
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 92
RESMETHRINS -- RESMETHRIN BIORESMETHRIN CISRESMETHRIN
This report contains the collective views of an international
group of experts and does not necessarily represent the
decisions or the stated policy of the United Nations Environment
Programme, the International Labour Organisation, or the World
Health Organization
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation,
and the World Health Organization
World Health Organization Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
ISBN 92 4 154292 6
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
ENVIRONMENTAL HEALTH CRITERIA FOR RESMETHRINS
INTRODUCTION
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical
methods
1.2 Production and uses
1.3 Residues in food
1.4 Environmental fate
1.5 Kinetics and metabolism
1.6 Effects on experimental animals and in vitro test
systems
1.7 Effects on the environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF ENVIRONMENTAL POLLUTION AND ENVIRONMENTAL LEVELS
3.1 Industrial production
3.2 Use patterns
3.3 Residues in food
3.4 Fate and residues in domestic animals
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Photodegradation
4.2 Degradation in soil
4.3 Degradation on plants
5. KINETICS AND METABOLISM
5.1 Metabolism in mammals
5.2 Enzymatic systems for biotransformation
6. EFFECTS ON THE ENVIRONMENT
6.1 Aquatic organisms
6.2 Terrestrial organisms
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Acute toxicity
7.2 Short-term exposure
7.2.1 Oral administration
7.2.2 Inhalation
7.2.3 Dermal application
7.3 Primary irritation and sensitization
7.4 Long-term exposure and carcinogenicity
7.5 Mutagenicity
7.6 Reproductive effects, embryotoxicity, and teratogenicity
7.7 Immunotoxicity
7.8 Neurotoxicity
7.9 Mechanism of toxicity (mode of action)
7.10 Potentiation
8. EFFECTS ON MAN
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human health risks
9.2 Effects on the environment
10. CONCLUSIONS
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALLETHRINS AND
RESMETHRINS
Members
Dr L.A. Albert-Palacios, National Institute of Biological Resources
Research, Xalapa, Veracruz, Mexicoa
Dr V. Benes, Institute of Hygiene and Epidemiology, Prague,
Czechoslovakia
Dr A.H. El-Sabae, Faculty of Agriculture, Alexandria University,
Alexandria, Egypt
Dr Y. Hayashi, National Institute of Hygienic Sciences, Tokyo, Japan
Dr S. Johnson, US Environmental Protection Agency, Hazard Evaluation
Division, Washington DC, USA
Dr S.K. Kashyap, National Institute of Occupational Health, Ahmedabad,
India (Vice-Chairman)
Dr J.H. Koeman, Agricultural University, Wageningen, Netherlandsa
Dr Yu. I. Kundiev, Research Institute of Labour, Hygiene and
Occupational Diseases, Kiev, USSR (Chairman)
Dr J.P. Leahey, ICI Agrochemicals Division, Jealotts Hill Research
Station, Bracknell, Berkshire, United Kingdom (Rapporteur)
Dr M. Matsuo, Sumitomo Chemical Co. Ltd, Takarazuka Research Center,
Takarazuka, Hyogo, Japan
Dr G.U. Oleru, College of Medicine, University of Lagos, Lagos,
Nigeria
Observers
Mr J.-M. Pochon, International Group of National Associations of
Agrochemical Manufacturers, Brussels, Belgium
Dr L.M. Sasynovitch, Research Institute of Hygiene and Toxicology of
Pesticides, Polymers and Plastics, Kiev, USSR
a Invited but unable to attend.
Secretariat
Dr Z.P. Grigorevskaja, Centre for International Projects, Moscow, USSR
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr J. Sekizawa, National Institute of Hygienic Sciences, Tokyo, Japan
(Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400 -
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR RESMETHRINS
A WHO Task Group on Environmental Health Criteria for Allethrins
and Resmethrins met in Moscow from 16 to 20 November 1987. The meeting
was convened with the financial assistance of the United Nations
Environment Programme (UNEP) and was hosted by the Centre for
International Projects of the USSR State Committee on Science and
Technology. On behalf of the USSR Commission for UNEP (UNEPCOM), Dr
M.I. Gunar opened the Meeting and welcomed the participants. Dr K.W.
Jager welcomed the participants on behalf of the Heads of the three
IPCS cooperating organizations (UNEP/ILO/WHO). The group reviewed and
revised the draft Environmental Health Criteria and Health and Safety
Guides and made an evaluation of the risks for human health and the
environment from exposure to allethrins and resmethrins.
The first drafts of the documents were prepared by Dr J. Miyamoto
and Dr M. Matsuo of Sumitomo Chemical Co. Ltd, with the assistance of
the staff of the National Institute of Hygienic Sciences, Tokyo,
Japan. Dr I. Yamamoto of the Tokyo University of Agriculture and Dr M.
Eto of Kyushu University, Japan, assisted in the finalization of the
draft.
The second draft was prepared by Dr J. Sekizawa of the National
Institute of Hygienic Sciences, Tokyo, incorporating comments received
following the circulation of the first draft to the IPCS contact
points for Environmental Health Criteria documents.
The help of the Sumitomo Chemical Company Ltd, Japan and Roussel
Uclaf, France in making their toxicological proprietary information on
allethrins and resmethrins available to the IPCS and the Task Group is
gratefully acknowledged. This enabled the Task Group to make its
evaluation on the basis of more complete data.
The efforts of all who helped in the preparation and finalization
of the documents are gratefully acknowledged.
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of Health
and Human Services, through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA -- a WHO collaborating Centre for Environmental Health Effects.
The United Nations Environment Programme (UNEP) generously supported
the costs of printing.
NOTE: The proprietary information contained in this document cannot
replace documentation for registration purposes, because the
latter has to be closely linked to the source, the
manufacturing route, and the purity/impurities of the
substance to be registered. The data should be used in
accordance with paragraphs 82-84 and recommendations paragraph
90 of the Second FAO Government Consultation (1982).
INTRODUCTION
SYNTHETIC PYRETHROIDS -- A PROFILE
1. During investigations to modify the chemical structures of natural
pyrethrins, a certain number of synthetic pyrethroids were
produced with improved physical and chemical properties and
greater biological activity. Several of the earlier synthetic
pyrethroids were successfully commercialized, mainly for the
control of household insects. Other more recent pyrethroids have
been introduced as agricultural insecticides because of their
excellent activity against a wide range of insect pests and their
non-persistence in the environment.
2. The pyrethroids constitute another group of insecticides in
addition to organochlorine, organophosphorus, carbamate, and other
compounds. Pyrethroids commercially available to date include
allethrin, resmethrin, d-phenothrin, and tetramethrin (for insects
of public health importance), and cypermethrin, deltamethrin,
fenvalerate, and permethrin (mainly for agricultural insects).
Other pyrethroids are also available including furamethrin,
kadethrin, and tellallethrin (usually for household insects),
fenpropathrin, tralomethrin, cyhalothrin, lambda-cyhalothrin,
tefluthrin, cyfluthrin, flucythrinate, fluvalinate, and biphenate
(for agricultural insects).
3. Toxicological evaluations of several synthetic pyrethroids have
been performed by the FAO/WHO Joint Meeting on Pesticide Residues
(JMPR). The acceptable daily intake (ADI) or temporary ADI has
been estimated by the JMPR for cypermethrin, deltamethrin,
fenvalerate, permethrin, phenothrin, cyfluthrin, cyhalothrin, and
flucythrinate.
4. Chemically, synthetic pyrethroids are esters of specific acids
(e.g., chrysanthemic acid, halo-substituted chrysanthemic acid,
2-(4-chlorophenyl)-3-methylbutyric acid) and alcohols (e.g.,
allethrolone, 3-phenoxybenzyl alcohol). For certain pyrethroids,
the asymmetric centre(s) exist in the acid and/or alcohol moiety,
and the commercial products sometimes consist of a mixture of both
optical (1R/1S or d/1) and geometric (cis/trans)-isomers.
However, most of the insecticidal activity of such products may
reside in only one or two isomers. Some of the products (e.g.,
d-phenothrin, deltamethrin) consist only of such active isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the axons in the
peripheral and central nervous systems by interacting with sodium
channels in mammals and/or insects. A single dose produces toxic
signs in mammals, such as tremors, hyperexcitability, salivation,
choreoathetosis, and paralysis. The signs disappear fairly
rapidly, and the animals recover, generally within a week. At
near-lethal dose levels, synthetic pyrethroids cause transient
changes in the nervous system, such as axonal swelling and/or
breaks and myelin degeneration in sciatic nerves. They are not
considered to cause delayed neurotoxicity of the kind induced by
some organophosphorus compounds. The mechanism of toxicity of
synthetic pyrethroids and their classification into two types are
discussed in the Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate, flucythrinate,
and cypermethrin) may cause a transient itching and/or burning
sensation in exposed human skin.
7. Synthetic pyrethroids are generally metabolized in mammals through
ester hydrolysis, oxidation, and conjugation, and there is no
tendency to accumulate in tissues. In the environment, synthetic
pyrethroids are fairly rapidly degraded in soil and in plants.
Ester hydrolysis and oxidation at various sites on the molecule
are the major degradation processes. The pyrethroids are strongly
adsorbed on soil and sediments, and hardly eluted with water.
There is little tendency for bioaccumulation in organisms.
8. Because of low application rates and rapid degradation in the
environment, residues in food are generally low.
9. Synthetic pyrethroids have been shown to be toxic for fish,
aquatic arthropods, and honey-bees in laboratory tests. But, in
practical usage, no serious adverse effects have been noticed,
because of the low rates of application and lack of persistence in
the environment. The toxicity of synthetic pyrethroids in birds
and domestic animals is low.
10. In addition to the evaluation documents of FAO/WHO, there are
several reviews and books on the chemistry, metabolism, mammalian
toxicity, environmental effects, etc. of synthetic pyrethroids,
including those by Elliot (1977), Miyamoto (1981), Miyamoto &
Kearney (1983), and Leahey (1985).
1. SUMMARY
1.1 Identity, Physical and Chemical Properties, Analytical Methods
Resmethrin was first produced in 1967 and marketed in 1969.
Chemically, it is an ester of chrysanthemic acid (CA),
2,2-dimethyl-3-(2,2-dimethylvinyl) cyclopropanecarboxylic acid and
5-benzyl-3-furylmethyl alcohol (BFA). It is a racemic mixture of 4
optical isomers: [1R, trans]-, [1R, cis]-, [1S, trans]- and
[1S, cis]-isomer. The composition ratio in technical products is
roughly 4:1:4:1. The [1R, trans]-isomer is called bioresmethrin and
the [1R, cis]-isomer is cismethrin. Among the isomers, the
[1R, trans]-isomer has the highest insecticidal activity followed by
the [1R, cis]-isomer.
Technical grade resmethrin is a colourless waxy solid with a
melting point of 43-48°C and a boiling point of 180°C at 0.01 mmHg.
The specific gravity is 1.050 at 20°C. The vapour pressure is
1.1 × 10-8 mmHg at 30°C. It is insoluble in water (< 1 mg/litre at
30°C), but soluble in organic solvents, such as hexane, kerosene, and
xylene. It is not stable in air, light, or in alkaline media. The
n-octanol/water partition coefficient is 2.9 × 103 for resmethrin
and 6.2 × 104 for bioresmethrin.
Analysis of environmental samples and determination of residues
can be carried out by a high-performance liquid chromatograph equipped
with a UV-detector (206 nm) detecting levels as low as 0.05 mg/kg. A
gas chromatograph with flame ionization detector is used for the
analysis of technical products.
Vapourized resmethrin can be efficiently recovered for analysis by
using Porpak C18 or Chromosorb 102 sorbents as the trap.
1.2 Production and Uses
It is estimated that about 20-30 tonnes of resmethrin are produced
and used per year. Resmethrin is used mainly for the control of
household and public health insects. It is formulated as an aerosol,
oil formulation, or emulsifiable concentrate. Formulations are also
prepared containing other insecticides and/or synergists.
Bioresmethrin is used on stored grain and for the control of white
fly in greenhouses, as well as for household and public health insect
control.
1.3 Residues in Food
Resmethrin on treated crops (tomato, lettuce) degraded so that
residues were negligible after 3 days on tomato and < 0.1 mg/kg after
7 days on lettuce. Bioresmethrin on stored grain was fairly stable, a
residue of 4 mg/kg declining to 1.1 mg/kg over a period of 6 months.
White bread made from treated grain does not contain any residues of
resmethrin, but residues may occur in wholemeal bread.
When the cis- and trans-isomers of radiolabelled resmethrin
were given orally to White Leghorn laying hens at a dose level of
10 mg/kg, more than 90% of the radioactivity was eliminated in the
excreta 24 h after treatment. Residues in the egg white and yolk, and
in the body tissues were low, the highest levels occurring in the
liver and kidney.
Lactating Jersey cows treated orally with radiolabelled resmethrin
at 10 mg/kg body weight rapidly absorbed, metabolized, and excreted
the chemical. The cis-isomer was eliminated primarily in the faeces
and the trans-isomer was eliminated mainly in the urine. Tissue
residues 48 h after treatment were low (< 1 µg/g) except in the liver
and kidney, and only very low levels of radioactivity were secreted in
the milk.
1.4 Environmental Fate
Resmethrin is rapidly photodegraded on silica gel plates, as a
thin film on glass plates, and in aqueous solution. In sunlight,
aqueous solutions have a half-life of 47 min (pure water) and 20 min
(sea water). A range of photoproducts is formed from ester cleavage
and oxidation reactions.
Resmethrin is also very rapidly degraded in soil, 2% of the
applied parent compound remaining after 16 days. Complete
mineralization to carbon dioxide is a very important degradation
process (38% after 16 days).
Rapid degradation occurs on plants (tomato, lettuce, and wheat).
After 5 days, no resmethrin is detected on plants and a very complex
mixture of, at least, 31 break-down products is formed. The alcohol
formed by ester cleavage, benzaldehyde, phenylacetic acid, benzoic
acid, and benzyl alcohol were identified as break-down products, each
being present at low levels (3% of the total residue).
1.5 Kinetics and Metabolism
When rats were administered 14C-(alcohol labelled)-[1RS
trans]-resmethrin orally at the rate of 500 mg/kg body weight, the
radiocarbon was eliminated slowly in the urine (36%) and faeces (64%)
within 3 weeks. More than 50% of the 14C dose was secreted in the
bile in 72 h and enterohepatically circulated. The major metabolic
reactions were ester cleavage, oxidation at the trans-methyl of the
isobutenyl group to alcohol, aldehyde, and carboxylic acid and at the
4'-,alpha-, and 4-positions of 5-benzyl-3-furylmethyl alcohol (BFA),
and conjugation.
Rats fed 14C-(acid- or alcohol-labelled) bioresmethrin or
cismethrin at the rate of 1 mg/kg eliminated 5-benzyl-3-
furancarboxylic acid (BFCA), 4'-hydroxy-BFCA, and alpha-hydroxy-BFCA
together with 2-trans- hydroxymethyl- and 2-carboxyl derivatives of
chrysanthemic acid (CA). Cis/trans-isomerized CA derivatives were
also found. The residual metabolites in the body were derived from the
alcohol moiety of bioresmethrin.
1.6 Effects on Experimental Animals and In Vitro Test Systems
Resmethrin and bioresmethrin were weakly toxic for animals when
examined by various routes of exposure (the oral LD50 of resmethrin
ranged from 690 mg/kg for the mouse to >5 000 mg/kg for the rat). The
oral LD50s for bioresmethrin were 225 mg/kg for the rabbit,
480-10 000 mg/kg for the mouse, and 8800 mg/kg for the rat. Cismethrin
was moderately toxic for the mouse (oral LD50: 152-160 mg/kg).
Signs of poisoning were tremors, hyperactivity, and convulsion
(T-syndrome). Resmethrin belongs to the Type I pyrethroid group.
The acute toxicity (oral and intraperitoneal) of the metabolites
(e.g., CA, BFA, BFCA) was examined in mice and rats; CA and BFA were
more toxic than the parent compound in mice though toxic signs were
different. The acute toxicity (oral LD50) of the metabolites in rats
ranged from 997 mg/kg to > 4640 mg/kg, which is within the same range
of acute toxicity as the parent compound. While technical grade
resmethrin was found to be a slight irritant to the skin, it did not
cause sensitization and photochemical irritation in guinea-pigs and
New Zealand White rabbits.
Resmethrin was applied twice weekly, for 3 weeks, to the skin of
New Zealand White rabbits by fixing a piece of cotton cloth treated
with resmethrin (0.247 mg/cm2) over skin that had been pre-treated
with liquid, imitating sweat, or with resmethrin (10 g), or had not
been pre-treated. No significant changes were noted in body weight and
organ weight ratios on day 24 or in the clinical chemistry in any of
the groups up to day 24. No significant compound-related dermal
effects were observed.
When Sprague-Dawley or Long-Evans rats were fed resmethrin in the
diet at levels of up to 6000 mg/kg for 14 days, mortality was observed
at the highest dose level, and tremor and reduced body weight and food
consumption were noted at levels of 1500 mg/kg or more. The maximum
no-observed-adverse-effect dietary level was 188 mg/kg for Long-Evans
rats.
When Long-Evans rats were given resmethrin in the diet at levels
of up to 750 mg/kg (male) and up to 2400 mg/kg (female) for 90 days,
all females died at 2400 mg/kg and tremor and reduced body weight were
noted at 750 mg/kg. The maximum no-observed-adverse-effect dietary
level was 75 mg/kg for both female and male rats.
When rats were fed bioresmethrin in the diet at levels of up to
8000 mg/kg for 91 days, body weight was reduced at the highest level,
and was accompanied by changes in blood chemistry indicating liver
dysfunction. The no-observed-adverse-effect level in this study was
400 mg/kg diet, which corresponds to 32.8 mg/kg body weight and
36.1 mg/kg body weight for males and females, respectively.
Dogs (males and females) were administered bioresmethrin at levels
of up to 500 mg/kg body weight for 90 days. A no-observed-adverse-
effect level was observed of 80 mg/kg body weight.
Technical resmethrin was administered via inhalation to Wistar
rats for 6 h/day on 5 days of each week, for a period of 90 days, at
nominal exposure levels of 0.1, 0.3, or 1.0 g/m3. The no-observed-
adverse-effect level was at 0.1 g/m3.
Rats and rabbits inhaled aerosolized resmethrin formulations for
5 h/day on 5 consecutive days at levels of 2.9-3.2 mg active
ingredient/litre of air. Though clinical signs including rapid
breathing and nasal discharge were observed, there were no
compound-related effects on body weight and histopathological
findings, immediately and 7 and 14 days after exposure. No indication
of toxic effects other than irritation were observed in any of the
tests.
No oncogenic effects were seen when CD-1 mice were fed 0, 250,
500, or 1000 mg resmethrin/kg basal diet for 85 weeks.
Wistar rats fed resmethrin at levels of 0, 500, 2500, or
5000 mg/kg basal diet for 112 weeks did not show any signs of
oncogenicity and a no-observed-adverse-effect level of 500 mg/kg was
established for chronic toxicity. When resmethrin was administered to
Sprague-Dawley rats at dietary levels of 500, 1500, or 5000 mg/kg for
24 weeks, tremors, decreased body weight, and increased liver and
kidney weights were observed at 5000 mg/kg. A no-observed-adverse-
effect level of 1500 mg/kg was established.
Dogs fed resmethrin for 6 months showed increased liver weights at
30 mg/kg body weight per day, but did not show any adverse effects at
10 mg/kg body weight per day.
Resmethrin, bioresmethrin, and cismethrin were tested for
mutagenicity and/or chromosomal effects in several short-term test
systems, such as Escherichia coli and Salmonella reverse mutation
tests, primary DNA damage tests in eukaryotes and prokaryotes, and
chromosomal effects in Chinese hamster cells or mouse bone marrow
cells. Negative results were obtained with all tests.
In a teratogenicity study, pregnant Long-Evans rats were
administered resmethrin in the diet at levels of 188 or 1500 mg/kg,
from day 6 to day 16 of gestation. Though mortality, tremor, and
decreased food and water consumption were noted in the dams at
1500 mg/kg, gross abnormalities of the fetal skeleton and soft tissues
were not observed in the treated animals.
Resmethrin was administered orally (by gavage in corn oil) at
levels of 0, 20, 40, or 80 mg/kg body weight to female Sprague-Dawley
rats during the period of major organogenesis. Resmethrin was not
teratogenic at levels up to and including 80 mg/kg. The
no-observed-adverse-effect level for fetotoxicity was 40 mg/kg.
ICR mice were given (+),-[trans, cis]-resmethrin orally at
levels up to 100 mg/kg body weight. Sprague-Dawley rats received
levels up to 50 mg/kg body weight during the period of major
organogenesis. Embryo- and fetotoxicity and teratogenicity were not
observed in this study.
In another study, resmethrin was administered to New Zealand White
rabbits by oral intubation at dose levels of 0, 10, 30, or 100 mg/kg
body weight on day 6 to day 18 of gestation. Resmethrin was not
teratogenic at levels up to and including the 100 mg/kg dose.
In a 3-generation reproduction study, Sprague-Dawley rats were fed
resmethrin in the basal diet at 0, 500, 800, or 1250 mg/kg. A decrease
in pup weight and a slight increase in the number of pups cast dead
were observed at the 500 mg/kg.
1.7 Effects on the Environment
In laboratory studies, resmethrin is highly toxic for fish with
96-h LC50 values of 0.3-5.5 µg/litre. Among the isomers, cismethrin
is the most toxic for fish. However, under field conditions, it has
been shown that the hazard is significantly reduced because of the
rapid degradation and low water solubility of the compound. Resmethrin
is less toxic for arthropods with 48-h LC50 values of 2-25 µg/litre.
Both bioresmethrin and cismethrin are harmless for birds.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C22H26O3
Chemical structure:
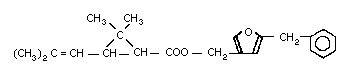 Resmethrin was first produced in 1967 by Elliott et al. (1967). It
is prepared by the esterification of [1RS, 3RS or 1RS, cis,
trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid
or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol.
Resmethrin is thus a mixture of 4 stereoisomers (see Fig. 1). The
cis:trans isomer ratio is reported to be 1:4 and the optical ratio
of 1R:1S is 1:1 (racemic). Thus, its composition will be roughly
4:1:4:1 of isomers (1), (2), (3), and (4).
Bioresmethrin is the [1R,trans]- or (1) isomer and cismethrin is
the [1R,cis]- or (2) isomer (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of resmethrins are given in
Table 2.
Resmethrin is decomposed rapidly on exposure to air or light and
in alkaline media (Elliot, 1976; FAO/WHO, 1976; Martin & Worthing,
1977; Meister et al., 1983; Puchalski, 1983; Worthing & Walker, 1983;
Devaux & Bolla, 1986).
2.3 Analytical Methods
Methods for the determination of residues, analysis of
environmental samples, and product analysis for both resmethrin and
bioresmethrin are summarized in Table 3. Procedures for extraction,
partition, and cleanup are listed together with analytical equipment
and conditions, minimum detectable concentrations, and percentage
recovery.
Technical grade resmethrin can be analysed by dissolving the
product and dicyclohexyl phthalate (an internal standard) in acetone
and injecting the solution in a gas chromatograph equipped with a
flame ionization detector (FID) (Murano, 1972).
Resmethrin was first produced in 1967 by Elliott et al. (1967). It
is prepared by the esterification of [1RS, 3RS or 1RS, cis,
trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid
or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol.
Resmethrin is thus a mixture of 4 stereoisomers (see Fig. 1). The
cis:trans isomer ratio is reported to be 1:4 and the optical ratio
of 1R:1S is 1:1 (racemic). Thus, its composition will be roughly
4:1:4:1 of isomers (1), (2), (3), and (4).
Bioresmethrin is the [1R,trans]- or (1) isomer and cismethrin is
the [1R,cis]- or (2) isomer (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of resmethrins are given in
Table 2.
Resmethrin is decomposed rapidly on exposure to air or light and
in alkaline media (Elliot, 1976; FAO/WHO, 1976; Martin & Worthing,
1977; Meister et al., 1983; Puchalski, 1983; Worthing & Walker, 1983;
Devaux & Bolla, 1986).
2.3 Analytical Methods
Methods for the determination of residues, analysis of
environmental samples, and product analysis for both resmethrin and
bioresmethrin are summarized in Table 3. Procedures for extraction,
partition, and cleanup are listed together with analytical equipment
and conditions, minimum detectable concentrations, and percentage
recovery.
Technical grade resmethrin can be analysed by dissolving the
product and dicyclohexyl phthalate (an internal standard) in acetone
and injecting the solution in a gas chromatograph equipped with a
flame ionization detector (FID) (Murano, 1972).
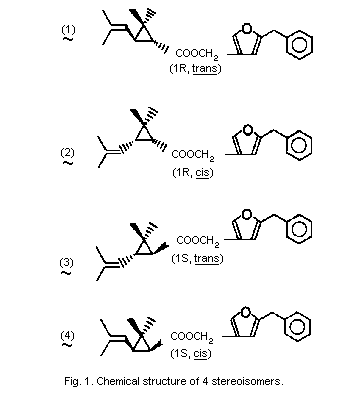 Table 1. Chemical identity of resmethrins of various stereoisomeric compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
Resmethrine [5-(phenylmethyl)-3-furanyl]methyl (1):(2):(3):(4) Benzofuroline,
10453-86-8 2,2-dimethyl-3-(2-methyl-1-propenyl) =4:1:4:1 Benzylfuroline,
GZ1310000 cyclo-propanecarboxylate (9CI) Chrysron, Chryson,
Synthrin, For-Syn,
5-benzyl-3-furylmethyl [1RS, cis, trans]- NIA17370, NRDC104,
2,2-dimethyl-3-(2,2-dimethylvinyl)- SBP-1382, ENT27474,
cyclopropanecarboxylate FMC17370, RU 48440,
or Pynosect, Pyretherm;
5-benzyl-3-furylmethyl [1RS, cis, trans]- Premgard
chrysanthemate
Bioresmethrine same as resmethrin (1) d-trans-Resmethrin,
28434-01-7 (+)-trans-Resmethrin,
GZ1310500 5-benzyl-3-furylmethyl [1R, trans]- NRDC107, RU 11484,
chrysanthemate
Cismethrin same as resmethrin (2) (+)-cis-Resmethrin,
35764-59-1 NRDC119
GZ1430000 5-benzyl-3-furylmethyl [1R, cis]-
chrysanthemate
Table 1 (contd).
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
(-)-trans-resmethrin same as resmethrin (3) --
GZ 1410000 5-benzyl-3-furylmethyl
[1S, trans]-chrysanthemate
(-)-cis-resmethrin same as above (4) --
GZ 1420000
5-benzyl-3-furylmethyl
[1S, cis]-chrysanthemate
a NIOSH (1983).
b (1R), d, (+) or (1S), 1, (-) in the acid part of resmethrin signifies the same stereospecific conformation,
respectively.
c Chrysanthemic acid is a name of the acid that forms the acid part of resmethrin.
d Numbers in the parentheses identify the structures shown in the figures of stereoisomers.
e ISO common name: Common names for pesticides and other agrochemicals approved by the Technical Committee
of the International Organization for Standardization.
The sampling efficiency for vapourized pesticides (chlordane,
chlorpyrifos, diazinone, propoxur, and resmethrin) was examined using
five trapping solid sorbents, i.e., polyurethane foam, dispo plugs,
Chromosorb 102, Porpak C18, Carbowax 20M on Gas Chrom Q, and Tenax GC
(Roper & Wright, 1984). Resmethrin was determined using HPLC. The
sampling efficiency of Porpak C18 was best (94%) for resmethrin;
however, that of Chromosorb 102 was best (95%) when means of the 5
sorbents with the different insecticides were compared.
Table 2. Some physical and chemical properties of resmethrins
Resmethrin Bioresmethrin Cismethrin
Physical state waxy solid Viscous oil --
(liquid or solid)
Colour colourless practically --
colourless
Relative molecular mass 338.48 338.48 338.48
Melting point (°C) 43-48 30-35 --
Boiling point 180°C (0.01 mmHg) 180°C (0.01 mmHg) --
Water solubility (30°C) < 1 mg/litre < 0.3 mg/litre --
Solubility in organic solublea soluble soluble
solvents
Density (20°C) 1.050 1.050
Vapour pressure (mmHg) 1.1 × 10-8 (30°C) 1.4 × 10-4 (25°C) --
n-Octanol/water 2.9 × 103 6.2 × 104
partition coefficient
a Methanol (81 g/kg), hexane (220 g/kg), xylene (> 1 kg/kg), kerosene (10%).
Very soluble in methylene chloride and aromatic petroleum hydrocarbons.
Table 3. Analytical methods for resmethrina
Sample Sample preparation Determination Limit % Recovery Reference
of fortification
Extraction Partition Clean-up GC or HPLC detection level (mg/kg)
solvent Column Elution condition, detector, (mg/kg)
carrier, flow,
column,
temperature, R.T.
Residue
analysis
Apple n-hexane/ extraction silica CH2Cl2 HPLC UV-206 nm, 0.05 37 (0.1), 60 (1.0) Baker &
Pear acetone solvent/ gel 25 cm ODS, propane 0.05 44 (0.1), 72 (1.0) Bottomley (1982)
Cabbage (1/1) H2O 2-ol, 1 ml/min 0.05 34 (0.1), 57 (1.0)
Potato 0.05 54 (0.1), 80 (1.0)
Corn pentane CH3CN/ Florisil EtOAc/ FID-GC, N2 86 0.2 80-87.5 (0.2-3.2) Simonaitis &
Cornmeal pentane pentane ml/min, 1.83 m, 75-86.2 (0.15-2.56) Cail (1975)
Flour (3 + 97) 10% UC-W 98, 80-88.7 (0.16-2.71)
Wheat 245°C, 5.5 min 79.6-86.2 (0.16-2.55)
Wheat n-hexane CI-MS/GC, m/e 171, 0.04 97 (0.2) Cave (1981)
(grain) methane, 20 ml/min, 98 (1.0)
1.5 m, 5% OV-101, 92 (2.0)
230°C, ca. 2.5 min
Rice petroleum colorimetric 96 (0.7) Desmarchelier
(cooked) ether method, 680 nm 93 (0.8) (1980)
acetone 93 (0.8)
acetone/
petroleum
ether
(2/8)
Table 3. (cont'd).
Sample Sample preparation Determination Limit % Recovery Reference
of fortification
Extraction Partition Clean-up GC or HPLC detection level (mg/kg)
solvent Column Elution condition, detector, (mg/kg)
carrier, flow,
column,
temperature, R.T.
Environmental
analysis
Dish n-hexane n-hexane/ HPTLC benzene/ dual-wavelength 88-98 (300 µg) Uno et al. (1982)
Apple CH3CN CCl4 densitometry 97 (300 µg)
Spinach (1 + 1) lambda1 = 370 nm; 98 (300 µg)
(dislodgeable Rf = 0.44 lambda2 = 230 nm
residue)
Product
analysis
Technial acetone FID-GC, H2 40
grade ml/min, 1 m, 2% Murano (1972)
DEGS, 190°C,
3.9 min
a HPLC = high-performance liquid chromatography; FID = Flame ionization detector; MS = mass spectrometry;
CC = column chromatography; GC = gas chromatography; UV = ultraviolet; HPTLC = high-performance thin-layer
chromatography; RT = retention time; CI = chemical ionization.
3. SOURCES OF ENVIRONMENTAL POLLUTION AND ENVIRONMENTAL LEVELS
3.1 Industrial Production
Resmethrin was first put on the market in 1969 and bioresmethrin,
in 1973. No information is available to the general public on
production volume. However, it has been estimated to amount to 20-30
tonnes yearly (Miyamoto, personal communication, 1986).
3.2 Use Patterns
Resmethrin is mainly used in aerosol formulations, but also in oil
formulations and emulsifiable concentrates, for the control of
household and public health insects. It is also used in combination
with other insecticides (e.g., tetramethrin, malathion).
Resmethrin is currently used for mosquito control (by aerial
application) in the USA, and it can also be used for the control of
white fly in greenhouses.
Bioresmethrin is mainly used as a post-harvest treatment to
control pests in stored grain (mostly in Australia). Bioresmethrin is
also widely used for white fly control in greenhouse-grown vegetables,
mostly in European countries. It is also used in aerosol formulation
for the control of household and public health insects, in combination
with synergists and/or other insecticides. It has been used in France
for cattle shed treatment (Battelle, 1982).
3.3 Residues in Food
Resmethrin residues on tomatoes and lettuce after greenhouse
treatment decreased very rapidly resulting in negligible residues on
tomatoes after 3 days and < 0.1 mg/kg on lettuce after 7 days (Burr,
1983a,b).
Field trials have been carried out with bioresmethrin on fruit and
vegetable crops, e.g., tomatoes and cucumbers. Tomato fruits were
treated with 14C-labelled bioresmethrin (1.25 mg/kg). The fruit was
harvested 7-72 h after application and amounts of the compound in the
flesh and skin, and on the skin surface were measured. At 72 h, 0.2%
of the applied compound was detected in the flesh, 4.65% in the skin,
and 15.5% on the surface (Buick & Flanagan, 1973).
There are many reports of residue studies, especially on
post-harvest treatment of grain for storage (FAO/WHO, 1976/1977).
Residues in the stored grains, e.g., wheat, have also been
determined. For example, wheat was treated with bioresmethrin at the
rate of 4 mg/kg and maintained in an unaerated silo at 24°C or in an
aerated silo at 14°C, for 6 months. At the end of 6 months, the
residue levels had declined to only 1.1 mg/kg (unaerated) and
1.9 mg/kg (aerated) (Bengston et al., 1975).
Wheat was treated with bioresmethrin (4 mg/kg) and subjected to
milling and baking processes. When it was used with 20 mg piperonyl
butoxide/kg, 4.0 mg bioresmethrin remained per kg flour, but no
residues were detected in bread (Ardley, 1975). In another study, some
stability of residues during baking were demonstrated, residues of
2.9 mg/kg in wheat resulted in residues of 1 mg/kg in wholemeal bread
(Desmarchelier, 1980).
3.4 Fate and Residues in Domestic Animals
White Leghorn laying hens were given the cis- and trans-
isomers of resmethrin, labelled with radiocarbon in either the
alcohol or acid moiety, at a dosage of 10 mg/kg body weight. With each
isomer and label position, more than 90% of the radiocarbon was
eliminated in the excreta within 24 h of the treatment. Radiocarbon
residues in the egg white and yolk fractions were low with peak levels
observed, respectively, 1 and 4-5 days after treatment. Radiocarbon
residues in tissues were low in birds sacrificed 12 h after treatment.
The highest levels were found in the liver and kidney (Christopher
et al., 1985).
Resmethrin labelled with radiocarbon in either the acid or alcohol
moiety and administered orally to lactating Jersey cows at 10 mg/kg
body weight was rapidly absorbed, metabolized, and excreted. The
cis-isomer was eliminated primarily in the faeces, but the
trans-isomer was eliminated primarily in the urine. Tissue residues
48 h after treatment were low (< 1 mg/kg), except in the liver,
ovary, and kidney, and were generally higher with the alcohol-labelled
compounds. Only very low levels of radiocarbon were secreted in the
milk. Unmetabolized resmethrin appeared in trace amounts in tissues
and as the major residue in milk and faeces. The major metabolites
from both isomers arise from ester hydrolysis and subsequent oxidation
of the hydrolysed products. They include: chrysanthemic acid (free and
conjugated with glucuronic acid), chrysanthemumdicarboxylic acid,
5-benzyl-3-furoic acid (free and conjugated with glucuronic acid or
glycine), and 5-(alpha-hydroxybenzyl)-3-furoic acid (Ridlen et al.,
1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Photodegradation
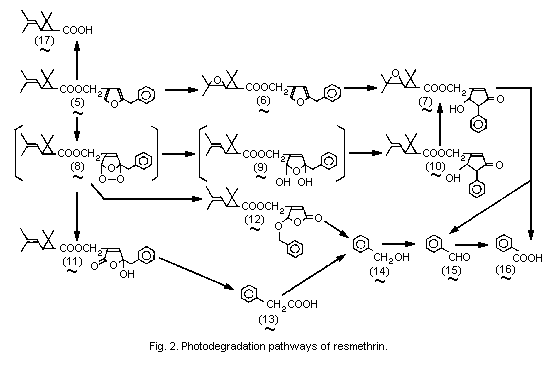
The photodegradation pathways of resmethrin are summarized in
Fig. 2.
On irradiation with a sunlamp, trans- and cis-isomers of
14C-resmethrin (5) labelled in the carboxy (acid or phenyl (alcohol)
group decomposed on silica gel plates with a half-life of about
160-190 min. After 420 min irradiation of 14C-bioresmethrin, only 5%
of the recovered radioactivity was resmethrin, 45% was ester
photoproducts, 14% was non-ester photoproducts, and 32% was not
identified. The photoproduct found in the largest amount was
5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl trans-chrysanthemate
(10) (about 24% of the applied 14C), which originated from the cyclic
ozonide-type peroxide intermediates (8,9) formed by oxidation of the
furan ring. A relatively large (about 5%) amount of trans-
chrysanthemic acid (17) was formed. In addition, small amounts of the
R and S isomers of trans-epoxyresmethrin (6) (about 1.5% each),
5-benzyl-5-hydroxy-2-oxo-2,5-dihydro-3-furylmethyl trans-
chrysanthemate (11) (about 3%) and 2-benzyloxy-5-oxo-2,5-dihydro-
3-furylmethyl trans-chrysanthemate (12) (about 1%) were
detected with the acid- and alcohol-labelled preparations, and benzyl
alcohol (14) (about 2%), benzoic acid (16) (about 1.5%), and
phenylacetic acid (13) (about 1%) were found with the alcohol-labelled
preparation. The photoproduct distribution was greatly dependent on
the supporting material or medium. Oxidation at the furan ring
occurred predominantly on silica gel plates, while epoxidation at the
isobutenyl substituent proceeded to a greater extent on a filter paper
or in water. When exposed to sunlight for 10 h, cismethrin on silica
gel plates (1.7 mg/cm2) gave a large amount of cis-chrysanthemic
acid (17) but not trans-acid, together with smaller amounts of
5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl cis-chrysanthemate
(10), 5-benzyl-5-hydroxy-2-oxo-2,5-dihydro-3-furylmethyl
cis-chrysanthemate (11), benzaldehyde (15), and phenylacetic acid
(13). The photodecomposition rates were compared for residual deposits
(14 µg/cm2) of bioresmethrin, trans-tetramethrin, and
S-Bioallethrin on silica gel plates under sunlight or a sunlamp.
S-Bioallethrin was the most stable of these compounds, and
trans-tetramethrin was more persistent than bioresmethrin (Ueda
et al., 1974).
Resmethrin and structurally related 5-benzyl-3-furylmethyl
derivatives undergo rapid oxidation when exposed to sunlight or a
sunlamp, either in aqueous medium or as a thin film on glass or silica
gel. One major photodecomposition route involves epoxidation at the
isobutenyl substituent to give the R- and S-epoxides. Formation of the
other major photoproducts is initiated by oxidation of the furan ring
to a cyclic ozonide-type peroxide (Holmstead et al., 1977).
Table 1. Chemical identity of resmethrins of various stereoisomeric compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
Resmethrine [5-(phenylmethyl)-3-furanyl]methyl (1):(2):(3):(4) Benzofuroline,
10453-86-8 2,2-dimethyl-3-(2-methyl-1-propenyl) =4:1:4:1 Benzylfuroline,
GZ1310000 cyclo-propanecarboxylate (9CI) Chrysron, Chryson,
Synthrin, For-Syn,
5-benzyl-3-furylmethyl [1RS, cis, trans]- NIA17370, NRDC104,
2,2-dimethyl-3-(2,2-dimethylvinyl)- SBP-1382, ENT27474,
cyclopropanecarboxylate FMC17370, RU 48440,
or Pynosect, Pyretherm;
5-benzyl-3-furylmethyl [1RS, cis, trans]- Premgard
chrysanthemate
Bioresmethrine same as resmethrin (1) d-trans-Resmethrin,
28434-01-7 (+)-trans-Resmethrin,
GZ1310500 5-benzyl-3-furylmethyl [1R, trans]- NRDC107, RU 11484,
chrysanthemate
Cismethrin same as resmethrin (2) (+)-cis-Resmethrin,
35764-59-1 NRDC119
GZ1430000 5-benzyl-3-furylmethyl [1R, cis]-
chrysanthemate
Table 1 (contd).
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
(-)-trans-resmethrin same as resmethrin (3) --
GZ 1410000 5-benzyl-3-furylmethyl
[1S, trans]-chrysanthemate
(-)-cis-resmethrin same as above (4) --
GZ 1420000
5-benzyl-3-furylmethyl
[1S, cis]-chrysanthemate
a NIOSH (1983).
b (1R), d, (+) or (1S), 1, (-) in the acid part of resmethrin signifies the same stereospecific conformation,
respectively.
c Chrysanthemic acid is a name of the acid that forms the acid part of resmethrin.
d Numbers in the parentheses identify the structures shown in the figures of stereoisomers.
e ISO common name: Common names for pesticides and other agrochemicals approved by the Technical Committee
of the International Organization for Standardization.
The sampling efficiency for vapourized pesticides (chlordane,
chlorpyrifos, diazinone, propoxur, and resmethrin) was examined using
five trapping solid sorbents, i.e., polyurethane foam, dispo plugs,
Chromosorb 102, Porpak C18, Carbowax 20M on Gas Chrom Q, and Tenax GC
(Roper & Wright, 1984). Resmethrin was determined using HPLC. The
sampling efficiency of Porpak C18 was best (94%) for resmethrin;
however, that of Chromosorb 102 was best (95%) when means of the 5
sorbents with the different insecticides were compared.
Table 2. Some physical and chemical properties of resmethrins
Resmethrin Bioresmethrin Cismethrin
Physical state waxy solid Viscous oil --
(liquid or solid)
Colour colourless practically --
colourless
Relative molecular mass 338.48 338.48 338.48
Melting point (°C) 43-48 30-35 --
Boiling point 180°C (0.01 mmHg) 180°C (0.01 mmHg) --
Water solubility (30°C) < 1 mg/litre < 0.3 mg/litre --
Solubility in organic solublea soluble soluble
solvents
Density (20°C) 1.050 1.050
Vapour pressure (mmHg) 1.1 × 10-8 (30°C) 1.4 × 10-4 (25°C) --
n-Octanol/water 2.9 × 103 6.2 × 104
partition coefficient
a Methanol (81 g/kg), hexane (220 g/kg), xylene (> 1 kg/kg), kerosene (10%).
Very soluble in methylene chloride and aromatic petroleum hydrocarbons.
Table 3. Analytical methods for resmethrina
Sample Sample preparation Determination Limit % Recovery Reference
of fortification
Extraction Partition Clean-up GC or HPLC detection level (mg/kg)
solvent Column Elution condition, detector, (mg/kg)
carrier, flow,
column,
temperature, R.T.
Residue
analysis
Apple n-hexane/ extraction silica CH2Cl2 HPLC UV-206 nm, 0.05 37 (0.1), 60 (1.0) Baker &
Pear acetone solvent/ gel 25 cm ODS, propane 0.05 44 (0.1), 72 (1.0) Bottomley (1982)
Cabbage (1/1) H2O 2-ol, 1 ml/min 0.05 34 (0.1), 57 (1.0)
Potato 0.05 54 (0.1), 80 (1.0)
Corn pentane CH3CN/ Florisil EtOAc/ FID-GC, N2 86 0.2 80-87.5 (0.2-3.2) Simonaitis &
Cornmeal pentane pentane ml/min, 1.83 m, 75-86.2 (0.15-2.56) Cail (1975)
Flour (3 + 97) 10% UC-W 98, 80-88.7 (0.16-2.71)
Wheat 245°C, 5.5 min 79.6-86.2 (0.16-2.55)
Wheat n-hexane CI-MS/GC, m/e 171, 0.04 97 (0.2) Cave (1981)
(grain) methane, 20 ml/min, 98 (1.0)
1.5 m, 5% OV-101, 92 (2.0)
230°C, ca. 2.5 min
Rice petroleum colorimetric 96 (0.7) Desmarchelier
(cooked) ether method, 680 nm 93 (0.8) (1980)
acetone 93 (0.8)
acetone/
petroleum
ether
(2/8)
Table 3. (cont'd).
Sample Sample preparation Determination Limit % Recovery Reference
of fortification
Extraction Partition Clean-up GC or HPLC detection level (mg/kg)
solvent Column Elution condition, detector, (mg/kg)
carrier, flow,
column,
temperature, R.T.
Environmental
analysis
Dish n-hexane n-hexane/ HPTLC benzene/ dual-wavelength 88-98 (300 µg) Uno et al. (1982)
Apple CH3CN CCl4 densitometry 97 (300 µg)
Spinach (1 + 1) lambda1 = 370 nm; 98 (300 µg)
(dislodgeable Rf = 0.44 lambda2 = 230 nm
residue)
Product
analysis
Technial acetone FID-GC, H2 40
grade ml/min, 1 m, 2% Murano (1972)
DEGS, 190°C,
3.9 min
a HPLC = high-performance liquid chromatography; FID = Flame ionization detector; MS = mass spectrometry;
CC = column chromatography; GC = gas chromatography; UV = ultraviolet; HPTLC = high-performance thin-layer
chromatography; RT = retention time; CI = chemical ionization.
3. SOURCES OF ENVIRONMENTAL POLLUTION AND ENVIRONMENTAL LEVELS
3.1 Industrial Production
Resmethrin was first put on the market in 1969 and bioresmethrin,
in 1973. No information is available to the general public on
production volume. However, it has been estimated to amount to 20-30
tonnes yearly (Miyamoto, personal communication, 1986).
3.2 Use Patterns
Resmethrin is mainly used in aerosol formulations, but also in oil
formulations and emulsifiable concentrates, for the control of
household and public health insects. It is also used in combination
with other insecticides (e.g., tetramethrin, malathion).
Resmethrin is currently used for mosquito control (by aerial
application) in the USA, and it can also be used for the control of
white fly in greenhouses.
Bioresmethrin is mainly used as a post-harvest treatment to
control pests in stored grain (mostly in Australia). Bioresmethrin is
also widely used for white fly control in greenhouse-grown vegetables,
mostly in European countries. It is also used in aerosol formulation
for the control of household and public health insects, in combination
with synergists and/or other insecticides. It has been used in France
for cattle shed treatment (Battelle, 1982).
3.3 Residues in Food
Resmethrin residues on tomatoes and lettuce after greenhouse
treatment decreased very rapidly resulting in negligible residues on
tomatoes after 3 days and < 0.1 mg/kg on lettuce after 7 days (Burr,
1983a,b).
Field trials have been carried out with bioresmethrin on fruit and
vegetable crops, e.g., tomatoes and cucumbers. Tomato fruits were
treated with 14C-labelled bioresmethrin (1.25 mg/kg). The fruit was
harvested 7-72 h after application and amounts of the compound in the
flesh and skin, and on the skin surface were measured. At 72 h, 0.2%
of the applied compound was detected in the flesh, 4.65% in the skin,
and 15.5% on the surface (Buick & Flanagan, 1973).
There are many reports of residue studies, especially on
post-harvest treatment of grain for storage (FAO/WHO, 1976/1977).
Residues in the stored grains, e.g., wheat, have also been
determined. For example, wheat was treated with bioresmethrin at the
rate of 4 mg/kg and maintained in an unaerated silo at 24°C or in an
aerated silo at 14°C, for 6 months. At the end of 6 months, the
residue levels had declined to only 1.1 mg/kg (unaerated) and
1.9 mg/kg (aerated) (Bengston et al., 1975).
Wheat was treated with bioresmethrin (4 mg/kg) and subjected to
milling and baking processes. When it was used with 20 mg piperonyl
butoxide/kg, 4.0 mg bioresmethrin remained per kg flour, but no
residues were detected in bread (Ardley, 1975). In another study, some
stability of residues during baking were demonstrated, residues of
2.9 mg/kg in wheat resulted in residues of 1 mg/kg in wholemeal bread
(Desmarchelier, 1980).
3.4 Fate and Residues in Domestic Animals
White Leghorn laying hens were given the cis- and trans-
isomers of resmethrin, labelled with radiocarbon in either the
alcohol or acid moiety, at a dosage of 10 mg/kg body weight. With each
isomer and label position, more than 90% of the radiocarbon was
eliminated in the excreta within 24 h of the treatment. Radiocarbon
residues in the egg white and yolk fractions were low with peak levels
observed, respectively, 1 and 4-5 days after treatment. Radiocarbon
residues in tissues were low in birds sacrificed 12 h after treatment.
The highest levels were found in the liver and kidney (Christopher
et al., 1985).
Resmethrin labelled with radiocarbon in either the acid or alcohol
moiety and administered orally to lactating Jersey cows at 10 mg/kg
body weight was rapidly absorbed, metabolized, and excreted. The
cis-isomer was eliminated primarily in the faeces, but the
trans-isomer was eliminated primarily in the urine. Tissue residues
48 h after treatment were low (< 1 mg/kg), except in the liver,
ovary, and kidney, and were generally higher with the alcohol-labelled
compounds. Only very low levels of radiocarbon were secreted in the
milk. Unmetabolized resmethrin appeared in trace amounts in tissues
and as the major residue in milk and faeces. The major metabolites
from both isomers arise from ester hydrolysis and subsequent oxidation
of the hydrolysed products. They include: chrysanthemic acid (free and
conjugated with glucuronic acid), chrysanthemumdicarboxylic acid,
5-benzyl-3-furoic acid (free and conjugated with glucuronic acid or
glycine), and 5-(alpha-hydroxybenzyl)-3-furoic acid (Ridlen et al.,
1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Photodegradation
The photodegradation pathways of resmethrin are summarized in
Fig. 2.
On irradiation with a sunlamp, trans- and cis-isomers of
14C-resmethrin (5) labelled in the carboxy (acid or phenyl (alcohol)
group decomposed on silica gel plates with a half-life of about
160-190 min. After 420 min irradiation of 14C-bioresmethrin, only 5%
of the recovered radioactivity was resmethrin, 45% was ester
photoproducts, 14% was non-ester photoproducts, and 32% was not
identified. The photoproduct found in the largest amount was
5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl trans-chrysanthemate
(10) (about 24% of the applied 14C), which originated from the cyclic
ozonide-type peroxide intermediates (8,9) formed by oxidation of the
furan ring. A relatively large (about 5%) amount of trans-
chrysanthemic acid (17) was formed. In addition, small amounts of the
R and S isomers of trans-epoxyresmethrin (6) (about 1.5% each),
5-benzyl-5-hydroxy-2-oxo-2,5-dihydro-3-furylmethyl trans-
chrysanthemate (11) (about 3%) and 2-benzyloxy-5-oxo-2,5-dihydro-
3-furylmethyl trans-chrysanthemate (12) (about 1%) were
detected with the acid- and alcohol-labelled preparations, and benzyl
alcohol (14) (about 2%), benzoic acid (16) (about 1.5%), and
phenylacetic acid (13) (about 1%) were found with the alcohol-labelled
preparation. The photoproduct distribution was greatly dependent on
the supporting material or medium. Oxidation at the furan ring
occurred predominantly on silica gel plates, while epoxidation at the
isobutenyl substituent proceeded to a greater extent on a filter paper
or in water. When exposed to sunlight for 10 h, cismethrin on silica
gel plates (1.7 mg/cm2) gave a large amount of cis-chrysanthemic
acid (17) but not trans-acid, together with smaller amounts of
5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl cis-chrysanthemate
(10), 5-benzyl-5-hydroxy-2-oxo-2,5-dihydro-3-furylmethyl
cis-chrysanthemate (11), benzaldehyde (15), and phenylacetic acid
(13). The photodecomposition rates were compared for residual deposits
(14 µg/cm2) of bioresmethrin, trans-tetramethrin, and
S-Bioallethrin on silica gel plates under sunlight or a sunlamp.
S-Bioallethrin was the most stable of these compounds, and
trans-tetramethrin was more persistent than bioresmethrin (Ueda
et al., 1974).
Resmethrin and structurally related 5-benzyl-3-furylmethyl
derivatives undergo rapid oxidation when exposed to sunlight or a
sunlamp, either in aqueous medium or as a thin film on glass or silica
gel. One major photodecomposition route involves epoxidation at the
isobutenyl substituent to give the R- and S-epoxides. Formation of the
other major photoproducts is initiated by oxidation of the furan ring
to a cyclic ozonide-type peroxide (Holmstead et al., 1977).
 In contrast, there was a 10% loss in the original weight of
resmethrin after 24 h compared with losses of 29% and 41% for
tetramethrin and allethrin, respectively, when exposed as a thin film
(500 mg/38.5 cm2) on glass to an incandescent lamp (Abe et al.,
1972).
The photodegradation of aqueous solutions (sterile water and
artificial sea-water) of resmethrin by natural sunlight has also been
investigated. The half-life was estimated to be 47 min in water and
20 min in sea water. The major degradation product was chrysanthemic
acid (Watson, 1984).
4.2 Degradation in Soil
The degradation under aerobic conditions of 14C-resmethrin
applied to soil at rates equivalent to 0.12 and 1.7 kg/ha has been
studied. The compound degraded very rapidly, so that less than 2% of
the applied 14C-resmethrin remained after 16 days. Complete
mineralisation to 14CO2 was a very important metabolic process
(38% of the applied radioactivity in 16 days). The radioactivity
remaining in the soil after 16 days was a complex mixture of more than
11 products resulting from ester cleavage and oxidation reactions
(Kaufman, 1986).
4.3 Degradation on Plants
Tomato and lettuce plants were treated with 14C-resmethrin, under
greenhouse conditions; wheat was similarly treated in the field. The
radioactivity was applied by spraying an aqueous suspension at a
concentration equivalent to 0.5 g/litre. The applied resmethrin
degraded very rapidly so that 55-82% had degraded within 2 h and no
unchanged resmethrin remained after 5 days. A very complex mixture of
degradation products were formed on the plants (up to 31 individual
compounds). No individual component in the mixture of products formed
exceeded 10% of the amount applied at any sampling interval up to 15
days after treatment. The alcohol formed by ester cleavage and
benzaldehyde (15), phenylacetic acid (13), benzoic acid (16), and
benzyl alcohol (14) were identified as degradation products. These
compounds were each present at levels < 3% of the radioactive residue
(Mumma, 1985).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
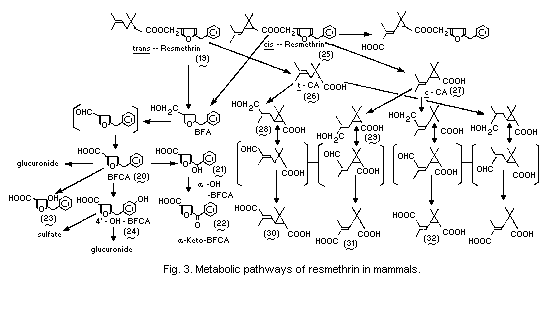
Metabolic pathways for resmethrin in mammals are summarized in
Fig. 3.
When 14C-[1RS,trans]-resmethrin (19) labelled in the alcohol
moiety was administered orally to Sprague-Dawley rats at 500 mg/kg,
the radiocarbon was rapidly absorbed from the gastrointestinal tract
and it took 3 weeks for the complete elimination of the radioactivity
in the urine (36% of the dose) and faeces (64%). A negligible amount
(less than 0.1%) of the radiocarbon was expired as 14CO2. The
radiocarbon was not completely excreted, even after 2 weeks, in rats
given an intravenous dose of 50 mg/kg; notably, appreciable amounts of
14C were found in faeces. The results of another study indicated that
the excretion of 14C via the bile duct was very rapid and that more
than 50% of the total dose was recovered from the bile within 72 h.
The urinary metabolites were mainly 5-benzyl-3-furancarboxylic acid
(20) (BFCA) in the free form (28% of the urinary 14C), the
glucuronide form (2%), and unknown conjugated (18%) forms. Other
significant metabolites present in the urine were hydroxy derivatives
of BFCA, such as alpha-(4-carboxy-2-furyl)-benzyl alcohol (21)
(alpha-OH-BFCA) (11%), 5-benzoyl-3-furancarboxylic acid (22)
(alpha-keto-BFCA) (1%), 5-benzyl-4-hydroxy-3-furancarboxylic acid (23)
(2%), and 5-(p-hydroxybenzyl)-3-furancarboxylic acid (24)
(4'-OH-BFCA) in the free form (3%), the sulfate form (12%) and the
glucuronide (2%) form (Miyamoto et al., 1971) (Fig. 3).
Ueda et al. (1975b) elucidated the metabolic fates of the acid and
alcohol moieties of bioresmethrin (19) and cismethrin (25) in rats
after a single oral dose (acid- and alcohol-labelled moieties) at
1 mg/kg. The predominant metabolites from the alcohol moiety were
BFCA, 4'-OH-BFCA, and alpha-OH-BFCA. The metabolites in the body were
derived from the alcohol moiety of bioresmethrin. On the other hand,
the isobutenyl group of the acid moiety was oxidized at either the
cis- or trans-methyl group of cismethrin, but only at the
trans-methyl group of bioresmethrin. The major metabolites from the
acid moiety were the hydroxymethyl (28, 29) or dicarboxylic acid
derivatives (30, 31) of chrysanthemic acid (26, 27) (CA). The aldehyde
intermediates of CA were not found. The major dicarboxylic acid
derivatives of CA were metabolites receiving oxidation at the
trans-methyl group of the isobutenyl moiety. Although the
trans-methyl group was predominantly oxidized in both isomers, the
cis-methyl group of the cis-isomer was also oxidized (32). An
anticipated metabolic pathway involves epimerization at C-3 of the
cyclopropane group leading to isomerized forms of dicarboxylic acid
derivatives of CA. The aldehyde derivatives of CA are the most likely
compounds for isomerization, because these derivatives readily undergo
cis- trans-isomerization under the alkaline conditions (Ueda et
al., 1975b).
In contrast, there was a 10% loss in the original weight of
resmethrin after 24 h compared with losses of 29% and 41% for
tetramethrin and allethrin, respectively, when exposed as a thin film
(500 mg/38.5 cm2) on glass to an incandescent lamp (Abe et al.,
1972).
The photodegradation of aqueous solutions (sterile water and
artificial sea-water) of resmethrin by natural sunlight has also been
investigated. The half-life was estimated to be 47 min in water and
20 min in sea water. The major degradation product was chrysanthemic
acid (Watson, 1984).
4.2 Degradation in Soil
The degradation under aerobic conditions of 14C-resmethrin
applied to soil at rates equivalent to 0.12 and 1.7 kg/ha has been
studied. The compound degraded very rapidly, so that less than 2% of
the applied 14C-resmethrin remained after 16 days. Complete
mineralisation to 14CO2 was a very important metabolic process
(38% of the applied radioactivity in 16 days). The radioactivity
remaining in the soil after 16 days was a complex mixture of more than
11 products resulting from ester cleavage and oxidation reactions
(Kaufman, 1986).
4.3 Degradation on Plants
Tomato and lettuce plants were treated with 14C-resmethrin, under
greenhouse conditions; wheat was similarly treated in the field. The
radioactivity was applied by spraying an aqueous suspension at a
concentration equivalent to 0.5 g/litre. The applied resmethrin
degraded very rapidly so that 55-82% had degraded within 2 h and no
unchanged resmethrin remained after 5 days. A very complex mixture of
degradation products were formed on the plants (up to 31 individual
compounds). No individual component in the mixture of products formed
exceeded 10% of the amount applied at any sampling interval up to 15
days after treatment. The alcohol formed by ester cleavage and
benzaldehyde (15), phenylacetic acid (13), benzoic acid (16), and
benzyl alcohol (14) were identified as degradation products. These
compounds were each present at levels < 3% of the radioactive residue
(Mumma, 1985).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Metabolic pathways for resmethrin in mammals are summarized in
Fig. 3.
When 14C-[1RS,trans]-resmethrin (19) labelled in the alcohol
moiety was administered orally to Sprague-Dawley rats at 500 mg/kg,
the radiocarbon was rapidly absorbed from the gastrointestinal tract
and it took 3 weeks for the complete elimination of the radioactivity
in the urine (36% of the dose) and faeces (64%). A negligible amount
(less than 0.1%) of the radiocarbon was expired as 14CO2. The
radiocarbon was not completely excreted, even after 2 weeks, in rats
given an intravenous dose of 50 mg/kg; notably, appreciable amounts of
14C were found in faeces. The results of another study indicated that
the excretion of 14C via the bile duct was very rapid and that more
than 50% of the total dose was recovered from the bile within 72 h.
The urinary metabolites were mainly 5-benzyl-3-furancarboxylic acid
(20) (BFCA) in the free form (28% of the urinary 14C), the
glucuronide form (2%), and unknown conjugated (18%) forms. Other
significant metabolites present in the urine were hydroxy derivatives
of BFCA, such as alpha-(4-carboxy-2-furyl)-benzyl alcohol (21)
(alpha-OH-BFCA) (11%), 5-benzoyl-3-furancarboxylic acid (22)
(alpha-keto-BFCA) (1%), 5-benzyl-4-hydroxy-3-furancarboxylic acid (23)
(2%), and 5-(p-hydroxybenzyl)-3-furancarboxylic acid (24)
(4'-OH-BFCA) in the free form (3%), the sulfate form (12%) and the
glucuronide (2%) form (Miyamoto et al., 1971) (Fig. 3).
Ueda et al. (1975b) elucidated the metabolic fates of the acid and
alcohol moieties of bioresmethrin (19) and cismethrin (25) in rats
after a single oral dose (acid- and alcohol-labelled moieties) at
1 mg/kg. The predominant metabolites from the alcohol moiety were
BFCA, 4'-OH-BFCA, and alpha-OH-BFCA. The metabolites in the body were
derived from the alcohol moiety of bioresmethrin. On the other hand,
the isobutenyl group of the acid moiety was oxidized at either the
cis- or trans-methyl group of cismethrin, but only at the
trans-methyl group of bioresmethrin. The major metabolites from the
acid moiety were the hydroxymethyl (28, 29) or dicarboxylic acid
derivatives (30, 31) of chrysanthemic acid (26, 27) (CA). The aldehyde
intermediates of CA were not found. The major dicarboxylic acid
derivatives of CA were metabolites receiving oxidation at the
trans-methyl group of the isobutenyl moiety. Although the
trans-methyl group was predominantly oxidized in both isomers, the
cis-methyl group of the cis-isomer was also oxidized (32). An
anticipated metabolic pathway involves epimerization at C-3 of the
cyclopropane group leading to isomerized forms of dicarboxylic acid
derivatives of CA. The aldehyde derivatives of CA are the most likely
compounds for isomerization, because these derivatives readily undergo
cis- trans-isomerization under the alkaline conditions (Ueda et
al., 1975b).
 5.2 Enzymatic Systems for Biotransformation
When 14C alcohol-labelled cismethrin, bioresmethrin, and
5-benzyl-3-furylmethyl alcohol (BFA) (18) were incubated with rat
liver S-9 homogenates or microsomes, a proportion of the radioactive
compounds was covalently bound to proteins. The covalent binding was
greater with phenobarbital-pretreated rats, and dependent on a
NADPH-generating system. When a S-9 homogenate was used, the bound
compounds were twice as high for cismethrin as for bioresmethrin and
BFA. Inversely, when microsomes were used, more covalent binding
occurred with bioresmethrin and BFA than with cismethrin. The
inhibition of esterases by tetraethyl pyrophosphate (TEPP) in a S-9
homogenate did not alter the amount of covalent binding to the three
compounds, whereas malathion inhibited this binding. However,
treatment of a S-9 homogenate with piperonyl butoxide greatly reduced
covalent binding. Covalent binding was inhibited when the microsomes
were incubated with carbon monoxide or modified by thermal
denaturation. It is suggested that oxidative metabolism was
responsible for the covalent binding (Hoellinger et al., 1985).
When four resmethrin isomers ([1R,trans]-, [1S,trans]-,
[1R,cis]-, and [1S,cis]-) were incubated with mouse and rat
microsomes in 50 mmol/litre tris-HCl buffer (pH 7.4) at 37°C for 1 h,
microsomal esterases readily cleaved the trans- but not the
cis-isomers. The ester linkage also appeared to undergo oxidative
cleavage when esterase attack was minimal. Ester metabolites were
detected in significant amounts only with [1R,cis]-resmethrin, in
which case oxidation had occurred at the isobutenyl moiety, with or
without oxidation at the benzylfuryl methyl group. Most of the
in vitro metabolites were identical with those in the excreta of
rats given resmethrin orally. The preferred site of oxidation in the
isobutenyl moiety varies with the resmethrin isomer and microsomal
source. Mouse microsomes predominantly oxidized the trans-methyl
group of both [1R,trans]- and [1S,trans]-resmethrin, the
selectivity, however, being the greatest with [1S,trans]-resmethrin.
Rat microsomes were relatively nonselective in attacking the
isobutenyl methyl groups of [1R,trans]-resmethrin (Ueda et al.,
1975a).
Plasma-esterases were equally active in hydrolysing cismethrin and
bioresmethrin (3.2-3.4 nmol benzylfurylmethanol/min per g) whereas
liver microsomal esterases hydrolysed bioresmethrin 10 times more
rapidly than cismethrin (White et al., 1976).
6. EFFECTS ON THE ENVIRONMENT
Data on the acute toxicity of resmethrin for aquatic and
terrestrial non-target organisms are summarized in Tables 4 and 5,
respectively.
6.1 Aquatic Organisms
Resmethrin is highly toxic for fish, and the toxicity is
negatively correlated with temperature (Mauck et al., 1976); the LC50
value for bluegill is 2.3 times greater at 22°C than at 12°C, as shown
in Table 4. The influence of both water hardness and pH on toxicity
ranged from slight to negligible. By keeping resmethrin solution at
12°C for 1 week, the toxicity for bluegill decreased 2-fold at each pH
value as the resmethrin solution aged.
Among the 4 isomers, the 1R, cis-isomer is most toxic for fish,
followed by the 1R, trans-, 1S, cis- and 1S, trans-, as observed
in pyrethroids. Contrary to other pyrethroids, resmethrin is less
toxic for arthropods than for fish, as shown in Table 4. The 48-h
LC50 values for arthropods are in the range of 2-25 µg/litre
(Nishiuchi, 1981).
Laboratory studies have shown that resmethrin is highly toxic for
fish but, because of the low water solubility of the compound and
rapid environmental degradation (photochemical and microbial), it
would be expected that, under field conditions, the toxic impact would
be greatly reduced. Such a reduction in toxic effects has been
confirmed in field studies carried out in the USA (Sjogren, 1985;
Norwood, 1986; Pierce, 1986).
In one of these studies, ponds were sprayed with resmethrin
formulations at the expected field rate for adult mosquito control
(0.01 kg/ha). Residue analysis confirmed that resmethrin levels fall
rapidly (1.1 ppb at zero time nondetectable after 96 h). Caged fish
showed good survival (82-100% for bluegill, 73-100% for goldfish) in
resmethrin-treated ponds. However, one fish species in this study
(white sucker) was susceptible (8% survival) when piperonyl
butoxide-synergised resmethrin was used, though good survival was
noted for this species with unsynergised resmethrin. In a second
study, synergised resmethrin was applied to a mangrove-fringed pond at
2-day intervals. The first two applications were at the normal field
rate, but the third was at 10 times this rate. In this study, both
fish species used to monitor fish survival (sheepshead minnow,
fingerling snook) showed good survival (100% at the normal rate and
88% at the 10-fold rate).
Table 4. Acute toxicity of resmethrin for non-target aquatic organisms
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish
Carp (Cyprinus 48-h LC50 44 technical static 25 Nishiuchi (1982)
carpio)
Killifish adult 48-h LC50 300 technical static 25 Miyamoto (1976)
(Oryzias adult 48-h LC50 16 (+)-trans- static 25 Miyamoto (1976)
latipes) adult 48-h LC50 8 (+)-cis- static 25 Miyamoto (1976)
adult 48-h LC50 > 10 000 (-)-trans- static 25 Miyamoto (1976)
adult 48-h LC50 3500 (-)-cis- static 25 Miyamoto (1976)
Rainbow trout 96-h LC50 2.2 technical static 12 Marking & Mauck
(Salmo gairdneri) (1975)
Coho salmon 96-h LC50 1.50 technical static 12 Mauck et al.
(Oncorhynchus (1976)
kisutch) 96-h LC50 0.277 technical flow- 12 Mauck et al.
through (1976)
Steelhead trout 96-h LC50 0.450 technical static 12 Mauck et al.
(Salmo gairdneri) (1976)
96-h LC50 0.275 technical flow- 12 Mauck et al.
through (1976)
Yellow perch 96-h LC50 2.36 technical static 12 Mauck et al.
(Perca flavescens) (1976)
96-h LC50 0.513 technical flow- 12 Mauck et al.
through (1976)
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Arthropods
Daphnia pulex 3-h LC50 15 000 technical static 25 Nishiuchi (1982)
3-h LC50 50 000 technical static 25 Miyamoto (1976)
3-h LC50 25 000 - (+)-trans- static 25 Miyamoto (1976)
50 000
3-h LC50 25 000 - (+)-cis- static 25 Miyamoto (1976)
50 000
3-h LC50 > 50 000 (-)-trans- static 25 Miyamoto (1976)
3-h LC50 > 50 000 (-)-cis- static 25 Miyamoto (1976)
Moina macrocopa 3-h LC50 14 000 technical static 25 Nishiuchi (1982)
Sigara substriata 0.59 cm; 48-h LC50 2 technical static 25 Nishiuchi (1981)
6.1 mg
Micronecta sedula 0.32 cm; 48-h LC50 3.3 technical static 25 Nishiuchi (1981)
1.8 mg
Cloeon dipterum 0.93 cm; 48-h LC50 4.5 technical static 25 Nishiuchi (1981)
5.6 mg
Orthetrum 2.3 cm; 48-h LC50 7.3 technical static 25 Nishiuchi (1981)
albistylum 0.62 g
speciosum
Eretes sticticus 1.5 cm; 48-h LC50 25 technical static 25 Nishiuchi (1981)
0.2 g
Sympetrum 2.1 cm; 48-h LC50 10 technical static 25 Nishiuchi (1981)
frequens 0.56 g
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish (contd).
Bluegill (Lepomis 96-h LC50 2.62 technical static 12 Mauck et al.
macrochirus) (1976)
96-h LC50 0.750 technical flow- 12 Mauck et al.
through (1976)
0.8 g 96-h LC50 5.46 technical static 22 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 4.28 technical static 17 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.33 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.53 technical static 12 6.6 10-13 Mauck et al.
(1976)
0.8 g 96-h LC50 2.54 technical static 12 7.8 160-180 Mauck et al.
(1976)
0.8 g 96-h LC50 2.06 technical static 12 8.2 280-320 Mauck et al.
(1976)
0.8 g 96-h LC50 2.29 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.46 technical static 12 9.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.89 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.70 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 3.13 technical static 12 8.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.68 technical static 12 9.5 40-48 Mauck et al.
(1976)
6.2 Terrestrial Organisms
Acute toxicity data show that both resmethrin and bioresmethrin
(Table 5) are harmless for birds, as are other pyrethroids.
In addition, a study has been carried out to assess the effects of
resmethrin on reproduction in the Mallard duck and Bobwhite quail. The
birds were given 12, 60, or 300 mg resmethrin/kg diet for 22 weeks. No
effects on reproduction were seen in either species at these dose
levels (Roberts et al., 1985a,b).
Table 5. Acute toxicity of resmethrin and bioresmethrin or non-target terrestrial
organisms
Species Compound Size Application Toxicity Reference
(days) (mg/kg)
Bird
Quail Resmethrin 14 diet LC50 Hill et al.
(Coturnix > 5000 (1975)
coturnix
japonica)
Mallard 10 diet LC50 Hill et al.
(Anas > 5000 (1975)
platyrhynchos)
Chicken Bioresmethrin oral LD50 Chesher &
> 10 000 Malone (1970a)
Wallwork
et al. (1970)
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Acute Toxicity
Acute toxic signs produced in experimental animals by exposure to
resmethrins include tremor, hyperexcitability, convulsive twitching,
prostration, and coma.
LD50 data on resmethrin isomers administered orally and by other
exposure routes are summarized in Tables 6 and 7, respectively.
These data show that resmethrins are weakly toxic, except for
cismethrin, the acute oral toxicity of which is moderate in the mouse.
The results of acute toxicity tests of the resmethrin metabolites
are given in Table 8.
Acute (1 h) inhalation studies were performed with 3 aerosol
formulations of resmethrin and 2 blank formulations. Groups of 20 male
rats and 4 male rabbits inhaled the active ingredient at concentration
levels of 12-13.7 mg/litre (value based on the weight loss from each
spray can). In addition, 10 exposed and 10 control rats were tested
for conditioned avoidance performance immediately following exposure.
Laboured breathing was seen generally in the animals. Several rabbits
died in both the resmethrin-treated and the blank-formulation groups.
In the rats, there were no effects on body weight, or gross or
histopathological lesions. However, the conditioned avoidance latency
in the resmethrin-treated rats was significantly greater than that in
the unexposed controls (Macko et al., 1979).
7.2 Short-term Exposure
7.2.1 Oral administration
Groups of 6 Sprague-Dawley rats from each sex were administered
resmethrin in the diet at concentrations of 0, 310-323, 630-660,
1230-1250, 1423-2670, or 1680-5100 mg/kg, for 14 days. Five out of 6
rats died at the highest exposure level in both sexes. Tremor was
observed and body weight gain and food utilization were reduced at
levels of 1230-1250 mg/kg or more. A significant increase in the
hepatic organ-to-body weight ratios was noted in all groups of females
and in groups of males fed 630-660 mg/kg or more. Compound-related
histopathological changes were not observed in any of the tissues and
organs examined. The maximum no-observed-adverse-effect dietary level
was 310 mg/kg for male rats only (Swentzel et al., 1977).
Table 6. Acute oral toxicity of resmethrin isomers
LD50 (mg/kg
Compound Animal Sex body weight) Reference
Resmethrin rat M > 5000 Miyamoto (1976)
rat F > 5000 Miyamoto (1976)
rat M 1244 Gaines & Linder (1986)
rat F 1721 Gaines & Linder (1986)
rat (weanling) M 1987 Gaines & Linder (1986)
rat ? > 2500 Worthing & Walker (1983)
rat - 960 Volkov et al. (1979)
mouse M 690 Miyamoto (1976)
mouse F 940 Miyamoto (1976)
Bioresmethrin rat M 8800 Glomot & Chevalier (1969)
rat F > 8000 Verschoyle & Barnes (1972)
rat F 7071 Wallwork et al. (1970)
rat - 840 Kholmatova (1984)
rat - 990 Volkov et al. (1979)
rat - 675 Kagan et al. (1986)
mouse M 590 Miyamoto (1976)
mouse F 800 Miyamoto (1976)
mouse M 3100 Ueda et al. (1975b)
mouse F 10000 Wallwork et al. (1970)
mouse - 520 Kholmatova (1984)
mouse - 480 Kagan et al. (1986)
rabbit - 225 Kholmatova (1984)
Cismethrin mouse M 152 Miyamoto (1976)
mouse F 160 Miyamoto (1976)
[1S,-trans]-resmethrin mouse M 500 Miyamoto (1976)
mouse F 600 Miyamoto (1976)
[1S,-cis]-resmethrin mouse M 3700 Miyamoto (1976)
mouse F 5000 Miyamoto (1976)
Table 7. Acute toxicity of resmethrins via other than oral exposure
LD50 LC50
Compound Animal Sex Route (mg/kg (mg/m3) Reference
body weight)
Resmethrin rat M dermal 2500 Gaines & Linder (1986)
rat F dermal 2500 Gaines & Linder (1986)
rabbit - dermal 2500 Green (1977)
rat - inhalation > 9490 Jackson & Hardy (1984)
(4-h exposure)
rat - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
rabbit - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
dog - inhalation > 420 Macko et al. (1979)
(4-h exposure)
Bioresmethrin rat F dermal > 10 000 Wallwork et al. (1970)
rat F intravenous 340 Verschoyle & Barnes (1972)
rat F intravenous 106-133 Chesher & Malone (1971b)
rat F intraperitoneal > 8000 Wallwork & Malone (1971)
rat F inhalation > 872 Wallwork & Malone (1972)
(24-h exposure)
mouse M intraperitoneal > 1500 Ueda et al. (1975b)
mouse F intraperitoneal > 5359 Wallwork et al. (1970)
Mixture of rat M,F inhalation > 1500 Miyamoto (1976)
bioresmethrin (4-h exposure)
& cismethrin mouse M,F inhalation > 1500 Miyamoto (1976)
(4-h exposure)
Table 8. Acute toxicity of metabolites of resmethrin
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
(bioresmethrin), (5-benzyl-3-furyl- mouse > 1500a 3100a Miyamoto (1975-1976)
methyl (+)-trans-chrysanthemate) (1) Ueda et al. (1975b)
1R or (+)-cis-resmethrin (5-benzyl-3-furyl- (2) mouse 320a Miyamoto (1975-1976)
methyl (+)-cis-chrysanthemate) Ueda et al. (1975b)
d,1-cis,trans-chrysanthemic acid (17) rat 2443 Reagan & Becci (1985a)
d,1-trans-chrysanthemic acid - rat 1598 Reagan & Becci (1985b)
(+)-trans-chrysanthemic acid, (+)-trans-CA (26) mouse 98a 280a Miyamoto (1975-1976)
(t-CA) Ueda et al. (1975b)
(+)-cis-chrysanthemic acid, (+)-cis-CA (c-CA) (27) mouse 600a Miyamoto (1975-1976)
Ueda et al. (1975b)
d-trans-chrysanthemic acid (26) rat 983 Reagan & Becci (1985c)
(+)-trans-chrysanthemumdicarboxylic acid, (30) mouse 408a Miyamoto (1975-1976)
(+)-trans-CDA (tE-CDA) Ueda et al. (1975b)
5-benzyl-3-furylmethanol (BFA) (18) mouse 75a 310a Miyamoto (1975-1976)
Ueda et al. (1975b)
Table 8. (cont'd).
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
5-benzyl-3-furoic acid (BFCA) (20) mouse 46a Miyamoto (1975-1976)
Ueda et al. (1975b)
5-benzyl-3-furoic acid (BFCA) (20) rat 997 Reagan & Becci (1985d)
ethyl chrysanthemate - rat > 4640 Stauffer Chemical Co.
(1984)
a Toxicity for male mice, 24 h after intraperitoneal injection or oral administration.
b Chemical identification numbers used in Table 1 and Fig. 2.
The same authors fed groups of 6 Long-Evans rats of each sex
resmethrin in the diet at concentrations of 0, 61-90, 148-180,
297-386, 584-669, 1080-1375, or 1266-2532 mg/kg body weight per day
for 14 days. Mortality was observed in the groups fed 1080 mg/kg or
more. Tremor was observed at levels of 386 mg/kg or more (females only
at 386 mg/kg). The terminal body weight and food utilization were
significantly lower in the groups fed 1080 mg/kg or more compared with
the controls. A significant increase in the hepatic organ-to-body
weight ratios was noted at levels of 297 mg/kg or more.
Compound-related histopathological changes were not observed in any of
the tissues and organs examined. The maximum no-observed-adverse-
effect dietary level was 148 mg/kg body weight per day for male and
180 mg/kg body weight per day for female rats (Swentzel et al., 1977).
In a third study by Swentzel et al. (1977), groups of 8-20
Long-Evans rats of each sex were fed resmethrin in the diet at
concentrations equivalent to 0, 22, 66, 211, or 679 mg/kg body weight
per day for males and of 0, 3, 7, 22, 67, 219, 724, or 2400 mg/kg body
weight per day for females, for 90 days. All the females fed the
highest level died. Tremor was observed at levels of 679 mg/kg or
more. The mean terminal body weight was significantly lower in the
group given 679-724 mg/kg than in the control group. A significant
increase in the hepatic and renal organ-to-body weight ratios was
noted in males fed 211 or 679 mg/kg; while, in females fed 219 or
724 mg/kg, only an increase in the hepatic ratio was observed. No
significant differences were observed in clinical chemistry values
(serum glutamic oxaloacetic transaminase (SGOT), serum glutamic
pyruvic transaminase (SGPT), total lactate dehydrogenase (LDH),
alkaline phosphatase (AP), gamma-glutamyl trans-peptidase (GGTP),
total bilirubin, total protein, blood-urea nitrogen (BUN)) between
test and control animals. Compound-related histopathological changes
were not observed in any of the tissues and organs examined. The
maximum no-observed-adverse-effect dietary level was 67 mg/kg body
weight per day for female and 66 mg/kg body weight per day for male
rats.
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage, daily, 6 days/week for 3 weeks at doses of 0, 1000,
or 2000 mg/kg body weight. Body weights were slightly reduced at
2000 mg/kg. A slight reduction was noted in haemoglobin content and
hematocrit value. Biochemical examination revealed an increase in
albumin and blood-urea nitrogen concentrations and a decrease in
serum-glutamic oxaloacetic transaminase (SGOT) activity. Increased
liver size and reduced thymus weight were observed at 1000 mg/kg.
Reduced prostate weight was noted only at the high dose.
Histopathological examination revealed thymic involution (Glomot,
unpublished report).a
a GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished report of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
Wallwork et al. (1971) reported a study in which groups of rats
(18 males and 18 females/group) were fed bioresmethrin in the diet at
concentrations of 0, 400, 1200, or 8000 mg/kg over 91 days. The group
receiving the highest dose was fed 4000 mg/kg for 30 days and
thereafter 8000 mg/kg. Body weights were reduced at the highest dose
level and there were changes in blood chemistry parameters indicating
liver dysfunction (an increase in serum-alkaline phosphatase (SAP),
SGOT, and urinary nitrogen and a decrease in glucose content). The red
blood cell count was depressed in the group receiving 1200 mg/kg. An
increase in liver weight and a decrease in the weights of several
other organs, such as the spleen and thymus, were observed at
4000 mg/kg. Fatty infiltration of the liver was noticed at 1200, 4000,
and 8000 mg/kg. The no-observed-adverse-effect level in this study was
400 mg/kg, equivalent to an average daily intake of 32.8 and
36.1 mg/kg body weight for males and females, respectively.
Bioresmethrin was administered to groups of dogs (2 of each
sex/group) by gavage, daily, at dose levels of 0 or 500 mg/kg for 7
days followed by an increased dose of 1000 mg/kg for a further 14
days. No effects were noted on mortality, behaviour, body weight,
haematology, blood chemistry, urinalysis, or electrocardiograms
(Malone & Chesher, 1970).
Groups of dogs (3 males and 3 females/group) were administered
bioresmethrin in gelatin capsule by gavage, daily for 90 days, at
dosage levels of 0, 25, 80, or 250 mg/kg body weight (the highest dose
was increased to 500 mg/kg body weight in week 7). There were no
effects on mortality, body weight, food consumption, ophthalmology, or
urinalysis. Red blood cell count, haemoglobin content, and packed cell
volume values were reduced at the highest dose level. Blood-urea
nitrogen was slightly increased at the highest dose after 12 weeks. No
adverse effects were observed on gross or histopathological
examination. The no-observed-adverse-effect level was 80 mg/kg body
weight, equivalent to an average of 1600 mg/kg diet (Noel et al.,
1971).
In summary, the no-observed-adverse-effect level for resmethrin as
determined in the 90-day study on rats was 66 mg/kg body weight per
day, whereas that for bioresmethrin was 33 mg/kg body weight per day
in the 91-day study on rats and 80 mg/kg body weight per day in the
90-day study on dogs.
7.2.2 Inhalation
Short-term inhalation studies were performed using three
resmethrin formulations and one blank formulation. Aerosol spray
formulations (frequency median diameter of the particles: 1.5-2.0 µm,
volume median diameter 10-18.5 µm) were introduced into the top of a
pyramid-shaped sealed chamber and dispersed for 30-second intervals
every 30 min. Groups of 25 male rats, 10 female rats, and 4 male
rabbits were exposed to the inhalations 10 times a day, over a period
of 5 h, for 5 consecutive days. Daily active ingredient concentrations
of each formulation in the chamber were 2.9-3.2 mg/litre, based on the
weight loss from each spray can. The clinical signs included increased
preening, ruffled pelt, rapid breathing, and slight nasal discharge.
All signs disappeared overnight, but recurred after each daily
exposure. In rats, there were no effects on body weight. No gross or
histopathological lesions related to exposure to the formulations were
observed in rats necropsied immediately after the last 5-h exposure
and 7 and 14 days after exposure. No indication of toxic effects,
other than irritation, was observed in any test (Macko et al., 1979).
When ICR mice and Sprague-Dawley rats were exposed to [1R,
trans, cis]-resmethrin (at concentrations of 0, 23, 47, or
210 mg/m3) for 4 h/day, 5 days/week over 4 weeks, no toxic effects
were observed on behaviour, food intake, haematology, clinical
biochemistry, mortality, or histopathology (Miyamoto, 1976).
When groups of 16 male and 16 female Wistar rats were exposed
through inhalation for 6 h/day on 5 days of each week, over a period
of 90 days, to technical resmethrin at target exposure levels of 0.1,
0.3, or 1.0 g/m3, the no-observed-adverse-effect level was
established at 0.1 g/m3. At 0.3 g/m3, there were minor effects on
some clinical pathology parameters and also signs of irritation.
However, microscopic pathological examination did not reveal any
treatment-related changes in the lungs or other organs of rats exposed
at this level. At 1 g/m3, there were clinical signs indicative of
irritation, minor neurobehavioural changes, a reduced rate of weight
gain, and some changes in clinical pathology parameters. Microscopic
examination at this level showed minimal changes in the larynx, liver,
and thyroid, but these were reversible during the recovery period.
There were no treatment-related lung changes (Coombs et al., 1985).
7.2.3 Dermal application
Resmethrin was applied twice a week for 3 weeks to the shaved skin
of 4 groups of 10 male New Zealand White rabbits. Cotton cloth treated
with resmethrin at 0.247 mg/cm3 was applied over 1 ml of liquid
(imitating sweat) to rabbits in the first group. In the second group,
cotton cloth treated with resmethrin was applied without the sweat,
and in the third group, the cotton cloth was fixed to skin that had
been pretreated with 10 g of technical grade resmethrin. In the fourth
group, untreated cotton cloth was fixed over skin pre-treated with
pyrax powder containing 1% resmethrin at the rate of 1 g/kg of body
weight. The 3 control groups received cotton cloth treated with
acetone, cotton cloth treated with acetone over 1 ml of the sweat, and
untreated cotton cloth over 1 g pyrax powder/kg, respectively. No
significant changes were noted, on day 24 of the test, in rabbit body
weights and organ-to-body weight ratios of liver, lung, kidney,
testis, and spleen. Average dermal irritation scores for
resmethrin-treated rabbits were not significantly higher than those
for the control groups and did not increase during the test. No
significant trends compared with the controls were seen in clinical
chemistry values (serum-glutamic oxalo-acetic transaminase,
serum-glutamic pyruvic transaminase, lactic dehydrogenase, alkaline
phosphatase, blood-urea nitrogen) on days 5, 12, 19, and 24 of the
test. There were no compound-related lesions of the skin or of any of
the other tissues and organs examined at the termination of the study
(Swentzel et al., 1977).
7.3 Primary Irritation and Sensitization
Technical grade resmethrin was found to be a slight irritant in
rabbits in a 24 day dorsal/ventral rabbit ear test. Dermal irritation
was evident on both intact and abraded skin at 72 h and up to 7 days.
Resmethrin did not cause sensitization reactions in guinea-pigs, or
photochemical irritation in New Zealand White rabbits. Repeated daily
applications of 0.1 g of the technical grade compound to one ear of
each of 5 New Zealand White rabbits were carried out for 30
consecutive days as well as applications of compound-impregnated
cotton sateen cloth (0.247 mg/cm2) with artificial sweat for 24 days.
In this study, resmethrin did not produce acne-form dermatitis
(Swentzel et al., 1977).
Groups of adult guinea-pigs (6 males/group) were treated with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro
benzene (DNCB) (0.1 ml of a 1% (w/v) formulation in mineral oil) to
assess the sensitization properties. The test substance was applied to
the ears for 4 days. On day 7, 0.2 ml was applied dermally and the
degree of irritation recorded. As expected, the DNCB was an irritant
while bioresmethrin showed only traces of erythema suggesting a low
potential for sensitization and irritation (Chesher & Malone, 1970b).
Instillation of technical bioresmethrin into the eye of 6 rabbits
(0.1 ml) did not produce any irritation or corneal damage, or any
indication of ocular hazard (Chesher & Malone, 1970c).
7.4 Long-term Exposure and Carcinogenicity
When [1R, trans, cis]-resmethrin was fed to Sprague-Dawley
rats (male and female) at dietary levels of 500, 1500, or 5000 mg/kg
for 24 weeks, toxic symptoms, such as tremors and decreased body
weight, increased liver and kidney weights and an increase in alkaline
phosphatase activity were observed at 5000 mg/kg. The no-observed-
adverse-effect level was 1500 mg/kg (77.7 mg/kg per day for males and
86.6 mg/kg per day for females) (Miyamoto, 1976).
Resmethrin fed to Wistar rats at dosage levels of 0, 500, 2500, or
5000 mg/kg in the basal diet over a 112-week period, was determined
not to be oncogenic up to, and including, 5000 mg/kg, which was the
highest dose tested. The no-observed-adverse-effect level of 500 mg/kg
for toxic effects, was the lowest effect level for hypertrophy of
hepatocytes, which was not considered a definite toxic response
(Knickerbocker et al., 1980; Hess et al., 1982).
Cox et al. (1979a) fed CD-1 outbred albino mice with 0, 250, 500,
or 1000 mg resmethrin/kg basal diet for an 85-week period. No
oncogenicity was observed at any doses up to and including 1000 mg/kg.
When Beagle dogs were administered 0, 10, 30, or 300 mg
resmethrin/kg body weight for 6 months, the no-observed-adverse-effect
level was 10 mg/kg per day. On day 57 of the study, the highest dose
level was increased from 100 mg/kg per day to 300 mg/kg per day.
Increased liver weights were noted at 30 mg/kg body weight per day
(Gephart et al., 1980).
An acceptable daily intake (ADI) was established by the USA EPA at
0.125 mg/kg body weight per day based on no-observed-adverse-effect
levels in long-term toxicity studies. A food additive tolerance has
been established by the US EPA, permitting resmethrin residues of up
to 3 ppm, in or on food commodities, resulting from use in food
handling areas (US Environmental Protection Agency, 1983).
7.5 Mutagenicity
Saccharomyces cerevisiae D4 and five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) were used to
evaluate the mutagenic potential of resmethrin. The compound was
tested in the absence or presence of liver microsomal enzyme
preparations from rats pre-treated with Aroclor 1254. Resmethrin was
not mutagenic to any of the indicator organisms under both conditions
(Swentzel et al., 1977).
Resmethrin, bioresmethrin, and cismethrin were tested for
mutagenicity in several test systems. Miyamoto (1976) and Suzuki
(1975) used the Escherichia coli and S.typhimurium reverse
mutation tests. Garret et al. (1986) summarized results of point/gene
mutations in prokaryotes or eukaryotes, primary DNA damage in
prokaryotes or eukaryotes, chromosomal effects in Chinese hamster
ovary cells, mouse bone marrow, and cardiac blood cells of the mouse.
Pluijmen et al. (1984) used reverse mutation tests in S.typhimurium
TA98 or TA100, or mutagenicity tests with V79 Chinese hamster cells to
test seven synthetic pyrethroids including resmethrin, bioresmethrin,
and cismethrin. They all gave negative results.
Bioresmethrin was also tested in a metaphase chromosome analysis
of cultured human lymphocytes, an autoradiographic assessment of
unscheduled DNA synthesis in mammalian cells, and a mouse micronucleus
test. They also gave negative results (Allen et al., 1986; Allen &
Proudlock, 1986; Vannier & Fournex, 1986).
7.6 Reproductive effects, Embryotoxicity, and Teratogenicity
Three groups of 30 pregnant Long-Evans rats were administered
resmethrin in the diet at concentrations of 0, 188, or 1500 mg/kg from
day 6 to day 16 of gestation. The dams showed tremors and decreased
food and water consumption at 1500 mg/kg and 2 dams died; the lower
fetal weight seen at this dose and the resorption of embryos and
fetuses in 15 out of 30 dams were probably due to maternal toxicity.
No gross abnormalities of fetal skeletons and soft tissues were
observed in the treated groups. Thus, the consumption of resmethrin in
ground feed was not teratogenic at 188 and 1500 mg/kg (Swentzel
et al., 1977).
In a 3-generation study, Sprague-Dawley rats were fed resmethrin
in the diet at 0, 500, 800, or 1250 mg/kg (Schwartz et al., 1979c). A
slight increase in the number of pups cast dead and a decrease in pup
weights were noted at the 500 mg/kg level. A no-observed-adverse-
effect level of < 500 mg/kg was established for pups cast dead and
reduced pup weights at 21 days.
Sprague-Dawley female albino rats were given resmethrin in corn
oil, by gavage, at dose levels of 0, 20, 40, or 80 mg/kg during the
period of major organogenesis (days 6-15 of gestation). Resmethrin was
not teratogenic in rats at levels up to, and including, 80 mg/kg. The
no-observed-adverse-effect level for fetotoxicity was 40 mg/kg (Machi
et al., 1979).
Pregnant New Zealand White Minnikin rabbits were given resmethrin
by oral intubation at 0, 10, 30, or 100 mg/kg body weight per day on
days 6-18 of gestation (Becci et al., 1979). On day 29 of gestation,
all animals were killed for examination. No teratogenic effects were
seen at dose levels up to and including 100 mg/kg.
When [1R, trans, cis]-resmethrin was administered orally to
female ICR mice (10, 30, or 100 mg/kg, daily) and Sprague-Dawley rats
(10, 20, or 50 mg/kg daily) during the period of gestation, to examine
maternal and embryotoxic effects, no significant adverse effects, such
as abortion, resorption of fetuses or embryos, external or skeletal
abnormalities of pups, and abnormalities in growth or organ
differentiation, were observed at any doses (Miyamoto, 1976).
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin at doses of 0, 10, 20, 40, or 80 mg/kg, by gavage,
daily, from day 8 to day 16 of gestation. The does were sacrificed on
day 28 and examined for implantation, live and dead fetuses,
resorption sites, and abnormalities. There was no apparent effect on
parents in the study as growth and gestation were unaffected. There
was an increase in the numbers of dead fetuses at the highest dose and
a large number of resorption sites were noted at all treatment levels.
A number of deformed fetuses were observed, but the total numbers were
not sufficient for an adequate statistical evaluation. The deformities
included straight tail, crossed hind limbs, and unilateral union of
6th and 7th ribs at the sternal end. An overall fetal loss was
observed at all dose levels (primarily because of the large number of
resorption sites recorded) (Waldron, 1969).
7.7 Immunotoxicity
The influence of various pesticides on the humoral and cellular
immune response to fluorescein-labelled ovalbumin has been analysed.
Resmethrin was administered intragastrically in corn oil as a single
dose (one half of LD50) before primary immunization. Control groups
included animals treated with corn oil alone, or immunosuppressed with
methotrexate. Booster immunizations and test bleedings were scheduled
to follow at weekly intervals. The cellular immune response was
quantified by redness and swelling, histological examination, and by
differential temperature measurements of the foot pads after antigen
challenge. The concentration, binding affinity, and heterogeneity of
the serum antibody were determined by fluorescence polarization
measurements. Resmethrin gave an early, sometimes very marked,
stimulation of the cellular immune response (Danliker et al., 1979).
7.8 Neurotoxicity
The neurotoxicity of resmethrin was evaluated in three short-term
dietary studies on Sprague-Dawley rats designed to examine the gross
and histopathological changes to the central and peripheral nervous
system. Resmethrin was not neurotoxic when administered at 1250 mg/kg
for 32 weeks, 5000 mg/kg for 30 days, or 12 640 mg/kg for 7 days (Cox
et al., 1979b; Schwartz et al., 1979a,b).
In order to better characterize the behavioural effects of
pyrethroid insecticides, comparisons were made of the effects of
cismethrin and deltamethrin exposure on motor activity and the
acoustic startle response in male Long-Evans rats (Crofton & Reiter,
1984). Acute dose effect, acute time course, and 30-day
repeated-exposure determinations of 1 h motor activity were made using
figure-eight mazes. The acoustic startle response to a 13-kHz, 120-dB,
40-msecond tone was measured at each of three background white noise
levels (50, 65, and 80 dB). Deltamethrin (0, 2, 4, 6, or 8 mg/kg) or
cismethrin (0, 6, 12, 18, or 24 mg/kg) was administered orally in
0.2 ml corn oil/kg. Both compounds produced similar dose-dependent
decreases in motor activity. The time course of onset and recovery for
this decreased activity was rapid (1-4 h). No cumulative effects on
motor activity were found of a 30-day exposure to 2 mg deltamethrin/kg
per day or 6 mg cismethrin/kg per day. The effects of cismethrin and
deltamethrin on the acoustic startle response differed. Deltamethrin
produced a dose-dependent decrease in amplitude and an increase in
latency, and cismethrin produced an increase in amplitude and no
change in latency. The differential effects of cismethrin (Type I
pyrethroids) and deltamethrin (Type II pyrethroids) on the acoustic
startle response may be related to the contrasting effects previously
shown with neurophysiological and/or neurochemical techniques (see
Annex).
The isolated rat neurohypophysis, which shows a calcium-dependent
hormone release when depolarized in vitro, was used as a model
system to investigate the effects of the pyrethroids deltamethrin and
resmethrin on mammalian nervous tissue (Dyball, 1982). Both compounds
inhibited neurohypophysial hormone release in response to electrical
stimulation, deltamethrin being more potent than resmethrin.
Deltamethrin reduced the hormone content of the neurohypophysis.
Resmethrin did not reduce stored hormone significantly and its effects
on release were dose dependent. They could be mimicked by raising the
Na+ of the medium, but not by lowering the Ca2+. Resmethrin did not
have any effects on the release of hormone following depolarization of
the tissue with a raised K+. The results are consistent with the
suggestion that the compounds do not act on the potential-dependent
secretion process but rather on the mechanism linking depolarization
of the secretory terminals with the arrival of action potentials,
possibly by interfering with sodium-channel activation and
inactivation.
The neurological effects of four synthetic pyrethroids resmethrin,
permethrin, cypermethrin, and deltamethrin were investigated in the
rat to establish whether there is a correlation between the
clinical-functional status of the animal and peripheral nerve damage,
as measured biochemically (Rose & Dewar, 1983). Neuromuscular
dysfunction was assessed by means of the inclined plane test and
peripheral nerve damage by reference to ß-glucuronidase and
ß-galactosidase activity increases in nerve tissue homogenates from
treated and control animals. A transient functional impairment was
found in animals treated with any one of the four pyrethroids tested
and, in all cases, this was maximal at the end of the 7-day dosing
regimen (resmethrin doses of 500-2000 mg/kg per day). Significant
increases in ß-glucuronidase and ß-galactosidase were found, 3-4 weeks
after the start of dosing in the distal portion of the
sciatic/posterior tibial nerves of animals treated with permethrin,
cypermethrin, or deltamethrin; however, no changes were found in
resmethrin-treated animals. Thus, it is concluded that there is no
direct correlation between the time-course of the neuromuscular
dysfunction and the neurobiochemical changes. This suggests that these
pyrethroids have at least two distinct actions, a short-term
pharmacological effect and, at near-lethal dose levels, a more chronic
neurotoxic effect that results in sparse axonal nerve damage.
Resmethrin (30 µmol/litre)-induced release of transmitters was not
affected by manipulation of the Na+ current with either choline or
tetrodotoxin agents, which readily reversed the effects of
veratridine, deltamethrin, and cypermethrin (Doherty et al., 1986).
Resmethrin (I50: 2.2 µmol/litre) inhibited the ATP-dependent uptake
of Ca2+ but deltamethrin and cypermethrin were much less effective.
Resmethrin also displaced Ca2+ from crude synaptosomal membranes. The
release-promoting effects of resmethrin in rat brain in vitro are
better explained by its effects on Ca2+ rather than by a specific
effect on the Na+ channel. In contrast, deltamethrin and
cypermethrin promote transmitter release by a Na+ dependent process.
Glickman & Casida (1982) discussed species and structural
variations affecting pyrethroid neurotoxicity. They concluded that the
mammalian nervous system, or at least the brain, appears to lack sites
sensitive to bioresmethrin and to a lesser extent to [1R,
trans]-permethrin, yet small structural changes restore the toxicity
(e.g., [1R,trans]-ethano-resmethrin and [1R, cis]-resmethrin. The
authors reported that birds and mammals in general respond to cis-
but not to trans-resmethrin in contrast to insects, crustaceans, and
fish, which are highly sensitive to both isomers. Furthermore, common
green lacewing larvae are very tolerant to pyrethroids, suggesting
possible involvement of nerve insensitivity in addition to
detoxification in this species. These examples of insensitivity may be
associated with modified sites of action or perhaps an increased
stabilization of nerve membranes making them more resistant to
pyrethroid-induced excitation.
7.9 Mechanism of Toxicity (Mode of Action)
Cismethrin is toxic for both insects and mammals. Bioresmethrin is
about 50 times less toxic than cismethrin and produces few symptoms in
mice and rats, even at extremely high doses. The poisoning syndrome of
cismethrin and bioresmethrin was characterized by whole body tremors
(T-syndrome) and both compounds were therefore classified as Type I
pyrethroids (Verschoyle & Aldridge, 1980; Lawrence & Casida, 1982,
Annex).
Tremors started when the concentrations of cismethrin and
bioresmethrin in the brain were 0.5-1 mg/kg and 4-5 mg/kg,
respectively. At death, brain levels of cismethrin were 3.9-5.1 mg/kg,
and those of bioresmethrin were 23-35 mg/kg. As liver microsomal
esterases hydrolysed bioresmethrin 10 times more rapidly than
cismethrin, the lower toxicity of bioresmethrin was partly attributed
to its faster metabolism and intrinsically lower toxicity at the
critical site of action in the nervous system (White et al., 1976).
Cismethrin given intravenously produced repetitive activity, after
external stimulation, in the spinal cord of rabbit (Carlton, 1977). As
gamma-aminobutyric acid (GABA) blockers, such as bicuculline and
picrotoxin, produced convulsions similar to those of cismethrin, the
effects of the compounds on dorsal root potentials were examined in
the rat. Bicuculline reduced the amplitude of the potential to 67% of
the control values, but cismethrin enhanced the potential to 142% of
the control values. The convulsions associated with cismethrin
poisoning, therefore, are not produced by an antagonism of
GABA-mediated inhibitory transmission (Smith, 1980).
Administration of cismethrin into the lateral ventricle of the
brain or into the spinal cord causes signs similar to those observed
after intravenous administration. The onset of tremors after
intraventricular administration of cismethrin was delayed for about 15
min while, after intraspinal injection, the symptoms occurred within
2-5 min, indicating that cismethrin may act directly on the spinal
cord rather than in the brain (Gray et al., 1980b).
After intravenous administration of [14C]-alcohol-labelled
cismethrin or bioresmethrin to rats, the parent pyrethroid was rapidly
cleared from the blood and liver, and both isomers rapidly entered the
central nervous system (CNS) reaching peak concentrations within 2-5
min. Cismethrin concentrations in the brain exceeding 3.5 nmol/g were
associated only with animals showing tremors. These levels of
cismethrin were maintained for up to 30 min, but bioresmethrin was
depleted more rapidly, possibly due to brain metabolism. It is
concluded that the low toxicity of bioresmethrin is possibly because
of its inability to interact with the site of action in the CNS and to
its rapid metabolism in the liver (Gray et al., 1980a).
Cismethrin produced repetitive firing in the flight muscles and
uncoupling of motor unit activity in the housefly (Miller & Adams,
1977), and repetitive firing in a cercal sensory nerve of the
cockroach (Gammon et al., 1981). On the basis of the
electrophysiological effects and symptomatology in insects as well as
in mammals, cismethrin is classified as a Type I pyrethroid,
characterized by a mainly peripheral nervous system action (Gammon et
al., 1981).
7.10 Potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin, and/or piperonyl butoxide, alone or in
combinations, at doses approximating the acute intraperitoneal LD50
value. No potentiation of the acute toxicity was observed in this
study. In all cases with bioresmethrin combinations, the observed
LD50 values were equal to, or exceeded, the expected value (Wallwork
& Malone, 1971).
8. EFFECTS ON MAN
Although the resmethrins have been used for many years, no data
have been reported on their toxicity for human beings.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Health Risks
Resmethrin, consisting of four stereo-isomers, is an effective
insecticide mainly used to control household insects and other public
health insects, with applications in the food handling and storage
areas.
Bioresmethrin and cismethrin are composed of a selected isomer(s).
As with resmethrin, bioresmethrin is used to control household insects
and other public health insects. In addition, bioresmethrin is used as
a post-harvest insecticide to control stored grain pest. Human
exposure to the resmethrins will be mainly via inhalation, when the
formulations are sprayed in the form of a mist, though the use in food
handling and storage areas as well as post-harvest treatment may
result in dietary residues. Air levels following conventional
household aerosol spraying with the resmethrins are not expected to
exceed 0.5 mg/ms.
The only significant potential dietary exposure will result from
the use of resmethrin on stored grain. Residues of up to 4 mg/kg might
be present in grain, but this would be reduced to zero in white bread.
However, reduced residues may be present in wholemeal bread. A food
additive tolerance has been established by the US Environmental
Protection Agency (US EPA) permitting resmethrin residues of up to
3 mg/kg (3 ppm), in or on food commodities, resulting from use in food
handling areas. An ADI was established by the US EPA at 0.1250 mg/kg
body weight per day, based on no-observed-adverse-effect levels in
long-term toxicity studies. No data are available on occupational
exposure to the resmethrins. Although the resmethrins have been used
for many years, no data have been reported concerning their toxicity
for human beings. Thus, to determine the potential toxicity for human
beings, extrapolation from animal data and in vitro studies has been
used.
The result of short-term studies on experimental animals suggest
that resmethrins are weakly toxic when administered by various routes
of exposure (oral LD50 of resmethrin: 690 mg/kg (mouse) - >
5000 mg/kg (rat); those of bioresmethrin: 225 mg/kg (rabbit),
480-10 000 mg/kg (mouse), 8800 mg/kg (rats)). Cismethrin showed
moderate toxicity for the mouse (oral LD50 152-160 mg/kg). The acute
toxicity of resmethrin metabolites were in the same range in the rat,
but somewhat more toxic in the mouse.
While technical grade resmethrin is a slight skin irritant, it is
not a sensitizer.
Resmethrins are not mutagenic in a variety of test systems,
including gene mutations, DNA damage and DNA repair, and chromosomal
effects.
Resmethrin was not carcinogenic for mice or rats, when fed at
dietary levels of up to 1000 mg/kg for 85 weeks and 5000 mg/kg for 112
weeks, respectively.
Resmethrin was not teratogenic in the rat, mouse, or rabbit, up to
dose levels of 100 mg/kg body weight. A level of 40 mg/kg body weight
appeared to be the no-observed-adverse-effect level for fetotoxicity
in the rat.
Resmethrins at near lethal doses are likely to cause
hyperactivity, tremors, and convulsions and have been classified as
Type I pyrethroids.
For resmethrin, a no-observed-adverse-effect level was established
in a 90-day rat study to be 66-67 mg/kg body weight per day, whereas,
in another 2-year rat study, the lowest effect level appeared to be
500 mg/kg diet, corresponding to 25 mg/kg body weight per day. In a
6-month feeding study on dogs, the no-observed-adverse-effect level
was 10 mg/kg body weight per day.
In a 90-day inhalation study on rats, a no-observed-adverse-effect
level was established of 0.1 g/m3.
The no-observed-adverse-effect level for bioresmethrin in a 9-day
feeding study on the rat was 33-36 mg/kg body weight per day; and in a
90-day study on dogs, it was 80 mg/kg body weight per day.
In a 24-week feeding study on rats, the no-observed-adverse-effect
level for [1R, trans, cis]-resmethrin was 1500 mg/kg diet
corresponding to 75 mg/kg body weight.
9.2 Effects on the Environment
Resmethrins are used mainly indoors for the control of household
insects and also for stored grain protection. They are also used in
greenhouses and can be used outdoors for mosquito control. However,
under outdoor conditions, rapid photodegradation and microbial
degradation in the soil ensure that residues will not persist to any
extent in the environment.
In laboratory studies, resmethrins have been shown to be very
toxic for fish (96-h LC50 values: 0.3-5.5 µg/litre) but less toxic
for Daphnia and aquatic insect larvae. However, under field
conditions the low water solubility of the resmethrins and their ready
degradation greatly alleviate the effects that might be predicted from
the laboratory studies. The toxicity of resmethrins for birds is low
(LD50 > 5000 mg/kg) and they do not produce any effects on avian
reproduction.
10. CONCLUSIONS
General population: Under recommended conditions of household
and other public health use, the exposure of the general population to
resmethrins is negligible and is unlikely to present a hazard. Under
recommended conditions of use in food handling and storage areas as
well as in post-harvest treatment, the exposure of the general
population to resmethrins in the diet is unlikely to exceed the ADI
established by the US EPA.
Occupational exposure: With reasonable work practices, hygienic
measures, and safety precautions, the use of resmethrins is unlikely
to present a hazard to those occupationally exposed to it.
Environment: With recommended application rates, it is unlikely
that resmethrins or their degradation products will attain levels of
environmental significance. In spite of the fact that resmethrins are
highly toxic for fish, this is only likely to cause a problem in the
case of spillage or over-spraying.
11. RECOMMENDATIONS
- In order to fully assess the potential dietary exposure from
current uses of resmethrins, it is suggested that the degradation
pathway on stored grain be studied, and the terminal residues on
grain and bread be defined.
- It is considered that resmethrin, despite its high octanol-water
partition coefficient, is very unlikely to bioaccumulate in
non-target species, because of its ready degradation. However,
experimental confirmation of this in fish might be useful.
- A further multigeneration reproduction study to define a
no-observed-adverse-effect level should be considered.
- Over many years of use, no adverse effects have been reported as a
result of human exposure to resmethrins, but it is still necessary
to maintain observations on human exposure.
- The labelling of resmethrins for household use should include
adequate instructions for use and storage and, where appropriate,
a warning of flammability.
- Efforts should be made to obtain a more precise estimate of the
total global usage of resmethrins.
12. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The Joint FAO/WHO Expert Committee on Pesticide Residues (JMPR)
discussed and evaluated bioresmethrin at its meetings in 1975 and 1976
(FAO/WHO, 1976, 1977). An acceptable daily intake (ADI) has not been
established.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified bioresmethrin as a technical
product unlikely to present an acute hazard in normal use, and
resmethrin and cismethrin as slightly hazardous (WHO, 1986). A Data
Sheet on bioresmethrin (No. 34) has been issued (WHO/FAO, 1978).
REFERENCES
ABBOTT, D.D. (1971) Total degradation products of SBP-1382. Acute
oral LD50/mice, 6 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
ABE, Y., TSUDA, K., & FUJITA, Y. (1972) Studies on pyrethroidal
compounds. Part III. Photostability of pyrethroidal compounds.
Botyu-Kagaku, 37: 102-110.
ALLEN, J.A. & PROUDLOCK, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after
exposure to bioresmethrin, Huntingdon, United Kingdom,
Huntingdon Research Centre, 18 pp (Report No.
RSL-BRM-698/851420/A) (Unpublished proprietary data supplied by
Roussel Uclaf).
ALLEN, J.A., BROOKER, P.C., & HOWELL, A. (1986) Bioresmethrin
metaphase chromosome analysis of human lymphocytes cultures
in vitro, Huntingdon, United Kingdom, Huntingdon Research Centre,
15 pp (Report No. RSL-BRM-697/851370/A) (Unpublished proprietary
data supplied by Roussel Uclaf).
ARDLEY J.H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat (Unpublished report of Wellcome
Australia Ltd).
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and
high-performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Battelle Institute.
BECCI, P.J., KNICKERBOCKER, M., & PARENT, R.A. (1979) Teratological
evaluation of SBP-1382 in albino rabbits, Waverly, New York,
Food and Drug Research Laboratories, 17 pp (Report No. 6288)
(Unpublished proprietary data supplied by Roussel Uclaf).
BENGSTON, M., COOPER, L.M., & GRANT-TAYLOR, F.J. (1975) A comparison
of bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in wheat.
Queensland J. Agric. anim. Sci., 32: 51-78.
BUICK, A.R. & FLANAGAN, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes (Report No. HEGH 73/1) (Unpublished
data of Wellcome Foundation Ltd).
BURT, M.E. (1983a) Resmethrin residues in tomato and cucumber, New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
BURT, M.E. (1983b) Resmethrin residues in lettuce (greenhouse), New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
CARLTON, M. (1977) Some effects of cismethrin on the rabbit nervous
system. Pestic. Sci., 8: 700-712.
CAVE, S.L. (1981) Simultaneous estimation of bioresmethrin and
piperonylbutoxide by gas-liquid chromatography with
chemical-ionization mass spectrometry. Pestic. Sci.,
12: 156-160.
CHAPMAN, R.A. & HARRIS, C.R. (1978) Extraction and liquid-solid
chromatography cleanup procedures for the direct analysis of four
pyrethroid insecticides in crops by gas-liquid chromatography.
J. Chromatogr., 166: 513-518.
CHESHER, B.C. & MALONE, J.C. (1970a) Acute toxicity tests with NRDC
107 in hens (Report No. B 236-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970b) Sensitisation study with NRDC
107 on guinea pigs (Report B 198-70) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits (Report No. B 217-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1971a) Toxicity to dogs of NRDC 107
(continuation) (Report No. B 27-71) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER B.C. & MALONE J.C. (1971b) Acute toxicity of NRDC 107 by the
intravenous route. (Report No. B 329-71) (Unpublished data of
Wellcome Foundation Ltd).
CHRISTOPHER, R.J., MUNGER, C.E., & WAYNE IRVIE, G. (1985) Distribution
and depletion of [14C]-resmethrin isomers administered orally to
laying hens. Pestic. Sci., 16: 378-382.
COOMBS, D.W., HARDY, C.J., CLARK, G.C., STREET, A.E., GOPINATH, C.,
LEWIS, D.J., & RAO, R.S. (1985) Resmethrin 90-day inhalation
toxicity study in the rat, Huntingdon, United Kingdom,
Huntingdon Research Centre, 80 pp (Report No. SBP 6/84997)
(Unpublished proprietary data supplied by Roussel Uclaf).
COX, G.E., KNICKERBOCKER, M., & PARENT, R.A. (1979a) Evaluation of
dietary administration of SBP-1382 in CD-1 outbred albino mice
over an 85-week period, Waverly, New York, Food and Drug
Research Laboratories, 52 pp (Report No. 5270) (Unpublished
proprietary data supplied by Roussel Uclaf).
COX, G.E., BECCI, P.J., & PARENT, R.A. (1979b) Supplementary report
for evaluation of the neurotoxic effects of SBP-1382 in albino
rats - phase I, Waverly, New York, Food and Drug Research
Laboratories, 7 pp (Report No. 6362) (Unpublished proprietary data
supplied by Roussel Uclaf).
CROFTON, K.M. & REITER, L W. (1984) Effects of two pyrethroid
insecticides on motor activity and the acoustic startle response
in the rat. Toxicol. appl. Pharmacol., 75: 318-328.
DANLIKER, W.B., HICKS, A.N., LEVISON, S.A., STEWART, K., & BROWN, R.J.
(1979) Effects of pesticides on immume response, Washington, DC,
US Environmental Protection Agency (EPA-600/1-79-039-PB
80-1308 34).
DESMARCHELIER, J.M. (1980) Comparative study of analytical methods for
bioresmethrin, phenothrin, d-phenothrin, pyrethrum I, carbaryl,
fenitrothion, methacrifos, pirimifos-methyl and dichlorfos on
various grains. J. Pestic. Sci., 5: 521-532.
DEVAUX, Ph. & BOLLA, P. (1986) Bioresmethrin n-octanol/water
partition coefficient and water solubility, Romainville, France,
Roussel Uclaf, 4 pp (Report No. RU-BRM-142.06.86. STE/A)
(Unpublished proprietary data).
DEVAUX, Ph. & TILLIER, C. (1986) Vapor pressure of bioallethrin and
bioresmethrin, Romainville, France, Roussel Uclaf, 8 pp (Report
No. RU-BRM/BA-150.06.86.STE/A) (Unpublished proprietary data).
DOHERTY, J.D., LAUTER, C.J., & SALEM, N., Jr (1986) Synaptic effects
on the synthetic pyrethroid resmethrin in rat brain in vitro.
Comp. Biochem. Physiol., C84: 373-379.
DYBALL, R.E.J. (1982) Inhibition by decamethrin and resmethrin of
hormone release from the isolated rat neurohypophysis: A model
mammalian neurosecretory system. Pestic. Biochem. Physiol.,
17: 42-47.
ELLIOT, M. (1976) Properties and applications of pyrethroids.
Environ. Health Perspect., 14: 3-13.
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series, No. 42).
ELLIOTT, M., FARNMAN, A.W., JONES. N.F., NEEDHAM, P.H., & PEARSON,
B.C. (1967) 5-Benzyl-3-furylmethyl chrysanthemate: a new potent
insecticide. Nature (Lond.), 213: 493-494.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome,
11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
FAO/WHO (1976) Bioresmethrin. In: 1975 Evaluation of some pesticide
residues in food, Geneva, World Health Organization, pp. 17-40
(WHO Pesticide Residues Series, No. 5).
FAO/WHO (1977) Bioresmethrin. In: 1976 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of the
United Nations, pp. 55-78 (AGP: 1976/M/14).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
GAINES, T.B. & LINDER, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. appl. Toxicol., 7: 299-308.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular
junction. Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol.,
15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. appl. Pharmacol.,
66: 290-296.
GARRET, N.E., FRANK STACK, H., & WATERS, D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res.,
168: 301-325.
GEPHART, L.A., JOHNSON, W.D., BECCI, P.J., & PARENT, R.A. (1980)
180-day subchronic oral dosing study with resmethrin in Beagle
dogs, Waverly, New York, Food and Drug Research Laboratories,
86 pp (Report No. 6289) (Unpublished proprietary data supplied by
Roussel Uclaf).
GLICKMAN, A.H. & CASIDA, J.E., (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4: 793-799.
GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished data of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
GLOMOT, R. & CHEVALLIER, B. (1969) Etude de la toxicité aiguë du
RU11484 (NRDC 107) Romainville, France, Roussel Uclaf (Rapport
Toxico 11386) (Unpublished data).
GRAY, A.J., CONNORS, T.A., HOELLINGER, H., & NGUYEN-HOANG-NAM (1980a)
Relationship between the pharmacokinetics of intravenous
cismethrin and bioresmethrin and their mammalian toxicity.
Pestic. Biochem. Physiol., 13: 281-293.
GRAY, A.J., CONNORS, T.A., & RICKARD, J. (1980b) Mechanism of
mammalian toxicity of the pyrethroids. In: Littaner, V.Z., Dudai,
Y., Silman, I. Teichberg, V.I., & Vogel, Z., ed.
Neurotransmitters and their receptors, New York, John Wiley &
Sons Ltd, pp. 565-568.
GREEN, W.M. (1977) Acute dermal LD50 toxicity study on technical
resmethrin, Moorestown, New Jersey, Bio-Toxicology Laboratory,
8 pp (Unpublished proprietary data supplied by Roussel Uclaf).
HESS, F.G., THOMPSON, S.W., & BECCI, P.J. (1982) A lifetime
evaluation of the dietary administration of SBP-1382 to Wistar
albino rats/amendment, Waverly, New York, Food and Drug Research
Laboratory, 28 pp (Supplemental report No. 5271) (Unpublished
proprietary data supplied by Roussel Uclaf).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Bureau of Sport
Fishery and Wildlife, pp. 1-61 (Report No. 191).
HOELLINGER, H., SONNIER, M., GRAY, A.J., CONNORS, T.A., PICHON, J., &
NGUYEN-HOANG-NAM (1985) In vitro covalent binding of cismethrin,
bioresmethrin and their common alcohol to hepatic proteins.
Toxicol. appl. Pharmacol., 77: 11-18.
HOLMSTEAD, R.L., CASIDA, J.E., & RUZO, L.O. (1977) Photochemical
reactions of pyrethroid insecticides. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society
(ACS Symposium Series, No. 42).
JACKSON, G.C. & HARDY, C.J. (1984) SBP-1382 acute inhalation toxicity
in rats 4-hour exposure, Huntingdon, United Kingdom, Huntingdon
Research Centre, 24 pp (Report No. SBP 4a/831042) (Unpublished
proprietary data supplied by Roussel Uclaf).
KAGAN, U.S., PANSHINA, T.N. & SASINOVITCH, L.M. (1986) [Biochemical
effects of synthetic pyrethroid toxic action.] Gig. i Sanit.,
1: 7 (in Russian).
KAUFMAN, D.D. (1986) Degradation and movement of cis- and
trans-resmethrin in soil, Beltsville, US Department of
Agriculture, 36 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
KHOLMATOVA, M. (1984) Hygiene and toxicology of Dravin 755 and
Isotrine new pesticides used in agriculture, Alma Ata (Résumé of
doctorate thesis).
KNICKERBOCKER, M., BECCI, P.J., COX, G.E., & PARENT, R.A. (1980)
A lifetime evaluation of the dietary administration of SBP-1382
to Wistar albino rats, Waverly, New York, Food and Drug Research
Laboratories, 110 pp (Report No. 5271) (Unpublished proprietary
data supplied by Roussel Uclaf).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor and
Francis Ltd, 440 pp.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem.
Physiol., 20: 203-216.
MACHI, R.A., KAM, C., GALLO, M.A., STEVENS, K.R., & GAGLIARDI, J.J.
(1979) Teratological evaluation of SBP-1382 technical in the
albino rat, Florham Park, New Jersey, Booz, Allen & Hamilton,
Inc., 44 pp (Report No. 2054-066) (Unpublished proprietary data
supplied by Roussel Uclaf).
MACKO, J.A., HARVEY, J.G., POPE, C.R., & MCCREESH, A.H. (1979) Hazard
evaluation of aerosol formulations containing the synthetic
pyrethroid insecticide resmethrin, (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(SBP-1382), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency, 38 pp (Report No. 75-51-0848-79).
MALONE, J.C. & CHESHER, B.C. (1970) Toxicity to dogs of NRDC 107,
Berkhamsted, Wellcome Foundation Ltd (Report No. B 204-70)
(Unpublished data).
MARKING, L.L. & MAUCK, W.L. (1975) Toxicity of paired mixtures of
candidate forest insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 13: 518-523.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, 461 pp.
MAUCK, W.L., OLSON, L.E., & MARKING, L.L. (1976) Toxicity of natural
pyrethroids and five pyrethroids to fish. Arch. environ. Contam.
Toxicol., 4: 18-29.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemical handbook, Section C: Pesticide dictionary,
Willoughby, Meister Publishing Co., pp. C205-C206.
MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 98-115 (ACS Symposium Series, No. 42).
MIYAMOTO, J. (1975-76) Terminal residues of bioresmethrin.
Submission to IUPAC Terminal Residue Commission, Madrid, September
1975, Leverkusen, September 1976.
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4
September, 1982, Oxford, Pergamon Press (in 4 volumes).
MIYAMOTO, J., NISHIDA, T., & UEDA, K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furylmethyl d1-trans-chrysanthemate in the
rat. Pestic. Biochem. Physiol., 1: 293-306.
MUMMA, R.O. (1985) Persistence of metabolism of 14C-resmethrin on
greenhouse grown tomato fruit, lettuce and field grown wheat,
Part III, Pennsylvania State University, 33 pp Unpublished
proprietary data supplied by Roussel Uclaf).
MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem.,
36: 2203-2211.
NIOSH (1983) In: Takten, R.L. & Lewis, R.J., Jr, ed. Registry of
toxic effects of chemical substances, 1981-82 edition,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 3057 pp (in 3 volumes).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitai-Kagaku, 4: 31-46 (in Japanese).
NISHIUCHI, Y. (1982) [Toxicity of pesticides to fish.]
Kongetsuno-Noyaku, 26: 105-110 (in Japanese).
NOEL, P.R.B., RIVETT, K.F., CHESTERMAN, H., STREET, A.E., & SPICER,
E.J.F. (1971) Oral toxicity study in Beagle dogs (repeated dosage
for 3 months), 76 pp (Report No. 3900/71/58) (Unpublished data
of Huntingdon Research Centre, United Kingdom).
NORWOOD, T.E. (1986) Preliminary statistical report on assessment of
the effects of SBP-1382 40MF (resmethrin) and Scourge (18%
resmethrin plus 54% piperonyl butoxide) on non target, aquatic
organisms when used for adult mosquito control: results of a
field validation study in fresh water ponds in Minnesota, Summer
1984, North Oaks, Minnesota, Mosquito Control Technology, 20 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
PIERCE, R.H. (1986) Acute toxicity of Scourge on select estuarine
organisms during field applications, Sarasota, Florida, Mote
Marine Laboratory, 16 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C., HAUTEFEUILLE,
A., & BARTSCH, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
PUCHALSKI, R. (1983) Water solubility and octanol/water partition
coefficients of SBP-1382, Lyndhurst, New Jersey, Penick
Corporation, 5 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985a) Acute oral LD50 study of
D-L-cis-trans-chrysanthemic acid in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 10 pp
(Report No. 8350A) (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985b) Acute oral LD50 study of
D-L-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350B) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985c) Acute oral LD50 study of
D-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350C) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985d) Acute oral LD50 study of BFCA
99.6% Sprague-Dawley rats, Waverly, New York, Food and Drug
Research Laboratories, 10 pp (Report No. 8725A) (Unpublished
proprietary data supplied by Roussel Uclaf).
RIDLEN, R.L., CHRISTOPHER, R.J., WAYNE IRVIE, G., BEIER, R.C., & CAMP,
B.J. (1984) Distribution and metabolism of cis and
trans-resmethrin in lactating Jersey cows. J. agric. food
Chem., 32: 1211-1217.
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O.
(1985a) The effects of dietary inclusion of resmethrin on
reproduction in the bobwhite quail, Huntingdon, United Kingdom,
Huntingdon Research Centre, 34 pp (Report No. SBP 8BT/85957)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O,
(1985b) The effects of dietary inclusion of resmethrin on
reproduction in the mallard duck, Huntingdon, United Kingdom,
Huntingdon Research Centre, 28 pp (Report No. SBP 7BT/85550)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROPER, E.M. & WRIGHT, G.C. (1984) Sampling efficiency of five solid
sorbents for trapping airborne pesticides. Bull. environ. Contam.
Toxicol., 33: 476-483.
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four synthetic
pyrethroids fail to show any correlation between neuromuscular
dysfunction and neurobiochemical abnormalities in rats. Arch.
Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979a) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase I, Waverly,
New York, Food and Drug Research Laboratories, 18 pp (Report
No. 6067) (Unpublished proprietary data supplied by Roussel
Uclaf).
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979b) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase II,
Waverly, New York, Food and Drug Research Laboratories, 17 pp
(Report No. 6068) (Unpublished proprietary data supplied by
Roussel Uclaf).
SCHWARTZ, C.S., GEPHART, L., BECCI, P.J., & PARENT R.A. (1979c) The
evaluation of the effects of SBP-1382 following dietary
administration through three generations in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 70 pp
(Report No. 5739) (Unpublished proprietary data supplied by
Roussel Uclaf).
SEABER, J.A. (1985) Delayed contact hypersensitivity in the guinea
pig with SBP-1382 insecticide, Huntingdon, United Kingdom,
Huntingdon Research Centre, 14 pp (Report No. 84936D/SBP 10/ss)
(Unpublished proprietary data supplied by Roussel Uclaf).
SIMONAITIS, R.A. & CAIL, R.S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour, and wheat.
J. Assoc. Off. Anal. Chem., 58: 1032-1036.
SJOGREN, R.D. (1985) Assessment of the effects of SBP-1382 40MF
(resmethrin) and Scourge (18% resmethrin plus 54% piperonyl
butoxide) on non target, aquatic organisms when used for adult
mosquito control: results of a field validation study in fresh
water ponds in Minnesota, Summer 1984, North Oaks, Minnesota,
Mosquito Control Technology, 105 pp (Unpublished proprietary data
supplied by Roussel Uclaf).
SMITH, P.R. (1980) The effect of cismethrin on the rat dorsal root
potentials. Eur. J. Pharmacol., 66: 125-128.
STAUFFER (1984) Ethyl chrysanthemate/Product safety information,
Westport, USA, Stauffer Chemical Co., 5 pp.
SWENTZEL, K.L., ANGERHOFER, R.A., & HAIGHT, E.A. (1977) Toxicological
evaluation of pyrethroid insecticide (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(resmethrin), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency (Report No. 51-0830-77).
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1974) Photodecomposition of
resmethrin and related pyrethroids. J. agric. food Chem.,
22: 212-220.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pestic. Biochem.
Physiol., 5: 280-294.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975b) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food
Chem., 23: 106-115.
UNO, M., OKADA, T., OHMAE, T., & TANIGAWA, K. (1982) [Determination of
pyrethroid insecticides by dual-wavelength densitometry.]
Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
US ENVIRONMENTAL PROTECTION AGENCY (1983) Tolerances for pesticides in
food administered by EPA: Resmethrin. Fed. Reg.,
48: 36246-36247.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973)
DDT-likeaction of allethrin in the sensory nervous system of
Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B A., & AKKERMANS, L.M.A. (1979) Effect
of insecticides on the sensory nervous system. In: Narahashi, T.,
ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 182-210.
VANNIER, B. & FOURNEX, R. (1986) Bioresmethrin. Detection of a
mutagenic potency (micronucleus test in the mouse), Romainville,
France, Roussel Uclaf, 16 pp (Report No. RU-BRM-85612-BB/178/A)
(Unpublished proprietary data).
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol.,
45: 325-329.
VERSCHOYLE, R.D. & BARNES, J.M. (1972) Toxicity of natural and
synthetic pyrethrins in rats. Pestic. Biochem. Physiol.,
2(3): 308-311.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature and structure-dependent interaction
of pyrethroids with the sodium channels in frog node of Ranvier.
Biochim. Biophys. Acta, 728: 73-82.
VOLKOV, U.P., STRELETS, I.P., & TIULENEV, K.L. (1979) [Progress in
the field of pyrethroids and their synergists. Collection of
articles on household chemistry.] Moscow, Volume 7, p. 125
(in Russian).
WALDRON, M.M. (1969) Foetal toxicity study -- 95 H 56 -- given orally
in the rabbit (Report Path 51) (Unpublished data of Wellcome
Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide (Report
No. B 47-71) (Unpublished data of Wellcome Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1972) Bioresmethrin. Preliminary 24
hour rat inhalation study (Report No. B 290-72) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CHESHER, B.C., & MALONE J.C. (1970) Toxicity of NRDC
107 to laboratory animals (Report No. B 199-70) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study (Report No. B 199-71) (Unpublished
data of Wellcome Foundation Ltd).
WATSON, S.C. (1984) Photolysis study of SBP-1382 resmethrin (final
report), Columbus, Ohio, Battelle Columbus Laboratory, 10 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
WEEKS, M.H., LELAND, T.M., BOLDT, R.E., MELLICK, P.W., & STEINBERG, M.
(1972) Toxicological evaluation of pyrethroid insecticide
(5-benzyl-3-furyl) methyl-2,2-dimethyl-3-(2-methyl-propenyl)
cyclopropanecarboxylate (SBP-1382), Aberdeen Proving Ground, US
Army Environmental Hygiene Agency, 44 pp (Special study
No. 51-127-71/72).
WHITE, I.N.H., VERSCHOYLE, R.D., MORADIAN, M.H., & BARNES, J.M. (1976)
The relationship between brain levels of cismethrin and
bioresmethrin in female rats and neurotoxic effects. Pestic.
Biochem. Physiol., 6: 491-500.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-1987, Geneva, World Health
Organization, p. 38 (VBC/86.1, Rev. 1).
WHO/FAO (1978) Data sheet on pesticides No. 34: Bioresmethrin,
Geneva, World Health Organization (WHO/VBC/DS/78. 34).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, 485 pp.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YOSHIOKA, H. (1980) [Chemistry and action of the recent synthetic
pyrethroids and their stereoisomers.] J. synth. org. Chem.
Japan, 38: 1151-1162 (in Japanese).
APPENDIX
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs (Xenopus laevis; Rana temporaria, and Rana
esculenta), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches by Gammon et al. (1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982; Gammon
& Casida, 1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the insecticide
on neurotransmitter release or on the sensitivity of the subsynaptic
membrane or the muscle fibre membrane. Presynaptic repetitive firing
was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres,
the pyrethroid-induced repetitive activity increases dramatically as
the temperature is lowered, and a decrease of 5°C in temperature may
cause a more than 3-fold increase in the number of repetitive impulses
per train. This effect is easily reversed by raising the temperature.
The origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that underlies
the generation and conduction of each nerve impulse. The transitional
state of the sodium channel is controlled by 2 separately acting
gating mechanisms, referred to as the activation gate and the
inactivation gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the activation
gate and the slow closing of the inactivation gate proceed normally.
However, once the sodium channel is open, the activation gate is
restrained in the open position by the pyrethroid molecule. While all
pyrethroids have essentially the same basic mechanism of action, the
rate of relaxation differs substantially for the various pyrethroids
(Flannigan & Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more
than 100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the Type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing afterpotentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral-line organ in the form of
long-lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train increase
markedly on lowering the temperature. Cypermethrin does not cause
repetitive activity in myelinated nerve fibres. Instead, this
pyrethroid causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of the nerve
membrane as a result of the summation of depolarizing after-potentials
during train stimulation (Vijverberg & Van den Bercken, 1979;
Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a
long-lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]-t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an absolute
correlation, without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, [1R,cis]-cypermethrin,
[1R,trans]-cypermethrin, and [2S, alphaS]-fenvalerate were
inhibitors, but their non-toxic stereoisomers were not; non-cyano
pyrethroids were much less potent or were inactive (Lawrence & Casida,
1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The
[3H]-dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of pyrethroids are
mediated, at least in part, through an interaction with a
GABA-regulated chloride ionophore-associated binding site. Moreover,
studies with [3H]-Ro 5-4864 support this hypothesis and, in addition,
indicate that the pyrethroid-binding site may be very closely related
to the convulsant benzodiazepine site of action (Lawrence et al.,
1985).
The Type II pyrethroids (deltamethrin, [1R,cis]-cypermethrin and
[2S, alpha2]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two
non-insecticidal stereoisomers and Type I pyrethroids (permethrin,
resmethrin, allethrin) were inactive. Therefore, cyanophenoxybenzyl
pyrethroids appear to act on the GABA receptor-ionophore complex
(Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action
potentials without presynaptic repetitive activity. However, an
intermediate group of pyrethroids (1R-permethrin, cyphenothrin, and
fenvalerate) caused both types of effect. Thus, in muscle or nerve
membrane the pyrethroid induced repetitive activities due to a
prolongation of the sodium current. But no clear distinction was
observed between non-cyano and alpha-cyano pyrethroids (Ruigt & Van
den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is
the sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group (allethrin, d-phenothrin, permethrin, and
cismethrin) cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation. This
results in relatively short trains of repetitive nerve impulses in
sense organs, sensory (afferent) nerve fibres, and, in effect, nerve
terminals. On the other hand, the alpha-cyano pyrethroids cause a
long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin and cypermethrin, which
have identical molecular structures except for the presence of an
alpha-cyano group on the phenoxybenzyl alcohol, indicates that it is
this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids,
the ultimate lesion that causes death will depend on the animal
species, environmental conditions, and on the chemical structure and
physical characteristics of the pyrethroid molecule (Vijverberg & Van
den Bercken, 1982).
5.2 Enzymatic Systems for Biotransformation
When 14C alcohol-labelled cismethrin, bioresmethrin, and
5-benzyl-3-furylmethyl alcohol (BFA) (18) were incubated with rat
liver S-9 homogenates or microsomes, a proportion of the radioactive
compounds was covalently bound to proteins. The covalent binding was
greater with phenobarbital-pretreated rats, and dependent on a
NADPH-generating system. When a S-9 homogenate was used, the bound
compounds were twice as high for cismethrin as for bioresmethrin and
BFA. Inversely, when microsomes were used, more covalent binding
occurred with bioresmethrin and BFA than with cismethrin. The
inhibition of esterases by tetraethyl pyrophosphate (TEPP) in a S-9
homogenate did not alter the amount of covalent binding to the three
compounds, whereas malathion inhibited this binding. However,
treatment of a S-9 homogenate with piperonyl butoxide greatly reduced
covalent binding. Covalent binding was inhibited when the microsomes
were incubated with carbon monoxide or modified by thermal
denaturation. It is suggested that oxidative metabolism was
responsible for the covalent binding (Hoellinger et al., 1985).
When four resmethrin isomers ([1R,trans]-, [1S,trans]-,
[1R,cis]-, and [1S,cis]-) were incubated with mouse and rat
microsomes in 50 mmol/litre tris-HCl buffer (pH 7.4) at 37°C for 1 h,
microsomal esterases readily cleaved the trans- but not the
cis-isomers. The ester linkage also appeared to undergo oxidative
cleavage when esterase attack was minimal. Ester metabolites were
detected in significant amounts only with [1R,cis]-resmethrin, in
which case oxidation had occurred at the isobutenyl moiety, with or
without oxidation at the benzylfuryl methyl group. Most of the
in vitro metabolites were identical with those in the excreta of
rats given resmethrin orally. The preferred site of oxidation in the
isobutenyl moiety varies with the resmethrin isomer and microsomal
source. Mouse microsomes predominantly oxidized the trans-methyl
group of both [1R,trans]- and [1S,trans]-resmethrin, the
selectivity, however, being the greatest with [1S,trans]-resmethrin.
Rat microsomes were relatively nonselective in attacking the
isobutenyl methyl groups of [1R,trans]-resmethrin (Ueda et al.,
1975a).
Plasma-esterases were equally active in hydrolysing cismethrin and
bioresmethrin (3.2-3.4 nmol benzylfurylmethanol/min per g) whereas
liver microsomal esterases hydrolysed bioresmethrin 10 times more
rapidly than cismethrin (White et al., 1976).
6. EFFECTS ON THE ENVIRONMENT
Data on the acute toxicity of resmethrin for aquatic and
terrestrial non-target organisms are summarized in Tables 4 and 5,
respectively.
6.1 Aquatic Organisms
Resmethrin is highly toxic for fish, and the toxicity is
negatively correlated with temperature (Mauck et al., 1976); the LC50
value for bluegill is 2.3 times greater at 22°C than at 12°C, as shown
in Table 4. The influence of both water hardness and pH on toxicity
ranged from slight to negligible. By keeping resmethrin solution at
12°C for 1 week, the toxicity for bluegill decreased 2-fold at each pH
value as the resmethrin solution aged.
Among the 4 isomers, the 1R, cis-isomer is most toxic for fish,
followed by the 1R, trans-, 1S, cis- and 1S, trans-, as observed
in pyrethroids. Contrary to other pyrethroids, resmethrin is less
toxic for arthropods than for fish, as shown in Table 4. The 48-h
LC50 values for arthropods are in the range of 2-25 µg/litre
(Nishiuchi, 1981).
Laboratory studies have shown that resmethrin is highly toxic for
fish but, because of the low water solubility of the compound and
rapid environmental degradation (photochemical and microbial), it
would be expected that, under field conditions, the toxic impact would
be greatly reduced. Such a reduction in toxic effects has been
confirmed in field studies carried out in the USA (Sjogren, 1985;
Norwood, 1986; Pierce, 1986).
In one of these studies, ponds were sprayed with resmethrin
formulations at the expected field rate for adult mosquito control
(0.01 kg/ha). Residue analysis confirmed that resmethrin levels fall
rapidly (1.1 ppb at zero time nondetectable after 96 h). Caged fish
showed good survival (82-100% for bluegill, 73-100% for goldfish) in
resmethrin-treated ponds. However, one fish species in this study
(white sucker) was susceptible (8% survival) when piperonyl
butoxide-synergised resmethrin was used, though good survival was
noted for this species with unsynergised resmethrin. In a second
study, synergised resmethrin was applied to a mangrove-fringed pond at
2-day intervals. The first two applications were at the normal field
rate, but the third was at 10 times this rate. In this study, both
fish species used to monitor fish survival (sheepshead minnow,
fingerling snook) showed good survival (100% at the normal rate and
88% at the 10-fold rate).
Table 4. Acute toxicity of resmethrin for non-target aquatic organisms
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish
Carp (Cyprinus 48-h LC50 44 technical static 25 Nishiuchi (1982)
carpio)
Killifish adult 48-h LC50 300 technical static 25 Miyamoto (1976)
(Oryzias adult 48-h LC50 16 (+)-trans- static 25 Miyamoto (1976)
latipes) adult 48-h LC50 8 (+)-cis- static 25 Miyamoto (1976)
adult 48-h LC50 > 10 000 (-)-trans- static 25 Miyamoto (1976)
adult 48-h LC50 3500 (-)-cis- static 25 Miyamoto (1976)
Rainbow trout 96-h LC50 2.2 technical static 12 Marking & Mauck
(Salmo gairdneri) (1975)
Coho salmon 96-h LC50 1.50 technical static 12 Mauck et al.
(Oncorhynchus (1976)
kisutch) 96-h LC50 0.277 technical flow- 12 Mauck et al.
through (1976)
Steelhead trout 96-h LC50 0.450 technical static 12 Mauck et al.
(Salmo gairdneri) (1976)
96-h LC50 0.275 technical flow- 12 Mauck et al.
through (1976)
Yellow perch 96-h LC50 2.36 technical static 12 Mauck et al.
(Perca flavescens) (1976)
96-h LC50 0.513 technical flow- 12 Mauck et al.
through (1976)
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Arthropods
Daphnia pulex 3-h LC50 15 000 technical static 25 Nishiuchi (1982)
3-h LC50 50 000 technical static 25 Miyamoto (1976)
3-h LC50 25 000 - (+)-trans- static 25 Miyamoto (1976)
50 000
3-h LC50 25 000 - (+)-cis- static 25 Miyamoto (1976)
50 000
3-h LC50 > 50 000 (-)-trans- static 25 Miyamoto (1976)
3-h LC50 > 50 000 (-)-cis- static 25 Miyamoto (1976)
Moina macrocopa 3-h LC50 14 000 technical static 25 Nishiuchi (1982)
Sigara substriata 0.59 cm; 48-h LC50 2 technical static 25 Nishiuchi (1981)
6.1 mg
Micronecta sedula 0.32 cm; 48-h LC50 3.3 technical static 25 Nishiuchi (1981)
1.8 mg
Cloeon dipterum 0.93 cm; 48-h LC50 4.5 technical static 25 Nishiuchi (1981)
5.6 mg
Orthetrum 2.3 cm; 48-h LC50 7.3 technical static 25 Nishiuchi (1981)
albistylum 0.62 g
speciosum
Eretes sticticus 1.5 cm; 48-h LC50 25 technical static 25 Nishiuchi (1981)
0.2 g
Sympetrum 2.1 cm; 48-h LC50 10 technical static 25 Nishiuchi (1981)
frequens 0.56 g
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish (contd).
Bluegill (Lepomis 96-h LC50 2.62 technical static 12 Mauck et al.
macrochirus) (1976)
96-h LC50 0.750 technical flow- 12 Mauck et al.
through (1976)
0.8 g 96-h LC50 5.46 technical static 22 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 4.28 technical static 17 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.33 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.53 technical static 12 6.6 10-13 Mauck et al.
(1976)
0.8 g 96-h LC50 2.54 technical static 12 7.8 160-180 Mauck et al.
(1976)
0.8 g 96-h LC50 2.06 technical static 12 8.2 280-320 Mauck et al.
(1976)
0.8 g 96-h LC50 2.29 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.46 technical static 12 9.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.89 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.70 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 3.13 technical static 12 8.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.68 technical static 12 9.5 40-48 Mauck et al.
(1976)
6.2 Terrestrial Organisms
Acute toxicity data show that both resmethrin and bioresmethrin
(Table 5) are harmless for birds, as are other pyrethroids.
In addition, a study has been carried out to assess the effects of
resmethrin on reproduction in the Mallard duck and Bobwhite quail. The
birds were given 12, 60, or 300 mg resmethrin/kg diet for 22 weeks. No
effects on reproduction were seen in either species at these dose
levels (Roberts et al., 1985a,b).
Table 5. Acute toxicity of resmethrin and bioresmethrin or non-target terrestrial
organisms
Species Compound Size Application Toxicity Reference
(days) (mg/kg)
Bird
Quail Resmethrin 14 diet LC50 Hill et al.
(Coturnix > 5000 (1975)
coturnix
japonica)
Mallard 10 diet LC50 Hill et al.
(Anas > 5000 (1975)
platyrhynchos)
Chicken Bioresmethrin oral LD50 Chesher &
> 10 000 Malone (1970a)
Wallwork
et al. (1970)
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Acute Toxicity
Acute toxic signs produced in experimental animals by exposure to
resmethrins include tremor, hyperexcitability, convulsive twitching,
prostration, and coma.
LD50 data on resmethrin isomers administered orally and by other
exposure routes are summarized in Tables 6 and 7, respectively.
These data show that resmethrins are weakly toxic, except for
cismethrin, the acute oral toxicity of which is moderate in the mouse.
The results of acute toxicity tests of the resmethrin metabolites
are given in Table 8.
Acute (1 h) inhalation studies were performed with 3 aerosol
formulations of resmethrin and 2 blank formulations. Groups of 20 male
rats and 4 male rabbits inhaled the active ingredient at concentration
levels of 12-13.7 mg/litre (value based on the weight loss from each
spray can). In addition, 10 exposed and 10 control rats were tested
for conditioned avoidance performance immediately following exposure.
Laboured breathing was seen generally in the animals. Several rabbits
died in both the resmethrin-treated and the blank-formulation groups.
In the rats, there were no effects on body weight, or gross or
histopathological lesions. However, the conditioned avoidance latency
in the resmethrin-treated rats was significantly greater than that in
the unexposed controls (Macko et al., 1979).
7.2 Short-term Exposure
7.2.1 Oral administration
Groups of 6 Sprague-Dawley rats from each sex were administered
resmethrin in the diet at concentrations of 0, 310-323, 630-660,
1230-1250, 1423-2670, or 1680-5100 mg/kg, for 14 days. Five out of 6
rats died at the highest exposure level in both sexes. Tremor was
observed and body weight gain and food utilization were reduced at
levels of 1230-1250 mg/kg or more. A significant increase in the
hepatic organ-to-body weight ratios was noted in all groups of females
and in groups of males fed 630-660 mg/kg or more. Compound-related
histopathological changes were not observed in any of the tissues and
organs examined. The maximum no-observed-adverse-effect dietary level
was 310 mg/kg for male rats only (Swentzel et al., 1977).
Table 6. Acute oral toxicity of resmethrin isomers
LD50 (mg/kg
Compound Animal Sex body weight) Reference
Resmethrin rat M > 5000 Miyamoto (1976)
rat F > 5000 Miyamoto (1976)
rat M 1244 Gaines & Linder (1986)
rat F 1721 Gaines & Linder (1986)
rat (weanling) M 1987 Gaines & Linder (1986)
rat ? > 2500 Worthing & Walker (1983)
rat - 960 Volkov et al. (1979)
mouse M 690 Miyamoto (1976)
mouse F 940 Miyamoto (1976)
Bioresmethrin rat M 8800 Glomot & Chevalier (1969)
rat F > 8000 Verschoyle & Barnes (1972)
rat F 7071 Wallwork et al. (1970)
rat - 840 Kholmatova (1984)
rat - 990 Volkov et al. (1979)
rat - 675 Kagan et al. (1986)
mouse M 590 Miyamoto (1976)
mouse F 800 Miyamoto (1976)
mouse M 3100 Ueda et al. (1975b)
mouse F 10000 Wallwork et al. (1970)
mouse - 520 Kholmatova (1984)
mouse - 480 Kagan et al. (1986)
rabbit - 225 Kholmatova (1984)
Cismethrin mouse M 152 Miyamoto (1976)
mouse F 160 Miyamoto (1976)
[1S,-trans]-resmethrin mouse M 500 Miyamoto (1976)
mouse F 600 Miyamoto (1976)
[1S,-cis]-resmethrin mouse M 3700 Miyamoto (1976)
mouse F 5000 Miyamoto (1976)
Table 7. Acute toxicity of resmethrins via other than oral exposure
LD50 LC50
Compound Animal Sex Route (mg/kg (mg/m3) Reference
body weight)
Resmethrin rat M dermal 2500 Gaines & Linder (1986)
rat F dermal 2500 Gaines & Linder (1986)
rabbit - dermal 2500 Green (1977)
rat - inhalation > 9490 Jackson & Hardy (1984)
(4-h exposure)
rat - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
rabbit - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
dog - inhalation > 420 Macko et al. (1979)
(4-h exposure)
Bioresmethrin rat F dermal > 10 000 Wallwork et al. (1970)
rat F intravenous 340 Verschoyle & Barnes (1972)
rat F intravenous 106-133 Chesher & Malone (1971b)
rat F intraperitoneal > 8000 Wallwork & Malone (1971)
rat F inhalation > 872 Wallwork & Malone (1972)
(24-h exposure)
mouse M intraperitoneal > 1500 Ueda et al. (1975b)
mouse F intraperitoneal > 5359 Wallwork et al. (1970)
Mixture of rat M,F inhalation > 1500 Miyamoto (1976)
bioresmethrin (4-h exposure)
& cismethrin mouse M,F inhalation > 1500 Miyamoto (1976)
(4-h exposure)
Table 8. Acute toxicity of metabolites of resmethrin
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
(bioresmethrin), (5-benzyl-3-furyl- mouse > 1500a 3100a Miyamoto (1975-1976)
methyl (+)-trans-chrysanthemate) (1) Ueda et al. (1975b)
1R or (+)-cis-resmethrin (5-benzyl-3-furyl- (2) mouse 320a Miyamoto (1975-1976)
methyl (+)-cis-chrysanthemate) Ueda et al. (1975b)
d,1-cis,trans-chrysanthemic acid (17) rat 2443 Reagan & Becci (1985a)
d,1-trans-chrysanthemic acid - rat 1598 Reagan & Becci (1985b)
(+)-trans-chrysanthemic acid, (+)-trans-CA (26) mouse 98a 280a Miyamoto (1975-1976)
(t-CA) Ueda et al. (1975b)
(+)-cis-chrysanthemic acid, (+)-cis-CA (c-CA) (27) mouse 600a Miyamoto (1975-1976)
Ueda et al. (1975b)
d-trans-chrysanthemic acid (26) rat 983 Reagan & Becci (1985c)
(+)-trans-chrysanthemumdicarboxylic acid, (30) mouse 408a Miyamoto (1975-1976)
(+)-trans-CDA (tE-CDA) Ueda et al. (1975b)
5-benzyl-3-furylmethanol (BFA) (18) mouse 75a 310a Miyamoto (1975-1976)
Ueda et al. (1975b)
Table 8. (cont'd).
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
5-benzyl-3-furoic acid (BFCA) (20) mouse 46a Miyamoto (1975-1976)
Ueda et al. (1975b)
5-benzyl-3-furoic acid (BFCA) (20) rat 997 Reagan & Becci (1985d)
ethyl chrysanthemate - rat > 4640 Stauffer Chemical Co.
(1984)
a Toxicity for male mice, 24 h after intraperitoneal injection or oral administration.
b Chemical identification numbers used in Table 1 and Fig. 2.
The same authors fed groups of 6 Long-Evans rats of each sex
resmethrin in the diet at concentrations of 0, 61-90, 148-180,
297-386, 584-669, 1080-1375, or 1266-2532 mg/kg body weight per day
for 14 days. Mortality was observed in the groups fed 1080 mg/kg or
more. Tremor was observed at levels of 386 mg/kg or more (females only
at 386 mg/kg). The terminal body weight and food utilization were
significantly lower in the groups fed 1080 mg/kg or more compared with
the controls. A significant increase in the hepatic organ-to-body
weight ratios was noted at levels of 297 mg/kg or more.
Compound-related histopathological changes were not observed in any of
the tissues and organs examined. The maximum no-observed-adverse-
effect dietary level was 148 mg/kg body weight per day for male and
180 mg/kg body weight per day for female rats (Swentzel et al., 1977).
In a third study by Swentzel et al. (1977), groups of 8-20
Long-Evans rats of each sex were fed resmethrin in the diet at
concentrations equivalent to 0, 22, 66, 211, or 679 mg/kg body weight
per day for males and of 0, 3, 7, 22, 67, 219, 724, or 2400 mg/kg body
weight per day for females, for 90 days. All the females fed the
highest level died. Tremor was observed at levels of 679 mg/kg or
more. The mean terminal body weight was significantly lower in the
group given 679-724 mg/kg than in the control group. A significant
increase in the hepatic and renal organ-to-body weight ratios was
noted in males fed 211 or 679 mg/kg; while, in females fed 219 or
724 mg/kg, only an increase in the hepatic ratio was observed. No
significant differences were observed in clinical chemistry values
(serum glutamic oxaloacetic transaminase (SGOT), serum glutamic
pyruvic transaminase (SGPT), total lactate dehydrogenase (LDH),
alkaline phosphatase (AP), gamma-glutamyl trans-peptidase (GGTP),
total bilirubin, total protein, blood-urea nitrogen (BUN)) between
test and control animals. Compound-related histopathological changes
were not observed in any of the tissues and organs examined. The
maximum no-observed-adverse-effect dietary level was 67 mg/kg body
weight per day for female and 66 mg/kg body weight per day for male
rats.
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage, daily, 6 days/week for 3 weeks at doses of 0, 1000,
or 2000 mg/kg body weight. Body weights were slightly reduced at
2000 mg/kg. A slight reduction was noted in haemoglobin content and
hematocrit value. Biochemical examination revealed an increase in
albumin and blood-urea nitrogen concentrations and a decrease in
serum-glutamic oxaloacetic transaminase (SGOT) activity. Increased
liver size and reduced thymus weight were observed at 1000 mg/kg.
Reduced prostate weight was noted only at the high dose.
Histopathological examination revealed thymic involution (Glomot,
unpublished report).a
a GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished report of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
Wallwork et al. (1971) reported a study in which groups of rats
(18 males and 18 females/group) were fed bioresmethrin in the diet at
concentrations of 0, 400, 1200, or 8000 mg/kg over 91 days. The group
receiving the highest dose was fed 4000 mg/kg for 30 days and
thereafter 8000 mg/kg. Body weights were reduced at the highest dose
level and there were changes in blood chemistry parameters indicating
liver dysfunction (an increase in serum-alkaline phosphatase (SAP),
SGOT, and urinary nitrogen and a decrease in glucose content). The red
blood cell count was depressed in the group receiving 1200 mg/kg. An
increase in liver weight and a decrease in the weights of several
other organs, such as the spleen and thymus, were observed at
4000 mg/kg. Fatty infiltration of the liver was noticed at 1200, 4000,
and 8000 mg/kg. The no-observed-adverse-effect level in this study was
400 mg/kg, equivalent to an average daily intake of 32.8 and
36.1 mg/kg body weight for males and females, respectively.
Bioresmethrin was administered to groups of dogs (2 of each
sex/group) by gavage, daily, at dose levels of 0 or 500 mg/kg for 7
days followed by an increased dose of 1000 mg/kg for a further 14
days. No effects were noted on mortality, behaviour, body weight,
haematology, blood chemistry, urinalysis, or electrocardiograms
(Malone & Chesher, 1970).
Groups of dogs (3 males and 3 females/group) were administered
bioresmethrin in gelatin capsule by gavage, daily for 90 days, at
dosage levels of 0, 25, 80, or 250 mg/kg body weight (the highest dose
was increased to 500 mg/kg body weight in week 7). There were no
effects on mortality, body weight, food consumption, ophthalmology, or
urinalysis. Red blood cell count, haemoglobin content, and packed cell
volume values were reduced at the highest dose level. Blood-urea
nitrogen was slightly increased at the highest dose after 12 weeks. No
adverse effects were observed on gross or histopathological
examination. The no-observed-adverse-effect level was 80 mg/kg body
weight, equivalent to an average of 1600 mg/kg diet (Noel et al.,
1971).
In summary, the no-observed-adverse-effect level for resmethrin as
determined in the 90-day study on rats was 66 mg/kg body weight per
day, whereas that for bioresmethrin was 33 mg/kg body weight per day
in the 91-day study on rats and 80 mg/kg body weight per day in the
90-day study on dogs.
7.2.2 Inhalation
Short-term inhalation studies were performed using three
resmethrin formulations and one blank formulation. Aerosol spray
formulations (frequency median diameter of the particles: 1.5-2.0 µm,
volume median diameter 10-18.5 µm) were introduced into the top of a
pyramid-shaped sealed chamber and dispersed for 30-second intervals
every 30 min. Groups of 25 male rats, 10 female rats, and 4 male
rabbits were exposed to the inhalations 10 times a day, over a period
of 5 h, for 5 consecutive days. Daily active ingredient concentrations
of each formulation in the chamber were 2.9-3.2 mg/litre, based on the
weight loss from each spray can. The clinical signs included increased
preening, ruffled pelt, rapid breathing, and slight nasal discharge.
All signs disappeared overnight, but recurred after each daily
exposure. In rats, there were no effects on body weight. No gross or
histopathological lesions related to exposure to the formulations were
observed in rats necropsied immediately after the last 5-h exposure
and 7 and 14 days after exposure. No indication of toxic effects,
other than irritation, was observed in any test (Macko et al., 1979).
When ICR mice and Sprague-Dawley rats were exposed to [1R,
trans, cis]-resmethrin (at concentrations of 0, 23, 47, or
210 mg/m3) for 4 h/day, 5 days/week over 4 weeks, no toxic effects
were observed on behaviour, food intake, haematology, clinical
biochemistry, mortality, or histopathology (Miyamoto, 1976).
When groups of 16 male and 16 female Wistar rats were exposed
through inhalation for 6 h/day on 5 days of each week, over a period
of 90 days, to technical resmethrin at target exposure levels of 0.1,
0.3, or 1.0 g/m3, the no-observed-adverse-effect level was
established at 0.1 g/m3. At 0.3 g/m3, there were minor effects on
some clinical pathology parameters and also signs of irritation.
However, microscopic pathological examination did not reveal any
treatment-related changes in the lungs or other organs of rats exposed
at this level. At 1 g/m3, there were clinical signs indicative of
irritation, minor neurobehavioural changes, a reduced rate of weight
gain, and some changes in clinical pathology parameters. Microscopic
examination at this level showed minimal changes in the larynx, liver,
and thyroid, but these were reversible during the recovery period.
There were no treatment-related lung changes (Coombs et al., 1985).
7.2.3 Dermal application
Resmethrin was applied twice a week for 3 weeks to the shaved skin
of 4 groups of 10 male New Zealand White rabbits. Cotton cloth treated
with resmethrin at 0.247 mg/cm3 was applied over 1 ml of liquid
(imitating sweat) to rabbits in the first group. In the second group,
cotton cloth treated with resmethrin was applied without the sweat,
and in the third group, the cotton cloth was fixed to skin that had
been pretreated with 10 g of technical grade resmethrin. In the fourth
group, untreated cotton cloth was fixed over skin pre-treated with
pyrax powder containing 1% resmethrin at the rate of 1 g/kg of body
weight. The 3 control groups received cotton cloth treated with
acetone, cotton cloth treated with acetone over 1 ml of the sweat, and
untreated cotton cloth over 1 g pyrax powder/kg, respectively. No
significant changes were noted, on day 24 of the test, in rabbit body
weights and organ-to-body weight ratios of liver, lung, kidney,
testis, and spleen. Average dermal irritation scores for
resmethrin-treated rabbits were not significantly higher than those
for the control groups and did not increase during the test. No
significant trends compared with the controls were seen in clinical
chemistry values (serum-glutamic oxalo-acetic transaminase,
serum-glutamic pyruvic transaminase, lactic dehydrogenase, alkaline
phosphatase, blood-urea nitrogen) on days 5, 12, 19, and 24 of the
test. There were no compound-related lesions of the skin or of any of
the other tissues and organs examined at the termination of the study
(Swentzel et al., 1977).
7.3 Primary Irritation and Sensitization
Technical grade resmethrin was found to be a slight irritant in
rabbits in a 24 day dorsal/ventral rabbit ear test. Dermal irritation
was evident on both intact and abraded skin at 72 h and up to 7 days.
Resmethrin did not cause sensitization reactions in guinea-pigs, or
photochemical irritation in New Zealand White rabbits. Repeated daily
applications of 0.1 g of the technical grade compound to one ear of
each of 5 New Zealand White rabbits were carried out for 30
consecutive days as well as applications of compound-impregnated
cotton sateen cloth (0.247 mg/cm2) with artificial sweat for 24 days.
In this study, resmethrin did not produce acne-form dermatitis
(Swentzel et al., 1977).
Groups of adult guinea-pigs (6 males/group) were treated with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro
benzene (DNCB) (0.1 ml of a 1% (w/v) formulation in mineral oil) to
assess the sensitization properties. The test substance was applied to
the ears for 4 days. On day 7, 0.2 ml was applied dermally and the
degree of irritation recorded. As expected, the DNCB was an irritant
while bioresmethrin showed only traces of erythema suggesting a low
potential for sensitization and irritation (Chesher & Malone, 1970b).
Instillation of technical bioresmethrin into the eye of 6 rabbits
(0.1 ml) did not produce any irritation or corneal damage, or any
indication of ocular hazard (Chesher & Malone, 1970c).
7.4 Long-term Exposure and Carcinogenicity
When [1R, trans, cis]-resmethrin was fed to Sprague-Dawley
rats (male and female) at dietary levels of 500, 1500, or 5000 mg/kg
for 24 weeks, toxic symptoms, such as tremors and decreased body
weight, increased liver and kidney weights and an increase in alkaline
phosphatase activity were observed at 5000 mg/kg. The no-observed-
adverse-effect level was 1500 mg/kg (77.7 mg/kg per day for males and
86.6 mg/kg per day for females) (Miyamoto, 1976).
Resmethrin fed to Wistar rats at dosage levels of 0, 500, 2500, or
5000 mg/kg in the basal diet over a 112-week period, was determined
not to be oncogenic up to, and including, 5000 mg/kg, which was the
highest dose tested. The no-observed-adverse-effect level of 500 mg/kg
for toxic effects, was the lowest effect level for hypertrophy of
hepatocytes, which was not considered a definite toxic response
(Knickerbocker et al., 1980; Hess et al., 1982).
Cox et al. (1979a) fed CD-1 outbred albino mice with 0, 250, 500,
or 1000 mg resmethrin/kg basal diet for an 85-week period. No
oncogenicity was observed at any doses up to and including 1000 mg/kg.
When Beagle dogs were administered 0, 10, 30, or 300 mg
resmethrin/kg body weight for 6 months, the no-observed-adverse-effect
level was 10 mg/kg per day. On day 57 of the study, the highest dose
level was increased from 100 mg/kg per day to 300 mg/kg per day.
Increased liver weights were noted at 30 mg/kg body weight per day
(Gephart et al., 1980).
An acceptable daily intake (ADI) was established by the USA EPA at
0.125 mg/kg body weight per day based on no-observed-adverse-effect
levels in long-term toxicity studies. A food additive tolerance has
been established by the US EPA, permitting resmethrin residues of up
to 3 ppm, in or on food commodities, resulting from use in food
handling areas (US Environmental Protection Agency, 1983).
7.5 Mutagenicity
Saccharomyces cerevisiae D4 and five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) were used to
evaluate the mutagenic potential of resmethrin. The compound was
tested in the absence or presence of liver microsomal enzyme
preparations from rats pre-treated with Aroclor 1254. Resmethrin was
not mutagenic to any of the indicator organisms under both conditions
(Swentzel et al., 1977).
Resmethrin, bioresmethrin, and cismethrin were tested for
mutagenicity in several test systems. Miyamoto (1976) and Suzuki
(1975) used the Escherichia coli and S.typhimurium reverse
mutation tests. Garret et al. (1986) summarized results of point/gene
mutations in prokaryotes or eukaryotes, primary DNA damage in
prokaryotes or eukaryotes, chromosomal effects in Chinese hamster
ovary cells, mouse bone marrow, and cardiac blood cells of the mouse.
Pluijmen et al. (1984) used reverse mutation tests in S.typhimurium
TA98 or TA100, or mutagenicity tests with V79 Chinese hamster cells to
test seven synthetic pyrethroids including resmethrin, bioresmethrin,
and cismethrin. They all gave negative results.
Bioresmethrin was also tested in a metaphase chromosome analysis
of cultured human lymphocytes, an autoradiographic assessment of
unscheduled DNA synthesis in mammalian cells, and a mouse micronucleus
test. They also gave negative results (Allen et al., 1986; Allen &
Proudlock, 1986; Vannier & Fournex, 1986).
7.6 Reproductive effects, Embryotoxicity, and Teratogenicity
Three groups of 30 pregnant Long-Evans rats were administered
resmethrin in the diet at concentrations of 0, 188, or 1500 mg/kg from
day 6 to day 16 of gestation. The dams showed tremors and decreased
food and water consumption at 1500 mg/kg and 2 dams died; the lower
fetal weight seen at this dose and the resorption of embryos and
fetuses in 15 out of 30 dams were probably due to maternal toxicity.
No gross abnormalities of fetal skeletons and soft tissues were
observed in the treated groups. Thus, the consumption of resmethrin in
ground feed was not teratogenic at 188 and 1500 mg/kg (Swentzel
et al., 1977).
In a 3-generation study, Sprague-Dawley rats were fed resmethrin
in the diet at 0, 500, 800, or 1250 mg/kg (Schwartz et al., 1979c). A
slight increase in the number of pups cast dead and a decrease in pup
weights were noted at the 500 mg/kg level. A no-observed-adverse-
effect level of < 500 mg/kg was established for pups cast dead and
reduced pup weights at 21 days.
Sprague-Dawley female albino rats were given resmethrin in corn
oil, by gavage, at dose levels of 0, 20, 40, or 80 mg/kg during the
period of major organogenesis (days 6-15 of gestation). Resmethrin was
not teratogenic in rats at levels up to, and including, 80 mg/kg. The
no-observed-adverse-effect level for fetotoxicity was 40 mg/kg (Machi
et al., 1979).
Pregnant New Zealand White Minnikin rabbits were given resmethrin
by oral intubation at 0, 10, 30, or 100 mg/kg body weight per day on
days 6-18 of gestation (Becci et al., 1979). On day 29 of gestation,
all animals were killed for examination. No teratogenic effects were
seen at dose levels up to and including 100 mg/kg.
When [1R, trans, cis]-resmethrin was administered orally to
female ICR mice (10, 30, or 100 mg/kg, daily) and Sprague-Dawley rats
(10, 20, or 50 mg/kg daily) during the period of gestation, to examine
maternal and embryotoxic effects, no significant adverse effects, such
as abortion, resorption of fetuses or embryos, external or skeletal
abnormalities of pups, and abnormalities in growth or organ
differentiation, were observed at any doses (Miyamoto, 1976).
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin at doses of 0, 10, 20, 40, or 80 mg/kg, by gavage,
daily, from day 8 to day 16 of gestation. The does were sacrificed on
day 28 and examined for implantation, live and dead fetuses,
resorption sites, and abnormalities. There was no apparent effect on
parents in the study as growth and gestation were unaffected. There
was an increase in the numbers of dead fetuses at the highest dose and
a large number of resorption sites were noted at all treatment levels.
A number of deformed fetuses were observed, but the total numbers were
not sufficient for an adequate statistical evaluation. The deformities
included straight tail, crossed hind limbs, and unilateral union of
6th and 7th ribs at the sternal end. An overall fetal loss was
observed at all dose levels (primarily because of the large number of
resorption sites recorded) (Waldron, 1969).
7.7 Immunotoxicity
The influence of various pesticides on the humoral and cellular
immune response to fluorescein-labelled ovalbumin has been analysed.
Resmethrin was administered intragastrically in corn oil as a single
dose (one half of LD50) before primary immunization. Control groups
included animals treated with corn oil alone, or immunosuppressed with
methotrexate. Booster immunizations and test bleedings were scheduled
to follow at weekly intervals. The cellular immune response was
quantified by redness and swelling, histological examination, and by
differential temperature measurements of the foot pads after antigen
challenge. The concentration, binding affinity, and heterogeneity of
the serum antibody were determined by fluorescence polarization
measurements. Resmethrin gave an early, sometimes very marked,
stimulation of the cellular immune response (Danliker et al., 1979).
7.8 Neurotoxicity
The neurotoxicity of resmethrin was evaluated in three short-term
dietary studies on Sprague-Dawley rats designed to examine the gross
and histopathological changes to the central and peripheral nervous
system. Resmethrin was not neurotoxic when administered at 1250 mg/kg
for 32 weeks, 5000 mg/kg for 30 days, or 12 640 mg/kg for 7 days (Cox
et al., 1979b; Schwartz et al., 1979a,b).
In order to better characterize the behavioural effects of
pyrethroid insecticides, comparisons were made of the effects of
cismethrin and deltamethrin exposure on motor activity and the
acoustic startle response in male Long-Evans rats (Crofton & Reiter,
1984). Acute dose effect, acute time course, and 30-day
repeated-exposure determinations of 1 h motor activity were made using
figure-eight mazes. The acoustic startle response to a 13-kHz, 120-dB,
40-msecond tone was measured at each of three background white noise
levels (50, 65, and 80 dB). Deltamethrin (0, 2, 4, 6, or 8 mg/kg) or
cismethrin (0, 6, 12, 18, or 24 mg/kg) was administered orally in
0.2 ml corn oil/kg. Both compounds produced similar dose-dependent
decreases in motor activity. The time course of onset and recovery for
this decreased activity was rapid (1-4 h). No cumulative effects on
motor activity were found of a 30-day exposure to 2 mg deltamethrin/kg
per day or 6 mg cismethrin/kg per day. The effects of cismethrin and
deltamethrin on the acoustic startle response differed. Deltamethrin
produced a dose-dependent decrease in amplitude and an increase in
latency, and cismethrin produced an increase in amplitude and no
change in latency. The differential effects of cismethrin (Type I
pyrethroids) and deltamethrin (Type II pyrethroids) on the acoustic
startle response may be related to the contrasting effects previously
shown with neurophysiological and/or neurochemical techniques (see
Annex).
The isolated rat neurohypophysis, which shows a calcium-dependent
hormone release when depolarized in vitro, was used as a model
system to investigate the effects of the pyrethroids deltamethrin and
resmethrin on mammalian nervous tissue (Dyball, 1982). Both compounds
inhibited neurohypophysial hormone release in response to electrical
stimulation, deltamethrin being more potent than resmethrin.
Deltamethrin reduced the hormone content of the neurohypophysis.
Resmethrin did not reduce stored hormone significantly and its effects
on release were dose dependent. They could be mimicked by raising the
Na+ of the medium, but not by lowering the Ca2+. Resmethrin did not
have any effects on the release of hormone following depolarization of
the tissue with a raised K+. The results are consistent with the
suggestion that the compounds do not act on the potential-dependent
secretion process but rather on the mechanism linking depolarization
of the secretory terminals with the arrival of action potentials,
possibly by interfering with sodium-channel activation and
inactivation.
The neurological effects of four synthetic pyrethroids resmethrin,
permethrin, cypermethrin, and deltamethrin were investigated in the
rat to establish whether there is a correlation between the
clinical-functional status of the animal and peripheral nerve damage,
as measured biochemically (Rose & Dewar, 1983). Neuromuscular
dysfunction was assessed by means of the inclined plane test and
peripheral nerve damage by reference to ß-glucuronidase and
ß-galactosidase activity increases in nerve tissue homogenates from
treated and control animals. A transient functional impairment was
found in animals treated with any one of the four pyrethroids tested
and, in all cases, this was maximal at the end of the 7-day dosing
regimen (resmethrin doses of 500-2000 mg/kg per day). Significant
increases in ß-glucuronidase and ß-galactosidase were found, 3-4 weeks
after the start of dosing in the distal portion of the
sciatic/posterior tibial nerves of animals treated with permethrin,
cypermethrin, or deltamethrin; however, no changes were found in
resmethrin-treated animals. Thus, it is concluded that there is no
direct correlation between the time-course of the neuromuscular
dysfunction and the neurobiochemical changes. This suggests that these
pyrethroids have at least two distinct actions, a short-term
pharmacological effect and, at near-lethal dose levels, a more chronic
neurotoxic effect that results in sparse axonal nerve damage.
Resmethrin (30 µmol/litre)-induced release of transmitters was not
affected by manipulation of the Na+ current with either choline or
tetrodotoxin agents, which readily reversed the effects of
veratridine, deltamethrin, and cypermethrin (Doherty et al., 1986).
Resmethrin (I50: 2.2 µmol/litre) inhibited the ATP-dependent uptake
of Ca2+ but deltamethrin and cypermethrin were much less effective.
Resmethrin also displaced Ca2+ from crude synaptosomal membranes. The
release-promoting effects of resmethrin in rat brain in vitro are
better explained by its effects on Ca2+ rather than by a specific
effect on the Na+ channel. In contrast, deltamethrin and
cypermethrin promote transmitter release by a Na+ dependent process.
Glickman & Casida (1982) discussed species and structural
variations affecting pyrethroid neurotoxicity. They concluded that the
mammalian nervous system, or at least the brain, appears to lack sites
sensitive to bioresmethrin and to a lesser extent to [1R,
trans]-permethrin, yet small structural changes restore the toxicity
(e.g., [1R,trans]-ethano-resmethrin and [1R, cis]-resmethrin. The
authors reported that birds and mammals in general respond to cis-
but not to trans-resmethrin in contrast to insects, crustaceans, and
fish, which are highly sensitive to both isomers. Furthermore, common
green lacewing larvae are very tolerant to pyrethroids, suggesting
possible involvement of nerve insensitivity in addition to
detoxification in this species. These examples of insensitivity may be
associated with modified sites of action or perhaps an increased
stabilization of nerve membranes making them more resistant to
pyrethroid-induced excitation.
7.9 Mechanism of Toxicity (Mode of Action)
Cismethrin is toxic for both insects and mammals. Bioresmethrin is
about 50 times less toxic than cismethrin and produces few symptoms in
mice and rats, even at extremely high doses. The poisoning syndrome of
cismethrin and bioresmethrin was characterized by whole body tremors
(T-syndrome) and both compounds were therefore classified as Type I
pyrethroids (Verschoyle & Aldridge, 1980; Lawrence & Casida, 1982,
Annex).
Tremors started when the concentrations of cismethrin and
bioresmethrin in the brain were 0.5-1 mg/kg and 4-5 mg/kg,
respectively. At death, brain levels of cismethrin were 3.9-5.1 mg/kg,
and those of bioresmethrin were 23-35 mg/kg. As liver microsomal
esterases hydrolysed bioresmethrin 10 times more rapidly than
cismethrin, the lower toxicity of bioresmethrin was partly attributed
to its faster metabolism and intrinsically lower toxicity at the
critical site of action in the nervous system (White et al., 1976).
Cismethrin given intravenously produced repetitive activity, after
external stimulation, in the spinal cord of rabbit (Carlton, 1977). As
gamma-aminobutyric acid (GABA) blockers, such as bicuculline and
picrotoxin, produced convulsions similar to those of cismethrin, the
effects of the compounds on dorsal root potentials were examined in
the rat. Bicuculline reduced the amplitude of the potential to 67% of
the control values, but cismethrin enhanced the potential to 142% of
the control values. The convulsions associated with cismethrin
poisoning, therefore, are not produced by an antagonism of
GABA-mediated inhibitory transmission (Smith, 1980).
Administration of cismethrin into the lateral ventricle of the
brain or into the spinal cord causes signs similar to those observed
after intravenous administration. The onset of tremors after
intraventricular administration of cismethrin was delayed for about 15
min while, after intraspinal injection, the symptoms occurred within
2-5 min, indicating that cismethrin may act directly on the spinal
cord rather than in the brain (Gray et al., 1980b).
After intravenous administration of [14C]-alcohol-labelled
cismethrin or bioresmethrin to rats, the parent pyrethroid was rapidly
cleared from the blood and liver, and both isomers rapidly entered the
central nervous system (CNS) reaching peak concentrations within 2-5
min. Cismethrin concentrations in the brain exceeding 3.5 nmol/g were
associated only with animals showing tremors. These levels of
cismethrin were maintained for up to 30 min, but bioresmethrin was
depleted more rapidly, possibly due to brain metabolism. It is
concluded that the low toxicity of bioresmethrin is possibly because
of its inability to interact with the site of action in the CNS and to
its rapid metabolism in the liver (Gray et al., 1980a).
Cismethrin produced repetitive firing in the flight muscles and
uncoupling of motor unit activity in the housefly (Miller & Adams,
1977), and repetitive firing in a cercal sensory nerve of the
cockroach (Gammon et al., 1981). On the basis of the
electrophysiological effects and symptomatology in insects as well as
in mammals, cismethrin is classified as a Type I pyrethroid,
characterized by a mainly peripheral nervous system action (Gammon et
al., 1981).
7.10 Potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin, and/or piperonyl butoxide, alone or in
combinations, at doses approximating the acute intraperitoneal LD50
value. No potentiation of the acute toxicity was observed in this
study. In all cases with bioresmethrin combinations, the observed
LD50 values were equal to, or exceeded, the expected value (Wallwork
& Malone, 1971).
8. EFFECTS ON MAN
Although the resmethrins have been used for many years, no data
have been reported on their toxicity for human beings.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Health Risks
Resmethrin, consisting of four stereo-isomers, is an effective
insecticide mainly used to control household insects and other public
health insects, with applications in the food handling and storage
areas.
Bioresmethrin and cismethrin are composed of a selected isomer(s).
As with resmethrin, bioresmethrin is used to control household insects
and other public health insects. In addition, bioresmethrin is used as
a post-harvest insecticide to control stored grain pest. Human
exposure to the resmethrins will be mainly via inhalation, when the
formulations are sprayed in the form of a mist, though the use in food
handling and storage areas as well as post-harvest treatment may
result in dietary residues. Air levels following conventional
household aerosol spraying with the resmethrins are not expected to
exceed 0.5 mg/ms.
The only significant potential dietary exposure will result from
the use of resmethrin on stored grain. Residues of up to 4 mg/kg might
be present in grain, but this would be reduced to zero in white bread.
However, reduced residues may be present in wholemeal bread. A food
additive tolerance has been established by the US Environmental
Protection Agency (US EPA) permitting resmethrin residues of up to
3 mg/kg (3 ppm), in or on food commodities, resulting from use in food
handling areas. An ADI was established by the US EPA at 0.1250 mg/kg
body weight per day, based on no-observed-adverse-effect levels in
long-term toxicity studies. No data are available on occupational
exposure to the resmethrins. Although the resmethrins have been used
for many years, no data have been reported concerning their toxicity
for human beings. Thus, to determine the potential toxicity for human
beings, extrapolation from animal data and in vitro studies has been
used.
The result of short-term studies on experimental animals suggest
that resmethrins are weakly toxic when administered by various routes
of exposure (oral LD50 of resmethrin: 690 mg/kg (mouse) - >
5000 mg/kg (rat); those of bioresmethrin: 225 mg/kg (rabbit),
480-10 000 mg/kg (mouse), 8800 mg/kg (rats)). Cismethrin showed
moderate toxicity for the mouse (oral LD50 152-160 mg/kg). The acute
toxicity of resmethrin metabolites were in the same range in the rat,
but somewhat more toxic in the mouse.
While technical grade resmethrin is a slight skin irritant, it is
not a sensitizer.
Resmethrins are not mutagenic in a variety of test systems,
including gene mutations, DNA damage and DNA repair, and chromosomal
effects.
Resmethrin was not carcinogenic for mice or rats, when fed at
dietary levels of up to 1000 mg/kg for 85 weeks and 5000 mg/kg for 112
weeks, respectively.
Resmethrin was not teratogenic in the rat, mouse, or rabbit, up to
dose levels of 100 mg/kg body weight. A level of 40 mg/kg body weight
appeared to be the no-observed-adverse-effect level for fetotoxicity
in the rat.
Resmethrins at near lethal doses are likely to cause
hyperactivity, tremors, and convulsions and have been classified as
Type I pyrethroids.
For resmethrin, a no-observed-adverse-effect level was established
in a 90-day rat study to be 66-67 mg/kg body weight per day, whereas,
in another 2-year rat study, the lowest effect level appeared to be
500 mg/kg diet, corresponding to 25 mg/kg body weight per day. In a
6-month feeding study on dogs, the no-observed-adverse-effect level
was 10 mg/kg body weight per day.
In a 90-day inhalation study on rats, a no-observed-adverse-effect
level was established of 0.1 g/m3.
The no-observed-adverse-effect level for bioresmethrin in a 9-day
feeding study on the rat was 33-36 mg/kg body weight per day; and in a
90-day study on dogs, it was 80 mg/kg body weight per day.
In a 24-week feeding study on rats, the no-observed-adverse-effect
level for [1R, trans, cis]-resmethrin was 1500 mg/kg diet
corresponding to 75 mg/kg body weight.
9.2 Effects on the Environment
Resmethrins are used mainly indoors for the control of household
insects and also for stored grain protection. They are also used in
greenhouses and can be used outdoors for mosquito control. However,
under outdoor conditions, rapid photodegradation and microbial
degradation in the soil ensure that residues will not persist to any
extent in the environment.
In laboratory studies, resmethrins have been shown to be very
toxic for fish (96-h LC50 values: 0.3-5.5 µg/litre) but less toxic
for Daphnia and aquatic insect larvae. However, under field
conditions the low water solubility of the resmethrins and their ready
degradation greatly alleviate the effects that might be predicted from
the laboratory studies. The toxicity of resmethrins for birds is low
(LD50 > 5000 mg/kg) and they do not produce any effects on avian
reproduction.
10. CONCLUSIONS
General population: Under recommended conditions of household
and other public health use, the exposure of the general population to
resmethrins is negligible and is unlikely to present a hazard. Under
recommended conditions of use in food handling and storage areas as
well as in post-harvest treatment, the exposure of the general
population to resmethrins in the diet is unlikely to exceed the ADI
established by the US EPA.
Occupational exposure: With reasonable work practices, hygienic
measures, and safety precautions, the use of resmethrins is unlikely
to present a hazard to those occupationally exposed to it.
Environment: With recommended application rates, it is unlikely
that resmethrins or their degradation products will attain levels of
environmental significance. In spite of the fact that resmethrins are
highly toxic for fish, this is only likely to cause a problem in the
case of spillage or over-spraying.
11. RECOMMENDATIONS
- In order to fully assess the potential dietary exposure from
current uses of resmethrins, it is suggested that the degradation
pathway on stored grain be studied, and the terminal residues on
grain and bread be defined.
- It is considered that resmethrin, despite its high octanol-water
partition coefficient, is very unlikely to bioaccumulate in
non-target species, because of its ready degradation. However,
experimental confirmation of this in fish might be useful.
- A further multigeneration reproduction study to define a
no-observed-adverse-effect level should be considered.
- Over many years of use, no adverse effects have been reported as a
result of human exposure to resmethrins, but it is still necessary
to maintain observations on human exposure.
- The labelling of resmethrins for household use should include
adequate instructions for use and storage and, where appropriate,
a warning of flammability.
- Efforts should be made to obtain a more precise estimate of the
total global usage of resmethrins.
12. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The Joint FAO/WHO Expert Committee on Pesticide Residues (JMPR)
discussed and evaluated bioresmethrin at its meetings in 1975 and 1976
(FAO/WHO, 1976, 1977). An acceptable daily intake (ADI) has not been
established.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified bioresmethrin as a technical
product unlikely to present an acute hazard in normal use, and
resmethrin and cismethrin as slightly hazardous (WHO, 1986). A Data
Sheet on bioresmethrin (No. 34) has been issued (WHO/FAO, 1978).
REFERENCES
ABBOTT, D.D. (1971) Total degradation products of SBP-1382. Acute
oral LD50/mice, 6 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
ABE, Y., TSUDA, K., & FUJITA, Y. (1972) Studies on pyrethroidal
compounds. Part III. Photostability of pyrethroidal compounds.
Botyu-Kagaku, 37: 102-110.
ALLEN, J.A. & PROUDLOCK, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after
exposure to bioresmethrin, Huntingdon, United Kingdom,
Huntingdon Research Centre, 18 pp (Report No.
RSL-BRM-698/851420/A) (Unpublished proprietary data supplied by
Roussel Uclaf).
ALLEN, J.A., BROOKER, P.C., & HOWELL, A. (1986) Bioresmethrin
metaphase chromosome analysis of human lymphocytes cultures
in vitro, Huntingdon, United Kingdom, Huntingdon Research Centre,
15 pp (Report No. RSL-BRM-697/851370/A) (Unpublished proprietary
data supplied by Roussel Uclaf).
ARDLEY J.H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat (Unpublished report of Wellcome
Australia Ltd).
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and
high-performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Battelle Institute.
BECCI, P.J., KNICKERBOCKER, M., & PARENT, R.A. (1979) Teratological
evaluation of SBP-1382 in albino rabbits, Waverly, New York,
Food and Drug Research Laboratories, 17 pp (Report No. 6288)
(Unpublished proprietary data supplied by Roussel Uclaf).
BENGSTON, M., COOPER, L.M., & GRANT-TAYLOR, F.J. (1975) A comparison
of bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in wheat.
Queensland J. Agric. anim. Sci., 32: 51-78.
BUICK, A.R. & FLANAGAN, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes (Report No. HEGH 73/1) (Unpublished
data of Wellcome Foundation Ltd).
BURT, M.E. (1983a) Resmethrin residues in tomato and cucumber, New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
BURT, M.E. (1983b) Resmethrin residues in lettuce (greenhouse), New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
CARLTON, M. (1977) Some effects of cismethrin on the rabbit nervous
system. Pestic. Sci., 8: 700-712.
CAVE, S.L. (1981) Simultaneous estimation of bioresmethrin and
piperonylbutoxide by gas-liquid chromatography with
chemical-ionization mass spectrometry. Pestic. Sci.,
12: 156-160.
CHAPMAN, R.A. & HARRIS, C.R. (1978) Extraction and liquid-solid
chromatography cleanup procedures for the direct analysis of four
pyrethroid insecticides in crops by gas-liquid chromatography.
J. Chromatogr., 166: 513-518.
CHESHER, B.C. & MALONE, J.C. (1970a) Acute toxicity tests with NRDC
107 in hens (Report No. B 236-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970b) Sensitisation study with NRDC
107 on guinea pigs (Report B 198-70) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits (Report No. B 217-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1971a) Toxicity to dogs of NRDC 107
(continuation) (Report No. B 27-71) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER B.C. & MALONE J.C. (1971b) Acute toxicity of NRDC 107 by the
intravenous route. (Report No. B 329-71) (Unpublished data of
Wellcome Foundation Ltd).
CHRISTOPHER, R.J., MUNGER, C.E., & WAYNE IRVIE, G. (1985) Distribution
and depletion of [14C]-resmethrin isomers administered orally to
laying hens. Pestic. Sci., 16: 378-382.
COOMBS, D.W., HARDY, C.J., CLARK, G.C., STREET, A.E., GOPINATH, C.,
LEWIS, D.J., & RAO, R.S. (1985) Resmethrin 90-day inhalation
toxicity study in the rat, Huntingdon, United Kingdom,
Huntingdon Research Centre, 80 pp (Report No. SBP 6/84997)
(Unpublished proprietary data supplied by Roussel Uclaf).
COX, G.E., KNICKERBOCKER, M., & PARENT, R.A. (1979a) Evaluation of
dietary administration of SBP-1382 in CD-1 outbred albino mice
over an 85-week period, Waverly, New York, Food and Drug
Research Laboratories, 52 pp (Report No. 5270) (Unpublished
proprietary data supplied by Roussel Uclaf).
COX, G.E., BECCI, P.J., & PARENT, R.A. (1979b) Supplementary report
for evaluation of the neurotoxic effects of SBP-1382 in albino
rats - phase I, Waverly, New York, Food and Drug Research
Laboratories, 7 pp (Report No. 6362) (Unpublished proprietary data
supplied by Roussel Uclaf).
CROFTON, K.M. & REITER, L W. (1984) Effects of two pyrethroid
insecticides on motor activity and the acoustic startle response
in the rat. Toxicol. appl. Pharmacol., 75: 318-328.
DANLIKER, W.B., HICKS, A.N., LEVISON, S.A., STEWART, K., & BROWN, R.J.
(1979) Effects of pesticides on immume response, Washington, DC,
US Environmental Protection Agency (EPA-600/1-79-039-PB
80-1308 34).
DESMARCHELIER, J.M. (1980) Comparative study of analytical methods for
bioresmethrin, phenothrin, d-phenothrin, pyrethrum I, carbaryl,
fenitrothion, methacrifos, pirimifos-methyl and dichlorfos on
various grains. J. Pestic. Sci., 5: 521-532.
DEVAUX, Ph. & BOLLA, P. (1986) Bioresmethrin n-octanol/water
partition coefficient and water solubility, Romainville, France,
Roussel Uclaf, 4 pp (Report No. RU-BRM-142.06.86. STE/A)
(Unpublished proprietary data).
DEVAUX, Ph. & TILLIER, C. (1986) Vapor pressure of bioallethrin and
bioresmethrin, Romainville, France, Roussel Uclaf, 8 pp (Report
No. RU-BRM/BA-150.06.86.STE/A) (Unpublished proprietary data).
DOHERTY, J.D., LAUTER, C.J., & SALEM, N., Jr (1986) Synaptic effects
on the synthetic pyrethroid resmethrin in rat brain in vitro.
Comp. Biochem. Physiol., C84: 373-379.
DYBALL, R.E.J. (1982) Inhibition by decamethrin and resmethrin of
hormone release from the isolated rat neurohypophysis: A model
mammalian neurosecretory system. Pestic. Biochem. Physiol.,
17: 42-47.
ELLIOT, M. (1976) Properties and applications of pyrethroids.
Environ. Health Perspect., 14: 3-13.
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series, No. 42).
ELLIOTT, M., FARNMAN, A.W., JONES. N.F., NEEDHAM, P.H., & PEARSON,
B.C. (1967) 5-Benzyl-3-furylmethyl chrysanthemate: a new potent
insecticide. Nature (Lond.), 213: 493-494.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome,
11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
FAO/WHO (1976) Bioresmethrin. In: 1975 Evaluation of some pesticide
residues in food, Geneva, World Health Organization, pp. 17-40
(WHO Pesticide Residues Series, No. 5).
FAO/WHO (1977) Bioresmethrin. In: 1976 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of the
United Nations, pp. 55-78 (AGP: 1976/M/14).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
GAINES, T.B. & LINDER, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. appl. Toxicol., 7: 299-308.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular
junction. Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol.,
15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. appl. Pharmacol.,
66: 290-296.
GARRET, N.E., FRANK STACK, H., & WATERS, D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res.,
168: 301-325.
GEPHART, L.A., JOHNSON, W.D., BECCI, P.J., & PARENT, R.A. (1980)
180-day subchronic oral dosing study with resmethrin in Beagle
dogs, Waverly, New York, Food and Drug Research Laboratories,
86 pp (Report No. 6289) (Unpublished proprietary data supplied by
Roussel Uclaf).
GLICKMAN, A.H. & CASIDA, J.E., (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4: 793-799.
GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished data of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
GLOMOT, R. & CHEVALLIER, B. (1969) Etude de la toxicité aiguë du
RU11484 (NRDC 107) Romainville, France, Roussel Uclaf (Rapport
Toxico 11386) (Unpublished data).
GRAY, A.J., CONNORS, T.A., HOELLINGER, H., & NGUYEN-HOANG-NAM (1980a)
Relationship between the pharmacokinetics of intravenous
cismethrin and bioresmethrin and their mammalian toxicity.
Pestic. Biochem. Physiol., 13: 281-293.
GRAY, A.J., CONNORS, T.A., & RICKARD, J. (1980b) Mechanism of
mammalian toxicity of the pyrethroids. In: Littaner, V.Z., Dudai,
Y., Silman, I. Teichberg, V.I., & Vogel, Z., ed.
Neurotransmitters and their receptors, New York, John Wiley &
Sons Ltd, pp. 565-568.
GREEN, W.M. (1977) Acute dermal LD50 toxicity study on technical
resmethrin, Moorestown, New Jersey, Bio-Toxicology Laboratory,
8 pp (Unpublished proprietary data supplied by Roussel Uclaf).
HESS, F.G., THOMPSON, S.W., & BECCI, P.J. (1982) A lifetime
evaluation of the dietary administration of SBP-1382 to Wistar
albino rats/amendment, Waverly, New York, Food and Drug Research
Laboratory, 28 pp (Supplemental report No. 5271) (Unpublished
proprietary data supplied by Roussel Uclaf).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Bureau of Sport
Fishery and Wildlife, pp. 1-61 (Report No. 191).
HOELLINGER, H., SONNIER, M., GRAY, A.J., CONNORS, T.A., PICHON, J., &
NGUYEN-HOANG-NAM (1985) In vitro covalent binding of cismethrin,
bioresmethrin and their common alcohol to hepatic proteins.
Toxicol. appl. Pharmacol., 77: 11-18.
HOLMSTEAD, R.L., CASIDA, J.E., & RUZO, L.O. (1977) Photochemical
reactions of pyrethroid insecticides. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society
(ACS Symposium Series, No. 42).
JACKSON, G.C. & HARDY, C.J. (1984) SBP-1382 acute inhalation toxicity
in rats 4-hour exposure, Huntingdon, United Kingdom, Huntingdon
Research Centre, 24 pp (Report No. SBP 4a/831042) (Unpublished
proprietary data supplied by Roussel Uclaf).
KAGAN, U.S., PANSHINA, T.N. & SASINOVITCH, L.M. (1986) [Biochemical
effects of synthetic pyrethroid toxic action.] Gig. i Sanit.,
1: 7 (in Russian).
KAUFMAN, D.D. (1986) Degradation and movement of cis- and
trans-resmethrin in soil, Beltsville, US Department of
Agriculture, 36 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
KHOLMATOVA, M. (1984) Hygiene and toxicology of Dravin 755 and
Isotrine new pesticides used in agriculture, Alma Ata (Résumé of
doctorate thesis).
KNICKERBOCKER, M., BECCI, P.J., COX, G.E., & PARENT, R.A. (1980)
A lifetime evaluation of the dietary administration of SBP-1382
to Wistar albino rats, Waverly, New York, Food and Drug Research
Laboratories, 110 pp (Report No. 5271) (Unpublished proprietary
data supplied by Roussel Uclaf).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor and
Francis Ltd, 440 pp.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem.
Physiol., 20: 203-216.
MACHI, R.A., KAM, C., GALLO, M.A., STEVENS, K.R., & GAGLIARDI, J.J.
(1979) Teratological evaluation of SBP-1382 technical in the
albino rat, Florham Park, New Jersey, Booz, Allen & Hamilton,
Inc., 44 pp (Report No. 2054-066) (Unpublished proprietary data
supplied by Roussel Uclaf).
MACKO, J.A., HARVEY, J.G., POPE, C.R., & MCCREESH, A.H. (1979) Hazard
evaluation of aerosol formulations containing the synthetic
pyrethroid insecticide resmethrin, (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(SBP-1382), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency, 38 pp (Report No. 75-51-0848-79).
MALONE, J.C. & CHESHER, B.C. (1970) Toxicity to dogs of NRDC 107,
Berkhamsted, Wellcome Foundation Ltd (Report No. B 204-70)
(Unpublished data).
MARKING, L.L. & MAUCK, W.L. (1975) Toxicity of paired mixtures of
candidate forest insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 13: 518-523.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, 461 pp.
MAUCK, W.L., OLSON, L.E., & MARKING, L.L. (1976) Toxicity of natural
pyrethroids and five pyrethroids to fish. Arch. environ. Contam.
Toxicol., 4: 18-29.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemical handbook, Section C: Pesticide dictionary,
Willoughby, Meister Publishing Co., pp. C205-C206.
MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 98-115 (ACS Symposium Series, No. 42).
MIYAMOTO, J. (1975-76) Terminal residues of bioresmethrin.
Submission to IUPAC Terminal Residue Commission, Madrid, September
1975, Leverkusen, September 1976.
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4
September, 1982, Oxford, Pergamon Press (in 4 volumes).
MIYAMOTO, J., NISHIDA, T., & UEDA, K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furylmethyl d1-trans-chrysanthemate in the
rat. Pestic. Biochem. Physiol., 1: 293-306.
MUMMA, R.O. (1985) Persistence of metabolism of 14C-resmethrin on
greenhouse grown tomato fruit, lettuce and field grown wheat,
Part III, Pennsylvania State University, 33 pp Unpublished
proprietary data supplied by Roussel Uclaf).
MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem.,
36: 2203-2211.
NIOSH (1983) In: Takten, R.L. & Lewis, R.J., Jr, ed. Registry of
toxic effects of chemical substances, 1981-82 edition,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 3057 pp (in 3 volumes).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitai-Kagaku, 4: 31-46 (in Japanese).
NISHIUCHI, Y. (1982) [Toxicity of pesticides to fish.]
Kongetsuno-Noyaku, 26: 105-110 (in Japanese).
NOEL, P.R.B., RIVETT, K.F., CHESTERMAN, H., STREET, A.E., & SPICER,
E.J.F. (1971) Oral toxicity study in Beagle dogs (repeated dosage
for 3 months), 76 pp (Report No. 3900/71/58) (Unpublished data
of Huntingdon Research Centre, United Kingdom).
NORWOOD, T.E. (1986) Preliminary statistical report on assessment of
the effects of SBP-1382 40MF (resmethrin) and Scourge (18%
resmethrin plus 54% piperonyl butoxide) on non target, aquatic
organisms when used for adult mosquito control: results of a
field validation study in fresh water ponds in Minnesota, Summer
1984, North Oaks, Minnesota, Mosquito Control Technology, 20 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
PIERCE, R.H. (1986) Acute toxicity of Scourge on select estuarine
organisms during field applications, Sarasota, Florida, Mote
Marine Laboratory, 16 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C., HAUTEFEUILLE,
A., & BARTSCH, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
PUCHALSKI, R. (1983) Water solubility and octanol/water partition
coefficients of SBP-1382, Lyndhurst, New Jersey, Penick
Corporation, 5 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985a) Acute oral LD50 study of
D-L-cis-trans-chrysanthemic acid in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 10 pp
(Report No. 8350A) (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985b) Acute oral LD50 study of
D-L-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350B) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985c) Acute oral LD50 study of
D-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350C) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985d) Acute oral LD50 study of BFCA
99.6% Sprague-Dawley rats, Waverly, New York, Food and Drug
Research Laboratories, 10 pp (Report No. 8725A) (Unpublished
proprietary data supplied by Roussel Uclaf).
RIDLEN, R.L., CHRISTOPHER, R.J., WAYNE IRVIE, G., BEIER, R.C., & CAMP,
B.J. (1984) Distribution and metabolism of cis and
trans-resmethrin in lactating Jersey cows. J. agric. food
Chem., 32: 1211-1217.
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O.
(1985a) The effects of dietary inclusion of resmethrin on
reproduction in the bobwhite quail, Huntingdon, United Kingdom,
Huntingdon Research Centre, 34 pp (Report No. SBP 8BT/85957)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O,
(1985b) The effects of dietary inclusion of resmethrin on
reproduction in the mallard duck, Huntingdon, United Kingdom,
Huntingdon Research Centre, 28 pp (Report No. SBP 7BT/85550)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROPER, E.M. & WRIGHT, G.C. (1984) Sampling efficiency of five solid
sorbents for trapping airborne pesticides. Bull. environ. Contam.
Toxicol., 33: 476-483.
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four synthetic
pyrethroids fail to show any correlation between neuromuscular
dysfunction and neurobiochemical abnormalities in rats. Arch.
Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979a) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase I, Waverly,
New York, Food and Drug Research Laboratories, 18 pp (Report
No. 6067) (Unpublished proprietary data supplied by Roussel
Uclaf).
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979b) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase II,
Waverly, New York, Food and Drug Research Laboratories, 17 pp
(Report No. 6068) (Unpublished proprietary data supplied by
Roussel Uclaf).
SCHWARTZ, C.S., GEPHART, L., BECCI, P.J., & PARENT R.A. (1979c) The
evaluation of the effects of SBP-1382 following dietary
administration through three generations in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 70 pp
(Report No. 5739) (Unpublished proprietary data supplied by
Roussel Uclaf).
SEABER, J.A. (1985) Delayed contact hypersensitivity in the guinea
pig with SBP-1382 insecticide, Huntingdon, United Kingdom,
Huntingdon Research Centre, 14 pp (Report No. 84936D/SBP 10/ss)
(Unpublished proprietary data supplied by Roussel Uclaf).
SIMONAITIS, R.A. & CAIL, R.S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour, and wheat.
J. Assoc. Off. Anal. Chem., 58: 1032-1036.
SJOGREN, R.D. (1985) Assessment of the effects of SBP-1382 40MF
(resmethrin) and Scourge (18% resmethrin plus 54% piperonyl
butoxide) on non target, aquatic organisms when used for adult
mosquito control: results of a field validation study in fresh
water ponds in Minnesota, Summer 1984, North Oaks, Minnesota,
Mosquito Control Technology, 105 pp (Unpublished proprietary data
supplied by Roussel Uclaf).
SMITH, P.R. (1980) The effect of cismethrin on the rat dorsal root
potentials. Eur. J. Pharmacol., 66: 125-128.
STAUFFER (1984) Ethyl chrysanthemate/Product safety information,
Westport, USA, Stauffer Chemical Co., 5 pp.
SWENTZEL, K.L., ANGERHOFER, R.A., & HAIGHT, E.A. (1977) Toxicological
evaluation of pyrethroid insecticide (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(resmethrin), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency (Report No. 51-0830-77).
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1974) Photodecomposition of
resmethrin and related pyrethroids. J. agric. food Chem.,
22: 212-220.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pestic. Biochem.
Physiol., 5: 280-294.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975b) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food
Chem., 23: 106-115.
UNO, M., OKADA, T., OHMAE, T., & TANIGAWA, K. (1982) [Determination of
pyrethroid insecticides by dual-wavelength densitometry.]
Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
US ENVIRONMENTAL PROTECTION AGENCY (1983) Tolerances for pesticides in
food administered by EPA: Resmethrin. Fed. Reg.,
48: 36246-36247.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973)
DDT-likeaction of allethrin in the sensory nervous system of
Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B A., & AKKERMANS, L.M.A. (1979) Effect
of insecticides on the sensory nervous system. In: Narahashi, T.,
ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 182-210.
VANNIER, B. & FOURNEX, R. (1986) Bioresmethrin. Detection of a
mutagenic potency (micronucleus test in the mouse), Romainville,
France, Roussel Uclaf, 16 pp (Report No. RU-BRM-85612-BB/178/A)
(Unpublished proprietary data).
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol.,
45: 325-329.
VERSCHOYLE, R.D. & BARNES, J.M. (1972) Toxicity of natural and
synthetic pyrethrins in rats. Pestic. Biochem. Physiol.,
2(3): 308-311.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature and structure-dependent interaction
of pyrethroids with the sodium channels in frog node of Ranvier.
Biochim. Biophys. Acta, 728: 73-82.
VOLKOV, U.P., STRELETS, I.P., & TIULENEV, K.L. (1979) [Progress in
the field of pyrethroids and their synergists. Collection of
articles on household chemistry.] Moscow, Volume 7, p. 125
(in Russian).
WALDRON, M.M. (1969) Foetal toxicity study -- 95 H 56 -- given orally
in the rabbit (Report Path 51) (Unpublished data of Wellcome
Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide (Report
No. B 47-71) (Unpublished data of Wellcome Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1972) Bioresmethrin. Preliminary 24
hour rat inhalation study (Report No. B 290-72) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CHESHER, B.C., & MALONE J.C. (1970) Toxicity of NRDC
107 to laboratory animals (Report No. B 199-70) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study (Report No. B 199-71) (Unpublished
data of Wellcome Foundation Ltd).
WATSON, S.C. (1984) Photolysis study of SBP-1382 resmethrin (final
report), Columbus, Ohio, Battelle Columbus Laboratory, 10 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
WEEKS, M.H., LELAND, T.M., BOLDT, R.E., MELLICK, P.W., & STEINBERG, M.
(1972) Toxicological evaluation of pyrethroid insecticide
(5-benzyl-3-furyl) methyl-2,2-dimethyl-3-(2-methyl-propenyl)
cyclopropanecarboxylate (SBP-1382), Aberdeen Proving Ground, US
Army Environmental Hygiene Agency, 44 pp (Special study
No. 51-127-71/72).
WHITE, I.N.H., VERSCHOYLE, R.D., MORADIAN, M.H., & BARNES, J.M. (1976)
The relationship between brain levels of cismethrin and
bioresmethrin in female rats and neurotoxic effects. Pestic.
Biochem. Physiol., 6: 491-500.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-1987, Geneva, World Health
Organization, p. 38 (VBC/86.1, Rev. 1).
WHO/FAO (1978) Data sheet on pesticides No. 34: Bioresmethrin,
Geneva, World Health Organization (WHO/VBC/DS/78. 34).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, 485 pp.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YOSHIOKA, H. (1980) [Chemistry and action of the recent synthetic
pyrethroids and their stereoisomers.] J. synth. org. Chem.
Japan, 38: 1151-1162 (in Japanese).
APPENDIX
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs (Xenopus laevis; Rana temporaria, and Rana
esculenta), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches by Gammon et al. (1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982; Gammon
& Casida, 1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the insecticide
on neurotransmitter release or on the sensitivity of the subsynaptic
membrane or the muscle fibre membrane. Presynaptic repetitive firing
was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres,
the pyrethroid-induced repetitive activity increases dramatically as
the temperature is lowered, and a decrease of 5°C in temperature may
cause a more than 3-fold increase in the number of repetitive impulses
per train. This effect is easily reversed by raising the temperature.
The origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that underlies
the generation and conduction of each nerve impulse. The transitional
state of the sodium channel is controlled by 2 separately acting
gating mechanisms, referred to as the activation gate and the
inactivation gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the activation
gate and the slow closing of the inactivation gate proceed normally.
However, once the sodium channel is open, the activation gate is
restrained in the open position by the pyrethroid molecule. While all
pyrethroids have essentially the same basic mechanism of action, the
rate of relaxation differs substantially for the various pyrethroids
(Flannigan & Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more
than 100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the Type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing afterpotentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral-line organ in the form of
long-lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train increase
markedly on lowering the temperature. Cypermethrin does not cause
repetitive activity in myelinated nerve fibres. Instead, this
pyrethroid causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of the nerve
membrane as a result of the summation of depolarizing after-potentials
during train stimulation (Vijverberg & Van den Bercken, 1979;
Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a
long-lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]-t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an absolute
correlation, without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, [1R,cis]-cypermethrin,
[1R,trans]-cypermethrin, and [2S, alphaS]-fenvalerate were
inhibitors, but their non-toxic stereoisomers were not; non-cyano
pyrethroids were much less potent or were inactive (Lawrence & Casida,
1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The
[3H]-dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of pyrethroids are
mediated, at least in part, through an interaction with a
GABA-regulated chloride ionophore-associated binding site. Moreover,
studies with [3H]-Ro 5-4864 support this hypothesis and, in addition,
indicate that the pyrethroid-binding site may be very closely related
to the convulsant benzodiazepine site of action (Lawrence et al.,
1985).
The Type II pyrethroids (deltamethrin, [1R,cis]-cypermethrin and
[2S, alpha2]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two
non-insecticidal stereoisomers and Type I pyrethroids (permethrin,
resmethrin, allethrin) were inactive. Therefore, cyanophenoxybenzyl
pyrethroids appear to act on the GABA receptor-ionophore complex
(Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action
potentials without presynaptic repetitive activity. However, an
intermediate group of pyrethroids (1R-permethrin, cyphenothrin, and
fenvalerate) caused both types of effect. Thus, in muscle or nerve
membrane the pyrethroid induced repetitive activities due to a
prolongation of the sodium current. But no clear distinction was
observed between non-cyano and alpha-cyano pyrethroids (Ruigt & Van
den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is
the sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group (allethrin, d-phenothrin, permethrin, and
cismethrin) cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation. This
results in relatively short trains of repetitive nerve impulses in
sense organs, sensory (afferent) nerve fibres, and, in effect, nerve
terminals. On the other hand, the alpha-cyano pyrethroids cause a
long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin and cypermethrin, which
have identical molecular structures except for the presence of an
alpha-cyano group on the phenoxybenzyl alcohol, indicates that it is
this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids,
the ultimate lesion that causes death will depend on the animal
species, environmental conditions, and on the chemical structure and
physical characteristics of the pyrethroid molecule (Vijverberg & Van
den Bercken, 1982).
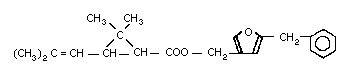 Resmethrin was first produced in 1967 by Elliott et al. (1967). It
is prepared by the esterification of [1RS, 3RS or 1RS, cis,
trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid
or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol.
Resmethrin is thus a mixture of 4 stereoisomers (see Fig. 1). The
cis:trans isomer ratio is reported to be 1:4 and the optical ratio
of 1R:1S is 1:1 (racemic). Thus, its composition will be roughly
4:1:4:1 of isomers (1), (2), (3), and (4).
Bioresmethrin is the [1R,trans]- or (1) isomer and cismethrin is
the [1R,cis]- or (2) isomer (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of resmethrins are given in
Table 2.
Resmethrin is decomposed rapidly on exposure to air or light and
in alkaline media (Elliot, 1976; FAO/WHO, 1976; Martin & Worthing,
1977; Meister et al., 1983; Puchalski, 1983; Worthing & Walker, 1983;
Devaux & Bolla, 1986).
2.3 Analytical Methods
Methods for the determination of residues, analysis of
environmental samples, and product analysis for both resmethrin and
bioresmethrin are summarized in Table 3. Procedures for extraction,
partition, and cleanup are listed together with analytical equipment
and conditions, minimum detectable concentrations, and percentage
recovery.
Technical grade resmethrin can be analysed by dissolving the
product and dicyclohexyl phthalate (an internal standard) in acetone
and injecting the solution in a gas chromatograph equipped with a
flame ionization detector (FID) (Murano, 1972).
Resmethrin was first produced in 1967 by Elliott et al. (1967). It
is prepared by the esterification of [1RS, 3RS or 1RS, cis,
trans]-2,2-dimethyl-3-(2,2-dimethylvinyl)cyclopropanecarboxylic acid
or chrysanthemic acid with 5-benzyl-3-furyl-methyl alcohol.
Resmethrin is thus a mixture of 4 stereoisomers (see Fig. 1). The
cis:trans isomer ratio is reported to be 1:4 and the optical ratio
of 1R:1S is 1:1 (racemic). Thus, its composition will be roughly
4:1:4:1 of isomers (1), (2), (3), and (4).
Bioresmethrin is the [1R,trans]- or (1) isomer and cismethrin is
the [1R,cis]- or (2) isomer (Table 1).
2.2 Physical and Chemical Properties
Some physical and chemical properties of resmethrins are given in
Table 2.
Resmethrin is decomposed rapidly on exposure to air or light and
in alkaline media (Elliot, 1976; FAO/WHO, 1976; Martin & Worthing,
1977; Meister et al., 1983; Puchalski, 1983; Worthing & Walker, 1983;
Devaux & Bolla, 1986).
2.3 Analytical Methods
Methods for the determination of residues, analysis of
environmental samples, and product analysis for both resmethrin and
bioresmethrin are summarized in Table 3. Procedures for extraction,
partition, and cleanup are listed together with analytical equipment
and conditions, minimum detectable concentrations, and percentage
recovery.
Technical grade resmethrin can be analysed by dissolving the
product and dicyclohexyl phthalate (an internal standard) in acetone
and injecting the solution in a gas chromatograph equipped with a
flame ionization detector (FID) (Murano, 1972).
 Table 1. Chemical identity of resmethrins of various stereoisomeric compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
Resmethrine [5-(phenylmethyl)-3-furanyl]methyl (1):(2):(3):(4) Benzofuroline,
Table 1. Chemical identity of resmethrins of various stereoisomeric compositions
Common name/ CAS Index name (9CI) Stereoisomeric Synonyms and
CAS Registry No./ compositiond trade names
RTECS Registry No.a Stereospecific nameb,c
Resmethrine [5-(phenylmethyl)-3-furanyl]methyl (1):(2):(3):(4) Benzofuroline,
 In contrast, there was a 10% loss in the original weight of
resmethrin after 24 h compared with losses of 29% and 41% for
tetramethrin and allethrin, respectively, when exposed as a thin film
(500 mg/38.5 cm2) on glass to an incandescent lamp (Abe et al.,
1972).
The photodegradation of aqueous solutions (sterile water and
artificial sea-water) of resmethrin by natural sunlight has also been
investigated. The half-life was estimated to be 47 min in water and
20 min in sea water. The major degradation product was chrysanthemic
acid (Watson, 1984).
4.2 Degradation in Soil
The degradation under aerobic conditions of 14C-resmethrin
applied to soil at rates equivalent to 0.12 and 1.7 kg/ha has been
studied. The compound degraded very rapidly, so that less than 2% of
the applied 14C-resmethrin remained after 16 days. Complete
mineralisation to 14CO2 was a very important metabolic process
(38% of the applied radioactivity in 16 days). The radioactivity
remaining in the soil after 16 days was a complex mixture of more than
11 products resulting from ester cleavage and oxidation reactions
(Kaufman, 1986).
4.3 Degradation on Plants
Tomato and lettuce plants were treated with 14C-resmethrin, under
greenhouse conditions; wheat was similarly treated in the field. The
radioactivity was applied by spraying an aqueous suspension at a
concentration equivalent to 0.5 g/litre. The applied resmethrin
degraded very rapidly so that 55-82% had degraded within 2 h and no
unchanged resmethrin remained after 5 days. A very complex mixture of
degradation products were formed on the plants (up to 31 individual
compounds). No individual component in the mixture of products formed
exceeded 10% of the amount applied at any sampling interval up to 15
days after treatment. The alcohol formed by ester cleavage and
benzaldehyde (15), phenylacetic acid (13), benzoic acid (16), and
benzyl alcohol (14) were identified as degradation products. These
compounds were each present at levels < 3% of the radioactive residue
(Mumma, 1985).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Metabolic pathways for resmethrin in mammals are summarized in
Fig. 3.
When 14C-[1RS,trans]-resmethrin (19) labelled in the alcohol
moiety was administered orally to Sprague-Dawley rats at 500 mg/kg,
the radiocarbon was rapidly absorbed from the gastrointestinal tract
and it took 3 weeks for the complete elimination of the radioactivity
in the urine (36% of the dose) and faeces (64%). A negligible amount
(less than 0.1%) of the radiocarbon was expired as 14CO2. The
radiocarbon was not completely excreted, even after 2 weeks, in rats
given an intravenous dose of 50 mg/kg; notably, appreciable amounts of
14C were found in faeces. The results of another study indicated that
the excretion of 14C via the bile duct was very rapid and that more
than 50% of the total dose was recovered from the bile within 72 h.
The urinary metabolites were mainly 5-benzyl-3-furancarboxylic acid
(20) (BFCA) in the free form (28% of the urinary 14C), the
glucuronide form (2%), and unknown conjugated (18%) forms. Other
significant metabolites present in the urine were hydroxy derivatives
of BFCA, such as alpha-(4-carboxy-2-furyl)-benzyl alcohol (21)
(alpha-OH-BFCA) (11%), 5-benzoyl-3-furancarboxylic acid (22)
(alpha-keto-BFCA) (1%), 5-benzyl-4-hydroxy-3-furancarboxylic acid (23)
(2%), and 5-(p-hydroxybenzyl)-3-furancarboxylic acid (24)
(4'-OH-BFCA) in the free form (3%), the sulfate form (12%) and the
glucuronide (2%) form (Miyamoto et al., 1971) (Fig. 3).
Ueda et al. (1975b) elucidated the metabolic fates of the acid and
alcohol moieties of bioresmethrin (19) and cismethrin (25) in rats
after a single oral dose (acid- and alcohol-labelled moieties) at
1 mg/kg. The predominant metabolites from the alcohol moiety were
BFCA, 4'-OH-BFCA, and alpha-OH-BFCA. The metabolites in the body were
derived from the alcohol moiety of bioresmethrin. On the other hand,
the isobutenyl group of the acid moiety was oxidized at either the
cis- or trans-methyl group of cismethrin, but only at the
trans-methyl group of bioresmethrin. The major metabolites from the
acid moiety were the hydroxymethyl (28, 29) or dicarboxylic acid
derivatives (30, 31) of chrysanthemic acid (26, 27) (CA). The aldehyde
intermediates of CA were not found. The major dicarboxylic acid
derivatives of CA were metabolites receiving oxidation at the
trans-methyl group of the isobutenyl moiety. Although the
trans-methyl group was predominantly oxidized in both isomers, the
cis-methyl group of the cis-isomer was also oxidized (32). An
anticipated metabolic pathway involves epimerization at C-3 of the
cyclopropane group leading to isomerized forms of dicarboxylic acid
derivatives of CA. The aldehyde derivatives of CA are the most likely
compounds for isomerization, because these derivatives readily undergo
cis- trans-isomerization under the alkaline conditions (Ueda et
al., 1975b).
In contrast, there was a 10% loss in the original weight of
resmethrin after 24 h compared with losses of 29% and 41% for
tetramethrin and allethrin, respectively, when exposed as a thin film
(500 mg/38.5 cm2) on glass to an incandescent lamp (Abe et al.,
1972).
The photodegradation of aqueous solutions (sterile water and
artificial sea-water) of resmethrin by natural sunlight has also been
investigated. The half-life was estimated to be 47 min in water and
20 min in sea water. The major degradation product was chrysanthemic
acid (Watson, 1984).
4.2 Degradation in Soil
The degradation under aerobic conditions of 14C-resmethrin
applied to soil at rates equivalent to 0.12 and 1.7 kg/ha has been
studied. The compound degraded very rapidly, so that less than 2% of
the applied 14C-resmethrin remained after 16 days. Complete
mineralisation to 14CO2 was a very important metabolic process
(38% of the applied radioactivity in 16 days). The radioactivity
remaining in the soil after 16 days was a complex mixture of more than
11 products resulting from ester cleavage and oxidation reactions
(Kaufman, 1986).
4.3 Degradation on Plants
Tomato and lettuce plants were treated with 14C-resmethrin, under
greenhouse conditions; wheat was similarly treated in the field. The
radioactivity was applied by spraying an aqueous suspension at a
concentration equivalent to 0.5 g/litre. The applied resmethrin
degraded very rapidly so that 55-82% had degraded within 2 h and no
unchanged resmethrin remained after 5 days. A very complex mixture of
degradation products were formed on the plants (up to 31 individual
compounds). No individual component in the mixture of products formed
exceeded 10% of the amount applied at any sampling interval up to 15
days after treatment. The alcohol formed by ester cleavage and
benzaldehyde (15), phenylacetic acid (13), benzoic acid (16), and
benzyl alcohol (14) were identified as degradation products. These
compounds were each present at levels < 3% of the radioactive residue
(Mumma, 1985).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Metabolic pathways for resmethrin in mammals are summarized in
Fig. 3.
When 14C-[1RS,trans]-resmethrin (19) labelled in the alcohol
moiety was administered orally to Sprague-Dawley rats at 500 mg/kg,
the radiocarbon was rapidly absorbed from the gastrointestinal tract
and it took 3 weeks for the complete elimination of the radioactivity
in the urine (36% of the dose) and faeces (64%). A negligible amount
(less than 0.1%) of the radiocarbon was expired as 14CO2. The
radiocarbon was not completely excreted, even after 2 weeks, in rats
given an intravenous dose of 50 mg/kg; notably, appreciable amounts of
14C were found in faeces. The results of another study indicated that
the excretion of 14C via the bile duct was very rapid and that more
than 50% of the total dose was recovered from the bile within 72 h.
The urinary metabolites were mainly 5-benzyl-3-furancarboxylic acid
(20) (BFCA) in the free form (28% of the urinary 14C), the
glucuronide form (2%), and unknown conjugated (18%) forms. Other
significant metabolites present in the urine were hydroxy derivatives
of BFCA, such as alpha-(4-carboxy-2-furyl)-benzyl alcohol (21)
(alpha-OH-BFCA) (11%), 5-benzoyl-3-furancarboxylic acid (22)
(alpha-keto-BFCA) (1%), 5-benzyl-4-hydroxy-3-furancarboxylic acid (23)
(2%), and 5-(p-hydroxybenzyl)-3-furancarboxylic acid (24)
(4'-OH-BFCA) in the free form (3%), the sulfate form (12%) and the
glucuronide (2%) form (Miyamoto et al., 1971) (Fig. 3).
Ueda et al. (1975b) elucidated the metabolic fates of the acid and
alcohol moieties of bioresmethrin (19) and cismethrin (25) in rats
after a single oral dose (acid- and alcohol-labelled moieties) at
1 mg/kg. The predominant metabolites from the alcohol moiety were
BFCA, 4'-OH-BFCA, and alpha-OH-BFCA. The metabolites in the body were
derived from the alcohol moiety of bioresmethrin. On the other hand,
the isobutenyl group of the acid moiety was oxidized at either the
cis- or trans-methyl group of cismethrin, but only at the
trans-methyl group of bioresmethrin. The major metabolites from the
acid moiety were the hydroxymethyl (28, 29) or dicarboxylic acid
derivatives (30, 31) of chrysanthemic acid (26, 27) (CA). The aldehyde
intermediates of CA were not found. The major dicarboxylic acid
derivatives of CA were metabolites receiving oxidation at the
trans-methyl group of the isobutenyl moiety. Although the
trans-methyl group was predominantly oxidized in both isomers, the
cis-methyl group of the cis-isomer was also oxidized (32). An
anticipated metabolic pathway involves epimerization at C-3 of the
cyclopropane group leading to isomerized forms of dicarboxylic acid
derivatives of CA. The aldehyde derivatives of CA are the most likely
compounds for isomerization, because these derivatives readily undergo
cis- trans-isomerization under the alkaline conditions (Ueda et
al., 1975b).
 5.2 Enzymatic Systems for Biotransformation
When 14C alcohol-labelled cismethrin, bioresmethrin, and
5-benzyl-3-furylmethyl alcohol (BFA) (18) were incubated with rat
liver S-9 homogenates or microsomes, a proportion of the radioactive
compounds was covalently bound to proteins. The covalent binding was
greater with phenobarbital-pretreated rats, and dependent on a
NADPH-generating system. When a S-9 homogenate was used, the bound
compounds were twice as high for cismethrin as for bioresmethrin and
BFA. Inversely, when microsomes were used, more covalent binding
occurred with bioresmethrin and BFA than with cismethrin. The
inhibition of esterases by tetraethyl pyrophosphate (TEPP) in a S-9
homogenate did not alter the amount of covalent binding to the three
compounds, whereas malathion inhibited this binding. However,
treatment of a S-9 homogenate with piperonyl butoxide greatly reduced
covalent binding. Covalent binding was inhibited when the microsomes
were incubated with carbon monoxide or modified by thermal
denaturation. It is suggested that oxidative metabolism was
responsible for the covalent binding (Hoellinger et al., 1985).
When four resmethrin isomers ([1R,trans]-, [1S,trans]-,
[1R,cis]-, and [1S,cis]-) were incubated with mouse and rat
microsomes in 50 mmol/litre tris-HCl buffer (pH 7.4) at 37°C for 1 h,
microsomal esterases readily cleaved the trans- but not the
cis-isomers. The ester linkage also appeared to undergo oxidative
cleavage when esterase attack was minimal. Ester metabolites were
detected in significant amounts only with [1R,cis]-resmethrin, in
which case oxidation had occurred at the isobutenyl moiety, with or
without oxidation at the benzylfuryl methyl group. Most of the
in vitro metabolites were identical with those in the excreta of
rats given resmethrin orally. The preferred site of oxidation in the
isobutenyl moiety varies with the resmethrin isomer and microsomal
source. Mouse microsomes predominantly oxidized the trans-methyl
group of both [1R,trans]- and [1S,trans]-resmethrin, the
selectivity, however, being the greatest with [1S,trans]-resmethrin.
Rat microsomes were relatively nonselective in attacking the
isobutenyl methyl groups of [1R,trans]-resmethrin (Ueda et al.,
1975a).
Plasma-esterases were equally active in hydrolysing cismethrin and
bioresmethrin (3.2-3.4 nmol benzylfurylmethanol/min per g) whereas
liver microsomal esterases hydrolysed bioresmethrin 10 times more
rapidly than cismethrin (White et al., 1976).
6. EFFECTS ON THE ENVIRONMENT
Data on the acute toxicity of resmethrin for aquatic and
terrestrial non-target organisms are summarized in Tables 4 and 5,
respectively.
6.1 Aquatic Organisms
Resmethrin is highly toxic for fish, and the toxicity is
negatively correlated with temperature (Mauck et al., 1976); the LC50
value for bluegill is 2.3 times greater at 22°C than at 12°C, as shown
in Table 4. The influence of both water hardness and pH on toxicity
ranged from slight to negligible. By keeping resmethrin solution at
12°C for 1 week, the toxicity for bluegill decreased 2-fold at each pH
value as the resmethrin solution aged.
Among the 4 isomers, the 1R, cis-isomer is most toxic for fish,
followed by the 1R, trans-, 1S, cis- and 1S, trans-, as observed
in pyrethroids. Contrary to other pyrethroids, resmethrin is less
toxic for arthropods than for fish, as shown in Table 4. The 48-h
LC50 values for arthropods are in the range of 2-25 µg/litre
(Nishiuchi, 1981).
Laboratory studies have shown that resmethrin is highly toxic for
fish but, because of the low water solubility of the compound and
rapid environmental degradation (photochemical and microbial), it
would be expected that, under field conditions, the toxic impact would
be greatly reduced. Such a reduction in toxic effects has been
confirmed in field studies carried out in the USA (Sjogren, 1985;
Norwood, 1986; Pierce, 1986).
In one of these studies, ponds were sprayed with resmethrin
formulations at the expected field rate for adult mosquito control
(0.01 kg/ha). Residue analysis confirmed that resmethrin levels fall
rapidly (1.1 ppb at zero time nondetectable after 96 h). Caged fish
showed good survival (82-100% for bluegill, 73-100% for goldfish) in
resmethrin-treated ponds. However, one fish species in this study
(white sucker) was susceptible (8% survival) when piperonyl
butoxide-synergised resmethrin was used, though good survival was
noted for this species with unsynergised resmethrin. In a second
study, synergised resmethrin was applied to a mangrove-fringed pond at
2-day intervals. The first two applications were at the normal field
rate, but the third was at 10 times this rate. In this study, both
fish species used to monitor fish survival (sheepshead minnow,
fingerling snook) showed good survival (100% at the normal rate and
88% at the 10-fold rate).
Table 4. Acute toxicity of resmethrin for non-target aquatic organisms
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish
Carp (Cyprinus 48-h LC50 44 technical static 25 Nishiuchi (1982)
carpio)
Killifish adult 48-h LC50 300 technical static 25 Miyamoto (1976)
(Oryzias adult 48-h LC50 16 (+)-trans- static 25 Miyamoto (1976)
latipes) adult 48-h LC50 8 (+)-cis- static 25 Miyamoto (1976)
adult 48-h LC50 > 10 000 (-)-trans- static 25 Miyamoto (1976)
adult 48-h LC50 3500 (-)-cis- static 25 Miyamoto (1976)
Rainbow trout 96-h LC50 2.2 technical static 12 Marking & Mauck
(Salmo gairdneri) (1975)
Coho salmon 96-h LC50 1.50 technical static 12 Mauck et al.
(Oncorhynchus (1976)
kisutch) 96-h LC50 0.277 technical flow- 12 Mauck et al.
through (1976)
Steelhead trout 96-h LC50 0.450 technical static 12 Mauck et al.
(Salmo gairdneri) (1976)
96-h LC50 0.275 technical flow- 12 Mauck et al.
through (1976)
Yellow perch 96-h LC50 2.36 technical static 12 Mauck et al.
(Perca flavescens) (1976)
96-h LC50 0.513 technical flow- 12 Mauck et al.
through (1976)
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Arthropods
Daphnia pulex 3-h LC50 15 000 technical static 25 Nishiuchi (1982)
3-h LC50 50 000 technical static 25 Miyamoto (1976)
3-h LC50 25 000 - (+)-trans- static 25 Miyamoto (1976)
50 000
3-h LC50 25 000 - (+)-cis- static 25 Miyamoto (1976)
50 000
3-h LC50 > 50 000 (-)-trans- static 25 Miyamoto (1976)
3-h LC50 > 50 000 (-)-cis- static 25 Miyamoto (1976)
Moina macrocopa 3-h LC50 14 000 technical static 25 Nishiuchi (1982)
Sigara substriata 0.59 cm; 48-h LC50 2 technical static 25 Nishiuchi (1981)
6.1 mg
Micronecta sedula 0.32 cm; 48-h LC50 3.3 technical static 25 Nishiuchi (1981)
1.8 mg
Cloeon dipterum 0.93 cm; 48-h LC50 4.5 technical static 25 Nishiuchi (1981)
5.6 mg
Orthetrum 2.3 cm; 48-h LC50 7.3 technical static 25 Nishiuchi (1981)
albistylum 0.62 g
speciosum
Eretes sticticus 1.5 cm; 48-h LC50 25 technical static 25 Nishiuchi (1981)
0.2 g
Sympetrum 2.1 cm; 48-h LC50 10 technical static 25 Nishiuchi (1981)
frequens 0.56 g
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish (contd).
Bluegill (Lepomis 96-h LC50 2.62 technical static 12 Mauck et al.
macrochirus) (1976)
96-h LC50 0.750 technical flow- 12 Mauck et al.
through (1976)
0.8 g 96-h LC50 5.46 technical static 22 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 4.28 technical static 17 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.33 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.53 technical static 12 6.6 10-13 Mauck et al.
(1976)
0.8 g 96-h LC50 2.54 technical static 12 7.8 160-180 Mauck et al.
(1976)
0.8 g 96-h LC50 2.06 technical static 12 8.2 280-320 Mauck et al.
(1976)
0.8 g 96-h LC50 2.29 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.46 technical static 12 9.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.89 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.70 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 3.13 technical static 12 8.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.68 technical static 12 9.5 40-48 Mauck et al.
(1976)
6.2 Terrestrial Organisms
Acute toxicity data show that both resmethrin and bioresmethrin
(Table 5) are harmless for birds, as are other pyrethroids.
In addition, a study has been carried out to assess the effects of
resmethrin on reproduction in the Mallard duck and Bobwhite quail. The
birds were given 12, 60, or 300 mg resmethrin/kg diet for 22 weeks. No
effects on reproduction were seen in either species at these dose
levels (Roberts et al., 1985a,b).
Table 5. Acute toxicity of resmethrin and bioresmethrin or non-target terrestrial
organisms
Species Compound Size Application Toxicity Reference
(days) (mg/kg)
Bird
Quail Resmethrin 14 diet LC50 Hill et al.
(Coturnix > 5000 (1975)
coturnix
japonica)
Mallard 10 diet LC50 Hill et al.
(Anas > 5000 (1975)
platyrhynchos)
Chicken Bioresmethrin oral LD50 Chesher &
> 10 000 Malone (1970a)
Wallwork
et al. (1970)
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Acute Toxicity
Acute toxic signs produced in experimental animals by exposure to
resmethrins include tremor, hyperexcitability, convulsive twitching,
prostration, and coma.
LD50 data on resmethrin isomers administered orally and by other
exposure routes are summarized in Tables 6 and 7, respectively.
These data show that resmethrins are weakly toxic, except for
cismethrin, the acute oral toxicity of which is moderate in the mouse.
The results of acute toxicity tests of the resmethrin metabolites
are given in Table 8.
Acute (1 h) inhalation studies were performed with 3 aerosol
formulations of resmethrin and 2 blank formulations. Groups of 20 male
rats and 4 male rabbits inhaled the active ingredient at concentration
levels of 12-13.7 mg/litre (value based on the weight loss from each
spray can). In addition, 10 exposed and 10 control rats were tested
for conditioned avoidance performance immediately following exposure.
Laboured breathing was seen generally in the animals. Several rabbits
died in both the resmethrin-treated and the blank-formulation groups.
In the rats, there were no effects on body weight, or gross or
histopathological lesions. However, the conditioned avoidance latency
in the resmethrin-treated rats was significantly greater than that in
the unexposed controls (Macko et al., 1979).
7.2 Short-term Exposure
7.2.1 Oral administration
Groups of 6 Sprague-Dawley rats from each sex were administered
resmethrin in the diet at concentrations of 0, 310-323, 630-660,
1230-1250, 1423-2670, or 1680-5100 mg/kg, for 14 days. Five out of 6
rats died at the highest exposure level in both sexes. Tremor was
observed and body weight gain and food utilization were reduced at
levels of 1230-1250 mg/kg or more. A significant increase in the
hepatic organ-to-body weight ratios was noted in all groups of females
and in groups of males fed 630-660 mg/kg or more. Compound-related
histopathological changes were not observed in any of the tissues and
organs examined. The maximum no-observed-adverse-effect dietary level
was 310 mg/kg for male rats only (Swentzel et al., 1977).
Table 6. Acute oral toxicity of resmethrin isomers
LD50 (mg/kg
Compound Animal Sex body weight) Reference
Resmethrin rat M > 5000 Miyamoto (1976)
rat F > 5000 Miyamoto (1976)
rat M 1244 Gaines & Linder (1986)
rat F 1721 Gaines & Linder (1986)
rat (weanling) M 1987 Gaines & Linder (1986)
rat ? > 2500 Worthing & Walker (1983)
rat - 960 Volkov et al. (1979)
mouse M 690 Miyamoto (1976)
mouse F 940 Miyamoto (1976)
Bioresmethrin rat M 8800 Glomot & Chevalier (1969)
rat F > 8000 Verschoyle & Barnes (1972)
rat F 7071 Wallwork et al. (1970)
rat - 840 Kholmatova (1984)
rat - 990 Volkov et al. (1979)
rat - 675 Kagan et al. (1986)
mouse M 590 Miyamoto (1976)
mouse F 800 Miyamoto (1976)
mouse M 3100 Ueda et al. (1975b)
mouse F 10000 Wallwork et al. (1970)
mouse - 520 Kholmatova (1984)
mouse - 480 Kagan et al. (1986)
rabbit - 225 Kholmatova (1984)
Cismethrin mouse M 152 Miyamoto (1976)
mouse F 160 Miyamoto (1976)
[1S,-trans]-resmethrin mouse M 500 Miyamoto (1976)
mouse F 600 Miyamoto (1976)
[1S,-cis]-resmethrin mouse M 3700 Miyamoto (1976)
mouse F 5000 Miyamoto (1976)
Table 7. Acute toxicity of resmethrins via other than oral exposure
LD50 LC50
Compound Animal Sex Route (mg/kg (mg/m3) Reference
body weight)
Resmethrin rat M dermal 2500 Gaines & Linder (1986)
rat F dermal 2500 Gaines & Linder (1986)
rabbit - dermal 2500 Green (1977)
rat - inhalation > 9490 Jackson & Hardy (1984)
(4-h exposure)
rat - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
rabbit - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
dog - inhalation > 420 Macko et al. (1979)
(4-h exposure)
Bioresmethrin rat F dermal > 10 000 Wallwork et al. (1970)
rat F intravenous 340 Verschoyle & Barnes (1972)
rat F intravenous 106-133 Chesher & Malone (1971b)
rat F intraperitoneal > 8000 Wallwork & Malone (1971)
rat F inhalation > 872 Wallwork & Malone (1972)
(24-h exposure)
mouse M intraperitoneal > 1500 Ueda et al. (1975b)
mouse F intraperitoneal > 5359 Wallwork et al. (1970)
Mixture of rat M,F inhalation > 1500 Miyamoto (1976)
bioresmethrin (4-h exposure)
& cismethrin mouse M,F inhalation > 1500 Miyamoto (1976)
(4-h exposure)
Table 8. Acute toxicity of metabolites of resmethrin
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
(bioresmethrin), (5-benzyl-3-furyl- mouse > 1500a 3100a Miyamoto (1975-1976)
methyl (+)-trans-chrysanthemate) (1) Ueda et al. (1975b)
1R or (+)-cis-resmethrin (5-benzyl-3-furyl- (2) mouse 320a Miyamoto (1975-1976)
methyl (+)-cis-chrysanthemate) Ueda et al. (1975b)
d,1-cis,trans-chrysanthemic acid (17) rat 2443 Reagan & Becci (1985a)
d,1-trans-chrysanthemic acid - rat 1598 Reagan & Becci (1985b)
(+)-trans-chrysanthemic acid, (+)-trans-CA (26) mouse 98a 280a Miyamoto (1975-1976)
(t-CA) Ueda et al. (1975b)
(+)-cis-chrysanthemic acid, (+)-cis-CA (c-CA) (27) mouse 600a Miyamoto (1975-1976)
Ueda et al. (1975b)
d-trans-chrysanthemic acid (26) rat 983 Reagan & Becci (1985c)
(+)-trans-chrysanthemumdicarboxylic acid, (30) mouse 408a Miyamoto (1975-1976)
(+)-trans-CDA (tE-CDA) Ueda et al. (1975b)
5-benzyl-3-furylmethanol (BFA) (18) mouse 75a 310a Miyamoto (1975-1976)
Ueda et al. (1975b)
Table 8. (cont'd).
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
5-benzyl-3-furoic acid (BFCA) (20) mouse 46a Miyamoto (1975-1976)
Ueda et al. (1975b)
5-benzyl-3-furoic acid (BFCA) (20) rat 997 Reagan & Becci (1985d)
ethyl chrysanthemate - rat > 4640 Stauffer Chemical Co.
(1984)
a Toxicity for male mice, 24 h after intraperitoneal injection or oral administration.
b Chemical identification numbers used in Table 1 and Fig. 2.
The same authors fed groups of 6 Long-Evans rats of each sex
resmethrin in the diet at concentrations of 0, 61-90, 148-180,
297-386, 584-669, 1080-1375, or 1266-2532 mg/kg body weight per day
for 14 days. Mortality was observed in the groups fed 1080 mg/kg or
more. Tremor was observed at levels of 386 mg/kg or more (females only
at 386 mg/kg). The terminal body weight and food utilization were
significantly lower in the groups fed 1080 mg/kg or more compared with
the controls. A significant increase in the hepatic organ-to-body
weight ratios was noted at levels of 297 mg/kg or more.
Compound-related histopathological changes were not observed in any of
the tissues and organs examined. The maximum no-observed-adverse-
effect dietary level was 148 mg/kg body weight per day for male and
180 mg/kg body weight per day for female rats (Swentzel et al., 1977).
In a third study by Swentzel et al. (1977), groups of 8-20
Long-Evans rats of each sex were fed resmethrin in the diet at
concentrations equivalent to 0, 22, 66, 211, or 679 mg/kg body weight
per day for males and of 0, 3, 7, 22, 67, 219, 724, or 2400 mg/kg body
weight per day for females, for 90 days. All the females fed the
highest level died. Tremor was observed at levels of 679 mg/kg or
more. The mean terminal body weight was significantly lower in the
group given 679-724 mg/kg than in the control group. A significant
increase in the hepatic and renal organ-to-body weight ratios was
noted in males fed 211 or 679 mg/kg; while, in females fed 219 or
724 mg/kg, only an increase in the hepatic ratio was observed. No
significant differences were observed in clinical chemistry values
(serum glutamic oxaloacetic transaminase (SGOT), serum glutamic
pyruvic transaminase (SGPT), total lactate dehydrogenase (LDH),
alkaline phosphatase (AP), gamma-glutamyl trans-peptidase (GGTP),
total bilirubin, total protein, blood-urea nitrogen (BUN)) between
test and control animals. Compound-related histopathological changes
were not observed in any of the tissues and organs examined. The
maximum no-observed-adverse-effect dietary level was 67 mg/kg body
weight per day for female and 66 mg/kg body weight per day for male
rats.
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage, daily, 6 days/week for 3 weeks at doses of 0, 1000,
or 2000 mg/kg body weight. Body weights were slightly reduced at
2000 mg/kg. A slight reduction was noted in haemoglobin content and
hematocrit value. Biochemical examination revealed an increase in
albumin and blood-urea nitrogen concentrations and a decrease in
serum-glutamic oxaloacetic transaminase (SGOT) activity. Increased
liver size and reduced thymus weight were observed at 1000 mg/kg.
Reduced prostate weight was noted only at the high dose.
Histopathological examination revealed thymic involution (Glomot,
unpublished report).a
a GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished report of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
Wallwork et al. (1971) reported a study in which groups of rats
(18 males and 18 females/group) were fed bioresmethrin in the diet at
concentrations of 0, 400, 1200, or 8000 mg/kg over 91 days. The group
receiving the highest dose was fed 4000 mg/kg for 30 days and
thereafter 8000 mg/kg. Body weights were reduced at the highest dose
level and there were changes in blood chemistry parameters indicating
liver dysfunction (an increase in serum-alkaline phosphatase (SAP),
SGOT, and urinary nitrogen and a decrease in glucose content). The red
blood cell count was depressed in the group receiving 1200 mg/kg. An
increase in liver weight and a decrease in the weights of several
other organs, such as the spleen and thymus, were observed at
4000 mg/kg. Fatty infiltration of the liver was noticed at 1200, 4000,
and 8000 mg/kg. The no-observed-adverse-effect level in this study was
400 mg/kg, equivalent to an average daily intake of 32.8 and
36.1 mg/kg body weight for males and females, respectively.
Bioresmethrin was administered to groups of dogs (2 of each
sex/group) by gavage, daily, at dose levels of 0 or 500 mg/kg for 7
days followed by an increased dose of 1000 mg/kg for a further 14
days. No effects were noted on mortality, behaviour, body weight,
haematology, blood chemistry, urinalysis, or electrocardiograms
(Malone & Chesher, 1970).
Groups of dogs (3 males and 3 females/group) were administered
bioresmethrin in gelatin capsule by gavage, daily for 90 days, at
dosage levels of 0, 25, 80, or 250 mg/kg body weight (the highest dose
was increased to 500 mg/kg body weight in week 7). There were no
effects on mortality, body weight, food consumption, ophthalmology, or
urinalysis. Red blood cell count, haemoglobin content, and packed cell
volume values were reduced at the highest dose level. Blood-urea
nitrogen was slightly increased at the highest dose after 12 weeks. No
adverse effects were observed on gross or histopathological
examination. The no-observed-adverse-effect level was 80 mg/kg body
weight, equivalent to an average of 1600 mg/kg diet (Noel et al.,
1971).
In summary, the no-observed-adverse-effect level for resmethrin as
determined in the 90-day study on rats was 66 mg/kg body weight per
day, whereas that for bioresmethrin was 33 mg/kg body weight per day
in the 91-day study on rats and 80 mg/kg body weight per day in the
90-day study on dogs.
7.2.2 Inhalation
Short-term inhalation studies were performed using three
resmethrin formulations and one blank formulation. Aerosol spray
formulations (frequency median diameter of the particles: 1.5-2.0 µm,
volume median diameter 10-18.5 µm) were introduced into the top of a
pyramid-shaped sealed chamber and dispersed for 30-second intervals
every 30 min. Groups of 25 male rats, 10 female rats, and 4 male
rabbits were exposed to the inhalations 10 times a day, over a period
of 5 h, for 5 consecutive days. Daily active ingredient concentrations
of each formulation in the chamber were 2.9-3.2 mg/litre, based on the
weight loss from each spray can. The clinical signs included increased
preening, ruffled pelt, rapid breathing, and slight nasal discharge.
All signs disappeared overnight, but recurred after each daily
exposure. In rats, there were no effects on body weight. No gross or
histopathological lesions related to exposure to the formulations were
observed in rats necropsied immediately after the last 5-h exposure
and 7 and 14 days after exposure. No indication of toxic effects,
other than irritation, was observed in any test (Macko et al., 1979).
When ICR mice and Sprague-Dawley rats were exposed to [1R,
trans, cis]-resmethrin (at concentrations of 0, 23, 47, or
210 mg/m3) for 4 h/day, 5 days/week over 4 weeks, no toxic effects
were observed on behaviour, food intake, haematology, clinical
biochemistry, mortality, or histopathology (Miyamoto, 1976).
When groups of 16 male and 16 female Wistar rats were exposed
through inhalation for 6 h/day on 5 days of each week, over a period
of 90 days, to technical resmethrin at target exposure levels of 0.1,
0.3, or 1.0 g/m3, the no-observed-adverse-effect level was
established at 0.1 g/m3. At 0.3 g/m3, there were minor effects on
some clinical pathology parameters and also signs of irritation.
However, microscopic pathological examination did not reveal any
treatment-related changes in the lungs or other organs of rats exposed
at this level. At 1 g/m3, there were clinical signs indicative of
irritation, minor neurobehavioural changes, a reduced rate of weight
gain, and some changes in clinical pathology parameters. Microscopic
examination at this level showed minimal changes in the larynx, liver,
and thyroid, but these were reversible during the recovery period.
There were no treatment-related lung changes (Coombs et al., 1985).
7.2.3 Dermal application
Resmethrin was applied twice a week for 3 weeks to the shaved skin
of 4 groups of 10 male New Zealand White rabbits. Cotton cloth treated
with resmethrin at 0.247 mg/cm3 was applied over 1 ml of liquid
(imitating sweat) to rabbits in the first group. In the second group,
cotton cloth treated with resmethrin was applied without the sweat,
and in the third group, the cotton cloth was fixed to skin that had
been pretreated with 10 g of technical grade resmethrin. In the fourth
group, untreated cotton cloth was fixed over skin pre-treated with
pyrax powder containing 1% resmethrin at the rate of 1 g/kg of body
weight. The 3 control groups received cotton cloth treated with
acetone, cotton cloth treated with acetone over 1 ml of the sweat, and
untreated cotton cloth over 1 g pyrax powder/kg, respectively. No
significant changes were noted, on day 24 of the test, in rabbit body
weights and organ-to-body weight ratios of liver, lung, kidney,
testis, and spleen. Average dermal irritation scores for
resmethrin-treated rabbits were not significantly higher than those
for the control groups and did not increase during the test. No
significant trends compared with the controls were seen in clinical
chemistry values (serum-glutamic oxalo-acetic transaminase,
serum-glutamic pyruvic transaminase, lactic dehydrogenase, alkaline
phosphatase, blood-urea nitrogen) on days 5, 12, 19, and 24 of the
test. There were no compound-related lesions of the skin or of any of
the other tissues and organs examined at the termination of the study
(Swentzel et al., 1977).
7.3 Primary Irritation and Sensitization
Technical grade resmethrin was found to be a slight irritant in
rabbits in a 24 day dorsal/ventral rabbit ear test. Dermal irritation
was evident on both intact and abraded skin at 72 h and up to 7 days.
Resmethrin did not cause sensitization reactions in guinea-pigs, or
photochemical irritation in New Zealand White rabbits. Repeated daily
applications of 0.1 g of the technical grade compound to one ear of
each of 5 New Zealand White rabbits were carried out for 30
consecutive days as well as applications of compound-impregnated
cotton sateen cloth (0.247 mg/cm2) with artificial sweat for 24 days.
In this study, resmethrin did not produce acne-form dermatitis
(Swentzel et al., 1977).
Groups of adult guinea-pigs (6 males/group) were treated with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro
benzene (DNCB) (0.1 ml of a 1% (w/v) formulation in mineral oil) to
assess the sensitization properties. The test substance was applied to
the ears for 4 days. On day 7, 0.2 ml was applied dermally and the
degree of irritation recorded. As expected, the DNCB was an irritant
while bioresmethrin showed only traces of erythema suggesting a low
potential for sensitization and irritation (Chesher & Malone, 1970b).
Instillation of technical bioresmethrin into the eye of 6 rabbits
(0.1 ml) did not produce any irritation or corneal damage, or any
indication of ocular hazard (Chesher & Malone, 1970c).
7.4 Long-term Exposure and Carcinogenicity
When [1R, trans, cis]-resmethrin was fed to Sprague-Dawley
rats (male and female) at dietary levels of 500, 1500, or 5000 mg/kg
for 24 weeks, toxic symptoms, such as tremors and decreased body
weight, increased liver and kidney weights and an increase in alkaline
phosphatase activity were observed at 5000 mg/kg. The no-observed-
adverse-effect level was 1500 mg/kg (77.7 mg/kg per day for males and
86.6 mg/kg per day for females) (Miyamoto, 1976).
Resmethrin fed to Wistar rats at dosage levels of 0, 500, 2500, or
5000 mg/kg in the basal diet over a 112-week period, was determined
not to be oncogenic up to, and including, 5000 mg/kg, which was the
highest dose tested. The no-observed-adverse-effect level of 500 mg/kg
for toxic effects, was the lowest effect level for hypertrophy of
hepatocytes, which was not considered a definite toxic response
(Knickerbocker et al., 1980; Hess et al., 1982).
Cox et al. (1979a) fed CD-1 outbred albino mice with 0, 250, 500,
or 1000 mg resmethrin/kg basal diet for an 85-week period. No
oncogenicity was observed at any doses up to and including 1000 mg/kg.
When Beagle dogs were administered 0, 10, 30, or 300 mg
resmethrin/kg body weight for 6 months, the no-observed-adverse-effect
level was 10 mg/kg per day. On day 57 of the study, the highest dose
level was increased from 100 mg/kg per day to 300 mg/kg per day.
Increased liver weights were noted at 30 mg/kg body weight per day
(Gephart et al., 1980).
An acceptable daily intake (ADI) was established by the USA EPA at
0.125 mg/kg body weight per day based on no-observed-adverse-effect
levels in long-term toxicity studies. A food additive tolerance has
been established by the US EPA, permitting resmethrin residues of up
to 3 ppm, in or on food commodities, resulting from use in food
handling areas (US Environmental Protection Agency, 1983).
7.5 Mutagenicity
Saccharomyces cerevisiae D4 and five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) were used to
evaluate the mutagenic potential of resmethrin. The compound was
tested in the absence or presence of liver microsomal enzyme
preparations from rats pre-treated with Aroclor 1254. Resmethrin was
not mutagenic to any of the indicator organisms under both conditions
(Swentzel et al., 1977).
Resmethrin, bioresmethrin, and cismethrin were tested for
mutagenicity in several test systems. Miyamoto (1976) and Suzuki
(1975) used the Escherichia coli and S.typhimurium reverse
mutation tests. Garret et al. (1986) summarized results of point/gene
mutations in prokaryotes or eukaryotes, primary DNA damage in
prokaryotes or eukaryotes, chromosomal effects in Chinese hamster
ovary cells, mouse bone marrow, and cardiac blood cells of the mouse.
Pluijmen et al. (1984) used reverse mutation tests in S.typhimurium
TA98 or TA100, or mutagenicity tests with V79 Chinese hamster cells to
test seven synthetic pyrethroids including resmethrin, bioresmethrin,
and cismethrin. They all gave negative results.
Bioresmethrin was also tested in a metaphase chromosome analysis
of cultured human lymphocytes, an autoradiographic assessment of
unscheduled DNA synthesis in mammalian cells, and a mouse micronucleus
test. They also gave negative results (Allen et al., 1986; Allen &
Proudlock, 1986; Vannier & Fournex, 1986).
7.6 Reproductive effects, Embryotoxicity, and Teratogenicity
Three groups of 30 pregnant Long-Evans rats were administered
resmethrin in the diet at concentrations of 0, 188, or 1500 mg/kg from
day 6 to day 16 of gestation. The dams showed tremors and decreased
food and water consumption at 1500 mg/kg and 2 dams died; the lower
fetal weight seen at this dose and the resorption of embryos and
fetuses in 15 out of 30 dams were probably due to maternal toxicity.
No gross abnormalities of fetal skeletons and soft tissues were
observed in the treated groups. Thus, the consumption of resmethrin in
ground feed was not teratogenic at 188 and 1500 mg/kg (Swentzel
et al., 1977).
In a 3-generation study, Sprague-Dawley rats were fed resmethrin
in the diet at 0, 500, 800, or 1250 mg/kg (Schwartz et al., 1979c). A
slight increase in the number of pups cast dead and a decrease in pup
weights were noted at the 500 mg/kg level. A no-observed-adverse-
effect level of < 500 mg/kg was established for pups cast dead and
reduced pup weights at 21 days.
Sprague-Dawley female albino rats were given resmethrin in corn
oil, by gavage, at dose levels of 0, 20, 40, or 80 mg/kg during the
period of major organogenesis (days 6-15 of gestation). Resmethrin was
not teratogenic in rats at levels up to, and including, 80 mg/kg. The
no-observed-adverse-effect level for fetotoxicity was 40 mg/kg (Machi
et al., 1979).
Pregnant New Zealand White Minnikin rabbits were given resmethrin
by oral intubation at 0, 10, 30, or 100 mg/kg body weight per day on
days 6-18 of gestation (Becci et al., 1979). On day 29 of gestation,
all animals were killed for examination. No teratogenic effects were
seen at dose levels up to and including 100 mg/kg.
When [1R, trans, cis]-resmethrin was administered orally to
female ICR mice (10, 30, or 100 mg/kg, daily) and Sprague-Dawley rats
(10, 20, or 50 mg/kg daily) during the period of gestation, to examine
maternal and embryotoxic effects, no significant adverse effects, such
as abortion, resorption of fetuses or embryos, external or skeletal
abnormalities of pups, and abnormalities in growth or organ
differentiation, were observed at any doses (Miyamoto, 1976).
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin at doses of 0, 10, 20, 40, or 80 mg/kg, by gavage,
daily, from day 8 to day 16 of gestation. The does were sacrificed on
day 28 and examined for implantation, live and dead fetuses,
resorption sites, and abnormalities. There was no apparent effect on
parents in the study as growth and gestation were unaffected. There
was an increase in the numbers of dead fetuses at the highest dose and
a large number of resorption sites were noted at all treatment levels.
A number of deformed fetuses were observed, but the total numbers were
not sufficient for an adequate statistical evaluation. The deformities
included straight tail, crossed hind limbs, and unilateral union of
6th and 7th ribs at the sternal end. An overall fetal loss was
observed at all dose levels (primarily because of the large number of
resorption sites recorded) (Waldron, 1969).
7.7 Immunotoxicity
The influence of various pesticides on the humoral and cellular
immune response to fluorescein-labelled ovalbumin has been analysed.
Resmethrin was administered intragastrically in corn oil as a single
dose (one half of LD50) before primary immunization. Control groups
included animals treated with corn oil alone, or immunosuppressed with
methotrexate. Booster immunizations and test bleedings were scheduled
to follow at weekly intervals. The cellular immune response was
quantified by redness and swelling, histological examination, and by
differential temperature measurements of the foot pads after antigen
challenge. The concentration, binding affinity, and heterogeneity of
the serum antibody were determined by fluorescence polarization
measurements. Resmethrin gave an early, sometimes very marked,
stimulation of the cellular immune response (Danliker et al., 1979).
7.8 Neurotoxicity
The neurotoxicity of resmethrin was evaluated in three short-term
dietary studies on Sprague-Dawley rats designed to examine the gross
and histopathological changes to the central and peripheral nervous
system. Resmethrin was not neurotoxic when administered at 1250 mg/kg
for 32 weeks, 5000 mg/kg for 30 days, or 12 640 mg/kg for 7 days (Cox
et al., 1979b; Schwartz et al., 1979a,b).
In order to better characterize the behavioural effects of
pyrethroid insecticides, comparisons were made of the effects of
cismethrin and deltamethrin exposure on motor activity and the
acoustic startle response in male Long-Evans rats (Crofton & Reiter,
1984). Acute dose effect, acute time course, and 30-day
repeated-exposure determinations of 1 h motor activity were made using
figure-eight mazes. The acoustic startle response to a 13-kHz, 120-dB,
40-msecond tone was measured at each of three background white noise
levels (50, 65, and 80 dB). Deltamethrin (0, 2, 4, 6, or 8 mg/kg) or
cismethrin (0, 6, 12, 18, or 24 mg/kg) was administered orally in
0.2 ml corn oil/kg. Both compounds produced similar dose-dependent
decreases in motor activity. The time course of onset and recovery for
this decreased activity was rapid (1-4 h). No cumulative effects on
motor activity were found of a 30-day exposure to 2 mg deltamethrin/kg
per day or 6 mg cismethrin/kg per day. The effects of cismethrin and
deltamethrin on the acoustic startle response differed. Deltamethrin
produced a dose-dependent decrease in amplitude and an increase in
latency, and cismethrin produced an increase in amplitude and no
change in latency. The differential effects of cismethrin (Type I
pyrethroids) and deltamethrin (Type II pyrethroids) on the acoustic
startle response may be related to the contrasting effects previously
shown with neurophysiological and/or neurochemical techniques (see
Annex).
The isolated rat neurohypophysis, which shows a calcium-dependent
hormone release when depolarized in vitro, was used as a model
system to investigate the effects of the pyrethroids deltamethrin and
resmethrin on mammalian nervous tissue (Dyball, 1982). Both compounds
inhibited neurohypophysial hormone release in response to electrical
stimulation, deltamethrin being more potent than resmethrin.
Deltamethrin reduced the hormone content of the neurohypophysis.
Resmethrin did not reduce stored hormone significantly and its effects
on release were dose dependent. They could be mimicked by raising the
Na+ of the medium, but not by lowering the Ca2+. Resmethrin did not
have any effects on the release of hormone following depolarization of
the tissue with a raised K+. The results are consistent with the
suggestion that the compounds do not act on the potential-dependent
secretion process but rather on the mechanism linking depolarization
of the secretory terminals with the arrival of action potentials,
possibly by interfering with sodium-channel activation and
inactivation.
The neurological effects of four synthetic pyrethroids resmethrin,
permethrin, cypermethrin, and deltamethrin were investigated in the
rat to establish whether there is a correlation between the
clinical-functional status of the animal and peripheral nerve damage,
as measured biochemically (Rose & Dewar, 1983). Neuromuscular
dysfunction was assessed by means of the inclined plane test and
peripheral nerve damage by reference to ß-glucuronidase and
ß-galactosidase activity increases in nerve tissue homogenates from
treated and control animals. A transient functional impairment was
found in animals treated with any one of the four pyrethroids tested
and, in all cases, this was maximal at the end of the 7-day dosing
regimen (resmethrin doses of 500-2000 mg/kg per day). Significant
increases in ß-glucuronidase and ß-galactosidase were found, 3-4 weeks
after the start of dosing in the distal portion of the
sciatic/posterior tibial nerves of animals treated with permethrin,
cypermethrin, or deltamethrin; however, no changes were found in
resmethrin-treated animals. Thus, it is concluded that there is no
direct correlation between the time-course of the neuromuscular
dysfunction and the neurobiochemical changes. This suggests that these
pyrethroids have at least two distinct actions, a short-term
pharmacological effect and, at near-lethal dose levels, a more chronic
neurotoxic effect that results in sparse axonal nerve damage.
Resmethrin (30 µmol/litre)-induced release of transmitters was not
affected by manipulation of the Na+ current with either choline or
tetrodotoxin agents, which readily reversed the effects of
veratridine, deltamethrin, and cypermethrin (Doherty et al., 1986).
Resmethrin (I50: 2.2 µmol/litre) inhibited the ATP-dependent uptake
of Ca2+ but deltamethrin and cypermethrin were much less effective.
Resmethrin also displaced Ca2+ from crude synaptosomal membranes. The
release-promoting effects of resmethrin in rat brain in vitro are
better explained by its effects on Ca2+ rather than by a specific
effect on the Na+ channel. In contrast, deltamethrin and
cypermethrin promote transmitter release by a Na+ dependent process.
Glickman & Casida (1982) discussed species and structural
variations affecting pyrethroid neurotoxicity. They concluded that the
mammalian nervous system, or at least the brain, appears to lack sites
sensitive to bioresmethrin and to a lesser extent to [1R,
trans]-permethrin, yet small structural changes restore the toxicity
(e.g., [1R,trans]-ethano-resmethrin and [1R, cis]-resmethrin. The
authors reported that birds and mammals in general respond to cis-
but not to trans-resmethrin in contrast to insects, crustaceans, and
fish, which are highly sensitive to both isomers. Furthermore, common
green lacewing larvae are very tolerant to pyrethroids, suggesting
possible involvement of nerve insensitivity in addition to
detoxification in this species. These examples of insensitivity may be
associated with modified sites of action or perhaps an increased
stabilization of nerve membranes making them more resistant to
pyrethroid-induced excitation.
7.9 Mechanism of Toxicity (Mode of Action)
Cismethrin is toxic for both insects and mammals. Bioresmethrin is
about 50 times less toxic than cismethrin and produces few symptoms in
mice and rats, even at extremely high doses. The poisoning syndrome of
cismethrin and bioresmethrin was characterized by whole body tremors
(T-syndrome) and both compounds were therefore classified as Type I
pyrethroids (Verschoyle & Aldridge, 1980; Lawrence & Casida, 1982,
Annex).
Tremors started when the concentrations of cismethrin and
bioresmethrin in the brain were 0.5-1 mg/kg and 4-5 mg/kg,
respectively. At death, brain levels of cismethrin were 3.9-5.1 mg/kg,
and those of bioresmethrin were 23-35 mg/kg. As liver microsomal
esterases hydrolysed bioresmethrin 10 times more rapidly than
cismethrin, the lower toxicity of bioresmethrin was partly attributed
to its faster metabolism and intrinsically lower toxicity at the
critical site of action in the nervous system (White et al., 1976).
Cismethrin given intravenously produced repetitive activity, after
external stimulation, in the spinal cord of rabbit (Carlton, 1977). As
gamma-aminobutyric acid (GABA) blockers, such as bicuculline and
picrotoxin, produced convulsions similar to those of cismethrin, the
effects of the compounds on dorsal root potentials were examined in
the rat. Bicuculline reduced the amplitude of the potential to 67% of
the control values, but cismethrin enhanced the potential to 142% of
the control values. The convulsions associated with cismethrin
poisoning, therefore, are not produced by an antagonism of
GABA-mediated inhibitory transmission (Smith, 1980).
Administration of cismethrin into the lateral ventricle of the
brain or into the spinal cord causes signs similar to those observed
after intravenous administration. The onset of tremors after
intraventricular administration of cismethrin was delayed for about 15
min while, after intraspinal injection, the symptoms occurred within
2-5 min, indicating that cismethrin may act directly on the spinal
cord rather than in the brain (Gray et al., 1980b).
After intravenous administration of [14C]-alcohol-labelled
cismethrin or bioresmethrin to rats, the parent pyrethroid was rapidly
cleared from the blood and liver, and both isomers rapidly entered the
central nervous system (CNS) reaching peak concentrations within 2-5
min. Cismethrin concentrations in the brain exceeding 3.5 nmol/g were
associated only with animals showing tremors. These levels of
cismethrin were maintained for up to 30 min, but bioresmethrin was
depleted more rapidly, possibly due to brain metabolism. It is
concluded that the low toxicity of bioresmethrin is possibly because
of its inability to interact with the site of action in the CNS and to
its rapid metabolism in the liver (Gray et al., 1980a).
Cismethrin produced repetitive firing in the flight muscles and
uncoupling of motor unit activity in the housefly (Miller & Adams,
1977), and repetitive firing in a cercal sensory nerve of the
cockroach (Gammon et al., 1981). On the basis of the
electrophysiological effects and symptomatology in insects as well as
in mammals, cismethrin is classified as a Type I pyrethroid,
characterized by a mainly peripheral nervous system action (Gammon et
al., 1981).
7.10 Potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin, and/or piperonyl butoxide, alone or in
combinations, at doses approximating the acute intraperitoneal LD50
value. No potentiation of the acute toxicity was observed in this
study. In all cases with bioresmethrin combinations, the observed
LD50 values were equal to, or exceeded, the expected value (Wallwork
& Malone, 1971).
8. EFFECTS ON MAN
Although the resmethrins have been used for many years, no data
have been reported on their toxicity for human beings.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Health Risks
Resmethrin, consisting of four stereo-isomers, is an effective
insecticide mainly used to control household insects and other public
health insects, with applications in the food handling and storage
areas.
Bioresmethrin and cismethrin are composed of a selected isomer(s).
As with resmethrin, bioresmethrin is used to control household insects
and other public health insects. In addition, bioresmethrin is used as
a post-harvest insecticide to control stored grain pest. Human
exposure to the resmethrins will be mainly via inhalation, when the
formulations are sprayed in the form of a mist, though the use in food
handling and storage areas as well as post-harvest treatment may
result in dietary residues. Air levels following conventional
household aerosol spraying with the resmethrins are not expected to
exceed 0.5 mg/ms.
The only significant potential dietary exposure will result from
the use of resmethrin on stored grain. Residues of up to 4 mg/kg might
be present in grain, but this would be reduced to zero in white bread.
However, reduced residues may be present in wholemeal bread. A food
additive tolerance has been established by the US Environmental
Protection Agency (US EPA) permitting resmethrin residues of up to
3 mg/kg (3 ppm), in or on food commodities, resulting from use in food
handling areas. An ADI was established by the US EPA at 0.1250 mg/kg
body weight per day, based on no-observed-adverse-effect levels in
long-term toxicity studies. No data are available on occupational
exposure to the resmethrins. Although the resmethrins have been used
for many years, no data have been reported concerning their toxicity
for human beings. Thus, to determine the potential toxicity for human
beings, extrapolation from animal data and in vitro studies has been
used.
The result of short-term studies on experimental animals suggest
that resmethrins are weakly toxic when administered by various routes
of exposure (oral LD50 of resmethrin: 690 mg/kg (mouse) - >
5000 mg/kg (rat); those of bioresmethrin: 225 mg/kg (rabbit),
480-10 000 mg/kg (mouse), 8800 mg/kg (rats)). Cismethrin showed
moderate toxicity for the mouse (oral LD50 152-160 mg/kg). The acute
toxicity of resmethrin metabolites were in the same range in the rat,
but somewhat more toxic in the mouse.
While technical grade resmethrin is a slight skin irritant, it is
not a sensitizer.
Resmethrins are not mutagenic in a variety of test systems,
including gene mutations, DNA damage and DNA repair, and chromosomal
effects.
Resmethrin was not carcinogenic for mice or rats, when fed at
dietary levels of up to 1000 mg/kg for 85 weeks and 5000 mg/kg for 112
weeks, respectively.
Resmethrin was not teratogenic in the rat, mouse, or rabbit, up to
dose levels of 100 mg/kg body weight. A level of 40 mg/kg body weight
appeared to be the no-observed-adverse-effect level for fetotoxicity
in the rat.
Resmethrins at near lethal doses are likely to cause
hyperactivity, tremors, and convulsions and have been classified as
Type I pyrethroids.
For resmethrin, a no-observed-adverse-effect level was established
in a 90-day rat study to be 66-67 mg/kg body weight per day, whereas,
in another 2-year rat study, the lowest effect level appeared to be
500 mg/kg diet, corresponding to 25 mg/kg body weight per day. In a
6-month feeding study on dogs, the no-observed-adverse-effect level
was 10 mg/kg body weight per day.
In a 90-day inhalation study on rats, a no-observed-adverse-effect
level was established of 0.1 g/m3.
The no-observed-adverse-effect level for bioresmethrin in a 9-day
feeding study on the rat was 33-36 mg/kg body weight per day; and in a
90-day study on dogs, it was 80 mg/kg body weight per day.
In a 24-week feeding study on rats, the no-observed-adverse-effect
level for [1R, trans, cis]-resmethrin was 1500 mg/kg diet
corresponding to 75 mg/kg body weight.
9.2 Effects on the Environment
Resmethrins are used mainly indoors for the control of household
insects and also for stored grain protection. They are also used in
greenhouses and can be used outdoors for mosquito control. However,
under outdoor conditions, rapid photodegradation and microbial
degradation in the soil ensure that residues will not persist to any
extent in the environment.
In laboratory studies, resmethrins have been shown to be very
toxic for fish (96-h LC50 values: 0.3-5.5 µg/litre) but less toxic
for Daphnia and aquatic insect larvae. However, under field
conditions the low water solubility of the resmethrins and their ready
degradation greatly alleviate the effects that might be predicted from
the laboratory studies. The toxicity of resmethrins for birds is low
(LD50 > 5000 mg/kg) and they do not produce any effects on avian
reproduction.
10. CONCLUSIONS
General population: Under recommended conditions of household
and other public health use, the exposure of the general population to
resmethrins is negligible and is unlikely to present a hazard. Under
recommended conditions of use in food handling and storage areas as
well as in post-harvest treatment, the exposure of the general
population to resmethrins in the diet is unlikely to exceed the ADI
established by the US EPA.
Occupational exposure: With reasonable work practices, hygienic
measures, and safety precautions, the use of resmethrins is unlikely
to present a hazard to those occupationally exposed to it.
Environment: With recommended application rates, it is unlikely
that resmethrins or their degradation products will attain levels of
environmental significance. In spite of the fact that resmethrins are
highly toxic for fish, this is only likely to cause a problem in the
case of spillage or over-spraying.
11. RECOMMENDATIONS
- In order to fully assess the potential dietary exposure from
current uses of resmethrins, it is suggested that the degradation
pathway on stored grain be studied, and the terminal residues on
grain and bread be defined.
- It is considered that resmethrin, despite its high octanol-water
partition coefficient, is very unlikely to bioaccumulate in
non-target species, because of its ready degradation. However,
experimental confirmation of this in fish might be useful.
- A further multigeneration reproduction study to define a
no-observed-adverse-effect level should be considered.
- Over many years of use, no adverse effects have been reported as a
result of human exposure to resmethrins, but it is still necessary
to maintain observations on human exposure.
- The labelling of resmethrins for household use should include
adequate instructions for use and storage and, where appropriate,
a warning of flammability.
- Efforts should be made to obtain a more precise estimate of the
total global usage of resmethrins.
12. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The Joint FAO/WHO Expert Committee on Pesticide Residues (JMPR)
discussed and evaluated bioresmethrin at its meetings in 1975 and 1976
(FAO/WHO, 1976, 1977). An acceptable daily intake (ADI) has not been
established.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified bioresmethrin as a technical
product unlikely to present an acute hazard in normal use, and
resmethrin and cismethrin as slightly hazardous (WHO, 1986). A Data
Sheet on bioresmethrin (No. 34) has been issued (WHO/FAO, 1978).
REFERENCES
ABBOTT, D.D. (1971) Total degradation products of SBP-1382. Acute
oral LD50/mice, 6 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
ABE, Y., TSUDA, K., & FUJITA, Y. (1972) Studies on pyrethroidal
compounds. Part III. Photostability of pyrethroidal compounds.
Botyu-Kagaku, 37: 102-110.
ALLEN, J.A. & PROUDLOCK, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after
exposure to bioresmethrin, Huntingdon, United Kingdom,
Huntingdon Research Centre, 18 pp (Report No.
RSL-BRM-698/851420/A) (Unpublished proprietary data supplied by
Roussel Uclaf).
ALLEN, J.A., BROOKER, P.C., & HOWELL, A. (1986) Bioresmethrin
metaphase chromosome analysis of human lymphocytes cultures
in vitro, Huntingdon, United Kingdom, Huntingdon Research Centre,
15 pp (Report No. RSL-BRM-697/851370/A) (Unpublished proprietary
data supplied by Roussel Uclaf).
ARDLEY J.H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat (Unpublished report of Wellcome
Australia Ltd).
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and
high-performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Battelle Institute.
BECCI, P.J., KNICKERBOCKER, M., & PARENT, R.A. (1979) Teratological
evaluation of SBP-1382 in albino rabbits, Waverly, New York,
Food and Drug Research Laboratories, 17 pp (Report No. 6288)
(Unpublished proprietary data supplied by Roussel Uclaf).
BENGSTON, M., COOPER, L.M., & GRANT-TAYLOR, F.J. (1975) A comparison
of bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in wheat.
Queensland J. Agric. anim. Sci., 32: 51-78.
BUICK, A.R. & FLANAGAN, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes (Report No. HEGH 73/1) (Unpublished
data of Wellcome Foundation Ltd).
BURT, M.E. (1983a) Resmethrin residues in tomato and cucumber, New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
BURT, M.E. (1983b) Resmethrin residues in lettuce (greenhouse), New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
CARLTON, M. (1977) Some effects of cismethrin on the rabbit nervous
system. Pestic. Sci., 8: 700-712.
CAVE, S.L. (1981) Simultaneous estimation of bioresmethrin and
piperonylbutoxide by gas-liquid chromatography with
chemical-ionization mass spectrometry. Pestic. Sci.,
12: 156-160.
CHAPMAN, R.A. & HARRIS, C.R. (1978) Extraction and liquid-solid
chromatography cleanup procedures for the direct analysis of four
pyrethroid insecticides in crops by gas-liquid chromatography.
J. Chromatogr., 166: 513-518.
CHESHER, B.C. & MALONE, J.C. (1970a) Acute toxicity tests with NRDC
107 in hens (Report No. B 236-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970b) Sensitisation study with NRDC
107 on guinea pigs (Report B 198-70) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits (Report No. B 217-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1971a) Toxicity to dogs of NRDC 107
(continuation) (Report No. B 27-71) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER B.C. & MALONE J.C. (1971b) Acute toxicity of NRDC 107 by the
intravenous route. (Report No. B 329-71) (Unpublished data of
Wellcome Foundation Ltd).
CHRISTOPHER, R.J., MUNGER, C.E., & WAYNE IRVIE, G. (1985) Distribution
and depletion of [14C]-resmethrin isomers administered orally to
laying hens. Pestic. Sci., 16: 378-382.
COOMBS, D.W., HARDY, C.J., CLARK, G.C., STREET, A.E., GOPINATH, C.,
LEWIS, D.J., & RAO, R.S. (1985) Resmethrin 90-day inhalation
toxicity study in the rat, Huntingdon, United Kingdom,
Huntingdon Research Centre, 80 pp (Report No. SBP 6/84997)
(Unpublished proprietary data supplied by Roussel Uclaf).
COX, G.E., KNICKERBOCKER, M., & PARENT, R.A. (1979a) Evaluation of
dietary administration of SBP-1382 in CD-1 outbred albino mice
over an 85-week period, Waverly, New York, Food and Drug
Research Laboratories, 52 pp (Report No. 5270) (Unpublished
proprietary data supplied by Roussel Uclaf).
COX, G.E., BECCI, P.J., & PARENT, R.A. (1979b) Supplementary report
for evaluation of the neurotoxic effects of SBP-1382 in albino
rats - phase I, Waverly, New York, Food and Drug Research
Laboratories, 7 pp (Report No. 6362) (Unpublished proprietary data
supplied by Roussel Uclaf).
CROFTON, K.M. & REITER, L W. (1984) Effects of two pyrethroid
insecticides on motor activity and the acoustic startle response
in the rat. Toxicol. appl. Pharmacol., 75: 318-328.
DANLIKER, W.B., HICKS, A.N., LEVISON, S.A., STEWART, K., & BROWN, R.J.
(1979) Effects of pesticides on immume response, Washington, DC,
US Environmental Protection Agency (EPA-600/1-79-039-PB
80-1308 34).
DESMARCHELIER, J.M. (1980) Comparative study of analytical methods for
bioresmethrin, phenothrin, d-phenothrin, pyrethrum I, carbaryl,
fenitrothion, methacrifos, pirimifos-methyl and dichlorfos on
various grains. J. Pestic. Sci., 5: 521-532.
DEVAUX, Ph. & BOLLA, P. (1986) Bioresmethrin n-octanol/water
partition coefficient and water solubility, Romainville, France,
Roussel Uclaf, 4 pp (Report No. RU-BRM-142.06.86. STE/A)
(Unpublished proprietary data).
DEVAUX, Ph. & TILLIER, C. (1986) Vapor pressure of bioallethrin and
bioresmethrin, Romainville, France, Roussel Uclaf, 8 pp (Report
No. RU-BRM/BA-150.06.86.STE/A) (Unpublished proprietary data).
DOHERTY, J.D., LAUTER, C.J., & SALEM, N., Jr (1986) Synaptic effects
on the synthetic pyrethroid resmethrin in rat brain in vitro.
Comp. Biochem. Physiol., C84: 373-379.
DYBALL, R.E.J. (1982) Inhibition by decamethrin and resmethrin of
hormone release from the isolated rat neurohypophysis: A model
mammalian neurosecretory system. Pestic. Biochem. Physiol.,
17: 42-47.
ELLIOT, M. (1976) Properties and applications of pyrethroids.
Environ. Health Perspect., 14: 3-13.
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series, No. 42).
ELLIOTT, M., FARNMAN, A.W., JONES. N.F., NEEDHAM, P.H., & PEARSON,
B.C. (1967) 5-Benzyl-3-furylmethyl chrysanthemate: a new potent
insecticide. Nature (Lond.), 213: 493-494.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome,
11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
FAO/WHO (1976) Bioresmethrin. In: 1975 Evaluation of some pesticide
residues in food, Geneva, World Health Organization, pp. 17-40
(WHO Pesticide Residues Series, No. 5).
FAO/WHO (1977) Bioresmethrin. In: 1976 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of the
United Nations, pp. 55-78 (AGP: 1976/M/14).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
GAINES, T.B. & LINDER, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. appl. Toxicol., 7: 299-308.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular
junction. Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol.,
15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. appl. Pharmacol.,
66: 290-296.
GARRET, N.E., FRANK STACK, H., & WATERS, D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res.,
168: 301-325.
GEPHART, L.A., JOHNSON, W.D., BECCI, P.J., & PARENT, R.A. (1980)
180-day subchronic oral dosing study with resmethrin in Beagle
dogs, Waverly, New York, Food and Drug Research Laboratories,
86 pp (Report No. 6289) (Unpublished proprietary data supplied by
Roussel Uclaf).
GLICKMAN, A.H. & CASIDA, J.E., (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4: 793-799.
GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished data of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
GLOMOT, R. & CHEVALLIER, B. (1969) Etude de la toxicité aiguë du
RU11484 (NRDC 107) Romainville, France, Roussel Uclaf (Rapport
Toxico 11386) (Unpublished data).
GRAY, A.J., CONNORS, T.A., HOELLINGER, H., & NGUYEN-HOANG-NAM (1980a)
Relationship between the pharmacokinetics of intravenous
cismethrin and bioresmethrin and their mammalian toxicity.
Pestic. Biochem. Physiol., 13: 281-293.
GRAY, A.J., CONNORS, T.A., & RICKARD, J. (1980b) Mechanism of
mammalian toxicity of the pyrethroids. In: Littaner, V.Z., Dudai,
Y., Silman, I. Teichberg, V.I., & Vogel, Z., ed.
Neurotransmitters and their receptors, New York, John Wiley &
Sons Ltd, pp. 565-568.
GREEN, W.M. (1977) Acute dermal LD50 toxicity study on technical
resmethrin, Moorestown, New Jersey, Bio-Toxicology Laboratory,
8 pp (Unpublished proprietary data supplied by Roussel Uclaf).
HESS, F.G., THOMPSON, S.W., & BECCI, P.J. (1982) A lifetime
evaluation of the dietary administration of SBP-1382 to Wistar
albino rats/amendment, Waverly, New York, Food and Drug Research
Laboratory, 28 pp (Supplemental report No. 5271) (Unpublished
proprietary data supplied by Roussel Uclaf).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Bureau of Sport
Fishery and Wildlife, pp. 1-61 (Report No. 191).
HOELLINGER, H., SONNIER, M., GRAY, A.J., CONNORS, T.A., PICHON, J., &
NGUYEN-HOANG-NAM (1985) In vitro covalent binding of cismethrin,
bioresmethrin and their common alcohol to hepatic proteins.
Toxicol. appl. Pharmacol., 77: 11-18.
HOLMSTEAD, R.L., CASIDA, J.E., & RUZO, L.O. (1977) Photochemical
reactions of pyrethroid insecticides. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society
(ACS Symposium Series, No. 42).
JACKSON, G.C. & HARDY, C.J. (1984) SBP-1382 acute inhalation toxicity
in rats 4-hour exposure, Huntingdon, United Kingdom, Huntingdon
Research Centre, 24 pp (Report No. SBP 4a/831042) (Unpublished
proprietary data supplied by Roussel Uclaf).
KAGAN, U.S., PANSHINA, T.N. & SASINOVITCH, L.M. (1986) [Biochemical
effects of synthetic pyrethroid toxic action.] Gig. i Sanit.,
1: 7 (in Russian).
KAUFMAN, D.D. (1986) Degradation and movement of cis- and
trans-resmethrin in soil, Beltsville, US Department of
Agriculture, 36 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
KHOLMATOVA, M. (1984) Hygiene and toxicology of Dravin 755 and
Isotrine new pesticides used in agriculture, Alma Ata (Résumé of
doctorate thesis).
KNICKERBOCKER, M., BECCI, P.J., COX, G.E., & PARENT, R.A. (1980)
A lifetime evaluation of the dietary administration of SBP-1382
to Wistar albino rats, Waverly, New York, Food and Drug Research
Laboratories, 110 pp (Report No. 5271) (Unpublished proprietary
data supplied by Roussel Uclaf).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor and
Francis Ltd, 440 pp.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem.
Physiol., 20: 203-216.
MACHI, R.A., KAM, C., GALLO, M.A., STEVENS, K.R., & GAGLIARDI, J.J.
(1979) Teratological evaluation of SBP-1382 technical in the
albino rat, Florham Park, New Jersey, Booz, Allen & Hamilton,
Inc., 44 pp (Report No. 2054-066) (Unpublished proprietary data
supplied by Roussel Uclaf).
MACKO, J.A., HARVEY, J.G., POPE, C.R., & MCCREESH, A.H. (1979) Hazard
evaluation of aerosol formulations containing the synthetic
pyrethroid insecticide resmethrin, (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(SBP-1382), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency, 38 pp (Report No. 75-51-0848-79).
MALONE, J.C. & CHESHER, B.C. (1970) Toxicity to dogs of NRDC 107,
Berkhamsted, Wellcome Foundation Ltd (Report No. B 204-70)
(Unpublished data).
MARKING, L.L. & MAUCK, W.L. (1975) Toxicity of paired mixtures of
candidate forest insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 13: 518-523.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, 461 pp.
MAUCK, W.L., OLSON, L.E., & MARKING, L.L. (1976) Toxicity of natural
pyrethroids and five pyrethroids to fish. Arch. environ. Contam.
Toxicol., 4: 18-29.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemical handbook, Section C: Pesticide dictionary,
Willoughby, Meister Publishing Co., pp. C205-C206.
MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 98-115 (ACS Symposium Series, No. 42).
MIYAMOTO, J. (1975-76) Terminal residues of bioresmethrin.
Submission to IUPAC Terminal Residue Commission, Madrid, September
1975, Leverkusen, September 1976.
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4
September, 1982, Oxford, Pergamon Press (in 4 volumes).
MIYAMOTO, J., NISHIDA, T., & UEDA, K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furylmethyl d1-trans-chrysanthemate in the
rat. Pestic. Biochem. Physiol., 1: 293-306.
MUMMA, R.O. (1985) Persistence of metabolism of 14C-resmethrin on
greenhouse grown tomato fruit, lettuce and field grown wheat,
Part III, Pennsylvania State University, 33 pp Unpublished
proprietary data supplied by Roussel Uclaf).
MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem.,
36: 2203-2211.
NIOSH (1983) In: Takten, R.L. & Lewis, R.J., Jr, ed. Registry of
toxic effects of chemical substances, 1981-82 edition,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 3057 pp (in 3 volumes).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitai-Kagaku, 4: 31-46 (in Japanese).
NISHIUCHI, Y. (1982) [Toxicity of pesticides to fish.]
Kongetsuno-Noyaku, 26: 105-110 (in Japanese).
NOEL, P.R.B., RIVETT, K.F., CHESTERMAN, H., STREET, A.E., & SPICER,
E.J.F. (1971) Oral toxicity study in Beagle dogs (repeated dosage
for 3 months), 76 pp (Report No. 3900/71/58) (Unpublished data
of Huntingdon Research Centre, United Kingdom).
NORWOOD, T.E. (1986) Preliminary statistical report on assessment of
the effects of SBP-1382 40MF (resmethrin) and Scourge (18%
resmethrin plus 54% piperonyl butoxide) on non target, aquatic
organisms when used for adult mosquito control: results of a
field validation study in fresh water ponds in Minnesota, Summer
1984, North Oaks, Minnesota, Mosquito Control Technology, 20 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
PIERCE, R.H. (1986) Acute toxicity of Scourge on select estuarine
organisms during field applications, Sarasota, Florida, Mote
Marine Laboratory, 16 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C., HAUTEFEUILLE,
A., & BARTSCH, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
PUCHALSKI, R. (1983) Water solubility and octanol/water partition
coefficients of SBP-1382, Lyndhurst, New Jersey, Penick
Corporation, 5 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985a) Acute oral LD50 study of
D-L-cis-trans-chrysanthemic acid in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 10 pp
(Report No. 8350A) (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985b) Acute oral LD50 study of
D-L-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350B) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985c) Acute oral LD50 study of
D-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350C) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985d) Acute oral LD50 study of BFCA
99.6% Sprague-Dawley rats, Waverly, New York, Food and Drug
Research Laboratories, 10 pp (Report No. 8725A) (Unpublished
proprietary data supplied by Roussel Uclaf).
RIDLEN, R.L., CHRISTOPHER, R.J., WAYNE IRVIE, G., BEIER, R.C., & CAMP,
B.J. (1984) Distribution and metabolism of cis and
trans-resmethrin in lactating Jersey cows. J. agric. food
Chem., 32: 1211-1217.
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O.
(1985a) The effects of dietary inclusion of resmethrin on
reproduction in the bobwhite quail, Huntingdon, United Kingdom,
Huntingdon Research Centre, 34 pp (Report No. SBP 8BT/85957)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O,
(1985b) The effects of dietary inclusion of resmethrin on
reproduction in the mallard duck, Huntingdon, United Kingdom,
Huntingdon Research Centre, 28 pp (Report No. SBP 7BT/85550)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROPER, E.M. & WRIGHT, G.C. (1984) Sampling efficiency of five solid
sorbents for trapping airborne pesticides. Bull. environ. Contam.
Toxicol., 33: 476-483.
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four synthetic
pyrethroids fail to show any correlation between neuromuscular
dysfunction and neurobiochemical abnormalities in rats. Arch.
Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979a) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase I, Waverly,
New York, Food and Drug Research Laboratories, 18 pp (Report
No. 6067) (Unpublished proprietary data supplied by Roussel
Uclaf).
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979b) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase II,
Waverly, New York, Food and Drug Research Laboratories, 17 pp
(Report No. 6068) (Unpublished proprietary data supplied by
Roussel Uclaf).
SCHWARTZ, C.S., GEPHART, L., BECCI, P.J., & PARENT R.A. (1979c) The
evaluation of the effects of SBP-1382 following dietary
administration through three generations in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 70 pp
(Report No. 5739) (Unpublished proprietary data supplied by
Roussel Uclaf).
SEABER, J.A. (1985) Delayed contact hypersensitivity in the guinea
pig with SBP-1382 insecticide, Huntingdon, United Kingdom,
Huntingdon Research Centre, 14 pp (Report No. 84936D/SBP 10/ss)
(Unpublished proprietary data supplied by Roussel Uclaf).
SIMONAITIS, R.A. & CAIL, R.S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour, and wheat.
J. Assoc. Off. Anal. Chem., 58: 1032-1036.
SJOGREN, R.D. (1985) Assessment of the effects of SBP-1382 40MF
(resmethrin) and Scourge (18% resmethrin plus 54% piperonyl
butoxide) on non target, aquatic organisms when used for adult
mosquito control: results of a field validation study in fresh
water ponds in Minnesota, Summer 1984, North Oaks, Minnesota,
Mosquito Control Technology, 105 pp (Unpublished proprietary data
supplied by Roussel Uclaf).
SMITH, P.R. (1980) The effect of cismethrin on the rat dorsal root
potentials. Eur. J. Pharmacol., 66: 125-128.
STAUFFER (1984) Ethyl chrysanthemate/Product safety information,
Westport, USA, Stauffer Chemical Co., 5 pp.
SWENTZEL, K.L., ANGERHOFER, R.A., & HAIGHT, E.A. (1977) Toxicological
evaluation of pyrethroid insecticide (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(resmethrin), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency (Report No. 51-0830-77).
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1974) Photodecomposition of
resmethrin and related pyrethroids. J. agric. food Chem.,
22: 212-220.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pestic. Biochem.
Physiol., 5: 280-294.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975b) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food
Chem., 23: 106-115.
UNO, M., OKADA, T., OHMAE, T., & TANIGAWA, K. (1982) [Determination of
pyrethroid insecticides by dual-wavelength densitometry.]
Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
US ENVIRONMENTAL PROTECTION AGENCY (1983) Tolerances for pesticides in
food administered by EPA: Resmethrin. Fed. Reg.,
48: 36246-36247.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973)
DDT-likeaction of allethrin in the sensory nervous system of
Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B A., & AKKERMANS, L.M.A. (1979) Effect
of insecticides on the sensory nervous system. In: Narahashi, T.,
ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 182-210.
VANNIER, B. & FOURNEX, R. (1986) Bioresmethrin. Detection of a
mutagenic potency (micronucleus test in the mouse), Romainville,
France, Roussel Uclaf, 16 pp (Report No. RU-BRM-85612-BB/178/A)
(Unpublished proprietary data).
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol.,
45: 325-329.
VERSCHOYLE, R.D. & BARNES, J.M. (1972) Toxicity of natural and
synthetic pyrethrins in rats. Pestic. Biochem. Physiol.,
2(3): 308-311.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature and structure-dependent interaction
of pyrethroids with the sodium channels in frog node of Ranvier.
Biochim. Biophys. Acta, 728: 73-82.
VOLKOV, U.P., STRELETS, I.P., & TIULENEV, K.L. (1979) [Progress in
the field of pyrethroids and their synergists. Collection of
articles on household chemistry.] Moscow, Volume 7, p. 125
(in Russian).
WALDRON, M.M. (1969) Foetal toxicity study -- 95 H 56 -- given orally
in the rabbit (Report Path 51) (Unpublished data of Wellcome
Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide (Report
No. B 47-71) (Unpublished data of Wellcome Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1972) Bioresmethrin. Preliminary 24
hour rat inhalation study (Report No. B 290-72) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CHESHER, B.C., & MALONE J.C. (1970) Toxicity of NRDC
107 to laboratory animals (Report No. B 199-70) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study (Report No. B 199-71) (Unpublished
data of Wellcome Foundation Ltd).
WATSON, S.C. (1984) Photolysis study of SBP-1382 resmethrin (final
report), Columbus, Ohio, Battelle Columbus Laboratory, 10 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
WEEKS, M.H., LELAND, T.M., BOLDT, R.E., MELLICK, P.W., & STEINBERG, M.
(1972) Toxicological evaluation of pyrethroid insecticide
(5-benzyl-3-furyl) methyl-2,2-dimethyl-3-(2-methyl-propenyl)
cyclopropanecarboxylate (SBP-1382), Aberdeen Proving Ground, US
Army Environmental Hygiene Agency, 44 pp (Special study
No. 51-127-71/72).
WHITE, I.N.H., VERSCHOYLE, R.D., MORADIAN, M.H., & BARNES, J.M. (1976)
The relationship between brain levels of cismethrin and
bioresmethrin in female rats and neurotoxic effects. Pestic.
Biochem. Physiol., 6: 491-500.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-1987, Geneva, World Health
Organization, p. 38 (VBC/86.1, Rev. 1).
WHO/FAO (1978) Data sheet on pesticides No. 34: Bioresmethrin,
Geneva, World Health Organization (WHO/VBC/DS/78. 34).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, 485 pp.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YOSHIOKA, H. (1980) [Chemistry and action of the recent synthetic
pyrethroids and their stereoisomers.] J. synth. org. Chem.
Japan, 38: 1151-1162 (in Japanese).
APPENDIX
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs (Xenopus laevis; Rana temporaria, and Rana
esculenta), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches by Gammon et al. (1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982; Gammon
& Casida, 1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the insecticide
on neurotransmitter release or on the sensitivity of the subsynaptic
membrane or the muscle fibre membrane. Presynaptic repetitive firing
was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres,
the pyrethroid-induced repetitive activity increases dramatically as
the temperature is lowered, and a decrease of 5°C in temperature may
cause a more than 3-fold increase in the number of repetitive impulses
per train. This effect is easily reversed by raising the temperature.
The origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that underlies
the generation and conduction of each nerve impulse. The transitional
state of the sodium channel is controlled by 2 separately acting
gating mechanisms, referred to as the activation gate and the
inactivation gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the activation
gate and the slow closing of the inactivation gate proceed normally.
However, once the sodium channel is open, the activation gate is
restrained in the open position by the pyrethroid molecule. While all
pyrethroids have essentially the same basic mechanism of action, the
rate of relaxation differs substantially for the various pyrethroids
(Flannigan & Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more
than 100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the Type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing afterpotentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral-line organ in the form of
long-lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train increase
markedly on lowering the temperature. Cypermethrin does not cause
repetitive activity in myelinated nerve fibres. Instead, this
pyrethroid causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of the nerve
membrane as a result of the summation of depolarizing after-potentials
during train stimulation (Vijverberg & Van den Bercken, 1979;
Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a
long-lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]-t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an absolute
correlation, without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, [1R,cis]-cypermethrin,
[1R,trans]-cypermethrin, and [2S, alphaS]-fenvalerate were
inhibitors, but their non-toxic stereoisomers were not; non-cyano
pyrethroids were much less potent or were inactive (Lawrence & Casida,
1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The
[3H]-dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of pyrethroids are
mediated, at least in part, through an interaction with a
GABA-regulated chloride ionophore-associated binding site. Moreover,
studies with [3H]-Ro 5-4864 support this hypothesis and, in addition,
indicate that the pyrethroid-binding site may be very closely related
to the convulsant benzodiazepine site of action (Lawrence et al.,
1985).
The Type II pyrethroids (deltamethrin, [1R,cis]-cypermethrin and
[2S, alpha2]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two
non-insecticidal stereoisomers and Type I pyrethroids (permethrin,
resmethrin, allethrin) were inactive. Therefore, cyanophenoxybenzyl
pyrethroids appear to act on the GABA receptor-ionophore complex
(Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action
potentials without presynaptic repetitive activity. However, an
intermediate group of pyrethroids (1R-permethrin, cyphenothrin, and
fenvalerate) caused both types of effect. Thus, in muscle or nerve
membrane the pyrethroid induced repetitive activities due to a
prolongation of the sodium current. But no clear distinction was
observed between non-cyano and alpha-cyano pyrethroids (Ruigt & Van
den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is
the sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group (allethrin, d-phenothrin, permethrin, and
cismethrin) cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation. This
results in relatively short trains of repetitive nerve impulses in
sense organs, sensory (afferent) nerve fibres, and, in effect, nerve
terminals. On the other hand, the alpha-cyano pyrethroids cause a
long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin and cypermethrin, which
have identical molecular structures except for the presence of an
alpha-cyano group on the phenoxybenzyl alcohol, indicates that it is
this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids,
the ultimate lesion that causes death will depend on the animal
species, environmental conditions, and on the chemical structure and
physical characteristics of the pyrethroid molecule (Vijverberg & Van
den Bercken, 1982).
5.2 Enzymatic Systems for Biotransformation
When 14C alcohol-labelled cismethrin, bioresmethrin, and
5-benzyl-3-furylmethyl alcohol (BFA) (18) were incubated with rat
liver S-9 homogenates or microsomes, a proportion of the radioactive
compounds was covalently bound to proteins. The covalent binding was
greater with phenobarbital-pretreated rats, and dependent on a
NADPH-generating system. When a S-9 homogenate was used, the bound
compounds were twice as high for cismethrin as for bioresmethrin and
BFA. Inversely, when microsomes were used, more covalent binding
occurred with bioresmethrin and BFA than with cismethrin. The
inhibition of esterases by tetraethyl pyrophosphate (TEPP) in a S-9
homogenate did not alter the amount of covalent binding to the three
compounds, whereas malathion inhibited this binding. However,
treatment of a S-9 homogenate with piperonyl butoxide greatly reduced
covalent binding. Covalent binding was inhibited when the microsomes
were incubated with carbon monoxide or modified by thermal
denaturation. It is suggested that oxidative metabolism was
responsible for the covalent binding (Hoellinger et al., 1985).
When four resmethrin isomers ([1R,trans]-, [1S,trans]-,
[1R,cis]-, and [1S,cis]-) were incubated with mouse and rat
microsomes in 50 mmol/litre tris-HCl buffer (pH 7.4) at 37°C for 1 h,
microsomal esterases readily cleaved the trans- but not the
cis-isomers. The ester linkage also appeared to undergo oxidative
cleavage when esterase attack was minimal. Ester metabolites were
detected in significant amounts only with [1R,cis]-resmethrin, in
which case oxidation had occurred at the isobutenyl moiety, with or
without oxidation at the benzylfuryl methyl group. Most of the
in vitro metabolites were identical with those in the excreta of
rats given resmethrin orally. The preferred site of oxidation in the
isobutenyl moiety varies with the resmethrin isomer and microsomal
source. Mouse microsomes predominantly oxidized the trans-methyl
group of both [1R,trans]- and [1S,trans]-resmethrin, the
selectivity, however, being the greatest with [1S,trans]-resmethrin.
Rat microsomes were relatively nonselective in attacking the
isobutenyl methyl groups of [1R,trans]-resmethrin (Ueda et al.,
1975a).
Plasma-esterases were equally active in hydrolysing cismethrin and
bioresmethrin (3.2-3.4 nmol benzylfurylmethanol/min per g) whereas
liver microsomal esterases hydrolysed bioresmethrin 10 times more
rapidly than cismethrin (White et al., 1976).
6. EFFECTS ON THE ENVIRONMENT
Data on the acute toxicity of resmethrin for aquatic and
terrestrial non-target organisms are summarized in Tables 4 and 5,
respectively.
6.1 Aquatic Organisms
Resmethrin is highly toxic for fish, and the toxicity is
negatively correlated with temperature (Mauck et al., 1976); the LC50
value for bluegill is 2.3 times greater at 22°C than at 12°C, as shown
in Table 4. The influence of both water hardness and pH on toxicity
ranged from slight to negligible. By keeping resmethrin solution at
12°C for 1 week, the toxicity for bluegill decreased 2-fold at each pH
value as the resmethrin solution aged.
Among the 4 isomers, the 1R, cis-isomer is most toxic for fish,
followed by the 1R, trans-, 1S, cis- and 1S, trans-, as observed
in pyrethroids. Contrary to other pyrethroids, resmethrin is less
toxic for arthropods than for fish, as shown in Table 4. The 48-h
LC50 values for arthropods are in the range of 2-25 µg/litre
(Nishiuchi, 1981).
Laboratory studies have shown that resmethrin is highly toxic for
fish but, because of the low water solubility of the compound and
rapid environmental degradation (photochemical and microbial), it
would be expected that, under field conditions, the toxic impact would
be greatly reduced. Such a reduction in toxic effects has been
confirmed in field studies carried out in the USA (Sjogren, 1985;
Norwood, 1986; Pierce, 1986).
In one of these studies, ponds were sprayed with resmethrin
formulations at the expected field rate for adult mosquito control
(0.01 kg/ha). Residue analysis confirmed that resmethrin levels fall
rapidly (1.1 ppb at zero time nondetectable after 96 h). Caged fish
showed good survival (82-100% for bluegill, 73-100% for goldfish) in
resmethrin-treated ponds. However, one fish species in this study
(white sucker) was susceptible (8% survival) when piperonyl
butoxide-synergised resmethrin was used, though good survival was
noted for this species with unsynergised resmethrin. In a second
study, synergised resmethrin was applied to a mangrove-fringed pond at
2-day intervals. The first two applications were at the normal field
rate, but the third was at 10 times this rate. In this study, both
fish species used to monitor fish survival (sheepshead minnow,
fingerling snook) showed good survival (100% at the normal rate and
88% at the 10-fold rate).
Table 4. Acute toxicity of resmethrin for non-target aquatic organisms
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish
Carp (Cyprinus 48-h LC50 44 technical static 25 Nishiuchi (1982)
carpio)
Killifish adult 48-h LC50 300 technical static 25 Miyamoto (1976)
(Oryzias adult 48-h LC50 16 (+)-trans- static 25 Miyamoto (1976)
latipes) adult 48-h LC50 8 (+)-cis- static 25 Miyamoto (1976)
adult 48-h LC50 > 10 000 (-)-trans- static 25 Miyamoto (1976)
adult 48-h LC50 3500 (-)-cis- static 25 Miyamoto (1976)
Rainbow trout 96-h LC50 2.2 technical static 12 Marking & Mauck
(Salmo gairdneri) (1975)
Coho salmon 96-h LC50 1.50 technical static 12 Mauck et al.
(Oncorhynchus (1976)
kisutch) 96-h LC50 0.277 technical flow- 12 Mauck et al.
through (1976)
Steelhead trout 96-h LC50 0.450 technical static 12 Mauck et al.
(Salmo gairdneri) (1976)
96-h LC50 0.275 technical flow- 12 Mauck et al.
through (1976)
Yellow perch 96-h LC50 2.36 technical static 12 Mauck et al.
(Perca flavescens) (1976)
96-h LC50 0.513 technical flow- 12 Mauck et al.
through (1976)
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Arthropods
Daphnia pulex 3-h LC50 15 000 technical static 25 Nishiuchi (1982)
3-h LC50 50 000 technical static 25 Miyamoto (1976)
3-h LC50 25 000 - (+)-trans- static 25 Miyamoto (1976)
50 000
3-h LC50 25 000 - (+)-cis- static 25 Miyamoto (1976)
50 000
3-h LC50 > 50 000 (-)-trans- static 25 Miyamoto (1976)
3-h LC50 > 50 000 (-)-cis- static 25 Miyamoto (1976)
Moina macrocopa 3-h LC50 14 000 technical static 25 Nishiuchi (1982)
Sigara substriata 0.59 cm; 48-h LC50 2 technical static 25 Nishiuchi (1981)
6.1 mg
Micronecta sedula 0.32 cm; 48-h LC50 3.3 technical static 25 Nishiuchi (1981)
1.8 mg
Cloeon dipterum 0.93 cm; 48-h LC50 4.5 technical static 25 Nishiuchi (1981)
5.6 mg
Orthetrum 2.3 cm; 48-h LC50 7.3 technical static 25 Nishiuchi (1981)
albistylum 0.62 g
speciosum
Eretes sticticus 1.5 cm; 48-h LC50 25 technical static 25 Nishiuchi (1981)
0.2 g
Sympetrum 2.1 cm; 48-h LC50 10 technical static 25 Nishiuchi (1981)
frequens 0.56 g
Table 4. (cont'd).
Species Size Parameter Concentration Formulation System Temperature pH Hardness (mg Reference
(µg/litre) (°C) CaCO3/litre)
Fish (contd).
Bluegill (Lepomis 96-h LC50 2.62 technical static 12 Mauck et al.
macrochirus) (1976)
96-h LC50 0.750 technical flow- 12 Mauck et al.
through (1976)
0.8 g 96-h LC50 5.46 technical static 22 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 4.28 technical static 17 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.33 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.53 technical static 12 6.6 10-13 Mauck et al.
(1976)
0.8 g 96-h LC50 2.54 technical static 12 7.8 160-180 Mauck et al.
(1976)
0.8 g 96-h LC50 2.06 technical static 12 8.2 280-320 Mauck et al.
(1976)
0.8 g 96-h LC50 2.29 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.46 technical static 12 9.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.89 technical static 12 6.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.70 technical static 12 7.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 3.13 technical static 12 8.5 40-48 Mauck et al.
(1976)
0.8 g 96-h LC50 2.68 technical static 12 9.5 40-48 Mauck et al.
(1976)
6.2 Terrestrial Organisms
Acute toxicity data show that both resmethrin and bioresmethrin
(Table 5) are harmless for birds, as are other pyrethroids.
In addition, a study has been carried out to assess the effects of
resmethrin on reproduction in the Mallard duck and Bobwhite quail. The
birds were given 12, 60, or 300 mg resmethrin/kg diet for 22 weeks. No
effects on reproduction were seen in either species at these dose
levels (Roberts et al., 1985a,b).
Table 5. Acute toxicity of resmethrin and bioresmethrin or non-target terrestrial
organisms
Species Compound Size Application Toxicity Reference
(days) (mg/kg)
Bird
Quail Resmethrin 14 diet LC50 Hill et al.
(Coturnix > 5000 (1975)
coturnix
japonica)
Mallard 10 diet LC50 Hill et al.
(Anas > 5000 (1975)
platyrhynchos)
Chicken Bioresmethrin oral LD50 Chesher &
> 10 000 Malone (1970a)
Wallwork
et al. (1970)
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 Acute Toxicity
Acute toxic signs produced in experimental animals by exposure to
resmethrins include tremor, hyperexcitability, convulsive twitching,
prostration, and coma.
LD50 data on resmethrin isomers administered orally and by other
exposure routes are summarized in Tables 6 and 7, respectively.
These data show that resmethrins are weakly toxic, except for
cismethrin, the acute oral toxicity of which is moderate in the mouse.
The results of acute toxicity tests of the resmethrin metabolites
are given in Table 8.
Acute (1 h) inhalation studies were performed with 3 aerosol
formulations of resmethrin and 2 blank formulations. Groups of 20 male
rats and 4 male rabbits inhaled the active ingredient at concentration
levels of 12-13.7 mg/litre (value based on the weight loss from each
spray can). In addition, 10 exposed and 10 control rats were tested
for conditioned avoidance performance immediately following exposure.
Laboured breathing was seen generally in the animals. Several rabbits
died in both the resmethrin-treated and the blank-formulation groups.
In the rats, there were no effects on body weight, or gross or
histopathological lesions. However, the conditioned avoidance latency
in the resmethrin-treated rats was significantly greater than that in
the unexposed controls (Macko et al., 1979).
7.2 Short-term Exposure
7.2.1 Oral administration
Groups of 6 Sprague-Dawley rats from each sex were administered
resmethrin in the diet at concentrations of 0, 310-323, 630-660,
1230-1250, 1423-2670, or 1680-5100 mg/kg, for 14 days. Five out of 6
rats died at the highest exposure level in both sexes. Tremor was
observed and body weight gain and food utilization were reduced at
levels of 1230-1250 mg/kg or more. A significant increase in the
hepatic organ-to-body weight ratios was noted in all groups of females
and in groups of males fed 630-660 mg/kg or more. Compound-related
histopathological changes were not observed in any of the tissues and
organs examined. The maximum no-observed-adverse-effect dietary level
was 310 mg/kg for male rats only (Swentzel et al., 1977).
Table 6. Acute oral toxicity of resmethrin isomers
LD50 (mg/kg
Compound Animal Sex body weight) Reference
Resmethrin rat M > 5000 Miyamoto (1976)
rat F > 5000 Miyamoto (1976)
rat M 1244 Gaines & Linder (1986)
rat F 1721 Gaines & Linder (1986)
rat (weanling) M 1987 Gaines & Linder (1986)
rat ? > 2500 Worthing & Walker (1983)
rat - 960 Volkov et al. (1979)
mouse M 690 Miyamoto (1976)
mouse F 940 Miyamoto (1976)
Bioresmethrin rat M 8800 Glomot & Chevalier (1969)
rat F > 8000 Verschoyle & Barnes (1972)
rat F 7071 Wallwork et al. (1970)
rat - 840 Kholmatova (1984)
rat - 990 Volkov et al. (1979)
rat - 675 Kagan et al. (1986)
mouse M 590 Miyamoto (1976)
mouse F 800 Miyamoto (1976)
mouse M 3100 Ueda et al. (1975b)
mouse F 10000 Wallwork et al. (1970)
mouse - 520 Kholmatova (1984)
mouse - 480 Kagan et al. (1986)
rabbit - 225 Kholmatova (1984)
Cismethrin mouse M 152 Miyamoto (1976)
mouse F 160 Miyamoto (1976)
[1S,-trans]-resmethrin mouse M 500 Miyamoto (1976)
mouse F 600 Miyamoto (1976)
[1S,-cis]-resmethrin mouse M 3700 Miyamoto (1976)
mouse F 5000 Miyamoto (1976)
Table 7. Acute toxicity of resmethrins via other than oral exposure
LD50 LC50
Compound Animal Sex Route (mg/kg (mg/m3) Reference
body weight)
Resmethrin rat M dermal 2500 Gaines & Linder (1986)
rat F dermal 2500 Gaines & Linder (1986)
rabbit - dermal 2500 Green (1977)
rat - inhalation > 9490 Jackson & Hardy (1984)
(4-h exposure)
rat - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
rabbit - inhalation > 12 000 Macko et al. (1979)
(1-h exposure)
dog - inhalation > 420 Macko et al. (1979)
(4-h exposure)
Bioresmethrin rat F dermal > 10 000 Wallwork et al. (1970)
rat F intravenous 340 Verschoyle & Barnes (1972)
rat F intravenous 106-133 Chesher & Malone (1971b)
rat F intraperitoneal > 8000 Wallwork & Malone (1971)
rat F inhalation > 872 Wallwork & Malone (1972)
(24-h exposure)
mouse M intraperitoneal > 1500 Ueda et al. (1975b)
mouse F intraperitoneal > 5359 Wallwork et al. (1970)
Mixture of rat M,F inhalation > 1500 Miyamoto (1976)
bioresmethrin (4-h exposure)
& cismethrin mouse M,F inhalation > 1500 Miyamoto (1976)
(4-h exposure)
Table 8. Acute toxicity of metabolites of resmethrin
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
(bioresmethrin), (5-benzyl-3-furyl- mouse > 1500a 3100a Miyamoto (1975-1976)
methyl (+)-trans-chrysanthemate) (1) Ueda et al. (1975b)
1R or (+)-cis-resmethrin (5-benzyl-3-furyl- (2) mouse 320a Miyamoto (1975-1976)
methyl (+)-cis-chrysanthemate) Ueda et al. (1975b)
d,1-cis,trans-chrysanthemic acid (17) rat 2443 Reagan & Becci (1985a)
d,1-trans-chrysanthemic acid - rat 1598 Reagan & Becci (1985b)
(+)-trans-chrysanthemic acid, (+)-trans-CA (26) mouse 98a 280a Miyamoto (1975-1976)
(t-CA) Ueda et al. (1975b)
(+)-cis-chrysanthemic acid, (+)-cis-CA (c-CA) (27) mouse 600a Miyamoto (1975-1976)
Ueda et al. (1975b)
d-trans-chrysanthemic acid (26) rat 983 Reagan & Becci (1985c)
(+)-trans-chrysanthemumdicarboxylic acid, (30) mouse 408a Miyamoto (1975-1976)
(+)-trans-CDA (tE-CDA) Ueda et al. (1975b)
5-benzyl-3-furylmethanol (BFA) (18) mouse 75a 310a Miyamoto (1975-1976)
Ueda et al. (1975b)
Table 8. (cont'd).
Metabolite Nob Animal LD50 (mg/kg) Reference
ip oral
5-benzyl-3-furoic acid (BFCA) (20) mouse 46a Miyamoto (1975-1976)
Ueda et al. (1975b)
5-benzyl-3-furoic acid (BFCA) (20) rat 997 Reagan & Becci (1985d)
ethyl chrysanthemate - rat > 4640 Stauffer Chemical Co.
(1984)
a Toxicity for male mice, 24 h after intraperitoneal injection or oral administration.
b Chemical identification numbers used in Table 1 and Fig. 2.
The same authors fed groups of 6 Long-Evans rats of each sex
resmethrin in the diet at concentrations of 0, 61-90, 148-180,
297-386, 584-669, 1080-1375, or 1266-2532 mg/kg body weight per day
for 14 days. Mortality was observed in the groups fed 1080 mg/kg or
more. Tremor was observed at levels of 386 mg/kg or more (females only
at 386 mg/kg). The terminal body weight and food utilization were
significantly lower in the groups fed 1080 mg/kg or more compared with
the controls. A significant increase in the hepatic organ-to-body
weight ratios was noted at levels of 297 mg/kg or more.
Compound-related histopathological changes were not observed in any of
the tissues and organs examined. The maximum no-observed-adverse-
effect dietary level was 148 mg/kg body weight per day for male and
180 mg/kg body weight per day for female rats (Swentzel et al., 1977).
In a third study by Swentzel et al. (1977), groups of 8-20
Long-Evans rats of each sex were fed resmethrin in the diet at
concentrations equivalent to 0, 22, 66, 211, or 679 mg/kg body weight
per day for males and of 0, 3, 7, 22, 67, 219, 724, or 2400 mg/kg body
weight per day for females, for 90 days. All the females fed the
highest level died. Tremor was observed at levels of 679 mg/kg or
more. The mean terminal body weight was significantly lower in the
group given 679-724 mg/kg than in the control group. A significant
increase in the hepatic and renal organ-to-body weight ratios was
noted in males fed 211 or 679 mg/kg; while, in females fed 219 or
724 mg/kg, only an increase in the hepatic ratio was observed. No
significant differences were observed in clinical chemistry values
(serum glutamic oxaloacetic transaminase (SGOT), serum glutamic
pyruvic transaminase (SGPT), total lactate dehydrogenase (LDH),
alkaline phosphatase (AP), gamma-glutamyl trans-peptidase (GGTP),
total bilirubin, total protein, blood-urea nitrogen (BUN)) between
test and control animals. Compound-related histopathological changes
were not observed in any of the tissues and organs examined. The
maximum no-observed-adverse-effect dietary level was 67 mg/kg body
weight per day for female and 66 mg/kg body weight per day for male
rats.
Groups of rats (10 males/group) were administered bioresmethrin
orally by gavage, daily, 6 days/week for 3 weeks at doses of 0, 1000,
or 2000 mg/kg body weight. Body weights were slightly reduced at
2000 mg/kg. A slight reduction was noted in haemoglobin content and
hematocrit value. Biochemical examination revealed an increase in
albumin and blood-urea nitrogen concentrations and a decrease in
serum-glutamic oxaloacetic transaminase (SGOT) activity. Increased
liver size and reduced thymus weight were observed at 1000 mg/kg.
Reduced prostate weight was noted only at the high dose.
Histopathological examination revealed thymic involution (Glomot,
unpublished report).a
a GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished report of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
Wallwork et al. (1971) reported a study in which groups of rats
(18 males and 18 females/group) were fed bioresmethrin in the diet at
concentrations of 0, 400, 1200, or 8000 mg/kg over 91 days. The group
receiving the highest dose was fed 4000 mg/kg for 30 days and
thereafter 8000 mg/kg. Body weights were reduced at the highest dose
level and there were changes in blood chemistry parameters indicating
liver dysfunction (an increase in serum-alkaline phosphatase (SAP),
SGOT, and urinary nitrogen and a decrease in glucose content). The red
blood cell count was depressed in the group receiving 1200 mg/kg. An
increase in liver weight and a decrease in the weights of several
other organs, such as the spleen and thymus, were observed at
4000 mg/kg. Fatty infiltration of the liver was noticed at 1200, 4000,
and 8000 mg/kg. The no-observed-adverse-effect level in this study was
400 mg/kg, equivalent to an average daily intake of 32.8 and
36.1 mg/kg body weight for males and females, respectively.
Bioresmethrin was administered to groups of dogs (2 of each
sex/group) by gavage, daily, at dose levels of 0 or 500 mg/kg for 7
days followed by an increased dose of 1000 mg/kg for a further 14
days. No effects were noted on mortality, behaviour, body weight,
haematology, blood chemistry, urinalysis, or electrocardiograms
(Malone & Chesher, 1970).
Groups of dogs (3 males and 3 females/group) were administered
bioresmethrin in gelatin capsule by gavage, daily for 90 days, at
dosage levels of 0, 25, 80, or 250 mg/kg body weight (the highest dose
was increased to 500 mg/kg body weight in week 7). There were no
effects on mortality, body weight, food consumption, ophthalmology, or
urinalysis. Red blood cell count, haemoglobin content, and packed cell
volume values were reduced at the highest dose level. Blood-urea
nitrogen was slightly increased at the highest dose after 12 weeks. No
adverse effects were observed on gross or histopathological
examination. The no-observed-adverse-effect level was 80 mg/kg body
weight, equivalent to an average of 1600 mg/kg diet (Noel et al.,
1971).
In summary, the no-observed-adverse-effect level for resmethrin as
determined in the 90-day study on rats was 66 mg/kg body weight per
day, whereas that for bioresmethrin was 33 mg/kg body weight per day
in the 91-day study on rats and 80 mg/kg body weight per day in the
90-day study on dogs.
7.2.2 Inhalation
Short-term inhalation studies were performed using three
resmethrin formulations and one blank formulation. Aerosol spray
formulations (frequency median diameter of the particles: 1.5-2.0 µm,
volume median diameter 10-18.5 µm) were introduced into the top of a
pyramid-shaped sealed chamber and dispersed for 30-second intervals
every 30 min. Groups of 25 male rats, 10 female rats, and 4 male
rabbits were exposed to the inhalations 10 times a day, over a period
of 5 h, for 5 consecutive days. Daily active ingredient concentrations
of each formulation in the chamber were 2.9-3.2 mg/litre, based on the
weight loss from each spray can. The clinical signs included increased
preening, ruffled pelt, rapid breathing, and slight nasal discharge.
All signs disappeared overnight, but recurred after each daily
exposure. In rats, there were no effects on body weight. No gross or
histopathological lesions related to exposure to the formulations were
observed in rats necropsied immediately after the last 5-h exposure
and 7 and 14 days after exposure. No indication of toxic effects,
other than irritation, was observed in any test (Macko et al., 1979).
When ICR mice and Sprague-Dawley rats were exposed to [1R,
trans, cis]-resmethrin (at concentrations of 0, 23, 47, or
210 mg/m3) for 4 h/day, 5 days/week over 4 weeks, no toxic effects
were observed on behaviour, food intake, haematology, clinical
biochemistry, mortality, or histopathology (Miyamoto, 1976).
When groups of 16 male and 16 female Wistar rats were exposed
through inhalation for 6 h/day on 5 days of each week, over a period
of 90 days, to technical resmethrin at target exposure levels of 0.1,
0.3, or 1.0 g/m3, the no-observed-adverse-effect level was
established at 0.1 g/m3. At 0.3 g/m3, there were minor effects on
some clinical pathology parameters and also signs of irritation.
However, microscopic pathological examination did not reveal any
treatment-related changes in the lungs or other organs of rats exposed
at this level. At 1 g/m3, there were clinical signs indicative of
irritation, minor neurobehavioural changes, a reduced rate of weight
gain, and some changes in clinical pathology parameters. Microscopic
examination at this level showed minimal changes in the larynx, liver,
and thyroid, but these were reversible during the recovery period.
There were no treatment-related lung changes (Coombs et al., 1985).
7.2.3 Dermal application
Resmethrin was applied twice a week for 3 weeks to the shaved skin
of 4 groups of 10 male New Zealand White rabbits. Cotton cloth treated
with resmethrin at 0.247 mg/cm3 was applied over 1 ml of liquid
(imitating sweat) to rabbits in the first group. In the second group,
cotton cloth treated with resmethrin was applied without the sweat,
and in the third group, the cotton cloth was fixed to skin that had
been pretreated with 10 g of technical grade resmethrin. In the fourth
group, untreated cotton cloth was fixed over skin pre-treated with
pyrax powder containing 1% resmethrin at the rate of 1 g/kg of body
weight. The 3 control groups received cotton cloth treated with
acetone, cotton cloth treated with acetone over 1 ml of the sweat, and
untreated cotton cloth over 1 g pyrax powder/kg, respectively. No
significant changes were noted, on day 24 of the test, in rabbit body
weights and organ-to-body weight ratios of liver, lung, kidney,
testis, and spleen. Average dermal irritation scores for
resmethrin-treated rabbits were not significantly higher than those
for the control groups and did not increase during the test. No
significant trends compared with the controls were seen in clinical
chemistry values (serum-glutamic oxalo-acetic transaminase,
serum-glutamic pyruvic transaminase, lactic dehydrogenase, alkaline
phosphatase, blood-urea nitrogen) on days 5, 12, 19, and 24 of the
test. There were no compound-related lesions of the skin or of any of
the other tissues and organs examined at the termination of the study
(Swentzel et al., 1977).
7.3 Primary Irritation and Sensitization
Technical grade resmethrin was found to be a slight irritant in
rabbits in a 24 day dorsal/ventral rabbit ear test. Dermal irritation
was evident on both intact and abraded skin at 72 h and up to 7 days.
Resmethrin did not cause sensitization reactions in guinea-pigs, or
photochemical irritation in New Zealand White rabbits. Repeated daily
applications of 0.1 g of the technical grade compound to one ear of
each of 5 New Zealand White rabbits were carried out for 30
consecutive days as well as applications of compound-impregnated
cotton sateen cloth (0.247 mg/cm2) with artificial sweat for 24 days.
In this study, resmethrin did not produce acne-form dermatitis
(Swentzel et al., 1977).
Groups of adult guinea-pigs (6 males/group) were treated with
bioresmethrin (0.1 ml of a 5% (w/v) formulation) or 2,4-dinitrochloro
benzene (DNCB) (0.1 ml of a 1% (w/v) formulation in mineral oil) to
assess the sensitization properties. The test substance was applied to
the ears for 4 days. On day 7, 0.2 ml was applied dermally and the
degree of irritation recorded. As expected, the DNCB was an irritant
while bioresmethrin showed only traces of erythema suggesting a low
potential for sensitization and irritation (Chesher & Malone, 1970b).
Instillation of technical bioresmethrin into the eye of 6 rabbits
(0.1 ml) did not produce any irritation or corneal damage, or any
indication of ocular hazard (Chesher & Malone, 1970c).
7.4 Long-term Exposure and Carcinogenicity
When [1R, trans, cis]-resmethrin was fed to Sprague-Dawley
rats (male and female) at dietary levels of 500, 1500, or 5000 mg/kg
for 24 weeks, toxic symptoms, such as tremors and decreased body
weight, increased liver and kidney weights and an increase in alkaline
phosphatase activity were observed at 5000 mg/kg. The no-observed-
adverse-effect level was 1500 mg/kg (77.7 mg/kg per day for males and
86.6 mg/kg per day for females) (Miyamoto, 1976).
Resmethrin fed to Wistar rats at dosage levels of 0, 500, 2500, or
5000 mg/kg in the basal diet over a 112-week period, was determined
not to be oncogenic up to, and including, 5000 mg/kg, which was the
highest dose tested. The no-observed-adverse-effect level of 500 mg/kg
for toxic effects, was the lowest effect level for hypertrophy of
hepatocytes, which was not considered a definite toxic response
(Knickerbocker et al., 1980; Hess et al., 1982).
Cox et al. (1979a) fed CD-1 outbred albino mice with 0, 250, 500,
or 1000 mg resmethrin/kg basal diet for an 85-week period. No
oncogenicity was observed at any doses up to and including 1000 mg/kg.
When Beagle dogs were administered 0, 10, 30, or 300 mg
resmethrin/kg body weight for 6 months, the no-observed-adverse-effect
level was 10 mg/kg per day. On day 57 of the study, the highest dose
level was increased from 100 mg/kg per day to 300 mg/kg per day.
Increased liver weights were noted at 30 mg/kg body weight per day
(Gephart et al., 1980).
An acceptable daily intake (ADI) was established by the USA EPA at
0.125 mg/kg body weight per day based on no-observed-adverse-effect
levels in long-term toxicity studies. A food additive tolerance has
been established by the US EPA, permitting resmethrin residues of up
to 3 ppm, in or on food commodities, resulting from use in food
handling areas (US Environmental Protection Agency, 1983).
7.5 Mutagenicity
Saccharomyces cerevisiae D4 and five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) were used to
evaluate the mutagenic potential of resmethrin. The compound was
tested in the absence or presence of liver microsomal enzyme
preparations from rats pre-treated with Aroclor 1254. Resmethrin was
not mutagenic to any of the indicator organisms under both conditions
(Swentzel et al., 1977).
Resmethrin, bioresmethrin, and cismethrin were tested for
mutagenicity in several test systems. Miyamoto (1976) and Suzuki
(1975) used the Escherichia coli and S.typhimurium reverse
mutation tests. Garret et al. (1986) summarized results of point/gene
mutations in prokaryotes or eukaryotes, primary DNA damage in
prokaryotes or eukaryotes, chromosomal effects in Chinese hamster
ovary cells, mouse bone marrow, and cardiac blood cells of the mouse.
Pluijmen et al. (1984) used reverse mutation tests in S.typhimurium
TA98 or TA100, or mutagenicity tests with V79 Chinese hamster cells to
test seven synthetic pyrethroids including resmethrin, bioresmethrin,
and cismethrin. They all gave negative results.
Bioresmethrin was also tested in a metaphase chromosome analysis
of cultured human lymphocytes, an autoradiographic assessment of
unscheduled DNA synthesis in mammalian cells, and a mouse micronucleus
test. They also gave negative results (Allen et al., 1986; Allen &
Proudlock, 1986; Vannier & Fournex, 1986).
7.6 Reproductive effects, Embryotoxicity, and Teratogenicity
Three groups of 30 pregnant Long-Evans rats were administered
resmethrin in the diet at concentrations of 0, 188, or 1500 mg/kg from
day 6 to day 16 of gestation. The dams showed tremors and decreased
food and water consumption at 1500 mg/kg and 2 dams died; the lower
fetal weight seen at this dose and the resorption of embryos and
fetuses in 15 out of 30 dams were probably due to maternal toxicity.
No gross abnormalities of fetal skeletons and soft tissues were
observed in the treated groups. Thus, the consumption of resmethrin in
ground feed was not teratogenic at 188 and 1500 mg/kg (Swentzel
et al., 1977).
In a 3-generation study, Sprague-Dawley rats were fed resmethrin
in the diet at 0, 500, 800, or 1250 mg/kg (Schwartz et al., 1979c). A
slight increase in the number of pups cast dead and a decrease in pup
weights were noted at the 500 mg/kg level. A no-observed-adverse-
effect level of < 500 mg/kg was established for pups cast dead and
reduced pup weights at 21 days.
Sprague-Dawley female albino rats were given resmethrin in corn
oil, by gavage, at dose levels of 0, 20, 40, or 80 mg/kg during the
period of major organogenesis (days 6-15 of gestation). Resmethrin was
not teratogenic in rats at levels up to, and including, 80 mg/kg. The
no-observed-adverse-effect level for fetotoxicity was 40 mg/kg (Machi
et al., 1979).
Pregnant New Zealand White Minnikin rabbits were given resmethrin
by oral intubation at 0, 10, 30, or 100 mg/kg body weight per day on
days 6-18 of gestation (Becci et al., 1979). On day 29 of gestation,
all animals were killed for examination. No teratogenic effects were
seen at dose levels up to and including 100 mg/kg.
When [1R, trans, cis]-resmethrin was administered orally to
female ICR mice (10, 30, or 100 mg/kg, daily) and Sprague-Dawley rats
(10, 20, or 50 mg/kg daily) during the period of gestation, to examine
maternal and embryotoxic effects, no significant adverse effects, such
as abortion, resorption of fetuses or embryos, external or skeletal
abnormalities of pups, and abnormalities in growth or organ
differentiation, were observed at any doses (Miyamoto, 1976).
Groups of pregnant rabbits (4-6 rabbits/group) were administered
bioresmethrin at doses of 0, 10, 20, 40, or 80 mg/kg, by gavage,
daily, from day 8 to day 16 of gestation. The does were sacrificed on
day 28 and examined for implantation, live and dead fetuses,
resorption sites, and abnormalities. There was no apparent effect on
parents in the study as growth and gestation were unaffected. There
was an increase in the numbers of dead fetuses at the highest dose and
a large number of resorption sites were noted at all treatment levels.
A number of deformed fetuses were observed, but the total numbers were
not sufficient for an adequate statistical evaluation. The deformities
included straight tail, crossed hind limbs, and unilateral union of
6th and 7th ribs at the sternal end. An overall fetal loss was
observed at all dose levels (primarily because of the large number of
resorption sites recorded) (Waldron, 1969).
7.7 Immunotoxicity
The influence of various pesticides on the humoral and cellular
immune response to fluorescein-labelled ovalbumin has been analysed.
Resmethrin was administered intragastrically in corn oil as a single
dose (one half of LD50) before primary immunization. Control groups
included animals treated with corn oil alone, or immunosuppressed with
methotrexate. Booster immunizations and test bleedings were scheduled
to follow at weekly intervals. The cellular immune response was
quantified by redness and swelling, histological examination, and by
differential temperature measurements of the foot pads after antigen
challenge. The concentration, binding affinity, and heterogeneity of
the serum antibody were determined by fluorescence polarization
measurements. Resmethrin gave an early, sometimes very marked,
stimulation of the cellular immune response (Danliker et al., 1979).
7.8 Neurotoxicity
The neurotoxicity of resmethrin was evaluated in three short-term
dietary studies on Sprague-Dawley rats designed to examine the gross
and histopathological changes to the central and peripheral nervous
system. Resmethrin was not neurotoxic when administered at 1250 mg/kg
for 32 weeks, 5000 mg/kg for 30 days, or 12 640 mg/kg for 7 days (Cox
et al., 1979b; Schwartz et al., 1979a,b).
In order to better characterize the behavioural effects of
pyrethroid insecticides, comparisons were made of the effects of
cismethrin and deltamethrin exposure on motor activity and the
acoustic startle response in male Long-Evans rats (Crofton & Reiter,
1984). Acute dose effect, acute time course, and 30-day
repeated-exposure determinations of 1 h motor activity were made using
figure-eight mazes. The acoustic startle response to a 13-kHz, 120-dB,
40-msecond tone was measured at each of three background white noise
levels (50, 65, and 80 dB). Deltamethrin (0, 2, 4, 6, or 8 mg/kg) or
cismethrin (0, 6, 12, 18, or 24 mg/kg) was administered orally in
0.2 ml corn oil/kg. Both compounds produced similar dose-dependent
decreases in motor activity. The time course of onset and recovery for
this decreased activity was rapid (1-4 h). No cumulative effects on
motor activity were found of a 30-day exposure to 2 mg deltamethrin/kg
per day or 6 mg cismethrin/kg per day. The effects of cismethrin and
deltamethrin on the acoustic startle response differed. Deltamethrin
produced a dose-dependent decrease in amplitude and an increase in
latency, and cismethrin produced an increase in amplitude and no
change in latency. The differential effects of cismethrin (Type I
pyrethroids) and deltamethrin (Type II pyrethroids) on the acoustic
startle response may be related to the contrasting effects previously
shown with neurophysiological and/or neurochemical techniques (see
Annex).
The isolated rat neurohypophysis, which shows a calcium-dependent
hormone release when depolarized in vitro, was used as a model
system to investigate the effects of the pyrethroids deltamethrin and
resmethrin on mammalian nervous tissue (Dyball, 1982). Both compounds
inhibited neurohypophysial hormone release in response to electrical
stimulation, deltamethrin being more potent than resmethrin.
Deltamethrin reduced the hormone content of the neurohypophysis.
Resmethrin did not reduce stored hormone significantly and its effects
on release were dose dependent. They could be mimicked by raising the
Na+ of the medium, but not by lowering the Ca2+. Resmethrin did not
have any effects on the release of hormone following depolarization of
the tissue with a raised K+. The results are consistent with the
suggestion that the compounds do not act on the potential-dependent
secretion process but rather on the mechanism linking depolarization
of the secretory terminals with the arrival of action potentials,
possibly by interfering with sodium-channel activation and
inactivation.
The neurological effects of four synthetic pyrethroids resmethrin,
permethrin, cypermethrin, and deltamethrin were investigated in the
rat to establish whether there is a correlation between the
clinical-functional status of the animal and peripheral nerve damage,
as measured biochemically (Rose & Dewar, 1983). Neuromuscular
dysfunction was assessed by means of the inclined plane test and
peripheral nerve damage by reference to ß-glucuronidase and
ß-galactosidase activity increases in nerve tissue homogenates from
treated and control animals. A transient functional impairment was
found in animals treated with any one of the four pyrethroids tested
and, in all cases, this was maximal at the end of the 7-day dosing
regimen (resmethrin doses of 500-2000 mg/kg per day). Significant
increases in ß-glucuronidase and ß-galactosidase were found, 3-4 weeks
after the start of dosing in the distal portion of the
sciatic/posterior tibial nerves of animals treated with permethrin,
cypermethrin, or deltamethrin; however, no changes were found in
resmethrin-treated animals. Thus, it is concluded that there is no
direct correlation between the time-course of the neuromuscular
dysfunction and the neurobiochemical changes. This suggests that these
pyrethroids have at least two distinct actions, a short-term
pharmacological effect and, at near-lethal dose levels, a more chronic
neurotoxic effect that results in sparse axonal nerve damage.
Resmethrin (30 µmol/litre)-induced release of transmitters was not
affected by manipulation of the Na+ current with either choline or
tetrodotoxin agents, which readily reversed the effects of
veratridine, deltamethrin, and cypermethrin (Doherty et al., 1986).
Resmethrin (I50: 2.2 µmol/litre) inhibited the ATP-dependent uptake
of Ca2+ but deltamethrin and cypermethrin were much less effective.
Resmethrin also displaced Ca2+ from crude synaptosomal membranes. The
release-promoting effects of resmethrin in rat brain in vitro are
better explained by its effects on Ca2+ rather than by a specific
effect on the Na+ channel. In contrast, deltamethrin and
cypermethrin promote transmitter release by a Na+ dependent process.
Glickman & Casida (1982) discussed species and structural
variations affecting pyrethroid neurotoxicity. They concluded that the
mammalian nervous system, or at least the brain, appears to lack sites
sensitive to bioresmethrin and to a lesser extent to [1R,
trans]-permethrin, yet small structural changes restore the toxicity
(e.g., [1R,trans]-ethano-resmethrin and [1R, cis]-resmethrin. The
authors reported that birds and mammals in general respond to cis-
but not to trans-resmethrin in contrast to insects, crustaceans, and
fish, which are highly sensitive to both isomers. Furthermore, common
green lacewing larvae are very tolerant to pyrethroids, suggesting
possible involvement of nerve insensitivity in addition to
detoxification in this species. These examples of insensitivity may be
associated with modified sites of action or perhaps an increased
stabilization of nerve membranes making them more resistant to
pyrethroid-induced excitation.
7.9 Mechanism of Toxicity (Mode of Action)
Cismethrin is toxic for both insects and mammals. Bioresmethrin is
about 50 times less toxic than cismethrin and produces few symptoms in
mice and rats, even at extremely high doses. The poisoning syndrome of
cismethrin and bioresmethrin was characterized by whole body tremors
(T-syndrome) and both compounds were therefore classified as Type I
pyrethroids (Verschoyle & Aldridge, 1980; Lawrence & Casida, 1982,
Annex).
Tremors started when the concentrations of cismethrin and
bioresmethrin in the brain were 0.5-1 mg/kg and 4-5 mg/kg,
respectively. At death, brain levels of cismethrin were 3.9-5.1 mg/kg,
and those of bioresmethrin were 23-35 mg/kg. As liver microsomal
esterases hydrolysed bioresmethrin 10 times more rapidly than
cismethrin, the lower toxicity of bioresmethrin was partly attributed
to its faster metabolism and intrinsically lower toxicity at the
critical site of action in the nervous system (White et al., 1976).
Cismethrin given intravenously produced repetitive activity, after
external stimulation, in the spinal cord of rabbit (Carlton, 1977). As
gamma-aminobutyric acid (GABA) blockers, such as bicuculline and
picrotoxin, produced convulsions similar to those of cismethrin, the
effects of the compounds on dorsal root potentials were examined in
the rat. Bicuculline reduced the amplitude of the potential to 67% of
the control values, but cismethrin enhanced the potential to 142% of
the control values. The convulsions associated with cismethrin
poisoning, therefore, are not produced by an antagonism of
GABA-mediated inhibitory transmission (Smith, 1980).
Administration of cismethrin into the lateral ventricle of the
brain or into the spinal cord causes signs similar to those observed
after intravenous administration. The onset of tremors after
intraventricular administration of cismethrin was delayed for about 15
min while, after intraspinal injection, the symptoms occurred within
2-5 min, indicating that cismethrin may act directly on the spinal
cord rather than in the brain (Gray et al., 1980b).
After intravenous administration of [14C]-alcohol-labelled
cismethrin or bioresmethrin to rats, the parent pyrethroid was rapidly
cleared from the blood and liver, and both isomers rapidly entered the
central nervous system (CNS) reaching peak concentrations within 2-5
min. Cismethrin concentrations in the brain exceeding 3.5 nmol/g were
associated only with animals showing tremors. These levels of
cismethrin were maintained for up to 30 min, but bioresmethrin was
depleted more rapidly, possibly due to brain metabolism. It is
concluded that the low toxicity of bioresmethrin is possibly because
of its inability to interact with the site of action in the CNS and to
its rapid metabolism in the liver (Gray et al., 1980a).
Cismethrin produced repetitive firing in the flight muscles and
uncoupling of motor unit activity in the housefly (Miller & Adams,
1977), and repetitive firing in a cercal sensory nerve of the
cockroach (Gammon et al., 1981). On the basis of the
electrophysiological effects and symptomatology in insects as well as
in mammals, cismethrin is classified as a Type I pyrethroid,
characterized by a mainly peripheral nervous system action (Gammon et
al., 1981).
7.10 Potentiation
Groups of rats (6 female rats/group) were administered
bioresmethrin, bioallethrin, and/or piperonyl butoxide, alone or in
combinations, at doses approximating the acute intraperitoneal LD50
value. No potentiation of the acute toxicity was observed in this
study. In all cases with bioresmethrin combinations, the observed
LD50 values were equal to, or exceeded, the expected value (Wallwork
& Malone, 1971).
8. EFFECTS ON MAN
Although the resmethrins have been used for many years, no data
have been reported on their toxicity for human beings.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Human Health Risks
Resmethrin, consisting of four stereo-isomers, is an effective
insecticide mainly used to control household insects and other public
health insects, with applications in the food handling and storage
areas.
Bioresmethrin and cismethrin are composed of a selected isomer(s).
As with resmethrin, bioresmethrin is used to control household insects
and other public health insects. In addition, bioresmethrin is used as
a post-harvest insecticide to control stored grain pest. Human
exposure to the resmethrins will be mainly via inhalation, when the
formulations are sprayed in the form of a mist, though the use in food
handling and storage areas as well as post-harvest treatment may
result in dietary residues. Air levels following conventional
household aerosol spraying with the resmethrins are not expected to
exceed 0.5 mg/ms.
The only significant potential dietary exposure will result from
the use of resmethrin on stored grain. Residues of up to 4 mg/kg might
be present in grain, but this would be reduced to zero in white bread.
However, reduced residues may be present in wholemeal bread. A food
additive tolerance has been established by the US Environmental
Protection Agency (US EPA) permitting resmethrin residues of up to
3 mg/kg (3 ppm), in or on food commodities, resulting from use in food
handling areas. An ADI was established by the US EPA at 0.1250 mg/kg
body weight per day, based on no-observed-adverse-effect levels in
long-term toxicity studies. No data are available on occupational
exposure to the resmethrins. Although the resmethrins have been used
for many years, no data have been reported concerning their toxicity
for human beings. Thus, to determine the potential toxicity for human
beings, extrapolation from animal data and in vitro studies has been
used.
The result of short-term studies on experimental animals suggest
that resmethrins are weakly toxic when administered by various routes
of exposure (oral LD50 of resmethrin: 690 mg/kg (mouse) - >
5000 mg/kg (rat); those of bioresmethrin: 225 mg/kg (rabbit),
480-10 000 mg/kg (mouse), 8800 mg/kg (rats)). Cismethrin showed
moderate toxicity for the mouse (oral LD50 152-160 mg/kg). The acute
toxicity of resmethrin metabolites were in the same range in the rat,
but somewhat more toxic in the mouse.
While technical grade resmethrin is a slight skin irritant, it is
not a sensitizer.
Resmethrins are not mutagenic in a variety of test systems,
including gene mutations, DNA damage and DNA repair, and chromosomal
effects.
Resmethrin was not carcinogenic for mice or rats, when fed at
dietary levels of up to 1000 mg/kg for 85 weeks and 5000 mg/kg for 112
weeks, respectively.
Resmethrin was not teratogenic in the rat, mouse, or rabbit, up to
dose levels of 100 mg/kg body weight. A level of 40 mg/kg body weight
appeared to be the no-observed-adverse-effect level for fetotoxicity
in the rat.
Resmethrins at near lethal doses are likely to cause
hyperactivity, tremors, and convulsions and have been classified as
Type I pyrethroids.
For resmethrin, a no-observed-adverse-effect level was established
in a 90-day rat study to be 66-67 mg/kg body weight per day, whereas,
in another 2-year rat study, the lowest effect level appeared to be
500 mg/kg diet, corresponding to 25 mg/kg body weight per day. In a
6-month feeding study on dogs, the no-observed-adverse-effect level
was 10 mg/kg body weight per day.
In a 90-day inhalation study on rats, a no-observed-adverse-effect
level was established of 0.1 g/m3.
The no-observed-adverse-effect level for bioresmethrin in a 9-day
feeding study on the rat was 33-36 mg/kg body weight per day; and in a
90-day study on dogs, it was 80 mg/kg body weight per day.
In a 24-week feeding study on rats, the no-observed-adverse-effect
level for [1R, trans, cis]-resmethrin was 1500 mg/kg diet
corresponding to 75 mg/kg body weight.
9.2 Effects on the Environment
Resmethrins are used mainly indoors for the control of household
insects and also for stored grain protection. They are also used in
greenhouses and can be used outdoors for mosquito control. However,
under outdoor conditions, rapid photodegradation and microbial
degradation in the soil ensure that residues will not persist to any
extent in the environment.
In laboratory studies, resmethrins have been shown to be very
toxic for fish (96-h LC50 values: 0.3-5.5 µg/litre) but less toxic
for Daphnia and aquatic insect larvae. However, under field
conditions the low water solubility of the resmethrins and their ready
degradation greatly alleviate the effects that might be predicted from
the laboratory studies. The toxicity of resmethrins for birds is low
(LD50 > 5000 mg/kg) and they do not produce any effects on avian
reproduction.
10. CONCLUSIONS
General population: Under recommended conditions of household
and other public health use, the exposure of the general population to
resmethrins is negligible and is unlikely to present a hazard. Under
recommended conditions of use in food handling and storage areas as
well as in post-harvest treatment, the exposure of the general
population to resmethrins in the diet is unlikely to exceed the ADI
established by the US EPA.
Occupational exposure: With reasonable work practices, hygienic
measures, and safety precautions, the use of resmethrins is unlikely
to present a hazard to those occupationally exposed to it.
Environment: With recommended application rates, it is unlikely
that resmethrins or their degradation products will attain levels of
environmental significance. In spite of the fact that resmethrins are
highly toxic for fish, this is only likely to cause a problem in the
case of spillage or over-spraying.
11. RECOMMENDATIONS
- In order to fully assess the potential dietary exposure from
current uses of resmethrins, it is suggested that the degradation
pathway on stored grain be studied, and the terminal residues on
grain and bread be defined.
- It is considered that resmethrin, despite its high octanol-water
partition coefficient, is very unlikely to bioaccumulate in
non-target species, because of its ready degradation. However,
experimental confirmation of this in fish might be useful.
- A further multigeneration reproduction study to define a
no-observed-adverse-effect level should be considered.
- Over many years of use, no adverse effects have been reported as a
result of human exposure to resmethrins, but it is still necessary
to maintain observations on human exposure.
- The labelling of resmethrins for household use should include
adequate instructions for use and storage and, where appropriate,
a warning of flammability.
- Efforts should be made to obtain a more precise estimate of the
total global usage of resmethrins.
12. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The Joint FAO/WHO Expert Committee on Pesticide Residues (JMPR)
discussed and evaluated bioresmethrin at its meetings in 1975 and 1976
(FAO/WHO, 1976, 1977). An acceptable daily intake (ADI) has not been
established.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified bioresmethrin as a technical
product unlikely to present an acute hazard in normal use, and
resmethrin and cismethrin as slightly hazardous (WHO, 1986). A Data
Sheet on bioresmethrin (No. 34) has been issued (WHO/FAO, 1978).
REFERENCES
ABBOTT, D.D. (1971) Total degradation products of SBP-1382. Acute
oral LD50/mice, 6 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
ABE, Y., TSUDA, K., & FUJITA, Y. (1972) Studies on pyrethroidal
compounds. Part III. Photostability of pyrethroidal compounds.
Botyu-Kagaku, 37: 102-110.
ALLEN, J.A. & PROUDLOCK, R.J. (1986) Autoradiographic assessment of
unscheduled DNA repair synthesis in mammalian cells after
exposure to bioresmethrin, Huntingdon, United Kingdom,
Huntingdon Research Centre, 18 pp (Report No.
RSL-BRM-698/851420/A) (Unpublished proprietary data supplied by
Roussel Uclaf).
ALLEN, J.A., BROOKER, P.C., & HOWELL, A. (1986) Bioresmethrin
metaphase chromosome analysis of human lymphocytes cultures
in vitro, Huntingdon, United Kingdom, Huntingdon Research Centre,
15 pp (Report No. RSL-BRM-697/851370/A) (Unpublished proprietary
data supplied by Roussel Uclaf).
ARDLEY J.H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat (Unpublished report of Wellcome
Australia Ltd).
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and
high-performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Battelle Institute.
BECCI, P.J., KNICKERBOCKER, M., & PARENT, R.A. (1979) Teratological
evaluation of SBP-1382 in albino rabbits, Waverly, New York,
Food and Drug Research Laboratories, 17 pp (Report No. 6288)
(Unpublished proprietary data supplied by Roussel Uclaf).
BENGSTON, M., COOPER, L.M., & GRANT-TAYLOR, F.J. (1975) A comparison
of bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as
grain protectants against malathion-resistant insects in wheat.
Queensland J. Agric. anim. Sci., 32: 51-78.
BUICK, A.R. & FLANAGAN, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes (Report No. HEGH 73/1) (Unpublished
data of Wellcome Foundation Ltd).
BURT, M.E. (1983a) Resmethrin residues in tomato and cucumber, New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
BURT, M.E. (1983b) Resmethrin residues in lettuce (greenhouse), New
Brunswick, Rutgers State University of New Jersey, Agricultural
Experiment Station, 18 pp (Unpublished proprietary data supplied
by Roussel Uclaf).
CARLTON, M. (1977) Some effects of cismethrin on the rabbit nervous
system. Pestic. Sci., 8: 700-712.
CAVE, S.L. (1981) Simultaneous estimation of bioresmethrin and
piperonylbutoxide by gas-liquid chromatography with
chemical-ionization mass spectrometry. Pestic. Sci.,
12: 156-160.
CHAPMAN, R.A. & HARRIS, C.R. (1978) Extraction and liquid-solid
chromatography cleanup procedures for the direct analysis of four
pyrethroid insecticides in crops by gas-liquid chromatography.
J. Chromatogr., 166: 513-518.
CHESHER, B.C. & MALONE, J.C. (1970a) Acute toxicity tests with NRDC
107 in hens (Report No. B 236-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970b) Sensitisation study with NRDC
107 on guinea pigs (Report B 198-70) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1970c) Ocular irritancy of NRDC 107 in
rabbits (Report No. B 217-70) (Unpublished data of Wellcome
Foundation Ltd).
CHESHER, B.C. & MALONE, J.C. (1971a) Toxicity to dogs of NRDC 107
(continuation) (Report No. B 27-71) (Unpublished data of
Wellcome Foundation Ltd).
CHESHER B.C. & MALONE J.C. (1971b) Acute toxicity of NRDC 107 by the
intravenous route. (Report No. B 329-71) (Unpublished data of
Wellcome Foundation Ltd).
CHRISTOPHER, R.J., MUNGER, C.E., & WAYNE IRVIE, G. (1985) Distribution
and depletion of [14C]-resmethrin isomers administered orally to
laying hens. Pestic. Sci., 16: 378-382.
COOMBS, D.W., HARDY, C.J., CLARK, G.C., STREET, A.E., GOPINATH, C.,
LEWIS, D.J., & RAO, R.S. (1985) Resmethrin 90-day inhalation
toxicity study in the rat, Huntingdon, United Kingdom,
Huntingdon Research Centre, 80 pp (Report No. SBP 6/84997)
(Unpublished proprietary data supplied by Roussel Uclaf).
COX, G.E., KNICKERBOCKER, M., & PARENT, R.A. (1979a) Evaluation of
dietary administration of SBP-1382 in CD-1 outbred albino mice
over an 85-week period, Waverly, New York, Food and Drug
Research Laboratories, 52 pp (Report No. 5270) (Unpublished
proprietary data supplied by Roussel Uclaf).
COX, G.E., BECCI, P.J., & PARENT, R.A. (1979b) Supplementary report
for evaluation of the neurotoxic effects of SBP-1382 in albino
rats - phase I, Waverly, New York, Food and Drug Research
Laboratories, 7 pp (Report No. 6362) (Unpublished proprietary data
supplied by Roussel Uclaf).
CROFTON, K.M. & REITER, L W. (1984) Effects of two pyrethroid
insecticides on motor activity and the acoustic startle response
in the rat. Toxicol. appl. Pharmacol., 75: 318-328.
DANLIKER, W.B., HICKS, A.N., LEVISON, S.A., STEWART, K., & BROWN, R.J.
(1979) Effects of pesticides on immume response, Washington, DC,
US Environmental Protection Agency (EPA-600/1-79-039-PB
80-1308 34).
DESMARCHELIER, J.M. (1980) Comparative study of analytical methods for
bioresmethrin, phenothrin, d-phenothrin, pyrethrum I, carbaryl,
fenitrothion, methacrifos, pirimifos-methyl and dichlorfos on
various grains. J. Pestic. Sci., 5: 521-532.
DEVAUX, Ph. & BOLLA, P. (1986) Bioresmethrin n-octanol/water
partition coefficient and water solubility, Romainville, France,
Roussel Uclaf, 4 pp (Report No. RU-BRM-142.06.86. STE/A)
(Unpublished proprietary data).
DEVAUX, Ph. & TILLIER, C. (1986) Vapor pressure of bioallethrin and
bioresmethrin, Romainville, France, Roussel Uclaf, 8 pp (Report
No. RU-BRM/BA-150.06.86.STE/A) (Unpublished proprietary data).
DOHERTY, J.D., LAUTER, C.J., & SALEM, N., Jr (1986) Synaptic effects
on the synthetic pyrethroid resmethrin in rat brain in vitro.
Comp. Biochem. Physiol., C84: 373-379.
DYBALL, R.E.J. (1982) Inhibition by decamethrin and resmethrin of
hormone release from the isolated rat neurohypophysis: A model
mammalian neurosecretory system. Pestic. Biochem. Physiol.,
17: 42-47.
ELLIOT, M. (1976) Properties and applications of pyrethroids.
Environ. Health Perspect., 14: 3-13.
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series, No. 42).
ELLIOTT, M., FARNMAN, A.W., JONES. N.F., NEEDHAM, P.H., & PEARSON,
B.C. (1967) 5-Benzyl-3-furylmethyl chrysanthemate: a new potent
insecticide. Nature (Lond.), 213: 493-494.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid. Pestic. Biochem. Physiol., 6: 547-550.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome,
11-15 October, 1982, Rome, Food and Agriculture Organization of
the United Nations.
FAO/WHO (1976) Bioresmethrin. In: 1975 Evaluation of some pesticide
residues in food, Geneva, World Health Organization, pp. 17-40
(WHO Pesticide Residues Series, No. 5).
FAO/WHO (1977) Bioresmethrin. In: 1976 Evaluation of some pesticide
residues in food, Rome, Food and Agriculture Organization of the
United Nations, pp. 55-78 (AGP: 1976/M/14).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous sensation
between synthetic pyrethroid insecticides. Contact Dermatitis,
13: 140-147.
GAINES, T.B. & LINDER, R.E. (1986) Acute toxicity of pesticides in
adult and weanling rats. Fundam. appl. Toxicol., 7: 299-308.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular
junction. Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol.,
15: 181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. appl. Pharmacol.,
66: 290-296.
GARRET, N.E., FRANK STACK, H., & WATERS, D. (1986) Evaluation of the
genetic activity profiles of 65 pesticides. Mutat. Res.,
168: 301-325.
GEPHART, L.A., JOHNSON, W.D., BECCI, P.J., & PARENT, R.A. (1980)
180-day subchronic oral dosing study with resmethrin in Beagle
dogs, Waverly, New York, Food and Drug Research Laboratories,
86 pp (Report No. 6289) (Unpublished proprietary data supplied by
Roussel Uclaf).
GLICKMAN, A.H. & CASIDA, J.E., (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4: 793-799.
GLOMOT, R. (undated) Etude de la toxicité chronique de 3 semaines
chez le rat du RU11484 (NRDC 107) (Unpublished data of Roussel
Uclaf submitted to WHO by Wellcome Foundation Ltd).
GLOMOT, R. & CHEVALLIER, B. (1969) Etude de la toxicité aiguë du
RU11484 (NRDC 107) Romainville, France, Roussel Uclaf (Rapport
Toxico 11386) (Unpublished data).
GRAY, A.J., CONNORS, T.A., HOELLINGER, H., & NGUYEN-HOANG-NAM (1980a)
Relationship between the pharmacokinetics of intravenous
cismethrin and bioresmethrin and their mammalian toxicity.
Pestic. Biochem. Physiol., 13: 281-293.
GRAY, A.J., CONNORS, T.A., & RICKARD, J. (1980b) Mechanism of
mammalian toxicity of the pyrethroids. In: Littaner, V.Z., Dudai,
Y., Silman, I. Teichberg, V.I., & Vogel, Z., ed.
Neurotransmitters and their receptors, New York, John Wiley &
Sons Ltd, pp. 565-568.
GREEN, W.M. (1977) Acute dermal LD50 toxicity study on technical
resmethrin, Moorestown, New Jersey, Bio-Toxicology Laboratory,
8 pp (Unpublished proprietary data supplied by Roussel Uclaf).
HESS, F.G., THOMPSON, S.W., & BECCI, P.J. (1982) A lifetime
evaluation of the dietary administration of SBP-1382 to Wistar
albino rats/amendment, Waverly, New York, Food and Drug Research
Laboratory, 28 pp (Supplemental report No. 5271) (Unpublished
proprietary data supplied by Roussel Uclaf).
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Bureau of Sport
Fishery and Wildlife, pp. 1-61 (Report No. 191).
HOELLINGER, H., SONNIER, M., GRAY, A.J., CONNORS, T.A., PICHON, J., &
NGUYEN-HOANG-NAM (1985) In vitro covalent binding of cismethrin,
bioresmethrin and their common alcohol to hepatic proteins.
Toxicol. appl. Pharmacol., 77: 11-18.
HOLMSTEAD, R.L., CASIDA, J.E., & RUZO, L.O. (1977) Photochemical
reactions of pyrethroid insecticides. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society
(ACS Symposium Series, No. 42).
JACKSON, G.C. & HARDY, C.J. (1984) SBP-1382 acute inhalation toxicity
in rats 4-hour exposure, Huntingdon, United Kingdom, Huntingdon
Research Centre, 24 pp (Report No. SBP 4a/831042) (Unpublished
proprietary data supplied by Roussel Uclaf).
KAGAN, U.S., PANSHINA, T.N. & SASINOVITCH, L.M. (1986) [Biochemical
effects of synthetic pyrethroid toxic action.] Gig. i Sanit.,
1: 7 (in Russian).
KAUFMAN, D.D. (1986) Degradation and movement of cis- and
trans-resmethrin in soil, Beltsville, US Department of
Agriculture, 36 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
KHOLMATOVA, M. (1984) Hygiene and toxicology of Dravin 755 and
Isotrine new pesticides used in agriculture, Alma Ata (Résumé of
doctorate thesis).
KNICKERBOCKER, M., BECCI, P.J., COX, G.E., & PARENT, R.A. (1980)
A lifetime evaluation of the dietary administration of SBP-1382
to Wistar albino rats, Waverly, New York, Food and Drug Research
Laboratories, 110 pp (Report No. 5271) (Unpublished proprietary
data supplied by Roussel Uclaf).
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem.
Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor and
Francis Ltd, 440 pp.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem.
Physiol., 20: 203-216.
MACHI, R.A., KAM, C., GALLO, M.A., STEVENS, K.R., & GAGLIARDI, J.J.
(1979) Teratological evaluation of SBP-1382 technical in the
albino rat, Florham Park, New Jersey, Booz, Allen & Hamilton,
Inc., 44 pp (Report No. 2054-066) (Unpublished proprietary data
supplied by Roussel Uclaf).
MACKO, J.A., HARVEY, J.G., POPE, C.R., & MCCREESH, A.H. (1979) Hazard
evaluation of aerosol formulations containing the synthetic
pyrethroid insecticide resmethrin, (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(SBP-1382), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency, 38 pp (Report No. 75-51-0848-79).
MALONE, J.C. & CHESHER, B.C. (1970) Toxicity to dogs of NRDC 107,
Berkhamsted, Wellcome Foundation Ltd (Report No. B 204-70)
(Unpublished data).
MARKING, L.L. & MAUCK, W.L. (1975) Toxicity of paired mixtures of
candidate forest insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 13: 518-523.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th ed.,
Croydon, British Crop Protection Council, 461 pp.
MAUCK, W.L., OLSON, L.E., & MARKING, L.L. (1976) Toxicity of natural
pyrethroids and five pyrethroids to fish. Arch. environ. Contam.
Toxicol., 4: 18-29.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemical handbook, Section C: Pesticide dictionary,
Willoughby, Meister Publishing Co., pp. C205-C206.
MILLER, T.A. & ADAMS, M.E. (1977) Central vs peripheral action of
pyrethroids on the housefly nervous system. In: Elliot, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical
Society, pp. 98-115 (ACS Symposium Series, No. 42).
MIYAMOTO, J. (1975-76) Terminal residues of bioresmethrin.
Submission to IUPAC Terminal Residue Commission, Madrid, September
1975, Leverkusen, September 1976.
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry metabolism and residue analysis of
synthetic pyrethroids. Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International
Congress of Pesticide Chemistry, Kyoto, Japan, 29 August-4
September, 1982, Oxford, Pergamon Press (in 4 volumes).
MIYAMOTO, J., NISHIDA, T., & UEDA, K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furylmethyl d1-trans-chrysanthemate in the
rat. Pestic. Biochem. Physiol., 1: 293-306.
MUMMA, R.O. (1985) Persistence of metabolism of 14C-resmethrin on
greenhouse grown tomato fruit, lettuce and field grown wheat,
Part III, Pennsylvania State University, 33 pp Unpublished
proprietary data supplied by Roussel Uclaf).
MURANO, A. (1972) Determination of the optical isomers of insecticidal
pyrethroids by gas-liquid chromatography. Agric. biol. Chem.,
36: 2203-2211.
NIOSH (1983) In: Takten, R.L. & Lewis, R.J., Jr, ed. Registry of
toxic effects of chemical substances, 1981-82 edition,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 3057 pp (in 3 volumes).
NISHIUCHI, Y. (1981) [Toxicity of pesticides to some aquatic
organisms. I. Toxicity of pesticides to some aquatic insects.]
Seitai-Kagaku, 4: 31-46 (in Japanese).
NISHIUCHI, Y. (1982) [Toxicity of pesticides to fish.]
Kongetsuno-Noyaku, 26: 105-110 (in Japanese).
NOEL, P.R.B., RIVETT, K.F., CHESTERMAN, H., STREET, A.E., & SPICER,
E.J.F. (1971) Oral toxicity study in Beagle dogs (repeated dosage
for 3 months), 76 pp (Report No. 3900/71/58) (Unpublished data
of Huntingdon Research Centre, United Kingdom).
NORWOOD, T.E. (1986) Preliminary statistical report on assessment of
the effects of SBP-1382 40MF (resmethrin) and Scourge (18%
resmethrin plus 54% piperonyl butoxide) on non target, aquatic
organisms when used for adult mosquito control: results of a
field validation study in fresh water ponds in Minnesota, Summer
1984, North Oaks, Minnesota, Mosquito Control Technology, 20 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
PIERCE, R.H. (1986) Acute toxicity of Scourge on select estuarine
organisms during field applications, Sarasota, Florida, Mote
Marine Laboratory, 16 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C., HAUTEFEUILLE,
A., & BARTSCH, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
PUCHALSKI, R. (1983) Water solubility and octanol/water partition
coefficients of SBP-1382, Lyndhurst, New Jersey, Penick
Corporation, 5 pp (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985a) Acute oral LD50 study of
D-L-cis-trans-chrysanthemic acid in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 10 pp
(Report No. 8350A) (Unpublished proprietary data supplied by
Roussel Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985b) Acute oral LD50 study of
D-L-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350B) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985c) Acute oral LD50 study of
D-trans-chrysanthemic acid in Sprague-Dawley rats, Waverly,
New York, Food and Drug Research Laboratories, 10 pp (Report
No. 8350C) (Unpublished proprietary data supplied by Roussel
Uclaf).
REAGEN, E.L. & BECCI, P.J. (1985d) Acute oral LD50 study of BFCA
99.6% Sprague-Dawley rats, Waverly, New York, Food and Drug
Research Laboratories, 10 pp (Report No. 8725A) (Unpublished
proprietary data supplied by Roussel Uclaf).
RIDLEN, R.L., CHRISTOPHER, R.J., WAYNE IRVIE, G., BEIER, R.C., & CAMP,
B.J. (1984) Distribution and metabolism of cis and
trans-resmethrin in lactating Jersey cows. J. agric. food
Chem., 32: 1211-1217.
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O.
(1985a) The effects of dietary inclusion of resmethrin on
reproduction in the bobwhite quail, Huntingdon, United Kingdom,
Huntingdon Research Centre, 34 pp (Report No. SBP 8BT/85957)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROBERTS, N.L., FAIRLEY, C., ANDERSON, A., DAWE, S., & CHANTER, D.O,
(1985b) The effects of dietary inclusion of resmethrin on
reproduction in the mallard duck, Huntingdon, United Kingdom,
Huntingdon Research Centre, 28 pp (Report No. SBP 7BT/85550)
(Unpublished proprietary data supplied by Roussel Uclaf).
ROPER, E.M. & WRIGHT, G.C. (1984) Sampling efficiency of five solid
sorbents for trapping airborne pesticides. Bull. environ. Contam.
Toxicol., 33: 476-483.
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four synthetic
pyrethroids fail to show any correlation between neuromuscular
dysfunction and neurobiochemical abnormalities in rats. Arch.
Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis.
Pestic. Biochem. Physiol., 25: 176-187.
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979a) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase I, Waverly,
New York, Food and Drug Research Laboratories, 18 pp (Report
No. 6067) (Unpublished proprietary data supplied by Roussel
Uclaf).
SCHWARTZ, C.S., BECCI, P.J., & PARENT, R.A. (1979b) Evaluation of the
neurotoxic effects of SBP-1382 in albino rats. Phase II,
Waverly, New York, Food and Drug Research Laboratories, 17 pp
(Report No. 6068) (Unpublished proprietary data supplied by
Roussel Uclaf).
SCHWARTZ, C.S., GEPHART, L., BECCI, P.J., & PARENT R.A. (1979c) The
evaluation of the effects of SBP-1382 following dietary
administration through three generations in Sprague-Dawley rats,
Waverly, New York, Food and Drug Research Laboratories, 70 pp
(Report No. 5739) (Unpublished proprietary data supplied by
Roussel Uclaf).
SEABER, J.A. (1985) Delayed contact hypersensitivity in the guinea
pig with SBP-1382 insecticide, Huntingdon, United Kingdom,
Huntingdon Research Centre, 14 pp (Report No. 84936D/SBP 10/ss)
(Unpublished proprietary data supplied by Roussel Uclaf).
SIMONAITIS, R.A. & CAIL, R.S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour, and wheat.
J. Assoc. Off. Anal. Chem., 58: 1032-1036.
SJOGREN, R.D. (1985) Assessment of the effects of SBP-1382 40MF
(resmethrin) and Scourge (18% resmethrin plus 54% piperonyl
butoxide) on non target, aquatic organisms when used for adult
mosquito control: results of a field validation study in fresh
water ponds in Minnesota, Summer 1984, North Oaks, Minnesota,
Mosquito Control Technology, 105 pp (Unpublished proprietary data
supplied by Roussel Uclaf).
SMITH, P.R. (1980) The effect of cismethrin on the rat dorsal root
potentials. Eur. J. Pharmacol., 66: 125-128.
STAUFFER (1984) Ethyl chrysanthemate/Product safety information,
Westport, USA, Stauffer Chemical Co., 5 pp.
SWENTZEL, K.L., ANGERHOFER, R.A., & HAIGHT, E.A. (1977) Toxicological
evaluation of pyrethroid insecticide (5-benzyl-1,3-furyl)
methyl-2,2-dimethyl-3-(2-methylpropenyl)cyclopropane-carboxylate
(resmethrin), Aberdeen Proving Ground, US Army Environmental
Hygiene Agency (Report No. 51-0830-77).
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1974) Photodecomposition of
resmethrin and related pyrethroids. J. agric. food Chem.,
22: 212-220.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975a) Metabolism of four
resmethrin isomers by liver microsomes. Pestic. Biochem.
Physiol., 5: 280-294.
UEDA, K., GAUGHAN, L.C., & CASIDA, J.E. (1975b) Metabolism of
(+)-trans- and (+)-cis-resmethrin in rats. J. agric. food
Chem., 23: 106-115.
UNO, M., OKADA, T., OHMAE, T., & TANIGAWA, K. (1982) [Determination of
pyrethroid insecticides by dual-wavelength densitometry.]
Shokuhin Eiseigaku Zasshi, 23: 191-195 (in Japanese).
US ENVIRONMENTAL PROTECTION AGENCY (1983) Tolerances for pesticides in
food administered by EPA: Resmethrin. Fed. Reg.,
48: 36246-36247.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Voltage clamp studies
on the effects of allethrin and DDT on the sodium channels in frog
myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M. (1973)
DDT-likeaction of allethrin in the sensory nervous system of
Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B A., & AKKERMANS, L.M.A. (1979) Effect
of insecticides on the sensory nervous system. In: Narahashi, T.,
ed. Neurotoxicology of insecticides and pheromones, New York,
London, Plenum Press, pp. 182-210.
VANNIER, B. & FOURNEX, R. (1986) Bioresmethrin. Detection of a
mutagenic potency (micronucleus test in the mouse), Romainville,
France, Roussel Uclaf, 16 pp (Report No. RU-BRM-85612-BB/178/A)
(Unpublished proprietary data).
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethroids in rats. Arch. Toxicol.,
45: 325-329.
VERSCHOYLE, R.D. & BARNES, J.M. (1972) Toxicity of natural and
synthetic pyrethrins in rats. Pestic. Biochem. Physiol.,
2(3): 308-311.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency dependent
effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of pyrethroid
insecticides on the vertebrate nervous system. Neuropathol. appl.
Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the
lateral-line sense organ and on peripheral nerves of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J. (1982b)
Similar mode of action of pyrethroids and DDT on sodium channel
gating in myelinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., & VAN DEN
BERCKEN, J. (1983) Temperature and structure-dependent interaction
of pyrethroids with the sodium channels in frog node of Ranvier.
Biochim. Biophys. Acta, 728: 73-82.
VOLKOV, U.P., STRELETS, I.P., & TIULENEV, K.L. (1979) [Progress in
the field of pyrethroids and their synergists. Collection of
articles on household chemistry.] Moscow, Volume 7, p. 125
(in Russian).
WALDRON, M.M. (1969) Foetal toxicity study -- 95 H 56 -- given orally
in the rabbit (Report Path 51) (Unpublished data of Wellcome
Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide (Report
No. B 47-71) (Unpublished data of Wellcome Foundation Ltd).
WALLWORK, L.M. & MALONE, J.C. (1972) Bioresmethrin. Preliminary 24
hour rat inhalation study (Report No. B 290-72) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CHESHER, B.C., & MALONE J.C. (1970) Toxicity of NRDC
107 to laboratory animals (Report No. B 199-70) (Unpublished
data of Wellcome Foundation Ltd).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1971) NRDC 107, rat
oral 91 day toxicity study (Report No. B 199-71) (Unpublished
data of Wellcome Foundation Ltd).
WATSON, S.C. (1984) Photolysis study of SBP-1382 resmethrin (final
report), Columbus, Ohio, Battelle Columbus Laboratory, 10 pp
(Unpublished proprietary data supplied by Roussel Uclaf).
WEEKS, M.H., LELAND, T.M., BOLDT, R.E., MELLICK, P.W., & STEINBERG, M.
(1972) Toxicological evaluation of pyrethroid insecticide
(5-benzyl-3-furyl) methyl-2,2-dimethyl-3-(2-methyl-propenyl)
cyclopropanecarboxylate (SBP-1382), Aberdeen Proving Ground, US
Army Environmental Hygiene Agency, 44 pp (Special study
No. 51-127-71/72).
WHITE, I.N.H., VERSCHOYLE, R.D., MORADIAN, M.H., & BARNES, J.M. (1976)
The relationship between brain levels of cismethrin and
bioresmethrin in female rats and neurotoxic effects. Pestic.
Biochem. Physiol., 6: 491-500.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23.
WHO (1986) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1986-1987, Geneva, World Health
Organization, p. 38 (VBC/86.1, Rev. 1).
WHO/FAO (1978) Data sheet on pesticides No. 34: Bioresmethrin,
Geneva, World Health Organization (WHO/VBC/DS/78. 34).
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, 485 pp.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
YOSHIOKA, H. (1980) [Chemistry and action of the recent synthetic
pyrethroids and their stereoisomers.] J. synth. org. Chem.
Japan, 38: 1151-1162 (in Japanese).
APPENDIX
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs (Xenopus laevis; Rana temporaria, and Rana
esculenta), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches by Gammon et al. (1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982; Gammon
& Casida, 1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the insecticide
on neurotransmitter release or on the sensitivity of the subsynaptic
membrane or the muscle fibre membrane. Presynaptic repetitive firing
was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres,
the pyrethroid-induced repetitive activity increases dramatically as
the temperature is lowered, and a decrease of 5°C in temperature may
cause a more than 3-fold increase in the number of repetitive impulses
per train. This effect is easily reversed by raising the temperature.
The origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that underlies
the generation and conduction of each nerve impulse. The transitional
state of the sodium channel is controlled by 2 separately acting
gating mechanisms, referred to as the activation gate and the
inactivation gate. Since pyrethroids only appear to affect the sodium
current during depolarization, the rapid opening of the activation
gate and the slow closing of the inactivation gate proceed normally.
However, once the sodium channel is open, the activation gate is
restrained in the open position by the pyrethroid molecule. While all
pyrethroids have essentially the same basic mechanism of action, the
rate of relaxation differs substantially for the various pyrethroids
(Flannigan & Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more
than 100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the Type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing afterpotentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral-line organ in the form of
long-lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses. The
duration of the trains and the number of impulses per train increase
markedly on lowering the temperature. Cypermethrin does not cause
repetitive activity in myelinated nerve fibres. Instead, this
pyrethroid causes a frequency-dependent depression of the nervous
impulse, brought about by a progressive depolarization of the nerve
membrane as a result of the summation of depolarizing after-potentials
during train stimulation (Vijverberg & Van den Bercken, 1979;
Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a
long-lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]-t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an absolute
correlation, without any false positive or negative, between mouse
intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, [1R,cis]-cypermethrin,
[1R,trans]-cypermethrin, and [2S, alphaS]-fenvalerate were
inhibitors, but their non-toxic stereoisomers were not; non-cyano
pyrethroids were much less potent or were inactive (Lawrence & Casida,
1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The
[3H]-dihydropicrotoxin and [35S]-TBPS binding studies with
pyrethroids strongly indicated that Type II effects of pyrethroids are
mediated, at least in part, through an interaction with a
GABA-regulated chloride ionophore-associated binding site. Moreover,
studies with [3H]-Ro 5-4864 support this hypothesis and, in addition,
indicate that the pyrethroid-binding site may be very closely related
to the convulsant benzodiazepine site of action (Lawrence et al.,
1985).
The Type II pyrethroids (deltamethrin, [1R,cis]-cypermethrin and
[2S, alpha2]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two
non-insecticidal stereoisomers and Type I pyrethroids (permethrin,
resmethrin, allethrin) were inactive. Therefore, cyanophenoxybenzyl
pyrethroids appear to act on the GABA receptor-ionophore complex
(Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog (Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action
potentials without presynaptic repetitive activity. However, an
intermediate group of pyrethroids (1R-permethrin, cyphenothrin, and
fenvalerate) caused both types of effect. Thus, in muscle or nerve
membrane the pyrethroid induced repetitive activities due to a
prolongation of the sodium current. But no clear distinction was
observed between non-cyano and alpha-cyano pyrethroids (Ruigt & Van
den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is
the sodium channel in the nerve membrane. Pyrethroids without an
alpha-cyano group (allethrin, d-phenothrin, permethrin, and
cismethrin) cause a moderate prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation. This
results in relatively short trains of repetitive nerve impulses in
sense organs, sensory (afferent) nerve fibres, and, in effect, nerve
terminals. On the other hand, the alpha-cyano pyrethroids cause a
long-lasting prolongation of the transient increase in sodium
permeability of the nerve membrane during excitation. This results in
long-lasting trains of repetitive impulses in sense organs and a
frequency-dependent depression of the nerve impulse in nerve fibres.
The difference in effects between permethrin and cypermethrin, which
have identical molecular structures except for the presence of an
alpha-cyano group on the phenoxybenzyl alcohol, indicates that it is
this alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids, is
not necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more
advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids,
the ultimate lesion that causes death will depend on the animal
species, environmental conditions, and on the chemical structure and
physical characteristics of the pyrethroid molecule (Vijverberg & Van
den Bercken, 1982).
