
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 102
1-PROPANOL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
1-Propanol.
(Environmental health criteria ; 102)
1.Alcohol,propyl
I.Series
ISBN 92 4 157102 0 (NLM Classification: QD 305.A4)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposures
1.5. Kinetics and metabolism
1.6. Effects on organisms in the environment
1.7. Effects on experimental animals and in vitro test systems
1.8. Health effects on human beings
1.9. Summary of evaluation
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Waste disposal
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.3. Biotransformation
4.3.1. Biodegradation
4.3.2. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Animals
6.1.2. Human beings
6.2. Distribution
6.2.1. Animals
6.2.2. Human beings
6.3. Metabolic transformation
6.3.1. Animals
6.4. Elimination and excretion
6.4.1. Animals
6.4.2. Human beings
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic organisms
7.2. Terrestrial organisms
7.2.1. Insects
7.2.2. Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Mortality
8.1.2. Signs of intoxication
8.1.3. Skin, eye, and respiratory tract irritation;
sensitization
8.2. Repeated exposures
8.3. Neurotoxic and behavioural effects
8.4. Biochemical effects
8.4.1. Effects on lipids in the liver and blood
8.4.2. Effects on microsomal enzymes
8.4.3. Other biochemical findings
8.5. Reproduction, embryotoxicity, and teratogenicity
8.6. Mutagenicity
8.6.1. Bacteria
8.6.2. Mammalian cells in vitro
8.7. Carcinogenicity
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Poisoning incidents
9.1.2. Controlled human studies
9.2. Occupational exposure
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
1-PROPANOL
Members
Dr R. Drew, Department of Clinical Pharmacology, Flinders
University of South Australia, Bedford Park, South Australia,
Australia
Dr B. Gilbert, Company for Development of Technology Transfer
(CODETEC), City University, Campinas, Brazil (Rapporteur)
Dr B. Hardin, Document Development Branch, Division of Standards
Development and Technology Transfer, National Institute for
Occupational Safety and Health, Cincinnati, Ohio, USA (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Professor M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, University of Alexandria,
Alexandria, Egypt
Dr L. Rosenstein, Office of Toxic Substances, US Environmental
Protection Agency, Washington, DC, USA
Professor I.V. Sanotsky, Chief, Department of Toxicology Institute
of Industrial Hygiene and Occupational Diseases, Moscow, USSR
(Vice-Chairman)
Dr J. Sokal, Division of Industrial Toxicology, Institute of
Occupational Medicine, Lodz, Poland
Dr H.J. Wiegand, Toxicology Department, Huls AG, Marl, Federal
Republic of Germany
Dr K. Woodward, Department of Health, Medical Toxicology and
Environmental Health Division, London, United Kingdom
Observers
Dr K. Miller (Representing International Commission on Occupational
Health (ICOH)), British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Secretariat
Professor F. Valic , Consultant, IPCS, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Yugoslavia (Secretary)
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, Holland
Host Organization
Dr S.D. Gangolli, British Industrial Biological Research
Association, Carshalton, Surrey, United Kingdom
Dr D. Anderson, British Industrial Biological Research Association,
Carshalton, Surrey, United Kingdom
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400/
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 1-PROPANOL
A WHO Task Group on Environmental Health Criteria for
1-Propanol met at the British Industrial Biological Research
Association (BIBRA), Carshalton, Surrey, United Kingdom, from 10 to
14 April 1989. Dr S.D. Gangolli, who opened the meeting, welcomed
the participants on behalf of the Department of Health, and Dr D.
Anderson on behalf of BIBRA, the host institution. Dr F. Valic
greeted the participants on behalf of the heads of the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed
and revised the draft criteria document and made an evaluation of
the human health risks and effects on the environment of exposure
to 1-propanol. The drafts of this document were prepared by Dr T.
VERMEIRE, National Institute of Public Health and Environmental
Hygiene, Bilthoven, Netherlands. Dr F. VALIC was responsible for
the overall scientific content of the document and Mrs M.O. HEAD of
Oxford, England, for the editing.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and
Social Security generously supported the cost of printing.
1. SUMMARY
1.1 Identity, Physical and Chemical Properties, Analytical Methods
1-Propanol is a colourless, highly flammable liquid that is
volatile at room temperature and normal atmospheric pressure. It
is miscible with water and organic solvents. Analytical methods
for propanol include gas chromatography, which can detect
5 x 10-5 mg/m3 in air, 1 x 10-4 mg/litre in water, and 0.002
mg/litre in blood, serum, or urine, when suitable extraction or
concentration procedures are used with the sample.
1.2 Sources of Human and Environmental Exposure
The annual world production capacity in 1979 exceeded 130 000
tonnes. It is produced in nature by the decomposition of organic
materials by a variety of microorganisms, and occurs in plants and
fuel oil. 1-Propanol is produced from ethene by reaction with
carbon monoxide and hydrogen to give propionaldehyde, which is then
hydrogenated. It is also a by-product of methanol manufacture and
may be produced from propane directly or from acrolein. The major
use of 1-propanol is as a multi-purpose solvent in industry and in
the home. It is used in flexographic printing ink and textile
applications, products for personal use, such as cosmetics and
lotions, and in window cleaning, polishing, and antiseptic
formulations. Second in importance is its use as an intermediate
in the manufacture of a variety of chemical compounds.
1.3 Environmental Transport, Distribution and Transformation
The main pathway of entry of 1-propanol into the environment is
through its emission into the atmosphere during production,
processing, storage, transport, use, and waste disposal. Emissions
into water and soil also occur. Because the main use of 1-propanol
is as a volatile solvent, much of the production volume is
eventually released into the environment.
1-Propanol rapidly disappears from the atmosphere by reaction
with hydroxyl radicals and through rain-out. It is readily
biodegradable, both aerobically and anaerobically, and, because of
these chemical and biological removal mechanisms, measurable levels
are not normally encountered in the environment. However, the
compound has been detected in urban air, at waste-disposal sites,
and also in water leaching from a landfill. Soil permeability for
1-propanol is probably high and the compound enhances permeability
for some aromatic solvents.
1-Propanol has a log n-octanol/water partition coefficient of
0.34 and a bioconcentration factor of 0.7, which render
bioaccumulation highly unlikely.
1.4 Environmental Levels and Human Exposures
Exposure of the general population may occur through accidental
ingestion, through inhalation during use, and through ingestion via
food (containing 1-propanol as a natural or added flavour volatile
or as a solvent residue) and non-alcoholic as well as alcoholic
beverages. For example, beer contains up to 195 mg/litre, wine up
to 116 mg/litre, and various types of spirit up to 3520 mg/litre.
Exposure of the general population to 1-propanol via inhalation and
drinking-water is low (in the USA the average concentration in
urban air samples was 0.00005 mg/m3 and that in drinking-water,
0.001 mg/litre). Workers are potentially exposed through
inhalation during manufacture, processing, and use. However, no
data are available to quantify such exposures.
1.5 Kinetics and Metabolism
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Data on the absorption rate following
inhalation and dermal exposures are lacking. 1-Propanol is
metabolized by alcohol dehydrogenase (ADH) to propionic acid via
the aldehyde and may enter the tricarboxylic acid cycle. This
oxidation is a rate-limiting step of 1-propanol metabolism. In
vitro, rat and rabbit microsomal oxidases are also capable of
oxidizing 1-propanol to propionic aldehyde. The relative affinity
of ADH and the microsomal oxidizing systems for 1-propanol is much
higher than that of ethanol; therefore 1-propanol is rapidly
eliminated from the organism. In the rat, the half-life after an
oral dose of 1000 mg/kg was 45 min.
In both animals and man, 1-propanol may be eliminated from the
body in the expired air or in urine. In human beings administered
an oral dose of 1-propanol of 3.75 mg/kg body weight and 1200 mg
ethanol/kg body weight, the total urinary excretion of 1-propanol
was 2.1% of the dose. The urinary levels of 1-propanol were lower
the lower the amount of simultaneously ingested ethanol, showing
competition for ADH between 1-propanol and the ethanol overdose.
1.6 Effects on Organisms in the Environment
At concentrations normally encountered in the environment,
1-propanol is not toxic for aquatic organisms, insects, or plants.
The inhibitory threshold for cell multiplication of three of the
more sensitive aquatic species (3 protozoa) was 38 - 568 mg/litre.
For the higher organisms, the lethal concentration was about 5000
mg/litre, varying remarkably little from one phylum to another and
exhibiting a very steep dose-response curve. Some bacteria and
microorganisms in waste-water and activated sludge are able to
adapt to concentrations greater than 17 000 mg/litre.
Seed germination may be inhibited or stimulated by 1-propanol
depending on the concentration in water used and conditions of
exposure. The compound increases nitrite accumulation in maize,
peas, and wheat.
1.7 Effects on Experimental Animals and In Vitro Test Systems
The acute toxicity of 1-propanol for mammals (based on
mortality) is low, whether exposure is via the dermal, oral, or the
respiratory route. Oral LD50 values for several animal species
have been reported to range between 1870 and 6800 mg/kg body
weight. However, an oral LD50 of 560 - 660 mg/kg body weight was
reported for very young rats. The principal toxic effect of
1-propanol following a single exposure is depression of the central
nervous system. The available evidence for 1-propanol suggests
that its effects on the central nervous system are similar to those
of ethanol; however, 1-propanol appears to be more neurotoxic. The
ED50 values for narcosis in rabbits and loss of righting reflex in
mice were, respectively, 1440 mg/kg body weight orally, and
1478 mg/kg body weight intraperitoneally; these are approximately
four times lower than those for ethanol. In the tilted plane test,
1-propanol was 2.5 times as potent as ethanol in rats.
Single oral doses of 3000 or 6000 mg/kg body weight resulted in
a reversible accumulation of triglycerides in the liver of rats.
High vapour concentrations caused irritation of the respiratory
tract in mice. The respiratory rate in mice was decreased by 50%
at concentrations of approximately 30 000 mg/m3.
Data on eye and skin irritation are not available. No
sensitization was observed in one reported skin sensitization test
on CF1 mice.
There was limited evidence, in male rats exposed for 6 weeks to
15 220 mg/m3, that 1-propanol impairs reproductive function. No
effect was noted after a similar exposure to 8610 mg/m3. When
pregnant rats were exposed to 1-propanol, maternal and
developmental toxicity were evident at 23 968 and 14 893 mg/m3
(9743 and 6054 ppm); there was no toxicity at 9001 mg/m3
(3659 ppm). No evidence was seen of behavioural defects in the
offspring of male rats exposed for 6 weeks to 8610 or 15 220 mg
1-propanol/m3, or in offspring of rats exposed during pregnancy to
the same concentrations. However, when 5 to 8-day-old rats were
orally dosed with 3000 - 7800 mg 1-propanol/kg, per day, there was
evidence of CNS depression during dosing and signs of withdrawal
when dosing ended. The brains of these rats were examined when
they were 18 days old; reductions were found in the absolute and
relative brain weights and in the contents of DNA as well as
regional decreases in cholesterol and protein levels.
1-Propanol gave negative results in 2 assays for point
mutations using Salmonella typhimurium and in a reverse mutation
test with Escherichia coli CA-274. Negative results were obtained
in tests for the induction of sister chromatid exchange or
micronuclei in mammalian cells in vitro. No other mutagenicity
data were available.
In a carcinogenicity study on small groups of Wistar rats
exposed throughout their lifetime to oral doses of 240 mg/kg or to
subcutaneous doses of 48 mg/kg, a significant increase in the
incidence of liver sarcoma was noted in the group dosed
subcutaneously. However, the study was inadequate for the
assessment of carcinogenicity for a number of reasons including
lack of experimental detail, too few animals, and the use of a high
single dose inducing liver toxicity.
1.8 Health Effects on Human Beings
There are no reports of adverse health effects in the general
population or in occupational groups. In the only fatal poisoning
case reported, it was recorded that a woman was found unconscious
and died 4 - 5 h after ingestion. Autopsy revealed a "swollen
brain" and lung oedema. In a study on skin irritation and
sensitization, allergic reactions were reported in a laboratory
worker. In another group of 12 volunteers, erythema lasting for at
least 60 min was observed in 9 individuals following a 5-min
application of filter papers containing 0.025 ml of a 75% solution
of 1-propanol in water on the forearms. No other reports on
adverse health effects following occupational exposure to
1-propanol are available.
No epidemiological studies are available to assess the long-
term effects, including the carcinogenicity, of 1-propanol in human
beings.
1.9 Summary of Evaluation
Exposure of human beings to 1-propanol may occur through the
ingestion of food or beverages containing 1-propanol. Inhalation
exposure may occur during household use and occupationally during
manufacture, processing, and use. The very limited data on the
level of 1-propanol in the ambient air and water suggest that
concentrations are very low.
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Absorption following inhalation is
expected to be rapid and dermal absorption is expected to be slow.
The acute toxicity of 1-Propanol for animals is low whether
exposed via the dermal, oral, or the respiratory route. Exposure
of members of the general population to potentially lethal levels
may occur through accidental or intentional ingestion. However,
only one case of lethal poisoning by 1-propanol has been reported.
The most likely acute effects of 1-propanol in man are alcoholic
intoxication and narcosis. The results of animal studies indicate
that 1-propanol is 2 - 4 times as intoxicating as ethanol.
1-Propanol may be irritating to hydrated skin.
Animal toxicity data are not adequate to make an evaluation of
the human health risks associated with repeated or long-term
exposure to 1-propanol. However, limited short-term rat studies
suggest that oral exposure to 1-propanol is unlikely to pose a
serious health hazard under the usual conditions of human exposure.
Inhalation exposure to a concentration of 15 220 mg/m3 caused
impaired reproductive performance in male rats, but exposure to
8610 mg/m3 did not. In pregnant rats, 9001 mg/m3 (3659 ppm) was a
no-observed-effect level (NOEL) and 14 893 mg/m3 (6054 ppm) was a
lowest-observed-effect level (LOEL) for both maternal and
developmental toxicity. Thus, inhalation exposure to high
concentrations of 1-propanol produced reproductive and
developmental toxicity in male and female rats in the presence of
overt toxicity in the exposed animals. The concentrations required
to produce these effects in rats were higher than those likely to
be encountered under normal conditions of human exposure.
1-Propanol was negative in assays for point mutations in
bacteria. Although these findings suggest that the substance does
not have any genotoxic potential, an adequate assessment of
mutagenicity cannot be made on the basis of the limited data
available. The available study is inadequate to evaluate the
carcinogenicity of 1-propanol in experimental animals. No data are
available on the long-term exposure of human populations to
1-propanol. Hence the carcinogenicity of 1-propanol for human
beings cannot be evaluated.
Apart from one case of fatal poisoning following ingestion of
half a litre of 1-propanol, there are practically no reports on the
adverse health effects from exposure to 1-propanol, either in the
general population or in occupational groups. The Task Group
considers it unlikely that 1-propanol will pose a serious health
risk for the general population under normal exposure conditions.
1-Propanol can be released into the environment during
production, processing, storage, transport, use, and waste
disposal. Because of its primary use as a volatile solvent, most
of the production volume is eventually released into the
atmosphere. However, by reacting with hydroxyl radicals and
through rain-out, 1-propanol will disappear rapidly from the
atmosphere, with a residence time of less than 3 days. Removal of
1-propanol from water and soil also occurs rapidly so that
measurable levels are rarely found in any of the three
compartments. Adsorption of 1-propanol on soil particles is poor,
but it is mobile in soil and it has been shown to increase the
permeability of soil to some aromatic hydrocarbons.
In view of the physical properties of 1-propanol,
bioaccumulation is unlikely and, except in the case of accident or
inappropriate disposal, 1-propanol does not present a risk for
aquatic organisms, insects, and plants at concentrations that
usually occur in the environment.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C3H8O
Chemical structure: H H H
| | |
H - C - C - C - OH
| | |
H H H
Common name: n-propyl alcohol
Abbreviation: NPA
Common synonyms: ethyl carbinol, 1-hydroxypropane, propanol,
n-propanol (IUPAC name), 1-propanol (CAS
name), propan-1-ol
Common trade names: Albacol, Optal, Osmosol extra, UN 1274
CAS registry number: 71-23-8
Specifications: commercial 1-propanol contains typically
99.85% of the compound and, as main
impurities, water (< 0.1% by weight),
aldehydes (< 0.2% by weight), ethanol
(< 10 mg/kg), and methanol (< 100 mg/kg)
[35, 104-84].
Conversion factors: 1 ppm 1-propanol = 2.46 mg/m3 air; and
1 mg 1-propanol/m3 air = 0.41 ppm, at 25 °C
and 101.3 kPa (760 mmHg).
2.2 Physical and Chemical Properties
1-Propanol is a highly flammable, volatile, colourless liquid
at room temperature and standard atmospheric pressure. Its odour
is described as alcohol-like, sweet, and pleasant [83]. Continuous
exposure can result in loss of sensitivity to the odour (olfactory
adaptation) [182]. The compound is completely miscible with water
and with most organic solvents. It undergoes all chemical
reactions typical of primary alcohols. 1-Propanol reacts violently
with oxidizing agents.
Some physical and chemical data on 1-propanol are given in
Table 1.
2.3 Analytical Methods
A summary of methods for the determination of 1-propanol in
air, water, and biological media is presented in Table 2.
The sensitivity of the gas chromatographic determination of
alcohols with electron capture or photoionization detection can be
greatly improved by prior derivatization with pentafluorophenyl-
dimethylsilyl chloride [109].
Table 1. Some physical and chemical properties of 1-propanol
--------------------------------------------------------------
Physical state liquid
Colour colourless
Relative molecular mass: 60.09
Odour perception threshold <0.07-100 mg/m3a
Odour recognition threshold 0.32-150 mg/m3b
Melting point (°C) -127
Boiling point (°C) 97
Water solubility infinite
Log n-octanol/water partition 0.34c
coefficient
Specific density (20 °C) 0.804
Relative vapour density 2.07
Vapour pressure (20 °C) 1.9 kPa (14.5 mmHg)
Flash point
(open cup) 25 °C
(closed cup) 15 °C
Flammability limits 2.1-13.5% by volume
--------------------------------------------------------------
a From: May [122]; Corbit & Engen [42]; Oelert & Florian
[138]; Stone et al. [182]; Dravnieks [50]; Hellman & Small
[83]; Laing [111]; and Punter [151].
b From: May [122] and Hellman & Small [83].
c Experimentally derived by Hansch & Anderson [80].
Ramsey & Flanagan [154] reported a method for the detection and
identification of 1-propanol and other volatile organic compounds
in the headspace of blood, plasma, or serum, using gas
chromatography with flame ionization and electron capture
detection. The method is applicable to samples obtained from
victims of poisoning, for which a high sensitivity is not
desirable. After preincubation of the samples with a proteolytic
enzyme, the method can be used for the analysis of tissues.
Gas chromatographic methods, using flame ionization detection,
are available for the determination of 1-propanol in milk and milk
products [142], in alcoholic beverages [91, 71, 64, 148], in
foodstuffs [148], in food packaging [54], in digestive contents,
silage juices, and microorganism growth cultures [98], and in drug
raw materials [121]. Methods for the identification of 1-propanol
as flavour volatile have also been described (see Table 4, section
5.2).
Table 2. Sampling and analysis of 1-propanol
-----------------------------------------------------------------------------------------------------
Medium Sampling method Analytical Detection Sample Comments Reference
method limit size
-----------------------------------------------------------------------------------------------------
Air sampling on gas chromatography 0.01 mg/ 1-10 suitable for [195]
charcoal, desorption with flame ionization sample litre personal and
by carbon disulfide detection area monitoring;
working range,
50-900 mg/m3
Air sampling on gas chromatography 0.25 24 suitable for [112]
charcoal, desorption with flame ionization mg/m3 litre area monitoring,
by a 1:1 mixture of detection, packed applicable mixtures
carbon disulfide and with Oronite NIW on of both polar and
water Carbopack B non-polar solvents
Air condensation, pre- gas chromatography 5 x 10-5 suitable for [174]
concentration by with flame ionization mg/m3 analysis of
microdistillation, detection, packed oxygenated
purging by nitrogen, with Poropack QS organic compounds
trapping on porous and S in ambient air
polymer, desorption
by heating
Water concentration by gas chromatography 0.0001 60 ml suitable for [174]
microdistillation, with flame ionization mg/litre analysis of
purging by nitrogen, detection, packed oxygenated
trapping on porous with Poropack QS organic compounds
polymer, desorption and S in water
by heating
Water direct injection gas chromatography 1 mg/ 0.001 suitable for [106]
with flame ionization litre ml analysis of a
detection, packed mixture of a
with porous polymer wide variety
Tenax GC of compounds
-----------------------------------------------------------------------------------------------------
Table 2. (contd.)
-----------------------------------------------------------------------------------------------------
Medium Sampling method Analytical Detection Sample Comments Reference
method limit size
-----------------------------------------------------------------------------------------------------
Water direct injection gas chromatography 0.04 mg/ 0.002 suitable for [194]
with steam as litre ml analysis of
carrier and flame a mixture of
ionization detection, aliphatic
packed with Chromosorb compounds
PAW modified with
phosphoric acid
Water derivatization by paper electrophoresis 40 mg/ 0.1 ml suitable for [8]
2-fluoro-1-methyl- with detection by litre analysis of
pyridinium p-toluene Dragendorff's reagent mixtures of
sulfonate in primary and
presence of secondary
tridodecylamine alcohols, such as
in alcoholic
beverages
Water derivatization with TLC (silica gel G) 0.05 mg/ 0.005 [208]
4-(6-methylbenzo- or HPTLC (RP-18) or litre ml
thiazol-2-yl)phenyl HPLC (Silicagel Si 60 (TLC)
iso-cyanate in or Li-Chrosorb RP-18)
presence of with fluorimetric
triethylene-diamine detection
in xylene
Water direct application spot test detection 2.5 x a qualitative [162]
using 0.1% vanadium 10-2 mg/ method with
(V)- N-phenylbenzo- drop interference
hydroxamate in by other alcohols,
alcohol free phenols, cresols,
chloroform dioxane, methyliso-
butyl ketone,
acetone, reaction
is immediate
-----------------------------------------------------------------------------------------------------
Table 2. (contd.)
-----------------------------------------------------------------------------------------------------
Medium Sampling method Analytical Detection Sample Comments Reference
method limit size
-----------------------------------------------------------------------------------------------------
Serum, extraction by gas chromatography 0.002 1 ml suitable for [114]
urine dichloromethane with mass mg/litre determination
spectrometric of aliphatic
detection, column alcohols
was coated with
Emulphor ON-870
Blood, addition of gas chromatography 0.01 mg/ 1.1 ml whole blood is [21, 13,
urine, potassium carbonate; with flame ionization litre pretreated with 110]
tissue headspace sampling detection; split sodium fluoride
columns packed with or perchloric acid;
polypropylene glycol the method is
on Chromosorb W NAW applicable to
and SP1000 on tissue after
Carbopack, equilibration with
respectively water
Blood addition of sodium gas chromatography 0.01 mg/ 0.1 ml [209]
sulfate; headspace with flame ionization litre
sampling detection, split fused
silica columns: DB
1701 and CP Sil 8 CB
-----------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
1-propanol occurs in fuel oils. It has been identified as a
metabolic product of microorganisms and as a flavour volatile in
foodstuffs (section 5) [104]. Other potential sources of
atmospheric alcohols are photochemical reactions of hydrocarbons,
combustion, and, perhaps, oceans [174].
3.2 Man-Made Sources
3.2.1 Production levels and processes
The global capacity for the production of 1-propanol in 1979
exceeded 130 000 tonnes with most of this capacity in the USA
[104]. In 1975, the total USA production amounted to 57 000
tonnes, and 6600 tonnes were exported [176]. In 1979, 85 000
tonnes were produced [104]. The production in the countries of the
European Economic Community was estimated at 5100 tonnes in 1979
and 3300 tonnes over the first 9 months of 1983. The imports from
the USA rose from 4000 tonnes in 1979 to 8700 tonnes over the first
9 months of 1983 [5]. 1-Propanol was not manufactured in eastern
Europe or in the Far East in 1979, but one company in Japan was
reported to produce this compound by Kirk & Othmer [104].
1-Propanol is manufactured by the hydroformylation of ethene
(reaction with carbon monoxide and hydrogen) to propionaldehyde,
which is subsequently hydrogenated to 1-propanol [104]. The
compound can also be recovered commercially as a by-product of the
high pressure synthesis of methanol from carbon monoxide and
hydrogen [35]. It has been produced by the vapour-phase oxidation
of propane [104] and during the reduction of propene-derived
acrolein [4, 35]. Earlier, 1-propanol was fractionally distilled
from the fuel oils that form in the yeast fermentation process for
the manufacture of ethanol [35].
3.2.2 Uses
The major use of 1-propanol is as a solvent. It is used as
carrier and extraction solvent for natural products, such as
flavourings, vegetable oils, resins, waxes, and gums, and as a
solvent for synthetic polymers, such as polyvinyl butyral,
cellulose esters, lacquers, and PVC adhesives. Other solvent
applications include the use of 1-propanol in the polymerization
and spinning of acrylonitrile, in flexographic printing inks, and
in the dyeing of wool. 1-Propanol is used for both its solvent and
antiseptic properties in drugs and cosmetics, such as lotions,
soaps, and nail polishes. It is also used as a chemical
intermediate, e.g., in the manufacture of propanal, 1-bromopropane,
O,O-dipropylphosphoro-dithioic acid, n-propyl amines, esters
(propyl acetate, propyl carbamate), alcoholates, and xanthates.
Miscellaneous uses include the application of 1-propanol in
degreasing agents, polishing compounds (window cleaners, floor
polishes), and brake fluid, as coupling and dispersing agents, and
as a ruminant feed supplement. It improves the water tolerance of
motor fuels [82, 104, 35, 198].
3.2.3 Waste disposal
1-Propanol may enter the atmosphere, water, and/or soil
following waste disposal (section 4.1). At landfill sites,
1-propanol has been identified in the air and leachates (section
5.1). Emission of 1-propanol via waste gases and waste water
occurs in industry, and diffuse airborne emissions occur during the
use of the compound (section 4.1).
1-Propanol can be removed from waste water by biodegradation
(section 4.3.1). Activated carbon adsorption is not feasible,
because the compound is poorly adsorbed [69]. Removal of the
compound from waste water by reverse osmosis (hyperfiltration) may
be successful, depending on the type of membrane. Cellulose
acetate membranes yielded an average of 40% separation of
1-propanol [53], while cross-linked polyethyleneimine membranes
yielded 60 - 85% separation for a primary alcohol, such as
ethanol [55]. Ozonization of 1-propanol appears to be too slow a
process to be of any significance for water treatment [90].
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and Distribution Between Media
In view of the physical properties and the use pattern of
1-propanol, it can be concluded that the main pathway of entry of
this compound into the environment is through its emission into the
atmosphere during production, handling, storage, transport, and
use, and following waste disposal. Second in importance is its
emission into water and soil. In the USA, industrial losses into
the environment were estimated at 1.5% of the production in 1976,
and 75% of the 1-propanol produced was estimated to be eventually
released into the atmosphere [49].
Intercompartmental transfer of 1-propanol can occur between
water, soil or waste, and air, and between soil or waste and water.
Volatilization of the compound will be considerable in view of its
rather high vapour pressure. Transport of 1-propanol from the
atmosphere to soil or water will occur via rain-out, as it is
highly soluble in water. Data on the behaviour of 1-propanol in
soil are scarce. With respect to adsorption, there is one study
showing that the compound is poorly adsorbed on activated carbon
[198]. Since 1-propanol is completely miscible with water, it can
be expected to be mobile in the soil. It has also been shown to
increase the permeability of soil to aromatic hydrocarbons [57].
4.2 Abiotic Degradation
Once in the atmosphere, 1-propanol is mainly degraded by
hydroxyl radicals. It is not expected to react at appreciable
rates with other reactive species, such as ozone, and hydroperoxy-,
alkyl-, and alkoxy-radicals. Since the compound does not absorb
ultraviolet radiation within the solar spectrum, photodegradation
is not expected [34]. Experimentally determined rate constants
for the reaction between 1-propanol and hydroxyl radicals are
0.43 x 10-11 cm3/molecule per second at 19 °C [32], and 0.53 x
10-11 cm3/molecule per second at 23 °C [141]. Atmospheric
residence times of 2.7 and 2.2 days, respectively, can be calculated
on the basis of these rate constants [44]. These short lifetimes
will prevent migration of the chemical to the stratosphere.
The initial reaction product of 1-propanol with a hydroxyl
radical is an alpha-hydroxypropyl. By analogy with the irradiation
of ethanol in an NOx-air atmosphere, these radicals are expected to
react with oxygen, almost exclusively with hydrogen abstraction
from the hydroxyl group to produce propionaldehyde [34].
Hydrolysis or light-induced degradation of 1-propanol in water
cannot be expected. No data are available on abiotic degradation
in soil.
4.3 Biotransformation
4.3.1 Biodegradation
The results of the determination of the biological oxygen
demand (BOD) of 1-propanol in various sources at 20 °C, using
dilution methods, are summarized in Table 3. Unless otherwise
stated, they are expressed as a percentage of the theoretical
oxygen demand (ThOD), which is 2.40 g oxygen/g 1-propanol. The
chemical oxygen demand (COD) was reported to be 91% of the ThOD
[149].
Table 3. BOD of 1-propanol
-------------------------------------------------------------------------------
Dilution water Source or seed material Adaptation BODxa Value Reference
(+/-) (% ThOD)
-------------------------------------------------------------------------------
Fresh domestic waste water BOD5 64 [149]
BOD20 75
domestic waste water BOD5 93 [202]
synthetic waste water BOD5 97 [202]
activated sludge + BOD5 99b [144]
Salt domestic waste water BOD5 43 [149]
- BOD20 73
-------------------------------------------------------------------------------
a BODx = biological oxygen demand after x days of incubation.
b Expressed as percentage of the COD.
Gerhold & Malaney [66] added 1-propanol to undiluted activated
sludge and found an oxygen uptake of 37% of the ThOD in 24 h.
There are two reports on anaerobic biodegradation. Typical
1-propanol removal efficiencies for an anaerobic lagoon treatment
facility with a retention time of 15 days were 77% and 81% after
loading with concentrated wastes [92]. In closed bottle studies,
1-propanol was completely degraded anaerobically by an acetate-
enriched culture, derived from a seed of domestic sludge. The
culture started to utilize cross-fed, 1-propanol after 4 days, at a
rate of 110 mg/litre per day. In a mixed reactor with a 20-day
retention time, seeded by the same culture, 41% removal was
achieved in the 20 days following 70 days of acclimation to give a
final 1-propanol concentration of 10 000 mg/litre [38].
4.3.2 Bioaccumulation
1-Propanol is completely miscible with water. Its log
n-octanol/water partition coefficient is 0.34 [80]. A
bioconcentration factor of 0.7 can be calculated using the formula
of Veith & Kosian [197]. In addition, the compound is
biodegradable. In view of these data, no bioaccumulation is
expected.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental Levels
The rapid chemical and physical removal of 1-propanol from air
and water is reflected in the few reports indicating its presence
in these compartments. No data are available on the occurrence of
the compound in soil.
In 11 samples of air from a city in the USA in 1982, the
average concentration of 1-propanol was 0.00005 mg/m3, while the
compound was not detected in 18 rural samples [174].
1-Propanol at a concentration of 73 mg/m3 was detected in the
air beneath the surface of 1 out of 6 landfill sites sampled in the
United Kingdom. This particular site was used for the disposal of
domestic waste [216]. 1-Propanol was also detected in the leachate
from two sanitary landfill sites in the USA. This would, at least
partly, have originated from the anaerobic degradation of organic
compounds by microorganisms [30, 102]. 1-Propanol was identified
as a product of the bacterial fermentation of dead blue-green algae
[214], fish spoilage bacteria [3], and Kluyveromyces lactis yeast
[78]. The compound was measured in fresh swine manure [215].
5.2 General Population Exposure
1-Propanol was detected in drinking-water samples in the USA at
a concentration of 0.001 mg/litre [165].
Alcoholic beverages nearly always contain 1-propanol as a
product of fermentation. Beer contains up to 195 mg/litre [17],
wine up to 116 mg/litre [18], various types of spirit up to
3520 mg/litre [130], and neat ethanol up to 2910 mg/litre [9, 19,
146, 140, 186, 148].
Studies summarized in Table 4 show the presence of low levels
of 1-propanol as a flavour volatile in a variety of foodstuffs and
non-alcoholic drinks. According to Stofberg & Grundschober [179],
most of the 1-propanol that they found in the foodstuffs and drinks
was of natural origin, not added.
5.3 Occupational Exposure
Workers are potentially exposed to 1-propanol during the
production of the compound itself or its derivatives, or during its
use in solvent-type applications. No data are available on levels
of exposure.
Table 4. 1-Propanol as a flavour volatile in foodstuffs and
non-alcoholic drinksa
-------------------------------------------------------------------
Foodstuff/drink Reference
Common name Scientific name
-------------------------------------------------------------------
Kefir culture [142]
Cream culture [142]
Filberts (roasted) Corylus avellana [103]
Raw milk [97]
Heat-treated milk [97]
Kumazasa Sasa [134]
albomarginata
Heated trioleinb [123]
Boiled buckwheat flour Fagopyrum esculentum [211]
Ripe tomato, tomato Lycopersicon esculentum [39]
juice, puree, and paste
Kogyoku apple [212]
Apple and apple juice [179]
Tomato Lycopersicon esculentum [179]
White bread [179]
Butter [179]
Cheddar/Swiss cheese [179]
Swiss Gruyere cheese [22]
Soy sauce (Shoyu) [135]
Fish sauce (Patis) [163]
Pigweed Amaranthus retroflexus [58]
Winged bean (raw/roasted) Psophocarpus tetragonalobus [47]
Soybean (raw, roasted) Glycine max [47]
Potato tuber Solanum tuberosum [205, 206]
Roasted watermelon seeds Citrullus colocynthis [175]
Babaco fruit Carica pentagona [168]
Tilsit cheese [133]
Endive Cichorium endivia [73]
Valancia orange juice [127]
-------------------------------------------------------------------
a Detected by GC/FI, GC/FP, or GC/MS.
b The triolein was heated at 185 °C with periodic injection of
steam, during 75 h.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Animals
Data on absorption following inhalation or dermal exposure are
not available.
Oral exposure of Wistar rats to one dose of 3004 mg 1-propanol/kg
body weight in water resulted in a maximum blood concentration of
1860 mg 1-propanol/litre, 1.5 h after exposure [11].
In rabbits receiving single intraperitoneal doses of 800, 1200,
or 1600 mg 1-propanol/kg body weight in saline, maximum blood
concentrations, attained within 0.5 h, were proportional to the
dose [139].
Blood levels of 1-propanol were determined in groups of 3 adult
(200 - 300 g) Sprague-Dawley rats following 1, 10, or 19 consecutive
7-h daily exposures to measured concentrations of 9001 or 14 893 mg/m3
(3659 or 6054 ppm), and after a single exposure to 23 968 mg/m3
(9743 ppm). Immature (110 - 120 g) females of the same strain were
also evaluated following a single 7-h exposure to 23 968 mg/m3
(9743 ppm). In the immature females, the blood level of 1-propanol
was 1640 mg/litre. The blood levels in adult rats following a single
exposure were 26 mg/litre (9001 mg/m3), 42 mg/litre (14 893 mg/m3),
and 66 mg/litre (23 968 mg/m3). Blood levels in adults were not
detected following 10 and 19 exposures to 9001 mg/m3 (3659 ppm), and
were 49 and 43 mg/litre after exposure to 14 893 mg/m3 (6054 ppm) [132].
6.1.2 Human beings
Ten human volunteers drank 1-propanol in ethanolic orange juice
at doses of 3.75 mg 1-propanol and 1200 mg ethanol/kg body weight
over a period of 2 h. At the end of this period, the average peak
blood concentration of 1-propanol was 0.85 ± 0.17 mg/litre (mean ±
standard deviation). When the blood samples taken at comparable
times were analysed after incubation with aryl sulfatase (EC
3.1.6.1), an average peak concentration of 0.92 ± 0.19 mg/litre was
measured, just after exposure [19]. These data suggest that
1-propanol is not extensively sulfate conjugated.
6.2 Distribution
6.2.1 Animals
1-Propanol, a compound that is infinitely soluble in water, is
rapidly distributed throughout the body of various species [1, 157].
When 14C-1-propanol was administered intraperitoneally to rats
in a single dose of 450 mg/kg body weight, the concentrations of
1-propanol and/or its metabolites in the blood, liver, and brain
were similar 5 min after administration. Radioactivity was
detected in the nuclear and mitochondrial fractions of liver and
brain homogenates. Maximum levels were reached later in these
subcellular fractions than in the whole organs [125].
6.2.2 Human beings
In the presence of other aliphatic alcohols, after oral
ingestion of an alcoholic beverage, 1-propanol appears to be widely
distributed throughout the human body [14, 15].
1-Propanol was shown in vitro to bind to human alpha-fetoprotein
with a higher affinity than either methanol or ethanol, which is in
accordance with its higher hydrophobicity [87].
6.3 Metabolic Transformation
6.3.1 Animals
The metabolic fate of 1-propanol is shown in Fig. 1.
1-Propanol is primarily oxidized to propionaldehyde by the non-
specific cytosolic enzyme alcohol dehydrogenase (ADH) (EC 1.1.1.1)
followed by conversion to propionic acid [139]. ADH activity is
known to be the rate-limiting factor in the elimination of
aliphatic alcohols. The Michaelis-Menten constant (Km) of ADH
purified from rat, dog, horse, or human liver with 1-propanol as
substrate, is lower than the Km for ethanol or 2-propanol [45, 7,
72]. Hence, 1-propanol is a better substrate for ADH than ethanol
or 2-propanol and retards the elimination of the latter compounds.
It has been shown in vitro that rat and rabbit liver microsomal
oxidases (EC 1.14.14.1) are also capable of oxidizing 1-propanol to
propionaldehyde [188, 126]. The relative affinity of the
microsomal ethanol oxidizing system (MEOS) for 1-propanol is about
three times higher than for ethanol and is in accordance with their
relative hydrophobicities. In rabbits, cytochrome P-450 isozyme 3a
is responsible for the microsomal metabolism of alcohols [126], in
rats, it is isozyme P-450j, and in human liver, isozyme P-450 HLj
[160]. These forms of cytochrome P-450 are inducible by ethanol
[126, 160], it may therefore be expected that in individuals who
regularly consume ethanol, the MEOS will contribute to the overall
oxidation of 1-propanol. The metabolism of N-nitrosodimethylamine
and 1-propanol is mediated by the same isozyme of cytochrome P-450
[160]. Tomera et al., [191] showed that 1-propanol inhibited the
metabolism of N-nitrodimethylamine in isolated perfused rat livers.
As in the case of propionic acid formed from the catabolism of
odd chain fatty acids, propionic acid arising from the oxidation of
1-propanol can form a coenzyme A (CoA) conjugate [157] catalysed by
acyl CoA synthetase (EC 6.2.1.3). A number of different pathways
for the further metabolism of propionyl-CoA (Fig. 1) are discussed.
However, the relative contribution of each of these to the overall
elimination of 1-propanol is not known.
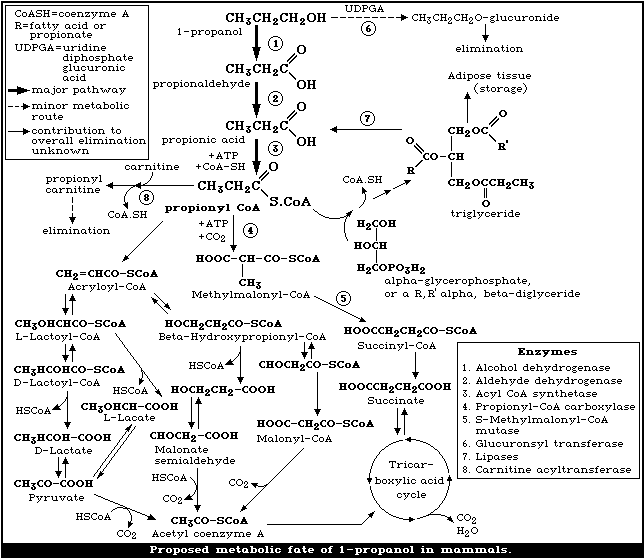 (i) In the methylmalonyl pathway, propionyl-CoA is carboxylated to
methylmalonyl-CoA, this is followed by trans-carboxylation to
succinyl-CoA, which subsequently enters the tricarboxcylic acid
cycle to be metabolized to carbon dioxide and water.
(ii) In the lactate pathway, the propionyl-CoA is dehydrogentated
to acrylolyl-CoA, alpha-hydration gives L-lactoyl-CoA, which is
hydrolysed to lactate.
(iii) In another pathway the acrylolyl-CoA is hydrated to
3-hydroxypropionyl-CoA, deacylation and oxidation result in the
formation of malonic acid semialdehyde, which is converted to
acetyl-CoA. These reactions, which constitute the major pathways
for propionic acid metabolism in plant mitochondria, also occur in
animals.
(iv) The propionyl CoA may also participate in triglyceride
synthesis.
(v) The obligatory formation of propionyl carnitine required for
the transport of propionic acid into mitochrondria may also be an
excretory pathway under conditions of high carnitine and propionic
acid concentrations [158, 153].
Propionic acid and/or propionyl-CoA are potent inhibitors of
several mitochondrial enzymes required for fatty acid oxidation,
gluconeogenesis, and ureagenesis; their inhibitory effects can be
reversed with carnitine [24, 23]. The formation of propionyl-CoA,
its metabolism and effects on oxidative metabolism provide an
explanation for the hepatic effects observed in rats after high
oral exposure to 1-propanol (section 8.2) and for the biochemical
effects seen in some studies (section 8.3.1). Indeed the
accumulation of acyl-CoA esters, including propionyl-CoA, is
implicated in the pathogenesis of Reye syndrome [43].
6.4 Elimination and Excretion
Aliphatic alcohols may be eliminated from the body via expired
air or the urine. Theoretically, urinary metabolites may arise
from oxidation or from conjugation with glucuronic acid or sulfate.
There are no reports of the excretion of unchanged 1-propanol in
expired air or urine and, following an oral dose to rabbits of
800 mg/kg, only 0.9% was found in the urine as propyl-glucuronide
and none as a sulfate conjugate [100].
6.4.1 Animals
Available in vivo data, reviewed by Rietbrock & Abshagen [157],
showed that the elimination of 1-propanol was dose independent
above a single oral dose of 1000 mg/kg body weight in rats and
above a single intraperitoneal dose of 1200 mg/kg body weight in
rabbits [139, 1, 11]. The rate of the zero-order elimination of
the compound from the blood of rats that had received a single oral
dose of 3000 mg/kg body weight was found to be 510 mg/kg body
weight per hour [11]. At lower doses, the elimination rate was
first order. When rats were given a single oral dose of 1000 mg/kg
body weight, the half-life of 1-propanol was 45 min [1]. The
overall metabolism and elimination of 1-propanol are described in
section 6.3.1. In mice, a half-life of 57 min was estimated for
the exponential elimination phase following a single oral exposure
[1]. This should be considered an approximation because there were
only 2 or 3 time points per dose.
In vitro, the elimination of 1-propanol from the perfusate of
rat liver was also shown to be saturable, a zero-order phase being
succeeded below a concentration of 78 mg/litre by an exponential
phase with a half-life of 14 min [7].
6.4.2 Human beings
No data were available describing the elimination kinetics of
1-propanol in human beings.
When 10 volunteers drank 1-propanol in ethanolic orange juice
at doses of 3.75 mg 1-propanol and 1200 mg ethanol/kg body weight
over a period of 2 h, the compound was detected in the blood and in
the urine, partly as glucuronide. The total urinary excretion of
1-propanol was 2.1% of the dose. The urinary levels of 1-propanol
were lower when the amount of simultaneously ingested ethanol was
less, showing competition for ADH between 1-propanol and the
ethanol overdose [19, 20].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table
5. In none of these studies was the concentration of 1-propanol
reported to have been measured. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
open-system studies occurred at lower concentrations than the
nominal ones.
Several short-term studies have also been conducted. Seiler et
al. [167] determined the breakpoint of bioinhibition for a total
of 20 strains of several bacterial groups prevalent in a waste-water
treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
1-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 10 000 - 30 000 mg/litre of medium.
No analysis for the compound was reported.
Inhibition of cell multiplication of blue algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) reached 100%
after 8 days of static exposure to 255 and 3100 mg 1-propanol/litre
water, respectively, in a closed system at 27 °C and a pH of 7
[25, 27].
7.2 Terrestrial Organisms
7.2.1 Insects
The toxicity of 1-propanol for insect larvae is summarized in
Table 5. In static tests, the 48-h LC50 values for adults of the
fruit fly strains of Drosophila melanogaster and Drosophila
simulans were between 18 490 and 24 120 mg/litre of nutrient medium
and 11 260 and 12 860 mg/litre of nutrient medium, respectively [46].
7.2.2 Plants
The effects of 1-propanol on the rate of seed germination
have been investigated on several occasions. Total inhibition of
the germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers absorbing a solution containing 8050
mg 1-propanol/litre water [40]. The germination of white amaranth
(Amaranthus albus) seeds was stimulated in a dose-related manner
after 5 h incubation at 25 °C on filter papers moistened with a
solution containing 3600 - 36 050 mg 1-propanol/litre water [36].
Reynolds [36] measured 50% inhibition of germination of lettuce
(Lactuca sativa) seeds after incubation for 3 days at 30 °C on agar
containing 3065 mg 1-propanol/litre. The percentage germination
and the axis length of soya bean (Glycine max) seeds, with the
testa removed, were not reduced after exposure to pure 1-propanol
for 2 h. After treatment with a 50% (v/v) 1-propanol/water mixture
for 2 min, germination was almost completely inhibited and axis
length was reduced [150].
Table 5. Acute aquatic toxicity of 1-propanol
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria
Pseudomonas putida 25 7 closed 16 h TTb 2 700 [27]
Microorganisms
Activated sludge 21 7.4-8 closed 3 h 50% inhibition 1 000 [105]
of respiration
Acclimated mixed 30 6.8 closed 1.2 h 50% inhibition 19 085 [196]
waste-water culture of respiration
Protozoa
Entosiphon sulcatum 25 6.9 closed 72 h TTb 38 [26]
Chilomonas paramecium 20 6.9 closed 48 h TTb 175 [29]
Uronema parduczi 25 6.9 closed 20 h TTb 568 [28]
Algae
Selenastrum capricornutum 25-26 closed 96 h NOAECc 2 000 [172]
Scenedesmus pannonicus 25-26 closed 48 h NOAECc 2 900 [172]
Chlorella pyrenoidosa 25-26 closed 48 h NOAECc 1 150 [172]
Coelenterate
Hydra oligactis 17 8.2-8.4 >5 closed 48 h LC50 6 800 [170]
Worms
Flatworm (Dugesia) 20 8.2-8.4 >5 closed 48 h LC50 4 700 [170]
Tubificid worm 20 8.2-8.4 >5 closed 48 h LC50 9 200 [170]
Molluscs
Giant pond snail 20 8.2-8.4 >5 closed 48 h LC50 6 500 [170]
(Lymnea stagnalis)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Crustaceans
Water flea 20 8 >2 250 open 24 h EC50d 4 415 [27]
(Daphnia magna)e EC0 3 336
EC100 5 909
19 open 48 h LC50 7 080 [33]
Water flea 19 open 48 h LC50 3 025 [33]
(Daphnia pulex)e
Water flea 19 open 48 h LC50 5 820 [33]
(Daphnia cucullata)e
Isopod 20 8.2-8.4 >5 209 closed 48 h LC50 2 500 [170]
(Asellus aquaticus)
Scud (Gammarus pulex) 20 8.2-8.4 >5 209 closed 48 h LC50 1 000 [170]
Insects
Mosquito larvae 22-24 open 4 h LC50 10 450 [107]
(Aedes aegypti)
Mosquito larvae (Aedes 26 8.2-8.4 >5 209 open 48 h LC50 4 400, 4 800 [172]
aegypti, Culex pipiens) LC0 3 200, 3 600
Midge larvae 20 8.2-8.4 >5 209 closed 48 h LC50 2 350 [170]
(Chironomus gr. thummi)
Leech larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 400 [170]
(Eropdella octoculata)
Dragon fly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 4 200 [170]
(Ischnura elegans)
Stonefly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 520 [170]
(Nemoura cinerea)
Mayfly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 3 110 [170]
(Cloeon dipterum)
Corixa punctata (larvae) 20 8.2-8.4 >5 209 closed 48 h LC50 2 000 [170]
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Creek chub 15-21 8.3 98 open 24 h LC0 200 [68]
(Semotitus atromaculatus)
Golden orfe 20 7-8 >5 200-300 48 h LC50 4 320, 4 560 [99]
(Leuciscus idus melanotus) LC0 3 600, 4 000
Fathead minnow 20 8.2-8.4 >5 209 open 48 h LC50 5 000 [172]
(Pimephales promelas) LC0 2 600
Rainbow trout 15 7-8 >5 98 open 48 h LC50 3 200 [172]
(Salmo gairdneri) LC0 2 000
Paddy fish 24 8.2-8.4 >5 209 open 48 h LC50 5 900 [172]
(Oryzias latipes) LC0 4 400
Amphibia
South African clawed toad 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Xenopus laevis)
Mexican axolotl 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Ambystoma mexicanum)
SEA WATER
Bacteria
Photobacterium 15 15 min 50% light 8 686 [84]
phosphorerum closed reduction
5 5 min 50% light 17 700 [48]
closed 15 min reduction 18 400
Crustacea
Brine shrimp 24 24 h LC50 4 200 [149]f
(Artemia salina) open
Harpacticoid copepod 21 7.9 >5 96 h LC50 2 300 [12]g
(Nitocra spinipes)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Bleak (Alburnus alburnus) 10 7.9 >5 open 96 h LC50 3 800 [12]g
---------------------------------------------------------------------------------------------------------------------------------
a Static systems used in all experiments reported.
b TT = toxic threshold for inhibition of cell multiplication.
c NOAEC = no-observed-adverse-effect-concentration; effect is growth inhibition.
d Effect is complete immobilization.
e Age of Daphnia was 24 h for Daphnia magna and Daphnia pulex, and 11 ± 1 day for Daphnia cucullata.
f Salinity was 2.8%.
g Salinity was 0.7%.
1-Propanol was marginally effective in breaking the dormancy of
seeds of genetically pure dormant lines of wild oat (Avena fatua)
after 5 days of exposure to solutions containing up to 1202 mg/litre.
Seed viability was affected at higher concentrations [2].
When excised seedling roots of maize (Zea mays) were treated by
vacuum infiltration in a 5% solution of 1-propanol in water, 3
times for 60 seconds, and then incubated anaerobically at 28 °C,
nitrite accumulation increased by 10 times or more, the utilization
of nitrate increased, and the utilization of exogenous nitrite was
inhibited. These effects were enhanced under aerobic conditions
[75]. Dry et al. [51] observed that stimulation of nitrite
accumulation in pea and wheat roots under aerobic conditions was
accompanied by a decline in the cellular levels of glucose-6-
phosphate. It was suggested by Gray & Cresswell [75] that an
increase in the utilization of nitrate was related to increased
access of nitrate to the site of nitrate metabolism as a result of
an increase in membrane permeability.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
The available LD50s for various animal species are summarized
in Table 6. Based on mortality estimates, 1-propanol exhibits low
toxicity, except in very young rats. Oral LD50 values for several
animal species range between 1870 and 6800 mg/kg body weight. For
very young rats, oral LD50s of 560 - 660 mg/kg body weight have
been reported [152].
An intraperitoneal dose of 785 mg 1-propanol/kg was lethal to 10
out of 10 C57B/6J and 10 out of 10 DBa/2J mice, but a dose of 392 mg/
kg did not cause any deaths in either strain [183]. An LD16 of
450 mg/kg was reported in rats after intraperitoneal administration.
When rats were exposed to 1-propanol vapour for 4 h at a
concentration of approximately 9840 mg/m3, 2 out of 6 died within
14 days [173].
8.1.2 Signs of intoxication
Osborne-Mendel or Sherman rats of both sexes receiving a lethal
oral dose of undiluted 1-propanol became comatose within a few
minutes [187]. Deep narcosis occurred in mice exposed through
inhalation of 1-propanol at a concentration of 50 mg/litre for 2 h.
Very young rats (60 - 100 g) of an unspecified strain and of
both sexes, received a single oral dose of between 150 and 3000 mg
undiluted 1-propanol/kg body weight. Animals that died showed
hyperaemia, vacuolation, and dilated sinusoids in the liver, and
hyperaemia, tubular cloudy swelling, and tubular necrosis in the
kidneys [152].
When anaesthetized Sprague-Dawley rats were made to inspire
160 mg of the undiluted compound, all 9 exposed rats died within
165 min, 6 of them dying immediately from respiratory arrest. All
controls survived and were killed 24 h later. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight of the exposed rats was increased by 92%. The lungs
showed oedema and small areas of focal haemorrhage [65].
Special studies on neurotoxic and behavioural effects, and on
biochemical effects are described in sections 8.3 and 8.4.
Table 6. LD50s for 1-propanol
---------------------------------------------------------------------------------------------------
Species Sex Route of Observation LD50 (mg/kg Comments Reference
exposure period body weight)
---------------------------------------------------------------------------------------------------
Wistar rat (non- male oral 14 days 1870 vehicle: water [173]
fasted)
Osborne-Mendel male, oral until 6500 undiluted [187]
or Sherman rat female recovery
CD mouse not oral 3 days 6800 undiluted [164]
reported
Rabbit male, oral 1 day 2820 [130]
female
Wistar rat male intravenous 5 days 590 vehicle: water [190]
H mouse male intravenous 5 days 697 vehicle: water [190]
female intravenous not 1090 vehicle: water [40]
reported
Chinchilla rabbit male, intravenous 5 days 483 vehicle: water [190]
female
Wistar rat male intraperitoneal 5 days 2247 vehicle: water [190]
H mouse male intraperitoneal 5 days 3695 vehicle: water [190]
Syrian hamster male intraperitoneal 5 days 2337 vehicle: water [190]
Guinea-pig intraperitoneal 5 days 1208 vehicle: water [190]
New Zealand male dermal 14 days 4050 1/10 of body [173]
rabbit surface exposed
under cover for
24 h
---------------------------------------------------------------------------------------------------
8.1.3 Skin, eye, and respiratory tract irritation; sensitization
Data for skin and eye irritation were not available. One skin
sensitization test has been reported concerning an ear-swelling
test on CF1 mice. No sensitization was observed [61]. Although
the test requires further validation, it correctly discriminated
between a number of known positive and negative human sensitizing
agents. The authors claim it to be an accurate, sensitive, and
efficient method for evaluating delayed contact sensitization.
The sensory irritation of 1-propanol was investigated using a
50% reflex decrease in the respiratory rate of mice (RD50) as an
index. Only the heads of the mice were exposed. An exposure-
related effect was found with RD50 values for the first 10 min of
exposure of 31 252 mg/m3 for Swiss Webster mice [101] and
33 604 mg/m3 for CF-1 mice [108]. The potential of 1-propanol as
a respiratory irritant is therefore low.
8.2 Repeated Exposures
Only a few data are available concerning the oral exposure of
animals.
When 3 male and 3 female rats of unspecified strain were
exposed to 4 daily oral doses of 2160 mg undiluted 1-propanol, no
deaths occurred and no gross pathological signs were seen in the
liver [187].
In a group of 6 male rats of unspecified strain, receiving
drinking-water containing 1-propanol at a concentration of 60 090
mg/litre for 4 months, food consumption, body weight gain, and
liver histopathology were comparable to those of the control group.
It should be noted that the authors reported a dose rate of 3 mg/kg
body weight per day, while a dose rate of approximately 3000 mg/kg
body weight per day seems more appropriate, assuming a water
consumption of 20 ml/day and a body weight of 400 g [85].
Groups of 10 Wistar rats were exposed to 1-propanol in the
drinking-water at a concentration of 320 000 mg/litre (calculated
by the Task Group to be equivalent to approximately 16 000 mg/kg
body weight per day, on the basis of the assumptions made above)
for 5, 9, or 13 weeks. Control groups comprised 10 rats each. The
exposed rats gradually became weak, losing their appetites and
showing a decreased body weight gain. Electron microscopic studies
of the liver showed irregularly shaped megamitochondria with few
cristae, and normally sized but irregularly shaped mitochondria
with a decreased number of cristae. Biochemical changes included a
decreased state 3 respiration using glutamate as a substrate and
decreased specific activities of cytochrome c oxidase (EC 1.9.3.1)
and monoamine oxidase (EC 1.4.3.4) [203].
8.3 Neurotoxic and Behavioural Effects
In one study on anaesthetized mongrel dogs, it was shown that
1-propanol, as well as other primary alcohols, could increase the
permeability of the blood-brain barrier. The dogs received a
sodium fluorescein solution and 0.578 mg 1-propanol in saline,
intravenously. The concentration of sodium fluorescein in the
cerebrospinal fluid rose to a maximum within 10 min and returned to
control levels, 3 h after exposure [78].
The oral ED50 (1440 mg/kg body weight) for narcosis in rabbits
exposed to 1-propanol was 4 times lower than that for ethanol
[129]. Deep narcosis occurred in mice exposed through inhalation
to 50 mg 1-propanol/litre for 2 h, and a 40-min exposure to 2.3 mg/
litre reduced the unconditioned flexor response in rabbits. When
rabbits were intravenously infused with 1-propanol at a rate of 9 -
30 mg/min per kg body weight, positional nystagmus with an
inhibited rotatory response was observed at and above a blood
concentration of 900 mg/litre [137].
The intraperitoneal ED50 for loss of righting reflex in Swiss
Webster mice administered 1478 mg 1-propanol/kg body weight was 2.8
times lower than that for ethanol [117]. When C57BL/6J or DBA/2J
mice were given a single dose of 1-propanol intraperitoneally, both
strains showed decreased activity in the open field test at
392 mg/kg body weight, but the decrease was not significant. All
mice given 785 and 1570 mg/kg body weight died [183]. The rotarod
performance of Swiss-Cox mice decreased in a dose-related manner
after single oral doses of 1-propanol of 2000 or 4000 mg/kg body
weight. A dose of 1000 mg/kg body weight did not cause behavioural
impairment. When the study was repeated on days 4, 6, 7, and 8
after the first trial, tolerance did not develop [1].
The threshold for the induction of ataxia in Sprague-Dawley
rats following intraperitoneal exposure was 799 mg/kg body weight
[119]. In a tilted plane test, the performance of rats decreased
by an average of 71% after oral exposure to 2000 mg/kg body weight.
On a molar basis, 1-propanol was 2.5 times as intoxicating as
ethanol [204].
According to several investigators, depression of the central
nervous system by 1-propanol was related to interactions with
neuronal membranes. Lyon et al. [117] observed a high correlation
between the narcotic potencies of the aliphatic alcohols, including
1-propanol, in mice and their ability to disorder the brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was in turn related to membrane
solubility. A change in membrane fluidity was shown to occur in
isolated synaptosomal plasma membranes of rat cortex in vitro by a
decrease in 1,6-diphenyl-1,3,5-hexatriene fluorescence polarization
[81].
Functional loss due to disruption of membrane integrity by
1-propanol was observed in vitro. The action potentials of the
sciatic nerves of the toad (Bufo marinus) [155] and of giant axons
of the squid (Loligo forbesi) [143] were decreased by 1-propanol.
In isolated rat phrenic nerve-diaphragm, 1-propanol increased the
amplitudes of end-plate and miniature end-plate potentials and the
number of quanta of acetylcholine of end-plate potentials [62].
The compound also affected the rate of decay of postsynaptic
currents in the neuromuscular junction of the crayfish (Cherax
destructor) [200], and in the phrenic nerve-diaphragm of the rat
[62].
Effects on the ionic currents underlying the changes in
excitability described above were also investigated in vitro.
1-Propanol inhibited both the K+-stimulated and the Na+-dependent
influx of Ca2+ ions into isolated rat brain synaptosomes [80, 81,
124], and the influx of Na+ ions into rat brain synaptosomes [128].
It decreased the Na+ and K+ currents in the giant axons of the
squid (Loligo forbesi) [143], and in sciatic nerve fibres of the
clawed toad (Xenopus laevis) [6]. The interference of 1-propanol
with the transport of Ca2+ ions across biological membranes was
also shown in vitro by the inhibition of Ca2+ ion-induced
contractions of guinea-pig ileum [213], and in vivo, in rats, by a
decrease in regional brain Ca2+ ion levels, 30 min after one
intraperitoneal dose of 2000 mg/kg body weight [159].
The disruption of neuronal membranes by 1-propanol was also
thought to explain its inhibitory action on the binding of
dihydromorphine to isolated mouse brain caudate membranes [185] and
on membrane-bound guanylate cyclase (EC 4.6.1.2) in intact murine
neuroblastoma N1E-115 cells [172]. The activation by 1-propanol of
membrane-bound adenylate cyclase (EC 4.6.1.1) from isolated mouse
striatal membranes, in the presence of guanine nucleotides, was
also suggested to be the result of membrane perturbation [116].
8.4 Biochemical Effects
8.4.1 Effects on lipids in the liver and blood
Oral administration of single doses of 3000 or 6000 mg
1-propanol/kg body weight to Wistar rats caused a transient
increase in hepatic triglycerides, which was related to the
duration of an elevated blood-1-propanol concentration [10, 11].
Gaillard & Derache [63] did not observe an increase in hepatic
triglycerides in Wistar rats, 17 h after a single dose of 6000 mg
1-propanol/kg body weight.
Factors possibly responsible for hepatic triglyceride
accumulation include: an increase in hepatic uptake of labelled
palmitate [11], an increased esterification of palmitate to form
liver triglycerides [10, 11], and decreased palmitate oxidation
[11]. The decrease in palmitate oxidation was related to an
increase in the hepatic alpha-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [11].
An increase in extramitochondrial reducing equivalents, indicated
by an increased lactate/pyruvate ratio, was observed in vitro by
Forsander [60], but not in vivo by Beaugé et al. [11].
The effects of 1-propanol on palmitate incorporation into
triglycerides appear to depend on the dose, high doses causing
inhibition and lower ones leading to an increase. The
incorporation of palmitate into serum triglycerides and serum and
liver phospholipids, 4.5 h after a single oral dose of 6000 mg
1-propanol/kg body weight to rats, was found to be inhibited, while
an increase in hepatic triglyceride accumulation was only observed
8 h after dosing [10]. Three hours after a dose of 3000 mg/kg body
weight, the incorporation of palmitate in blood triglycerides was
increased concomitantly with an increase in hepatic triglycerides
while levels of phospholipids in the liver and blood were unaffected
[11]. Similar effects have been noted with ethanol [11].
8.4.2 Effects on microsomal enzymes
The effects of 1-propanol on microsomal enzymes (EC 1.14.14.1)
in vivo was investigated by Powis [147], who administered a single
oral dose of 960 mg/kg body weight to Wistar rats. Twenty four
hours after exposure, no effects were observed on the activities of
aniline hydroxylase and aminopyrine demethylase in liver
microsomes. This study is inadequate to demonstrate an inductive
effect of 1-propanol on the microsomal mixed function oxidase
system. In vitro, 1-propanol inhibited aldrin epoxidase and
p-aniline hydroxylase in isolated rat liver microsomes via an
interaction with cytochrome P-450, which causes a reverse Type I
spectral change [41, 210, 189, 161]. At high concentrations, the
inhibition of the mixed function monoxygenase system by aliphatic
alcohols correlates directly with the lipophilicity of the
alcohols, and is probably the result of unspecific effects on the
membrane (see section 8.3). The compound did not affect the levels
of hepatic microsomal cytochrome P-450, haem, cytochrome b5, and
NADPH-cytochrome c reductase (EC 1.6.2.4) in phenobarbital-induced
rats [96]. 1-Propanol increased the levels of cytochrome P-450 in
cultured chick embryo hepatocytes. The activities of benzphetamine
demethylase and UDP-glucuronosyl transferase (EC 2.4.1.17) were
also increased [169].
8.4.3 Other biochemical findings
The glutathione level in the liver of Wistar rats administered
a single dose of 1660 mg 1-propanol/kg body weight, orally, had
decreased by 20%, 6 h after exposure. Lipid peroxidation, as
indicated by diene conjugates formation, was increased; ethanol in
equivalent doses produced similar effects [199].
The activities of liver ornithine decarboxylase (EC 4.1.1.17)
and liver tyrosine aminotransferase (EC 2.6.1.5) increased in
partially hepatectomized rats 4 h after one oral dose of 2300 mg
1-propanol/kg body weight or an equivalent dose of ethanol. No
effects were observed on levels of alanine aminotransferase
(EC 2.6.1.2) in the liver and kidneys, and on levels of ornithine
decarboxylase in the kidneys and brain [145].
The effects of 1-propanol on neuronal membrane-bound adenylate
cyclase (EC 4.6.1.1) and guanylate cyclase (EC 4.6.1.2) in vitro
are discussed in section 8.3. 1-Propanol and ethanol, can have
different effects on the activity of adenylate cyclase, depending
on the concentration of alcohol and the biological system being
investigated [178, 192, 93].
When Sprague-Dawley rats inhaled 1-propanol for 6 h at a
concentration of 490 mg/m3, the serum level of testosterone was
decreased by 42% immediately after exposure, but not 18 h after
exposure. When this exposure regimen was repeated daily over one
week, no effects on serum testosterone levels were observed. Serum
levels of luteinizing hormone and corticosterone were unchanged at
all times [31].
When a crude homogenate of dispersed acinar cells, prepared
from guinea-pig pancreas, was incubated with 1-propanol and
secretin, the secretin-stimulated activities of adenylate cyclase
(EC 4.6.1.1) and cellular cyclic adenosine 3',5'-monophosphate were
potentiated at low concentrations of 1-propanol, but the
potentiation was reversible. Irreversible inhibition occurred at
higher concentrations [192].
8.5 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 15 male Sprague-Dawley rats were exposed to
1-propanol at measured concentrations of 8610 or 15 220 mg/m3 for
7 h/day over 6 weeks. Beginning on the third day after the last
exposure, males were mated for a maximum of 5 days with unexposed
females. There was no apparent effect of exposure to 8610 mg/m3 on
mating performance or fertility. After exposure to 15 220 mg/m3,
17 out of 18 males copulated (as evidenced by the presence of a
vaginal plug), but only 2 of 17 mated females became pregnant. The
offspring of the exposed males were evaluated postnatally in a
battery of behavioural tests. There was no evidence of any
exposure-related effect [132].
These investigators also exposed groups of 15 pregnant Sprague-
Dawley rats to the same concentrations of 1-propanol on gestation
days 1 - 20. Pregnant females exposed to 15 220 mg/m3 showed
significantly reduced weight gain and food consumption. Their
female offspring also showed reduced weight gain up to 3 weeks of
age, but there was no consistent effect on male offspring. Litter
sizes were not affected. "Several" of the offspring from dams
exposed to 15 220 mg/m3 had crooked tails. Behavioural testing of
offspring did not reveal any evidence of an exposure-related
effect, though there was an increase in total external, visceral,
and skeletal malformations at 23 968 mg/m3 (9743 ppm) and in total
skeletal malformation at 14 893 mg/m3 (6054 ppm) [132].
The effects of 1-propanol on brain development in the neonatal
rat were also studied. A group of 21, 5-day-old Long-Evans rats
was exposed to 1-propanol via an artificial milk formula, which was
administered through an intragastric catheter for 4 consecutive
days. The rats received 12 feeds daily, each lasting 20 min.
Doses were 3800, 7500, 3000, or 7800 mg/kg body weight on day
5 - 8, respectively. Controls received the milk formula only.
During the exposure, the exposed pups frequently showed an impaired
righting response. After the last exposure, withdrawal symptoms
were displayed. Pups were killed at 18 days of age, at which time
there was no effect on body weight or on absolute weight of
kidneys, heart, or liver. However, the absolute and relative brain
weights were decreased in the exposed pups. Biochemical analysis
showed that the exposed pups had a decreased amount of DNA in all
brain areas examined. Cholesterol levels were decreased in the
forebrain and cerebellar samples, while protein levels were
decreased only in the forebrain samples [74].
8.6 Mutagenicity
8.6.1 Bacteria
1-Propanol was tested for mutagenic activity using Ames test
without S9; up to 100-µmol/plate was negative with Salmonella
typhimurium TA-100 [181]. Negative results were also reported in
TA-100 and TA-98 with or without metabolic activation, following
standard Ames test protocol [94].
In a reverse mutation assay with Escherchia coli CA-274
following a pre-incubation protocol, a 5-fold increase in the
number of revertants was observed at a concentration of 4.5%
1-propanol. No metabolic activation system was used [86].
8.6.2 Mammalian cells in vitro
1-Propanol (100 mg/litre once a day for 7 days) did not
increase the number of sister chromatid exchanges in Chinese
hamster ovary cells [136], or in V79 Chinese hamster lung
fibroblasts at 6000 mg/litre for 3 h (with activation) and 28 h
(without activation) [200]. It did not increase the number of
micronuclei in V79 Chinese hamster lung fibroblasts at 40 200 mg/
litre for 1 h [113].
A dose-related increase in the inhibition of metabolic
cooperation between hamster V79 cells, a phenomenon believed to
reflect carcinogenic promotion ability and not be indicative of
genotoxic potential, was observed by Chen et al. [37]. This may be
due to the membrane effects of 2-propanol.
8.7 Carcinogenicity
A group of 18 Wistar rats of both sexes received doses of
240 mg 1-propanol/kg body weight, by gavage, twice a week, for
their lifetime. Another group of 31 Wistar rats of both sexes
received subcutaneous injections of 48 mg compound/kg body weight,
twice a week, for their lifetime. Control groups, comprising 25
rats for each route, received saline. It was not reported whether
the analytical grade, double-distilled test compound was analysed
for the presence of impurities. The average survival time was 570
days for the orally exposed rats, 666 days for the subcutaneously
exposed rats, and 643 days for both control groups. The tumour
incidence is reported in Table 7. The data were not statistically
analysed. It was reported that "nearly all rats" showed liver
damage including congestion, steatosis, necrosis, fibrosis, and
metaplasia and hyperplasia of the haematopoietic bone marrow
parenchyma. However, the incidence of these lesions were not
reported [67].
Table 7. Tumour incidence in Wistar rats exposed orally or
subcutaneously to 1-propanol for lifetimea
-------------------------------------------------------------------
Organ/ Tumour type Incidence
tissue oral exposure sc exposure
affected exposed controls exposed controls
-------------------------------------------------------------------
Blood myeloid leukemia 2/18 0/25 4/31 0/25
Liver carcinoma 1/18 0/25 0/31 0/25
Liver sarcoma 2/18 0/25 5/31 0/25
Other carcinoma 0/18 0/25 3/31b 0/25
sarcoma 0/18 0/25 2/31c 0/25
benign tumoursd 10/18 3/25 7/31 2/25
-------------------------------------------------------------------
a From: Gibel et al. [67].
b One carcinoma each in kidney, bladder, and uterus.
c One sarcoma each in spleen and at injection site.
d Mostly papillomas and mammary fibroadenomas.
Although there was an apparent increase in the incidence of
liver sarcoma, the study is inadequate for the assessment of
carcinogenicity. The dosing schedule did not conform to standard
protocol. Too few animals were used in each dose group, the sex
ratio of each group was unclear, no data were provided on the
histological type of liver sarcoma, no statistical analysis was
conducted, the maximum tolerated dose was exceeded, as evidenced by
the reported liver damage, and only single dose levels were used.
In the case of subcutaneous administration, the exposure route was
inappropriate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
One case of poisoning by 1-propanol has been reported. It
concerned a 46-year-old woman who was estimated to have consumed
approximately half a litre of the compound as a solvent in a
cosmetic preparation, probably a hair lotion. It was pointed out
that the woman could have ingested this preparation more than once
in the past. The woman was found unconscious. She died 4 - 5 h
after ingestion. No other signs or symptoms were reported.
Autopsy revealed a "swollen brain" and lung oedema [52].
9.1.2 Controlled human studies
Filter papers moistened with 0.025 ml of a 75% solution of
1-propanol in water were placed on the forearms of a group of 12
volunteers following immersion of the forearms in water at 23 °C
for 10 min. The patches were covered for 5 min and then gently
blotted. Nine of the 12 persons showed erythema for at least 60
min following exposure. The cutaneous reaction was totally blocked
in 4 out of 4 persons after pretreatment with 40% 4-methylpyrazole
in hydrophilic ointment 1 h before the challenge, showing,
according to the authors, that 1-propanol must be metabolized to
propanal before vasoactivity occurs [207].
9.2 Occupational Exposure
A laboratory worker in a company manufacturing hair cosmetics
developed allergic reactions in patch tests with chemically pure
1-propanol solutions in water (10 - 99.5% by volume). This person
also reacted to 2-propanol, 1-butanol, 2-butanol, and formaldehyde,
but not to ethanol and methanol. Controls were not tested [115].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 1-propanol may occur through
ingestion of food and alcoholic beverages containing 1-propanol
(e.g., wine and beer 100 - 200 mg/litre, spirits up to 3500
mg/litre). Inhalation exposure may occur during household use and
occupationally during manufacture and processing. Exposure of the
general population via inhalation and drinking-water is very low
(average concentrations in urban air and drinking-water in the USA,
0.00005 mg/m3 and drinking-water 0.001 mg/litre, respectively)
(section 5).
10.1.2 Health effects
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Data on the absorption rate following
inhalation are lacking but, in view of the physical properties of
the compound, it is also expected to be rapid. Dermal absorption
is expected to be slow (section 6).
1-Propanol exhibits low acute toxicity for animals (based on
lethality estimates), whether exposed via the dermal, oral, or
respiratory route (section 8.1). Exposure to potentially lethal
levels may occur in the general population through accidental or
intentional ingestion. However, only one case of lethal poisoning
by 1-propanol has been reported, which probably reflects its low
toxicity and limited use by the public (section 9.1.1). The
principal toxic effect of 1-propanol following a single exposure is
depression of the central nervous system. Quantitative exposure-
effect data on human beings are not available. The most likely
acute effects of 1-propanol in man are alcoholic intoxication and
narcosis. Animal studies indicate that 1-propanol is 2 - 4 times
as intoxicating as ethanol.
A controlled human study has indicated that 1-propanol may be
irritating to hydrated skin. However, the potential of 1-propanol
as a respiratory irritant is low (section 8.1.3). Data are
inadequate for evaluation of the irritating properties of this
compound for the skin, eye, and respiratory tract in human beings,
or for evaluation of its sensitizing potential.
The results of limited drinking-water studies on animals
suggest that oral exposure to 1-propanol is unlikely to pose a
serious health hazard under the usual conditions of human exposure
(section 8.2).
Inhalation exposure to a concentration of 15 220 mg/m3 caused
impaired reproductive performance in male rats, but exposure to
8610 mg/m3 did not. In pregnant rats, 9001 mg/m3 (3659 ppm) was a
NOEL and 14 893 mg/m3 (6054 ppm) was a LOEL for both maternal and
developmental toxicity. Behavioural effects were not detected in
offspring whose mothers were exposed during pregnancy to 15 220
mg/m3, but oral dosing of neonatal rats produced biochemical
changes in the brain that were detected 10 days after the last
treatment (section 8.5). Inhalation exposure to high concentrations
of 1-propanol produced reproductive and developmental toxic effects
in male and female rats. These effects occurred in the presence of
other overt signs of toxicity in the exposed animals and 1-propanol
does not appear to be selectively toxic to male or female
reproductive processes. The concentrations required to produce
these effects in rats were higher than those likely to be
encountered under normal conditions of human exposure.
1-Propanol was negative in assays for point mutations in
bacteria. It did not increase the incidence of sister chromatid
exchange or micronuclei in mammalian cells in vitro. Although
these findings suggest that the substance does not have any
genotoxic potential, no adequate assessment of mutagenicity can be
made on the basis of the limited data available. The results of an
in vitro test said to predict promotional activity were negative
(section 8.6). The available study is inadequate to evaluate the
carcinogenicity of 1-propanol in experimental animals (section
8.7). No data are available on the long-term exposure of human
populations to 1-propanol. Hence, the carcinogenicity of
1-propanol in human beings cannot be evaluated. Apart from one
case of fatal poisoning following ingestion of half a litre of
1-propanol, there are practically no reports on adverse health
effects from exposure to 1-propanol either in the general
population or in occupational groups (section 9).
The Task Group considers it unlikely that 1-propanol will pose
a serious health risk for the general population under normal
exposure conditions.
10.2 Evaluation of Effects on the Environment
1-Propanol can be released into the environment during
production, processing, storage, transport, use, and waste disposal
(section 3). It is transferred from water, soil, and waste
disposal sites to the atmosphere by volatilization, from the
atmosphere to water and soil by rain-out, and from soil and waste
disposal sites to ground water by leaching. It is difficult to
estimate its emission into each compartment. Because of its
primary use as a volatile solvent, most of the production volume is
eventually released into the atmosphere (section 4.1).
By reacting with hydroxyl radicals and through rain-out,
1-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 3 days (section 4.2). Thus, measurable
atmospheric levels of 1-propanol are not usually encountered.
Hydrolysis and photolysis are not expected to be important in
the removal of 1-propanol from water and soil, but removal occurs
rapidly by aerobic and anaerobic biodegradation (section 4.3.1) so
that measurable levels are rarely found. Adsorption of 1-propanol
on soil particles is poor but it is likely to be mobile in soil and
it has been shown to increase the permeability of soil to some
aromatic hydrocarbons (section 4.1).
In view of the physical properties of 1-propanol, its potential
for bioaccumulation is low (section 4.3.2). Except in the case of
accident or inappropriate disposal, 1-propanol does not present a
risk for aquatic organisms, insects, or plants at concentrations
that usually occur in the environment. However, 1-propanol at
concentrations of around 5000 mg/litre in water is lethal to
oxygen-using aquatic organisms, indicating that its emission into
surface water at this level may result in serious alteration of the
local ecosystem (section 7).
11. RECOMMENDATIONS
1. 1-Propanol has not shown mutagenic potential in the small
number of assays performed. A full array of modern
genotoxicity tests should be carried out.
2. A single published report suggests carcinogenic activity by
1-propanol, but this study is seriously flawed and cannot be
used to evaluate the potential carcinogenicity of 1-propanol.
The desirability of a carcinogenesis bioassay of 1-propanol
should be considered, on the basis of the outcome of
genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
1-propanol produced reproductive and developmental toxicity in
experimental animals. In view of the potential for
environmental and drinking-water contamination, reproductive
and developmental toxicity should be investigated using oral
dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
1-propanol.
5. The unusually uniform level of toxicity in diverse types of
aquatic organisms that consume gaseous oxygen, and the
exceptionally steep dose-effect curve observed, suggest a
nonspecific effect that may not be restricted to 1-propanol.
These effects merit investigation.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1-Propanol was considered by the Joint FAO/WHO Expert
Committee on Food Additives (JECFA) in its twenty-third report.
Specifications were formulated, but no toxicological monograph was
prepared, and the substance could not be evaluated on the basis of
the data available. Additional toxicological information was
available to JECFA at a later meeting, including the results of a
limited study in rats suggesting a carcinogenic potential for
1-propanol. In its twenty-fifth report,a JECFA noted that life-
time feeding studies in rodents were required to resolve the
problem of carcinogenicity. A toxicological monograph was
prepared. The existing specifications were revised and designated
as "tentative", but no ADI was established.
-------------------------------------------------------------------
a WHO Technical Report Series, No. 669, 1981 ( Evaluation of
certain food additives: Twenty-fifth Report of the Joint
FAO/WHO Expert Committee on Food Additives).
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
2. ADKINS, S.W., NAYLOR, J.M., & SIMPSON, G.M. (1984) The
physiological basis of seed dormancy in Avena fatua. V.
Action of ethanol and other organic compounds. Physiol.
Plant., 62: 18-24.
3. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
4. ALLINGER, N.L., CAVA, M.P., DE JONGH, D.C., JOHNSON, C.R.,
LEBEL, N.A., & STEVENS, C.L. (1971) Organic chemistry, New
York, Worth Publishers, Inc.
5. ANON. (1984) Propyl alcohol complaint settled by US
companies. Chem. marketing Rep., April 23: 3, 17.
6. ARHEM, P. & VAN HELDEN, D. (1983) Effects of aliphatic
alcohols on myelinated nerve membrane. Acta physiol. Scand.,
119: 105-107.
7. AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl and isoamyl alcohols by the isolated
perfused rat liver. J. Pharmacol. exp. Ther., 197: 669-674.
8. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of 2-
halo-pyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
9. BEAUD, P. & RAMUZ, A. (1978) Dosage simultané des alcools
supérieures, et de l'acetate d'éthyle dans les eaux-de-vie
par chromatographie gaz-liquide-solide. Trav. chim. Aliment.
Hyg., 69: 423-430.
10. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1974)
Perturbations du métabolisme hépatique du palmitate [1-14C]
déterminée par l'administration de n-propanol chez le rat.
Biochimie, 56: 1157-1159.
11. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1979)
Comparative effects of ethanol, n-propanol and isopropanol on
lipid disposal by rat liver. Chem.-biol. Interact., 26:
155-166.
12. BENGTSSON, B.-E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13
aliphatic alcohols. Chemosphere, 13: 613-622.
13. BILZER, N. & GRUNER, O. (1983) [Critical assessment regarding
determination of aliphatic alcohols (congeners in alcoholic
drinks) in blood with the aid of head-space analysis.]
Blutalkohol, 20: 411-421 (in German).
14. BILZER, N. & PENNERS, B.-M. (1985) [Concerning the velocity
of reduction and excretion of the attendant substance
propanol-1 and isobutanol after drinking whisky of the trade
mark Chivas Regal.] Blutalkohol, 22: 140-145 (in German).
15. BILZER, N., PENNERS, B.-M., & GRUNER, O. (1985) [Studies
about the course of concentration in blood for congener
propanol-1 and isobutanol after drinking overseas rum
("Captain Morgan").] Blutalkohol, 22: 146-151 (in German).
16. BONTE, W. (1978) [Congener content of wine and similar
beverages.] Blutalkohol, 15: 392-404 (in German).
17. BONTE, W. (1979) [Congener content of German and foreign
beers.] Blutalkohol, 16: 108-124 (in German).
18. BONTE, W., DECKER, J., & BUSSE, J. (1978) [Congener content
of highproof alcoholic beverages.] Blutalkohol, 15: 323-338
(in German).
19. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981a) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human blood.]
Blutalkohol, 18: 399-411 (in German).
20. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human urine.]
Blutalkohol, 18: 412-426 (in German).
21. BONTE, W., STOPPELMAN, G., RUDELL, E., & SPRUNG, R. (1981c)
[Computerized detection of congeners of alcoholic beverages
in body fluids.] Blutalkohol, 18: 303-310 (in German).
22. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
23. BRASS, E.P. & BEYERINCK, R.A. (1987) Interactions of
propionate and carnitine metabolism in isolated hepatocytes.
Metabolism, 36: 781-787.
24. BRASS, E.P., FENNESSEY, P.V. & MILLER, L.V. (1986) Inhibition
of oxidative metabolism by propionic acid and its reversal by
carnitine in isolated rat hepatocytes. Biochem. J., 236:
131-136.
25. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
26. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
27. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.]
Z. Wasser-Abwasser Forsch., 10: 87-98 (in German).
28. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
29. BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.]
Z. Wasser-Abwasser Forsch., 13: 170-173 (in German).
30. BURROWS, W.D. & ROWE, R.S. (1975) Ether soluble constituents
of landfill leachate. J. Water Pollut. Control Fed., 47:
921-923.
31. CAMERON, A.M., ZAHLSEN, K., HAUG, E., NILSEN, O.G., & EIK-
NES, K.B. (1985) Circulating steroids in male rats following
inhalation of n-alcohols. Arch. Toxicol., Suppl., 8: 422-424.
32. CAMPBELL, I.M., MCLAUGHLIN, D.G., & HANDY, B.J. (1976) Rate
constants for reactions of hydroxyl radicals with alcohol
vapours at 292 K. Chem. Phys. Lett., 38: 362-364.
33. CANTON, J.H. & ADEMA, D.M.M. (1978) Reproducibility of short-
term and reproduction toxicity experiments with Daphnia magna
and comparison of the sensitivity of Daphnia magna with
Daphnia pulex and Daphnia cucullata in short-term experiments.
Hydrobiologia, 59: 135-140.
34. CARTER, W.P.L., DARNALL, K.R. GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4-hydroxy radicals
with oxygen. J. phys. Chem., 83: 2305-2311.
35. CEC (1982) Propan-1-ol chemico-physical data, toxicity data,
environmental occurrence, and permissible levels. In: Report
of the Scientific Committee for Food on extraction solvents,
Brussels, Commission of the European Communities, Directorate
General for Internal Market and Industrial Affairs, pp. 27-45.
36. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination: interaction
with phytochrome. Physiol. Plant, 65: 451-454.
37. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese hamster
V79 cells by various organic solvents and simple compounds.
Cell Biol. Toxicol., 1: 155-171.
38. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation
and degradation of petrochemical wastewater components by
methane fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
39. CHUNG, T.-Y., HAYASE, F., & KATO, H. (1983) Volatile
components of ripe tomatoes and their juices, purees and
pastes. Agric. biol. Chem., 47: 343-351.
40. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962) Influence
of alcohols and potassium salts of xanthogenic acids on
various biological objects. Arch. int. Pharmacodyn. Ther.,
135: 330-343.
41. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol.,
9: 383-397.
42. CORBIT, T.E. & ENGEN, T. (1971) Facilitation of olfactory
detection. Perception Psychophysiol., 10: 433-436.
43. CORKEY, B.E., HALE, D.E., GLENNON, M.C., KELLEY, R.I.,
COATES, P.M. KILPATRIK, L. & STANLEY, C.A. (1988)
Relationship between unusual hepatic acyl coenzyme A profiles
and the pathogenesis of Reye syndrome. J. clin. Invest.
82: 782-788.
44. CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No. 600/
3-80-084, PB 80-221948).
45. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100:
34-46.
46. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species:
D. melanogaster and D. simulans. Comp. Biochem. Physiol.,
54C: 71-74.
47. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
48. DE ZWART, D. & SLOOFF, W. (1983) The Microtox as an
alternative assay in the acute toxicity assessment of water
pollutants. Aquat. Toxicol., 4: 129-138.
49. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity of
selected synthetic organic chemicals, The MITRE Corporation
(MITRE Technical Report MTR-7248, Rev. 1, Appendix III).
50. DRAVNIEKS, A. (1974) A building-block model for the
characterization of odorant molecules and their odors. Ann.
N.Y. Acad. Sci., 237: 144-163.
51. DRY, I., WALLACE, W., & NICHOLAS, D.J.D. (1981) Role of ATP
in nitrite reduction in roots of wheat and pea. Planta,
152: 234-238.
52. DURWALD, W. & DEGEN, W. (1956) [A fatal poisoning by n-propyl
alcohol.] Arch. Toxikol., 16: 84-88 (in German).
53. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
54. EICEMAN, G.A. & KARASEK, F.W. (1981) Identification of
residual organic compounds in food packages. J. Chromatogr.,
210: 93-103.
55. FANG, H.H.P. & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
56. FAO/WHO (1980) Toxicological evaluation of certain food
additives. Report of the Joint FAO/WHO Expert Committee on
Food Additives, Geneva, World Health Organization,
pp. 162-168 (WHO Food Additive Series 16).
57. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. geotech. J., 22: 205-214.
58. FLATH, R.A., ALTIERI, M.A., & MON, T.R. (1984) Volatile
constituents of Amaranthus retroflexus L. J. agric. food
Chem., 32: 92-94.
59. FLICK, E.W. (1985) Industrial solvents handbook. New Jersey,
Noyes Data Corp., pp. 220-223.
60. FORSANDER, O.A. (1967) Influence of some aliphatic alcohols
on the metabolism of rat liver slices. Biochem. J., 105:
93-97.
61. GAD, S.C., DUNN ,B.J., DOBBS, D.W., REILLY, C., & WALSH, R.D.
(1986) Development and validation of an alternative dermal
sensitization test: the mouse ear swelling test (MEST).
Toxicol. appl. Pharmacol., 84: 93-114.
62. GAGE, P.W. (1965) The effect of methyl, ethyl and n-propyl
alcohol on neuromuscular transmission in the rat.
J. Pharmacol. exp. Ther., 150: 236-243.
63. GAILLARD, D. & DERACHE, R. (1966) Action de quelques alcools
aliphatiques sur la mobilisation de différentes fractions
lipidiques chez le rat. Food Cosmet. Toxicol., 4: 515-520.
64. GELSOMINI, N. (1985) Head-space analysis with capillary
columns in quality control of wines. In: Proceedings of the
6th International Symposium on Capillary Chromatography,
pp. 515-519.
65. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
66. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants
in the oxidation of aliphatic compounds by activated sludge.
J. Water Pollut. Control Fed., 38: 562-579.
67. GIBEL, W., LOHS, K., & WILDNER, G.P. (1975) [Experimental
study on the cancerogenic activity of propanol-1, 2-methyl-
propanol-1 and 3-methylbutanol. I.] Arch. Geschwulstforsch.,
45: 19-24 (in German).
68. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal
of a chemical waste problem by fish toxicity tests. Sewage
ind. Waste, 24: 1397-1401.
69. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut. Control
Fed., 46: 947-965.
70. GLASGOW, A.M. & CHASE, H.P. (1976) Effect of propionic acid
on fatty acid oxidation and ureagenesis. Pediatr. Res.,
10: 683-686.
71. GOODMAN, D.E. & RAO, R.M. (1984) GC determination of fusel
alcohols in distilled alcoholic beverages. Am. Lab., 16:
100-103.
72. GORESKY, C.A., GORDON, E.R., & BACH, G.G. (1983) Uptake of
monohydric alcohols by liver: demonstration of a shared
enzymic space. Am. J. Physiol., 244: G198-G214.
73. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
74. GRANT, K.A. & SAMSON, H.H. (1984) n-Propanol induced
microcephaly in the neonatal rat. Neurobehav. Toxicol.
Teratol., 6: 165-169.
75. GRAY, V.M. & CRESSWELL, C.F. (1983) The effect of respiratory
inhibitors and alcohols on nitrate utilization and nitrite
accumulation in excised roots of Z. mays L. Z. Pflanzenphysiol.,
112: 207-214.
76. GREGERSEN, N. (1979) Studies on the effects of saturated and
unsaturated short-chain monocarboxylic acids on the energy
metabolism of rat liver mitochondria. Pediat. Res., 14:
1227-1230.
77. GREGERSEN, N. (1981) The specific inhibition of the pyruvate
dehydrogenase complex from pig kidney by propionyl-CoA and
isovaleryl-CoA. Biochem. Med. III, 26: 20-27.
78. GULATI, A., NATH, C., SHANKER, K., SRIMAL, R.C., DHAWAN, K.N.,
& BHARGAVA, K.P. (1985) Effects of alcohols on the
permeability of blood-brain barrier. Pharmacol. Res. Commun.,
17: 85-93.
79. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
80. HANSCH, C. & ANDERSON, S. (1967) The effect of intramolecular
hydrophobic bonding on partition coefficients. J. org. Chem.,
32: 2583-2586.
81. HARRIS, R.A. (1983) Ethanol, membrane perturbation, and
synaptosomal ion transport. Proc. West. Pharmacol. Soc.,
26: 255-257.
82. HAWLEY, G.G. (1981) Condensed chemical dictionary, 10th ed.,
Melbourne, Van Nostrand Reinhold Company Inc.
83. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
84. HERMENS, J., BUSSER, F., LEEUWANGH, P., & MUSCH, A. (1985)
Quantitative structure-activity relationships and mixture
toxicity of organic chemicals in Photobacterium phosphoreum:
the Microtox 85 test. Ecotoxicol. environ. Saf., 9: 17-25.
85. HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974)
Effects of chronic ingestion of some lower aliphatic alcohols
in rats. Res. Commun. chem. Pathol. Pharmacol., 9: 177-180.
86. HILSCHER, H., GEISSLER, E., LOHS, K., & GIBEL, W. (1969)
[Studies on the toxicity and mutagenicity of single fusel oil
components on E. coli.] Acta biol. med. Germ., 23: 843-852
(in German).
87. HIRANO, K., WATANABE, Y., ADACHI, T., ITO, Y., & SUGIURA, M.
(1985) Drug-binding properties of human alpha-foetoprotein.
Biochem J., 231: 189-191.
88. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509
(in German).
89. HODGE, H.C. & STERNER, J.H. (1943) Am. ind. Hyg. Assoc. Q.,
10: 93.
90. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
91. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
161 pp.
92. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
93. HUANG, R.-D, SMITH, M.F., & ZAHLER, W.L. (1982) Inhibition of
Forskolin-activated adenylate cyclase by ethanol and other
solvents. J. cyclic Nucleotide Res., 8: 385-394.
94. HUDOLEI, V.V., MIZGIREV, I.V., & PLISS, G.B. (1987)
[Evaluation of mutagenic activity of carcinogens and other
chemical agents with Salmonella typhimurium assays.] Vopr.
Onkol., 32: 73-80 (in Russian).
95. IRPTC (1987) Data profile on n-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
96. IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ,
I.D., & BRADSHAW, J.J. (1978) Organic compounds. Their
interaction with and degradation of hepatic microsomal drug-
metabolizing enzymes in vitro. Drug Metab. Dispos., 6:
218-225.
97. JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavour volatiles in heat-treated milks.
J. dairy Res., 45: 391-403.
98. JOUANY, J.-P. (1982) Volatile fatty acid and alcohol
determination in digestive contents, silage juices, bacterial
cultures and anaerobic fermentor contents. Sci. Aliment.,
2: 131-144.
99. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
100. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J.,
53: 129-136.
101. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents.
Am. Ind. Hyg. Assoc. J., 41: 451-455.
102. KHARE, M. & DONDERO, N.C. (1977) Fractionation and
concentration from water of volatiles and organics on high
vacuum system: examination of sanitary landfill leachate.
Environ. Sci. Technol., 11: 814-819.
103. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted filberts.
J. agric. food Chem., 20: 1021-1028.
104. KIRK, R.E & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
105. KLECKA, G.M., LANDI, L.P., & BODNER, K.M. (1985) Evaluation
of the OECD activated sludge, respiration inhibition test.
Chemosphere, 14: 1239-1251.
106. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
107. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegypti
larvae. J. Invertebr. Pathol., 42: 285-287.
108. KRISTIANSEN, U., HANSEN, L. NIELSEN, G.A., & HOLST, E. (1986)
Sensory irritation and pulmonary irritation of cumene and
n-propanol: Mechanisms of receptor activation and
desensitization. Acta pharmacol. toxicol., 59: 60-72.
109. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
110. KUHNHOLZ, B. (1985) [Reflections concerning the analysis of
free aliphatic alcohols in tissue.] Blutalkohol, 22: 455-461
(in German).
111. LAING, D.G. (1975) A comparative study of the olfactory
sensitivity of humans and rats. Chem. Senses Flavor, 1:
257-269.
112. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
113. LASNE, C., GU, Z.W., VENEGAS, W., & CHOUROULINKOV, I. (1984)
The in vitro micronucleus assay for detection of cytogenetic
effects induced by mutagen-carcinogens: comparison with the
in vitro sister-chromatid exchange assay. Mutat. Res.,
130: 273-282.
114. LIEBICH, H.M., BUELOW, H.J., & KALLMAYER, R. (1982)
Quantification of endogenous aliphatic alcohols in serum and
urine. J. Chromatogr., 239: 343-349.
115. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermatit., 3: 240-244.
116. LUTHIN, G.R. & TABAKOFF, B. (1984) Activation of adenylate
cyclase by alcohols requires the nucleotide-binding protein.
J. Pharmacol. exp. Ther., 228: 579-587.
117. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
118. MAICKEL, R.P. & NASH, J.F. (1985) Differing effects of
short-chain alcohols on body temperature and coordinated
muscular activity in mice. Neuropharmacology, 24: 83-89.
119. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology,
17: 451-461.
120. MANKES, R.F., LEFEVRE, R., RENAK,V., FIESHER, J., & ABRAHAM,
R. (1985) Reproductive effects of some solvent alcohols with
differing partition coefficients. Teratology, 31: 67A.
121. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
122. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft,
26: 385-389 (in German).
123. MAY, W.A., PETERSON, R.J., & CHANG, S.S. (1983) Chemical
reactions involved in the deep-fat frying of foods. IX.
Identification of the volatile decomposition products.
J. Am. Oil Chem. Soc., 60: 990-995.
124. MICHAELIS, M.L. & MICHAELIS, E.K. (1983) Alcohol and local
anesthetic effects on Na+-dependent Ca2+-fluxes in brain
synaptic membrane vesicles. Biochem. Pharmacol., 32:
963-969.
125. MIKHEEV, M.J., & FROLOVA, A.D. (1978) [Toxicokinetics of
certain representatives of a homologous series of alcohols.]
Gig. i Sanit., 6:33-36 (in Russian).
126. MORGAN, E.T., KOOP, D.R., & COON, M.J. (1982) Catalytic
activity of cytochrome P-450 isozyme 3a isolated from liver
microsomes of ethanol-treated rabbits. J. biol. Chem.,
257: 13951-13957.
127. MOSHONAS, M.G. & SHAW, P.E. (1987) Quantitative analysis of
orange juice flavor volatiles by direct-injection gas
chromatography. J. Agric. food Chem., 35:161-165.
129. MULLIN, M.J. & HUNT, W.A. (1985) Actions of ethanol on
voltage-sensitive sodium channels: effects on neurotoxin-
stimulated sodium uptake in synaptosomes. J. Pharmacol. exp.
Ther., 232: 413-419.
130. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
131. MURPHREE, H.B., GREENBERG, L.A., & CARROLL, R.B. (1967)
Neuropharmacological effects of substances other than ethanol
in alcoholic beverages. Fed. Proc., 26: 1468-1473.
132. NELSON, B.K., BRIGHTWELL, W.S., & BURG, J.R. (1985)
Comparison of behavioural teratogenic effects of ethanol and
n-propanol administered by inhalation to rats. Neurobehav.
Toxicol Teratol., 7: 770-783.
132. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W. & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentration to rats. Food Chem. Toxicol.,
26(3): 247-254.
133. NEY, K.H. (1985) [Flavour of Tilsit cheese.] Fette Seifen
Anstrichmittel, 87: 289-293 (in German).
134. NGUYEN, V.C. & KATO, H. (1982) Volatile flavor components of
Kumazasa (Sasa albo-marginata). Agric. Biol. Chem., 46:
2795-2801.
135. NUNOMURA, N., SASAKI, M., & YOKOTSUKA, T. (1984) Shoyu
(soy sauce) flavor components: neutral fraction. Agric. Biol.
Chem., 48: 1753-1762.
136. OBE, G. & RISTOW, H. (1977) Acetaldehyde, but not ethanol,
induces sister chromatid exchanges in Chinese hamster cells
in vitro. Mutat. Res., 56: 211-213.
137. ODKVIST, L.M., LARSBY, B., THAM, R., & ASCHAN, G. (1979) On
the mechanism of vestibular disturbances caused by industrial
solvents. Adv. Oto-Rhino-Laryng., 25: 167-172.
138. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub. Reinhalt. Luft, 32: 400-407 (in German).
139. OERSKOV, S.I. (1950) Experiments on the oxydation of propyl
alcohol in rabbits. Acta physiol. Scand., 20: 258-262.
140. OTSUK141.A, K., IKI, I., & YAMASHITA, T. (1979) [Relationship
between type of whisky and volatile component.] Hakkokogaku,
57: 20-30 (in Japanese).
141. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. 4. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 K. J. phys. Chem., 82: 1329-1333.
142. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
143. PATERNOSTRE, M., PICHON, Y., & DUPEYRAT, M. (1983) Effects
of n-alcohols on ionic transmembrane currents in the squid
giant axon. Stud. phys. thero. Chem., 24: 515-522.
144. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
145. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols
of the stimulated activity of ornithine decarboxylase and
tyrosine aminotransferase occurring in regenerating rat liver.
Biochem. Pharmacol., 29: 2799-2803.
146. POSTEL, W. & ADAM, L. (1978) [Gas chromatographic
characterization of whisky. III. Communication: Irish
whiskey.] Branntweinwirtsch., 118: 404-407 (in German).
147. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
148. PREUSS, A. & ZIPFEL, K. (1985) [Headspace-gas chromatographic
identification of alcoholic beverages and foodstuffs.]
Lebensmittelchem. gerichtl. Chem., 39: 97-99 (in German).
149. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
150. PRIESTLEY, D.A. & LEOPOLD, A.C. (1980) Alcohol stress on
soya bean seeds. Ann. Bot., 45: 39-45.
151. PUNTER, P.H. (1983) Measurement of human olfactory thresholds
for several groups of structurally related compounds. Chem.
Senses, 7: 215-235.
152. PURCHASE, I.F.H. (1969) Studies in kaffircorn malting and
brewing. XXII. The acute toxicity of some fusel oils found in
Bantu beer. S.A. med. J., 43: 795-798.
153. QUISTAD, G.B., STAIGER, L.E. & SCHOOLEY, D.A. (1986) The
role of carnitine in the conjugation of acidic xenobiotics.
Drug Metab. Dispos., 14:521-525.
154. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
155. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. membr. Biol., 84: 229-238.
156. REYNOLDS, T. (1977) Comparative effects of aliphatic
compounds on inhibition of lettuce fruit germination. Ann.
Bot., 41: 637-648.
157. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch., 21:
1309-1319 (in German).
158. ROE, C.R., MILLINGTON, D.S., MALTBY, D.A., BOHAN, T.P. &
HOPPEL, C.L. (1984) L-Carnitine enhances excretion of
propionyl coenzyme A as propionylcarnitine in propionic
acidemia. J. clin. Invest., 73: 1785-1788.
159. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
160. RYAN, D.E., THOMAS, P.E., WRIGHTON, S.A., GUZELIAN, P.S. &
LEVIN, W. (1987) Ethanol-inducible rat and human liver
cytochrome P-450: characterization and role as high affinity
N-nitrosodimethylamine demethylamine demethylases. In:
Miners, J., Birkett, D.J., Drew, R., May, B., & McManus, ed.
Microsomes and drug oxidations, London, Taylor & Francis,
pp. 3-11.
161. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
162. SAHU, B.R. & TANDON, U. (1983) A novel method for spot test
detection of alcohols. J. Indian Chem. Soc., 60: 615-616.
163. SANCEDA, N., KURATA, T., & ARAKAWA, N. (1984) Fractionation
and identification of volatile compounds in Patis, a
Phillipine fish sauce. Agric. Biol. Chem., 48: 3047-3052.
164. SAVINI, E.C. (1968) Estimation of the LD50 in mol/kg. Proc.
Eur. Soc. Study Drug Toxicol., 9: 276-278.
165. SCHEIMAN, M.A., SAUNDERS, R.A., & SAALFELD, F.E. (1974)
Organic contaminants in the District of Columbia water supply.
Biomed. Mass Spectrom., 1: 209-211.
166. SCOTT, T. & EAGLESON, M. (1983) Concise encyclopedia of
biochemistry. New York, W. de Gruyter, pp. 158-159.
167. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
168. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona Heilborn).
J. agric. food Chem., 33: 795-797.
169. SINCLAIR, J.F., SMITH, L., BEMENT, W.J., SINCLAIR, P.R., &
BONKOWSKY, H.L. (1982) Increases in cytochrome P-450 in
cultured hepatocytes mediated by 3- and 4-carbon alcohols.
Biochem. Pharmacol., 31: 2811-2815.
170. SLOOFF, W. (1983) Benthic macroinvertebrates and water
quality assessment: some toxicological considerations. Aquat.
Toxicol., 4 : 73-82.
171. SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the
usefulness of the Mexican Axolotl (Ambystoma mexicanum) and
the clawed toad (Xenopus laevis) in toxicological bioassays.
Bull. environ. Contam. Toxicol., 24: 439-443.
172. SLOOFF, W., CANTON, J.H., & HERMENS, J.L.M. (1983) Comparison
of the susceptibility of 22 freshwater species to 15 chemical
compounds. I. (Sub)acute toxicity tests. Aquat. Toxicol.,
4: 113-128.
173. SMYTH, H.F., CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data. Arch. ind. Hyg. occup.
Med., 10: 61-68.
174. SNIDER, J.B. & DAWSON, G.A. (1985) Tropospheric light
alcohols, carbonyls, and acetonitrile: concentrations in the
southwestern United States and Henry's law data. J. geophys.
Res., 90: 3797-3805.
175. SOLIMAN, M.A., EL SAWY, A.A., FADEL, H.M., OSMAN, F., & GAD,
A.M. (1985) Volatile components of roasted Citrillus
colocynthis, var. Colocynthoides. Agric. Biol. Chem.,
49: 269-275.
176. SRI (1984) n-Propanol: record number 0115 (last revision
date 01-06-84). In: Toxicology data bank, Palo Alto,
California, Stanford Research Institute, Bethesda, Maryland,
National Library of Medicine.
177. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
178. STOCK, K. & SCHMIDT, M. (1978) Effects of short-chain
alcohols on adenylate cyclase in plasma membranes of rat
adipocytes. Naunyn-Schmiedeberg Arch. Pharmacol., 302: 37-43.
179. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials: second cumulative
series. Perfumer Flavorist, 9: 53-83.
180. STOKES, J.A. & HARRIS, R.A. (1982) Alcohols and synaptosomal
calcium transport. Mol. Pharmacol., 22: 99-104.
181. STOLZENBERG, S.J. & HINE, C.H. (1979) Mutagenicity of
halogenated and oxygenated three-carbon compounds. J. Toxicol.
environ. Health, 5: 1149-1158.
182. STONE, H., PRYOR, G.T., & STEINMETZ, G. (1972) A comparison
of olfactory adaptation among seven odorants and their
relationship with several physico-chemical properties.
Perception Psychophys., 12: 501-504.
183. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
184. STUMPF, D.A., MCAFEE, J., PARKS, J.K., & EGUREN, L. (1986)
Propionate inhibition of succinate: CoA ligase (GDP) and the
citric acid cycle in mitochondria. Pediatr. Res., 14:
1127-1131.
185. TABAKOFF, B. & HOFFMAN, P.L. (1983) Alcohol interactions with
brain opiate receptors. Life Sci., 32: 197-204.
186. TANDOI, P., GUIDOTTI, M., & STACCHINI, P. (1984) [On the
composition of brandies.] Riv. Soc. Ital. Sci. Aliment.,
13: 69-76 (in Italian).
187. TAYLOR, J.M., JENNER, P.M., & JONES, W.I. (1964) A comparison
of the toxicity of some allyl, propenyl, and propyl compounds
in the rat. Toxicol. appl. Pharmacol., 6: 378-387.
188. TESCHKE, R., HASUMURA, Y., & LIEBER, C.S. (1975) Hepatic
microsomal alcohol-oxidizing system. Affinity for methanol,
ethanol, propanol, and butanol. J. biol. Chem., 250:
7397-7403.
189. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
190. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
191. TOMERA, J.F., SKIPPER, P.L., WISHNOK, J.S., TANNEBAUM, S.R.,
& BRUNENGRABER, H. (1984) Inhibition of N-nitroso-
dimethylamine metabolism by ethanol and other inhibitors in
the isolated perfused rat liver. Carcinogenesis, 5: 113-116.
192. UHLEMANN, E.R., ROBBERECHT, P., & GARDNER, J.D. (1979)
Effects of alcohols on the actions of VIP and secretin on
acinar cells from guinea-pig pancreas. Gastroenterology,
76: 917-925.
193. UNRUH, J.D. & SPINICELLI, L. (1981) Propyl alcohols, n-propyl
alcohol. In: Kirk, R.E. & Othmer, D.F., ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience,
Vol. 19, pp. 221-227, 194.
194. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
195. US NIOSH (1984) Method 1401. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1,
pp. 1401-1-1401-4.
196. VAISHNAV, D.D. & LOPAS, D.M. (1985) Relationship between
lipophilicity and biodegradation inhibition of selected
industrial chemicals. Dev. ind. Microbiol., 26: 557-565.
197. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
198. VERSCHUEREN, K. (1983) Handbook of environmental data on
organic chemicals, 2nd ed., Melbourne, Van Nostrand Reinhold
Company Inc.
199. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
200. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER, B.,
& BASLER, A. (1987) Genotoxicity of three carbon compounds
evaluated in the SCE test in vitro. Environ. Mutagen.,
9: 401-410.
201. WACHTEL, R.E. (1984) Aliphatic alcohols increase the decay
rate of glutamate-activated currents at the crayfish
neuromuscular junction. Br. J. Pharmacol., 83: 393-397.
202. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265.
203. WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
IIJIMA, M. (1984) Induction of megamitochondria in the rat
liver by n-propyl alcohol and n-butyl alcohol. Acta pathol.
Jpn., 34: 471-480.
204. WALLGREN, H. (1960) Relative intoxicating effects on rats
of ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
205. WATERER, D.R. & PRITCHARD, M.K. (1985a) Volatile production
by wounded Russet Burbank and Norland potatoes. Sci. Aliments,
5: 205-216.
206. WATERER, D.R. & PRITCHARD, M.K. (1985b) Production of
volatile metabolites in potatoes infected by Erwinia
carotovora var. carotovora and E. carotovora var. atroseptica.
Can. J. plant Pathol., 7: 47-51.
207. WILKIN, J.K. & FORTNER, G. (1985) Cutaneous vascular
sensitivity to lower aliphatic alcohols and aldehydes in
orientals. Alcoholism clin. exp. Res., 9: 522-525.
208. WINTERSTEIGER, R., GAMSE, G., & PACHA, W. (1982)
[Quantification of alcoholic compounds and amines with 4-(6-
methylbenzo-thiazol-2-yl)phenyl isocyanate. Fresenius Z.
anal. Chem., 312: 455-461.
209. WOLF, M., URBAN, R., WELLER, J.-P., & TROGER, H.D. (1985)
[The analysis of congeners of alcoholic beverages in blood.
First communication: the application of capillary gas
chromatography in micro head-space analysis.] Blutalkohol,
22: 321-332 (in German).
210. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
211. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., UCHIDA,
H., & HAYASHI, K. (1983) Volatile flavor compounds of boiled
buckwheat flour. Agric. Biol. Chem., 47: 729-738.
212. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., &
HAYASHI, K. (1984) Volatile flavor components of Kogyoku
apples. Agric. Biol. Chem., 48: 849-855.
213. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
214. YASUHARA, A. & FUWA, K. (1982) Characterization of odorous
compounds in rotten blue-green algae. Agric. Biol. Chem.,
46: 1761-1766.
215. YASUHARA, A., FUWA, K., & JIMBU, M. (1984) Identification of
odorous compounds in fresh and rotten swine manure. Agric.
Biol. Chem., 48: 3001-3010.
216. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Manag. Res., 1: 213-226.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-1 est un liquide incolore, très inflammable, qui
est volatil à la température ambiante et sous la pression
atmosphérique normale. Il est miscible à l'eau et aux solvants
organiques. Parmi les méthodes d'analyse, on peut citer la
chromatographie en phase gazeuse, qui permet de déceler jusqu'à
5 x 10-5 mg/m3 dans l'air, 1 x 10-4 mg/litre dans l'eau et
0,002 mg/litre dans le sang, le sérum ou les urines lorsque
l'échantillon a été extrait ou concentré de façon satisfaisante.
2. Sources d'exposition humaine et environnementale
La capacité de production mondiale a dépassé 130 000 tonnes en
1979. Le propanol-1 d'origine naturelle résulte de la
décomposition de matériaux organiques par divers microorganismes et
on le rencontre également dans les végétaux et le mazout.
Industriellement, on le produit par réaction de l'éthylène sur
l'oxyde de carbone et l'hydrogène qui donne du propionaldéhyde,
celui-ci étant ensuite hydrogéné en propanol. C'est également un
sous-produit de la fabrication du méthanol et il peut être obtenu
directement à partir du propane ou de l'acroléine. Le propanol-1
est essentiellement un solvant à tout faire, à usage industriel ou
domestique. Il entre dans la composition des encres d'imprimerie
flexographiques et il est également utilisé dans l'industrie
textile, dans des produits à usage personnel tels que les produits
cosmétiques, les lotions ainsi que dans les produits pour le
nettoyage des vitres, les encaustiques et les antiseptiques. En
second lieu par ordre d'importance, on peut citer son utilisation
comme produit intermédiaire dans la fabrication de divers composés
chimiques.
3. Transport, distribution et transformation dans l'environnement
La principale voie de pénétration de propanol-1 dans
l'environnement est constituée par les émissions dans l'atmosphère
qui se produisent au cours de la production, de la transformation,
du stockage, du transport et de l'utilisation de ce composé ou du
rejet de déchets qui en contiennent. Il peut y avoir également des
décharges dans l'eau et le sol. Le propanol-1 étant principalement
utilisé comme solvant volatil, il finit par se dissiper en grande
partie dans l'environnement.
Le propanol-1 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et par les précipitations. Il
est facilement biodégradable tant par voie aérobie que par voie
anaérobie et du fait de l'existence de ces mécanismes d'élimination
chimique et biologique, on ne le rencontre normalement pas en
quantité mesurable dans l'environnement. Toutefois on en a décelé
la présence dans l'air des agglomérations urbaines, dans des
décharges, ainsi que dans les eaux s'échappant de zones
d'enfouissement de déchets.
Le logarithme du coefficient de partage n-octanol/eau du
propanol-1 est de 0,34 et son facteur de bioconcentration a une
valeur de 0,7, ce qui en rend la bioaccumulation très improbable.
4. Niveau dans l'environnement et exposition humaine
La population en général peut être exposée au propanol par
suite d'une ingestion accidentelle, par inhalation lors de
l'utilisation du produit ou par absorption avec la nourriture
(propanol d'origine naturelle, additif d'aromatisation ou reste de
solvant) ou des boissons alcoolisées ou non. Par exemple la bière
en contient jusqu'à 195 mg/litre, le vin jusqu'à 116 mg/litre et
certains spiritueux jusqu'à 3500 mg/litre. L'exposition de la
population par suite d'inhalation ou de la consommation d'eau de
boisson est faible (aux Etats-Unis la concentration moyenne dans
des échantillons d'air urbain se situait à 0,00005 mg/m3 et dans
des échantillons d'eau de boisson, à 0,001 mg/litre). Les
travailleurs courent un risque d'exposition par inhalation lors de
la production, de la transformation et de l'utilisation du produit.
Toutefois, on ne dispose d'aucune donnée qui permettrait de
chiffrer ce type d'exposition.
5. Cinétique et métabolisme
Après ingestion, le propanol-1 est rapidement absorbé et
distribué dans l'ensemble de l'organisme. On manque de données sur
la vitesse d'absorption après inhalation et exposition cutanée. Le
propanol-1 est métabolisé par l'alcool déshydrogénase (ADH) en
aldéhyde puis acide propionique et peut entrer dans le cycle de
Krebs. Cette oxydation constitue l'étape limitante du métabolisme
du propanol-1. In vitro, les oxydases microsomiques du rat et du
lapin sont également capables d'oxyder le propanol-1 en aldéhyde
propionique. L'ADH et des systèmes d'oxydation microsomiques ont
une affinité beaucoup plus importante pour le propanol-1 que pour
l'éthanol; aussi le propanol-1 est-il rapidement éliminé de
l'organisme. Chez le rat, sa demi-vie après administration par
voie orale d'une dose de 1000 mg/kg est de 45 minutes.
Chez l'animal et chez l'homme, le propanol-1 peut être éliminé
de l'organisme dans l'air expiré ou dans les urines. Chez des
êtres humains ayant reçu par voie orale une dose de 3,75 mg de
propanol-1 par kg de poids corporel et de 1200 mg d'éthanol par kg
de poids corporel, l'excrétion urinaire totale du propanol-1 a été
de 2,1 % de la dose. Les concentrations urinaires de propanol-1
étaient d'autant plus basses que la quantité d'éthanol ingérée
simultanément était basse, ce qui montre qu'il y avait compétition
pour l'ADH entre le propanol-1 et la surdose d'éthanol.
6. Effets sur les êtres vivant dans leur milieu naturel
Aux concentrations normalement présentes dans l'environnement,
le propanol-1 n'est pas toxique pour la vie aquatique, les insectes
ou les végétaux. Chez trois des espèces aquatiques les plus
sensibles (trois protozoaires), le seuil l'inhibition de la
multiplication cellulaire se situait entre 38 et 568 mg/litre.
Dans le cas d'organismes plus évolués, la concentration létale
était d'environ 5000 mg/litre, avec des variations remarquablement
faibles d'un phylum à l'autre et une courbe dose-réponse de très
forte pente. Certaines bactéries et micro-organismes qui vivent
dans les eaux résiduaires et les boues activées sont capables de
s'adapter à des concentrations supérieures à 17 000 mg/litre.
Le propanol-1 peut inhiber ou au contraire stimuler la
germination des semences selon sa concentration dans l'eau
d'arrosage et les conditions d'exposition. Le composé accroît
l'accumulation de nitrites dans le maïs, les pois et le froment.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in vitro
Le propanol-1 présente une faible toxicité aiguë pour les
mammifères (mesurée d'après la mortalité), que l'exposition se
fasse par voie percutanée, orale ou respiratoire. On a fait état
de valeurs allant de 1870 à 6800 mg par kg de poids corporel pour
la DL50 par voie orale chez plusieurs espèces animales. Toutefois
pour de très jeunes rats, on donne une DL50 orale de 560 à 660 mg
par kg de poids corporel. Après une seule exposition, le principal
effet toxique du propanol-1 consiste dans la dépression du système
nerveux central. Selon les données disponibles, le propanol-1
exercerait sur le système nerveux central des effets analogues à
ceux de l'éthanol; toutefois il semblerait que la neurotoxicité du
propanol-1 soit plus importante. Les DE50 pour l'anesthésie chez
le lapin et la perte du réflexe de redressement chez la souris se
situaient respectivement à 1440 mg par kg de poids corporel par
voie orale et à 1478 mg par kg de poids corporel par voie intra-
péritonéale; ces doses sont environ quatre fois plus faibles que
dans le cas de l'éthanol. Dans l'épreuve du plan incliné, le
propanol-1 s'est révélé 2,5 fois plus actif que l'éthanol chez le
rat.
Des doses uniques de 3000 ou 6000 mg par kg de poids corporel
administrées par voie orale à des rats ont provoqué une
accumulation réversible de triglycérides dans le foie. Les vapeurs
fortement concentrées provoquent une irritation des voies
respiratoires chez la souris. Aux concentrations d'environ 30 000
mg/m3 on note une réduction de 50 % du rythme respiratoire chez la
souris.
On ne dispose de données ni sur l'irritation oculaire ni sur
l'irritation cutanée. Aucun effet n'a été observé lors d'une
épreuve de sensibilisation cutanée sur des souris CF1.
Chez des rats mâles exposés pendant six semaines à une dose de
15 220 mg/m3 de propanol-1, on a relevé quelques signes d'une
action nocive possible sur la fonction de reproduction. En
revanche aucun effet n'a été noté à la dose de 8610 mg/m3. Après
exposition de rattes gravides au propanol-1, on a observé des
signes patents de toxicité pour les mères et les foetus aux doses
de 23 968 et 14 893 mg/m3 (9743 et 6054 ppm respectivement); aucun
signe de toxicité n'a été noté à 9001 mg/m3 (3659 ppm). On n'a
observé aucune anomalie comportementale parmi les descendants de
rats mâles exposés pendant six semaines à 8610 ou 15 220 mg de
propanol-1 par m3, ni dans la descendance de rattes exposées
pendant leur gestation aux mêmes concentrations. Toutefois, en
administrant à des ratons de 5 à 8 jours des doses orales de 3000 à
7800 mg de propanol-1 par kg et par jour, on constatait des signes
de dépression du SNC pendant l'administration et un syndrome de
sevrage à la cessation du traitement. Le cerveau de ces rats a été
examiné à l'âge de 18 jours; on a constaté une réduction du poids
de cet organe tant en valeur absolue qu'en valeur relative, avec
diminution de la teneur en ADN et réduction localisée des taux de
cholestérol et de protéine.
La recherche de mutations ponctuelles au moyen de 2 épreuves
utilisant Salmonella typhimurium n'a donné que des résultats
négatifs, de même la recherche de mutations réverses sur
Escherichia coli CA-274. Les résultats ont été également négatifs
en ce qui concerne les échanges entre chromatides soeurs ou la
présence de micro-noyaux dans les cellules mammaliennes in vitro.
Il n'existe pas d' autres données relatives à la mutagénicité.
Lors d'une étude de cancérogénicité portant sur de petits
groupes de rats Wistar exposés tout au long de leur existence par
voie orale à des doses de 240 mg/kg ou par voie sous cutanée à des
doses de 48 mg/kg, on a constaté un accroissement sensible de
l'incidence des sarcomes du foie dans le groupe recevant le produit
par voie sous cutanée. Toutefois cette étude ne permet pas
d'apprécier la cancérogénicité du propanol-1, notamment à cause de
l'absence de détails expérimentaux, du nombre trop restreint
d'animaux et de l'utilisation d'une dose hépatotoxique unique très
élevée.
8. Effets sur la santé humaine
On n'a pas signalé d'effets nocifs sur la santé humaine dans la
population en général ou parmi des groupes professionnels. Le seul
cas d'intoxication mortelle qui ait été signalé est celui d'une
femme, retrouvée inconsciente et qui est décédé 4 à 5 heures après
l'ingestion de propanol. L'autopsie a révélé un oedème cérébral et
pulmonaire. Lors d'une étude sur l'irritation et la sensibilisation
cutanées, on a signalé des réactions allergiques chez un membre du
personnel du laboratoire. Chez neuf volontaires sur 12, on a
observé un érythème qui a duré au moins 1 h après 5 minutes
d'application sur les avant-bras de papiers filtres imprégnés de
0,025 ml d'une solution aqueuse à 75 % de propanol-1. On ne
dispose d'aucun autre rapport concernant d'éventuels effets
toxiques après exposition professionnelle au propanol-1.
Il n'existe pas d'études épidémiologiques qui permettent
d'établir les effets à long terme, et notamment la cancérogénicité,
du propanol-1 chez l'homme.
9. Résumé de l'évaluation
Il peut y avoir exposition humaine au propanol-1 à la suite de
l'absorption de nourriture ou de boissons qui en contiennent. Une
exposition par inhalation peut se produire lors de l'utilisation de
ce produit à des fins ménagères, ou professionnelles, au cours de
la fabrication, de la transformation et de l'utilisation de ce
produit. Les données très limitées dont on dispose sur la teneur
de l'air ambiant et de l'eau en propanol-1 indiquent que ces
teneurs sont très faibles.
Le propanol-1 est rapidement absorbé et se répartit dans tout
l'organisme après ingestion. Après inhalation, l'absorption est
également rapide mais la résorption percutanée devrait être lente.
Chez l'animal, la toxicité aiguë du propanol-1 est faible, que
l'exposition ait lieu par voie percutanée, par voir orale ou par
voie respiratoire. L'exposition de personnes appartenant à la
population générale à des teneurs potentiellement mortelles peut se
produire à la suite d'une ingestion accidentelle ou volontaire.
Toutefois, un seul cas d'intoxication mortelle par le propanol-1 a
été signalé jusqu'ici. Les effets aigus les plus probables chez
l'homme sont une intoxication de type alcoolique pouvant entraîner
une narcose. L'expérimentation animale montre que le propanol-1
est 2 à 4 fois plus toxique que l'éthanol.
Le propanol-1 peut être irritant pour la peau mouillée.
On ne dispose pas de données suffisantes sur la toxicité chez
l'animal pour procéder à une évaluation des risques pour la santé
humaine qui découleraient d'une exposition répétée ou prolongée au
propanol-1. Toutefois, un certain nombre d'études à court terme
sur le rat, bien que limitées, indiquent que dans les conditions où
se produit habituellement l'exposition humaine, il est peu probable
que le risque encouru soit très grave.
Une exposition par inhalation à une concentration de 15 220
mg/m3 a affecté la fonction de reproduction de rats mâles, ce qui
n'a pas été le cas à la dose de 8610 mg/m3. Chez des rattes
gravides, la dose sans effet observable se situait à 9001 mg/3
(3659 ppm); quant à la dose la plus faible donnant lieu à un effet
observable, elle était de 14 893 mg/m3 (6054 ppm), en ce qui
concerne la toxicité pour la mère et le foetus. L'exposition par
inhalation à de fortes concentrations de propanol-1 a donc des
effets nocifs sur la reproduction et le développement des rats
mâles et femelles lorsque les concentrations utilisées sont
manifestement toxiques pour ces animaux. Pour obtenir ces effets,
il a fallu utiliser des concentrations plus élevées que celles
auxquelles l'homme pourrait être normalement exposé.
Différentes épreuves ont montré que le propanol-1 ne provoquait
pas de mutations ponctuelles chez les bactéries. Toutefois, si ces
résultats donnent à penser que le produit n'est pas génotoxique,
les données disponibles sont trop limitées pour qu'on puisse en
évaluer correctement le pouvoir mutagène. La seule étude dont on
possède les résultats ne permet pas d'évaluer convenablement la
cancérogénicité du propanol-1 chez l'animal d'expérience. On ne
dispose d'aucune donnée sur l'exposition à long terme des
populations humaines à ce produit, de sorte qu'on ne peut pas se
prononcer sur son pouvoir cancérogène chez l'homme.
A part un cas d'intoxication mortelle consécutif à l'ingestion
d'un demi-litre de propanol-1, il n'existe pratiquement aucun
rapport concernant d'éventuels effets nocifs découlant d'une
exposition au propanol-1, qu'il s'agisse de la population générale
ou de groupes professionnels. Le Groupe spécial estime qu'il est
improbable que le propanol-1 presente des risques graves pour la
population générale dans les conditions normales d'exposition.
Du propanol-1 peut être libéré dans l'environnement lors de la
production, de la transformation, du stockage, du transport, de
l'utilisation ou de rejet de ce produit. Comme il est
essentiellement utilisé comme solvant volatil, l'essentiel de la
production finit par aboutir dans l'atmosphère. Toutefois, par
réaction avec les radicaux hydroxyles et entraînement par les
précipitations, le propanol-1 est rapidement éliminé de
l'atmosphère, d'où il disparaît en moins de trois jours. Le
propanol-1 s'élimine également rapidement de l'eau et du sol de
sorte que l'on ne le rencontre que rarement en concentrations
mesurables dans l'air, l'eau et la terre. Le propanol-1 est peu
absorbé par les particules du sol où il se révèle mobile et dont il
accroît la perméabilité à certains hydrocarbures aromatiques.
Compte tenu des propriétés physiques du propanol-1, il est peu
probable qu'il donne lieu à une bioaccumulation et sauf accident ou
rejet négligent, le propanol-1 ne présente aucun risque pour la
faune ou la flore aquatique aux concentrations où on le rencontre
habituellement dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 1-propanol es un líquido incoloro y sumamente inflamable,
volátil a temperatura ambiente y presión atmosférica normal. Es
miscible con el agua y los disolventes orgánicos. Entre los
métodos analíticos para el propanol figuran la cromatografía de
gases, que puede detectar hasta 5 x 10-5 mg/m3 en el aire,
1 x 10-4 mg/litro en el agua y 0,002 mg/litro en la sangre, el
suero o la orina cuando se utilizan con la muestra procedimientos
adecuados de extracción o concentración.
2. Fuentes de exposición humana y ambiental
En 1979, la capacidad de producción mundial al año superó las
130 000 toneladas. En la naturaleza se produce por descomposición
de material orgánico por diversos micro-organismos, y se halla en
las plantas y en los aceites combustibles. El 1-propanol se
produce a partir del eteno por reacción con el monóxido de carbono
y el hidrógeno para dar propionaldehído, que a continuación se
hidrogena. Aparece también como sub-producto en la fabricación del
metanol y puede producirse a partir del propano directamente o a
partir de la acroleína. El uso principal del 1-propanol es como
disolvente de uso múltiple en la industria y el hogar. Se utiliza
en las tintas de impresión flexográfica y en aplicaciones textiles,
productos de uso personal como cosméticos y lociones, en productos
para limpiar cristales, en abrillantadores y en fórmulas
antisépticas. Le sigue en importancia su uso como producto
intermedio en la fabricación de diversos compuestos químicos.
3. Transporte, distribución y transformación en el medio ambiente
La principal vía de entrada del 1-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación de
desechos. También se producen emisiones al agua y al suelo.
Puesto que el uso principal del 1-propanol es como disolvente
volátil, gran parte del volumen de producción acaba en el medio
ambiente.
El 1-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y por el lavado con la lluvia. Es
fácilmente biodegradable, tanto en condiciones aerobias como
anaerobias y, a causa de estos mecanismos de eliminación química y
biológica, no suelen encontrarse niveles medibles de la sustancia
en el medio ambiente. No obstante, se ha detectado en la atmósfera
urbana, en vertederos de desechos y también en las aguas que se
rezumaban de un terraplenado. La permeabilidad del suelo al
1-propanol es probablemente elevada y el compuesto aumenta la
permeabilidad a ciertos disolventes aromáticos.
El 1-propanol tiene un coeficiente de reparto log
n-octanol/agua de 0,34 y un factor de bioconcentración de 0,7, lo
que hace muy poco probable su bioacumulación.
4. Niveles ambientales y exposición humana
La exposición de la población general puede producirse por
ingestión accidental, por inhalación durante el uso y por ingestión
junto con los alimentos (que contengan 1-propanol como aromatizante
volátil natural o añadido o como residuo de disolvente) y bebidas
alcohólicas y no alcohólicas. Por ejemplo, la cerveza contiene
hasta 195 mg/litro, el vino hasta 116 mg/litro y los diversos tipos
de licores hasta 3520 mg/litro. La exposición de la población
general al 1-propanol por inhalación y en el agua de bebida es baja
(en los Estados Unidos, la concentración media en muestras de aire
urbano fue de 0,00005 mg/m3 y la correspondiente al agua de bebida
0,001 mg/litro). Aunque los trabajadores están potencialmente
expuestos por la inhalación durante la fabricación, el tratamiento
y la utilización, no se dispone de datos para cuantificar esas
exposiciones.
5. Cinética y metabolismo
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se carece de datos sobre la tasa de
absorción tras la inhalación y la exposición dérmica. El
1-propanol es metabolizado por la deshidrogenasa alcohólica para
dar ácido propiónico por intermedio del aldehído y puede entrar en
el ciclo del ácido tricarboxílico. Esta oxidación es una etapa
limitativa de la velocidad del metabolismo del 1-propanol. In
vitro, las oxidasas microsómicas de rata y conejo también son
capaces de oxidar el 1-propanol a aldehído propiónico. La afinidad
relativa de la deshidrogenasa alcohólica y los sistemas de
oxidación microsómicos por el 1-propanol es mucho más elevada que
en el caso del etanol; así pues, el 1-propanol se elimina
rápidamente del organismo. En la rata, el periodo de
semieliminación de una dosis oral de 1000 mg/kg fue de 45 minutos.
Tanto en animales como en el hombre, el 1-propanol puede ser
eliminado del organismo en el aire exhalado o en la orina. A
sujetos a los que se administró una dosis de 1-propanol de 3,75 mg
por kg de peso corporal y 1200 mg de etanol por kg de peso corporal
por vía oral, la excreción urinaria total de 1-propanol fue del
2,1% de la dosis. Los niveles urinarios de 1-propanol fueron más
bajos cuanto más baja era la cantidad de etanol ingerida
simultáneamente, lo que demuestra la competencia por la
deshidrogenasa alcohólica entre el 1-propanol y la sobredosis de
etanol.
6. Efectos en los organismos en el medio ambiente
A las concentraciones en que normalmente se encuentra en el
medio ambiente, el 1-propanol no resulta tóxico para los organismos
acuáticos, los insectos ni las plantas. El umbral de inhibición
para la multiplicación celular de tres de las especies acuáticas
más sensibles (tres protozoos) fue de 38 - 568 mg/litro. Para los
organismos superiores, la concentración letal fue de unos 5000
mg/litro, y variaba notablemente poco de un filum a otro y exhibía
una curva dosis-respuesta sumamente pronunciada. Algunas bacterias
y microorganismos de aguas residuales y cienos activados son
capaces de adaptarse a concentraciones superiores a 17 000
mg/litro.
La germinación de semillas puede verse inhibida o estimulada
por el 1-propanol según la concentración en el agua utilizada y las
condiciones de exposición. El compuesto aumenta la acumulación de
nitritos en el maíz, los guisantes y el trigo.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 1-propanol para los mamíferos (a juzgar
por la mortalidad) es baja, por cualquiera de las vías de
exposición: cutánea, oral o respiratoria. Se ha comunicado que
los valores de la DL50 por vía oral para varias especies animales
varían entre 1870 y 6800 mg/kg de peso corporal. No obstante, en
ratas muy jóvenes se notificó una DL50 por vía oral de 560 - 660
mg/kg de peso corporal. El principal efecto tóxico del 1-propanol
tras una exposición única es la depresión del sistema nervioso
central. Los datos de que se dispone sobre el 1-propanol parecen
indicar que sus efectos sobre el sistema nervioso central son
semejantes a los del etanol; no obstante, el 1-propanol parece ser
más neurotóxico. Los valores de la DE50 para la narcosis en el
conejo y la pérdida del reflejo de enderezamiento en el ratón
fueron, respectivamente, de 1440 mg/kg de peso corporal por vía
oral, y de 1478 mg/kg de peso corporal por vía intraperitoneal;
estos valores son unas cuatro veces más bajos que los
correspondientes al etanol. En el ensayo del plano inclinado, el
1-propanol fue 2,5 veces más potente que el etanol en la rata.
La administración de dosis únicas por vía oral de 3000 ó
6000 mg/kg de peso corporal tuvo como resultado una acumulación
reversible de triglicéridos en el hígado de la rata. Las
concentraciones elevadas de vapor provocaron irritaciones en el
tracto respiratorio del ratón. El ritmo respiratorio del ratón se
vio disminuido en un 50% a concentraciones de aproximadamente
30 000 mg/m3.
No se dispone de datos sobre irritación ocular y cutánea. No
se observó sensibilización en una prueba de sensibilización cutánea
en ratones CF1.
En machos de rata expuestos durante seis semanas a 15 220
mg/m3, se obtuvieron pruebas limitadas de que el 1-propanol
disminuye la capacidad reproductora. No se observaron efectos tras
una exposición similar a 8610 mg/m3. Cuando se expusieron ratas
gestantes al 1-propanol, se observó toxicidad materna y fetal a
23 968 y 14 893 mg/m3 (9743 y 6054 ppm); no se observó toxicidad a
9001 mg/m3 (3659 ppm). No se observaron defectos de comportamiento
en la progenie de machos de rata expuestos durante seis semanas a
8610 ó 15 220 mg de 1-propanol/m3, ni en la de ratas expuestas
durante la gestación a las mismas concentraciones. No obstante,
cuando se administró a ratas de 5 a 8 días de edad 3000 - 7800 mg
de 1-propanol/kg por vía oral al día, se observó depresión del
sistema nervioso central durante la dosificación y síntomas de
privación cuando se retiraba la dosis. Cuando las ratas cumplieron
18 días se examinaron sus cerebros y se observaron reducciones en
los pesos cerebrales absoluto y relativo y en el contenido de ADN,
así como disminuciones regionales de los niveles de colesterol y de
proteínas.
El 1-propanol dio resultados negativos en dos ensayos de
detección de mutaciones puntuales utilizando Salmonella typhimurium
y en un ensayo de mutación inversa realizado con Escherichia coli
CA-274. Se obtuvieron resultados negativos en ensayos para inducir
intercambio de cromátidas hermanas o micronúcleos en células de
mamíferos in vitro. No se obtuvieron otros datos sobre
mutagenicidad.
En un estudio de carcinogenicidad realizado en grupos reducidos
de ratas Wistar expuestas durante toda su vida a dosis orales de
240 mg/kg o a dosis subcutáneas de 48 mg/kg, se observó un aumento
significativo de la incidencia de sarcoma hepático en el grupo en
el que la dosis se administraba por vía subcutánea. No obstante,
el estudio resultó insuficiente para evaluar la carcino-genicidad
por diversos motivos, entre ellos la falta de detalle experimental,
el reducido número de animales y el empleo de una sola dosis
elevada para inducir toxicidad hepática.
8. Efectos en la salud humana
No se tiene noticia de efectos adversos para la salud en la
población general o en grupos profesionales. El único caso
notificado de intoxicación mortal, fue el de una mujer que perdió
el conocimiento y falleció a las 4 - 5 h de la ingestión. La
autopsia reveló "inflamación cerebral" y edema pulmonar. En un
estudio sobre irritación cutánea y sensibilización, se comunicó la
aparición de reacciones alérgicas en un operario de laboratorio.
En otro grupo de 12 voluntarios, se observó en 9 individuos un
eritema que duró al menos 60 minutos tras la aplicación durante 5
minutos de papeles de filtro impregnados con 0,025 ml de una
solución al 75% de 1-propanol en agua aplicados sobre los
antebrazos.
No se dispone de más informes sobre efectos adversos para la
salud tras la exposición profesional al 1-propanol. No se dispone
de estudios epidemiológicos para evaluar los efectos a largo plazo,
incluida la carcinogenicidad, del 1-propanol en el ser humano.
9. Resumen de la evaluación
La exposición del ser humano al 1-propanol puede producirse por
la ingestión de alimentos o bebidas que lo contengan. La
exposición por inhalación puede tener lugar durante el uso
doméstico de la sustancia y en el ámbito laboral durante su
fabricación, tratamiento y uso. Los muy limitados datos de que se
dispone sobre el nivel de 1-propanol en el aire y el agua parecen
indicar que las concentraciones son muy reducidas.
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se cree que la absorción tras la
inhalación es rápida y la absorción por vía cutánea lenta.
La toxicidad aguda del 1-propanol para los animales es baja
en toda exposición, ya sea por vía cutánea, oral o respiratoria.
La exposición de miembros de la población general a niveles
potencialmente letales puede producirse por ingestión accidental o
intencionada. No obstante, sólo se ha notificado un caso de
envenenamiento mortal por 1-propanol. Los efectos agudos más
probables del 1-propanol en el hombre son la intoxicación
alcohólica y la narcosis. Los resultados de los estudios en
animales indican que el 1-propanol es 2 - 4 veces más tóxico que
el etanol.
El 1-propanol puede ser irritante para la piel hidratada.
Los datos sobre toxicidad animal no bastan para evaluar los
riesgos para la salud humana asociados a la exposición repetida o
duradera al 1-propanol. No obstante, estudios limitados a corto
plazo sobre la rata indican que la exposición por vía oral al
1-propanol tiene pocas probabilidades de suponer un riesgo grave
para la salud en las condiciones habituales de exposición humana.
En la rata la exposición por inhalación a una concentración de
15 220 mg/m3 redujo la capacidad reproductiva del macho, pero no
así la exposición a 8610 mg/m3. En la rata gestante, 9001 mg/m3
(3659 ppm) fue el nivel de efectos no observados y 14 893 mg/m3
(6054 ppm) el nivel más bajo de observación de efectos tanto
respecto a la toxicidad materna como a la fetal. Así pues, la
exposición por inhalación a concentraciones elevadas de 1-propanol
produjo toxicidad reproductiva y embriológica en el macho y la
hembra en presencia de toxicidad manifiesta en los animales
expuestos. Las concentraciones necesarias para producir esos
efectos en la rata fueron más elevadas que las que probablemente
se observen en condiciones normales de exposición humana.
El 1-propanol dio resultados negativos en los ensayos de
detección de mutaciones puntuales en bacterias. Aunque esto indica
que la sustancia no tiene ningún potencial genotóxico, no puede
evaluarse adecuadamente la mutagenicidad basándose en los limitados
datos disponibles. El estudio realizado no basta para evaluar la
carcino-genicidad del 1-propanol en animales de experimentación.
No se dispone de datos sobre la exposición a largo plazo de
poblaciones humanas al 1-propanol. Por todo ello, no puede
evaluarse la carcinogenicidad del 1-propanol en el ser humano.
Aparte de un caso de intoxicación mortal tras la ingestión de
medio litro de 1-propanol, no se tiene prácticamente noticia de
efectos adversos para la salud producidos por la exposición al
1-propanol, ni en la población general ni en grupos profesionales.
El Grupo Especial de Trabajo considera poco probable que el
1-propanol plantee un riesgo grave para la salud de la población
general en condiciones normales de exposición.
El 1-propanol puede liberarse al medio ambiente durante la
producción, el tratamiento, el almacenamiento, el transporte, el
uso y la evacuación de desechos. A causa de su uso principal como
disolvente volátil, la mayoría del volumen de producción acaba por
ser liberado a la atmósfera. No obstante, por reacciones con
radicales hidroxilo y por lavado pluvial, el 1-propanol
desaparecerá rápidamente de la atmósfera, siendo su tiempo de
presencia en ella inferior a 3 días. La eliminación del 1-propanol
del agua y del suelo también se produce rápidamente, de modo que
raras veces se detectan niveles medibles en cualquiera de estos
tres compartimientos. La adsorción del 1-propanol en las
partículas del suelo es escasa, pero la sustancia es móvil en el
suelo y se ha demostrado que aumenta la permeabilidad del mismo a
ciertos hidrocarburos aromáticos.
En vista de las propiedades físicas del 1-propanol, la bio-
acumulación es poco probable y, salvo en el caso de evacuación
accidental o inadecuada, el 1-propanol no constituye un riesgo para
los organismos acuáticos, los insectos y las plantas en las
concentraciones que por lo general se encuentran en el medio
ambiente.
(i) In the methylmalonyl pathway, propionyl-CoA is carboxylated to
methylmalonyl-CoA, this is followed by trans-carboxylation to
succinyl-CoA, which subsequently enters the tricarboxcylic acid
cycle to be metabolized to carbon dioxide and water.
(ii) In the lactate pathway, the propionyl-CoA is dehydrogentated
to acrylolyl-CoA, alpha-hydration gives L-lactoyl-CoA, which is
hydrolysed to lactate.
(iii) In another pathway the acrylolyl-CoA is hydrated to
3-hydroxypropionyl-CoA, deacylation and oxidation result in the
formation of malonic acid semialdehyde, which is converted to
acetyl-CoA. These reactions, which constitute the major pathways
for propionic acid metabolism in plant mitochondria, also occur in
animals.
(iv) The propionyl CoA may also participate in triglyceride
synthesis.
(v) The obligatory formation of propionyl carnitine required for
the transport of propionic acid into mitochrondria may also be an
excretory pathway under conditions of high carnitine and propionic
acid concentrations [158, 153].
Propionic acid and/or propionyl-CoA are potent inhibitors of
several mitochondrial enzymes required for fatty acid oxidation,
gluconeogenesis, and ureagenesis; their inhibitory effects can be
reversed with carnitine [24, 23]. The formation of propionyl-CoA,
its metabolism and effects on oxidative metabolism provide an
explanation for the hepatic effects observed in rats after high
oral exposure to 1-propanol (section 8.2) and for the biochemical
effects seen in some studies (section 8.3.1). Indeed the
accumulation of acyl-CoA esters, including propionyl-CoA, is
implicated in the pathogenesis of Reye syndrome [43].
6.4 Elimination and Excretion
Aliphatic alcohols may be eliminated from the body via expired
air or the urine. Theoretically, urinary metabolites may arise
from oxidation or from conjugation with glucuronic acid or sulfate.
There are no reports of the excretion of unchanged 1-propanol in
expired air or urine and, following an oral dose to rabbits of
800 mg/kg, only 0.9% was found in the urine as propyl-glucuronide
and none as a sulfate conjugate [100].
6.4.1 Animals
Available in vivo data, reviewed by Rietbrock & Abshagen [157],
showed that the elimination of 1-propanol was dose independent
above a single oral dose of 1000 mg/kg body weight in rats and
above a single intraperitoneal dose of 1200 mg/kg body weight in
rabbits [139, 1, 11]. The rate of the zero-order elimination of
the compound from the blood of rats that had received a single oral
dose of 3000 mg/kg body weight was found to be 510 mg/kg body
weight per hour [11]. At lower doses, the elimination rate was
first order. When rats were given a single oral dose of 1000 mg/kg
body weight, the half-life of 1-propanol was 45 min [1]. The
overall metabolism and elimination of 1-propanol are described in
section 6.3.1. In mice, a half-life of 57 min was estimated for
the exponential elimination phase following a single oral exposure
[1]. This should be considered an approximation because there were
only 2 or 3 time points per dose.
In vitro, the elimination of 1-propanol from the perfusate of
rat liver was also shown to be saturable, a zero-order phase being
succeeded below a concentration of 78 mg/litre by an exponential
phase with a half-life of 14 min [7].
6.4.2 Human beings
No data were available describing the elimination kinetics of
1-propanol in human beings.
When 10 volunteers drank 1-propanol in ethanolic orange juice
at doses of 3.75 mg 1-propanol and 1200 mg ethanol/kg body weight
over a period of 2 h, the compound was detected in the blood and in
the urine, partly as glucuronide. The total urinary excretion of
1-propanol was 2.1% of the dose. The urinary levels of 1-propanol
were lower when the amount of simultaneously ingested ethanol was
less, showing competition for ADH between 1-propanol and the
ethanol overdose [19, 20].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table
5. In none of these studies was the concentration of 1-propanol
reported to have been measured. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
open-system studies occurred at lower concentrations than the
nominal ones.
Several short-term studies have also been conducted. Seiler et
al. [167] determined the breakpoint of bioinhibition for a total
of 20 strains of several bacterial groups prevalent in a waste-water
treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
1-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 10 000 - 30 000 mg/litre of medium.
No analysis for the compound was reported.
Inhibition of cell multiplication of blue algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) reached 100%
after 8 days of static exposure to 255 and 3100 mg 1-propanol/litre
water, respectively, in a closed system at 27 °C and a pH of 7
[25, 27].
7.2 Terrestrial Organisms
7.2.1 Insects
The toxicity of 1-propanol for insect larvae is summarized in
Table 5. In static tests, the 48-h LC50 values for adults of the
fruit fly strains of Drosophila melanogaster and Drosophila
simulans were between 18 490 and 24 120 mg/litre of nutrient medium
and 11 260 and 12 860 mg/litre of nutrient medium, respectively [46].
7.2.2 Plants
The effects of 1-propanol on the rate of seed germination
have been investigated on several occasions. Total inhibition of
the germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers absorbing a solution containing 8050
mg 1-propanol/litre water [40]. The germination of white amaranth
(Amaranthus albus) seeds was stimulated in a dose-related manner
after 5 h incubation at 25 °C on filter papers moistened with a
solution containing 3600 - 36 050 mg 1-propanol/litre water [36].
Reynolds [36] measured 50% inhibition of germination of lettuce
(Lactuca sativa) seeds after incubation for 3 days at 30 °C on agar
containing 3065 mg 1-propanol/litre. The percentage germination
and the axis length of soya bean (Glycine max) seeds, with the
testa removed, were not reduced after exposure to pure 1-propanol
for 2 h. After treatment with a 50% (v/v) 1-propanol/water mixture
for 2 min, germination was almost completely inhibited and axis
length was reduced [150].
Table 5. Acute aquatic toxicity of 1-propanol
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria
Pseudomonas putida 25 7 closed 16 h TTb 2 700 [27]
Microorganisms
Activated sludge 21 7.4-8 closed 3 h 50% inhibition 1 000 [105]
of respiration
Acclimated mixed 30 6.8 closed 1.2 h 50% inhibition 19 085 [196]
waste-water culture of respiration
Protozoa
Entosiphon sulcatum 25 6.9 closed 72 h TTb 38 [26]
Chilomonas paramecium 20 6.9 closed 48 h TTb 175 [29]
Uronema parduczi 25 6.9 closed 20 h TTb 568 [28]
Algae
Selenastrum capricornutum 25-26 closed 96 h NOAECc 2 000 [172]
Scenedesmus pannonicus 25-26 closed 48 h NOAECc 2 900 [172]
Chlorella pyrenoidosa 25-26 closed 48 h NOAECc 1 150 [172]
Coelenterate
Hydra oligactis 17 8.2-8.4 >5 closed 48 h LC50 6 800 [170]
Worms
Flatworm (Dugesia) 20 8.2-8.4 >5 closed 48 h LC50 4 700 [170]
Tubificid worm 20 8.2-8.4 >5 closed 48 h LC50 9 200 [170]
Molluscs
Giant pond snail 20 8.2-8.4 >5 closed 48 h LC50 6 500 [170]
(Lymnea stagnalis)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Crustaceans
Water flea 20 8 >2 250 open 24 h EC50d 4 415 [27]
(Daphnia magna)e EC0 3 336
EC100 5 909
19 open 48 h LC50 7 080 [33]
Water flea 19 open 48 h LC50 3 025 [33]
(Daphnia pulex)e
Water flea 19 open 48 h LC50 5 820 [33]
(Daphnia cucullata)e
Isopod 20 8.2-8.4 >5 209 closed 48 h LC50 2 500 [170]
(Asellus aquaticus)
Scud (Gammarus pulex) 20 8.2-8.4 >5 209 closed 48 h LC50 1 000 [170]
Insects
Mosquito larvae 22-24 open 4 h LC50 10 450 [107]
(Aedes aegypti)
Mosquito larvae (Aedes 26 8.2-8.4 >5 209 open 48 h LC50 4 400, 4 800 [172]
aegypti, Culex pipiens) LC0 3 200, 3 600
Midge larvae 20 8.2-8.4 >5 209 closed 48 h LC50 2 350 [170]
(Chironomus gr. thummi)
Leech larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 400 [170]
(Eropdella octoculata)
Dragon fly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 4 200 [170]
(Ischnura elegans)
Stonefly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 520 [170]
(Nemoura cinerea)
Mayfly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 3 110 [170]
(Cloeon dipterum)
Corixa punctata (larvae) 20 8.2-8.4 >5 209 closed 48 h LC50 2 000 [170]
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Creek chub 15-21 8.3 98 open 24 h LC0 200 [68]
(Semotitus atromaculatus)
Golden orfe 20 7-8 >5 200-300 48 h LC50 4 320, 4 560 [99]
(Leuciscus idus melanotus) LC0 3 600, 4 000
Fathead minnow 20 8.2-8.4 >5 209 open 48 h LC50 5 000 [172]
(Pimephales promelas) LC0 2 600
Rainbow trout 15 7-8 >5 98 open 48 h LC50 3 200 [172]
(Salmo gairdneri) LC0 2 000
Paddy fish 24 8.2-8.4 >5 209 open 48 h LC50 5 900 [172]
(Oryzias latipes) LC0 4 400
Amphibia
South African clawed toad 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Xenopus laevis)
Mexican axolotl 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Ambystoma mexicanum)
SEA WATER
Bacteria
Photobacterium 15 15 min 50% light 8 686 [84]
phosphorerum closed reduction
5 5 min 50% light 17 700 [48]
closed 15 min reduction 18 400
Crustacea
Brine shrimp 24 24 h LC50 4 200 [149]f
(Artemia salina) open
Harpacticoid copepod 21 7.9 >5 96 h LC50 2 300 [12]g
(Nitocra spinipes)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Bleak (Alburnus alburnus) 10 7.9 >5 open 96 h LC50 3 800 [12]g
---------------------------------------------------------------------------------------------------------------------------------
a Static systems used in all experiments reported.
b TT = toxic threshold for inhibition of cell multiplication.
c NOAEC = no-observed-adverse-effect-concentration; effect is growth inhibition.
d Effect is complete immobilization.
e Age of Daphnia was 24 h for Daphnia magna and Daphnia pulex, and 11 ± 1 day for Daphnia cucullata.
f Salinity was 2.8%.
g Salinity was 0.7%.
1-Propanol was marginally effective in breaking the dormancy of
seeds of genetically pure dormant lines of wild oat (Avena fatua)
after 5 days of exposure to solutions containing up to 1202 mg/litre.
Seed viability was affected at higher concentrations [2].
When excised seedling roots of maize (Zea mays) were treated by
vacuum infiltration in a 5% solution of 1-propanol in water, 3
times for 60 seconds, and then incubated anaerobically at 28 °C,
nitrite accumulation increased by 10 times or more, the utilization
of nitrate increased, and the utilization of exogenous nitrite was
inhibited. These effects were enhanced under aerobic conditions
[75]. Dry et al. [51] observed that stimulation of nitrite
accumulation in pea and wheat roots under aerobic conditions was
accompanied by a decline in the cellular levels of glucose-6-
phosphate. It was suggested by Gray & Cresswell [75] that an
increase in the utilization of nitrate was related to increased
access of nitrate to the site of nitrate metabolism as a result of
an increase in membrane permeability.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
The available LD50s for various animal species are summarized
in Table 6. Based on mortality estimates, 1-propanol exhibits low
toxicity, except in very young rats. Oral LD50 values for several
animal species range between 1870 and 6800 mg/kg body weight. For
very young rats, oral LD50s of 560 - 660 mg/kg body weight have
been reported [152].
An intraperitoneal dose of 785 mg 1-propanol/kg was lethal to 10
out of 10 C57B/6J and 10 out of 10 DBa/2J mice, but a dose of 392 mg/
kg did not cause any deaths in either strain [183]. An LD16 of
450 mg/kg was reported in rats after intraperitoneal administration.
When rats were exposed to 1-propanol vapour for 4 h at a
concentration of approximately 9840 mg/m3, 2 out of 6 died within
14 days [173].
8.1.2 Signs of intoxication
Osborne-Mendel or Sherman rats of both sexes receiving a lethal
oral dose of undiluted 1-propanol became comatose within a few
minutes [187]. Deep narcosis occurred in mice exposed through
inhalation of 1-propanol at a concentration of 50 mg/litre for 2 h.
Very young rats (60 - 100 g) of an unspecified strain and of
both sexes, received a single oral dose of between 150 and 3000 mg
undiluted 1-propanol/kg body weight. Animals that died showed
hyperaemia, vacuolation, and dilated sinusoids in the liver, and
hyperaemia, tubular cloudy swelling, and tubular necrosis in the
kidneys [152].
When anaesthetized Sprague-Dawley rats were made to inspire
160 mg of the undiluted compound, all 9 exposed rats died within
165 min, 6 of them dying immediately from respiratory arrest. All
controls survived and were killed 24 h later. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight of the exposed rats was increased by 92%. The lungs
showed oedema and small areas of focal haemorrhage [65].
Special studies on neurotoxic and behavioural effects, and on
biochemical effects are described in sections 8.3 and 8.4.
Table 6. LD50s for 1-propanol
---------------------------------------------------------------------------------------------------
Species Sex Route of Observation LD50 (mg/kg Comments Reference
exposure period body weight)
---------------------------------------------------------------------------------------------------
Wistar rat (non- male oral 14 days 1870 vehicle: water [173]
fasted)
Osborne-Mendel male, oral until 6500 undiluted [187]
or Sherman rat female recovery
CD mouse not oral 3 days 6800 undiluted [164]
reported
Rabbit male, oral 1 day 2820 [130]
female
Wistar rat male intravenous 5 days 590 vehicle: water [190]
H mouse male intravenous 5 days 697 vehicle: water [190]
female intravenous not 1090 vehicle: water [40]
reported
Chinchilla rabbit male, intravenous 5 days 483 vehicle: water [190]
female
Wistar rat male intraperitoneal 5 days 2247 vehicle: water [190]
H mouse male intraperitoneal 5 days 3695 vehicle: water [190]
Syrian hamster male intraperitoneal 5 days 2337 vehicle: water [190]
Guinea-pig intraperitoneal 5 days 1208 vehicle: water [190]
New Zealand male dermal 14 days 4050 1/10 of body [173]
rabbit surface exposed
under cover for
24 h
---------------------------------------------------------------------------------------------------
8.1.3 Skin, eye, and respiratory tract irritation; sensitization
Data for skin and eye irritation were not available. One skin
sensitization test has been reported concerning an ear-swelling
test on CF1 mice. No sensitization was observed [61]. Although
the test requires further validation, it correctly discriminated
between a number of known positive and negative human sensitizing
agents. The authors claim it to be an accurate, sensitive, and
efficient method for evaluating delayed contact sensitization.
The sensory irritation of 1-propanol was investigated using a
50% reflex decrease in the respiratory rate of mice (RD50) as an
index. Only the heads of the mice were exposed. An exposure-
related effect was found with RD50 values for the first 10 min of
exposure of 31 252 mg/m3 for Swiss Webster mice [101] and
33 604 mg/m3 for CF-1 mice [108]. The potential of 1-propanol as
a respiratory irritant is therefore low.
8.2 Repeated Exposures
Only a few data are available concerning the oral exposure of
animals.
When 3 male and 3 female rats of unspecified strain were
exposed to 4 daily oral doses of 2160 mg undiluted 1-propanol, no
deaths occurred and no gross pathological signs were seen in the
liver [187].
In a group of 6 male rats of unspecified strain, receiving
drinking-water containing 1-propanol at a concentration of 60 090
mg/litre for 4 months, food consumption, body weight gain, and
liver histopathology were comparable to those of the control group.
It should be noted that the authors reported a dose rate of 3 mg/kg
body weight per day, while a dose rate of approximately 3000 mg/kg
body weight per day seems more appropriate, assuming a water
consumption of 20 ml/day and a body weight of 400 g [85].
Groups of 10 Wistar rats were exposed to 1-propanol in the
drinking-water at a concentration of 320 000 mg/litre (calculated
by the Task Group to be equivalent to approximately 16 000 mg/kg
body weight per day, on the basis of the assumptions made above)
for 5, 9, or 13 weeks. Control groups comprised 10 rats each. The
exposed rats gradually became weak, losing their appetites and
showing a decreased body weight gain. Electron microscopic studies
of the liver showed irregularly shaped megamitochondria with few
cristae, and normally sized but irregularly shaped mitochondria
with a decreased number of cristae. Biochemical changes included a
decreased state 3 respiration using glutamate as a substrate and
decreased specific activities of cytochrome c oxidase (EC 1.9.3.1)
and monoamine oxidase (EC 1.4.3.4) [203].
8.3 Neurotoxic and Behavioural Effects
In one study on anaesthetized mongrel dogs, it was shown that
1-propanol, as well as other primary alcohols, could increase the
permeability of the blood-brain barrier. The dogs received a
sodium fluorescein solution and 0.578 mg 1-propanol in saline,
intravenously. The concentration of sodium fluorescein in the
cerebrospinal fluid rose to a maximum within 10 min and returned to
control levels, 3 h after exposure [78].
The oral ED50 (1440 mg/kg body weight) for narcosis in rabbits
exposed to 1-propanol was 4 times lower than that for ethanol
[129]. Deep narcosis occurred in mice exposed through inhalation
to 50 mg 1-propanol/litre for 2 h, and a 40-min exposure to 2.3 mg/
litre reduced the unconditioned flexor response in rabbits. When
rabbits were intravenously infused with 1-propanol at a rate of 9 -
30 mg/min per kg body weight, positional nystagmus with an
inhibited rotatory response was observed at and above a blood
concentration of 900 mg/litre [137].
The intraperitoneal ED50 for loss of righting reflex in Swiss
Webster mice administered 1478 mg 1-propanol/kg body weight was 2.8
times lower than that for ethanol [117]. When C57BL/6J or DBA/2J
mice were given a single dose of 1-propanol intraperitoneally, both
strains showed decreased activity in the open field test at
392 mg/kg body weight, but the decrease was not significant. All
mice given 785 and 1570 mg/kg body weight died [183]. The rotarod
performance of Swiss-Cox mice decreased in a dose-related manner
after single oral doses of 1-propanol of 2000 or 4000 mg/kg body
weight. A dose of 1000 mg/kg body weight did not cause behavioural
impairment. When the study was repeated on days 4, 6, 7, and 8
after the first trial, tolerance did not develop [1].
The threshold for the induction of ataxia in Sprague-Dawley
rats following intraperitoneal exposure was 799 mg/kg body weight
[119]. In a tilted plane test, the performance of rats decreased
by an average of 71% after oral exposure to 2000 mg/kg body weight.
On a molar basis, 1-propanol was 2.5 times as intoxicating as
ethanol [204].
According to several investigators, depression of the central
nervous system by 1-propanol was related to interactions with
neuronal membranes. Lyon et al. [117] observed a high correlation
between the narcotic potencies of the aliphatic alcohols, including
1-propanol, in mice and their ability to disorder the brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was in turn related to membrane
solubility. A change in membrane fluidity was shown to occur in
isolated synaptosomal plasma membranes of rat cortex in vitro by a
decrease in 1,6-diphenyl-1,3,5-hexatriene fluorescence polarization
[81].
Functional loss due to disruption of membrane integrity by
1-propanol was observed in vitro. The action potentials of the
sciatic nerves of the toad (Bufo marinus) [155] and of giant axons
of the squid (Loligo forbesi) [143] were decreased by 1-propanol.
In isolated rat phrenic nerve-diaphragm, 1-propanol increased the
amplitudes of end-plate and miniature end-plate potentials and the
number of quanta of acetylcholine of end-plate potentials [62].
The compound also affected the rate of decay of postsynaptic
currents in the neuromuscular junction of the crayfish (Cherax
destructor) [200], and in the phrenic nerve-diaphragm of the rat
[62].
Effects on the ionic currents underlying the changes in
excitability described above were also investigated in vitro.
1-Propanol inhibited both the K+-stimulated and the Na+-dependent
influx of Ca2+ ions into isolated rat brain synaptosomes [80, 81,
124], and the influx of Na+ ions into rat brain synaptosomes [128].
It decreased the Na+ and K+ currents in the giant axons of the
squid (Loligo forbesi) [143], and in sciatic nerve fibres of the
clawed toad (Xenopus laevis) [6]. The interference of 1-propanol
with the transport of Ca2+ ions across biological membranes was
also shown in vitro by the inhibition of Ca2+ ion-induced
contractions of guinea-pig ileum [213], and in vivo, in rats, by a
decrease in regional brain Ca2+ ion levels, 30 min after one
intraperitoneal dose of 2000 mg/kg body weight [159].
The disruption of neuronal membranes by 1-propanol was also
thought to explain its inhibitory action on the binding of
dihydromorphine to isolated mouse brain caudate membranes [185] and
on membrane-bound guanylate cyclase (EC 4.6.1.2) in intact murine
neuroblastoma N1E-115 cells [172]. The activation by 1-propanol of
membrane-bound adenylate cyclase (EC 4.6.1.1) from isolated mouse
striatal membranes, in the presence of guanine nucleotides, was
also suggested to be the result of membrane perturbation [116].
8.4 Biochemical Effects
8.4.1 Effects on lipids in the liver and blood
Oral administration of single doses of 3000 or 6000 mg
1-propanol/kg body weight to Wistar rats caused a transient
increase in hepatic triglycerides, which was related to the
duration of an elevated blood-1-propanol concentration [10, 11].
Gaillard & Derache [63] did not observe an increase in hepatic
triglycerides in Wistar rats, 17 h after a single dose of 6000 mg
1-propanol/kg body weight.
Factors possibly responsible for hepatic triglyceride
accumulation include: an increase in hepatic uptake of labelled
palmitate [11], an increased esterification of palmitate to form
liver triglycerides [10, 11], and decreased palmitate oxidation
[11]. The decrease in palmitate oxidation was related to an
increase in the hepatic alpha-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [11].
An increase in extramitochondrial reducing equivalents, indicated
by an increased lactate/pyruvate ratio, was observed in vitro by
Forsander [60], but not in vivo by Beaugé et al. [11].
The effects of 1-propanol on palmitate incorporation into
triglycerides appear to depend on the dose, high doses causing
inhibition and lower ones leading to an increase. The
incorporation of palmitate into serum triglycerides and serum and
liver phospholipids, 4.5 h after a single oral dose of 6000 mg
1-propanol/kg body weight to rats, was found to be inhibited, while
an increase in hepatic triglyceride accumulation was only observed
8 h after dosing [10]. Three hours after a dose of 3000 mg/kg body
weight, the incorporation of palmitate in blood triglycerides was
increased concomitantly with an increase in hepatic triglycerides
while levels of phospholipids in the liver and blood were unaffected
[11]. Similar effects have been noted with ethanol [11].
8.4.2 Effects on microsomal enzymes
The effects of 1-propanol on microsomal enzymes (EC 1.14.14.1)
in vivo was investigated by Powis [147], who administered a single
oral dose of 960 mg/kg body weight to Wistar rats. Twenty four
hours after exposure, no effects were observed on the activities of
aniline hydroxylase and aminopyrine demethylase in liver
microsomes. This study is inadequate to demonstrate an inductive
effect of 1-propanol on the microsomal mixed function oxidase
system. In vitro, 1-propanol inhibited aldrin epoxidase and
p-aniline hydroxylase in isolated rat liver microsomes via an
interaction with cytochrome P-450, which causes a reverse Type I
spectral change [41, 210, 189, 161]. At high concentrations, the
inhibition of the mixed function monoxygenase system by aliphatic
alcohols correlates directly with the lipophilicity of the
alcohols, and is probably the result of unspecific effects on the
membrane (see section 8.3). The compound did not affect the levels
of hepatic microsomal cytochrome P-450, haem, cytochrome b5, and
NADPH-cytochrome c reductase (EC 1.6.2.4) in phenobarbital-induced
rats [96]. 1-Propanol increased the levels of cytochrome P-450 in
cultured chick embryo hepatocytes. The activities of benzphetamine
demethylase and UDP-glucuronosyl transferase (EC 2.4.1.17) were
also increased [169].
8.4.3 Other biochemical findings
The glutathione level in the liver of Wistar rats administered
a single dose of 1660 mg 1-propanol/kg body weight, orally, had
decreased by 20%, 6 h after exposure. Lipid peroxidation, as
indicated by diene conjugates formation, was increased; ethanol in
equivalent doses produced similar effects [199].
The activities of liver ornithine decarboxylase (EC 4.1.1.17)
and liver tyrosine aminotransferase (EC 2.6.1.5) increased in
partially hepatectomized rats 4 h after one oral dose of 2300 mg
1-propanol/kg body weight or an equivalent dose of ethanol. No
effects were observed on levels of alanine aminotransferase
(EC 2.6.1.2) in the liver and kidneys, and on levels of ornithine
decarboxylase in the kidneys and brain [145].
The effects of 1-propanol on neuronal membrane-bound adenylate
cyclase (EC 4.6.1.1) and guanylate cyclase (EC 4.6.1.2) in vitro
are discussed in section 8.3. 1-Propanol and ethanol, can have
different effects on the activity of adenylate cyclase, depending
on the concentration of alcohol and the biological system being
investigated [178, 192, 93].
When Sprague-Dawley rats inhaled 1-propanol for 6 h at a
concentration of 490 mg/m3, the serum level of testosterone was
decreased by 42% immediately after exposure, but not 18 h after
exposure. When this exposure regimen was repeated daily over one
week, no effects on serum testosterone levels were observed. Serum
levels of luteinizing hormone and corticosterone were unchanged at
all times [31].
When a crude homogenate of dispersed acinar cells, prepared
from guinea-pig pancreas, was incubated with 1-propanol and
secretin, the secretin-stimulated activities of adenylate cyclase
(EC 4.6.1.1) and cellular cyclic adenosine 3',5'-monophosphate were
potentiated at low concentrations of 1-propanol, but the
potentiation was reversible. Irreversible inhibition occurred at
higher concentrations [192].
8.5 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 15 male Sprague-Dawley rats were exposed to
1-propanol at measured concentrations of 8610 or 15 220 mg/m3 for
7 h/day over 6 weeks. Beginning on the third day after the last
exposure, males were mated for a maximum of 5 days with unexposed
females. There was no apparent effect of exposure to 8610 mg/m3 on
mating performance or fertility. After exposure to 15 220 mg/m3,
17 out of 18 males copulated (as evidenced by the presence of a
vaginal plug), but only 2 of 17 mated females became pregnant. The
offspring of the exposed males were evaluated postnatally in a
battery of behavioural tests. There was no evidence of any
exposure-related effect [132].
These investigators also exposed groups of 15 pregnant Sprague-
Dawley rats to the same concentrations of 1-propanol on gestation
days 1 - 20. Pregnant females exposed to 15 220 mg/m3 showed
significantly reduced weight gain and food consumption. Their
female offspring also showed reduced weight gain up to 3 weeks of
age, but there was no consistent effect on male offspring. Litter
sizes were not affected. "Several" of the offspring from dams
exposed to 15 220 mg/m3 had crooked tails. Behavioural testing of
offspring did not reveal any evidence of an exposure-related
effect, though there was an increase in total external, visceral,
and skeletal malformations at 23 968 mg/m3 (9743 ppm) and in total
skeletal malformation at 14 893 mg/m3 (6054 ppm) [132].
The effects of 1-propanol on brain development in the neonatal
rat were also studied. A group of 21, 5-day-old Long-Evans rats
was exposed to 1-propanol via an artificial milk formula, which was
administered through an intragastric catheter for 4 consecutive
days. The rats received 12 feeds daily, each lasting 20 min.
Doses were 3800, 7500, 3000, or 7800 mg/kg body weight on day
5 - 8, respectively. Controls received the milk formula only.
During the exposure, the exposed pups frequently showed an impaired
righting response. After the last exposure, withdrawal symptoms
were displayed. Pups were killed at 18 days of age, at which time
there was no effect on body weight or on absolute weight of
kidneys, heart, or liver. However, the absolute and relative brain
weights were decreased in the exposed pups. Biochemical analysis
showed that the exposed pups had a decreased amount of DNA in all
brain areas examined. Cholesterol levels were decreased in the
forebrain and cerebellar samples, while protein levels were
decreased only in the forebrain samples [74].
8.6 Mutagenicity
8.6.1 Bacteria
1-Propanol was tested for mutagenic activity using Ames test
without S9; up to 100-µmol/plate was negative with Salmonella
typhimurium TA-100 [181]. Negative results were also reported in
TA-100 and TA-98 with or without metabolic activation, following
standard Ames test protocol [94].
In a reverse mutation assay with Escherchia coli CA-274
following a pre-incubation protocol, a 5-fold increase in the
number of revertants was observed at a concentration of 4.5%
1-propanol. No metabolic activation system was used [86].
8.6.2 Mammalian cells in vitro
1-Propanol (100 mg/litre once a day for 7 days) did not
increase the number of sister chromatid exchanges in Chinese
hamster ovary cells [136], or in V79 Chinese hamster lung
fibroblasts at 6000 mg/litre for 3 h (with activation) and 28 h
(without activation) [200]. It did not increase the number of
micronuclei in V79 Chinese hamster lung fibroblasts at 40 200 mg/
litre for 1 h [113].
A dose-related increase in the inhibition of metabolic
cooperation between hamster V79 cells, a phenomenon believed to
reflect carcinogenic promotion ability and not be indicative of
genotoxic potential, was observed by Chen et al. [37]. This may be
due to the membrane effects of 2-propanol.
8.7 Carcinogenicity
A group of 18 Wistar rats of both sexes received doses of
240 mg 1-propanol/kg body weight, by gavage, twice a week, for
their lifetime. Another group of 31 Wistar rats of both sexes
received subcutaneous injections of 48 mg compound/kg body weight,
twice a week, for their lifetime. Control groups, comprising 25
rats for each route, received saline. It was not reported whether
the analytical grade, double-distilled test compound was analysed
for the presence of impurities. The average survival time was 570
days for the orally exposed rats, 666 days for the subcutaneously
exposed rats, and 643 days for both control groups. The tumour
incidence is reported in Table 7. The data were not statistically
analysed. It was reported that "nearly all rats" showed liver
damage including congestion, steatosis, necrosis, fibrosis, and
metaplasia and hyperplasia of the haematopoietic bone marrow
parenchyma. However, the incidence of these lesions were not
reported [67].
Table 7. Tumour incidence in Wistar rats exposed orally or
subcutaneously to 1-propanol for lifetimea
-------------------------------------------------------------------
Organ/ Tumour type Incidence
tissue oral exposure sc exposure
affected exposed controls exposed controls
-------------------------------------------------------------------
Blood myeloid leukemia 2/18 0/25 4/31 0/25
Liver carcinoma 1/18 0/25 0/31 0/25
Liver sarcoma 2/18 0/25 5/31 0/25
Other carcinoma 0/18 0/25 3/31b 0/25
sarcoma 0/18 0/25 2/31c 0/25
benign tumoursd 10/18 3/25 7/31 2/25
-------------------------------------------------------------------
a From: Gibel et al. [67].
b One carcinoma each in kidney, bladder, and uterus.
c One sarcoma each in spleen and at injection site.
d Mostly papillomas and mammary fibroadenomas.
Although there was an apparent increase in the incidence of
liver sarcoma, the study is inadequate for the assessment of
carcinogenicity. The dosing schedule did not conform to standard
protocol. Too few animals were used in each dose group, the sex
ratio of each group was unclear, no data were provided on the
histological type of liver sarcoma, no statistical analysis was
conducted, the maximum tolerated dose was exceeded, as evidenced by
the reported liver damage, and only single dose levels were used.
In the case of subcutaneous administration, the exposure route was
inappropriate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
One case of poisoning by 1-propanol has been reported. It
concerned a 46-year-old woman who was estimated to have consumed
approximately half a litre of the compound as a solvent in a
cosmetic preparation, probably a hair lotion. It was pointed out
that the woman could have ingested this preparation more than once
in the past. The woman was found unconscious. She died 4 - 5 h
after ingestion. No other signs or symptoms were reported.
Autopsy revealed a "swollen brain" and lung oedema [52].
9.1.2 Controlled human studies
Filter papers moistened with 0.025 ml of a 75% solution of
1-propanol in water were placed on the forearms of a group of 12
volunteers following immersion of the forearms in water at 23 °C
for 10 min. The patches were covered for 5 min and then gently
blotted. Nine of the 12 persons showed erythema for at least 60
min following exposure. The cutaneous reaction was totally blocked
in 4 out of 4 persons after pretreatment with 40% 4-methylpyrazole
in hydrophilic ointment 1 h before the challenge, showing,
according to the authors, that 1-propanol must be metabolized to
propanal before vasoactivity occurs [207].
9.2 Occupational Exposure
A laboratory worker in a company manufacturing hair cosmetics
developed allergic reactions in patch tests with chemically pure
1-propanol solutions in water (10 - 99.5% by volume). This person
also reacted to 2-propanol, 1-butanol, 2-butanol, and formaldehyde,
but not to ethanol and methanol. Controls were not tested [115].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 1-propanol may occur through
ingestion of food and alcoholic beverages containing 1-propanol
(e.g., wine and beer 100 - 200 mg/litre, spirits up to 3500
mg/litre). Inhalation exposure may occur during household use and
occupationally during manufacture and processing. Exposure of the
general population via inhalation and drinking-water is very low
(average concentrations in urban air and drinking-water in the USA,
0.00005 mg/m3 and drinking-water 0.001 mg/litre, respectively)
(section 5).
10.1.2 Health effects
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Data on the absorption rate following
inhalation are lacking but, in view of the physical properties of
the compound, it is also expected to be rapid. Dermal absorption
is expected to be slow (section 6).
1-Propanol exhibits low acute toxicity for animals (based on
lethality estimates), whether exposed via the dermal, oral, or
respiratory route (section 8.1). Exposure to potentially lethal
levels may occur in the general population through accidental or
intentional ingestion. However, only one case of lethal poisoning
by 1-propanol has been reported, which probably reflects its low
toxicity and limited use by the public (section 9.1.1). The
principal toxic effect of 1-propanol following a single exposure is
depression of the central nervous system. Quantitative exposure-
effect data on human beings are not available. The most likely
acute effects of 1-propanol in man are alcoholic intoxication and
narcosis. Animal studies indicate that 1-propanol is 2 - 4 times
as intoxicating as ethanol.
A controlled human study has indicated that 1-propanol may be
irritating to hydrated skin. However, the potential of 1-propanol
as a respiratory irritant is low (section 8.1.3). Data are
inadequate for evaluation of the irritating properties of this
compound for the skin, eye, and respiratory tract in human beings,
or for evaluation of its sensitizing potential.
The results of limited drinking-water studies on animals
suggest that oral exposure to 1-propanol is unlikely to pose a
serious health hazard under the usual conditions of human exposure
(section 8.2).
Inhalation exposure to a concentration of 15 220 mg/m3 caused
impaired reproductive performance in male rats, but exposure to
8610 mg/m3 did not. In pregnant rats, 9001 mg/m3 (3659 ppm) was a
NOEL and 14 893 mg/m3 (6054 ppm) was a LOEL for both maternal and
developmental toxicity. Behavioural effects were not detected in
offspring whose mothers were exposed during pregnancy to 15 220
mg/m3, but oral dosing of neonatal rats produced biochemical
changes in the brain that were detected 10 days after the last
treatment (section 8.5). Inhalation exposure to high concentrations
of 1-propanol produced reproductive and developmental toxic effects
in male and female rats. These effects occurred in the presence of
other overt signs of toxicity in the exposed animals and 1-propanol
does not appear to be selectively toxic to male or female
reproductive processes. The concentrations required to produce
these effects in rats were higher than those likely to be
encountered under normal conditions of human exposure.
1-Propanol was negative in assays for point mutations in
bacteria. It did not increase the incidence of sister chromatid
exchange or micronuclei in mammalian cells in vitro. Although
these findings suggest that the substance does not have any
genotoxic potential, no adequate assessment of mutagenicity can be
made on the basis of the limited data available. The results of an
in vitro test said to predict promotional activity were negative
(section 8.6). The available study is inadequate to evaluate the
carcinogenicity of 1-propanol in experimental animals (section
8.7). No data are available on the long-term exposure of human
populations to 1-propanol. Hence, the carcinogenicity of
1-propanol in human beings cannot be evaluated. Apart from one
case of fatal poisoning following ingestion of half a litre of
1-propanol, there are practically no reports on adverse health
effects from exposure to 1-propanol either in the general
population or in occupational groups (section 9).
The Task Group considers it unlikely that 1-propanol will pose
a serious health risk for the general population under normal
exposure conditions.
10.2 Evaluation of Effects on the Environment
1-Propanol can be released into the environment during
production, processing, storage, transport, use, and waste disposal
(section 3). It is transferred from water, soil, and waste
disposal sites to the atmosphere by volatilization, from the
atmosphere to water and soil by rain-out, and from soil and waste
disposal sites to ground water by leaching. It is difficult to
estimate its emission into each compartment. Because of its
primary use as a volatile solvent, most of the production volume is
eventually released into the atmosphere (section 4.1).
By reacting with hydroxyl radicals and through rain-out,
1-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 3 days (section 4.2). Thus, measurable
atmospheric levels of 1-propanol are not usually encountered.
Hydrolysis and photolysis are not expected to be important in
the removal of 1-propanol from water and soil, but removal occurs
rapidly by aerobic and anaerobic biodegradation (section 4.3.1) so
that measurable levels are rarely found. Adsorption of 1-propanol
on soil particles is poor but it is likely to be mobile in soil and
it has been shown to increase the permeability of soil to some
aromatic hydrocarbons (section 4.1).
In view of the physical properties of 1-propanol, its potential
for bioaccumulation is low (section 4.3.2). Except in the case of
accident or inappropriate disposal, 1-propanol does not present a
risk for aquatic organisms, insects, or plants at concentrations
that usually occur in the environment. However, 1-propanol at
concentrations of around 5000 mg/litre in water is lethal to
oxygen-using aquatic organisms, indicating that its emission into
surface water at this level may result in serious alteration of the
local ecosystem (section 7).
11. RECOMMENDATIONS
1. 1-Propanol has not shown mutagenic potential in the small
number of assays performed. A full array of modern
genotoxicity tests should be carried out.
2. A single published report suggests carcinogenic activity by
1-propanol, but this study is seriously flawed and cannot be
used to evaluate the potential carcinogenicity of 1-propanol.
The desirability of a carcinogenesis bioassay of 1-propanol
should be considered, on the basis of the outcome of
genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
1-propanol produced reproductive and developmental toxicity in
experimental animals. In view of the potential for
environmental and drinking-water contamination, reproductive
and developmental toxicity should be investigated using oral
dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
1-propanol.
5. The unusually uniform level of toxicity in diverse types of
aquatic organisms that consume gaseous oxygen, and the
exceptionally steep dose-effect curve observed, suggest a
nonspecific effect that may not be restricted to 1-propanol.
These effects merit investigation.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1-Propanol was considered by the Joint FAO/WHO Expert
Committee on Food Additives (JECFA) in its twenty-third report.
Specifications were formulated, but no toxicological monograph was
prepared, and the substance could not be evaluated on the basis of
the data available. Additional toxicological information was
available to JECFA at a later meeting, including the results of a
limited study in rats suggesting a carcinogenic potential for
1-propanol. In its twenty-fifth report,a JECFA noted that life-
time feeding studies in rodents were required to resolve the
problem of carcinogenicity. A toxicological monograph was
prepared. The existing specifications were revised and designated
as "tentative", but no ADI was established.
-------------------------------------------------------------------
a WHO Technical Report Series, No. 669, 1981 ( Evaluation of
certain food additives: Twenty-fifth Report of the Joint
FAO/WHO Expert Committee on Food Additives).
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
2. ADKINS, S.W., NAYLOR, J.M., & SIMPSON, G.M. (1984) The
physiological basis of seed dormancy in Avena fatua. V.
Action of ethanol and other organic compounds. Physiol.
Plant., 62: 18-24.
3. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
4. ALLINGER, N.L., CAVA, M.P., DE JONGH, D.C., JOHNSON, C.R.,
LEBEL, N.A., & STEVENS, C.L. (1971) Organic chemistry, New
York, Worth Publishers, Inc.
5. ANON. (1984) Propyl alcohol complaint settled by US
companies. Chem. marketing Rep., April 23: 3, 17.
6. ARHEM, P. & VAN HELDEN, D. (1983) Effects of aliphatic
alcohols on myelinated nerve membrane. Acta physiol. Scand.,
119: 105-107.
7. AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl and isoamyl alcohols by the isolated
perfused rat liver. J. Pharmacol. exp. Ther., 197: 669-674.
8. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of 2-
halo-pyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
9. BEAUD, P. & RAMUZ, A. (1978) Dosage simultané des alcools
supérieures, et de l'acetate d'éthyle dans les eaux-de-vie
par chromatographie gaz-liquide-solide. Trav. chim. Aliment.
Hyg., 69: 423-430.
10. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1974)
Perturbations du métabolisme hépatique du palmitate [1-14C]
déterminée par l'administration de n-propanol chez le rat.
Biochimie, 56: 1157-1159.
11. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1979)
Comparative effects of ethanol, n-propanol and isopropanol on
lipid disposal by rat liver. Chem.-biol. Interact., 26:
155-166.
12. BENGTSSON, B.-E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13
aliphatic alcohols. Chemosphere, 13: 613-622.
13. BILZER, N. & GRUNER, O. (1983) [Critical assessment regarding
determination of aliphatic alcohols (congeners in alcoholic
drinks) in blood with the aid of head-space analysis.]
Blutalkohol, 20: 411-421 (in German).
14. BILZER, N. & PENNERS, B.-M. (1985) [Concerning the velocity
of reduction and excretion of the attendant substance
propanol-1 and isobutanol after drinking whisky of the trade
mark Chivas Regal.] Blutalkohol, 22: 140-145 (in German).
15. BILZER, N., PENNERS, B.-M., & GRUNER, O. (1985) [Studies
about the course of concentration in blood for congener
propanol-1 and isobutanol after drinking overseas rum
("Captain Morgan").] Blutalkohol, 22: 146-151 (in German).
16. BONTE, W. (1978) [Congener content of wine and similar
beverages.] Blutalkohol, 15: 392-404 (in German).
17. BONTE, W. (1979) [Congener content of German and foreign
beers.] Blutalkohol, 16: 108-124 (in German).
18. BONTE, W., DECKER, J., & BUSSE, J. (1978) [Congener content
of highproof alcoholic beverages.] Blutalkohol, 15: 323-338
(in German).
19. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981a) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human blood.]
Blutalkohol, 18: 399-411 (in German).
20. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human urine.]
Blutalkohol, 18: 412-426 (in German).
21. BONTE, W., STOPPELMAN, G., RUDELL, E., & SPRUNG, R. (1981c)
[Computerized detection of congeners of alcoholic beverages
in body fluids.] Blutalkohol, 18: 303-310 (in German).
22. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
23. BRASS, E.P. & BEYERINCK, R.A. (1987) Interactions of
propionate and carnitine metabolism in isolated hepatocytes.
Metabolism, 36: 781-787.
24. BRASS, E.P., FENNESSEY, P.V. & MILLER, L.V. (1986) Inhibition
of oxidative metabolism by propionic acid and its reversal by
carnitine in isolated rat hepatocytes. Biochem. J., 236:
131-136.
25. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
26. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
27. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.]
Z. Wasser-Abwasser Forsch., 10: 87-98 (in German).
28. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
29. BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.]
Z. Wasser-Abwasser Forsch., 13: 170-173 (in German).
30. BURROWS, W.D. & ROWE, R.S. (1975) Ether soluble constituents
of landfill leachate. J. Water Pollut. Control Fed., 47:
921-923.
31. CAMERON, A.M., ZAHLSEN, K., HAUG, E., NILSEN, O.G., & EIK-
NES, K.B. (1985) Circulating steroids in male rats following
inhalation of n-alcohols. Arch. Toxicol., Suppl., 8: 422-424.
32. CAMPBELL, I.M., MCLAUGHLIN, D.G., & HANDY, B.J. (1976) Rate
constants for reactions of hydroxyl radicals with alcohol
vapours at 292 K. Chem. Phys. Lett., 38: 362-364.
33. CANTON, J.H. & ADEMA, D.M.M. (1978) Reproducibility of short-
term and reproduction toxicity experiments with Daphnia magna
and comparison of the sensitivity of Daphnia magna with
Daphnia pulex and Daphnia cucullata in short-term experiments.
Hydrobiologia, 59: 135-140.
34. CARTER, W.P.L., DARNALL, K.R. GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4-hydroxy radicals
with oxygen. J. phys. Chem., 83: 2305-2311.
35. CEC (1982) Propan-1-ol chemico-physical data, toxicity data,
environmental occurrence, and permissible levels. In: Report
of the Scientific Committee for Food on extraction solvents,
Brussels, Commission of the European Communities, Directorate
General for Internal Market and Industrial Affairs, pp. 27-45.
36. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination: interaction
with phytochrome. Physiol. Plant, 65: 451-454.
37. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese hamster
V79 cells by various organic solvents and simple compounds.
Cell Biol. Toxicol., 1: 155-171.
38. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation
and degradation of petrochemical wastewater components by
methane fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
39. CHUNG, T.-Y., HAYASE, F., & KATO, H. (1983) Volatile
components of ripe tomatoes and their juices, purees and
pastes. Agric. biol. Chem., 47: 343-351.
40. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962) Influence
of alcohols and potassium salts of xanthogenic acids on
various biological objects. Arch. int. Pharmacodyn. Ther.,
135: 330-343.
41. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol.,
9: 383-397.
42. CORBIT, T.E. & ENGEN, T. (1971) Facilitation of olfactory
detection. Perception Psychophysiol., 10: 433-436.
43. CORKEY, B.E., HALE, D.E., GLENNON, M.C., KELLEY, R.I.,
COATES, P.M. KILPATRIK, L. & STANLEY, C.A. (1988)
Relationship between unusual hepatic acyl coenzyme A profiles
and the pathogenesis of Reye syndrome. J. clin. Invest.
82: 782-788.
44. CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No. 600/
3-80-084, PB 80-221948).
45. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100:
34-46.
46. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species:
D. melanogaster and D. simulans. Comp. Biochem. Physiol.,
54C: 71-74.
47. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
48. DE ZWART, D. & SLOOFF, W. (1983) The Microtox as an
alternative assay in the acute toxicity assessment of water
pollutants. Aquat. Toxicol., 4: 129-138.
49. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity of
selected synthetic organic chemicals, The MITRE Corporation
(MITRE Technical Report MTR-7248, Rev. 1, Appendix III).
50. DRAVNIEKS, A. (1974) A building-block model for the
characterization of odorant molecules and their odors. Ann.
N.Y. Acad. Sci., 237: 144-163.
51. DRY, I., WALLACE, W., & NICHOLAS, D.J.D. (1981) Role of ATP
in nitrite reduction in roots of wheat and pea. Planta,
152: 234-238.
52. DURWALD, W. & DEGEN, W. (1956) [A fatal poisoning by n-propyl
alcohol.] Arch. Toxikol., 16: 84-88 (in German).
53. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
54. EICEMAN, G.A. & KARASEK, F.W. (1981) Identification of
residual organic compounds in food packages. J. Chromatogr.,
210: 93-103.
55. FANG, H.H.P. & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
56. FAO/WHO (1980) Toxicological evaluation of certain food
additives. Report of the Joint FAO/WHO Expert Committee on
Food Additives, Geneva, World Health Organization,
pp. 162-168 (WHO Food Additive Series 16).
57. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. geotech. J., 22: 205-214.
58. FLATH, R.A., ALTIERI, M.A., & MON, T.R. (1984) Volatile
constituents of Amaranthus retroflexus L. J. agric. food
Chem., 32: 92-94.
59. FLICK, E.W. (1985) Industrial solvents handbook. New Jersey,
Noyes Data Corp., pp. 220-223.
60. FORSANDER, O.A. (1967) Influence of some aliphatic alcohols
on the metabolism of rat liver slices. Biochem. J., 105:
93-97.
61. GAD, S.C., DUNN ,B.J., DOBBS, D.W., REILLY, C., & WALSH, R.D.
(1986) Development and validation of an alternative dermal
sensitization test: the mouse ear swelling test (MEST).
Toxicol. appl. Pharmacol., 84: 93-114.
62. GAGE, P.W. (1965) The effect of methyl, ethyl and n-propyl
alcohol on neuromuscular transmission in the rat.
J. Pharmacol. exp. Ther., 150: 236-243.
63. GAILLARD, D. & DERACHE, R. (1966) Action de quelques alcools
aliphatiques sur la mobilisation de différentes fractions
lipidiques chez le rat. Food Cosmet. Toxicol., 4: 515-520.
64. GELSOMINI, N. (1985) Head-space analysis with capillary
columns in quality control of wines. In: Proceedings of the
6th International Symposium on Capillary Chromatography,
pp. 515-519.
65. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
66. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants
in the oxidation of aliphatic compounds by activated sludge.
J. Water Pollut. Control Fed., 38: 562-579.
67. GIBEL, W., LOHS, K., & WILDNER, G.P. (1975) [Experimental
study on the cancerogenic activity of propanol-1, 2-methyl-
propanol-1 and 3-methylbutanol. I.] Arch. Geschwulstforsch.,
45: 19-24 (in German).
68. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal
of a chemical waste problem by fish toxicity tests. Sewage
ind. Waste, 24: 1397-1401.
69. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut. Control
Fed., 46: 947-965.
70. GLASGOW, A.M. & CHASE, H.P. (1976) Effect of propionic acid
on fatty acid oxidation and ureagenesis. Pediatr. Res.,
10: 683-686.
71. GOODMAN, D.E. & RAO, R.M. (1984) GC determination of fusel
alcohols in distilled alcoholic beverages. Am. Lab., 16:
100-103.
72. GORESKY, C.A., GORDON, E.R., & BACH, G.G. (1983) Uptake of
monohydric alcohols by liver: demonstration of a shared
enzymic space. Am. J. Physiol., 244: G198-G214.
73. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
74. GRANT, K.A. & SAMSON, H.H. (1984) n-Propanol induced
microcephaly in the neonatal rat. Neurobehav. Toxicol.
Teratol., 6: 165-169.
75. GRAY, V.M. & CRESSWELL, C.F. (1983) The effect of respiratory
inhibitors and alcohols on nitrate utilization and nitrite
accumulation in excised roots of Z. mays L. Z. Pflanzenphysiol.,
112: 207-214.
76. GREGERSEN, N. (1979) Studies on the effects of saturated and
unsaturated short-chain monocarboxylic acids on the energy
metabolism of rat liver mitochondria. Pediat. Res., 14:
1227-1230.
77. GREGERSEN, N. (1981) The specific inhibition of the pyruvate
dehydrogenase complex from pig kidney by propionyl-CoA and
isovaleryl-CoA. Biochem. Med. III, 26: 20-27.
78. GULATI, A., NATH, C., SHANKER, K., SRIMAL, R.C., DHAWAN, K.N.,
& BHARGAVA, K.P. (1985) Effects of alcohols on the
permeability of blood-brain barrier. Pharmacol. Res. Commun.,
17: 85-93.
79. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
80. HANSCH, C. & ANDERSON, S. (1967) The effect of intramolecular
hydrophobic bonding on partition coefficients. J. org. Chem.,
32: 2583-2586.
81. HARRIS, R.A. (1983) Ethanol, membrane perturbation, and
synaptosomal ion transport. Proc. West. Pharmacol. Soc.,
26: 255-257.
82. HAWLEY, G.G. (1981) Condensed chemical dictionary, 10th ed.,
Melbourne, Van Nostrand Reinhold Company Inc.
83. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
84. HERMENS, J., BUSSER, F., LEEUWANGH, P., & MUSCH, A. (1985)
Quantitative structure-activity relationships and mixture
toxicity of organic chemicals in Photobacterium phosphoreum:
the Microtox 85 test. Ecotoxicol. environ. Saf., 9: 17-25.
85. HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974)
Effects of chronic ingestion of some lower aliphatic alcohols
in rats. Res. Commun. chem. Pathol. Pharmacol., 9: 177-180.
86. HILSCHER, H., GEISSLER, E., LOHS, K., & GIBEL, W. (1969)
[Studies on the toxicity and mutagenicity of single fusel oil
components on E. coli.] Acta biol. med. Germ., 23: 843-852
(in German).
87. HIRANO, K., WATANABE, Y., ADACHI, T., ITO, Y., & SUGIURA, M.
(1985) Drug-binding properties of human alpha-foetoprotein.
Biochem J., 231: 189-191.
88. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509
(in German).
89. HODGE, H.C. & STERNER, J.H. (1943) Am. ind. Hyg. Assoc. Q.,
10: 93.
90. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
91. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
161 pp.
92. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
93. HUANG, R.-D, SMITH, M.F., & ZAHLER, W.L. (1982) Inhibition of
Forskolin-activated adenylate cyclase by ethanol and other
solvents. J. cyclic Nucleotide Res., 8: 385-394.
94. HUDOLEI, V.V., MIZGIREV, I.V., & PLISS, G.B. (1987)
[Evaluation of mutagenic activity of carcinogens and other
chemical agents with Salmonella typhimurium assays.] Vopr.
Onkol., 32: 73-80 (in Russian).
95. IRPTC (1987) Data profile on n-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
96. IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ,
I.D., & BRADSHAW, J.J. (1978) Organic compounds. Their
interaction with and degradation of hepatic microsomal drug-
metabolizing enzymes in vitro. Drug Metab. Dispos., 6:
218-225.
97. JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavour volatiles in heat-treated milks.
J. dairy Res., 45: 391-403.
98. JOUANY, J.-P. (1982) Volatile fatty acid and alcohol
determination in digestive contents, silage juices, bacterial
cultures and anaerobic fermentor contents. Sci. Aliment.,
2: 131-144.
99. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
100. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J.,
53: 129-136.
101. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents.
Am. Ind. Hyg. Assoc. J., 41: 451-455.
102. KHARE, M. & DONDERO, N.C. (1977) Fractionation and
concentration from water of volatiles and organics on high
vacuum system: examination of sanitary landfill leachate.
Environ. Sci. Technol., 11: 814-819.
103. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted filberts.
J. agric. food Chem., 20: 1021-1028.
104. KIRK, R.E & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
105. KLECKA, G.M., LANDI, L.P., & BODNER, K.M. (1985) Evaluation
of the OECD activated sludge, respiration inhibition test.
Chemosphere, 14: 1239-1251.
106. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
107. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegypti
larvae. J. Invertebr. Pathol., 42: 285-287.
108. KRISTIANSEN, U., HANSEN, L. NIELSEN, G.A., & HOLST, E. (1986)
Sensory irritation and pulmonary irritation of cumene and
n-propanol: Mechanisms of receptor activation and
desensitization. Acta pharmacol. toxicol., 59: 60-72.
109. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
110. KUHNHOLZ, B. (1985) [Reflections concerning the analysis of
free aliphatic alcohols in tissue.] Blutalkohol, 22: 455-461
(in German).
111. LAING, D.G. (1975) A comparative study of the olfactory
sensitivity of humans and rats. Chem. Senses Flavor, 1:
257-269.
112. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
113. LASNE, C., GU, Z.W., VENEGAS, W., & CHOUROULINKOV, I. (1984)
The in vitro micronucleus assay for detection of cytogenetic
effects induced by mutagen-carcinogens: comparison with the
in vitro sister-chromatid exchange assay. Mutat. Res.,
130: 273-282.
114. LIEBICH, H.M., BUELOW, H.J., & KALLMAYER, R. (1982)
Quantification of endogenous aliphatic alcohols in serum and
urine. J. Chromatogr., 239: 343-349.
115. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermatit., 3: 240-244.
116. LUTHIN, G.R. & TABAKOFF, B. (1984) Activation of adenylate
cyclase by alcohols requires the nucleotide-binding protein.
J. Pharmacol. exp. Ther., 228: 579-587.
117. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
118. MAICKEL, R.P. & NASH, J.F. (1985) Differing effects of
short-chain alcohols on body temperature and coordinated
muscular activity in mice. Neuropharmacology, 24: 83-89.
119. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology,
17: 451-461.
120. MANKES, R.F., LEFEVRE, R., RENAK,V., FIESHER, J., & ABRAHAM,
R. (1985) Reproductive effects of some solvent alcohols with
differing partition coefficients. Teratology, 31: 67A.
121. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
122. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft,
26: 385-389 (in German).
123. MAY, W.A., PETERSON, R.J., & CHANG, S.S. (1983) Chemical
reactions involved in the deep-fat frying of foods. IX.
Identification of the volatile decomposition products.
J. Am. Oil Chem. Soc., 60: 990-995.
124. MICHAELIS, M.L. & MICHAELIS, E.K. (1983) Alcohol and local
anesthetic effects on Na+-dependent Ca2+-fluxes in brain
synaptic membrane vesicles. Biochem. Pharmacol., 32:
963-969.
125. MIKHEEV, M.J., & FROLOVA, A.D. (1978) [Toxicokinetics of
certain representatives of a homologous series of alcohols.]
Gig. i Sanit., 6:33-36 (in Russian).
126. MORGAN, E.T., KOOP, D.R., & COON, M.J. (1982) Catalytic
activity of cytochrome P-450 isozyme 3a isolated from liver
microsomes of ethanol-treated rabbits. J. biol. Chem.,
257: 13951-13957.
127. MOSHONAS, M.G. & SHAW, P.E. (1987) Quantitative analysis of
orange juice flavor volatiles by direct-injection gas
chromatography. J. Agric. food Chem., 35:161-165.
129. MULLIN, M.J. & HUNT, W.A. (1985) Actions of ethanol on
voltage-sensitive sodium channels: effects on neurotoxin-
stimulated sodium uptake in synaptosomes. J. Pharmacol. exp.
Ther., 232: 413-419.
130. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
131. MURPHREE, H.B., GREENBERG, L.A., & CARROLL, R.B. (1967)
Neuropharmacological effects of substances other than ethanol
in alcoholic beverages. Fed. Proc., 26: 1468-1473.
132. NELSON, B.K., BRIGHTWELL, W.S., & BURG, J.R. (1985)
Comparison of behavioural teratogenic effects of ethanol and
n-propanol administered by inhalation to rats. Neurobehav.
Toxicol Teratol., 7: 770-783.
132. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W. & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentration to rats. Food Chem. Toxicol.,
26(3): 247-254.
133. NEY, K.H. (1985) [Flavour of Tilsit cheese.] Fette Seifen
Anstrichmittel, 87: 289-293 (in German).
134. NGUYEN, V.C. & KATO, H. (1982) Volatile flavor components of
Kumazasa (Sasa albo-marginata). Agric. Biol. Chem., 46:
2795-2801.
135. NUNOMURA, N., SASAKI, M., & YOKOTSUKA, T. (1984) Shoyu
(soy sauce) flavor components: neutral fraction. Agric. Biol.
Chem., 48: 1753-1762.
136. OBE, G. & RISTOW, H. (1977) Acetaldehyde, but not ethanol,
induces sister chromatid exchanges in Chinese hamster cells
in vitro. Mutat. Res., 56: 211-213.
137. ODKVIST, L.M., LARSBY, B., THAM, R., & ASCHAN, G. (1979) On
the mechanism of vestibular disturbances caused by industrial
solvents. Adv. Oto-Rhino-Laryng., 25: 167-172.
138. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub. Reinhalt. Luft, 32: 400-407 (in German).
139. OERSKOV, S.I. (1950) Experiments on the oxydation of propyl
alcohol in rabbits. Acta physiol. Scand., 20: 258-262.
140. OTSUK141.A, K., IKI, I., & YAMASHITA, T. (1979) [Relationship
between type of whisky and volatile component.] Hakkokogaku,
57: 20-30 (in Japanese).
141. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. 4. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 K. J. phys. Chem., 82: 1329-1333.
142. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
143. PATERNOSTRE, M., PICHON, Y., & DUPEYRAT, M. (1983) Effects
of n-alcohols on ionic transmembrane currents in the squid
giant axon. Stud. phys. thero. Chem., 24: 515-522.
144. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
145. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols
of the stimulated activity of ornithine decarboxylase and
tyrosine aminotransferase occurring in regenerating rat liver.
Biochem. Pharmacol., 29: 2799-2803.
146. POSTEL, W. & ADAM, L. (1978) [Gas chromatographic
characterization of whisky. III. Communication: Irish
whiskey.] Branntweinwirtsch., 118: 404-407 (in German).
147. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
148. PREUSS, A. & ZIPFEL, K. (1985) [Headspace-gas chromatographic
identification of alcoholic beverages and foodstuffs.]
Lebensmittelchem. gerichtl. Chem., 39: 97-99 (in German).
149. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
150. PRIESTLEY, D.A. & LEOPOLD, A.C. (1980) Alcohol stress on
soya bean seeds. Ann. Bot., 45: 39-45.
151. PUNTER, P.H. (1983) Measurement of human olfactory thresholds
for several groups of structurally related compounds. Chem.
Senses, 7: 215-235.
152. PURCHASE, I.F.H. (1969) Studies in kaffircorn malting and
brewing. XXII. The acute toxicity of some fusel oils found in
Bantu beer. S.A. med. J., 43: 795-798.
153. QUISTAD, G.B., STAIGER, L.E. & SCHOOLEY, D.A. (1986) The
role of carnitine in the conjugation of acidic xenobiotics.
Drug Metab. Dispos., 14:521-525.
154. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
155. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. membr. Biol., 84: 229-238.
156. REYNOLDS, T. (1977) Comparative effects of aliphatic
compounds on inhibition of lettuce fruit germination. Ann.
Bot., 41: 637-648.
157. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch., 21:
1309-1319 (in German).
158. ROE, C.R., MILLINGTON, D.S., MALTBY, D.A., BOHAN, T.P. &
HOPPEL, C.L. (1984) L-Carnitine enhances excretion of
propionyl coenzyme A as propionylcarnitine in propionic
acidemia. J. clin. Invest., 73: 1785-1788.
159. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
160. RYAN, D.E., THOMAS, P.E., WRIGHTON, S.A., GUZELIAN, P.S. &
LEVIN, W. (1987) Ethanol-inducible rat and human liver
cytochrome P-450: characterization and role as high affinity
N-nitrosodimethylamine demethylamine demethylases. In:
Miners, J., Birkett, D.J., Drew, R., May, B., & McManus, ed.
Microsomes and drug oxidations, London, Taylor & Francis,
pp. 3-11.
161. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
162. SAHU, B.R. & TANDON, U. (1983) A novel method for spot test
detection of alcohols. J. Indian Chem. Soc., 60: 615-616.
163. SANCEDA, N., KURATA, T., & ARAKAWA, N. (1984) Fractionation
and identification of volatile compounds in Patis, a
Phillipine fish sauce. Agric. Biol. Chem., 48: 3047-3052.
164. SAVINI, E.C. (1968) Estimation of the LD50 in mol/kg. Proc.
Eur. Soc. Study Drug Toxicol., 9: 276-278.
165. SCHEIMAN, M.A., SAUNDERS, R.A., & SAALFELD, F.E. (1974)
Organic contaminants in the District of Columbia water supply.
Biomed. Mass Spectrom., 1: 209-211.
166. SCOTT, T. & EAGLESON, M. (1983) Concise encyclopedia of
biochemistry. New York, W. de Gruyter, pp. 158-159.
167. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
168. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona Heilborn).
J. agric. food Chem., 33: 795-797.
169. SINCLAIR, J.F., SMITH, L., BEMENT, W.J., SINCLAIR, P.R., &
BONKOWSKY, H.L. (1982) Increases in cytochrome P-450 in
cultured hepatocytes mediated by 3- and 4-carbon alcohols.
Biochem. Pharmacol., 31: 2811-2815.
170. SLOOFF, W. (1983) Benthic macroinvertebrates and water
quality assessment: some toxicological considerations. Aquat.
Toxicol., 4 : 73-82.
171. SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the
usefulness of the Mexican Axolotl (Ambystoma mexicanum) and
the clawed toad (Xenopus laevis) in toxicological bioassays.
Bull. environ. Contam. Toxicol., 24: 439-443.
172. SLOOFF, W., CANTON, J.H., & HERMENS, J.L.M. (1983) Comparison
of the susceptibility of 22 freshwater species to 15 chemical
compounds. I. (Sub)acute toxicity tests. Aquat. Toxicol.,
4: 113-128.
173. SMYTH, H.F., CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data. Arch. ind. Hyg. occup.
Med., 10: 61-68.
174. SNIDER, J.B. & DAWSON, G.A. (1985) Tropospheric light
alcohols, carbonyls, and acetonitrile: concentrations in the
southwestern United States and Henry's law data. J. geophys.
Res., 90: 3797-3805.
175. SOLIMAN, M.A., EL SAWY, A.A., FADEL, H.M., OSMAN, F., & GAD,
A.M. (1985) Volatile components of roasted Citrillus
colocynthis, var. Colocynthoides. Agric. Biol. Chem.,
49: 269-275.
176. SRI (1984) n-Propanol: record number 0115 (last revision
date 01-06-84). In: Toxicology data bank, Palo Alto,
California, Stanford Research Institute, Bethesda, Maryland,
National Library of Medicine.
177. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
178. STOCK, K. & SCHMIDT, M. (1978) Effects of short-chain
alcohols on adenylate cyclase in plasma membranes of rat
adipocytes. Naunyn-Schmiedeberg Arch. Pharmacol., 302: 37-43.
179. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials: second cumulative
series. Perfumer Flavorist, 9: 53-83.
180. STOKES, J.A. & HARRIS, R.A. (1982) Alcohols and synaptosomal
calcium transport. Mol. Pharmacol., 22: 99-104.
181. STOLZENBERG, S.J. & HINE, C.H. (1979) Mutagenicity of
halogenated and oxygenated three-carbon compounds. J. Toxicol.
environ. Health, 5: 1149-1158.
182. STONE, H., PRYOR, G.T., & STEINMETZ, G. (1972) A comparison
of olfactory adaptation among seven odorants and their
relationship with several physico-chemical properties.
Perception Psychophys., 12: 501-504.
183. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
184. STUMPF, D.A., MCAFEE, J., PARKS, J.K., & EGUREN, L. (1986)
Propionate inhibition of succinate: CoA ligase (GDP) and the
citric acid cycle in mitochondria. Pediatr. Res., 14:
1127-1131.
185. TABAKOFF, B. & HOFFMAN, P.L. (1983) Alcohol interactions with
brain opiate receptors. Life Sci., 32: 197-204.
186. TANDOI, P., GUIDOTTI, M., & STACCHINI, P. (1984) [On the
composition of brandies.] Riv. Soc. Ital. Sci. Aliment.,
13: 69-76 (in Italian).
187. TAYLOR, J.M., JENNER, P.M., & JONES, W.I. (1964) A comparison
of the toxicity of some allyl, propenyl, and propyl compounds
in the rat. Toxicol. appl. Pharmacol., 6: 378-387.
188. TESCHKE, R., HASUMURA, Y., & LIEBER, C.S. (1975) Hepatic
microsomal alcohol-oxidizing system. Affinity for methanol,
ethanol, propanol, and butanol. J. biol. Chem., 250:
7397-7403.
189. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
190. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
191. TOMERA, J.F., SKIPPER, P.L., WISHNOK, J.S., TANNEBAUM, S.R.,
& BRUNENGRABER, H. (1984) Inhibition of N-nitroso-
dimethylamine metabolism by ethanol and other inhibitors in
the isolated perfused rat liver. Carcinogenesis, 5: 113-116.
192. UHLEMANN, E.R., ROBBERECHT, P., & GARDNER, J.D. (1979)
Effects of alcohols on the actions of VIP and secretin on
acinar cells from guinea-pig pancreas. Gastroenterology,
76: 917-925.
193. UNRUH, J.D. & SPINICELLI, L. (1981) Propyl alcohols, n-propyl
alcohol. In: Kirk, R.E. & Othmer, D.F., ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience,
Vol. 19, pp. 221-227, 194.
194. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
195. US NIOSH (1984) Method 1401. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1,
pp. 1401-1-1401-4.
196. VAISHNAV, D.D. & LOPAS, D.M. (1985) Relationship between
lipophilicity and biodegradation inhibition of selected
industrial chemicals. Dev. ind. Microbiol., 26: 557-565.
197. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
198. VERSCHUEREN, K. (1983) Handbook of environmental data on
organic chemicals, 2nd ed., Melbourne, Van Nostrand Reinhold
Company Inc.
199. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
200. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER, B.,
& BASLER, A. (1987) Genotoxicity of three carbon compounds
evaluated in the SCE test in vitro. Environ. Mutagen.,
9: 401-410.
201. WACHTEL, R.E. (1984) Aliphatic alcohols increase the decay
rate of glutamate-activated currents at the crayfish
neuromuscular junction. Br. J. Pharmacol., 83: 393-397.
202. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265.
203. WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
IIJIMA, M. (1984) Induction of megamitochondria in the rat
liver by n-propyl alcohol and n-butyl alcohol. Acta pathol.
Jpn., 34: 471-480.
204. WALLGREN, H. (1960) Relative intoxicating effects on rats
of ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
205. WATERER, D.R. & PRITCHARD, M.K. (1985a) Volatile production
by wounded Russet Burbank and Norland potatoes. Sci. Aliments,
5: 205-216.
206. WATERER, D.R. & PRITCHARD, M.K. (1985b) Production of
volatile metabolites in potatoes infected by Erwinia
carotovora var. carotovora and E. carotovora var. atroseptica.
Can. J. plant Pathol., 7: 47-51.
207. WILKIN, J.K. & FORTNER, G. (1985) Cutaneous vascular
sensitivity to lower aliphatic alcohols and aldehydes in
orientals. Alcoholism clin. exp. Res., 9: 522-525.
208. WINTERSTEIGER, R., GAMSE, G., & PACHA, W. (1982)
[Quantification of alcoholic compounds and amines with 4-(6-
methylbenzo-thiazol-2-yl)phenyl isocyanate. Fresenius Z.
anal. Chem., 312: 455-461.
209. WOLF, M., URBAN, R., WELLER, J.-P., & TROGER, H.D. (1985)
[The analysis of congeners of alcoholic beverages in blood.
First communication: the application of capillary gas
chromatography in micro head-space analysis.] Blutalkohol,
22: 321-332 (in German).
210. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
211. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., UCHIDA,
H., & HAYASHI, K. (1983) Volatile flavor compounds of boiled
buckwheat flour. Agric. Biol. Chem., 47: 729-738.
212. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., &
HAYASHI, K. (1984) Volatile flavor components of Kogyoku
apples. Agric. Biol. Chem., 48: 849-855.
213. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
214. YASUHARA, A. & FUWA, K. (1982) Characterization of odorous
compounds in rotten blue-green algae. Agric. Biol. Chem.,
46: 1761-1766.
215. YASUHARA, A., FUWA, K., & JIMBU, M. (1984) Identification of
odorous compounds in fresh and rotten swine manure. Agric.
Biol. Chem., 48: 3001-3010.
216. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Manag. Res., 1: 213-226.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-1 est un liquide incolore, très inflammable, qui
est volatil à la température ambiante et sous la pression
atmosphérique normale. Il est miscible à l'eau et aux solvants
organiques. Parmi les méthodes d'analyse, on peut citer la
chromatographie en phase gazeuse, qui permet de déceler jusqu'à
5 x 10-5 mg/m3 dans l'air, 1 x 10-4 mg/litre dans l'eau et
0,002 mg/litre dans le sang, le sérum ou les urines lorsque
l'échantillon a été extrait ou concentré de façon satisfaisante.
2. Sources d'exposition humaine et environnementale
La capacité de production mondiale a dépassé 130 000 tonnes en
1979. Le propanol-1 d'origine naturelle résulte de la
décomposition de matériaux organiques par divers microorganismes et
on le rencontre également dans les végétaux et le mazout.
Industriellement, on le produit par réaction de l'éthylène sur
l'oxyde de carbone et l'hydrogène qui donne du propionaldéhyde,
celui-ci étant ensuite hydrogéné en propanol. C'est également un
sous-produit de la fabrication du méthanol et il peut être obtenu
directement à partir du propane ou de l'acroléine. Le propanol-1
est essentiellement un solvant à tout faire, à usage industriel ou
domestique. Il entre dans la composition des encres d'imprimerie
flexographiques et il est également utilisé dans l'industrie
textile, dans des produits à usage personnel tels que les produits
cosmétiques, les lotions ainsi que dans les produits pour le
nettoyage des vitres, les encaustiques et les antiseptiques. En
second lieu par ordre d'importance, on peut citer son utilisation
comme produit intermédiaire dans la fabrication de divers composés
chimiques.
3. Transport, distribution et transformation dans l'environnement
La principale voie de pénétration de propanol-1 dans
l'environnement est constituée par les émissions dans l'atmosphère
qui se produisent au cours de la production, de la transformation,
du stockage, du transport et de l'utilisation de ce composé ou du
rejet de déchets qui en contiennent. Il peut y avoir également des
décharges dans l'eau et le sol. Le propanol-1 étant principalement
utilisé comme solvant volatil, il finit par se dissiper en grande
partie dans l'environnement.
Le propanol-1 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et par les précipitations. Il
est facilement biodégradable tant par voie aérobie que par voie
anaérobie et du fait de l'existence de ces mécanismes d'élimination
chimique et biologique, on ne le rencontre normalement pas en
quantité mesurable dans l'environnement. Toutefois on en a décelé
la présence dans l'air des agglomérations urbaines, dans des
décharges, ainsi que dans les eaux s'échappant de zones
d'enfouissement de déchets.
Le logarithme du coefficient de partage n-octanol/eau du
propanol-1 est de 0,34 et son facteur de bioconcentration a une
valeur de 0,7, ce qui en rend la bioaccumulation très improbable.
4. Niveau dans l'environnement et exposition humaine
La population en général peut être exposée au propanol par
suite d'une ingestion accidentelle, par inhalation lors de
l'utilisation du produit ou par absorption avec la nourriture
(propanol d'origine naturelle, additif d'aromatisation ou reste de
solvant) ou des boissons alcoolisées ou non. Par exemple la bière
en contient jusqu'à 195 mg/litre, le vin jusqu'à 116 mg/litre et
certains spiritueux jusqu'à 3500 mg/litre. L'exposition de la
population par suite d'inhalation ou de la consommation d'eau de
boisson est faible (aux Etats-Unis la concentration moyenne dans
des échantillons d'air urbain se situait à 0,00005 mg/m3 et dans
des échantillons d'eau de boisson, à 0,001 mg/litre). Les
travailleurs courent un risque d'exposition par inhalation lors de
la production, de la transformation et de l'utilisation du produit.
Toutefois, on ne dispose d'aucune donnée qui permettrait de
chiffrer ce type d'exposition.
5. Cinétique et métabolisme
Après ingestion, le propanol-1 est rapidement absorbé et
distribué dans l'ensemble de l'organisme. On manque de données sur
la vitesse d'absorption après inhalation et exposition cutanée. Le
propanol-1 est métabolisé par l'alcool déshydrogénase (ADH) en
aldéhyde puis acide propionique et peut entrer dans le cycle de
Krebs. Cette oxydation constitue l'étape limitante du métabolisme
du propanol-1. In vitro, les oxydases microsomiques du rat et du
lapin sont également capables d'oxyder le propanol-1 en aldéhyde
propionique. L'ADH et des systèmes d'oxydation microsomiques ont
une affinité beaucoup plus importante pour le propanol-1 que pour
l'éthanol; aussi le propanol-1 est-il rapidement éliminé de
l'organisme. Chez le rat, sa demi-vie après administration par
voie orale d'une dose de 1000 mg/kg est de 45 minutes.
Chez l'animal et chez l'homme, le propanol-1 peut être éliminé
de l'organisme dans l'air expiré ou dans les urines. Chez des
êtres humains ayant reçu par voie orale une dose de 3,75 mg de
propanol-1 par kg de poids corporel et de 1200 mg d'éthanol par kg
de poids corporel, l'excrétion urinaire totale du propanol-1 a été
de 2,1 % de la dose. Les concentrations urinaires de propanol-1
étaient d'autant plus basses que la quantité d'éthanol ingérée
simultanément était basse, ce qui montre qu'il y avait compétition
pour l'ADH entre le propanol-1 et la surdose d'éthanol.
6. Effets sur les êtres vivant dans leur milieu naturel
Aux concentrations normalement présentes dans l'environnement,
le propanol-1 n'est pas toxique pour la vie aquatique, les insectes
ou les végétaux. Chez trois des espèces aquatiques les plus
sensibles (trois protozoaires), le seuil l'inhibition de la
multiplication cellulaire se situait entre 38 et 568 mg/litre.
Dans le cas d'organismes plus évolués, la concentration létale
était d'environ 5000 mg/litre, avec des variations remarquablement
faibles d'un phylum à l'autre et une courbe dose-réponse de très
forte pente. Certaines bactéries et micro-organismes qui vivent
dans les eaux résiduaires et les boues activées sont capables de
s'adapter à des concentrations supérieures à 17 000 mg/litre.
Le propanol-1 peut inhiber ou au contraire stimuler la
germination des semences selon sa concentration dans l'eau
d'arrosage et les conditions d'exposition. Le composé accroît
l'accumulation de nitrites dans le maïs, les pois et le froment.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in vitro
Le propanol-1 présente une faible toxicité aiguë pour les
mammifères (mesurée d'après la mortalité), que l'exposition se
fasse par voie percutanée, orale ou respiratoire. On a fait état
de valeurs allant de 1870 à 6800 mg par kg de poids corporel pour
la DL50 par voie orale chez plusieurs espèces animales. Toutefois
pour de très jeunes rats, on donne une DL50 orale de 560 à 660 mg
par kg de poids corporel. Après une seule exposition, le principal
effet toxique du propanol-1 consiste dans la dépression du système
nerveux central. Selon les données disponibles, le propanol-1
exercerait sur le système nerveux central des effets analogues à
ceux de l'éthanol; toutefois il semblerait que la neurotoxicité du
propanol-1 soit plus importante. Les DE50 pour l'anesthésie chez
le lapin et la perte du réflexe de redressement chez la souris se
situaient respectivement à 1440 mg par kg de poids corporel par
voie orale et à 1478 mg par kg de poids corporel par voie intra-
péritonéale; ces doses sont environ quatre fois plus faibles que
dans le cas de l'éthanol. Dans l'épreuve du plan incliné, le
propanol-1 s'est révélé 2,5 fois plus actif que l'éthanol chez le
rat.
Des doses uniques de 3000 ou 6000 mg par kg de poids corporel
administrées par voie orale à des rats ont provoqué une
accumulation réversible de triglycérides dans le foie. Les vapeurs
fortement concentrées provoquent une irritation des voies
respiratoires chez la souris. Aux concentrations d'environ 30 000
mg/m3 on note une réduction de 50 % du rythme respiratoire chez la
souris.
On ne dispose de données ni sur l'irritation oculaire ni sur
l'irritation cutanée. Aucun effet n'a été observé lors d'une
épreuve de sensibilisation cutanée sur des souris CF1.
Chez des rats mâles exposés pendant six semaines à une dose de
15 220 mg/m3 de propanol-1, on a relevé quelques signes d'une
action nocive possible sur la fonction de reproduction. En
revanche aucun effet n'a été noté à la dose de 8610 mg/m3. Après
exposition de rattes gravides au propanol-1, on a observé des
signes patents de toxicité pour les mères et les foetus aux doses
de 23 968 et 14 893 mg/m3 (9743 et 6054 ppm respectivement); aucun
signe de toxicité n'a été noté à 9001 mg/m3 (3659 ppm). On n'a
observé aucune anomalie comportementale parmi les descendants de
rats mâles exposés pendant six semaines à 8610 ou 15 220 mg de
propanol-1 par m3, ni dans la descendance de rattes exposées
pendant leur gestation aux mêmes concentrations. Toutefois, en
administrant à des ratons de 5 à 8 jours des doses orales de 3000 à
7800 mg de propanol-1 par kg et par jour, on constatait des signes
de dépression du SNC pendant l'administration et un syndrome de
sevrage à la cessation du traitement. Le cerveau de ces rats a été
examiné à l'âge de 18 jours; on a constaté une réduction du poids
de cet organe tant en valeur absolue qu'en valeur relative, avec
diminution de la teneur en ADN et réduction localisée des taux de
cholestérol et de protéine.
La recherche de mutations ponctuelles au moyen de 2 épreuves
utilisant Salmonella typhimurium n'a donné que des résultats
négatifs, de même la recherche de mutations réverses sur
Escherichia coli CA-274. Les résultats ont été également négatifs
en ce qui concerne les échanges entre chromatides soeurs ou la
présence de micro-noyaux dans les cellules mammaliennes in vitro.
Il n'existe pas d' autres données relatives à la mutagénicité.
Lors d'une étude de cancérogénicité portant sur de petits
groupes de rats Wistar exposés tout au long de leur existence par
voie orale à des doses de 240 mg/kg ou par voie sous cutanée à des
doses de 48 mg/kg, on a constaté un accroissement sensible de
l'incidence des sarcomes du foie dans le groupe recevant le produit
par voie sous cutanée. Toutefois cette étude ne permet pas
d'apprécier la cancérogénicité du propanol-1, notamment à cause de
l'absence de détails expérimentaux, du nombre trop restreint
d'animaux et de l'utilisation d'une dose hépatotoxique unique très
élevée.
8. Effets sur la santé humaine
On n'a pas signalé d'effets nocifs sur la santé humaine dans la
population en général ou parmi des groupes professionnels. Le seul
cas d'intoxication mortelle qui ait été signalé est celui d'une
femme, retrouvée inconsciente et qui est décédé 4 à 5 heures après
l'ingestion de propanol. L'autopsie a révélé un oedème cérébral et
pulmonaire. Lors d'une étude sur l'irritation et la sensibilisation
cutanées, on a signalé des réactions allergiques chez un membre du
personnel du laboratoire. Chez neuf volontaires sur 12, on a
observé un érythème qui a duré au moins 1 h après 5 minutes
d'application sur les avant-bras de papiers filtres imprégnés de
0,025 ml d'une solution aqueuse à 75 % de propanol-1. On ne
dispose d'aucun autre rapport concernant d'éventuels effets
toxiques après exposition professionnelle au propanol-1.
Il n'existe pas d'études épidémiologiques qui permettent
d'établir les effets à long terme, et notamment la cancérogénicité,
du propanol-1 chez l'homme.
9. Résumé de l'évaluation
Il peut y avoir exposition humaine au propanol-1 à la suite de
l'absorption de nourriture ou de boissons qui en contiennent. Une
exposition par inhalation peut se produire lors de l'utilisation de
ce produit à des fins ménagères, ou professionnelles, au cours de
la fabrication, de la transformation et de l'utilisation de ce
produit. Les données très limitées dont on dispose sur la teneur
de l'air ambiant et de l'eau en propanol-1 indiquent que ces
teneurs sont très faibles.
Le propanol-1 est rapidement absorbé et se répartit dans tout
l'organisme après ingestion. Après inhalation, l'absorption est
également rapide mais la résorption percutanée devrait être lente.
Chez l'animal, la toxicité aiguë du propanol-1 est faible, que
l'exposition ait lieu par voie percutanée, par voir orale ou par
voie respiratoire. L'exposition de personnes appartenant à la
population générale à des teneurs potentiellement mortelles peut se
produire à la suite d'une ingestion accidentelle ou volontaire.
Toutefois, un seul cas d'intoxication mortelle par le propanol-1 a
été signalé jusqu'ici. Les effets aigus les plus probables chez
l'homme sont une intoxication de type alcoolique pouvant entraîner
une narcose. L'expérimentation animale montre que le propanol-1
est 2 à 4 fois plus toxique que l'éthanol.
Le propanol-1 peut être irritant pour la peau mouillée.
On ne dispose pas de données suffisantes sur la toxicité chez
l'animal pour procéder à une évaluation des risques pour la santé
humaine qui découleraient d'une exposition répétée ou prolongée au
propanol-1. Toutefois, un certain nombre d'études à court terme
sur le rat, bien que limitées, indiquent que dans les conditions où
se produit habituellement l'exposition humaine, il est peu probable
que le risque encouru soit très grave.
Une exposition par inhalation à une concentration de 15 220
mg/m3 a affecté la fonction de reproduction de rats mâles, ce qui
n'a pas été le cas à la dose de 8610 mg/m3. Chez des rattes
gravides, la dose sans effet observable se situait à 9001 mg/3
(3659 ppm); quant à la dose la plus faible donnant lieu à un effet
observable, elle était de 14 893 mg/m3 (6054 ppm), en ce qui
concerne la toxicité pour la mère et le foetus. L'exposition par
inhalation à de fortes concentrations de propanol-1 a donc des
effets nocifs sur la reproduction et le développement des rats
mâles et femelles lorsque les concentrations utilisées sont
manifestement toxiques pour ces animaux. Pour obtenir ces effets,
il a fallu utiliser des concentrations plus élevées que celles
auxquelles l'homme pourrait être normalement exposé.
Différentes épreuves ont montré que le propanol-1 ne provoquait
pas de mutations ponctuelles chez les bactéries. Toutefois, si ces
résultats donnent à penser que le produit n'est pas génotoxique,
les données disponibles sont trop limitées pour qu'on puisse en
évaluer correctement le pouvoir mutagène. La seule étude dont on
possède les résultats ne permet pas d'évaluer convenablement la
cancérogénicité du propanol-1 chez l'animal d'expérience. On ne
dispose d'aucune donnée sur l'exposition à long terme des
populations humaines à ce produit, de sorte qu'on ne peut pas se
prononcer sur son pouvoir cancérogène chez l'homme.
A part un cas d'intoxication mortelle consécutif à l'ingestion
d'un demi-litre de propanol-1, il n'existe pratiquement aucun
rapport concernant d'éventuels effets nocifs découlant d'une
exposition au propanol-1, qu'il s'agisse de la population générale
ou de groupes professionnels. Le Groupe spécial estime qu'il est
improbable que le propanol-1 presente des risques graves pour la
population générale dans les conditions normales d'exposition.
Du propanol-1 peut être libéré dans l'environnement lors de la
production, de la transformation, du stockage, du transport, de
l'utilisation ou de rejet de ce produit. Comme il est
essentiellement utilisé comme solvant volatil, l'essentiel de la
production finit par aboutir dans l'atmosphère. Toutefois, par
réaction avec les radicaux hydroxyles et entraînement par les
précipitations, le propanol-1 est rapidement éliminé de
l'atmosphère, d'où il disparaît en moins de trois jours. Le
propanol-1 s'élimine également rapidement de l'eau et du sol de
sorte que l'on ne le rencontre que rarement en concentrations
mesurables dans l'air, l'eau et la terre. Le propanol-1 est peu
absorbé par les particules du sol où il se révèle mobile et dont il
accroît la perméabilité à certains hydrocarbures aromatiques.
Compte tenu des propriétés physiques du propanol-1, il est peu
probable qu'il donne lieu à une bioaccumulation et sauf accident ou
rejet négligent, le propanol-1 ne présente aucun risque pour la
faune ou la flore aquatique aux concentrations où on le rencontre
habituellement dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 1-propanol es un líquido incoloro y sumamente inflamable,
volátil a temperatura ambiente y presión atmosférica normal. Es
miscible con el agua y los disolventes orgánicos. Entre los
métodos analíticos para el propanol figuran la cromatografía de
gases, que puede detectar hasta 5 x 10-5 mg/m3 en el aire,
1 x 10-4 mg/litro en el agua y 0,002 mg/litro en la sangre, el
suero o la orina cuando se utilizan con la muestra procedimientos
adecuados de extracción o concentración.
2. Fuentes de exposición humana y ambiental
En 1979, la capacidad de producción mundial al año superó las
130 000 toneladas. En la naturaleza se produce por descomposición
de material orgánico por diversos micro-organismos, y se halla en
las plantas y en los aceites combustibles. El 1-propanol se
produce a partir del eteno por reacción con el monóxido de carbono
y el hidrógeno para dar propionaldehído, que a continuación se
hidrogena. Aparece también como sub-producto en la fabricación del
metanol y puede producirse a partir del propano directamente o a
partir de la acroleína. El uso principal del 1-propanol es como
disolvente de uso múltiple en la industria y el hogar. Se utiliza
en las tintas de impresión flexográfica y en aplicaciones textiles,
productos de uso personal como cosméticos y lociones, en productos
para limpiar cristales, en abrillantadores y en fórmulas
antisépticas. Le sigue en importancia su uso como producto
intermedio en la fabricación de diversos compuestos químicos.
3. Transporte, distribución y transformación en el medio ambiente
La principal vía de entrada del 1-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación de
desechos. También se producen emisiones al agua y al suelo.
Puesto que el uso principal del 1-propanol es como disolvente
volátil, gran parte del volumen de producción acaba en el medio
ambiente.
El 1-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y por el lavado con la lluvia. Es
fácilmente biodegradable, tanto en condiciones aerobias como
anaerobias y, a causa de estos mecanismos de eliminación química y
biológica, no suelen encontrarse niveles medibles de la sustancia
en el medio ambiente. No obstante, se ha detectado en la atmósfera
urbana, en vertederos de desechos y también en las aguas que se
rezumaban de un terraplenado. La permeabilidad del suelo al
1-propanol es probablemente elevada y el compuesto aumenta la
permeabilidad a ciertos disolventes aromáticos.
El 1-propanol tiene un coeficiente de reparto log
n-octanol/agua de 0,34 y un factor de bioconcentración de 0,7, lo
que hace muy poco probable su bioacumulación.
4. Niveles ambientales y exposición humana
La exposición de la población general puede producirse por
ingestión accidental, por inhalación durante el uso y por ingestión
junto con los alimentos (que contengan 1-propanol como aromatizante
volátil natural o añadido o como residuo de disolvente) y bebidas
alcohólicas y no alcohólicas. Por ejemplo, la cerveza contiene
hasta 195 mg/litro, el vino hasta 116 mg/litro y los diversos tipos
de licores hasta 3520 mg/litro. La exposición de la población
general al 1-propanol por inhalación y en el agua de bebida es baja
(en los Estados Unidos, la concentración media en muestras de aire
urbano fue de 0,00005 mg/m3 y la correspondiente al agua de bebida
0,001 mg/litro). Aunque los trabajadores están potencialmente
expuestos por la inhalación durante la fabricación, el tratamiento
y la utilización, no se dispone de datos para cuantificar esas
exposiciones.
5. Cinética y metabolismo
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se carece de datos sobre la tasa de
absorción tras la inhalación y la exposición dérmica. El
1-propanol es metabolizado por la deshidrogenasa alcohólica para
dar ácido propiónico por intermedio del aldehído y puede entrar en
el ciclo del ácido tricarboxílico. Esta oxidación es una etapa
limitativa de la velocidad del metabolismo del 1-propanol. In
vitro, las oxidasas microsómicas de rata y conejo también son
capaces de oxidar el 1-propanol a aldehído propiónico. La afinidad
relativa de la deshidrogenasa alcohólica y los sistemas de
oxidación microsómicos por el 1-propanol es mucho más elevada que
en el caso del etanol; así pues, el 1-propanol se elimina
rápidamente del organismo. En la rata, el periodo de
semieliminación de una dosis oral de 1000 mg/kg fue de 45 minutos.
Tanto en animales como en el hombre, el 1-propanol puede ser
eliminado del organismo en el aire exhalado o en la orina. A
sujetos a los que se administró una dosis de 1-propanol de 3,75 mg
por kg de peso corporal y 1200 mg de etanol por kg de peso corporal
por vía oral, la excreción urinaria total de 1-propanol fue del
2,1% de la dosis. Los niveles urinarios de 1-propanol fueron más
bajos cuanto más baja era la cantidad de etanol ingerida
simultáneamente, lo que demuestra la competencia por la
deshidrogenasa alcohólica entre el 1-propanol y la sobredosis de
etanol.
6. Efectos en los organismos en el medio ambiente
A las concentraciones en que normalmente se encuentra en el
medio ambiente, el 1-propanol no resulta tóxico para los organismos
acuáticos, los insectos ni las plantas. El umbral de inhibición
para la multiplicación celular de tres de las especies acuáticas
más sensibles (tres protozoos) fue de 38 - 568 mg/litro. Para los
organismos superiores, la concentración letal fue de unos 5000
mg/litro, y variaba notablemente poco de un filum a otro y exhibía
una curva dosis-respuesta sumamente pronunciada. Algunas bacterias
y microorganismos de aguas residuales y cienos activados son
capaces de adaptarse a concentraciones superiores a 17 000
mg/litro.
La germinación de semillas puede verse inhibida o estimulada
por el 1-propanol según la concentración en el agua utilizada y las
condiciones de exposición. El compuesto aumenta la acumulación de
nitritos en el maíz, los guisantes y el trigo.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 1-propanol para los mamíferos (a juzgar
por la mortalidad) es baja, por cualquiera de las vías de
exposición: cutánea, oral o respiratoria. Se ha comunicado que
los valores de la DL50 por vía oral para varias especies animales
varían entre 1870 y 6800 mg/kg de peso corporal. No obstante, en
ratas muy jóvenes se notificó una DL50 por vía oral de 560 - 660
mg/kg de peso corporal. El principal efecto tóxico del 1-propanol
tras una exposición única es la depresión del sistema nervioso
central. Los datos de que se dispone sobre el 1-propanol parecen
indicar que sus efectos sobre el sistema nervioso central son
semejantes a los del etanol; no obstante, el 1-propanol parece ser
más neurotóxico. Los valores de la DE50 para la narcosis en el
conejo y la pérdida del reflejo de enderezamiento en el ratón
fueron, respectivamente, de 1440 mg/kg de peso corporal por vía
oral, y de 1478 mg/kg de peso corporal por vía intraperitoneal;
estos valores son unas cuatro veces más bajos que los
correspondientes al etanol. En el ensayo del plano inclinado, el
1-propanol fue 2,5 veces más potente que el etanol en la rata.
La administración de dosis únicas por vía oral de 3000 ó
6000 mg/kg de peso corporal tuvo como resultado una acumulación
reversible de triglicéridos en el hígado de la rata. Las
concentraciones elevadas de vapor provocaron irritaciones en el
tracto respiratorio del ratón. El ritmo respiratorio del ratón se
vio disminuido en un 50% a concentraciones de aproximadamente
30 000 mg/m3.
No se dispone de datos sobre irritación ocular y cutánea. No
se observó sensibilización en una prueba de sensibilización cutánea
en ratones CF1.
En machos de rata expuestos durante seis semanas a 15 220
mg/m3, se obtuvieron pruebas limitadas de que el 1-propanol
disminuye la capacidad reproductora. No se observaron efectos tras
una exposición similar a 8610 mg/m3. Cuando se expusieron ratas
gestantes al 1-propanol, se observó toxicidad materna y fetal a
23 968 y 14 893 mg/m3 (9743 y 6054 ppm); no se observó toxicidad a
9001 mg/m3 (3659 ppm). No se observaron defectos de comportamiento
en la progenie de machos de rata expuestos durante seis semanas a
8610 ó 15 220 mg de 1-propanol/m3, ni en la de ratas expuestas
durante la gestación a las mismas concentraciones. No obstante,
cuando se administró a ratas de 5 a 8 días de edad 3000 - 7800 mg
de 1-propanol/kg por vía oral al día, se observó depresión del
sistema nervioso central durante la dosificación y síntomas de
privación cuando se retiraba la dosis. Cuando las ratas cumplieron
18 días se examinaron sus cerebros y se observaron reducciones en
los pesos cerebrales absoluto y relativo y en el contenido de ADN,
así como disminuciones regionales de los niveles de colesterol y de
proteínas.
El 1-propanol dio resultados negativos en dos ensayos de
detección de mutaciones puntuales utilizando Salmonella typhimurium
y en un ensayo de mutación inversa realizado con Escherichia coli
CA-274. Se obtuvieron resultados negativos en ensayos para inducir
intercambio de cromátidas hermanas o micronúcleos en células de
mamíferos in vitro. No se obtuvieron otros datos sobre
mutagenicidad.
En un estudio de carcinogenicidad realizado en grupos reducidos
de ratas Wistar expuestas durante toda su vida a dosis orales de
240 mg/kg o a dosis subcutáneas de 48 mg/kg, se observó un aumento
significativo de la incidencia de sarcoma hepático en el grupo en
el que la dosis se administraba por vía subcutánea. No obstante,
el estudio resultó insuficiente para evaluar la carcino-genicidad
por diversos motivos, entre ellos la falta de detalle experimental,
el reducido número de animales y el empleo de una sola dosis
elevada para inducir toxicidad hepática.
8. Efectos en la salud humana
No se tiene noticia de efectos adversos para la salud en la
población general o en grupos profesionales. El único caso
notificado de intoxicación mortal, fue el de una mujer que perdió
el conocimiento y falleció a las 4 - 5 h de la ingestión. La
autopsia reveló "inflamación cerebral" y edema pulmonar. En un
estudio sobre irritación cutánea y sensibilización, se comunicó la
aparición de reacciones alérgicas en un operario de laboratorio.
En otro grupo de 12 voluntarios, se observó en 9 individuos un
eritema que duró al menos 60 minutos tras la aplicación durante 5
minutos de papeles de filtro impregnados con 0,025 ml de una
solución al 75% de 1-propanol en agua aplicados sobre los
antebrazos.
No se dispone de más informes sobre efectos adversos para la
salud tras la exposición profesional al 1-propanol. No se dispone
de estudios epidemiológicos para evaluar los efectos a largo plazo,
incluida la carcinogenicidad, del 1-propanol en el ser humano.
9. Resumen de la evaluación
La exposición del ser humano al 1-propanol puede producirse por
la ingestión de alimentos o bebidas que lo contengan. La
exposición por inhalación puede tener lugar durante el uso
doméstico de la sustancia y en el ámbito laboral durante su
fabricación, tratamiento y uso. Los muy limitados datos de que se
dispone sobre el nivel de 1-propanol en el aire y el agua parecen
indicar que las concentraciones son muy reducidas.
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se cree que la absorción tras la
inhalación es rápida y la absorción por vía cutánea lenta.
La toxicidad aguda del 1-propanol para los animales es baja
en toda exposición, ya sea por vía cutánea, oral o respiratoria.
La exposición de miembros de la población general a niveles
potencialmente letales puede producirse por ingestión accidental o
intencionada. No obstante, sólo se ha notificado un caso de
envenenamiento mortal por 1-propanol. Los efectos agudos más
probables del 1-propanol en el hombre son la intoxicación
alcohólica y la narcosis. Los resultados de los estudios en
animales indican que el 1-propanol es 2 - 4 veces más tóxico que
el etanol.
El 1-propanol puede ser irritante para la piel hidratada.
Los datos sobre toxicidad animal no bastan para evaluar los
riesgos para la salud humana asociados a la exposición repetida o
duradera al 1-propanol. No obstante, estudios limitados a corto
plazo sobre la rata indican que la exposición por vía oral al
1-propanol tiene pocas probabilidades de suponer un riesgo grave
para la salud en las condiciones habituales de exposición humana.
En la rata la exposición por inhalación a una concentración de
15 220 mg/m3 redujo la capacidad reproductiva del macho, pero no
así la exposición a 8610 mg/m3. En la rata gestante, 9001 mg/m3
(3659 ppm) fue el nivel de efectos no observados y 14 893 mg/m3
(6054 ppm) el nivel más bajo de observación de efectos tanto
respecto a la toxicidad materna como a la fetal. Así pues, la
exposición por inhalación a concentraciones elevadas de 1-propanol
produjo toxicidad reproductiva y embriológica en el macho y la
hembra en presencia de toxicidad manifiesta en los animales
expuestos. Las concentraciones necesarias para producir esos
efectos en la rata fueron más elevadas que las que probablemente
se observen en condiciones normales de exposición humana.
El 1-propanol dio resultados negativos en los ensayos de
detección de mutaciones puntuales en bacterias. Aunque esto indica
que la sustancia no tiene ningún potencial genotóxico, no puede
evaluarse adecuadamente la mutagenicidad basándose en los limitados
datos disponibles. El estudio realizado no basta para evaluar la
carcino-genicidad del 1-propanol en animales de experimentación.
No se dispone de datos sobre la exposición a largo plazo de
poblaciones humanas al 1-propanol. Por todo ello, no puede
evaluarse la carcinogenicidad del 1-propanol en el ser humano.
Aparte de un caso de intoxicación mortal tras la ingestión de
medio litro de 1-propanol, no se tiene prácticamente noticia de
efectos adversos para la salud producidos por la exposición al
1-propanol, ni en la población general ni en grupos profesionales.
El Grupo Especial de Trabajo considera poco probable que el
1-propanol plantee un riesgo grave para la salud de la población
general en condiciones normales de exposición.
El 1-propanol puede liberarse al medio ambiente durante la
producción, el tratamiento, el almacenamiento, el transporte, el
uso y la evacuación de desechos. A causa de su uso principal como
disolvente volátil, la mayoría del volumen de producción acaba por
ser liberado a la atmósfera. No obstante, por reacciones con
radicales hidroxilo y por lavado pluvial, el 1-propanol
desaparecerá rápidamente de la atmósfera, siendo su tiempo de
presencia en ella inferior a 3 días. La eliminación del 1-propanol
del agua y del suelo también se produce rápidamente, de modo que
raras veces se detectan niveles medibles en cualquiera de estos
tres compartimientos. La adsorción del 1-propanol en las
partículas del suelo es escasa, pero la sustancia es móvil en el
suelo y se ha demostrado que aumenta la permeabilidad del mismo a
ciertos hidrocarburos aromáticos.
En vista de las propiedades físicas del 1-propanol, la bio-
acumulación es poco probable y, salvo en el caso de evacuación
accidental o inadecuada, el 1-propanol no constituye un riesgo para
los organismos acuáticos, los insectos y las plantas en las
concentraciones que por lo general se encuentran en el medio
ambiente.
 (i) In the methylmalonyl pathway, propionyl-CoA is carboxylated to
methylmalonyl-CoA, this is followed by trans-carboxylation to
succinyl-CoA, which subsequently enters the tricarboxcylic acid
cycle to be metabolized to carbon dioxide and water.
(ii) In the lactate pathway, the propionyl-CoA is dehydrogentated
to acrylolyl-CoA, alpha-hydration gives L-lactoyl-CoA, which is
hydrolysed to lactate.
(iii) In another pathway the acrylolyl-CoA is hydrated to
3-hydroxypropionyl-CoA, deacylation and oxidation result in the
formation of malonic acid semialdehyde, which is converted to
acetyl-CoA. These reactions, which constitute the major pathways
for propionic acid metabolism in plant mitochondria, also occur in
animals.
(iv) The propionyl CoA may also participate in triglyceride
synthesis.
(v) The obligatory formation of propionyl carnitine required for
the transport of propionic acid into mitochrondria may also be an
excretory pathway under conditions of high carnitine and propionic
acid concentrations [158, 153].
Propionic acid and/or propionyl-CoA are potent inhibitors of
several mitochondrial enzymes required for fatty acid oxidation,
gluconeogenesis, and ureagenesis; their inhibitory effects can be
reversed with carnitine [24, 23]. The formation of propionyl-CoA,
its metabolism and effects on oxidative metabolism provide an
explanation for the hepatic effects observed in rats after high
oral exposure to 1-propanol (section 8.2) and for the biochemical
effects seen in some studies (section 8.3.1). Indeed the
accumulation of acyl-CoA esters, including propionyl-CoA, is
implicated in the pathogenesis of Reye syndrome [43].
6.4 Elimination and Excretion
Aliphatic alcohols may be eliminated from the body via expired
air or the urine. Theoretically, urinary metabolites may arise
from oxidation or from conjugation with glucuronic acid or sulfate.
There are no reports of the excretion of unchanged 1-propanol in
expired air or urine and, following an oral dose to rabbits of
800 mg/kg, only 0.9% was found in the urine as propyl-glucuronide
and none as a sulfate conjugate [100].
6.4.1 Animals
Available in vivo data, reviewed by Rietbrock & Abshagen [157],
showed that the elimination of 1-propanol was dose independent
above a single oral dose of 1000 mg/kg body weight in rats and
above a single intraperitoneal dose of 1200 mg/kg body weight in
rabbits [139, 1, 11]. The rate of the zero-order elimination of
the compound from the blood of rats that had received a single oral
dose of 3000 mg/kg body weight was found to be 510 mg/kg body
weight per hour [11]. At lower doses, the elimination rate was
first order. When rats were given a single oral dose of 1000 mg/kg
body weight, the half-life of 1-propanol was 45 min [1]. The
overall metabolism and elimination of 1-propanol are described in
section 6.3.1. In mice, a half-life of 57 min was estimated for
the exponential elimination phase following a single oral exposure
[1]. This should be considered an approximation because there were
only 2 or 3 time points per dose.
In vitro, the elimination of 1-propanol from the perfusate of
rat liver was also shown to be saturable, a zero-order phase being
succeeded below a concentration of 78 mg/litre by an exponential
phase with a half-life of 14 min [7].
6.4.2 Human beings
No data were available describing the elimination kinetics of
1-propanol in human beings.
When 10 volunteers drank 1-propanol in ethanolic orange juice
at doses of 3.75 mg 1-propanol and 1200 mg ethanol/kg body weight
over a period of 2 h, the compound was detected in the blood and in
the urine, partly as glucuronide. The total urinary excretion of
1-propanol was 2.1% of the dose. The urinary levels of 1-propanol
were lower when the amount of simultaneously ingested ethanol was
less, showing competition for ADH between 1-propanol and the
ethanol overdose [19, 20].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table
5. In none of these studies was the concentration of 1-propanol
reported to have been measured. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
open-system studies occurred at lower concentrations than the
nominal ones.
Several short-term studies have also been conducted. Seiler et
al. [167] determined the breakpoint of bioinhibition for a total
of 20 strains of several bacterial groups prevalent in a waste-water
treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
1-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 10 000 - 30 000 mg/litre of medium.
No analysis for the compound was reported.
Inhibition of cell multiplication of blue algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) reached 100%
after 8 days of static exposure to 255 and 3100 mg 1-propanol/litre
water, respectively, in a closed system at 27 °C and a pH of 7
[25, 27].
7.2 Terrestrial Organisms
7.2.1 Insects
The toxicity of 1-propanol for insect larvae is summarized in
Table 5. In static tests, the 48-h LC50 values for adults of the
fruit fly strains of Drosophila melanogaster and Drosophila
simulans were between 18 490 and 24 120 mg/litre of nutrient medium
and 11 260 and 12 860 mg/litre of nutrient medium, respectively [46].
7.2.2 Plants
The effects of 1-propanol on the rate of seed germination
have been investigated on several occasions. Total inhibition of
the germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers absorbing a solution containing 8050
mg 1-propanol/litre water [40]. The germination of white amaranth
(Amaranthus albus) seeds was stimulated in a dose-related manner
after 5 h incubation at 25 °C on filter papers moistened with a
solution containing 3600 - 36 050 mg 1-propanol/litre water [36].
Reynolds [36] measured 50% inhibition of germination of lettuce
(Lactuca sativa) seeds after incubation for 3 days at 30 °C on agar
containing 3065 mg 1-propanol/litre. The percentage germination
and the axis length of soya bean (Glycine max) seeds, with the
testa removed, were not reduced after exposure to pure 1-propanol
for 2 h. After treatment with a 50% (v/v) 1-propanol/water mixture
for 2 min, germination was almost completely inhibited and axis
length was reduced [150].
Table 5. Acute aquatic toxicity of 1-propanol
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria
Pseudomonas putida 25 7 closed 16 h TTb 2 700 [27]
Microorganisms
Activated sludge 21 7.4-8 closed 3 h 50% inhibition 1 000 [105]
of respiration
Acclimated mixed 30 6.8 closed 1.2 h 50% inhibition 19 085 [196]
waste-water culture of respiration
Protozoa
Entosiphon sulcatum 25 6.9 closed 72 h TTb 38 [26]
Chilomonas paramecium 20 6.9 closed 48 h TTb 175 [29]
Uronema parduczi 25 6.9 closed 20 h TTb 568 [28]
Algae
Selenastrum capricornutum 25-26 closed 96 h NOAECc 2 000 [172]
Scenedesmus pannonicus 25-26 closed 48 h NOAECc 2 900 [172]
Chlorella pyrenoidosa 25-26 closed 48 h NOAECc 1 150 [172]
Coelenterate
Hydra oligactis 17 8.2-8.4 >5 closed 48 h LC50 6 800 [170]
Worms
Flatworm (Dugesia) 20 8.2-8.4 >5 closed 48 h LC50 4 700 [170]
Tubificid worm 20 8.2-8.4 >5 closed 48 h LC50 9 200 [170]
Molluscs
Giant pond snail 20 8.2-8.4 >5 closed 48 h LC50 6 500 [170]
(Lymnea stagnalis)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Crustaceans
Water flea 20 8 >2 250 open 24 h EC50d 4 415 [27]
(Daphnia magna)e EC0 3 336
EC100 5 909
19 open 48 h LC50 7 080 [33]
Water flea 19 open 48 h LC50 3 025 [33]
(Daphnia pulex)e
Water flea 19 open 48 h LC50 5 820 [33]
(Daphnia cucullata)e
Isopod 20 8.2-8.4 >5 209 closed 48 h LC50 2 500 [170]
(Asellus aquaticus)
Scud (Gammarus pulex) 20 8.2-8.4 >5 209 closed 48 h LC50 1 000 [170]
Insects
Mosquito larvae 22-24 open 4 h LC50 10 450 [107]
(Aedes aegypti)
Mosquito larvae (Aedes 26 8.2-8.4 >5 209 open 48 h LC50 4 400, 4 800 [172]
aegypti, Culex pipiens) LC0 3 200, 3 600
Midge larvae 20 8.2-8.4 >5 209 closed 48 h LC50 2 350 [170]
(Chironomus gr. thummi)
Leech larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 400 [170]
(Eropdella octoculata)
Dragon fly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 4 200 [170]
(Ischnura elegans)
Stonefly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 520 [170]
(Nemoura cinerea)
Mayfly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 3 110 [170]
(Cloeon dipterum)
Corixa punctata (larvae) 20 8.2-8.4 >5 209 closed 48 h LC50 2 000 [170]
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Creek chub 15-21 8.3 98 open 24 h LC0 200 [68]
(Semotitus atromaculatus)
Golden orfe 20 7-8 >5 200-300 48 h LC50 4 320, 4 560 [99]
(Leuciscus idus melanotus) LC0 3 600, 4 000
Fathead minnow 20 8.2-8.4 >5 209 open 48 h LC50 5 000 [172]
(Pimephales promelas) LC0 2 600
Rainbow trout 15 7-8 >5 98 open 48 h LC50 3 200 [172]
(Salmo gairdneri) LC0 2 000
Paddy fish 24 8.2-8.4 >5 209 open 48 h LC50 5 900 [172]
(Oryzias latipes) LC0 4 400
Amphibia
South African clawed toad 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Xenopus laevis)
Mexican axolotl 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Ambystoma mexicanum)
SEA WATER
Bacteria
Photobacterium 15 15 min 50% light 8 686 [84]
phosphorerum closed reduction
5 5 min 50% light 17 700 [48]
closed 15 min reduction 18 400
Crustacea
Brine shrimp 24 24 h LC50 4 200 [149]f
(Artemia salina) open
Harpacticoid copepod 21 7.9 >5 96 h LC50 2 300 [12]g
(Nitocra spinipes)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Bleak (Alburnus alburnus) 10 7.9 >5 open 96 h LC50 3 800 [12]g
---------------------------------------------------------------------------------------------------------------------------------
a Static systems used in all experiments reported.
b TT = toxic threshold for inhibition of cell multiplication.
c NOAEC = no-observed-adverse-effect-concentration; effect is growth inhibition.
d Effect is complete immobilization.
e Age of Daphnia was 24 h for Daphnia magna and Daphnia pulex, and 11 ± 1 day for Daphnia cucullata.
f Salinity was 2.8%.
g Salinity was 0.7%.
1-Propanol was marginally effective in breaking the dormancy of
seeds of genetically pure dormant lines of wild oat (Avena fatua)
after 5 days of exposure to solutions containing up to 1202 mg/litre.
Seed viability was affected at higher concentrations [2].
When excised seedling roots of maize (Zea mays) were treated by
vacuum infiltration in a 5% solution of 1-propanol in water, 3
times for 60 seconds, and then incubated anaerobically at 28 °C,
nitrite accumulation increased by 10 times or more, the utilization
of nitrate increased, and the utilization of exogenous nitrite was
inhibited. These effects were enhanced under aerobic conditions
[75]. Dry et al. [51] observed that stimulation of nitrite
accumulation in pea and wheat roots under aerobic conditions was
accompanied by a decline in the cellular levels of glucose-6-
phosphate. It was suggested by Gray & Cresswell [75] that an
increase in the utilization of nitrate was related to increased
access of nitrate to the site of nitrate metabolism as a result of
an increase in membrane permeability.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
The available LD50s for various animal species are summarized
in Table 6. Based on mortality estimates, 1-propanol exhibits low
toxicity, except in very young rats. Oral LD50 values for several
animal species range between 1870 and 6800 mg/kg body weight. For
very young rats, oral LD50s of 560 - 660 mg/kg body weight have
been reported [152].
An intraperitoneal dose of 785 mg 1-propanol/kg was lethal to 10
out of 10 C57B/6J and 10 out of 10 DBa/2J mice, but a dose of 392 mg/
kg did not cause any deaths in either strain [183]. An LD16 of
450 mg/kg was reported in rats after intraperitoneal administration.
When rats were exposed to 1-propanol vapour for 4 h at a
concentration of approximately 9840 mg/m3, 2 out of 6 died within
14 days [173].
8.1.2 Signs of intoxication
Osborne-Mendel or Sherman rats of both sexes receiving a lethal
oral dose of undiluted 1-propanol became comatose within a few
minutes [187]. Deep narcosis occurred in mice exposed through
inhalation of 1-propanol at a concentration of 50 mg/litre for 2 h.
Very young rats (60 - 100 g) of an unspecified strain and of
both sexes, received a single oral dose of between 150 and 3000 mg
undiluted 1-propanol/kg body weight. Animals that died showed
hyperaemia, vacuolation, and dilated sinusoids in the liver, and
hyperaemia, tubular cloudy swelling, and tubular necrosis in the
kidneys [152].
When anaesthetized Sprague-Dawley rats were made to inspire
160 mg of the undiluted compound, all 9 exposed rats died within
165 min, 6 of them dying immediately from respiratory arrest. All
controls survived and were killed 24 h later. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight of the exposed rats was increased by 92%. The lungs
showed oedema and small areas of focal haemorrhage [65].
Special studies on neurotoxic and behavioural effects, and on
biochemical effects are described in sections 8.3 and 8.4.
Table 6. LD50s for 1-propanol
---------------------------------------------------------------------------------------------------
Species Sex Route of Observation LD50 (mg/kg Comments Reference
exposure period body weight)
---------------------------------------------------------------------------------------------------
Wistar rat (non- male oral 14 days 1870 vehicle: water [173]
fasted)
Osborne-Mendel male, oral until 6500 undiluted [187]
or Sherman rat female recovery
CD mouse not oral 3 days 6800 undiluted [164]
reported
Rabbit male, oral 1 day 2820 [130]
female
Wistar rat male intravenous 5 days 590 vehicle: water [190]
H mouse male intravenous 5 days 697 vehicle: water [190]
female intravenous not 1090 vehicle: water [40]
reported
Chinchilla rabbit male, intravenous 5 days 483 vehicle: water [190]
female
Wistar rat male intraperitoneal 5 days 2247 vehicle: water [190]
H mouse male intraperitoneal 5 days 3695 vehicle: water [190]
Syrian hamster male intraperitoneal 5 days 2337 vehicle: water [190]
Guinea-pig intraperitoneal 5 days 1208 vehicle: water [190]
New Zealand male dermal 14 days 4050 1/10 of body [173]
rabbit surface exposed
under cover for
24 h
---------------------------------------------------------------------------------------------------
8.1.3 Skin, eye, and respiratory tract irritation; sensitization
Data for skin and eye irritation were not available. One skin
sensitization test has been reported concerning an ear-swelling
test on CF1 mice. No sensitization was observed [61]. Although
the test requires further validation, it correctly discriminated
between a number of known positive and negative human sensitizing
agents. The authors claim it to be an accurate, sensitive, and
efficient method for evaluating delayed contact sensitization.
The sensory irritation of 1-propanol was investigated using a
50% reflex decrease in the respiratory rate of mice (RD50) as an
index. Only the heads of the mice were exposed. An exposure-
related effect was found with RD50 values for the first 10 min of
exposure of 31 252 mg/m3 for Swiss Webster mice [101] and
33 604 mg/m3 for CF-1 mice [108]. The potential of 1-propanol as
a respiratory irritant is therefore low.
8.2 Repeated Exposures
Only a few data are available concerning the oral exposure of
animals.
When 3 male and 3 female rats of unspecified strain were
exposed to 4 daily oral doses of 2160 mg undiluted 1-propanol, no
deaths occurred and no gross pathological signs were seen in the
liver [187].
In a group of 6 male rats of unspecified strain, receiving
drinking-water containing 1-propanol at a concentration of 60 090
mg/litre for 4 months, food consumption, body weight gain, and
liver histopathology were comparable to those of the control group.
It should be noted that the authors reported a dose rate of 3 mg/kg
body weight per day, while a dose rate of approximately 3000 mg/kg
body weight per day seems more appropriate, assuming a water
consumption of 20 ml/day and a body weight of 400 g [85].
Groups of 10 Wistar rats were exposed to 1-propanol in the
drinking-water at a concentration of 320 000 mg/litre (calculated
by the Task Group to be equivalent to approximately 16 000 mg/kg
body weight per day, on the basis of the assumptions made above)
for 5, 9, or 13 weeks. Control groups comprised 10 rats each. The
exposed rats gradually became weak, losing their appetites and
showing a decreased body weight gain. Electron microscopic studies
of the liver showed irregularly shaped megamitochondria with few
cristae, and normally sized but irregularly shaped mitochondria
with a decreased number of cristae. Biochemical changes included a
decreased state 3 respiration using glutamate as a substrate and
decreased specific activities of cytochrome c oxidase (EC 1.9.3.1)
and monoamine oxidase (EC 1.4.3.4) [203].
8.3 Neurotoxic and Behavioural Effects
In one study on anaesthetized mongrel dogs, it was shown that
1-propanol, as well as other primary alcohols, could increase the
permeability of the blood-brain barrier. The dogs received a
sodium fluorescein solution and 0.578 mg 1-propanol in saline,
intravenously. The concentration of sodium fluorescein in the
cerebrospinal fluid rose to a maximum within 10 min and returned to
control levels, 3 h after exposure [78].
The oral ED50 (1440 mg/kg body weight) for narcosis in rabbits
exposed to 1-propanol was 4 times lower than that for ethanol
[129]. Deep narcosis occurred in mice exposed through inhalation
to 50 mg 1-propanol/litre for 2 h, and a 40-min exposure to 2.3 mg/
litre reduced the unconditioned flexor response in rabbits. When
rabbits were intravenously infused with 1-propanol at a rate of 9 -
30 mg/min per kg body weight, positional nystagmus with an
inhibited rotatory response was observed at and above a blood
concentration of 900 mg/litre [137].
The intraperitoneal ED50 for loss of righting reflex in Swiss
Webster mice administered 1478 mg 1-propanol/kg body weight was 2.8
times lower than that for ethanol [117]. When C57BL/6J or DBA/2J
mice were given a single dose of 1-propanol intraperitoneally, both
strains showed decreased activity in the open field test at
392 mg/kg body weight, but the decrease was not significant. All
mice given 785 and 1570 mg/kg body weight died [183]. The rotarod
performance of Swiss-Cox mice decreased in a dose-related manner
after single oral doses of 1-propanol of 2000 or 4000 mg/kg body
weight. A dose of 1000 mg/kg body weight did not cause behavioural
impairment. When the study was repeated on days 4, 6, 7, and 8
after the first trial, tolerance did not develop [1].
The threshold for the induction of ataxia in Sprague-Dawley
rats following intraperitoneal exposure was 799 mg/kg body weight
[119]. In a tilted plane test, the performance of rats decreased
by an average of 71% after oral exposure to 2000 mg/kg body weight.
On a molar basis, 1-propanol was 2.5 times as intoxicating as
ethanol [204].
According to several investigators, depression of the central
nervous system by 1-propanol was related to interactions with
neuronal membranes. Lyon et al. [117] observed a high correlation
between the narcotic potencies of the aliphatic alcohols, including
1-propanol, in mice and their ability to disorder the brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was in turn related to membrane
solubility. A change in membrane fluidity was shown to occur in
isolated synaptosomal plasma membranes of rat cortex in vitro by a
decrease in 1,6-diphenyl-1,3,5-hexatriene fluorescence polarization
[81].
Functional loss due to disruption of membrane integrity by
1-propanol was observed in vitro. The action potentials of the
sciatic nerves of the toad (Bufo marinus) [155] and of giant axons
of the squid (Loligo forbesi) [143] were decreased by 1-propanol.
In isolated rat phrenic nerve-diaphragm, 1-propanol increased the
amplitudes of end-plate and miniature end-plate potentials and the
number of quanta of acetylcholine of end-plate potentials [62].
The compound also affected the rate of decay of postsynaptic
currents in the neuromuscular junction of the crayfish (Cherax
destructor) [200], and in the phrenic nerve-diaphragm of the rat
[62].
Effects on the ionic currents underlying the changes in
excitability described above were also investigated in vitro.
1-Propanol inhibited both the K+-stimulated and the Na+-dependent
influx of Ca2+ ions into isolated rat brain synaptosomes [80, 81,
124], and the influx of Na+ ions into rat brain synaptosomes [128].
It decreased the Na+ and K+ currents in the giant axons of the
squid (Loligo forbesi) [143], and in sciatic nerve fibres of the
clawed toad (Xenopus laevis) [6]. The interference of 1-propanol
with the transport of Ca2+ ions across biological membranes was
also shown in vitro by the inhibition of Ca2+ ion-induced
contractions of guinea-pig ileum [213], and in vivo, in rats, by a
decrease in regional brain Ca2+ ion levels, 30 min after one
intraperitoneal dose of 2000 mg/kg body weight [159].
The disruption of neuronal membranes by 1-propanol was also
thought to explain its inhibitory action on the binding of
dihydromorphine to isolated mouse brain caudate membranes [185] and
on membrane-bound guanylate cyclase (EC 4.6.1.2) in intact murine
neuroblastoma N1E-115 cells [172]. The activation by 1-propanol of
membrane-bound adenylate cyclase (EC 4.6.1.1) from isolated mouse
striatal membranes, in the presence of guanine nucleotides, was
also suggested to be the result of membrane perturbation [116].
8.4 Biochemical Effects
8.4.1 Effects on lipids in the liver and blood
Oral administration of single doses of 3000 or 6000 mg
1-propanol/kg body weight to Wistar rats caused a transient
increase in hepatic triglycerides, which was related to the
duration of an elevated blood-1-propanol concentration [10, 11].
Gaillard & Derache [63] did not observe an increase in hepatic
triglycerides in Wistar rats, 17 h after a single dose of 6000 mg
1-propanol/kg body weight.
Factors possibly responsible for hepatic triglyceride
accumulation include: an increase in hepatic uptake of labelled
palmitate [11], an increased esterification of palmitate to form
liver triglycerides [10, 11], and decreased palmitate oxidation
[11]. The decrease in palmitate oxidation was related to an
increase in the hepatic alpha-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [11].
An increase in extramitochondrial reducing equivalents, indicated
by an increased lactate/pyruvate ratio, was observed in vitro by
Forsander [60], but not in vivo by Beaugé et al. [11].
The effects of 1-propanol on palmitate incorporation into
triglycerides appear to depend on the dose, high doses causing
inhibition and lower ones leading to an increase. The
incorporation of palmitate into serum triglycerides and serum and
liver phospholipids, 4.5 h after a single oral dose of 6000 mg
1-propanol/kg body weight to rats, was found to be inhibited, while
an increase in hepatic triglyceride accumulation was only observed
8 h after dosing [10]. Three hours after a dose of 3000 mg/kg body
weight, the incorporation of palmitate in blood triglycerides was
increased concomitantly with an increase in hepatic triglycerides
while levels of phospholipids in the liver and blood were unaffected
[11]. Similar effects have been noted with ethanol [11].
8.4.2 Effects on microsomal enzymes
The effects of 1-propanol on microsomal enzymes (EC 1.14.14.1)
in vivo was investigated by Powis [147], who administered a single
oral dose of 960 mg/kg body weight to Wistar rats. Twenty four
hours after exposure, no effects were observed on the activities of
aniline hydroxylase and aminopyrine demethylase in liver
microsomes. This study is inadequate to demonstrate an inductive
effect of 1-propanol on the microsomal mixed function oxidase
system. In vitro, 1-propanol inhibited aldrin epoxidase and
p-aniline hydroxylase in isolated rat liver microsomes via an
interaction with cytochrome P-450, which causes a reverse Type I
spectral change [41, 210, 189, 161]. At high concentrations, the
inhibition of the mixed function monoxygenase system by aliphatic
alcohols correlates directly with the lipophilicity of the
alcohols, and is probably the result of unspecific effects on the
membrane (see section 8.3). The compound did not affect the levels
of hepatic microsomal cytochrome P-450, haem, cytochrome b5, and
NADPH-cytochrome c reductase (EC 1.6.2.4) in phenobarbital-induced
rats [96]. 1-Propanol increased the levels of cytochrome P-450 in
cultured chick embryo hepatocytes. The activities of benzphetamine
demethylase and UDP-glucuronosyl transferase (EC 2.4.1.17) were
also increased [169].
8.4.3 Other biochemical findings
The glutathione level in the liver of Wistar rats administered
a single dose of 1660 mg 1-propanol/kg body weight, orally, had
decreased by 20%, 6 h after exposure. Lipid peroxidation, as
indicated by diene conjugates formation, was increased; ethanol in
equivalent doses produced similar effects [199].
The activities of liver ornithine decarboxylase (EC 4.1.1.17)
and liver tyrosine aminotransferase (EC 2.6.1.5) increased in
partially hepatectomized rats 4 h after one oral dose of 2300 mg
1-propanol/kg body weight or an equivalent dose of ethanol. No
effects were observed on levels of alanine aminotransferase
(EC 2.6.1.2) in the liver and kidneys, and on levels of ornithine
decarboxylase in the kidneys and brain [145].
The effects of 1-propanol on neuronal membrane-bound adenylate
cyclase (EC 4.6.1.1) and guanylate cyclase (EC 4.6.1.2) in vitro
are discussed in section 8.3. 1-Propanol and ethanol, can have
different effects on the activity of adenylate cyclase, depending
on the concentration of alcohol and the biological system being
investigated [178, 192, 93].
When Sprague-Dawley rats inhaled 1-propanol for 6 h at a
concentration of 490 mg/m3, the serum level of testosterone was
decreased by 42% immediately after exposure, but not 18 h after
exposure. When this exposure regimen was repeated daily over one
week, no effects on serum testosterone levels were observed. Serum
levels of luteinizing hormone and corticosterone were unchanged at
all times [31].
When a crude homogenate of dispersed acinar cells, prepared
from guinea-pig pancreas, was incubated with 1-propanol and
secretin, the secretin-stimulated activities of adenylate cyclase
(EC 4.6.1.1) and cellular cyclic adenosine 3',5'-monophosphate were
potentiated at low concentrations of 1-propanol, but the
potentiation was reversible. Irreversible inhibition occurred at
higher concentrations [192].
8.5 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 15 male Sprague-Dawley rats were exposed to
1-propanol at measured concentrations of 8610 or 15 220 mg/m3 for
7 h/day over 6 weeks. Beginning on the third day after the last
exposure, males were mated for a maximum of 5 days with unexposed
females. There was no apparent effect of exposure to 8610 mg/m3 on
mating performance or fertility. After exposure to 15 220 mg/m3,
17 out of 18 males copulated (as evidenced by the presence of a
vaginal plug), but only 2 of 17 mated females became pregnant. The
offspring of the exposed males were evaluated postnatally in a
battery of behavioural tests. There was no evidence of any
exposure-related effect [132].
These investigators also exposed groups of 15 pregnant Sprague-
Dawley rats to the same concentrations of 1-propanol on gestation
days 1 - 20. Pregnant females exposed to 15 220 mg/m3 showed
significantly reduced weight gain and food consumption. Their
female offspring also showed reduced weight gain up to 3 weeks of
age, but there was no consistent effect on male offspring. Litter
sizes were not affected. "Several" of the offspring from dams
exposed to 15 220 mg/m3 had crooked tails. Behavioural testing of
offspring did not reveal any evidence of an exposure-related
effect, though there was an increase in total external, visceral,
and skeletal malformations at 23 968 mg/m3 (9743 ppm) and in total
skeletal malformation at 14 893 mg/m3 (6054 ppm) [132].
The effects of 1-propanol on brain development in the neonatal
rat were also studied. A group of 21, 5-day-old Long-Evans rats
was exposed to 1-propanol via an artificial milk formula, which was
administered through an intragastric catheter for 4 consecutive
days. The rats received 12 feeds daily, each lasting 20 min.
Doses were 3800, 7500, 3000, or 7800 mg/kg body weight on day
5 - 8, respectively. Controls received the milk formula only.
During the exposure, the exposed pups frequently showed an impaired
righting response. After the last exposure, withdrawal symptoms
were displayed. Pups were killed at 18 days of age, at which time
there was no effect on body weight or on absolute weight of
kidneys, heart, or liver. However, the absolute and relative brain
weights were decreased in the exposed pups. Biochemical analysis
showed that the exposed pups had a decreased amount of DNA in all
brain areas examined. Cholesterol levels were decreased in the
forebrain and cerebellar samples, while protein levels were
decreased only in the forebrain samples [74].
8.6 Mutagenicity
8.6.1 Bacteria
1-Propanol was tested for mutagenic activity using Ames test
without S9; up to 100-µmol/plate was negative with Salmonella
typhimurium TA-100 [181]. Negative results were also reported in
TA-100 and TA-98 with or without metabolic activation, following
standard Ames test protocol [94].
In a reverse mutation assay with Escherchia coli CA-274
following a pre-incubation protocol, a 5-fold increase in the
number of revertants was observed at a concentration of 4.5%
1-propanol. No metabolic activation system was used [86].
8.6.2 Mammalian cells in vitro
1-Propanol (100 mg/litre once a day for 7 days) did not
increase the number of sister chromatid exchanges in Chinese
hamster ovary cells [136], or in V79 Chinese hamster lung
fibroblasts at 6000 mg/litre for 3 h (with activation) and 28 h
(without activation) [200]. It did not increase the number of
micronuclei in V79 Chinese hamster lung fibroblasts at 40 200 mg/
litre for 1 h [113].
A dose-related increase in the inhibition of metabolic
cooperation between hamster V79 cells, a phenomenon believed to
reflect carcinogenic promotion ability and not be indicative of
genotoxic potential, was observed by Chen et al. [37]. This may be
due to the membrane effects of 2-propanol.
8.7 Carcinogenicity
A group of 18 Wistar rats of both sexes received doses of
240 mg 1-propanol/kg body weight, by gavage, twice a week, for
their lifetime. Another group of 31 Wistar rats of both sexes
received subcutaneous injections of 48 mg compound/kg body weight,
twice a week, for their lifetime. Control groups, comprising 25
rats for each route, received saline. It was not reported whether
the analytical grade, double-distilled test compound was analysed
for the presence of impurities. The average survival time was 570
days for the orally exposed rats, 666 days for the subcutaneously
exposed rats, and 643 days for both control groups. The tumour
incidence is reported in Table 7. The data were not statistically
analysed. It was reported that "nearly all rats" showed liver
damage including congestion, steatosis, necrosis, fibrosis, and
metaplasia and hyperplasia of the haematopoietic bone marrow
parenchyma. However, the incidence of these lesions were not
reported [67].
Table 7. Tumour incidence in Wistar rats exposed orally or
subcutaneously to 1-propanol for lifetimea
-------------------------------------------------------------------
Organ/ Tumour type Incidence
tissue oral exposure sc exposure
affected exposed controls exposed controls
-------------------------------------------------------------------
Blood myeloid leukemia 2/18 0/25 4/31 0/25
Liver carcinoma 1/18 0/25 0/31 0/25
Liver sarcoma 2/18 0/25 5/31 0/25
Other carcinoma 0/18 0/25 3/31b 0/25
sarcoma 0/18 0/25 2/31c 0/25
benign tumoursd 10/18 3/25 7/31 2/25
-------------------------------------------------------------------
a From: Gibel et al. [67].
b One carcinoma each in kidney, bladder, and uterus.
c One sarcoma each in spleen and at injection site.
d Mostly papillomas and mammary fibroadenomas.
Although there was an apparent increase in the incidence of
liver sarcoma, the study is inadequate for the assessment of
carcinogenicity. The dosing schedule did not conform to standard
protocol. Too few animals were used in each dose group, the sex
ratio of each group was unclear, no data were provided on the
histological type of liver sarcoma, no statistical analysis was
conducted, the maximum tolerated dose was exceeded, as evidenced by
the reported liver damage, and only single dose levels were used.
In the case of subcutaneous administration, the exposure route was
inappropriate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
One case of poisoning by 1-propanol has been reported. It
concerned a 46-year-old woman who was estimated to have consumed
approximately half a litre of the compound as a solvent in a
cosmetic preparation, probably a hair lotion. It was pointed out
that the woman could have ingested this preparation more than once
in the past. The woman was found unconscious. She died 4 - 5 h
after ingestion. No other signs or symptoms were reported.
Autopsy revealed a "swollen brain" and lung oedema [52].
9.1.2 Controlled human studies
Filter papers moistened with 0.025 ml of a 75% solution of
1-propanol in water were placed on the forearms of a group of 12
volunteers following immersion of the forearms in water at 23 °C
for 10 min. The patches were covered for 5 min and then gently
blotted. Nine of the 12 persons showed erythema for at least 60
min following exposure. The cutaneous reaction was totally blocked
in 4 out of 4 persons after pretreatment with 40% 4-methylpyrazole
in hydrophilic ointment 1 h before the challenge, showing,
according to the authors, that 1-propanol must be metabolized to
propanal before vasoactivity occurs [207].
9.2 Occupational Exposure
A laboratory worker in a company manufacturing hair cosmetics
developed allergic reactions in patch tests with chemically pure
1-propanol solutions in water (10 - 99.5% by volume). This person
also reacted to 2-propanol, 1-butanol, 2-butanol, and formaldehyde,
but not to ethanol and methanol. Controls were not tested [115].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 1-propanol may occur through
ingestion of food and alcoholic beverages containing 1-propanol
(e.g., wine and beer 100 - 200 mg/litre, spirits up to 3500
mg/litre). Inhalation exposure may occur during household use and
occupationally during manufacture and processing. Exposure of the
general population via inhalation and drinking-water is very low
(average concentrations in urban air and drinking-water in the USA,
0.00005 mg/m3 and drinking-water 0.001 mg/litre, respectively)
(section 5).
10.1.2 Health effects
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Data on the absorption rate following
inhalation are lacking but, in view of the physical properties of
the compound, it is also expected to be rapid. Dermal absorption
is expected to be slow (section 6).
1-Propanol exhibits low acute toxicity for animals (based on
lethality estimates), whether exposed via the dermal, oral, or
respiratory route (section 8.1). Exposure to potentially lethal
levels may occur in the general population through accidental or
intentional ingestion. However, only one case of lethal poisoning
by 1-propanol has been reported, which probably reflects its low
toxicity and limited use by the public (section 9.1.1). The
principal toxic effect of 1-propanol following a single exposure is
depression of the central nervous system. Quantitative exposure-
effect data on human beings are not available. The most likely
acute effects of 1-propanol in man are alcoholic intoxication and
narcosis. Animal studies indicate that 1-propanol is 2 - 4 times
as intoxicating as ethanol.
A controlled human study has indicated that 1-propanol may be
irritating to hydrated skin. However, the potential of 1-propanol
as a respiratory irritant is low (section 8.1.3). Data are
inadequate for evaluation of the irritating properties of this
compound for the skin, eye, and respiratory tract in human beings,
or for evaluation of its sensitizing potential.
The results of limited drinking-water studies on animals
suggest that oral exposure to 1-propanol is unlikely to pose a
serious health hazard under the usual conditions of human exposure
(section 8.2).
Inhalation exposure to a concentration of 15 220 mg/m3 caused
impaired reproductive performance in male rats, but exposure to
8610 mg/m3 did not. In pregnant rats, 9001 mg/m3 (3659 ppm) was a
NOEL and 14 893 mg/m3 (6054 ppm) was a LOEL for both maternal and
developmental toxicity. Behavioural effects were not detected in
offspring whose mothers were exposed during pregnancy to 15 220
mg/m3, but oral dosing of neonatal rats produced biochemical
changes in the brain that were detected 10 days after the last
treatment (section 8.5). Inhalation exposure to high concentrations
of 1-propanol produced reproductive and developmental toxic effects
in male and female rats. These effects occurred in the presence of
other overt signs of toxicity in the exposed animals and 1-propanol
does not appear to be selectively toxic to male or female
reproductive processes. The concentrations required to produce
these effects in rats were higher than those likely to be
encountered under normal conditions of human exposure.
1-Propanol was negative in assays for point mutations in
bacteria. It did not increase the incidence of sister chromatid
exchange or micronuclei in mammalian cells in vitro. Although
these findings suggest that the substance does not have any
genotoxic potential, no adequate assessment of mutagenicity can be
made on the basis of the limited data available. The results of an
in vitro test said to predict promotional activity were negative
(section 8.6). The available study is inadequate to evaluate the
carcinogenicity of 1-propanol in experimental animals (section
8.7). No data are available on the long-term exposure of human
populations to 1-propanol. Hence, the carcinogenicity of
1-propanol in human beings cannot be evaluated. Apart from one
case of fatal poisoning following ingestion of half a litre of
1-propanol, there are practically no reports on adverse health
effects from exposure to 1-propanol either in the general
population or in occupational groups (section 9).
The Task Group considers it unlikely that 1-propanol will pose
a serious health risk for the general population under normal
exposure conditions.
10.2 Evaluation of Effects on the Environment
1-Propanol can be released into the environment during
production, processing, storage, transport, use, and waste disposal
(section 3). It is transferred from water, soil, and waste
disposal sites to the atmosphere by volatilization, from the
atmosphere to water and soil by rain-out, and from soil and waste
disposal sites to ground water by leaching. It is difficult to
estimate its emission into each compartment. Because of its
primary use as a volatile solvent, most of the production volume is
eventually released into the atmosphere (section 4.1).
By reacting with hydroxyl radicals and through rain-out,
1-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 3 days (section 4.2). Thus, measurable
atmospheric levels of 1-propanol are not usually encountered.
Hydrolysis and photolysis are not expected to be important in
the removal of 1-propanol from water and soil, but removal occurs
rapidly by aerobic and anaerobic biodegradation (section 4.3.1) so
that measurable levels are rarely found. Adsorption of 1-propanol
on soil particles is poor but it is likely to be mobile in soil and
it has been shown to increase the permeability of soil to some
aromatic hydrocarbons (section 4.1).
In view of the physical properties of 1-propanol, its potential
for bioaccumulation is low (section 4.3.2). Except in the case of
accident or inappropriate disposal, 1-propanol does not present a
risk for aquatic organisms, insects, or plants at concentrations
that usually occur in the environment. However, 1-propanol at
concentrations of around 5000 mg/litre in water is lethal to
oxygen-using aquatic organisms, indicating that its emission into
surface water at this level may result in serious alteration of the
local ecosystem (section 7).
11. RECOMMENDATIONS
1. 1-Propanol has not shown mutagenic potential in the small
number of assays performed. A full array of modern
genotoxicity tests should be carried out.
2. A single published report suggests carcinogenic activity by
1-propanol, but this study is seriously flawed and cannot be
used to evaluate the potential carcinogenicity of 1-propanol.
The desirability of a carcinogenesis bioassay of 1-propanol
should be considered, on the basis of the outcome of
genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
1-propanol produced reproductive and developmental toxicity in
experimental animals. In view of the potential for
environmental and drinking-water contamination, reproductive
and developmental toxicity should be investigated using oral
dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
1-propanol.
5. The unusually uniform level of toxicity in diverse types of
aquatic organisms that consume gaseous oxygen, and the
exceptionally steep dose-effect curve observed, suggest a
nonspecific effect that may not be restricted to 1-propanol.
These effects merit investigation.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1-Propanol was considered by the Joint FAO/WHO Expert
Committee on Food Additives (JECFA) in its twenty-third report.
Specifications were formulated, but no toxicological monograph was
prepared, and the substance could not be evaluated on the basis of
the data available. Additional toxicological information was
available to JECFA at a later meeting, including the results of a
limited study in rats suggesting a carcinogenic potential for
1-propanol. In its twenty-fifth report,a JECFA noted that life-
time feeding studies in rodents were required to resolve the
problem of carcinogenicity. A toxicological monograph was
prepared. The existing specifications were revised and designated
as "tentative", but no ADI was established.
-------------------------------------------------------------------
a WHO Technical Report Series, No. 669, 1981 ( Evaluation of
certain food additives: Twenty-fifth Report of the Joint
FAO/WHO Expert Committee on Food Additives).
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
2. ADKINS, S.W., NAYLOR, J.M., & SIMPSON, G.M. (1984) The
physiological basis of seed dormancy in Avena fatua. V.
Action of ethanol and other organic compounds. Physiol.
Plant., 62: 18-24.
3. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
4. ALLINGER, N.L., CAVA, M.P., DE JONGH, D.C., JOHNSON, C.R.,
LEBEL, N.A., & STEVENS, C.L. (1971) Organic chemistry, New
York, Worth Publishers, Inc.
5. ANON. (1984) Propyl alcohol complaint settled by US
companies. Chem. marketing Rep., April 23: 3, 17.
6. ARHEM, P. & VAN HELDEN, D. (1983) Effects of aliphatic
alcohols on myelinated nerve membrane. Acta physiol. Scand.,
119: 105-107.
7. AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl and isoamyl alcohols by the isolated
perfused rat liver. J. Pharmacol. exp. Ther., 197: 669-674.
8. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of 2-
halo-pyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
9. BEAUD, P. & RAMUZ, A. (1978) Dosage simultané des alcools
supérieures, et de l'acetate d'éthyle dans les eaux-de-vie
par chromatographie gaz-liquide-solide. Trav. chim. Aliment.
Hyg., 69: 423-430.
10. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1974)
Perturbations du métabolisme hépatique du palmitate [1-14C]
déterminée par l'administration de n-propanol chez le rat.
Biochimie, 56: 1157-1159.
11. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1979)
Comparative effects of ethanol, n-propanol and isopropanol on
lipid disposal by rat liver. Chem.-biol. Interact., 26:
155-166.
12. BENGTSSON, B.-E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13
aliphatic alcohols. Chemosphere, 13: 613-622.
13. BILZER, N. & GRUNER, O. (1983) [Critical assessment regarding
determination of aliphatic alcohols (congeners in alcoholic
drinks) in blood with the aid of head-space analysis.]
Blutalkohol, 20: 411-421 (in German).
14. BILZER, N. & PENNERS, B.-M. (1985) [Concerning the velocity
of reduction and excretion of the attendant substance
propanol-1 and isobutanol after drinking whisky of the trade
mark Chivas Regal.] Blutalkohol, 22: 140-145 (in German).
15. BILZER, N., PENNERS, B.-M., & GRUNER, O. (1985) [Studies
about the course of concentration in blood for congener
propanol-1 and isobutanol after drinking overseas rum
("Captain Morgan").] Blutalkohol, 22: 146-151 (in German).
16. BONTE, W. (1978) [Congener content of wine and similar
beverages.] Blutalkohol, 15: 392-404 (in German).
17. BONTE, W. (1979) [Congener content of German and foreign
beers.] Blutalkohol, 16: 108-124 (in German).
18. BONTE, W., DECKER, J., & BUSSE, J. (1978) [Congener content
of highproof alcoholic beverages.] Blutalkohol, 15: 323-338
(in German).
19. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981a) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human blood.]
Blutalkohol, 18: 399-411 (in German).
20. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human urine.]
Blutalkohol, 18: 412-426 (in German).
21. BONTE, W., STOPPELMAN, G., RUDELL, E., & SPRUNG, R. (1981c)
[Computerized detection of congeners of alcoholic beverages
in body fluids.] Blutalkohol, 18: 303-310 (in German).
22. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
23. BRASS, E.P. & BEYERINCK, R.A. (1987) Interactions of
propionate and carnitine metabolism in isolated hepatocytes.
Metabolism, 36: 781-787.
24. BRASS, E.P., FENNESSEY, P.V. & MILLER, L.V. (1986) Inhibition
of oxidative metabolism by propionic acid and its reversal by
carnitine in isolated rat hepatocytes. Biochem. J., 236:
131-136.
25. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
26. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
27. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.]
Z. Wasser-Abwasser Forsch., 10: 87-98 (in German).
28. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
29. BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.]
Z. Wasser-Abwasser Forsch., 13: 170-173 (in German).
30. BURROWS, W.D. & ROWE, R.S. (1975) Ether soluble constituents
of landfill leachate. J. Water Pollut. Control Fed., 47:
921-923.
31. CAMERON, A.M., ZAHLSEN, K., HAUG, E., NILSEN, O.G., & EIK-
NES, K.B. (1985) Circulating steroids in male rats following
inhalation of n-alcohols. Arch. Toxicol., Suppl., 8: 422-424.
32. CAMPBELL, I.M., MCLAUGHLIN, D.G., & HANDY, B.J. (1976) Rate
constants for reactions of hydroxyl radicals with alcohol
vapours at 292 K. Chem. Phys. Lett., 38: 362-364.
33. CANTON, J.H. & ADEMA, D.M.M. (1978) Reproducibility of short-
term and reproduction toxicity experiments with Daphnia magna
and comparison of the sensitivity of Daphnia magna with
Daphnia pulex and Daphnia cucullata in short-term experiments.
Hydrobiologia, 59: 135-140.
34. CARTER, W.P.L., DARNALL, K.R. GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4-hydroxy radicals
with oxygen. J. phys. Chem., 83: 2305-2311.
35. CEC (1982) Propan-1-ol chemico-physical data, toxicity data,
environmental occurrence, and permissible levels. In: Report
of the Scientific Committee for Food on extraction solvents,
Brussels, Commission of the European Communities, Directorate
General for Internal Market and Industrial Affairs, pp. 27-45.
36. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination: interaction
with phytochrome. Physiol. Plant, 65: 451-454.
37. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese hamster
V79 cells by various organic solvents and simple compounds.
Cell Biol. Toxicol., 1: 155-171.
38. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation
and degradation of petrochemical wastewater components by
methane fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
39. CHUNG, T.-Y., HAYASE, F., & KATO, H. (1983) Volatile
components of ripe tomatoes and their juices, purees and
pastes. Agric. biol. Chem., 47: 343-351.
40. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962) Influence
of alcohols and potassium salts of xanthogenic acids on
various biological objects. Arch. int. Pharmacodyn. Ther.,
135: 330-343.
41. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol.,
9: 383-397.
42. CORBIT, T.E. & ENGEN, T. (1971) Facilitation of olfactory
detection. Perception Psychophysiol., 10: 433-436.
43. CORKEY, B.E., HALE, D.E., GLENNON, M.C., KELLEY, R.I.,
COATES, P.M. KILPATRIK, L. & STANLEY, C.A. (1988)
Relationship between unusual hepatic acyl coenzyme A profiles
and the pathogenesis of Reye syndrome. J. clin. Invest.
82: 782-788.
44. CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No. 600/
3-80-084, PB 80-221948).
45. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100:
34-46.
46. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species:
D. melanogaster and D. simulans. Comp. Biochem. Physiol.,
54C: 71-74.
47. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
48. DE ZWART, D. & SLOOFF, W. (1983) The Microtox as an
alternative assay in the acute toxicity assessment of water
pollutants. Aquat. Toxicol., 4: 129-138.
49. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity of
selected synthetic organic chemicals, The MITRE Corporation
(MITRE Technical Report MTR-7248, Rev. 1, Appendix III).
50. DRAVNIEKS, A. (1974) A building-block model for the
characterization of odorant molecules and their odors. Ann.
N.Y. Acad. Sci., 237: 144-163.
51. DRY, I., WALLACE, W., & NICHOLAS, D.J.D. (1981) Role of ATP
in nitrite reduction in roots of wheat and pea. Planta,
152: 234-238.
52. DURWALD, W. & DEGEN, W. (1956) [A fatal poisoning by n-propyl
alcohol.] Arch. Toxikol., 16: 84-88 (in German).
53. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
54. EICEMAN, G.A. & KARASEK, F.W. (1981) Identification of
residual organic compounds in food packages. J. Chromatogr.,
210: 93-103.
55. FANG, H.H.P. & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
56. FAO/WHO (1980) Toxicological evaluation of certain food
additives. Report of the Joint FAO/WHO Expert Committee on
Food Additives, Geneva, World Health Organization,
pp. 162-168 (WHO Food Additive Series 16).
57. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. geotech. J., 22: 205-214.
58. FLATH, R.A., ALTIERI, M.A., & MON, T.R. (1984) Volatile
constituents of Amaranthus retroflexus L. J. agric. food
Chem., 32: 92-94.
59. FLICK, E.W. (1985) Industrial solvents handbook. New Jersey,
Noyes Data Corp., pp. 220-223.
60. FORSANDER, O.A. (1967) Influence of some aliphatic alcohols
on the metabolism of rat liver slices. Biochem. J., 105:
93-97.
61. GAD, S.C., DUNN ,B.J., DOBBS, D.W., REILLY, C., & WALSH, R.D.
(1986) Development and validation of an alternative dermal
sensitization test: the mouse ear swelling test (MEST).
Toxicol. appl. Pharmacol., 84: 93-114.
62. GAGE, P.W. (1965) The effect of methyl, ethyl and n-propyl
alcohol on neuromuscular transmission in the rat.
J. Pharmacol. exp. Ther., 150: 236-243.
63. GAILLARD, D. & DERACHE, R. (1966) Action de quelques alcools
aliphatiques sur la mobilisation de différentes fractions
lipidiques chez le rat. Food Cosmet. Toxicol., 4: 515-520.
64. GELSOMINI, N. (1985) Head-space analysis with capillary
columns in quality control of wines. In: Proceedings of the
6th International Symposium on Capillary Chromatography,
pp. 515-519.
65. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
66. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants
in the oxidation of aliphatic compounds by activated sludge.
J. Water Pollut. Control Fed., 38: 562-579.
67. GIBEL, W., LOHS, K., & WILDNER, G.P. (1975) [Experimental
study on the cancerogenic activity of propanol-1, 2-methyl-
propanol-1 and 3-methylbutanol. I.] Arch. Geschwulstforsch.,
45: 19-24 (in German).
68. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal
of a chemical waste problem by fish toxicity tests. Sewage
ind. Waste, 24: 1397-1401.
69. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut. Control
Fed., 46: 947-965.
70. GLASGOW, A.M. & CHASE, H.P. (1976) Effect of propionic acid
on fatty acid oxidation and ureagenesis. Pediatr. Res.,
10: 683-686.
71. GOODMAN, D.E. & RAO, R.M. (1984) GC determination of fusel
alcohols in distilled alcoholic beverages. Am. Lab., 16:
100-103.
72. GORESKY, C.A., GORDON, E.R., & BACH, G.G. (1983) Uptake of
monohydric alcohols by liver: demonstration of a shared
enzymic space. Am. J. Physiol., 244: G198-G214.
73. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
74. GRANT, K.A. & SAMSON, H.H. (1984) n-Propanol induced
microcephaly in the neonatal rat. Neurobehav. Toxicol.
Teratol., 6: 165-169.
75. GRAY, V.M. & CRESSWELL, C.F. (1983) The effect of respiratory
inhibitors and alcohols on nitrate utilization and nitrite
accumulation in excised roots of Z. mays L. Z. Pflanzenphysiol.,
112: 207-214.
76. GREGERSEN, N. (1979) Studies on the effects of saturated and
unsaturated short-chain monocarboxylic acids on the energy
metabolism of rat liver mitochondria. Pediat. Res., 14:
1227-1230.
77. GREGERSEN, N. (1981) The specific inhibition of the pyruvate
dehydrogenase complex from pig kidney by propionyl-CoA and
isovaleryl-CoA. Biochem. Med. III, 26: 20-27.
78. GULATI, A., NATH, C., SHANKER, K., SRIMAL, R.C., DHAWAN, K.N.,
& BHARGAVA, K.P. (1985) Effects of alcohols on the
permeability of blood-brain barrier. Pharmacol. Res. Commun.,
17: 85-93.
79. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
80. HANSCH, C. & ANDERSON, S. (1967) The effect of intramolecular
hydrophobic bonding on partition coefficients. J. org. Chem.,
32: 2583-2586.
81. HARRIS, R.A. (1983) Ethanol, membrane perturbation, and
synaptosomal ion transport. Proc. West. Pharmacol. Soc.,
26: 255-257.
82. HAWLEY, G.G. (1981) Condensed chemical dictionary, 10th ed.,
Melbourne, Van Nostrand Reinhold Company Inc.
83. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
84. HERMENS, J., BUSSER, F., LEEUWANGH, P., & MUSCH, A. (1985)
Quantitative structure-activity relationships and mixture
toxicity of organic chemicals in Photobacterium phosphoreum:
the Microtox 85 test. Ecotoxicol. environ. Saf., 9: 17-25.
85. HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974)
Effects of chronic ingestion of some lower aliphatic alcohols
in rats. Res. Commun. chem. Pathol. Pharmacol., 9: 177-180.
86. HILSCHER, H., GEISSLER, E., LOHS, K., & GIBEL, W. (1969)
[Studies on the toxicity and mutagenicity of single fusel oil
components on E. coli.] Acta biol. med. Germ., 23: 843-852
(in German).
87. HIRANO, K., WATANABE, Y., ADACHI, T., ITO, Y., & SUGIURA, M.
(1985) Drug-binding properties of human alpha-foetoprotein.
Biochem J., 231: 189-191.
88. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509
(in German).
89. HODGE, H.C. & STERNER, J.H. (1943) Am. ind. Hyg. Assoc. Q.,
10: 93.
90. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
91. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
161 pp.
92. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
93. HUANG, R.-D, SMITH, M.F., & ZAHLER, W.L. (1982) Inhibition of
Forskolin-activated adenylate cyclase by ethanol and other
solvents. J. cyclic Nucleotide Res., 8: 385-394.
94. HUDOLEI, V.V., MIZGIREV, I.V., & PLISS, G.B. (1987)
[Evaluation of mutagenic activity of carcinogens and other
chemical agents with Salmonella typhimurium assays.] Vopr.
Onkol., 32: 73-80 (in Russian).
95. IRPTC (1987) Data profile on n-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
96. IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ,
I.D., & BRADSHAW, J.J. (1978) Organic compounds. Their
interaction with and degradation of hepatic microsomal drug-
metabolizing enzymes in vitro. Drug Metab. Dispos., 6:
218-225.
97. JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavour volatiles in heat-treated milks.
J. dairy Res., 45: 391-403.
98. JOUANY, J.-P. (1982) Volatile fatty acid and alcohol
determination in digestive contents, silage juices, bacterial
cultures and anaerobic fermentor contents. Sci. Aliment.,
2: 131-144.
99. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
100. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J.,
53: 129-136.
101. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents.
Am. Ind. Hyg. Assoc. J., 41: 451-455.
102. KHARE, M. & DONDERO, N.C. (1977) Fractionation and
concentration from water of volatiles and organics on high
vacuum system: examination of sanitary landfill leachate.
Environ. Sci. Technol., 11: 814-819.
103. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted filberts.
J. agric. food Chem., 20: 1021-1028.
104. KIRK, R.E & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
105. KLECKA, G.M., LANDI, L.P., & BODNER, K.M. (1985) Evaluation
of the OECD activated sludge, respiration inhibition test.
Chemosphere, 14: 1239-1251.
106. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
107. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegypti
larvae. J. Invertebr. Pathol., 42: 285-287.
108. KRISTIANSEN, U., HANSEN, L. NIELSEN, G.A., & HOLST, E. (1986)
Sensory irritation and pulmonary irritation of cumene and
n-propanol: Mechanisms of receptor activation and
desensitization. Acta pharmacol. toxicol., 59: 60-72.
109. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
110. KUHNHOLZ, B. (1985) [Reflections concerning the analysis of
free aliphatic alcohols in tissue.] Blutalkohol, 22: 455-461
(in German).
111. LAING, D.G. (1975) A comparative study of the olfactory
sensitivity of humans and rats. Chem. Senses Flavor, 1:
257-269.
112. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
113. LASNE, C., GU, Z.W., VENEGAS, W., & CHOUROULINKOV, I. (1984)
The in vitro micronucleus assay for detection of cytogenetic
effects induced by mutagen-carcinogens: comparison with the
in vitro sister-chromatid exchange assay. Mutat. Res.,
130: 273-282.
114. LIEBICH, H.M., BUELOW, H.J., & KALLMAYER, R. (1982)
Quantification of endogenous aliphatic alcohols in serum and
urine. J. Chromatogr., 239: 343-349.
115. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermatit., 3: 240-244.
116. LUTHIN, G.R. & TABAKOFF, B. (1984) Activation of adenylate
cyclase by alcohols requires the nucleotide-binding protein.
J. Pharmacol. exp. Ther., 228: 579-587.
117. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
118. MAICKEL, R.P. & NASH, J.F. (1985) Differing effects of
short-chain alcohols on body temperature and coordinated
muscular activity in mice. Neuropharmacology, 24: 83-89.
119. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology,
17: 451-461.
120. MANKES, R.F., LEFEVRE, R., RENAK,V., FIESHER, J., & ABRAHAM,
R. (1985) Reproductive effects of some solvent alcohols with
differing partition coefficients. Teratology, 31: 67A.
121. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
122. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft,
26: 385-389 (in German).
123. MAY, W.A., PETERSON, R.J., & CHANG, S.S. (1983) Chemical
reactions involved in the deep-fat frying of foods. IX.
Identification of the volatile decomposition products.
J. Am. Oil Chem. Soc., 60: 990-995.
124. MICHAELIS, M.L. & MICHAELIS, E.K. (1983) Alcohol and local
anesthetic effects on Na+-dependent Ca2+-fluxes in brain
synaptic membrane vesicles. Biochem. Pharmacol., 32:
963-969.
125. MIKHEEV, M.J., & FROLOVA, A.D. (1978) [Toxicokinetics of
certain representatives of a homologous series of alcohols.]
Gig. i Sanit., 6:33-36 (in Russian).
126. MORGAN, E.T., KOOP, D.R., & COON, M.J. (1982) Catalytic
activity of cytochrome P-450 isozyme 3a isolated from liver
microsomes of ethanol-treated rabbits. J. biol. Chem.,
257: 13951-13957.
127. MOSHONAS, M.G. & SHAW, P.E. (1987) Quantitative analysis of
orange juice flavor volatiles by direct-injection gas
chromatography. J. Agric. food Chem., 35:161-165.
129. MULLIN, M.J. & HUNT, W.A. (1985) Actions of ethanol on
voltage-sensitive sodium channels: effects on neurotoxin-
stimulated sodium uptake in synaptosomes. J. Pharmacol. exp.
Ther., 232: 413-419.
130. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
131. MURPHREE, H.B., GREENBERG, L.A., & CARROLL, R.B. (1967)
Neuropharmacological effects of substances other than ethanol
in alcoholic beverages. Fed. Proc., 26: 1468-1473.
132. NELSON, B.K., BRIGHTWELL, W.S., & BURG, J.R. (1985)
Comparison of behavioural teratogenic effects of ethanol and
n-propanol administered by inhalation to rats. Neurobehav.
Toxicol Teratol., 7: 770-783.
132. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W. & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentration to rats. Food Chem. Toxicol.,
26(3): 247-254.
133. NEY, K.H. (1985) [Flavour of Tilsit cheese.] Fette Seifen
Anstrichmittel, 87: 289-293 (in German).
134. NGUYEN, V.C. & KATO, H. (1982) Volatile flavor components of
Kumazasa (Sasa albo-marginata). Agric. Biol. Chem., 46:
2795-2801.
135. NUNOMURA, N., SASAKI, M., & YOKOTSUKA, T. (1984) Shoyu
(soy sauce) flavor components: neutral fraction. Agric. Biol.
Chem., 48: 1753-1762.
136. OBE, G. & RISTOW, H. (1977) Acetaldehyde, but not ethanol,
induces sister chromatid exchanges in Chinese hamster cells
in vitro. Mutat. Res., 56: 211-213.
137. ODKVIST, L.M., LARSBY, B., THAM, R., & ASCHAN, G. (1979) On
the mechanism of vestibular disturbances caused by industrial
solvents. Adv. Oto-Rhino-Laryng., 25: 167-172.
138. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub. Reinhalt. Luft, 32: 400-407 (in German).
139. OERSKOV, S.I. (1950) Experiments on the oxydation of propyl
alcohol in rabbits. Acta physiol. Scand., 20: 258-262.
140. OTSUK141.A, K., IKI, I., & YAMASHITA, T. (1979) [Relationship
between type of whisky and volatile component.] Hakkokogaku,
57: 20-30 (in Japanese).
141. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. 4. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 K. J. phys. Chem., 82: 1329-1333.
142. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
143. PATERNOSTRE, M., PICHON, Y., & DUPEYRAT, M. (1983) Effects
of n-alcohols on ionic transmembrane currents in the squid
giant axon. Stud. phys. thero. Chem., 24: 515-522.
144. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
145. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols
of the stimulated activity of ornithine decarboxylase and
tyrosine aminotransferase occurring in regenerating rat liver.
Biochem. Pharmacol., 29: 2799-2803.
146. POSTEL, W. & ADAM, L. (1978) [Gas chromatographic
characterization of whisky. III. Communication: Irish
whiskey.] Branntweinwirtsch., 118: 404-407 (in German).
147. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
148. PREUSS, A. & ZIPFEL, K. (1985) [Headspace-gas chromatographic
identification of alcoholic beverages and foodstuffs.]
Lebensmittelchem. gerichtl. Chem., 39: 97-99 (in German).
149. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
150. PRIESTLEY, D.A. & LEOPOLD, A.C. (1980) Alcohol stress on
soya bean seeds. Ann. Bot., 45: 39-45.
151. PUNTER, P.H. (1983) Measurement of human olfactory thresholds
for several groups of structurally related compounds. Chem.
Senses, 7: 215-235.
152. PURCHASE, I.F.H. (1969) Studies in kaffircorn malting and
brewing. XXII. The acute toxicity of some fusel oils found in
Bantu beer. S.A. med. J., 43: 795-798.
153. QUISTAD, G.B., STAIGER, L.E. & SCHOOLEY, D.A. (1986) The
role of carnitine in the conjugation of acidic xenobiotics.
Drug Metab. Dispos., 14:521-525.
154. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
155. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. membr. Biol., 84: 229-238.
156. REYNOLDS, T. (1977) Comparative effects of aliphatic
compounds on inhibition of lettuce fruit germination. Ann.
Bot., 41: 637-648.
157. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch., 21:
1309-1319 (in German).
158. ROE, C.R., MILLINGTON, D.S., MALTBY, D.A., BOHAN, T.P. &
HOPPEL, C.L. (1984) L-Carnitine enhances excretion of
propionyl coenzyme A as propionylcarnitine in propionic
acidemia. J. clin. Invest., 73: 1785-1788.
159. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
160. RYAN, D.E., THOMAS, P.E., WRIGHTON, S.A., GUZELIAN, P.S. &
LEVIN, W. (1987) Ethanol-inducible rat and human liver
cytochrome P-450: characterization and role as high affinity
N-nitrosodimethylamine demethylamine demethylases. In:
Miners, J., Birkett, D.J., Drew, R., May, B., & McManus, ed.
Microsomes and drug oxidations, London, Taylor & Francis,
pp. 3-11.
161. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
162. SAHU, B.R. & TANDON, U. (1983) A novel method for spot test
detection of alcohols. J. Indian Chem. Soc., 60: 615-616.
163. SANCEDA, N., KURATA, T., & ARAKAWA, N. (1984) Fractionation
and identification of volatile compounds in Patis, a
Phillipine fish sauce. Agric. Biol. Chem., 48: 3047-3052.
164. SAVINI, E.C. (1968) Estimation of the LD50 in mol/kg. Proc.
Eur. Soc. Study Drug Toxicol., 9: 276-278.
165. SCHEIMAN, M.A., SAUNDERS, R.A., & SAALFELD, F.E. (1974)
Organic contaminants in the District of Columbia water supply.
Biomed. Mass Spectrom., 1: 209-211.
166. SCOTT, T. & EAGLESON, M. (1983) Concise encyclopedia of
biochemistry. New York, W. de Gruyter, pp. 158-159.
167. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
168. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona Heilborn).
J. agric. food Chem., 33: 795-797.
169. SINCLAIR, J.F., SMITH, L., BEMENT, W.J., SINCLAIR, P.R., &
BONKOWSKY, H.L. (1982) Increases in cytochrome P-450 in
cultured hepatocytes mediated by 3- and 4-carbon alcohols.
Biochem. Pharmacol., 31: 2811-2815.
170. SLOOFF, W. (1983) Benthic macroinvertebrates and water
quality assessment: some toxicological considerations. Aquat.
Toxicol., 4 : 73-82.
171. SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the
usefulness of the Mexican Axolotl (Ambystoma mexicanum) and
the clawed toad (Xenopus laevis) in toxicological bioassays.
Bull. environ. Contam. Toxicol., 24: 439-443.
172. SLOOFF, W., CANTON, J.H., & HERMENS, J.L.M. (1983) Comparison
of the susceptibility of 22 freshwater species to 15 chemical
compounds. I. (Sub)acute toxicity tests. Aquat. Toxicol.,
4: 113-128.
173. SMYTH, H.F., CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data. Arch. ind. Hyg. occup.
Med., 10: 61-68.
174. SNIDER, J.B. & DAWSON, G.A. (1985) Tropospheric light
alcohols, carbonyls, and acetonitrile: concentrations in the
southwestern United States and Henry's law data. J. geophys.
Res., 90: 3797-3805.
175. SOLIMAN, M.A., EL SAWY, A.A., FADEL, H.M., OSMAN, F., & GAD,
A.M. (1985) Volatile components of roasted Citrillus
colocynthis, var. Colocynthoides. Agric. Biol. Chem.,
49: 269-275.
176. SRI (1984) n-Propanol: record number 0115 (last revision
date 01-06-84). In: Toxicology data bank, Palo Alto,
California, Stanford Research Institute, Bethesda, Maryland,
National Library of Medicine.
177. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
178. STOCK, K. & SCHMIDT, M. (1978) Effects of short-chain
alcohols on adenylate cyclase in plasma membranes of rat
adipocytes. Naunyn-Schmiedeberg Arch. Pharmacol., 302: 37-43.
179. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials: second cumulative
series. Perfumer Flavorist, 9: 53-83.
180. STOKES, J.A. & HARRIS, R.A. (1982) Alcohols and synaptosomal
calcium transport. Mol. Pharmacol., 22: 99-104.
181. STOLZENBERG, S.J. & HINE, C.H. (1979) Mutagenicity of
halogenated and oxygenated three-carbon compounds. J. Toxicol.
environ. Health, 5: 1149-1158.
182. STONE, H., PRYOR, G.T., & STEINMETZ, G. (1972) A comparison
of olfactory adaptation among seven odorants and their
relationship with several physico-chemical properties.
Perception Psychophys., 12: 501-504.
183. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
184. STUMPF, D.A., MCAFEE, J., PARKS, J.K., & EGUREN, L. (1986)
Propionate inhibition of succinate: CoA ligase (GDP) and the
citric acid cycle in mitochondria. Pediatr. Res., 14:
1127-1131.
185. TABAKOFF, B. & HOFFMAN, P.L. (1983) Alcohol interactions with
brain opiate receptors. Life Sci., 32: 197-204.
186. TANDOI, P., GUIDOTTI, M., & STACCHINI, P. (1984) [On the
composition of brandies.] Riv. Soc. Ital. Sci. Aliment.,
13: 69-76 (in Italian).
187. TAYLOR, J.M., JENNER, P.M., & JONES, W.I. (1964) A comparison
of the toxicity of some allyl, propenyl, and propyl compounds
in the rat. Toxicol. appl. Pharmacol., 6: 378-387.
188. TESCHKE, R., HASUMURA, Y., & LIEBER, C.S. (1975) Hepatic
microsomal alcohol-oxidizing system. Affinity for methanol,
ethanol, propanol, and butanol. J. biol. Chem., 250:
7397-7403.
189. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
190. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
191. TOMERA, J.F., SKIPPER, P.L., WISHNOK, J.S., TANNEBAUM, S.R.,
& BRUNENGRABER, H. (1984) Inhibition of N-nitroso-
dimethylamine metabolism by ethanol and other inhibitors in
the isolated perfused rat liver. Carcinogenesis, 5: 113-116.
192. UHLEMANN, E.R., ROBBERECHT, P., & GARDNER, J.D. (1979)
Effects of alcohols on the actions of VIP and secretin on
acinar cells from guinea-pig pancreas. Gastroenterology,
76: 917-925.
193. UNRUH, J.D. & SPINICELLI, L. (1981) Propyl alcohols, n-propyl
alcohol. In: Kirk, R.E. & Othmer, D.F., ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience,
Vol. 19, pp. 221-227, 194.
194. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
195. US NIOSH (1984) Method 1401. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1,
pp. 1401-1-1401-4.
196. VAISHNAV, D.D. & LOPAS, D.M. (1985) Relationship between
lipophilicity and biodegradation inhibition of selected
industrial chemicals. Dev. ind. Microbiol., 26: 557-565.
197. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
198. VERSCHUEREN, K. (1983) Handbook of environmental data on
organic chemicals, 2nd ed., Melbourne, Van Nostrand Reinhold
Company Inc.
199. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
200. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER, B.,
& BASLER, A. (1987) Genotoxicity of three carbon compounds
evaluated in the SCE test in vitro. Environ. Mutagen.,
9: 401-410.
201. WACHTEL, R.E. (1984) Aliphatic alcohols increase the decay
rate of glutamate-activated currents at the crayfish
neuromuscular junction. Br. J. Pharmacol., 83: 393-397.
202. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265.
203. WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
IIJIMA, M. (1984) Induction of megamitochondria in the rat
liver by n-propyl alcohol and n-butyl alcohol. Acta pathol.
Jpn., 34: 471-480.
204. WALLGREN, H. (1960) Relative intoxicating effects on rats
of ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
205. WATERER, D.R. & PRITCHARD, M.K. (1985a) Volatile production
by wounded Russet Burbank and Norland potatoes. Sci. Aliments,
5: 205-216.
206. WATERER, D.R. & PRITCHARD, M.K. (1985b) Production of
volatile metabolites in potatoes infected by Erwinia
carotovora var. carotovora and E. carotovora var. atroseptica.
Can. J. plant Pathol., 7: 47-51.
207. WILKIN, J.K. & FORTNER, G. (1985) Cutaneous vascular
sensitivity to lower aliphatic alcohols and aldehydes in
orientals. Alcoholism clin. exp. Res., 9: 522-525.
208. WINTERSTEIGER, R., GAMSE, G., & PACHA, W. (1982)
[Quantification of alcoholic compounds and amines with 4-(6-
methylbenzo-thiazol-2-yl)phenyl isocyanate. Fresenius Z.
anal. Chem., 312: 455-461.
209. WOLF, M., URBAN, R., WELLER, J.-P., & TROGER, H.D. (1985)
[The analysis of congeners of alcoholic beverages in blood.
First communication: the application of capillary gas
chromatography in micro head-space analysis.] Blutalkohol,
22: 321-332 (in German).
210. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
211. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., UCHIDA,
H., & HAYASHI, K. (1983) Volatile flavor compounds of boiled
buckwheat flour. Agric. Biol. Chem., 47: 729-738.
212. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., &
HAYASHI, K. (1984) Volatile flavor components of Kogyoku
apples. Agric. Biol. Chem., 48: 849-855.
213. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
214. YASUHARA, A. & FUWA, K. (1982) Characterization of odorous
compounds in rotten blue-green algae. Agric. Biol. Chem.,
46: 1761-1766.
215. YASUHARA, A., FUWA, K., & JIMBU, M. (1984) Identification of
odorous compounds in fresh and rotten swine manure. Agric.
Biol. Chem., 48: 3001-3010.
216. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Manag. Res., 1: 213-226.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-1 est un liquide incolore, très inflammable, qui
est volatil à la température ambiante et sous la pression
atmosphérique normale. Il est miscible à l'eau et aux solvants
organiques. Parmi les méthodes d'analyse, on peut citer la
chromatographie en phase gazeuse, qui permet de déceler jusqu'à
5 x 10-5 mg/m3 dans l'air, 1 x 10-4 mg/litre dans l'eau et
0,002 mg/litre dans le sang, le sérum ou les urines lorsque
l'échantillon a été extrait ou concentré de façon satisfaisante.
2. Sources d'exposition humaine et environnementale
La capacité de production mondiale a dépassé 130 000 tonnes en
1979. Le propanol-1 d'origine naturelle résulte de la
décomposition de matériaux organiques par divers microorganismes et
on le rencontre également dans les végétaux et le mazout.
Industriellement, on le produit par réaction de l'éthylène sur
l'oxyde de carbone et l'hydrogène qui donne du propionaldéhyde,
celui-ci étant ensuite hydrogéné en propanol. C'est également un
sous-produit de la fabrication du méthanol et il peut être obtenu
directement à partir du propane ou de l'acroléine. Le propanol-1
est essentiellement un solvant à tout faire, à usage industriel ou
domestique. Il entre dans la composition des encres d'imprimerie
flexographiques et il est également utilisé dans l'industrie
textile, dans des produits à usage personnel tels que les produits
cosmétiques, les lotions ainsi que dans les produits pour le
nettoyage des vitres, les encaustiques et les antiseptiques. En
second lieu par ordre d'importance, on peut citer son utilisation
comme produit intermédiaire dans la fabrication de divers composés
chimiques.
3. Transport, distribution et transformation dans l'environnement
La principale voie de pénétration de propanol-1 dans
l'environnement est constituée par les émissions dans l'atmosphère
qui se produisent au cours de la production, de la transformation,
du stockage, du transport et de l'utilisation de ce composé ou du
rejet de déchets qui en contiennent. Il peut y avoir également des
décharges dans l'eau et le sol. Le propanol-1 étant principalement
utilisé comme solvant volatil, il finit par se dissiper en grande
partie dans l'environnement.
Le propanol-1 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et par les précipitations. Il
est facilement biodégradable tant par voie aérobie que par voie
anaérobie et du fait de l'existence de ces mécanismes d'élimination
chimique et biologique, on ne le rencontre normalement pas en
quantité mesurable dans l'environnement. Toutefois on en a décelé
la présence dans l'air des agglomérations urbaines, dans des
décharges, ainsi que dans les eaux s'échappant de zones
d'enfouissement de déchets.
Le logarithme du coefficient de partage n-octanol/eau du
propanol-1 est de 0,34 et son facteur de bioconcentration a une
valeur de 0,7, ce qui en rend la bioaccumulation très improbable.
4. Niveau dans l'environnement et exposition humaine
La population en général peut être exposée au propanol par
suite d'une ingestion accidentelle, par inhalation lors de
l'utilisation du produit ou par absorption avec la nourriture
(propanol d'origine naturelle, additif d'aromatisation ou reste de
solvant) ou des boissons alcoolisées ou non. Par exemple la bière
en contient jusqu'à 195 mg/litre, le vin jusqu'à 116 mg/litre et
certains spiritueux jusqu'à 3500 mg/litre. L'exposition de la
population par suite d'inhalation ou de la consommation d'eau de
boisson est faible (aux Etats-Unis la concentration moyenne dans
des échantillons d'air urbain se situait à 0,00005 mg/m3 et dans
des échantillons d'eau de boisson, à 0,001 mg/litre). Les
travailleurs courent un risque d'exposition par inhalation lors de
la production, de la transformation et de l'utilisation du produit.
Toutefois, on ne dispose d'aucune donnée qui permettrait de
chiffrer ce type d'exposition.
5. Cinétique et métabolisme
Après ingestion, le propanol-1 est rapidement absorbé et
distribué dans l'ensemble de l'organisme. On manque de données sur
la vitesse d'absorption après inhalation et exposition cutanée. Le
propanol-1 est métabolisé par l'alcool déshydrogénase (ADH) en
aldéhyde puis acide propionique et peut entrer dans le cycle de
Krebs. Cette oxydation constitue l'étape limitante du métabolisme
du propanol-1. In vitro, les oxydases microsomiques du rat et du
lapin sont également capables d'oxyder le propanol-1 en aldéhyde
propionique. L'ADH et des systèmes d'oxydation microsomiques ont
une affinité beaucoup plus importante pour le propanol-1 que pour
l'éthanol; aussi le propanol-1 est-il rapidement éliminé de
l'organisme. Chez le rat, sa demi-vie après administration par
voie orale d'une dose de 1000 mg/kg est de 45 minutes.
Chez l'animal et chez l'homme, le propanol-1 peut être éliminé
de l'organisme dans l'air expiré ou dans les urines. Chez des
êtres humains ayant reçu par voie orale une dose de 3,75 mg de
propanol-1 par kg de poids corporel et de 1200 mg d'éthanol par kg
de poids corporel, l'excrétion urinaire totale du propanol-1 a été
de 2,1 % de la dose. Les concentrations urinaires de propanol-1
étaient d'autant plus basses que la quantité d'éthanol ingérée
simultanément était basse, ce qui montre qu'il y avait compétition
pour l'ADH entre le propanol-1 et la surdose d'éthanol.
6. Effets sur les êtres vivant dans leur milieu naturel
Aux concentrations normalement présentes dans l'environnement,
le propanol-1 n'est pas toxique pour la vie aquatique, les insectes
ou les végétaux. Chez trois des espèces aquatiques les plus
sensibles (trois protozoaires), le seuil l'inhibition de la
multiplication cellulaire se situait entre 38 et 568 mg/litre.
Dans le cas d'organismes plus évolués, la concentration létale
était d'environ 5000 mg/litre, avec des variations remarquablement
faibles d'un phylum à l'autre et une courbe dose-réponse de très
forte pente. Certaines bactéries et micro-organismes qui vivent
dans les eaux résiduaires et les boues activées sont capables de
s'adapter à des concentrations supérieures à 17 000 mg/litre.
Le propanol-1 peut inhiber ou au contraire stimuler la
germination des semences selon sa concentration dans l'eau
d'arrosage et les conditions d'exposition. Le composé accroît
l'accumulation de nitrites dans le maïs, les pois et le froment.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in vitro
Le propanol-1 présente une faible toxicité aiguë pour les
mammifères (mesurée d'après la mortalité), que l'exposition se
fasse par voie percutanée, orale ou respiratoire. On a fait état
de valeurs allant de 1870 à 6800 mg par kg de poids corporel pour
la DL50 par voie orale chez plusieurs espèces animales. Toutefois
pour de très jeunes rats, on donne une DL50 orale de 560 à 660 mg
par kg de poids corporel. Après une seule exposition, le principal
effet toxique du propanol-1 consiste dans la dépression du système
nerveux central. Selon les données disponibles, le propanol-1
exercerait sur le système nerveux central des effets analogues à
ceux de l'éthanol; toutefois il semblerait que la neurotoxicité du
propanol-1 soit plus importante. Les DE50 pour l'anesthésie chez
le lapin et la perte du réflexe de redressement chez la souris se
situaient respectivement à 1440 mg par kg de poids corporel par
voie orale et à 1478 mg par kg de poids corporel par voie intra-
péritonéale; ces doses sont environ quatre fois plus faibles que
dans le cas de l'éthanol. Dans l'épreuve du plan incliné, le
propanol-1 s'est révélé 2,5 fois plus actif que l'éthanol chez le
rat.
Des doses uniques de 3000 ou 6000 mg par kg de poids corporel
administrées par voie orale à des rats ont provoqué une
accumulation réversible de triglycérides dans le foie. Les vapeurs
fortement concentrées provoquent une irritation des voies
respiratoires chez la souris. Aux concentrations d'environ 30 000
mg/m3 on note une réduction de 50 % du rythme respiratoire chez la
souris.
On ne dispose de données ni sur l'irritation oculaire ni sur
l'irritation cutanée. Aucun effet n'a été observé lors d'une
épreuve de sensibilisation cutanée sur des souris CF1.
Chez des rats mâles exposés pendant six semaines à une dose de
15 220 mg/m3 de propanol-1, on a relevé quelques signes d'une
action nocive possible sur la fonction de reproduction. En
revanche aucun effet n'a été noté à la dose de 8610 mg/m3. Après
exposition de rattes gravides au propanol-1, on a observé des
signes patents de toxicité pour les mères et les foetus aux doses
de 23 968 et 14 893 mg/m3 (9743 et 6054 ppm respectivement); aucun
signe de toxicité n'a été noté à 9001 mg/m3 (3659 ppm). On n'a
observé aucune anomalie comportementale parmi les descendants de
rats mâles exposés pendant six semaines à 8610 ou 15 220 mg de
propanol-1 par m3, ni dans la descendance de rattes exposées
pendant leur gestation aux mêmes concentrations. Toutefois, en
administrant à des ratons de 5 à 8 jours des doses orales de 3000 à
7800 mg de propanol-1 par kg et par jour, on constatait des signes
de dépression du SNC pendant l'administration et un syndrome de
sevrage à la cessation du traitement. Le cerveau de ces rats a été
examiné à l'âge de 18 jours; on a constaté une réduction du poids
de cet organe tant en valeur absolue qu'en valeur relative, avec
diminution de la teneur en ADN et réduction localisée des taux de
cholestérol et de protéine.
La recherche de mutations ponctuelles au moyen de 2 épreuves
utilisant Salmonella typhimurium n'a donné que des résultats
négatifs, de même la recherche de mutations réverses sur
Escherichia coli CA-274. Les résultats ont été également négatifs
en ce qui concerne les échanges entre chromatides soeurs ou la
présence de micro-noyaux dans les cellules mammaliennes in vitro.
Il n'existe pas d' autres données relatives à la mutagénicité.
Lors d'une étude de cancérogénicité portant sur de petits
groupes de rats Wistar exposés tout au long de leur existence par
voie orale à des doses de 240 mg/kg ou par voie sous cutanée à des
doses de 48 mg/kg, on a constaté un accroissement sensible de
l'incidence des sarcomes du foie dans le groupe recevant le produit
par voie sous cutanée. Toutefois cette étude ne permet pas
d'apprécier la cancérogénicité du propanol-1, notamment à cause de
l'absence de détails expérimentaux, du nombre trop restreint
d'animaux et de l'utilisation d'une dose hépatotoxique unique très
élevée.
8. Effets sur la santé humaine
On n'a pas signalé d'effets nocifs sur la santé humaine dans la
population en général ou parmi des groupes professionnels. Le seul
cas d'intoxication mortelle qui ait été signalé est celui d'une
femme, retrouvée inconsciente et qui est décédé 4 à 5 heures après
l'ingestion de propanol. L'autopsie a révélé un oedème cérébral et
pulmonaire. Lors d'une étude sur l'irritation et la sensibilisation
cutanées, on a signalé des réactions allergiques chez un membre du
personnel du laboratoire. Chez neuf volontaires sur 12, on a
observé un érythème qui a duré au moins 1 h après 5 minutes
d'application sur les avant-bras de papiers filtres imprégnés de
0,025 ml d'une solution aqueuse à 75 % de propanol-1. On ne
dispose d'aucun autre rapport concernant d'éventuels effets
toxiques après exposition professionnelle au propanol-1.
Il n'existe pas d'études épidémiologiques qui permettent
d'établir les effets à long terme, et notamment la cancérogénicité,
du propanol-1 chez l'homme.
9. Résumé de l'évaluation
Il peut y avoir exposition humaine au propanol-1 à la suite de
l'absorption de nourriture ou de boissons qui en contiennent. Une
exposition par inhalation peut se produire lors de l'utilisation de
ce produit à des fins ménagères, ou professionnelles, au cours de
la fabrication, de la transformation et de l'utilisation de ce
produit. Les données très limitées dont on dispose sur la teneur
de l'air ambiant et de l'eau en propanol-1 indiquent que ces
teneurs sont très faibles.
Le propanol-1 est rapidement absorbé et se répartit dans tout
l'organisme après ingestion. Après inhalation, l'absorption est
également rapide mais la résorption percutanée devrait être lente.
Chez l'animal, la toxicité aiguë du propanol-1 est faible, que
l'exposition ait lieu par voie percutanée, par voir orale ou par
voie respiratoire. L'exposition de personnes appartenant à la
population générale à des teneurs potentiellement mortelles peut se
produire à la suite d'une ingestion accidentelle ou volontaire.
Toutefois, un seul cas d'intoxication mortelle par le propanol-1 a
été signalé jusqu'ici. Les effets aigus les plus probables chez
l'homme sont une intoxication de type alcoolique pouvant entraîner
une narcose. L'expérimentation animale montre que le propanol-1
est 2 à 4 fois plus toxique que l'éthanol.
Le propanol-1 peut être irritant pour la peau mouillée.
On ne dispose pas de données suffisantes sur la toxicité chez
l'animal pour procéder à une évaluation des risques pour la santé
humaine qui découleraient d'une exposition répétée ou prolongée au
propanol-1. Toutefois, un certain nombre d'études à court terme
sur le rat, bien que limitées, indiquent que dans les conditions où
se produit habituellement l'exposition humaine, il est peu probable
que le risque encouru soit très grave.
Une exposition par inhalation à une concentration de 15 220
mg/m3 a affecté la fonction de reproduction de rats mâles, ce qui
n'a pas été le cas à la dose de 8610 mg/m3. Chez des rattes
gravides, la dose sans effet observable se situait à 9001 mg/3
(3659 ppm); quant à la dose la plus faible donnant lieu à un effet
observable, elle était de 14 893 mg/m3 (6054 ppm), en ce qui
concerne la toxicité pour la mère et le foetus. L'exposition par
inhalation à de fortes concentrations de propanol-1 a donc des
effets nocifs sur la reproduction et le développement des rats
mâles et femelles lorsque les concentrations utilisées sont
manifestement toxiques pour ces animaux. Pour obtenir ces effets,
il a fallu utiliser des concentrations plus élevées que celles
auxquelles l'homme pourrait être normalement exposé.
Différentes épreuves ont montré que le propanol-1 ne provoquait
pas de mutations ponctuelles chez les bactéries. Toutefois, si ces
résultats donnent à penser que le produit n'est pas génotoxique,
les données disponibles sont trop limitées pour qu'on puisse en
évaluer correctement le pouvoir mutagène. La seule étude dont on
possède les résultats ne permet pas d'évaluer convenablement la
cancérogénicité du propanol-1 chez l'animal d'expérience. On ne
dispose d'aucune donnée sur l'exposition à long terme des
populations humaines à ce produit, de sorte qu'on ne peut pas se
prononcer sur son pouvoir cancérogène chez l'homme.
A part un cas d'intoxication mortelle consécutif à l'ingestion
d'un demi-litre de propanol-1, il n'existe pratiquement aucun
rapport concernant d'éventuels effets nocifs découlant d'une
exposition au propanol-1, qu'il s'agisse de la population générale
ou de groupes professionnels. Le Groupe spécial estime qu'il est
improbable que le propanol-1 presente des risques graves pour la
population générale dans les conditions normales d'exposition.
Du propanol-1 peut être libéré dans l'environnement lors de la
production, de la transformation, du stockage, du transport, de
l'utilisation ou de rejet de ce produit. Comme il est
essentiellement utilisé comme solvant volatil, l'essentiel de la
production finit par aboutir dans l'atmosphère. Toutefois, par
réaction avec les radicaux hydroxyles et entraînement par les
précipitations, le propanol-1 est rapidement éliminé de
l'atmosphère, d'où il disparaît en moins de trois jours. Le
propanol-1 s'élimine également rapidement de l'eau et du sol de
sorte que l'on ne le rencontre que rarement en concentrations
mesurables dans l'air, l'eau et la terre. Le propanol-1 est peu
absorbé par les particules du sol où il se révèle mobile et dont il
accroît la perméabilité à certains hydrocarbures aromatiques.
Compte tenu des propriétés physiques du propanol-1, il est peu
probable qu'il donne lieu à une bioaccumulation et sauf accident ou
rejet négligent, le propanol-1 ne présente aucun risque pour la
faune ou la flore aquatique aux concentrations où on le rencontre
habituellement dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 1-propanol es un líquido incoloro y sumamente inflamable,
volátil a temperatura ambiente y presión atmosférica normal. Es
miscible con el agua y los disolventes orgánicos. Entre los
métodos analíticos para el propanol figuran la cromatografía de
gases, que puede detectar hasta 5 x 10-5 mg/m3 en el aire,
1 x 10-4 mg/litro en el agua y 0,002 mg/litro en la sangre, el
suero o la orina cuando se utilizan con la muestra procedimientos
adecuados de extracción o concentración.
2. Fuentes de exposición humana y ambiental
En 1979, la capacidad de producción mundial al año superó las
130 000 toneladas. En la naturaleza se produce por descomposición
de material orgánico por diversos micro-organismos, y se halla en
las plantas y en los aceites combustibles. El 1-propanol se
produce a partir del eteno por reacción con el monóxido de carbono
y el hidrógeno para dar propionaldehído, que a continuación se
hidrogena. Aparece también como sub-producto en la fabricación del
metanol y puede producirse a partir del propano directamente o a
partir de la acroleína. El uso principal del 1-propanol es como
disolvente de uso múltiple en la industria y el hogar. Se utiliza
en las tintas de impresión flexográfica y en aplicaciones textiles,
productos de uso personal como cosméticos y lociones, en productos
para limpiar cristales, en abrillantadores y en fórmulas
antisépticas. Le sigue en importancia su uso como producto
intermedio en la fabricación de diversos compuestos químicos.
3. Transporte, distribución y transformación en el medio ambiente
La principal vía de entrada del 1-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación de
desechos. También se producen emisiones al agua y al suelo.
Puesto que el uso principal del 1-propanol es como disolvente
volátil, gran parte del volumen de producción acaba en el medio
ambiente.
El 1-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y por el lavado con la lluvia. Es
fácilmente biodegradable, tanto en condiciones aerobias como
anaerobias y, a causa de estos mecanismos de eliminación química y
biológica, no suelen encontrarse niveles medibles de la sustancia
en el medio ambiente. No obstante, se ha detectado en la atmósfera
urbana, en vertederos de desechos y también en las aguas que se
rezumaban de un terraplenado. La permeabilidad del suelo al
1-propanol es probablemente elevada y el compuesto aumenta la
permeabilidad a ciertos disolventes aromáticos.
El 1-propanol tiene un coeficiente de reparto log
n-octanol/agua de 0,34 y un factor de bioconcentración de 0,7, lo
que hace muy poco probable su bioacumulación.
4. Niveles ambientales y exposición humana
La exposición de la población general puede producirse por
ingestión accidental, por inhalación durante el uso y por ingestión
junto con los alimentos (que contengan 1-propanol como aromatizante
volátil natural o añadido o como residuo de disolvente) y bebidas
alcohólicas y no alcohólicas. Por ejemplo, la cerveza contiene
hasta 195 mg/litro, el vino hasta 116 mg/litro y los diversos tipos
de licores hasta 3520 mg/litro. La exposición de la población
general al 1-propanol por inhalación y en el agua de bebida es baja
(en los Estados Unidos, la concentración media en muestras de aire
urbano fue de 0,00005 mg/m3 y la correspondiente al agua de bebida
0,001 mg/litro). Aunque los trabajadores están potencialmente
expuestos por la inhalación durante la fabricación, el tratamiento
y la utilización, no se dispone de datos para cuantificar esas
exposiciones.
5. Cinética y metabolismo
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se carece de datos sobre la tasa de
absorción tras la inhalación y la exposición dérmica. El
1-propanol es metabolizado por la deshidrogenasa alcohólica para
dar ácido propiónico por intermedio del aldehído y puede entrar en
el ciclo del ácido tricarboxílico. Esta oxidación es una etapa
limitativa de la velocidad del metabolismo del 1-propanol. In
vitro, las oxidasas microsómicas de rata y conejo también son
capaces de oxidar el 1-propanol a aldehído propiónico. La afinidad
relativa de la deshidrogenasa alcohólica y los sistemas de
oxidación microsómicos por el 1-propanol es mucho más elevada que
en el caso del etanol; así pues, el 1-propanol se elimina
rápidamente del organismo. En la rata, el periodo de
semieliminación de una dosis oral de 1000 mg/kg fue de 45 minutos.
Tanto en animales como en el hombre, el 1-propanol puede ser
eliminado del organismo en el aire exhalado o en la orina. A
sujetos a los que se administró una dosis de 1-propanol de 3,75 mg
por kg de peso corporal y 1200 mg de etanol por kg de peso corporal
por vía oral, la excreción urinaria total de 1-propanol fue del
2,1% de la dosis. Los niveles urinarios de 1-propanol fueron más
bajos cuanto más baja era la cantidad de etanol ingerida
simultáneamente, lo que demuestra la competencia por la
deshidrogenasa alcohólica entre el 1-propanol y la sobredosis de
etanol.
6. Efectos en los organismos en el medio ambiente
A las concentraciones en que normalmente se encuentra en el
medio ambiente, el 1-propanol no resulta tóxico para los organismos
acuáticos, los insectos ni las plantas. El umbral de inhibición
para la multiplicación celular de tres de las especies acuáticas
más sensibles (tres protozoos) fue de 38 - 568 mg/litro. Para los
organismos superiores, la concentración letal fue de unos 5000
mg/litro, y variaba notablemente poco de un filum a otro y exhibía
una curva dosis-respuesta sumamente pronunciada. Algunas bacterias
y microorganismos de aguas residuales y cienos activados son
capaces de adaptarse a concentraciones superiores a 17 000
mg/litro.
La germinación de semillas puede verse inhibida o estimulada
por el 1-propanol según la concentración en el agua utilizada y las
condiciones de exposición. El compuesto aumenta la acumulación de
nitritos en el maíz, los guisantes y el trigo.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 1-propanol para los mamíferos (a juzgar
por la mortalidad) es baja, por cualquiera de las vías de
exposición: cutánea, oral o respiratoria. Se ha comunicado que
los valores de la DL50 por vía oral para varias especies animales
varían entre 1870 y 6800 mg/kg de peso corporal. No obstante, en
ratas muy jóvenes se notificó una DL50 por vía oral de 560 - 660
mg/kg de peso corporal. El principal efecto tóxico del 1-propanol
tras una exposición única es la depresión del sistema nervioso
central. Los datos de que se dispone sobre el 1-propanol parecen
indicar que sus efectos sobre el sistema nervioso central son
semejantes a los del etanol; no obstante, el 1-propanol parece ser
más neurotóxico. Los valores de la DE50 para la narcosis en el
conejo y la pérdida del reflejo de enderezamiento en el ratón
fueron, respectivamente, de 1440 mg/kg de peso corporal por vía
oral, y de 1478 mg/kg de peso corporal por vía intraperitoneal;
estos valores son unas cuatro veces más bajos que los
correspondientes al etanol. En el ensayo del plano inclinado, el
1-propanol fue 2,5 veces más potente que el etanol en la rata.
La administración de dosis únicas por vía oral de 3000 ó
6000 mg/kg de peso corporal tuvo como resultado una acumulación
reversible de triglicéridos en el hígado de la rata. Las
concentraciones elevadas de vapor provocaron irritaciones en el
tracto respiratorio del ratón. El ritmo respiratorio del ratón se
vio disminuido en un 50% a concentraciones de aproximadamente
30 000 mg/m3.
No se dispone de datos sobre irritación ocular y cutánea. No
se observó sensibilización en una prueba de sensibilización cutánea
en ratones CF1.
En machos de rata expuestos durante seis semanas a 15 220
mg/m3, se obtuvieron pruebas limitadas de que el 1-propanol
disminuye la capacidad reproductora. No se observaron efectos tras
una exposición similar a 8610 mg/m3. Cuando se expusieron ratas
gestantes al 1-propanol, se observó toxicidad materna y fetal a
23 968 y 14 893 mg/m3 (9743 y 6054 ppm); no se observó toxicidad a
9001 mg/m3 (3659 ppm). No se observaron defectos de comportamiento
en la progenie de machos de rata expuestos durante seis semanas a
8610 ó 15 220 mg de 1-propanol/m3, ni en la de ratas expuestas
durante la gestación a las mismas concentraciones. No obstante,
cuando se administró a ratas de 5 a 8 días de edad 3000 - 7800 mg
de 1-propanol/kg por vía oral al día, se observó depresión del
sistema nervioso central durante la dosificación y síntomas de
privación cuando se retiraba la dosis. Cuando las ratas cumplieron
18 días se examinaron sus cerebros y se observaron reducciones en
los pesos cerebrales absoluto y relativo y en el contenido de ADN,
así como disminuciones regionales de los niveles de colesterol y de
proteínas.
El 1-propanol dio resultados negativos en dos ensayos de
detección de mutaciones puntuales utilizando Salmonella typhimurium
y en un ensayo de mutación inversa realizado con Escherichia coli
CA-274. Se obtuvieron resultados negativos en ensayos para inducir
intercambio de cromátidas hermanas o micronúcleos en células de
mamíferos in vitro. No se obtuvieron otros datos sobre
mutagenicidad.
En un estudio de carcinogenicidad realizado en grupos reducidos
de ratas Wistar expuestas durante toda su vida a dosis orales de
240 mg/kg o a dosis subcutáneas de 48 mg/kg, se observó un aumento
significativo de la incidencia de sarcoma hepático en el grupo en
el que la dosis se administraba por vía subcutánea. No obstante,
el estudio resultó insuficiente para evaluar la carcino-genicidad
por diversos motivos, entre ellos la falta de detalle experimental,
el reducido número de animales y el empleo de una sola dosis
elevada para inducir toxicidad hepática.
8. Efectos en la salud humana
No se tiene noticia de efectos adversos para la salud en la
población general o en grupos profesionales. El único caso
notificado de intoxicación mortal, fue el de una mujer que perdió
el conocimiento y falleció a las 4 - 5 h de la ingestión. La
autopsia reveló "inflamación cerebral" y edema pulmonar. En un
estudio sobre irritación cutánea y sensibilización, se comunicó la
aparición de reacciones alérgicas en un operario de laboratorio.
En otro grupo de 12 voluntarios, se observó en 9 individuos un
eritema que duró al menos 60 minutos tras la aplicación durante 5
minutos de papeles de filtro impregnados con 0,025 ml de una
solución al 75% de 1-propanol en agua aplicados sobre los
antebrazos.
No se dispone de más informes sobre efectos adversos para la
salud tras la exposición profesional al 1-propanol. No se dispone
de estudios epidemiológicos para evaluar los efectos a largo plazo,
incluida la carcinogenicidad, del 1-propanol en el ser humano.
9. Resumen de la evaluación
La exposición del ser humano al 1-propanol puede producirse por
la ingestión de alimentos o bebidas que lo contengan. La
exposición por inhalación puede tener lugar durante el uso
doméstico de la sustancia y en el ámbito laboral durante su
fabricación, tratamiento y uso. Los muy limitados datos de que se
dispone sobre el nivel de 1-propanol en el aire y el agua parecen
indicar que las concentraciones son muy reducidas.
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se cree que la absorción tras la
inhalación es rápida y la absorción por vía cutánea lenta.
La toxicidad aguda del 1-propanol para los animales es baja
en toda exposición, ya sea por vía cutánea, oral o respiratoria.
La exposición de miembros de la población general a niveles
potencialmente letales puede producirse por ingestión accidental o
intencionada. No obstante, sólo se ha notificado un caso de
envenenamiento mortal por 1-propanol. Los efectos agudos más
probables del 1-propanol en el hombre son la intoxicación
alcohólica y la narcosis. Los resultados de los estudios en
animales indican que el 1-propanol es 2 - 4 veces más tóxico que
el etanol.
El 1-propanol puede ser irritante para la piel hidratada.
Los datos sobre toxicidad animal no bastan para evaluar los
riesgos para la salud humana asociados a la exposición repetida o
duradera al 1-propanol. No obstante, estudios limitados a corto
plazo sobre la rata indican que la exposición por vía oral al
1-propanol tiene pocas probabilidades de suponer un riesgo grave
para la salud en las condiciones habituales de exposición humana.
En la rata la exposición por inhalación a una concentración de
15 220 mg/m3 redujo la capacidad reproductiva del macho, pero no
así la exposición a 8610 mg/m3. En la rata gestante, 9001 mg/m3
(3659 ppm) fue el nivel de efectos no observados y 14 893 mg/m3
(6054 ppm) el nivel más bajo de observación de efectos tanto
respecto a la toxicidad materna como a la fetal. Así pues, la
exposición por inhalación a concentraciones elevadas de 1-propanol
produjo toxicidad reproductiva y embriológica en el macho y la
hembra en presencia de toxicidad manifiesta en los animales
expuestos. Las concentraciones necesarias para producir esos
efectos en la rata fueron más elevadas que las que probablemente
se observen en condiciones normales de exposición humana.
El 1-propanol dio resultados negativos en los ensayos de
detección de mutaciones puntuales en bacterias. Aunque esto indica
que la sustancia no tiene ningún potencial genotóxico, no puede
evaluarse adecuadamente la mutagenicidad basándose en los limitados
datos disponibles. El estudio realizado no basta para evaluar la
carcino-genicidad del 1-propanol en animales de experimentación.
No se dispone de datos sobre la exposición a largo plazo de
poblaciones humanas al 1-propanol. Por todo ello, no puede
evaluarse la carcinogenicidad del 1-propanol en el ser humano.
Aparte de un caso de intoxicación mortal tras la ingestión de
medio litro de 1-propanol, no se tiene prácticamente noticia de
efectos adversos para la salud producidos por la exposición al
1-propanol, ni en la población general ni en grupos profesionales.
El Grupo Especial de Trabajo considera poco probable que el
1-propanol plantee un riesgo grave para la salud de la población
general en condiciones normales de exposición.
El 1-propanol puede liberarse al medio ambiente durante la
producción, el tratamiento, el almacenamiento, el transporte, el
uso y la evacuación de desechos. A causa de su uso principal como
disolvente volátil, la mayoría del volumen de producción acaba por
ser liberado a la atmósfera. No obstante, por reacciones con
radicales hidroxilo y por lavado pluvial, el 1-propanol
desaparecerá rápidamente de la atmósfera, siendo su tiempo de
presencia en ella inferior a 3 días. La eliminación del 1-propanol
del agua y del suelo también se produce rápidamente, de modo que
raras veces se detectan niveles medibles en cualquiera de estos
tres compartimientos. La adsorción del 1-propanol en las
partículas del suelo es escasa, pero la sustancia es móvil en el
suelo y se ha demostrado que aumenta la permeabilidad del mismo a
ciertos hidrocarburos aromáticos.
En vista de las propiedades físicas del 1-propanol, la bio-
acumulación es poco probable y, salvo en el caso de evacuación
accidental o inadecuada, el 1-propanol no constituye un riesgo para
los organismos acuáticos, los insectos y las plantas en las
concentraciones que por lo general se encuentran en el medio
ambiente.
(i) In the methylmalonyl pathway, propionyl-CoA is carboxylated to
methylmalonyl-CoA, this is followed by trans-carboxylation to
succinyl-CoA, which subsequently enters the tricarboxcylic acid
cycle to be metabolized to carbon dioxide and water.
(ii) In the lactate pathway, the propionyl-CoA is dehydrogentated
to acrylolyl-CoA, alpha-hydration gives L-lactoyl-CoA, which is
hydrolysed to lactate.
(iii) In another pathway the acrylolyl-CoA is hydrated to
3-hydroxypropionyl-CoA, deacylation and oxidation result in the
formation of malonic acid semialdehyde, which is converted to
acetyl-CoA. These reactions, which constitute the major pathways
for propionic acid metabolism in plant mitochondria, also occur in
animals.
(iv) The propionyl CoA may also participate in triglyceride
synthesis.
(v) The obligatory formation of propionyl carnitine required for
the transport of propionic acid into mitochrondria may also be an
excretory pathway under conditions of high carnitine and propionic
acid concentrations [158, 153].
Propionic acid and/or propionyl-CoA are potent inhibitors of
several mitochondrial enzymes required for fatty acid oxidation,
gluconeogenesis, and ureagenesis; their inhibitory effects can be
reversed with carnitine [24, 23]. The formation of propionyl-CoA,
its metabolism and effects on oxidative metabolism provide an
explanation for the hepatic effects observed in rats after high
oral exposure to 1-propanol (section 8.2) and for the biochemical
effects seen in some studies (section 8.3.1). Indeed the
accumulation of acyl-CoA esters, including propionyl-CoA, is
implicated in the pathogenesis of Reye syndrome [43].
6.4 Elimination and Excretion
Aliphatic alcohols may be eliminated from the body via expired
air or the urine. Theoretically, urinary metabolites may arise
from oxidation or from conjugation with glucuronic acid or sulfate.
There are no reports of the excretion of unchanged 1-propanol in
expired air or urine and, following an oral dose to rabbits of
800 mg/kg, only 0.9% was found in the urine as propyl-glucuronide
and none as a sulfate conjugate [100].
6.4.1 Animals
Available in vivo data, reviewed by Rietbrock & Abshagen [157],
showed that the elimination of 1-propanol was dose independent
above a single oral dose of 1000 mg/kg body weight in rats and
above a single intraperitoneal dose of 1200 mg/kg body weight in
rabbits [139, 1, 11]. The rate of the zero-order elimination of
the compound from the blood of rats that had received a single oral
dose of 3000 mg/kg body weight was found to be 510 mg/kg body
weight per hour [11]. At lower doses, the elimination rate was
first order. When rats were given a single oral dose of 1000 mg/kg
body weight, the half-life of 1-propanol was 45 min [1]. The
overall metabolism and elimination of 1-propanol are described in
section 6.3.1. In mice, a half-life of 57 min was estimated for
the exponential elimination phase following a single oral exposure
[1]. This should be considered an approximation because there were
only 2 or 3 time points per dose.
In vitro, the elimination of 1-propanol from the perfusate of
rat liver was also shown to be saturable, a zero-order phase being
succeeded below a concentration of 78 mg/litre by an exponential
phase with a half-life of 14 min [7].
6.4.2 Human beings
No data were available describing the elimination kinetics of
1-propanol in human beings.
When 10 volunteers drank 1-propanol in ethanolic orange juice
at doses of 3.75 mg 1-propanol and 1200 mg ethanol/kg body weight
over a period of 2 h, the compound was detected in the blood and in
the urine, partly as glucuronide. The total urinary excretion of
1-propanol was 2.1% of the dose. The urinary levels of 1-propanol
were lower when the amount of simultaneously ingested ethanol was
less, showing competition for ADH between 1-propanol and the
ethanol overdose [19, 20].
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Aquatic Organisms
A summary of acute aquatic toxicity data is presented in Table
5. In none of these studies was the concentration of 1-propanol
reported to have been measured. In view of the volatility of the
compound, it can be expected that the toxic effects observed in the
open-system studies occurred at lower concentrations than the
nominal ones.
Several short-term studies have also been conducted. Seiler et
al. [167] determined the breakpoint of bioinhibition for a total
of 20 strains of several bacterial groups prevalent in a waste-water
treatment plant in the chemical industry, i.e., Zoogloea,
Alcaligenes, and Pseudomonas. After one week of static exposure to
1-propanol in an open system at 30 °C, 100% growth inhibition
occurred at concentrations of 10 000 - 30 000 mg/litre of medium.
No analysis for the compound was reported.
Inhibition of cell multiplication of blue algae (Microcystis
aeruginosa) and green algae (Scenedesmus quadricauda) reached 100%
after 8 days of static exposure to 255 and 3100 mg 1-propanol/litre
water, respectively, in a closed system at 27 °C and a pH of 7
[25, 27].
7.2 Terrestrial Organisms
7.2.1 Insects
The toxicity of 1-propanol for insect larvae is summarized in
Table 5. In static tests, the 48-h LC50 values for adults of the
fruit fly strains of Drosophila melanogaster and Drosophila
simulans were between 18 490 and 24 120 mg/litre of nutrient medium
and 11 260 and 12 860 mg/litre of nutrient medium, respectively [46].
7.2.2 Plants
The effects of 1-propanol on the rate of seed germination
have been investigated on several occasions. Total inhibition of
the germination of barley grains was reached after incubation for 4
days at 18 °C on filter papers absorbing a solution containing 8050
mg 1-propanol/litre water [40]. The germination of white amaranth
(Amaranthus albus) seeds was stimulated in a dose-related manner
after 5 h incubation at 25 °C on filter papers moistened with a
solution containing 3600 - 36 050 mg 1-propanol/litre water [36].
Reynolds [36] measured 50% inhibition of germination of lettuce
(Lactuca sativa) seeds after incubation for 3 days at 30 °C on agar
containing 3065 mg 1-propanol/litre. The percentage germination
and the axis length of soya bean (Glycine max) seeds, with the
testa removed, were not reduced after exposure to pure 1-propanol
for 2 h. After treatment with a 50% (v/v) 1-propanol/water mixture
for 2 min, germination was almost completely inhibited and axis
length was reduced [150].
Table 5. Acute aquatic toxicity of 1-propanol
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
FRESHWATER
Bacteria
Pseudomonas putida 25 7 closed 16 h TTb 2 700 [27]
Microorganisms
Activated sludge 21 7.4-8 closed 3 h 50% inhibition 1 000 [105]
of respiration
Acclimated mixed 30 6.8 closed 1.2 h 50% inhibition 19 085 [196]
waste-water culture of respiration
Protozoa
Entosiphon sulcatum 25 6.9 closed 72 h TTb 38 [26]
Chilomonas paramecium 20 6.9 closed 48 h TTb 175 [29]
Uronema parduczi 25 6.9 closed 20 h TTb 568 [28]
Algae
Selenastrum capricornutum 25-26 closed 96 h NOAECc 2 000 [172]
Scenedesmus pannonicus 25-26 closed 48 h NOAECc 2 900 [172]
Chlorella pyrenoidosa 25-26 closed 48 h NOAECc 1 150 [172]
Coelenterate
Hydra oligactis 17 8.2-8.4 >5 closed 48 h LC50 6 800 [170]
Worms
Flatworm (Dugesia) 20 8.2-8.4 >5 closed 48 h LC50 4 700 [170]
Tubificid worm 20 8.2-8.4 >5 closed 48 h LC50 9 200 [170]
Molluscs
Giant pond snail 20 8.2-8.4 >5 closed 48 h LC50 6 500 [170]
(Lymnea stagnalis)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Crustaceans
Water flea 20 8 >2 250 open 24 h EC50d 4 415 [27]
(Daphnia magna)e EC0 3 336
EC100 5 909
19 open 48 h LC50 7 080 [33]
Water flea 19 open 48 h LC50 3 025 [33]
(Daphnia pulex)e
Water flea 19 open 48 h LC50 5 820 [33]
(Daphnia cucullata)e
Isopod 20 8.2-8.4 >5 209 closed 48 h LC50 2 500 [170]
(Asellus aquaticus)
Scud (Gammarus pulex) 20 8.2-8.4 >5 209 closed 48 h LC50 1 000 [170]
Insects
Mosquito larvae 22-24 open 4 h LC50 10 450 [107]
(Aedes aegypti)
Mosquito larvae (Aedes 26 8.2-8.4 >5 209 open 48 h LC50 4 400, 4 800 [172]
aegypti, Culex pipiens) LC0 3 200, 3 600
Midge larvae 20 8.2-8.4 >5 209 closed 48 h LC50 2 350 [170]
(Chironomus gr. thummi)
Leech larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 400 [170]
(Eropdella octoculata)
Dragon fly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 4 200 [170]
(Ischnura elegans)
Stonefly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 1 520 [170]
(Nemoura cinerea)
Mayfly larvae 20 8.2-8.4 >5 209 closed 48 h LC50 3 110 [170]
(Cloeon dipterum)
Corixa punctata (larvae) 20 8.2-8.4 >5 209 closed 48 h LC50 2 000 [170]
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Creek chub 15-21 8.3 98 open 24 h LC0 200 [68]
(Semotitus atromaculatus)
Golden orfe 20 7-8 >5 200-300 48 h LC50 4 320, 4 560 [99]
(Leuciscus idus melanotus) LC0 3 600, 4 000
Fathead minnow 20 8.2-8.4 >5 209 open 48 h LC50 5 000 [172]
(Pimephales promelas) LC0 2 600
Rainbow trout 15 7-8 >5 98 open 48 h LC50 3 200 [172]
(Salmo gairdneri) LC0 2 000
Paddy fish 24 8.2-8.4 >5 209 open 48 h LC50 5 900 [172]
(Oryzias latipes) LC0 4 400
Amphibia
South African clawed toad 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Xenopus laevis)
Mexican axolotl 20 8.2-8.4 >5 209 open 48 h LC50 4 000 [171]
(Ambystoma mexicanum)
SEA WATER
Bacteria
Photobacterium 15 15 min 50% light 8 686 [84]
phosphorerum closed reduction
5 5 min 50% light 17 700 [48]
closed 15 min reduction 18 400
Crustacea
Brine shrimp 24 24 h LC50 4 200 [149]f
(Artemia salina) open
Harpacticoid copepod 21 7.9 >5 96 h LC50 2 300 [12]g
(Nitocra spinipes)
---------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------------------------------
Organism Temper- pH Dissolved Hardness Systema Exposure Parameter Nominal Reference
ature oxygen (mg/CaCO3/ period concentration
(°C) (mg/litre) litre) (mg/litre)
---------------------------------------------------------------------------------------------------------------------------------
Fish
Bleak (Alburnus alburnus) 10 7.9 >5 open 96 h LC50 3 800 [12]g
---------------------------------------------------------------------------------------------------------------------------------
a Static systems used in all experiments reported.
b TT = toxic threshold for inhibition of cell multiplication.
c NOAEC = no-observed-adverse-effect-concentration; effect is growth inhibition.
d Effect is complete immobilization.
e Age of Daphnia was 24 h for Daphnia magna and Daphnia pulex, and 11 ± 1 day for Daphnia cucullata.
f Salinity was 2.8%.
g Salinity was 0.7%.
1-Propanol was marginally effective in breaking the dormancy of
seeds of genetically pure dormant lines of wild oat (Avena fatua)
after 5 days of exposure to solutions containing up to 1202 mg/litre.
Seed viability was affected at higher concentrations [2].
When excised seedling roots of maize (Zea mays) were treated by
vacuum infiltration in a 5% solution of 1-propanol in water, 3
times for 60 seconds, and then incubated anaerobically at 28 °C,
nitrite accumulation increased by 10 times or more, the utilization
of nitrate increased, and the utilization of exogenous nitrite was
inhibited. These effects were enhanced under aerobic conditions
[75]. Dry et al. [51] observed that stimulation of nitrite
accumulation in pea and wheat roots under aerobic conditions was
accompanied by a decline in the cellular levels of glucose-6-
phosphate. It was suggested by Gray & Cresswell [75] that an
increase in the utilization of nitrate was related to increased
access of nitrate to the site of nitrate metabolism as a result of
an increase in membrane permeability.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single Exposures
8.1.1 Mortality
The available LD50s for various animal species are summarized
in Table 6. Based on mortality estimates, 1-propanol exhibits low
toxicity, except in very young rats. Oral LD50 values for several
animal species range between 1870 and 6800 mg/kg body weight. For
very young rats, oral LD50s of 560 - 660 mg/kg body weight have
been reported [152].
An intraperitoneal dose of 785 mg 1-propanol/kg was lethal to 10
out of 10 C57B/6J and 10 out of 10 DBa/2J mice, but a dose of 392 mg/
kg did not cause any deaths in either strain [183]. An LD16 of
450 mg/kg was reported in rats after intraperitoneal administration.
When rats were exposed to 1-propanol vapour for 4 h at a
concentration of approximately 9840 mg/m3, 2 out of 6 died within
14 days [173].
8.1.2 Signs of intoxication
Osborne-Mendel or Sherman rats of both sexes receiving a lethal
oral dose of undiluted 1-propanol became comatose within a few
minutes [187]. Deep narcosis occurred in mice exposed through
inhalation of 1-propanol at a concentration of 50 mg/litre for 2 h.
Very young rats (60 - 100 g) of an unspecified strain and of
both sexes, received a single oral dose of between 150 and 3000 mg
undiluted 1-propanol/kg body weight. Animals that died showed
hyperaemia, vacuolation, and dilated sinusoids in the liver, and
hyperaemia, tubular cloudy swelling, and tubular necrosis in the
kidneys [152].
When anaesthetized Sprague-Dawley rats were made to inspire
160 mg of the undiluted compound, all 9 exposed rats died within
165 min, 6 of them dying immediately from respiratory arrest. All
controls survived and were killed 24 h later. It was not reported
whether the latter were sham-exposed or not. The average absolute
lung weight of the exposed rats was increased by 92%. The lungs
showed oedema and small areas of focal haemorrhage [65].
Special studies on neurotoxic and behavioural effects, and on
biochemical effects are described in sections 8.3 and 8.4.
Table 6. LD50s for 1-propanol
---------------------------------------------------------------------------------------------------
Species Sex Route of Observation LD50 (mg/kg Comments Reference
exposure period body weight)
---------------------------------------------------------------------------------------------------
Wistar rat (non- male oral 14 days 1870 vehicle: water [173]
fasted)
Osborne-Mendel male, oral until 6500 undiluted [187]
or Sherman rat female recovery
CD mouse not oral 3 days 6800 undiluted [164]
reported
Rabbit male, oral 1 day 2820 [130]
female
Wistar rat male intravenous 5 days 590 vehicle: water [190]
H mouse male intravenous 5 days 697 vehicle: water [190]
female intravenous not 1090 vehicle: water [40]
reported
Chinchilla rabbit male, intravenous 5 days 483 vehicle: water [190]
female
Wistar rat male intraperitoneal 5 days 2247 vehicle: water [190]
H mouse male intraperitoneal 5 days 3695 vehicle: water [190]
Syrian hamster male intraperitoneal 5 days 2337 vehicle: water [190]
Guinea-pig intraperitoneal 5 days 1208 vehicle: water [190]
New Zealand male dermal 14 days 4050 1/10 of body [173]
rabbit surface exposed
under cover for
24 h
---------------------------------------------------------------------------------------------------
8.1.3 Skin, eye, and respiratory tract irritation; sensitization
Data for skin and eye irritation were not available. One skin
sensitization test has been reported concerning an ear-swelling
test on CF1 mice. No sensitization was observed [61]. Although
the test requires further validation, it correctly discriminated
between a number of known positive and negative human sensitizing
agents. The authors claim it to be an accurate, sensitive, and
efficient method for evaluating delayed contact sensitization.
The sensory irritation of 1-propanol was investigated using a
50% reflex decrease in the respiratory rate of mice (RD50) as an
index. Only the heads of the mice were exposed. An exposure-
related effect was found with RD50 values for the first 10 min of
exposure of 31 252 mg/m3 for Swiss Webster mice [101] and
33 604 mg/m3 for CF-1 mice [108]. The potential of 1-propanol as
a respiratory irritant is therefore low.
8.2 Repeated Exposures
Only a few data are available concerning the oral exposure of
animals.
When 3 male and 3 female rats of unspecified strain were
exposed to 4 daily oral doses of 2160 mg undiluted 1-propanol, no
deaths occurred and no gross pathological signs were seen in the
liver [187].
In a group of 6 male rats of unspecified strain, receiving
drinking-water containing 1-propanol at a concentration of 60 090
mg/litre for 4 months, food consumption, body weight gain, and
liver histopathology were comparable to those of the control group.
It should be noted that the authors reported a dose rate of 3 mg/kg
body weight per day, while a dose rate of approximately 3000 mg/kg
body weight per day seems more appropriate, assuming a water
consumption of 20 ml/day and a body weight of 400 g [85].
Groups of 10 Wistar rats were exposed to 1-propanol in the
drinking-water at a concentration of 320 000 mg/litre (calculated
by the Task Group to be equivalent to approximately 16 000 mg/kg
body weight per day, on the basis of the assumptions made above)
for 5, 9, or 13 weeks. Control groups comprised 10 rats each. The
exposed rats gradually became weak, losing their appetites and
showing a decreased body weight gain. Electron microscopic studies
of the liver showed irregularly shaped megamitochondria with few
cristae, and normally sized but irregularly shaped mitochondria
with a decreased number of cristae. Biochemical changes included a
decreased state 3 respiration using glutamate as a substrate and
decreased specific activities of cytochrome c oxidase (EC 1.9.3.1)
and monoamine oxidase (EC 1.4.3.4) [203].
8.3 Neurotoxic and Behavioural Effects
In one study on anaesthetized mongrel dogs, it was shown that
1-propanol, as well as other primary alcohols, could increase the
permeability of the blood-brain barrier. The dogs received a
sodium fluorescein solution and 0.578 mg 1-propanol in saline,
intravenously. The concentration of sodium fluorescein in the
cerebrospinal fluid rose to a maximum within 10 min and returned to
control levels, 3 h after exposure [78].
The oral ED50 (1440 mg/kg body weight) for narcosis in rabbits
exposed to 1-propanol was 4 times lower than that for ethanol
[129]. Deep narcosis occurred in mice exposed through inhalation
to 50 mg 1-propanol/litre for 2 h, and a 40-min exposure to 2.3 mg/
litre reduced the unconditioned flexor response in rabbits. When
rabbits were intravenously infused with 1-propanol at a rate of 9 -
30 mg/min per kg body weight, positional nystagmus with an
inhibited rotatory response was observed at and above a blood
concentration of 900 mg/litre [137].
The intraperitoneal ED50 for loss of righting reflex in Swiss
Webster mice administered 1478 mg 1-propanol/kg body weight was 2.8
times lower than that for ethanol [117]. When C57BL/6J or DBA/2J
mice were given a single dose of 1-propanol intraperitoneally, both
strains showed decreased activity in the open field test at
392 mg/kg body weight, but the decrease was not significant. All
mice given 785 and 1570 mg/kg body weight died [183]. The rotarod
performance of Swiss-Cox mice decreased in a dose-related manner
after single oral doses of 1-propanol of 2000 or 4000 mg/kg body
weight. A dose of 1000 mg/kg body weight did not cause behavioural
impairment. When the study was repeated on days 4, 6, 7, and 8
after the first trial, tolerance did not develop [1].
The threshold for the induction of ataxia in Sprague-Dawley
rats following intraperitoneal exposure was 799 mg/kg body weight
[119]. In a tilted plane test, the performance of rats decreased
by an average of 71% after oral exposure to 2000 mg/kg body weight.
On a molar basis, 1-propanol was 2.5 times as intoxicating as
ethanol [204].
According to several investigators, depression of the central
nervous system by 1-propanol was related to interactions with
neuronal membranes. Lyon et al. [117] observed a high correlation
between the narcotic potencies of the aliphatic alcohols, including
1-propanol, in mice and their ability to disorder the brain
synaptosomal plasma membrane in vitro, as measured by electron
paramagnetic resonance, which was in turn related to membrane
solubility. A change in membrane fluidity was shown to occur in
isolated synaptosomal plasma membranes of rat cortex in vitro by a
decrease in 1,6-diphenyl-1,3,5-hexatriene fluorescence polarization
[81].
Functional loss due to disruption of membrane integrity by
1-propanol was observed in vitro. The action potentials of the
sciatic nerves of the toad (Bufo marinus) [155] and of giant axons
of the squid (Loligo forbesi) [143] were decreased by 1-propanol.
In isolated rat phrenic nerve-diaphragm, 1-propanol increased the
amplitudes of end-plate and miniature end-plate potentials and the
number of quanta of acetylcholine of end-plate potentials [62].
The compound also affected the rate of decay of postsynaptic
currents in the neuromuscular junction of the crayfish (Cherax
destructor) [200], and in the phrenic nerve-diaphragm of the rat
[62].
Effects on the ionic currents underlying the changes in
excitability described above were also investigated in vitro.
1-Propanol inhibited both the K+-stimulated and the Na+-dependent
influx of Ca2+ ions into isolated rat brain synaptosomes [80, 81,
124], and the influx of Na+ ions into rat brain synaptosomes [128].
It decreased the Na+ and K+ currents in the giant axons of the
squid (Loligo forbesi) [143], and in sciatic nerve fibres of the
clawed toad (Xenopus laevis) [6]. The interference of 1-propanol
with the transport of Ca2+ ions across biological membranes was
also shown in vitro by the inhibition of Ca2+ ion-induced
contractions of guinea-pig ileum [213], and in vivo, in rats, by a
decrease in regional brain Ca2+ ion levels, 30 min after one
intraperitoneal dose of 2000 mg/kg body weight [159].
The disruption of neuronal membranes by 1-propanol was also
thought to explain its inhibitory action on the binding of
dihydromorphine to isolated mouse brain caudate membranes [185] and
on membrane-bound guanylate cyclase (EC 4.6.1.2) in intact murine
neuroblastoma N1E-115 cells [172]. The activation by 1-propanol of
membrane-bound adenylate cyclase (EC 4.6.1.1) from isolated mouse
striatal membranes, in the presence of guanine nucleotides, was
also suggested to be the result of membrane perturbation [116].
8.4 Biochemical Effects
8.4.1 Effects on lipids in the liver and blood
Oral administration of single doses of 3000 or 6000 mg
1-propanol/kg body weight to Wistar rats caused a transient
increase in hepatic triglycerides, which was related to the
duration of an elevated blood-1-propanol concentration [10, 11].
Gaillard & Derache [63] did not observe an increase in hepatic
triglycerides in Wistar rats, 17 h after a single dose of 6000 mg
1-propanol/kg body weight.
Factors possibly responsible for hepatic triglyceride
accumulation include: an increase in hepatic uptake of labelled
palmitate [11], an increased esterification of palmitate to form
liver triglycerides [10, 11], and decreased palmitate oxidation
[11]. The decrease in palmitate oxidation was related to an
increase in the hepatic alpha-hydroxybutyrate/acetoacetate ratio,
implying a decrease in the intramitochondrial NAD+/NADH ratio [11].
An increase in extramitochondrial reducing equivalents, indicated
by an increased lactate/pyruvate ratio, was observed in vitro by
Forsander [60], but not in vivo by Beaugé et al. [11].
The effects of 1-propanol on palmitate incorporation into
triglycerides appear to depend on the dose, high doses causing
inhibition and lower ones leading to an increase. The
incorporation of palmitate into serum triglycerides and serum and
liver phospholipids, 4.5 h after a single oral dose of 6000 mg
1-propanol/kg body weight to rats, was found to be inhibited, while
an increase in hepatic triglyceride accumulation was only observed
8 h after dosing [10]. Three hours after a dose of 3000 mg/kg body
weight, the incorporation of palmitate in blood triglycerides was
increased concomitantly with an increase in hepatic triglycerides
while levels of phospholipids in the liver and blood were unaffected
[11]. Similar effects have been noted with ethanol [11].
8.4.2 Effects on microsomal enzymes
The effects of 1-propanol on microsomal enzymes (EC 1.14.14.1)
in vivo was investigated by Powis [147], who administered a single
oral dose of 960 mg/kg body weight to Wistar rats. Twenty four
hours after exposure, no effects were observed on the activities of
aniline hydroxylase and aminopyrine demethylase in liver
microsomes. This study is inadequate to demonstrate an inductive
effect of 1-propanol on the microsomal mixed function oxidase
system. In vitro, 1-propanol inhibited aldrin epoxidase and
p-aniline hydroxylase in isolated rat liver microsomes via an
interaction with cytochrome P-450, which causes a reverse Type I
spectral change [41, 210, 189, 161]. At high concentrations, the
inhibition of the mixed function monoxygenase system by aliphatic
alcohols correlates directly with the lipophilicity of the
alcohols, and is probably the result of unspecific effects on the
membrane (see section 8.3). The compound did not affect the levels
of hepatic microsomal cytochrome P-450, haem, cytochrome b5, and
NADPH-cytochrome c reductase (EC 1.6.2.4) in phenobarbital-induced
rats [96]. 1-Propanol increased the levels of cytochrome P-450 in
cultured chick embryo hepatocytes. The activities of benzphetamine
demethylase and UDP-glucuronosyl transferase (EC 2.4.1.17) were
also increased [169].
8.4.3 Other biochemical findings
The glutathione level in the liver of Wistar rats administered
a single dose of 1660 mg 1-propanol/kg body weight, orally, had
decreased by 20%, 6 h after exposure. Lipid peroxidation, as
indicated by diene conjugates formation, was increased; ethanol in
equivalent doses produced similar effects [199].
The activities of liver ornithine decarboxylase (EC 4.1.1.17)
and liver tyrosine aminotransferase (EC 2.6.1.5) increased in
partially hepatectomized rats 4 h after one oral dose of 2300 mg
1-propanol/kg body weight or an equivalent dose of ethanol. No
effects were observed on levels of alanine aminotransferase
(EC 2.6.1.2) in the liver and kidneys, and on levels of ornithine
decarboxylase in the kidneys and brain [145].
The effects of 1-propanol on neuronal membrane-bound adenylate
cyclase (EC 4.6.1.1) and guanylate cyclase (EC 4.6.1.2) in vitro
are discussed in section 8.3. 1-Propanol and ethanol, can have
different effects on the activity of adenylate cyclase, depending
on the concentration of alcohol and the biological system being
investigated [178, 192, 93].
When Sprague-Dawley rats inhaled 1-propanol for 6 h at a
concentration of 490 mg/m3, the serum level of testosterone was
decreased by 42% immediately after exposure, but not 18 h after
exposure. When this exposure regimen was repeated daily over one
week, no effects on serum testosterone levels were observed. Serum
levels of luteinizing hormone and corticosterone were unchanged at
all times [31].
When a crude homogenate of dispersed acinar cells, prepared
from guinea-pig pancreas, was incubated with 1-propanol and
secretin, the secretin-stimulated activities of adenylate cyclase
(EC 4.6.1.1) and cellular cyclic adenosine 3',5'-monophosphate were
potentiated at low concentrations of 1-propanol, but the
potentiation was reversible. Irreversible inhibition occurred at
higher concentrations [192].
8.5 Reproduction, Embryotoxicity, and Teratogenicity
Groups of 15 male Sprague-Dawley rats were exposed to
1-propanol at measured concentrations of 8610 or 15 220 mg/m3 for
7 h/day over 6 weeks. Beginning on the third day after the last
exposure, males were mated for a maximum of 5 days with unexposed
females. There was no apparent effect of exposure to 8610 mg/m3 on
mating performance or fertility. After exposure to 15 220 mg/m3,
17 out of 18 males copulated (as evidenced by the presence of a
vaginal plug), but only 2 of 17 mated females became pregnant. The
offspring of the exposed males were evaluated postnatally in a
battery of behavioural tests. There was no evidence of any
exposure-related effect [132].
These investigators also exposed groups of 15 pregnant Sprague-
Dawley rats to the same concentrations of 1-propanol on gestation
days 1 - 20. Pregnant females exposed to 15 220 mg/m3 showed
significantly reduced weight gain and food consumption. Their
female offspring also showed reduced weight gain up to 3 weeks of
age, but there was no consistent effect on male offspring. Litter
sizes were not affected. "Several" of the offspring from dams
exposed to 15 220 mg/m3 had crooked tails. Behavioural testing of
offspring did not reveal any evidence of an exposure-related
effect, though there was an increase in total external, visceral,
and skeletal malformations at 23 968 mg/m3 (9743 ppm) and in total
skeletal malformation at 14 893 mg/m3 (6054 ppm) [132].
The effects of 1-propanol on brain development in the neonatal
rat were also studied. A group of 21, 5-day-old Long-Evans rats
was exposed to 1-propanol via an artificial milk formula, which was
administered through an intragastric catheter for 4 consecutive
days. The rats received 12 feeds daily, each lasting 20 min.
Doses were 3800, 7500, 3000, or 7800 mg/kg body weight on day
5 - 8, respectively. Controls received the milk formula only.
During the exposure, the exposed pups frequently showed an impaired
righting response. After the last exposure, withdrawal symptoms
were displayed. Pups were killed at 18 days of age, at which time
there was no effect on body weight or on absolute weight of
kidneys, heart, or liver. However, the absolute and relative brain
weights were decreased in the exposed pups. Biochemical analysis
showed that the exposed pups had a decreased amount of DNA in all
brain areas examined. Cholesterol levels were decreased in the
forebrain and cerebellar samples, while protein levels were
decreased only in the forebrain samples [74].
8.6 Mutagenicity
8.6.1 Bacteria
1-Propanol was tested for mutagenic activity using Ames test
without S9; up to 100-µmol/plate was negative with Salmonella
typhimurium TA-100 [181]. Negative results were also reported in
TA-100 and TA-98 with or without metabolic activation, following
standard Ames test protocol [94].
In a reverse mutation assay with Escherchia coli CA-274
following a pre-incubation protocol, a 5-fold increase in the
number of revertants was observed at a concentration of 4.5%
1-propanol. No metabolic activation system was used [86].
8.6.2 Mammalian cells in vitro
1-Propanol (100 mg/litre once a day for 7 days) did not
increase the number of sister chromatid exchanges in Chinese
hamster ovary cells [136], or in V79 Chinese hamster lung
fibroblasts at 6000 mg/litre for 3 h (with activation) and 28 h
(without activation) [200]. It did not increase the number of
micronuclei in V79 Chinese hamster lung fibroblasts at 40 200 mg/
litre for 1 h [113].
A dose-related increase in the inhibition of metabolic
cooperation between hamster V79 cells, a phenomenon believed to
reflect carcinogenic promotion ability and not be indicative of
genotoxic potential, was observed by Chen et al. [37]. This may be
due to the membrane effects of 2-propanol.
8.7 Carcinogenicity
A group of 18 Wistar rats of both sexes received doses of
240 mg 1-propanol/kg body weight, by gavage, twice a week, for
their lifetime. Another group of 31 Wistar rats of both sexes
received subcutaneous injections of 48 mg compound/kg body weight,
twice a week, for their lifetime. Control groups, comprising 25
rats for each route, received saline. It was not reported whether
the analytical grade, double-distilled test compound was analysed
for the presence of impurities. The average survival time was 570
days for the orally exposed rats, 666 days for the subcutaneously
exposed rats, and 643 days for both control groups. The tumour
incidence is reported in Table 7. The data were not statistically
analysed. It was reported that "nearly all rats" showed liver
damage including congestion, steatosis, necrosis, fibrosis, and
metaplasia and hyperplasia of the haematopoietic bone marrow
parenchyma. However, the incidence of these lesions were not
reported [67].
Table 7. Tumour incidence in Wistar rats exposed orally or
subcutaneously to 1-propanol for lifetimea
-------------------------------------------------------------------
Organ/ Tumour type Incidence
tissue oral exposure sc exposure
affected exposed controls exposed controls
-------------------------------------------------------------------
Blood myeloid leukemia 2/18 0/25 4/31 0/25
Liver carcinoma 1/18 0/25 0/31 0/25
Liver sarcoma 2/18 0/25 5/31 0/25
Other carcinoma 0/18 0/25 3/31b 0/25
sarcoma 0/18 0/25 2/31c 0/25
benign tumoursd 10/18 3/25 7/31 2/25
-------------------------------------------------------------------
a From: Gibel et al. [67].
b One carcinoma each in kidney, bladder, and uterus.
c One sarcoma each in spleen and at injection site.
d Mostly papillomas and mammary fibroadenomas.
Although there was an apparent increase in the incidence of
liver sarcoma, the study is inadequate for the assessment of
carcinogenicity. The dosing schedule did not conform to standard
protocol. Too few animals were used in each dose group, the sex
ratio of each group was unclear, no data were provided on the
histological type of liver sarcoma, no statistical analysis was
conducted, the maximum tolerated dose was exceeded, as evidenced by
the reported liver damage, and only single dose levels were used.
In the case of subcutaneous administration, the exposure route was
inappropriate.
9. EFFECTS ON MAN
9.1 General Population Exposure
9.1.1 Poisoning incidents
One case of poisoning by 1-propanol has been reported. It
concerned a 46-year-old woman who was estimated to have consumed
approximately half a litre of the compound as a solvent in a
cosmetic preparation, probably a hair lotion. It was pointed out
that the woman could have ingested this preparation more than once
in the past. The woman was found unconscious. She died 4 - 5 h
after ingestion. No other signs or symptoms were reported.
Autopsy revealed a "swollen brain" and lung oedema [52].
9.1.2 Controlled human studies
Filter papers moistened with 0.025 ml of a 75% solution of
1-propanol in water were placed on the forearms of a group of 12
volunteers following immersion of the forearms in water at 23 °C
for 10 min. The patches were covered for 5 min and then gently
blotted. Nine of the 12 persons showed erythema for at least 60
min following exposure. The cutaneous reaction was totally blocked
in 4 out of 4 persons after pretreatment with 40% 4-methylpyrazole
in hydrophilic ointment 1 h before the challenge, showing,
according to the authors, that 1-propanol must be metabolized to
propanal before vasoactivity occurs [207].
9.2 Occupational Exposure
A laboratory worker in a company manufacturing hair cosmetics
developed allergic reactions in patch tests with chemically pure
1-propanol solutions in water (10 - 99.5% by volume). This person
also reacted to 2-propanol, 1-butanol, 2-butanol, and formaldehyde,
but not to ethanol and methanol. Controls were not tested [115].
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of Human Health Risks
10.1.1 Exposure
Exposure of human beings to 1-propanol may occur through
ingestion of food and alcoholic beverages containing 1-propanol
(e.g., wine and beer 100 - 200 mg/litre, spirits up to 3500
mg/litre). Inhalation exposure may occur during household use and
occupationally during manufacture and processing. Exposure of the
general population via inhalation and drinking-water is very low
(average concentrations in urban air and drinking-water in the USA,
0.00005 mg/m3 and drinking-water 0.001 mg/litre, respectively)
(section 5).
10.1.2 Health effects
1-Propanol is rapidly absorbed and distributed throughout the
body following ingestion. Data on the absorption rate following
inhalation are lacking but, in view of the physical properties of
the compound, it is also expected to be rapid. Dermal absorption
is expected to be slow (section 6).
1-Propanol exhibits low acute toxicity for animals (based on
lethality estimates), whether exposed via the dermal, oral, or
respiratory route (section 8.1). Exposure to potentially lethal
levels may occur in the general population through accidental or
intentional ingestion. However, only one case of lethal poisoning
by 1-propanol has been reported, which probably reflects its low
toxicity and limited use by the public (section 9.1.1). The
principal toxic effect of 1-propanol following a single exposure is
depression of the central nervous system. Quantitative exposure-
effect data on human beings are not available. The most likely
acute effects of 1-propanol in man are alcoholic intoxication and
narcosis. Animal studies indicate that 1-propanol is 2 - 4 times
as intoxicating as ethanol.
A controlled human study has indicated that 1-propanol may be
irritating to hydrated skin. However, the potential of 1-propanol
as a respiratory irritant is low (section 8.1.3). Data are
inadequate for evaluation of the irritating properties of this
compound for the skin, eye, and respiratory tract in human beings,
or for evaluation of its sensitizing potential.
The results of limited drinking-water studies on animals
suggest that oral exposure to 1-propanol is unlikely to pose a
serious health hazard under the usual conditions of human exposure
(section 8.2).
Inhalation exposure to a concentration of 15 220 mg/m3 caused
impaired reproductive performance in male rats, but exposure to
8610 mg/m3 did not. In pregnant rats, 9001 mg/m3 (3659 ppm) was a
NOEL and 14 893 mg/m3 (6054 ppm) was a LOEL for both maternal and
developmental toxicity. Behavioural effects were not detected in
offspring whose mothers were exposed during pregnancy to 15 220
mg/m3, but oral dosing of neonatal rats produced biochemical
changes in the brain that were detected 10 days after the last
treatment (section 8.5). Inhalation exposure to high concentrations
of 1-propanol produced reproductive and developmental toxic effects
in male and female rats. These effects occurred in the presence of
other overt signs of toxicity in the exposed animals and 1-propanol
does not appear to be selectively toxic to male or female
reproductive processes. The concentrations required to produce
these effects in rats were higher than those likely to be
encountered under normal conditions of human exposure.
1-Propanol was negative in assays for point mutations in
bacteria. It did not increase the incidence of sister chromatid
exchange or micronuclei in mammalian cells in vitro. Although
these findings suggest that the substance does not have any
genotoxic potential, no adequate assessment of mutagenicity can be
made on the basis of the limited data available. The results of an
in vitro test said to predict promotional activity were negative
(section 8.6). The available study is inadequate to evaluate the
carcinogenicity of 1-propanol in experimental animals (section
8.7). No data are available on the long-term exposure of human
populations to 1-propanol. Hence, the carcinogenicity of
1-propanol in human beings cannot be evaluated. Apart from one
case of fatal poisoning following ingestion of half a litre of
1-propanol, there are practically no reports on adverse health
effects from exposure to 1-propanol either in the general
population or in occupational groups (section 9).
The Task Group considers it unlikely that 1-propanol will pose
a serious health risk for the general population under normal
exposure conditions.
10.2 Evaluation of Effects on the Environment
1-Propanol can be released into the environment during
production, processing, storage, transport, use, and waste disposal
(section 3). It is transferred from water, soil, and waste
disposal sites to the atmosphere by volatilization, from the
atmosphere to water and soil by rain-out, and from soil and waste
disposal sites to ground water by leaching. It is difficult to
estimate its emission into each compartment. Because of its
primary use as a volatile solvent, most of the production volume is
eventually released into the atmosphere (section 4.1).
By reacting with hydroxyl radicals and through rain-out,
1-propanol will disappear rapidly from the atmosphere, with a
residence time of less than 3 days (section 4.2). Thus, measurable
atmospheric levels of 1-propanol are not usually encountered.
Hydrolysis and photolysis are not expected to be important in
the removal of 1-propanol from water and soil, but removal occurs
rapidly by aerobic and anaerobic biodegradation (section 4.3.1) so
that measurable levels are rarely found. Adsorption of 1-propanol
on soil particles is poor but it is likely to be mobile in soil and
it has been shown to increase the permeability of soil to some
aromatic hydrocarbons (section 4.1).
In view of the physical properties of 1-propanol, its potential
for bioaccumulation is low (section 4.3.2). Except in the case of
accident or inappropriate disposal, 1-propanol does not present a
risk for aquatic organisms, insects, or plants at concentrations
that usually occur in the environment. However, 1-propanol at
concentrations of around 5000 mg/litre in water is lethal to
oxygen-using aquatic organisms, indicating that its emission into
surface water at this level may result in serious alteration of the
local ecosystem (section 7).
11. RECOMMENDATIONS
1. 1-Propanol has not shown mutagenic potential in the small
number of assays performed. A full array of modern
genotoxicity tests should be carried out.
2. A single published report suggests carcinogenic activity by
1-propanol, but this study is seriously flawed and cannot be
used to evaluate the potential carcinogenicity of 1-propanol.
The desirability of a carcinogenesis bioassay of 1-propanol
should be considered, on the basis of the outcome of
genotoxicity tests.
3. Inhalation exposure to overtly toxic concentrations of
1-propanol produced reproductive and developmental toxicity in
experimental animals. In view of the potential for
environmental and drinking-water contamination, reproductive
and developmental toxicity should be investigated using oral
dosing.
4. Epidemiological studies including precise exposure data would
assist in an assessment of the occupational hazards from
1-propanol.
5. The unusually uniform level of toxicity in diverse types of
aquatic organisms that consume gaseous oxygen, and the
exceptionally steep dose-effect curve observed, suggest a
nonspecific effect that may not be restricted to 1-propanol.
These effects merit investigation.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1-Propanol was considered by the Joint FAO/WHO Expert
Committee on Food Additives (JECFA) in its twenty-third report.
Specifications were formulated, but no toxicological monograph was
prepared, and the substance could not be evaluated on the basis of
the data available. Additional toxicological information was
available to JECFA at a later meeting, including the results of a
limited study in rats suggesting a carcinogenic potential for
1-propanol. In its twenty-fifth report,a JECFA noted that life-
time feeding studies in rodents were required to resolve the
problem of carcinogenicity. A toxicological monograph was
prepared. The existing specifications were revised and designated
as "tentative", but no ADI was established.
-------------------------------------------------------------------
a WHO Technical Report Series, No. 669, 1981 ( Evaluation of
certain food additives: Twenty-fifth Report of the Joint
FAO/WHO Expert Committee on Food Additives).
REFERENCES
1. ABSHAGEN, U. & RIETBROCK, N. (1970) [The mechanism of the
2-propanol oxidation.] Naunyn-Schmiedebergs Arch. Pharmakol.
exp. Pathol., 265: 411-424 (in German).
2. ADKINS, S.W., NAYLOR, J.M., & SIMPSON, G.M. (1984) The
physiological basis of seed dormancy in Avena fatua. V.
Action of ethanol and other organic compounds. Physiol.
Plant., 62: 18-24.
3. AHAMED, A. & MATCHES, J.R. (1983) Alcohol production by
fish spoilage bacteria. J. food Prot., 46: 1055-1059.
4. ALLINGER, N.L., CAVA, M.P., DE JONGH, D.C., JOHNSON, C.R.,
LEBEL, N.A., & STEVENS, C.L. (1971) Organic chemistry, New
York, Worth Publishers, Inc.
5. ANON. (1984) Propyl alcohol complaint settled by US
companies. Chem. marketing Rep., April 23: 3, 17.
6. ARHEM, P. & VAN HELDEN, D. (1983) Effects of aliphatic
alcohols on myelinated nerve membrane. Acta physiol. Scand.,
119: 105-107.
7. AUTY, R.M. & BRANCH, R.A. (1976) The elimination of ethyl,
n-propyl, n-butyl and isoamyl alcohols by the isolated
perfused rat liver. J. Pharmacol. exp. Ther., 197: 669-674.
8. BALD, E. & MAZURKIEWICZ, B. (1980) Analytical utility of 2-
halo-pyridinium salts. Part III. Paper electrophoretic
characterization of alcohols as 2-alkoxy-1-methylpyridinium
p-toluenesulfonates. Chromatographia, 13: 295-297.
9. BEAUD, P. & RAMUZ, A. (1978) Dosage simultané des alcools
supérieures, et de l'acetate d'éthyle dans les eaux-de-vie
par chromatographie gaz-liquide-solide. Trav. chim. Aliment.
Hyg., 69: 423-430.
10. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1974)
Perturbations du métabolisme hépatique du palmitate [1-14C]
déterminée par l'administration de n-propanol chez le rat.
Biochimie, 56: 1157-1159.
11. BEAUGE, F., CLEMENT, M., NORDMANN, J., & NORDMANN, R. (1979)
Comparative effects of ethanol, n-propanol and isopropanol on
lipid disposal by rat liver. Chem.-biol. Interact., 26:
155-166.
12. BENGTSSON, B.-E., RENBERG, L., & TARKPEA, M. (1984) Molecular
structure and aquatic toxicity: an example with C1-C13
aliphatic alcohols. Chemosphere, 13: 613-622.
13. BILZER, N. & GRUNER, O. (1983) [Critical assessment regarding
determination of aliphatic alcohols (congeners in alcoholic
drinks) in blood with the aid of head-space analysis.]
Blutalkohol, 20: 411-421 (in German).
14. BILZER, N. & PENNERS, B.-M. (1985) [Concerning the velocity
of reduction and excretion of the attendant substance
propanol-1 and isobutanol after drinking whisky of the trade
mark Chivas Regal.] Blutalkohol, 22: 140-145 (in German).
15. BILZER, N., PENNERS, B.-M., & GRUNER, O. (1985) [Studies
about the course of concentration in blood for congener
propanol-1 and isobutanol after drinking overseas rum
("Captain Morgan").] Blutalkohol, 22: 146-151 (in German).
16. BONTE, W. (1978) [Congener content of wine and similar
beverages.] Blutalkohol, 15: 392-404 (in German).
17. BONTE, W. (1979) [Congener content of German and foreign
beers.] Blutalkohol, 16: 108-124 (in German).
18. BONTE, W., DECKER, J., & BUSSE, J. (1978) [Congener content
of highproof alcoholic beverages.] Blutalkohol, 15: 323-338
(in German).
19. BONTE, W., RUDELL, E., SPRUNG, R., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981a) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human blood.]
Blutalkohol, 18: 399-411 (in German).
20. BONTE, W., SPRUNG, R., RUDELL, E., FRAUENRATH, C., BLANKE, E.,
KUPILAS, G., WOCHNIK, J., & ZAH, G. (1981b) [Experimental
investigations concerning the analytical detection of small
doses of higher aliphatic alcohols in human urine.]
Blutalkohol, 18: 412-426 (in German).
21. BONTE, W., STOPPELMAN, G., RUDELL, E., & SPRUNG, R. (1981c)
[Computerized detection of congeners of alcoholic beverages
in body fluids.] Blutalkohol, 18: 303-310 (in German).
22. BOSSET, J.O. & LIARDON, R. (1984) The aroma composition of
Swiss Gruyere cheese. II. The neutral volatile components.
Lebensm.-Wiss. Technol., 17: 359-362.
23. BRASS, E.P. & BEYERINCK, R.A. (1987) Interactions of
propionate and carnitine metabolism in isolated hepatocytes.
Metabolism, 36: 781-787.
24. BRASS, E.P., FENNESSEY, P.V. & MILLER, L.V. (1986) Inhibition
of oxidative metabolism by propionic acid and its reversal by
carnitine in isolated rat hepatocytes. Biochem. J., 236:
131-136.
25. BRINGMANN, G. (1975) [Determination of the harmful
biological action of water-endangering substances through
inhibition of cell multiplication in the blue alga
Microcystis.] Ges.-Ing., 96: 238-241 (in German).
26. BRINGMANN, G. (1978) [Determination of the harmful
biological action of water-endangering substances on
protozoa. I. Bacteria fed flagellates.] Z. Wasser-Abwasser
Forsch., 11: 210-215 (in German).
27. BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the
harmful action of water-endangering substances on bacteria
(Pseudomonas putida) and green algae (Scenedesmus
quadricauda) in the cell multiplication inhibition test.]
Z. Wasser-Abwasser Forsch., 10: 87-98 (in German).
28. BRINGMANN, G. & KUHN, R. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa. II. Bacteria fed ciliates.] Z. Wasser-Abwasser
Forsch., 13: 26-31 (in German).
29. BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination
of the harmful biological action of water-endangering
substances on protozoa. III. Saprozoic flagellates.]
Z. Wasser-Abwasser Forsch., 13: 170-173 (in German).
30. BURROWS, W.D. & ROWE, R.S. (1975) Ether soluble constituents
of landfill leachate. J. Water Pollut. Control Fed., 47:
921-923.
31. CAMERON, A.M., ZAHLSEN, K., HAUG, E., NILSEN, O.G., & EIK-
NES, K.B. (1985) Circulating steroids in male rats following
inhalation of n-alcohols. Arch. Toxicol., Suppl., 8: 422-424.
32. CAMPBELL, I.M., MCLAUGHLIN, D.G., & HANDY, B.J. (1976) Rate
constants for reactions of hydroxyl radicals with alcohol
vapours at 292 K. Chem. Phys. Lett., 38: 362-364.
33. CANTON, J.H. & ADEMA, D.M.M. (1978) Reproducibility of short-
term and reproduction toxicity experiments with Daphnia magna
and comparison of the sensitivity of Daphnia magna with
Daphnia pulex and Daphnia cucullata in short-term experiments.
Hydrobiologia, 59: 135-140.
34. CARTER, W.P.L., DARNALL, K.R. GRAHAM, R.A., WINER, A.M., &
PITTS, J.N. (1979) Reactions of C2 and C4-hydroxy radicals
with oxygen. J. phys. Chem., 83: 2305-2311.
35. CEC (1982) Propan-1-ol chemico-physical data, toxicity data,
environmental occurrence, and permissible levels. In: Report
of the Scientific Committee for Food on extraction solvents,
Brussels, Commission of the European Communities, Directorate
General for Internal Market and Industrial Affairs, pp. 27-45.
36. CHADOEUF-HANNEL, R. & TAYLORSON, R.B. (1985) Anaesthetic
stimulation of Amaranthus albus seed germination: interaction
with phytochrome. Physiol. Plant, 65: 451-454.
37. CHEN, T.-H., KAVANAGH, T.J., CHANG, C.C., & TROSKO, J.E.
(1984) Inhibition of metabolic cooperation in Chinese hamster
V79 cells by various organic solvents and simple compounds.
Cell Biol. Toxicol., 1: 155-171.
38. CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation
and degradation of petrochemical wastewater components by
methane fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
39. CHUNG, T.-Y., HAYASE, F., & KATO, H. (1983) Volatile
components of ripe tomatoes and their juices, purees and
pastes. Agric. biol. Chem., 47: 343-351.
40. CHVAPIL, M., ZAHRADNIK, R., & CMUCHALOVA, B. (1962) Influence
of alcohols and potassium salts of xanthogenic acids on
various biological objects. Arch. int. Pharmacodyn. Ther.,
135: 330-343.
41. COHEN, G.M. & MANNERING, G.J. (1973) Involvement of a
hydrophobic site in the inhibition of the microsomal
p-hydroxylation of aniline by alcohols. Mol. Pharmacol.,
9: 383-397.
42. CORBIT, T.E. & ENGEN, T. (1971) Facilitation of olfactory
detection. Perception Psychophysiol., 10: 433-436.
43. CORKEY, B.E., HALE, D.E., GLENNON, M.C., KELLEY, R.I.,
COATES, P.M. KILPATRIK, L. & STANLEY, C.A. (1988)
Relationship between unusual hepatic acyl coenzyme A profiles
and the pathogenesis of Reye syndrome. J. clin. Invest.
82: 782-788.
44. CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina,
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA No. 600/
3-80-084, PB 80-221948).
45. DALZIEL, K. & DICKINSON, F.M. (1966) The kinetics and
mechanism of liver alcohol dehydrogenase with primary and
secondary alcohols as substrates. Biochem. J., 100:
34-46.
46. DAVID, J. & BOCQUET, C. (1976) Compared toxicities of
different alcohols for two Drosophila sibling species:
D. melanogaster and D. simulans. Comp. Biochem. Physiol.,
54C: 71-74.
47. DEL ROSARIO, R., DE LUMEN, B.O., HABU, T., FLATH, R.A., MON,
T.R., & TERANISHI, R. (1984) Comparison of headspace
volatiles from winged beans and soybeans. J. agric. food
Chem., 32: 1011-1015.
48. DE ZWART, D. & SLOOFF, W. (1983) The Microtox as an
alternative assay in the acute toxicity assessment of water
pollutants. Aquat. Toxicol., 4: 129-138.
49. DORIGAN, J., FULLER, B., & DUFFY, R. (1976) Scoring of
organic air pollutants. Chemistry, production and toxicity of
selected synthetic organic chemicals, The MITRE Corporation
(MITRE Technical Report MTR-7248, Rev. 1, Appendix III).
50. DRAVNIEKS, A. (1974) A building-block model for the
characterization of odorant molecules and their odors. Ann.
N.Y. Acad. Sci., 237: 144-163.
51. DRY, I., WALLACE, W., & NICHOLAS, D.J.D. (1981) Role of ATP
in nitrite reduction in roots of wheat and pea. Planta,
152: 234-238.
52. DURWALD, W. & DEGEN, W. (1956) [A fatal poisoning by n-propyl
alcohol.] Arch. Toxikol., 16: 84-88 (in German).
53. DUVEL, W.A. & HELFGOTT, T. (1975) Removal of wastewater
organics by reverse osmosis. J. Water Pollut. Control Fed.,
47: 57-65.
54. EICEMAN, G.A. & KARASEK, F.W. (1981) Identification of
residual organic compounds in food packages. J. Chromatogr.,
210: 93-103.
55. FANG, H.H.P. & CHIAN, E.S.K. (1976) Reverse osmosis
separation of polar organic compounds in aqueous solution.
Environ. Sci. Technol., 10: 364-369.
56. FAO/WHO (1980) Toxicological evaluation of certain food
additives. Report of the Joint FAO/WHO Expert Committee on
Food Additives, Geneva, World Health Organization,
pp. 162-168 (WHO Food Additive Series 16).
57. FERNANDEZ, F. & QUIGLEY, R.M. (1985) Hydraulic conductivity
of natural clays permeated with simple liquid hydrocarbons.
Can. geotech. J., 22: 205-214.
58. FLATH, R.A., ALTIERI, M.A., & MON, T.R. (1984) Volatile
constituents of Amaranthus retroflexus L. J. agric. food
Chem., 32: 92-94.
59. FLICK, E.W. (1985) Industrial solvents handbook. New Jersey,
Noyes Data Corp., pp. 220-223.
60. FORSANDER, O.A. (1967) Influence of some aliphatic alcohols
on the metabolism of rat liver slices. Biochem. J., 105:
93-97.
61. GAD, S.C., DUNN ,B.J., DOBBS, D.W., REILLY, C., & WALSH, R.D.
(1986) Development and validation of an alternative dermal
sensitization test: the mouse ear swelling test (MEST).
Toxicol. appl. Pharmacol., 84: 93-114.
62. GAGE, P.W. (1965) The effect of methyl, ethyl and n-propyl
alcohol on neuromuscular transmission in the rat.
J. Pharmacol. exp. Ther., 150: 236-243.
63. GAILLARD, D. & DERACHE, R. (1966) Action de quelques alcools
aliphatiques sur la mobilisation de différentes fractions
lipidiques chez le rat. Food Cosmet. Toxicol., 4: 515-520.
64. GELSOMINI, N. (1985) Head-space analysis with capillary
columns in quality control of wines. In: Proceedings of the
6th International Symposium on Capillary Chromatography,
pp. 515-519.
65. GERARDE, H.W., AHLSTROM, D.B., & LINDEN, N.J. (1966) The
aspiration hazard and toxicity of a homologous series of
alcohols. Arch. environ. Health, 13: 457-461.
66. GERHOLD, R.M. & MALANEY, G.W. (1966) Structural determinants
in the oxidation of aliphatic compounds by activated sludge.
J. Water Pollut. Control Fed., 38: 562-579.
67. GIBEL, W., LOHS, K., & WILDNER, G.P. (1975) [Experimental
study on the cancerogenic activity of propanol-1, 2-methyl-
propanol-1 and 3-methylbutanol. I.] Arch. Geschwulstforsch.,
45: 19-24 (in German).
68. GILLETTE, L.A., MILLER, D.L., & REDMAN, H.E. (1952) Appraisal
of a chemical waste problem by fish toxicity tests. Sewage
ind. Waste, 24: 1397-1401.
69. GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated
carbon adsorption of petrochemicals. J. Water Pollut. Control
Fed., 46: 947-965.
70. GLASGOW, A.M. & CHASE, H.P. (1976) Effect of propionic acid
on fatty acid oxidation and ureagenesis. Pediatr. Res.,
10: 683-686.
71. GOODMAN, D.E. & RAO, R.M. (1984) GC determination of fusel
alcohols in distilled alcoholic beverages. Am. Lab., 16:
100-103.
72. GORESKY, C.A., GORDON, E.R., & BACH, G.G. (1983) Uptake of
monohydric alcohols by liver: demonstration of a shared
enzymic space. Am. J. Physiol., 244: G198-G214.
73. GOTZ-SCHMIDT, E.-M. & SCHREIER, P. (1986) Neutral volatiles
from blended endive ( Cichorium endivia L.). J. agric. food
Chem., 34: 212-215.
74. GRANT, K.A. & SAMSON, H.H. (1984) n-Propanol induced
microcephaly in the neonatal rat. Neurobehav. Toxicol.
Teratol., 6: 165-169.
75. GRAY, V.M. & CRESSWELL, C.F. (1983) The effect of respiratory
inhibitors and alcohols on nitrate utilization and nitrite
accumulation in excised roots of Z. mays L. Z. Pflanzenphysiol.,
112: 207-214.
76. GREGERSEN, N. (1979) Studies on the effects of saturated and
unsaturated short-chain monocarboxylic acids on the energy
metabolism of rat liver mitochondria. Pediat. Res., 14:
1227-1230.
77. GREGERSEN, N. (1981) The specific inhibition of the pyruvate
dehydrogenase complex from pig kidney by propionyl-CoA and
isovaleryl-CoA. Biochem. Med. III, 26: 20-27.
78. GULATI, A., NATH, C., SHANKER, K., SRIMAL, R.C., DHAWAN, K.N.,
& BHARGAVA, K.P. (1985) Effects of alcohols on the
permeability of blood-brain barrier. Pharmacol. Res. Commun.,
17: 85-93.
79. HANSSEN, H.-P., SPRECHER, E., & KLINGENBERG, A. (1984)
Accumulation of volatile flavour compounds in liquid cultures
of Kluyveromyces lactis strains. Z. Naturforsch., 39c:
1030-1033.
80. HANSCH, C. & ANDERSON, S. (1967) The effect of intramolecular
hydrophobic bonding on partition coefficients. J. org. Chem.,
32: 2583-2586.
81. HARRIS, R.A. (1983) Ethanol, membrane perturbation, and
synaptosomal ion transport. Proc. West. Pharmacol. Soc.,
26: 255-257.
82. HAWLEY, G.G. (1981) Condensed chemical dictionary, 10th ed.,
Melbourne, Van Nostrand Reinhold Company Inc.
83. HELLMAN, T.M. & SMALL, F.H. (1974) Characterization of the
odor properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
84. HERMENS, J., BUSSER, F., LEEUWANGH, P., & MUSCH, A. (1985)
Quantitative structure-activity relationships and mixture
toxicity of organic chemicals in Photobacterium phosphoreum:
the Microtox 85 test. Ecotoxicol. environ. Saf., 9: 17-25.
85. HILLBOM, M.E., FRANSSILA, K., & FORSANDER, O.A. (1974)
Effects of chronic ingestion of some lower aliphatic alcohols
in rats. Res. Commun. chem. Pathol. Pharmacol., 9: 177-180.
86. HILSCHER, H., GEISSLER, E., LOHS, K., & GIBEL, W. (1969)
[Studies on the toxicity and mutagenicity of single fusel oil
components on E. coli.] Acta biol. med. Germ., 23: 843-852
(in German).
87. HIRANO, K., WATANABE, Y., ADACHI, T., ITO, Y., & SUGIURA, M.
(1985) Drug-binding properties of human alpha-foetoprotein.
Biochem J., 231: 189-191.
88. HO, Y.H., SCHWARZE, I., & SOEHRING, K. (1970) [The influence
of low aliphatic alcohols on the chloral hydrate metabolism
in rat liver sections.] Arzneim.-Forsch., 20: 1507-1509
(in German).
89. HODGE, H.C. & STERNER, J.H. (1943) Am. ind. Hyg. Assoc. Q.,
10: 93.
90. HOIGNE, J. & BADER, H. (1983) Rate constants of reactions of
ozone with organic and inorganic compounds in water. I. Non-
dissociating organic compounds. Water Res., 17: 173-183.
91. HORWITZ, W., ed. (1975) Official methods of analysis of the
Association of Official Analytical Chemists, 12th ed.,
Washington DC, Association of Official Analytical Chemists,
161 pp.
92. HOVIOUS, J.C., CONWAY, R.A., & GANZE, C.W. (1973) Anaerobic
lagoon pretreatment of petrochemical wastes. J. Water Pollut.
Control Fed., 45: 71-84.
93. HUANG, R.-D, SMITH, M.F., & ZAHLER, W.L. (1982) Inhibition of
Forskolin-activated adenylate cyclase by ethanol and other
solvents. J. cyclic Nucleotide Res., 8: 385-394.
94. HUDOLEI, V.V., MIZGIREV, I.V., & PLISS, G.B. (1987)
[Evaluation of mutagenic activity of carcinogens and other
chemical agents with Salmonella typhimurium assays.] Vopr.
Onkol., 32: 73-80 (in Russian).
95. IRPTC (1987) Data profile on n-propanol, Geneva,
Switzerland, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
96. IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ,
I.D., & BRADSHAW, J.J. (1978) Organic compounds. Their
interaction with and degradation of hepatic microsomal drug-
metabolizing enzymes in vitro. Drug Metab. Dispos., 6:
218-225.
97. JADDOU, H.A., PAVEY, J.A., & MANNING, D.J. (1978) Chemical
analysis of flavour volatiles in heat-treated milks.
J. dairy Res., 45: 391-403.
98. JOUANY, J.-P. (1982) Volatile fatty acid and alcohol
determination in digestive contents, silage juices, bacterial
cultures and anaerobic fermentor contents. Sci. Aliment.,
2: 131-144.
99. JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of
200 chemical compounds for acute toxicity towards fish by
means of the golden orfe test.] Z. Wasser-Abwasser Forsch.,
11: 161-164 (in German).
100. KAMIL, I.A., SMITH, J.N., & WILLIAMS, R.T. (1953) The
metabolism of aliphatic alcohols. The glucuronic acid
conjugation of acyclic aliphatic alcohols. Biochem. J.,
53: 129-136.
101. KANE, L.E., DOMBROSKE, R., & ALARIE, Y. (1980) Evaluation of
sensory irritation from some common industrial solvents.
Am. Ind. Hyg. Assoc. J., 41: 451-455.
102. KHARE, M. & DONDERO, N.C. (1977) Fractionation and
concentration from water of volatiles and organics on high
vacuum system: examination of sanitary landfill leachate.
Environ. Sci. Technol., 11: 814-819.
103. KINLIN, T.E., MURALIDHARA, R., PITTET, A.O., SANDERSON, A., &
WALRADT, J.P. (1972) Volatile components of roasted filberts.
J. agric. food Chem., 20: 1021-1028.
104. KIRK, R.E & OTHMER, D.F., ed. (1978-1984) Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience.
105. KLECKA, G.M., LANDI, L.P., & BODNER, K.M. (1985) Evaluation
of the OECD activated sludge, respiration inhibition test.
Chemosphere, 14: 1239-1251.
106. KNUTH, M.L. & HOGLUND, M.D. (1984) Quantitative analysis of
68 polar compounds from ten chemical classes by direct
aqueous injection gas chromatography. J. Chromatogr., 285:
153-160.
107. KRAMER, V.C., SCHNELL, D.J., & NICKERSON, K.W. (1983)
Relative toxicity of organic solvents to Aedes aegypti
larvae. J. Invertebr. Pathol., 42: 285-287.
108. KRISTIANSEN, U., HANSEN, L. NIELSEN, G.A., & HOLST, E. (1986)
Sensory irritation and pulmonary irritation of cumene and
n-propanol: Mechanisms of receptor activation and
desensitization. Acta pharmacol. toxicol., 59: 60-72.
109. KRULL, I.S., SWARTZ, M., & DRISCOLL, J.N. (1984)
Derivatizations for improved detection of alcohols by gas
chromatography and photoionization detection. Anal. Lett.,
17(A20): 2369-2384.
110. KUHNHOLZ, B. (1985) [Reflections concerning the analysis of
free aliphatic alcohols in tissue.] Blutalkohol, 22: 455-461
(in German).
111. LAING, D.G. (1975) A comparative study of the olfactory
sensitivity of humans and rats. Chem. Senses Flavor, 1:
257-269.
112. LANGVARDT, P.W. & MELCHER, R. (1979) Simultaneous
determination of polar and non-polar solvents in air using a
two-phase desorption from charcoal. Am. Ind. Hyg. Assoc. J.,
40: 1006-1012.
113. LASNE, C., GU, Z.W., VENEGAS, W., & CHOUROULINKOV, I. (1984)
The in vitro micronucleus assay for detection of cytogenetic
effects induced by mutagen-carcinogens: comparison with the
in vitro sister-chromatid exchange assay. Mutat. Res.,
130: 273-282.
114. LIEBICH, H.M., BUELOW, H.J., & KALLMAYER, R. (1982)
Quantification of endogenous aliphatic alcohols in serum and
urine. J. Chromatogr., 239: 343-349.
115. LUDWIG, E. & HAUSEN, B.M. (1977) Sensitivity to isopropyl
alcohol. Contact Dermatit., 3: 240-244.
116. LUTHIN, G.R. & TABAKOFF, B. (1984) Activation of adenylate
cyclase by alcohols requires the nucleotide-binding protein.
J. Pharmacol. exp. Ther., 228: 579-587.
117. LYON, R.C., MCCOMB, J.A., SCHREURS, J., & GOLDSTEIN, D.B.
(1981) A relationship between alcohol intoxication and the
disordering of brain membranes by a series of short-chain
alcohols. J. Pharmacol. exp. Ther., 218: 669-675.
118. MAICKEL, R.P. & NASH, J.F. (1985) Differing effects of
short-chain alcohols on body temperature and coordinated
muscular activity in mice. Neuropharmacology, 24: 83-89.
119. MCCREERY, M.J. & HUNT, W.A. (1978) Physico-chemical
correlates of alcohol intoxication. Neuropharmacology,
17: 451-461.
120. MANKES, R.F., LEFEVRE, R., RENAK,V., FIESHER, J., & ABRAHAM,
R. (1985) Reproductive effects of some solvent alcohols with
differing partition coefficients. Teratology, 31: 67A.
121. MATSUI, F., LOVERING, E.G., WATSON, J.R., BLACK, D.B., &
SEARS, R.W. (1984) Gas chromatographic method for solvent
residues in drug raw materials. J. pharm. Sci., 73:
1664-1666.
122. MAY, J. (1966) [Odour thresholds of solvents for the
judgement of solvent odour in air.] Staub Reinhalt. Luft,
26: 385-389 (in German).
123. MAY, W.A., PETERSON, R.J., & CHANG, S.S. (1983) Chemical
reactions involved in the deep-fat frying of foods. IX.
Identification of the volatile decomposition products.
J. Am. Oil Chem. Soc., 60: 990-995.
124. MICHAELIS, M.L. & MICHAELIS, E.K. (1983) Alcohol and local
anesthetic effects on Na+-dependent Ca2+-fluxes in brain
synaptic membrane vesicles. Biochem. Pharmacol., 32:
963-969.
125. MIKHEEV, M.J., & FROLOVA, A.D. (1978) [Toxicokinetics of
certain representatives of a homologous series of alcohols.]
Gig. i Sanit., 6:33-36 (in Russian).
126. MORGAN, E.T., KOOP, D.R., & COON, M.J. (1982) Catalytic
activity of cytochrome P-450 isozyme 3a isolated from liver
microsomes of ethanol-treated rabbits. J. biol. Chem.,
257: 13951-13957.
127. MOSHONAS, M.G. & SHAW, P.E. (1987) Quantitative analysis of
orange juice flavor volatiles by direct-injection gas
chromatography. J. Agric. food Chem., 35:161-165.
129. MULLIN, M.J. & HUNT, W.A. (1985) Actions of ethanol on
voltage-sensitive sodium channels: effects on neurotoxin-
stimulated sodium uptake in synaptosomes. J. Pharmacol. exp.
Ther., 232: 413-419.
130. MUNCH, J.C. (1972) Aliphatic alcohols and alkyl esters:
narcotic and lethal potencies to tadpoles and to rabbits.
Ind. Med., 41: 31-33.
131. MURPHREE, H.B., GREENBERG, L.A., & CARROLL, R.B. (1967)
Neuropharmacological effects of substances other than ethanol
in alcoholic beverages. Fed. Proc., 26: 1468-1473.
132. NELSON, B.K., BRIGHTWELL, W.S., & BURG, J.R. (1985)
Comparison of behavioural teratogenic effects of ethanol and
n-propanol administered by inhalation to rats. Neurobehav.
Toxicol Teratol., 7: 770-783.
132. NELSON, B.K., BRIGHTWELL, W.S., MACKENZIE-TAYLOR, D.R., KHAN,
A., BURG, J.R., WEIGEL, W.W. & GOAD, P.T. (1988)
Teratogenicity of n-propanol and isopropanol administered at
high inhalation concentration to rats. Food Chem. Toxicol.,
26(3): 247-254.
133. NEY, K.H. (1985) [Flavour of Tilsit cheese.] Fette Seifen
Anstrichmittel, 87: 289-293 (in German).
134. NGUYEN, V.C. & KATO, H. (1982) Volatile flavor components of
Kumazasa (Sasa albo-marginata). Agric. Biol. Chem., 46:
2795-2801.
135. NUNOMURA, N., SASAKI, M., & YOKOTSUKA, T. (1984) Shoyu
(soy sauce) flavor components: neutral fraction. Agric. Biol.
Chem., 48: 1753-1762.
136. OBE, G. & RISTOW, H. (1977) Acetaldehyde, but not ethanol,
induces sister chromatid exchanges in Chinese hamster cells
in vitro. Mutat. Res., 56: 211-213.
137. ODKVIST, L.M., LARSBY, B., THAM, R., & ASCHAN, G. (1979) On
the mechanism of vestibular disturbances caused by industrial
solvents. Adv. Oto-Rhino-Laryng., 25: 167-172.
138. OELERT, H.H. & FLORIAN, T. (1972) [Recording and valuation
of the inconvenience caused by odours from diesel exhaust.]
Staub. Reinhalt. Luft, 32: 400-407 (in German).
139. OERSKOV, S.I. (1950) Experiments on the oxydation of propyl
alcohol in rabbits. Acta physiol. Scand., 20: 258-262.
140. OTSUK141.A, K., IKI, I., & YAMASHITA, T. (1979) [Relationship
between type of whisky and volatile component.] Hakkokogaku,
57: 20-30 (in Japanese).
141. OVEREND, R. & PARASKEVOPOULOS, G. (1978) Rates of OH radical
reactions. 4. Reactions with methanol, ethanol, 1-propanol,
and 2-propanol at 296 K. J. phys. Chem., 82: 1329-1333.
142. PALO, V. & ILKOVA, H. (1970) Direct gas chromatographic
estimation of lower alcohols, acetaldehyde, acetone and
diacetyl in milk products. J. Chromatogr., 53: 363-367.
143. PATERNOSTRE, M., PICHON, Y., & DUPEYRAT, M. (1983) Effects
of n-alcohols on ionic transmembrane currents in the squid
giant axon. Stud. phys. thero. Chem., 24: 515-522.
144. PITTER, P. (1976) Determination of biological degradability
of organic substances. Water Res., 10: 231-235.
145. POSO, H. & POSO, A.R. (1980) Inhibition by aliphatic alcohols
of the stimulated activity of ornithine decarboxylase and
tyrosine aminotransferase occurring in regenerating rat liver.
Biochem. Pharmacol., 29: 2799-2803.
146. POSTEL, W. & ADAM, L. (1978) [Gas chromatographic
characterization of whisky. III. Communication: Irish
whiskey.] Branntweinwirtsch., 118: 404-407 (in German).
147. POWIS, G. (1975) Effect of a single oral dose of methanol,
ethanol and propan-2-ol on the hepatic microsomal metabolism
of foreign compounds in the rat. Biochem. J., 148: 269-277.
148. PREUSS, A. & ZIPFEL, K. (1985) [Headspace-gas chromatographic
identification of alcoholic beverages and foodstuffs.]
Lebensmittelchem. gerichtl. Chem., 39: 97-99 (in German).
149. PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
150. PRIESTLEY, D.A. & LEOPOLD, A.C. (1980) Alcohol stress on
soya bean seeds. Ann. Bot., 45: 39-45.
151. PUNTER, P.H. (1983) Measurement of human olfactory thresholds
for several groups of structurally related compounds. Chem.
Senses, 7: 215-235.
152. PURCHASE, I.F.H. (1969) Studies in kaffircorn malting and
brewing. XXII. The acute toxicity of some fusel oils found in
Bantu beer. S.A. med. J., 43: 795-798.
153. QUISTAD, G.B., STAIGER, L.E. & SCHOOLEY, D.A. (1986) The
role of carnitine in the conjugation of acidic xenobiotics.
Drug Metab. Dispos., 14:521-525.
154. RAMSEY, J.D. & FLANAGAN, R.J. (1982) Detection and
identification of volatile organic compounds in blood by
headspace gas chromatograpy as an aid to the diagnosis of
solvent abuse. J. Chromatogr., 240: 423-444.
155. REQUENA, J., VELAZ, M.E., GUERRERO, J.R., & MEDINA, J.D.
(1985) Isomers of long-chain alkane derivatives and nervous
impulse blockage. J. membr. Biol., 84: 229-238.
156. REYNOLDS, T. (1977) Comparative effects of aliphatic
compounds on inhibition of lettuce fruit germination. Ann.
Bot., 41: 637-648.
157. RIETBROCK, N. & ABSHAGEN, U. (1971) [Pharmacokinetics and
metabolism of aliphatic alcohols.] Arzneim.-Forsch., 21:
1309-1319 (in German).
158. ROE, C.R., MILLINGTON, D.S., MALTBY, D.A., BOHAN, T.P. &
HOPPEL, C.L. (1984) L-Carnitine enhances excretion of
propionyl coenzyme A as propionylcarnitine in propionic
acidemia. J. clin. Invest., 73: 1785-1788.
159. ROSS, D.H. (1976) Selective action of alcohols on cerebral
calcium levels. Ann. N.Y. Acad. Sci., 273: 280-294.
160. RYAN, D.E., THOMAS, P.E., WRIGHTON, S.A., GUZELIAN, P.S. &
LEVIN, W. (1987) Ethanol-inducible rat and human liver
cytochrome P-450: characterization and role as high affinity
N-nitrosodimethylamine demethylamine demethylases. In:
Miners, J., Birkett, D.J., Drew, R., May, B., & McManus, ed.
Microsomes and drug oxidations, London, Taylor & Francis,
pp. 3-11.
161. SABLJIC, A. & PROTIC-SABLIC, M. (1983) Quantitative
structure-activity study on the mechanism of inhibition of
microsomal p-hydroxylation of aniline by alcohols. Mol.
Pharmacol., 23: 213-218.
162. SAHU, B.R. & TANDON, U. (1983) A novel method for spot test
detection of alcohols. J. Indian Chem. Soc., 60: 615-616.
163. SANCEDA, N., KURATA, T., & ARAKAWA, N. (1984) Fractionation
and identification of volatile compounds in Patis, a
Phillipine fish sauce. Agric. Biol. Chem., 48: 3047-3052.
164. SAVINI, E.C. (1968) Estimation of the LD50 in mol/kg. Proc.
Eur. Soc. Study Drug Toxicol., 9: 276-278.
165. SCHEIMAN, M.A., SAUNDERS, R.A., & SAALFELD, F.E. (1974)
Organic contaminants in the District of Columbia water supply.
Biomed. Mass Spectrom., 1: 209-211.
166. SCOTT, T. & EAGLESON, M. (1983) Concise encyclopedia of
biochemistry. New York, W. de Gruyter, pp. 158-159.
167. SEILER, H., BLAIM, H., & BUSSE, M. (1984) [Antibacterial
effects on predominant taxa in the activated sludge system of
a chemical combine.] Z. Wasser-Abwasser Forsch., 17: 127-133
(in German).
168. SHAW, G.J., ALLEN, J.M., & VISSER, F.R. (1985) Volatile
flavor components of Babaco fruit ( Carica pentagona Heilborn).
J. agric. food Chem., 33: 795-797.
169. SINCLAIR, J.F., SMITH, L., BEMENT, W.J., SINCLAIR, P.R., &
BONKOWSKY, H.L. (1982) Increases in cytochrome P-450 in
cultured hepatocytes mediated by 3- and 4-carbon alcohols.
Biochem. Pharmacol., 31: 2811-2815.
170. SLOOFF, W. (1983) Benthic macroinvertebrates and water
quality assessment: some toxicological considerations. Aquat.
Toxicol., 4 : 73-82.
171. SLOOFF, W. & BAERSELMAN, R. (1980) Comparison of the
usefulness of the Mexican Axolotl (Ambystoma mexicanum) and
the clawed toad (Xenopus laevis) in toxicological bioassays.
Bull. environ. Contam. Toxicol., 24: 439-443.
172. SLOOFF, W., CANTON, J.H., & HERMENS, J.L.M. (1983) Comparison
of the susceptibility of 22 freshwater species to 15 chemical
compounds. I. (Sub)acute toxicity tests. Aquat. Toxicol.,
4: 113-128.
173. SMYTH, H.F., CARPENTER, C.P., WEIL, C.S., & POZZANI, U.C.
(1954) Range-finding toxicity data. Arch. ind. Hyg. occup.
Med., 10: 61-68.
174. SNIDER, J.B. & DAWSON, G.A. (1985) Tropospheric light
alcohols, carbonyls, and acetonitrile: concentrations in the
southwestern United States and Henry's law data. J. geophys.
Res., 90: 3797-3805.
175. SOLIMAN, M.A., EL SAWY, A.A., FADEL, H.M., OSMAN, F., & GAD,
A.M. (1985) Volatile components of roasted Citrillus
colocynthis, var. Colocynthoides. Agric. Biol. Chem.,
49: 269-275.
176. SRI (1984) n-Propanol: record number 0115 (last revision
date 01-06-84). In: Toxicology data bank, Palo Alto,
California, Stanford Research Institute, Bethesda, Maryland,
National Library of Medicine.
177. STENSTROM, S., ENLOE, L., PFENNING, M., & RICHELSON, E.
(1986) Acute effects of ethanol and other short-chain
alcohols on the guanylate cyclase system of murine
neuroblastoma cells (clone N1E-115). J. Pharmacol. exp.
Ther., 236: 458-463.
178. STOCK, K. & SCHMIDT, M. (1978) Effects of short-chain
alcohols on adenylate cyclase in plasma membranes of rat
adipocytes. Naunyn-Schmiedeberg Arch. Pharmacol., 302: 37-43.
179. STOFBERG, J. & GRUNDSCHOBER, F. (1984) Consumption ratio and
food predominance of flavoring materials: second cumulative
series. Perfumer Flavorist, 9: 53-83.
180. STOKES, J.A. & HARRIS, R.A. (1982) Alcohols and synaptosomal
calcium transport. Mol. Pharmacol., 22: 99-104.
181. STOLZENBERG, S.J. & HINE, C.H. (1979) Mutagenicity of
halogenated and oxygenated three-carbon compounds. J. Toxicol.
environ. Health, 5: 1149-1158.
182. STONE, H., PRYOR, G.T., & STEINMETZ, G. (1972) A comparison
of olfactory adaptation among seven odorants and their
relationship with several physico-chemical properties.
Perception Psychophys., 12: 501-504.
183. STRANGE, A.W., SCHNEIDER, C.W., & GOLDBORT, R. (1976)
Selection of C3 alcohols by high and low ethanol selecting
mouse strains and the effects on open field activity.
Pharmacol. Biochem. Behav., 4: 527-530.
184. STUMPF, D.A., MCAFEE, J., PARKS, J.K., & EGUREN, L. (1986)
Propionate inhibition of succinate: CoA ligase (GDP) and the
citric acid cycle in mitochondria. Pediatr. Res., 14:
1127-1131.
185. TABAKOFF, B. & HOFFMAN, P.L. (1983) Alcohol interactions with
brain opiate receptors. Life Sci., 32: 197-204.
186. TANDOI, P., GUIDOTTI, M., & STACCHINI, P. (1984) [On the
composition of brandies.] Riv. Soc. Ital. Sci. Aliment.,
13: 69-76 (in Italian).
187. TAYLOR, J.M., JENNER, P.M., & JONES, W.I. (1964) A comparison
of the toxicity of some allyl, propenyl, and propyl compounds
in the rat. Toxicol. appl. Pharmacol., 6: 378-387.
188. TESCHKE, R., HASUMURA, Y., & LIEBER, C.S. (1975) Hepatic
microsomal alcohol-oxidizing system. Affinity for methanol,
ethanol, propanol, and butanol. J. biol. Chem., 250:
7397-7403.
189. TESTA, B. (1981) Structural and electronic factors
influencing the inhibition of aniline hydroxylation by
alcohols and their binding to cytochrome P-450. Chem.-biol.
Interact., 34: 287-300.
190. TICHY, M., TRCKA, V. ROTH, Z., & KRIVUCOVA, M. (1985) QSAR
analysis and data extrapolation among mammals in a series of
aliphatic alcohols. Environ. Health Perspect., 61: 321-328.
191. TOMERA, J.F., SKIPPER, P.L., WISHNOK, J.S., TANNEBAUM, S.R.,
& BRUNENGRABER, H. (1984) Inhibition of N-nitroso-
dimethylamine metabolism by ethanol and other inhibitors in
the isolated perfused rat liver. Carcinogenesis, 5: 113-116.
192. UHLEMANN, E.R., ROBBERECHT, P., & GARDNER, J.D. (1979)
Effects of alcohols on the actions of VIP and secretin on
acinar cells from guinea-pig pancreas. Gastroenterology,
76: 917-925.
193. UNRUH, J.D. & SPINICELLI, L. (1981) Propyl alcohols, n-propyl
alcohol. In: Kirk, R.E. & Othmer, D.F., ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience,
Vol. 19, pp. 221-227, 194.
194. URANO, K., OGURA, K., & WADA, H. (1981) Direct analytical
method for aliphatic compounds in water by steam carrier gas
chromatography. Water Res., 15: 225-231.
195. US NIOSH (1984) Method 1401. In: Eller, P.M., ed. NIOSH
Manual of analytical methods, 3rd ed., Cincinnati, Ohio,
National Institute for Occupational Safety and Health, Vol. 1,
pp. 1401-1-1401-4.
196. VAISHNAV, D.D. & LOPAS, D.M. (1985) Relationship between
lipophilicity and biodegradation inhibition of selected
industrial chemicals. Dev. ind. Microbiol., 26: 557-565.
197. VEITH, G.D. & KOSIAN, P. (1983) Estimating bioconcentration
potential from octanol/water partition coefficients. In:
Mackay et al., ed. Physical behaviour of PCBs in the Great
Lakes, Ann Arbor, Michigan, Ann Arbor Science, pp. 269-282.
198. VERSCHUEREN, K. (1983) Handbook of environmental data on
organic chemicals, 2nd ed., Melbourne, Van Nostrand Reinhold
Company Inc.
199. VIDELA, L.A., FERNANDEZ, V., & DE MARINIS, A. (1982) Liver
peroxidative pressure and glutathione status following
acetaldehyde and aliphatic alcohols pretreatment in the rat.
Biochem. Biophys. Res. Commun., 104: 965-970.
200. VON DER HUDE, W., SCHEUTWINKEL, M., GRAMLICH, U., FISSLER, B.,
& BASLER, A. (1987) Genotoxicity of three carbon compounds
evaluated in the SCE test in vitro. Environ. Mutagen.,
9: 401-410.
201. WACHTEL, R.E. (1984) Aliphatic alcohols increase the decay
rate of glutamate-activated currents at the crayfish
neuromuscular junction. Br. J. Pharmacol., 83: 393-397.
202. WAGNER, R. (1976) [Investigations into the degradation
behaviour of organic compounds using the respirometric
dilution method. II. The degradation kinetics of the test
compounds.] Vom Wasser, 47: 241-265.
203. WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
IIJIMA, M. (1984) Induction of megamitochondria in the rat
liver by n-propyl alcohol and n-butyl alcohol. Acta pathol.
Jpn., 34: 471-480.
204. WALLGREN, H. (1960) Relative intoxicating effects on rats
of ethyl, propyl and butyl alcohols. Acta pharmacol. toxicol.,
16: 217-222.
205. WATERER, D.R. & PRITCHARD, M.K. (1985a) Volatile production
by wounded Russet Burbank and Norland potatoes. Sci. Aliments,
5: 205-216.
206. WATERER, D.R. & PRITCHARD, M.K. (1985b) Production of
volatile metabolites in potatoes infected by Erwinia
carotovora var. carotovora and E. carotovora var. atroseptica.
Can. J. plant Pathol., 7: 47-51.
207. WILKIN, J.K. & FORTNER, G. (1985) Cutaneous vascular
sensitivity to lower aliphatic alcohols and aldehydes in
orientals. Alcoholism clin. exp. Res., 9: 522-525.
208. WINTERSTEIGER, R., GAMSE, G., & PACHA, W. (1982)
[Quantification of alcoholic compounds and amines with 4-(6-
methylbenzo-thiazol-2-yl)phenyl isocyanate. Fresenius Z.
anal. Chem., 312: 455-461.
209. WOLF, M., URBAN, R., WELLER, J.-P., & TROGER, H.D. (1985)
[The analysis of congeners of alcoholic beverages in blood.
First communication: the application of capillary gas
chromatography in micro head-space analysis.] Blutalkohol,
22: 321-332 (in German).
210. WOLFF, T. (1978) In vitro inhibition of monooxygenase
dependent reactions by organic solvents. Int. Congr. Ser.
Excerpta Med., 440: 196-199.
211. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., UCHIDA,
H., & HAYASHI, K. (1983) Volatile flavor compounds of boiled
buckwheat flour. Agric. Biol. Chem., 47: 729-738.
212. YAJIMA, I., YANAI, T., NAKAMURA, M., SAKAKIBARA, H., &
HAYASHI, K. (1984) Volatile flavor components of Kogyoku
apples. Agric. Biol. Chem., 48: 849-855.
213. YASHUDA, Y., CABRAL, A.M., & ANTONIO, A. (1976) Inhibitory
action of aliphatic alcohols on smooth muscle contraction.
Pharmacology, 14: 473-478.
214. YASUHARA, A. & FUWA, K. (1982) Characterization of odorous
compounds in rotten blue-green algae. Agric. Biol. Chem.,
46: 1761-1766.
215. YASUHARA, A., FUWA, K., & JIMBU, M. (1984) Identification of
odorous compounds in fresh and rotten swine manure. Agric.
Biol. Chem., 48: 3001-3010.
216. YOUNG, P.J. & PARKER, A. (1983) The identification and
possible environmental impact of trace gases and vapours in
landfill gas. Waste Manag. Res., 1: 213-226.
RESUME
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le propanol-1 est un liquide incolore, très inflammable, qui
est volatil à la température ambiante et sous la pression
atmosphérique normale. Il est miscible à l'eau et aux solvants
organiques. Parmi les méthodes d'analyse, on peut citer la
chromatographie en phase gazeuse, qui permet de déceler jusqu'à
5 x 10-5 mg/m3 dans l'air, 1 x 10-4 mg/litre dans l'eau et
0,002 mg/litre dans le sang, le sérum ou les urines lorsque
l'échantillon a été extrait ou concentré de façon satisfaisante.
2. Sources d'exposition humaine et environnementale
La capacité de production mondiale a dépassé 130 000 tonnes en
1979. Le propanol-1 d'origine naturelle résulte de la
décomposition de matériaux organiques par divers microorganismes et
on le rencontre également dans les végétaux et le mazout.
Industriellement, on le produit par réaction de l'éthylène sur
l'oxyde de carbone et l'hydrogène qui donne du propionaldéhyde,
celui-ci étant ensuite hydrogéné en propanol. C'est également un
sous-produit de la fabrication du méthanol et il peut être obtenu
directement à partir du propane ou de l'acroléine. Le propanol-1
est essentiellement un solvant à tout faire, à usage industriel ou
domestique. Il entre dans la composition des encres d'imprimerie
flexographiques et il est également utilisé dans l'industrie
textile, dans des produits à usage personnel tels que les produits
cosmétiques, les lotions ainsi que dans les produits pour le
nettoyage des vitres, les encaustiques et les antiseptiques. En
second lieu par ordre d'importance, on peut citer son utilisation
comme produit intermédiaire dans la fabrication de divers composés
chimiques.
3. Transport, distribution et transformation dans l'environnement
La principale voie de pénétration de propanol-1 dans
l'environnement est constituée par les émissions dans l'atmosphère
qui se produisent au cours de la production, de la transformation,
du stockage, du transport et de l'utilisation de ce composé ou du
rejet de déchets qui en contiennent. Il peut y avoir également des
décharges dans l'eau et le sol. Le propanol-1 étant principalement
utilisé comme solvant volatil, il finit par se dissiper en grande
partie dans l'environnement.
Le propanol-1 est rapidement éliminé de l'atmosphère par
réaction sur les radicaux hydroxyles et par les précipitations. Il
est facilement biodégradable tant par voie aérobie que par voie
anaérobie et du fait de l'existence de ces mécanismes d'élimination
chimique et biologique, on ne le rencontre normalement pas en
quantité mesurable dans l'environnement. Toutefois on en a décelé
la présence dans l'air des agglomérations urbaines, dans des
décharges, ainsi que dans les eaux s'échappant de zones
d'enfouissement de déchets.
Le logarithme du coefficient de partage n-octanol/eau du
propanol-1 est de 0,34 et son facteur de bioconcentration a une
valeur de 0,7, ce qui en rend la bioaccumulation très improbable.
4. Niveau dans l'environnement et exposition humaine
La population en général peut être exposée au propanol par
suite d'une ingestion accidentelle, par inhalation lors de
l'utilisation du produit ou par absorption avec la nourriture
(propanol d'origine naturelle, additif d'aromatisation ou reste de
solvant) ou des boissons alcoolisées ou non. Par exemple la bière
en contient jusqu'à 195 mg/litre, le vin jusqu'à 116 mg/litre et
certains spiritueux jusqu'à 3500 mg/litre. L'exposition de la
population par suite d'inhalation ou de la consommation d'eau de
boisson est faible (aux Etats-Unis la concentration moyenne dans
des échantillons d'air urbain se situait à 0,00005 mg/m3 et dans
des échantillons d'eau de boisson, à 0,001 mg/litre). Les
travailleurs courent un risque d'exposition par inhalation lors de
la production, de la transformation et de l'utilisation du produit.
Toutefois, on ne dispose d'aucune donnée qui permettrait de
chiffrer ce type d'exposition.
5. Cinétique et métabolisme
Après ingestion, le propanol-1 est rapidement absorbé et
distribué dans l'ensemble de l'organisme. On manque de données sur
la vitesse d'absorption après inhalation et exposition cutanée. Le
propanol-1 est métabolisé par l'alcool déshydrogénase (ADH) en
aldéhyde puis acide propionique et peut entrer dans le cycle de
Krebs. Cette oxydation constitue l'étape limitante du métabolisme
du propanol-1. In vitro, les oxydases microsomiques du rat et du
lapin sont également capables d'oxyder le propanol-1 en aldéhyde
propionique. L'ADH et des systèmes d'oxydation microsomiques ont
une affinité beaucoup plus importante pour le propanol-1 que pour
l'éthanol; aussi le propanol-1 est-il rapidement éliminé de
l'organisme. Chez le rat, sa demi-vie après administration par
voie orale d'une dose de 1000 mg/kg est de 45 minutes.
Chez l'animal et chez l'homme, le propanol-1 peut être éliminé
de l'organisme dans l'air expiré ou dans les urines. Chez des
êtres humains ayant reçu par voie orale une dose de 3,75 mg de
propanol-1 par kg de poids corporel et de 1200 mg d'éthanol par kg
de poids corporel, l'excrétion urinaire totale du propanol-1 a été
de 2,1 % de la dose. Les concentrations urinaires de propanol-1
étaient d'autant plus basses que la quantité d'éthanol ingérée
simultanément était basse, ce qui montre qu'il y avait compétition
pour l'ADH entre le propanol-1 et la surdose d'éthanol.
6. Effets sur les êtres vivant dans leur milieu naturel
Aux concentrations normalement présentes dans l'environnement,
le propanol-1 n'est pas toxique pour la vie aquatique, les insectes
ou les végétaux. Chez trois des espèces aquatiques les plus
sensibles (trois protozoaires), le seuil l'inhibition de la
multiplication cellulaire se situait entre 38 et 568 mg/litre.
Dans le cas d'organismes plus évolués, la concentration létale
était d'environ 5000 mg/litre, avec des variations remarquablement
faibles d'un phylum à l'autre et une courbe dose-réponse de très
forte pente. Certaines bactéries et micro-organismes qui vivent
dans les eaux résiduaires et les boues activées sont capables de
s'adapter à des concentrations supérieures à 17 000 mg/litre.
Le propanol-1 peut inhiber ou au contraire stimuler la
germination des semences selon sa concentration dans l'eau
d'arrosage et les conditions d'exposition. Le composé accroît
l'accumulation de nitrites dans le maïs, les pois et le froment.
7. Effets sur les animaux d'expérience et sur les systèmes d'épreuve
in vitro
Le propanol-1 présente une faible toxicité aiguë pour les
mammifères (mesurée d'après la mortalité), que l'exposition se
fasse par voie percutanée, orale ou respiratoire. On a fait état
de valeurs allant de 1870 à 6800 mg par kg de poids corporel pour
la DL50 par voie orale chez plusieurs espèces animales. Toutefois
pour de très jeunes rats, on donne une DL50 orale de 560 à 660 mg
par kg de poids corporel. Après une seule exposition, le principal
effet toxique du propanol-1 consiste dans la dépression du système
nerveux central. Selon les données disponibles, le propanol-1
exercerait sur le système nerveux central des effets analogues à
ceux de l'éthanol; toutefois il semblerait que la neurotoxicité du
propanol-1 soit plus importante. Les DE50 pour l'anesthésie chez
le lapin et la perte du réflexe de redressement chez la souris se
situaient respectivement à 1440 mg par kg de poids corporel par
voie orale et à 1478 mg par kg de poids corporel par voie intra-
péritonéale; ces doses sont environ quatre fois plus faibles que
dans le cas de l'éthanol. Dans l'épreuve du plan incliné, le
propanol-1 s'est révélé 2,5 fois plus actif que l'éthanol chez le
rat.
Des doses uniques de 3000 ou 6000 mg par kg de poids corporel
administrées par voie orale à des rats ont provoqué une
accumulation réversible de triglycérides dans le foie. Les vapeurs
fortement concentrées provoquent une irritation des voies
respiratoires chez la souris. Aux concentrations d'environ 30 000
mg/m3 on note une réduction de 50 % du rythme respiratoire chez la
souris.
On ne dispose de données ni sur l'irritation oculaire ni sur
l'irritation cutanée. Aucun effet n'a été observé lors d'une
épreuve de sensibilisation cutanée sur des souris CF1.
Chez des rats mâles exposés pendant six semaines à une dose de
15 220 mg/m3 de propanol-1, on a relevé quelques signes d'une
action nocive possible sur la fonction de reproduction. En
revanche aucun effet n'a été noté à la dose de 8610 mg/m3. Après
exposition de rattes gravides au propanol-1, on a observé des
signes patents de toxicité pour les mères et les foetus aux doses
de 23 968 et 14 893 mg/m3 (9743 et 6054 ppm respectivement); aucun
signe de toxicité n'a été noté à 9001 mg/m3 (3659 ppm). On n'a
observé aucune anomalie comportementale parmi les descendants de
rats mâles exposés pendant six semaines à 8610 ou 15 220 mg de
propanol-1 par m3, ni dans la descendance de rattes exposées
pendant leur gestation aux mêmes concentrations. Toutefois, en
administrant à des ratons de 5 à 8 jours des doses orales de 3000 à
7800 mg de propanol-1 par kg et par jour, on constatait des signes
de dépression du SNC pendant l'administration et un syndrome de
sevrage à la cessation du traitement. Le cerveau de ces rats a été
examiné à l'âge de 18 jours; on a constaté une réduction du poids
de cet organe tant en valeur absolue qu'en valeur relative, avec
diminution de la teneur en ADN et réduction localisée des taux de
cholestérol et de protéine.
La recherche de mutations ponctuelles au moyen de 2 épreuves
utilisant Salmonella typhimurium n'a donné que des résultats
négatifs, de même la recherche de mutations réverses sur
Escherichia coli CA-274. Les résultats ont été également négatifs
en ce qui concerne les échanges entre chromatides soeurs ou la
présence de micro-noyaux dans les cellules mammaliennes in vitro.
Il n'existe pas d' autres données relatives à la mutagénicité.
Lors d'une étude de cancérogénicité portant sur de petits
groupes de rats Wistar exposés tout au long de leur existence par
voie orale à des doses de 240 mg/kg ou par voie sous cutanée à des
doses de 48 mg/kg, on a constaté un accroissement sensible de
l'incidence des sarcomes du foie dans le groupe recevant le produit
par voie sous cutanée. Toutefois cette étude ne permet pas
d'apprécier la cancérogénicité du propanol-1, notamment à cause de
l'absence de détails expérimentaux, du nombre trop restreint
d'animaux et de l'utilisation d'une dose hépatotoxique unique très
élevée.
8. Effets sur la santé humaine
On n'a pas signalé d'effets nocifs sur la santé humaine dans la
population en général ou parmi des groupes professionnels. Le seul
cas d'intoxication mortelle qui ait été signalé est celui d'une
femme, retrouvée inconsciente et qui est décédé 4 à 5 heures après
l'ingestion de propanol. L'autopsie a révélé un oedème cérébral et
pulmonaire. Lors d'une étude sur l'irritation et la sensibilisation
cutanées, on a signalé des réactions allergiques chez un membre du
personnel du laboratoire. Chez neuf volontaires sur 12, on a
observé un érythème qui a duré au moins 1 h après 5 minutes
d'application sur les avant-bras de papiers filtres imprégnés de
0,025 ml d'une solution aqueuse à 75 % de propanol-1. On ne
dispose d'aucun autre rapport concernant d'éventuels effets
toxiques après exposition professionnelle au propanol-1.
Il n'existe pas d'études épidémiologiques qui permettent
d'établir les effets à long terme, et notamment la cancérogénicité,
du propanol-1 chez l'homme.
9. Résumé de l'évaluation
Il peut y avoir exposition humaine au propanol-1 à la suite de
l'absorption de nourriture ou de boissons qui en contiennent. Une
exposition par inhalation peut se produire lors de l'utilisation de
ce produit à des fins ménagères, ou professionnelles, au cours de
la fabrication, de la transformation et de l'utilisation de ce
produit. Les données très limitées dont on dispose sur la teneur
de l'air ambiant et de l'eau en propanol-1 indiquent que ces
teneurs sont très faibles.
Le propanol-1 est rapidement absorbé et se répartit dans tout
l'organisme après ingestion. Après inhalation, l'absorption est
également rapide mais la résorption percutanée devrait être lente.
Chez l'animal, la toxicité aiguë du propanol-1 est faible, que
l'exposition ait lieu par voie percutanée, par voir orale ou par
voie respiratoire. L'exposition de personnes appartenant à la
population générale à des teneurs potentiellement mortelles peut se
produire à la suite d'une ingestion accidentelle ou volontaire.
Toutefois, un seul cas d'intoxication mortelle par le propanol-1 a
été signalé jusqu'ici. Les effets aigus les plus probables chez
l'homme sont une intoxication de type alcoolique pouvant entraîner
une narcose. L'expérimentation animale montre que le propanol-1
est 2 à 4 fois plus toxique que l'éthanol.
Le propanol-1 peut être irritant pour la peau mouillée.
On ne dispose pas de données suffisantes sur la toxicité chez
l'animal pour procéder à une évaluation des risques pour la santé
humaine qui découleraient d'une exposition répétée ou prolongée au
propanol-1. Toutefois, un certain nombre d'études à court terme
sur le rat, bien que limitées, indiquent que dans les conditions où
se produit habituellement l'exposition humaine, il est peu probable
que le risque encouru soit très grave.
Une exposition par inhalation à une concentration de 15 220
mg/m3 a affecté la fonction de reproduction de rats mâles, ce qui
n'a pas été le cas à la dose de 8610 mg/m3. Chez des rattes
gravides, la dose sans effet observable se situait à 9001 mg/3
(3659 ppm); quant à la dose la plus faible donnant lieu à un effet
observable, elle était de 14 893 mg/m3 (6054 ppm), en ce qui
concerne la toxicité pour la mère et le foetus. L'exposition par
inhalation à de fortes concentrations de propanol-1 a donc des
effets nocifs sur la reproduction et le développement des rats
mâles et femelles lorsque les concentrations utilisées sont
manifestement toxiques pour ces animaux. Pour obtenir ces effets,
il a fallu utiliser des concentrations plus élevées que celles
auxquelles l'homme pourrait être normalement exposé.
Différentes épreuves ont montré que le propanol-1 ne provoquait
pas de mutations ponctuelles chez les bactéries. Toutefois, si ces
résultats donnent à penser que le produit n'est pas génotoxique,
les données disponibles sont trop limitées pour qu'on puisse en
évaluer correctement le pouvoir mutagène. La seule étude dont on
possède les résultats ne permet pas d'évaluer convenablement la
cancérogénicité du propanol-1 chez l'animal d'expérience. On ne
dispose d'aucune donnée sur l'exposition à long terme des
populations humaines à ce produit, de sorte qu'on ne peut pas se
prononcer sur son pouvoir cancérogène chez l'homme.
A part un cas d'intoxication mortelle consécutif à l'ingestion
d'un demi-litre de propanol-1, il n'existe pratiquement aucun
rapport concernant d'éventuels effets nocifs découlant d'une
exposition au propanol-1, qu'il s'agisse de la population générale
ou de groupes professionnels. Le Groupe spécial estime qu'il est
improbable que le propanol-1 presente des risques graves pour la
population générale dans les conditions normales d'exposition.
Du propanol-1 peut être libéré dans l'environnement lors de la
production, de la transformation, du stockage, du transport, de
l'utilisation ou de rejet de ce produit. Comme il est
essentiellement utilisé comme solvant volatil, l'essentiel de la
production finit par aboutir dans l'atmosphère. Toutefois, par
réaction avec les radicaux hydroxyles et entraînement par les
précipitations, le propanol-1 est rapidement éliminé de
l'atmosphère, d'où il disparaît en moins de trois jours. Le
propanol-1 s'élimine également rapidement de l'eau et du sol de
sorte que l'on ne le rencontre que rarement en concentrations
mesurables dans l'air, l'eau et la terre. Le propanol-1 est peu
absorbé par les particules du sol où il se révèle mobile et dont il
accroît la perméabilité à certains hydrocarbures aromatiques.
Compte tenu des propriétés physiques du propanol-1, il est peu
probable qu'il donne lieu à une bioaccumulation et sauf accident ou
rejet négligent, le propanol-1 ne présente aucun risque pour la
faune ou la flore aquatique aux concentrations où on le rencontre
habituellement dans l'environnement.
RESUMEN
1. Identidad, propiedades físicas y químicas, métodos analíticos
El 1-propanol es un líquido incoloro y sumamente inflamable,
volátil a temperatura ambiente y presión atmosférica normal. Es
miscible con el agua y los disolventes orgánicos. Entre los
métodos analíticos para el propanol figuran la cromatografía de
gases, que puede detectar hasta 5 x 10-5 mg/m3 en el aire,
1 x 10-4 mg/litro en el agua y 0,002 mg/litro en la sangre, el
suero o la orina cuando se utilizan con la muestra procedimientos
adecuados de extracción o concentración.
2. Fuentes de exposición humana y ambiental
En 1979, la capacidad de producción mundial al año superó las
130 000 toneladas. En la naturaleza se produce por descomposición
de material orgánico por diversos micro-organismos, y se halla en
las plantas y en los aceites combustibles. El 1-propanol se
produce a partir del eteno por reacción con el monóxido de carbono
y el hidrógeno para dar propionaldehído, que a continuación se
hidrogena. Aparece también como sub-producto en la fabricación del
metanol y puede producirse a partir del propano directamente o a
partir de la acroleína. El uso principal del 1-propanol es como
disolvente de uso múltiple en la industria y el hogar. Se utiliza
en las tintas de impresión flexográfica y en aplicaciones textiles,
productos de uso personal como cosméticos y lociones, en productos
para limpiar cristales, en abrillantadores y en fórmulas
antisépticas. Le sigue en importancia su uso como producto
intermedio en la fabricación de diversos compuestos químicos.
3. Transporte, distribución y transformación en el medio ambiente
La principal vía de entrada del 1-propanol en el medio ambiente
es su emisión a la atmósfera durante la producción, el tratamiento,
el almacenamiento, el transporte, el uso y la evacuación de
desechos. También se producen emisiones al agua y al suelo.
Puesto que el uso principal del 1-propanol es como disolvente
volátil, gran parte del volumen de producción acaba en el medio
ambiente.
El 1-propanol desaparece rápidamente de la atmósfera por
reacción con radicales hidroxilo y por el lavado con la lluvia. Es
fácilmente biodegradable, tanto en condiciones aerobias como
anaerobias y, a causa de estos mecanismos de eliminación química y
biológica, no suelen encontrarse niveles medibles de la sustancia
en el medio ambiente. No obstante, se ha detectado en la atmósfera
urbana, en vertederos de desechos y también en las aguas que se
rezumaban de un terraplenado. La permeabilidad del suelo al
1-propanol es probablemente elevada y el compuesto aumenta la
permeabilidad a ciertos disolventes aromáticos.
El 1-propanol tiene un coeficiente de reparto log
n-octanol/agua de 0,34 y un factor de bioconcentración de 0,7, lo
que hace muy poco probable su bioacumulación.
4. Niveles ambientales y exposición humana
La exposición de la población general puede producirse por
ingestión accidental, por inhalación durante el uso y por ingestión
junto con los alimentos (que contengan 1-propanol como aromatizante
volátil natural o añadido o como residuo de disolvente) y bebidas
alcohólicas y no alcohólicas. Por ejemplo, la cerveza contiene
hasta 195 mg/litro, el vino hasta 116 mg/litro y los diversos tipos
de licores hasta 3520 mg/litro. La exposición de la población
general al 1-propanol por inhalación y en el agua de bebida es baja
(en los Estados Unidos, la concentración media en muestras de aire
urbano fue de 0,00005 mg/m3 y la correspondiente al agua de bebida
0,001 mg/litro). Aunque los trabajadores están potencialmente
expuestos por la inhalación durante la fabricación, el tratamiento
y la utilización, no se dispone de datos para cuantificar esas
exposiciones.
5. Cinética y metabolismo
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se carece de datos sobre la tasa de
absorción tras la inhalación y la exposición dérmica. El
1-propanol es metabolizado por la deshidrogenasa alcohólica para
dar ácido propiónico por intermedio del aldehído y puede entrar en
el ciclo del ácido tricarboxílico. Esta oxidación es una etapa
limitativa de la velocidad del metabolismo del 1-propanol. In
vitro, las oxidasas microsómicas de rata y conejo también son
capaces de oxidar el 1-propanol a aldehído propiónico. La afinidad
relativa de la deshidrogenasa alcohólica y los sistemas de
oxidación microsómicos por el 1-propanol es mucho más elevada que
en el caso del etanol; así pues, el 1-propanol se elimina
rápidamente del organismo. En la rata, el periodo de
semieliminación de una dosis oral de 1000 mg/kg fue de 45 minutos.
Tanto en animales como en el hombre, el 1-propanol puede ser
eliminado del organismo en el aire exhalado o en la orina. A
sujetos a los que se administró una dosis de 1-propanol de 3,75 mg
por kg de peso corporal y 1200 mg de etanol por kg de peso corporal
por vía oral, la excreción urinaria total de 1-propanol fue del
2,1% de la dosis. Los niveles urinarios de 1-propanol fueron más
bajos cuanto más baja era la cantidad de etanol ingerida
simultáneamente, lo que demuestra la competencia por la
deshidrogenasa alcohólica entre el 1-propanol y la sobredosis de
etanol.
6. Efectos en los organismos en el medio ambiente
A las concentraciones en que normalmente se encuentra en el
medio ambiente, el 1-propanol no resulta tóxico para los organismos
acuáticos, los insectos ni las plantas. El umbral de inhibición
para la multiplicación celular de tres de las especies acuáticas
más sensibles (tres protozoos) fue de 38 - 568 mg/litro. Para los
organismos superiores, la concentración letal fue de unos 5000
mg/litro, y variaba notablemente poco de un filum a otro y exhibía
una curva dosis-respuesta sumamente pronunciada. Algunas bacterias
y microorganismos de aguas residuales y cienos activados son
capaces de adaptarse a concentraciones superiores a 17 000
mg/litro.
La germinación de semillas puede verse inhibida o estimulada
por el 1-propanol según la concentración en el agua utilizada y las
condiciones de exposición. El compuesto aumenta la acumulación de
nitritos en el maíz, los guisantes y el trigo.
7. Efectos en animales de experimentación y en sistemas de ensayo
in vitro
La toxicidad aguda del 1-propanol para los mamíferos (a juzgar
por la mortalidad) es baja, por cualquiera de las vías de
exposición: cutánea, oral o respiratoria. Se ha comunicado que
los valores de la DL50 por vía oral para varias especies animales
varían entre 1870 y 6800 mg/kg de peso corporal. No obstante, en
ratas muy jóvenes se notificó una DL50 por vía oral de 560 - 660
mg/kg de peso corporal. El principal efecto tóxico del 1-propanol
tras una exposición única es la depresión del sistema nervioso
central. Los datos de que se dispone sobre el 1-propanol parecen
indicar que sus efectos sobre el sistema nervioso central son
semejantes a los del etanol; no obstante, el 1-propanol parece ser
más neurotóxico. Los valores de la DE50 para la narcosis en el
conejo y la pérdida del reflejo de enderezamiento en el ratón
fueron, respectivamente, de 1440 mg/kg de peso corporal por vía
oral, y de 1478 mg/kg de peso corporal por vía intraperitoneal;
estos valores son unas cuatro veces más bajos que los
correspondientes al etanol. En el ensayo del plano inclinado, el
1-propanol fue 2,5 veces más potente que el etanol en la rata.
La administración de dosis únicas por vía oral de 3000 ó
6000 mg/kg de peso corporal tuvo como resultado una acumulación
reversible de triglicéridos en el hígado de la rata. Las
concentraciones elevadas de vapor provocaron irritaciones en el
tracto respiratorio del ratón. El ritmo respiratorio del ratón se
vio disminuido en un 50% a concentraciones de aproximadamente
30 000 mg/m3.
No se dispone de datos sobre irritación ocular y cutánea. No
se observó sensibilización en una prueba de sensibilización cutánea
en ratones CF1.
En machos de rata expuestos durante seis semanas a 15 220
mg/m3, se obtuvieron pruebas limitadas de que el 1-propanol
disminuye la capacidad reproductora. No se observaron efectos tras
una exposición similar a 8610 mg/m3. Cuando se expusieron ratas
gestantes al 1-propanol, se observó toxicidad materna y fetal a
23 968 y 14 893 mg/m3 (9743 y 6054 ppm); no se observó toxicidad a
9001 mg/m3 (3659 ppm). No se observaron defectos de comportamiento
en la progenie de machos de rata expuestos durante seis semanas a
8610 ó 15 220 mg de 1-propanol/m3, ni en la de ratas expuestas
durante la gestación a las mismas concentraciones. No obstante,
cuando se administró a ratas de 5 a 8 días de edad 3000 - 7800 mg
de 1-propanol/kg por vía oral al día, se observó depresión del
sistema nervioso central durante la dosificación y síntomas de
privación cuando se retiraba la dosis. Cuando las ratas cumplieron
18 días se examinaron sus cerebros y se observaron reducciones en
los pesos cerebrales absoluto y relativo y en el contenido de ADN,
así como disminuciones regionales de los niveles de colesterol y de
proteínas.
El 1-propanol dio resultados negativos en dos ensayos de
detección de mutaciones puntuales utilizando Salmonella typhimurium
y en un ensayo de mutación inversa realizado con Escherichia coli
CA-274. Se obtuvieron resultados negativos en ensayos para inducir
intercambio de cromátidas hermanas o micronúcleos en células de
mamíferos in vitro. No se obtuvieron otros datos sobre
mutagenicidad.
En un estudio de carcinogenicidad realizado en grupos reducidos
de ratas Wistar expuestas durante toda su vida a dosis orales de
240 mg/kg o a dosis subcutáneas de 48 mg/kg, se observó un aumento
significativo de la incidencia de sarcoma hepático en el grupo en
el que la dosis se administraba por vía subcutánea. No obstante,
el estudio resultó insuficiente para evaluar la carcino-genicidad
por diversos motivos, entre ellos la falta de detalle experimental,
el reducido número de animales y el empleo de una sola dosis
elevada para inducir toxicidad hepática.
8. Efectos en la salud humana
No se tiene noticia de efectos adversos para la salud en la
población general o en grupos profesionales. El único caso
notificado de intoxicación mortal, fue el de una mujer que perdió
el conocimiento y falleció a las 4 - 5 h de la ingestión. La
autopsia reveló "inflamación cerebral" y edema pulmonar. En un
estudio sobre irritación cutánea y sensibilización, se comunicó la
aparición de reacciones alérgicas en un operario de laboratorio.
En otro grupo de 12 voluntarios, se observó en 9 individuos un
eritema que duró al menos 60 minutos tras la aplicación durante 5
minutos de papeles de filtro impregnados con 0,025 ml de una
solución al 75% de 1-propanol en agua aplicados sobre los
antebrazos.
No se dispone de más informes sobre efectos adversos para la
salud tras la exposición profesional al 1-propanol. No se dispone
de estudios epidemiológicos para evaluar los efectos a largo plazo,
incluida la carcinogenicidad, del 1-propanol en el ser humano.
9. Resumen de la evaluación
La exposición del ser humano al 1-propanol puede producirse por
la ingestión de alimentos o bebidas que lo contengan. La
exposición por inhalación puede tener lugar durante el uso
doméstico de la sustancia y en el ámbito laboral durante su
fabricación, tratamiento y uso. Los muy limitados datos de que se
dispone sobre el nivel de 1-propanol en el aire y el agua parecen
indicar que las concentraciones son muy reducidas.
El 1-propanol se absorbe y distribuye rápidamente por todo el
organismo tras la ingestión. Se cree que la absorción tras la
inhalación es rápida y la absorción por vía cutánea lenta.
La toxicidad aguda del 1-propanol para los animales es baja
en toda exposición, ya sea por vía cutánea, oral o respiratoria.
La exposición de miembros de la población general a niveles
potencialmente letales puede producirse por ingestión accidental o
intencionada. No obstante, sólo se ha notificado un caso de
envenenamiento mortal por 1-propanol. Los efectos agudos más
probables del 1-propanol en el hombre son la intoxicación
alcohólica y la narcosis. Los resultados de los estudios en
animales indican que el 1-propanol es 2 - 4 veces más tóxico que
el etanol.
El 1-propanol puede ser irritante para la piel hidratada.
Los datos sobre toxicidad animal no bastan para evaluar los
riesgos para la salud humana asociados a la exposición repetida o
duradera al 1-propanol. No obstante, estudios limitados a corto
plazo sobre la rata indican que la exposición por vía oral al
1-propanol tiene pocas probabilidades de suponer un riesgo grave
para la salud en las condiciones habituales de exposición humana.
En la rata la exposición por inhalación a una concentración de
15 220 mg/m3 redujo la capacidad reproductiva del macho, pero no
así la exposición a 8610 mg/m3. En la rata gestante, 9001 mg/m3
(3659 ppm) fue el nivel de efectos no observados y 14 893 mg/m3
(6054 ppm) el nivel más bajo de observación de efectos tanto
respecto a la toxicidad materna como a la fetal. Así pues, la
exposición por inhalación a concentraciones elevadas de 1-propanol
produjo toxicidad reproductiva y embriológica en el macho y la
hembra en presencia de toxicidad manifiesta en los animales
expuestos. Las concentraciones necesarias para producir esos
efectos en la rata fueron más elevadas que las que probablemente
se observen en condiciones normales de exposición humana.
El 1-propanol dio resultados negativos en los ensayos de
detección de mutaciones puntuales en bacterias. Aunque esto indica
que la sustancia no tiene ningún potencial genotóxico, no puede
evaluarse adecuadamente la mutagenicidad basándose en los limitados
datos disponibles. El estudio realizado no basta para evaluar la
carcino-genicidad del 1-propanol en animales de experimentación.
No se dispone de datos sobre la exposición a largo plazo de
poblaciones humanas al 1-propanol. Por todo ello, no puede
evaluarse la carcinogenicidad del 1-propanol en el ser humano.
Aparte de un caso de intoxicación mortal tras la ingestión de
medio litro de 1-propanol, no se tiene prácticamente noticia de
efectos adversos para la salud producidos por la exposición al
1-propanol, ni en la población general ni en grupos profesionales.
El Grupo Especial de Trabajo considera poco probable que el
1-propanol plantee un riesgo grave para la salud de la población
general en condiciones normales de exposición.
El 1-propanol puede liberarse al medio ambiente durante la
producción, el tratamiento, el almacenamiento, el transporte, el
uso y la evacuación de desechos. A causa de su uso principal como
disolvente volátil, la mayoría del volumen de producción acaba por
ser liberado a la atmósfera. No obstante, por reacciones con
radicales hidroxilo y por lavado pluvial, el 1-propanol
desaparecerá rápidamente de la atmósfera, siendo su tiempo de
presencia en ella inferior a 3 días. La eliminación del 1-propanol
del agua y del suelo también se produce rápidamente, de modo que
raras veces se detectan niveles medibles en cualquiera de estos
tres compartimientos. La adsorción del 1-propanol en las
partículas del suelo es escasa, pero la sustancia es móvil en el
suelo y se ha demostrado que aumenta la permeabilidad del mismo a
ciertos hidrocarburos aromáticos.
En vista de las propiedades físicas del 1-propanol, la bio-
acumulación es poco probable y, salvo en el caso de evacuación
accidental o inadecuada, el 1-propanol no constituye un riesgo para
los organismos acuáticos, los insectos y las plantas en las
concentraciones que por lo general se encuentran en el medio
ambiente.
