
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 110
TRICRESYL PHOSPHATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Tricresyl phosphate.
(Environmental health criteria ; 110)
1.Tritolyl phosphates - adverse effects 2.Tritolyl phosphates -
toxicity I.Series
ISBN 92 4 157110 1 (NLM Classification: QV 627)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR TRICRESYL PHOSPHATE
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution, and transformation
1.4. Environmental levels and human exposure
1.5. Effects on organisms in the environment
1.6. Kinetics and metabolism
1.7. Effects on experimental animals and in vitro test systems
1.8. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Tricresyl phosphate
2.1.2. Tri- o-cresyl phosphate
2.1.3. Tri- m-cresyl phosphate
2.1.4. Tri- p-cresyl phosphate
2.2. Physical and chemical properties
2.3. Conversion factor
2.4. Analytical methods
2.4.1. Extraction and concentration
2.4.2. Clean-up procedures
2.4.3. Gas chromatography and mass spectrometry
2.4.4. Contamination of analytical reagents
2.4.5. Other analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production levels and processes
3.1.1. Accidental release
3.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and transformation in the environment
4.1.1. Release to the environment
4.1.2. Fate in water and sediment
4.1.3. Biodegradation
4.1.4. Water treatment
4.2. Bioaccumulation and biomagnification
4.2.1. Fish
4.2.2. Plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Sediment
5.2. General population exposure
5.2.1. Drinking-water
5.2.2. Fish
5.2.3. Human tissues
5.3. Occupational exposure
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Unicellular algae
6.2. Aquatic organisms
6.3. Insects
6.4. Plants
7. KINETICS AND METABOLISM
7.1. Absorption
7.2. Distribution
7.3. Metabolic transformation
7.4. Excretion
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Short-term exposure
8.3. Skin and eye irritation
8.4. Teratogenicity
8.5. Reproduction
8.6. Mutagenicity and carcinogenicity
8.7. Neurotoxicity
8.7.1. Experimental neuropathology
8.7.2. Neurochemistry
8.7.3. Interspecies sensitivity and variability to OPIDN
8.7.4. Neurophysiology
9. EFFECTS ON HUMANS
9.1. Historical background
9.2. Occupational exposure
9.3. Clinical features
9.4. Prognosis
9.5. Neurophysiological investigations
9.6. Pathological investigations
9.7. Laboratory investigations
9.8. Treatment
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure levels
10.1.2. Toxic effects
10.2. Evaluation of effects on the environment
10.2.1. Exposure levels
10.2.2. Toxic effects
11. RECOMMENDATIONS
REFERENCES
RESUME
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
RECOMMANDATIONS
RESUMEN
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN EL
MEDIO AMBIENTE
RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR TRICRESYL PHOSPHATE
Members
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon,
Cambridgeshire, England ( Chairman)
Dr S. Fairhurst, Medical Division, Health and Safety
Executive, Bootle, Merseyside, England ( Joint Rapporteur)
Ms N. Kanoh, Division of Information on Chemical Safety,
National Institute of Hygienic Sciences, Setagaya-ku,
Tokyo, Japan
Dr A. Nakamura, Division of Medical Devices, National
Institute of Hygienic Sciences, Setagaya-ku, Tokyo,
Japan
Dr M. Tasheva, Department of Toxicology, Institute of
Hygiene and Occupational Health, Sofia, Bulgaria
Dr B. Veronesi, Neurotoxicology Division, US Environmental
Protection Agency, Research Triangle Park, North
Carolina, USA
Mr W.D. Wagner, Division of Standards Development and
Technology Transfer, National Institute for
Occupational Safety and Health, Cincinnati, Ohio, USA
Dr R. Wallentowicz, Exposure Assessment Application
Branch, US Environmental Protection Agency, Washington,
DC, USA ( Joint Rapporteur)
Dr Shen-Zhi Zhang, Beijing Municipal Centre for Hygiene
and Epidemic Control, Beijing, China
Observers
Dr M. Beth, Berufsgenossenschaft der Chemischen Industrie
(BG Chemie), Heidelberg, Federal Republic of Germany
Dr R. Kleinstück, Bayer AG, Leverkusen, Federal Republic
of Germany
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety,
Division of Environmental Health, World Health Organiz-
ation, Switzerland ( Secretary)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be
obtained from the International Register of Potentially
Toxic Chemicals, Palais des Nations, 1211 Geneva 10,
Switzerland (Telephone No. 7988400 or 7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR TRICRESYL PHOSPHATE
A WHO Task Group meeting on Environmental Health
Criteria for Tricresyl Phosphate was held at the British
Industrial Biological Research Association (BIBRA),
Carshalton, United Kingdom, from 9 to 13 October 1989.
Dr S.D. Gangolli, Director, BIBRA, welcomed the partici-
pants on behalf of the host institution and Dr M. Gilbert
opened the meeting on behalf of the three cooperating
organizations of the IPCS (ILO, UNEP, WHO). The Task Group
reviewed and revised the draft criteria document and made
an evaluation of the risks for human health and the
environment from exposure to tricresyl phosphate.
The first draft of this document was prepared by
DR A. NAKAMURA, National Institute for Hygienic Sciences,
Japan. Dr M. Gilbert and Dr P.G. Jenkins, both members of
the IPCS Central Unit, were responsible for the overall
scientific content and editing, respectively.
ABBREVIATIONS
ACh acetylcholine
AChE acetylcholinesterase
BCF bioconcentration factor
CNS central nervous system
FPD flame photometric detector
GC gas chromatography
GLC gas liquid chromatography
GPC gel permeation chromatography
IC50 inhibition concentration, median
LC50 lethal concentration, median
MS mass spectrometry
NOEL no-observed-effect level
NPD nitrogen-phosphorus sensitive detector
NTE neurotoxic esterase
OPIDN organophosphate-induced delayed neuropathy
2-PAM
chloride pralidoxine (2-pyridine aldoxime methyl) chloride
PVC polyvinyl chloride
TAP triaryl phosphate
TBP tributyl phosphate
TCP tricresyl phosphate
TLC thin-layer chromatography
TMCP tri- m-cresyl phosphate
TOCP tri- o-cresyl phosphate
TPCP tri- p-cresyl phosphate
TPP triphenyl phosphate
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
Tricresyl phosphate (TCP) is a non-flammable, non-
explosive, colourless, viscous liquid. Its partition coef-
ficient between octanol and water (log Pow) is 5.1. It
is easily hydrolysed in an alkaline medium to produce
dicresyl phosphate and cresol, but it is stable in neutral
and acidic media at normal temperatures.
The analytical method of choice is gas chromatography
with a nitrogen-phosphorus sensitive detector or a flame
photometric detector. The detection limit in a water
sample is approximately 1 ng/litre. TCP is easily ex-
tracted from aqueous solution with various organic sol-
vents. Florisil column chromatography is usually used for
clean-up, but it is difficult to separate TCP from lipids
by this method. Other clean-up methods (GPC, activated
charcoal chromatography and Sep-pak C-18) have been rec-
ommended for the purpose. Analytical reagents are often
contaminated with traces of TCP because of its widespread
use. Therefore, care must be taken in order to obtain
reliable data in trace analysis of TCP.
1.2 Sources of human and environmental exposure
TCP is usually produced by the reaction of cresols
with phosphorus oxychloride. There are two industrial
sources of cresols: "cresylic acid" obtained as a residue
from coke ovens and petroleum refining; and "synthetic
cresols" prepared from cymene via oxidation and degrad-
ation. As a result, TCP is a mixture of various triaryl
phosphates.
TCP is used as a plasticizer in vinyl plastics, as a
flame-retardant, as an additive to extreme pressure lubri-
cants, and as a non-flammable fluid in hydraulic systems.
1.3 Environmental transport, distribution, and transformation
The release of TCP to the environment derives mainly
from end-point use, little release occurring during
production. The total release to the environment in the
USA was estimated at 32 800 tonnes in 1977.
Because of its low water solubility and high adsorp-
tion to particulates, TCP is rapidly adsorbed onto river
or lake sediment and soil. Its biodegradation in the
aquatic environment is rapid, being almost complete in
river water within 5 days. The ortho isomer is degraded
slightly faster than the meta or para isomers. TCP is
readily biodegraded in sewage sludge with a half-life of
7.5 h, the degradation within 24 h being up to 99%.
Abiotic degradation is slower with a half-life of 96 days.
Bioconcentration factors (BCF) of 165-2768 were
measured for several fish species in the laboratory using
radiolabelled TCP. The radioactivity was lost rapidly on
cessation of exposure, depuration half-lives ranging
between 25.8 and 90 h.
1.4 Environmental levels and human exposure
TCP has been measured in air at concentrations up to
70 ng/m3 in Japan but reached a maximum of only 2 ng/m3
at a production site in the USA. Workplace air in the USA
contained less than 0.8 mg/m3 at a lubrication oil bar-
rel-filling operation and 0.15 mg/m3 (total phosphates)
in an automobile zinc die-casting plant. Concentrations
of TCP measured in drinking-water in Canada were low (0.4
to 4.3 ng/litre) and TCP was undetectable in well-water.
Levels in river and lake waters are frequently consider-
ably higher. However, this is due to the presence of sus-
pended sediment to which TCP is strongly adsorbed.
Concentrations in sediment are higher with up to 1300
ng/g in river sediment and 2160 ng/g in marine sediment.
Levels in soil and vegetation measured within the
perimeter of production plants were elevated.
Residues in fish and shellfish of up to 40 ng/g have
been reported but the majority of sampled animals con-
tained no detectable residues.
1.5 Effects on organisms in the environment
The primary productivity of cultures of freshwater
green algae was reduced to 50% by tri- o-cresyl phosphate
(TOCP) at 1.5 to 4.2 ng/litre, depending on the species,
whereas the meta and para isomers were less toxic. There
are limited data on the acute toxicity of TCP to aquatic
invertebrates: the 48-h LC50 for Daphnia is 5.6 ng/litre;
the 24-h LC50 for nematodes is 400 ng/litre; the 2-week
NOEL for Daphnia (mortality, growth, reproduction) is 0.1
mg/litre. The 96-h LC50 values for three fish species
were between 4.0 and 8700 mg/litre. Rainbow trout showed
approximately 30% mortality after a 4-month exposure to
0.9 ng/litre IMOL S-140 (2% tri- o-cresyl phosphate,
TOCP) and minor effects within 14 days.
The exposure levels used in these experiments were
much greater than likely water concentrations in the en-
vironment and, in most cases, greatly exceed the solu-
bility of the compounds.
1.6 Kinetics and metabolism
The absorption, distribution, metabolism, and elimin-
ation of organophosphates are critical to the delayed
neuropathic effects of these compounds.
Dermal absorption of TOCP in humans appears to be at
least an order of magnitude faster than that in dogs.
Significant dermal absorption also appears to occur in
cats. Oral absorption of the compound has been reported
in rabbits. There is no direct information on absorption
via the inhalation route.
In cat studies, absorbed TOCP was widely distributed
throughout the body, the highest concentration being found
in the sciatic nerve, a target tissue. Other tissues with
high concentration of TOCP and its metabolites were the
liver, kidney, and gall bladder.
TOCP is metabolized via three pathways. The first is
the hydroxylation of one or more of the methyl groups, and
the second is dearylation of the o-cresyl groups. The
third is further oxidation of the hydroxymethyl to alde-
hyde and carboxylic acid. The hydroxylation step is criti-
cal because the hydroxymethyl TOCP is cyclized to form
saligenin cyclic o-tolyl phosphate, the relatively
unstable neurotoxic metabolite.
TOCP and its metabolites are eliminated via the urine
and faeces, together with small amounts in the expired
air.
1.7 Effects on experimental animals and in vitro test systems
Of the three isomers of TCP, TOCP is by far the most
toxic in acute and short-term exposure. It is the only
isomer that produces delayed neurotoxicity.
There is wide interspecies variability for the various
toxic end-points (e.g., acute lethality, delayed neurotox-
icity) of TOCP exposure, the chicken being one of the most
sensitive species.
Organophosphate-induced delayed neuropathy (OPIDN) has
been produced with both single and repeated exposure
regimes in a wide range of experimental species and it is
classified as a "dying-back neuropathy". Degenerative
changes occur in the distal axon and extends with time
towards the cell body.
Clinical signs are paralysis of the hindlegs after a
characteristic delay of 2-3 weeks after exposure. A single
oral dose of 50-500 mg TOCP/kg induced delayed neuropathy
in chickens, whereas doses of 840 mg/kg or more were
necessary to produce spinal cord degeneration in Long-
Evans rats. The metabolite saligenin cyclic o-tolyl phos-
phate is the active neurotoxic agents. Species sensitivity
is inversely correlated with rate of further metabolism.
Inhibition of "neurotoxic esterase" is thought to be
the biochemical lesion leading to OPIDN; inhibition by
more than 65% shortly after exposure to TOCP presages sub-
sequent neuropathy. Factors other than metabolism (e.g.,
route of exposure, age, sex, strain) influence variability
in response to TOCP neurotoxicity. A clear no-observed-
effect level for delayed neuropathy is not apparent from
the data available.
Reproduction studies in rats and mice receiving
repeated oral exposure to TOCP showed histopathological
damage in the testes and ovaries, morphological changes in
sperm, decreased fertility in both sexes, and decreased
litter size and viability. A clear no-effect level for the
reproductive effects of TOCP was not apparent from the
data available. A teratogenicity study in rats, using
oral doses producing maternal toxicity, yielded negative
results.
Little information is available on mutagenicity and
none on carcinogenicity.
1.8 Effects on humans
Accidental ingestion is the main cause of intoxi-
cation. Since the end of the nineteenth century, numerous
cases of poisoning due to contamination of drink, food,
or drugs have been reported. Occupational exposure is
principally via dermal absorption or inhalation, and some
cases of poisoning have been reported. Ingestion of prep-
arations contaminated by TOCP may be followed by gastro-
intestinal symptoms (nausea, vomiting, and diarrhoea),
although in some cases polyneuropathy is the first evi-
dence of poisoning. The neurological symptoms are charac-
teristically delayed. The initial symptoms are pain and
paraesthesia in the lower extremities. A mild impairment
of cutaneous sensations and sometimes an impairment of
vibratory sense may be present. In most cases the muscle
weakness progresses rapidly to a striking paralysis of the
lower extremities with or without an involvement of the
upper extremities. Severe cases show pyramidal signs.
Fatalities are rare, but recovery from the neurological
signs and symptoms can be extremely slow and extend over a
number of months or years. Histopathological findings
show axonal degeneration. Routine laboratory examinations
show no abnormal findings, but an increase of protein con-
centration in the cerebrospinal fluid may be seen. First
aid should reduce exposure by inducing vomiting immedi-
ately after ingestion, providing the patient is conscious.
The cardinal long-term therapy is physical rehabilitation
and no specific antidote is known. There is considerable
variation between individuals both in response to TCP and
recovery from the toxic effects. Severe symptoms have
been reported following the ingestion of 0.15 g of TCP,
while other individuals failed to show any toxic effect
after ingesting 1-2 g. Some patients show complete
recovery, whereas others retain marked effects for a
considerable period.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Tricresyl phosphate (commercial product: mixture of isomers)
Chemical structure:
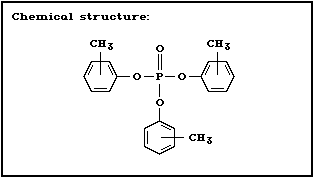 Molecular formula: C21H21O4P
Relative molecular mass: 368.4
CAS chemical name: phosphoric acid, tritolyl ester
CAS registry number: 1330-78-5
RTECS registry number: TD0175000
Synonyms: tricresylphosphate, tricresyl phos-
phate, TCP, tritolyl phosphate, tri-
methylphenyl phosphate
Trade name: Kronitex-TCP(R), Santicizer 140(R),
Pliabrac 521(R), Phosflex 179(R),
Disflamoll TKP(R), Lindol(R),
Kolflex 5050(R), PX.917(R),
Celluflex 179C(R),
Manufacturers and suppliers (Modern Plastics Encyclopedia,
1975):
Albright & Wilson Ltd., Ashland Chemical Co., Bayer AG,
Celanese Co., East Coast Chemicals Co., F.M.C. Corp.,
Harwick Chemical Corp., Kolker Chemical Co., McKesson
Chemical Co., Mobay Chemical Co., Pittsburgh Chemical
Co., Rhone-Poulenc Co., Sobin Chemical Co., Stauffer
Chemical Co., Daihachi Chemical Ind. Co. Ltd., Kyowa
Hakko Kogyo Co. Ltd., Hodogaya Chemical Co. Ltd.,
Mitsubishi Gas Chemical Co. Inc., Kurogane Kasei Co.
Ltd., Kashima Ind. Co.
2.1.2 Tri- o-cresyl phosphate
Chemical structure:
Molecular formula: C21H21O4P
Relative molecular mass: 368.4
CAS chemical name: phosphoric acid, tritolyl ester
CAS registry number: 1330-78-5
RTECS registry number: TD0175000
Synonyms: tricresylphosphate, tricresyl phos-
phate, TCP, tritolyl phosphate, tri-
methylphenyl phosphate
Trade name: Kronitex-TCP(R), Santicizer 140(R),
Pliabrac 521(R), Phosflex 179(R),
Disflamoll TKP(R), Lindol(R),
Kolflex 5050(R), PX.917(R),
Celluflex 179C(R),
Manufacturers and suppliers (Modern Plastics Encyclopedia,
1975):
Albright & Wilson Ltd., Ashland Chemical Co., Bayer AG,
Celanese Co., East Coast Chemicals Co., F.M.C. Corp.,
Harwick Chemical Corp., Kolker Chemical Co., McKesson
Chemical Co., Mobay Chemical Co., Pittsburgh Chemical
Co., Rhone-Poulenc Co., Sobin Chemical Co., Stauffer
Chemical Co., Daihachi Chemical Ind. Co. Ltd., Kyowa
Hakko Kogyo Co. Ltd., Hodogaya Chemical Co. Ltd.,
Mitsubishi Gas Chemical Co. Inc., Kurogane Kasei Co.
Ltd., Kashima Ind. Co.
2.1.2 Tri- o-cresyl phosphate
Chemical structure:
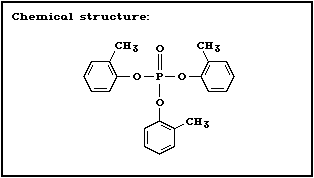 CAS chemical name: phosphoric acid, tri- o-tolyl ester
CAS registry number: 78-30-8
RTECS registry number: TD0350000
Synonyms: tri- o-cresyl phosphate, tri- o-
cresylphosphate, phosphoric acid
tris(2-methylphenyl) ester, o-TCP,
TOCP, TOTP, tri- o-tolyl phosphate,
tri-2-tolyl phosphate, tri-2-methyl-
phenyl phosphate
2.1.3 Tri- m-cresyl phosphate
Chemical structure:
CAS chemical name: phosphoric acid, tri- o-tolyl ester
CAS registry number: 78-30-8
RTECS registry number: TD0350000
Synonyms: tri- o-cresyl phosphate, tri- o-
cresylphosphate, phosphoric acid
tris(2-methylphenyl) ester, o-TCP,
TOCP, TOTP, tri- o-tolyl phosphate,
tri-2-tolyl phosphate, tri-2-methyl-
phenyl phosphate
2.1.3 Tri- m-cresyl phosphate
Chemical structure:
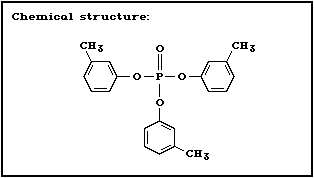 CAS chemical name: phosphoric acid, tri- m-tolyl ester
CAS registry number: 563-04-2
Synonyms: tri- m-cresylphosphate, phosphoric
acid tris(3-methylphenyl) ester,
m-TCP, tri- m-tolyl phosphate,
tri-3-tolyl phosphate, tri-3-methyl-
phenyl phosphate
2.1.4 Tri- p-cresyl phosphate
Chemical structure:
CAS chemical name: phosphoric acid, tri- m-tolyl ester
CAS registry number: 563-04-2
Synonyms: tri- m-cresylphosphate, phosphoric
acid tris(3-methylphenyl) ester,
m-TCP, tri- m-tolyl phosphate,
tri-3-tolyl phosphate, tri-3-methyl-
phenyl phosphate
2.1.4 Tri- p-cresyl phosphate
Chemical structure:
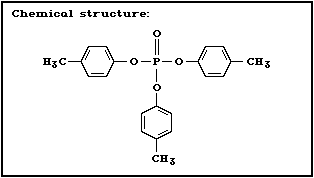 CAS chemical name: phosphoric acid, tri- p-tolyl ester
CAS registry number: 78-32-0
Synonyms: tri- p-cresylphosphate, phosphoric
acid tris(4-methylphenyl) ester,
p-TCP, tri- p-tolyl phosphate,
tri-4-tolyl phosphate, tri-4-methyl-
phenyl phosphate
2.2 Physical and chemical properties
The physical properties of tricresyl phosphate (TCP)
are listed in Table 1.
Table 1. Physical properties of tricresyl phosphate and isomers
________________________________________________________________________________________________________
Physical properties Tricresyl phosphate Tri- o- cresyl Tri- m- cresyl Tri- p- cresyl
(mixtures of isomers) phosphate phosphate phosphate
_________________________________________________________________________________________________________
Physical state liquid liquid half-solid crystalline
solid
Colour colourless colourless colourless colourless
Odour very slightly very slightly very slightly very slightly
aromatic aromatic aromatic aromatic
Melting or -33b 11a 25.6a 77-78a
freezing point (°C)
Boiling point or 241-255 (4 mmHg)b
range (°C) 190-200 (0.5-10 mmHg)c 410 (760 mmHg)a 260 (15 mmHg)a 244 (3.5 mmHg)a
Specific gravity 1.160-1.175 (25 °C)b; 1.1955a 1.150a 1.237a
(density) 1.165c
Refractive index 1.553-1.556 (25 °C)b; 1.5575a 1.5575a
1.556 (20 °)c
Viscosity (cSt) 60 (25 °C), 4.0 (100 °C)c
Flash point (°C) 257c
Vapour pressure (mmHg) 1 x 10-4 (20 °C)c 10 (265 °C)a
Henry's Law constant 1.1-2.8 x 10-6 atm-m3/mold
Solubility in water 0.36e; 0.34 ± 0.04f 0.074g
(mg/litre)
Octanol-water partition 5.11e
coefficient (log Pow) 5.12h
________________________________________________________________________________________________________
a Hine et al. (1981).
b Modern Plastics Encyclopedia (1975).
c Lefaux (1972).
d Boethling & Cooper (1985).
e Saeger et al. (1979).
f Ofstad & Sletten (1985).
g Hollifield (1979).
h Kenmotsu (1980b).
TCP is non-flammable and non-explosive. When the para
isomer was heated at 370 °C with air for 30 min, 99% of
the compound was recovered. The main volatile products
obtained were water, carbon dioxide, toluene, and cresols
(Paciorek et al., 1978). No data on pyrolysis or combus-
tion of TCP at higher temperatures are available (at about
600 °C, triphenyl phosphate begins to decompose, yielding
some aromatic hydrocarbons, some oxygenated aromatic com-
pounds, and phosphoric oxides). Its partition coefficient
between octanol and water (log Pow) is approximately
5.11-5.12.
Hydrolysis of TCP is thought to proceed in an anal-
ogous manner to triphenyl phosphate. It hydrolyses rapidly
in an alkaline solution. Despite a lack of data, neutral
or acidic hydrolysis of TCP, by analogy to TPP, is assumed
to be very slow. The hydrolysis rate constants and half-
lives reported are summarized in Table 2. Formation of
dicresyl phosphate during alkaline hydrolysis would be
expected, but no data are available (Wolfe, 1980).
Table 2. Hydrolysis rate constant (2nd order, K2) and half-lives in
aqueous solution
________________________________________________________________________________
Temper- Rate
Compound Solution ature pH constant Half- Reference
(°C) (M-1.sec-1) life
________________________________________________________________________________
Tri- p-cresyl Water 27 alkaline 2.5 x 10-1 Wolfe (1980)
phosphate 0.2N NaOH/ 22 13 1.66 h Muir et al.
acetone (1983)
(1 : 1)
Tri- m-cresyl 0.1N NaOH/ 22 13 1.31 h Muir et al.
phosphate acetone (1983)
(1 : 1)
________________________________________________________________________________
The photolysis of TAPs in ethanol yielded the corre-
sponding monoaryl phosphate and diphenyl derivatives
(Finnegan, 1972). The results are summarized in Table 3.
2.3 Conversion factor
Tricresyl phosphate 1 ppm = 15.07 mg/m3 air
Table 3. Photolysis of symmetrical triaryl phosphatesa
________________________________________________________________________
Starting Resulting compounds Recovered Quantum yield
compound ---------------------- ester (%) for biaryl
Ar-Ar (%) ArOPO3H2(%) formation
________________________________________________________________________
Phenyl 2 48 6 x 10-4
p-Tolyl 35-51 2-10 13-20 190 x 10-4
p-t-butylphenyl 51 55 24 44 x 10-4
Mesityl 4 7 7 not determined
________________________________________________________________________
a From: Finnegan & Matson (1972).
The esters were irradiated, at a concentration of 0.02 mol/litre
ethanol, using a 450W Hanovia arc lamp, for 5 h.
2.4 Analytical methods
Analytical methods for determining TCP in air, water,
sediment, fish, biological tissues, and edible oils are
summarized in Table 4. The method of choice is gas chro-
matography (GC) with a nitrogen-phosphorus sensitive de-
tector (GC/NPD) or a flame photometric detector (GC/FPD).
The detection limit in water samples is at the ng/litre
level. Using GC, TCP and other trialkyl/aryl phosphates,
such as triphenyl phosphate (TPP), trioctyl phosphate, and
trixylenyl phosphate, can be simultaneously determined.
High-performance liquid chromatography (HPLC) and thin-
layer chromatography (TLC) are sometimes used for deter-
mining TCP, but these are not widely applicable.
It should be noted that the behaviour of TCP in ana-
lytical processes and in its environmental distribution is
similar to that of other TAPs, lipids, and phthalic acid
esters, owing to analogous physical and chemical proper-
ties.
2.4.1 Extraction and concentration
TCP is easily extracted from aqueous solution with
methylene chloride, hexane, or benzene (Kenmotsu et al.,
1980a; Muir et al., 1981). Low levels of TCP in water can
be successfully concentrated on an Amberlite XAD-2 resin
column (Lebel et al., 1981; Lebel & Williams, 1983). TCP
has been extracted from sediment with various polar sol-
vents, such as aqueous methanol (Muir et al., 1980, 1981),
acetonitrile (Kenmotsu et al., 1980a), or acetone
(Ishikawa et al., 1985). The extraction method established
by the US Association of Official Analytical Chemists
(AOAC) for organochlorine and organophosphorus pesticides
is also applicable for the extraction of TCP from fat-
containing foods and fish (Lombardo & Egry, 1979). Hexane
(Lombardo & Egry, 1979), methanol (Muir et al., 1980,
1981; Muir & Grift, 1983), acetonitrile and methylene
chloride (Kenmotsu et al., 1979), and acetone-hexane
(Lebel & Williams, 1983) have been used for the extraction
of TCP from fish or adipose tissue. Workplace airborne
samples can be collected on Millipore(R) filters and the
particulate TCP analysed (US NIOSH, 1977, 1979, 1982).
Vapour phase and particulate TCP in the atmosphere have
been simultaneously collected on glycerol-coated
Florisil(R) columns and 96% of the TCP recovered (Yasuda,
1980). The Midwest Research Institute (MRI/USA) has used
high-volume air filter pads and activated carbon filters
to sample ambient air (MRI, 1979).
2.4.2 Clean-up procedures
Florisil column chromatography has been used routinely
for clean-up of TCP (Lombardo & Egry, 1979; Kenmotsu et
al., 1980a; Lebel & Williams, 1983). The separation of TCP
from tributyl phosphate (TBP) and parathion is possible by
this procedure but is more difficult than for other TAPs
such as trixylenyl phosphate (Kenmotsu et al., 1981b;
Lebel & Williams, 1983). Sulfur-containing compounds,
which often exist in sediment samples and interfere with
the analysis of TCP by GC/FPD, can easily be separated by
elution with hexane from Florisil columns (Kenmotsu et
al., 1980a). Partitioning between acetonitrile and pet-
roleum ether is useful to separate TCP from fish fat
(Lombardo & Egry, 1979; Kenmotsu et al., 1980a). Since
the polarity of TCP is similar to that of lipids in bio-
logical tissues, it is difficult to separate TCP from
lipids by Florisil column chromatography. Gel permeation
chromatography (GPC) is useful in this case (Muir et al.,
1981), the elution volume varying according to the
type of phosphate ester, i.e. trialkyl-, triaryl-, or
tri(haloalkyl) phosphates (Lebel & Williams, 1983). Acti-
vated charcoal column chromatography (Kenmotsu et al.,
1980a), alumina column chromatography (Muir et al., 1980,
1981), and C-18 bonded silica cartridge (Sep-pak C-18)
(Muir et al., 1980; Muir & Grift, 1983) have also been
used to separate TCP from co-extracting compounds in vari-
ous samples.
Table 4. Methods for the determination of TCP and TPP
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
Workplace collect with Millipore GC/FPD 1 µg per TCP and TPP US NIOSH (1982)
air filter, extract with sample
ethanol
Environmental trap with glycerol- GC/FPD 1 ng/m3 simultaneous Yasuda (1980)
air Florisil column, eluate method for
with methanol, add trialkyl/aryl
water, and extract with phosphates
hexane
Air collect by aspiration TLC 5 ng/plate TCP and TPP Druyan (1975)
through ethanol,
hydrolyse with NaOH; the
resultant phenols are
reacted with
p-O2NC6H4N2+ and
separated with silica
gel plate
Drinking-water adsorb with XAD-2 GC/NPD 1 ng/litre method for Lebel et al.
resin, eluate with GC/MS low level (1979, 1981)
acetone-hexane or trialkyl/aryl
acetone phosphates
River or sea extract with GC/NPD 0.02 µg/litre simultaneous Kenmotsu et al.
water methylene chloride GC/FPD (TPP) method for (1980a, 1981b,
or benzene GC/MS 0.05 µg/litre trialkyl/aryl 1982b)
(TCP) phosphates Muir et
al. (1981)
Ishikawa et
al. (1985)
Farm pond reflux with methanol- GC/NPD 1 ng/g simultaneous Muir et al.
sediment water (9+1) or method for (1980, 1981)
methylene chloride- triaryl
methanol (1+1), phosphates
clean-up by acid
alumina column
chromatography
Table 4. (contd.)
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
River or sea extract with GC/FPD 5 ng/g simultaneous Kenmotsu et al.
sediment acetonitrile or GC/MS method for (1980a, 1981b,
acetone, clean-up by trialkyl/aryl 1982a, 1982b,
charcoal or Florisil phosphates 1983)
column chromatography Ishikawa et al.
(1985)
Fish extract with hexane GC/NPD 1 ng/g simultaneous Muir et al.
or methanol, clean-up GC/MS method for (1980, 1981,
by gel permeation triaryl 1983)
column chromatography phosphates
and acid alumina
column chromatograpy
Fish extract with GC/FPD 5 ng/g simultaneous Kenmotsu et
acetonitrile and GC/MS method for al. (1980a)
methylene chloride, trialkyl/aryl
clean-up by phosphates
acetonitrile-hexane
partitioning,
charcoal column
chromatography,
concentrated sulfuric
acid extraction and
Florisil column
chromatography
Human adipose extract with benzene GC/NPD 1 ng/g simultaneous Lebel & Williams
tissues or acetone-hexane (15 GC/FPD method for (1983)
+ 85), clean-up by GC/MS trialkyl/aryl
gel permeation phosphates
chromatography and
Florisil column
chromatography
Table 4. (contd.)
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
Plasma extract with ethyl HPLC 50 ng/injection TCP and its Nomeir &
ether, filter with (254 nm) metabolites Abou-Donia
0.45-µm nylon filter (1983)
Edible oils extract with ethanol, colori- 0.01% simple method Vaswani et
hydrolyse with NaOH; metric for TCP al. (1983)
the resultant cresol determination
is coupled with 2,6-
dichlorobenzoquinone
Edible oils separate with silica TLC simple method Bhattacharyya
gel G thin-layer (UV) for TCP et al.(1974)
plate; spray determination
rhodamine B solution
________________________________________________________________________________________________________
2.4.3 Gas chromatography and mass spectrometry
To identify TCP in environmental samples by packed
column GLC, it is useful to compare retention times using
two types of liquid phase with different polarities. As a
low polarity liquid phase, 10% OV-1 (Kenmotsu et al.,
1980a), 3% SE-30 (Ramsey & Lee, 1980), 3% OV-17 (Lebel et
al., 1981), 3% OV-101 (Deo & Howard, 1978), SP-2100 (Muir
et al., 1980), and 5% DC-200 (Daft, 1982) have been used,
while 1% QF-1 (Bloom, 1973), 5% FFAP and 5% Thermon-3000
(Kenmotsu et al., 1980a), and 2% DEGS (Daft, 1982) have
been used as a higher polarity liquid phase.
TCP is often accompanied by other TAPs in environmen-
tal samples, which show multiple peaks in GC and occasion-
ally have the same retention indices as that of TCP
(Ramsey & Lee, 1980; Kenmotsu et al., 1982b). Therefore,
capillary GLC or GC-mass spectrometry (GC/MS) is preferred
(Lebel et al., 1981; Lebel & Williams, 1983; Kenmotsu et
al., 1983; Ofstad & Sletten, 1985). In electron impact
mass spectrometry, TCP gives a high intensity molecular
ion, as do other TAPs (Deo & Howard, 1978; Wightman &
Malaiyandi, 1983; Kenmotsu et al., 1982b). A selected ion
monitoring (SIM) technique is also useful for trace analy-
sis of TCP in environmental samples (Ishikawa et al.,
1985), but care must be taken to select suitable fragment
ions in order to avoid interference by other TAPs.
The phenolic components of TCP are confirmed by alka-
line hydrolysis, followed by GLC analysis of the resulting
phenols (Murray, 1975; Sugden et al., 1980).
2.4.4 Contamination of analytical reagents
The widespread use of TCP in plastics and hydraulic
fluids can cause contamination of analytical reagents.
Traces of TCP have been found in rubber O-rings and rubber
seals used in a Corning water supply system (Lebel et al.,
1981), Super Q water (Williams & Lebel, 1981), and aceto-
nitrile, methylene chloride, and hexane (Daft, 1982).
Trialkyl phosphates have also been found in cyclohexane
(Bowers et al., 1981), hexane (Hudec et al., 1981), and
analytical grade filters (Daft, 1982). Therefore, care
must be taken to avoid contamination of analytical re-
agents in order to obtain accurate data in trace analysis
of TCP.
2.4.5 Other analytical methods
A rapid colorimetric method has been developed for the
determination of TCP in edible oil (Vaswani et al., 1983),
but no information about the interference with other TAPs
is available. Silica gel TLC has been used for deter-
mining TCP in edible oil (Bhattacharyya et al., 1974;
Krishnamurthy et al., 1985). Reversed phase TLC has also
been used (Renberg et al., 1980). However, separating TAPs
from each other by TLC is not sufficient (Bloom, 1973).
HPLC with a C-18 bonded column has been used for deter-
mining TCP in plasma, while size exclusion HPLC has been
used in the case of machine oil (Majors & Johnson, 1978).
An ultraviolet spectrometric detector is usually used in
HPLC, but it is not specific for TAPs. Tittarelli &
Mascherpa (1981) described a highly specific HPLC detec-
tor for TAPs using a graphite furnace atomic absorption
spectrometer.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production levels and processes
Tricresyl phosphate does not occur naturally in the
environment. Figures concerning the total world production
are not available. In Japan, 33 000 tonnes were produced
in 1984a. In the USA, approximately 54 000 tonnes of TAP
including 10 400 tonnes of TCP were produced in 1977
(Boethling & Cooper, 1985). About 800-1000 tonnes TCP per
year is now produced in China.
TCP is usually produced by the reaction of cresols
with phosphorus oxychloride. One of the industrial sources
of cresols is the so-called cresylic acid or tar acid,
which is a mixture of isomers of cresol and varying
amounts of xylenols, phenol, and other high-boiling phe-
nolic fractions obtained as a residue from coke ovens and
petroleum refining (Duke, 1978). Another source is
"synthetic cresol", prepared from cymene via oxidation
and catalytic degradation (Association of the Plasticizer
Industry of Japan, 1976), and this has been used for pro-
duction of TCP in Japan since 1971. The composition of
some cresylic acids and synthetic cresol is shown in
Table 5.
TCP derived from these alkylphenols is, therefore, a
complex mixture of various TAPs, i.e. tri- o-cresyl phos-
phate, tri- m-cresyl phosphate, tri- p-cresyl phosphate,
di- m-cresyl- p-cresyl phosphate, di- p-cresyl- m-cresyl phos-
phate, etc. The very toxic tri- o-cresyl phosphate
(TOCP) is usually excluded as much as possible. In some
cases, commercial tricresyl phosphate (TCP) has been
reported to contain a small amount of TPP (Daft, 1982;
Ofstad & Sletten, 1985).
The most noteworthy trend in aryl phosphate manufac-
ture and use in the USA has been the replacement of tri-
phenyl, tricresyl, and trixylenyl phosphates derived from
petroleum-based feedstocks by aryl phosphates derived from
synthetic precursors. The production of cresyl diphenyl
phosphate, a petroleum-based aryl phosphate, was discon-
tinued in 1979 in the USA. The mixed trialkyl/aryl phos-
phates are replacing TPP and TCP as a plasticizer, whereas
the synthetic TAPs are replacing TCP and trixylenyl phos-
phate in functional fluids (Boethling & Cooper, 1985).
______________
a Personal communication from the Association of the
Plasticizer Industry of Japan, 1985.
Table 5. Composition of some commercial cresylic acidsa and
"synthetic cresol"b
______________________________________________________________
Composition (%)
Boiling Cresylic Acids Synthetic
Constituents point Sample Sample Sample Cresol
(°C) A B C
______________________________________________________________
o- cresol 191.0 3 0 0 0.1%
2,6-Xylenol 201.0 6 6 0
m- Cresol 202.2 42 43 47 \
p- Cresol 202.3 30 31 34 /99%
o- Ethylphenol 204.5 3 3 0
2,4-/2,5-Xylenol 211.5 16 17 19
______________________________________________________________
a From: Bondy et al. (1960).
b From: Association of the Plasticizer Industry of Japan
(1976).
3.1.1 Accidental release
Liquid TCP and hydraulic fluid and lubricant oil con-
taining phosphate esters are transported by tank trucks,
rail cars, and to a lesser extent in barrels (US NIOSH,
1979). Occasionally, empty barrels (or drums) previously
containing hydraulic fluid or lubricant oil have been
reused to store or to transport edible oil (or water), and
this has resulted in poisoning of humans and cattle
(Susser & Stein, 1957; Smith & Spalding, 1959; Chaudhuri,
1965; Nicholson, 1974; Senanayake, 1981). Another case of
poisoning involved flour contaminated with oil from a leak
during shipping (Sorokin, 1969).
In a report by Beck et al. (1977), accidental spillage
of TAPs intended for use in pipeline pumping stations oc-
curred and resulted in poisoning of cattle. Effluent from
an evaporation pond overflowed onto the pasture during
spring run-off. Sampling showed concentrations of TAPs
from 0.304% to 3.44% by weight in soil, grass, and water
near the plant. Thirty days later TAPs were still present
in the evaporation pond but not in the soil samples
(Chemical and Geological Laboratories Ltd., 1971).
Beck et al. (1977) described in his report: "Mass
poisonings are possible because large quantities of tri-
aryl phosphate are used as lubricants and coolants in jet
engines in pipeline compressor stations. If an emergency
arises as much as 1200 gallons of this material can be
expelled into the atmosphere within 20 seconds. The con-
struction of pipelines over thousands of miles, with
manned or unmanned compressors every 100 miles, consti-
tutes an environmental hazard to both domestic livestock
and wildlife."; and "Natural leaching of the ground by
weather conditions probably removed the poison from the
soil, but repeated spills of large quantities of a stable
compound could contaminate ground water supplies".
3.2 Uses
TCP is used as a plasticizer in vinyl plastic manufac-
ture, as a flame-retardant, a solvent for nitrocellulose,
in cellulosic molding compositions, as an additive to
extreme pressure lubricants, and as a non-flammable fluid
in hydraulic systems (Windholz, 1983). The main market for
PVC-based products plasticized with organic phosphate
esters is in the manufacture of automobile and other motor
vehicle interiors in the USA (Lapp, 1976). In Japan, ap-
proximately 2500 tonnes of TCP was used in 1984 as a plas-
ticizer in PVC film for agricultural use, 400 tonnes as
non-flammable plasticizer in floor and wall covering, and
100 tonnes for miscellaneous purposes (Association of the
Plasticizer Industry of Japan, 1985).
The fastest growing use of organic phosphate esters is
in the manufacture of fire-resistant hydraulic fluids and
lubricants in the USA (Lapp, 1976). The two types of
organic phosphate hydraulic fluids being manufactured are
phosphate ester oil blends and "pure synthetics". The
phosphate ester oil blends contain between 30% and 50%
organic phosphate esters in addition to petroleum oil and
coupling agents; the "pure synthetics" contain a mixture
of organic phosphate esters. For example, a typical syn-
thetic organic phosphate fluid contains TCP, trixylenyl
phosphate, and other TAPs. The compositions of several
commercial synthetic organic phosphate fluids are listed
in Table 6. Organic phosphate ester lubricant additives
are usually of three general types: extreme pressure
agents, anti-wear agents, and stick-slip moderators. The
first two types are used in systems with some type of
gears and account for over 80% of all organic phosphate
lubricant additives. These agents are also used in cut-
ting oils, machine oils, transmission fluids, and cooling
lubricants (Lapp, 1976).
In Japan, approximately 300 tonnes of TCP was used for
lubricant additives in 1985 (Association of the Plasti-
cizer Industry of Japan, 1985), and approximately 1320
tonnes of TAPs was used in 1976 in Ontario, Canada (Muir
et al., 1980).
There are other minor uses of TCP: additives in making
synthetic leather (Franchini et al., 1978), shoes (Pegum,
1966), and polyvinyl acetate products (Anon., 1986); sol-
vent for acrylate lacquers and varnishes (Anon., 1986); in
non-smudge carbon paper (Hjorth, 1962; Pegum, 1966).
Table 6. Composition of various commercial organophosphorus hydraulic fluids and lubricants
________________________________________________________________________________________________________
Component (%)
________________________________________________________
Name TPP TCP Others Producer Reference
________________________________________________________________________________________________________
IMOL S-140 1 2 (ortho) Tris(dimethylphenyl)- (18) Imperial Oil Ltd. Lockhart
42 (meta) Tris(ethylphenyl)- (6) et al.
31 (para) Tris(trimethylphenyl)- (1975)
and unknown (1)
Pydraul 50E 36 nonylphenyl diphenyl phosphate (40) Monsanto Co. Nevins &
cumylphenyl diphenyl phosphate (22) Johnson
(1978)
Pydraul 115E 7 nonylphenyl diphenyl phosphate (29)
cumylphenyl diphenyl phosphate (62)
Pydraul 50E 18.4 nonylphenyl diphenyl (52.8) Monsanto Co. Deo &
cumylphenyl diphenyl (24.0) Howard
(1978)
Kronitex TCP 20.7 (meta) dicresyl xylenyl (9.2) FMC Corp. Deo &
38.8 (di- Howard
meta, para) (1978)
30.4 (di-
para, meta)
Santicizer- 14.7 19.4 m-cresyl diphenyl (18.6) Monsanto Co. Deo &
140 CDP p-cresyl diphenyl (14.4) Howard
phenyl dicresyl (29.4) (1978)
Fyrquel GT 19.2 m-cresyl diphenyl (2.1) Stauffer Chem. Co. Deo &
phenyl dicresyl (3.2) Howard
dicresyl xylenyl (36.2) (1978)
di(C3-phenyl) xylenyl (37.1)
Phosflex 41-P 11.9 40.8 m-cresyl diphenyl (2.1) Stauffer Chem. Co. Deo &
trixylenyl (9.4) Howard
(C3-phenyl)3 (28.7) (1978)
________________________________________________________________________________________________________
________________________________________________________________________________________________________
Component (%)
_______________________________________________________
Name TPP TCP Others Producer Reference
________________________________________________________________________________________________________
Fyrquel 220 phosphates derived from Imperial Oil Ltd. Pickard et
phenol (2.6); o-cresol (0.5); m- and al. (1975)
p-cresol (13.6); 2-ethylphenol (0.6)
2,4- and 2.5-xylenol (22.3); mixed
xylenol (49.2); 3,4-xylenol (8.6);
6-9 phenolics (1.3); 2,4,6-trimethyl
phenol (1.4)
Kronitex 100 18 diphenyl 2-isopropylphenyl (27) FMC Corp. Nobile et
diphenyl 4-isopropylphenyl (11) al. (1980)
tris(2-isopropylphenyl) (11)
phenyl di-(2-isopropylphenyl) (7)
phenyl di-(4-isopropylphenyl) (5)
Kronitex 50 33 diphenyl 2-isopropylphenyl (21) FMC Corp. Nobile et
diphenyl 4-isopropylphenyl (12) al. (1980)
tris(2-isopropylphenyl) (8)
phenyl di-(2-isopropylphenyl) (6)
phenyl di-(4-isopropylphenyl) (2)
________________________________________________________________________________________________________
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Summary
The majority of TCP release to the environment is accounted
for by end-point use rather than production. Total release to
the environment in the USA was estimated at 32 800 tonnes in
1977.
TCP released into water is readily adsorbed on to sediment
particles, and little or none remains in solution.
TCP is readily biodegraded in sewage sludge with a half-
life of 7.5 h, the degradation within 24 h being up to 99%. TCP
is almost completely degraded within 5 days in river water. The
ortho isomer is degraded slightly faster than the meta or para
isomers. Abiotic degradation is slower with a half-life of 96
days.
TCP has, because of its physico-chemical properties, a high
potential for bioaccumulation. Laboratory studies of continuous
exposure to high concentrations (which are environmentally
unrealistic) of radiolabelled TCP have shown high bioconcen-
tration factors (BCF). However, these studies failed to show
that the isotope was still associated with the original com-
pound. Taking into account the ready biodegradability of TCP,
these data should be viewed as probable overestimates, and it
is suggested that little bioaccumulation would occur with
environmentally realistic TCP exposure.
4.1 Transport and transformation in the environment
4.1.1 Release to the environment
Total losses of aryl phosphates to the environment in
the USA from production in 1977 were estimated as 2585
tonnes, the main source being land disposal of manufactur-
ing wastes (2540 tonnes). Releases from end-product use
(32 800 tonnes in the USA in 1977) are estimated to be
much greater than from production. The amount of volatil-
ization and leaching from plastic items was 16 300 tonnes
and that of leakage of hydraulic fluids and lubricants
13 400 tonnes (Boethling & Cooper, 1985). One major hy-
draulic fluid manufacturer estimated that as much as 80%
of the annual consumption of aryl phosphate hydraulic
fluids is used to make up for leakage (MRI, 1979).
No data are available on the release to the atmosphere
of TCP from production processes. However, open, high-tem-
perature processes such as roll milling, calendering, and
extrusion of plasticized polymers may result in signifi-
cant gaseous emissions of aryl phosphates (Boethling &
Cooper, 1985).
Yasuda (1980) detected significant levels of TCP in
urban air and in the atmosphere over coastal waters near
industrialized areas. Details are described in section
5.1.1.
The results of a study by the US Environmental Protec-
tion Agency (MRI, 1979) showed that TCP can evaporate from
automotive upholstery fabric and condense on the interior
surface of a relatively cool window.
The emission of TCP from waste incineration plants may
also be a pathway to the atmosphere. In a study by Vick et
al. (1978), TCP was not detected in vapour samples taken
before and after the dust collectors of incinerators.
4.1.2 Fate in water and sediment
The solubility of TCP in water is low (Table 1). Moni-
toring studies have shown trialkyl/triaryl phosphates to
be present in water and sediment sampled near major indus-
trialized areas (Konasewich et al., 1978; Sheldon & Hites,
1978, 1979; Mayer et al., 1981; Williams & Lebel, 1981;
Williams et al., 1982; Ishikawa et al., 1985; Fukushima &
Kawai, 1986). However, TCP was only occasionally detected
in water samples, whereas TPP was often detected (Mayer et
al., 1981; Williams & Lebel, 1981; Williams et al., 1982;
Ishikawa et al., 1985). The total concentrations of
Pydraul (Table 6) components in river (0.24 µg/litre) and
lake sediments (570 µg/kg) in the USA revealed a water-
sediment difference of more than 3 orders of magnitude
(Mayer et al., 1981). Equilibrium of TCP with the bottom
sediment in a shallow (0.5-m depth) pond would be expected
to be reached rapidly, as in the case of TPP (Muir et al.,
1982). The adsorption coefficient of TCP on marine sedi-
ment was found to be 420 (Kenmotsu et al., 1980b).
It is apparent from the following data that TCP is
rapidly adsorbed onto river sediment: the level of total
aryl phosphate in the sediment of the Kanawha River (USA)
was 229 mg/kg at the FMC plant outfall but only 4.4 mg/kg
13 km downstream (Boethling & Cooper, 1985).
Wagemann et al. (1974) and Wagemann (1975) reported
that a commercial synthetic lubricating oil, IMOL S-140
(Table 6), degraded in sterilized river water under lab-
oratory fluorescent light and under sunlight at 25 °C, and
that the first order rate constant and half-life were,
respectively, 9 x 10-3 days-1 and 96 days (Wagemann,
1975).
4.1.3 Biodegradation
River die-away studies by Saeger et al. (1979) on nine
phosphate esters demonstrated that these esters, exposed
to the natural microbial population of the river, under-
went primary biodegradation at moderate to rapid rates. A
200-µg portion of TCP was completely degraded within 4
days in 200 ml of Mississippi River (USA) water at room
temperature. Hattori et al. (1981) also investigated the
degradation of TCP in Neya and Oh River water (Osaka,
Japan). After a lag period of 1-2 days, the TCP (1 mg per
litre) was almost completely degraded within 5 days under
non-sterilized conditions, whereas no degradation in heat-
sterilized water occurred during 15 days. In clear non-
sterilized sea water, however, the degradation was very
slow. Saeger et al. (1979) also found that in sterile
river water there was no significant evidence of non-
biological degradation or loss. Among the isomers of TCP,
the ortho isomer degraded in river water slightly faster
than the meta isomer and both isomers degraded faster than
the para isomer, which degraded about as fast as TPP
(Howard & Deo, 1979).
Primary biodegradation rates from semicontinuous acti-
vated sludge (SCAS) studies (US Soap and Detergent Assoc.,
1965; Mausner et al.,1969) showed generally the same trend
in degradation rates as river die-away studies. At a 24-h
feed level of 3-13 mg/litre, TCP showed 99% degradation.
The ultimate biodegradability of TCP was measured by
Saeger et al. (1979) using the apparatus and procedure
developed by Thompson & Duthie (1968) and modified by
Sturm (1973). At 26.4 mg TCP/litre, the carbon dioxide
evolution reached 82% of its theoretical value.
For alkyl-aryl and triaryl phosphates, increasing the
number and size of substituent groups on the phenyl mol-
ecule decreases the biodegradability (Saeger et al.,
1979).
The degradation pathway for TCP most probably involves
stepwise enzymatic hydrolysis to orthophosphate and
phenolic moieties (Barrett et al., 1969; Pickard et al.,
1975). The phenol would then be expected to undergo
further degradation. Dagley & Patel (1957) demonstrated
that p-cresol is oxidized to p-hydroxybenzoic acid by a
species of Pseudomonas. Ku & Alvarez (1982) studied the
biodegradation of [14C]-tri- p-cresyl phosphate in a
laboratory model sewage treatment system, and, in 24-h
experiments, found that 70-80% of the TCP (added at
1 mg/litre) was degraded, with a half-life of 7.5 h. The
major metabolite extracted with ethyl ether from the
aqueous phase was identified as p-hydroxybenzoic acid by
thin-layer chromatography and gas chromatography-mass
spectrometry, while two other radioactive spots remained
unidentified.
4.1.4 Water treatment
Data from FMC Corporation (USA) show that TCP (6.23
mg/litre) in waste water was reduced to 0.23 mg/litre in
the effluent water by biological treatment, whereas the
aryl phosphates with higher relative molecular mass (>452)
(and, therefore, more highly substituted) were not easily
removed (Boethling & Cooper, 1985). Fukushima & Kawai
(1986) reported that TCP (0.186-9.31 µg/litre) in raw
water was reduced to 0.078 µg/litre or less in treated
water by conventional waste water treatment. Filtration of
effluent samples through 1-µm pore size filters resulted
in a further removal of 93% of total aryl phosphates,
again demonstrating the adsorptive behavior of these com-
pounds (Boethling & Cooper, 1985).
4.2 Bioaccumulation and biomagnification
4.2.1 Fish
Data on the bioconcentration and depuration of TCP are
given in Table 7. None of the exposures were considered to
be representative of realistic environmental levels. More-
over the bioconcentration factor (BCF) measured in the
laboratory must be considered as a bioaccumulation poten-
tial rather than an absolute bioaccumulation factor (Veith
et al., 1979).
Several equations have been used in attempts to
predict the BCF of organic chemicals in various fish
strains using the octanol-water partition coefficient
(Pow) or water solubility values (Neely et al., 1974; Lu
& Metcalf, 1975; Kanazawa, 1978; Veith et al., 1979;
Sasaki et al., 1982).
The clearance of tri- m-cresyl phosphate has been
shown to be biphasic, with higher rates of clearance in
the first 6 days after transfer to clean water, especially
for rainbow trout. The clearance rate constants for rain-
bow trout were about 50% more than those for fathead
minnows (Muir et al., 1983).
4.2.2 Plants
The uptake and translocation of tri- p-cresyl phos-
phate by soybean plants has been studied by Casterline et
al. (1985), the initial concentration in soil being 10 mg
per kg. Approximately 70% of the compound had disappeared
from the soil within 90 days (when the plants were har-
vested). At that time, the amount per plant was 34 µg
(0.17% of the applied TCP). Of this total plant content,
74% was found in the stem, 24% in the leaves, and 2% in
the pods. The seeds contained no detectable tri- p-
cresyl phosphate.
Table 7. Bioaccumulation and clearance of tricresyl phosphate by fish
________________________________________________________________________________________________________
Flow/ Analy- Exposure Uptake Clearance Depura-
Species Com- stat tical BCF concent- rate rate tion Reference
pound (temp.) methoda (K1/K2) ration (k1, (k2 x half-life
(mg/litre) h-1) 103,h-1) (hr)
________________________________________________________________________________________________________
Rainbow para stat TR 2768 ± 641b 0.005-0.05 9.6-13.3 72.2 Muir
trout isomer (10°C) TR 1420 ± 42c 14.0 et al.
( Salmo TR 1466 ± 138d 17.0 (1983)
gairdneri ) HER 770 ± 24c 65.4
meta TR 1162 ± 313b 11.5-24.2 30.3
isomer TR 784 ± 82c 18.5
TR 1102 ± 137d 21.2
HER 310 ± 52c 25.8
Fathead para stat TR 2199 ± 227b 0.005-0.05 7.0-9.6 90.0 Muir
minnows isomer (10°C) TR 928 ± 78c 4.9 et al.
( Pimephales TR 588 ± 129d 9.6 (1983)
promelas ) HER 709 ± 76c 73.7
meta TR 1653 ± 232b 8.5-14.7 59.2
isomer TR 596 ± 103c 7.9
TR 385 ± 92d 8.7
HER 62 ± 3c 53.3
commer- flow GC- 165 0.0316 Veith et
cial (25°C) FPD al. (1979)
Bluegill TCP
( Leptomis para TR 1589 Sitthich-
macrochirus ) isomer aikasem
(1978)
________________________________________________________________________________________________________
a GC-FPD = gas chromatography (flame photometric detector) after suitable extraction;
TR = total radioactivity; HER = hexane-extractable radioactivity.
b BCF was calculated by the "initial rate method".
c The static test method was used (Zitko, 1980).
d k1 and k2 were derived by non-linear regression calculation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Summary
TCP has been measured in the atmosphere in Japan at concen-
trations up to 70 ng/m3 but only reached 2 ng/m3 at a pro-
duction site in the USA. Workplace air in the USA contained
less than 0.8 mg/m3 at a lubrication oil barrel-filling oper-
ation and 0.15 mg/m3 (total phosphates) in an automobile zinc
die-casting plant. The concentrations of TCP measured in
drinking-water in Canada were low (0.4 to 4.3 ng/litre), and
TCP was undetectable in well-water. Levels in river and lake
water are frequently considerably higher. However, this is due
to the presence of suspended sediment to which TCP is strongly
adsorbed. Concentrations up to 1300 µg/kg in river sediment
and 2160 µg/kg in marine sediment have been measured.
Elevated TOCP levels in soil and vegetation have been found
within the perimeter of production plants.
Residues of TOCP in fish and shellfish up to 40 µg/kg have
been reported but the majority of animals sampled contained no
detectable amounts.
5.1 Environmental levels
TCP has been found in air, water, soil, sediment, and
aquatic organisms. However, the levels of TCP in environ-
mental samples are low (Table 8), except in soil and sedi-
ment collected in heavily industrialized areas (Table 9).
5.1.1 Air
Yasuda (1980) measured the distribution of various
organic phosphorus compounds in the atmosphere over the
eastern Seto Inland Sea, Japan. Near the heavily industri-
alized cities (Fukuyama, Akashi, Osaka), 11.5-21.4 ng
TCP/m3 was detected. Yasuda (1980) also measured levels
of phosphate esters in the atmosphere above the Dogo Plain
and the Ozu Basin agricultural area of Western Shikoku.
TCP was detected only in the urban air of Matsuyama, where
the level was 26.7-70.3 ng/m3. TCP levels of 0.01-2 ng/m3
in air collected at production sites in the USA have been
reported (MRI, 1979).
Table 8. Concentration of TCP in environmental air, water, sediment, and fish at various locations
______________________________________________________________________________________________________
Locations Year Sample Concentration Number of Reference
samples
(detected/
analysed)
______________________________________________________________________________________________________
Shikoku (Japan) 1976 atmosphere 26.7-70.3 ng/m3 (3/19) Yasuda
(1980)
Eastern Seto 1977 atmosphere 11.5-21.4 ng/m3 (3/4)
Inland Sea (Japan)
Japan (various 1975 river and sea water ND (50-1500 ng/litre)a (0/100) EAJ (1977)
locations) river and sea sediment 150 ng/g (1/100)
fish ND (20-250 ng/g)a (0/100
Japan (various 1978 river and sea water ND (50-2500 ng/litre)a (0/114) EAJ (1979)
locations) river and sea sediment 1060-2160 ng/g (3/114)
fish ND (0.25-150 ng/g)a (0/98)
Osaka (Japan) 1976 river water 100-9500 ng/litre (11/13) Kawai et
al. (1978)
Eastern Ontario 1978 drinking-water 0.3 ng/litre (1/12) Lebel et
water treatment al. (1981)
plant (Canada)
Tokyo (Japan) 1978 river water ND (50 µg/litre)a (0/12) Wakabayashi
sea water ND (50 µg/litre)a (0/3) (1980)
river sediment 7-370 ng/g (9/10)
sea sediment 4 ng/g (1/3)
Canada (various 1979 drinking-water 0.7-4.3 ng/litre (7/60) Williams &
locations) Lebel (1981)
Great Lake 1980 drinking-water 0.4-1.8 ng/litre (5/12) Williams et
(Canada) al. (1982)
Kitakyushu 1980 river water 67-259 ng/litre (3/16) Ishikawa et
City (Japan) sea water ND (20 ng/litre)a (0/9) al. (1985)
sea sediment ND (10 ng/g)a (0/6)
Seto Inland 1980 fish and shell fish 1-19 ng/g (4/41) Kenmotsu et
Sea (Japan) al. (1981a)
_____________________________________________________________________________________________________
a Range of detection limits due to analytical methods used; ND = not detected.
Table 9. Concentration of TCP detected near the producers and users of trialkyl/aryl phosphates
_____________________________________________________________________________________________
Locations Year Sample Concentration Number of Reference
samples
(detected/
analysed)
_____________________________________________________________________________________________
Near TAPs manufacturing fish 2-5 ng/g Muir (1984)
plants (USA)
Columbia River (USA) fish (sturgeon) 40 ng/g Lombardo &
Egry (1979)
Kanawha River (USA) 1978 river water 20 000 ng/litre Boethling &
Cooper (1985)
FMC Corp., Nitro, MV 1979 air (HV)a 2 ng/m3 Boethling &
(USA) Cooper (1985)
Stauffer Chemical Co., 1979 air (HV)a 0.01-0.05 ng/m3 (2/4) Boethling &
Gallipolis Ferry, MV vegetation 1000-20 000 ng/g (4/4) Cooper (1985)
(USA) soil 1000-4000 ng/g (4/4)
FMC Corp. Plant (USA) 1980 waste water 6.23 mg/litre Boethling &
effluent water 0.23 mg/litre Cooper (1985)
Baltimore Harbour 1983 sediment 400-600 ng/g (2/3) Boethling &
(USA) Cooper (1985)
Detroit River, mouth 1983 sediment 230-1300 ng/g (2/2) Boethling &
(USA) Cooper (1985)
_____________________________________________________________________________________________
a HV = High volume filter pad (air sampler).
5.1.2 Water
Although there have been many monitoring studies for
TAPs in water, TCP has not often been detected in natural
water. Where present, it is only at low levels. According
to the annual reports of the Environment Agency of Japan,
TCP has not been detected in river or sea water at any
sampling points. Due to the variety of analytical methods
and procedures used, the detection limits varied between
5 and 2500 ng/litre between different laboratories (EAJ,
1977; 1979; 1981). Kawai et al. (1978) detected TCP at
100-9500 ng/litre in river water sampled in Osaka (Japan),
and found that the concentration of TCP in river water
tended to parallel the concentration of suspended solid.
Ishikawa et al. (1985) detected TCP levels of 67-259
ng/litre in 3 out of 16 samples of river water in
Kitakyushu City (Japan), but not in sea water. Both cities
are located in the most heavily industrialized areas of
Japan.
Relatively high concentrations of TAPs have frequently
been detected in river water sampled near producer or user
sites: 20 µg TCP/litre was detected in the Kanawha River
(USA) 8 miles downstream from the plant outfall (Boethling
& Cooper, 1985).
5.1.3 Soil
There has been only one report of TCP in soil (from
Stauffer Chemical Co. at Gallipolis Ferry (USA)), the
level being 1.0-4.0 mg/kg (Boethling & Cooper, 1985). The
high concentration of total TAPs (26 550 mg/kg) in this
sample was thought to reflect product accumulation in the
area, which was subject to frequent spills.
5.1.4 Sediment
Because of the high sediment adsorption coefficient,
higher levels of TCP have frequently been detected in
sediment than in water. TCP was detected at 400-600 ng/g
in sediment in Baltimore harbour (USA) and at 230-1300
ng/g in the Detroit River (USA) (Boethling & Cooper,
1985). According to the annual reports of the Environment
Agency of Japan, a level of 150 ng/g was found (Mitaki
River, Japan) in one out of 100 sediment samples in 1975,
whereas 1060-2160 ng/g (Doukai Bay, Japan) was found in 3
out of 114 samples in 1978 (EAJ, 1977; 1979; 1981).
Wakabayashi (1980) detected 7-370 ng/g in nine out of ten
river sediment samples, and 4 ng/g in one out of three sea
sediment samples in Tokyo.
5.2 General population exposure
5.2.1 Drinking-water
TCP levels in drinking-water are very low. Lebel et
al. (1981) analysed TAPs in drinking-water sampled from
eastern Ontario water treatment plants and found TCP (at
0.3 ng/litre) in only one out of 12 samples. In an
extended survey of drinking-water conducted in Canada
(Williams & Lebel, 1981), TCP was detected at 0.7-4.3
ng/litre in 7 out of 60 samples of treated potable water
obtained at the treatment plants of 29 municipalities.
In a study by Williams et al. (1982), TCP was detected
in river and lake water but not in well-water. TCP was
also found, at concentrations of 0.4 to 1.8 ng/litre, in 5
out of 12 samples of drinking-water obtained from 12 water
treatment plants located around the Great Lakes (USA).
In general, the TCP concentration in drinking-water is
lower (by factors of 10-2 to 10-3) than that in river
water. This is due to the efficient removal of TCP at
water treatment plants by infiltration using activated
carbon with a high adsorption coefficient.
5.2.2 Fish
Lombardo & Egry (1979) found a TCP concentration of
40 ng/g in sturgeon caught in the Columbia River (USA),
where many metal-processing plants were located upstream
from the sampling point. Muir (1984) found 2-5 ng/g in
fish caught near TAP manufacturing plants. According to
the annual reports of the Environment Agency of Japan, TCP
was not detected in fish caught at any sampling points
(EAJ, 1977; 1979; 1981). The analytical detection limits
varied from 0.25 to 250 ng/g. Kenmotsu et al. (1981a)
found 1-19 ng/g in 4 out of 41 samples of fish and shell-
fish collected in the Seto Inland Sea, Japan.
5.2.3 Human tissues
There has been only one report of TAPs in human adi-
pose tissues (Lebel & Williams, 1983). Although there was
no history of TCP exposure in these patients, tris(1,3-
dichloroisopropyl) phosphate and tributoxyethyl phosphate
were detected at levels of 0.5-110 ng/g and 4.0-26.8 ng/g,
respectively.
5.3 Occupational exposure
The National Institute for Occupational Safety and
Health, USA (US NIOSH) has monitored workplace air, and
found that air samples collected near barrel-filling
operations where lubricating oil was produced by blending
TCP contained less than 0.8 mg TAP/m3 (US NIOSH, 1979).
Air collected near the zinc die-casting machine in auto-
mobile manufacturing contained a total phosphate ester
level of 0.15 mg/m3 (US NIOSH, 1980). Airborne TOCP
resulting from the production of heavy-duty radiators has
been investigated by NIOSH, but the concentration was
below the limit of detection (US NIOSH, 1982). Triaryl
phosphates (at approximately 0.1 ppm) were detected in the
air of the aircraft elevator machinery spaces on the
carrier USS Leyte (CVS-32) where a triaryl phosphate oil
was used as a hydraulic fluid (Baldridge et al., 1959).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Summary
The primary productivity of cultures of freshwater green
algae was reduced to 50% by TOCP at levels of 1.5 to 4.2 mg per
litre, depending on the species. The meta and para isomers were
less toxic. There are few data on the acute toxicity of TCP to
aquatic invertebrates: a 48-h LC50 for Daphnia of 5.6 mg per
litre, a 24-h LC50 for a nematode of 400 mg/litre, and a
2-week NOEL for Daphnia (mortality, growth, reproduction) of
0.1 mg/litre. The 96-h LC50 values for three fish species
varied between 4.0 and 8700 mg TCP/litre. Rainbow trout showed
approximately 30% mortality after a 4-month exposure to IMOL
S-140 (2% TOCP) at 0.9 mg/litre and minor effects within 14
days. The exposure levels used in these studies were much
higher than likely water concentrations in the environment and,
in most cases, greatly exceeded the solubility of the compounds.
There is no information on the bioavailability or toxicity
to burrowing or bottom-living organisms of TCP bound to sedi-
ment.
There is an indication that crop plants can be affected by
TOCP released from plastic coverings, but there is no infor-
mation concerning the effects on wild plant species.
6.1 Unicellular algae
Data on the toxicity of TCP compounds to unicellular
algae are given in Table 10.
The toxicity of TCP compounds to freshwater algae
depends on their chemical structure. Substitution of the
hydrogen by a methyl group in the benzene ring decreases
the toxicity (Wong & Chau, 1984). Of the TCP isomers, the
ortho isomer was the most toxic for the primary pro-
ductivity of Ankistradesmus falcatus, followed by the
meta and para isomers (Wong & Chau, 1984).
6.2 Aquatic organisms
Data on the toxicity of TCP to aquatic organisms are
presented in Table 11.
Table 10. Toxicity of TCP to freshwater unicellular algae
________________________________________________________________________________________________________
Organism Isomer Temper- Species Effect Concent- Reference
ature ration
(°C) (mg/litre)
________________________________________________________________________________________________________
Alga ortho 20 Ankistrodesmus falcatus 24-h IC50 for primary 2.5 Wong & Chau
meta 20 var. acicularis productivity >5.0 (1984)
para 20 >5.0
Green alga ortho 20 Scenedesmus 24-h IC50 for primary 4.2 Wong & Chau
meta 20 quadricaudata productivity >5.0 (1984)
para 20 >5.0
Lake Ontario ortho 20 24-h IC50 for primary 1.7 Wong & Chau
phytoplankton meta 20 productivity 4.1 (1984)
para 20 >5.0
Green alga Scenedesmus 4-day EC50 1.5 Adema et al.
pannonicus (1983)a
________________________________________________________________________________________________________
a Tests were performed according to or in line with standardized procedures (OECD, 1981).
EC50: 50% effective concentration; IC50: 50% inhibition concentration.
Table 11. Toxicity of TCP to aquatic organisms
________________________________________________________________________________________________________
Organisms Chem- Size/ Temp. Flow/ Hard- End-point Parameter Concent- Reference
icals weight (°C) stat ness or criteria ration
(mg/ (mg/
litre) litre)
________________________________________________________________________________________________________
Tidewater TCPa 40-100 20 stat 96-h LC50 8700 Dawson et
silverside mm al. (1977)
( Menidia
beryllina )
Bluegill TCPa 35-75 23 55 96-h LC50 7000 Dawson et
( Lepomis mm al. (1977)
macrochirus ) 0.60 g 12 flow- 44 96-h LC50 0.26 Mayer &
through 314 0.061 Ellersieck
(1986)
Guppy TCPa stat
( Poecilia 96-h LC50 4.0 Adema et
reticulata ) mortality, 96-h NOEL 1.0 al. (1983)b
swimming
behaviour,
colour
mortality, 4-week 1.0 Adema et
growth, NOEL al. (1983)b
swimming
behaviour
IMOL stat mortality, 24-h NOEL > 57 Wagemann
S-140 visible (1975)
effects
Flagfish TCPa egg-larval 6-week 0.01 Adema et
( Jordanella development, NOEL al. (1983)b
floridae ) mortality,
growth,
swimming
behaviour,
colour
________________________________________________________________________________________________________
Table 11. (contd.)
________________________________________________________________________________________________________
Organisms Chem- Size/ Temp. Flow/ Hard- End-point Parameter Concent- Reference
icals weight (°C) stat ness or criteria ration
(mg/ (mg/
litre) litre)
________________________________________________________________________________________________________
Rainbow trout IMOL flow- ate less, condition 0.9 Wagemann
( Salmo S-140 through less active after 8 (1975)
gairdneri ) days
ceased condition 0.9 Wagemann
surface after 14 (1975)
feeding days
mortality condition 0.9 Wagemann
(5/16) after 4 (1975)
months
TCPa 0.23 g 12 flow- 44 96-h LC50 0.26 Mayer &
0.50 g through 0.40 Ellersieck
(1986)
Waterflea TCPa flow- mortality 48-h LC50 5.6 Adema et
( Daphnia through al. (1983)b
magna ) mortality, 2-week NOEL 0.1 Adema et
reproduction, al. (1983)b
growth
Channel catfish 1.30 g 12 flow- 44 96-h LC50 0.80 Mayer &
( Ictalurus through Ellersieck
punctatus ) (1986)
Yellow perch 0.79 g 292
________________________________________________________________________________________________________
a No descriptions of isomeric compositions were given in the references.
b Tests were performed according to or in line with standardized procedures (OECD, 1981).
Measurement of the acute toxicity (96-h LC50) of TCP
to fish range from 8700 mg/litre in tidewater silversides
(Dawson et al., 1977) to 4 mg/litre in guppies (Adema et
al., 1983). The composition of the materials that were
used in these experiments was not given.
Tests on guppies showed that a saturated solution of
IMOL S-140 (see Table 6) in water (14 mg/litre) was not
acutely toxic (96-h exposure), but exposures of 4 months
or more at concentrations of 0.3-0.9 mg/litre caused symp-
toms of chronic poisoning in rainbow trout. Initially only
feeding habits and behaviour changed, but later swimming
ability was impaired, and the fish eventually died
(Wagemann et al., 1974; Wagemann, 1975). Fatty tissue
turned a blue-grey colour, the liver enlargened, and the
activities of lactate dehydrogenase (LDH) and glutamic-
oxaloacetic transaminase (GOT) increased (Wagemann et al.,
1974; Wagemann, 1975; Lockhart et al., 1975).
Phosflex 179-C (TOCP) slightly inhibited the acetyl-
cholinesterase activity of an electric ray (Torpedo
electroplax) but did not interfere with binding of
acetylcholine to its receptor (Eldefrawi et al., 1977).
Fish or frogs that received IMOL S-140 or TOCP did not
show, under the test conditions, significant reduction of
brain cholinesterase activity (Lockhart et al., 1975). A
similar observation was made by Cohen & Murphy (1970) on
mice and quail.
6.3 Insects
The toxicity of TCP to insects is presented in Table
12. Most of these data were obtained in the course of
studies on the synergism of TCP or TPP with organophos-
phorus insecticides or juvenile insect hormone mimics.
6.4 Plants
The effects of gaseous TCP on crops covered with vinyl
film have been investigated. TCP emitted from the film
caused a certain amount of leaf shrinking (Inden &
Tachibana, 1975).
The active metabolite of TOCP, saligenin cyclic o-
tolyl phosphate, caused decreased germination of kidney
beans and wheat (Eto et al., 1962).
Table 12. Toxicity of TCP to insects
____________________________________________________________________________________________________
Species TCP Application Age Effecta Concentration Reference
isomer method
____________________________________________________________________________________________________
Mosquito larva ortho in water early 4th 5-day LD13 0.1 mg/litre Quinstad et
( Aedes aegypti) instar [± 19] al. (1975)
(LD2[± 3]
in control)
Mosquito larva in water 4th instar 5-day LD7 0.1 mg/litre Quinstad et
( Culex pipiens [± 7] al. (1975)
quinquefasciatus) (LD5[± 5]
in control)
Mosquito larva in water 4-h instar 24-h LC50 > 1 mg/litre Plapp & Tong
( Culex tarsalis) (1966)
Housefly larva topical 3rd instar 7-day LD12 0.1 mg/g Quinstad et
( Musca domestica) treatment [± 6] al. (1975)
(LD8[± 10]
in control)
Housefly contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Housefly meta contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Mosquito larva in water 4th instar 24-h LD50 > 1 mg/litre Plapp & Tong
( Culex tarsaris) (1966)
Housefly para contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Mosquito larva in water 4th instar 24-h LD50 > 1 mg/litre Plapp & Tong
( Culex tarsaris) (1966)
____________________________________________________________________________________________________
a Values in square brackets are standard deviations.
7. KINETICS AND METABOLISM
Summary
The absorption, distribution, metabolism, and elimination
of organophosphates are critical to the delayed neuropathic
effects of these compounds. In addition, other factors (e.g.,
route of administration, sex, age, strain) affect their meta-
bolic fate and subsequent neurotoxic expression. Variability in
these factors may underline the interspecies variation in the
sensitivity to TOCP-induced delayed neuropathy (i.e. OPIDN).
This correlation has been demonstrated with other OPIDN com-
pounds, but relevant studies on TOCP itself are limited.
Dermal absorption of TOCP in humans appears to be at least
an order of magnitude faster than in dogs. Significant dermal
absorption also appears to occur in cats. Oral absorption of
the compound has been reported in rabbits. There is no direct
information on absorption via the inhalation route.
In cat studies, absorbed TOCP was widely distributed
throughout the body, the highest concentration being found in
the sciatic nerve, a target tissue. Other tissues with high
concentrations of TOCP and its metabolites are the liver, kid-
ney, and gall bladder.
TOCP is metabolized via three pathways. The first is the
hydroxylation of one or more of the methyl groups, and the sec-
ond is dearylation of the o-cresyl groups. The third is further
oxidation of the hydroxymethyl to aldehyde and carboxylic acid.
The hydroxylation step is critical because the hydroxymethyl
TOCP is cyclized to form saligenin cyclic o-tolyl phosphate,
the relatively unstable neurotoxic metabolite.
TOCP and its metabolites are eliminated via the urine and
faeces, together with small amounts in the expired air.
7.1 Absorption
TOCP absorption has been studied in a variety of
species using oral or dermal administration. No infor-
mation is available on absorption following inhalation.
Gross & Grosse (1932) reported that TOCP given orally
(0.1 g/kg in olive oil) was absorbed by rabbits.
Hodge & Sterner (1943) demonstrated poor absorption of
32P-labelled TOCP in a dog after administration of a
single dermal dose of 200 mg/kg. The rate of transfer
(dose: 2-4 mg [32P]-TOCP/kg) through intact human palm
skin appeared to be about 100 times faster than that
through the abdominal skin of the dog. This was based on
urinary excretion and surface area considerations.
Another species, the cat, showed even greater absorp-
tion. When [14C]-TOCP (50 mg/kg) was dermally applied to
adult male cats, the disappearance of radioactivity from
the application site was bi-exponential. In the first
phase, 73% of the TOCP disappeared within 12 h, while the
second phase half-life was 2 days (Nomeir & Abou-Donia,
1984; 1986b).
Studies by Kurebayashi et al. (1985) indicated incom-
plete absorption of tri- p-cresyl phosphate (TPCP) from
the intestine of rats after a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg) in 1.5 ml of dimethyl
sulfoxide. Much of the radioactivity was recovered in the
faeces, predominantly in the form of unchanged TCP.
7.2 Distribution
After a single oral dose of [32P]-TOCP (770 mg/kg)
to chickens, the total radioactivity in liver increased
consistently throughout 72 h. The levels of radioactivity
in the plasma were consistently lower than those in liver;
at 24 h the plasma levels were 5% of those in liver. The
radioactivity was predominantly associated with TOCP
metabolites in liver but with unmetabolized TOCP in blood
(Sharma & Watanabe, 1974).
Following a single dose of [32P]-TOCP (200 mg/kg) to
the abdominal skin of the dog, the radioactivity in the
blood within 24 h was equivalent to an average value of
80 µg/litre and was distributed throughout the visceral
organs, muscle, brain, and bone. The levels of radioac-
tivity in tissues were in the following descending order:
liver > blood > kidney > lung > muscle or spinal
cord > brain or sciatic nerve (Hodge & Sterner, 1943).
In cats given a single dermal dose of 50 mg [14C]-
TOCP/kg, the chemical was absorbed from the skin and sub-
sequently distributed throughout the body. TOCP reached
its highest concentration in plasma at 12 h, and its
metabolites attained their maximum concentration between
24-48 h. The relative residence values of unmetabolized
TOCP in various tissues, relative to the plasma, were:
brain, 0.09; spinal cord, 0.18; sciatic nerve, 2.1; liver,
0.44; kidney, 0.55; lung, 1.27. Parent TOCP was the pre-
dominant compound in the brain, spinal cord, and sciatic
nerve, while the metabolites o- hydroxybenzoic acid and
di- o-cresyl phosphate were predominant in the liver,
kidney, and lung (Nomeir & Abou-Donia, 1984). In contrast,
when measuring total radioactivity sampled 1-10 days post
exposure, highest levels were found in the bile, gall
bladder, urinary bladder, kidney, and liver, with only low
levels in the spinal cord and brain (Nomeir & Abou-Donia,
1986b).
Gross & Grosse (1932) reported that most of the cresol
ester was recovered from the liver (5%) and intestine
(67%) within 2 h after an intravenous injection (0.5 g/kg)
of TOCP into rabbits.
At 24, 72, and 168 h after oral administration of
[14C]-TPCP to rats, the concentrations of radioactivity
in adipose tissue, liver, and kidney were higher than
those in other tissues (Kurebayashi et al., 1985).
7.3 Metabolic transformation
TOCP is metabolized in rats, rabbits, mice, and
chickens to form a neurotoxic esterase inhibitor (Davison,
1953; Aldridge, 1954; Aldridge & Barnes, 1961; Casida,
1961). In rats injected intraperitoneally with TOCP, this
esterase inhibitor was located mainly in the intestine and
liver (Myers et al., 1955). The neurotoxic metabolite was
isolated from the intestine and liver of rats following
TOCP administration and was identified as saligenin cyclic
o-tolyl phosphate [2-( o-cresyl)-4H-1:3:2-benzodioxaphos-
phoran-2-one] (M-1); M2 and M3 in Fig. 1 are also possible
metabolites (Casida et al., 1961; Eto et al., 1962). The
saligenin cyclic o-tolyl phosphate was also found in
chickens (Eto et al., 1962; Sharma & Watanabe, 1974) and
in cats (Taylor & Buttar, 1967; Nomeir & Abou-Donia, 1984;
1986a,b). Although quantitative data are not available,
indirect evidence suggests that cats metabolize this
neurotoxic compound more efficiently than chickens
(Taylor & Buttar, 1967). Two intermediate metabolites,
di-( o-cresyl) mono- o-hydroxymethylphenyl phosphate
[mono-hydroxymethyl TOCP] and di-( o-hydroxymethylphenyl)
mono- o-cresyl phosphate, [di-hydroxymethyl TOCP],
transform to saligenin cyclic o-tolyl phosphate (Eto
et al., 1962; 1967), which is relatively unstable and is
rapidly hydrolysed to inactive metabolic products.
CAS chemical name: phosphoric acid, tri- p-tolyl ester
CAS registry number: 78-32-0
Synonyms: tri- p-cresylphosphate, phosphoric
acid tris(4-methylphenyl) ester,
p-TCP, tri- p-tolyl phosphate,
tri-4-tolyl phosphate, tri-4-methyl-
phenyl phosphate
2.2 Physical and chemical properties
The physical properties of tricresyl phosphate (TCP)
are listed in Table 1.
Table 1. Physical properties of tricresyl phosphate and isomers
________________________________________________________________________________________________________
Physical properties Tricresyl phosphate Tri- o- cresyl Tri- m- cresyl Tri- p- cresyl
(mixtures of isomers) phosphate phosphate phosphate
_________________________________________________________________________________________________________
Physical state liquid liquid half-solid crystalline
solid
Colour colourless colourless colourless colourless
Odour very slightly very slightly very slightly very slightly
aromatic aromatic aromatic aromatic
Melting or -33b 11a 25.6a 77-78a
freezing point (°C)
Boiling point or 241-255 (4 mmHg)b
range (°C) 190-200 (0.5-10 mmHg)c 410 (760 mmHg)a 260 (15 mmHg)a 244 (3.5 mmHg)a
Specific gravity 1.160-1.175 (25 °C)b; 1.1955a 1.150a 1.237a
(density) 1.165c
Refractive index 1.553-1.556 (25 °C)b; 1.5575a 1.5575a
1.556 (20 °)c
Viscosity (cSt) 60 (25 °C), 4.0 (100 °C)c
Flash point (°C) 257c
Vapour pressure (mmHg) 1 x 10-4 (20 °C)c 10 (265 °C)a
Henry's Law constant 1.1-2.8 x 10-6 atm-m3/mold
Solubility in water 0.36e; 0.34 ± 0.04f 0.074g
(mg/litre)
Octanol-water partition 5.11e
coefficient (log Pow) 5.12h
________________________________________________________________________________________________________
a Hine et al. (1981).
b Modern Plastics Encyclopedia (1975).
c Lefaux (1972).
d Boethling & Cooper (1985).
e Saeger et al. (1979).
f Ofstad & Sletten (1985).
g Hollifield (1979).
h Kenmotsu (1980b).
TCP is non-flammable and non-explosive. When the para
isomer was heated at 370 °C with air for 30 min, 99% of
the compound was recovered. The main volatile products
obtained were water, carbon dioxide, toluene, and cresols
(Paciorek et al., 1978). No data on pyrolysis or combus-
tion of TCP at higher temperatures are available (at about
600 °C, triphenyl phosphate begins to decompose, yielding
some aromatic hydrocarbons, some oxygenated aromatic com-
pounds, and phosphoric oxides). Its partition coefficient
between octanol and water (log Pow) is approximately
5.11-5.12.
Hydrolysis of TCP is thought to proceed in an anal-
ogous manner to triphenyl phosphate. It hydrolyses rapidly
in an alkaline solution. Despite a lack of data, neutral
or acidic hydrolysis of TCP, by analogy to TPP, is assumed
to be very slow. The hydrolysis rate constants and half-
lives reported are summarized in Table 2. Formation of
dicresyl phosphate during alkaline hydrolysis would be
expected, but no data are available (Wolfe, 1980).
Table 2. Hydrolysis rate constant (2nd order, K2) and half-lives in
aqueous solution
________________________________________________________________________________
Temper- Rate
Compound Solution ature pH constant Half- Reference
(°C) (M-1.sec-1) life
________________________________________________________________________________
Tri- p-cresyl Water 27 alkaline 2.5 x 10-1 Wolfe (1980)
phosphate 0.2N NaOH/ 22 13 1.66 h Muir et al.
acetone (1983)
(1 : 1)
Tri- m-cresyl 0.1N NaOH/ 22 13 1.31 h Muir et al.
phosphate acetone (1983)
(1 : 1)
________________________________________________________________________________
The photolysis of TAPs in ethanol yielded the corre-
sponding monoaryl phosphate and diphenyl derivatives
(Finnegan, 1972). The results are summarized in Table 3.
2.3 Conversion factor
Tricresyl phosphate 1 ppm = 15.07 mg/m3 air
Table 3. Photolysis of symmetrical triaryl phosphatesa
________________________________________________________________________
Starting Resulting compounds Recovered Quantum yield
compound ---------------------- ester (%) for biaryl
Ar-Ar (%) ArOPO3H2(%) formation
________________________________________________________________________
Phenyl 2 48 6 x 10-4
p-Tolyl 35-51 2-10 13-20 190 x 10-4
p-t-butylphenyl 51 55 24 44 x 10-4
Mesityl 4 7 7 not determined
________________________________________________________________________
a From: Finnegan & Matson (1972).
The esters were irradiated, at a concentration of 0.02 mol/litre
ethanol, using a 450W Hanovia arc lamp, for 5 h.
2.4 Analytical methods
Analytical methods for determining TCP in air, water,
sediment, fish, biological tissues, and edible oils are
summarized in Table 4. The method of choice is gas chro-
matography (GC) with a nitrogen-phosphorus sensitive de-
tector (GC/NPD) or a flame photometric detector (GC/FPD).
The detection limit in water samples is at the ng/litre
level. Using GC, TCP and other trialkyl/aryl phosphates,
such as triphenyl phosphate (TPP), trioctyl phosphate, and
trixylenyl phosphate, can be simultaneously determined.
High-performance liquid chromatography (HPLC) and thin-
layer chromatography (TLC) are sometimes used for deter-
mining TCP, but these are not widely applicable.
It should be noted that the behaviour of TCP in ana-
lytical processes and in its environmental distribution is
similar to that of other TAPs, lipids, and phthalic acid
esters, owing to analogous physical and chemical proper-
ties.
2.4.1 Extraction and concentration
TCP is easily extracted from aqueous solution with
methylene chloride, hexane, or benzene (Kenmotsu et al.,
1980a; Muir et al., 1981). Low levels of TCP in water can
be successfully concentrated on an Amberlite XAD-2 resin
column (Lebel et al., 1981; Lebel & Williams, 1983). TCP
has been extracted from sediment with various polar sol-
vents, such as aqueous methanol (Muir et al., 1980, 1981),
acetonitrile (Kenmotsu et al., 1980a), or acetone
(Ishikawa et al., 1985). The extraction method established
by the US Association of Official Analytical Chemists
(AOAC) for organochlorine and organophosphorus pesticides
is also applicable for the extraction of TCP from fat-
containing foods and fish (Lombardo & Egry, 1979). Hexane
(Lombardo & Egry, 1979), methanol (Muir et al., 1980,
1981; Muir & Grift, 1983), acetonitrile and methylene
chloride (Kenmotsu et al., 1979), and acetone-hexane
(Lebel & Williams, 1983) have been used for the extraction
of TCP from fish or adipose tissue. Workplace airborne
samples can be collected on Millipore(R) filters and the
particulate TCP analysed (US NIOSH, 1977, 1979, 1982).
Vapour phase and particulate TCP in the atmosphere have
been simultaneously collected on glycerol-coated
Florisil(R) columns and 96% of the TCP recovered (Yasuda,
1980). The Midwest Research Institute (MRI/USA) has used
high-volume air filter pads and activated carbon filters
to sample ambient air (MRI, 1979).
2.4.2 Clean-up procedures
Florisil column chromatography has been used routinely
for clean-up of TCP (Lombardo & Egry, 1979; Kenmotsu et
al., 1980a; Lebel & Williams, 1983). The separation of TCP
from tributyl phosphate (TBP) and parathion is possible by
this procedure but is more difficult than for other TAPs
such as trixylenyl phosphate (Kenmotsu et al., 1981b;
Lebel & Williams, 1983). Sulfur-containing compounds,
which often exist in sediment samples and interfere with
the analysis of TCP by GC/FPD, can easily be separated by
elution with hexane from Florisil columns (Kenmotsu et
al., 1980a). Partitioning between acetonitrile and pet-
roleum ether is useful to separate TCP from fish fat
(Lombardo & Egry, 1979; Kenmotsu et al., 1980a). Since
the polarity of TCP is similar to that of lipids in bio-
logical tissues, it is difficult to separate TCP from
lipids by Florisil column chromatography. Gel permeation
chromatography (GPC) is useful in this case (Muir et al.,
1981), the elution volume varying according to the
type of phosphate ester, i.e. trialkyl-, triaryl-, or
tri(haloalkyl) phosphates (Lebel & Williams, 1983). Acti-
vated charcoal column chromatography (Kenmotsu et al.,
1980a), alumina column chromatography (Muir et al., 1980,
1981), and C-18 bonded silica cartridge (Sep-pak C-18)
(Muir et al., 1980; Muir & Grift, 1983) have also been
used to separate TCP from co-extracting compounds in vari-
ous samples.
Table 4. Methods for the determination of TCP and TPP
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
Workplace collect with Millipore GC/FPD 1 µg per TCP and TPP US NIOSH (1982)
air filter, extract with sample
ethanol
Environmental trap with glycerol- GC/FPD 1 ng/m3 simultaneous Yasuda (1980)
air Florisil column, eluate method for
with methanol, add trialkyl/aryl
water, and extract with phosphates
hexane
Air collect by aspiration TLC 5 ng/plate TCP and TPP Druyan (1975)
through ethanol,
hydrolyse with NaOH; the
resultant phenols are
reacted with
p-O2NC6H4N2+ and
separated with silica
gel plate
Drinking-water adsorb with XAD-2 GC/NPD 1 ng/litre method for Lebel et al.
resin, eluate with GC/MS low level (1979, 1981)
acetone-hexane or trialkyl/aryl
acetone phosphates
River or sea extract with GC/NPD 0.02 µg/litre simultaneous Kenmotsu et al.
water methylene chloride GC/FPD (TPP) method for (1980a, 1981b,
or benzene GC/MS 0.05 µg/litre trialkyl/aryl 1982b)
(TCP) phosphates Muir et
al. (1981)
Ishikawa et
al. (1985)
Farm pond reflux with methanol- GC/NPD 1 ng/g simultaneous Muir et al.
sediment water (9+1) or method for (1980, 1981)
methylene chloride- triaryl
methanol (1+1), phosphates
clean-up by acid
alumina column
chromatography
Table 4. (contd.)
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
River or sea extract with GC/FPD 5 ng/g simultaneous Kenmotsu et al.
sediment acetonitrile or GC/MS method for (1980a, 1981b,
acetone, clean-up by trialkyl/aryl 1982a, 1982b,
charcoal or Florisil phosphates 1983)
column chromatography Ishikawa et al.
(1985)
Fish extract with hexane GC/NPD 1 ng/g simultaneous Muir et al.
or methanol, clean-up GC/MS method for (1980, 1981,
by gel permeation triaryl 1983)
column chromatography phosphates
and acid alumina
column chromatograpy
Fish extract with GC/FPD 5 ng/g simultaneous Kenmotsu et
acetonitrile and GC/MS method for al. (1980a)
methylene chloride, trialkyl/aryl
clean-up by phosphates
acetonitrile-hexane
partitioning,
charcoal column
chromatography,
concentrated sulfuric
acid extraction and
Florisil column
chromatography
Human adipose extract with benzene GC/NPD 1 ng/g simultaneous Lebel & Williams
tissues or acetone-hexane (15 GC/FPD method for (1983)
+ 85), clean-up by GC/MS trialkyl/aryl
gel permeation phosphates
chromatography and
Florisil column
chromatography
Table 4. (contd.)
________________________________________________________________________________________________________
Sample type Sampling method Analytical Limit of Applicability Reference
extraction/clean-up method detection
________________________________________________________________________________________________________
Plasma extract with ethyl HPLC 50 ng/injection TCP and its Nomeir &
ether, filter with (254 nm) metabolites Abou-Donia
0.45-µm nylon filter (1983)
Edible oils extract with ethanol, colori- 0.01% simple method Vaswani et
hydrolyse with NaOH; metric for TCP al. (1983)
the resultant cresol determination
is coupled with 2,6-
dichlorobenzoquinone
Edible oils separate with silica TLC simple method Bhattacharyya
gel G thin-layer (UV) for TCP et al.(1974)
plate; spray determination
rhodamine B solution
________________________________________________________________________________________________________
2.4.3 Gas chromatography and mass spectrometry
To identify TCP in environmental samples by packed
column GLC, it is useful to compare retention times using
two types of liquid phase with different polarities. As a
low polarity liquid phase, 10% OV-1 (Kenmotsu et al.,
1980a), 3% SE-30 (Ramsey & Lee, 1980), 3% OV-17 (Lebel et
al., 1981), 3% OV-101 (Deo & Howard, 1978), SP-2100 (Muir
et al., 1980), and 5% DC-200 (Daft, 1982) have been used,
while 1% QF-1 (Bloom, 1973), 5% FFAP and 5% Thermon-3000
(Kenmotsu et al., 1980a), and 2% DEGS (Daft, 1982) have
been used as a higher polarity liquid phase.
TCP is often accompanied by other TAPs in environmen-
tal samples, which show multiple peaks in GC and occasion-
ally have the same retention indices as that of TCP
(Ramsey & Lee, 1980; Kenmotsu et al., 1982b). Therefore,
capillary GLC or GC-mass spectrometry (GC/MS) is preferred
(Lebel et al., 1981; Lebel & Williams, 1983; Kenmotsu et
al., 1983; Ofstad & Sletten, 1985). In electron impact
mass spectrometry, TCP gives a high intensity molecular
ion, as do other TAPs (Deo & Howard, 1978; Wightman &
Malaiyandi, 1983; Kenmotsu et al., 1982b). A selected ion
monitoring (SIM) technique is also useful for trace analy-
sis of TCP in environmental samples (Ishikawa et al.,
1985), but care must be taken to select suitable fragment
ions in order to avoid interference by other TAPs.
The phenolic components of TCP are confirmed by alka-
line hydrolysis, followed by GLC analysis of the resulting
phenols (Murray, 1975; Sugden et al., 1980).
2.4.4 Contamination of analytical reagents
The widespread use of TCP in plastics and hydraulic
fluids can cause contamination of analytical reagents.
Traces of TCP have been found in rubber O-rings and rubber
seals used in a Corning water supply system (Lebel et al.,
1981), Super Q water (Williams & Lebel, 1981), and aceto-
nitrile, methylene chloride, and hexane (Daft, 1982).
Trialkyl phosphates have also been found in cyclohexane
(Bowers et al., 1981), hexane (Hudec et al., 1981), and
analytical grade filters (Daft, 1982). Therefore, care
must be taken to avoid contamination of analytical re-
agents in order to obtain accurate data in trace analysis
of TCP.
2.4.5 Other analytical methods
A rapid colorimetric method has been developed for the
determination of TCP in edible oil (Vaswani et al., 1983),
but no information about the interference with other TAPs
is available. Silica gel TLC has been used for deter-
mining TCP in edible oil (Bhattacharyya et al., 1974;
Krishnamurthy et al., 1985). Reversed phase TLC has also
been used (Renberg et al., 1980). However, separating TAPs
from each other by TLC is not sufficient (Bloom, 1973).
HPLC with a C-18 bonded column has been used for deter-
mining TCP in plasma, while size exclusion HPLC has been
used in the case of machine oil (Majors & Johnson, 1978).
An ultraviolet spectrometric detector is usually used in
HPLC, but it is not specific for TAPs. Tittarelli &
Mascherpa (1981) described a highly specific HPLC detec-
tor for TAPs using a graphite furnace atomic absorption
spectrometer.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production levels and processes
Tricresyl phosphate does not occur naturally in the
environment. Figures concerning the total world production
are not available. In Japan, 33 000 tonnes were produced
in 1984a. In the USA, approximately 54 000 tonnes of TAP
including 10 400 tonnes of TCP were produced in 1977
(Boethling & Cooper, 1985). About 800-1000 tonnes TCP per
year is now produced in China.
TCP is usually produced by the reaction of cresols
with phosphorus oxychloride. One of the industrial sources
of cresols is the so-called cresylic acid or tar acid,
which is a mixture of isomers of cresol and varying
amounts of xylenols, phenol, and other high-boiling phe-
nolic fractions obtained as a residue from coke ovens and
petroleum refining (Duke, 1978). Another source is
"synthetic cresol", prepared from cymene via oxidation
and catalytic degradation (Association of the Plasticizer
Industry of Japan, 1976), and this has been used for pro-
duction of TCP in Japan since 1971. The composition of
some cresylic acids and synthetic cresol is shown in
Table 5.
TCP derived from these alkylphenols is, therefore, a
complex mixture of various TAPs, i.e. tri- o-cresyl phos-
phate, tri- m-cresyl phosphate, tri- p-cresyl phosphate,
di- m-cresyl- p-cresyl phosphate, di- p-cresyl- m-cresyl phos-
phate, etc. The very toxic tri- o-cresyl phosphate
(TOCP) is usually excluded as much as possible. In some
cases, commercial tricresyl phosphate (TCP) has been
reported to contain a small amount of TPP (Daft, 1982;
Ofstad & Sletten, 1985).
The most noteworthy trend in aryl phosphate manufac-
ture and use in the USA has been the replacement of tri-
phenyl, tricresyl, and trixylenyl phosphates derived from
petroleum-based feedstocks by aryl phosphates derived from
synthetic precursors. The production of cresyl diphenyl
phosphate, a petroleum-based aryl phosphate, was discon-
tinued in 1979 in the USA. The mixed trialkyl/aryl phos-
phates are replacing TPP and TCP as a plasticizer, whereas
the synthetic TAPs are replacing TCP and trixylenyl phos-
phate in functional fluids (Boethling & Cooper, 1985).
______________
a Personal communication from the Association of the
Plasticizer Industry of Japan, 1985.
Table 5. Composition of some commercial cresylic acidsa and
"synthetic cresol"b
______________________________________________________________
Composition (%)
Boiling Cresylic Acids Synthetic
Constituents point Sample Sample Sample Cresol
(°C) A B C
______________________________________________________________
o- cresol 191.0 3 0 0 0.1%
2,6-Xylenol 201.0 6 6 0
m- Cresol 202.2 42 43 47 \
p- Cresol 202.3 30 31 34 /99%
o- Ethylphenol 204.5 3 3 0
2,4-/2,5-Xylenol 211.5 16 17 19
______________________________________________________________
a From: Bondy et al. (1960).
b From: Association of the Plasticizer Industry of Japan
(1976).
3.1.1 Accidental release
Liquid TCP and hydraulic fluid and lubricant oil con-
taining phosphate esters are transported by tank trucks,
rail cars, and to a lesser extent in barrels (US NIOSH,
1979). Occasionally, empty barrels (or drums) previously
containing hydraulic fluid or lubricant oil have been
reused to store or to transport edible oil (or water), and
this has resulted in poisoning of humans and cattle
(Susser & Stein, 1957; Smith & Spalding, 1959; Chaudhuri,
1965; Nicholson, 1974; Senanayake, 1981). Another case of
poisoning involved flour contaminated with oil from a leak
during shipping (Sorokin, 1969).
In a report by Beck et al. (1977), accidental spillage
of TAPs intended for use in pipeline pumping stations oc-
curred and resulted in poisoning of cattle. Effluent from
an evaporation pond overflowed onto the pasture during
spring run-off. Sampling showed concentrations of TAPs
from 0.304% to 3.44% by weight in soil, grass, and water
near the plant. Thirty days later TAPs were still present
in the evaporation pond but not in the soil samples
(Chemical and Geological Laboratories Ltd., 1971).
Beck et al. (1977) described in his report: "Mass
poisonings are possible because large quantities of tri-
aryl phosphate are used as lubricants and coolants in jet
engines in pipeline compressor stations. If an emergency
arises as much as 1200 gallons of this material can be
expelled into the atmosphere within 20 seconds. The con-
struction of pipelines over thousands of miles, with
manned or unmanned compressors every 100 miles, consti-
tutes an environmental hazard to both domestic livestock
and wildlife."; and "Natural leaching of the ground by
weather conditions probably removed the poison from the
soil, but repeated spills of large quantities of a stable
compound could contaminate ground water supplies".
3.2 Uses
TCP is used as a plasticizer in vinyl plastic manufac-
ture, as a flame-retardant, a solvent for nitrocellulose,
in cellulosic molding compositions, as an additive to
extreme pressure lubricants, and as a non-flammable fluid
in hydraulic systems (Windholz, 1983). The main market for
PVC-based products plasticized with organic phosphate
esters is in the manufacture of automobile and other motor
vehicle interiors in the USA (Lapp, 1976). In Japan, ap-
proximately 2500 tonnes of TCP was used in 1984 as a plas-
ticizer in PVC film for agricultural use, 400 tonnes as
non-flammable plasticizer in floor and wall covering, and
100 tonnes for miscellaneous purposes (Association of the
Plasticizer Industry of Japan, 1985).
The fastest growing use of organic phosphate esters is
in the manufacture of fire-resistant hydraulic fluids and
lubricants in the USA (Lapp, 1976). The two types of
organic phosphate hydraulic fluids being manufactured are
phosphate ester oil blends and "pure synthetics". The
phosphate ester oil blends contain between 30% and 50%
organic phosphate esters in addition to petroleum oil and
coupling agents; the "pure synthetics" contain a mixture
of organic phosphate esters. For example, a typical syn-
thetic organic phosphate fluid contains TCP, trixylenyl
phosphate, and other TAPs. The compositions of several
commercial synthetic organic phosphate fluids are listed
in Table 6. Organic phosphate ester lubricant additives
are usually of three general types: extreme pressure
agents, anti-wear agents, and stick-slip moderators. The
first two types are used in systems with some type of
gears and account for over 80% of all organic phosphate
lubricant additives. These agents are also used in cut-
ting oils, machine oils, transmission fluids, and cooling
lubricants (Lapp, 1976).
In Japan, approximately 300 tonnes of TCP was used for
lubricant additives in 1985 (Association of the Plasti-
cizer Industry of Japan, 1985), and approximately 1320
tonnes of TAPs was used in 1976 in Ontario, Canada (Muir
et al., 1980).
There are other minor uses of TCP: additives in making
synthetic leather (Franchini et al., 1978), shoes (Pegum,
1966), and polyvinyl acetate products (Anon., 1986); sol-
vent for acrylate lacquers and varnishes (Anon., 1986); in
non-smudge carbon paper (Hjorth, 1962; Pegum, 1966).
Table 6. Composition of various commercial organophosphorus hydraulic fluids and lubricants
________________________________________________________________________________________________________
Component (%)
________________________________________________________
Name TPP TCP Others Producer Reference
________________________________________________________________________________________________________
IMOL S-140 1 2 (ortho) Tris(dimethylphenyl)- (18) Imperial Oil Ltd. Lockhart
42 (meta) Tris(ethylphenyl)- (6) et al.
31 (para) Tris(trimethylphenyl)- (1975)
and unknown (1)
Pydraul 50E 36 nonylphenyl diphenyl phosphate (40) Monsanto Co. Nevins &
cumylphenyl diphenyl phosphate (22) Johnson
(1978)
Pydraul 115E 7 nonylphenyl diphenyl phosphate (29)
cumylphenyl diphenyl phosphate (62)
Pydraul 50E 18.4 nonylphenyl diphenyl (52.8) Monsanto Co. Deo &
cumylphenyl diphenyl (24.0) Howard
(1978)
Kronitex TCP 20.7 (meta) dicresyl xylenyl (9.2) FMC Corp. Deo &
38.8 (di- Howard
meta, para) (1978)
30.4 (di-
para, meta)
Santicizer- 14.7 19.4 m-cresyl diphenyl (18.6) Monsanto Co. Deo &
140 CDP p-cresyl diphenyl (14.4) Howard
phenyl dicresyl (29.4) (1978)
Fyrquel GT 19.2 m-cresyl diphenyl (2.1) Stauffer Chem. Co. Deo &
phenyl dicresyl (3.2) Howard
dicresyl xylenyl (36.2) (1978)
di(C3-phenyl) xylenyl (37.1)
Phosflex 41-P 11.9 40.8 m-cresyl diphenyl (2.1) Stauffer Chem. Co. Deo &
trixylenyl (9.4) Howard
(C3-phenyl)3 (28.7) (1978)
________________________________________________________________________________________________________
________________________________________________________________________________________________________
Component (%)
_______________________________________________________
Name TPP TCP Others Producer Reference
________________________________________________________________________________________________________
Fyrquel 220 phosphates derived from Imperial Oil Ltd. Pickard et
phenol (2.6); o-cresol (0.5); m- and al. (1975)
p-cresol (13.6); 2-ethylphenol (0.6)
2,4- and 2.5-xylenol (22.3); mixed
xylenol (49.2); 3,4-xylenol (8.6);
6-9 phenolics (1.3); 2,4,6-trimethyl
phenol (1.4)
Kronitex 100 18 diphenyl 2-isopropylphenyl (27) FMC Corp. Nobile et
diphenyl 4-isopropylphenyl (11) al. (1980)
tris(2-isopropylphenyl) (11)
phenyl di-(2-isopropylphenyl) (7)
phenyl di-(4-isopropylphenyl) (5)
Kronitex 50 33 diphenyl 2-isopropylphenyl (21) FMC Corp. Nobile et
diphenyl 4-isopropylphenyl (12) al. (1980)
tris(2-isopropylphenyl) (8)
phenyl di-(2-isopropylphenyl) (6)
phenyl di-(4-isopropylphenyl) (2)
________________________________________________________________________________________________________
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Summary
The majority of TCP release to the environment is accounted
for by end-point use rather than production. Total release to
the environment in the USA was estimated at 32 800 tonnes in
1977.
TCP released into water is readily adsorbed on to sediment
particles, and little or none remains in solution.
TCP is readily biodegraded in sewage sludge with a half-
life of 7.5 h, the degradation within 24 h being up to 99%. TCP
is almost completely degraded within 5 days in river water. The
ortho isomer is degraded slightly faster than the meta or para
isomers. Abiotic degradation is slower with a half-life of 96
days.
TCP has, because of its physico-chemical properties, a high
potential for bioaccumulation. Laboratory studies of continuous
exposure to high concentrations (which are environmentally
unrealistic) of radiolabelled TCP have shown high bioconcen-
tration factors (BCF). However, these studies failed to show
that the isotope was still associated with the original com-
pound. Taking into account the ready biodegradability of TCP,
these data should be viewed as probable overestimates, and it
is suggested that little bioaccumulation would occur with
environmentally realistic TCP exposure.
4.1 Transport and transformation in the environment
4.1.1 Release to the environment
Total losses of aryl phosphates to the environment in
the USA from production in 1977 were estimated as 2585
tonnes, the main source being land disposal of manufactur-
ing wastes (2540 tonnes). Releases from end-product use
(32 800 tonnes in the USA in 1977) are estimated to be
much greater than from production. The amount of volatil-
ization and leaching from plastic items was 16 300 tonnes
and that of leakage of hydraulic fluids and lubricants
13 400 tonnes (Boethling & Cooper, 1985). One major hy-
draulic fluid manufacturer estimated that as much as 80%
of the annual consumption of aryl phosphate hydraulic
fluids is used to make up for leakage (MRI, 1979).
No data are available on the release to the atmosphere
of TCP from production processes. However, open, high-tem-
perature processes such as roll milling, calendering, and
extrusion of plasticized polymers may result in signifi-
cant gaseous emissions of aryl phosphates (Boethling &
Cooper, 1985).
Yasuda (1980) detected significant levels of TCP in
urban air and in the atmosphere over coastal waters near
industrialized areas. Details are described in section
5.1.1.
The results of a study by the US Environmental Protec-
tion Agency (MRI, 1979) showed that TCP can evaporate from
automotive upholstery fabric and condense on the interior
surface of a relatively cool window.
The emission of TCP from waste incineration plants may
also be a pathway to the atmosphere. In a study by Vick et
al. (1978), TCP was not detected in vapour samples taken
before and after the dust collectors of incinerators.
4.1.2 Fate in water and sediment
The solubility of TCP in water is low (Table 1). Moni-
toring studies have shown trialkyl/triaryl phosphates to
be present in water and sediment sampled near major indus-
trialized areas (Konasewich et al., 1978; Sheldon & Hites,
1978, 1979; Mayer et al., 1981; Williams & Lebel, 1981;
Williams et al., 1982; Ishikawa et al., 1985; Fukushima &
Kawai, 1986). However, TCP was only occasionally detected
in water samples, whereas TPP was often detected (Mayer et
al., 1981; Williams & Lebel, 1981; Williams et al., 1982;
Ishikawa et al., 1985). The total concentrations of
Pydraul (Table 6) components in river (0.24 µg/litre) and
lake sediments (570 µg/kg) in the USA revealed a water-
sediment difference of more than 3 orders of magnitude
(Mayer et al., 1981). Equilibrium of TCP with the bottom
sediment in a shallow (0.5-m depth) pond would be expected
to be reached rapidly, as in the case of TPP (Muir et al.,
1982). The adsorption coefficient of TCP on marine sedi-
ment was found to be 420 (Kenmotsu et al., 1980b).
It is apparent from the following data that TCP is
rapidly adsorbed onto river sediment: the level of total
aryl phosphate in the sediment of the Kanawha River (USA)
was 229 mg/kg at the FMC plant outfall but only 4.4 mg/kg
13 km downstream (Boethling & Cooper, 1985).
Wagemann et al. (1974) and Wagemann (1975) reported
that a commercial synthetic lubricating oil, IMOL S-140
(Table 6), degraded in sterilized river water under lab-
oratory fluorescent light and under sunlight at 25 °C, and
that the first order rate constant and half-life were,
respectively, 9 x 10-3 days-1 and 96 days (Wagemann,
1975).
4.1.3 Biodegradation
River die-away studies by Saeger et al. (1979) on nine
phosphate esters demonstrated that these esters, exposed
to the natural microbial population of the river, under-
went primary biodegradation at moderate to rapid rates. A
200-µg portion of TCP was completely degraded within 4
days in 200 ml of Mississippi River (USA) water at room
temperature. Hattori et al. (1981) also investigated the
degradation of TCP in Neya and Oh River water (Osaka,
Japan). After a lag period of 1-2 days, the TCP (1 mg per
litre) was almost completely degraded within 5 days under
non-sterilized conditions, whereas no degradation in heat-
sterilized water occurred during 15 days. In clear non-
sterilized sea water, however, the degradation was very
slow. Saeger et al. (1979) also found that in sterile
river water there was no significant evidence of non-
biological degradation or loss. Among the isomers of TCP,
the ortho isomer degraded in river water slightly faster
than the meta isomer and both isomers degraded faster than
the para isomer, which degraded about as fast as TPP
(Howard & Deo, 1979).
Primary biodegradation rates from semicontinuous acti-
vated sludge (SCAS) studies (US Soap and Detergent Assoc.,
1965; Mausner et al.,1969) showed generally the same trend
in degradation rates as river die-away studies. At a 24-h
feed level of 3-13 mg/litre, TCP showed 99% degradation.
The ultimate biodegradability of TCP was measured by
Saeger et al. (1979) using the apparatus and procedure
developed by Thompson & Duthie (1968) and modified by
Sturm (1973). At 26.4 mg TCP/litre, the carbon dioxide
evolution reached 82% of its theoretical value.
For alkyl-aryl and triaryl phosphates, increasing the
number and size of substituent groups on the phenyl mol-
ecule decreases the biodegradability (Saeger et al.,
1979).
The degradation pathway for TCP most probably involves
stepwise enzymatic hydrolysis to orthophosphate and
phenolic moieties (Barrett et al., 1969; Pickard et al.,
1975). The phenol would then be expected to undergo
further degradation. Dagley & Patel (1957) demonstrated
that p-cresol is oxidized to p-hydroxybenzoic acid by a
species of Pseudomonas. Ku & Alvarez (1982) studied the
biodegradation of [14C]-tri- p-cresyl phosphate in a
laboratory model sewage treatment system, and, in 24-h
experiments, found that 70-80% of the TCP (added at
1 mg/litre) was degraded, with a half-life of 7.5 h. The
major metabolite extracted with ethyl ether from the
aqueous phase was identified as p-hydroxybenzoic acid by
thin-layer chromatography and gas chromatography-mass
spectrometry, while two other radioactive spots remained
unidentified.
4.1.4 Water treatment
Data from FMC Corporation (USA) show that TCP (6.23
mg/litre) in waste water was reduced to 0.23 mg/litre in
the effluent water by biological treatment, whereas the
aryl phosphates with higher relative molecular mass (>452)
(and, therefore, more highly substituted) were not easily
removed (Boethling & Cooper, 1985). Fukushima & Kawai
(1986) reported that TCP (0.186-9.31 µg/litre) in raw
water was reduced to 0.078 µg/litre or less in treated
water by conventional waste water treatment. Filtration of
effluent samples through 1-µm pore size filters resulted
in a further removal of 93% of total aryl phosphates,
again demonstrating the adsorptive behavior of these com-
pounds (Boethling & Cooper, 1985).
4.2 Bioaccumulation and biomagnification
4.2.1 Fish
Data on the bioconcentration and depuration of TCP are
given in Table 7. None of the exposures were considered to
be representative of realistic environmental levels. More-
over the bioconcentration factor (BCF) measured in the
laboratory must be considered as a bioaccumulation poten-
tial rather than an absolute bioaccumulation factor (Veith
et al., 1979).
Several equations have been used in attempts to
predict the BCF of organic chemicals in various fish
strains using the octanol-water partition coefficient
(Pow) or water solubility values (Neely et al., 1974; Lu
& Metcalf, 1975; Kanazawa, 1978; Veith et al., 1979;
Sasaki et al., 1982).
The clearance of tri- m-cresyl phosphate has been
shown to be biphasic, with higher rates of clearance in
the first 6 days after transfer to clean water, especially
for rainbow trout. The clearance rate constants for rain-
bow trout were about 50% more than those for fathead
minnows (Muir et al., 1983).
4.2.2 Plants
The uptake and translocation of tri- p-cresyl phos-
phate by soybean plants has been studied by Casterline et
al. (1985), the initial concentration in soil being 10 mg
per kg. Approximately 70% of the compound had disappeared
from the soil within 90 days (when the plants were har-
vested). At that time, the amount per plant was 34 µg
(0.17% of the applied TCP). Of this total plant content,
74% was found in the stem, 24% in the leaves, and 2% in
the pods. The seeds contained no detectable tri- p-
cresyl phosphate.
Table 7. Bioaccumulation and clearance of tricresyl phosphate by fish
________________________________________________________________________________________________________
Flow/ Analy- Exposure Uptake Clearance Depura-
Species Com- stat tical BCF concent- rate rate tion Reference
pound (temp.) methoda (K1/K2) ration (k1, (k2 x half-life
(mg/litre) h-1) 103,h-1) (hr)
________________________________________________________________________________________________________
Rainbow para stat TR 2768 ± 641b 0.005-0.05 9.6-13.3 72.2 Muir
trout isomer (10°C) TR 1420 ± 42c 14.0 et al.
( Salmo TR 1466 ± 138d 17.0 (1983)
gairdneri ) HER 770 ± 24c 65.4
meta TR 1162 ± 313b 11.5-24.2 30.3
isomer TR 784 ± 82c 18.5
TR 1102 ± 137d 21.2
HER 310 ± 52c 25.8
Fathead para stat TR 2199 ± 227b 0.005-0.05 7.0-9.6 90.0 Muir
minnows isomer (10°C) TR 928 ± 78c 4.9 et al.
( Pimephales TR 588 ± 129d 9.6 (1983)
promelas ) HER 709 ± 76c 73.7
meta TR 1653 ± 232b 8.5-14.7 59.2
isomer TR 596 ± 103c 7.9
TR 385 ± 92d 8.7
HER 62 ± 3c 53.3
commer- flow GC- 165 0.0316 Veith et
cial (25°C) FPD al. (1979)
Bluegill TCP
( Leptomis para TR 1589 Sitthich-
macrochirus ) isomer aikasem
(1978)
________________________________________________________________________________________________________
a GC-FPD = gas chromatography (flame photometric detector) after suitable extraction;
TR = total radioactivity; HER = hexane-extractable radioactivity.
b BCF was calculated by the "initial rate method".
c The static test method was used (Zitko, 1980).
d k1 and k2 were derived by non-linear regression calculation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Summary
TCP has been measured in the atmosphere in Japan at concen-
trations up to 70 ng/m3 but only reached 2 ng/m3 at a pro-
duction site in the USA. Workplace air in the USA contained
less than 0.8 mg/m3 at a lubrication oil barrel-filling oper-
ation and 0.15 mg/m3 (total phosphates) in an automobile zinc
die-casting plant. The concentrations of TCP measured in
drinking-water in Canada were low (0.4 to 4.3 ng/litre), and
TCP was undetectable in well-water. Levels in river and lake
water are frequently considerably higher. However, this is due
to the presence of suspended sediment to which TCP is strongly
adsorbed. Concentrations up to 1300 µg/kg in river sediment
and 2160 µg/kg in marine sediment have been measured.
Elevated TOCP levels in soil and vegetation have been found
within the perimeter of production plants.
Residues of TOCP in fish and shellfish up to 40 µg/kg have
been reported but the majority of animals sampled contained no
detectable amounts.
5.1 Environmental levels
TCP has been found in air, water, soil, sediment, and
aquatic organisms. However, the levels of TCP in environ-
mental samples are low (Table 8), except in soil and sedi-
ment collected in heavily industrialized areas (Table 9).
5.1.1 Air
Yasuda (1980) measured the distribution of various
organic phosphorus compounds in the atmosphere over the
eastern Seto Inland Sea, Japan. Near the heavily industri-
alized cities (Fukuyama, Akashi, Osaka), 11.5-21.4 ng
TCP/m3 was detected. Yasuda (1980) also measured levels
of phosphate esters in the atmosphere above the Dogo Plain
and the Ozu Basin agricultural area of Western Shikoku.
TCP was detected only in the urban air of Matsuyama, where
the level was 26.7-70.3 ng/m3. TCP levels of 0.01-2 ng/m3
in air collected at production sites in the USA have been
reported (MRI, 1979).
Table 8. Concentration of TCP in environmental air, water, sediment, and fish at various locations
______________________________________________________________________________________________________
Locations Year Sample Concentration Number of Reference
samples
(detected/
analysed)
______________________________________________________________________________________________________
Shikoku (Japan) 1976 atmosphere 26.7-70.3 ng/m3 (3/19) Yasuda
(1980)
Eastern Seto 1977 atmosphere 11.5-21.4 ng/m3 (3/4)
Inland Sea (Japan)
Japan (various 1975 river and sea water ND (50-1500 ng/litre)a (0/100) EAJ (1977)
locations) river and sea sediment 150 ng/g (1/100)
fish ND (20-250 ng/g)a (0/100
Japan (various 1978 river and sea water ND (50-2500 ng/litre)a (0/114) EAJ (1979)
locations) river and sea sediment 1060-2160 ng/g (3/114)
fish ND (0.25-150 ng/g)a (0/98)
Osaka (Japan) 1976 river water 100-9500 ng/litre (11/13) Kawai et
al. (1978)
Eastern Ontario 1978 drinking-water 0.3 ng/litre (1/12) Lebel et
water treatment al. (1981)
plant (Canada)
Tokyo (Japan) 1978 river water ND (50 µg/litre)a (0/12) Wakabayashi
sea water ND (50 µg/litre)a (0/3) (1980)
river sediment 7-370 ng/g (9/10)
sea sediment 4 ng/g (1/3)
Canada (various 1979 drinking-water 0.7-4.3 ng/litre (7/60) Williams &
locations) Lebel (1981)
Great Lake 1980 drinking-water 0.4-1.8 ng/litre (5/12) Williams et
(Canada) al. (1982)
Kitakyushu 1980 river water 67-259 ng/litre (3/16) Ishikawa et
City (Japan) sea water ND (20 ng/litre)a (0/9) al. (1985)
sea sediment ND (10 ng/g)a (0/6)
Seto Inland 1980 fish and shell fish 1-19 ng/g (4/41) Kenmotsu et
Sea (Japan) al. (1981a)
_____________________________________________________________________________________________________
a Range of detection limits due to analytical methods used; ND = not detected.
Table 9. Concentration of TCP detected near the producers and users of trialkyl/aryl phosphates
_____________________________________________________________________________________________
Locations Year Sample Concentration Number of Reference
samples
(detected/
analysed)
_____________________________________________________________________________________________
Near TAPs manufacturing fish 2-5 ng/g Muir (1984)
plants (USA)
Columbia River (USA) fish (sturgeon) 40 ng/g Lombardo &
Egry (1979)
Kanawha River (USA) 1978 river water 20 000 ng/litre Boethling &
Cooper (1985)
FMC Corp., Nitro, MV 1979 air (HV)a 2 ng/m3 Boethling &
(USA) Cooper (1985)
Stauffer Chemical Co., 1979 air (HV)a 0.01-0.05 ng/m3 (2/4) Boethling &
Gallipolis Ferry, MV vegetation 1000-20 000 ng/g (4/4) Cooper (1985)
(USA) soil 1000-4000 ng/g (4/4)
FMC Corp. Plant (USA) 1980 waste water 6.23 mg/litre Boethling &
effluent water 0.23 mg/litre Cooper (1985)
Baltimore Harbour 1983 sediment 400-600 ng/g (2/3) Boethling &
(USA) Cooper (1985)
Detroit River, mouth 1983 sediment 230-1300 ng/g (2/2) Boethling &
(USA) Cooper (1985)
_____________________________________________________________________________________________
a HV = High volume filter pad (air sampler).
5.1.2 Water
Although there have been many monitoring studies for
TAPs in water, TCP has not often been detected in natural
water. Where present, it is only at low levels. According
to the annual reports of the Environment Agency of Japan,
TCP has not been detected in river or sea water at any
sampling points. Due to the variety of analytical methods
and procedures used, the detection limits varied between
5 and 2500 ng/litre between different laboratories (EAJ,
1977; 1979; 1981). Kawai et al. (1978) detected TCP at
100-9500 ng/litre in river water sampled in Osaka (Japan),
and found that the concentration of TCP in river water
tended to parallel the concentration of suspended solid.
Ishikawa et al. (1985) detected TCP levels of 67-259
ng/litre in 3 out of 16 samples of river water in
Kitakyushu City (Japan), but not in sea water. Both cities
are located in the most heavily industrialized areas of
Japan.
Relatively high concentrations of TAPs have frequently
been detected in river water sampled near producer or user
sites: 20 µg TCP/litre was detected in the Kanawha River
(USA) 8 miles downstream from the plant outfall (Boethling
& Cooper, 1985).
5.1.3 Soil
There has been only one report of TCP in soil (from
Stauffer Chemical Co. at Gallipolis Ferry (USA)), the
level being 1.0-4.0 mg/kg (Boethling & Cooper, 1985). The
high concentration of total TAPs (26 550 mg/kg) in this
sample was thought to reflect product accumulation in the
area, which was subject to frequent spills.
5.1.4 Sediment
Because of the high sediment adsorption coefficient,
higher levels of TCP have frequently been detected in
sediment than in water. TCP was detected at 400-600 ng/g
in sediment in Baltimore harbour (USA) and at 230-1300
ng/g in the Detroit River (USA) (Boethling & Cooper,
1985). According to the annual reports of the Environment
Agency of Japan, a level of 150 ng/g was found (Mitaki
River, Japan) in one out of 100 sediment samples in 1975,
whereas 1060-2160 ng/g (Doukai Bay, Japan) was found in 3
out of 114 samples in 1978 (EAJ, 1977; 1979; 1981).
Wakabayashi (1980) detected 7-370 ng/g in nine out of ten
river sediment samples, and 4 ng/g in one out of three sea
sediment samples in Tokyo.
5.2 General population exposure
5.2.1 Drinking-water
TCP levels in drinking-water are very low. Lebel et
al. (1981) analysed TAPs in drinking-water sampled from
eastern Ontario water treatment plants and found TCP (at
0.3 ng/litre) in only one out of 12 samples. In an
extended survey of drinking-water conducted in Canada
(Williams & Lebel, 1981), TCP was detected at 0.7-4.3
ng/litre in 7 out of 60 samples of treated potable water
obtained at the treatment plants of 29 municipalities.
In a study by Williams et al. (1982), TCP was detected
in river and lake water but not in well-water. TCP was
also found, at concentrations of 0.4 to 1.8 ng/litre, in 5
out of 12 samples of drinking-water obtained from 12 water
treatment plants located around the Great Lakes (USA).
In general, the TCP concentration in drinking-water is
lower (by factors of 10-2 to 10-3) than that in river
water. This is due to the efficient removal of TCP at
water treatment plants by infiltration using activated
carbon with a high adsorption coefficient.
5.2.2 Fish
Lombardo & Egry (1979) found a TCP concentration of
40 ng/g in sturgeon caught in the Columbia River (USA),
where many metal-processing plants were located upstream
from the sampling point. Muir (1984) found 2-5 ng/g in
fish caught near TAP manufacturing plants. According to
the annual reports of the Environment Agency of Japan, TCP
was not detected in fish caught at any sampling points
(EAJ, 1977; 1979; 1981). The analytical detection limits
varied from 0.25 to 250 ng/g. Kenmotsu et al. (1981a)
found 1-19 ng/g in 4 out of 41 samples of fish and shell-
fish collected in the Seto Inland Sea, Japan.
5.2.3 Human tissues
There has been only one report of TAPs in human adi-
pose tissues (Lebel & Williams, 1983). Although there was
no history of TCP exposure in these patients, tris(1,3-
dichloroisopropyl) phosphate and tributoxyethyl phosphate
were detected at levels of 0.5-110 ng/g and 4.0-26.8 ng/g,
respectively.
5.3 Occupational exposure
The National Institute for Occupational Safety and
Health, USA (US NIOSH) has monitored workplace air, and
found that air samples collected near barrel-filling
operations where lubricating oil was produced by blending
TCP contained less than 0.8 mg TAP/m3 (US NIOSH, 1979).
Air collected near the zinc die-casting machine in auto-
mobile manufacturing contained a total phosphate ester
level of 0.15 mg/m3 (US NIOSH, 1980). Airborne TOCP
resulting from the production of heavy-duty radiators has
been investigated by NIOSH, but the concentration was
below the limit of detection (US NIOSH, 1982). Triaryl
phosphates (at approximately 0.1 ppm) were detected in the
air of the aircraft elevator machinery spaces on the
carrier USS Leyte (CVS-32) where a triaryl phosphate oil
was used as a hydraulic fluid (Baldridge et al., 1959).
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Summary
The primary productivity of cultures of freshwater green
algae was reduced to 50% by TOCP at levels of 1.5 to 4.2 mg per
litre, depending on the species. The meta and para isomers were
less toxic. There are few data on the acute toxicity of TCP to
aquatic invertebrates: a 48-h LC50 for Daphnia of 5.6 mg per
litre, a 24-h LC50 for a nematode of 400 mg/litre, and a
2-week NOEL for Daphnia (mortality, growth, reproduction) of
0.1 mg/litre. The 96-h LC50 values for three fish species
varied between 4.0 and 8700 mg TCP/litre. Rainbow trout showed
approximately 30% mortality after a 4-month exposure to IMOL
S-140 (2% TOCP) at 0.9 mg/litre and minor effects within 14
days. The exposure levels used in these studies were much
higher than likely water concentrations in the environment and,
in most cases, greatly exceeded the solubility of the compounds.
There is no information on the bioavailability or toxicity
to burrowing or bottom-living organisms of TCP bound to sedi-
ment.
There is an indication that crop plants can be affected by
TOCP released from plastic coverings, but there is no infor-
mation concerning the effects on wild plant species.
6.1 Unicellular algae
Data on the toxicity of TCP compounds to unicellular
algae are given in Table 10.
The toxicity of TCP compounds to freshwater algae
depends on their chemical structure. Substitution of the
hydrogen by a methyl group in the benzene ring decreases
the toxicity (Wong & Chau, 1984). Of the TCP isomers, the
ortho isomer was the most toxic for the primary pro-
ductivity of Ankistradesmus falcatus, followed by the
meta and para isomers (Wong & Chau, 1984).
6.2 Aquatic organisms
Data on the toxicity of TCP to aquatic organisms are
presented in Table 11.
Table 10. Toxicity of TCP to freshwater unicellular algae
________________________________________________________________________________________________________
Organism Isomer Temper- Species Effect Concent- Reference
ature ration
(°C) (mg/litre)
________________________________________________________________________________________________________
Alga ortho 20 Ankistrodesmus falcatus 24-h IC50 for primary 2.5 Wong & Chau
meta 20 var. acicularis productivity >5.0 (1984)
para 20 >5.0
Green alga ortho 20 Scenedesmus 24-h IC50 for primary 4.2 Wong & Chau
meta 20 quadricaudata productivity >5.0 (1984)
para 20 >5.0
Lake Ontario ortho 20 24-h IC50 for primary 1.7 Wong & Chau
phytoplankton meta 20 productivity 4.1 (1984)
para 20 >5.0
Green alga Scenedesmus 4-day EC50 1.5 Adema et al.
pannonicus (1983)a
________________________________________________________________________________________________________
a Tests were performed according to or in line with standardized procedures (OECD, 1981).
EC50: 50% effective concentration; IC50: 50% inhibition concentration.
Table 11. Toxicity of TCP to aquatic organisms
________________________________________________________________________________________________________
Organisms Chem- Size/ Temp. Flow/ Hard- End-point Parameter Concent- Reference
icals weight (°C) stat ness or criteria ration
(mg/ (mg/
litre) litre)
________________________________________________________________________________________________________
Tidewater TCPa 40-100 20 stat 96-h LC50 8700 Dawson et
silverside mm al. (1977)
( Menidia
beryllina )
Bluegill TCPa 35-75 23 55 96-h LC50 7000 Dawson et
( Lepomis mm al. (1977)
macrochirus ) 0.60 g 12 flow- 44 96-h LC50 0.26 Mayer &
through 314 0.061 Ellersieck
(1986)
Guppy TCPa stat
( Poecilia 96-h LC50 4.0 Adema et
reticulata ) mortality, 96-h NOEL 1.0 al. (1983)b
swimming
behaviour,
colour
mortality, 4-week 1.0 Adema et
growth, NOEL al. (1983)b
swimming
behaviour
IMOL stat mortality, 24-h NOEL > 57 Wagemann
S-140 visible (1975)
effects
Flagfish TCPa egg-larval 6-week 0.01 Adema et
( Jordanella development, NOEL al. (1983)b
floridae ) mortality,
growth,
swimming
behaviour,
colour
________________________________________________________________________________________________________
Table 11. (contd.)
________________________________________________________________________________________________________
Organisms Chem- Size/ Temp. Flow/ Hard- End-point Parameter Concent- Reference
icals weight (°C) stat ness or criteria ration
(mg/ (mg/
litre) litre)
________________________________________________________________________________________________________
Rainbow trout IMOL flow- ate less, condition 0.9 Wagemann
( Salmo S-140 through less active after 8 (1975)
gairdneri ) days
ceased condition 0.9 Wagemann
surface after 14 (1975)
feeding days
mortality condition 0.9 Wagemann
(5/16) after 4 (1975)
months
TCPa 0.23 g 12 flow- 44 96-h LC50 0.26 Mayer &
0.50 g through 0.40 Ellersieck
(1986)
Waterflea TCPa flow- mortality 48-h LC50 5.6 Adema et
( Daphnia through al. (1983)b
magna ) mortality, 2-week NOEL 0.1 Adema et
reproduction, al. (1983)b
growth
Channel catfish 1.30 g 12 flow- 44 96-h LC50 0.80 Mayer &
( Ictalurus through Ellersieck
punctatus ) (1986)
Yellow perch 0.79 g 292
________________________________________________________________________________________________________
a No descriptions of isomeric compositions were given in the references.
b Tests were performed according to or in line with standardized procedures (OECD, 1981).
Measurement of the acute toxicity (96-h LC50) of TCP
to fish range from 8700 mg/litre in tidewater silversides
(Dawson et al., 1977) to 4 mg/litre in guppies (Adema et
al., 1983). The composition of the materials that were
used in these experiments was not given.
Tests on guppies showed that a saturated solution of
IMOL S-140 (see Table 6) in water (14 mg/litre) was not
acutely toxic (96-h exposure), but exposures of 4 months
or more at concentrations of 0.3-0.9 mg/litre caused symp-
toms of chronic poisoning in rainbow trout. Initially only
feeding habits and behaviour changed, but later swimming
ability was impaired, and the fish eventually died
(Wagemann et al., 1974; Wagemann, 1975). Fatty tissue
turned a blue-grey colour, the liver enlargened, and the
activities of lactate dehydrogenase (LDH) and glutamic-
oxaloacetic transaminase (GOT) increased (Wagemann et al.,
1974; Wagemann, 1975; Lockhart et al., 1975).
Phosflex 179-C (TOCP) slightly inhibited the acetyl-
cholinesterase activity of an electric ray (Torpedo
electroplax) but did not interfere with binding of
acetylcholine to its receptor (Eldefrawi et al., 1977).
Fish or frogs that received IMOL S-140 or TOCP did not
show, under the test conditions, significant reduction of
brain cholinesterase activity (Lockhart et al., 1975). A
similar observation was made by Cohen & Murphy (1970) on
mice and quail.
6.3 Insects
The toxicity of TCP to insects is presented in Table
12. Most of these data were obtained in the course of
studies on the synergism of TCP or TPP with organophos-
phorus insecticides or juvenile insect hormone mimics.
6.4 Plants
The effects of gaseous TCP on crops covered with vinyl
film have been investigated. TCP emitted from the film
caused a certain amount of leaf shrinking (Inden &
Tachibana, 1975).
The active metabolite of TOCP, saligenin cyclic o-
tolyl phosphate, caused decreased germination of kidney
beans and wheat (Eto et al., 1962).
Table 12. Toxicity of TCP to insects
____________________________________________________________________________________________________
Species TCP Application Age Effecta Concentration Reference
isomer method
____________________________________________________________________________________________________
Mosquito larva ortho in water early 4th 5-day LD13 0.1 mg/litre Quinstad et
( Aedes aegypti) instar [± 19] al. (1975)
(LD2[± 3]
in control)
Mosquito larva in water 4th instar 5-day LD7 0.1 mg/litre Quinstad et
( Culex pipiens [± 7] al. (1975)
quinquefasciatus) (LD5[± 5]
in control)
Mosquito larva in water 4-h instar 24-h LC50 > 1 mg/litre Plapp & Tong
( Culex tarsalis) (1966)
Housefly larva topical 3rd instar 7-day LD12 0.1 mg/g Quinstad et
( Musca domestica) treatment [± 6] al. (1975)
(LD8[± 10]
in control)
Housefly contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Housefly meta contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Mosquito larva in water 4th instar 24-h LD50 > 1 mg/litre Plapp & Tong
( Culex tarsaris) (1966)
Housefly para contact 2-5 days old 24-h LD50 > 1 mg/jar Plapp & Tong
( Musca domestica) method (1966)
Mosquito larva in water 4th instar 24-h LD50 > 1 mg/litre Plapp & Tong
( Culex tarsaris) (1966)
____________________________________________________________________________________________________
a Values in square brackets are standard deviations.
7. KINETICS AND METABOLISM
Summary
The absorption, distribution, metabolism, and elimination
of organophosphates are critical to the delayed neuropathic
effects of these compounds. In addition, other factors (e.g.,
route of administration, sex, age, strain) affect their meta-
bolic fate and subsequent neurotoxic expression. Variability in
these factors may underline the interspecies variation in the
sensitivity to TOCP-induced delayed neuropathy (i.e. OPIDN).
This correlation has been demonstrated with other OPIDN com-
pounds, but relevant studies on TOCP itself are limited.
Dermal absorption of TOCP in humans appears to be at least
an order of magnitude faster than in dogs. Significant dermal
absorption also appears to occur in cats. Oral absorption of
the compound has been reported in rabbits. There is no direct
information on absorption via the inhalation route.
In cat studies, absorbed TOCP was widely distributed
throughout the body, the highest concentration being found in
the sciatic nerve, a target tissue. Other tissues with high
concentrations of TOCP and its metabolites are the liver, kid-
ney, and gall bladder.
TOCP is metabolized via three pathways. The first is the
hydroxylation of one or more of the methyl groups, and the sec-
ond is dearylation of the o-cresyl groups. The third is further
oxidation of the hydroxymethyl to aldehyde and carboxylic acid.
The hydroxylation step is critical because the hydroxymethyl
TOCP is cyclized to form saligenin cyclic o-tolyl phosphate,
the relatively unstable neurotoxic metabolite.
TOCP and its metabolites are eliminated via the urine and
faeces, together with small amounts in the expired air.
7.1 Absorption
TOCP absorption has been studied in a variety of
species using oral or dermal administration. No infor-
mation is available on absorption following inhalation.
Gross & Grosse (1932) reported that TOCP given orally
(0.1 g/kg in olive oil) was absorbed by rabbits.
Hodge & Sterner (1943) demonstrated poor absorption of
32P-labelled TOCP in a dog after administration of a
single dermal dose of 200 mg/kg. The rate of transfer
(dose: 2-4 mg [32P]-TOCP/kg) through intact human palm
skin appeared to be about 100 times faster than that
through the abdominal skin of the dog. This was based on
urinary excretion and surface area considerations.
Another species, the cat, showed even greater absorp-
tion. When [14C]-TOCP (50 mg/kg) was dermally applied to
adult male cats, the disappearance of radioactivity from
the application site was bi-exponential. In the first
phase, 73% of the TOCP disappeared within 12 h, while the
second phase half-life was 2 days (Nomeir & Abou-Donia,
1984; 1986b).
Studies by Kurebayashi et al. (1985) indicated incom-
plete absorption of tri- p-cresyl phosphate (TPCP) from
the intestine of rats after a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg) in 1.5 ml of dimethyl
sulfoxide. Much of the radioactivity was recovered in the
faeces, predominantly in the form of unchanged TCP.
7.2 Distribution
After a single oral dose of [32P]-TOCP (770 mg/kg)
to chickens, the total radioactivity in liver increased
consistently throughout 72 h. The levels of radioactivity
in the plasma were consistently lower than those in liver;
at 24 h the plasma levels were 5% of those in liver. The
radioactivity was predominantly associated with TOCP
metabolites in liver but with unmetabolized TOCP in blood
(Sharma & Watanabe, 1974).
Following a single dose of [32P]-TOCP (200 mg/kg) to
the abdominal skin of the dog, the radioactivity in the
blood within 24 h was equivalent to an average value of
80 µg/litre and was distributed throughout the visceral
organs, muscle, brain, and bone. The levels of radioac-
tivity in tissues were in the following descending order:
liver > blood > kidney > lung > muscle or spinal
cord > brain or sciatic nerve (Hodge & Sterner, 1943).
In cats given a single dermal dose of 50 mg [14C]-
TOCP/kg, the chemical was absorbed from the skin and sub-
sequently distributed throughout the body. TOCP reached
its highest concentration in plasma at 12 h, and its
metabolites attained their maximum concentration between
24-48 h. The relative residence values of unmetabolized
TOCP in various tissues, relative to the plasma, were:
brain, 0.09; spinal cord, 0.18; sciatic nerve, 2.1; liver,
0.44; kidney, 0.55; lung, 1.27. Parent TOCP was the pre-
dominant compound in the brain, spinal cord, and sciatic
nerve, while the metabolites o- hydroxybenzoic acid and
di- o-cresyl phosphate were predominant in the liver,
kidney, and lung (Nomeir & Abou-Donia, 1984). In contrast,
when measuring total radioactivity sampled 1-10 days post
exposure, highest levels were found in the bile, gall
bladder, urinary bladder, kidney, and liver, with only low
levels in the spinal cord and brain (Nomeir & Abou-Donia,
1986b).
Gross & Grosse (1932) reported that most of the cresol
ester was recovered from the liver (5%) and intestine
(67%) within 2 h after an intravenous injection (0.5 g/kg)
of TOCP into rabbits.
At 24, 72, and 168 h after oral administration of
[14C]-TPCP to rats, the concentrations of radioactivity
in adipose tissue, liver, and kidney were higher than
those in other tissues (Kurebayashi et al., 1985).
7.3 Metabolic transformation
TOCP is metabolized in rats, rabbits, mice, and
chickens to form a neurotoxic esterase inhibitor (Davison,
1953; Aldridge, 1954; Aldridge & Barnes, 1961; Casida,
1961). In rats injected intraperitoneally with TOCP, this
esterase inhibitor was located mainly in the intestine and
liver (Myers et al., 1955). The neurotoxic metabolite was
isolated from the intestine and liver of rats following
TOCP administration and was identified as saligenin cyclic
o-tolyl phosphate [2-( o-cresyl)-4H-1:3:2-benzodioxaphos-
phoran-2-one] (M-1); M2 and M3 in Fig. 1 are also possible
metabolites (Casida et al., 1961; Eto et al., 1962). The
saligenin cyclic o-tolyl phosphate was also found in
chickens (Eto et al., 1962; Sharma & Watanabe, 1974) and
in cats (Taylor & Buttar, 1967; Nomeir & Abou-Donia, 1984;
1986a,b). Although quantitative data are not available,
indirect evidence suggests that cats metabolize this
neurotoxic compound more efficiently than chickens
(Taylor & Buttar, 1967). Two intermediate metabolites,
di-( o-cresyl) mono- o-hydroxymethylphenyl phosphate
[mono-hydroxymethyl TOCP] and di-( o-hydroxymethylphenyl)
mono- o-cresyl phosphate, [di-hydroxymethyl TOCP],
transform to saligenin cyclic o-tolyl phosphate (Eto
et al., 1962; 1967), which is relatively unstable and is
rapidly hydrolysed to inactive metabolic products.
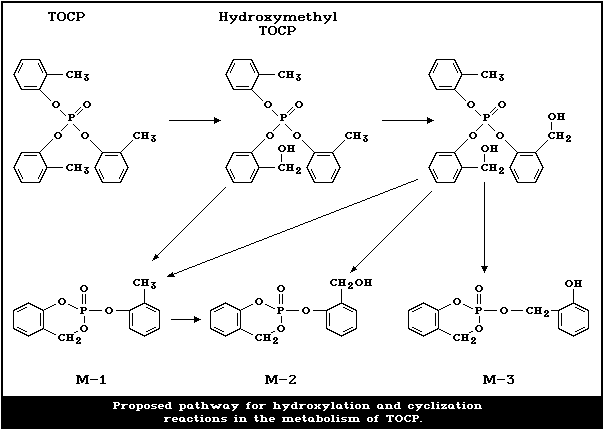 TOCP is metabolized via three essential pathways. The
first is the hydroxylation of one or more of the methyl
groups to hydroxymethyl, which is responsible for the for-
mation of mono- and di-hydroxymethyl TOCP and o-hydroxy-
benzyl alcohol. This reaction is known to be catalysed by
the microsomal mixed-function oxidase system (Eto et al.,
1967). The hydroxymethyl TOCP is cyclized to form sal-
igenin cyclic o-tolyl phosphate with spontaneous release
of o-cresol, this being catalysed by the reaction of
plasma albumin or other components (Eto et al., 1967). The
cyclic phosphate metabolite is relatively unstable and is
rapidly hydrolysed to inactive metabolic products (Eto et
al., 1967). The second pathway is the dearylation of one
or more of the o-cresyl groups of TOCP, resulting in the
formation of o-cresol, di- o-cresyl phosphate, o-cresyl
phosphate, and phosphoric acid. The third pathway is
further oxidation of hydroxymethyl to aldehyde and car-
boxylic acid. These oxidation reactions are most likely to
be mediated by alcohol and aldehyde dehydrogenases.
Studies with [32P]-TOCP in rats have shown that
hydrolysis leads to the rapid excretion in the urine of
diaryl phosphates, monoaryl phosphates, and phosphoric
acid (Casida et al., 1961).
Nomeir & Abou-Donia (1984; 1986a,b) clearly identified
the metabolites of TOCP in male cats. Mono- and di-
hydroxymethyl TOCPs and saligenin cyclic- o-tolyl phos-
phates were present in most tissues, but their concen-
trations were low compared with those of other metab-
olites. The major metabolite of TOCP in the liver, kidney,
lung, and urine of cats was o-hydroxybenzoic acid; di-
o-cresyl phosphate, o-cresyl phosphate, o-cresol,
o-hydroxy-benzyl alcohol, and o-hydroxybenzaldehyde
were also identified. However,the brain, spinal cord,
sciatic nerve, and faeces contained predominantly
unchanged TOCP.
Johnson (1975a) compared the metabolic pathways of the
three isomers of TCP. The main observations, which con-
cerned several organophosphorus esters, were as follows:
(i) Provided that the o-alkyl group has at least one
hydrogen on the alpha-carbon atom, cyclic derivatives
can be obtained that are often highly neurotoxic.
(ii) At the para position, a substituent requires two
hydrogen atoms on the alpha-carbon atom in order to
produce a neurotoxic metabolite inhibiting NTE.
(iii) Substituents at the meta position may be metab-
olized but do not yield inhibitory products.
The major urinary metabolites of TPCP in rats were
p- hydroxybenzoic acid, di- p- cresyl phosphate, and
p- cresyl p- carboxyphenyl phosphate. Mono- (or di-) p-
cresyl di- (or mono-) p- carboxyphenyl phosphate was
identified as the intermediate metabolite in the bile
(Kurebayashi et al., 1985).
7.4 Excretion
After a single oral dose of [32P]-TOCP (770 mg/kg)
to hens, 26.5% of the total radioactivity was eliminated
in the combined urinary-faecal excreta over 72 h, mostly
as TOCP (Sharma & Watanabe, 1974).
After a single dose of [14C]-TOCP (50 mg/kg) to male
cats, approximately 28% of the applied dose was excreted
in the urine and 20% via the bile into the faeces within
10 days (Nomeir & Abou-Donia, 1986b). After this exposure,
the disappearance of TOCP and its metabolites from the
plasma followed monoexponential kinetics. The apparent
half-lives of TOCP and its metabolites (in days) in the
plasma were: TOCP, 1.20; saligenin cyclic- o- tolyl phos-
phate, 2.47; di- o- cresyl phosphate, 4.50; o- cresyl
phosphate, 4.30; o- cresol, 2.65; o- hydroxybenzyl
alcohol, 14.0; o- hydroxy-benzaldehyde, 5.70; o-
hydroxybenzoic acid, 6.00; monohydroxymethyl TOCP, 2.20.
The apparent half-lives of TOCP and its metabolites
reflected the rates of all processes involving the
conversion, clearance, and/or redistribution of these
metabolites (Nomeir & Abou-Donia, 1984).
Elimination via the bile has been demonstrated after
intravenous injection into rabbits (Gross & Grosse, 1932)
and intraperitoneal injection into rats (Myers et al.,
1955). Smith et al. (1932) measured the urinary phenol
excretion in cats given subcutaneous doses of 0.4 to 1.0
ml TOCP/kg. Little if any increase in the urinary phenol
excretion was found either before or after the onset of
paralysis of the hindlimbs.
After a single oral dose (500 mg/kg) of tri- m-cresyl
phosphate (TMCP) or TPCP to rabbits, 92% of TMCP and 95%
of TPCP was eliminated in the faeces within 4 days (Gross
& Grosse, 1932). After a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg), about 90% or 76%,
respectively, of the radioactivity was eliminated in the
urine and faeces within 24 h (Kurebayashi et al., 1985).
The apparent half-lives of the radioactivity in tissues
ranged from 14 h for blood to 26 h for lung and brain. At
the lower dose level, about 28% of the dose was eliminated
via the bile within 24 h. The expiratory excretion as
14CO2 over 3 days amounted to 18% of the radioactivity,
which was reduced to 3% when the rats were treated with
neomycin. The authors suggested that the enterohepatic
circulation and intestinal microflora play an important
role in the degradation of TPCP biliary metabolites.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Summary
Of the three isomers of TCP, TOCP is by far the most toxic
and is the only isomer that produces delayed neuropathy.
A wide animal interspecies variability exists for the
various end-points (e.g., lethality, neuropathology, ataxia,
enzyme inhibition) of TOCP exposure, the chicken being one of
the most sensitive species to delayed neuropathy (i.e. OPIDN).
Species sensitivity to the lethal effects of TOCP adminis-
tration is highly variable, chickens and cats being more sensi-
tive than rats and mice. A single oral dose of 50-500 mg TOCP
per kg induced delayed neuropathy in chickens, whereas doses of
840 mg/kg or more were necessary to produce spinal cord degen-
eration in Long-Evans rats.
No effects were reported in skin and eye irritation studies
on rabbits.
Reproduction studies on rats and mice receiving repeated
oral exposure to TOCP showed histopathological damage in the
testes and ovaries, morphological changes in sperm, decreased
fertility in both sexes, and decreased litter size and
viability. However, no reproductive effects were seen in a
study with TPCP. A clear no-observed-effect level for the
reproductive effects of TOCP was not apparent from the data
available. A study in rats, using oral doses that produced
maternal toxicity, failed to show any teratogenicity.
Little or no information is available on mutagenicity and
carcinogenicity.
Delayed neuropathy has been produced with both single and
repeated exposure regimes in a wide range of experimental
species, and it is classified as a "dying-back neuropathy".
"Neurotoxic esterase" is thought to be the biochemical target
of OPIDN and its inhibition by more than 65% shortly after
exposure to TOCP presages subsequent neuropathy. Factors other
than metabolism (e.g., route of exposure, age, sex, species,
strain) influence variability in sensitivity to OPIDN.
Electrophysiology studies have been performed in cats and
chickens exposed to TOCP.
In the chicken, single exposures below 58 mg/kg or short-
term (i.e. 90-day exposure) daily doses of less than 5 mg/kg
appear to be no-observed-effect levels for delayed neuropathy.
8.1 Single exposure
The acute toxicity of TCP to different species is
summarized in Table 13.
Table 13. LD50 values for TCP and its isomers
____________________________________________________________________
Compounds Route Species LD50 Reference
of (mg/kg)
admin.
____________________________________________________________________
Tricresyl oral rat 5190 Marhold (1972)
phosphate oral rat >4640 Stauffer (1988)a
(mixed oral rat >15 800 Johannsen (1977)
isomers) oral mouse 3900 Izmerov (1982)
oral chicken >10 000 Johannsen et al. (1977)
dermal rabbit >7900 Johannsen et al. (1977)
dermal cat 1500 Abou-Donia et al. (1980)
Tri- o- cresyl oral rat 8400 Johannsen et al. (1977)
phosphate oral rat 1160 Veronesi et al. (1984a)
oral rabbit 3700 Johannsen et al. (1977)
oral chicken 500 Kimmerle & Loeser (1974)
oral chicken 100-200 Smith et al. (1932)
Tri- p- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate chicken >1000 Smith et al. (1932)
Tri- m- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate oral chicken >2000 Smith et al. (1932)
____________________________________________________________________
a Personal communication to the IPCS from Stauffer Chem. Co. (1988)
entitled: Test procedures and data summaries for t-butyl phenyl
diphenyl phosphate, tricresyl phosphate, trixylenyl phosphate,
mixed triaryl phosphate and isopropyl phenyl diphenyl.
The acute symptoms of intoxication are typical of
organophosphorus poisoning. The most toxic compound
appears to be TOCP; the acute toxicity of TCP depends on
the relative proportions of the different isomers.
The chicken, guinea-pig, and rabbit are the most sen-
sitive species, death occurring at an oral dose of 100
mg/kg (Smith et al., 1932); rats and mice are the least
sensitive species.
Sheep given oral doses (100, 200, or 400 mg/kg) of
TOCP exhibited acute intoxication characterized by diar-
rhoea, dehydration, metabolic acidosis, and death within
6 days. Pigs dosed with 100 to 1600 mg TOCP/kg showed
minimal signs of acute intoxication, but developed severe
signs of delayed neuropathy approximately 15 days after
administration (Wilson et al., 1982).
8.2 Short-term exposure
Saito et al. (1974) conducted a 3-month study in rats
with TCP consisting of 60-65% TMCP and 35-40% TPCP. The
compound was suspended in water with 5% gum arabic and
given orally to SD rats at 30, 100, 300, or 1000 mg/kg per
day. Histopathological examination revealed no notable
changes associated with the compound. Based on those
observations, the authors concluded that TCP was of low
short-term toxicity.
Oishi et al. (1982) reported that Wistar rats fed a
pellet diet containing a mixture of TCP isomers at a con-
centration of 5 g/kg diet for 9 weeks developed increases
in absolute and relative liver weights. Haematological
examination revealed no notable changes, but, in the
plasma, total protein, urea, cholesterol and glutamate-
pyruvate transaminase were significantly increased. Slight
liver histopathology included cytoplasmic vacuolization,
increase in the number of binucleated cells, and enlarge-
ment of cell size.
Chapin et al. (1988) exposed male and female CD-1 mice
to diets containing 0, 0.437, 0.875, 1.75, 3.5, or 7.0%
TCP (a mixture of ortho, meta, and para isomers) for
14 days. No clinical signs of toxicity were observed in
the animals at doses up to 0.875%. All animals in the
groups given 1.75, 3.5, or 7.0% exhibited piloerection,
tremors, and diarrhoea, and were lethargic before death
during the 14-day exposure.
8.3 Skin and eye irritation
No published information is available.
8.4 Teratogenicity
Mele & Jensh (1977) reported that no abnormalities
were found in fetuses from pregnant Wistar rats treated
with 500 or 750 mg TOCP/kg of on the 18th and 19th days of
gestation. This study was primarily designed to investi-
gate the effects of prenatal treatment with TOCP on post-
natal behaviour. As such, it cannot be regarded as a true
teratogenicity study.
Tocco et al. (1987) tested the teratogenicity of TOCP
in Long-Evans rats treated with 87.5, 175, and 350 mg/kg
per day throughout organogenesis from gestation days 6 to
18. No maternal deaths or toxicity were observed at the
low or medium dose levels. Maternal lethality in the high-
dose group was higher than that in the control groups.
Numerous soft tissue and skeletal malformations were
observed in both control and TOCP-treated groups, but
there were no significant differences in the frequency of
malformations between the treated and control animals.
8.5 Reproduction
In studies by Somkuti et al. (1987a), TOCP was tested
for effects on the male reproductive tract in male Fisher-
344 rats. Animals were dosed daily for 63 days at dose
levels ranging from 10 to 100 mg/kg per day. Vehicle-
treated animals served as controls. As judged by enzy-
matic, hormonal, and sperm motility, density, and mor-
phology investigations, the minimum effective (threshold)
dose for observable testicular toxicity was 10-25 mg/kg
per day. The data suggested that TOCP interfered directly
with spermatogenic processes and sperm motility and not
via androgenic mechanisms or decreased vitamin E avail-
ability. Testicular pathological changes were seen at
doses above 25 mg/kg per day and included the following:
PAS-positive droplets, immature germ cells, and multinu-
cleate giant cells in the lumen. TPCP produced a decrease
in sperm density but no other testicular effects at a dose
level of 100 mg/kg per day.
To study the time course of the TOCP-induced testicu-
lar lesion in F-344 rats, the onset of possible changes in
sperm numbers and production, serum hormones, and various
enzyme activities was followed. Rats were administered
TOCP daily (150 mg/kg) for periods of 3, 7, 10, 14, or 21
days, while vehicle-treated animals served as controls.
Both sperm motility and the number of sperm per mg of
cauda epididymis were lower in treated animals by day 10.
The ratio of testicular to body weight was significantly
decreased only those rats treated for 21 days. Testicular
neurotoxic esterase and nonspecific esterase activities
were also inhibited, while beta-glucuronidase activity was
not affected. Luteinizing and follicle-stimulating hormone
levels were normal, as were both serum and interstitial
fluid testosterone concentrations. Sertoli cell fluid
secretion, as measured by testis weight increase after
efferent duct ligation, showed no significant changes.
Other organs (spleen, liver, kidney, pancreas, small
intestine, and adrenal and pituitary glands) revealed no
overt signs of pathology as observed by light microscopy
in animals treated for 21 days. A separate group of ani-
mals was treated for 21 days and subsequently examined
after 98 days of observation (two cycles of the rat semin-
iferous epithelium). Normal spermatogenesis did not
return, indicating that the toxicity was irreversible at
the dose used. The effects noted in these studies further
define the testicular lesion produced by TOCP, and show
that a dose level 150 mg/kg per day for 21 days produces
irreversible testicular toxicity (Somkuti et al., 1987b).
The testicular effects of TOCP have also been studied
in the rooster, 100 mg/kg per day being administered
orally to ten adult leghorn roosters for 18 consecutive
days. By days 7-10 of the study, TOCP-treated birds
exhibited limb paralysis characteristic of OPIDN. Enzyme
analyses at the end of the study revealed significant
inhibition of neurotoxic esterase (NTE) activity in both
brain and testis and a slight decrease in brain acetyl-
cholinesterase (AChE) activity. Sperm motility was shown
to be greatly decreased. In addition, sections of
formalin-fixed, methacrylate-embedded testes from TOCP-
treated birds showed vacuolation and disorganization in
the seminiferous epithelium. The marginal body weight
decrease (17%) in treated animals was not considered to
contribute to the testicular toxicity induced by TOCP
(Somkuti et al., 1987c).
TCP (isomer mixture not known) was tested in sperm
morphology and vaginal cytology examination (SMVCE)
studies in groups of 25 male and female Fisher-344 rats
and 25 male and female Swiss CD-1 mice. Treatment lasted
for 13 weeks at dose levels of 1700, 3300, or 6600 mg/kg
diet or 50, 100, 200, 400, or 800 mg/kg body weight via
gavage in the rats, and 500, 1000, or 2100 mg/kg diet or
50, 100, or 200 mg/kg body weight via gavage in the mice.
Effects were seen in treated animals, but no details were
given (Morrissey et al., 1988a). In a follow-up continuous
breeding reproduction study in Swiss CD-1 mice, male and
female fertility was reduced (Morrissey et al., 1988b).
Carlton et al. (1987) examined the reproductive
effects of TCP (mixed isomers; < 9% TOCP). Male Long-Evans
rats received 0, 100, or 200 mg/kg and females received 0,
200, or 400 mg/kg in corn oil by gavage. The low-dose
males were mated with low-dose females, and the high-dose
males with high-dose females. Males were dosed for 56 days
and females for 14 days prior to breeding and throughout
the breeding period, gestation, and lactation. Sperm con-
centration, motility, and progressive movement were
decreased in the high-dose males, and there was a dose-
dependent increase in abnormal sperm morphology. The
number of females delivering live young was severely
reduced by TCP exposure. Litter size and pup viability
were decreased in the high-dose group, but pup body weight
and developmental landmarks were unaffected by TCP
exposure. Histological changes were observed in the testes
and epididymides of treated males (i.e. necrosis, degener-
ation, early sperm granulomas in seminiferous tubules, and
epididymal hypospermia) and in the ovaries of treated
females (i.e. diffuse vacuolar cytoplasmic alteration of
interstitial cells and increased numbers of follicles and
corpora lutea).
In a continuous breeding study, Chapin et al. (1988)
fed Swiss CD-1 mice a diet containing TCP (mixed o-, m-,
p-isomers) at a level of 0, 0.5, 1, or 2 g/kg diet over
98 days. Although the fertility index was not changed in
the high-dose groups, the number of litters per pair
decreased in a dose-dependent fashion, and the proportion
of pups born alive and their weight were significantly
decreased. Histopathological examination of the parental
generation revealed dose-related seminiferous tubule
atrophy and decreased testis and epididymal weights in the
high-dose males, but the female reproductive tract showed
no histopathological changes. A crossover mating trial
revealed impaired fertility in both males and females
exposed to 2 g/kg diet.
8.6 Mutagenicity and carcinogenicity
Haworth et al. (1983) reported a negative result with
TCP (substitution pattern not known) in the Salmonella
mutagenicity test.
8.7 Neurotoxicity
8.7.1 Experimental neuropathology
TOCP first gained notoriety as the culpable neurotoxic
agent of the "Ginger Jake" epidemic (Jeter, 1930;
Goodale & Humphreys, 1931; Vonderahe, 1931). Since then
several experimental studies have modelled TOCP neuropathy
in various species.
Smith & Lillie (1931) produced delayed paralysis in
various animals, including dogs, monkeys, cats, and
chickens. These animal models were used to describe the
functional and morphological features of TOCP neuropathy.
After a delay period of 2-3 weeks following exposure to
single or multiple doses, paralysis of the hindlegs
developed in these species in response to the TOCP. Neuro-
pathologically, degeneration was confined to the spinal
cord and peripheral nerve fibres. These changes were
essentially similar to the lesions reported in human
victims of the "Ginger Jake" epidemic.
The delayed neuropathy associated with TOCP and other
organophosphorous compounds has been termed OPIDN.
Cavanagh (1964) showed that, in cats and chickens,
degeneration affected the long fibres in the spinal cord
and peripheral nerves; moreover, in cats, fibre diameter
seemed to be important in determining the onset and
severity of the peripheral nerve lesions. He categorized
this delayed neuropathy as a "dying-back". Thus, the
lesions of the ascending tracts are seen in the cervical
region, especially in the dorsal columns (i.e. fasciculus
gracilis), while those of the descending tracts are seen
in the thoracic and lumbosacral regions of the spinal
cord. He also showed that the peripheral and central nerve
lesions were the result of axonal degeneration and did not
reflect primary demyelination. The affected nerve axons
degenerate in a "dying-back" fashion towards the cell
body, i.e. axonal degeneration begins at the most distal
portion of the axon and proceeds towards the cell body.
Prineas (1969) described axons with accumulated
tubulovesicular membranes within myelinated motor nerve
terminals in the foot muscles of cats dosed with TOCP.
Similar early axoplasmic changes have been noted in TOCP-
treated chickens (Bischoff, 1967, 1970; Spoerri & Glees,
1979, 1980).
At the ultrastructural level, the nerve endings are
characterized by a marked proliferation and distension of
vesicular elements of the endoplasmic reticulum and a
coinciding disintegration of the filamentous and tubular
organelles (Bischoff, 1977). Bischoff also noted that the
presynaptic nerve terminals and boutons terminaux appeared
particularly sensitive in TOCP intoxication.
OPIDN has also been produced in rats dosed with TOCP
(Veronesi, 1984). The pathological changes developed in
the absence of discernable ataxia after a 2-week exposure
to acute and multiple oral doses of TOCP (>840 mg/kg). In
the rat, dorsal column degeneration of the cervical cord
and a selective vulnerability of large diameter tibial
branches supported a "dying-back" neuropathy, as in
other species.
OPIDN has also been described in European ferrets
( Mustela putorius furo) administered a single oral or
dermal dose of 250, 500, or 1000 mg TOCP/kg body weight.
Five animals per group were sacrificed 48 h after dosing,
the others being observed for another 54 days. All ferrets
dosed dermally with 1000 mg/kg developed neurological
signs ranging from ataxia to partial paralysis. Dermal
doses of 250 and 500 mg/kg produced variable degrees of
hind limb weakness and ataxia. Of the animals dosed
orally, only those treated with 1000 mg/kg showed neuro-
logical signs, which did not progress beyond mild ataxia.
Slight axonal degeneration was noted in the dorsolateral
part of the lateral funicilus and in the fasciculus gra-
cilis of spinal cords in ferrets receiving a dermal dose
of 1000 mg/kg. Whole brain neurotoxic esterase activity
was maximally inhibited (46%) at this dose level. The
study demonstrated that in the ferret dermal exposure was
more effective than oral exposure at the same dose level
(Stumpf et al., 1989).
8.7.2 Neurochemistry
Johnson (1969) found that approximately 6% of brain
esterases are not affected by non-neuropathic compounds
but are specifically inhibited, irreversibly, by neuro-
pathic ones, such as TOCP. Johnson used the term "neuro-
toxic esterase" (NTE) and proposed that NTE is the pri-
mary target of the organophosphorus esters causing OPIDN
(Johnson, 1975a,b). There appears to be a strong corre-
lation in the chicken between NTE inhibition above 70%
shortly after exposure and subsequent neuropathy for a
large number of tested organophosphorous compounds
(Johnson, 1974).
Padilla & Veronesi (1985) demonstrated the relevance
of NTE to the rodent model of OPIDN by exposing Long-Evans
rats to single doses of TOCP ranging from 290 to 3480
mg/kg. High NTE inhibition in the spinal cord (>72%) and
the brain (> 66%) produced severe spinal cord damage in
over 90% of exposed rats, indicating that NTE depression
could predict OPIDN damage in rats acutely exposed to
organophosphates.
Concerning the role of lipids in TOCP neuropathy,
Morazain & Rosenberg (1970) showed that there was a rise
of 25-50% in the cholesterol level in the sciatic nerve
and a 50% decrease in its triglyceride content in chickens
orally dosed with 1 ml TOCP/kg. Phospholipids, diglycer-
ides, cholesterol esters, proteolipids, and tissue phos-
pholipases were not affected by TOCP. The possible
involvement of lipids in the production of TOCP-induced
delayed neuropathy has not yet been resolved.
Other toxic effects have been demonstrated. Brown &
Sharma (1975) found that neural membrane ATPases were
inhibited by organophosphates. Cohen & Murphy (1970)
reported that TOCP potentiates the anticholinesterase
action of malathion by 29-fold in mice, 17-fold in quail,
and 11-fold in sunfish.
8.7.3 Interspecies sensitivity and variability to OPIDN
Certain animal species (e.g., cats, dogs, cows, and
chickens) are susceptible to OPIDN-related paralysis,
whereas others (e.g., rats and mice) are less susceptible
to the ataxia but very susceptible to the pathological
changes. Species susceptibility to delayed neurotoxicity
induced by TOCP shows an inverse correlation with the rate
of metabolic conversion to the neurotoxic metabolite (see
section 7). Because of its high susceptibility to ataxia,
the adult chicken has been used as an experimental model
to study OPIDN.
TOCP is metabolized to the more potent neurotoxic
agent, saligenin cyclic o-tolyl phosphate, which is at
least five times more neurotoxic than TOCP after oral
administration to chickens: a metabolite level of 40 mg/kg
caused ataxia equivalent to that resulting from 200 mg
TOCP/kg (Bleiberg & Johnson, 1965).
It has been shown that a single oral dose of TOCP in
the range of 58 to 580 mg/kg (i.e. 0.05-0.5 ml/kg) induces
mild to severe paralysis in the hen ( Gallus domesticus)
(Cavanagh, 1954; Hine et al., 1956).
Johannsen et al. (1977) showed that chickens adminis-
tered cumulative doses of TCP (60 000 mg/kg) or TOCP (1500
mg/kg) developed both the ataxic and neuropathological
symptoms of OPIDN.
In 90-day studies in hens, obvious functional and
morphological neuropathological changes were found at
daily oral dose levels of 5 to 20 mg TOCP/kg body weight,
but not at lower dosages (Smith et al., 1932; Prentice &
Majeed, 1983; Roberts et al., 1983).
In a subchronic feeding study, Haggerty et al. (1986)
exposed rats to TCP (isomeric substitution pattern not
known) for 13 weeks at dose levels of 0, 900, 1700, 3300,
6600, or 13 000 mg/kg. Decreased hindlimb grip strength
was observed in male rats (at 13 000 mg/kg), but not in
female rats. In mice exposed to 0, 250, 500, 1000, 2100,
or 4200 mg/kg, decrements in both fore- and hindlimb grip
strength were observed in males (at 4200 mg/kg) and
females (at 2100 and 4200 mg/kg). A reduction of body
weight was seen both in rats and mice at the two highest
dose levels. Preliminary histopathological diagnosis
indicated demyelination and axonal degeneration of the
sciatic nerve in male and female mice only.
Freeman et al. (1988) tested the neurotoxicity of TCP
(substitution pattern not known) to F-344 rats in a short-
term study. After 13 weeks of dosing with TCP in the feed,
hindlimb grip strength decreased in male rats at 300 and
600 mg/kg, but not in female rats. Serum cholinesterase
was reduced at 300 and 600 mg/kg in both males and
females. All effects observed with 600 mg/kg were appar-
ently reversible during the recovery period.
In a 13-week short-term feeding study, Irwin et al.
(1987) exposed F-344 rats to TCP (0, 75, 150, 300, or 600
mg/kg; substitution pattern not known), while B6C3F1 mice
receive 0, 60, 125, or 250 mg/kg. After 13 weeks of
dosing, forelimb grip strength was unaffected by TCP in
both mice and rats. Hindlimb grip strength decreased in
male rats (at 300 and 600 mg/kg) but not in female rats.
In mice, decrements in hindlimb grip strength were
observed in males (250 mg/kg) and females (125 and 250
mg/kg). Serum cholinesterase levels showed a dose-depen-
dent reduction in both rats and mice. TCP had no effect on
food consumption in either species. All groups exhibited
normal body weight values.
Factors such as age, sex, and strain figure promi-
nently in the expression of OPIDN. The young of most
species are non-susceptible to TOCP-induced delayed neuro-
pathy (Johnson & Barnes, 1970), which could be due to poor
absorption of TOCP. However, recent experiments (Olson &
Bursian, 1988) have suggested that factors (e.g., route of
administration) other than absorption are more critical to
this lack of susceptibility.
Strain differences in the susceptibility of rats to
TOCP-delayed neuropathy have been reported. Although OPIDN
can be readily produced in Long-Evans and Sprague-Dawley
rats after acute oral doses of > 840 mg/kg (Veronesi &
Abou-Donia, 1982; Veronesi, 1984), repeated doses of TOCP
(10-100 mg/kg) failed to produce neuropathy in Fischer-344
rats (Somkuti et al., 1988). Variations in TOCP inhibition
of brain AChE and NTE have also been reported in these
three strains (Carrington & Abou-Donia, 1988).
8.7.4 Neurophysiology
Robertson et al. (1987) investigated electrophysio-
logical changes in the adult hen following single oral
doses of 30 or 750 mg TOCP/kg. At the higher dose, the
birds demonstrated clinical signs of toxicity 12 days
after dosing that included gait abnormalities, which
became progressively more severe and in some cases led to
complete ataxia. Lymphocyte NTE was inhibited by more
than 70%. The lower dose produced no clinical signs of
toxicity and only 54% lymphocyte NTE inhibition. Both
treated groups displayed significant action potential
disruption in both the tibial and sciatic nerves that
resulted in decreased refractoriness in the tibial nerve,
increased refractoriness in the sciatic nerve, and
elevated strength duration threshold for both nerves.
Electrophysiological changes have been investigated in
cats following single dermal doses ranging from 250 to
2000 mg TOCP/kg and 90-day dermal administration of 1 to
100 mg/kg (Abou-Donia et al., 1986). In contrast to the
hen, the clinical signs of TOCP neurotoxicity appear in
the cat 21-26 days before the electrophysiological effects
on the gastrocnemius muscle. Recovery of the cat from
delayed neurotoxicity symptoms was more marked than that
of the hen. No effects on peripheral nerve transmission
or on neuromuscular junction functioning were seen in the
cat.
9. EFFECTS ON HUMANS
Summary
There have been many reported cases of human poisoning,
mostly from accidental or irresponsible contamination of food-
stuffs. Occupational poisoning, usually resulting from dermal
exposure, has also been reported. The ortho isomer of TCP is
the responsible toxic agent.
Though short-term symptoms of ingestion might involve
vomiting, abdominal pain, and diarrhoea, characteristically
delayed, longer-term symptoms are neurological, frequently
leading to paralysis and pyramidal signs (spasticity, etc.).
There is considerable variation in the sensitivity of indi-
viduals to TOCP; severe symptoms were reported with a TOCP dose
of 0.15 g in one individual, while others were unaffected by 1
to 2 g. There is also considerable variation in the rate of
recovery from poisoning, some patients recovering completely
and others still severely affected years later, after appar-
ently similar exposure.
First-aid treatment involves the induction of vomiting or
pumping of the stomach. The patient should be hospitalized as
soon as possible. Atropine or 2-PAM may be used as an effective
antidotal treatment against cholinergic symptoms. Long-term,
antispastic drugs may be useful, though physical rehabilitation
is the cardinal therapy.
9.1 Historical background
Of the tricresyl phosphate isomers, the ortho (TOCP)
is by far the most toxic and alone gives rise to the major
neurotoxicity in man. It is considered that the toxicity
of the commercial products depends on the concentration of
the ortho isomer, but the mixed o-cresyl esters in these
products are also toxic and contribute to the neurotoxic
action.
It is well known that TOCP produces delayed effects on
the central and peripheral nervous systems. TOCP poisoning
has occurred throughout the world (Inoue et al., 1988);
the major outbreaks are indicated in Table 14.
Table 14. Major outbreaks of TOCP poisoning
___________________________________________________________________________
Year Place Number Vehicles of TOCP Reference
of cases
___________________________________________________________________________
1898 France 6 phospho-creosote Lorot (1899)
1900-1928 Europe 43 phospho-creosote Roger & Recordier
(15% TOCP) (1934)
1930-1931 USA 50 000 ginger extract Morgan (1982)
1931 Europe several Apiol pill Susser & Stein
hundred (1957)
1938 South Africa 68 cooking oil Sampson (1942)
1940 Switzerland 80 cooking oil Walthard (1945)
1941-1945 Germany more than cooking oil Mertens (1948)
200
1945 England 17 cooking oil Hotston (1946)
1955 South Africa 11 water or solvent Susser & Stein
(1957)
1957 Morocco about cooking oil Smith & Spalding
10 000 (1959)
1960 India 58 solid food Vora et al. (1962)
1962 India more than flour Chaudhuri (1965)
400
1967 Fiji 56 flour Sorokin (1969)
1980 Romania 12 alcohol Vasilescu & Florescu
(1980)
1981 Sri Lanka more than cooking oil Senanayake &
20 (0.56%) Jeyaratnam (1981)
___________________________________________________________________________
In 1899, Lorot initially reported six cases of poly-
neuropathy out of 41 cases of pulmonary tuberculosis
treated with phospho-creosote. Later it was shown that the
phospho-creosote contained 15% TOCP. In the next 30 years,
43 additional cases caused by the drug were reported in
various parts of continental Europe (Roger & Recordier,
1934).
In the spring of 1930, in mid-western and south-
western USA, an outbreak of paralysis characterized by
bilateral foot- and wrist-drop appeared suddenly (Burley,
1930; Merritt & Moor, 1930). Ultimately 50 000 people were
poisoned by a popular substitute for alcohol called
"Ginger Jake" (Morgan, 1982). Smith et al. (1930) proved
that the adulterated beverage contained about 2% TOCP and
that this caused the paralysis.
In 1931, several hundred women in Europe (especially
in Germany, The Netherlands, Yugoslavia, and France) were
poisoned by the TOCP contained in Apiol pills and taken as
an abortifacient (Roger & Recordier, 1934; Susser & Stein,
1957). The TOCP was presumably included as an additional
stimulus to abortion (Ter Braak & Carrillo, 1932).
An outbreak involving 11 people occurred in Durban,
South Africa, in 1955 (Susser & Stein, 1957). From the
epidemiological survey it was suggested that water, as
well as solvents, may have been a vehicle for the TOCP.
In 1959, about 10 000 Moroccan people were intoxicated
by TOCP: jet engine oil had been illegally mixed into
their cooking oil (Smith & Spalding, 1959; Svennilson,
1960). Accidental poisoning by TOCP contamination of solid
food occurred in 1960 in Bombay, India, where 58 victims
were recorded (Vora et al., 1962).
During the period April-June, 1962, more than 400
cases of paralysis occurred in the Malda district in
India. The cause of this disease proved to be the consump-
tion of flour contaminated with TOCP (Chaudhuri, 1965).
In 1967, similar poisoning was recorded in Fiji, where
56 people showed neuropathy (Sorokin, 1969). The cause was
stated to be contamination of dry sharps flour by TOCP
through the sacking material.
Vasilescu & Florescu (1980) in Romania reported 12
patients with toxic neuropathy following accidental inges-
tion of alcohol contaminated by TOCP.
An outbreak of acute polyneuropathy in over 20 young
females occurred in Sri Lanka during 1977-1978 (Senanayake
& Jeyaratnam, 1981). The cause of the neuropathy was
traced to TCP found as a contaminant in a special cooking
oil (gingili oil). Contamination probably occurred during
transport of the oil in containers previously used for
storing mineral oils.
9.2 Occupational exposure
Gartner & Elsaesser (1943) reported the case of a
worker who developed pyramidal signs after exposure to
TOCP for two years in a German chemical plant. In this
case, percutaneous absorption was considered to be the
main route of exposure.
In 1944, three cases of toxic polyneuropathy among
workers who had worked for six to eight months in a plant
manufacturing TCP in England were reported (Hunter et al.,
1944). Skin penetration and inhalation were thought to be
the main causes of the occupational poisoning.
Parnitzke (1946) reported a case of TOCP poisoning
after 3 years of exposure in a German plant and stated
that TOCP had been absorbed through the skin and presum-
ably the gastrointestinal tract.
Since 1958, a high prevalence of polyneuropathy among
shoe factory workers has been reported in Italy. The cause
has been attributed to TCP (Cavalleri & Cosi, 1978).
Although this is possible, this cause-effect relationship
has not so far been based on unquestionable evidence. This
polyneuropathy might have various aetiological factors
(including n-hexane) or be produced by a combination of
them (Leveque, 1983).
9.3 Clinical features
Goldstein et al. (1988) reported a case of severe
intoxication in a 4-year-old child following ingestion of
a lubricant containing TCP (substitution pattern not
known). The clinical findings were acute gastrointestinal
symptoms, delayed cholinergic crisis, and neurological
toxicity.
The severity of signs and symptoms after poisoning
with TOCP seems not always to be proportional to the
dosage (Staehelin, 1941). In a Swiss Army outbreak of
poisoning among more than 80 young men, toxic symptoms
appeared in once case after eating food containing only
0.15 g TOCP. Severe neurological disturbance developed in
three men from the intake of 0.5 to 0.7 g, whereas in two
other cases the intake of 1.5-2 g did not lead to any
symptoms. This leads to the conclusion that individual
susceptibility varies greatly (Staehelin, 1941).
In general, the signs and symptoms of TOCP poisoning
are distinctive, whereas the symptomatology varies
somewhat according to whether a single relatively large
dose or small cumulative doses are taken. In the former
case, the initial symptoms are gastrointestinal, ranging
from slight to severe nausea and vomiting, sometimes
accompanied by abdominal pain and diarrhoea. Among these
symptoms, vomiting is most frequently observed (Staehelin,
1941). These symptoms are usually transient, lasting from
a few hours to a few days (Walthard, 1945; Susser & Stein,
1957).
In cases of chronic low level exposure, the above
symptoms may not be present and the major symptoms are
neurological (Parnitzke, 1946). The clinical features of
acute exposure to TOCP were described by Staehelin (1941).
A latent period of 3-28 days is observed after acute
exposure, and clear "delayed neurotoxicity" then gradu-
ally appears. The initial neurological symptoms are sharp,
cramp-like pains in the calves, and some numbness and
tingling in the feet and sometimes the hands. Within a few
hours or a day or two at most, these pains are followed by
increasing weakness of the lower limbs, and soon the
patient becomes unsteady and then unable to maintain bal-
ance. The cramp-like pains may cease with the onset of
weakness, or persist for some days. One or two weeks after
the onset in the lower limbs and while paralysis may still
be progressing, the weakness spreads to the hands. While
some patients show complete wrist drop and total loss of
power in the hands, sometimes with weakness up to the
elbows, the predominant neurological abnormalities are
observed in the lower limbs. Bilateral foot drop with com-
plete loss of power from the ankle down is a common
finding. Depending on the severity of the affection, the
patient may have weakness in the knees, less at the hips,
and, only in the most severe cases, weakness of the trunk.
About three weeks or more after the onset of paralysis, a
most striking and rapid wasting may be observed. While the
small muscles of the feet, calves, the anterior tibials,
and the thighs do not escape this wasting, in so far as
they are involved in the disease, the change is most
obvious in the small muscles.
In the initial stage, the ankle jerks are absent and
knee reflexes may be normal or occasionally depressed.
Plantar reflexes are unobtainable. Mild cases do not show
any upper motor neuron signs. On the other hand, in the
more severe cases, even at the early stage, knee jerks may
be exaggerated, presaging the development of upper motor
neuron involvement. In general, upper motor neuron signs,
e.g., pyramidal signs, gradually become evident at about
the third week or later. Knee jerks become exaggerated and
so also may the biceps, triceps, and supinator jerks
(Cavanagh, 1964). A finger flexor reflex appears for the
first time or increases (Susser & Stein, 1957). As the
pyramidal tract lesion becomes evident, involuntary flexor
withdrawal of the whole limbs follows gentle plantar
stimulation. Babinski responses are observed much later.
Muscle tonus of the limbs gradually increases. In severe
cases, the signs of upper motor involvement are delayed,
probably masked by the gross flaccid muscle weakness.
Several authors state emphatically that sensory dis-
turbances do not occur. Sampson (1942) reported sensory
disturbances although these were admittedly unobtrusive,
in contrast to motor dysfunction. Reports of muscle and
peripheral nerve tenderness are fairly frequent. If the
sensory disturbances are observed, there is hypoesthesia
with loss of pin-prick and temperature sense; the ability
to detect vibration is sometimes affected distally. The
sensory disturbances vary in extent from merely the soles
of the feet to the whole of the limbs.
Usually cranial nerves are not involved. In general,
mental signs are rare, but transient euphoria and con-
fusion have been observed in the early stage (Schwab,
1948).
9.4 Prognosis
Following exposure, muscle weakness progresses over
several weeks, sometimes even months. Sensory changes
often begin to regress during the early weeks, the rap-
idity depending on the severity of the case, and then
muscle strength gradually returns in patients who are only
mildly affected. Improvement begins with the return of
sensation, then muscle strength in the hands, and eventu-
ally strength in the lower limbs. In cases of pyramidal
signs, recovery is generally poor. Zeligs (1938),
reporting eight years after the 1930 mid-western and
south-western USA outbreak, surveyed the records of 316
patients. He was able to follow up 60 patients, all of
whom were disabled and still in institutions. Aring (1942)
examined more than 100 patients in the 10 years following
this outbreak and they appeared still to be affected.
Morgan & Penovich (1978) followed up 11 survivors in the
47 years after the same outbreak; the principal findings
were the spasticity and abnormal reflexes of an upper
motor neuron syndrome.
Of the 80 patients in the 1940 Swiss army accident,
14 were quite well after five years, 15 were totally inca-
pacitated, and 38 showed spasticity (Walthard 1945).
In the 1938 Durban outbreak, all the patients showed
some symptoms of the disease 18 years later (Susser &
Stein, 1957). The mildest case had slight weakness at the
ankle, while the most severely affected had foot drop,
muscle atrophy, and pyramidal signs (spasticity, ankle
clonus, and positive Babinski sign).
The residual signs and symptoms are mainly confined to
the lower limbs. They consist of weakness and muscle atro-
phy of varying degrees in the feet and the small muscles
of the hand. Disability is principally related to the
pyramidal signs with resultant spasticity of the lower
extremities.
9.5 Neurophysiological investigations
There have been very few electrophysiological studies
on human TOCP poisoning. Svennilson (1960) reported an
electromyographic study on 65 patients in the 1959 Morocco
poisoning. These cases showed varying degrees of dener-
vation and polyphasic abnormal potentials in the paralysed
muscles. The clinically healthy proximal groups of muscles
also showed marked polyphasic action potentials but not
denervation.
Vasilescu & Florescu (1980) reported detailed studies
on 12 patients from the 1980 accident in Romania. They
observed > 50% decrease in the muscle evoked potential
amplitude, fibrillation potentials in the same muscles at
rest, and decreased motor nerve conduction velocity.
In neurophysiological studies by Senanayake (1981) in
Sri Lanka, the main findings were widespread neurotoxic
patterns and prolongation of terminal latencies with rela-
tively mild slowing of motor nerve conduction velocities.
These studies confirmed the evidence of axonal degener-
ation.
9.6 Pathological investigations
Numerous pathological studies have been made on biopsy
or autopsy samples since the Jamaica ginger accidents in
1930. In 1930, Goldfain described some changes observed
in the peripheral nerves and spinal cord, quoting Jeter's
autopsy report. Histopathological investigations by
Goodale & Humphreys (1931) indicated degeneration of
myelin sheaths and axis cylinders in the radial, sciatic,
and tibial nerves in all cases examined. Vonderahe (1931)
found marked degenerative changes in the anterior horn
cells, characterized by swelling, central chromatolysis
(disappearance of the Nissl substance), excentric nuclei,
and shrinking of the cells. The pathological studies also
revealed degeneration in the radial and anterior tibial
nerves, and degenerative changes in the anterior roots.
There were no pathological signs of inflammation. Accord-
ing to Smith & Lillie (1931), the paralysis due to Jamaica
ginger was essentially a degeneration of the myelin
sheaths of the peripheral nerves, with a variable amount
of relatively moderate central degenerative changes
affecting the anterior horn cells throughout the spinal
cord, but more often in the lumber and cervical regions.
In the 1938 Durban (South Africa) epidemic, Sampson (1942)
also reported that degeneration of the anterior horn cells
occurred in some instances and that peripheral nerves
showed axonal degeneration. Aring (1942) described degen-
eration in the posterior and lateral columns in later
investigations of survivors of the outbreak, thereby con-
firming the origin of some of the spinal symptoms. It is
noteworthy that the latter changes were evident in the
lumbar region, while the dorsal column changes, in which
only the fasciculus gracilis was involved, concerned the
cervical region.
Muscle biopsy studies of patients from the 1959
Moroccan poisoning showed a moderate degree of muscle
atrophy and a slight increase of the muscle fibre nuclei.
Spherical axonal swelling and terminal knobs were noted as
a sign of peripheral nerve degeneration in muscles
(Svennilson, 1960). Similar changes were also noted in
the poisoning cases reported in Malda, India, in 1962
(Chaudhuri et al., 1962). These effects on muscle suggest
denervation.
9.7 Laboratory investigations
Little information has been obtained from laboratory
examinations of exposed humans. There is no significant
change in the urine or blood (Sampson, 1942; Senanayake &
Jeyaratnam, 1981), but the cerebrospinal fluid may show an
increase in protein concentration, with or without pleo-
cytosis (Sampson, 1942; Mertens, 1948; Susser & Stein,
1957).
Vora et al. (1962) measured blood cholinesterase
levels in patients admitted to hospitals during the 1960
Bombay poisoning and demonstrated that plasma cholinester-
ase was increased one month after exposure. The erythro-
cyte cholinesterase level was considerably diminished
(50%) quite early after the onset of symptoms. Both plasma
and erythrocyte cholinesterase activities returned to
normal within about 3 months.
In a factory manufacturing TAP, about half of the
workers examined showed significant decreases in pseudo-
cholinesterase (cholinesterase other than AChE), and many
of them had minor signs and symptoms (Tabershaw et al.,
1957).
Morgan & Hughes (1981) also investigated cholinester-
ase activity in workers in a plant manufacturing TAP plas-
ticizers. They found that plasma cholinesterase estimation
in workers exposed to TAP compounds cannot be used as a
sensitive barometer of organic phosphate absorption; thus
routine regular estimations serve no useful purpose. Its
value is chiefly as a screening method at the pre-employ-
ment medical examination to exclude personnel at risk, as
a baseline in the event of massive exposure, and as a
means of diagnosis in cases of CNS disease that simulate
TAP poisoning.
9.8 Treatment
In the event of skin contact with TCP, contaminated
clothing should be rapidly removed and affected body areas
copiously irrigated with water. The ingestion of food or
drink contaminated with TOCP should be treated by inducing
vomiting, unless the patient is unconscious. Atropine or
pralidoxine (2-PAM) chloride may be required to counteract
cholinergic effects. No specific antidote is available.
Medical therapy should begin as soon as possible, even
though the results of medical therapeutic measures have
been disappointing. B-complex vitamins and corticosteroids
may protect nervous tissue against further involvement
(Geoffroy et al., 1960). The cardinal therapy is physical
rehabilitation. Administration of anti-spastic drugs may
be required.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human poisoning involving the accidental ingestion of
tri- o-cresyl phosphate (TOCP) or occupational exposure
of workers has frequently been reported. The likely route
of occupational exposure is cutaneous absorption. The
neurotoxic symptoms involve initial inhibition of cholin-
esterases and subsequent delayed neuropathy characterized
by severe paralysis.
Because of considerable variation among individuals in
sensitivity to TOCP, it is not possible to establish a
safe level of exposure. Symptoms have been reported from
the ingestion of 0.15 g of an isomeric mixture with a low
proportion of TOCP; the minimum effective dose of the
ortho isomer is, therefore, much lower than this. Animal
studies show considerable variation among species in the
response to TOCP, and humans appear to be particularly
sensitive.
Irritant and allergic dermatitis have been reported.
Both the pure ortho isomer and isomeric mixtures con-
taining TOCP are, therefore, considered major hazards to
human health.
There is no safe level for ingestion. Exposure to the
compound through dermal contact or inhalation should be
minimized.
10.1.1 Exposure levels
Exposure of the general population to tricresyl phos-
phate (TCP) through various environmental media, including
drinking-water, can be regarded as minimal. TCP has been
detected at relatively higher concentrations in urban air
than in air collected at production sites, although the
levels are usually low. TCP was not detected in human
adipose tissue samples in a survey conducted in the USA.
There have been many cases of accidental human poisoning
through the ingestion of medicines, food, flour, cooking
oil, and beverages contaminated with hydraulic fluid or
lubricant oil containing TCP produced from "cresylic
acid". The toxic symptoms can be observed after ingestion
of only 0.15 g of tri- o-cresyl phosphate, a component of
TCP from cresylic acid. The contamination has usually
happened when empty barrels or drums, previously used for
hydraulic fluid or lubricating oil storage, have been
reused.
10.1.2 Toxic effects
Accidental human exposure to a single large dose re-
sults in gastrointestinal disturbance varying from slight
to severe nausea and vomiting, accompanied by abdominal
pain and diarrhoea. In the case of exposure to small cumu-
lative doses, "delayed neurotoxicity" gradually proceeds
after a latent period of 3-28 days. In most cases, the
muscle weakness changes rapidly to a striking paralysis of
the lower limbs, with or without an involvement of the
hands. In severe cases, pyramidal signs gradually become
evident. Some neurophysiological studies indicate wide-
spread neurotoxic patterns and prolongation of terminal
latencies with relatively small decreases of motor nerve
conduction velocities. This confirms the evidence of
axonal degeneration, which is the main feature observed in
pathological investigations.
The neurotoxic metabolite of TCP has been identified
as saligenin cyclic o-tolyl phosphate, which is derived
from o-hydroxymethyl metabolites. Thus, it seems that at
least one o-tolyl group among the three phenolic moieties
of TCP is necessary to induce neurotoxic effects. TCP pro-
duced from synthetic cresol, which contains less than 0.1%
of o-cresol, is therefore not neurotoxic.
Subchronic animal studies on TCP derived from syn-
thetic cresol indicate that the target organs are liver
and kidney, but this was not confirmed in the case of
human intoxication. No adequate data are available on
mutagenicity and carcinogenicity. TCP is not toxic to
chick embryos.
10.2 Evaluation of effects on the environment
The measurement of environmental concentrations of TCP
in water has shown only low levels of contamination. This
reflects the low water solubility and ready degradability
of the compound. Since the acute toxicity of TCP to
aquatic organisms is also low, it is unlikely that it
poses a threat to such organisms.
As a consequence of the physico-chemical properties of
TCP, there is a high potential for bioaccumulation. How-
ever, this does not occur in practice, owing to low con-
centrations of TOCP in the environment and living organ-
isms and to its rapid degradation.
TCP bound to sediment accumulates in the environment,
and levels measured in river, estuarine, and marine sedi-
ments have been high. Since there is no information either
on the bioavailability of these residues to burrowing or
bottom-living organisms or on their hazards, the possi-
bility of effects on such species cannot be discounted.
TCP spillage leads to hazard for the local environ-
ment.
10.2.1 Exposure levels
TCP is found in air, surface water, soil, sediment,
and aquatic organisms near heavily industrialized areas,
although concentrations are usually low. Owing to the high
biodegradation rate of TCP in aqueous environment, it is
not considered to affect aquatic organisms adversely. One
report showed an extremely high concentration of total
triaryl phosphate (26.55 g/kg) in a soil sample obtained
from a production plant yard. This suggests the need for
land waste disposal.
10.2.2 Toxic effects
Freshwater algae are relatively sensitive to TCP, the
50% growth inhibitory concentration ranging from 1.5 to
5.0 mg/litre. Among fish species, the rainbow trout is
adversely affected by TCP concentrations below 1 mg/litre
(0.3-0.9 mg/litre), with sign of chronic poisoning, but
the tidewater silverside is more resistant (LC50 is 8700
mg/litre). TCP does not inhibit cholinesterase activity in
fish or frogs, but it has a synergistic effect on organo-
phosphorus insecticide activity.
11. RECOMMENDATIONS
When tri-substituted cresols are used in the synthesis
and manufacture of other compounds, the purified meta and
para isomers should be used in order to avoid the acciden-
tal synthesis of ortho-substituted products.
REFERENCES
ABOU-DONIA, M.B., VARGA, P.A., GRAHAM, D.G., & KINNES, C.G. (1980)
Delayed neurotoxicity of a single dermal administration of o-ethyl
o-4-nitro-phenyl phenylphosphonothioate and tri-o-cresyl phosphate
to cats. Toxicol. Lett., 1 (Special issue): 141.
ABOU-DONIA, M.B., TROFATTER, L.P., GRAHAM, D.G., & LAPADULA, D.M.
(1986) Electromyographic, neuropathologic and functional
correlates in the cat as the result of tri-o-cresyl phosphate
delayed neurotoxicity. Toxicol. appl. Pharmacol., 83(1): 126-141.
ADEMA, D.M.M., KUIPER, J., HANSTVEIT, A.O., & CANTON, H.H. (1983)
Consecutive system of tests for assessment of the effects of
chemical agents in the aquatic environment. In: Pesticide
chemistry-Human welfare and the environment. Proceedings of the
Fifth International Congress on Pesticide Chemistry,Kyoto, Japan,
29 August - 4 September, 1982, Oxford, New York, Pergamon Press,
Vol. 3, pp. 537-544.
ALDRIDGE, W.N. (1954) Tricresyl phosphates and cholinesterase.
Biochem. J., 56: 185-189.
ALDRIDGE, W.N. & BARNES, J.M. (1961) Neurotoxic and biochemical
properties of some triaryl phosphates. Biochem. Pharmacol., 6:
177-188.
ANONYMOUS (1986) Handbook of commodity chemicals, Tokyo, Kagaku-
Kogyo-Nipposha.
ARING, C.D. (1942) The systemic nervous affinity of triorthocresyl
phosphate (Jamaica ginger palsy). Brain, 65: 34-47.
ASSOCIATION OF THE PLASTICIZER INDUSTRY OF JAPAN (1976) Safety of
the plasticizer TCP, tricresyl phosphate, Tokyo, Association of
the Plasticizer Industry of Japan.
BALDRIDGE, H.D., JENDEN, D.J., KNIGHT, C.E., PREZIOSI, T.J., &
TUREMAN, J.R. (1959) Toxicology of a triaryl phosphate Oil. III.
Human exposure in operational use aboard ship. Am Med. Assoc. Arch.
ind. Hyg., 20: 258-261.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., &
WELCH, V.A. (1961) The reactions of organic phosphates. Part V.
The hydrolysis of triphenyl and trimethyl phosphates. J. Chem. Soc.
(B), 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER,
B., VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic
phosphates. Part VI. The hydrolysis of aryl phosphates. J. Chem.
Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphoryl-enzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BECK, B.E., WOOD, C.D., & WHENHAM, G.R. (1977) Triaryl phosphate
poisoning in cattle. Vet. Pathol., 14: 128-137.
BELL, J.D. & RUSVINE, J.R. (1967) Synergism of organophosphates in
Musca domestica and Chrysomya putoria. Entomol. exp. appl., 10:
263-269.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY,
S.K. (1974) Detection and estimation of tricresyl phosphate in
mustard oil. Forensic Sci., 3: 263-270.
BISCHOFF, A. (1967) The ultrastructure of tri-ortho-cresyl
phosphate poisoning. I. Studies on myelin and axonal alterations in
the sciatic nerve. Acta neuropathol., 9: 158-174.
BISCHOFF, A. (1970) The ultrastructure of tri-ortho-cresyl
phosphate poisoning in the chicken. II. Studies on spinal cord
alterations. Acta neuropathol., 15: 142-155.
BISCHOFF, A. (1977) Tri-ortho-cresyl phosphate neurotoxicity. In:
Roisin, L., Shiraki, H., & Grcevic, N., ed. Neurotoxicology, New
York, Raven Press, pp. 431-441.
BLEIBERG, M.J. & JOHNSON, H. (1965) Effects of certain
metabolically active drugs and oximes on tri- o-cresyl phosphate
toxicity. Toxicol. appl. Pharmacol., 7: 227-235.
BLOOM, P.J. (1973) Application des chromatographies sur couche
mince et gaz-liquide à l'analyse qualitative et quantitative des
esters des acides phosphorique et phosphoreux. J. Chromatogr.,
75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and
effects of triaryl and tri-alkyl/aryl phosphate esters. Residue
Rev., 94: 49-99.
BONDY, H.F., FIELD, E.J., WORDEN, A.N., & HUGHES, J.P.W. A study of
the acute toxicity of the tri-aryl phosphates used as plasticizers.
(1960) Br. J. ind. Med., 17: 190-200.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., &
KARASEK, F.W. (1981) Trace impurities in solvents commonly used for
gas chromatographic analysis of environmental samples. J.
Chromatogr., 206: 279-288.
BROWN H.R. & SHARMA, R.P. (1975) Inhibition of neural membrane
adenosine-triphosphatases by organophosphates. Toxicol. appl.
Pharmacol., 33: 140.
BURLEY, B.T. (1930) The 1930 type of polyneuritis. New Engl. J.
Med., 202: 1139-1142.
CARLTON, B.D., HASARAN, A.H., MEZZA, L.E., & SMITH, M.K. (1987)
Examination of the reproductive effects of tricresyl phosphate
administered to Long-Evans rats. Toxicology, 46: 321-328.
CARRINGTON, C.D. & ABOU-DONIA, M.B. (1988) Variation between three
strains of rat: inhibition of neurotoxic esterase and
acetylcholinesterase by tri-o-cresyl phosphate. J. Toxicol.
environ. Health, 25(3): 259-268.
CASIDA, J.E. (1961) Specificity of substituted phenyl phosphorus
compounds for esterase inhibition in mice. Biochem. Pharmacol., 5:
332-342.
CASIDA, J.E., ETO, M., & BARON R.L. (1961) Biological activity of a
tri-o-cresyl phosphate metabolite. Nature (Lond.), 191: 1396-1397.
CASTERLINE, J.L., Jr., KU, Y., & BARNETT, N.M. (1985) Uptake of
tri-p-cresyl phosphate in soybean plants. Bull. environ. Contam.
Toxicol., 35: 209-212.
CAVALLERI, A. & COSI, V. (1978) Polyneuritis incidence in shoe
factory workers: Case report and etiological considerations. Arch.
environ. Health, 33: 192-197.
CAVANAGH, J.B.(1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system. An experimental study in hens. J.
Neurol. Neurosurg. Psychiatry, 17: 163-172.
CAVANAGH, J.B. (1964) The significance of the "dying back" process
in experimental and human neurological disease. Int. Rev. exp.
Pathol., 3: 219-267.
CHAPIN, R.E., GEORGE, J.D., & LAMB, J.C., IV (1988) Reproductive
toxicity of tricresyl phosphate in a continuous breeding protocol
in Swiss (CD-1) mice. Fundam. appl. Toxicol., 10: 344-354.
CHAUDHURI, R.N. (1965) Paralytic disease caused by consumption of
flour contaminated with tricresyl phosphate. Trans. R. Soc. Trop.
Med. Hyg., 59: 98-102.
CHAUDHURI, R.N., SEN GUPTA, P.C., CHAKRAVARTI, R.N., MUKHERJEE,
A.M., GHOSH, S.M., CHATTERJEA, J.B., SARKAR, J.K., BASU, S.P.,
ADHYA, R.N., MITRA, N.K., SAHA, T.K., & MITRA, P. (1962) Recent
outbreak of a paralytic disease in Malda, West Bengal. Bull.
Calcutta Sch. Trop. Med., 10: 141-152.
CHEMICAL AND GEOLOGICAL LABORATORIES LTD (1971) Calgary, Alberta,
Chemical and Geological Laboratories (Laboratory report No. E71-
5360).
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
COHEN, S.D. & MURPHY, S.D. (1970) Comparative potentiation of
malathion by triorthotolyl phosphate in four classes of
vertebrates. Toxicol. appl. Pharmacol., 16: 701-708.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues
in foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAGLEY, S. & PATEL, M.D. (1957) Oxidation of p-cresol and related
compounds by a Pseudomonas. Biochem. J., 66: 227-233.
DAVISON, A.N. (1953) The cholinesterases of the central nervous
system after the administration of organo-phosphorus compounds.
Brit. J. Pharmacol., 8: 212-216.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977)
The acute toxicity of 47 industrial chemicals to fresh and salt
water fishes. J. hazard. Mater., 1: 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic
mass spectrometric analysis of some commercial aryl phosphate oils.
J. Assoc. Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by
thin-layer chromatography.] Gig. i Sanit. , 10: 62-65 (in Russian).
DUKE, A.J. (1978) The significance of isomerism and complexity of
composition on the performance of triaryl phosphate plasticisers in
PVC. Chimia, 32: 457-463.
EAJ (1977) [ Environmental monitoring of chemicals ], Tokyo,
Environment Agency Japan, pp. 212-214 (Environmental Survey Report
Series, No. 3) (in Japanese).
EAJ (1979) [ Chemicals in the environment], Tokyo, Environment
Agency Japan, pp. 61, 78, 92, 93 (Office of Health Studies Report
Series, No. 5) (in Japanese).
EAJ (1981) [ Environmental monitoring of chemicals: Environmental
Survey Report of 1978, 1979 F.Y. ], Tokyo, Environment Agency
Japan, pp. 116-117 (in Japanese).
ELDEFRAWI, A.T., MANSOUR, N.A., BRATTSTEN, L.B., AHRENS, V.D., &
LISK, D.J. (1977) Further toxicologic studies with commercial and
candidate flame retardant chemicals. Part II. Bull. environ.
Contam. Toxicol., 17: 720-726.
ETO, M., CASHIDA, J.E., & ETO, T. (1962) Hydroxylation and
cyclization reactions involved in the metabolism of tri- o-cresyl
phosphate. Biochem. Pharmacol., 11: 337-352.
ETO, M., OSHIMA, Y., & CASHIDA, J.E. (1967) Plasma albumin as a
catalyst in cyclization of diaryl-o-(a-hydroxy)tolyl phosphates.
Biochem. Pharmacol., 16: 295-308.
ETO, M., HASHIMOTO, Y., OZAKI, K., KASAI, T., & SASAKI Y. (1975)
Fungitoxicity and insecticide synergism of monothioquinol phosphate
esters and related compounds. Botyu Kagaku, 40: 110-117.
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl
phosphate esters. A new photochemical coupling reaction. J. Am.
Chem. Soc., 94: 4780-4782.
FRANCHINI, I., CAVATORTA, A., D'ERRICO, M., DE SANTIS, M., ROMITA,
G., GATTI, R., JUVARRA, G., & PALLA, G. (1978) Studies on the
etiology of the experimental neuropathy from industrial adhesives
(glues). Experientia (Basel), 34: 250-252.
FREEMAN, G.B., IRWIN, R., TREJO, R., HEJTMANCIK, M., RYAN, M., &
PETERS, A.C. (1988) Reversibility and tolerance to tricresyl
phosphate-induced neurotoxic effects in F344 rats. Toxicologist,
8: 76.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GARTNER, W. & ELSAESSER, K.H. (1943) [Commercial ortho-
tricresylphosphate poisoning.] Arch. Gewerbepathol. Gewerbehyg.,
12: 1-9 (in German).
GEOFFROY, H., SLOMIC, A., BENEBADJI, M., & PASCAL, P. (1960)
Myèlopoly-néurites tri-crésyl phosphatées. Toxi-épidémie marocaine
de septembre-octobre 1959. World Neurol., 1: 294-315.
GOLDFAIN, E. (1930) Jamaica ginger multiple neuritis. J. Oklahoma
State Med. Assoc., 23: 191-193.
GOLDSTEIN, D.A., MCGUIGAN, M.A., & RIPLEY, B.D. (1988) Acute
tricresyl phosphate intoxication in childhood. Hum. Toxicol., 7:
179-182.
GOODALE, R.H. & HUMPHREYS, M.B. (1931) Jamaica ginger paralysis.
Autopsy observations. J. Am. Med. Assoc., 96: 14-16.
GROSS, E. & GROSSE, A. (1932) [A contribution to the toxicology of
ortho-tricresylphosphates.] Arch. exp. Pathol. Pharmakol., 168:
473-514 (in German).
HAGGERTY, G.J., HEJTMANCIK, M., DESKIN, R., RYAN, M.J., PETERS,
A.C., & IRWIN, R. (1986) Effects of subchronic exposure to
tricresyl phosphate in F344 rats and B6C3F1 mice: behavioural and
morphological correlates. Toxicologist, 6: 218.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Shuishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, N., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HINE, C.H., DUNLAP, M.K., RICE, E.G., COURSEY, M.M., GROSS, R.M., &
ANDERSON, H.H. (1956) The neurotoxicity and anticholinesterase
properties of some substituted phenyl phosphates. J. Pharmacol.
exp. Ther., 116: 227-236.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD,
G.T. (1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, 3rd revised ed., New York,
Wiley-Interscience, Vol. 2A, pp. 2362-2363.
HJORTH, N. (1962) Contact dermatitis from cellulose acetate film.
Cross-sensitization between tricresylphosphate (TCP) and
triphenylphosphate (TPP). Contact dermatitis, 2: 86-100.
HODGE, H.C. & STERNER, J.H. (1943) The skin absorption of
triorthocresyl phosphate as shown by radioactive phosphorus. J.
Pharmacol. exp. Ther., 79: 225-234.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOTSTON, R.D. (1946) Outbreak of polyneuritis due to orthotricresyl
phosphate poisoning. Lancet, 1: 207.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. Bull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(di-
chloropropyl)phosphate, a mutagenic flame retardant: Frequent
occurrence in human seminal plasma. Science, 211: 951-952.
HUNTER, D., PERRY, K.M.A., & EVANS, R.B. (1944) Toxic polyneuritis
arising during the manufacture of tricresyl phosphate. Br. J. ind.
Med., 1: 227-231.
INDEN, T. & TACHIBANA, S. (1975) [Damage of crops by gases from the
plastic materials under covering conditions.] Bull. Fac. Agric. Mie
Univ., 1975: 1-10 (in Japanese).
INOUE, N., FUJISHIRO, K., MORI, K., & MATSUOKA, M. (1988)
Triorthocresyl phosphate poisoning: A review of human cases.
Sangyo-Ika-Daigaku-Zasshi, 10(4): 433-442.
IRWIN, R., FREEMAN, G.B., TREJO, R., HEJTMANCIK, M., & PETERS, A.
(1987) Effects of chronic exposure to tricresyl phosphate in F344
rats and B6C3F1 mice: behavioural and biochemical correlates. Soc.
Neurosci. Abstr., 13: 90.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water
Res., 19: 119-125.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982) Toxicometric
parameters of industrial toxic chemicals under single exposure,
Moscow, Centre of International Projects, GKNT, p. 114.
JETER, H. (1930) Autopsy report of a case of so-called "Jake
Paralysis". J. Am. Med. Assoc., 95: 112-113.
JOHANSSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J.,
RADUE, R.W., & GRAHAM, P.R. (1977) Evaluation of delayed
neurotoxicity and dose-response relationships of phosphate esters
in the adult hen. Toxicol. appl. Pharmacol., 41: 291-304.
JOHNSON, M.K. (1969) Delayed neurotoxic action of some
organophosphorus compounds. Br. med. Bull., 25: 231-235.
JOHNSON, M.K. (1974) The primary biochemical lesion leading to the
delayed neurotoxicity of some organophosphorus esters. J.
Neurochem., 23: 785-789.
JOHNSON, M.K. (1975a) Organophosphorus esters causing neurotoxic
effects. Mechanism of action and structure/activity studies. Arch.
Toxicol., 34: 259-288.
JOHNSON, M.K. (1975b) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. & BARNES, J.M. (1970) The sensitivity of chicks to
the delayed neurotoxic effects of some organophosphorous compounds.
Biochem. Pharmacol., 19: 3045.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water
pollution caused by organophosphorus compounds.] Kankyo Gijyutsu,
7: 668-675 (in Japanese).
KENMOTSU, K., SAITO, N., OGINO, N., & MATSUNAGA, K. (1979) [An
environ-mental survey of chemicals. XI. Analytical methods of
organic phosphoric acid triesters.] Okayama-ken Kankyo Hoken Senta
Nempo, 3: 175-191 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment
and sea water.] J. Food Hyg. Soc. Jpn., 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental
pollutants. V. Environmental fate of organic phosphoric acid
triesters.] Okayama-ken Kankyo Hoken Senta Nempo, 4: 103-110 (in
Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.]
Okayama-ken Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination
of organic phosphate esters in environment samples.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982a) [An environmental survey of chemicals. XIX. Determination
of organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken
Senta Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982b) [Studies on the biological toxicity of several pollutants
in environments. VII. GC/MS Spectrometric determination of
organophosphoric acid triesters in sediment.] Okayama-ken Kankyo
Hoken Senta Nempo, 6: 142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemicals. XXII. GC/MS
spectrometric determination of organophosphoric acid triesters in
sediment (2).] Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in
Japanese).
KIMMERLE, G. & LOESER, E. (1974) Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environ. Qual. Saf., 3: 174-178.
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on
organic and heavy metal contaminants in the Lakes Erie, Michigan,
Huron and Superior basins. Great Lakes Water Qual. Bd.
KRISHNAMURTHY, M.N., RAJALAKSHMI, S., & PRAKASH KAPUR, O. (1985)
Detection of tricresyl phosphates and determination of tri-o-cresyl
phosphate in edible oils. J. Assoc. Off. Anal. Chem., 68(6):
1074-1076.
KU, Y. & ALVAREZ, G.H. (1982) Biodegradation of (14C) tri- p-cresyl
phosphate in a laboratory activated-sludge system. Appl. environ.
Microbiol., 43: 619-622.
KUREBAYASHI, H., TANAKA, A., & YAMAHA, T. (1985) Metabolism and
dispo-sition of the flame retardant plasticizer, tri-p-cresyl
phosphate, in the rat. Toxicol. appl. Pharmacol., 77: 395-404.
LAPP, T.W. (1976) Study on chemical substances from information
concerning the manufacture, distribution, use, disposal,
alternatives, and magnitude of exposure to the environment and
man: Task 1 - The manufacture and use of selected aryl and alkyl
phosphate esters, Springfield, Virginia, National Technical
Information Service, 129 pp (EPA contract No. 68-01-2687) (NTIS PB-
251819).
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic
phosphate triesters in human adipose tissue. J. Assoc. Off. Anal.
Chem., 66: 691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from
drinking water at the ng/L level, using macroreticular resin. J.
Assoc. Off. Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas
chromatographic determination of trialkyl/aryl phosphates in
drinking water, following isolation using macroreticular resin. J.
Assoc. Off. Anal. Chem., 64: 991-998.
LEFAUX, R. (1968) Practical toxicology of plastics, London, Iliffe
Books Ltd, p. 580.
LEFAUX, R. (1972) Industrial toxicology. Phosphoric esters. In:
Practical toxicology of plastics, London, Iliffe Books Ltd, pp.
121-127.
LEVEQUE, J. (1983) Tricresyl phosphates. In: Encyclopaedia of
occupational health safety, 3rd ed., Geneva, International Labour
Office, Vol. 2, pp. 2216-2218.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY,
D. (1975) Chronic toxicity of a synthetic tri-aryl phosphate oil to
fish. Environ. Physiol. Biochem., 5: 361-369.
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62: 47-51.
LOROT, C. (1899) Les combinations de la créosote dans le traitement
de la tuberculose pulmonaire, Paris (Thèse de Paris No. 25).
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Perspect., 10: 269-284.
MAJORS, R.E. & JOHNSON, E.L. ( 1978) High-performance exclusion
chromatography of low-molecular-weight additives. J. Chromatogr.,
167: 17-30.
MARHOLD, J.V. (1972) Collection of results of toxicological
assessments of compounds and formulations tested at the Department
of Toxicology, Research Institute for Organic Syntheses, Prague,
Institute for Management Training in the Chemical Industry.
MAUSNER, M., BENEDICT, J.H., BOOMAN, K.A., BRENNER, T.E., CONWAY,
R.A., DUTHIE, J.R., GARRISON, L.J., HENDRIX, C.D., & SHEWMAKER,
J.E. (1969) The status of biodegradability testing of nonionic
surfactants. J. Am. Oil Chem. Soc., 46: 432-440.
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Springfield, Virginia, National Technical
Information Service.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE,
P.M., & SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An
aquatic environmental assessment of Pydrauls 50E and 115E. In:
Branson R. & Dickson, K.L., ed. Aquatic Toxicology and Hazard
Assessment: Fourth Conference, Philadelphia, American Society for
Testing and Materials, pp. 103-123 (ASTM STP 737).
MELE, J.M. & JENSH, R.P. (1977) Teratogenic effects of orally
administered tri-o-cresyl phosphate on Wistar albino rats.
Teratology, 15: 32A.
MERRITT, H.H. & MOOR, M. (1930) Peripheral neuritis associated with
ginger extract ingestion. New Engl. J. Med., 203: 4-12.
MERTENS, H.G. (1948) [Clinical aspects of tri-orthocrésyl phosphate
poisoning (including a report on several cases of poisoning in an
Igelit factory).] Arch. Psychiatr. Nervenkr., 179: 458-482
(in German).
MRI (1979) Assessment of the need for limitation on triaryl and
trialkyl/aryl phosphates. Draft final report, Kansas City, Midwest
Research Institute (EPA Contract 68-01-4313).
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising
Supplement 52(10A), p 697, New York, McGraw-Hill Inc.
MORAZAIN, R. & ROSENBERG, P. (1970) Lipid changes in tri-o-cresyl-
phosphate-induced neuropathy. Toxicol. appl. Pharmacol., 16: 461-474.
MORGAN, A.A. & HUGHES, J.P.W. (1981) An investigation into the
value of cholinesterase estimations of workers in a plant
manufacturing tri-aryl phosphate plasticizers. J. Soc. Occup. Med.,
31: 69-75.
MORGAN, J.P. (1982) The Jamaica Ginger Paralysis. J. Am. Med.
Assoc., 248: 1864-1867.
MORGAN, J.P. & PENOVICH, P. (1978) Jamaica Ginger Paralysis. Forty-
seven-year follow-up. Arch. Neurol., 35: 530-532.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988a) Evaluation of rodent sperm, vaginal
cytology, and reproductive organ weight data from National
Toxicology Program 13-week study. Fundam. appl. Toxicol., 11:
343-358.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988b) Association of sperm, vaginal
cytology, and reproductive organ weight data with results of
continuous breeding reproductive studies in Swiss CD-1 mice.
Fundam. appl. Toxicol., 11: 359-371.
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G. & GRIFT, N.P. (1983) Extraction and cleanup procedures
for determination of diarylphosphates in fish, sediment, and water
samples. J. Assoc. Anal. Off. Chem., 66: 684-690.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980) Determination of
several triarylphosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and
cleanup of fish, sediment, and water for determination of triaryl
phosphates by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Technol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983) Environmental
dynamics of phosphate esters. III. Comparison of the
bioconcentration of four triaryl phosphates by fish. Chemosphere,
12: 155-166.
MURRAY, D.A.J. (1975) Analysis of tri-aryl phosphate esters and the
determination of IMOL S-140 in fish tissue and water samples by gas
chromatography. J. Fish Res. Board Can., 32: 457-460.
MYERS, D.K., REBEL, J.B.J., VEEGER, C., KEMP, A., & SIMONS, E.G.L.
(1955) Metabolism of triaryl phosphates in rodents. Nature (Lond.),
176: 259-260.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition
coefficient to measure the bioconcentration potential of organic
chemicals in fish. Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate
ester mixtures to invertebrates and fish. Bull. environ. Contam.
Toxicol., 19: 250-256.
NICHOLSON, S.S. (1974) Bovine posterior paralysis due to
organophosphate poisoning. J. Am. Vet. Med. Assoc., 165: 280-281.
NOBILE, E.R., PAGE, S.W., & LOMBARDO, P. (1980) Characterization of
four commercial flame retardant aryl phosphates. Bull. environ.
Contam. Toxicol., 25: 755-761.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performance liquid
chromato-graphic analysis on radial compression column of the
neurotoxic tri-o-cresyl phosphate and metabolites. Anal. Biochem.,
135: 296-303.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1984) Disposition of (14C)tri-o-
cresyl phosphate and its metabolites in various tissues of the male
cat following a single dermal application. Drug Metab. Disp., 12
(6): 705-711.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986a) Studies on the metabolism
of the neurotoxic tri-o-cresyl phosphate synthesis and
identification by infrared, proton nuclear magnetic resonance and
mass spectrometry of five of its metabolites. Toxicology, 38: 1-13.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986b) Studies on the metabolism
of the neurotoxic tri- o-cresyl phosphate, distribution, excretion,
and metabolism in male cats after a single, dermal application.
Toxicology, 38: 15-33.
OECD (1981) OECD guidelines for testing of chemicals. Section 2:
Effects on biotic systems, Paris, Organization for Economic
Cooperation and Development, Publications Office.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresylphosphate. Sci. total
Environ., 43: 233-241.
OISHI, H., OISHI, S., & HIRAGA, K. (1982) Toxicity of several
phosphoric acid esters in rats. Toxicol. Lett., 13: 29-34.
OLSON, B.A. & BURSIAN, S.J. (1988) Effect of route of
administration on the development of organophosphate-induced
delayed neurotoxicity in 4-week-old chicks. J. Toxicol. environ.
Health, 23(4): 499-505.
PACIOREK, K.J.L., KRATZER, R.H., KAUFMAN, J., NAKAHARA, J.H.,
CHRISTOS, T., & HARTSTEIN, A.M. (1978) Thermal oxidative
degradation studies of phosphate esters. Am. Ind. Hyg. Assoc. J.,
39: 633-639.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78(1): 78-87.
PARNITZKE, K.H. (1946) [On the recent accumulation of ortho-
tricresylphosphate poisoning (sources and clinical course).] Dtsch.
Gesundheitswes., 1: 666-670 (in German).
PEGUM, J.S. (1966) Contact dermatitis from plastics containing tri-
aryl phosphates. Br. J. Dermatol., 78: 626-631.
PENMAN, D.R & OSBORNE, G.O. (1976) Trialkyl phosphates and related
compounds as antifertility agents of the twospotted spider mite. J.
econ. Entomol., 69(2): 266-268.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975)
Utilization of triaryl phosphates by a mixed bacterial population.
Can. J. Microbiol., 21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with
synergistic properties. J. econ. Entomol., 59(1): 11-15.
PRENTICE, D.E. & MAJEED, S.K. (1983) A subchronic study (90 days)
using multiple dose levels of tri-ortho-cresyl phosphate (TOCP):
some neuropathological observations in the domestic hen.
Neurotoxicology, 4(2): 277-282.
PRINEAS, J.S. (1969) The pathogenesis of dying-back
polyneuropathies. Part I. An ultrastructural study of experimental
tri-ortho-cresyl phosphate intoxication in the cat. J. Neuropathol.
exp. Neurol., 28: 571-597.
QUINSTAD, G.B., STAIGER, L.E., & SCHOOLEY, D.A. (1975)
Environmental degradation of the insect growth regulator
methoprene. V. Metabolism by house-flies and mosquitoes. Pestic.
Biochem. Physiol., 5: 233-241.
RAMSEY, J.D. & LEE, T.D. (1980) Gas-liquid chromatographic
retention indices of 296 non-drug substances on SE-30 or OV-1
likely to be encountered in toxicological analyses. J. Chromatogr.,
184: 185-206.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin
layer chromatography. Evaluation of methods and application on
phosphate esters, polychlorinated paraffins and some PCB-
substitutes. Chemosphere, 9: 683-691.
ROBERTS, N.L., FAIRLEY, C., & PHILLIPS, C. (1983) Measurements and
evaluation of clinical signs following use of the hen as the animal
model for screening acute delayed and subchronic neurotoxicity
studies. Neurotoxicology, 4(2): 263-270.
ROBERTSON, D.G., SCHWAB, B.W., SILLS, R.D., RICHARDSON, R.J., &
ANDERSON, R.J. (1987) Electrophysiologic changes following
treatment with organophosphorus-induced delayed neuropathy-
producing agents in the adult hen. Toxicol. appl. Pharmacol., 87:
420-429.
ROGER, H. & RECORDIER, M. (1934) Les polynéurites
phosphocréosotiques (phosphate de créosote, ginger paralysis,
apiol). Ann. Med., 35: 44-63.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P.,
& TUCKER, E.S. (1979) Environmental fate of selected phosphate
esters. Environ. Sci. Technol., 13: 840-844.
SAITO, C., KATO, T., TANIGUCHI, H., FUJITA, T., WADA, H., & MORI,
Y. (1974) [Subacute toxicity of tricresylphosphate (TCP) in rats.]
Pharmacometrics, 8: 107-118 (in Japanese).
SAMPSON, B.F. (1942) The strange Durban epidemic of 1937. S. Afr.
med. J., 16: 1-9.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by
Killifish (Oryzeas latipes). Bull. environ. Contam. Toxicol., 28:
752-759.
SCHWAB, H. (1948) [Psychic disturbance by triorthocresylphosphate
poisoning.] Dtsch. Med. Wochenschr., 73: 124-125 (in German).
SENANAYAKE, N. (1981) Tri-cresyl phosphate neuropathy in Sri Lanka:
a clinical and neurophysiological study with a three year follow
up. J. Neurol. Neurosurg. Psychiatry, 44: 775-780.
SENANAYAKE, N. & JEYARATNAM, J. (1981) Toxic polyneuropathy due to
gingili oil contaminated with tri-cresyl phosphate affecting
adolescent girls in Sri Lanka. Lancet, 10 January: 88-89.
SHARMA, R.P. & WATANABE, P.G. (1974) Time related disposition of
tri-o-tolyl phosphate (TOTP) and metabolites in chicken. Pharmacol.
Res. Commun., 6(5): 475-484.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the
Delaware River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, H.V. & SPALDING, J.M.K. (1959) Outbreak of paralysis in
Morocco due to ortho-cresyl phosphate poisoning. Lancet, 2:
1019-1021.
SMITH, M.I. & LILLIE, R.D. (1931) The histopathology of
triorthocresyl phosphate poisoning. Arch. Neurol Psychiatry, 26:
976-992.
SMITH, M.I., ELVOVE, E., & FRAZIER, W.H. (1930) The pharmacological
action of certain phenol esters, with special reference to the
etiolgy of so-called ginger paralysis. Public Health Rep., 45:
2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies
on the pharmacology of certain phenol esters with special reference
to the relation of chemical constitution and physiologic action.
Natl. Inst. Health Bull., 160: 1-53.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987a) Reproductive tract lesions resulting from
subchronic administration (63 days) of tri-o-cresyl phosphate in
male rats. Toxicol. appl. Pharmacol., 89: 49-63.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987b) Time course of the tri-o-cresyl phosphate-
induced testicular lesion in F-344 rats: enzymatic, hormonal, and
sperm parameter studies. Toxicol. appl. Pharmacol., 89: 64-72.
SOMKUTI, S.G., LAPADULA, D.M., CHAPIN, R.E., & ABOU-DONIA, M.B.
(1987c) Testicular toxicity following oral administration of tri-o-
cresyl phosphate (TOCP) in roosters. Toxicol. Lett., 37: 279-290.
SOMKUTI, S.G., TILSON, H.A., BROWN, H.R., CAMPBELL, G.A.,
LAPADULA, D.M., & ABOU-DONIA, M.B. (1988) Lack of delayed
neurotoxic effect after tri-ortho-cresyl phosphate treatment in
male Fischer-344 rats: bio-chemical, neurobehavioural, and
neuropathological studies. Fundam. appl. Toxicol., 10: 199-205.
SOROKIN, M. (1969) Orthocresyl phosphate neuropathy: Report of an
outbreak in Fiji. Med. J. Aust., 1: 506-508.
SPOERRI, P.E. & GLEES, P. (1979) Ultrastructural reactions of
spinal ganglia to tri-ortho-cresyl phosphate: Effects of
neurotoxicity. Cell Tissue Res., 199: 409-414.
SPOERRI, P.E. & GLEES, P. (1980) Ultrastructural changes in neurons
and neuroglia of the avian telencephalon (Hyperstriatum
Accessorium) following tri-ortho-cresyl phosphate intoxication.
Cell Tissue Res., 206: 203-210.
STAEHELIN, R. (1941) [On the triorthocresylphosphate poisonings.]
Schweiz. Med. Wochenschr., 71: 1-5 (in German).
STUMPF, A.M., TANAKA, D.JR., AULERIOH, A.J., & BURSIAN, S.J. (1989)
Delayed neurotoxic effects of tri-o-tolyl phosphate in the European
ferret. J. Toxicol. environ. Health, 26: 61-73.
STURM, R.N. (1973) Biodegradability of nonionic surfactants:
Screening test for predicting rate and ultimate biodegradation. J.
Am. Oil Chem. Soc., 50: 159-167.
SUGDEN, E.A., GREENHALGH, R., & PETTIT, J.R. (1980)
Characterization of delayed neurotoxic triaryl phosphates by
analysis of trifluoroacetylated phenolic moieties. Environ. Sci.
Technol., 14(12): 1498-1501.
SUSSER, M. & STEIN, Z. (1957) An outbreak of tri-ortho-cresyl
phosphate (TOCP) poisoning in Durban. Br. J. ind. Med., 14:
111-120.
SVENNILSON, E. (1960) Studies of triorthocresyl phosphate
neuropathy, Morocco 1960. Acta psychiatr. Scand., 150(Suppl):
334-336.
TABERSHAW, I.R., KLEINFELD, M., & FEINER, B. (1957) Manufacture of
tricresyl phosphate and other alkyl phenyl phosphates: An
industrial hygiene study. Am. Med. Assoc. Arch. ind. Health, 15:
537-540.
TAYLOR, J.D. & BUTTAR, H.S. (1967) Evidence for the presence of 2-
(o-cresyl)-4 H-1:3:2-benzodioxaphosphoran-2-one in cat intestine
following tri-o-cresyl phosphate administration. Toxicol. appl.
Pharmacol., 11: 529-537.
TER BRAAK, J.W.G. & CARRILLO, R. (1932) [Polyneuritis after usage
of an abortifacient (tri-ortho-cresyl-phosphate-poisoning).] Dtsch.
Z. Nervenheilkd., 125: 86-116 (in German).
THOMPSON, J.E. & DUTHIE, J.R. (1968) The biodegradability and
treatability of NTA. J. Water Pollut. Control Fed., 40: 306-319.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorus compounds. Anal. Chem., 53: 1466-1469.
TOCCO, D.R., RANDALL, J.L., YORK, R.G., & SMITH, M.K. (1987)
Evaluation of the teratogenic effects of tri-ortho-cresyl phosphate
in the Long-Evans hooded rat. Fundam. appl. Toxicol., 8(3): 291-297.
US NIOSH (1977) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Vol. 3, P & CAM, pp. S/209-S/210 (DHEW (NIOSH) Publication
No. 7-157C).
US NIOSH (1979) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS PB-82-216268.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division,
General Motors Corp., 1000 Lexington Avenue, Rochester, New York,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (PB82-104530, NTIS).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorous exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations and
Field Studies (Report No. IWA-89.10).
US SOAP AND DETERGENT ASSOCIATION (1965) A procedure and standards
for the determination of the biodegradability of alkyl benzene
sulfonate and linear alkylate sulfonate (Report of the Subcommittee
on Biodegradation Test Methods). J. Am. Oil Chem. Soc., 42: 986-993.
VASILESCU, C. & FLORESCU, A. (1980) Clinical and
electrophysiological study of neuropathy after organophosphorus
compound poisoning. Arch. Toxicol., 43: 305-315.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A
simple colorimetric method for estimation of tricresyl phosphate in
edible oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J.
Fish Res. Board Can., 36: 1040-1048.
VERONESI, B. (1984) A rodent-model of organophosphorus-induced
delayed neuropathy: distribution of central (spinal cord) and
peripheral nerve damage. Neuropathol. appl. Neurobiol., 10(6):
357-368.
VERONESI, B. & ABOU-DONIA, M.B. (1982) Central and peripheral
neuropathology induced in rats by tri-o-cresyl phosphate (TOCP).
Vet. hum. Toxicol., 24: 3 (abstract).
VERONESI, B., NEWLAND, D., & INMAN, A. (1984) The effect of
metabolic interference on rodent-sensitivity to tri-ortho-cresyl
phosphate. Toxicologist, 4: 55.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J.J., & SVEC, H.J.
(1978) Organic emissions from combustion of combination coal/refuse
to produce electricity. Chemosphere, 7: 893-902.
VONDERAHE, A.R. (1931) Pathologic changes in paralysis caused by
drinking Jamaica Ginger. Arch. Neurol. Psychiatry, 25: 29-43.
VORA, D.D., DASTUR, D.K., BRAGANCA, B.M., PARIHAR, L.M., IYER,
C.G.S., FONDEKAR, R.B., & PRABHAKARAN, K. (1962) Toxic polyneuritis
in Bombay due to ortho-cresyl-phosphate poisoning. J. Neurol.
Neurosurg. Psychiatry, 25: 234-242.
WAGEMANN, R. (1975) Some environmental and toxicological aspects of
a tri-aryl phosphate synthetic oil. Verh. Int. Ver. Limnol., 19:
2178-2184.
WAGEMANN, R., GRAHAM, R., & LOCKHART, W.L. (1974) Studies on
chemical degradation and fish toxicity of a synthetic tri-aryl
phosphate lubrication oil, IMOL S-140. Ottawa, Environment Canada,
Fisheries and Marine Service, (Technical Report, No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution caused by
organophosphoric fire-proofing plasticizers.] Annu. Rep. Tokyo
Metrop. Res. Inst. Environ. Prot., 11: 110-113 (in Japanese).
WALTHARD, K.M. (1945) Aperçu des résultats obtenus lors des
derniers examens des malades intoxiqués en 1940 par le phosphate
triorthocrésilique. Schweiz. Arch. Neurol. Psychiatry, 58: 189-194.
WIGHTMAN, R.H. & MALAIYANDI, M. (1983) Physical properties of some
synthetic trialkyl/aryl phosphate commonly found in environmental
samples. Environ. Sci. Technol., 17: 256-261.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of
tri(haloalkyl)-, trialkyl-, and triarylphosphates in Canadian
drinking water. Bull. environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON,
R. (1982) Determination of mutagenic potential and organic
contaminants of Great Lakes drinking water. Chemosphere, 11: 263-276.
WILSON, R.D., ROWE, L.D., LOVERING, S.L., & WITZEL, D.A. (1982)
Acute toxicity of tri-ortho-cresyl phosphate in sheep and swine.
Am. J. vet. Res., 43(11): 1954-1957.
WINDHOLZ, M., ed. (1983) The Merck index, 10th ed., Rahway, New
Jersey, Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate
esters: Application of linear free energy relationships to estimate
hydrolysis rate constants for use in environmental fate assessment.
Chemosphere, 9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-165.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides
in the atmosphere above the Dogo plain and Ozu basin.] J. Chem.
Soc. Jpn, 1980(4): 645-653 (in Japanese).
ZELIGS, M.A. (1938) Upper motor neuron sequelae in "Jake" paralysis
: A clinical follow up study. J. nerv. ment. Dis., 87: 464-470.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop. Can. Tech. Rep. fish aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de tricrésyle (TCP) est un liquide
visqueux, ininflammable, inexplosible et incolore. Son
coefficient de partage entre l'octanol et l'eau (log de
Pow) est égal à 5,1. Il s'hydrolyse facilement en
milieu alcalin pour donner du phosphate de dicrésyle, mais
il est stable en milieu neutre ou acide à température
normale.
Du point de vue analytique, la méthode de choix est la
chromatographie en phase gazeuse avec détection par un
dispositif sensible à l'azote/phosphore ou par photométrie
de flamme. La limite de détection dans les échantillons
aqueux est d'environ 1 mg/litre. Le TCP s'extrait facile-
ment des solutions aqueuses au moyen de divers solvants
organiques. Pour la purification, on emploie habituelle-
ment une colonne chromatographique de Florisil, mais il
est difficile de séparer le TCP des lipides par cette
méthode. D'autres méthodes de purification ont été re-
commandées à cette fin (chromatographie en phase gazeuse,
chromatographie sur charbon activé ou Sep-pack C-18). Les
réactifs analytiques sont souvent contaminés par des
traces de TCP en raison de la très large utilisation faite
de ce produit. Aussi faut-il prendre certaines précautions
si l'on veut que l'analyse des traces de TCP soit fiable.
2. Sources d'exposition humaine et environnementale
Le TCP est généralement produit par réaction des cré-
sols sur l'oxychlorure de phosphore. Il y a deux sources
de production industrielle de crésols: l'"acide crésy-
lique", résidu des fours à coke et du raffinage du
pétrole, et les "crésols de synthèse" préparés par oxy-
dation et dégradation du cymène. Le TCP est donc un
mélange de divers phosphates de triaryle.
Le TCP est utilisé comme plastifiant des matières
plastiques vinyliques, comme retardateur d'inflammation,
comme additif pour les lubrifiants à très haute pression
et comme liquide ininflammable dans les systèmes hydrau-
liques.
3. Transport, distribution et transformation dans l'environnement
La libération de TCP dans l'environnement est peu
importante au cours de la production et se produit essen-
tiellement lors de l'utilisation finale du produit. On
estime qu'en 1977, 32 800 tonnes en ont été libérées au
total dans l'atmosphère aux Etats-Unis.
En raison de sa faible solubilité dans l'eau et de sa
forte adsorption aux particules, le TCP s'adsorbe rapide-
ment aux sédiments des rivières et des lacs et aux parti-
cules du sol. Il est rapidement biodégradé en milieu
aquatique, sa décomposition étant pratiquement complète
dans les cours d'eau en l'espace de cinq jours. L'isomère
ortho se décompose légèrement plus vite que les isomères
méta et para. Le TCP est facilement biodégradé dans les
boues d'égouts, avec une demi-vie de 7,5 heures, la dégra-
dation atteignant 99% en l'espace de 24 heures. La dégra-
dation abiotique est plus lente, puisque dans ce cas la
demi-vie est de 96 jours.
Des facteurs de bioconcentration de 165-2768 ont été
mesurés en laboratoire sur plusieurs espèces de poissons à
l'aide de TCP radio-marqué. La radioactivité a rapidement
disparu après cessation de l'exposition, la demi-vie de
dépuration allant de 25,8 à 90 heures.
4. Niveaux dans l'environnement et exposition humaine
On a relevé dans l'air des concentration de TCP allant
jusqu'à 70 ng/m3 au Japon, les concentrations maximales
dans une unité de production des Etats-Unis d'Amérique
n'étant que de 2 ng/m3. Dans un atelier de remplissage
de fûts d'huile lubrifiante, aux Etats-Unis d'Amérique, on
a constaté que l'air ne contenait que 0,8 mg/m3 de TCP,
la concentration ne dépassant pas 0,15 mg/m3 en phos-
phates totaux, dans une unité de moulage de zinc d'une
usine d'automobiles. Les concentrations mesurées dans
l'eau de boisson au Canada se sont révélées faibles (0,4 à
4,3 ng/litre) et le TCP n'a pas pu être décelé dans l'eau
des puits. Dans les rivières et les lacs, les concen-
trations sont souvent beaucoup plus élevées. Toutefois on
peut attribuer cet état de choses à la présence de sédi-
ments en suspension auxquels le TCP est fortement adsorbé.
Les concentrations sédimentaires sont plus élevées
puisqu'elles peuvent atteindre 1300 ng/g dans le sédiments
de cours d'eau et 2160 ng/g par les sédiments marins.
Des concentrations élevées ont été mesurées dans le
sol et la végétation aux alentours d'unités de production.
On a fait état de résidus dans des poissons et des
fruits de mer allant jusqu'à 49 ng/g, mais la majorité des
échantillons n'en contenaient pas de quantités décelables.
5. Effets sur les êtres vivants dans leur milieu naturel
On a révélé une réduction de 50% de la productivité
des cultures d'algues vertes d'eau douce, en présence de
concentrations de phosphate de tri- o-crésyle (TOCP)
allant de 1,5 à 4,2 ng/litre, selon les espèces, les iso-
mères méta et para étant moins toxiques. On ne dispose que
de données limitées sur la toxicité aiguë du TCP vis-à-vis
des invertébrés aquatiques: la CL50 à 48 heures pour la
daphnie est de 5,6 ng/litre; la CL50 à 24 heures pour les
nématodes est de 400 ng/litre; la dose sans effet obser-
vable à 2 semaines pour la daphnie (mortalité, croissance,
reproduction) est de 0,1 mg/litre. Pour trois espèces de
poissons, les valeurs de la CL50 à 96 heures se situaient
entre 4,0 et 8700 mg/litre. Chez des truites arc-en-ciel
on a constaté une mortalité d'environ 30% après exposition
de quatre mois à 0,9 ng/litre de IMOL S-140 (phosphate de
tri- o-crésyle à 2%) et des effets plus légers sur une
période de 14 jours.
Les niveaux d'exposition au cours de ces expériences
étaient beaucoup plus élevés que les concentrations sus-
ceptibles d'être rencontrées dans le milieu naturel et
dans la plupart des cas, les valeurs étaient très
supérieures à la solubilité des composés.
6. Cinétique et métabolisme
L'absorption, la distribution, le métabolisme et
l'élimination des organophosphorés jouent un rôle déter-
minant dans les effets neuropathologiques retardés de ces
composés.
Chez l'homme, l'absorption percutanée du TOCP semble
être au moins dix fois plus rapide que chez le chien. On
observe également une importante absorption par cette voie
chez le chat. L'absorption par voie orale a été observée
chez le lapin. On ne possède aucune donnée de première
main sur l'absorption par la voie respiratoire.
Chez le chat, on a constaté qu'après absorption, le
TOCP se répartissait largement dans l'ensemble de l'orga-
nisme, la concentration la plus élevée se situant dans le
nerf sciatique, qui constitue un tissu cible. De fortes
concentrations de TOCP ou de ses métabolites se rencon-
traient également au niveau du foie, des reins et de la
vésicule bilaire.
La métabolisation du TOCP s'effectue selon trois
voies. La première consiste dans l'hydroxylation d'un ou
plusieurs groupes méthyles et la seconde comporte la
désarylation des groupements orthocrésyles. Dans la
troisième, il y a encore oxydation du groupement hydroxy-
méthyle en aldéhyde et en acide carboxylique. L'hydroxy-
lation constitue une étape déterminante, car le TOCP
hydroxyméthylé est cyclisé pour former du phosphate
cyclique d' o-tolyle et de saligénol, un métabolite
neurotoxique relativement instable.
Le TOCP et ses métabolites sont éliminés dans les
urines et les matières fécales ainsi que, en petites
quantités, dans l'air expiré.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuves
in vitro
Des trois isomères du TCP, le TOCP est de loin celui
qui présente la toxicité aiguë la plus forte et qui se
révèle également le plus toxique en cas d'exposition
brève. Il est le seul à déterminer une neurotoxicité
retardée.
Les différents paramètres toxicologiques varient
beaucoup selon l'espèce (qu'il s'agisse par exemple de la
mortalité aiguë ou de la neurotoxicité retardée). Le
poulet est l'une des espèces les plus sensibles.
On a pu obtenir chez des espèces d'animaux de labora-
toire très variées une neuropathie retardée induite par un
organophosphoré (NRIOP) tant à la suite d'une exposition
unique qu'à la suite d'expositions répétées. Il s'agit
d'une neuropathie qui se traduit par des altérations
dégénératives au niveau de la partie distale de l'axone et
qui s'étend peu à peu à l'ensemble du neurone.
Elle se manifeste cliniquement par une paralysie des
pattes postérieures après une période de latence caracté-
ristique de deux à trois semaines suivant l'exposition.
Une dose orale unique de 50 à 500 mg de TOCP/kg a produit
une neuropathie retardée chez des poulets, des doses de
840 mg/kg ou davantage étant nécessaires pour produire une
dégénérescence de la moëlle épinière chez des rats Long-
Evans. C'est l'un des métabolites du TOCP, le phosphate
cyclique d' o-tolyle et de saligénol, qui constitue
l'agent neurotoxique actif. La sensibilité des espèces
varie en sens inverse de la vitesse de métabolisation
ultérieure.
On pense que la lésion biochimique qui conduit à la
neuropathie consiste dans l'inhibition de l'"estérase
neurotoxique". Un taux d'inhibition de plus de 65% peu de
temps après une exposition au TOCP fait présager l'appari-
tion ultérieure d'une neuropathie. La variabilité dans la
réponse neurotoxique dépend également d'autres facteurs
(par exemple la voie d'exposition, l'âge, le sexe, la
souche). Les données disponibles ne permettent pas de
définir clairement une dose sans effet observé pour cette
neuropathie.
Les études de reproduction effectuées sur des rats et
des souris qui recevaient des doses répétées de TOCP par
voie orale, ont révélé la présence de lésions histopatho-
logiques au niveau des testicules et des ovaires, de modi-
fications dans la morphologie des spermatozoïdes, d'une
moindre fécondité chez les deux sexes ainsi que d'une
diminution de la taille des portées et de la viabilité des
ratons et des souriceaux. Les données disponibles n'ont
pas permis de déterminer la dose sans effet pour ce type
d'anomalies imputables au TOCP. Une étude de tératogéni-
cité effectuée sur des rats, avec des doses orales
toxiques pour la mère, n'a pas donné de résultats
positifs.
On n'a guère de données sur la mutagénicité et aucune
sur la cancérogénicité de cette substance.
8. Effets sur l'homme
L'ingestion accidentelle constitue la cause principale
d'intoxication. Depuis la fin du dix-neuvième siècle, de
nombreux cas d'intoxication dus à la contamination de
boissons, de denrées alimentaires ou de produits pharma-
ceutiques ont été signalés. L'exposition professionnelle
tient essentiellement à une absorption percutanée ou à une
inhalation et certains cas d'intoxication de ce type ont
été signalés. L'ingestion de préparations contaminées par
du TOCP peut produire des symptômes digestifs (nausée,
vomissements et diarrhées), encore que dans certains cas,
c'est la polyneuropathie qui constitue le premier signe
d'intoxication. Les symptômes neurologiques sont carac-
térisés par une période de latence. Les premiers symptômes
consistent en douleurs et paresthésie aux extrémités des
membres inférieurs. Il y a une légère diminution de la
sensibilité cutanée et quelques fois réduction de la sen-
sibilité vibratoire. Dans la plupart des cas, la faiblesse
musculaire évolue rapidement vers une paralysie des
extrémités inférieures avec ou sans extension aux membres
supérieurs. Dans les cas graves, apparaissent des signes
d'atteinte pyramidale. Les cas mortels sont rares mais
les symptômes neurologiques peuvent être très lents à dis-
paraître et la guérison peut prendre des mois, voire des
années. L'examen histopathologique révèle une dégénéres-
cence des axones. Les examens classiques de laboratoire
ne révèlent pas d'anomalies si ce n'est parfois une
augmentation de la teneur en protéines du liquide céphalo-
rachidien. Les premiers soins consistent à réduire
l'exposition en faisant vomir immédiatement le malade,
dans la mesure où celui-ci est encore conscient. A plus
long terme, le traitement consiste essentiellement en une
réadaptation physique car on ne connaît pas d'antidote
spécifique. La réaction au phosphate de tricrésyle varie
considérablement d'un individu à l'autre de même que les
possibilités de guérison à la suite d'une intoxication.
On a signalé l'apparition de symptômes graves après inges-
tion de 0,15 g de TCP, alors que chez d'autres personnes,
l'ingestion de quantités atteignant 1 à 2 g n'a produit
aucun effet toxique. Certains malades guérissent complète-
ment alors que d'autres présentent des séquelles marquées
pendant de très longues périodes.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
On a souvent signalé des cas d'intoxication humaine
par suite d'une ingestion accidentelle de phosphate de
tri- o-crésyle ou par suite d'une exposition profession-
nelle. Les symptômes neurotoxiques correspondent tout
d'abord à l'inhibition de la cholinestérase puis à une
neuropathie retardée caractérisée par une paralysie
grave.
Etant donné les variations considérables de sensi-
bilité selon les individus, il n'est pas possible de
déterminer quel est le seuil de sécurité. Des symptômes
ont été signalés après ingestion de 0,15 g d'un mélange
d'isomères contenant une faible proportion de TOCP; la
dose minimale efficace d'isomères ortho est donc beaucoup
plus faible encore. L'expérimentation animale fait égale-
ment ressortir de très importantes variations selon les
espèces pour ce qui concerne la réaction au TOCP et
l'homme semble être particulièrement sensible.
Des cas de dermatite d'irritation et de dermatites
allergiques ont été rapportés.
On peut donc considérer que l'isomère ortho et les
mélanges d'isomères qui en contiennent constituent un
risque de première importance pour la santé humaine.
Aucune dose n'est sans danger si elle est ingérée. Il
convient de réduire au minimum l'exposition cutanée ou
respiratoire.
1.1 Niveaux d'exposition
On peut considérer comme minimale l'exposition de la
population générale au phosphate de tricrésyle par
l'intermédiaire des divers compartiments du milieu
ambiant, notamment l'eau de consommation. On a décelé du
phosphate de tricrésyle à des concentrations plus fortes
dans l'air urbain que dans l'air prélevé au niveau des
sites de production, encore que ces valeurs soient
généralement faibles. Lors d'une enquête effectuée aux
Etats-Unis d'Amérique on n'a pas décelé de TCP dans des
échantillons de tissu adipeux humain. Il y a eu de
nombreux cas d'intoxication humaine accidentelle dus à
l'ingestion de médicaments, de nourriture, de farine,
d'huile de cuisine et de boissons contaminés par des
fluides hydrauliques ou des lubrifiants à base de TCP
produits à partir d'acide crésylique. Des symptômes
toxiques peuvent s'observer après ingestion de doses ne
dépassant pas 0,15 g de TOCP, substance qui est présente
dans le TCP produit à partir de l'acide crésylique. La
contamination se produit généralement lors de la
réutilisation de fûts ou de tonneaux vides qui avaient
contenu un liquide hydraulique ou de l'huile lubrifiante.
1.2 Effets toxiques
L'absorption accidentelle d'une forte dose entraîne
chez l'homme des troubles digestifs, à savoir une nausée
d'intensité variable, pouvant aller jusqu'aux vomisse-
ments, accompagnée de douleurs abdominales et de diar-
rhées. En cas d'exposition à de faibles doses cumulées,
une "neuropathie retardée" s'installe progressivement
après une période de latence de 3 à 28 jours. Dans la
plupart des cas, la faiblesse musculaire fait rapidement
place à une paralysie des membres inférieurs qui peut
parfois s'étendre aux mains. Dans les cas graves, on voit
peu à peu appraître des signes d'atteinte pyramidale.
Certaines études neurophysiologiques révèlent l'existence
d'une neurotoxicité généralisée avec prolongation du temps
de latence terminale et réduction relativement faible de
la vitesse de conduction des nerfs moteurs. Ces consta-
tations confirment la dégénérescence axonale qui est la
principale caractéristique relevée lors des examens histo-
pathologiques.
Le métabolite neurotoxique du TCP a été identifié; il
s'agit du phosphate cyclique de o-tolyle et de saligénol
qui provient lui-même des métabolites o-hydroxyméthylés.
Il semble donc que la présence d'au moins un groupement
o-tolyle soit nécessaire parmi les trois restes phéno-
liques du TCP pour qu'apparaissent des effets neuro-
toxiques. Le TCP produit à partir du crésol de synthèse,
qui contient moins de 0,1% d'orthocrésol, n'est donc pas
neurotoxique.
Les résultats d'études de toxicité subchronique
effectuées sur des animaux d'expérience avec du TCP pré-
paré à partir de crésol de synthèse, montrent que les
organes cibles sont le foie et le rein; toutefois cette
observation n'a pas été confirmée chez l'homme. On ne
dispose pas de données suffisantes sur la mutagénicité et
la cancérogénicité du TCP. On sait néanmoins que le TCP
n'est pas toxique pour l'embryon de poulet.
2. Evaluation des effets sur l'environnement
Le dosage du TCP dans l'eau montre que la contami-
nation est faible. Cet état de choses tient à la faible
solubilité dans l'eau du TCP et à l'aisance avec laquelle
il se décompose. Etant donnée la faible toxicité aiguë du
TCP pour les organismes aquatiques, il est peu probable
qu'il constitue une menace pour ces organismes.
Du fait de ses propriétés physico-chimiques, le TCP a
une forte tendance à la bioaccumulation. Toutefois celle-
ci ne se produit pas dans la pratique en raison de la
faible concentration du TOCP dans l'environnement et les
êtres vivants et de la dégradation rapide de cette
substance.
Les TCP fixés aux sédiments s'accumulent dans
l'environnement et on en a relevé des concentrations
élevées dans les sédiments des cours d'eau et des estu-
aires ainsi que dans les sédiments marins. Du fait que
l'on ne possède aucune information sur la biodisponibilité
de ces résidus pour les organismes fouisseurs ou ben-
thiques, ni sur les dangers qu'ils pourraient représenter,
on ne peut écarter à priori la possibilité d'effets
nocifs.
Il faut également mentionner la possibilité de risques
localisés pour l'environnement dus à un déversement acci-
dentel de TCP.
2.1 Niveaux d'exposition
Les TCP sont présents dans l'air, dans les eaux super-
ficielles, dans le sol, les sédiments et les organismes
aquatiques, à proximité des zones très industrialisées,
encore que leurs concentrations y soient généralement
faibles. En raison de la vitesse élevée de biodégradation
de ces substances en milieu aqueux, il ne semble pas
qu'elles puissent avoir des effets nocifs sur la faune
aquatique. Il a été fait état d'une concentration extrême-
ment élevée en phosphates totaux de triaryle (25,55 g/kg)
dans un échantillon de sol provenant d'une plantation.
Cette observation montre qu'il est nécessaire de procéder
à l'enfouissement des déchets.
2.2 Effets toxiques
Les algues d'eau douce sont relativement sensibles aux
TCP, la concentration inhibant à 50% la croissance com-
prise entre 1,5 et 5,0 ml/litre. En ce qui concerne les
poissons, des concentrations de TCP inférieures à
1 mg/litre (0,3-0,9 mg/litre) provoquent des signes
d'intoxication chronique chez la truite arc-en-ciel, mais
Menidia notata est plus résistant (CL50 de 8700
mg/litre). Les TCP n'inhibent pas l'activité cholinesté-
rasique des poissons ou des grenouilles mais ils poten-
tialisent l'effet des insecticides organophosphorés.
RECOMMANDATIONS
Lorsqu'on utilise des crésols tri-substitués pour la
synthèse et la préparation d'autres composés, il est
préférable d'utiliser des isomères para et méta purifiés
afin d'éviter toute synthèse accidentelle de dérivés
orthosubstitués.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El fosfato de tricresilo (FTC) es un líquido ininflam-
able, no explosivo, incoloro y viscoso. Su coeficiente de
partición entre el octanol y el agua (log Pow) es de
5,1. Se hidroliza con facilidad en un medio alcalino dando
fosfato de dicresilo y cresol, pero es estable en medios
neutros y ácidos a temperaturas normales.
El método analítico de elección es la cromatografía de
gases con un detector sensible al nitrógeno-fósforo o un
detector fotométrico de llama. El límite de detección en
una muestra de agua es de 1 ng/litro aproximadamente. El
FTC se extrae con facilidad de las soluciones acuosas con
distintos disolventes orgánicos. Se utiliza habitualmente
para la extracción la cromatografía en columna de flori-
sil, pero es difícil separar el FTC de los lípidos con
este método. Se han recomendado para esa finalidad otros
métodos de extracción (GPC, cromatografía en carbón vege-
tal activado y Sep-pak C-18). Los reactivos analíticos
están contaminados a menudo con cantidades infinitesimales
de FTC debido a su amplio uso. Por consiguiente, la obten-
ción de datos fiables en el análisis de cantidades infini-
tesimales de FTC requiere un procedimiento cuidadoso.
2. Fuentes de exposición humana y ambiental
El FTC se produce habitualmente por reacción de
cresoles con oxicloruro de fósforo. Existen dos fuentes
industriales de cresoles: el "ácido cresílico", obtenido
como residuo de los hornos de carbón de coque y del refino
del petróleo; y los "cresoles sintéticos", preparados a
partir del cimeno por oxidación y degradación. Como resul-
tado, el FTC es una mezcla de varios fosfatos triaríli-
cos.
El FTC se utiliza como plastificante en los plásticos
vinílicos, y también como pirorretardante, aditivo para
lubricantes de presión extrema y líquido ininflamable en
los sistemas hidráulicos.
3. Transporte, distribución y transformación en el medio ambiente
El paso de FTC al medio ambiente se debe principal-
mente a su uso final, pues la liberación en el curso de la
fabricación es escasa. En 1977 se calculó que el paso
total al medio ambiente en los Estados Unidos de América
fue de 32 800 toneladas.
Debido a su escasa hidrosolubilidad y a su elevada
adsorción por los materiales en partículas, el FTC se
absorbe con rapidez en los sedimentos de ríos o lagos y en
el suelo. Su biodegradación en el medio acuático es
rápida, quedando casi terminada en el agua de río en cinco
días. El isómero orto se degrada con una rapidez ligera-
mente mayor que los isómeros meta o para. El FTC se biode-
grada con facilidad en el fango de los alcantarillados,
presentando una semivida de 7,5 horas; la degradación en
24 horas alcanza el 99%. La degradación abiótica es más
lenta, dando una semivida de 96 días.
Se midieron los factores de bioconcentración de 165-
2768 en varias especies de peces en el laboratorio utili-
zando FTC radiomarcado. La radiactividad desapareció
rápidamente al cesar la exposición, observándose semividas
de depuración comprendidas entre 25,8 y 90 horas.
4. Niveles medioambientales y exposición humana
En el Japón se han medido concentraciones atmosféricas
de FTC de hasta 70 ng/m3, pero alcanzaron un máximo de
sólo 2 ng/m3 en una instalación de fabricación de los
Estados Unidos de América. En este país, el aire del medio
laboral contenía menos de 0,8 mg/m3 en una nave de
llenado de barriles de aceite lubricante y 0,15 mg/m3
(fosfatos totales) en una planta de troquelado de zinc
para automóviles. En el Canadá, las concentraciones de
FTC medidas en el agua potable fueron bajas (0,4 a
4,3 ng/litro) y el producto resultó indetectable en el
agua de pozo. Las concentraciones observadas en las aguas
de ríos y lagos son con frecuencia apreciablemente
mayores. Sin embargo, ello se debe a la presencia de sedi-
mentos en suspensión en los que queda fuertemente absor-
bido el FTC.
Las concentraciones en los sedimentos son altas en los
ríos y en el mar, habiéndose observado valores de hasta
1300 ng/g y 2160 ng/g, respectivamente.
Se observaron concentraciones altas en el suelo y la
vegetación en los alrededores de instalaciones de fabri-
cación.
Se han señalado restos en peces y mariscos de hasta
40 ng/g, pero la mayor parte de los animales examinados no
contenían residuos apreciables.
5. Efectos sobre los seres vivos del medio ambiente
La productividad primaria de cultivos de algas verdes
de agua dulce quedó reducida en el 50% mediante la adición
de fosfato de tri- o-cresilo a razón de 1,5 a 4,2
ng/litro, según las especies, mientras que los isómeros
meta y para resultaban menos tóxicos. Son limitados los
datos referentes a la toxicidad aguda del FTC para los
invertebrados acuáticos: la CL50 en 48 horas para Daphnia
es de 5,6 ng/litro y la CL50 en 24 horas para los nemato-
dos es de 400 ng/litro; la concentración NOEL (mortalidad,
crecimiento, reproducción) en dos semanas para Daphnia es
de 0,1 mg/litro. Los valores de CL50 en 96 horas para
tres especies de peces se hallaban comprendidos entre 4,0
y 8700 mg/litro. La trucha irisada presentó una mortalidad
del 30% aproximadamente después de una exposición de
cuatro meses a una concentración de 0,9 ng/litro de IMOL
S-140 (fosfato de tri- o-cresilo al 2%) y efectos menores
en un periodo de 14 días.
Los niveles de exposición utilizados en esos experi-
mentos fueron mucho mayores que las concentraciones que
probablemente pueden hallarse en el agua en el medio
ambiente y, en la mayoría de los casos, excedían en gran
manera a la solubilidad de los productos.
6. Cinética y metabolismo
La absorción, distribución, metabolismo y eliminación
de los organofosfatos son elementos críticos en los
efectos neuropáticos tardíos de estos productos.
La absorción cutánea del FTOC en el hombre parece ser
por lo menos de un orden de magnitud más rápida que en los
perros. También se observa una absorción cutánea signifi-
cativa en el gato. En el conejo se ha señalado la absor-
ción oral del producto. No hay información directa sobre
la absorción por inhalación.
En estudios efectuados en gatos se observó que el FTOC
absorbido se distribuye ampliamente por todo el organismo,
hallándose la máxima concentración en el nervio ciático,
que es el tejido diana. Otros órganos en los que se encu-
entran altas concentraciones de FTOC y de sus metabolitos
son el hígado, los riñones y la vesícula biliar.
El metabolismo del FTOC sigue tres vías. La primera
es la hidroxidación de uno o más grupos metílicos y la
segunda es la desarilación de los grupos o-cresilo. La
tercera vía es la oxidación ulterior del grupo hidroxi-
metilo para dar aldehído y ácido carboxílico. La etapa de
hidroxilación es decisiva porque el FTOC hidroximetílico
forma un producto cíclico, el fosfato cíclico de o-
tolilo saligenina, metabolito neurotóxico relativamente
ines-table.
El FOTC y sus metabolitos se eliminan por la orina en
las heces, junto con pequeñas cantidades en el aire
espirado.
7. Efectos en los animales de experimentación y en sistemas de
prueba in vitro
Entre los tres isómeros del FTC, el FOTC es con gran
diferencia el más tóxico en la exposición aguda y a corto
plazo. Es el único isómero que produce neurotoxicidad
tardía.
Existe una amplia variabilidad entre especies en lo
que respecta a los distintos puntos finales tóxicos (por
ejemplo, letalidad aguda, neurotoxicidad tardía) de la
exposición al FOTC, siendo el pollo una de las especies
más sensibles.
Se ha producido neuropatía tardía inducida por organo-
fosfatos mediante la exposición única y repetida en una
amplia gama de animales de experimentación; este trastorno
se clasifica como una "neuropatía de muerte sobre el
dorso". Se producen lesiones degenerativas en el axón
distal, que se extienden con el tiempo hacia el cuerpo de
la célula.
Los signos clínicos consisten en la parálisis de las
patas traseras después de un intervalo característico de
2-3 semanas a partir de la exposición. Una sola dosis
oral de 50-500 mg de FOTC/kg produjo la neuropatía tardía
en pollos, mientras que se necesitaron dosis de 840 mg/kg
o más para producir la degeneración de la médula espinal
en ratas Long-Evans. El metabolito fosfato cíclico de
o-tolilo saligenina es el agente neurotóxico activo. La
sensibilidad de las especies guarda correlación inversa
con la tasa de metabolismo ulterior.
Se cree que la inhibición de la "esterasa de la
neurotoxicidad" es la lesión bioquímica que conduce a la
neuropatía tardía inducida por organofosfatos; la inhi-
bición de más del 65% poco después de la exposición al
FOTC permite prever una neuropatía ulterior. En la vari-
abilidad de la respuesta neurotóxica al FOTC influyen
factores distintos del metabolismo (por ejemplo, vías de
exposición, edad, sexo, estirpe). Los datos disponibles no
permiten establecer un nivel neto sin efectos observados
para la neuropatía tardía.
Los estudios de reproducción en ratas y ratones some-
tidos a una exposición oral repetida al FOTC mostraron
lesiones histopatológicas de los testículos y los ovarios,
alteraciones morfológicas del esperma, disminución de la
fecundidad en ambos sexos, y descenso del tamaño y
viabilidad de las crías. Los datos disponibles no permiten
establecer un nivel neto sin efectos en lo que respecta a
la acción del FOTC sobre la reproducción. Un estudio de
teratogenicidad en ratas, utilizando dosis orales que
producían toxicidad materna, dio resultados negativos.
Se dispone de escasa información sobre la mutageni-
cidad y de ninguna sobre la cancerogenicidad.
8. Efectos en la especie humana
La ingestión accidental es la principal causa de
intoxicación. Desde fines del siglo XIX se han observado
numerosos casos de intoxicación por contaminación de bebi-
das, alimentos o medicamentos. La exposición profesional
se produce principalmente por absorción cutánea o inha-
lación, habiéndose registrado algunos casos de envenena-
miento. La ingestión de preparaciones que contienen FOTC
puede ir seguida de síntomas gastrointestinales (náuseas,
vómitos y diarrea), aunque en algunos casos la polineuro-
patía es el primer signo de intoxicación. Los síntomas
neurológicos suelen ser tardíos. Los síntomas iniciales
consisten en dolor y parestesia de las extremidades
inferiores. Puede observarse una alteración moderada de
las sensaciones cutáneas y a veces del sentido de la
vibración. En la mayoría de los casos, la debilidad
muscular evoluciona rápidamente hasta dar una marcada
parálisis de las extremidades inferiores, con o sin parti-
cipación de las superiores. En los casos graves aparecen
signos piramidales. Son raras las defunciones, pero la
recuperación de los síntomas y signos neurológicos puede
ser extremadamente lenta y durar varios meses o años. El
examen histopatológico muestra la presencia de degener-
ación del axón. Los análisis corrientes de laboratorio no
muestran hallazgos anormales, pero cabe observar un
aumento de la concentración de las proteínas en el líquido
cefalorraquídeo. Los primeros auxilios consisten en la
reducción de la exposición provocando el vómito inmediata-
mente después de la ingestión, siempre que el paciente
esté consciente. El tratamiento fundamental a largo plazo
consiste en la rehabilitación física, no conociéndose
ningún antídoto específico. Existen grandes variaciones
entre las personas en la respuesta al FTC y en la recuper-
ación después de producirse los efectos tóxicos. Se han
registrado síntomas graves después de la ingestión de
0,15 g de FTC, mientras que otras personas no presentaron
efecto tóxico alguno después de ingerir 1-2 g. Ciertos
enfermos se recuperan por completo, mientras que otros
conservan efectos marcados durante un periodo apreciable.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Se han registrado con frecuencia intoxicaciones
humanas por ingestión accidental de fosfato de tri- o-
cresilo (FTOC) o por exposición laboral de trabajadores.
La vía probable de la exposición laboral es la absorción
cutánea. Entre los síntomas neurotóxicos figuran la inhi-
bición inicial de las colinesterasas y la neuropatía
tardía ulterior caracterizada por parálisis grave.
Debido a la considerable variación existente en la
sensibilidad de las personas al FTOC, no puede estable-
cerse un nivel de exposición sin riesgo. Se han señalado
síntomas por ingestión de 0,15 g de una mezcla de isómeros
con baja proporción de FTOC; así pues, la dosis efectiva
mínima del ortoisómero es muy inferior. Los estudios
efectuados en animales muestran considerables variaciones
entre especies en la respuesta al FTOC, y las personas
parecen ser especialmente sensibles.
Se han señalado casos de dermatitis irritante y
alérgica.
En consecuencia, tanto el ortoisómero puro como las
mezclas de isómeros que contienen FTOC se consideran
riesgos importantes para la salud humana.
No existe un nivel inocuo de ingestión. La exposición
al producto por contacto cutáneo o inhalación debe
reducirse al mínimo.
1.1 Niveles de exposición
Puede considerarse mínima la exposición de la pobla-
ción general al fosfato de tricresilo (FTC) por conducto
de distintos medios ambientales, incluida el agua de
beber. Se han observado concentraciones de FTC relativa-
mente más altas en el aire urbano que en el aire recogido
en los emplazamientos de fabricación, aunque los niveles
suelen ser bajos. En un estudio efectuado en los Estados
Unidos de América no se detectó el FTC en muestras de
tejido adiposo humano. Se han observado numerosos casos
de intoxicación humana accidental por ingestión de medi-
camentos, alimentos tales como harina y aceite de cocinar,
y bebidas contaminados con líquido hidráulico o aceite
lubricante que contenían FTC producido a partir del
"ácido cresílico". Pueden observarse síntomas tóxicos
después de la ingestión de sólo 0,15 g de fosfato de tri-
o-cresilo, componente del FTC obtenido a partir de ácido
cresílico. El origen habitual de la contaminación ha con-
sistido en la reutilización de barriles o bidones vacíos
utilizados previamente para contener líquido hidráulico o
aceite lubricante.
1.2 Efectos tóxicos
La exposición humana accidental a una sola dosis alta
ocasiona trastornos gastrointestinales que varían de las
náuseas ligeras a las intensas con vómitos, dolor abdomi-
nal y diarrea. En el caso de la exposición a pequeñas
dosis acumuladas, aparece progresivamente la "neurotoxi-
cidad tardía" después de un periodo latente de 3-28 días.
En la mayor parte de los casos, la debilidad muscular
pasa rápidamente a ser una marcada parálisis de las ex-
tremidades inferiores, con o sin afectación de las manos.
En los casos graves aparecen progresivamente signos
piramidales. Algunos estudios neurofisiológicos muestran
fenómenos extendidos de neurotoxicidad y prolongación de
las latencias terminales, con disminución relativamente
pequeña de la velocidad de conducción de los nervios
motores. Ello confirma los signos de degeneración del
axón, que es la característica principal observada en los
exámenes histopatológicos.
Se ha identificado el metabolito neurotóxico del FTC
como el fosfato cíclico de o-tolilo saligenina, derivado
de los metabolitos o-hidroximetílicos. Parece pues que
los efectos neurotóxicos exigen la presencia por lo menos
de un grupo o-tolilo entre las tres porciones fenólicas
del FTC. Ello significa que no es neurotóxico el FTC
producido a partir de cresol sintético, que contiene menos
del 0,1% de o-cresol.
Los estudios de toxicidad subcrónica efectuados en
animales con FTC obtenido de cresol sintético muestran que
los órganos diana son el hígado y los riñones, pero esa
observación no se ha confirmado en los casos de intoxi-
cación humana. No se dispone de datos apropiados sobre la
mutagenicidad y la cancerogenicidad. El FTC no es tóxico
para los embriones de pollo.
2. Evaluación de los efectos en el medio ambiente
La medición de las concentraciones de FTC en el agua
ambiental ha mostrado que existen sólo niveles bajos de
contaminación. Ese hecho refleja la escasa hidrosolu-
bilidad y la fácil degradabilidad del producto. Dado que
la toxicidad aguda del FTC para los seres acuáticos es
también baja, es improbable que represente una amenaza
para ellos.
Debido a las propiedades fisicoquímicas del FTC
existen altas posibilidades de bioacumulación. Sin
embargo, ello no se produce en la práctica porque las con-
centraciones de FOTC en el medio ambiente y en los organ-
ismos vivos son bajas y porque los productos se degradan
con rapidez.
El FTC fijado por los sedimentos se acumula en el
medio ambiente, habiéndose medido concentraciones altas en
los sedimentos de ríos, estuarios y mares. Dado que se
carece de información sobre la biodisponibilidad de esos
residuos para los seres vivos que se hallan en madrigueras
o en el fondo o sobre sus riesgos, no puede descartarse la
posibilidad de que aparezcan efectos en tales especies.
La fuga de FTC suscita riesgos para el medio ambiente
local.
2.1 Niveles de exposición
El FTC se halla en el aire, las aguas de superficie,
el suelo, los sedimentos y los seres vivos acuáticos cerca
de las zonas muy industrializadas, aunque en concen-
traciones habitualmente bajas. Debido a la alta tasa de
biodegradación del FTC en el medio acuoso, no se considera
que afecta adversamente a los seres vivos acuáticos. En un
estudio se encontró una concentración extremadamente alta
de fosfatos triarílicos totales (26,55 g/kg) en una
muestra de suelo obtenida en el patio de una planta de
producción. Ello sugiere la necesidad de eliminar los
residuos por relleno de terrenos.
2.2 Efectos tóxicos
Las algas de agua dulce son relativamente sensibles al
FTC, cuya concentración inhibidora del 50% del crecimiento
es de 1,5 a 5,0 mg/litro. Entre las especies de peces, la
trucha irisada sufre el efecto de concentraciones de FTC
inferiores a 1 mg/litro (0,3-0,9 mg/litro), con signos de
intoxicación crónica, pero el pez argentado de las mareas
es más resistente (CL50 de 8700 mg/litro). El FTC no
inhibe la actividad colinesterásica seca de peces y ranas,
pero tiene un efecto sinérgico con la actividad
insecticida de los órganos fosforados.
RECOMENDACIONES
Cuando se utilizan cresoles trisustituidos en la
síntesis y fabricación de otros productos, es necesario
emplear isómeros meta y para purificados para evitar la
síntesis accidental de productos orto-sustituidos.
TOCP is metabolized via three essential pathways. The
first is the hydroxylation of one or more of the methyl
groups to hydroxymethyl, which is responsible for the for-
mation of mono- and di-hydroxymethyl TOCP and o-hydroxy-
benzyl alcohol. This reaction is known to be catalysed by
the microsomal mixed-function oxidase system (Eto et al.,
1967). The hydroxymethyl TOCP is cyclized to form sal-
igenin cyclic o-tolyl phosphate with spontaneous release
of o-cresol, this being catalysed by the reaction of
plasma albumin or other components (Eto et al., 1967). The
cyclic phosphate metabolite is relatively unstable and is
rapidly hydrolysed to inactive metabolic products (Eto et
al., 1967). The second pathway is the dearylation of one
or more of the o-cresyl groups of TOCP, resulting in the
formation of o-cresol, di- o-cresyl phosphate, o-cresyl
phosphate, and phosphoric acid. The third pathway is
further oxidation of hydroxymethyl to aldehyde and car-
boxylic acid. These oxidation reactions are most likely to
be mediated by alcohol and aldehyde dehydrogenases.
Studies with [32P]-TOCP in rats have shown that
hydrolysis leads to the rapid excretion in the urine of
diaryl phosphates, monoaryl phosphates, and phosphoric
acid (Casida et al., 1961).
Nomeir & Abou-Donia (1984; 1986a,b) clearly identified
the metabolites of TOCP in male cats. Mono- and di-
hydroxymethyl TOCPs and saligenin cyclic- o-tolyl phos-
phates were present in most tissues, but their concen-
trations were low compared with those of other metab-
olites. The major metabolite of TOCP in the liver, kidney,
lung, and urine of cats was o-hydroxybenzoic acid; di-
o-cresyl phosphate, o-cresyl phosphate, o-cresol,
o-hydroxy-benzyl alcohol, and o-hydroxybenzaldehyde
were also identified. However,the brain, spinal cord,
sciatic nerve, and faeces contained predominantly
unchanged TOCP.
Johnson (1975a) compared the metabolic pathways of the
three isomers of TCP. The main observations, which con-
cerned several organophosphorus esters, were as follows:
(i) Provided that the o-alkyl group has at least one
hydrogen on the alpha-carbon atom, cyclic derivatives
can be obtained that are often highly neurotoxic.
(ii) At the para position, a substituent requires two
hydrogen atoms on the alpha-carbon atom in order to
produce a neurotoxic metabolite inhibiting NTE.
(iii) Substituents at the meta position may be metab-
olized but do not yield inhibitory products.
The major urinary metabolites of TPCP in rats were
p- hydroxybenzoic acid, di- p- cresyl phosphate, and
p- cresyl p- carboxyphenyl phosphate. Mono- (or di-) p-
cresyl di- (or mono-) p- carboxyphenyl phosphate was
identified as the intermediate metabolite in the bile
(Kurebayashi et al., 1985).
7.4 Excretion
After a single oral dose of [32P]-TOCP (770 mg/kg)
to hens, 26.5% of the total radioactivity was eliminated
in the combined urinary-faecal excreta over 72 h, mostly
as TOCP (Sharma & Watanabe, 1974).
After a single dose of [14C]-TOCP (50 mg/kg) to male
cats, approximately 28% of the applied dose was excreted
in the urine and 20% via the bile into the faeces within
10 days (Nomeir & Abou-Donia, 1986b). After this exposure,
the disappearance of TOCP and its metabolites from the
plasma followed monoexponential kinetics. The apparent
half-lives of TOCP and its metabolites (in days) in the
plasma were: TOCP, 1.20; saligenin cyclic- o- tolyl phos-
phate, 2.47; di- o- cresyl phosphate, 4.50; o- cresyl
phosphate, 4.30; o- cresol, 2.65; o- hydroxybenzyl
alcohol, 14.0; o- hydroxy-benzaldehyde, 5.70; o-
hydroxybenzoic acid, 6.00; monohydroxymethyl TOCP, 2.20.
The apparent half-lives of TOCP and its metabolites
reflected the rates of all processes involving the
conversion, clearance, and/or redistribution of these
metabolites (Nomeir & Abou-Donia, 1984).
Elimination via the bile has been demonstrated after
intravenous injection into rabbits (Gross & Grosse, 1932)
and intraperitoneal injection into rats (Myers et al.,
1955). Smith et al. (1932) measured the urinary phenol
excretion in cats given subcutaneous doses of 0.4 to 1.0
ml TOCP/kg. Little if any increase in the urinary phenol
excretion was found either before or after the onset of
paralysis of the hindlimbs.
After a single oral dose (500 mg/kg) of tri- m-cresyl
phosphate (TMCP) or TPCP to rabbits, 92% of TMCP and 95%
of TPCP was eliminated in the faeces within 4 days (Gross
& Grosse, 1932). After a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg), about 90% or 76%,
respectively, of the radioactivity was eliminated in the
urine and faeces within 24 h (Kurebayashi et al., 1985).
The apparent half-lives of the radioactivity in tissues
ranged from 14 h for blood to 26 h for lung and brain. At
the lower dose level, about 28% of the dose was eliminated
via the bile within 24 h. The expiratory excretion as
14CO2 over 3 days amounted to 18% of the radioactivity,
which was reduced to 3% when the rats were treated with
neomycin. The authors suggested that the enterohepatic
circulation and intestinal microflora play an important
role in the degradation of TPCP biliary metabolites.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Summary
Of the three isomers of TCP, TOCP is by far the most toxic
and is the only isomer that produces delayed neuropathy.
A wide animal interspecies variability exists for the
various end-points (e.g., lethality, neuropathology, ataxia,
enzyme inhibition) of TOCP exposure, the chicken being one of
the most sensitive species to delayed neuropathy (i.e. OPIDN).
Species sensitivity to the lethal effects of TOCP adminis-
tration is highly variable, chickens and cats being more sensi-
tive than rats and mice. A single oral dose of 50-500 mg TOCP
per kg induced delayed neuropathy in chickens, whereas doses of
840 mg/kg or more were necessary to produce spinal cord degen-
eration in Long-Evans rats.
No effects were reported in skin and eye irritation studies
on rabbits.
Reproduction studies on rats and mice receiving repeated
oral exposure to TOCP showed histopathological damage in the
testes and ovaries, morphological changes in sperm, decreased
fertility in both sexes, and decreased litter size and
viability. However, no reproductive effects were seen in a
study with TPCP. A clear no-observed-effect level for the
reproductive effects of TOCP was not apparent from the data
available. A study in rats, using oral doses that produced
maternal toxicity, failed to show any teratogenicity.
Little or no information is available on mutagenicity and
carcinogenicity.
Delayed neuropathy has been produced with both single and
repeated exposure regimes in a wide range of experimental
species, and it is classified as a "dying-back neuropathy".
"Neurotoxic esterase" is thought to be the biochemical target
of OPIDN and its inhibition by more than 65% shortly after
exposure to TOCP presages subsequent neuropathy. Factors other
than metabolism (e.g., route of exposure, age, sex, species,
strain) influence variability in sensitivity to OPIDN.
Electrophysiology studies have been performed in cats and
chickens exposed to TOCP.
In the chicken, single exposures below 58 mg/kg or short-
term (i.e. 90-day exposure) daily doses of less than 5 mg/kg
appear to be no-observed-effect levels for delayed neuropathy.
8.1 Single exposure
The acute toxicity of TCP to different species is
summarized in Table 13.
Table 13. LD50 values for TCP and its isomers
____________________________________________________________________
Compounds Route Species LD50 Reference
of (mg/kg)
admin.
____________________________________________________________________
Tricresyl oral rat 5190 Marhold (1972)
phosphate oral rat >4640 Stauffer (1988)a
(mixed oral rat >15 800 Johannsen (1977)
isomers) oral mouse 3900 Izmerov (1982)
oral chicken >10 000 Johannsen et al. (1977)
dermal rabbit >7900 Johannsen et al. (1977)
dermal cat 1500 Abou-Donia et al. (1980)
Tri- o- cresyl oral rat 8400 Johannsen et al. (1977)
phosphate oral rat 1160 Veronesi et al. (1984a)
oral rabbit 3700 Johannsen et al. (1977)
oral chicken 500 Kimmerle & Loeser (1974)
oral chicken 100-200 Smith et al. (1932)
Tri- p- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate chicken >1000 Smith et al. (1932)
Tri- m- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate oral chicken >2000 Smith et al. (1932)
____________________________________________________________________
a Personal communication to the IPCS from Stauffer Chem. Co. (1988)
entitled: Test procedures and data summaries for t-butyl phenyl
diphenyl phosphate, tricresyl phosphate, trixylenyl phosphate,
mixed triaryl phosphate and isopropyl phenyl diphenyl.
The acute symptoms of intoxication are typical of
organophosphorus poisoning. The most toxic compound
appears to be TOCP; the acute toxicity of TCP depends on
the relative proportions of the different isomers.
The chicken, guinea-pig, and rabbit are the most sen-
sitive species, death occurring at an oral dose of 100
mg/kg (Smith et al., 1932); rats and mice are the least
sensitive species.
Sheep given oral doses (100, 200, or 400 mg/kg) of
TOCP exhibited acute intoxication characterized by diar-
rhoea, dehydration, metabolic acidosis, and death within
6 days. Pigs dosed with 100 to 1600 mg TOCP/kg showed
minimal signs of acute intoxication, but developed severe
signs of delayed neuropathy approximately 15 days after
administration (Wilson et al., 1982).
8.2 Short-term exposure
Saito et al. (1974) conducted a 3-month study in rats
with TCP consisting of 60-65% TMCP and 35-40% TPCP. The
compound was suspended in water with 5% gum arabic and
given orally to SD rats at 30, 100, 300, or 1000 mg/kg per
day. Histopathological examination revealed no notable
changes associated with the compound. Based on those
observations, the authors concluded that TCP was of low
short-term toxicity.
Oishi et al. (1982) reported that Wistar rats fed a
pellet diet containing a mixture of TCP isomers at a con-
centration of 5 g/kg diet for 9 weeks developed increases
in absolute and relative liver weights. Haematological
examination revealed no notable changes, but, in the
plasma, total protein, urea, cholesterol and glutamate-
pyruvate transaminase were significantly increased. Slight
liver histopathology included cytoplasmic vacuolization,
increase in the number of binucleated cells, and enlarge-
ment of cell size.
Chapin et al. (1988) exposed male and female CD-1 mice
to diets containing 0, 0.437, 0.875, 1.75, 3.5, or 7.0%
TCP (a mixture of ortho, meta, and para isomers) for
14 days. No clinical signs of toxicity were observed in
the animals at doses up to 0.875%. All animals in the
groups given 1.75, 3.5, or 7.0% exhibited piloerection,
tremors, and diarrhoea, and were lethargic before death
during the 14-day exposure.
8.3 Skin and eye irritation
No published information is available.
8.4 Teratogenicity
Mele & Jensh (1977) reported that no abnormalities
were found in fetuses from pregnant Wistar rats treated
with 500 or 750 mg TOCP/kg of on the 18th and 19th days of
gestation. This study was primarily designed to investi-
gate the effects of prenatal treatment with TOCP on post-
natal behaviour. As such, it cannot be regarded as a true
teratogenicity study.
Tocco et al. (1987) tested the teratogenicity of TOCP
in Long-Evans rats treated with 87.5, 175, and 350 mg/kg
per day throughout organogenesis from gestation days 6 to
18. No maternal deaths or toxicity were observed at the
low or medium dose levels. Maternal lethality in the high-
dose group was higher than that in the control groups.
Numerous soft tissue and skeletal malformations were
observed in both control and TOCP-treated groups, but
there were no significant differences in the frequency of
malformations between the treated and control animals.
8.5 Reproduction
In studies by Somkuti et al. (1987a), TOCP was tested
for effects on the male reproductive tract in male Fisher-
344 rats. Animals were dosed daily for 63 days at dose
levels ranging from 10 to 100 mg/kg per day. Vehicle-
treated animals served as controls. As judged by enzy-
matic, hormonal, and sperm motility, density, and mor-
phology investigations, the minimum effective (threshold)
dose for observable testicular toxicity was 10-25 mg/kg
per day. The data suggested that TOCP interfered directly
with spermatogenic processes and sperm motility and not
via androgenic mechanisms or decreased vitamin E avail-
ability. Testicular pathological changes were seen at
doses above 25 mg/kg per day and included the following:
PAS-positive droplets, immature germ cells, and multinu-
cleate giant cells in the lumen. TPCP produced a decrease
in sperm density but no other testicular effects at a dose
level of 100 mg/kg per day.
To study the time course of the TOCP-induced testicu-
lar lesion in F-344 rats, the onset of possible changes in
sperm numbers and production, serum hormones, and various
enzyme activities was followed. Rats were administered
TOCP daily (150 mg/kg) for periods of 3, 7, 10, 14, or 21
days, while vehicle-treated animals served as controls.
Both sperm motility and the number of sperm per mg of
cauda epididymis were lower in treated animals by day 10.
The ratio of testicular to body weight was significantly
decreased only those rats treated for 21 days. Testicular
neurotoxic esterase and nonspecific esterase activities
were also inhibited, while beta-glucuronidase activity was
not affected. Luteinizing and follicle-stimulating hormone
levels were normal, as were both serum and interstitial
fluid testosterone concentrations. Sertoli cell fluid
secretion, as measured by testis weight increase after
efferent duct ligation, showed no significant changes.
Other organs (spleen, liver, kidney, pancreas, small
intestine, and adrenal and pituitary glands) revealed no
overt signs of pathology as observed by light microscopy
in animals treated for 21 days. A separate group of ani-
mals was treated for 21 days and subsequently examined
after 98 days of observation (two cycles of the rat semin-
iferous epithelium). Normal spermatogenesis did not
return, indicating that the toxicity was irreversible at
the dose used. The effects noted in these studies further
define the testicular lesion produced by TOCP, and show
that a dose level 150 mg/kg per day for 21 days produces
irreversible testicular toxicity (Somkuti et al., 1987b).
The testicular effects of TOCP have also been studied
in the rooster, 100 mg/kg per day being administered
orally to ten adult leghorn roosters for 18 consecutive
days. By days 7-10 of the study, TOCP-treated birds
exhibited limb paralysis characteristic of OPIDN. Enzyme
analyses at the end of the study revealed significant
inhibition of neurotoxic esterase (NTE) activity in both
brain and testis and a slight decrease in brain acetyl-
cholinesterase (AChE) activity. Sperm motility was shown
to be greatly decreased. In addition, sections of
formalin-fixed, methacrylate-embedded testes from TOCP-
treated birds showed vacuolation and disorganization in
the seminiferous epithelium. The marginal body weight
decrease (17%) in treated animals was not considered to
contribute to the testicular toxicity induced by TOCP
(Somkuti et al., 1987c).
TCP (isomer mixture not known) was tested in sperm
morphology and vaginal cytology examination (SMVCE)
studies in groups of 25 male and female Fisher-344 rats
and 25 male and female Swiss CD-1 mice. Treatment lasted
for 13 weeks at dose levels of 1700, 3300, or 6600 mg/kg
diet or 50, 100, 200, 400, or 800 mg/kg body weight via
gavage in the rats, and 500, 1000, or 2100 mg/kg diet or
50, 100, or 200 mg/kg body weight via gavage in the mice.
Effects were seen in treated animals, but no details were
given (Morrissey et al., 1988a). In a follow-up continuous
breeding reproduction study in Swiss CD-1 mice, male and
female fertility was reduced (Morrissey et al., 1988b).
Carlton et al. (1987) examined the reproductive
effects of TCP (mixed isomers; < 9% TOCP). Male Long-Evans
rats received 0, 100, or 200 mg/kg and females received 0,
200, or 400 mg/kg in corn oil by gavage. The low-dose
males were mated with low-dose females, and the high-dose
males with high-dose females. Males were dosed for 56 days
and females for 14 days prior to breeding and throughout
the breeding period, gestation, and lactation. Sperm con-
centration, motility, and progressive movement were
decreased in the high-dose males, and there was a dose-
dependent increase in abnormal sperm morphology. The
number of females delivering live young was severely
reduced by TCP exposure. Litter size and pup viability
were decreased in the high-dose group, but pup body weight
and developmental landmarks were unaffected by TCP
exposure. Histological changes were observed in the testes
and epididymides of treated males (i.e. necrosis, degener-
ation, early sperm granulomas in seminiferous tubules, and
epididymal hypospermia) and in the ovaries of treated
females (i.e. diffuse vacuolar cytoplasmic alteration of
interstitial cells and increased numbers of follicles and
corpora lutea).
In a continuous breeding study, Chapin et al. (1988)
fed Swiss CD-1 mice a diet containing TCP (mixed o-, m-,
p-isomers) at a level of 0, 0.5, 1, or 2 g/kg diet over
98 days. Although the fertility index was not changed in
the high-dose groups, the number of litters per pair
decreased in a dose-dependent fashion, and the proportion
of pups born alive and their weight were significantly
decreased. Histopathological examination of the parental
generation revealed dose-related seminiferous tubule
atrophy and decreased testis and epididymal weights in the
high-dose males, but the female reproductive tract showed
no histopathological changes. A crossover mating trial
revealed impaired fertility in both males and females
exposed to 2 g/kg diet.
8.6 Mutagenicity and carcinogenicity
Haworth et al. (1983) reported a negative result with
TCP (substitution pattern not known) in the Salmonella
mutagenicity test.
8.7 Neurotoxicity
8.7.1 Experimental neuropathology
TOCP first gained notoriety as the culpable neurotoxic
agent of the "Ginger Jake" epidemic (Jeter, 1930;
Goodale & Humphreys, 1931; Vonderahe, 1931). Since then
several experimental studies have modelled TOCP neuropathy
in various species.
Smith & Lillie (1931) produced delayed paralysis in
various animals, including dogs, monkeys, cats, and
chickens. These animal models were used to describe the
functional and morphological features of TOCP neuropathy.
After a delay period of 2-3 weeks following exposure to
single or multiple doses, paralysis of the hindlegs
developed in these species in response to the TOCP. Neuro-
pathologically, degeneration was confined to the spinal
cord and peripheral nerve fibres. These changes were
essentially similar to the lesions reported in human
victims of the "Ginger Jake" epidemic.
The delayed neuropathy associated with TOCP and other
organophosphorous compounds has been termed OPIDN.
Cavanagh (1964) showed that, in cats and chickens,
degeneration affected the long fibres in the spinal cord
and peripheral nerves; moreover, in cats, fibre diameter
seemed to be important in determining the onset and
severity of the peripheral nerve lesions. He categorized
this delayed neuropathy as a "dying-back". Thus, the
lesions of the ascending tracts are seen in the cervical
region, especially in the dorsal columns (i.e. fasciculus
gracilis), while those of the descending tracts are seen
in the thoracic and lumbosacral regions of the spinal
cord. He also showed that the peripheral and central nerve
lesions were the result of axonal degeneration and did not
reflect primary demyelination. The affected nerve axons
degenerate in a "dying-back" fashion towards the cell
body, i.e. axonal degeneration begins at the most distal
portion of the axon and proceeds towards the cell body.
Prineas (1969) described axons with accumulated
tubulovesicular membranes within myelinated motor nerve
terminals in the foot muscles of cats dosed with TOCP.
Similar early axoplasmic changes have been noted in TOCP-
treated chickens (Bischoff, 1967, 1970; Spoerri & Glees,
1979, 1980).
At the ultrastructural level, the nerve endings are
characterized by a marked proliferation and distension of
vesicular elements of the endoplasmic reticulum and a
coinciding disintegration of the filamentous and tubular
organelles (Bischoff, 1977). Bischoff also noted that the
presynaptic nerve terminals and boutons terminaux appeared
particularly sensitive in TOCP intoxication.
OPIDN has also been produced in rats dosed with TOCP
(Veronesi, 1984). The pathological changes developed in
the absence of discernable ataxia after a 2-week exposure
to acute and multiple oral doses of TOCP (>840 mg/kg). In
the rat, dorsal column degeneration of the cervical cord
and a selective vulnerability of large diameter tibial
branches supported a "dying-back" neuropathy, as in
other species.
OPIDN has also been described in European ferrets
( Mustela putorius furo) administered a single oral or
dermal dose of 250, 500, or 1000 mg TOCP/kg body weight.
Five animals per group were sacrificed 48 h after dosing,
the others being observed for another 54 days. All ferrets
dosed dermally with 1000 mg/kg developed neurological
signs ranging from ataxia to partial paralysis. Dermal
doses of 250 and 500 mg/kg produced variable degrees of
hind limb weakness and ataxia. Of the animals dosed
orally, only those treated with 1000 mg/kg showed neuro-
logical signs, which did not progress beyond mild ataxia.
Slight axonal degeneration was noted in the dorsolateral
part of the lateral funicilus and in the fasciculus gra-
cilis of spinal cords in ferrets receiving a dermal dose
of 1000 mg/kg. Whole brain neurotoxic esterase activity
was maximally inhibited (46%) at this dose level. The
study demonstrated that in the ferret dermal exposure was
more effective than oral exposure at the same dose level
(Stumpf et al., 1989).
8.7.2 Neurochemistry
Johnson (1969) found that approximately 6% of brain
esterases are not affected by non-neuropathic compounds
but are specifically inhibited, irreversibly, by neuro-
pathic ones, such as TOCP. Johnson used the term "neuro-
toxic esterase" (NTE) and proposed that NTE is the pri-
mary target of the organophosphorus esters causing OPIDN
(Johnson, 1975a,b). There appears to be a strong corre-
lation in the chicken between NTE inhibition above 70%
shortly after exposure and subsequent neuropathy for a
large number of tested organophosphorous compounds
(Johnson, 1974).
Padilla & Veronesi (1985) demonstrated the relevance
of NTE to the rodent model of OPIDN by exposing Long-Evans
rats to single doses of TOCP ranging from 290 to 3480
mg/kg. High NTE inhibition in the spinal cord (>72%) and
the brain (> 66%) produced severe spinal cord damage in
over 90% of exposed rats, indicating that NTE depression
could predict OPIDN damage in rats acutely exposed to
organophosphates.
Concerning the role of lipids in TOCP neuropathy,
Morazain & Rosenberg (1970) showed that there was a rise
of 25-50% in the cholesterol level in the sciatic nerve
and a 50% decrease in its triglyceride content in chickens
orally dosed with 1 ml TOCP/kg. Phospholipids, diglycer-
ides, cholesterol esters, proteolipids, and tissue phos-
pholipases were not affected by TOCP. The possible
involvement of lipids in the production of TOCP-induced
delayed neuropathy has not yet been resolved.
Other toxic effects have been demonstrated. Brown &
Sharma (1975) found that neural membrane ATPases were
inhibited by organophosphates. Cohen & Murphy (1970)
reported that TOCP potentiates the anticholinesterase
action of malathion by 29-fold in mice, 17-fold in quail,
and 11-fold in sunfish.
8.7.3 Interspecies sensitivity and variability to OPIDN
Certain animal species (e.g., cats, dogs, cows, and
chickens) are susceptible to OPIDN-related paralysis,
whereas others (e.g., rats and mice) are less susceptible
to the ataxia but very susceptible to the pathological
changes. Species susceptibility to delayed neurotoxicity
induced by TOCP shows an inverse correlation with the rate
of metabolic conversion to the neurotoxic metabolite (see
section 7). Because of its high susceptibility to ataxia,
the adult chicken has been used as an experimental model
to study OPIDN.
TOCP is metabolized to the more potent neurotoxic
agent, saligenin cyclic o-tolyl phosphate, which is at
least five times more neurotoxic than TOCP after oral
administration to chickens: a metabolite level of 40 mg/kg
caused ataxia equivalent to that resulting from 200 mg
TOCP/kg (Bleiberg & Johnson, 1965).
It has been shown that a single oral dose of TOCP in
the range of 58 to 580 mg/kg (i.e. 0.05-0.5 ml/kg) induces
mild to severe paralysis in the hen ( Gallus domesticus)
(Cavanagh, 1954; Hine et al., 1956).
Johannsen et al. (1977) showed that chickens adminis-
tered cumulative doses of TCP (60 000 mg/kg) or TOCP (1500
mg/kg) developed both the ataxic and neuropathological
symptoms of OPIDN.
In 90-day studies in hens, obvious functional and
morphological neuropathological changes were found at
daily oral dose levels of 5 to 20 mg TOCP/kg body weight,
but not at lower dosages (Smith et al., 1932; Prentice &
Majeed, 1983; Roberts et al., 1983).
In a subchronic feeding study, Haggerty et al. (1986)
exposed rats to TCP (isomeric substitution pattern not
known) for 13 weeks at dose levels of 0, 900, 1700, 3300,
6600, or 13 000 mg/kg. Decreased hindlimb grip strength
was observed in male rats (at 13 000 mg/kg), but not in
female rats. In mice exposed to 0, 250, 500, 1000, 2100,
or 4200 mg/kg, decrements in both fore- and hindlimb grip
strength were observed in males (at 4200 mg/kg) and
females (at 2100 and 4200 mg/kg). A reduction of body
weight was seen both in rats and mice at the two highest
dose levels. Preliminary histopathological diagnosis
indicated demyelination and axonal degeneration of the
sciatic nerve in male and female mice only.
Freeman et al. (1988) tested the neurotoxicity of TCP
(substitution pattern not known) to F-344 rats in a short-
term study. After 13 weeks of dosing with TCP in the feed,
hindlimb grip strength decreased in male rats at 300 and
600 mg/kg, but not in female rats. Serum cholinesterase
was reduced at 300 and 600 mg/kg in both males and
females. All effects observed with 600 mg/kg were appar-
ently reversible during the recovery period.
In a 13-week short-term feeding study, Irwin et al.
(1987) exposed F-344 rats to TCP (0, 75, 150, 300, or 600
mg/kg; substitution pattern not known), while B6C3F1 mice
receive 0, 60, 125, or 250 mg/kg. After 13 weeks of
dosing, forelimb grip strength was unaffected by TCP in
both mice and rats. Hindlimb grip strength decreased in
male rats (at 300 and 600 mg/kg) but not in female rats.
In mice, decrements in hindlimb grip strength were
observed in males (250 mg/kg) and females (125 and 250
mg/kg). Serum cholinesterase levels showed a dose-depen-
dent reduction in both rats and mice. TCP had no effect on
food consumption in either species. All groups exhibited
normal body weight values.
Factors such as age, sex, and strain figure promi-
nently in the expression of OPIDN. The young of most
species are non-susceptible to TOCP-induced delayed neuro-
pathy (Johnson & Barnes, 1970), which could be due to poor
absorption of TOCP. However, recent experiments (Olson &
Bursian, 1988) have suggested that factors (e.g., route of
administration) other than absorption are more critical to
this lack of susceptibility.
Strain differences in the susceptibility of rats to
TOCP-delayed neuropathy have been reported. Although OPIDN
can be readily produced in Long-Evans and Sprague-Dawley
rats after acute oral doses of > 840 mg/kg (Veronesi &
Abou-Donia, 1982; Veronesi, 1984), repeated doses of TOCP
(10-100 mg/kg) failed to produce neuropathy in Fischer-344
rats (Somkuti et al., 1988). Variations in TOCP inhibition
of brain AChE and NTE have also been reported in these
three strains (Carrington & Abou-Donia, 1988).
8.7.4 Neurophysiology
Robertson et al. (1987) investigated electrophysio-
logical changes in the adult hen following single oral
doses of 30 or 750 mg TOCP/kg. At the higher dose, the
birds demonstrated clinical signs of toxicity 12 days
after dosing that included gait abnormalities, which
became progressively more severe and in some cases led to
complete ataxia. Lymphocyte NTE was inhibited by more
than 70%. The lower dose produced no clinical signs of
toxicity and only 54% lymphocyte NTE inhibition. Both
treated groups displayed significant action potential
disruption in both the tibial and sciatic nerves that
resulted in decreased refractoriness in the tibial nerve,
increased refractoriness in the sciatic nerve, and
elevated strength duration threshold for both nerves.
Electrophysiological changes have been investigated in
cats following single dermal doses ranging from 250 to
2000 mg TOCP/kg and 90-day dermal administration of 1 to
100 mg/kg (Abou-Donia et al., 1986). In contrast to the
hen, the clinical signs of TOCP neurotoxicity appear in
the cat 21-26 days before the electrophysiological effects
on the gastrocnemius muscle. Recovery of the cat from
delayed neurotoxicity symptoms was more marked than that
of the hen. No effects on peripheral nerve transmission
or on neuromuscular junction functioning were seen in the
cat.
9. EFFECTS ON HUMANS
Summary
There have been many reported cases of human poisoning,
mostly from accidental or irresponsible contamination of food-
stuffs. Occupational poisoning, usually resulting from dermal
exposure, has also been reported. The ortho isomer of TCP is
the responsible toxic agent.
Though short-term symptoms of ingestion might involve
vomiting, abdominal pain, and diarrhoea, characteristically
delayed, longer-term symptoms are neurological, frequently
leading to paralysis and pyramidal signs (spasticity, etc.).
There is considerable variation in the sensitivity of indi-
viduals to TOCP; severe symptoms were reported with a TOCP dose
of 0.15 g in one individual, while others were unaffected by 1
to 2 g. There is also considerable variation in the rate of
recovery from poisoning, some patients recovering completely
and others still severely affected years later, after appar-
ently similar exposure.
First-aid treatment involves the induction of vomiting or
pumping of the stomach. The patient should be hospitalized as
soon as possible. Atropine or 2-PAM may be used as an effective
antidotal treatment against cholinergic symptoms. Long-term,
antispastic drugs may be useful, though physical rehabilitation
is the cardinal therapy.
9.1 Historical background
Of the tricresyl phosphate isomers, the ortho (TOCP)
is by far the most toxic and alone gives rise to the major
neurotoxicity in man. It is considered that the toxicity
of the commercial products depends on the concentration of
the ortho isomer, but the mixed o-cresyl esters in these
products are also toxic and contribute to the neurotoxic
action.
It is well known that TOCP produces delayed effects on
the central and peripheral nervous systems. TOCP poisoning
has occurred throughout the world (Inoue et al., 1988);
the major outbreaks are indicated in Table 14.
Table 14. Major outbreaks of TOCP poisoning
___________________________________________________________________________
Year Place Number Vehicles of TOCP Reference
of cases
___________________________________________________________________________
1898 France 6 phospho-creosote Lorot (1899)
1900-1928 Europe 43 phospho-creosote Roger & Recordier
(15% TOCP) (1934)
1930-1931 USA 50 000 ginger extract Morgan (1982)
1931 Europe several Apiol pill Susser & Stein
hundred (1957)
1938 South Africa 68 cooking oil Sampson (1942)
1940 Switzerland 80 cooking oil Walthard (1945)
1941-1945 Germany more than cooking oil Mertens (1948)
200
1945 England 17 cooking oil Hotston (1946)
1955 South Africa 11 water or solvent Susser & Stein
(1957)
1957 Morocco about cooking oil Smith & Spalding
10 000 (1959)
1960 India 58 solid food Vora et al. (1962)
1962 India more than flour Chaudhuri (1965)
400
1967 Fiji 56 flour Sorokin (1969)
1980 Romania 12 alcohol Vasilescu & Florescu
(1980)
1981 Sri Lanka more than cooking oil Senanayake &
20 (0.56%) Jeyaratnam (1981)
___________________________________________________________________________
In 1899, Lorot initially reported six cases of poly-
neuropathy out of 41 cases of pulmonary tuberculosis
treated with phospho-creosote. Later it was shown that the
phospho-creosote contained 15% TOCP. In the next 30 years,
43 additional cases caused by the drug were reported in
various parts of continental Europe (Roger & Recordier,
1934).
In the spring of 1930, in mid-western and south-
western USA, an outbreak of paralysis characterized by
bilateral foot- and wrist-drop appeared suddenly (Burley,
1930; Merritt & Moor, 1930). Ultimately 50 000 people were
poisoned by a popular substitute for alcohol called
"Ginger Jake" (Morgan, 1982). Smith et al. (1930) proved
that the adulterated beverage contained about 2% TOCP and
that this caused the paralysis.
In 1931, several hundred women in Europe (especially
in Germany, The Netherlands, Yugoslavia, and France) were
poisoned by the TOCP contained in Apiol pills and taken as
an abortifacient (Roger & Recordier, 1934; Susser & Stein,
1957). The TOCP was presumably included as an additional
stimulus to abortion (Ter Braak & Carrillo, 1932).
An outbreak involving 11 people occurred in Durban,
South Africa, in 1955 (Susser & Stein, 1957). From the
epidemiological survey it was suggested that water, as
well as solvents, may have been a vehicle for the TOCP.
In 1959, about 10 000 Moroccan people were intoxicated
by TOCP: jet engine oil had been illegally mixed into
their cooking oil (Smith & Spalding, 1959; Svennilson,
1960). Accidental poisoning by TOCP contamination of solid
food occurred in 1960 in Bombay, India, where 58 victims
were recorded (Vora et al., 1962).
During the period April-June, 1962, more than 400
cases of paralysis occurred in the Malda district in
India. The cause of this disease proved to be the consump-
tion of flour contaminated with TOCP (Chaudhuri, 1965).
In 1967, similar poisoning was recorded in Fiji, where
56 people showed neuropathy (Sorokin, 1969). The cause was
stated to be contamination of dry sharps flour by TOCP
through the sacking material.
Vasilescu & Florescu (1980) in Romania reported 12
patients with toxic neuropathy following accidental inges-
tion of alcohol contaminated by TOCP.
An outbreak of acute polyneuropathy in over 20 young
females occurred in Sri Lanka during 1977-1978 (Senanayake
& Jeyaratnam, 1981). The cause of the neuropathy was
traced to TCP found as a contaminant in a special cooking
oil (gingili oil). Contamination probably occurred during
transport of the oil in containers previously used for
storing mineral oils.
9.2 Occupational exposure
Gartner & Elsaesser (1943) reported the case of a
worker who developed pyramidal signs after exposure to
TOCP for two years in a German chemical plant. In this
case, percutaneous absorption was considered to be the
main route of exposure.
In 1944, three cases of toxic polyneuropathy among
workers who had worked for six to eight months in a plant
manufacturing TCP in England were reported (Hunter et al.,
1944). Skin penetration and inhalation were thought to be
the main causes of the occupational poisoning.
Parnitzke (1946) reported a case of TOCP poisoning
after 3 years of exposure in a German plant and stated
that TOCP had been absorbed through the skin and presum-
ably the gastrointestinal tract.
Since 1958, a high prevalence of polyneuropathy among
shoe factory workers has been reported in Italy. The cause
has been attributed to TCP (Cavalleri & Cosi, 1978).
Although this is possible, this cause-effect relationship
has not so far been based on unquestionable evidence. This
polyneuropathy might have various aetiological factors
(including n-hexane) or be produced by a combination of
them (Leveque, 1983).
9.3 Clinical features
Goldstein et al. (1988) reported a case of severe
intoxication in a 4-year-old child following ingestion of
a lubricant containing TCP (substitution pattern not
known). The clinical findings were acute gastrointestinal
symptoms, delayed cholinergic crisis, and neurological
toxicity.
The severity of signs and symptoms after poisoning
with TOCP seems not always to be proportional to the
dosage (Staehelin, 1941). In a Swiss Army outbreak of
poisoning among more than 80 young men, toxic symptoms
appeared in once case after eating food containing only
0.15 g TOCP. Severe neurological disturbance developed in
three men from the intake of 0.5 to 0.7 g, whereas in two
other cases the intake of 1.5-2 g did not lead to any
symptoms. This leads to the conclusion that individual
susceptibility varies greatly (Staehelin, 1941).
In general, the signs and symptoms of TOCP poisoning
are distinctive, whereas the symptomatology varies
somewhat according to whether a single relatively large
dose or small cumulative doses are taken. In the former
case, the initial symptoms are gastrointestinal, ranging
from slight to severe nausea and vomiting, sometimes
accompanied by abdominal pain and diarrhoea. Among these
symptoms, vomiting is most frequently observed (Staehelin,
1941). These symptoms are usually transient, lasting from
a few hours to a few days (Walthard, 1945; Susser & Stein,
1957).
In cases of chronic low level exposure, the above
symptoms may not be present and the major symptoms are
neurological (Parnitzke, 1946). The clinical features of
acute exposure to TOCP were described by Staehelin (1941).
A latent period of 3-28 days is observed after acute
exposure, and clear "delayed neurotoxicity" then gradu-
ally appears. The initial neurological symptoms are sharp,
cramp-like pains in the calves, and some numbness and
tingling in the feet and sometimes the hands. Within a few
hours or a day or two at most, these pains are followed by
increasing weakness of the lower limbs, and soon the
patient becomes unsteady and then unable to maintain bal-
ance. The cramp-like pains may cease with the onset of
weakness, or persist for some days. One or two weeks after
the onset in the lower limbs and while paralysis may still
be progressing, the weakness spreads to the hands. While
some patients show complete wrist drop and total loss of
power in the hands, sometimes with weakness up to the
elbows, the predominant neurological abnormalities are
observed in the lower limbs. Bilateral foot drop with com-
plete loss of power from the ankle down is a common
finding. Depending on the severity of the affection, the
patient may have weakness in the knees, less at the hips,
and, only in the most severe cases, weakness of the trunk.
About three weeks or more after the onset of paralysis, a
most striking and rapid wasting may be observed. While the
small muscles of the feet, calves, the anterior tibials,
and the thighs do not escape this wasting, in so far as
they are involved in the disease, the change is most
obvious in the small muscles.
In the initial stage, the ankle jerks are absent and
knee reflexes may be normal or occasionally depressed.
Plantar reflexes are unobtainable. Mild cases do not show
any upper motor neuron signs. On the other hand, in the
more severe cases, even at the early stage, knee jerks may
be exaggerated, presaging the development of upper motor
neuron involvement. In general, upper motor neuron signs,
e.g., pyramidal signs, gradually become evident at about
the third week or later. Knee jerks become exaggerated and
so also may the biceps, triceps, and supinator jerks
(Cavanagh, 1964). A finger flexor reflex appears for the
first time or increases (Susser & Stein, 1957). As the
pyramidal tract lesion becomes evident, involuntary flexor
withdrawal of the whole limbs follows gentle plantar
stimulation. Babinski responses are observed much later.
Muscle tonus of the limbs gradually increases. In severe
cases, the signs of upper motor involvement are delayed,
probably masked by the gross flaccid muscle weakness.
Several authors state emphatically that sensory dis-
turbances do not occur. Sampson (1942) reported sensory
disturbances although these were admittedly unobtrusive,
in contrast to motor dysfunction. Reports of muscle and
peripheral nerve tenderness are fairly frequent. If the
sensory disturbances are observed, there is hypoesthesia
with loss of pin-prick and temperature sense; the ability
to detect vibration is sometimes affected distally. The
sensory disturbances vary in extent from merely the soles
of the feet to the whole of the limbs.
Usually cranial nerves are not involved. In general,
mental signs are rare, but transient euphoria and con-
fusion have been observed in the early stage (Schwab,
1948).
9.4 Prognosis
Following exposure, muscle weakness progresses over
several weeks, sometimes even months. Sensory changes
often begin to regress during the early weeks, the rap-
idity depending on the severity of the case, and then
muscle strength gradually returns in patients who are only
mildly affected. Improvement begins with the return of
sensation, then muscle strength in the hands, and eventu-
ally strength in the lower limbs. In cases of pyramidal
signs, recovery is generally poor. Zeligs (1938),
reporting eight years after the 1930 mid-western and
south-western USA outbreak, surveyed the records of 316
patients. He was able to follow up 60 patients, all of
whom were disabled and still in institutions. Aring (1942)
examined more than 100 patients in the 10 years following
this outbreak and they appeared still to be affected.
Morgan & Penovich (1978) followed up 11 survivors in the
47 years after the same outbreak; the principal findings
were the spasticity and abnormal reflexes of an upper
motor neuron syndrome.
Of the 80 patients in the 1940 Swiss army accident,
14 were quite well after five years, 15 were totally inca-
pacitated, and 38 showed spasticity (Walthard 1945).
In the 1938 Durban outbreak, all the patients showed
some symptoms of the disease 18 years later (Susser &
Stein, 1957). The mildest case had slight weakness at the
ankle, while the most severely affected had foot drop,
muscle atrophy, and pyramidal signs (spasticity, ankle
clonus, and positive Babinski sign).
The residual signs and symptoms are mainly confined to
the lower limbs. They consist of weakness and muscle atro-
phy of varying degrees in the feet and the small muscles
of the hand. Disability is principally related to the
pyramidal signs with resultant spasticity of the lower
extremities.
9.5 Neurophysiological investigations
There have been very few electrophysiological studies
on human TOCP poisoning. Svennilson (1960) reported an
electromyographic study on 65 patients in the 1959 Morocco
poisoning. These cases showed varying degrees of dener-
vation and polyphasic abnormal potentials in the paralysed
muscles. The clinically healthy proximal groups of muscles
also showed marked polyphasic action potentials but not
denervation.
Vasilescu & Florescu (1980) reported detailed studies
on 12 patients from the 1980 accident in Romania. They
observed > 50% decrease in the muscle evoked potential
amplitude, fibrillation potentials in the same muscles at
rest, and decreased motor nerve conduction velocity.
In neurophysiological studies by Senanayake (1981) in
Sri Lanka, the main findings were widespread neurotoxic
patterns and prolongation of terminal latencies with rela-
tively mild slowing of motor nerve conduction velocities.
These studies confirmed the evidence of axonal degener-
ation.
9.6 Pathological investigations
Numerous pathological studies have been made on biopsy
or autopsy samples since the Jamaica ginger accidents in
1930. In 1930, Goldfain described some changes observed
in the peripheral nerves and spinal cord, quoting Jeter's
autopsy report. Histopathological investigations by
Goodale & Humphreys (1931) indicated degeneration of
myelin sheaths and axis cylinders in the radial, sciatic,
and tibial nerves in all cases examined. Vonderahe (1931)
found marked degenerative changes in the anterior horn
cells, characterized by swelling, central chromatolysis
(disappearance of the Nissl substance), excentric nuclei,
and shrinking of the cells. The pathological studies also
revealed degeneration in the radial and anterior tibial
nerves, and degenerative changes in the anterior roots.
There were no pathological signs of inflammation. Accord-
ing to Smith & Lillie (1931), the paralysis due to Jamaica
ginger was essentially a degeneration of the myelin
sheaths of the peripheral nerves, with a variable amount
of relatively moderate central degenerative changes
affecting the anterior horn cells throughout the spinal
cord, but more often in the lumber and cervical regions.
In the 1938 Durban (South Africa) epidemic, Sampson (1942)
also reported that degeneration of the anterior horn cells
occurred in some instances and that peripheral nerves
showed axonal degeneration. Aring (1942) described degen-
eration in the posterior and lateral columns in later
investigations of survivors of the outbreak, thereby con-
firming the origin of some of the spinal symptoms. It is
noteworthy that the latter changes were evident in the
lumbar region, while the dorsal column changes, in which
only the fasciculus gracilis was involved, concerned the
cervical region.
Muscle biopsy studies of patients from the 1959
Moroccan poisoning showed a moderate degree of muscle
atrophy and a slight increase of the muscle fibre nuclei.
Spherical axonal swelling and terminal knobs were noted as
a sign of peripheral nerve degeneration in muscles
(Svennilson, 1960). Similar changes were also noted in
the poisoning cases reported in Malda, India, in 1962
(Chaudhuri et al., 1962). These effects on muscle suggest
denervation.
9.7 Laboratory investigations
Little information has been obtained from laboratory
examinations of exposed humans. There is no significant
change in the urine or blood (Sampson, 1942; Senanayake &
Jeyaratnam, 1981), but the cerebrospinal fluid may show an
increase in protein concentration, with or without pleo-
cytosis (Sampson, 1942; Mertens, 1948; Susser & Stein,
1957).
Vora et al. (1962) measured blood cholinesterase
levels in patients admitted to hospitals during the 1960
Bombay poisoning and demonstrated that plasma cholinester-
ase was increased one month after exposure. The erythro-
cyte cholinesterase level was considerably diminished
(50%) quite early after the onset of symptoms. Both plasma
and erythrocyte cholinesterase activities returned to
normal within about 3 months.
In a factory manufacturing TAP, about half of the
workers examined showed significant decreases in pseudo-
cholinesterase (cholinesterase other than AChE), and many
of them had minor signs and symptoms (Tabershaw et al.,
1957).
Morgan & Hughes (1981) also investigated cholinester-
ase activity in workers in a plant manufacturing TAP plas-
ticizers. They found that plasma cholinesterase estimation
in workers exposed to TAP compounds cannot be used as a
sensitive barometer of organic phosphate absorption; thus
routine regular estimations serve no useful purpose. Its
value is chiefly as a screening method at the pre-employ-
ment medical examination to exclude personnel at risk, as
a baseline in the event of massive exposure, and as a
means of diagnosis in cases of CNS disease that simulate
TAP poisoning.
9.8 Treatment
In the event of skin contact with TCP, contaminated
clothing should be rapidly removed and affected body areas
copiously irrigated with water. The ingestion of food or
drink contaminated with TOCP should be treated by inducing
vomiting, unless the patient is unconscious. Atropine or
pralidoxine (2-PAM) chloride may be required to counteract
cholinergic effects. No specific antidote is available.
Medical therapy should begin as soon as possible, even
though the results of medical therapeutic measures have
been disappointing. B-complex vitamins and corticosteroids
may protect nervous tissue against further involvement
(Geoffroy et al., 1960). The cardinal therapy is physical
rehabilitation. Administration of anti-spastic drugs may
be required.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human poisoning involving the accidental ingestion of
tri- o-cresyl phosphate (TOCP) or occupational exposure
of workers has frequently been reported. The likely route
of occupational exposure is cutaneous absorption. The
neurotoxic symptoms involve initial inhibition of cholin-
esterases and subsequent delayed neuropathy characterized
by severe paralysis.
Because of considerable variation among individuals in
sensitivity to TOCP, it is not possible to establish a
safe level of exposure. Symptoms have been reported from
the ingestion of 0.15 g of an isomeric mixture with a low
proportion of TOCP; the minimum effective dose of the
ortho isomer is, therefore, much lower than this. Animal
studies show considerable variation among species in the
response to TOCP, and humans appear to be particularly
sensitive.
Irritant and allergic dermatitis have been reported.
Both the pure ortho isomer and isomeric mixtures con-
taining TOCP are, therefore, considered major hazards to
human health.
There is no safe level for ingestion. Exposure to the
compound through dermal contact or inhalation should be
minimized.
10.1.1 Exposure levels
Exposure of the general population to tricresyl phos-
phate (TCP) through various environmental media, including
drinking-water, can be regarded as minimal. TCP has been
detected at relatively higher concentrations in urban air
than in air collected at production sites, although the
levels are usually low. TCP was not detected in human
adipose tissue samples in a survey conducted in the USA.
There have been many cases of accidental human poisoning
through the ingestion of medicines, food, flour, cooking
oil, and beverages contaminated with hydraulic fluid or
lubricant oil containing TCP produced from "cresylic
acid". The toxic symptoms can be observed after ingestion
of only 0.15 g of tri- o-cresyl phosphate, a component of
TCP from cresylic acid. The contamination has usually
happened when empty barrels or drums, previously used for
hydraulic fluid or lubricating oil storage, have been
reused.
10.1.2 Toxic effects
Accidental human exposure to a single large dose re-
sults in gastrointestinal disturbance varying from slight
to severe nausea and vomiting, accompanied by abdominal
pain and diarrhoea. In the case of exposure to small cumu-
lative doses, "delayed neurotoxicity" gradually proceeds
after a latent period of 3-28 days. In most cases, the
muscle weakness changes rapidly to a striking paralysis of
the lower limbs, with or without an involvement of the
hands. In severe cases, pyramidal signs gradually become
evident. Some neurophysiological studies indicate wide-
spread neurotoxic patterns and prolongation of terminal
latencies with relatively small decreases of motor nerve
conduction velocities. This confirms the evidence of
axonal degeneration, which is the main feature observed in
pathological investigations.
The neurotoxic metabolite of TCP has been identified
as saligenin cyclic o-tolyl phosphate, which is derived
from o-hydroxymethyl metabolites. Thus, it seems that at
least one o-tolyl group among the three phenolic moieties
of TCP is necessary to induce neurotoxic effects. TCP pro-
duced from synthetic cresol, which contains less than 0.1%
of o-cresol, is therefore not neurotoxic.
Subchronic animal studies on TCP derived from syn-
thetic cresol indicate that the target organs are liver
and kidney, but this was not confirmed in the case of
human intoxication. No adequate data are available on
mutagenicity and carcinogenicity. TCP is not toxic to
chick embryos.
10.2 Evaluation of effects on the environment
The measurement of environmental concentrations of TCP
in water has shown only low levels of contamination. This
reflects the low water solubility and ready degradability
of the compound. Since the acute toxicity of TCP to
aquatic organisms is also low, it is unlikely that it
poses a threat to such organisms.
As a consequence of the physico-chemical properties of
TCP, there is a high potential for bioaccumulation. How-
ever, this does not occur in practice, owing to low con-
centrations of TOCP in the environment and living organ-
isms and to its rapid degradation.
TCP bound to sediment accumulates in the environment,
and levels measured in river, estuarine, and marine sedi-
ments have been high. Since there is no information either
on the bioavailability of these residues to burrowing or
bottom-living organisms or on their hazards, the possi-
bility of effects on such species cannot be discounted.
TCP spillage leads to hazard for the local environ-
ment.
10.2.1 Exposure levels
TCP is found in air, surface water, soil, sediment,
and aquatic organisms near heavily industrialized areas,
although concentrations are usually low. Owing to the high
biodegradation rate of TCP in aqueous environment, it is
not considered to affect aquatic organisms adversely. One
report showed an extremely high concentration of total
triaryl phosphate (26.55 g/kg) in a soil sample obtained
from a production plant yard. This suggests the need for
land waste disposal.
10.2.2 Toxic effects
Freshwater algae are relatively sensitive to TCP, the
50% growth inhibitory concentration ranging from 1.5 to
5.0 mg/litre. Among fish species, the rainbow trout is
adversely affected by TCP concentrations below 1 mg/litre
(0.3-0.9 mg/litre), with sign of chronic poisoning, but
the tidewater silverside is more resistant (LC50 is 8700
mg/litre). TCP does not inhibit cholinesterase activity in
fish or frogs, but it has a synergistic effect on organo-
phosphorus insecticide activity.
11. RECOMMENDATIONS
When tri-substituted cresols are used in the synthesis
and manufacture of other compounds, the purified meta and
para isomers should be used in order to avoid the acciden-
tal synthesis of ortho-substituted products.
REFERENCES
ABOU-DONIA, M.B., VARGA, P.A., GRAHAM, D.G., & KINNES, C.G. (1980)
Delayed neurotoxicity of a single dermal administration of o-ethyl
o-4-nitro-phenyl phenylphosphonothioate and tri-o-cresyl phosphate
to cats. Toxicol. Lett., 1 (Special issue): 141.
ABOU-DONIA, M.B., TROFATTER, L.P., GRAHAM, D.G., & LAPADULA, D.M.
(1986) Electromyographic, neuropathologic and functional
correlates in the cat as the result of tri-o-cresyl phosphate
delayed neurotoxicity. Toxicol. appl. Pharmacol., 83(1): 126-141.
ADEMA, D.M.M., KUIPER, J., HANSTVEIT, A.O., & CANTON, H.H. (1983)
Consecutive system of tests for assessment of the effects of
chemical agents in the aquatic environment. In: Pesticide
chemistry-Human welfare and the environment. Proceedings of the
Fifth International Congress on Pesticide Chemistry,Kyoto, Japan,
29 August - 4 September, 1982, Oxford, New York, Pergamon Press,
Vol. 3, pp. 537-544.
ALDRIDGE, W.N. (1954) Tricresyl phosphates and cholinesterase.
Biochem. J., 56: 185-189.
ALDRIDGE, W.N. & BARNES, J.M. (1961) Neurotoxic and biochemical
properties of some triaryl phosphates. Biochem. Pharmacol., 6:
177-188.
ANONYMOUS (1986) Handbook of commodity chemicals, Tokyo, Kagaku-
Kogyo-Nipposha.
ARING, C.D. (1942) The systemic nervous affinity of triorthocresyl
phosphate (Jamaica ginger palsy). Brain, 65: 34-47.
ASSOCIATION OF THE PLASTICIZER INDUSTRY OF JAPAN (1976) Safety of
the plasticizer TCP, tricresyl phosphate, Tokyo, Association of
the Plasticizer Industry of Japan.
BALDRIDGE, H.D., JENDEN, D.J., KNIGHT, C.E., PREZIOSI, T.J., &
TUREMAN, J.R. (1959) Toxicology of a triaryl phosphate Oil. III.
Human exposure in operational use aboard ship. Am Med. Assoc. Arch.
ind. Hyg., 20: 258-261.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., &
WELCH, V.A. (1961) The reactions of organic phosphates. Part V.
The hydrolysis of triphenyl and trimethyl phosphates. J. Chem. Soc.
(B), 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER,
B., VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic
phosphates. Part VI. The hydrolysis of aryl phosphates. J. Chem.
Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphoryl-enzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BECK, B.E., WOOD, C.D., & WHENHAM, G.R. (1977) Triaryl phosphate
poisoning in cattle. Vet. Pathol., 14: 128-137.
BELL, J.D. & RUSVINE, J.R. (1967) Synergism of organophosphates in
Musca domestica and Chrysomya putoria. Entomol. exp. appl., 10:
263-269.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY,
S.K. (1974) Detection and estimation of tricresyl phosphate in
mustard oil. Forensic Sci., 3: 263-270.
BISCHOFF, A. (1967) The ultrastructure of tri-ortho-cresyl
phosphate poisoning. I. Studies on myelin and axonal alterations in
the sciatic nerve. Acta neuropathol., 9: 158-174.
BISCHOFF, A. (1970) The ultrastructure of tri-ortho-cresyl
phosphate poisoning in the chicken. II. Studies on spinal cord
alterations. Acta neuropathol., 15: 142-155.
BISCHOFF, A. (1977) Tri-ortho-cresyl phosphate neurotoxicity. In:
Roisin, L., Shiraki, H., & Grcevic, N., ed. Neurotoxicology, New
York, Raven Press, pp. 431-441.
BLEIBERG, M.J. & JOHNSON, H. (1965) Effects of certain
metabolically active drugs and oximes on tri- o-cresyl phosphate
toxicity. Toxicol. appl. Pharmacol., 7: 227-235.
BLOOM, P.J. (1973) Application des chromatographies sur couche
mince et gaz-liquide à l'analyse qualitative et quantitative des
esters des acides phosphorique et phosphoreux. J. Chromatogr.,
75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and
effects of triaryl and tri-alkyl/aryl phosphate esters. Residue
Rev., 94: 49-99.
BONDY, H.F., FIELD, E.J., WORDEN, A.N., & HUGHES, J.P.W. A study of
the acute toxicity of the tri-aryl phosphates used as plasticizers.
(1960) Br. J. ind. Med., 17: 190-200.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., &
KARASEK, F.W. (1981) Trace impurities in solvents commonly used for
gas chromatographic analysis of environmental samples. J.
Chromatogr., 206: 279-288.
BROWN H.R. & SHARMA, R.P. (1975) Inhibition of neural membrane
adenosine-triphosphatases by organophosphates. Toxicol. appl.
Pharmacol., 33: 140.
BURLEY, B.T. (1930) The 1930 type of polyneuritis. New Engl. J.
Med., 202: 1139-1142.
CARLTON, B.D., HASARAN, A.H., MEZZA, L.E., & SMITH, M.K. (1987)
Examination of the reproductive effects of tricresyl phosphate
administered to Long-Evans rats. Toxicology, 46: 321-328.
CARRINGTON, C.D. & ABOU-DONIA, M.B. (1988) Variation between three
strains of rat: inhibition of neurotoxic esterase and
acetylcholinesterase by tri-o-cresyl phosphate. J. Toxicol.
environ. Health, 25(3): 259-268.
CASIDA, J.E. (1961) Specificity of substituted phenyl phosphorus
compounds for esterase inhibition in mice. Biochem. Pharmacol., 5:
332-342.
CASIDA, J.E., ETO, M., & BARON R.L. (1961) Biological activity of a
tri-o-cresyl phosphate metabolite. Nature (Lond.), 191: 1396-1397.
CASTERLINE, J.L., Jr., KU, Y., & BARNETT, N.M. (1985) Uptake of
tri-p-cresyl phosphate in soybean plants. Bull. environ. Contam.
Toxicol., 35: 209-212.
CAVALLERI, A. & COSI, V. (1978) Polyneuritis incidence in shoe
factory workers: Case report and etiological considerations. Arch.
environ. Health, 33: 192-197.
CAVANAGH, J.B.(1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system. An experimental study in hens. J.
Neurol. Neurosurg. Psychiatry, 17: 163-172.
CAVANAGH, J.B. (1964) The significance of the "dying back" process
in experimental and human neurological disease. Int. Rev. exp.
Pathol., 3: 219-267.
CHAPIN, R.E., GEORGE, J.D., & LAMB, J.C., IV (1988) Reproductive
toxicity of tricresyl phosphate in a continuous breeding protocol
in Swiss (CD-1) mice. Fundam. appl. Toxicol., 10: 344-354.
CHAUDHURI, R.N. (1965) Paralytic disease caused by consumption of
flour contaminated with tricresyl phosphate. Trans. R. Soc. Trop.
Med. Hyg., 59: 98-102.
CHAUDHURI, R.N., SEN GUPTA, P.C., CHAKRAVARTI, R.N., MUKHERJEE,
A.M., GHOSH, S.M., CHATTERJEA, J.B., SARKAR, J.K., BASU, S.P.,
ADHYA, R.N., MITRA, N.K., SAHA, T.K., & MITRA, P. (1962) Recent
outbreak of a paralytic disease in Malda, West Bengal. Bull.
Calcutta Sch. Trop. Med., 10: 141-152.
CHEMICAL AND GEOLOGICAL LABORATORIES LTD (1971) Calgary, Alberta,
Chemical and Geological Laboratories (Laboratory report No. E71-
5360).
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
COHEN, S.D. & MURPHY, S.D. (1970) Comparative potentiation of
malathion by triorthotolyl phosphate in four classes of
vertebrates. Toxicol. appl. Pharmacol., 16: 701-708.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues
in foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAGLEY, S. & PATEL, M.D. (1957) Oxidation of p-cresol and related
compounds by a Pseudomonas. Biochem. J., 66: 227-233.
DAVISON, A.N. (1953) The cholinesterases of the central nervous
system after the administration of organo-phosphorus compounds.
Brit. J. Pharmacol., 8: 212-216.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977)
The acute toxicity of 47 industrial chemicals to fresh and salt
water fishes. J. hazard. Mater., 1: 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic
mass spectrometric analysis of some commercial aryl phosphate oils.
J. Assoc. Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by
thin-layer chromatography.] Gig. i Sanit. , 10: 62-65 (in Russian).
DUKE, A.J. (1978) The significance of isomerism and complexity of
composition on the performance of triaryl phosphate plasticisers in
PVC. Chimia, 32: 457-463.
EAJ (1977) [ Environmental monitoring of chemicals ], Tokyo,
Environment Agency Japan, pp. 212-214 (Environmental Survey Report
Series, No. 3) (in Japanese).
EAJ (1979) [ Chemicals in the environment], Tokyo, Environment
Agency Japan, pp. 61, 78, 92, 93 (Office of Health Studies Report
Series, No. 5) (in Japanese).
EAJ (1981) [ Environmental monitoring of chemicals: Environmental
Survey Report of 1978, 1979 F.Y. ], Tokyo, Environment Agency
Japan, pp. 116-117 (in Japanese).
ELDEFRAWI, A.T., MANSOUR, N.A., BRATTSTEN, L.B., AHRENS, V.D., &
LISK, D.J. (1977) Further toxicologic studies with commercial and
candidate flame retardant chemicals. Part II. Bull. environ.
Contam. Toxicol., 17: 720-726.
ETO, M., CASHIDA, J.E., & ETO, T. (1962) Hydroxylation and
cyclization reactions involved in the metabolism of tri- o-cresyl
phosphate. Biochem. Pharmacol., 11: 337-352.
ETO, M., OSHIMA, Y., & CASHIDA, J.E. (1967) Plasma albumin as a
catalyst in cyclization of diaryl-o-(a-hydroxy)tolyl phosphates.
Biochem. Pharmacol., 16: 295-308.
ETO, M., HASHIMOTO, Y., OZAKI, K., KASAI, T., & SASAKI Y. (1975)
Fungitoxicity and insecticide synergism of monothioquinol phosphate
esters and related compounds. Botyu Kagaku, 40: 110-117.
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl
phosphate esters. A new photochemical coupling reaction. J. Am.
Chem. Soc., 94: 4780-4782.
FRANCHINI, I., CAVATORTA, A., D'ERRICO, M., DE SANTIS, M., ROMITA,
G., GATTI, R., JUVARRA, G., & PALLA, G. (1978) Studies on the
etiology of the experimental neuropathy from industrial adhesives
(glues). Experientia (Basel), 34: 250-252.
FREEMAN, G.B., IRWIN, R., TREJO, R., HEJTMANCIK, M., RYAN, M., &
PETERS, A.C. (1988) Reversibility and tolerance to tricresyl
phosphate-induced neurotoxic effects in F344 rats. Toxicologist,
8: 76.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GARTNER, W. & ELSAESSER, K.H. (1943) [Commercial ortho-
tricresylphosphate poisoning.] Arch. Gewerbepathol. Gewerbehyg.,
12: 1-9 (in German).
GEOFFROY, H., SLOMIC, A., BENEBADJI, M., & PASCAL, P. (1960)
Myèlopoly-néurites tri-crésyl phosphatées. Toxi-épidémie marocaine
de septembre-octobre 1959. World Neurol., 1: 294-315.
GOLDFAIN, E. (1930) Jamaica ginger multiple neuritis. J. Oklahoma
State Med. Assoc., 23: 191-193.
GOLDSTEIN, D.A., MCGUIGAN, M.A., & RIPLEY, B.D. (1988) Acute
tricresyl phosphate intoxication in childhood. Hum. Toxicol., 7:
179-182.
GOODALE, R.H. & HUMPHREYS, M.B. (1931) Jamaica ginger paralysis.
Autopsy observations. J. Am. Med. Assoc., 96: 14-16.
GROSS, E. & GROSSE, A. (1932) [A contribution to the toxicology of
ortho-tricresylphosphates.] Arch. exp. Pathol. Pharmakol., 168:
473-514 (in German).
HAGGERTY, G.J., HEJTMANCIK, M., DESKIN, R., RYAN, M.J., PETERS,
A.C., & IRWIN, R. (1986) Effects of subchronic exposure to
tricresyl phosphate in F344 rats and B6C3F1 mice: behavioural and
morphological correlates. Toxicologist, 6: 218.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Shuishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, N., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HINE, C.H., DUNLAP, M.K., RICE, E.G., COURSEY, M.M., GROSS, R.M., &
ANDERSON, H.H. (1956) The neurotoxicity and anticholinesterase
properties of some substituted phenyl phosphates. J. Pharmacol.
exp. Ther., 116: 227-236.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD,
G.T. (1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, 3rd revised ed., New York,
Wiley-Interscience, Vol. 2A, pp. 2362-2363.
HJORTH, N. (1962) Contact dermatitis from cellulose acetate film.
Cross-sensitization between tricresylphosphate (TCP) and
triphenylphosphate (TPP). Contact dermatitis, 2: 86-100.
HODGE, H.C. & STERNER, J.H. (1943) The skin absorption of
triorthocresyl phosphate as shown by radioactive phosphorus. J.
Pharmacol. exp. Ther., 79: 225-234.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOTSTON, R.D. (1946) Outbreak of polyneuritis due to orthotricresyl
phosphate poisoning. Lancet, 1: 207.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. Bull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(di-
chloropropyl)phosphate, a mutagenic flame retardant: Frequent
occurrence in human seminal plasma. Science, 211: 951-952.
HUNTER, D., PERRY, K.M.A., & EVANS, R.B. (1944) Toxic polyneuritis
arising during the manufacture of tricresyl phosphate. Br. J. ind.
Med., 1: 227-231.
INDEN, T. & TACHIBANA, S. (1975) [Damage of crops by gases from the
plastic materials under covering conditions.] Bull. Fac. Agric. Mie
Univ., 1975: 1-10 (in Japanese).
INOUE, N., FUJISHIRO, K., MORI, K., & MATSUOKA, M. (1988)
Triorthocresyl phosphate poisoning: A review of human cases.
Sangyo-Ika-Daigaku-Zasshi, 10(4): 433-442.
IRWIN, R., FREEMAN, G.B., TREJO, R., HEJTMANCIK, M., & PETERS, A.
(1987) Effects of chronic exposure to tricresyl phosphate in F344
rats and B6C3F1 mice: behavioural and biochemical correlates. Soc.
Neurosci. Abstr., 13: 90.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water
Res., 19: 119-125.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982) Toxicometric
parameters of industrial toxic chemicals under single exposure,
Moscow, Centre of International Projects, GKNT, p. 114.
JETER, H. (1930) Autopsy report of a case of so-called "Jake
Paralysis". J. Am. Med. Assoc., 95: 112-113.
JOHANSSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J.,
RADUE, R.W., & GRAHAM, P.R. (1977) Evaluation of delayed
neurotoxicity and dose-response relationships of phosphate esters
in the adult hen. Toxicol. appl. Pharmacol., 41: 291-304.
JOHNSON, M.K. (1969) Delayed neurotoxic action of some
organophosphorus compounds. Br. med. Bull., 25: 231-235.
JOHNSON, M.K. (1974) The primary biochemical lesion leading to the
delayed neurotoxicity of some organophosphorus esters. J.
Neurochem., 23: 785-789.
JOHNSON, M.K. (1975a) Organophosphorus esters causing neurotoxic
effects. Mechanism of action and structure/activity studies. Arch.
Toxicol., 34: 259-288.
JOHNSON, M.K. (1975b) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. & BARNES, J.M. (1970) The sensitivity of chicks to
the delayed neurotoxic effects of some organophosphorous compounds.
Biochem. Pharmacol., 19: 3045.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water
pollution caused by organophosphorus compounds.] Kankyo Gijyutsu,
7: 668-675 (in Japanese).
KENMOTSU, K., SAITO, N., OGINO, N., & MATSUNAGA, K. (1979) [An
environ-mental survey of chemicals. XI. Analytical methods of
organic phosphoric acid triesters.] Okayama-ken Kankyo Hoken Senta
Nempo, 3: 175-191 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment
and sea water.] J. Food Hyg. Soc. Jpn., 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental
pollutants. V. Environmental fate of organic phosphoric acid
triesters.] Okayama-ken Kankyo Hoken Senta Nempo, 4: 103-110 (in
Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.]
Okayama-ken Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination
of organic phosphate esters in environment samples.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982a) [An environmental survey of chemicals. XIX. Determination
of organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken
Senta Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982b) [Studies on the biological toxicity of several pollutants
in environments. VII. GC/MS Spectrometric determination of
organophosphoric acid triesters in sediment.] Okayama-ken Kankyo
Hoken Senta Nempo, 6: 142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemicals. XXII. GC/MS
spectrometric determination of organophosphoric acid triesters in
sediment (2).] Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in
Japanese).
KIMMERLE, G. & LOESER, E. (1974) Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environ. Qual. Saf., 3: 174-178.
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on
organic and heavy metal contaminants in the Lakes Erie, Michigan,
Huron and Superior basins. Great Lakes Water Qual. Bd.
KRISHNAMURTHY, M.N., RAJALAKSHMI, S., & PRAKASH KAPUR, O. (1985)
Detection of tricresyl phosphates and determination of tri-o-cresyl
phosphate in edible oils. J. Assoc. Off. Anal. Chem., 68(6):
1074-1076.
KU, Y. & ALVAREZ, G.H. (1982) Biodegradation of (14C) tri- p-cresyl
phosphate in a laboratory activated-sludge system. Appl. environ.
Microbiol., 43: 619-622.
KUREBAYASHI, H., TANAKA, A., & YAMAHA, T. (1985) Metabolism and
dispo-sition of the flame retardant plasticizer, tri-p-cresyl
phosphate, in the rat. Toxicol. appl. Pharmacol., 77: 395-404.
LAPP, T.W. (1976) Study on chemical substances from information
concerning the manufacture, distribution, use, disposal,
alternatives, and magnitude of exposure to the environment and
man: Task 1 - The manufacture and use of selected aryl and alkyl
phosphate esters, Springfield, Virginia, National Technical
Information Service, 129 pp (EPA contract No. 68-01-2687) (NTIS PB-
251819).
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic
phosphate triesters in human adipose tissue. J. Assoc. Off. Anal.
Chem., 66: 691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from
drinking water at the ng/L level, using macroreticular resin. J.
Assoc. Off. Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas
chromatographic determination of trialkyl/aryl phosphates in
drinking water, following isolation using macroreticular resin. J.
Assoc. Off. Anal. Chem., 64: 991-998.
LEFAUX, R. (1968) Practical toxicology of plastics, London, Iliffe
Books Ltd, p. 580.
LEFAUX, R. (1972) Industrial toxicology. Phosphoric esters. In:
Practical toxicology of plastics, London, Iliffe Books Ltd, pp.
121-127.
LEVEQUE, J. (1983) Tricresyl phosphates. In: Encyclopaedia of
occupational health safety, 3rd ed., Geneva, International Labour
Office, Vol. 2, pp. 2216-2218.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY,
D. (1975) Chronic toxicity of a synthetic tri-aryl phosphate oil to
fish. Environ. Physiol. Biochem., 5: 361-369.
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62: 47-51.
LOROT, C. (1899) Les combinations de la créosote dans le traitement
de la tuberculose pulmonaire, Paris (Thèse de Paris No. 25).
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Perspect., 10: 269-284.
MAJORS, R.E. & JOHNSON, E.L. ( 1978) High-performance exclusion
chromatography of low-molecular-weight additives. J. Chromatogr.,
167: 17-30.
MARHOLD, J.V. (1972) Collection of results of toxicological
assessments of compounds and formulations tested at the Department
of Toxicology, Research Institute for Organic Syntheses, Prague,
Institute for Management Training in the Chemical Industry.
MAUSNER, M., BENEDICT, J.H., BOOMAN, K.A., BRENNER, T.E., CONWAY,
R.A., DUTHIE, J.R., GARRISON, L.J., HENDRIX, C.D., & SHEWMAKER,
J.E. (1969) The status of biodegradability testing of nonionic
surfactants. J. Am. Oil Chem. Soc., 46: 432-440.
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Springfield, Virginia, National Technical
Information Service.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE,
P.M., & SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An
aquatic environmental assessment of Pydrauls 50E and 115E. In:
Branson R. & Dickson, K.L., ed. Aquatic Toxicology and Hazard
Assessment: Fourth Conference, Philadelphia, American Society for
Testing and Materials, pp. 103-123 (ASTM STP 737).
MELE, J.M. & JENSH, R.P. (1977) Teratogenic effects of orally
administered tri-o-cresyl phosphate on Wistar albino rats.
Teratology, 15: 32A.
MERRITT, H.H. & MOOR, M. (1930) Peripheral neuritis associated with
ginger extract ingestion. New Engl. J. Med., 203: 4-12.
MERTENS, H.G. (1948) [Clinical aspects of tri-orthocrésyl phosphate
poisoning (including a report on several cases of poisoning in an
Igelit factory).] Arch. Psychiatr. Nervenkr., 179: 458-482
(in German).
MRI (1979) Assessment of the need for limitation on triaryl and
trialkyl/aryl phosphates. Draft final report, Kansas City, Midwest
Research Institute (EPA Contract 68-01-4313).
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising
Supplement 52(10A), p 697, New York, McGraw-Hill Inc.
MORAZAIN, R. & ROSENBERG, P. (1970) Lipid changes in tri-o-cresyl-
phosphate-induced neuropathy. Toxicol. appl. Pharmacol., 16: 461-474.
MORGAN, A.A. & HUGHES, J.P.W. (1981) An investigation into the
value of cholinesterase estimations of workers in a plant
manufacturing tri-aryl phosphate plasticizers. J. Soc. Occup. Med.,
31: 69-75.
MORGAN, J.P. (1982) The Jamaica Ginger Paralysis. J. Am. Med.
Assoc., 248: 1864-1867.
MORGAN, J.P. & PENOVICH, P. (1978) Jamaica Ginger Paralysis. Forty-
seven-year follow-up. Arch. Neurol., 35: 530-532.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988a) Evaluation of rodent sperm, vaginal
cytology, and reproductive organ weight data from National
Toxicology Program 13-week study. Fundam. appl. Toxicol., 11:
343-358.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988b) Association of sperm, vaginal
cytology, and reproductive organ weight data with results of
continuous breeding reproductive studies in Swiss CD-1 mice.
Fundam. appl. Toxicol., 11: 359-371.
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G. & GRIFT, N.P. (1983) Extraction and cleanup procedures
for determination of diarylphosphates in fish, sediment, and water
samples. J. Assoc. Anal. Off. Chem., 66: 684-690.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980) Determination of
several triarylphosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and
cleanup of fish, sediment, and water for determination of triaryl
phosphates by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Technol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983) Environmental
dynamics of phosphate esters. III. Comparison of the
bioconcentration of four triaryl phosphates by fish. Chemosphere,
12: 155-166.
MURRAY, D.A.J. (1975) Analysis of tri-aryl phosphate esters and the
determination of IMOL S-140 in fish tissue and water samples by gas
chromatography. J. Fish Res. Board Can., 32: 457-460.
MYERS, D.K., REBEL, J.B.J., VEEGER, C., KEMP, A., & SIMONS, E.G.L.
(1955) Metabolism of triaryl phosphates in rodents. Nature (Lond.),
176: 259-260.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition
coefficient to measure the bioconcentration potential of organic
chemicals in fish. Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate
ester mixtures to invertebrates and fish. Bull. environ. Contam.
Toxicol., 19: 250-256.
NICHOLSON, S.S. (1974) Bovine posterior paralysis due to
organophosphate poisoning. J. Am. Vet. Med. Assoc., 165: 280-281.
NOBILE, E.R., PAGE, S.W., & LOMBARDO, P. (1980) Characterization of
four commercial flame retardant aryl phosphates. Bull. environ.
Contam. Toxicol., 25: 755-761.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performance liquid
chromato-graphic analysis on radial compression column of the
neurotoxic tri-o-cresyl phosphate and metabolites. Anal. Biochem.,
135: 296-303.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1984) Disposition of (14C)tri-o-
cresyl phosphate and its metabolites in various tissues of the male
cat following a single dermal application. Drug Metab. Disp., 12
(6): 705-711.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986a) Studies on the metabolism
of the neurotoxic tri-o-cresyl phosphate synthesis and
identification by infrared, proton nuclear magnetic resonance and
mass spectrometry of five of its metabolites. Toxicology, 38: 1-13.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986b) Studies on the metabolism
of the neurotoxic tri- o-cresyl phosphate, distribution, excretion,
and metabolism in male cats after a single, dermal application.
Toxicology, 38: 15-33.
OECD (1981) OECD guidelines for testing of chemicals. Section 2:
Effects on biotic systems, Paris, Organization for Economic
Cooperation and Development, Publications Office.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresylphosphate. Sci. total
Environ., 43: 233-241.
OISHI, H., OISHI, S., & HIRAGA, K. (1982) Toxicity of several
phosphoric acid esters in rats. Toxicol. Lett., 13: 29-34.
OLSON, B.A. & BURSIAN, S.J. (1988) Effect of route of
administration on the development of organophosphate-induced
delayed neurotoxicity in 4-week-old chicks. J. Toxicol. environ.
Health, 23(4): 499-505.
PACIOREK, K.J.L., KRATZER, R.H., KAUFMAN, J., NAKAHARA, J.H.,
CHRISTOS, T., & HARTSTEIN, A.M. (1978) Thermal oxidative
degradation studies of phosphate esters. Am. Ind. Hyg. Assoc. J.,
39: 633-639.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78(1): 78-87.
PARNITZKE, K.H. (1946) [On the recent accumulation of ortho-
tricresylphosphate poisoning (sources and clinical course).] Dtsch.
Gesundheitswes., 1: 666-670 (in German).
PEGUM, J.S. (1966) Contact dermatitis from plastics containing tri-
aryl phosphates. Br. J. Dermatol., 78: 626-631.
PENMAN, D.R & OSBORNE, G.O. (1976) Trialkyl phosphates and related
compounds as antifertility agents of the twospotted spider mite. J.
econ. Entomol., 69(2): 266-268.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975)
Utilization of triaryl phosphates by a mixed bacterial population.
Can. J. Microbiol., 21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with
synergistic properties. J. econ. Entomol., 59(1): 11-15.
PRENTICE, D.E. & MAJEED, S.K. (1983) A subchronic study (90 days)
using multiple dose levels of tri-ortho-cresyl phosphate (TOCP):
some neuropathological observations in the domestic hen.
Neurotoxicology, 4(2): 277-282.
PRINEAS, J.S. (1969) The pathogenesis of dying-back
polyneuropathies. Part I. An ultrastructural study of experimental
tri-ortho-cresyl phosphate intoxication in the cat. J. Neuropathol.
exp. Neurol., 28: 571-597.
QUINSTAD, G.B., STAIGER, L.E., & SCHOOLEY, D.A. (1975)
Environmental degradation of the insect growth regulator
methoprene. V. Metabolism by house-flies and mosquitoes. Pestic.
Biochem. Physiol., 5: 233-241.
RAMSEY, J.D. & LEE, T.D. (1980) Gas-liquid chromatographic
retention indices of 296 non-drug substances on SE-30 or OV-1
likely to be encountered in toxicological analyses. J. Chromatogr.,
184: 185-206.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin
layer chromatography. Evaluation of methods and application on
phosphate esters, polychlorinated paraffins and some PCB-
substitutes. Chemosphere, 9: 683-691.
ROBERTS, N.L., FAIRLEY, C., & PHILLIPS, C. (1983) Measurements and
evaluation of clinical signs following use of the hen as the animal
model for screening acute delayed and subchronic neurotoxicity
studies. Neurotoxicology, 4(2): 263-270.
ROBERTSON, D.G., SCHWAB, B.W., SILLS, R.D., RICHARDSON, R.J., &
ANDERSON, R.J. (1987) Electrophysiologic changes following
treatment with organophosphorus-induced delayed neuropathy-
producing agents in the adult hen. Toxicol. appl. Pharmacol., 87:
420-429.
ROGER, H. & RECORDIER, M. (1934) Les polynéurites
phosphocréosotiques (phosphate de créosote, ginger paralysis,
apiol). Ann. Med., 35: 44-63.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P.,
& TUCKER, E.S. (1979) Environmental fate of selected phosphate
esters. Environ. Sci. Technol., 13: 840-844.
SAITO, C., KATO, T., TANIGUCHI, H., FUJITA, T., WADA, H., & MORI,
Y. (1974) [Subacute toxicity of tricresylphosphate (TCP) in rats.]
Pharmacometrics, 8: 107-118 (in Japanese).
SAMPSON, B.F. (1942) The strange Durban epidemic of 1937. S. Afr.
med. J., 16: 1-9.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by
Killifish (Oryzeas latipes). Bull. environ. Contam. Toxicol., 28:
752-759.
SCHWAB, H. (1948) [Psychic disturbance by triorthocresylphosphate
poisoning.] Dtsch. Med. Wochenschr., 73: 124-125 (in German).
SENANAYAKE, N. (1981) Tri-cresyl phosphate neuropathy in Sri Lanka:
a clinical and neurophysiological study with a three year follow
up. J. Neurol. Neurosurg. Psychiatry, 44: 775-780.
SENANAYAKE, N. & JEYARATNAM, J. (1981) Toxic polyneuropathy due to
gingili oil contaminated with tri-cresyl phosphate affecting
adolescent girls in Sri Lanka. Lancet, 10 January: 88-89.
SHARMA, R.P. & WATANABE, P.G. (1974) Time related disposition of
tri-o-tolyl phosphate (TOTP) and metabolites in chicken. Pharmacol.
Res. Commun., 6(5): 475-484.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the
Delaware River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, H.V. & SPALDING, J.M.K. (1959) Outbreak of paralysis in
Morocco due to ortho-cresyl phosphate poisoning. Lancet, 2:
1019-1021.
SMITH, M.I. & LILLIE, R.D. (1931) The histopathology of
triorthocresyl phosphate poisoning. Arch. Neurol Psychiatry, 26:
976-992.
SMITH, M.I., ELVOVE, E., & FRAZIER, W.H. (1930) The pharmacological
action of certain phenol esters, with special reference to the
etiolgy of so-called ginger paralysis. Public Health Rep., 45:
2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies
on the pharmacology of certain phenol esters with special reference
to the relation of chemical constitution and physiologic action.
Natl. Inst. Health Bull., 160: 1-53.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987a) Reproductive tract lesions resulting from
subchronic administration (63 days) of tri-o-cresyl phosphate in
male rats. Toxicol. appl. Pharmacol., 89: 49-63.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987b) Time course of the tri-o-cresyl phosphate-
induced testicular lesion in F-344 rats: enzymatic, hormonal, and
sperm parameter studies. Toxicol. appl. Pharmacol., 89: 64-72.
SOMKUTI, S.G., LAPADULA, D.M., CHAPIN, R.E., & ABOU-DONIA, M.B.
(1987c) Testicular toxicity following oral administration of tri-o-
cresyl phosphate (TOCP) in roosters. Toxicol. Lett., 37: 279-290.
SOMKUTI, S.G., TILSON, H.A., BROWN, H.R., CAMPBELL, G.A.,
LAPADULA, D.M., & ABOU-DONIA, M.B. (1988) Lack of delayed
neurotoxic effect after tri-ortho-cresyl phosphate treatment in
male Fischer-344 rats: bio-chemical, neurobehavioural, and
neuropathological studies. Fundam. appl. Toxicol., 10: 199-205.
SOROKIN, M. (1969) Orthocresyl phosphate neuropathy: Report of an
outbreak in Fiji. Med. J. Aust., 1: 506-508.
SPOERRI, P.E. & GLEES, P. (1979) Ultrastructural reactions of
spinal ganglia to tri-ortho-cresyl phosphate: Effects of
neurotoxicity. Cell Tissue Res., 199: 409-414.
SPOERRI, P.E. & GLEES, P. (1980) Ultrastructural changes in neurons
and neuroglia of the avian telencephalon (Hyperstriatum
Accessorium) following tri-ortho-cresyl phosphate intoxication.
Cell Tissue Res., 206: 203-210.
STAEHELIN, R. (1941) [On the triorthocresylphosphate poisonings.]
Schweiz. Med. Wochenschr., 71: 1-5 (in German).
STUMPF, A.M., TANAKA, D.JR., AULERIOH, A.J., & BURSIAN, S.J. (1989)
Delayed neurotoxic effects of tri-o-tolyl phosphate in the European
ferret. J. Toxicol. environ. Health, 26: 61-73.
STURM, R.N. (1973) Biodegradability of nonionic surfactants:
Screening test for predicting rate and ultimate biodegradation. J.
Am. Oil Chem. Soc., 50: 159-167.
SUGDEN, E.A., GREENHALGH, R., & PETTIT, J.R. (1980)
Characterization of delayed neurotoxic triaryl phosphates by
analysis of trifluoroacetylated phenolic moieties. Environ. Sci.
Technol., 14(12): 1498-1501.
SUSSER, M. & STEIN, Z. (1957) An outbreak of tri-ortho-cresyl
phosphate (TOCP) poisoning in Durban. Br. J. ind. Med., 14:
111-120.
SVENNILSON, E. (1960) Studies of triorthocresyl phosphate
neuropathy, Morocco 1960. Acta psychiatr. Scand., 150(Suppl):
334-336.
TABERSHAW, I.R., KLEINFELD, M., & FEINER, B. (1957) Manufacture of
tricresyl phosphate and other alkyl phenyl phosphates: An
industrial hygiene study. Am. Med. Assoc. Arch. ind. Health, 15:
537-540.
TAYLOR, J.D. & BUTTAR, H.S. (1967) Evidence for the presence of 2-
(o-cresyl)-4 H-1:3:2-benzodioxaphosphoran-2-one in cat intestine
following tri-o-cresyl phosphate administration. Toxicol. appl.
Pharmacol., 11: 529-537.
TER BRAAK, J.W.G. & CARRILLO, R. (1932) [Polyneuritis after usage
of an abortifacient (tri-ortho-cresyl-phosphate-poisoning).] Dtsch.
Z. Nervenheilkd., 125: 86-116 (in German).
THOMPSON, J.E. & DUTHIE, J.R. (1968) The biodegradability and
treatability of NTA. J. Water Pollut. Control Fed., 40: 306-319.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorus compounds. Anal. Chem., 53: 1466-1469.
TOCCO, D.R., RANDALL, J.L., YORK, R.G., & SMITH, M.K. (1987)
Evaluation of the teratogenic effects of tri-ortho-cresyl phosphate
in the Long-Evans hooded rat. Fundam. appl. Toxicol., 8(3): 291-297.
US NIOSH (1977) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Vol. 3, P & CAM, pp. S/209-S/210 (DHEW (NIOSH) Publication
No. 7-157C).
US NIOSH (1979) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS PB-82-216268.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division,
General Motors Corp., 1000 Lexington Avenue, Rochester, New York,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (PB82-104530, NTIS).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorous exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations and
Field Studies (Report No. IWA-89.10).
US SOAP AND DETERGENT ASSOCIATION (1965) A procedure and standards
for the determination of the biodegradability of alkyl benzene
sulfonate and linear alkylate sulfonate (Report of the Subcommittee
on Biodegradation Test Methods). J. Am. Oil Chem. Soc., 42: 986-993.
VASILESCU, C. & FLORESCU, A. (1980) Clinical and
electrophysiological study of neuropathy after organophosphorus
compound poisoning. Arch. Toxicol., 43: 305-315.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A
simple colorimetric method for estimation of tricresyl phosphate in
edible oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J.
Fish Res. Board Can., 36: 1040-1048.
VERONESI, B. (1984) A rodent-model of organophosphorus-induced
delayed neuropathy: distribution of central (spinal cord) and
peripheral nerve damage. Neuropathol. appl. Neurobiol., 10(6):
357-368.
VERONESI, B. & ABOU-DONIA, M.B. (1982) Central and peripheral
neuropathology induced in rats by tri-o-cresyl phosphate (TOCP).
Vet. hum. Toxicol., 24: 3 (abstract).
VERONESI, B., NEWLAND, D., & INMAN, A. (1984) The effect of
metabolic interference on rodent-sensitivity to tri-ortho-cresyl
phosphate. Toxicologist, 4: 55.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J.J., & SVEC, H.J.
(1978) Organic emissions from combustion of combination coal/refuse
to produce electricity. Chemosphere, 7: 893-902.
VONDERAHE, A.R. (1931) Pathologic changes in paralysis caused by
drinking Jamaica Ginger. Arch. Neurol. Psychiatry, 25: 29-43.
VORA, D.D., DASTUR, D.K., BRAGANCA, B.M., PARIHAR, L.M., IYER,
C.G.S., FONDEKAR, R.B., & PRABHAKARAN, K. (1962) Toxic polyneuritis
in Bombay due to ortho-cresyl-phosphate poisoning. J. Neurol.
Neurosurg. Psychiatry, 25: 234-242.
WAGEMANN, R. (1975) Some environmental and toxicological aspects of
a tri-aryl phosphate synthetic oil. Verh. Int. Ver. Limnol., 19:
2178-2184.
WAGEMANN, R., GRAHAM, R., & LOCKHART, W.L. (1974) Studies on
chemical degradation and fish toxicity of a synthetic tri-aryl
phosphate lubrication oil, IMOL S-140. Ottawa, Environment Canada,
Fisheries and Marine Service, (Technical Report, No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution caused by
organophosphoric fire-proofing plasticizers.] Annu. Rep. Tokyo
Metrop. Res. Inst. Environ. Prot., 11: 110-113 (in Japanese).
WALTHARD, K.M. (1945) Aperçu des résultats obtenus lors des
derniers examens des malades intoxiqués en 1940 par le phosphate
triorthocrésilique. Schweiz. Arch. Neurol. Psychiatry, 58: 189-194.
WIGHTMAN, R.H. & MALAIYANDI, M. (1983) Physical properties of some
synthetic trialkyl/aryl phosphate commonly found in environmental
samples. Environ. Sci. Technol., 17: 256-261.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of
tri(haloalkyl)-, trialkyl-, and triarylphosphates in Canadian
drinking water. Bull. environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON,
R. (1982) Determination of mutagenic potential and organic
contaminants of Great Lakes drinking water. Chemosphere, 11: 263-276.
WILSON, R.D., ROWE, L.D., LOVERING, S.L., & WITZEL, D.A. (1982)
Acute toxicity of tri-ortho-cresyl phosphate in sheep and swine.
Am. J. vet. Res., 43(11): 1954-1957.
WINDHOLZ, M., ed. (1983) The Merck index, 10th ed., Rahway, New
Jersey, Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate
esters: Application of linear free energy relationships to estimate
hydrolysis rate constants for use in environmental fate assessment.
Chemosphere, 9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-165.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides
in the atmosphere above the Dogo plain and Ozu basin.] J. Chem.
Soc. Jpn, 1980(4): 645-653 (in Japanese).
ZELIGS, M.A. (1938) Upper motor neuron sequelae in "Jake" paralysis
: A clinical follow up study. J. nerv. ment. Dis., 87: 464-470.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop. Can. Tech. Rep. fish aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de tricrésyle (TCP) est un liquide
visqueux, ininflammable, inexplosible et incolore. Son
coefficient de partage entre l'octanol et l'eau (log de
Pow) est égal à 5,1. Il s'hydrolyse facilement en
milieu alcalin pour donner du phosphate de dicrésyle, mais
il est stable en milieu neutre ou acide à température
normale.
Du point de vue analytique, la méthode de choix est la
chromatographie en phase gazeuse avec détection par un
dispositif sensible à l'azote/phosphore ou par photométrie
de flamme. La limite de détection dans les échantillons
aqueux est d'environ 1 mg/litre. Le TCP s'extrait facile-
ment des solutions aqueuses au moyen de divers solvants
organiques. Pour la purification, on emploie habituelle-
ment une colonne chromatographique de Florisil, mais il
est difficile de séparer le TCP des lipides par cette
méthode. D'autres méthodes de purification ont été re-
commandées à cette fin (chromatographie en phase gazeuse,
chromatographie sur charbon activé ou Sep-pack C-18). Les
réactifs analytiques sont souvent contaminés par des
traces de TCP en raison de la très large utilisation faite
de ce produit. Aussi faut-il prendre certaines précautions
si l'on veut que l'analyse des traces de TCP soit fiable.
2. Sources d'exposition humaine et environnementale
Le TCP est généralement produit par réaction des cré-
sols sur l'oxychlorure de phosphore. Il y a deux sources
de production industrielle de crésols: l'"acide crésy-
lique", résidu des fours à coke et du raffinage du
pétrole, et les "crésols de synthèse" préparés par oxy-
dation et dégradation du cymène. Le TCP est donc un
mélange de divers phosphates de triaryle.
Le TCP est utilisé comme plastifiant des matières
plastiques vinyliques, comme retardateur d'inflammation,
comme additif pour les lubrifiants à très haute pression
et comme liquide ininflammable dans les systèmes hydrau-
liques.
3. Transport, distribution et transformation dans l'environnement
La libération de TCP dans l'environnement est peu
importante au cours de la production et se produit essen-
tiellement lors de l'utilisation finale du produit. On
estime qu'en 1977, 32 800 tonnes en ont été libérées au
total dans l'atmosphère aux Etats-Unis.
En raison de sa faible solubilité dans l'eau et de sa
forte adsorption aux particules, le TCP s'adsorbe rapide-
ment aux sédiments des rivières et des lacs et aux parti-
cules du sol. Il est rapidement biodégradé en milieu
aquatique, sa décomposition étant pratiquement complète
dans les cours d'eau en l'espace de cinq jours. L'isomère
ortho se décompose légèrement plus vite que les isomères
méta et para. Le TCP est facilement biodégradé dans les
boues d'égouts, avec une demi-vie de 7,5 heures, la dégra-
dation atteignant 99% en l'espace de 24 heures. La dégra-
dation abiotique est plus lente, puisque dans ce cas la
demi-vie est de 96 jours.
Des facteurs de bioconcentration de 165-2768 ont été
mesurés en laboratoire sur plusieurs espèces de poissons à
l'aide de TCP radio-marqué. La radioactivité a rapidement
disparu après cessation de l'exposition, la demi-vie de
dépuration allant de 25,8 à 90 heures.
4. Niveaux dans l'environnement et exposition humaine
On a relevé dans l'air des concentration de TCP allant
jusqu'à 70 ng/m3 au Japon, les concentrations maximales
dans une unité de production des Etats-Unis d'Amérique
n'étant que de 2 ng/m3. Dans un atelier de remplissage
de fûts d'huile lubrifiante, aux Etats-Unis d'Amérique, on
a constaté que l'air ne contenait que 0,8 mg/m3 de TCP,
la concentration ne dépassant pas 0,15 mg/m3 en phos-
phates totaux, dans une unité de moulage de zinc d'une
usine d'automobiles. Les concentrations mesurées dans
l'eau de boisson au Canada se sont révélées faibles (0,4 à
4,3 ng/litre) et le TCP n'a pas pu être décelé dans l'eau
des puits. Dans les rivières et les lacs, les concen-
trations sont souvent beaucoup plus élevées. Toutefois on
peut attribuer cet état de choses à la présence de sédi-
ments en suspension auxquels le TCP est fortement adsorbé.
Les concentrations sédimentaires sont plus élevées
puisqu'elles peuvent atteindre 1300 ng/g dans le sédiments
de cours d'eau et 2160 ng/g par les sédiments marins.
Des concentrations élevées ont été mesurées dans le
sol et la végétation aux alentours d'unités de production.
On a fait état de résidus dans des poissons et des
fruits de mer allant jusqu'à 49 ng/g, mais la majorité des
échantillons n'en contenaient pas de quantités décelables.
5. Effets sur les êtres vivants dans leur milieu naturel
On a révélé une réduction de 50% de la productivité
des cultures d'algues vertes d'eau douce, en présence de
concentrations de phosphate de tri- o-crésyle (TOCP)
allant de 1,5 à 4,2 ng/litre, selon les espèces, les iso-
mères méta et para étant moins toxiques. On ne dispose que
de données limitées sur la toxicité aiguë du TCP vis-à-vis
des invertébrés aquatiques: la CL50 à 48 heures pour la
daphnie est de 5,6 ng/litre; la CL50 à 24 heures pour les
nématodes est de 400 ng/litre; la dose sans effet obser-
vable à 2 semaines pour la daphnie (mortalité, croissance,
reproduction) est de 0,1 mg/litre. Pour trois espèces de
poissons, les valeurs de la CL50 à 96 heures se situaient
entre 4,0 et 8700 mg/litre. Chez des truites arc-en-ciel
on a constaté une mortalité d'environ 30% après exposition
de quatre mois à 0,9 ng/litre de IMOL S-140 (phosphate de
tri- o-crésyle à 2%) et des effets plus légers sur une
période de 14 jours.
Les niveaux d'exposition au cours de ces expériences
étaient beaucoup plus élevés que les concentrations sus-
ceptibles d'être rencontrées dans le milieu naturel et
dans la plupart des cas, les valeurs étaient très
supérieures à la solubilité des composés.
6. Cinétique et métabolisme
L'absorption, la distribution, le métabolisme et
l'élimination des organophosphorés jouent un rôle déter-
minant dans les effets neuropathologiques retardés de ces
composés.
Chez l'homme, l'absorption percutanée du TOCP semble
être au moins dix fois plus rapide que chez le chien. On
observe également une importante absorption par cette voie
chez le chat. L'absorption par voie orale a été observée
chez le lapin. On ne possède aucune donnée de première
main sur l'absorption par la voie respiratoire.
Chez le chat, on a constaté qu'après absorption, le
TOCP se répartissait largement dans l'ensemble de l'orga-
nisme, la concentration la plus élevée se situant dans le
nerf sciatique, qui constitue un tissu cible. De fortes
concentrations de TOCP ou de ses métabolites se rencon-
traient également au niveau du foie, des reins et de la
vésicule bilaire.
La métabolisation du TOCP s'effectue selon trois
voies. La première consiste dans l'hydroxylation d'un ou
plusieurs groupes méthyles et la seconde comporte la
désarylation des groupements orthocrésyles. Dans la
troisième, il y a encore oxydation du groupement hydroxy-
méthyle en aldéhyde et en acide carboxylique. L'hydroxy-
lation constitue une étape déterminante, car le TOCP
hydroxyméthylé est cyclisé pour former du phosphate
cyclique d' o-tolyle et de saligénol, un métabolite
neurotoxique relativement instable.
Le TOCP et ses métabolites sont éliminés dans les
urines et les matières fécales ainsi que, en petites
quantités, dans l'air expiré.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuves
in vitro
Des trois isomères du TCP, le TOCP est de loin celui
qui présente la toxicité aiguë la plus forte et qui se
révèle également le plus toxique en cas d'exposition
brève. Il est le seul à déterminer une neurotoxicité
retardée.
Les différents paramètres toxicologiques varient
beaucoup selon l'espèce (qu'il s'agisse par exemple de la
mortalité aiguë ou de la neurotoxicité retardée). Le
poulet est l'une des espèces les plus sensibles.
On a pu obtenir chez des espèces d'animaux de labora-
toire très variées une neuropathie retardée induite par un
organophosphoré (NRIOP) tant à la suite d'une exposition
unique qu'à la suite d'expositions répétées. Il s'agit
d'une neuropathie qui se traduit par des altérations
dégénératives au niveau de la partie distale de l'axone et
qui s'étend peu à peu à l'ensemble du neurone.
Elle se manifeste cliniquement par une paralysie des
pattes postérieures après une période de latence caracté-
ristique de deux à trois semaines suivant l'exposition.
Une dose orale unique de 50 à 500 mg de TOCP/kg a produit
une neuropathie retardée chez des poulets, des doses de
840 mg/kg ou davantage étant nécessaires pour produire une
dégénérescence de la moëlle épinière chez des rats Long-
Evans. C'est l'un des métabolites du TOCP, le phosphate
cyclique d' o-tolyle et de saligénol, qui constitue
l'agent neurotoxique actif. La sensibilité des espèces
varie en sens inverse de la vitesse de métabolisation
ultérieure.
On pense que la lésion biochimique qui conduit à la
neuropathie consiste dans l'inhibition de l'"estérase
neurotoxique". Un taux d'inhibition de plus de 65% peu de
temps après une exposition au TOCP fait présager l'appari-
tion ultérieure d'une neuropathie. La variabilité dans la
réponse neurotoxique dépend également d'autres facteurs
(par exemple la voie d'exposition, l'âge, le sexe, la
souche). Les données disponibles ne permettent pas de
définir clairement une dose sans effet observé pour cette
neuropathie.
Les études de reproduction effectuées sur des rats et
des souris qui recevaient des doses répétées de TOCP par
voie orale, ont révélé la présence de lésions histopatho-
logiques au niveau des testicules et des ovaires, de modi-
fications dans la morphologie des spermatozoïdes, d'une
moindre fécondité chez les deux sexes ainsi que d'une
diminution de la taille des portées et de la viabilité des
ratons et des souriceaux. Les données disponibles n'ont
pas permis de déterminer la dose sans effet pour ce type
d'anomalies imputables au TOCP. Une étude de tératogéni-
cité effectuée sur des rats, avec des doses orales
toxiques pour la mère, n'a pas donné de résultats
positifs.
On n'a guère de données sur la mutagénicité et aucune
sur la cancérogénicité de cette substance.
8. Effets sur l'homme
L'ingestion accidentelle constitue la cause principale
d'intoxication. Depuis la fin du dix-neuvième siècle, de
nombreux cas d'intoxication dus à la contamination de
boissons, de denrées alimentaires ou de produits pharma-
ceutiques ont été signalés. L'exposition professionnelle
tient essentiellement à une absorption percutanée ou à une
inhalation et certains cas d'intoxication de ce type ont
été signalés. L'ingestion de préparations contaminées par
du TOCP peut produire des symptômes digestifs (nausée,
vomissements et diarrhées), encore que dans certains cas,
c'est la polyneuropathie qui constitue le premier signe
d'intoxication. Les symptômes neurologiques sont carac-
térisés par une période de latence. Les premiers symptômes
consistent en douleurs et paresthésie aux extrémités des
membres inférieurs. Il y a une légère diminution de la
sensibilité cutanée et quelques fois réduction de la sen-
sibilité vibratoire. Dans la plupart des cas, la faiblesse
musculaire évolue rapidement vers une paralysie des
extrémités inférieures avec ou sans extension aux membres
supérieurs. Dans les cas graves, apparaissent des signes
d'atteinte pyramidale. Les cas mortels sont rares mais
les symptômes neurologiques peuvent être très lents à dis-
paraître et la guérison peut prendre des mois, voire des
années. L'examen histopathologique révèle une dégénéres-
cence des axones. Les examens classiques de laboratoire
ne révèlent pas d'anomalies si ce n'est parfois une
augmentation de la teneur en protéines du liquide céphalo-
rachidien. Les premiers soins consistent à réduire
l'exposition en faisant vomir immédiatement le malade,
dans la mesure où celui-ci est encore conscient. A plus
long terme, le traitement consiste essentiellement en une
réadaptation physique car on ne connaît pas d'antidote
spécifique. La réaction au phosphate de tricrésyle varie
considérablement d'un individu à l'autre de même que les
possibilités de guérison à la suite d'une intoxication.
On a signalé l'apparition de symptômes graves après inges-
tion de 0,15 g de TCP, alors que chez d'autres personnes,
l'ingestion de quantités atteignant 1 à 2 g n'a produit
aucun effet toxique. Certains malades guérissent complète-
ment alors que d'autres présentent des séquelles marquées
pendant de très longues périodes.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
On a souvent signalé des cas d'intoxication humaine
par suite d'une ingestion accidentelle de phosphate de
tri- o-crésyle ou par suite d'une exposition profession-
nelle. Les symptômes neurotoxiques correspondent tout
d'abord à l'inhibition de la cholinestérase puis à une
neuropathie retardée caractérisée par une paralysie
grave.
Etant donné les variations considérables de sensi-
bilité selon les individus, il n'est pas possible de
déterminer quel est le seuil de sécurité. Des symptômes
ont été signalés après ingestion de 0,15 g d'un mélange
d'isomères contenant une faible proportion de TOCP; la
dose minimale efficace d'isomères ortho est donc beaucoup
plus faible encore. L'expérimentation animale fait égale-
ment ressortir de très importantes variations selon les
espèces pour ce qui concerne la réaction au TOCP et
l'homme semble être particulièrement sensible.
Des cas de dermatite d'irritation et de dermatites
allergiques ont été rapportés.
On peut donc considérer que l'isomère ortho et les
mélanges d'isomères qui en contiennent constituent un
risque de première importance pour la santé humaine.
Aucune dose n'est sans danger si elle est ingérée. Il
convient de réduire au minimum l'exposition cutanée ou
respiratoire.
1.1 Niveaux d'exposition
On peut considérer comme minimale l'exposition de la
population générale au phosphate de tricrésyle par
l'intermédiaire des divers compartiments du milieu
ambiant, notamment l'eau de consommation. On a décelé du
phosphate de tricrésyle à des concentrations plus fortes
dans l'air urbain que dans l'air prélevé au niveau des
sites de production, encore que ces valeurs soient
généralement faibles. Lors d'une enquête effectuée aux
Etats-Unis d'Amérique on n'a pas décelé de TCP dans des
échantillons de tissu adipeux humain. Il y a eu de
nombreux cas d'intoxication humaine accidentelle dus à
l'ingestion de médicaments, de nourriture, de farine,
d'huile de cuisine et de boissons contaminés par des
fluides hydrauliques ou des lubrifiants à base de TCP
produits à partir d'acide crésylique. Des symptômes
toxiques peuvent s'observer après ingestion de doses ne
dépassant pas 0,15 g de TOCP, substance qui est présente
dans le TCP produit à partir de l'acide crésylique. La
contamination se produit généralement lors de la
réutilisation de fûts ou de tonneaux vides qui avaient
contenu un liquide hydraulique ou de l'huile lubrifiante.
1.2 Effets toxiques
L'absorption accidentelle d'une forte dose entraîne
chez l'homme des troubles digestifs, à savoir une nausée
d'intensité variable, pouvant aller jusqu'aux vomisse-
ments, accompagnée de douleurs abdominales et de diar-
rhées. En cas d'exposition à de faibles doses cumulées,
une "neuropathie retardée" s'installe progressivement
après une période de latence de 3 à 28 jours. Dans la
plupart des cas, la faiblesse musculaire fait rapidement
place à une paralysie des membres inférieurs qui peut
parfois s'étendre aux mains. Dans les cas graves, on voit
peu à peu appraître des signes d'atteinte pyramidale.
Certaines études neurophysiologiques révèlent l'existence
d'une neurotoxicité généralisée avec prolongation du temps
de latence terminale et réduction relativement faible de
la vitesse de conduction des nerfs moteurs. Ces consta-
tations confirment la dégénérescence axonale qui est la
principale caractéristique relevée lors des examens histo-
pathologiques.
Le métabolite neurotoxique du TCP a été identifié; il
s'agit du phosphate cyclique de o-tolyle et de saligénol
qui provient lui-même des métabolites o-hydroxyméthylés.
Il semble donc que la présence d'au moins un groupement
o-tolyle soit nécessaire parmi les trois restes phéno-
liques du TCP pour qu'apparaissent des effets neuro-
toxiques. Le TCP produit à partir du crésol de synthèse,
qui contient moins de 0,1% d'orthocrésol, n'est donc pas
neurotoxique.
Les résultats d'études de toxicité subchronique
effectuées sur des animaux d'expérience avec du TCP pré-
paré à partir de crésol de synthèse, montrent que les
organes cibles sont le foie et le rein; toutefois cette
observation n'a pas été confirmée chez l'homme. On ne
dispose pas de données suffisantes sur la mutagénicité et
la cancérogénicité du TCP. On sait néanmoins que le TCP
n'est pas toxique pour l'embryon de poulet.
2. Evaluation des effets sur l'environnement
Le dosage du TCP dans l'eau montre que la contami-
nation est faible. Cet état de choses tient à la faible
solubilité dans l'eau du TCP et à l'aisance avec laquelle
il se décompose. Etant donnée la faible toxicité aiguë du
TCP pour les organismes aquatiques, il est peu probable
qu'il constitue une menace pour ces organismes.
Du fait de ses propriétés physico-chimiques, le TCP a
une forte tendance à la bioaccumulation. Toutefois celle-
ci ne se produit pas dans la pratique en raison de la
faible concentration du TOCP dans l'environnement et les
êtres vivants et de la dégradation rapide de cette
substance.
Les TCP fixés aux sédiments s'accumulent dans
l'environnement et on en a relevé des concentrations
élevées dans les sédiments des cours d'eau et des estu-
aires ainsi que dans les sédiments marins. Du fait que
l'on ne possède aucune information sur la biodisponibilité
de ces résidus pour les organismes fouisseurs ou ben-
thiques, ni sur les dangers qu'ils pourraient représenter,
on ne peut écarter à priori la possibilité d'effets
nocifs.
Il faut également mentionner la possibilité de risques
localisés pour l'environnement dus à un déversement acci-
dentel de TCP.
2.1 Niveaux d'exposition
Les TCP sont présents dans l'air, dans les eaux super-
ficielles, dans le sol, les sédiments et les organismes
aquatiques, à proximité des zones très industrialisées,
encore que leurs concentrations y soient généralement
faibles. En raison de la vitesse élevée de biodégradation
de ces substances en milieu aqueux, il ne semble pas
qu'elles puissent avoir des effets nocifs sur la faune
aquatique. Il a été fait état d'une concentration extrême-
ment élevée en phosphates totaux de triaryle (25,55 g/kg)
dans un échantillon de sol provenant d'une plantation.
Cette observation montre qu'il est nécessaire de procéder
à l'enfouissement des déchets.
2.2 Effets toxiques
Les algues d'eau douce sont relativement sensibles aux
TCP, la concentration inhibant à 50% la croissance com-
prise entre 1,5 et 5,0 ml/litre. En ce qui concerne les
poissons, des concentrations de TCP inférieures à
1 mg/litre (0,3-0,9 mg/litre) provoquent des signes
d'intoxication chronique chez la truite arc-en-ciel, mais
Menidia notata est plus résistant (CL50 de 8700
mg/litre). Les TCP n'inhibent pas l'activité cholinesté-
rasique des poissons ou des grenouilles mais ils poten-
tialisent l'effet des insecticides organophosphorés.
RECOMMANDATIONS
Lorsqu'on utilise des crésols tri-substitués pour la
synthèse et la préparation d'autres composés, il est
préférable d'utiliser des isomères para et méta purifiés
afin d'éviter toute synthèse accidentelle de dérivés
orthosubstitués.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El fosfato de tricresilo (FTC) es un líquido ininflam-
able, no explosivo, incoloro y viscoso. Su coeficiente de
partición entre el octanol y el agua (log Pow) es de
5,1. Se hidroliza con facilidad en un medio alcalino dando
fosfato de dicresilo y cresol, pero es estable en medios
neutros y ácidos a temperaturas normales.
El método analítico de elección es la cromatografía de
gases con un detector sensible al nitrógeno-fósforo o un
detector fotométrico de llama. El límite de detección en
una muestra de agua es de 1 ng/litro aproximadamente. El
FTC se extrae con facilidad de las soluciones acuosas con
distintos disolventes orgánicos. Se utiliza habitualmente
para la extracción la cromatografía en columna de flori-
sil, pero es difícil separar el FTC de los lípidos con
este método. Se han recomendado para esa finalidad otros
métodos de extracción (GPC, cromatografía en carbón vege-
tal activado y Sep-pak C-18). Los reactivos analíticos
están contaminados a menudo con cantidades infinitesimales
de FTC debido a su amplio uso. Por consiguiente, la obten-
ción de datos fiables en el análisis de cantidades infini-
tesimales de FTC requiere un procedimiento cuidadoso.
2. Fuentes de exposición humana y ambiental
El FTC se produce habitualmente por reacción de
cresoles con oxicloruro de fósforo. Existen dos fuentes
industriales de cresoles: el "ácido cresílico", obtenido
como residuo de los hornos de carbón de coque y del refino
del petróleo; y los "cresoles sintéticos", preparados a
partir del cimeno por oxidación y degradación. Como resul-
tado, el FTC es una mezcla de varios fosfatos triaríli-
cos.
El FTC se utiliza como plastificante en los plásticos
vinílicos, y también como pirorretardante, aditivo para
lubricantes de presión extrema y líquido ininflamable en
los sistemas hidráulicos.
3. Transporte, distribución y transformación en el medio ambiente
El paso de FTC al medio ambiente se debe principal-
mente a su uso final, pues la liberación en el curso de la
fabricación es escasa. En 1977 se calculó que el paso
total al medio ambiente en los Estados Unidos de América
fue de 32 800 toneladas.
Debido a su escasa hidrosolubilidad y a su elevada
adsorción por los materiales en partículas, el FTC se
absorbe con rapidez en los sedimentos de ríos o lagos y en
el suelo. Su biodegradación en el medio acuático es
rápida, quedando casi terminada en el agua de río en cinco
días. El isómero orto se degrada con una rapidez ligera-
mente mayor que los isómeros meta o para. El FTC se biode-
grada con facilidad en el fango de los alcantarillados,
presentando una semivida de 7,5 horas; la degradación en
24 horas alcanza el 99%. La degradación abiótica es más
lenta, dando una semivida de 96 días.
Se midieron los factores de bioconcentración de 165-
2768 en varias especies de peces en el laboratorio utili-
zando FTC radiomarcado. La radiactividad desapareció
rápidamente al cesar la exposición, observándose semividas
de depuración comprendidas entre 25,8 y 90 horas.
4. Niveles medioambientales y exposición humana
En el Japón se han medido concentraciones atmosféricas
de FTC de hasta 70 ng/m3, pero alcanzaron un máximo de
sólo 2 ng/m3 en una instalación de fabricación de los
Estados Unidos de América. En este país, el aire del medio
laboral contenía menos de 0,8 mg/m3 en una nave de
llenado de barriles de aceite lubricante y 0,15 mg/m3
(fosfatos totales) en una planta de troquelado de zinc
para automóviles. En el Canadá, las concentraciones de
FTC medidas en el agua potable fueron bajas (0,4 a
4,3 ng/litro) y el producto resultó indetectable en el
agua de pozo. Las concentraciones observadas en las aguas
de ríos y lagos son con frecuencia apreciablemente
mayores. Sin embargo, ello se debe a la presencia de sedi-
mentos en suspensión en los que queda fuertemente absor-
bido el FTC.
Las concentraciones en los sedimentos son altas en los
ríos y en el mar, habiéndose observado valores de hasta
1300 ng/g y 2160 ng/g, respectivamente.
Se observaron concentraciones altas en el suelo y la
vegetación en los alrededores de instalaciones de fabri-
cación.
Se han señalado restos en peces y mariscos de hasta
40 ng/g, pero la mayor parte de los animales examinados no
contenían residuos apreciables.
5. Efectos sobre los seres vivos del medio ambiente
La productividad primaria de cultivos de algas verdes
de agua dulce quedó reducida en el 50% mediante la adición
de fosfato de tri- o-cresilo a razón de 1,5 a 4,2
ng/litro, según las especies, mientras que los isómeros
meta y para resultaban menos tóxicos. Son limitados los
datos referentes a la toxicidad aguda del FTC para los
invertebrados acuáticos: la CL50 en 48 horas para Daphnia
es de 5,6 ng/litro y la CL50 en 24 horas para los nemato-
dos es de 400 ng/litro; la concentración NOEL (mortalidad,
crecimiento, reproducción) en dos semanas para Daphnia es
de 0,1 mg/litro. Los valores de CL50 en 96 horas para
tres especies de peces se hallaban comprendidos entre 4,0
y 8700 mg/litro. La trucha irisada presentó una mortalidad
del 30% aproximadamente después de una exposición de
cuatro meses a una concentración de 0,9 ng/litro de IMOL
S-140 (fosfato de tri- o-cresilo al 2%) y efectos menores
en un periodo de 14 días.
Los niveles de exposición utilizados en esos experi-
mentos fueron mucho mayores que las concentraciones que
probablemente pueden hallarse en el agua en el medio
ambiente y, en la mayoría de los casos, excedían en gran
manera a la solubilidad de los productos.
6. Cinética y metabolismo
La absorción, distribución, metabolismo y eliminación
de los organofosfatos son elementos críticos en los
efectos neuropáticos tardíos de estos productos.
La absorción cutánea del FTOC en el hombre parece ser
por lo menos de un orden de magnitud más rápida que en los
perros. También se observa una absorción cutánea signifi-
cativa en el gato. En el conejo se ha señalado la absor-
ción oral del producto. No hay información directa sobre
la absorción por inhalación.
En estudios efectuados en gatos se observó que el FTOC
absorbido se distribuye ampliamente por todo el organismo,
hallándose la máxima concentración en el nervio ciático,
que es el tejido diana. Otros órganos en los que se encu-
entran altas concentraciones de FTOC y de sus metabolitos
son el hígado, los riñones y la vesícula biliar.
El metabolismo del FTOC sigue tres vías. La primera
es la hidroxidación de uno o más grupos metílicos y la
segunda es la desarilación de los grupos o-cresilo. La
tercera vía es la oxidación ulterior del grupo hidroxi-
metilo para dar aldehído y ácido carboxílico. La etapa de
hidroxilación es decisiva porque el FTOC hidroximetílico
forma un producto cíclico, el fosfato cíclico de o-
tolilo saligenina, metabolito neurotóxico relativamente
ines-table.
El FOTC y sus metabolitos se eliminan por la orina en
las heces, junto con pequeñas cantidades en el aire
espirado.
7. Efectos en los animales de experimentación y en sistemas de
prueba in vitro
Entre los tres isómeros del FTC, el FOTC es con gran
diferencia el más tóxico en la exposición aguda y a corto
plazo. Es el único isómero que produce neurotoxicidad
tardía.
Existe una amplia variabilidad entre especies en lo
que respecta a los distintos puntos finales tóxicos (por
ejemplo, letalidad aguda, neurotoxicidad tardía) de la
exposición al FOTC, siendo el pollo una de las especies
más sensibles.
Se ha producido neuropatía tardía inducida por organo-
fosfatos mediante la exposición única y repetida en una
amplia gama de animales de experimentación; este trastorno
se clasifica como una "neuropatía de muerte sobre el
dorso". Se producen lesiones degenerativas en el axón
distal, que se extienden con el tiempo hacia el cuerpo de
la célula.
Los signos clínicos consisten en la parálisis de las
patas traseras después de un intervalo característico de
2-3 semanas a partir de la exposición. Una sola dosis
oral de 50-500 mg de FOTC/kg produjo la neuropatía tardía
en pollos, mientras que se necesitaron dosis de 840 mg/kg
o más para producir la degeneración de la médula espinal
en ratas Long-Evans. El metabolito fosfato cíclico de
o-tolilo saligenina es el agente neurotóxico activo. La
sensibilidad de las especies guarda correlación inversa
con la tasa de metabolismo ulterior.
Se cree que la inhibición de la "esterasa de la
neurotoxicidad" es la lesión bioquímica que conduce a la
neuropatía tardía inducida por organofosfatos; la inhi-
bición de más del 65% poco después de la exposición al
FOTC permite prever una neuropatía ulterior. En la vari-
abilidad de la respuesta neurotóxica al FOTC influyen
factores distintos del metabolismo (por ejemplo, vías de
exposición, edad, sexo, estirpe). Los datos disponibles no
permiten establecer un nivel neto sin efectos observados
para la neuropatía tardía.
Los estudios de reproducción en ratas y ratones some-
tidos a una exposición oral repetida al FOTC mostraron
lesiones histopatológicas de los testículos y los ovarios,
alteraciones morfológicas del esperma, disminución de la
fecundidad en ambos sexos, y descenso del tamaño y
viabilidad de las crías. Los datos disponibles no permiten
establecer un nivel neto sin efectos en lo que respecta a
la acción del FOTC sobre la reproducción. Un estudio de
teratogenicidad en ratas, utilizando dosis orales que
producían toxicidad materna, dio resultados negativos.
Se dispone de escasa información sobre la mutageni-
cidad y de ninguna sobre la cancerogenicidad.
8. Efectos en la especie humana
La ingestión accidental es la principal causa de
intoxicación. Desde fines del siglo XIX se han observado
numerosos casos de intoxicación por contaminación de bebi-
das, alimentos o medicamentos. La exposición profesional
se produce principalmente por absorción cutánea o inha-
lación, habiéndose registrado algunos casos de envenena-
miento. La ingestión de preparaciones que contienen FOTC
puede ir seguida de síntomas gastrointestinales (náuseas,
vómitos y diarrea), aunque en algunos casos la polineuro-
patía es el primer signo de intoxicación. Los síntomas
neurológicos suelen ser tardíos. Los síntomas iniciales
consisten en dolor y parestesia de las extremidades
inferiores. Puede observarse una alteración moderada de
las sensaciones cutáneas y a veces del sentido de la
vibración. En la mayoría de los casos, la debilidad
muscular evoluciona rápidamente hasta dar una marcada
parálisis de las extremidades inferiores, con o sin parti-
cipación de las superiores. En los casos graves aparecen
signos piramidales. Son raras las defunciones, pero la
recuperación de los síntomas y signos neurológicos puede
ser extremadamente lenta y durar varios meses o años. El
examen histopatológico muestra la presencia de degener-
ación del axón. Los análisis corrientes de laboratorio no
muestran hallazgos anormales, pero cabe observar un
aumento de la concentración de las proteínas en el líquido
cefalorraquídeo. Los primeros auxilios consisten en la
reducción de la exposición provocando el vómito inmediata-
mente después de la ingestión, siempre que el paciente
esté consciente. El tratamiento fundamental a largo plazo
consiste en la rehabilitación física, no conociéndose
ningún antídoto específico. Existen grandes variaciones
entre las personas en la respuesta al FTC y en la recuper-
ación después de producirse los efectos tóxicos. Se han
registrado síntomas graves después de la ingestión de
0,15 g de FTC, mientras que otras personas no presentaron
efecto tóxico alguno después de ingerir 1-2 g. Ciertos
enfermos se recuperan por completo, mientras que otros
conservan efectos marcados durante un periodo apreciable.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Se han registrado con frecuencia intoxicaciones
humanas por ingestión accidental de fosfato de tri- o-
cresilo (FTOC) o por exposición laboral de trabajadores.
La vía probable de la exposición laboral es la absorción
cutánea. Entre los síntomas neurotóxicos figuran la inhi-
bición inicial de las colinesterasas y la neuropatía
tardía ulterior caracterizada por parálisis grave.
Debido a la considerable variación existente en la
sensibilidad de las personas al FTOC, no puede estable-
cerse un nivel de exposición sin riesgo. Se han señalado
síntomas por ingestión de 0,15 g de una mezcla de isómeros
con baja proporción de FTOC; así pues, la dosis efectiva
mínima del ortoisómero es muy inferior. Los estudios
efectuados en animales muestran considerables variaciones
entre especies en la respuesta al FTOC, y las personas
parecen ser especialmente sensibles.
Se han señalado casos de dermatitis irritante y
alérgica.
En consecuencia, tanto el ortoisómero puro como las
mezclas de isómeros que contienen FTOC se consideran
riesgos importantes para la salud humana.
No existe un nivel inocuo de ingestión. La exposición
al producto por contacto cutáneo o inhalación debe
reducirse al mínimo.
1.1 Niveles de exposición
Puede considerarse mínima la exposición de la pobla-
ción general al fosfato de tricresilo (FTC) por conducto
de distintos medios ambientales, incluida el agua de
beber. Se han observado concentraciones de FTC relativa-
mente más altas en el aire urbano que en el aire recogido
en los emplazamientos de fabricación, aunque los niveles
suelen ser bajos. En un estudio efectuado en los Estados
Unidos de América no se detectó el FTC en muestras de
tejido adiposo humano. Se han observado numerosos casos
de intoxicación humana accidental por ingestión de medi-
camentos, alimentos tales como harina y aceite de cocinar,
y bebidas contaminados con líquido hidráulico o aceite
lubricante que contenían FTC producido a partir del
"ácido cresílico". Pueden observarse síntomas tóxicos
después de la ingestión de sólo 0,15 g de fosfato de tri-
o-cresilo, componente del FTC obtenido a partir de ácido
cresílico. El origen habitual de la contaminación ha con-
sistido en la reutilización de barriles o bidones vacíos
utilizados previamente para contener líquido hidráulico o
aceite lubricante.
1.2 Efectos tóxicos
La exposición humana accidental a una sola dosis alta
ocasiona trastornos gastrointestinales que varían de las
náuseas ligeras a las intensas con vómitos, dolor abdomi-
nal y diarrea. En el caso de la exposición a pequeñas
dosis acumuladas, aparece progresivamente la "neurotoxi-
cidad tardía" después de un periodo latente de 3-28 días.
En la mayor parte de los casos, la debilidad muscular
pasa rápidamente a ser una marcada parálisis de las ex-
tremidades inferiores, con o sin afectación de las manos.
En los casos graves aparecen progresivamente signos
piramidales. Algunos estudios neurofisiológicos muestran
fenómenos extendidos de neurotoxicidad y prolongación de
las latencias terminales, con disminución relativamente
pequeña de la velocidad de conducción de los nervios
motores. Ello confirma los signos de degeneración del
axón, que es la característica principal observada en los
exámenes histopatológicos.
Se ha identificado el metabolito neurotóxico del FTC
como el fosfato cíclico de o-tolilo saligenina, derivado
de los metabolitos o-hidroximetílicos. Parece pues que
los efectos neurotóxicos exigen la presencia por lo menos
de un grupo o-tolilo entre las tres porciones fenólicas
del FTC. Ello significa que no es neurotóxico el FTC
producido a partir de cresol sintético, que contiene menos
del 0,1% de o-cresol.
Los estudios de toxicidad subcrónica efectuados en
animales con FTC obtenido de cresol sintético muestran que
los órganos diana son el hígado y los riñones, pero esa
observación no se ha confirmado en los casos de intoxi-
cación humana. No se dispone de datos apropiados sobre la
mutagenicidad y la cancerogenicidad. El FTC no es tóxico
para los embriones de pollo.
2. Evaluación de los efectos en el medio ambiente
La medición de las concentraciones de FTC en el agua
ambiental ha mostrado que existen sólo niveles bajos de
contaminación. Ese hecho refleja la escasa hidrosolu-
bilidad y la fácil degradabilidad del producto. Dado que
la toxicidad aguda del FTC para los seres acuáticos es
también baja, es improbable que represente una amenaza
para ellos.
Debido a las propiedades fisicoquímicas del FTC
existen altas posibilidades de bioacumulación. Sin
embargo, ello no se produce en la práctica porque las con-
centraciones de FOTC en el medio ambiente y en los organ-
ismos vivos son bajas y porque los productos se degradan
con rapidez.
El FTC fijado por los sedimentos se acumula en el
medio ambiente, habiéndose medido concentraciones altas en
los sedimentos de ríos, estuarios y mares. Dado que se
carece de información sobre la biodisponibilidad de esos
residuos para los seres vivos que se hallan en madrigueras
o en el fondo o sobre sus riesgos, no puede descartarse la
posibilidad de que aparezcan efectos en tales especies.
La fuga de FTC suscita riesgos para el medio ambiente
local.
2.1 Niveles de exposición
El FTC se halla en el aire, las aguas de superficie,
el suelo, los sedimentos y los seres vivos acuáticos cerca
de las zonas muy industrializadas, aunque en concen-
traciones habitualmente bajas. Debido a la alta tasa de
biodegradación del FTC en el medio acuoso, no se considera
que afecta adversamente a los seres vivos acuáticos. En un
estudio se encontró una concentración extremadamente alta
de fosfatos triarílicos totales (26,55 g/kg) en una
muestra de suelo obtenida en el patio de una planta de
producción. Ello sugiere la necesidad de eliminar los
residuos por relleno de terrenos.
2.2 Efectos tóxicos
Las algas de agua dulce son relativamente sensibles al
FTC, cuya concentración inhibidora del 50% del crecimiento
es de 1,5 a 5,0 mg/litro. Entre las especies de peces, la
trucha irisada sufre el efecto de concentraciones de FTC
inferiores a 1 mg/litro (0,3-0,9 mg/litro), con signos de
intoxicación crónica, pero el pez argentado de las mareas
es más resistente (CL50 de 8700 mg/litro). El FTC no
inhibe la actividad colinesterásica seca de peces y ranas,
pero tiene un efecto sinérgico con la actividad
insecticida de los órganos fosforados.
RECOMENDACIONES
Cuando se utilizan cresoles trisustituidos en la
síntesis y fabricación de otros productos, es necesario
emplear isómeros meta y para purificados para evitar la
síntesis accidental de productos orto-sustituidos.
 Molecular formula: C21H21O4P
Relative molecular mass: 368.4
CAS chemical name: phosphoric acid, tritolyl ester
CAS registry number:
Molecular formula: C21H21O4P
Relative molecular mass: 368.4
CAS chemical name: phosphoric acid, tritolyl ester
CAS registry number:  CAS chemical name: phosphoric acid, tri- o-tolyl ester
CAS registry number:
CAS chemical name: phosphoric acid, tri- o-tolyl ester
CAS registry number:  CAS chemical name: phosphoric acid, tri- m-tolyl ester
CAS registry number:
CAS chemical name: phosphoric acid, tri- m-tolyl ester
CAS registry number:  CAS chemical name: phosphoric acid, tri- p-tolyl ester
CAS registry number:
CAS chemical name: phosphoric acid, tri- p-tolyl ester
CAS registry number:  TOCP is metabolized via three essential pathways. The
first is the hydroxylation of one or more of the methyl
groups to hydroxymethyl, which is responsible for the for-
mation of mono- and di-hydroxymethyl TOCP and o-hydroxy-
benzyl alcohol. This reaction is known to be catalysed by
the microsomal mixed-function oxidase system (Eto et al.,
1967). The hydroxymethyl TOCP is cyclized to form sal-
igenin cyclic o-tolyl phosphate with spontaneous release
of o-cresol, this being catalysed by the reaction of
plasma albumin or other components (Eto et al., 1967). The
cyclic phosphate metabolite is relatively unstable and is
rapidly hydrolysed to inactive metabolic products (Eto et
al., 1967). The second pathway is the dearylation of one
or more of the o-cresyl groups of TOCP, resulting in the
formation of o-cresol, di- o-cresyl phosphate, o-cresyl
phosphate, and phosphoric acid. The third pathway is
further oxidation of hydroxymethyl to aldehyde and car-
boxylic acid. These oxidation reactions are most likely to
be mediated by alcohol and aldehyde dehydrogenases.
Studies with [32P]-TOCP in rats have shown that
hydrolysis leads to the rapid excretion in the urine of
diaryl phosphates, monoaryl phosphates, and phosphoric
acid (Casida et al., 1961).
Nomeir & Abou-Donia (1984; 1986a,b) clearly identified
the metabolites of TOCP in male cats. Mono- and di-
hydroxymethyl TOCPs and saligenin cyclic- o-tolyl phos-
phates were present in most tissues, but their concen-
trations were low compared with those of other metab-
olites. The major metabolite of TOCP in the liver, kidney,
lung, and urine of cats was o-hydroxybenzoic acid; di-
o-cresyl phosphate, o-cresyl phosphate, o-cresol,
o-hydroxy-benzyl alcohol, and o-hydroxybenzaldehyde
were also identified. However,the brain, spinal cord,
sciatic nerve, and faeces contained predominantly
unchanged TOCP.
Johnson (1975a) compared the metabolic pathways of the
three isomers of TCP. The main observations, which con-
cerned several organophosphorus esters, were as follows:
(i) Provided that the o-alkyl group has at least one
hydrogen on the alpha-carbon atom, cyclic derivatives
can be obtained that are often highly neurotoxic.
(ii) At the para position, a substituent requires two
hydrogen atoms on the alpha-carbon atom in order to
produce a neurotoxic metabolite inhibiting NTE.
(iii) Substituents at the meta position may be metab-
olized but do not yield inhibitory products.
The major urinary metabolites of TPCP in rats were
p- hydroxybenzoic acid, di- p- cresyl phosphate, and
p- cresyl p- carboxyphenyl phosphate. Mono- (or di-) p-
cresyl di- (or mono-) p- carboxyphenyl phosphate was
identified as the intermediate metabolite in the bile
(Kurebayashi et al., 1985).
7.4 Excretion
After a single oral dose of [32P]-TOCP (770 mg/kg)
to hens, 26.5% of the total radioactivity was eliminated
in the combined urinary-faecal excreta over 72 h, mostly
as TOCP (Sharma & Watanabe, 1974).
After a single dose of [14C]-TOCP (50 mg/kg) to male
cats, approximately 28% of the applied dose was excreted
in the urine and 20% via the bile into the faeces within
10 days (Nomeir & Abou-Donia, 1986b). After this exposure,
the disappearance of TOCP and its metabolites from the
plasma followed monoexponential kinetics. The apparent
half-lives of TOCP and its metabolites (in days) in the
plasma were: TOCP, 1.20; saligenin cyclic- o- tolyl phos-
phate, 2.47; di- o- cresyl phosphate, 4.50; o- cresyl
phosphate, 4.30; o- cresol, 2.65; o- hydroxybenzyl
alcohol, 14.0; o- hydroxy-benzaldehyde, 5.70; o-
hydroxybenzoic acid, 6.00; monohydroxymethyl TOCP, 2.20.
The apparent half-lives of TOCP and its metabolites
reflected the rates of all processes involving the
conversion, clearance, and/or redistribution of these
metabolites (Nomeir & Abou-Donia, 1984).
Elimination via the bile has been demonstrated after
intravenous injection into rabbits (Gross & Grosse, 1932)
and intraperitoneal injection into rats (Myers et al.,
1955). Smith et al. (1932) measured the urinary phenol
excretion in cats given subcutaneous doses of 0.4 to 1.0
ml TOCP/kg. Little if any increase in the urinary phenol
excretion was found either before or after the onset of
paralysis of the hindlimbs.
After a single oral dose (500 mg/kg) of tri- m-cresyl
phosphate (TMCP) or TPCP to rabbits, 92% of TMCP and 95%
of TPCP was eliminated in the faeces within 4 days (Gross
& Grosse, 1932). After a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg), about 90% or 76%,
respectively, of the radioactivity was eliminated in the
urine and faeces within 24 h (Kurebayashi et al., 1985).
The apparent half-lives of the radioactivity in tissues
ranged from 14 h for blood to 26 h for lung and brain. At
the lower dose level, about 28% of the dose was eliminated
via the bile within 24 h. The expiratory excretion as
14CO2 over 3 days amounted to 18% of the radioactivity,
which was reduced to 3% when the rats were treated with
neomycin. The authors suggested that the enterohepatic
circulation and intestinal microflora play an important
role in the degradation of TPCP biliary metabolites.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Summary
Of the three isomers of TCP, TOCP is by far the most toxic
and is the only isomer that produces delayed neuropathy.
A wide animal interspecies variability exists for the
various end-points (e.g., lethality, neuropathology, ataxia,
enzyme inhibition) of TOCP exposure, the chicken being one of
the most sensitive species to delayed neuropathy (i.e. OPIDN).
Species sensitivity to the lethal effects of TOCP adminis-
tration is highly variable, chickens and cats being more sensi-
tive than rats and mice. A single oral dose of 50-500 mg TOCP
per kg induced delayed neuropathy in chickens, whereas doses of
840 mg/kg or more were necessary to produce spinal cord degen-
eration in Long-Evans rats.
No effects were reported in skin and eye irritation studies
on rabbits.
Reproduction studies on rats and mice receiving repeated
oral exposure to TOCP showed histopathological damage in the
testes and ovaries, morphological changes in sperm, decreased
fertility in both sexes, and decreased litter size and
viability. However, no reproductive effects were seen in a
study with TPCP. A clear no-observed-effect level for the
reproductive effects of TOCP was not apparent from the data
available. A study in rats, using oral doses that produced
maternal toxicity, failed to show any teratogenicity.
Little or no information is available on mutagenicity and
carcinogenicity.
Delayed neuropathy has been produced with both single and
repeated exposure regimes in a wide range of experimental
species, and it is classified as a "dying-back neuropathy".
"Neurotoxic esterase" is thought to be the biochemical target
of OPIDN and its inhibition by more than 65% shortly after
exposure to TOCP presages subsequent neuropathy. Factors other
than metabolism (e.g., route of exposure, age, sex, species,
strain) influence variability in sensitivity to OPIDN.
Electrophysiology studies have been performed in cats and
chickens exposed to TOCP.
In the chicken, single exposures below 58 mg/kg or short-
term (i.e. 90-day exposure) daily doses of less than 5 mg/kg
appear to be no-observed-effect levels for delayed neuropathy.
8.1 Single exposure
The acute toxicity of TCP to different species is
summarized in Table 13.
Table 13. LD50 values for TCP and its isomers
____________________________________________________________________
Compounds Route Species LD50 Reference
of (mg/kg)
admin.
____________________________________________________________________
Tricresyl oral rat 5190 Marhold (1972)
phosphate oral rat >4640 Stauffer (1988)a
(mixed oral rat >15 800 Johannsen (1977)
isomers) oral mouse 3900 Izmerov (1982)
oral chicken >10 000 Johannsen et al. (1977)
dermal rabbit >7900 Johannsen et al. (1977)
dermal cat 1500 Abou-Donia et al. (1980)
Tri- o- cresyl oral rat 8400 Johannsen et al. (1977)
phosphate oral rat 1160 Veronesi et al. (1984a)
oral rabbit 3700 Johannsen et al. (1977)
oral chicken 500 Kimmerle & Loeser (1974)
oral chicken 100-200 Smith et al. (1932)
Tri- p- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate chicken >1000 Smith et al. (1932)
Tri- m- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate oral chicken >2000 Smith et al. (1932)
____________________________________________________________________
a Personal communication to the IPCS from Stauffer Chem. Co. (1988)
entitled: Test procedures and data summaries for t-butyl phenyl
diphenyl phosphate, tricresyl phosphate, trixylenyl phosphate,
mixed triaryl phosphate and isopropyl phenyl diphenyl.
The acute symptoms of intoxication are typical of
organophosphorus poisoning. The most toxic compound
appears to be TOCP; the acute toxicity of TCP depends on
the relative proportions of the different isomers.
The chicken, guinea-pig, and rabbit are the most sen-
sitive species, death occurring at an oral dose of 100
mg/kg (Smith et al., 1932); rats and mice are the least
sensitive species.
Sheep given oral doses (100, 200, or 400 mg/kg) of
TOCP exhibited acute intoxication characterized by diar-
rhoea, dehydration, metabolic acidosis, and death within
6 days. Pigs dosed with 100 to 1600 mg TOCP/kg showed
minimal signs of acute intoxication, but developed severe
signs of delayed neuropathy approximately 15 days after
administration (Wilson et al., 1982).
8.2 Short-term exposure
Saito et al. (1974) conducted a 3-month study in rats
with TCP consisting of 60-65% TMCP and 35-40% TPCP. The
compound was suspended in water with 5% gum arabic and
given orally to SD rats at 30, 100, 300, or 1000 mg/kg per
day. Histopathological examination revealed no notable
changes associated with the compound. Based on those
observations, the authors concluded that TCP was of low
short-term toxicity.
Oishi et al. (1982) reported that Wistar rats fed a
pellet diet containing a mixture of TCP isomers at a con-
centration of 5 g/kg diet for 9 weeks developed increases
in absolute and relative liver weights. Haematological
examination revealed no notable changes, but, in the
plasma, total protein, urea, cholesterol and glutamate-
pyruvate transaminase were significantly increased. Slight
liver histopathology included cytoplasmic vacuolization,
increase in the number of binucleated cells, and enlarge-
ment of cell size.
Chapin et al. (1988) exposed male and female CD-1 mice
to diets containing 0, 0.437, 0.875, 1.75, 3.5, or 7.0%
TCP (a mixture of ortho, meta, and para isomers) for
14 days. No clinical signs of toxicity were observed in
the animals at doses up to 0.875%. All animals in the
groups given 1.75, 3.5, or 7.0% exhibited piloerection,
tremors, and diarrhoea, and were lethargic before death
during the 14-day exposure.
8.3 Skin and eye irritation
No published information is available.
8.4 Teratogenicity
Mele & Jensh (1977) reported that no abnormalities
were found in fetuses from pregnant Wistar rats treated
with 500 or 750 mg TOCP/kg of on the 18th and 19th days of
gestation. This study was primarily designed to investi-
gate the effects of prenatal treatment with TOCP on post-
natal behaviour. As such, it cannot be regarded as a true
teratogenicity study.
Tocco et al. (1987) tested the teratogenicity of TOCP
in Long-Evans rats treated with 87.5, 175, and 350 mg/kg
per day throughout organogenesis from gestation days 6 to
18. No maternal deaths or toxicity were observed at the
low or medium dose levels. Maternal lethality in the high-
dose group was higher than that in the control groups.
Numerous soft tissue and skeletal malformations were
observed in both control and TOCP-treated groups, but
there were no significant differences in the frequency of
malformations between the treated and control animals.
8.5 Reproduction
In studies by Somkuti et al. (1987a), TOCP was tested
for effects on the male reproductive tract in male Fisher-
344 rats. Animals were dosed daily for 63 days at dose
levels ranging from 10 to 100 mg/kg per day. Vehicle-
treated animals served as controls. As judged by enzy-
matic, hormonal, and sperm motility, density, and mor-
phology investigations, the minimum effective (threshold)
dose for observable testicular toxicity was 10-25 mg/kg
per day. The data suggested that TOCP interfered directly
with spermatogenic processes and sperm motility and not
via androgenic mechanisms or decreased vitamin E avail-
ability. Testicular pathological changes were seen at
doses above 25 mg/kg per day and included the following:
PAS-positive droplets, immature germ cells, and multinu-
cleate giant cells in the lumen. TPCP produced a decrease
in sperm density but no other testicular effects at a dose
level of 100 mg/kg per day.
To study the time course of the TOCP-induced testicu-
lar lesion in F-344 rats, the onset of possible changes in
sperm numbers and production, serum hormones, and various
enzyme activities was followed. Rats were administered
TOCP daily (150 mg/kg) for periods of 3, 7, 10, 14, or 21
days, while vehicle-treated animals served as controls.
Both sperm motility and the number of sperm per mg of
cauda epididymis were lower in treated animals by day 10.
The ratio of testicular to body weight was significantly
decreased only those rats treated for 21 days. Testicular
neurotoxic esterase and nonspecific esterase activities
were also inhibited, while beta-glucuronidase activity was
not affected. Luteinizing and follicle-stimulating hormone
levels were normal, as were both serum and interstitial
fluid testosterone concentrations. Sertoli cell fluid
secretion, as measured by testis weight increase after
efferent duct ligation, showed no significant changes.
Other organs (spleen, liver, kidney, pancreas, small
intestine, and adrenal and pituitary glands) revealed no
overt signs of pathology as observed by light microscopy
in animals treated for 21 days. A separate group of ani-
mals was treated for 21 days and subsequently examined
after 98 days of observation (two cycles of the rat semin-
iferous epithelium). Normal spermatogenesis did not
return, indicating that the toxicity was irreversible at
the dose used. The effects noted in these studies further
define the testicular lesion produced by TOCP, and show
that a dose level 150 mg/kg per day for 21 days produces
irreversible testicular toxicity (Somkuti et al., 1987b).
The testicular effects of TOCP have also been studied
in the rooster, 100 mg/kg per day being administered
orally to ten adult leghorn roosters for 18 consecutive
days. By days 7-10 of the study, TOCP-treated birds
exhibited limb paralysis characteristic of OPIDN. Enzyme
analyses at the end of the study revealed significant
inhibition of neurotoxic esterase (NTE) activity in both
brain and testis and a slight decrease in brain acetyl-
cholinesterase (AChE) activity. Sperm motility was shown
to be greatly decreased. In addition, sections of
formalin-fixed, methacrylate-embedded testes from TOCP-
treated birds showed vacuolation and disorganization in
the seminiferous epithelium. The marginal body weight
decrease (17%) in treated animals was not considered to
contribute to the testicular toxicity induced by TOCP
(Somkuti et al., 1987c).
TCP (isomer mixture not known) was tested in sperm
morphology and vaginal cytology examination (SMVCE)
studies in groups of 25 male and female Fisher-344 rats
and 25 male and female Swiss CD-1 mice. Treatment lasted
for 13 weeks at dose levels of 1700, 3300, or 6600 mg/kg
diet or 50, 100, 200, 400, or 800 mg/kg body weight via
gavage in the rats, and 500, 1000, or 2100 mg/kg diet or
50, 100, or 200 mg/kg body weight via gavage in the mice.
Effects were seen in treated animals, but no details were
given (Morrissey et al., 1988a). In a follow-up continuous
breeding reproduction study in Swiss CD-1 mice, male and
female fertility was reduced (Morrissey et al., 1988b).
Carlton et al. (1987) examined the reproductive
effects of TCP (mixed isomers; < 9% TOCP). Male Long-Evans
rats received 0, 100, or 200 mg/kg and females received 0,
200, or 400 mg/kg in corn oil by gavage. The low-dose
males were mated with low-dose females, and the high-dose
males with high-dose females. Males were dosed for 56 days
and females for 14 days prior to breeding and throughout
the breeding period, gestation, and lactation. Sperm con-
centration, motility, and progressive movement were
decreased in the high-dose males, and there was a dose-
dependent increase in abnormal sperm morphology. The
number of females delivering live young was severely
reduced by TCP exposure. Litter size and pup viability
were decreased in the high-dose group, but pup body weight
and developmental landmarks were unaffected by TCP
exposure. Histological changes were observed in the testes
and epididymides of treated males (i.e. necrosis, degener-
ation, early sperm granulomas in seminiferous tubules, and
epididymal hypospermia) and in the ovaries of treated
females (i.e. diffuse vacuolar cytoplasmic alteration of
interstitial cells and increased numbers of follicles and
corpora lutea).
In a continuous breeding study, Chapin et al. (1988)
fed Swiss CD-1 mice a diet containing TCP (mixed o-, m-,
p-isomers) at a level of 0, 0.5, 1, or 2 g/kg diet over
98 days. Although the fertility index was not changed in
the high-dose groups, the number of litters per pair
decreased in a dose-dependent fashion, and the proportion
of pups born alive and their weight were significantly
decreased. Histopathological examination of the parental
generation revealed dose-related seminiferous tubule
atrophy and decreased testis and epididymal weights in the
high-dose males, but the female reproductive tract showed
no histopathological changes. A crossover mating trial
revealed impaired fertility in both males and females
exposed to 2 g/kg diet.
8.6 Mutagenicity and carcinogenicity
Haworth et al. (1983) reported a negative result with
TCP (substitution pattern not known) in the Salmonella
mutagenicity test.
8.7 Neurotoxicity
8.7.1 Experimental neuropathology
TOCP first gained notoriety as the culpable neurotoxic
agent of the "Ginger Jake" epidemic (Jeter, 1930;
Goodale & Humphreys, 1931; Vonderahe, 1931). Since then
several experimental studies have modelled TOCP neuropathy
in various species.
Smith & Lillie (1931) produced delayed paralysis in
various animals, including dogs, monkeys, cats, and
chickens. These animal models were used to describe the
functional and morphological features of TOCP neuropathy.
After a delay period of 2-3 weeks following exposure to
single or multiple doses, paralysis of the hindlegs
developed in these species in response to the TOCP. Neuro-
pathologically, degeneration was confined to the spinal
cord and peripheral nerve fibres. These changes were
essentially similar to the lesions reported in human
victims of the "Ginger Jake" epidemic.
The delayed neuropathy associated with TOCP and other
organophosphorous compounds has been termed OPIDN.
Cavanagh (1964) showed that, in cats and chickens,
degeneration affected the long fibres in the spinal cord
and peripheral nerves; moreover, in cats, fibre diameter
seemed to be important in determining the onset and
severity of the peripheral nerve lesions. He categorized
this delayed neuropathy as a "dying-back". Thus, the
lesions of the ascending tracts are seen in the cervical
region, especially in the dorsal columns (i.e. fasciculus
gracilis), while those of the descending tracts are seen
in the thoracic and lumbosacral regions of the spinal
cord. He also showed that the peripheral and central nerve
lesions were the result of axonal degeneration and did not
reflect primary demyelination. The affected nerve axons
degenerate in a "dying-back" fashion towards the cell
body, i.e. axonal degeneration begins at the most distal
portion of the axon and proceeds towards the cell body.
Prineas (1969) described axons with accumulated
tubulovesicular membranes within myelinated motor nerve
terminals in the foot muscles of cats dosed with TOCP.
Similar early axoplasmic changes have been noted in TOCP-
treated chickens (Bischoff, 1967, 1970; Spoerri & Glees,
1979, 1980).
At the ultrastructural level, the nerve endings are
characterized by a marked proliferation and distension of
vesicular elements of the endoplasmic reticulum and a
coinciding disintegration of the filamentous and tubular
organelles (Bischoff, 1977). Bischoff also noted that the
presynaptic nerve terminals and boutons terminaux appeared
particularly sensitive in TOCP intoxication.
OPIDN has also been produced in rats dosed with TOCP
(Veronesi, 1984). The pathological changes developed in
the absence of discernable ataxia after a 2-week exposure
to acute and multiple oral doses of TOCP (>840 mg/kg). In
the rat, dorsal column degeneration of the cervical cord
and a selective vulnerability of large diameter tibial
branches supported a "dying-back" neuropathy, as in
other species.
OPIDN has also been described in European ferrets
( Mustela putorius furo) administered a single oral or
dermal dose of 250, 500, or 1000 mg TOCP/kg body weight.
Five animals per group were sacrificed 48 h after dosing,
the others being observed for another 54 days. All ferrets
dosed dermally with 1000 mg/kg developed neurological
signs ranging from ataxia to partial paralysis. Dermal
doses of 250 and 500 mg/kg produced variable degrees of
hind limb weakness and ataxia. Of the animals dosed
orally, only those treated with 1000 mg/kg showed neuro-
logical signs, which did not progress beyond mild ataxia.
Slight axonal degeneration was noted in the dorsolateral
part of the lateral funicilus and in the fasciculus gra-
cilis of spinal cords in ferrets receiving a dermal dose
of 1000 mg/kg. Whole brain neurotoxic esterase activity
was maximally inhibited (46%) at this dose level. The
study demonstrated that in the ferret dermal exposure was
more effective than oral exposure at the same dose level
(Stumpf et al., 1989).
8.7.2 Neurochemistry
Johnson (1969) found that approximately 6% of brain
esterases are not affected by non-neuropathic compounds
but are specifically inhibited, irreversibly, by neuro-
pathic ones, such as TOCP. Johnson used the term "neuro-
toxic esterase" (NTE) and proposed that NTE is the pri-
mary target of the organophosphorus esters causing OPIDN
(Johnson, 1975a,b). There appears to be a strong corre-
lation in the chicken between NTE inhibition above 70%
shortly after exposure and subsequent neuropathy for a
large number of tested organophosphorous compounds
(Johnson, 1974).
Padilla & Veronesi (1985) demonstrated the relevance
of NTE to the rodent model of OPIDN by exposing Long-Evans
rats to single doses of TOCP ranging from 290 to 3480
mg/kg. High NTE inhibition in the spinal cord (>72%) and
the brain (> 66%) produced severe spinal cord damage in
over 90% of exposed rats, indicating that NTE depression
could predict OPIDN damage in rats acutely exposed to
organophosphates.
Concerning the role of lipids in TOCP neuropathy,
Morazain & Rosenberg (1970) showed that there was a rise
of 25-50% in the cholesterol level in the sciatic nerve
and a 50% decrease in its triglyceride content in chickens
orally dosed with 1 ml TOCP/kg. Phospholipids, diglycer-
ides, cholesterol esters, proteolipids, and tissue phos-
pholipases were not affected by TOCP. The possible
involvement of lipids in the production of TOCP-induced
delayed neuropathy has not yet been resolved.
Other toxic effects have been demonstrated. Brown &
Sharma (1975) found that neural membrane ATPases were
inhibited by organophosphates. Cohen & Murphy (1970)
reported that TOCP potentiates the anticholinesterase
action of malathion by 29-fold in mice, 17-fold in quail,
and 11-fold in sunfish.
8.7.3 Interspecies sensitivity and variability to OPIDN
Certain animal species (e.g., cats, dogs, cows, and
chickens) are susceptible to OPIDN-related paralysis,
whereas others (e.g., rats and mice) are less susceptible
to the ataxia but very susceptible to the pathological
changes. Species susceptibility to delayed neurotoxicity
induced by TOCP shows an inverse correlation with the rate
of metabolic conversion to the neurotoxic metabolite (see
section 7). Because of its high susceptibility to ataxia,
the adult chicken has been used as an experimental model
to study OPIDN.
TOCP is metabolized to the more potent neurotoxic
agent, saligenin cyclic o-tolyl phosphate, which is at
least five times more neurotoxic than TOCP after oral
administration to chickens: a metabolite level of 40 mg/kg
caused ataxia equivalent to that resulting from 200 mg
TOCP/kg (Bleiberg & Johnson, 1965).
It has been shown that a single oral dose of TOCP in
the range of 58 to 580 mg/kg (i.e. 0.05-0.5 ml/kg) induces
mild to severe paralysis in the hen ( Gallus domesticus)
(Cavanagh, 1954; Hine et al., 1956).
Johannsen et al. (1977) showed that chickens adminis-
tered cumulative doses of TCP (60 000 mg/kg) or TOCP (1500
mg/kg) developed both the ataxic and neuropathological
symptoms of OPIDN.
In 90-day studies in hens, obvious functional and
morphological neuropathological changes were found at
daily oral dose levels of 5 to 20 mg TOCP/kg body weight,
but not at lower dosages (Smith et al., 1932; Prentice &
Majeed, 1983; Roberts et al., 1983).
In a subchronic feeding study, Haggerty et al. (1986)
exposed rats to TCP (isomeric substitution pattern not
known) for 13 weeks at dose levels of 0, 900, 1700, 3300,
6600, or 13 000 mg/kg. Decreased hindlimb grip strength
was observed in male rats (at 13 000 mg/kg), but not in
female rats. In mice exposed to 0, 250, 500, 1000, 2100,
or 4200 mg/kg, decrements in both fore- and hindlimb grip
strength were observed in males (at 4200 mg/kg) and
females (at 2100 and 4200 mg/kg). A reduction of body
weight was seen both in rats and mice at the two highest
dose levels. Preliminary histopathological diagnosis
indicated demyelination and axonal degeneration of the
sciatic nerve in male and female mice only.
Freeman et al. (1988) tested the neurotoxicity of TCP
(substitution pattern not known) to F-344 rats in a short-
term study. After 13 weeks of dosing with TCP in the feed,
hindlimb grip strength decreased in male rats at 300 and
600 mg/kg, but not in female rats. Serum cholinesterase
was reduced at 300 and 600 mg/kg in both males and
females. All effects observed with 600 mg/kg were appar-
ently reversible during the recovery period.
In a 13-week short-term feeding study, Irwin et al.
(1987) exposed F-344 rats to TCP (0, 75, 150, 300, or 600
mg/kg; substitution pattern not known), while B6C3F1 mice
receive 0, 60, 125, or 250 mg/kg. After 13 weeks of
dosing, forelimb grip strength was unaffected by TCP in
both mice and rats. Hindlimb grip strength decreased in
male rats (at 300 and 600 mg/kg) but not in female rats.
In mice, decrements in hindlimb grip strength were
observed in males (250 mg/kg) and females (125 and 250
mg/kg). Serum cholinesterase levels showed a dose-depen-
dent reduction in both rats and mice. TCP had no effect on
food consumption in either species. All groups exhibited
normal body weight values.
Factors such as age, sex, and strain figure promi-
nently in the expression of OPIDN. The young of most
species are non-susceptible to TOCP-induced delayed neuro-
pathy (Johnson & Barnes, 1970), which could be due to poor
absorption of TOCP. However, recent experiments (Olson &
Bursian, 1988) have suggested that factors (e.g., route of
administration) other than absorption are more critical to
this lack of susceptibility.
Strain differences in the susceptibility of rats to
TOCP-delayed neuropathy have been reported. Although OPIDN
can be readily produced in Long-Evans and Sprague-Dawley
rats after acute oral doses of > 840 mg/kg (Veronesi &
Abou-Donia, 1982; Veronesi, 1984), repeated doses of TOCP
(10-100 mg/kg) failed to produce neuropathy in Fischer-344
rats (Somkuti et al., 1988). Variations in TOCP inhibition
of brain AChE and NTE have also been reported in these
three strains (Carrington & Abou-Donia, 1988).
8.7.4 Neurophysiology
Robertson et al. (1987) investigated electrophysio-
logical changes in the adult hen following single oral
doses of 30 or 750 mg TOCP/kg. At the higher dose, the
birds demonstrated clinical signs of toxicity 12 days
after dosing that included gait abnormalities, which
became progressively more severe and in some cases led to
complete ataxia. Lymphocyte NTE was inhibited by more
than 70%. The lower dose produced no clinical signs of
toxicity and only 54% lymphocyte NTE inhibition. Both
treated groups displayed significant action potential
disruption in both the tibial and sciatic nerves that
resulted in decreased refractoriness in the tibial nerve,
increased refractoriness in the sciatic nerve, and
elevated strength duration threshold for both nerves.
Electrophysiological changes have been investigated in
cats following single dermal doses ranging from 250 to
2000 mg TOCP/kg and 90-day dermal administration of 1 to
100 mg/kg (Abou-Donia et al., 1986). In contrast to the
hen, the clinical signs of TOCP neurotoxicity appear in
the cat 21-26 days before the electrophysiological effects
on the gastrocnemius muscle. Recovery of the cat from
delayed neurotoxicity symptoms was more marked than that
of the hen. No effects on peripheral nerve transmission
or on neuromuscular junction functioning were seen in the
cat.
9. EFFECTS ON HUMANS
Summary
There have been many reported cases of human poisoning,
mostly from accidental or irresponsible contamination of food-
stuffs. Occupational poisoning, usually resulting from dermal
exposure, has also been reported. The ortho isomer of TCP is
the responsible toxic agent.
Though short-term symptoms of ingestion might involve
vomiting, abdominal pain, and diarrhoea, characteristically
delayed, longer-term symptoms are neurological, frequently
leading to paralysis and pyramidal signs (spasticity, etc.).
There is considerable variation in the sensitivity of indi-
viduals to TOCP; severe symptoms were reported with a TOCP dose
of 0.15 g in one individual, while others were unaffected by 1
to 2 g. There is also considerable variation in the rate of
recovery from poisoning, some patients recovering completely
and others still severely affected years later, after appar-
ently similar exposure.
First-aid treatment involves the induction of vomiting or
pumping of the stomach. The patient should be hospitalized as
soon as possible. Atropine or 2-PAM may be used as an effective
antidotal treatment against cholinergic symptoms. Long-term,
antispastic drugs may be useful, though physical rehabilitation
is the cardinal therapy.
9.1 Historical background
Of the tricresyl phosphate isomers, the ortho (TOCP)
is by far the most toxic and alone gives rise to the major
neurotoxicity in man. It is considered that the toxicity
of the commercial products depends on the concentration of
the ortho isomer, but the mixed o-cresyl esters in these
products are also toxic and contribute to the neurotoxic
action.
It is well known that TOCP produces delayed effects on
the central and peripheral nervous systems. TOCP poisoning
has occurred throughout the world (Inoue et al., 1988);
the major outbreaks are indicated in Table 14.
Table 14. Major outbreaks of TOCP poisoning
___________________________________________________________________________
Year Place Number Vehicles of TOCP Reference
of cases
___________________________________________________________________________
1898 France 6 phospho-creosote Lorot (1899)
1900-1928 Europe 43 phospho-creosote Roger & Recordier
(15% TOCP) (1934)
1930-1931 USA 50 000 ginger extract Morgan (1982)
1931 Europe several Apiol pill Susser & Stein
hundred (1957)
1938 South Africa 68 cooking oil Sampson (1942)
1940 Switzerland 80 cooking oil Walthard (1945)
1941-1945 Germany more than cooking oil Mertens (1948)
200
1945 England 17 cooking oil Hotston (1946)
1955 South Africa 11 water or solvent Susser & Stein
(1957)
1957 Morocco about cooking oil Smith & Spalding
10 000 (1959)
1960 India 58 solid food Vora et al. (1962)
1962 India more than flour Chaudhuri (1965)
400
1967 Fiji 56 flour Sorokin (1969)
1980 Romania 12 alcohol Vasilescu & Florescu
(1980)
1981 Sri Lanka more than cooking oil Senanayake &
20 (0.56%) Jeyaratnam (1981)
___________________________________________________________________________
In 1899, Lorot initially reported six cases of poly-
neuropathy out of 41 cases of pulmonary tuberculosis
treated with phospho-creosote. Later it was shown that the
phospho-creosote contained 15% TOCP. In the next 30 years,
43 additional cases caused by the drug were reported in
various parts of continental Europe (Roger & Recordier,
1934).
In the spring of 1930, in mid-western and south-
western USA, an outbreak of paralysis characterized by
bilateral foot- and wrist-drop appeared suddenly (Burley,
1930; Merritt & Moor, 1930). Ultimately 50 000 people were
poisoned by a popular substitute for alcohol called
"Ginger Jake" (Morgan, 1982). Smith et al. (1930) proved
that the adulterated beverage contained about 2% TOCP and
that this caused the paralysis.
In 1931, several hundred women in Europe (especially
in Germany, The Netherlands, Yugoslavia, and France) were
poisoned by the TOCP contained in Apiol pills and taken as
an abortifacient (Roger & Recordier, 1934; Susser & Stein,
1957). The TOCP was presumably included as an additional
stimulus to abortion (Ter Braak & Carrillo, 1932).
An outbreak involving 11 people occurred in Durban,
South Africa, in 1955 (Susser & Stein, 1957). From the
epidemiological survey it was suggested that water, as
well as solvents, may have been a vehicle for the TOCP.
In 1959, about 10 000 Moroccan people were intoxicated
by TOCP: jet engine oil had been illegally mixed into
their cooking oil (Smith & Spalding, 1959; Svennilson,
1960). Accidental poisoning by TOCP contamination of solid
food occurred in 1960 in Bombay, India, where 58 victims
were recorded (Vora et al., 1962).
During the period April-June, 1962, more than 400
cases of paralysis occurred in the Malda district in
India. The cause of this disease proved to be the consump-
tion of flour contaminated with TOCP (Chaudhuri, 1965).
In 1967, similar poisoning was recorded in Fiji, where
56 people showed neuropathy (Sorokin, 1969). The cause was
stated to be contamination of dry sharps flour by TOCP
through the sacking material.
Vasilescu & Florescu (1980) in Romania reported 12
patients with toxic neuropathy following accidental inges-
tion of alcohol contaminated by TOCP.
An outbreak of acute polyneuropathy in over 20 young
females occurred in Sri Lanka during 1977-1978 (Senanayake
& Jeyaratnam, 1981). The cause of the neuropathy was
traced to TCP found as a contaminant in a special cooking
oil (gingili oil). Contamination probably occurred during
transport of the oil in containers previously used for
storing mineral oils.
9.2 Occupational exposure
Gartner & Elsaesser (1943) reported the case of a
worker who developed pyramidal signs after exposure to
TOCP for two years in a German chemical plant. In this
case, percutaneous absorption was considered to be the
main route of exposure.
In 1944, three cases of toxic polyneuropathy among
workers who had worked for six to eight months in a plant
manufacturing TCP in England were reported (Hunter et al.,
1944). Skin penetration and inhalation were thought to be
the main causes of the occupational poisoning.
Parnitzke (1946) reported a case of TOCP poisoning
after 3 years of exposure in a German plant and stated
that TOCP had been absorbed through the skin and presum-
ably the gastrointestinal tract.
Since 1958, a high prevalence of polyneuropathy among
shoe factory workers has been reported in Italy. The cause
has been attributed to TCP (Cavalleri & Cosi, 1978).
Although this is possible, this cause-effect relationship
has not so far been based on unquestionable evidence. This
polyneuropathy might have various aetiological factors
(including n-hexane) or be produced by a combination of
them (Leveque, 1983).
9.3 Clinical features
Goldstein et al. (1988) reported a case of severe
intoxication in a 4-year-old child following ingestion of
a lubricant containing TCP (substitution pattern not
known). The clinical findings were acute gastrointestinal
symptoms, delayed cholinergic crisis, and neurological
toxicity.
The severity of signs and symptoms after poisoning
with TOCP seems not always to be proportional to the
dosage (Staehelin, 1941). In a Swiss Army outbreak of
poisoning among more than 80 young men, toxic symptoms
appeared in once case after eating food containing only
0.15 g TOCP. Severe neurological disturbance developed in
three men from the intake of 0.5 to 0.7 g, whereas in two
other cases the intake of 1.5-2 g did not lead to any
symptoms. This leads to the conclusion that individual
susceptibility varies greatly (Staehelin, 1941).
In general, the signs and symptoms of TOCP poisoning
are distinctive, whereas the symptomatology varies
somewhat according to whether a single relatively large
dose or small cumulative doses are taken. In the former
case, the initial symptoms are gastrointestinal, ranging
from slight to severe nausea and vomiting, sometimes
accompanied by abdominal pain and diarrhoea. Among these
symptoms, vomiting is most frequently observed (Staehelin,
1941). These symptoms are usually transient, lasting from
a few hours to a few days (Walthard, 1945; Susser & Stein,
1957).
In cases of chronic low level exposure, the above
symptoms may not be present and the major symptoms are
neurological (Parnitzke, 1946). The clinical features of
acute exposure to TOCP were described by Staehelin (1941).
A latent period of 3-28 days is observed after acute
exposure, and clear "delayed neurotoxicity" then gradu-
ally appears. The initial neurological symptoms are sharp,
cramp-like pains in the calves, and some numbness and
tingling in the feet and sometimes the hands. Within a few
hours or a day or two at most, these pains are followed by
increasing weakness of the lower limbs, and soon the
patient becomes unsteady and then unable to maintain bal-
ance. The cramp-like pains may cease with the onset of
weakness, or persist for some days. One or two weeks after
the onset in the lower limbs and while paralysis may still
be progressing, the weakness spreads to the hands. While
some patients show complete wrist drop and total loss of
power in the hands, sometimes with weakness up to the
elbows, the predominant neurological abnormalities are
observed in the lower limbs. Bilateral foot drop with com-
plete loss of power from the ankle down is a common
finding. Depending on the severity of the affection, the
patient may have weakness in the knees, less at the hips,
and, only in the most severe cases, weakness of the trunk.
About three weeks or more after the onset of paralysis, a
most striking and rapid wasting may be observed. While the
small muscles of the feet, calves, the anterior tibials,
and the thighs do not escape this wasting, in so far as
they are involved in the disease, the change is most
obvious in the small muscles.
In the initial stage, the ankle jerks are absent and
knee reflexes may be normal or occasionally depressed.
Plantar reflexes are unobtainable. Mild cases do not show
any upper motor neuron signs. On the other hand, in the
more severe cases, even at the early stage, knee jerks may
be exaggerated, presaging the development of upper motor
neuron involvement. In general, upper motor neuron signs,
e.g., pyramidal signs, gradually become evident at about
the third week or later. Knee jerks become exaggerated and
so also may the biceps, triceps, and supinator jerks
(Cavanagh, 1964). A finger flexor reflex appears for the
first time or increases (Susser & Stein, 1957). As the
pyramidal tract lesion becomes evident, involuntary flexor
withdrawal of the whole limbs follows gentle plantar
stimulation. Babinski responses are observed much later.
Muscle tonus of the limbs gradually increases. In severe
cases, the signs of upper motor involvement are delayed,
probably masked by the gross flaccid muscle weakness.
Several authors state emphatically that sensory dis-
turbances do not occur. Sampson (1942) reported sensory
disturbances although these were admittedly unobtrusive,
in contrast to motor dysfunction. Reports of muscle and
peripheral nerve tenderness are fairly frequent. If the
sensory disturbances are observed, there is hypoesthesia
with loss of pin-prick and temperature sense; the ability
to detect vibration is sometimes affected distally. The
sensory disturbances vary in extent from merely the soles
of the feet to the whole of the limbs.
Usually cranial nerves are not involved. In general,
mental signs are rare, but transient euphoria and con-
fusion have been observed in the early stage (Schwab,
1948).
9.4 Prognosis
Following exposure, muscle weakness progresses over
several weeks, sometimes even months. Sensory changes
often begin to regress during the early weeks, the rap-
idity depending on the severity of the case, and then
muscle strength gradually returns in patients who are only
mildly affected. Improvement begins with the return of
sensation, then muscle strength in the hands, and eventu-
ally strength in the lower limbs. In cases of pyramidal
signs, recovery is generally poor. Zeligs (1938),
reporting eight years after the 1930 mid-western and
south-western USA outbreak, surveyed the records of 316
patients. He was able to follow up 60 patients, all of
whom were disabled and still in institutions. Aring (1942)
examined more than 100 patients in the 10 years following
this outbreak and they appeared still to be affected.
Morgan & Penovich (1978) followed up 11 survivors in the
47 years after the same outbreak; the principal findings
were the spasticity and abnormal reflexes of an upper
motor neuron syndrome.
Of the 80 patients in the 1940 Swiss army accident,
14 were quite well after five years, 15 were totally inca-
pacitated, and 38 showed spasticity (Walthard 1945).
In the 1938 Durban outbreak, all the patients showed
some symptoms of the disease 18 years later (Susser &
Stein, 1957). The mildest case had slight weakness at the
ankle, while the most severely affected had foot drop,
muscle atrophy, and pyramidal signs (spasticity, ankle
clonus, and positive Babinski sign).
The residual signs and symptoms are mainly confined to
the lower limbs. They consist of weakness and muscle atro-
phy of varying degrees in the feet and the small muscles
of the hand. Disability is principally related to the
pyramidal signs with resultant spasticity of the lower
extremities.
9.5 Neurophysiological investigations
There have been very few electrophysiological studies
on human TOCP poisoning. Svennilson (1960) reported an
electromyographic study on 65 patients in the 1959 Morocco
poisoning. These cases showed varying degrees of dener-
vation and polyphasic abnormal potentials in the paralysed
muscles. The clinically healthy proximal groups of muscles
also showed marked polyphasic action potentials but not
denervation.
Vasilescu & Florescu (1980) reported detailed studies
on 12 patients from the 1980 accident in Romania. They
observed > 50% decrease in the muscle evoked potential
amplitude, fibrillation potentials in the same muscles at
rest, and decreased motor nerve conduction velocity.
In neurophysiological studies by Senanayake (1981) in
Sri Lanka, the main findings were widespread neurotoxic
patterns and prolongation of terminal latencies with rela-
tively mild slowing of motor nerve conduction velocities.
These studies confirmed the evidence of axonal degener-
ation.
9.6 Pathological investigations
Numerous pathological studies have been made on biopsy
or autopsy samples since the Jamaica ginger accidents in
1930. In 1930, Goldfain described some changes observed
in the peripheral nerves and spinal cord, quoting Jeter's
autopsy report. Histopathological investigations by
Goodale & Humphreys (1931) indicated degeneration of
myelin sheaths and axis cylinders in the radial, sciatic,
and tibial nerves in all cases examined. Vonderahe (1931)
found marked degenerative changes in the anterior horn
cells, characterized by swelling, central chromatolysis
(disappearance of the Nissl substance), excentric nuclei,
and shrinking of the cells. The pathological studies also
revealed degeneration in the radial and anterior tibial
nerves, and degenerative changes in the anterior roots.
There were no pathological signs of inflammation. Accord-
ing to Smith & Lillie (1931), the paralysis due to Jamaica
ginger was essentially a degeneration of the myelin
sheaths of the peripheral nerves, with a variable amount
of relatively moderate central degenerative changes
affecting the anterior horn cells throughout the spinal
cord, but more often in the lumber and cervical regions.
In the 1938 Durban (South Africa) epidemic, Sampson (1942)
also reported that degeneration of the anterior horn cells
occurred in some instances and that peripheral nerves
showed axonal degeneration. Aring (1942) described degen-
eration in the posterior and lateral columns in later
investigations of survivors of the outbreak, thereby con-
firming the origin of some of the spinal symptoms. It is
noteworthy that the latter changes were evident in the
lumbar region, while the dorsal column changes, in which
only the fasciculus gracilis was involved, concerned the
cervical region.
Muscle biopsy studies of patients from the 1959
Moroccan poisoning showed a moderate degree of muscle
atrophy and a slight increase of the muscle fibre nuclei.
Spherical axonal swelling and terminal knobs were noted as
a sign of peripheral nerve degeneration in muscles
(Svennilson, 1960). Similar changes were also noted in
the poisoning cases reported in Malda, India, in 1962
(Chaudhuri et al., 1962). These effects on muscle suggest
denervation.
9.7 Laboratory investigations
Little information has been obtained from laboratory
examinations of exposed humans. There is no significant
change in the urine or blood (Sampson, 1942; Senanayake &
Jeyaratnam, 1981), but the cerebrospinal fluid may show an
increase in protein concentration, with or without pleo-
cytosis (Sampson, 1942; Mertens, 1948; Susser & Stein,
1957).
Vora et al. (1962) measured blood cholinesterase
levels in patients admitted to hospitals during the 1960
Bombay poisoning and demonstrated that plasma cholinester-
ase was increased one month after exposure. The erythro-
cyte cholinesterase level was considerably diminished
(50%) quite early after the onset of symptoms. Both plasma
and erythrocyte cholinesterase activities returned to
normal within about 3 months.
In a factory manufacturing TAP, about half of the
workers examined showed significant decreases in pseudo-
cholinesterase (cholinesterase other than AChE), and many
of them had minor signs and symptoms (Tabershaw et al.,
1957).
Morgan & Hughes (1981) also investigated cholinester-
ase activity in workers in a plant manufacturing TAP plas-
ticizers. They found that plasma cholinesterase estimation
in workers exposed to TAP compounds cannot be used as a
sensitive barometer of organic phosphate absorption; thus
routine regular estimations serve no useful purpose. Its
value is chiefly as a screening method at the pre-employ-
ment medical examination to exclude personnel at risk, as
a baseline in the event of massive exposure, and as a
means of diagnosis in cases of CNS disease that simulate
TAP poisoning.
9.8 Treatment
In the event of skin contact with TCP, contaminated
clothing should be rapidly removed and affected body areas
copiously irrigated with water. The ingestion of food or
drink contaminated with TOCP should be treated by inducing
vomiting, unless the patient is unconscious. Atropine or
pralidoxine (2-PAM) chloride may be required to counteract
cholinergic effects. No specific antidote is available.
Medical therapy should begin as soon as possible, even
though the results of medical therapeutic measures have
been disappointing. B-complex vitamins and corticosteroids
may protect nervous tissue against further involvement
(Geoffroy et al., 1960). The cardinal therapy is physical
rehabilitation. Administration of anti-spastic drugs may
be required.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human poisoning involving the accidental ingestion of
tri- o-cresyl phosphate (TOCP) or occupational exposure
of workers has frequently been reported. The likely route
of occupational exposure is cutaneous absorption. The
neurotoxic symptoms involve initial inhibition of cholin-
esterases and subsequent delayed neuropathy characterized
by severe paralysis.
Because of considerable variation among individuals in
sensitivity to TOCP, it is not possible to establish a
safe level of exposure. Symptoms have been reported from
the ingestion of 0.15 g of an isomeric mixture with a low
proportion of TOCP; the minimum effective dose of the
ortho isomer is, therefore, much lower than this. Animal
studies show considerable variation among species in the
response to TOCP, and humans appear to be particularly
sensitive.
Irritant and allergic dermatitis have been reported.
Both the pure ortho isomer and isomeric mixtures con-
taining TOCP are, therefore, considered major hazards to
human health.
There is no safe level for ingestion. Exposure to the
compound through dermal contact or inhalation should be
minimized.
10.1.1 Exposure levels
Exposure of the general population to tricresyl phos-
phate (TCP) through various environmental media, including
drinking-water, can be regarded as minimal. TCP has been
detected at relatively higher concentrations in urban air
than in air collected at production sites, although the
levels are usually low. TCP was not detected in human
adipose tissue samples in a survey conducted in the USA.
There have been many cases of accidental human poisoning
through the ingestion of medicines, food, flour, cooking
oil, and beverages contaminated with hydraulic fluid or
lubricant oil containing TCP produced from "cresylic
acid". The toxic symptoms can be observed after ingestion
of only 0.15 g of tri- o-cresyl phosphate, a component of
TCP from cresylic acid. The contamination has usually
happened when empty barrels or drums, previously used for
hydraulic fluid or lubricating oil storage, have been
reused.
10.1.2 Toxic effects
Accidental human exposure to a single large dose re-
sults in gastrointestinal disturbance varying from slight
to severe nausea and vomiting, accompanied by abdominal
pain and diarrhoea. In the case of exposure to small cumu-
lative doses, "delayed neurotoxicity" gradually proceeds
after a latent period of 3-28 days. In most cases, the
muscle weakness changes rapidly to a striking paralysis of
the lower limbs, with or without an involvement of the
hands. In severe cases, pyramidal signs gradually become
evident. Some neurophysiological studies indicate wide-
spread neurotoxic patterns and prolongation of terminal
latencies with relatively small decreases of motor nerve
conduction velocities. This confirms the evidence of
axonal degeneration, which is the main feature observed in
pathological investigations.
The neurotoxic metabolite of TCP has been identified
as saligenin cyclic o-tolyl phosphate, which is derived
from o-hydroxymethyl metabolites. Thus, it seems that at
least one o-tolyl group among the three phenolic moieties
of TCP is necessary to induce neurotoxic effects. TCP pro-
duced from synthetic cresol, which contains less than 0.1%
of o-cresol, is therefore not neurotoxic.
Subchronic animal studies on TCP derived from syn-
thetic cresol indicate that the target organs are liver
and kidney, but this was not confirmed in the case of
human intoxication. No adequate data are available on
mutagenicity and carcinogenicity. TCP is not toxic to
chick embryos.
10.2 Evaluation of effects on the environment
The measurement of environmental concentrations of TCP
in water has shown only low levels of contamination. This
reflects the low water solubility and ready degradability
of the compound. Since the acute toxicity of TCP to
aquatic organisms is also low, it is unlikely that it
poses a threat to such organisms.
As a consequence of the physico-chemical properties of
TCP, there is a high potential for bioaccumulation. How-
ever, this does not occur in practice, owing to low con-
centrations of TOCP in the environment and living organ-
isms and to its rapid degradation.
TCP bound to sediment accumulates in the environment,
and levels measured in river, estuarine, and marine sedi-
ments have been high. Since there is no information either
on the bioavailability of these residues to burrowing or
bottom-living organisms or on their hazards, the possi-
bility of effects on such species cannot be discounted.
TCP spillage leads to hazard for the local environ-
ment.
10.2.1 Exposure levels
TCP is found in air, surface water, soil, sediment,
and aquatic organisms near heavily industrialized areas,
although concentrations are usually low. Owing to the high
biodegradation rate of TCP in aqueous environment, it is
not considered to affect aquatic organisms adversely. One
report showed an extremely high concentration of total
triaryl phosphate (26.55 g/kg) in a soil sample obtained
from a production plant yard. This suggests the need for
land waste disposal.
10.2.2 Toxic effects
Freshwater algae are relatively sensitive to TCP, the
50% growth inhibitory concentration ranging from 1.5 to
5.0 mg/litre. Among fish species, the rainbow trout is
adversely affected by TCP concentrations below 1 mg/litre
(0.3-0.9 mg/litre), with sign of chronic poisoning, but
the tidewater silverside is more resistant (LC50 is 8700
mg/litre). TCP does not inhibit cholinesterase activity in
fish or frogs, but it has a synergistic effect on organo-
phosphorus insecticide activity.
11. RECOMMENDATIONS
When tri-substituted cresols are used in the synthesis
and manufacture of other compounds, the purified meta and
para isomers should be used in order to avoid the acciden-
tal synthesis of ortho-substituted products.
REFERENCES
ABOU-DONIA, M.B., VARGA, P.A., GRAHAM, D.G., & KINNES, C.G. (1980)
Delayed neurotoxicity of a single dermal administration of o-ethyl
o-4-nitro-phenyl phenylphosphonothioate and tri-o-cresyl phosphate
to cats. Toxicol. Lett., 1 (Special issue): 141.
ABOU-DONIA, M.B., TROFATTER, L.P., GRAHAM, D.G., & LAPADULA, D.M.
(1986) Electromyographic, neuropathologic and functional
correlates in the cat as the result of tri-o-cresyl phosphate
delayed neurotoxicity. Toxicol. appl. Pharmacol., 83(1): 126-141.
ADEMA, D.M.M., KUIPER, J., HANSTVEIT, A.O., & CANTON, H.H. (1983)
Consecutive system of tests for assessment of the effects of
chemical agents in the aquatic environment. In: Pesticide
chemistry-Human welfare and the environment. Proceedings of the
Fifth International Congress on Pesticide Chemistry,Kyoto, Japan,
29 August - 4 September, 1982, Oxford, New York, Pergamon Press,
Vol. 3, pp. 537-544.
ALDRIDGE, W.N. (1954) Tricresyl phosphates and cholinesterase.
Biochem. J., 56: 185-189.
ALDRIDGE, W.N. & BARNES, J.M. (1961) Neurotoxic and biochemical
properties of some triaryl phosphates. Biochem. Pharmacol., 6:
177-188.
ANONYMOUS (1986) Handbook of commodity chemicals, Tokyo, Kagaku-
Kogyo-Nipposha.
ARING, C.D. (1942) The systemic nervous affinity of triorthocresyl
phosphate (Jamaica ginger palsy). Brain, 65: 34-47.
ASSOCIATION OF THE PLASTICIZER INDUSTRY OF JAPAN (1976) Safety of
the plasticizer TCP, tricresyl phosphate, Tokyo, Association of
the Plasticizer Industry of Japan.
BALDRIDGE, H.D., JENDEN, D.J., KNIGHT, C.E., PREZIOSI, T.J., &
TUREMAN, J.R. (1959) Toxicology of a triaryl phosphate Oil. III.
Human exposure in operational use aboard ship. Am Med. Assoc. Arch.
ind. Hyg., 20: 258-261.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., &
WELCH, V.A. (1961) The reactions of organic phosphates. Part V.
The hydrolysis of triphenyl and trimethyl phosphates. J. Chem. Soc.
(B), 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER,
B., VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic
phosphates. Part VI. The hydrolysis of aryl phosphates. J. Chem.
Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphoryl-enzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BECK, B.E., WOOD, C.D., & WHENHAM, G.R. (1977) Triaryl phosphate
poisoning in cattle. Vet. Pathol., 14: 128-137.
BELL, J.D. & RUSVINE, J.R. (1967) Synergism of organophosphates in
Musca domestica and Chrysomya putoria. Entomol. exp. appl., 10:
263-269.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY,
S.K. (1974) Detection and estimation of tricresyl phosphate in
mustard oil. Forensic Sci., 3: 263-270.
BISCHOFF, A. (1967) The ultrastructure of tri-ortho-cresyl
phosphate poisoning. I. Studies on myelin and axonal alterations in
the sciatic nerve. Acta neuropathol., 9: 158-174.
BISCHOFF, A. (1970) The ultrastructure of tri-ortho-cresyl
phosphate poisoning in the chicken. II. Studies on spinal cord
alterations. Acta neuropathol., 15: 142-155.
BISCHOFF, A. (1977) Tri-ortho-cresyl phosphate neurotoxicity. In:
Roisin, L., Shiraki, H., & Grcevic, N., ed. Neurotoxicology, New
York, Raven Press, pp. 431-441.
BLEIBERG, M.J. & JOHNSON, H. (1965) Effects of certain
metabolically active drugs and oximes on tri- o-cresyl phosphate
toxicity. Toxicol. appl. Pharmacol., 7: 227-235.
BLOOM, P.J. (1973) Application des chromatographies sur couche
mince et gaz-liquide à l'analyse qualitative et quantitative des
esters des acides phosphorique et phosphoreux. J. Chromatogr.,
75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and
effects of triaryl and tri-alkyl/aryl phosphate esters. Residue
Rev., 94: 49-99.
BONDY, H.F., FIELD, E.J., WORDEN, A.N., & HUGHES, J.P.W. A study of
the acute toxicity of the tri-aryl phosphates used as plasticizers.
(1960) Br. J. ind. Med., 17: 190-200.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., &
KARASEK, F.W. (1981) Trace impurities in solvents commonly used for
gas chromatographic analysis of environmental samples. J.
Chromatogr., 206: 279-288.
BROWN H.R. & SHARMA, R.P. (1975) Inhibition of neural membrane
adenosine-triphosphatases by organophosphates. Toxicol. appl.
Pharmacol., 33: 140.
BURLEY, B.T. (1930) The 1930 type of polyneuritis. New Engl. J.
Med., 202: 1139-1142.
CARLTON, B.D., HASARAN, A.H., MEZZA, L.E., & SMITH, M.K. (1987)
Examination of the reproductive effects of tricresyl phosphate
administered to Long-Evans rats. Toxicology, 46: 321-328.
CARRINGTON, C.D. & ABOU-DONIA, M.B. (1988) Variation between three
strains of rat: inhibition of neurotoxic esterase and
acetylcholinesterase by tri-o-cresyl phosphate. J. Toxicol.
environ. Health, 25(3): 259-268.
CASIDA, J.E. (1961) Specificity of substituted phenyl phosphorus
compounds for esterase inhibition in mice. Biochem. Pharmacol., 5:
332-342.
CASIDA, J.E., ETO, M., & BARON R.L. (1961) Biological activity of a
tri-o-cresyl phosphate metabolite. Nature (Lond.), 191: 1396-1397.
CASTERLINE, J.L., Jr., KU, Y., & BARNETT, N.M. (1985) Uptake of
tri-p-cresyl phosphate in soybean plants. Bull. environ. Contam.
Toxicol., 35: 209-212.
CAVALLERI, A. & COSI, V. (1978) Polyneuritis incidence in shoe
factory workers: Case report and etiological considerations. Arch.
environ. Health, 33: 192-197.
CAVANAGH, J.B.(1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system. An experimental study in hens. J.
Neurol. Neurosurg. Psychiatry, 17: 163-172.
CAVANAGH, J.B. (1964) The significance of the "dying back" process
in experimental and human neurological disease. Int. Rev. exp.
Pathol., 3: 219-267.
CHAPIN, R.E., GEORGE, J.D., & LAMB, J.C., IV (1988) Reproductive
toxicity of tricresyl phosphate in a continuous breeding protocol
in Swiss (CD-1) mice. Fundam. appl. Toxicol., 10: 344-354.
CHAUDHURI, R.N. (1965) Paralytic disease caused by consumption of
flour contaminated with tricresyl phosphate. Trans. R. Soc. Trop.
Med. Hyg., 59: 98-102.
CHAUDHURI, R.N., SEN GUPTA, P.C., CHAKRAVARTI, R.N., MUKHERJEE,
A.M., GHOSH, S.M., CHATTERJEA, J.B., SARKAR, J.K., BASU, S.P.,
ADHYA, R.N., MITRA, N.K., SAHA, T.K., & MITRA, P. (1962) Recent
outbreak of a paralytic disease in Malda, West Bengal. Bull.
Calcutta Sch. Trop. Med., 10: 141-152.
CHEMICAL AND GEOLOGICAL LABORATORIES LTD (1971) Calgary, Alberta,
Chemical and Geological Laboratories (Laboratory report No. E71-
5360).
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
COHEN, S.D. & MURPHY, S.D. (1970) Comparative potentiation of
malathion by triorthotolyl phosphate in four classes of
vertebrates. Toxicol. appl. Pharmacol., 16: 701-708.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues
in foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAGLEY, S. & PATEL, M.D. (1957) Oxidation of p-cresol and related
compounds by a Pseudomonas. Biochem. J., 66: 227-233.
DAVISON, A.N. (1953) The cholinesterases of the central nervous
system after the administration of organo-phosphorus compounds.
Brit. J. Pharmacol., 8: 212-216.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977)
The acute toxicity of 47 industrial chemicals to fresh and salt
water fishes. J. hazard. Mater., 1: 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic
mass spectrometric analysis of some commercial aryl phosphate oils.
J. Assoc. Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by
thin-layer chromatography.] Gig. i Sanit. , 10: 62-65 (in Russian).
DUKE, A.J. (1978) The significance of isomerism and complexity of
composition on the performance of triaryl phosphate plasticisers in
PVC. Chimia, 32: 457-463.
EAJ (1977) [ Environmental monitoring of chemicals ], Tokyo,
Environment Agency Japan, pp. 212-214 (Environmental Survey Report
Series, No. 3) (in Japanese).
EAJ (1979) [ Chemicals in the environment], Tokyo, Environment
Agency Japan, pp. 61, 78, 92, 93 (Office of Health Studies Report
Series, No. 5) (in Japanese).
EAJ (1981) [ Environmental monitoring of chemicals: Environmental
Survey Report of 1978, 1979 F.Y. ], Tokyo, Environment Agency
Japan, pp. 116-117 (in Japanese).
ELDEFRAWI, A.T., MANSOUR, N.A., BRATTSTEN, L.B., AHRENS, V.D., &
LISK, D.J. (1977) Further toxicologic studies with commercial and
candidate flame retardant chemicals. Part II. Bull. environ.
Contam. Toxicol., 17: 720-726.
ETO, M., CASHIDA, J.E., & ETO, T. (1962) Hydroxylation and
cyclization reactions involved in the metabolism of tri- o-cresyl
phosphate. Biochem. Pharmacol., 11: 337-352.
ETO, M., OSHIMA, Y., & CASHIDA, J.E. (1967) Plasma albumin as a
catalyst in cyclization of diaryl-o-(a-hydroxy)tolyl phosphates.
Biochem. Pharmacol., 16: 295-308.
ETO, M., HASHIMOTO, Y., OZAKI, K., KASAI, T., & SASAKI Y. (1975)
Fungitoxicity and insecticide synergism of monothioquinol phosphate
esters and related compounds. Botyu Kagaku, 40: 110-117.
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl
phosphate esters. A new photochemical coupling reaction. J. Am.
Chem. Soc., 94: 4780-4782.
FRANCHINI, I., CAVATORTA, A., D'ERRICO, M., DE SANTIS, M., ROMITA,
G., GATTI, R., JUVARRA, G., & PALLA, G. (1978) Studies on the
etiology of the experimental neuropathy from industrial adhesives
(glues). Experientia (Basel), 34: 250-252.
FREEMAN, G.B., IRWIN, R., TREJO, R., HEJTMANCIK, M., RYAN, M., &
PETERS, A.C. (1988) Reversibility and tolerance to tricresyl
phosphate-induced neurotoxic effects in F344 rats. Toxicologist,
8: 76.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GARTNER, W. & ELSAESSER, K.H. (1943) [Commercial ortho-
tricresylphosphate poisoning.] Arch. Gewerbepathol. Gewerbehyg.,
12: 1-9 (in German).
GEOFFROY, H., SLOMIC, A., BENEBADJI, M., & PASCAL, P. (1960)
Myèlopoly-néurites tri-crésyl phosphatées. Toxi-épidémie marocaine
de septembre-octobre 1959. World Neurol., 1: 294-315.
GOLDFAIN, E. (1930) Jamaica ginger multiple neuritis. J. Oklahoma
State Med. Assoc., 23: 191-193.
GOLDSTEIN, D.A., MCGUIGAN, M.A., & RIPLEY, B.D. (1988) Acute
tricresyl phosphate intoxication in childhood. Hum. Toxicol., 7:
179-182.
GOODALE, R.H. & HUMPHREYS, M.B. (1931) Jamaica ginger paralysis.
Autopsy observations. J. Am. Med. Assoc., 96: 14-16.
GROSS, E. & GROSSE, A. (1932) [A contribution to the toxicology of
ortho-tricresylphosphates.] Arch. exp. Pathol. Pharmakol., 168:
473-514 (in German).
HAGGERTY, G.J., HEJTMANCIK, M., DESKIN, R., RYAN, M.J., PETERS,
A.C., & IRWIN, R. (1986) Effects of subchronic exposure to
tricresyl phosphate in F344 rats and B6C3F1 mice: behavioural and
morphological correlates. Toxicologist, 6: 218.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Shuishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, N., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HINE, C.H., DUNLAP, M.K., RICE, E.G., COURSEY, M.M., GROSS, R.M., &
ANDERSON, H.H. (1956) The neurotoxicity and anticholinesterase
properties of some substituted phenyl phosphates. J. Pharmacol.
exp. Ther., 116: 227-236.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD,
G.T. (1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, 3rd revised ed., New York,
Wiley-Interscience, Vol. 2A, pp. 2362-2363.
HJORTH, N. (1962) Contact dermatitis from cellulose acetate film.
Cross-sensitization between tricresylphosphate (TCP) and
triphenylphosphate (TPP). Contact dermatitis, 2: 86-100.
HODGE, H.C. & STERNER, J.H. (1943) The skin absorption of
triorthocresyl phosphate as shown by radioactive phosphorus. J.
Pharmacol. exp. Ther., 79: 225-234.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOTSTON, R.D. (1946) Outbreak of polyneuritis due to orthotricresyl
phosphate poisoning. Lancet, 1: 207.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. Bull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(di-
chloropropyl)phosphate, a mutagenic flame retardant: Frequent
occurrence in human seminal plasma. Science, 211: 951-952.
HUNTER, D., PERRY, K.M.A., & EVANS, R.B. (1944) Toxic polyneuritis
arising during the manufacture of tricresyl phosphate. Br. J. ind.
Med., 1: 227-231.
INDEN, T. & TACHIBANA, S. (1975) [Damage of crops by gases from the
plastic materials under covering conditions.] Bull. Fac. Agric. Mie
Univ., 1975: 1-10 (in Japanese).
INOUE, N., FUJISHIRO, K., MORI, K., & MATSUOKA, M. (1988)
Triorthocresyl phosphate poisoning: A review of human cases.
Sangyo-Ika-Daigaku-Zasshi, 10(4): 433-442.
IRWIN, R., FREEMAN, G.B., TREJO, R., HEJTMANCIK, M., & PETERS, A.
(1987) Effects of chronic exposure to tricresyl phosphate in F344
rats and B6C3F1 mice: behavioural and biochemical correlates. Soc.
Neurosci. Abstr., 13: 90.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water
Res., 19: 119-125.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982) Toxicometric
parameters of industrial toxic chemicals under single exposure,
Moscow, Centre of International Projects, GKNT, p. 114.
JETER, H. (1930) Autopsy report of a case of so-called "Jake
Paralysis". J. Am. Med. Assoc., 95: 112-113.
JOHANSSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J.,
RADUE, R.W., & GRAHAM, P.R. (1977) Evaluation of delayed
neurotoxicity and dose-response relationships of phosphate esters
in the adult hen. Toxicol. appl. Pharmacol., 41: 291-304.
JOHNSON, M.K. (1969) Delayed neurotoxic action of some
organophosphorus compounds. Br. med. Bull., 25: 231-235.
JOHNSON, M.K. (1974) The primary biochemical lesion leading to the
delayed neurotoxicity of some organophosphorus esters. J.
Neurochem., 23: 785-789.
JOHNSON, M.K. (1975a) Organophosphorus esters causing neurotoxic
effects. Mechanism of action and structure/activity studies. Arch.
Toxicol., 34: 259-288.
JOHNSON, M.K. (1975b) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. & BARNES, J.M. (1970) The sensitivity of chicks to
the delayed neurotoxic effects of some organophosphorous compounds.
Biochem. Pharmacol., 19: 3045.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water
pollution caused by organophosphorus compounds.] Kankyo Gijyutsu,
7: 668-675 (in Japanese).
KENMOTSU, K., SAITO, N., OGINO, N., & MATSUNAGA, K. (1979) [An
environ-mental survey of chemicals. XI. Analytical methods of
organic phosphoric acid triesters.] Okayama-ken Kankyo Hoken Senta
Nempo, 3: 175-191 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment
and sea water.] J. Food Hyg. Soc. Jpn., 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental
pollutants. V. Environmental fate of organic phosphoric acid
triesters.] Okayama-ken Kankyo Hoken Senta Nempo, 4: 103-110 (in
Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.]
Okayama-ken Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination
of organic phosphate esters in environment samples.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982a) [An environmental survey of chemicals. XIX. Determination
of organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken
Senta Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982b) [Studies on the biological toxicity of several pollutants
in environments. VII. GC/MS Spectrometric determination of
organophosphoric acid triesters in sediment.] Okayama-ken Kankyo
Hoken Senta Nempo, 6: 142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemicals. XXII. GC/MS
spectrometric determination of organophosphoric acid triesters in
sediment (2).] Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in
Japanese).
KIMMERLE, G. & LOESER, E. (1974) Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environ. Qual. Saf., 3: 174-178.
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on
organic and heavy metal contaminants in the Lakes Erie, Michigan,
Huron and Superior basins. Great Lakes Water Qual. Bd.
KRISHNAMURTHY, M.N., RAJALAKSHMI, S., & PRAKASH KAPUR, O. (1985)
Detection of tricresyl phosphates and determination of tri-o-cresyl
phosphate in edible oils. J. Assoc. Off. Anal. Chem., 68(6):
1074-1076.
KU, Y. & ALVAREZ, G.H. (1982) Biodegradation of (14C) tri- p-cresyl
phosphate in a laboratory activated-sludge system. Appl. environ.
Microbiol., 43: 619-622.
KUREBAYASHI, H., TANAKA, A., & YAMAHA, T. (1985) Metabolism and
dispo-sition of the flame retardant plasticizer, tri-p-cresyl
phosphate, in the rat. Toxicol. appl. Pharmacol., 77: 395-404.
LAPP, T.W. (1976) Study on chemical substances from information
concerning the manufacture, distribution, use, disposal,
alternatives, and magnitude of exposure to the environment and
man: Task 1 - The manufacture and use of selected aryl and alkyl
phosphate esters, Springfield, Virginia, National Technical
Information Service, 129 pp (EPA contract No. 68-01-2687) (NTIS PB-
251819).
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic
phosphate triesters in human adipose tissue. J. Assoc. Off. Anal.
Chem., 66: 691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from
drinking water at the ng/L level, using macroreticular resin. J.
Assoc. Off. Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas
chromatographic determination of trialkyl/aryl phosphates in
drinking water, following isolation using macroreticular resin. J.
Assoc. Off. Anal. Chem., 64: 991-998.
LEFAUX, R. (1968) Practical toxicology of plastics, London, Iliffe
Books Ltd, p. 580.
LEFAUX, R. (1972) Industrial toxicology. Phosphoric esters. In:
Practical toxicology of plastics, London, Iliffe Books Ltd, pp.
121-127.
LEVEQUE, J. (1983) Tricresyl phosphates. In: Encyclopaedia of
occupational health safety, 3rd ed., Geneva, International Labour
Office, Vol. 2, pp. 2216-2218.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY,
D. (1975) Chronic toxicity of a synthetic tri-aryl phosphate oil to
fish. Environ. Physiol. Biochem., 5: 361-369.
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62: 47-51.
LOROT, C. (1899) Les combinations de la créosote dans le traitement
de la tuberculose pulmonaire, Paris (Thèse de Paris No. 25).
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Perspect., 10: 269-284.
MAJORS, R.E. & JOHNSON, E.L. ( 1978) High-performance exclusion
chromatography of low-molecular-weight additives. J. Chromatogr.,
167: 17-30.
MARHOLD, J.V. (1972) Collection of results of toxicological
assessments of compounds and formulations tested at the Department
of Toxicology, Research Institute for Organic Syntheses, Prague,
Institute for Management Training in the Chemical Industry.
MAUSNER, M., BENEDICT, J.H., BOOMAN, K.A., BRENNER, T.E., CONWAY,
R.A., DUTHIE, J.R., GARRISON, L.J., HENDRIX, C.D., & SHEWMAKER,
J.E. (1969) The status of biodegradability testing of nonionic
surfactants. J. Am. Oil Chem. Soc., 46: 432-440.
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Springfield, Virginia, National Technical
Information Service.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE,
P.M., & SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An
aquatic environmental assessment of Pydrauls 50E and 115E. In:
Branson R. & Dickson, K.L., ed. Aquatic Toxicology and Hazard
Assessment: Fourth Conference, Philadelphia, American Society for
Testing and Materials, pp. 103-123 (ASTM STP 737).
MELE, J.M. & JENSH, R.P. (1977) Teratogenic effects of orally
administered tri-o-cresyl phosphate on Wistar albino rats.
Teratology, 15: 32A.
MERRITT, H.H. & MOOR, M. (1930) Peripheral neuritis associated with
ginger extract ingestion. New Engl. J. Med., 203: 4-12.
MERTENS, H.G. (1948) [Clinical aspects of tri-orthocrésyl phosphate
poisoning (including a report on several cases of poisoning in an
Igelit factory).] Arch. Psychiatr. Nervenkr., 179: 458-482
(in German).
MRI (1979) Assessment of the need for limitation on triaryl and
trialkyl/aryl phosphates. Draft final report, Kansas City, Midwest
Research Institute (EPA Contract 68-01-4313).
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising
Supplement 52(10A), p 697, New York, McGraw-Hill Inc.
MORAZAIN, R. & ROSENBERG, P. (1970) Lipid changes in tri-o-cresyl-
phosphate-induced neuropathy. Toxicol. appl. Pharmacol., 16: 461-474.
MORGAN, A.A. & HUGHES, J.P.W. (1981) An investigation into the
value of cholinesterase estimations of workers in a plant
manufacturing tri-aryl phosphate plasticizers. J. Soc. Occup. Med.,
31: 69-75.
MORGAN, J.P. (1982) The Jamaica Ginger Paralysis. J. Am. Med.
Assoc., 248: 1864-1867.
MORGAN, J.P. & PENOVICH, P. (1978) Jamaica Ginger Paralysis. Forty-
seven-year follow-up. Arch. Neurol., 35: 530-532.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988a) Evaluation of rodent sperm, vaginal
cytology, and reproductive organ weight data from National
Toxicology Program 13-week study. Fundam. appl. Toxicol., 11:
343-358.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988b) Association of sperm, vaginal
cytology, and reproductive organ weight data with results of
continuous breeding reproductive studies in Swiss CD-1 mice.
Fundam. appl. Toxicol., 11: 359-371.
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G. & GRIFT, N.P. (1983) Extraction and cleanup procedures
for determination of diarylphosphates in fish, sediment, and water
samples. J. Assoc. Anal. Off. Chem., 66: 684-690.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980) Determination of
several triarylphosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and
cleanup of fish, sediment, and water for determination of triaryl
phosphates by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Technol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983) Environmental
dynamics of phosphate esters. III. Comparison of the
bioconcentration of four triaryl phosphates by fish. Chemosphere,
12: 155-166.
MURRAY, D.A.J. (1975) Analysis of tri-aryl phosphate esters and the
determination of IMOL S-140 in fish tissue and water samples by gas
chromatography. J. Fish Res. Board Can., 32: 457-460.
MYERS, D.K., REBEL, J.B.J., VEEGER, C., KEMP, A., & SIMONS, E.G.L.
(1955) Metabolism of triaryl phosphates in rodents. Nature (Lond.),
176: 259-260.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition
coefficient to measure the bioconcentration potential of organic
chemicals in fish. Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate
ester mixtures to invertebrates and fish. Bull. environ. Contam.
Toxicol., 19: 250-256.
NICHOLSON, S.S. (1974) Bovine posterior paralysis due to
organophosphate poisoning. J. Am. Vet. Med. Assoc., 165: 280-281.
NOBILE, E.R., PAGE, S.W., & LOMBARDO, P. (1980) Characterization of
four commercial flame retardant aryl phosphates. Bull. environ.
Contam. Toxicol., 25: 755-761.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performance liquid
chromato-graphic analysis on radial compression column of the
neurotoxic tri-o-cresyl phosphate and metabolites. Anal. Biochem.,
135: 296-303.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1984) Disposition of (14C)tri-o-
cresyl phosphate and its metabolites in various tissues of the male
cat following a single dermal application. Drug Metab. Disp., 12
(6): 705-711.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986a) Studies on the metabolism
of the neurotoxic tri-o-cresyl phosphate synthesis and
identification by infrared, proton nuclear magnetic resonance and
mass spectrometry of five of its metabolites. Toxicology, 38: 1-13.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986b) Studies on the metabolism
of the neurotoxic tri- o-cresyl phosphate, distribution, excretion,
and metabolism in male cats after a single, dermal application.
Toxicology, 38: 15-33.
OECD (1981) OECD guidelines for testing of chemicals. Section 2:
Effects on biotic systems, Paris, Organization for Economic
Cooperation and Development, Publications Office.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresylphosphate. Sci. total
Environ., 43: 233-241.
OISHI, H., OISHI, S., & HIRAGA, K. (1982) Toxicity of several
phosphoric acid esters in rats. Toxicol. Lett., 13: 29-34.
OLSON, B.A. & BURSIAN, S.J. (1988) Effect of route of
administration on the development of organophosphate-induced
delayed neurotoxicity in 4-week-old chicks. J. Toxicol. environ.
Health, 23(4): 499-505.
PACIOREK, K.J.L., KRATZER, R.H., KAUFMAN, J., NAKAHARA, J.H.,
CHRISTOS, T., & HARTSTEIN, A.M. (1978) Thermal oxidative
degradation studies of phosphate esters. Am. Ind. Hyg. Assoc. J.,
39: 633-639.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78(1): 78-87.
PARNITZKE, K.H. (1946) [On the recent accumulation of ortho-
tricresylphosphate poisoning (sources and clinical course).] Dtsch.
Gesundheitswes., 1: 666-670 (in German).
PEGUM, J.S. (1966) Contact dermatitis from plastics containing tri-
aryl phosphates. Br. J. Dermatol., 78: 626-631.
PENMAN, D.R & OSBORNE, G.O. (1976) Trialkyl phosphates and related
compounds as antifertility agents of the twospotted spider mite. J.
econ. Entomol., 69(2): 266-268.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975)
Utilization of triaryl phosphates by a mixed bacterial population.
Can. J. Microbiol., 21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with
synergistic properties. J. econ. Entomol., 59(1): 11-15.
PRENTICE, D.E. & MAJEED, S.K. (1983) A subchronic study (90 days)
using multiple dose levels of tri-ortho-cresyl phosphate (TOCP):
some neuropathological observations in the domestic hen.
Neurotoxicology, 4(2): 277-282.
PRINEAS, J.S. (1969) The pathogenesis of dying-back
polyneuropathies. Part I. An ultrastructural study of experimental
tri-ortho-cresyl phosphate intoxication in the cat. J. Neuropathol.
exp. Neurol., 28: 571-597.
QUINSTAD, G.B., STAIGER, L.E., & SCHOOLEY, D.A. (1975)
Environmental degradation of the insect growth regulator
methoprene. V. Metabolism by house-flies and mosquitoes. Pestic.
Biochem. Physiol., 5: 233-241.
RAMSEY, J.D. & LEE, T.D. (1980) Gas-liquid chromatographic
retention indices of 296 non-drug substances on SE-30 or OV-1
likely to be encountered in toxicological analyses. J. Chromatogr.,
184: 185-206.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin
layer chromatography. Evaluation of methods and application on
phosphate esters, polychlorinated paraffins and some PCB-
substitutes. Chemosphere, 9: 683-691.
ROBERTS, N.L., FAIRLEY, C., & PHILLIPS, C. (1983) Measurements and
evaluation of clinical signs following use of the hen as the animal
model for screening acute delayed and subchronic neurotoxicity
studies. Neurotoxicology, 4(2): 263-270.
ROBERTSON, D.G., SCHWAB, B.W., SILLS, R.D., RICHARDSON, R.J., &
ANDERSON, R.J. (1987) Electrophysiologic changes following
treatment with organophosphorus-induced delayed neuropathy-
producing agents in the adult hen. Toxicol. appl. Pharmacol., 87:
420-429.
ROGER, H. & RECORDIER, M. (1934) Les polynéurites
phosphocréosotiques (phosphate de créosote, ginger paralysis,
apiol). Ann. Med., 35: 44-63.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P.,
& TUCKER, E.S. (1979) Environmental fate of selected phosphate
esters. Environ. Sci. Technol., 13: 840-844.
SAITO, C., KATO, T., TANIGUCHI, H., FUJITA, T., WADA, H., & MORI,
Y. (1974) [Subacute toxicity of tricresylphosphate (TCP) in rats.]
Pharmacometrics, 8: 107-118 (in Japanese).
SAMPSON, B.F. (1942) The strange Durban epidemic of 1937. S. Afr.
med. J., 16: 1-9.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by
Killifish (Oryzeas latipes). Bull. environ. Contam. Toxicol., 28:
752-759.
SCHWAB, H. (1948) [Psychic disturbance by triorthocresylphosphate
poisoning.] Dtsch. Med. Wochenschr., 73: 124-125 (in German).
SENANAYAKE, N. (1981) Tri-cresyl phosphate neuropathy in Sri Lanka:
a clinical and neurophysiological study with a three year follow
up. J. Neurol. Neurosurg. Psychiatry, 44: 775-780.
SENANAYAKE, N. & JEYARATNAM, J. (1981) Toxic polyneuropathy due to
gingili oil contaminated with tri-cresyl phosphate affecting
adolescent girls in Sri Lanka. Lancet, 10 January: 88-89.
SHARMA, R.P. & WATANABE, P.G. (1974) Time related disposition of
tri-o-tolyl phosphate (TOTP) and metabolites in chicken. Pharmacol.
Res. Commun., 6(5): 475-484.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the
Delaware River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, H.V. & SPALDING, J.M.K. (1959) Outbreak of paralysis in
Morocco due to ortho-cresyl phosphate poisoning. Lancet, 2:
1019-1021.
SMITH, M.I. & LILLIE, R.D. (1931) The histopathology of
triorthocresyl phosphate poisoning. Arch. Neurol Psychiatry, 26:
976-992.
SMITH, M.I., ELVOVE, E., & FRAZIER, W.H. (1930) The pharmacological
action of certain phenol esters, with special reference to the
etiolgy of so-called ginger paralysis. Public Health Rep., 45:
2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies
on the pharmacology of certain phenol esters with special reference
to the relation of chemical constitution and physiologic action.
Natl. Inst. Health Bull., 160: 1-53.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987a) Reproductive tract lesions resulting from
subchronic administration (63 days) of tri-o-cresyl phosphate in
male rats. Toxicol. appl. Pharmacol., 89: 49-63.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987b) Time course of the tri-o-cresyl phosphate-
induced testicular lesion in F-344 rats: enzymatic, hormonal, and
sperm parameter studies. Toxicol. appl. Pharmacol., 89: 64-72.
SOMKUTI, S.G., LAPADULA, D.M., CHAPIN, R.E., & ABOU-DONIA, M.B.
(1987c) Testicular toxicity following oral administration of tri-o-
cresyl phosphate (TOCP) in roosters. Toxicol. Lett., 37: 279-290.
SOMKUTI, S.G., TILSON, H.A., BROWN, H.R., CAMPBELL, G.A.,
LAPADULA, D.M., & ABOU-DONIA, M.B. (1988) Lack of delayed
neurotoxic effect after tri-ortho-cresyl phosphate treatment in
male Fischer-344 rats: bio-chemical, neurobehavioural, and
neuropathological studies. Fundam. appl. Toxicol., 10: 199-205.
SOROKIN, M. (1969) Orthocresyl phosphate neuropathy: Report of an
outbreak in Fiji. Med. J. Aust., 1: 506-508.
SPOERRI, P.E. & GLEES, P. (1979) Ultrastructural reactions of
spinal ganglia to tri-ortho-cresyl phosphate: Effects of
neurotoxicity. Cell Tissue Res., 199: 409-414.
SPOERRI, P.E. & GLEES, P. (1980) Ultrastructural changes in neurons
and neuroglia of the avian telencephalon (Hyperstriatum
Accessorium) following tri-ortho-cresyl phosphate intoxication.
Cell Tissue Res., 206: 203-210.
STAEHELIN, R. (1941) [On the triorthocresylphosphate poisonings.]
Schweiz. Med. Wochenschr., 71: 1-5 (in German).
STUMPF, A.M., TANAKA, D.JR., AULERIOH, A.J., & BURSIAN, S.J. (1989)
Delayed neurotoxic effects of tri-o-tolyl phosphate in the European
ferret. J. Toxicol. environ. Health, 26: 61-73.
STURM, R.N. (1973) Biodegradability of nonionic surfactants:
Screening test for predicting rate and ultimate biodegradation. J.
Am. Oil Chem. Soc., 50: 159-167.
SUGDEN, E.A., GREENHALGH, R., & PETTIT, J.R. (1980)
Characterization of delayed neurotoxic triaryl phosphates by
analysis of trifluoroacetylated phenolic moieties. Environ. Sci.
Technol., 14(12): 1498-1501.
SUSSER, M. & STEIN, Z. (1957) An outbreak of tri-ortho-cresyl
phosphate (TOCP) poisoning in Durban. Br. J. ind. Med., 14:
111-120.
SVENNILSON, E. (1960) Studies of triorthocresyl phosphate
neuropathy, Morocco 1960. Acta psychiatr. Scand., 150(Suppl):
334-336.
TABERSHAW, I.R., KLEINFELD, M., & FEINER, B. (1957) Manufacture of
tricresyl phosphate and other alkyl phenyl phosphates: An
industrial hygiene study. Am. Med. Assoc. Arch. ind. Health, 15:
537-540.
TAYLOR, J.D. & BUTTAR, H.S. (1967) Evidence for the presence of 2-
(o-cresyl)-4 H-1:3:2-benzodioxaphosphoran-2-one in cat intestine
following tri-o-cresyl phosphate administration. Toxicol. appl.
Pharmacol., 11: 529-537.
TER BRAAK, J.W.G. & CARRILLO, R. (1932) [Polyneuritis after usage
of an abortifacient (tri-ortho-cresyl-phosphate-poisoning).] Dtsch.
Z. Nervenheilkd., 125: 86-116 (in German).
THOMPSON, J.E. & DUTHIE, J.R. (1968) The biodegradability and
treatability of NTA. J. Water Pollut. Control Fed., 40: 306-319.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorus compounds. Anal. Chem., 53: 1466-1469.
TOCCO, D.R., RANDALL, J.L., YORK, R.G., & SMITH, M.K. (1987)
Evaluation of the teratogenic effects of tri-ortho-cresyl phosphate
in the Long-Evans hooded rat. Fundam. appl. Toxicol., 8(3): 291-297.
US NIOSH (1977) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Vol. 3, P & CAM, pp. S/209-S/210 (DHEW (NIOSH) Publication
No. 7-157C).
US NIOSH (1979) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS PB-82-216268.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division,
General Motors Corp., 1000 Lexington Avenue, Rochester, New York,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (PB82-104530, NTIS).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorous exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations and
Field Studies (Report No. IWA-89.10).
US SOAP AND DETERGENT ASSOCIATION (1965) A procedure and standards
for the determination of the biodegradability of alkyl benzene
sulfonate and linear alkylate sulfonate (Report of the Subcommittee
on Biodegradation Test Methods). J. Am. Oil Chem. Soc., 42: 986-993.
VASILESCU, C. & FLORESCU, A. (1980) Clinical and
electrophysiological study of neuropathy after organophosphorus
compound poisoning. Arch. Toxicol., 43: 305-315.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A
simple colorimetric method for estimation of tricresyl phosphate in
edible oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J.
Fish Res. Board Can., 36: 1040-1048.
VERONESI, B. (1984) A rodent-model of organophosphorus-induced
delayed neuropathy: distribution of central (spinal cord) and
peripheral nerve damage. Neuropathol. appl. Neurobiol., 10(6):
357-368.
VERONESI, B. & ABOU-DONIA, M.B. (1982) Central and peripheral
neuropathology induced in rats by tri-o-cresyl phosphate (TOCP).
Vet. hum. Toxicol., 24: 3 (abstract).
VERONESI, B., NEWLAND, D., & INMAN, A. (1984) The effect of
metabolic interference on rodent-sensitivity to tri-ortho-cresyl
phosphate. Toxicologist, 4: 55.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J.J., & SVEC, H.J.
(1978) Organic emissions from combustion of combination coal/refuse
to produce electricity. Chemosphere, 7: 893-902.
VONDERAHE, A.R. (1931) Pathologic changes in paralysis caused by
drinking Jamaica Ginger. Arch. Neurol. Psychiatry, 25: 29-43.
VORA, D.D., DASTUR, D.K., BRAGANCA, B.M., PARIHAR, L.M., IYER,
C.G.S., FONDEKAR, R.B., & PRABHAKARAN, K. (1962) Toxic polyneuritis
in Bombay due to ortho-cresyl-phosphate poisoning. J. Neurol.
Neurosurg. Psychiatry, 25: 234-242.
WAGEMANN, R. (1975) Some environmental and toxicological aspects of
a tri-aryl phosphate synthetic oil. Verh. Int. Ver. Limnol., 19:
2178-2184.
WAGEMANN, R., GRAHAM, R., & LOCKHART, W.L. (1974) Studies on
chemical degradation and fish toxicity of a synthetic tri-aryl
phosphate lubrication oil, IMOL S-140. Ottawa, Environment Canada,
Fisheries and Marine Service, (Technical Report, No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution caused by
organophosphoric fire-proofing plasticizers.] Annu. Rep. Tokyo
Metrop. Res. Inst. Environ. Prot., 11: 110-113 (in Japanese).
WALTHARD, K.M. (1945) Aperçu des résultats obtenus lors des
derniers examens des malades intoxiqués en 1940 par le phosphate
triorthocrésilique. Schweiz. Arch. Neurol. Psychiatry, 58: 189-194.
WIGHTMAN, R.H. & MALAIYANDI, M. (1983) Physical properties of some
synthetic trialkyl/aryl phosphate commonly found in environmental
samples. Environ. Sci. Technol., 17: 256-261.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of
tri(haloalkyl)-, trialkyl-, and triarylphosphates in Canadian
drinking water. Bull. environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON,
R. (1982) Determination of mutagenic potential and organic
contaminants of Great Lakes drinking water. Chemosphere, 11: 263-276.
WILSON, R.D., ROWE, L.D., LOVERING, S.L., & WITZEL, D.A. (1982)
Acute toxicity of tri-ortho-cresyl phosphate in sheep and swine.
Am. J. vet. Res., 43(11): 1954-1957.
WINDHOLZ, M., ed. (1983) The Merck index, 10th ed., Rahway, New
Jersey, Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate
esters: Application of linear free energy relationships to estimate
hydrolysis rate constants for use in environmental fate assessment.
Chemosphere, 9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-165.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides
in the atmosphere above the Dogo plain and Ozu basin.] J. Chem.
Soc. Jpn, 1980(4): 645-653 (in Japanese).
ZELIGS, M.A. (1938) Upper motor neuron sequelae in "Jake" paralysis
: A clinical follow up study. J. nerv. ment. Dis., 87: 464-470.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop. Can. Tech. Rep. fish aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de tricrésyle (TCP) est un liquide
visqueux, ininflammable, inexplosible et incolore. Son
coefficient de partage entre l'octanol et l'eau (log de
Pow) est égal à 5,1. Il s'hydrolyse facilement en
milieu alcalin pour donner du phosphate de dicrésyle, mais
il est stable en milieu neutre ou acide à température
normale.
Du point de vue analytique, la méthode de choix est la
chromatographie en phase gazeuse avec détection par un
dispositif sensible à l'azote/phosphore ou par photométrie
de flamme. La limite de détection dans les échantillons
aqueux est d'environ 1 mg/litre. Le TCP s'extrait facile-
ment des solutions aqueuses au moyen de divers solvants
organiques. Pour la purification, on emploie habituelle-
ment une colonne chromatographique de Florisil, mais il
est difficile de séparer le TCP des lipides par cette
méthode. D'autres méthodes de purification ont été re-
commandées à cette fin (chromatographie en phase gazeuse,
chromatographie sur charbon activé ou Sep-pack C-18). Les
réactifs analytiques sont souvent contaminés par des
traces de TCP en raison de la très large utilisation faite
de ce produit. Aussi faut-il prendre certaines précautions
si l'on veut que l'analyse des traces de TCP soit fiable.
2. Sources d'exposition humaine et environnementale
Le TCP est généralement produit par réaction des cré-
sols sur l'oxychlorure de phosphore. Il y a deux sources
de production industrielle de crésols: l'"acide crésy-
lique", résidu des fours à coke et du raffinage du
pétrole, et les "crésols de synthèse" préparés par oxy-
dation et dégradation du cymène. Le TCP est donc un
mélange de divers phosphates de triaryle.
Le TCP est utilisé comme plastifiant des matières
plastiques vinyliques, comme retardateur d'inflammation,
comme additif pour les lubrifiants à très haute pression
et comme liquide ininflammable dans les systèmes hydrau-
liques.
3. Transport, distribution et transformation dans l'environnement
La libération de TCP dans l'environnement est peu
importante au cours de la production et se produit essen-
tiellement lors de l'utilisation finale du produit. On
estime qu'en 1977, 32 800 tonnes en ont été libérées au
total dans l'atmosphère aux Etats-Unis.
En raison de sa faible solubilité dans l'eau et de sa
forte adsorption aux particules, le TCP s'adsorbe rapide-
ment aux sédiments des rivières et des lacs et aux parti-
cules du sol. Il est rapidement biodégradé en milieu
aquatique, sa décomposition étant pratiquement complète
dans les cours d'eau en l'espace de cinq jours. L'isomère
ortho se décompose légèrement plus vite que les isomères
méta et para. Le TCP est facilement biodégradé dans les
boues d'égouts, avec une demi-vie de 7,5 heures, la dégra-
dation atteignant 99% en l'espace de 24 heures. La dégra-
dation abiotique est plus lente, puisque dans ce cas la
demi-vie est de 96 jours.
Des facteurs de bioconcentration de 165-2768 ont été
mesurés en laboratoire sur plusieurs espèces de poissons à
l'aide de TCP radio-marqué. La radioactivité a rapidement
disparu après cessation de l'exposition, la demi-vie de
dépuration allant de 25,8 à 90 heures.
4. Niveaux dans l'environnement et exposition humaine
On a relevé dans l'air des concentration de TCP allant
jusqu'à 70 ng/m3 au Japon, les concentrations maximales
dans une unité de production des Etats-Unis d'Amérique
n'étant que de 2 ng/m3. Dans un atelier de remplissage
de fûts d'huile lubrifiante, aux Etats-Unis d'Amérique, on
a constaté que l'air ne contenait que 0,8 mg/m3 de TCP,
la concentration ne dépassant pas 0,15 mg/m3 en phos-
phates totaux, dans une unité de moulage de zinc d'une
usine d'automobiles. Les concentrations mesurées dans
l'eau de boisson au Canada se sont révélées faibles (0,4 à
4,3 ng/litre) et le TCP n'a pas pu être décelé dans l'eau
des puits. Dans les rivières et les lacs, les concen-
trations sont souvent beaucoup plus élevées. Toutefois on
peut attribuer cet état de choses à la présence de sédi-
ments en suspension auxquels le TCP est fortement adsorbé.
Les concentrations sédimentaires sont plus élevées
puisqu'elles peuvent atteindre 1300 ng/g dans le sédiments
de cours d'eau et 2160 ng/g par les sédiments marins.
Des concentrations élevées ont été mesurées dans le
sol et la végétation aux alentours d'unités de production.
On a fait état de résidus dans des poissons et des
fruits de mer allant jusqu'à 49 ng/g, mais la majorité des
échantillons n'en contenaient pas de quantités décelables.
5. Effets sur les êtres vivants dans leur milieu naturel
On a révélé une réduction de 50% de la productivité
des cultures d'algues vertes d'eau douce, en présence de
concentrations de phosphate de tri- o-crésyle (TOCP)
allant de 1,5 à 4,2 ng/litre, selon les espèces, les iso-
mères méta et para étant moins toxiques. On ne dispose que
de données limitées sur la toxicité aiguë du TCP vis-à-vis
des invertébrés aquatiques: la CL50 à 48 heures pour la
daphnie est de 5,6 ng/litre; la CL50 à 24 heures pour les
nématodes est de 400 ng/litre; la dose sans effet obser-
vable à 2 semaines pour la daphnie (mortalité, croissance,
reproduction) est de 0,1 mg/litre. Pour trois espèces de
poissons, les valeurs de la CL50 à 96 heures se situaient
entre 4,0 et 8700 mg/litre. Chez des truites arc-en-ciel
on a constaté une mortalité d'environ 30% après exposition
de quatre mois à 0,9 ng/litre de IMOL S-140 (phosphate de
tri- o-crésyle à 2%) et des effets plus légers sur une
période de 14 jours.
Les niveaux d'exposition au cours de ces expériences
étaient beaucoup plus élevés que les concentrations sus-
ceptibles d'être rencontrées dans le milieu naturel et
dans la plupart des cas, les valeurs étaient très
supérieures à la solubilité des composés.
6. Cinétique et métabolisme
L'absorption, la distribution, le métabolisme et
l'élimination des organophosphorés jouent un rôle déter-
minant dans les effets neuropathologiques retardés de ces
composés.
Chez l'homme, l'absorption percutanée du TOCP semble
être au moins dix fois plus rapide que chez le chien. On
observe également une importante absorption par cette voie
chez le chat. L'absorption par voie orale a été observée
chez le lapin. On ne possède aucune donnée de première
main sur l'absorption par la voie respiratoire.
Chez le chat, on a constaté qu'après absorption, le
TOCP se répartissait largement dans l'ensemble de l'orga-
nisme, la concentration la plus élevée se situant dans le
nerf sciatique, qui constitue un tissu cible. De fortes
concentrations de TOCP ou de ses métabolites se rencon-
traient également au niveau du foie, des reins et de la
vésicule bilaire.
La métabolisation du TOCP s'effectue selon trois
voies. La première consiste dans l'hydroxylation d'un ou
plusieurs groupes méthyles et la seconde comporte la
désarylation des groupements orthocrésyles. Dans la
troisième, il y a encore oxydation du groupement hydroxy-
méthyle en aldéhyde et en acide carboxylique. L'hydroxy-
lation constitue une étape déterminante, car le TOCP
hydroxyméthylé est cyclisé pour former du phosphate
cyclique d' o-tolyle et de saligénol, un métabolite
neurotoxique relativement instable.
Le TOCP et ses métabolites sont éliminés dans les
urines et les matières fécales ainsi que, en petites
quantités, dans l'air expiré.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuves
in vitro
Des trois isomères du TCP, le TOCP est de loin celui
qui présente la toxicité aiguë la plus forte et qui se
révèle également le plus toxique en cas d'exposition
brève. Il est le seul à déterminer une neurotoxicité
retardée.
Les différents paramètres toxicologiques varient
beaucoup selon l'espèce (qu'il s'agisse par exemple de la
mortalité aiguë ou de la neurotoxicité retardée). Le
poulet est l'une des espèces les plus sensibles.
On a pu obtenir chez des espèces d'animaux de labora-
toire très variées une neuropathie retardée induite par un
organophosphoré (NRIOP) tant à la suite d'une exposition
unique qu'à la suite d'expositions répétées. Il s'agit
d'une neuropathie qui se traduit par des altérations
dégénératives au niveau de la partie distale de l'axone et
qui s'étend peu à peu à l'ensemble du neurone.
Elle se manifeste cliniquement par une paralysie des
pattes postérieures après une période de latence caracté-
ristique de deux à trois semaines suivant l'exposition.
Une dose orale unique de 50 à 500 mg de TOCP/kg a produit
une neuropathie retardée chez des poulets, des doses de
840 mg/kg ou davantage étant nécessaires pour produire une
dégénérescence de la moëlle épinière chez des rats Long-
Evans. C'est l'un des métabolites du TOCP, le phosphate
cyclique d' o-tolyle et de saligénol, qui constitue
l'agent neurotoxique actif. La sensibilité des espèces
varie en sens inverse de la vitesse de métabolisation
ultérieure.
On pense que la lésion biochimique qui conduit à la
neuropathie consiste dans l'inhibition de l'"estérase
neurotoxique". Un taux d'inhibition de plus de 65% peu de
temps après une exposition au TOCP fait présager l'appari-
tion ultérieure d'une neuropathie. La variabilité dans la
réponse neurotoxique dépend également d'autres facteurs
(par exemple la voie d'exposition, l'âge, le sexe, la
souche). Les données disponibles ne permettent pas de
définir clairement une dose sans effet observé pour cette
neuropathie.
Les études de reproduction effectuées sur des rats et
des souris qui recevaient des doses répétées de TOCP par
voie orale, ont révélé la présence de lésions histopatho-
logiques au niveau des testicules et des ovaires, de modi-
fications dans la morphologie des spermatozoïdes, d'une
moindre fécondité chez les deux sexes ainsi que d'une
diminution de la taille des portées et de la viabilité des
ratons et des souriceaux. Les données disponibles n'ont
pas permis de déterminer la dose sans effet pour ce type
d'anomalies imputables au TOCP. Une étude de tératogéni-
cité effectuée sur des rats, avec des doses orales
toxiques pour la mère, n'a pas donné de résultats
positifs.
On n'a guère de données sur la mutagénicité et aucune
sur la cancérogénicité de cette substance.
8. Effets sur l'homme
L'ingestion accidentelle constitue la cause principale
d'intoxication. Depuis la fin du dix-neuvième siècle, de
nombreux cas d'intoxication dus à la contamination de
boissons, de denrées alimentaires ou de produits pharma-
ceutiques ont été signalés. L'exposition professionnelle
tient essentiellement à une absorption percutanée ou à une
inhalation et certains cas d'intoxication de ce type ont
été signalés. L'ingestion de préparations contaminées par
du TOCP peut produire des symptômes digestifs (nausée,
vomissements et diarrhées), encore que dans certains cas,
c'est la polyneuropathie qui constitue le premier signe
d'intoxication. Les symptômes neurologiques sont carac-
térisés par une période de latence. Les premiers symptômes
consistent en douleurs et paresthésie aux extrémités des
membres inférieurs. Il y a une légère diminution de la
sensibilité cutanée et quelques fois réduction de la sen-
sibilité vibratoire. Dans la plupart des cas, la faiblesse
musculaire évolue rapidement vers une paralysie des
extrémités inférieures avec ou sans extension aux membres
supérieurs. Dans les cas graves, apparaissent des signes
d'atteinte pyramidale. Les cas mortels sont rares mais
les symptômes neurologiques peuvent être très lents à dis-
paraître et la guérison peut prendre des mois, voire des
années. L'examen histopathologique révèle une dégénéres-
cence des axones. Les examens classiques de laboratoire
ne révèlent pas d'anomalies si ce n'est parfois une
augmentation de la teneur en protéines du liquide céphalo-
rachidien. Les premiers soins consistent à réduire
l'exposition en faisant vomir immédiatement le malade,
dans la mesure où celui-ci est encore conscient. A plus
long terme, le traitement consiste essentiellement en une
réadaptation physique car on ne connaît pas d'antidote
spécifique. La réaction au phosphate de tricrésyle varie
considérablement d'un individu à l'autre de même que les
possibilités de guérison à la suite d'une intoxication.
On a signalé l'apparition de symptômes graves après inges-
tion de 0,15 g de TCP, alors que chez d'autres personnes,
l'ingestion de quantités atteignant 1 à 2 g n'a produit
aucun effet toxique. Certains malades guérissent complète-
ment alors que d'autres présentent des séquelles marquées
pendant de très longues périodes.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
On a souvent signalé des cas d'intoxication humaine
par suite d'une ingestion accidentelle de phosphate de
tri- o-crésyle ou par suite d'une exposition profession-
nelle. Les symptômes neurotoxiques correspondent tout
d'abord à l'inhibition de la cholinestérase puis à une
neuropathie retardée caractérisée par une paralysie
grave.
Etant donné les variations considérables de sensi-
bilité selon les individus, il n'est pas possible de
déterminer quel est le seuil de sécurité. Des symptômes
ont été signalés après ingestion de 0,15 g d'un mélange
d'isomères contenant une faible proportion de TOCP; la
dose minimale efficace d'isomères ortho est donc beaucoup
plus faible encore. L'expérimentation animale fait égale-
ment ressortir de très importantes variations selon les
espèces pour ce qui concerne la réaction au TOCP et
l'homme semble être particulièrement sensible.
Des cas de dermatite d'irritation et de dermatites
allergiques ont été rapportés.
On peut donc considérer que l'isomère ortho et les
mélanges d'isomères qui en contiennent constituent un
risque de première importance pour la santé humaine.
Aucune dose n'est sans danger si elle est ingérée. Il
convient de réduire au minimum l'exposition cutanée ou
respiratoire.
1.1 Niveaux d'exposition
On peut considérer comme minimale l'exposition de la
population générale au phosphate de tricrésyle par
l'intermédiaire des divers compartiments du milieu
ambiant, notamment l'eau de consommation. On a décelé du
phosphate de tricrésyle à des concentrations plus fortes
dans l'air urbain que dans l'air prélevé au niveau des
sites de production, encore que ces valeurs soient
généralement faibles. Lors d'une enquête effectuée aux
Etats-Unis d'Amérique on n'a pas décelé de TCP dans des
échantillons de tissu adipeux humain. Il y a eu de
nombreux cas d'intoxication humaine accidentelle dus à
l'ingestion de médicaments, de nourriture, de farine,
d'huile de cuisine et de boissons contaminés par des
fluides hydrauliques ou des lubrifiants à base de TCP
produits à partir d'acide crésylique. Des symptômes
toxiques peuvent s'observer après ingestion de doses ne
dépassant pas 0,15 g de TOCP, substance qui est présente
dans le TCP produit à partir de l'acide crésylique. La
contamination se produit généralement lors de la
réutilisation de fûts ou de tonneaux vides qui avaient
contenu un liquide hydraulique ou de l'huile lubrifiante.
1.2 Effets toxiques
L'absorption accidentelle d'une forte dose entraîne
chez l'homme des troubles digestifs, à savoir une nausée
d'intensité variable, pouvant aller jusqu'aux vomisse-
ments, accompagnée de douleurs abdominales et de diar-
rhées. En cas d'exposition à de faibles doses cumulées,
une "neuropathie retardée" s'installe progressivement
après une période de latence de 3 à 28 jours. Dans la
plupart des cas, la faiblesse musculaire fait rapidement
place à une paralysie des membres inférieurs qui peut
parfois s'étendre aux mains. Dans les cas graves, on voit
peu à peu appraître des signes d'atteinte pyramidale.
Certaines études neurophysiologiques révèlent l'existence
d'une neurotoxicité généralisée avec prolongation du temps
de latence terminale et réduction relativement faible de
la vitesse de conduction des nerfs moteurs. Ces consta-
tations confirment la dégénérescence axonale qui est la
principale caractéristique relevée lors des examens histo-
pathologiques.
Le métabolite neurotoxique du TCP a été identifié; il
s'agit du phosphate cyclique de o-tolyle et de saligénol
qui provient lui-même des métabolites o-hydroxyméthylés.
Il semble donc que la présence d'au moins un groupement
o-tolyle soit nécessaire parmi les trois restes phéno-
liques du TCP pour qu'apparaissent des effets neuro-
toxiques. Le TCP produit à partir du crésol de synthèse,
qui contient moins de 0,1% d'orthocrésol, n'est donc pas
neurotoxique.
Les résultats d'études de toxicité subchronique
effectuées sur des animaux d'expérience avec du TCP pré-
paré à partir de crésol de synthèse, montrent que les
organes cibles sont le foie et le rein; toutefois cette
observation n'a pas été confirmée chez l'homme. On ne
dispose pas de données suffisantes sur la mutagénicité et
la cancérogénicité du TCP. On sait néanmoins que le TCP
n'est pas toxique pour l'embryon de poulet.
2. Evaluation des effets sur l'environnement
Le dosage du TCP dans l'eau montre que la contami-
nation est faible. Cet état de choses tient à la faible
solubilité dans l'eau du TCP et à l'aisance avec laquelle
il se décompose. Etant donnée la faible toxicité aiguë du
TCP pour les organismes aquatiques, il est peu probable
qu'il constitue une menace pour ces organismes.
Du fait de ses propriétés physico-chimiques, le TCP a
une forte tendance à la bioaccumulation. Toutefois celle-
ci ne se produit pas dans la pratique en raison de la
faible concentration du TOCP dans l'environnement et les
êtres vivants et de la dégradation rapide de cette
substance.
Les TCP fixés aux sédiments s'accumulent dans
l'environnement et on en a relevé des concentrations
élevées dans les sédiments des cours d'eau et des estu-
aires ainsi que dans les sédiments marins. Du fait que
l'on ne possède aucune information sur la biodisponibilité
de ces résidus pour les organismes fouisseurs ou ben-
thiques, ni sur les dangers qu'ils pourraient représenter,
on ne peut écarter à priori la possibilité d'effets
nocifs.
Il faut également mentionner la possibilité de risques
localisés pour l'environnement dus à un déversement acci-
dentel de TCP.
2.1 Niveaux d'exposition
Les TCP sont présents dans l'air, dans les eaux super-
ficielles, dans le sol, les sédiments et les organismes
aquatiques, à proximité des zones très industrialisées,
encore que leurs concentrations y soient généralement
faibles. En raison de la vitesse élevée de biodégradation
de ces substances en milieu aqueux, il ne semble pas
qu'elles puissent avoir des effets nocifs sur la faune
aquatique. Il a été fait état d'une concentration extrême-
ment élevée en phosphates totaux de triaryle (25,55 g/kg)
dans un échantillon de sol provenant d'une plantation.
Cette observation montre qu'il est nécessaire de procéder
à l'enfouissement des déchets.
2.2 Effets toxiques
Les algues d'eau douce sont relativement sensibles aux
TCP, la concentration inhibant à 50% la croissance com-
prise entre 1,5 et 5,0 ml/litre. En ce qui concerne les
poissons, des concentrations de TCP inférieures à
1 mg/litre (0,3-0,9 mg/litre) provoquent des signes
d'intoxication chronique chez la truite arc-en-ciel, mais
Menidia notata est plus résistant (CL50 de 8700
mg/litre). Les TCP n'inhibent pas l'activité cholinesté-
rasique des poissons ou des grenouilles mais ils poten-
tialisent l'effet des insecticides organophosphorés.
RECOMMANDATIONS
Lorsqu'on utilise des crésols tri-substitués pour la
synthèse et la préparation d'autres composés, il est
préférable d'utiliser des isomères para et méta purifiés
afin d'éviter toute synthèse accidentelle de dérivés
orthosubstitués.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El fosfato de tricresilo (FTC) es un líquido ininflam-
able, no explosivo, incoloro y viscoso. Su coeficiente de
partición entre el octanol y el agua (log Pow) es de
5,1. Se hidroliza con facilidad en un medio alcalino dando
fosfato de dicresilo y cresol, pero es estable en medios
neutros y ácidos a temperaturas normales.
El método analítico de elección es la cromatografía de
gases con un detector sensible al nitrógeno-fósforo o un
detector fotométrico de llama. El límite de detección en
una muestra de agua es de 1 ng/litro aproximadamente. El
FTC se extrae con facilidad de las soluciones acuosas con
distintos disolventes orgánicos. Se utiliza habitualmente
para la extracción la cromatografía en columna de flori-
sil, pero es difícil separar el FTC de los lípidos con
este método. Se han recomendado para esa finalidad otros
métodos de extracción (GPC, cromatografía en carbón vege-
tal activado y Sep-pak C-18). Los reactivos analíticos
están contaminados a menudo con cantidades infinitesimales
de FTC debido a su amplio uso. Por consiguiente, la obten-
ción de datos fiables en el análisis de cantidades infini-
tesimales de FTC requiere un procedimiento cuidadoso.
2. Fuentes de exposición humana y ambiental
El FTC se produce habitualmente por reacción de
cresoles con oxicloruro de fósforo. Existen dos fuentes
industriales de cresoles: el "ácido cresílico", obtenido
como residuo de los hornos de carbón de coque y del refino
del petróleo; y los "cresoles sintéticos", preparados a
partir del cimeno por oxidación y degradación. Como resul-
tado, el FTC es una mezcla de varios fosfatos triaríli-
cos.
El FTC se utiliza como plastificante en los plásticos
vinílicos, y también como pirorretardante, aditivo para
lubricantes de presión extrema y líquido ininflamable en
los sistemas hidráulicos.
3. Transporte, distribución y transformación en el medio ambiente
El paso de FTC al medio ambiente se debe principal-
mente a su uso final, pues la liberación en el curso de la
fabricación es escasa. En 1977 se calculó que el paso
total al medio ambiente en los Estados Unidos de América
fue de 32 800 toneladas.
Debido a su escasa hidrosolubilidad y a su elevada
adsorción por los materiales en partículas, el FTC se
absorbe con rapidez en los sedimentos de ríos o lagos y en
el suelo. Su biodegradación en el medio acuático es
rápida, quedando casi terminada en el agua de río en cinco
días. El isómero orto se degrada con una rapidez ligera-
mente mayor que los isómeros meta o para. El FTC se biode-
grada con facilidad en el fango de los alcantarillados,
presentando una semivida de 7,5 horas; la degradación en
24 horas alcanza el 99%. La degradación abiótica es más
lenta, dando una semivida de 96 días.
Se midieron los factores de bioconcentración de 165-
2768 en varias especies de peces en el laboratorio utili-
zando FTC radiomarcado. La radiactividad desapareció
rápidamente al cesar la exposición, observándose semividas
de depuración comprendidas entre 25,8 y 90 horas.
4. Niveles medioambientales y exposición humana
En el Japón se han medido concentraciones atmosféricas
de FTC de hasta 70 ng/m3, pero alcanzaron un máximo de
sólo 2 ng/m3 en una instalación de fabricación de los
Estados Unidos de América. En este país, el aire del medio
laboral contenía menos de 0,8 mg/m3 en una nave de
llenado de barriles de aceite lubricante y 0,15 mg/m3
(fosfatos totales) en una planta de troquelado de zinc
para automóviles. En el Canadá, las concentraciones de
FTC medidas en el agua potable fueron bajas (0,4 a
4,3 ng/litro) y el producto resultó indetectable en el
agua de pozo. Las concentraciones observadas en las aguas
de ríos y lagos son con frecuencia apreciablemente
mayores. Sin embargo, ello se debe a la presencia de sedi-
mentos en suspensión en los que queda fuertemente absor-
bido el FTC.
Las concentraciones en los sedimentos son altas en los
ríos y en el mar, habiéndose observado valores de hasta
1300 ng/g y 2160 ng/g, respectivamente.
Se observaron concentraciones altas en el suelo y la
vegetación en los alrededores de instalaciones de fabri-
cación.
Se han señalado restos en peces y mariscos de hasta
40 ng/g, pero la mayor parte de los animales examinados no
contenían residuos apreciables.
5. Efectos sobre los seres vivos del medio ambiente
La productividad primaria de cultivos de algas verdes
de agua dulce quedó reducida en el 50% mediante la adición
de fosfato de tri- o-cresilo a razón de 1,5 a 4,2
ng/litro, según las especies, mientras que los isómeros
meta y para resultaban menos tóxicos. Son limitados los
datos referentes a la toxicidad aguda del FTC para los
invertebrados acuáticos: la CL50 en 48 horas para Daphnia
es de 5,6 ng/litro y la CL50 en 24 horas para los nemato-
dos es de 400 ng/litro; la concentración NOEL (mortalidad,
crecimiento, reproducción) en dos semanas para Daphnia es
de 0,1 mg/litro. Los valores de CL50 en 96 horas para
tres especies de peces se hallaban comprendidos entre 4,0
y 8700 mg/litro. La trucha irisada presentó una mortalidad
del 30% aproximadamente después de una exposición de
cuatro meses a una concentración de 0,9 ng/litro de IMOL
S-140 (fosfato de tri- o-cresilo al 2%) y efectos menores
en un periodo de 14 días.
Los niveles de exposición utilizados en esos experi-
mentos fueron mucho mayores que las concentraciones que
probablemente pueden hallarse en el agua en el medio
ambiente y, en la mayoría de los casos, excedían en gran
manera a la solubilidad de los productos.
6. Cinética y metabolismo
La absorción, distribución, metabolismo y eliminación
de los organofosfatos son elementos críticos en los
efectos neuropáticos tardíos de estos productos.
La absorción cutánea del FTOC en el hombre parece ser
por lo menos de un orden de magnitud más rápida que en los
perros. También se observa una absorción cutánea signifi-
cativa en el gato. En el conejo se ha señalado la absor-
ción oral del producto. No hay información directa sobre
la absorción por inhalación.
En estudios efectuados en gatos se observó que el FTOC
absorbido se distribuye ampliamente por todo el organismo,
hallándose la máxima concentración en el nervio ciático,
que es el tejido diana. Otros órganos en los que se encu-
entran altas concentraciones de FTOC y de sus metabolitos
son el hígado, los riñones y la vesícula biliar.
El metabolismo del FTOC sigue tres vías. La primera
es la hidroxidación de uno o más grupos metílicos y la
segunda es la desarilación de los grupos o-cresilo. La
tercera vía es la oxidación ulterior del grupo hidroxi-
metilo para dar aldehído y ácido carboxílico. La etapa de
hidroxilación es decisiva porque el FTOC hidroximetílico
forma un producto cíclico, el fosfato cíclico de o-
tolilo saligenina, metabolito neurotóxico relativamente
ines-table.
El FOTC y sus metabolitos se eliminan por la orina en
las heces, junto con pequeñas cantidades en el aire
espirado.
7. Efectos en los animales de experimentación y en sistemas de
prueba in vitro
Entre los tres isómeros del FTC, el FOTC es con gran
diferencia el más tóxico en la exposición aguda y a corto
plazo. Es el único isómero que produce neurotoxicidad
tardía.
Existe una amplia variabilidad entre especies en lo
que respecta a los distintos puntos finales tóxicos (por
ejemplo, letalidad aguda, neurotoxicidad tardía) de la
exposición al FOTC, siendo el pollo una de las especies
más sensibles.
Se ha producido neuropatía tardía inducida por organo-
fosfatos mediante la exposición única y repetida en una
amplia gama de animales de experimentación; este trastorno
se clasifica como una "neuropatía de muerte sobre el
dorso". Se producen lesiones degenerativas en el axón
distal, que se extienden con el tiempo hacia el cuerpo de
la célula.
Los signos clínicos consisten en la parálisis de las
patas traseras después de un intervalo característico de
2-3 semanas a partir de la exposición. Una sola dosis
oral de 50-500 mg de FOTC/kg produjo la neuropatía tardía
en pollos, mientras que se necesitaron dosis de 840 mg/kg
o más para producir la degeneración de la médula espinal
en ratas Long-Evans. El metabolito fosfato cíclico de
o-tolilo saligenina es el agente neurotóxico activo. La
sensibilidad de las especies guarda correlación inversa
con la tasa de metabolismo ulterior.
Se cree que la inhibición de la "esterasa de la
neurotoxicidad" es la lesión bioquímica que conduce a la
neuropatía tardía inducida por organofosfatos; la inhi-
bición de más del 65% poco después de la exposición al
FOTC permite prever una neuropatía ulterior. En la vari-
abilidad de la respuesta neurotóxica al FOTC influyen
factores distintos del metabolismo (por ejemplo, vías de
exposición, edad, sexo, estirpe). Los datos disponibles no
permiten establecer un nivel neto sin efectos observados
para la neuropatía tardía.
Los estudios de reproducción en ratas y ratones some-
tidos a una exposición oral repetida al FOTC mostraron
lesiones histopatológicas de los testículos y los ovarios,
alteraciones morfológicas del esperma, disminución de la
fecundidad en ambos sexos, y descenso del tamaño y
viabilidad de las crías. Los datos disponibles no permiten
establecer un nivel neto sin efectos en lo que respecta a
la acción del FOTC sobre la reproducción. Un estudio de
teratogenicidad en ratas, utilizando dosis orales que
producían toxicidad materna, dio resultados negativos.
Se dispone de escasa información sobre la mutageni-
cidad y de ninguna sobre la cancerogenicidad.
8. Efectos en la especie humana
La ingestión accidental es la principal causa de
intoxicación. Desde fines del siglo XIX se han observado
numerosos casos de intoxicación por contaminación de bebi-
das, alimentos o medicamentos. La exposición profesional
se produce principalmente por absorción cutánea o inha-
lación, habiéndose registrado algunos casos de envenena-
miento. La ingestión de preparaciones que contienen FOTC
puede ir seguida de síntomas gastrointestinales (náuseas,
vómitos y diarrea), aunque en algunos casos la polineuro-
patía es el primer signo de intoxicación. Los síntomas
neurológicos suelen ser tardíos. Los síntomas iniciales
consisten en dolor y parestesia de las extremidades
inferiores. Puede observarse una alteración moderada de
las sensaciones cutáneas y a veces del sentido de la
vibración. En la mayoría de los casos, la debilidad
muscular evoluciona rápidamente hasta dar una marcada
parálisis de las extremidades inferiores, con o sin parti-
cipación de las superiores. En los casos graves aparecen
signos piramidales. Son raras las defunciones, pero la
recuperación de los síntomas y signos neurológicos puede
ser extremadamente lenta y durar varios meses o años. El
examen histopatológico muestra la presencia de degener-
ación del axón. Los análisis corrientes de laboratorio no
muestran hallazgos anormales, pero cabe observar un
aumento de la concentración de las proteínas en el líquido
cefalorraquídeo. Los primeros auxilios consisten en la
reducción de la exposición provocando el vómito inmediata-
mente después de la ingestión, siempre que el paciente
esté consciente. El tratamiento fundamental a largo plazo
consiste en la rehabilitación física, no conociéndose
ningún antídoto específico. Existen grandes variaciones
entre las personas en la respuesta al FTC y en la recuper-
ación después de producirse los efectos tóxicos. Se han
registrado síntomas graves después de la ingestión de
0,15 g de FTC, mientras que otras personas no presentaron
efecto tóxico alguno después de ingerir 1-2 g. Ciertos
enfermos se recuperan por completo, mientras que otros
conservan efectos marcados durante un periodo apreciable.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Se han registrado con frecuencia intoxicaciones
humanas por ingestión accidental de fosfato de tri- o-
cresilo (FTOC) o por exposición laboral de trabajadores.
La vía probable de la exposición laboral es la absorción
cutánea. Entre los síntomas neurotóxicos figuran la inhi-
bición inicial de las colinesterasas y la neuropatía
tardía ulterior caracterizada por parálisis grave.
Debido a la considerable variación existente en la
sensibilidad de las personas al FTOC, no puede estable-
cerse un nivel de exposición sin riesgo. Se han señalado
síntomas por ingestión de 0,15 g de una mezcla de isómeros
con baja proporción de FTOC; así pues, la dosis efectiva
mínima del ortoisómero es muy inferior. Los estudios
efectuados en animales muestran considerables variaciones
entre especies en la respuesta al FTOC, y las personas
parecen ser especialmente sensibles.
Se han señalado casos de dermatitis irritante y
alérgica.
En consecuencia, tanto el ortoisómero puro como las
mezclas de isómeros que contienen FTOC se consideran
riesgos importantes para la salud humana.
No existe un nivel inocuo de ingestión. La exposición
al producto por contacto cutáneo o inhalación debe
reducirse al mínimo.
1.1 Niveles de exposición
Puede considerarse mínima la exposición de la pobla-
ción general al fosfato de tricresilo (FTC) por conducto
de distintos medios ambientales, incluida el agua de
beber. Se han observado concentraciones de FTC relativa-
mente más altas en el aire urbano que en el aire recogido
en los emplazamientos de fabricación, aunque los niveles
suelen ser bajos. En un estudio efectuado en los Estados
Unidos de América no se detectó el FTC en muestras de
tejido adiposo humano. Se han observado numerosos casos
de intoxicación humana accidental por ingestión de medi-
camentos, alimentos tales como harina y aceite de cocinar,
y bebidas contaminados con líquido hidráulico o aceite
lubricante que contenían FTC producido a partir del
"ácido cresílico". Pueden observarse síntomas tóxicos
después de la ingestión de sólo 0,15 g de fosfato de tri-
o-cresilo, componente del FTC obtenido a partir de ácido
cresílico. El origen habitual de la contaminación ha con-
sistido en la reutilización de barriles o bidones vacíos
utilizados previamente para contener líquido hidráulico o
aceite lubricante.
1.2 Efectos tóxicos
La exposición humana accidental a una sola dosis alta
ocasiona trastornos gastrointestinales que varían de las
náuseas ligeras a las intensas con vómitos, dolor abdomi-
nal y diarrea. En el caso de la exposición a pequeñas
dosis acumuladas, aparece progresivamente la "neurotoxi-
cidad tardía" después de un periodo latente de 3-28 días.
En la mayor parte de los casos, la debilidad muscular
pasa rápidamente a ser una marcada parálisis de las ex-
tremidades inferiores, con o sin afectación de las manos.
En los casos graves aparecen progresivamente signos
piramidales. Algunos estudios neurofisiológicos muestran
fenómenos extendidos de neurotoxicidad y prolongación de
las latencias terminales, con disminución relativamente
pequeña de la velocidad de conducción de los nervios
motores. Ello confirma los signos de degeneración del
axón, que es la característica principal observada en los
exámenes histopatológicos.
Se ha identificado el metabolito neurotóxico del FTC
como el fosfato cíclico de o-tolilo saligenina, derivado
de los metabolitos o-hidroximetílicos. Parece pues que
los efectos neurotóxicos exigen la presencia por lo menos
de un grupo o-tolilo entre las tres porciones fenólicas
del FTC. Ello significa que no es neurotóxico el FTC
producido a partir de cresol sintético, que contiene menos
del 0,1% de o-cresol.
Los estudios de toxicidad subcrónica efectuados en
animales con FTC obtenido de cresol sintético muestran que
los órganos diana son el hígado y los riñones, pero esa
observación no se ha confirmado en los casos de intoxi-
cación humana. No se dispone de datos apropiados sobre la
mutagenicidad y la cancerogenicidad. El FTC no es tóxico
para los embriones de pollo.
2. Evaluación de los efectos en el medio ambiente
La medición de las concentraciones de FTC en el agua
ambiental ha mostrado que existen sólo niveles bajos de
contaminación. Ese hecho refleja la escasa hidrosolu-
bilidad y la fácil degradabilidad del producto. Dado que
la toxicidad aguda del FTC para los seres acuáticos es
también baja, es improbable que represente una amenaza
para ellos.
Debido a las propiedades fisicoquímicas del FTC
existen altas posibilidades de bioacumulación. Sin
embargo, ello no se produce en la práctica porque las con-
centraciones de FOTC en el medio ambiente y en los organ-
ismos vivos son bajas y porque los productos se degradan
con rapidez.
El FTC fijado por los sedimentos se acumula en el
medio ambiente, habiéndose medido concentraciones altas en
los sedimentos de ríos, estuarios y mares. Dado que se
carece de información sobre la biodisponibilidad de esos
residuos para los seres vivos que se hallan en madrigueras
o en el fondo o sobre sus riesgos, no puede descartarse la
posibilidad de que aparezcan efectos en tales especies.
La fuga de FTC suscita riesgos para el medio ambiente
local.
2.1 Niveles de exposición
El FTC se halla en el aire, las aguas de superficie,
el suelo, los sedimentos y los seres vivos acuáticos cerca
de las zonas muy industrializadas, aunque en concen-
traciones habitualmente bajas. Debido a la alta tasa de
biodegradación del FTC en el medio acuoso, no se considera
que afecta adversamente a los seres vivos acuáticos. En un
estudio se encontró una concentración extremadamente alta
de fosfatos triarílicos totales (26,55 g/kg) en una
muestra de suelo obtenida en el patio de una planta de
producción. Ello sugiere la necesidad de eliminar los
residuos por relleno de terrenos.
2.2 Efectos tóxicos
Las algas de agua dulce son relativamente sensibles al
FTC, cuya concentración inhibidora del 50% del crecimiento
es de 1,5 a 5,0 mg/litro. Entre las especies de peces, la
trucha irisada sufre el efecto de concentraciones de FTC
inferiores a 1 mg/litro (0,3-0,9 mg/litro), con signos de
intoxicación crónica, pero el pez argentado de las mareas
es más resistente (CL50 de 8700 mg/litro). El FTC no
inhibe la actividad colinesterásica seca de peces y ranas,
pero tiene un efecto sinérgico con la actividad
insecticida de los órganos fosforados.
RECOMENDACIONES
Cuando se utilizan cresoles trisustituidos en la
síntesis y fabricación de otros productos, es necesario
emplear isómeros meta y para purificados para evitar la
síntesis accidental de productos orto-sustituidos.
TOCP is metabolized via three essential pathways. The
first is the hydroxylation of one or more of the methyl
groups to hydroxymethyl, which is responsible for the for-
mation of mono- and di-hydroxymethyl TOCP and o-hydroxy-
benzyl alcohol. This reaction is known to be catalysed by
the microsomal mixed-function oxidase system (Eto et al.,
1967). The hydroxymethyl TOCP is cyclized to form sal-
igenin cyclic o-tolyl phosphate with spontaneous release
of o-cresol, this being catalysed by the reaction of
plasma albumin or other components (Eto et al., 1967). The
cyclic phosphate metabolite is relatively unstable and is
rapidly hydrolysed to inactive metabolic products (Eto et
al., 1967). The second pathway is the dearylation of one
or more of the o-cresyl groups of TOCP, resulting in the
formation of o-cresol, di- o-cresyl phosphate, o-cresyl
phosphate, and phosphoric acid. The third pathway is
further oxidation of hydroxymethyl to aldehyde and car-
boxylic acid. These oxidation reactions are most likely to
be mediated by alcohol and aldehyde dehydrogenases.
Studies with [32P]-TOCP in rats have shown that
hydrolysis leads to the rapid excretion in the urine of
diaryl phosphates, monoaryl phosphates, and phosphoric
acid (Casida et al., 1961).
Nomeir & Abou-Donia (1984; 1986a,b) clearly identified
the metabolites of TOCP in male cats. Mono- and di-
hydroxymethyl TOCPs and saligenin cyclic- o-tolyl phos-
phates were present in most tissues, but their concen-
trations were low compared with those of other metab-
olites. The major metabolite of TOCP in the liver, kidney,
lung, and urine of cats was o-hydroxybenzoic acid; di-
o-cresyl phosphate, o-cresyl phosphate, o-cresol,
o-hydroxy-benzyl alcohol, and o-hydroxybenzaldehyde
were also identified. However,the brain, spinal cord,
sciatic nerve, and faeces contained predominantly
unchanged TOCP.
Johnson (1975a) compared the metabolic pathways of the
three isomers of TCP. The main observations, which con-
cerned several organophosphorus esters, were as follows:
(i) Provided that the o-alkyl group has at least one
hydrogen on the alpha-carbon atom, cyclic derivatives
can be obtained that are often highly neurotoxic.
(ii) At the para position, a substituent requires two
hydrogen atoms on the alpha-carbon atom in order to
produce a neurotoxic metabolite inhibiting NTE.
(iii) Substituents at the meta position may be metab-
olized but do not yield inhibitory products.
The major urinary metabolites of TPCP in rats were
p- hydroxybenzoic acid, di- p- cresyl phosphate, and
p- cresyl p- carboxyphenyl phosphate. Mono- (or di-) p-
cresyl di- (or mono-) p- carboxyphenyl phosphate was
identified as the intermediate metabolite in the bile
(Kurebayashi et al., 1985).
7.4 Excretion
After a single oral dose of [32P]-TOCP (770 mg/kg)
to hens, 26.5% of the total radioactivity was eliminated
in the combined urinary-faecal excreta over 72 h, mostly
as TOCP (Sharma & Watanabe, 1974).
After a single dose of [14C]-TOCP (50 mg/kg) to male
cats, approximately 28% of the applied dose was excreted
in the urine and 20% via the bile into the faeces within
10 days (Nomeir & Abou-Donia, 1986b). After this exposure,
the disappearance of TOCP and its metabolites from the
plasma followed monoexponential kinetics. The apparent
half-lives of TOCP and its metabolites (in days) in the
plasma were: TOCP, 1.20; saligenin cyclic- o- tolyl phos-
phate, 2.47; di- o- cresyl phosphate, 4.50; o- cresyl
phosphate, 4.30; o- cresol, 2.65; o- hydroxybenzyl
alcohol, 14.0; o- hydroxy-benzaldehyde, 5.70; o-
hydroxybenzoic acid, 6.00; monohydroxymethyl TOCP, 2.20.
The apparent half-lives of TOCP and its metabolites
reflected the rates of all processes involving the
conversion, clearance, and/or redistribution of these
metabolites (Nomeir & Abou-Donia, 1984).
Elimination via the bile has been demonstrated after
intravenous injection into rabbits (Gross & Grosse, 1932)
and intraperitoneal injection into rats (Myers et al.,
1955). Smith et al. (1932) measured the urinary phenol
excretion in cats given subcutaneous doses of 0.4 to 1.0
ml TOCP/kg. Little if any increase in the urinary phenol
excretion was found either before or after the onset of
paralysis of the hindlimbs.
After a single oral dose (500 mg/kg) of tri- m-cresyl
phosphate (TMCP) or TPCP to rabbits, 92% of TMCP and 95%
of TPCP was eliminated in the faeces within 4 days (Gross
& Grosse, 1932). After a single oral dose of [methyl-
14C]-TPCP (7.8 or 89.6 mg/kg), about 90% or 76%,
respectively, of the radioactivity was eliminated in the
urine and faeces within 24 h (Kurebayashi et al., 1985).
The apparent half-lives of the radioactivity in tissues
ranged from 14 h for blood to 26 h for lung and brain. At
the lower dose level, about 28% of the dose was eliminated
via the bile within 24 h. The expiratory excretion as
14CO2 over 3 days amounted to 18% of the radioactivity,
which was reduced to 3% when the rats were treated with
neomycin. The authors suggested that the enterohepatic
circulation and intestinal microflora play an important
role in the degradation of TPCP biliary metabolites.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Summary
Of the three isomers of TCP, TOCP is by far the most toxic
and is the only isomer that produces delayed neuropathy.
A wide animal interspecies variability exists for the
various end-points (e.g., lethality, neuropathology, ataxia,
enzyme inhibition) of TOCP exposure, the chicken being one of
the most sensitive species to delayed neuropathy (i.e. OPIDN).
Species sensitivity to the lethal effects of TOCP adminis-
tration is highly variable, chickens and cats being more sensi-
tive than rats and mice. A single oral dose of 50-500 mg TOCP
per kg induced delayed neuropathy in chickens, whereas doses of
840 mg/kg or more were necessary to produce spinal cord degen-
eration in Long-Evans rats.
No effects were reported in skin and eye irritation studies
on rabbits.
Reproduction studies on rats and mice receiving repeated
oral exposure to TOCP showed histopathological damage in the
testes and ovaries, morphological changes in sperm, decreased
fertility in both sexes, and decreased litter size and
viability. However, no reproductive effects were seen in a
study with TPCP. A clear no-observed-effect level for the
reproductive effects of TOCP was not apparent from the data
available. A study in rats, using oral doses that produced
maternal toxicity, failed to show any teratogenicity.
Little or no information is available on mutagenicity and
carcinogenicity.
Delayed neuropathy has been produced with both single and
repeated exposure regimes in a wide range of experimental
species, and it is classified as a "dying-back neuropathy".
"Neurotoxic esterase" is thought to be the biochemical target
of OPIDN and its inhibition by more than 65% shortly after
exposure to TOCP presages subsequent neuropathy. Factors other
than metabolism (e.g., route of exposure, age, sex, species,
strain) influence variability in sensitivity to OPIDN.
Electrophysiology studies have been performed in cats and
chickens exposed to TOCP.
In the chicken, single exposures below 58 mg/kg or short-
term (i.e. 90-day exposure) daily doses of less than 5 mg/kg
appear to be no-observed-effect levels for delayed neuropathy.
8.1 Single exposure
The acute toxicity of TCP to different species is
summarized in Table 13.
Table 13. LD50 values for TCP and its isomers
____________________________________________________________________
Compounds Route Species LD50 Reference
of (mg/kg)
admin.
____________________________________________________________________
Tricresyl oral rat 5190 Marhold (1972)
phosphate oral rat >4640 Stauffer (1988)a
(mixed oral rat >15 800 Johannsen (1977)
isomers) oral mouse 3900 Izmerov (1982)
oral chicken >10 000 Johannsen et al. (1977)
dermal rabbit >7900 Johannsen et al. (1977)
dermal cat 1500 Abou-Donia et al. (1980)
Tri- o- cresyl oral rat 8400 Johannsen et al. (1977)
phosphate oral rat 1160 Veronesi et al. (1984a)
oral rabbit 3700 Johannsen et al. (1977)
oral chicken 500 Kimmerle & Loeser (1974)
oral chicken 100-200 Smith et al. (1932)
Tri- p- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate chicken >1000 Smith et al. (1932)
Tri- m- cresyl oral rabbit >3000 Smith et al. (1932)
phosphate oral chicken >2000 Smith et al. (1932)
____________________________________________________________________
a Personal communication to the IPCS from Stauffer Chem. Co. (1988)
entitled: Test procedures and data summaries for t-butyl phenyl
diphenyl phosphate, tricresyl phosphate, trixylenyl phosphate,
mixed triaryl phosphate and isopropyl phenyl diphenyl.
The acute symptoms of intoxication are typical of
organophosphorus poisoning. The most toxic compound
appears to be TOCP; the acute toxicity of TCP depends on
the relative proportions of the different isomers.
The chicken, guinea-pig, and rabbit are the most sen-
sitive species, death occurring at an oral dose of 100
mg/kg (Smith et al., 1932); rats and mice are the least
sensitive species.
Sheep given oral doses (100, 200, or 400 mg/kg) of
TOCP exhibited acute intoxication characterized by diar-
rhoea, dehydration, metabolic acidosis, and death within
6 days. Pigs dosed with 100 to 1600 mg TOCP/kg showed
minimal signs of acute intoxication, but developed severe
signs of delayed neuropathy approximately 15 days after
administration (Wilson et al., 1982).
8.2 Short-term exposure
Saito et al. (1974) conducted a 3-month study in rats
with TCP consisting of 60-65% TMCP and 35-40% TPCP. The
compound was suspended in water with 5% gum arabic and
given orally to SD rats at 30, 100, 300, or 1000 mg/kg per
day. Histopathological examination revealed no notable
changes associated with the compound. Based on those
observations, the authors concluded that TCP was of low
short-term toxicity.
Oishi et al. (1982) reported that Wistar rats fed a
pellet diet containing a mixture of TCP isomers at a con-
centration of 5 g/kg diet for 9 weeks developed increases
in absolute and relative liver weights. Haematological
examination revealed no notable changes, but, in the
plasma, total protein, urea, cholesterol and glutamate-
pyruvate transaminase were significantly increased. Slight
liver histopathology included cytoplasmic vacuolization,
increase in the number of binucleated cells, and enlarge-
ment of cell size.
Chapin et al. (1988) exposed male and female CD-1 mice
to diets containing 0, 0.437, 0.875, 1.75, 3.5, or 7.0%
TCP (a mixture of ortho, meta, and para isomers) for
14 days. No clinical signs of toxicity were observed in
the animals at doses up to 0.875%. All animals in the
groups given 1.75, 3.5, or 7.0% exhibited piloerection,
tremors, and diarrhoea, and were lethargic before death
during the 14-day exposure.
8.3 Skin and eye irritation
No published information is available.
8.4 Teratogenicity
Mele & Jensh (1977) reported that no abnormalities
were found in fetuses from pregnant Wistar rats treated
with 500 or 750 mg TOCP/kg of on the 18th and 19th days of
gestation. This study was primarily designed to investi-
gate the effects of prenatal treatment with TOCP on post-
natal behaviour. As such, it cannot be regarded as a true
teratogenicity study.
Tocco et al. (1987) tested the teratogenicity of TOCP
in Long-Evans rats treated with 87.5, 175, and 350 mg/kg
per day throughout organogenesis from gestation days 6 to
18. No maternal deaths or toxicity were observed at the
low or medium dose levels. Maternal lethality in the high-
dose group was higher than that in the control groups.
Numerous soft tissue and skeletal malformations were
observed in both control and TOCP-treated groups, but
there were no significant differences in the frequency of
malformations between the treated and control animals.
8.5 Reproduction
In studies by Somkuti et al. (1987a), TOCP was tested
for effects on the male reproductive tract in male Fisher-
344 rats. Animals were dosed daily for 63 days at dose
levels ranging from 10 to 100 mg/kg per day. Vehicle-
treated animals served as controls. As judged by enzy-
matic, hormonal, and sperm motility, density, and mor-
phology investigations, the minimum effective (threshold)
dose for observable testicular toxicity was 10-25 mg/kg
per day. The data suggested that TOCP interfered directly
with spermatogenic processes and sperm motility and not
via androgenic mechanisms or decreased vitamin E avail-
ability. Testicular pathological changes were seen at
doses above 25 mg/kg per day and included the following:
PAS-positive droplets, immature germ cells, and multinu-
cleate giant cells in the lumen. TPCP produced a decrease
in sperm density but no other testicular effects at a dose
level of 100 mg/kg per day.
To study the time course of the TOCP-induced testicu-
lar lesion in F-344 rats, the onset of possible changes in
sperm numbers and production, serum hormones, and various
enzyme activities was followed. Rats were administered
TOCP daily (150 mg/kg) for periods of 3, 7, 10, 14, or 21
days, while vehicle-treated animals served as controls.
Both sperm motility and the number of sperm per mg of
cauda epididymis were lower in treated animals by day 10.
The ratio of testicular to body weight was significantly
decreased only those rats treated for 21 days. Testicular
neurotoxic esterase and nonspecific esterase activities
were also inhibited, while beta-glucuronidase activity was
not affected. Luteinizing and follicle-stimulating hormone
levels were normal, as were both serum and interstitial
fluid testosterone concentrations. Sertoli cell fluid
secretion, as measured by testis weight increase after
efferent duct ligation, showed no significant changes.
Other organs (spleen, liver, kidney, pancreas, small
intestine, and adrenal and pituitary glands) revealed no
overt signs of pathology as observed by light microscopy
in animals treated for 21 days. A separate group of ani-
mals was treated for 21 days and subsequently examined
after 98 days of observation (two cycles of the rat semin-
iferous epithelium). Normal spermatogenesis did not
return, indicating that the toxicity was irreversible at
the dose used. The effects noted in these studies further
define the testicular lesion produced by TOCP, and show
that a dose level 150 mg/kg per day for 21 days produces
irreversible testicular toxicity (Somkuti et al., 1987b).
The testicular effects of TOCP have also been studied
in the rooster, 100 mg/kg per day being administered
orally to ten adult leghorn roosters for 18 consecutive
days. By days 7-10 of the study, TOCP-treated birds
exhibited limb paralysis characteristic of OPIDN. Enzyme
analyses at the end of the study revealed significant
inhibition of neurotoxic esterase (NTE) activity in both
brain and testis and a slight decrease in brain acetyl-
cholinesterase (AChE) activity. Sperm motility was shown
to be greatly decreased. In addition, sections of
formalin-fixed, methacrylate-embedded testes from TOCP-
treated birds showed vacuolation and disorganization in
the seminiferous epithelium. The marginal body weight
decrease (17%) in treated animals was not considered to
contribute to the testicular toxicity induced by TOCP
(Somkuti et al., 1987c).
TCP (isomer mixture not known) was tested in sperm
morphology and vaginal cytology examination (SMVCE)
studies in groups of 25 male and female Fisher-344 rats
and 25 male and female Swiss CD-1 mice. Treatment lasted
for 13 weeks at dose levels of 1700, 3300, or 6600 mg/kg
diet or 50, 100, 200, 400, or 800 mg/kg body weight via
gavage in the rats, and 500, 1000, or 2100 mg/kg diet or
50, 100, or 200 mg/kg body weight via gavage in the mice.
Effects were seen in treated animals, but no details were
given (Morrissey et al., 1988a). In a follow-up continuous
breeding reproduction study in Swiss CD-1 mice, male and
female fertility was reduced (Morrissey et al., 1988b).
Carlton et al. (1987) examined the reproductive
effects of TCP (mixed isomers; < 9% TOCP). Male Long-Evans
rats received 0, 100, or 200 mg/kg and females received 0,
200, or 400 mg/kg in corn oil by gavage. The low-dose
males were mated with low-dose females, and the high-dose
males with high-dose females. Males were dosed for 56 days
and females for 14 days prior to breeding and throughout
the breeding period, gestation, and lactation. Sperm con-
centration, motility, and progressive movement were
decreased in the high-dose males, and there was a dose-
dependent increase in abnormal sperm morphology. The
number of females delivering live young was severely
reduced by TCP exposure. Litter size and pup viability
were decreased in the high-dose group, but pup body weight
and developmental landmarks were unaffected by TCP
exposure. Histological changes were observed in the testes
and epididymides of treated males (i.e. necrosis, degener-
ation, early sperm granulomas in seminiferous tubules, and
epididymal hypospermia) and in the ovaries of treated
females (i.e. diffuse vacuolar cytoplasmic alteration of
interstitial cells and increased numbers of follicles and
corpora lutea).
In a continuous breeding study, Chapin et al. (1988)
fed Swiss CD-1 mice a diet containing TCP (mixed o-, m-,
p-isomers) at a level of 0, 0.5, 1, or 2 g/kg diet over
98 days. Although the fertility index was not changed in
the high-dose groups, the number of litters per pair
decreased in a dose-dependent fashion, and the proportion
of pups born alive and their weight were significantly
decreased. Histopathological examination of the parental
generation revealed dose-related seminiferous tubule
atrophy and decreased testis and epididymal weights in the
high-dose males, but the female reproductive tract showed
no histopathological changes. A crossover mating trial
revealed impaired fertility in both males and females
exposed to 2 g/kg diet.
8.6 Mutagenicity and carcinogenicity
Haworth et al. (1983) reported a negative result with
TCP (substitution pattern not known) in the Salmonella
mutagenicity test.
8.7 Neurotoxicity
8.7.1 Experimental neuropathology
TOCP first gained notoriety as the culpable neurotoxic
agent of the "Ginger Jake" epidemic (Jeter, 1930;
Goodale & Humphreys, 1931; Vonderahe, 1931). Since then
several experimental studies have modelled TOCP neuropathy
in various species.
Smith & Lillie (1931) produced delayed paralysis in
various animals, including dogs, monkeys, cats, and
chickens. These animal models were used to describe the
functional and morphological features of TOCP neuropathy.
After a delay period of 2-3 weeks following exposure to
single or multiple doses, paralysis of the hindlegs
developed in these species in response to the TOCP. Neuro-
pathologically, degeneration was confined to the spinal
cord and peripheral nerve fibres. These changes were
essentially similar to the lesions reported in human
victims of the "Ginger Jake" epidemic.
The delayed neuropathy associated with TOCP and other
organophosphorous compounds has been termed OPIDN.
Cavanagh (1964) showed that, in cats and chickens,
degeneration affected the long fibres in the spinal cord
and peripheral nerves; moreover, in cats, fibre diameter
seemed to be important in determining the onset and
severity of the peripheral nerve lesions. He categorized
this delayed neuropathy as a "dying-back". Thus, the
lesions of the ascending tracts are seen in the cervical
region, especially in the dorsal columns (i.e. fasciculus
gracilis), while those of the descending tracts are seen
in the thoracic and lumbosacral regions of the spinal
cord. He also showed that the peripheral and central nerve
lesions were the result of axonal degeneration and did not
reflect primary demyelination. The affected nerve axons
degenerate in a "dying-back" fashion towards the cell
body, i.e. axonal degeneration begins at the most distal
portion of the axon and proceeds towards the cell body.
Prineas (1969) described axons with accumulated
tubulovesicular membranes within myelinated motor nerve
terminals in the foot muscles of cats dosed with TOCP.
Similar early axoplasmic changes have been noted in TOCP-
treated chickens (Bischoff, 1967, 1970; Spoerri & Glees,
1979, 1980).
At the ultrastructural level, the nerve endings are
characterized by a marked proliferation and distension of
vesicular elements of the endoplasmic reticulum and a
coinciding disintegration of the filamentous and tubular
organelles (Bischoff, 1977). Bischoff also noted that the
presynaptic nerve terminals and boutons terminaux appeared
particularly sensitive in TOCP intoxication.
OPIDN has also been produced in rats dosed with TOCP
(Veronesi, 1984). The pathological changes developed in
the absence of discernable ataxia after a 2-week exposure
to acute and multiple oral doses of TOCP (>840 mg/kg). In
the rat, dorsal column degeneration of the cervical cord
and a selective vulnerability of large diameter tibial
branches supported a "dying-back" neuropathy, as in
other species.
OPIDN has also been described in European ferrets
( Mustela putorius furo) administered a single oral or
dermal dose of 250, 500, or 1000 mg TOCP/kg body weight.
Five animals per group were sacrificed 48 h after dosing,
the others being observed for another 54 days. All ferrets
dosed dermally with 1000 mg/kg developed neurological
signs ranging from ataxia to partial paralysis. Dermal
doses of 250 and 500 mg/kg produced variable degrees of
hind limb weakness and ataxia. Of the animals dosed
orally, only those treated with 1000 mg/kg showed neuro-
logical signs, which did not progress beyond mild ataxia.
Slight axonal degeneration was noted in the dorsolateral
part of the lateral funicilus and in the fasciculus gra-
cilis of spinal cords in ferrets receiving a dermal dose
of 1000 mg/kg. Whole brain neurotoxic esterase activity
was maximally inhibited (46%) at this dose level. The
study demonstrated that in the ferret dermal exposure was
more effective than oral exposure at the same dose level
(Stumpf et al., 1989).
8.7.2 Neurochemistry
Johnson (1969) found that approximately 6% of brain
esterases are not affected by non-neuropathic compounds
but are specifically inhibited, irreversibly, by neuro-
pathic ones, such as TOCP. Johnson used the term "neuro-
toxic esterase" (NTE) and proposed that NTE is the pri-
mary target of the organophosphorus esters causing OPIDN
(Johnson, 1975a,b). There appears to be a strong corre-
lation in the chicken between NTE inhibition above 70%
shortly after exposure and subsequent neuropathy for a
large number of tested organophosphorous compounds
(Johnson, 1974).
Padilla & Veronesi (1985) demonstrated the relevance
of NTE to the rodent model of OPIDN by exposing Long-Evans
rats to single doses of TOCP ranging from 290 to 3480
mg/kg. High NTE inhibition in the spinal cord (>72%) and
the brain (> 66%) produced severe spinal cord damage in
over 90% of exposed rats, indicating that NTE depression
could predict OPIDN damage in rats acutely exposed to
organophosphates.
Concerning the role of lipids in TOCP neuropathy,
Morazain & Rosenberg (1970) showed that there was a rise
of 25-50% in the cholesterol level in the sciatic nerve
and a 50% decrease in its triglyceride content in chickens
orally dosed with 1 ml TOCP/kg. Phospholipids, diglycer-
ides, cholesterol esters, proteolipids, and tissue phos-
pholipases were not affected by TOCP. The possible
involvement of lipids in the production of TOCP-induced
delayed neuropathy has not yet been resolved.
Other toxic effects have been demonstrated. Brown &
Sharma (1975) found that neural membrane ATPases were
inhibited by organophosphates. Cohen & Murphy (1970)
reported that TOCP potentiates the anticholinesterase
action of malathion by 29-fold in mice, 17-fold in quail,
and 11-fold in sunfish.
8.7.3 Interspecies sensitivity and variability to OPIDN
Certain animal species (e.g., cats, dogs, cows, and
chickens) are susceptible to OPIDN-related paralysis,
whereas others (e.g., rats and mice) are less susceptible
to the ataxia but very susceptible to the pathological
changes. Species susceptibility to delayed neurotoxicity
induced by TOCP shows an inverse correlation with the rate
of metabolic conversion to the neurotoxic metabolite (see
section 7). Because of its high susceptibility to ataxia,
the adult chicken has been used as an experimental model
to study OPIDN.
TOCP is metabolized to the more potent neurotoxic
agent, saligenin cyclic o-tolyl phosphate, which is at
least five times more neurotoxic than TOCP after oral
administration to chickens: a metabolite level of 40 mg/kg
caused ataxia equivalent to that resulting from 200 mg
TOCP/kg (Bleiberg & Johnson, 1965).
It has been shown that a single oral dose of TOCP in
the range of 58 to 580 mg/kg (i.e. 0.05-0.5 ml/kg) induces
mild to severe paralysis in the hen ( Gallus domesticus)
(Cavanagh, 1954; Hine et al., 1956).
Johannsen et al. (1977) showed that chickens adminis-
tered cumulative doses of TCP (60 000 mg/kg) or TOCP (1500
mg/kg) developed both the ataxic and neuropathological
symptoms of OPIDN.
In 90-day studies in hens, obvious functional and
morphological neuropathological changes were found at
daily oral dose levels of 5 to 20 mg TOCP/kg body weight,
but not at lower dosages (Smith et al., 1932; Prentice &
Majeed, 1983; Roberts et al., 1983).
In a subchronic feeding study, Haggerty et al. (1986)
exposed rats to TCP (isomeric substitution pattern not
known) for 13 weeks at dose levels of 0, 900, 1700, 3300,
6600, or 13 000 mg/kg. Decreased hindlimb grip strength
was observed in male rats (at 13 000 mg/kg), but not in
female rats. In mice exposed to 0, 250, 500, 1000, 2100,
or 4200 mg/kg, decrements in both fore- and hindlimb grip
strength were observed in males (at 4200 mg/kg) and
females (at 2100 and 4200 mg/kg). A reduction of body
weight was seen both in rats and mice at the two highest
dose levels. Preliminary histopathological diagnosis
indicated demyelination and axonal degeneration of the
sciatic nerve in male and female mice only.
Freeman et al. (1988) tested the neurotoxicity of TCP
(substitution pattern not known) to F-344 rats in a short-
term study. After 13 weeks of dosing with TCP in the feed,
hindlimb grip strength decreased in male rats at 300 and
600 mg/kg, but not in female rats. Serum cholinesterase
was reduced at 300 and 600 mg/kg in both males and
females. All effects observed with 600 mg/kg were appar-
ently reversible during the recovery period.
In a 13-week short-term feeding study, Irwin et al.
(1987) exposed F-344 rats to TCP (0, 75, 150, 300, or 600
mg/kg; substitution pattern not known), while B6C3F1 mice
receive 0, 60, 125, or 250 mg/kg. After 13 weeks of
dosing, forelimb grip strength was unaffected by TCP in
both mice and rats. Hindlimb grip strength decreased in
male rats (at 300 and 600 mg/kg) but not in female rats.
In mice, decrements in hindlimb grip strength were
observed in males (250 mg/kg) and females (125 and 250
mg/kg). Serum cholinesterase levels showed a dose-depen-
dent reduction in both rats and mice. TCP had no effect on
food consumption in either species. All groups exhibited
normal body weight values.
Factors such as age, sex, and strain figure promi-
nently in the expression of OPIDN. The young of most
species are non-susceptible to TOCP-induced delayed neuro-
pathy (Johnson & Barnes, 1970), which could be due to poor
absorption of TOCP. However, recent experiments (Olson &
Bursian, 1988) have suggested that factors (e.g., route of
administration) other than absorption are more critical to
this lack of susceptibility.
Strain differences in the susceptibility of rats to
TOCP-delayed neuropathy have been reported. Although OPIDN
can be readily produced in Long-Evans and Sprague-Dawley
rats after acute oral doses of > 840 mg/kg (Veronesi &
Abou-Donia, 1982; Veronesi, 1984), repeated doses of TOCP
(10-100 mg/kg) failed to produce neuropathy in Fischer-344
rats (Somkuti et al., 1988). Variations in TOCP inhibition
of brain AChE and NTE have also been reported in these
three strains (Carrington & Abou-Donia, 1988).
8.7.4 Neurophysiology
Robertson et al. (1987) investigated electrophysio-
logical changes in the adult hen following single oral
doses of 30 or 750 mg TOCP/kg. At the higher dose, the
birds demonstrated clinical signs of toxicity 12 days
after dosing that included gait abnormalities, which
became progressively more severe and in some cases led to
complete ataxia. Lymphocyte NTE was inhibited by more
than 70%. The lower dose produced no clinical signs of
toxicity and only 54% lymphocyte NTE inhibition. Both
treated groups displayed significant action potential
disruption in both the tibial and sciatic nerves that
resulted in decreased refractoriness in the tibial nerve,
increased refractoriness in the sciatic nerve, and
elevated strength duration threshold for both nerves.
Electrophysiological changes have been investigated in
cats following single dermal doses ranging from 250 to
2000 mg TOCP/kg and 90-day dermal administration of 1 to
100 mg/kg (Abou-Donia et al., 1986). In contrast to the
hen, the clinical signs of TOCP neurotoxicity appear in
the cat 21-26 days before the electrophysiological effects
on the gastrocnemius muscle. Recovery of the cat from
delayed neurotoxicity symptoms was more marked than that
of the hen. No effects on peripheral nerve transmission
or on neuromuscular junction functioning were seen in the
cat.
9. EFFECTS ON HUMANS
Summary
There have been many reported cases of human poisoning,
mostly from accidental or irresponsible contamination of food-
stuffs. Occupational poisoning, usually resulting from dermal
exposure, has also been reported. The ortho isomer of TCP is
the responsible toxic agent.
Though short-term symptoms of ingestion might involve
vomiting, abdominal pain, and diarrhoea, characteristically
delayed, longer-term symptoms are neurological, frequently
leading to paralysis and pyramidal signs (spasticity, etc.).
There is considerable variation in the sensitivity of indi-
viduals to TOCP; severe symptoms were reported with a TOCP dose
of 0.15 g in one individual, while others were unaffected by 1
to 2 g. There is also considerable variation in the rate of
recovery from poisoning, some patients recovering completely
and others still severely affected years later, after appar-
ently similar exposure.
First-aid treatment involves the induction of vomiting or
pumping of the stomach. The patient should be hospitalized as
soon as possible. Atropine or 2-PAM may be used as an effective
antidotal treatment against cholinergic symptoms. Long-term,
antispastic drugs may be useful, though physical rehabilitation
is the cardinal therapy.
9.1 Historical background
Of the tricresyl phosphate isomers, the ortho (TOCP)
is by far the most toxic and alone gives rise to the major
neurotoxicity in man. It is considered that the toxicity
of the commercial products depends on the concentration of
the ortho isomer, but the mixed o-cresyl esters in these
products are also toxic and contribute to the neurotoxic
action.
It is well known that TOCP produces delayed effects on
the central and peripheral nervous systems. TOCP poisoning
has occurred throughout the world (Inoue et al., 1988);
the major outbreaks are indicated in Table 14.
Table 14. Major outbreaks of TOCP poisoning
___________________________________________________________________________
Year Place Number Vehicles of TOCP Reference
of cases
___________________________________________________________________________
1898 France 6 phospho-creosote Lorot (1899)
1900-1928 Europe 43 phospho-creosote Roger & Recordier
(15% TOCP) (1934)
1930-1931 USA 50 000 ginger extract Morgan (1982)
1931 Europe several Apiol pill Susser & Stein
hundred (1957)
1938 South Africa 68 cooking oil Sampson (1942)
1940 Switzerland 80 cooking oil Walthard (1945)
1941-1945 Germany more than cooking oil Mertens (1948)
200
1945 England 17 cooking oil Hotston (1946)
1955 South Africa 11 water or solvent Susser & Stein
(1957)
1957 Morocco about cooking oil Smith & Spalding
10 000 (1959)
1960 India 58 solid food Vora et al. (1962)
1962 India more than flour Chaudhuri (1965)
400
1967 Fiji 56 flour Sorokin (1969)
1980 Romania 12 alcohol Vasilescu & Florescu
(1980)
1981 Sri Lanka more than cooking oil Senanayake &
20 (0.56%) Jeyaratnam (1981)
___________________________________________________________________________
In 1899, Lorot initially reported six cases of poly-
neuropathy out of 41 cases of pulmonary tuberculosis
treated with phospho-creosote. Later it was shown that the
phospho-creosote contained 15% TOCP. In the next 30 years,
43 additional cases caused by the drug were reported in
various parts of continental Europe (Roger & Recordier,
1934).
In the spring of 1930, in mid-western and south-
western USA, an outbreak of paralysis characterized by
bilateral foot- and wrist-drop appeared suddenly (Burley,
1930; Merritt & Moor, 1930). Ultimately 50 000 people were
poisoned by a popular substitute for alcohol called
"Ginger Jake" (Morgan, 1982). Smith et al. (1930) proved
that the adulterated beverage contained about 2% TOCP and
that this caused the paralysis.
In 1931, several hundred women in Europe (especially
in Germany, The Netherlands, Yugoslavia, and France) were
poisoned by the TOCP contained in Apiol pills and taken as
an abortifacient (Roger & Recordier, 1934; Susser & Stein,
1957). The TOCP was presumably included as an additional
stimulus to abortion (Ter Braak & Carrillo, 1932).
An outbreak involving 11 people occurred in Durban,
South Africa, in 1955 (Susser & Stein, 1957). From the
epidemiological survey it was suggested that water, as
well as solvents, may have been a vehicle for the TOCP.
In 1959, about 10 000 Moroccan people were intoxicated
by TOCP: jet engine oil had been illegally mixed into
their cooking oil (Smith & Spalding, 1959; Svennilson,
1960). Accidental poisoning by TOCP contamination of solid
food occurred in 1960 in Bombay, India, where 58 victims
were recorded (Vora et al., 1962).
During the period April-June, 1962, more than 400
cases of paralysis occurred in the Malda district in
India. The cause of this disease proved to be the consump-
tion of flour contaminated with TOCP (Chaudhuri, 1965).
In 1967, similar poisoning was recorded in Fiji, where
56 people showed neuropathy (Sorokin, 1969). The cause was
stated to be contamination of dry sharps flour by TOCP
through the sacking material.
Vasilescu & Florescu (1980) in Romania reported 12
patients with toxic neuropathy following accidental inges-
tion of alcohol contaminated by TOCP.
An outbreak of acute polyneuropathy in over 20 young
females occurred in Sri Lanka during 1977-1978 (Senanayake
& Jeyaratnam, 1981). The cause of the neuropathy was
traced to TCP found as a contaminant in a special cooking
oil (gingili oil). Contamination probably occurred during
transport of the oil in containers previously used for
storing mineral oils.
9.2 Occupational exposure
Gartner & Elsaesser (1943) reported the case of a
worker who developed pyramidal signs after exposure to
TOCP for two years in a German chemical plant. In this
case, percutaneous absorption was considered to be the
main route of exposure.
In 1944, three cases of toxic polyneuropathy among
workers who had worked for six to eight months in a plant
manufacturing TCP in England were reported (Hunter et al.,
1944). Skin penetration and inhalation were thought to be
the main causes of the occupational poisoning.
Parnitzke (1946) reported a case of TOCP poisoning
after 3 years of exposure in a German plant and stated
that TOCP had been absorbed through the skin and presum-
ably the gastrointestinal tract.
Since 1958, a high prevalence of polyneuropathy among
shoe factory workers has been reported in Italy. The cause
has been attributed to TCP (Cavalleri & Cosi, 1978).
Although this is possible, this cause-effect relationship
has not so far been based on unquestionable evidence. This
polyneuropathy might have various aetiological factors
(including n-hexane) or be produced by a combination of
them (Leveque, 1983).
9.3 Clinical features
Goldstein et al. (1988) reported a case of severe
intoxication in a 4-year-old child following ingestion of
a lubricant containing TCP (substitution pattern not
known). The clinical findings were acute gastrointestinal
symptoms, delayed cholinergic crisis, and neurological
toxicity.
The severity of signs and symptoms after poisoning
with TOCP seems not always to be proportional to the
dosage (Staehelin, 1941). In a Swiss Army outbreak of
poisoning among more than 80 young men, toxic symptoms
appeared in once case after eating food containing only
0.15 g TOCP. Severe neurological disturbance developed in
three men from the intake of 0.5 to 0.7 g, whereas in two
other cases the intake of 1.5-2 g did not lead to any
symptoms. This leads to the conclusion that individual
susceptibility varies greatly (Staehelin, 1941).
In general, the signs and symptoms of TOCP poisoning
are distinctive, whereas the symptomatology varies
somewhat according to whether a single relatively large
dose or small cumulative doses are taken. In the former
case, the initial symptoms are gastrointestinal, ranging
from slight to severe nausea and vomiting, sometimes
accompanied by abdominal pain and diarrhoea. Among these
symptoms, vomiting is most frequently observed (Staehelin,
1941). These symptoms are usually transient, lasting from
a few hours to a few days (Walthard, 1945; Susser & Stein,
1957).
In cases of chronic low level exposure, the above
symptoms may not be present and the major symptoms are
neurological (Parnitzke, 1946). The clinical features of
acute exposure to TOCP were described by Staehelin (1941).
A latent period of 3-28 days is observed after acute
exposure, and clear "delayed neurotoxicity" then gradu-
ally appears. The initial neurological symptoms are sharp,
cramp-like pains in the calves, and some numbness and
tingling in the feet and sometimes the hands. Within a few
hours or a day or two at most, these pains are followed by
increasing weakness of the lower limbs, and soon the
patient becomes unsteady and then unable to maintain bal-
ance. The cramp-like pains may cease with the onset of
weakness, or persist for some days. One or two weeks after
the onset in the lower limbs and while paralysis may still
be progressing, the weakness spreads to the hands. While
some patients show complete wrist drop and total loss of
power in the hands, sometimes with weakness up to the
elbows, the predominant neurological abnormalities are
observed in the lower limbs. Bilateral foot drop with com-
plete loss of power from the ankle down is a common
finding. Depending on the severity of the affection, the
patient may have weakness in the knees, less at the hips,
and, only in the most severe cases, weakness of the trunk.
About three weeks or more after the onset of paralysis, a
most striking and rapid wasting may be observed. While the
small muscles of the feet, calves, the anterior tibials,
and the thighs do not escape this wasting, in so far as
they are involved in the disease, the change is most
obvious in the small muscles.
In the initial stage, the ankle jerks are absent and
knee reflexes may be normal or occasionally depressed.
Plantar reflexes are unobtainable. Mild cases do not show
any upper motor neuron signs. On the other hand, in the
more severe cases, even at the early stage, knee jerks may
be exaggerated, presaging the development of upper motor
neuron involvement. In general, upper motor neuron signs,
e.g., pyramidal signs, gradually become evident at about
the third week or later. Knee jerks become exaggerated and
so also may the biceps, triceps, and supinator jerks
(Cavanagh, 1964). A finger flexor reflex appears for the
first time or increases (Susser & Stein, 1957). As the
pyramidal tract lesion becomes evident, involuntary flexor
withdrawal of the whole limbs follows gentle plantar
stimulation. Babinski responses are observed much later.
Muscle tonus of the limbs gradually increases. In severe
cases, the signs of upper motor involvement are delayed,
probably masked by the gross flaccid muscle weakness.
Several authors state emphatically that sensory dis-
turbances do not occur. Sampson (1942) reported sensory
disturbances although these were admittedly unobtrusive,
in contrast to motor dysfunction. Reports of muscle and
peripheral nerve tenderness are fairly frequent. If the
sensory disturbances are observed, there is hypoesthesia
with loss of pin-prick and temperature sense; the ability
to detect vibration is sometimes affected distally. The
sensory disturbances vary in extent from merely the soles
of the feet to the whole of the limbs.
Usually cranial nerves are not involved. In general,
mental signs are rare, but transient euphoria and con-
fusion have been observed in the early stage (Schwab,
1948).
9.4 Prognosis
Following exposure, muscle weakness progresses over
several weeks, sometimes even months. Sensory changes
often begin to regress during the early weeks, the rap-
idity depending on the severity of the case, and then
muscle strength gradually returns in patients who are only
mildly affected. Improvement begins with the return of
sensation, then muscle strength in the hands, and eventu-
ally strength in the lower limbs. In cases of pyramidal
signs, recovery is generally poor. Zeligs (1938),
reporting eight years after the 1930 mid-western and
south-western USA outbreak, surveyed the records of 316
patients. He was able to follow up 60 patients, all of
whom were disabled and still in institutions. Aring (1942)
examined more than 100 patients in the 10 years following
this outbreak and they appeared still to be affected.
Morgan & Penovich (1978) followed up 11 survivors in the
47 years after the same outbreak; the principal findings
were the spasticity and abnormal reflexes of an upper
motor neuron syndrome.
Of the 80 patients in the 1940 Swiss army accident,
14 were quite well after five years, 15 were totally inca-
pacitated, and 38 showed spasticity (Walthard 1945).
In the 1938 Durban outbreak, all the patients showed
some symptoms of the disease 18 years later (Susser &
Stein, 1957). The mildest case had slight weakness at the
ankle, while the most severely affected had foot drop,
muscle atrophy, and pyramidal signs (spasticity, ankle
clonus, and positive Babinski sign).
The residual signs and symptoms are mainly confined to
the lower limbs. They consist of weakness and muscle atro-
phy of varying degrees in the feet and the small muscles
of the hand. Disability is principally related to the
pyramidal signs with resultant spasticity of the lower
extremities.
9.5 Neurophysiological investigations
There have been very few electrophysiological studies
on human TOCP poisoning. Svennilson (1960) reported an
electromyographic study on 65 patients in the 1959 Morocco
poisoning. These cases showed varying degrees of dener-
vation and polyphasic abnormal potentials in the paralysed
muscles. The clinically healthy proximal groups of muscles
also showed marked polyphasic action potentials but not
denervation.
Vasilescu & Florescu (1980) reported detailed studies
on 12 patients from the 1980 accident in Romania. They
observed > 50% decrease in the muscle evoked potential
amplitude, fibrillation potentials in the same muscles at
rest, and decreased motor nerve conduction velocity.
In neurophysiological studies by Senanayake (1981) in
Sri Lanka, the main findings were widespread neurotoxic
patterns and prolongation of terminal latencies with rela-
tively mild slowing of motor nerve conduction velocities.
These studies confirmed the evidence of axonal degener-
ation.
9.6 Pathological investigations
Numerous pathological studies have been made on biopsy
or autopsy samples since the Jamaica ginger accidents in
1930. In 1930, Goldfain described some changes observed
in the peripheral nerves and spinal cord, quoting Jeter's
autopsy report. Histopathological investigations by
Goodale & Humphreys (1931) indicated degeneration of
myelin sheaths and axis cylinders in the radial, sciatic,
and tibial nerves in all cases examined. Vonderahe (1931)
found marked degenerative changes in the anterior horn
cells, characterized by swelling, central chromatolysis
(disappearance of the Nissl substance), excentric nuclei,
and shrinking of the cells. The pathological studies also
revealed degeneration in the radial and anterior tibial
nerves, and degenerative changes in the anterior roots.
There were no pathological signs of inflammation. Accord-
ing to Smith & Lillie (1931), the paralysis due to Jamaica
ginger was essentially a degeneration of the myelin
sheaths of the peripheral nerves, with a variable amount
of relatively moderate central degenerative changes
affecting the anterior horn cells throughout the spinal
cord, but more often in the lumber and cervical regions.
In the 1938 Durban (South Africa) epidemic, Sampson (1942)
also reported that degeneration of the anterior horn cells
occurred in some instances and that peripheral nerves
showed axonal degeneration. Aring (1942) described degen-
eration in the posterior and lateral columns in later
investigations of survivors of the outbreak, thereby con-
firming the origin of some of the spinal symptoms. It is
noteworthy that the latter changes were evident in the
lumbar region, while the dorsal column changes, in which
only the fasciculus gracilis was involved, concerned the
cervical region.
Muscle biopsy studies of patients from the 1959
Moroccan poisoning showed a moderate degree of muscle
atrophy and a slight increase of the muscle fibre nuclei.
Spherical axonal swelling and terminal knobs were noted as
a sign of peripheral nerve degeneration in muscles
(Svennilson, 1960). Similar changes were also noted in
the poisoning cases reported in Malda, India, in 1962
(Chaudhuri et al., 1962). These effects on muscle suggest
denervation.
9.7 Laboratory investigations
Little information has been obtained from laboratory
examinations of exposed humans. There is no significant
change in the urine or blood (Sampson, 1942; Senanayake &
Jeyaratnam, 1981), but the cerebrospinal fluid may show an
increase in protein concentration, with or without pleo-
cytosis (Sampson, 1942; Mertens, 1948; Susser & Stein,
1957).
Vora et al. (1962) measured blood cholinesterase
levels in patients admitted to hospitals during the 1960
Bombay poisoning and demonstrated that plasma cholinester-
ase was increased one month after exposure. The erythro-
cyte cholinesterase level was considerably diminished
(50%) quite early after the onset of symptoms. Both plasma
and erythrocyte cholinesterase activities returned to
normal within about 3 months.
In a factory manufacturing TAP, about half of the
workers examined showed significant decreases in pseudo-
cholinesterase (cholinesterase other than AChE), and many
of them had minor signs and symptoms (Tabershaw et al.,
1957).
Morgan & Hughes (1981) also investigated cholinester-
ase activity in workers in a plant manufacturing TAP plas-
ticizers. They found that plasma cholinesterase estimation
in workers exposed to TAP compounds cannot be used as a
sensitive barometer of organic phosphate absorption; thus
routine regular estimations serve no useful purpose. Its
value is chiefly as a screening method at the pre-employ-
ment medical examination to exclude personnel at risk, as
a baseline in the event of massive exposure, and as a
means of diagnosis in cases of CNS disease that simulate
TAP poisoning.
9.8 Treatment
In the event of skin contact with TCP, contaminated
clothing should be rapidly removed and affected body areas
copiously irrigated with water. The ingestion of food or
drink contaminated with TOCP should be treated by inducing
vomiting, unless the patient is unconscious. Atropine or
pralidoxine (2-PAM) chloride may be required to counteract
cholinergic effects. No specific antidote is available.
Medical therapy should begin as soon as possible, even
though the results of medical therapeutic measures have
been disappointing. B-complex vitamins and corticosteroids
may protect nervous tissue against further involvement
(Geoffroy et al., 1960). The cardinal therapy is physical
rehabilitation. Administration of anti-spastic drugs may
be required.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human poisoning involving the accidental ingestion of
tri- o-cresyl phosphate (TOCP) or occupational exposure
of workers has frequently been reported. The likely route
of occupational exposure is cutaneous absorption. The
neurotoxic symptoms involve initial inhibition of cholin-
esterases and subsequent delayed neuropathy characterized
by severe paralysis.
Because of considerable variation among individuals in
sensitivity to TOCP, it is not possible to establish a
safe level of exposure. Symptoms have been reported from
the ingestion of 0.15 g of an isomeric mixture with a low
proportion of TOCP; the minimum effective dose of the
ortho isomer is, therefore, much lower than this. Animal
studies show considerable variation among species in the
response to TOCP, and humans appear to be particularly
sensitive.
Irritant and allergic dermatitis have been reported.
Both the pure ortho isomer and isomeric mixtures con-
taining TOCP are, therefore, considered major hazards to
human health.
There is no safe level for ingestion. Exposure to the
compound through dermal contact or inhalation should be
minimized.
10.1.1 Exposure levels
Exposure of the general population to tricresyl phos-
phate (TCP) through various environmental media, including
drinking-water, can be regarded as minimal. TCP has been
detected at relatively higher concentrations in urban air
than in air collected at production sites, although the
levels are usually low. TCP was not detected in human
adipose tissue samples in a survey conducted in the USA.
There have been many cases of accidental human poisoning
through the ingestion of medicines, food, flour, cooking
oil, and beverages contaminated with hydraulic fluid or
lubricant oil containing TCP produced from "cresylic
acid". The toxic symptoms can be observed after ingestion
of only 0.15 g of tri- o-cresyl phosphate, a component of
TCP from cresylic acid. The contamination has usually
happened when empty barrels or drums, previously used for
hydraulic fluid or lubricating oil storage, have been
reused.
10.1.2 Toxic effects
Accidental human exposure to a single large dose re-
sults in gastrointestinal disturbance varying from slight
to severe nausea and vomiting, accompanied by abdominal
pain and diarrhoea. In the case of exposure to small cumu-
lative doses, "delayed neurotoxicity" gradually proceeds
after a latent period of 3-28 days. In most cases, the
muscle weakness changes rapidly to a striking paralysis of
the lower limbs, with or without an involvement of the
hands. In severe cases, pyramidal signs gradually become
evident. Some neurophysiological studies indicate wide-
spread neurotoxic patterns and prolongation of terminal
latencies with relatively small decreases of motor nerve
conduction velocities. This confirms the evidence of
axonal degeneration, which is the main feature observed in
pathological investigations.
The neurotoxic metabolite of TCP has been identified
as saligenin cyclic o-tolyl phosphate, which is derived
from o-hydroxymethyl metabolites. Thus, it seems that at
least one o-tolyl group among the three phenolic moieties
of TCP is necessary to induce neurotoxic effects. TCP pro-
duced from synthetic cresol, which contains less than 0.1%
of o-cresol, is therefore not neurotoxic.
Subchronic animal studies on TCP derived from syn-
thetic cresol indicate that the target organs are liver
and kidney, but this was not confirmed in the case of
human intoxication. No adequate data are available on
mutagenicity and carcinogenicity. TCP is not toxic to
chick embryos.
10.2 Evaluation of effects on the environment
The measurement of environmental concentrations of TCP
in water has shown only low levels of contamination. This
reflects the low water solubility and ready degradability
of the compound. Since the acute toxicity of TCP to
aquatic organisms is also low, it is unlikely that it
poses a threat to such organisms.
As a consequence of the physico-chemical properties of
TCP, there is a high potential for bioaccumulation. How-
ever, this does not occur in practice, owing to low con-
centrations of TOCP in the environment and living organ-
isms and to its rapid degradation.
TCP bound to sediment accumulates in the environment,
and levels measured in river, estuarine, and marine sedi-
ments have been high. Since there is no information either
on the bioavailability of these residues to burrowing or
bottom-living organisms or on their hazards, the possi-
bility of effects on such species cannot be discounted.
TCP spillage leads to hazard for the local environ-
ment.
10.2.1 Exposure levels
TCP is found in air, surface water, soil, sediment,
and aquatic organisms near heavily industrialized areas,
although concentrations are usually low. Owing to the high
biodegradation rate of TCP in aqueous environment, it is
not considered to affect aquatic organisms adversely. One
report showed an extremely high concentration of total
triaryl phosphate (26.55 g/kg) in a soil sample obtained
from a production plant yard. This suggests the need for
land waste disposal.
10.2.2 Toxic effects
Freshwater algae are relatively sensitive to TCP, the
50% growth inhibitory concentration ranging from 1.5 to
5.0 mg/litre. Among fish species, the rainbow trout is
adversely affected by TCP concentrations below 1 mg/litre
(0.3-0.9 mg/litre), with sign of chronic poisoning, but
the tidewater silverside is more resistant (LC50 is 8700
mg/litre). TCP does not inhibit cholinesterase activity in
fish or frogs, but it has a synergistic effect on organo-
phosphorus insecticide activity.
11. RECOMMENDATIONS
When tri-substituted cresols are used in the synthesis
and manufacture of other compounds, the purified meta and
para isomers should be used in order to avoid the acciden-
tal synthesis of ortho-substituted products.
REFERENCES
ABOU-DONIA, M.B., VARGA, P.A., GRAHAM, D.G., & KINNES, C.G. (1980)
Delayed neurotoxicity of a single dermal administration of o-ethyl
o-4-nitro-phenyl phenylphosphonothioate and tri-o-cresyl phosphate
to cats. Toxicol. Lett., 1 (Special issue): 141.
ABOU-DONIA, M.B., TROFATTER, L.P., GRAHAM, D.G., & LAPADULA, D.M.
(1986) Electromyographic, neuropathologic and functional
correlates in the cat as the result of tri-o-cresyl phosphate
delayed neurotoxicity. Toxicol. appl. Pharmacol., 83(1): 126-141.
ADEMA, D.M.M., KUIPER, J., HANSTVEIT, A.O., & CANTON, H.H. (1983)
Consecutive system of tests for assessment of the effects of
chemical agents in the aquatic environment. In: Pesticide
chemistry-Human welfare and the environment. Proceedings of the
Fifth International Congress on Pesticide Chemistry,Kyoto, Japan,
29 August - 4 September, 1982, Oxford, New York, Pergamon Press,
Vol. 3, pp. 537-544.
ALDRIDGE, W.N. (1954) Tricresyl phosphates and cholinesterase.
Biochem. J., 56: 185-189.
ALDRIDGE, W.N. & BARNES, J.M. (1961) Neurotoxic and biochemical
properties of some triaryl phosphates. Biochem. Pharmacol., 6:
177-188.
ANONYMOUS (1986) Handbook of commodity chemicals, Tokyo, Kagaku-
Kogyo-Nipposha.
ARING, C.D. (1942) The systemic nervous affinity of triorthocresyl
phosphate (Jamaica ginger palsy). Brain, 65: 34-47.
ASSOCIATION OF THE PLASTICIZER INDUSTRY OF JAPAN (1976) Safety of
the plasticizer TCP, tricresyl phosphate, Tokyo, Association of
the Plasticizer Industry of Japan.
BALDRIDGE, H.D., JENDEN, D.J., KNIGHT, C.E., PREZIOSI, T.J., &
TUREMAN, J.R. (1959) Toxicology of a triaryl phosphate Oil. III.
Human exposure in operational use aboard ship. Am Med. Assoc. Arch.
ind. Hyg., 20: 258-261.
BARNARD, P.W.C., BUNTON, C.A., LLEWELLYN, D.R., VERNON, C.A., &
WELCH, V.A. (1961) The reactions of organic phosphates. Part V.
The hydrolysis of triphenyl and trimethyl phosphates. J. Chem. Soc.
(B), 1961: 2670-2676.
BARNARD, P.W.C., BUNTON, C.A., KELLERMAN, D., MHALA, M.M., SILVER,
B., VERNON, C.A., & WELCH, V.A. (1966) Reactions of organic
phosphates. Part VI. The hydrolysis of aryl phosphates. J. Chem.
Soc. (B), 1966: 227-235.
BARRETT, H., BUTLER, R., & WILSON, I.B. (1969) Evidence for a
phosphoryl-enzyme intermediate in alkaline phosphatase catalyzed
reactions. Biochemistry, 8: 1042-1047.
BECK, B.E., WOOD, C.D., & WHENHAM, G.R. (1977) Triaryl phosphate
poisoning in cattle. Vet. Pathol., 14: 128-137.
BELL, J.D. & RUSVINE, J.R. (1967) Synergism of organophosphates in
Musca domestica and Chrysomya putoria. Entomol. exp. appl., 10:
263-269.
BHATTACHARYYA, J., BHATTACHARYYA, K., SENGUPTA, P.K., & GANGULY,
S.K. (1974) Detection and estimation of tricresyl phosphate in
mustard oil. Forensic Sci., 3: 263-270.
BISCHOFF, A. (1967) The ultrastructure of tri-ortho-cresyl
phosphate poisoning. I. Studies on myelin and axonal alterations in
the sciatic nerve. Acta neuropathol., 9: 158-174.
BISCHOFF, A. (1970) The ultrastructure of tri-ortho-cresyl
phosphate poisoning in the chicken. II. Studies on spinal cord
alterations. Acta neuropathol., 15: 142-155.
BISCHOFF, A. (1977) Tri-ortho-cresyl phosphate neurotoxicity. In:
Roisin, L., Shiraki, H., & Grcevic, N., ed. Neurotoxicology, New
York, Raven Press, pp. 431-441.
BLEIBERG, M.J. & JOHNSON, H. (1965) Effects of certain
metabolically active drugs and oximes on tri- o-cresyl phosphate
toxicity. Toxicol. appl. Pharmacol., 7: 227-235.
BLOOM, P.J. (1973) Application des chromatographies sur couche
mince et gaz-liquide à l'analyse qualitative et quantitative des
esters des acides phosphorique et phosphoreux. J. Chromatogr.,
75: 261-269.
BOETHLING, R.S. & COOPER, J.C. (1985) Environmental fate and
effects of triaryl and tri-alkyl/aryl phosphate esters. Residue
Rev., 94: 49-99.
BONDY, H.F., FIELD, E.J., WORDEN, A.N., & HUGHES, J.P.W. A study of
the acute toxicity of the tri-aryl phosphates used as plasticizers.
(1960) Br. J. ind. Med., 17: 190-200.
BOWERS, W.D., PARSONS, M.L., CLEMENT, R.E., EICEMAN, G.A., &
KARASEK, F.W. (1981) Trace impurities in solvents commonly used for
gas chromatographic analysis of environmental samples. J.
Chromatogr., 206: 279-288.
BROWN H.R. & SHARMA, R.P. (1975) Inhibition of neural membrane
adenosine-triphosphatases by organophosphates. Toxicol. appl.
Pharmacol., 33: 140.
BURLEY, B.T. (1930) The 1930 type of polyneuritis. New Engl. J.
Med., 202: 1139-1142.
CARLTON, B.D., HASARAN, A.H., MEZZA, L.E., & SMITH, M.K. (1987)
Examination of the reproductive effects of tricresyl phosphate
administered to Long-Evans rats. Toxicology, 46: 321-328.
CARRINGTON, C.D. & ABOU-DONIA, M.B. (1988) Variation between three
strains of rat: inhibition of neurotoxic esterase and
acetylcholinesterase by tri-o-cresyl phosphate. J. Toxicol.
environ. Health, 25(3): 259-268.
CASIDA, J.E. (1961) Specificity of substituted phenyl phosphorus
compounds for esterase inhibition in mice. Biochem. Pharmacol., 5:
332-342.
CASIDA, J.E., ETO, M., & BARON R.L. (1961) Biological activity of a
tri-o-cresyl phosphate metabolite. Nature (Lond.), 191: 1396-1397.
CASTERLINE, J.L., Jr., KU, Y., & BARNETT, N.M. (1985) Uptake of
tri-p-cresyl phosphate in soybean plants. Bull. environ. Contam.
Toxicol., 35: 209-212.
CAVALLERI, A. & COSI, V. (1978) Polyneuritis incidence in shoe
factory workers: Case report and etiological considerations. Arch.
environ. Health, 33: 192-197.
CAVANAGH, J.B.(1954) The toxic effects of tri-ortho-cresyl
phosphate on the nervous system. An experimental study in hens. J.
Neurol. Neurosurg. Psychiatry, 17: 163-172.
CAVANAGH, J.B. (1964) The significance of the "dying back" process
in experimental and human neurological disease. Int. Rev. exp.
Pathol., 3: 219-267.
CHAPIN, R.E., GEORGE, J.D., & LAMB, J.C., IV (1988) Reproductive
toxicity of tricresyl phosphate in a continuous breeding protocol
in Swiss (CD-1) mice. Fundam. appl. Toxicol., 10: 344-354.
CHAUDHURI, R.N. (1965) Paralytic disease caused by consumption of
flour contaminated with tricresyl phosphate. Trans. R. Soc. Trop.
Med. Hyg., 59: 98-102.
CHAUDHURI, R.N., SEN GUPTA, P.C., CHAKRAVARTI, R.N., MUKHERJEE,
A.M., GHOSH, S.M., CHATTERJEA, J.B., SARKAR, J.K., BASU, S.P.,
ADHYA, R.N., MITRA, N.K., SAHA, T.K., & MITRA, P. (1962) Recent
outbreak of a paralytic disease in Malda, West Bengal. Bull.
Calcutta Sch. Trop. Med., 10: 141-152.
CHEMICAL AND GEOLOGICAL LABORATORIES LTD (1971) Calgary, Alberta,
Chemical and Geological Laboratories (Laboratory report No. E71-
5360).
CLAYTON, G.D. & CLAYTON, F.E., ed. (1981) Patty's industrial
hygiene and toxicology, New York, Wiley-Interscience, Vol. 2A,
pp. 2362-2363.
COHEN, S.D. & MURPHY, S.D. (1970) Comparative potentiation of
malathion by triorthotolyl phosphate in four classes of
vertebrates. Toxicol. appl. Pharmacol., 16: 701-708.
DAFT, J.L. (1982) Identification of aryl/alkyl phosphate residues
in foods. Bull. environ. Contam. Toxicol., 29: 221-227.
DAGLEY, S. & PATEL, M.D. (1957) Oxidation of p-cresol and related
compounds by a Pseudomonas. Biochem. J., 66: 227-233.
DAVISON, A.N. (1953) The cholinesterases of the central nervous
system after the administration of organo-phosphorus compounds.
Brit. J. Pharmacol., 8: 212-216.
DAWSON, G.W., JENNINGS, A.L., DROZDOWSKI, D., & RIDER, E. (1977)
The acute toxicity of 47 industrial chemicals to fresh and salt
water fishes. J. hazard. Mater., 1: 303-318.
DEO, P.G. & HOWARD, P.H. (1978) Combined gas-liquid chromatographic
mass spectrometric analysis of some commercial aryl phosphate oils.
J. Assoc. Off. Anal. Chem., 61: 266-271.
DRUYAN, E.A. (1975) [Separation and determination of tricresyl
phosphate, triphenyl phosphate, phenol, o-, m-, and p-cresol by
thin-layer chromatography.] Gig. i Sanit. , 10: 62-65 (in Russian).
DUKE, A.J. (1978) The significance of isomerism and complexity of
composition on the performance of triaryl phosphate plasticisers in
PVC. Chimia, 32: 457-463.
EAJ (1977) [ Environmental monitoring of chemicals ], Tokyo,
Environment Agency Japan, pp. 212-214 (Environmental Survey Report
Series, No. 3) (in Japanese).
EAJ (1979) [ Chemicals in the environment], Tokyo, Environment
Agency Japan, pp. 61, 78, 92, 93 (Office of Health Studies Report
Series, No. 5) (in Japanese).
EAJ (1981) [ Environmental monitoring of chemicals: Environmental
Survey Report of 1978, 1979 F.Y. ], Tokyo, Environment Agency
Japan, pp. 116-117 (in Japanese).
ELDEFRAWI, A.T., MANSOUR, N.A., BRATTSTEN, L.B., AHRENS, V.D., &
LISK, D.J. (1977) Further toxicologic studies with commercial and
candidate flame retardant chemicals. Part II. Bull. environ.
Contam. Toxicol., 17: 720-726.
ETO, M., CASHIDA, J.E., & ETO, T. (1962) Hydroxylation and
cyclization reactions involved in the metabolism of tri- o-cresyl
phosphate. Biochem. Pharmacol., 11: 337-352.
ETO, M., OSHIMA, Y., & CASHIDA, J.E. (1967) Plasma albumin as a
catalyst in cyclization of diaryl-o-(a-hydroxy)tolyl phosphates.
Biochem. Pharmacol., 16: 295-308.
ETO, M., HASHIMOTO, Y., OZAKI, K., KASAI, T., & SASAKI Y. (1975)
Fungitoxicity and insecticide synergism of monothioquinol phosphate
esters and related compounds. Botyu Kagaku, 40: 110-117.
FINNEGAN, R.A. & MATSON, J.A. (1972) Irradiation of triaryl
phosphate esters. A new photochemical coupling reaction. J. Am.
Chem. Soc., 94: 4780-4782.
FRANCHINI, I., CAVATORTA, A., D'ERRICO, M., DE SANTIS, M., ROMITA,
G., GATTI, R., JUVARRA, G., & PALLA, G. (1978) Studies on the
etiology of the experimental neuropathy from industrial adhesives
(glues). Experientia (Basel), 34: 250-252.
FREEMAN, G.B., IRWIN, R., TREJO, R., HEJTMANCIK, M., RYAN, M., &
PETERS, A.C. (1988) Reversibility and tolerance to tricresyl
phosphate-induced neurotoxic effects in F344 rats. Toxicologist,
8: 76.
FUKUSHIMA, M. & KAWAI, S. (1986) [Present status and transition of
selected organophosphoric acid triesters in the water area of Osaka
city.] Seitai Kagaku, 8: 13-24 (in Japanese).
GARTNER, W. & ELSAESSER, K.H. (1943) [Commercial ortho-
tricresylphosphate poisoning.] Arch. Gewerbepathol. Gewerbehyg.,
12: 1-9 (in German).
GEOFFROY, H., SLOMIC, A., BENEBADJI, M., & PASCAL, P. (1960)
Myèlopoly-néurites tri-crésyl phosphatées. Toxi-épidémie marocaine
de septembre-octobre 1959. World Neurol., 1: 294-315.
GOLDFAIN, E. (1930) Jamaica ginger multiple neuritis. J. Oklahoma
State Med. Assoc., 23: 191-193.
GOLDSTEIN, D.A., MCGUIGAN, M.A., & RIPLEY, B.D. (1988) Acute
tricresyl phosphate intoxication in childhood. Hum. Toxicol., 7:
179-182.
GOODALE, R.H. & HUMPHREYS, M.B. (1931) Jamaica ginger paralysis.
Autopsy observations. J. Am. Med. Assoc., 96: 14-16.
GROSS, E. & GROSSE, A. (1932) [A contribution to the toxicology of
ortho-tricresylphosphates.] Arch. exp. Pathol. Pharmakol., 168:
473-514 (in German).
HAGGERTY, G.J., HEJTMANCIK, M., DESKIN, R., RYAN, M.J., PETERS,
A.C., & IRWIN, R. (1986) Effects of subchronic exposure to
tricresyl phosphate in F344 rats and B6C3F1 mice: behavioural and
morphological correlates. Toxicologist, 6: 218.
HATTORI, Y., ISHITANI, H., KUGE, Y., & NAKAMOTO, M. (1981)
[Environmental fate of organic phosphate esters.] Shuishitu Odaku
Kenkyu, 4: 137-141 (in Japanese).
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, N., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HINE, C.H., DUNLAP, M.K., RICE, E.G., COURSEY, M.M., GROSS, R.M., &
ANDERSON, H.H. (1956) The neurotoxicity and anticholinesterase
properties of some substituted phenyl phosphates. J. Pharmacol.
exp. Ther., 116: 227-236.
HINE, C., ROWE, V.K., WHITE, E.R., DARMER, K.I., Jr, & YOUNGBLOOD,
G.T. (1981) In: Clayton, G.D. & Clayton, F.E., ed. Patty's
industrial hygiene and toxicology, 3rd revised ed., New York,
Wiley-Interscience, Vol. 2A, pp. 2362-2363.
HJORTH, N. (1962) Contact dermatitis from cellulose acetate film.
Cross-sensitization between tricresylphosphate (TCP) and
triphenylphosphate (TPP). Contact dermatitis, 2: 86-100.
HODGE, H.C. & STERNER, J.H. (1943) The skin absorption of
triorthocresyl phosphate as shown by radioactive phosphorus. J.
Pharmacol. exp. Ther., 79: 225-234.
HOLLIFIELD, H.C. (1979) Rapid nephelometric estimate of water
solubility of highly insoluble organic chemicals of environmental
interest. Bull. environ. Contam. Toxicol., 23: 579-586.
HOTSTON, R.D. (1946) Outbreak of polyneuritis due to orthotricresyl
phosphate poisoning. Lancet, 1: 207.
HOWARD, P.H. & DEO, P.G. (1979) Degradation of aryl phosphates in
aquatic environments. Bull. environ. Contam. Toxicol., 22: 337-344.
HUDEC, T., THEAN, J., KUEHL, D., & DOUDHERTY, R.C. (1981) Tris(di-
chloropropyl)phosphate, a mutagenic flame retardant: Frequent
occurrence in human seminal plasma. Science, 211: 951-952.
HUNTER, D., PERRY, K.M.A., & EVANS, R.B. (1944) Toxic polyneuritis
arising during the manufacture of tricresyl phosphate. Br. J. ind.
Med., 1: 227-231.
INDEN, T. & TACHIBANA, S. (1975) [Damage of crops by gases from the
plastic materials under covering conditions.] Bull. Fac. Agric. Mie
Univ., 1975: 1-10 (in Japanese).
INOUE, N., FUJISHIRO, K., MORI, K., & MATSUOKA, M. (1988)
Triorthocresyl phosphate poisoning: A review of human cases.
Sangyo-Ika-Daigaku-Zasshi, 10(4): 433-442.
IRWIN, R., FREEMAN, G.B., TREJO, R., HEJTMANCIK, M., & PETERS, A.
(1987) Effects of chronic exposure to tricresyl phosphate in F344
rats and B6C3F1 mice: behavioural and biochemical correlates. Soc.
Neurosci. Abstr., 13: 90.
ISHIKAWA, S., TAKETOMI, M., & SHINOHARA, R. (1985) Determination of
trialkyl and triaryl phosphates in environmental samples. Water
Res., 19: 119-125.
IZMEROV, N.F., SANOTSKY, I.V., & SIDOROV, K.K. (1982) Toxicometric
parameters of industrial toxic chemicals under single exposure,
Moscow, Centre of International Projects, GKNT, p. 114.
JETER, H. (1930) Autopsy report of a case of so-called "Jake
Paralysis". J. Am. Med. Assoc., 95: 112-113.
JOHANSSEN, F.R., WRIGHT, P.L., GORDON, D.E., LEVINSKAS, G.J.,
RADUE, R.W., & GRAHAM, P.R. (1977) Evaluation of delayed
neurotoxicity and dose-response relationships of phosphate esters
in the adult hen. Toxicol. appl. Pharmacol., 41: 291-304.
JOHNSON, M.K. (1969) Delayed neurotoxic action of some
organophosphorus compounds. Br. med. Bull., 25: 231-235.
JOHNSON, M.K. (1974) The primary biochemical lesion leading to the
delayed neurotoxicity of some organophosphorus esters. J.
Neurochem., 23: 785-789.
JOHNSON, M.K. (1975a) Organophosphorus esters causing neurotoxic
effects. Mechanism of action and structure/activity studies. Arch.
Toxicol., 34: 259-288.
JOHNSON, M.K. (1975b) The delayed neuropathy caused by some
organophosphorus esters: mechanism and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. & BARNES, J.M. (1970) The sensitivity of chicks to
the delayed neurotoxic effects of some organophosphorous compounds.
Biochem. Pharmacol., 19: 3045.
KANAZAWA, J. (1978) Studies on formulation and residue analysis of
pesticides. J. pestic. Sci., 3: 185-193.
KAWAI, S., FUKUSHIMA, M., ODA, K., & UNO, G. (1978) [Water
pollution caused by organophosphorus compounds.] Kankyo Gijyutsu,
7: 668-675 (in Japanese).
KENMOTSU, K., SAITO, N., OGINO, N., & MATSUNAGA, K. (1979) [An
environ-mental survey of chemicals. XI. Analytical methods of
organic phosphoric acid triesters.] Okayama-ken Kankyo Hoken Senta
Nempo, 3: 175-191 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980a) [Multiresidue
determination of phosphoric acid triesters in fish, sea sediment
and sea water.] J. Food Hyg. Soc. Jpn., 21: 18-31 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1980b) [Studies on the
mechanisms of biological activities of various environmental
pollutants. V. Environmental fate of organic phosphoric acid
triesters.] Okayama-ken Kankyo Hoken Senta Nempo, 4: 103-110 (in
Japanese).
KENMOTSU, K., MATSUNAGA, K., & ISHIDA, T. (1981a) [Studies on the
biological toxicity of several pollutants in environments.]
Okayama-ken Kankyo Hoken Senta Nempo, 5: 167-175 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., & OGINO, Y. (1981b) [An
environmental survey of chemicals. XVII. Multiresidue determination
of organic phosphate esters in environment samples.] Okayama-ken
Kankyo Hoken Senta Nempo, 5: 145-156 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982a) [An environmental survey of chemicals. XIX. Determination
of organophosphoric acid triesters (2).] Okayama-ken Kankyo Hoken
Senta Nempo, 6: 126-132 (in Japanese).
KENMOTSU, K., MATSUNAGA, K., SAITO, N., OGINO, Y., & ISHIDA, T.
(1982b) [Studies on the biological toxicity of several pollutants
in environments. VII. GC/MS Spectrometric determination of
organophosphoric acid triesters in sediment.] Okayama-ken Kankyo
Hoken Senta Nempo, 6: 142-152 (in Japanese).
KENMOTSU, K., NAKAGIRI, M., OGINO, Y., MATSUNAGA, K., & ISHIDA, T.
(1983) [An environmental survey of chemicals. XXII. GC/MS
spectrometric determination of organophosphoric acid triesters in
sediment (2).] Okayama-ken Kankyo Hoken Senta Nempo, 7: 143-149 (in
Japanese).
KIMMERLE, G. & LOESER, E. (1974) Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environ. Qual. Saf., 3: 174-178.
KONASEWICH, D., TRAVERSY, W., & ZAR, H. (1978) Status report on
organic and heavy metal contaminants in the Lakes Erie, Michigan,
Huron and Superior basins. Great Lakes Water Qual. Bd.
KRISHNAMURTHY, M.N., RAJALAKSHMI, S., & PRAKASH KAPUR, O. (1985)
Detection of tricresyl phosphates and determination of tri-o-cresyl
phosphate in edible oils. J. Assoc. Off. Anal. Chem., 68(6):
1074-1076.
KU, Y. & ALVAREZ, G.H. (1982) Biodegradation of (14C) tri- p-cresyl
phosphate in a laboratory activated-sludge system. Appl. environ.
Microbiol., 43: 619-622.
KUREBAYASHI, H., TANAKA, A., & YAMAHA, T. (1985) Metabolism and
dispo-sition of the flame retardant plasticizer, tri-p-cresyl
phosphate, in the rat. Toxicol. appl. Pharmacol., 77: 395-404.
LAPP, T.W. (1976) Study on chemical substances from information
concerning the manufacture, distribution, use, disposal,
alternatives, and magnitude of exposure to the environment and
man: Task 1 - The manufacture and use of selected aryl and alkyl
phosphate esters, Springfield, Virginia, National Technical
Information Service, 129 pp (EPA contract No. 68-01-2687) (NTIS PB-
251819).
LEBEL, G.L. & WILLIAMS, D.T. (1983) Determination of organic
phosphate triesters in human adipose tissue. J. Assoc. Off. Anal.
Chem., 66: 691-699.
LEBEL, G.L., WILLIAMS, D.T., GRIFFITH, G., & BENOIT, F.M. (1979)
Isolation and concentration of organophosphorus pesticides from
drinking water at the ng/L level, using macroreticular resin. J.
Assoc. Off. Anal. Chem., 62: 241-249.
LEBEL, G.L., WILLIAMS, D.T., & BENOIT, F.M. (1981) Gas
chromatographic determination of trialkyl/aryl phosphates in
drinking water, following isolation using macroreticular resin. J.
Assoc. Off. Anal. Chem., 64: 991-998.
LEFAUX, R. (1968) Practical toxicology of plastics, London, Iliffe
Books Ltd, p. 580.
LEFAUX, R. (1972) Industrial toxicology. Phosphoric esters. In:
Practical toxicology of plastics, London, Iliffe Books Ltd, pp.
121-127.
LEVEQUE, J. (1983) Tricresyl phosphates. In: Encyclopaedia of
occupational health safety, 3rd ed., Geneva, International Labour
Office, Vol. 2, pp. 2216-2218.
LOCKHART, W.L., WAGEMANN, R., CLAYTON, J.W., GRAHAM, B., & MURRAY,
D. (1975) Chronic toxicity of a synthetic tri-aryl phosphate oil to
fish. Environ. Physiol. Biochem., 5: 361-369.
LOMBARDO, P. & EGRY, I.J. (1979) Identification and gas-liquid
chromatographic determination of aryl phosphate residues in
environmental samples. J. Assoc. Off. Anal. Chem., 62: 47-51.
LOROT, C. (1899) Les combinations de la créosote dans le traitement
de la tuberculose pulmonaire, Paris (Thèse de Paris No. 25).
LU, P.-Y. & METCALF, R.L. (1975) Environmental fate and
biodegradability of benzene derivatives as studied in a model
aquatic ecosystem. Environ. Health Perspect., 10: 269-284.
MAJORS, R.E. & JOHNSON, E.L. ( 1978) High-performance exclusion
chromatography of low-molecular-weight additives. J. Chromatogr.,
167: 17-30.
MARHOLD, J.V. (1972) Collection of results of toxicological
assessments of compounds and formulations tested at the Department
of Toxicology, Research Institute for Organic Syntheses, Prague,
Institute for Management Training in the Chemical Industry.
MAUSNER, M., BENEDICT, J.H., BOOMAN, K.A., BRENNER, T.E., CONWAY,
R.A., DUTHIE, J.R., GARRISON, L.J., HENDRIX, C.D., & SHEWMAKER,
J.E. (1969) The status of biodegradability testing of nonionic
surfactants. J. Am. Oil Chem. Soc., 46: 432-440.
MAYER, F.L. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Springfield, Virginia, National Technical
Information Service.
MAYER, F.L., ADAMS, W.J., FINLEY, M.T., MICHAEL, P.R., MEHRLE,
P.M., & SAEGER, V.W. (1981) Phosphate ester hydraulic fluids: An
aquatic environmental assessment of Pydrauls 50E and 115E. In:
Branson R. & Dickson, K.L., ed. Aquatic Toxicology and Hazard
Assessment: Fourth Conference, Philadelphia, American Society for
Testing and Materials, pp. 103-123 (ASTM STP 737).
MELE, J.M. & JENSH, R.P. (1977) Teratogenic effects of orally
administered tri-o-cresyl phosphate on Wistar albino rats.
Teratology, 15: 32A.
MERRITT, H.H. & MOOR, M. (1930) Peripheral neuritis associated with
ginger extract ingestion. New Engl. J. Med., 203: 4-12.
MERTENS, H.G. (1948) [Clinical aspects of tri-orthocrésyl phosphate
poisoning (including a report on several cases of poisoning in an
Igelit factory).] Arch. Psychiatr. Nervenkr., 179: 458-482
(in German).
MRI (1979) Assessment of the need for limitation on triaryl and
trialkyl/aryl phosphates. Draft final report, Kansas City, Midwest
Research Institute (EPA Contract 68-01-4313).
MODERN PLASTICS ENCYCLOPEDIA (1975) International Advertising
Supplement 52(10A), p 697, New York, McGraw-Hill Inc.
MORAZAIN, R. & ROSENBERG, P. (1970) Lipid changes in tri-o-cresyl-
phosphate-induced neuropathy. Toxicol. appl. Pharmacol., 16: 461-474.
MORGAN, A.A. & HUGHES, J.P.W. (1981) An investigation into the
value of cholinesterase estimations of workers in a plant
manufacturing tri-aryl phosphate plasticizers. J. Soc. Occup. Med.,
31: 69-75.
MORGAN, J.P. (1982) The Jamaica Ginger Paralysis. J. Am. Med.
Assoc., 248: 1864-1867.
MORGAN, J.P. & PENOVICH, P. (1978) Jamaica Ginger Paralysis. Forty-
seven-year follow-up. Arch. Neurol., 35: 530-532.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988a) Evaluation of rodent sperm, vaginal
cytology, and reproductive organ weight data from National
Toxicology Program 13-week study. Fundam. appl. Toxicol., 11:
343-358.
MORRISSEY, R.E., SCHWETZ, B.A., LAMB, J.C., IV, ROSS, M.D., TEAGUE,
J.L., & MORRIS, R.W. (1988b) Association of sperm, vaginal
cytology, and reproductive organ weight data with results of
continuous breeding reproductive studies in Swiss CD-1 mice.
Fundam. appl. Toxicol., 11: 359-371.
MUIR, D.C.G. (1984) Phosphate esters. In: Hutzinger, O., ed. The
handbook of environmental chemistry, Berlin, Heidelberg, New York,
Tokyo, Springer-Verlag, Vol. 3, Part C, pp. 41-66.
MUIR, D.C.G. & GRIFT, N.P. (1983) Extraction and cleanup procedures
for determination of diarylphosphates in fish, sediment, and water
samples. J. Assoc. Anal. Off. Chem., 66: 684-690.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1980) Determination of
several triarylphosphates in fish and sediment samples. Can. Plains
Proc., 9: 1-12.
MUIR, D.C.G., GRIFT, N.P., & SOLOMON, J. (1981) Extraction and
cleanup of fish, sediment, and water for determination of triaryl
phosphates by gas-liquid chromatography. J. Assoc. Off. Anal.
Chem., 64: 79-84.
MUIR, D.C.G., GRIFT, N.P., & LOCKHART, W.L. (1982) Comparison of
laboratory and field results for prediction of the environmental
behavior of phosphate esters. Environ. Technol. Chem., 1: 113-119.
MUIR, D.C.G., YARECHEWSKI, A.L., & GRIFT, N.P. (1983) Environmental
dynamics of phosphate esters. III. Comparison of the
bioconcentration of four triaryl phosphates by fish. Chemosphere,
12: 155-166.
MURRAY, D.A.J. (1975) Analysis of tri-aryl phosphate esters and the
determination of IMOL S-140 in fish tissue and water samples by gas
chromatography. J. Fish Res. Board Can., 32: 457-460.
MYERS, D.K., REBEL, J.B.J., VEEGER, C., KEMP, A., & SIMONS, E.G.L.
(1955) Metabolism of triaryl phosphates in rodents. Nature (Lond.),
176: 259-260.
NEELY, W.B., BRANSON, D.R., & BLAU, G.E. (1974) Partition
coefficient to measure the bioconcentration potential of organic
chemicals in fish. Environ. Sci. Technol., 8: 1113-1115.
NEVINS, M.J. & JOHNSON, W.W. (1978) Acute toxicity of phosphate
ester mixtures to invertebrates and fish. Bull. environ. Contam.
Toxicol., 19: 250-256.
NICHOLSON, S.S. (1974) Bovine posterior paralysis due to
organophosphate poisoning. J. Am. Vet. Med. Assoc., 165: 280-281.
NOBILE, E.R., PAGE, S.W., & LOMBARDO, P. (1980) Characterization of
four commercial flame retardant aryl phosphates. Bull. environ.
Contam. Toxicol., 25: 755-761.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1983) High-performance liquid
chromato-graphic analysis on radial compression column of the
neurotoxic tri-o-cresyl phosphate and metabolites. Anal. Biochem.,
135: 296-303.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1984) Disposition of (14C)tri-o-
cresyl phosphate and its metabolites in various tissues of the male
cat following a single dermal application. Drug Metab. Disp., 12
(6): 705-711.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986a) Studies on the metabolism
of the neurotoxic tri-o-cresyl phosphate synthesis and
identification by infrared, proton nuclear magnetic resonance and
mass spectrometry of five of its metabolites. Toxicology, 38: 1-13.
NOMEIR, A.A. & ABOU-DONIA, M.B. (1986b) Studies on the metabolism
of the neurotoxic tri- o-cresyl phosphate, distribution, excretion,
and metabolism in male cats after a single, dermal application.
Toxicology, 38: 15-33.
OECD (1981) OECD guidelines for testing of chemicals. Section 2:
Effects on biotic systems, Paris, Organization for Economic
Cooperation and Development, Publications Office.
OFSTAD, E.B. & SLETTEN, T. (1985) Composition and water solubility
determination of a commercial tricresylphosphate. Sci. total
Environ., 43: 233-241.
OISHI, H., OISHI, S., & HIRAGA, K. (1982) Toxicity of several
phosphoric acid esters in rats. Toxicol. Lett., 13: 29-34.
OLSON, B.A. & BURSIAN, S.J. (1988) Effect of route of
administration on the development of organophosphate-induced
delayed neurotoxicity in 4-week-old chicks. J. Toxicol. environ.
Health, 23(4): 499-505.
PACIOREK, K.J.L., KRATZER, R.H., KAUFMAN, J., NAKAHARA, J.H.,
CHRISTOS, T., & HARTSTEIN, A.M. (1978) Thermal oxidative
degradation studies of phosphate esters. Am. Ind. Hyg. Assoc. J.,
39: 633-639.
PADILLA, S. & VERONESI, B. (1985) The relationship between
neurological damage and neurotoxic esterase inhibition in rats
acutely exposed to tri-ortho-cresyl phosphate. Toxicol. appl.
Pharmacol., 78(1): 78-87.
PARNITZKE, K.H. (1946) [On the recent accumulation of ortho-
tricresylphosphate poisoning (sources and clinical course).] Dtsch.
Gesundheitswes., 1: 666-670 (in German).
PEGUM, J.S. (1966) Contact dermatitis from plastics containing tri-
aryl phosphates. Br. J. Dermatol., 78: 626-631.
PENMAN, D.R & OSBORNE, G.O. (1976) Trialkyl phosphates and related
compounds as antifertility agents of the twospotted spider mite. J.
econ. Entomol., 69(2): 266-268.
PICKARD, M.A., WHELIHAN, J.A., & WESTLAKE, D.W.S. (1975)
Utilization of triaryl phosphates by a mixed bacterial population.
Can. J. Microbiol., 21: 140-145.
PLAPP, F.W., Jr & TONG, H.H.C. (1966) Synergism of malathion and
parathion against resistant insects: Phosphorus esters with
synergistic properties. J. econ. Entomol., 59(1): 11-15.
PRENTICE, D.E. & MAJEED, S.K. (1983) A subchronic study (90 days)
using multiple dose levels of tri-ortho-cresyl phosphate (TOCP):
some neuropathological observations in the domestic hen.
Neurotoxicology, 4(2): 277-282.
PRINEAS, J.S. (1969) The pathogenesis of dying-back
polyneuropathies. Part I. An ultrastructural study of experimental
tri-ortho-cresyl phosphate intoxication in the cat. J. Neuropathol.
exp. Neurol., 28: 571-597.
QUINSTAD, G.B., STAIGER, L.E., & SCHOOLEY, D.A. (1975)
Environmental degradation of the insect growth regulator
methoprene. V. Metabolism by house-flies and mosquitoes. Pestic.
Biochem. Physiol., 5: 233-241.
RAMSEY, J.D. & LEE, T.D. (1980) Gas-liquid chromatographic
retention indices of 296 non-drug substances on SE-30 or OV-1
likely to be encountered in toxicological analyses. J. Chromatogr.,
184: 185-206.
RENBERG, L., SUNDSTROM, G., & SUNDH-NYGARD, K. (1980) Partition
coefficients of organic chemicals derived from reversed phase thin
layer chromatography. Evaluation of methods and application on
phosphate esters, polychlorinated paraffins and some PCB-
substitutes. Chemosphere, 9: 683-691.
ROBERTS, N.L., FAIRLEY, C., & PHILLIPS, C. (1983) Measurements and
evaluation of clinical signs following use of the hen as the animal
model for screening acute delayed and subchronic neurotoxicity
studies. Neurotoxicology, 4(2): 263-270.
ROBERTSON, D.G., SCHWAB, B.W., SILLS, R.D., RICHARDSON, R.J., &
ANDERSON, R.J. (1987) Electrophysiologic changes following
treatment with organophosphorus-induced delayed neuropathy-
producing agents in the adult hen. Toxicol. appl. Pharmacol., 87:
420-429.
ROGER, H. & RECORDIER, M. (1934) Les polynéurites
phosphocréosotiques (phosphate de créosote, ginger paralysis,
apiol). Ann. Med., 35: 44-63.
SAEGER, V.W., HICKS, O., KALEY, R.G., MICHAEL, P.R., MIEURE, J.P.,
& TUCKER, E.S. (1979) Environmental fate of selected phosphate
esters. Environ. Sci. Technol., 13: 840-844.
SAITO, C., KATO, T., TANIGUCHI, H., FUJITA, T., WADA, H., & MORI,
Y. (1974) [Subacute toxicity of tricresylphosphate (TCP) in rats.]
Pharmacometrics, 8: 107-118 (in Japanese).
SAMPSON, B.F. (1942) The strange Durban epidemic of 1937. S. Afr.
med. J., 16: 1-9.
SASAKI, K., SUZUKI, T., TAKEDA, M., & UCHIYAMA, M. (1982)
Bioconcentration and excretion of phosphoric acid triesters by
Killifish (Oryzeas latipes). Bull. environ. Contam. Toxicol., 28:
752-759.
SCHWAB, H. (1948) [Psychic disturbance by triorthocresylphosphate
poisoning.] Dtsch. Med. Wochenschr., 73: 124-125 (in German).
SENANAYAKE, N. (1981) Tri-cresyl phosphate neuropathy in Sri Lanka:
a clinical and neurophysiological study with a three year follow
up. J. Neurol. Neurosurg. Psychiatry, 44: 775-780.
SENANAYAKE, N. & JEYARATNAM, J. (1981) Toxic polyneuropathy due to
gingili oil contaminated with tri-cresyl phosphate affecting
adolescent girls in Sri Lanka. Lancet, 10 January: 88-89.
SHARMA, R.P. & WATANABE, P.G. (1974) Time related disposition of
tri-o-tolyl phosphate (TOTP) and metabolites in chicken. Pharmacol.
Res. Commun., 6(5): 475-484.
SHELDON, L.S. & HITES, R.A. (1978) Organic compounds in the
Delaware River. Environ. Sci. Technol., 12: 1188-1194.
SHELDON, L.S. & HITES, R.A. (1979) Sources and movement of organic
chemicals in the Delaware River. Environ. Sci. Technol., 13: 574-579.
SITTHICHAIKASEM, S. (1978) Some toxicological effects of phosphate
esters on rainbow trout and bluegill, Iowa State University (Ph. D.
Thesis).
SMITH, H.V. & SPALDING, J.M.K. (1959) Outbreak of paralysis in
Morocco due to ortho-cresyl phosphate poisoning. Lancet, 2:
1019-1021.
SMITH, M.I. & LILLIE, R.D. (1931) The histopathology of
triorthocresyl phosphate poisoning. Arch. Neurol Psychiatry, 26:
976-992.
SMITH, M.I., ELVOVE, E., & FRAZIER, W.H. (1930) The pharmacological
action of certain phenol esters, with special reference to the
etiolgy of so-called ginger paralysis. Public Health Rep., 45:
2509-2524.
SMITH, M.I., ENGEL, E.W., & STOHLMAN, F.F. (1932) Further studies
on the pharmacology of certain phenol esters with special reference
to the relation of chemical constitution and physiologic action.
Natl. Inst. Health Bull., 160: 1-53.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987a) Reproductive tract lesions resulting from
subchronic administration (63 days) of tri-o-cresyl phosphate in
male rats. Toxicol. appl. Pharmacol., 89: 49-63.
SOMKUTI, S.C., LAPADULA, D.M., CHAPIN, R.E., LAMB, J.C., IV, &
ABOU-DONIA, M.B. (1987b) Time course of the tri-o-cresyl phosphate-
induced testicular lesion in F-344 rats: enzymatic, hormonal, and
sperm parameter studies. Toxicol. appl. Pharmacol., 89: 64-72.
SOMKUTI, S.G., LAPADULA, D.M., CHAPIN, R.E., & ABOU-DONIA, M.B.
(1987c) Testicular toxicity following oral administration of tri-o-
cresyl phosphate (TOCP) in roosters. Toxicol. Lett., 37: 279-290.
SOMKUTI, S.G., TILSON, H.A., BROWN, H.R., CAMPBELL, G.A.,
LAPADULA, D.M., & ABOU-DONIA, M.B. (1988) Lack of delayed
neurotoxic effect after tri-ortho-cresyl phosphate treatment in
male Fischer-344 rats: bio-chemical, neurobehavioural, and
neuropathological studies. Fundam. appl. Toxicol., 10: 199-205.
SOROKIN, M. (1969) Orthocresyl phosphate neuropathy: Report of an
outbreak in Fiji. Med. J. Aust., 1: 506-508.
SPOERRI, P.E. & GLEES, P. (1979) Ultrastructural reactions of
spinal ganglia to tri-ortho-cresyl phosphate: Effects of
neurotoxicity. Cell Tissue Res., 199: 409-414.
SPOERRI, P.E. & GLEES, P. (1980) Ultrastructural changes in neurons
and neuroglia of the avian telencephalon (Hyperstriatum
Accessorium) following tri-ortho-cresyl phosphate intoxication.
Cell Tissue Res., 206: 203-210.
STAEHELIN, R. (1941) [On the triorthocresylphosphate poisonings.]
Schweiz. Med. Wochenschr., 71: 1-5 (in German).
STUMPF, A.M., TANAKA, D.JR., AULERIOH, A.J., & BURSIAN, S.J. (1989)
Delayed neurotoxic effects of tri-o-tolyl phosphate in the European
ferret. J. Toxicol. environ. Health, 26: 61-73.
STURM, R.N. (1973) Biodegradability of nonionic surfactants:
Screening test for predicting rate and ultimate biodegradation. J.
Am. Oil Chem. Soc., 50: 159-167.
SUGDEN, E.A., GREENHALGH, R., & PETTIT, J.R. (1980)
Characterization of delayed neurotoxic triaryl phosphates by
analysis of trifluoroacetylated phenolic moieties. Environ. Sci.
Technol., 14(12): 1498-1501.
SUSSER, M. & STEIN, Z. (1957) An outbreak of tri-ortho-cresyl
phosphate (TOCP) poisoning in Durban. Br. J. ind. Med., 14:
111-120.
SVENNILSON, E. (1960) Studies of triorthocresyl phosphate
neuropathy, Morocco 1960. Acta psychiatr. Scand., 150(Suppl):
334-336.
TABERSHAW, I.R., KLEINFELD, M., & FEINER, B. (1957) Manufacture of
tricresyl phosphate and other alkyl phenyl phosphates: An
industrial hygiene study. Am. Med. Assoc. Arch. ind. Health, 15:
537-540.
TAYLOR, J.D. & BUTTAR, H.S. (1967) Evidence for the presence of 2-
(o-cresyl)-4 H-1:3:2-benzodioxaphosphoran-2-one in cat intestine
following tri-o-cresyl phosphate administration. Toxicol. appl.
Pharmacol., 11: 529-537.
TER BRAAK, J.W.G. & CARRILLO, R. (1932) [Polyneuritis after usage
of an abortifacient (tri-ortho-cresyl-phosphate-poisoning).] Dtsch.
Z. Nervenheilkd., 125: 86-116 (in German).
THOMPSON, J.E. & DUTHIE, J.R. (1968) The biodegradability and
treatability of NTA. J. Water Pollut. Control Fed., 40: 306-319.
TITTARELLI, P. & MASCHERPA, A. (1981) Liquid chromatography with
graphite furnace atomic absorption spectrophotometric detector for
speciation of organophosphorus compounds. Anal. Chem., 53: 1466-1469.
TOCCO, D.R., RANDALL, J.L., YORK, R.G., & SMITH, M.K. (1987)
Evaluation of the teratogenic effects of tri-ortho-cresyl phosphate
in the Long-Evans hooded rat. Fundam. appl. Toxicol., 8(3): 291-297.
US NIOSH (1977) NIOSH manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, Vol. 3, P & CAM, pp. S/209-S/210 (DHEW (NIOSH) Publication
No. 7-157C).
US NIOSH (1979) Industrial hygiene walk-through survey report on
organophosphorus exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS PB-82-216268.
US NIOSH (1980) Industrial hygiene walk-through survey report on
organophosphorus exposures at Rochester products division,
General Motors Corp., 1000 Lexington Avenue, Rochester, New York,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (PB82-104530, NTIS).
US NIOSH (1982) Industrial hygiene walk-through survey report on
organophosphorous exposures at Chevron Chemical, Belle Chase,
Louisiana, Cincinnati, Ohio, National Institute for Occupational
Safety and Health, Division of Surveillance, Hazard Evaluations and
Field Studies (Report No. IWA-89.10).
US SOAP AND DETERGENT ASSOCIATION (1965) A procedure and standards
for the determination of the biodegradability of alkyl benzene
sulfonate and linear alkylate sulfonate (Report of the Subcommittee
on Biodegradation Test Methods). J. Am. Oil Chem. Soc., 42: 986-993.
VASILESCU, C. & FLORESCU, A. (1980) Clinical and
electrophysiological study of neuropathy after organophosphorus
compound poisoning. Arch. Toxicol., 43: 305-315.
VASWANI, M., MAHAJAN, P.M., SETIA, R.C., & BHIDE, N.K. (1983) A
simple colorimetric method for estimation of tricresyl phosphate in
edible oils. J. Oil Technol. Assoc. India, 15(1): 12-13.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring and
estimating the bioconcentration factor of chemicals in fish. J.
Fish Res. Board Can., 36: 1040-1048.
VERONESI, B. (1984) A rodent-model of organophosphorus-induced
delayed neuropathy: distribution of central (spinal cord) and
peripheral nerve damage. Neuropathol. appl. Neurobiol., 10(6):
357-368.
VERONESI, B. & ABOU-DONIA, M.B. (1982) Central and peripheral
neuropathology induced in rats by tri-o-cresyl phosphate (TOCP).
Vet. hum. Toxicol., 24: 3 (abstract).
VERONESI, B., NEWLAND, D., & INMAN, A. (1984) The effect of
metabolic interference on rodent-sensitivity to tri-ortho-cresyl
phosphate. Toxicologist, 4: 55.
VICK, R.D., JUNK, G.A., AVERY, M.J., RICHARD, J.J., & SVEC, H.J.
(1978) Organic emissions from combustion of combination coal/refuse
to produce electricity. Chemosphere, 7: 893-902.
VONDERAHE, A.R. (1931) Pathologic changes in paralysis caused by
drinking Jamaica Ginger. Arch. Neurol. Psychiatry, 25: 29-43.
VORA, D.D., DASTUR, D.K., BRAGANCA, B.M., PARIHAR, L.M., IYER,
C.G.S., FONDEKAR, R.B., & PRABHAKARAN, K. (1962) Toxic polyneuritis
in Bombay due to ortho-cresyl-phosphate poisoning. J. Neurol.
Neurosurg. Psychiatry, 25: 234-242.
WAGEMANN, R. (1975) Some environmental and toxicological aspects of
a tri-aryl phosphate synthetic oil. Verh. Int. Ver. Limnol., 19:
2178-2184.
WAGEMANN, R., GRAHAM, R., & LOCKHART, W.L. (1974) Studies on
chemical degradation and fish toxicity of a synthetic tri-aryl
phosphate lubrication oil, IMOL S-140. Ottawa, Environment Canada,
Fisheries and Marine Service, (Technical Report, No. 486).
WAKABAYASHI, A. (1980) [Environmental pollution caused by
organophosphoric fire-proofing plasticizers.] Annu. Rep. Tokyo
Metrop. Res. Inst. Environ. Prot., 11: 110-113 (in Japanese).
WALTHARD, K.M. (1945) Aperçu des résultats obtenus lors des
derniers examens des malades intoxiqués en 1940 par le phosphate
triorthocrésilique. Schweiz. Arch. Neurol. Psychiatry, 58: 189-194.
WIGHTMAN, R.H. & MALAIYANDI, M. (1983) Physical properties of some
synthetic trialkyl/aryl phosphate commonly found in environmental
samples. Environ. Sci. Technol., 17: 256-261.
WILLIAMS, D.T. & LEBEL, G.L. (1981) A national survey of
tri(haloalkyl)-, trialkyl-, and triarylphosphates in Canadian
drinking water. Bull. environ. Contam. Toxicol., 27: 450-457.
WILLIAMS, D.T., NESTMANN, E.R., LEBEL, G.L., BENOIT, F.M., & OTSON,
R. (1982) Determination of mutagenic potential and organic
contaminants of Great Lakes drinking water. Chemosphere, 11: 263-276.
WILSON, R.D., ROWE, L.D., LOVERING, S.L., & WITZEL, D.A. (1982)
Acute toxicity of tri-ortho-cresyl phosphate in sheep and swine.
Am. J. vet. Res., 43(11): 1954-1957.
WINDHOLZ, M., ed. (1983) The Merck index, 10th ed., Rahway, New
Jersey, Merck and Co., Inc.
WOLFE, N.L. (1980) Organophosphate and organophosphorothionate
esters: Application of linear free energy relationships to estimate
hydrolysis rate constants for use in environmental fate assessment.
Chemosphere, 9: 571-579.
WONG, P.T.S. & CHAU, Y.K. (1984) Structure-toxicity of triaryl
phosphates in freshwater algae. Sci. total Environ., 32(2): 157-165.
YASUDA, H. (1980) [Concentration of organic phosphorus pesticides
in the atmosphere above the Dogo plain and Ozu basin.] J. Chem.
Soc. Jpn, 1980(4): 645-653 (in Japanese).
ZELIGS, M.A. (1938) Upper motor neuron sequelae in "Jake" paralysis
: A clinical follow up study. J. nerv. ment. Dis., 87: 464-470.
ZITKO, V. (1980) Proceedings of the 6th Annual Aquatic Toxicity
Workshop. Can. Tech. Rep. fish aquat. Sci., 575: 234-265.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le phosphate de tricrésyle (TCP) est un liquide
visqueux, ininflammable, inexplosible et incolore. Son
coefficient de partage entre l'octanol et l'eau (log de
Pow) est égal à 5,1. Il s'hydrolyse facilement en
milieu alcalin pour donner du phosphate de dicrésyle, mais
il est stable en milieu neutre ou acide à température
normale.
Du point de vue analytique, la méthode de choix est la
chromatographie en phase gazeuse avec détection par un
dispositif sensible à l'azote/phosphore ou par photométrie
de flamme. La limite de détection dans les échantillons
aqueux est d'environ 1 mg/litre. Le TCP s'extrait facile-
ment des solutions aqueuses au moyen de divers solvants
organiques. Pour la purification, on emploie habituelle-
ment une colonne chromatographique de Florisil, mais il
est difficile de séparer le TCP des lipides par cette
méthode. D'autres méthodes de purification ont été re-
commandées à cette fin (chromatographie en phase gazeuse,
chromatographie sur charbon activé ou Sep-pack C-18). Les
réactifs analytiques sont souvent contaminés par des
traces de TCP en raison de la très large utilisation faite
de ce produit. Aussi faut-il prendre certaines précautions
si l'on veut que l'analyse des traces de TCP soit fiable.
2. Sources d'exposition humaine et environnementale
Le TCP est généralement produit par réaction des cré-
sols sur l'oxychlorure de phosphore. Il y a deux sources
de production industrielle de crésols: l'"acide crésy-
lique", résidu des fours à coke et du raffinage du
pétrole, et les "crésols de synthèse" préparés par oxy-
dation et dégradation du cymène. Le TCP est donc un
mélange de divers phosphates de triaryle.
Le TCP est utilisé comme plastifiant des matières
plastiques vinyliques, comme retardateur d'inflammation,
comme additif pour les lubrifiants à très haute pression
et comme liquide ininflammable dans les systèmes hydrau-
liques.
3. Transport, distribution et transformation dans l'environnement
La libération de TCP dans l'environnement est peu
importante au cours de la production et se produit essen-
tiellement lors de l'utilisation finale du produit. On
estime qu'en 1977, 32 800 tonnes en ont été libérées au
total dans l'atmosphère aux Etats-Unis.
En raison de sa faible solubilité dans l'eau et de sa
forte adsorption aux particules, le TCP s'adsorbe rapide-
ment aux sédiments des rivières et des lacs et aux parti-
cules du sol. Il est rapidement biodégradé en milieu
aquatique, sa décomposition étant pratiquement complète
dans les cours d'eau en l'espace de cinq jours. L'isomère
ortho se décompose légèrement plus vite que les isomères
méta et para. Le TCP est facilement biodégradé dans les
boues d'égouts, avec une demi-vie de 7,5 heures, la dégra-
dation atteignant 99% en l'espace de 24 heures. La dégra-
dation abiotique est plus lente, puisque dans ce cas la
demi-vie est de 96 jours.
Des facteurs de bioconcentration de 165-2768 ont été
mesurés en laboratoire sur plusieurs espèces de poissons à
l'aide de TCP radio-marqué. La radioactivité a rapidement
disparu après cessation de l'exposition, la demi-vie de
dépuration allant de 25,8 à 90 heures.
4. Niveaux dans l'environnement et exposition humaine
On a relevé dans l'air des concentration de TCP allant
jusqu'à 70 ng/m3 au Japon, les concentrations maximales
dans une unité de production des Etats-Unis d'Amérique
n'étant que de 2 ng/m3. Dans un atelier de remplissage
de fûts d'huile lubrifiante, aux Etats-Unis d'Amérique, on
a constaté que l'air ne contenait que 0,8 mg/m3 de TCP,
la concentration ne dépassant pas 0,15 mg/m3 en phos-
phates totaux, dans une unité de moulage de zinc d'une
usine d'automobiles. Les concentrations mesurées dans
l'eau de boisson au Canada se sont révélées faibles (0,4 à
4,3 ng/litre) et le TCP n'a pas pu être décelé dans l'eau
des puits. Dans les rivières et les lacs, les concen-
trations sont souvent beaucoup plus élevées. Toutefois on
peut attribuer cet état de choses à la présence de sédi-
ments en suspension auxquels le TCP est fortement adsorbé.
Les concentrations sédimentaires sont plus élevées
puisqu'elles peuvent atteindre 1300 ng/g dans le sédiments
de cours d'eau et 2160 ng/g par les sédiments marins.
Des concentrations élevées ont été mesurées dans le
sol et la végétation aux alentours d'unités de production.
On a fait état de résidus dans des poissons et des
fruits de mer allant jusqu'à 49 ng/g, mais la majorité des
échantillons n'en contenaient pas de quantités décelables.
5. Effets sur les êtres vivants dans leur milieu naturel
On a révélé une réduction de 50% de la productivité
des cultures d'algues vertes d'eau douce, en présence de
concentrations de phosphate de tri- o-crésyle (TOCP)
allant de 1,5 à 4,2 ng/litre, selon les espèces, les iso-
mères méta et para étant moins toxiques. On ne dispose que
de données limitées sur la toxicité aiguë du TCP vis-à-vis
des invertébrés aquatiques: la CL50 à 48 heures pour la
daphnie est de 5,6 ng/litre; la CL50 à 24 heures pour les
nématodes est de 400 ng/litre; la dose sans effet obser-
vable à 2 semaines pour la daphnie (mortalité, croissance,
reproduction) est de 0,1 mg/litre. Pour trois espèces de
poissons, les valeurs de la CL50 à 96 heures se situaient
entre 4,0 et 8700 mg/litre. Chez des truites arc-en-ciel
on a constaté une mortalité d'environ 30% après exposition
de quatre mois à 0,9 ng/litre de IMOL S-140 (phosphate de
tri- o-crésyle à 2%) et des effets plus légers sur une
période de 14 jours.
Les niveaux d'exposition au cours de ces expériences
étaient beaucoup plus élevés que les concentrations sus-
ceptibles d'être rencontrées dans le milieu naturel et
dans la plupart des cas, les valeurs étaient très
supérieures à la solubilité des composés.
6. Cinétique et métabolisme
L'absorption, la distribution, le métabolisme et
l'élimination des organophosphorés jouent un rôle déter-
minant dans les effets neuropathologiques retardés de ces
composés.
Chez l'homme, l'absorption percutanée du TOCP semble
être au moins dix fois plus rapide que chez le chien. On
observe également une importante absorption par cette voie
chez le chat. L'absorption par voie orale a été observée
chez le lapin. On ne possède aucune donnée de première
main sur l'absorption par la voie respiratoire.
Chez le chat, on a constaté qu'après absorption, le
TOCP se répartissait largement dans l'ensemble de l'orga-
nisme, la concentration la plus élevée se situant dans le
nerf sciatique, qui constitue un tissu cible. De fortes
concentrations de TOCP ou de ses métabolites se rencon-
traient également au niveau du foie, des reins et de la
vésicule bilaire.
La métabolisation du TOCP s'effectue selon trois
voies. La première consiste dans l'hydroxylation d'un ou
plusieurs groupes méthyles et la seconde comporte la
désarylation des groupements orthocrésyles. Dans la
troisième, il y a encore oxydation du groupement hydroxy-
méthyle en aldéhyde et en acide carboxylique. L'hydroxy-
lation constitue une étape déterminante, car le TOCP
hydroxyméthylé est cyclisé pour former du phosphate
cyclique d' o-tolyle et de saligénol, un métabolite
neurotoxique relativement instable.
Le TOCP et ses métabolites sont éliminés dans les
urines et les matières fécales ainsi que, en petites
quantités, dans l'air expiré.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuves
in vitro
Des trois isomères du TCP, le TOCP est de loin celui
qui présente la toxicité aiguë la plus forte et qui se
révèle également le plus toxique en cas d'exposition
brève. Il est le seul à déterminer une neurotoxicité
retardée.
Les différents paramètres toxicologiques varient
beaucoup selon l'espèce (qu'il s'agisse par exemple de la
mortalité aiguë ou de la neurotoxicité retardée). Le
poulet est l'une des espèces les plus sensibles.
On a pu obtenir chez des espèces d'animaux de labora-
toire très variées une neuropathie retardée induite par un
organophosphoré (NRIOP) tant à la suite d'une exposition
unique qu'à la suite d'expositions répétées. Il s'agit
d'une neuropathie qui se traduit par des altérations
dégénératives au niveau de la partie distale de l'axone et
qui s'étend peu à peu à l'ensemble du neurone.
Elle se manifeste cliniquement par une paralysie des
pattes postérieures après une période de latence caracté-
ristique de deux à trois semaines suivant l'exposition.
Une dose orale unique de 50 à 500 mg de TOCP/kg a produit
une neuropathie retardée chez des poulets, des doses de
840 mg/kg ou davantage étant nécessaires pour produire une
dégénérescence de la moëlle épinière chez des rats Long-
Evans. C'est l'un des métabolites du TOCP, le phosphate
cyclique d' o-tolyle et de saligénol, qui constitue
l'agent neurotoxique actif. La sensibilité des espèces
varie en sens inverse de la vitesse de métabolisation
ultérieure.
On pense que la lésion biochimique qui conduit à la
neuropathie consiste dans l'inhibition de l'"estérase
neurotoxique". Un taux d'inhibition de plus de 65% peu de
temps après une exposition au TOCP fait présager l'appari-
tion ultérieure d'une neuropathie. La variabilité dans la
réponse neurotoxique dépend également d'autres facteurs
(par exemple la voie d'exposition, l'âge, le sexe, la
souche). Les données disponibles ne permettent pas de
définir clairement une dose sans effet observé pour cette
neuropathie.
Les études de reproduction effectuées sur des rats et
des souris qui recevaient des doses répétées de TOCP par
voie orale, ont révélé la présence de lésions histopatho-
logiques au niveau des testicules et des ovaires, de modi-
fications dans la morphologie des spermatozoïdes, d'une
moindre fécondité chez les deux sexes ainsi que d'une
diminution de la taille des portées et de la viabilité des
ratons et des souriceaux. Les données disponibles n'ont
pas permis de déterminer la dose sans effet pour ce type
d'anomalies imputables au TOCP. Une étude de tératogéni-
cité effectuée sur des rats, avec des doses orales
toxiques pour la mère, n'a pas donné de résultats
positifs.
On n'a guère de données sur la mutagénicité et aucune
sur la cancérogénicité de cette substance.
8. Effets sur l'homme
L'ingestion accidentelle constitue la cause principale
d'intoxication. Depuis la fin du dix-neuvième siècle, de
nombreux cas d'intoxication dus à la contamination de
boissons, de denrées alimentaires ou de produits pharma-
ceutiques ont été signalés. L'exposition professionnelle
tient essentiellement à une absorption percutanée ou à une
inhalation et certains cas d'intoxication de ce type ont
été signalés. L'ingestion de préparations contaminées par
du TOCP peut produire des symptômes digestifs (nausée,
vomissements et diarrhées), encore que dans certains cas,
c'est la polyneuropathie qui constitue le premier signe
d'intoxication. Les symptômes neurologiques sont carac-
térisés par une période de latence. Les premiers symptômes
consistent en douleurs et paresthésie aux extrémités des
membres inférieurs. Il y a une légère diminution de la
sensibilité cutanée et quelques fois réduction de la sen-
sibilité vibratoire. Dans la plupart des cas, la faiblesse
musculaire évolue rapidement vers une paralysie des
extrémités inférieures avec ou sans extension aux membres
supérieurs. Dans les cas graves, apparaissent des signes
d'atteinte pyramidale. Les cas mortels sont rares mais
les symptômes neurologiques peuvent être très lents à dis-
paraître et la guérison peut prendre des mois, voire des
années. L'examen histopathologique révèle une dégénéres-
cence des axones. Les examens classiques de laboratoire
ne révèlent pas d'anomalies si ce n'est parfois une
augmentation de la teneur en protéines du liquide céphalo-
rachidien. Les premiers soins consistent à réduire
l'exposition en faisant vomir immédiatement le malade,
dans la mesure où celui-ci est encore conscient. A plus
long terme, le traitement consiste essentiellement en une
réadaptation physique car on ne connaît pas d'antidote
spécifique. La réaction au phosphate de tricrésyle varie
considérablement d'un individu à l'autre de même que les
possibilités de guérison à la suite d'une intoxication.
On a signalé l'apparition de symptômes graves après inges-
tion de 0,15 g de TCP, alors que chez d'autres personnes,
l'ingestion de quantités atteignant 1 à 2 g n'a produit
aucun effet toxique. Certains malades guérissent complète-
ment alors que d'autres présentent des séquelles marquées
pendant de très longues périodes.
EVALUATION DES RISQUES POUR LA SANTE HUMAINE ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des risques pour la santé humaine
On a souvent signalé des cas d'intoxication humaine
par suite d'une ingestion accidentelle de phosphate de
tri- o-crésyle ou par suite d'une exposition profession-
nelle. Les symptômes neurotoxiques correspondent tout
d'abord à l'inhibition de la cholinestérase puis à une
neuropathie retardée caractérisée par une paralysie
grave.
Etant donné les variations considérables de sensi-
bilité selon les individus, il n'est pas possible de
déterminer quel est le seuil de sécurité. Des symptômes
ont été signalés après ingestion de 0,15 g d'un mélange
d'isomères contenant une faible proportion de TOCP; la
dose minimale efficace d'isomères ortho est donc beaucoup
plus faible encore. L'expérimentation animale fait égale-
ment ressortir de très importantes variations selon les
espèces pour ce qui concerne la réaction au TOCP et
l'homme semble être particulièrement sensible.
Des cas de dermatite d'irritation et de dermatites
allergiques ont été rapportés.
On peut donc considérer que l'isomère ortho et les
mélanges d'isomères qui en contiennent constituent un
risque de première importance pour la santé humaine.
Aucune dose n'est sans danger si elle est ingérée. Il
convient de réduire au minimum l'exposition cutanée ou
respiratoire.
1.1 Niveaux d'exposition
On peut considérer comme minimale l'exposition de la
population générale au phosphate de tricrésyle par
l'intermédiaire des divers compartiments du milieu
ambiant, notamment l'eau de consommation. On a décelé du
phosphate de tricrésyle à des concentrations plus fortes
dans l'air urbain que dans l'air prélevé au niveau des
sites de production, encore que ces valeurs soient
généralement faibles. Lors d'une enquête effectuée aux
Etats-Unis d'Amérique on n'a pas décelé de TCP dans des
échantillons de tissu adipeux humain. Il y a eu de
nombreux cas d'intoxication humaine accidentelle dus à
l'ingestion de médicaments, de nourriture, de farine,
d'huile de cuisine et de boissons contaminés par des
fluides hydrauliques ou des lubrifiants à base de TCP
produits à partir d'acide crésylique. Des symptômes
toxiques peuvent s'observer après ingestion de doses ne
dépassant pas 0,15 g de TOCP, substance qui est présente
dans le TCP produit à partir de l'acide crésylique. La
contamination se produit généralement lors de la
réutilisation de fûts ou de tonneaux vides qui avaient
contenu un liquide hydraulique ou de l'huile lubrifiante.
1.2 Effets toxiques
L'absorption accidentelle d'une forte dose entraîne
chez l'homme des troubles digestifs, à savoir une nausée
d'intensité variable, pouvant aller jusqu'aux vomisse-
ments, accompagnée de douleurs abdominales et de diar-
rhées. En cas d'exposition à de faibles doses cumulées,
une "neuropathie retardée" s'installe progressivement
après une période de latence de 3 à 28 jours. Dans la
plupart des cas, la faiblesse musculaire fait rapidement
place à une paralysie des membres inférieurs qui peut
parfois s'étendre aux mains. Dans les cas graves, on voit
peu à peu appraître des signes d'atteinte pyramidale.
Certaines études neurophysiologiques révèlent l'existence
d'une neurotoxicité généralisée avec prolongation du temps
de latence terminale et réduction relativement faible de
la vitesse de conduction des nerfs moteurs. Ces consta-
tations confirment la dégénérescence axonale qui est la
principale caractéristique relevée lors des examens histo-
pathologiques.
Le métabolite neurotoxique du TCP a été identifié; il
s'agit du phosphate cyclique de o-tolyle et de saligénol
qui provient lui-même des métabolites o-hydroxyméthylés.
Il semble donc que la présence d'au moins un groupement
o-tolyle soit nécessaire parmi les trois restes phéno-
liques du TCP pour qu'apparaissent des effets neuro-
toxiques. Le TCP produit à partir du crésol de synthèse,
qui contient moins de 0,1% d'orthocrésol, n'est donc pas
neurotoxique.
Les résultats d'études de toxicité subchronique
effectuées sur des animaux d'expérience avec du TCP pré-
paré à partir de crésol de synthèse, montrent que les
organes cibles sont le foie et le rein; toutefois cette
observation n'a pas été confirmée chez l'homme. On ne
dispose pas de données suffisantes sur la mutagénicité et
la cancérogénicité du TCP. On sait néanmoins que le TCP
n'est pas toxique pour l'embryon de poulet.
2. Evaluation des effets sur l'environnement
Le dosage du TCP dans l'eau montre que la contami-
nation est faible. Cet état de choses tient à la faible
solubilité dans l'eau du TCP et à l'aisance avec laquelle
il se décompose. Etant donnée la faible toxicité aiguë du
TCP pour les organismes aquatiques, il est peu probable
qu'il constitue une menace pour ces organismes.
Du fait de ses propriétés physico-chimiques, le TCP a
une forte tendance à la bioaccumulation. Toutefois celle-
ci ne se produit pas dans la pratique en raison de la
faible concentration du TOCP dans l'environnement et les
êtres vivants et de la dégradation rapide de cette
substance.
Les TCP fixés aux sédiments s'accumulent dans
l'environnement et on en a relevé des concentrations
élevées dans les sédiments des cours d'eau et des estu-
aires ainsi que dans les sédiments marins. Du fait que
l'on ne possède aucune information sur la biodisponibilité
de ces résidus pour les organismes fouisseurs ou ben-
thiques, ni sur les dangers qu'ils pourraient représenter,
on ne peut écarter à priori la possibilité d'effets
nocifs.
Il faut également mentionner la possibilité de risques
localisés pour l'environnement dus à un déversement acci-
dentel de TCP.
2.1 Niveaux d'exposition
Les TCP sont présents dans l'air, dans les eaux super-
ficielles, dans le sol, les sédiments et les organismes
aquatiques, à proximité des zones très industrialisées,
encore que leurs concentrations y soient généralement
faibles. En raison de la vitesse élevée de biodégradation
de ces substances en milieu aqueux, il ne semble pas
qu'elles puissent avoir des effets nocifs sur la faune
aquatique. Il a été fait état d'une concentration extrême-
ment élevée en phosphates totaux de triaryle (25,55 g/kg)
dans un échantillon de sol provenant d'une plantation.
Cette observation montre qu'il est nécessaire de procéder
à l'enfouissement des déchets.
2.2 Effets toxiques
Les algues d'eau douce sont relativement sensibles aux
TCP, la concentration inhibant à 50% la croissance com-
prise entre 1,5 et 5,0 ml/litre. En ce qui concerne les
poissons, des concentrations de TCP inférieures à
1 mg/litre (0,3-0,9 mg/litre) provoquent des signes
d'intoxication chronique chez la truite arc-en-ciel, mais
Menidia notata est plus résistant (CL50 de 8700
mg/litre). Les TCP n'inhibent pas l'activité cholinesté-
rasique des poissons ou des grenouilles mais ils poten-
tialisent l'effet des insecticides organophosphorés.
RECOMMANDATIONS
Lorsqu'on utilise des crésols tri-substitués pour la
synthèse et la préparation d'autres composés, il est
préférable d'utiliser des isomères para et méta purifiés
afin d'éviter toute synthèse accidentelle de dérivés
orthosubstitués.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El fosfato de tricresilo (FTC) es un líquido ininflam-
able, no explosivo, incoloro y viscoso. Su coeficiente de
partición entre el octanol y el agua (log Pow) es de
5,1. Se hidroliza con facilidad en un medio alcalino dando
fosfato de dicresilo y cresol, pero es estable en medios
neutros y ácidos a temperaturas normales.
El método analítico de elección es la cromatografía de
gases con un detector sensible al nitrógeno-fósforo o un
detector fotométrico de llama. El límite de detección en
una muestra de agua es de 1 ng/litro aproximadamente. El
FTC se extrae con facilidad de las soluciones acuosas con
distintos disolventes orgánicos. Se utiliza habitualmente
para la extracción la cromatografía en columna de flori-
sil, pero es difícil separar el FTC de los lípidos con
este método. Se han recomendado para esa finalidad otros
métodos de extracción (GPC, cromatografía en carbón vege-
tal activado y Sep-pak C-18). Los reactivos analíticos
están contaminados a menudo con cantidades infinitesimales
de FTC debido a su amplio uso. Por consiguiente, la obten-
ción de datos fiables en el análisis de cantidades infini-
tesimales de FTC requiere un procedimiento cuidadoso.
2. Fuentes de exposición humana y ambiental
El FTC se produce habitualmente por reacción de
cresoles con oxicloruro de fósforo. Existen dos fuentes
industriales de cresoles: el "ácido cresílico", obtenido
como residuo de los hornos de carbón de coque y del refino
del petróleo; y los "cresoles sintéticos", preparados a
partir del cimeno por oxidación y degradación. Como resul-
tado, el FTC es una mezcla de varios fosfatos triaríli-
cos.
El FTC se utiliza como plastificante en los plásticos
vinílicos, y también como pirorretardante, aditivo para
lubricantes de presión extrema y líquido ininflamable en
los sistemas hidráulicos.
3. Transporte, distribución y transformación en el medio ambiente
El paso de FTC al medio ambiente se debe principal-
mente a su uso final, pues la liberación en el curso de la
fabricación es escasa. En 1977 se calculó que el paso
total al medio ambiente en los Estados Unidos de América
fue de 32 800 toneladas.
Debido a su escasa hidrosolubilidad y a su elevada
adsorción por los materiales en partículas, el FTC se
absorbe con rapidez en los sedimentos de ríos o lagos y en
el suelo. Su biodegradación en el medio acuático es
rápida, quedando casi terminada en el agua de río en cinco
días. El isómero orto se degrada con una rapidez ligera-
mente mayor que los isómeros meta o para. El FTC se biode-
grada con facilidad en el fango de los alcantarillados,
presentando una semivida de 7,5 horas; la degradación en
24 horas alcanza el 99%. La degradación abiótica es más
lenta, dando una semivida de 96 días.
Se midieron los factores de bioconcentración de 165-
2768 en varias especies de peces en el laboratorio utili-
zando FTC radiomarcado. La radiactividad desapareció
rápidamente al cesar la exposición, observándose semividas
de depuración comprendidas entre 25,8 y 90 horas.
4. Niveles medioambientales y exposición humana
En el Japón se han medido concentraciones atmosféricas
de FTC de hasta 70 ng/m3, pero alcanzaron un máximo de
sólo 2 ng/m3 en una instalación de fabricación de los
Estados Unidos de América. En este país, el aire del medio
laboral contenía menos de 0,8 mg/m3 en una nave de
llenado de barriles de aceite lubricante y 0,15 mg/m3
(fosfatos totales) en una planta de troquelado de zinc
para automóviles. En el Canadá, las concentraciones de
FTC medidas en el agua potable fueron bajas (0,4 a
4,3 ng/litro) y el producto resultó indetectable en el
agua de pozo. Las concentraciones observadas en las aguas
de ríos y lagos son con frecuencia apreciablemente
mayores. Sin embargo, ello se debe a la presencia de sedi-
mentos en suspensión en los que queda fuertemente absor-
bido el FTC.
Las concentraciones en los sedimentos son altas en los
ríos y en el mar, habiéndose observado valores de hasta
1300 ng/g y 2160 ng/g, respectivamente.
Se observaron concentraciones altas en el suelo y la
vegetación en los alrededores de instalaciones de fabri-
cación.
Se han señalado restos en peces y mariscos de hasta
40 ng/g, pero la mayor parte de los animales examinados no
contenían residuos apreciables.
5. Efectos sobre los seres vivos del medio ambiente
La productividad primaria de cultivos de algas verdes
de agua dulce quedó reducida en el 50% mediante la adición
de fosfato de tri- o-cresilo a razón de 1,5 a 4,2
ng/litro, según las especies, mientras que los isómeros
meta y para resultaban menos tóxicos. Son limitados los
datos referentes a la toxicidad aguda del FTC para los
invertebrados acuáticos: la CL50 en 48 horas para Daphnia
es de 5,6 ng/litro y la CL50 en 24 horas para los nemato-
dos es de 400 ng/litro; la concentración NOEL (mortalidad,
crecimiento, reproducción) en dos semanas para Daphnia es
de 0,1 mg/litro. Los valores de CL50 en 96 horas para
tres especies de peces se hallaban comprendidos entre 4,0
y 8700 mg/litro. La trucha irisada presentó una mortalidad
del 30% aproximadamente después de una exposición de
cuatro meses a una concentración de 0,9 ng/litro de IMOL
S-140 (fosfato de tri- o-cresilo al 2%) y efectos menores
en un periodo de 14 días.
Los niveles de exposición utilizados en esos experi-
mentos fueron mucho mayores que las concentraciones que
probablemente pueden hallarse en el agua en el medio
ambiente y, en la mayoría de los casos, excedían en gran
manera a la solubilidad de los productos.
6. Cinética y metabolismo
La absorción, distribución, metabolismo y eliminación
de los organofosfatos son elementos críticos en los
efectos neuropáticos tardíos de estos productos.
La absorción cutánea del FTOC en el hombre parece ser
por lo menos de un orden de magnitud más rápida que en los
perros. También se observa una absorción cutánea signifi-
cativa en el gato. En el conejo se ha señalado la absor-
ción oral del producto. No hay información directa sobre
la absorción por inhalación.
En estudios efectuados en gatos se observó que el FTOC
absorbido se distribuye ampliamente por todo el organismo,
hallándose la máxima concentración en el nervio ciático,
que es el tejido diana. Otros órganos en los que se encu-
entran altas concentraciones de FTOC y de sus metabolitos
son el hígado, los riñones y la vesícula biliar.
El metabolismo del FTOC sigue tres vías. La primera
es la hidroxidación de uno o más grupos metílicos y la
segunda es la desarilación de los grupos o-cresilo. La
tercera vía es la oxidación ulterior del grupo hidroxi-
metilo para dar aldehído y ácido carboxílico. La etapa de
hidroxilación es decisiva porque el FTOC hidroximetílico
forma un producto cíclico, el fosfato cíclico de o-
tolilo saligenina, metabolito neurotóxico relativamente
ines-table.
El FOTC y sus metabolitos se eliminan por la orina en
las heces, junto con pequeñas cantidades en el aire
espirado.
7. Efectos en los animales de experimentación y en sistemas de
prueba in vitro
Entre los tres isómeros del FTC, el FOTC es con gran
diferencia el más tóxico en la exposición aguda y a corto
plazo. Es el único isómero que produce neurotoxicidad
tardía.
Existe una amplia variabilidad entre especies en lo
que respecta a los distintos puntos finales tóxicos (por
ejemplo, letalidad aguda, neurotoxicidad tardía) de la
exposición al FOTC, siendo el pollo una de las especies
más sensibles.
Se ha producido neuropatía tardía inducida por organo-
fosfatos mediante la exposición única y repetida en una
amplia gama de animales de experimentación; este trastorno
se clasifica como una "neuropatía de muerte sobre el
dorso". Se producen lesiones degenerativas en el axón
distal, que se extienden con el tiempo hacia el cuerpo de
la célula.
Los signos clínicos consisten en la parálisis de las
patas traseras después de un intervalo característico de
2-3 semanas a partir de la exposición. Una sola dosis
oral de 50-500 mg de FOTC/kg produjo la neuropatía tardía
en pollos, mientras que se necesitaron dosis de 840 mg/kg
o más para producir la degeneración de la médula espinal
en ratas Long-Evans. El metabolito fosfato cíclico de
o-tolilo saligenina es el agente neurotóxico activo. La
sensibilidad de las especies guarda correlación inversa
con la tasa de metabolismo ulterior.
Se cree que la inhibición de la "esterasa de la
neurotoxicidad" es la lesión bioquímica que conduce a la
neuropatía tardía inducida por organofosfatos; la inhi-
bición de más del 65% poco después de la exposición al
FOTC permite prever una neuropatía ulterior. En la vari-
abilidad de la respuesta neurotóxica al FOTC influyen
factores distintos del metabolismo (por ejemplo, vías de
exposición, edad, sexo, estirpe). Los datos disponibles no
permiten establecer un nivel neto sin efectos observados
para la neuropatía tardía.
Los estudios de reproducción en ratas y ratones some-
tidos a una exposición oral repetida al FOTC mostraron
lesiones histopatológicas de los testículos y los ovarios,
alteraciones morfológicas del esperma, disminución de la
fecundidad en ambos sexos, y descenso del tamaño y
viabilidad de las crías. Los datos disponibles no permiten
establecer un nivel neto sin efectos en lo que respecta a
la acción del FOTC sobre la reproducción. Un estudio de
teratogenicidad en ratas, utilizando dosis orales que
producían toxicidad materna, dio resultados negativos.
Se dispone de escasa información sobre la mutageni-
cidad y de ninguna sobre la cancerogenicidad.
8. Efectos en la especie humana
La ingestión accidental es la principal causa de
intoxicación. Desde fines del siglo XIX se han observado
numerosos casos de intoxicación por contaminación de bebi-
das, alimentos o medicamentos. La exposición profesional
se produce principalmente por absorción cutánea o inha-
lación, habiéndose registrado algunos casos de envenena-
miento. La ingestión de preparaciones que contienen FOTC
puede ir seguida de síntomas gastrointestinales (náuseas,
vómitos y diarrea), aunque en algunos casos la polineuro-
patía es el primer signo de intoxicación. Los síntomas
neurológicos suelen ser tardíos. Los síntomas iniciales
consisten en dolor y parestesia de las extremidades
inferiores. Puede observarse una alteración moderada de
las sensaciones cutáneas y a veces del sentido de la
vibración. En la mayoría de los casos, la debilidad
muscular evoluciona rápidamente hasta dar una marcada
parálisis de las extremidades inferiores, con o sin parti-
cipación de las superiores. En los casos graves aparecen
signos piramidales. Son raras las defunciones, pero la
recuperación de los síntomas y signos neurológicos puede
ser extremadamente lenta y durar varios meses o años. El
examen histopatológico muestra la presencia de degener-
ación del axón. Los análisis corrientes de laboratorio no
muestran hallazgos anormales, pero cabe observar un
aumento de la concentración de las proteínas en el líquido
cefalorraquídeo. Los primeros auxilios consisten en la
reducción de la exposición provocando el vómito inmediata-
mente después de la ingestión, siempre que el paciente
esté consciente. El tratamiento fundamental a largo plazo
consiste en la rehabilitación física, no conociéndose
ningún antídoto específico. Existen grandes variaciones
entre las personas en la respuesta al FTC y en la recuper-
ación después de producirse los efectos tóxicos. Se han
registrado síntomas graves después de la ingestión de
0,15 g de FTC, mientras que otras personas no presentaron
efecto tóxico alguno después de ingerir 1-2 g. Ciertos
enfermos se recuperan por completo, mientras que otros
conservan efectos marcados durante un periodo apreciable.
EVALUACION DE LOS RIESGOS PARA LA SALUD HUMANA Y DE LOS EFECTOS EN
EL MEDIO AMBIENTE
1. Evaluación de los riesgos para la salud humana
Se han registrado con frecuencia intoxicaciones
humanas por ingestión accidental de fosfato de tri- o-
cresilo (FTOC) o por exposición laboral de trabajadores.
La vía probable de la exposición laboral es la absorción
cutánea. Entre los síntomas neurotóxicos figuran la inhi-
bición inicial de las colinesterasas y la neuropatía
tardía ulterior caracterizada por parálisis grave.
Debido a la considerable variación existente en la
sensibilidad de las personas al FTOC, no puede estable-
cerse un nivel de exposición sin riesgo. Se han señalado
síntomas por ingestión de 0,15 g de una mezcla de isómeros
con baja proporción de FTOC; así pues, la dosis efectiva
mínima del ortoisómero es muy inferior. Los estudios
efectuados en animales muestran considerables variaciones
entre especies en la respuesta al FTOC, y las personas
parecen ser especialmente sensibles.
Se han señalado casos de dermatitis irritante y
alérgica.
En consecuencia, tanto el ortoisómero puro como las
mezclas de isómeros que contienen FTOC se consideran
riesgos importantes para la salud humana.
No existe un nivel inocuo de ingestión. La exposición
al producto por contacto cutáneo o inhalación debe
reducirse al mínimo.
1.1 Niveles de exposición
Puede considerarse mínima la exposición de la pobla-
ción general al fosfato de tricresilo (FTC) por conducto
de distintos medios ambientales, incluida el agua de
beber. Se han observado concentraciones de FTC relativa-
mente más altas en el aire urbano que en el aire recogido
en los emplazamientos de fabricación, aunque los niveles
suelen ser bajos. En un estudio efectuado en los Estados
Unidos de América no se detectó el FTC en muestras de
tejido adiposo humano. Se han observado numerosos casos
de intoxicación humana accidental por ingestión de medi-
camentos, alimentos tales como harina y aceite de cocinar,
y bebidas contaminados con líquido hidráulico o aceite
lubricante que contenían FTC producido a partir del
"ácido cresílico". Pueden observarse síntomas tóxicos
después de la ingestión de sólo 0,15 g de fosfato de tri-
o-cresilo, componente del FTC obtenido a partir de ácido
cresílico. El origen habitual de la contaminación ha con-
sistido en la reutilización de barriles o bidones vacíos
utilizados previamente para contener líquido hidráulico o
aceite lubricante.
1.2 Efectos tóxicos
La exposición humana accidental a una sola dosis alta
ocasiona trastornos gastrointestinales que varían de las
náuseas ligeras a las intensas con vómitos, dolor abdomi-
nal y diarrea. En el caso de la exposición a pequeñas
dosis acumuladas, aparece progresivamente la "neurotoxi-
cidad tardía" después de un periodo latente de 3-28 días.
En la mayor parte de los casos, la debilidad muscular
pasa rápidamente a ser una marcada parálisis de las ex-
tremidades inferiores, con o sin afectación de las manos.
En los casos graves aparecen progresivamente signos
piramidales. Algunos estudios neurofisiológicos muestran
fenómenos extendidos de neurotoxicidad y prolongación de
las latencias terminales, con disminución relativamente
pequeña de la velocidad de conducción de los nervios
motores. Ello confirma los signos de degeneración del
axón, que es la característica principal observada en los
exámenes histopatológicos.
Se ha identificado el metabolito neurotóxico del FTC
como el fosfato cíclico de o-tolilo saligenina, derivado
de los metabolitos o-hidroximetílicos. Parece pues que
los efectos neurotóxicos exigen la presencia por lo menos
de un grupo o-tolilo entre las tres porciones fenólicas
del FTC. Ello significa que no es neurotóxico el FTC
producido a partir de cresol sintético, que contiene menos
del 0,1% de o-cresol.
Los estudios de toxicidad subcrónica efectuados en
animales con FTC obtenido de cresol sintético muestran que
los órganos diana son el hígado y los riñones, pero esa
observación no se ha confirmado en los casos de intoxi-
cación humana. No se dispone de datos apropiados sobre la
mutagenicidad y la cancerogenicidad. El FTC no es tóxico
para los embriones de pollo.
2. Evaluación de los efectos en el medio ambiente
La medición de las concentraciones de FTC en el agua
ambiental ha mostrado que existen sólo niveles bajos de
contaminación. Ese hecho refleja la escasa hidrosolu-
bilidad y la fácil degradabilidad del producto. Dado que
la toxicidad aguda del FTC para los seres acuáticos es
también baja, es improbable que represente una amenaza
para ellos.
Debido a las propiedades fisicoquímicas del FTC
existen altas posibilidades de bioacumulación. Sin
embargo, ello no se produce en la práctica porque las con-
centraciones de FOTC en el medio ambiente y en los organ-
ismos vivos son bajas y porque los productos se degradan
con rapidez.
El FTC fijado por los sedimentos se acumula en el
medio ambiente, habiéndose medido concentraciones altas en
los sedimentos de ríos, estuarios y mares. Dado que se
carece de información sobre la biodisponibilidad de esos
residuos para los seres vivos que se hallan en madrigueras
o en el fondo o sobre sus riesgos, no puede descartarse la
posibilidad de que aparezcan efectos en tales especies.
La fuga de FTC suscita riesgos para el medio ambiente
local.
2.1 Niveles de exposición
El FTC se halla en el aire, las aguas de superficie,
el suelo, los sedimentos y los seres vivos acuáticos cerca
de las zonas muy industrializadas, aunque en concen-
traciones habitualmente bajas. Debido a la alta tasa de
biodegradación del FTC en el medio acuoso, no se considera
que afecta adversamente a los seres vivos acuáticos. En un
estudio se encontró una concentración extremadamente alta
de fosfatos triarílicos totales (26,55 g/kg) en una
muestra de suelo obtenida en el patio de una planta de
producción. Ello sugiere la necesidad de eliminar los
residuos por relleno de terrenos.
2.2 Efectos tóxicos
Las algas de agua dulce son relativamente sensibles al
FTC, cuya concentración inhibidora del 50% del crecimiento
es de 1,5 a 5,0 mg/litro. Entre las especies de peces, la
trucha irisada sufre el efecto de concentraciones de FTC
inferiores a 1 mg/litro (0,3-0,9 mg/litro), con signos de
intoxicación crónica, pero el pez argentado de las mareas
es más resistente (CL50 de 8700 mg/litro). El FTC no
inhibe la actividad colinesterásica seca de peces y ranas,
pero tiene un efecto sinérgico con la actividad
insecticida de los órganos fosforados.
RECOMENDACIONES
Cuando se utilizan cresoles trisustituidos en la
síntesis y fabricación de otros productos, es necesario
emplear isómeros meta y para purificados para evitar la
síntesis accidental de productos orto-sustituidos.
