > 1000 (1981)
Cyanobacterium TPP 20 Anabaena flos-aquae 28-h Inhibition of 5 Wong &
(blue-green alga) 61% of control acetylene reduction Chau
(1984)
Lake Ontario TPP 20 IC50 for primary 0.2 Wong &
phytoplankton productivity Chau
(1984)
---------------------------------------------------------------------------------------------------------
Table 13. Toxicity of TPP for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organisms Age/size Temper- pH Flow/ Hard- End-point Parameter Concent- Reference
ature stat ness or ration
(°C) (mg/ criteria (mg/litre)
litre) used
---------------------------------------------------------------------------------------------------------
Rainbow trout Fry: 0.11 g, stat 96-h LC50 0.36 Palawski
(Salmo 24 mm, 12 days et al.
gairdneri) past swim-up (1983)
stage
Fry: 0.11 g, stat immobility, 96-h LC50 0.30 Palawski
24 mm, 12 days mortality, et al.
past swim-up loss of (1983)
stage equilibrium
Fry 12(±1) 7.2 stat 272 mortality 96-h LC50 0.40 Mayer et
flow 272 and growth 90-d EC0 >0.0014 al. (1981)
inhibition
0.60 g 12 7.4 stat 40 96-h LC50 0.37 Mayer &
Ellersieck
(1986)
Fathead minnow stat 96-h LC50 0.66 Mayer
(Pimephales Egg and fry flow mortality 90-d EC0 0.087-0.27 et al.
promelas) Egg and fry flow and growth 90-d EC0 >0.23 (1981)
inhibition
1.00 g 22 7.3 stat 44 96-h LC50 1.0 Mayer &
Ellersieck
(1986)
Sheepshead minnow stat 96-h LC50 0.32-0.56 Mayer et
(Cyprinodon al. (1981)
viriegatus)
Bluegill 33-75 mm 23 7.6- stat 55 96-h LC50 290 Dawson et
(Leptomis 7.9 al. (1977)
macrochirus)
Killifish 0.1-0.2 g 25 stat 96-h LC50 1.2 Sasaki et
(Oryzias al. (1981)
latipes)
Table 13. Toxicity of TPP for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organisms Age/size Temper- pH Flow/ Hard- End-point Parameter Concent- Reference
ature stat ness or ration
(°C) (mg/ criteria (mg/litre)
litre) used
---------------------------------------------------------------------------------------------------------
Goldfish 0.8-2.8 g stat 96-h LC50 0.70 Sasaki et
(Carassius al. (1981)
auratus)
Channel catfish 0.23 g 22 7.5 stat 38 96-h LC50 0.42 Mayer &
(Ictalurus Ellersieck
punctatus) (1986)
Tidewater 40-100 mm 20 stat 96-h LC50 95 Dawson
silverside et al.
(Menidia (1977)
beryllina)
Mysid shrimp stat 96-h LC50 0.18-0.32 Mayer
(Mysidopsis et al.
bahia) (1981)
Water flea stat 48-h EC50 1.0 Mayer
et al.
(1981)
---------------------------------------------------------------------------------------------------------
The 96-h LC50 values for pure TPP to fish range from 0.36 mg/litre
for the rainbow trout (Palawski et al., 1983) to 290 mg/litre for the
bluegill (Dawson et al., 1977).
The growth and survival of rainbow trout fry were not affected when
they were exposed to TPP at a concentration of 0.0014 mg/litre (Mayer et
al., 1981). At 0.23 mg/litre, the survival of fathead minnow fry was
significantly reduced, but neither the growth of the survivors nor
hatchability was affected (Mayer et al., 1981; Palawski et al., 1983).
Sublethal effects of TPP on fish include morphological and
behavioural abnormalities (Wagemann et al., 1974; Lockhart et al.,
1975). Spinal curvature was observed in surviving rainbow trout exposed
for 24-72 h at concentrations near the LC50 (Sasaki et al., 1981;
Palawski et al., 1983).
Death of goldfish occurred in a 20-litre water tank in which a piece
(18 x 38 cm) of car seat upholstery containing TPP had been immersed
(Ahrens et al., 1978). Goldfish exposed to TPP (concentration not
stated) showed histopathological lesions characterized by congestion,
degeneration, and haemorrhage of the smaller blood vessels, principally
venules and capillaries. Such vascular pathology was most pronounced in
the gills. Similar but less pronounced congestion of the smaller blood
vessels was noted in the brain, spinal cord, pseudobranch and kidneys
(Ahrens et al., 1978). Immobility of fish exposed to 0.21-0.29 mg
TPP/litre disappeared within 7 days after exposure had stopped
(Palawski, et al., 1983).
Exposure of aquatic organisms to TPP would normally arise from
spillage of hydraulic fluids containing this compound. Studies have been
made of the effects of various products, especially Pydraul 50E and
115E, Houghtosafe 1120, and Santicizer 154, on fish and aquatic
invertebrates. Where comparisons have been made between different
components of the fluids, TPP has been shown to be responsible for most
of the acute toxicity observed. However, certain characteristic
sublethal symptoms seen with these hydraulic fluids (such as cataracts
of the eye lens, effects on bone development and collagen content,
haemorrhagic lesions of the dorsal and gill regions, and vertebral
deformity) do not occur after exposure to TPP. They are therefore caused
by other fluid components (Wagemann et al., 1974; Dawson et al., 1977;
Nevins & Johnson, 1978; Mayer et al., 1981; Adams et al., 1983).
6.3. Insects
In studies on 5th-instar small brown planthopper larvae (Laodelphax
striatellus), the chemical being applied by contact, the 21-h LD50 was
570.2 µg TPP per tube, but TPP-OH was without effect (Eto et al., 1975).
The 24-h LD50 in similar studies on adult female (2-5 days old) house
flies (Musca domestica) was >1000 µg TPP per jar (Plapp & Tong, 1966).
When adult female (4-5 days old) green rice leafhoppers were treated
topically, the 24-h LD50 for TPP was 4.6 mg/g and for TPP-OH was 11.53
mg/g (Eto et al., 1975).
7. KINETICS AND METABOLISM
No data on the kinetics and metabolism of TPP in experimental
animals are available. Eto et al. (1975) reported that treated
houseflies transform TPP into diphenyl p -hydroxyphenyl phosphate
(TPP-OH) in vivo.
8. EFFECTS ON ANIMALS AND IN VITRO SYSTEMS
Summary
Acute toxicity data exist for several species of animals and
indicate low toxicity via the oral and dermal routes (1320 to 10 800
mg/kg and >7900 mg/kg, respectively). No inhalation data are
available. TPP also exhibits low toxicity in short-term studies and is
not irritant to mouse skin. In rats, no effects were seen in mothers or
offspring following repeated dietary exposure of 166-690 mg/kg per day
for a period of 91 days, including mating and gestation periods.
The neurotoxicity of TPP has been debated since the early studies of
Smith et al. (1930, 1932), which reported delayed neuropathy in cats and
monkeys exposed to TPP in acute and short-term studies. However, Wills
et al. (1979) could demonstrate no ataxia or neuropathic damage in cats
exposed to 99.9%-pure TPP. Consequently, the validity of the Smith
studies has been questioned. Other toxicity studies using behavioural
and morphological end-points have demonstrated that TPP administered
short-term to cats and chickens fails to produce neuro-toxic changes. A
mixture of triaryl (including cresyl and phenyl) phosphates produced
neurochemical changes and minor peripheral nerve pathology in the caudal
nerve of rats; acute intraperitoneal injection of 150 mg or less
produced neither biochemical nor morphological change.
Negative results have been reported for several in vitro
mutagenicity studies. No satisfactory studies are available on
carcinogenicity.
8.1. Single exposure
Acute toxicity data resulting from single exposure to TPP are
summarized in Table 14. Little information is available on the acute
signs of toxicity.
8.2. Short-term exposure
Sutton et al. (1960) reported a 35-day feeding study in male
Holtzman rats with TPP at doses of 1 and 5 g/kg. Depression of body
weight gain and an increase of liver weight were observed in the high-
dose group. No haematological changes were found.
Table 14. Acute toxicity of triphenyl phosphate
-------------------------------------------------------------
Species Route of LD50 Reference
adminis- (mg/kg)
tration
-------------------------------------------------------------
Rat oral 3500 Hierholzer et al. (1957)
Rat oral 3800 Antonyuk (1974)
Rat oral 10 800 Johannsen et al. (1977)
Rat oral > 5000 US EPA (1986)
Mouse oral > 3000 Sutton et al. (1960)
Mouse oral 1320 Antonyuk (1974)
Mouse oral > 5000 US EPA (1986)
Guinea-pig oral > 4000 Sutton et al. (1960)
Chicken oral > 2000 Smith et al. (1932)
Chicken oral > 5000 Johannsen et al. (1977)
Rabbit dermal > 7900 Johannsen et al. (1977)
-------------------------------------------------------------
In studies by Hinton et al. (1987), TPP was fed to weanling Sprague-
Dawley rats (10 of each sex per group) at dose levels of 0, 2.5, 5, 7.5,
or 10 g/kg for 120 days. The immunotoxicity evaluation included total
protein analysis, electrophoretic analysis of serum proteins, lymphoid
organ weights in relation to growth, and histopathology, together with
expanded immunohistochemical evaluation of B- and T-lymphocyte regions
in the spleen, thymus, and lymph nodes using immunoperoxidase staining.
Assessment was made of the humoral response to a T-lymphocyte-dependent
antigen, sheep red blood cells; it began at mid-term of the feeding
period for the primary response and was followed by secondary and
tertiary booster immunizations at intervals of 3 weeks. The kinetics of
the response were measured by haemolysin assay of relative antibody
titres at days 3, 4, 5, and 6 post injection. No significant effects on
the response were noted for either sex at any of the dose levels tested.
The only effects noted were a decreased rate of growth at high levels of
TPP and increased levels of alpha- and ß-globulins (Hinton et al.,
1987).
When Antonyuk (1974) administered TPP orally for 3 months to rats at
doses of 380 or 1900 mg/kg, there were no deaths, no abnormal growths,
and no inhibition of cholinesterase activity. In another study,
Antonyuk (1974) administered 650 to 1900 mg/kg orally to rats for 3
months with no significant toxic effects.
8.3. Skin irritation
No significant skin irritation was observed when a gauze pad soaked
with approximately 0.5 ml of a 70% solution of TPP in alcohol was
applied to the skin of mice for 72 h (Sutton et al., 1960).
8.4. Reproduction
In studies by Welsh et al. (1987), male and female Sprague-Dawley
(Spartan) rats (40 of each sex per group) were fed dietary levels of 0,
2.5, 5, 7.5, or 10 mg TPP/kg (from 4 weeks post weaning for 91 days,
through mating and gestation). At these dietary levels, the daily
intake of TPP during pregnancy was 0, 166, 341, 516, and 690 mg/kg body
weight, respectively. TPP exposure had no toxic effects on mothers or
offspring at these dosages. The types of developmental anomalies were
similar in both treated and control animals, and no significant increase
in the incidence of anomalies was seen in the treated groups as compared
to control values. TPP was not teratogenic in Sprague-Dawley rats at the
levels tested.
8.5. Mutagenicity
Szybalski (1958) reported negative results with TPP in a paper disk
method using streptomycin-dependent mutants of E.coli.
TPP did not demonstrate mutagenic activity in microbial assays
employing Salmonella typhimurium (TA 1535, TA 1537, TA 1538, TA 98, and
TA 100 strains) and Saccharomyces cerevisiae (D4 strain) indicator
organisms. All studies were carried out both in the presence and
absence of metabolic activation (Monsanto, 1979).
Negative results were also reported in Ames tests conducted with
Salmonella typhimurium strains TA 98, TA 100, TA 1535, and TA 1537, in
the absence or presence of rat liver S9 (Zeiger et al., 1987).
TPP was tested for its ability to induce mutations at the thymidine
kinase (TK) locus in cultured L5178Y mouse lymphoma cells. When tested
with or without metabolic activation, TPP did not induce significant
mutations at the TK locus (Monsanto, 1979).
8.6. Carcinogenicity
Theiss et al. (1977) studied the occurrence of lung adenomas in
strain A/St male mice, 6 to 8 weeks old, using doses of 80, 40, or 20 mg
TPP/kg injected intraperitoneally 1, 3, and 18 times, respectively, into
groups of 20 mice. Twenty-four weeks after the first injection, the
animals were sacrificed, and the frequency of lung tumours was compared
with that in the control group of 50 animals treated with tricarpylin
(vehicle). The pulmonary adenoma response to TPP was not significantly
greater than the response of the control mice. This study was
considered inadequate due to the low survival of animals in two of the
three experimental groups and the short duration of the study.
8.7. Neurotoxicity
In 1930, Smith and his associates found that single and multiple
doses of technical grade TPP produced generalized delayed paralysis in
cats and monkeys but not in chickens or rabbits (Smith et al., 1930).
Smith et al. (1932) attempted to ascertain the minimum lethal dose
of TPP. Rabbits survived after an intramuscular injection of 1 g/kg;
chickens also were unaffected after oral administrations of 0.5 to 2
g/kg. In cats, the minimum toxic dose by subcutaneous injection was
about 0.2 g/kg and the reaction was of the delayed type; a neurotoxic
action and flaccid paralysis were followed by death.
Johannsen et al. (1977) dosed chickens with cumulative doses of 60
g/kg but failed to produce ataxia or neuropathology suggestive of
organophosphate-induced delayed neuropathy (OPIDN).
In an attempt to re-evaluate the delayed neurotoxicity, Wills et al.
(1979) reported that 99.9%-pure TPP did not produce any evidence of
axonal degeneration, demyelination, or any other pathological changes at
11 levels of the nervous system (from the cerebral cortex to peripheral
nerves) when subcutaneously injected into cats at doses of 0.4, 0.7, or
1.0 g/kg. Prostration occurred at the higher doses. Wills et al. (1979)
suggested that the samples of TPP used by Smith et al. (1930) may have
contained impurities that were capable of producing axonal degeneration
and demyelination.
Sobotka et al. (1986) fed young male Sprague-Dawley rats (10 per
group) diets containing TPP at levels of 0, 2.5, 5, 7.5, or 10 g/kg, for
4 months. Treatment-related decreases in growth rate, in the absence of
changes in food consumption, were found at all dietary levels above 2.5
g/kg. There was no evidence of neuromotor toxicity following subchronic
dietary exposure to TPP.
In a study by Vainiotalo et al. (1987), a commercial cresyl diphenyl
phosphate preparation was analysed and found to contain approximately
35% triphenyl phosphate, 45% cresyl diphenyl phosphates, 18% dicresyl
phenyl phosphates, and 2% tricresyl phosphates. The product was almost
free of the o-cresyl isomers, as revealed by the analysis of its
alkaline hydrolysis products. A single intraperitoneal injection (150 or
300 mg/kg) of this mixture caused the induction of microsomal cytochrome
P-450 in the liver of Wistar rats, a concomitant increase in the
activities of mixed function monooxygenases, and proliferation of smooth
endoplasmic reticulum 24 h after the treatment. The activity of
pseudocholinesterase in blood was inhibited 4 h and 24 h after the
injection but the effect leveled off. Treatment with 300 mg/kg
inhibited brain 2',3'-cyclic-nucleotide 3'-phosphohydrolase through the
2-week observation period associated with demyelination in peripheral
nerves.
8.8. In Vitro studies
In vitro TPP was found to cause significant direct inhibition of
monocyte antigen presentation at non-cytotoxic concentrations as low as
1 µmol/litre (Esa et al., 1988).
9. EFFECTS ON HUMANS
Sutton et al. (1960) found no evidence of neurological disease or
other abnormalities in 32 workers exposed to TPP vapour, mist, or dust
(at a time-weighted air concentration of 3.5 mg/m3) for an average of
7.4 years. In six of these workers, who were exposed more regularly to
TPP, there was a statistically significant asymptomatic reduction in
erythrocyte cholinesterase values, but no plasma cholinesterase
depression.
In 39 workers exposed to an organophosphate ester mixture with about
30% TPP and 70% different isopropyl TPPs, a significantly lower level of
serum IgM and a lower activity (of borderline significance; p = 0.05) of
erythrocyte cholinesterase, compared to controls, were reported.
However, plasma cholinesterase activity and the other observed
parameters were not significantly affected (Emmett et al., 1984).
A few individuals have been reported to show positive reactions in
patch tests using cellulose acetate film containing both TCP and TPP.
However, the causative agent could not be identified and may have been
TCP (Hjorth, 1964). A single case of allergy to spectacle frames could
also have been due to TCP (Carlsen et al., 1986).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
Animal data indicate that TPP has low toxicity. It produces no
irritant effect on animal skin. Despite an early report to the contrary,
TPP is not considered neurotoxic in animals or man. The no-observed-
adverse-effect level on mothers and offspring from a 90-day rat study
was at 690 mg/kg per day. Both exposure of the general population and
occupational exposure to TPP are low.
TPP is not mutagenic.
The available data indicate no hazard to humans.
10.1.1. Exposure levels
Exposure of the general population to TPP through various
environmental media, including drinking-water, is likely. The
concentrations of TPP measured in drinking-water in Canada and the USA
are extremely low. TPP has often been detected in urban air, although
the levels are low. Vaporization of TPP from heated vinyl automotive
upholstery under hot weather has been suggested, but no data on
concentrations in cars are available. In a survey of TAPs in human
adipose tissues, TPP was not detected. There are insufficient data to
evaluate the significance of the general population exposure to TPP.
Significant air concentrations (0.5-29.6 mg/m3) have been reported
in a TPP manufacturing plant, but recent figures are not available.
More data on occupational exposure to TPP in manufacturing plants are
required.
10.1.2. Toxic effects
The toxicity profile of TPP is quite inadequate for a full
evaluation of its hazard.
There is no evidence that TPP has mutagenic activity in bacteria or
that it has carcinogenic activity, based on a study in one animal
species. No evidence that TPP causes delayed neurotoxicity has so far
been obtained in animal experiments. In a 35-day feeding study in rats,
depression of body weight gain and increase in liver weight were
observed at a dose of 5 g/kg. No adequate data are available on the
effects of TPP on reproduction, i.e. function of gonads, fertility,
parturition, and growth and development of offspring.
Contact dermatitis due to TPP has been described.
10.2. Evaluation of effects on the environment
Water concentrations of TPP in the environment are low and toxic
effects on aquatic organisms are unlikely. Spills of hydraulic fluids
containing TPP would be expected to cause local kills. Since TPP is
removed rapidly from the tissues of fish when exposure ends and
bioconcentration factors are moderate, bioaccumulation is not considered
to be a hazard.
High concentrations of TPP in sediment near production plants have
been reported. TPP bound to sediment has been shown to be bioavailable
to one organism living in sediment, but no toxicity data on sediment-
living or sediment-ingesting species exist. There is, therefore, the
possibility of effects on aquatic communities.
10.2.1. Exposure levels
TPP is found in air, surface water, soil, sediment, and aquatic
organisms sampled in heavily industrialized areas. The highest reported
concentration of TPP in industrial water effluent is 16 µg/litre, while
that in river water is 7.9 µg/litre. Taking into account the rapid
biodegradation of TPP in aqueous environments, normal concentrations of
TPP in aqueous environments are unlikely to adversely affect aquatic
organisms. However, disposal of TPP-treated vinyl fabric upholstery
into a pond would result in a sufficiently high concentration of TPP to
kill fish.
10.2.2. Toxic effects
Among the various triaryl phosphates, TPP is the most acutely toxic
compound to fish, shrimps, and daphnids. The 96-h LC50 of TPP for fish
ranges from 0.36 mg/litre in rainbow trout to 290 mg/litre in bluegill.
Salmonids are generally sensitive to TPP, but the growth and survival of
rainbow trout fry were not affected when they were exposed to TPP at a
concentration of 0.0014 mg/litre. Histopathological lesions in goldfish
exposed to TPP consist of congestion, degeneration, and haemorrhage of
the small blood vessels, principally venules and capillaries. Such
vascular pathology is most pronounced in the gills.
The growth of algae was completely inhibited at TPP concentrations
of 1 mg/litre or more but was stimulated at lower concentrations (0.1
and 0.05 mg/litre). The nitrogenase activity of Anabaena flos-aquae was
significantly reduced, even at 0.1 mg/litre.
11. RECOMMENDATIONS
11.1. Recommendations for further research
There is a need for skin sensitization, in vitro cytogenicity, and
pharmacokinetic studies on triphenyl phosphate.
REFERENCES
ADAMS, W.J., KIMERLE, R.A., HEIDOLPH, B.B., & MICHAEL, P.R. (1983) Field
comparison of laboratory-derived acute and chronic toxicity data,
Philadelphia, American Society of Testing and Materials, pp. 367-385
(ASTM STP 802).
AHRENS, V.D., HENION, J.D., MAYLIN, G.A., LEIBOVITZ, L., ST. JOHN, L.E.,
Jr, & LISK, D.J. (1978) A water-extractable toxic compound in vinyl
upholstery fabric. Bull. environ. Contam. Toxicol., 20: 418-422.
ALDOUS, C.N. (1982) Alterations in rat brain norepinephrine and dopamine
levels and synthesis rates in response to five neurotoxic chemicals:
acrylamide, 2,5-hexanedione, tri-o-tolyl phosphate, leptophos, and
methyl mercuric chloride. Diss. Abstr. Int., 43(5): 1441B.
ANTONYUK, O.K. (1974) Hygienic evaluation of the plasticizer triphenyl
phosphate added to polymer composition. Gig. i Sanit., 8: 98-99.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., & WELCH,
V.A. (1961) The reactions of organic phosphates. Part V. The hydrolysis
of triphenyl and trimethyl phosphates. J. Chem. Soc., 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER, B.,
VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic phosphates. Part
VI. The hydrolysis of aryl phosphates. J. Chem. Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphorylenzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY, S.K.
(1974) Detection and estimation of tricresyl phosphate in mustard oil.
Forensic Sci., 3: 263-270.
BLOOM, P.J. (1973) Application des chromatographies sur couch mince et
gazliquide à l'analyse qualitative et quantitative des esters des acides
phosphorique et phosphoreux. J. Chromatogr., 75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and effects of
triaryl and trialkyl/aryl phosphate esters. Residue Rev., 94: 49-99.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., & KARASEK,
F.W. (1981) Trace impurities in solvents commonly used for gas
chromatographic analysis of environmental samples. J. Chromatogr., 206:
279-288.
BRAUN, D. (1965) [Qualitative analysis of plasticizers by using thin-
layer chromatography.] Chimia, 19(2): 77-82 (in German).
CAINES, L.A. & HOLDEN, A.V. (1976) Stream pollution by an organomercury
compound. Bull. environ. Contam. Toxicol., 16(4): 483-485.
CARLSEN, L., ANDERSEN, K.E., & EGSGAARD, H. (1986) Triphenyl phosphate
allergy from spectacle frames. Contact dermatitis, 15: 274-277.
Ciba-Geigy Corporation: Acute oral toxicity to rats and mice, EPA-OTS
document 86-870000069.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues in
foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977) The
acute toxicity of 47 industrial chemicals to fresh and saltwater fishes.
J. hazard. Mater., 1(4): 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic mass
spectrometric analysis of some commercial aryl phosphate oils. J. Assoc.
Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by thin-
layer chromatography.] Gig. i Sanit., 10: 62-65 (in Russian).
EAJ (1977) [Environmental monitoring of chemicals.] Tokyo, Environment
Agency Japan, pp. 212-214 (Environmental Survey Report Series No. 3) (in
Japanese).
EAJ (1981) Environmental monitoring of chemicals: Environmental survey
report of 1978, 1979 F.Y., Tokyo, Environment Agency Japan, pp. 171-183.
EMMETT, E.A., DANNENBERG, A.M., LEWIS, P.G., FOX, R., BLEECKER, M.,
TANAKA, F., SYNKOWSKI, D.R., & LEVINE, M.S. (1984) A clinical study of
industrial workers exposed to organophosphate esters and a comparison
group, Pittsfield, Massachusetts, General Electric Company (Prepared for
the US Environmental Protection Agency, Washington).
EMMETT, E.A., LEWIS, P.G., TANAKA, F., BLEECKER, M., FOX, R.,
DARLINGTON, A.C., SYNKOWSKI, D.R., DANNENBERG, A.M., Jr, TAYLOR, W.J., &
LEVINE, M.S. (1985) Industrial exposure to organophosphorus compounds.
Studies of a group of workers with a decrease in esterase-staining
monocytes. J. occup. Med., 27(12): 905-914.
ESA, A.H., WARR, G.A., & NEWCOMBE, D.S. (1988) Immunotoxicity of
organophosphorous compounds. Clin. Immunol. Immunopathol., 49: 41-52.
ETO, M., HASHIMOTO, Y., OZAKI, K., & SASAKI, Y. (1975) [Fungitoxicity
and insecticide synergism of monothioquinol phosphate esters and related
compounds.] Botyu Kagaku, 40: 110-117 (in Japanese).
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl phosphate
esters. A new photochemical coupling reaction. J. Am. Chem. Soc., 94:
4780-4782.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GILBERT, J., SHEPHERD, M.J., WALLWORK, M.A., & SHARMAN, M. (1986) A
survey of trialkyl and triaryl phosphates in the United Kingdom total
diet samples. Food Addit. Contam., 3(2): 113-122.
GUNDERSON, E.L. (1988) FDA total diet study, April 1982 - April 1984,
dietary intakes of pesticides, selected elements and other chemicals. J.
Assoc. Off. Anal. Chem., 71(6): 1200-1209.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Suishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HIERHOLZER, K., NOETZEL, H., & SCHMIDT, L. (1957) [Comparative
toxicological study on triphenyl phosphate and tricresyl phosphate.]
Arzneimittelforschung, 7: 585-588.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD, G.T.
(1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's industrial hygiene
and toxicology, 3rd revised ed., New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
HINTON, D.M., JESSOP, J.J., ARNOLD, A., ALBERT, R.H., & HINES, F.A.
(1987) Evaluation of immunotoxicity in a subchronic feeding study of
triphenyl phosphate. Toxicol. ind. Health, 3(1): 71-89.
HJORTH, N. (1964) Contact dermatitis from cellulose acetate film. Cross-
sensitization between tricresyl phosphate (TCP) and triphenyl phosphate
(TPP). Contact dermatitis, 12: 86-100.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water solubility
of highly insoluble organic chemicals of environmental interest. Bull.
environ. Contam. Toxicol., 23: 579-586.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. B ull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(dichloro-
propyl)phosphate, a mutagenic flame retardant: Frequent occurrence in
human seminal plasma. Science, 211: 951-952.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water Res.,
19: 119-125.
JOHANNSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J., RADUE,
R.W., & GRAHAM, P.R. (1977) Evaluation of delayed neurotoxicity and
dose-response relationships of phosphate esters in the adult hen.
Toxicol. appl. Pharmacol., 41: 291-304.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. Pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water pollution
caused by organophosphoric compounds.] Kankyo Gijyutsu, 7: 668-675
(in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment and sea
water.] J. Food Hyg, Soc. Jpn, 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental pollutants.
V: Environmental fate of organic phosphoric acid triesters.] Okayama-ken
Kankyo Hoken Senta Nemnpo, 4: 103-110 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination of
organic phosphate esters in environment samples.] Okayama-ken Kankyo
Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T. (1982a)
[An environmental survey of chemicals. XIX. Determination of
organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken Senta
Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T. (1982b)
[Studies on the biological toxicity of several pollutants in
environments. VII. GC/MS Spectrometric determination of organophosphoric
acid triesters in sediment.] Okayama-ken Kankyo Hoken Senta Nempo, 6:
142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemical. XXII. GC/MS spectrometric
determination of organophosphoric acid triesters in sediment (2).]
Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in Japanese).
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on organic
and heavy metal contaminants in the Lakes Erie, Michigan, Huron and
Superior basins. Great Lakes Water Qual. Bd.
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic phosphate
triesters in human adipose tissue. J. Assoc. Off. Anal. Chem., 66:
691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from drinking
water at the ng/L level, using macroreticular resin. J. Assoc. Off.
Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas chromatographic
determination of trialkyl/aryl phosphates in drinking water, following
isolation using macroreticular resin. J. Assoc. Off. Anal. Chem.,
64: 991-998.
LHOMME, V., BRUNEAU, C., SOYER, N., & BRAULT, A. (1984) Thermal behavior
of some organic phosphates. Ind. Eng. Chem. Prod. Res. Dev., 23:
98-102.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY, D.
(1975) Chronic toxicity of a synthetic triaryl phosphate oil to fish.
Environ. Physiol. Biochem., 5: 361-369.
LOCKHART, W.L., METNER, D.A., BLOUW, A.P., & MUIR, D.C.G. (1982)
Prediction of biological availability of organic chemical pollutants to
aquatic animals and plants. In: Pearson, J.G., Foster, R.B., & Bishop,
W.E., ed. Aquatic toxicology and hazard assessment: Fifth conference,
Philadelphia, American Society for Testing and Materials, pp. 259-272
(ASTM STP 766).
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62(1): 47-51.
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and biodegradability
of benzene derivatives as studied in a model aquatic ecosystem. Environ.
Health Perspect., 10: 269-284.
MAYER, F.L., Jr, MAYER, K.S., & ELLERSIECK, M.R. (1986) Relation of
survival to other endpoints in chronic toxicity tests with fish.
Environ. Toxicol. Chem., 5(8): 737-748.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE, P.M., &
SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An aquatic
environmental assessment of Pydrauls 50E and 115E, In: Branson, D.R. &
Dickson, K.L., ed. Aquatic Toxicology and Hazard Assessment: Fourth
Conference, Philadelphia, American Society for Testing and Materials,
pp. 103-123 (ASTM STP 737).
MOCHIDA, K., GOMYODA, M., FUJITA, T., & YAMAGATA, K. (1988) Tricresyl
phosphate and triphenyl phosphate are toxic to cultured human, monkey,
and dog cells. Zentralbl. Bakterial. Mikrobiol. Hyg., B185(4-5):
427-429.
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising Supplement
52: 10A, p. 697, New York McGraw-Hill Inc.
MONSANTO INDUSTRIAL CHEMICALS CO. (1979) Summary of the mutagenicity
study, neurotoxicity study, teratology study, long-term feeding study
and 90-day inhalation study which Monsanto has on the aryl phosphate,
Saint-Louis, Missouri, Monsanto Industrial Chemicals Co. (Prepared for
the US Environmental Protection Agency) (EPA-OTS document No. 40-
7942057).
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980a) Determination of
several triaryl phosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., BLOUW, A.P., & LOCKHART, W.L. (1980b)
Environmental dynamics of phosphate esters. I. Uptake and
bioaccumulation of triphenyl phosphate by rainbow trout. Chemosphere,
9: 525-532.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and cleanup
of fish, sediment, and water for determination of triaryl phosphates by
gas-liquid chromatography. J. Assoc. Off. Anal. Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Toxicol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983a) Environmental
dynamics of phosphate esters. III. Comparison of the bioconcentration of
four triaryl phosphates by fish. Chemosphere, 12: 155-166.
MUIR, D.C.G., TOWNSEND, B.E., & LOCKHART, W.L. (1983b) Bioavailability
of six organic chemicals to Chironous tentans larvae in sediment and
water. Environ. Toxicol. Chem., 2: 269-281.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition coefficient to
measure bioconcentration potential of organic chemicals in fish.
Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate ester
mixtures to invertebrates and fish. Bull. environ. Contam. Toxicol.,
19: 250-256.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performanace liquid
chromatographic analysis on radial compression column of the neurotoxic
tri-o-cresyl phosphate and metabolites. Anal. Biochem., 135: 296-303.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresyl phosphate. Sci. total Environ.,
43: 233-241.
PALAWSKI, D., BUCKLER, D.R., & MAYER, F.L. (1983) Survival and condition
of Rainbow trout (Salmo gairdneri) after acute exposures to methyl
parathion, triphenyl phosphate, and DEF. Bull. environ. Contam.
Toxicol., 30: 614-620.
PEEREBOOM, J.W.C. (1960) The analysis of plasticizers by micro-
adsorption chromatography. J. Chromatogr., 4: 323-328.
PEGUM, J.S. (1966) Contact dermatitis from plastics containing triaryl
phosphtes. Br. J. Dermatol., 78: 626-631.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975) Utilization of
triaryl phosphates by a mixed bacterial population. Can. J. Microbiol.,
21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with synergistic
properties. J. econ. Entomol., 59(1): 11-15.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin layer
chromatography. Evaluation of methods and application on phosphate
esters, polychlorinated paraffins and some PCB-substitutes. Chemosphere,
9: 683-691.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P., &
TUCKER, E.S. (1979) Environmental fate of selected phosphate esters.
Environ. Sci. Technol., 13: 840-844.
SASAKI, K., TAKEDA, M., & UCHIYAMA, M. (1981) Toxicity, absorption and
elimination of phosphoric acid triesters by Killifish and Goldfish.
Bull. environ. Contam. Toxicol., 27: 775-782.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by Killifish
(Oryzeas laptipes). Bull. environ. Contam. Toxicol., 28: 752-759.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the Delaware
River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, M.I., EVOLVE, E., & FRAZIER, W.H. (1930) Pharmacological action
of certain phenol esters with special reference to the etiology of so-
called ginger paralysis. Public Health Rep., 45: 2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies on the
pharmacology of certain phenol esters with special reference to the
relation of chemical constitution and physiologic action. Natl. Inst.
Health Bull., 160: 1-53.
SOBOTKA, T.J., BRODIE, R.E., ARNOLD, A., WEST, G.L., & O'DONNELL, M.W.
(1986) Neuromotor function in rats during subchronic dietary exposure to
triphenyl phosphate. Neurobehav. Toxicol. Teratol., 8: 7-10.
SUGIYAMA, H. & TANAKA, K. (1982) [Investigation of trace organic
chemicals in the sea water of the Tokyo Bay by gas chromatography mass-
spectrometry.] Bull. Kanagawa Prefect. environ. Center, 4: 33-38 (in
Japanese).
SUTTON, W.L., TERHAAR, C.J., MILLER, F.A., SCHERBERGER, R.F., RILEY,
E.C., ROUDABUSH, R.L., & FASSETT, D.W. (1960) Studies on the industrial
hygiene and toxicology of triphenyl phosphate. Arch. environ. Health,
1: 45-58.
SZYBALSKI, W. (1958) Special microbiological systems. II. Observations
on chemical mutagenesis in microorganisms. Ann. N.Y. Acad. Sci., 76:
475-489.
THEISS, J.C., STONER, G.D., SHIMKIN, M.B., & WEISBURGER, E.K. (1977)
Test for carcinogenicity of organic contaminants of United States
drinking waters by pulmonary tumor response in strain A mice. Cancer
Res., 37: 2717-2720.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorous compounds. Anal. Chem., 53: 1466-1499.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division, General
Motors Corporation, Rochester, Cincinnati, Ohio, National Institute for
Occupational Safety and Health (PB82-104530).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chasse, Louisiana,
Cincinnati, Ohio, National Institute for Occupational Safety and Health
(Report No. IWA-89-10).
VAINIOTALO, S., VERKKALA, E., SAVOLAINEN, H., NICKELS, J., & ZITTING, A.
(1987) Acute biological effects of commercial cresyl diphenyl phosphate
in rats. Toxicology, 44(1): 31-44.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A simple
colorimetric method for estimation of tricresyl phosphate in edible
oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J. Fish
Res. Board Can., 36: 1040-1048.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J., & SVEC, H.J. (1978)
Organic emissions from combustion of combination coal/refuse to produce
electricity. Chemosphere, 7: 893-902.
WAGEMANN, R., GRAHAM, B., & LOCKHART, W.L. (1974) Studies on chemical
degradation and fish toxicity of a synthetic triaryl phosphate
lubrication oil, IMOL S-140, Ottawa, Environment Canada, Fisheries and
Marine Service (Technical Report No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution and toxicological
aspects of a triaryl phosphate synthetic oil]. Annual Report of the
Tokyo Metropolitan Research Institute for Environmental Protection, 11:
110-113 (in Japanese).
WELSH, J.J., COLLINS, T.F.X., WHITBY, K.E., BLACK, T.N., & ARNOLD, A.
(1987) Teratogenic potential of triphenyl phosphate in Sprague-Dawley
(Spartan) rats. Toxicol. ind. Health, 3(3): 357-369.
WHO (1990) Environmental Health Criteria 110: Tricresyl phosphate,
Geneva, World Health Organization.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of tri(haloalkyl)-
, trialkyl-, and triaryl phosphates in Canadian drinking water. Bull.
environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON, R.
(1982) Determination of mutagenic potential and organic contaminants of
Great Lakes drinking water. Chemosphere, 11: 263-276.
WILLS, J.H., BARRON, K., GROBLEWSKI, G.E., BENITZ, K.F., & JOHNSON, M.K.
(1979) Does triphenyl phosphate produce delayed neurotoxic effects?
Toxicol. Lett., 4: 21-24.
WINDHOLZ, M., ed. (1983) The Merck Index, 10th ed., Rahway, New Jersey,
Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate esters:
Application of linear free energy relationships to estimate hydrolysis
rate constants for use in environmental fate assessment. Chemosphere,
9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-65.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides in the
atmosphere above the Dogo plain and Ozu basin.] J. Chem. Soc. Jpn, 1980:
645-653 (in Japanese).
ZEIGER, E., ANDERSON, B., HAWORTH, S., LAWLOR, T., MORTELMANS, K., &
SPECK, W. (1987) Salmonella mutagenicity tests: III. Results from the
testing of 255 chemicals. Environ. Mutagen., 9(Suppl. 9): 1-110.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop, Can. Tech. Rep. Fish. aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de triphényle (TPP) est une substance cristalline,
ininflammable, inexplosible et incolore. Son coefficient de partage
entre l'octanol et l'eau (log de Pow) est de 4,61-4,76. A la température
ambiante ordinaire, il s'hydrolyse rapidement en milieu alcalin pour
donner du phosphate de diphényle et du phénol, mais l'hydrolyse est très
lente en milieu acide ou neutre.
Pour l'analyse, la méthode choix est la chromatographie gaz-liquide,
avec détection au moyen d'un dispositif sensible à l'azote/phosphore ou
par photométrie de flamme. La limite de détection dans l'eau est
d'environ 20 ng/litre.
2. Sources d'exposition humaine et environnementale
Le phosphate de triphényle est produit à partir de l'oxychlorure de
phosphore et du phénol. Il est utilisé comme retardateur de flamme dans
les résines phénoliques et les résines à base d'oxydes de phénylène que
l'on utilise pour la fabrication de pièces d'automobiles et de
l'appareillage électrique; on l'emploie également comme plastifiant
ininflammable dans l'acétate de cellulose servant à la confection des
pellicules photographiques. Il entre également dans la compositision des
liquides hydrauliques et des huiles lubrifiantes à côté d'un certain
nombre d'autres usages de moindre importance.
On peut considérer qu'en utilisation normale, la population dans son
ensemble n'encourt qu'une exposition minime.
3. Transport, distribution et transformation dans l'environnement
Les phosphates de triaryle pénètrent dans le milieu aquatique par
suite de fuites de liquides hydrauliques, de la lixiviation de certains
plastiques et en faibles quantités, lors des divers processus de
fabrication. En raison de sa faible solubilité dans l'eau et de son
coefficient d'adsorption au sol relativement élevé, le phosphate de
triphényle se fixe rapidement sur les sédiments de rivières ou des
étangs. En milieu aquatique, il subit une biodégradation rapide.
La dégradation du phosphate de triphényle comporte une hydrolyse
enzymatique par étapes en orthophosphate et phénol.
Les facteurs de bioconcentration mesurés chez plusieurs espèces de
poissons vont de 6 à 18 900 et la demi-vie d'élimination varie de 1,2 à
49,6 heures.
La libération de cette substance dans l'air des unités de production
constitue une source d'exposition humaine sur les lieux de travail. La
combustion des matières plastiques et la volatilisation du phosphate de
triphényle à partir de ces substances ou de la surface de l'eau peut
également constituer une voie importante de pénétration dans
l'atmosphère.
4. Niveaux dans l'environnement et exposition humaine
On trouve un peu partout du phosphate de triphényle dans l'air,
l'eau, les sédiments et les organismes aquatiques, mais les prélèvements
effectués n'en contiennent que de faibles quantités. Les teneurs les
plus fortes qui aient été signalées sont de 23,2 ng/m3 dans l'air, 7900
ng/litre dans des cours d'eau, 4000 ng/g dans des sédiments et de 600
ng/g dans le poisson.
5. Effets sur les êtres vivants dans leur milieu naturel
La croissance des algues est complètement inhibée à des
concentrations de 1 mg/litre ou davantage mais elle est en revanche
stimulée à des concentrations plus faibles (0,1 et 0,05 mg/litre).
L'activité de la nitrogénase d' Anabaena flos-aquae diminue à mesure que
la dose augmente, passant de 84% à 0,1 mg/litre à 68% à 5,0 mg/litre.
Le phosphate de triphényle est, parmi les phosphates de triaryle,
celui qui présente la plus forte toxicité aiguë vis-à-vis des poissons,
des crevettes et des daphnies. L'indice de toxicité aiguë de ce
phosphate pour le poisson (CL50 à 96 h) va de 290 mg/litre pour Lepomis
macrochirus, à 0,36 mg/litre pour la truite arc-en-ciel. La grande
différence entre la truite et les vairons du genre Pimephales en ce qui
concerne les valeurs de la CL50, pourraient être due à des différences
dans leur aptitude à métaboliser le phosphate de triphényle. Parmi les
effets sublétaux observés chez les poissons, on peut citer des anomalies
morphologiques telles que congestion, dégénérescence et hémorragie au
niveau des petits vaisseaux sanguins (essentiellement branchiaux) ainsi
que des anomalies de comportement. L'immobilité des poissons exposés à
0,21-0,29 mg/litre de phosphate de triphényle a complètement disparu
dans les sept jours qui ont suivi le changement d'eau.
6. Effets sur les animaux d'expérience et les systèmes d'épreuves in vitro
On estime que la DL50 par voie orale est supérieure à 6,4 g/kg chez
le rat et à 2,0 g/kg chez le poulet.
Des doses de phosphate de triphényle allant de 0,5 à 2 g/kg ont été
bien tolérées par des lapins après injection intramusculaire et par des
poulets après administration orale. Lors d'une étude d'alimentation de
35 jours, on a observé après administration de cette substance à des
rats Holtzman mâles, une réduction du gain de poids corporel et une
augmentation du poids du foie.
On n'a pas observé d'effets tératogènes chez des rats Sprague-Dawley
à des doses allant jusqu'à 690 mg/kg de poids corporel. On n'a pas
publié d'études concernant la reproduction.
On ne dispose pas de données sur la mutagénicité du phosphate de
triphényle qui résultent d'épreuves correctement validées et il n'y a
pas eu non plus d'études de cancérogénicité convenables.
Après des injections sous-cutanées de phosphate de triphényle à des
chats (jusqu'à 1 g/kg) on n'a pas observé de neurotoxicité retardée; on
n'en a pas observé non plus après une étude de 4 mois sur des rats
Sprague-Dawley qui en recevaient dans leur alimentation des doses allant
jusqu'à 1% de la ration.
Aucun effet immunotoxique n'a été signalé après une étude de 120
jours pendant laquelle des rats ont reçu du phosphate de triphényle dans
leur nourriture à des doses allant jusqu'à 1%.
7. Effets sur l'homme
On a signalé une réduction statistiquement significative de la
cholinestérase érythrocytaire chez certains travailleurs, mais aucun
signe d'affection neurologique n'a été relevé chez des ouvriers qui
travaillaient dans une unité de production de phosphate de triphényle.
On n'a pas signalé non plus de neurotoxicité retardée parmi les cas
d'intoxication par le phosphate de triphényle. On a décrit des cas de
dermatite de contact dus au phosphate de triphényle.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
Les données tirées des études sur l'animal montrent que le phosphate
de triphényle est peu toxique. Appliqué sur la peau d'animaux de
laboratoire, il ne produit pas d'irritation. On estime que le phosphate
de triphényle n'est pas neurotoxique pour l'homme ni l'animal, bien
qu'un premier rapport ait pu affirmer le contraire. Lors d'une étude de
90 jours sur des rats, on a évalué à 690 mg/kg par jour la dose sans
effet nocif observable pour les mères et leur descendance. L'exposition
professionnelle et l'exposition de la population dans son ensemble
demeurent à un faible niveau.
Le phosphate de triphényle n'est pas mutagène.
Selon des données disponibles, il ne présente aucun danger pour
l'homme.
1.1 Niveaux d'exposition
Il y a probablement un risque d'exposition de la population générale
au phosphate de triphényle par l'intermédiaire des divers compartiments
de l'environnement et notamment par l'eau de consommation. Toutefois les
concentrations de phosphate de triphényle mesurées dans de l'eau de
boisson au Canada et aux Etats-Unis se sont révélées extrêmement
faibles. On en a souvent décelé la présence dans l'air des villes mais à
faibles concentrations. On a pu craindre que l'échauffement des sièges
d'automobiles en vinyle, lorsque la température extérieure est très
élevée, puisse conduire à la vaporisation du phosphate de triphényle
utilisé comme plastifiant, mais on ne dispose d'aucune donnée sur les
concentrations présentes à l'intérieur des véhicules. Lors d'une enquête
portant sur la teneur des tissus adipeux humains en phosphates de
triaryle, on n'a pas décelé la présence de phosphate de triphényle. On
ne dispose pas de données suffisantes pour se faire une idée de
l'importance de l'exposition de la population générale au phosphate de
triphényle. On a signalé des concentrations importantes de ce produit
dans l'air d'une unité de production (0,5 à 29,6 mg/m3) mais on ne
dispose pas de chiffres récents. Il serait bon d'avoir davantage de
données sur l'exposition professionnelle au phosphate de triphényle dans
les unités de production.
1.2 Effets toxiques
Le profil de toxicité du phosphate de triphényle ne permet guère
d'évaluer de façon complète le danger qu'il représente.
On a noté aucun signe d'activité mutagène chez les bactéries ni
d'ailleurs d'activité cancérogène, en se basant pour cela sur une étude
relative à une seule espèce animale. L'expérimentation animale n'a pas
pu jusqu'ici, mettre en évidence une neurotoxicité retardée attribuable
à cette substance. Lors d'une étude d'alimentation de 35 jours sur des
rats, on a relevé à la dose de 5 g/kg, une réduction du gain de poids
corporel et une augmentation du poids du foie. On ne possède pas de
données suffisantes sur les effets qu'il pourrait exercer sur la
fonction de reproduction (gonades, fécondité, parturitions, croissance
et développement de la progéniture).
On a décrit des cas de dermatite de contact attribuable au phosphate
de triphényle.
2. Evaluation des effets sur l'environnement
Dans l'eau, la concentration du phosphate de triphényle est faible et
il est peu probable qu'il exerce des effets toxiques sur les organismes
aquatiques. Il peut y avoir mortalité locale par suite du déversement
accidentel de liquides hydrauliques contenant du phosphate de
triphényle. Cependant, comme ce phosphate s'élimine rapidement des
tissus pisciaires lorsque cesse l'exposition et que les facteurs de
bioconcentration sont moyens, on ne pense pas qu'il y ait véritablement
risque de bioaccumulation.
On a fait état de fortes concentrations de phosphate de triphényle
dans les sédiments proches des unités de production. Il a été montré en
outre que le phosphate de triphényle lié aux sédiments pouvait être fixé
par un organisme qui y était présent mais on ne possède aucune donnée de
toxicité sur les espèces qui vivent dans les sédiments ou qui s'en
nourrissent. Reste que des effets sur les populations aquatiques sont
possibles.
2.1 Niveaux d'exposition
Dans les régions très industrialisées, les prélèvements effectués
dans l'air, dans les eaux superficielles, dans le sol, les sédiments et
parmi les organismes aquatiques indiquent la présence de phosphate de
triphényle. La concentration la plus élevée qui ait été signalée dans
des effluents industriels était de 16 µg/litre; dans un cours d'eau,
elle était de 7,9 µg/litre. Si l'on prend en considération la
biodégradation rapide du phosphate de triphényle dans le milieu
aquatique, il est peu probable que les concentrations que l'on rencontre
normalement puissent se révéler nocives pour les organismes qui y
vivent. Toutefois la décharge dans des mares de déchets de garnitures de
sièges en vinyle traité par du phosphate de triphényle, pourrait donner
lieu à des concentrations mortelles pour les poissons.
2.2 Effets toxiques
Parmi les divers phosphates de triaryle, le phosphate de triphényle
est celui dont la toxicité aiguë est la plus forte pour les poissons,
les crevettes et les daphnies. La CL50 à 96 h varie de 0,36 mg/litre
pour la truite arc-en-ciel à 290 mg/litre pour Lepomis macrochirus. Les
salmonidés sont en général sensibles au phosphate de triphényle mais on
a constaté qui ni la croissance ni la survie des alevins de truite arc-
en-ciel ne souffraient d'une exposition à cette substance à la
concentration de 0,0014 mg/litre. Chez des poissons rouges exposés à du
phosphate de triphényle, on a constaté un certain nombre d'anomalies
histologiques: congestion, dégénérescence et hémorragie au niveau des
petits vaisseaux sanguins, principalement les veinules et les
capillaires. Cette pathologie vasculaire est plus prononcée au niveau
des branchies.
La présence de concentrations de phosphate de triphényle de l'ordre
de 1 mg/litre ou davantage a complètement inhibé la croissance de
certaines algues alors que des concentrations plus faibles (0,1 et 0,05
mg/litre) avaient l'effet contraire. L'activité de la nitrogénase
d' Anabaena flos-aquae a été sensiblement réduite, même à la
concentration de 0,1 mg/litre.
RECOMMANDATIONS
1. Recommandations relatives aux recherches à effectuer
a) Etudes à entreprendre sur la sensibilisation cutanée.
b) Nécessité d'une étude de cytogénicité in vitro.
c) Nécessité d'études pharmacocinétiques selon les différentes voies
d'absorption.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El trifenilfosfato (TFF) es una sustancia no inflamable, no
explosiva, incolora y cristalina. Su coeficiente de reparto en octanol y
agua (log Poa) es de 4,61-4,76. A temperatura ambiente normal se
hidroliza rápidamente en solución alcalina, dando difenilfosfato y
fenol, y muy lentamente en soluciones ácidas o neutras.
El método analítico más apropiado es la cromatografía gas-líquido con
un detector sensible al nitrógeno-fósforo o uno fotométrico de llama. El
límite de detección en el agua es de unos 20 ng/litro.
2. Fuentes de exposición humana y ambiental
El TFF se fabrica a partir de oxicloruro de fósforo y fenol. Se
utiliza como pirorretardante en resinas fenólicas y de óxido de fenileno
en la producción de componentes eléctricos y del automóvil y como
plastificante no inflamable en acetato de celulosa para películas
fotográficas. También es un componente de fluidos hidráulicos o aceites
lubricantes y tiene otros usos de menor importancia.
La exposición de la población general por el uso normal puede
considerarse mínima.
3. Transporte, distribución y transformación en el medio ambiente
Los triarilfosfatos entran en el medio acuático principalmente por
escapes de fluidos hidráulicos, así como por lixiviación a partir de los
plásticos y, en menor medida, a partir de los procesos de fabricación. A
causa de su baja solubilidad en agua y su coeficiente de adsorción en el
suelo relativamente alto, el TFF se adsorbe con rapidez en los
sedimentos de los ríos (o de las charcas). Su biodegradación en el medio
acuoso es rápida.
El TFF se degrada mediante una hidrólisis enzimática escalonada que
lo divide en ortofosfato y componentes fenólicos.
Los factores de bioconcentración (FBC) medidos en varias especies de
peces oscilan entre 6 y 18 900, y la semivida de depuración va de 1,2 a
49,6 h.
La liberación de TFF desde los lugares de producción al aire
representa una fuente de exposición humana en el ambiente de trabajo. La
combustión de plásticos y la volatilización a partir de ellos o de las
superficies acuáticas también pueden ser importantes vías de ingreso en
la atmósfera.
4. Niveles medioambientales y exposición humana
El TFF se ha detectado con frecuencia en el aire, el agua, los
sedimentos y los organismos acuáticos, pero los niveles en muestras
medioambientales son bajos. Los niveles máximos detectados son de 23,2
ng/m3 en el aire, 7900 ng/litro en agua de río, 4000 ng/g en sedimentos
y 600 ng/g en peces.
5. Efectos sobre los seres vivos del medio ambiente
Las concentraciones de TFF iguales o superiores a 1 mg/litro inhiben
completamente el crecimiento de las algas, pero la concentraciones más
bajas (0,1 y 0,05 mg/litro) lo estimulan. La actividad de la nitrogenasa
de Anabaena flos-aquae decrece en función de la dosis desde 84% a 0,1
mg/litro hasta 68% a 5,0 mg/litro.
De los distintos triarilfosfatos, el TFF es el más tóxico para los
peces, los camarones y los dáfnidos. El índice de toxicidad aguda del
TFF para los peces (CL50 96 h) oscila entre 290 mg/litro en Lepomis
macrochirus y 0,36 mg/litro en la trucha arco iris. La gran diferencia
entre los valores de CE0 de la trucha y de Pimephales promelas puede
deberse a su distinta capacidad para metabolizar el TFF. Entre los
efectos subletales que produce en los peces figuran anomalías
morfológicas como congestión, degeneración y hemorragias de los vasos
sanguíneos más pequeños (principalmente de las branquias) y anomalías en
el comportamiento. La inmovilidad de los peces expuestos a
concentraciones de 0,21-0,29 mg/litro desapareció totalmente al cabo de
siete días cuando se los puso en agua limpia.
6. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
Se ha calculado que por vía oral la DL50 del TFF en ratas es > 6,4
g/kg y en pollos es > 2,0 g/kg.
Los conejos y los pollos toleraron bien dosis de TFF de 0,5 a 2,0
g/kg por vía intramuscular y oral respectivamente. En un estudio de
alimentación de 35 días en machos de rata Holtzman, con una dosis de TFF
se observó una disminución en la ganancia de peso corporal y un aumento
del peso del hígado.
Con dosis de TFF de hasta 690 mg/kg de peso corporal no se produjeron
efectos teratogénicos en ratas Sprague-Dawley. No se conocen estudios
sobre la reproducción.
No hay datos acerca de la capacidad mutagénica del TFF obtenidos en
ensayos bien contrastados y no se han hecho estudios adecuados de
carcinogenicidad.
La aplicación a gatos de una única dosis subcutánea de TFF (de hasta
1 g/kg) no causó neurotoxicidad diferida; tampoco se observó en un
estudio de cuatro meses en ratas Sprague-Dawley con dosis de hasta el 1%
en el alimento.
No se comunicaron efectos inmunotóxicos tras un estudio de 120 días
en ratas a las que se administraron dosis de hasta el 1% en el alimento.
7. Efectos en la especie humana
Se ha comunicado que, si bien algunos trabajadores de instalaciones
de producción de TFF han mostrado una reducción estadísticamente
significativa de la colinesterasa eritrocítica, no hay manifestaciones
de enfermedades neurológicas. No hay informes de neurotoxicidad diferida
en casos de intoxicación por TFF. Se han descrito casos de dermatitis de
contacto debida al TFF.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Los datos obtenidos en animales indican que el TFF tiene una
toxicidad baja. No produce efectos irritantes en la piel de los
animales. A pesar de un primer informe en sentido contrario, no se
considera que el TFF sea neurotóxico para los animales o el hombre. El
nivel sin efecto adverso observado fue, en las madres y en las crías, de
690 mg/kg al día en un estudio de 90 días realizado en ratas. La
exposición al TFF es baja tanto en la población general como en los
trabajadores.
El TFF no tiene efectos mutagénicos.
Los datos disponibles indican que no entraña peligro para los seres
humanos.
1.1 Niveles de exposición
Puede considerarse probable la exposición de la población general al
TFF por conducto de distintos medios ambientales, incluida el agua de
bebida. Se han medido concentraciones extraordinariamente bajas de TFF
en el agua de bebida del Canadá y los EE.UU. Con frecuencia se ha
detectado TFF en el aire urbano, aunque los niveles son bajos. Se ha
hablado de vaporización de TFF al calentarse el vinilo de la tapicería
de los automóviles, pero no se dispone de datos sobre la concentración
en los coches. En un estudio de los triarilfosfatos en el tejido adiposo
humano no se detectó TFF. Estos datos no bastan para evaluar la
importancia de la exposición de la población general al TFF.
Se ha comunicado la existencia de concentraciones importantes (0,5-
29,6 mg/m3) en el aire de unas instalaciones de producción de TFF, pero
no se dispone de cifras recientes. Se necesitan más datos sobre la
exposición profesional al TFF en los lugares de fabricación.
1.2 Efectos tóxicos
Los datos de que se dispone sobre la toxicidad del TFF son totalmente
insuficientes para una valoración completa del riesgo que representa.
No hay pruebas de que el TFF tenga actividad mutagénica en bacterias
ni de que tenga actividad carcinogénica, de acuerdo con un estudio sobre
una especie animal. De momento no se han obtenido pruebas de que el TFF
cause neurotoxicidad diferida en animales de experimentación. En un
estudio de alimentación de 35 días en ratas se observó una disminución
en la ganancia de peso corporal y un aumento de peso del hígado con una
dosis de 5 g/kg. No se dispone de datos adecuados en cuanto a los
efectos del TFF en la reproducción, es decir, en la función de las
gónadas, la fertilidad, el parto y el crecimiento y desarrollo de la
descendencia.
Se han descrito casos de dermatitis de contacto causada por el TFF.
2. Evaluación de los efectos en el medio ambiente
Las concentraciones de TFF en el agua ambiental son bajas y los
efectos tóxicos en los organismos acuáticos son poco probables. Los
vertidos de fluidos hidráulicos con TFF podrían tener efectos letales a
nivel local. Puesto que el TFF se elimina rápidamente de los tejidos de
los peces al terminar la exposición y los factores de bioconcentración
son moderados, no se considera que la bioacumulación sea un peligro.
Se ha informado de la presencia de altas concentraciones de TFF en
sedimentos cercanos a instalaciones de producción. Se ha demostrado que
cierto organismo que vive en los sedimentos puede utilizar el TFF fijado
por éstos, pero no hay datos de toxicidad sobre las especies que viven
en los sedimentos o se alimentan de ellos. Existe, por consiguiente, la
posibilidad de efectos en las comunidades acuáticas.
2.1 Niveles de exposición
El TFF se halla en el aire, el agua superficial, el suelo, los
sedimentos y los organismos acuáticos recogidos en zonas muy
industrializadas. La concentración más alta de TFF en efluentes de aguas
industriales que se ha comunicado es de 16 µg/litro, mientras que en
aguas fluviales es de 7,9 µg/litro. Teniendo en cuenta la rápida bio-
degradación del TFF en el medio acuoso, es poco probable que las
concentraciones normales de TFF en él afecten de manera adversa a los
organismos acuáticos. Sin embargo, si se arrojase a una charca tejido de
tapicería de vinilo tratado con TFF se produciría una concentración
suficientemente alta para matar a los peces.
2.2 Efectos tóxicos
Entre los diferentes triarilfosfatos, el TFF es el compuesto más
tóxico para peces, camarones y dáfnidos. Los valores de la CL50 en 96
horas de TFF para los peces varían entre 0,36 mg/litro en la trucha arco
iris y 290 mg/litro en Lepomis macrochirus. Aunque los salmónidos en
general son sensibles al TFF, el crecimiento y la supervivencia de los
alevines de trucha arco iris no se vieron afectados cuando éstos se
expusieron a una concentración de TFF de 0,0014 mg/litro. Los ejemplares
de Carassius auratus expuestos al TFF presentaron lesiones
histopatológicas consistentes en congestión, degeneración y hemorragia
de los vasos sanguíneos pequeños, principalmente vénulas y capilares.
Esta patología vascular es más pronunciada en las branquias.
Las concentraciones de TFF iguales o superiores a 1 mg/litro
inhibieron completamente el crecimento de las algas, pero a
concentraciones más bajas (0,1 y 0,05 mg/litro) lo estimularonn. La
actividad de la nitrogenasa de Anabaena flos-aquae se redujo de forma
significativa, incluso a una concentración de 0,1 mg/litro.
RECOMENDACIONES
1. Recomendaciones para futuras investigaciones
a) Se deberían realizar estudios de sensibilización cutánea.
b) Es necesario realizar un estudio de citogenicidad in vitro.
c) Se precisan estudios farmacocinéticos de las diferentes vías.
See Also:
Toxicological Abbreviations
Triphenyl phosphate (ICSC)
 Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number:
Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number: 
 Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number:
Molecular formula: C18H15O4P
Relative molecular mass: 326.3
CAS chemical name: Phosphoric acid, triphenyl ester
CAS registry number: