
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 109
SUMMARY REPORT ON THE EVALUATION OF
SHORT-TERM TESTS FOR CARCINOGENS
(COLLABORATIVE STUDY ON IN VIVO TESTS)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Summary report on the evaluation of short-term tests for
carcinogens : (collaborative study on in vivo tests).
(Environmental health criteria ; 109)
1.Carcinogens - analysis 2.Mutagens - analysis
3.Mutagenicity tests 4. Evaluation studies I.Series
ISBN 92 4 157109 8 (NLM Classification: QZ 202)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
SYNOPSIS
1. INTRODUCTION
2. THE COLLABORATIVE STUDY ON SHORT-TERM IN VIVO TESTS FOR MUTAGENS AND
CARCINOGENS (CSSTT/2) 1983-85
3. OVERALL AIMS OF THE STUDY AND CRITERIA FOR THE SELECTION OF AN
APPROPRIATE SHORT-TERM IN VIVO TEST
3.1. The use of short-term tests for the primary identification of
genotoxic chemicals
3.2. The use of short term in vivo assays for assessing the hazard
associated with exposure to in vitro genotoxins
3.3. The role of short-term in vitro tests in research into the
mechanisms of cancer
3.4. Assays for the detection of germ cell mutagens
4. CRITERIA FOR THE SELECTION OF THE FOUR TEST CHEMICALS
4.1. Activity of the four test chemicals in short-term in vitro tests
4.2. Summary of carcinogenicity data on the test chemicals
5. SOURCE AND PURITY OF THE TEST CHEMICALS
6. SHORT-TERM IN VIVO ASSAYS
6.1. Cytogenetic assays
6.2. Assays in rodent liver cells
6.3. Miscellaneous assays
6.4. The mouse spot test
6.5. Mammalian germ cell studies
6.6. Drosophila assays
7. RESULTS
7.1. Benzo [a] pyrene and pyrene
7.1.1. Cytogenetic studies
7.1.2. Liver-specific assays
7.1.3. Miscellaneous assays
7.1.4. Mouse spot tests
7.1.5. Mammalian germ cell assays
7.1.6. Drosophila assays
7.2. 2-Acetylaminofluorene and 4-acetylaminofluorene
7.2.1. Cytogenetic studies
7.2.2. Liver-specific assays
7.2.3. Miscellaneous assays
7.2.4. Mouse spot tests
7.2.5. Mammalian germ cell assays
7.2.6. Drosophila assays
7.3. Summary of the in vivo genotoxicity of the four chemicals
8. ASSESSMENT OF THE PERFORMANCE OF THE ASSAYS
8.1. Cytogenetic assays
8.1.1. Chromosome aberrations
8.1.2. Micronuclei
8.1.3. Sister chromatid exchange
8.2. Liver assays
8.2.1. Initiation and promotion
8.2.2. Unscheduled DNA synthesis and S-phase analysis
8.2.3. DNA strand breaks
8.2.4. Cytogenetics
8.3. Miscellaneous assays
8.3.1. Specific carcinogenicity assays
8.3.2. Supplementary assays
8.3.3. Immunotoxicity assays
8.3.4. Host-mediated assays and urine mutagenicity tests
8.4. Mouse spot tests
8.5. Assays in mammalian germ cells
8.5.1. Dominant lethal and unscheduled DNA synthesis assay
8.5.2. Sperm abnormality tests
8.6. Drosophila assays
9. SELECTION OF THE MOST EFFECTIVE IN VIVO ASSAYS IN RELATION TO
THEIR PERFORMANCE
9.1. Assays that are not considered appropriate for routine in vivo
testing of chemicals for genotoxic activity
9.2. Assays that satisfy some or all of the criteria for an acceptable
in vivo short-term test
9.2.1. Assays currently in general use
9.2.2. Assays that show promise forfuture development
9.3. The detection of germ cell mutagens
9.4. Influence of route of administration of the test chemicals
10. CONCLUSIONS
REFERENCES
ETUDE COLLECTIVE POUR L'EVALUATION ET LA VALIDATION DES EPREUVES DE COURTE
DUREE RELATIVES AUX CANCEROGENES
ESTUDIO EN COLABORACION SOBRE EVALUACION Y COMPROBACION DE PRUEBAS A CORTO
PLAZO PARA SUSTANCIAS CARCINOGENAS
PARTICIPANTS IN THE COLLABORATIVE STUDY
Dr I. Adler, Mammalian Genetics Institute, Association for
Radiation and Environmental Research, Neuherberg, Federal
Republic of Germany
Dr R. Albanese, Pharmaceuticals Division, Imperial Chemical
Industries PLC, Macclesfield, Cheshire, England
Dr J.W. Allen, Genetic Toxicology Division, US Environmental
Protection Agency, Research Triangle Park, North
Carolina, USA
Dr J.A. Allen, Department of Mutagenesis and Cell Biology,
Huntingdon Research Centre Ltd., Huntingdon, Cambridge-
shire, England
Dr O. Andersen, Odense University, Institute of Community
Health, Department of Environmental Medicine, Odense,
Denmark
Dr D. Anderson, British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Dr J. Arany, Institut d'Hygiène et d'Epidémiologie,
Brussels, Belgium
Dr J. Ashby, Central Toxicology Laboratory, Imperial Chemi-
cal Industries PLC, Macclesfield, Cheshire, United
Kingdom
Dr R.A. Baan, Medical Biological Laboratory TNO, Rijswijk,
Netherlands
Dr P. Bannasch, Cytopathology Department, Institute of
Experimental Pathology, German Cancer Research Centre,
Heidelberg, Federal Republic of Germany
Dr G.C. Becking, International Programme on Chemical Safety,
World Health Organization, Research Triangle Park, North
Carolina, USA
Dr B. Beije, Department of Genetic and Cellular Toxicology,
Wallenberg Laboratory, Stockholm University, Stockholm,
Sweden
Dr J. Benes, Institute of Nuclear Biology and Radiochemis-
try, Prague, Czechoslovakia
Dr E. Bermudez, Department of Genetic Toxicology, Chemical
Industry Institute of Toxicology, Research Triangle Park,
North Carolina, USA
Dr H.C. Birnboim, Department of Experimental Oncology,
Ottawa Regional Cancer Centre, Ottawa, Ontario, Canada
Dr J.B. Bishop, Cellular and Genetic Toxicology Branch,
Toxicology Research and Testing Program, National Insti-
tute of Environmental Health Sciences, Research Triangle
Park, North Carolina, USA
Dr D.H. Blakey, Mutagenesis Section, Environmental Health
Centre, Department of National Health and Welfare,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr R. Braum, Central Institute for Genetics and for Research
on Cultivated Plants, Academy of Science of the German
Democratic Republic, Gatersleben, German Democratic
Republic
Dr G. Bronzetti, National Research Council, Institute of
Mutagenesis and Differentiation, Pisa, Italy
Dr B.E. Butterworth, Chemical Industry Institute of Toxi-
cology, Research Triangle Park, North Carolina, USA
Dr P.S. Chauhan, Bio-Medical Group, Bhabha Atomic Research
Centre, Bombay, India
Dr I. Chouroulinkov, Unité de Cancérogénèse Expérimentale et
de Toxicologie Génétique (E.R. 304) I.R.S.C.-C.N.R.S.,
Villejuif, France
Dr M.G. Clare, Shell Research Ltd, Sittingbourne, Kent,
United Kingdom
Dr R.D. Combes, School of Biological Sciences, Portsmouth
Polytechnic, Portsmouth, United Kingdom
Dr C. Coton, Mammalian Genetics Laboratory, Department of
Biology, European Nuclear Centre, Mol, Belgium
Dr R. Crebelli, Higher Institute of Health, Viale Regina
Elena, Rome, Italy
Dr B.J. Dean, Upchurch, Sittingbourne, Kent, United Kingdom
Dr G.M. Decad, Department of Materials Toxicology, IBM
Corporation, San Jose, California, USA
Dr F.J. de Serres, National Institute of Environmental
Health Sciences, Research Triangle Park, North Carolina,
USA
Dr D.J. Doolittle, Toxicology Research, Bowman Gray
Technical Center, R.J. Reynolds Co., Winston-Salem, North
Carolina, USA
Dr U.H. Ehling, Mammalian Genetics Institute, Association
for Radiation and Environmental Research, Neuherberg,
Federal Republic of Germany
Dr B.M. Elliott, Genetic Toxicology Section, Imperial Chemi-
cal Industries PLC, Macclesfield, Cheshire, United
Kingdom
Dr R. Fahrig, Fraunhofer Institute for Research on Toxi-
cology and Aerosols, Hannover, Federal Republic of
Germany
Dr R. Forster, Life Science Research, Rome Toxicology
Centre, Pomezia, Rome, Italy
Dr K. Fujikawa, Drug Safety Evaluation Laboratories, Central
Research Division, Takeda Chemical Industries Ltd, Osaka,
Japan
Dr C. Furihata, Department of Molecular Oncology, Institute
of Medical Science, University of Tokyo, Tokyo, Japan
Dr S.M. Galloway, Merck Sharp & Dohme Research Labora-
tories, West Point, Pennsylvania, USA
Dr W.M. Generoso, Biology Division, Oak Ridge National
Laboratory, Oak Ridge, Tennessee, USA
Dr H.P. Glauert, McArdle Laboratory for Cancer Research,
University of Wisconsin, Madison, Wisconsin, USA
Dr U. Graf, Toxicology Institute, Zurich Federal Polytechnic
and University, Zurich, Switzerland
Dr B.L. Harper, Division of Environmental Toxicology, Uni-
versity of Texas Medical Branch, Galveston, Texas, USA
Dr G.G. Hatch, Toxicology Division, Northrop Services Inc.,
Environmental Sciences, Research Triangle Park, North
Carolina, USA
Dr M. Hayashi, Biological Safety Research Centre, National
Institute of Hygienic Sciences, Tokyo 158, Japan
Dr R.M. Hicks, School of Pathology, Middlesex Hospital
Medical School, London, United Kingdom
Dr J.M. Hunt, Department of Pathology and Laboratory Medi-
cine, University of Texas Medical School, Houston, Texas,
USA
Dr N. Inui, Biological Research Centre, Japan Tobacco Inc.,
Kanagawa, Japan
Dr M. Ishidate, Jr., Biological Safety Research Centre,
National Institute of Hygienic Sciences, Tokyo, Japan
Dr V.I. Ivanov, Institute of Medical Genetics, Academy of
Medical Sciences, Moscow, USSR
Dr J.C. Jensen, National Food Institute, Institute of Toxi-
cology, Copenhagen, Denmark
Dr D. Jenssen, Department of Genetic Toxicology, Wallenberg
Laboratory, University of Stockholm, Stockholm, Sweden
Dr B.J. Kilbey, Institute of Animal Genetics, University of
Edinburgh, Edinburgh, United Kingdom
Dr I. Kimber, Central Toxicology Laboratory, Imperial Chemi-
cal Industries PLC, Macclesfield, Cheshire, United King-
dom
Dr U. Kliesch, Mammalian Genetics Institute, Association for
Radiation and Environmental Research, Neuherberg, Federal
Republic of Germany
Dr A.D. Kligerman, Environmental Health Research and
Testing Inc., Research Triangle Park, North Carolina,
USA
Dr D. Kornbrust, Merck Sharp & Dohme Research Laboratories,
Department of Safety Assessment, West Point,
Pennsylvania, USA
Dr C. Lasne, Unité de Cancérogénèse Expérimentale et de
Toxicologie Génétique (ER-304) I.R.S.C.-C.N.R.S.,
Villejuif, France
Dr A. Léonard, Mammalian Genetics Laboratory, Department
of Biology, European Nuclear Centre, Mol, Belgium
Dr C.A. Luke, Medical Department, Brookhaven National Lab-
oratory, Upton, New York, USA
Dr J.T. MacGregor, US Department of Agriculture, Western
Regional Research Center, Berkeley, California, USA
Dr A.M. Malashenko, Scientific Research Laboratory of Exper-
imental Biological Models of the Academy of Medical
Sciences of the USSR, Moscow Region, USSR
Dr C. Malaveille, International Agency for Research on
Cancer, Lyon, France
Dr B.H. Margolin, Biometry and Risk Assessment Program,
National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA
Dr D. McGregor, Developmental Toxicology, Inveresk Research
International Ltd, Musselburgh, United Kingdom
Dr A.L. Meyer, Shell Research Ltd, Sittingbourne, Kent,
United Kingdom
Dr J.C. Mirsalis, Cellular and Genetic Toxicology Depart-
ment, SRI International, Menlo Park, California, USA
Dr N. Nashed, Johann Wolfgang Goethe-Universität, Frankfurt
am Main, Federal Republic of Germany
Dr S.B. Neal, Toxicology Division, Lilly Research Labora-
tory, Greenfield, Indiana, USA
Dr A. Neuhäuser-Klaus, Mammalian Genetics Institute,
Association for Radiation and Environmental Research,
Neuherberg, Federal Republic of Germany
Dr D.A. Pagano, Cellular and Genetic Toxicology Branch,
Toxicology Research and Testing Program, National Insti-
tute of Environmental Health Sciences, Research Triangle
Park, North Carolina, USA
Dr F. Palitti, Evolutionary Genetics Centre of the National
Research Council, Genetics and Molecular Biology Depart-
ment, University City, Rome, Italy
Dr S. Parodi, Chemical Carcinogenesis Laboratory, National
Cancer Research Institute, Genoa, Italy
Dr M. Pereira, Health Effects Research Laboratory, US
Environmental Protection Agency, Cincinnati, Ohio, USA
Dr J. Pot-Deprun, Unité de Cancérogénèse Expérimentale et
Toxicologie Génétique (ER-304) I.R.S.C.- C.N.R.S.
Laboratoires de Recherche Appliquée sur le Cancer,
Villejuif, France
Dr G.S. Probst, Toxicology Division, Lilly Research Labora-
tories, Greenfield, Indiana, USA
Dr C. Ramel, Wallenberg Laboratory, University of Stockholm,
Stockholm, Sweden
Dr K. Randerath, Baylor College of Medicine, Department of
Pharmacology, Houston, Texas, USA
Dr H.S. Rosenkranz, Department of Environmental Health
Sciences, Case Western Reserve University, Cleveland,
Ohio, USA
Dr P. Russo, National Cancer Research Institute, Genoa,
Italy
Dr M.F. Salamone, Moe-Biohazard Laboratory, Rexdale,
Ontario, Canada
Dr C.B. Salocks, Department of Materials Toxicology, IBM
Corporation, San Jose, California, USA
Dr J. Schöneich, Central Institute for Genetics and for
Research on Cultivated Plants, Academy of Science of the
German Democratic Republic, Gatersleben, German
Democratic Republic
Dr A.G. Searle, Medical Research Council Radiobiology Unit,
Harwell, Didcot, United Kingdom
Dr G.A Sega, Biology Division, Oak Ridge National Labora-
tory, Oak Ridge, Tennessee, USA
Dr M.D. Shelby, Cellular and Genetic Toxicology Branch,
Toxicology Research and Testing Program, National Insti-
tute of Environmental Health Sciences, Research Triangle
Park, North Carolina, USA
Dr H. Shibuya, Laboratory of Genetic Toxicology, Hatano
Research Institute, Food and Drug Safety Center,
Kanagawa, Japan
Dr T. Shibuya, Laboratory of Genetics, Hatano Research
Institute, Food and Drug Safety Center, Kanagawa, Japan
Dr R.H. Stevens, Radiation Research Laboratory, Department
of Radiation, University of Iowa, Iowa, USA
Dr G.D. Stoner, Department of Pathology, Medical College of
Ohio, Toledo, Ohio, USA
Dr J.A. Styles, Central Toxicology Laboratory, Imperial
Chemical Industries PLC, Macclesfield, Cheshire, United
Kingdom
Dr K.E. Suter, Preclinical Research, Toxicology Department,
Sandoz Limited, Basel, Switzerland
Dr A. D. Tates, Department of Radiation Genetics and
Chemical Mutagenesis, State University of Leiden, Leiden,
Netherlands
Dr R.R. Tice, Medical Department, Brookhaven National
Laboratory, Upton, New York, USA
Dr H. Tsuda, First Department of Pathology, Nagoya City,
University Medical School, Nagoya, Japan
Dr R. Valencia, Department of Zoology, University of
Wisconsin, Zoology Research Building, Madison, Wisconsin,
USA
Dr A. Vlachos, Haskell Laboratory for Toxicology and Indus-
trial Medicine, E.I. Du Pont de Nemours & Co. Inc.,
Newark, Delaware, USA
Dr E.W. Vogel, Department of Radiation Genetics and Chemical
Mutagenesis, State University of Leiden, Leiden, Nether-
lands
Dr P.A. Watkins, Pharmaceuticals Division, Imperial Chemical
Industries PLC, Macclesfield, Cheshire, United Kingdom
Dr G.A. Wickramaratne, Central Toxicology Laboratory,
Imperial Chemical Industries PLC, Macclesfield, Cheshire,
United Kingdom
Dr D. Wild, Pharmacology and Toxicology Institute, Würzburg
University, Würzburg, Federal Republic of Germany
Dr P.K. Working, Department of Genetic Toxicology, Chemical
Industry Institute of Toxicology, Research Triangle Park,
North Carolina, USA
Dr V.S. Zhurkov, A. N. Sysin Institute of General and
Environmental Hygiene, Moscow, USSR
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication. In the interest of all
users of the environmental health criteria documents,
readers are kindly requested to communicate any errors
that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland, in order that they may be included in
corrigenda, which will appear in subsequent volumes.
ABBREVIATIONS
AAF Acetylaminofluorene
BP Benzo [a] pyrene
CSSTT/1 Collaborative study on short-term tests for
genotoxicity and carcinogenicity
CSSTT/2 Collaborative study on short-term in vivo
tests for mutagens and carcinogens
GGT Gamma-glutamyltranspeptidase
IPESTTC International Collaborative Programme for the
Evaluation of Short-Term Tests for Carcinogens
NK Natural killer
PYR Pyrene
SCE Sister chromatid exchange
SSB Single strand breaks
UDS Unscheduled DNA synthesis
SYNOPSIS
THE INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY (IPCS) COLLABORATIVE STUDY
ON THE ASSESSMENT AND VALIDATION OF SHORT-TERM TESTS FOR CARCINOGENS.
The first part of this project, dealing with in vitro
studies, was published in 1985 (Ashby et al., 1985) and
was summarized in Environmental Health Criteria 47 (WHO,
1985). The second part, which is the subject of this
report, was published in 1988 (Ashby et al., 1988a).
The need for inter-laboratory collaborative studies
on an international scale arose from the necessity to
investigate the value of short-term tests for detecting
mutagenic and carcinogenic chemicals. Short-term assays
were proposed as alternatives or supplementary procedures
to traditional long-term rodent bioassays. Concern about
the choice of short-term tests and their reliability and
sensitivity led to the instigation of the first major
international collaborative exercise, the International
Collaborative Programme for the Evaluation of Short-Term
Tests for Carcinogens (IPESTTC) (de Serres & Ashby, 1981).
The results of this study confirmed the value of the
salmonella mutation test as a reliable and practicable
assay for the primary identification of carcinogens and
mutagens. It was also observed that, in the salmonella
test, some known rodent carcinogens were either not
detected or only detected with considerable difficulty.
Several other assays represented in the IPESTTC study were
able to detect some of the rodent carcinogens that were
negative in the salmonella assay. The supporting data
base was too small, however, to permit the recommendation
of an assay that would complement the salmonella mutation
test.
It was apparent, from the results of the IPESTTC
study, that a further collaborative exercise would be
required to establish a) the most effective combination of
in vitro assays for primary screening of chemicals for
genotoxic activity and b) the most useful short-term in
vivo tests for confirming mammalian genotoxicity and car-
cinogenic potential. The Collaborative Study on the
Assessment and Validation of Short-Term Tests for Geno-
toxicity and Carcinogenicity (CSSTT) was proposed by the
International Programme on Chemical Safety (IPCS) and the
National Institute of Environmental Health Sciences
(NIEHS) of the USA (a Participating Institution of the
IPCS). Because of the complexity of the organization and
the magnitude of the project, it was divided into two
discrete studies: the Collaborative Study on Short-Term
Tests for Genotoxicity and Carcinogenicity (CSSTT/1) and
the Collaborative Study on Short-Term In Vivo Tests for
Mutagens and Carcinogens (CSSTT/2).
In CSSTT/1, a comprehensive data base was assembled
from a wide range of in vitro assays conducted with ten
carefully selected organic chemicals. These included eight
established rodent carcinogens that were either negative
or difficult to detect in the salmonella assay and two
chemicals that were regarded as non-carcinogenic. Data
were evaluated from almost 90 sets of assays conducted by
some 60 participating scientists. Four types of assays
performed well enough to be considered as possible comp-
lementary tests to the salmonella assay. These included
tests for chromosomal aberrations, gene mutations and neo-
plastic transformation in cultured mammalian cells, and an
assay for aneuploidy in yeast. With the exception of the
chromosomal aberration assay, it was apparent that proto-
cols in general use for these assays required further
evaluation before they could be considered fully accept-
able.
The major conclusion of the CSSTT/1 study on in vitro
assays was that the use of chromosomal aberration assays
in conjunction with the salmonella mutation test may pro-
vide an efficient primary screen for possible new carcino-
gens.
In the IPESTTC study, seven of the fourteen presumed
non-carcinogens gave positive results in many of the in
vitro assays. The limited in vivo data available from that
study suggested that these seven chemicals were inactive
in in vivo short-term tests. The non-carcinogens in two of
the carcinogen/non-carcinogen pairs in IPESTTC, i.e.
benzo [a] pyrene/pyrene (BP/PYR) and 2-acetylamino-fluorene/
4-acetylaminofluorene (2AAF/4AAF), provided good examples
of these different responses, and these pairs of chemicals
were selected for the in vivo part of the collaborative
study (CSSTT/2). There was, however, a question mark
against the presumed non-carcinogenicity of 4AAF, and a
vital part of the study was the initiation of long-term
cancer bioassays of 2AAF and 4AAF in rats.
The objective of CSSTT/2, therefore, was to generate a
comprehensive data profile from a broad range of short-
term in vivo tests as a means of understanding how various
genetic endpoints in key target tissues respond to chemi-
cals defined as genotoxic in vitro. The ultimate goal was
to identify which in vivo assays could be used to deter-
mine the in vivo activity of established genotoxins.
Ninety-seven investigators from sixteen countries par-
ticipated in the in vivo project and data were presented
from some fifty separate in vivo techniques. The results
were evaluated at a meeting of investigators held at Cap
d'Agde, France, in May 1985. A series of reports were
prepared comprising an assessment of each group of assays,
summary reports on the germ cell assays and the liver-
specific tests, and summary reports on the total data base
on each pair of chemicals. Subsequently, an overview of
the whole in vivo study was prepared in readiness for
final publication.
The criteria defining an acceptable short-term in vivo
test were satisfied by only a small proportion of the
assays represented in CSSTT/2. However, the assays
included in the study were not limited to those most
likely to meet the criteria. Thus, although data were
submitted from assays not designed primarily to identify
in vivo genotoxicity, they provided information on a
broad spectrum of biological effects of the four
chemicals. Most of the in vivo somatic cell assays for
genotoxicity discriminated between the two carcinogen/non-
carcinogen pairs although, in some cases, weak activity
was detected in tests with the non-carcinogens,
particularly with 4AAF. The insensitivity of some assays
to one or other of the two pairs of chemicals support the
concept that negative in vivo data should be obtained from
at least two assays in different tissues before a chemical
can be accepted as non-genotoxic in vivo.
The mouse bone marrow micronucleus test was confirmed
as a robust, sensitive, and reproducible assay and was
recommended for primary in vivo testing of in vitro geno-
toxins. The overall performance of the rat liver assay for
unscheduled DNA synthesis suggested that it could be comp-
lementary to the micronucleus test, although certain
aspects of the sensitivity and selectivity of this assay
require additional investigation. Some widely advocated
assays including the host-mediated and urine mutagenicity
assays and tests using drosophila were concluded to be
inappropriate for hazard-assessment purposes.
The results of the CSSTT/2 study confirmed that short-
term in vivo tests have a vital role to play in hazard
assessment and that this role is to identify those chemi-
cals, shown to be genotoxic in vitro, that are active in
vivo and, thus, are most likely to present a carcinogenic/
mutagenic hazard to mammals, including humans.
1. INTRODUCTION
For many years it has been known that some environ-
mental chemicals are associated with an increased inci-
dence of cancer in humans. The danger posed by exposure to
established human carcinogens such as ß-naphthylamine and
vinyl chloride was originally recognized from epidemi-
ological evidence. The main purpose of investigating
chemical carcinogenesis, however, is to prevent environ-
mentally induced cancer by identifying such chemicals
before they are released into the environment. Over the
past two or three decades, carcinogenic activity has
usually been determined by the ability of a chemical to
produce tumours in laboratory animals during lifetime
exposure to the chemical. Long-term animal studies of
this kind may last for two or three years and utilize
scarce resources and expertise. In consequence, it is
only feasible to test a very small proportion of the new
chemicals produced each year in animal bioassays.
Many attempts were made in the late 1960s and early
1970s to detect potentially carcinogenic chemicals in
tests using bacteria or cultured mammalian cells. It was
suspected that many cancers resulted from changes to the
informational macromolecules of cells, i.e. deoxyribo-
nucleic acid (DNA), and, in general, the tests were based
on the induction of genetic changes to the test cells such
as gene mutations and chromosomal aberrations. Little
progress was made until it was realized that the majority
of carcinogenic chemicals required biotransformation by
mammalian enzymes before they were in a molecular form
capable of interaction with DNA. This observation led to
the development, by Professor Bruce Ames and his col-
leagues at the University of California, USA, of a bac-
terial test for mutagens that incorporated essential
aspects of mammalian metabolism in the form of an enzyme-
rich fraction of mammalian liver. In this system, known
as the "salmonella assay" or the "Ames test", it was
shown that a number of chemicals, known to be carcinogenic
in laboratory animal studies, were metabolized by the
incorporated mammalian enzymes to reactive molecules that
induced mutations in the Salmonella typhimurium tester
strains. In a series of validation studies with the
salmonella assay conducted during the mid-1970s and
totalling about 500 chemicals, a high percentage of
carcinogenic chemicals induced mutations in the bacteria
and a high proportion of non-carcinogens were negative.
Considering that the salmonella assay could produce
results within a week (compared with the two or three
years for a rodent bioassay), it is not surprising that it
was soon being used extensively throughout the world. Many
hundreds of chemicals of diverse structure were tested
and, in many cases, interpreted in human health terms
without full comprehension of the biological principles
involved in extrapolating data from a simple bacterial
assay to a complex organism like man. It became apparent
at this time that a number of established animal carcino-
gens consistently gave negative results in the salmonella
assay and, similarly, some chemicals, considered to be
non-carcinogenic, were shown to be mutagenic in bacterial
tests. This observation confirmed that no single short-
term assay could be relied on to detect all carcinogens
and also confirmed the value of the practice of using
short-term in vitro and in vivo tests in batteries or in
tier systems. The variety of such packages proliferated,
leading to a great deal of confusion and conflict regard-
ing the most appropriate tests to investigate chemicals
for possible carcinogenic activity.
Concern about the reliability, sensitivity and the
choice of short-term tests resulted in the instigation of
the International Collaborative Programme for the Evalu-
ation of Short-Term Tests for Carcinogens (IPESTTC). This
project, completed in 1981, involved investigators from
more than 50 laboratories, and some 30 in vitro and in vivo
assays were evaluated for their ability to discriminate
between carcinogenic and non-carcinogenic chemicals (de
Serres and Ashby, 1981). Twenty-five known carcinogens and
17 chemicals considered to be non-carcinogenic, including
14 pairs of carcinogen/non-carcinogen analogues, were
tested in most of the assays. An evaluation of the data
indicated that the salmonella mutation assay gave the best
overall performance, producing reliable results in a large
number of laboratories. Other assays that also appeared
to discriminate between carcinogens and non-carcinogens
included in vitro tests for chromosomal aberrations and
unscheduled DNA synthesis and, although fewer chemicals
were tested, results from drosophila tests, mammalian cell
gene mutation assays, and rodent bone marrow cytogenetic
studies suggested that they, too, were useful components
of a testing battery. Following a critical evaluation of
the data from the IPESTTC project, it was concluded that,
although the value of the salmonella assay was confirmed,
there were still some rodent carcinogens that were either
reproducibly negative in this assay or detected only with
difficulty. A proportion of these chemicals were detected
in some of the other assays but the data base was
insufficient to identify which in vitro or in vivo test(s)
were the most effective complementary assay(s) to the
salmonella test.
It was apparent from the IPESTTC study that a further
collaborative exercise would be necessary to establish a)
the most effective combination of in vitro assays for pri-
mary screening of chemicals for carcinogenic potential and
b) the most useful short-term in vivo procedures for con-
firming mammalian genotoxicity and carcinogenic potential.
A collaborative programme designed to investigate these
two questions was proposed by the International Programme
on Chemical Safety (IPCS) and the National Institute of
Environmental Health Sciences (NIEHS) of the USA. Because
of the logistical problems involved in organizing and
managing international collaborative projects, this pro-
gramme was divided into two discrete studies. The first,
referred to as the Collaborative Study on Short-Term Tests
for Genotoxicity and Carcinogenicity (CSSTT/1) was pub-
lished in 1985 (Ashby et al., 1985) and summarized in
Environmental Health Criteria 47 (WHO, 1985). The second
part of the programme was the Collaborative Study on
Short-Term In Vivo Tests for Mutagens and Carcinogens
(CSSTT/2) and is the subject of this report.
It was decided very early in the planning stage that,
whereas 42 chemicals were investigated in the IPESTTC
project, the CSSTT/1 study would concentrate on generating
a more comprehensive data base on a smaller number of
chemicals rather than an incomplete set of data from a
large number. Thus, ten organic chemicals were selected
comprising eight established carcinogens that were either
negative or difficult to detect in the salmonella assay
and two chemicals that had not shown any evidence of car-
cinogenicity in rodent cancer bioassays. The results of
the study were evaluated at a meeting of investigators in
1983 at which data from nearly 90 individual sets of
assays conducted by some 60 participating scientists were
scrutinised. Almost all the in vitro assays in use at that
time were represented and four assays, in particular, per-
formed well enough to be considered as possible complemen-
tary tests to the salmonella assay. These were tests for
chromosomal aberrations, gene mutations and neoplastic
transformation in cultured mammalian cells, and an assay
for aneuploidy induction in yeast. An important component
of the project was to identify protocol variations that
might explain differences in results between investigators
using, ostensibly, the same assay. This assessment indi-
cated that the protocols in general use for gene mutation
and transformation assays in mammalian cells and
aneuploidy in yeast require further evaluation before they
can be considered fully reproducible between laboratories.
The data provided by the investigators using chromosomal
aberration assays allowed the working group to identify
certain critical factors in the protocols that appeared to
influence sensitivity and selectivity in response to
carcinogens. The in vitro test for chromosomal aberrations
was, therefore, considered to be the most appropriate
complementary assay to the salmonella test and it was con-
cluded that a combination of these two assays may provide
an efficient primary screen for possible new carcinogens
and mammalian mutagens.
2. THE COLLABORATIVE STUDY ON SHORT-TERM IN VIVO TESTS
FOR MUTAGENS AND CARCINOGENS (CSSTT/2) 1983-1985
In general terms, the in vitro part of the collabor-
ative study (CSSTT/1) achieved its targets by indicating a
small group of assays that show promise as complementary
tests to the salmonella mutation assay. Detailed scrutiny
of the data also identified critical aspects of the proto-
cols of these assays that required modification in order
to reach the levels of reproducibility, sensitivity, and
selectivity already achieved by the salmonella assay.
Even so, the IPESTTC study had shown that 7 of 14 presumed
non-carcinogens elicited positive responses in many of the
in vitro assays. In the same study, these seven non-car-
cinogens were predominantly inactive in a series of short-
term in vivo tests. The in vivo test data presented in
the IPESTTC study were limited, i.e. only five investi-
gators provided results from mammalian in vivo tests and
only a proportion of the chemicals were tested by each
investigator. The results suggested that, although some
non-carcinogens were positive in vitro, they were inac-
tive in in vivo short-term tests. The non-carcinogens in
two of the carcinogen/non-carcinogen pairs, i.e. benzo-
[a] pyrene/pyrene (BP/PYR) and 2-acetylaminofluorene/4-
acetylaminofluorene (2AAF/4AAF), provided good examples of
these different responses.
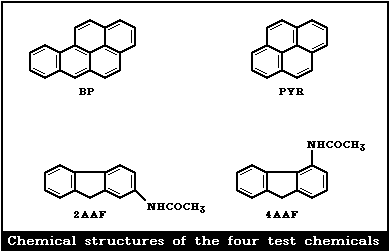 These observations were the origin of the present
study, the object of which was to generate a comprehensive
data profile from a broad range of short-term in vivo
assays as a means of understanding how various genetic
end-points in key target tissues respond to chemicals
defined as genotoxic in vitro. The objectives and design
of the collaborative study on short-term in vivo tests
(CSSTT/2) were outlined by an ad hoc Working Groupa at a
meeting organized by the IPCS in Geneva on 30 April 1981,
and the plans were consolidated by an IPCS Working
Groupb in Geneva, on 13-14 November 1981. The sub-
sequent coordination of the collaborative study was the
responsibility of a Steering Committeec derived primar-
ily from the Working Group.
The four test chemicals selected for the in vivo pro-
ject were the two carcinogen/non-carcinogen pairs, i.e.
BP/PYR and 2AAF/4AAF, that provided the initial impetus to
the study, and the first samples were distributed to
investigators in March 1983. During 1983, additional par-
ticipants joined the study to provide data on new in vivo
assays and nine coordinatorsd were appointed to oversee
the work being conducted by investigators performing
identical or similar assays. In all, 97 investigators from
16 countries participated in CSSTT/2. Progress was
reviewed at a meeting of the Steering Group with the
coordinators held in Brussels on 12-13 January 1984. At
this meeting a plan was developed to enter the data from
the study into a computer at NIEHS. It was envisaged that
such a plan would allow a comprehensive statistical
analysis using common statistical techniques for each kind
of assay and provide a comparison of results from
different laboratories performing the same assay.
Initially, it was expected that all the studies would be
completed by October 1984, but by September 1984 it was
evident that additional time would be required for many of
the investigators to complete their evaluation of the four
chemicals. At a second meeting of the Steering Group and
coordinators, held on 14-17 November 1984, status reports
prepared by the coordinators were reviewed in detail and
deadlines were set for the provision of final reports from
each investigator. Two additional coordinators were ap-
pointed at this meeting to oversee the rodent dominant
lethal assay (Dr W. Generoso) and the mouse coat colour
spot test (Dr R. Fahrig). Following this progress review,
a meet-ing of investigators was planned for May 1985, for
the presentation, collation, and assessment of the results
and the preparation of final reports on the study.
--------------------------------------------------------------------------------
a Participants: Dr J. Ashby, Professor N.P. Bochkov, Dr B.E. Matter,
Professor T. Matsushima, Dr F.J. de Serres, Dr M. Shelby, and Professor
F.H. Sobels.
b Participants: Dr J. Ashby, Dr G.R. Douglas, Dr M. Ishidate, Jr., Dr A.
Leonard, Dr N. Loprieno, Dr B.E. Matter, Professor T. Matsushima, Dr R.
Montesano, Dr F.J. de Serres, Dr M. Shelby, Professor F.H. Sobels, Dr M.
Stoltz, and Dr M. Waters.
c Steering Committee: Dr F.J. de Serres (Chairman), Dr J. Ashby, Dr. M.
Ishidate, Jr., Dr B. Margolin, Dr M. Shelby, Dr M. Draper, and Dr G.C.
Becking.
d Coordinators: Dr W. Vogel, Dr W.M. Generoso, Dr I.-D. Adler, Dr D. Wild,
Dr. T. Tice, Dr B.E. Butterworth, Dr D. McGregor, Dr M. Salamone, and
Dr R. Fahrig.
The meeting of investigators was held during 15-21 May
l985 at Cap d'Agde, France. Representatives from each par-
ticipating laboratory met to prepare a series of reports
comprising a) an assessment of each group of assays, b)
summary reports on the germ cell assays and the liver-
specific assays, and c) summary reports on the total data
base on each pair of chemicals, i.e. BP/PYR and 2AAF/
4AAF. During the meeting, draft reports were prepared and
were to be finalized during the following two months. At
a meeting of the Editorsa held at NIEHS from 24 July to 6
August 1985, the work group and investigators' reports
were reviewed, and a time-table was developed for the com-
pletion of all reports and for the preparation of the
Introduction and Overview of the study in readiness for
final publication of the in vivo study.
A feature of both the CSSTT studies and the earlier
IPESTTC project was the voluntary participation of a large
number of scientists together with support from their
parent institutions. The organization of CSSTT/1 and
CSSTT/2 was financed largely by IPCS and some of its par-
ticipating institutions. In most cases, funding of the
experimental work was by individual investigators, many of
whom incorporated the studies into their research pro-
grammes. This, of course, required the goodwill and
support of senior management of the participating labora-
tories from universities, research institutions, and in-
dustrial research facilities throughout the world. Ad-
ditional financial assistance was provided by a number of
governments that support IPCS, including Belgium, Italy,
Japan, The Netherlands, the United Kingdom, and the USA.
The United Kingdom Department of Health and Social Secur-
ity funded the rat carcinogenicity studies with 2AAF and
4AAF. The Belgian government and its National Institute
of Hygiene and Epidemiology financed meetings of the
Steering Committee and coordinators in Brussels. The
meeting of investigators held in Cap d'Agde was organized
by the French government and cosponsored by the French
Ministry of Health and the Commission of European
Communities.
--------------------------------------------------------------------------------
a Editors: Dr J. Ashby, Dr F.J. de Serres, Dr M.D. Shelby, Dr B.H.
Margolin, Dr M. Ishidate, Jr., and Dr G.C. Becking.
3. OVERALL AIMS OF THE STUDY AND CRITERIA FOR THE SELECTION OF
APPROPRIATE SHORT-TERM IN VIVO TESTS
Because of their relative simplicity, reproducibility,
and reliability, short-term in vitro tests are the methods
of choice for the initial testing of chemicals for geno-
toxic activity. The role and usefulness of genotoxicity
assays using whole animals, i.e. short-term in vivo
tests, are less clearly defined despite the fact that
they are widely used and are an integral part of most
legislative guidelines for the conduct of mutagenicity
tests. In general, in vivo tests are more resource-
consuming than their in vitro counterparts and the use of
animals for experiments for which there is an acceptable
in vitro alternative is to be discouraged. As these
observations suggest, in vivo assays should be designed to
answer questions that cannot be investigated adequately
with in vitro tests.
3.1 The use of short-term tests for the primary identification
of genotoxic chemicals
Certain short-term in vivo assays, such as the rodent
bone marrow chromosome assay, the rodent dominant lethal
test, and the host-mediated assay, were used in the early
1970s in primary screens for the identification of muta-
genic chemicals. With the introduction of reliable and
valid short-term in vitro tests for mutagens in the mid-
1970s, the use of in vivo procedures for initial screen-
ing of chemicals declined considerably. Certain legislat-
ive authorities still recommend a combination of in vitro
tests reserved for confirmatory or supplementary use or
for providing data for hazard assessment. These different
roles for whole animal procedures have led to the accept-
ance of test protocols of varying complexity, i.e. less
rigorous protocols for screening modes and more comprehen-
sive protocols when the test is required to assess hazard
potential.
Implicit in the concept of the two IPCS studies is the
assumption that chemicals shown to be genotoxic in vivo
also exhibit genotoxic activity in a properly designed
and conducted in vitro primary screen. This principle is
well-established in the scientific literature and was con-
firmed in the IPESTTC and CSSTT/1 studies. Thus, it was
an integral principle in the study design that the primary
screening of chemicals could be adequately served by in
vitro tests alone and that short-term in vivo assays have
no role to play when screening for genotoxic activity. In
vivo procedures will, therefore, be reserved for more
specific applications such as investigating the activity
of in vitro genotoxins in the whole animal and to assist
in the assessment of the mutagenic and carcinogenic poten-
tial associated with exposure of humans to in vitro geno-
toxins.
3.2 The use of short-term in vivo assays for assessing the
hazard associated with exposure to in vitro genotoxins
It is apparent from the last paragraph that the major
objective of the study was to investigate the activity in
the whole mammal of chemicals identified as genotoxic in
vitro and to establish which in vivo tests are the most
useful for this purpose. The major criterion for an ac-
ceptable short-term in vivo test, therefore, rests with
its ability to differentiate between carcinogenic and non-
carcinogenic chemicals, particularly those that have been
identified as genotoxic in an in vitro primary screen. To
extend this criterion further, an acceptable assay should
be capable of separating carcinogen/non-carcinogen ana-
logues, e.g., 2AAF/4AAF, in which, in some cases, small
differences in chemical structure are responsible for
dramatic differences in carcinogenic potential. Other
important criteria, some of which were more clearly
characterized during the course of the study, include the
following:
* data should be reproducible between different labora-
tories,
* the assays should not require too high a degree of
technical and scientific expertise to be conducted on
a routine, every-day basis,
* the genotoxic changes or end-points of the assays
should be clear and unambiguous,
* there should be agreed, valid statistical techniques
to differentiate between positive and negative data.
It is relevant to the performance of in vivo tests to
consider the metabolic fate of carcinogenic chemicals.
As a generalization and depending on the route of exposure
of the animal to the chemicals, reactive metabolites of
many carcinogens are generated mainly in the liver as a
by-product of a predominantly detoxifying process. These
metabolites may be capable of interaction with the genetic
material, DNA, in the liver cells, they may be transported
in an active form to other tissues, or they may be further
modified in those tissues to forms able to interact with
DNA. Thus, a study of the genotoxic activity of a chemical
in the whole animal should be able to detect the genotoxic
effects of chemicals or their metabolites in tissues out-
side the liver and of those whose main genotoxic reac-
tivity may be confined to the liver itself. For example,
assays based on genetic alterations to cells in the bone
marrow, which is readily accessible to chemicals or their
metabolites circulating in the blood, respond to a wide
range of chemical carcinogens. However, where reactive
metabolites are not readily transported from the liver,
bone marrow-type tests would be of little value and assays
capable of detecting the genotoxicity in liver cells
should be available.
3.3 The role of short-term in vivo tests in research
into the mechanisms of cancer
Chemical carcinogenesis is generally recognized as a
multistage process that begins with initiation, usually
considered to involve changes in DNA structure leading to
mutations, followed by promotion of the lesion to a pre-
malignant state and progression to overt cancer. Thus, the
majority of current short-term tests, designed to detect
the consequences of DNA interaction, will only respond to
chemicals that may induce tumours by a predominantly geno-
toxic mechanism or induce the initial phase of the car-
cinogenic process.
A feature of the literature on short-term tests for
chemical carcinogens is the occasional reference to chemi-
cals, shown to be associated with the induction of tumours
in laboratory animals, that consistently fail to be de-
tected in short-term in vitro or in vivo assays for geno-
toxicity. Such negative observations with established car-
cinogens have been explained by a lack of sensitivity of
the particular assays. If these chemicals (diethylhexyl-
phthalate is an example) are truly non-DNA-reactive, how-
ever, then negative data in assays for genotoxicity would
be the expected result. The acceptance of the existence
of so-called "non-genotoxic carcinogens" is of critical
importance to the future of short-term testing and one
consideration during the assessment of the CSSTT/2 study
was the identification of in vivo procedures that may be
useful for investigating such chemicals with the eventual
objective of developing specific short-term tests for non-
DNA-reactive carcinogens.
3.4 Assays for the detection of germ cell mutagens
As a general principle of genetic toxicology, genetic
damage induced by chemicals in somatic cells results in
hazard only to the affected individual, while genetic
effects in male or female germ cells may cause heritable
disease or malformation in the immediate progeny or in
future descendants of the affected individual. The ma-
jority of the assays represented in the CSSTT/2 study were
conducted in cells from somatic tissues and are, there-
fore, only directly interpretable in terms of somatic
mutation and carcinogenic potential. Although there have
been attempts to extrapolate data derived from somatic
cell assays to assessment of the probability of a chemical
inducing germ cell mutations, there are a number of
assays, some of which were represented in this study, that
measure genetic damage directly in the germ cells. The
objectives behind the inclusion of germ cell assays in the
present study were to determine:
* which kind of germ cell procedure effectively ident-
ifies germ cell mutagens;
* what is the relationship between the induction of
changes in sperm morphology and unscheduled DNA syn-
thesis in germ cells and the formation of true heri-
table mutations;
* which of the four in vitro genotoxins induce genetic
damage in mammalian germ cells.
4. CRITERIA FOR THE SELECTION OF THE FOUR TEST CHEMICALS
Selection of the two pairs of chemicals, benzo [a] pyrene/
pyrene (BP/PYR) and 2-acetylaminofluorene/4-acetylamino-
fluorene (2AAF/4AAF), was based, primarily, on their per-
formance in the IPESTTC study. In that study, seven of
the fourteen chemicals believed to be non-carcinogens
exhibited a high frequency of positive responses in in
vitro tests while being predominantly or totally negative
in a series of short-term in vivo tests. Although the in
vivo data base was limited, the non-carcinogens in the
BP/PYR and 2AAF/4AAF pairs provided good examples of such
differences in response and these four chemicals were
selected as useful representative candidates for investi-
gating the in vivo behaviour of chemicals known to be in
vitro genotoxins.
4.1 Activity of the four test chemicals in short-term in vitro tests
The majority of chemical carcinogens, including BP and
2AAF, require some form of enzyme-mediated biotransform-
ation for the formation of reactive metabolites. Most
target cells, whether bacteria, yeast or cultured mam-
malian cells, used in in vitro tests lack the appropriate
enzyme activity and this is usually provided in the form
of an enzyme-rich fraction derived from homogenates of rat
liver, referred to as the S9 fraction. Liver enzyme
activity is usually stimulated by pre-treatment of the
animals with an enzyme-inducer such as Aroclor 1254.
Both the carcinogens, BP and 2AAF, are consistently
positive in bacterial assays and other in vitro short-term
tests and, with the exception of certain mammalian cell
assays, demonstration of genotoxic activity requires the
incorporation of a rodent liver enzyme system. Positive
results have also be reported for the two non-carcinogens,
PYR and 4AAF, in a number of in vitro systems but not on
the scale nor usually with the same potency as their
carcinogenic analogues. Thus, both PYR and 4AAF induce
mutations in bacteria, gene conversion in yeast, and
unscheduled DNA synthesis (UDS) and gene mutation in
cultured mammalian cells. They do not, apparently, induce
mutations in yeast or chromosome aberrations in cultured
mammalian cells. 4AAF, but not PYR, can induce UDS in
cultured primary hepatocytes.
In the salmonella mutation assay, separation of the
carcinogen/non-carcinogen pairs is influenced signifi-
cantly by the nature of the rodent liver activation system
employed in the test. When the liver enzyme suspension is
derived from uninduced rodents, BP and 2AAF give positive
results and the two non-carcinogens, PYR and 4AAF, are
generally negative. If Aroclor-induced animals are used
as a source of the activation system, however, the ability
of the assay to discriminate between the carcinogens and
non-carcinogens is lost.
A comprehensive tabulation of the activities of the
four chemicals in short-term in vitro tests is provided by
McGregor (1988b).
4.2 Summary of carcinogenicity data on the test chemicals
The rodent carcinogenicity data for the four test
chemicals is reviewed in Hicks et al. (1988).
Both BP and 2AAF are well established and potent
carcinogens in rodents. Fewer studies have been conducted
with the non-carcinogens, PYR and 4AAF, and their presumed
non-carcinogenicity, although derived from limited rodent
bioassays, must remain tentative pending more comprehen-
sive testing.
BP is a locally-acting carcinogen in rats and mice,
producing skin tumours after dermal application and
tumours of the stomach when administered orally. In mouse
skin, it has been shown to have both initiating and
promoting activity. BP is also a lung carcinogen in mice
and induces mammary gland tumours in rats. There is no
evidence that BP is hepatocarcinogenic in rats and only a
single, unconfirmed report of the induction of hepatomas
in mice. On balance, the available evidence suggests that
BP does not induce liver tumours in rodents.
PYR has not been tested for carcinogenic activity in
any species other than the mouse and even then, only by
dermal application. These studies have shown fairly con-
clusively that PYR is not carcinogenic by this route.
There is some evidence from promotion/initiation studies
on mouse skin that it may have weak initiating activity.
Because data on systemic carcinogenesis in the mouse and
information from other species are lacking, the carcino-
genicity of PYR is somewhat equivocal. However, the fact
that its analogue, BP, is such a potent skin carcinogen,
whereas the data obtained with PYR in fairly comprehensive
mouse skin studies are negative, suggests that the
presumed non-carcinogenic status of PYR is justified.
2AAF is a potent carcinogen in both rats and mice. It
produces tumours in the liver and bladder in both species
after oral administration and in the rat it is carcino-
genic in Zymbal's glands and mammary glands. Little
information is available about its activity when adminis-
tered by other routes.
4AAF has only been tested in rats by incorporation in
the diet, and its activity in mice has not been investi-
gated. In rat feeding experiments, 4AAF has been consist-
ently non-carcinogenic though there are limited data to
suggest that its metabolite, N -OH-4AAF, may have some
weak carcinogenic activity. If 4AAF does prove to be
carcinogenic, its activity is clearly far less potent than
that of 2AAF and further long-term studies are required
before 4AAF can be unequivocally regarded as non-carcino-
genic. The question may be resolved when the results of
the rat carcinogenicity study, initiated as part of the
CSSTT/2 project, are made available.
5. SOURCE AND PURITY OF THE TEST CHEMICALS
Samples of the four test chemicals were obtained from
commercial sources: BP, 2AAF, and 4AAF were supplied by
Lancaster Synthesis Ltd., Eastgate, Whiteland, Morecambe,
Lancashire, United Kingdom, and PYR was obtained from
Aldrich Chemicals, Gillingham, Dorset, United Kingdom.
4AAF was synthesized by a route that avoided contamination
with 2AAF. The purity of the chemicals was determined by
the supplying laboratories before dispatch to the
participants. All the chemicals were greater than 99.5%
pure and full analytical details are provided by Paton and
Ashby (1988).
6. SHORT-TERM IN VIVO ASSAYS
For convenience the assays are divided into six groups:
* cytogenetic (chromosome) assays conducted on cells from
rodent bone marrow or other non-hepatic tissues;
* assays investigating a variety of effects in cells from
rodent liver;
* miscellaneous assays that utilize other tissues or body
fluids;
* mouse coat colour spot tests;
* assays in mammalian germ cells;
* tests utilizing the fruit fly, Drosophila melanogaster.
6.1 Cytogenetic assays
Assays for the induction of chromosome aberrations,
i.e., microscopically visible alterations in chromosome
structure, were conducted in bone marrow cells from rats,
mice, and Chinese hamsters and also in mouse ascites
tumour cells.
Micronucleus tests were conducted on cells from rat
and mouse bone marrow and on erythrocytes in circulating
blood from mice. Micronuclei are chromosome fragments or
intact chromosomes excluded from the cell nucleus during
mitosis. They are considered to be evidence of induced
chromosome breakage or chromosome loss and are usually
analysed in developing or mature erythrocytes.
Sister chromatid exchanges (SCE) can be demonstrated
in metaphase chromosomes by a differential staining tech-
nique and occur as a consequence of the exchange of repli-
cating DNA between chromatids at apparently homologous
loci. Although considered to result from DNA breakage and
reunion, the mechanism of the formation of SCE is not
fully understood. Assays for the induction of SCE were
conducted in bone marrow cells from rats, mice, and
Chinese hamsters, in circulating blood leucocytes from
mice, and in Chinese hamster intestinal epithelium.
Table 1. Assays employed in the CSSTT/2 study
-------------------------------------------------------------------------
1. Cytogenetic studies
Chromosome aberrations
Mouse bone marrow
Mouse ascites tumour cells
Rat bone marrow
Chinese hamster bone marrow
Micronucleus tests
Mouse bone marrow
Mouse circulation blood cells
Rat bone marrow
Sister chromatid exchanges
Mouse bone marrow
Mouse circulating blood lymphocytes
Rat bone marrow
Chinese hamster bone marrow
Chinese hamster intestinal epithelial cells
2. Liver-specific assays
Tests for initiation/promotion: altered enzyme foci
Unscheduled DNA synthesis (UDS)
UDS in mouse liver
UDS in neonatal, weanling and adult rat liver
Frequency of S-phase hepatocytes
S-phase in mouse liver
S-phase in weanling and adult rat liver
Cytogenetic tests
Aberrations in rat liver epithelial-like cells
SCE in rat liver epithelial-like cells
Micronuclei in hepatocytes
Diploid/tetraploid ratio in rat and mouse hepatocytes
Primary changes in DNA
Alkaline elution assay for DNA strand damage
DNA/protein cross-links
DNA unwinding assay for strand damage
3. Miscellaneous assays
Specific carcinogenicity assays
Two-year oral dosing study in rats
Mouse lung adenoma assay
Quail egg tumour-induction
UDS in rat fore-stomach
Sebaceous gland suppression assay
Observation of dermal epithelial hyperplasia
Transformation of rat peritoneal macrophages
6-Thioguanine-resistant mutations in Syrian hamster lung cells
Measurement of DNA adducts
32P-post-labelling in rat and mouse tissues
Immunochemical detection of DNA adducts
Radiolabelled test chemicals - rat liver
-----------------------------------------------------------------------------
Table 1. (contd.)
-----------------------------------------------------------------------------
3. Miscellaneous assays (contd.)
Immunotoxicity assays
Natural Killer (NK) cell and T-cell cytotoxicity in rats
Host-mediated assays
Mutation of salmonella in mouse tissues
Genetic changes in yeast cells in mouse tissues
Urine mutagenicity tests in rats, mice and guinea pigs
4. Mouse spot tests
Mouse coat colour spot test
Mouse melanocyte assay
5. Mammalian germ cell studies
Dominant lethal assays with male and female mice and male rats
Morphological abnormalities in mouse and rat spermatozoa
Unscheduled DNA synthesis in rat and mouse male germ cells
6. Drosophila assays
Sex-linked recessive lethal mutations in germ cells
Chromosome loss in germ cells
Somatic mutation and recombination: mosaic spots in eyes
and wings
-----------------------------------------------------------------------------
6.2 Assays in rodent liver cells
A variety of liver-specific assays were represented in
the study, including the demonstration of enzyme changes,
a number of different tests on DNA, and cytogenetic
assays.
The rat liver assay for altered enzyme foci employs a
histochemical technique that identifies groups of cells or
foci that have elevated levels of the marker enzyme gamma-
glutamyltranspeptidase (GGT). The observation of GGT-
positive foci often precedes the appearance of liver car-
cinoma and the test is used to attempt to identify early
stages of carcinogenesis in the liver. Protocols have been
devised to investigate both initiation and promotion
stages of carcinogenesis.
A number of investigators used assays that measure
directly or indirectly the response of DNA to chemical
damage. These included tests for breaks in single strands
of DNA using the alkaline elution technique, an assay for
the induction of crosslinks between DNA and protein mol-
ecules, and a measure of single strand breaks based on the
degree of unwinding of DNA molecules. One consequence of
DNA breakage is the initiation of the enzyme-mediated
repair process which involves the synthesis of new, rela-
tively short strands of DNA to repair the break. This type
of repair is referred to as unscheduled DNA synthesis or
UDS (to differentiate from the normal or S-phase DNA syn-
thesis that occurs during cell replication). DNA synthesis
can be measured by observing the uptake of tritiated thy-
midine by the newly synthesized strands using autoradio-
graphical techniques. Assays for the induction of UDS and
to measure changes in the numbers of S-phase cells in
rodent liver were included in the study.
The cytogenetic methods included the analysis of meta-
phase chromosome aberrations, micronuclei and SCE in rat
liver cells, and the determination of the ratio of diploid
to tetraploid cells in rat and mouse liver.
6.3 Miscellaneous assays
The first group of assays to be considered under this
heading are those that are directly related to the induc-
tion of tumours in rodents. The most important of these
is a 2-year oral dosing study in rats to compare the car-
cinogenicity of 2AAF and 4AAF. Although the study was not
completed at the time of this report, preliminary infor-
mation from a small group of rats examined after 26 weeks
of dosing is available. Also included in this group was
a) the mouse lung tumour assay that determined the effects
of each of the four test chemicals on the incidence of
lung adenoma in mice and b) a test in Japanese quail in
which the test chemicals were introduced into the yolk
sacs of quail eggs. The birds were then examined for
evidence of tumours at various intervals after hatching.
The sebaceous gland suppression test is based on the
observation of morphological changes to the sebaceous
glands in histological preparations of mouse skin after
dermal exposure to the test chemicals. The presence of
epidermal hyperplasia can also be detected histologically
and both methods have been shown to respond to skin car-
cinogens, particularly polycyclic hydrocarbons.
Genotoxic carcinogens can bind covalently to biologi-
cal macromolecules such as DNA either before or after
metabolic biotransformation. The products of DNA-binding
are referred to as DNA adducts and a number of investi-
gators provided data on the detection and measurement of
DNA adducts with the test chemicals in tissues from rats
and mice. Three different methods were represented:
* the enzyme-linked immunoabsorbent assay (ELISA);
* the 32P-post-labelling assay;
* an assay for radiolabelled chemicals bound to DNA.
Two similar assays for the investigation of the immune
response of animals to exposure to carcinogens were rep-
resented. Natural Killer (NK) cells are leucocytes with
the ability to lyse a variety of `foreign' cells, e.g.,
those with a malignant phenotype. The NK cell assay was
used to investigate the capacity of NK cells, derived from
the splenic mononuclear leucocytes of rats treated with
2AAF or 4AAF, to lyse human erythroleukaemic cells in
vitro. The basis of the T-cell assay is that immunocom-
petent T-cells are able to react to tissue changes induced
by carcinogenic chemicals in rats. The changes in T-cell
activity can be determined by their cytotoxicity towards
target cells derived from rat intestinal adenocarcinoma.
The rat peritoneal cell transformation test is based
on the observation that mitogen-stimulated peritoneal
cells, harvested from rats dosed with carcinogens, undergo
a form of transformation that enables them to proliferate
and produce colonies in soft agar culture.
Host-mediated assays were used fairly widely in the
early l970s as primary screening tests for mutagenic
chemicals. In its original form, a suspension of microbial
target cells, i.e. yeast or bacteria, was introduced into
the peritoneal cavities of mice. The mice were treated
with the suspect chemical and, after an appropriate inter-
val, the target cells were harvested and changes in the
mutation frequency or other genetic end-points were deter-
mined in in vitro culture. Since that time, there have
been several modifications to the procedure, the most
significant being the injection of the target organisms
into the circulating blood of the host. In this way, the
bacterial or yeast target cells are distributed in various
organs and can, for example, be harvested from the liver,
lungs, and kidney. The target cells can, in principle, be
affected by reactive metabolites generated from the test
chemical in these organs. Intra-sanguinous host-mediated
assays using either Salmonella typhimurium or the yeast,
Saccharomyces cerevisiae, were represented in the study.
Urine and other body fluids can be collected from
rodents treated with test chemicals and assayed for muta-
genic activity. For example, after appropriate treatment
to release conjugated metabolites, urine can be tested for
the presence of mutagens in a conventional in vitro bac-
terial mutation assay. In principle, the urine assay is
capable of detecting mutagenic chemicals excreted un-
changed or after metabolic activation.
6.4 The mouse spot test
The mouse spot test detects mutations induced in mel-
anocyte precurser cells in the embryos of specially de-
rived strains of mice. The mouse strain carries recessive
mutations at a number of specific coat colour loci and the
embryos are, thus, heterozygous at these loci. Mutations
induced at the wild-type or normal alleles of the coat
colour loci result in the development of clones of the
mutant melanocytes. The young mice are examined after
birth and the mutations are expressed as patches of con-
trasting coloured fur. The mutations can result from one
of a number of genetic events including gene mutations,
chromosome deletions, mitotic crossing-over, and loss of
whole chromosomes. In addition to the conventional tech-
nique of observing the appearance of coloured spots in the
fur, a modification of the spot test allows the micro-
scopic recognition of mutant melanocytes in preparations
of embryonic skin.
6.5 Mammalian germ cell studies
The classical dominant lethal assay in male rodents
involves the treatment of the animals with the test chemi-
cal and then mating with groups of females at intervals to
cover the complete spermatogenic cycle. Dominant lethal
mutations induced at any stage of spermatogenesis can be
detected by dissection of the uterine contents of each
female and are characterized by dead fetuses or a re-
duction in the numbers of fetal implantations. Dominant
treatment of male rats, male mice, or female mice.
Two other assays represented in this group were the
abnormal sperm morphology assay and the unscheduled DNA
synthesis (UDS) test. The former assay monitors mature
spermatazoa for irregularities in morphology at intervals
after treatment of rodents with the test chemicals. The
UDS test measures DNA repair in developing spermatocytes
and spermatids.
6.6 Drosophila assays
Although data from drosophila tests cannot be regarded
as appropriate alternatives to mammalian data for assess-
ing the potential hazard to humans from exposure to geno-
toxic chemicals, drosophila are, in fact, intact, complex,
eukaryotic organisms whose metabolic and genetic charac-
teristics have some parallels with those of the whole mam-
mal. Their main value in genotoxicity testing lies with
the availability of assays to study the effects of chemi-
cals on both somatic and germ cells. The tests represented
in the CSSTT/2 study were:
* the sex-linked recessive lethal mutation assay in
germ cells;
* tests for chromosome loss from germ cells;
* assays for somatic mutation and recombination using
the induction of mosaic spots in either the wings or
the eyes.
7. RESULTS
The investigators met at Cap d'Agde, France, from 15
to 21 May 1985, to assess the results of the study. Each
group of investigators presenting data from a particular
type of assay discussed their data and individual results
were assessed and agreed. This led to the preparation of
consensus reports on the response of each assay to the
four test chemicals. These consensus views were then
incorporated into coordinators' summary reports on each
group of assays. Summary reports were presented and criti-
cally discussed in open plenary discussions, thereby
allowing the overall conclusions and recommendations
resulting from the study to be formulated. Reports of
individual investigators, the coordinators summary
reports, and certain technical appendices form the main
text of the final publication together with an editorial
overview of the study (Ashby et al., 1988).
The purpose of this chapter of the report is to
present the results of the in vivo studies with the four
test chemicals and to construct a profile, in qualitative
terms, of their genotoxic activity in the whole animal.
Quantitative differences in response in relation to sex,
species, route of exposure, and technical variations are
considered in more detail in the next section, which also
includes a comprehensive tabulation of the data generated
by individual investigators (Table 4). However, as many
of the assays were replicated in a number of laboratories,
a simplified table of results is presented here (Table 2).
It must be emphasized that Table 2 contains the consensus
views of the Working Groups assessing the individual
results and performance of each assay and that, in some
cases, there were conflicting results on the same assay
from participating laboratories.
Table 2. Results of the short-term in vivo tests summarized
by Working Groups a
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
1. Cytogenetic studies
Chromosomal aberrations
Mouse bone marrow P N ? N
Mouse ascites cells P NT N N
Rat bone marrow P N ? N
Chinese hamster bone marrow P N P N
Micronucleus tests
Mouse bone marrow P N P N
Mouse blood - maternal P N N N
Mouse blood - fetal P N ? N
Rat bone marrow P N P N
Sister chromatid exchange
Mouse bone marrow P W P W
Mouse blood P N P N
Rat bone marrow P W P W
Chinese hamster bone marrow P N P N
Chinese hamster intestinal cells P N P N
2. Liver-specific assays
Altered enzyme foci - rat P N P W
Unscheduled DNA synthesis
Mouse liver N N N N
Rat liver N N P W
S-phase hepatocytes
Mouse liver NT NT W P
Rat liver - adult W N ? P
Rat liver - weanling P NT NT NT
Cytogenetic tests
Metaphase aberrations - rat W N W N
Micronuclei - rat N N P N
SCE - rat W N ? N
Ploidy - rat P N P N
Ploidy - mouse P N P N
Primary DNA changes
Alkaline elution N N ? ?
DNA/protein cross-links P N P NT
DNA unwinding N N P N
with repair inhibitor P N P N
3. Miscellaneous assays
Carcinogenicity
Oral dosing - rat NT NT P ?
Mouse lung adenoma P N P N
Quail egg tumours ? ? ? ?
UDS in rat fore-stomach ? N N N
--------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
3. Miscellaneous assays (contd.)
Sebacious gland suppression - mouse P N NT NT
Epithelial hyperplasia - mouse P N NT NT
Peritoneal macrophages - rat N N N N
Syrian hamster lung - mutation P N ? N
DNA adducts
32P-post-labelling - rat P N P W
Immunochemical (ELISA) NT NT ? ?
Radiolabelled chemicals NT NT P P
Immunotoxicity
Natural Killer (NK) cells NT NT P N
T-cell cytoxicity P N P W
Host-mediated assays - mouse
Salmonella typhimurium N N N N
Saccharomyces (liver) P P P P
Saccharomyces (lung) N N N N
Urine mutagenicity
Rats P P P P
Mice W W P P
Guinea-pig NT NT P NT
4. Mouse spot tests
Coat colour spots P N P N
Melanocyte assays NT NT P N
5. Mammalian germ cells
Dominant lethal assays
Male mice P N N N
Female mice P N N N
Male rats NT NT N N
Sperm abnormalities
Mice P N P N
Rats NT NT N N
UDS in germ cells
Mice N N N N
Rats N N N N
6. Drosophila assays
Sex-linked recessives N N N N
Chromosome loss N N N N
Somatic mutation
Eye spots P N P N
Wing spots P N W ?
--------------------------------------------------------------------------
a P = positive
W = weak positive
N = negative
? = results inconclusive or not yet reported
NT = not tested
7.1 Benzo [a] pyrene (BP) and pyrene (PYR)
7.1.1 Cytogenetic studies
Assays for the induction of structural chromosome
aberrations or micronuclei in rodent bone marrow cells
were consistently positive with BP and negative with PYR.
Among a number of experimental variables between different
investigators, neither the species, strain, sex, solvent,
nor route of exposure affected the qualitative result. BP
increased the incidence of sister chromatid exchanges
(SCE) in mouse, rat, and Chinese hamster bone marrow in
every study. The results with PYR, however, were less
clear, and this presumed non-carcinogen induced SCE at
high dose levels in mice after oral or intraperitoneal
dosing and in male rats after oral administration, i.e. 3
of 9 SCE studies reported a weak positive result. Analysis
of micronuclei in circulating blood erythrocytes from
maternal, fetal, or weanling mice, and SCE in circulating
blood lymphocytes from adult mice clearly discriminated
between BP and PYR.
7.1.2 Liver-specific assays
Five investigators provided data from observations of
altered enzyme foci in rat liver under the general heading
of initiation/promotion assays. There were, however,
significant protocol variations between investigators.
Two protocols required administration of the rodent tumour
promotor, phenobarbitone, in the drinking-water or diet
for some weeks after dosing in order to determine
initiating activity of the test chemicals. The promoting
activity of the test chemicals was studied by two other
workers by producing initiation events in the liver before
treatment with the test chemicals. The fifth protocol
tested the ability of the chemicals to induce both
initiation and promotion without discriminating between
the two phases. In all cases, the evidence for induced
pre-cancerous changes was the observation of foci or
clones of cells with altered enzyme characteristics. BP
was shown to have significant initiating activity in rat
liver in two studies and, in a third study, was shown
capable of promoting nitrosamine-induced initiation.
Using a fourth, essentially experimental, protocol that
involved the transplantation of donor liver cells to the
host animal, BP failed to show promoting activity. No
evidence of tumour initiating or promoting activity was
observed in any of the studies with PYR.
The rodent liver assay for unscheduled DNA synthesis
measures the induction of repairable lesion in the DNA of
hepatocytes and, theoretically, should be capable of
detecting chemicals that are metabolized in the liver to
produce genotoxic (i.e. DNA-interactive) metabolites.
Both BP and PYR were reproducibly negative in mouse
hepatocytes and in hepatocytes from adult, weanling, or
neonatal rats using either oral or intraperitoneal dosing.
The induction of S-phase DNA synthesis was also
investigated and BP was shown to increase the incidence of
S-phase cells in weanling rats and, in one of two
experiments, a slight increase in S-phase cells was
observed in adult rats. In mice, BP had no significant
effect on the incidence of S-phase, and PYR showed no
evidence of S-phase synthesis induction in either
species.
Limited data were presented on the cytogenetic effects
of the chemicals in liver-derived cells. In epithelial-
like cells from weanling rats, BP was observed to induce a
small increase in the incidence of structural chromosome
aberrations and SCE; tests with PYR were negative. These
findings, however, require confirmation. No evidence of
micronucleus-induction was detected in rats treated with
BP or PYR after partial hepatectomy. Using a cytofluoro-
metric method, an increase in the ratio of diploid to
tetraploid cells was observed in liver-derived cells after
treatment of rats with BP, but not after dosing with PYR.
Three different assays were used to study the ability
of the chemicals to induce single-strand breaks (SSB) in
liver cell DNA. Both BP and PYR were uniformly negative
in the alkaline elution assay for SSB conducted in three
laboratories. BP, however, was shown to induce crosslinks
between DNA and protein, while PYR was negative in this
assay. The third assay in this group was a measure of SSB
based on the degree of alkali-induced unwinding of DNA
molecules. When the DNA-repair inhibitor, adenosine
arabinoside was incorporated, BP was shown to induce SSB
in mouse liver cell DNA using this technique. In the
absence of the repair inhibitor, SSB were not observed.
PYR produced negative results in the DNA-unwinding assay.
The results from this group of assays suggest that BP is
capable of inducing SSB in the DNA of mouse liver cells
that are effectively repaired in the absence of a DNA-
repair inhibitor. In rats, the observation of DNA/protein
crosslinks tentatively suggests the induction of SSB in
rat liver cells, but additional studies are required to
assess the DNA effects of BP in rat liver.
7.1.3 Miscellaneous assays
Two investigators are producing data that are directly
related to the induction of tumours. In the lung adenoma
assay, mice were injected with BP or PYR by the intra-
peritoneal route, 2-3 times each week, for up to 8 weeks.
The mice were examined 23 weeks after the start of the
study and the numbers of adenoma on the surface of the
pulmonary lobes were recorded. BP induced a significant
increase in adenomas compared with the untreated control
group while no increase in the induction of these tumours
was observed in mice injected with PYR. This assay
differentiated very clearly between the carcinogenic
activity of BP and PYR. The second study in this category
involved injecting the test material into the yolk sac of
quail eggs and then observing the birds for the presence
of tumours at intervals after hatching. In birds examined
at approximately 3 months, there was no evidence of tumour
induction by either BP or PYR. Surviving birds will be
maintained for up to 12 months before being examined for
the presence of tumours and, therefore, a definitive
conclusion on the carcinogenicity of BP and PYR to quail
is not yet possible.
One investigator studied the induction of UDS in the
fore-stomach of rats after oral dosing; the data with BP
were considered to be equivocal while PYR gave negative
results.
Two assays involved the application of the test chemi-
cals to mouse skin followed by histological examination of
skin sections. BP induced epithelial hyperplasia and
suppression of sebaceous glands, both of which have been
correlated with polycyclic hydrocarbon-induced skin
carcinogenesis. PYR failed to elicit either of these
responses.
The transformation of rat peritoneal macrophages has
been advocated as a short-term in vivo test for poten-
tially carcinogenic chemicals. Macrophages isolated from
rats dosed with BP or PYR, however, failed to show any
evidence of transformation in experiments conducted in
three laboratories.
Somatic mutation data on cells isolated from Syrian
hamster lung tissue, after dosing with the test chemicals,
were presented by one investigator. Cultures of cells
from animals dosed with BP showed a higher frequency of
mutations at the 6-thioguanine locus than those from
untreated hamsters, suggesting the induction of somatic
mutations in lung cells from this species. PYR produced
negative mutation data in this assay.
32P-labelling of purified DNA was used to detect DNA
adducts in tissues from rats and mice treated intraperito-
neally with the test chemicals. Measurable levels of PYR
adducts were not detected in DNA from mouse liver, lung,
or kidney, or from rat liver or lung tissues. BP adducts
were identified in all of these tissues.
Only one of the two assays for immunotoxicity was con-
ducted with the BP/PYR pair. Thus, the T-cell assay showed
that BP, but not PYR, was capable of inducing a cytotoxic
response in rat T-cells.
Two investigators provided data from host-mediated
assays in mice dosed with BP or PYR. In tests with the
yeast, Saccharomyces cerevisiae, a significant increase
in mitotic gene conversion was detected in yeast cells
isolated from the liver of mice dosed with either BP or
PYR, while yeast cells isolated from lung tissue produced
negative data with both chemicals. No evidence of the
induction of mutations was observed in Salmonella
typhimurium isolated from liver tissues of mice dosed with
BP or PYR.
Assays for the detection of mutagens in urine were
conducted with both rats and mice. Mutagenic activity was
detected in urine samples from both species after dosing
with BP or PYR and, therefore, the test failed to differ-
entiate between the two chemicals.
7.1.4 Mouse spot tests
Only one investigator provided mouse spot test data on
BP and PYR. Using the observation of coat colour spots in
off-spring as evidence of somatic mutation, BP was clearly
positive and PYR negative.
7.1.5 Mammalian germ cell assays
BP induced dominant lethal mutations in both male and
female mice after intraperitoneal injections but not after
oral dosing. The response in the male was highly stage-
specific; only the mature spermatozoa were sensitive and
spermatid and spermatocyte stages were unaffected. Domi-
nant lethal assays with PYR were reproducibly negative.
Studies of morphological abnormalities in mature
spermatozoa were conducted in mice after oral or
intraperitoneal dosing. BP was uniformly positive in this
assay, regardless of the route of exposure. Although a
weak positive response was observed in one of seven
experiments with PYR, this observation was not confirmed
and the consensus view was that PYR did not induce sperm
abnormalities. In studies of the induction of unscheduled
DNA synthesis in rat and mouse male germ cells, negative
results were obtained with both BP and PYR.
7.1.6 Drosophila assays
In all, ten studies were conducted with BP and PYR in
drosophila and in each case the oral route of treatment
was used. Germ cell studies, i.e. the sex-linked
recessive lethal mutation assay and the test for chromo-
some loss, were uniformly negative with both chemicals.
In contrast, tests for somatic mutation and recombination,
based on the observation of eye or wing spots, showed that
BP was able to induce genetic changes in somatic cells in
each of three studies. PYR was consistently negative.
7.2 2-Acetylaminofluorene and 4-acetylaminofluorene
7.2.1 Cytogenetic studies
Seven investigators provided data from analysis of
metaphase chromosomes in mouse bone marrow cells. 2AAF
induced a significant increase in the incidence of
structural chromosome aberrations in only two of seven
assays, one of which was considered to be inconclusive.
The results of assays with 4AAF were negative with the
exception of two assays in which the data were inconclus-
ive. In contrast, 2AAF was reproducibly positive and 4AAF
consistently negative in 15 of 16 micronucleus tests
conducted in mouse bone marrow erythrocytes. The route of
exposure, i.e. oral dosing or intraperitoneal injection,
did not appear to influence the outcome of these studies.
Micronuclei were produced by 2AAF in rat bone marrow cells
and a small increase was also recorded in one of two
assays with 4AAF. Two workers investigated the influence
of these two chemicals on the incidence of structural
chromosome aberrations in Chinese hamster bone marrow;
2AAF was positive in this species and 4AAF gave a weak
positive result in one of the two studies. Both chemicals
failed to induce detectable chromosome damage in mouse
ascites tumour cells. Four micronucleus tests were
conducted with mouse blood erythrocytes. An increase in
micronuclei was recorded by one worker in blood cells from
weanling mice dosed with 2AAF, although when samples of
maternal or fetal blood were examined, increases in
micronuclei were not apparent. However, studies conducted
in another laboratory showed that 2AAF was capable of
increasing the incidence of micronuclei in fetal blood.
Thus, 2AAF is clearly clastogenic to rodent bone marrow
cells while 4AAF was considered by most investigators to
be devoid of clastogenic activity.
Nine assays for sister chromatid exchanges (SCE) were
conducted in bone marrow cells from mice, rats, or Chinese
hamsters. 2AAF was clearly positive in this test and the
consensus view was that 4AAF, although negative in four
assays, was capable of inducing a small (but significant)
increase in SCE. In studies with mouse lymphocytes and
Chinese hamster intestinal epithelial cells, 2AAF was
positive and 4AAF was considered to be negative.
7.2.2 Liver-specific assays
As discussed in section 7.1.2, there were a number of
variations between the five protocols used in the
initiation/promotion assays. Significant initiation and
promotion activity was demonstrated in studies with 2AAF
based on the observation of foci of cells with altered
enzyme characteristics in rat liver. These assays failed
to discriminate clearly between the two chemicals, since
4AAF was shown to be an initiator in the hands of one
investigator and had weak promoting activity in another
study. Both chemicals, therefore, proved capable of
producing these pre-cancerous changes in rat liver tissue
though with different degrees of potency.
2AAF and 4AAF failed to induce detectable unscheduled
DNA synthesis (UDS) in mouse hepatocytes after oral
dosing. Quite different results were obtained in rat
liver, however, and 2AAF was shown to induce UDS in rat
hepatocytes in each of the seven laboratories that sub-
mitted data. Three of these laboratories also reported the
detection of a small increase in UDS in rats after dosing
with 4AAF. The other four laboratories obtained negative
data. Thus, 2AAF induced UDS in hepatocytes from rats but
not mice, and 4AAF elicited a weak UDS response in the rat
and was negative in mouse cells.
The results of the analysis of S-phase DNA synthesis
indicated that the presumed non-carcinogen, 4AAF, was a
very potent inducer of S-phase cells in the liver of both
rats and mice. 2AAF, on the other hand, induced only a
small increase in S-phase cells in the mouse and three
studies in rat liver produced one clear positive and two
negative results.
In cytogenetic experiments in cells derived from rat
liver, 2AAF appeared to induce a small increase in the
incidence of structural aberrations and, in partially
hepatectomised rats, a significant increase in micro-
nuclei. 4AAF gave negative results in tests for chromo-
somal aberrations, micronuclei, and SCE in rat liver
cells. When the ratio of diploid to tetraploid cells was
investigated in rat and mouse liver, an increase in the
ratio was observed in both species after dosing with 2AAF
but not in response to 4AAF.
The alkaline elution assay for single strand breaks
(SSB) produced conflicting results between three labora-
tories. Two investigators presented negative data from
assays with both chemicals while a third observed that
both 2AAF and 4AAF produced SSB in this test. Using the
detection of DNA/DNA or DNA/protein crosslinks as a
measure of SSB, 2AAF was shown to produce SSB. No data
were presented on 4AAF. Another investigator calculated
SSB from the amount of DNA unwinding induced by the chemi-
cals. 2AAF and 4AAF gave positive and negative results,
respectively, either in the presence or absence of a DNA
repair inhibitor. These data suggest that, unlike 4AAF,
2AAF is capable of producing SSB in rat liver DNA under
appropriate experimental conditions.
7.2.3 Miscellaneous assays
A 2-year oral dosing carcinogenicity study in rats
with 2AAF and 4AAF is still in progress. Preliminary
findings in a small number of animals examined after 26
weeks of dosing showed that an increase in liver tumours
was already apparent in animals dosed with 2AAF; there was
no evidence of tumour induction in the 4AAF-dosed ani-
mals.
The lung adenoma assay, in which mice were given
intraperitoneal injections of the test chemicals 2-3 times
a week for up to 8 weeks, clearly separated 2AAF and 4AAF.
2AAF induced a significant increase in adenomas while the
incidence in mice dosed with 4AAF was comparable with that
of untreated mice.
There was no evidence of the induction of UDS in rat
fore-stomach after oral dosing with either chemical, and
the assay for the induction of somatic mutations in Syrian
hamster lung cells produced inconclusive data with 2AAF
and was negative in tests with 4AAF. The rat macrophage
test was reproducibly negative with both 2AAF and 4AAF.
Three techniques were applied to detect the presence
of DNA adducts formed in various tissues after dosing
rodents with the test chemicals. Data from the ELISA
technique, which requires the use of monoclonal antibodies
to detect specific adducts, are not yet available. How-
ever, the 32P-post-labelling method indicated that 2AAF
formed adducts with DNA in rat liver and lung and in mouse
liver, lung, and kidney after intraperitoneal dosing. No
data were presented from mice dosed with 4AAF. In the
rat, however, 4AAF formed adducts in liver and lung DNA
though at a much lower rate than 2AAF. In studies using
radiolabelled test materials, both chemicals were shown to
form adducts with DNA in the liver and lungs of rats after
intraperitoneal administration, although adduct formation
was considerably less with 4AAF than with 2AAF.
2AAF produced positive results in both immunotoxicity
assays. However, although 4AAF was negative in the
Natural Killer (NK) cell assay, data from the T-cell
cytotoxicity test suggested that it was capable of
inducing a measurable cytotoxic response in rat T-Cells.
Nine sets of data were considered from mouse host-
mediated assays. In eight of these, including assays for
genetic changes in yeast isolated from liver, lung and
kidney, and for mutations in salmonella isolated from
liver tissue, 4AAF was uniformly negative. 2AAF produced
two weak positive responses and five negative results in
the same eight studies. The ninth assay, however, indi-
cated that both chemicals induced mitotic gene conversion
in yeast isolated from liver tissue. In addition, muta-
genic materials were detected in urine samples from rats
and mice after dosing with either isomer and from guinea-
pigs after dosing with 2AAF. 4AAF data on guinea-pig
urine were not available.
7.2.4 Mouse spot tests
Six separate mouse spot tests were conducted with 2AAF
and the results were equally divided between positive and
negative. An increase in coat colour spots was detected
in two studies while in two others, no evidence of the
induction of coat colour spots was observed. Similarly,
in the melanocyte assay, one positive result and one
negative were reported. Four of five spot tests conducted
with 4AAF were negative and the data were inconclusive in
the fifth.
7.2.5 Mammalian germ cell assays
Dominant lethal mutation assays conducted in male or
female mice or male rats and tests for the induction of
UDS in rat or mouse male germ cells were uniformly nega-
tive with both compounds. In the test for morphological
abnormalities in mature spermatozoa, two assays in which
2AAF was given orally to mice or rats were also negative.
After intraperitoneal dosing, however, 2AAF induced a
significant increase in the incidence of abnormal mouse
sperm. 4AAF was negative in both species regardless of
the route of exposure. These data suggest that, although
2AAF or its metabolites appear capable of penetrating the
testes and adversely affecting sperm morphology after
intraperitoneal dosing, there is no evidence for interac-
tion of 2AAF metabolites with germ cell DNA.
7.2.6 Drosophila assays
The mutagenic effects of the two chemicals on
drosophila germ cells were studied in tests for sex-linked
recessive lethal mutations in five laboratories and for
chromosome loss in two laboratories. Data from six of
these studies indicated that neither chemical produced
genetic damage in drosophila. In the seventh laboratory,
however, 2AAF was clearly mutagenic in the sex-linked
recessive lethal assay; 4AAF produced a weak positive
result in this study.
2AAF induced eye or wing spots in somatic mutation and
recombination assays in drosophila, although the response
was weak in two of the studies. The somatic mutation data
generated from tests with 4AAF were ambiguous, producing
one weak positive result, one inconclusive result and one
negative.
7.3 Summary of the in vivo genotoxicity of the four chemicals
A comprehensive in vivo genotoxicity profile has been
generated on the four test chemicals, each of which had
previously shown evidence of mutagenic activity in in
vitro screening tests. It is relevant, in this section,
to consider the mammalian genotoxicity in relation to the
hazard assessment procedures recommended by many regulat-
ory bodies. Table 3 shows the mutagenic activity of the
chemicals in the in vivo tests common to most legislative
guidelines. Based on these results, and in the absence of
pharmacokinetic and metabolic data, BP would be considered
to present a potential mutagenic and carcinogenic hazard
and 2AAF would be regarded as a potential carcinogen.
These observations are, of course, corroborated by the
established carcinogenic activity of these two chemicals.
The abbreviated data base shown in Table 3 suggests
that neither 4AAF nor PYR present a significant genotoxic
hazard and it is useful to consider whether the compre-
hensive data generated in CSSTT/2 support this initial
prediction. The summarized consensus results (Table 2)
show that conclusive data for PYR were presented from 48
distinct in vivo tests and PYR was considered to be nega-
tive in 43 of these. The remaining five tests suggested a
weak induction of SCE in rodent bone marrow that was not
reproduced in all laboratories, a single positive host-
mediated assay, and evidence of the excretion of mutagenic
metabolites in rodent urine. None of these data seriously
question the validity of the original prediction that PYR
is not a significant genotoxic hazard.
Table 3. Activity of the four test chemicals in established
in vivo mutagenicity assays
------------------------------------------------------------------------
BP PYR 2AAF 4AAF
Somatic cell tests
Metaphase chromosome analysis P N Pa N
Micronucleus test P N P N
Mouse spot test P N Pa N
Germ cell tests
Dominant lethal assay P N N N
Heritable translocation test NT NT NT NT
Mammalian germ cell cytogenetics NT NT NT NT
------------------------------------------------------------------------
a Data not consistent between laboratories
NT = not tested
P = positive
N = negative
A similar analysis of the 4AAF data indicates that
conclusive results were obtained from 50 different types
of in vivo tests. Of these, 38 were regarded as negative
and the remaining twelve assays produced positive or weak
positive results. Like BP, 4AAF produced a positive
result in a host-mediated assay and in tests for mutagenic
activity in rodent urine. Other observations, however,
suggest that 4AAF may not be devoid of in vivo genotox-
icity. For example, 4AAF induced detectable unscheduled
DNA synthesis in rat liver hepatocytes that was reproduced
in three different laboratories. One investigator reported
the induction of micronuclei in rat bone marrow cells
after intraperitoneal dosing with 4AAF, and five of seven
studies conducted in rat or mouse bone marrow recorded
small, but significant, increases in SCE. There was also
data to suggest that 4AAF was capable of adduct formation
with liver cell DNA in rats. All the germ cell data on
4AAF were negative. The positive findings in somatic
tissues cannot be dismissed as inconsequential and they
raise serious doubts about the prediction, based on the
data shown in Table 3, that 4AAF does not present a
significant genotoxic hazard. The outcome of the 2-year
rat carcinogenicity study with 4AAF is crucial to the
assessment of the validity of this prediction and, in
fact, to the evaluation of the whole CSSTT/2 data base.
8. ASSESSMENT OF THE PERFORMANCE OF THE ASSAYS
The purpose of this section is to present an objective
assessment of the performance of the in vivo tests as it
relates to the carcinogenic activity of the test chemi-
cals. (A detailed tabulation of the qualitative results of
each assay is presented in Table 4). Data from a study of
only four chemicals do not, of course, provide sufficient
information on which to judge the overall value of a par-
ticular assay for discriminating between non-carcinogenic
and potentially carcinogenic chemicals. However, when a
data base of this magnitude is available, in which many
tests were replicated in a number of different labora-
tories, then a unique insight into the utility, reproduc-
ibility, and reliability, as well as the accuracy of the
tests, can be obtained. Thus, data from the current study,
together with the considerable experience of the investi-
gators and assessors, enable the performance of many of
the assays to be comprehensively evaluated.
Although a high proportion of test systems performed
reasonably well with these four chemicals, some assays,
not surprisingly, failed to meet the predetermined cri-
teria for acceptable performance (section 4). The rodent
host-mediated and urine mutagenicity assays are good
examples. They involve either the exposure of marker
organisms to the chemicals or its metabolites in vivo or
the detection of mutagenic excretory products in urine of
treated rodents. Both types of tests were conducted in
several laboratories and neither proved capable of ident-
ifying the two carcinogens among the four in vitro muta-
gens, i.e. the host-mediated assays were generally nega-
tive with the four test chemicals while the urine tests
were uniformly positive. These observations suggest that
neither of these two classes of tests has any practical
value in evaluating the in vivo genotoxicity of chemicals
known to be genotoxic in vitro. The results obtained from
the peritoneal macrophage transformation assay suggest a
similar conclusion. This is a form of host-mediated assay
in which the transformation of macrophages, isolated from
the peritoneum of treated rats, is investigated in in
vitro culture. The four test chemicals gave negative
results in each of three laboratories.
Table 4. IPCS CSSTT in vivo study - summary of qualitative
results from individual investigatorsa
-------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
-------------------------------------------------------------------------------------------------
1. CYTOGENETICS
1.1 Chromosomal aberrations
1.1.1 Mouse bone marrow 8 P N NT NT o
8 NT NT P ? ip
1.1.2 Mouse bone marrow 9 P N NT NT o
9 NT NT N N ip
1.1.3 Mouse bone marrow 10 P N N N o
1.1.4 Mouse bone marrow 11 P N N N o
1.1.5 Mouse bone marrow 12 P N P ? o
1.1.6 Mouse bone marrow 13 P NT N N o
1.1.7 Mouse bone marrow 14 P N ? N ip
1.1.8 Mouse ascites tumour cells 13 P NT N N o
1.1.9 Rat bone marrow 15 P N NT NT o
15 NT NT ? N ip
1.1.10 Chinese hamster bone marrow 16 P N P W o
1.1.11 Chinese hamster bone marrow 17 W N W N o
1.2 Micronuclei
1.2.1 Mouse bone marrow 19 P N P N o
1.2.2 Mouse bone marrow 20 P N P N o
1.2.3 Mouse bone marrow 21 P N P N o
1.2.4 Mouse bone marrow 22 P N N N o
1.2.5 Mouse bone marrow 23 P N P N o
1.2.6 Mouse bone marrow 24 P W P W o
1.2.7 Mouse bone marrow 25 P N NT NT o
1.2.8 Mouse bone marrow 26 P N P N o
1.2.9 Mouse bone marrow 27 P N P N o
1.2.10 Mouse bone marrow 28 P N NT NT o
28 NT NT P N ip
1.2.11 Mouse bone marrow 29 P N P N ip
1.2.12 Mouse bone marrow 29 P N NT NT o
1.2.13 Mouse bone marrow 30 P N P N o
30 P N P N ip
1.2.14 Mouse bone marrow 31 P N P N ip
1.2.15 Mouse bone marrow 32 P N P N o
1.2.16 Mouse bone marrow 33 P N P N o
1.2.17 Mouse blood - weanling 34 P N P ? o
1.2.18 Mouse blood - fetal 34 P N N N o
1.2.19 Mouse blood - maternal 34 P N N N o
1.2.20 Mouse blood - fetal 32 P N P N o
1.2.21 Rat bone marrow 35 NT NT P N o
1.2.22 Rat bone marrow 36 P N NT NT o
36 NT NT P W ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
1.3 Sister chromatid exchange
1.3.1 Mouse bone marrow 38 P N P W ip
1.3.2 Mouse bone marrow 39 P W P W o
1.3.3 Mouse bone marrow 40 P N P W o
1.3.4 Mouse bone marrow 41 P W P W ip
1.3.5 Mouse bone marrow 42 P N P N o
1.3.6 Mouse bone marrow 43 P N W N o
1.3.7 Rat bone marrow 44 P W P W o
1.3.8 Mouse lymphocytes 45 P N P N o
1.3.9 Chinese hamster bone marrow 46 P N P N ip
Chinese hamster bone marrow 46 P N P N o
1.3.10 Chinese hamster intestinal cells 46 P N P N o
2. LIVER-SPECIFIC ASSAYS
2.1 Initiation/promotion
2.1.1 Altered enzyme foci 50 NT NT N N o
2.1.2 GGT-positive foci 51 P N P N o
2.1.3 GGT-positive foci 52 P N P P o
2.1.4 GGT-positive foci - promotion 53 P N P W o
2.1.5 GGT-positive foci 54 N N P N o
2.1.6 GGT-positive foci 73 NT NT P N o
2.2 Unscheduled DNA synthesis (UDS)
2.2.1 UDS in mouse liver 56 N N N N o
2.2.2 UDS in rat liver 56 N N P N o
2.2.3 UDS in rat liver 57 NT NT P W o
2.2.4 UDS in rat liver 58 N N P W o
2.2.5 UDS in mouse liver 58 NT NT N N o
2.2.6 UDS in rat liver 59 NT NT P N o
2.2.7 UDS in rat liver 60 NT NT P W o
2.2.8 UDS in rat liver 61 NT NT P N o
2.2.9 UDS in rat liver 62 NT NT P N o
2.2.10 UDS in weanling rat liver 63 N NT NT NT o
2.2.11 UDS - neonate rat 61 NT NT W NT o
2.3 S-phase hepatocytes
2.3.1 Rat 56 W NT NT P o
2.3.2 Mouse 56 NT NT NT W o
2.3.3 Rat 58 N N P P o
2.3.4 Mouse 58 NT NT W P o
2.3.5 Rat 59 NT NT NT P o
2.3.6 Rat - weanling 63 P NT NT NT o
2.3.7 Rat 60 NT NT N P o
2.3.8 Rat 62 NT NT N P o
2.3.9 Rat 61 NT NT NT P o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
2.4 Cytogenetic analysis
2.4.1 Aberrations in liver 65 W N W N o
epitheloid cells
2.4.2 SCE in liver epitheloid cells 65 W N ? N o
2.4.3 Micronuclei in hepatocytes 66 N N P N o
2.5 DNA strand breaks
2.5.1 Alkaline elution 68 N N N N o
2.5.2 Alkaline elution 69 N N N N o
2.5.3 Alkaline elution 70 N W P P o
2.5.4 DNA-DNA/DNA-protein crosslinks 70 P N P NT ip
2.5.5 DNA unwinding 71 N N P N o
2.5.6 DNA unwinding (with araA) 71 P N P N o
3. MISCELLANEOUS ASSAYS
3.1 Carcinogenesis
3.1.1 2-year rat study 73 NT NT P * o
3.1.2 Mouse lung adenoma 74 P N P N o
3.1.3 Quail egg 75 * * * *
3.2 Supplementary assay
3.2.1 Rat fore-stomach UDS 76 ? N N N o
3.2.2 Sebaceous gland suppression 77 P N NT NT der
3.2.3 Epithelial hyperplasia 77 P N NT NT der
3.2.4 Rat hepatocyte ploidy 78 P N P N o
3.2.5 Mouse hepatocyte ploidy 78 P N P N o
3.2.6 Syrian hamster 6-TG-resistant 79 P N ? N ip
lung cells
3.2.7a 32P-post labelling - rat liver 80 P N P W ip
3.2.7b 32P-post labelling - rat lung 80 P N P W ip
3.2.7c 32P-post labelling - mouse 80 P N P NT ip
liver
3.2.7d 32P-post labelling - mouse 80 P N P NT ip
lung
3.2.7e 32P-post labelling - mouse 80 P N P NT ip
kidney
3.2.8 Radiolabelled test compounds 81 NT NT P P ip
3.2.9 ELISA rat 82 NT NT * * o
3.3 Immunotoxicity
3.3.1 T-cells - rat 83 P N P W ip
3.3.2 Natural Killer cells - rat 84 NT NT P N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
3.4 Transformation of peritoneal macrophages
3.4.1 Rat macrophages 85 N N N N o
3.4.2 Rat macrophages 85 N N N N o
3.4.3 Rat macrophages 85 N N N N o
3.5 Host-mediated assays
3.5.1a Mouse/yeast - mitotic 86 NT NT N N o
recombination - liver
3.5.1b Mouse/yeast - mitotic 86 NT NT N N o
recombination - lung
3.5.1c Mouse/yeast - mitotic 86 NT NT N N o
recombination - kidney
3.5.1d Mouse/yeast - mutation - 86 NT NT W N o
liver
3.5.1e Mouse/yeast - mutation - 86 NT NT N N o
lung
3.5.1f Mouse/yeast - mutation - 86 NT NT W N o
kidney
3.5.2a Mouse/yeast - gene conversion 87 P P P P o
liver
3.5.2b Mouse/yeast - gene conversion 87 N N N N o
lung
3.5.3 Mouse/salmonella - liver 88 N N N N o
3.6 Urine mutagenicity
3.6.1 Rat urine extract 89 P P P P ip
3.6.2 Rat urine extract 90 P P P P ip
3.6.3 Mouse urine extract 91 W W P P o
3.6.4a Rat urine 92 P P P P ip
92 P P P P o
3.6.4b Guinea-pig 92 NT NT P NT ip
3.6.5 Rat urine 93 P P P P ip
4. MOUSE SPOT TEST
4.1.1 Coat colour 95 NT NT P N ip
4.1.2 Coat colour 96 NT NT N N o
96 NT NT N NT ip
4.1.3 Coat colour 97 P N P N ip
4.1.4 Melanocytes 98 NT NT P ? ip
4.1.5 Melanocytes 99 NT NT N N ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
5. MAMMALIAN GERM CELL STUDIES
5.1 Dominant lethal
5.1.1 Female mice 102 P N N N ip
5.1.2 Male mice 103 P N N N ip
5.1.3 Male mice 104 P N N N ip
104 N ? NT NT o
5.1.4 Male mice 105 P N N N ip
105 N NT NT NT o
5.1.5 Male rat 106 NT NT N N o
5.2 Sperm abnormalities
5.2.1 Mouse 108 P N P N ip
5.2.2 Mouse 109 W N N N o
5.2.3 Mouse 110 P W P N ip
5.2.4 Mouse 111 P N N N o
5.2.5 Rat 112 NT NT N N o
5.3 Unscheduled DNA synthesis
5.3.1 Rat spermatocytes 113 N N N N o
5.3.2 Mouse sperm 114 N N N N ip
114 N NT NT NT o
6. DROSPHILA ASSAYS
6.1 Sex-linked recessive lethal
6.1.1 SLRL assay 116 N N P W o
6.1.2 SLRL assay 117 N N N N o
6.1.3 SLRL assay 118 N N N N o
6.1.4 SLRL assay 119 N N N N o
6.1.5 SLRL assay 120 N N N N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
6.2 Chromosome loss
6.2.1 Germ cell 121 N N N N o
6.2.2 Germ cell 116 N NT N NT o
6.3 Somatic mutation and recombination
6.3.1 Eye spots 116 P N W W o
6.3.2 Eye spots 122 P N P N o
6.3.3 Wing spots 118 P N W ? o
--------------------------------------------------------------------------------------------------------------
a From: Ashby et al. (1988)
b Numbers refer to chapters in Ashby et al. (1988)
P Positive
NT Not tested
N Negative
? Inconclusive
W Weak positive
* Results not available
8.1 Cytogenetic assays
Assays that utilize target cells in rodent bone marrow
for detecting chromosomal effects, i.e., chromosomal aber-
rations, micronuclei, or sister chromatid exchanges, are
widely advocated for investigating the activity of in
vitro genotoxins in the intact animal and represent the
largest group of tests in this study.
8.1.1 Chromosomal aberrations
Data from metaphase chromosome studies in mouse bone
marrow were provided by seven investigators. Each investi-
gator successfully discriminated between BP and PYR, and
five sets of data determined 4AAF to be negative while two
were inconclusive. The results of chromosome studies with
2AAF, however, were conflicting. Two laboratories identi-
fied 2AAF as a clastogen, one set of data were inconclus-
ive and four investigators reported 2AAF as negative.
There appears to be no simple explanation for these dis-
crepancies, which were not associated with differences in
mouse stain, route of treatment, sampling interval, or
other aspects of experimental design. The mouse micro-
nucleus assay (section 8.1.2) showed 2AAF to be an in vivo
clastogen (though less potent than BP) and it is clear
that the dosing schedules used in the metaphase chromosome
assays were more than adequate to induce chromosome aber-
rations, i.e., 2AAF would be expected to be detected as a
clastogen in the metaphase chromosome assay. One expla-
nation for the negative findings in four laboratories may
be the resolving power of the sample sizes used. Most
standard protocols for the bone marrow metaphase assay
require at least 50 cells per animal from 10 animals per
dose/time group (i.e., 500 cells) to be analysed for aber-
rations at each data point. This recommended minimum fig-
ure was only achieved by three of the seven investigators
and three others presented data on only 250 cells per
dose/time group. These observations suggest that, with
samples of this size, almost a 10-fold increase in
aberrations over the control incidence would be required
to demonstrate a significant induction of aberrations by
2AAF.
Two investigators conducted studies in Chinese hamster
bone marrow cells. PYR was negative in both studies. In
experiments in which 500 cells per dose/time group were
analysed, BP and 2AAF were clearly positive while, when
only 300 cells were used, the results were classified as
weak positive with both carcinogens. It is interesting to
note that 4AAF induced a weak positive response in Chinese
hamster bone marrow in one study.
One set of data from rat bone marrow was presented and
clearly separated BP from PYR. 2AAF, however, gave
unambiguous data and 4AAF was negative.
The performance of the bone marrow cytogenetic assays
did not, in general, meet the criteria for a reliable and
reproducible short-term in vivo test. There are, however,
serious reservations regarding the numbers of animals and
the numbers of cells analysed in many of the studies and
the results suggest that protocols designed to give an
acceptable resolving power should be strictly adhered to.
8.1.2 Micronuclei
Eighteen laboratories conducted micronucleus tests; 16
laboratories performed assays on bone marrow cells from
mice and two investigators used rats. Data were also
presented on the incidence of micronuclei in circulating
blood cells from adult and fetal mice.
Mouse bone marrow micronucleus assays were conducted
to a common protocol with only minor deviations. At least
5 mice were used for each dose/time interval with 1000
cells being analysed from each animal. The data from these
assays showed a high level of agreement between labora-
tories. Seventeen sets of data on BP were uniformly posi-
tive and 16 sets on PYR were negative. Similarly, 14
investigators showed 2AAF to be capable of inducing an
increase in micronuclei and 4AAF was negative in 14 cases.
There were only occasional anomalous results. For example,
one laboratory failed to detect 2AAF, and another investi-
gator recorded weak positive results from both PYR and
4AAF. These anomalies do not detract from the general
accuracy and reproducibility of mouse micronucleus tests
with the four test chemicals.
One investigator used erythrocytes from mouse periph-
eral blood to assay the incidence of micronuclei. The
results were similar to those obtained in the bone marrow
studies, i.e. BP and 2AAF were positive while PYR and 4AAF
were considered to be negative by the criteria adopted for
a positive response. In two laboratories, fetal blood was
obtained from mice on day 15 of pregnancy, 30 or 48 h
after oral dosing of the mother. BP was positive and PYR
was negative in each of these studies. The fetal assay was
less sensitive to the effects of 2AAF, one laboratory
observing a small increase in micronuclei and the other
finding no increase. 4AAF was inactive in fetal cells.
Two micronucleus studies were conducted in rat bone
marrow cells and the results suggest that the response in
the rat was weaker than that in the mouse. In one
laboratory, 2AAF was positive and 4AAF negative; BP and
PYR were not tested. The second investigator reported a
positive response with BP and a negative one with PYR.
This investigator's result with 2AAF is shown as positive
in Table 4. However, using criteria decided by the
micronucleus assay working group, it was concluded that
2AAF was negative in this assay. It was also considered
that the protocol used in the rat studies was not designed
for the optimum detection of micronuclei.
The results from the micronucleus tests confirm the
value of the assay in mouse bone marrow as an accurate and
reproducible means of qualitative discrimination between
carcinogenic and non-carcinogenic chemicals.
8.1.3 Sister chromatid exchange
Nine investigators presented data on the induction of
sister chromatid exchanges (SCE) in vivo using mice, rats,
or Chinese hamsters. The test chemicals were administered
by gavage or intraperitoneal injection and most investi-
gators analysed SCE in bone marrow cells. One set of data
were presented on SCE in mouse peripheral lymphocytes and
another set on Chinese hamster intestinal epithelial
cells. The two established carcinogens, BP and 2AAF,
induced an increase in SCE in each of the six studies
conducted in mouse bone marrow. In two of these studies,
PYR induced a weak positive response and 4AAF was weakly
positive in four of the six assays. Weak positive results
were also obtained in rat bone marrow assays with the two
presumed non-carcinogens, while BP and 2AAF were clearly
positive. In experiments conducted in blood lymphocytes
in mice and in bone marrow cells and intestinal epithelial
cells in Chinese hamsters, BP and 2AAF gave positive
results while PYR and 4AAF were negative. None of the
experiments suggested that the route of administration of
the test chemicals significantly influenced the qualitat-
ive results.
Both the established carcinogens were consistently
positive in tests for the induction of SCE in three rodent
species, suggesting that the SCE assay is a sensitive and
reproducible in vivo test for genotoxic carcinogens. PYR
or 4AAF, however, induced small (but significant) in-
creases in the incidence of SCE in 8 of 22 tests, although
the presumed non-carcinogens were much less potent
inducers of SCE than their carcinogenic analogues. These
observations raise a number of questions regarding the
significance of small increases in SCE in in vivo tests.
In the first instance, if 4AAF is confirmed to be non-
carcinogenic in the current rodent bioassay, then these
weak responses have to be regarded as `false positives'.
If, however, 4AAF is shown to have weak carcinogenic
activity, then the weak positive responses obtained in
four of the six mouse bone marrow studies can be inter-
preted as accurately reflecting the genotoxicity of 4AAF.
In the report of the SCE Working Group (Tice et al., 1988)
the biological relevance of statistically significant
increases in SCE at unrealistically high doses was con-
sidered. It was suggested that multiple mechanisms are
involved in the formation of SCE and that one of these may
be a response to stress, either at the cell or animal
level, caused by a toxic response to high doses of a
chemical. The small induction of SCE observed after high
doses of PYR or 4AAF, therefore, may not be related
mechanistically to the larger increases in SCE seen after
low doses of BP or 2AAF. Thus, the final assessment of
the performance of the in vivo SCE assays depends, to a
large extent, on the outcome of the 2-year bioassay with
the AAF analogues, i.e. a negative result with 4AAF will
cast serious doubts on the value of SCE tests for investi-
gating the in vivo activity of in vitro genotoxins.
8.2 Liver assays
The activity of certain genotoxic chemicals appears to
be confined to the liver. Such chemicals do not, of
course, induce detectable genetic changes in bone marrow
cells or other extra-hepatic tissues and this has led to a
need to develop techniques for investigating genotoxic or
related changes in liver cells. The main thrust of this
research has centred on the demonstration of unscheduled
DNA synthesis in hepatocytes but data were also presented
from a variety of other assays that used target cells in
the liver.
8.2.1 Initiation and promotion
Two of the assays used in this group were specifically
aimed at detecting initiating activity of the test chemi-
cals. The chemicals were administered after partial hepa-
tectomy, and the mice were then treated with the promotor,
phenobarbital, for several weeks before liver sections
were examined for the occurrence of pre-cancerous foci,
i.e. groups of cells having elevated levels of the marker
enzyme, gamma-glutamyl-transpeptidase (GGT). Both these
protocols have been used successfully with many test
chemicals and, in the present study, successfully separ-
ated BP and 2AAF from PYR and 4AAF. A third protocol was
used to investigate the promoting activity of the test
chemicals after initiation by pre-treatment with dimethyl-
nitrosamine. This, too, successfully separated BP from
PYR, but, although 2AAF produced an increase in GGT-
positive foci, a weak positive response was also observed
with 4AAF. The two other procedures used to study
initiation/promotion events based on altered enzyme foci
failed to detect BP in one assay and 2AAF in the second
assay and are considered to be, primarily, research
techniques and not yet suitable for routine application.
The two better-established assays showed quite clearly
that both BP and 2AAF initiated altered enzyme foci in rat
liver. The two presumed non-carcinogens were completely
negative and these tests, although they cannot be regarded
as "short-term" in the true sense, have a useful role in
investigating liver carcinogenesis.
8.2.2 Unscheduled DNA synthesis and S-phase analysis
The investigators in this group used the detection of
unscheduled DNA synthesis (UDS) as a measure of DNA repair
initiated in response to chemical-induced damage to the
DNA. In each study, UDS was detected using autoradio-
graphic techniques that also allowed simultaneous measure-
ment of S-phase cells, i.e. those cells undergoing normal
DNA synthesis as a prelude to mitosis. Seven investi-
gators examined 2AAF and 4AAF for the induction of UDS and
S-phase cells in hepatocytes of male rats, and in two
laboratories all four test chemicals were investigated in
rats and mice. The induction of UDS and S-phase cells in
rat fore-stomach was investigated in another laboratory.
Although there were minor protocol variations, the UDS
working group concluded that none of these significantly
affected the results and there was excellent agreement in
the observations and conclusions of the investigators.
2AAF was shown to be a potent inducer of UDS in rat
hepatocytes and also caused proliferation of hepatic cells
as shown by an increase in the number of cells in S-phase.
In mice, however, 2AAF failed to induce SCE in either of
two laboratories, though there was evidence of a weak
induction of S-phase cells. Only low doses were examined,
i.e. up to 50mg/kg, but the results indicate an important
species difference in the response to 2AAF.
Administration of 4AAF to rats produced negative UDS
results in four laboratories and weak positive results in
three laboratories. Although the three weak positive
results were initially determined without recourse to
statistical analysis, a comprehensive analysis of the data
by Margolin and Risko (1988) confirmed the initial
interpretation. The six investigators who recorded the
number of S-phase cells observed a marked increase in
hepatic cell proliferation in rats dosed with 4AAF. This
compound failed to induce UDS in mouse liver but did cause
an increase in S-phase cells.
For comparative purposes, two laboratories also con-
ducted UDS tests on hepatocytes in vitro. Both 2AAF and
4AAF induced UDS in rat hepatocytes but were negative in
mouse cells.
BP and PYR were tested in rats by two investigators
and one assay was conducted in mice. Both chemicals
failed to induce UDS in either species after oral dosing.
In contrast, BP gave positive results in both rat and
mouse hepatocytes treated in vitro.
In an investigation of the UDS activity of the four
chemicals in rat fore-stomach, 2AAF, 4AAF, and PYR failed
to induce UDS while BP gave equivocal results.
The performance of the UDS assay in rodents dosed with
2AAF or 4AAF is worthy of further consideration. 2AAF is
a potent rat hepatocarcinogen and a potent inducer of UDS
in rat hepatocytes. In the mouse, however, 2AAF did not
induce UDS and, on balance, available data suggest that it
does not produce liver tumours. Thus, for this chemical,
the UDS results accurately reflect its hepatocarcinogenic
potential. The current lack of cancer data on 4AAF pre-
cludes this kind of comparison, but the UDS data suggest
that if 4AAF is carcinogenic, it is markedly less so than
2AAF.
Although fewer tests were conducted with BP and PYR,
the data indicate that neither chemical induces UDS in rat
or mouse liver, although BP was positive in in vitro UDS
tests. The reason for this negative response to BP has an
important bearing on the validity of the UDS assay for
detecting potentially carcinogenic chemicals. Although BP
is an established carcinogen, it has never been shown to
produce tumours in rodent liver. There is sufficient
evidence, however, to suggest that DNA-reactive adducts
are formed in liver cells after dosing rodents with BP.
For example, most of the data that show BP to be genotoxic
in vitro are derived from tests in which metabolic acti-
vation is provided by an enzyme mixture derived from rat
liver. In addition, BP clearly induces altered enzyme
foci in rat liver, there is tentative evidence for the
induction of DNA strand breakage based on DNA-DNA/DNA-
protein crosslinks, and 32P-post-labelling techniques in-
dicate the presence of DNA adducts of BP metabolites in
both rat and mouse liver. Therefore, although BP is not a
hepatocarcinogen in rodents, there are data to suggest
that (a) BP is metabolized in rodent liver to DNA-reactive
metabolites, (b) interaction of DNA with BP metabolites
does occur, and (c) based on data from the DNA unwinding
test, some repair of the DNA lesions takes place. On this
basis, the liver UDS assay should, theoretically, be
capable of detecting BP-induced lesions in the liver. All
these observations indicate that such factors as the
distribution of BP to the liver after oral dosing, the
rate of formation of genotoxic metabolites, the rate of
detoxification, and the rate and nature of the DNA repair
process yield an insufficient number of DNA adducts to
produce a detectable UDS response.
In practice, of course, BP is easily detected in other
in vivo tests such as those conducted in bone marrow
cells. However, the results with BP and, to a lesser
extent, 4AAF, suggest the need for additional evaluation
of the performance of the in vivo UDS assay with liver-
specific genotoxins.
8.2.3 DNA strand breaks
Data were considered from three different assay sys-
tems used to detect single-strand breaks in DNA. Results
from the alkaline elution assay, in which two laboratories
found no activity with any of the four test chemicals,
indicate that this assay is of little value in investigat-
ing in vivo genotoxicity. The test for DNA-DNA/DNA-protein
crosslinks and the DNA unwinding assay in rodent liver
cells both gave very promising results and warrant further
evaluation.
8.2.4 Cytogenetics
Three sets of data were considered in this group.
Micronucleus assays were conducted in rat liver cells
following partial hepatectomy. The results were similar to
those obtained in the rat liver UDS assay, i.e. a dose-
dependant response was obtained with 2AAF, while the
remaining three chemicals gave negative results. The
other two data sets were generated from analyses of
chromosome aberrations and SCE in epithelial-like cells
derived from weanling rat liver. Although the assays are
relatively simple with clearly defined end-points, the
results were either equivocal or negative and the sample
sizes, i.e. the numbers of animals/cells per dose group,
were too small to permit an effective assessment. Both
the micronucleus test and the chromosome assays are worthy
of further evaluation.
8.3 Miscellaneous assays
This section contains a wide variety of assays, some
of which have been in use for many years while others, few
of which were undertaken in more than one laboratory, are
relatively new or in the process of development.
8.3.1 Specific carcinogenicity assays
Neither of the two procedures considered here can be
regarded as short-term tests and final results from one of
them, the assay in quail eggs, were not available at the
time of the assessment. The results of the mouse adenoma
assay, however, were available and showed a decisive dis-
crimination between BP and PYR and a much smaller and less
convincing difference between 2AAF and 4AAF.
8.3.2 Supplementary assays
Data from the sebaceous gland suppression test and the
epidermal hyperplasia assay have shown a good correlation
with skin carcinogenicity and have proved useful for com-
paring the carcinogenic potential of polycyclic hydrocar-
bons. Their value in this area was confirmed by the re-
sults of assays with BP and PYR but the application of
these assays to other classes of chemicals is of uncertain
value.
The hepatocytes of rodents include mono- and binu-
cleated cells and nuclei that may be diploid, tetraploid,
or octaploid. The ratio of cells with different levels of
ploidy in liver varies with species and age and can be
altered by exposure to certain chemicals. There have been
references to the effects of various chemicals on liver
ploidy ratios over a number of years, but there appears to
be no consistent relationship between ploidy changes and
chemical carcinogenesis. Thus the observation that the two
carcinogens, BP and 2AAF, increase the ratio of diploid to
tetraploid cells is of uncertain significance. The assay
may be of value in studying liver cell kinetics but its
value in the detection of in vivo genotoxicity is debat-
able.
The general principles of the in vivo/in vitro somatic
mutation assay were developed in the late 1970s and showed
initial promise as a test for investigating organ-specific
mutations induced by chemical carcinogens. One
investigator provided data from a modification of this
technique in which cells isolated from lung tissue of
treated Syrian hamsters were cultured in vitro and the
induction of mutations was determined. A reproducible
increase in mutations was observed in lung cells after
dosing with BP, but the results with 2AAF and the two non-
carcinogens were considered to be negative. Although this
is a logical and potentially useful approach to studying
in vivo genotoxicity, the method requires much wider
evaluation before it can be considered as an acceptable
assay.
The detection and identification of DNA adducts
provides conclusive evidence that a metabolite of the
administered chemical has interacted with one of the
important target macromolecules of carcinogenesis. Theor-
etical considerations indicate that, for many chemical
classes, the formation of DNA adducts is a prerequisite
for permanent genetic changes, such as mutations, and for
the initiation stage of carcinogenesis. Research has shown
a direct relationship between the administered dose of a
chemical and DNA-adduct formation, so that precise dosi-
metry in specific organs is possible. Three approaches
were used in the study, though results from a novel tech-
nique using monoclonal antibodies to identify specific
adducts are not yet available. One study involved the use
of radiolabelled 2AAF and 4AAF; while binding to liver DNA
was detected with both compounds, the covalent binding of
2AAF was approximately seven times that of 4AAF. These
data correlate well with the results of the UDS assay in
rat liver, which showed 2AAF to be a potent inducer of UDS
while 4AAF was, at best, a weak inducer.
The above assay has the disadvantage of requiring
radiolabelled test chemicals, and a significant advance in
the field was the introduction of techniques in which
animals are dosed with unlabelled test chemicals and the
DNA is radiolabelled after isolation and purification. The
32P-labelled normal nucleotides and nucleotide adducts
are separated by thin-layer chromatography and detected by
autoradiography, and a comprehensive, organospecific DNA
adduct profile is obtained. The data presented in the
current study showed that 2AAF adducts were detected at
significant levels in the DNA of mouse liver, lung, and
kidney and in rat liver and lung. 4AAF adducts were not
detected in mouse tissue and at only very low levels in
rat liver. PYR adducts were not detected in either rat or
mouse tissues and BP produced adducts with DNA in liver
and lung of both species and in mouse kidney.
The detection and identification of DNA adducts is
potentially an extremely powerful tool in the investi-
gation of in vivo genotoxicity. With the establishment of
`cold' techniques, i.e. those not requiring radiolabelled
test chemicals, this type of assay can be applied to most
chemical classes. Although further development is re-
quired, particularly with regard to the genotoxic signifi-
cance of different types of adduct, the method shows great
promise for the future.
8.3.3 Immunotoxicity assays
In the Natural Killer (NK) cell assay, the activity of
NK cells was measured at intervals after dosing rats with
either 2AAF or 4AAF. The carcinogen, 2AAF, induced a
significant change in NK cell cytotoxicity that was not
observed after dosing with 4AAF. The T-cell assay, in
which immunocompetent T-cells are able to react to
carcinogen-induced tissue changes, responded positively to
BP and 2AAF; PYR failed to induce any change in reactivity
and 4AAF gave a weak positive response. The results of
both types of assay closely reflected the carcinogenic
activity of the test chemicals. The relevance of the
observed immunological changes to the carcinogenic process
is unclear and, thus, their potential value in detecting
precarcinogenic changes caused by genotoxic and non-
genotoxic carcinogens remains to be fully evaluated.
Before they can be accepted as anything more than a useful
research tool, further investigations of the specificity
of the immunological reactions and a wider evaluation are
imperative.
8.3.4 Host-mediated assays and urine mutagenicity tests
As described at the beginning of Section 8, both these
classes of test failed to meet the criteria for an accept-
able short-term in vivo assay. The host-mediated assay was
a favoured in vivo procedure in the early 1970s, though in
recent years questions have been raised concerning its
sensitivity. Although the intrasanguinous modification to
the original intraperitoneal technique offered theoretical
advantages, the results of the present study confirmed the
inadequacy of the assay. It is concluded that both these
procedures are not appropriate for the investigation of in
vitro genotoxins.
8.4 Mouse spot tests
Although data were presented from two types of mouse
spot test, they are, in effect, different means of observ-
ing the same genetic alterations, i.e. the melanocyte test
detects the change at the cellular level while the coat
colour test demonstrates the expression of the cellular
changes.
BP and PYR were only tested in one laboratory and the
spot test successfully identified the carcinogen. In tests
with 4AAF, one investigator's results were considered to
be inconclusive and four other sets of data, including one
after oral dosing and three after intraperitoneal admin-
istration, were negative. Six laboratories tested 2AAF;
there were three positive results and two negatives after
intraperitoneal administration. 2AAF was also negative
after oral dosing. Examination of the protocols indicated
that 2AAF induced detectable genetic changes only after
administration of the material as a solution in dimethyl-
sulfoxide, i.e. negative results followed the admin-
istration of corn oil formulations of 2AAF, illustrating
the critical effect of the vehicle on the absorption of
the test chemical.
An overview of the mouse spot test data indicates that
intraperitoneal administration of an appropriate formu-
lation of the test chemicals produces results that reflect
their carcinogenic activity. The original coat colour
procedure appears to be more reliable than the melanocyte
technique, which may be influenced by sporadic mutational
events and inadequate melanization of the hair follicles.
However, the dependence on the intraperitoneal route of
administration may be a disadvantage, and the mouse spot
test appears to add very little to the information gained
more easily from bone marrow cell cytogenetic assays.
8.5 Assays in mammalian germ cells
For assessment purposes the germ cell assays have been
separated into two groups: those that are considered to
involve a direct interaction with DNA, and the assay for
sperm abnormalities, the genetic significance of which is
uncertain.
8.5.1 Dominant lethal and unscheduled DNA synthesis assays
Classical dominant lethal tests were conducted in male
or female mice and in male rats. PYR, 2AAF, and 4AAF gave
unequivocally negative results after intraperitoneal
dosing. In an oral dosing study in male mice, BP was nega-
tive and PYR produced inconclusive data. After intraper-
itoneal administration of BP, however, there was conclus-
ive evidence of dominant lethal induction in male and
female mice. In the male, dominant lethality was confined
to the late spermatid/spermatozoa stages of spermatogen-
esis.
Results of UDS assays in rat spermatocytes after oral
dosing and in mouse spermatocytes after oral or intraper-
itoneal administration were negative with all four chemi-
cals. The negative data with BP are not altogether sur-
prising as dominant lethality was only observed in late
spermatids/spermatozoa, in which the DNA is highly con-
densed and in which UDS does not occur.
Based on the dominant lethal test results, BP is a
confirmed, though relatively weak, germ cell mutagen in
mice after intraperitoneal dosing. Published data indicate
that BP does not induce heritable translocations at doses
that produce dominant lethality (Generoso et al., 1982),
although this may be due, in part, to the fact that BP
appears to induce mainly chromatid deletions in bone
marrow cell chromosomes, with very few exchange-type aber-
rations.
8.5.2 Sperm abnormality tests
This assay is based on the observation of morphologi-
cal alterations in mature spermatozoa after treatment of
animals at all stages of spermatogenesis with the test
chemicals. BP induced an increase in the incidence of
abnormal sperm in mice after oral or intraperitoneal
dosing and 2AAF was also positive following oral gavage.
Tests with 4AAF were uniformly negative and PYR produced a
weak positive response in one of four assays. Thus, both
carcinogens produced sperm abnormalities under appropriate
experimental conditions.
The important questions raised by these observations
relate to the relevance of morphological abnormalities in
sperm to germ cell mutations and to carcinogenesis.
Although changes in sperm head shape may be genetically
determined, it is uncertain whether they are due to geno-
toxicity or to other toxic phenomena. A positive result
in the test, therefore, indicates that the test material
or its metabolites are able to reach the germ cells in
sufficient quantities to induce an adverse biological
effect. Although a good correlation has been shown between
the induction of sperm abnormalities and carcinogenic
activity (Wyrobek et al., 1983), the relevance of this
correlation to short-term testing strategies is doubtful
as more appropriate in vivo tests are available, e.g.,
bone marrow cytogenetic assays. Equally doubtful is the
relevance of the test to heritable genetic damage, as
there is no clear-cut evidence that abnormal sperm are a
consequence of induced DNA damage.
8.6 Drosophila assays
The sex-linked recessive lethal assay and the test for
chromosome loss in drosophila germ cells failed to show a
consistent separation of BP and 2AAF from PYR and 4AAF,
respectively. Three tests based on somatic cell changes
were positive with BP and negative with PYR but the
results of tests with the acetylaminofluorenes were less
decisive. 2AAF produced an unambiguous positive result
and two weak positives, while the three results with 4AAF
were weak positive, negative and inconclusive. Thus, the
drosophila germ cell assays were insensitive to the four
in vitro genotoxins and, although the somatic cell pro-
cedures appeared to be more sensitive, it was concluded
that the main value of drosophila tests is to provide com-
prehensive analyses of the genetic mechanisms involved in
the induction of mutations by chemicals.
9. SELECTION OF THE MOST EFFECTIVE IN VIVO ASSAYS IN RELATION
TO THEIR PERFORMANCE
In a study of this nature it is inevitable that some
assays, even well-established procedures, will fail to
perform well. Indeed, the recognition of inadequate tests
is as important a goal as the identification of those that
are useful and acceptable. Other assays produced results
that correlated well with the presumed carcinogenic status
of the test chemicals but are not widely used or available
or are still in the process of development. Yet other
tests that performed adequately in the collaborative study
cannot be regarded as practicable short-term tests either
because of their technical complexity or because of the
length of time required to produce results. Most of the
latter group of tests have important research roles in
carcinogenesis.
9.1 Assays that are not considered appropriate for routine
in vivo testing of chemicals for genotoxic activity
Host-mediated and urine mutagenicity assays (section
8.3.4), the peritoneal macrophage transformation assay,
and the alkaline elution assay for single strand breaks
(section 8.2.3) proved incapable of separating the car-
cinogen/non-carcinogen pairs and appear to have little
value in investigating in vivo genotoxicity. The in
vivo/in vitro somatic mutation procedure in Syrian
hamsters (section 8.3.2) also falls into this category,
although this conclusion should not detract from the
initial promise of this approach in Chinese hamsters
(McGregor, 1988a).
Drosophila tests (section 8.6) have also proved gener-
ally inadequate in the identification of in vivo geno-
toxins and it is concluded that their main value in gen-
etic toxicology is to provide analysis of the genetic
mechanisms involved in the induction of mutations. Similar
reservations apply to the initiation/promotion techniques
using liver foci (section 8.2.1) and the analysis of
ploidy levels in liver tissue (section 8.3.2). Both assays
are more appropriate to investigation of the carcinogenic
process than to the identification of in vivo genotoxins.
The results of the immunological assays (section 8.3.3)
closely reflected the carcinogenic potential of the
chemicals, but they can only be regarded as research tools
pending a greater understanding of the specificity of the
immunological reactions and a wider evaluation of the
procedures.
Two assays conducted in mouse skin, i.e. the seb-
aceous gland suppression test and the observation of epi-
dermal hyperplasia, confirmed their value in the detection
of carcinogenic polycyclic hydrocarbons such as BP. Both
tests failed to respond to 2AAF when applied dermally and
are not considered to be suitable for investigating in
vivo genotoxicity in general.
9.2 Assays that satisfy some or all of the criteria for an
acceptable short-term in vivo test
9.2.1 Assays currently in general use
The mouse micronucleus test (section 8.1.2) was the
most widely represented single assay in the study and
produced the best overall performance with only occasional
anomalous data. Qualitative results were not affected
significantly by the route of exposure, strain or sex of
mouse, or treatment schedule. The mouse bone marrow
micronucleus assay was confirmed as a robust and sensitive
short-term in vivo test for genotoxic chemicals.
Both the micronucleus test and the analysis of meta-
phase chromosome aberrations in rodent bone marrow respond
to similar chromosome breakage events and they would be
expected to produce qualitatively similar results with the
four test compounds. Table 4 shows that this was not the
case and the metaphase chromosome procedure was signifi-
cantly less sensitive than the micronucleus test. Poss-
ible explanations for this lack of sensitivity are dis-
cussed above (section 8.1.1) and suggest that the use of
metaphase chromosome analysis should not be undervalued on
the basis of these data.
The sister chromatid exchange (SCE) procedure repro-
ducibly detected the two carcinogens, but also produced a
number of weak positive results with 4AAF and PYR. Taken
at face value, therefore, the in vivo SCE results clearly
reflect the relative potency of the two chemical pairs in
in vitro tests. As discussed in section 8.1.3, however,
very weak SCE responses may be caused by non-genotoxic
mechanisms and a final judgement on the significance of
the weak activity of 4AAF must be delayed until its true
carcinogenic status is defined.
After considering the data from the three classes of
bone marrow assay, the conclusion of the Steering Group
(Ashby et al., 1988) was - `the mouse micronucleus assay
provides the simplest and most effective measure of geno-
toxicity in the bone marrow and, as such, it is rec-
ommended here as a primary in vivo genotoxicity test'.
Despite the successful performance of the micronucleus
test with these four chemicals, it is apparent from the
literature that certain liver carcinogens do not induce
detectable chromosomal effects in the bone marrow. In an
acceptable testing strategy, therefore, negative results
in the micronucleus test should be supplemented with
evidence of non-genotoxicity in the liver. This concept
led to the extensive evaluation of liver-specific assays
in the CSSTT/2 study and the main candidate in this group
of tests was the rat liver UDS assay (section 8.2.2).
The liver carcinogen, 2AAF, was a potent inducer of
UDS in rat liver while most investigators defined its
presumed non-carcinogenic analogue, 4AAF, as a weak
genotoxin in this system. Unlike the micronucleus test
result, BP was devoid of activity in liver UDS assays.
This is consistent with its lack of carcinogenicity in rat
liver.
Although the significance of the weak liver UDS
activity of 4AAF will only be resolved when definitive
carcinogenicity data are available, the findings raise
certain problems of interpretation that are common, in
part, to the weak bone marrow activity of 4AAF and PYR.
In the UDS assays, 2AAF was a potent inducer of UDS at
doses between 5 and 100 mg/kg while 4AAF showed only weak
activity between 50 and 1000 mg/kg, and then only in some
experiments. In general, the activity of 4AAF was within
the range of control variability and, as a result, the
UDS Working Group questioned its biological significance
(Mirsalis, 1988). A detailed analysis, however, indicated
the high statistical significance of the 4AAF data
(Margolin and Risko, 1988). Thus, although the rat liver
UDS assay discriminated quantitatively between 2AAF and
4AAF, the significance of the weak activity of 4AAF
remains unresolved. These observations suggest that
qualitative determinations in terms of `positive' and
`negative' are too simplistic for hazard assessment
purposes and one of the most useful points raised by the
present study is the need for appropriate positive test
criteria, in biological and statistical terms, for widely
used in vivo tests.
Thus, the mouse bone marrow micronucleus assay is the
method of choice for primary in vivo testing of in vitro
genotoxins and the rat liver UDS assay, providing the
above problems can be resolved, appears to be the most
promising complementary liver-specific test.
9.2.2 Assays that show promise for future development
Accepting that bone marrow assays are suitable for
detecting extra-hepatic somatic cell genotoxins and that
the rat liver UDS assay can, with certain reservations
(see sections 8.2.2 and 9.2.1), be used to investigate
liver-specific genotoxins, it is useful to consider which
other assays evaluated in this study may have a role in
the assessment of in vivo genotoxicity.
Development of assays to detect single-strand breaks
in DNA and to detect and identify DNA adducts will,
undoubtedly, continue. It will be interesting to follow
the progress of the assays for DNA-DNA/DNA-protein cross-
links and DNA unwinding, although it is probable that
their technical complexity will limit widespread use and
acceptance. Of the DNA adduct techniques represented, the
post-labelling method appears to hold most promise. The
need for radiolabelled test compounds or specific mono-
clonal antibodies is eliminated and the assay provides
information on the direct interaction of test chemicals
with DNA in a variety of target organs, including the
liver. Providing a reliable and robust protocol can be
established and pending the provision of more information
regarding the relationship between DNA adduct formation
and genotoxic changes to the DNA, this assay could have an
important role to play in the assessment of genotoxic
hazard.
The performance of the cytogenetic assays in liver
cells was disappointing but, theoretically, they could,
with further development, prove to be of value in the
assessment of liver-specific genotoxicity. The reliance
of the current micronucleus procedure on partial hepa-
tectomy is a disadvantage, but the development of alterna-
tive means of stimulating hepatic mitotic activity, either
in vivo or in short-term culture, could be the basis of a
useful short-term liver-specific assay. Cytogenetic
assays on circulating blood cells performed reasonably
well and the theoretical and practical advantages of tests
for SCE or micronuclei in circulating blood should
encourage further development of these tests.
The mouse spot test, in its original form (section
6.4), successfully separated the carcinogen/non-carcinogen
pairs and, providing care is taken in the selection of an
appropriate solvent or formulation, it appears to be a
useful and reproducible assay. Its availability is limited
by the need to maintain specific mouse strains and its
overall sensitivity, compared to the micronucleus test, is
suspect, as positive results in the present study were
dependant on intraperitoneal dosing.
9.3 The detection of germ cell mutagens
The performance and significance of the three germ
cell assays represented in the study are discussed above
(section 8.5). Only BP was clearly identified as a germ
cell mutagen in the dominant lethal assay. The failure of
the sperm abnormality test to discriminate between BP and
2AAF suggests that this assay is affected by non-genetic
toxic effects and is, therefore, of doubtful value in
predicting the induction of mutations in mammalian germ
cells.
Although there are theoretical reasons why BP should
not induce UDS in germ cells (section 8.5.1), this nega-
tive result raises questions similar to those resulting
from the failure of BP to induce UDS in rat liver (section
8.2.2), i.e. why does BP not induce detectable UDS in
target cells where there is evidence, from other assays,
of genotoxic activity?
As concluded by Ashby et al. (1988) `the germ cell
activities of BP have served to focus several important,
but unresolved, issues associated with the conduct of, and
the interpretation of data derived from, germ cell
genotoxicity and mutagenicity assays'.
9.4 Influence of the route of administration of the test chemicals
A feature of the micronucleus assays was that their
qualitative separation of BP and 2AAF from PYR and 4AAF
was not influenced by the route of administration of the
chemicals. Dominant lethal mutations, however, were only
detected after intraperitoneal dosing with BP; oral dosing
was ineffective. Similarly, the mouse spot test was
dependant on the intraperitoneal route of administration
for the demonstration of mutagenic effects. The intro-
duction of the test material in the vicinity of the uterus
in the spot test circumvents the natural pharmacokinetics
and metabolic routes of the test animal, and it can be
argued that this unrealistic pattern of exposure does not
reflect true in vivo genotoxicity. It can be similarly
argued that the purpose of in vivo tests is to achieve
maximum exposure of the target cells and that intraper-
itoneal dosing can be justified for this reason. It may
be, as suggested by Shelby (1986), that intraperitoneal
dosing should be reserved for assays used in a screening
mode, i.e. for the detection of genotoxic activity, and
that for investigating the in vivo activity of established
genotoxins, a route of administration should be chosen
that reflects possible human exposure. Arguments such as
these must be resolved before strategies for testing
chemicals for genotoxic activity can be fully harmonized.
10. CONCLUSIONS
Of the fifty or so separate in vivo techniques rep-
resented in the CSSTT/2 study, only a small number satis-
fied the criteria defined for an acceptable short-term in
vivo test (section 3.2). These criteria, however, defined
a very specific purpose, i.e. the identification of in
vivo activity of established in vitro genotoxins. In order
to generate a comprehensive data base on the four test
chemicals, assays included in the study were not limited
to, for example, those that were considered most likely to
meet the criteria. As a result, some investigators sub-
mitted data from assays that were not designed for detec-
ting in vivo genotoxins but, nevertheless, provided valu-
able information on a broad spectrum of biological effects
of the test chemicals. The failure of such tests to
satisfy the narrow selection criteria used in CSSTT/2
should not be regarded as a reflection on the validity of
the procedures for specific research purposes. With these
considerations in mind, and based on the concept (section
3.2) that short-term assays are required to investigate
both liver-specific and extra-hepatic genotoxicity, the
following conclusions appear appropriate.
1. Most of the in vivo somatic cell assays for genotox-
icity were able to discriminate between the two carcino-
gen/non-carcinogen pairs, although, in some instances,
weak activity was observed in tests with the non-
carcinogens, particularly with 4AAF.
2. The weak genotoxicity of 4AAF in some studies
suggested that qualitative determinations in terms of
`positive' and `negative' are too simplistic for hazard
assessment purposes and highlights the need for appropri-
ate positive test criteria, in biological and statistical
terms, for widely used in vivo tests.
3. The insensitivity of some assays to one or other of
the two pairs of compounds supports the concept that nega-
tive in vivo data should be produced from at least two
assays in different tissues, i.e. one in extra-hepatic
somatic tissues and one in the liver, if they are to be
used for assessing genotoxic hazard.
4. The mouse bone marrow micronucleus test was confirmed
as a robust and sensitive assay and is the assay of choice
for primary in vivo testing of in vitro genotoxins.
5. The overall performance of the rat liver UDS assay
suggests that it could be complementary to the micro-
nucleus test providing the reservations raised above
(sections 8.2.2 and 9.2.1) can be satisfied. Development
and evaluation of alternative procedures for investigating
liver genotoxicity should be actively pursued.
6. Certain widely advocated assays, including the host-
mediated and urine mutation assays and tests using
drosophila are concluded to be inappropriate for hazard-
assessment purposes.
7. BP and 2AAF showed genotoxic activity in the organs in
which they also produce tumours. Their genotoxic activity
in other tissues, however, precludes the use of data from
short-term tests for making predictions of organ-specific
carcinogenicity.
8. The majority of the positive responses observed in
this study were obtained after oral administration of the
test chemicals and at doses substantially below lethal
levels, suggesting that short-term in vivo assays are not
intrinsically insensitive, as is sometimes inferred. Posi-
tive results were obtained in the dominant lethal assay
and the mouse spot test after intraperitoneal dosing only;
both assays produced negative results after oral admin-
istration. These facts suggest that the intraperitoneal
route should be reserved for the detection of genotoxic
activity and that, for hazard-assessment purposes, a route
of administration should be chosen that is relevant to the
perceived route(s) of human exposure.
9. The germ cell mutagenicity exhibited by BP was
adequately predicted by the somatic cell mutagenicity
assays, adding weight to the suggestion that tests on
mammalian germ cells should only be conducted to gain
further information on the mutagenic spectrum of estab-
lished in vivo somatic cell mutagens.
10. The occurrence of chemical carcinogens that fail to
show evidence of genotoxicity in in vitro and in vivo
assays indicates the existence of a class of chemicals
that induce cancer by a mechanism that is not a conse-
quence of a direct interaction with DNA. The acceptance
of the validity of the concept of non-genotoxic mechanisms
of cancer induction is considered to be a crucial question
in the future deployment of short-term tests in carcino-
genesis.
11. The overall conclusion from the CSSTT/2 study is that
short-term in vivo tests have a vital role to play in
hazard assessment. This role is to define which
chemicals, identified as genotoxic from in vitro tests,
are active in vivo and, thus, are those most likely to
present a carcinogenic/mutagenic hazard to mammals,
including humans.
REFERENCES
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr,
MARGOLIN, B.H., MATTER, B., & SHELBY, M.D., ed. (1985) Evaluation of
short-term tests for carcinogens, Amsterdam, Oxford, New York, Elsevier
Science Publishers (Progress in Mutation Research, Vol. 5).
ASHBY, J., DE SERRES, F.J., SHELBY, M.D., MARGOLIN, B.H.,
ISHIDATE, M., Jr, & BECKING, G.C., ed. (1988a) Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 1.
ASHBY, J., SHELBY, M.D., & DE SERRES, F.J. (1988b) Overview of the IPCS
collaborative study on in vivo assay systems and conclusions. In: Ashby,
J., de Serres, F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, &
Becking, G.C., ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative study on
in vivo assays, Cambridge, United Kingdom, Cambridge University Press, Vol.
1, pp. 6-28.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-term tests for
carcinogens: Report of the international collaborative study, Amsterdam,
Oxford, New York, Elsevier Science Publishers (Progress in Mutation
Research, Vol. 1).
GENEROSO, W.M., CAIN, K.T., HELLWIG, C.S. & CACHEIRO, N.L.A. (1982)
Lack of association between induction of dominant-lethal mutations and
induction of heritable translocations with benzo (a) pyrene in postmeiotic
germ cells of male mice. Mutat. Res., 94: 155-163.
HICKS, M.R., ASHBY, J., & PENMAN, M. (1988) Review of the rodent
carcinogenicity data for the four test chemicals. In: Ashby, J., de Serres,
F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C.,
ed. Evaluation of short-term tests for carcinogens: Report of the
International Programme on Chemical Safety's collaborative study on in vivo
assays, Cambridge, United Kingdom, Cambridge University Press, Vol. 2, pp.
351-365.
MCGREGOR, D. (1988a) Summary report on the performance of the
miscellaneous group of in vivo assays (carcinogenicity, DNA adducts,
immunological, host-mediated mutation, urinary mutation assays, and limited
metabolic assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 2, pp. 3-21.
MCGREGOR, D. (1988b) The activities of 2AAF, 4AAF, BP and PYR in in
vitro assays for genetic toxicity. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 2, pp. 345-350.
MARGOLIN, B.H. & RISKO, K.J. (1988) The statistical analysis of in
vivo genotoxicity data: case studies of the rat hepatocyte UDS and mouse
bone marrow micronucleus assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 29-42.
MIRSALIS, J.C. (1988) Summary report on the performance of the in vivo
DNA repair assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests
for carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 1, pp. 345-351.
PATON, D. & ASHBY, J. (1988) Source and analytical details of the test
chemicals. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin, B.H.,
Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests for
carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 2, pp. 341-344.
SHELBY, M.D. (1986) A case for the continued use of the intraperitoneal
route of exposure. Mutat. Res., 70: 169-171.
TICE, R.R. (1988) Summary report on the performance of the in vivo
sister chromatid exchange assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 233-253.
WHO (1985) Environmental Health Criteria 47: Summary report on the
evaluation of short-term tests for carcinogens (collaborative study on in
vitro tests), Geneva, World Health Organization, 77 pp.
WYROBEK, A.J., GORDON, L.A., BURKHART, J.G., FRANCIS, M.W.,
KAPP, R.W., LETZ, G., MALLING, H.V., TOPHAM, J.C., & WHORTON, M.D.
(1983) An evaluation of the mouse sperm morphology test and other tests in
non-human mammals: A report of the United States Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 115: 1-7.
RESUME
ETUDE COLLECTIVE DU PROGRAMME INTERNATIONAL SUR LA SECURITE DES
SUBSTANCES CHIMIQUES (IPCS) POUR L'EVALUATION ET LA VALIDATION
DES EPREUVES DE COURTE DUREE RELATIVES AUX CANCEROGENES
La première partie de ce projet, qui traite des études
in vitro, a été publiée en 1985 (Ashby et al., 1985); on
en trouvera un résumé dans le No 47 de la série Critères
d'hygiène de l'environnement (OMS 1985). La seconde
partie, qui fait l'objet du présent rapport, a été publieé
en 1988 (Ashby et al., 1988).
Devant la nécessité d'évaluer l'intérêt des épreuves
de courte durée pour la recherche des substances mutagènes
et cancérogènes, il devenait indispensable d'organiser des
études collectives inter-laboratoires à l'échelle inter-
nationale. Ces épreuves de courte durée devaient compléter
les épreuves classiques à long terme sur rongeurs ou s'y
substituer. C'est le problème du choix ainsi que de la
fiabilité et de la sensibilité de ces épreuves qui a
conduit à la première grande entreprise de collaboration
au niveau international, à savoir le Programme coopératif
international pour l'évaluation des épreuves de courte
durée relatives aux cancérogènes (IPESTTC) (de Serres &
Ashby, 1981). Les résultats de cette étude ont confirmé
la fiabilité et la faisabilité de l'épreuve de mutation
des salmonelles pour une première identification des sub-
stances cancérogènes et mutagènes. On a également con-
staté qu'avec cette épreuve, certains produits notoirement
cancérogènes chez les rongeurs étaient impossibles ou tout
du moins très difficiles à mettre en évidence. Un certain
nombre d'autres épreuves examinées dans le cadre de
l'étude IPESTTC se sont révélées capables de mettre en
évidence quelques-uns des cancérogènes murins pour
lesquels l'épreuve sur salmonelle donnait un résultat
négatif. Toutefois, la base de données était trop res-
treinte pour qu'on puisse recommander une épreuve capable
de compléter l'épreuve de mutation des salmonelles.
Au vu des résultats de l'étude IPESTTC, il est apparu
nécessaire de poursuivre ce type de coopération afin
d'établir a) quel est l'ensemble d'épreuves in vitro le
plus efficace pour un premier tri des substances chimiques
sur la base de leur activité génotoxique et b) quelles
sont les épreuves in vivo de courte durée les plus utiles
pour confirmer la génotoxicité et le pouvoir cancérogène
des substances chimiques chez les mammifères. L'étude
collective pour l'évaluation et la validation des épreuves
de génotoxicité et de cancérogénicité de courte durée
(CSSTT), a été proposée par le Programme international sur
la sécurité des substances chimiques (IPCS) et le National
Institute of Environmental Health Sciences (NIEHS) des
Etats-Unis d'Amérique, l'une des institutions qui
participent au Programme IPCS. Devant l'ampleur de cette
entreprise et du fait des problèmes logistiques que posent
l'organisation et la gestion de ce genre d'études inter-
nationales, le projet a été divisé en deux études
distinctes: l'Etude collective sur les épreuves de géno-
toxicité et de cancérogénicité de courte durée in vitro
(CSSTT/1) et l'Etude collective sur les épreuves de
mutagénicité et de cancérogénicité de courte durée in vivo
(CSSTT/2).
L'Etude CSSTT/1 a permis de constituer une vaste base
de données à partir d'épreuves in vitro très diverses
portant sur dix produits chimiques organiques. Parmi eux
figuraient huit substances de cancérogénicité reconnue
pour les rongeurs qui s'étaient révélées soit négatives à
l'épreuve sur salmonelle soit difficiles à reconnaître de
cette manière, ainsi que deux substances considérées comme
non cancérogènes. On a procédé à l'évaluation de données
tirées de 90 groupes d'épreuves effectuées par quelque
60 scientifiques. Quatre types d'épreuves ont donné des
résultats suffisamment bons pour qu'on puisse envisager de
les utiliser en complément à l'épreuve sur salmonelle. Il
s'agissait de la recherche des aberrations chromosomiques,
des mutations géniques et des transformations néoplasiques
en culture de cellules mammaliennes ainsi que de l'épreuve
d'aneuploïdie des levures. Sauf dans le cas de la
recherche des aberrations chromosomiques, on a constaté
que les protocoles généralement utilisés pour ces épreuves
devaient être étudiés plus à fond avant d'être considérés
comme tout à fait acceptables.
La principale conclusion de l'étude CSSTT/1 relative
aux épreuves in vitro, c'est que en combinant l'épreuve
de recherche des aberrations chromosomiques à l'épreuve de
mutation des salmonelles, on peut procéder à un premier
criblage efficace des substances cancérogènes.
Lors de l'étude IPESTTC, sept substances sur 14 sup-
posées non cancérogènes ont donné des résultats positifs
dans un grand nombre d'épreuves in vitro. D'après les
données in vivo limitées qu'a fournies cette étude, il
semblerait que ces sept produits soient inactifs dans les
épreuves de courte durée in vivo. Les produits non can-
cérogènes de deux des couples cancérogène/non-cancérogène
soumis à l'étude IPESTTC, à savoir le couple benzo [a] py-
rène/pyrène (BP/PYR) et le couple acétyl-2 aminofluorène/
acétyl-4 aminofluorène (2AAF/4AAF), constituent de bons
exemples de ces réactions différentes et d'ailleurs, ces
couples de produits avaient été retenus pour la partie in
vivo de l'étude collective (CSSTT/2). Toutefois, il y
avait doute quant à la non cancérogénicité supposée du
4AAF et une part très importante de l'étude a été con-
sacrée à la mise en route d'études de cancérogéniticé à
long terme sur le 2AAF et le 4AAF chez le rat.
L'objectif de l'étude CSSTT/2 était donc de produire
un profil de données complet à partir d'une large gamme
d'épreuve in vivo de courte durée afin de voir comment les
différents paramètres génétiques des tissus-cibles se com-
portent vis-à-vis de produits chimiques classés comme
génotoxiques d'après les épreuves in vitro. La finalité
de l'étude était de recenser les épreuves in vivo suscep-
tibles d'être utilisées pour déterminer l'activité in vivo
de substances notoirement génotoxiques.
Quatre-vingt-dix-sept chercheurs de 16 pays ont par-
ticipé à l'étude in vivo, les données présentées portant
sur une cinquantaine de techniques distinctes. Les résul-
tats ont été évalués lors d'une réunion de ces chercheurs
qui s'est tenue au Cap d'Agde (France) en mai 1985. On a
préparé une série de rapports comportant une évaluation de
chaque groupe d'épreuves, des rapports récapitulatifs sur
les épreuves relatives aux cellules germinales et les
épreuves d'hépatotoxicité et des rapports résumant toute
la base de données relative à chaque couple de produits.
Par la suite, on a préparé un récapitulatif de toute
l'étude in vivo en vue de sa publication définitive.
Seule une faible proportion des épreuves examinées
lors de l'étude CSSTT/2 ont satisfait aux critères
d'acceptabilité comme épreuves in vivo de courte durée.
Toutefois les épreuves examinées lors de cette étude ne se
limitaient pas à celles jugées a priori les plus conformes
aux critères. De la sorte, on a pu tirer des données
provenant d'épreuves qui, à l'origine, n'étaient pas des
épreuves de génotoxicité in vivo, des renseignements sur
une large gamme d'effets biologiques produits par ces
quatre substances. La plupart des épreuves de génotoxicité
in vivo portant sur des cellules somatiques permettaient
de distinguer les deux couples cancérogène/non-cancérogène
encore que dans certains cas, les produits non cancéro-
gènes, en particulier le 4AAF aient présenté une faible
activité. L'insensibilité de certaines épreuves à l'un ou
à l'autre des deux couples de produits chimiques, corrob-
orent l'idée selon laquelle il faut obtenir des résultats
négatifs in vivo dans au moins deux épreuves pratiquées
sur des tissus différents avant de considérer qu'un
produit chimique n'est pas génotoxique in vivo.
Il a été confirmé que l'épreuve des micro-noyaux sur
moëlle osseuse de souris est robuste, sensible et repro-
ductible et on la recommande pour une première recherche
de la génotoxicité in vivo et in vitro. Les résultats
généraux obtenus au moyen de l'épreuve sur foie de rat
pour la synthèse anarchique de l'ADN incitent à penser
qu'elle pourrait compléter l'épreuve des micro-noyaux,
mais il faut en étudier encore la sensibilité et la
sélectivité. Certaines épreuves pourtant largement pré-
conisées, notamment les épreuves par passage sur hôte, les
épreuves de mutagénicité des urines et les épreuves sur
drosophile, se sont révelées impropres à une évaluation du
risque.
Les résultats de l'étude CSSTT/2 ont confirmé que les
épreuves in vivo de courte durée ont un rôle capital à
jouer dans l'évaluation du risque, rôle qui consiste dans
l'identification des produits chimiques qui s'étant
révélés génotoxiques in vitro, sont également actifs in
vivo et comportent donc vraisemblablement un risque de
cancérogénicité ou de mutagénicité pour les mammifères en
général et l'homme en particulier.
RESUMEN
PROGRAMA INTERNACIONAL DE SEGURIDAD DE LAS SUSTANCIAS QUIMICAS (IPCS):
ESTUDIO EN COLABORACION SOBRE EVALUACION Y COMPROBACION DE PRUEBAS
A CORTO PLAZO PARA SUSTANCIAS CARCINOGENAS
La primera parte del presente proyecto, relativa a
estudios in vitro, se publicó en 1985 (Ashby et al., 1985) y se
resumió en la publicación 47 de la serie "Environmental
Health Criteria" (OMS, 1985). La segunda parte es el
tema del presente informe y se publicó en 1988 (Ashby et
al., 1988).
La necesidad de estudios en colaboración entre
laboratorios en escala internacional surgió de que era
imprescindible investigar el valor de las pruebas a corto
plazo para detectar las sustancias químicas mutágenas y
carcinógenas. Se propusieron los ensayos a corto plazo
como métodos que sustituyeran o complementaran a los
tradicionales ensayos biológicos a largo plazo en
roedores. La preocupación por la elección de las pruebas
a corto plazo y por su fiabilidad y sensibilidad condujo a
que se realizara el primer ejercicio importante de
colaboración internacional: el programa internacional en
colaboración sobre evaluación de las pruebas a corto plazo
para sustancias carcinógenas (IPESTTC) (de Serres y Ashby,
1981). Los resultados de ese estudio confirmaron el valor
de la prueba de mutación de salmonelas como ensayo fiable
y factible para la identificación primaria de sustancias
carcinógenas y mutágenas. Se observó también que en la
prueba de salmonelas no se detectaban o sólo se detectaban
con grandes dificultades algunas sustancias de cancerogen-
icidad conocida en los roedores. Otros ensayos incluidos
en el estudio IPESTTC pudieron detectar sustancias
carcinógenas para roedores que eran negativas en el ensayo
de salmonelas. Sin embargo, la base de datos de apoyo era
demasiado pequeña para permitir la recomendación de un
ensayo que complementara la prueba de mutación de
salmonelas.
Los resultados del estudio IPESTTC pusieron de
manifiesto que se necesitaría un nuevo ejercicio en
colaboración para determinar: a) la combinación más
eficaz de ensayos in vitro para la detección primaria de
la actividad genotóxica de sustancias químicas, y b) las
pruebas in vivo a corto plazo más útiles para confirmar la
posibilidad de genotoxicidad y cancerogenicidad en mamí-
feros. El Programa Internacional sobre Seguridad de las
Sustancias Químicas (IPCS) y el Instituto Nacional de
Ciencias de Higiene del Medio (NIHS) de los Estados Unidos
de América, como institución participante en el IPCS,
propusieron el estudio en colaboración sobre evaluación y
comprobación de las pruebas a corto plazo para la geno-
toxicidad y la cancerogenicidad (CSSTT). Dadas la magnitud
prevista del proyecto y la logística de la organización y
administración de los proyectos internacionales, el
proyecto se dividió en dos estudios independientes: el
estudio en colaboración sobre pruebas a corto plazo para
la genotoxicidad y la cancerogenicidad (CSSTT/1) y el
estudio en colaboración sobre pruebas in vivo a corto
plazo para sustancias mutágenas y carcinógenas (CSSTT/2).
En el estudio CSSTT/1 se reunió una abarcante base de
datos procedentes de una amplia gama de ensayos in vitro
realizados con diez productos químicos orgánicos cuidad-
osamente seleccionados. Incluían ocho sustancias de
cancerogenicidad probada para roedores, que resultaban
negativas o difíciles de detectar en el ensayo de
salmonelas y dos sustancias químicas consideradas como no
carcinógenas. Se evaluaron los datos procedentes de casi
90 series de ensayos efectuados por unos 60 científicos
participantes. El rendimiento de cuatro tipos de ensayos
se estimó suficiente para considerarlos como posibles
pruebas complementarias del ensayo de salmonelas.
Incluyeron las pruebas para la determinación de aberra-
ciones cromosómicas, mutaciones génicas y transformaciones
neoplásicas en células de mamífero en cultivo, y un ensayo
para determinar la aneuploidia en levaduras. Con la
excepción del ensayo de aberraciones cromosómicas, resultó
evidente que los protocolos utilizados en general para
esas pruebas exigían evaluación adicional antes de poder
considerarlos plenamente aceptables.
La principal conclusión del estudio CSSTT/1 sobre las
pruebas in vitro fue que el empleo de los ensayos de
aberraciones cromosómicas en asociación con la prueba de
mutación de salmonelas podía resultar un detector primario
eficaz para posibles sustancias carcinógenas nuevas.
En el estudio IPESTTC, siete de las catorce sustancias
no carcinógenas presuntas dieron resultados positivos en
muchas de las pruebas in vitro. Los limitados datos de
pruebas in vivo disponibles procedentes de ese estudio
permiten pensar que esas siete sustancias químicas eran
inactivas en las pruebas a corto plazo in vivo. En dos
pares de sustancias carcinógenas/no carcinógenas de
IPESTTC (benzo [a] pireno/pireno (BP/PIR) y 2-acetilamino-
fluoreno/4-acetilamino-fluoreno (2AAF/4AAF), las sustan-
cias no carcinógenas proporcionaron buenos ejemplos de
tales respuestas diferentes, de modo que se seleccion-aron
esos pares de sustancias químicas para la parte in vivo
del estudio en colaboración (CSSTT/2). Sin embargo, hubo
una fuerte duda respecto a la presunta falta de cancero-
genicidad del 4AAF, y una parte crucial del estudio fue el
comienzo de bioensayos de cancerogenicidad a largo plazo
del 2AAF y el 4AAF en ratas.
Por consiguiente, el objetivo del estudio CSSTT/2 fue
producir un abarcante perfil de datos procedentes de una
amplia gama de pruebas in vivo a corto plazo, como medio
de conocer el mecanismo por el que los distintos puntos
finales genéticos de los tejidos destinatarios fundamen-
tales responden a sustancias químicas definidas como geno-
tóxicas in vitro. La meta final consistía en identificar
qué ensayos in vivo podrían usarse para determinar la
actividad in vivo de los productos genotóxicos probados.
Participaron en el proyecto de pruebas in vivo 97
investigadores de 16 países y se presentaron datos de unas
cincuenta técnicas in vivo distintas. Los resultados se
evaluaron en una reunión de investigadores celebrada en
Cap d'Agde (Francia) en mayo de 1985. Se preparó una
serie de informes que comprendían una evaluación de cada
grupo de ensayos, informes resumidos sobre los ensayos en
células germinales y las pruebas hepáticas específicas, e
informes resumidos sobre la base total de datos corres-
pondiente a cada par de sustancias químicas. Después se
preparó un examen general de todo el estudio in vivo listo
para la publicación final.
Sólo una pequeña proporción de los ensayos represent-
ados en el estudio CSSTT/2 satisficieron los criterios que
definen una prueba in vivo a corto plazo aceptable. Sin
embargo, los ensayos incluidos en el estudio no se
limitaron a los que podían cumplir con más probabilidad
los criterios. Así, aunque los datos procedían de ensayos
que no estaban destinados fundamentalmente a identificar
la genotoxicidad in vivo, proporcionaron información
sobre un amplio espectro de efectos biológicos de las
cuatro sustancias químicas. La mayoría de los ensayos en
células sométicas in vivo para determinar la genotoxicidad
discriminaron entre las sustancias carcinógenas/no carcin-
ógenas de los dos pares, aunque en algunos casos se
detectó una actividad débil en las pruebas con sustancias
no carcinógenas, en particular con el 4AAF. La insensib-
ilidad de algunos ensayos a uno u otro de los dos pares de
productos químicos apoya el concepto de que deben obtener-
se datos in vivo negativos en dos ensayos por lo menos en
diferentes tejidos antes de que pueda aceptarse que una
sustancia química carece de genotoxicidad in vivo.
Se confirmó que la prueba de micronúcleos de médula
ósea de ratón es un ensayo potente, sensible y repro-
ducible, que se recomienda para las pruebas in vivo
primarias de las sustancias genotóxicas in vitro. El
rendimiento general del ensayo en células hepáticas de
rata para la síntesis no programada de ADN permite pensar
que puede ser complementario de la prueba de micronúcleos,
aunque requieren investigación adicional ciertos aspectos
de sensibilidad y selectividad de ese ensayo. Se llegó a
la conclusión de que ciertos ensayos ampliamente susten-
tados, que incluyen los de mutagenicidad por orina y
mediada por el huésped y las pruebas que utilizan la mosca
drosófila, son inapropiados para la evaluación de
riesgos.
Los resultados del estudio CSSTT/2 confirmaron que las
pruebas in vivo a corto plazo tienen que desempeñar una
función crucial en la evaluación de los riesgos y que esa
función consiste en identificar los productos químicos
que, una vez demostrada la genotoxicidad in vitro, son
activos in vivo y tienen así mayores probabilidades de
presentar un riesgo de cancerogenicidad o mutagenicidad
para los mamíferos, incluido el hombre.
These observations were the origin of the present
study, the object of which was to generate a comprehensive
data profile from a broad range of short-term in vivo
assays as a means of understanding how various genetic
end-points in key target tissues respond to chemicals
defined as genotoxic in vitro. The objectives and design
of the collaborative study on short-term in vivo tests
(CSSTT/2) were outlined by an ad hoc Working Groupa at a
meeting organized by the IPCS in Geneva on 30 April 1981,
and the plans were consolidated by an IPCS Working
Groupb in Geneva, on 13-14 November 1981. The sub-
sequent coordination of the collaborative study was the
responsibility of a Steering Committeec derived primar-
ily from the Working Group.
The four test chemicals selected for the in vivo pro-
ject were the two carcinogen/non-carcinogen pairs, i.e.
BP/PYR and 2AAF/4AAF, that provided the initial impetus to
the study, and the first samples were distributed to
investigators in March 1983. During 1983, additional par-
ticipants joined the study to provide data on new in vivo
assays and nine coordinatorsd were appointed to oversee
the work being conducted by investigators performing
identical or similar assays. In all, 97 investigators from
16 countries participated in CSSTT/2. Progress was
reviewed at a meeting of the Steering Group with the
coordinators held in Brussels on 12-13 January 1984. At
this meeting a plan was developed to enter the data from
the study into a computer at NIEHS. It was envisaged that
such a plan would allow a comprehensive statistical
analysis using common statistical techniques for each kind
of assay and provide a comparison of results from
different laboratories performing the same assay.
Initially, it was expected that all the studies would be
completed by October 1984, but by September 1984 it was
evident that additional time would be required for many of
the investigators to complete their evaluation of the four
chemicals. At a second meeting of the Steering Group and
coordinators, held on 14-17 November 1984, status reports
prepared by the coordinators were reviewed in detail and
deadlines were set for the provision of final reports from
each investigator. Two additional coordinators were ap-
pointed at this meeting to oversee the rodent dominant
lethal assay (Dr W. Generoso) and the mouse coat colour
spot test (Dr R. Fahrig). Following this progress review,
a meet-ing of investigators was planned for May 1985, for
the presentation, collation, and assessment of the results
and the preparation of final reports on the study.
--------------------------------------------------------------------------------
a Participants: Dr J. Ashby, Professor N.P. Bochkov, Dr B.E. Matter,
Professor T. Matsushima, Dr F.J. de Serres, Dr M. Shelby, and Professor
F.H. Sobels.
b Participants: Dr J. Ashby, Dr G.R. Douglas, Dr M. Ishidate, Jr., Dr A.
Leonard, Dr N. Loprieno, Dr B.E. Matter, Professor T. Matsushima, Dr R.
Montesano, Dr F.J. de Serres, Dr M. Shelby, Professor F.H. Sobels, Dr M.
Stoltz, and Dr M. Waters.
c Steering Committee: Dr F.J. de Serres (Chairman), Dr J. Ashby, Dr. M.
Ishidate, Jr., Dr B. Margolin, Dr M. Shelby, Dr M. Draper, and Dr G.C.
Becking.
d Coordinators: Dr W. Vogel, Dr W.M. Generoso, Dr I.-D. Adler, Dr D. Wild,
Dr. T. Tice, Dr B.E. Butterworth, Dr D. McGregor, Dr M. Salamone, and
Dr R. Fahrig.
The meeting of investigators was held during 15-21 May
l985 at Cap d'Agde, France. Representatives from each par-
ticipating laboratory met to prepare a series of reports
comprising a) an assessment of each group of assays, b)
summary reports on the germ cell assays and the liver-
specific assays, and c) summary reports on the total data
base on each pair of chemicals, i.e. BP/PYR and 2AAF/
4AAF. During the meeting, draft reports were prepared and
were to be finalized during the following two months. At
a meeting of the Editorsa held at NIEHS from 24 July to 6
August 1985, the work group and investigators' reports
were reviewed, and a time-table was developed for the com-
pletion of all reports and for the preparation of the
Introduction and Overview of the study in readiness for
final publication of the in vivo study.
A feature of both the CSSTT studies and the earlier
IPESTTC project was the voluntary participation of a large
number of scientists together with support from their
parent institutions. The organization of CSSTT/1 and
CSSTT/2 was financed largely by IPCS and some of its par-
ticipating institutions. In most cases, funding of the
experimental work was by individual investigators, many of
whom incorporated the studies into their research pro-
grammes. This, of course, required the goodwill and
support of senior management of the participating labora-
tories from universities, research institutions, and in-
dustrial research facilities throughout the world. Ad-
ditional financial assistance was provided by a number of
governments that support IPCS, including Belgium, Italy,
Japan, The Netherlands, the United Kingdom, and the USA.
The United Kingdom Department of Health and Social Secur-
ity funded the rat carcinogenicity studies with 2AAF and
4AAF. The Belgian government and its National Institute
of Hygiene and Epidemiology financed meetings of the
Steering Committee and coordinators in Brussels. The
meeting of investigators held in Cap d'Agde was organized
by the French government and cosponsored by the French
Ministry of Health and the Commission of European
Communities.
--------------------------------------------------------------------------------
a Editors: Dr J. Ashby, Dr F.J. de Serres, Dr M.D. Shelby, Dr B.H.
Margolin, Dr M. Ishidate, Jr., and Dr G.C. Becking.
3. OVERALL AIMS OF THE STUDY AND CRITERIA FOR THE SELECTION OF
APPROPRIATE SHORT-TERM IN VIVO TESTS
Because of their relative simplicity, reproducibility,
and reliability, short-term in vitro tests are the methods
of choice for the initial testing of chemicals for geno-
toxic activity. The role and usefulness of genotoxicity
assays using whole animals, i.e. short-term in vivo
tests, are less clearly defined despite the fact that
they are widely used and are an integral part of most
legislative guidelines for the conduct of mutagenicity
tests. In general, in vivo tests are more resource-
consuming than their in vitro counterparts and the use of
animals for experiments for which there is an acceptable
in vitro alternative is to be discouraged. As these
observations suggest, in vivo assays should be designed to
answer questions that cannot be investigated adequately
with in vitro tests.
3.1 The use of short-term tests for the primary identification
of genotoxic chemicals
Certain short-term in vivo assays, such as the rodent
bone marrow chromosome assay, the rodent dominant lethal
test, and the host-mediated assay, were used in the early
1970s in primary screens for the identification of muta-
genic chemicals. With the introduction of reliable and
valid short-term in vitro tests for mutagens in the mid-
1970s, the use of in vivo procedures for initial screen-
ing of chemicals declined considerably. Certain legislat-
ive authorities still recommend a combination of in vitro
tests reserved for confirmatory or supplementary use or
for providing data for hazard assessment. These different
roles for whole animal procedures have led to the accept-
ance of test protocols of varying complexity, i.e. less
rigorous protocols for screening modes and more comprehen-
sive protocols when the test is required to assess hazard
potential.
Implicit in the concept of the two IPCS studies is the
assumption that chemicals shown to be genotoxic in vivo
also exhibit genotoxic activity in a properly designed
and conducted in vitro primary screen. This principle is
well-established in the scientific literature and was con-
firmed in the IPESTTC and CSSTT/1 studies. Thus, it was
an integral principle in the study design that the primary
screening of chemicals could be adequately served by in
vitro tests alone and that short-term in vivo assays have
no role to play when screening for genotoxic activity. In
vivo procedures will, therefore, be reserved for more
specific applications such as investigating the activity
of in vitro genotoxins in the whole animal and to assist
in the assessment of the mutagenic and carcinogenic poten-
tial associated with exposure of humans to in vitro geno-
toxins.
3.2 The use of short-term in vivo assays for assessing the
hazard associated with exposure to in vitro genotoxins
It is apparent from the last paragraph that the major
objective of the study was to investigate the activity in
the whole mammal of chemicals identified as genotoxic in
vitro and to establish which in vivo tests are the most
useful for this purpose. The major criterion for an ac-
ceptable short-term in vivo test, therefore, rests with
its ability to differentiate between carcinogenic and non-
carcinogenic chemicals, particularly those that have been
identified as genotoxic in an in vitro primary screen. To
extend this criterion further, an acceptable assay should
be capable of separating carcinogen/non-carcinogen ana-
logues, e.g., 2AAF/4AAF, in which, in some cases, small
differences in chemical structure are responsible for
dramatic differences in carcinogenic potential. Other
important criteria, some of which were more clearly
characterized during the course of the study, include the
following:
* data should be reproducible between different labora-
tories,
* the assays should not require too high a degree of
technical and scientific expertise to be conducted on
a routine, every-day basis,
* the genotoxic changes or end-points of the assays
should be clear and unambiguous,
* there should be agreed, valid statistical techniques
to differentiate between positive and negative data.
It is relevant to the performance of in vivo tests to
consider the metabolic fate of carcinogenic chemicals.
As a generalization and depending on the route of exposure
of the animal to the chemicals, reactive metabolites of
many carcinogens are generated mainly in the liver as a
by-product of a predominantly detoxifying process. These
metabolites may be capable of interaction with the genetic
material, DNA, in the liver cells, they may be transported
in an active form to other tissues, or they may be further
modified in those tissues to forms able to interact with
DNA. Thus, a study of the genotoxic activity of a chemical
in the whole animal should be able to detect the genotoxic
effects of chemicals or their metabolites in tissues out-
side the liver and of those whose main genotoxic reac-
tivity may be confined to the liver itself. For example,
assays based on genetic alterations to cells in the bone
marrow, which is readily accessible to chemicals or their
metabolites circulating in the blood, respond to a wide
range of chemical carcinogens. However, where reactive
metabolites are not readily transported from the liver,
bone marrow-type tests would be of little value and assays
capable of detecting the genotoxicity in liver cells
should be available.
3.3 The role of short-term in vivo tests in research
into the mechanisms of cancer
Chemical carcinogenesis is generally recognized as a
multistage process that begins with initiation, usually
considered to involve changes in DNA structure leading to
mutations, followed by promotion of the lesion to a pre-
malignant state and progression to overt cancer. Thus, the
majority of current short-term tests, designed to detect
the consequences of DNA interaction, will only respond to
chemicals that may induce tumours by a predominantly geno-
toxic mechanism or induce the initial phase of the car-
cinogenic process.
A feature of the literature on short-term tests for
chemical carcinogens is the occasional reference to chemi-
cals, shown to be associated with the induction of tumours
in laboratory animals, that consistently fail to be de-
tected in short-term in vitro or in vivo assays for geno-
toxicity. Such negative observations with established car-
cinogens have been explained by a lack of sensitivity of
the particular assays. If these chemicals (diethylhexyl-
phthalate is an example) are truly non-DNA-reactive, how-
ever, then negative data in assays for genotoxicity would
be the expected result. The acceptance of the existence
of so-called "non-genotoxic carcinogens" is of critical
importance to the future of short-term testing and one
consideration during the assessment of the CSSTT/2 study
was the identification of in vivo procedures that may be
useful for investigating such chemicals with the eventual
objective of developing specific short-term tests for non-
DNA-reactive carcinogens.
3.4 Assays for the detection of germ cell mutagens
As a general principle of genetic toxicology, genetic
damage induced by chemicals in somatic cells results in
hazard only to the affected individual, while genetic
effects in male or female germ cells may cause heritable
disease or malformation in the immediate progeny or in
future descendants of the affected individual. The ma-
jority of the assays represented in the CSSTT/2 study were
conducted in cells from somatic tissues and are, there-
fore, only directly interpretable in terms of somatic
mutation and carcinogenic potential. Although there have
been attempts to extrapolate data derived from somatic
cell assays to assessment of the probability of a chemical
inducing germ cell mutations, there are a number of
assays, some of which were represented in this study, that
measure genetic damage directly in the germ cells. The
objectives behind the inclusion of germ cell assays in the
present study were to determine:
* which kind of germ cell procedure effectively ident-
ifies germ cell mutagens;
* what is the relationship between the induction of
changes in sperm morphology and unscheduled DNA syn-
thesis in germ cells and the formation of true heri-
table mutations;
* which of the four in vitro genotoxins induce genetic
damage in mammalian germ cells.
4. CRITERIA FOR THE SELECTION OF THE FOUR TEST CHEMICALS
Selection of the two pairs of chemicals, benzo [a] pyrene/
pyrene (BP/PYR) and 2-acetylaminofluorene/4-acetylamino-
fluorene (2AAF/4AAF), was based, primarily, on their per-
formance in the IPESTTC study. In that study, seven of
the fourteen chemicals believed to be non-carcinogens
exhibited a high frequency of positive responses in in
vitro tests while being predominantly or totally negative
in a series of short-term in vivo tests. Although the in
vivo data base was limited, the non-carcinogens in the
BP/PYR and 2AAF/4AAF pairs provided good examples of such
differences in response and these four chemicals were
selected as useful representative candidates for investi-
gating the in vivo behaviour of chemicals known to be in
vitro genotoxins.
4.1 Activity of the four test chemicals in short-term in vitro tests
The majority of chemical carcinogens, including BP and
2AAF, require some form of enzyme-mediated biotransform-
ation for the formation of reactive metabolites. Most
target cells, whether bacteria, yeast or cultured mam-
malian cells, used in in vitro tests lack the appropriate
enzyme activity and this is usually provided in the form
of an enzyme-rich fraction derived from homogenates of rat
liver, referred to as the S9 fraction. Liver enzyme
activity is usually stimulated by pre-treatment of the
animals with an enzyme-inducer such as Aroclor 1254.
Both the carcinogens, BP and 2AAF, are consistently
positive in bacterial assays and other in vitro short-term
tests and, with the exception of certain mammalian cell
assays, demonstration of genotoxic activity requires the
incorporation of a rodent liver enzyme system. Positive
results have also be reported for the two non-carcinogens,
PYR and 4AAF, in a number of in vitro systems but not on
the scale nor usually with the same potency as their
carcinogenic analogues. Thus, both PYR and 4AAF induce
mutations in bacteria, gene conversion in yeast, and
unscheduled DNA synthesis (UDS) and gene mutation in
cultured mammalian cells. They do not, apparently, induce
mutations in yeast or chromosome aberrations in cultured
mammalian cells. 4AAF, but not PYR, can induce UDS in
cultured primary hepatocytes.
In the salmonella mutation assay, separation of the
carcinogen/non-carcinogen pairs is influenced signifi-
cantly by the nature of the rodent liver activation system
employed in the test. When the liver enzyme suspension is
derived from uninduced rodents, BP and 2AAF give positive
results and the two non-carcinogens, PYR and 4AAF, are
generally negative. If Aroclor-induced animals are used
as a source of the activation system, however, the ability
of the assay to discriminate between the carcinogens and
non-carcinogens is lost.
A comprehensive tabulation of the activities of the
four chemicals in short-term in vitro tests is provided by
McGregor (1988b).
4.2 Summary of carcinogenicity data on the test chemicals
The rodent carcinogenicity data for the four test
chemicals is reviewed in Hicks et al. (1988).
Both BP and 2AAF are well established and potent
carcinogens in rodents. Fewer studies have been conducted
with the non-carcinogens, PYR and 4AAF, and their presumed
non-carcinogenicity, although derived from limited rodent
bioassays, must remain tentative pending more comprehen-
sive testing.
BP is a locally-acting carcinogen in rats and mice,
producing skin tumours after dermal application and
tumours of the stomach when administered orally. In mouse
skin, it has been shown to have both initiating and
promoting activity. BP is also a lung carcinogen in mice
and induces mammary gland tumours in rats. There is no
evidence that BP is hepatocarcinogenic in rats and only a
single, unconfirmed report of the induction of hepatomas
in mice. On balance, the available evidence suggests that
BP does not induce liver tumours in rodents.
PYR has not been tested for carcinogenic activity in
any species other than the mouse and even then, only by
dermal application. These studies have shown fairly con-
clusively that PYR is not carcinogenic by this route.
There is some evidence from promotion/initiation studies
on mouse skin that it may have weak initiating activity.
Because data on systemic carcinogenesis in the mouse and
information from other species are lacking, the carcino-
genicity of PYR is somewhat equivocal. However, the fact
that its analogue, BP, is such a potent skin carcinogen,
whereas the data obtained with PYR in fairly comprehensive
mouse skin studies are negative, suggests that the
presumed non-carcinogenic status of PYR is justified.
2AAF is a potent carcinogen in both rats and mice. It
produces tumours in the liver and bladder in both species
after oral administration and in the rat it is carcino-
genic in Zymbal's glands and mammary glands. Little
information is available about its activity when adminis-
tered by other routes.
4AAF has only been tested in rats by incorporation in
the diet, and its activity in mice has not been investi-
gated. In rat feeding experiments, 4AAF has been consist-
ently non-carcinogenic though there are limited data to
suggest that its metabolite, N -OH-4AAF, may have some
weak carcinogenic activity. If 4AAF does prove to be
carcinogenic, its activity is clearly far less potent than
that of 2AAF and further long-term studies are required
before 4AAF can be unequivocally regarded as non-carcino-
genic. The question may be resolved when the results of
the rat carcinogenicity study, initiated as part of the
CSSTT/2 project, are made available.
5. SOURCE AND PURITY OF THE TEST CHEMICALS
Samples of the four test chemicals were obtained from
commercial sources: BP, 2AAF, and 4AAF were supplied by
Lancaster Synthesis Ltd., Eastgate, Whiteland, Morecambe,
Lancashire, United Kingdom, and PYR was obtained from
Aldrich Chemicals, Gillingham, Dorset, United Kingdom.
4AAF was synthesized by a route that avoided contamination
with 2AAF. The purity of the chemicals was determined by
the supplying laboratories before dispatch to the
participants. All the chemicals were greater than 99.5%
pure and full analytical details are provided by Paton and
Ashby (1988).
6. SHORT-TERM IN VIVO ASSAYS
For convenience the assays are divided into six groups:
* cytogenetic (chromosome) assays conducted on cells from
rodent bone marrow or other non-hepatic tissues;
* assays investigating a variety of effects in cells from
rodent liver;
* miscellaneous assays that utilize other tissues or body
fluids;
* mouse coat colour spot tests;
* assays in mammalian germ cells;
* tests utilizing the fruit fly, Drosophila melanogaster.
6.1 Cytogenetic assays
Assays for the induction of chromosome aberrations,
i.e., microscopically visible alterations in chromosome
structure, were conducted in bone marrow cells from rats,
mice, and Chinese hamsters and also in mouse ascites
tumour cells.
Micronucleus tests were conducted on cells from rat
and mouse bone marrow and on erythrocytes in circulating
blood from mice. Micronuclei are chromosome fragments or
intact chromosomes excluded from the cell nucleus during
mitosis. They are considered to be evidence of induced
chromosome breakage or chromosome loss and are usually
analysed in developing or mature erythrocytes.
Sister chromatid exchanges (SCE) can be demonstrated
in metaphase chromosomes by a differential staining tech-
nique and occur as a consequence of the exchange of repli-
cating DNA between chromatids at apparently homologous
loci. Although considered to result from DNA breakage and
reunion, the mechanism of the formation of SCE is not
fully understood. Assays for the induction of SCE were
conducted in bone marrow cells from rats, mice, and
Chinese hamsters, in circulating blood leucocytes from
mice, and in Chinese hamster intestinal epithelium.
Table 1. Assays employed in the CSSTT/2 study
-------------------------------------------------------------------------
1. Cytogenetic studies
Chromosome aberrations
Mouse bone marrow
Mouse ascites tumour cells
Rat bone marrow
Chinese hamster bone marrow
Micronucleus tests
Mouse bone marrow
Mouse circulation blood cells
Rat bone marrow
Sister chromatid exchanges
Mouse bone marrow
Mouse circulating blood lymphocytes
Rat bone marrow
Chinese hamster bone marrow
Chinese hamster intestinal epithelial cells
2. Liver-specific assays
Tests for initiation/promotion: altered enzyme foci
Unscheduled DNA synthesis (UDS)
UDS in mouse liver
UDS in neonatal, weanling and adult rat liver
Frequency of S-phase hepatocytes
S-phase in mouse liver
S-phase in weanling and adult rat liver
Cytogenetic tests
Aberrations in rat liver epithelial-like cells
SCE in rat liver epithelial-like cells
Micronuclei in hepatocytes
Diploid/tetraploid ratio in rat and mouse hepatocytes
Primary changes in DNA
Alkaline elution assay for DNA strand damage
DNA/protein cross-links
DNA unwinding assay for strand damage
3. Miscellaneous assays
Specific carcinogenicity assays
Two-year oral dosing study in rats
Mouse lung adenoma assay
Quail egg tumour-induction
UDS in rat fore-stomach
Sebaceous gland suppression assay
Observation of dermal epithelial hyperplasia
Transformation of rat peritoneal macrophages
6-Thioguanine-resistant mutations in Syrian hamster lung cells
Measurement of DNA adducts
32P-post-labelling in rat and mouse tissues
Immunochemical detection of DNA adducts
Radiolabelled test chemicals - rat liver
-----------------------------------------------------------------------------
Table 1. (contd.)
-----------------------------------------------------------------------------
3. Miscellaneous assays (contd.)
Immunotoxicity assays
Natural Killer (NK) cell and T-cell cytotoxicity in rats
Host-mediated assays
Mutation of salmonella in mouse tissues
Genetic changes in yeast cells in mouse tissues
Urine mutagenicity tests in rats, mice and guinea pigs
4. Mouse spot tests
Mouse coat colour spot test
Mouse melanocyte assay
5. Mammalian germ cell studies
Dominant lethal assays with male and female mice and male rats
Morphological abnormalities in mouse and rat spermatozoa
Unscheduled DNA synthesis in rat and mouse male germ cells
6. Drosophila assays
Sex-linked recessive lethal mutations in germ cells
Chromosome loss in germ cells
Somatic mutation and recombination: mosaic spots in eyes
and wings
-----------------------------------------------------------------------------
6.2 Assays in rodent liver cells
A variety of liver-specific assays were represented in
the study, including the demonstration of enzyme changes,
a number of different tests on DNA, and cytogenetic
assays.
The rat liver assay for altered enzyme foci employs a
histochemical technique that identifies groups of cells or
foci that have elevated levels of the marker enzyme gamma-
glutamyltranspeptidase (GGT). The observation of GGT-
positive foci often precedes the appearance of liver car-
cinoma and the test is used to attempt to identify early
stages of carcinogenesis in the liver. Protocols have been
devised to investigate both initiation and promotion
stages of carcinogenesis.
A number of investigators used assays that measure
directly or indirectly the response of DNA to chemical
damage. These included tests for breaks in single strands
of DNA using the alkaline elution technique, an assay for
the induction of crosslinks between DNA and protein mol-
ecules, and a measure of single strand breaks based on the
degree of unwinding of DNA molecules. One consequence of
DNA breakage is the initiation of the enzyme-mediated
repair process which involves the synthesis of new, rela-
tively short strands of DNA to repair the break. This type
of repair is referred to as unscheduled DNA synthesis or
UDS (to differentiate from the normal or S-phase DNA syn-
thesis that occurs during cell replication). DNA synthesis
can be measured by observing the uptake of tritiated thy-
midine by the newly synthesized strands using autoradio-
graphical techniques. Assays for the induction of UDS and
to measure changes in the numbers of S-phase cells in
rodent liver were included in the study.
The cytogenetic methods included the analysis of meta-
phase chromosome aberrations, micronuclei and SCE in rat
liver cells, and the determination of the ratio of diploid
to tetraploid cells in rat and mouse liver.
6.3 Miscellaneous assays
The first group of assays to be considered under this
heading are those that are directly related to the induc-
tion of tumours in rodents. The most important of these
is a 2-year oral dosing study in rats to compare the car-
cinogenicity of 2AAF and 4AAF. Although the study was not
completed at the time of this report, preliminary infor-
mation from a small group of rats examined after 26 weeks
of dosing is available. Also included in this group was
a) the mouse lung tumour assay that determined the effects
of each of the four test chemicals on the incidence of
lung adenoma in mice and b) a test in Japanese quail in
which the test chemicals were introduced into the yolk
sacs of quail eggs. The birds were then examined for
evidence of tumours at various intervals after hatching.
The sebaceous gland suppression test is based on the
observation of morphological changes to the sebaceous
glands in histological preparations of mouse skin after
dermal exposure to the test chemicals. The presence of
epidermal hyperplasia can also be detected histologically
and both methods have been shown to respond to skin car-
cinogens, particularly polycyclic hydrocarbons.
Genotoxic carcinogens can bind covalently to biologi-
cal macromolecules such as DNA either before or after
metabolic biotransformation. The products of DNA-binding
are referred to as DNA adducts and a number of investi-
gators provided data on the detection and measurement of
DNA adducts with the test chemicals in tissues from rats
and mice. Three different methods were represented:
* the enzyme-linked immunoabsorbent assay (ELISA);
* the 32P-post-labelling assay;
* an assay for radiolabelled chemicals bound to DNA.
Two similar assays for the investigation of the immune
response of animals to exposure to carcinogens were rep-
resented. Natural Killer (NK) cells are leucocytes with
the ability to lyse a variety of `foreign' cells, e.g.,
those with a malignant phenotype. The NK cell assay was
used to investigate the capacity of NK cells, derived from
the splenic mononuclear leucocytes of rats treated with
2AAF or 4AAF, to lyse human erythroleukaemic cells in
vitro. The basis of the T-cell assay is that immunocom-
petent T-cells are able to react to tissue changes induced
by carcinogenic chemicals in rats. The changes in T-cell
activity can be determined by their cytotoxicity towards
target cells derived from rat intestinal adenocarcinoma.
The rat peritoneal cell transformation test is based
on the observation that mitogen-stimulated peritoneal
cells, harvested from rats dosed with carcinogens, undergo
a form of transformation that enables them to proliferate
and produce colonies in soft agar culture.
Host-mediated assays were used fairly widely in the
early l970s as primary screening tests for mutagenic
chemicals. In its original form, a suspension of microbial
target cells, i.e. yeast or bacteria, was introduced into
the peritoneal cavities of mice. The mice were treated
with the suspect chemical and, after an appropriate inter-
val, the target cells were harvested and changes in the
mutation frequency or other genetic end-points were deter-
mined in in vitro culture. Since that time, there have
been several modifications to the procedure, the most
significant being the injection of the target organisms
into the circulating blood of the host. In this way, the
bacterial or yeast target cells are distributed in various
organs and can, for example, be harvested from the liver,
lungs, and kidney. The target cells can, in principle, be
affected by reactive metabolites generated from the test
chemical in these organs. Intra-sanguinous host-mediated
assays using either Salmonella typhimurium or the yeast,
Saccharomyces cerevisiae, were represented in the study.
Urine and other body fluids can be collected from
rodents treated with test chemicals and assayed for muta-
genic activity. For example, after appropriate treatment
to release conjugated metabolites, urine can be tested for
the presence of mutagens in a conventional in vitro bac-
terial mutation assay. In principle, the urine assay is
capable of detecting mutagenic chemicals excreted un-
changed or after metabolic activation.
6.4 The mouse spot test
The mouse spot test detects mutations induced in mel-
anocyte precurser cells in the embryos of specially de-
rived strains of mice. The mouse strain carries recessive
mutations at a number of specific coat colour loci and the
embryos are, thus, heterozygous at these loci. Mutations
induced at the wild-type or normal alleles of the coat
colour loci result in the development of clones of the
mutant melanocytes. The young mice are examined after
birth and the mutations are expressed as patches of con-
trasting coloured fur. The mutations can result from one
of a number of genetic events including gene mutations,
chromosome deletions, mitotic crossing-over, and loss of
whole chromosomes. In addition to the conventional tech-
nique of observing the appearance of coloured spots in the
fur, a modification of the spot test allows the micro-
scopic recognition of mutant melanocytes in preparations
of embryonic skin.
6.5 Mammalian germ cell studies
The classical dominant lethal assay in male rodents
involves the treatment of the animals with the test chemi-
cal and then mating with groups of females at intervals to
cover the complete spermatogenic cycle. Dominant lethal
mutations induced at any stage of spermatogenesis can be
detected by dissection of the uterine contents of each
female and are characterized by dead fetuses or a re-
duction in the numbers of fetal implantations. Dominant
treatment of male rats, male mice, or female mice.
Two other assays represented in this group were the
abnormal sperm morphology assay and the unscheduled DNA
synthesis (UDS) test. The former assay monitors mature
spermatazoa for irregularities in morphology at intervals
after treatment of rodents with the test chemicals. The
UDS test measures DNA repair in developing spermatocytes
and spermatids.
6.6 Drosophila assays
Although data from drosophila tests cannot be regarded
as appropriate alternatives to mammalian data for assess-
ing the potential hazard to humans from exposure to geno-
toxic chemicals, drosophila are, in fact, intact, complex,
eukaryotic organisms whose metabolic and genetic charac-
teristics have some parallels with those of the whole mam-
mal. Their main value in genotoxicity testing lies with
the availability of assays to study the effects of chemi-
cals on both somatic and germ cells. The tests represented
in the CSSTT/2 study were:
* the sex-linked recessive lethal mutation assay in
germ cells;
* tests for chromosome loss from germ cells;
* assays for somatic mutation and recombination using
the induction of mosaic spots in either the wings or
the eyes.
7. RESULTS
The investigators met at Cap d'Agde, France, from 15
to 21 May 1985, to assess the results of the study. Each
group of investigators presenting data from a particular
type of assay discussed their data and individual results
were assessed and agreed. This led to the preparation of
consensus reports on the response of each assay to the
four test chemicals. These consensus views were then
incorporated into coordinators' summary reports on each
group of assays. Summary reports were presented and criti-
cally discussed in open plenary discussions, thereby
allowing the overall conclusions and recommendations
resulting from the study to be formulated. Reports of
individual investigators, the coordinators summary
reports, and certain technical appendices form the main
text of the final publication together with an editorial
overview of the study (Ashby et al., 1988).
The purpose of this chapter of the report is to
present the results of the in vivo studies with the four
test chemicals and to construct a profile, in qualitative
terms, of their genotoxic activity in the whole animal.
Quantitative differences in response in relation to sex,
species, route of exposure, and technical variations are
considered in more detail in the next section, which also
includes a comprehensive tabulation of the data generated
by individual investigators (Table 4). However, as many
of the assays were replicated in a number of laboratories,
a simplified table of results is presented here (Table 2).
It must be emphasized that Table 2 contains the consensus
views of the Working Groups assessing the individual
results and performance of each assay and that, in some
cases, there were conflicting results on the same assay
from participating laboratories.
Table 2. Results of the short-term in vivo tests summarized
by Working Groups a
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
1. Cytogenetic studies
Chromosomal aberrations
Mouse bone marrow P N ? N
Mouse ascites cells P NT N N
Rat bone marrow P N ? N
Chinese hamster bone marrow P N P N
Micronucleus tests
Mouse bone marrow P N P N
Mouse blood - maternal P N N N
Mouse blood - fetal P N ? N
Rat bone marrow P N P N
Sister chromatid exchange
Mouse bone marrow P W P W
Mouse blood P N P N
Rat bone marrow P W P W
Chinese hamster bone marrow P N P N
Chinese hamster intestinal cells P N P N
2. Liver-specific assays
Altered enzyme foci - rat P N P W
Unscheduled DNA synthesis
Mouse liver N N N N
Rat liver N N P W
S-phase hepatocytes
Mouse liver NT NT W P
Rat liver - adult W N ? P
Rat liver - weanling P NT NT NT
Cytogenetic tests
Metaphase aberrations - rat W N W N
Micronuclei - rat N N P N
SCE - rat W N ? N
Ploidy - rat P N P N
Ploidy - mouse P N P N
Primary DNA changes
Alkaline elution N N ? ?
DNA/protein cross-links P N P NT
DNA unwinding N N P N
with repair inhibitor P N P N
3. Miscellaneous assays
Carcinogenicity
Oral dosing - rat NT NT P ?
Mouse lung adenoma P N P N
Quail egg tumours ? ? ? ?
UDS in rat fore-stomach ? N N N
--------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
3. Miscellaneous assays (contd.)
Sebacious gland suppression - mouse P N NT NT
Epithelial hyperplasia - mouse P N NT NT
Peritoneal macrophages - rat N N N N
Syrian hamster lung - mutation P N ? N
DNA adducts
32P-post-labelling - rat P N P W
Immunochemical (ELISA) NT NT ? ?
Radiolabelled chemicals NT NT P P
Immunotoxicity
Natural Killer (NK) cells NT NT P N
T-cell cytoxicity P N P W
Host-mediated assays - mouse
Salmonella typhimurium N N N N
Saccharomyces (liver) P P P P
Saccharomyces (lung) N N N N
Urine mutagenicity
Rats P P P P
Mice W W P P
Guinea-pig NT NT P NT
4. Mouse spot tests
Coat colour spots P N P N
Melanocyte assays NT NT P N
5. Mammalian germ cells
Dominant lethal assays
Male mice P N N N
Female mice P N N N
Male rats NT NT N N
Sperm abnormalities
Mice P N P N
Rats NT NT N N
UDS in germ cells
Mice N N N N
Rats N N N N
6. Drosophila assays
Sex-linked recessives N N N N
Chromosome loss N N N N
Somatic mutation
Eye spots P N P N
Wing spots P N W ?
--------------------------------------------------------------------------
a P = positive
W = weak positive
N = negative
? = results inconclusive or not yet reported
NT = not tested
7.1 Benzo [a] pyrene (BP) and pyrene (PYR)
7.1.1 Cytogenetic studies
Assays for the induction of structural chromosome
aberrations or micronuclei in rodent bone marrow cells
were consistently positive with BP and negative with PYR.
Among a number of experimental variables between different
investigators, neither the species, strain, sex, solvent,
nor route of exposure affected the qualitative result. BP
increased the incidence of sister chromatid exchanges
(SCE) in mouse, rat, and Chinese hamster bone marrow in
every study. The results with PYR, however, were less
clear, and this presumed non-carcinogen induced SCE at
high dose levels in mice after oral or intraperitoneal
dosing and in male rats after oral administration, i.e. 3
of 9 SCE studies reported a weak positive result. Analysis
of micronuclei in circulating blood erythrocytes from
maternal, fetal, or weanling mice, and SCE in circulating
blood lymphocytes from adult mice clearly discriminated
between BP and PYR.
7.1.2 Liver-specific assays
Five investigators provided data from observations of
altered enzyme foci in rat liver under the general heading
of initiation/promotion assays. There were, however,
significant protocol variations between investigators.
Two protocols required administration of the rodent tumour
promotor, phenobarbitone, in the drinking-water or diet
for some weeks after dosing in order to determine
initiating activity of the test chemicals. The promoting
activity of the test chemicals was studied by two other
workers by producing initiation events in the liver before
treatment with the test chemicals. The fifth protocol
tested the ability of the chemicals to induce both
initiation and promotion without discriminating between
the two phases. In all cases, the evidence for induced
pre-cancerous changes was the observation of foci or
clones of cells with altered enzyme characteristics. BP
was shown to have significant initiating activity in rat
liver in two studies and, in a third study, was shown
capable of promoting nitrosamine-induced initiation.
Using a fourth, essentially experimental, protocol that
involved the transplantation of donor liver cells to the
host animal, BP failed to show promoting activity. No
evidence of tumour initiating or promoting activity was
observed in any of the studies with PYR.
The rodent liver assay for unscheduled DNA synthesis
measures the induction of repairable lesion in the DNA of
hepatocytes and, theoretically, should be capable of
detecting chemicals that are metabolized in the liver to
produce genotoxic (i.e. DNA-interactive) metabolites.
Both BP and PYR were reproducibly negative in mouse
hepatocytes and in hepatocytes from adult, weanling, or
neonatal rats using either oral or intraperitoneal dosing.
The induction of S-phase DNA synthesis was also
investigated and BP was shown to increase the incidence of
S-phase cells in weanling rats and, in one of two
experiments, a slight increase in S-phase cells was
observed in adult rats. In mice, BP had no significant
effect on the incidence of S-phase, and PYR showed no
evidence of S-phase synthesis induction in either
species.
Limited data were presented on the cytogenetic effects
of the chemicals in liver-derived cells. In epithelial-
like cells from weanling rats, BP was observed to induce a
small increase in the incidence of structural chromosome
aberrations and SCE; tests with PYR were negative. These
findings, however, require confirmation. No evidence of
micronucleus-induction was detected in rats treated with
BP or PYR after partial hepatectomy. Using a cytofluoro-
metric method, an increase in the ratio of diploid to
tetraploid cells was observed in liver-derived cells after
treatment of rats with BP, but not after dosing with PYR.
Three different assays were used to study the ability
of the chemicals to induce single-strand breaks (SSB) in
liver cell DNA. Both BP and PYR were uniformly negative
in the alkaline elution assay for SSB conducted in three
laboratories. BP, however, was shown to induce crosslinks
between DNA and protein, while PYR was negative in this
assay. The third assay in this group was a measure of SSB
based on the degree of alkali-induced unwinding of DNA
molecules. When the DNA-repair inhibitor, adenosine
arabinoside was incorporated, BP was shown to induce SSB
in mouse liver cell DNA using this technique. In the
absence of the repair inhibitor, SSB were not observed.
PYR produced negative results in the DNA-unwinding assay.
The results from this group of assays suggest that BP is
capable of inducing SSB in the DNA of mouse liver cells
that are effectively repaired in the absence of a DNA-
repair inhibitor. In rats, the observation of DNA/protein
crosslinks tentatively suggests the induction of SSB in
rat liver cells, but additional studies are required to
assess the DNA effects of BP in rat liver.
7.1.3 Miscellaneous assays
Two investigators are producing data that are directly
related to the induction of tumours. In the lung adenoma
assay, mice were injected with BP or PYR by the intra-
peritoneal route, 2-3 times each week, for up to 8 weeks.
The mice were examined 23 weeks after the start of the
study and the numbers of adenoma on the surface of the
pulmonary lobes were recorded. BP induced a significant
increase in adenomas compared with the untreated control
group while no increase in the induction of these tumours
was observed in mice injected with PYR. This assay
differentiated very clearly between the carcinogenic
activity of BP and PYR. The second study in this category
involved injecting the test material into the yolk sac of
quail eggs and then observing the birds for the presence
of tumours at intervals after hatching. In birds examined
at approximately 3 months, there was no evidence of tumour
induction by either BP or PYR. Surviving birds will be
maintained for up to 12 months before being examined for
the presence of tumours and, therefore, a definitive
conclusion on the carcinogenicity of BP and PYR to quail
is not yet possible.
One investigator studied the induction of UDS in the
fore-stomach of rats after oral dosing; the data with BP
were considered to be equivocal while PYR gave negative
results.
Two assays involved the application of the test chemi-
cals to mouse skin followed by histological examination of
skin sections. BP induced epithelial hyperplasia and
suppression of sebaceous glands, both of which have been
correlated with polycyclic hydrocarbon-induced skin
carcinogenesis. PYR failed to elicit either of these
responses.
The transformation of rat peritoneal macrophages has
been advocated as a short-term in vivo test for poten-
tially carcinogenic chemicals. Macrophages isolated from
rats dosed with BP or PYR, however, failed to show any
evidence of transformation in experiments conducted in
three laboratories.
Somatic mutation data on cells isolated from Syrian
hamster lung tissue, after dosing with the test chemicals,
were presented by one investigator. Cultures of cells
from animals dosed with BP showed a higher frequency of
mutations at the 6-thioguanine locus than those from
untreated hamsters, suggesting the induction of somatic
mutations in lung cells from this species. PYR produced
negative mutation data in this assay.
32P-labelling of purified DNA was used to detect DNA
adducts in tissues from rats and mice treated intraperito-
neally with the test chemicals. Measurable levels of PYR
adducts were not detected in DNA from mouse liver, lung,
or kidney, or from rat liver or lung tissues. BP adducts
were identified in all of these tissues.
Only one of the two assays for immunotoxicity was con-
ducted with the BP/PYR pair. Thus, the T-cell assay showed
that BP, but not PYR, was capable of inducing a cytotoxic
response in rat T-cells.
Two investigators provided data from host-mediated
assays in mice dosed with BP or PYR. In tests with the
yeast, Saccharomyces cerevisiae, a significant increase
in mitotic gene conversion was detected in yeast cells
isolated from the liver of mice dosed with either BP or
PYR, while yeast cells isolated from lung tissue produced
negative data with both chemicals. No evidence of the
induction of mutations was observed in Salmonella
typhimurium isolated from liver tissues of mice dosed with
BP or PYR.
Assays for the detection of mutagens in urine were
conducted with both rats and mice. Mutagenic activity was
detected in urine samples from both species after dosing
with BP or PYR and, therefore, the test failed to differ-
entiate between the two chemicals.
7.1.4 Mouse spot tests
Only one investigator provided mouse spot test data on
BP and PYR. Using the observation of coat colour spots in
off-spring as evidence of somatic mutation, BP was clearly
positive and PYR negative.
7.1.5 Mammalian germ cell assays
BP induced dominant lethal mutations in both male and
female mice after intraperitoneal injections but not after
oral dosing. The response in the male was highly stage-
specific; only the mature spermatozoa were sensitive and
spermatid and spermatocyte stages were unaffected. Domi-
nant lethal assays with PYR were reproducibly negative.
Studies of morphological abnormalities in mature
spermatozoa were conducted in mice after oral or
intraperitoneal dosing. BP was uniformly positive in this
assay, regardless of the route of exposure. Although a
weak positive response was observed in one of seven
experiments with PYR, this observation was not confirmed
and the consensus view was that PYR did not induce sperm
abnormalities. In studies of the induction of unscheduled
DNA synthesis in rat and mouse male germ cells, negative
results were obtained with both BP and PYR.
7.1.6 Drosophila assays
In all, ten studies were conducted with BP and PYR in
drosophila and in each case the oral route of treatment
was used. Germ cell studies, i.e. the sex-linked
recessive lethal mutation assay and the test for chromo-
some loss, were uniformly negative with both chemicals.
In contrast, tests for somatic mutation and recombination,
based on the observation of eye or wing spots, showed that
BP was able to induce genetic changes in somatic cells in
each of three studies. PYR was consistently negative.
7.2 2-Acetylaminofluorene and 4-acetylaminofluorene
7.2.1 Cytogenetic studies
Seven investigators provided data from analysis of
metaphase chromosomes in mouse bone marrow cells. 2AAF
induced a significant increase in the incidence of
structural chromosome aberrations in only two of seven
assays, one of which was considered to be inconclusive.
The results of assays with 4AAF were negative with the
exception of two assays in which the data were inconclus-
ive. In contrast, 2AAF was reproducibly positive and 4AAF
consistently negative in 15 of 16 micronucleus tests
conducted in mouse bone marrow erythrocytes. The route of
exposure, i.e. oral dosing or intraperitoneal injection,
did not appear to influence the outcome of these studies.
Micronuclei were produced by 2AAF in rat bone marrow cells
and a small increase was also recorded in one of two
assays with 4AAF. Two workers investigated the influence
of these two chemicals on the incidence of structural
chromosome aberrations in Chinese hamster bone marrow;
2AAF was positive in this species and 4AAF gave a weak
positive result in one of the two studies. Both chemicals
failed to induce detectable chromosome damage in mouse
ascites tumour cells. Four micronucleus tests were
conducted with mouse blood erythrocytes. An increase in
micronuclei was recorded by one worker in blood cells from
weanling mice dosed with 2AAF, although when samples of
maternal or fetal blood were examined, increases in
micronuclei were not apparent. However, studies conducted
in another laboratory showed that 2AAF was capable of
increasing the incidence of micronuclei in fetal blood.
Thus, 2AAF is clearly clastogenic to rodent bone marrow
cells while 4AAF was considered by most investigators to
be devoid of clastogenic activity.
Nine assays for sister chromatid exchanges (SCE) were
conducted in bone marrow cells from mice, rats, or Chinese
hamsters. 2AAF was clearly positive in this test and the
consensus view was that 4AAF, although negative in four
assays, was capable of inducing a small (but significant)
increase in SCE. In studies with mouse lymphocytes and
Chinese hamster intestinal epithelial cells, 2AAF was
positive and 4AAF was considered to be negative.
7.2.2 Liver-specific assays
As discussed in section 7.1.2, there were a number of
variations between the five protocols used in the
initiation/promotion assays. Significant initiation and
promotion activity was demonstrated in studies with 2AAF
based on the observation of foci of cells with altered
enzyme characteristics in rat liver. These assays failed
to discriminate clearly between the two chemicals, since
4AAF was shown to be an initiator in the hands of one
investigator and had weak promoting activity in another
study. Both chemicals, therefore, proved capable of
producing these pre-cancerous changes in rat liver tissue
though with different degrees of potency.
2AAF and 4AAF failed to induce detectable unscheduled
DNA synthesis (UDS) in mouse hepatocytes after oral
dosing. Quite different results were obtained in rat
liver, however, and 2AAF was shown to induce UDS in rat
hepatocytes in each of the seven laboratories that sub-
mitted data. Three of these laboratories also reported the
detection of a small increase in UDS in rats after dosing
with 4AAF. The other four laboratories obtained negative
data. Thus, 2AAF induced UDS in hepatocytes from rats but
not mice, and 4AAF elicited a weak UDS response in the rat
and was negative in mouse cells.
The results of the analysis of S-phase DNA synthesis
indicated that the presumed non-carcinogen, 4AAF, was a
very potent inducer of S-phase cells in the liver of both
rats and mice. 2AAF, on the other hand, induced only a
small increase in S-phase cells in the mouse and three
studies in rat liver produced one clear positive and two
negative results.
In cytogenetic experiments in cells derived from rat
liver, 2AAF appeared to induce a small increase in the
incidence of structural aberrations and, in partially
hepatectomised rats, a significant increase in micro-
nuclei. 4AAF gave negative results in tests for chromo-
somal aberrations, micronuclei, and SCE in rat liver
cells. When the ratio of diploid to tetraploid cells was
investigated in rat and mouse liver, an increase in the
ratio was observed in both species after dosing with 2AAF
but not in response to 4AAF.
The alkaline elution assay for single strand breaks
(SSB) produced conflicting results between three labora-
tories. Two investigators presented negative data from
assays with both chemicals while a third observed that
both 2AAF and 4AAF produced SSB in this test. Using the
detection of DNA/DNA or DNA/protein crosslinks as a
measure of SSB, 2AAF was shown to produce SSB. No data
were presented on 4AAF. Another investigator calculated
SSB from the amount of DNA unwinding induced by the chemi-
cals. 2AAF and 4AAF gave positive and negative results,
respectively, either in the presence or absence of a DNA
repair inhibitor. These data suggest that, unlike 4AAF,
2AAF is capable of producing SSB in rat liver DNA under
appropriate experimental conditions.
7.2.3 Miscellaneous assays
A 2-year oral dosing carcinogenicity study in rats
with 2AAF and 4AAF is still in progress. Preliminary
findings in a small number of animals examined after 26
weeks of dosing showed that an increase in liver tumours
was already apparent in animals dosed with 2AAF; there was
no evidence of tumour induction in the 4AAF-dosed ani-
mals.
The lung adenoma assay, in which mice were given
intraperitoneal injections of the test chemicals 2-3 times
a week for up to 8 weeks, clearly separated 2AAF and 4AAF.
2AAF induced a significant increase in adenomas while the
incidence in mice dosed with 4AAF was comparable with that
of untreated mice.
There was no evidence of the induction of UDS in rat
fore-stomach after oral dosing with either chemical, and
the assay for the induction of somatic mutations in Syrian
hamster lung cells produced inconclusive data with 2AAF
and was negative in tests with 4AAF. The rat macrophage
test was reproducibly negative with both 2AAF and 4AAF.
Three techniques were applied to detect the presence
of DNA adducts formed in various tissues after dosing
rodents with the test chemicals. Data from the ELISA
technique, which requires the use of monoclonal antibodies
to detect specific adducts, are not yet available. How-
ever, the 32P-post-labelling method indicated that 2AAF
formed adducts with DNA in rat liver and lung and in mouse
liver, lung, and kidney after intraperitoneal dosing. No
data were presented from mice dosed with 4AAF. In the
rat, however, 4AAF formed adducts in liver and lung DNA
though at a much lower rate than 2AAF. In studies using
radiolabelled test materials, both chemicals were shown to
form adducts with DNA in the liver and lungs of rats after
intraperitoneal administration, although adduct formation
was considerably less with 4AAF than with 2AAF.
2AAF produced positive results in both immunotoxicity
assays. However, although 4AAF was negative in the
Natural Killer (NK) cell assay, data from the T-cell
cytotoxicity test suggested that it was capable of
inducing a measurable cytotoxic response in rat T-Cells.
Nine sets of data were considered from mouse host-
mediated assays. In eight of these, including assays for
genetic changes in yeast isolated from liver, lung and
kidney, and for mutations in salmonella isolated from
liver tissue, 4AAF was uniformly negative. 2AAF produced
two weak positive responses and five negative results in
the same eight studies. The ninth assay, however, indi-
cated that both chemicals induced mitotic gene conversion
in yeast isolated from liver tissue. In addition, muta-
genic materials were detected in urine samples from rats
and mice after dosing with either isomer and from guinea-
pigs after dosing with 2AAF. 4AAF data on guinea-pig
urine were not available.
7.2.4 Mouse spot tests
Six separate mouse spot tests were conducted with 2AAF
and the results were equally divided between positive and
negative. An increase in coat colour spots was detected
in two studies while in two others, no evidence of the
induction of coat colour spots was observed. Similarly,
in the melanocyte assay, one positive result and one
negative were reported. Four of five spot tests conducted
with 4AAF were negative and the data were inconclusive in
the fifth.
7.2.5 Mammalian germ cell assays
Dominant lethal mutation assays conducted in male or
female mice or male rats and tests for the induction of
UDS in rat or mouse male germ cells were uniformly nega-
tive with both compounds. In the test for morphological
abnormalities in mature spermatozoa, two assays in which
2AAF was given orally to mice or rats were also negative.
After intraperitoneal dosing, however, 2AAF induced a
significant increase in the incidence of abnormal mouse
sperm. 4AAF was negative in both species regardless of
the route of exposure. These data suggest that, although
2AAF or its metabolites appear capable of penetrating the
testes and adversely affecting sperm morphology after
intraperitoneal dosing, there is no evidence for interac-
tion of 2AAF metabolites with germ cell DNA.
7.2.6 Drosophila assays
The mutagenic effects of the two chemicals on
drosophila germ cells were studied in tests for sex-linked
recessive lethal mutations in five laboratories and for
chromosome loss in two laboratories. Data from six of
these studies indicated that neither chemical produced
genetic damage in drosophila. In the seventh laboratory,
however, 2AAF was clearly mutagenic in the sex-linked
recessive lethal assay; 4AAF produced a weak positive
result in this study.
2AAF induced eye or wing spots in somatic mutation and
recombination assays in drosophila, although the response
was weak in two of the studies. The somatic mutation data
generated from tests with 4AAF were ambiguous, producing
one weak positive result, one inconclusive result and one
negative.
7.3 Summary of the in vivo genotoxicity of the four chemicals
A comprehensive in vivo genotoxicity profile has been
generated on the four test chemicals, each of which had
previously shown evidence of mutagenic activity in in
vitro screening tests. It is relevant, in this section,
to consider the mammalian genotoxicity in relation to the
hazard assessment procedures recommended by many regulat-
ory bodies. Table 3 shows the mutagenic activity of the
chemicals in the in vivo tests common to most legislative
guidelines. Based on these results, and in the absence of
pharmacokinetic and metabolic data, BP would be considered
to present a potential mutagenic and carcinogenic hazard
and 2AAF would be regarded as a potential carcinogen.
These observations are, of course, corroborated by the
established carcinogenic activity of these two chemicals.
The abbreviated data base shown in Table 3 suggests
that neither 4AAF nor PYR present a significant genotoxic
hazard and it is useful to consider whether the compre-
hensive data generated in CSSTT/2 support this initial
prediction. The summarized consensus results (Table 2)
show that conclusive data for PYR were presented from 48
distinct in vivo tests and PYR was considered to be nega-
tive in 43 of these. The remaining five tests suggested a
weak induction of SCE in rodent bone marrow that was not
reproduced in all laboratories, a single positive host-
mediated assay, and evidence of the excretion of mutagenic
metabolites in rodent urine. None of these data seriously
question the validity of the original prediction that PYR
is not a significant genotoxic hazard.
Table 3. Activity of the four test chemicals in established
in vivo mutagenicity assays
------------------------------------------------------------------------
BP PYR 2AAF 4AAF
Somatic cell tests
Metaphase chromosome analysis P N Pa N
Micronucleus test P N P N
Mouse spot test P N Pa N
Germ cell tests
Dominant lethal assay P N N N
Heritable translocation test NT NT NT NT
Mammalian germ cell cytogenetics NT NT NT NT
------------------------------------------------------------------------
a Data not consistent between laboratories
NT = not tested
P = positive
N = negative
A similar analysis of the 4AAF data indicates that
conclusive results were obtained from 50 different types
of in vivo tests. Of these, 38 were regarded as negative
and the remaining twelve assays produced positive or weak
positive results. Like BP, 4AAF produced a positive
result in a host-mediated assay and in tests for mutagenic
activity in rodent urine. Other observations, however,
suggest that 4AAF may not be devoid of in vivo genotox-
icity. For example, 4AAF induced detectable unscheduled
DNA synthesis in rat liver hepatocytes that was reproduced
in three different laboratories. One investigator reported
the induction of micronuclei in rat bone marrow cells
after intraperitoneal dosing with 4AAF, and five of seven
studies conducted in rat or mouse bone marrow recorded
small, but significant, increases in SCE. There was also
data to suggest that 4AAF was capable of adduct formation
with liver cell DNA in rats. All the germ cell data on
4AAF were negative. The positive findings in somatic
tissues cannot be dismissed as inconsequential and they
raise serious doubts about the prediction, based on the
data shown in Table 3, that 4AAF does not present a
significant genotoxic hazard. The outcome of the 2-year
rat carcinogenicity study with 4AAF is crucial to the
assessment of the validity of this prediction and, in
fact, to the evaluation of the whole CSSTT/2 data base.
8. ASSESSMENT OF THE PERFORMANCE OF THE ASSAYS
The purpose of this section is to present an objective
assessment of the performance of the in vivo tests as it
relates to the carcinogenic activity of the test chemi-
cals. (A detailed tabulation of the qualitative results of
each assay is presented in Table 4). Data from a study of
only four chemicals do not, of course, provide sufficient
information on which to judge the overall value of a par-
ticular assay for discriminating between non-carcinogenic
and potentially carcinogenic chemicals. However, when a
data base of this magnitude is available, in which many
tests were replicated in a number of different labora-
tories, then a unique insight into the utility, reproduc-
ibility, and reliability, as well as the accuracy of the
tests, can be obtained. Thus, data from the current study,
together with the considerable experience of the investi-
gators and assessors, enable the performance of many of
the assays to be comprehensively evaluated.
Although a high proportion of test systems performed
reasonably well with these four chemicals, some assays,
not surprisingly, failed to meet the predetermined cri-
teria for acceptable performance (section 4). The rodent
host-mediated and urine mutagenicity assays are good
examples. They involve either the exposure of marker
organisms to the chemicals or its metabolites in vivo or
the detection of mutagenic excretory products in urine of
treated rodents. Both types of tests were conducted in
several laboratories and neither proved capable of ident-
ifying the two carcinogens among the four in vitro muta-
gens, i.e. the host-mediated assays were generally nega-
tive with the four test chemicals while the urine tests
were uniformly positive. These observations suggest that
neither of these two classes of tests has any practical
value in evaluating the in vivo genotoxicity of chemicals
known to be genotoxic in vitro. The results obtained from
the peritoneal macrophage transformation assay suggest a
similar conclusion. This is a form of host-mediated assay
in which the transformation of macrophages, isolated from
the peritoneum of treated rats, is investigated in in
vitro culture. The four test chemicals gave negative
results in each of three laboratories.
Table 4. IPCS CSSTT in vivo study - summary of qualitative
results from individual investigatorsa
-------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
-------------------------------------------------------------------------------------------------
1. CYTOGENETICS
1.1 Chromosomal aberrations
1.1.1 Mouse bone marrow 8 P N NT NT o
8 NT NT P ? ip
1.1.2 Mouse bone marrow 9 P N NT NT o
9 NT NT N N ip
1.1.3 Mouse bone marrow 10 P N N N o
1.1.4 Mouse bone marrow 11 P N N N o
1.1.5 Mouse bone marrow 12 P N P ? o
1.1.6 Mouse bone marrow 13 P NT N N o
1.1.7 Mouse bone marrow 14 P N ? N ip
1.1.8 Mouse ascites tumour cells 13 P NT N N o
1.1.9 Rat bone marrow 15 P N NT NT o
15 NT NT ? N ip
1.1.10 Chinese hamster bone marrow 16 P N P W o
1.1.11 Chinese hamster bone marrow 17 W N W N o
1.2 Micronuclei
1.2.1 Mouse bone marrow 19 P N P N o
1.2.2 Mouse bone marrow 20 P N P N o
1.2.3 Mouse bone marrow 21 P N P N o
1.2.4 Mouse bone marrow 22 P N N N o
1.2.5 Mouse bone marrow 23 P N P N o
1.2.6 Mouse bone marrow 24 P W P W o
1.2.7 Mouse bone marrow 25 P N NT NT o
1.2.8 Mouse bone marrow 26 P N P N o
1.2.9 Mouse bone marrow 27 P N P N o
1.2.10 Mouse bone marrow 28 P N NT NT o
28 NT NT P N ip
1.2.11 Mouse bone marrow 29 P N P N ip
1.2.12 Mouse bone marrow 29 P N NT NT o
1.2.13 Mouse bone marrow 30 P N P N o
30 P N P N ip
1.2.14 Mouse bone marrow 31 P N P N ip
1.2.15 Mouse bone marrow 32 P N P N o
1.2.16 Mouse bone marrow 33 P N P N o
1.2.17 Mouse blood - weanling 34 P N P ? o
1.2.18 Mouse blood - fetal 34 P N N N o
1.2.19 Mouse blood - maternal 34 P N N N o
1.2.20 Mouse blood - fetal 32 P N P N o
1.2.21 Rat bone marrow 35 NT NT P N o
1.2.22 Rat bone marrow 36 P N NT NT o
36 NT NT P W ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
1.3 Sister chromatid exchange
1.3.1 Mouse bone marrow 38 P N P W ip
1.3.2 Mouse bone marrow 39 P W P W o
1.3.3 Mouse bone marrow 40 P N P W o
1.3.4 Mouse bone marrow 41 P W P W ip
1.3.5 Mouse bone marrow 42 P N P N o
1.3.6 Mouse bone marrow 43 P N W N o
1.3.7 Rat bone marrow 44 P W P W o
1.3.8 Mouse lymphocytes 45 P N P N o
1.3.9 Chinese hamster bone marrow 46 P N P N ip
Chinese hamster bone marrow 46 P N P N o
1.3.10 Chinese hamster intestinal cells 46 P N P N o
2. LIVER-SPECIFIC ASSAYS
2.1 Initiation/promotion
2.1.1 Altered enzyme foci 50 NT NT N N o
2.1.2 GGT-positive foci 51 P N P N o
2.1.3 GGT-positive foci 52 P N P P o
2.1.4 GGT-positive foci - promotion 53 P N P W o
2.1.5 GGT-positive foci 54 N N P N o
2.1.6 GGT-positive foci 73 NT NT P N o
2.2 Unscheduled DNA synthesis (UDS)
2.2.1 UDS in mouse liver 56 N N N N o
2.2.2 UDS in rat liver 56 N N P N o
2.2.3 UDS in rat liver 57 NT NT P W o
2.2.4 UDS in rat liver 58 N N P W o
2.2.5 UDS in mouse liver 58 NT NT N N o
2.2.6 UDS in rat liver 59 NT NT P N o
2.2.7 UDS in rat liver 60 NT NT P W o
2.2.8 UDS in rat liver 61 NT NT P N o
2.2.9 UDS in rat liver 62 NT NT P N o
2.2.10 UDS in weanling rat liver 63 N NT NT NT o
2.2.11 UDS - neonate rat 61 NT NT W NT o
2.3 S-phase hepatocytes
2.3.1 Rat 56 W NT NT P o
2.3.2 Mouse 56 NT NT NT W o
2.3.3 Rat 58 N N P P o
2.3.4 Mouse 58 NT NT W P o
2.3.5 Rat 59 NT NT NT P o
2.3.6 Rat - weanling 63 P NT NT NT o
2.3.7 Rat 60 NT NT N P o
2.3.8 Rat 62 NT NT N P o
2.3.9 Rat 61 NT NT NT P o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
2.4 Cytogenetic analysis
2.4.1 Aberrations in liver 65 W N W N o
epitheloid cells
2.4.2 SCE in liver epitheloid cells 65 W N ? N o
2.4.3 Micronuclei in hepatocytes 66 N N P N o
2.5 DNA strand breaks
2.5.1 Alkaline elution 68 N N N N o
2.5.2 Alkaline elution 69 N N N N o
2.5.3 Alkaline elution 70 N W P P o
2.5.4 DNA-DNA/DNA-protein crosslinks 70 P N P NT ip
2.5.5 DNA unwinding 71 N N P N o
2.5.6 DNA unwinding (with araA) 71 P N P N o
3. MISCELLANEOUS ASSAYS
3.1 Carcinogenesis
3.1.1 2-year rat study 73 NT NT P * o
3.1.2 Mouse lung adenoma 74 P N P N o
3.1.3 Quail egg 75 * * * *
3.2 Supplementary assay
3.2.1 Rat fore-stomach UDS 76 ? N N N o
3.2.2 Sebaceous gland suppression 77 P N NT NT der
3.2.3 Epithelial hyperplasia 77 P N NT NT der
3.2.4 Rat hepatocyte ploidy 78 P N P N o
3.2.5 Mouse hepatocyte ploidy 78 P N P N o
3.2.6 Syrian hamster 6-TG-resistant 79 P N ? N ip
lung cells
3.2.7a 32P-post labelling - rat liver 80 P N P W ip
3.2.7b 32P-post labelling - rat lung 80 P N P W ip
3.2.7c 32P-post labelling - mouse 80 P N P NT ip
liver
3.2.7d 32P-post labelling - mouse 80 P N P NT ip
lung
3.2.7e 32P-post labelling - mouse 80 P N P NT ip
kidney
3.2.8 Radiolabelled test compounds 81 NT NT P P ip
3.2.9 ELISA rat 82 NT NT * * o
3.3 Immunotoxicity
3.3.1 T-cells - rat 83 P N P W ip
3.3.2 Natural Killer cells - rat 84 NT NT P N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
3.4 Transformation of peritoneal macrophages
3.4.1 Rat macrophages 85 N N N N o
3.4.2 Rat macrophages 85 N N N N o
3.4.3 Rat macrophages 85 N N N N o
3.5 Host-mediated assays
3.5.1a Mouse/yeast - mitotic 86 NT NT N N o
recombination - liver
3.5.1b Mouse/yeast - mitotic 86 NT NT N N o
recombination - lung
3.5.1c Mouse/yeast - mitotic 86 NT NT N N o
recombination - kidney
3.5.1d Mouse/yeast - mutation - 86 NT NT W N o
liver
3.5.1e Mouse/yeast - mutation - 86 NT NT N N o
lung
3.5.1f Mouse/yeast - mutation - 86 NT NT W N o
kidney
3.5.2a Mouse/yeast - gene conversion 87 P P P P o
liver
3.5.2b Mouse/yeast - gene conversion 87 N N N N o
lung
3.5.3 Mouse/salmonella - liver 88 N N N N o
3.6 Urine mutagenicity
3.6.1 Rat urine extract 89 P P P P ip
3.6.2 Rat urine extract 90 P P P P ip
3.6.3 Mouse urine extract 91 W W P P o
3.6.4a Rat urine 92 P P P P ip
92 P P P P o
3.6.4b Guinea-pig 92 NT NT P NT ip
3.6.5 Rat urine 93 P P P P ip
4. MOUSE SPOT TEST
4.1.1 Coat colour 95 NT NT P N ip
4.1.2 Coat colour 96 NT NT N N o
96 NT NT N NT ip
4.1.3 Coat colour 97 P N P N ip
4.1.4 Melanocytes 98 NT NT P ? ip
4.1.5 Melanocytes 99 NT NT N N ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
5. MAMMALIAN GERM CELL STUDIES
5.1 Dominant lethal
5.1.1 Female mice 102 P N N N ip
5.1.2 Male mice 103 P N N N ip
5.1.3 Male mice 104 P N N N ip
104 N ? NT NT o
5.1.4 Male mice 105 P N N N ip
105 N NT NT NT o
5.1.5 Male rat 106 NT NT N N o
5.2 Sperm abnormalities
5.2.1 Mouse 108 P N P N ip
5.2.2 Mouse 109 W N N N o
5.2.3 Mouse 110 P W P N ip
5.2.4 Mouse 111 P N N N o
5.2.5 Rat 112 NT NT N N o
5.3 Unscheduled DNA synthesis
5.3.1 Rat spermatocytes 113 N N N N o
5.3.2 Mouse sperm 114 N N N N ip
114 N NT NT NT o
6. DROSPHILA ASSAYS
6.1 Sex-linked recessive lethal
6.1.1 SLRL assay 116 N N P W o
6.1.2 SLRL assay 117 N N N N o
6.1.3 SLRL assay 118 N N N N o
6.1.4 SLRL assay 119 N N N N o
6.1.5 SLRL assay 120 N N N N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
6.2 Chromosome loss
6.2.1 Germ cell 121 N N N N o
6.2.2 Germ cell 116 N NT N NT o
6.3 Somatic mutation and recombination
6.3.1 Eye spots 116 P N W W o
6.3.2 Eye spots 122 P N P N o
6.3.3 Wing spots 118 P N W ? o
--------------------------------------------------------------------------------------------------------------
a From: Ashby et al. (1988)
b Numbers refer to chapters in Ashby et al. (1988)
P Positive
NT Not tested
N Negative
? Inconclusive
W Weak positive
* Results not available
8.1 Cytogenetic assays
Assays that utilize target cells in rodent bone marrow
for detecting chromosomal effects, i.e., chromosomal aber-
rations, micronuclei, or sister chromatid exchanges, are
widely advocated for investigating the activity of in
vitro genotoxins in the intact animal and represent the
largest group of tests in this study.
8.1.1 Chromosomal aberrations
Data from metaphase chromosome studies in mouse bone
marrow were provided by seven investigators. Each investi-
gator successfully discriminated between BP and PYR, and
five sets of data determined 4AAF to be negative while two
were inconclusive. The results of chromosome studies with
2AAF, however, were conflicting. Two laboratories identi-
fied 2AAF as a clastogen, one set of data were inconclus-
ive and four investigators reported 2AAF as negative.
There appears to be no simple explanation for these dis-
crepancies, which were not associated with differences in
mouse stain, route of treatment, sampling interval, or
other aspects of experimental design. The mouse micro-
nucleus assay (section 8.1.2) showed 2AAF to be an in vivo
clastogen (though less potent than BP) and it is clear
that the dosing schedules used in the metaphase chromosome
assays were more than adequate to induce chromosome aber-
rations, i.e., 2AAF would be expected to be detected as a
clastogen in the metaphase chromosome assay. One expla-
nation for the negative findings in four laboratories may
be the resolving power of the sample sizes used. Most
standard protocols for the bone marrow metaphase assay
require at least 50 cells per animal from 10 animals per
dose/time group (i.e., 500 cells) to be analysed for aber-
rations at each data point. This recommended minimum fig-
ure was only achieved by three of the seven investigators
and three others presented data on only 250 cells per
dose/time group. These observations suggest that, with
samples of this size, almost a 10-fold increase in
aberrations over the control incidence would be required
to demonstrate a significant induction of aberrations by
2AAF.
Two investigators conducted studies in Chinese hamster
bone marrow cells. PYR was negative in both studies. In
experiments in which 500 cells per dose/time group were
analysed, BP and 2AAF were clearly positive while, when
only 300 cells were used, the results were classified as
weak positive with both carcinogens. It is interesting to
note that 4AAF induced a weak positive response in Chinese
hamster bone marrow in one study.
One set of data from rat bone marrow was presented and
clearly separated BP from PYR. 2AAF, however, gave
unambiguous data and 4AAF was negative.
The performance of the bone marrow cytogenetic assays
did not, in general, meet the criteria for a reliable and
reproducible short-term in vivo test. There are, however,
serious reservations regarding the numbers of animals and
the numbers of cells analysed in many of the studies and
the results suggest that protocols designed to give an
acceptable resolving power should be strictly adhered to.
8.1.2 Micronuclei
Eighteen laboratories conducted micronucleus tests; 16
laboratories performed assays on bone marrow cells from
mice and two investigators used rats. Data were also
presented on the incidence of micronuclei in circulating
blood cells from adult and fetal mice.
Mouse bone marrow micronucleus assays were conducted
to a common protocol with only minor deviations. At least
5 mice were used for each dose/time interval with 1000
cells being analysed from each animal. The data from these
assays showed a high level of agreement between labora-
tories. Seventeen sets of data on BP were uniformly posi-
tive and 16 sets on PYR were negative. Similarly, 14
investigators showed 2AAF to be capable of inducing an
increase in micronuclei and 4AAF was negative in 14 cases.
There were only occasional anomalous results. For example,
one laboratory failed to detect 2AAF, and another investi-
gator recorded weak positive results from both PYR and
4AAF. These anomalies do not detract from the general
accuracy and reproducibility of mouse micronucleus tests
with the four test chemicals.
One investigator used erythrocytes from mouse periph-
eral blood to assay the incidence of micronuclei. The
results were similar to those obtained in the bone marrow
studies, i.e. BP and 2AAF were positive while PYR and 4AAF
were considered to be negative by the criteria adopted for
a positive response. In two laboratories, fetal blood was
obtained from mice on day 15 of pregnancy, 30 or 48 h
after oral dosing of the mother. BP was positive and PYR
was negative in each of these studies. The fetal assay was
less sensitive to the effects of 2AAF, one laboratory
observing a small increase in micronuclei and the other
finding no increase. 4AAF was inactive in fetal cells.
Two micronucleus studies were conducted in rat bone
marrow cells and the results suggest that the response in
the rat was weaker than that in the mouse. In one
laboratory, 2AAF was positive and 4AAF negative; BP and
PYR were not tested. The second investigator reported a
positive response with BP and a negative one with PYR.
This investigator's result with 2AAF is shown as positive
in Table 4. However, using criteria decided by the
micronucleus assay working group, it was concluded that
2AAF was negative in this assay. It was also considered
that the protocol used in the rat studies was not designed
for the optimum detection of micronuclei.
The results from the micronucleus tests confirm the
value of the assay in mouse bone marrow as an accurate and
reproducible means of qualitative discrimination between
carcinogenic and non-carcinogenic chemicals.
8.1.3 Sister chromatid exchange
Nine investigators presented data on the induction of
sister chromatid exchanges (SCE) in vivo using mice, rats,
or Chinese hamsters. The test chemicals were administered
by gavage or intraperitoneal injection and most investi-
gators analysed SCE in bone marrow cells. One set of data
were presented on SCE in mouse peripheral lymphocytes and
another set on Chinese hamster intestinal epithelial
cells. The two established carcinogens, BP and 2AAF,
induced an increase in SCE in each of the six studies
conducted in mouse bone marrow. In two of these studies,
PYR induced a weak positive response and 4AAF was weakly
positive in four of the six assays. Weak positive results
were also obtained in rat bone marrow assays with the two
presumed non-carcinogens, while BP and 2AAF were clearly
positive. In experiments conducted in blood lymphocytes
in mice and in bone marrow cells and intestinal epithelial
cells in Chinese hamsters, BP and 2AAF gave positive
results while PYR and 4AAF were negative. None of the
experiments suggested that the route of administration of
the test chemicals significantly influenced the qualitat-
ive results.
Both the established carcinogens were consistently
positive in tests for the induction of SCE in three rodent
species, suggesting that the SCE assay is a sensitive and
reproducible in vivo test for genotoxic carcinogens. PYR
or 4AAF, however, induced small (but significant) in-
creases in the incidence of SCE in 8 of 22 tests, although
the presumed non-carcinogens were much less potent
inducers of SCE than their carcinogenic analogues. These
observations raise a number of questions regarding the
significance of small increases in SCE in in vivo tests.
In the first instance, if 4AAF is confirmed to be non-
carcinogenic in the current rodent bioassay, then these
weak responses have to be regarded as `false positives'.
If, however, 4AAF is shown to have weak carcinogenic
activity, then the weak positive responses obtained in
four of the six mouse bone marrow studies can be inter-
preted as accurately reflecting the genotoxicity of 4AAF.
In the report of the SCE Working Group (Tice et al., 1988)
the biological relevance of statistically significant
increases in SCE at unrealistically high doses was con-
sidered. It was suggested that multiple mechanisms are
involved in the formation of SCE and that one of these may
be a response to stress, either at the cell or animal
level, caused by a toxic response to high doses of a
chemical. The small induction of SCE observed after high
doses of PYR or 4AAF, therefore, may not be related
mechanistically to the larger increases in SCE seen after
low doses of BP or 2AAF. Thus, the final assessment of
the performance of the in vivo SCE assays depends, to a
large extent, on the outcome of the 2-year bioassay with
the AAF analogues, i.e. a negative result with 4AAF will
cast serious doubts on the value of SCE tests for investi-
gating the in vivo activity of in vitro genotoxins.
8.2 Liver assays
The activity of certain genotoxic chemicals appears to
be confined to the liver. Such chemicals do not, of
course, induce detectable genetic changes in bone marrow
cells or other extra-hepatic tissues and this has led to a
need to develop techniques for investigating genotoxic or
related changes in liver cells. The main thrust of this
research has centred on the demonstration of unscheduled
DNA synthesis in hepatocytes but data were also presented
from a variety of other assays that used target cells in
the liver.
8.2.1 Initiation and promotion
Two of the assays used in this group were specifically
aimed at detecting initiating activity of the test chemi-
cals. The chemicals were administered after partial hepa-
tectomy, and the mice were then treated with the promotor,
phenobarbital, for several weeks before liver sections
were examined for the occurrence of pre-cancerous foci,
i.e. groups of cells having elevated levels of the marker
enzyme, gamma-glutamyl-transpeptidase (GGT). Both these
protocols have been used successfully with many test
chemicals and, in the present study, successfully separ-
ated BP and 2AAF from PYR and 4AAF. A third protocol was
used to investigate the promoting activity of the test
chemicals after initiation by pre-treatment with dimethyl-
nitrosamine. This, too, successfully separated BP from
PYR, but, although 2AAF produced an increase in GGT-
positive foci, a weak positive response was also observed
with 4AAF. The two other procedures used to study
initiation/promotion events based on altered enzyme foci
failed to detect BP in one assay and 2AAF in the second
assay and are considered to be, primarily, research
techniques and not yet suitable for routine application.
The two better-established assays showed quite clearly
that both BP and 2AAF initiated altered enzyme foci in rat
liver. The two presumed non-carcinogens were completely
negative and these tests, although they cannot be regarded
as "short-term" in the true sense, have a useful role in
investigating liver carcinogenesis.
8.2.2 Unscheduled DNA synthesis and S-phase analysis
The investigators in this group used the detection of
unscheduled DNA synthesis (UDS) as a measure of DNA repair
initiated in response to chemical-induced damage to the
DNA. In each study, UDS was detected using autoradio-
graphic techniques that also allowed simultaneous measure-
ment of S-phase cells, i.e. those cells undergoing normal
DNA synthesis as a prelude to mitosis. Seven investi-
gators examined 2AAF and 4AAF for the induction of UDS and
S-phase cells in hepatocytes of male rats, and in two
laboratories all four test chemicals were investigated in
rats and mice. The induction of UDS and S-phase cells in
rat fore-stomach was investigated in another laboratory.
Although there were minor protocol variations, the UDS
working group concluded that none of these significantly
affected the results and there was excellent agreement in
the observations and conclusions of the investigators.
2AAF was shown to be a potent inducer of UDS in rat
hepatocytes and also caused proliferation of hepatic cells
as shown by an increase in the number of cells in S-phase.
In mice, however, 2AAF failed to induce SCE in either of
two laboratories, though there was evidence of a weak
induction of S-phase cells. Only low doses were examined,
i.e. up to 50mg/kg, but the results indicate an important
species difference in the response to 2AAF.
Administration of 4AAF to rats produced negative UDS
results in four laboratories and weak positive results in
three laboratories. Although the three weak positive
results were initially determined without recourse to
statistical analysis, a comprehensive analysis of the data
by Margolin and Risko (1988) confirmed the initial
interpretation. The six investigators who recorded the
number of S-phase cells observed a marked increase in
hepatic cell proliferation in rats dosed with 4AAF. This
compound failed to induce UDS in mouse liver but did cause
an increase in S-phase cells.
For comparative purposes, two laboratories also con-
ducted UDS tests on hepatocytes in vitro. Both 2AAF and
4AAF induced UDS in rat hepatocytes but were negative in
mouse cells.
BP and PYR were tested in rats by two investigators
and one assay was conducted in mice. Both chemicals
failed to induce UDS in either species after oral dosing.
In contrast, BP gave positive results in both rat and
mouse hepatocytes treated in vitro.
In an investigation of the UDS activity of the four
chemicals in rat fore-stomach, 2AAF, 4AAF, and PYR failed
to induce UDS while BP gave equivocal results.
The performance of the UDS assay in rodents dosed with
2AAF or 4AAF is worthy of further consideration. 2AAF is
a potent rat hepatocarcinogen and a potent inducer of UDS
in rat hepatocytes. In the mouse, however, 2AAF did not
induce UDS and, on balance, available data suggest that it
does not produce liver tumours. Thus, for this chemical,
the UDS results accurately reflect its hepatocarcinogenic
potential. The current lack of cancer data on 4AAF pre-
cludes this kind of comparison, but the UDS data suggest
that if 4AAF is carcinogenic, it is markedly less so than
2AAF.
Although fewer tests were conducted with BP and PYR,
the data indicate that neither chemical induces UDS in rat
or mouse liver, although BP was positive in in vitro UDS
tests. The reason for this negative response to BP has an
important bearing on the validity of the UDS assay for
detecting potentially carcinogenic chemicals. Although BP
is an established carcinogen, it has never been shown to
produce tumours in rodent liver. There is sufficient
evidence, however, to suggest that DNA-reactive adducts
are formed in liver cells after dosing rodents with BP.
For example, most of the data that show BP to be genotoxic
in vitro are derived from tests in which metabolic acti-
vation is provided by an enzyme mixture derived from rat
liver. In addition, BP clearly induces altered enzyme
foci in rat liver, there is tentative evidence for the
induction of DNA strand breakage based on DNA-DNA/DNA-
protein crosslinks, and 32P-post-labelling techniques in-
dicate the presence of DNA adducts of BP metabolites in
both rat and mouse liver. Therefore, although BP is not a
hepatocarcinogen in rodents, there are data to suggest
that (a) BP is metabolized in rodent liver to DNA-reactive
metabolites, (b) interaction of DNA with BP metabolites
does occur, and (c) based on data from the DNA unwinding
test, some repair of the DNA lesions takes place. On this
basis, the liver UDS assay should, theoretically, be
capable of detecting BP-induced lesions in the liver. All
these observations indicate that such factors as the
distribution of BP to the liver after oral dosing, the
rate of formation of genotoxic metabolites, the rate of
detoxification, and the rate and nature of the DNA repair
process yield an insufficient number of DNA adducts to
produce a detectable UDS response.
In practice, of course, BP is easily detected in other
in vivo tests such as those conducted in bone marrow
cells. However, the results with BP and, to a lesser
extent, 4AAF, suggest the need for additional evaluation
of the performance of the in vivo UDS assay with liver-
specific genotoxins.
8.2.3 DNA strand breaks
Data were considered from three different assay sys-
tems used to detect single-strand breaks in DNA. Results
from the alkaline elution assay, in which two laboratories
found no activity with any of the four test chemicals,
indicate that this assay is of little value in investigat-
ing in vivo genotoxicity. The test for DNA-DNA/DNA-protein
crosslinks and the DNA unwinding assay in rodent liver
cells both gave very promising results and warrant further
evaluation.
8.2.4 Cytogenetics
Three sets of data were considered in this group.
Micronucleus assays were conducted in rat liver cells
following partial hepatectomy. The results were similar to
those obtained in the rat liver UDS assay, i.e. a dose-
dependant response was obtained with 2AAF, while the
remaining three chemicals gave negative results. The
other two data sets were generated from analyses of
chromosome aberrations and SCE in epithelial-like cells
derived from weanling rat liver. Although the assays are
relatively simple with clearly defined end-points, the
results were either equivocal or negative and the sample
sizes, i.e. the numbers of animals/cells per dose group,
were too small to permit an effective assessment. Both
the micronucleus test and the chromosome assays are worthy
of further evaluation.
8.3 Miscellaneous assays
This section contains a wide variety of assays, some
of which have been in use for many years while others, few
of which were undertaken in more than one laboratory, are
relatively new or in the process of development.
8.3.1 Specific carcinogenicity assays
Neither of the two procedures considered here can be
regarded as short-term tests and final results from one of
them, the assay in quail eggs, were not available at the
time of the assessment. The results of the mouse adenoma
assay, however, were available and showed a decisive dis-
crimination between BP and PYR and a much smaller and less
convincing difference between 2AAF and 4AAF.
8.3.2 Supplementary assays
Data from the sebaceous gland suppression test and the
epidermal hyperplasia assay have shown a good correlation
with skin carcinogenicity and have proved useful for com-
paring the carcinogenic potential of polycyclic hydrocar-
bons. Their value in this area was confirmed by the re-
sults of assays with BP and PYR but the application of
these assays to other classes of chemicals is of uncertain
value.
The hepatocytes of rodents include mono- and binu-
cleated cells and nuclei that may be diploid, tetraploid,
or octaploid. The ratio of cells with different levels of
ploidy in liver varies with species and age and can be
altered by exposure to certain chemicals. There have been
references to the effects of various chemicals on liver
ploidy ratios over a number of years, but there appears to
be no consistent relationship between ploidy changes and
chemical carcinogenesis. Thus the observation that the two
carcinogens, BP and 2AAF, increase the ratio of diploid to
tetraploid cells is of uncertain significance. The assay
may be of value in studying liver cell kinetics but its
value in the detection of in vivo genotoxicity is debat-
able.
The general principles of the in vivo/in vitro somatic
mutation assay were developed in the late 1970s and showed
initial promise as a test for investigating organ-specific
mutations induced by chemical carcinogens. One
investigator provided data from a modification of this
technique in which cells isolated from lung tissue of
treated Syrian hamsters were cultured in vitro and the
induction of mutations was determined. A reproducible
increase in mutations was observed in lung cells after
dosing with BP, but the results with 2AAF and the two non-
carcinogens were considered to be negative. Although this
is a logical and potentially useful approach to studying
in vivo genotoxicity, the method requires much wider
evaluation before it can be considered as an acceptable
assay.
The detection and identification of DNA adducts
provides conclusive evidence that a metabolite of the
administered chemical has interacted with one of the
important target macromolecules of carcinogenesis. Theor-
etical considerations indicate that, for many chemical
classes, the formation of DNA adducts is a prerequisite
for permanent genetic changes, such as mutations, and for
the initiation stage of carcinogenesis. Research has shown
a direct relationship between the administered dose of a
chemical and DNA-adduct formation, so that precise dosi-
metry in specific organs is possible. Three approaches
were used in the study, though results from a novel tech-
nique using monoclonal antibodies to identify specific
adducts are not yet available. One study involved the use
of radiolabelled 2AAF and 4AAF; while binding to liver DNA
was detected with both compounds, the covalent binding of
2AAF was approximately seven times that of 4AAF. These
data correlate well with the results of the UDS assay in
rat liver, which showed 2AAF to be a potent inducer of UDS
while 4AAF was, at best, a weak inducer.
The above assay has the disadvantage of requiring
radiolabelled test chemicals, and a significant advance in
the field was the introduction of techniques in which
animals are dosed with unlabelled test chemicals and the
DNA is radiolabelled after isolation and purification. The
32P-labelled normal nucleotides and nucleotide adducts
are separated by thin-layer chromatography and detected by
autoradiography, and a comprehensive, organospecific DNA
adduct profile is obtained. The data presented in the
current study showed that 2AAF adducts were detected at
significant levels in the DNA of mouse liver, lung, and
kidney and in rat liver and lung. 4AAF adducts were not
detected in mouse tissue and at only very low levels in
rat liver. PYR adducts were not detected in either rat or
mouse tissues and BP produced adducts with DNA in liver
and lung of both species and in mouse kidney.
The detection and identification of DNA adducts is
potentially an extremely powerful tool in the investi-
gation of in vivo genotoxicity. With the establishment of
`cold' techniques, i.e. those not requiring radiolabelled
test chemicals, this type of assay can be applied to most
chemical classes. Although further development is re-
quired, particularly with regard to the genotoxic signifi-
cance of different types of adduct, the method shows great
promise for the future.
8.3.3 Immunotoxicity assays
In the Natural Killer (NK) cell assay, the activity of
NK cells was measured at intervals after dosing rats with
either 2AAF or 4AAF. The carcinogen, 2AAF, induced a
significant change in NK cell cytotoxicity that was not
observed after dosing with 4AAF. The T-cell assay, in
which immunocompetent T-cells are able to react to
carcinogen-induced tissue changes, responded positively to
BP and 2AAF; PYR failed to induce any change in reactivity
and 4AAF gave a weak positive response. The results of
both types of assay closely reflected the carcinogenic
activity of the test chemicals. The relevance of the
observed immunological changes to the carcinogenic process
is unclear and, thus, their potential value in detecting
precarcinogenic changes caused by genotoxic and non-
genotoxic carcinogens remains to be fully evaluated.
Before they can be accepted as anything more than a useful
research tool, further investigations of the specificity
of the immunological reactions and a wider evaluation are
imperative.
8.3.4 Host-mediated assays and urine mutagenicity tests
As described at the beginning of Section 8, both these
classes of test failed to meet the criteria for an accept-
able short-term in vivo assay. The host-mediated assay was
a favoured in vivo procedure in the early 1970s, though in
recent years questions have been raised concerning its
sensitivity. Although the intrasanguinous modification to
the original intraperitoneal technique offered theoretical
advantages, the results of the present study confirmed the
inadequacy of the assay. It is concluded that both these
procedures are not appropriate for the investigation of in
vitro genotoxins.
8.4 Mouse spot tests
Although data were presented from two types of mouse
spot test, they are, in effect, different means of observ-
ing the same genetic alterations, i.e. the melanocyte test
detects the change at the cellular level while the coat
colour test demonstrates the expression of the cellular
changes.
BP and PYR were only tested in one laboratory and the
spot test successfully identified the carcinogen. In tests
with 4AAF, one investigator's results were considered to
be inconclusive and four other sets of data, including one
after oral dosing and three after intraperitoneal admin-
istration, were negative. Six laboratories tested 2AAF;
there were three positive results and two negatives after
intraperitoneal administration. 2AAF was also negative
after oral dosing. Examination of the protocols indicated
that 2AAF induced detectable genetic changes only after
administration of the material as a solution in dimethyl-
sulfoxide, i.e. negative results followed the admin-
istration of corn oil formulations of 2AAF, illustrating
the critical effect of the vehicle on the absorption of
the test chemical.
An overview of the mouse spot test data indicates that
intraperitoneal administration of an appropriate formu-
lation of the test chemicals produces results that reflect
their carcinogenic activity. The original coat colour
procedure appears to be more reliable than the melanocyte
technique, which may be influenced by sporadic mutational
events and inadequate melanization of the hair follicles.
However, the dependence on the intraperitoneal route of
administration may be a disadvantage, and the mouse spot
test appears to add very little to the information gained
more easily from bone marrow cell cytogenetic assays.
8.5 Assays in mammalian germ cells
For assessment purposes the germ cell assays have been
separated into two groups: those that are considered to
involve a direct interaction with DNA, and the assay for
sperm abnormalities, the genetic significance of which is
uncertain.
8.5.1 Dominant lethal and unscheduled DNA synthesis assays
Classical dominant lethal tests were conducted in male
or female mice and in male rats. PYR, 2AAF, and 4AAF gave
unequivocally negative results after intraperitoneal
dosing. In an oral dosing study in male mice, BP was nega-
tive and PYR produced inconclusive data. After intraper-
itoneal administration of BP, however, there was conclus-
ive evidence of dominant lethal induction in male and
female mice. In the male, dominant lethality was confined
to the late spermatid/spermatozoa stages of spermatogen-
esis.
Results of UDS assays in rat spermatocytes after oral
dosing and in mouse spermatocytes after oral or intraper-
itoneal administration were negative with all four chemi-
cals. The negative data with BP are not altogether sur-
prising as dominant lethality was only observed in late
spermatids/spermatozoa, in which the DNA is highly con-
densed and in which UDS does not occur.
Based on the dominant lethal test results, BP is a
confirmed, though relatively weak, germ cell mutagen in
mice after intraperitoneal dosing. Published data indicate
that BP does not induce heritable translocations at doses
that produce dominant lethality (Generoso et al., 1982),
although this may be due, in part, to the fact that BP
appears to induce mainly chromatid deletions in bone
marrow cell chromosomes, with very few exchange-type aber-
rations.
8.5.2 Sperm abnormality tests
This assay is based on the observation of morphologi-
cal alterations in mature spermatozoa after treatment of
animals at all stages of spermatogenesis with the test
chemicals. BP induced an increase in the incidence of
abnormal sperm in mice after oral or intraperitoneal
dosing and 2AAF was also positive following oral gavage.
Tests with 4AAF were uniformly negative and PYR produced a
weak positive response in one of four assays. Thus, both
carcinogens produced sperm abnormalities under appropriate
experimental conditions.
The important questions raised by these observations
relate to the relevance of morphological abnormalities in
sperm to germ cell mutations and to carcinogenesis.
Although changes in sperm head shape may be genetically
determined, it is uncertain whether they are due to geno-
toxicity or to other toxic phenomena. A positive result
in the test, therefore, indicates that the test material
or its metabolites are able to reach the germ cells in
sufficient quantities to induce an adverse biological
effect. Although a good correlation has been shown between
the induction of sperm abnormalities and carcinogenic
activity (Wyrobek et al., 1983), the relevance of this
correlation to short-term testing strategies is doubtful
as more appropriate in vivo tests are available, e.g.,
bone marrow cytogenetic assays. Equally doubtful is the
relevance of the test to heritable genetic damage, as
there is no clear-cut evidence that abnormal sperm are a
consequence of induced DNA damage.
8.6 Drosophila assays
The sex-linked recessive lethal assay and the test for
chromosome loss in drosophila germ cells failed to show a
consistent separation of BP and 2AAF from PYR and 4AAF,
respectively. Three tests based on somatic cell changes
were positive with BP and negative with PYR but the
results of tests with the acetylaminofluorenes were less
decisive. 2AAF produced an unambiguous positive result
and two weak positives, while the three results with 4AAF
were weak positive, negative and inconclusive. Thus, the
drosophila germ cell assays were insensitive to the four
in vitro genotoxins and, although the somatic cell pro-
cedures appeared to be more sensitive, it was concluded
that the main value of drosophila tests is to provide com-
prehensive analyses of the genetic mechanisms involved in
the induction of mutations by chemicals.
9. SELECTION OF THE MOST EFFECTIVE IN VIVO ASSAYS IN RELATION
TO THEIR PERFORMANCE
In a study of this nature it is inevitable that some
assays, even well-established procedures, will fail to
perform well. Indeed, the recognition of inadequate tests
is as important a goal as the identification of those that
are useful and acceptable. Other assays produced results
that correlated well with the presumed carcinogenic status
of the test chemicals but are not widely used or available
or are still in the process of development. Yet other
tests that performed adequately in the collaborative study
cannot be regarded as practicable short-term tests either
because of their technical complexity or because of the
length of time required to produce results. Most of the
latter group of tests have important research roles in
carcinogenesis.
9.1 Assays that are not considered appropriate for routine
in vivo testing of chemicals for genotoxic activity
Host-mediated and urine mutagenicity assays (section
8.3.4), the peritoneal macrophage transformation assay,
and the alkaline elution assay for single strand breaks
(section 8.2.3) proved incapable of separating the car-
cinogen/non-carcinogen pairs and appear to have little
value in investigating in vivo genotoxicity. The in
vivo/in vitro somatic mutation procedure in Syrian
hamsters (section 8.3.2) also falls into this category,
although this conclusion should not detract from the
initial promise of this approach in Chinese hamsters
(McGregor, 1988a).
Drosophila tests (section 8.6) have also proved gener-
ally inadequate in the identification of in vivo geno-
toxins and it is concluded that their main value in gen-
etic toxicology is to provide analysis of the genetic
mechanisms involved in the induction of mutations. Similar
reservations apply to the initiation/promotion techniques
using liver foci (section 8.2.1) and the analysis of
ploidy levels in liver tissue (section 8.3.2). Both assays
are more appropriate to investigation of the carcinogenic
process than to the identification of in vivo genotoxins.
The results of the immunological assays (section 8.3.3)
closely reflected the carcinogenic potential of the
chemicals, but they can only be regarded as research tools
pending a greater understanding of the specificity of the
immunological reactions and a wider evaluation of the
procedures.
Two assays conducted in mouse skin, i.e. the seb-
aceous gland suppression test and the observation of epi-
dermal hyperplasia, confirmed their value in the detection
of carcinogenic polycyclic hydrocarbons such as BP. Both
tests failed to respond to 2AAF when applied dermally and
are not considered to be suitable for investigating in
vivo genotoxicity in general.
9.2 Assays that satisfy some or all of the criteria for an
acceptable short-term in vivo test
9.2.1 Assays currently in general use
The mouse micronucleus test (section 8.1.2) was the
most widely represented single assay in the study and
produced the best overall performance with only occasional
anomalous data. Qualitative results were not affected
significantly by the route of exposure, strain or sex of
mouse, or treatment schedule. The mouse bone marrow
micronucleus assay was confirmed as a robust and sensitive
short-term in vivo test for genotoxic chemicals.
Both the micronucleus test and the analysis of meta-
phase chromosome aberrations in rodent bone marrow respond
to similar chromosome breakage events and they would be
expected to produce qualitatively similar results with the
four test compounds. Table 4 shows that this was not the
case and the metaphase chromosome procedure was signifi-
cantly less sensitive than the micronucleus test. Poss-
ible explanations for this lack of sensitivity are dis-
cussed above (section 8.1.1) and suggest that the use of
metaphase chromosome analysis should not be undervalued on
the basis of these data.
The sister chromatid exchange (SCE) procedure repro-
ducibly detected the two carcinogens, but also produced a
number of weak positive results with 4AAF and PYR. Taken
at face value, therefore, the in vivo SCE results clearly
reflect the relative potency of the two chemical pairs in
in vitro tests. As discussed in section 8.1.3, however,
very weak SCE responses may be caused by non-genotoxic
mechanisms and a final judgement on the significance of
the weak activity of 4AAF must be delayed until its true
carcinogenic status is defined.
After considering the data from the three classes of
bone marrow assay, the conclusion of the Steering Group
(Ashby et al., 1988) was - `the mouse micronucleus assay
provides the simplest and most effective measure of geno-
toxicity in the bone marrow and, as such, it is rec-
ommended here as a primary in vivo genotoxicity test'.
Despite the successful performance of the micronucleus
test with these four chemicals, it is apparent from the
literature that certain liver carcinogens do not induce
detectable chromosomal effects in the bone marrow. In an
acceptable testing strategy, therefore, negative results
in the micronucleus test should be supplemented with
evidence of non-genotoxicity in the liver. This concept
led to the extensive evaluation of liver-specific assays
in the CSSTT/2 study and the main candidate in this group
of tests was the rat liver UDS assay (section 8.2.2).
The liver carcinogen, 2AAF, was a potent inducer of
UDS in rat liver while most investigators defined its
presumed non-carcinogenic analogue, 4AAF, as a weak
genotoxin in this system. Unlike the micronucleus test
result, BP was devoid of activity in liver UDS assays.
This is consistent with its lack of carcinogenicity in rat
liver.
Although the significance of the weak liver UDS
activity of 4AAF will only be resolved when definitive
carcinogenicity data are available, the findings raise
certain problems of interpretation that are common, in
part, to the weak bone marrow activity of 4AAF and PYR.
In the UDS assays, 2AAF was a potent inducer of UDS at
doses between 5 and 100 mg/kg while 4AAF showed only weak
activity between 50 and 1000 mg/kg, and then only in some
experiments. In general, the activity of 4AAF was within
the range of control variability and, as a result, the
UDS Working Group questioned its biological significance
(Mirsalis, 1988). A detailed analysis, however, indicated
the high statistical significance of the 4AAF data
(Margolin and Risko, 1988). Thus, although the rat liver
UDS assay discriminated quantitatively between 2AAF and
4AAF, the significance of the weak activity of 4AAF
remains unresolved. These observations suggest that
qualitative determinations in terms of `positive' and
`negative' are too simplistic for hazard assessment
purposes and one of the most useful points raised by the
present study is the need for appropriate positive test
criteria, in biological and statistical terms, for widely
used in vivo tests.
Thus, the mouse bone marrow micronucleus assay is the
method of choice for primary in vivo testing of in vitro
genotoxins and the rat liver UDS assay, providing the
above problems can be resolved, appears to be the most
promising complementary liver-specific test.
9.2.2 Assays that show promise for future development
Accepting that bone marrow assays are suitable for
detecting extra-hepatic somatic cell genotoxins and that
the rat liver UDS assay can, with certain reservations
(see sections 8.2.2 and 9.2.1), be used to investigate
liver-specific genotoxins, it is useful to consider which
other assays evaluated in this study may have a role in
the assessment of in vivo genotoxicity.
Development of assays to detect single-strand breaks
in DNA and to detect and identify DNA adducts will,
undoubtedly, continue. It will be interesting to follow
the progress of the assays for DNA-DNA/DNA-protein cross-
links and DNA unwinding, although it is probable that
their technical complexity will limit widespread use and
acceptance. Of the DNA adduct techniques represented, the
post-labelling method appears to hold most promise. The
need for radiolabelled test compounds or specific mono-
clonal antibodies is eliminated and the assay provides
information on the direct interaction of test chemicals
with DNA in a variety of target organs, including the
liver. Providing a reliable and robust protocol can be
established and pending the provision of more information
regarding the relationship between DNA adduct formation
and genotoxic changes to the DNA, this assay could have an
important role to play in the assessment of genotoxic
hazard.
The performance of the cytogenetic assays in liver
cells was disappointing but, theoretically, they could,
with further development, prove to be of value in the
assessment of liver-specific genotoxicity. The reliance
of the current micronucleus procedure on partial hepa-
tectomy is a disadvantage, but the development of alterna-
tive means of stimulating hepatic mitotic activity, either
in vivo or in short-term culture, could be the basis of a
useful short-term liver-specific assay. Cytogenetic
assays on circulating blood cells performed reasonably
well and the theoretical and practical advantages of tests
for SCE or micronuclei in circulating blood should
encourage further development of these tests.
The mouse spot test, in its original form (section
6.4), successfully separated the carcinogen/non-carcinogen
pairs and, providing care is taken in the selection of an
appropriate solvent or formulation, it appears to be a
useful and reproducible assay. Its availability is limited
by the need to maintain specific mouse strains and its
overall sensitivity, compared to the micronucleus test, is
suspect, as positive results in the present study were
dependant on intraperitoneal dosing.
9.3 The detection of germ cell mutagens
The performance and significance of the three germ
cell assays represented in the study are discussed above
(section 8.5). Only BP was clearly identified as a germ
cell mutagen in the dominant lethal assay. The failure of
the sperm abnormality test to discriminate between BP and
2AAF suggests that this assay is affected by non-genetic
toxic effects and is, therefore, of doubtful value in
predicting the induction of mutations in mammalian germ
cells.
Although there are theoretical reasons why BP should
not induce UDS in germ cells (section 8.5.1), this nega-
tive result raises questions similar to those resulting
from the failure of BP to induce UDS in rat liver (section
8.2.2), i.e. why does BP not induce detectable UDS in
target cells where there is evidence, from other assays,
of genotoxic activity?
As concluded by Ashby et al. (1988) `the germ cell
activities of BP have served to focus several important,
but unresolved, issues associated with the conduct of, and
the interpretation of data derived from, germ cell
genotoxicity and mutagenicity assays'.
9.4 Influence of the route of administration of the test chemicals
A feature of the micronucleus assays was that their
qualitative separation of BP and 2AAF from PYR and 4AAF
was not influenced by the route of administration of the
chemicals. Dominant lethal mutations, however, were only
detected after intraperitoneal dosing with BP; oral dosing
was ineffective. Similarly, the mouse spot test was
dependant on the intraperitoneal route of administration
for the demonstration of mutagenic effects. The intro-
duction of the test material in the vicinity of the uterus
in the spot test circumvents the natural pharmacokinetics
and metabolic routes of the test animal, and it can be
argued that this unrealistic pattern of exposure does not
reflect true in vivo genotoxicity. It can be similarly
argued that the purpose of in vivo tests is to achieve
maximum exposure of the target cells and that intraper-
itoneal dosing can be justified for this reason. It may
be, as suggested by Shelby (1986), that intraperitoneal
dosing should be reserved for assays used in a screening
mode, i.e. for the detection of genotoxic activity, and
that for investigating the in vivo activity of established
genotoxins, a route of administration should be chosen
that reflects possible human exposure. Arguments such as
these must be resolved before strategies for testing
chemicals for genotoxic activity can be fully harmonized.
10. CONCLUSIONS
Of the fifty or so separate in vivo techniques rep-
resented in the CSSTT/2 study, only a small number satis-
fied the criteria defined for an acceptable short-term in
vivo test (section 3.2). These criteria, however, defined
a very specific purpose, i.e. the identification of in
vivo activity of established in vitro genotoxins. In order
to generate a comprehensive data base on the four test
chemicals, assays included in the study were not limited
to, for example, those that were considered most likely to
meet the criteria. As a result, some investigators sub-
mitted data from assays that were not designed for detec-
ting in vivo genotoxins but, nevertheless, provided valu-
able information on a broad spectrum of biological effects
of the test chemicals. The failure of such tests to
satisfy the narrow selection criteria used in CSSTT/2
should not be regarded as a reflection on the validity of
the procedures for specific research purposes. With these
considerations in mind, and based on the concept (section
3.2) that short-term assays are required to investigate
both liver-specific and extra-hepatic genotoxicity, the
following conclusions appear appropriate.
1. Most of the in vivo somatic cell assays for genotox-
icity were able to discriminate between the two carcino-
gen/non-carcinogen pairs, although, in some instances,
weak activity was observed in tests with the non-
carcinogens, particularly with 4AAF.
2. The weak genotoxicity of 4AAF in some studies
suggested that qualitative determinations in terms of
`positive' and `negative' are too simplistic for hazard
assessment purposes and highlights the need for appropri-
ate positive test criteria, in biological and statistical
terms, for widely used in vivo tests.
3. The insensitivity of some assays to one or other of
the two pairs of compounds supports the concept that nega-
tive in vivo data should be produced from at least two
assays in different tissues, i.e. one in extra-hepatic
somatic tissues and one in the liver, if they are to be
used for assessing genotoxic hazard.
4. The mouse bone marrow micronucleus test was confirmed
as a robust and sensitive assay and is the assay of choice
for primary in vivo testing of in vitro genotoxins.
5. The overall performance of the rat liver UDS assay
suggests that it could be complementary to the micro-
nucleus test providing the reservations raised above
(sections 8.2.2 and 9.2.1) can be satisfied. Development
and evaluation of alternative procedures for investigating
liver genotoxicity should be actively pursued.
6. Certain widely advocated assays, including the host-
mediated and urine mutation assays and tests using
drosophila are concluded to be inappropriate for hazard-
assessment purposes.
7. BP and 2AAF showed genotoxic activity in the organs in
which they also produce tumours. Their genotoxic activity
in other tissues, however, precludes the use of data from
short-term tests for making predictions of organ-specific
carcinogenicity.
8. The majority of the positive responses observed in
this study were obtained after oral administration of the
test chemicals and at doses substantially below lethal
levels, suggesting that short-term in vivo assays are not
intrinsically insensitive, as is sometimes inferred. Posi-
tive results were obtained in the dominant lethal assay
and the mouse spot test after intraperitoneal dosing only;
both assays produced negative results after oral admin-
istration. These facts suggest that the intraperitoneal
route should be reserved for the detection of genotoxic
activity and that, for hazard-assessment purposes, a route
of administration should be chosen that is relevant to the
perceived route(s) of human exposure.
9. The germ cell mutagenicity exhibited by BP was
adequately predicted by the somatic cell mutagenicity
assays, adding weight to the suggestion that tests on
mammalian germ cells should only be conducted to gain
further information on the mutagenic spectrum of estab-
lished in vivo somatic cell mutagens.
10. The occurrence of chemical carcinogens that fail to
show evidence of genotoxicity in in vitro and in vivo
assays indicates the existence of a class of chemicals
that induce cancer by a mechanism that is not a conse-
quence of a direct interaction with DNA. The acceptance
of the validity of the concept of non-genotoxic mechanisms
of cancer induction is considered to be a crucial question
in the future deployment of short-term tests in carcino-
genesis.
11. The overall conclusion from the CSSTT/2 study is that
short-term in vivo tests have a vital role to play in
hazard assessment. This role is to define which
chemicals, identified as genotoxic from in vitro tests,
are active in vivo and, thus, are those most likely to
present a carcinogenic/mutagenic hazard to mammals,
including humans.
REFERENCES
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr,
MARGOLIN, B.H., MATTER, B., & SHELBY, M.D., ed. (1985) Evaluation of
short-term tests for carcinogens, Amsterdam, Oxford, New York, Elsevier
Science Publishers (Progress in Mutation Research, Vol. 5).
ASHBY, J., DE SERRES, F.J., SHELBY, M.D., MARGOLIN, B.H.,
ISHIDATE, M., Jr, & BECKING, G.C., ed. (1988a) Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 1.
ASHBY, J., SHELBY, M.D., & DE SERRES, F.J. (1988b) Overview of the IPCS
collaborative study on in vivo assay systems and conclusions. In: Ashby,
J., de Serres, F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, &
Becking, G.C., ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative study on
in vivo assays, Cambridge, United Kingdom, Cambridge University Press, Vol.
1, pp. 6-28.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-term tests for
carcinogens: Report of the international collaborative study, Amsterdam,
Oxford, New York, Elsevier Science Publishers (Progress in Mutation
Research, Vol. 1).
GENEROSO, W.M., CAIN, K.T., HELLWIG, C.S. & CACHEIRO, N.L.A. (1982)
Lack of association between induction of dominant-lethal mutations and
induction of heritable translocations with benzo (a) pyrene in postmeiotic
germ cells of male mice. Mutat. Res., 94: 155-163.
HICKS, M.R., ASHBY, J., & PENMAN, M. (1988) Review of the rodent
carcinogenicity data for the four test chemicals. In: Ashby, J., de Serres,
F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C.,
ed. Evaluation of short-term tests for carcinogens: Report of the
International Programme on Chemical Safety's collaborative study on in vivo
assays, Cambridge, United Kingdom, Cambridge University Press, Vol. 2, pp.
351-365.
MCGREGOR, D. (1988a) Summary report on the performance of the
miscellaneous group of in vivo assays (carcinogenicity, DNA adducts,
immunological, host-mediated mutation, urinary mutation assays, and limited
metabolic assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 2, pp. 3-21.
MCGREGOR, D. (1988b) The activities of 2AAF, 4AAF, BP and PYR in in
vitro assays for genetic toxicity. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 2, pp. 345-350.
MARGOLIN, B.H. & RISKO, K.J. (1988) The statistical analysis of in
vivo genotoxicity data: case studies of the rat hepatocyte UDS and mouse
bone marrow micronucleus assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 29-42.
MIRSALIS, J.C. (1988) Summary report on the performance of the in vivo
DNA repair assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests
for carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 1, pp. 345-351.
PATON, D. & ASHBY, J. (1988) Source and analytical details of the test
chemicals. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin, B.H.,
Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests for
carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 2, pp. 341-344.
SHELBY, M.D. (1986) A case for the continued use of the intraperitoneal
route of exposure. Mutat. Res., 70: 169-171.
TICE, R.R. (1988) Summary report on the performance of the in vivo
sister chromatid exchange assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 233-253.
WHO (1985) Environmental Health Criteria 47: Summary report on the
evaluation of short-term tests for carcinogens (collaborative study on in
vitro tests), Geneva, World Health Organization, 77 pp.
WYROBEK, A.J., GORDON, L.A., BURKHART, J.G., FRANCIS, M.W.,
KAPP, R.W., LETZ, G., MALLING, H.V., TOPHAM, J.C., & WHORTON, M.D.
(1983) An evaluation of the mouse sperm morphology test and other tests in
non-human mammals: A report of the United States Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 115: 1-7.
RESUME
ETUDE COLLECTIVE DU PROGRAMME INTERNATIONAL SUR LA SECURITE DES
SUBSTANCES CHIMIQUES (IPCS) POUR L'EVALUATION ET LA VALIDATION
DES EPREUVES DE COURTE DUREE RELATIVES AUX CANCEROGENES
La première partie de ce projet, qui traite des études
in vitro, a été publiée en 1985 (Ashby et al., 1985); on
en trouvera un résumé dans le No 47 de la série Critères
d'hygiène de l'environnement (OMS 1985). La seconde
partie, qui fait l'objet du présent rapport, a été publieé
en 1988 (Ashby et al., 1988).
Devant la nécessité d'évaluer l'intérêt des épreuves
de courte durée pour la recherche des substances mutagènes
et cancérogènes, il devenait indispensable d'organiser des
études collectives inter-laboratoires à l'échelle inter-
nationale. Ces épreuves de courte durée devaient compléter
les épreuves classiques à long terme sur rongeurs ou s'y
substituer. C'est le problème du choix ainsi que de la
fiabilité et de la sensibilité de ces épreuves qui a
conduit à la première grande entreprise de collaboration
au niveau international, à savoir le Programme coopératif
international pour l'évaluation des épreuves de courte
durée relatives aux cancérogènes (IPESTTC) (de Serres &
Ashby, 1981). Les résultats de cette étude ont confirmé
la fiabilité et la faisabilité de l'épreuve de mutation
des salmonelles pour une première identification des sub-
stances cancérogènes et mutagènes. On a également con-
staté qu'avec cette épreuve, certains produits notoirement
cancérogènes chez les rongeurs étaient impossibles ou tout
du moins très difficiles à mettre en évidence. Un certain
nombre d'autres épreuves examinées dans le cadre de
l'étude IPESTTC se sont révélées capables de mettre en
évidence quelques-uns des cancérogènes murins pour
lesquels l'épreuve sur salmonelle donnait un résultat
négatif. Toutefois, la base de données était trop res-
treinte pour qu'on puisse recommander une épreuve capable
de compléter l'épreuve de mutation des salmonelles.
Au vu des résultats de l'étude IPESTTC, il est apparu
nécessaire de poursuivre ce type de coopération afin
d'établir a) quel est l'ensemble d'épreuves in vitro le
plus efficace pour un premier tri des substances chimiques
sur la base de leur activité génotoxique et b) quelles
sont les épreuves in vivo de courte durée les plus utiles
pour confirmer la génotoxicité et le pouvoir cancérogène
des substances chimiques chez les mammifères. L'étude
collective pour l'évaluation et la validation des épreuves
de génotoxicité et de cancérogénicité de courte durée
(CSSTT), a été proposée par le Programme international sur
la sécurité des substances chimiques (IPCS) et le National
Institute of Environmental Health Sciences (NIEHS) des
Etats-Unis d'Amérique, l'une des institutions qui
participent au Programme IPCS. Devant l'ampleur de cette
entreprise et du fait des problèmes logistiques que posent
l'organisation et la gestion de ce genre d'études inter-
nationales, le projet a été divisé en deux études
distinctes: l'Etude collective sur les épreuves de géno-
toxicité et de cancérogénicité de courte durée in vitro
(CSSTT/1) et l'Etude collective sur les épreuves de
mutagénicité et de cancérogénicité de courte durée in vivo
(CSSTT/2).
L'Etude CSSTT/1 a permis de constituer une vaste base
de données à partir d'épreuves in vitro très diverses
portant sur dix produits chimiques organiques. Parmi eux
figuraient huit substances de cancérogénicité reconnue
pour les rongeurs qui s'étaient révélées soit négatives à
l'épreuve sur salmonelle soit difficiles à reconnaître de
cette manière, ainsi que deux substances considérées comme
non cancérogènes. On a procédé à l'évaluation de données
tirées de 90 groupes d'épreuves effectuées par quelque
60 scientifiques. Quatre types d'épreuves ont donné des
résultats suffisamment bons pour qu'on puisse envisager de
les utiliser en complément à l'épreuve sur salmonelle. Il
s'agissait de la recherche des aberrations chromosomiques,
des mutations géniques et des transformations néoplasiques
en culture de cellules mammaliennes ainsi que de l'épreuve
d'aneuploïdie des levures. Sauf dans le cas de la
recherche des aberrations chromosomiques, on a constaté
que les protocoles généralement utilisés pour ces épreuves
devaient être étudiés plus à fond avant d'être considérés
comme tout à fait acceptables.
La principale conclusion de l'étude CSSTT/1 relative
aux épreuves in vitro, c'est que en combinant l'épreuve
de recherche des aberrations chromosomiques à l'épreuve de
mutation des salmonelles, on peut procéder à un premier
criblage efficace des substances cancérogènes.
Lors de l'étude IPESTTC, sept substances sur 14 sup-
posées non cancérogènes ont donné des résultats positifs
dans un grand nombre d'épreuves in vitro. D'après les
données in vivo limitées qu'a fournies cette étude, il
semblerait que ces sept produits soient inactifs dans les
épreuves de courte durée in vivo. Les produits non can-
cérogènes de deux des couples cancérogène/non-cancérogène
soumis à l'étude IPESTTC, à savoir le couple benzo [a] py-
rène/pyrène (BP/PYR) et le couple acétyl-2 aminofluorène/
acétyl-4 aminofluorène (2AAF/4AAF), constituent de bons
exemples de ces réactions différentes et d'ailleurs, ces
couples de produits avaient été retenus pour la partie in
vivo de l'étude collective (CSSTT/2). Toutefois, il y
avait doute quant à la non cancérogénicité supposée du
4AAF et une part très importante de l'étude a été con-
sacrée à la mise en route d'études de cancérogéniticé à
long terme sur le 2AAF et le 4AAF chez le rat.
L'objectif de l'étude CSSTT/2 était donc de produire
un profil de données complet à partir d'une large gamme
d'épreuve in vivo de courte durée afin de voir comment les
différents paramètres génétiques des tissus-cibles se com-
portent vis-à-vis de produits chimiques classés comme
génotoxiques d'après les épreuves in vitro. La finalité
de l'étude était de recenser les épreuves in vivo suscep-
tibles d'être utilisées pour déterminer l'activité in vivo
de substances notoirement génotoxiques.
Quatre-vingt-dix-sept chercheurs de 16 pays ont par-
ticipé à l'étude in vivo, les données présentées portant
sur une cinquantaine de techniques distinctes. Les résul-
tats ont été évalués lors d'une réunion de ces chercheurs
qui s'est tenue au Cap d'Agde (France) en mai 1985. On a
préparé une série de rapports comportant une évaluation de
chaque groupe d'épreuves, des rapports récapitulatifs sur
les épreuves relatives aux cellules germinales et les
épreuves d'hépatotoxicité et des rapports résumant toute
la base de données relative à chaque couple de produits.
Par la suite, on a préparé un récapitulatif de toute
l'étude in vivo en vue de sa publication définitive.
Seule une faible proportion des épreuves examinées
lors de l'étude CSSTT/2 ont satisfait aux critères
d'acceptabilité comme épreuves in vivo de courte durée.
Toutefois les épreuves examinées lors de cette étude ne se
limitaient pas à celles jugées a priori les plus conformes
aux critères. De la sorte, on a pu tirer des données
provenant d'épreuves qui, à l'origine, n'étaient pas des
épreuves de génotoxicité in vivo, des renseignements sur
une large gamme d'effets biologiques produits par ces
quatre substances. La plupart des épreuves de génotoxicité
in vivo portant sur des cellules somatiques permettaient
de distinguer les deux couples cancérogène/non-cancérogène
encore que dans certains cas, les produits non cancéro-
gènes, en particulier le 4AAF aient présenté une faible
activité. L'insensibilité de certaines épreuves à l'un ou
à l'autre des deux couples de produits chimiques, corrob-
orent l'idée selon laquelle il faut obtenir des résultats
négatifs in vivo dans au moins deux épreuves pratiquées
sur des tissus différents avant de considérer qu'un
produit chimique n'est pas génotoxique in vivo.
Il a été confirmé que l'épreuve des micro-noyaux sur
moëlle osseuse de souris est robuste, sensible et repro-
ductible et on la recommande pour une première recherche
de la génotoxicité in vivo et in vitro. Les résultats
généraux obtenus au moyen de l'épreuve sur foie de rat
pour la synthèse anarchique de l'ADN incitent à penser
qu'elle pourrait compléter l'épreuve des micro-noyaux,
mais il faut en étudier encore la sensibilité et la
sélectivité. Certaines épreuves pourtant largement pré-
conisées, notamment les épreuves par passage sur hôte, les
épreuves de mutagénicité des urines et les épreuves sur
drosophile, se sont révelées impropres à une évaluation du
risque.
Les résultats de l'étude CSSTT/2 ont confirmé que les
épreuves in vivo de courte durée ont un rôle capital à
jouer dans l'évaluation du risque, rôle qui consiste dans
l'identification des produits chimiques qui s'étant
révélés génotoxiques in vitro, sont également actifs in
vivo et comportent donc vraisemblablement un risque de
cancérogénicité ou de mutagénicité pour les mammifères en
général et l'homme en particulier.
RESUMEN
PROGRAMA INTERNACIONAL DE SEGURIDAD DE LAS SUSTANCIAS QUIMICAS (IPCS):
ESTUDIO EN COLABORACION SOBRE EVALUACION Y COMPROBACION DE PRUEBAS
A CORTO PLAZO PARA SUSTANCIAS CARCINOGENAS
La primera parte del presente proyecto, relativa a
estudios in vitro, se publicó en 1985 (Ashby et al., 1985) y se
resumió en la publicación 47 de la serie "Environmental
Health Criteria" (OMS, 1985). La segunda parte es el
tema del presente informe y se publicó en 1988 (Ashby et
al., 1988).
La necesidad de estudios en colaboración entre
laboratorios en escala internacional surgió de que era
imprescindible investigar el valor de las pruebas a corto
plazo para detectar las sustancias químicas mutágenas y
carcinógenas. Se propusieron los ensayos a corto plazo
como métodos que sustituyeran o complementaran a los
tradicionales ensayos biológicos a largo plazo en
roedores. La preocupación por la elección de las pruebas
a corto plazo y por su fiabilidad y sensibilidad condujo a
que se realizara el primer ejercicio importante de
colaboración internacional: el programa internacional en
colaboración sobre evaluación de las pruebas a corto plazo
para sustancias carcinógenas (IPESTTC) (de Serres y Ashby,
1981). Los resultados de ese estudio confirmaron el valor
de la prueba de mutación de salmonelas como ensayo fiable
y factible para la identificación primaria de sustancias
carcinógenas y mutágenas. Se observó también que en la
prueba de salmonelas no se detectaban o sólo se detectaban
con grandes dificultades algunas sustancias de cancerogen-
icidad conocida en los roedores. Otros ensayos incluidos
en el estudio IPESTTC pudieron detectar sustancias
carcinógenas para roedores que eran negativas en el ensayo
de salmonelas. Sin embargo, la base de datos de apoyo era
demasiado pequeña para permitir la recomendación de un
ensayo que complementara la prueba de mutación de
salmonelas.
Los resultados del estudio IPESTTC pusieron de
manifiesto que se necesitaría un nuevo ejercicio en
colaboración para determinar: a) la combinación más
eficaz de ensayos in vitro para la detección primaria de
la actividad genotóxica de sustancias químicas, y b) las
pruebas in vivo a corto plazo más útiles para confirmar la
posibilidad de genotoxicidad y cancerogenicidad en mamí-
feros. El Programa Internacional sobre Seguridad de las
Sustancias Químicas (IPCS) y el Instituto Nacional de
Ciencias de Higiene del Medio (NIHS) de los Estados Unidos
de América, como institución participante en el IPCS,
propusieron el estudio en colaboración sobre evaluación y
comprobación de las pruebas a corto plazo para la geno-
toxicidad y la cancerogenicidad (CSSTT). Dadas la magnitud
prevista del proyecto y la logística de la organización y
administración de los proyectos internacionales, el
proyecto se dividió en dos estudios independientes: el
estudio en colaboración sobre pruebas a corto plazo para
la genotoxicidad y la cancerogenicidad (CSSTT/1) y el
estudio en colaboración sobre pruebas in vivo a corto
plazo para sustancias mutágenas y carcinógenas (CSSTT/2).
En el estudio CSSTT/1 se reunió una abarcante base de
datos procedentes de una amplia gama de ensayos in vitro
realizados con diez productos químicos orgánicos cuidad-
osamente seleccionados. Incluían ocho sustancias de
cancerogenicidad probada para roedores, que resultaban
negativas o difíciles de detectar en el ensayo de
salmonelas y dos sustancias químicas consideradas como no
carcinógenas. Se evaluaron los datos procedentes de casi
90 series de ensayos efectuados por unos 60 científicos
participantes. El rendimiento de cuatro tipos de ensayos
se estimó suficiente para considerarlos como posibles
pruebas complementarias del ensayo de salmonelas.
Incluyeron las pruebas para la determinación de aberra-
ciones cromosómicas, mutaciones génicas y transformaciones
neoplásicas en células de mamífero en cultivo, y un ensayo
para determinar la aneuploidia en levaduras. Con la
excepción del ensayo de aberraciones cromosómicas, resultó
evidente que los protocolos utilizados en general para
esas pruebas exigían evaluación adicional antes de poder
considerarlos plenamente aceptables.
La principal conclusión del estudio CSSTT/1 sobre las
pruebas in vitro fue que el empleo de los ensayos de
aberraciones cromosómicas en asociación con la prueba de
mutación de salmonelas podía resultar un detector primario
eficaz para posibles sustancias carcinógenas nuevas.
En el estudio IPESTTC, siete de las catorce sustancias
no carcinógenas presuntas dieron resultados positivos en
muchas de las pruebas in vitro. Los limitados datos de
pruebas in vivo disponibles procedentes de ese estudio
permiten pensar que esas siete sustancias químicas eran
inactivas en las pruebas a corto plazo in vivo. En dos
pares de sustancias carcinógenas/no carcinógenas de
IPESTTC (benzo [a] pireno/pireno (BP/PIR) y 2-acetilamino-
fluoreno/4-acetilamino-fluoreno (2AAF/4AAF), las sustan-
cias no carcinógenas proporcionaron buenos ejemplos de
tales respuestas diferentes, de modo que se seleccion-aron
esos pares de sustancias químicas para la parte in vivo
del estudio en colaboración (CSSTT/2). Sin embargo, hubo
una fuerte duda respecto a la presunta falta de cancero-
genicidad del 4AAF, y una parte crucial del estudio fue el
comienzo de bioensayos de cancerogenicidad a largo plazo
del 2AAF y el 4AAF en ratas.
Por consiguiente, el objetivo del estudio CSSTT/2 fue
producir un abarcante perfil de datos procedentes de una
amplia gama de pruebas in vivo a corto plazo, como medio
de conocer el mecanismo por el que los distintos puntos
finales genéticos de los tejidos destinatarios fundamen-
tales responden a sustancias químicas definidas como geno-
tóxicas in vitro. La meta final consistía en identificar
qué ensayos in vivo podrían usarse para determinar la
actividad in vivo de los productos genotóxicos probados.
Participaron en el proyecto de pruebas in vivo 97
investigadores de 16 países y se presentaron datos de unas
cincuenta técnicas in vivo distintas. Los resultados se
evaluaron en una reunión de investigadores celebrada en
Cap d'Agde (Francia) en mayo de 1985. Se preparó una
serie de informes que comprendían una evaluación de cada
grupo de ensayos, informes resumidos sobre los ensayos en
células germinales y las pruebas hepáticas específicas, e
informes resumidos sobre la base total de datos corres-
pondiente a cada par de sustancias químicas. Después se
preparó un examen general de todo el estudio in vivo listo
para la publicación final.
Sólo una pequeña proporción de los ensayos represent-
ados en el estudio CSSTT/2 satisficieron los criterios que
definen una prueba in vivo a corto plazo aceptable. Sin
embargo, los ensayos incluidos en el estudio no se
limitaron a los que podían cumplir con más probabilidad
los criterios. Así, aunque los datos procedían de ensayos
que no estaban destinados fundamentalmente a identificar
la genotoxicidad in vivo, proporcionaron información
sobre un amplio espectro de efectos biológicos de las
cuatro sustancias químicas. La mayoría de los ensayos en
células sométicas in vivo para determinar la genotoxicidad
discriminaron entre las sustancias carcinógenas/no carcin-
ógenas de los dos pares, aunque en algunos casos se
detectó una actividad débil en las pruebas con sustancias
no carcinógenas, en particular con el 4AAF. La insensib-
ilidad de algunos ensayos a uno u otro de los dos pares de
productos químicos apoya el concepto de que deben obtener-
se datos in vivo negativos en dos ensayos por lo menos en
diferentes tejidos antes de que pueda aceptarse que una
sustancia química carece de genotoxicidad in vivo.
Se confirmó que la prueba de micronúcleos de médula
ósea de ratón es un ensayo potente, sensible y repro-
ducible, que se recomienda para las pruebas in vivo
primarias de las sustancias genotóxicas in vitro. El
rendimiento general del ensayo en células hepáticas de
rata para la síntesis no programada de ADN permite pensar
que puede ser complementario de la prueba de micronúcleos,
aunque requieren investigación adicional ciertos aspectos
de sensibilidad y selectividad de ese ensayo. Se llegó a
la conclusión de que ciertos ensayos ampliamente susten-
tados, que incluyen los de mutagenicidad por orina y
mediada por el huésped y las pruebas que utilizan la mosca
drosófila, son inapropiados para la evaluación de
riesgos.
Los resultados del estudio CSSTT/2 confirmaron que las
pruebas in vivo a corto plazo tienen que desempeñar una
función crucial en la evaluación de los riesgos y que esa
función consiste en identificar los productos químicos
que, una vez demostrada la genotoxicidad in vitro, son
activos in vivo y tienen así mayores probabilidades de
presentar un riesgo de cancerogenicidad o mutagenicidad
para los mamíferos, incluido el hombre.
 These observations were the origin of the present
study, the object of which was to generate a comprehensive
data profile from a broad range of short-term in vivo
assays as a means of understanding how various genetic
end-points in key target tissues respond to chemicals
defined as genotoxic in vitro. The objectives and design
of the collaborative study on short-term in vivo tests
(CSSTT/2) were outlined by an ad hoc Working Groupa at a
meeting organized by the IPCS in Geneva on 30 April 1981,
and the plans were consolidated by an IPCS Working
Groupb in Geneva, on 13-14 November 1981. The sub-
sequent coordination of the collaborative study was the
responsibility of a Steering Committeec derived primar-
ily from the Working Group.
The four test chemicals selected for the in vivo pro-
ject were the two carcinogen/non-carcinogen pairs, i.e.
BP/PYR and 2AAF/4AAF, that provided the initial impetus to
the study, and the first samples were distributed to
investigators in March 1983. During 1983, additional par-
ticipants joined the study to provide data on new in vivo
assays and nine coordinatorsd were appointed to oversee
the work being conducted by investigators performing
identical or similar assays. In all, 97 investigators from
16 countries participated in CSSTT/2. Progress was
reviewed at a meeting of the Steering Group with the
coordinators held in Brussels on 12-13 January 1984. At
this meeting a plan was developed to enter the data from
the study into a computer at NIEHS. It was envisaged that
such a plan would allow a comprehensive statistical
analysis using common statistical techniques for each kind
of assay and provide a comparison of results from
different laboratories performing the same assay.
Initially, it was expected that all the studies would be
completed by October 1984, but by September 1984 it was
evident that additional time would be required for many of
the investigators to complete their evaluation of the four
chemicals. At a second meeting of the Steering Group and
coordinators, held on 14-17 November 1984, status reports
prepared by the coordinators were reviewed in detail and
deadlines were set for the provision of final reports from
each investigator. Two additional coordinators were ap-
pointed at this meeting to oversee the rodent dominant
lethal assay (Dr W. Generoso) and the mouse coat colour
spot test (Dr R. Fahrig). Following this progress review,
a meet-ing of investigators was planned for May 1985, for
the presentation, collation, and assessment of the results
and the preparation of final reports on the study.
--------------------------------------------------------------------------------
a Participants: Dr J. Ashby, Professor N.P. Bochkov, Dr B.E. Matter,
Professor T. Matsushima, Dr F.J. de Serres, Dr M. Shelby, and Professor
F.H. Sobels.
b Participants: Dr J. Ashby, Dr G.R. Douglas, Dr M. Ishidate, Jr., Dr A.
Leonard, Dr N. Loprieno, Dr B.E. Matter, Professor T. Matsushima, Dr R.
Montesano, Dr F.J. de Serres, Dr M. Shelby, Professor F.H. Sobels, Dr M.
Stoltz, and Dr M. Waters.
c Steering Committee: Dr F.J. de Serres (Chairman), Dr J. Ashby, Dr. M.
Ishidate, Jr., Dr B. Margolin, Dr M. Shelby, Dr M. Draper, and Dr G.C.
Becking.
d Coordinators: Dr W. Vogel, Dr W.M. Generoso, Dr I.-D. Adler, Dr D. Wild,
Dr. T. Tice, Dr B.E. Butterworth, Dr D. McGregor, Dr M. Salamone, and
Dr R. Fahrig.
The meeting of investigators was held during 15-21 May
l985 at Cap d'Agde, France. Representatives from each par-
ticipating laboratory met to prepare a series of reports
comprising a) an assessment of each group of assays, b)
summary reports on the germ cell assays and the liver-
specific assays, and c) summary reports on the total data
base on each pair of chemicals, i.e. BP/PYR and 2AAF/
4AAF. During the meeting, draft reports were prepared and
were to be finalized during the following two months. At
a meeting of the Editorsa held at NIEHS from 24 July to 6
August 1985, the work group and investigators' reports
were reviewed, and a time-table was developed for the com-
pletion of all reports and for the preparation of the
Introduction and Overview of the study in readiness for
final publication of the in vivo study.
A feature of both the CSSTT studies and the earlier
IPESTTC project was the voluntary participation of a large
number of scientists together with support from their
parent institutions. The organization of CSSTT/1 and
CSSTT/2 was financed largely by IPCS and some of its par-
ticipating institutions. In most cases, funding of the
experimental work was by individual investigators, many of
whom incorporated the studies into their research pro-
grammes. This, of course, required the goodwill and
support of senior management of the participating labora-
tories from universities, research institutions, and in-
dustrial research facilities throughout the world. Ad-
ditional financial assistance was provided by a number of
governments that support IPCS, including Belgium, Italy,
Japan, The Netherlands, the United Kingdom, and the USA.
The United Kingdom Department of Health and Social Secur-
ity funded the rat carcinogenicity studies with 2AAF and
4AAF. The Belgian government and its National Institute
of Hygiene and Epidemiology financed meetings of the
Steering Committee and coordinators in Brussels. The
meeting of investigators held in Cap d'Agde was organized
by the French government and cosponsored by the French
Ministry of Health and the Commission of European
Communities.
--------------------------------------------------------------------------------
a Editors: Dr J. Ashby, Dr F.J. de Serres, Dr M.D. Shelby, Dr B.H.
Margolin, Dr M. Ishidate, Jr., and Dr G.C. Becking.
3. OVERALL AIMS OF THE STUDY AND CRITERIA FOR THE SELECTION OF
APPROPRIATE SHORT-TERM IN VIVO TESTS
Because of their relative simplicity, reproducibility,
and reliability, short-term in vitro tests are the methods
of choice for the initial testing of chemicals for geno-
toxic activity. The role and usefulness of genotoxicity
assays using whole animals, i.e. short-term in vivo
tests, are less clearly defined despite the fact that
they are widely used and are an integral part of most
legislative guidelines for the conduct of mutagenicity
tests. In general, in vivo tests are more resource-
consuming than their in vitro counterparts and the use of
animals for experiments for which there is an acceptable
in vitro alternative is to be discouraged. As these
observations suggest, in vivo assays should be designed to
answer questions that cannot be investigated adequately
with in vitro tests.
3.1 The use of short-term tests for the primary identification
of genotoxic chemicals
Certain short-term in vivo assays, such as the rodent
bone marrow chromosome assay, the rodent dominant lethal
test, and the host-mediated assay, were used in the early
1970s in primary screens for the identification of muta-
genic chemicals. With the introduction of reliable and
valid short-term in vitro tests for mutagens in the mid-
1970s, the use of in vivo procedures for initial screen-
ing of chemicals declined considerably. Certain legislat-
ive authorities still recommend a combination of in vitro
tests reserved for confirmatory or supplementary use or
for providing data for hazard assessment. These different
roles for whole animal procedures have led to the accept-
ance of test protocols of varying complexity, i.e. less
rigorous protocols for screening modes and more comprehen-
sive protocols when the test is required to assess hazard
potential.
Implicit in the concept of the two IPCS studies is the
assumption that chemicals shown to be genotoxic in vivo
also exhibit genotoxic activity in a properly designed
and conducted in vitro primary screen. This principle is
well-established in the scientific literature and was con-
firmed in the IPESTTC and CSSTT/1 studies. Thus, it was
an integral principle in the study design that the primary
screening of chemicals could be adequately served by in
vitro tests alone and that short-term in vivo assays have
no role to play when screening for genotoxic activity. In
vivo procedures will, therefore, be reserved for more
specific applications such as investigating the activity
of in vitro genotoxins in the whole animal and to assist
in the assessment of the mutagenic and carcinogenic poten-
tial associated with exposure of humans to in vitro geno-
toxins.
3.2 The use of short-term in vivo assays for assessing the
hazard associated with exposure to in vitro genotoxins
It is apparent from the last paragraph that the major
objective of the study was to investigate the activity in
the whole mammal of chemicals identified as genotoxic in
vitro and to establish which in vivo tests are the most
useful for this purpose. The major criterion for an ac-
ceptable short-term in vivo test, therefore, rests with
its ability to differentiate between carcinogenic and non-
carcinogenic chemicals, particularly those that have been
identified as genotoxic in an in vitro primary screen. To
extend this criterion further, an acceptable assay should
be capable of separating carcinogen/non-carcinogen ana-
logues, e.g., 2AAF/4AAF, in which, in some cases, small
differences in chemical structure are responsible for
dramatic differences in carcinogenic potential. Other
important criteria, some of which were more clearly
characterized during the course of the study, include the
following:
* data should be reproducible between different labora-
tories,
* the assays should not require too high a degree of
technical and scientific expertise to be conducted on
a routine, every-day basis,
* the genotoxic changes or end-points of the assays
should be clear and unambiguous,
* there should be agreed, valid statistical techniques
to differentiate between positive and negative data.
It is relevant to the performance of in vivo tests to
consider the metabolic fate of carcinogenic chemicals.
As a generalization and depending on the route of exposure
of the animal to the chemicals, reactive metabolites of
many carcinogens are generated mainly in the liver as a
by-product of a predominantly detoxifying process. These
metabolites may be capable of interaction with the genetic
material, DNA, in the liver cells, they may be transported
in an active form to other tissues, or they may be further
modified in those tissues to forms able to interact with
DNA. Thus, a study of the genotoxic activity of a chemical
in the whole animal should be able to detect the genotoxic
effects of chemicals or their metabolites in tissues out-
side the liver and of those whose main genotoxic reac-
tivity may be confined to the liver itself. For example,
assays based on genetic alterations to cells in the bone
marrow, which is readily accessible to chemicals or their
metabolites circulating in the blood, respond to a wide
range of chemical carcinogens. However, where reactive
metabolites are not readily transported from the liver,
bone marrow-type tests would be of little value and assays
capable of detecting the genotoxicity in liver cells
should be available.
3.3 The role of short-term in vivo tests in research
into the mechanisms of cancer
Chemical carcinogenesis is generally recognized as a
multistage process that begins with initiation, usually
considered to involve changes in DNA structure leading to
mutations, followed by promotion of the lesion to a pre-
malignant state and progression to overt cancer. Thus, the
majority of current short-term tests, designed to detect
the consequences of DNA interaction, will only respond to
chemicals that may induce tumours by a predominantly geno-
toxic mechanism or induce the initial phase of the car-
cinogenic process.
A feature of the literature on short-term tests for
chemical carcinogens is the occasional reference to chemi-
cals, shown to be associated with the induction of tumours
in laboratory animals, that consistently fail to be de-
tected in short-term in vitro or in vivo assays for geno-
toxicity. Such negative observations with established car-
cinogens have been explained by a lack of sensitivity of
the particular assays. If these chemicals (diethylhexyl-
phthalate is an example) are truly non-DNA-reactive, how-
ever, then negative data in assays for genotoxicity would
be the expected result. The acceptance of the existence
of so-called "non-genotoxic carcinogens" is of critical
importance to the future of short-term testing and one
consideration during the assessment of the CSSTT/2 study
was the identification of in vivo procedures that may be
useful for investigating such chemicals with the eventual
objective of developing specific short-term tests for non-
DNA-reactive carcinogens.
3.4 Assays for the detection of germ cell mutagens
As a general principle of genetic toxicology, genetic
damage induced by chemicals in somatic cells results in
hazard only to the affected individual, while genetic
effects in male or female germ cells may cause heritable
disease or malformation in the immediate progeny or in
future descendants of the affected individual. The ma-
jority of the assays represented in the CSSTT/2 study were
conducted in cells from somatic tissues and are, there-
fore, only directly interpretable in terms of somatic
mutation and carcinogenic potential. Although there have
been attempts to extrapolate data derived from somatic
cell assays to assessment of the probability of a chemical
inducing germ cell mutations, there are a number of
assays, some of which were represented in this study, that
measure genetic damage directly in the germ cells. The
objectives behind the inclusion of germ cell assays in the
present study were to determine:
* which kind of germ cell procedure effectively ident-
ifies germ cell mutagens;
* what is the relationship between the induction of
changes in sperm morphology and unscheduled DNA syn-
thesis in germ cells and the formation of true heri-
table mutations;
* which of the four in vitro genotoxins induce genetic
damage in mammalian germ cells.
4. CRITERIA FOR THE SELECTION OF THE FOUR TEST CHEMICALS
Selection of the two pairs of chemicals, benzo [a] pyrene/
pyrene (BP/PYR) and 2-acetylaminofluorene/4-acetylamino-
fluorene (2AAF/4AAF), was based, primarily, on their per-
formance in the IPESTTC study. In that study, seven of
the fourteen chemicals believed to be non-carcinogens
exhibited a high frequency of positive responses in in
vitro tests while being predominantly or totally negative
in a series of short-term in vivo tests. Although the in
vivo data base was limited, the non-carcinogens in the
BP/PYR and 2AAF/4AAF pairs provided good examples of such
differences in response and these four chemicals were
selected as useful representative candidates for investi-
gating the in vivo behaviour of chemicals known to be in
vitro genotoxins.
4.1 Activity of the four test chemicals in short-term in vitro tests
The majority of chemical carcinogens, including BP and
2AAF, require some form of enzyme-mediated biotransform-
ation for the formation of reactive metabolites. Most
target cells, whether bacteria, yeast or cultured mam-
malian cells, used in in vitro tests lack the appropriate
enzyme activity and this is usually provided in the form
of an enzyme-rich fraction derived from homogenates of rat
liver, referred to as the S9 fraction. Liver enzyme
activity is usually stimulated by pre-treatment of the
animals with an enzyme-inducer such as Aroclor 1254.
Both the carcinogens, BP and 2AAF, are consistently
positive in bacterial assays and other in vitro short-term
tests and, with the exception of certain mammalian cell
assays, demonstration of genotoxic activity requires the
incorporation of a rodent liver enzyme system. Positive
results have also be reported for the two non-carcinogens,
PYR and 4AAF, in a number of in vitro systems but not on
the scale nor usually with the same potency as their
carcinogenic analogues. Thus, both PYR and 4AAF induce
mutations in bacteria, gene conversion in yeast, and
unscheduled DNA synthesis (UDS) and gene mutation in
cultured mammalian cells. They do not, apparently, induce
mutations in yeast or chromosome aberrations in cultured
mammalian cells. 4AAF, but not PYR, can induce UDS in
cultured primary hepatocytes.
In the salmonella mutation assay, separation of the
carcinogen/non-carcinogen pairs is influenced signifi-
cantly by the nature of the rodent liver activation system
employed in the test. When the liver enzyme suspension is
derived from uninduced rodents, BP and 2AAF give positive
results and the two non-carcinogens, PYR and 4AAF, are
generally negative. If Aroclor-induced animals are used
as a source of the activation system, however, the ability
of the assay to discriminate between the carcinogens and
non-carcinogens is lost.
A comprehensive tabulation of the activities of the
four chemicals in short-term in vitro tests is provided by
McGregor (1988b).
4.2 Summary of carcinogenicity data on the test chemicals
The rodent carcinogenicity data for the four test
chemicals is reviewed in Hicks et al. (1988).
Both BP and 2AAF are well established and potent
carcinogens in rodents. Fewer studies have been conducted
with the non-carcinogens, PYR and 4AAF, and their presumed
non-carcinogenicity, although derived from limited rodent
bioassays, must remain tentative pending more comprehen-
sive testing.
BP is a locally-acting carcinogen in rats and mice,
producing skin tumours after dermal application and
tumours of the stomach when administered orally. In mouse
skin, it has been shown to have both initiating and
promoting activity. BP is also a lung carcinogen in mice
and induces mammary gland tumours in rats. There is no
evidence that BP is hepatocarcinogenic in rats and only a
single, unconfirmed report of the induction of hepatomas
in mice. On balance, the available evidence suggests that
BP does not induce liver tumours in rodents.
PYR has not been tested for carcinogenic activity in
any species other than the mouse and even then, only by
dermal application. These studies have shown fairly con-
clusively that PYR is not carcinogenic by this route.
There is some evidence from promotion/initiation studies
on mouse skin that it may have weak initiating activity.
Because data on systemic carcinogenesis in the mouse and
information from other species are lacking, the carcino-
genicity of PYR is somewhat equivocal. However, the fact
that its analogue, BP, is such a potent skin carcinogen,
whereas the data obtained with PYR in fairly comprehensive
mouse skin studies are negative, suggests that the
presumed non-carcinogenic status of PYR is justified.
2AAF is a potent carcinogen in both rats and mice. It
produces tumours in the liver and bladder in both species
after oral administration and in the rat it is carcino-
genic in Zymbal's glands and mammary glands. Little
information is available about its activity when adminis-
tered by other routes.
4AAF has only been tested in rats by incorporation in
the diet, and its activity in mice has not been investi-
gated. In rat feeding experiments, 4AAF has been consist-
ently non-carcinogenic though there are limited data to
suggest that its metabolite, N -OH-4AAF, may have some
weak carcinogenic activity. If 4AAF does prove to be
carcinogenic, its activity is clearly far less potent than
that of 2AAF and further long-term studies are required
before 4AAF can be unequivocally regarded as non-carcino-
genic. The question may be resolved when the results of
the rat carcinogenicity study, initiated as part of the
CSSTT/2 project, are made available.
5. SOURCE AND PURITY OF THE TEST CHEMICALS
Samples of the four test chemicals were obtained from
commercial sources: BP, 2AAF, and 4AAF were supplied by
Lancaster Synthesis Ltd., Eastgate, Whiteland, Morecambe,
Lancashire, United Kingdom, and PYR was obtained from
Aldrich Chemicals, Gillingham, Dorset, United Kingdom.
4AAF was synthesized by a route that avoided contamination
with 2AAF. The purity of the chemicals was determined by
the supplying laboratories before dispatch to the
participants. All the chemicals were greater than 99.5%
pure and full analytical details are provided by Paton and
Ashby (1988).
6. SHORT-TERM IN VIVO ASSAYS
For convenience the assays are divided into six groups:
* cytogenetic (chromosome) assays conducted on cells from
rodent bone marrow or other non-hepatic tissues;
* assays investigating a variety of effects in cells from
rodent liver;
* miscellaneous assays that utilize other tissues or body
fluids;
* mouse coat colour spot tests;
* assays in mammalian germ cells;
* tests utilizing the fruit fly, Drosophila melanogaster.
6.1 Cytogenetic assays
Assays for the induction of chromosome aberrations,
i.e., microscopically visible alterations in chromosome
structure, were conducted in bone marrow cells from rats,
mice, and Chinese hamsters and also in mouse ascites
tumour cells.
Micronucleus tests were conducted on cells from rat
and mouse bone marrow and on erythrocytes in circulating
blood from mice. Micronuclei are chromosome fragments or
intact chromosomes excluded from the cell nucleus during
mitosis. They are considered to be evidence of induced
chromosome breakage or chromosome loss and are usually
analysed in developing or mature erythrocytes.
Sister chromatid exchanges (SCE) can be demonstrated
in metaphase chromosomes by a differential staining tech-
nique and occur as a consequence of the exchange of repli-
cating DNA between chromatids at apparently homologous
loci. Although considered to result from DNA breakage and
reunion, the mechanism of the formation of SCE is not
fully understood. Assays for the induction of SCE were
conducted in bone marrow cells from rats, mice, and
Chinese hamsters, in circulating blood leucocytes from
mice, and in Chinese hamster intestinal epithelium.
Table 1. Assays employed in the CSSTT/2 study
-------------------------------------------------------------------------
1. Cytogenetic studies
Chromosome aberrations
Mouse bone marrow
Mouse ascites tumour cells
Rat bone marrow
Chinese hamster bone marrow
Micronucleus tests
Mouse bone marrow
Mouse circulation blood cells
Rat bone marrow
Sister chromatid exchanges
Mouse bone marrow
Mouse circulating blood lymphocytes
Rat bone marrow
Chinese hamster bone marrow
Chinese hamster intestinal epithelial cells
2. Liver-specific assays
Tests for initiation/promotion: altered enzyme foci
Unscheduled DNA synthesis (UDS)
UDS in mouse liver
UDS in neonatal, weanling and adult rat liver
Frequency of S-phase hepatocytes
S-phase in mouse liver
S-phase in weanling and adult rat liver
Cytogenetic tests
Aberrations in rat liver epithelial-like cells
SCE in rat liver epithelial-like cells
Micronuclei in hepatocytes
Diploid/tetraploid ratio in rat and mouse hepatocytes
Primary changes in DNA
Alkaline elution assay for DNA strand damage
DNA/protein cross-links
DNA unwinding assay for strand damage
3. Miscellaneous assays
Specific carcinogenicity assays
Two-year oral dosing study in rats
Mouse lung adenoma assay
Quail egg tumour-induction
UDS in rat fore-stomach
Sebaceous gland suppression assay
Observation of dermal epithelial hyperplasia
Transformation of rat peritoneal macrophages
6-Thioguanine-resistant mutations in Syrian hamster lung cells
Measurement of DNA adducts
32P-post-labelling in rat and mouse tissues
Immunochemical detection of DNA adducts
Radiolabelled test chemicals - rat liver
-----------------------------------------------------------------------------
Table 1. (contd.)
-----------------------------------------------------------------------------
3. Miscellaneous assays (contd.)
Immunotoxicity assays
Natural Killer (NK) cell and T-cell cytotoxicity in rats
Host-mediated assays
Mutation of salmonella in mouse tissues
Genetic changes in yeast cells in mouse tissues
Urine mutagenicity tests in rats, mice and guinea pigs
4. Mouse spot tests
Mouse coat colour spot test
Mouse melanocyte assay
5. Mammalian germ cell studies
Dominant lethal assays with male and female mice and male rats
Morphological abnormalities in mouse and rat spermatozoa
Unscheduled DNA synthesis in rat and mouse male germ cells
6. Drosophila assays
Sex-linked recessive lethal mutations in germ cells
Chromosome loss in germ cells
Somatic mutation and recombination: mosaic spots in eyes
and wings
-----------------------------------------------------------------------------
6.2 Assays in rodent liver cells
A variety of liver-specific assays were represented in
the study, including the demonstration of enzyme changes,
a number of different tests on DNA, and cytogenetic
assays.
The rat liver assay for altered enzyme foci employs a
histochemical technique that identifies groups of cells or
foci that have elevated levels of the marker enzyme gamma-
glutamyltranspeptidase (GGT). The observation of GGT-
positive foci often precedes the appearance of liver car-
cinoma and the test is used to attempt to identify early
stages of carcinogenesis in the liver. Protocols have been
devised to investigate both initiation and promotion
stages of carcinogenesis.
A number of investigators used assays that measure
directly or indirectly the response of DNA to chemical
damage. These included tests for breaks in single strands
of DNA using the alkaline elution technique, an assay for
the induction of crosslinks between DNA and protein mol-
ecules, and a measure of single strand breaks based on the
degree of unwinding of DNA molecules. One consequence of
DNA breakage is the initiation of the enzyme-mediated
repair process which involves the synthesis of new, rela-
tively short strands of DNA to repair the break. This type
of repair is referred to as unscheduled DNA synthesis or
UDS (to differentiate from the normal or S-phase DNA syn-
thesis that occurs during cell replication). DNA synthesis
can be measured by observing the uptake of tritiated thy-
midine by the newly synthesized strands using autoradio-
graphical techniques. Assays for the induction of UDS and
to measure changes in the numbers of S-phase cells in
rodent liver were included in the study.
The cytogenetic methods included the analysis of meta-
phase chromosome aberrations, micronuclei and SCE in rat
liver cells, and the determination of the ratio of diploid
to tetraploid cells in rat and mouse liver.
6.3 Miscellaneous assays
The first group of assays to be considered under this
heading are those that are directly related to the induc-
tion of tumours in rodents. The most important of these
is a 2-year oral dosing study in rats to compare the car-
cinogenicity of 2AAF and 4AAF. Although the study was not
completed at the time of this report, preliminary infor-
mation from a small group of rats examined after 26 weeks
of dosing is available. Also included in this group was
a) the mouse lung tumour assay that determined the effects
of each of the four test chemicals on the incidence of
lung adenoma in mice and b) a test in Japanese quail in
which the test chemicals were introduced into the yolk
sacs of quail eggs. The birds were then examined for
evidence of tumours at various intervals after hatching.
The sebaceous gland suppression test is based on the
observation of morphological changes to the sebaceous
glands in histological preparations of mouse skin after
dermal exposure to the test chemicals. The presence of
epidermal hyperplasia can also be detected histologically
and both methods have been shown to respond to skin car-
cinogens, particularly polycyclic hydrocarbons.
Genotoxic carcinogens can bind covalently to biologi-
cal macromolecules such as DNA either before or after
metabolic biotransformation. The products of DNA-binding
are referred to as DNA adducts and a number of investi-
gators provided data on the detection and measurement of
DNA adducts with the test chemicals in tissues from rats
and mice. Three different methods were represented:
* the enzyme-linked immunoabsorbent assay (ELISA);
* the 32P-post-labelling assay;
* an assay for radiolabelled chemicals bound to DNA.
Two similar assays for the investigation of the immune
response of animals to exposure to carcinogens were rep-
resented. Natural Killer (NK) cells are leucocytes with
the ability to lyse a variety of `foreign' cells, e.g.,
those with a malignant phenotype. The NK cell assay was
used to investigate the capacity of NK cells, derived from
the splenic mononuclear leucocytes of rats treated with
2AAF or 4AAF, to lyse human erythroleukaemic cells in
vitro. The basis of the T-cell assay is that immunocom-
petent T-cells are able to react to tissue changes induced
by carcinogenic chemicals in rats. The changes in T-cell
activity can be determined by their cytotoxicity towards
target cells derived from rat intestinal adenocarcinoma.
The rat peritoneal cell transformation test is based
on the observation that mitogen-stimulated peritoneal
cells, harvested from rats dosed with carcinogens, undergo
a form of transformation that enables them to proliferate
and produce colonies in soft agar culture.
Host-mediated assays were used fairly widely in the
early l970s as primary screening tests for mutagenic
chemicals. In its original form, a suspension of microbial
target cells, i.e. yeast or bacteria, was introduced into
the peritoneal cavities of mice. The mice were treated
with the suspect chemical and, after an appropriate inter-
val, the target cells were harvested and changes in the
mutation frequency or other genetic end-points were deter-
mined in in vitro culture. Since that time, there have
been several modifications to the procedure, the most
significant being the injection of the target organisms
into the circulating blood of the host. In this way, the
bacterial or yeast target cells are distributed in various
organs and can, for example, be harvested from the liver,
lungs, and kidney. The target cells can, in principle, be
affected by reactive metabolites generated from the test
chemical in these organs. Intra-sanguinous host-mediated
assays using either Salmonella typhimurium or the yeast,
Saccharomyces cerevisiae, were represented in the study.
Urine and other body fluids can be collected from
rodents treated with test chemicals and assayed for muta-
genic activity. For example, after appropriate treatment
to release conjugated metabolites, urine can be tested for
the presence of mutagens in a conventional in vitro bac-
terial mutation assay. In principle, the urine assay is
capable of detecting mutagenic chemicals excreted un-
changed or after metabolic activation.
6.4 The mouse spot test
The mouse spot test detects mutations induced in mel-
anocyte precurser cells in the embryos of specially de-
rived strains of mice. The mouse strain carries recessive
mutations at a number of specific coat colour loci and the
embryos are, thus, heterozygous at these loci. Mutations
induced at the wild-type or normal alleles of the coat
colour loci result in the development of clones of the
mutant melanocytes. The young mice are examined after
birth and the mutations are expressed as patches of con-
trasting coloured fur. The mutations can result from one
of a number of genetic events including gene mutations,
chromosome deletions, mitotic crossing-over, and loss of
whole chromosomes. In addition to the conventional tech-
nique of observing the appearance of coloured spots in the
fur, a modification of the spot test allows the micro-
scopic recognition of mutant melanocytes in preparations
of embryonic skin.
6.5 Mammalian germ cell studies
The classical dominant lethal assay in male rodents
involves the treatment of the animals with the test chemi-
cal and then mating with groups of females at intervals to
cover the complete spermatogenic cycle. Dominant lethal
mutations induced at any stage of spermatogenesis can be
detected by dissection of the uterine contents of each
female and are characterized by dead fetuses or a re-
duction in the numbers of fetal implantations. Dominant
treatment of male rats, male mice, or female mice.
Two other assays represented in this group were the
abnormal sperm morphology assay and the unscheduled DNA
synthesis (UDS) test. The former assay monitors mature
spermatazoa for irregularities in morphology at intervals
after treatment of rodents with the test chemicals. The
UDS test measures DNA repair in developing spermatocytes
and spermatids.
6.6 Drosophila assays
Although data from drosophila tests cannot be regarded
as appropriate alternatives to mammalian data for assess-
ing the potential hazard to humans from exposure to geno-
toxic chemicals, drosophila are, in fact, intact, complex,
eukaryotic organisms whose metabolic and genetic charac-
teristics have some parallels with those of the whole mam-
mal. Their main value in genotoxicity testing lies with
the availability of assays to study the effects of chemi-
cals on both somatic and germ cells. The tests represented
in the CSSTT/2 study were:
* the sex-linked recessive lethal mutation assay in
germ cells;
* tests for chromosome loss from germ cells;
* assays for somatic mutation and recombination using
the induction of mosaic spots in either the wings or
the eyes.
7. RESULTS
The investigators met at Cap d'Agde, France, from 15
to 21 May 1985, to assess the results of the study. Each
group of investigators presenting data from a particular
type of assay discussed their data and individual results
were assessed and agreed. This led to the preparation of
consensus reports on the response of each assay to the
four test chemicals. These consensus views were then
incorporated into coordinators' summary reports on each
group of assays. Summary reports were presented and criti-
cally discussed in open plenary discussions, thereby
allowing the overall conclusions and recommendations
resulting from the study to be formulated. Reports of
individual investigators, the coordinators summary
reports, and certain technical appendices form the main
text of the final publication together with an editorial
overview of the study (Ashby et al., 1988).
The purpose of this chapter of the report is to
present the results of the in vivo studies with the four
test chemicals and to construct a profile, in qualitative
terms, of their genotoxic activity in the whole animal.
Quantitative differences in response in relation to sex,
species, route of exposure, and technical variations are
considered in more detail in the next section, which also
includes a comprehensive tabulation of the data generated
by individual investigators (Table 4). However, as many
of the assays were replicated in a number of laboratories,
a simplified table of results is presented here (Table 2).
It must be emphasized that Table 2 contains the consensus
views of the Working Groups assessing the individual
results and performance of each assay and that, in some
cases, there were conflicting results on the same assay
from participating laboratories.
Table 2. Results of the short-term in vivo tests summarized
by Working Groups a
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
1. Cytogenetic studies
Chromosomal aberrations
Mouse bone marrow P N ? N
Mouse ascites cells P NT N N
Rat bone marrow P N ? N
Chinese hamster bone marrow P N P N
Micronucleus tests
Mouse bone marrow P N P N
Mouse blood - maternal P N N N
Mouse blood - fetal P N ? N
Rat bone marrow P N P N
Sister chromatid exchange
Mouse bone marrow P W P W
Mouse blood P N P N
Rat bone marrow P W P W
Chinese hamster bone marrow P N P N
Chinese hamster intestinal cells P N P N
2. Liver-specific assays
Altered enzyme foci - rat P N P W
Unscheduled DNA synthesis
Mouse liver N N N N
Rat liver N N P W
S-phase hepatocytes
Mouse liver NT NT W P
Rat liver - adult W N ? P
Rat liver - weanling P NT NT NT
Cytogenetic tests
Metaphase aberrations - rat W N W N
Micronuclei - rat N N P N
SCE - rat W N ? N
Ploidy - rat P N P N
Ploidy - mouse P N P N
Primary DNA changes
Alkaline elution N N ? ?
DNA/protein cross-links P N P NT
DNA unwinding N N P N
with repair inhibitor P N P N
3. Miscellaneous assays
Carcinogenicity
Oral dosing - rat NT NT P ?
Mouse lung adenoma P N P N
Quail egg tumours ? ? ? ?
UDS in rat fore-stomach ? N N N
--------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
3. Miscellaneous assays (contd.)
Sebacious gland suppression - mouse P N NT NT
Epithelial hyperplasia - mouse P N NT NT
Peritoneal macrophages - rat N N N N
Syrian hamster lung - mutation P N ? N
DNA adducts
32P-post-labelling - rat P N P W
Immunochemical (ELISA) NT NT ? ?
Radiolabelled chemicals NT NT P P
Immunotoxicity
Natural Killer (NK) cells NT NT P N
T-cell cytoxicity P N P W
Host-mediated assays - mouse
Salmonella typhimurium N N N N
Saccharomyces (liver) P P P P
Saccharomyces (lung) N N N N
Urine mutagenicity
Rats P P P P
Mice W W P P
Guinea-pig NT NT P NT
4. Mouse spot tests
Coat colour spots P N P N
Melanocyte assays NT NT P N
5. Mammalian germ cells
Dominant lethal assays
Male mice P N N N
Female mice P N N N
Male rats NT NT N N
Sperm abnormalities
Mice P N P N
Rats NT NT N N
UDS in germ cells
Mice N N N N
Rats N N N N
6. Drosophila assays
Sex-linked recessives N N N N
Chromosome loss N N N N
Somatic mutation
Eye spots P N P N
Wing spots P N W ?
--------------------------------------------------------------------------
a P = positive
W = weak positive
N = negative
? = results inconclusive or not yet reported
NT = not tested
7.1 Benzo [a] pyrene (BP) and pyrene (PYR)
7.1.1 Cytogenetic studies
Assays for the induction of structural chromosome
aberrations or micronuclei in rodent bone marrow cells
were consistently positive with BP and negative with PYR.
Among a number of experimental variables between different
investigators, neither the species, strain, sex, solvent,
nor route of exposure affected the qualitative result. BP
increased the incidence of sister chromatid exchanges
(SCE) in mouse, rat, and Chinese hamster bone marrow in
every study. The results with PYR, however, were less
clear, and this presumed non-carcinogen induced SCE at
high dose levels in mice after oral or intraperitoneal
dosing and in male rats after oral administration, i.e. 3
of 9 SCE studies reported a weak positive result. Analysis
of micronuclei in circulating blood erythrocytes from
maternal, fetal, or weanling mice, and SCE in circulating
blood lymphocytes from adult mice clearly discriminated
between BP and PYR.
7.1.2 Liver-specific assays
Five investigators provided data from observations of
altered enzyme foci in rat liver under the general heading
of initiation/promotion assays. There were, however,
significant protocol variations between investigators.
Two protocols required administration of the rodent tumour
promotor, phenobarbitone, in the drinking-water or diet
for some weeks after dosing in order to determine
initiating activity of the test chemicals. The promoting
activity of the test chemicals was studied by two other
workers by producing initiation events in the liver before
treatment with the test chemicals. The fifth protocol
tested the ability of the chemicals to induce both
initiation and promotion without discriminating between
the two phases. In all cases, the evidence for induced
pre-cancerous changes was the observation of foci or
clones of cells with altered enzyme characteristics. BP
was shown to have significant initiating activity in rat
liver in two studies and, in a third study, was shown
capable of promoting nitrosamine-induced initiation.
Using a fourth, essentially experimental, protocol that
involved the transplantation of donor liver cells to the
host animal, BP failed to show promoting activity. No
evidence of tumour initiating or promoting activity was
observed in any of the studies with PYR.
The rodent liver assay for unscheduled DNA synthesis
measures the induction of repairable lesion in the DNA of
hepatocytes and, theoretically, should be capable of
detecting chemicals that are metabolized in the liver to
produce genotoxic (i.e. DNA-interactive) metabolites.
Both BP and PYR were reproducibly negative in mouse
hepatocytes and in hepatocytes from adult, weanling, or
neonatal rats using either oral or intraperitoneal dosing.
The induction of S-phase DNA synthesis was also
investigated and BP was shown to increase the incidence of
S-phase cells in weanling rats and, in one of two
experiments, a slight increase in S-phase cells was
observed in adult rats. In mice, BP had no significant
effect on the incidence of S-phase, and PYR showed no
evidence of S-phase synthesis induction in either
species.
Limited data were presented on the cytogenetic effects
of the chemicals in liver-derived cells. In epithelial-
like cells from weanling rats, BP was observed to induce a
small increase in the incidence of structural chromosome
aberrations and SCE; tests with PYR were negative. These
findings, however, require confirmation. No evidence of
micronucleus-induction was detected in rats treated with
BP or PYR after partial hepatectomy. Using a cytofluoro-
metric method, an increase in the ratio of diploid to
tetraploid cells was observed in liver-derived cells after
treatment of rats with BP, but not after dosing with PYR.
Three different assays were used to study the ability
of the chemicals to induce single-strand breaks (SSB) in
liver cell DNA. Both BP and PYR were uniformly negative
in the alkaline elution assay for SSB conducted in three
laboratories. BP, however, was shown to induce crosslinks
between DNA and protein, while PYR was negative in this
assay. The third assay in this group was a measure of SSB
based on the degree of alkali-induced unwinding of DNA
molecules. When the DNA-repair inhibitor, adenosine
arabinoside was incorporated, BP was shown to induce SSB
in mouse liver cell DNA using this technique. In the
absence of the repair inhibitor, SSB were not observed.
PYR produced negative results in the DNA-unwinding assay.
The results from this group of assays suggest that BP is
capable of inducing SSB in the DNA of mouse liver cells
that are effectively repaired in the absence of a DNA-
repair inhibitor. In rats, the observation of DNA/protein
crosslinks tentatively suggests the induction of SSB in
rat liver cells, but additional studies are required to
assess the DNA effects of BP in rat liver.
7.1.3 Miscellaneous assays
Two investigators are producing data that are directly
related to the induction of tumours. In the lung adenoma
assay, mice were injected with BP or PYR by the intra-
peritoneal route, 2-3 times each week, for up to 8 weeks.
The mice were examined 23 weeks after the start of the
study and the numbers of adenoma on the surface of the
pulmonary lobes were recorded. BP induced a significant
increase in adenomas compared with the untreated control
group while no increase in the induction of these tumours
was observed in mice injected with PYR. This assay
differentiated very clearly between the carcinogenic
activity of BP and PYR. The second study in this category
involved injecting the test material into the yolk sac of
quail eggs and then observing the birds for the presence
of tumours at intervals after hatching. In birds examined
at approximately 3 months, there was no evidence of tumour
induction by either BP or PYR. Surviving birds will be
maintained for up to 12 months before being examined for
the presence of tumours and, therefore, a definitive
conclusion on the carcinogenicity of BP and PYR to quail
is not yet possible.
One investigator studied the induction of UDS in the
fore-stomach of rats after oral dosing; the data with BP
were considered to be equivocal while PYR gave negative
results.
Two assays involved the application of the test chemi-
cals to mouse skin followed by histological examination of
skin sections. BP induced epithelial hyperplasia and
suppression of sebaceous glands, both of which have been
correlated with polycyclic hydrocarbon-induced skin
carcinogenesis. PYR failed to elicit either of these
responses.
The transformation of rat peritoneal macrophages has
been advocated as a short-term in vivo test for poten-
tially carcinogenic chemicals. Macrophages isolated from
rats dosed with BP or PYR, however, failed to show any
evidence of transformation in experiments conducted in
three laboratories.
Somatic mutation data on cells isolated from Syrian
hamster lung tissue, after dosing with the test chemicals,
were presented by one investigator. Cultures of cells
from animals dosed with BP showed a higher frequency of
mutations at the 6-thioguanine locus than those from
untreated hamsters, suggesting the induction of somatic
mutations in lung cells from this species. PYR produced
negative mutation data in this assay.
32P-labelling of purified DNA was used to detect DNA
adducts in tissues from rats and mice treated intraperito-
neally with the test chemicals. Measurable levels of PYR
adducts were not detected in DNA from mouse liver, lung,
or kidney, or from rat liver or lung tissues. BP adducts
were identified in all of these tissues.
Only one of the two assays for immunotoxicity was con-
ducted with the BP/PYR pair. Thus, the T-cell assay showed
that BP, but not PYR, was capable of inducing a cytotoxic
response in rat T-cells.
Two investigators provided data from host-mediated
assays in mice dosed with BP or PYR. In tests with the
yeast, Saccharomyces cerevisiae, a significant increase
in mitotic gene conversion was detected in yeast cells
isolated from the liver of mice dosed with either BP or
PYR, while yeast cells isolated from lung tissue produced
negative data with both chemicals. No evidence of the
induction of mutations was observed in Salmonella
typhimurium isolated from liver tissues of mice dosed with
BP or PYR.
Assays for the detection of mutagens in urine were
conducted with both rats and mice. Mutagenic activity was
detected in urine samples from both species after dosing
with BP or PYR and, therefore, the test failed to differ-
entiate between the two chemicals.
7.1.4 Mouse spot tests
Only one investigator provided mouse spot test data on
BP and PYR. Using the observation of coat colour spots in
off-spring as evidence of somatic mutation, BP was clearly
positive and PYR negative.
7.1.5 Mammalian germ cell assays
BP induced dominant lethal mutations in both male and
female mice after intraperitoneal injections but not after
oral dosing. The response in the male was highly stage-
specific; only the mature spermatozoa were sensitive and
spermatid and spermatocyte stages were unaffected. Domi-
nant lethal assays with PYR were reproducibly negative.
Studies of morphological abnormalities in mature
spermatozoa were conducted in mice after oral or
intraperitoneal dosing. BP was uniformly positive in this
assay, regardless of the route of exposure. Although a
weak positive response was observed in one of seven
experiments with PYR, this observation was not confirmed
and the consensus view was that PYR did not induce sperm
abnormalities. In studies of the induction of unscheduled
DNA synthesis in rat and mouse male germ cells, negative
results were obtained with both BP and PYR.
7.1.6 Drosophila assays
In all, ten studies were conducted with BP and PYR in
drosophila and in each case the oral route of treatment
was used. Germ cell studies, i.e. the sex-linked
recessive lethal mutation assay and the test for chromo-
some loss, were uniformly negative with both chemicals.
In contrast, tests for somatic mutation and recombination,
based on the observation of eye or wing spots, showed that
BP was able to induce genetic changes in somatic cells in
each of three studies. PYR was consistently negative.
7.2 2-Acetylaminofluorene and 4-acetylaminofluorene
7.2.1 Cytogenetic studies
Seven investigators provided data from analysis of
metaphase chromosomes in mouse bone marrow cells. 2AAF
induced a significant increase in the incidence of
structural chromosome aberrations in only two of seven
assays, one of which was considered to be inconclusive.
The results of assays with 4AAF were negative with the
exception of two assays in which the data were inconclus-
ive. In contrast, 2AAF was reproducibly positive and 4AAF
consistently negative in 15 of 16 micronucleus tests
conducted in mouse bone marrow erythrocytes. The route of
exposure, i.e. oral dosing or intraperitoneal injection,
did not appear to influence the outcome of these studies.
Micronuclei were produced by 2AAF in rat bone marrow cells
and a small increase was also recorded in one of two
assays with 4AAF. Two workers investigated the influence
of these two chemicals on the incidence of structural
chromosome aberrations in Chinese hamster bone marrow;
2AAF was positive in this species and 4AAF gave a weak
positive result in one of the two studies. Both chemicals
failed to induce detectable chromosome damage in mouse
ascites tumour cells. Four micronucleus tests were
conducted with mouse blood erythrocytes. An increase in
micronuclei was recorded by one worker in blood cells from
weanling mice dosed with 2AAF, although when samples of
maternal or fetal blood were examined, increases in
micronuclei were not apparent. However, studies conducted
in another laboratory showed that 2AAF was capable of
increasing the incidence of micronuclei in fetal blood.
Thus, 2AAF is clearly clastogenic to rodent bone marrow
cells while 4AAF was considered by most investigators to
be devoid of clastogenic activity.
Nine assays for sister chromatid exchanges (SCE) were
conducted in bone marrow cells from mice, rats, or Chinese
hamsters. 2AAF was clearly positive in this test and the
consensus view was that 4AAF, although negative in four
assays, was capable of inducing a small (but significant)
increase in SCE. In studies with mouse lymphocytes and
Chinese hamster intestinal epithelial cells, 2AAF was
positive and 4AAF was considered to be negative.
7.2.2 Liver-specific assays
As discussed in section 7.1.2, there were a number of
variations between the five protocols used in the
initiation/promotion assays. Significant initiation and
promotion activity was demonstrated in studies with 2AAF
based on the observation of foci of cells with altered
enzyme characteristics in rat liver. These assays failed
to discriminate clearly between the two chemicals, since
4AAF was shown to be an initiator in the hands of one
investigator and had weak promoting activity in another
study. Both chemicals, therefore, proved capable of
producing these pre-cancerous changes in rat liver tissue
though with different degrees of potency.
2AAF and 4AAF failed to induce detectable unscheduled
DNA synthesis (UDS) in mouse hepatocytes after oral
dosing. Quite different results were obtained in rat
liver, however, and 2AAF was shown to induce UDS in rat
hepatocytes in each of the seven laboratories that sub-
mitted data. Three of these laboratories also reported the
detection of a small increase in UDS in rats after dosing
with 4AAF. The other four laboratories obtained negative
data. Thus, 2AAF induced UDS in hepatocytes from rats but
not mice, and 4AAF elicited a weak UDS response in the rat
and was negative in mouse cells.
The results of the analysis of S-phase DNA synthesis
indicated that the presumed non-carcinogen, 4AAF, was a
very potent inducer of S-phase cells in the liver of both
rats and mice. 2AAF, on the other hand, induced only a
small increase in S-phase cells in the mouse and three
studies in rat liver produced one clear positive and two
negative results.
In cytogenetic experiments in cells derived from rat
liver, 2AAF appeared to induce a small increase in the
incidence of structural aberrations and, in partially
hepatectomised rats, a significant increase in micro-
nuclei. 4AAF gave negative results in tests for chromo-
somal aberrations, micronuclei, and SCE in rat liver
cells. When the ratio of diploid to tetraploid cells was
investigated in rat and mouse liver, an increase in the
ratio was observed in both species after dosing with 2AAF
but not in response to 4AAF.
The alkaline elution assay for single strand breaks
(SSB) produced conflicting results between three labora-
tories. Two investigators presented negative data from
assays with both chemicals while a third observed that
both 2AAF and 4AAF produced SSB in this test. Using the
detection of DNA/DNA or DNA/protein crosslinks as a
measure of SSB, 2AAF was shown to produce SSB. No data
were presented on 4AAF. Another investigator calculated
SSB from the amount of DNA unwinding induced by the chemi-
cals. 2AAF and 4AAF gave positive and negative results,
respectively, either in the presence or absence of a DNA
repair inhibitor. These data suggest that, unlike 4AAF,
2AAF is capable of producing SSB in rat liver DNA under
appropriate experimental conditions.
7.2.3 Miscellaneous assays
A 2-year oral dosing carcinogenicity study in rats
with 2AAF and 4AAF is still in progress. Preliminary
findings in a small number of animals examined after 26
weeks of dosing showed that an increase in liver tumours
was already apparent in animals dosed with 2AAF; there was
no evidence of tumour induction in the 4AAF-dosed ani-
mals.
The lung adenoma assay, in which mice were given
intraperitoneal injections of the test chemicals 2-3 times
a week for up to 8 weeks, clearly separated 2AAF and 4AAF.
2AAF induced a significant increase in adenomas while the
incidence in mice dosed with 4AAF was comparable with that
of untreated mice.
There was no evidence of the induction of UDS in rat
fore-stomach after oral dosing with either chemical, and
the assay for the induction of somatic mutations in Syrian
hamster lung cells produced inconclusive data with 2AAF
and was negative in tests with 4AAF. The rat macrophage
test was reproducibly negative with both 2AAF and 4AAF.
Three techniques were applied to detect the presence
of DNA adducts formed in various tissues after dosing
rodents with the test chemicals. Data from the ELISA
technique, which requires the use of monoclonal antibodies
to detect specific adducts, are not yet available. How-
ever, the 32P-post-labelling method indicated that 2AAF
formed adducts with DNA in rat liver and lung and in mouse
liver, lung, and kidney after intraperitoneal dosing. No
data were presented from mice dosed with 4AAF. In the
rat, however, 4AAF formed adducts in liver and lung DNA
though at a much lower rate than 2AAF. In studies using
radiolabelled test materials, both chemicals were shown to
form adducts with DNA in the liver and lungs of rats after
intraperitoneal administration, although adduct formation
was considerably less with 4AAF than with 2AAF.
2AAF produced positive results in both immunotoxicity
assays. However, although 4AAF was negative in the
Natural Killer (NK) cell assay, data from the T-cell
cytotoxicity test suggested that it was capable of
inducing a measurable cytotoxic response in rat T-Cells.
Nine sets of data were considered from mouse host-
mediated assays. In eight of these, including assays for
genetic changes in yeast isolated from liver, lung and
kidney, and for mutations in salmonella isolated from
liver tissue, 4AAF was uniformly negative. 2AAF produced
two weak positive responses and five negative results in
the same eight studies. The ninth assay, however, indi-
cated that both chemicals induced mitotic gene conversion
in yeast isolated from liver tissue. In addition, muta-
genic materials were detected in urine samples from rats
and mice after dosing with either isomer and from guinea-
pigs after dosing with 2AAF. 4AAF data on guinea-pig
urine were not available.
7.2.4 Mouse spot tests
Six separate mouse spot tests were conducted with 2AAF
and the results were equally divided between positive and
negative. An increase in coat colour spots was detected
in two studies while in two others, no evidence of the
induction of coat colour spots was observed. Similarly,
in the melanocyte assay, one positive result and one
negative were reported. Four of five spot tests conducted
with 4AAF were negative and the data were inconclusive in
the fifth.
7.2.5 Mammalian germ cell assays
Dominant lethal mutation assays conducted in male or
female mice or male rats and tests for the induction of
UDS in rat or mouse male germ cells were uniformly nega-
tive with both compounds. In the test for morphological
abnormalities in mature spermatozoa, two assays in which
2AAF was given orally to mice or rats were also negative.
After intraperitoneal dosing, however, 2AAF induced a
significant increase in the incidence of abnormal mouse
sperm. 4AAF was negative in both species regardless of
the route of exposure. These data suggest that, although
2AAF or its metabolites appear capable of penetrating the
testes and adversely affecting sperm morphology after
intraperitoneal dosing, there is no evidence for interac-
tion of 2AAF metabolites with germ cell DNA.
7.2.6 Drosophila assays
The mutagenic effects of the two chemicals on
drosophila germ cells were studied in tests for sex-linked
recessive lethal mutations in five laboratories and for
chromosome loss in two laboratories. Data from six of
these studies indicated that neither chemical produced
genetic damage in drosophila. In the seventh laboratory,
however, 2AAF was clearly mutagenic in the sex-linked
recessive lethal assay; 4AAF produced a weak positive
result in this study.
2AAF induced eye or wing spots in somatic mutation and
recombination assays in drosophila, although the response
was weak in two of the studies. The somatic mutation data
generated from tests with 4AAF were ambiguous, producing
one weak positive result, one inconclusive result and one
negative.
7.3 Summary of the in vivo genotoxicity of the four chemicals
A comprehensive in vivo genotoxicity profile has been
generated on the four test chemicals, each of which had
previously shown evidence of mutagenic activity in in
vitro screening tests. It is relevant, in this section,
to consider the mammalian genotoxicity in relation to the
hazard assessment procedures recommended by many regulat-
ory bodies. Table 3 shows the mutagenic activity of the
chemicals in the in vivo tests common to most legislative
guidelines. Based on these results, and in the absence of
pharmacokinetic and metabolic data, BP would be considered
to present a potential mutagenic and carcinogenic hazard
and 2AAF would be regarded as a potential carcinogen.
These observations are, of course, corroborated by the
established carcinogenic activity of these two chemicals.
The abbreviated data base shown in Table 3 suggests
that neither 4AAF nor PYR present a significant genotoxic
hazard and it is useful to consider whether the compre-
hensive data generated in CSSTT/2 support this initial
prediction. The summarized consensus results (Table 2)
show that conclusive data for PYR were presented from 48
distinct in vivo tests and PYR was considered to be nega-
tive in 43 of these. The remaining five tests suggested a
weak induction of SCE in rodent bone marrow that was not
reproduced in all laboratories, a single positive host-
mediated assay, and evidence of the excretion of mutagenic
metabolites in rodent urine. None of these data seriously
question the validity of the original prediction that PYR
is not a significant genotoxic hazard.
Table 3. Activity of the four test chemicals in established
in vivo mutagenicity assays
------------------------------------------------------------------------
BP PYR 2AAF 4AAF
Somatic cell tests
Metaphase chromosome analysis P N Pa N
Micronucleus test P N P N
Mouse spot test P N Pa N
Germ cell tests
Dominant lethal assay P N N N
Heritable translocation test NT NT NT NT
Mammalian germ cell cytogenetics NT NT NT NT
------------------------------------------------------------------------
a Data not consistent between laboratories
NT = not tested
P = positive
N = negative
A similar analysis of the 4AAF data indicates that
conclusive results were obtained from 50 different types
of in vivo tests. Of these, 38 were regarded as negative
and the remaining twelve assays produced positive or weak
positive results. Like BP, 4AAF produced a positive
result in a host-mediated assay and in tests for mutagenic
activity in rodent urine. Other observations, however,
suggest that 4AAF may not be devoid of in vivo genotox-
icity. For example, 4AAF induced detectable unscheduled
DNA synthesis in rat liver hepatocytes that was reproduced
in three different laboratories. One investigator reported
the induction of micronuclei in rat bone marrow cells
after intraperitoneal dosing with 4AAF, and five of seven
studies conducted in rat or mouse bone marrow recorded
small, but significant, increases in SCE. There was also
data to suggest that 4AAF was capable of adduct formation
with liver cell DNA in rats. All the germ cell data on
4AAF were negative. The positive findings in somatic
tissues cannot be dismissed as inconsequential and they
raise serious doubts about the prediction, based on the
data shown in Table 3, that 4AAF does not present a
significant genotoxic hazard. The outcome of the 2-year
rat carcinogenicity study with 4AAF is crucial to the
assessment of the validity of this prediction and, in
fact, to the evaluation of the whole CSSTT/2 data base.
8. ASSESSMENT OF THE PERFORMANCE OF THE ASSAYS
The purpose of this section is to present an objective
assessment of the performance of the in vivo tests as it
relates to the carcinogenic activity of the test chemi-
cals. (A detailed tabulation of the qualitative results of
each assay is presented in Table 4). Data from a study of
only four chemicals do not, of course, provide sufficient
information on which to judge the overall value of a par-
ticular assay for discriminating between non-carcinogenic
and potentially carcinogenic chemicals. However, when a
data base of this magnitude is available, in which many
tests were replicated in a number of different labora-
tories, then a unique insight into the utility, reproduc-
ibility, and reliability, as well as the accuracy of the
tests, can be obtained. Thus, data from the current study,
together with the considerable experience of the investi-
gators and assessors, enable the performance of many of
the assays to be comprehensively evaluated.
Although a high proportion of test systems performed
reasonably well with these four chemicals, some assays,
not surprisingly, failed to meet the predetermined cri-
teria for acceptable performance (section 4). The rodent
host-mediated and urine mutagenicity assays are good
examples. They involve either the exposure of marker
organisms to the chemicals or its metabolites in vivo or
the detection of mutagenic excretory products in urine of
treated rodents. Both types of tests were conducted in
several laboratories and neither proved capable of ident-
ifying the two carcinogens among the four in vitro muta-
gens, i.e. the host-mediated assays were generally nega-
tive with the four test chemicals while the urine tests
were uniformly positive. These observations suggest that
neither of these two classes of tests has any practical
value in evaluating the in vivo genotoxicity of chemicals
known to be genotoxic in vitro. The results obtained from
the peritoneal macrophage transformation assay suggest a
similar conclusion. This is a form of host-mediated assay
in which the transformation of macrophages, isolated from
the peritoneum of treated rats, is investigated in in
vitro culture. The four test chemicals gave negative
results in each of three laboratories.
Table 4. IPCS CSSTT in vivo study - summary of qualitative
results from individual investigatorsa
-------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
-------------------------------------------------------------------------------------------------
1. CYTOGENETICS
1.1 Chromosomal aberrations
1.1.1 Mouse bone marrow 8 P N NT NT o
8 NT NT P ? ip
1.1.2 Mouse bone marrow 9 P N NT NT o
9 NT NT N N ip
1.1.3 Mouse bone marrow 10 P N N N o
1.1.4 Mouse bone marrow 11 P N N N o
1.1.5 Mouse bone marrow 12 P N P ? o
1.1.6 Mouse bone marrow 13 P NT N N o
1.1.7 Mouse bone marrow 14 P N ? N ip
1.1.8 Mouse ascites tumour cells 13 P NT N N o
1.1.9 Rat bone marrow 15 P N NT NT o
15 NT NT ? N ip
1.1.10 Chinese hamster bone marrow 16 P N P W o
1.1.11 Chinese hamster bone marrow 17 W N W N o
1.2 Micronuclei
1.2.1 Mouse bone marrow 19 P N P N o
1.2.2 Mouse bone marrow 20 P N P N o
1.2.3 Mouse bone marrow 21 P N P N o
1.2.4 Mouse bone marrow 22 P N N N o
1.2.5 Mouse bone marrow 23 P N P N o
1.2.6 Mouse bone marrow 24 P W P W o
1.2.7 Mouse bone marrow 25 P N NT NT o
1.2.8 Mouse bone marrow 26 P N P N o
1.2.9 Mouse bone marrow 27 P N P N o
1.2.10 Mouse bone marrow 28 P N NT NT o
28 NT NT P N ip
1.2.11 Mouse bone marrow 29 P N P N ip
1.2.12 Mouse bone marrow 29 P N NT NT o
1.2.13 Mouse bone marrow 30 P N P N o
30 P N P N ip
1.2.14 Mouse bone marrow 31 P N P N ip
1.2.15 Mouse bone marrow 32 P N P N o
1.2.16 Mouse bone marrow 33 P N P N o
1.2.17 Mouse blood - weanling 34 P N P ? o
1.2.18 Mouse blood - fetal 34 P N N N o
1.2.19 Mouse blood - maternal 34 P N N N o
1.2.20 Mouse blood - fetal 32 P N P N o
1.2.21 Rat bone marrow 35 NT NT P N o
1.2.22 Rat bone marrow 36 P N NT NT o
36 NT NT P W ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
1.3 Sister chromatid exchange
1.3.1 Mouse bone marrow 38 P N P W ip
1.3.2 Mouse bone marrow 39 P W P W o
1.3.3 Mouse bone marrow 40 P N P W o
1.3.4 Mouse bone marrow 41 P W P W ip
1.3.5 Mouse bone marrow 42 P N P N o
1.3.6 Mouse bone marrow 43 P N W N o
1.3.7 Rat bone marrow 44 P W P W o
1.3.8 Mouse lymphocytes 45 P N P N o
1.3.9 Chinese hamster bone marrow 46 P N P N ip
Chinese hamster bone marrow 46 P N P N o
1.3.10 Chinese hamster intestinal cells 46 P N P N o
2. LIVER-SPECIFIC ASSAYS
2.1 Initiation/promotion
2.1.1 Altered enzyme foci 50 NT NT N N o
2.1.2 GGT-positive foci 51 P N P N o
2.1.3 GGT-positive foci 52 P N P P o
2.1.4 GGT-positive foci - promotion 53 P N P W o
2.1.5 GGT-positive foci 54 N N P N o
2.1.6 GGT-positive foci 73 NT NT P N o
2.2 Unscheduled DNA synthesis (UDS)
2.2.1 UDS in mouse liver 56 N N N N o
2.2.2 UDS in rat liver 56 N N P N o
2.2.3 UDS in rat liver 57 NT NT P W o
2.2.4 UDS in rat liver 58 N N P W o
2.2.5 UDS in mouse liver 58 NT NT N N o
2.2.6 UDS in rat liver 59 NT NT P N o
2.2.7 UDS in rat liver 60 NT NT P W o
2.2.8 UDS in rat liver 61 NT NT P N o
2.2.9 UDS in rat liver 62 NT NT P N o
2.2.10 UDS in weanling rat liver 63 N NT NT NT o
2.2.11 UDS - neonate rat 61 NT NT W NT o
2.3 S-phase hepatocytes
2.3.1 Rat 56 W NT NT P o
2.3.2 Mouse 56 NT NT NT W o
2.3.3 Rat 58 N N P P o
2.3.4 Mouse 58 NT NT W P o
2.3.5 Rat 59 NT NT NT P o
2.3.6 Rat - weanling 63 P NT NT NT o
2.3.7 Rat 60 NT NT N P o
2.3.8 Rat 62 NT NT N P o
2.3.9 Rat 61 NT NT NT P o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
2.4 Cytogenetic analysis
2.4.1 Aberrations in liver 65 W N W N o
epitheloid cells
2.4.2 SCE in liver epitheloid cells 65 W N ? N o
2.4.3 Micronuclei in hepatocytes 66 N N P N o
2.5 DNA strand breaks
2.5.1 Alkaline elution 68 N N N N o
2.5.2 Alkaline elution 69 N N N N o
2.5.3 Alkaline elution 70 N W P P o
2.5.4 DNA-DNA/DNA-protein crosslinks 70 P N P NT ip
2.5.5 DNA unwinding 71 N N P N o
2.5.6 DNA unwinding (with araA) 71 P N P N o
3. MISCELLANEOUS ASSAYS
3.1 Carcinogenesis
3.1.1 2-year rat study 73 NT NT P * o
3.1.2 Mouse lung adenoma 74 P N P N o
3.1.3 Quail egg 75 * * * *
3.2 Supplementary assay
3.2.1 Rat fore-stomach UDS 76 ? N N N o
3.2.2 Sebaceous gland suppression 77 P N NT NT der
3.2.3 Epithelial hyperplasia 77 P N NT NT der
3.2.4 Rat hepatocyte ploidy 78 P N P N o
3.2.5 Mouse hepatocyte ploidy 78 P N P N o
3.2.6 Syrian hamster 6-TG-resistant 79 P N ? N ip
lung cells
3.2.7a 32P-post labelling - rat liver 80 P N P W ip
3.2.7b 32P-post labelling - rat lung 80 P N P W ip
3.2.7c 32P-post labelling - mouse 80 P N P NT ip
liver
3.2.7d 32P-post labelling - mouse 80 P N P NT ip
lung
3.2.7e 32P-post labelling - mouse 80 P N P NT ip
kidney
3.2.8 Radiolabelled test compounds 81 NT NT P P ip
3.2.9 ELISA rat 82 NT NT * * o
3.3 Immunotoxicity
3.3.1 T-cells - rat 83 P N P W ip
3.3.2 Natural Killer cells - rat 84 NT NT P N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
3.4 Transformation of peritoneal macrophages
3.4.1 Rat macrophages 85 N N N N o
3.4.2 Rat macrophages 85 N N N N o
3.4.3 Rat macrophages 85 N N N N o
3.5 Host-mediated assays
3.5.1a Mouse/yeast - mitotic 86 NT NT N N o
recombination - liver
3.5.1b Mouse/yeast - mitotic 86 NT NT N N o
recombination - lung
3.5.1c Mouse/yeast - mitotic 86 NT NT N N o
recombination - kidney
3.5.1d Mouse/yeast - mutation - 86 NT NT W N o
liver
3.5.1e Mouse/yeast - mutation - 86 NT NT N N o
lung
3.5.1f Mouse/yeast - mutation - 86 NT NT W N o
kidney
3.5.2a Mouse/yeast - gene conversion 87 P P P P o
liver
3.5.2b Mouse/yeast - gene conversion 87 N N N N o
lung
3.5.3 Mouse/salmonella - liver 88 N N N N o
3.6 Urine mutagenicity
3.6.1 Rat urine extract 89 P P P P ip
3.6.2 Rat urine extract 90 P P P P ip
3.6.3 Mouse urine extract 91 W W P P o
3.6.4a Rat urine 92 P P P P ip
92 P P P P o
3.6.4b Guinea-pig 92 NT NT P NT ip
3.6.5 Rat urine 93 P P P P ip
4. MOUSE SPOT TEST
4.1.1 Coat colour 95 NT NT P N ip
4.1.2 Coat colour 96 NT NT N N o
96 NT NT N NT ip
4.1.3 Coat colour 97 P N P N ip
4.1.4 Melanocytes 98 NT NT P ? ip
4.1.5 Melanocytes 99 NT NT N N ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
5. MAMMALIAN GERM CELL STUDIES
5.1 Dominant lethal
5.1.1 Female mice 102 P N N N ip
5.1.2 Male mice 103 P N N N ip
5.1.3 Male mice 104 P N N N ip
104 N ? NT NT o
5.1.4 Male mice 105 P N N N ip
105 N NT NT NT o
5.1.5 Male rat 106 NT NT N N o
5.2 Sperm abnormalities
5.2.1 Mouse 108 P N P N ip
5.2.2 Mouse 109 W N N N o
5.2.3 Mouse 110 P W P N ip
5.2.4 Mouse 111 P N N N o
5.2.5 Rat 112 NT NT N N o
5.3 Unscheduled DNA synthesis
5.3.1 Rat spermatocytes 113 N N N N o
5.3.2 Mouse sperm 114 N N N N ip
114 N NT NT NT o
6. DROSPHILA ASSAYS
6.1 Sex-linked recessive lethal
6.1.1 SLRL assay 116 N N P W o
6.1.2 SLRL assay 117 N N N N o
6.1.3 SLRL assay 118 N N N N o
6.1.4 SLRL assay 119 N N N N o
6.1.5 SLRL assay 120 N N N N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
6.2 Chromosome loss
6.2.1 Germ cell 121 N N N N o
6.2.2 Germ cell 116 N NT N NT o
6.3 Somatic mutation and recombination
6.3.1 Eye spots 116 P N W W o
6.3.2 Eye spots 122 P N P N o
6.3.3 Wing spots 118 P N W ? o
--------------------------------------------------------------------------------------------------------------
a From: Ashby et al. (1988)
b Numbers refer to chapters in Ashby et al. (1988)
P Positive
NT Not tested
N Negative
? Inconclusive
W Weak positive
* Results not available
8.1 Cytogenetic assays
Assays that utilize target cells in rodent bone marrow
for detecting chromosomal effects, i.e., chromosomal aber-
rations, micronuclei, or sister chromatid exchanges, are
widely advocated for investigating the activity of in
vitro genotoxins in the intact animal and represent the
largest group of tests in this study.
8.1.1 Chromosomal aberrations
Data from metaphase chromosome studies in mouse bone
marrow were provided by seven investigators. Each investi-
gator successfully discriminated between BP and PYR, and
five sets of data determined 4AAF to be negative while two
were inconclusive. The results of chromosome studies with
2AAF, however, were conflicting. Two laboratories identi-
fied 2AAF as a clastogen, one set of data were inconclus-
ive and four investigators reported 2AAF as negative.
There appears to be no simple explanation for these dis-
crepancies, which were not associated with differences in
mouse stain, route of treatment, sampling interval, or
other aspects of experimental design. The mouse micro-
nucleus assay (section 8.1.2) showed 2AAF to be an in vivo
clastogen (though less potent than BP) and it is clear
that the dosing schedules used in the metaphase chromosome
assays were more than adequate to induce chromosome aber-
rations, i.e., 2AAF would be expected to be detected as a
clastogen in the metaphase chromosome assay. One expla-
nation for the negative findings in four laboratories may
be the resolving power of the sample sizes used. Most
standard protocols for the bone marrow metaphase assay
require at least 50 cells per animal from 10 animals per
dose/time group (i.e., 500 cells) to be analysed for aber-
rations at each data point. This recommended minimum fig-
ure was only achieved by three of the seven investigators
and three others presented data on only 250 cells per
dose/time group. These observations suggest that, with
samples of this size, almost a 10-fold increase in
aberrations over the control incidence would be required
to demonstrate a significant induction of aberrations by
2AAF.
Two investigators conducted studies in Chinese hamster
bone marrow cells. PYR was negative in both studies. In
experiments in which 500 cells per dose/time group were
analysed, BP and 2AAF were clearly positive while, when
only 300 cells were used, the results were classified as
weak positive with both carcinogens. It is interesting to
note that 4AAF induced a weak positive response in Chinese
hamster bone marrow in one study.
One set of data from rat bone marrow was presented and
clearly separated BP from PYR. 2AAF, however, gave
unambiguous data and 4AAF was negative.
The performance of the bone marrow cytogenetic assays
did not, in general, meet the criteria for a reliable and
reproducible short-term in vivo test. There are, however,
serious reservations regarding the numbers of animals and
the numbers of cells analysed in many of the studies and
the results suggest that protocols designed to give an
acceptable resolving power should be strictly adhered to.
8.1.2 Micronuclei
Eighteen laboratories conducted micronucleus tests; 16
laboratories performed assays on bone marrow cells from
mice and two investigators used rats. Data were also
presented on the incidence of micronuclei in circulating
blood cells from adult and fetal mice.
Mouse bone marrow micronucleus assays were conducted
to a common protocol with only minor deviations. At least
5 mice were used for each dose/time interval with 1000
cells being analysed from each animal. The data from these
assays showed a high level of agreement between labora-
tories. Seventeen sets of data on BP were uniformly posi-
tive and 16 sets on PYR were negative. Similarly, 14
investigators showed 2AAF to be capable of inducing an
increase in micronuclei and 4AAF was negative in 14 cases.
There were only occasional anomalous results. For example,
one laboratory failed to detect 2AAF, and another investi-
gator recorded weak positive results from both PYR and
4AAF. These anomalies do not detract from the general
accuracy and reproducibility of mouse micronucleus tests
with the four test chemicals.
One investigator used erythrocytes from mouse periph-
eral blood to assay the incidence of micronuclei. The
results were similar to those obtained in the bone marrow
studies, i.e. BP and 2AAF were positive while PYR and 4AAF
were considered to be negative by the criteria adopted for
a positive response. In two laboratories, fetal blood was
obtained from mice on day 15 of pregnancy, 30 or 48 h
after oral dosing of the mother. BP was positive and PYR
was negative in each of these studies. The fetal assay was
less sensitive to the effects of 2AAF, one laboratory
observing a small increase in micronuclei and the other
finding no increase. 4AAF was inactive in fetal cells.
Two micronucleus studies were conducted in rat bone
marrow cells and the results suggest that the response in
the rat was weaker than that in the mouse. In one
laboratory, 2AAF was positive and 4AAF negative; BP and
PYR were not tested. The second investigator reported a
positive response with BP and a negative one with PYR.
This investigator's result with 2AAF is shown as positive
in Table 4. However, using criteria decided by the
micronucleus assay working group, it was concluded that
2AAF was negative in this assay. It was also considered
that the protocol used in the rat studies was not designed
for the optimum detection of micronuclei.
The results from the micronucleus tests confirm the
value of the assay in mouse bone marrow as an accurate and
reproducible means of qualitative discrimination between
carcinogenic and non-carcinogenic chemicals.
8.1.3 Sister chromatid exchange
Nine investigators presented data on the induction of
sister chromatid exchanges (SCE) in vivo using mice, rats,
or Chinese hamsters. The test chemicals were administered
by gavage or intraperitoneal injection and most investi-
gators analysed SCE in bone marrow cells. One set of data
were presented on SCE in mouse peripheral lymphocytes and
another set on Chinese hamster intestinal epithelial
cells. The two established carcinogens, BP and 2AAF,
induced an increase in SCE in each of the six studies
conducted in mouse bone marrow. In two of these studies,
PYR induced a weak positive response and 4AAF was weakly
positive in four of the six assays. Weak positive results
were also obtained in rat bone marrow assays with the two
presumed non-carcinogens, while BP and 2AAF were clearly
positive. In experiments conducted in blood lymphocytes
in mice and in bone marrow cells and intestinal epithelial
cells in Chinese hamsters, BP and 2AAF gave positive
results while PYR and 4AAF were negative. None of the
experiments suggested that the route of administration of
the test chemicals significantly influenced the qualitat-
ive results.
Both the established carcinogens were consistently
positive in tests for the induction of SCE in three rodent
species, suggesting that the SCE assay is a sensitive and
reproducible in vivo test for genotoxic carcinogens. PYR
or 4AAF, however, induced small (but significant) in-
creases in the incidence of SCE in 8 of 22 tests, although
the presumed non-carcinogens were much less potent
inducers of SCE than their carcinogenic analogues. These
observations raise a number of questions regarding the
significance of small increases in SCE in in vivo tests.
In the first instance, if 4AAF is confirmed to be non-
carcinogenic in the current rodent bioassay, then these
weak responses have to be regarded as `false positives'.
If, however, 4AAF is shown to have weak carcinogenic
activity, then the weak positive responses obtained in
four of the six mouse bone marrow studies can be inter-
preted as accurately reflecting the genotoxicity of 4AAF.
In the report of the SCE Working Group (Tice et al., 1988)
the biological relevance of statistically significant
increases in SCE at unrealistically high doses was con-
sidered. It was suggested that multiple mechanisms are
involved in the formation of SCE and that one of these may
be a response to stress, either at the cell or animal
level, caused by a toxic response to high doses of a
chemical. The small induction of SCE observed after high
doses of PYR or 4AAF, therefore, may not be related
mechanistically to the larger increases in SCE seen after
low doses of BP or 2AAF. Thus, the final assessment of
the performance of the in vivo SCE assays depends, to a
large extent, on the outcome of the 2-year bioassay with
the AAF analogues, i.e. a negative result with 4AAF will
cast serious doubts on the value of SCE tests for investi-
gating the in vivo activity of in vitro genotoxins.
8.2 Liver assays
The activity of certain genotoxic chemicals appears to
be confined to the liver. Such chemicals do not, of
course, induce detectable genetic changes in bone marrow
cells or other extra-hepatic tissues and this has led to a
need to develop techniques for investigating genotoxic or
related changes in liver cells. The main thrust of this
research has centred on the demonstration of unscheduled
DNA synthesis in hepatocytes but data were also presented
from a variety of other assays that used target cells in
the liver.
8.2.1 Initiation and promotion
Two of the assays used in this group were specifically
aimed at detecting initiating activity of the test chemi-
cals. The chemicals were administered after partial hepa-
tectomy, and the mice were then treated with the promotor,
phenobarbital, for several weeks before liver sections
were examined for the occurrence of pre-cancerous foci,
i.e. groups of cells having elevated levels of the marker
enzyme, gamma-glutamyl-transpeptidase (GGT). Both these
protocols have been used successfully with many test
chemicals and, in the present study, successfully separ-
ated BP and 2AAF from PYR and 4AAF. A third protocol was
used to investigate the promoting activity of the test
chemicals after initiation by pre-treatment with dimethyl-
nitrosamine. This, too, successfully separated BP from
PYR, but, although 2AAF produced an increase in GGT-
positive foci, a weak positive response was also observed
with 4AAF. The two other procedures used to study
initiation/promotion events based on altered enzyme foci
failed to detect BP in one assay and 2AAF in the second
assay and are considered to be, primarily, research
techniques and not yet suitable for routine application.
The two better-established assays showed quite clearly
that both BP and 2AAF initiated altered enzyme foci in rat
liver. The two presumed non-carcinogens were completely
negative and these tests, although they cannot be regarded
as "short-term" in the true sense, have a useful role in
investigating liver carcinogenesis.
8.2.2 Unscheduled DNA synthesis and S-phase analysis
The investigators in this group used the detection of
unscheduled DNA synthesis (UDS) as a measure of DNA repair
initiated in response to chemical-induced damage to the
DNA. In each study, UDS was detected using autoradio-
graphic techniques that also allowed simultaneous measure-
ment of S-phase cells, i.e. those cells undergoing normal
DNA synthesis as a prelude to mitosis. Seven investi-
gators examined 2AAF and 4AAF for the induction of UDS and
S-phase cells in hepatocytes of male rats, and in two
laboratories all four test chemicals were investigated in
rats and mice. The induction of UDS and S-phase cells in
rat fore-stomach was investigated in another laboratory.
Although there were minor protocol variations, the UDS
working group concluded that none of these significantly
affected the results and there was excellent agreement in
the observations and conclusions of the investigators.
2AAF was shown to be a potent inducer of UDS in rat
hepatocytes and also caused proliferation of hepatic cells
as shown by an increase in the number of cells in S-phase.
In mice, however, 2AAF failed to induce SCE in either of
two laboratories, though there was evidence of a weak
induction of S-phase cells. Only low doses were examined,
i.e. up to 50mg/kg, but the results indicate an important
species difference in the response to 2AAF.
Administration of 4AAF to rats produced negative UDS
results in four laboratories and weak positive results in
three laboratories. Although the three weak positive
results were initially determined without recourse to
statistical analysis, a comprehensive analysis of the data
by Margolin and Risko (1988) confirmed the initial
interpretation. The six investigators who recorded the
number of S-phase cells observed a marked increase in
hepatic cell proliferation in rats dosed with 4AAF. This
compound failed to induce UDS in mouse liver but did cause
an increase in S-phase cells.
For comparative purposes, two laboratories also con-
ducted UDS tests on hepatocytes in vitro. Both 2AAF and
4AAF induced UDS in rat hepatocytes but were negative in
mouse cells.
BP and PYR were tested in rats by two investigators
and one assay was conducted in mice. Both chemicals
failed to induce UDS in either species after oral dosing.
In contrast, BP gave positive results in both rat and
mouse hepatocytes treated in vitro.
In an investigation of the UDS activity of the four
chemicals in rat fore-stomach, 2AAF, 4AAF, and PYR failed
to induce UDS while BP gave equivocal results.
The performance of the UDS assay in rodents dosed with
2AAF or 4AAF is worthy of further consideration. 2AAF is
a potent rat hepatocarcinogen and a potent inducer of UDS
in rat hepatocytes. In the mouse, however, 2AAF did not
induce UDS and, on balance, available data suggest that it
does not produce liver tumours. Thus, for this chemical,
the UDS results accurately reflect its hepatocarcinogenic
potential. The current lack of cancer data on 4AAF pre-
cludes this kind of comparison, but the UDS data suggest
that if 4AAF is carcinogenic, it is markedly less so than
2AAF.
Although fewer tests were conducted with BP and PYR,
the data indicate that neither chemical induces UDS in rat
or mouse liver, although BP was positive in in vitro UDS
tests. The reason for this negative response to BP has an
important bearing on the validity of the UDS assay for
detecting potentially carcinogenic chemicals. Although BP
is an established carcinogen, it has never been shown to
produce tumours in rodent liver. There is sufficient
evidence, however, to suggest that DNA-reactive adducts
are formed in liver cells after dosing rodents with BP.
For example, most of the data that show BP to be genotoxic
in vitro are derived from tests in which metabolic acti-
vation is provided by an enzyme mixture derived from rat
liver. In addition, BP clearly induces altered enzyme
foci in rat liver, there is tentative evidence for the
induction of DNA strand breakage based on DNA-DNA/DNA-
protein crosslinks, and 32P-post-labelling techniques in-
dicate the presence of DNA adducts of BP metabolites in
both rat and mouse liver. Therefore, although BP is not a
hepatocarcinogen in rodents, there are data to suggest
that (a) BP is metabolized in rodent liver to DNA-reactive
metabolites, (b) interaction of DNA with BP metabolites
does occur, and (c) based on data from the DNA unwinding
test, some repair of the DNA lesions takes place. On this
basis, the liver UDS assay should, theoretically, be
capable of detecting BP-induced lesions in the liver. All
these observations indicate that such factors as the
distribution of BP to the liver after oral dosing, the
rate of formation of genotoxic metabolites, the rate of
detoxification, and the rate and nature of the DNA repair
process yield an insufficient number of DNA adducts to
produce a detectable UDS response.
In practice, of course, BP is easily detected in other
in vivo tests such as those conducted in bone marrow
cells. However, the results with BP and, to a lesser
extent, 4AAF, suggest the need for additional evaluation
of the performance of the in vivo UDS assay with liver-
specific genotoxins.
8.2.3 DNA strand breaks
Data were considered from three different assay sys-
tems used to detect single-strand breaks in DNA. Results
from the alkaline elution assay, in which two laboratories
found no activity with any of the four test chemicals,
indicate that this assay is of little value in investigat-
ing in vivo genotoxicity. The test for DNA-DNA/DNA-protein
crosslinks and the DNA unwinding assay in rodent liver
cells both gave very promising results and warrant further
evaluation.
8.2.4 Cytogenetics
Three sets of data were considered in this group.
Micronucleus assays were conducted in rat liver cells
following partial hepatectomy. The results were similar to
those obtained in the rat liver UDS assay, i.e. a dose-
dependant response was obtained with 2AAF, while the
remaining three chemicals gave negative results. The
other two data sets were generated from analyses of
chromosome aberrations and SCE in epithelial-like cells
derived from weanling rat liver. Although the assays are
relatively simple with clearly defined end-points, the
results were either equivocal or negative and the sample
sizes, i.e. the numbers of animals/cells per dose group,
were too small to permit an effective assessment. Both
the micronucleus test and the chromosome assays are worthy
of further evaluation.
8.3 Miscellaneous assays
This section contains a wide variety of assays, some
of which have been in use for many years while others, few
of which were undertaken in more than one laboratory, are
relatively new or in the process of development.
8.3.1 Specific carcinogenicity assays
Neither of the two procedures considered here can be
regarded as short-term tests and final results from one of
them, the assay in quail eggs, were not available at the
time of the assessment. The results of the mouse adenoma
assay, however, were available and showed a decisive dis-
crimination between BP and PYR and a much smaller and less
convincing difference between 2AAF and 4AAF.
8.3.2 Supplementary assays
Data from the sebaceous gland suppression test and the
epidermal hyperplasia assay have shown a good correlation
with skin carcinogenicity and have proved useful for com-
paring the carcinogenic potential of polycyclic hydrocar-
bons. Their value in this area was confirmed by the re-
sults of assays with BP and PYR but the application of
these assays to other classes of chemicals is of uncertain
value.
The hepatocytes of rodents include mono- and binu-
cleated cells and nuclei that may be diploid, tetraploid,
or octaploid. The ratio of cells with different levels of
ploidy in liver varies with species and age and can be
altered by exposure to certain chemicals. There have been
references to the effects of various chemicals on liver
ploidy ratios over a number of years, but there appears to
be no consistent relationship between ploidy changes and
chemical carcinogenesis. Thus the observation that the two
carcinogens, BP and 2AAF, increase the ratio of diploid to
tetraploid cells is of uncertain significance. The assay
may be of value in studying liver cell kinetics but its
value in the detection of in vivo genotoxicity is debat-
able.
The general principles of the in vivo/in vitro somatic
mutation assay were developed in the late 1970s and showed
initial promise as a test for investigating organ-specific
mutations induced by chemical carcinogens. One
investigator provided data from a modification of this
technique in which cells isolated from lung tissue of
treated Syrian hamsters were cultured in vitro and the
induction of mutations was determined. A reproducible
increase in mutations was observed in lung cells after
dosing with BP, but the results with 2AAF and the two non-
carcinogens were considered to be negative. Although this
is a logical and potentially useful approach to studying
in vivo genotoxicity, the method requires much wider
evaluation before it can be considered as an acceptable
assay.
The detection and identification of DNA adducts
provides conclusive evidence that a metabolite of the
administered chemical has interacted with one of the
important target macromolecules of carcinogenesis. Theor-
etical considerations indicate that, for many chemical
classes, the formation of DNA adducts is a prerequisite
for permanent genetic changes, such as mutations, and for
the initiation stage of carcinogenesis. Research has shown
a direct relationship between the administered dose of a
chemical and DNA-adduct formation, so that precise dosi-
metry in specific organs is possible. Three approaches
were used in the study, though results from a novel tech-
nique using monoclonal antibodies to identify specific
adducts are not yet available. One study involved the use
of radiolabelled 2AAF and 4AAF; while binding to liver DNA
was detected with both compounds, the covalent binding of
2AAF was approximately seven times that of 4AAF. These
data correlate well with the results of the UDS assay in
rat liver, which showed 2AAF to be a potent inducer of UDS
while 4AAF was, at best, a weak inducer.
The above assay has the disadvantage of requiring
radiolabelled test chemicals, and a significant advance in
the field was the introduction of techniques in which
animals are dosed with unlabelled test chemicals and the
DNA is radiolabelled after isolation and purification. The
32P-labelled normal nucleotides and nucleotide adducts
are separated by thin-layer chromatography and detected by
autoradiography, and a comprehensive, organospecific DNA
adduct profile is obtained. The data presented in the
current study showed that 2AAF adducts were detected at
significant levels in the DNA of mouse liver, lung, and
kidney and in rat liver and lung. 4AAF adducts were not
detected in mouse tissue and at only very low levels in
rat liver. PYR adducts were not detected in either rat or
mouse tissues and BP produced adducts with DNA in liver
and lung of both species and in mouse kidney.
The detection and identification of DNA adducts is
potentially an extremely powerful tool in the investi-
gation of in vivo genotoxicity. With the establishment of
`cold' techniques, i.e. those not requiring radiolabelled
test chemicals, this type of assay can be applied to most
chemical classes. Although further development is re-
quired, particularly with regard to the genotoxic signifi-
cance of different types of adduct, the method shows great
promise for the future.
8.3.3 Immunotoxicity assays
In the Natural Killer (NK) cell assay, the activity of
NK cells was measured at intervals after dosing rats with
either 2AAF or 4AAF. The carcinogen, 2AAF, induced a
significant change in NK cell cytotoxicity that was not
observed after dosing with 4AAF. The T-cell assay, in
which immunocompetent T-cells are able to react to
carcinogen-induced tissue changes, responded positively to
BP and 2AAF; PYR failed to induce any change in reactivity
and 4AAF gave a weak positive response. The results of
both types of assay closely reflected the carcinogenic
activity of the test chemicals. The relevance of the
observed immunological changes to the carcinogenic process
is unclear and, thus, their potential value in detecting
precarcinogenic changes caused by genotoxic and non-
genotoxic carcinogens remains to be fully evaluated.
Before they can be accepted as anything more than a useful
research tool, further investigations of the specificity
of the immunological reactions and a wider evaluation are
imperative.
8.3.4 Host-mediated assays and urine mutagenicity tests
As described at the beginning of Section 8, both these
classes of test failed to meet the criteria for an accept-
able short-term in vivo assay. The host-mediated assay was
a favoured in vivo procedure in the early 1970s, though in
recent years questions have been raised concerning its
sensitivity. Although the intrasanguinous modification to
the original intraperitoneal technique offered theoretical
advantages, the results of the present study confirmed the
inadequacy of the assay. It is concluded that both these
procedures are not appropriate for the investigation of in
vitro genotoxins.
8.4 Mouse spot tests
Although data were presented from two types of mouse
spot test, they are, in effect, different means of observ-
ing the same genetic alterations, i.e. the melanocyte test
detects the change at the cellular level while the coat
colour test demonstrates the expression of the cellular
changes.
BP and PYR were only tested in one laboratory and the
spot test successfully identified the carcinogen. In tests
with 4AAF, one investigator's results were considered to
be inconclusive and four other sets of data, including one
after oral dosing and three after intraperitoneal admin-
istration, were negative. Six laboratories tested 2AAF;
there were three positive results and two negatives after
intraperitoneal administration. 2AAF was also negative
after oral dosing. Examination of the protocols indicated
that 2AAF induced detectable genetic changes only after
administration of the material as a solution in dimethyl-
sulfoxide, i.e. negative results followed the admin-
istration of corn oil formulations of 2AAF, illustrating
the critical effect of the vehicle on the absorption of
the test chemical.
An overview of the mouse spot test data indicates that
intraperitoneal administration of an appropriate formu-
lation of the test chemicals produces results that reflect
their carcinogenic activity. The original coat colour
procedure appears to be more reliable than the melanocyte
technique, which may be influenced by sporadic mutational
events and inadequate melanization of the hair follicles.
However, the dependence on the intraperitoneal route of
administration may be a disadvantage, and the mouse spot
test appears to add very little to the information gained
more easily from bone marrow cell cytogenetic assays.
8.5 Assays in mammalian germ cells
For assessment purposes the germ cell assays have been
separated into two groups: those that are considered to
involve a direct interaction with DNA, and the assay for
sperm abnormalities, the genetic significance of which is
uncertain.
8.5.1 Dominant lethal and unscheduled DNA synthesis assays
Classical dominant lethal tests were conducted in male
or female mice and in male rats. PYR, 2AAF, and 4AAF gave
unequivocally negative results after intraperitoneal
dosing. In an oral dosing study in male mice, BP was nega-
tive and PYR produced inconclusive data. After intraper-
itoneal administration of BP, however, there was conclus-
ive evidence of dominant lethal induction in male and
female mice. In the male, dominant lethality was confined
to the late spermatid/spermatozoa stages of spermatogen-
esis.
Results of UDS assays in rat spermatocytes after oral
dosing and in mouse spermatocytes after oral or intraper-
itoneal administration were negative with all four chemi-
cals. The negative data with BP are not altogether sur-
prising as dominant lethality was only observed in late
spermatids/spermatozoa, in which the DNA is highly con-
densed and in which UDS does not occur.
Based on the dominant lethal test results, BP is a
confirmed, though relatively weak, germ cell mutagen in
mice after intraperitoneal dosing. Published data indicate
that BP does not induce heritable translocations at doses
that produce dominant lethality (Generoso et al., 1982),
although this may be due, in part, to the fact that BP
appears to induce mainly chromatid deletions in bone
marrow cell chromosomes, with very few exchange-type aber-
rations.
8.5.2 Sperm abnormality tests
This assay is based on the observation of morphologi-
cal alterations in mature spermatozoa after treatment of
animals at all stages of spermatogenesis with the test
chemicals. BP induced an increase in the incidence of
abnormal sperm in mice after oral or intraperitoneal
dosing and 2AAF was also positive following oral gavage.
Tests with 4AAF were uniformly negative and PYR produced a
weak positive response in one of four assays. Thus, both
carcinogens produced sperm abnormalities under appropriate
experimental conditions.
The important questions raised by these observations
relate to the relevance of morphological abnormalities in
sperm to germ cell mutations and to carcinogenesis.
Although changes in sperm head shape may be genetically
determined, it is uncertain whether they are due to geno-
toxicity or to other toxic phenomena. A positive result
in the test, therefore, indicates that the test material
or its metabolites are able to reach the germ cells in
sufficient quantities to induce an adverse biological
effect. Although a good correlation has been shown between
the induction of sperm abnormalities and carcinogenic
activity (Wyrobek et al., 1983), the relevance of this
correlation to short-term testing strategies is doubtful
as more appropriate in vivo tests are available, e.g.,
bone marrow cytogenetic assays. Equally doubtful is the
relevance of the test to heritable genetic damage, as
there is no clear-cut evidence that abnormal sperm are a
consequence of induced DNA damage.
8.6 Drosophila assays
The sex-linked recessive lethal assay and the test for
chromosome loss in drosophila germ cells failed to show a
consistent separation of BP and 2AAF from PYR and 4AAF,
respectively. Three tests based on somatic cell changes
were positive with BP and negative with PYR but the
results of tests with the acetylaminofluorenes were less
decisive. 2AAF produced an unambiguous positive result
and two weak positives, while the three results with 4AAF
were weak positive, negative and inconclusive. Thus, the
drosophila germ cell assays were insensitive to the four
in vitro genotoxins and, although the somatic cell pro-
cedures appeared to be more sensitive, it was concluded
that the main value of drosophila tests is to provide com-
prehensive analyses of the genetic mechanisms involved in
the induction of mutations by chemicals.
9. SELECTION OF THE MOST EFFECTIVE IN VIVO ASSAYS IN RELATION
TO THEIR PERFORMANCE
In a study of this nature it is inevitable that some
assays, even well-established procedures, will fail to
perform well. Indeed, the recognition of inadequate tests
is as important a goal as the identification of those that
are useful and acceptable. Other assays produced results
that correlated well with the presumed carcinogenic status
of the test chemicals but are not widely used or available
or are still in the process of development. Yet other
tests that performed adequately in the collaborative study
cannot be regarded as practicable short-term tests either
because of their technical complexity or because of the
length of time required to produce results. Most of the
latter group of tests have important research roles in
carcinogenesis.
9.1 Assays that are not considered appropriate for routine
in vivo testing of chemicals for genotoxic activity
Host-mediated and urine mutagenicity assays (section
8.3.4), the peritoneal macrophage transformation assay,
and the alkaline elution assay for single strand breaks
(section 8.2.3) proved incapable of separating the car-
cinogen/non-carcinogen pairs and appear to have little
value in investigating in vivo genotoxicity. The in
vivo/in vitro somatic mutation procedure in Syrian
hamsters (section 8.3.2) also falls into this category,
although this conclusion should not detract from the
initial promise of this approach in Chinese hamsters
(McGregor, 1988a).
Drosophila tests (section 8.6) have also proved gener-
ally inadequate in the identification of in vivo geno-
toxins and it is concluded that their main value in gen-
etic toxicology is to provide analysis of the genetic
mechanisms involved in the induction of mutations. Similar
reservations apply to the initiation/promotion techniques
using liver foci (section 8.2.1) and the analysis of
ploidy levels in liver tissue (section 8.3.2). Both assays
are more appropriate to investigation of the carcinogenic
process than to the identification of in vivo genotoxins.
The results of the immunological assays (section 8.3.3)
closely reflected the carcinogenic potential of the
chemicals, but they can only be regarded as research tools
pending a greater understanding of the specificity of the
immunological reactions and a wider evaluation of the
procedures.
Two assays conducted in mouse skin, i.e. the seb-
aceous gland suppression test and the observation of epi-
dermal hyperplasia, confirmed their value in the detection
of carcinogenic polycyclic hydrocarbons such as BP. Both
tests failed to respond to 2AAF when applied dermally and
are not considered to be suitable for investigating in
vivo genotoxicity in general.
9.2 Assays that satisfy some or all of the criteria for an
acceptable short-term in vivo test
9.2.1 Assays currently in general use
The mouse micronucleus test (section 8.1.2) was the
most widely represented single assay in the study and
produced the best overall performance with only occasional
anomalous data. Qualitative results were not affected
significantly by the route of exposure, strain or sex of
mouse, or treatment schedule. The mouse bone marrow
micronucleus assay was confirmed as a robust and sensitive
short-term in vivo test for genotoxic chemicals.
Both the micronucleus test and the analysis of meta-
phase chromosome aberrations in rodent bone marrow respond
to similar chromosome breakage events and they would be
expected to produce qualitatively similar results with the
four test compounds. Table 4 shows that this was not the
case and the metaphase chromosome procedure was signifi-
cantly less sensitive than the micronucleus test. Poss-
ible explanations for this lack of sensitivity are dis-
cussed above (section 8.1.1) and suggest that the use of
metaphase chromosome analysis should not be undervalued on
the basis of these data.
The sister chromatid exchange (SCE) procedure repro-
ducibly detected the two carcinogens, but also produced a
number of weak positive results with 4AAF and PYR. Taken
at face value, therefore, the in vivo SCE results clearly
reflect the relative potency of the two chemical pairs in
in vitro tests. As discussed in section 8.1.3, however,
very weak SCE responses may be caused by non-genotoxic
mechanisms and a final judgement on the significance of
the weak activity of 4AAF must be delayed until its true
carcinogenic status is defined.
After considering the data from the three classes of
bone marrow assay, the conclusion of the Steering Group
(Ashby et al., 1988) was - `the mouse micronucleus assay
provides the simplest and most effective measure of geno-
toxicity in the bone marrow and, as such, it is rec-
ommended here as a primary in vivo genotoxicity test'.
Despite the successful performance of the micronucleus
test with these four chemicals, it is apparent from the
literature that certain liver carcinogens do not induce
detectable chromosomal effects in the bone marrow. In an
acceptable testing strategy, therefore, negative results
in the micronucleus test should be supplemented with
evidence of non-genotoxicity in the liver. This concept
led to the extensive evaluation of liver-specific assays
in the CSSTT/2 study and the main candidate in this group
of tests was the rat liver UDS assay (section 8.2.2).
The liver carcinogen, 2AAF, was a potent inducer of
UDS in rat liver while most investigators defined its
presumed non-carcinogenic analogue, 4AAF, as a weak
genotoxin in this system. Unlike the micronucleus test
result, BP was devoid of activity in liver UDS assays.
This is consistent with its lack of carcinogenicity in rat
liver.
Although the significance of the weak liver UDS
activity of 4AAF will only be resolved when definitive
carcinogenicity data are available, the findings raise
certain problems of interpretation that are common, in
part, to the weak bone marrow activity of 4AAF and PYR.
In the UDS assays, 2AAF was a potent inducer of UDS at
doses between 5 and 100 mg/kg while 4AAF showed only weak
activity between 50 and 1000 mg/kg, and then only in some
experiments. In general, the activity of 4AAF was within
the range of control variability and, as a result, the
UDS Working Group questioned its biological significance
(Mirsalis, 1988). A detailed analysis, however, indicated
the high statistical significance of the 4AAF data
(Margolin and Risko, 1988). Thus, although the rat liver
UDS assay discriminated quantitatively between 2AAF and
4AAF, the significance of the weak activity of 4AAF
remains unresolved. These observations suggest that
qualitative determinations in terms of `positive' and
`negative' are too simplistic for hazard assessment
purposes and one of the most useful points raised by the
present study is the need for appropriate positive test
criteria, in biological and statistical terms, for widely
used in vivo tests.
Thus, the mouse bone marrow micronucleus assay is the
method of choice for primary in vivo testing of in vitro
genotoxins and the rat liver UDS assay, providing the
above problems can be resolved, appears to be the most
promising complementary liver-specific test.
9.2.2 Assays that show promise for future development
Accepting that bone marrow assays are suitable for
detecting extra-hepatic somatic cell genotoxins and that
the rat liver UDS assay can, with certain reservations
(see sections 8.2.2 and 9.2.1), be used to investigate
liver-specific genotoxins, it is useful to consider which
other assays evaluated in this study may have a role in
the assessment of in vivo genotoxicity.
Development of assays to detect single-strand breaks
in DNA and to detect and identify DNA adducts will,
undoubtedly, continue. It will be interesting to follow
the progress of the assays for DNA-DNA/DNA-protein cross-
links and DNA unwinding, although it is probable that
their technical complexity will limit widespread use and
acceptance. Of the DNA adduct techniques represented, the
post-labelling method appears to hold most promise. The
need for radiolabelled test compounds or specific mono-
clonal antibodies is eliminated and the assay provides
information on the direct interaction of test chemicals
with DNA in a variety of target organs, including the
liver. Providing a reliable and robust protocol can be
established and pending the provision of more information
regarding the relationship between DNA adduct formation
and genotoxic changes to the DNA, this assay could have an
important role to play in the assessment of genotoxic
hazard.
The performance of the cytogenetic assays in liver
cells was disappointing but, theoretically, they could,
with further development, prove to be of value in the
assessment of liver-specific genotoxicity. The reliance
of the current micronucleus procedure on partial hepa-
tectomy is a disadvantage, but the development of alterna-
tive means of stimulating hepatic mitotic activity, either
in vivo or in short-term culture, could be the basis of a
useful short-term liver-specific assay. Cytogenetic
assays on circulating blood cells performed reasonably
well and the theoretical and practical advantages of tests
for SCE or micronuclei in circulating blood should
encourage further development of these tests.
The mouse spot test, in its original form (section
6.4), successfully separated the carcinogen/non-carcinogen
pairs and, providing care is taken in the selection of an
appropriate solvent or formulation, it appears to be a
useful and reproducible assay. Its availability is limited
by the need to maintain specific mouse strains and its
overall sensitivity, compared to the micronucleus test, is
suspect, as positive results in the present study were
dependant on intraperitoneal dosing.
9.3 The detection of germ cell mutagens
The performance and significance of the three germ
cell assays represented in the study are discussed above
(section 8.5). Only BP was clearly identified as a germ
cell mutagen in the dominant lethal assay. The failure of
the sperm abnormality test to discriminate between BP and
2AAF suggests that this assay is affected by non-genetic
toxic effects and is, therefore, of doubtful value in
predicting the induction of mutations in mammalian germ
cells.
Although there are theoretical reasons why BP should
not induce UDS in germ cells (section 8.5.1), this nega-
tive result raises questions similar to those resulting
from the failure of BP to induce UDS in rat liver (section
8.2.2), i.e. why does BP not induce detectable UDS in
target cells where there is evidence, from other assays,
of genotoxic activity?
As concluded by Ashby et al. (1988) `the germ cell
activities of BP have served to focus several important,
but unresolved, issues associated with the conduct of, and
the interpretation of data derived from, germ cell
genotoxicity and mutagenicity assays'.
9.4 Influence of the route of administration of the test chemicals
A feature of the micronucleus assays was that their
qualitative separation of BP and 2AAF from PYR and 4AAF
was not influenced by the route of administration of the
chemicals. Dominant lethal mutations, however, were only
detected after intraperitoneal dosing with BP; oral dosing
was ineffective. Similarly, the mouse spot test was
dependant on the intraperitoneal route of administration
for the demonstration of mutagenic effects. The intro-
duction of the test material in the vicinity of the uterus
in the spot test circumvents the natural pharmacokinetics
and metabolic routes of the test animal, and it can be
argued that this unrealistic pattern of exposure does not
reflect true in vivo genotoxicity. It can be similarly
argued that the purpose of in vivo tests is to achieve
maximum exposure of the target cells and that intraper-
itoneal dosing can be justified for this reason. It may
be, as suggested by Shelby (1986), that intraperitoneal
dosing should be reserved for assays used in a screening
mode, i.e. for the detection of genotoxic activity, and
that for investigating the in vivo activity of established
genotoxins, a route of administration should be chosen
that reflects possible human exposure. Arguments such as
these must be resolved before strategies for testing
chemicals for genotoxic activity can be fully harmonized.
10. CONCLUSIONS
Of the fifty or so separate in vivo techniques rep-
resented in the CSSTT/2 study, only a small number satis-
fied the criteria defined for an acceptable short-term in
vivo test (section 3.2). These criteria, however, defined
a very specific purpose, i.e. the identification of in
vivo activity of established in vitro genotoxins. In order
to generate a comprehensive data base on the four test
chemicals, assays included in the study were not limited
to, for example, those that were considered most likely to
meet the criteria. As a result, some investigators sub-
mitted data from assays that were not designed for detec-
ting in vivo genotoxins but, nevertheless, provided valu-
able information on a broad spectrum of biological effects
of the test chemicals. The failure of such tests to
satisfy the narrow selection criteria used in CSSTT/2
should not be regarded as a reflection on the validity of
the procedures for specific research purposes. With these
considerations in mind, and based on the concept (section
3.2) that short-term assays are required to investigate
both liver-specific and extra-hepatic genotoxicity, the
following conclusions appear appropriate.
1. Most of the in vivo somatic cell assays for genotox-
icity were able to discriminate between the two carcino-
gen/non-carcinogen pairs, although, in some instances,
weak activity was observed in tests with the non-
carcinogens, particularly with 4AAF.
2. The weak genotoxicity of 4AAF in some studies
suggested that qualitative determinations in terms of
`positive' and `negative' are too simplistic for hazard
assessment purposes and highlights the need for appropri-
ate positive test criteria, in biological and statistical
terms, for widely used in vivo tests.
3. The insensitivity of some assays to one or other of
the two pairs of compounds supports the concept that nega-
tive in vivo data should be produced from at least two
assays in different tissues, i.e. one in extra-hepatic
somatic tissues and one in the liver, if they are to be
used for assessing genotoxic hazard.
4. The mouse bone marrow micronucleus test was confirmed
as a robust and sensitive assay and is the assay of choice
for primary in vivo testing of in vitro genotoxins.
5. The overall performance of the rat liver UDS assay
suggests that it could be complementary to the micro-
nucleus test providing the reservations raised above
(sections 8.2.2 and 9.2.1) can be satisfied. Development
and evaluation of alternative procedures for investigating
liver genotoxicity should be actively pursued.
6. Certain widely advocated assays, including the host-
mediated and urine mutation assays and tests using
drosophila are concluded to be inappropriate for hazard-
assessment purposes.
7. BP and 2AAF showed genotoxic activity in the organs in
which they also produce tumours. Their genotoxic activity
in other tissues, however, precludes the use of data from
short-term tests for making predictions of organ-specific
carcinogenicity.
8. The majority of the positive responses observed in
this study were obtained after oral administration of the
test chemicals and at doses substantially below lethal
levels, suggesting that short-term in vivo assays are not
intrinsically insensitive, as is sometimes inferred. Posi-
tive results were obtained in the dominant lethal assay
and the mouse spot test after intraperitoneal dosing only;
both assays produced negative results after oral admin-
istration. These facts suggest that the intraperitoneal
route should be reserved for the detection of genotoxic
activity and that, for hazard-assessment purposes, a route
of administration should be chosen that is relevant to the
perceived route(s) of human exposure.
9. The germ cell mutagenicity exhibited by BP was
adequately predicted by the somatic cell mutagenicity
assays, adding weight to the suggestion that tests on
mammalian germ cells should only be conducted to gain
further information on the mutagenic spectrum of estab-
lished in vivo somatic cell mutagens.
10. The occurrence of chemical carcinogens that fail to
show evidence of genotoxicity in in vitro and in vivo
assays indicates the existence of a class of chemicals
that induce cancer by a mechanism that is not a conse-
quence of a direct interaction with DNA. The acceptance
of the validity of the concept of non-genotoxic mechanisms
of cancer induction is considered to be a crucial question
in the future deployment of short-term tests in carcino-
genesis.
11. The overall conclusion from the CSSTT/2 study is that
short-term in vivo tests have a vital role to play in
hazard assessment. This role is to define which
chemicals, identified as genotoxic from in vitro tests,
are active in vivo and, thus, are those most likely to
present a carcinogenic/mutagenic hazard to mammals,
including humans.
REFERENCES
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr,
MARGOLIN, B.H., MATTER, B., & SHELBY, M.D., ed. (1985) Evaluation of
short-term tests for carcinogens, Amsterdam, Oxford, New York, Elsevier
Science Publishers (Progress in Mutation Research, Vol. 5).
ASHBY, J., DE SERRES, F.J., SHELBY, M.D., MARGOLIN, B.H.,
ISHIDATE, M., Jr, & BECKING, G.C., ed. (1988a) Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 1.
ASHBY, J., SHELBY, M.D., & DE SERRES, F.J. (1988b) Overview of the IPCS
collaborative study on in vivo assay systems and conclusions. In: Ashby,
J., de Serres, F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, &
Becking, G.C., ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative study on
in vivo assays, Cambridge, United Kingdom, Cambridge University Press, Vol.
1, pp. 6-28.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-term tests for
carcinogens: Report of the international collaborative study, Amsterdam,
Oxford, New York, Elsevier Science Publishers (Progress in Mutation
Research, Vol. 1).
GENEROSO, W.M., CAIN, K.T., HELLWIG, C.S. & CACHEIRO, N.L.A. (1982)
Lack of association between induction of dominant-lethal mutations and
induction of heritable translocations with benzo (a) pyrene in postmeiotic
germ cells of male mice. Mutat. Res., 94: 155-163.
HICKS, M.R., ASHBY, J., & PENMAN, M. (1988) Review of the rodent
carcinogenicity data for the four test chemicals. In: Ashby, J., de Serres,
F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C.,
ed. Evaluation of short-term tests for carcinogens: Report of the
International Programme on Chemical Safety's collaborative study on in vivo
assays, Cambridge, United Kingdom, Cambridge University Press, Vol. 2, pp.
351-365.
MCGREGOR, D. (1988a) Summary report on the performance of the
miscellaneous group of in vivo assays (carcinogenicity, DNA adducts,
immunological, host-mediated mutation, urinary mutation assays, and limited
metabolic assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 2, pp. 3-21.
MCGREGOR, D. (1988b) The activities of 2AAF, 4AAF, BP and PYR in in
vitro assays for genetic toxicity. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 2, pp. 345-350.
MARGOLIN, B.H. & RISKO, K.J. (1988) The statistical analysis of in
vivo genotoxicity data: case studies of the rat hepatocyte UDS and mouse
bone marrow micronucleus assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 29-42.
MIRSALIS, J.C. (1988) Summary report on the performance of the in vivo
DNA repair assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests
for carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 1, pp. 345-351.
PATON, D. & ASHBY, J. (1988) Source and analytical details of the test
chemicals. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin, B.H.,
Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests for
carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 2, pp. 341-344.
SHELBY, M.D. (1986) A case for the continued use of the intraperitoneal
route of exposure. Mutat. Res., 70: 169-171.
TICE, R.R. (1988) Summary report on the performance of the in vivo
sister chromatid exchange assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 233-253.
WHO (1985) Environmental Health Criteria 47: Summary report on the
evaluation of short-term tests for carcinogens (collaborative study on in
vitro tests), Geneva, World Health Organization, 77 pp.
WYROBEK, A.J., GORDON, L.A., BURKHART, J.G., FRANCIS, M.W.,
KAPP, R.W., LETZ, G., MALLING, H.V., TOPHAM, J.C., & WHORTON, M.D.
(1983) An evaluation of the mouse sperm morphology test and other tests in
non-human mammals: A report of the United States Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 115: 1-7.
RESUME
ETUDE COLLECTIVE DU PROGRAMME INTERNATIONAL SUR LA SECURITE DES
SUBSTANCES CHIMIQUES (IPCS) POUR L'EVALUATION ET LA VALIDATION
DES EPREUVES DE COURTE DUREE RELATIVES AUX CANCEROGENES
La première partie de ce projet, qui traite des études
in vitro, a été publiée en 1985 (Ashby et al., 1985); on
en trouvera un résumé dans le No 47 de la série Critères
d'hygiène de l'environnement (OMS 1985). La seconde
partie, qui fait l'objet du présent rapport, a été publieé
en 1988 (Ashby et al., 1988).
Devant la nécessité d'évaluer l'intérêt des épreuves
de courte durée pour la recherche des substances mutagènes
et cancérogènes, il devenait indispensable d'organiser des
études collectives inter-laboratoires à l'échelle inter-
nationale. Ces épreuves de courte durée devaient compléter
les épreuves classiques à long terme sur rongeurs ou s'y
substituer. C'est le problème du choix ainsi que de la
fiabilité et de la sensibilité de ces épreuves qui a
conduit à la première grande entreprise de collaboration
au niveau international, à savoir le Programme coopératif
international pour l'évaluation des épreuves de courte
durée relatives aux cancérogènes (IPESTTC) (de Serres &
Ashby, 1981). Les résultats de cette étude ont confirmé
la fiabilité et la faisabilité de l'épreuve de mutation
des salmonelles pour une première identification des sub-
stances cancérogènes et mutagènes. On a également con-
staté qu'avec cette épreuve, certains produits notoirement
cancérogènes chez les rongeurs étaient impossibles ou tout
du moins très difficiles à mettre en évidence. Un certain
nombre d'autres épreuves examinées dans le cadre de
l'étude IPESTTC se sont révélées capables de mettre en
évidence quelques-uns des cancérogènes murins pour
lesquels l'épreuve sur salmonelle donnait un résultat
négatif. Toutefois, la base de données était trop res-
treinte pour qu'on puisse recommander une épreuve capable
de compléter l'épreuve de mutation des salmonelles.
Au vu des résultats de l'étude IPESTTC, il est apparu
nécessaire de poursuivre ce type de coopération afin
d'établir a) quel est l'ensemble d'épreuves in vitro le
plus efficace pour un premier tri des substances chimiques
sur la base de leur activité génotoxique et b) quelles
sont les épreuves in vivo de courte durée les plus utiles
pour confirmer la génotoxicité et le pouvoir cancérogène
des substances chimiques chez les mammifères. L'étude
collective pour l'évaluation et la validation des épreuves
de génotoxicité et de cancérogénicité de courte durée
(CSSTT), a été proposée par le Programme international sur
la sécurité des substances chimiques (IPCS) et le National
Institute of Environmental Health Sciences (NIEHS) des
Etats-Unis d'Amérique, l'une des institutions qui
participent au Programme IPCS. Devant l'ampleur de cette
entreprise et du fait des problèmes logistiques que posent
l'organisation et la gestion de ce genre d'études inter-
nationales, le projet a été divisé en deux études
distinctes: l'Etude collective sur les épreuves de géno-
toxicité et de cancérogénicité de courte durée in vitro
(CSSTT/1) et l'Etude collective sur les épreuves de
mutagénicité et de cancérogénicité de courte durée in vivo
(CSSTT/2).
L'Etude CSSTT/1 a permis de constituer une vaste base
de données à partir d'épreuves in vitro très diverses
portant sur dix produits chimiques organiques. Parmi eux
figuraient huit substances de cancérogénicité reconnue
pour les rongeurs qui s'étaient révélées soit négatives à
l'épreuve sur salmonelle soit difficiles à reconnaître de
cette manière, ainsi que deux substances considérées comme
non cancérogènes. On a procédé à l'évaluation de données
tirées de 90 groupes d'épreuves effectuées par quelque
60 scientifiques. Quatre types d'épreuves ont donné des
résultats suffisamment bons pour qu'on puisse envisager de
les utiliser en complément à l'épreuve sur salmonelle. Il
s'agissait de la recherche des aberrations chromosomiques,
des mutations géniques et des transformations néoplasiques
en culture de cellules mammaliennes ainsi que de l'épreuve
d'aneuploïdie des levures. Sauf dans le cas de la
recherche des aberrations chromosomiques, on a constaté
que les protocoles généralement utilisés pour ces épreuves
devaient être étudiés plus à fond avant d'être considérés
comme tout à fait acceptables.
La principale conclusion de l'étude CSSTT/1 relative
aux épreuves in vitro, c'est que en combinant l'épreuve
de recherche des aberrations chromosomiques à l'épreuve de
mutation des salmonelles, on peut procéder à un premier
criblage efficace des substances cancérogènes.
Lors de l'étude IPESTTC, sept substances sur 14 sup-
posées non cancérogènes ont donné des résultats positifs
dans un grand nombre d'épreuves in vitro. D'après les
données in vivo limitées qu'a fournies cette étude, il
semblerait que ces sept produits soient inactifs dans les
épreuves de courte durée in vivo. Les produits non can-
cérogènes de deux des couples cancérogène/non-cancérogène
soumis à l'étude IPESTTC, à savoir le couple benzo [a] py-
rène/pyrène (BP/PYR) et le couple acétyl-2 aminofluorène/
acétyl-4 aminofluorène (2AAF/4AAF), constituent de bons
exemples de ces réactions différentes et d'ailleurs, ces
couples de produits avaient été retenus pour la partie in
vivo de l'étude collective (CSSTT/2). Toutefois, il y
avait doute quant à la non cancérogénicité supposée du
4AAF et une part très importante de l'étude a été con-
sacrée à la mise en route d'études de cancérogéniticé à
long terme sur le 2AAF et le 4AAF chez le rat.
L'objectif de l'étude CSSTT/2 était donc de produire
un profil de données complet à partir d'une large gamme
d'épreuve in vivo de courte durée afin de voir comment les
différents paramètres génétiques des tissus-cibles se com-
portent vis-à-vis de produits chimiques classés comme
génotoxiques d'après les épreuves in vitro. La finalité
de l'étude était de recenser les épreuves in vivo suscep-
tibles d'être utilisées pour déterminer l'activité in vivo
de substances notoirement génotoxiques.
Quatre-vingt-dix-sept chercheurs de 16 pays ont par-
ticipé à l'étude in vivo, les données présentées portant
sur une cinquantaine de techniques distinctes. Les résul-
tats ont été évalués lors d'une réunion de ces chercheurs
qui s'est tenue au Cap d'Agde (France) en mai 1985. On a
préparé une série de rapports comportant une évaluation de
chaque groupe d'épreuves, des rapports récapitulatifs sur
les épreuves relatives aux cellules germinales et les
épreuves d'hépatotoxicité et des rapports résumant toute
la base de données relative à chaque couple de produits.
Par la suite, on a préparé un récapitulatif de toute
l'étude in vivo en vue de sa publication définitive.
Seule une faible proportion des épreuves examinées
lors de l'étude CSSTT/2 ont satisfait aux critères
d'acceptabilité comme épreuves in vivo de courte durée.
Toutefois les épreuves examinées lors de cette étude ne se
limitaient pas à celles jugées a priori les plus conformes
aux critères. De la sorte, on a pu tirer des données
provenant d'épreuves qui, à l'origine, n'étaient pas des
épreuves de génotoxicité in vivo, des renseignements sur
une large gamme d'effets biologiques produits par ces
quatre substances. La plupart des épreuves de génotoxicité
in vivo portant sur des cellules somatiques permettaient
de distinguer les deux couples cancérogène/non-cancérogène
encore que dans certains cas, les produits non cancéro-
gènes, en particulier le 4AAF aient présenté une faible
activité. L'insensibilité de certaines épreuves à l'un ou
à l'autre des deux couples de produits chimiques, corrob-
orent l'idée selon laquelle il faut obtenir des résultats
négatifs in vivo dans au moins deux épreuves pratiquées
sur des tissus différents avant de considérer qu'un
produit chimique n'est pas génotoxique in vivo.
Il a été confirmé que l'épreuve des micro-noyaux sur
moëlle osseuse de souris est robuste, sensible et repro-
ductible et on la recommande pour une première recherche
de la génotoxicité in vivo et in vitro. Les résultats
généraux obtenus au moyen de l'épreuve sur foie de rat
pour la synthèse anarchique de l'ADN incitent à penser
qu'elle pourrait compléter l'épreuve des micro-noyaux,
mais il faut en étudier encore la sensibilité et la
sélectivité. Certaines épreuves pourtant largement pré-
conisées, notamment les épreuves par passage sur hôte, les
épreuves de mutagénicité des urines et les épreuves sur
drosophile, se sont révelées impropres à une évaluation du
risque.
Les résultats de l'étude CSSTT/2 ont confirmé que les
épreuves in vivo de courte durée ont un rôle capital à
jouer dans l'évaluation du risque, rôle qui consiste dans
l'identification des produits chimiques qui s'étant
révélés génotoxiques in vitro, sont également actifs in
vivo et comportent donc vraisemblablement un risque de
cancérogénicité ou de mutagénicité pour les mammifères en
général et l'homme en particulier.
RESUMEN
PROGRAMA INTERNACIONAL DE SEGURIDAD DE LAS SUSTANCIAS QUIMICAS (IPCS):
ESTUDIO EN COLABORACION SOBRE EVALUACION Y COMPROBACION DE PRUEBAS
A CORTO PLAZO PARA SUSTANCIAS CARCINOGENAS
La primera parte del presente proyecto, relativa a
estudios in vitro, se publicó en 1985 (Ashby et al., 1985) y se
resumió en la publicación 47 de la serie "Environmental
Health Criteria" (OMS, 1985). La segunda parte es el
tema del presente informe y se publicó en 1988 (Ashby et
al., 1988).
La necesidad de estudios en colaboración entre
laboratorios en escala internacional surgió de que era
imprescindible investigar el valor de las pruebas a corto
plazo para detectar las sustancias químicas mutágenas y
carcinógenas. Se propusieron los ensayos a corto plazo
como métodos que sustituyeran o complementaran a los
tradicionales ensayos biológicos a largo plazo en
roedores. La preocupación por la elección de las pruebas
a corto plazo y por su fiabilidad y sensibilidad condujo a
que se realizara el primer ejercicio importante de
colaboración internacional: el programa internacional en
colaboración sobre evaluación de las pruebas a corto plazo
para sustancias carcinógenas (IPESTTC) (de Serres y Ashby,
1981). Los resultados de ese estudio confirmaron el valor
de la prueba de mutación de salmonelas como ensayo fiable
y factible para la identificación primaria de sustancias
carcinógenas y mutágenas. Se observó también que en la
prueba de salmonelas no se detectaban o sólo se detectaban
con grandes dificultades algunas sustancias de cancerogen-
icidad conocida en los roedores. Otros ensayos incluidos
en el estudio IPESTTC pudieron detectar sustancias
carcinógenas para roedores que eran negativas en el ensayo
de salmonelas. Sin embargo, la base de datos de apoyo era
demasiado pequeña para permitir la recomendación de un
ensayo que complementara la prueba de mutación de
salmonelas.
Los resultados del estudio IPESTTC pusieron de
manifiesto que se necesitaría un nuevo ejercicio en
colaboración para determinar: a) la combinación más
eficaz de ensayos in vitro para la detección primaria de
la actividad genotóxica de sustancias químicas, y b) las
pruebas in vivo a corto plazo más útiles para confirmar la
posibilidad de genotoxicidad y cancerogenicidad en mamí-
feros. El Programa Internacional sobre Seguridad de las
Sustancias Químicas (IPCS) y el Instituto Nacional de
Ciencias de Higiene del Medio (NIHS) de los Estados Unidos
de América, como institución participante en el IPCS,
propusieron el estudio en colaboración sobre evaluación y
comprobación de las pruebas a corto plazo para la geno-
toxicidad y la cancerogenicidad (CSSTT). Dadas la magnitud
prevista del proyecto y la logística de la organización y
administración de los proyectos internacionales, el
proyecto se dividió en dos estudios independientes: el
estudio en colaboración sobre pruebas a corto plazo para
la genotoxicidad y la cancerogenicidad (CSSTT/1) y el
estudio en colaboración sobre pruebas in vivo a corto
plazo para sustancias mutágenas y carcinógenas (CSSTT/2).
En el estudio CSSTT/1 se reunió una abarcante base de
datos procedentes de una amplia gama de ensayos in vitro
realizados con diez productos químicos orgánicos cuidad-
osamente seleccionados. Incluían ocho sustancias de
cancerogenicidad probada para roedores, que resultaban
negativas o difíciles de detectar en el ensayo de
salmonelas y dos sustancias químicas consideradas como no
carcinógenas. Se evaluaron los datos procedentes de casi
90 series de ensayos efectuados por unos 60 científicos
participantes. El rendimiento de cuatro tipos de ensayos
se estimó suficiente para considerarlos como posibles
pruebas complementarias del ensayo de salmonelas.
Incluyeron las pruebas para la determinación de aberra-
ciones cromosómicas, mutaciones génicas y transformaciones
neoplásicas en células de mamífero en cultivo, y un ensayo
para determinar la aneuploidia en levaduras. Con la
excepción del ensayo de aberraciones cromosómicas, resultó
evidente que los protocolos utilizados en general para
esas pruebas exigían evaluación adicional antes de poder
considerarlos plenamente aceptables.
La principal conclusión del estudio CSSTT/1 sobre las
pruebas in vitro fue que el empleo de los ensayos de
aberraciones cromosómicas en asociación con la prueba de
mutación de salmonelas podía resultar un detector primario
eficaz para posibles sustancias carcinógenas nuevas.
En el estudio IPESTTC, siete de las catorce sustancias
no carcinógenas presuntas dieron resultados positivos en
muchas de las pruebas in vitro. Los limitados datos de
pruebas in vivo disponibles procedentes de ese estudio
permiten pensar que esas siete sustancias químicas eran
inactivas en las pruebas a corto plazo in vivo. En dos
pares de sustancias carcinógenas/no carcinógenas de
IPESTTC (benzo [a] pireno/pireno (BP/PIR) y 2-acetilamino-
fluoreno/4-acetilamino-fluoreno (2AAF/4AAF), las sustan-
cias no carcinógenas proporcionaron buenos ejemplos de
tales respuestas diferentes, de modo que se seleccion-aron
esos pares de sustancias químicas para la parte in vivo
del estudio en colaboración (CSSTT/2). Sin embargo, hubo
una fuerte duda respecto a la presunta falta de cancero-
genicidad del 4AAF, y una parte crucial del estudio fue el
comienzo de bioensayos de cancerogenicidad a largo plazo
del 2AAF y el 4AAF en ratas.
Por consiguiente, el objetivo del estudio CSSTT/2 fue
producir un abarcante perfil de datos procedentes de una
amplia gama de pruebas in vivo a corto plazo, como medio
de conocer el mecanismo por el que los distintos puntos
finales genéticos de los tejidos destinatarios fundamen-
tales responden a sustancias químicas definidas como geno-
tóxicas in vitro. La meta final consistía en identificar
qué ensayos in vivo podrían usarse para determinar la
actividad in vivo de los productos genotóxicos probados.
Participaron en el proyecto de pruebas in vivo 97
investigadores de 16 países y se presentaron datos de unas
cincuenta técnicas in vivo distintas. Los resultados se
evaluaron en una reunión de investigadores celebrada en
Cap d'Agde (Francia) en mayo de 1985. Se preparó una
serie de informes que comprendían una evaluación de cada
grupo de ensayos, informes resumidos sobre los ensayos en
células germinales y las pruebas hepáticas específicas, e
informes resumidos sobre la base total de datos corres-
pondiente a cada par de sustancias químicas. Después se
preparó un examen general de todo el estudio in vivo listo
para la publicación final.
Sólo una pequeña proporción de los ensayos represent-
ados en el estudio CSSTT/2 satisficieron los criterios que
definen una prueba in vivo a corto plazo aceptable. Sin
embargo, los ensayos incluidos en el estudio no se
limitaron a los que podían cumplir con más probabilidad
los criterios. Así, aunque los datos procedían de ensayos
que no estaban destinados fundamentalmente a identificar
la genotoxicidad in vivo, proporcionaron información
sobre un amplio espectro de efectos biológicos de las
cuatro sustancias químicas. La mayoría de los ensayos en
células sométicas in vivo para determinar la genotoxicidad
discriminaron entre las sustancias carcinógenas/no carcin-
ógenas de los dos pares, aunque en algunos casos se
detectó una actividad débil en las pruebas con sustancias
no carcinógenas, en particular con el 4AAF. La insensib-
ilidad de algunos ensayos a uno u otro de los dos pares de
productos químicos apoya el concepto de que deben obtener-
se datos in vivo negativos en dos ensayos por lo menos en
diferentes tejidos antes de que pueda aceptarse que una
sustancia química carece de genotoxicidad in vivo.
Se confirmó que la prueba de micronúcleos de médula
ósea de ratón es un ensayo potente, sensible y repro-
ducible, que se recomienda para las pruebas in vivo
primarias de las sustancias genotóxicas in vitro. El
rendimiento general del ensayo en células hepáticas de
rata para la síntesis no programada de ADN permite pensar
que puede ser complementario de la prueba de micronúcleos,
aunque requieren investigación adicional ciertos aspectos
de sensibilidad y selectividad de ese ensayo. Se llegó a
la conclusión de que ciertos ensayos ampliamente susten-
tados, que incluyen los de mutagenicidad por orina y
mediada por el huésped y las pruebas que utilizan la mosca
drosófila, son inapropiados para la evaluación de
riesgos.
Los resultados del estudio CSSTT/2 confirmaron que las
pruebas in vivo a corto plazo tienen que desempeñar una
función crucial en la evaluación de los riesgos y que esa
función consiste en identificar los productos químicos
que, una vez demostrada la genotoxicidad in vitro, son
activos in vivo y tienen así mayores probabilidades de
presentar un riesgo de cancerogenicidad o mutagenicidad
para los mamíferos, incluido el hombre.
These observations were the origin of the present
study, the object of which was to generate a comprehensive
data profile from a broad range of short-term in vivo
assays as a means of understanding how various genetic
end-points in key target tissues respond to chemicals
defined as genotoxic in vitro. The objectives and design
of the collaborative study on short-term in vivo tests
(CSSTT/2) were outlined by an ad hoc Working Groupa at a
meeting organized by the IPCS in Geneva on 30 April 1981,
and the plans were consolidated by an IPCS Working
Groupb in Geneva, on 13-14 November 1981. The sub-
sequent coordination of the collaborative study was the
responsibility of a Steering Committeec derived primar-
ily from the Working Group.
The four test chemicals selected for the in vivo pro-
ject were the two carcinogen/non-carcinogen pairs, i.e.
BP/PYR and 2AAF/4AAF, that provided the initial impetus to
the study, and the first samples were distributed to
investigators in March 1983. During 1983, additional par-
ticipants joined the study to provide data on new in vivo
assays and nine coordinatorsd were appointed to oversee
the work being conducted by investigators performing
identical or similar assays. In all, 97 investigators from
16 countries participated in CSSTT/2. Progress was
reviewed at a meeting of the Steering Group with the
coordinators held in Brussels on 12-13 January 1984. At
this meeting a plan was developed to enter the data from
the study into a computer at NIEHS. It was envisaged that
such a plan would allow a comprehensive statistical
analysis using common statistical techniques for each kind
of assay and provide a comparison of results from
different laboratories performing the same assay.
Initially, it was expected that all the studies would be
completed by October 1984, but by September 1984 it was
evident that additional time would be required for many of
the investigators to complete their evaluation of the four
chemicals. At a second meeting of the Steering Group and
coordinators, held on 14-17 November 1984, status reports
prepared by the coordinators were reviewed in detail and
deadlines were set for the provision of final reports from
each investigator. Two additional coordinators were ap-
pointed at this meeting to oversee the rodent dominant
lethal assay (Dr W. Generoso) and the mouse coat colour
spot test (Dr R. Fahrig). Following this progress review,
a meet-ing of investigators was planned for May 1985, for
the presentation, collation, and assessment of the results
and the preparation of final reports on the study.
--------------------------------------------------------------------------------
a Participants: Dr J. Ashby, Professor N.P. Bochkov, Dr B.E. Matter,
Professor T. Matsushima, Dr F.J. de Serres, Dr M. Shelby, and Professor
F.H. Sobels.
b Participants: Dr J. Ashby, Dr G.R. Douglas, Dr M. Ishidate, Jr., Dr A.
Leonard, Dr N. Loprieno, Dr B.E. Matter, Professor T. Matsushima, Dr R.
Montesano, Dr F.J. de Serres, Dr M. Shelby, Professor F.H. Sobels, Dr M.
Stoltz, and Dr M. Waters.
c Steering Committee: Dr F.J. de Serres (Chairman), Dr J. Ashby, Dr. M.
Ishidate, Jr., Dr B. Margolin, Dr M. Shelby, Dr M. Draper, and Dr G.C.
Becking.
d Coordinators: Dr W. Vogel, Dr W.M. Generoso, Dr I.-D. Adler, Dr D. Wild,
Dr. T. Tice, Dr B.E. Butterworth, Dr D. McGregor, Dr M. Salamone, and
Dr R. Fahrig.
The meeting of investigators was held during 15-21 May
l985 at Cap d'Agde, France. Representatives from each par-
ticipating laboratory met to prepare a series of reports
comprising a) an assessment of each group of assays, b)
summary reports on the germ cell assays and the liver-
specific assays, and c) summary reports on the total data
base on each pair of chemicals, i.e. BP/PYR and 2AAF/
4AAF. During the meeting, draft reports were prepared and
were to be finalized during the following two months. At
a meeting of the Editorsa held at NIEHS from 24 July to 6
August 1985, the work group and investigators' reports
were reviewed, and a time-table was developed for the com-
pletion of all reports and for the preparation of the
Introduction and Overview of the study in readiness for
final publication of the in vivo study.
A feature of both the CSSTT studies and the earlier
IPESTTC project was the voluntary participation of a large
number of scientists together with support from their
parent institutions. The organization of CSSTT/1 and
CSSTT/2 was financed largely by IPCS and some of its par-
ticipating institutions. In most cases, funding of the
experimental work was by individual investigators, many of
whom incorporated the studies into their research pro-
grammes. This, of course, required the goodwill and
support of senior management of the participating labora-
tories from universities, research institutions, and in-
dustrial research facilities throughout the world. Ad-
ditional financial assistance was provided by a number of
governments that support IPCS, including Belgium, Italy,
Japan, The Netherlands, the United Kingdom, and the USA.
The United Kingdom Department of Health and Social Secur-
ity funded the rat carcinogenicity studies with 2AAF and
4AAF. The Belgian government and its National Institute
of Hygiene and Epidemiology financed meetings of the
Steering Committee and coordinators in Brussels. The
meeting of investigators held in Cap d'Agde was organized
by the French government and cosponsored by the French
Ministry of Health and the Commission of European
Communities.
--------------------------------------------------------------------------------
a Editors: Dr J. Ashby, Dr F.J. de Serres, Dr M.D. Shelby, Dr B.H.
Margolin, Dr M. Ishidate, Jr., and Dr G.C. Becking.
3. OVERALL AIMS OF THE STUDY AND CRITERIA FOR THE SELECTION OF
APPROPRIATE SHORT-TERM IN VIVO TESTS
Because of their relative simplicity, reproducibility,
and reliability, short-term in vitro tests are the methods
of choice for the initial testing of chemicals for geno-
toxic activity. The role and usefulness of genotoxicity
assays using whole animals, i.e. short-term in vivo
tests, are less clearly defined despite the fact that
they are widely used and are an integral part of most
legislative guidelines for the conduct of mutagenicity
tests. In general, in vivo tests are more resource-
consuming than their in vitro counterparts and the use of
animals for experiments for which there is an acceptable
in vitro alternative is to be discouraged. As these
observations suggest, in vivo assays should be designed to
answer questions that cannot be investigated adequately
with in vitro tests.
3.1 The use of short-term tests for the primary identification
of genotoxic chemicals
Certain short-term in vivo assays, such as the rodent
bone marrow chromosome assay, the rodent dominant lethal
test, and the host-mediated assay, were used in the early
1970s in primary screens for the identification of muta-
genic chemicals. With the introduction of reliable and
valid short-term in vitro tests for mutagens in the mid-
1970s, the use of in vivo procedures for initial screen-
ing of chemicals declined considerably. Certain legislat-
ive authorities still recommend a combination of in vitro
tests reserved for confirmatory or supplementary use or
for providing data for hazard assessment. These different
roles for whole animal procedures have led to the accept-
ance of test protocols of varying complexity, i.e. less
rigorous protocols for screening modes and more comprehen-
sive protocols when the test is required to assess hazard
potential.
Implicit in the concept of the two IPCS studies is the
assumption that chemicals shown to be genotoxic in vivo
also exhibit genotoxic activity in a properly designed
and conducted in vitro primary screen. This principle is
well-established in the scientific literature and was con-
firmed in the IPESTTC and CSSTT/1 studies. Thus, it was
an integral principle in the study design that the primary
screening of chemicals could be adequately served by in
vitro tests alone and that short-term in vivo assays have
no role to play when screening for genotoxic activity. In
vivo procedures will, therefore, be reserved for more
specific applications such as investigating the activity
of in vitro genotoxins in the whole animal and to assist
in the assessment of the mutagenic and carcinogenic poten-
tial associated with exposure of humans to in vitro geno-
toxins.
3.2 The use of short-term in vivo assays for assessing the
hazard associated with exposure to in vitro genotoxins
It is apparent from the last paragraph that the major
objective of the study was to investigate the activity in
the whole mammal of chemicals identified as genotoxic in
vitro and to establish which in vivo tests are the most
useful for this purpose. The major criterion for an ac-
ceptable short-term in vivo test, therefore, rests with
its ability to differentiate between carcinogenic and non-
carcinogenic chemicals, particularly those that have been
identified as genotoxic in an in vitro primary screen. To
extend this criterion further, an acceptable assay should
be capable of separating carcinogen/non-carcinogen ana-
logues, e.g., 2AAF/4AAF, in which, in some cases, small
differences in chemical structure are responsible for
dramatic differences in carcinogenic potential. Other
important criteria, some of which were more clearly
characterized during the course of the study, include the
following:
* data should be reproducible between different labora-
tories,
* the assays should not require too high a degree of
technical and scientific expertise to be conducted on
a routine, every-day basis,
* the genotoxic changes or end-points of the assays
should be clear and unambiguous,
* there should be agreed, valid statistical techniques
to differentiate between positive and negative data.
It is relevant to the performance of in vivo tests to
consider the metabolic fate of carcinogenic chemicals.
As a generalization and depending on the route of exposure
of the animal to the chemicals, reactive metabolites of
many carcinogens are generated mainly in the liver as a
by-product of a predominantly detoxifying process. These
metabolites may be capable of interaction with the genetic
material, DNA, in the liver cells, they may be transported
in an active form to other tissues, or they may be further
modified in those tissues to forms able to interact with
DNA. Thus, a study of the genotoxic activity of a chemical
in the whole animal should be able to detect the genotoxic
effects of chemicals or their metabolites in tissues out-
side the liver and of those whose main genotoxic reac-
tivity may be confined to the liver itself. For example,
assays based on genetic alterations to cells in the bone
marrow, which is readily accessible to chemicals or their
metabolites circulating in the blood, respond to a wide
range of chemical carcinogens. However, where reactive
metabolites are not readily transported from the liver,
bone marrow-type tests would be of little value and assays
capable of detecting the genotoxicity in liver cells
should be available.
3.3 The role of short-term in vivo tests in research
into the mechanisms of cancer
Chemical carcinogenesis is generally recognized as a
multistage process that begins with initiation, usually
considered to involve changes in DNA structure leading to
mutations, followed by promotion of the lesion to a pre-
malignant state and progression to overt cancer. Thus, the
majority of current short-term tests, designed to detect
the consequences of DNA interaction, will only respond to
chemicals that may induce tumours by a predominantly geno-
toxic mechanism or induce the initial phase of the car-
cinogenic process.
A feature of the literature on short-term tests for
chemical carcinogens is the occasional reference to chemi-
cals, shown to be associated with the induction of tumours
in laboratory animals, that consistently fail to be de-
tected in short-term in vitro or in vivo assays for geno-
toxicity. Such negative observations with established car-
cinogens have been explained by a lack of sensitivity of
the particular assays. If these chemicals (diethylhexyl-
phthalate is an example) are truly non-DNA-reactive, how-
ever, then negative data in assays for genotoxicity would
be the expected result. The acceptance of the existence
of so-called "non-genotoxic carcinogens" is of critical
importance to the future of short-term testing and one
consideration during the assessment of the CSSTT/2 study
was the identification of in vivo procedures that may be
useful for investigating such chemicals with the eventual
objective of developing specific short-term tests for non-
DNA-reactive carcinogens.
3.4 Assays for the detection of germ cell mutagens
As a general principle of genetic toxicology, genetic
damage induced by chemicals in somatic cells results in
hazard only to the affected individual, while genetic
effects in male or female germ cells may cause heritable
disease or malformation in the immediate progeny or in
future descendants of the affected individual. The ma-
jority of the assays represented in the CSSTT/2 study were
conducted in cells from somatic tissues and are, there-
fore, only directly interpretable in terms of somatic
mutation and carcinogenic potential. Although there have
been attempts to extrapolate data derived from somatic
cell assays to assessment of the probability of a chemical
inducing germ cell mutations, there are a number of
assays, some of which were represented in this study, that
measure genetic damage directly in the germ cells. The
objectives behind the inclusion of germ cell assays in the
present study were to determine:
* which kind of germ cell procedure effectively ident-
ifies germ cell mutagens;
* what is the relationship between the induction of
changes in sperm morphology and unscheduled DNA syn-
thesis in germ cells and the formation of true heri-
table mutations;
* which of the four in vitro genotoxins induce genetic
damage in mammalian germ cells.
4. CRITERIA FOR THE SELECTION OF THE FOUR TEST CHEMICALS
Selection of the two pairs of chemicals, benzo [a] pyrene/
pyrene (BP/PYR) and 2-acetylaminofluorene/4-acetylamino-
fluorene (2AAF/4AAF), was based, primarily, on their per-
formance in the IPESTTC study. In that study, seven of
the fourteen chemicals believed to be non-carcinogens
exhibited a high frequency of positive responses in in
vitro tests while being predominantly or totally negative
in a series of short-term in vivo tests. Although the in
vivo data base was limited, the non-carcinogens in the
BP/PYR and 2AAF/4AAF pairs provided good examples of such
differences in response and these four chemicals were
selected as useful representative candidates for investi-
gating the in vivo behaviour of chemicals known to be in
vitro genotoxins.
4.1 Activity of the four test chemicals in short-term in vitro tests
The majority of chemical carcinogens, including BP and
2AAF, require some form of enzyme-mediated biotransform-
ation for the formation of reactive metabolites. Most
target cells, whether bacteria, yeast or cultured mam-
malian cells, used in in vitro tests lack the appropriate
enzyme activity and this is usually provided in the form
of an enzyme-rich fraction derived from homogenates of rat
liver, referred to as the S9 fraction. Liver enzyme
activity is usually stimulated by pre-treatment of the
animals with an enzyme-inducer such as Aroclor 1254.
Both the carcinogens, BP and 2AAF, are consistently
positive in bacterial assays and other in vitro short-term
tests and, with the exception of certain mammalian cell
assays, demonstration of genotoxic activity requires the
incorporation of a rodent liver enzyme system. Positive
results have also be reported for the two non-carcinogens,
PYR and 4AAF, in a number of in vitro systems but not on
the scale nor usually with the same potency as their
carcinogenic analogues. Thus, both PYR and 4AAF induce
mutations in bacteria, gene conversion in yeast, and
unscheduled DNA synthesis (UDS) and gene mutation in
cultured mammalian cells. They do not, apparently, induce
mutations in yeast or chromosome aberrations in cultured
mammalian cells. 4AAF, but not PYR, can induce UDS in
cultured primary hepatocytes.
In the salmonella mutation assay, separation of the
carcinogen/non-carcinogen pairs is influenced signifi-
cantly by the nature of the rodent liver activation system
employed in the test. When the liver enzyme suspension is
derived from uninduced rodents, BP and 2AAF give positive
results and the two non-carcinogens, PYR and 4AAF, are
generally negative. If Aroclor-induced animals are used
as a source of the activation system, however, the ability
of the assay to discriminate between the carcinogens and
non-carcinogens is lost.
A comprehensive tabulation of the activities of the
four chemicals in short-term in vitro tests is provided by
McGregor (1988b).
4.2 Summary of carcinogenicity data on the test chemicals
The rodent carcinogenicity data for the four test
chemicals is reviewed in Hicks et al. (1988).
Both BP and 2AAF are well established and potent
carcinogens in rodents. Fewer studies have been conducted
with the non-carcinogens, PYR and 4AAF, and their presumed
non-carcinogenicity, although derived from limited rodent
bioassays, must remain tentative pending more comprehen-
sive testing.
BP is a locally-acting carcinogen in rats and mice,
producing skin tumours after dermal application and
tumours of the stomach when administered orally. In mouse
skin, it has been shown to have both initiating and
promoting activity. BP is also a lung carcinogen in mice
and induces mammary gland tumours in rats. There is no
evidence that BP is hepatocarcinogenic in rats and only a
single, unconfirmed report of the induction of hepatomas
in mice. On balance, the available evidence suggests that
BP does not induce liver tumours in rodents.
PYR has not been tested for carcinogenic activity in
any species other than the mouse and even then, only by
dermal application. These studies have shown fairly con-
clusively that PYR is not carcinogenic by this route.
There is some evidence from promotion/initiation studies
on mouse skin that it may have weak initiating activity.
Because data on systemic carcinogenesis in the mouse and
information from other species are lacking, the carcino-
genicity of PYR is somewhat equivocal. However, the fact
that its analogue, BP, is such a potent skin carcinogen,
whereas the data obtained with PYR in fairly comprehensive
mouse skin studies are negative, suggests that the
presumed non-carcinogenic status of PYR is justified.
2AAF is a potent carcinogen in both rats and mice. It
produces tumours in the liver and bladder in both species
after oral administration and in the rat it is carcino-
genic in Zymbal's glands and mammary glands. Little
information is available about its activity when adminis-
tered by other routes.
4AAF has only been tested in rats by incorporation in
the diet, and its activity in mice has not been investi-
gated. In rat feeding experiments, 4AAF has been consist-
ently non-carcinogenic though there are limited data to
suggest that its metabolite, N -OH-4AAF, may have some
weak carcinogenic activity. If 4AAF does prove to be
carcinogenic, its activity is clearly far less potent than
that of 2AAF and further long-term studies are required
before 4AAF can be unequivocally regarded as non-carcino-
genic. The question may be resolved when the results of
the rat carcinogenicity study, initiated as part of the
CSSTT/2 project, are made available.
5. SOURCE AND PURITY OF THE TEST CHEMICALS
Samples of the four test chemicals were obtained from
commercial sources: BP, 2AAF, and 4AAF were supplied by
Lancaster Synthesis Ltd., Eastgate, Whiteland, Morecambe,
Lancashire, United Kingdom, and PYR was obtained from
Aldrich Chemicals, Gillingham, Dorset, United Kingdom.
4AAF was synthesized by a route that avoided contamination
with 2AAF. The purity of the chemicals was determined by
the supplying laboratories before dispatch to the
participants. All the chemicals were greater than 99.5%
pure and full analytical details are provided by Paton and
Ashby (1988).
6. SHORT-TERM IN VIVO ASSAYS
For convenience the assays are divided into six groups:
* cytogenetic (chromosome) assays conducted on cells from
rodent bone marrow or other non-hepatic tissues;
* assays investigating a variety of effects in cells from
rodent liver;
* miscellaneous assays that utilize other tissues or body
fluids;
* mouse coat colour spot tests;
* assays in mammalian germ cells;
* tests utilizing the fruit fly, Drosophila melanogaster.
6.1 Cytogenetic assays
Assays for the induction of chromosome aberrations,
i.e., microscopically visible alterations in chromosome
structure, were conducted in bone marrow cells from rats,
mice, and Chinese hamsters and also in mouse ascites
tumour cells.
Micronucleus tests were conducted on cells from rat
and mouse bone marrow and on erythrocytes in circulating
blood from mice. Micronuclei are chromosome fragments or
intact chromosomes excluded from the cell nucleus during
mitosis. They are considered to be evidence of induced
chromosome breakage or chromosome loss and are usually
analysed in developing or mature erythrocytes.
Sister chromatid exchanges (SCE) can be demonstrated
in metaphase chromosomes by a differential staining tech-
nique and occur as a consequence of the exchange of repli-
cating DNA between chromatids at apparently homologous
loci. Although considered to result from DNA breakage and
reunion, the mechanism of the formation of SCE is not
fully understood. Assays for the induction of SCE were
conducted in bone marrow cells from rats, mice, and
Chinese hamsters, in circulating blood leucocytes from
mice, and in Chinese hamster intestinal epithelium.
Table 1. Assays employed in the CSSTT/2 study
-------------------------------------------------------------------------
1. Cytogenetic studies
Chromosome aberrations
Mouse bone marrow
Mouse ascites tumour cells
Rat bone marrow
Chinese hamster bone marrow
Micronucleus tests
Mouse bone marrow
Mouse circulation blood cells
Rat bone marrow
Sister chromatid exchanges
Mouse bone marrow
Mouse circulating blood lymphocytes
Rat bone marrow
Chinese hamster bone marrow
Chinese hamster intestinal epithelial cells
2. Liver-specific assays
Tests for initiation/promotion: altered enzyme foci
Unscheduled DNA synthesis (UDS)
UDS in mouse liver
UDS in neonatal, weanling and adult rat liver
Frequency of S-phase hepatocytes
S-phase in mouse liver
S-phase in weanling and adult rat liver
Cytogenetic tests
Aberrations in rat liver epithelial-like cells
SCE in rat liver epithelial-like cells
Micronuclei in hepatocytes
Diploid/tetraploid ratio in rat and mouse hepatocytes
Primary changes in DNA
Alkaline elution assay for DNA strand damage
DNA/protein cross-links
DNA unwinding assay for strand damage
3. Miscellaneous assays
Specific carcinogenicity assays
Two-year oral dosing study in rats
Mouse lung adenoma assay
Quail egg tumour-induction
UDS in rat fore-stomach
Sebaceous gland suppression assay
Observation of dermal epithelial hyperplasia
Transformation of rat peritoneal macrophages
6-Thioguanine-resistant mutations in Syrian hamster lung cells
Measurement of DNA adducts
32P-post-labelling in rat and mouse tissues
Immunochemical detection of DNA adducts
Radiolabelled test chemicals - rat liver
-----------------------------------------------------------------------------
Table 1. (contd.)
-----------------------------------------------------------------------------
3. Miscellaneous assays (contd.)
Immunotoxicity assays
Natural Killer (NK) cell and T-cell cytotoxicity in rats
Host-mediated assays
Mutation of salmonella in mouse tissues
Genetic changes in yeast cells in mouse tissues
Urine mutagenicity tests in rats, mice and guinea pigs
4. Mouse spot tests
Mouse coat colour spot test
Mouse melanocyte assay
5. Mammalian germ cell studies
Dominant lethal assays with male and female mice and male rats
Morphological abnormalities in mouse and rat spermatozoa
Unscheduled DNA synthesis in rat and mouse male germ cells
6. Drosophila assays
Sex-linked recessive lethal mutations in germ cells
Chromosome loss in germ cells
Somatic mutation and recombination: mosaic spots in eyes
and wings
-----------------------------------------------------------------------------
6.2 Assays in rodent liver cells
A variety of liver-specific assays were represented in
the study, including the demonstration of enzyme changes,
a number of different tests on DNA, and cytogenetic
assays.
The rat liver assay for altered enzyme foci employs a
histochemical technique that identifies groups of cells or
foci that have elevated levels of the marker enzyme gamma-
glutamyltranspeptidase (GGT). The observation of GGT-
positive foci often precedes the appearance of liver car-
cinoma and the test is used to attempt to identify early
stages of carcinogenesis in the liver. Protocols have been
devised to investigate both initiation and promotion
stages of carcinogenesis.
A number of investigators used assays that measure
directly or indirectly the response of DNA to chemical
damage. These included tests for breaks in single strands
of DNA using the alkaline elution technique, an assay for
the induction of crosslinks between DNA and protein mol-
ecules, and a measure of single strand breaks based on the
degree of unwinding of DNA molecules. One consequence of
DNA breakage is the initiation of the enzyme-mediated
repair process which involves the synthesis of new, rela-
tively short strands of DNA to repair the break. This type
of repair is referred to as unscheduled DNA synthesis or
UDS (to differentiate from the normal or S-phase DNA syn-
thesis that occurs during cell replication). DNA synthesis
can be measured by observing the uptake of tritiated thy-
midine by the newly synthesized strands using autoradio-
graphical techniques. Assays for the induction of UDS and
to measure changes in the numbers of S-phase cells in
rodent liver were included in the study.
The cytogenetic methods included the analysis of meta-
phase chromosome aberrations, micronuclei and SCE in rat
liver cells, and the determination of the ratio of diploid
to tetraploid cells in rat and mouse liver.
6.3 Miscellaneous assays
The first group of assays to be considered under this
heading are those that are directly related to the induc-
tion of tumours in rodents. The most important of these
is a 2-year oral dosing study in rats to compare the car-
cinogenicity of 2AAF and 4AAF. Although the study was not
completed at the time of this report, preliminary infor-
mation from a small group of rats examined after 26 weeks
of dosing is available. Also included in this group was
a) the mouse lung tumour assay that determined the effects
of each of the four test chemicals on the incidence of
lung adenoma in mice and b) a test in Japanese quail in
which the test chemicals were introduced into the yolk
sacs of quail eggs. The birds were then examined for
evidence of tumours at various intervals after hatching.
The sebaceous gland suppression test is based on the
observation of morphological changes to the sebaceous
glands in histological preparations of mouse skin after
dermal exposure to the test chemicals. The presence of
epidermal hyperplasia can also be detected histologically
and both methods have been shown to respond to skin car-
cinogens, particularly polycyclic hydrocarbons.
Genotoxic carcinogens can bind covalently to biologi-
cal macromolecules such as DNA either before or after
metabolic biotransformation. The products of DNA-binding
are referred to as DNA adducts and a number of investi-
gators provided data on the detection and measurement of
DNA adducts with the test chemicals in tissues from rats
and mice. Three different methods were represented:
* the enzyme-linked immunoabsorbent assay (ELISA);
* the 32P-post-labelling assay;
* an assay for radiolabelled chemicals bound to DNA.
Two similar assays for the investigation of the immune
response of animals to exposure to carcinogens were rep-
resented. Natural Killer (NK) cells are leucocytes with
the ability to lyse a variety of `foreign' cells, e.g.,
those with a malignant phenotype. The NK cell assay was
used to investigate the capacity of NK cells, derived from
the splenic mononuclear leucocytes of rats treated with
2AAF or 4AAF, to lyse human erythroleukaemic cells in
vitro. The basis of the T-cell assay is that immunocom-
petent T-cells are able to react to tissue changes induced
by carcinogenic chemicals in rats. The changes in T-cell
activity can be determined by their cytotoxicity towards
target cells derived from rat intestinal adenocarcinoma.
The rat peritoneal cell transformation test is based
on the observation that mitogen-stimulated peritoneal
cells, harvested from rats dosed with carcinogens, undergo
a form of transformation that enables them to proliferate
and produce colonies in soft agar culture.
Host-mediated assays were used fairly widely in the
early l970s as primary screening tests for mutagenic
chemicals. In its original form, a suspension of microbial
target cells, i.e. yeast or bacteria, was introduced into
the peritoneal cavities of mice. The mice were treated
with the suspect chemical and, after an appropriate inter-
val, the target cells were harvested and changes in the
mutation frequency or other genetic end-points were deter-
mined in in vitro culture. Since that time, there have
been several modifications to the procedure, the most
significant being the injection of the target organisms
into the circulating blood of the host. In this way, the
bacterial or yeast target cells are distributed in various
organs and can, for example, be harvested from the liver,
lungs, and kidney. The target cells can, in principle, be
affected by reactive metabolites generated from the test
chemical in these organs. Intra-sanguinous host-mediated
assays using either Salmonella typhimurium or the yeast,
Saccharomyces cerevisiae, were represented in the study.
Urine and other body fluids can be collected from
rodents treated with test chemicals and assayed for muta-
genic activity. For example, after appropriate treatment
to release conjugated metabolites, urine can be tested for
the presence of mutagens in a conventional in vitro bac-
terial mutation assay. In principle, the urine assay is
capable of detecting mutagenic chemicals excreted un-
changed or after metabolic activation.
6.4 The mouse spot test
The mouse spot test detects mutations induced in mel-
anocyte precurser cells in the embryos of specially de-
rived strains of mice. The mouse strain carries recessive
mutations at a number of specific coat colour loci and the
embryos are, thus, heterozygous at these loci. Mutations
induced at the wild-type or normal alleles of the coat
colour loci result in the development of clones of the
mutant melanocytes. The young mice are examined after
birth and the mutations are expressed as patches of con-
trasting coloured fur. The mutations can result from one
of a number of genetic events including gene mutations,
chromosome deletions, mitotic crossing-over, and loss of
whole chromosomes. In addition to the conventional tech-
nique of observing the appearance of coloured spots in the
fur, a modification of the spot test allows the micro-
scopic recognition of mutant melanocytes in preparations
of embryonic skin.
6.5 Mammalian germ cell studies
The classical dominant lethal assay in male rodents
involves the treatment of the animals with the test chemi-
cal and then mating with groups of females at intervals to
cover the complete spermatogenic cycle. Dominant lethal
mutations induced at any stage of spermatogenesis can be
detected by dissection of the uterine contents of each
female and are characterized by dead fetuses or a re-
duction in the numbers of fetal implantations. Dominant
treatment of male rats, male mice, or female mice.
Two other assays represented in this group were the
abnormal sperm morphology assay and the unscheduled DNA
synthesis (UDS) test. The former assay monitors mature
spermatazoa for irregularities in morphology at intervals
after treatment of rodents with the test chemicals. The
UDS test measures DNA repair in developing spermatocytes
and spermatids.
6.6 Drosophila assays
Although data from drosophila tests cannot be regarded
as appropriate alternatives to mammalian data for assess-
ing the potential hazard to humans from exposure to geno-
toxic chemicals, drosophila are, in fact, intact, complex,
eukaryotic organisms whose metabolic and genetic charac-
teristics have some parallels with those of the whole mam-
mal. Their main value in genotoxicity testing lies with
the availability of assays to study the effects of chemi-
cals on both somatic and germ cells. The tests represented
in the CSSTT/2 study were:
* the sex-linked recessive lethal mutation assay in
germ cells;
* tests for chromosome loss from germ cells;
* assays for somatic mutation and recombination using
the induction of mosaic spots in either the wings or
the eyes.
7. RESULTS
The investigators met at Cap d'Agde, France, from 15
to 21 May 1985, to assess the results of the study. Each
group of investigators presenting data from a particular
type of assay discussed their data and individual results
were assessed and agreed. This led to the preparation of
consensus reports on the response of each assay to the
four test chemicals. These consensus views were then
incorporated into coordinators' summary reports on each
group of assays. Summary reports were presented and criti-
cally discussed in open plenary discussions, thereby
allowing the overall conclusions and recommendations
resulting from the study to be formulated. Reports of
individual investigators, the coordinators summary
reports, and certain technical appendices form the main
text of the final publication together with an editorial
overview of the study (Ashby et al., 1988).
The purpose of this chapter of the report is to
present the results of the in vivo studies with the four
test chemicals and to construct a profile, in qualitative
terms, of their genotoxic activity in the whole animal.
Quantitative differences in response in relation to sex,
species, route of exposure, and technical variations are
considered in more detail in the next section, which also
includes a comprehensive tabulation of the data generated
by individual investigators (Table 4). However, as many
of the assays were replicated in a number of laboratories,
a simplified table of results is presented here (Table 2).
It must be emphasized that Table 2 contains the consensus
views of the Working Groups assessing the individual
results and performance of each assay and that, in some
cases, there were conflicting results on the same assay
from participating laboratories.
Table 2. Results of the short-term in vivo tests summarized
by Working Groups a
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
1. Cytogenetic studies
Chromosomal aberrations
Mouse bone marrow P N ? N
Mouse ascites cells P NT N N
Rat bone marrow P N ? N
Chinese hamster bone marrow P N P N
Micronucleus tests
Mouse bone marrow P N P N
Mouse blood - maternal P N N N
Mouse blood - fetal P N ? N
Rat bone marrow P N P N
Sister chromatid exchange
Mouse bone marrow P W P W
Mouse blood P N P N
Rat bone marrow P W P W
Chinese hamster bone marrow P N P N
Chinese hamster intestinal cells P N P N
2. Liver-specific assays
Altered enzyme foci - rat P N P W
Unscheduled DNA synthesis
Mouse liver N N N N
Rat liver N N P W
S-phase hepatocytes
Mouse liver NT NT W P
Rat liver - adult W N ? P
Rat liver - weanling P NT NT NT
Cytogenetic tests
Metaphase aberrations - rat W N W N
Micronuclei - rat N N P N
SCE - rat W N ? N
Ploidy - rat P N P N
Ploidy - mouse P N P N
Primary DNA changes
Alkaline elution N N ? ?
DNA/protein cross-links P N P NT
DNA unwinding N N P N
with repair inhibitor P N P N
3. Miscellaneous assays
Carcinogenicity
Oral dosing - rat NT NT P ?
Mouse lung adenoma P N P N
Quail egg tumours ? ? ? ?
UDS in rat fore-stomach ? N N N
--------------------------------------------------------------------------
Table 2. (contd.)
----------------------------------------------------------------------------
BP PYR 2AAF 4AAF
3. Miscellaneous assays (contd.)
Sebacious gland suppression - mouse P N NT NT
Epithelial hyperplasia - mouse P N NT NT
Peritoneal macrophages - rat N N N N
Syrian hamster lung - mutation P N ? N
DNA adducts
32P-post-labelling - rat P N P W
Immunochemical (ELISA) NT NT ? ?
Radiolabelled chemicals NT NT P P
Immunotoxicity
Natural Killer (NK) cells NT NT P N
T-cell cytoxicity P N P W
Host-mediated assays - mouse
Salmonella typhimurium N N N N
Saccharomyces (liver) P P P P
Saccharomyces (lung) N N N N
Urine mutagenicity
Rats P P P P
Mice W W P P
Guinea-pig NT NT P NT
4. Mouse spot tests
Coat colour spots P N P N
Melanocyte assays NT NT P N
5. Mammalian germ cells
Dominant lethal assays
Male mice P N N N
Female mice P N N N
Male rats NT NT N N
Sperm abnormalities
Mice P N P N
Rats NT NT N N
UDS in germ cells
Mice N N N N
Rats N N N N
6. Drosophila assays
Sex-linked recessives N N N N
Chromosome loss N N N N
Somatic mutation
Eye spots P N P N
Wing spots P N W ?
--------------------------------------------------------------------------
a P = positive
W = weak positive
N = negative
? = results inconclusive or not yet reported
NT = not tested
7.1 Benzo [a] pyrene (BP) and pyrene (PYR)
7.1.1 Cytogenetic studies
Assays for the induction of structural chromosome
aberrations or micronuclei in rodent bone marrow cells
were consistently positive with BP and negative with PYR.
Among a number of experimental variables between different
investigators, neither the species, strain, sex, solvent,
nor route of exposure affected the qualitative result. BP
increased the incidence of sister chromatid exchanges
(SCE) in mouse, rat, and Chinese hamster bone marrow in
every study. The results with PYR, however, were less
clear, and this presumed non-carcinogen induced SCE at
high dose levels in mice after oral or intraperitoneal
dosing and in male rats after oral administration, i.e. 3
of 9 SCE studies reported a weak positive result. Analysis
of micronuclei in circulating blood erythrocytes from
maternal, fetal, or weanling mice, and SCE in circulating
blood lymphocytes from adult mice clearly discriminated
between BP and PYR.
7.1.2 Liver-specific assays
Five investigators provided data from observations of
altered enzyme foci in rat liver under the general heading
of initiation/promotion assays. There were, however,
significant protocol variations between investigators.
Two protocols required administration of the rodent tumour
promotor, phenobarbitone, in the drinking-water or diet
for some weeks after dosing in order to determine
initiating activity of the test chemicals. The promoting
activity of the test chemicals was studied by two other
workers by producing initiation events in the liver before
treatment with the test chemicals. The fifth protocol
tested the ability of the chemicals to induce both
initiation and promotion without discriminating between
the two phases. In all cases, the evidence for induced
pre-cancerous changes was the observation of foci or
clones of cells with altered enzyme characteristics. BP
was shown to have significant initiating activity in rat
liver in two studies and, in a third study, was shown
capable of promoting nitrosamine-induced initiation.
Using a fourth, essentially experimental, protocol that
involved the transplantation of donor liver cells to the
host animal, BP failed to show promoting activity. No
evidence of tumour initiating or promoting activity was
observed in any of the studies with PYR.
The rodent liver assay for unscheduled DNA synthesis
measures the induction of repairable lesion in the DNA of
hepatocytes and, theoretically, should be capable of
detecting chemicals that are metabolized in the liver to
produce genotoxic (i.e. DNA-interactive) metabolites.
Both BP and PYR were reproducibly negative in mouse
hepatocytes and in hepatocytes from adult, weanling, or
neonatal rats using either oral or intraperitoneal dosing.
The induction of S-phase DNA synthesis was also
investigated and BP was shown to increase the incidence of
S-phase cells in weanling rats and, in one of two
experiments, a slight increase in S-phase cells was
observed in adult rats. In mice, BP had no significant
effect on the incidence of S-phase, and PYR showed no
evidence of S-phase synthesis induction in either
species.
Limited data were presented on the cytogenetic effects
of the chemicals in liver-derived cells. In epithelial-
like cells from weanling rats, BP was observed to induce a
small increase in the incidence of structural chromosome
aberrations and SCE; tests with PYR were negative. These
findings, however, require confirmation. No evidence of
micronucleus-induction was detected in rats treated with
BP or PYR after partial hepatectomy. Using a cytofluoro-
metric method, an increase in the ratio of diploid to
tetraploid cells was observed in liver-derived cells after
treatment of rats with BP, but not after dosing with PYR.
Three different assays were used to study the ability
of the chemicals to induce single-strand breaks (SSB) in
liver cell DNA. Both BP and PYR were uniformly negative
in the alkaline elution assay for SSB conducted in three
laboratories. BP, however, was shown to induce crosslinks
between DNA and protein, while PYR was negative in this
assay. The third assay in this group was a measure of SSB
based on the degree of alkali-induced unwinding of DNA
molecules. When the DNA-repair inhibitor, adenosine
arabinoside was incorporated, BP was shown to induce SSB
in mouse liver cell DNA using this technique. In the
absence of the repair inhibitor, SSB were not observed.
PYR produced negative results in the DNA-unwinding assay.
The results from this group of assays suggest that BP is
capable of inducing SSB in the DNA of mouse liver cells
that are effectively repaired in the absence of a DNA-
repair inhibitor. In rats, the observation of DNA/protein
crosslinks tentatively suggests the induction of SSB in
rat liver cells, but additional studies are required to
assess the DNA effects of BP in rat liver.
7.1.3 Miscellaneous assays
Two investigators are producing data that are directly
related to the induction of tumours. In the lung adenoma
assay, mice were injected with BP or PYR by the intra-
peritoneal route, 2-3 times each week, for up to 8 weeks.
The mice were examined 23 weeks after the start of the
study and the numbers of adenoma on the surface of the
pulmonary lobes were recorded. BP induced a significant
increase in adenomas compared with the untreated control
group while no increase in the induction of these tumours
was observed in mice injected with PYR. This assay
differentiated very clearly between the carcinogenic
activity of BP and PYR. The second study in this category
involved injecting the test material into the yolk sac of
quail eggs and then observing the birds for the presence
of tumours at intervals after hatching. In birds examined
at approximately 3 months, there was no evidence of tumour
induction by either BP or PYR. Surviving birds will be
maintained for up to 12 months before being examined for
the presence of tumours and, therefore, a definitive
conclusion on the carcinogenicity of BP and PYR to quail
is not yet possible.
One investigator studied the induction of UDS in the
fore-stomach of rats after oral dosing; the data with BP
were considered to be equivocal while PYR gave negative
results.
Two assays involved the application of the test chemi-
cals to mouse skin followed by histological examination of
skin sections. BP induced epithelial hyperplasia and
suppression of sebaceous glands, both of which have been
correlated with polycyclic hydrocarbon-induced skin
carcinogenesis. PYR failed to elicit either of these
responses.
The transformation of rat peritoneal macrophages has
been advocated as a short-term in vivo test for poten-
tially carcinogenic chemicals. Macrophages isolated from
rats dosed with BP or PYR, however, failed to show any
evidence of transformation in experiments conducted in
three laboratories.
Somatic mutation data on cells isolated from Syrian
hamster lung tissue, after dosing with the test chemicals,
were presented by one investigator. Cultures of cells
from animals dosed with BP showed a higher frequency of
mutations at the 6-thioguanine locus than those from
untreated hamsters, suggesting the induction of somatic
mutations in lung cells from this species. PYR produced
negative mutation data in this assay.
32P-labelling of purified DNA was used to detect DNA
adducts in tissues from rats and mice treated intraperito-
neally with the test chemicals. Measurable levels of PYR
adducts were not detected in DNA from mouse liver, lung,
or kidney, or from rat liver or lung tissues. BP adducts
were identified in all of these tissues.
Only one of the two assays for immunotoxicity was con-
ducted with the BP/PYR pair. Thus, the T-cell assay showed
that BP, but not PYR, was capable of inducing a cytotoxic
response in rat T-cells.
Two investigators provided data from host-mediated
assays in mice dosed with BP or PYR. In tests with the
yeast, Saccharomyces cerevisiae, a significant increase
in mitotic gene conversion was detected in yeast cells
isolated from the liver of mice dosed with either BP or
PYR, while yeast cells isolated from lung tissue produced
negative data with both chemicals. No evidence of the
induction of mutations was observed in Salmonella
typhimurium isolated from liver tissues of mice dosed with
BP or PYR.
Assays for the detection of mutagens in urine were
conducted with both rats and mice. Mutagenic activity was
detected in urine samples from both species after dosing
with BP or PYR and, therefore, the test failed to differ-
entiate between the two chemicals.
7.1.4 Mouse spot tests
Only one investigator provided mouse spot test data on
BP and PYR. Using the observation of coat colour spots in
off-spring as evidence of somatic mutation, BP was clearly
positive and PYR negative.
7.1.5 Mammalian germ cell assays
BP induced dominant lethal mutations in both male and
female mice after intraperitoneal injections but not after
oral dosing. The response in the male was highly stage-
specific; only the mature spermatozoa were sensitive and
spermatid and spermatocyte stages were unaffected. Domi-
nant lethal assays with PYR were reproducibly negative.
Studies of morphological abnormalities in mature
spermatozoa were conducted in mice after oral or
intraperitoneal dosing. BP was uniformly positive in this
assay, regardless of the route of exposure. Although a
weak positive response was observed in one of seven
experiments with PYR, this observation was not confirmed
and the consensus view was that PYR did not induce sperm
abnormalities. In studies of the induction of unscheduled
DNA synthesis in rat and mouse male germ cells, negative
results were obtained with both BP and PYR.
7.1.6 Drosophila assays
In all, ten studies were conducted with BP and PYR in
drosophila and in each case the oral route of treatment
was used. Germ cell studies, i.e. the sex-linked
recessive lethal mutation assay and the test for chromo-
some loss, were uniformly negative with both chemicals.
In contrast, tests for somatic mutation and recombination,
based on the observation of eye or wing spots, showed that
BP was able to induce genetic changes in somatic cells in
each of three studies. PYR was consistently negative.
7.2 2-Acetylaminofluorene and 4-acetylaminofluorene
7.2.1 Cytogenetic studies
Seven investigators provided data from analysis of
metaphase chromosomes in mouse bone marrow cells. 2AAF
induced a significant increase in the incidence of
structural chromosome aberrations in only two of seven
assays, one of which was considered to be inconclusive.
The results of assays with 4AAF were negative with the
exception of two assays in which the data were inconclus-
ive. In contrast, 2AAF was reproducibly positive and 4AAF
consistently negative in 15 of 16 micronucleus tests
conducted in mouse bone marrow erythrocytes. The route of
exposure, i.e. oral dosing or intraperitoneal injection,
did not appear to influence the outcome of these studies.
Micronuclei were produced by 2AAF in rat bone marrow cells
and a small increase was also recorded in one of two
assays with 4AAF. Two workers investigated the influence
of these two chemicals on the incidence of structural
chromosome aberrations in Chinese hamster bone marrow;
2AAF was positive in this species and 4AAF gave a weak
positive result in one of the two studies. Both chemicals
failed to induce detectable chromosome damage in mouse
ascites tumour cells. Four micronucleus tests were
conducted with mouse blood erythrocytes. An increase in
micronuclei was recorded by one worker in blood cells from
weanling mice dosed with 2AAF, although when samples of
maternal or fetal blood were examined, increases in
micronuclei were not apparent. However, studies conducted
in another laboratory showed that 2AAF was capable of
increasing the incidence of micronuclei in fetal blood.
Thus, 2AAF is clearly clastogenic to rodent bone marrow
cells while 4AAF was considered by most investigators to
be devoid of clastogenic activity.
Nine assays for sister chromatid exchanges (SCE) were
conducted in bone marrow cells from mice, rats, or Chinese
hamsters. 2AAF was clearly positive in this test and the
consensus view was that 4AAF, although negative in four
assays, was capable of inducing a small (but significant)
increase in SCE. In studies with mouse lymphocytes and
Chinese hamster intestinal epithelial cells, 2AAF was
positive and 4AAF was considered to be negative.
7.2.2 Liver-specific assays
As discussed in section 7.1.2, there were a number of
variations between the five protocols used in the
initiation/promotion assays. Significant initiation and
promotion activity was demonstrated in studies with 2AAF
based on the observation of foci of cells with altered
enzyme characteristics in rat liver. These assays failed
to discriminate clearly between the two chemicals, since
4AAF was shown to be an initiator in the hands of one
investigator and had weak promoting activity in another
study. Both chemicals, therefore, proved capable of
producing these pre-cancerous changes in rat liver tissue
though with different degrees of potency.
2AAF and 4AAF failed to induce detectable unscheduled
DNA synthesis (UDS) in mouse hepatocytes after oral
dosing. Quite different results were obtained in rat
liver, however, and 2AAF was shown to induce UDS in rat
hepatocytes in each of the seven laboratories that sub-
mitted data. Three of these laboratories also reported the
detection of a small increase in UDS in rats after dosing
with 4AAF. The other four laboratories obtained negative
data. Thus, 2AAF induced UDS in hepatocytes from rats but
not mice, and 4AAF elicited a weak UDS response in the rat
and was negative in mouse cells.
The results of the analysis of S-phase DNA synthesis
indicated that the presumed non-carcinogen, 4AAF, was a
very potent inducer of S-phase cells in the liver of both
rats and mice. 2AAF, on the other hand, induced only a
small increase in S-phase cells in the mouse and three
studies in rat liver produced one clear positive and two
negative results.
In cytogenetic experiments in cells derived from rat
liver, 2AAF appeared to induce a small increase in the
incidence of structural aberrations and, in partially
hepatectomised rats, a significant increase in micro-
nuclei. 4AAF gave negative results in tests for chromo-
somal aberrations, micronuclei, and SCE in rat liver
cells. When the ratio of diploid to tetraploid cells was
investigated in rat and mouse liver, an increase in the
ratio was observed in both species after dosing with 2AAF
but not in response to 4AAF.
The alkaline elution assay for single strand breaks
(SSB) produced conflicting results between three labora-
tories. Two investigators presented negative data from
assays with both chemicals while a third observed that
both 2AAF and 4AAF produced SSB in this test. Using the
detection of DNA/DNA or DNA/protein crosslinks as a
measure of SSB, 2AAF was shown to produce SSB. No data
were presented on 4AAF. Another investigator calculated
SSB from the amount of DNA unwinding induced by the chemi-
cals. 2AAF and 4AAF gave positive and negative results,
respectively, either in the presence or absence of a DNA
repair inhibitor. These data suggest that, unlike 4AAF,
2AAF is capable of producing SSB in rat liver DNA under
appropriate experimental conditions.
7.2.3 Miscellaneous assays
A 2-year oral dosing carcinogenicity study in rats
with 2AAF and 4AAF is still in progress. Preliminary
findings in a small number of animals examined after 26
weeks of dosing showed that an increase in liver tumours
was already apparent in animals dosed with 2AAF; there was
no evidence of tumour induction in the 4AAF-dosed ani-
mals.
The lung adenoma assay, in which mice were given
intraperitoneal injections of the test chemicals 2-3 times
a week for up to 8 weeks, clearly separated 2AAF and 4AAF.
2AAF induced a significant increase in adenomas while the
incidence in mice dosed with 4AAF was comparable with that
of untreated mice.
There was no evidence of the induction of UDS in rat
fore-stomach after oral dosing with either chemical, and
the assay for the induction of somatic mutations in Syrian
hamster lung cells produced inconclusive data with 2AAF
and was negative in tests with 4AAF. The rat macrophage
test was reproducibly negative with both 2AAF and 4AAF.
Three techniques were applied to detect the presence
of DNA adducts formed in various tissues after dosing
rodents with the test chemicals. Data from the ELISA
technique, which requires the use of monoclonal antibodies
to detect specific adducts, are not yet available. How-
ever, the 32P-post-labelling method indicated that 2AAF
formed adducts with DNA in rat liver and lung and in mouse
liver, lung, and kidney after intraperitoneal dosing. No
data were presented from mice dosed with 4AAF. In the
rat, however, 4AAF formed adducts in liver and lung DNA
though at a much lower rate than 2AAF. In studies using
radiolabelled test materials, both chemicals were shown to
form adducts with DNA in the liver and lungs of rats after
intraperitoneal administration, although adduct formation
was considerably less with 4AAF than with 2AAF.
2AAF produced positive results in both immunotoxicity
assays. However, although 4AAF was negative in the
Natural Killer (NK) cell assay, data from the T-cell
cytotoxicity test suggested that it was capable of
inducing a measurable cytotoxic response in rat T-Cells.
Nine sets of data were considered from mouse host-
mediated assays. In eight of these, including assays for
genetic changes in yeast isolated from liver, lung and
kidney, and for mutations in salmonella isolated from
liver tissue, 4AAF was uniformly negative. 2AAF produced
two weak positive responses and five negative results in
the same eight studies. The ninth assay, however, indi-
cated that both chemicals induced mitotic gene conversion
in yeast isolated from liver tissue. In addition, muta-
genic materials were detected in urine samples from rats
and mice after dosing with either isomer and from guinea-
pigs after dosing with 2AAF. 4AAF data on guinea-pig
urine were not available.
7.2.4 Mouse spot tests
Six separate mouse spot tests were conducted with 2AAF
and the results were equally divided between positive and
negative. An increase in coat colour spots was detected
in two studies while in two others, no evidence of the
induction of coat colour spots was observed. Similarly,
in the melanocyte assay, one positive result and one
negative were reported. Four of five spot tests conducted
with 4AAF were negative and the data were inconclusive in
the fifth.
7.2.5 Mammalian germ cell assays
Dominant lethal mutation assays conducted in male or
female mice or male rats and tests for the induction of
UDS in rat or mouse male germ cells were uniformly nega-
tive with both compounds. In the test for morphological
abnormalities in mature spermatozoa, two assays in which
2AAF was given orally to mice or rats were also negative.
After intraperitoneal dosing, however, 2AAF induced a
significant increase in the incidence of abnormal mouse
sperm. 4AAF was negative in both species regardless of
the route of exposure. These data suggest that, although
2AAF or its metabolites appear capable of penetrating the
testes and adversely affecting sperm morphology after
intraperitoneal dosing, there is no evidence for interac-
tion of 2AAF metabolites with germ cell DNA.
7.2.6 Drosophila assays
The mutagenic effects of the two chemicals on
drosophila germ cells were studied in tests for sex-linked
recessive lethal mutations in five laboratories and for
chromosome loss in two laboratories. Data from six of
these studies indicated that neither chemical produced
genetic damage in drosophila. In the seventh laboratory,
however, 2AAF was clearly mutagenic in the sex-linked
recessive lethal assay; 4AAF produced a weak positive
result in this study.
2AAF induced eye or wing spots in somatic mutation and
recombination assays in drosophila, although the response
was weak in two of the studies. The somatic mutation data
generated from tests with 4AAF were ambiguous, producing
one weak positive result, one inconclusive result and one
negative.
7.3 Summary of the in vivo genotoxicity of the four chemicals
A comprehensive in vivo genotoxicity profile has been
generated on the four test chemicals, each of which had
previously shown evidence of mutagenic activity in in
vitro screening tests. It is relevant, in this section,
to consider the mammalian genotoxicity in relation to the
hazard assessment procedures recommended by many regulat-
ory bodies. Table 3 shows the mutagenic activity of the
chemicals in the in vivo tests common to most legislative
guidelines. Based on these results, and in the absence of
pharmacokinetic and metabolic data, BP would be considered
to present a potential mutagenic and carcinogenic hazard
and 2AAF would be regarded as a potential carcinogen.
These observations are, of course, corroborated by the
established carcinogenic activity of these two chemicals.
The abbreviated data base shown in Table 3 suggests
that neither 4AAF nor PYR present a significant genotoxic
hazard and it is useful to consider whether the compre-
hensive data generated in CSSTT/2 support this initial
prediction. The summarized consensus results (Table 2)
show that conclusive data for PYR were presented from 48
distinct in vivo tests and PYR was considered to be nega-
tive in 43 of these. The remaining five tests suggested a
weak induction of SCE in rodent bone marrow that was not
reproduced in all laboratories, a single positive host-
mediated assay, and evidence of the excretion of mutagenic
metabolites in rodent urine. None of these data seriously
question the validity of the original prediction that PYR
is not a significant genotoxic hazard.
Table 3. Activity of the four test chemicals in established
in vivo mutagenicity assays
------------------------------------------------------------------------
BP PYR 2AAF 4AAF
Somatic cell tests
Metaphase chromosome analysis P N Pa N
Micronucleus test P N P N
Mouse spot test P N Pa N
Germ cell tests
Dominant lethal assay P N N N
Heritable translocation test NT NT NT NT
Mammalian germ cell cytogenetics NT NT NT NT
------------------------------------------------------------------------
a Data not consistent between laboratories
NT = not tested
P = positive
N = negative
A similar analysis of the 4AAF data indicates that
conclusive results were obtained from 50 different types
of in vivo tests. Of these, 38 were regarded as negative
and the remaining twelve assays produced positive or weak
positive results. Like BP, 4AAF produced a positive
result in a host-mediated assay and in tests for mutagenic
activity in rodent urine. Other observations, however,
suggest that 4AAF may not be devoid of in vivo genotox-
icity. For example, 4AAF induced detectable unscheduled
DNA synthesis in rat liver hepatocytes that was reproduced
in three different laboratories. One investigator reported
the induction of micronuclei in rat bone marrow cells
after intraperitoneal dosing with 4AAF, and five of seven
studies conducted in rat or mouse bone marrow recorded
small, but significant, increases in SCE. There was also
data to suggest that 4AAF was capable of adduct formation
with liver cell DNA in rats. All the germ cell data on
4AAF were negative. The positive findings in somatic
tissues cannot be dismissed as inconsequential and they
raise serious doubts about the prediction, based on the
data shown in Table 3, that 4AAF does not present a
significant genotoxic hazard. The outcome of the 2-year
rat carcinogenicity study with 4AAF is crucial to the
assessment of the validity of this prediction and, in
fact, to the evaluation of the whole CSSTT/2 data base.
8. ASSESSMENT OF THE PERFORMANCE OF THE ASSAYS
The purpose of this section is to present an objective
assessment of the performance of the in vivo tests as it
relates to the carcinogenic activity of the test chemi-
cals. (A detailed tabulation of the qualitative results of
each assay is presented in Table 4). Data from a study of
only four chemicals do not, of course, provide sufficient
information on which to judge the overall value of a par-
ticular assay for discriminating between non-carcinogenic
and potentially carcinogenic chemicals. However, when a
data base of this magnitude is available, in which many
tests were replicated in a number of different labora-
tories, then a unique insight into the utility, reproduc-
ibility, and reliability, as well as the accuracy of the
tests, can be obtained. Thus, data from the current study,
together with the considerable experience of the investi-
gators and assessors, enable the performance of many of
the assays to be comprehensively evaluated.
Although a high proportion of test systems performed
reasonably well with these four chemicals, some assays,
not surprisingly, failed to meet the predetermined cri-
teria for acceptable performance (section 4). The rodent
host-mediated and urine mutagenicity assays are good
examples. They involve either the exposure of marker
organisms to the chemicals or its metabolites in vivo or
the detection of mutagenic excretory products in urine of
treated rodents. Both types of tests were conducted in
several laboratories and neither proved capable of ident-
ifying the two carcinogens among the four in vitro muta-
gens, i.e. the host-mediated assays were generally nega-
tive with the four test chemicals while the urine tests
were uniformly positive. These observations suggest that
neither of these two classes of tests has any practical
value in evaluating the in vivo genotoxicity of chemicals
known to be genotoxic in vitro. The results obtained from
the peritoneal macrophage transformation assay suggest a
similar conclusion. This is a form of host-mediated assay
in which the transformation of macrophages, isolated from
the peritoneum of treated rats, is investigated in in
vitro culture. The four test chemicals gave negative
results in each of three laboratories.
Table 4. IPCS CSSTT in vivo study - summary of qualitative
results from individual investigatorsa
-------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
-------------------------------------------------------------------------------------------------
1. CYTOGENETICS
1.1 Chromosomal aberrations
1.1.1 Mouse bone marrow 8 P N NT NT o
8 NT NT P ? ip
1.1.2 Mouse bone marrow 9 P N NT NT o
9 NT NT N N ip
1.1.3 Mouse bone marrow 10 P N N N o
1.1.4 Mouse bone marrow 11 P N N N o
1.1.5 Mouse bone marrow 12 P N P ? o
1.1.6 Mouse bone marrow 13 P NT N N o
1.1.7 Mouse bone marrow 14 P N ? N ip
1.1.8 Mouse ascites tumour cells 13 P NT N N o
1.1.9 Rat bone marrow 15 P N NT NT o
15 NT NT ? N ip
1.1.10 Chinese hamster bone marrow 16 P N P W o
1.1.11 Chinese hamster bone marrow 17 W N W N o
1.2 Micronuclei
1.2.1 Mouse bone marrow 19 P N P N o
1.2.2 Mouse bone marrow 20 P N P N o
1.2.3 Mouse bone marrow 21 P N P N o
1.2.4 Mouse bone marrow 22 P N N N o
1.2.5 Mouse bone marrow 23 P N P N o
1.2.6 Mouse bone marrow 24 P W P W o
1.2.7 Mouse bone marrow 25 P N NT NT o
1.2.8 Mouse bone marrow 26 P N P N o
1.2.9 Mouse bone marrow 27 P N P N o
1.2.10 Mouse bone marrow 28 P N NT NT o
28 NT NT P N ip
1.2.11 Mouse bone marrow 29 P N P N ip
1.2.12 Mouse bone marrow 29 P N NT NT o
1.2.13 Mouse bone marrow 30 P N P N o
30 P N P N ip
1.2.14 Mouse bone marrow 31 P N P N ip
1.2.15 Mouse bone marrow 32 P N P N o
1.2.16 Mouse bone marrow 33 P N P N o
1.2.17 Mouse blood - weanling 34 P N P ? o
1.2.18 Mouse blood - fetal 34 P N N N o
1.2.19 Mouse blood - maternal 34 P N N N o
1.2.20 Mouse blood - fetal 32 P N P N o
1.2.21 Rat bone marrow 35 NT NT P N o
1.2.22 Rat bone marrow 36 P N NT NT o
36 NT NT P W ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
1.3 Sister chromatid exchange
1.3.1 Mouse bone marrow 38 P N P W ip
1.3.2 Mouse bone marrow 39 P W P W o
1.3.3 Mouse bone marrow 40 P N P W o
1.3.4 Mouse bone marrow 41 P W P W ip
1.3.5 Mouse bone marrow 42 P N P N o
1.3.6 Mouse bone marrow 43 P N W N o
1.3.7 Rat bone marrow 44 P W P W o
1.3.8 Mouse lymphocytes 45 P N P N o
1.3.9 Chinese hamster bone marrow 46 P N P N ip
Chinese hamster bone marrow 46 P N P N o
1.3.10 Chinese hamster intestinal cells 46 P N P N o
2. LIVER-SPECIFIC ASSAYS
2.1 Initiation/promotion
2.1.1 Altered enzyme foci 50 NT NT N N o
2.1.2 GGT-positive foci 51 P N P N o
2.1.3 GGT-positive foci 52 P N P P o
2.1.4 GGT-positive foci - promotion 53 P N P W o
2.1.5 GGT-positive foci 54 N N P N o
2.1.6 GGT-positive foci 73 NT NT P N o
2.2 Unscheduled DNA synthesis (UDS)
2.2.1 UDS in mouse liver 56 N N N N o
2.2.2 UDS in rat liver 56 N N P N o
2.2.3 UDS in rat liver 57 NT NT P W o
2.2.4 UDS in rat liver 58 N N P W o
2.2.5 UDS in mouse liver 58 NT NT N N o
2.2.6 UDS in rat liver 59 NT NT P N o
2.2.7 UDS in rat liver 60 NT NT P W o
2.2.8 UDS in rat liver 61 NT NT P N o
2.2.9 UDS in rat liver 62 NT NT P N o
2.2.10 UDS in weanling rat liver 63 N NT NT NT o
2.2.11 UDS - neonate rat 61 NT NT W NT o
2.3 S-phase hepatocytes
2.3.1 Rat 56 W NT NT P o
2.3.2 Mouse 56 NT NT NT W o
2.3.3 Rat 58 N N P P o
2.3.4 Mouse 58 NT NT W P o
2.3.5 Rat 59 NT NT NT P o
2.3.6 Rat - weanling 63 P NT NT NT o
2.3.7 Rat 60 NT NT N P o
2.3.8 Rat 62 NT NT N P o
2.3.9 Rat 61 NT NT NT P o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
2.4 Cytogenetic analysis
2.4.1 Aberrations in liver 65 W N W N o
epitheloid cells
2.4.2 SCE in liver epitheloid cells 65 W N ? N o
2.4.3 Micronuclei in hepatocytes 66 N N P N o
2.5 DNA strand breaks
2.5.1 Alkaline elution 68 N N N N o
2.5.2 Alkaline elution 69 N N N N o
2.5.3 Alkaline elution 70 N W P P o
2.5.4 DNA-DNA/DNA-protein crosslinks 70 P N P NT ip
2.5.5 DNA unwinding 71 N N P N o
2.5.6 DNA unwinding (with araA) 71 P N P N o
3. MISCELLANEOUS ASSAYS
3.1 Carcinogenesis
3.1.1 2-year rat study 73 NT NT P * o
3.1.2 Mouse lung adenoma 74 P N P N o
3.1.3 Quail egg 75 * * * *
3.2 Supplementary assay
3.2.1 Rat fore-stomach UDS 76 ? N N N o
3.2.2 Sebaceous gland suppression 77 P N NT NT der
3.2.3 Epithelial hyperplasia 77 P N NT NT der
3.2.4 Rat hepatocyte ploidy 78 P N P N o
3.2.5 Mouse hepatocyte ploidy 78 P N P N o
3.2.6 Syrian hamster 6-TG-resistant 79 P N ? N ip
lung cells
3.2.7a 32P-post labelling - rat liver 80 P N P W ip
3.2.7b 32P-post labelling - rat lung 80 P N P W ip
3.2.7c 32P-post labelling - mouse 80 P N P NT ip
liver
3.2.7d 32P-post labelling - mouse 80 P N P NT ip
lung
3.2.7e 32P-post labelling - mouse 80 P N P NT ip
kidney
3.2.8 Radiolabelled test compounds 81 NT NT P P ip
3.2.9 ELISA rat 82 NT NT * * o
3.3 Immunotoxicity
3.3.1 T-cells - rat 83 P N P W ip
3.3.2 Natural Killer cells - rat 84 NT NT P N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
3.4 Transformation of peritoneal macrophages
3.4.1 Rat macrophages 85 N N N N o
3.4.2 Rat macrophages 85 N N N N o
3.4.3 Rat macrophages 85 N N N N o
3.5 Host-mediated assays
3.5.1a Mouse/yeast - mitotic 86 NT NT N N o
recombination - liver
3.5.1b Mouse/yeast - mitotic 86 NT NT N N o
recombination - lung
3.5.1c Mouse/yeast - mitotic 86 NT NT N N o
recombination - kidney
3.5.1d Mouse/yeast - mutation - 86 NT NT W N o
liver
3.5.1e Mouse/yeast - mutation - 86 NT NT N N o
lung
3.5.1f Mouse/yeast - mutation - 86 NT NT W N o
kidney
3.5.2a Mouse/yeast - gene conversion 87 P P P P o
liver
3.5.2b Mouse/yeast - gene conversion 87 N N N N o
lung
3.5.3 Mouse/salmonella - liver 88 N N N N o
3.6 Urine mutagenicity
3.6.1 Rat urine extract 89 P P P P ip
3.6.2 Rat urine extract 90 P P P P ip
3.6.3 Mouse urine extract 91 W W P P o
3.6.4a Rat urine 92 P P P P ip
92 P P P P o
3.6.4b Guinea-pig 92 NT NT P NT ip
3.6.5 Rat urine 93 P P P P ip
4. MOUSE SPOT TEST
4.1.1 Coat colour 95 NT NT P N ip
4.1.2 Coat colour 96 NT NT N N o
96 NT NT N NT ip
4.1.3 Coat colour 97 P N P N ip
4.1.4 Melanocytes 98 NT NT P ? ip
4.1.5 Melanocytes 99 NT NT N N ip
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
5. MAMMALIAN GERM CELL STUDIES
5.1 Dominant lethal
5.1.1 Female mice 102 P N N N ip
5.1.2 Male mice 103 P N N N ip
5.1.3 Male mice 104 P N N N ip
104 N ? NT NT o
5.1.4 Male mice 105 P N N N ip
105 N NT NT NT o
5.1.5 Male rat 106 NT NT N N o
5.2 Sperm abnormalities
5.2.1 Mouse 108 P N P N ip
5.2.2 Mouse 109 W N N N o
5.2.3 Mouse 110 P W P N ip
5.2.4 Mouse 111 P N N N o
5.2.5 Rat 112 NT NT N N o
5.3 Unscheduled DNA synthesis
5.3.1 Rat spermatocytes 113 N N N N o
5.3.2 Mouse sperm 114 N N N N ip
114 N NT NT NT o
6. DROSPHILA ASSAYS
6.1 Sex-linked recessive lethal
6.1.1 SLRL assay 116 N N P W o
6.1.2 SLRL assay 117 N N N N o
6.1.3 SLRL assay 118 N N N N o
6.1.4 SLRL assay 119 N N N N o
6.1.5 SLRL assay 120 N N N N o
--------------------------------------------------------------------------------------------------------------
Table 4. (contd.)
--------------------------------------------------------------------------------------------------------------
Assay Chapter BP PYR 2AAF 4AAF Route of
numbersb exposure
--------------------------------------------------------------------------------------------------------------
6.2 Chromosome loss
6.2.1 Germ cell 121 N N N N o
6.2.2 Germ cell 116 N NT N NT o
6.3 Somatic mutation and recombination
6.3.1 Eye spots 116 P N W W o
6.3.2 Eye spots 122 P N P N o
6.3.3 Wing spots 118 P N W ? o
--------------------------------------------------------------------------------------------------------------
a From: Ashby et al. (1988)
b Numbers refer to chapters in Ashby et al. (1988)
P Positive
NT Not tested
N Negative
? Inconclusive
W Weak positive
* Results not available
8.1 Cytogenetic assays
Assays that utilize target cells in rodent bone marrow
for detecting chromosomal effects, i.e., chromosomal aber-
rations, micronuclei, or sister chromatid exchanges, are
widely advocated for investigating the activity of in
vitro genotoxins in the intact animal and represent the
largest group of tests in this study.
8.1.1 Chromosomal aberrations
Data from metaphase chromosome studies in mouse bone
marrow were provided by seven investigators. Each investi-
gator successfully discriminated between BP and PYR, and
five sets of data determined 4AAF to be negative while two
were inconclusive. The results of chromosome studies with
2AAF, however, were conflicting. Two laboratories identi-
fied 2AAF as a clastogen, one set of data were inconclus-
ive and four investigators reported 2AAF as negative.
There appears to be no simple explanation for these dis-
crepancies, which were not associated with differences in
mouse stain, route of treatment, sampling interval, or
other aspects of experimental design. The mouse micro-
nucleus assay (section 8.1.2) showed 2AAF to be an in vivo
clastogen (though less potent than BP) and it is clear
that the dosing schedules used in the metaphase chromosome
assays were more than adequate to induce chromosome aber-
rations, i.e., 2AAF would be expected to be detected as a
clastogen in the metaphase chromosome assay. One expla-
nation for the negative findings in four laboratories may
be the resolving power of the sample sizes used. Most
standard protocols for the bone marrow metaphase assay
require at least 50 cells per animal from 10 animals per
dose/time group (i.e., 500 cells) to be analysed for aber-
rations at each data point. This recommended minimum fig-
ure was only achieved by three of the seven investigators
and three others presented data on only 250 cells per
dose/time group. These observations suggest that, with
samples of this size, almost a 10-fold increase in
aberrations over the control incidence would be required
to demonstrate a significant induction of aberrations by
2AAF.
Two investigators conducted studies in Chinese hamster
bone marrow cells. PYR was negative in both studies. In
experiments in which 500 cells per dose/time group were
analysed, BP and 2AAF were clearly positive while, when
only 300 cells were used, the results were classified as
weak positive with both carcinogens. It is interesting to
note that 4AAF induced a weak positive response in Chinese
hamster bone marrow in one study.
One set of data from rat bone marrow was presented and
clearly separated BP from PYR. 2AAF, however, gave
unambiguous data and 4AAF was negative.
The performance of the bone marrow cytogenetic assays
did not, in general, meet the criteria for a reliable and
reproducible short-term in vivo test. There are, however,
serious reservations regarding the numbers of animals and
the numbers of cells analysed in many of the studies and
the results suggest that protocols designed to give an
acceptable resolving power should be strictly adhered to.
8.1.2 Micronuclei
Eighteen laboratories conducted micronucleus tests; 16
laboratories performed assays on bone marrow cells from
mice and two investigators used rats. Data were also
presented on the incidence of micronuclei in circulating
blood cells from adult and fetal mice.
Mouse bone marrow micronucleus assays were conducted
to a common protocol with only minor deviations. At least
5 mice were used for each dose/time interval with 1000
cells being analysed from each animal. The data from these
assays showed a high level of agreement between labora-
tories. Seventeen sets of data on BP were uniformly posi-
tive and 16 sets on PYR were negative. Similarly, 14
investigators showed 2AAF to be capable of inducing an
increase in micronuclei and 4AAF was negative in 14 cases.
There were only occasional anomalous results. For example,
one laboratory failed to detect 2AAF, and another investi-
gator recorded weak positive results from both PYR and
4AAF. These anomalies do not detract from the general
accuracy and reproducibility of mouse micronucleus tests
with the four test chemicals.
One investigator used erythrocytes from mouse periph-
eral blood to assay the incidence of micronuclei. The
results were similar to those obtained in the bone marrow
studies, i.e. BP and 2AAF were positive while PYR and 4AAF
were considered to be negative by the criteria adopted for
a positive response. In two laboratories, fetal blood was
obtained from mice on day 15 of pregnancy, 30 or 48 h
after oral dosing of the mother. BP was positive and PYR
was negative in each of these studies. The fetal assay was
less sensitive to the effects of 2AAF, one laboratory
observing a small increase in micronuclei and the other
finding no increase. 4AAF was inactive in fetal cells.
Two micronucleus studies were conducted in rat bone
marrow cells and the results suggest that the response in
the rat was weaker than that in the mouse. In one
laboratory, 2AAF was positive and 4AAF negative; BP and
PYR were not tested. The second investigator reported a
positive response with BP and a negative one with PYR.
This investigator's result with 2AAF is shown as positive
in Table 4. However, using criteria decided by the
micronucleus assay working group, it was concluded that
2AAF was negative in this assay. It was also considered
that the protocol used in the rat studies was not designed
for the optimum detection of micronuclei.
The results from the micronucleus tests confirm the
value of the assay in mouse bone marrow as an accurate and
reproducible means of qualitative discrimination between
carcinogenic and non-carcinogenic chemicals.
8.1.3 Sister chromatid exchange
Nine investigators presented data on the induction of
sister chromatid exchanges (SCE) in vivo using mice, rats,
or Chinese hamsters. The test chemicals were administered
by gavage or intraperitoneal injection and most investi-
gators analysed SCE in bone marrow cells. One set of data
were presented on SCE in mouse peripheral lymphocytes and
another set on Chinese hamster intestinal epithelial
cells. The two established carcinogens, BP and 2AAF,
induced an increase in SCE in each of the six studies
conducted in mouse bone marrow. In two of these studies,
PYR induced a weak positive response and 4AAF was weakly
positive in four of the six assays. Weak positive results
were also obtained in rat bone marrow assays with the two
presumed non-carcinogens, while BP and 2AAF were clearly
positive. In experiments conducted in blood lymphocytes
in mice and in bone marrow cells and intestinal epithelial
cells in Chinese hamsters, BP and 2AAF gave positive
results while PYR and 4AAF were negative. None of the
experiments suggested that the route of administration of
the test chemicals significantly influenced the qualitat-
ive results.
Both the established carcinogens were consistently
positive in tests for the induction of SCE in three rodent
species, suggesting that the SCE assay is a sensitive and
reproducible in vivo test for genotoxic carcinogens. PYR
or 4AAF, however, induced small (but significant) in-
creases in the incidence of SCE in 8 of 22 tests, although
the presumed non-carcinogens were much less potent
inducers of SCE than their carcinogenic analogues. These
observations raise a number of questions regarding the
significance of small increases in SCE in in vivo tests.
In the first instance, if 4AAF is confirmed to be non-
carcinogenic in the current rodent bioassay, then these
weak responses have to be regarded as `false positives'.
If, however, 4AAF is shown to have weak carcinogenic
activity, then the weak positive responses obtained in
four of the six mouse bone marrow studies can be inter-
preted as accurately reflecting the genotoxicity of 4AAF.
In the report of the SCE Working Group (Tice et al., 1988)
the biological relevance of statistically significant
increases in SCE at unrealistically high doses was con-
sidered. It was suggested that multiple mechanisms are
involved in the formation of SCE and that one of these may
be a response to stress, either at the cell or animal
level, caused by a toxic response to high doses of a
chemical. The small induction of SCE observed after high
doses of PYR or 4AAF, therefore, may not be related
mechanistically to the larger increases in SCE seen after
low doses of BP or 2AAF. Thus, the final assessment of
the performance of the in vivo SCE assays depends, to a
large extent, on the outcome of the 2-year bioassay with
the AAF analogues, i.e. a negative result with 4AAF will
cast serious doubts on the value of SCE tests for investi-
gating the in vivo activity of in vitro genotoxins.
8.2 Liver assays
The activity of certain genotoxic chemicals appears to
be confined to the liver. Such chemicals do not, of
course, induce detectable genetic changes in bone marrow
cells or other extra-hepatic tissues and this has led to a
need to develop techniques for investigating genotoxic or
related changes in liver cells. The main thrust of this
research has centred on the demonstration of unscheduled
DNA synthesis in hepatocytes but data were also presented
from a variety of other assays that used target cells in
the liver.
8.2.1 Initiation and promotion
Two of the assays used in this group were specifically
aimed at detecting initiating activity of the test chemi-
cals. The chemicals were administered after partial hepa-
tectomy, and the mice were then treated with the promotor,
phenobarbital, for several weeks before liver sections
were examined for the occurrence of pre-cancerous foci,
i.e. groups of cells having elevated levels of the marker
enzyme, gamma-glutamyl-transpeptidase (GGT). Both these
protocols have been used successfully with many test
chemicals and, in the present study, successfully separ-
ated BP and 2AAF from PYR and 4AAF. A third protocol was
used to investigate the promoting activity of the test
chemicals after initiation by pre-treatment with dimethyl-
nitrosamine. This, too, successfully separated BP from
PYR, but, although 2AAF produced an increase in GGT-
positive foci, a weak positive response was also observed
with 4AAF. The two other procedures used to study
initiation/promotion events based on altered enzyme foci
failed to detect BP in one assay and 2AAF in the second
assay and are considered to be, primarily, research
techniques and not yet suitable for routine application.
The two better-established assays showed quite clearly
that both BP and 2AAF initiated altered enzyme foci in rat
liver. The two presumed non-carcinogens were completely
negative and these tests, although they cannot be regarded
as "short-term" in the true sense, have a useful role in
investigating liver carcinogenesis.
8.2.2 Unscheduled DNA synthesis and S-phase analysis
The investigators in this group used the detection of
unscheduled DNA synthesis (UDS) as a measure of DNA repair
initiated in response to chemical-induced damage to the
DNA. In each study, UDS was detected using autoradio-
graphic techniques that also allowed simultaneous measure-
ment of S-phase cells, i.e. those cells undergoing normal
DNA synthesis as a prelude to mitosis. Seven investi-
gators examined 2AAF and 4AAF for the induction of UDS and
S-phase cells in hepatocytes of male rats, and in two
laboratories all four test chemicals were investigated in
rats and mice. The induction of UDS and S-phase cells in
rat fore-stomach was investigated in another laboratory.
Although there were minor protocol variations, the UDS
working group concluded that none of these significantly
affected the results and there was excellent agreement in
the observations and conclusions of the investigators.
2AAF was shown to be a potent inducer of UDS in rat
hepatocytes and also caused proliferation of hepatic cells
as shown by an increase in the number of cells in S-phase.
In mice, however, 2AAF failed to induce SCE in either of
two laboratories, though there was evidence of a weak
induction of S-phase cells. Only low doses were examined,
i.e. up to 50mg/kg, but the results indicate an important
species difference in the response to 2AAF.
Administration of 4AAF to rats produced negative UDS
results in four laboratories and weak positive results in
three laboratories. Although the three weak positive
results were initially determined without recourse to
statistical analysis, a comprehensive analysis of the data
by Margolin and Risko (1988) confirmed the initial
interpretation. The six investigators who recorded the
number of S-phase cells observed a marked increase in
hepatic cell proliferation in rats dosed with 4AAF. This
compound failed to induce UDS in mouse liver but did cause
an increase in S-phase cells.
For comparative purposes, two laboratories also con-
ducted UDS tests on hepatocytes in vitro. Both 2AAF and
4AAF induced UDS in rat hepatocytes but were negative in
mouse cells.
BP and PYR were tested in rats by two investigators
and one assay was conducted in mice. Both chemicals
failed to induce UDS in either species after oral dosing.
In contrast, BP gave positive results in both rat and
mouse hepatocytes treated in vitro.
In an investigation of the UDS activity of the four
chemicals in rat fore-stomach, 2AAF, 4AAF, and PYR failed
to induce UDS while BP gave equivocal results.
The performance of the UDS assay in rodents dosed with
2AAF or 4AAF is worthy of further consideration. 2AAF is
a potent rat hepatocarcinogen and a potent inducer of UDS
in rat hepatocytes. In the mouse, however, 2AAF did not
induce UDS and, on balance, available data suggest that it
does not produce liver tumours. Thus, for this chemical,
the UDS results accurately reflect its hepatocarcinogenic
potential. The current lack of cancer data on 4AAF pre-
cludes this kind of comparison, but the UDS data suggest
that if 4AAF is carcinogenic, it is markedly less so than
2AAF.
Although fewer tests were conducted with BP and PYR,
the data indicate that neither chemical induces UDS in rat
or mouse liver, although BP was positive in in vitro UDS
tests. The reason for this negative response to BP has an
important bearing on the validity of the UDS assay for
detecting potentially carcinogenic chemicals. Although BP
is an established carcinogen, it has never been shown to
produce tumours in rodent liver. There is sufficient
evidence, however, to suggest that DNA-reactive adducts
are formed in liver cells after dosing rodents with BP.
For example, most of the data that show BP to be genotoxic
in vitro are derived from tests in which metabolic acti-
vation is provided by an enzyme mixture derived from rat
liver. In addition, BP clearly induces altered enzyme
foci in rat liver, there is tentative evidence for the
induction of DNA strand breakage based on DNA-DNA/DNA-
protein crosslinks, and 32P-post-labelling techniques in-
dicate the presence of DNA adducts of BP metabolites in
both rat and mouse liver. Therefore, although BP is not a
hepatocarcinogen in rodents, there are data to suggest
that (a) BP is metabolized in rodent liver to DNA-reactive
metabolites, (b) interaction of DNA with BP metabolites
does occur, and (c) based on data from the DNA unwinding
test, some repair of the DNA lesions takes place. On this
basis, the liver UDS assay should, theoretically, be
capable of detecting BP-induced lesions in the liver. All
these observations indicate that such factors as the
distribution of BP to the liver after oral dosing, the
rate of formation of genotoxic metabolites, the rate of
detoxification, and the rate and nature of the DNA repair
process yield an insufficient number of DNA adducts to
produce a detectable UDS response.
In practice, of course, BP is easily detected in other
in vivo tests such as those conducted in bone marrow
cells. However, the results with BP and, to a lesser
extent, 4AAF, suggest the need for additional evaluation
of the performance of the in vivo UDS assay with liver-
specific genotoxins.
8.2.3 DNA strand breaks
Data were considered from three different assay sys-
tems used to detect single-strand breaks in DNA. Results
from the alkaline elution assay, in which two laboratories
found no activity with any of the four test chemicals,
indicate that this assay is of little value in investigat-
ing in vivo genotoxicity. The test for DNA-DNA/DNA-protein
crosslinks and the DNA unwinding assay in rodent liver
cells both gave very promising results and warrant further
evaluation.
8.2.4 Cytogenetics
Three sets of data were considered in this group.
Micronucleus assays were conducted in rat liver cells
following partial hepatectomy. The results were similar to
those obtained in the rat liver UDS assay, i.e. a dose-
dependant response was obtained with 2AAF, while the
remaining three chemicals gave negative results. The
other two data sets were generated from analyses of
chromosome aberrations and SCE in epithelial-like cells
derived from weanling rat liver. Although the assays are
relatively simple with clearly defined end-points, the
results were either equivocal or negative and the sample
sizes, i.e. the numbers of animals/cells per dose group,
were too small to permit an effective assessment. Both
the micronucleus test and the chromosome assays are worthy
of further evaluation.
8.3 Miscellaneous assays
This section contains a wide variety of assays, some
of which have been in use for many years while others, few
of which were undertaken in more than one laboratory, are
relatively new or in the process of development.
8.3.1 Specific carcinogenicity assays
Neither of the two procedures considered here can be
regarded as short-term tests and final results from one of
them, the assay in quail eggs, were not available at the
time of the assessment. The results of the mouse adenoma
assay, however, were available and showed a decisive dis-
crimination between BP and PYR and a much smaller and less
convincing difference between 2AAF and 4AAF.
8.3.2 Supplementary assays
Data from the sebaceous gland suppression test and the
epidermal hyperplasia assay have shown a good correlation
with skin carcinogenicity and have proved useful for com-
paring the carcinogenic potential of polycyclic hydrocar-
bons. Their value in this area was confirmed by the re-
sults of assays with BP and PYR but the application of
these assays to other classes of chemicals is of uncertain
value.
The hepatocytes of rodents include mono- and binu-
cleated cells and nuclei that may be diploid, tetraploid,
or octaploid. The ratio of cells with different levels of
ploidy in liver varies with species and age and can be
altered by exposure to certain chemicals. There have been
references to the effects of various chemicals on liver
ploidy ratios over a number of years, but there appears to
be no consistent relationship between ploidy changes and
chemical carcinogenesis. Thus the observation that the two
carcinogens, BP and 2AAF, increase the ratio of diploid to
tetraploid cells is of uncertain significance. The assay
may be of value in studying liver cell kinetics but its
value in the detection of in vivo genotoxicity is debat-
able.
The general principles of the in vivo/in vitro somatic
mutation assay were developed in the late 1970s and showed
initial promise as a test for investigating organ-specific
mutations induced by chemical carcinogens. One
investigator provided data from a modification of this
technique in which cells isolated from lung tissue of
treated Syrian hamsters were cultured in vitro and the
induction of mutations was determined. A reproducible
increase in mutations was observed in lung cells after
dosing with BP, but the results with 2AAF and the two non-
carcinogens were considered to be negative. Although this
is a logical and potentially useful approach to studying
in vivo genotoxicity, the method requires much wider
evaluation before it can be considered as an acceptable
assay.
The detection and identification of DNA adducts
provides conclusive evidence that a metabolite of the
administered chemical has interacted with one of the
important target macromolecules of carcinogenesis. Theor-
etical considerations indicate that, for many chemical
classes, the formation of DNA adducts is a prerequisite
for permanent genetic changes, such as mutations, and for
the initiation stage of carcinogenesis. Research has shown
a direct relationship between the administered dose of a
chemical and DNA-adduct formation, so that precise dosi-
metry in specific organs is possible. Three approaches
were used in the study, though results from a novel tech-
nique using monoclonal antibodies to identify specific
adducts are not yet available. One study involved the use
of radiolabelled 2AAF and 4AAF; while binding to liver DNA
was detected with both compounds, the covalent binding of
2AAF was approximately seven times that of 4AAF. These
data correlate well with the results of the UDS assay in
rat liver, which showed 2AAF to be a potent inducer of UDS
while 4AAF was, at best, a weak inducer.
The above assay has the disadvantage of requiring
radiolabelled test chemicals, and a significant advance in
the field was the introduction of techniques in which
animals are dosed with unlabelled test chemicals and the
DNA is radiolabelled after isolation and purification. The
32P-labelled normal nucleotides and nucleotide adducts
are separated by thin-layer chromatography and detected by
autoradiography, and a comprehensive, organospecific DNA
adduct profile is obtained. The data presented in the
current study showed that 2AAF adducts were detected at
significant levels in the DNA of mouse liver, lung, and
kidney and in rat liver and lung. 4AAF adducts were not
detected in mouse tissue and at only very low levels in
rat liver. PYR adducts were not detected in either rat or
mouse tissues and BP produced adducts with DNA in liver
and lung of both species and in mouse kidney.
The detection and identification of DNA adducts is
potentially an extremely powerful tool in the investi-
gation of in vivo genotoxicity. With the establishment of
`cold' techniques, i.e. those not requiring radiolabelled
test chemicals, this type of assay can be applied to most
chemical classes. Although further development is re-
quired, particularly with regard to the genotoxic signifi-
cance of different types of adduct, the method shows great
promise for the future.
8.3.3 Immunotoxicity assays
In the Natural Killer (NK) cell assay, the activity of
NK cells was measured at intervals after dosing rats with
either 2AAF or 4AAF. The carcinogen, 2AAF, induced a
significant change in NK cell cytotoxicity that was not
observed after dosing with 4AAF. The T-cell assay, in
which immunocompetent T-cells are able to react to
carcinogen-induced tissue changes, responded positively to
BP and 2AAF; PYR failed to induce any change in reactivity
and 4AAF gave a weak positive response. The results of
both types of assay closely reflected the carcinogenic
activity of the test chemicals. The relevance of the
observed immunological changes to the carcinogenic process
is unclear and, thus, their potential value in detecting
precarcinogenic changes caused by genotoxic and non-
genotoxic carcinogens remains to be fully evaluated.
Before they can be accepted as anything more than a useful
research tool, further investigations of the specificity
of the immunological reactions and a wider evaluation are
imperative.
8.3.4 Host-mediated assays and urine mutagenicity tests
As described at the beginning of Section 8, both these
classes of test failed to meet the criteria for an accept-
able short-term in vivo assay. The host-mediated assay was
a favoured in vivo procedure in the early 1970s, though in
recent years questions have been raised concerning its
sensitivity. Although the intrasanguinous modification to
the original intraperitoneal technique offered theoretical
advantages, the results of the present study confirmed the
inadequacy of the assay. It is concluded that both these
procedures are not appropriate for the investigation of in
vitro genotoxins.
8.4 Mouse spot tests
Although data were presented from two types of mouse
spot test, they are, in effect, different means of observ-
ing the same genetic alterations, i.e. the melanocyte test
detects the change at the cellular level while the coat
colour test demonstrates the expression of the cellular
changes.
BP and PYR were only tested in one laboratory and the
spot test successfully identified the carcinogen. In tests
with 4AAF, one investigator's results were considered to
be inconclusive and four other sets of data, including one
after oral dosing and three after intraperitoneal admin-
istration, were negative. Six laboratories tested 2AAF;
there were three positive results and two negatives after
intraperitoneal administration. 2AAF was also negative
after oral dosing. Examination of the protocols indicated
that 2AAF induced detectable genetic changes only after
administration of the material as a solution in dimethyl-
sulfoxide, i.e. negative results followed the admin-
istration of corn oil formulations of 2AAF, illustrating
the critical effect of the vehicle on the absorption of
the test chemical.
An overview of the mouse spot test data indicates that
intraperitoneal administration of an appropriate formu-
lation of the test chemicals produces results that reflect
their carcinogenic activity. The original coat colour
procedure appears to be more reliable than the melanocyte
technique, which may be influenced by sporadic mutational
events and inadequate melanization of the hair follicles.
However, the dependence on the intraperitoneal route of
administration may be a disadvantage, and the mouse spot
test appears to add very little to the information gained
more easily from bone marrow cell cytogenetic assays.
8.5 Assays in mammalian germ cells
For assessment purposes the germ cell assays have been
separated into two groups: those that are considered to
involve a direct interaction with DNA, and the assay for
sperm abnormalities, the genetic significance of which is
uncertain.
8.5.1 Dominant lethal and unscheduled DNA synthesis assays
Classical dominant lethal tests were conducted in male
or female mice and in male rats. PYR, 2AAF, and 4AAF gave
unequivocally negative results after intraperitoneal
dosing. In an oral dosing study in male mice, BP was nega-
tive and PYR produced inconclusive data. After intraper-
itoneal administration of BP, however, there was conclus-
ive evidence of dominant lethal induction in male and
female mice. In the male, dominant lethality was confined
to the late spermatid/spermatozoa stages of spermatogen-
esis.
Results of UDS assays in rat spermatocytes after oral
dosing and in mouse spermatocytes after oral or intraper-
itoneal administration were negative with all four chemi-
cals. The negative data with BP are not altogether sur-
prising as dominant lethality was only observed in late
spermatids/spermatozoa, in which the DNA is highly con-
densed and in which UDS does not occur.
Based on the dominant lethal test results, BP is a
confirmed, though relatively weak, germ cell mutagen in
mice after intraperitoneal dosing. Published data indicate
that BP does not induce heritable translocations at doses
that produce dominant lethality (Generoso et al., 1982),
although this may be due, in part, to the fact that BP
appears to induce mainly chromatid deletions in bone
marrow cell chromosomes, with very few exchange-type aber-
rations.
8.5.2 Sperm abnormality tests
This assay is based on the observation of morphologi-
cal alterations in mature spermatozoa after treatment of
animals at all stages of spermatogenesis with the test
chemicals. BP induced an increase in the incidence of
abnormal sperm in mice after oral or intraperitoneal
dosing and 2AAF was also positive following oral gavage.
Tests with 4AAF were uniformly negative and PYR produced a
weak positive response in one of four assays. Thus, both
carcinogens produced sperm abnormalities under appropriate
experimental conditions.
The important questions raised by these observations
relate to the relevance of morphological abnormalities in
sperm to germ cell mutations and to carcinogenesis.
Although changes in sperm head shape may be genetically
determined, it is uncertain whether they are due to geno-
toxicity or to other toxic phenomena. A positive result
in the test, therefore, indicates that the test material
or its metabolites are able to reach the germ cells in
sufficient quantities to induce an adverse biological
effect. Although a good correlation has been shown between
the induction of sperm abnormalities and carcinogenic
activity (Wyrobek et al., 1983), the relevance of this
correlation to short-term testing strategies is doubtful
as more appropriate in vivo tests are available, e.g.,
bone marrow cytogenetic assays. Equally doubtful is the
relevance of the test to heritable genetic damage, as
there is no clear-cut evidence that abnormal sperm are a
consequence of induced DNA damage.
8.6 Drosophila assays
The sex-linked recessive lethal assay and the test for
chromosome loss in drosophila germ cells failed to show a
consistent separation of BP and 2AAF from PYR and 4AAF,
respectively. Three tests based on somatic cell changes
were positive with BP and negative with PYR but the
results of tests with the acetylaminofluorenes were less
decisive. 2AAF produced an unambiguous positive result
and two weak positives, while the three results with 4AAF
were weak positive, negative and inconclusive. Thus, the
drosophila germ cell assays were insensitive to the four
in vitro genotoxins and, although the somatic cell pro-
cedures appeared to be more sensitive, it was concluded
that the main value of drosophila tests is to provide com-
prehensive analyses of the genetic mechanisms involved in
the induction of mutations by chemicals.
9. SELECTION OF THE MOST EFFECTIVE IN VIVO ASSAYS IN RELATION
TO THEIR PERFORMANCE
In a study of this nature it is inevitable that some
assays, even well-established procedures, will fail to
perform well. Indeed, the recognition of inadequate tests
is as important a goal as the identification of those that
are useful and acceptable. Other assays produced results
that correlated well with the presumed carcinogenic status
of the test chemicals but are not widely used or available
or are still in the process of development. Yet other
tests that performed adequately in the collaborative study
cannot be regarded as practicable short-term tests either
because of their technical complexity or because of the
length of time required to produce results. Most of the
latter group of tests have important research roles in
carcinogenesis.
9.1 Assays that are not considered appropriate for routine
in vivo testing of chemicals for genotoxic activity
Host-mediated and urine mutagenicity assays (section
8.3.4), the peritoneal macrophage transformation assay,
and the alkaline elution assay for single strand breaks
(section 8.2.3) proved incapable of separating the car-
cinogen/non-carcinogen pairs and appear to have little
value in investigating in vivo genotoxicity. The in
vivo/in vitro somatic mutation procedure in Syrian
hamsters (section 8.3.2) also falls into this category,
although this conclusion should not detract from the
initial promise of this approach in Chinese hamsters
(McGregor, 1988a).
Drosophila tests (section 8.6) have also proved gener-
ally inadequate in the identification of in vivo geno-
toxins and it is concluded that their main value in gen-
etic toxicology is to provide analysis of the genetic
mechanisms involved in the induction of mutations. Similar
reservations apply to the initiation/promotion techniques
using liver foci (section 8.2.1) and the analysis of
ploidy levels in liver tissue (section 8.3.2). Both assays
are more appropriate to investigation of the carcinogenic
process than to the identification of in vivo genotoxins.
The results of the immunological assays (section 8.3.3)
closely reflected the carcinogenic potential of the
chemicals, but they can only be regarded as research tools
pending a greater understanding of the specificity of the
immunological reactions and a wider evaluation of the
procedures.
Two assays conducted in mouse skin, i.e. the seb-
aceous gland suppression test and the observation of epi-
dermal hyperplasia, confirmed their value in the detection
of carcinogenic polycyclic hydrocarbons such as BP. Both
tests failed to respond to 2AAF when applied dermally and
are not considered to be suitable for investigating in
vivo genotoxicity in general.
9.2 Assays that satisfy some or all of the criteria for an
acceptable short-term in vivo test
9.2.1 Assays currently in general use
The mouse micronucleus test (section 8.1.2) was the
most widely represented single assay in the study and
produced the best overall performance with only occasional
anomalous data. Qualitative results were not affected
significantly by the route of exposure, strain or sex of
mouse, or treatment schedule. The mouse bone marrow
micronucleus assay was confirmed as a robust and sensitive
short-term in vivo test for genotoxic chemicals.
Both the micronucleus test and the analysis of meta-
phase chromosome aberrations in rodent bone marrow respond
to similar chromosome breakage events and they would be
expected to produce qualitatively similar results with the
four test compounds. Table 4 shows that this was not the
case and the metaphase chromosome procedure was signifi-
cantly less sensitive than the micronucleus test. Poss-
ible explanations for this lack of sensitivity are dis-
cussed above (section 8.1.1) and suggest that the use of
metaphase chromosome analysis should not be undervalued on
the basis of these data.
The sister chromatid exchange (SCE) procedure repro-
ducibly detected the two carcinogens, but also produced a
number of weak positive results with 4AAF and PYR. Taken
at face value, therefore, the in vivo SCE results clearly
reflect the relative potency of the two chemical pairs in
in vitro tests. As discussed in section 8.1.3, however,
very weak SCE responses may be caused by non-genotoxic
mechanisms and a final judgement on the significance of
the weak activity of 4AAF must be delayed until its true
carcinogenic status is defined.
After considering the data from the three classes of
bone marrow assay, the conclusion of the Steering Group
(Ashby et al., 1988) was - `the mouse micronucleus assay
provides the simplest and most effective measure of geno-
toxicity in the bone marrow and, as such, it is rec-
ommended here as a primary in vivo genotoxicity test'.
Despite the successful performance of the micronucleus
test with these four chemicals, it is apparent from the
literature that certain liver carcinogens do not induce
detectable chromosomal effects in the bone marrow. In an
acceptable testing strategy, therefore, negative results
in the micronucleus test should be supplemented with
evidence of non-genotoxicity in the liver. This concept
led to the extensive evaluation of liver-specific assays
in the CSSTT/2 study and the main candidate in this group
of tests was the rat liver UDS assay (section 8.2.2).
The liver carcinogen, 2AAF, was a potent inducer of
UDS in rat liver while most investigators defined its
presumed non-carcinogenic analogue, 4AAF, as a weak
genotoxin in this system. Unlike the micronucleus test
result, BP was devoid of activity in liver UDS assays.
This is consistent with its lack of carcinogenicity in rat
liver.
Although the significance of the weak liver UDS
activity of 4AAF will only be resolved when definitive
carcinogenicity data are available, the findings raise
certain problems of interpretation that are common, in
part, to the weak bone marrow activity of 4AAF and PYR.
In the UDS assays, 2AAF was a potent inducer of UDS at
doses between 5 and 100 mg/kg while 4AAF showed only weak
activity between 50 and 1000 mg/kg, and then only in some
experiments. In general, the activity of 4AAF was within
the range of control variability and, as a result, the
UDS Working Group questioned its biological significance
(Mirsalis, 1988). A detailed analysis, however, indicated
the high statistical significance of the 4AAF data
(Margolin and Risko, 1988). Thus, although the rat liver
UDS assay discriminated quantitatively between 2AAF and
4AAF, the significance of the weak activity of 4AAF
remains unresolved. These observations suggest that
qualitative determinations in terms of `positive' and
`negative' are too simplistic for hazard assessment
purposes and one of the most useful points raised by the
present study is the need for appropriate positive test
criteria, in biological and statistical terms, for widely
used in vivo tests.
Thus, the mouse bone marrow micronucleus assay is the
method of choice for primary in vivo testing of in vitro
genotoxins and the rat liver UDS assay, providing the
above problems can be resolved, appears to be the most
promising complementary liver-specific test.
9.2.2 Assays that show promise for future development
Accepting that bone marrow assays are suitable for
detecting extra-hepatic somatic cell genotoxins and that
the rat liver UDS assay can, with certain reservations
(see sections 8.2.2 and 9.2.1), be used to investigate
liver-specific genotoxins, it is useful to consider which
other assays evaluated in this study may have a role in
the assessment of in vivo genotoxicity.
Development of assays to detect single-strand breaks
in DNA and to detect and identify DNA adducts will,
undoubtedly, continue. It will be interesting to follow
the progress of the assays for DNA-DNA/DNA-protein cross-
links and DNA unwinding, although it is probable that
their technical complexity will limit widespread use and
acceptance. Of the DNA adduct techniques represented, the
post-labelling method appears to hold most promise. The
need for radiolabelled test compounds or specific mono-
clonal antibodies is eliminated and the assay provides
information on the direct interaction of test chemicals
with DNA in a variety of target organs, including the
liver. Providing a reliable and robust protocol can be
established and pending the provision of more information
regarding the relationship between DNA adduct formation
and genotoxic changes to the DNA, this assay could have an
important role to play in the assessment of genotoxic
hazard.
The performance of the cytogenetic assays in liver
cells was disappointing but, theoretically, they could,
with further development, prove to be of value in the
assessment of liver-specific genotoxicity. The reliance
of the current micronucleus procedure on partial hepa-
tectomy is a disadvantage, but the development of alterna-
tive means of stimulating hepatic mitotic activity, either
in vivo or in short-term culture, could be the basis of a
useful short-term liver-specific assay. Cytogenetic
assays on circulating blood cells performed reasonably
well and the theoretical and practical advantages of tests
for SCE or micronuclei in circulating blood should
encourage further development of these tests.
The mouse spot test, in its original form (section
6.4), successfully separated the carcinogen/non-carcinogen
pairs and, providing care is taken in the selection of an
appropriate solvent or formulation, it appears to be a
useful and reproducible assay. Its availability is limited
by the need to maintain specific mouse strains and its
overall sensitivity, compared to the micronucleus test, is
suspect, as positive results in the present study were
dependant on intraperitoneal dosing.
9.3 The detection of germ cell mutagens
The performance and significance of the three germ
cell assays represented in the study are discussed above
(section 8.5). Only BP was clearly identified as a germ
cell mutagen in the dominant lethal assay. The failure of
the sperm abnormality test to discriminate between BP and
2AAF suggests that this assay is affected by non-genetic
toxic effects and is, therefore, of doubtful value in
predicting the induction of mutations in mammalian germ
cells.
Although there are theoretical reasons why BP should
not induce UDS in germ cells (section 8.5.1), this nega-
tive result raises questions similar to those resulting
from the failure of BP to induce UDS in rat liver (section
8.2.2), i.e. why does BP not induce detectable UDS in
target cells where there is evidence, from other assays,
of genotoxic activity?
As concluded by Ashby et al. (1988) `the germ cell
activities of BP have served to focus several important,
but unresolved, issues associated with the conduct of, and
the interpretation of data derived from, germ cell
genotoxicity and mutagenicity assays'.
9.4 Influence of the route of administration of the test chemicals
A feature of the micronucleus assays was that their
qualitative separation of BP and 2AAF from PYR and 4AAF
was not influenced by the route of administration of the
chemicals. Dominant lethal mutations, however, were only
detected after intraperitoneal dosing with BP; oral dosing
was ineffective. Similarly, the mouse spot test was
dependant on the intraperitoneal route of administration
for the demonstration of mutagenic effects. The intro-
duction of the test material in the vicinity of the uterus
in the spot test circumvents the natural pharmacokinetics
and metabolic routes of the test animal, and it can be
argued that this unrealistic pattern of exposure does not
reflect true in vivo genotoxicity. It can be similarly
argued that the purpose of in vivo tests is to achieve
maximum exposure of the target cells and that intraper-
itoneal dosing can be justified for this reason. It may
be, as suggested by Shelby (1986), that intraperitoneal
dosing should be reserved for assays used in a screening
mode, i.e. for the detection of genotoxic activity, and
that for investigating the in vivo activity of established
genotoxins, a route of administration should be chosen
that reflects possible human exposure. Arguments such as
these must be resolved before strategies for testing
chemicals for genotoxic activity can be fully harmonized.
10. CONCLUSIONS
Of the fifty or so separate in vivo techniques rep-
resented in the CSSTT/2 study, only a small number satis-
fied the criteria defined for an acceptable short-term in
vivo test (section 3.2). These criteria, however, defined
a very specific purpose, i.e. the identification of in
vivo activity of established in vitro genotoxins. In order
to generate a comprehensive data base on the four test
chemicals, assays included in the study were not limited
to, for example, those that were considered most likely to
meet the criteria. As a result, some investigators sub-
mitted data from assays that were not designed for detec-
ting in vivo genotoxins but, nevertheless, provided valu-
able information on a broad spectrum of biological effects
of the test chemicals. The failure of such tests to
satisfy the narrow selection criteria used in CSSTT/2
should not be regarded as a reflection on the validity of
the procedures for specific research purposes. With these
considerations in mind, and based on the concept (section
3.2) that short-term assays are required to investigate
both liver-specific and extra-hepatic genotoxicity, the
following conclusions appear appropriate.
1. Most of the in vivo somatic cell assays for genotox-
icity were able to discriminate between the two carcino-
gen/non-carcinogen pairs, although, in some instances,
weak activity was observed in tests with the non-
carcinogens, particularly with 4AAF.
2. The weak genotoxicity of 4AAF in some studies
suggested that qualitative determinations in terms of
`positive' and `negative' are too simplistic for hazard
assessment purposes and highlights the need for appropri-
ate positive test criteria, in biological and statistical
terms, for widely used in vivo tests.
3. The insensitivity of some assays to one or other of
the two pairs of compounds supports the concept that nega-
tive in vivo data should be produced from at least two
assays in different tissues, i.e. one in extra-hepatic
somatic tissues and one in the liver, if they are to be
used for assessing genotoxic hazard.
4. The mouse bone marrow micronucleus test was confirmed
as a robust and sensitive assay and is the assay of choice
for primary in vivo testing of in vitro genotoxins.
5. The overall performance of the rat liver UDS assay
suggests that it could be complementary to the micro-
nucleus test providing the reservations raised above
(sections 8.2.2 and 9.2.1) can be satisfied. Development
and evaluation of alternative procedures for investigating
liver genotoxicity should be actively pursued.
6. Certain widely advocated assays, including the host-
mediated and urine mutation assays and tests using
drosophila are concluded to be inappropriate for hazard-
assessment purposes.
7. BP and 2AAF showed genotoxic activity in the organs in
which they also produce tumours. Their genotoxic activity
in other tissues, however, precludes the use of data from
short-term tests for making predictions of organ-specific
carcinogenicity.
8. The majority of the positive responses observed in
this study were obtained after oral administration of the
test chemicals and at doses substantially below lethal
levels, suggesting that short-term in vivo assays are not
intrinsically insensitive, as is sometimes inferred. Posi-
tive results were obtained in the dominant lethal assay
and the mouse spot test after intraperitoneal dosing only;
both assays produced negative results after oral admin-
istration. These facts suggest that the intraperitoneal
route should be reserved for the detection of genotoxic
activity and that, for hazard-assessment purposes, a route
of administration should be chosen that is relevant to the
perceived route(s) of human exposure.
9. The germ cell mutagenicity exhibited by BP was
adequately predicted by the somatic cell mutagenicity
assays, adding weight to the suggestion that tests on
mammalian germ cells should only be conducted to gain
further information on the mutagenic spectrum of estab-
lished in vivo somatic cell mutagens.
10. The occurrence of chemical carcinogens that fail to
show evidence of genotoxicity in in vitro and in vivo
assays indicates the existence of a class of chemicals
that induce cancer by a mechanism that is not a conse-
quence of a direct interaction with DNA. The acceptance
of the validity of the concept of non-genotoxic mechanisms
of cancer induction is considered to be a crucial question
in the future deployment of short-term tests in carcino-
genesis.
11. The overall conclusion from the CSSTT/2 study is that
short-term in vivo tests have a vital role to play in
hazard assessment. This role is to define which
chemicals, identified as genotoxic from in vitro tests,
are active in vivo and, thus, are those most likely to
present a carcinogenic/mutagenic hazard to mammals,
including humans.
REFERENCES
ASHBY, J., DE SERRES, F.J., DRAPER, M., ISHIDATE, M., Jr,
MARGOLIN, B.H., MATTER, B., & SHELBY, M.D., ed. (1985) Evaluation of
short-term tests for carcinogens, Amsterdam, Oxford, New York, Elsevier
Science Publishers (Progress in Mutation Research, Vol. 5).
ASHBY, J., DE SERRES, F.J., SHELBY, M.D., MARGOLIN, B.H.,
ISHIDATE, M., Jr, & BECKING, G.C., ed. (1988a) Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 1.
ASHBY, J., SHELBY, M.D., & DE SERRES, F.J. (1988b) Overview of the IPCS
collaborative study on in vivo assay systems and conclusions. In: Ashby,
J., de Serres, F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, &
Becking, G.C., ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative study on
in vivo assays, Cambridge, United Kingdom, Cambridge University Press, Vol.
1, pp. 6-28.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-term tests for
carcinogens: Report of the international collaborative study, Amsterdam,
Oxford, New York, Elsevier Science Publishers (Progress in Mutation
Research, Vol. 1).
GENEROSO, W.M., CAIN, K.T., HELLWIG, C.S. & CACHEIRO, N.L.A. (1982)
Lack of association between induction of dominant-lethal mutations and
induction of heritable translocations with benzo (a) pyrene in postmeiotic
germ cells of male mice. Mutat. Res., 94: 155-163.
HICKS, M.R., ASHBY, J., & PENMAN, M. (1988) Review of the rodent
carcinogenicity data for the four test chemicals. In: Ashby, J., de Serres,
F.J., Shelby, M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C.,
ed. Evaluation of short-term tests for carcinogens: Report of the
International Programme on Chemical Safety's collaborative study on in vivo
assays, Cambridge, United Kingdom, Cambridge University Press, Vol. 2, pp.
351-365.
MCGREGOR, D. (1988a) Summary report on the performance of the
miscellaneous group of in vivo assays (carcinogenicity, DNA adducts,
immunological, host-mediated mutation, urinary mutation assays, and limited
metabolic assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term
tests for carcinogens: Report of the International Programme on Chemical
Safety's collaborative study on in vivo assays, Cambridge, United Kingdom,
Cambridge University Press, Vol. 2, pp. 3-21.
MCGREGOR, D. (1988b) The activities of 2AAF, 4AAF, BP and PYR in in
vitro assays for genetic toxicity. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 2, pp. 345-350.
MARGOLIN, B.H. & RISKO, K.J. (1988) The statistical analysis of in
vivo genotoxicity data: case studies of the rat hepatocyte UDS and mouse
bone marrow micronucleus assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 29-42.
MIRSALIS, J.C. (1988) Summary report on the performance of the in vivo
DNA repair assays. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin,
B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests
for carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 1, pp. 345-351.
PATON, D. & ASHBY, J. (1988) Source and analytical details of the test
chemicals. In: Ashby, J., de Serres, F.J., Shelby, M.D., Margolin, B.H.,
Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of short-term tests for
carcinogens: Report of the International Programme on Chemical Safety's
collaborative study on in vivo assays, Cambridge, United Kingdom, Cambridge
University Press, Vol. 2, pp. 341-344.
SHELBY, M.D. (1986) A case for the continued use of the intraperitoneal
route of exposure. Mutat. Res., 70: 169-171.
TICE, R.R. (1988) Summary report on the performance of the in vivo
sister chromatid exchange assays. In: Ashby, J., de Serres, F.J., Shelby,
M.D., Margolin, B.H., Ishidate, M., Jr, & Becking, G.C., ed. Evaluation of
short-term tests for carcinogens: Report of the International Programme on
Chemical Safety's collaborative study on in vivo assays, Cambridge, United
Kingdom, Cambridge University Press, Vol. 1, pp. 233-253.
WHO (1985) Environmental Health Criteria 47: Summary report on the
evaluation of short-term tests for carcinogens (collaborative study on in
vitro tests), Geneva, World Health Organization, 77 pp.
WYROBEK, A.J., GORDON, L.A., BURKHART, J.G., FRANCIS, M.W.,
KAPP, R.W., LETZ, G., MALLING, H.V., TOPHAM, J.C., & WHORTON, M.D.
(1983) An evaluation of the mouse sperm morphology test and other tests in
non-human mammals: A report of the United States Environmental Protection
Agency Gene-Tox Program. Mutat. Res., 115: 1-7.
RESUME
ETUDE COLLECTIVE DU PROGRAMME INTERNATIONAL SUR LA SECURITE DES
SUBSTANCES CHIMIQUES (IPCS) POUR L'EVALUATION ET LA VALIDATION
DES EPREUVES DE COURTE DUREE RELATIVES AUX CANCEROGENES
La première partie de ce projet, qui traite des études
in vitro, a été publiée en 1985 (Ashby et al., 1985); on
en trouvera un résumé dans le No 47 de la série Critères
d'hygiène de l'environnement (OMS 1985). La seconde
partie, qui fait l'objet du présent rapport, a été publieé
en 1988 (Ashby et al., 1988).
Devant la nécessité d'évaluer l'intérêt des épreuves
de courte durée pour la recherche des substances mutagènes
et cancérogènes, il devenait indispensable d'organiser des
études collectives inter-laboratoires à l'échelle inter-
nationale. Ces épreuves de courte durée devaient compléter
les épreuves classiques à long terme sur rongeurs ou s'y
substituer. C'est le problème du choix ainsi que de la
fiabilité et de la sensibilité de ces épreuves qui a
conduit à la première grande entreprise de collaboration
au niveau international, à savoir le Programme coopératif
international pour l'évaluation des épreuves de courte
durée relatives aux cancérogènes (IPESTTC) (de Serres &
Ashby, 1981). Les résultats de cette étude ont confirmé
la fiabilité et la faisabilité de l'épreuve de mutation
des salmonelles pour une première identification des sub-
stances cancérogènes et mutagènes. On a également con-
staté qu'avec cette épreuve, certains produits notoirement
cancérogènes chez les rongeurs étaient impossibles ou tout
du moins très difficiles à mettre en évidence. Un certain
nombre d'autres épreuves examinées dans le cadre de
l'étude IPESTTC se sont révélées capables de mettre en
évidence quelques-uns des cancérogènes murins pour
lesquels l'épreuve sur salmonelle donnait un résultat
négatif. Toutefois, la base de données était trop res-
treinte pour qu'on puisse recommander une épreuve capable
de compléter l'épreuve de mutation des salmonelles.
Au vu des résultats de l'étude IPESTTC, il est apparu
nécessaire de poursuivre ce type de coopération afin
d'établir a) quel est l'ensemble d'épreuves in vitro le
plus efficace pour un premier tri des substances chimiques
sur la base de leur activité génotoxique et b) quelles
sont les épreuves in vivo de courte durée les plus utiles
pour confirmer la génotoxicité et le pouvoir cancérogène
des substances chimiques chez les mammifères. L'étude
collective pour l'évaluation et la validation des épreuves
de génotoxicité et de cancérogénicité de courte durée
(CSSTT), a été proposée par le Programme international sur
la sécurité des substances chimiques (IPCS) et le National
Institute of Environmental Health Sciences (NIEHS) des
Etats-Unis d'Amérique, l'une des institutions qui
participent au Programme IPCS. Devant l'ampleur de cette
entreprise et du fait des problèmes logistiques que posent
l'organisation et la gestion de ce genre d'études inter-
nationales, le projet a été divisé en deux études
distinctes: l'Etude collective sur les épreuves de géno-
toxicité et de cancérogénicité de courte durée in vitro
(CSSTT/1) et l'Etude collective sur les épreuves de
mutagénicité et de cancérogénicité de courte durée in vivo
(CSSTT/2).
L'Etude CSSTT/1 a permis de constituer une vaste base
de données à partir d'épreuves in vitro très diverses
portant sur dix produits chimiques organiques. Parmi eux
figuraient huit substances de cancérogénicité reconnue
pour les rongeurs qui s'étaient révélées soit négatives à
l'épreuve sur salmonelle soit difficiles à reconnaître de
cette manière, ainsi que deux substances considérées comme
non cancérogènes. On a procédé à l'évaluation de données
tirées de 90 groupes d'épreuves effectuées par quelque
60 scientifiques. Quatre types d'épreuves ont donné des
résultats suffisamment bons pour qu'on puisse envisager de
les utiliser en complément à l'épreuve sur salmonelle. Il
s'agissait de la recherche des aberrations chromosomiques,
des mutations géniques et des transformations néoplasiques
en culture de cellules mammaliennes ainsi que de l'épreuve
d'aneuploïdie des levures. Sauf dans le cas de la
recherche des aberrations chromosomiques, on a constaté
que les protocoles généralement utilisés pour ces épreuves
devaient être étudiés plus à fond avant d'être considérés
comme tout à fait acceptables.
La principale conclusion de l'étude CSSTT/1 relative
aux épreuves in vitro, c'est que en combinant l'épreuve
de recherche des aberrations chromosomiques à l'épreuve de
mutation des salmonelles, on peut procéder à un premier
criblage efficace des substances cancérogènes.
Lors de l'étude IPESTTC, sept substances sur 14 sup-
posées non cancérogènes ont donné des résultats positifs
dans un grand nombre d'épreuves in vitro. D'après les
données in vivo limitées qu'a fournies cette étude, il
semblerait que ces sept produits soient inactifs dans les
épreuves de courte durée in vivo. Les produits non can-
cérogènes de deux des couples cancérogène/non-cancérogène
soumis à l'étude IPESTTC, à savoir le couple benzo [a] py-
rène/pyrène (BP/PYR) et le couple acétyl-2 aminofluorène/
acétyl-4 aminofluorène (2AAF/4AAF), constituent de bons
exemples de ces réactions différentes et d'ailleurs, ces
couples de produits avaient été retenus pour la partie in
vivo de l'étude collective (CSSTT/2). Toutefois, il y
avait doute quant à la non cancérogénicité supposée du
4AAF et une part très importante de l'étude a été con-
sacrée à la mise en route d'études de cancérogéniticé à
long terme sur le 2AAF et le 4AAF chez le rat.
L'objectif de l'étude CSSTT/2 était donc de produire
un profil de données complet à partir d'une large gamme
d'épreuve in vivo de courte durée afin de voir comment les
différents paramètres génétiques des tissus-cibles se com-
portent vis-à-vis de produits chimiques classés comme
génotoxiques d'après les épreuves in vitro. La finalité
de l'étude était de recenser les épreuves in vivo suscep-
tibles d'être utilisées pour déterminer l'activité in vivo
de substances notoirement génotoxiques.
Quatre-vingt-dix-sept chercheurs de 16 pays ont par-
ticipé à l'étude in vivo, les données présentées portant
sur une cinquantaine de techniques distinctes. Les résul-
tats ont été évalués lors d'une réunion de ces chercheurs
qui s'est tenue au Cap d'Agde (France) en mai 1985. On a
préparé une série de rapports comportant une évaluation de
chaque groupe d'épreuves, des rapports récapitulatifs sur
les épreuves relatives aux cellules germinales et les
épreuves d'hépatotoxicité et des rapports résumant toute
la base de données relative à chaque couple de produits.
Par la suite, on a préparé un récapitulatif de toute
l'étude in vivo en vue de sa publication définitive.
Seule une faible proportion des épreuves examinées
lors de l'étude CSSTT/2 ont satisfait aux critères
d'acceptabilité comme épreuves in vivo de courte durée.
Toutefois les épreuves examinées lors de cette étude ne se
limitaient pas à celles jugées a priori les plus conformes
aux critères. De la sorte, on a pu tirer des données
provenant d'épreuves qui, à l'origine, n'étaient pas des
épreuves de génotoxicité in vivo, des renseignements sur
une large gamme d'effets biologiques produits par ces
quatre substances. La plupart des épreuves de génotoxicité
in vivo portant sur des cellules somatiques permettaient
de distinguer les deux couples cancérogène/non-cancérogène
encore que dans certains cas, les produits non cancéro-
gènes, en particulier le 4AAF aient présenté une faible
activité. L'insensibilité de certaines épreuves à l'un ou
à l'autre des deux couples de produits chimiques, corrob-
orent l'idée selon laquelle il faut obtenir des résultats
négatifs in vivo dans au moins deux épreuves pratiquées
sur des tissus différents avant de considérer qu'un
produit chimique n'est pas génotoxique in vivo.
Il a été confirmé que l'épreuve des micro-noyaux sur
moëlle osseuse de souris est robuste, sensible et repro-
ductible et on la recommande pour une première recherche
de la génotoxicité in vivo et in vitro. Les résultats
généraux obtenus au moyen de l'épreuve sur foie de rat
pour la synthèse anarchique de l'ADN incitent à penser
qu'elle pourrait compléter l'épreuve des micro-noyaux,
mais il faut en étudier encore la sensibilité et la
sélectivité. Certaines épreuves pourtant largement pré-
conisées, notamment les épreuves par passage sur hôte, les
épreuves de mutagénicité des urines et les épreuves sur
drosophile, se sont révelées impropres à une évaluation du
risque.
Les résultats de l'étude CSSTT/2 ont confirmé que les
épreuves in vivo de courte durée ont un rôle capital à
jouer dans l'évaluation du risque, rôle qui consiste dans
l'identification des produits chimiques qui s'étant
révélés génotoxiques in vitro, sont également actifs in
vivo et comportent donc vraisemblablement un risque de
cancérogénicité ou de mutagénicité pour les mammifères en
général et l'homme en particulier.
RESUMEN
PROGRAMA INTERNACIONAL DE SEGURIDAD DE LAS SUSTANCIAS QUIMICAS (IPCS):
ESTUDIO EN COLABORACION SOBRE EVALUACION Y COMPROBACION DE PRUEBAS
A CORTO PLAZO PARA SUSTANCIAS CARCINOGENAS
La primera parte del presente proyecto, relativa a
estudios in vitro, se publicó en 1985 (Ashby et al., 1985) y se
resumió en la publicación 47 de la serie "Environmental
Health Criteria" (OMS, 1985). La segunda parte es el
tema del presente informe y se publicó en 1988 (Ashby et
al., 1988).
La necesidad de estudios en colaboración entre
laboratorios en escala internacional surgió de que era
imprescindible investigar el valor de las pruebas a corto
plazo para detectar las sustancias químicas mutágenas y
carcinógenas. Se propusieron los ensayos a corto plazo
como métodos que sustituyeran o complementaran a los
tradicionales ensayos biológicos a largo plazo en
roedores. La preocupación por la elección de las pruebas
a corto plazo y por su fiabilidad y sensibilidad condujo a
que se realizara el primer ejercicio importante de
colaboración internacional: el programa internacional en
colaboración sobre evaluación de las pruebas a corto plazo
para sustancias carcinógenas (IPESTTC) (de Serres y Ashby,
1981). Los resultados de ese estudio confirmaron el valor
de la prueba de mutación de salmonelas como ensayo fiable
y factible para la identificación primaria de sustancias
carcinógenas y mutágenas. Se observó también que en la
prueba de salmonelas no se detectaban o sólo se detectaban
con grandes dificultades algunas sustancias de cancerogen-
icidad conocida en los roedores. Otros ensayos incluidos
en el estudio IPESTTC pudieron detectar sustancias
carcinógenas para roedores que eran negativas en el ensayo
de salmonelas. Sin embargo, la base de datos de apoyo era
demasiado pequeña para permitir la recomendación de un
ensayo que complementara la prueba de mutación de
salmonelas.
Los resultados del estudio IPESTTC pusieron de
manifiesto que se necesitaría un nuevo ejercicio en
colaboración para determinar: a) la combinación más
eficaz de ensayos in vitro para la detección primaria de
la actividad genotóxica de sustancias químicas, y b) las
pruebas in vivo a corto plazo más útiles para confirmar la
posibilidad de genotoxicidad y cancerogenicidad en mamí-
feros. El Programa Internacional sobre Seguridad de las
Sustancias Químicas (IPCS) y el Instituto Nacional de
Ciencias de Higiene del Medio (NIHS) de los Estados Unidos
de América, como institución participante en el IPCS,
propusieron el estudio en colaboración sobre evaluación y
comprobación de las pruebas a corto plazo para la geno-
toxicidad y la cancerogenicidad (CSSTT). Dadas la magnitud
prevista del proyecto y la logística de la organización y
administración de los proyectos internacionales, el
proyecto se dividió en dos estudios independientes: el
estudio en colaboración sobre pruebas a corto plazo para
la genotoxicidad y la cancerogenicidad (CSSTT/1) y el
estudio en colaboración sobre pruebas in vivo a corto
plazo para sustancias mutágenas y carcinógenas (CSSTT/2).
En el estudio CSSTT/1 se reunió una abarcante base de
datos procedentes de una amplia gama de ensayos in vitro
realizados con diez productos químicos orgánicos cuidad-
osamente seleccionados. Incluían ocho sustancias de
cancerogenicidad probada para roedores, que resultaban
negativas o difíciles de detectar en el ensayo de
salmonelas y dos sustancias químicas consideradas como no
carcinógenas. Se evaluaron los datos procedentes de casi
90 series de ensayos efectuados por unos 60 científicos
participantes. El rendimiento de cuatro tipos de ensayos
se estimó suficiente para considerarlos como posibles
pruebas complementarias del ensayo de salmonelas.
Incluyeron las pruebas para la determinación de aberra-
ciones cromosómicas, mutaciones génicas y transformaciones
neoplásicas en células de mamífero en cultivo, y un ensayo
para determinar la aneuploidia en levaduras. Con la
excepción del ensayo de aberraciones cromosómicas, resultó
evidente que los protocolos utilizados en general para
esas pruebas exigían evaluación adicional antes de poder
considerarlos plenamente aceptables.
La principal conclusión del estudio CSSTT/1 sobre las
pruebas in vitro fue que el empleo de los ensayos de
aberraciones cromosómicas en asociación con la prueba de
mutación de salmonelas podía resultar un detector primario
eficaz para posibles sustancias carcinógenas nuevas.
En el estudio IPESTTC, siete de las catorce sustancias
no carcinógenas presuntas dieron resultados positivos en
muchas de las pruebas in vitro. Los limitados datos de
pruebas in vivo disponibles procedentes de ese estudio
permiten pensar que esas siete sustancias químicas eran
inactivas en las pruebas a corto plazo in vivo. En dos
pares de sustancias carcinógenas/no carcinógenas de
IPESTTC (benzo [a] pireno/pireno (BP/PIR) y 2-acetilamino-
fluoreno/4-acetilamino-fluoreno (2AAF/4AAF), las sustan-
cias no carcinógenas proporcionaron buenos ejemplos de
tales respuestas diferentes, de modo que se seleccion-aron
esos pares de sustancias químicas para la parte in vivo
del estudio en colaboración (CSSTT/2). Sin embargo, hubo
una fuerte duda respecto a la presunta falta de cancero-
genicidad del 4AAF, y una parte crucial del estudio fue el
comienzo de bioensayos de cancerogenicidad a largo plazo
del 2AAF y el 4AAF en ratas.
Por consiguiente, el objetivo del estudio CSSTT/2 fue
producir un abarcante perfil de datos procedentes de una
amplia gama de pruebas in vivo a corto plazo, como medio
de conocer el mecanismo por el que los distintos puntos
finales genéticos de los tejidos destinatarios fundamen-
tales responden a sustancias químicas definidas como geno-
tóxicas in vitro. La meta final consistía en identificar
qué ensayos in vivo podrían usarse para determinar la
actividad in vivo de los productos genotóxicos probados.
Participaron en el proyecto de pruebas in vivo 97
investigadores de 16 países y se presentaron datos de unas
cincuenta técnicas in vivo distintas. Los resultados se
evaluaron en una reunión de investigadores celebrada en
Cap d'Agde (Francia) en mayo de 1985. Se preparó una
serie de informes que comprendían una evaluación de cada
grupo de ensayos, informes resumidos sobre los ensayos en
células germinales y las pruebas hepáticas específicas, e
informes resumidos sobre la base total de datos corres-
pondiente a cada par de sustancias químicas. Después se
preparó un examen general de todo el estudio in vivo listo
para la publicación final.
Sólo una pequeña proporción de los ensayos represent-
ados en el estudio CSSTT/2 satisficieron los criterios que
definen una prueba in vivo a corto plazo aceptable. Sin
embargo, los ensayos incluidos en el estudio no se
limitaron a los que podían cumplir con más probabilidad
los criterios. Así, aunque los datos procedían de ensayos
que no estaban destinados fundamentalmente a identificar
la genotoxicidad in vivo, proporcionaron información
sobre un amplio espectro de efectos biológicos de las
cuatro sustancias químicas. La mayoría de los ensayos en
células sométicas in vivo para determinar la genotoxicidad
discriminaron entre las sustancias carcinógenas/no carcin-
ógenas de los dos pares, aunque en algunos casos se
detectó una actividad débil en las pruebas con sustancias
no carcinógenas, en particular con el 4AAF. La insensib-
ilidad de algunos ensayos a uno u otro de los dos pares de
productos químicos apoya el concepto de que deben obtener-
se datos in vivo negativos en dos ensayos por lo menos en
diferentes tejidos antes de que pueda aceptarse que una
sustancia química carece de genotoxicidad in vivo.
Se confirmó que la prueba de micronúcleos de médula
ósea de ratón es un ensayo potente, sensible y repro-
ducible, que se recomienda para las pruebas in vivo
primarias de las sustancias genotóxicas in vitro. El
rendimiento general del ensayo en células hepáticas de
rata para la síntesis no programada de ADN permite pensar
que puede ser complementario de la prueba de micronúcleos,
aunque requieren investigación adicional ciertos aspectos
de sensibilidad y selectividad de ese ensayo. Se llegó a
la conclusión de que ciertos ensayos ampliamente susten-
tados, que incluyen los de mutagenicidad por orina y
mediada por el huésped y las pruebas que utilizan la mosca
drosófila, son inapropiados para la evaluación de
riesgos.
Los resultados del estudio CSSTT/2 confirmaron que las
pruebas in vivo a corto plazo tienen que desempeñar una
función crucial en la evaluación de los riesgos y que esa
función consiste en identificar los productos químicos
que, una vez demostrada la genotoxicidad in vitro, son
activos in vivo y tienen así mayores probabilidades de
presentar un riesgo de cancerogenicidad o mutagenicidad
para los mamíferos, incluido el hombre.
