
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 20
SELECTED PETROLEUM PRODUCTS
This report contains the collective views of an
international group of experts and does not
necessarily represent the decisions or the stated
policy of the United Nations Environment Programme,
the International Labour Organisation, or the World
Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1982
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
ISBN 92 4 154080 X
(c) World Health Organization 1982
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED PETROLEUM PRODUCTS
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Properties and analytical methods
1.1.1.1 Properties
1.1.1.2 Analytical methods
1.1.2. Sources of environmental pollution
1.1.3. Environmental concentrations and levels of exposure
1.1.3.1 General population exposure
1.1.3.2 Occupational exposure
1.1.4. Effects on experimental animals
1.1.5. Clinical and epidemiological studies in man
1.1.6. Evaluation of health risks
1.1.7. Control measures
1.2. Recommendations for further studies
1.2.1. Analytical aspects
1.2.2. Sources and levels in the environment
1.2.3. Studies on experimental animals
1.2.4. Human studies
2. CRUDE OILS
2.1. Properties and analytical methods
2.1.1. Chemical composition and properties
2.1.2. Methods of sampling and analysis
2.1.2.1 Gases and vapours
2.1.2.2 Aerosols
2.2. Sources of environmental pollution
2.2.1. Natural occurrence
2.2.2. Man-made sources
2.2.2.1 Production
2.2.2.2 Uses
2.2.2.3 Disposal of waste
2.3. Toxicological effects of crude oils
2.3.1. Effects on experimental animals
2.3.2. Effects on man
3. PETROLEUM SOLVENTS
3.1. Properties and analytical methods
3.1.1. Chemical composition and properties
3.1.1.1 Special boiling point solvents (SBPs)
3.1.1.2 White spirits
3.1.1.3 High boiling aromatic solvents
3.1.2. Purity of petroleum solvents
3.1.3. Methods of sampling and analysis
3.2. Sources of environmental pollution
3.2.1. Natural occurrence
3.2.2. Man-made sources
3.2.2.1 Production
3.2.2.2 Uses
3.3. Environmental exposure levels
3.4. Environmental distribution and transformation
3.5. Metabolism
3.5.1. Absorption
3.5.2. Distribution in the body
3.5.3. Biotransformation
3.5.4. Elimination
3.6. Effects on experimental animals
3.6.1. Short-term exposure
3.6.2. Long-term exposure
3.6.3. Mutagenicity, teratogenicity, and carcinogenicity
3.6.3.1 Mutagenicity
3.6.3.2 Teratogenicity
3.6.3.3 Carcinogenicity
3.7. Effects on man
3.7.1. Controlled exposures
3.7.1.1 Effects of dermal exposure
3.7.1.2 Effects of inhalation
3.7.2. Epidemiological studies
3.7.2.1 Occupational exposure
3.7.2.2 General population exposure
3.7.3. Clinical studies
3.7.3.1 Effects of dermal exposure
3.7.3.2 Effects of inhalation
3.7.3.3 Effects of ingestion
4. LUBRICATING BASE OILS AND RELATED OILS, GREASES, AND WAXES
4.1. Properties and analytical methods
4.1.1. Chemical and physical properties
4.1.1.1 Purity of product
4.1.2. Methods of sampling and analysis
4.2. Sources of environmental pollution
4.2.1. Natural occurrence
4.2.2. Man-made sources
4.2.2.1 Production
4.2.2.2 Uses
4.2.2.3 Disposal of waste
4.3. Environmental exposure levels
4.4. Environmental distribution and transformation
4.5. Metabolism
4.6. Effects on experimental animals
4.6.1. Short-term exposure
4.6.1.1 Effects of dermal exposure
4.6.2. Long-term exposure
4.6.2.1 Carcinogenic effects
4.6.2.2 Effects of dermal exposure and
subcutaneous administration
4.6.2.3 Effects of inhalation and intratracheal
exposures
4.6.2.4 Dietary studies
4.7. Effects on man
4.7.1. Occupational exposure
4.7.1.1 Skin disorders
4.7.1.2 Skin carcinogenicity
4.7.1.3 Effects of off mist exposure
4.8. Clinical studies
5. BITUMEN
5.1. Properties and analytical methods
5.1.1. Chemical and physical properties
5.1.2. Methods of sampling and analysis
5.2. Sources of environmental pollution
5.2.1. Natural sources
5.2.2. Man-made sources
5.2.2.1 Production
5.2.2.2 Uses
5.3. Environmental exposure levels
5.4. Environmental distribution and transformation
5.5. Metabolism
5.6. Effects on experimental animals
5.6.1. Short-term exposure
5.6.2. Long-term exposure
5.7. Effects on man
5.7.1. Epidemiological studies
5.7.1.1 Occupational exposure
5.7.1.2 General population exposure
5.7.1.3 High (accidental) exposure
5.7.2. Clinical studies
6. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO CRUDE OILS AND
SELECTED PETROLEUM PRODUCTS
6.1. Crude oils
6.2. Petroleum solvents
6.3. Lubricating base oils, greases, and waxes
6.4. Bitumen
7. CONTROL MEASURES
7.1. General
7.2. Petroleum solvents
7.3. Lubricating base oils, greases, and waxes
7.4. Bitumen
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
theft publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED PETROLEUM
PRODUCTS
Members
Dr D. A. Akintonwa, Department of Biochemistry, Faculty of Medicine,
University of Calabar, Calabar, Nigeria
Dr L. Boniforti, Department of Contaminants, Laboratory of Toxicology,
Institute of Health, Rome, Italy
Dr K. W. Jager, Shell Internationale Research, Maatschappij B.V., The
Hague, Netherlands (Rapporteur)
Professor L. Jirásek, 1st Dermatological Clinic, Charles University,
Prague, Czechoslovakia
Professor A. A. Kasparov, Institute of Industrial Hygiene and
Occupational Diseases, Academy of Medical Sciences, Moscow, USSR
(Vice-Chairman)
Professor W. O. Phoon, Department of Social Medicine and Public
Health, Faculty of Medicine, University of Singapore, Singapore
(Chairman)
Dr M. Rouhani, Institute of Occupational Safety and Health, Ministry
of Labour and Social Affairs, Teheran, Iran (Present address:
Nice, France)
Dr E. Schmidt, Directorate of Malariology and Environmental
Sanitation, Ministry of Health and Welfare, Caracas, Venezuela
Dr N. K. Weaver, American Petroleum Institute, Washington DC, USA
Representatives of other organizations
Dr P. V. C. Pinnagoda, International Labour Organisation, Geneva,
Switzerland
Professor L. Parmeggiani, Permanent Commission and International
Association on Occupational Health
Dr J. W. Huismans, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Mr J. Wilbourn, International Agency for Research on Cancer, Lyons,
France
Secretariat
Dr A. David, Medical Officer, Office of Occupational Health, World
Health Organization, Geneva, Switzerland (Co-Secretary)
Dr M. A. El Batawi, Chief Medical Officer, Office of Occupational
Health, World Health Organization, Geneva, Switzerland
Mrs B. Goelzer, Scientist, Office of Occupational Health, World Health
Organization, Geneva, Switzerland (Co-Secretary)
Dr Y. Hasegawa, Medical Officer, Environmental Health Criteria and
Standards, World Health Organization, Geneva, Switzerland
Dr M. Sharratt, Senior Toxicologist, BP Group Occupational Health
Centre, Middlesex, England (Temporary Adviser)
ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED PETROLEUM PRODUCTS
Further to the recommendations of the Stockholm United Nations
Conference on the Human Environment in 1972, and in response to a
number of World Health Assembly Resolutions and the recommendation of
the Governing Council of the United Nations Environment Programme, a
programme on the integrated assessment of the health effects of
environmental pollution was initiated in 1973. The programme, known as
the WHO Environmental Health Criteria Programme, has been implemented
with the support of the Environment Fund of the United Nations
Environment Programme. In 1980, the Environmental Health Criteria
Programme was incorporated into the International Programme on
Chemical Safety. The result of the Environmental Health Criteria
Programme is a series of criteria documents.
The Office of Occupational Health, WHO, was the unit responsible
for the development of the Environmental Health Criteria document on
Selected Petroleum Products.
The Task Group for this document met in Geneva from 15-19 October
1979. The meeting was opened by Dr M. A. El Batawi, Chief, Office of
Occupational Health, who welcomed the participants and the
representatives of other international organizations on behalf of the
Director-General.
The Task Group reviewed and revised the second draft criteria
document and made an evaluation of the health risks of exposure to
selected petroleum products.
The first and second drafts were prepared by Dr K. W. Jager,
Shell Internationale Research, Maatschappij B. V., The Hague,
Netherlands. Comments on the second draft, which have been
incorporated in this report, were received from the national focal
points for the WHO Environmental Health Criteria Programme in
Australia, the Federal Republic of Germany, Mexico, the United
Kingdom, and the USA, and from the WHO Collaborating Centres of
Occupational Health in: Chile, Finland, Indonesia, Netherlands,
Singapore, Sweden, Switzerland, the United Kingdom, and the USSR.
Additional comments were received from Dr. R. E. Eckardt (USA), Dr M.
Rouhani (Iran), from the International Petroleum Industry
Environmental Conservation Association, and from the American
Petroleum Institute.
The collaboration of these national institutions, international
organizations, and individual experts is gratefully acknowledged. The
Secretariat also wishes to thank Dr K. W. Jager and Dr M. Sharratt for
their invaluable assistance in the final stages of the preparation of
the document.
As the final text of the evaluation could not be distributed at
the meeting, it was circulated to all participants in November 1978.
The comments received were then considered by the Rapporteur and some
members of the Secretariat, and suggested alterations were included.
Later, section 2.1.2, Methods of sampling and analysis, was completely
rewritten by Mr. T. P. C. M. van Dongen of the Shell Laboratory
(Amsterdam) and Dr K. W. Jager, the Rapporteur.
The document has been based, primarily, on original publications
listed in the reference section. However, several recent reviews of
health aspects of petroleum products have also been used, including:
Petroleum Handbook (1966); API Toxicology Reviews (API, 1965, 1967,
1969); US DHEW (1970); and Lazarev & Levina (1976).
The purpose of this document is to review and evaluate available
information on the biological effects of some petroleum products, and
to provide a scientific basis for decisions aimed at the protection of
human health from the adverse consequences of exposure to these
substances in both the occupational and general environments.
It was only feasible to discuss several groups of related
products, and to select priorities among them. Thus, non-fuel products
derived from crude oils are considered in three broad groups, i.e.,
petroleum solvents, lubricating base-oils, and bitumens. These have
been selected as priorities, because of their widespread use and
because large sub-groups of the population may come into close contact
with them through occupational or domestic use. Moreover, adverse
health effects are known to occur from occupational exposure to some
of these products.
Base chemicals derived from the cracking of crude oil fractions,
such as ethylene, propylene, and other olefins, and fuels derived from
crude oils ranging from gasoline to heavy fuel oil, are not discussed
in this document. As fuels and non-fuels of a similar boiling range
may have similar effects, e.g., on the skin or, after aspiration, on
the respiratory tract, most toxicological data discussed in this
review are more or less relevant to crude oil-derived fuels of a
similar boiling range. In fact, it is impossible to make a strict
division between data relating to fuels and non-fuels and they have
been considered together, whenever relevant.
The published literature and other available information have
been critically evaluated and where possible, an attempt has been made
to establish whether or not, under certain conditions, a potential
risk to man exists. Suggestions for avoiding established risks and for
further studies have also been made.
The environmental impact, if any, of the products has only been
considered where it is directly related to the health of man.
Details of the WHO Environmental Health Criteria Programme
including some terms frequently used in the document may be found in
the general introduction to the Environmental Health Criteria
Programme published together with the environmental health criteria
document on mercury (Environmental Health Criteria 1, Mercury, Geneva,
World Health Organization, 1976), now also available as a reprint.
Financial support for the publication of this criteria document
was kindly provided by the United States Department of Health and
Human Services through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA -- a WHO Collaborating Centre for Environmental Health Effects.
The following conversion factors have been used in the present
document:
benzene 1 ppm = 3.0 mg/m3
gasoline 1 ppm = 4.5 mg/m3a
heptane 1 ppm = 4.0 mg/m3
hexane 1 ppm = 3.6 mg/m3
octane 1 ppm = 4.85 mg/m3
pentane 1 ppm = 3.0 mg/m3
toluene 1 ppm = 3.75 mg/m3
xylene 1 ppm = 4.35 mg/m3
a A conversion factor for gasoline of 1 ppm = 4.5 mg/m3 has been
used throughout the document, though this factor normally varies
according to the composition of the gasoline.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
1.1.1 Properties and analytical methods
1.1.1.1 Properties
(a) Crude oils are a complex mixture of straight and branched
chain paraffinic, cycloparaffinic, aromatic and polynuclear aromatic
hydrocarbons together with small amounts of sulfur and nitrogen
compounds. The composition of crude oils varies considerably with
geographical origin. They can be broadly divided into paraffinic,
asphaltic, and mixed crude oils. Paraffinic crude oils provide large
amounts of paraffinic hydrocarbons, paraffin wax, and high grade oils,
while asphaltic crude oils province more cycloparaffins and high
viscosity lubricating oils.
(b) Petroleum solvents, produced by the distillation of crude
oils, are also complex mixtures of hydrocarbons. They are generally
classified on the basis of distillation ranges. Special boiling-point
solvents, such as petroleum ether and rubber solvent, are mixtures of
C-5 to C-9 normal- and branched-chain paraffins and cycloparaffins
with a boiling-range of 30-160°C. With solvents such as Stoddard
solvent, mineral spirits, and low aromatic white spirits, the chain
lengths are longer (C-7 to C-12) and the boiling-range higher
(150-220°C) and they contain various amounts of aromatic compounds.
Higher boiling-point solvents (B.P. 160-300°C) containing more than 9
carbon atoms per molecule are also produced.
(c) Lubricant base oils, greases, and waxes are products with
boiling-points in the range of 300-700°C that are normally produced by
high-vacuum distillation of the residues of the initial distillation.
(d) Bitumen, the solid and semi-solid residue of the
distillation process, varies from a highly viscous liquid to a brittle
solid, at ambient temperatures, and consists of a mixture of
asphaltenes (high relative molecular mass aromatic and heterocyclic
hydrocarbons), resins (polymers formed from unsaturated hydrocarbons
during processing), together with saturated hydrocarbons and aromatic
hydrocarbons containing one or more benzene rings per molecule
(including polynuclear aromatic hydrocarbons).
1.1.1.2 Analytical methods
A vast and specialized literature on sampling methods and
analytical techniques is available for petroleum products. Many
techniques have proved useful, e.g., infrared spectroscopy, thin-layer
chromatography, ultraviolet fluorescent spectrometry, capillary
gaschromatography, and chromatography combined with mass spectrometry.
1.1.2 Sources of environmental pollution
(a) Crude oil is normally transported in large volumes in
tankers and pipelines. Breakdown or leakage of these may cause a major
and sudden environmental hazard. Less significant degrees of pollution
have resulted from the cleaning out of oil tankers. Certain volatile
components, especially hydrogen sulfide but also other sulfur
compounds, acids, and hydrocarbons may contaminate the atmosphere near
oilfields and refineries.
(b) As a rule, petroleum solvents do not present serious
pollution problems for the general population, since they are mainly
used in industry and seldom domestically. Spillage or use in poorly
ventilated rooms or without proper control measures may cause serious
work-place pollution. Solvents containing n-hexane or benzene may
present particular hazards with respect to health.
(c) Because of theft nature and uses, lubricating base oils,
greases, and waxes rarely present problems for the general population
though spillage may create localized environmental problems. However,
in industry, some of these products, especially the metal working
oils, may produce marked contamination of the workplace and equipment.
(d) From the available evidence, it appears that bitumen is not
a significant source of environmental pollution but, under certain
conditions, occupational exposure may occur.
1.1.3 Environmental concentrations and levels of exposure
1.1.3.1 General population exposure
Little information is available concerning the concentrations of
petroleum products in air, water, or food. Most of the crude oils are
produced from deep wells, but natural seepage occurs on land and on
the sea-bed. Natural bitumen and asphalt deposits occur in several
parts of the world. There are not sufficient data available to
estimate the total environmental exposure of human beings to these
petroleum products. On occasions, the general population may be
exposed for short periods to fumes from heated bitumen used in road
building or roofing. Small amounts of hydrocarbons, probably derived
from petroleum hydrocarbons, have been found in shell fish. Volatile
petroleum components may contribute to atmospheric pollution near
refineries, and storage and pumping areas.
1.1.3.2 Occupational exposure
(a) Crude oil is usually handled in closed systems from oil
well to refinery, so that workers are not exposed to it, unless a
serious breakdown or leakage occurs. However, volatile components can
escape at well heads, pump glands, or through vents in storage tanks
and tanks on ships.
(b) Petroleum solvents are extensively manufactured and are
widely used in many occupations. Because of their volatility,
industrial exposure to "special-boiling-point" spirits can sometimes
be high. Excessive exposure has occurred and has caused ill health in
workshops where ventilation was insufficient. With white spirits, skin
contact is usually of greater importance than inhalation, at least at
ambient temperatures. Skin contact is particularly important in
relation to high aromatic solvents, since the aromatic moieties tend
to penetrate skin readily. Both skin contact and exposure to fumes or
mists of high boiling-point aromatic solvents can occur
occupationally.
(c) The extent of occupational exposure to lubricating oils,
greases, and waxes depends on the occupation and on the precautions
adopted. Some lubricants and transformer oils are handled only
occasionally, while work with automatic lathes of old design can
result not only in direct contamination of clothes and exposed skin,
but also in the inhalation of oil mist that may be produced by the
machine and will further contaminate the skin and clothing. Moreover,
other equipment, floors, and even roofs may become contaminated.
(d) Extensive exposure to bitumen may occur in such occupations
as roadbuilding and repairing, roofing, and flooring.
1.1.4 Effects on experimental animals
(a) Crude oil
Toxicological studies on mice and rabbits have shown that, in
general, the tumorogenicity of crude oils is lower than that of some
distilled fractions.
(b) Petroleum solvents
The few data available suggest that solvents are readily absorbed
when inhaled or ingested and that excretion is also rapid. The
metabolic products of benzene and n-hexane are well established but
the metabolism of other petroleum solvents is not well documented.
Animal studies have been complicated by the fact that mixtures
have generally been studied and that the composition of superficially
similar products can vary greatly. However, studies on representative
samples have demonstrated that solvents present a low oral and
percutaneous hazard for rats. Skin is severely damaged only on
prolonged, repeated contact; "short-chain" solvents mainly have a
defatting action, while dermatotoxic effects are found with
"longer-chain" solvents. In general, the higher the aromatic content
of the solvents, the more intense the effects, whatever the route of
exposure. In short-term exposure (4-8 h) of rats, atmospheric
concentrations causing the death of 50% of animals (LC50) ranged
mainly from approximately 1000-15 000 ppm. The main signs of poisoning
were respiratory tract irritation, depression of the central nervous
system (CNS), and coma, followed rapidly by death.
The presence of small volumes of solvent in the respiratory tract
led to chemical pneumonitis in all species tested. The degree of
injury depended on the viscosity rather than on the chemical nature of
the materials; the higher the viscosity, the lower the possibility of
aspiration into the deeper parts of the lungs.
Repeated exposure of rats, cats, and dogs to the vapours of a
wide range of petroleum solvents showed that the toxicity was
consistently low. However, exposure to n-hexane resulted in
pathological changes similar to those associated with peripheral
neuropathy in man. The maximum no-observed-adverse-effect level for
n-hexane is not yet certain. Results of teratogenicity studies on a
wide range of hydrocarbon solvents have been essentially negative.
Benzene and the aromatic extracts are the only well-defined
petroleum solvents for which carcinogenicity has been reported.
(c) Lubricating base oils, greases, and waxes
These substances are of low acute oral and dermal toxicity,
though high oral doses have a laxative effect.
In long-term studies on mice, rats, guineapigs, and rabbits, it
has been demonstrated that the carcinogenic activity of these products
resides in the polynuclear aromatic hydrocarbon fraction. By suitable
refining, oils, greases, and waxes can be obtained that consistently
give negative results in skin-painting tests. The most potentially
carcinogenic substances have been found among the 4,5, and 6 condensed
ring polynuclear compounds with relative molecular masses ranging from
230 to 330. Experimental evidence suggests that some long-chain
aliphatic, alicyclic, and alkylaromatic hydrocarbons may act as
co-carcinogens, when applied to the skin together with the
carcinogenic fraction.
It has been shown that washing the skin of animals after
application of carcinogenic oils decreases both the number and rate of
appearance of tumours. The degree of reduction is related to the time
between application and washing. A lowering of the frequency of
application of the oils also reduces the rate of tumour development.
Carcinogenic activity has been demonstrated in certain
metal-working and textile oil formulations and there is evidence that
carcinogenic polynuclear aromatic compounds may be produced, when oil
products are subjected to high temperatures.
Aspiration of oils has been shown to induce a foreign body
reaction in animal lungs as well as lipid pneumonia. However, when
animals were exposed to oil mist, very little was retained in the
lungs, and lipid pneumonia did not occur, even at high exposure
levels. From studies on the mouse, rat, hamster, rabbit, and dog, it
would appear that atmospheric exposure to 5 mg/m3 of oil mist is
without risk.
Oral administration of food-grade mineral oils and waxes to rats
did not result in any carcinogenic or chronic toxic effects.
(d) Bitumens
Although some bitumens applied to the skin of mice exhibit
carcinogenic activity, it is low compared with that of coal tar, and
it is generally accepted that the toxicity of bitumens is low.
1.1.5 Clinical and epidemiological studies in man
(a) Crude oils
Many cases of keratotic changes and epithelioma on exposed parts
of the skin have been reported in workers exposed to crude oils. The
relative roles of the oil and of other factors, e.g., sunlight, is
uncertain.
(b) Petroleum solvents
Petroleum solvents with boiling-ranges up to 230°C are primary
irritants, though their irritant and defatting actions decrease as the
boiling-range increases. Solvents of naphthenic origin or with a high
aromatic content tend to be the most irritant. On repeated contact,
the keratin layer of the skin is damaged, making the skin more
susceptible to other irritants, sensitizing agents, and bacteria.
Acute occupational poisoning by gasoline vapour has usually been
the result of entering unpurged gasoline tanks or other premises,
where high concentrations of gasoline vapour have accumulated. With
increasing concentrations of gasoline vapour, exposed subjects may
experience drowsiness, dullness, numbness, and headache followed by
dizziness, ataxia, and nausea. Exposure to higher concentrations of
vapour, or for a longer period, may lead to loss of consciousness
followed by death, which may be preceded by convulsions.
In the last 15 years, an increasing number of cases of
polyneuropathy have been reported in workers exposed to high
concentrations of volatile petroleum solvents, mainly consisting of
technical hexane. Though n-hexane seems to play a major role, the
possibility that other components of the solvents may have a similar
or synergistic action cannot be ruled out.
Ingestion of large volumes of solvent is usually well tolerated,
unless aspiration occurs. Small volumes (1-2 ml) of kerosene will, if
aspirated, cause acute chemical pneumonitis, which is often fatal. The
prognosis of chemical aspiration pneumonitis has improved over the
past years with improved methods of treatment. Where no aspiration
occurs, the symptoms are similar to those following over-exposure to
vapour.
Long-term exposure to low vapour concentrations has been reported
to produce non-specific symptoms such as nervousness, loss of
appetite, and nausea. Other symptoms referable to the peripheral and
central nervous systems, the gastrointestinal tract, the lungs, eyes,
and reproductive system have also been described. No
dose-concentration effect relationships can be derived from present
knowledge either for short-term or long-term exposures. It is
considered probable that blood abnormalities, previously reported
following exposure to solvents, were, in fact, due to the presence of
benzene in the solvents.
(c) Lubricating oils, greases, and waxes
Exposure of the skin to these products can induce several types
of disorder including primary irritation, oil ache, hyperkeratosis,
and photosensitivity. The degree of severity of these disorders
depends on the nature of the oil, the integrity of skin, the frequency
and length of contact, and individual susceptibility. In general,
lower-boiling-point materials have a more pronounced defatting effect,
while the higher-boiling-point materials induce the formation of acne.
In many cases, additives or contaminants in the oils are responsible
for the disorders, rather than the oil itself.
Prolonged exposure to non-solvent, refined mineral oils has been
associated with the induction of cancer of the scrotum, e.g., in
machine operators and those involved in spinning operations. Less
frequently, cancer at other sites, including the hand and forearm,
lung, and bronchus have been associated with exposure to oils
containing significant concentrations of polynuclear aromatic
compounds. Results of epidemiological studies have suggested an
association between exposure to oil mist and an increased incidence of
pulmonary cancer. However, the exact levels of exposure to the oils
and polynuclear aromatic compounds in these studies is not known. Very
rarely, cases have been reported of lipid pneumonia associated with
prolonged exposure to high concentrations of oil mist. Whether there
was a causal relationship is uncertain.
(d) Bitumen
Evidence from epidemiological studies on workers in oil
refineries, highway construction, roofing industries, and bitumen
transport firms strongly suggests that petroleum-based bitumens do not
present a significant health hazard.
The possibility that bitumen and the vapours emanating from it
might contribute to the overall incidence of cancer of the skin and of
the respiratory tract has to be considered in view of their content of
polynuclear aromatic compounds, but there are no data to substantiate
this.
1.1.6 Evaluation of health risks
Available information indicates that the health risks for the
general population from the production of crude oil and the
manufacture and use of petroleum products are very low. Under normal
circumstances, there is, at the most, a nuisance because of pollution
of the air and/or water.
The major risks are related to the health of workers involved in
the manufacture or handling of these products.
Exposure to high concentrations of the vapour of petroleum
solvents can produce narcotic effects. Long-term exposures to low
concentrations have been reported to produce non-specific symptoms.
The no-observed-adverse-effect level of exposure has not been
established for these products. Prolonged exposure to n-hexane has
resulted in the development of polyneuropathies most of which have
proved reversible on cessation of exposure. In the case of solvents
containing benzene, the possibility of bone marrow depression and
leukaemogenesis must be borne in mind. Prolonged skin contact with
petroleum solvents can lead to contact irritative dermatitis, but only
rarely to contact allergic dermatitis.
Both types of skin disease occur more frequently in professions
using products derived from base oils, especially metal-working oils.
Such diseases may cause considerable distress, they affect the general
well-being and reduce the capacity to work. Skin cancer has been
described in workers after prolonged and intensive exposure to less
refined base oil derivatives, e.g., the metal-working oils formerly in
use. Practically all these skin diseases appeared in occupations where
hygiene and working conditions were poor. These factors were as
important as the intrinsic toxicity of the oils.
Exposure to low concentrations of mists of highly refined oils
appears to be without serious health hazards; this is not necessarily
the case with less refined oils, which have been reported to cause an
increased incidence of cancer of the respiratory tract, after
prolonged high-level exposure.
There is no evidence to suggest that the production and use of
bitumens presents a health hazard for the general population and for
workers (other than burns from splashes of hot bitumen).
1.1.7 Control measures
Every effort should be made to avoid the contamination of
workers, the workplace, or the general environment with petroleum
products. This can be achieved by appropriate technological measures
and good work practice.
As far as possible, products containing highly toxic compounds
should be avoided and alternatives sought.
Where contact is unavoidable, suitable protective equipment
should be used. Health education of employers and workers should be
promoted emphasizing the necessity for maintaining high standards of
personal hygiene. When necessary, pre-employment, and regular periodic
medical examinations should be carried out on exposed workers.
Adequate control programmes should be implemented, including the
disposal of many types of waste oil products.
1.2 Recommendations for further studies
1.2.1 Analytical aspects
A major problem in assessing the health hazards of petroleum
products is that the majority have been developed and specified
according to their physical properties such as the boiling-point and
viscosity rather than their chemical composition. Products with the
same physical properties may vary considerably in chemical composition
(e.g., different proportions of isomers) and, hence, biological
properties. It is, therefore, important for future experimental animal
and human studies that analytical methods should be available to
establish the chemical structure of the products to which subjects are
exposed, and research into suitable methods should continue.
Analytical methods suitable for determining low concentrations of
solvents and oil products and their individual components in the
environment should continue to be developed and some consideration
should be given to the development of simple control techniques at the
work-site level.
1.2.2 Sources and levels in the environment
In some cases, the use of aromatic extracts and highly aromatic
base oils should be reconsidered and alternatives sought, where there
might be a risk of carcinogenic effects on the skin and respiratory
tract.
More information is needed on the concentrations of petroleum
products and their constituents in the work-place and the general
environment, especially in the neighbourhood of refineries and
petrochemical plants. Such data would result in more meaningful
epidemiological studies and would be of use in the development of
suitable measures to control pollution and the exposure of the general
population.
There is a need to understand more fully the factors responsible
for the production of oil mists and the importance to health of
inhalation of particles of various sizes. Most oil mists contain
chemical additives and the possible effects of these, when inhaled by
man, must be considered.
Improved methods for quantifying human exposure to petroleum
products in the working environment are required. While inhalation
exposure can be estimated from atmospheric monitoring, the extent of
exposure through skin contact has rarely, if ever, been examined.
International cooperation is needed in the elaboration and
clarification of exposure limits for petroleum products and their
components in water, air, and the working environment. These should be
based on adequate evaluation of their risks.
1.2.3 Studies on experimental animals
More studies are needed of the mechanisms by which petroleum
products produce injury in experimental animals. Little information is
available on the metabolism and pharmacokinetics of the components of
oils. In particular, elucidation of the dose/time/effect relationships
of exposure of animals to n-hexane would be of value in assessing
acceptable human exposure levels. Information on the neurotoxicity of
other components of petroleum solvents and on their ability to act
synergistically with n-hexane should also be sought. The possible
effects of petroleum solvents on aspects of the reproductive
processes, not already studied in depth, should be examined. A quick
and reliable analytical method for determining 4, 5, and 6 condensed
ring polynuclear aromatic compounds needs to be developed and its
predictive value in assessing carcinogenic potential examined.
Similarly, a short-term biological test for carcinogenicity,
applicable to oil products, would be of great value in providing a
method for the rapid assessment of the potential carcinogenicity of
oils.
1.2.4 Human studies
Further studies to determine the dose-effect relationships of
exposure to a wide range of petroleum oil and solvent products would
be of value, particularly in relation to long-term exposure. In such
studies, the possibility that any adverse effect produced by exposure
might be influenced by working conditions (e.g., general work
environment, heat, stress, and noise) should be considered and, if
necessary, investigated. As well as studying general health, possible
specific actions on the cardiovascular, gastrointestinal, and central
and peripheral nervous systems should be considered. Possible
susceptible groups, and factors such as age, sex, state of health, and
genetic background should also be taken into consideration. There is a
need to assess the extent of health problems caused by the use of
petroleum products in the developing countries, where exposure
conditions may be less well controlled; relatively few studies
relating to these problems have been carried out.
Efforts should be made to develop common criteria for the
detection and definition of health effects in order to allow
comparison of findings between different research workers and
institutes throughout the world.
2. CRUDE OILS
2.1 Properties and Analytical Methods
2.1.1 Chemical composition and properties
Crude oils originate from the decomposition and transformation of
aquatic, mainly marine, animals and plants that became buried under
successive layers of mud and silt some 15-500 million years ago; they
are essentially very complex mixtures of many thousands of different
hydrocarbons. Depending on the source, the oils contain various
proportions of straight and branched-chain paraffins, cycloparaffins,
and naphthenic, aromatic, and polynuclear aromatic hydrocarbons. The
younger oils are characterized by their more asphaltic nature. As many
"paraffins" of high relative molecular mass may contain naphthenic
and/or aromatic rings, this should not be understood as a sharp
division between defined chemical entities.
The hydrocarbons may be gaseous, liquid, or solid, under normal
conditions of temperature and pressure, depending on the number and
arrangement of carbon atoms in the molecules. As a general rule, at
ambient temperatures, compounds with molecules containing up to 4
carbon atoms are gaseous; those with 5-20 carbon atoms, liquid; and
those with more than 20 carbon atoms, solid. In crude oil, gaseous and
solid compounds occur dissolved in the liquid fraction. Solidification
of crude oils is caused by the presence of waxy normal paraffins of
high relative molecular mass. Unsaturated hydrocarbons such as olefins
and alkynes do not occur in crude oils.
Crude oils are similar to coal in that they are greatly enriched
in carbon and hydrogen compared with the average composition of the
earth's crust. Both are excellent sources of carbon for chemical
synthesis.
The sulfur content of crude oil ranges from less than 2 to
60 g/kg, depending on the origin of the oil. The sulfur is present not
only as sulfide but also as mercaptans, thiophenes, and more complex
organic sulfur compounds. The level of organic nitrogen compounds in
most crude oils is less than 1 g/kg, but some may occasionally contain
as much as 20 g/kg. Nitrogen compounds in crude oil are complex and
mostly unidentified structures, which, through thermal decomposition
during the distillation process of crude oil, are converted to simpler
structures. Crude oils may also contain some naphthenic acids and
phenolic compounds (Petroleum Handbook, 1966).
As crude oils are the decomposition products of former aquatic
animal and plant organisms, it is not surprising that they contain
most, if not all, of the known elements. These are mainly present in
few small quantities, i.e., only in mg/kg or small fractions of mg/kg.
However, nickel, molybdenum, and mercury levels are sometimes as high
as 10 mg/kg and vanadium levels, 50 mg/kg (Mason, 1966; Bertine &
Goldberg, 1971). More complete coverage of crude oil trace elements
can be found in BP (1975).
Crude oils vary widely in appearance and consistency from country
to country and from field to field. They range from yellowish brown,
mobile liquids to black, viscous semi-solids. The differences are due
to the different proportions of the various molecular types and sizes
of hydrocarbons. One crude oil may contain mostly paraffins, another
mostly naphthenes. Whether paraffinic or naphthenic, one may contain a
large quantity of lower hydrocarbons and be mobile or contain a lot of
dissolved gas; another may consist mainly of higher hydrocarbons and
be highly viscous, with little or no dissolved gas. The nature of the
crude oil governs, to a certain extent, the nature of the products
that can be manufactured from it and their suitability for special
applications. A naphthenic crude oil will be more suitable for the
production of asphaltic bitumen, a paraffinic crude oil for wax. A
naphthenic crude oil, and even more so an aromatic one, will yield
lubricating oils with viscosities that are sensitive to temperature.
However, with modern refining methods there is greater flexibility in
the use of crude oils to produce any desired type of product. Crude
oils are usually classified into three groups, according to the nature
of the hydrocarbons they contain:
(a) Paraffin base crude oils
These contain paraffin wax, but little or no asphaltic matter.
They consist mainly of paraffinic hydrocarbons and usually give good
yields of paraffin wax and high-grade lubricating oils.
(b) Asphaltic base crude oils
These contain little or no paraffin wax, but asphaltic matter is
usually present in large proportions. They consist mainly of
naphthenes and yield lubricating oils that are more viscosity
sensitive to temperature than those from paraffin base crude oils.
These crude oils are now often referred to as naphthene base crude
oils.
(c) Mixed base crude oils
These contain substantial amounts of both paraffin wax and
asphaltic matter. Both paraffins and naphthenes are present together
with a certain proportion of aromatic hydrocarbons.
This classification is a rough-and-ready division into types and
should not be used too strictly. Most crude oils exhibit considerable
overlapping of the types described and by far the majority are of the
mixed base type (Petroleum Handbook, 1966).
A useful compilation of the various characteristics and
approximate composition of most relevant crude oils is given in Anon
(1973).
2.1.2 Methods of sampling and analysis
As the methods of sampling and analysis are the same for crude
oils, petroleum solvents, and lubricant base oils, a general
discussion follows.
The petroleum products dealt with in this document are mostly
complex mixtures of closely related chemical compounds, identified as
a product on the basis of certain physical and chemical
characteristics related to their intended use. Because of the complex
nature of these products, only some of the relatively simple,
low-boiling components can be determined individually, and even these
cannot be selectively monitored in the working area without
appreciable expense. Thus monitoring for groups of compounds such as
"total hydrocarbons", etc. is often unavoidable. The objective of the
analysis will, in general, be to determine the concentration of any
particular suspected component class rather than to identify the
product. Moreover, because of differences in the volatility,
solubility, etc. of the components, the product will lose its
"identity" the moment is escapes from its original confinement and
enters the environment.
Potential health hazards associated with handling petroleum
products mainly arise from skin contact and inhalation. By proper
precautionary measures, the risk of skin contact can easily be
controlled. The occurrence of air contaminants, however, quite often
escapes human perception and this section will be devoted to ways of
assessing levels of contaminants in air.
Based on their different toxicological behaviour, 3 classes of
air contaminants can be distinguished, namely: gases and vapours
(from, e.g., solvents, petrol); mists (from, e.g., higher-boiling
refined oils); and fumes (from, e.g., high-boiling aromatic extracts,
bitumens).
Sampling and analysis for these 3 classes will be discussed
separately and particular attention will be given to single components
at present considered to be the most hazardous, such as benzene,
n-hexane, and polycyclic hydrocarbons.
Though, in the context of this Environmental Health Criteria
document, methods for the monitoring of both the air in the workplace
and the ambient air are relevant, only methods for work-place
monitoring will be briefly reviewed. The most sensitive methods for
monitoring work-place air could also be used for monitoring the
generally much lower levels in the ambient air.
The most frequent reason for sampling the air in the workplace is
to measure the concentration of hazardous agents to which the worker
may be exposed. The preferred way of assessing the exposure level is
to determine the time-weighted average (TWA) concentration for a
normal 8-h working day in the breathing zone of an individual worker.
For area monitoring, fixed station or portable monitors are used. Data
obtained in this way are independent of the presence and movement
pattern of the worker.
A detailed description of sampling strategy is given, for
instance, in NIOSH (1977a).
An alternative method for the determination of the amount
absorbed by a worker is biological monitoring, i.e., assessment of the
absorbed substance or its metabolites in biological material (urine,
blood, expired air). Such methods are available for many substances,
but unfortunately not for petroleum products, with the exception of
benzene and its homologues and, to a certain extent, n-hexane. The
principles of biological monitoring have been reviewed by many
authors, e.g., Piotrowski (1977).
The types of pollutants that occur in the work-place can be
divided into 2 broad categories, based on their physical state,
namely: gaseous pollutants and aerosols. Methods for sampling gaseous
pollutants are different from those for aerosols.
2.1.2.1 Gases and vapours
For personal monitoring, sampling and analysis are usually
performed in 2 separate steps. Samples are collected, mostly over a
prolonged period of time, from the breathing zone of the worker by
passing the contaminated air at a flow rate of 50-200 ml per min
(using a personal sampling pump carried by the worker) through a small
tube containing a suitable adsorbent (NIOSH, 1973; Clayton & Clayton,
1978; Voborsky, 1980). For hydrocarbon vapours, activated charcoal is
one of the best adsorbents.
Recently, passive dosimeters, based on diffusion of the substance
into an adsorbing layer, have been developed and marketed. Though
laboratory studies have shown these dosimeters to be as accurate as
adsorbent tubes using sampling pumps, more field data are needed to
prove their validity.
For grab samples, the contaminated air may also be collected in
Tedlar, Mylar, or Saran bags or in gas pipettes. Such samples must be
analysed as soon as possible, because of possible sample losses.
The techniques used for personal monitoring can also be used for
area monitoring. In many instances, however, the high specificity and
accuracy that can be obtained by the sophisticated methods used for
the analysis of personal monitoring samples is not required and
relatively simple, direct reading instruments can often be used when
searching for leakages, when monitoring areas with only a single
substance as a contaminant, or when monitoring areas where the total
hydrocarbon level is generally below the exposure limit for any of the
individual substances of concern.
The most simple direct reading instrument is the colorimetric
indicator tube, usually used with a hand pump, a wide variety of which
are available. However, while it is true that colorimetric indicator
tubes are of low initial cost and simple and convenient to use, there
are distinct limitations and potential errors inherent in this method.
A manual describing the applications and limitations of these devices
is available (AIHA, 1976). Other, commercially available, direct
reading instruments include portable infrared instruments, portable
gas chromatographs, and non-specific analysers, such as total
hydrocarbon analysers (ACGIH, 1978b).
An analytical procedure may, however, include several of the
following steps: sample recovery, concentration, pre-separation,
derivatization, and analysis.
The sample can be recovered from solid collection media by
solvent extraction or by thermal desorption. When a liquid absorbent
is used, a concentration step may be required.
Very many analytical techniques are available. However, as the
quantities of organic material to be determined are generally minute
and concealed in a matrix of many other substances, some analytical
techniques are especially suitable, such as gas chromatography (GC),
gas chromatography and mass spectrometry (GC/MS), and high-pressure
liquid chromatography (HPLC) with ultraviolet or fluorescence
detection.
Criteria for the choice of analytical technique include:
specificity required; quantities involved; ease of operation;
suitability for automation; and cost per analysis.
The principles of the analytical techniques mentioned are
described extensively in many monographs. For example a short
description of all relevant analytical techniques is given in NIOSH
(1973). Thus, only those for total hydrocarbons, n-hexane, and
benzene will be discussed here.
(a) Total hydrocarbons
Colorimetric indicator tubes are available from most
manufacturers for the determination of total hydrocarbons in the
work-place air. These tubes normally cover the range from about
100 ppm to several thousand ppm (corresponding to gasoline levels
ranging from 450 mg/m3 to several grams per m3 if a conversion
factor of 4.5 is applied). Many commercial instruments are also
available (ACGIH, 1978), the most reliable being those based on flame
ionization detection. These methods are generic in nature and the
instruments have to be calibrated, e.g., against methane or
n-octane. The read-out is not absolute, as the detector response
differs according to the composition of the hydrocarbons.
(b) n-Hexane
Depending on the situation, one of the 2 following approaches can
be applied in analysing specifically for n-hexane:
(i) n-Hexane as the main contaminant: direct area monitoring
can be performed using either a flame ionizing detector, without
previous separation (total hydrocarbon detector), or the total
hydrocarbon or low range n-hexane colorimetric indicator tubes
( n-hexane tubes are non-specific and react to all hydrocarbons; the
range is from about 20 mg/m3 upwards).
The NIOSH method S-90 (NIOSH, 1977-79), using the charcoal
tube/carbon disulfide desorption method with GC-analysis on packed
columns is suitable for personal monitoring.
(ii) n-Hexane present as one of the constituents of a
hydrocarbon mixture: In this case the matrix is very complicated. It
is more or less a prerequisite to use capillary GC to obtain a
satisfactory separation. Sample recovery is preferably carried out
with a 2-step thermal desorption, though solvent desorption using a
solvent with a longer retention time on the GC column (e.g., decane)
could be used.
Recently, it has been suggested that the urinary excretion of
hexane metabolites could be used for monitoring occupational exposure
to n-hexane and its isomers (Perbellini et al., 1981).
(c) Benzene
If benzene is the main pollutant, total hydrocarbon analysers,
or, even better, the benzene colorimetric indicator tubes can be used
(ranges available: from 0.15 to 150 mg/m3, sensitive to other
aromatic compounds, somewhat sensitive to hydrocarbons).
In all cases, the personal monitoring charcoal-tube/carbon-di-
sulfide-desorption/GC-analysis method can be used, i.e., NIOSH method
S-311 (NIOSH, 1977-79).
A detailed description of the determination of benzene in work
environments can be found in CONCAWE (1981a).
Biological monitoring for benzene exposure is carried out by
measuring the elimination of phenol (metabolite of benzene) in urine.
Several colorimetric methods (using 2,6-dibromo- N-chloro-
p-benzoquinoneimine-Gibbs reagent, 2,6-dibromoquinone-4-chlorimide,
diazo- p-nitroaniline or 4-dimethylamino-2,3-dimethyl-l-phenyl-
3-pyrazolin-5-one (4-aminopyrine)) or gas chromatographic methods are
available. A concentration of phenol in urine of more than 25 mg/litre
indicates some exposure to benzene (Truhaut & Murray, 1978).
2.1.2.2 Aerosols
The sampling of aerosols is performed by drawing a measured
volume of air through a filter, an impaction or impingement device, or
an electrostatic or thermal precipitator. The most common method,
especially for personal monitoring, consists of drawing air, at a
well-defined rate, through a filter. For personal monitoring, a
portable pump and a suitable filter in a filter-holder, located in the
worker's breathing zone, is used.
For area monitoring, some direct reading instruments for grab
sampling are also available based on, e.g., light-scattering,
attenuation of beta radiation, and changes in the resonant frequency
of a piezoelectric quartz crystal (ACGIH, 1978).
In some cases, size-selective sampling is necessary. This can be
accomplished by placing a cyclone or elutriator in front of the
sampler, or by the use of special-size selective sampling devices.
When, however, the aerosol also presents a hazard through absorption
via the gastrointestinal tract, total particulate matter should be
sampled.
In many instances, the total particulate concentration in air is
the only information needed, in which case, a gravimetric
determination of the material collected is all that is required.
On the other hand, if it is necessary to determine the
benzene-soluble matter present in the total particulate matter
collected, the collected matter must first be extracted with benzene.
The extract must then be evaporated to dryness and the residue weighed
(NIOSH, 1977-79). When more detail is required concerning the
composition of the aerosol collected, the benzene extract should be
analysed for the substances of concern.
Mists
Aerosols generated from refined oils and oils with a relatively
low aromatic content are often referred to as mists. The methods of
analysis most frequently used for mists consist of drawing air, at a
well-defined rate, through a preweighed and preconditioned glass-fibre
filter and recording the weight gain. If the weight gain indicates
that the total particulate concentration in the work atmosphere is
well below the appropriate exposure limits, no further analytical
action is required for the air sample. However, when concentrations in
excess of such levels are found, investigators invariably require
determination of the oil content of the filter.
For this purpose, the filter is extracted with a suitable solvent
and the oil content of the extract determined, either gravimetrically
(after evaporation of the solvent) or spectrophotometrically, using
ultraviolet or infrared adsorption or fluorescence spectrophotometry
(CONCAWE, 1981b; NIOSH, 1977-79).
The exposure limits for mists are mainly established as total
particulate oil mist and, for general investigations and control work,
it is recommended that sampling should be designed to take this into
account. Nevertheless, there may be some occasions when the
investigator feels it necessary to assess the concentration of
respirable particles in the mist, and special sampling techniques,
e.g., using a cyclone, will need to be employed. Experience, however,
does suggest that, in general, the equivalent diameter of particles '
in oil mists in engineering workshops is well below 5 µm and hence
they may be regarded as respirable. Thus, it is common industrial
practice to sample for total particulate matter.
For area monitoring, one of the direct reading devices mentioned
earlier could also be used.
Fumes
Aerosols generated from high-boiling aromatic extract oils and
bitumens are called fumes.
Where exposure to fumes from materials containing significant
concentrations of polycyclic aromatic hydrocarbons, such as aromatic
extract oils, is likely to occur, some guidance can be gained from the
AGGIH TLV-TWA of 0.2 mg/m3 for particulate polycyclic aromatic
hydrocarbons (as benzene-soluble material BSM). Coal tar pitch
volatiles include the fused polycyclic hydrocarbons that volatilize
from the distillation residues of coal, petroleum, wood, and other
organic matter. In the case of aromatic extract oils, the fact that a
major part of the BSM consists of non-polycyclic aromatic compounds
should be taken into account (NIOSH, 1977b).
With regard to the present standard for BSM, the analytical
method is as follows: total particulate matter suspended in air is
collected on a glass-fibre filter, with a silver membrane back-up
filter. The filter is extracted with benzene, using ultrasonic
agitation. An aliquot of the extract is evaporated to dryness and the
residue is weighed (NIOSH, 1977-79).
If it is felt necessary to characterize more fully the polycyclic
aromatic hydrocarbons (PAHs) present in the benzene extract of the
fume samples. further analysis of these extracts can be performed as
follows:
(a) GC method (Grimmer & Böhnke, 1972; Grimmer, 1979): The filter
extract is treated in several steps to isolate a fraction,
enriched in PAHS. This fraction is then analysed by capillary
GC/MS. For very complex products, the aerosol composition might
be too complicated to obtain a reasonable chromatogram, even
after all the pre-separation steps.
(b) HPLC separation with fluorescence detection (Das & Thomas, 1978;
Belinky, 1980).
The filter extract is evaporated and dissolved in 0.5-1 ml of
benzene. This extract is directly injected into the HPLC
instrument. As the fluorescence detector only records the highly
unsaturated molecules, the larger part of the matrix does not
give any signal at all on the detector. Only the
alkyl-substituted and unsubstituted polycyclic aromatic compounds
give rise to a detector signal.
Specificity for selected substances can be increased
substantially by a proper choice of excitation and emission
wavelengths. For the more volatile polycyclic aromatic
hydrocarbons, like pyrene, some losses may occur during sampling,
due to volatilization. If these more volatile polycyclic aromatic
hydrocarbons are also of interest, the filter collector could be
backed up by a silicagel tube. The silicagel from this tube is
then treated in the same way as the filter.
2.2 Sources of Environmental Pollution
2.2.1 Natural occurrence
Crude oils are exclusively natural products, most of which are
produced from artificial wells. Natural seepage of crude oils occurs
in various parts of the world, not only on land, but also on the
sea-bed; however, this represents only a minor source of environmental
pollution in comparison with man-made sources.
2.2.2 Man-made sources
2.2.2.1 Production
Taking world-wide figures, total crude oil production for 1973
was about 2900 million tonnes, i.e., approximately 10 times the crude
oil production in 1938. The rate of growth of production has declined
since 1973 (the 1979 level was just over 3200 million tonnes), and
very little overall increase, if any, is expected in the near future.
2.2.2.2 Uses
In some areas, e.g., Japan, certain unrefined crude oils are used
as fuels.
Negligible amounts of unrefined oils are used for such
applications as road construction and malaria control.
In some areas, where crude oils come to the surface in natural
seepage, they have been used by the local population, since
prehistoric times, for a number of purposes, but mainly for heating
and lighting.
Nearly. all the crude oil produced is processed in refineries
into various fuel and non-fuel fractions.
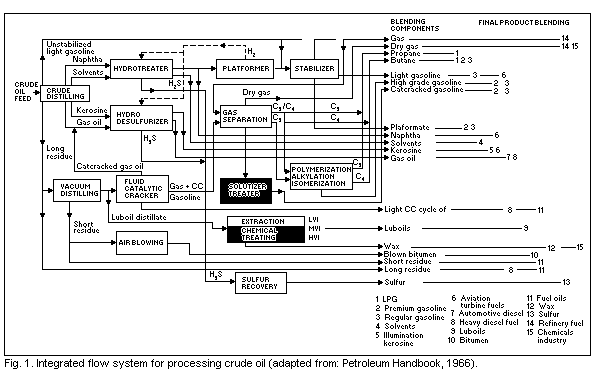
An example of an integrated flow scheme for the processing of
crude oil is shown in Fig. 1. The crude oil distillation (a
straightforward distillation process) and subsequent
vacuum-distillation (distillation under high vacuum) of the residues
of the first process splits crude oil into its basic fractions which,
after further treatment, purification, and sometimes blending with
additives, are used as commercial products. The major petroleum
fractions are listed in Table 1 in broad categories according to
increasing boiling-point.
Certain petroleum fractions, such as naphtha or wax can be
submitted to various thermal or catalytic cracking processes and to
other refinery processes such as alkylation, and isomerization. In the
course of these processes, long-chain paraffinic hydrocarbon molecules
are broken down into smaller molecules including unsaturated
(olefinic) compounds. Some of these olefins may stay in the
end-product of the cracking process, others, especially if under the
influence of high temperatures and catalysts, will react among each
other and form more complex structures ranging from iso-octanes to
polynuclear aromatic hydrocarbons (Badger, 1962).
The products obtained from cracking processes can be distilled
into various fractions in a similar way to crude oils, though
obviously the composition of the fractions is different. For instance,
they contain a certain percentage of olefins that are highly valued as
base materials for the chemical industry. By suitable choice of
cracking procedures, the yield of special compounds such as gasoline
components or olefins can be boosted. On the other hand, fractions
derived from the cracking of petroleum products contain a higher
percentage of polynuclear aromatic hydrocarbons than corresponding
straight-run crude oil fractions. The implications of this will be
discussed later.
TABLE 1. Range of major petroleum fractions
Fuels Boiling range Non fuels
(approximate)
natural gas
refinery gas
liquefied petroleum <10 °C
gas (LPG)
gasolines 35 °C petroleum solvents
kerosenes naphtha
gas oils 300 °C
heavy fuel oils 300 °C base oils also used for
lubricating, metal
working and textile oils
petrolatum
700 °C petroleum waxes
>700 °C bitumens, coke
Examples of olefinic base chemicals derived from cracking
processes are: ethylene, acetylene, propylene, butylenes, pentenes,
and higher aliphatic olefins, such as butadiene, isoprene.
It is outside the scope of this review to give further details
and other refining processes.
 2.2.2.3 Disposal of waste
In a refining process, the release of oil into refinery effluents
is practically negligible and of a lower order of magnitude than
tanker washings in tankers that do not use the "load-on-top" system.
Waste gas in production fields is generally burnt on the spot. In
refineries and chemical plants, it may be necessary to burn some gas
at a flare for reasons of safety, and some oil and gas is consumed as
refinery fuel. Atmospheric pollutants in and around refineries
basically consist of saturated and unsaturated hydrocarbons, carbon
monoxide, hydrogen sulfide, and sulfur dioxide (Poliansky &
Musserskaja, 1971; Krasovitskaja, 1976). Sulfur dioxide, hydrogen
sulfide, and mercaptan emissions are not discussed in this review and
emissions of hydrocarbon vapours into the atmosphere from storage
terminals, filling stations, and cars will be covered in another
document.
2.3 Toxicological Effects of Crude Oils
As, in this document, crude oils are discussed only to provide
background information for the petroleum solvents, lubricating base
oils, and bitumens derived from them, no detailed discussion will
follow concerning environmental exposure levels, environmental
distribution and transport, physiological factors relating to
mammalian uptake, dose-response relationships, and maximum permissible
levels. Most of the relevant aspects will, however, be covered in the
sections on fractions derived from the crude oils. This also applies
to toxicological effects on experimental animals and man, with the
exception of a very few studies that are related to crude oil exposure
only.
The toxicological and nuisance aspects of hydrogen sulfide and
mercaptans have been reviewed in detail by Miner (1969) and Sullivan
(1969). A review on hydrogen sulfide has been prepared by NIOSH
(1977c) and an Environmental Health Criteria document on hydrogen
sulfide has recently been published (WHO, 1982).
2.3.1 Effects on experimental animals
Leitch (1924) examined 16 untreated crude oils from various parts
of the world for their carcinogenicity by applying them 3 times a week
to the skin of mice and found significant differences in
tumorigenicity among these oils. Similar results were reported by
Hieger & Woodhouse (1952) in skin tests on mice and rabbits. The
tumorigenicity of the crude oils they examined was low in comparison
with that of some of the distilled fractions. Skin tests were also
carried out on mice and rabbits by Antonov & Lints (1960), who found
that Saratov oil possessed weak carcinogenic properties. The main
causes of death in these tests, however, were pneumonia and general
intoxication, probably from absorption of oil components through the
skin. The authors found that rabbits were more sensitive than mice, as
did Hieger & Woodhouse (1952).
Batt-Neal & Wolman (1977) demonstrated skin tumorigenicity and
amyloid deposition following skin exposure of mice to saturated
acetone extracts of various oils collected from beaches.
2.3.2 Effects on man
Examination of 743 oilfield workers exposed to California crude
oil and excessive sunlight revealed that 7 of them had epitheliomas on
exposed parts of the body and that nearly 20% had keratotic changes on
the hands, forearms, face, and neck. Five of the 7 subjects, who
developed epitheliomas, were blonds, though blonds were in the
minority in this group of workers (Schwartz et al., 1947).
During 1938-39, Schwartz saw 189 cases of carcinomas on exposed
parts of the skin; 128 were in males, 71 of whom were oilfield workers
20 others being workers exposed to excessive sunlight only. Emmett
(1975) mentions the strong potentiating effect of UV radiation on
other potentially carcinogenic exposures. In southern Texas, however,
the incidence of skin carcinomas in 330 oilfield workers was low,
which underlines the fact that Texas and Pennsylvania oils are known
to be less carcinogenic than California oil (Twort & Ing, 1928).
In a study on 50 volunteer operators, who had not previously been
in contact with oil and petroleum products, crude oil was applied to
the skin of the inner surface of the forearm, for periods of 3-6 h. An
inflammatory reaction of the skin developed with moderate erythema,
oedema, and slight burning. Changes in the thermosensitive threshold
were noted, as well as an increase in the permeability of the
epidermis (Gusein-Zade, 1975).
3. PETROLEUM SOLVENTS
3.1 Properties and Analytical Methods
3.1.1 Chemical composition and properties
Only solvents consisting of hydrogen and carbon alone and
produced from petroleum will be considered in this review. It should
be noted, however, that similar solvents are also produced from coal.
Petroleum solvents consist of complex mixtures of hydrocarbons
reflecting the hydrocarbon constituents of the crude oil or, more
usually, the intermediate refinery streams from which they are
distilled. Because of their complex nature, classification is a
problem and no standard, worldwide-accepted nomenclature exists.
However, providing that it is recognized that considerable overlapping
and many exceptions occur, they can be classified into 3 broad
subdivisions, based on distillation ranges:
(a) special-boiling-point solvents (SBPs) - grades with narrow or
wide distillation ranges within the main limits of 30-160°C;
(b) white spirits - grades distilling within the main range
150-220°C, the boiling-points of individual grades usually
ranging over more than 20°C;
(c) high-boiling aromatic solvents - grades distilling in the range
160-300°C with final boiling-points above 220°C.
Benzene, toluene, and the xylene isomers occur as components of
petroleum solvents, but as they fall more naturally into the category
of chemical intermediates, they will be referred to here only in so
far as they are important as components of the mixtures being
discussed.
Two further clarifications can be made. Firstly, it is common
industrial practice to ascribe the name of the predominant isomer
present to the petroleum solvent; thus the descriptions pentane,
isopentane, hexane, isohexane, and heptane are commonly met. However,
in almost all cases, the amount of the named isomer present in an
industrial scale product will not exceed 95% v/v of the solvent and
may be as small as 30% v/v.
Second, most petroleum solvents are marketed on the basis of
typical physical properties rather than on chemical specifications,
because of the limitations during refining of controlling the complex
mixtures of isomers that make up the petroleum solvents. As production
techniques become more sophisticated, greater control is possible and
more properties can be specified within narrower limits. However, even
when such narrow limits are met, the mixture of components present may
vary, because of variations in the types of crude oil being processed
and alterations in conditions in processing units.
To meet the wide range of properties required by the market,
several different processes are used. Distillation is the common
process setting the volatility range. Chemical conversion techniques,
including reforming, alkylation, and hydrogenation, alter the chemical
composition and hence the solvency, as do physical conversion
techniques such as solvent extraction and molecular sieve separation.
Specific treatments such as caustic soda and sulfuric acid washing and
clay percolation are frequently applied to remove odourous substances,
chiefly sulfur compounds.
The reader is referred to Boenheim & Pearson (1973) for detailed
discussions of the chemical and physical composition and uses of
petroleum solvents.
3.1.1.1 Special-boiling-point solvents (SBPs)
These are highly purified naphtha fractions with specially
selected boiling ranges. The boiling range may be narrow or wide, and
generally falls within the limits of 30-160°C: SBPs are classified
according to their boiling range, e.g., SBP 62/82. Petroleum ether,
lighter fluid, spot remover, and rubber solvent are consumer products
in this range. Generally, SBPs consist of a mixture of hydrocarbons in
the C-5 to C-9 range: normal and branched paraffins, cycloparaffins,
and aromatic compounds. They contain only traces of olefins. An
example of the composition of a typical sample of straight run (i.e.,
non-dearomatized) SBP 80/110 is given in Table 2.
3.1.1.2 White spirits
The boiling-range of this group of solvents falls within the
limits 150-220°C (intermediate between gasoline and kerosene). These
solvents can be classified into low-aromatic grades (approximately
15-20% aromatic hydrocarbons) and high-aromatic grades (45% or more
aromatic hydrocarbons). They generally consist of hydrocarbons in the
C-7 to C-12 range, again including normal and branched paraffins as
well as naphthenic (cycloparaffins) and aromatic compounds. Olefins
are present in trace amounts only. Stoddard solvent, mineral spirits,
low-aromatic white spirits (LAWS) and turpentine-substitute are
well-known examples from this range.
3.1.1.3 High-boiling aromatic solvents
Aromatic hydrocarbons occur naturally in certain crude oils in
widely varying concentrations. They are also formed during secondary
processes such as thermal and catalytic reforming. They can be
concentrated and extracted by solvent extraction.
Apart from benzene, toluene, and xylene, which will not be
discussed separately in this review, this group includes solvents with
an aromatic content of 80-100%, and a wide boiling-range from 160 to
300°C. High-boiling aromatic solvents are obtained by distillation or
solvent extraction from refinery fractions such as kerosene and
lubricating base oils, and consist of very complex mixtures of
hydrocarbons with more than 9 carbon atoms per molecule. The
composition of a typical sample of one of these aromatic hydrocarbons
from the middle range (distillation range approximately 192-203°C) is
given in Table 3.
Most aromatic solvents are highly purified "white" solvents.
Those in the higher boiling range, derived from lubricating base oil
stocks by solvent extraction, may be less pure and coloured. They are
often by-products and are used as solvents for various technical
purposes. In many cases, they are referred to as "processing oils"
instead of solvents, and considered under lubricating oils.
3.1.2 Purity of petroleum solvents
In these complex mixtures, impurity is, of course, a matter of
definition. Components that are taken out in the course of the various
refining and treating processes used to obtain the more pure solvents
could be regarded as such. The major impurities would then be sulfur
compounds such as hydrogen sulfide, mercaptans, and thiophens, as well
as, olefins and other reactive unsaturated hydrocarbons.
A second category of impurities includes the hydrocarbons that
have been demonstrated to be carcinogenic in animals and man, such as
benzene, the polynuclear hydrocarbons and related heterocyclic
compounds containing nitrogen or sulfur.
TABLE 2. Composition of typical sample of SBP 80/110a
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
normal n-pentane 0.2 36.2
paraffins n-hexane 8.2 69.0
n-heptane 17.2 98.4
branched 2 methyl butane Tc 0.1 27.9
paraffins 2,2 dimethyl butane T trace 49.7
2,3 dimethyl butane T 0.3 58.0
2 methyl pentane 1.5 60.3
3 methyl pentane 1.6 63.3
2,2 dimethyl pentane 1.0 79.2
2,4 dimethyl pentane 1.3 80.5
2,2,3 trimethyl butane T 0.3 80.9
2,3 dimethyl pentane 9.7 89.8
3 methyl hexane 9.2 91.9
3 ethyl pentane 3.1 93.5
2,2,4 trimethyl pentane trace 99.2
2,2 dimethyl hexane trace 106.8
2,5 dimethyl hexane 0.6 109.1
3,3 dimethyl hexane T trace 112.0
2,3 dimethyl hexane 0.8 115.66
3,4 dimethyl hexane trace 117.7
3 methyl heptane 0.5 118.9
cyclo C-6 cyclohexane 8.4 80.7
paraffins methyl cyclohexane 14.2 100.9
cyclo C-5 cyclopentane T trace 49.3
paraffins methyl cyclopentane 4.7 71.8
1,1 dimethyl cyclopentane T 2.9 87.9
1-cis-3-dimethyl 1.9 90.8
cyclopentane T
TABLE 2. (contd).
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
cyclo C-5 1-trans-3-dimethyl 2.7 91.7
paraffins cyclopentane T
contd. 1-trans-2-dimethyl 0.5 91.9
cyclopentane T
1-cis-2-dimethyl cyclopentane T 0.5 99.5
ethyl cyclopentane 0.6 103.5
1,1,3 trimethyl cyclopentane T 0.8 104.9
1-trans-2-cis-4-trimethyl 0.4 109.3
cyclopentane T
1-trans-2-cis-3-trimethyl 0.4 110.2
cyclopentane T
1,1,2 trimethyl cyclopentane T 0.3 113.7
unidentified Probably
paraffins 1.1 110.0
aromatic benzene 0.7 80.1
compounds toluene 3.9 110.6
olefins 0.4
a From: Shell International Petroleum Co., London (unpublished data).
b Average of duplicate analyses.
c T = tentative identification.
TABLE 3. Composition of typical sample of Solvesso 150a
Hydrocarbon % v/v of solvent
n-butylbenzene 2.47
sec-butylbenzene 0.08
tert-butylbenzene 0.05
m-cymene 0.13
o-cymene 0.01
p-cymene 0.52
1.2-diethylbenzene 1.72
1.3-diethylbenzene 1.10
1.4-diethylbenzene 0.56
1.2-dimethyl-3-ethylbenzene 2.86
1.2-dimethyl-4-ethylbenzene 6.64
1.3-dimethyl-2-ethylbenzene 0.71
1.3-dimethyl-4-ethylbenzene 4.17
1.3-dimethyl-5-ethylbenzene 2.80
1.4-dimethyl-2-ethylbenzene 3.26
m-ethyltoluene 0.37
o-ethyltoluene 0.02
p-ethyltoluene 0.01
indane 0.46
isobutylbenzene 0.32
isopropylbenzene 0.01
1-methyl-3-t-butylbenzene 0.76
1-methyl-2-n-propylbenzene 1.26
1-methyl-3-n-propylbenzene 2.08
1-methyl-4-n-propylbenzene 1.93
1-methylindane 0.91
2-methylindane 2.43
4-methylindane 9.28
5-methylindane 2.02
naphthalene 4.03
n-propylbenzene 0.00
1.2,3,4-tetramethylbenzene 3.66
1.2,3,5-tetramethylbenzene 8.84
1.2,4,5-tetramethylbenzene 5.53
toluene 0.02
1.2,3-trimethylbenzene 0.10
1.2,4-trimethylbenzene 0.05
1,3,5-trimethylbenzene 0.01
a Courtesy Esso Standard Oil Company, New York, N.Y.,
USA (From: Gerarde, 1960).
TABLE 3. (contd).
Hydrocarbon % v/v of solvent
m-xylene 0.05
o-xylene 0.03
p-xylene 0.03
C-11-naphthalenes 0.31
C-11-indanes 3.58
C-11-alkylbenzene 18.27
C-12-alkylbenzene 0.73
C-12-indanes 0.08
C-13-alkylbenzene 0.02
C-10-indenes 0.10
C-11-indenes 0.07
C-12-naphthalenes trace
C-13-naphthalenes
C-12-indenes 0.10
aromatic compounds Total 94.55
Generally, the total sulfur content, the olefin content, and the
total aromatic content are specified for commercial petroleum
solvents. Where special products such as food-grade materials are
concerned, the benzene content is specified as well as the UV
absorption limits at certain wavelengths, as a measure of the
polynuclear aromatic hydrocarbon content.
3.1.3 Methods of sampling and analysis
See section 2.1.2.
3.2 Sources of Environmental Pollution
3.2.1 Natural occurrence
Petroleum solvents do not occur in nature as such, but only as
components of the crude oils from which they are derived.
Environmental pollution is always man-made and related to the use of
the solvents.
3.2.2 Man-made sources
3.2.2.1 Production
Because there is no uniform system of definition and
classification of petroleum solvents, firm statistics concerning the
magnitude of production of this group of materials do not exist. The
best estimate of the world-wide production of the group of solvents
would be 9 million tonnes for the year 1979.
3.2.2.2 Uses
It is not feasible to give more than a general outline of the
uses of the range of petroleum solvents.
(a) SpeciaL-boiling-point solvents (SBPs)
SBPs are mainly used as: solvents and thinners in lacquers and
paints; extraction solvents for perfumes, for vegetable oils and oil
and fats of animal origin; quick-drying solvents in printing-ink,
coatings, and adhesives; lighter fuel; and for dry-cleaning and
degreasing purposes.
(b) White spirits
White spirits are mainly used as: solvents and thinners for
lacquers, paints, resins, and printing-ink; solvents in formulations
of chemical products, e.g., pesticides; and for metal degreasing, wool
degreasing, and dry-cleaning.
(c) Aromatic extracts
The higher-boiling and less-purified aromatic extracts have very
good solvent properties for many polymers and are used as
ex-tender-oils in rubber, plastics, and bitumens, and also as solvents
in printing-ink and pesticide formulations. Furthermore, they can be
used as base-materials in the manufacture of carbon black.
3.3 Environmental Exposure Levels
Specific data are not available concerning levels of petroleum
solvents in air, water, food, or other environmental media. However,
low concentrations of hydrocarbons found in mussels have probably been
derived from petroleum hydrocarbons present in the environment
(Ehrhardt & Heineman, 1975).
Because of the relatively low boiling-range of these solvents,
industrial exposure to vapour may sometimes be high. This is known to
occur, especially in small workshops with insufficient ventilation,
where, for example, adhesives are used routinely. Although a lot of
consumer products may contain these solvents, excessive domestic
exposure would not normally be expected unless neat solvent were used
for cleaning purposes, indoors. Very limited, indirect exposure of the
general population is possible following the use of these solvents as
extractants in the production of food-grade vegetable oils.
Exposure to the higher-boiling and less-purified aromatic
extracts is mainly confined to occupational situations, where
excessive skin-contact may occur, or exposure to vapour in processes
carried out at elevated temperatures or with high-speed machines that
could give rise to fumes or mists. This will be considered in detail
under lubricating base oils.
3.4 Environmental Distribution and Transformation
Data on the distribution between media, environmental
transformation and degradation, interaction with physical, chemical,
or biological factors and bioconcentration, are not available for
petroleum solvents.
However some information exists concerning the behaviour and
degradation of crude oil in water (Floodgate, 1972, Hellmann & Zehle,
1972), and of hydrocarbons in general (Walker et al., 1975), and there
is much information on the microbial degradation of individual
petroleum hydrocarbons (Van der Linden & Thysse, 1965; Haines &
Alexander, 1974).
From these publications it can be seen that the subject is highly
complex and many factors have to be taken into account, such as the
composition of the oil product, the extent of dispersion into the
medium, and climatic conditions.
3.5 Metabolism
3.5.1 Absorption
The kinetics are determined by diffusion rates, solubility in
fat, and the concentration gradients in the individual compartments of
the body.
The highly volatile C-5, C-6, and C-7 paraffins, cycloparaffins,
and aromatic hydrocarbons readily pass across the alveolar membrane
into the bloodstream and are transported within minutes to the central
nervous system. Longer-chain homologues can, to a certain extent, also
pass the alveolar membrane, but their principal effect is local. This
was shown by Gerarde (1963) in studies on rats.
The alveolar air and blood concentrations of white spirit have
been measured in man following inhalation (Åstrand et al., 1975).
Aromatic hydrocarbons were absorbed to a greater extent into the
bloodstream than aliphatic hydrocarbons (approximate values being 62%
and 50%, respectively). Similar uptake values in man were shown for
the aromatic hydrocarbons, benzene and toluene, by Nomiyama & Nomiyama
(1974), for xylene by Sedivec & Flek (1976), Åstrand et al. (1978),
and Riihimäki et al. (1979), and for ethylbenzene by Bardodej &
Bardodejová (1970). Nomiyama & Nomiyama (1974) demonstrated a much
lower pulmonary absorption for n-hexane, the only aliphatic compound
that they tested; it was also rapidly excreted.
The skin is only permeable to hydrocarbons of a certain size.
With paraffinic substances, the maximum chain length appeared to be up
to 14 C-atoms (Scheuplein & Blank, 1971). Aromatic compounds have a
more compact structure and, in studies on guineapigs, Hoekstra &
Phillips (1967) showed that compounds from this group with a higher
number of C atoms could still pass the skin barrier.
The absorption of vapours through the skin is of minor
importance. For example, in man, whole body skin exposure to
2250 mg/m3 (600 ppm) of toluene was equivalent to an inhalation
exposure of less than 37.5 mg/m3 (10 ppm) (Riihimäki & Pfäffli,
1978). However, absorption during immersion in liquid solvents may be
considerable. Percutaneous absorption during immersion of both hands
in pure xylene was equal to an inhalation exposure of 435 mg/m3
(100 ppm) (Engström et al., 1977). The permeation of xylene is thus
about 20 nmol/min per cm2 (Engström et al., 1977; Riihimäki, 1979)
and that for toluene, 3 µmol/min per cm2 (Cohr & Stockholm, 1979).
Cutaneous exposure was probably a major route of absorption in 2 cases
of acute renal failure with oliguria, caused by exposure to diesel oil
(Barrientos et al., 1977; Crisp et al., 1979).
Data for absorption in the intestinal tract are not available,
but it is presumed that it would resemble absorption in the alveoli
rather than that through the skin.
3.5.2 Distribution in the body
Tissue hexane levels in rats, following inhalation of anaesthetic
concentrations, were measured by Böhlen et al. (1973). The tissue
distribution generally depended on exposure time and was proportional
to the lipid content of an organ until saturation occurred. The liver
was a special case for, as its lipid level changed rapidly, the
saturation level varied. Hexane was also apparently bound to some
blood components.
Women working at conveyor belts gluing parts of rubber footwear
had concentrations of petroleum solvents (no details on
physicochemical properties given) in the blood ranging from 2 . 35 ±
0.4 up to 4.6 ± 0.6 mg/litre at concentrations in the air of
100-300 mg/ m3. The solvent concentration in the blood increased with
increasing length of the working period from 1.6 mg/litre in the first
year to 2.5 mg/litre after 3.5 years and 3.4 mg/litre after 7-8 years
of service.
Wistar rats were exposed to the solvents used in the factory at
concentrations in air of 300-1000 mg/m3 for 30-45 days, 4 h/day. The
concentration of solvent in the blood amounted to 0.45 ± 0.05 -
1.2 ± 0.01 mg/litre (Lipovskij et al., 1977a).
Transfer of petroleum solvents through the placenta was studied
in 85 pregnant women workers in the rubber industry, who came into
contact with petroleum solvents during work (physicochemical
properties of the solvents not defined, concentration in the air of
the operating premises 300 ± 10 mg/m3). The average level of solvents
in the blood of 46 pregnant women, on whom abortion was performed, was
1.27 ± 0.3 mg/litre. A level of 3.29 ± 0.6 mg/kg was found in the
tissue of the embryo. Women giving birth to a child (39 women) had a
level of solvents in the blood of 2.5 ± 0.3 g/litre, while the content
in the blood of the umbilical cord was 3.5 ± 0.3 g/litre. The
concentration of solvents in the blood of the newborn infants was
twice that of the mothers.
Pregnant Wistar rats were exposed to the same solvent at a
concentration of 300 ± 10 mg/m3, for 48 days, 4 h per day. The
solvent was present in the blood, brain, liver, placenta, uterus, and
fetal tissues (Lipovskij et al., 1979).
3.5.3 Biotransformation
In both man and animals, the aliphatic hydrocarbons are generally
considered to be biochemically inert and excreted in the same form
(Williams, 1959). However, it has been shown that some normal alkanes
will, at least in part, be oxidized by the mammalian organism. For
example, Ichihara et al. (1969) demonstrated the oxidation of decane
in animals such as mice and rats, and the oxidative pathway of
n-hexane to hexane-2,5-dione and hexane-2,5-diol via
methyl- n-butylketone has been well established (see for example
Spencer et al., 1978).
As far as the metabolism of the cycloparaffins and aromatic
hydrocarbons is concerned, the half-life, form, and rate of excretion
of each component of the solvent has to be considered. It should be
mentioned, however, that the metabolism of individual compounds will
not be discussed in this document and readers are referred to the
reviews by Williams (1959) and Gerarde (1960).
The carcinogenicity of the solvents is thought to be due to the
presence of benzene and some of the polynuclear aromatic compounds.
3.5.4 Elimination
The elimination of the lower-boiling solvents (SBP type) in both
animals and man is usually rapid and mainly occurs via the respiratory
tract. However, in the case of ingestion of the heavier solvents
(white spirits), elimination mainly takes place with the faeces
(Browning, 1965).
3.6 Effects on Experimental Animals
It has been mentioned in section 3.1.1, that the petroleum
solvents under discussion in this document are more or less complex
mixtures of a range of hydrocarbons. For the commercial products, the
specification given generally includes the specific gravity,
boiling-range, and total content of aromatic hydrocarbons. The
concentrations of individual components vary, within certain limits,
with the source of the crude oil from which the solvent is derived,
and with the processes by which it is produced. These facts should be
kept in mind because:
(a) the toxicity data developed for a certain solvent-specification
indicate the order of magnitude of the toxicity of this type of
product;
(b) in practice it would be impossible and impracticable to carry out
complete toxicity testing on every single solvent on the market.
It is only sensible to develop toxicity data for typical
representative samples of a certain boiling range and within a
certain specification of aromatic content. In the evaluation of
the results, however, the analytical composition of the material
- especially its contents of n-hexane, benzene, and polynuclear
aromatic hydrocarbons should be taken into account.
3.6.1 Short-term exposure
Hine & Zuidema (1970) examined various aspects of the acute
toxicity of 10 samples of petroleum solvents that contained components
representative of the range of hydrocarbons found in commercial
petroleum solvents. Four were aromatic solvents containing at least
98% aromatic hydrocarbons (coded A) and 6 were non-aromatic solvents
containing less than 1% aromatic hydrocarbons (coded S). The boiling
ranges and principal components of the samples examined are given in
Table 4.
Acute oral, inhalation, and percutaneous toxicity and skin and
eye irritancy were examined for all samples. Intratracheal aspiration
was simulated with 2 samples and repeated skin irritation tests were
carried out using 5 of the samples. Undiluted samples were used for
the investigations, all of which were carried out on rats with the
exception of skin and eye irritancy and skin toxicity rests in which
rabbits were used.
TABLE 4. The boiling-range and principal components of solvents examined for
acute toxicitya
Sample Boiling-range Principal components
A-1 281-286°F (138-141°C) C-8 aromatic compounds (ortho, meta, and
paraxylene; ethyl benzene)
A-2 362-398°F (163-203°C) C-9, C-10 and C-11 aromatic compounds
A-3 364-408°F (188-209°C) C-10 and C-11 aromatic compounds
A-4 384-507°F (196-264°C) C-11 to C-14 aromatic compounds
S-1 149-166°F (65-75°C) C-6 normal and isoparaffins (hexanes) and
naphthenes (cyclohexane, methylcyclopentane)
S-2 196-220°F (91-104°C) C-7 normal and isoparaffins (heptanes) and naphthenes
(methylcyclohexane, dimethylcyclopentane)
S-3 313-356°F (156-180°C) C-9 and C-10 normal and isoparaffins and naphthenes
S-4 368-395°F (187-212°C) C-11 and C-12 normal and isoparaffins and naphthenes
S-5 345-402°F (174-216°C) C-12 isoparaffins
S-6 384-500°F (195-260°C) C-13 to C-16 normal and isoparaffins and naphthenes
a From: Hine & Zuidema (1970).
The findings of Hine & Zuidema (1970) which are summarized in
Table 5, showed that all the solvents tested could be considered of
low hazard to health unless aspirated or inhaled in extremely high
concentrations. Aromatic solvents were more toxic than non-aromatic
materials, the dose of solvent required to kill 50% of rats, when
administered orally or percutaneously, being lower for aromatic than
for non-aromatic solvents. Skin and eye irritancy were also greater
with aromatic solvents. The toxicity of the vapours could not be
compared, because the volatility of samples varied greatly. All
solvents induced similar toxic effects, whatever the route of
administration, including central nervous system depression
(characterized by incoordination, prostration, and coma) followed by
death. Convulsions sometimes occurred. All solvents caused skin and
eye irritation though, in general, as the chain length of the
non-aromatic solvents increased their irritant properties decreased.
Repeated skin exposure led to skin irritation and necrosis with all
solvents.
Hoekstra & Phillips (1963) found that light mineral oils, when
applied topically to the skin of guineapigs, caused epidermal
hypertrophy, hyperplasia, hyperkeratosis, and depilation. Examination
of the effects of various oil fractions demonstrated that the main
effect of the short-chain volatile paraffins was to defat the skin,
while longer-chain and aromatic hydrocarbons had a dermatoxic effect
that was related to the permeability of the skin to these compounds.
The maximum dermatoxic effect was seen with hydrocarbons containing
14-19 carbon atoms, while a transition to non-dermatoxicity occurred
around 21-23 carbon atoms. This was confirmed with pure n-paraffins,
but variations may exist with other types of hydrocarbons.
Simultaneous application of innocuous long-chain substances together
with irritant short-chain substances greatly reduced their toxicity,
though this effect was less marked with aromatic solvents.
In further studies on the effects of inhaling the vapours of
hydrocarbon solvents (Carpenter et al. 1977a, b, c), the acute (4-h
exposure) LC50 and no-observed-adverse-effect concentrations were
studied in rats cats, and dogs. Results are summarized in Table 6.
These studies confirmed the occurrence of central nervous system
depression and there was also evidence of respiratory tract irritancy.
There were no marked or consistent differences between the species
examined. The major factor determining the acute inhalation hazard was
the volatility of the solvent, those containing 9 or more carbon atoms
tending to be insufficiently volatile to produce concentrations high
enough to be lethal over a short period of exposure. One exception was
a "high naphthenic" solvent, which was peculiar also in that
depression was not preceded by signs of irritation of the respiratory
tract, so that there was no warning of overexposure. Increased
aromatic content did not consistently result in increased inhalation
toxicity, though earlier work (Lazarew, 1929) suggested that the acute
inhalation toxicity of gasoline vapours increased with increasing
contents of cycloparaffins and aromatic hydrocarbons. The narcotic
action was also found to increase in each step by a factor of 3 in the
series - pentane, hexane, heptane, and octane (Fühner, 1921). Swann et
al (1974) found that anaesthesia occurred with these compounds at
concentrations of 32 000 ppm or more and that respiratory tract
irritation increased with chain length. Full anaesthesia can be
produced with gasoline (Haggard, 1921), but anaesthetic concentrations
are little lower than those that cause convulsions and death
(Browning, 1965).
TABLE 5. Toxicity of solvents. Summary of resultsa
Test Sample Result Classification
Oral A-1 10.0(7.5-13.3) practically non-toxic
LD50 A-2 4.5(3.0-6.8) slightly toxic
(ml/kg) A-3 13.3(7.5-23.7) practically non-toxic
A-4 12.3(8.1-18.7) practically non-toxic
S-1 >25.0b relatively harmless
S-2 >25.0b relatively harmless
S-3 >25.0b relatively harmless
S-4 >25.0b relatively harmless
S-5 >25.0b relatively harmless
S-6 >25.0b relatively harmless
Vapour A-1 6 350(4 670-8 640) slightly toxice
exposure A-2 >2 450b SVNTARTd
LC50 in ppm A-3 >580c SVNTART
for 4 h A-4 >553c SVNTART
S-1 73 680(66 310-79 940) practically non-toxic
S-2 14 000-16 000 practically non-toxic
S-3 2 000-2 600 slightly toxic
S-4 >710 SVNTART
S-5 >792 SVNTART
S-6 >263 SVNTART
Aspiration A-4 5/10 hazardous
(mortality) S-6 5/10 hazardous
Primary A-1 2.21 moderately irritating
skin A-2 2.04 moderately irritating
irritation A-3 2.17 moderately irritating
A-4 2.79 moderately irritating
S-1 1.92 slightly irritating
S-2 1.13 slightly irritating
S-3 2.38 moderately irritating
S-4 1.04 slightly irritating
S-5 1.29 slightly irritating
S-6 0.75 minimally irritating
Eye A-1 6.33 moderately irritating
irritation A-2 6.0 moderately irritating
A-3 4.33 moderately irritating
A-4 3.67 slightly irritating
S-1 0.33 minimally irritating
S-2 1.0 minimally irritating
S-3 2.0 minimally irritating
S-4 0 minimally irritating
S-5 0 minimally irritating
S-6 0 minimally irritating
TABLE 5. (contd).
Test Sample Result Classification
4-h A-1 approx. 5.0 practically non-toxic
percutaneous A-2 approx. 5.0 practically non-toxic
LD50 rangefind A-3 approx. 5.0 practically non-toxic
(ml/kg) A-4 approx. 5.0 practically non-toxic
S-1 >5.0 practically non-toxic
S-2 >5.0 practically non-toxice
S-3 >5.0 practically non-toxice
S-4 approx. 5.0 practically non-toxice
S-5 5.0 practically non-toxice
S-6 5.0 practically non-toxice
Repeated benzene 3.6
skin toluene 3.5
irritationf A-1 3.3
a From: Hine & Zuidema (1970).
b Doses above this amount not practical for testing.
c Maximum concentration obtainable at 25 °C.
d SVNTART = Saturated vapours not toxic at room temperature.
e Lowest toxicity classification may be "relatively harmless"
f Scored according to the method of Draize.
The greatest health hazard arises when hydrocarbon solvents are
aspirated into the lungs. This rapidly induces acute chemical
pneumonitis, which is characterized by pulmonary oedema and
haemorrhage, and is generally fatal (Waring, 1933; Lesser et al.,
1943; Gerarde, 1959). Gerarde (1959) demonstrated that the ratio of
the oral and intratracheal LD50s was 140:1 for kerosene, the
intra-tracheal LD50 being 0.2 ml for rats. This and other evidence
demonstrated that pulmonary injury was caused by direct contact with
the solvent and not by solvent present in the blood, following its
absorption through the gastrointestinal tract.
TABLE 6. Toxicity of solventsa
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
V.M. & P. 118-151 55.4 32.7 11.9 C-7 to C-10 16 >8 >19 2.8 2.8 0.7-7 2.1 2.0
Naphtha (3400) (>1700) (>4100) (600) (800) (0.15- 1.5) (450) (430)
Stoddard 153-194 47.7 37.6 14.7 C-8 to C-12 >8.2 >2.9 > 10 1.1 1.9 0.5 - 5 7.85 1.15
solvent (>1400) (>510) (>1700) (190) (330) (09-.9) (>150) (200)
Rubber 75-112 41.4 53.6 4.9 C-6 to C-7 61 >5.9 >49 7.9 7.9 40 1.7 1.7
solvent (15000) (>1500) (>12000) (2000) (2000) (10) (430) (430)
Mixed 138-141 - - 100 C-8 29 >5.4 <41 3.5 3.5 0.6-6 >0.46 0.46
xylenes (6700) (>1200) (<9500) (810) (180) (.14-1.4 (>110) (110)
'60' 128-159 c28.8 c21.4 c49.5 C-8 to C-9 24 >9.5 <20 0.44 1.4 10 C. 1.3 0.44
Solvent (4900) (>1900) (<4100) (90) (280) (2) (260) (90)
'70' 157-211 16.5 15.7 67.8 C-9 to C-11 >4.4 >2.4 >2 1.1 1.1 4 0.32 0.32
Solvent (>810) (>440) (>370) (200) (200) (0.7) (59) (59)
'140' 184-205 60.8 35.7 3.4 C-5 to C-12 >0.27 >0.21> > >0.23 >0.23 4 0.31 0.23
Flash (sic) (>43) (>33) saturated (>37) (>37) (0.6) (49) (37)
aliphatic vapour
solvent'
'80' 96-142 9.7 18.9 71.4 C-6 to C-8 27 >2.1 >24 > 1.7 > 1.7 4 0.45 0.45
Thinner (6200) (>480) (>5500) (>390) (>390) (0.9) (100) (100)
'50' 98- 105 66.3 0.6 33.1 C-7 33 >3.4 >30 2.4 2.4 10 1.7 1.7
Thinner (8300) (>600) (>7600) (600) (600) (2.5) (430) (430)
Deodorised 208-272 55.2 40.~9 3.9 not stated >0.1 This value applies for all toxicity evaluation and is 0.1
kerosene probably (14) a saturated atmosphere at room temperature. (14)
C 12 to
C-15
TABLE 6. (contd)
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
'40' 187-231 35.4 32.9 31.5 C-9 to C-13 >0.2 >0.25 >7 0.22 0,22 1 0.21 0.15
Thinner (>33) (>41) (aerosol) (36) (36) (0.17) (35) (25)
Toluene 95-110 38.7 15.4 45.9 C-6 to C-7 35 >3 >31 3.9 3.9 10 1.9 1.9
concentrate (8800) (>760) (>7800) (980) (980) (2.5) (480) (480)
'High 184-206 0.3 0.8 98.9 C-9 to C-12 >0.38 This value applies for all animal 0.4 0.15 0.15
aromatic (>66) toxicity evaluations and is a (0.07) (26) (26)
solvent saturated atmosphere at room temperature
'High 157- 183 29.0 69.9 1.1 not stated 5.3 3.8 - 2.1 - - - 2.1
naphthenic probably (960) (>690) (380) (380)
solvent' C-9 to
C-11
'Naphthenic 151-200 24.7 37.2 38.1 C-9 to C-11 >10 - - 2.2 - - - 2.2
aromatic (some (380) (380)
solvent' aerosol
present)
a Summary of data from Carpenter et al. (1977a,b,c).
b p = paraffins, N = naphthenes, A = aromatic compounds.
c For details of actual length of LC50 exposure, see original papers.
d NEL/No-ill effect/exposures 6th/day, 5 days/week for 13 weeks.
The same author found that viscosity appeared to be the property
that determined the aspiration hazard of liquid hydrocarbons.
Lower-boiling-point solvents (B.P. up to 100°C) evaporated so rapidly
in the mouth of anaesthetized rats that death was due to CNS
depression following absorption of the vapours. Higher-boiling-point
solvents tended to induce chemical aspiration pneumonitis. With
alkanes, the aspiration hazard decreased sharply with solvents
containing 16 or more carbon atoms, probably because the viscosity of
such solvents prevented aspiration into the alveoli. In the aromatic
series, side-chains containing more than 6 carbon atoms also tended to
diminish the aspiration hazard, as did blending with more viscous
lubricants (Gerarde, 1963).
3.6.2 Long-term exposure
The repeated skin tests of Hoekstra & Phillips (1963) and Hine &
Zuidema (1970) have already been mentioned in section 3.6.1.
Smyth & Smyth (1928) exposed guineapigs for 4 h/day, 6 days/
week, for a total of 65 exposures to a gasoline-type solvent
(boiling-range 145-183°C) at a concentration of 6750 mg/m3. During
the earlier exposures, the animals appeared to be restless. This was
followed by slight narcotic effects. Diarrhoea and albuminuria
developed temporarily, but blood, lung, and other changes were absent.
In a recent series of inhalation studies on various hydrocarbon
fractions, Carpenter et al. (1977a, b, c) provided much new
information on the toxicity of a wide range of solvents. Physical
properties and the general chemical constitution are detailed in the
papers and the approximate compositions are given in Table 6. The
no-observed-adverse-effect levels of individual solvents following
inhalation studies over 13 weeks in rats and dogs is also tabulated,
together with the human sensory response (section 3.7.1.2). In
general, these studies were remarkable in the lack of toxicity that
they indicated. The few toxic effects that did occur, were usually
kidney damage in rats and minor haematological variations.
Other long-term animal studies have been carried out with the
lower-boiling fractions and more specifically with technical hexane
and technical heptane, because of their possible neurotoxic effects
and to establish a safe level of industrial exposure (TLV).
Mature female Wistar rats were exposed to petroleum solvent
vapour (physicochemical properties not given) at a concentration of
300 ± 8.2 mg/m3 for 30-45 days, for 4 h/day. The serotonin content of
the myometrium in exposed rats equalled 75.7 ± 2.6 µg/kg compared with
68.47 ± 2.5 µg/kg in the control group. Uterine contractions were more
numerous and stronger in exposed animals. The level of solvent in the
venous blood was 2.0 ± 0.4 mg/litre. In the uterine tissues it was
almost twice as high (3.8 ± 0.6 mg/kg). The increase in serotonin
content in the organism could cause disturbances in the transport of
the fertilized egg cell and the nidation, and subsequently, early
abortion (Lipovskij, 1978).
Miyagaki (1967) exposed 5 groups of 10 male mice to vapour
concentrations of technical hexane of 360, 900, 1800, 3600, and
7200 mg/m3. There was a sixth control group. The exposures lasted
24 h/day, 6 days/week, over a period of one year. The composition of
the hexane used is uncertain. The measured vapour concentrations
correlated well with those calculated. Electromyographical studies
were carried out and strength-duration curves, electrical reaction
time, flexor/extensor ratios, and gait-posture were observed. The
muscular atrophy of the hind legs was measured and in some of the
animals, histological examination of the distal mucles of the hind
legs was carried out. Evidence of neurogenic muscular atrophy was only
seen in the group receiving the highest exposure. At the lowest
exposure level (360 mg/m3), no changes were found in any of the
variables studied. At the other levels, changes were found related to
the severity and duration of exposure. Based on their results, the
authors proposed a reduction in the TLV to 360 mg/m3.
In a different study, which included a group of 5 rats (sex and
age not stated) exposed to a hexane concentration of 3060 mg/m3, over
a period of 143 days (no other details given) and a control group, no
significant differences were found in average body weights and blood
values (haematocrit, total serum protein, and protein fractions)
between treated and control animals. Histological examination of
"many" organs revealed only a "slight reaction of the RES in the
spleen" in the exposed animals. In the sciatic nerve and its
ramifications, injuries such as degeneration of myelin and axon
cylinders were seen. The myoneural junction remained unaffected. The
exposed group manifested some decrease in nocturnal activity (Kurita,
1967).
Truhaut et al. (1973) published the results of
electrophysiological studies on rats exposed to technical hexane or
technical heptane. The air concentration of hexane was 7200 ±
720 mg/m3 and that of heptane, 6000 ± 600 mg/m3 (calculated and
expressed as n-hexane and n-heptane). The duration of exposure was
5 h/day, 5 days/week for 1-6 months. The initial weight of the male
and female rats was 150g. No data on the growth-weight curves were
included. The composition of technical hexane was given as:
n-pentane 0.3%
methyl-2-pentane and cyclopentane 25.1%
methyl-3-pentane 18.4%
n-hexane 45.8%
methylcyclopentane 8 %
methylhexanes 1.2%
benzene 1.2%
and that of technical heptane as:
methyl-2-hexane plus dimethyl 2,3 pentane plus cyclohexane 9.8%
methyl-3-hexane 16.2%
n-heptane 52.4%
dimethyl 2,4 hexane plus methylcyclohexane 15.4%
methylheptane 3.3%
benzene 0.1%
toluene 2.8%
Electrophysiological studies were carried out on isolated sciatic
and saphenous nerves taken from anaesthetized rats. During the first 2
months of exposure, no changes were found in any of the treatments
but, in the succeeding months, symptoms of neural involvement were
detected, in general, increasing with the duration of exposure. The
signs were: decrease in conduction-velocity, increase in refractory
period, and decrease in excitability. Histopathological examination
did not reveal clear-cut demyelination, but early indications of this
type of change were certainly found. Truhaut has recently cast doubt
on these findings, stating that his experimental method was not
sufficiently rigorous to allow his earlier conclusions to be made
(Truhaut, 1978).
Numerous studies on the neuropathological effects of n-hexane
have been published including a review by Schaumburg & Spencer (1976).
Rats exposed for 24 h/day to an n-hexane concentration of
1440-2160 mg/m3 for about 20 weeks developed peripheral neuropathy.
Toxicity data on the presumptive in vivo neurotoxic oxidation
products of n-hexane have also been published (Spencer et al.,
1978). It should be mentioned that many authors have used the term
" n-hexane" loosely; the tested material often really consisted of
various C-6 hydrocarbons.
Some other hydrocarbon solvents have been implicated as possibly
causing neuropathy, following prolonged exposure to high
concentrations. As these reports are generally related to human
exposure, they will be considered in section 3.7.2.1. Of relevance is
the observation that toluene inhaled by rats over a period of one year
induced electrophysiological changes at concentrations of 7500 mg/m3
and 750 mg/m3 but not at 375 mg/m3 (Matsumoto, 1971). Fournas & Hine
(1958) exposed rats to high concentrations of various alkyl aromatic
hydrocarbons and found some clinical evidence of neurotoxicity with
most of the compounds tested; p-t-butyltoluene was shown to induce
CNS damage in rats by Ungar et al. (1955).
3.6.3 Mutagenicity, teratogenicity, and carcinogenicity
3.6.3.1 Mutagenicity
The potential mutagenic activity of selected petroleum products
was assessed using a battery of in vivo and in vitro bioassays,
including the dominant lethal test, the Ames' test (with and without
activation), the mouse lymphoma cell transformation test, and
observations on cytogenicity. The following products elicited negative
responses in one or more tests: VM&P Naphtha, Stoddard Solvent, mixed
xylenes, "60 Solvent", "70 Solvent", 140° Flash Aliphatic Solvent, "50
Thinner", kerosene, toluene, high solvency naphtha- unleaded petrol,
and No. 2 heating oil (40% aromatic compounds). Positive mouse
lumphoma cell transformation tests were elicited by benzene, diesel
fuel, No. 2 heating oil, and jet A fuel; positive results in
cytogenetic tests (clastogenic responses) were obtained with rubber
solvents, "60 Solvent", high aromatic solvent, No. 2 heating oil, and
jet A fuel. For each of these products the Ames bacterial bioassay was
negative (API, 1974, 1975b, c, 1977a, 1978a, d, f, 1979d).
3.6.3.2 Teratogenicity
Tests for teratogenicity induced by inhalation of high and low
doses of benzene, Stoddard Solvent, toluene, mixed xylenes, unleaded
petrol, high aromatic solvent, n-hexane, diesel fuel, VM&P naphtha,
kerosene, rubber solvent, jet fuel A, and No. 2 heating oil were all
negative. However, benzene exposure at 120 mg/m3 induced a
statistically significant increase in fetal resorptions in rats (API,
1974, 1975a, b, c, 1977 a, b, 1978a-f, 1979a-d).
3.6.3.3 Carcinogenicity
Carcinogenicity tests have only been conducted on the group of
high-boiling aromatic extracts derived from the solvent refining of
lubricating base oils. These studies will be considered together with
long-term studies on lubricating base oils, but, in short, practically
the whole of the carcinogenic polynuclear aromatic hydrocarbons were
found in the extracts from the base oils. However, the carcinogenic
activity of the extracts was considerably less than that of
coal-tar-derived products.
Similar carcinogenicity studies have not been carried out with
the lower boiling aromatic solvents (white spirits). Such studies
would be indicated, though the content of potentially carcinogenic 4,
5 and 6 condensed ring polynuclear aromatic hydrocarbons in these
aromatic solvents is probably much lower than in high boiling aromatic
solvents, because of the lower boiling range. Lijinsky & Raha (1961)
mention, however, that no commercial distillation procedure will
completely remove all traces of polynuclear aromatic hydrocarbons from
petroleum solvents and that special treatment is necessary to achieve
this. Though, in most cases, the authors found very low concentrations
(in the µg/m3-range), they are of the opinion that these levels
should be investigated, particularly in food-grade material.
3.7 Effects on Man
3.7.1 Controlled exposures
3.7.1.1 Effects of dermal exposure
Klauder & Brille (1947) patch-tested petroleum solvents of
various boiling ranges on the skin of human volunteers. They found a
correlation between the boiling ranges of the petroleum products of
paraffinic origin and their irritant and defatting action on the skin.
Both effects decreased, the higher the boiling range. Petroleum
solvents with boiling ranges up to and including that of kerosene
(approximately 230°C) were found to be primary irritants. Petroleum
solvents of naphthenic origin or with a high aromatic content were
more irritant than solvents of paraffinic origin of the same boiling
range. The skin of Negroes showed a higher tolerance than that of
Caucasians.
Pre-existing skin disease may increase the susceptibility of the
skin to the effects of contact with petroleum solvents and will also
facilitate uptake by this route (Klauder & Brille, 1947; Riihimäki &
Pfäffli, 1978).
The effects of various solvents on the horny layer of the skin
were examined by Malten et al. (1968) and Spruit et al. (1970). They
found that petroleum ether (SBP 40/65) caused serious irritation of
human forearm skin, when applied for periods of 10-30 min. When
applied for 15 min on 6 successive days, injury occurred in the horny
layer. Recovery -- as measured by water vapour loss -- could take up
to 6 weeks. The skin irritation and the changes in the composition of
the horny layer were independent phenomena.
Tagami & Ogino (1973) applied undiluted refined kerosene to the
arm of a volunteer in an occluded patch-test. After 1 h, a burning
sensation developed, slight erythema appeared after 2 h, and after
7 h, the skin was tender and very red, even beyond the patch-test
site. After 12 h, the burning sensation had subsided but a large tense
bulla had appeared surrounded by small scattered vesicles. This
changed into a large flaccid purulent bulla 24 h later, which easily
broke, leaving a raw surface. The main differences between the test
situations just described were: (a) a marked difference in boiling
range (volatility and skin penetration) of the products tested; and
(b) the length of exposure, the effect increasing with increasing
duration of exposure.
The same authors then applied 85%, 70%, 55%, and 40% dilutions of
the kerosene in mineral oil in covered skin test on 34-adult male
Negro and Caucasian volunteers. The skin of all the subjects reacted
to 85% kerosene solution, the 70% solution caused skin irritation in
29 subjects, the 55% solution in 8, and the 40% solution was
completely without effect. The skin of Negroes appeared to be less
irritated by kerosene than that of Caucasians; the influence of age
was not clear. No definite correlation was found between individual
response to kerosene and the permeability of the horny layer of the
skin (Tagami & Ogino, 1973).
3.7.1.2 Effects of inhalation
Inhalation of air containing petrol at concentrations of
1350-3150 mg/m3 for 18 min by human volunteers did not cause any
symptoms; a 14-min exposure to 12 600-31 500 mg/m3 induced dizziness
(Fieldner et al., 1921). Davis et al. (1960) exposed human volunteers
for 30 min to concentrations of petrol of 900, 2250, or 4500 mg/m3 in
air. Very few symptoms were noted. Itching and burning of the eyes was
apparent in most subjects in the highest exposure group.
The human odour- and sensory irritation thresholds were measured
as part of the toxicity evaluation of solvents carried out by
Carpenter et al. (1977a) (Table 6). It can be seen that vapours of
hydrocarbon solvents can be detected at rather low concentrations, but
that unpleasant odour and irritation only become apparent at much
higher concentrations. Nevertheless, in establishing an exposure limit
for each solvent, human sensory data were often limiting factors.
In volunteer studies, psychological functions were affected by a
50-min exposure to a concentration of white spirit of 4000 mg/m3
resulting in a prolonged reaction time and impaired short-term memory.
Lower concentrations did not have any effect (Gamberale et al., 1975).
3.7.2 Epidemiological studies
A distinction between epidemiological and clinical studies has
always been difficult in view of the inadequacy of reporting. The
various aspects will therefore be discussed under headings relevant to
the information available.
3.7.2.1 Occupational exposure
(a) Haematological effects
Benzene is unquestionably the most dangerous hydrocarbon used in
industry and the benzene content of some petroleum solvents presents a
major long-term hazard for man. In particular, special boiling
solvents in the lower-boiling range may contain a considerable
percentage of benzene (Gerarde, 1960). In this review, it is
impossible to consider in detail the effects of benzene, which
basically causes bone-marrow depression and has a leukaemogenic
action. Excellent reports on all aspects of benzene toxicity can be
found in: ILO (1968); Deutsche Forschungsgemeinschaft (1970); IARC
(1974); and Laskin & Goldstein (1977).
In the past, benzene-like effects have also been ascribed to
other low-boiling petroleum solvents. However, there now seems to be
general agreement that these effects only occur, when benzene is
present in the mixture (Browning, 1959, 1965).
(b) Neuropathological effects
The neuropathological effects of inhalation of petroleum solvents
in man have been discussed in a number of reviews including those of
Cavanagh (1973), Allen (1975), Seppäläinen (1975), Comstock (1977),
and Savolainen (1977). The reviews include discussions of methods used
to evaluate human neuropathy.
Over the past 15 years, an increasing number of cases of
poly-neuropathy and other neurotoxic effects have been described in
workers exposed to hydrocarbon solvents. Many of these cases have been
associated with prolonged and repeated exposure to high concentrations
of n-hexane. Frequently, such exposure has resulted from poor
ventilation in a workroom in which solvents containing high
concentrations of hexane have been used. Miyagaki (1967) reported
hexane concentrations in air of 1800-3600 mg/m3 in the workroom and
7200 mg/m3 near the source in a work-place where 17 cases of
peripheral neuropathy occurred. Yamamura (1967) and Inoue et al.
(1970) found concentrations ranging from 1800-9000 mg/m3 in a work
place where, over a 9-year period, 93 out of the 1662 workers suffered
from polyneuritis. These workers used glue in the manufacture of vinyl
sandals for 8-14 h daily, probably for more than 5 days each week,
over long periods, in poorly ventilated rooms. The presence of hexane
in glues is reported to have caused similar incidents in Italy and
Iran (Capellini et al., 1968; Scrima & de Rosa, 1973; Ghazai, 1974).
In most cases, the extent of exposure to hexane was uncertain, either
because air concentrations were not measured or because excessive skin
contamination occurred; workers frequently handled the glues and
washed residues off the skin with hexane-containing solvents. In other
cases, air concentrations were measured. Hexane concentrations of
approximately 2340 mg/m3 (but at times up to 4680 mg/m3) were found
in a small, poorly ventilated workroom in which 3 women, who developed
polyneuropathy, had worked; again excessive skin contact took place
(Herskowitz et al, 1971; Ishii et al., 1972). Glue was also the source
of hexane concentrations of 1000-4000 mg/m3 found by Paulson &
Waylonis (1976) in the air of a work-place where 8 out of 50 employees
developed mild neuropathy.
The main clinical manifestation in these cases was polyneuropathy
of the glove-and-stocking type with sensations of numbness and cold.
This was accompanied by muscular weakness and headaches. Gradual
recovery was usual, when exposure ceased, with the exception of some
subjects who had severe muscular atrophy. Peripheral nerve biopsy
showed demyelination with relative preservation of the axon. Iida
et al. (1969) reported that in 44 cases there was a reasonable, but
not exact, correlation between clinical findings and electromyography
and nerve conduction velocity data.
Optic involvement was observed by Inoue et al. (1970). The
neuro-ophthalmological function was studied by Raitta et al. (1978) in
15 workers who had been exposed at work over periods of 5-21 years to
hexane concentrations of 1800-3600 mg/m3, with occasional levels of
10 800 mg/m3. Defective colour discrimination was found in 12 of the
workers and slight macular changes in 11 out of 15. The visually
evoked potentials and electroretinograms were interpreted by
Seppäläinen et al. (1979) as indicating cerebral dysfunction, probably
a conduction block in intracerebral axons.
Polyneuropathy attributable to the inhalation of hexane has also
been observed in "glue sniffers" -- subjects who inhale solvent
vapours to induce elation or other states of mind. Cases including
very rare cases following medicinal use, were described by Schwarz
(1933), Browning (1965), Karani (1966), Gonzales & Downey (1972),
Matsumura et al. (1972), Taher et al. (1974) and Korobkin et al.
(1975). Other incidents have been reported or reviewed by Shirabe et
al. (1974), and Poklis & Burkett (1977). While hexane appears to have
played a major role, a direct or synergistic action of other
components cannot be ruled out.
Some cases have been described that were thought to arise from
the long-term, continuous domestic use of kerosene stoves in poorly
ventilated rooms (Contamin et al., 1960). Under simulated conditions,
hexane concentrations of about 1440 mg/m3 were produced in
experimental rooms by Lièvre et al. (1967).
The possibility of the production of peripheral neuropathy by
components other than hexane, or the intensification of the activity
of hexane, is suggested by some clinical reports. Cargill (1972)
(cited by Gaultier et al., 1973) described peripheral neuritis in
workers using glue containing cyclohexane, gasoline "c", and methyl
ketone, and Franco et al. (1979) reported sensory and peripheral motor
conduction disturbances, where exposure to cyclohexane had occurred.
The condition was described by Gaultier et al. (1973) in subjects who
had been in prolonged contact with a glue solvent containing 80%
pentane, 5% hexane, and 14% heptane. Gasoline concentrations of
2250 mg/m3 were found in the workshop atmosphere. Atmospheric
gasoline concentrations of up to 5625 mg/m3 (mainly n-pentane,
n-hexane, and n-heptane) were found in workshops where workers had
developed polyneuropathy and had complained of insomnia, irritability,
and other non-specific CNS symptoms. White spirit has also been
implicated as a cause of peripheral neuropathy (Gaultier et al.,
1973). Heavy exposure to jet fuel vapours was reported in workers who
experienced dizziness, palpitations, nausea, and headaches, later
followed by signs and symptoms of polyneuropathy; higher vibration
thresholds, compared with unexposed controls were also found. (Knave
et al., 1976).
Other symptoms besides those of polyneuropathy have been
described in subjects exposed to hydrocarbon solvents. Sterner (1941)
reported headaches, nausea, mental depression, anorexia, inability to
concentrate and sustain activity, and slight anaemia, in workers
exposed to gasoline vapours (used to dilute spray paint) containing
5-10% aromatic hydrocarbons and producing concentrations of total
aromatic hydrocarbons in air of 300-800 ppma. Knave et al. (1978)
compared 30 workers, occupationally exposed to jet fuel, with
unexposed controls. The average period of exposure was 17 years and
the estimated TWA exposure was 300 mg/m3. Significant differences
were found between exposed and unexposed groups in the incidence and
prevalence of psychiatric symptoms, psychological test results,
especially attention and sensorimotor speed, and in
electroencephalograms. Exposure of car painters, over many years, to
low concentrations (31.8% of the Finnish TLV on average) of solvent
mixtures containing toluene, xylene, butyl acetate, and "white
spirit", was found to be associated with an increased incidence of
sleep disturbance, absentmindedness, falling asleep while watching the
television, and headaches. Lower peripheral nerve conduction
velocities, psychomotor impairment, and personality changes were more
common in exposed than in control subjects. Further studies are needed
to elucidate the significance of such findings (Hänninen et al., 1976;
Seppäläinen et al., 1978).
In summary, the most serious adverse neurological effect of
hydrocarbon solvents in man is the production of peripheral
neuropathy. Observations in man and studies on experimental animals
support the view that exposure to n-hexane is the principal cause.
However, the synergistic activity of other hydrocarbons is possible;
such a phenomenon has been reported following the sniffing of a glue
thinner containing both the neuropathic solvent, methyl- n-butyl
ketone, and the non-neuropathic solvent, methyl ethyl ketone
(Altenkirch et al., 1977). At present, the possibility that other
hydrocarbons also have some neurotoxic activity cannot be ruled out.
Peripheral neuropathy has occurred only in conditions of prolonged and
repeated exposure to high concentrations of hydro-carbon solvent
vapour in air; in many cases, there was also excessive skin contact.
Atmospheric exposure levels in excess of 2 g/m3 are usually
encountered where peripheral neuropathy is seen, but more studies are
required to clarify the situation, and until this is done, close
supervision is needed to ensure that the TLV for solvents is not
exceeded. Further studies are also necessary to determine the
significance of psychological disturbances and of neurophysiological
findings in workers exposed to lower levels of the hydrocarbons.
(c) Effects on reproductive functions
Examination of 408 female workers in petroleum refineries, who
had been subjected to long-term exposure to hydrocarbons, hydrogen
sulfide, and other products related to the treatment of sulfurous
crude, revealed disturbances in the menstrual function, mainly in the
form of hypomenstruation and pre-menstrual syndromes. According to
Suhanova & Melnikova (1974), such disturbance of the menstrual
function in female workers in refineries and in persons suffering from
chronic intoxication from petroleum products (36 persons in the age
bracket 30-39 years) is caused by the hypo-functioning of the ovaries.
a Since the composition is not given, it is not possible to
transform this concentration into mg/m3, the adopted SI unit.
Beskrovnaja et al. (1979) studied the gynaecological disease rate
in more than 5000 female operators in plants producing rubber articles
(petroleum solvent vapour concentration in the air of 250-350 mg/m3).
They observed disturbances in the menstrual cycle in workers with more
than 5 years' service and a high frequency of metrorrhagia. As the
period of service increased, a reduction in the frequency of
miscarriages was noticed, which was interpreted by the authors as
possible adaptation. A disturbance of the ovarian function was noted
in 24.4% of the workers examined, mostly in the form of a functional
deficiency of corpus luteum.
Investigations of vaginal smears of 184 female gluers in the
rubber industry in the age bracket 18-38 years revealed a disturbance
of the ovarian function with reduced estrogen stimulation in 21.7% of
the women (10.4% in the control group). This figure was related to the
period of service. After 10 years service, the percentage was twice as
high as after 5 years' service.
Women who had been in contact with petroleum solvents were found
to have a reduced estrogen level in the blood (22.2 µg/day compared
with 29.6 µg/day in the control group). Essentially, no changes were
observed in the excretion of the follicle-stimulating and luteinizing
hormone pregnanediol. The authors of this study assumed that the
sensitivity of the ovaries towards the stimulating effect of the
gonadotrophins was reduced (Hrustaleva et al., 1979).
Novikov et. al. (1979) studied lactation in 332 nursing mothers
288 of whom worked in the rubber industry (vulcanizers, pressers,
gluers). The concentration of petroleum solvents (the physico-chemical
properties of which are not described) in the air of the operating
premises was predominantly 300 mg/m3. Hypolactation, found in 23.8%
of the women compared with 6.7% in the control group was related to
length of service. Hydrocarbon solvents were found in the milk of all
the persons examined (71) in concentrations of 0.50 ± 0.05 mg to
0.60 ± 0.09 mg/litre. The serotonin content of the blood of these
women was significantly lower than in the control group. It is assumed
that hypolactation was the result of the effect of solvents on the
lactation control mechanism via the hypothalamus and the
serotoninergic system.
(d) Effects on the skin
Skin can also be affected by exposure to solvents. In a study on
skin conditions and other diseases in 54 gasoline and diesel station
workers, skin conditions related to exposure to gasoline and diesel
fuel were minimal and did not interfere with capacity to work. Only
dryness, chapping, and reddening of the skin were found. The dark skin
of Indonesians seemed to be more resistant to these effects. The
gasoline vapour in the working environment caused some non-specific
symptoms in addition to those resulting from heavy out-door work
(Suma'mur & Susianti Wenas, 1979).
3.7.2.2 General population exposure
Data are not available concerning the exposure of the general
population to petroleum solvents with the exception of that of
"sniffers" and addicts already mentioned in section 3.7.2.1.
3.7.3 Clinical studies
It should be noted that literature surveys of clinical studies
and clinical effects have been published by Gerarde (1960), Browning
(1965), and Levina (1976).
3.7.3.1 Effects of dermal exposure
In general, petroleum solvents have a defatting action on the
skin and, on repeated contact, cause injury to the horny layer
(section 3.7.1.1). This makes the skin more susceptible to other
irritants, sensitizing agents, and bacteria. It may also result in
progressive dermatitis, characterized successively by dryness,
redness, chapping and scaling, which could lead, by sensitization, to
eczema. These stages of dermatitis may be seen in workers in garages
or automobile repair shops, who wash their hands with solvents, petrol
or kerosene (Tagami & Ogino 1973). The more aromatic solvents, in
particular, can cause a significant degree of primary skin irritation.
The defatting action and primary irritation caused by petroleum
solvents decrease, the higher the boiling range section 3.6.1).
Cases in which gasoline or kerosene remains in contact with the
skin for prolonged periods mainly occur in children or in unconscious
accident-cases, when clothing has become soaked with the solvent. In
such cases, the lesions start with a burning sensation and erythema,
followed by the formation of small or large vesicles, blisters, or
even extensive epidermolysis. The vesicles and blisters may become
mucopurulent in a few days. The acute picture is that of a chemical
burn, (Helbling, 1950; Aidin, 1958; Ainsworth, 1960; Stewart, 1960;
Browning, 1965; Hunter, 1968; Tagami & Ogino 1973).
Gasoline may occasionally be absorbed through the skin in toxic
quantities if large areas of skin such as the hands and forearms are
regularly exposed (Hayhurst, 1936), or in cases of extensive
epidermolysis in contact with gasoline-soaked clothing. However
inhalation of vapour plays a significant additional role in all these
cases and generally is the main route of absorption (Browning, 1965).
3.7.3.2 Effects of inhalation
The acute effect of massive overexposure to gasoline vapour is
mainly narcosis with loss of consciousness and possibly convulsions,
which may be fatal (Browning, 1965). Octane causes rapid and deep
narcosis, pentane and hexane are less powerful narcotics, but they and
heptane exert a paralytic effect on the central nervous system and its
respiratory centre.
In more gradual overexposure, the symptoms just described may be
preceded by eye irritation, irritation of the respiratory tract,
dizziness, headache, and a sense of drunkenness.
A gasoline concentration of 9000 mg/m3 can be breathed without
significant ill-effects by most people but susceptible subjects may
show symptoms after exposure to 1350-2250 mg/m3 (Ainsworth, 1960). At
31 500 mg/m3, dizziness and symptoms of drunkenness may appear.
Exposures in excess of 45 000 mg/m3 soon become intolerable and may
rapidly prove fatal (Machle, 1941, Aidin, 1958). Lower and higher
values have been quoted, obviously depending on the composition of the
gasoline (Browning, 1965). The margin of safety between narcosis and
respiratory arrest is very narrow in exposures to high concentrations
of gasoline (Wang, 1961).
Absorption of gasoline vapour by inhalation may be very rapid if
the concentration is high, especially with the lower members of the
paraffinic range, and symptoms can appear within a few minutes.
Excretion, probably of unchanged vapours, takes place mainly via the
lungs (Browning, 1965).
Acute occupational poisoning by gasoline vapour is mostly caused
by entering unpurged gasoline tanks or other premises where high
concentrations of gasoline may have accumulated. Exposure to high
concentrations may also occur in car accidents, when victims are
trapped and/or unconscious.
Histopathological changes found in subjects who have died after
exposure to high concentrations of gasoline vapour include: hyperaemia
and petechial haemorrhages in the lungs, and, sometimes, necrosis of
the alveolar walls. There may be haemorrhages or effusions in internal
organs and serous cavities (Helbling, 1950; Ainsworth, 1960; Browning,
1965). Liver and kidney may show fatty degeneration. Hyperaemia and
oedema of the brain are common in this condition and myelin swelling
may occur (Machle, 1941).
Long-term exposure to low (unspecified) vapour concentrations may
cause nonspecific symptoms of the nervous system and digestive tract
(Zielhuis, 1961; Browning, 1965; Muhametova & Podrez, 1975; Sehtman et
al., 1979), including changes in liver function and in the visual
organ. In women, the reproductive organs may be affected (Muhametova &
Podrez, 1975; Sehtman et al., 1979). Neurological disturbances, as
described in section 3.7.2.1 may develop (Hayhurst, 1936; Machle,
1941; Browning, 1965). Browning (1965) considered that significant
changes observed in the blood count were caused by the presence of
benzene in the solvent.
This chronic form of toxicity may be found in small,
insufficiently ventilated workshops where solvents, or products
containing them, are handled in an unsatisfactory way (section
3.7.2.1) and when these materials are used in substantial amounts in
enclosed spaces, particularly for cleaning or degreasing (Anon.,
1974).
3.7.3.3 Effects of ingestion
Accidental ingestion of petroleum distillates in the range of
petroleum solvents is an important cause of poisoning in children
(Waring, 1933; Nunn & Martin, 1934; Lesser et al., 1943; Carithers,
1955; Daeschner et al., 1957; Gerarde, 1959, 1963). Most cases,
however, are caused by gasoline and kerosene and fewer by petroleum
solvents. The symptomatology is the same in all cases.
Coughing, choking, and gagging are often noted at the time of
ingestion of these substances. Respiratory embarrassment may be
present early, indicating that aspiration has taken place. Epigastric
discomfort may develop, followed by vomiting with a further risk of
aspiration. Aspiration by one mechanism or another is reported to
occur in up to 95% of cases in children, but this depends on the
situation and on the type (boiling-range) of the solvent involved.
Aspiration is less common in adults, but may occur when trying to
siphon gasoline from a tank. Small amounts of kerosene of the order of
1-2 ml, if aspirated, can cause severe and even fatal pulmonary
changes. On the other hand, if aspiration does not occur, much larger
quantities can be tolerated (Daeschner et al., 1957; Hensen, 1959;
Browning, 1965). Browning (1965) quotes a value of 7.5 g/kg body
weight, but this may depend on the type of solvent. Children appear to
be more susceptible to the toxic effects than adults (Siwe, 1932). In
cases where aspiration does not take place, and especially with the
lower-boiling solvents, central nervous system symptoms may develop
such as lethargy, convulsions, and coma. With smaller doses, the
symptoms include vertigo, headache, and signs of drunkenness. Nausea,
vomiting, and diarrhoea may occur and the stools may be blood-tainted.
In uncomplicated cases, the gastrointestinal symptoms will
disappear within 48 h. Pulmonary symptomatology will not develop, if
aspiration has not occurred and if there was no massive exposure to
vapours.
The syndrome of acute chemical aspiration pneumonitis will be
described in a wider context in section 4.8. Human experience is in
accordance with the results of experimental animal studies (section
3.6.1). Chemical pneumonitis with pulmonary oedema and haemorrhagic
frothy sputum may develop extremely rapidly following aspiration of
petroleum solvents. Roentgenographic changes may be seen within a few
hours, especially at the lung bases. Later, bacterial pneumonia can
complicate the situation (Daeschner et al., 1957; Gerarde, 1963).
The prognosis of aspiration of hydrocarbon solvents appears to
have improved with better treatment in the course of time. Siwe (1932)
reported that 50% of the cases he reported were fatal, Nunn & Martin
(1934) reported 28% mortality following the ingestion of gasoline and
9.2% following kerosene-ingestion, and Blattner (1951) reported that
death resulted in 10-14% of cases of kerosene poisoning. Recently, 3
cases have been described, where exposure to diesel oil seemed to
cause acute renal failure with oliguria (Reidenberg et al., 1964;
Barrientos et al., 1977; Crisp et al., 1979). However, in 2 cases,
absorption seemed to have been mainly through the skin (section
3.5.1).
4. LUBRICATING BASE OILS AND RELATED OILS,
GREASES, AND WAXES
4.1 Properties and Analytical Methods
4.1.1 Chemical and physical properties
Base oils are a limited group of petroleum products in the
boiling-range of 300-700°C, normally derived from the high-vacuum
distillation of the residues of the crude-distilling process. These
oils undergo a further refining process before being used.
Lubricating oils, metal-working oils, and related products are
produced by blending base oils in order to obtain the desired physical
properties. Chemical additives are frequently added, usually in small
amounts (a few mg to a few g/kg) to improve the performance of the
lubricant. In some special cases, higher concentrations are necessary.
Additives belong to the following general categories: viscosity-index
improver, emulsifiers, wetting agents, antioxidants, dispersants,
antiwear additives, extreme pressure additives, rust-inhibitors,
antifoam agents, pourpoint depressants, and germicides.
Greases based on mineral oil consist of solid or semisolid
dispersions of metallic soaps and other thickeners in a mineral oil
base.
Petroleum waxes are crystalline solids at normal temperature.
"Slack wax", a soft, impure paraffin-wax is obtained in the
manufacture of lubricating base oils from paraffinic crude oils by a
dewaxing process. Slack wax can be refined into 2 types of commercial
wax: paraffin wax and microcrystalline wax.
Base oils are very complex mixtures of hundreds to thousands of
different hydrocarbons, containing straight-chain and branched
paraffins, cycloparaffins, naphthenic, aromatic, and polynuclear
aromatic hydrocarbons in the range of C-17 and higher. The separate
molecules are so large, that any molecule may, for instance, contain
one or more aromatic rings with one or more long side-chains.
The actual composition depends on the source of the crude oil,
from which the product is derived, and the manufacturing and treating
processes used. They range from thin, easily flowing "spindle oils" to
thick "cylinder oils". A limited number of base oils are used for
blending to obtain the commercial products. As the relation between
viscosity and temperature is an important factor in this field, base
oil grades are characterized by their viscosity and viscosity index
(VI). The higher the viscosity index, the less the change in viscosity
with temperature.
Low viscosity index (LVI) oils are used whenever the viscosity
temperature characteristics and oxidation stability are of less
importance. In general, they are derived from naphthenic oils and
undergo treatment with sulfuric acid and clay or are given a
hydro-finishing treatment.
Medium viscosity index (MVI) oils, can be used as a base for
general-purpose lubricants. They can be derived from naphthenic (MVIN)
or paraffinic (MVIP) feedstocks. MVIP has to be dewaxed and can also
be solvent refined, acid- and clay-treated or hydro-treated.
High viscosity index (HVI) oils, are prepared by both solvent
refining and dewaxing of paraffinic feedstocks. They are used for
gasoline and diesel engine oils and for turbine lubricants, because
they are, in general, more oxidation-stable than other base oils and
have appropriate viscosity/temperature characteristics.
White oils are generally produced by more drastic refining of MVI
oils, in order to remove unsaturated compounds, aromatic compounds,
and other constituents that influence colour, odour, taste, and
acceptability as food-grade material. Solvent extraction followed by
repeated treatment with oleum and alkali is used. Hydrogenation is
another means of producing such oils.
Medicinal oil is the highest purified grade, which complies with
the requirements of the various national pharmacopoeias and
regulations on food-grade material. As liquid paraffin, the same grade
is used as a lubricant for food-handling machines and as an ingredient
in pharmaceutical preparations and cosmetics.
Technical white oils, less rigidly purified than medicinal oil,
are non-carcinogenic oils that can be used for the lubrication of
textile machinery, spinning mules, etc., but are mainly used in the
cosmetic industry in the manufacture of hair-oils, creams, etc.
Aromatic extracts, which are obtained in the solvent-refining
process, have already been discussed in section 3.1.1.
Petroleum waxes consist essentially of high relative molecular
mass paraffinic hydrocarbons with approximately 20-40 carbon atoms per
molecule. Paraffin waxes consist mainly of normal paraffins together
with some iso and cycloparaffins. They are macrocrystalline with a
melting-point of 43-71°C.
Microcrystalline waxes on the other hand, consist mainly of iso
and cycloparaffins with some alkylated aromatic hydrocarbons. They are
mostly soft materials, but may sometimes be a hard brittle solid.
When highly purified, the colour may be white, however, it is
usually yellow or amber, sometimes even black. The melting-point is
60-90°C. The paraffin oil content ranges from 5 to 50 g/kg.
Petrolatum is also known as petroleum jelly and is a
micro-crystalline wax with a high oil content.
4.1.1.1 Purity of Product
As these oils and waxes are complex mixtures, the nature and
proportion of potentially undesirable "impurities" depends to a large
extent on the definition of the term and on the degree of refining. In
the range from the highest refined oil -- for example, white medicinal
oil -- to the least refined oil, "impurities" could include
polynuclear aromatic hydrocarbons and unsaturated hydrocarbon
compounds.
It is clear that highly purified products can be obtained at the
cost of extra refining and treating processes. Paraffin waxes are an
example of progressive refining: fully refined paraffin wax is a white
solid material containing an oil concentration of less than 5 g/kg. It
is odourless and tasteless and has a melting-point of 50-70°C. Candle
waxes range from fully refined paraffin wax to less refined waxes
containing oil levels of up to 15 g/kg. This type of wax is not
completely colourless and odourless. Scale wax and match wax contain
an oil residue of up to 30 g/kg.
4.1.2 Methods of sampling and analysis
See section 2.1.2.
4.2 Sources of Environmental Pollution
4.2.1 Natural occurrence
Base oils occur in nature only as components of the crude oils
from which they are derived.
4.2.2 Man-made sources
4.2.2.1 Production
As mentioned earlier, lubricating base oils are a group of
petroleum distillates and residues in the boiling-range 300-700°C,
derived by high-vacuum distillation of the residues obtained in crude
oil distillation (section 4.1.1). In order to obtain base oils with
the qualities and specifications needed for various applications,
further refining and treatment of the various distillate fractions is
needed. The main processes used are:
(a) Solvent extraction
This is a process by which aromatic hydrocarbons can be extracted
from oil fractions, thus obtaining a low aromatic or aromatic-free
raffinate, and a high aromatic extract. Liquid sulfur dioxide,
sulfolane, benzene, phenol, or furfural can be used as solvents for
this purpose. The raffinate can be used for producing a lubricating
base oil, the extract can be used as a solvent (see under petroleum
solvents).
(b) Dewaxing process
In this process, "slack wax" is separated (crystallized) from
base oil fractions obtained from paraffinic crude oil residues. This
is done by cooling, followed by filter-pressing. The separation of wax
and oil under chilled conditions is facilitated by the addition of
solvent. The resulting wax may still have an oil content of up to
100-400 g/kg.
(c) Acid treatment
The oil is mixed with 98% sulfuric acid and the acid sludge is
centrifuged off. This is usually followed by clay treatment in which
the oil is mixed with absorbent clay, followed by filtration. This
neutralizes the oil and improves colour, odour, and stability.
(d) Hydrofinishing
This is a catalytic hydrogen treatment at elevated temperature
and pressure. Improved stability and colour of the oil is obtained by
the hydrogenation of unstable compounds, which removes sulfur and
saturated olefins, diolefins, and aromatic components.
As the definition and classification of lubricating base oils are
not clear, it is difficult to give precise production data for this
group of products. The best current estimate is 35-38 million tonnes
per year. Though there was a decrease in the annual production after
1973, lubricant production has subsequently increased, but at a lower
rate.
4.2.2.2 Uses
In general, the main functions of lubricating oils and
metal-working oils are: to reduce friction, to remove generated heat,
to remove debris from the contact area, and to protect against
corrosion. In addition, mineral oils are used as hydraulic media in a
wide variety of applications. Very general remarks have already been
made concerning the composition of, and the various additives in,
lubrication oils, metal-working oils, and greases. The following is a
list of the most important categories of products on the market, from
which it is clear that the great majority are handled in industrial
situations, and that the general population has regular contact with
only a few such products:
(a) Industrial lubricating oils, which can vary from thin spindle-oil
to the very viscous oils used in steam engines;
(b) Lubricants for internal combustion engines of various types;
(c) Crankcase, compressor, gear, and turbine oils;
(d) Greases for bearings and other purposes;
(e) Hydraulic, transformer, insulating, heat-transmission oils;
(f) Cooling, quenching, anticorrosion, and mould oils;
(g) Metal-working oils: cutting, grinding, rolling, drilling,
drawing-oil, a multitude of products for specific purposes. They
consist in general of complex mixtures with various additives,
for use in the neat state and also as water extendible fluids or
emulsions;
(h) Textile oils: spindle, hatching, technical white lubricating oil;
(i) Process oils: used in printing-inks, as rubber extenders, and as
technical white oils in cosmetics, etc;
(j) Waxes of various grades and purity;
(k) Medicinal white oil used for medicinal and food-grade
applications.
The manufacturing, treating, and purification processes used in
the preparation of the base oils vary according to the future
application of the commercial product.
Many of the products can undergo considerable changes during use.
This should be borne in mind in extrapolating from used oils to the
original product in the case of adverse health effects, e.g., in the
case of skin sensitization. The following are a few examples of
changes that may occur:
(a) During use, the chemical and physical characteristics of
lubricating oils change, mainly because of contamination, oxidation,
and polymerization. Metal particles, airborne dust, water, and, in the
case of internal combustion engine oils, small quantities of fuel,
acids, and soot, are the main contaminants. All this can result in the
formation of sludge.
(b) Metal-working oils become contaminated in use with a wide
variety of foreign matter, in particular metal particles (and ions).
Water-based metal-working emulsions can also be affected by bacteria,
yeasts, and fungi. Oxidation and heat cracking may occur at the
application point, causing smoke, steam, and oil mist to be emitted
into the working environment. Nitrite may be added to most water-based
cutting oils, thus these sometimes contain carcinogenic nitrosamines
(Zingmark & Rappe, 1977).
Cutting oils, lubricating oils, and greases can also contain
diethanolamine, which may react with nitrites and nitrogen oxides in
the air to form nitrosamines.
(c) Quenching oils are used for hardening steel. Oxidation and
cracking of the oil can occur because of the high temperatures
involved. The amount of benzo (alpha) pyrene in lubricating oils
increases considerably when they are exposed to heat during use, as in
motor-car engines, metal quenching, and other processes (Thony et al.,
1975).
4.2.2.3 Disposal of waste
Without a proper method for the disposal of used lubricating and
other oils, severe environmental contamination and hazards may occur.
There is a vast literature on methods for the disposal, reuse,
and recovery of industrial lubricants and much research is being done
in this field. It would be out of context in this document to go into
a detailed discussion of the various aspects. For this, the reader is
referred to Concawe Report 9/73 (Concawe Task Force, 1973) and API
publication No. 4036 (API, 1969), which contain discussions on the
situations in Western Europe and the USA, respectively, as well as on
more general aspects.
At present, the main ways of disposal are:
(a) dumping in sewage systems, waterways, etc; this is illegal in
many countries and ecologically undesirable;
(b) dumping into the ground, in garbage dumps, in dry wells, if
approved by local authorities;
(c) using as road oils, etc;
(d) controlled incineration or burning for heat value;
(e) re-refining and other recycling methods.
4.3 Environmental Exposure Levels
Specific data are not available concerning levels of this group
of products in air, water, food, or other environmental media, levels
of possible contamination, and uptake by man.
Depending on occupation and on the hygiene precautions adopted,
skin exposure in workers can vary from minimal to very high. No
systematic measurements have been carried out in this field. Exposure
to oil mists occurs in certain manufacturing processes and
applications, such as the operation of automatic lathes. This type of
exposure has been measured by various authors and will be discussed in
section 4.7.1.2. Secondary oral intake of unknown quantities of
mineral oil can take place under these conditions.
When "sulphofrezol" (approximate composition: 50-60% "goudron"
(?flux oil), 40-50% spindle oil distillate, and 1.7% sulfur) was used
as a lubricating and cooling liquid on metal-working lathes, the air
of the operating premises was found to contain an oily aerosol in
concentrations of 1-50 mg/m3, hydrocarbons at 26- 150 mg/m3, carbon
monoxide at 8-12 mg/m3, formaldehyde at 0.05-1.2 mg/m3, sulfur
dioxide at 2-20 mg/ms, and benzo(alpha)pyrene at 0.01-0.2 mg/m3.
When multicomponent lubricating-cooling liquids containing chlorine
and fluorine compounds are used as additives, hydrogen chloride and
other substances may also find their way into the workroom air. It is
therefore essential to provide the metal-working lathes with
vapour-extracting facilities (Medved' et al., 1976).
4.4 Environmental Distribution and Transformation
Data on lubricating base oils concerned with distribution among
media, environmental transformation and degradation, interaction with
physical, chemical, or biological factors, and bioconcentration are
not available. However, as mentioned in section 3.4, there is a lot of
information concerning the microbial degradation of individual
petroleum hydrocarbons. For example, it has been shown that certain
polynuclear aromatic hydrocarbons in lubricating oils can be oxidized
by bacteria; benzo(alpha)pyrene and benzo(alpha)anthracene have been
shown to be oxidized to cis-dihydrodiols. The oxidation is not thought
to reach the stage of carcinogenic activation found in mammals, as
this would result in a trans-configuration (Gibson et al., 1975).
4.5 Metabolism
Because of the complex and variable composition of compounds in
this group, only a few factual data relating to their metabolism can
be given.
The skin barrier is permeable only to hydrocarbons of a certain
relative molecular mass and structure. For paraffinic substances this
appears to be up to 20 carbon atoms. However, from their studies on
guineapigs, Hoekstra & Phillips (1963) reported that, because aromatic
compounds have a more compact structure, compounds in this group with
a higher number of carbon atoms might still pass through the skin
barrier.
In studies on mice, rats, hamsters, rabbits, and dogs, mineral
oil droplets were phagocytosed in the lungs and clinical observations
in man have shown that deposition takes place in the hilar nodes, from
where they may be transported to other organs such as the spleen
(Sante, 1949; Proudfit et al., 1950; Shoshkes et al., 1950; Wagner et
al., 1964). Almost all (95-99%) of ingested food-grade mineral oil
leaves the body unchanged in the faeces, 1-5% being absorbed as such
via the intestinal mucosa. Phagocytosis may play a part in this. The
ingested part of the oil is transported throughout the body via the
lymphatics and the bloodstream. Storage takes place in adipose tissue
or in the fat in organs. After excessive exposure, mineral oil
droplets have been identified in mesenteric and portal lymph nodes,
and also in liver, spleen, and adipose tissue in man (Stryker, 1941;
Ebert et al., 1966; Boitnott & Margolis, 1966b).
Data relating to food-grade mineral oil have been reviewed in WHO
(1974). Very few data are available on the biotransformation and rate
of elimination of this group of products but data concerning some of
the components are referred to in section 3.5.
Injections of 0.5 ml of various mineral oils were made in the
peritoneal cavity of mice and the oil recovered after 7-24 days (Twort
et al., 1937). Both the refractive index and density of the oil had
decreased proportionally, probably as the result of chemical
transformation in the body. Treatment of mineral oil with sulfuric
acid and clay, or solvent extraction had a similar effect. The solvent
extracts, however, showed increased refractive indices and densities.
The authors suggested that both parameters might be useful indicators
of the carcinogenicity of the mineral oil.
4.6 Effects on Experimental Animals
4.6.1 Short-term exposure
Data are not available concerning the LD50s of base oils but the
oral and dermal LD50 data available for commercial lubricating oils
indicate that, in general, these products are only moderately or
slightly toxic.
Acute no-observed-adverse-effect levels have not been determined.
Oral studies with food-grade mineral oils show that these are
laxatives. The same applies to non-food-grade commercial mineral oils,
though it is impossible to predict whether in a certain case a toxic
or laxative effect will prevail.
4.6.1.1 Effects of dermal exposure
More factual information is available on the acute or short-term
effects of mineral base oils on the skin. In a very thorough study
Hoekstra & Phillips (1963) examined the effects of mineral oils and
their fractions on the skin of guineapigs. It appeared that very
short-chain paraffins had a mainly defatting action on the skin and
that the effects of longer-chain and aromatic hydrocarbons were
closely related to the permeability of the skin to these compounds. We
can do no better than cite the summary of this work:
"A number of light mineral oils applied topically to the skin of
guinea-pigs caused a marked epidermal hypertrophy, hyperplasia,
hyperkeratosis, and subsequent depilation. This dermatoxic effect
could not be closely related to source of the crude oil or its
viscosity, degree of refinement, or the acid used in refinement.
" n-Paraffin, isoparaffin, naphthene and aromatic fractions
separated from light mineral oil each produced the dermatoxic effect
as did highly purified individual paraffins from C-12 to C-18. Oleic
acid caused only a slight dermatoxic effect. Fractional distillation
of an aromatic-free mineral oil demonstrated that while all
lower-boiling fractions were dermatoxic, a distillation range was
reached at which the fractions were innocuous to the skin. This was
also true for n-paraffin-, isoparaffin-, monocyclic naphthene-, and
polycyclic naphthene-rich fractions derived from a mineral oil.
Fractional distillation of the aromatic hydrocarbons from mineral oil
representing the same distillation range did not yield fractions
without skin-damaging effect. Crude estimates of the molecular size of
the n-paraffins, isoparaffins, monocyclic naphthenes, and polycyclic
naphthenes by comparison with the boiling-temperatures of known,
homologous series of hydrocarbons indicated that the maximum skin
damage resulted from hydrocarbons of about 14 to 19 carbon atoms. The
transition point to non-dermatoxic hydrocarbons occurred at about
21-23 carbon atoms. This was verified with purified n-paraffins.
Variations may exist for the different types of hydrocarbons.
"Simultaneous application of the innocuous "higher-boiling"
mineral oil fractions with dermatoxic "lower-boiling" fractions or
with hexadecane greatly reduced or eliminated the skin-damaging
effects. The alleviation of the skin damage from aromatic fractions by
simultaneous application of the "higher-boiling", non-aromatic
fractions was much less pronounced.
"The hyperkeratotic reaction of the skin to petroleum
hydrocarbons appears to he a very general response to lipid solvents
and is not related to any specific reactive group or type of
structure. Molecular size seems to be very important in determining
the dermatoxic properties of hydrocarbons, the larger molecules being
innocuous. Under the conditions of the experiments the very
low-molecular weight hydrocarbons also had little or no dermatoxic
properties, presumably because of their volatility. It is postulated
that the skin barrier is permeable only to hydrocarbons of a certain
maximal effective size and that penetration into or through the
barrier is essential for the initiation of the hyperkeratotic
response. The alleviation of the dermatoxic effect of hydrocarbons by
admixture with innocuous, higher molecular weight hydrocarbons, is
likewise explained by a reduction in penetration of the dermatoxic
component through the Skin barrier" (Hoekstra & Phillips, 1963)
4.6.2 Long-term exposure
Most long-term studies with mineral oils have been concerned with
the carcinogenicity of the compounds. Data concerning mutagenicity,
embryotoxicity, and teratogenicity are lacking.
4.6.2.1 Carcinogenic effects
The carcinogenic activity of certain polynuclear aromatic
hydrocarbons is attributed to electronic structural features of the
molecule. The various theories on these mechanisms are discussed in
detail by Arcos & Argus (1974), Bergel (1974), and Jerina & Daly
(1974). Carcinogenic polynuclear aromatic hydrocarbons have regions of
high-electron density in their molecules, the so-called K-regions that
are readily epoxidized by tissue mixed-function oxidases and more
specifically by the enzyme aryl hydrocarbon hydroxylase.
Polynuclear aromatic hydrocarbons are in fact precarcinogens,
which, though harmless in themselves, are metabolized in the body, by
the combined action of mixed function oxidase and epoxide hydratase
(4.2.1.63), to biologically reactive intermediates that constitute the
ultimate carcinogens (Sims et al., 1974). The enzymes responsible are
active in the liver, and in other organs such as the skin, lungs, and
intestines.
Whether, in fact this metabolism leads to detoxication or to
"lethal synthesis" may differ with various polynuclear aromatic
hydrocarbons and may depend on many other factors (Anon., 1975). The
short-lived epoxides resulting from the metabolism of at least some of
the polynuclear aromatic hydrocarbons are probably the activated
metabolites with the real carcinogenic effects.
Evidence is now accumulating that it is the 7,8 dihydroxy-
benzpyrene - 9,10 oxide of 3,4 benzo(alpha)pyrene that is the
ultimate carcinogen and, consequently, this has now superseded the
K-region epoxide in biological importance with regard to
carcinogenicity (Yang et al., 1976; Thakker et al., 1977; Yagi et al.,
1977; Koreeda et al., 1978). The enzyme aryl hydrocarbon monooxygenase
(according to recent international rules of nomenclature) in the case
of 3,4 benzo(alpha)pyrene, is referred to as benzopyrene
3-monooxy-genase (EC 1.14.14.2) (de Pierre & Ernster, 1978).
There are indications that the tissue concentrations of the
enzyme aryl hydrocarbon hydroxylase vary considerably with the age,
sex, species, strain, and environment of the animal (Nebert & Gelboin,
1969). Outside agents, such as polynuclear aromatic hydrocarbons, can
induce the activity of this enzyme (Wattenberg, 1972). It seems that
the inducibility depends on genetic factors in both mice and human
subjects (Nebert et al., 1972; Kellerman et al., 1973). In mice, it
seems to depend on a single dominant autosomal gene (Nebert et al,
1972).
4.6.2.2 Effects of dermal exposure and subcutaneous
administration
Leitch (1922) repeatedly painted crude shale oil on the skin of
mice. He obtained skin tumours in a substantial percentage of the
mice. Similar tests were carried out by Twort & Ing (1928) and Twort &
Twort (1931) using a range of crude oil as well as more refined
products of both shale oil and petroleum oil. They found that
petroleum oils had a lower and sometimes negligible carcinogenic
activity compared with shale oils. Naphthenic oils were less active
than oils with a high aromatic content. The more potent fractions
could be extracted with solvents from the oils.
Acid treatment of the oils decreased the carcinogenic activity
and heavier-grade oils were less potent than spindle oils.
Oils with a boiling-point above 370°C, derived from fluid
catalytic cracking, appeared to be carcinogenic to the skin of mice.
The most potent fraction distilled between 430 and 550°C, and the
carcinogenic activity was contained in the aromatic components of
these oils.
Woodhouse (1934) compared the carcinogenicity of sulfur-
dioxide-solvent-extracts of spindle distillates, derived from
crude oils from different parts of the world, with that of the
unrefined spindle oils and coal-tar, using a mouse-skin-painting
technique. He found that in all cases, the carcinogenic activity,
which was more or less characterized by its UV-fluorescence spectrum,
was concentrated in the solvent extract. Potency varied according to
the source of the crude oil. The potency of the most active extract,
however, was lower than the activity of coal-tar. By thorough
sulfur-dioxide-solvent refining, the carcinogenicity of the spindle
oil was almost completely removed. The same author (Woodhouse, 1950)
reported studies on the carcinogenic activity of various petroleum
fractions and extracts. Results were in line with his previous
findings and with those of other workers. Three white oils including
medicinal liquid paraffin gave negative results.
More recent studies have been aimed at identifying the
carcinogenic substances.
Dilution of carcinogenic oils with non-carcinogenic oils more
than proportionally reduced the carcinogenic activity, which at times
disappeared completely. Mild hydrogenation or treatment with active
absorbents reduced, but did not abolish carcinogenicity. Mild acid
treatment, on the other hand, did not have such an effect (Smith et
al., 1951). Eight samples of unrefined "slack waxes" obtained from
pressing operations -- still containing 12-29% of oil -- were painted
3 times a week on the skin of white mice (Smith et al., 1951).
Aromatic extracts, obtained by the solvent refining of these waxes,
were examined in the same way. After skin-painting for almost the life
span of the animals, some benign rumours were found with all samples
and in a few cases, skin carcinomas developed. The aromatic extracts,
however, were much more active. The authors concluded that the
aromatic components which were removed by further refining of the
waxes, were the cause of the carcinogenic activity of "slack waxes".
Fischer et al. (1951) described methods for the determination of
polynuclear aromatic hydrocarbons: the ultraviolet absorption method,
the caffein extraction method, the chromatographic ref-ractometric
method, and the maleic anhydride method. Levels, determined by any of
these methods, were fairly well correlated with the tumorigenic
potency of these oils as established in animal experiments. These
analytical techniques are relatively simple and much more rapid than
long-term animal experiments. They are considered to have some
predictive value. A further development is the DMSO
extraction/refractive index method.
The correlation between the analytical data and the carcinogenic
activity in skin-painting studies on mice is now under investigation
(Shell Toxicology Laboratory -- private communication) and encouraging
results have been obtained with the lower- and higher- range
polynuclear hydrocarbons. The compounds in the middle range are still
under investigation.
Auld (1940) considered that it was not the general
UV-fluorescence that was characteristic of carcinogenicity but the
pattern of the spectrum and its classification. However, in a report
by the Analytical Subcommittee of the Institute of Petroleum
(Catchpole et al., 1971a), it was concluded that, at the time of the
report, there was no suitable, easy and quick method for determining
either the total polynuclear aromatic hydrocarbons or individual
carcinogens in them.
The tumorigenic activity of various concentrations of a large
number of pure aromatic hydrocarbons was examined in different strains
and species of animals by Hartwell (1951), who found that the potent
substances contained 4,5, or 6 condensed aromatic rings with
relative-molecular masses ranging from 230 to 320.
Cook et al. (1958) tried to isolate individual carcinogens from 3
Kuwait crude oils by fractional distillation and various treatment
processes. They tested the fractions obtained on the skin of mice and
rabbits and found that more of the carcinogenic activity was contained
in the fraction distilling between 350 and 400°C and that this
activity was associated with the aromatic constituents. Solvent
extraction with aqueous acetone or furfural removed the activity,
which passed into the extract (20% of the original fraction). Several
polynuclear aromatic hydrocarbons such as di-, tri-, and tetramethyl-
phenanthrenes, tetramethylfluorene, 1-methylpyrene, 1,2-benzofluorene
and 8-methyl-1,2-benzofluorene could be identified in these extracts
as well as some complex organic sulfur compounds such as
dibenzothiophen derivatives and polycyclic thiophens, a number of
which are known to have carcinogenic activity. Pentamethyl-carbazole
was identified as an example of a complex organic nitrogen compound.
Similar findings were obtained by Bogovsky et al. (1960) on
fractionating Estonian shale oil.
Approximately 100 fractions were separated from a catalytically
cracked oil by various techniques (Tye et al., 1966). Selected
fractions were chemically analysed and assayed for carcinogenicity by
repeated application to the skin of C3H mice. In the highest-boiling
fractions (438-455°C), the major carcinogens were 4 and 5 ringed
polynuclear aromatic hydrocarbons, benzo(alpha)pyrene as a
characteristic carcinogen was present at a concentration of 0.4%. In
the intermediate boiling range (404-438°C), unsubstituted and
methyl-substituted 4-ringed polynuclear aromatic hydrocarbons formed
the major carcinogens, benz (a)anthracene and its alkyl homologues
being present at a concentration of 0.4%. In the low-boiling fraction
(339-404°C), assorted smaller molecules including alkyl-3-ringed
polynuclear aromatic hydrocarbons were present.
Benzo (c)phenanthrenes, the most dominant carcinogens in this
fraction, were present at a concentration of 0.01%. The carcinogenic
potency in the bioassay was found in the intermediate- and
high-boiling fraction (boiling-range 404-455°C); fractions boiling at
under 349°C did not appear to be carcinogenic.
Generally, where the alkyl-substituted 4-6 condensed-ring
polynuclear aromatic hydrocarbons were concerned, the longer the
side-chain, the lower the carcinogenic activity (Auld, 1950).
These findings were confirmed by the United Kingdom Medical
Research Council (1968), who reported similar studies. Uncracked crude
oil fractions boiling at above 350°C, which had the highest
carcinogenic activity in skin tests on mice and rabbits, contained the
same or similar polynuclear aromatic hydrocarbons; no other new
carcinogens were found. After solvent extraction, the carcinogenic
activity of the extract was much higher than that of the raffinate,
which was very low.
The carcinogenicity of individual polynuclear aromatic
hydrocarbons will not be discussed in this document. For this, the
reader should refer to IARC (1973).
The fact that various cracking processes considerably increase
the contents of polynuclear aromatic hydrocarbons in mineral oils has
been demonstrated by Kennaway (1925), Twort & Fulton (1930), Twort &
Twort (1935), Pates (1952), and Dietz et al. (1952). Kennaway (1925)
discovered that skin cancer of mice could be produced with synthetic
tars obtained by pyrolising substances like acetylene, isoprene,
yeast, human skin, and a non-carcinogenic petroleum. Badger (1962)
pyrolysed a whole range of aliphatic and simple aromatic hydrocarbons
at 700°C. It was concluded from an analysis of the resulting tars
that, at high temperatures, polycyclic aromatic hydrocarbons were
formed from simpler hydrocarbons via primary radicals formed by
carbon-hydrogen and carbon-carbon fission. The same process occurred
at 550°C, but to a much lesser extent.
Skin tests on C3H-mice using 15 base oils with known content of
polynuclear aromatic hydrocarbons, which, in some cases, only differed
in theft refining history, revealed that solvent-refining removed the
carcinogenic components to such an extent that none of the oils
treated in this manner induced carcinomas (Bingham et al., 1965).
Solvent extraction was carried out with the usual solvents including
phenol, cresol, and furfural. Conventional acid and clay treatment
only removed certain of the polynuclear aromatic hydrocarbons and all
oils treated in this way still retained some carcinogenic activity. In
these tests, a general correlation was again found between the content
of 4 and 5 ring polynuclear aromatic hydrocarbons and the
carcinogenicity of the base oils examined.
Catchpole et al. (1971b) compared mass spectrometry analysis of
an untreated distillate with its solvent-treated and its hydrogenated
derivatives. The decrease in polynuclear aromatic hydrocarbons
thiophenes, and sulfur in both treated samples compared with the
untreated sample was quite marked. Smaller differences were found
between the levels in the two refined samples.
In studies by Boehme & Huehnermann (1966), the relative amounts
of aromatic compounds present in some of the more purified mineral oil
products were determined from their UV absorption spectra. The
relative concentrations were:
medicinal white oil 1
paraffin waxes 100
micro crystalline waxes 300
white petrolatum 1000
yellow petrolatum 10 000
The carcinogenicity of a sample of amber petrolatum was tested on
the skin of mice. A 15% solution of the petrolatum in iso-octane, did
not show any significant carcinogenicity. The solvent-extracted
aromatic fraction (1.2% of the original material) tested in iso-octane
solution at 50 times its concentration in the petrolatum did not
produce any carcinogenic effects on mouse skin (Lijinski et al.,
1966). Studies are reported by Oser et al. (1965) in which 3 kinds of
pharmaceutical and food-grade petrolatum were administered to mice in
the form of a single subcutaneous injection of 100 mg of petrolatum
per mouse. Observations over 18 months did not reveal any carcinogenic
or other toxic effects.
Liquid paraffin was found to be non-carcinogenic in long-term skin
testing on mice by Twort & Twort (1931) and Woodhouse (1934). White
mineral oil was also found to be non-carcinogenic in mice after
long-term skin application (WHO, 1974).
Shubik et al. (1962) examined 36 samples of petroleum wax. Eight
samples contained identifiable polynuclear aromatic hydrocarbons; the
highest concentration was 0.64 mg/kg. The results of skin and
subcutaneous testing follow in the author's words:
"Five petroleum waxes were tested by repeated skin application in
benzene solution to mice and rabbits. In addition one of the test
waxes was fractionated and its aromatic and non-aromatic components
were tested separately on mice, also by repeated skin applications.
Solvent-treated controls were kept. No carcinogenic effects were
detected. Five petroleum waxes were tested in mice by subcutaneous
implantation in disc form. Fibrosarcomas developed around the implants
with incidences correlated to the melting points of the waxes. In
addition, one of the test waxes was fractionated and its aromatic and
non-aromatic components tested separately; the same wax was also
tested subcutaneously in powdered form. The findings indicate that the
subcutaneous sarcomas occurred as a result of the physical rather than
the chemical properties of the materials."
From the various studies, it would seen that there is no relation
between the carcinogenicity of a lubricating base oil and its
potential to cause dermatitis.
In all the previously mentioned studies and others by Horton et
al. (1963) and Bingham & Horton (1966), there is general agreement
that the carcinogenicity of a mineral oil is largely related to the
aromatic fraction with a boiling-point above 370°C, and more
particularly to the polynuclear aromatic hydrocarbons containing 4-6
condensed benzene rings. However, some long-chain, aliphatic,
alicyclic, and alkyl-aromatic hydrocarbons from a lower boiling-range
with 10-20 carbon atoms, such as n-dodecane, cyclohexyl-decane, and
dodecyl-benzene may act as accelerators or co-carcinogens. Though
completely non-carcinogenic themselves, they were found to increase
tumour incidence and reduce the time of appearance, when carcinogenic
fractions dissolved in them were applied, to the skin of mice (Horton
et al., 1963; Bingham & Horton, 1966). However, in order to act as a
cocarcinogen, n-dodecane must be present in a concentration of
20-30% or more. Such solutions in accelerating solvents show an
unusual capacity to spread upon the skin (Horton et al., 1957, 1965).
In a further study on the effects of co-carcinogenic compounds on
the carcinogenic action of benzo(alpha)pyrene and benz (a)anthracene
on the skin of mice (Bingham & Falk, 1969), the co-carcinogenicity of
n-dodecane was confirmed. However, when the authors claim "that
there is a 1000-fold increase in the enhancement of potency of low
concentrations of benzo(alpha)pyrene and benz (a)anthracene when
dodecane is the diluent", this statement may not be fully justified by
the results obtained in relatively small numbers of animals per test
group. Nevertheless 50% n-dodecane in the solvent decaline doubled
or trebled the tumour incidence in the highest dose-group, and tumours
were found at much higher dilutions than when decaline alone was used
as a solvent. When 2-dodecanol and 2-phenyldodecane were used in
various concentrations in the solvent of benzo(alpha)pyrene, higher
concentrations of these 2 substances shortened the interval of
appearance of tumours rather than increasing the total incidence of
rumours.
From this it would appear that these accelerators would not have
any effect, when present in an intrinsically non-carcinogenic oil, and
that they would only have an effect in other oils, when present in
appreciable concentrations. This effect seems to be due, at least in
part, to the spreading power of the solvent causing more intimate
contact with a greater skin area. On the other hand, a more plausible
explanation could be an increase in P-450 enzyme induced by dodecane,
which possibly does not occur with 2-dodecanol and 2-phenyldodecane.
3,4 Benzo(alpha)pyrene can induce P-450 microsomal mixed function
oxidase and this can also catalyse arylhydrocarbon (AH) monooxygenase
activity (de Pierre & Ernster, 1978). The increase in P-450 induced by
dodecane will accelerate and increase the formation of the ultimate
carcinogen 3,4 benzo(alpha)pyrene and thus increase the total
incidence of tumours. However, these substances will not normally be
present in base oils and related products, because they fall into a
different boiling range.
Apart from this, some frequently used additives - which in
themselves are non-carcinogenic - such as elemental sulfur and some
sulfur-containing compounds such as benzyldisulfide, ditertiary
butylpolysulfide and ditertiary octylpolysulfide (Horton et al., 1965;
Bingham et al., 1965; Bingham & Horton 1966) as well as certain
phenols (Boutwell & Bosch 1959) have also been shown to enhance the
carcinogenic activity of polynuclear aromatic hydrocarbons on the
mouse skin. Addition of sulfur to a non-carcinogenic oil did not, of
course, have any effect on the carcinogenicity (Bingham et al., 1965).
Baldwin et al. (1964) reported that additives such as lead naphthenate
did not have an enhancing effect and there are indications that other
components of complex mineral oils with a boiling-range above 370°C
act as inhibitors of the carcinogenic effect of polynuclear aromatic
hydrocarbons, e.g., the saturated (probably cyclic) hydrocarbons
(Bingham & Horton, 1966).
Sunderland et al. (1951) reported skin tests of mineral oil
fractions on mice, under various conditions. They found that washing
the skin with soap and water, after the application of the oil,
reduced both the number of tumours and the rate of appearance. The
reduction was related to the length of the interval between oil
application and washing. Painting once, instead of twice, weekly
greatly reduced the rate of tumour appearance.
From human experience (section 4.7.1.1), it is known that the
ultraviolet radiation of sunshine has a potentiating effect on the
carcinogenicity of mineral oils. Similar indications exist from animal
studies (Emmett, 1975). It has also been demonstrated that certain
co-carcinogenic n-alkanes may increase the carcinogenic potential of
certain wavelengths of UV light on mouse skin (Bingham & Word, 1977).
In comparative studies with various mineral oil fractions on the
skin of mice and rabbits, it became apparent that the rabbit skin was
more sensitive than the mouse skin according to the type, number, and
growth rate of tumours (Cruickshank & Squire 1950; Hieger & Woodhouse
1952; Antonov & Lints 1960; UK Medical Research Council, 1968). In
some instances, mineral oil fractions were non-carcinogenic to mouse
skin, but positive on the skin of rabbits (Shubik & Saffiotti), 1954).
While, rats and guineapigs are apparently less sensitive than mice
(Sugiura et al., 1949; Desoille et al., 1973) Rhesus monkeys appear to
be fairly sensitive (Sugiura et al., 1949).
As to the suspected causes of intra- and inter- species
differences in susceptibility to polynuclear aromatic hydrocarbons,
the levels of the enzymes forming and detoxifying the biological
reactive intermediates, the ultimate carcinogens, in the tissue of the
various strains and species might be one of the important factors
(section 4.6.2.1). Clear intra-species differences in tumorigenic
response have been reported by, for example, Smith & Sunderland (1951)
and Gilman & Vesselinovitch (1955).
In addition to these studies on "pure" mineral oil fractions and
various additives, some studies with metal working oils and textile
oils have been published.
Two cutting oils based on sulfurized mineral oils, were diluted
with water and were tested in various concentrations on the skin of 3
strains of mice. Both were found to be carcinogenic. A marked
reduction in incidence and an increase in induction time of tumours
was found at a dilution of 1:8 compared with 1:4 (Gilman &
Vesselinovitch, 1955). The same authors (Gilman & Vesselinovitch,
1956) compared a straight cutting oil and a water-soluble cutting
fluid in 2 different strains of mice. They found a consistent,
comparable but low incidence of skin tumours in 3 separate trials.
In a later study, 3 commercial additive-containing cutting oils,
one of which was an emulsifiable oil, were repeatedly applied to mouse
skin for up to 31 weeks. Carcinogenic skin changes were observed with
all these oils but were, possibly, less marked with the emulsifiable
oil. In addition to these carcinogenic effects, focal necrosis of the
liver associated with amyloid deposition and amyloidosis of the skin,
spleen, and kidneys were observed. The additives may have had a
contributory effect in some of the pathological changes observed
(Jepson et al., 1977).
In the case of cutting oils, the temperatures to which the oils
are exposed at the cutting edges of the tools are such that cracking
of the oil might conceivably occur and theoretically a
non-carcinogenic cutting oil might become carcinogenic during use. An
unspecified cutting oil from the sump of a machine was tested on the
skin of mice and rabbits. It induced benign tumours in rabbits only
(Cruickshank & Squire, 1950). As the fresh oil was not examined, no
conclusion can be drawn concerning the previously-mentioned
assumption.
Unused cutting oil, used cutting oil, and residue from the sump
were compared in skin tests on 2 strains of mice by Dargent et al.
(1967). Both used and unused cutting oil caused a high incidence of
skin ulceration. Hyperkeratosis and papillomas were more frequent in
the case of used oils and a single case of skin cancer occurred in the
groups of mice treated with used oil and sump residue.
Cutting and quenching oils, fresh and used, were tested on the
skin of rats, guineapigs, and mice. All tests on rats and guineapigs
were negative. In mice, skin tumours were more numerous and more
malignant with used oils than with fresh oil. Only used oils produced
tumours in organs distant from the site of application. The unused
quenching oil was found to contain a benzo(alpha)pyrene level of
0.6-0.8 mg/kg (ppm); in the used oil, this fraction had increased to
20 mg/kg, which is in agreement with the finding of its increased
carcinogenicity. The increase in carcinogenic poly-nuclear aromatic
hydrocarbons may be the result of thermal cracking due to the heat of
the process (Desoille et al., 1973). The studies of Thony et al.
(1975) indicated that the increase in poly-nuclear aromatic
hydrocarbons in cutting oils was very small and considerably less than
for quenching oils and engine oils. The benzo(alpha)pyrene content of
new cutting oils ranged from 0 to 150 mg/kg, compared with 0 to
250 mg/kg for used cutting oils.
A sample of commercial jute batching oil of unspecified origin and
composition, containing a concentration of 3,4-benzopyrene of less
than 1 mg/kg, was tested for carcinogenic activity on the skin of
mice. The oil in question induced malignant skin tumours in 6 out of
24 test animals and proved to be a potent tumour promotor in mice
pre-treated with 7,12-dimethylbenz (a)anthracene (DMBA) (Roe et al.,
1967).
To summarize, it may be concluded that: (a) the carcinogenic
activity of mineral oils seems to be related mainly to the presence
and concentration of certain polynuclear aromatic hydrocarbons,
containing 4,5, or 6 condensed rings; and (b) cracking processes
tend to increase the polynuclear aromatic hydrocarbon contents in
petroleum products. For example such compounds may accumulate in a
metal working oil in contact with the host cutting edge of a tool.
The polynuclear aromatic hydrocarbon content of mineral oils can
be decreased by solvent extraction and/or by hydrogenation. Acid
treatment is less effective for this purpose.
Analytical methods for the determination of total polynuclear
aromatic hydrocarbons should provide a useful, quick, and cheap tool
for predictive screening of mineral oils for carcinogenicity. These
methods should be biologically validated. The correlation will not
always be good, because the carcinogenicity of polynuclear aromatic
hydrocarbons varies considerably and because of the unpredictable
effects of cocarcinogens, inhibitors, and accelerators.
Various substances that are used as additives such as sulfur
compounds, as well as substances that may normally be present in
mineral oils, such as n-dodecane, may act as cocarcinogens. Other
saturated hydrocarbons, normally present, may act as inhibitors of the
same effect.
It is clear from animal studies that washing with soap and water
substantially reduces the hazard to the skin of repeated and long
contact with potentially carcinogenic oils.
4.6.2.3 Effects of inhalation and intratracheal exposures
The aspiration hazard and toxicity for animals of a number of
hydrocarbons and hydrocarbon mixtures was determined by Gerarde
(1963). This study has been discussed in more detail in section 3.6.1.
He found that the aspiration hazard decreased with increased viscosity
of the product and with mixtures containing low viscosity products.
Mice were exposed to mists of various mineral and vegetable oils
with an average droplet size of 2.5 µm, 80% of the particles retained
in all areas of the lungs were 2.5 µm or less in diameter. The highest
concentration of retained particles was found around the terminal
bronchioles and alveolar ducts in all parts of both lungs. Oil
particles were immediately phagocytosed, a process that was
essentially completed within 48 h, unless prolonged exposures (2-4
weeks) had been given. The initial concentration of retained oil
droplets was similar for all types of oil mists. During a 90-h
follow-up, however, the concentration of vegetable oil droplets
decreased progressively, whereas the concentration of mineral oil
droplets remained practically unchanged. After 2-4 weeks of exposure,
mineral oil droplet retention gave rise to localized slight
foreign-body reactions as well as to a few patches of lipoid
pneumonia. Of the other oils, only cod liver oil caused a moderate
foreign-body reaction (Shoshkes et al., 1950).
The effects of prolonged inhalation of oil mists (mineral oil
levels in air of 63-132 mg/m3) were observed in mice, rats, rabbits,
and monkeys. Ordinary automobile lubricating oil and a smoke-screening
oil, were tested for periods varying from 100 to 365 consecutive days
(Lushbaugh et al., 1950) The authors found that surprisingly little
oil accumulated in the lungs and that whatever was retained was
rapidly phagocytosed and transferred to the pulmonary connective
tissue and the hilar lymph nodes. Lipoid pneumonia was not found to be
a hazard at these dosages, though the incidence of infectious
pneumonia in the exposed monkeys greatly increased. Many exposed
monkeys died of a hyperplastic gastritis, probably because much of the
inhaled oil was initially deposited in the nasal passages and
subsequently swallowed. Hyper-plastic gastritis was especially evident
with the shale oil (which is different from petroleum oil) used as
smoke screening oil (Lushbaugh, 1947). No significant increase in
tumour incidence or reduction in the latent period of tumour
production was found in the mouse study.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Rats and guineapigs were exposed for 6 h/day, 4 days a
week, for up to 2 years, to a cutting oil mist consisting of a 10:1
mixture of neutral paraffin and prime lard oil. In some cases, this
caused multi-focal adenomatosis in the lungs of the animals. A
carcinoma of the lung was found in only 1 of 105 exposed rats and not
in any of 65 guineapigs.
In tests on mice, Wagner et al. (1961) found that inhalation of
either mineral or motor oil mist reduced the acute lethal effects of
respired oxidants such as ozone and nitrogen dioxide. The effect was
demonstrable only after a latent period of up to 8-9 days following
oil mist exposure and was thought to result from the formation of a
thin film of oil on the alveolar surfaces.
Long-term inhalation toxicity studies using a highly purified
white mineral oil, composed of naphthene-based saturated hydrocarbons,
were reported by Wagner et al. (1964). Five species of laboratory
animals (dog, rabbit, rat, hamster, mouse) were exposed daily, for
periods from one year to 26 months, to a petroleum-base mineral oil
mist at concentrations of 5 mg/m3, the current USA threshold limit
value, and 100 mg/m3. Histological evaluation of tissues of the dogs
and rats exposed to 100 mg/m3 showed significant pulmonary alveolar
and hilar lymph node oil deposition and/or lipid granuloma formation
after 12 months of exposure. In addition, these animals showed
significant increases in the activities of basic and
magnesium-activated phosphatases.
Body-weight gain, haematological variables, and respiratory
function values did not deviate significantly from the control data at
any of the exposure levels. Studies with a spontaneous
pulmonary-tumour-susceptible strain of mouse presented equivocal
evidence of an increased rate of tumour formation at the 100 mg/m3
concentration. These findings suggest that prolonged exposure to a
mineral oil mist concentration of 5 mg/m3 would not present any toxic
hazard. It would appear, however, that protracted exposure at
approximately 100 mg/m3 would, in time, produce harmful physiological
effects.
The toxicity of aerosols of various petroleum oils (industrial,
transformer, and compressor distillates) differed only slightly in a
long-term experiment by Lutov (1974). White rats were exposed to
concentrations of 13, 30, and 60 mg/m3 for 5 h/day, for 6 months.
Changes in the electrocardiogram, and reductions in arterial pressure,
respiratory frequency, and immunological reactivity were seen even at
12-13 mg/m3, which was considered to be just above the threshold
level. A concentration of 5 mg/m3 is recommended as a maximum
permissible concentration for petroleum oil aerosols without
additives.
In a further long-term study on white rats, Lutov et al. (1976)
studied the toxicity of petroleum oil aerosols in concentrations
ranging from 11.4 ± 1.5 to 61.4 ± 5.2 mg/m3 in combination with
products obtained by thermo-decomposition, i.e., hydrocarbons in
concentrations ranging from 200.0 ± 5.8 to 410.0 ± 12.8 mg/m3 and
carbon monoxide in concentrations ranging from 11.2 ± 1.5 to
35.4 ± 4.6 mg/m3. White female rats were exposed for, 5 h/day over a
6-month period. The combined exposure was found to produce more marked
effects on the parameters tested in the earlier experiment.
The minimum concentrations of oil aerosol that caused functional
changes in the respiratory system with a single inhalation were in the
range of 860-1200 mg/m3. When concentrations of 53 and 60 mg/m3 were
used in the long-term experiment, physical growth was impaired and the
functional condition of the nervous system and the liver changed.
There were morphological changes in the lungs including a
catarrhal-desquamative bronchitis, a swelling of the alveolar
membranes, formation of oleogranules, and the presence of a large
quantity of oleophages and oil droplets in the lymphatic vessels and
the peribronchial and bronchopulmonary lymphatic nodules. At
concentrations of 10-17 mg/m3, the changes were slight and
reversible. A maximum admissible concentration in the air of a work
place of 5 mg/m3 is the official USSR exposure limit (Ivanov et al.,
1978).
In tests on rabbits, Laughlen (1925) demonstrated that mineral oil
given by mouth or nose can enter the trachea. Corper & Freed (1922),
injected olive oil and medicinal mineral oil intratracheally into
rabbits in amounts of 0.5-1.0 ml. The oil was readily aspirated into
the finest pulmonary divisions and into the alveoli. It was retained
for months and caused a mild proliferative reaction typical of a
foreign body reaction. In studies by Pinkerton (1928), rabbits and
puppies were injected intratracheally with animal, vegetable, and
medicinal oil. Complete removal of the oil from the lungs took several
months in all cases. Neutral vegetable oil produced practically no
reaction, animal oils caused giant cell formation and rapid and marked
pulmonary fibrosis. Mineral oil was rapidly phagocytosed, giant cell
formation and slight fibrosis were apparent after 2-3 months, and
storage of the oil took place in the bronchial lymph nodes.
4.6.2.4 Dietary studies
Long-term oral studies have not been conducted with lubricating
base oils.
Lushbaugh & Hackett (1948/1949) carried out a study in which rats
received an average of 0.2 ml/rat per day of a highly refined
diesel-engine lubricating oil, used as smoke screening oil, in theft
diet for a period of 14 months. In this group of 40 rats, 2 developed
foci of colonic mucosal hyperplasia and one developed colonic
adeno-carcinoma.
Results of various dietary studies using food-grade and
pharma-ceutical-grade materials, such as medicinal and food-grade
mineral oil, and food-grade paraffin waxes are included in the
following summary, though these studies with highly purified material
are not representative for the less purified grades.
Schmahl & Reiter (1933) fed 2% (w/v) mineral oil in the diet of
rats for 500 days without adverse effects. In studies by Daniel et al.
(1953), rats were kept for 15 months on diets supplemented with 10%
liquid paraffin, without adverse effects. Five kinds of petroleum
waxes were tested by feeding rats a diet containing 10% of the wax for
2 years. The rats were then observed until natural death. No toxic or
carcinogenic effects were found (Shubik et al., 1962). In the studies
by Oser et al. (1965), already reported in section 4.6.2.2, 3 kinds of
petrolatum were also administered to rats in the diet at 50 mg/kg
diet. Observations over 2 years did not reveal any carcinogenic or
other toxic effects.
4.7 Effects on Man
The general population does not have any contact with base oils;
contact with the commercial products derived from them will, at most,
be occasional and of a lower order of magnitude than occupational
exposure. Cases of accidental ingestion are exceptions.
However, pharmaceutical grade liquid paraffin in the form of
medicinal oil is widely used as a laxative (faecal softener).
Occupational exposure to base oils is restricted to the small
group of workers who manufacture them in oil refineries and to workers
in blending units, who mix and blend them with additives in order to
produce commercial products such as lubricating oils, greases,
metal-working oils, and textile oils. These activities are normally
carried out in closed systems in which case occupational contact is
minimal and discontinuous, usually accidental. This also applies, when
the finished product is put into containers.
4.7.1 Occupational exposure
Epidemiological and clinical data concerning base oils are almost
non-existent. Thus, in this section, it is only possible to analyse
epidemiological studies of workers exposed to products containing base
oils, such as lubricating oils, metal-working oils, and textile oils.
From the list of lubricating oil products given in section 4.2.2,
it is clear that various groups of workers are occupationally exposed
to these products, the most widely-used being the metal-working oils
and textile oils.
The magnitude of occupational exposure varies considerably. In the
case of some lubricants and transformer oils, handling is only
occasional, and, even then, exposure is minimal. In other situations,
such as in the earlier years of mule spinning and in work with
automatic lathes of old design, extremely high exposure did - and
sometimes still does occur. Not only is there continuous direct
contamination of clothes and exposed parts of the skin, but the rapid
movement of the machinery may turn the oil into an aerosol and thus
generate an oil mist that can be inhaled and further contaminate skin
and clothing. Equipment and floors may become covered with an oil-film
and situations have been described where oil even dripped from the
roof.
A certain degree of oil mist generation may also occur in the
printing and rubber industries and with pneumatic equipment (e.g.,
drills), especially under conditions of limited ventilation such as
are found underground.
Even under the best technical conditions, it may sometimes be
difficult to completely avoid contamination. For example, in the case
of toolsetters, the wearing of protective clothing impedes easy
movement, and clothing, including underwear, may become soaked with
oil.
Henry (1946/1947) gave an excellent insight into the types and
magnitude of occupational exposure up to the time of the report. It is
known that some of these conditions had continued for a period of
about 20 years. However, in many countries, great improvements have
been made since then. An understanding of the range of such exposures
is needed to put epidemiological studies of later years into
perspective, especially in view of the long latency of carcinogenic
effects. In the evaluation of such studies, it has to be kept in mind
that today's findings may reflect the industrial hygiene situation of
20-30 years ago.
4.7.1.1 Skin disorders
A great variety of skin disorders attributed to working with
mineral oil or with products based on mineral oils, such as metal
working fluids, are mentioned in the medical literature. This is not
surprising considering the many factors that influence theft
development (van Raalte, 1963; Key et al., 1966; Kipling, 1968;
Hodgson, 1970, 1973).
The main factors include: (a) the degree of intrinsic potential
of a mineral oil product to damage the skin; (b) the integrity of
the skin; (c) the degree and continuity of contact between oil and
skin; and (d) individual predisposition.
The situation, however, is more complex than this, for, in actual
practice, a multitude of other factors may influence the main
conditions to such an extent and in such a complex way, that a
cause-and-effect relationship may become obscured. Most authors agree
that practically all skin disorders attributed to exposure to mineral
oil products can be prevented entirely by adequate industrial and
personal hygiene practices, and that the majority of cases have
occurred in workshops where inadequate conditions prevailed.
Some of the more important factors in the complex interplay are:
(a) Factors related to the mineral oil product used:
(i) Base oil:
the lower the boiling-point of the oil, the more
pronounced the solvent action of the product; this causes
defatting of the skin and leads to dryness, chapping,
scaling, and cracking;
the higher the boiling-point, the more blocking of skin
pores occurs, giving rise to acne formation;
some mineral oils are more irritant than others; the
lower-boiling fractions are sometimes, but not always,
more irritant than higher-boiling fractions (section
4.6.1);
mineral oils themselves do not appear to be sensitizers.
(ii) Additives:
these may be primary irritants or sensitizers in theft own
right (e.g., chromium salts), though manufacturers try to
avoid the use of such additives;
solvents and detergents increase the defatting effect of
the lower boiling-range oils;
some chlorinated additives, such as chlorinated
naphthalenes, may cause chloracne. The use of chlorinated
naphthalenes was discontinued in most countries, many
years ago.
(iii) Changes in the composition of the oil that may occur
during its use:
cracking of oil fractions may occur due to heat;
reactions may occur between components of the mixture or
between them and materials that are added at a later
stage;
metal salts or ions may be formed in the mixture or
solution in the case of metal-working oils; in the case
of, for example, chromium and nickel, this can cause skin
reactions in sensitized workers.
(iv) Impurities of all kinds may accumulate in, for instance,
metal-working oil baths in which the oil is not regularly
changed. Important among these impurities are: metal
particles, which may cause microtraumata of the skin; and
microorganisms, which may cause inflammation of the skin
by way of infection or exotoxins; bacteria, yeasts, or
fungi may be present in mono- or mixed culture, and some
of these organisms can degrade an oil and form potentially
irritating compounds.
(b) Factors related to the work situation:
(i) design of workshop and equipment determine to a large
extent the magnitude and duration of exposure to the oil,
and whether this exposure is continuous or intermittent;
(ii) availability of general and exhaust ventilation of
sufficient design and capacity may determine whether there
is exposure to oil mist or not;
(iii) use of protective clothing such as gloves, will diminish
duration and intensity of skin contact;
(iv) general hygienic facilities such as wash basins near the
work-place, shower facilities, frequent changing and
laundering of work clothing, all of which help in limiting
the extent and duration of exposure;
(v) hands and contaminated parts of the body should be washed
with fatty toilet soap and, after washing the skin should
be treated with a suitable emollient cream; use of
solvents, and alkaline or abrasive soaps for washing can
damage the skin and contribute to the occurrence of skin
diseases.
(c) Individual factors:
(i) general and hereditary conditions of the skin may
predispose to adverse skin effects from all sorts of
chemicals;
(ii) work discipline and safety-mindedness can influence the
duration and intensity of the exposure;
(iii) proper use of safety equipment and general hygiene
facilities, as well as personal hygiene in the form of
frequent bathing and changing of underwear, will help to
avoid over- and prolonged exposure.
Contamination of the skin may often occur despite all precautions
taken, particularly in the case of metal-working oils. Wiping with
oil-soaked rags is an additional source of contamination and may cause
skin lesions. Continuous wearing of oil-contaminated clothing is an
important factor in the etiology of scrotal cancer. Rapidly rotating
machinery may generate oil foams and mists.
The most common form of skin disorder is acute or chronic contact
irritative (toxic) dermatitis caused by the irritative action of
various components, additives, and/or impurities in mineral oils.
Dermatitis may be preceded by defatting and/or maceration of the skin.
Mechanical irritation, microtraumata, and skin cuts may play a role in
its origin. Clinical signs of contact irritative dermatitis are, in
order of severity, erythema, oedema, bullae or necrosis, and sharp
demarcation of the affected areas from unaffected ones.
The other type of dermatitis is contact allergic dermatitis
(synonym: contact eczema) caused by allergic sensitization to various
allergens. Additives or impurities are the most allergenic components
of oil formulations. The signs of eczema are more variable then those
of toxic dermatitis and include erythema, papulae, vesiculae, bullae,
scales, hyperkeratoses, and rhagades. These lesions have a tendency to
spread into areas that have not been in direct contact with the
allergen. Eczema may be preceded by contact irritative dermatitis,
from which it develops by secondary sensitization.
Oil folliculitis, or oil acne is characterized by the triad
comedones, folliculitis, and follicular scars. The lesions are found
on the parts of the body with the greatest exposure. Friction from
clothing and machinery rubbing the oil into the exposed parts of the
skin, is an important additional factor. First there is plugging of
the hair follicles and pores of the skin by follicular hyperkeratosis,
cell debris, and oil with its impurities, followed by blackheads and
secondary infection. Poor personal hygiene is the main cause. Oil acne
occurs more often in the earlier years of exposure to oil. Later,
there appears to be a gradual change in the reaction of the skin to
the oil (Kinnear et al., 1954). Chlorinated naphthalenes and related
compounds have given rise to chloracne.
Photosensitivity is an abnormal sensitivity of the skin to
sunlight caused by certain constituents of coal-tar, but also
sometimes by mineral oil constituents. Related to this is melanosis,
the general darkening of the skin that may follow acute
photodermatitis, as well as toxic melanoderma, which develop after
long-term exposure to oils containing certain anthracene fractions.
Both photosensitivity and melanosis rarely result from exposure to
mineral oil.
Hyperkeratosis may occur either together with dermatitis and oil
acne of long standing, or in isolation -- mostly on the forearms or
other heavily exposed parts of the body. Two forms of hyperkeratosis
can be distinguished: (a) circular, white and flat hyperkeratotic
areas of a few mm in diameter, sometimes in the form of smooth
plaques; these may occur in small clusters and are slightly raised
above the level of the surrounding skin; and (b) a second form,
which may occur at the same time, and consists of rugose, pigmented
warts that are considerably raised above the surrounding tissue level.
The pattern is generally irregular, but may be round or oval.
Precarcinogenic changes may be present in the hyperkeratotic
plaques in the form of rough, slightly raised patches, which sometimes
may take the form of horns or warts. In themselves these forms are
still harmless, but they have a tendency to become malignant. As soon
as these forms contain some malignant cells they are called
keratoacanthomata. After growing for a certain period, they may be
shed from the skin. Another form of precarcinogenic change that may be
encountered is the shark- or shagreen-skin, a pigmented, atrophic
skin, beset with small horns and warts.
A basal cell carcinoma (basalioma) is a very slowly enlarging
tumour of the skin. It may ulcerate, or invade the area round it, but,
in general, it does not metastasize. The most common form of malignant
tumour is the squamous cell carcinoma (spinalioma), starting as a
small tumour, that may arise from a keratosis or in apparently healthy
skin. It continues to grow, starts ulcerating, invades surrounding
tissues and eventually may metastasize. None of these conditions is
limited to mineral oil exposure. Similar changes may occur as a result
of UV-light exposure, excessive doses of X-rays, exposure to pitch
coal-tar, or ingestion of arsenic. A specific localization, however,
is the epithelium of the scrotum. In this case "it is reasonable to
assume that it could be caused by occupational exposure to soot, tar,
pitch, or oil" (Kipling, 1968).
A combination of various potentiating factors, such as mineral oil
contact and exposure to sunlight (UV-radiation) increases the tendency
to develop skin cancer (Schwartz et al., 1947; Smiley, 1951; Kinnear
et al., 1954; Emmett, 1975). This is especially pronounced in persons
with fair hair.
4.7.1.2 Skin carcinogenicity
The history of the development of skin cancer as a result of
exposure to mineral oil, as well as the major epidemiological
literature related to the subject have been reviewed by the
International Agency for Research on Cancer (IARC, 1973). To quote
theft findings:
"Volkmann (1875) described scrotal cancers among workers producing
paraffin by the distillation of coal-tar. Subsequently, Liebe (1892)
noted the absence of such hazard among workers exposed to pure
paraffin. Several investigators have since shown that cancers among
paraffin workers are not due to the paraffin but to impurities in oils
produced during processing (Leitch, 1922; Hendricks et al., 1959).
Refined paraffin is free of PAH and does not induce skin cancer in
mice (Shubik et al., 1962).
"Bell (1876) first described cancer of the scrotum in a Scottish
shale oil worker. In a 23-year period, 49 Scottish paraffin workers
developed skin cancer of which 13 were scrotal (Henry, 1946).
"The cotton mule spinning industry in Great Britain originally
used shale oil for the lubrication of the spindles (Henry, 1946). The
first case of death from scrotal cancer in a worker who used shale oil
in mule spinning occurred in 1923 (Bridge & Henry, 1928). In the years
1920 to 1943, there were 1303 legally notified cases of skin cancer in
the British mule spinning industry, including 824 of the scrotum.
There were 575 fatal cases of scrotal cancer recorded between 1911 and
1938 (Henry, 1946).
"In Great Britain, the Mule Spinning Regulations have ensured that
since 1953 only oil drastically refined with sulphuric acid shall be
used in mule spinning and that mule spinners shall be medically
examined every six months. These measures, together with the marked
decline of the process of mule spinning, have produced a sustained
fall in the incidence of cancer of the scrotum in Great Britain.
"Cutting oils used by workers to cut metals were found to increase
the risk of skin cancer in Birmingham, England (Cruickshank & Squire,
1950; Cruickshank & Gourevitch, 1952), particularly among workers in
automatic machine shops. Between 1950 and 1967, 187 cases of scrotal
cancer occurred in this region, of which at least two-thirds could be
attributed to oil (Waterhouse, 1971).
"At the present time toolsetters and setter operators in automatic
shops who use neat cutting oil have an increased risk of cancer. The
work requires constant contact with the machines and consequent
contamination with the oil. In the Birmingham area of England, a high
frequency of skin and scrotal cancer from oil has occurred,
particularly among bar automatic machine workers; but other
engineering practices also present a cancer hazard, e.g., metal
rolling, tube drawing, metal hardening and machine operating. Although
the major risk is from exposure to undiluted oils, emulsions have been
incriminated occasionally. The industries most affected are those with
automatic shops, such as nut and bolt manufacturers. Workers have also
been affected after exposure during the changing of transformer oil in
electrical sub-stations and during the painting or spraying of mould
oil for brick- and tile-making or concrete moulding, in drop forging,
rubber mixing, wire drawing, rope making and in the jute industry and
from grease in metal working (Kipling, 1968).
"In France, in the valley of the river Arve in the Savoy Alps,
there have occurred since 1955 at least 60 cases of cancer of the
scrotum together with many cases of cancer of the skin among the bar
automatic machine workers (décolleteurs). The very high frequency in
the relatively small population of the valley was observed mainly
among the self-employed and workers in small premises (Thony & Thony,
1970). They were in contact with undiluted cutting oils.
"Cancers of the larynx, lung and stomach have also been attributed
to oil mist (Southam, 1928); and recently evidence has been produced
that persons who developed cancer of the scrotum are significantly
more liable to develop cancers at other sites, e.g., in the
respiratory tract or upper digestive tract (Holmes et al., 1970)."
Kinnear et al. (1954, 1955) published the results of an extensive
epidemiological study of skin disease in jute workers in relation to
mineral oil exposure. They found a high incidence of premalignant
changes on the skin of the exposed parts of the body in long-term,
older workers and isolated cases of scrotal carcinoma.
Bingham & Horton (1966) estimated the latent period for skin
cancer caused by mineral oil exposure to be 50-54 years (range 4-75
years). In the case of crude paraffin oil, they mentioned an average
of 15-18 years (range 3-35 years). In these cases, the range may be
more important than the average, though the average indicates that, in
general, the latent period is very long. A considerable proportion of
the cases of skin epithelioma caused by mineral oil exposure had such
a long latency period that the disease only appeared after retirement
from active work (Cruickshank & Gourevitch, 1952; Kinnear et al.,
1955).
Five cases of squamous cell carcinoma of the hand and forearm and
one of the scrotum occurred in machine operators at a plant in
Ontario, Canada (Mastromatteo, 1955). These workers had been exposed,
for an average of 21 years, to cutting fluids that were subsequently
demonstrated to be carcinogenic in animal tests (Gilman &
Vesselinovitch, 1955).
Milne (1970) traced 5 cases of carcinoma of the scrotum, as
registered in the Central Cancer Registry of Victoria, Australia, and
found that, in all cases, the carcinomas occurred during or after the
seventh decade of life. Three of the subjects had had intensive
contact with mineral oils throughout theft working life, one was a
stoker in a gas-works, and the fifth had always been involved in
administrative work.
In the Netherlands, a recent survey demonstrated that scrotal
cancer occurred only sporadically and was not correlated with
occupational exposure to mineral oil (Pruyn & Reijnierse, 1972;
Fokkens et al., 1972; van Raalte, 1972).
Eight cases of scrotal cancer were discovered by Avellan et al.
(1967), over a period of 24 years, among 250 automatic lathe operators
in Gothenburg, Sweden. Diagnosis was made when the operators were
between 54 and 66 years of age and after periods of exposure to
mineral oil ranging from 19 to 43 years. In the words of the authors:
"All of the cases occurred among operators who began their work
during the era when the exposure, as a result of the prevailing
machine construction, was considerable and before the regulations and
the controls, which were instituted after the discovery of the first
cases, had been set up."
Wahlberg (1974) analysed 34 cases of scrotal cancer reported to
the Swedish Cancer Registry between 1958 and 1970. Seven cases (21%)
had been heavily exposed, occupationally, in the past to oil. and oil
mist, e.g., as automatic lathe operators. One of this group developed
a primary lung cancer.
A retrospective study of 298 cases of scrotal cancer registered in
the Birmingham region in the United Kingdom between 1936 and 1972 was
reported by Brown et al. (1975). The incidence of scrotal cancer was
5-6 cases per million males per year, whereas for the whole of the
United Kingdom, the incidence is 1-2 cases per million males per year.
The patients or relatives were interviewed in 109 cases: 94 had been
exposed to mineral oil (mainly tool-setters and machine operators, and
all cases had been exposed to cutting oil, 14 had been exposed to
pitch or tar, and, in 7 cases, there was no apparent occupational
exposure. In 298 cases of scrotal cancer, 52 other primary rumours
were noted; of these, 42 arose following the scrotal cancer including
15 skin tumours and 12 bronchial tumours. The incidence of both these
types of cancers was much in excess of what would be expected
statistically. The majority of the group with second primary tumours
were machine operators and tool-setters (75% in the case of bronchial
carcinoma). The authors postulated that in these cases both primary
rumours were initiated by the same carcinogen.
After studying all the available literature, Desoille et al.
(1973) concluded that, in general, and with the exception of certain
extreme situations, the number of tumours caused by exposure to
mineral oils was low. According to Auld (1950) and Eckhardt (1957),
though certain cutting oils and other mineral oils are carcinogenic to
the skin, the degree of carcinogenicity is very low compared with that
of coal-tar, pitch, and shale oil. In their opinion, and that of most
other authors, e.g., Avellan et al. (1967), even this level of
carcinogenicity would disappear, if a minimum of industrial and
personal hygiene measures were adhered to. On the other hand, Auld
(1950), Desoille et al. (1973), Thony et al. (1975) and many others
urge that alternative products should be developed that do not expose
the workers to a tumorigenic hazard.
In a further report on the skin cancer epidemic in the French Arve
Valley, Thony et al. (1975) recorded 133 epithelioma cases in 15
years, mostly of the scrotum. The incidence in these workers was 36
times that expected in the general population. In workers in small
workshops with poor industrial and personal hygiene, the incidence was
found to be 3 times higher than in the larger plants. The average age
was 54 years (range 35-75 years) and the average exposure 30 years
(range 15-50 years), at the time of clinical diagnosis. In addition,
an increased incidence of bronchopulmonary tumours was found,
especially in the décolleteurs. Officially until 1947, but in
practice possibly until 1950, coaltar-derived anthracene oils were
used, which originally contained benzo(alpha)pyrene levels of up to
1000 mg/kg. The level was later reduced to 10 mg/kg. These oils may be
responsible for many of the previously mentioned cases, but skin
cancer also occurred in some of the workers exposed only to the
petroleum-derived oils that were used exclusively later on. Fresh
cutting oils, as delivered to the users, contained benzo(alpha)pyrene
levels ranging from 0.5 to 145 µg/litre and the concentration of
carcinogens was found to increase during use, though not to the same
extent as that in quenching oils (2-100 times with an average of 30
times), and in lubricating oils for internal combustion engines.
4.7.1.3 Effects of oil mist exposure
Southam (1928) was the first to attribute cancers of the larynx,
lung, and stomach to oil mist exposure.
In a report by Huguenin et al. (1950), 32 out of 144 patients with
lung cancer had been in prolonged and intense contact with oil mist in
the past. Old metal-working machines with improper protection against
oil mist, processes where oil vapour arose from contact with hot
metal, and high-pressure cleaning of machines with oil, were found in
the places where these patients had worked. Hendricks et al. (1962)
considered the observations of Huguenin et al. (1950) to be of
doubtful significance in view of the high incidence of this disease in
non-oil-mist-exposed groups. Nevertheless, the conditions in these
cases may have been such, that a causal relationship, in at least some
of them, cannot altogether be excluded.
More printing industry workers were found among cases of bronchial
carcinoma in a Stockholm clinic (8 out of 125) than would have been
expected (Ask-Upmark, 1955). Certain industrial processes where oil
mist might occur were studied by Hendricks et al. (1962), who gave an
indication of possible exposures in their table of actual exposures
measured in various industries (Table 7).
The average particle size in the oil mists varied according to the
generating process and was found to be about 1.0-5.0 µm.
In 241 Kodak workers exposed to oil mist (Ely et al., 1970), no
significant differences were found, either in mortality, respiratory
symptoms, and disease, or in lung function compared with a control
group.
In the same year, Holmes et al. (1970) produced evidence, that
persons who developed cancer of the scrotum were significantly more
liable to develop cancers of the respiratory tract and the upper
digestive tract.
Similar findings have been reported in a preliminary paper by
Waterhouse (1972). An excess of primary tumours at other sites (skin,
respiratory and upper alimentary tracts) was found in men with scrotal
epitheliomas.
From animal studies, it appears that oil mist particles larger
than 5 µm will not easily penetrate into the lungs, but will be
retained mainly in the nasopharynx and upper respiratory tract.
Smaller particles, especially those of 2.5 µm and less, will readily
pass into the alveoli, where they will be phagocytosed and passed on
to the lymph nodes. When this mechanism cannot cope with the
situation, in cases of continuously repeated high exposure to oil
mists, a chemical pneumonitis and chronic lipid pneumonia may develop
(Proudfit et al., 1950; Foe & Bigham, 1954), but this appears to be an
extremely exceptional condition. Clinical details and examples of this
will be discussed in section 4.8.
TABLE 7. Exposure to oil mist in selected industries
Type of industry Observations Exposure range
oil mist mg/m3
brass and aluminium production 5 1.4-20.7
copper mining 7 5.4-22.0
automobile manufacture 37 1.0-56.5
manufacture of steel products 33 0.8-50.0
newspaper (press room) 8 2.0-16.6
screw manufacture 6 1.0-14.2
From: Hendricks et al. (1962).
In 12 out of 19 workers with oil mist exposures ranging from 9 to
18 years, Jones (1961) found a marked linear and reticular pattern in
the radiograms of the lungs. Exposures ranged from 1 to 9 mg/m3 with
70% of the particles of the order of 1 µm.
Various groups of research workers have studied different aspects
of the printing industry. Printing-ink consists chiefly of a
suspension of carbon black in mineral oil or aromatic extracts of
mineral oil. Modern high-speed newsprint presses can generate fairly
high concentrations of ink-mist aerosol. Most particles, however, are
outside the respirable range and may end up in the stomach, rather
than in the lungs of exposed workers. Lippmann & Goldstein (1970)
found an average droplet size of 14 µm (ranging up to 30 µm) in the
press rooms of the New York Times. The time-weighted average
concentration of respirable mist particles was found to be 1.4 mg/m3
against a total time-weighted average mist concentration of
8.6 mg/m3. In a parallel epidemiological study in the same firm,
mortality and morbidity data over a 15-year period were compared for
pressmen and compositors. No significant differences in respiratory
mortality or morbidity were found (Goldstein & Benoit, 1970).
In a similar study by Pasternak & Ehrlich (1972), there was no
increase in respiratory symptoms or decrement in respiratory
performance in 778 New York pressmen compared with 1207 compositors.
No significant differences in death rates, were found in those who
were less than 40 years old when first employed, even if they had
worked for more than 20 years. In those first employed when they were
more than 40 years old and with more than 20 years of employment,
there was a significantly higher death rate in pressmen compared with
compositors. The reason for this finding was not clear. The mean oil
mist concentration measured in the 3 workshops concerned was
3.7-5.2 mg/m3.
Moss et al. (1972) analysed the causes of death of 3485 former
full-time printing industry workers from London and Manchester, who
died in the period 1952-66; Greenberg (1972) studied the death
certificates of 670 male printing workers who died between 1954 and
1966. In both studies, an excess number of cancers of the lung and
bronchus were found. This excess was more marked in Manchester and
greatest in machine-room men. The authors concluded that the slight
excess found might or might not be due to occupation. They considered
that an occupational cause was more likely in the case of the greater
excess in the Manchester machine-room men.
These studies in the printing industry have been mentioned in
relation to mineral oil exposure. However, the same data could be
related to carbon black and even to lead exposure. Similar
considerations should be kept in mind in the evaluation of the data in
other occupations where many other factors might also play a role,
either individually or combined with the mineral oil used, as, for
instance, chromium in the metal-working industry.
In studies in 34 metal-working firms in Baden-Würtemberg (FGR),
the frequency of respiratory complaints (cough, expectoration, and
dyspnoea) in 443 workers exposed to oil mist for long periods was
compared with that in 398 unexposed controls, matched for age. Mineral
oil mist exposures from cutting oils had ranged from 40 to 150 mg/m3
over long periods. The highest incidence of complaints was found among
unexposed smokers, the lowest incidence amongst non-smoking,
oil-mist-exposed workers. The authors did not find any signs of
irritative effects from oil-mist exposure, but a significant
protective effect against the well-known irritant effects of smoking
(Drasche et al., 1974). This finding is in line with findings in
experimental animals, that previous exposure to oil mist reduces the
lethal effects of respiratory oxidants such as ozone and nitrogen
dioxide in mice (Wagner et al., 1961). The authors stated that
conclusions could not be drawn from this study in relation to the
carcinogenicity of these oil mists in the respiratory tract.
During the cold processing of metal, the concentration of mineral
oil mist in the air (spindle oil aerosol) fluctuated from 3 to
40 mg/m3 (on average 10 mg/m3). During an examination of 77 lathe
operators (men and women), functional disturbances found to occur in
the respiratory system, in particular after a period of service of
more than 10 years, included a reduction in the active volume and the
maximum ventilation of the lungs and an increase in oxygen requirement
and in its coefficient of utilization (Bruskin & Demcenko, 1975).
A 30-year retrospective cancer mortality study was carried out by
Decoufle (1976) on 5189 workers engaged in metal-working for at least
one year. No significant differences in cancer mortality were
observed, when compared with the general population. Indications of
increased incidences of respiratory and digestive cancer, observed
when comparison was made on an age group or exposure basis, were not
statistically significant.
Decoufle (1978) published a further study on a group of 2485 male
workers employed between 1938 and 1967 in jobs exposing them to
various levels of cutting oil mists. Compared with the total death
rate of the US male population, no significant differences were
observed for 15 cancer site categories. However, a 2-fold risk of
cancers of the stomach and large intestine (combined) was seen after
20 years of follow-up in the subgroup of men with 5 or more years'
exposure to cutting oil mists, prior to 1938. Deaths from nonmalignant
respiratory disease were significantly fewer than expected. These
results suggested that occupational exposure to soluble and insoluble
cutting oil mists, during various metal machining processes, did not
pose a health hazard in terms of respiratory cancer and fatal
nonmalignant respiratory disease, but might be associated with certain
forms of gastrointestinal cancer.
To summarize, it can be concluded from the literature that
oil-mist exposure can give rise to pulmonary disease, but only after
prolonged exposure in workplaces with unsatisfactory hygienic
conditions. If oils with a low content of polynuclear aromatic
hydrocarbons are used in situations where oil mists can be generated
and the TLV for oil mists is not exceeded, this problem is unlikely to
arise.
4.8 Clinical Studies
Clinical studies on the effects of mineral base oils and products
derived from them on the skin have been discussed together with
epidemiological studies in sections 4.7.1.1 and 4.7.1.2. It was felt,
that compilation of individual case-histories from the literature on
this subject was superfluous. Adverse effects resulting from the
surgical use of paraffin for cosmetic purposes, and those following
grease-gun accidents have not been considered in this review.
Clinical studies on the effects of these products after ingestion
show that the main effects are caused by aspiration, which may be a
complication of ingestion, and usually occurs during subsequent
spontaneous or induced vomiting. Otherwise, there is general agreement
that mineral base oils, lubricating oils, and greases have a low order
of toxicity, when ingested (Gerarde, 1960). At the most, some
gastrointestinal symptoms, such as abdominal cramps and diarrhoea,
result.
There was evidence from a clinical case study that prolonged
ingestion of mineral oil over a number of years could result in oil
deposition in the small intestine, abdominal lymph nodes, liver, and
spleen and lungs. This produced significant structural and functional
abnormalities that were considered to have contributed to the
patient's death (Nochomovitz et al., 1975).
White mineral oil (pharmaceutical grade) is a base oil
specification intended for oral use. It is a highly purified base oil
distillate, mainly containing saturated paraffinic fractions, and it
has to be free of polynuclear aromatic hydrocarbons. It is used as a
laxative, in pharmaceutical formulations, and as a food-grade
lubricant. From studies with radio-opaque oil, it appears possible
that a small amount of mineral oil, when taken orally as a laxative
just before retiring, may gain entrance into the lungs. Though this is
not of any consequence if it occurs only once, a cumulative effect in
the lungs might result, if it occurs repeatedly, day after day, over
several years (Sante, 1949; Miller et al, 1962).
Clinical experience of the effects of inhalation of mineral oil in
the lungs is in agreement with the results of animal studies (section
4.6.2.3). In man, mineral oil is rapidly phagocytosed and transferred
to the regional lymph nodes. In contrast with vegetable oil and oil of
animal origin, however, mineral oil cannot be metabolized and is
deposited in the interstitial tissue and in the lymph nodes, where it
induces a cellular reaction, with the appearance of giant cells that
may result in fibrosis of more or less extensive lung areas. The
resulting clinical and pathological picture depends to a large extent
on whether there has been massive, acute over-exposure, with the
body's defence mechanisms overwhelmed (see (a)), or whether long-term,
low-level exposure has occurred, where the defence mechanisms can cope
with the daily exposure and no disease will become apparent, until the
signs and symptoms of the secondary fibrotic reaction appear (see
(b)), (Pinkerton, 1927, 1928; Cannon, 1940; Freiman et al., 1940; Moel
& Taylor, 1943).
A few typical clinical entities can be recognized:
(a) Diffuse acute mineral oil pneumonia is practically always
the result of an accidental massive aspiration of mineral oil (Sante,
1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham, 1954;
Gerarde, 1960). It is, in fact, a chemical pneumonitis frequently with
a superimposed secondary infection, progressing into an interstitial
proliferative inflammation. Cases resulting from aspiration following
accidental ingestion of products containing gasoline, kerosene, or
other petroleum solvents in the same boiling-range are well-known from
the clinical literature. Many cases have occurred as a result of
aspiration of seawater contaminated with diesel oil by survivors of
sinking ships (Weissman, 1951).
Aspiration of mineral oils in the boiling-range under discussion
may have similar results. This can happen when choking occurs, while
taking white medicinal oil. This acute type of mineral oil pneumonitis
is not likely to occur as a result of occupational exposure to oil
mist. Clinically, all signs and symptoms of an acute massive
pneumonitis are present with elevated temperature and a chest X-ray
typical for this condition. Oil droplets may be found in the sputum
and, histologically, the condition is characterized by intra-alveolar
accumulation of oil-laden phagocytes and inflammatory cells. Acute
mineral oil pneumonia is a special form of lipoid pneumonia, which
presents a similar clinical picture and is the result of aspiration of
vegetable or animal oil (mainly cod liver oil, but also egg-yolk and
milk). Though in these cases a similar severe acute pneumonia may
develop, the oil can be metabolized and disappears completely after a
certain time (Sante, 1949). With oils of animal origin, such as cod
liver oil, however, the reaction of the body (lungs and other internal
organs) can be much more serious than with mineral oils (Young et al.,
1939).
(b) Diffuse chronic mineral oil pneumonia occurs as a result of
gradually developing fibrotic and proliferative changes in both lungs.
It may follow years after the acute form, or without an acute
beginning after a long, practically asymptomatic period as a result of
repeated smaller "aspirations", such as those resulting from regular
massive use of mineral-oil-based laxatives, nose-drops, or nose-sprays
(Pinkerton, 1927; Bishop, 1940; Freiman et al., 1940). The apparently
rare cases of mineral oil pneumonitis following prolonged occupational
exposure to excessive oil mist concentrations fall into this category
(Sante, 1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham,
1954; Gerarde, 1960). Reports from the literature suggest that in some
of these cases, at least, a predisposing factor was present in the
form of a pre-existent or concomitant lung disease (Freiman et al.,
1940; Weissman, 1951; Forbes & Markham, 1967). Increasing dyspnoea and
productive cough are the most important symptoms. The chest X-ray
generally shows increased perihilar opacities with signs of lung
fibrosis and diffuse patchy opacities. Oil droplets or oil-laden
phagocytes may be found in sputum or in tissue derived from lung
biopsy (Goodwin, 1934; Bishop, 1940; Freiman et al., 1940; Rossier &
Bühlmann, 1949; Sante, 1949; Proudfit et al., 1950; Borrie & Gwynne,
1973).
(c) Paraffinomas are large fibrous nodules or globules of liquid
mineral oil embedded in dense hyaline fibrous tissue. They represent a
separate form of the chronic mineral oil pneumonitis in which
oil-laden phagocytes, destroyed by pressure, atrophy in the fibrous
scar tissue formed. Paraffinomas may be found singly or in clusters
around the large bronchial branches or at the site of the hilar lymph
nodes. It may be difficult to differentiate a paraffinoma from a lung
tumour radiologically (Brown & Biskind, 1941; Wood, 1943; Sante, 1949;
Proudfit et al., 1950; Weissman, 1951; Bryan & Boitnott, 1969; Borrie
& Gwynne, 1973). Aspiration biopsy may help in the differential
diagnosis (Nathanson et al., 1943).
Boitnott & Margolis (1966a) have described analytical methods for
the identification of the various oils in human tissues. Mineral oil
droplets may pass from the hilar nodes via the thoracic duct into the
systemic circulation (Pinkerton, 1927; Young et al., 1939; Freiman et
al., 1940; Boitnott & Margolis, 1966b). As a result of this
transmission, oil droplets have been found in the liver, spleen, and
other organs (Pinkerton & Moragues, 1940; Rewell, 1947).
The general pattern found in young children is slightly different
from that found in adults. Pinkerton (1927) described 6 cases of
lipoid pneumonia in children and data on 25 cases in children have
been summarized by Goodwin (1934). Seven cases have been reported by
Ikeda (1935) and 27 cases by Bromer & Wolman (1939). There is general
agreement that fats and oils of animal origin play a more important
role than mineral oil in lipoid pneumonia in infants and young
children. Furthermore, the acute massive aspiration type is more
frequently seen in children than the chronic form. The main causal
factors in these observations are: false passage of a gavage tube,
false deglutition in bottle-feeding with the baby lying on its back --
especially in cases of debilitating disease, and also aspiration
following forced administration of milk or cod liver oil with or
without vomiting or choking (Freiman et al, 1949).
With regard to adults, Ikeda (1937) summarized 106 cases from the
literature, Graef (1939) 22 cases, Bishop (1940) 136 cases, Freiman et
al. (1940) 58 cases, and Moel & Taylor (1943) 20 cases. They concluded
that lipid pneumonia, especially the diffuse chronic form, occurs more
frequently in adults than is generally believed. Liquid paraffin is by
far the most important etiological agent in the adult. Debilitated
states, dysphagia and impaired cough reflexes, because of neurological
or other disorders, are important predisposing factors. The authors
stress, however, that mineral oil is widely used without evident harm,
even in elderly persons. Wherever there is a real indication for this
type of medication, they see no reason to discontinue it. Extensive
use, especially self-medicated, of liquid paraffin intranasally or via
the oral route by debilitated or dysphagic patients should, however,
be discouraged (Bishop, 1940; Freiman et al., 1940).
As a matter of interest, a few cases of lipoid pneumonia have been
described in relation to the intratracheal administration of
mineral-oil-based mixtures by opera singers, prior to every
performance on the stage, in order to improve the quality of the voice
(Even, 1947; Facquet & Langeard, 1947; Meyer, 1976). A similar case
was described by Garvin (1939) as a result of intratracheal
self-medication.
From the literature, it is apparent that with the recognition of
the causal factors and the change from oil-based to water-based
nose-drops, the incidence of the type of lipid pneumonia just
described has drastically decreased, since the end of the forties.
The following more or less typical cases, in which there was -- or
might have been -- a relation between occupational exposure to oil
mist and the occurrence of lipid pneumonia, were found in the
literature. Proudfit et al. (1950) reported a case of chronic lipid
pneumonia in a 40-year-old man who had been spraying mineral oil for
17 years; he had a typical chest X-ray. The chief complaints were
cough, shortness of breath, and fatigue. Mineral oil droplets were
identified in the sputum; 3 years later the condition had progressed
slightly. A case of lipid pneumonia was described by Weissman (1951)
in which the disease apparently developed on the basis of
long-standing pulmonary fibrosis as the result of blast-spraying of
machine parts with mineral oil; no mask had been used as protection
against the inhalation of nebulized oil. Foe & Bigham (1954) reported
the case of a 30-year-old aircraft mechanic who complained of fatigue,
shortness of breath on exertion, and frequent chest colds. Lipid
pneumonia was diagnosed from a lung biopsy. The mechanic had been
spray-cleaning aircraft engines with a mixture of 50% kerosene and 50%
vegetable-oil-soap.
Two cases of progressive respiratory disease, which developed in
the fifth decade of life were described by Forbes & Markham (1967).
Both patients had a moderate to heavy smoking history; one, in
addition to this, had a family history of asthma; dyspnoea and
wheezing were the major signs in each case. Both reacted well to
treatment, but recurrence of signs and symptoms was related to working
with cutting oils, the composition of which was not mentioned.
The epidemiological data on the possible relationship between
cancer of the respiratory tract and long-term occupational exposure to
oil mist has been discussed in section 4.7.1.2. There is some evidence
that in cases where an unsatisfactory industrial hygiene situation
coincided with the use of an oil with probable carcinogenic
properties, such a causal relationship might exist. On the other hand,
various extensive studies have shown that, in general, it is certainly
not a major problem.
However, Wood (1943) reported a fatal case of extensive lipid
pneumonia in a house-maid who had used oily nose-drops in large
quantities for recurrent sinusitis over a period of 10 years. At
postmortem, an alveolar-cell carcinoma was found in the lungs. The
author suggested that there might have been a causal relationship
between the 2 diseases, though he assumed that this occurrence would
be rare. Two cases of bronchogenic carcinoma were described by Sante
(1949). In the first case, the malignant epithelial tumour was
situated in an area of dense fibrotic tissue, typical of a
paraffinoma. In the other case, a squamous cell carcinoma was found
together with lipid pneumonia in an early stage of organization. The
author felt that there might have been an etiological relationship in
the first case, but considered this less likely in the second case.
Both patients had taken a tablespoon of mineral oil as a laxative,
just before retiring, for years. Volk (1964) studied a series of more
than 100 cases of mineral oil pneumonia and noted that not one case of
bronchogenic carcinoma occurred in this group. In addition, he
reviewed 114 consecutive autopsies of adenocarcinoma of the lung in
which he did not find any lesions that might be associated with
mineral oil pneumonia.
A rapidly lethal case of multifocal alveolar cell carcinoma of the
lung was reported in a 63-year-old man who had centrifuged used
cutting oil for 23 years and had been exposed continuously to oil mist
of this type during that period (Despierres et al, 1965). Wahlberg
(1974) described one case of primary lung cancer in 7 men who
developed scrotal cancer following heavy occupational exposure to oil
and oil mist in, for example, automatic lathe operation. A case in
which achalasia led to chronic mineral oil pneumonia was reported by
Bryan & Boitnott (1969). An adenocarcinoma developed in the area of
scarring resulting from the mineral oil and caused death. On the basis
of a literature study and theft own findings, the authors concluded
that the pathogenesis might be related to the pulmonary scarring
rather than directly to the mineral oil (see also Yokoo & Suckow,
1961). However, the authors also considered that there was no reason
to suppose that carcinomas were more likely to arise in a scar induced
by mineral oil than in a scar of a different origin (Bryan & Boitnott,
1969).
5. BITUMEN
5.1 Properties and Analytical Methods
5.1.1 Chemical and physical properties
The term bitumen is applied to solid and semi-solid residues from
the distillation of suitable crude oils. This product is known as
"asphalt" in the USA. In most other countries, the term asphalt is
reserved for certain natural deposits and for mechanically made
mixtures of bitumen and mineral matter.
Bitumen is the residue obtained by atmospheric and vacuum
distillation of certain types of crude oil. It is generally a black or
dark-brown material, ranging from a highly viscous liquid to a solid
and brittle substance at normal ambient temperatures, depending on the
proportion of light fractions removed. On heating, bitumen softens
gradually and eventually becomes fluid. Grades are characterized by
their "penetration" and "softening" point. According to the Petroleum
Handbook (1966), bitumen can be considered as a colloidal system of
highly condensed aromatic particles in an oil with ring-type
molecules. From this statement, it is clear that bitumen is a very
complex mixture of mainly high-boiling hydrocarbons. Its composition
varies widely with the geographical source of the crude oil and the
process of manufacture. For example, a mixture of 6 samples was found
to contain:
(a) 32% asphaltenes: high-relative-molecular-mass aromatic compounds
and heterocyclic hydrocarbons of which some are unsaturated. They
are soluble in carbon disulfide but insoluble in petroleum
naphtha;
(b) 32% resins: polymers resulting from the processing of unsaturated
hydrocarbons;
(c) 14% saturated hydrocarbons: hydrocarbons in which the carbon atoms
are connected by a single bond; and
(d) 22% aromatic hydrocarbons: hydrocarbons containing one or more
benzene rings per molecule, including condensed polycyclic
aromatic hydrocarbons (Simmers et al., 1959; Simmers, 1964).
While the appearance and engineering applications of bitumens and
asphalts are similar to those of coal-tars and pitches, fundamental
differences exist between these 2 classes of materials (Puzinauskas &
Corbett, 1978). Bitumen is generally derived from crude oil by a
process that does not involve cracking or thermal conversion, and
coal-tars and pitches are obtained by high-temperature carbonization
of bituminous coal. Chemically, coal-tar materials are mainly composed
of highly condensed-ring aromatic and heterocyclic hydrocarbons.
Bitumens, on the other hand, contain a much higher proportion of high
relative molecular mass paraffinic and naphthenic hydrocarbons and
their derivatives. Under comparable heating during application and
use, coal-tars generate substantially higher emissions of polynuclear
aromatic hydrocarbons than bitumen. While epidemiological surveys of
workers engaged in the production of coal-tar have revealed an
increased incidence of lung cancer, no increases in cancer or other
adverse effects have been observed in studies on workers involved in
the manufacture and application of asphalt.
The benzo(alpha)pyrene content of petroleum bitumens derived from
various Russian crude oils was determined by Janyseva et al. (1963).
They demonstrated that the benzo(alpha)pyrene content of straight-run
bitumen was considerably lower (of the order of 0.6 mg/kg) than that
of bitumens derived from cracking residues (of the order of
4-272 mg/kg). Schamp & van Wassenhove (1972) reported
benzo(alpha)-pyrene levels of 3-5 mg/kg in bitumens.
5.1.2 Methods of sampling and analysis
See section 2.1.2.
5.2 Sources of Environmental Pollution
5.2.1 Natural sources
Natural bitumen and asphalt deposits occur in various parts of the
world, mainly as a result of mineral oil seepage from the ground. The
most well-known natural asphalt deposit is the Trinidad Lake, which
contains a mixture of about 39% bitumen, 32% mineral matter, and 29%
water and gas.
5.2.2 Man-made sources
5.2.2.1 Production
Total world-wide bitumen production reached 90 million tonnes in
1973. As with crude oil, this was approximately 10 times the immediate
pre-war level. Bitumen production rose to 100 million tonnes in 1979
and is expected to continue to increase in the future, although at a
lower rate of growth than in the past.
The following types of bitumen are produced by refining and
treatment:
"Straight" bitumen
The residue of atmospheric or vacuum distillation of
asphaltic-based crude oils. For special applications, very hard
pitch-type bitumen residues can be obtained by distilling cracked
oils.
"Blown" bitumen
Manufactured by feeding air-bubbles countercurrent through a
column of hot molten straight bitumen. Oxidation reactions occur
leading to dehydrogenation and polymerization of the unsaturated and
aromatic components. In this process, large condensed aromatic nuclei
may also formed.
"Cutback" bitumen (or more fluid bitumen grades)
Obtained by mixing bitumen with petroleum solvents or mineral oil,
sometimes with coal-tar or high aromatic extracts.
Bitumen emulsion
Made by emulsifying 50-65% of bitumen in water in the presence of
0.5-1.0% of an emulsifier, usually soap and generally used cold for
both roadmaking and industrial purposes.
5.2.2.2 Uses
The main use of bitumen is for paving roads. It is also used in:
(a) lining irrigation canals, water reservoirs, dams, and dykes;
(b) mastic asphalt for industrial flooring;
(c) bituminized felts for roofing;
(d) protective coatings, for walls, motor-cars, water mains;
(e) adhesives for the building industry;
(f) coal briquetting;
(g) electrical insulation; and
(h) battery-making.
5.3 Environmental Exposure Levels
Apart from walking or riding on bituminous pavements and roads,
the general population will not normally come into contact with
bitumen, except in the form of protective coatings and coal
briquettes. On the other hand, the general population can, on
occasion, be exposed to fumes from heated bitumens for short periods
of time during road-building or the covering of roofs. Emissions from
asphalt roads have been mentioned in the literature as a possible
source of exposure for the general population, but such exposure is
considered to be as negligible. Recent evidence from the Federal
Republic of Germany and the USA confirms this (Hettche, 1963).
Occupational exposure to bitumen may be much more intensive in
certain professions and may range from accidental splashing with hot
bitumen to repeated and prolonged contact of the skin with the more
liquid bitumen grades or to exposure to fumes from heated bitumen.
5.4 Environmental Distribution and Transformation
No specific data relating to bitumens are available in relation to
distribution among media, environmental transformation and
degradation, interaction with physical, chemical, or biological
factors, and bioconcentration. On the other hand, there is a lot of
information on the microbial degradation of individual petroleum
hydrocarbons (section 3.4).
5.5 Metabolism
Uptake and storage of the light fractions contained in bitumens
may occur; however, no specific data exist on this subject.
Bitumens occur in a variety of commercial products. The
composition of these products may vary widely depending on the
geographical origin of the crude oil used and the manufacturing
process applied. These facts may influence the results of metabolic
studies, all of which are concerned with the exposure of experimental
animals. Human data are lacking.
5.6 Effects on Experimental Animals
5.6.1 Short-term exposure
No data are available on acute toxicity, exposures related to
adverse effects, interactions, and species comparisons. It is
generally accepted that the acute toxicity of bitumens is low.
5.6.2 Long-term exposure
No data appear to have been published on toxic effects in specific
organs, teratogenicity, or reproduction. The data on mutagenicity are
limited. On the other hand, substantial experimental work has been
carried out concerning the carcinogenic effects of bitumens on skin.
Twort & Fulton (1930) examined the carcinogenic effects of various
synthetic tars and their fractions on the skin of mice. The
carcinogenic activity varied according to the compound used and with
the temperature of pyrolysis. Results of these studies confirmed the
earlier finding of Kennaway (1925), that the temperature at which the
tar formed was an important factor in the production of carcinogenic
substances. Cancer of the skin of mice was induced by applications of
the synthetic residues obtained from heating substances such as
acetylene, isoprene, a noncarcinogenic petroleum, yeast, and human
skin. The yield of carcinogens decreased when carbonization had
occurred at temperatures above 950°C. The yield was greatest between
850 and 870°C; less between 600 and 750°C, and negligible at 500°C.
Furthermore, the authors found that the carcinogenic activity of the
synthetic residues could be reduced considerably by oxidation or
reduction (by various methods) or by dilution with oleic acid.
A wide range of aliphatic and simple aromatic hydrocarbons were
pyrolysed by Badger (1962), who showed that polynuclear aromatic
hydrocarbons were formed from simpler hydrocarbons at 700°C via
primary radicals formed by carbon-hydrogen and carbon-carbon fission
(cracking) at this elevated temperature. The same process occurred to
a much lesser extent at 550°C.
According to Bogovski et al. (1963), thermal distillation of a tar
to coke decreased the carcinogenic activity by the formation of
irreversible condensation products from polynuclear aromatic
hydrocarbons, together with other high molecular compounds. In
long-term, skin-painting experiments on white mice, the authors showed
that such a coking process reduced the tumour incidence from 68% to
3.7% in the case of shale-oil tar. As shale-oil tars have a much
higher carcinogenic activity than petroleum residues (Twort & Twort,
1931), it might be expected that coking of petroleum residues would
reduce the carcinogenicity of the material still further.
The carcinogenic effects of bitumen on the skin of C-57 black mice
was studied by Simmers and co-workers (1959). In a first series of
tests, they used a mixture of 6 samples from Southern Californian
refineries, in which both steam- and aft-blown bitumens were mixed.
Painted twice weekly on the skin for a lifetime, the mixture caused 12
dermoid carcinomas in 68 animals, compared with none in the untreated
control group. Formation of cancer was preceded by hair loss, dryness
and scaling of the skin, and papilloma formation. After subcutaneous
injection, 8 sarcomas occurred in 62 animals at the site of injection
compared with none in the control group. However, the relevance of
results from such subcutaneous studies is questionable.
In an inhalation study on the same pooled sample and using the
same strain of mice, the animals inhaled an aerosol of bitumen
droplets suspended in moist air for 30 min/day, 5 days per week, for
up to nearly 17 months (Simmers, 1964). Changes found on microscopic
examination were minimal and included occasional congestion, acute
bronchitis, pneumonitis, bronchial dilatation, and some peribronchial
round-cell infiltration. In a second inhalation study, the animals
were exposed to cooled smoke from bitumen at 120°C for 6-7´ h/day, 5
days a week, for up to 21 months. In this study, peribronchial
round-cell infiltration, bronchitis, pneumonitis, abscess formation,
loss of cilia, epithelial atrophy, and necrosis were more common.
Squamous cell metaplasia was rare, but hyperplasia was more commonly
seen. The changes in both experiments were patchy, rather
non-specific, and similar to those described as a result of exposure
to other air pollutants.
In his next series of tests, Simmers (1965a) compared the
carcinogenicity of straightrun and air-blown bitumen in prolonged
regular skin application tests and after single or repeated
subcutaneous injection. Undiluted air-blown bitumen did not induce
tumours, when applied to the skin, probably because the bitumen was
too hard; when dissolved in toluene the incidence of skin cancers
increased to 45%. This may be the result of better contact with, or
penetration into the skin. On the other hand, the polynuclear aromatic
hydrocarbons might be concentrated by the solvent. In a similar
skin-painting study with straight-run bitumen, skin cancers occurred
in only 14%. The same trend was apparent after subcutaneous injection,
where 0 and 13% tumours were found at the site of injection with
straight-run and air-blown bitumen, respectively. The author presumed
that this difference in response resulted from a difference in
chemical composition. Although the aromatic fraction of air-blown
bitumen was lower than that of straight-run bitumen, it contained more
complex aromatic hydrocarbons due to polymerization and condensation
caused by the air-blowing.
Straight-run bitumen was separated into 4 fractions, which were
painted 3 times a week, continuously, on the skin of the same strain
of mice (Simmers 1965b). The fraction containing most of the saturated
compounds and aromatic hydrocarbons -- which also showed practically
all the UV light fluorescence -- induced considerably more skin
rumours (43.3%) than straight-run and air-blown bitumens had done in
earlier studies. The author pointed out that, though fluorescence was
not a guarantee of carcinogenic activity, polynuclear aromatic
hydrocarbons known to be carcinogenic are fluorescent. In this
context, both Kennaway & Heiger (1930) and Berenblum et al. (1947)
suggested that these compounds -- even in very small quantities --
might be discovered by this method. The 4 bitumen fractions used in
the previous study were injected subcutaneously -- once or repeatedly
-- in the same strain of mice, at various doses (Simmers, 1966). A
variety of benign and malignant tumours resulted, both at the
injection site and in distant organs. The dose seemed to be more
important for tumour formation than the duration of exposure.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Four road-bitumens of different geographical and
manufacturing origin were applied to the skin of, or injected
intra-muscularly into, mice and rats. In some of the test-animals,
tumours were found at the site of application. Fumes from heated
coal-tar and from a blown bitumen used as a roofing bitumen did not
induce cancers of the lungs in rats and guineapigs in inhalation
experiments lasting up to 2 years. However, the general typical
reactions found in lung-tissue were the same as those described by
Simmers (1964). Condensates of the coal-tar fumes were highly
carcinogenic when applied to the skin and intramuscularly in mice;
condensates of the blown bitumens fumes were non-carcinogenic to the
skin of mice and rabbits in similar tests.
Petroleum residues derived from cracking were painted 3 times a
week for a lifetime on the skin of albino mice. Depending on the
cracking process, the residues exhibited various degrees of
carcinogenic activity, but none were as active as a higher temperature
generated coal-tar. Only those fractions distilled from the tar above
370°C showed carcinogenic activity. Blending with a noncarcinogenic
oil increased the carcinogenic activity of one bitumen, possibly
because of better skin penetration (Smith et al., 1951).
Kireeva (1968) painted groups of white SS-57 mice, once a week
throughout the life span, with 40% solutions in benzene of various
bitumens derived from Ukrainian crudes and coal-tar pitch. The
control-group was painted with benzene only. With coal-tar pitch, skin
tumours appeared in 88.4% of the animals; with bitumens prepared from
cracking residues, they occurred in 9.5-18.4%, and with straight-run
bitumens, depending on its origin, in only 0.0-4.6% of the animals.
Benzene solutions of 8 bitumens of different geographical origin
and 2 coal-tar pitches were applied twice weekly to the skin of Swiss
albino mice throughout their lifetime. Benzene alone was applied to
control animals (Wallcave et al., 1971). The polynuclear aromatic
hydrocarbon content of the coal-tar pitches was found to be several
orders of magnitude greater than that of the bitumens examined. Only
one carcinoma and 5 papillomas were observed in 218 mice treated with
bitumens, whereas over 90% of the coal-tar pitch treated animals
developed such tumours. Again, it was suggested that the tumour
incidence depended on the polynuclear aromatic hydrocarbon content of
the product tested.
Benzene solutions of the bitumen or polynuclear aromatic fractions
of Athabasca tar sands were not mutagenic for Salmonella typhimurium.
This may have been a reflection of the complex interactions occurring
with such hydrocarbon mixtures (Shahin & Fournier, 1978).
To summarize, in animal studies, some bitumens have been shown to
possess some carcinogenic activity, when applied to the skin, while
inhalation studies with bitumen vapours have proved to be negative.
The carcinogenicity of bitumens depends to a certain degree on the
method of production (cracking, blowing) or mixing. "Cutback" with
high aromatic oil or coal-tar facilitates skin-contact and increases
the carcinogenicity of the mixture. The carcinogenic activity,
however, is low in comparison with that of coal-tar. Moreover, as Siou
(1972) in a literature study on bitumens concluded, it is difficult to
extrapolate from these animal data to man, because the contact with
bitumen, even in occupational exposures, is of a quite different order
to regularly repeated skin application throughout the life span of a
test animal.
5.7 Effects on Man
5.7.1 Epidemiological studies
5.7.1.1 Occupational exposure
Henry (1947), in his analysis of 3753 cases of skin cancer, found
only one case in which bitumen might have been involved. The man had
worked for 23 years as a road-worker and had been exposed to coal-tar
in the earlier years.
The health and the causes of death of 96 workers who had worked in
a bitumen plant since it opened, including those who had left the
company was reviewed by Hoogendam (1962). Exposures ranged up to 40
years. Thirty-nine workers had been exposed for more than 20 years and
at least 15 had been exposed for more than 30 years. No significant
differences were found in theft general health pattern and no cases of
skin tumours were found. One man, who had worked for 40 years in this
plant died at the age of 55 of bronchial carcinoma. It is impossible
to determine whether there was a relation with his work in this case.
Vital capacities and forced expiratory volume (FEV) of those still
working did not differ from those of a control group.
The mortality rate of refinery workers was compared with those of
other workers in a study on 15 437 employees of an oil refinery over
the 29-year period 1935-63. A comparison was also made with data from
a similar group of the outside population. The incidence of lung
cancer was no greater in the refinery operators than in the other
groups (Baird, 1967).
Baylor & Weaver (1968) compared the health of 462 asphalt workers
in 25 oil refineries with that of 379 controls. Each of the asphalt
workers had been engaged in this type of work for at least 5 years
with an average of 15.1 years, service. No significant differences in
health were found between the 2 groups. The authors also studied the
medical literature of the 20 years preceding theft publication and
were unable to find one single case of lung or skin cancer that could
be attributed to petroleum bitumens. Furthermore, an extensive search
for information on the health of workers was carried out with road
construction firms (31 companies in 24 states with 11 478 man-years of
asphalt working and 15 boards of health of the state highway
commissions), roofing industries (3 firms with over 1100 long-term
bitumen workers), and bitumen trucking firms (with over 5000 drivers).
This gave no indication that bitumen constituted a health hazard or
that there were cancers of the skin or lungs in these groups that
could be attributed to working with bitumen. Similar indirect
information was obtained from 6 large insurance companies. The authors
concluded that the bitumens in current use did not present a
significant health hazard and that the study indicated that the
carcinogenic or other harmful properties of most commercial asphalts
under the present commercial usage -- if present at all -- are likely
to be of a very low order.
Studies conducted on behalf of the API did not reveal any
occupational health hazards for a "medium-exposure group" of petroleum
refinery workers. This group included asphalt workers, grease, and
lubricant workers (Tabershaw/Cooper Associates Inc. 1974/75).
Hermann (1975) and Hettche (1963) drew similar conclusions to
those of Baylor & Weaver (1968) in inferring that bitumens including
blown grades, were biologically inactive and that vapours from hot mix
plants were not carcinogenic.
5.7.1.2 General population exposure
Measurement of emissions of polynuclear aromatic hydrocarbons from
various industrial processes, including bitumen "blowing" and the
manufacture of asphalt hot-road-mixture, revealed that these
industrial processes were not major sources of benzo(alpha)pyrene
emissions (used as an indicator for polynuclear aromatic hydrocarbons
emission) and certainly emitted less than residential and small
industrial coal-burning furnaces (Von Lehmden et al., 1965).
In the literature, the possibility is mentioned that vapours
emanating from asphalt, or dust arising from it might contribute to
the overall incidence o f cancer of the respiratory tract (Hueper,
1961; Berge, 1969). There are not, however, any data to substantiate
this assumption and, in extrapolating the results of animal testing on
bitumen-vapour inhalation, the chances of such an effect occurring
among the general population seem quite remote. It can be presumed
that a lot of the confusion has been generated by the former use of
coaltar and mixed grades for this purpose, a fact which clearly has
not always been taken into consideration by those working in the
field.
In the USSR, however, the use of "carcinogenic" road material
(that is material containing benzo (a) pyrene) as the upper layer of
roads is forbidden in heavily populated areas. In practice, this means
that coal-tar must not be used for this purpose and that petroleum
bitumen should be used instead (Gorbov & Fomenko, 1962).
5.7.1.3 High (accidental) exposure
Burrell (1957) found a very high incidence of oesophageal cancer
in a group of Bantu confined to one location in East London (South
Africa). The author related this to the long-term preparation and use
of the soporific alcoholic concoction "cidiviki", which was locally
fermented in drums still containing a ¨-inch coating of"cutback"
bitumen. The author presumed that enough carcinogenic material could
have leached out into the brew to have at least acted as a
cocarcinogen.
The most common accidental over-exposure in handling bitumen is
the occurrence of burns from hot bitumen splashes. A few cases,
however, have been described, in which a skin tumour developed within
1-3 months of a burn caused by hot bitumen, coal-tar, or crude oil
(Bang, 1923; Huguenin, 1925; Gunsett, 1930; Sträuli 1957). Considering
the total number of such bums, only a very small proportion develop
into rumours and these cases relate to combinations of such burns with
fresh scar tissue and/or lesions of the mucous membranes. Tumours have
been described in similar situations following heat burns by wood,
welding electrodes, etc. (Gunsett, 1930; Sträuli, 1957). Such skin
tumours can occur in burn scars and are often multifactorial (Emmett,
1975).
In relation to the vast amount of bitumen that has been used for
many decades, the reports of tumours are extremely scarce. In fact, it
can be postulated that, though, from animal testing, it is known that
some bitumens are weak carcinogens, there is no evidence from normal
occupational exposure to indicate that these compounds are a
carcinogenic hazard to the skin or the respiratory tract. This would
also indicate that the present pattern of use of bitumens would not
cause any hazard, whatsoever, for the general population.
The Asphalt Institute studied emissions from the hot-mix process
for the manufacture of paving asphalts (Puzinauskas & Corbett, 1975).
The concentration of polynuclear aromatic hydrocarbons in the
particulate emission was low at 340 mg/m3 and the average
concentration of benzo(alpha)pyrene was very low at 13 ng/m3.
5.7.2 Clinical studies
These relate mainly to bitumen skin burns and will not be
considered here. Other clinical data have been included in the
description of the epidemiological data.
The skin of fair-haired persons might be more prone to react
adversely to repeated prolonged exposure to UV-radiation in sunlight
in combination with bitumen exposure (Smiley, 1951; Kinnear et al.
1954; Emmett, 1975).
6. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO CRUDE OILS AND
SELECTED PETROLEUM PRODUCTS
6.1 Crude Oils
Crude oil is normally handled in a closed system from the oil
well, via storage tanks, pipelines, and shipment by tankers to the
refineries. Under these conditions, health hazards to, and death of
workers involved in these operations will occur only when a serious
breakdown or leakage occurs. Volatile components escaping at well
heads, at pump glands, or through vents in storage tanks and ships'
tanks may, under certain conditions, constitute a similar health
hazard; hydrogen sulfide, if present, is the most acutely toxic
component but detailed consideration is outside the scope of this
review. These volatile components may also contribute to the pollution
of the atmosphere in storage or pumping areas. To the general
population, this is mainly a nuisance problem, because of the odour
from the hydrogen sulfide and mercaptans involved. Crude oil pollution
of seas and inland waterways as a result of accidents with crude oil
tankers or pipelines may, at times, cause a major and sudden
environmental hazard. Tank washings from tankers not using the
"load-on-top" system are another source of pollution of the sea with
crude oils. However, this particular aspect falls outside the scope of
this review.
Atmospheric concentrations of the volatile components of crude oil
were found to be lower near marine drilling rigs than land-based rigs.
Differences in temperature and air movement have been shown to be the
main causes.
The lower temperature of marine pipelines encourages
solidification of waxy compounds of the crude oil on their inner
surfaces. During their repair, the clothes and skin of workers may be
contaminated by these compounds (Alekperov et al., 1974).
6.2 Petroleum Solvents
Though petroleum products are widely used, they do not generally
present a health risk for the general population, as their volatility
prevents them accumulating in the ambient air in concentrations high
enough to cause adverse health effects. However, if improperly used in
closed, poorly ventilated rooms, they may become a cause of accidental
acute poisoning. Under these conditions, the lower-boiling solvents
may also present a fire hazard.
Cases of accidental ingestion may occur, especially in children.
Serious and even fatal lung disease may develop when aspiration in the
lungs occurs. Absorption from the gut, however, is generally not a
serious health hazard.
Substances with a strong odour may cause a nuisance, when present
in the ambient air, even in very low concentrations. The same applies
to the possible contamination of drinking-water through leakage of
petroleum solvents from containers.
Health impairment due to occupational exposure to petroleum
solvents occurs only infrequently in normal work practice. Repeated
skin contact may result in contact irritative dermatitis and only
rarely in contact allergic dermatitis. Continuous day-to-day exposure
to excessive vapour concentrations (which usually occur in poorly
ventilated workshops) may give rise to general non-specific symptoms
of ill-health. Some solvents, however, have a specific systemic
effect, e.g., benzene (bone marrow depression, leukaemogenesis) and
n-hexane (polyneuropathies). Polyneuropathies also occur in cases of
abusive use of petroleum solvents by "sniffers" and addicts.
Accidental exposure to excessive vapour concentration may cause
narcosis, which can be followed very rapidly by respiratory arrest and
death. Accidental over-exposure of the skin, especially if the solvent
is allowed to remain in contact with the skin, may cause skin
irritation, possibly leading to chemical burns.
Blood dyscrasia does not occur through exposure to petroleum
solvents that do not contain benzene. The specific long-term health
hazards of exposure to benzene and petroleum solvents that contain a
substantial percentage of benzene will not be discussed in this
document.
The high aromatic residues that are still used, occasionally, as
solvents for specific purposes may pose a carcinogenic risk to the
skin or on inhalation, if such contact is intensive and prolonged.
6.3 Lubricating Base Oils, Greases, and Waxes
This group of products is unlikely to present a health risk for
the general population, even with gross accidental over-exposure.
Unrestricted and indiscriminate oral or nasal administration of white
medicinal oil can occasionally give rise to the occurrence of lipid
pneumonia, when pre-existent disease or other conditions predispose to
the entry of mineral oil into the respiratory tract.
Prolonged and intensive contact of the skin with metal-working
oils during occupational exposure results in a high incidence of skin
disorders and relatively more cases of skin cancer have occurred in
such occupations. Improvements in both industrial and personal hygiene
have reduced the incidence of malignant and non-malignant diseases of
the skin. Results from animal studies indicate that substitution of
the oils, formerly used, by more refined products may significantly
contribute to the prevention of skin cancer caused by exposure to oil
under poor standards of industrial hygiene. Because chronic lung
disease following exposure to oil mist is extremely rare and an
increase in the incidence of pulmonary carcinoma has only been
reported under exposure conditions where skin cancer occurs, it seems
reasonable to assume that these occupational diseases can be prevented
by suitable preventive measures.
6.4 Bitumen
The health risks associated with bitumen appear to be minimal,
because contact is unlikely. Occupational exposure is not associated
with an increase in cancer of the skin or the respiratory tract. The
most significant occupational hazard is burning of the skin from
splashes of heated bitumen.
Certain bitumen grades, however, such as those derived from
cracked oils, and those mixed with coal-tar or high aromatic extracts,
may have a carcinogenic potential and, therefore, require special
precautions in handling.
7. CONTROL MEASURES
7.1 General
Every effort should be made not to contaminate workers, the work
place, or the general environment with petroleum products. Proper
design of machinery, equipment and the workshop, enclosure of
processes, adequate ventilation, provision of protective clothing and
adequate facilities for personal hygiene, suitable education and
supervision, and development of safe working procedures are essential
basic requirements; good hygienic work practices can achieve a great
deal in protecting the worker.
Care should be taken, when developing products, to ensure that
levels of the most toxic components, e.g., benzene, n-hexane, and
polynuclear aromatic hydrocarbons are known, so that proper controls
can be devised for the use of the products.
Products containing such highly toxic components should, when
possible, be avoided and alternatives sought, where exposure is
unavoidable or is likely to occur. Products which, during use, may
generate more and highly toxic contaminants should be identified and
efforts made to reduce contamination. Care should be exercised when
handling and using such products and consideration given to the need
for renewal of products at appropriate intervals. In relation to
metal-working, the danger of repeatedly "topping-up" machinery over
long periods with a product, and of mixing different products in one
machine, is emphasized.
Where contact is unavoidable, suitable protective equipment should
be used, although this should always be used as the last resort. Where
skin contact is inevitable, efforts should be made to limit or avoid
not only benzene, n-hexane, and polynuclear aromatic hydrocarbons
but also any additives that may have an adverse effect on the skin.
The use of abrasives and solvents in cleansing the skin should be
discouraged.
In the less developed countries, there is often a lack of
awareness concerning the need for proper control measures when
handling petroleum products. In these countries, the health education
of employers and workers should be promoted with reference to the
products. Adequate control programmes should be implemented using
known techniques. Whenever necessary, these should be modified to fit
the particular circumstances of the country.
In addition, proper planning is necessary to control the disposal
of the many types of waste oil products so that environmental
contamination is avoided. Thought must also be given to the need to
control the siting of housing and amenities in relation to petroleum
refineries and petrochemical plants.
7.2 Petroleum Solvents
Excessive exposures to these products should be avoided. Proper
education of manufacturers and users and appropriate labelling of
consumer products are important. Regular control of workroom
atmospheric concentrations of petroleum solvents is indicated,
especially in smaller workshops.
Petroleum solvents are, with the exception of benzene and
n-hexane, less toxic than most other solvents and, with suitable
precautions, can be safely used. For this reason, and because they are
cheap, they are widely used in industry and their replacement by other
solvents is unlikely in the foreseeable future.
7.3 Lubricating Base Oils, Greases, and Waxes
The only way in which members of the general population could,
under normal circumstances, be over-exposed to any of this group of
compounds would be in the indiscriminate use of medicinal liquid
paraffin. Excessive use -- especially self-administered -- of this
material, intranasally or via the oral route, by debilitated or
dysphagic patients should be discouraged.
The main possibilities of over-exposure occur in the occupational
situation. Without going into detail, the following factors should be
considered with the aim of avoiding hazardous exposures:
(a) Avoid contact by technological means including: proper design
of equipment, machinery, and workshop; proper design and operation of
general and exhaust ventilation systems in places where oil mist
generation cannot be avoided; use of base oils with the lowest content
of polynuclear aromatic hydrocarbons that can practically be achieved
by suitable refining processes; limiting of the concent of additives
that have adverse effects on the skin; frequent changing of oil in
situations where carcinogenic compounds might be generated by heat;
avoidance of the reuse of used oils, without refining, for other
purposes where they might cause a health hazard; education and
instruction in all these safe-working procedures, and supervision of
their proper execution;
(b) Use of safe-working procedures and, where required, protective
clothing and proper protective equipment; availability of general
hygienic facilities including wash-basins in or near the work-place,
shower facilities, lockers for private clothing outside the
contaminated area; regular changing and laundering of working clothes
and underwear; the use of suitable emollient skin creams and in some
instances barrier creams. Abrasives and solvents should not be used
for cleaning the skin.
(c) Periodic medical examinations.
These precautions have been compiled and adapted from the
following references: Kipling (1968); UK Medical Research Council
(1968); Cruickshank (1969); Desoille et al. (1973); BRMA (undated).
Manufacturer can contribute by ensuring that these precautions are
observed in their own premises and by giving the proper information
and guidance to the consumer (see also Eckardt, 1967).
Government actions vary from country to country depending on local
legislation and practice. They may range from supervision and control
of the measures taken by industry to the issue of directives or codes
of practice. Cautionary notices can be issued (e.g., HMSO, 1979),
which have to be displayed in the working place, as well as leaflets
for the workers (e.g., HMSO, 1967). Diseases caused by mineral oil may
be notifiable as occupational diseases, according to national
legislation.
Consumer education on the possible hazards of the products used
and their safe handling can best be done by the manufacturing
industries, when possible in cooperation with relevant Government
agencies.
Medical action in this field would consist of cooperating in the
team supervising the industrial hygiene of the operation, in
pre-placement examination and selection, and later in the periodic
medical follow-up of the exposed workers.
Various other organic liquids meet requirements for lubricating
oils and greases. Carboxylic esters are generally used. These
synthetic lubricants, however, are not in abundant supply, are
expensive, and are not likely to be widely used outside their specific
field of application. Replacement products are sometimes more
hazardous. For instance, cutting oils may be replaced by mixtures
containing ethanolamine that give rise, under certain conditions, to
the formation of nitrosamines.
When safe lubricating base oils are used there is, on the other
hand, no reason from an occupational health point of view to use such
alternatives, not even in places where long-term intensive skin
contact and/or oil mist exposure might take place. In these cases,
industrial hygiene should be improved and personal hygiene should be
closely supervised, regardless of the product used, to avoid excess
exposure.
7.4 Bitumen
In road-building which is the major application of bitumen,
alternative materials such as plastics and resins are far too
expensive for general use. Coal-tar, which was used previously,
presents a greater environmental health hazard than bitumen. Other
alternatives would be concrete and bricks.
In various other applications, such as flooring, roofing,
protective coatings, and adhesives, alternative materials are
available, but these are not always superior in quality and/or
competitive in price.
REFERENCES
ABBRITTI, G., SIRACUSA, A., CIANCHETTI, C., COLI, C. A., CURRADI, F.,
PERTICONI, G. F., & De ROSA, F. (1976) Shoe-makers' polyneuropathy
in Italy: The aetiological problem. Br. J. ind. Med., 33: 92-99.
ACGIH (1971a) Documentation of the threshold limit values,
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 19-20.
ACGIH (1971b) Documentation for TLV -- Supplements for those
substances added or changed since 1971, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists, p. 341.
ACGIH (1974) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1974, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978a) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1978, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978b) Air sampling instruments for evaluation of atmospheric
contaminants. 5th ed., Cincinnatti, OH, USA, American Conference
of Governmental Industrial Hygienists.
ADLARD, E. R. (1972) A review of the methods for the identification of
persistent hydrocarbon pollutants on seas and beaches. J. Inst.
Petrol., 58: 63-74.
AIDIN, R. (1958) Petrol-vapour poisoning. Br. med. J., ii: 369-370.
AIHA (1976) Direct reading colorimetric indicator tubes manual,
Akron, OH, USA, American Industrial Hygiene Association.
AINSWORTH, R. W. (1960) Petrol-vapour poisoning. Br. med. J.,
i: 1547-1548.
ALEKPEROV, I. I., AMIROV, R. O., POVOVAROV, V. D., & LOSEVA, I. E.
(1974) [Hygiene of work at exploitation of marine oil deposits.]
Moscow, Idz. "Nedra" (in Russian).
ALLEN, N. (1975) Chemical neurotoxins in industry and the environment.
In: Tower, D. B., ed. The nervous system, Vol. 2, The clinical
neurosciences, New York, Raven, pp. 235-248.
ALTENKIRCH, H., MAGER, J., STOLTENBURG, G., & HELMBREHT, J. (1977)
Toxic polyneuropathies after sniffing a glue thinner. J. Neurol.,
214: 137.
ANDRÉ, M. L. & O'NEAL, M. J. (1959) Mass spectrometric analyses of
medium viscosity lubricating oils. Anal. Chem., 31: 164-169.
ANON, (1973) Evaluations of world's important crudes. Oklahoma, USA,
The Petroleum Publishing Company (Reprinted from Oil Gas J.).
ANON (1974) Exposure to petrol fumes. Br. med. J., ii: 115.
ANON (1975) Benzpyrene hydroxylase - innate determinant of PAH
toxicity? Food cosmet. Toxicol., 12: 264-268.
ANTONOV, A.M. & LINTS, A.M. (1960) The blastomogenic action of natural
Saratov oil. Problems Oncol., 6: 1629-1634.
API (1965) Toxicological review. Aromatic petroleum naphtha. 2nd
ed., New York, American Petroleum Institute, pp. 5.
API (1967a) Toxicological review. Gasoline, 1st ed., New York,
American Petroleum Institute, pp. 5.
API (1967b) Toxicological review. Kerosine, 2nd ed., New York,
American Petroleum Institute, pp. 4.
API (1969a) Final Report of the task force on used oil disposal.
Washington, DC, American Petroleum Institute, pp. 44.
API (1969b) Toxicological review. Petroleum naphthas, 1st ed., New
York, American Petroleum Institute, pp. 7.
API (1974) Mutagenicity study of thirteen petroleum fractions,
Washington, DC, American Petroleum Institute (API U-150-14, The
Hine Laboratories Inc. Report No. 4).
API (1975a) Review of analytical methods for determination of oil and
grease in waters produced from oil and gas extraction operations.
Prepared by the Task Group on Analytical Methods July 14th 1975,
New York, American Petroleum Institute, pp. 38.
API (1975b) Mutagenic evaluation of rubber solvent (CHF-32-263),
W/filtered rubber solvent (Summary and analysis), Washington,
DC, American Petroleum Institute (Litton Bionetics Inc. Report).
API (1975c) Research report. Mutagenic evaluation of 60 solvent
(CHF-33-148) (Summary and Analysis), Washington, DC, American
Petroleum Institute (Litton Bionetics Inc. Report).
API (1977a) Research report. Mutagenicity evaluation of kerosene.
Final report (Summary and Analysis), Washington, DC, American
Petroleum Institute, 3 pp. (Litton Bionetics Inc. Project
No. 2697).
API (1977b) Research report. Teratology study in rats: Stoddard
solvent. Final report (Summary and Analysis), Washington, DC,
American Petroleum Institute, 5 pp. (Litton Bionetics Inc. Project
No. 20698-2).
API (1978a) Mutagenicity evaluation of Stoddard solvent (Summary),
Washington, DC, American Petroleum Institute (Litton Bionetics
Inc. Report no. API-PS-4).
API (1978b) Research report - Teratology study in rats: Rubber
solvent. Final report. Litton, API (Full Report and Analyses).
API (1978c) Teratology study in rats: Xylene. Final Report,
Washington, DC, American Petroleum Institute, 39 pp. (Litton
Bionetics Inc. Project No. 20698-5).
API (1978d) Research report. Mutagenicity of xylene. Final report
(Full report and Analytical).
API (1978e) Teratology study in rats: Toluene. Final report (Summary
and Analysis), Washington, DC, American Petroleum Institute, 2 pp.
(Litton Bionetics Inc. Project No, 20698-4).
(1978f) Mutagenicity evaluation of toluene (Summary and Analysis),
Washington, DC, American Petroleum Institute, 3 pp. (Litton
Bionetics Inc. Project No. 20847).
API (1979a) Research report. Teratology study in rats: Kerosene.
Final report (Summary and Analytical), Washington, DC, American
Petroleum Institute 2 pp. (Litton Bionetics Inc. Project
No. 20698-10).
API (1979b) Research report. Inhalation teratology study in rats: VM
and P naphtha, Final report, Washington, DC, American Petroleum
Institute, 18 pp. (Litton Bionetics Inc. Project No. 21035-02).
API (1979c) Research report. Teratology study in rats: n-Hexane.
Final report, Washington, DC, American Petroleum Institute,
24 pp. (Litton Bionetics Inc. Project No. 20698-9).
API (1979d) In vitro and in vivo mutagenicity studies: New oil
composite. Final report, Washington, DC, American Petroleum
Institute (Hazleton report).
ARCOS, J. C. & ARGUS, M. F. (1974) Chemical induction of
cancer-structural bases and biological mechanisms, Vol. 11A,
New York and London, Academic Press.
ASK-UPMARK, E. (1955) Bronchial carcinoma in printing workers. Dis.
Chest., 27: 427-235.
ASTM D2820 (1972) Standard method of test for Cl through C5
hydrocarbons in the atmosphere by gas chromatography,
Philadelphia, Am. Soc. for Testing and Materials.
ASTM D1605-60 (1973) Standard recommended practices for sampling
atmospheres for analysis of gases and vapours, Philadelphia,
Am. Soc. For Testing and Materials.
ASTM D510 (1974) Standard methods of sampling water, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2682 (1974) Standard method of test for polynuclear
aromatic hydrocarbons in air particulate matter, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2778 (1974) Standard method of test for solvent extraction or
organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ASTM D3370 (1976) Standard method of test for solvent extraction
of organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ÅSTRAND, I., KILBOM, A., & ÖVRUM, P. (1975) Exposure to white spirit.
I. Concentration in alveolar, air and blood during rest and
exercise. Scand. J. Work environ. Health, 1: 15-30.
ÅSTRAND, L., ENGSTRÖM, J., & OVRUM, P. (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man.
Scand. J. Work Environ. Health, 4: 185-194.
AULD, S. J. M. (1950) Environmental cancer and petroleum. J. Inst.
Petrol., 36: 235-253.
AVELLAN, L., JACOBSON, B., & JOHANSON, B. (1967) Carcinoma of scrotum
induced by mineral oil. Scand. J. plast. reconstr. Surg.,
1: 135-140.
AXELSON, O., HANE, M., & HOGSTEDT, C. (1976) [Case descriptions of
chronic psycho-organic syndrome in house painters.]
Läkartidningen, 73: 317-318 (in Swedish).
BADGER, G. M. (1962) Mode of formation of carcinogens in human
environment. Nat. Cancer Inst. Monogr., 9: 1-16.
BAIRD, V. C. (1967) Effects of atmospheric contamination on cancer
mortality in petroleum refinery employees. J. occup. Med.,
9: 415-420.
BALDWIN, R. W., CUNNIGHAM, G. J., & PRATT, D. (1964) Carcinogenic
action of motor engine oil additives. Br. J. Cancer,
18: 503-507.
BANG, F. (1923) Contribution a l'étude de la cancérisation de la
cellule et du temps d'éclosion des tumeurs malignes. A propos d'un
cas de "cancer aigu" du goudron chez un ouvrier. Bull. Assoc. Fr.
Étud. Cancer, 12: 184-199.
BARANOVA, V. G. & LOGINOVA, N. K. (1977) [Determining cyclopentadiene
in the air of industrial premises.] Gig. Tr. prof. Zabol.,
No. 3, pp. 54-55 (in Russian).
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of ethyl
benzene, styrene and alpha-methylstyrene in man. Am. Ind. Hyg.
Assoc. J., 31: 206-209.
BARRIENTOS, A., ORTUNO, M. T., MORALES, J. M., TELLO, F. M., &
RODICIO, J. L. (1977) Acute renal failure after use of diesel fuel
as shampoo. Arch. intern. Med., 137: 1217.
BATT-NEAL, L. & WOLMAN, M. (1977) Tumors and amyloidosis in mice
painted with crude oil found on bathing beaches. Bull. environ.
Contam. Toxicol., 18: 385-391.
BAYLOR, C. H. & WEAVER, N. K. (1968) A health survey of petroleum
asphalt workers. Arch. environ. Health, 17: 210-214.
BELINKY, B. R. (1980) In: Dolberg, D. D. & Verstuyft, ed. Analytical
techniques in occupational health chemistry. Washington, DC,
ACS, (ACS Symposium Series 120).
BELL, J. (1876) Paraffin epithelioma of the scrotum. Edin. med. J.,
22: 135.
BERENBLUM, I., HOLIDAY, E. R., & JOPE, E. M. (1947) Some physical
methods of investigating carcinogenic hydrocarbons. Br. med.
Bull., 4: 326-331.
BERGE, H. (1969) [Harmful emissions in and around cities.]
Vitalstoffe, 14: 161-163 (in German).
BERGEL, F. (1974) Carcinogenic hazards in natural and man-made
environments. Proc. R. Soc. (London), 185: 165-181.
BERTINE, K. K. & GOLDBERG, E. E. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BESKROVNAJA, N. I., HRUSTALEVA, G. F., ZIGULINA, G. A., & DAVYDKINA,
T. I. (1979) [The gynaecological sick rate of female workers in
the rubber industry.] Gig. Tr. prof. Zabol., No. 8, pp. 36-38
(in Russian).
BILIMAIER, D., YEE, H. T., ALLEN, N., CRAFT, B., WILLIAMS, N.,
EPSTEIN, S., & FONTAINE, R. (1947) Peripheral neuropathy in a
coated fabrics plant. J. occup. Med., 16: 665-671.
BINGHAM, E. & FALK, H. L. (1969) Environmental carcinogens. The
modifying effect of co-carcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BINGHAM, E. & HORTON, A. W. (1966) Environmental carcinogenesis:
experimental observations related to occupational cancer.
Adv. Biol. Skin, 7: 183-193.
BINGHAM, E. & WORD, P. J. (1977) Co-carcinogenic effects of n-alkanes
and ultraviolet light on mice. J. Natl Cancer Inst.,
38(4): 1099-1101.
BINGHAM, E., HORTON, A. W, & TYE, R. (1965) The carcinogenic potency
of certain oils. Arch. environ. Health, 10: 449-451.
BISHOP, P. G. C. (1940) Oil aspiration pneumonia and pneumolipoidosis.
Ann. int. Med., 13: 1327-1359.
BLATTNER, R. J. (1951) Kerosene poisoning. J. Pediatr., 39: 391-392.
BOEHME, H. & HUEHNERMANN, W. (1966) Examination of mineral oil
products in the (West) German Pharmacopoeia, 7th edition. Arch.
Pharm., 299: 368-80D.
BOENHEIM, A. F. & PEARSON, A. J. (1973) Petroleum hydrocarbon
solvents. In: Modern petroleum technology, 4th ed., Barking,
Applied Science Publishers Ltd.
BOGOVSKI, P. A., EISEN, O. G., & ARRO, I. H. (1960) [On the
carcinogenic action of some chromatographic fractions of
chamber-oven tar obtained from Estonian oil-shale.] Vop. Onkol.,
6: 34-42 (in Russian).
BOGOVSKI, P. A., GORTALUM, G. M., & KOZHEVNIKOV, A. V. (1963)
Decancerigenization of some shale processing products. Acta Un.
Int. Cancer, 19: 481-482.
BOHLEN, P., SCHLUNEGGER, U. P., & LÄUPPI, E. (1973) Uptake and
distribution of hexane in tissues. Toxicol. appl. Pharmacol.,
25: 242.
BOITNOTT, J. K. & MARGOLIS, S. (1966a) Mineral oil in human tissues.
I. Detection of saturated hydrocarbons using thin-layer
chromatography. Bull. John Hopkins Hosp., 118: 402-713.
BOITNOTT, J. K. & MARGOLIS, S. (1966b) Mineral oils in human tissues,
II. Oil droplets in lymphnodes of the porta hepatis. Bull. John
Hopkins Hosp., 118: 414-422.
BORRIE, J. & GWYNNE, J. F. (1973) Paraffinoma of lung: Lipoid
pneumonia. Report of two cases. Thorax, 28: 214-221.
BOURNE, L. B. (1967) Skin diseases from oils - causes and prevention,
London, British Safety Council, 11 pp.
BOUTWELL, R. K. & BOSCH, D. K. (1959) The tumor-promoting action of
phenol and related compounds for mouse skin. Cancer Res.,
19: 413-424.
BP (1973) Health guide to BP petroleum products, London, The British
Petroleum Company Ltd, pp. 26.
BP (1975) Trace elements and other elements in crude oil:
A literature review, Sunbury, The British Petroleum Company Ltd.
BRIDGE, J. C. & HENRY, S. A. (1928) Industrial cancers. In: Report of
the International Conference on Cancer, London, Bristol, John
Wright, pp. 258-268.
BRMA (undated) Mineral oils, London, British Rubber Manufacturers
Association Ltd.
BROMER, R. S. & WOLMAN, I. J. (1939) Lipoid pneumonia in infants and
children. Radiology, 32: 1-7.
BROWN, A. J., WALDRON, H. A., & WATERHOUSE, J. A. H. (1975) Report on
a study of occupational skin cancer with special reference to
scrotal cancer, Birmingham, UK, University of Birmingham.
BROWN, A. L. & BISKIND, G. R. (1941) Differential diagnosis between
lipid pneumonia and pulmonary neoplasm. J. Am. Med. Assoc.,
117: 4-6.
BROWNING, E. (1959) Toxic solvents: a review. Br. J. ind. Med.,
16: 23-39.
BROWNING, E. (1965) Toxicity and metabolism of industrial solvents,
Amsterdam, London, New York, Elsevier Publishing Company.
BRUSKIN, Z. Z. & DEMCENKO, V. G. (1975) [External respiration and gas
exchange of workers who are subjected to the effect of lubricating
oil aerosols.] Gig. Tr. prof. Zabol., No. 4, pp. 28-30
(in Russian).
BRYAN, C. S. & BOITNOTT, J. K. (1969) Adenocarcinoma of the lung with
chronic mineral oil pneumonia. Am. Rev. Respir. Dis.,
99: 272-274.
BUKOVSKIJ, M. I., ZUKOV, V. L, KOZUHOVA, T. V., SANOCKIJ, I. V., &
SIDOROV, K. K., ed. (1978) [Maximum permissible concentrations
and guidelines for safety levels of hazardous substances in the
environment.] Severodoneck, All-Union Research Institute of
Safety Technique in the Chemical Industry, and Research Institute
of Labour Hygiene and Occupational Diseases, Academy of Medical
Sciences of USSR (in Russian).
BURRELL, R. J. W. (1957) Oesophageal cancer in the Bantu. S. A.
Tydskrif vir Geneeskunde, 31: 401-409.
BÜTTNER, G. (1974) Benzene in the work environment. Deutsche
Forschungsgemeinschaft, Bonn, FRG, Harald Boldt Verlag K. G.
Boppard, 63 pp.
CANNON, P. R. (1940) The problem of lipid pneumonia. J. Am. Med.
Assoc., 115: 2156-2179.
CAPELLINI, A., CHIAPPINO, G., & ZURIO, N. (1968) [Clinical and
experimental observations on so-called tricresylphosphate
polyneuritis.] Med. Lav., 59: 721-759 (in Italian with English
summary).
CARGILL, C. (1972) Polynévrites dans l'industrie du cuir et de la
chaussure: toxicité du n-hexane, Thèse, Paris VII.
CARITHERS, H. A. (1955) Accident prevention in childhood -- the
kerosene hazard. J. Am. Med. Assoc., 159: 109-11.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977a) Petroleum hydrocarbon
toxicity studies. XIV. Animal and human response to vapors of
"high aromatic solvent". Toxicol. appl. Pharmacol.,
41: 235-241.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977b) Petroleum hydrocarbon
toxicity studies. XV. Animal response to vapors of "high
naphthenic solvent". Toxicol. appl. Pharmacol., 41: 251-260.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977c) Petroleum hydrocarbon
toxicity studies. XVI. Animal response to vapours of Naphthenic
Aromatic Solvent. Toxicol. appl. Pharmacol., 41: 261-270
(contains references to the other studies in this series).
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971a) Specifications
for cutting oils with special reference to carcinogenicity. Ann.
occup. Hyg., 14: 171-179.
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971b) Specifications
for cutting oils with special reference to carcinogenicity.
J. Inst. Petrol., 57: 247-260.
CAVANAGH, J. B. (1973) Peripheral neuropathy caused by chemical
agents. In: C.R.C. Crit. Rev. Toxicol., 2: 365-417.
CIANCHETTI, C., ABBRITTI, G., PERTICONI, G., SIRACUSA, A., & CURRADI,
A. (1976) Toxic polyneuropathy of shoe-industry workers: A study
of 122 cases. J. Neurol. Neurosurg. Psychiatr., 39: 1151-1161.
CLAYTON, G. D. & CLAYTON F. E. (1978) Patty's industrial hygiene and
toxicology. 3rd revised ed., New York, Wiley & Sons.
COHR, K.-H. & STOCKHOLM, J. (1979) Toluene. A toxicological review.
Scand. J. Work Environ. Health, 5: 71-90.
COMSTOCK, B. S. (1977) A review of psychological measurements relevant
to central nervous system toxicity with special reference to
solvent inhalation. Clin. Toxicol, 11: 317-324.
CONCAWE (1981a) Exposure to atmospheric benzene vapour associated
with motor gasoline. The Hague (Concawe report No. 2/81).
CONCAWE (1981b) Guidelines for the determination of atmospheric
concentrations of oil-mists. The Hague (Concawe report
No. 1/81).
CONCAWE STUDY GROUP (1972) Methods for the analysis of oil in water
and soil, The Hague, Stichting Concawe, pp. 66 (Concawe report
No. 9/72).
CONCAWE TASK FORCE (1973) Disposal of used lubricating oil in Western
Europe, The Hague, Stichting Concawe, 59 pp. (Concawe report
No. 9/73).
CONCAWE TASK FORCE (1976) Methods for the determination of
hydro-carbons in the atmosphere, The Hague, Stichting Concawe,
pp. 23 (Concawe report No. 3/76).
CONTAMIN, F., GOULON, M., & MARGAIRAZ, A. (1960) Polynévrites
observées chez des sujets utilisant comme moyen de chauffage des
appareils à combusion catalytique de l'essence. Rev Neurol.,
103: 341-345.
COOK, J., CARRUTHERS, W., & WOODHOUSE, D. L. (1958) Carcinogenicity of
mineral oil fractions. Br. med. Bull., 14: 132-135.
CORPER, H. J. & FREED, H. (1922) The intratracheal injection of oils
for diagnostic and therapeutic purposes. J. Am. Med. Assoc.,
79: 1739-1743.
CRISP, A. J, BHALLA, A. K., & HOFFBRAND, B. I. (1979) Acute tubular
necrosis after exposure to diesel oil. Br. med. J.,
2(6183): 177.
CRUICKSHANK, C. N. D. (1969) Occupational cancer of the scrotum.
Br. med. j., 26: 249-250.
CRUICKSHANK, C. N. D. & GOUREVITCH, A. (1952) Skin cancer of the hand
and forearm. Br. J. ind. Med., 9: 74-79.
CRUICKSHANK, C. N. D. & SQUIRE, J. R. (1950) Skin cancer in the
engineering industry from the use of mineral oil. Br. J. ind.
Med., 7: 1-11.
DAESCHNER, C. W. Jr, BLATTNER, R. J., & COLLINGS, V. P. (1957)
Hydrocarbon pneumonitis. Pediatr. Clin. North Am., resp.
Disorders. February: 243-253, Philadelphia.
DANIEL, J. W., FRAZER, A. C., FRENCH, J. M., & SAMMONS, H. G. (1953)
The intestinal absorption of liquid paraffin in the rat.
Biochem. J., 54: 37p.
DARGENT, M., MARCEAU, M., JOSEPH, P., & FAUCON, M. (1967) Etude
experimentale sur les effets tumorigènes des lubrifiants utilisés
dans le décolletage des métaux. Rev. Lyonnaise Méd.,
16: 409-420.
DAS, B. S. & THOMAS, G. H. (1978) Fluorescence detection in high
performance liquid chromatographic determination of polycyclic
aromatic hydrocarbons. Anal. Chem., 50: 967-973.
DAVIS, A., SCHAFER, L. J., & BELL, Z. G. (1960) The effect in human
volunteers of exposure to air containing gasoline vapour. Arch.
environ. Health, 1: 548-554.
DECOUFLE, P. (1976) Cancer mortality among workers exposed to cutting
oil mist. Ann. N.Y. Acad. Sci., 271: 94-101.
DECOUFLE, P. (1978) Further analysis of cancer mortality patterns
among workers exposed to cutting oil mist. J. Natl Cancer Inst.,
61: 1025-1030.
De PIERRE, J. W. & ERNSTER, L. (1978) The metabolism of polycyclic
hydrocarbons and its relationship to cancer. Biochim. Biophys.
Acta., 473: 149-186.
DESOILLE, H., PHILBERT, M., RIPAULT, G., CAVIGNEAUX, A., & ROSSIGNOLI,
H. (1973) Action cancérogène des huiles minérales utilisées en
métallurgie. Arch. Mal. prof. 34: 669-680.
DESPIERRES, G., BENNET, P. A., PHELIP, H., & PLANTE, M. (1965) Cancer
alveolaire et inhalation d'huiles industrielles. J. Fr. Med.
Chir. Thorac., 19: 561-567.
Deutsche Forschungsgemeinschaft (1970) [Health hazardous substances
in the workplace.] Weinheim, Verlag. Chemie (in German).
DIETZ, W. A., KING, W. H. Jr, PRIESTLEY, W. Jr, & REHNER, J. Jr (1952)
Properties of high boiling petroleum products. Ind. Eng. Chem.,
44: 1818-1827.
DRASCHE, H., FINZEL, L., MARTSCHEI, H., & MEYER, R. (1974)
[Occupational health considerations in persons exposed to oil
mist.] Zbl. Arbeitsmed., 24: 305-312 (in German).
EBERT, A. G., SCHLEIFER, C. R., & HESS, S. M. (1966) Absorption,
disposition, and excretion of 3H-mineral oil in rats. J. Pharm.
Sci., 55: 923 -929.
ECKARDT, R. E. (1957) Carcinogenicity of petroleum products with
particular reference to the automotive industry. Ind. med. Surg.,
26: 396 -398.
ECKARDT, R. E. (1967) Cancer Prevention in the petroleum industry.
Int. J. Cancer, 2: 656-661.
EHRHARDT, M. & HEINEMAN, J. (1975) Hydrocarbons in blue mussels from
the Kiel Bight. Environ. Pollut., 9: 263-281.
ELY, T. S., PEDLEY, S. F., HEARNE, F. T., & STILLE, W. T. (1970) A
study of mortality symptoms and respiratory function in humans
occupationally exposed to oil mist. J. occup. Med.,
12: 253-261.
EMMETT, E. A. (1975) Occupational skin cancer: A review. J. occup.
Med., 17: 44-49.
ENGSTROM, K., HUSMAN, K., & RIIHIMÄKI, V. (1977) Percutaneous
absorption of m-xylene in man. Int. Arch. occup. environ. Health,
39: 181-189.
ESSO RESEARCH (1960) Unpublished report in: Toxicological evaluation
of some food additives including anticaking agents, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series,
No. 5, World Health Organization, Geneva.
ESSO (undated) This is about health-lubricating oils and cutting
fluids, London, Esso.
EVEN, R. (1947) Les pneumopathies huileuses de l'adulte. Soc. Med.
Hop. Paris, 63: 78-80.
EYRES, A. R. (1973) Codes of practice relating to metal working
fluids: the oil suppliers' view. J. Inst. Petrol., 59: 9-14.
FACQUET, M. J. & LANGEARD, Mme (1947) Deux cas d'opacités pulmonaires
du lobe moyen chez des chanteurs (pneumonie huileuse probable).
Bull. Mém. Soc. Méd. Hop. Paris, 63: 200-204.
FAO/WHO (1970) Toxicological evaluation of some extraction solvents
and certain other substances (Nutrition Meetings Report Series
No. 48a; WHO/Food Add./70.39).
FAO/WHO (1971) Specifications for the identity and purity of some
extraction solvents and certain other substances (Nutrition
Meetings Report Series No. 48b; WHO/Food Add./70.40).
FIELDNER, A. C., KATZ, S. H., & KINNEY, S. P. (1921) Permeation of
oxygen breathing apparatus by gases and vapours, Washington,
United States Bureau of Mines (Technical Paper 272).
FISCHER, H. G. M., PRIESTLEY, W. Jr, EBY, L. T., WANLESS, G. G., &
REHNER, J. Jr (1951) Properties of high boiling petroleum
products. Arch. ind. Hyg. occup. Med., 4: 315-324.
FLOODGATE, G. D. (1972) Microbiol degradation of oil. Mar. Pollut.
Bull., 3: 41-43.
FOE, R. B. & BIGHAM, R. S. (1954) Lipid pneumonia following
occupational exposure to oil spray. J. Am. Med. Assoc.,
155: 33-34.
FOKKENS, W., HOUWEN, J. W., HUENDER, H. G. P., KARPIAK-VAN-DRIEL, J.,
LIEM, T. H., & De WAARD, F. (1972) [Scrotal cancer and mineral
oils.] T. Soc. Geneeskd., 50: 343 (in Dutch).
FORBES, J. D. & MARKHAM, T. N. (1967) Cutting and grinding fluids in
chronic pulmonary airway disease. J. occup. Med., 9: 421-423.
FOURNAS, D. W. & HINE, C. H. (1958) Neurotoxicity of some selected
hydrocarbons. Arch. ind. Health, 18: 9-15.
FRANCO, G., MOGLIA, A., & GHITTORI, S. (1979) [Occupational neuropathy
due to cyclohexane.] Med. Lav., 70(2): 118-124
(in Italian).
FREIMAN, D. G., ENGELBERG, H., & MERRIT, W. H. (1940) Oil aspiration
(lipoid) pneumonia in adults -- A clinicopathologic study of
forty-seven cases. Arch. int. Med., 66: 11-38.
FÜHNER, H. (1921) [Narcotic action of gasoline and its components
(pentane, hexane, octane).] Biochem. Zeitschr., 115: 235-261
(in German).
GAMBERALE, F., ANNWALL, G., & HULTENGREN, M. (1975) Exposure to white
spirit. II. Psychological functions. Scand. J. Work Enrivon.
Health, 1: 31-39.
GARVIN, C. G. (1939) Lipoid pneumonia. Arch. int. Med., 64: 586-589.
GAULTIER, M., RANCUREL, G., PIRA, C., & EFTHYMIOU, M.-L. (1973)
Polynévrites et hydrocarbures aliphatiques. J. eur. Toxicol.,
6: 294-296.
GERARDE, H. W. (1959) Toxicological studies on hydrocarbons. V.
Kerosine. Toxicol. appl. Pharmacol., 1: 462-474.
GERARDE, H. W. (1960) Toxicology and biochemistry of aromatic
hydrocarbons in commercial solvents, Amsterdam, London, New
York, Princeton, Elsevier Publishing Company.
GERARDE, H. W. (1963) Toxicological studies on hydrocarbons. IX. The
aspiration hazard and toxicity of hydrocarbons and hydrocarbon
mixtures. Arch. environ. Health, 6: 329-341.
GERARDE, H. W., & GERARDE, D. F. (1961/1962) The ubiquitous
hydrocarbons (Reprinted from Assoc. Food Drug Off. United
States, Vols XXV and XXVI, 1961/1962).
GHAZAI, S. (1974) Sept cas de polynévrite dans une usine de chaussures
à Teheran. Arch. Mal. prof., 35: 552-553.
GIBSON, D. T., MAHADERAN, V., JERINA, D. M., YAGI, H., & YEH, H. J. C.
(1975) Oxidation of the carcinogens benzo(a)pyrene and
benzo(a)-anthracene to dihydrodiols by a bacterium. Science,
189: 295-297.
GILCHRIST, C. A., LYNES, A., STEEL, G., & WHITHAM, B. T. (1972) The
determination of polycyclic aromatic hydrocarbons in mineral oils
by thin-layer chromatography and mass spectrometry. Analyst,
97: 880-888.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1955) Cutting oils and
squamous-cell carcinoma. Part II: An experimental study of the
carcinogenicity of two types of cutting oils. Br. J. ind. Med,
12: 244-248.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1956) An evaluation of the
relative carcinogenicity of two types of cutting oils. A.M.A.
Arch. ind. Health, 14: 341-345.
GOLDSTEIN, D. H. & BENOIT, J. N. (1970) An epidemiologic study of an
oil mist exposure. Arch. environ. Health, 21: 600-603.
GONZALEZ, E.G. & DOWNEY, J. A. (1972) Polyneuropathy in a glue
sniffer. Arch. Phys. Med. Rehabil., 53: 333-337.
GOODWIN, T. C. (1934) Lipoid cell pneumonia. Am. J. Dis. Child.,
48: 309 -326.
GORBOV, V. A. & FOMENKO, V. N. (1962) [On the hygienic evaluation of
asphalt coverings as a possible source of air pollution with
carcinogens.] Gig. i Sanit., 27: 100-101 (in Russian).
GRAEF, I. (1939) Studies in lipid pneumonia. Arch. Pathol.,
28: 613-667.
GREENBERG, M. (1972) A proportional mortality study of a group of
newspaper workers. Br. J. in Med., 29: 15-20.
GRIMMER, G. (1972) [The quantitative determination of polycyclic
aromatics by capillary gas chromatography.] Erdöl Kohle,
25: 339-343 (in German).
GRIMMER, G. (1979) Distribution of polycylic dromatic hydrocarbons in
environmental samples. In: Egan, H., ed. Environmental
carcinogens, selected methods of analysis. Vol. 3 - Analysis
of polycyclic aromatic hydrocarbons in environmental samples,
Lyons, IARC pp. 55-61 (WHO/IARC Publication No. 29).
GRIMMER, G. & BÖHNKE, H. (1976) [Enrichment and gas chromatographic
profile analysis of polycyclic aromatic hydrocarbons in
lubricating oils.] Chromatographia, 9: 30-40 (in German).
GRIMMER, G. & BÖHNKE, H. (1978) [Polycyclic aromatic hydrocarbons and
heterocyclic compounds.] Erdöl Koble, 31: 272-277 (in German).
GRIMMER, G. & BÖHNKE, H. (1972) [Determination of total content of
polycyclic aromatic hydrocarbons in air dust and automobile
exhaust by capillary GC.] Z. anal. Chem., 261: 310-314 (in
German).
GUNSETT, A. (1930) Un cas de cancer aigu, épithélioma
spino-cellulaire, developpé après traumatisme par de l'asphalte
enflammé. Bull. Assoc. Etud. Cancer, 19: 459-462.
GUSEIN-ZADE, K. M. (1975) [Reactions of the skin on the effect of
Apsheron commercial oils.] Gig. Tr. prof. Zabol., No. 7,
pp. 51-52 (in Russian).
HAENNI, E. O. & HALL, M. A. (1960) Tentative ultraviolet absorption
limits for mineral oils. J.A.O.A.C., 43: 92-95.
HAENNI, E. O., JOE, F. L. Jr, HOWARD, J. W. & LEIBEL, R. L. (1962a) A
more sensitive and selective ultraviolet absorption criterion for
mineral oil. J.A.O.A.C., 45:(1): 59-66.
HAENNI, E. O., HOWARD, J. W., & JOE, F. L. Jr (1962b) Dimethyl
sulfoxide: A superior analytical extraction solvent for
polynuclear hydrocarbons and for some highly chlorinated
hydrocarbons. J.A.O.A.C., 45: 67 -70.
HAGGARD, H. W. (1921) The anesthetic and convulsant effects of
gasoline vapour. J. Pharmacol. Exper. Ther., 16: 401-404.
HAINES, J. R. & ALEXANDER, M. (1974) Microbial degradation of high
molecularweight alkanes. Appl. Microbiol., 28(6): 1084-1085.
HÄNNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioral effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HARTWELL, J. L. (1951) Survey of compounds which have been tested for
carcinogenic activity. 2nd ed. Washington, DC, Federal Security
Agency (Public Health Service Pub. No. 149).
HAYHURST, E. R. (1936) Poisoning by petroleum distillates. Ind. med.
Surg., 5: 53.
HELBLING, V. (1950) [A case of acute lethal percutaneous and
inhallatory poisoning by petrol.] Z. Unfallmed. Berufskr.,
43: 218-228 (in German).
HELLMANN, H. & ZEHLE, H. (1972) [Long-term investigation on
behavioural of crude oil on water.] Dtsch. Gewaesserkd. Mitt.,
16: 46-52 (in German).
HENDRICKS, N. V., BERRY, C. M., LIONE, J. G., & THORPE, J. J. (1959)
Cancer of the scrotum in wax pressman. I. Epidemiology. Arch.
ind. Health, 19: 524-529.
HENDRICKS, N. V., COLLINGS, G. H., DOOLEY, A. E., GARRETT, J. T., &
RATHER, J. B. Jr (1962) A review of exposure to oil mist. Arch.
environ. Health, 4: 139-145.
HENRY, S. A. (1946) Cancer of the scrotum in relation to occupation,
London, Oxford Medical Publications, Oxford University Press,
100 pp.
HENRY, S. A. (1946/1947) Occupational cutaneous cancer attributable to
certain chemicals in industry. Br. reed. Bull., 4: 389-401.
HENRY, S. A. (1947) Symposium on industrial skin cancer. Br. J.
Radiol., 20: 149-157.
HENSEN, E. V. (1959) Toxicology of some of the aliphatic and alicyclic
hydrocarbons. J. occup. Med., 1: 105-112.
HERMANN, H. (1975) Handling of crudes and petroleum products ...
industrial hygiene. Erdoel Kohle, Erdgas, Petrochem.
Brennst.-Chem., 28(6): 282-286.
HERSKOWITZ, A., ISHI, N., & SCHAUMBERG, H. (1971) n-Hexane
neuropathy; a syndrome occurring as a result of industrial
exposure. New Engl. J. Med., 285-2: 82-85.
HETTCHE, H. O. (1963) [The effects of bitumen and its vapours on human
health.] Städtehygiene, 14: 65-68 (in German).
HIEGER, I. & WOODHOUSE, D. L. (1952) The value of the rabbit for
carcinogenicity tests on petroleum fractions. Br. J. Cancer,
6: 293-299.
HINE, C. H. & ZUIDEMA, H. H. (1970) The toxicological properties of
hydrocarbon solvents. Ind. Med., 39(5): 215-220.
HMSO (1967) Cancer of the skin caused by oil, London, Her Majesty's
Stationary Office, pp. (SHW295/295A).
HMSO (1979) Cautionary Notice: Effects of mineral oil on the skin,
London, HMSO, (SHW397 revised 1979).
HODGSON, G. (1970) Cutaneous hazards of lubricants. Ind. Med.,
39(2): 68-73.
HODGSON, G. (1973) Health problems arising from contact and exposure
of workers to metal working fluids. J. Inst. Petr.,
59(565): 1-8.
HOEKSTRA, W. G. & PHILLIPS, P. H. (1963) Effects of topically applied
mineral oil fractions on the skin of guinea-pigs. J. invest.
Dermatol., 40(2): 79-88.
HOLMES, J. G., KIPLING, M.D., & WATERHOUSE, J. A. H. (1970) Subsequent
malignancies in men with scrotal epithelioma. Lancet,
ii: 214-215.
HOOGENDAM, I. (1962) Health checks on asphalt workers. In:
Proceedings of the Shell Industrial Doctors Meeting, 22-25 May,
1962, The Hague, Med. Div. Shell Int. Petrol. Co. Ltd. (Internal
Report).
HORTON, A. W., DENMAN, D. T., & TROSSET, R. P. (1957) Carcinogenesis
of the skin. II. The accelerating properties of aliphatic and
related hydrocarbons. Cancer Res., 17: 758-766.
HORTON, A. W., BURTON, M. J., TYE, R., & BINGHAM, E. L. (1963)
Composition versus carcinogenicity of distillate oils. Am. Chem.
Soc. Div. Pet. Chem. Prepr., 8: No. 4-C: 59-65.
HORTON, A. W., BINGHAM, E. L., BURTON, M. J. G., & TYE, R. (1965)
Carcinogenesis of the skin. III. The contribution of elemental
sulfur and of organic sulfur compounds. Cancer Res.,
25: 1759-1763.
HRUSTALEVA, G. F., CEMBARTSEVA, A. N., SVECNIKOVA, F. A., BESKROVNAJA,
N. I., ROTIN, V. V., & LESEHEV, S. S. (1979) [Hypophyseal-ovarian
relationships of women coming into contact with petroleum solvents
in industry.] Gig. Tr. prof. Zabol., No. 7, pp. 31-33
(in Russian).
HUEPER, W. C. (1961) Carcinogens in the human environment. Arch.
Pathol., 71: 355-380.
HUEPER, W. C. & PAYNE, W. W. (1960) Carcinogenic studies on petroleum
asphalt, cooling oil, and coal tar. Arch. Pathol., 70: 372-384.
HUGUENIN, R. (1925) Cancer aigu consécutif à une brûlure par le
mazout. Bull. Assoc. Fr. Etud. Cancer, 14: 403-404.
HUGUENIN, R., FAUVET, F., & MAZABRAUD, M. (1950) Rôle éventuel des
nébulisations d'huiles industrielles dans la pathogénie des
cancers broncho-pulmonaires. Arch. Mal. prof., 11: 48-51.
HUNTER, G. A. (1968) Chemical burns of the skin after contact with
petrol. Br. J. plast, Surg., 21: 337-341.
IARC (1973) Certain polycyclic aromatic hydrocarbons and heterocyclic
compounds. In: IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Lyons, International Agency for
Research on Cancer, Vol. 3, pp. 30-42.
IARC (1974) Some anti-thyroid and related substances, nitrofurans and
industrial chemicals. In: IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International
Agency for Research on Cancer, Vol. 7,326 pp.
ICHIHARA, K., KUSUNOSE, E., & KUSUNOSE, M. (1969) Microsomal
hydroxylation of decane. Biochim. Biophys. Acta, 176: 713-719.
IIDA, M., YAMAMURA, Y., & SOBUE, I. (1969) Electromyographic findings
and conduction velocity in n-hexane polyneuropathy.
Electromyography, 9: 247-261.
IKEDA, K. (1935) Oil aspiration pneumonia (lipoid pneumonia).
Am. J. Dis. Child. 49: 985-1006.
IKEDA K. (1937) Lipoid pneumonia of the adult type (paraffinoma of the
lung). Arch. Pathol., 23: 471-492.
ILO (1968) Benzene: uses, toxic effects, substitutes. Report of
expert meeting 1967, Geneva, International Labour Office, 287 pp.
INOUE, T., TAKEUCHI, Y., TAKEUCHI, S., YAMADE, S, SUZUKI, H.,
MATSUSHITE, T., MIYAGAKI, H., MAEDE, K., & MOTSUMOTO, T. (1970) A
health survey on vinyl sandal manufacturers where a high incidence
of n-hexane intoxication occurred. Jpn. J. ind. Health,
12: 5-16.
INSTITUTE OF PETROLEUM (1974) Petroleum statistics with 1973 totals,
London, Institute of Petroleum.
ISHII, N., HERSKOWITZ, A., & SCHAUMBERG, H. (1972) n-Hexane
poly-neuropathy: a clinical and experimental study.
J. Neuropathol. exp. Neurol., 31: 198.
IVANOV, N. G., POZDNJAKOV, V. S., & ROZOVA, T. A. (1978) [The maximum
admissible content of mineral (petroleum) oil aerosol in air of
working premises.] Gig. Tr. prof. Zabol., No. 5, pp. 28-31
(in Russian).
JANYSEVA, N. I., KIREEVA, I. S., & SERZANTOVA, N. N. (1963) [A propos
the content of 3,4-benzopyrene in petroleum bitumen.] Gig. i
Sanit., 28: 71-73 (in Russian).
JEPSON, J. R., STOVANOV, S., UNGER, M., CLAUSEN, J., & CHRISTENSEN, H.
E. (1977) Cutting fluids and their effects on the skin of mice.
Acta Pathol. Microbiol. Scand. (Section A), 85: 731-738.
JERINA, D. M. & DALY, J. W. (1974) Arene oxides: A new aspect of drug
metabolism. Science, 185: 573-582.
JONES, J. G. (1961) An investigation into the effects of exposure to
an oil mist on workers in a mill for the cold reduction of steel
strip. Ann. occup. Hyg., 3: 264-271.
KARANI, V. (1966) Peripheral neuritis after addiction to petrol.
Br. med. J., i: 216.
KELLERMAN, G., SHAW, C. R, & LUYTEN-KELLERMAN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KENNAWAY, E. L. (1925) Experiments on cancer-producing substances.
Br. meal J., ii: 1-6.
KENNAWAY, E. L. & HIEGER, I. (1930) Carcinogenic substances and their
fluorescence spectra. Br. med. J., 2: 1044-1046.
KEY, M. M., RITTER, E. J., & ARNDT, K. A. (1966) Cutting and grinding
fluids and their effects on the skin. Am. Ind. Hyg. Assoc. J.,
27: 423-427.
KINNEAR, J., ROGERS, J., & FINN, O. A. (1954) Degenerative changes in
the skin with special reference to jute-workers. Br. J.
Dermatol., 46: 344-349.
KINNEAR, J., ROGERS, J., FINN, O. A., & MAIR, A. (1955) Dermatoses in
jute-workers. Br. J. ind. Med., 12: 36-42.
KIPLING, M. D. (1968) Oil and the skin. Ann. Rep. of H.M. Chief
Inspector of Factories, 1967, London, HMSO, pp. 105-120.
KIREEVA, I. S. (1968) Carcinogenic properties of coal-tar pitch and
petroleum asphalts used as binders for coal briquettes. Hyg.
Sanit., 33(4, 5, 6): 180-186.
KLAUDER, J. V. & BRILLE, F. A. (1947) Correlation of boiling ranges of
some petroleum solvents with irritant action on the skin. Arch.
Dermatol. Syph., 56: 197-215.
KNAVE, B., PERSSON, H. E., GOLDBERG, J. M., & WESTERHOLM, P. (1976)
Long-term exposure to jet fuel. An investigation on
occupationally-exposed workers with special reference to the
nervous system. Scand. J. Work Environ. Health, 3: 152-164.
KNAVE, B., OLSEN, B. A., ELOFSON, S., GAMBERALE, F., ISAKSSON, A.,
MINDUS, P., PERSSON, H. E., STRUWE, G., WENNBERG, A., &
WESTERHOLM, P. (1978) Long-term exposure to jet fuel II. A
cross-sectional epidemiologic investigation on
occupationally-exposed industrial workers with special reference
to the nervous system. Scand. J. Work Environ. Health,
4: 19-45.
KOREEDA, M., MOORE, P. D., WISLOCKI, P. G., LEVIN, W., CONNEZ, A. H.,
YAGI, H., & JERINA, D. M. (1978) Binding of benzo (a)pyrene 7,8
diol -- 9,10 epoxides to DNA, RNA and protein of mouse skin occurs
with high stereoselectivity. Science, 199: 778-781.
KOROBKIN, R., ASBURY, A. K., SUMNER, A. J., & NIELSON, S. L. (1975)
Glue-sniffing neuropathy. Arch. Neurol., 32: 158-162.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KRASSOVITSKAJA, M. L. (1976) [Health protection of atmospheric air in
regions in which oil processing and petrochemical enterprises are
located. In: Guide to hygiene of the atmospheric air.] Moscow,
The Medicine Publishing House, pp. 273-304 (in Russian).
KURITA, H. (1967) Experimental studies on the effects of n-hexane on
albino rats. Jpn. J. ind. Health, 9: 24-36.
LASKIN, S. & GOLDSTEIN, B. D., ed. (1977) Benzene toxicity. A critical
evaluation Washington and London, Hemisphere Publishing Co.
LAUGHLEN, G. F. (1925) Studies on pneumonia following naso-pharyngeal
injections of oil. Am. J. Pathol., 1: 407-414.
LAZAREW, N. W. (1929) [The toxicology of benzenes.] Arch. Hyg.
Bakteriol., 102: 227-239 (in German).
LAZAREV, N. N. & LEVINA, E. A. (1976) [Harmful substances in
industry.] 7th ed., Leningrad, Publishing House "Khimia"
(in Russian).
LAZARINI, H.-J., DOIGNON, J., L'ÉPÉE, P., & MERILLON, H. (1974) Une
nouvelle observation de polynévrite par le n-hexane. Arch. Mal
prof., 35: 920-921.
LEE, M. L., BARTLE, K. D., & NOVOTNY, M. V. (1975) Profiles of the
polynuclear aromatic fraction from engine oils obtained by
capillary column gas -- liquid chromatography and
nitrogen-selective detection. Anal. Chem., 47(3): 540-543.
LEITCH, A. (1922) Paraffin cancer and its experimental production.
Br. med. J., ii: 1104-1009.
LEITCH, A. (1924) Mule spinners cancer and mineral oils. Br. med. J.,
ii: 941-943.
LEITHE, W. (1970) The analysis of air pollutants. Ann Arbor, Ann
Arbor-Humphrey Science Publishers, pp. 78-93.
LEITHE, W. (1972) [The analysis of organic pollutants in drinking,
industrially-used, and sewage water.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft MBH, pp. 119-132
(in German).
LESSER, L. I., WEENS, H. S., & McKEY, J. D. (1943) Pulmonary
manifestations following ingestion of kerosene. J. Pediatr.,
23: 352-364.
LEVINA, E. N. (1976) [Hydrocarbons.] In: Lazarev, N. V. & Levina, E.
N., ed. [Harmful substances in industry Volume I, Organic
substances.] 7th ed., Leningrad, Publishing House Himia, pp.
9-121 (in Russian).
LIEBE, G. (1892) [On tar or paraffin cancer.] Med. Jahrb., 236: 65
(in German).
LIEVRE, J., BENICHOU, C., & DESROY, M. (1967) Polynévrite par poêle à
combustion catalytique (trots sujets atteints dans une famille).
Bull. Soc. Méd. Hop. Paris, 118: 91-99.
LIJINSKY, W. (1960) Separation of polycyclic aromatic hydrocarbons in
complex mixtures: Chromatographic determination of trace amounts
in petroleum waxes. Anal. Chem., 32: 684-687.
LIJINSKY, W. & RAHA, C. R. (1961) Polycyclic aromatic hydrocarbons in
commercial solvents. Toxicol. appl. Pharmacol., 3: 469-473.
LIJINSKY, W., RAHA, C. R., & KEELING, J. (1961) Comparison of
procedures for the determination of polycyclic aromatic
hydrocarbons in waxes. Anal. Chem., 33: 810-812.
LIJINSKY, W, DOMSKY, I., MASON, G., RAMAHI, H. Y., & SAFARI, T. (1963)
The chromatographic determination of trace amounts of polynuclear
hydrocarbons in petrolatum, mineral oil, and coal-tar. Anal.
Chem., 33: 952-956.
LIJINSKY, W., SAFFIOTTI, U, & SHUBIK, P. (1966) Skin tumorigenesis by
an extract of amber petrolatum. Toxicol. appl. Pharm.,
8: 113-117.
LIPOVSKIJ, S. M. (1978) [The serotonin content in the tissue of uterus
and particulars on its contracting activity under the influence of
petroleum solvent vapours.] Gig. Tr. prof. Zabol., No. 7,
pp. 37-40 (in Russian).
LIPOVSKIJ, S. I., ELKIN, V. I., TOMAEVA, L. V., & ZORINA, V. V. (1977)
[The solvents content in the blood of workers in the rubber
industry.] Gig. Tr. prof. Zabol., No. 5, pp. 50-51 (in Russian).
LIPOVSKIJ, C. M., TOMAEVA, L. V., VARFOLOMEEV, D. I., FEDOSEEV, Ja.
E., & KARGANOVA, E. V. (1979) [The accumulation of solvents in the
tissues and organs of pregnant women.] Gig. Tr. prof. Zabol.,
No. 3, pp. 25-28 (in Russian).
LIPPMANN, M. & GOLDSTEIN, D. H. (1970) Oil.mist studies, environmental
evaluation and control. Arch. environ. Health, 21: 591-599.
LUSHBAUGH, C. C. (1947) Experimental hyperplastic gastritis and
gastric polyposis in monkeys. J. Natl Cancer Inst., 7: 313-320.
LUSHBAUGH, C. C. & HACKETT, A. (1948/1949) An infiltrating
aden-omatous lesion of the colon of rats ingesting motor
lubricating oil (S.F.G. No. 1 oil). J. Natl Cancer Inst.,
9: 159-172.
LUSHBAUGH, C. C., GREEN, J. W., & REDEMANN, C. E. (1950) Effects of
prolonged inhalation of oil fogs on experimental animals. Arch.
ind. Hyg. occup. Med., 1: 237-247.
LUTOV, V. A. (1974) [Materials for establishing the maximum admissible
concentration of petroleum oil aerosols without additives, used as
lubricating and cooling liquids.] Gig. Tr. prof. Zabol., No 10,
pp. 49-52 (in Russian).
LUTOV, V. Ya., CIRKIN, A. A., GRJADITSKIJ, Ju. E., & VASILENKO, N. I.
(1976) [The effect of petroleum oil aerosols and thermo-oxidizing
degradation products on the functional condition and immunologic
reactivity of the organism of experimental animals.] Gig. Tr.
Prof. Zabol., No. 2, pp. 33-37 (in Russian).
LYNCH, P. F. & BROWN, C. W. (1973) Identifying source of petroleum by
infrared spectrometry. Environ. Sci. Technol., 7: 1123-1127.
MACHLE, W. (1941) Gasoline intoxication. J. Am. Med. Assoc.,
117: 1965-1971.
MALTEN, K. E., SPRUIT, D., BOEMAARS, H. G. M., & De KEIZER, M. J. M.
(1968) Horny layer injury by solvents. Berufsdermatosen,
16: 135-147.
MASON, B. (1966) The concentration of minor elements in biogenic
deposits. Principles of geochemistry, 3rd ed., New York, John
Wiley & Sons Inc., pp. 241-243.
MASTROMATTEO, E. (1955) Cutting oils and squamous-cell carcinoma. Part
I. Incidence in a plant with a report of six cases. Br. J. ind.
Med., 12: 240-243.
MATHER, R. N. (1973) Codes of practice relating to metal-working
fluids -- the users' views. J. Inst. Petrol., 59: 15-17.
MATSUMOTO, T. (1971) Experimental studies on toluene poisoning, II.
Jpn. J. ind. Health, 13: 399-407.
MATSUMURA, M., INOUE, N., OHNISHI, A., SANTA, T., & GOTO, I. (1972)
Toxic polyneuropathy due to glue-sniffing. Clin. Neurol. (Japan),
12: 290-296.
MAZEE, W. M., GERSMANN, H. R., & VAN DER WIEL, A. (1966) Analysis of
polycyclic aromatic hydrocarbons in petroleum waxes and white
mineral oils with appraisal of merits of different methods of
analysis. Food cosmet. Toxicol., 4: 17-33.
McDERMOTT, H. J. & KILLIANY, S. E. Jr (1978) Quest for a gasoline TLV.
Am. Ind. Hyg. Assoc. J., 39(2): 110-117.
McKAY, J. F. & LATHAM, D. R. (1972) Fluorescence spectrometry in the
characterization of high boiling petroleum distillates. Anal.
Chem., 44(13): 2132-2137.
McKAY, J. F. & LATHAM, D. R. (1973) Polyaromatic hydrocarbons in high
boiling petroleum distillates. Anal. Chem., 45: 1050-1055.
Medical Research Council (1968) The carcinogenic action of mineral
oils. A chemical and biological study. London HMSO (Special Report
Series No. 306).
MEDVED', R. A., KUZINA, V. F, & KRIGMAN, B. I. (1976) [The hygienic
assessment of lubricating and cooling liquids introduced instead
of sulphofrezol for the cold processing of metal by cutting.]
Gig. Tr. prof. Zabol., No. 6, pp. 1-5 (in Russian).
MENDELL, J. R., SAIDA, K, GANANSIA, M. F., JACKSON, D. B., WEISS, H.,
GARDIER, R. W., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J.,
MARKS, B., & HETLAND, L. (1974) Toxic polyneuropathy produced by
methyl n-butyl ketone. Science, 185: 787-789.
MEYER, A. (1976) Huilome pulmonaire chez les chanteurs. Arch. Mal.
prof., 37(9): 629-631.
MILLER, A., BADER, R. A., BADER, M. E., TEIRSTEIN, A. S., & SELIKOFF,
I. J. (1962) Mineral oil pneumonia. Ann. intern. Med.,
57: 627-634.
MILNE, J. E. (1970) Carcinoma of the scrotum. Med. J. Aust.,
1: 13-16.
MINER, S. (1969) Air pollution aspects of hydrogen sulfide,
Bethesda, MD, Litton Systems Inc. (prepared for the United States
Department of Health, Education and Welfare Contract No.
PH-22-68025).
MIYAGAKI, H. (1967) Electrophysiological studies on the peripheral
neurotoxicity of n-hexane. Jpn. J. ind. Health, 9: 12-23.
MOEL, M. & TAYLOR, H. K. (1943) Oil aspiration pneumonia. Am. J.
Röntgenol. rad. Ther., 49(2): 177-184.
MOSS, E., SCOTT, T. S., & ATHERLEY, G. R. C. (1972) Mortality of
newspaper workers from lung cancer and bronchitis 1952-1966.
Br. J. ind. Med., 29(1): 1-14.
MUHAMETOVA, G. M. & PODREZ, Z. G. (1975) [Problems of the course,
pathogenesis and therapy of chronic patrol poisoning.] Ufa,
Baskirskoe kniznoe izdatelstvo (in Russian).
NATHANSON, L., FRANKEL, D., & JACOBI, M. (1943) Diagnosis of lipoid
pneumonia by aspiration biopsy. Arch. int. Med., 72: 627-634.
NEBERT, D. W. & GELBOIN, H. V. (1969) The in vivo and in vitro
induction of aryl hydrocarbon hydroxylase in mammalian cells of
different species, tissues, strains, and developmental and
hormonal states. Arch. Biochem, Biophys., 134: 76-89.
NEBERT, D. W., GOUJON, F. M., & GIELEN, J, E. (1972)Aryl hydrocarbon
hydroxylase induction by polycyclic hydrocarbons: simple autosomal
dominant trait in the mouse. Nature New Biology, 236: 107-109.
NELSON, L. B. & STORMONT, D. J. (1960) Determination of polynuclear
aromatic hydrocarbons in petroleum waxes. Chem. Ind., 21: 561.
NIOSH (1973) The industrial environment -- its evaluation and
control. Washington, DC, US Dept of Health, Education and
Welfare.
NIOSH (1977A) Occupational exposure sampling strategy manual.
Washington, DC, US Dept of Health, Education and Welfare (DHEW
(NIOSH) Publication No. 77-173).
NIOSH (1977B) Criteria for a recommended standard ... occupational
exposure to asphalt fumes, Washington DC, US Department of
Health, Education and Welfare (DHEW (NIOSH) Publ. No. 78-106).
NIOSH (1977C) Criteria for a recommended standard ... occupational
exposure to hydrogen sulphide, Washington DC, US Department of
Health, Education and Welfare.
NIOSH (1977D) Criteria for a recommended standard ... occupational
exposure to refined petroleum solvents, Washington DC, US
Department of Health, Education and Welfare, 247 pp. (DHEW (NIOSH)
Publ. No. 77-192).
NIOSH (1977-79) NIOSH manual of analytical methods. 2nd ed.
Cincinnati, OH, US Dept of Health, Education and Welfare, USA.
NIOSH (1979) Summary of NIOSH recommendations for occupational health
standards, Washington DC, US Department of Health and Welfare,
9 pp.
NOCHOMOVITZ, L. E., UYS, C. J., & EPSTEIN, S. (1975) Massive
deposition of mineral oil after prolonged ingestion. S.A. med.J.,
13 Dec., 2187-2190.
NOMIYAMA, K. & NOMIYAMA, H. (1974) Respiratory retention, uptake and
excretion of organic solvents in man. Int. Arch. Arbeitsmed.,
32: 75-91.
NOVIKOV, K. I., KOLODINA, L. N., LIPOVSKIJ, S. M., & BADALOVA, L. V.
(1979) [Lactation particulars in female workers of the chemical
(rubber) industry.] Gig. Tr. prof. Zabol., No. 2, pp. 45-48
(in Russian).
NUNN, J. A. & MARTIN, F. M. (1934) [Gasoline (petrolether) and
kerosine (petroleum) poisonings in children.] Samml.
Vergiftungsf., 5: 183-185 (in German).
OGATA, M., ASAHARA, H., & SAEKI, T. (1975) Sampling and analysis of
some aromatic aliphatic and chlorinated hydrocarbon vapours in
air, a gas-liquid chromatographic and colorimetric method.
Int. Arch. Arbeitsmed., 34: 25-37.
OSER, B. L., OSER, M., & CARSON, S. (1965) Toxicological studies of
petrolatum in mice and rats. Toxicol. appl. Pharmacol.,
7: 382-401.
PASTERNACK, B. & EHRLICH, L. (1972) Occupational exposure to an oil
mist atmosphere. A 12-year mortality study. Arch. environ.
Health, 25: 286-294.
PATES, M. M. (1952) [Blastomogenic effect of pyrolyse tars from
petroleum.] Tr. Akad. Med. Nauk., SSSR, 21: 157-163
(in Russian).
PAULSON, G. W. & WAYLONIS, G. W. (1976) Polyneuropathy due to
n-hexane. Arch. intern. Med., 136: 880-882.
PERBELLINI, L., BRUGNONE, F. & FAGGIONATO, G. (1981) Urinary excretion
of the metabolites of n-hexane and its isomers during
occupational exposure. Br. J. ind. Med., 38: 20-26.
PETROLEUM HANDBOOK (1966) London, Shell International Petroleum
Company Limited.
PINKERTON, H. (1927) Oils and fats. Their entrance into and fate in
the lungs of infants and children: A clinical and pathologic
report. Am. J. Dis. Child., 33: 259-285.
PINKERTON, H. (1928) The reaction to oils and fats in the lung. Arch.
Pathol, 5: 380-401.
PINKERTON, H. & MORAGUES, V. (1940) Paraffinoma of the lung with
secondary tuberclelike lesions in liver and spleen. Arch.
Pathol., 29: 691-699.
PIOTROWSKI, J. K. (1977) Exposure tests for organic compounds in
industrial toxicology. Cincinnati, OH, NIOSH, (DHEW (NIOSH)
Publication No. 77-144).
POKLIS, A. & BURKETT, C. D. (1977) Gasoline sniffing: A review. Clin.
Toxicol., 11: 35-41.
POLIANSKY, V. A. & MUSERSKAJA, A. N. (1971) [Pollution of the air
environment at modern oil processing plants and evaluation of the
implementation of recommendating.] In: [Occupational hygiene and
the protection of the health of workers in oil and petrochemical
industries.] Ufa, pp. 50-54 (in Russian).
PROCKOP, L. D. (1977) Multifocal nervous system damage from inhalation
of volatile hydrocarbons. J. occup. Med., 19: 139-140.
PROUDFIT, J.P., ORDSTRAND, H. S. VAN, & MILLER, C. W. (1950) Chronic
lipid pneumonia following occupational exposure. Arch. ind. Hyg.,
1: 105-111.
PRUYN, F. H. A.M. & REIJNIERSE, K. (1972) [Carcinoma of the scrotum
through mineral oils.] T. Soc. Geneesk., 50: 182-187 (in Dutch).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1975) Report on emissions from
asphalt hot mixes, Maryland, USA, The Asphalt Institute
(Research Report RR-75-IA).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1978) Differences between
petroleum asphalt, coal-tar pitch and road tar, Maryland, USA,
The Asphalt Institute (Research report RR-78-1).
RAALTE, H. G. S. VAN (1963) [Medico-toxicological aspects of oil used
in metal processing.] Tijdschr. Soc. Geneesk., 41: 611-617
(in Dutch).
RAALTE, H. G. S. VAN (1972) [Carcinoma of the scrotum through mineral
oils.] Tijdschr. Soc. Geneesk, 50: 604-605 (in Dutch).
RAITTA, C., SEPPÄLÄINEN, A. M., & HUUSKONEN, M. (1978) N-hexane
maculopathy in industried workers. Graefes Arch. klin. exp.
Ophthalmol., 209: 99-110.
REID, F. H. & HALPIN, W. R. (1968) Determination of halogenated and
aromatic hydrocarbons in air by charcoal tube and gas
chromatography. Am. Ind. Hyg. Assoc. J., 29: 390.
REIDENBERG, M. M., POWERS, D. V., & BELLO, C. T. (1964) Acute renal
failure due to nephrotoxins. Am. J. med. Sci., 59: 25-63.
REWELL, R. E. (1947) Lipoid pneumonia: A pitfall in diagnosis.
Br. med. J., 29: 409-411.
RIIHIMÄKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
Environ. Health, 5: 143-150.
RIIHIMÄKI, V. & PFÄFFLI, P. (1978) Percutaneous absorption of solvent
vapours in man. Scand. J. Work Environ. Health, 4: 73-85.
RIIHIMÄKI, V., PFÄFFLI, P., SAVOLAINEN, K., & PEKARI, K. (1979)
Kinetics of m-xylene in man. General features of absorption,
distribution, biotransformation and excretion in repetitive
inhalation exposure. Scand. J. Work Environ. Health,
5: 217-231.
ROBINSON, E. & ROBBINS, R. C. (1968) Sources, abundance, and fate of
gaseous atmospheric pollutants. Chapter VI; Hydrocarbons and
organics in the atmosphere, Huntsville, Alabama, Stanford
Research Institute (Report PR-6755 prepared for the American
Petroleum Institute).
ROE, F. J. C., CARTER, R. L., & TAYLOR, W. (1967) Cancer hazard from
mineral oil used in the processing of jute. Br. J. Cancer,
21: 694-702.
ROSSIER, P. H. & BÜHLMANN, A. (1949) [Clinical and laboratory studies.
An oil-aspiration pneumonia following the use of liquid paraffin
as nose drops for some years.] Schweiz. Med. Wochenschr.,
79: 685-686.
SANTE, L. R. (1949) The fate of oil particles in the lung and their
possible relationships to the development of bronchogenic
carcinoma. Am. J. Röntgenol., 62: 788-797.
SAVOLAINEN, H. (1977) Some aspects of the mechanisms by which
industrial solvents produce neurotoxic effects. Chem. biol.
Interactions, 18: 1-10.
SCHAMP, N. & VAN WASSENHOVE, F. (1972) Determination of
benzo(alpha)-pyrene in bitumen and plants. J. Chromatogr.,
69: 421-425.
SCHAUMBURG, H. H. & SPENCER, P.S. (1976) Degeneration in central and
peripheral nervous systems produced by n-hexane: An experimental
study. Brain, 99: 183-192.
SCHEUPLEIN, R. J. & BLANK, I. H. (1971) Permeability of the skin.
Physiol. Rev., 51: 702-747.
SCHMAHL, D. & REITER, A. (1933) [Experiments on cancer production by
liquid paraffin yellow vaseline and lanolin.] Arzneim. Forsch.,
3: 403-406 (in German).
SCHWARZ, H. G. (1933) [Petrol poisoning, chronic, iatrogenic.] Samml.
Vergiftungsf., 4: 247-248 (in German).
SCHWARTZ, L., JULIPAN, L., & PECK, S. (1947) Dermatoses caused by
petroleum. In: Occupational diseases of the skin, 2nd ed.,
Kimpton, London, pp. 232-258.
SCRIMA, M. & De ROSA, F. (1973) [Polyneuritis in upper shoe factories:
Environmental and concomitant causes.] Lav. Um., 25: 191-198
(in Italian).
SEDIVEC, V. & FLEK, J. (1976) The absorption, metabolism and excretion
of xylenes in man. Int. Arch. occup. Environ. Health,
37: 205-217.
SEHTMAN, B. A, SAMEDOR, I. G., & MUHAMETOVA, G. M. (1979)
[Work hygiene in the petroleum industry.] Moscow, Medicina Publ.
House (in Russian).
SEPPÄLÄINEN, A. M. (1975) Applications of neurophysiological methods
in occupational medicine: A review. Scand. J. Work Environ.
Health, 1: 1-14.
SEPPÄLÄINEN, A. M., HUSMAN, K., & MARTENSSON, C. (1978)
Neuro-physiological effects of long-term exposure to a mixture of
organic solvents. Scand. J. Work Environ. Health, 4: 304-314.
SEPPÄLÄINEN, A.M., RAITTA, C., & HUUSKONEN, M. (1979)
n-Hexane-induced changes in visual evoked potentials and
electroretinograms of industrial workers. Electroencephalogr.
clin. Neurophysiol., 47(4): 492-498.
SERRATRICE, G., CORREARD, R.-P., ARLAUD, J.-A., & JOUGLARD, J. (1973)
Polynévrite et manipulation de peinture pour bateaux. J. eur.
Toxicol., 6: 304-305.
SHAHIN, M. M. & FOURNIER, F. (1978) Suppression of mutation induction
and failure to detect mutagenic activity with Athabasca Tar sand
fractions. Mutat. Res., 58: 29-34.
SHIRABE, T., TSUDA, T., TERAO, A., & ARAKI, S. (1974) Toxic
poly-neuropathy due to glue-sniffing; report of two cases with
light and electron-microscopic study of the peripheral nerves and
muscles. J. Neurol. Sci., 21: 101-113.
SHOSHKES, M., BANFIELD, W., & ROSENBAUM, S. J. (1950) Distribution,
effect and fate of oil aerosol particles retained in the lungs of
mice. Arch. ind. Hyg. occup. Med., 1: 20-35.
SHUBIK, P. & SAFFIOTTI, U. (1954) Carcinogenic and promoting action of
low-boiling catalytically cracked oils. Proc. Am. Assoc. Cancer
Res., 1: 45.
SHUBIK, P., SAFFIOTTI, U. LIJINSKY, W., PIETRA, G., RAPPAPORT, H.,
TOTH, B., RAHA, C. R., TOMATIS, L., FELDMAN, R., & RAMAHI, H.
(1962) Studies on the toxicity of petroleum waxes, Toxicol. appl.
Pharmacol., 4 (Suppl,, Nov.): 1-62.
SIMMERS, M. H. (1964) Petroleum asphalt inhalation by mice, Arch,
environ, Hearth, 9(6): 727-734.
SIMMERS, M. H. (1965A) Cancers from air.refined and steam refined
asphalt. Ind. Med. Surg., 34(3): 244-261.
SIMMERS, M. H. (1965B) Cancers in mice from asphalt fractions,
Ind. Med. Surg., 34(7): 573-577.
SIMMERS, M. H. (1966) Tumors from asphalt fractions injected into
mice. Ind. Med. Surg., 35(10): 889-894.
SIMMERS, M. H., PODOLAK, E., & KINOSITA, R. (1959) Carcinogenic
effects of petroleum asphalt. Proc. Soc. exp. Blot. Med.,
10: 266-268.
SIMS, P., GROVER, P. L., SWAISLAND, A., PAL, K., & HEWER, A. (1974)
Metabolic activation of benzo(a)pyrene precedes by a diol-epoxide.
Nature (Lond.), 252: 326-328.
SIOU, G. (1972) Existe-t-il un risque de cancérisation par les
bitumes. Rev. Path. comp. Méd. exp., 72: 65-75.
SIWE, S. A. (1932) [The signs and symptoms of acute poisoning by
liquid hydrocarbons in the aliphatic series.] Monatschr.
Kinderheilkd., 55: 146-153 (in German).
SMILEY, J. A. (1951) The hazards of rope making. Br. J. ind. Med.,
8: 265-270.
SMITH, W. E. & SUNDERLAND, D. A. (1951) Responses of mice to
carcinogenic derivatives of petroleum. Cancer Res., 11: 281.
SMITH, W. E., SUNDERLAND, D. A., & SUGIURA, K. (1951) Experimental
analysis of the carcinogenic activity of certain petroleum
products. Arch. ind. Hyg. occup. Med., 4: 299-314.
SMS 2337-1 (1972) Determination of low concentrations of benzene in
air, The Hague, Shell Int. Petr. My. (Shell Method Series).
SMYTH, H. F. & SMYTH, H. F. (1928) Inhalation experiments with certain
lacquer solvents. J. ind. Hyg., 10: 261-271.
SOUTHAM, A. (1928) Mule-spinners' cancer. In: Report of the
International Conference on Cancer, London, Bristol, John
Wright, p. 28C.
SPENCER, P. S., BISCHOFF, M. C., & SCHAUMBURG, H. H. (1978) On the
specific molecular configuration of neurotoxic aliphatic
hexacarbon compounds causing peripheral distal axonopathy.
Toxicol. appl. Pharmacol., 44: 17-28.
SPRUIT, D., MALTEN, K. E., LIPMANN, E. W. R. M., & LIANG, T. P. (1970)
Horny layer injury by solvents. II. Can the irritancy of petroleum
either be diminished by pretreatment. Berufsdermatosen,
18: 269-280.
STERNER, J. H. (1941) Study of hazards in spray-painting with gasoline
as a diluent. J. ind. Hyg. Toxicol., 23: 437-448.
STEWART, I. (1960) Petrol vapour poisoning. Br. med. J.,
i: 1739-1740.
STRÄULI, P. (1957) [Acute tar cancer.] Oncologia, 10: 72-75
(in German).
STRYKER, W. A. (1941) Absorption of liquid petrolatum (mineral oil)
from the intestine. Arch. Pathol., 31: 670-692.
SUGIURA, K., SMITH, W. E., & SUNDERLAND, D. (1949) Carcinogenic action
of certain catalytically cracked oils with high boiling points.
Cancer Res., 9: 631.
SUHANOVA, V. A. & MEL'NIKOVA, V. V. (1974) [The menstruation function
of female workers at refineries and patients suffering from
chronic intoxication by petroleum products.] Gig. Tr. prof.
Zabol., No. 4, pp. 30-41 (in Russian).
SULLIVAN, R. J. (1969) Air pollution aspects of odorous compounds,
Bethesda, Maryland, Litton Systems Inc. (prepared for the US Dept.
of Health, Education and Welfare, Contract No. PH-22-68-25).
SUMA'MUR, P. K. & SUSIANTI WENAS. (1978/1979) Skin and other
affections in gasoline and/or diesel oil pump workers. Indones.
J. ind. Hyg. occup. Health, 11-12: 20-22 (XI No. 1, 2, 3, 4 and
XII No. 1 and 2).
SUNDERLAND, D. A., SMITH, W. E., & SUGIURA, K. (1951) The pathology of
growth behaviour of experimental tumours induced by certain
petroleum products. Cancer, 4: 1232-1245.
SUZUKI, T., SHIMBO, S., & NISHITANI, H. (1974) Muscular atrophy due to
glue sniffing. Int. Arch. Arbeitsmed., 33: 115-123.
SWANN, H. E. Jr., KWONN, B. K., HOGAN, G. K., & SNELLINGS, W. M.
(1974) Acute inhalation toxicology of volatile hydrocarbons.
Am. Ind. Hyg. Assoc. J., 35(9): 511-518.
Tabershaw/Cooper Association Inc. (1974/75) Morbidity study of
petroleum refinery workers. (Project OH-4, Medical Research
Report prepared for API).
TAGAMI, H. & OGINO, A. (1973) Kerosene dermatitis; factors affecting
skin irritability to kerosene. Dermatologica, 146: 123-131.
TAHER, S. M., ANDERSON, R. J., McCARTNEY, R, POPOVTZER, M. M., &
SCHRIER, R. W. (1974) Renal tubular acidosis associated with
toluene «sniffing». New Engl. J. Med., 290: 765-768.
TAKEUCHI, Y., MABUCHI, C., & TAKAGI, S. (1975) Polyneuropathy caused
by petroleum benzine. Int. Arch. Arbeitsmed., 34: 185-197.
THAKKER, D. R., YAGI, H, AKAGI, H, KOREEDA, M., LU, A. Y.-H., LERIM,
W., WOOD, A. W., CONNEY, A. H., & JERINA, D. M. (1977) Metabolism
of benzo(alpha)pyrene VI. Stereoselective metabolism of
benzo(alpha)-pyrene and benzo(alpha)pyrene 7,8-dihydrodiol to
diolepoxides. Chem. biol. Interaction 16: 281-300.
THONY, C. & THONY, J. (1970) Enquête épidémiologique sur le cancer des
huiles de coupe. In: Proceedings of the 16th International
Congress on Occupational Health, Tokyo, 1969, pp. 655-657.
THONY, C., THONY, J., LAFONTAINE, M., & LIMASSET, J. C. (1975)
Concentrations en hydrocarbures polycycliques aromatiques
cancérogènes de quelques huiles minerale étude due risque
correspondant. Arch. Mal. prof., 36: 37-52.
TRUHAUT, R. (1978) Introductory remarks. In: Meeting on MACs, XIX In.
International Congress on Occupational Health, 26 September 1978.
TRUHAUT, R. & MURRAY, R. (1978) International workshop on toxicology
of benzene, Paris, 9-11 November 1976. Int. Arch. Occup. Env,
41: 65-76.
TRUHAUT, R., LAGET, P., PIAT, G., DUTERTRE-CATELLA, P.-L. H., & HUYEN,
V. N. (1973) Premiers résultats électrophysiologiques après
intoxications experimentales par l'hexane et par l'heptane
techniques chez le rat blanc. Arch. Mal. prof.,
34(7-8): 417-426.
TWORT, C. C. & FULTON, J. D. (1930) Further experiments on the
carcinogenicity of synthetic tars and their fractions. J. Path.
Bacteriol., 32: 119-143.
TWORT, C. C. & ING, H. R. (1928) [Investigations on carcinogenic
agents.] Z. Krebsforsch., 27: 308-351 (in German).
TWORT, C. C. & TWORT, J. M. (1931) The carcinogenic potency of mineral
oils. J. And. Hyg. Toxicol., 13: 204-226.
TWORT, C. C. & TWORT, J. M. (1935) Induction of cancer by cracked
mineral oils. Lancet, November 30: 1226-1228.
TWORT, C. C., LYTH, R., & TWORT, J. M. (1937) The significance of
density, refractive index and viscosity of mineral oils in
relation to the type and degree of animal reaction. J. Hyg.,
37: 14-29.
TYE, R., BURTON, M. J., BINGHAM, E., BELL, Z., & HORTON, A. W. (1966)
Carcinogens in a cracked petroleum residuum. Arch. environ.
Health, 13: 202-207.
UK MEDICAL RESEARCH COUNCIL (1968) The carcinogenic action of mineral
oils. A chemical and biological study, London, Her Majesty's
Stationary Office, 8 pp. (Special Report Series No. 306).
UNGAR, H., HINE, C. H., KODAMA, J. K., & ANDERSON, H. H. (1955)
Neuropathology of rats experimentally poisoned with
p-tertiary-butyl-toluene. A.M.A. Arch. Pathol., 69: 139-149.
US DHEW (1970) Air quality criteria for hydrocarbons, Washington,
DC, United States Department of Health, Education and Welfare.
USSR (1972) [Sanitary norms for the planning of industrial
enterprises.] Moscow (in Russian).
VAN DER LINDEN, A. C. & THYSSE, G. J. E. (1965) The mechanisms of
microbial oxidations of petroleum hydrocarbons. Adv. Enzymol.,
27: 469-546.
VAUGHAN, C. G., WHEALS, B. B., & WHITEHOUSE, M. J. (1973) The use of
pressure-assisted liquid chromatography in the separation of
polynuclear hydrocarbons. J. Chromatogr., 78: 203-210.
VIGALJUK, R. V., ZAMALEEVA, Z. S., RUBINSKIJ, L. Ya., SOLODOVA, N. L.,
& LJUTAJA, T. I. (1977) [The chromatographic determination of the
total content of C1 C10 paraffin hydrocarbons and C6-C8
aromatics in the air of industrial plants.] Gig. Tr. prof.
Zabol., No. 6, pp. 57-57 (in Russian).
VOBORSKY, R. C. (1980 In: Dolberg, D. D. & Verstuyft, A. W, ed.
Analytical techniques in occupational health chemistry,
Washington, DC, ACS (ACS Symposium Series 120).
VOLK, B. W. (1964) Lipoid pneumonia: Clinical, pathologic and chemical
aspects. Biochem. Clin., 4: 187.
VOLKMANN, R. (1875) [On tar, paraffin, and soot cancer (chimneysweep's
cancer.] In: Beiträge zur Chirurgie, Leipzig, Breitkopf und
Hartel (in German).
VON LEHMDEN, D. J., VON HANGERBRAUCK, R. P., & MEEKER, J. E. (1965)
Polynuclear hydrocarbon emissions from selected industrial
process. J. Air. Pollut. Control Assoc., 15: 306-312.
WAGNER, W. D., DOBROGORSKI, O. J., & STOKINGER, H. E. (1961)
Antagonistic action of oil mists on air pollutants. Arch.
environ. Health, 2: 523-534.
WAGNER, W. D., WRIGHT, P. G., & STOKINGER, H. E. (1964) Inhalation
toxicology of oil mists. I. Chronic effects of white mineral oil.
Am. Ind. Hyg. Assoc. J., 25: 158-167.
WAHLBERG, J. E. (1974) Occupational and non-occupational scrotal
cancer in Sweden, 1958-1970. Acta Dermatovenerol., 54: 471-474.
WALKER, J. D, COLWELL, R. R., & PETRAKIS, I. (1975) A study of the
biodegradation of a South Louisiana crude oil employing
computerized mass spectrometry. In: Joint Conference on the
Prevention and Control of Oil Pollution, San Francisco, p. 197.
WALLCAVE, L, GARCIA, H., FELDMAN, R., LIJINSKY, W., & SHUBIK, P.
(1971) Skin tumorigenesis in mice by petroleum asphalts and
coaltar pitches of known polynuclear aromatic hydrocarbon content.
Toxicol. appl. Pharmacol., 18: 41-52.
WANG, C. C. (1961) Acute gasoline intoxication. Arch. environ.
Health, 2: 714-716.
WARING, J. I. (1933) Pneumonia in kerosene poisoning. Am. J. med.
Sci., 185: 325-330.
WATERHOUSE, J. A. H. (1971) Cutting oils and cancer. Ann. occup.
Hyg., 14: 161-170.
WATERHOUSE, J. A. H. (1972) Lung cancer and gastro-intestinal cancer
in mineral oil workers. Ann. occup. Hyg. 15: 43-44.
WATTENBERG, L. W. (1972) Dietary modification of intestinal and
pulmonary aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEISS, F. T. (1975) Activities of standardization groups in the
identification of waterborne oils. Paper read at National Bureau
of Standards Workshop - Santa Barbara, California, 6-7 October,
1975, 17 pp.
WEISSMAN, H. (1951) Lipoid pneumonia. Am. Rev. Tuberc., 64: 572-576.
WHO (1974) Toxicological evaluation of some food additives
including anti-caking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents, Geneva, World Health
Organization, pp. 417-422 (WHO Food Additive Series No. 5).
WHO (1982) Environmental health criteria 19: Hydrogen sulfide.
Geneva, World Health Organization.
WIEL, A. VAN DER (1975) Collaborative determination of precision of
DMSO extraction-refractive index method, Amsterdam,
Koninklijke/Shell Laboratory (Memorandum LFA/200/75).
WIEL, A. VAN DER (1979) Determination of potentially hazardous
poly-nuclear aromatics in petroleum fractions, Amsterdam,
Koninklijke/Shell Laboratory (Amsterdam Method Series, AMS 642-2).
WILLIAMS, R. T. (1959) Detoxication mechanisms, 2nd ed., New York,
John Wiley & Sons Inc.
WOOD, E. H. (1943) Unusual case of carcinoma of both lungs associated
with lipoid pneumonia. Radiology, 40: 193-195.
WOODHOUSE, D. L. (1934) The non-carcinogenicity of certain synthetic
lubricating oils and the action of some commercial spindle-oils,
solvent fractionated spindle oil distillates and tar oils, tested
under similar conditions. J. Inst. Petr. Technical.,
20: 1057-1063.
WOODHOUSE, D. L. (1950) The carcinogenic activity of some petroleum
fractions and extracts. J. Hyg. Camb., 48: 121-134.
YAGI, H., THAKKER, D. R., HERNANDEZ, O., KOREEDA, M., & JERINA, D. M.
(1977) Synthesis and reactions of the highly mutagenic 7, 8-diol
9, 10-epoxides of the carcinogen benzo-(a) pyrene. J. Am. Chem.
Soc., 99: 1604-1611.
YAMAMURA, Y. (1967) n-Hexane neuropathy. Folia psychiatr. neurol.
Jpn., 23: 45-57.
YANG, S. K., McCOURT, D. W., ROLLER, P. R., & GELBOIN, H. V. (1976)
Enzymatic conversion of benzo(alpha)pyrene leading predominantly
to the diol-epoxide r-7, t-8-dihydroxy-t-9, 10-oxy-7,8,9,10-
tetrahydrobenzo (a) pyrene through a single emantiomer of r-7,
t-8-dihydroxy-7, 8-dihydrobenzo-pyrene. Proc. Natl Acad. Sci.
US, 73: 2594-2598.
YOKOO, H. & SUCKOW, E. E. (1961) Peripheral lung cancers arising in
scars. Cancer, 14: 1205-1215.
YOUNG, A.M., APPLEBAUM, H. S., & WASSERMAN, P. B. (1939) Lipoid
pneumonia. J. Am. Med. Assoc., 112: 2406-2409.
ZANGGER, H. (1933) [Occupational accidents and hazards at work inside
enclosed spaces (reservoirs, tanks, and transport lorries.]
Arch. Gewerbepathol. Gewerbehyg., 4: 117-140 (in German).
ZIELHUIS, R. L. (1961) [Early complaints occurring on exposure to
organic solvents.] Tijdschr. Soc. Geneesk., 39: 595-600
(in Dutch).
ZINGMARK, P. A. & RAPPE, C. (1977) On the formation of
N-nitrosodi-ethanolamine in a grinding fluid concentrate after
storage. Ambio, 6: 237-238.
2.2.2.3 Disposal of waste
In a refining process, the release of oil into refinery effluents
is practically negligible and of a lower order of magnitude than
tanker washings in tankers that do not use the "load-on-top" system.
Waste gas in production fields is generally burnt on the spot. In
refineries and chemical plants, it may be necessary to burn some gas
at a flare for reasons of safety, and some oil and gas is consumed as
refinery fuel. Atmospheric pollutants in and around refineries
basically consist of saturated and unsaturated hydrocarbons, carbon
monoxide, hydrogen sulfide, and sulfur dioxide (Poliansky &
Musserskaja, 1971; Krasovitskaja, 1976). Sulfur dioxide, hydrogen
sulfide, and mercaptan emissions are not discussed in this review and
emissions of hydrocarbon vapours into the atmosphere from storage
terminals, filling stations, and cars will be covered in another
document.
2.3 Toxicological Effects of Crude Oils
As, in this document, crude oils are discussed only to provide
background information for the petroleum solvents, lubricating base
oils, and bitumens derived from them, no detailed discussion will
follow concerning environmental exposure levels, environmental
distribution and transport, physiological factors relating to
mammalian uptake, dose-response relationships, and maximum permissible
levels. Most of the relevant aspects will, however, be covered in the
sections on fractions derived from the crude oils. This also applies
to toxicological effects on experimental animals and man, with the
exception of a very few studies that are related to crude oil exposure
only.
The toxicological and nuisance aspects of hydrogen sulfide and
mercaptans have been reviewed in detail by Miner (1969) and Sullivan
(1969). A review on hydrogen sulfide has been prepared by NIOSH
(1977c) and an Environmental Health Criteria document on hydrogen
sulfide has recently been published (WHO, 1982).
2.3.1 Effects on experimental animals
Leitch (1924) examined 16 untreated crude oils from various parts
of the world for their carcinogenicity by applying them 3 times a week
to the skin of mice and found significant differences in
tumorigenicity among these oils. Similar results were reported by
Hieger & Woodhouse (1952) in skin tests on mice and rabbits. The
tumorigenicity of the crude oils they examined was low in comparison
with that of some of the distilled fractions. Skin tests were also
carried out on mice and rabbits by Antonov & Lints (1960), who found
that Saratov oil possessed weak carcinogenic properties. The main
causes of death in these tests, however, were pneumonia and general
intoxication, probably from absorption of oil components through the
skin. The authors found that rabbits were more sensitive than mice, as
did Hieger & Woodhouse (1952).
Batt-Neal & Wolman (1977) demonstrated skin tumorigenicity and
amyloid deposition following skin exposure of mice to saturated
acetone extracts of various oils collected from beaches.
2.3.2 Effects on man
Examination of 743 oilfield workers exposed to California crude
oil and excessive sunlight revealed that 7 of them had epitheliomas on
exposed parts of the body and that nearly 20% had keratotic changes on
the hands, forearms, face, and neck. Five of the 7 subjects, who
developed epitheliomas, were blonds, though blonds were in the
minority in this group of workers (Schwartz et al., 1947).
During 1938-39, Schwartz saw 189 cases of carcinomas on exposed
parts of the skin; 128 were in males, 71 of whom were oilfield workers
20 others being workers exposed to excessive sunlight only. Emmett
(1975) mentions the strong potentiating effect of UV radiation on
other potentially carcinogenic exposures. In southern Texas, however,
the incidence of skin carcinomas in 330 oilfield workers was low,
which underlines the fact that Texas and Pennsylvania oils are known
to be less carcinogenic than California oil (Twort & Ing, 1928).
In a study on 50 volunteer operators, who had not previously been
in contact with oil and petroleum products, crude oil was applied to
the skin of the inner surface of the forearm, for periods of 3-6 h. An
inflammatory reaction of the skin developed with moderate erythema,
oedema, and slight burning. Changes in the thermosensitive threshold
were noted, as well as an increase in the permeability of the
epidermis (Gusein-Zade, 1975).
3. PETROLEUM SOLVENTS
3.1 Properties and Analytical Methods
3.1.1 Chemical composition and properties
Only solvents consisting of hydrogen and carbon alone and
produced from petroleum will be considered in this review. It should
be noted, however, that similar solvents are also produced from coal.
Petroleum solvents consist of complex mixtures of hydrocarbons
reflecting the hydrocarbon constituents of the crude oil or, more
usually, the intermediate refinery streams from which they are
distilled. Because of their complex nature, classification is a
problem and no standard, worldwide-accepted nomenclature exists.
However, providing that it is recognized that considerable overlapping
and many exceptions occur, they can be classified into 3 broad
subdivisions, based on distillation ranges:
(a) special-boiling-point solvents (SBPs) - grades with narrow or
wide distillation ranges within the main limits of 30-160°C;
(b) white spirits - grades distilling within the main range
150-220°C, the boiling-points of individual grades usually
ranging over more than 20°C;
(c) high-boiling aromatic solvents - grades distilling in the range
160-300°C with final boiling-points above 220°C.
Benzene, toluene, and the xylene isomers occur as components of
petroleum solvents, but as they fall more naturally into the category
of chemical intermediates, they will be referred to here only in so
far as they are important as components of the mixtures being
discussed.
Two further clarifications can be made. Firstly, it is common
industrial practice to ascribe the name of the predominant isomer
present to the petroleum solvent; thus the descriptions pentane,
isopentane, hexane, isohexane, and heptane are commonly met. However,
in almost all cases, the amount of the named isomer present in an
industrial scale product will not exceed 95% v/v of the solvent and
may be as small as 30% v/v.
Second, most petroleum solvents are marketed on the basis of
typical physical properties rather than on chemical specifications,
because of the limitations during refining of controlling the complex
mixtures of isomers that make up the petroleum solvents. As production
techniques become more sophisticated, greater control is possible and
more properties can be specified within narrower limits. However, even
when such narrow limits are met, the mixture of components present may
vary, because of variations in the types of crude oil being processed
and alterations in conditions in processing units.
To meet the wide range of properties required by the market,
several different processes are used. Distillation is the common
process setting the volatility range. Chemical conversion techniques,
including reforming, alkylation, and hydrogenation, alter the chemical
composition and hence the solvency, as do physical conversion
techniques such as solvent extraction and molecular sieve separation.
Specific treatments such as caustic soda and sulfuric acid washing and
clay percolation are frequently applied to remove odourous substances,
chiefly sulfur compounds.
The reader is referred to Boenheim & Pearson (1973) for detailed
discussions of the chemical and physical composition and uses of
petroleum solvents.
3.1.1.1 Special-boiling-point solvents (SBPs)
These are highly purified naphtha fractions with specially
selected boiling ranges. The boiling range may be narrow or wide, and
generally falls within the limits of 30-160°C: SBPs are classified
according to their boiling range, e.g., SBP 62/82. Petroleum ether,
lighter fluid, spot remover, and rubber solvent are consumer products
in this range. Generally, SBPs consist of a mixture of hydrocarbons in
the C-5 to C-9 range: normal and branched paraffins, cycloparaffins,
and aromatic compounds. They contain only traces of olefins. An
example of the composition of a typical sample of straight run (i.e.,
non-dearomatized) SBP 80/110 is given in Table 2.
3.1.1.2 White spirits
The boiling-range of this group of solvents falls within the
limits 150-220°C (intermediate between gasoline and kerosene). These
solvents can be classified into low-aromatic grades (approximately
15-20% aromatic hydrocarbons) and high-aromatic grades (45% or more
aromatic hydrocarbons). They generally consist of hydrocarbons in the
C-7 to C-12 range, again including normal and branched paraffins as
well as naphthenic (cycloparaffins) and aromatic compounds. Olefins
are present in trace amounts only. Stoddard solvent, mineral spirits,
low-aromatic white spirits (LAWS) and turpentine-substitute are
well-known examples from this range.
3.1.1.3 High-boiling aromatic solvents
Aromatic hydrocarbons occur naturally in certain crude oils in
widely varying concentrations. They are also formed during secondary
processes such as thermal and catalytic reforming. They can be
concentrated and extracted by solvent extraction.
Apart from benzene, toluene, and xylene, which will not be
discussed separately in this review, this group includes solvents with
an aromatic content of 80-100%, and a wide boiling-range from 160 to
300°C. High-boiling aromatic solvents are obtained by distillation or
solvent extraction from refinery fractions such as kerosene and
lubricating base oils, and consist of very complex mixtures of
hydrocarbons with more than 9 carbon atoms per molecule. The
composition of a typical sample of one of these aromatic hydrocarbons
from the middle range (distillation range approximately 192-203°C) is
given in Table 3.
Most aromatic solvents are highly purified "white" solvents.
Those in the higher boiling range, derived from lubricating base oil
stocks by solvent extraction, may be less pure and coloured. They are
often by-products and are used as solvents for various technical
purposes. In many cases, they are referred to as "processing oils"
instead of solvents, and considered under lubricating oils.
3.1.2 Purity of petroleum solvents
In these complex mixtures, impurity is, of course, a matter of
definition. Components that are taken out in the course of the various
refining and treating processes used to obtain the more pure solvents
could be regarded as such. The major impurities would then be sulfur
compounds such as hydrogen sulfide, mercaptans, and thiophens, as well
as, olefins and other reactive unsaturated hydrocarbons.
A second category of impurities includes the hydrocarbons that
have been demonstrated to be carcinogenic in animals and man, such as
benzene, the polynuclear hydrocarbons and related heterocyclic
compounds containing nitrogen or sulfur.
TABLE 2. Composition of typical sample of SBP 80/110a
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
normal n-pentane 0.2 36.2
paraffins n-hexane 8.2 69.0
n-heptane 17.2 98.4
branched 2 methyl butane Tc 0.1 27.9
paraffins 2,2 dimethyl butane T trace 49.7
2,3 dimethyl butane T 0.3 58.0
2 methyl pentane 1.5 60.3
3 methyl pentane 1.6 63.3
2,2 dimethyl pentane 1.0 79.2
2,4 dimethyl pentane 1.3 80.5
2,2,3 trimethyl butane T 0.3 80.9
2,3 dimethyl pentane 9.7 89.8
3 methyl hexane 9.2 91.9
3 ethyl pentane 3.1 93.5
2,2,4 trimethyl pentane trace 99.2
2,2 dimethyl hexane trace 106.8
2,5 dimethyl hexane 0.6 109.1
3,3 dimethyl hexane T trace 112.0
2,3 dimethyl hexane 0.8 115.66
3,4 dimethyl hexane trace 117.7
3 methyl heptane 0.5 118.9
cyclo C-6 cyclohexane 8.4 80.7
paraffins methyl cyclohexane 14.2 100.9
cyclo C-5 cyclopentane T trace 49.3
paraffins methyl cyclopentane 4.7 71.8
1,1 dimethyl cyclopentane T 2.9 87.9
1-cis-3-dimethyl 1.9 90.8
cyclopentane T
TABLE 2. (contd).
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
cyclo C-5 1-trans-3-dimethyl 2.7 91.7
paraffins cyclopentane T
contd. 1-trans-2-dimethyl 0.5 91.9
cyclopentane T
1-cis-2-dimethyl cyclopentane T 0.5 99.5
ethyl cyclopentane 0.6 103.5
1,1,3 trimethyl cyclopentane T 0.8 104.9
1-trans-2-cis-4-trimethyl 0.4 109.3
cyclopentane T
1-trans-2-cis-3-trimethyl 0.4 110.2
cyclopentane T
1,1,2 trimethyl cyclopentane T 0.3 113.7
unidentified Probably
paraffins 1.1 110.0
aromatic benzene 0.7 80.1
compounds toluene 3.9 110.6
olefins 0.4
a From: Shell International Petroleum Co., London (unpublished data).
b Average of duplicate analyses.
c T = tentative identification.
TABLE 3. Composition of typical sample of Solvesso 150a
Hydrocarbon % v/v of solvent
n-butylbenzene 2.47
sec-butylbenzene 0.08
tert-butylbenzene 0.05
m-cymene 0.13
o-cymene 0.01
p-cymene 0.52
1.2-diethylbenzene 1.72
1.3-diethylbenzene 1.10
1.4-diethylbenzene 0.56
1.2-dimethyl-3-ethylbenzene 2.86
1.2-dimethyl-4-ethylbenzene 6.64
1.3-dimethyl-2-ethylbenzene 0.71
1.3-dimethyl-4-ethylbenzene 4.17
1.3-dimethyl-5-ethylbenzene 2.80
1.4-dimethyl-2-ethylbenzene 3.26
m-ethyltoluene 0.37
o-ethyltoluene 0.02
p-ethyltoluene 0.01
indane 0.46
isobutylbenzene 0.32
isopropylbenzene 0.01
1-methyl-3-t-butylbenzene 0.76
1-methyl-2-n-propylbenzene 1.26
1-methyl-3-n-propylbenzene 2.08
1-methyl-4-n-propylbenzene 1.93
1-methylindane 0.91
2-methylindane 2.43
4-methylindane 9.28
5-methylindane 2.02
naphthalene 4.03
n-propylbenzene 0.00
1.2,3,4-tetramethylbenzene 3.66
1.2,3,5-tetramethylbenzene 8.84
1.2,4,5-tetramethylbenzene 5.53
toluene 0.02
1.2,3-trimethylbenzene 0.10
1.2,4-trimethylbenzene 0.05
1,3,5-trimethylbenzene 0.01
a Courtesy Esso Standard Oil Company, New York, N.Y.,
USA (From: Gerarde, 1960).
TABLE 3. (contd).
Hydrocarbon % v/v of solvent
m-xylene 0.05
o-xylene 0.03
p-xylene 0.03
C-11-naphthalenes 0.31
C-11-indanes 3.58
C-11-alkylbenzene 18.27
C-12-alkylbenzene 0.73
C-12-indanes 0.08
C-13-alkylbenzene 0.02
C-10-indenes 0.10
C-11-indenes 0.07
C-12-naphthalenes trace
C-13-naphthalenes
C-12-indenes 0.10
aromatic compounds Total 94.55
Generally, the total sulfur content, the olefin content, and the
total aromatic content are specified for commercial petroleum
solvents. Where special products such as food-grade materials are
concerned, the benzene content is specified as well as the UV
absorption limits at certain wavelengths, as a measure of the
polynuclear aromatic hydrocarbon content.
3.1.3 Methods of sampling and analysis
See section 2.1.2.
3.2 Sources of Environmental Pollution
3.2.1 Natural occurrence
Petroleum solvents do not occur in nature as such, but only as
components of the crude oils from which they are derived.
Environmental pollution is always man-made and related to the use of
the solvents.
3.2.2 Man-made sources
3.2.2.1 Production
Because there is no uniform system of definition and
classification of petroleum solvents, firm statistics concerning the
magnitude of production of this group of materials do not exist. The
best estimate of the world-wide production of the group of solvents
would be 9 million tonnes for the year 1979.
3.2.2.2 Uses
It is not feasible to give more than a general outline of the
uses of the range of petroleum solvents.
(a) SpeciaL-boiling-point solvents (SBPs)
SBPs are mainly used as: solvents and thinners in lacquers and
paints; extraction solvents for perfumes, for vegetable oils and oil
and fats of animal origin; quick-drying solvents in printing-ink,
coatings, and adhesives; lighter fuel; and for dry-cleaning and
degreasing purposes.
(b) White spirits
White spirits are mainly used as: solvents and thinners for
lacquers, paints, resins, and printing-ink; solvents in formulations
of chemical products, e.g., pesticides; and for metal degreasing, wool
degreasing, and dry-cleaning.
(c) Aromatic extracts
The higher-boiling and less-purified aromatic extracts have very
good solvent properties for many polymers and are used as
ex-tender-oils in rubber, plastics, and bitumens, and also as solvents
in printing-ink and pesticide formulations. Furthermore, they can be
used as base-materials in the manufacture of carbon black.
3.3 Environmental Exposure Levels
Specific data are not available concerning levels of petroleum
solvents in air, water, food, or other environmental media. However,
low concentrations of hydrocarbons found in mussels have probably been
derived from petroleum hydrocarbons present in the environment
(Ehrhardt & Heineman, 1975).
Because of the relatively low boiling-range of these solvents,
industrial exposure to vapour may sometimes be high. This is known to
occur, especially in small workshops with insufficient ventilation,
where, for example, adhesives are used routinely. Although a lot of
consumer products may contain these solvents, excessive domestic
exposure would not normally be expected unless neat solvent were used
for cleaning purposes, indoors. Very limited, indirect exposure of the
general population is possible following the use of these solvents as
extractants in the production of food-grade vegetable oils.
Exposure to the higher-boiling and less-purified aromatic
extracts is mainly confined to occupational situations, where
excessive skin-contact may occur, or exposure to vapour in processes
carried out at elevated temperatures or with high-speed machines that
could give rise to fumes or mists. This will be considered in detail
under lubricating base oils.
3.4 Environmental Distribution and Transformation
Data on the distribution between media, environmental
transformation and degradation, interaction with physical, chemical,
or biological factors and bioconcentration, are not available for
petroleum solvents.
However some information exists concerning the behaviour and
degradation of crude oil in water (Floodgate, 1972, Hellmann & Zehle,
1972), and of hydrocarbons in general (Walker et al., 1975), and there
is much information on the microbial degradation of individual
petroleum hydrocarbons (Van der Linden & Thysse, 1965; Haines &
Alexander, 1974).
From these publications it can be seen that the subject is highly
complex and many factors have to be taken into account, such as the
composition of the oil product, the extent of dispersion into the
medium, and climatic conditions.
3.5 Metabolism
3.5.1 Absorption
The kinetics are determined by diffusion rates, solubility in
fat, and the concentration gradients in the individual compartments of
the body.
The highly volatile C-5, C-6, and C-7 paraffins, cycloparaffins,
and aromatic hydrocarbons readily pass across the alveolar membrane
into the bloodstream and are transported within minutes to the central
nervous system. Longer-chain homologues can, to a certain extent, also
pass the alveolar membrane, but their principal effect is local. This
was shown by Gerarde (1963) in studies on rats.
The alveolar air and blood concentrations of white spirit have
been measured in man following inhalation (Åstrand et al., 1975).
Aromatic hydrocarbons were absorbed to a greater extent into the
bloodstream than aliphatic hydrocarbons (approximate values being 62%
and 50%, respectively). Similar uptake values in man were shown for
the aromatic hydrocarbons, benzene and toluene, by Nomiyama & Nomiyama
(1974), for xylene by Sedivec & Flek (1976), Åstrand et al. (1978),
and Riihimäki et al. (1979), and for ethylbenzene by Bardodej &
Bardodejová (1970). Nomiyama & Nomiyama (1974) demonstrated a much
lower pulmonary absorption for n-hexane, the only aliphatic compound
that they tested; it was also rapidly excreted.
The skin is only permeable to hydrocarbons of a certain size.
With paraffinic substances, the maximum chain length appeared to be up
to 14 C-atoms (Scheuplein & Blank, 1971). Aromatic compounds have a
more compact structure and, in studies on guineapigs, Hoekstra &
Phillips (1967) showed that compounds from this group with a higher
number of C atoms could still pass the skin barrier.
The absorption of vapours through the skin is of minor
importance. For example, in man, whole body skin exposure to
2250 mg/m3 (600 ppm) of toluene was equivalent to an inhalation
exposure of less than 37.5 mg/m3 (10 ppm) (Riihimäki & Pfäffli,
1978). However, absorption during immersion in liquid solvents may be
considerable. Percutaneous absorption during immersion of both hands
in pure xylene was equal to an inhalation exposure of 435 mg/m3
(100 ppm) (Engström et al., 1977). The permeation of xylene is thus
about 20 nmol/min per cm2 (Engström et al., 1977; Riihimäki, 1979)
and that for toluene, 3 µmol/min per cm2 (Cohr & Stockholm, 1979).
Cutaneous exposure was probably a major route of absorption in 2 cases
of acute renal failure with oliguria, caused by exposure to diesel oil
(Barrientos et al., 1977; Crisp et al., 1979).
Data for absorption in the intestinal tract are not available,
but it is presumed that it would resemble absorption in the alveoli
rather than that through the skin.
3.5.2 Distribution in the body
Tissue hexane levels in rats, following inhalation of anaesthetic
concentrations, were measured by Böhlen et al. (1973). The tissue
distribution generally depended on exposure time and was proportional
to the lipid content of an organ until saturation occurred. The liver
was a special case for, as its lipid level changed rapidly, the
saturation level varied. Hexane was also apparently bound to some
blood components.
Women working at conveyor belts gluing parts of rubber footwear
had concentrations of petroleum solvents (no details on
physicochemical properties given) in the blood ranging from 2 . 35 ±
0.4 up to 4.6 ± 0.6 mg/litre at concentrations in the air of
100-300 mg/ m3. The solvent concentration in the blood increased with
increasing length of the working period from 1.6 mg/litre in the first
year to 2.5 mg/litre after 3.5 years and 3.4 mg/litre after 7-8 years
of service.
Wistar rats were exposed to the solvents used in the factory at
concentrations in air of 300-1000 mg/m3 for 30-45 days, 4 h/day. The
concentration of solvent in the blood amounted to 0.45 ± 0.05 -
1.2 ± 0.01 mg/litre (Lipovskij et al., 1977a).
Transfer of petroleum solvents through the placenta was studied
in 85 pregnant women workers in the rubber industry, who came into
contact with petroleum solvents during work (physicochemical
properties of the solvents not defined, concentration in the air of
the operating premises 300 ± 10 mg/m3). The average level of solvents
in the blood of 46 pregnant women, on whom abortion was performed, was
1.27 ± 0.3 mg/litre. A level of 3.29 ± 0.6 mg/kg was found in the
tissue of the embryo. Women giving birth to a child (39 women) had a
level of solvents in the blood of 2.5 ± 0.3 g/litre, while the content
in the blood of the umbilical cord was 3.5 ± 0.3 g/litre. The
concentration of solvents in the blood of the newborn infants was
twice that of the mothers.
Pregnant Wistar rats were exposed to the same solvent at a
concentration of 300 ± 10 mg/m3, for 48 days, 4 h per day. The
solvent was present in the blood, brain, liver, placenta, uterus, and
fetal tissues (Lipovskij et al., 1979).
3.5.3 Biotransformation
In both man and animals, the aliphatic hydrocarbons are generally
considered to be biochemically inert and excreted in the same form
(Williams, 1959). However, it has been shown that some normal alkanes
will, at least in part, be oxidized by the mammalian organism. For
example, Ichihara et al. (1969) demonstrated the oxidation of decane
in animals such as mice and rats, and the oxidative pathway of
n-hexane to hexane-2,5-dione and hexane-2,5-diol via
methyl- n-butylketone has been well established (see for example
Spencer et al., 1978).
As far as the metabolism of the cycloparaffins and aromatic
hydrocarbons is concerned, the half-life, form, and rate of excretion
of each component of the solvent has to be considered. It should be
mentioned, however, that the metabolism of individual compounds will
not be discussed in this document and readers are referred to the
reviews by Williams (1959) and Gerarde (1960).
The carcinogenicity of the solvents is thought to be due to the
presence of benzene and some of the polynuclear aromatic compounds.
3.5.4 Elimination
The elimination of the lower-boiling solvents (SBP type) in both
animals and man is usually rapid and mainly occurs via the respiratory
tract. However, in the case of ingestion of the heavier solvents
(white spirits), elimination mainly takes place with the faeces
(Browning, 1965).
3.6 Effects on Experimental Animals
It has been mentioned in section 3.1.1, that the petroleum
solvents under discussion in this document are more or less complex
mixtures of a range of hydrocarbons. For the commercial products, the
specification given generally includes the specific gravity,
boiling-range, and total content of aromatic hydrocarbons. The
concentrations of individual components vary, within certain limits,
with the source of the crude oil from which the solvent is derived,
and with the processes by which it is produced. These facts should be
kept in mind because:
(a) the toxicity data developed for a certain solvent-specification
indicate the order of magnitude of the toxicity of this type of
product;
(b) in practice it would be impossible and impracticable to carry out
complete toxicity testing on every single solvent on the market.
It is only sensible to develop toxicity data for typical
representative samples of a certain boiling range and within a
certain specification of aromatic content. In the evaluation of
the results, however, the analytical composition of the material
- especially its contents of n-hexane, benzene, and polynuclear
aromatic hydrocarbons should be taken into account.
3.6.1 Short-term exposure
Hine & Zuidema (1970) examined various aspects of the acute
toxicity of 10 samples of petroleum solvents that contained components
representative of the range of hydrocarbons found in commercial
petroleum solvents. Four were aromatic solvents containing at least
98% aromatic hydrocarbons (coded A) and 6 were non-aromatic solvents
containing less than 1% aromatic hydrocarbons (coded S). The boiling
ranges and principal components of the samples examined are given in
Table 4.
Acute oral, inhalation, and percutaneous toxicity and skin and
eye irritancy were examined for all samples. Intratracheal aspiration
was simulated with 2 samples and repeated skin irritation tests were
carried out using 5 of the samples. Undiluted samples were used for
the investigations, all of which were carried out on rats with the
exception of skin and eye irritancy and skin toxicity rests in which
rabbits were used.
TABLE 4. The boiling-range and principal components of solvents examined for
acute toxicitya
Sample Boiling-range Principal components
A-1 281-286°F (138-141°C) C-8 aromatic compounds (ortho, meta, and
paraxylene; ethyl benzene)
A-2 362-398°F (163-203°C) C-9, C-10 and C-11 aromatic compounds
A-3 364-408°F (188-209°C) C-10 and C-11 aromatic compounds
A-4 384-507°F (196-264°C) C-11 to C-14 aromatic compounds
S-1 149-166°F (65-75°C) C-6 normal and isoparaffins (hexanes) and
naphthenes (cyclohexane, methylcyclopentane)
S-2 196-220°F (91-104°C) C-7 normal and isoparaffins (heptanes) and naphthenes
(methylcyclohexane, dimethylcyclopentane)
S-3 313-356°F (156-180°C) C-9 and C-10 normal and isoparaffins and naphthenes
S-4 368-395°F (187-212°C) C-11 and C-12 normal and isoparaffins and naphthenes
S-5 345-402°F (174-216°C) C-12 isoparaffins
S-6 384-500°F (195-260°C) C-13 to C-16 normal and isoparaffins and naphthenes
a From: Hine & Zuidema (1970).
The findings of Hine & Zuidema (1970) which are summarized in
Table 5, showed that all the solvents tested could be considered of
low hazard to health unless aspirated or inhaled in extremely high
concentrations. Aromatic solvents were more toxic than non-aromatic
materials, the dose of solvent required to kill 50% of rats, when
administered orally or percutaneously, being lower for aromatic than
for non-aromatic solvents. Skin and eye irritancy were also greater
with aromatic solvents. The toxicity of the vapours could not be
compared, because the volatility of samples varied greatly. All
solvents induced similar toxic effects, whatever the route of
administration, including central nervous system depression
(characterized by incoordination, prostration, and coma) followed by
death. Convulsions sometimes occurred. All solvents caused skin and
eye irritation though, in general, as the chain length of the
non-aromatic solvents increased their irritant properties decreased.
Repeated skin exposure led to skin irritation and necrosis with all
solvents.
Hoekstra & Phillips (1963) found that light mineral oils, when
applied topically to the skin of guineapigs, caused epidermal
hypertrophy, hyperplasia, hyperkeratosis, and depilation. Examination
of the effects of various oil fractions demonstrated that the main
effect of the short-chain volatile paraffins was to defat the skin,
while longer-chain and aromatic hydrocarbons had a dermatoxic effect
that was related to the permeability of the skin to these compounds.
The maximum dermatoxic effect was seen with hydrocarbons containing
14-19 carbon atoms, while a transition to non-dermatoxicity occurred
around 21-23 carbon atoms. This was confirmed with pure n-paraffins,
but variations may exist with other types of hydrocarbons.
Simultaneous application of innocuous long-chain substances together
with irritant short-chain substances greatly reduced their toxicity,
though this effect was less marked with aromatic solvents.
In further studies on the effects of inhaling the vapours of
hydrocarbon solvents (Carpenter et al. 1977a, b, c), the acute (4-h
exposure) LC50 and no-observed-adverse-effect concentrations were
studied in rats cats, and dogs. Results are summarized in Table 6.
These studies confirmed the occurrence of central nervous system
depression and there was also evidence of respiratory tract irritancy.
There were no marked or consistent differences between the species
examined. The major factor determining the acute inhalation hazard was
the volatility of the solvent, those containing 9 or more carbon atoms
tending to be insufficiently volatile to produce concentrations high
enough to be lethal over a short period of exposure. One exception was
a "high naphthenic" solvent, which was peculiar also in that
depression was not preceded by signs of irritation of the respiratory
tract, so that there was no warning of overexposure. Increased
aromatic content did not consistently result in increased inhalation
toxicity, though earlier work (Lazarew, 1929) suggested that the acute
inhalation toxicity of gasoline vapours increased with increasing
contents of cycloparaffins and aromatic hydrocarbons. The narcotic
action was also found to increase in each step by a factor of 3 in the
series - pentane, hexane, heptane, and octane (Fühner, 1921). Swann et
al (1974) found that anaesthesia occurred with these compounds at
concentrations of 32 000 ppm or more and that respiratory tract
irritation increased with chain length. Full anaesthesia can be
produced with gasoline (Haggard, 1921), but anaesthetic concentrations
are little lower than those that cause convulsions and death
(Browning, 1965).
TABLE 5. Toxicity of solvents. Summary of resultsa
Test Sample Result Classification
Oral A-1 10.0(7.5-13.3) practically non-toxic
LD50 A-2 4.5(3.0-6.8) slightly toxic
(ml/kg) A-3 13.3(7.5-23.7) practically non-toxic
A-4 12.3(8.1-18.7) practically non-toxic
S-1 >25.0b relatively harmless
S-2 >25.0b relatively harmless
S-3 >25.0b relatively harmless
S-4 >25.0b relatively harmless
S-5 >25.0b relatively harmless
S-6 >25.0b relatively harmless
Vapour A-1 6 350(4 670-8 640) slightly toxice
exposure A-2 >2 450b SVNTARTd
LC50 in ppm A-3 >580c SVNTART
for 4 h A-4 >553c SVNTART
S-1 73 680(66 310-79 940) practically non-toxic
S-2 14 000-16 000 practically non-toxic
S-3 2 000-2 600 slightly toxic
S-4 >710 SVNTART
S-5 >792 SVNTART
S-6 >263 SVNTART
Aspiration A-4 5/10 hazardous
(mortality) S-6 5/10 hazardous
Primary A-1 2.21 moderately irritating
skin A-2 2.04 moderately irritating
irritation A-3 2.17 moderately irritating
A-4 2.79 moderately irritating
S-1 1.92 slightly irritating
S-2 1.13 slightly irritating
S-3 2.38 moderately irritating
S-4 1.04 slightly irritating
S-5 1.29 slightly irritating
S-6 0.75 minimally irritating
Eye A-1 6.33 moderately irritating
irritation A-2 6.0 moderately irritating
A-3 4.33 moderately irritating
A-4 3.67 slightly irritating
S-1 0.33 minimally irritating
S-2 1.0 minimally irritating
S-3 2.0 minimally irritating
S-4 0 minimally irritating
S-5 0 minimally irritating
S-6 0 minimally irritating
TABLE 5. (contd).
Test Sample Result Classification
4-h A-1 approx. 5.0 practically non-toxic
percutaneous A-2 approx. 5.0 practically non-toxic
LD50 rangefind A-3 approx. 5.0 practically non-toxic
(ml/kg) A-4 approx. 5.0 practically non-toxic
S-1 >5.0 practically non-toxic
S-2 >5.0 practically non-toxice
S-3 >5.0 practically non-toxice
S-4 approx. 5.0 practically non-toxice
S-5 5.0 practically non-toxice
S-6 5.0 practically non-toxice
Repeated benzene 3.6
skin toluene 3.5
irritationf A-1 3.3
a From: Hine & Zuidema (1970).
b Doses above this amount not practical for testing.
c Maximum concentration obtainable at 25 °C.
d SVNTART = Saturated vapours not toxic at room temperature.
e Lowest toxicity classification may be "relatively harmless"
f Scored according to the method of Draize.
The greatest health hazard arises when hydrocarbon solvents are
aspirated into the lungs. This rapidly induces acute chemical
pneumonitis, which is characterized by pulmonary oedema and
haemorrhage, and is generally fatal (Waring, 1933; Lesser et al.,
1943; Gerarde, 1959). Gerarde (1959) demonstrated that the ratio of
the oral and intratracheal LD50s was 140:1 for kerosene, the
intra-tracheal LD50 being 0.2 ml for rats. This and other evidence
demonstrated that pulmonary injury was caused by direct contact with
the solvent and not by solvent present in the blood, following its
absorption through the gastrointestinal tract.
TABLE 6. Toxicity of solventsa
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
V.M. & P. 118-151 55.4 32.7 11.9 C-7 to C-10 16 >8 >19 2.8 2.8 0.7-7 2.1 2.0
Naphtha (3400) (>1700) (>4100) (600) (800) (0.15- 1.5) (450) (430)
Stoddard 153-194 47.7 37.6 14.7 C-8 to C-12 >8.2 >2.9 > 10 1.1 1.9 0.5 - 5 7.85 1.15
solvent (>1400) (>510) (>1700) (190) (330) (09-.9) (>150) (200)
Rubber 75-112 41.4 53.6 4.9 C-6 to C-7 61 >5.9 >49 7.9 7.9 40 1.7 1.7
solvent (15000) (>1500) (>12000) (2000) (2000) (10) (430) (430)
Mixed 138-141 - - 100 C-8 29 >5.4 <41 3.5 3.5 0.6-6 >0.46 0.46
xylenes (6700) (>1200) (<9500) (810) (180) (.14-1.4 (>110) (110)
'60' 128-159 c28.8 c21.4 c49.5 C-8 to C-9 24 >9.5 <20 0.44 1.4 10 C. 1.3 0.44
Solvent (4900) (>1900) (<4100) (90) (280) (2) (260) (90)
'70' 157-211 16.5 15.7 67.8 C-9 to C-11 >4.4 >2.4 >2 1.1 1.1 4 0.32 0.32
Solvent (>810) (>440) (>370) (200) (200) (0.7) (59) (59)
'140' 184-205 60.8 35.7 3.4 C-5 to C-12 >0.27 >0.21> > >0.23 >0.23 4 0.31 0.23
Flash (sic) (>43) (>33) saturated (>37) (>37) (0.6) (49) (37)
aliphatic vapour
solvent'
'80' 96-142 9.7 18.9 71.4 C-6 to C-8 27 >2.1 >24 > 1.7 > 1.7 4 0.45 0.45
Thinner (6200) (>480) (>5500) (>390) (>390) (0.9) (100) (100)
'50' 98- 105 66.3 0.6 33.1 C-7 33 >3.4 >30 2.4 2.4 10 1.7 1.7
Thinner (8300) (>600) (>7600) (600) (600) (2.5) (430) (430)
Deodorised 208-272 55.2 40.~9 3.9 not stated >0.1 This value applies for all toxicity evaluation and is 0.1
kerosene probably (14) a saturated atmosphere at room temperature. (14)
C 12 to
C-15
TABLE 6. (contd)
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
'40' 187-231 35.4 32.9 31.5 C-9 to C-13 >0.2 >0.25 >7 0.22 0,22 1 0.21 0.15
Thinner (>33) (>41) (aerosol) (36) (36) (0.17) (35) (25)
Toluene 95-110 38.7 15.4 45.9 C-6 to C-7 35 >3 >31 3.9 3.9 10 1.9 1.9
concentrate (8800) (>760) (>7800) (980) (980) (2.5) (480) (480)
'High 184-206 0.3 0.8 98.9 C-9 to C-12 >0.38 This value applies for all animal 0.4 0.15 0.15
aromatic (>66) toxicity evaluations and is a (0.07) (26) (26)
solvent saturated atmosphere at room temperature
'High 157- 183 29.0 69.9 1.1 not stated 5.3 3.8 - 2.1 - - - 2.1
naphthenic probably (960) (>690) (380) (380)
solvent' C-9 to
C-11
'Naphthenic 151-200 24.7 37.2 38.1 C-9 to C-11 >10 - - 2.2 - - - 2.2
aromatic (some (380) (380)
solvent' aerosol
present)
a Summary of data from Carpenter et al. (1977a,b,c).
b p = paraffins, N = naphthenes, A = aromatic compounds.
c For details of actual length of LC50 exposure, see original papers.
d NEL/No-ill effect/exposures 6th/day, 5 days/week for 13 weeks.
The same author found that viscosity appeared to be the property
that determined the aspiration hazard of liquid hydrocarbons.
Lower-boiling-point solvents (B.P. up to 100°C) evaporated so rapidly
in the mouth of anaesthetized rats that death was due to CNS
depression following absorption of the vapours. Higher-boiling-point
solvents tended to induce chemical aspiration pneumonitis. With
alkanes, the aspiration hazard decreased sharply with solvents
containing 16 or more carbon atoms, probably because the viscosity of
such solvents prevented aspiration into the alveoli. In the aromatic
series, side-chains containing more than 6 carbon atoms also tended to
diminish the aspiration hazard, as did blending with more viscous
lubricants (Gerarde, 1963).
3.6.2 Long-term exposure
The repeated skin tests of Hoekstra & Phillips (1963) and Hine &
Zuidema (1970) have already been mentioned in section 3.6.1.
Smyth & Smyth (1928) exposed guineapigs for 4 h/day, 6 days/
week, for a total of 65 exposures to a gasoline-type solvent
(boiling-range 145-183°C) at a concentration of 6750 mg/m3. During
the earlier exposures, the animals appeared to be restless. This was
followed by slight narcotic effects. Diarrhoea and albuminuria
developed temporarily, but blood, lung, and other changes were absent.
In a recent series of inhalation studies on various hydrocarbon
fractions, Carpenter et al. (1977a, b, c) provided much new
information on the toxicity of a wide range of solvents. Physical
properties and the general chemical constitution are detailed in the
papers and the approximate compositions are given in Table 6. The
no-observed-adverse-effect levels of individual solvents following
inhalation studies over 13 weeks in rats and dogs is also tabulated,
together with the human sensory response (section 3.7.1.2). In
general, these studies were remarkable in the lack of toxicity that
they indicated. The few toxic effects that did occur, were usually
kidney damage in rats and minor haematological variations.
Other long-term animal studies have been carried out with the
lower-boiling fractions and more specifically with technical hexane
and technical heptane, because of their possible neurotoxic effects
and to establish a safe level of industrial exposure (TLV).
Mature female Wistar rats were exposed to petroleum solvent
vapour (physicochemical properties not given) at a concentration of
300 ± 8.2 mg/m3 for 30-45 days, for 4 h/day. The serotonin content of
the myometrium in exposed rats equalled 75.7 ± 2.6 µg/kg compared with
68.47 ± 2.5 µg/kg in the control group. Uterine contractions were more
numerous and stronger in exposed animals. The level of solvent in the
venous blood was 2.0 ± 0.4 mg/litre. In the uterine tissues it was
almost twice as high (3.8 ± 0.6 mg/kg). The increase in serotonin
content in the organism could cause disturbances in the transport of
the fertilized egg cell and the nidation, and subsequently, early
abortion (Lipovskij, 1978).
Miyagaki (1967) exposed 5 groups of 10 male mice to vapour
concentrations of technical hexane of 360, 900, 1800, 3600, and
7200 mg/m3. There was a sixth control group. The exposures lasted
24 h/day, 6 days/week, over a period of one year. The composition of
the hexane used is uncertain. The measured vapour concentrations
correlated well with those calculated. Electromyographical studies
were carried out and strength-duration curves, electrical reaction
time, flexor/extensor ratios, and gait-posture were observed. The
muscular atrophy of the hind legs was measured and in some of the
animals, histological examination of the distal mucles of the hind
legs was carried out. Evidence of neurogenic muscular atrophy was only
seen in the group receiving the highest exposure. At the lowest
exposure level (360 mg/m3), no changes were found in any of the
variables studied. At the other levels, changes were found related to
the severity and duration of exposure. Based on their results, the
authors proposed a reduction in the TLV to 360 mg/m3.
In a different study, which included a group of 5 rats (sex and
age not stated) exposed to a hexane concentration of 3060 mg/m3, over
a period of 143 days (no other details given) and a control group, no
significant differences were found in average body weights and blood
values (haematocrit, total serum protein, and protein fractions)
between treated and control animals. Histological examination of
"many" organs revealed only a "slight reaction of the RES in the
spleen" in the exposed animals. In the sciatic nerve and its
ramifications, injuries such as degeneration of myelin and axon
cylinders were seen. The myoneural junction remained unaffected. The
exposed group manifested some decrease in nocturnal activity (Kurita,
1967).
Truhaut et al. (1973) published the results of
electrophysiological studies on rats exposed to technical hexane or
technical heptane. The air concentration of hexane was 7200 ±
720 mg/m3 and that of heptane, 6000 ± 600 mg/m3 (calculated and
expressed as n-hexane and n-heptane). The duration of exposure was
5 h/day, 5 days/week for 1-6 months. The initial weight of the male
and female rats was 150g. No data on the growth-weight curves were
included. The composition of technical hexane was given as:
n-pentane 0.3%
methyl-2-pentane and cyclopentane 25.1%
methyl-3-pentane 18.4%
n-hexane 45.8%
methylcyclopentane 8 %
methylhexanes 1.2%
benzene 1.2%
and that of technical heptane as:
methyl-2-hexane plus dimethyl 2,3 pentane plus cyclohexane 9.8%
methyl-3-hexane 16.2%
n-heptane 52.4%
dimethyl 2,4 hexane plus methylcyclohexane 15.4%
methylheptane 3.3%
benzene 0.1%
toluene 2.8%
Electrophysiological studies were carried out on isolated sciatic
and saphenous nerves taken from anaesthetized rats. During the first 2
months of exposure, no changes were found in any of the treatments
but, in the succeeding months, symptoms of neural involvement were
detected, in general, increasing with the duration of exposure. The
signs were: decrease in conduction-velocity, increase in refractory
period, and decrease in excitability. Histopathological examination
did not reveal clear-cut demyelination, but early indications of this
type of change were certainly found. Truhaut has recently cast doubt
on these findings, stating that his experimental method was not
sufficiently rigorous to allow his earlier conclusions to be made
(Truhaut, 1978).
Numerous studies on the neuropathological effects of n-hexane
have been published including a review by Schaumburg & Spencer (1976).
Rats exposed for 24 h/day to an n-hexane concentration of
1440-2160 mg/m3 for about 20 weeks developed peripheral neuropathy.
Toxicity data on the presumptive in vivo neurotoxic oxidation
products of n-hexane have also been published (Spencer et al.,
1978). It should be mentioned that many authors have used the term
" n-hexane" loosely; the tested material often really consisted of
various C-6 hydrocarbons.
Some other hydrocarbon solvents have been implicated as possibly
causing neuropathy, following prolonged exposure to high
concentrations. As these reports are generally related to human
exposure, they will be considered in section 3.7.2.1. Of relevance is
the observation that toluene inhaled by rats over a period of one year
induced electrophysiological changes at concentrations of 7500 mg/m3
and 750 mg/m3 but not at 375 mg/m3 (Matsumoto, 1971). Fournas & Hine
(1958) exposed rats to high concentrations of various alkyl aromatic
hydrocarbons and found some clinical evidence of neurotoxicity with
most of the compounds tested; p-t-butyltoluene was shown to induce
CNS damage in rats by Ungar et al. (1955).
3.6.3 Mutagenicity, teratogenicity, and carcinogenicity
3.6.3.1 Mutagenicity
The potential mutagenic activity of selected petroleum products
was assessed using a battery of in vivo and in vitro bioassays,
including the dominant lethal test, the Ames' test (with and without
activation), the mouse lymphoma cell transformation test, and
observations on cytogenicity. The following products elicited negative
responses in one or more tests: VM&P Naphtha, Stoddard Solvent, mixed
xylenes, "60 Solvent", "70 Solvent", 140° Flash Aliphatic Solvent, "50
Thinner", kerosene, toluene, high solvency naphtha- unleaded petrol,
and No. 2 heating oil (40% aromatic compounds). Positive mouse
lumphoma cell transformation tests were elicited by benzene, diesel
fuel, No. 2 heating oil, and jet A fuel; positive results in
cytogenetic tests (clastogenic responses) were obtained with rubber
solvents, "60 Solvent", high aromatic solvent, No. 2 heating oil, and
jet A fuel. For each of these products the Ames bacterial bioassay was
negative (API, 1974, 1975b, c, 1977a, 1978a, d, f, 1979d).
3.6.3.2 Teratogenicity
Tests for teratogenicity induced by inhalation of high and low
doses of benzene, Stoddard Solvent, toluene, mixed xylenes, unleaded
petrol, high aromatic solvent, n-hexane, diesel fuel, VM&P naphtha,
kerosene, rubber solvent, jet fuel A, and No. 2 heating oil were all
negative. However, benzene exposure at 120 mg/m3 induced a
statistically significant increase in fetal resorptions in rats (API,
1974, 1975a, b, c, 1977 a, b, 1978a-f, 1979a-d).
3.6.3.3 Carcinogenicity
Carcinogenicity tests have only been conducted on the group of
high-boiling aromatic extracts derived from the solvent refining of
lubricating base oils. These studies will be considered together with
long-term studies on lubricating base oils, but, in short, practically
the whole of the carcinogenic polynuclear aromatic hydrocarbons were
found in the extracts from the base oils. However, the carcinogenic
activity of the extracts was considerably less than that of
coal-tar-derived products.
Similar carcinogenicity studies have not been carried out with
the lower boiling aromatic solvents (white spirits). Such studies
would be indicated, though the content of potentially carcinogenic 4,
5 and 6 condensed ring polynuclear aromatic hydrocarbons in these
aromatic solvents is probably much lower than in high boiling aromatic
solvents, because of the lower boiling range. Lijinsky & Raha (1961)
mention, however, that no commercial distillation procedure will
completely remove all traces of polynuclear aromatic hydrocarbons from
petroleum solvents and that special treatment is necessary to achieve
this. Though, in most cases, the authors found very low concentrations
(in the µg/m3-range), they are of the opinion that these levels
should be investigated, particularly in food-grade material.
3.7 Effects on Man
3.7.1 Controlled exposures
3.7.1.1 Effects of dermal exposure
Klauder & Brille (1947) patch-tested petroleum solvents of
various boiling ranges on the skin of human volunteers. They found a
correlation between the boiling ranges of the petroleum products of
paraffinic origin and their irritant and defatting action on the skin.
Both effects decreased, the higher the boiling range. Petroleum
solvents with boiling ranges up to and including that of kerosene
(approximately 230°C) were found to be primary irritants. Petroleum
solvents of naphthenic origin or with a high aromatic content were
more irritant than solvents of paraffinic origin of the same boiling
range. The skin of Negroes showed a higher tolerance than that of
Caucasians.
Pre-existing skin disease may increase the susceptibility of the
skin to the effects of contact with petroleum solvents and will also
facilitate uptake by this route (Klauder & Brille, 1947; Riihimäki &
Pfäffli, 1978).
The effects of various solvents on the horny layer of the skin
were examined by Malten et al. (1968) and Spruit et al. (1970). They
found that petroleum ether (SBP 40/65) caused serious irritation of
human forearm skin, when applied for periods of 10-30 min. When
applied for 15 min on 6 successive days, injury occurred in the horny
layer. Recovery -- as measured by water vapour loss -- could take up
to 6 weeks. The skin irritation and the changes in the composition of
the horny layer were independent phenomena.
Tagami & Ogino (1973) applied undiluted refined kerosene to the
arm of a volunteer in an occluded patch-test. After 1 h, a burning
sensation developed, slight erythema appeared after 2 h, and after
7 h, the skin was tender and very red, even beyond the patch-test
site. After 12 h, the burning sensation had subsided but a large tense
bulla had appeared surrounded by small scattered vesicles. This
changed into a large flaccid purulent bulla 24 h later, which easily
broke, leaving a raw surface. The main differences between the test
situations just described were: (a) a marked difference in boiling
range (volatility and skin penetration) of the products tested; and
(b) the length of exposure, the effect increasing with increasing
duration of exposure.
The same authors then applied 85%, 70%, 55%, and 40% dilutions of
the kerosene in mineral oil in covered skin test on 34-adult male
Negro and Caucasian volunteers. The skin of all the subjects reacted
to 85% kerosene solution, the 70% solution caused skin irritation in
29 subjects, the 55% solution in 8, and the 40% solution was
completely without effect. The skin of Negroes appeared to be less
irritated by kerosene than that of Caucasians; the influence of age
was not clear. No definite correlation was found between individual
response to kerosene and the permeability of the horny layer of the
skin (Tagami & Ogino, 1973).
3.7.1.2 Effects of inhalation
Inhalation of air containing petrol at concentrations of
1350-3150 mg/m3 for 18 min by human volunteers did not cause any
symptoms; a 14-min exposure to 12 600-31 500 mg/m3 induced dizziness
(Fieldner et al., 1921). Davis et al. (1960) exposed human volunteers
for 30 min to concentrations of petrol of 900, 2250, or 4500 mg/m3 in
air. Very few symptoms were noted. Itching and burning of the eyes was
apparent in most subjects in the highest exposure group.
The human odour- and sensory irritation thresholds were measured
as part of the toxicity evaluation of solvents carried out by
Carpenter et al. (1977a) (Table 6). It can be seen that vapours of
hydrocarbon solvents can be detected at rather low concentrations, but
that unpleasant odour and irritation only become apparent at much
higher concentrations. Nevertheless, in establishing an exposure limit
for each solvent, human sensory data were often limiting factors.
In volunteer studies, psychological functions were affected by a
50-min exposure to a concentration of white spirit of 4000 mg/m3
resulting in a prolonged reaction time and impaired short-term memory.
Lower concentrations did not have any effect (Gamberale et al., 1975).
3.7.2 Epidemiological studies
A distinction between epidemiological and clinical studies has
always been difficult in view of the inadequacy of reporting. The
various aspects will therefore be discussed under headings relevant to
the information available.
3.7.2.1 Occupational exposure
(a) Haematological effects
Benzene is unquestionably the most dangerous hydrocarbon used in
industry and the benzene content of some petroleum solvents presents a
major long-term hazard for man. In particular, special boiling
solvents in the lower-boiling range may contain a considerable
percentage of benzene (Gerarde, 1960). In this review, it is
impossible to consider in detail the effects of benzene, which
basically causes bone-marrow depression and has a leukaemogenic
action. Excellent reports on all aspects of benzene toxicity can be
found in: ILO (1968); Deutsche Forschungsgemeinschaft (1970); IARC
(1974); and Laskin & Goldstein (1977).
In the past, benzene-like effects have also been ascribed to
other low-boiling petroleum solvents. However, there now seems to be
general agreement that these effects only occur, when benzene is
present in the mixture (Browning, 1959, 1965).
(b) Neuropathological effects
The neuropathological effects of inhalation of petroleum solvents
in man have been discussed in a number of reviews including those of
Cavanagh (1973), Allen (1975), Seppäläinen (1975), Comstock (1977),
and Savolainen (1977). The reviews include discussions of methods used
to evaluate human neuropathy.
Over the past 15 years, an increasing number of cases of
poly-neuropathy and other neurotoxic effects have been described in
workers exposed to hydrocarbon solvents. Many of these cases have been
associated with prolonged and repeated exposure to high concentrations
of n-hexane. Frequently, such exposure has resulted from poor
ventilation in a workroom in which solvents containing high
concentrations of hexane have been used. Miyagaki (1967) reported
hexane concentrations in air of 1800-3600 mg/m3 in the workroom and
7200 mg/m3 near the source in a work-place where 17 cases of
peripheral neuropathy occurred. Yamamura (1967) and Inoue et al.
(1970) found concentrations ranging from 1800-9000 mg/m3 in a work
place where, over a 9-year period, 93 out of the 1662 workers suffered
from polyneuritis. These workers used glue in the manufacture of vinyl
sandals for 8-14 h daily, probably for more than 5 days each week,
over long periods, in poorly ventilated rooms. The presence of hexane
in glues is reported to have caused similar incidents in Italy and
Iran (Capellini et al., 1968; Scrima & de Rosa, 1973; Ghazai, 1974).
In most cases, the extent of exposure to hexane was uncertain, either
because air concentrations were not measured or because excessive skin
contamination occurred; workers frequently handled the glues and
washed residues off the skin with hexane-containing solvents. In other
cases, air concentrations were measured. Hexane concentrations of
approximately 2340 mg/m3 (but at times up to 4680 mg/m3) were found
in a small, poorly ventilated workroom in which 3 women, who developed
polyneuropathy, had worked; again excessive skin contact took place
(Herskowitz et al, 1971; Ishii et al., 1972). Glue was also the source
of hexane concentrations of 1000-4000 mg/m3 found by Paulson &
Waylonis (1976) in the air of a work-place where 8 out of 50 employees
developed mild neuropathy.
The main clinical manifestation in these cases was polyneuropathy
of the glove-and-stocking type with sensations of numbness and cold.
This was accompanied by muscular weakness and headaches. Gradual
recovery was usual, when exposure ceased, with the exception of some
subjects who had severe muscular atrophy. Peripheral nerve biopsy
showed demyelination with relative preservation of the axon. Iida
et al. (1969) reported that in 44 cases there was a reasonable, but
not exact, correlation between clinical findings and electromyography
and nerve conduction velocity data.
Optic involvement was observed by Inoue et al. (1970). The
neuro-ophthalmological function was studied by Raitta et al. (1978) in
15 workers who had been exposed at work over periods of 5-21 years to
hexane concentrations of 1800-3600 mg/m3, with occasional levels of
10 800 mg/m3. Defective colour discrimination was found in 12 of the
workers and slight macular changes in 11 out of 15. The visually
evoked potentials and electroretinograms were interpreted by
Seppäläinen et al. (1979) as indicating cerebral dysfunction, probably
a conduction block in intracerebral axons.
Polyneuropathy attributable to the inhalation of hexane has also
been observed in "glue sniffers" -- subjects who inhale solvent
vapours to induce elation or other states of mind. Cases including
very rare cases following medicinal use, were described by Schwarz
(1933), Browning (1965), Karani (1966), Gonzales & Downey (1972),
Matsumura et al. (1972), Taher et al. (1974) and Korobkin et al.
(1975). Other incidents have been reported or reviewed by Shirabe et
al. (1974), and Poklis & Burkett (1977). While hexane appears to have
played a major role, a direct or synergistic action of other
components cannot be ruled out.
Some cases have been described that were thought to arise from
the long-term, continuous domestic use of kerosene stoves in poorly
ventilated rooms (Contamin et al., 1960). Under simulated conditions,
hexane concentrations of about 1440 mg/m3 were produced in
experimental rooms by Lièvre et al. (1967).
The possibility of the production of peripheral neuropathy by
components other than hexane, or the intensification of the activity
of hexane, is suggested by some clinical reports. Cargill (1972)
(cited by Gaultier et al., 1973) described peripheral neuritis in
workers using glue containing cyclohexane, gasoline "c", and methyl
ketone, and Franco et al. (1979) reported sensory and peripheral motor
conduction disturbances, where exposure to cyclohexane had occurred.
The condition was described by Gaultier et al. (1973) in subjects who
had been in prolonged contact with a glue solvent containing 80%
pentane, 5% hexane, and 14% heptane. Gasoline concentrations of
2250 mg/m3 were found in the workshop atmosphere. Atmospheric
gasoline concentrations of up to 5625 mg/m3 (mainly n-pentane,
n-hexane, and n-heptane) were found in workshops where workers had
developed polyneuropathy and had complained of insomnia, irritability,
and other non-specific CNS symptoms. White spirit has also been
implicated as a cause of peripheral neuropathy (Gaultier et al.,
1973). Heavy exposure to jet fuel vapours was reported in workers who
experienced dizziness, palpitations, nausea, and headaches, later
followed by signs and symptoms of polyneuropathy; higher vibration
thresholds, compared with unexposed controls were also found. (Knave
et al., 1976).
Other symptoms besides those of polyneuropathy have been
described in subjects exposed to hydrocarbon solvents. Sterner (1941)
reported headaches, nausea, mental depression, anorexia, inability to
concentrate and sustain activity, and slight anaemia, in workers
exposed to gasoline vapours (used to dilute spray paint) containing
5-10% aromatic hydrocarbons and producing concentrations of total
aromatic hydrocarbons in air of 300-800 ppma. Knave et al. (1978)
compared 30 workers, occupationally exposed to jet fuel, with
unexposed controls. The average period of exposure was 17 years and
the estimated TWA exposure was 300 mg/m3. Significant differences
were found between exposed and unexposed groups in the incidence and
prevalence of psychiatric symptoms, psychological test results,
especially attention and sensorimotor speed, and in
electroencephalograms. Exposure of car painters, over many years, to
low concentrations (31.8% of the Finnish TLV on average) of solvent
mixtures containing toluene, xylene, butyl acetate, and "white
spirit", was found to be associated with an increased incidence of
sleep disturbance, absentmindedness, falling asleep while watching the
television, and headaches. Lower peripheral nerve conduction
velocities, psychomotor impairment, and personality changes were more
common in exposed than in control subjects. Further studies are needed
to elucidate the significance of such findings (Hänninen et al., 1976;
Seppäläinen et al., 1978).
In summary, the most serious adverse neurological effect of
hydrocarbon solvents in man is the production of peripheral
neuropathy. Observations in man and studies on experimental animals
support the view that exposure to n-hexane is the principal cause.
However, the synergistic activity of other hydrocarbons is possible;
such a phenomenon has been reported following the sniffing of a glue
thinner containing both the neuropathic solvent, methyl- n-butyl
ketone, and the non-neuropathic solvent, methyl ethyl ketone
(Altenkirch et al., 1977). At present, the possibility that other
hydrocarbons also have some neurotoxic activity cannot be ruled out.
Peripheral neuropathy has occurred only in conditions of prolonged and
repeated exposure to high concentrations of hydro-carbon solvent
vapour in air; in many cases, there was also excessive skin contact.
Atmospheric exposure levels in excess of 2 g/m3 are usually
encountered where peripheral neuropathy is seen, but more studies are
required to clarify the situation, and until this is done, close
supervision is needed to ensure that the TLV for solvents is not
exceeded. Further studies are also necessary to determine the
significance of psychological disturbances and of neurophysiological
findings in workers exposed to lower levels of the hydrocarbons.
(c) Effects on reproductive functions
Examination of 408 female workers in petroleum refineries, who
had been subjected to long-term exposure to hydrocarbons, hydrogen
sulfide, and other products related to the treatment of sulfurous
crude, revealed disturbances in the menstrual function, mainly in the
form of hypomenstruation and pre-menstrual syndromes. According to
Suhanova & Melnikova (1974), such disturbance of the menstrual
function in female workers in refineries and in persons suffering from
chronic intoxication from petroleum products (36 persons in the age
bracket 30-39 years) is caused by the hypo-functioning of the ovaries.
a Since the composition is not given, it is not possible to
transform this concentration into mg/m3, the adopted SI unit.
Beskrovnaja et al. (1979) studied the gynaecological disease rate
in more than 5000 female operators in plants producing rubber articles
(petroleum solvent vapour concentration in the air of 250-350 mg/m3).
They observed disturbances in the menstrual cycle in workers with more
than 5 years' service and a high frequency of metrorrhagia. As the
period of service increased, a reduction in the frequency of
miscarriages was noticed, which was interpreted by the authors as
possible adaptation. A disturbance of the ovarian function was noted
in 24.4% of the workers examined, mostly in the form of a functional
deficiency of corpus luteum.
Investigations of vaginal smears of 184 female gluers in the
rubber industry in the age bracket 18-38 years revealed a disturbance
of the ovarian function with reduced estrogen stimulation in 21.7% of
the women (10.4% in the control group). This figure was related to the
period of service. After 10 years service, the percentage was twice as
high as after 5 years' service.
Women who had been in contact with petroleum solvents were found
to have a reduced estrogen level in the blood (22.2 µg/day compared
with 29.6 µg/day in the control group). Essentially, no changes were
observed in the excretion of the follicle-stimulating and luteinizing
hormone pregnanediol. The authors of this study assumed that the
sensitivity of the ovaries towards the stimulating effect of the
gonadotrophins was reduced (Hrustaleva et al., 1979).
Novikov et. al. (1979) studied lactation in 332 nursing mothers
288 of whom worked in the rubber industry (vulcanizers, pressers,
gluers). The concentration of petroleum solvents (the physico-chemical
properties of which are not described) in the air of the operating
premises was predominantly 300 mg/m3. Hypolactation, found in 23.8%
of the women compared with 6.7% in the control group was related to
length of service. Hydrocarbon solvents were found in the milk of all
the persons examined (71) in concentrations of 0.50 ± 0.05 mg to
0.60 ± 0.09 mg/litre. The serotonin content of the blood of these
women was significantly lower than in the control group. It is assumed
that hypolactation was the result of the effect of solvents on the
lactation control mechanism via the hypothalamus and the
serotoninergic system.
(d) Effects on the skin
Skin can also be affected by exposure to solvents. In a study on
skin conditions and other diseases in 54 gasoline and diesel station
workers, skin conditions related to exposure to gasoline and diesel
fuel were minimal and did not interfere with capacity to work. Only
dryness, chapping, and reddening of the skin were found. The dark skin
of Indonesians seemed to be more resistant to these effects. The
gasoline vapour in the working environment caused some non-specific
symptoms in addition to those resulting from heavy out-door work
(Suma'mur & Susianti Wenas, 1979).
3.7.2.2 General population exposure
Data are not available concerning the exposure of the general
population to petroleum solvents with the exception of that of
"sniffers" and addicts already mentioned in section 3.7.2.1.
3.7.3 Clinical studies
It should be noted that literature surveys of clinical studies
and clinical effects have been published by Gerarde (1960), Browning
(1965), and Levina (1976).
3.7.3.1 Effects of dermal exposure
In general, petroleum solvents have a defatting action on the
skin and, on repeated contact, cause injury to the horny layer
(section 3.7.1.1). This makes the skin more susceptible to other
irritants, sensitizing agents, and bacteria. It may also result in
progressive dermatitis, characterized successively by dryness,
redness, chapping and scaling, which could lead, by sensitization, to
eczema. These stages of dermatitis may be seen in workers in garages
or automobile repair shops, who wash their hands with solvents, petrol
or kerosene (Tagami & Ogino 1973). The more aromatic solvents, in
particular, can cause a significant degree of primary skin irritation.
The defatting action and primary irritation caused by petroleum
solvents decrease, the higher the boiling range section 3.6.1).
Cases in which gasoline or kerosene remains in contact with the
skin for prolonged periods mainly occur in children or in unconscious
accident-cases, when clothing has become soaked with the solvent. In
such cases, the lesions start with a burning sensation and erythema,
followed by the formation of small or large vesicles, blisters, or
even extensive epidermolysis. The vesicles and blisters may become
mucopurulent in a few days. The acute picture is that of a chemical
burn, (Helbling, 1950; Aidin, 1958; Ainsworth, 1960; Stewart, 1960;
Browning, 1965; Hunter, 1968; Tagami & Ogino 1973).
Gasoline may occasionally be absorbed through the skin in toxic
quantities if large areas of skin such as the hands and forearms are
regularly exposed (Hayhurst, 1936), or in cases of extensive
epidermolysis in contact with gasoline-soaked clothing. However
inhalation of vapour plays a significant additional role in all these
cases and generally is the main route of absorption (Browning, 1965).
3.7.3.2 Effects of inhalation
The acute effect of massive overexposure to gasoline vapour is
mainly narcosis with loss of consciousness and possibly convulsions,
which may be fatal (Browning, 1965). Octane causes rapid and deep
narcosis, pentane and hexane are less powerful narcotics, but they and
heptane exert a paralytic effect on the central nervous system and its
respiratory centre.
In more gradual overexposure, the symptoms just described may be
preceded by eye irritation, irritation of the respiratory tract,
dizziness, headache, and a sense of drunkenness.
A gasoline concentration of 9000 mg/m3 can be breathed without
significant ill-effects by most people but susceptible subjects may
show symptoms after exposure to 1350-2250 mg/m3 (Ainsworth, 1960). At
31 500 mg/m3, dizziness and symptoms of drunkenness may appear.
Exposures in excess of 45 000 mg/m3 soon become intolerable and may
rapidly prove fatal (Machle, 1941, Aidin, 1958). Lower and higher
values have been quoted, obviously depending on the composition of the
gasoline (Browning, 1965). The margin of safety between narcosis and
respiratory arrest is very narrow in exposures to high concentrations
of gasoline (Wang, 1961).
Absorption of gasoline vapour by inhalation may be very rapid if
the concentration is high, especially with the lower members of the
paraffinic range, and symptoms can appear within a few minutes.
Excretion, probably of unchanged vapours, takes place mainly via the
lungs (Browning, 1965).
Acute occupational poisoning by gasoline vapour is mostly caused
by entering unpurged gasoline tanks or other premises where high
concentrations of gasoline may have accumulated. Exposure to high
concentrations may also occur in car accidents, when victims are
trapped and/or unconscious.
Histopathological changes found in subjects who have died after
exposure to high concentrations of gasoline vapour include: hyperaemia
and petechial haemorrhages in the lungs, and, sometimes, necrosis of
the alveolar walls. There may be haemorrhages or effusions in internal
organs and serous cavities (Helbling, 1950; Ainsworth, 1960; Browning,
1965). Liver and kidney may show fatty degeneration. Hyperaemia and
oedema of the brain are common in this condition and myelin swelling
may occur (Machle, 1941).
Long-term exposure to low (unspecified) vapour concentrations may
cause nonspecific symptoms of the nervous system and digestive tract
(Zielhuis, 1961; Browning, 1965; Muhametova & Podrez, 1975; Sehtman et
al., 1979), including changes in liver function and in the visual
organ. In women, the reproductive organs may be affected (Muhametova &
Podrez, 1975; Sehtman et al., 1979). Neurological disturbances, as
described in section 3.7.2.1 may develop (Hayhurst, 1936; Machle,
1941; Browning, 1965). Browning (1965) considered that significant
changes observed in the blood count were caused by the presence of
benzene in the solvent.
This chronic form of toxicity may be found in small,
insufficiently ventilated workshops where solvents, or products
containing them, are handled in an unsatisfactory way (section
3.7.2.1) and when these materials are used in substantial amounts in
enclosed spaces, particularly for cleaning or degreasing (Anon.,
1974).
3.7.3.3 Effects of ingestion
Accidental ingestion of petroleum distillates in the range of
petroleum solvents is an important cause of poisoning in children
(Waring, 1933; Nunn & Martin, 1934; Lesser et al., 1943; Carithers,
1955; Daeschner et al., 1957; Gerarde, 1959, 1963). Most cases,
however, are caused by gasoline and kerosene and fewer by petroleum
solvents. The symptomatology is the same in all cases.
Coughing, choking, and gagging are often noted at the time of
ingestion of these substances. Respiratory embarrassment may be
present early, indicating that aspiration has taken place. Epigastric
discomfort may develop, followed by vomiting with a further risk of
aspiration. Aspiration by one mechanism or another is reported to
occur in up to 95% of cases in children, but this depends on the
situation and on the type (boiling-range) of the solvent involved.
Aspiration is less common in adults, but may occur when trying to
siphon gasoline from a tank. Small amounts of kerosene of the order of
1-2 ml, if aspirated, can cause severe and even fatal pulmonary
changes. On the other hand, if aspiration does not occur, much larger
quantities can be tolerated (Daeschner et al., 1957; Hensen, 1959;
Browning, 1965). Browning (1965) quotes a value of 7.5 g/kg body
weight, but this may depend on the type of solvent. Children appear to
be more susceptible to the toxic effects than adults (Siwe, 1932). In
cases where aspiration does not take place, and especially with the
lower-boiling solvents, central nervous system symptoms may develop
such as lethargy, convulsions, and coma. With smaller doses, the
symptoms include vertigo, headache, and signs of drunkenness. Nausea,
vomiting, and diarrhoea may occur and the stools may be blood-tainted.
In uncomplicated cases, the gastrointestinal symptoms will
disappear within 48 h. Pulmonary symptomatology will not develop, if
aspiration has not occurred and if there was no massive exposure to
vapours.
The syndrome of acute chemical aspiration pneumonitis will be
described in a wider context in section 4.8. Human experience is in
accordance with the results of experimental animal studies (section
3.6.1). Chemical pneumonitis with pulmonary oedema and haemorrhagic
frothy sputum may develop extremely rapidly following aspiration of
petroleum solvents. Roentgenographic changes may be seen within a few
hours, especially at the lung bases. Later, bacterial pneumonia can
complicate the situation (Daeschner et al., 1957; Gerarde, 1963).
The prognosis of aspiration of hydrocarbon solvents appears to
have improved with better treatment in the course of time. Siwe (1932)
reported that 50% of the cases he reported were fatal, Nunn & Martin
(1934) reported 28% mortality following the ingestion of gasoline and
9.2% following kerosene-ingestion, and Blattner (1951) reported that
death resulted in 10-14% of cases of kerosene poisoning. Recently, 3
cases have been described, where exposure to diesel oil seemed to
cause acute renal failure with oliguria (Reidenberg et al., 1964;
Barrientos et al., 1977; Crisp et al., 1979). However, in 2 cases,
absorption seemed to have been mainly through the skin (section
3.5.1).
4. LUBRICATING BASE OILS AND RELATED OILS,
GREASES, AND WAXES
4.1 Properties and Analytical Methods
4.1.1 Chemical and physical properties
Base oils are a limited group of petroleum products in the
boiling-range of 300-700°C, normally derived from the high-vacuum
distillation of the residues of the crude-distilling process. These
oils undergo a further refining process before being used.
Lubricating oils, metal-working oils, and related products are
produced by blending base oils in order to obtain the desired physical
properties. Chemical additives are frequently added, usually in small
amounts (a few mg to a few g/kg) to improve the performance of the
lubricant. In some special cases, higher concentrations are necessary.
Additives belong to the following general categories: viscosity-index
improver, emulsifiers, wetting agents, antioxidants, dispersants,
antiwear additives, extreme pressure additives, rust-inhibitors,
antifoam agents, pourpoint depressants, and germicides.
Greases based on mineral oil consist of solid or semisolid
dispersions of metallic soaps and other thickeners in a mineral oil
base.
Petroleum waxes are crystalline solids at normal temperature.
"Slack wax", a soft, impure paraffin-wax is obtained in the
manufacture of lubricating base oils from paraffinic crude oils by a
dewaxing process. Slack wax can be refined into 2 types of commercial
wax: paraffin wax and microcrystalline wax.
Base oils are very complex mixtures of hundreds to thousands of
different hydrocarbons, containing straight-chain and branched
paraffins, cycloparaffins, naphthenic, aromatic, and polynuclear
aromatic hydrocarbons in the range of C-17 and higher. The separate
molecules are so large, that any molecule may, for instance, contain
one or more aromatic rings with one or more long side-chains.
The actual composition depends on the source of the crude oil,
from which the product is derived, and the manufacturing and treating
processes used. They range from thin, easily flowing "spindle oils" to
thick "cylinder oils". A limited number of base oils are used for
blending to obtain the commercial products. As the relation between
viscosity and temperature is an important factor in this field, base
oil grades are characterized by their viscosity and viscosity index
(VI). The higher the viscosity index, the less the change in viscosity
with temperature.
Low viscosity index (LVI) oils are used whenever the viscosity
temperature characteristics and oxidation stability are of less
importance. In general, they are derived from naphthenic oils and
undergo treatment with sulfuric acid and clay or are given a
hydro-finishing treatment.
Medium viscosity index (MVI) oils, can be used as a base for
general-purpose lubricants. They can be derived from naphthenic (MVIN)
or paraffinic (MVIP) feedstocks. MVIP has to be dewaxed and can also
be solvent refined, acid- and clay-treated or hydro-treated.
High viscosity index (HVI) oils, are prepared by both solvent
refining and dewaxing of paraffinic feedstocks. They are used for
gasoline and diesel engine oils and for turbine lubricants, because
they are, in general, more oxidation-stable than other base oils and
have appropriate viscosity/temperature characteristics.
White oils are generally produced by more drastic refining of MVI
oils, in order to remove unsaturated compounds, aromatic compounds,
and other constituents that influence colour, odour, taste, and
acceptability as food-grade material. Solvent extraction followed by
repeated treatment with oleum and alkali is used. Hydrogenation is
another means of producing such oils.
Medicinal oil is the highest purified grade, which complies with
the requirements of the various national pharmacopoeias and
regulations on food-grade material. As liquid paraffin, the same grade
is used as a lubricant for food-handling machines and as an ingredient
in pharmaceutical preparations and cosmetics.
Technical white oils, less rigidly purified than medicinal oil,
are non-carcinogenic oils that can be used for the lubrication of
textile machinery, spinning mules, etc., but are mainly used in the
cosmetic industry in the manufacture of hair-oils, creams, etc.
Aromatic extracts, which are obtained in the solvent-refining
process, have already been discussed in section 3.1.1.
Petroleum waxes consist essentially of high relative molecular
mass paraffinic hydrocarbons with approximately 20-40 carbon atoms per
molecule. Paraffin waxes consist mainly of normal paraffins together
with some iso and cycloparaffins. They are macrocrystalline with a
melting-point of 43-71°C.
Microcrystalline waxes on the other hand, consist mainly of iso
and cycloparaffins with some alkylated aromatic hydrocarbons. They are
mostly soft materials, but may sometimes be a hard brittle solid.
When highly purified, the colour may be white, however, it is
usually yellow or amber, sometimes even black. The melting-point is
60-90°C. The paraffin oil content ranges from 5 to 50 g/kg.
Petrolatum is also known as petroleum jelly and is a
micro-crystalline wax with a high oil content.
4.1.1.1 Purity of Product
As these oils and waxes are complex mixtures, the nature and
proportion of potentially undesirable "impurities" depends to a large
extent on the definition of the term and on the degree of refining. In
the range from the highest refined oil -- for example, white medicinal
oil -- to the least refined oil, "impurities" could include
polynuclear aromatic hydrocarbons and unsaturated hydrocarbon
compounds.
It is clear that highly purified products can be obtained at the
cost of extra refining and treating processes. Paraffin waxes are an
example of progressive refining: fully refined paraffin wax is a white
solid material containing an oil concentration of less than 5 g/kg. It
is odourless and tasteless and has a melting-point of 50-70°C. Candle
waxes range from fully refined paraffin wax to less refined waxes
containing oil levels of up to 15 g/kg. This type of wax is not
completely colourless and odourless. Scale wax and match wax contain
an oil residue of up to 30 g/kg.
4.1.2 Methods of sampling and analysis
See section 2.1.2.
4.2 Sources of Environmental Pollution
4.2.1 Natural occurrence
Base oils occur in nature only as components of the crude oils
from which they are derived.
4.2.2 Man-made sources
4.2.2.1 Production
As mentioned earlier, lubricating base oils are a group of
petroleum distillates and residues in the boiling-range 300-700°C,
derived by high-vacuum distillation of the residues obtained in crude
oil distillation (section 4.1.1). In order to obtain base oils with
the qualities and specifications needed for various applications,
further refining and treatment of the various distillate fractions is
needed. The main processes used are:
(a) Solvent extraction
This is a process by which aromatic hydrocarbons can be extracted
from oil fractions, thus obtaining a low aromatic or aromatic-free
raffinate, and a high aromatic extract. Liquid sulfur dioxide,
sulfolane, benzene, phenol, or furfural can be used as solvents for
this purpose. The raffinate can be used for producing a lubricating
base oil, the extract can be used as a solvent (see under petroleum
solvents).
(b) Dewaxing process
In this process, "slack wax" is separated (crystallized) from
base oil fractions obtained from paraffinic crude oil residues. This
is done by cooling, followed by filter-pressing. The separation of wax
and oil under chilled conditions is facilitated by the addition of
solvent. The resulting wax may still have an oil content of up to
100-400 g/kg.
(c) Acid treatment
The oil is mixed with 98% sulfuric acid and the acid sludge is
centrifuged off. This is usually followed by clay treatment in which
the oil is mixed with absorbent clay, followed by filtration. This
neutralizes the oil and improves colour, odour, and stability.
(d) Hydrofinishing
This is a catalytic hydrogen treatment at elevated temperature
and pressure. Improved stability and colour of the oil is obtained by
the hydrogenation of unstable compounds, which removes sulfur and
saturated olefins, diolefins, and aromatic components.
As the definition and classification of lubricating base oils are
not clear, it is difficult to give precise production data for this
group of products. The best current estimate is 35-38 million tonnes
per year. Though there was a decrease in the annual production after
1973, lubricant production has subsequently increased, but at a lower
rate.
4.2.2.2 Uses
In general, the main functions of lubricating oils and
metal-working oils are: to reduce friction, to remove generated heat,
to remove debris from the contact area, and to protect against
corrosion. In addition, mineral oils are used as hydraulic media in a
wide variety of applications. Very general remarks have already been
made concerning the composition of, and the various additives in,
lubrication oils, metal-working oils, and greases. The following is a
list of the most important categories of products on the market, from
which it is clear that the great majority are handled in industrial
situations, and that the general population has regular contact with
only a few such products:
(a) Industrial lubricating oils, which can vary from thin spindle-oil
to the very viscous oils used in steam engines;
(b) Lubricants for internal combustion engines of various types;
(c) Crankcase, compressor, gear, and turbine oils;
(d) Greases for bearings and other purposes;
(e) Hydraulic, transformer, insulating, heat-transmission oils;
(f) Cooling, quenching, anticorrosion, and mould oils;
(g) Metal-working oils: cutting, grinding, rolling, drilling,
drawing-oil, a multitude of products for specific purposes. They
consist in general of complex mixtures with various additives,
for use in the neat state and also as water extendible fluids or
emulsions;
(h) Textile oils: spindle, hatching, technical white lubricating oil;
(i) Process oils: used in printing-inks, as rubber extenders, and as
technical white oils in cosmetics, etc;
(j) Waxes of various grades and purity;
(k) Medicinal white oil used for medicinal and food-grade
applications.
The manufacturing, treating, and purification processes used in
the preparation of the base oils vary according to the future
application of the commercial product.
Many of the products can undergo considerable changes during use.
This should be borne in mind in extrapolating from used oils to the
original product in the case of adverse health effects, e.g., in the
case of skin sensitization. The following are a few examples of
changes that may occur:
(a) During use, the chemical and physical characteristics of
lubricating oils change, mainly because of contamination, oxidation,
and polymerization. Metal particles, airborne dust, water, and, in the
case of internal combustion engine oils, small quantities of fuel,
acids, and soot, are the main contaminants. All this can result in the
formation of sludge.
(b) Metal-working oils become contaminated in use with a wide
variety of foreign matter, in particular metal particles (and ions).
Water-based metal-working emulsions can also be affected by bacteria,
yeasts, and fungi. Oxidation and heat cracking may occur at the
application point, causing smoke, steam, and oil mist to be emitted
into the working environment. Nitrite may be added to most water-based
cutting oils, thus these sometimes contain carcinogenic nitrosamines
(Zingmark & Rappe, 1977).
Cutting oils, lubricating oils, and greases can also contain
diethanolamine, which may react with nitrites and nitrogen oxides in
the air to form nitrosamines.
(c) Quenching oils are used for hardening steel. Oxidation and
cracking of the oil can occur because of the high temperatures
involved. The amount of benzo (alpha) pyrene in lubricating oils
increases considerably when they are exposed to heat during use, as in
motor-car engines, metal quenching, and other processes (Thony et al.,
1975).
4.2.2.3 Disposal of waste
Without a proper method for the disposal of used lubricating and
other oils, severe environmental contamination and hazards may occur.
There is a vast literature on methods for the disposal, reuse,
and recovery of industrial lubricants and much research is being done
in this field. It would be out of context in this document to go into
a detailed discussion of the various aspects. For this, the reader is
referred to Concawe Report 9/73 (Concawe Task Force, 1973) and API
publication No. 4036 (API, 1969), which contain discussions on the
situations in Western Europe and the USA, respectively, as well as on
more general aspects.
At present, the main ways of disposal are:
(a) dumping in sewage systems, waterways, etc; this is illegal in
many countries and ecologically undesirable;
(b) dumping into the ground, in garbage dumps, in dry wells, if
approved by local authorities;
(c) using as road oils, etc;
(d) controlled incineration or burning for heat value;
(e) re-refining and other recycling methods.
4.3 Environmental Exposure Levels
Specific data are not available concerning levels of this group
of products in air, water, food, or other environmental media, levels
of possible contamination, and uptake by man.
Depending on occupation and on the hygiene precautions adopted,
skin exposure in workers can vary from minimal to very high. No
systematic measurements have been carried out in this field. Exposure
to oil mists occurs in certain manufacturing processes and
applications, such as the operation of automatic lathes. This type of
exposure has been measured by various authors and will be discussed in
section 4.7.1.2. Secondary oral intake of unknown quantities of
mineral oil can take place under these conditions.
When "sulphofrezol" (approximate composition: 50-60% "goudron"
(?flux oil), 40-50% spindle oil distillate, and 1.7% sulfur) was used
as a lubricating and cooling liquid on metal-working lathes, the air
of the operating premises was found to contain an oily aerosol in
concentrations of 1-50 mg/m3, hydrocarbons at 26- 150 mg/m3, carbon
monoxide at 8-12 mg/m3, formaldehyde at 0.05-1.2 mg/m3, sulfur
dioxide at 2-20 mg/ms, and benzo(alpha)pyrene at 0.01-0.2 mg/m3.
When multicomponent lubricating-cooling liquids containing chlorine
and fluorine compounds are used as additives, hydrogen chloride and
other substances may also find their way into the workroom air. It is
therefore essential to provide the metal-working lathes with
vapour-extracting facilities (Medved' et al., 1976).
4.4 Environmental Distribution and Transformation
Data on lubricating base oils concerned with distribution among
media, environmental transformation and degradation, interaction with
physical, chemical, or biological factors, and bioconcentration are
not available. However, as mentioned in section 3.4, there is a lot of
information concerning the microbial degradation of individual
petroleum hydrocarbons. For example, it has been shown that certain
polynuclear aromatic hydrocarbons in lubricating oils can be oxidized
by bacteria; benzo(alpha)pyrene and benzo(alpha)anthracene have been
shown to be oxidized to cis-dihydrodiols. The oxidation is not thought
to reach the stage of carcinogenic activation found in mammals, as
this would result in a trans-configuration (Gibson et al., 1975).
4.5 Metabolism
Because of the complex and variable composition of compounds in
this group, only a few factual data relating to their metabolism can
be given.
The skin barrier is permeable only to hydrocarbons of a certain
relative molecular mass and structure. For paraffinic substances this
appears to be up to 20 carbon atoms. However, from their studies on
guineapigs, Hoekstra & Phillips (1963) reported that, because aromatic
compounds have a more compact structure, compounds in this group with
a higher number of carbon atoms might still pass through the skin
barrier.
In studies on mice, rats, hamsters, rabbits, and dogs, mineral
oil droplets were phagocytosed in the lungs and clinical observations
in man have shown that deposition takes place in the hilar nodes, from
where they may be transported to other organs such as the spleen
(Sante, 1949; Proudfit et al., 1950; Shoshkes et al., 1950; Wagner et
al., 1964). Almost all (95-99%) of ingested food-grade mineral oil
leaves the body unchanged in the faeces, 1-5% being absorbed as such
via the intestinal mucosa. Phagocytosis may play a part in this. The
ingested part of the oil is transported throughout the body via the
lymphatics and the bloodstream. Storage takes place in adipose tissue
or in the fat in organs. After excessive exposure, mineral oil
droplets have been identified in mesenteric and portal lymph nodes,
and also in liver, spleen, and adipose tissue in man (Stryker, 1941;
Ebert et al., 1966; Boitnott & Margolis, 1966b).
Data relating to food-grade mineral oil have been reviewed in WHO
(1974). Very few data are available on the biotransformation and rate
of elimination of this group of products but data concerning some of
the components are referred to in section 3.5.
Injections of 0.5 ml of various mineral oils were made in the
peritoneal cavity of mice and the oil recovered after 7-24 days (Twort
et al., 1937). Both the refractive index and density of the oil had
decreased proportionally, probably as the result of chemical
transformation in the body. Treatment of mineral oil with sulfuric
acid and clay, or solvent extraction had a similar effect. The solvent
extracts, however, showed increased refractive indices and densities.
The authors suggested that both parameters might be useful indicators
of the carcinogenicity of the mineral oil.
4.6 Effects on Experimental Animals
4.6.1 Short-term exposure
Data are not available concerning the LD50s of base oils but the
oral and dermal LD50 data available for commercial lubricating oils
indicate that, in general, these products are only moderately or
slightly toxic.
Acute no-observed-adverse-effect levels have not been determined.
Oral studies with food-grade mineral oils show that these are
laxatives. The same applies to non-food-grade commercial mineral oils,
though it is impossible to predict whether in a certain case a toxic
or laxative effect will prevail.
4.6.1.1 Effects of dermal exposure
More factual information is available on the acute or short-term
effects of mineral base oils on the skin. In a very thorough study
Hoekstra & Phillips (1963) examined the effects of mineral oils and
their fractions on the skin of guineapigs. It appeared that very
short-chain paraffins had a mainly defatting action on the skin and
that the effects of longer-chain and aromatic hydrocarbons were
closely related to the permeability of the skin to these compounds. We
can do no better than cite the summary of this work:
"A number of light mineral oils applied topically to the skin of
guinea-pigs caused a marked epidermal hypertrophy, hyperplasia,
hyperkeratosis, and subsequent depilation. This dermatoxic effect
could not be closely related to source of the crude oil or its
viscosity, degree of refinement, or the acid used in refinement.
" n-Paraffin, isoparaffin, naphthene and aromatic fractions
separated from light mineral oil each produced the dermatoxic effect
as did highly purified individual paraffins from C-12 to C-18. Oleic
acid caused only a slight dermatoxic effect. Fractional distillation
of an aromatic-free mineral oil demonstrated that while all
lower-boiling fractions were dermatoxic, a distillation range was
reached at which the fractions were innocuous to the skin. This was
also true for n-paraffin-, isoparaffin-, monocyclic naphthene-, and
polycyclic naphthene-rich fractions derived from a mineral oil.
Fractional distillation of the aromatic hydrocarbons from mineral oil
representing the same distillation range did not yield fractions
without skin-damaging effect. Crude estimates of the molecular size of
the n-paraffins, isoparaffins, monocyclic naphthenes, and polycyclic
naphthenes by comparison with the boiling-temperatures of known,
homologous series of hydrocarbons indicated that the maximum skin
damage resulted from hydrocarbons of about 14 to 19 carbon atoms. The
transition point to non-dermatoxic hydrocarbons occurred at about
21-23 carbon atoms. This was verified with purified n-paraffins.
Variations may exist for the different types of hydrocarbons.
"Simultaneous application of the innocuous "higher-boiling"
mineral oil fractions with dermatoxic "lower-boiling" fractions or
with hexadecane greatly reduced or eliminated the skin-damaging
effects. The alleviation of the skin damage from aromatic fractions by
simultaneous application of the "higher-boiling", non-aromatic
fractions was much less pronounced.
"The hyperkeratotic reaction of the skin to petroleum
hydrocarbons appears to he a very general response to lipid solvents
and is not related to any specific reactive group or type of
structure. Molecular size seems to be very important in determining
the dermatoxic properties of hydrocarbons, the larger molecules being
innocuous. Under the conditions of the experiments the very
low-molecular weight hydrocarbons also had little or no dermatoxic
properties, presumably because of their volatility. It is postulated
that the skin barrier is permeable only to hydrocarbons of a certain
maximal effective size and that penetration into or through the
barrier is essential for the initiation of the hyperkeratotic
response. The alleviation of the dermatoxic effect of hydrocarbons by
admixture with innocuous, higher molecular weight hydrocarbons, is
likewise explained by a reduction in penetration of the dermatoxic
component through the Skin barrier" (Hoekstra & Phillips, 1963)
4.6.2 Long-term exposure
Most long-term studies with mineral oils have been concerned with
the carcinogenicity of the compounds. Data concerning mutagenicity,
embryotoxicity, and teratogenicity are lacking.
4.6.2.1 Carcinogenic effects
The carcinogenic activity of certain polynuclear aromatic
hydrocarbons is attributed to electronic structural features of the
molecule. The various theories on these mechanisms are discussed in
detail by Arcos & Argus (1974), Bergel (1974), and Jerina & Daly
(1974). Carcinogenic polynuclear aromatic hydrocarbons have regions of
high-electron density in their molecules, the so-called K-regions that
are readily epoxidized by tissue mixed-function oxidases and more
specifically by the enzyme aryl hydrocarbon hydroxylase.
Polynuclear aromatic hydrocarbons are in fact precarcinogens,
which, though harmless in themselves, are metabolized in the body, by
the combined action of mixed function oxidase and epoxide hydratase
(4.2.1.63), to biologically reactive intermediates that constitute the
ultimate carcinogens (Sims et al., 1974). The enzymes responsible are
active in the liver, and in other organs such as the skin, lungs, and
intestines.
Whether, in fact this metabolism leads to detoxication or to
"lethal synthesis" may differ with various polynuclear aromatic
hydrocarbons and may depend on many other factors (Anon., 1975). The
short-lived epoxides resulting from the metabolism of at least some of
the polynuclear aromatic hydrocarbons are probably the activated
metabolites with the real carcinogenic effects.
Evidence is now accumulating that it is the 7,8 dihydroxy-
benzpyrene - 9,10 oxide of 3,4 benzo(alpha)pyrene that is the
ultimate carcinogen and, consequently, this has now superseded the
K-region epoxide in biological importance with regard to
carcinogenicity (Yang et al., 1976; Thakker et al., 1977; Yagi et al.,
1977; Koreeda et al., 1978). The enzyme aryl hydrocarbon monooxygenase
(according to recent international rules of nomenclature) in the case
of 3,4 benzo(alpha)pyrene, is referred to as benzopyrene
3-monooxy-genase (EC 1.14.14.2) (de Pierre & Ernster, 1978).
There are indications that the tissue concentrations of the
enzyme aryl hydrocarbon hydroxylase vary considerably with the age,
sex, species, strain, and environment of the animal (Nebert & Gelboin,
1969). Outside agents, such as polynuclear aromatic hydrocarbons, can
induce the activity of this enzyme (Wattenberg, 1972). It seems that
the inducibility depends on genetic factors in both mice and human
subjects (Nebert et al., 1972; Kellerman et al., 1973). In mice, it
seems to depend on a single dominant autosomal gene (Nebert et al,
1972).
4.6.2.2 Effects of dermal exposure and subcutaneous
administration
Leitch (1922) repeatedly painted crude shale oil on the skin of
mice. He obtained skin tumours in a substantial percentage of the
mice. Similar tests were carried out by Twort & Ing (1928) and Twort &
Twort (1931) using a range of crude oil as well as more refined
products of both shale oil and petroleum oil. They found that
petroleum oils had a lower and sometimes negligible carcinogenic
activity compared with shale oils. Naphthenic oils were less active
than oils with a high aromatic content. The more potent fractions
could be extracted with solvents from the oils.
Acid treatment of the oils decreased the carcinogenic activity
and heavier-grade oils were less potent than spindle oils.
Oils with a boiling-point above 370°C, derived from fluid
catalytic cracking, appeared to be carcinogenic to the skin of mice.
The most potent fraction distilled between 430 and 550°C, and the
carcinogenic activity was contained in the aromatic components of
these oils.
Woodhouse (1934) compared the carcinogenicity of sulfur-
dioxide-solvent-extracts of spindle distillates, derived from
crude oils from different parts of the world, with that of the
unrefined spindle oils and coal-tar, using a mouse-skin-painting
technique. He found that in all cases, the carcinogenic activity,
which was more or less characterized by its UV-fluorescence spectrum,
was concentrated in the solvent extract. Potency varied according to
the source of the crude oil. The potency of the most active extract,
however, was lower than the activity of coal-tar. By thorough
sulfur-dioxide-solvent refining, the carcinogenicity of the spindle
oil was almost completely removed. The same author (Woodhouse, 1950)
reported studies on the carcinogenic activity of various petroleum
fractions and extracts. Results were in line with his previous
findings and with those of other workers. Three white oils including
medicinal liquid paraffin gave negative results.
More recent studies have been aimed at identifying the
carcinogenic substances.
Dilution of carcinogenic oils with non-carcinogenic oils more
than proportionally reduced the carcinogenic activity, which at times
disappeared completely. Mild hydrogenation or treatment with active
absorbents reduced, but did not abolish carcinogenicity. Mild acid
treatment, on the other hand, did not have such an effect (Smith et
al., 1951). Eight samples of unrefined "slack waxes" obtained from
pressing operations -- still containing 12-29% of oil -- were painted
3 times a week on the skin of white mice (Smith et al., 1951).
Aromatic extracts, obtained by the solvent refining of these waxes,
were examined in the same way. After skin-painting for almost the life
span of the animals, some benign rumours were found with all samples
and in a few cases, skin carcinomas developed. The aromatic extracts,
however, were much more active. The authors concluded that the
aromatic components which were removed by further refining of the
waxes, were the cause of the carcinogenic activity of "slack waxes".
Fischer et al. (1951) described methods for the determination of
polynuclear aromatic hydrocarbons: the ultraviolet absorption method,
the caffein extraction method, the chromatographic ref-ractometric
method, and the maleic anhydride method. Levels, determined by any of
these methods, were fairly well correlated with the tumorigenic
potency of these oils as established in animal experiments. These
analytical techniques are relatively simple and much more rapid than
long-term animal experiments. They are considered to have some
predictive value. A further development is the DMSO
extraction/refractive index method.
The correlation between the analytical data and the carcinogenic
activity in skin-painting studies on mice is now under investigation
(Shell Toxicology Laboratory -- private communication) and encouraging
results have been obtained with the lower- and higher- range
polynuclear hydrocarbons. The compounds in the middle range are still
under investigation.
Auld (1940) considered that it was not the general
UV-fluorescence that was characteristic of carcinogenicity but the
pattern of the spectrum and its classification. However, in a report
by the Analytical Subcommittee of the Institute of Petroleum
(Catchpole et al., 1971a), it was concluded that, at the time of the
report, there was no suitable, easy and quick method for determining
either the total polynuclear aromatic hydrocarbons or individual
carcinogens in them.
The tumorigenic activity of various concentrations of a large
number of pure aromatic hydrocarbons was examined in different strains
and species of animals by Hartwell (1951), who found that the potent
substances contained 4,5, or 6 condensed aromatic rings with
relative-molecular masses ranging from 230 to 320.
Cook et al. (1958) tried to isolate individual carcinogens from 3
Kuwait crude oils by fractional distillation and various treatment
processes. They tested the fractions obtained on the skin of mice and
rabbits and found that more of the carcinogenic activity was contained
in the fraction distilling between 350 and 400°C and that this
activity was associated with the aromatic constituents. Solvent
extraction with aqueous acetone or furfural removed the activity,
which passed into the extract (20% of the original fraction). Several
polynuclear aromatic hydrocarbons such as di-, tri-, and tetramethyl-
phenanthrenes, tetramethylfluorene, 1-methylpyrene, 1,2-benzofluorene
and 8-methyl-1,2-benzofluorene could be identified in these extracts
as well as some complex organic sulfur compounds such as
dibenzothiophen derivatives and polycyclic thiophens, a number of
which are known to have carcinogenic activity. Pentamethyl-carbazole
was identified as an example of a complex organic nitrogen compound.
Similar findings were obtained by Bogovsky et al. (1960) on
fractionating Estonian shale oil.
Approximately 100 fractions were separated from a catalytically
cracked oil by various techniques (Tye et al., 1966). Selected
fractions were chemically analysed and assayed for carcinogenicity by
repeated application to the skin of C3H mice. In the highest-boiling
fractions (438-455°C), the major carcinogens were 4 and 5 ringed
polynuclear aromatic hydrocarbons, benzo(alpha)pyrene as a
characteristic carcinogen was present at a concentration of 0.4%. In
the intermediate boiling range (404-438°C), unsubstituted and
methyl-substituted 4-ringed polynuclear aromatic hydrocarbons formed
the major carcinogens, benz (a)anthracene and its alkyl homologues
being present at a concentration of 0.4%. In the low-boiling fraction
(339-404°C), assorted smaller molecules including alkyl-3-ringed
polynuclear aromatic hydrocarbons were present.
Benzo (c)phenanthrenes, the most dominant carcinogens in this
fraction, were present at a concentration of 0.01%. The carcinogenic
potency in the bioassay was found in the intermediate- and
high-boiling fraction (boiling-range 404-455°C); fractions boiling at
under 349°C did not appear to be carcinogenic.
Generally, where the alkyl-substituted 4-6 condensed-ring
polynuclear aromatic hydrocarbons were concerned, the longer the
side-chain, the lower the carcinogenic activity (Auld, 1950).
These findings were confirmed by the United Kingdom Medical
Research Council (1968), who reported similar studies. Uncracked crude
oil fractions boiling at above 350°C, which had the highest
carcinogenic activity in skin tests on mice and rabbits, contained the
same or similar polynuclear aromatic hydrocarbons; no other new
carcinogens were found. After solvent extraction, the carcinogenic
activity of the extract was much higher than that of the raffinate,
which was very low.
The carcinogenicity of individual polynuclear aromatic
hydrocarbons will not be discussed in this document. For this, the
reader should refer to IARC (1973).
The fact that various cracking processes considerably increase
the contents of polynuclear aromatic hydrocarbons in mineral oils has
been demonstrated by Kennaway (1925), Twort & Fulton (1930), Twort &
Twort (1935), Pates (1952), and Dietz et al. (1952). Kennaway (1925)
discovered that skin cancer of mice could be produced with synthetic
tars obtained by pyrolising substances like acetylene, isoprene,
yeast, human skin, and a non-carcinogenic petroleum. Badger (1962)
pyrolysed a whole range of aliphatic and simple aromatic hydrocarbons
at 700°C. It was concluded from an analysis of the resulting tars
that, at high temperatures, polycyclic aromatic hydrocarbons were
formed from simpler hydrocarbons via primary radicals formed by
carbon-hydrogen and carbon-carbon fission. The same process occurred
at 550°C, but to a much lesser extent.
Skin tests on C3H-mice using 15 base oils with known content of
polynuclear aromatic hydrocarbons, which, in some cases, only differed
in theft refining history, revealed that solvent-refining removed the
carcinogenic components to such an extent that none of the oils
treated in this manner induced carcinomas (Bingham et al., 1965).
Solvent extraction was carried out with the usual solvents including
phenol, cresol, and furfural. Conventional acid and clay treatment
only removed certain of the polynuclear aromatic hydrocarbons and all
oils treated in this way still retained some carcinogenic activity. In
these tests, a general correlation was again found between the content
of 4 and 5 ring polynuclear aromatic hydrocarbons and the
carcinogenicity of the base oils examined.
Catchpole et al. (1971b) compared mass spectrometry analysis of
an untreated distillate with its solvent-treated and its hydrogenated
derivatives. The decrease in polynuclear aromatic hydrocarbons
thiophenes, and sulfur in both treated samples compared with the
untreated sample was quite marked. Smaller differences were found
between the levels in the two refined samples.
In studies by Boehme & Huehnermann (1966), the relative amounts
of aromatic compounds present in some of the more purified mineral oil
products were determined from their UV absorption spectra. The
relative concentrations were:
medicinal white oil 1
paraffin waxes 100
micro crystalline waxes 300
white petrolatum 1000
yellow petrolatum 10 000
The carcinogenicity of a sample of amber petrolatum was tested on
the skin of mice. A 15% solution of the petrolatum in iso-octane, did
not show any significant carcinogenicity. The solvent-extracted
aromatic fraction (1.2% of the original material) tested in iso-octane
solution at 50 times its concentration in the petrolatum did not
produce any carcinogenic effects on mouse skin (Lijinski et al.,
1966). Studies are reported by Oser et al. (1965) in which 3 kinds of
pharmaceutical and food-grade petrolatum were administered to mice in
the form of a single subcutaneous injection of 100 mg of petrolatum
per mouse. Observations over 18 months did not reveal any carcinogenic
or other toxic effects.
Liquid paraffin was found to be non-carcinogenic in long-term skin
testing on mice by Twort & Twort (1931) and Woodhouse (1934). White
mineral oil was also found to be non-carcinogenic in mice after
long-term skin application (WHO, 1974).
Shubik et al. (1962) examined 36 samples of petroleum wax. Eight
samples contained identifiable polynuclear aromatic hydrocarbons; the
highest concentration was 0.64 mg/kg. The results of skin and
subcutaneous testing follow in the author's words:
"Five petroleum waxes were tested by repeated skin application in
benzene solution to mice and rabbits. In addition one of the test
waxes was fractionated and its aromatic and non-aromatic components
were tested separately on mice, also by repeated skin applications.
Solvent-treated controls were kept. No carcinogenic effects were
detected. Five petroleum waxes were tested in mice by subcutaneous
implantation in disc form. Fibrosarcomas developed around the implants
with incidences correlated to the melting points of the waxes. In
addition, one of the test waxes was fractionated and its aromatic and
non-aromatic components tested separately; the same wax was also
tested subcutaneously in powdered form. The findings indicate that the
subcutaneous sarcomas occurred as a result of the physical rather than
the chemical properties of the materials."
From the various studies, it would seen that there is no relation
between the carcinogenicity of a lubricating base oil and its
potential to cause dermatitis.
In all the previously mentioned studies and others by Horton et
al. (1963) and Bingham & Horton (1966), there is general agreement
that the carcinogenicity of a mineral oil is largely related to the
aromatic fraction with a boiling-point above 370°C, and more
particularly to the polynuclear aromatic hydrocarbons containing 4-6
condensed benzene rings. However, some long-chain, aliphatic,
alicyclic, and alkyl-aromatic hydrocarbons from a lower boiling-range
with 10-20 carbon atoms, such as n-dodecane, cyclohexyl-decane, and
dodecyl-benzene may act as accelerators or co-carcinogens. Though
completely non-carcinogenic themselves, they were found to increase
tumour incidence and reduce the time of appearance, when carcinogenic
fractions dissolved in them were applied, to the skin of mice (Horton
et al., 1963; Bingham & Horton, 1966). However, in order to act as a
cocarcinogen, n-dodecane must be present in a concentration of
20-30% or more. Such solutions in accelerating solvents show an
unusual capacity to spread upon the skin (Horton et al., 1957, 1965).
In a further study on the effects of co-carcinogenic compounds on
the carcinogenic action of benzo(alpha)pyrene and benz (a)anthracene
on the skin of mice (Bingham & Falk, 1969), the co-carcinogenicity of
n-dodecane was confirmed. However, when the authors claim "that
there is a 1000-fold increase in the enhancement of potency of low
concentrations of benzo(alpha)pyrene and benz (a)anthracene when
dodecane is the diluent", this statement may not be fully justified by
the results obtained in relatively small numbers of animals per test
group. Nevertheless 50% n-dodecane in the solvent decaline doubled
or trebled the tumour incidence in the highest dose-group, and tumours
were found at much higher dilutions than when decaline alone was used
as a solvent. When 2-dodecanol and 2-phenyldodecane were used in
various concentrations in the solvent of benzo(alpha)pyrene, higher
concentrations of these 2 substances shortened the interval of
appearance of tumours rather than increasing the total incidence of
rumours.
From this it would appear that these accelerators would not have
any effect, when present in an intrinsically non-carcinogenic oil, and
that they would only have an effect in other oils, when present in
appreciable concentrations. This effect seems to be due, at least in
part, to the spreading power of the solvent causing more intimate
contact with a greater skin area. On the other hand, a more plausible
explanation could be an increase in P-450 enzyme induced by dodecane,
which possibly does not occur with 2-dodecanol and 2-phenyldodecane.
3,4 Benzo(alpha)pyrene can induce P-450 microsomal mixed function
oxidase and this can also catalyse arylhydrocarbon (AH) monooxygenase
activity (de Pierre & Ernster, 1978). The increase in P-450 induced by
dodecane will accelerate and increase the formation of the ultimate
carcinogen 3,4 benzo(alpha)pyrene and thus increase the total
incidence of tumours. However, these substances will not normally be
present in base oils and related products, because they fall into a
different boiling range.
Apart from this, some frequently used additives - which in
themselves are non-carcinogenic - such as elemental sulfur and some
sulfur-containing compounds such as benzyldisulfide, ditertiary
butylpolysulfide and ditertiary octylpolysulfide (Horton et al., 1965;
Bingham et al., 1965; Bingham & Horton 1966) as well as certain
phenols (Boutwell & Bosch 1959) have also been shown to enhance the
carcinogenic activity of polynuclear aromatic hydrocarbons on the
mouse skin. Addition of sulfur to a non-carcinogenic oil did not, of
course, have any effect on the carcinogenicity (Bingham et al., 1965).
Baldwin et al. (1964) reported that additives such as lead naphthenate
did not have an enhancing effect and there are indications that other
components of complex mineral oils with a boiling-range above 370°C
act as inhibitors of the carcinogenic effect of polynuclear aromatic
hydrocarbons, e.g., the saturated (probably cyclic) hydrocarbons
(Bingham & Horton, 1966).
Sunderland et al. (1951) reported skin tests of mineral oil
fractions on mice, under various conditions. They found that washing
the skin with soap and water, after the application of the oil,
reduced both the number of tumours and the rate of appearance. The
reduction was related to the length of the interval between oil
application and washing. Painting once, instead of twice, weekly
greatly reduced the rate of tumour appearance.
From human experience (section 4.7.1.1), it is known that the
ultraviolet radiation of sunshine has a potentiating effect on the
carcinogenicity of mineral oils. Similar indications exist from animal
studies (Emmett, 1975). It has also been demonstrated that certain
co-carcinogenic n-alkanes may increase the carcinogenic potential of
certain wavelengths of UV light on mouse skin (Bingham & Word, 1977).
In comparative studies with various mineral oil fractions on the
skin of mice and rabbits, it became apparent that the rabbit skin was
more sensitive than the mouse skin according to the type, number, and
growth rate of tumours (Cruickshank & Squire 1950; Hieger & Woodhouse
1952; Antonov & Lints 1960; UK Medical Research Council, 1968). In
some instances, mineral oil fractions were non-carcinogenic to mouse
skin, but positive on the skin of rabbits (Shubik & Saffiotti), 1954).
While, rats and guineapigs are apparently less sensitive than mice
(Sugiura et al., 1949; Desoille et al., 1973) Rhesus monkeys appear to
be fairly sensitive (Sugiura et al., 1949).
As to the suspected causes of intra- and inter- species
differences in susceptibility to polynuclear aromatic hydrocarbons,
the levels of the enzymes forming and detoxifying the biological
reactive intermediates, the ultimate carcinogens, in the tissue of the
various strains and species might be one of the important factors
(section 4.6.2.1). Clear intra-species differences in tumorigenic
response have been reported by, for example, Smith & Sunderland (1951)
and Gilman & Vesselinovitch (1955).
In addition to these studies on "pure" mineral oil fractions and
various additives, some studies with metal working oils and textile
oils have been published.
Two cutting oils based on sulfurized mineral oils, were diluted
with water and were tested in various concentrations on the skin of 3
strains of mice. Both were found to be carcinogenic. A marked
reduction in incidence and an increase in induction time of tumours
was found at a dilution of 1:8 compared with 1:4 (Gilman &
Vesselinovitch, 1955). The same authors (Gilman & Vesselinovitch,
1956) compared a straight cutting oil and a water-soluble cutting
fluid in 2 different strains of mice. They found a consistent,
comparable but low incidence of skin tumours in 3 separate trials.
In a later study, 3 commercial additive-containing cutting oils,
one of which was an emulsifiable oil, were repeatedly applied to mouse
skin for up to 31 weeks. Carcinogenic skin changes were observed with
all these oils but were, possibly, less marked with the emulsifiable
oil. In addition to these carcinogenic effects, focal necrosis of the
liver associated with amyloid deposition and amyloidosis of the skin,
spleen, and kidneys were observed. The additives may have had a
contributory effect in some of the pathological changes observed
(Jepson et al., 1977).
In the case of cutting oils, the temperatures to which the oils
are exposed at the cutting edges of the tools are such that cracking
of the oil might conceivably occur and theoretically a
non-carcinogenic cutting oil might become carcinogenic during use. An
unspecified cutting oil from the sump of a machine was tested on the
skin of mice and rabbits. It induced benign tumours in rabbits only
(Cruickshank & Squire, 1950). As the fresh oil was not examined, no
conclusion can be drawn concerning the previously-mentioned
assumption.
Unused cutting oil, used cutting oil, and residue from the sump
were compared in skin tests on 2 strains of mice by Dargent et al.
(1967). Both used and unused cutting oil caused a high incidence of
skin ulceration. Hyperkeratosis and papillomas were more frequent in
the case of used oils and a single case of skin cancer occurred in the
groups of mice treated with used oil and sump residue.
Cutting and quenching oils, fresh and used, were tested on the
skin of rats, guineapigs, and mice. All tests on rats and guineapigs
were negative. In mice, skin tumours were more numerous and more
malignant with used oils than with fresh oil. Only used oils produced
tumours in organs distant from the site of application. The unused
quenching oil was found to contain a benzo(alpha)pyrene level of
0.6-0.8 mg/kg (ppm); in the used oil, this fraction had increased to
20 mg/kg, which is in agreement with the finding of its increased
carcinogenicity. The increase in carcinogenic poly-nuclear aromatic
hydrocarbons may be the result of thermal cracking due to the heat of
the process (Desoille et al., 1973). The studies of Thony et al.
(1975) indicated that the increase in poly-nuclear aromatic
hydrocarbons in cutting oils was very small and considerably less than
for quenching oils and engine oils. The benzo(alpha)pyrene content of
new cutting oils ranged from 0 to 150 mg/kg, compared with 0 to
250 mg/kg for used cutting oils.
A sample of commercial jute batching oil of unspecified origin and
composition, containing a concentration of 3,4-benzopyrene of less
than 1 mg/kg, was tested for carcinogenic activity on the skin of
mice. The oil in question induced malignant skin tumours in 6 out of
24 test animals and proved to be a potent tumour promotor in mice
pre-treated with 7,12-dimethylbenz (a)anthracene (DMBA) (Roe et al.,
1967).
To summarize, it may be concluded that: (a) the carcinogenic
activity of mineral oils seems to be related mainly to the presence
and concentration of certain polynuclear aromatic hydrocarbons,
containing 4,5, or 6 condensed rings; and (b) cracking processes
tend to increase the polynuclear aromatic hydrocarbon contents in
petroleum products. For example such compounds may accumulate in a
metal working oil in contact with the host cutting edge of a tool.
The polynuclear aromatic hydrocarbon content of mineral oils can
be decreased by solvent extraction and/or by hydrogenation. Acid
treatment is less effective for this purpose.
Analytical methods for the determination of total polynuclear
aromatic hydrocarbons should provide a useful, quick, and cheap tool
for predictive screening of mineral oils for carcinogenicity. These
methods should be biologically validated. The correlation will not
always be good, because the carcinogenicity of polynuclear aromatic
hydrocarbons varies considerably and because of the unpredictable
effects of cocarcinogens, inhibitors, and accelerators.
Various substances that are used as additives such as sulfur
compounds, as well as substances that may normally be present in
mineral oils, such as n-dodecane, may act as cocarcinogens. Other
saturated hydrocarbons, normally present, may act as inhibitors of the
same effect.
It is clear from animal studies that washing with soap and water
substantially reduces the hazard to the skin of repeated and long
contact with potentially carcinogenic oils.
4.6.2.3 Effects of inhalation and intratracheal exposures
The aspiration hazard and toxicity for animals of a number of
hydrocarbons and hydrocarbon mixtures was determined by Gerarde
(1963). This study has been discussed in more detail in section 3.6.1.
He found that the aspiration hazard decreased with increased viscosity
of the product and with mixtures containing low viscosity products.
Mice were exposed to mists of various mineral and vegetable oils
with an average droplet size of 2.5 µm, 80% of the particles retained
in all areas of the lungs were 2.5 µm or less in diameter. The highest
concentration of retained particles was found around the terminal
bronchioles and alveolar ducts in all parts of both lungs. Oil
particles were immediately phagocytosed, a process that was
essentially completed within 48 h, unless prolonged exposures (2-4
weeks) had been given. The initial concentration of retained oil
droplets was similar for all types of oil mists. During a 90-h
follow-up, however, the concentration of vegetable oil droplets
decreased progressively, whereas the concentration of mineral oil
droplets remained practically unchanged. After 2-4 weeks of exposure,
mineral oil droplet retention gave rise to localized slight
foreign-body reactions as well as to a few patches of lipoid
pneumonia. Of the other oils, only cod liver oil caused a moderate
foreign-body reaction (Shoshkes et al., 1950).
The effects of prolonged inhalation of oil mists (mineral oil
levels in air of 63-132 mg/m3) were observed in mice, rats, rabbits,
and monkeys. Ordinary automobile lubricating oil and a smoke-screening
oil, were tested for periods varying from 100 to 365 consecutive days
(Lushbaugh et al., 1950) The authors found that surprisingly little
oil accumulated in the lungs and that whatever was retained was
rapidly phagocytosed and transferred to the pulmonary connective
tissue and the hilar lymph nodes. Lipoid pneumonia was not found to be
a hazard at these dosages, though the incidence of infectious
pneumonia in the exposed monkeys greatly increased. Many exposed
monkeys died of a hyperplastic gastritis, probably because much of the
inhaled oil was initially deposited in the nasal passages and
subsequently swallowed. Hyper-plastic gastritis was especially evident
with the shale oil (which is different from petroleum oil) used as
smoke screening oil (Lushbaugh, 1947). No significant increase in
tumour incidence or reduction in the latent period of tumour
production was found in the mouse study.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Rats and guineapigs were exposed for 6 h/day, 4 days a
week, for up to 2 years, to a cutting oil mist consisting of a 10:1
mixture of neutral paraffin and prime lard oil. In some cases, this
caused multi-focal adenomatosis in the lungs of the animals. A
carcinoma of the lung was found in only 1 of 105 exposed rats and not
in any of 65 guineapigs.
In tests on mice, Wagner et al. (1961) found that inhalation of
either mineral or motor oil mist reduced the acute lethal effects of
respired oxidants such as ozone and nitrogen dioxide. The effect was
demonstrable only after a latent period of up to 8-9 days following
oil mist exposure and was thought to result from the formation of a
thin film of oil on the alveolar surfaces.
Long-term inhalation toxicity studies using a highly purified
white mineral oil, composed of naphthene-based saturated hydrocarbons,
were reported by Wagner et al. (1964). Five species of laboratory
animals (dog, rabbit, rat, hamster, mouse) were exposed daily, for
periods from one year to 26 months, to a petroleum-base mineral oil
mist at concentrations of 5 mg/m3, the current USA threshold limit
value, and 100 mg/m3. Histological evaluation of tissues of the dogs
and rats exposed to 100 mg/m3 showed significant pulmonary alveolar
and hilar lymph node oil deposition and/or lipid granuloma formation
after 12 months of exposure. In addition, these animals showed
significant increases in the activities of basic and
magnesium-activated phosphatases.
Body-weight gain, haematological variables, and respiratory
function values did not deviate significantly from the control data at
any of the exposure levels. Studies with a spontaneous
pulmonary-tumour-susceptible strain of mouse presented equivocal
evidence of an increased rate of tumour formation at the 100 mg/m3
concentration. These findings suggest that prolonged exposure to a
mineral oil mist concentration of 5 mg/m3 would not present any toxic
hazard. It would appear, however, that protracted exposure at
approximately 100 mg/m3 would, in time, produce harmful physiological
effects.
The toxicity of aerosols of various petroleum oils (industrial,
transformer, and compressor distillates) differed only slightly in a
long-term experiment by Lutov (1974). White rats were exposed to
concentrations of 13, 30, and 60 mg/m3 for 5 h/day, for 6 months.
Changes in the electrocardiogram, and reductions in arterial pressure,
respiratory frequency, and immunological reactivity were seen even at
12-13 mg/m3, which was considered to be just above the threshold
level. A concentration of 5 mg/m3 is recommended as a maximum
permissible concentration for petroleum oil aerosols without
additives.
In a further long-term study on white rats, Lutov et al. (1976)
studied the toxicity of petroleum oil aerosols in concentrations
ranging from 11.4 ± 1.5 to 61.4 ± 5.2 mg/m3 in combination with
products obtained by thermo-decomposition, i.e., hydrocarbons in
concentrations ranging from 200.0 ± 5.8 to 410.0 ± 12.8 mg/m3 and
carbon monoxide in concentrations ranging from 11.2 ± 1.5 to
35.4 ± 4.6 mg/m3. White female rats were exposed for, 5 h/day over a
6-month period. The combined exposure was found to produce more marked
effects on the parameters tested in the earlier experiment.
The minimum concentrations of oil aerosol that caused functional
changes in the respiratory system with a single inhalation were in the
range of 860-1200 mg/m3. When concentrations of 53 and 60 mg/m3 were
used in the long-term experiment, physical growth was impaired and the
functional condition of the nervous system and the liver changed.
There were morphological changes in the lungs including a
catarrhal-desquamative bronchitis, a swelling of the alveolar
membranes, formation of oleogranules, and the presence of a large
quantity of oleophages and oil droplets in the lymphatic vessels and
the peribronchial and bronchopulmonary lymphatic nodules. At
concentrations of 10-17 mg/m3, the changes were slight and
reversible. A maximum admissible concentration in the air of a work
place of 5 mg/m3 is the official USSR exposure limit (Ivanov et al.,
1978).
In tests on rabbits, Laughlen (1925) demonstrated that mineral oil
given by mouth or nose can enter the trachea. Corper & Freed (1922),
injected olive oil and medicinal mineral oil intratracheally into
rabbits in amounts of 0.5-1.0 ml. The oil was readily aspirated into
the finest pulmonary divisions and into the alveoli. It was retained
for months and caused a mild proliferative reaction typical of a
foreign body reaction. In studies by Pinkerton (1928), rabbits and
puppies were injected intratracheally with animal, vegetable, and
medicinal oil. Complete removal of the oil from the lungs took several
months in all cases. Neutral vegetable oil produced practically no
reaction, animal oils caused giant cell formation and rapid and marked
pulmonary fibrosis. Mineral oil was rapidly phagocytosed, giant cell
formation and slight fibrosis were apparent after 2-3 months, and
storage of the oil took place in the bronchial lymph nodes.
4.6.2.4 Dietary studies
Long-term oral studies have not been conducted with lubricating
base oils.
Lushbaugh & Hackett (1948/1949) carried out a study in which rats
received an average of 0.2 ml/rat per day of a highly refined
diesel-engine lubricating oil, used as smoke screening oil, in theft
diet for a period of 14 months. In this group of 40 rats, 2 developed
foci of colonic mucosal hyperplasia and one developed colonic
adeno-carcinoma.
Results of various dietary studies using food-grade and
pharma-ceutical-grade materials, such as medicinal and food-grade
mineral oil, and food-grade paraffin waxes are included in the
following summary, though these studies with highly purified material
are not representative for the less purified grades.
Schmahl & Reiter (1933) fed 2% (w/v) mineral oil in the diet of
rats for 500 days without adverse effects. In studies by Daniel et al.
(1953), rats were kept for 15 months on diets supplemented with 10%
liquid paraffin, without adverse effects. Five kinds of petroleum
waxes were tested by feeding rats a diet containing 10% of the wax for
2 years. The rats were then observed until natural death. No toxic or
carcinogenic effects were found (Shubik et al., 1962). In the studies
by Oser et al. (1965), already reported in section 4.6.2.2, 3 kinds of
petrolatum were also administered to rats in the diet at 50 mg/kg
diet. Observations over 2 years did not reveal any carcinogenic or
other toxic effects.
4.7 Effects on Man
The general population does not have any contact with base oils;
contact with the commercial products derived from them will, at most,
be occasional and of a lower order of magnitude than occupational
exposure. Cases of accidental ingestion are exceptions.
However, pharmaceutical grade liquid paraffin in the form of
medicinal oil is widely used as a laxative (faecal softener).
Occupational exposure to base oils is restricted to the small
group of workers who manufacture them in oil refineries and to workers
in blending units, who mix and blend them with additives in order to
produce commercial products such as lubricating oils, greases,
metal-working oils, and textile oils. These activities are normally
carried out in closed systems in which case occupational contact is
minimal and discontinuous, usually accidental. This also applies, when
the finished product is put into containers.
4.7.1 Occupational exposure
Epidemiological and clinical data concerning base oils are almost
non-existent. Thus, in this section, it is only possible to analyse
epidemiological studies of workers exposed to products containing base
oils, such as lubricating oils, metal-working oils, and textile oils.
From the list of lubricating oil products given in section 4.2.2,
it is clear that various groups of workers are occupationally exposed
to these products, the most widely-used being the metal-working oils
and textile oils.
The magnitude of occupational exposure varies considerably. In the
case of some lubricants and transformer oils, handling is only
occasional, and, even then, exposure is minimal. In other situations,
such as in the earlier years of mule spinning and in work with
automatic lathes of old design, extremely high exposure did - and
sometimes still does occur. Not only is there continuous direct
contamination of clothes and exposed parts of the skin, but the rapid
movement of the machinery may turn the oil into an aerosol and thus
generate an oil mist that can be inhaled and further contaminate skin
and clothing. Equipment and floors may become covered with an oil-film
and situations have been described where oil even dripped from the
roof.
A certain degree of oil mist generation may also occur in the
printing and rubber industries and with pneumatic equipment (e.g.,
drills), especially under conditions of limited ventilation such as
are found underground.
Even under the best technical conditions, it may sometimes be
difficult to completely avoid contamination. For example, in the case
of toolsetters, the wearing of protective clothing impedes easy
movement, and clothing, including underwear, may become soaked with
oil.
Henry (1946/1947) gave an excellent insight into the types and
magnitude of occupational exposure up to the time of the report. It is
known that some of these conditions had continued for a period of
about 20 years. However, in many countries, great improvements have
been made since then. An understanding of the range of such exposures
is needed to put epidemiological studies of later years into
perspective, especially in view of the long latency of carcinogenic
effects. In the evaluation of such studies, it has to be kept in mind
that today's findings may reflect the industrial hygiene situation of
20-30 years ago.
4.7.1.1 Skin disorders
A great variety of skin disorders attributed to working with
mineral oil or with products based on mineral oils, such as metal
working fluids, are mentioned in the medical literature. This is not
surprising considering the many factors that influence theft
development (van Raalte, 1963; Key et al., 1966; Kipling, 1968;
Hodgson, 1970, 1973).
The main factors include: (a) the degree of intrinsic potential
of a mineral oil product to damage the skin; (b) the integrity of
the skin; (c) the degree and continuity of contact between oil and
skin; and (d) individual predisposition.
The situation, however, is more complex than this, for, in actual
practice, a multitude of other factors may influence the main
conditions to such an extent and in such a complex way, that a
cause-and-effect relationship may become obscured. Most authors agree
that practically all skin disorders attributed to exposure to mineral
oil products can be prevented entirely by adequate industrial and
personal hygiene practices, and that the majority of cases have
occurred in workshops where inadequate conditions prevailed.
Some of the more important factors in the complex interplay are:
(a) Factors related to the mineral oil product used:
(i) Base oil:
the lower the boiling-point of the oil, the more
pronounced the solvent action of the product; this causes
defatting of the skin and leads to dryness, chapping,
scaling, and cracking;
the higher the boiling-point, the more blocking of skin
pores occurs, giving rise to acne formation;
some mineral oils are more irritant than others; the
lower-boiling fractions are sometimes, but not always,
more irritant than higher-boiling fractions (section
4.6.1);
mineral oils themselves do not appear to be sensitizers.
(ii) Additives:
these may be primary irritants or sensitizers in theft own
right (e.g., chromium salts), though manufacturers try to
avoid the use of such additives;
solvents and detergents increase the defatting effect of
the lower boiling-range oils;
some chlorinated additives, such as chlorinated
naphthalenes, may cause chloracne. The use of chlorinated
naphthalenes was discontinued in most countries, many
years ago.
(iii) Changes in the composition of the oil that may occur
during its use:
cracking of oil fractions may occur due to heat;
reactions may occur between components of the mixture or
between them and materials that are added at a later
stage;
metal salts or ions may be formed in the mixture or
solution in the case of metal-working oils; in the case
of, for example, chromium and nickel, this can cause skin
reactions in sensitized workers.
(iv) Impurities of all kinds may accumulate in, for instance,
metal-working oil baths in which the oil is not regularly
changed. Important among these impurities are: metal
particles, which may cause microtraumata of the skin; and
microorganisms, which may cause inflammation of the skin
by way of infection or exotoxins; bacteria, yeasts, or
fungi may be present in mono- or mixed culture, and some
of these organisms can degrade an oil and form potentially
irritating compounds.
(b) Factors related to the work situation:
(i) design of workshop and equipment determine to a large
extent the magnitude and duration of exposure to the oil,
and whether this exposure is continuous or intermittent;
(ii) availability of general and exhaust ventilation of
sufficient design and capacity may determine whether there
is exposure to oil mist or not;
(iii) use of protective clothing such as gloves, will diminish
duration and intensity of skin contact;
(iv) general hygienic facilities such as wash basins near the
work-place, shower facilities, frequent changing and
laundering of work clothing, all of which help in limiting
the extent and duration of exposure;
(v) hands and contaminated parts of the body should be washed
with fatty toilet soap and, after washing the skin should
be treated with a suitable emollient cream; use of
solvents, and alkaline or abrasive soaps for washing can
damage the skin and contribute to the occurrence of skin
diseases.
(c) Individual factors:
(i) general and hereditary conditions of the skin may
predispose to adverse skin effects from all sorts of
chemicals;
(ii) work discipline and safety-mindedness can influence the
duration and intensity of the exposure;
(iii) proper use of safety equipment and general hygiene
facilities, as well as personal hygiene in the form of
frequent bathing and changing of underwear, will help to
avoid over- and prolonged exposure.
Contamination of the skin may often occur despite all precautions
taken, particularly in the case of metal-working oils. Wiping with
oil-soaked rags is an additional source of contamination and may cause
skin lesions. Continuous wearing of oil-contaminated clothing is an
important factor in the etiology of scrotal cancer. Rapidly rotating
machinery may generate oil foams and mists.
The most common form of skin disorder is acute or chronic contact
irritative (toxic) dermatitis caused by the irritative action of
various components, additives, and/or impurities in mineral oils.
Dermatitis may be preceded by defatting and/or maceration of the skin.
Mechanical irritation, microtraumata, and skin cuts may play a role in
its origin. Clinical signs of contact irritative dermatitis are, in
order of severity, erythema, oedema, bullae or necrosis, and sharp
demarcation of the affected areas from unaffected ones.
The other type of dermatitis is contact allergic dermatitis
(synonym: contact eczema) caused by allergic sensitization to various
allergens. Additives or impurities are the most allergenic components
of oil formulations. The signs of eczema are more variable then those
of toxic dermatitis and include erythema, papulae, vesiculae, bullae,
scales, hyperkeratoses, and rhagades. These lesions have a tendency to
spread into areas that have not been in direct contact with the
allergen. Eczema may be preceded by contact irritative dermatitis,
from which it develops by secondary sensitization.
Oil folliculitis, or oil acne is characterized by the triad
comedones, folliculitis, and follicular scars. The lesions are found
on the parts of the body with the greatest exposure. Friction from
clothing and machinery rubbing the oil into the exposed parts of the
skin, is an important additional factor. First there is plugging of
the hair follicles and pores of the skin by follicular hyperkeratosis,
cell debris, and oil with its impurities, followed by blackheads and
secondary infection. Poor personal hygiene is the main cause. Oil acne
occurs more often in the earlier years of exposure to oil. Later,
there appears to be a gradual change in the reaction of the skin to
the oil (Kinnear et al., 1954). Chlorinated naphthalenes and related
compounds have given rise to chloracne.
Photosensitivity is an abnormal sensitivity of the skin to
sunlight caused by certain constituents of coal-tar, but also
sometimes by mineral oil constituents. Related to this is melanosis,
the general darkening of the skin that may follow acute
photodermatitis, as well as toxic melanoderma, which develop after
long-term exposure to oils containing certain anthracene fractions.
Both photosensitivity and melanosis rarely result from exposure to
mineral oil.
Hyperkeratosis may occur either together with dermatitis and oil
acne of long standing, or in isolation -- mostly on the forearms or
other heavily exposed parts of the body. Two forms of hyperkeratosis
can be distinguished: (a) circular, white and flat hyperkeratotic
areas of a few mm in diameter, sometimes in the form of smooth
plaques; these may occur in small clusters and are slightly raised
above the level of the surrounding skin; and (b) a second form,
which may occur at the same time, and consists of rugose, pigmented
warts that are considerably raised above the surrounding tissue level.
The pattern is generally irregular, but may be round or oval.
Precarcinogenic changes may be present in the hyperkeratotic
plaques in the form of rough, slightly raised patches, which sometimes
may take the form of horns or warts. In themselves these forms are
still harmless, but they have a tendency to become malignant. As soon
as these forms contain some malignant cells they are called
keratoacanthomata. After growing for a certain period, they may be
shed from the skin. Another form of precarcinogenic change that may be
encountered is the shark- or shagreen-skin, a pigmented, atrophic
skin, beset with small horns and warts.
A basal cell carcinoma (basalioma) is a very slowly enlarging
tumour of the skin. It may ulcerate, or invade the area round it, but,
in general, it does not metastasize. The most common form of malignant
tumour is the squamous cell carcinoma (spinalioma), starting as a
small tumour, that may arise from a keratosis or in apparently healthy
skin. It continues to grow, starts ulcerating, invades surrounding
tissues and eventually may metastasize. None of these conditions is
limited to mineral oil exposure. Similar changes may occur as a result
of UV-light exposure, excessive doses of X-rays, exposure to pitch
coal-tar, or ingestion of arsenic. A specific localization, however,
is the epithelium of the scrotum. In this case "it is reasonable to
assume that it could be caused by occupational exposure to soot, tar,
pitch, or oil" (Kipling, 1968).
A combination of various potentiating factors, such as mineral oil
contact and exposure to sunlight (UV-radiation) increases the tendency
to develop skin cancer (Schwartz et al., 1947; Smiley, 1951; Kinnear
et al., 1954; Emmett, 1975). This is especially pronounced in persons
with fair hair.
4.7.1.2 Skin carcinogenicity
The history of the development of skin cancer as a result of
exposure to mineral oil, as well as the major epidemiological
literature related to the subject have been reviewed by the
International Agency for Research on Cancer (IARC, 1973). To quote
theft findings:
"Volkmann (1875) described scrotal cancers among workers producing
paraffin by the distillation of coal-tar. Subsequently, Liebe (1892)
noted the absence of such hazard among workers exposed to pure
paraffin. Several investigators have since shown that cancers among
paraffin workers are not due to the paraffin but to impurities in oils
produced during processing (Leitch, 1922; Hendricks et al., 1959).
Refined paraffin is free of PAH and does not induce skin cancer in
mice (Shubik et al., 1962).
"Bell (1876) first described cancer of the scrotum in a Scottish
shale oil worker. In a 23-year period, 49 Scottish paraffin workers
developed skin cancer of which 13 were scrotal (Henry, 1946).
"The cotton mule spinning industry in Great Britain originally
used shale oil for the lubrication of the spindles (Henry, 1946). The
first case of death from scrotal cancer in a worker who used shale oil
in mule spinning occurred in 1923 (Bridge & Henry, 1928). In the years
1920 to 1943, there were 1303 legally notified cases of skin cancer in
the British mule spinning industry, including 824 of the scrotum.
There were 575 fatal cases of scrotal cancer recorded between 1911 and
1938 (Henry, 1946).
"In Great Britain, the Mule Spinning Regulations have ensured that
since 1953 only oil drastically refined with sulphuric acid shall be
used in mule spinning and that mule spinners shall be medically
examined every six months. These measures, together with the marked
decline of the process of mule spinning, have produced a sustained
fall in the incidence of cancer of the scrotum in Great Britain.
"Cutting oils used by workers to cut metals were found to increase
the risk of skin cancer in Birmingham, England (Cruickshank & Squire,
1950; Cruickshank & Gourevitch, 1952), particularly among workers in
automatic machine shops. Between 1950 and 1967, 187 cases of scrotal
cancer occurred in this region, of which at least two-thirds could be
attributed to oil (Waterhouse, 1971).
"At the present time toolsetters and setter operators in automatic
shops who use neat cutting oil have an increased risk of cancer. The
work requires constant contact with the machines and consequent
contamination with the oil. In the Birmingham area of England, a high
frequency of skin and scrotal cancer from oil has occurred,
particularly among bar automatic machine workers; but other
engineering practices also present a cancer hazard, e.g., metal
rolling, tube drawing, metal hardening and machine operating. Although
the major risk is from exposure to undiluted oils, emulsions have been
incriminated occasionally. The industries most affected are those with
automatic shops, such as nut and bolt manufacturers. Workers have also
been affected after exposure during the changing of transformer oil in
electrical sub-stations and during the painting or spraying of mould
oil for brick- and tile-making or concrete moulding, in drop forging,
rubber mixing, wire drawing, rope making and in the jute industry and
from grease in metal working (Kipling, 1968).
"In France, in the valley of the river Arve in the Savoy Alps,
there have occurred since 1955 at least 60 cases of cancer of the
scrotum together with many cases of cancer of the skin among the bar
automatic machine workers (décolleteurs). The very high frequency in
the relatively small population of the valley was observed mainly
among the self-employed and workers in small premises (Thony & Thony,
1970). They were in contact with undiluted cutting oils.
"Cancers of the larynx, lung and stomach have also been attributed
to oil mist (Southam, 1928); and recently evidence has been produced
that persons who developed cancer of the scrotum are significantly
more liable to develop cancers at other sites, e.g., in the
respiratory tract or upper digestive tract (Holmes et al., 1970)."
Kinnear et al. (1954, 1955) published the results of an extensive
epidemiological study of skin disease in jute workers in relation to
mineral oil exposure. They found a high incidence of premalignant
changes on the skin of the exposed parts of the body in long-term,
older workers and isolated cases of scrotal carcinoma.
Bingham & Horton (1966) estimated the latent period for skin
cancer caused by mineral oil exposure to be 50-54 years (range 4-75
years). In the case of crude paraffin oil, they mentioned an average
of 15-18 years (range 3-35 years). In these cases, the range may be
more important than the average, though the average indicates that, in
general, the latent period is very long. A considerable proportion of
the cases of skin epithelioma caused by mineral oil exposure had such
a long latency period that the disease only appeared after retirement
from active work (Cruickshank & Gourevitch, 1952; Kinnear et al.,
1955).
Five cases of squamous cell carcinoma of the hand and forearm and
one of the scrotum occurred in machine operators at a plant in
Ontario, Canada (Mastromatteo, 1955). These workers had been exposed,
for an average of 21 years, to cutting fluids that were subsequently
demonstrated to be carcinogenic in animal tests (Gilman &
Vesselinovitch, 1955).
Milne (1970) traced 5 cases of carcinoma of the scrotum, as
registered in the Central Cancer Registry of Victoria, Australia, and
found that, in all cases, the carcinomas occurred during or after the
seventh decade of life. Three of the subjects had had intensive
contact with mineral oils throughout theft working life, one was a
stoker in a gas-works, and the fifth had always been involved in
administrative work.
In the Netherlands, a recent survey demonstrated that scrotal
cancer occurred only sporadically and was not correlated with
occupational exposure to mineral oil (Pruyn & Reijnierse, 1972;
Fokkens et al., 1972; van Raalte, 1972).
Eight cases of scrotal cancer were discovered by Avellan et al.
(1967), over a period of 24 years, among 250 automatic lathe operators
in Gothenburg, Sweden. Diagnosis was made when the operators were
between 54 and 66 years of age and after periods of exposure to
mineral oil ranging from 19 to 43 years. In the words of the authors:
"All of the cases occurred among operators who began their work
during the era when the exposure, as a result of the prevailing
machine construction, was considerable and before the regulations and
the controls, which were instituted after the discovery of the first
cases, had been set up."
Wahlberg (1974) analysed 34 cases of scrotal cancer reported to
the Swedish Cancer Registry between 1958 and 1970. Seven cases (21%)
had been heavily exposed, occupationally, in the past to oil. and oil
mist, e.g., as automatic lathe operators. One of this group developed
a primary lung cancer.
A retrospective study of 298 cases of scrotal cancer registered in
the Birmingham region in the United Kingdom between 1936 and 1972 was
reported by Brown et al. (1975). The incidence of scrotal cancer was
5-6 cases per million males per year, whereas for the whole of the
United Kingdom, the incidence is 1-2 cases per million males per year.
The patients or relatives were interviewed in 109 cases: 94 had been
exposed to mineral oil (mainly tool-setters and machine operators, and
all cases had been exposed to cutting oil, 14 had been exposed to
pitch or tar, and, in 7 cases, there was no apparent occupational
exposure. In 298 cases of scrotal cancer, 52 other primary rumours
were noted; of these, 42 arose following the scrotal cancer including
15 skin tumours and 12 bronchial tumours. The incidence of both these
types of cancers was much in excess of what would be expected
statistically. The majority of the group with second primary tumours
were machine operators and tool-setters (75% in the case of bronchial
carcinoma). The authors postulated that in these cases both primary
rumours were initiated by the same carcinogen.
After studying all the available literature, Desoille et al.
(1973) concluded that, in general, and with the exception of certain
extreme situations, the number of tumours caused by exposure to
mineral oils was low. According to Auld (1950) and Eckhardt (1957),
though certain cutting oils and other mineral oils are carcinogenic to
the skin, the degree of carcinogenicity is very low compared with that
of coal-tar, pitch, and shale oil. In their opinion, and that of most
other authors, e.g., Avellan et al. (1967), even this level of
carcinogenicity would disappear, if a minimum of industrial and
personal hygiene measures were adhered to. On the other hand, Auld
(1950), Desoille et al. (1973), Thony et al. (1975) and many others
urge that alternative products should be developed that do not expose
the workers to a tumorigenic hazard.
In a further report on the skin cancer epidemic in the French Arve
Valley, Thony et al. (1975) recorded 133 epithelioma cases in 15
years, mostly of the scrotum. The incidence in these workers was 36
times that expected in the general population. In workers in small
workshops with poor industrial and personal hygiene, the incidence was
found to be 3 times higher than in the larger plants. The average age
was 54 years (range 35-75 years) and the average exposure 30 years
(range 15-50 years), at the time of clinical diagnosis. In addition,
an increased incidence of bronchopulmonary tumours was found,
especially in the décolleteurs. Officially until 1947, but in
practice possibly until 1950, coaltar-derived anthracene oils were
used, which originally contained benzo(alpha)pyrene levels of up to
1000 mg/kg. The level was later reduced to 10 mg/kg. These oils may be
responsible for many of the previously mentioned cases, but skin
cancer also occurred in some of the workers exposed only to the
petroleum-derived oils that were used exclusively later on. Fresh
cutting oils, as delivered to the users, contained benzo(alpha)pyrene
levels ranging from 0.5 to 145 µg/litre and the concentration of
carcinogens was found to increase during use, though not to the same
extent as that in quenching oils (2-100 times with an average of 30
times), and in lubricating oils for internal combustion engines.
4.7.1.3 Effects of oil mist exposure
Southam (1928) was the first to attribute cancers of the larynx,
lung, and stomach to oil mist exposure.
In a report by Huguenin et al. (1950), 32 out of 144 patients with
lung cancer had been in prolonged and intense contact with oil mist in
the past. Old metal-working machines with improper protection against
oil mist, processes where oil vapour arose from contact with hot
metal, and high-pressure cleaning of machines with oil, were found in
the places where these patients had worked. Hendricks et al. (1962)
considered the observations of Huguenin et al. (1950) to be of
doubtful significance in view of the high incidence of this disease in
non-oil-mist-exposed groups. Nevertheless, the conditions in these
cases may have been such, that a causal relationship, in at least some
of them, cannot altogether be excluded.
More printing industry workers were found among cases of bronchial
carcinoma in a Stockholm clinic (8 out of 125) than would have been
expected (Ask-Upmark, 1955). Certain industrial processes where oil
mist might occur were studied by Hendricks et al. (1962), who gave an
indication of possible exposures in their table of actual exposures
measured in various industries (Table 7).
The average particle size in the oil mists varied according to the
generating process and was found to be about 1.0-5.0 µm.
In 241 Kodak workers exposed to oil mist (Ely et al., 1970), no
significant differences were found, either in mortality, respiratory
symptoms, and disease, or in lung function compared with a control
group.
In the same year, Holmes et al. (1970) produced evidence, that
persons who developed cancer of the scrotum were significantly more
liable to develop cancers of the respiratory tract and the upper
digestive tract.
Similar findings have been reported in a preliminary paper by
Waterhouse (1972). An excess of primary tumours at other sites (skin,
respiratory and upper alimentary tracts) was found in men with scrotal
epitheliomas.
From animal studies, it appears that oil mist particles larger
than 5 µm will not easily penetrate into the lungs, but will be
retained mainly in the nasopharynx and upper respiratory tract.
Smaller particles, especially those of 2.5 µm and less, will readily
pass into the alveoli, where they will be phagocytosed and passed on
to the lymph nodes. When this mechanism cannot cope with the
situation, in cases of continuously repeated high exposure to oil
mists, a chemical pneumonitis and chronic lipid pneumonia may develop
(Proudfit et al., 1950; Foe & Bigham, 1954), but this appears to be an
extremely exceptional condition. Clinical details and examples of this
will be discussed in section 4.8.
TABLE 7. Exposure to oil mist in selected industries
Type of industry Observations Exposure range
oil mist mg/m3
brass and aluminium production 5 1.4-20.7
copper mining 7 5.4-22.0
automobile manufacture 37 1.0-56.5
manufacture of steel products 33 0.8-50.0
newspaper (press room) 8 2.0-16.6
screw manufacture 6 1.0-14.2
From: Hendricks et al. (1962).
In 12 out of 19 workers with oil mist exposures ranging from 9 to
18 years, Jones (1961) found a marked linear and reticular pattern in
the radiograms of the lungs. Exposures ranged from 1 to 9 mg/m3 with
70% of the particles of the order of 1 µm.
Various groups of research workers have studied different aspects
of the printing industry. Printing-ink consists chiefly of a
suspension of carbon black in mineral oil or aromatic extracts of
mineral oil. Modern high-speed newsprint presses can generate fairly
high concentrations of ink-mist aerosol. Most particles, however, are
outside the respirable range and may end up in the stomach, rather
than in the lungs of exposed workers. Lippmann & Goldstein (1970)
found an average droplet size of 14 µm (ranging up to 30 µm) in the
press rooms of the New York Times. The time-weighted average
concentration of respirable mist particles was found to be 1.4 mg/m3
against a total time-weighted average mist concentration of
8.6 mg/m3. In a parallel epidemiological study in the same firm,
mortality and morbidity data over a 15-year period were compared for
pressmen and compositors. No significant differences in respiratory
mortality or morbidity were found (Goldstein & Benoit, 1970).
In a similar study by Pasternak & Ehrlich (1972), there was no
increase in respiratory symptoms or decrement in respiratory
performance in 778 New York pressmen compared with 1207 compositors.
No significant differences in death rates, were found in those who
were less than 40 years old when first employed, even if they had
worked for more than 20 years. In those first employed when they were
more than 40 years old and with more than 20 years of employment,
there was a significantly higher death rate in pressmen compared with
compositors. The reason for this finding was not clear. The mean oil
mist concentration measured in the 3 workshops concerned was
3.7-5.2 mg/m3.
Moss et al. (1972) analysed the causes of death of 3485 former
full-time printing industry workers from London and Manchester, who
died in the period 1952-66; Greenberg (1972) studied the death
certificates of 670 male printing workers who died between 1954 and
1966. In both studies, an excess number of cancers of the lung and
bronchus were found. This excess was more marked in Manchester and
greatest in machine-room men. The authors concluded that the slight
excess found might or might not be due to occupation. They considered
that an occupational cause was more likely in the case of the greater
excess in the Manchester machine-room men.
These studies in the printing industry have been mentioned in
relation to mineral oil exposure. However, the same data could be
related to carbon black and even to lead exposure. Similar
considerations should be kept in mind in the evaluation of the data in
other occupations where many other factors might also play a role,
either individually or combined with the mineral oil used, as, for
instance, chromium in the metal-working industry.
In studies in 34 metal-working firms in Baden-Würtemberg (FGR),
the frequency of respiratory complaints (cough, expectoration, and
dyspnoea) in 443 workers exposed to oil mist for long periods was
compared with that in 398 unexposed controls, matched for age. Mineral
oil mist exposures from cutting oils had ranged from 40 to 150 mg/m3
over long periods. The highest incidence of complaints was found among
unexposed smokers, the lowest incidence amongst non-smoking,
oil-mist-exposed workers. The authors did not find any signs of
irritative effects from oil-mist exposure, but a significant
protective effect against the well-known irritant effects of smoking
(Drasche et al., 1974). This finding is in line with findings in
experimental animals, that previous exposure to oil mist reduces the
lethal effects of respiratory oxidants such as ozone and nitrogen
dioxide in mice (Wagner et al., 1961). The authors stated that
conclusions could not be drawn from this study in relation to the
carcinogenicity of these oil mists in the respiratory tract.
During the cold processing of metal, the concentration of mineral
oil mist in the air (spindle oil aerosol) fluctuated from 3 to
40 mg/m3 (on average 10 mg/m3). During an examination of 77 lathe
operators (men and women), functional disturbances found to occur in
the respiratory system, in particular after a period of service of
more than 10 years, included a reduction in the active volume and the
maximum ventilation of the lungs and an increase in oxygen requirement
and in its coefficient of utilization (Bruskin & Demcenko, 1975).
A 30-year retrospective cancer mortality study was carried out by
Decoufle (1976) on 5189 workers engaged in metal-working for at least
one year. No significant differences in cancer mortality were
observed, when compared with the general population. Indications of
increased incidences of respiratory and digestive cancer, observed
when comparison was made on an age group or exposure basis, were not
statistically significant.
Decoufle (1978) published a further study on a group of 2485 male
workers employed between 1938 and 1967 in jobs exposing them to
various levels of cutting oil mists. Compared with the total death
rate of the US male population, no significant differences were
observed for 15 cancer site categories. However, a 2-fold risk of
cancers of the stomach and large intestine (combined) was seen after
20 years of follow-up in the subgroup of men with 5 or more years'
exposure to cutting oil mists, prior to 1938. Deaths from nonmalignant
respiratory disease were significantly fewer than expected. These
results suggested that occupational exposure to soluble and insoluble
cutting oil mists, during various metal machining processes, did not
pose a health hazard in terms of respiratory cancer and fatal
nonmalignant respiratory disease, but might be associated with certain
forms of gastrointestinal cancer.
To summarize, it can be concluded from the literature that
oil-mist exposure can give rise to pulmonary disease, but only after
prolonged exposure in workplaces with unsatisfactory hygienic
conditions. If oils with a low content of polynuclear aromatic
hydrocarbons are used in situations where oil mists can be generated
and the TLV for oil mists is not exceeded, this problem is unlikely to
arise.
4.8 Clinical Studies
Clinical studies on the effects of mineral base oils and products
derived from them on the skin have been discussed together with
epidemiological studies in sections 4.7.1.1 and 4.7.1.2. It was felt,
that compilation of individual case-histories from the literature on
this subject was superfluous. Adverse effects resulting from the
surgical use of paraffin for cosmetic purposes, and those following
grease-gun accidents have not been considered in this review.
Clinical studies on the effects of these products after ingestion
show that the main effects are caused by aspiration, which may be a
complication of ingestion, and usually occurs during subsequent
spontaneous or induced vomiting. Otherwise, there is general agreement
that mineral base oils, lubricating oils, and greases have a low order
of toxicity, when ingested (Gerarde, 1960). At the most, some
gastrointestinal symptoms, such as abdominal cramps and diarrhoea,
result.
There was evidence from a clinical case study that prolonged
ingestion of mineral oil over a number of years could result in oil
deposition in the small intestine, abdominal lymph nodes, liver, and
spleen and lungs. This produced significant structural and functional
abnormalities that were considered to have contributed to the
patient's death (Nochomovitz et al., 1975).
White mineral oil (pharmaceutical grade) is a base oil
specification intended for oral use. It is a highly purified base oil
distillate, mainly containing saturated paraffinic fractions, and it
has to be free of polynuclear aromatic hydrocarbons. It is used as a
laxative, in pharmaceutical formulations, and as a food-grade
lubricant. From studies with radio-opaque oil, it appears possible
that a small amount of mineral oil, when taken orally as a laxative
just before retiring, may gain entrance into the lungs. Though this is
not of any consequence if it occurs only once, a cumulative effect in
the lungs might result, if it occurs repeatedly, day after day, over
several years (Sante, 1949; Miller et al, 1962).
Clinical experience of the effects of inhalation of mineral oil in
the lungs is in agreement with the results of animal studies (section
4.6.2.3). In man, mineral oil is rapidly phagocytosed and transferred
to the regional lymph nodes. In contrast with vegetable oil and oil of
animal origin, however, mineral oil cannot be metabolized and is
deposited in the interstitial tissue and in the lymph nodes, where it
induces a cellular reaction, with the appearance of giant cells that
may result in fibrosis of more or less extensive lung areas. The
resulting clinical and pathological picture depends to a large extent
on whether there has been massive, acute over-exposure, with the
body's defence mechanisms overwhelmed (see (a)), or whether long-term,
low-level exposure has occurred, where the defence mechanisms can cope
with the daily exposure and no disease will become apparent, until the
signs and symptoms of the secondary fibrotic reaction appear (see
(b)), (Pinkerton, 1927, 1928; Cannon, 1940; Freiman et al., 1940; Moel
& Taylor, 1943).
A few typical clinical entities can be recognized:
(a) Diffuse acute mineral oil pneumonia is practically always
the result of an accidental massive aspiration of mineral oil (Sante,
1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham, 1954;
Gerarde, 1960). It is, in fact, a chemical pneumonitis frequently with
a superimposed secondary infection, progressing into an interstitial
proliferative inflammation. Cases resulting from aspiration following
accidental ingestion of products containing gasoline, kerosene, or
other petroleum solvents in the same boiling-range are well-known from
the clinical literature. Many cases have occurred as a result of
aspiration of seawater contaminated with diesel oil by survivors of
sinking ships (Weissman, 1951).
Aspiration of mineral oils in the boiling-range under discussion
may have similar results. This can happen when choking occurs, while
taking white medicinal oil. This acute type of mineral oil pneumonitis
is not likely to occur as a result of occupational exposure to oil
mist. Clinically, all signs and symptoms of an acute massive
pneumonitis are present with elevated temperature and a chest X-ray
typical for this condition. Oil droplets may be found in the sputum
and, histologically, the condition is characterized by intra-alveolar
accumulation of oil-laden phagocytes and inflammatory cells. Acute
mineral oil pneumonia is a special form of lipoid pneumonia, which
presents a similar clinical picture and is the result of aspiration of
vegetable or animal oil (mainly cod liver oil, but also egg-yolk and
milk). Though in these cases a similar severe acute pneumonia may
develop, the oil can be metabolized and disappears completely after a
certain time (Sante, 1949). With oils of animal origin, such as cod
liver oil, however, the reaction of the body (lungs and other internal
organs) can be much more serious than with mineral oils (Young et al.,
1939).
(b) Diffuse chronic mineral oil pneumonia occurs as a result of
gradually developing fibrotic and proliferative changes in both lungs.
It may follow years after the acute form, or without an acute
beginning after a long, practically asymptomatic period as a result of
repeated smaller "aspirations", such as those resulting from regular
massive use of mineral-oil-based laxatives, nose-drops, or nose-sprays
(Pinkerton, 1927; Bishop, 1940; Freiman et al., 1940). The apparently
rare cases of mineral oil pneumonitis following prolonged occupational
exposure to excessive oil mist concentrations fall into this category
(Sante, 1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham,
1954; Gerarde, 1960). Reports from the literature suggest that in some
of these cases, at least, a predisposing factor was present in the
form of a pre-existent or concomitant lung disease (Freiman et al.,
1940; Weissman, 1951; Forbes & Markham, 1967). Increasing dyspnoea and
productive cough are the most important symptoms. The chest X-ray
generally shows increased perihilar opacities with signs of lung
fibrosis and diffuse patchy opacities. Oil droplets or oil-laden
phagocytes may be found in sputum or in tissue derived from lung
biopsy (Goodwin, 1934; Bishop, 1940; Freiman et al., 1940; Rossier &
Bühlmann, 1949; Sante, 1949; Proudfit et al., 1950; Borrie & Gwynne,
1973).
(c) Paraffinomas are large fibrous nodules or globules of liquid
mineral oil embedded in dense hyaline fibrous tissue. They represent a
separate form of the chronic mineral oil pneumonitis in which
oil-laden phagocytes, destroyed by pressure, atrophy in the fibrous
scar tissue formed. Paraffinomas may be found singly or in clusters
around the large bronchial branches or at the site of the hilar lymph
nodes. It may be difficult to differentiate a paraffinoma from a lung
tumour radiologically (Brown & Biskind, 1941; Wood, 1943; Sante, 1949;
Proudfit et al., 1950; Weissman, 1951; Bryan & Boitnott, 1969; Borrie
& Gwynne, 1973). Aspiration biopsy may help in the differential
diagnosis (Nathanson et al., 1943).
Boitnott & Margolis (1966a) have described analytical methods for
the identification of the various oils in human tissues. Mineral oil
droplets may pass from the hilar nodes via the thoracic duct into the
systemic circulation (Pinkerton, 1927; Young et al., 1939; Freiman et
al., 1940; Boitnott & Margolis, 1966b). As a result of this
transmission, oil droplets have been found in the liver, spleen, and
other organs (Pinkerton & Moragues, 1940; Rewell, 1947).
The general pattern found in young children is slightly different
from that found in adults. Pinkerton (1927) described 6 cases of
lipoid pneumonia in children and data on 25 cases in children have
been summarized by Goodwin (1934). Seven cases have been reported by
Ikeda (1935) and 27 cases by Bromer & Wolman (1939). There is general
agreement that fats and oils of animal origin play a more important
role than mineral oil in lipoid pneumonia in infants and young
children. Furthermore, the acute massive aspiration type is more
frequently seen in children than the chronic form. The main causal
factors in these observations are: false passage of a gavage tube,
false deglutition in bottle-feeding with the baby lying on its back --
especially in cases of debilitating disease, and also aspiration
following forced administration of milk or cod liver oil with or
without vomiting or choking (Freiman et al, 1949).
With regard to adults, Ikeda (1937) summarized 106 cases from the
literature, Graef (1939) 22 cases, Bishop (1940) 136 cases, Freiman et
al. (1940) 58 cases, and Moel & Taylor (1943) 20 cases. They concluded
that lipid pneumonia, especially the diffuse chronic form, occurs more
frequently in adults than is generally believed. Liquid paraffin is by
far the most important etiological agent in the adult. Debilitated
states, dysphagia and impaired cough reflexes, because of neurological
or other disorders, are important predisposing factors. The authors
stress, however, that mineral oil is widely used without evident harm,
even in elderly persons. Wherever there is a real indication for this
type of medication, they see no reason to discontinue it. Extensive
use, especially self-medicated, of liquid paraffin intranasally or via
the oral route by debilitated or dysphagic patients should, however,
be discouraged (Bishop, 1940; Freiman et al., 1940).
As a matter of interest, a few cases of lipoid pneumonia have been
described in relation to the intratracheal administration of
mineral-oil-based mixtures by opera singers, prior to every
performance on the stage, in order to improve the quality of the voice
(Even, 1947; Facquet & Langeard, 1947; Meyer, 1976). A similar case
was described by Garvin (1939) as a result of intratracheal
self-medication.
From the literature, it is apparent that with the recognition of
the causal factors and the change from oil-based to water-based
nose-drops, the incidence of the type of lipid pneumonia just
described has drastically decreased, since the end of the forties.
The following more or less typical cases, in which there was -- or
might have been -- a relation between occupational exposure to oil
mist and the occurrence of lipid pneumonia, were found in the
literature. Proudfit et al. (1950) reported a case of chronic lipid
pneumonia in a 40-year-old man who had been spraying mineral oil for
17 years; he had a typical chest X-ray. The chief complaints were
cough, shortness of breath, and fatigue. Mineral oil droplets were
identified in the sputum; 3 years later the condition had progressed
slightly. A case of lipid pneumonia was described by Weissman (1951)
in which the disease apparently developed on the basis of
long-standing pulmonary fibrosis as the result of blast-spraying of
machine parts with mineral oil; no mask had been used as protection
against the inhalation of nebulized oil. Foe & Bigham (1954) reported
the case of a 30-year-old aircraft mechanic who complained of fatigue,
shortness of breath on exertion, and frequent chest colds. Lipid
pneumonia was diagnosed from a lung biopsy. The mechanic had been
spray-cleaning aircraft engines with a mixture of 50% kerosene and 50%
vegetable-oil-soap.
Two cases of progressive respiratory disease, which developed in
the fifth decade of life were described by Forbes & Markham (1967).
Both patients had a moderate to heavy smoking history; one, in
addition to this, had a family history of asthma; dyspnoea and
wheezing were the major signs in each case. Both reacted well to
treatment, but recurrence of signs and symptoms was related to working
with cutting oils, the composition of which was not mentioned.
The epidemiological data on the possible relationship between
cancer of the respiratory tract and long-term occupational exposure to
oil mist has been discussed in section 4.7.1.2. There is some evidence
that in cases where an unsatisfactory industrial hygiene situation
coincided with the use of an oil with probable carcinogenic
properties, such a causal relationship might exist. On the other hand,
various extensive studies have shown that, in general, it is certainly
not a major problem.
However, Wood (1943) reported a fatal case of extensive lipid
pneumonia in a house-maid who had used oily nose-drops in large
quantities for recurrent sinusitis over a period of 10 years. At
postmortem, an alveolar-cell carcinoma was found in the lungs. The
author suggested that there might have been a causal relationship
between the 2 diseases, though he assumed that this occurrence would
be rare. Two cases of bronchogenic carcinoma were described by Sante
(1949). In the first case, the malignant epithelial tumour was
situated in an area of dense fibrotic tissue, typical of a
paraffinoma. In the other case, a squamous cell carcinoma was found
together with lipid pneumonia in an early stage of organization. The
author felt that there might have been an etiological relationship in
the first case, but considered this less likely in the second case.
Both patients had taken a tablespoon of mineral oil as a laxative,
just before retiring, for years. Volk (1964) studied a series of more
than 100 cases of mineral oil pneumonia and noted that not one case of
bronchogenic carcinoma occurred in this group. In addition, he
reviewed 114 consecutive autopsies of adenocarcinoma of the lung in
which he did not find any lesions that might be associated with
mineral oil pneumonia.
A rapidly lethal case of multifocal alveolar cell carcinoma of the
lung was reported in a 63-year-old man who had centrifuged used
cutting oil for 23 years and had been exposed continuously to oil mist
of this type during that period (Despierres et al, 1965). Wahlberg
(1974) described one case of primary lung cancer in 7 men who
developed scrotal cancer following heavy occupational exposure to oil
and oil mist in, for example, automatic lathe operation. A case in
which achalasia led to chronic mineral oil pneumonia was reported by
Bryan & Boitnott (1969). An adenocarcinoma developed in the area of
scarring resulting from the mineral oil and caused death. On the basis
of a literature study and theft own findings, the authors concluded
that the pathogenesis might be related to the pulmonary scarring
rather than directly to the mineral oil (see also Yokoo & Suckow,
1961). However, the authors also considered that there was no reason
to suppose that carcinomas were more likely to arise in a scar induced
by mineral oil than in a scar of a different origin (Bryan & Boitnott,
1969).
5. BITUMEN
5.1 Properties and Analytical Methods
5.1.1 Chemical and physical properties
The term bitumen is applied to solid and semi-solid residues from
the distillation of suitable crude oils. This product is known as
"asphalt" in the USA. In most other countries, the term asphalt is
reserved for certain natural deposits and for mechanically made
mixtures of bitumen and mineral matter.
Bitumen is the residue obtained by atmospheric and vacuum
distillation of certain types of crude oil. It is generally a black or
dark-brown material, ranging from a highly viscous liquid to a solid
and brittle substance at normal ambient temperatures, depending on the
proportion of light fractions removed. On heating, bitumen softens
gradually and eventually becomes fluid. Grades are characterized by
their "penetration" and "softening" point. According to the Petroleum
Handbook (1966), bitumen can be considered as a colloidal system of
highly condensed aromatic particles in an oil with ring-type
molecules. From this statement, it is clear that bitumen is a very
complex mixture of mainly high-boiling hydrocarbons. Its composition
varies widely with the geographical source of the crude oil and the
process of manufacture. For example, a mixture of 6 samples was found
to contain:
(a) 32% asphaltenes: high-relative-molecular-mass aromatic compounds
and heterocyclic hydrocarbons of which some are unsaturated. They
are soluble in carbon disulfide but insoluble in petroleum
naphtha;
(b) 32% resins: polymers resulting from the processing of unsaturated
hydrocarbons;
(c) 14% saturated hydrocarbons: hydrocarbons in which the carbon atoms
are connected by a single bond; and
(d) 22% aromatic hydrocarbons: hydrocarbons containing one or more
benzene rings per molecule, including condensed polycyclic
aromatic hydrocarbons (Simmers et al., 1959; Simmers, 1964).
While the appearance and engineering applications of bitumens and
asphalts are similar to those of coal-tars and pitches, fundamental
differences exist between these 2 classes of materials (Puzinauskas &
Corbett, 1978). Bitumen is generally derived from crude oil by a
process that does not involve cracking or thermal conversion, and
coal-tars and pitches are obtained by high-temperature carbonization
of bituminous coal. Chemically, coal-tar materials are mainly composed
of highly condensed-ring aromatic and heterocyclic hydrocarbons.
Bitumens, on the other hand, contain a much higher proportion of high
relative molecular mass paraffinic and naphthenic hydrocarbons and
their derivatives. Under comparable heating during application and
use, coal-tars generate substantially higher emissions of polynuclear
aromatic hydrocarbons than bitumen. While epidemiological surveys of
workers engaged in the production of coal-tar have revealed an
increased incidence of lung cancer, no increases in cancer or other
adverse effects have been observed in studies on workers involved in
the manufacture and application of asphalt.
The benzo(alpha)pyrene content of petroleum bitumens derived from
various Russian crude oils was determined by Janyseva et al. (1963).
They demonstrated that the benzo(alpha)pyrene content of straight-run
bitumen was considerably lower (of the order of 0.6 mg/kg) than that
of bitumens derived from cracking residues (of the order of
4-272 mg/kg). Schamp & van Wassenhove (1972) reported
benzo(alpha)-pyrene levels of 3-5 mg/kg in bitumens.
5.1.2 Methods of sampling and analysis
See section 2.1.2.
5.2 Sources of Environmental Pollution
5.2.1 Natural sources
Natural bitumen and asphalt deposits occur in various parts of the
world, mainly as a result of mineral oil seepage from the ground. The
most well-known natural asphalt deposit is the Trinidad Lake, which
contains a mixture of about 39% bitumen, 32% mineral matter, and 29%
water and gas.
5.2.2 Man-made sources
5.2.2.1 Production
Total world-wide bitumen production reached 90 million tonnes in
1973. As with crude oil, this was approximately 10 times the immediate
pre-war level. Bitumen production rose to 100 million tonnes in 1979
and is expected to continue to increase in the future, although at a
lower rate of growth than in the past.
The following types of bitumen are produced by refining and
treatment:
"Straight" bitumen
The residue of atmospheric or vacuum distillation of
asphaltic-based crude oils. For special applications, very hard
pitch-type bitumen residues can be obtained by distilling cracked
oils.
"Blown" bitumen
Manufactured by feeding air-bubbles countercurrent through a
column of hot molten straight bitumen. Oxidation reactions occur
leading to dehydrogenation and polymerization of the unsaturated and
aromatic components. In this process, large condensed aromatic nuclei
may also formed.
"Cutback" bitumen (or more fluid bitumen grades)
Obtained by mixing bitumen with petroleum solvents or mineral oil,
sometimes with coal-tar or high aromatic extracts.
Bitumen emulsion
Made by emulsifying 50-65% of bitumen in water in the presence of
0.5-1.0% of an emulsifier, usually soap and generally used cold for
both roadmaking and industrial purposes.
5.2.2.2 Uses
The main use of bitumen is for paving roads. It is also used in:
(a) lining irrigation canals, water reservoirs, dams, and dykes;
(b) mastic asphalt for industrial flooring;
(c) bituminized felts for roofing;
(d) protective coatings, for walls, motor-cars, water mains;
(e) adhesives for the building industry;
(f) coal briquetting;
(g) electrical insulation; and
(h) battery-making.
5.3 Environmental Exposure Levels
Apart from walking or riding on bituminous pavements and roads,
the general population will not normally come into contact with
bitumen, except in the form of protective coatings and coal
briquettes. On the other hand, the general population can, on
occasion, be exposed to fumes from heated bitumens for short periods
of time during road-building or the covering of roofs. Emissions from
asphalt roads have been mentioned in the literature as a possible
source of exposure for the general population, but such exposure is
considered to be as negligible. Recent evidence from the Federal
Republic of Germany and the USA confirms this (Hettche, 1963).
Occupational exposure to bitumen may be much more intensive in
certain professions and may range from accidental splashing with hot
bitumen to repeated and prolonged contact of the skin with the more
liquid bitumen grades or to exposure to fumes from heated bitumen.
5.4 Environmental Distribution and Transformation
No specific data relating to bitumens are available in relation to
distribution among media, environmental transformation and
degradation, interaction with physical, chemical, or biological
factors, and bioconcentration. On the other hand, there is a lot of
information on the microbial degradation of individual petroleum
hydrocarbons (section 3.4).
5.5 Metabolism
Uptake and storage of the light fractions contained in bitumens
may occur; however, no specific data exist on this subject.
Bitumens occur in a variety of commercial products. The
composition of these products may vary widely depending on the
geographical origin of the crude oil used and the manufacturing
process applied. These facts may influence the results of metabolic
studies, all of which are concerned with the exposure of experimental
animals. Human data are lacking.
5.6 Effects on Experimental Animals
5.6.1 Short-term exposure
No data are available on acute toxicity, exposures related to
adverse effects, interactions, and species comparisons. It is
generally accepted that the acute toxicity of bitumens is low.
5.6.2 Long-term exposure
No data appear to have been published on toxic effects in specific
organs, teratogenicity, or reproduction. The data on mutagenicity are
limited. On the other hand, substantial experimental work has been
carried out concerning the carcinogenic effects of bitumens on skin.
Twort & Fulton (1930) examined the carcinogenic effects of various
synthetic tars and their fractions on the skin of mice. The
carcinogenic activity varied according to the compound used and with
the temperature of pyrolysis. Results of these studies confirmed the
earlier finding of Kennaway (1925), that the temperature at which the
tar formed was an important factor in the production of carcinogenic
substances. Cancer of the skin of mice was induced by applications of
the synthetic residues obtained from heating substances such as
acetylene, isoprene, a noncarcinogenic petroleum, yeast, and human
skin. The yield of carcinogens decreased when carbonization had
occurred at temperatures above 950°C. The yield was greatest between
850 and 870°C; less between 600 and 750°C, and negligible at 500°C.
Furthermore, the authors found that the carcinogenic activity of the
synthetic residues could be reduced considerably by oxidation or
reduction (by various methods) or by dilution with oleic acid.
A wide range of aliphatic and simple aromatic hydrocarbons were
pyrolysed by Badger (1962), who showed that polynuclear aromatic
hydrocarbons were formed from simpler hydrocarbons at 700°C via
primary radicals formed by carbon-hydrogen and carbon-carbon fission
(cracking) at this elevated temperature. The same process occurred to
a much lesser extent at 550°C.
According to Bogovski et al. (1963), thermal distillation of a tar
to coke decreased the carcinogenic activity by the formation of
irreversible condensation products from polynuclear aromatic
hydrocarbons, together with other high molecular compounds. In
long-term, skin-painting experiments on white mice, the authors showed
that such a coking process reduced the tumour incidence from 68% to
3.7% in the case of shale-oil tar. As shale-oil tars have a much
higher carcinogenic activity than petroleum residues (Twort & Twort,
1931), it might be expected that coking of petroleum residues would
reduce the carcinogenicity of the material still further.
The carcinogenic effects of bitumen on the skin of C-57 black mice
was studied by Simmers and co-workers (1959). In a first series of
tests, they used a mixture of 6 samples from Southern Californian
refineries, in which both steam- and aft-blown bitumens were mixed.
Painted twice weekly on the skin for a lifetime, the mixture caused 12
dermoid carcinomas in 68 animals, compared with none in the untreated
control group. Formation of cancer was preceded by hair loss, dryness
and scaling of the skin, and papilloma formation. After subcutaneous
injection, 8 sarcomas occurred in 62 animals at the site of injection
compared with none in the control group. However, the relevance of
results from such subcutaneous studies is questionable.
In an inhalation study on the same pooled sample and using the
same strain of mice, the animals inhaled an aerosol of bitumen
droplets suspended in moist air for 30 min/day, 5 days per week, for
up to nearly 17 months (Simmers, 1964). Changes found on microscopic
examination were minimal and included occasional congestion, acute
bronchitis, pneumonitis, bronchial dilatation, and some peribronchial
round-cell infiltration. In a second inhalation study, the animals
were exposed to cooled smoke from bitumen at 120°C for 6-7´ h/day, 5
days a week, for up to 21 months. In this study, peribronchial
round-cell infiltration, bronchitis, pneumonitis, abscess formation,
loss of cilia, epithelial atrophy, and necrosis were more common.
Squamous cell metaplasia was rare, but hyperplasia was more commonly
seen. The changes in both experiments were patchy, rather
non-specific, and similar to those described as a result of exposure
to other air pollutants.
In his next series of tests, Simmers (1965a) compared the
carcinogenicity of straightrun and air-blown bitumen in prolonged
regular skin application tests and after single or repeated
subcutaneous injection. Undiluted air-blown bitumen did not induce
tumours, when applied to the skin, probably because the bitumen was
too hard; when dissolved in toluene the incidence of skin cancers
increased to 45%. This may be the result of better contact with, or
penetration into the skin. On the other hand, the polynuclear aromatic
hydrocarbons might be concentrated by the solvent. In a similar
skin-painting study with straight-run bitumen, skin cancers occurred
in only 14%. The same trend was apparent after subcutaneous injection,
where 0 and 13% tumours were found at the site of injection with
straight-run and air-blown bitumen, respectively. The author presumed
that this difference in response resulted from a difference in
chemical composition. Although the aromatic fraction of air-blown
bitumen was lower than that of straight-run bitumen, it contained more
complex aromatic hydrocarbons due to polymerization and condensation
caused by the air-blowing.
Straight-run bitumen was separated into 4 fractions, which were
painted 3 times a week, continuously, on the skin of the same strain
of mice (Simmers 1965b). The fraction containing most of the saturated
compounds and aromatic hydrocarbons -- which also showed practically
all the UV light fluorescence -- induced considerably more skin
rumours (43.3%) than straight-run and air-blown bitumens had done in
earlier studies. The author pointed out that, though fluorescence was
not a guarantee of carcinogenic activity, polynuclear aromatic
hydrocarbons known to be carcinogenic are fluorescent. In this
context, both Kennaway & Heiger (1930) and Berenblum et al. (1947)
suggested that these compounds -- even in very small quantities --
might be discovered by this method. The 4 bitumen fractions used in
the previous study were injected subcutaneously -- once or repeatedly
-- in the same strain of mice, at various doses (Simmers, 1966). A
variety of benign and malignant tumours resulted, both at the
injection site and in distant organs. The dose seemed to be more
important for tumour formation than the duration of exposure.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Four road-bitumens of different geographical and
manufacturing origin were applied to the skin of, or injected
intra-muscularly into, mice and rats. In some of the test-animals,
tumours were found at the site of application. Fumes from heated
coal-tar and from a blown bitumen used as a roofing bitumen did not
induce cancers of the lungs in rats and guineapigs in inhalation
experiments lasting up to 2 years. However, the general typical
reactions found in lung-tissue were the same as those described by
Simmers (1964). Condensates of the coal-tar fumes were highly
carcinogenic when applied to the skin and intramuscularly in mice;
condensates of the blown bitumens fumes were non-carcinogenic to the
skin of mice and rabbits in similar tests.
Petroleum residues derived from cracking were painted 3 times a
week for a lifetime on the skin of albino mice. Depending on the
cracking process, the residues exhibited various degrees of
carcinogenic activity, but none were as active as a higher temperature
generated coal-tar. Only those fractions distilled from the tar above
370°C showed carcinogenic activity. Blending with a noncarcinogenic
oil increased the carcinogenic activity of one bitumen, possibly
because of better skin penetration (Smith et al., 1951).
Kireeva (1968) painted groups of white SS-57 mice, once a week
throughout the life span, with 40% solutions in benzene of various
bitumens derived from Ukrainian crudes and coal-tar pitch. The
control-group was painted with benzene only. With coal-tar pitch, skin
tumours appeared in 88.4% of the animals; with bitumens prepared from
cracking residues, they occurred in 9.5-18.4%, and with straight-run
bitumens, depending on its origin, in only 0.0-4.6% of the animals.
Benzene solutions of 8 bitumens of different geographical origin
and 2 coal-tar pitches were applied twice weekly to the skin of Swiss
albino mice throughout their lifetime. Benzene alone was applied to
control animals (Wallcave et al., 1971). The polynuclear aromatic
hydrocarbon content of the coal-tar pitches was found to be several
orders of magnitude greater than that of the bitumens examined. Only
one carcinoma and 5 papillomas were observed in 218 mice treated with
bitumens, whereas over 90% of the coal-tar pitch treated animals
developed such tumours. Again, it was suggested that the tumour
incidence depended on the polynuclear aromatic hydrocarbon content of
the product tested.
Benzene solutions of the bitumen or polynuclear aromatic fractions
of Athabasca tar sands were not mutagenic for Salmonella typhimurium.
This may have been a reflection of the complex interactions occurring
with such hydrocarbon mixtures (Shahin & Fournier, 1978).
To summarize, in animal studies, some bitumens have been shown to
possess some carcinogenic activity, when applied to the skin, while
inhalation studies with bitumen vapours have proved to be negative.
The carcinogenicity of bitumens depends to a certain degree on the
method of production (cracking, blowing) or mixing. "Cutback" with
high aromatic oil or coal-tar facilitates skin-contact and increases
the carcinogenicity of the mixture. The carcinogenic activity,
however, is low in comparison with that of coal-tar. Moreover, as Siou
(1972) in a literature study on bitumens concluded, it is difficult to
extrapolate from these animal data to man, because the contact with
bitumen, even in occupational exposures, is of a quite different order
to regularly repeated skin application throughout the life span of a
test animal.
5.7 Effects on Man
5.7.1 Epidemiological studies
5.7.1.1 Occupational exposure
Henry (1947), in his analysis of 3753 cases of skin cancer, found
only one case in which bitumen might have been involved. The man had
worked for 23 years as a road-worker and had been exposed to coal-tar
in the earlier years.
The health and the causes of death of 96 workers who had worked in
a bitumen plant since it opened, including those who had left the
company was reviewed by Hoogendam (1962). Exposures ranged up to 40
years. Thirty-nine workers had been exposed for more than 20 years and
at least 15 had been exposed for more than 30 years. No significant
differences were found in theft general health pattern and no cases of
skin tumours were found. One man, who had worked for 40 years in this
plant died at the age of 55 of bronchial carcinoma. It is impossible
to determine whether there was a relation with his work in this case.
Vital capacities and forced expiratory volume (FEV) of those still
working did not differ from those of a control group.
The mortality rate of refinery workers was compared with those of
other workers in a study on 15 437 employees of an oil refinery over
the 29-year period 1935-63. A comparison was also made with data from
a similar group of the outside population. The incidence of lung
cancer was no greater in the refinery operators than in the other
groups (Baird, 1967).
Baylor & Weaver (1968) compared the health of 462 asphalt workers
in 25 oil refineries with that of 379 controls. Each of the asphalt
workers had been engaged in this type of work for at least 5 years
with an average of 15.1 years, service. No significant differences in
health were found between the 2 groups. The authors also studied the
medical literature of the 20 years preceding theft publication and
were unable to find one single case of lung or skin cancer that could
be attributed to petroleum bitumens. Furthermore, an extensive search
for information on the health of workers was carried out with road
construction firms (31 companies in 24 states with 11 478 man-years of
asphalt working and 15 boards of health of the state highway
commissions), roofing industries (3 firms with over 1100 long-term
bitumen workers), and bitumen trucking firms (with over 5000 drivers).
This gave no indication that bitumen constituted a health hazard or
that there were cancers of the skin or lungs in these groups that
could be attributed to working with bitumen. Similar indirect
information was obtained from 6 large insurance companies. The authors
concluded that the bitumens in current use did not present a
significant health hazard and that the study indicated that the
carcinogenic or other harmful properties of most commercial asphalts
under the present commercial usage -- if present at all -- are likely
to be of a very low order.
Studies conducted on behalf of the API did not reveal any
occupational health hazards for a "medium-exposure group" of petroleum
refinery workers. This group included asphalt workers, grease, and
lubricant workers (Tabershaw/Cooper Associates Inc. 1974/75).
Hermann (1975) and Hettche (1963) drew similar conclusions to
those of Baylor & Weaver (1968) in inferring that bitumens including
blown grades, were biologically inactive and that vapours from hot mix
plants were not carcinogenic.
5.7.1.2 General population exposure
Measurement of emissions of polynuclear aromatic hydrocarbons from
various industrial processes, including bitumen "blowing" and the
manufacture of asphalt hot-road-mixture, revealed that these
industrial processes were not major sources of benzo(alpha)pyrene
emissions (used as an indicator for polynuclear aromatic hydrocarbons
emission) and certainly emitted less than residential and small
industrial coal-burning furnaces (Von Lehmden et al., 1965).
In the literature, the possibility is mentioned that vapours
emanating from asphalt, or dust arising from it might contribute to
the overall incidence o f cancer of the respiratory tract (Hueper,
1961; Berge, 1969). There are not, however, any data to substantiate
this assumption and, in extrapolating the results of animal testing on
bitumen-vapour inhalation, the chances of such an effect occurring
among the general population seem quite remote. It can be presumed
that a lot of the confusion has been generated by the former use of
coaltar and mixed grades for this purpose, a fact which clearly has
not always been taken into consideration by those working in the
field.
In the USSR, however, the use of "carcinogenic" road material
(that is material containing benzo (a) pyrene) as the upper layer of
roads is forbidden in heavily populated areas. In practice, this means
that coal-tar must not be used for this purpose and that petroleum
bitumen should be used instead (Gorbov & Fomenko, 1962).
5.7.1.3 High (accidental) exposure
Burrell (1957) found a very high incidence of oesophageal cancer
in a group of Bantu confined to one location in East London (South
Africa). The author related this to the long-term preparation and use
of the soporific alcoholic concoction "cidiviki", which was locally
fermented in drums still containing a ¨-inch coating of"cutback"
bitumen. The author presumed that enough carcinogenic material could
have leached out into the brew to have at least acted as a
cocarcinogen.
The most common accidental over-exposure in handling bitumen is
the occurrence of burns from hot bitumen splashes. A few cases,
however, have been described, in which a skin tumour developed within
1-3 months of a burn caused by hot bitumen, coal-tar, or crude oil
(Bang, 1923; Huguenin, 1925; Gunsett, 1930; Sträuli 1957). Considering
the total number of such bums, only a very small proportion develop
into rumours and these cases relate to combinations of such burns with
fresh scar tissue and/or lesions of the mucous membranes. Tumours have
been described in similar situations following heat burns by wood,
welding electrodes, etc. (Gunsett, 1930; Sträuli, 1957). Such skin
tumours can occur in burn scars and are often multifactorial (Emmett,
1975).
In relation to the vast amount of bitumen that has been used for
many decades, the reports of tumours are extremely scarce. In fact, it
can be postulated that, though, from animal testing, it is known that
some bitumens are weak carcinogens, there is no evidence from normal
occupational exposure to indicate that these compounds are a
carcinogenic hazard to the skin or the respiratory tract. This would
also indicate that the present pattern of use of bitumens would not
cause any hazard, whatsoever, for the general population.
The Asphalt Institute studied emissions from the hot-mix process
for the manufacture of paving asphalts (Puzinauskas & Corbett, 1975).
The concentration of polynuclear aromatic hydrocarbons in the
particulate emission was low at 340 mg/m3 and the average
concentration of benzo(alpha)pyrene was very low at 13 ng/m3.
5.7.2 Clinical studies
These relate mainly to bitumen skin burns and will not be
considered here. Other clinical data have been included in the
description of the epidemiological data.
The skin of fair-haired persons might be more prone to react
adversely to repeated prolonged exposure to UV-radiation in sunlight
in combination with bitumen exposure (Smiley, 1951; Kinnear et al.
1954; Emmett, 1975).
6. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO CRUDE OILS AND
SELECTED PETROLEUM PRODUCTS
6.1 Crude Oils
Crude oil is normally handled in a closed system from the oil
well, via storage tanks, pipelines, and shipment by tankers to the
refineries. Under these conditions, health hazards to, and death of
workers involved in these operations will occur only when a serious
breakdown or leakage occurs. Volatile components escaping at well
heads, at pump glands, or through vents in storage tanks and ships'
tanks may, under certain conditions, constitute a similar health
hazard; hydrogen sulfide, if present, is the most acutely toxic
component but detailed consideration is outside the scope of this
review. These volatile components may also contribute to the pollution
of the atmosphere in storage or pumping areas. To the general
population, this is mainly a nuisance problem, because of the odour
from the hydrogen sulfide and mercaptans involved. Crude oil pollution
of seas and inland waterways as a result of accidents with crude oil
tankers or pipelines may, at times, cause a major and sudden
environmental hazard. Tank washings from tankers not using the
"load-on-top" system are another source of pollution of the sea with
crude oils. However, this particular aspect falls outside the scope of
this review.
Atmospheric concentrations of the volatile components of crude oil
were found to be lower near marine drilling rigs than land-based rigs.
Differences in temperature and air movement have been shown to be the
main causes.
The lower temperature of marine pipelines encourages
solidification of waxy compounds of the crude oil on their inner
surfaces. During their repair, the clothes and skin of workers may be
contaminated by these compounds (Alekperov et al., 1974).
6.2 Petroleum Solvents
Though petroleum products are widely used, they do not generally
present a health risk for the general population, as their volatility
prevents them accumulating in the ambient air in concentrations high
enough to cause adverse health effects. However, if improperly used in
closed, poorly ventilated rooms, they may become a cause of accidental
acute poisoning. Under these conditions, the lower-boiling solvents
may also present a fire hazard.
Cases of accidental ingestion may occur, especially in children.
Serious and even fatal lung disease may develop when aspiration in the
lungs occurs. Absorption from the gut, however, is generally not a
serious health hazard.
Substances with a strong odour may cause a nuisance, when present
in the ambient air, even in very low concentrations. The same applies
to the possible contamination of drinking-water through leakage of
petroleum solvents from containers.
Health impairment due to occupational exposure to petroleum
solvents occurs only infrequently in normal work practice. Repeated
skin contact may result in contact irritative dermatitis and only
rarely in contact allergic dermatitis. Continuous day-to-day exposure
to excessive vapour concentrations (which usually occur in poorly
ventilated workshops) may give rise to general non-specific symptoms
of ill-health. Some solvents, however, have a specific systemic
effect, e.g., benzene (bone marrow depression, leukaemogenesis) and
n-hexane (polyneuropathies). Polyneuropathies also occur in cases of
abusive use of petroleum solvents by "sniffers" and addicts.
Accidental exposure to excessive vapour concentration may cause
narcosis, which can be followed very rapidly by respiratory arrest and
death. Accidental over-exposure of the skin, especially if the solvent
is allowed to remain in contact with the skin, may cause skin
irritation, possibly leading to chemical burns.
Blood dyscrasia does not occur through exposure to petroleum
solvents that do not contain benzene. The specific long-term health
hazards of exposure to benzene and petroleum solvents that contain a
substantial percentage of benzene will not be discussed in this
document.
The high aromatic residues that are still used, occasionally, as
solvents for specific purposes may pose a carcinogenic risk to the
skin or on inhalation, if such contact is intensive and prolonged.
6.3 Lubricating Base Oils, Greases, and Waxes
This group of products is unlikely to present a health risk for
the general population, even with gross accidental over-exposure.
Unrestricted and indiscriminate oral or nasal administration of white
medicinal oil can occasionally give rise to the occurrence of lipid
pneumonia, when pre-existent disease or other conditions predispose to
the entry of mineral oil into the respiratory tract.
Prolonged and intensive contact of the skin with metal-working
oils during occupational exposure results in a high incidence of skin
disorders and relatively more cases of skin cancer have occurred in
such occupations. Improvements in both industrial and personal hygiene
have reduced the incidence of malignant and non-malignant diseases of
the skin. Results from animal studies indicate that substitution of
the oils, formerly used, by more refined products may significantly
contribute to the prevention of skin cancer caused by exposure to oil
under poor standards of industrial hygiene. Because chronic lung
disease following exposure to oil mist is extremely rare and an
increase in the incidence of pulmonary carcinoma has only been
reported under exposure conditions where skin cancer occurs, it seems
reasonable to assume that these occupational diseases can be prevented
by suitable preventive measures.
6.4 Bitumen
The health risks associated with bitumen appear to be minimal,
because contact is unlikely. Occupational exposure is not associated
with an increase in cancer of the skin or the respiratory tract. The
most significant occupational hazard is burning of the skin from
splashes of heated bitumen.
Certain bitumen grades, however, such as those derived from
cracked oils, and those mixed with coal-tar or high aromatic extracts,
may have a carcinogenic potential and, therefore, require special
precautions in handling.
7. CONTROL MEASURES
7.1 General
Every effort should be made not to contaminate workers, the work
place, or the general environment with petroleum products. Proper
design of machinery, equipment and the workshop, enclosure of
processes, adequate ventilation, provision of protective clothing and
adequate facilities for personal hygiene, suitable education and
supervision, and development of safe working procedures are essential
basic requirements; good hygienic work practices can achieve a great
deal in protecting the worker.
Care should be taken, when developing products, to ensure that
levels of the most toxic components, e.g., benzene, n-hexane, and
polynuclear aromatic hydrocarbons are known, so that proper controls
can be devised for the use of the products.
Products containing such highly toxic components should, when
possible, be avoided and alternatives sought, where exposure is
unavoidable or is likely to occur. Products which, during use, may
generate more and highly toxic contaminants should be identified and
efforts made to reduce contamination. Care should be exercised when
handling and using such products and consideration given to the need
for renewal of products at appropriate intervals. In relation to
metal-working, the danger of repeatedly "topping-up" machinery over
long periods with a product, and of mixing different products in one
machine, is emphasized.
Where contact is unavoidable, suitable protective equipment should
be used, although this should always be used as the last resort. Where
skin contact is inevitable, efforts should be made to limit or avoid
not only benzene, n-hexane, and polynuclear aromatic hydrocarbons
but also any additives that may have an adverse effect on the skin.
The use of abrasives and solvents in cleansing the skin should be
discouraged.
In the less developed countries, there is often a lack of
awareness concerning the need for proper control measures when
handling petroleum products. In these countries, the health education
of employers and workers should be promoted with reference to the
products. Adequate control programmes should be implemented using
known techniques. Whenever necessary, these should be modified to fit
the particular circumstances of the country.
In addition, proper planning is necessary to control the disposal
of the many types of waste oil products so that environmental
contamination is avoided. Thought must also be given to the need to
control the siting of housing and amenities in relation to petroleum
refineries and petrochemical plants.
7.2 Petroleum Solvents
Excessive exposures to these products should be avoided. Proper
education of manufacturers and users and appropriate labelling of
consumer products are important. Regular control of workroom
atmospheric concentrations of petroleum solvents is indicated,
especially in smaller workshops.
Petroleum solvents are, with the exception of benzene and
n-hexane, less toxic than most other solvents and, with suitable
precautions, can be safely used. For this reason, and because they are
cheap, they are widely used in industry and their replacement by other
solvents is unlikely in the foreseeable future.
7.3 Lubricating Base Oils, Greases, and Waxes
The only way in which members of the general population could,
under normal circumstances, be over-exposed to any of this group of
compounds would be in the indiscriminate use of medicinal liquid
paraffin. Excessive use -- especially self-administered -- of this
material, intranasally or via the oral route, by debilitated or
dysphagic patients should be discouraged.
The main possibilities of over-exposure occur in the occupational
situation. Without going into detail, the following factors should be
considered with the aim of avoiding hazardous exposures:
(a) Avoid contact by technological means including: proper design
of equipment, machinery, and workshop; proper design and operation of
general and exhaust ventilation systems in places where oil mist
generation cannot be avoided; use of base oils with the lowest content
of polynuclear aromatic hydrocarbons that can practically be achieved
by suitable refining processes; limiting of the concent of additives
that have adverse effects on the skin; frequent changing of oil in
situations where carcinogenic compounds might be generated by heat;
avoidance of the reuse of used oils, without refining, for other
purposes where they might cause a health hazard; education and
instruction in all these safe-working procedures, and supervision of
their proper execution;
(b) Use of safe-working procedures and, where required, protective
clothing and proper protective equipment; availability of general
hygienic facilities including wash-basins in or near the work-place,
shower facilities, lockers for private clothing outside the
contaminated area; regular changing and laundering of working clothes
and underwear; the use of suitable emollient skin creams and in some
instances barrier creams. Abrasives and solvents should not be used
for cleaning the skin.
(c) Periodic medical examinations.
These precautions have been compiled and adapted from the
following references: Kipling (1968); UK Medical Research Council
(1968); Cruickshank (1969); Desoille et al. (1973); BRMA (undated).
Manufacturer can contribute by ensuring that these precautions are
observed in their own premises and by giving the proper information
and guidance to the consumer (see also Eckardt, 1967).
Government actions vary from country to country depending on local
legislation and practice. They may range from supervision and control
of the measures taken by industry to the issue of directives or codes
of practice. Cautionary notices can be issued (e.g., HMSO, 1979),
which have to be displayed in the working place, as well as leaflets
for the workers (e.g., HMSO, 1967). Diseases caused by mineral oil may
be notifiable as occupational diseases, according to national
legislation.
Consumer education on the possible hazards of the products used
and their safe handling can best be done by the manufacturing
industries, when possible in cooperation with relevant Government
agencies.
Medical action in this field would consist of cooperating in the
team supervising the industrial hygiene of the operation, in
pre-placement examination and selection, and later in the periodic
medical follow-up of the exposed workers.
Various other organic liquids meet requirements for lubricating
oils and greases. Carboxylic esters are generally used. These
synthetic lubricants, however, are not in abundant supply, are
expensive, and are not likely to be widely used outside their specific
field of application. Replacement products are sometimes more
hazardous. For instance, cutting oils may be replaced by mixtures
containing ethanolamine that give rise, under certain conditions, to
the formation of nitrosamines.
When safe lubricating base oils are used there is, on the other
hand, no reason from an occupational health point of view to use such
alternatives, not even in places where long-term intensive skin
contact and/or oil mist exposure might take place. In these cases,
industrial hygiene should be improved and personal hygiene should be
closely supervised, regardless of the product used, to avoid excess
exposure.
7.4 Bitumen
In road-building which is the major application of bitumen,
alternative materials such as plastics and resins are far too
expensive for general use. Coal-tar, which was used previously,
presents a greater environmental health hazard than bitumen. Other
alternatives would be concrete and bricks.
In various other applications, such as flooring, roofing,
protective coatings, and adhesives, alternative materials are
available, but these are not always superior in quality and/or
competitive in price.
REFERENCES
ABBRITTI, G., SIRACUSA, A., CIANCHETTI, C., COLI, C. A., CURRADI, F.,
PERTICONI, G. F., & De ROSA, F. (1976) Shoe-makers' polyneuropathy
in Italy: The aetiological problem. Br. J. ind. Med., 33: 92-99.
ACGIH (1971a) Documentation of the threshold limit values,
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 19-20.
ACGIH (1971b) Documentation for TLV -- Supplements for those
substances added or changed since 1971, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists, p. 341.
ACGIH (1974) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1974, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978a) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1978, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978b) Air sampling instruments for evaluation of atmospheric
contaminants. 5th ed., Cincinnatti, OH, USA, American Conference
of Governmental Industrial Hygienists.
ADLARD, E. R. (1972) A review of the methods for the identification of
persistent hydrocarbon pollutants on seas and beaches. J. Inst.
Petrol., 58: 63-74.
AIDIN, R. (1958) Petrol-vapour poisoning. Br. med. J., ii: 369-370.
AIHA (1976) Direct reading colorimetric indicator tubes manual,
Akron, OH, USA, American Industrial Hygiene Association.
AINSWORTH, R. W. (1960) Petrol-vapour poisoning. Br. med. J.,
i: 1547-1548.
ALEKPEROV, I. I., AMIROV, R. O., POVOVAROV, V. D., & LOSEVA, I. E.
(1974) [Hygiene of work at exploitation of marine oil deposits.]
Moscow, Idz. "Nedra" (in Russian).
ALLEN, N. (1975) Chemical neurotoxins in industry and the environment.
In: Tower, D. B., ed. The nervous system, Vol. 2, The clinical
neurosciences, New York, Raven, pp. 235-248.
ALTENKIRCH, H., MAGER, J., STOLTENBURG, G., & HELMBREHT, J. (1977)
Toxic polyneuropathies after sniffing a glue thinner. J. Neurol.,
214: 137.
ANDRÉ, M. L. & O'NEAL, M. J. (1959) Mass spectrometric analyses of
medium viscosity lubricating oils. Anal. Chem., 31: 164-169.
ANON, (1973) Evaluations of world's important crudes. Oklahoma, USA,
The Petroleum Publishing Company (Reprinted from Oil Gas J.).
ANON (1974) Exposure to petrol fumes. Br. med. J., ii: 115.
ANON (1975) Benzpyrene hydroxylase - innate determinant of PAH
toxicity? Food cosmet. Toxicol., 12: 264-268.
ANTONOV, A.M. & LINTS, A.M. (1960) The blastomogenic action of natural
Saratov oil. Problems Oncol., 6: 1629-1634.
API (1965) Toxicological review. Aromatic petroleum naphtha. 2nd
ed., New York, American Petroleum Institute, pp. 5.
API (1967a) Toxicological review. Gasoline, 1st ed., New York,
American Petroleum Institute, pp. 5.
API (1967b) Toxicological review. Kerosine, 2nd ed., New York,
American Petroleum Institute, pp. 4.
API (1969a) Final Report of the task force on used oil disposal.
Washington, DC, American Petroleum Institute, pp. 44.
API (1969b) Toxicological review. Petroleum naphthas, 1st ed., New
York, American Petroleum Institute, pp. 7.
API (1974) Mutagenicity study of thirteen petroleum fractions,
Washington, DC, American Petroleum Institute (API U-150-14, The
Hine Laboratories Inc. Report No. 4).
API (1975a) Review of analytical methods for determination of oil and
grease in waters produced from oil and gas extraction operations.
Prepared by the Task Group on Analytical Methods July 14th 1975,
New York, American Petroleum Institute, pp. 38.
API (1975b) Mutagenic evaluation of rubber solvent (CHF-32-263),
W/filtered rubber solvent (Summary and analysis), Washington,
DC, American Petroleum Institute (Litton Bionetics Inc. Report).
API (1975c) Research report. Mutagenic evaluation of 60 solvent
(CHF-33-148) (Summary and Analysis), Washington, DC, American
Petroleum Institute (Litton Bionetics Inc. Report).
API (1977a) Research report. Mutagenicity evaluation of kerosene.
Final report (Summary and Analysis), Washington, DC, American
Petroleum Institute, 3 pp. (Litton Bionetics Inc. Project
No. 2697).
API (1977b) Research report. Teratology study in rats: Stoddard
solvent. Final report (Summary and Analysis), Washington, DC,
American Petroleum Institute, 5 pp. (Litton Bionetics Inc. Project
No. 20698-2).
API (1978a) Mutagenicity evaluation of Stoddard solvent (Summary),
Washington, DC, American Petroleum Institute (Litton Bionetics
Inc. Report no. API-PS-4).
API (1978b) Research report - Teratology study in rats: Rubber
solvent. Final report. Litton, API (Full Report and Analyses).
API (1978c) Teratology study in rats: Xylene. Final Report,
Washington, DC, American Petroleum Institute, 39 pp. (Litton
Bionetics Inc. Project No. 20698-5).
API (1978d) Research report. Mutagenicity of xylene. Final report
(Full report and Analytical).
API (1978e) Teratology study in rats: Toluene. Final report (Summary
and Analysis), Washington, DC, American Petroleum Institute, 2 pp.
(Litton Bionetics Inc. Project No, 20698-4).
(1978f) Mutagenicity evaluation of toluene (Summary and Analysis),
Washington, DC, American Petroleum Institute, 3 pp. (Litton
Bionetics Inc. Project No. 20847).
API (1979a) Research report. Teratology study in rats: Kerosene.
Final report (Summary and Analytical), Washington, DC, American
Petroleum Institute 2 pp. (Litton Bionetics Inc. Project
No. 20698-10).
API (1979b) Research report. Inhalation teratology study in rats: VM
and P naphtha, Final report, Washington, DC, American Petroleum
Institute, 18 pp. (Litton Bionetics Inc. Project No. 21035-02).
API (1979c) Research report. Teratology study in rats: n-Hexane.
Final report, Washington, DC, American Petroleum Institute,
24 pp. (Litton Bionetics Inc. Project No. 20698-9).
API (1979d) In vitro and in vivo mutagenicity studies: New oil
composite. Final report, Washington, DC, American Petroleum
Institute (Hazleton report).
ARCOS, J. C. & ARGUS, M. F. (1974) Chemical induction of
cancer-structural bases and biological mechanisms, Vol. 11A,
New York and London, Academic Press.
ASK-UPMARK, E. (1955) Bronchial carcinoma in printing workers. Dis.
Chest., 27: 427-235.
ASTM D2820 (1972) Standard method of test for Cl through C5
hydrocarbons in the atmosphere by gas chromatography,
Philadelphia, Am. Soc. for Testing and Materials.
ASTM D1605-60 (1973) Standard recommended practices for sampling
atmospheres for analysis of gases and vapours, Philadelphia,
Am. Soc. For Testing and Materials.
ASTM D510 (1974) Standard methods of sampling water, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2682 (1974) Standard method of test for polynuclear
aromatic hydrocarbons in air particulate matter, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2778 (1974) Standard method of test for solvent extraction or
organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ASTM D3370 (1976) Standard method of test for solvent extraction
of organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ÅSTRAND, I., KILBOM, A., & ÖVRUM, P. (1975) Exposure to white spirit.
I. Concentration in alveolar, air and blood during rest and
exercise. Scand. J. Work environ. Health, 1: 15-30.
ÅSTRAND, L., ENGSTRÖM, J., & OVRUM, P. (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man.
Scand. J. Work Environ. Health, 4: 185-194.
AULD, S. J. M. (1950) Environmental cancer and petroleum. J. Inst.
Petrol., 36: 235-253.
AVELLAN, L., JACOBSON, B., & JOHANSON, B. (1967) Carcinoma of scrotum
induced by mineral oil. Scand. J. plast. reconstr. Surg.,
1: 135-140.
AXELSON, O., HANE, M., & HOGSTEDT, C. (1976) [Case descriptions of
chronic psycho-organic syndrome in house painters.]
Läkartidningen, 73: 317-318 (in Swedish).
BADGER, G. M. (1962) Mode of formation of carcinogens in human
environment. Nat. Cancer Inst. Monogr., 9: 1-16.
BAIRD, V. C. (1967) Effects of atmospheric contamination on cancer
mortality in petroleum refinery employees. J. occup. Med.,
9: 415-420.
BALDWIN, R. W., CUNNIGHAM, G. J., & PRATT, D. (1964) Carcinogenic
action of motor engine oil additives. Br. J. Cancer,
18: 503-507.
BANG, F. (1923) Contribution a l'étude de la cancérisation de la
cellule et du temps d'éclosion des tumeurs malignes. A propos d'un
cas de "cancer aigu" du goudron chez un ouvrier. Bull. Assoc. Fr.
Étud. Cancer, 12: 184-199.
BARANOVA, V. G. & LOGINOVA, N. K. (1977) [Determining cyclopentadiene
in the air of industrial premises.] Gig. Tr. prof. Zabol.,
No. 3, pp. 54-55 (in Russian).
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of ethyl
benzene, styrene and alpha-methylstyrene in man. Am. Ind. Hyg.
Assoc. J., 31: 206-209.
BARRIENTOS, A., ORTUNO, M. T., MORALES, J. M., TELLO, F. M., &
RODICIO, J. L. (1977) Acute renal failure after use of diesel fuel
as shampoo. Arch. intern. Med., 137: 1217.
BATT-NEAL, L. & WOLMAN, M. (1977) Tumors and amyloidosis in mice
painted with crude oil found on bathing beaches. Bull. environ.
Contam. Toxicol., 18: 385-391.
BAYLOR, C. H. & WEAVER, N. K. (1968) A health survey of petroleum
asphalt workers. Arch. environ. Health, 17: 210-214.
BELINKY, B. R. (1980) In: Dolberg, D. D. & Verstuyft, ed. Analytical
techniques in occupational health chemistry. Washington, DC,
ACS, (ACS Symposium Series 120).
BELL, J. (1876) Paraffin epithelioma of the scrotum. Edin. med. J.,
22: 135.
BERENBLUM, I., HOLIDAY, E. R., & JOPE, E. M. (1947) Some physical
methods of investigating carcinogenic hydrocarbons. Br. med.
Bull., 4: 326-331.
BERGE, H. (1969) [Harmful emissions in and around cities.]
Vitalstoffe, 14: 161-163 (in German).
BERGEL, F. (1974) Carcinogenic hazards in natural and man-made
environments. Proc. R. Soc. (London), 185: 165-181.
BERTINE, K. K. & GOLDBERG, E. E. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BESKROVNAJA, N. I., HRUSTALEVA, G. F., ZIGULINA, G. A., & DAVYDKINA,
T. I. (1979) [The gynaecological sick rate of female workers in
the rubber industry.] Gig. Tr. prof. Zabol., No. 8, pp. 36-38
(in Russian).
BILIMAIER, D., YEE, H. T., ALLEN, N., CRAFT, B., WILLIAMS, N.,
EPSTEIN, S., & FONTAINE, R. (1947) Peripheral neuropathy in a
coated fabrics plant. J. occup. Med., 16: 665-671.
BINGHAM, E. & FALK, H. L. (1969) Environmental carcinogens. The
modifying effect of co-carcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BINGHAM, E. & HORTON, A. W. (1966) Environmental carcinogenesis:
experimental observations related to occupational cancer.
Adv. Biol. Skin, 7: 183-193.
BINGHAM, E. & WORD, P. J. (1977) Co-carcinogenic effects of n-alkanes
and ultraviolet light on mice. J. Natl Cancer Inst.,
38(4): 1099-1101.
BINGHAM, E., HORTON, A. W, & TYE, R. (1965) The carcinogenic potency
of certain oils. Arch. environ. Health, 10: 449-451.
BISHOP, P. G. C. (1940) Oil aspiration pneumonia and pneumolipoidosis.
Ann. int. Med., 13: 1327-1359.
BLATTNER, R. J. (1951) Kerosene poisoning. J. Pediatr., 39: 391-392.
BOEHME, H. & HUEHNERMANN, W. (1966) Examination of mineral oil
products in the (West) German Pharmacopoeia, 7th edition. Arch.
Pharm., 299: 368-80D.
BOENHEIM, A. F. & PEARSON, A. J. (1973) Petroleum hydrocarbon
solvents. In: Modern petroleum technology, 4th ed., Barking,
Applied Science Publishers Ltd.
BOGOVSKI, P. A., EISEN, O. G., & ARRO, I. H. (1960) [On the
carcinogenic action of some chromatographic fractions of
chamber-oven tar obtained from Estonian oil-shale.] Vop. Onkol.,
6: 34-42 (in Russian).
BOGOVSKI, P. A., GORTALUM, G. M., & KOZHEVNIKOV, A. V. (1963)
Decancerigenization of some shale processing products. Acta Un.
Int. Cancer, 19: 481-482.
BOHLEN, P., SCHLUNEGGER, U. P., & LÄUPPI, E. (1973) Uptake and
distribution of hexane in tissues. Toxicol. appl. Pharmacol.,
25: 242.
BOITNOTT, J. K. & MARGOLIS, S. (1966a) Mineral oil in human tissues.
I. Detection of saturated hydrocarbons using thin-layer
chromatography. Bull. John Hopkins Hosp., 118: 402-713.
BOITNOTT, J. K. & MARGOLIS, S. (1966b) Mineral oils in human tissues,
II. Oil droplets in lymphnodes of the porta hepatis. Bull. John
Hopkins Hosp., 118: 414-422.
BORRIE, J. & GWYNNE, J. F. (1973) Paraffinoma of lung: Lipoid
pneumonia. Report of two cases. Thorax, 28: 214-221.
BOURNE, L. B. (1967) Skin diseases from oils - causes and prevention,
London, British Safety Council, 11 pp.
BOUTWELL, R. K. & BOSCH, D. K. (1959) The tumor-promoting action of
phenol and related compounds for mouse skin. Cancer Res.,
19: 413-424.
BP (1973) Health guide to BP petroleum products, London, The British
Petroleum Company Ltd, pp. 26.
BP (1975) Trace elements and other elements in crude oil:
A literature review, Sunbury, The British Petroleum Company Ltd.
BRIDGE, J. C. & HENRY, S. A. (1928) Industrial cancers. In: Report of
the International Conference on Cancer, London, Bristol, John
Wright, pp. 258-268.
BRMA (undated) Mineral oils, London, British Rubber Manufacturers
Association Ltd.
BROMER, R. S. & WOLMAN, I. J. (1939) Lipoid pneumonia in infants and
children. Radiology, 32: 1-7.
BROWN, A. J., WALDRON, H. A., & WATERHOUSE, J. A. H. (1975) Report on
a study of occupational skin cancer with special reference to
scrotal cancer, Birmingham, UK, University of Birmingham.
BROWN, A. L. & BISKIND, G. R. (1941) Differential diagnosis between
lipid pneumonia and pulmonary neoplasm. J. Am. Med. Assoc.,
117: 4-6.
BROWNING, E. (1959) Toxic solvents: a review. Br. J. ind. Med.,
16: 23-39.
BROWNING, E. (1965) Toxicity and metabolism of industrial solvents,
Amsterdam, London, New York, Elsevier Publishing Company.
BRUSKIN, Z. Z. & DEMCENKO, V. G. (1975) [External respiration and gas
exchange of workers who are subjected to the effect of lubricating
oil aerosols.] Gig. Tr. prof. Zabol., No. 4, pp. 28-30
(in Russian).
BRYAN, C. S. & BOITNOTT, J. K. (1969) Adenocarcinoma of the lung with
chronic mineral oil pneumonia. Am. Rev. Respir. Dis.,
99: 272-274.
BUKOVSKIJ, M. I., ZUKOV, V. L, KOZUHOVA, T. V., SANOCKIJ, I. V., &
SIDOROV, K. K., ed. (1978) [Maximum permissible concentrations
and guidelines for safety levels of hazardous substances in the
environment.] Severodoneck, All-Union Research Institute of
Safety Technique in the Chemical Industry, and Research Institute
of Labour Hygiene and Occupational Diseases, Academy of Medical
Sciences of USSR (in Russian).
BURRELL, R. J. W. (1957) Oesophageal cancer in the Bantu. S. A.
Tydskrif vir Geneeskunde, 31: 401-409.
BÜTTNER, G. (1974) Benzene in the work environment. Deutsche
Forschungsgemeinschaft, Bonn, FRG, Harald Boldt Verlag K. G.
Boppard, 63 pp.
CANNON, P. R. (1940) The problem of lipid pneumonia. J. Am. Med.
Assoc., 115: 2156-2179.
CAPELLINI, A., CHIAPPINO, G., & ZURIO, N. (1968) [Clinical and
experimental observations on so-called tricresylphosphate
polyneuritis.] Med. Lav., 59: 721-759 (in Italian with English
summary).
CARGILL, C. (1972) Polynévrites dans l'industrie du cuir et de la
chaussure: toxicité du n-hexane, Thèse, Paris VII.
CARITHERS, H. A. (1955) Accident prevention in childhood -- the
kerosene hazard. J. Am. Med. Assoc., 159: 109-11.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977a) Petroleum hydrocarbon
toxicity studies. XIV. Animal and human response to vapors of
"high aromatic solvent". Toxicol. appl. Pharmacol.,
41: 235-241.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977b) Petroleum hydrocarbon
toxicity studies. XV. Animal response to vapors of "high
naphthenic solvent". Toxicol. appl. Pharmacol., 41: 251-260.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977c) Petroleum hydrocarbon
toxicity studies. XVI. Animal response to vapours of Naphthenic
Aromatic Solvent. Toxicol. appl. Pharmacol., 41: 261-270
(contains references to the other studies in this series).
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971a) Specifications
for cutting oils with special reference to carcinogenicity. Ann.
occup. Hyg., 14: 171-179.
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971b) Specifications
for cutting oils with special reference to carcinogenicity.
J. Inst. Petrol., 57: 247-260.
CAVANAGH, J. B. (1973) Peripheral neuropathy caused by chemical
agents. In: C.R.C. Crit. Rev. Toxicol., 2: 365-417.
CIANCHETTI, C., ABBRITTI, G., PERTICONI, G., SIRACUSA, A., & CURRADI,
A. (1976) Toxic polyneuropathy of shoe-industry workers: A study
of 122 cases. J. Neurol. Neurosurg. Psychiatr., 39: 1151-1161.
CLAYTON, G. D. & CLAYTON F. E. (1978) Patty's industrial hygiene and
toxicology. 3rd revised ed., New York, Wiley & Sons.
COHR, K.-H. & STOCKHOLM, J. (1979) Toluene. A toxicological review.
Scand. J. Work Environ. Health, 5: 71-90.
COMSTOCK, B. S. (1977) A review of psychological measurements relevant
to central nervous system toxicity with special reference to
solvent inhalation. Clin. Toxicol, 11: 317-324.
CONCAWE (1981a) Exposure to atmospheric benzene vapour associated
with motor gasoline. The Hague (Concawe report No. 2/81).
CONCAWE (1981b) Guidelines for the determination of atmospheric
concentrations of oil-mists. The Hague (Concawe report
No. 1/81).
CONCAWE STUDY GROUP (1972) Methods for the analysis of oil in water
and soil, The Hague, Stichting Concawe, pp. 66 (Concawe report
No. 9/72).
CONCAWE TASK FORCE (1973) Disposal of used lubricating oil in Western
Europe, The Hague, Stichting Concawe, 59 pp. (Concawe report
No. 9/73).
CONCAWE TASK FORCE (1976) Methods for the determination of
hydro-carbons in the atmosphere, The Hague, Stichting Concawe,
pp. 23 (Concawe report No. 3/76).
CONTAMIN, F., GOULON, M., & MARGAIRAZ, A. (1960) Polynévrites
observées chez des sujets utilisant comme moyen de chauffage des
appareils à combusion catalytique de l'essence. Rev Neurol.,
103: 341-345.
COOK, J., CARRUTHERS, W., & WOODHOUSE, D. L. (1958) Carcinogenicity of
mineral oil fractions. Br. med. Bull., 14: 132-135.
CORPER, H. J. & FREED, H. (1922) The intratracheal injection of oils
for diagnostic and therapeutic purposes. J. Am. Med. Assoc.,
79: 1739-1743.
CRISP, A. J, BHALLA, A. K., & HOFFBRAND, B. I. (1979) Acute tubular
necrosis after exposure to diesel oil. Br. med. J.,
2(6183): 177.
CRUICKSHANK, C. N. D. (1969) Occupational cancer of the scrotum.
Br. med. j., 26: 249-250.
CRUICKSHANK, C. N. D. & GOUREVITCH, A. (1952) Skin cancer of the hand
and forearm. Br. J. ind. Med., 9: 74-79.
CRUICKSHANK, C. N. D. & SQUIRE, J. R. (1950) Skin cancer in the
engineering industry from the use of mineral oil. Br. J. ind.
Med., 7: 1-11.
DAESCHNER, C. W. Jr, BLATTNER, R. J., & COLLINGS, V. P. (1957)
Hydrocarbon pneumonitis. Pediatr. Clin. North Am., resp.
Disorders. February: 243-253, Philadelphia.
DANIEL, J. W., FRAZER, A. C., FRENCH, J. M., & SAMMONS, H. G. (1953)
The intestinal absorption of liquid paraffin in the rat.
Biochem. J., 54: 37p.
DARGENT, M., MARCEAU, M., JOSEPH, P., & FAUCON, M. (1967) Etude
experimentale sur les effets tumorigènes des lubrifiants utilisés
dans le décolletage des métaux. Rev. Lyonnaise Méd.,
16: 409-420.
DAS, B. S. & THOMAS, G. H. (1978) Fluorescence detection in high
performance liquid chromatographic determination of polycyclic
aromatic hydrocarbons. Anal. Chem., 50: 967-973.
DAVIS, A., SCHAFER, L. J., & BELL, Z. G. (1960) The effect in human
volunteers of exposure to air containing gasoline vapour. Arch.
environ. Health, 1: 548-554.
DECOUFLE, P. (1976) Cancer mortality among workers exposed to cutting
oil mist. Ann. N.Y. Acad. Sci., 271: 94-101.
DECOUFLE, P. (1978) Further analysis of cancer mortality patterns
among workers exposed to cutting oil mist. J. Natl Cancer Inst.,
61: 1025-1030.
De PIERRE, J. W. & ERNSTER, L. (1978) The metabolism of polycyclic
hydrocarbons and its relationship to cancer. Biochim. Biophys.
Acta., 473: 149-186.
DESOILLE, H., PHILBERT, M., RIPAULT, G., CAVIGNEAUX, A., & ROSSIGNOLI,
H. (1973) Action cancérogène des huiles minérales utilisées en
métallurgie. Arch. Mal. prof. 34: 669-680.
DESPIERRES, G., BENNET, P. A., PHELIP, H., & PLANTE, M. (1965) Cancer
alveolaire et inhalation d'huiles industrielles. J. Fr. Med.
Chir. Thorac., 19: 561-567.
Deutsche Forschungsgemeinschaft (1970) [Health hazardous substances
in the workplace.] Weinheim, Verlag. Chemie (in German).
DIETZ, W. A., KING, W. H. Jr, PRIESTLEY, W. Jr, & REHNER, J. Jr (1952)
Properties of high boiling petroleum products. Ind. Eng. Chem.,
44: 1818-1827.
DRASCHE, H., FINZEL, L., MARTSCHEI, H., & MEYER, R. (1974)
[Occupational health considerations in persons exposed to oil
mist.] Zbl. Arbeitsmed., 24: 305-312 (in German).
EBERT, A. G., SCHLEIFER, C. R., & HESS, S. M. (1966) Absorption,
disposition, and excretion of 3H-mineral oil in rats. J. Pharm.
Sci., 55: 923 -929.
ECKARDT, R. E. (1957) Carcinogenicity of petroleum products with
particular reference to the automotive industry. Ind. med. Surg.,
26: 396 -398.
ECKARDT, R. E. (1967) Cancer Prevention in the petroleum industry.
Int. J. Cancer, 2: 656-661.
EHRHARDT, M. & HEINEMAN, J. (1975) Hydrocarbons in blue mussels from
the Kiel Bight. Environ. Pollut., 9: 263-281.
ELY, T. S., PEDLEY, S. F., HEARNE, F. T., & STILLE, W. T. (1970) A
study of mortality symptoms and respiratory function in humans
occupationally exposed to oil mist. J. occup. Med.,
12: 253-261.
EMMETT, E. A. (1975) Occupational skin cancer: A review. J. occup.
Med., 17: 44-49.
ENGSTROM, K., HUSMAN, K., & RIIHIMÄKI, V. (1977) Percutaneous
absorption of m-xylene in man. Int. Arch. occup. environ. Health,
39: 181-189.
ESSO RESEARCH (1960) Unpublished report in: Toxicological evaluation
of some food additives including anticaking agents, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series,
No. 5, World Health Organization, Geneva.
ESSO (undated) This is about health-lubricating oils and cutting
fluids, London, Esso.
EVEN, R. (1947) Les pneumopathies huileuses de l'adulte. Soc. Med.
Hop. Paris, 63: 78-80.
EYRES, A. R. (1973) Codes of practice relating to metal working
fluids: the oil suppliers' view. J. Inst. Petrol., 59: 9-14.
FACQUET, M. J. & LANGEARD, Mme (1947) Deux cas d'opacités pulmonaires
du lobe moyen chez des chanteurs (pneumonie huileuse probable).
Bull. Mém. Soc. Méd. Hop. Paris, 63: 200-204.
FAO/WHO (1970) Toxicological evaluation of some extraction solvents
and certain other substances (Nutrition Meetings Report Series
No. 48a; WHO/Food Add./70.39).
FAO/WHO (1971) Specifications for the identity and purity of some
extraction solvents and certain other substances (Nutrition
Meetings Report Series No. 48b; WHO/Food Add./70.40).
FIELDNER, A. C., KATZ, S. H., & KINNEY, S. P. (1921) Permeation of
oxygen breathing apparatus by gases and vapours, Washington,
United States Bureau of Mines (Technical Paper 272).
FISCHER, H. G. M., PRIESTLEY, W. Jr, EBY, L. T., WANLESS, G. G., &
REHNER, J. Jr (1951) Properties of high boiling petroleum
products. Arch. ind. Hyg. occup. Med., 4: 315-324.
FLOODGATE, G. D. (1972) Microbiol degradation of oil. Mar. Pollut.
Bull., 3: 41-43.
FOE, R. B. & BIGHAM, R. S. (1954) Lipid pneumonia following
occupational exposure to oil spray. J. Am. Med. Assoc.,
155: 33-34.
FOKKENS, W., HOUWEN, J. W., HUENDER, H. G. P., KARPIAK-VAN-DRIEL, J.,
LIEM, T. H., & De WAARD, F. (1972) [Scrotal cancer and mineral
oils.] T. Soc. Geneeskd., 50: 343 (in Dutch).
FORBES, J. D. & MARKHAM, T. N. (1967) Cutting and grinding fluids in
chronic pulmonary airway disease. J. occup. Med., 9: 421-423.
FOURNAS, D. W. & HINE, C. H. (1958) Neurotoxicity of some selected
hydrocarbons. Arch. ind. Health, 18: 9-15.
FRANCO, G., MOGLIA, A., & GHITTORI, S. (1979) [Occupational neuropathy
due to cyclohexane.] Med. Lav., 70(2): 118-124
(in Italian).
FREIMAN, D. G., ENGELBERG, H., & MERRIT, W. H. (1940) Oil aspiration
(lipoid) pneumonia in adults -- A clinicopathologic study of
forty-seven cases. Arch. int. Med., 66: 11-38.
FÜHNER, H. (1921) [Narcotic action of gasoline and its components
(pentane, hexane, octane).] Biochem. Zeitschr., 115: 235-261
(in German).
GAMBERALE, F., ANNWALL, G., & HULTENGREN, M. (1975) Exposure to white
spirit. II. Psychological functions. Scand. J. Work Enrivon.
Health, 1: 31-39.
GARVIN, C. G. (1939) Lipoid pneumonia. Arch. int. Med., 64: 586-589.
GAULTIER, M., RANCUREL, G., PIRA, C., & EFTHYMIOU, M.-L. (1973)
Polynévrites et hydrocarbures aliphatiques. J. eur. Toxicol.,
6: 294-296.
GERARDE, H. W. (1959) Toxicological studies on hydrocarbons. V.
Kerosine. Toxicol. appl. Pharmacol., 1: 462-474.
GERARDE, H. W. (1960) Toxicology and biochemistry of aromatic
hydrocarbons in commercial solvents, Amsterdam, London, New
York, Princeton, Elsevier Publishing Company.
GERARDE, H. W. (1963) Toxicological studies on hydrocarbons. IX. The
aspiration hazard and toxicity of hydrocarbons and hydrocarbon
mixtures. Arch. environ. Health, 6: 329-341.
GERARDE, H. W., & GERARDE, D. F. (1961/1962) The ubiquitous
hydrocarbons (Reprinted from Assoc. Food Drug Off. United
States, Vols XXV and XXVI, 1961/1962).
GHAZAI, S. (1974) Sept cas de polynévrite dans une usine de chaussures
à Teheran. Arch. Mal. prof., 35: 552-553.
GIBSON, D. T., MAHADERAN, V., JERINA, D. M., YAGI, H., & YEH, H. J. C.
(1975) Oxidation of the carcinogens benzo(a)pyrene and
benzo(a)-anthracene to dihydrodiols by a bacterium. Science,
189: 295-297.
GILCHRIST, C. A., LYNES, A., STEEL, G., & WHITHAM, B. T. (1972) The
determination of polycyclic aromatic hydrocarbons in mineral oils
by thin-layer chromatography and mass spectrometry. Analyst,
97: 880-888.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1955) Cutting oils and
squamous-cell carcinoma. Part II: An experimental study of the
carcinogenicity of two types of cutting oils. Br. J. ind. Med,
12: 244-248.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1956) An evaluation of the
relative carcinogenicity of two types of cutting oils. A.M.A.
Arch. ind. Health, 14: 341-345.
GOLDSTEIN, D. H. & BENOIT, J. N. (1970) An epidemiologic study of an
oil mist exposure. Arch. environ. Health, 21: 600-603.
GONZALEZ, E.G. & DOWNEY, J. A. (1972) Polyneuropathy in a glue
sniffer. Arch. Phys. Med. Rehabil., 53: 333-337.
GOODWIN, T. C. (1934) Lipoid cell pneumonia. Am. J. Dis. Child.,
48: 309 -326.
GORBOV, V. A. & FOMENKO, V. N. (1962) [On the hygienic evaluation of
asphalt coverings as a possible source of air pollution with
carcinogens.] Gig. i Sanit., 27: 100-101 (in Russian).
GRAEF, I. (1939) Studies in lipid pneumonia. Arch. Pathol.,
28: 613-667.
GREENBERG, M. (1972) A proportional mortality study of a group of
newspaper workers. Br. J. in Med., 29: 15-20.
GRIMMER, G. (1972) [The quantitative determination of polycyclic
aromatics by capillary gas chromatography.] Erdöl Kohle,
25: 339-343 (in German).
GRIMMER, G. (1979) Distribution of polycylic dromatic hydrocarbons in
environmental samples. In: Egan, H., ed. Environmental
carcinogens, selected methods of analysis. Vol. 3 - Analysis
of polycyclic aromatic hydrocarbons in environmental samples,
Lyons, IARC pp. 55-61 (WHO/IARC Publication No. 29).
GRIMMER, G. & BÖHNKE, H. (1976) [Enrichment and gas chromatographic
profile analysis of polycyclic aromatic hydrocarbons in
lubricating oils.] Chromatographia, 9: 30-40 (in German).
GRIMMER, G. & BÖHNKE, H. (1978) [Polycyclic aromatic hydrocarbons and
heterocyclic compounds.] Erdöl Koble, 31: 272-277 (in German).
GRIMMER, G. & BÖHNKE, H. (1972) [Determination of total content of
polycyclic aromatic hydrocarbons in air dust and automobile
exhaust by capillary GC.] Z. anal. Chem., 261: 310-314 (in
German).
GUNSETT, A. (1930) Un cas de cancer aigu, épithélioma
spino-cellulaire, developpé après traumatisme par de l'asphalte
enflammé. Bull. Assoc. Etud. Cancer, 19: 459-462.
GUSEIN-ZADE, K. M. (1975) [Reactions of the skin on the effect of
Apsheron commercial oils.] Gig. Tr. prof. Zabol., No. 7,
pp. 51-52 (in Russian).
HAENNI, E. O. & HALL, M. A. (1960) Tentative ultraviolet absorption
limits for mineral oils. J.A.O.A.C., 43: 92-95.
HAENNI, E. O., JOE, F. L. Jr, HOWARD, J. W. & LEIBEL, R. L. (1962a) A
more sensitive and selective ultraviolet absorption criterion for
mineral oil. J.A.O.A.C., 45:(1): 59-66.
HAENNI, E. O., HOWARD, J. W., & JOE, F. L. Jr (1962b) Dimethyl
sulfoxide: A superior analytical extraction solvent for
polynuclear hydrocarbons and for some highly chlorinated
hydrocarbons. J.A.O.A.C., 45: 67 -70.
HAGGARD, H. W. (1921) The anesthetic and convulsant effects of
gasoline vapour. J. Pharmacol. Exper. Ther., 16: 401-404.
HAINES, J. R. & ALEXANDER, M. (1974) Microbial degradation of high
molecularweight alkanes. Appl. Microbiol., 28(6): 1084-1085.
HÄNNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioral effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HARTWELL, J. L. (1951) Survey of compounds which have been tested for
carcinogenic activity. 2nd ed. Washington, DC, Federal Security
Agency (Public Health Service Pub. No. 149).
HAYHURST, E. R. (1936) Poisoning by petroleum distillates. Ind. med.
Surg., 5: 53.
HELBLING, V. (1950) [A case of acute lethal percutaneous and
inhallatory poisoning by petrol.] Z. Unfallmed. Berufskr.,
43: 218-228 (in German).
HELLMANN, H. & ZEHLE, H. (1972) [Long-term investigation on
behavioural of crude oil on water.] Dtsch. Gewaesserkd. Mitt.,
16: 46-52 (in German).
HENDRICKS, N. V., BERRY, C. M., LIONE, J. G., & THORPE, J. J. (1959)
Cancer of the scrotum in wax pressman. I. Epidemiology. Arch.
ind. Health, 19: 524-529.
HENDRICKS, N. V., COLLINGS, G. H., DOOLEY, A. E., GARRETT, J. T., &
RATHER, J. B. Jr (1962) A review of exposure to oil mist. Arch.
environ. Health, 4: 139-145.
HENRY, S. A. (1946) Cancer of the scrotum in relation to occupation,
London, Oxford Medical Publications, Oxford University Press,
100 pp.
HENRY, S. A. (1946/1947) Occupational cutaneous cancer attributable to
certain chemicals in industry. Br. reed. Bull., 4: 389-401.
HENRY, S. A. (1947) Symposium on industrial skin cancer. Br. J.
Radiol., 20: 149-157.
HENSEN, E. V. (1959) Toxicology of some of the aliphatic and alicyclic
hydrocarbons. J. occup. Med., 1: 105-112.
HERMANN, H. (1975) Handling of crudes and petroleum products ...
industrial hygiene. Erdoel Kohle, Erdgas, Petrochem.
Brennst.-Chem., 28(6): 282-286.
HERSKOWITZ, A., ISHI, N., & SCHAUMBERG, H. (1971) n-Hexane
neuropathy; a syndrome occurring as a result of industrial
exposure. New Engl. J. Med., 285-2: 82-85.
HETTCHE, H. O. (1963) [The effects of bitumen and its vapours on human
health.] Städtehygiene, 14: 65-68 (in German).
HIEGER, I. & WOODHOUSE, D. L. (1952) The value of the rabbit for
carcinogenicity tests on petroleum fractions. Br. J. Cancer,
6: 293-299.
HINE, C. H. & ZUIDEMA, H. H. (1970) The toxicological properties of
hydrocarbon solvents. Ind. Med., 39(5): 215-220.
HMSO (1967) Cancer of the skin caused by oil, London, Her Majesty's
Stationary Office, pp. (SHW295/295A).
HMSO (1979) Cautionary Notice: Effects of mineral oil on the skin,
London, HMSO, (SHW397 revised 1979).
HODGSON, G. (1970) Cutaneous hazards of lubricants. Ind. Med.,
39(2): 68-73.
HODGSON, G. (1973) Health problems arising from contact and exposure
of workers to metal working fluids. J. Inst. Petr.,
59(565): 1-8.
HOEKSTRA, W. G. & PHILLIPS, P. H. (1963) Effects of topically applied
mineral oil fractions on the skin of guinea-pigs. J. invest.
Dermatol., 40(2): 79-88.
HOLMES, J. G., KIPLING, M.D., & WATERHOUSE, J. A. H. (1970) Subsequent
malignancies in men with scrotal epithelioma. Lancet,
ii: 214-215.
HOOGENDAM, I. (1962) Health checks on asphalt workers. In:
Proceedings of the Shell Industrial Doctors Meeting, 22-25 May,
1962, The Hague, Med. Div. Shell Int. Petrol. Co. Ltd. (Internal
Report).
HORTON, A. W., DENMAN, D. T., & TROSSET, R. P. (1957) Carcinogenesis
of the skin. II. The accelerating properties of aliphatic and
related hydrocarbons. Cancer Res., 17: 758-766.
HORTON, A. W., BURTON, M. J., TYE, R., & BINGHAM, E. L. (1963)
Composition versus carcinogenicity of distillate oils. Am. Chem.
Soc. Div. Pet. Chem. Prepr., 8: No. 4-C: 59-65.
HORTON, A. W., BINGHAM, E. L., BURTON, M. J. G., & TYE, R. (1965)
Carcinogenesis of the skin. III. The contribution of elemental
sulfur and of organic sulfur compounds. Cancer Res.,
25: 1759-1763.
HRUSTALEVA, G. F., CEMBARTSEVA, A. N., SVECNIKOVA, F. A., BESKROVNAJA,
N. I., ROTIN, V. V., & LESEHEV, S. S. (1979) [Hypophyseal-ovarian
relationships of women coming into contact with petroleum solvents
in industry.] Gig. Tr. prof. Zabol., No. 7, pp. 31-33
(in Russian).
HUEPER, W. C. (1961) Carcinogens in the human environment. Arch.
Pathol., 71: 355-380.
HUEPER, W. C. & PAYNE, W. W. (1960) Carcinogenic studies on petroleum
asphalt, cooling oil, and coal tar. Arch. Pathol., 70: 372-384.
HUGUENIN, R. (1925) Cancer aigu consécutif à une brûlure par le
mazout. Bull. Assoc. Fr. Etud. Cancer, 14: 403-404.
HUGUENIN, R., FAUVET, F., & MAZABRAUD, M. (1950) Rôle éventuel des
nébulisations d'huiles industrielles dans la pathogénie des
cancers broncho-pulmonaires. Arch. Mal. prof., 11: 48-51.
HUNTER, G. A. (1968) Chemical burns of the skin after contact with
petrol. Br. J. plast, Surg., 21: 337-341.
IARC (1973) Certain polycyclic aromatic hydrocarbons and heterocyclic
compounds. In: IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Lyons, International Agency for
Research on Cancer, Vol. 3, pp. 30-42.
IARC (1974) Some anti-thyroid and related substances, nitrofurans and
industrial chemicals. In: IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International
Agency for Research on Cancer, Vol. 7,326 pp.
ICHIHARA, K., KUSUNOSE, E., & KUSUNOSE, M. (1969) Microsomal
hydroxylation of decane. Biochim. Biophys. Acta, 176: 713-719.
IIDA, M., YAMAMURA, Y., & SOBUE, I. (1969) Electromyographic findings
and conduction velocity in n-hexane polyneuropathy.
Electromyography, 9: 247-261.
IKEDA, K. (1935) Oil aspiration pneumonia (lipoid pneumonia).
Am. J. Dis. Child. 49: 985-1006.
IKEDA K. (1937) Lipoid pneumonia of the adult type (paraffinoma of the
lung). Arch. Pathol., 23: 471-492.
ILO (1968) Benzene: uses, toxic effects, substitutes. Report of
expert meeting 1967, Geneva, International Labour Office, 287 pp.
INOUE, T., TAKEUCHI, Y., TAKEUCHI, S., YAMADE, S, SUZUKI, H.,
MATSUSHITE, T., MIYAGAKI, H., MAEDE, K., & MOTSUMOTO, T. (1970) A
health survey on vinyl sandal manufacturers where a high incidence
of n-hexane intoxication occurred. Jpn. J. ind. Health,
12: 5-16.
INSTITUTE OF PETROLEUM (1974) Petroleum statistics with 1973 totals,
London, Institute of Petroleum.
ISHII, N., HERSKOWITZ, A., & SCHAUMBERG, H. (1972) n-Hexane
poly-neuropathy: a clinical and experimental study.
J. Neuropathol. exp. Neurol., 31: 198.
IVANOV, N. G., POZDNJAKOV, V. S., & ROZOVA, T. A. (1978) [The maximum
admissible content of mineral (petroleum) oil aerosol in air of
working premises.] Gig. Tr. prof. Zabol., No. 5, pp. 28-31
(in Russian).
JANYSEVA, N. I., KIREEVA, I. S., & SERZANTOVA, N. N. (1963) [A propos
the content of 3,4-benzopyrene in petroleum bitumen.] Gig. i
Sanit., 28: 71-73 (in Russian).
JEPSON, J. R., STOVANOV, S., UNGER, M., CLAUSEN, J., & CHRISTENSEN, H.
E. (1977) Cutting fluids and their effects on the skin of mice.
Acta Pathol. Microbiol. Scand. (Section A), 85: 731-738.
JERINA, D. M. & DALY, J. W. (1974) Arene oxides: A new aspect of drug
metabolism. Science, 185: 573-582.
JONES, J. G. (1961) An investigation into the effects of exposure to
an oil mist on workers in a mill for the cold reduction of steel
strip. Ann. occup. Hyg., 3: 264-271.
KARANI, V. (1966) Peripheral neuritis after addiction to petrol.
Br. med. J., i: 216.
KELLERMAN, G., SHAW, C. R, & LUYTEN-KELLERMAN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KENNAWAY, E. L. (1925) Experiments on cancer-producing substances.
Br. meal J., ii: 1-6.
KENNAWAY, E. L. & HIEGER, I. (1930) Carcinogenic substances and their
fluorescence spectra. Br. med. J., 2: 1044-1046.
KEY, M. M., RITTER, E. J., & ARNDT, K. A. (1966) Cutting and grinding
fluids and their effects on the skin. Am. Ind. Hyg. Assoc. J.,
27: 423-427.
KINNEAR, J., ROGERS, J., & FINN, O. A. (1954) Degenerative changes in
the skin with special reference to jute-workers. Br. J.
Dermatol., 46: 344-349.
KINNEAR, J., ROGERS, J., FINN, O. A., & MAIR, A. (1955) Dermatoses in
jute-workers. Br. J. ind. Med., 12: 36-42.
KIPLING, M. D. (1968) Oil and the skin. Ann. Rep. of H.M. Chief
Inspector of Factories, 1967, London, HMSO, pp. 105-120.
KIREEVA, I. S. (1968) Carcinogenic properties of coal-tar pitch and
petroleum asphalts used as binders for coal briquettes. Hyg.
Sanit., 33(4, 5, 6): 180-186.
KLAUDER, J. V. & BRILLE, F. A. (1947) Correlation of boiling ranges of
some petroleum solvents with irritant action on the skin. Arch.
Dermatol. Syph., 56: 197-215.
KNAVE, B., PERSSON, H. E., GOLDBERG, J. M., & WESTERHOLM, P. (1976)
Long-term exposure to jet fuel. An investigation on
occupationally-exposed workers with special reference to the
nervous system. Scand. J. Work Environ. Health, 3: 152-164.
KNAVE, B., OLSEN, B. A., ELOFSON, S., GAMBERALE, F., ISAKSSON, A.,
MINDUS, P., PERSSON, H. E., STRUWE, G., WENNBERG, A., &
WESTERHOLM, P. (1978) Long-term exposure to jet fuel II. A
cross-sectional epidemiologic investigation on
occupationally-exposed industrial workers with special reference
to the nervous system. Scand. J. Work Environ. Health,
4: 19-45.
KOREEDA, M., MOORE, P. D., WISLOCKI, P. G., LEVIN, W., CONNEZ, A. H.,
YAGI, H., & JERINA, D. M. (1978) Binding of benzo (a)pyrene 7,8
diol -- 9,10 epoxides to DNA, RNA and protein of mouse skin occurs
with high stereoselectivity. Science, 199: 778-781.
KOROBKIN, R., ASBURY, A. K., SUMNER, A. J., & NIELSON, S. L. (1975)
Glue-sniffing neuropathy. Arch. Neurol., 32: 158-162.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KRASSOVITSKAJA, M. L. (1976) [Health protection of atmospheric air in
regions in which oil processing and petrochemical enterprises are
located. In: Guide to hygiene of the atmospheric air.] Moscow,
The Medicine Publishing House, pp. 273-304 (in Russian).
KURITA, H. (1967) Experimental studies on the effects of n-hexane on
albino rats. Jpn. J. ind. Health, 9: 24-36.
LASKIN, S. & GOLDSTEIN, B. D., ed. (1977) Benzene toxicity. A critical
evaluation Washington and London, Hemisphere Publishing Co.
LAUGHLEN, G. F. (1925) Studies on pneumonia following naso-pharyngeal
injections of oil. Am. J. Pathol., 1: 407-414.
LAZAREW, N. W. (1929) [The toxicology of benzenes.] Arch. Hyg.
Bakteriol., 102: 227-239 (in German).
LAZAREV, N. N. & LEVINA, E. A. (1976) [Harmful substances in
industry.] 7th ed., Leningrad, Publishing House "Khimia"
(in Russian).
LAZARINI, H.-J., DOIGNON, J., L'ÉPÉE, P., & MERILLON, H. (1974) Une
nouvelle observation de polynévrite par le n-hexane. Arch. Mal
prof., 35: 920-921.
LEE, M. L., BARTLE, K. D., & NOVOTNY, M. V. (1975) Profiles of the
polynuclear aromatic fraction from engine oils obtained by
capillary column gas -- liquid chromatography and
nitrogen-selective detection. Anal. Chem., 47(3): 540-543.
LEITCH, A. (1922) Paraffin cancer and its experimental production.
Br. med. J., ii: 1104-1009.
LEITCH, A. (1924) Mule spinners cancer and mineral oils. Br. med. J.,
ii: 941-943.
LEITHE, W. (1970) The analysis of air pollutants. Ann Arbor, Ann
Arbor-Humphrey Science Publishers, pp. 78-93.
LEITHE, W. (1972) [The analysis of organic pollutants in drinking,
industrially-used, and sewage water.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft MBH, pp. 119-132
(in German).
LESSER, L. I., WEENS, H. S., & McKEY, J. D. (1943) Pulmonary
manifestations following ingestion of kerosene. J. Pediatr.,
23: 352-364.
LEVINA, E. N. (1976) [Hydrocarbons.] In: Lazarev, N. V. & Levina, E.
N., ed. [Harmful substances in industry Volume I, Organic
substances.] 7th ed., Leningrad, Publishing House Himia, pp.
9-121 (in Russian).
LIEBE, G. (1892) [On tar or paraffin cancer.] Med. Jahrb., 236: 65
(in German).
LIEVRE, J., BENICHOU, C., & DESROY, M. (1967) Polynévrite par poêle à
combustion catalytique (trots sujets atteints dans une famille).
Bull. Soc. Méd. Hop. Paris, 118: 91-99.
LIJINSKY, W. (1960) Separation of polycyclic aromatic hydrocarbons in
complex mixtures: Chromatographic determination of trace amounts
in petroleum waxes. Anal. Chem., 32: 684-687.
LIJINSKY, W. & RAHA, C. R. (1961) Polycyclic aromatic hydrocarbons in
commercial solvents. Toxicol. appl. Pharmacol., 3: 469-473.
LIJINSKY, W., RAHA, C. R., & KEELING, J. (1961) Comparison of
procedures for the determination of polycyclic aromatic
hydrocarbons in waxes. Anal. Chem., 33: 810-812.
LIJINSKY, W, DOMSKY, I., MASON, G., RAMAHI, H. Y., & SAFARI, T. (1963)
The chromatographic determination of trace amounts of polynuclear
hydrocarbons in petrolatum, mineral oil, and coal-tar. Anal.
Chem., 33: 952-956.
LIJINSKY, W., SAFFIOTTI, U, & SHUBIK, P. (1966) Skin tumorigenesis by
an extract of amber petrolatum. Toxicol. appl. Pharm.,
8: 113-117.
LIPOVSKIJ, S. M. (1978) [The serotonin content in the tissue of uterus
and particulars on its contracting activity under the influence of
petroleum solvent vapours.] Gig. Tr. prof. Zabol., No. 7,
pp. 37-40 (in Russian).
LIPOVSKIJ, S. I., ELKIN, V. I., TOMAEVA, L. V., & ZORINA, V. V. (1977)
[The solvents content in the blood of workers in the rubber
industry.] Gig. Tr. prof. Zabol., No. 5, pp. 50-51 (in Russian).
LIPOVSKIJ, C. M., TOMAEVA, L. V., VARFOLOMEEV, D. I., FEDOSEEV, Ja.
E., & KARGANOVA, E. V. (1979) [The accumulation of solvents in the
tissues and organs of pregnant women.] Gig. Tr. prof. Zabol.,
No. 3, pp. 25-28 (in Russian).
LIPPMANN, M. & GOLDSTEIN, D. H. (1970) Oil.mist studies, environmental
evaluation and control. Arch. environ. Health, 21: 591-599.
LUSHBAUGH, C. C. (1947) Experimental hyperplastic gastritis and
gastric polyposis in monkeys. J. Natl Cancer Inst., 7: 313-320.
LUSHBAUGH, C. C. & HACKETT, A. (1948/1949) An infiltrating
aden-omatous lesion of the colon of rats ingesting motor
lubricating oil (S.F.G. No. 1 oil). J. Natl Cancer Inst.,
9: 159-172.
LUSHBAUGH, C. C., GREEN, J. W., & REDEMANN, C. E. (1950) Effects of
prolonged inhalation of oil fogs on experimental animals. Arch.
ind. Hyg. occup. Med., 1: 237-247.
LUTOV, V. A. (1974) [Materials for establishing the maximum admissible
concentration of petroleum oil aerosols without additives, used as
lubricating and cooling liquids.] Gig. Tr. prof. Zabol., No 10,
pp. 49-52 (in Russian).
LUTOV, V. Ya., CIRKIN, A. A., GRJADITSKIJ, Ju. E., & VASILENKO, N. I.
(1976) [The effect of petroleum oil aerosols and thermo-oxidizing
degradation products on the functional condition and immunologic
reactivity of the organism of experimental animals.] Gig. Tr.
Prof. Zabol., No. 2, pp. 33-37 (in Russian).
LYNCH, P. F. & BROWN, C. W. (1973) Identifying source of petroleum by
infrared spectrometry. Environ. Sci. Technol., 7: 1123-1127.
MACHLE, W. (1941) Gasoline intoxication. J. Am. Med. Assoc.,
117: 1965-1971.
MALTEN, K. E., SPRUIT, D., BOEMAARS, H. G. M., & De KEIZER, M. J. M.
(1968) Horny layer injury by solvents. Berufsdermatosen,
16: 135-147.
MASON, B. (1966) The concentration of minor elements in biogenic
deposits. Principles of geochemistry, 3rd ed., New York, John
Wiley & Sons Inc., pp. 241-243.
MASTROMATTEO, E. (1955) Cutting oils and squamous-cell carcinoma. Part
I. Incidence in a plant with a report of six cases. Br. J. ind.
Med., 12: 240-243.
MATHER, R. N. (1973) Codes of practice relating to metal-working
fluids -- the users' views. J. Inst. Petrol., 59: 15-17.
MATSUMOTO, T. (1971) Experimental studies on toluene poisoning, II.
Jpn. J. ind. Health, 13: 399-407.
MATSUMURA, M., INOUE, N., OHNISHI, A., SANTA, T., & GOTO, I. (1972)
Toxic polyneuropathy due to glue-sniffing. Clin. Neurol. (Japan),
12: 290-296.
MAZEE, W. M., GERSMANN, H. R., & VAN DER WIEL, A. (1966) Analysis of
polycyclic aromatic hydrocarbons in petroleum waxes and white
mineral oils with appraisal of merits of different methods of
analysis. Food cosmet. Toxicol., 4: 17-33.
McDERMOTT, H. J. & KILLIANY, S. E. Jr (1978) Quest for a gasoline TLV.
Am. Ind. Hyg. Assoc. J., 39(2): 110-117.
McKAY, J. F. & LATHAM, D. R. (1972) Fluorescence spectrometry in the
characterization of high boiling petroleum distillates. Anal.
Chem., 44(13): 2132-2137.
McKAY, J. F. & LATHAM, D. R. (1973) Polyaromatic hydrocarbons in high
boiling petroleum distillates. Anal. Chem., 45: 1050-1055.
Medical Research Council (1968) The carcinogenic action of mineral
oils. A chemical and biological study. London HMSO (Special Report
Series No. 306).
MEDVED', R. A., KUZINA, V. F, & KRIGMAN, B. I. (1976) [The hygienic
assessment of lubricating and cooling liquids introduced instead
of sulphofrezol for the cold processing of metal by cutting.]
Gig. Tr. prof. Zabol., No. 6, pp. 1-5 (in Russian).
MENDELL, J. R., SAIDA, K, GANANSIA, M. F., JACKSON, D. B., WEISS, H.,
GARDIER, R. W., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J.,
MARKS, B., & HETLAND, L. (1974) Toxic polyneuropathy produced by
methyl n-butyl ketone. Science, 185: 787-789.
MEYER, A. (1976) Huilome pulmonaire chez les chanteurs. Arch. Mal.
prof., 37(9): 629-631.
MILLER, A., BADER, R. A., BADER, M. E., TEIRSTEIN, A. S., & SELIKOFF,
I. J. (1962) Mineral oil pneumonia. Ann. intern. Med.,
57: 627-634.
MILNE, J. E. (1970) Carcinoma of the scrotum. Med. J. Aust.,
1: 13-16.
MINER, S. (1969) Air pollution aspects of hydrogen sulfide,
Bethesda, MD, Litton Systems Inc. (prepared for the United States
Department of Health, Education and Welfare Contract No.
PH-22-68025).
MIYAGAKI, H. (1967) Electrophysiological studies on the peripheral
neurotoxicity of n-hexane. Jpn. J. ind. Health, 9: 12-23.
MOEL, M. & TAYLOR, H. K. (1943) Oil aspiration pneumonia. Am. J.
Röntgenol. rad. Ther., 49(2): 177-184.
MOSS, E., SCOTT, T. S., & ATHERLEY, G. R. C. (1972) Mortality of
newspaper workers from lung cancer and bronchitis 1952-1966.
Br. J. ind. Med., 29(1): 1-14.
MUHAMETOVA, G. M. & PODREZ, Z. G. (1975) [Problems of the course,
pathogenesis and therapy of chronic patrol poisoning.] Ufa,
Baskirskoe kniznoe izdatelstvo (in Russian).
NATHANSON, L., FRANKEL, D., & JACOBI, M. (1943) Diagnosis of lipoid
pneumonia by aspiration biopsy. Arch. int. Med., 72: 627-634.
NEBERT, D. W. & GELBOIN, H. V. (1969) The in vivo and in vitro
induction of aryl hydrocarbon hydroxylase in mammalian cells of
different species, tissues, strains, and developmental and
hormonal states. Arch. Biochem, Biophys., 134: 76-89.
NEBERT, D. W., GOUJON, F. M., & GIELEN, J, E. (1972)Aryl hydrocarbon
hydroxylase induction by polycyclic hydrocarbons: simple autosomal
dominant trait in the mouse. Nature New Biology, 236: 107-109.
NELSON, L. B. & STORMONT, D. J. (1960) Determination of polynuclear
aromatic hydrocarbons in petroleum waxes. Chem. Ind., 21: 561.
NIOSH (1973) The industrial environment -- its evaluation and
control. Washington, DC, US Dept of Health, Education and
Welfare.
NIOSH (1977A) Occupational exposure sampling strategy manual.
Washington, DC, US Dept of Health, Education and Welfare (DHEW
(NIOSH) Publication No. 77-173).
NIOSH (1977B) Criteria for a recommended standard ... occupational
exposure to asphalt fumes, Washington DC, US Department of
Health, Education and Welfare (DHEW (NIOSH) Publ. No. 78-106).
NIOSH (1977C) Criteria for a recommended standard ... occupational
exposure to hydrogen sulphide, Washington DC, US Department of
Health, Education and Welfare.
NIOSH (1977D) Criteria for a recommended standard ... occupational
exposure to refined petroleum solvents, Washington DC, US
Department of Health, Education and Welfare, 247 pp. (DHEW (NIOSH)
Publ. No. 77-192).
NIOSH (1977-79) NIOSH manual of analytical methods. 2nd ed.
Cincinnati, OH, US Dept of Health, Education and Welfare, USA.
NIOSH (1979) Summary of NIOSH recommendations for occupational health
standards, Washington DC, US Department of Health and Welfare,
9 pp.
NOCHOMOVITZ, L. E., UYS, C. J., & EPSTEIN, S. (1975) Massive
deposition of mineral oil after prolonged ingestion. S.A. med.J.,
13 Dec., 2187-2190.
NOMIYAMA, K. & NOMIYAMA, H. (1974) Respiratory retention, uptake and
excretion of organic solvents in man. Int. Arch. Arbeitsmed.,
32: 75-91.
NOVIKOV, K. I., KOLODINA, L. N., LIPOVSKIJ, S. M., & BADALOVA, L. V.
(1979) [Lactation particulars in female workers of the chemical
(rubber) industry.] Gig. Tr. prof. Zabol., No. 2, pp. 45-48
(in Russian).
NUNN, J. A. & MARTIN, F. M. (1934) [Gasoline (petrolether) and
kerosine (petroleum) poisonings in children.] Samml.
Vergiftungsf., 5: 183-185 (in German).
OGATA, M., ASAHARA, H., & SAEKI, T. (1975) Sampling and analysis of
some aromatic aliphatic and chlorinated hydrocarbon vapours in
air, a gas-liquid chromatographic and colorimetric method.
Int. Arch. Arbeitsmed., 34: 25-37.
OSER, B. L., OSER, M., & CARSON, S. (1965) Toxicological studies of
petrolatum in mice and rats. Toxicol. appl. Pharmacol.,
7: 382-401.
PASTERNACK, B. & EHRLICH, L. (1972) Occupational exposure to an oil
mist atmosphere. A 12-year mortality study. Arch. environ.
Health, 25: 286-294.
PATES, M. M. (1952) [Blastomogenic effect of pyrolyse tars from
petroleum.] Tr. Akad. Med. Nauk., SSSR, 21: 157-163
(in Russian).
PAULSON, G. W. & WAYLONIS, G. W. (1976) Polyneuropathy due to
n-hexane. Arch. intern. Med., 136: 880-882.
PERBELLINI, L., BRUGNONE, F. & FAGGIONATO, G. (1981) Urinary excretion
of the metabolites of n-hexane and its isomers during
occupational exposure. Br. J. ind. Med., 38: 20-26.
PETROLEUM HANDBOOK (1966) London, Shell International Petroleum
Company Limited.
PINKERTON, H. (1927) Oils and fats. Their entrance into and fate in
the lungs of infants and children: A clinical and pathologic
report. Am. J. Dis. Child., 33: 259-285.
PINKERTON, H. (1928) The reaction to oils and fats in the lung. Arch.
Pathol, 5: 380-401.
PINKERTON, H. & MORAGUES, V. (1940) Paraffinoma of the lung with
secondary tuberclelike lesions in liver and spleen. Arch.
Pathol., 29: 691-699.
PIOTROWSKI, J. K. (1977) Exposure tests for organic compounds in
industrial toxicology. Cincinnati, OH, NIOSH, (DHEW (NIOSH)
Publication No. 77-144).
POKLIS, A. & BURKETT, C. D. (1977) Gasoline sniffing: A review. Clin.
Toxicol., 11: 35-41.
POLIANSKY, V. A. & MUSERSKAJA, A. N. (1971) [Pollution of the air
environment at modern oil processing plants and evaluation of the
implementation of recommendating.] In: [Occupational hygiene and
the protection of the health of workers in oil and petrochemical
industries.] Ufa, pp. 50-54 (in Russian).
PROCKOP, L. D. (1977) Multifocal nervous system damage from inhalation
of volatile hydrocarbons. J. occup. Med., 19: 139-140.
PROUDFIT, J.P., ORDSTRAND, H. S. VAN, & MILLER, C. W. (1950) Chronic
lipid pneumonia following occupational exposure. Arch. ind. Hyg.,
1: 105-111.
PRUYN, F. H. A.M. & REIJNIERSE, K. (1972) [Carcinoma of the scrotum
through mineral oils.] T. Soc. Geneesk., 50: 182-187 (in Dutch).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1975) Report on emissions from
asphalt hot mixes, Maryland, USA, The Asphalt Institute
(Research Report RR-75-IA).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1978) Differences between
petroleum asphalt, coal-tar pitch and road tar, Maryland, USA,
The Asphalt Institute (Research report RR-78-1).
RAALTE, H. G. S. VAN (1963) [Medico-toxicological aspects of oil used
in metal processing.] Tijdschr. Soc. Geneesk., 41: 611-617
(in Dutch).
RAALTE, H. G. S. VAN (1972) [Carcinoma of the scrotum through mineral
oils.] Tijdschr. Soc. Geneesk, 50: 604-605 (in Dutch).
RAITTA, C., SEPPÄLÄINEN, A. M., & HUUSKONEN, M. (1978) N-hexane
maculopathy in industried workers. Graefes Arch. klin. exp.
Ophthalmol., 209: 99-110.
REID, F. H. & HALPIN, W. R. (1968) Determination of halogenated and
aromatic hydrocarbons in air by charcoal tube and gas
chromatography. Am. Ind. Hyg. Assoc. J., 29: 390.
REIDENBERG, M. M., POWERS, D. V., & BELLO, C. T. (1964) Acute renal
failure due to nephrotoxins. Am. J. med. Sci., 59: 25-63.
REWELL, R. E. (1947) Lipoid pneumonia: A pitfall in diagnosis.
Br. med. J., 29: 409-411.
RIIHIMÄKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
Environ. Health, 5: 143-150.
RIIHIMÄKI, V. & PFÄFFLI, P. (1978) Percutaneous absorption of solvent
vapours in man. Scand. J. Work Environ. Health, 4: 73-85.
RIIHIMÄKI, V., PFÄFFLI, P., SAVOLAINEN, K., & PEKARI, K. (1979)
Kinetics of m-xylene in man. General features of absorption,
distribution, biotransformation and excretion in repetitive
inhalation exposure. Scand. J. Work Environ. Health,
5: 217-231.
ROBINSON, E. & ROBBINS, R. C. (1968) Sources, abundance, and fate of
gaseous atmospheric pollutants. Chapter VI; Hydrocarbons and
organics in the atmosphere, Huntsville, Alabama, Stanford
Research Institute (Report PR-6755 prepared for the American
Petroleum Institute).
ROE, F. J. C., CARTER, R. L., & TAYLOR, W. (1967) Cancer hazard from
mineral oil used in the processing of jute. Br. J. Cancer,
21: 694-702.
ROSSIER, P. H. & BÜHLMANN, A. (1949) [Clinical and laboratory studies.
An oil-aspiration pneumonia following the use of liquid paraffin
as nose drops for some years.] Schweiz. Med. Wochenschr.,
79: 685-686.
SANTE, L. R. (1949) The fate of oil particles in the lung and their
possible relationships to the development of bronchogenic
carcinoma. Am. J. Röntgenol., 62: 788-797.
SAVOLAINEN, H. (1977) Some aspects of the mechanisms by which
industrial solvents produce neurotoxic effects. Chem. biol.
Interactions, 18: 1-10.
SCHAMP, N. & VAN WASSENHOVE, F. (1972) Determination of
benzo(alpha)-pyrene in bitumen and plants. J. Chromatogr.,
69: 421-425.
SCHAUMBURG, H. H. & SPENCER, P.S. (1976) Degeneration in central and
peripheral nervous systems produced by n-hexane: An experimental
study. Brain, 99: 183-192.
SCHEUPLEIN, R. J. & BLANK, I. H. (1971) Permeability of the skin.
Physiol. Rev., 51: 702-747.
SCHMAHL, D. & REITER, A. (1933) [Experiments on cancer production by
liquid paraffin yellow vaseline and lanolin.] Arzneim. Forsch.,
3: 403-406 (in German).
SCHWARZ, H. G. (1933) [Petrol poisoning, chronic, iatrogenic.] Samml.
Vergiftungsf., 4: 247-248 (in German).
SCHWARTZ, L., JULIPAN, L., & PECK, S. (1947) Dermatoses caused by
petroleum. In: Occupational diseases of the skin, 2nd ed.,
Kimpton, London, pp. 232-258.
SCRIMA, M. & De ROSA, F. (1973) [Polyneuritis in upper shoe factories:
Environmental and concomitant causes.] Lav. Um., 25: 191-198
(in Italian).
SEDIVEC, V. & FLEK, J. (1976) The absorption, metabolism and excretion
of xylenes in man. Int. Arch. occup. Environ. Health,
37: 205-217.
SEHTMAN, B. A, SAMEDOR, I. G., & MUHAMETOVA, G. M. (1979)
[Work hygiene in the petroleum industry.] Moscow, Medicina Publ.
House (in Russian).
SEPPÄLÄINEN, A. M. (1975) Applications of neurophysiological methods
in occupational medicine: A review. Scand. J. Work Environ.
Health, 1: 1-14.
SEPPÄLÄINEN, A. M., HUSMAN, K., & MARTENSSON, C. (1978)
Neuro-physiological effects of long-term exposure to a mixture of
organic solvents. Scand. J. Work Environ. Health, 4: 304-314.
SEPPÄLÄINEN, A.M., RAITTA, C., & HUUSKONEN, M. (1979)
n-Hexane-induced changes in visual evoked potentials and
electroretinograms of industrial workers. Electroencephalogr.
clin. Neurophysiol., 47(4): 492-498.
SERRATRICE, G., CORREARD, R.-P., ARLAUD, J.-A., & JOUGLARD, J. (1973)
Polynévrite et manipulation de peinture pour bateaux. J. eur.
Toxicol., 6: 304-305.
SHAHIN, M. M. & FOURNIER, F. (1978) Suppression of mutation induction
and failure to detect mutagenic activity with Athabasca Tar sand
fractions. Mutat. Res., 58: 29-34.
SHIRABE, T., TSUDA, T., TERAO, A., & ARAKI, S. (1974) Toxic
poly-neuropathy due to glue-sniffing; report of two cases with
light and electron-microscopic study of the peripheral nerves and
muscles. J. Neurol. Sci., 21: 101-113.
SHOSHKES, M., BANFIELD, W., & ROSENBAUM, S. J. (1950) Distribution,
effect and fate of oil aerosol particles retained in the lungs of
mice. Arch. ind. Hyg. occup. Med., 1: 20-35.
SHUBIK, P. & SAFFIOTTI, U. (1954) Carcinogenic and promoting action of
low-boiling catalytically cracked oils. Proc. Am. Assoc. Cancer
Res., 1: 45.
SHUBIK, P., SAFFIOTTI, U. LIJINSKY, W., PIETRA, G., RAPPAPORT, H.,
TOTH, B., RAHA, C. R., TOMATIS, L., FELDMAN, R., & RAMAHI, H.
(1962) Studies on the toxicity of petroleum waxes, Toxicol. appl.
Pharmacol., 4 (Suppl,, Nov.): 1-62.
SIMMERS, M. H. (1964) Petroleum asphalt inhalation by mice, Arch,
environ, Hearth, 9(6): 727-734.
SIMMERS, M. H. (1965A) Cancers from air.refined and steam refined
asphalt. Ind. Med. Surg., 34(3): 244-261.
SIMMERS, M. H. (1965B) Cancers in mice from asphalt fractions,
Ind. Med. Surg., 34(7): 573-577.
SIMMERS, M. H. (1966) Tumors from asphalt fractions injected into
mice. Ind. Med. Surg., 35(10): 889-894.
SIMMERS, M. H., PODOLAK, E., & KINOSITA, R. (1959) Carcinogenic
effects of petroleum asphalt. Proc. Soc. exp. Blot. Med.,
10: 266-268.
SIMS, P., GROVER, P. L., SWAISLAND, A., PAL, K., & HEWER, A. (1974)
Metabolic activation of benzo(a)pyrene precedes by a diol-epoxide.
Nature (Lond.), 252: 326-328.
SIOU, G. (1972) Existe-t-il un risque de cancérisation par les
bitumes. Rev. Path. comp. Méd. exp., 72: 65-75.
SIWE, S. A. (1932) [The signs and symptoms of acute poisoning by
liquid hydrocarbons in the aliphatic series.] Monatschr.
Kinderheilkd., 55: 146-153 (in German).
SMILEY, J. A. (1951) The hazards of rope making. Br. J. ind. Med.,
8: 265-270.
SMITH, W. E. & SUNDERLAND, D. A. (1951) Responses of mice to
carcinogenic derivatives of petroleum. Cancer Res., 11: 281.
SMITH, W. E., SUNDERLAND, D. A., & SUGIURA, K. (1951) Experimental
analysis of the carcinogenic activity of certain petroleum
products. Arch. ind. Hyg. occup. Med., 4: 299-314.
SMS 2337-1 (1972) Determination of low concentrations of benzene in
air, The Hague, Shell Int. Petr. My. (Shell Method Series).
SMYTH, H. F. & SMYTH, H. F. (1928) Inhalation experiments with certain
lacquer solvents. J. ind. Hyg., 10: 261-271.
SOUTHAM, A. (1928) Mule-spinners' cancer. In: Report of the
International Conference on Cancer, London, Bristol, John
Wright, p. 28C.
SPENCER, P. S., BISCHOFF, M. C., & SCHAUMBURG, H. H. (1978) On the
specific molecular configuration of neurotoxic aliphatic
hexacarbon compounds causing peripheral distal axonopathy.
Toxicol. appl. Pharmacol., 44: 17-28.
SPRUIT, D., MALTEN, K. E., LIPMANN, E. W. R. M., & LIANG, T. P. (1970)
Horny layer injury by solvents. II. Can the irritancy of petroleum
either be diminished by pretreatment. Berufsdermatosen,
18: 269-280.
STERNER, J. H. (1941) Study of hazards in spray-painting with gasoline
as a diluent. J. ind. Hyg. Toxicol., 23: 437-448.
STEWART, I. (1960) Petrol vapour poisoning. Br. med. J.,
i: 1739-1740.
STRÄULI, P. (1957) [Acute tar cancer.] Oncologia, 10: 72-75
(in German).
STRYKER, W. A. (1941) Absorption of liquid petrolatum (mineral oil)
from the intestine. Arch. Pathol., 31: 670-692.
SUGIURA, K., SMITH, W. E., & SUNDERLAND, D. (1949) Carcinogenic action
of certain catalytically cracked oils with high boiling points.
Cancer Res., 9: 631.
SUHANOVA, V. A. & MEL'NIKOVA, V. V. (1974) [The menstruation function
of female workers at refineries and patients suffering from
chronic intoxication by petroleum products.] Gig. Tr. prof.
Zabol., No. 4, pp. 30-41 (in Russian).
SULLIVAN, R. J. (1969) Air pollution aspects of odorous compounds,
Bethesda, Maryland, Litton Systems Inc. (prepared for the US Dept.
of Health, Education and Welfare, Contract No. PH-22-68-25).
SUMA'MUR, P. K. & SUSIANTI WENAS. (1978/1979) Skin and other
affections in gasoline and/or diesel oil pump workers. Indones.
J. ind. Hyg. occup. Health, 11-12: 20-22 (XI No. 1, 2, 3, 4 and
XII No. 1 and 2).
SUNDERLAND, D. A., SMITH, W. E., & SUGIURA, K. (1951) The pathology of
growth behaviour of experimental tumours induced by certain
petroleum products. Cancer, 4: 1232-1245.
SUZUKI, T., SHIMBO, S., & NISHITANI, H. (1974) Muscular atrophy due to
glue sniffing. Int. Arch. Arbeitsmed., 33: 115-123.
SWANN, H. E. Jr., KWONN, B. K., HOGAN, G. K., & SNELLINGS, W. M.
(1974) Acute inhalation toxicology of volatile hydrocarbons.
Am. Ind. Hyg. Assoc. J., 35(9): 511-518.
Tabershaw/Cooper Association Inc. (1974/75) Morbidity study of
petroleum refinery workers. (Project OH-4, Medical Research
Report prepared for API).
TAGAMI, H. & OGINO, A. (1973) Kerosene dermatitis; factors affecting
skin irritability to kerosene. Dermatologica, 146: 123-131.
TAHER, S. M., ANDERSON, R. J., McCARTNEY, R, POPOVTZER, M. M., &
SCHRIER, R. W. (1974) Renal tubular acidosis associated with
toluene «sniffing». New Engl. J. Med., 290: 765-768.
TAKEUCHI, Y., MABUCHI, C., & TAKAGI, S. (1975) Polyneuropathy caused
by petroleum benzine. Int. Arch. Arbeitsmed., 34: 185-197.
THAKKER, D. R., YAGI, H, AKAGI, H, KOREEDA, M., LU, A. Y.-H., LERIM,
W., WOOD, A. W., CONNEY, A. H., & JERINA, D. M. (1977) Metabolism
of benzo(alpha)pyrene VI. Stereoselective metabolism of
benzo(alpha)-pyrene and benzo(alpha)pyrene 7,8-dihydrodiol to
diolepoxides. Chem. biol. Interaction 16: 281-300.
THONY, C. & THONY, J. (1970) Enquête épidémiologique sur le cancer des
huiles de coupe. In: Proceedings of the 16th International
Congress on Occupational Health, Tokyo, 1969, pp. 655-657.
THONY, C., THONY, J., LAFONTAINE, M., & LIMASSET, J. C. (1975)
Concentrations en hydrocarbures polycycliques aromatiques
cancérogènes de quelques huiles minerale étude due risque
correspondant. Arch. Mal. prof., 36: 37-52.
TRUHAUT, R. (1978) Introductory remarks. In: Meeting on MACs, XIX In.
International Congress on Occupational Health, 26 September 1978.
TRUHAUT, R. & MURRAY, R. (1978) International workshop on toxicology
of benzene, Paris, 9-11 November 1976. Int. Arch. Occup. Env,
41: 65-76.
TRUHAUT, R., LAGET, P., PIAT, G., DUTERTRE-CATELLA, P.-L. H., & HUYEN,
V. N. (1973) Premiers résultats électrophysiologiques après
intoxications experimentales par l'hexane et par l'heptane
techniques chez le rat blanc. Arch. Mal. prof.,
34(7-8): 417-426.
TWORT, C. C. & FULTON, J. D. (1930) Further experiments on the
carcinogenicity of synthetic tars and their fractions. J. Path.
Bacteriol., 32: 119-143.
TWORT, C. C. & ING, H. R. (1928) [Investigations on carcinogenic
agents.] Z. Krebsforsch., 27: 308-351 (in German).
TWORT, C. C. & TWORT, J. M. (1931) The carcinogenic potency of mineral
oils. J. And. Hyg. Toxicol., 13: 204-226.
TWORT, C. C. & TWORT, J. M. (1935) Induction of cancer by cracked
mineral oils. Lancet, November 30: 1226-1228.
TWORT, C. C., LYTH, R., & TWORT, J. M. (1937) The significance of
density, refractive index and viscosity of mineral oils in
relation to the type and degree of animal reaction. J. Hyg.,
37: 14-29.
TYE, R., BURTON, M. J., BINGHAM, E., BELL, Z., & HORTON, A. W. (1966)
Carcinogens in a cracked petroleum residuum. Arch. environ.
Health, 13: 202-207.
UK MEDICAL RESEARCH COUNCIL (1968) The carcinogenic action of mineral
oils. A chemical and biological study, London, Her Majesty's
Stationary Office, 8 pp. (Special Report Series No. 306).
UNGAR, H., HINE, C. H., KODAMA, J. K., & ANDERSON, H. H. (1955)
Neuropathology of rats experimentally poisoned with
p-tertiary-butyl-toluene. A.M.A. Arch. Pathol., 69: 139-149.
US DHEW (1970) Air quality criteria for hydrocarbons, Washington,
DC, United States Department of Health, Education and Welfare.
USSR (1972) [Sanitary norms for the planning of industrial
enterprises.] Moscow (in Russian).
VAN DER LINDEN, A. C. & THYSSE, G. J. E. (1965) The mechanisms of
microbial oxidations of petroleum hydrocarbons. Adv. Enzymol.,
27: 469-546.
VAUGHAN, C. G., WHEALS, B. B., & WHITEHOUSE, M. J. (1973) The use of
pressure-assisted liquid chromatography in the separation of
polynuclear hydrocarbons. J. Chromatogr., 78: 203-210.
VIGALJUK, R. V., ZAMALEEVA, Z. S., RUBINSKIJ, L. Ya., SOLODOVA, N. L.,
& LJUTAJA, T. I. (1977) [The chromatographic determination of the
total content of C1 C10 paraffin hydrocarbons and C6-C8
aromatics in the air of industrial plants.] Gig. Tr. prof.
Zabol., No. 6, pp. 57-57 (in Russian).
VOBORSKY, R. C. (1980 In: Dolberg, D. D. & Verstuyft, A. W, ed.
Analytical techniques in occupational health chemistry,
Washington, DC, ACS (ACS Symposium Series 120).
VOLK, B. W. (1964) Lipoid pneumonia: Clinical, pathologic and chemical
aspects. Biochem. Clin., 4: 187.
VOLKMANN, R. (1875) [On tar, paraffin, and soot cancer (chimneysweep's
cancer.] In: Beiträge zur Chirurgie, Leipzig, Breitkopf und
Hartel (in German).
VON LEHMDEN, D. J., VON HANGERBRAUCK, R. P., & MEEKER, J. E. (1965)
Polynuclear hydrocarbon emissions from selected industrial
process. J. Air. Pollut. Control Assoc., 15: 306-312.
WAGNER, W. D., DOBROGORSKI, O. J., & STOKINGER, H. E. (1961)
Antagonistic action of oil mists on air pollutants. Arch.
environ. Health, 2: 523-534.
WAGNER, W. D., WRIGHT, P. G., & STOKINGER, H. E. (1964) Inhalation
toxicology of oil mists. I. Chronic effects of white mineral oil.
Am. Ind. Hyg. Assoc. J., 25: 158-167.
WAHLBERG, J. E. (1974) Occupational and non-occupational scrotal
cancer in Sweden, 1958-1970. Acta Dermatovenerol., 54: 471-474.
WALKER, J. D, COLWELL, R. R., & PETRAKIS, I. (1975) A study of the
biodegradation of a South Louisiana crude oil employing
computerized mass spectrometry. In: Joint Conference on the
Prevention and Control of Oil Pollution, San Francisco, p. 197.
WALLCAVE, L, GARCIA, H., FELDMAN, R., LIJINSKY, W., & SHUBIK, P.
(1971) Skin tumorigenesis in mice by petroleum asphalts and
coaltar pitches of known polynuclear aromatic hydrocarbon content.
Toxicol. appl. Pharmacol., 18: 41-52.
WANG, C. C. (1961) Acute gasoline intoxication. Arch. environ.
Health, 2: 714-716.
WARING, J. I. (1933) Pneumonia in kerosene poisoning. Am. J. med.
Sci., 185: 325-330.
WATERHOUSE, J. A. H. (1971) Cutting oils and cancer. Ann. occup.
Hyg., 14: 161-170.
WATERHOUSE, J. A. H. (1972) Lung cancer and gastro-intestinal cancer
in mineral oil workers. Ann. occup. Hyg. 15: 43-44.
WATTENBERG, L. W. (1972) Dietary modification of intestinal and
pulmonary aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEISS, F. T. (1975) Activities of standardization groups in the
identification of waterborne oils. Paper read at National Bureau
of Standards Workshop - Santa Barbara, California, 6-7 October,
1975, 17 pp.
WEISSMAN, H. (1951) Lipoid pneumonia. Am. Rev. Tuberc., 64: 572-576.
WHO (1974) Toxicological evaluation of some food additives
including anti-caking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents, Geneva, World Health
Organization, pp. 417-422 (WHO Food Additive Series No. 5).
WHO (1982) Environmental health criteria 19: Hydrogen sulfide.
Geneva, World Health Organization.
WIEL, A. VAN DER (1975) Collaborative determination of precision of
DMSO extraction-refractive index method, Amsterdam,
Koninklijke/Shell Laboratory (Memorandum LFA/200/75).
WIEL, A. VAN DER (1979) Determination of potentially hazardous
poly-nuclear aromatics in petroleum fractions, Amsterdam,
Koninklijke/Shell Laboratory (Amsterdam Method Series, AMS 642-2).
WILLIAMS, R. T. (1959) Detoxication mechanisms, 2nd ed., New York,
John Wiley & Sons Inc.
WOOD, E. H. (1943) Unusual case of carcinoma of both lungs associated
with lipoid pneumonia. Radiology, 40: 193-195.
WOODHOUSE, D. L. (1934) The non-carcinogenicity of certain synthetic
lubricating oils and the action of some commercial spindle-oils,
solvent fractionated spindle oil distillates and tar oils, tested
under similar conditions. J. Inst. Petr. Technical.,
20: 1057-1063.
WOODHOUSE, D. L. (1950) The carcinogenic activity of some petroleum
fractions and extracts. J. Hyg. Camb., 48: 121-134.
YAGI, H., THAKKER, D. R., HERNANDEZ, O., KOREEDA, M., & JERINA, D. M.
(1977) Synthesis and reactions of the highly mutagenic 7, 8-diol
9, 10-epoxides of the carcinogen benzo-(a) pyrene. J. Am. Chem.
Soc., 99: 1604-1611.
YAMAMURA, Y. (1967) n-Hexane neuropathy. Folia psychiatr. neurol.
Jpn., 23: 45-57.
YANG, S. K., McCOURT, D. W., ROLLER, P. R., & GELBOIN, H. V. (1976)
Enzymatic conversion of benzo(alpha)pyrene leading predominantly
to the diol-epoxide r-7, t-8-dihydroxy-t-9, 10-oxy-7,8,9,10-
tetrahydrobenzo (a) pyrene through a single emantiomer of r-7,
t-8-dihydroxy-7, 8-dihydrobenzo-pyrene. Proc. Natl Acad. Sci.
US, 73: 2594-2598.
YOKOO, H. & SUCKOW, E. E. (1961) Peripheral lung cancers arising in
scars. Cancer, 14: 1205-1215.
YOUNG, A.M., APPLEBAUM, H. S., & WASSERMAN, P. B. (1939) Lipoid
pneumonia. J. Am. Med. Assoc., 112: 2406-2409.
ZANGGER, H. (1933) [Occupational accidents and hazards at work inside
enclosed spaces (reservoirs, tanks, and transport lorries.]
Arch. Gewerbepathol. Gewerbehyg., 4: 117-140 (in German).
ZIELHUIS, R. L. (1961) [Early complaints occurring on exposure to
organic solvents.] Tijdschr. Soc. Geneesk., 39: 595-600
(in Dutch).
ZINGMARK, P. A. & RAPPE, C. (1977) On the formation of
N-nitrosodi-ethanolamine in a grinding fluid concentrate after
storage. Ambio, 6: 237-238.
See Also:
Toxicological Abbreviations
 2.2.2.3 Disposal of waste
In a refining process, the release of oil into refinery effluents
is practically negligible and of a lower order of magnitude than
tanker washings in tankers that do not use the "load-on-top" system.
Waste gas in production fields is generally burnt on the spot. In
refineries and chemical plants, it may be necessary to burn some gas
at a flare for reasons of safety, and some oil and gas is consumed as
refinery fuel. Atmospheric pollutants in and around refineries
basically consist of saturated and unsaturated hydrocarbons, carbon
monoxide, hydrogen sulfide, and sulfur dioxide (Poliansky &
Musserskaja, 1971; Krasovitskaja, 1976). Sulfur dioxide, hydrogen
sulfide, and mercaptan emissions are not discussed in this review and
emissions of hydrocarbon vapours into the atmosphere from storage
terminals, filling stations, and cars will be covered in another
document.
2.3 Toxicological Effects of Crude Oils
As, in this document, crude oils are discussed only to provide
background information for the petroleum solvents, lubricating base
oils, and bitumens derived from them, no detailed discussion will
follow concerning environmental exposure levels, environmental
distribution and transport, physiological factors relating to
mammalian uptake, dose-response relationships, and maximum permissible
levels. Most of the relevant aspects will, however, be covered in the
sections on fractions derived from the crude oils. This also applies
to toxicological effects on experimental animals and man, with the
exception of a very few studies that are related to crude oil exposure
only.
The toxicological and nuisance aspects of hydrogen sulfide and
mercaptans have been reviewed in detail by Miner (1969) and Sullivan
(1969). A review on hydrogen sulfide has been prepared by NIOSH
(1977c) and an Environmental Health Criteria document on hydrogen
sulfide has recently been published (WHO, 1982).
2.3.1 Effects on experimental animals
Leitch (1924) examined 16 untreated crude oils from various parts
of the world for their carcinogenicity by applying them 3 times a week
to the skin of mice and found significant differences in
tumorigenicity among these oils. Similar results were reported by
Hieger & Woodhouse (1952) in skin tests on mice and rabbits. The
tumorigenicity of the crude oils they examined was low in comparison
with that of some of the distilled fractions. Skin tests were also
carried out on mice and rabbits by Antonov & Lints (1960), who found
that Saratov oil possessed weak carcinogenic properties. The main
causes of death in these tests, however, were pneumonia and general
intoxication, probably from absorption of oil components through the
skin. The authors found that rabbits were more sensitive than mice, as
did Hieger & Woodhouse (1952).
Batt-Neal & Wolman (1977) demonstrated skin tumorigenicity and
amyloid deposition following skin exposure of mice to saturated
acetone extracts of various oils collected from beaches.
2.3.2 Effects on man
Examination of 743 oilfield workers exposed to California crude
oil and excessive sunlight revealed that 7 of them had epitheliomas on
exposed parts of the body and that nearly 20% had keratotic changes on
the hands, forearms, face, and neck. Five of the 7 subjects, who
developed epitheliomas, were blonds, though blonds were in the
minority in this group of workers (Schwartz et al., 1947).
During 1938-39, Schwartz saw 189 cases of carcinomas on exposed
parts of the skin; 128 were in males, 71 of whom were oilfield workers
20 others being workers exposed to excessive sunlight only. Emmett
(1975) mentions the strong potentiating effect of UV radiation on
other potentially carcinogenic exposures. In southern Texas, however,
the incidence of skin carcinomas in 330 oilfield workers was low,
which underlines the fact that Texas and Pennsylvania oils are known
to be less carcinogenic than California oil (Twort & Ing, 1928).
In a study on 50 volunteer operators, who had not previously been
in contact with oil and petroleum products, crude oil was applied to
the skin of the inner surface of the forearm, for periods of 3-6 h. An
inflammatory reaction of the skin developed with moderate erythema,
oedema, and slight burning. Changes in the thermosensitive threshold
were noted, as well as an increase in the permeability of the
epidermis (Gusein-Zade, 1975).
3. PETROLEUM SOLVENTS
3.1 Properties and Analytical Methods
3.1.1 Chemical composition and properties
Only solvents consisting of hydrogen and carbon alone and
produced from petroleum will be considered in this review. It should
be noted, however, that similar solvents are also produced from coal.
Petroleum solvents consist of complex mixtures of hydrocarbons
reflecting the hydrocarbon constituents of the crude oil or, more
usually, the intermediate refinery streams from which they are
distilled. Because of their complex nature, classification is a
problem and no standard, worldwide-accepted nomenclature exists.
However, providing that it is recognized that considerable overlapping
and many exceptions occur, they can be classified into 3 broad
subdivisions, based on distillation ranges:
(a) special-boiling-point solvents (SBPs) - grades with narrow or
wide distillation ranges within the main limits of 30-160°C;
(b) white spirits - grades distilling within the main range
150-220°C, the boiling-points of individual grades usually
ranging over more than 20°C;
(c) high-boiling aromatic solvents - grades distilling in the range
160-300°C with final boiling-points above 220°C.
Benzene, toluene, and the xylene isomers occur as components of
petroleum solvents, but as they fall more naturally into the category
of chemical intermediates, they will be referred to here only in so
far as they are important as components of the mixtures being
discussed.
Two further clarifications can be made. Firstly, it is common
industrial practice to ascribe the name of the predominant isomer
present to the petroleum solvent; thus the descriptions pentane,
isopentane, hexane, isohexane, and heptane are commonly met. However,
in almost all cases, the amount of the named isomer present in an
industrial scale product will not exceed 95% v/v of the solvent and
may be as small as 30% v/v.
Second, most petroleum solvents are marketed on the basis of
typical physical properties rather than on chemical specifications,
because of the limitations during refining of controlling the complex
mixtures of isomers that make up the petroleum solvents. As production
techniques become more sophisticated, greater control is possible and
more properties can be specified within narrower limits. However, even
when such narrow limits are met, the mixture of components present may
vary, because of variations in the types of crude oil being processed
and alterations in conditions in processing units.
To meet the wide range of properties required by the market,
several different processes are used. Distillation is the common
process setting the volatility range. Chemical conversion techniques,
including reforming, alkylation, and hydrogenation, alter the chemical
composition and hence the solvency, as do physical conversion
techniques such as solvent extraction and molecular sieve separation.
Specific treatments such as caustic soda and sulfuric acid washing and
clay percolation are frequently applied to remove odourous substances,
chiefly sulfur compounds.
The reader is referred to Boenheim & Pearson (1973) for detailed
discussions of the chemical and physical composition and uses of
petroleum solvents.
3.1.1.1 Special-boiling-point solvents (SBPs)
These are highly purified naphtha fractions with specially
selected boiling ranges. The boiling range may be narrow or wide, and
generally falls within the limits of 30-160°C: SBPs are classified
according to their boiling range, e.g., SBP 62/82. Petroleum ether,
lighter fluid, spot remover, and rubber solvent are consumer products
in this range. Generally, SBPs consist of a mixture of hydrocarbons in
the C-5 to C-9 range: normal and branched paraffins, cycloparaffins,
and aromatic compounds. They contain only traces of olefins. An
example of the composition of a typical sample of straight run (i.e.,
non-dearomatized) SBP 80/110 is given in Table 2.
3.1.1.2 White spirits
The boiling-range of this group of solvents falls within the
limits 150-220°C (intermediate between gasoline and kerosene). These
solvents can be classified into low-aromatic grades (approximately
15-20% aromatic hydrocarbons) and high-aromatic grades (45% or more
aromatic hydrocarbons). They generally consist of hydrocarbons in the
C-7 to C-12 range, again including normal and branched paraffins as
well as naphthenic (cycloparaffins) and aromatic compounds. Olefins
are present in trace amounts only. Stoddard solvent, mineral spirits,
low-aromatic white spirits (LAWS) and turpentine-substitute are
well-known examples from this range.
3.1.1.3 High-boiling aromatic solvents
Aromatic hydrocarbons occur naturally in certain crude oils in
widely varying concentrations. They are also formed during secondary
processes such as thermal and catalytic reforming. They can be
concentrated and extracted by solvent extraction.
Apart from benzene, toluene, and xylene, which will not be
discussed separately in this review, this group includes solvents with
an aromatic content of 80-100%, and a wide boiling-range from 160 to
300°C. High-boiling aromatic solvents are obtained by distillation or
solvent extraction from refinery fractions such as kerosene and
lubricating base oils, and consist of very complex mixtures of
hydrocarbons with more than 9 carbon atoms per molecule. The
composition of a typical sample of one of these aromatic hydrocarbons
from the middle range (distillation range approximately 192-203°C) is
given in Table 3.
Most aromatic solvents are highly purified "white" solvents.
Those in the higher boiling range, derived from lubricating base oil
stocks by solvent extraction, may be less pure and coloured. They are
often by-products and are used as solvents for various technical
purposes. In many cases, they are referred to as "processing oils"
instead of solvents, and considered under lubricating oils.
3.1.2 Purity of petroleum solvents
In these complex mixtures, impurity is, of course, a matter of
definition. Components that are taken out in the course of the various
refining and treating processes used to obtain the more pure solvents
could be regarded as such. The major impurities would then be sulfur
compounds such as hydrogen sulfide, mercaptans, and thiophens, as well
as, olefins and other reactive unsaturated hydrocarbons.
A second category of impurities includes the hydrocarbons that
have been demonstrated to be carcinogenic in animals and man, such as
benzene, the polynuclear hydrocarbons and related heterocyclic
compounds containing nitrogen or sulfur.
TABLE 2. Composition of typical sample of SBP 80/110a
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
normal n-pentane 0.2 36.2
paraffins n-hexane 8.2 69.0
n-heptane 17.2 98.4
branched 2 methyl butane Tc 0.1 27.9
paraffins 2,2 dimethyl butane T trace 49.7
2,3 dimethyl butane T 0.3 58.0
2 methyl pentane 1.5 60.3
3 methyl pentane 1.6 63.3
2,2 dimethyl pentane 1.0 79.2
2,4 dimethyl pentane 1.3 80.5
2,2,3 trimethyl butane T 0.3 80.9
2,3 dimethyl pentane 9.7 89.8
3 methyl hexane 9.2 91.9
3 ethyl pentane 3.1 93.5
2,2,4 trimethyl pentane trace 99.2
2,2 dimethyl hexane trace 106.8
2,5 dimethyl hexane 0.6 109.1
3,3 dimethyl hexane T trace 112.0
2,3 dimethyl hexane 0.8 115.66
3,4 dimethyl hexane trace 117.7
3 methyl heptane 0.5 118.9
cyclo C-6 cyclohexane 8.4 80.7
paraffins methyl cyclohexane 14.2 100.9
cyclo C-5 cyclopentane T trace 49.3
paraffins methyl cyclopentane 4.7 71.8
1,1 dimethyl cyclopentane T 2.9 87.9
1-cis-3-dimethyl 1.9 90.8
cyclopentane T
TABLE 2. (contd).
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
cyclo C-5 1-trans-3-dimethyl 2.7 91.7
paraffins cyclopentane T
contd. 1-trans-2-dimethyl 0.5 91.9
cyclopentane T
1-cis-2-dimethyl cyclopentane T 0.5 99.5
ethyl cyclopentane 0.6 103.5
1,1,3 trimethyl cyclopentane T 0.8 104.9
1-trans-2-cis-4-trimethyl 0.4 109.3
cyclopentane T
1-trans-2-cis-3-trimethyl 0.4 110.2
cyclopentane T
1,1,2 trimethyl cyclopentane T 0.3 113.7
unidentified Probably
paraffins 1.1 110.0
aromatic benzene 0.7 80.1
compounds toluene 3.9 110.6
olefins 0.4
a From: Shell International Petroleum Co., London (unpublished data).
b Average of duplicate analyses.
c T = tentative identification.
TABLE 3. Composition of typical sample of Solvesso 150a
Hydrocarbon % v/v of solvent
n-butylbenzene 2.47
sec-butylbenzene 0.08
tert-butylbenzene 0.05
m-cymene 0.13
o-cymene 0.01
p-cymene 0.52
1.2-diethylbenzene 1.72
1.3-diethylbenzene 1.10
1.4-diethylbenzene 0.56
1.2-dimethyl-3-ethylbenzene 2.86
1.2-dimethyl-4-ethylbenzene 6.64
1.3-dimethyl-2-ethylbenzene 0.71
1.3-dimethyl-4-ethylbenzene 4.17
1.3-dimethyl-5-ethylbenzene 2.80
1.4-dimethyl-2-ethylbenzene 3.26
m-ethyltoluene 0.37
o-ethyltoluene 0.02
p-ethyltoluene 0.01
indane 0.46
isobutylbenzene 0.32
isopropylbenzene 0.01
1-methyl-3-t-butylbenzene 0.76
1-methyl-2-n-propylbenzene 1.26
1-methyl-3-n-propylbenzene 2.08
1-methyl-4-n-propylbenzene 1.93
1-methylindane 0.91
2-methylindane 2.43
4-methylindane 9.28
5-methylindane 2.02
naphthalene 4.03
n-propylbenzene 0.00
1.2,3,4-tetramethylbenzene 3.66
1.2,3,5-tetramethylbenzene 8.84
1.2,4,5-tetramethylbenzene 5.53
toluene 0.02
1.2,3-trimethylbenzene 0.10
1.2,4-trimethylbenzene 0.05
1,3,5-trimethylbenzene 0.01
a Courtesy Esso Standard Oil Company, New York, N.Y.,
USA (From: Gerarde, 1960).
TABLE 3. (contd).
Hydrocarbon % v/v of solvent
m-xylene 0.05
o-xylene 0.03
p-xylene 0.03
C-11-naphthalenes 0.31
C-11-indanes 3.58
C-11-alkylbenzene 18.27
C-12-alkylbenzene 0.73
C-12-indanes 0.08
C-13-alkylbenzene 0.02
C-10-indenes 0.10
C-11-indenes 0.07
C-12-naphthalenes trace
C-13-naphthalenes
C-12-indenes 0.10
aromatic compounds Total 94.55
Generally, the total sulfur content, the olefin content, and the
total aromatic content are specified for commercial petroleum
solvents. Where special products such as food-grade materials are
concerned, the benzene content is specified as well as the UV
absorption limits at certain wavelengths, as a measure of the
polynuclear aromatic hydrocarbon content.
3.1.3 Methods of sampling and analysis
See section 2.1.2.
3.2 Sources of Environmental Pollution
3.2.1 Natural occurrence
Petroleum solvents do not occur in nature as such, but only as
components of the crude oils from which they are derived.
Environmental pollution is always man-made and related to the use of
the solvents.
3.2.2 Man-made sources
3.2.2.1 Production
Because there is no uniform system of definition and
classification of petroleum solvents, firm statistics concerning the
magnitude of production of this group of materials do not exist. The
best estimate of the world-wide production of the group of solvents
would be 9 million tonnes for the year 1979.
3.2.2.2 Uses
It is not feasible to give more than a general outline of the
uses of the range of petroleum solvents.
(a) SpeciaL-boiling-point solvents (SBPs)
SBPs are mainly used as: solvents and thinners in lacquers and
paints; extraction solvents for perfumes, for vegetable oils and oil
and fats of animal origin; quick-drying solvents in printing-ink,
coatings, and adhesives; lighter fuel; and for dry-cleaning and
degreasing purposes.
(b) White spirits
White spirits are mainly used as: solvents and thinners for
lacquers, paints, resins, and printing-ink; solvents in formulations
of chemical products, e.g., pesticides; and for metal degreasing, wool
degreasing, and dry-cleaning.
(c) Aromatic extracts
The higher-boiling and less-purified aromatic extracts have very
good solvent properties for many polymers and are used as
ex-tender-oils in rubber, plastics, and bitumens, and also as solvents
in printing-ink and pesticide formulations. Furthermore, they can be
used as base-materials in the manufacture of carbon black.
3.3 Environmental Exposure Levels
Specific data are not available concerning levels of petroleum
solvents in air, water, food, or other environmental media. However,
low concentrations of hydrocarbons found in mussels have probably been
derived from petroleum hydrocarbons present in the environment
(Ehrhardt & Heineman, 1975).
Because of the relatively low boiling-range of these solvents,
industrial exposure to vapour may sometimes be high. This is known to
occur, especially in small workshops with insufficient ventilation,
where, for example, adhesives are used routinely. Although a lot of
consumer products may contain these solvents, excessive domestic
exposure would not normally be expected unless neat solvent were used
for cleaning purposes, indoors. Very limited, indirect exposure of the
general population is possible following the use of these solvents as
extractants in the production of food-grade vegetable oils.
Exposure to the higher-boiling and less-purified aromatic
extracts is mainly confined to occupational situations, where
excessive skin-contact may occur, or exposure to vapour in processes
carried out at elevated temperatures or with high-speed machines that
could give rise to fumes or mists. This will be considered in detail
under lubricating base oils.
3.4 Environmental Distribution and Transformation
Data on the distribution between media, environmental
transformation and degradation, interaction with physical, chemical,
or biological factors and bioconcentration, are not available for
petroleum solvents.
However some information exists concerning the behaviour and
degradation of crude oil in water (Floodgate, 1972, Hellmann & Zehle,
1972), and of hydrocarbons in general (Walker et al., 1975), and there
is much information on the microbial degradation of individual
petroleum hydrocarbons (Van der Linden & Thysse, 1965; Haines &
Alexander, 1974).
From these publications it can be seen that the subject is highly
complex and many factors have to be taken into account, such as the
composition of the oil product, the extent of dispersion into the
medium, and climatic conditions.
3.5 Metabolism
3.5.1 Absorption
The kinetics are determined by diffusion rates, solubility in
fat, and the concentration gradients in the individual compartments of
the body.
The highly volatile C-5, C-6, and C-7 paraffins, cycloparaffins,
and aromatic hydrocarbons readily pass across the alveolar membrane
into the bloodstream and are transported within minutes to the central
nervous system. Longer-chain homologues can, to a certain extent, also
pass the alveolar membrane, but their principal effect is local. This
was shown by Gerarde (1963) in studies on rats.
The alveolar air and blood concentrations of white spirit have
been measured in man following inhalation (Åstrand et al., 1975).
Aromatic hydrocarbons were absorbed to a greater extent into the
bloodstream than aliphatic hydrocarbons (approximate values being 62%
and 50%, respectively). Similar uptake values in man were shown for
the aromatic hydrocarbons, benzene and toluene, by Nomiyama & Nomiyama
(1974), for xylene by Sedivec & Flek (1976), Åstrand et al. (1978),
and Riihimäki et al. (1979), and for ethylbenzene by Bardodej &
Bardodejová (1970). Nomiyama & Nomiyama (1974) demonstrated a much
lower pulmonary absorption for n-hexane, the only aliphatic compound
that they tested; it was also rapidly excreted.
The skin is only permeable to hydrocarbons of a certain size.
With paraffinic substances, the maximum chain length appeared to be up
to 14 C-atoms (Scheuplein & Blank, 1971). Aromatic compounds have a
more compact structure and, in studies on guineapigs, Hoekstra &
Phillips (1967) showed that compounds from this group with a higher
number of C atoms could still pass the skin barrier.
The absorption of vapours through the skin is of minor
importance. For example, in man, whole body skin exposure to
2250 mg/m3 (600 ppm) of toluene was equivalent to an inhalation
exposure of less than 37.5 mg/m3 (10 ppm) (Riihimäki & Pfäffli,
1978). However, absorption during immersion in liquid solvents may be
considerable. Percutaneous absorption during immersion of both hands
in pure xylene was equal to an inhalation exposure of 435 mg/m3
(100 ppm) (Engström et al., 1977). The permeation of xylene is thus
about 20 nmol/min per cm2 (Engström et al., 1977; Riihimäki, 1979)
and that for toluene, 3 µmol/min per cm2 (Cohr & Stockholm, 1979).
Cutaneous exposure was probably a major route of absorption in 2 cases
of acute renal failure with oliguria, caused by exposure to diesel oil
(Barrientos et al., 1977; Crisp et al., 1979).
Data for absorption in the intestinal tract are not available,
but it is presumed that it would resemble absorption in the alveoli
rather than that through the skin.
3.5.2 Distribution in the body
Tissue hexane levels in rats, following inhalation of anaesthetic
concentrations, were measured by Böhlen et al. (1973). The tissue
distribution generally depended on exposure time and was proportional
to the lipid content of an organ until saturation occurred. The liver
was a special case for, as its lipid level changed rapidly, the
saturation level varied. Hexane was also apparently bound to some
blood components.
Women working at conveyor belts gluing parts of rubber footwear
had concentrations of petroleum solvents (no details on
physicochemical properties given) in the blood ranging from 2 . 35 ±
0.4 up to 4.6 ± 0.6 mg/litre at concentrations in the air of
100-300 mg/ m3. The solvent concentration in the blood increased with
increasing length of the working period from 1.6 mg/litre in the first
year to 2.5 mg/litre after 3.5 years and 3.4 mg/litre after 7-8 years
of service.
Wistar rats were exposed to the solvents used in the factory at
concentrations in air of 300-1000 mg/m3 for 30-45 days, 4 h/day. The
concentration of solvent in the blood amounted to 0.45 ± 0.05 -
1.2 ± 0.01 mg/litre (Lipovskij et al., 1977a).
Transfer of petroleum solvents through the placenta was studied
in 85 pregnant women workers in the rubber industry, who came into
contact with petroleum solvents during work (physicochemical
properties of the solvents not defined, concentration in the air of
the operating premises 300 ± 10 mg/m3). The average level of solvents
in the blood of 46 pregnant women, on whom abortion was performed, was
1.27 ± 0.3 mg/litre. A level of 3.29 ± 0.6 mg/kg was found in the
tissue of the embryo. Women giving birth to a child (39 women) had a
level of solvents in the blood of 2.5 ± 0.3 g/litre, while the content
in the blood of the umbilical cord was 3.5 ± 0.3 g/litre. The
concentration of solvents in the blood of the newborn infants was
twice that of the mothers.
Pregnant Wistar rats were exposed to the same solvent at a
concentration of 300 ± 10 mg/m3, for 48 days, 4 h per day. The
solvent was present in the blood, brain, liver, placenta, uterus, and
fetal tissues (Lipovskij et al., 1979).
3.5.3 Biotransformation
In both man and animals, the aliphatic hydrocarbons are generally
considered to be biochemically inert and excreted in the same form
(Williams, 1959). However, it has been shown that some normal alkanes
will, at least in part, be oxidized by the mammalian organism. For
example, Ichihara et al. (1969) demonstrated the oxidation of decane
in animals such as mice and rats, and the oxidative pathway of
n-hexane to hexane-2,5-dione and hexane-2,5-diol via
methyl- n-butylketone has been well established (see for example
Spencer et al., 1978).
As far as the metabolism of the cycloparaffins and aromatic
hydrocarbons is concerned, the half-life, form, and rate of excretion
of each component of the solvent has to be considered. It should be
mentioned, however, that the metabolism of individual compounds will
not be discussed in this document and readers are referred to the
reviews by Williams (1959) and Gerarde (1960).
The carcinogenicity of the solvents is thought to be due to the
presence of benzene and some of the polynuclear aromatic compounds.
3.5.4 Elimination
The elimination of the lower-boiling solvents (SBP type) in both
animals and man is usually rapid and mainly occurs via the respiratory
tract. However, in the case of ingestion of the heavier solvents
(white spirits), elimination mainly takes place with the faeces
(Browning, 1965).
3.6 Effects on Experimental Animals
It has been mentioned in section 3.1.1, that the petroleum
solvents under discussion in this document are more or less complex
mixtures of a range of hydrocarbons. For the commercial products, the
specification given generally includes the specific gravity,
boiling-range, and total content of aromatic hydrocarbons. The
concentrations of individual components vary, within certain limits,
with the source of the crude oil from which the solvent is derived,
and with the processes by which it is produced. These facts should be
kept in mind because:
(a) the toxicity data developed for a certain solvent-specification
indicate the order of magnitude of the toxicity of this type of
product;
(b) in practice it would be impossible and impracticable to carry out
complete toxicity testing on every single solvent on the market.
It is only sensible to develop toxicity data for typical
representative samples of a certain boiling range and within a
certain specification of aromatic content. In the evaluation of
the results, however, the analytical composition of the material
- especially its contents of n-hexane, benzene, and polynuclear
aromatic hydrocarbons should be taken into account.
3.6.1 Short-term exposure
Hine & Zuidema (1970) examined various aspects of the acute
toxicity of 10 samples of petroleum solvents that contained components
representative of the range of hydrocarbons found in commercial
petroleum solvents. Four were aromatic solvents containing at least
98% aromatic hydrocarbons (coded A) and 6 were non-aromatic solvents
containing less than 1% aromatic hydrocarbons (coded S). The boiling
ranges and principal components of the samples examined are given in
Table 4.
Acute oral, inhalation, and percutaneous toxicity and skin and
eye irritancy were examined for all samples. Intratracheal aspiration
was simulated with 2 samples and repeated skin irritation tests were
carried out using 5 of the samples. Undiluted samples were used for
the investigations, all of which were carried out on rats with the
exception of skin and eye irritancy and skin toxicity rests in which
rabbits were used.
TABLE 4. The boiling-range and principal components of solvents examined for
acute toxicitya
Sample Boiling-range Principal components
A-1 281-286°F (138-141°C) C-8 aromatic compounds (ortho, meta, and
paraxylene; ethyl benzene)
A-2 362-398°F (163-203°C) C-9, C-10 and C-11 aromatic compounds
A-3 364-408°F (188-209°C) C-10 and C-11 aromatic compounds
A-4 384-507°F (196-264°C) C-11 to C-14 aromatic compounds
S-1 149-166°F (65-75°C) C-6 normal and isoparaffins (hexanes) and
naphthenes (cyclohexane, methylcyclopentane)
S-2 196-220°F (91-104°C) C-7 normal and isoparaffins (heptanes) and naphthenes
(methylcyclohexane, dimethylcyclopentane)
S-3 313-356°F (156-180°C) C-9 and C-10 normal and isoparaffins and naphthenes
S-4 368-395°F (187-212°C) C-11 and C-12 normal and isoparaffins and naphthenes
S-5 345-402°F (174-216°C) C-12 isoparaffins
S-6 384-500°F (195-260°C) C-13 to C-16 normal and isoparaffins and naphthenes
a From: Hine & Zuidema (1970).
The findings of Hine & Zuidema (1970) which are summarized in
Table 5, showed that all the solvents tested could be considered of
low hazard to health unless aspirated or inhaled in extremely high
concentrations. Aromatic solvents were more toxic than non-aromatic
materials, the dose of solvent required to kill 50% of rats, when
administered orally or percutaneously, being lower for aromatic than
for non-aromatic solvents. Skin and eye irritancy were also greater
with aromatic solvents. The toxicity of the vapours could not be
compared, because the volatility of samples varied greatly. All
solvents induced similar toxic effects, whatever the route of
administration, including central nervous system depression
(characterized by incoordination, prostration, and coma) followed by
death. Convulsions sometimes occurred. All solvents caused skin and
eye irritation though, in general, as the chain length of the
non-aromatic solvents increased their irritant properties decreased.
Repeated skin exposure led to skin irritation and necrosis with all
solvents.
Hoekstra & Phillips (1963) found that light mineral oils, when
applied topically to the skin of guineapigs, caused epidermal
hypertrophy, hyperplasia, hyperkeratosis, and depilation. Examination
of the effects of various oil fractions demonstrated that the main
effect of the short-chain volatile paraffins was to defat the skin,
while longer-chain and aromatic hydrocarbons had a dermatoxic effect
that was related to the permeability of the skin to these compounds.
The maximum dermatoxic effect was seen with hydrocarbons containing
14-19 carbon atoms, while a transition to non-dermatoxicity occurred
around 21-23 carbon atoms. This was confirmed with pure n-paraffins,
but variations may exist with other types of hydrocarbons.
Simultaneous application of innocuous long-chain substances together
with irritant short-chain substances greatly reduced their toxicity,
though this effect was less marked with aromatic solvents.
In further studies on the effects of inhaling the vapours of
hydrocarbon solvents (Carpenter et al. 1977a, b, c), the acute (4-h
exposure) LC50 and no-observed-adverse-effect concentrations were
studied in rats cats, and dogs. Results are summarized in Table 6.
These studies confirmed the occurrence of central nervous system
depression and there was also evidence of respiratory tract irritancy.
There were no marked or consistent differences between the species
examined. The major factor determining the acute inhalation hazard was
the volatility of the solvent, those containing 9 or more carbon atoms
tending to be insufficiently volatile to produce concentrations high
enough to be lethal over a short period of exposure. One exception was
a "high naphthenic" solvent, which was peculiar also in that
depression was not preceded by signs of irritation of the respiratory
tract, so that there was no warning of overexposure. Increased
aromatic content did not consistently result in increased inhalation
toxicity, though earlier work (Lazarew, 1929) suggested that the acute
inhalation toxicity of gasoline vapours increased with increasing
contents of cycloparaffins and aromatic hydrocarbons. The narcotic
action was also found to increase in each step by a factor of 3 in the
series - pentane, hexane, heptane, and octane (Fühner, 1921). Swann et
al (1974) found that anaesthesia occurred with these compounds at
concentrations of 32 000 ppm or more and that respiratory tract
irritation increased with chain length. Full anaesthesia can be
produced with gasoline (Haggard, 1921), but anaesthetic concentrations
are little lower than those that cause convulsions and death
(Browning, 1965).
TABLE 5. Toxicity of solvents. Summary of resultsa
Test Sample Result Classification
Oral A-1 10.0(7.5-13.3) practically non-toxic
LD50 A-2 4.5(3.0-6.8) slightly toxic
(ml/kg) A-3 13.3(7.5-23.7) practically non-toxic
A-4 12.3(8.1-18.7) practically non-toxic
S-1 >25.0b relatively harmless
S-2 >25.0b relatively harmless
S-3 >25.0b relatively harmless
S-4 >25.0b relatively harmless
S-5 >25.0b relatively harmless
S-6 >25.0b relatively harmless
Vapour A-1 6 350(4 670-8 640) slightly toxice
exposure A-2 >2 450b SVNTARTd
LC50 in ppm A-3 >580c SVNTART
for 4 h A-4 >553c SVNTART
S-1 73 680(66 310-79 940) practically non-toxic
S-2 14 000-16 000 practically non-toxic
S-3 2 000-2 600 slightly toxic
S-4 >710 SVNTART
S-5 >792 SVNTART
S-6 >263 SVNTART
Aspiration A-4 5/10 hazardous
(mortality) S-6 5/10 hazardous
Primary A-1 2.21 moderately irritating
skin A-2 2.04 moderately irritating
irritation A-3 2.17 moderately irritating
A-4 2.79 moderately irritating
S-1 1.92 slightly irritating
S-2 1.13 slightly irritating
S-3 2.38 moderately irritating
S-4 1.04 slightly irritating
S-5 1.29 slightly irritating
S-6 0.75 minimally irritating
Eye A-1 6.33 moderately irritating
irritation A-2 6.0 moderately irritating
A-3 4.33 moderately irritating
A-4 3.67 slightly irritating
S-1 0.33 minimally irritating
S-2 1.0 minimally irritating
S-3 2.0 minimally irritating
S-4 0 minimally irritating
S-5 0 minimally irritating
S-6 0 minimally irritating
TABLE 5. (contd).
Test Sample Result Classification
4-h A-1 approx. 5.0 practically non-toxic
percutaneous A-2 approx. 5.0 practically non-toxic
LD50 rangefind A-3 approx. 5.0 practically non-toxic
(ml/kg) A-4 approx. 5.0 practically non-toxic
S-1 >5.0 practically non-toxic
S-2 >5.0 practically non-toxice
S-3 >5.0 practically non-toxice
S-4 approx. 5.0 practically non-toxice
S-5 5.0 practically non-toxice
S-6 5.0 practically non-toxice
Repeated benzene 3.6
skin toluene 3.5
irritationf A-1 3.3
a From: Hine & Zuidema (1970).
b Doses above this amount not practical for testing.
c Maximum concentration obtainable at 25 °C.
d SVNTART = Saturated vapours not toxic at room temperature.
e Lowest toxicity classification may be "relatively harmless"
f Scored according to the method of Draize.
The greatest health hazard arises when hydrocarbon solvents are
aspirated into the lungs. This rapidly induces acute chemical
pneumonitis, which is characterized by pulmonary oedema and
haemorrhage, and is generally fatal (Waring, 1933; Lesser et al.,
1943; Gerarde, 1959). Gerarde (1959) demonstrated that the ratio of
the oral and intratracheal LD50s was 140:1 for kerosene, the
intra-tracheal LD50 being 0.2 ml for rats. This and other evidence
demonstrated that pulmonary injury was caused by direct contact with
the solvent and not by solvent present in the blood, following its
absorption through the gastrointestinal tract.
TABLE 6. Toxicity of solventsa
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
V.M. & P. 118-151 55.4 32.7 11.9 C-7 to C-10 16 >8 >19 2.8 2.8 0.7-7 2.1 2.0
Naphtha (3400) (>1700) (>4100) (600) (800) (0.15- 1.5) (450) (430)
Stoddard 153-194 47.7 37.6 14.7 C-8 to C-12 >8.2 >2.9 > 10 1.1 1.9 0.5 - 5 7.85 1.15
solvent (>1400) (>510) (>1700) (190) (330) (09-.9) (>150) (200)
Rubber 75-112 41.4 53.6 4.9 C-6 to C-7 61 >5.9 >49 7.9 7.9 40 1.7 1.7
solvent (15000) (>1500) (>12000) (2000) (2000) (10) (430) (430)
Mixed 138-141 - - 100 C-8 29 >5.4 <41 3.5 3.5 0.6-6 >0.46 0.46
xylenes (6700) (>1200) (<9500) (810) (180) (.14-1.4 (>110) (110)
'60' 128-159 c28.8 c21.4 c49.5 C-8 to C-9 24 >9.5 <20 0.44 1.4 10 C. 1.3 0.44
Solvent (4900) (>1900) (<4100) (90) (280) (2) (260) (90)
'70' 157-211 16.5 15.7 67.8 C-9 to C-11 >4.4 >2.4 >2 1.1 1.1 4 0.32 0.32
Solvent (>810) (>440) (>370) (200) (200) (0.7) (59) (59)
'140' 184-205 60.8 35.7 3.4 C-5 to C-12 >0.27 >0.21> > >0.23 >0.23 4 0.31 0.23
Flash (sic) (>43) (>33) saturated (>37) (>37) (0.6) (49) (37)
aliphatic vapour
solvent'
'80' 96-142 9.7 18.9 71.4 C-6 to C-8 27 >2.1 >24 > 1.7 > 1.7 4 0.45 0.45
Thinner (6200) (>480) (>5500) (>390) (>390) (0.9) (100) (100)
'50' 98- 105 66.3 0.6 33.1 C-7 33 >3.4 >30 2.4 2.4 10 1.7 1.7
Thinner (8300) (>600) (>7600) (600) (600) (2.5) (430) (430)
Deodorised 208-272 55.2 40.~9 3.9 not stated >0.1 This value applies for all toxicity evaluation and is 0.1
kerosene probably (14) a saturated atmosphere at room temperature. (14)
C 12 to
C-15
TABLE 6. (contd)
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
'40' 187-231 35.4 32.9 31.5 C-9 to C-13 >0.2 >0.25 >7 0.22 0,22 1 0.21 0.15
Thinner (>33) (>41) (aerosol) (36) (36) (0.17) (35) (25)
Toluene 95-110 38.7 15.4 45.9 C-6 to C-7 35 >3 >31 3.9 3.9 10 1.9 1.9
concentrate (8800) (>760) (>7800) (980) (980) (2.5) (480) (480)
'High 184-206 0.3 0.8 98.9 C-9 to C-12 >0.38 This value applies for all animal 0.4 0.15 0.15
aromatic (>66) toxicity evaluations and is a (0.07) (26) (26)
solvent saturated atmosphere at room temperature
'High 157- 183 29.0 69.9 1.1 not stated 5.3 3.8 - 2.1 - - - 2.1
naphthenic probably (960) (>690) (380) (380)
solvent' C-9 to
C-11
'Naphthenic 151-200 24.7 37.2 38.1 C-9 to C-11 >10 - - 2.2 - - - 2.2
aromatic (some (380) (380)
solvent' aerosol
present)
a Summary of data from Carpenter et al. (1977a,b,c).
b p = paraffins, N = naphthenes, A = aromatic compounds.
c For details of actual length of LC50 exposure, see original papers.
d NEL/No-ill effect/exposures 6th/day, 5 days/week for 13 weeks.
The same author found that viscosity appeared to be the property
that determined the aspiration hazard of liquid hydrocarbons.
Lower-boiling-point solvents (B.P. up to 100°C) evaporated so rapidly
in the mouth of anaesthetized rats that death was due to CNS
depression following absorption of the vapours. Higher-boiling-point
solvents tended to induce chemical aspiration pneumonitis. With
alkanes, the aspiration hazard decreased sharply with solvents
containing 16 or more carbon atoms, probably because the viscosity of
such solvents prevented aspiration into the alveoli. In the aromatic
series, side-chains containing more than 6 carbon atoms also tended to
diminish the aspiration hazard, as did blending with more viscous
lubricants (Gerarde, 1963).
3.6.2 Long-term exposure
The repeated skin tests of Hoekstra & Phillips (1963) and Hine &
Zuidema (1970) have already been mentioned in section 3.6.1.
Smyth & Smyth (1928) exposed guineapigs for 4 h/day, 6 days/
week, for a total of 65 exposures to a gasoline-type solvent
(boiling-range 145-183°C) at a concentration of 6750 mg/m3. During
the earlier exposures, the animals appeared to be restless. This was
followed by slight narcotic effects. Diarrhoea and albuminuria
developed temporarily, but blood, lung, and other changes were absent.
In a recent series of inhalation studies on various hydrocarbon
fractions, Carpenter et al. (1977a, b, c) provided much new
information on the toxicity of a wide range of solvents. Physical
properties and the general chemical constitution are detailed in the
papers and the approximate compositions are given in Table 6. The
no-observed-adverse-effect levels of individual solvents following
inhalation studies over 13 weeks in rats and dogs is also tabulated,
together with the human sensory response (section 3.7.1.2). In
general, these studies were remarkable in the lack of toxicity that
they indicated. The few toxic effects that did occur, were usually
kidney damage in rats and minor haematological variations.
Other long-term animal studies have been carried out with the
lower-boiling fractions and more specifically with technical hexane
and technical heptane, because of their possible neurotoxic effects
and to establish a safe level of industrial exposure (TLV).
Mature female Wistar rats were exposed to petroleum solvent
vapour (physicochemical properties not given) at a concentration of
300 ± 8.2 mg/m3 for 30-45 days, for 4 h/day. The serotonin content of
the myometrium in exposed rats equalled 75.7 ± 2.6 µg/kg compared with
68.47 ± 2.5 µg/kg in the control group. Uterine contractions were more
numerous and stronger in exposed animals. The level of solvent in the
venous blood was 2.0 ± 0.4 mg/litre. In the uterine tissues it was
almost twice as high (3.8 ± 0.6 mg/kg). The increase in serotonin
content in the organism could cause disturbances in the transport of
the fertilized egg cell and the nidation, and subsequently, early
abortion (Lipovskij, 1978).
Miyagaki (1967) exposed 5 groups of 10 male mice to vapour
concentrations of technical hexane of 360, 900, 1800, 3600, and
7200 mg/m3. There was a sixth control group. The exposures lasted
24 h/day, 6 days/week, over a period of one year. The composition of
the hexane used is uncertain. The measured vapour concentrations
correlated well with those calculated. Electromyographical studies
were carried out and strength-duration curves, electrical reaction
time, flexor/extensor ratios, and gait-posture were observed. The
muscular atrophy of the hind legs was measured and in some of the
animals, histological examination of the distal mucles of the hind
legs was carried out. Evidence of neurogenic muscular atrophy was only
seen in the group receiving the highest exposure. At the lowest
exposure level (360 mg/m3), no changes were found in any of the
variables studied. At the other levels, changes were found related to
the severity and duration of exposure. Based on their results, the
authors proposed a reduction in the TLV to 360 mg/m3.
In a different study, which included a group of 5 rats (sex and
age not stated) exposed to a hexane concentration of 3060 mg/m3, over
a period of 143 days (no other details given) and a control group, no
significant differences were found in average body weights and blood
values (haematocrit, total serum protein, and protein fractions)
between treated and control animals. Histological examination of
"many" organs revealed only a "slight reaction of the RES in the
spleen" in the exposed animals. In the sciatic nerve and its
ramifications, injuries such as degeneration of myelin and axon
cylinders were seen. The myoneural junction remained unaffected. The
exposed group manifested some decrease in nocturnal activity (Kurita,
1967).
Truhaut et al. (1973) published the results of
electrophysiological studies on rats exposed to technical hexane or
technical heptane. The air concentration of hexane was 7200 ±
720 mg/m3 and that of heptane, 6000 ± 600 mg/m3 (calculated and
expressed as n-hexane and n-heptane). The duration of exposure was
5 h/day, 5 days/week for 1-6 months. The initial weight of the male
and female rats was 150g. No data on the growth-weight curves were
included. The composition of technical hexane was given as:
n-pentane 0.3%
methyl-2-pentane and cyclopentane 25.1%
methyl-3-pentane 18.4%
n-hexane 45.8%
methylcyclopentane 8 %
methylhexanes 1.2%
benzene 1.2%
and that of technical heptane as:
methyl-2-hexane plus dimethyl 2,3 pentane plus cyclohexane 9.8%
methyl-3-hexane 16.2%
n-heptane 52.4%
dimethyl 2,4 hexane plus methylcyclohexane 15.4%
methylheptane 3.3%
benzene 0.1%
toluene 2.8%
Electrophysiological studies were carried out on isolated sciatic
and saphenous nerves taken from anaesthetized rats. During the first 2
months of exposure, no changes were found in any of the treatments
but, in the succeeding months, symptoms of neural involvement were
detected, in general, increasing with the duration of exposure. The
signs were: decrease in conduction-velocity, increase in refractory
period, and decrease in excitability. Histopathological examination
did not reveal clear-cut demyelination, but early indications of this
type of change were certainly found. Truhaut has recently cast doubt
on these findings, stating that his experimental method was not
sufficiently rigorous to allow his earlier conclusions to be made
(Truhaut, 1978).
Numerous studies on the neuropathological effects of n-hexane
have been published including a review by Schaumburg & Spencer (1976).
Rats exposed for 24 h/day to an n-hexane concentration of
1440-2160 mg/m3 for about 20 weeks developed peripheral neuropathy.
Toxicity data on the presumptive in vivo neurotoxic oxidation
products of n-hexane have also been published (Spencer et al.,
1978). It should be mentioned that many authors have used the term
" n-hexane" loosely; the tested material often really consisted of
various C-6 hydrocarbons.
Some other hydrocarbon solvents have been implicated as possibly
causing neuropathy, following prolonged exposure to high
concentrations. As these reports are generally related to human
exposure, they will be considered in section 3.7.2.1. Of relevance is
the observation that toluene inhaled by rats over a period of one year
induced electrophysiological changes at concentrations of 7500 mg/m3
and 750 mg/m3 but not at 375 mg/m3 (Matsumoto, 1971). Fournas & Hine
(1958) exposed rats to high concentrations of various alkyl aromatic
hydrocarbons and found some clinical evidence of neurotoxicity with
most of the compounds tested; p-t-butyltoluene was shown to induce
CNS damage in rats by Ungar et al. (1955).
3.6.3 Mutagenicity, teratogenicity, and carcinogenicity
3.6.3.1 Mutagenicity
The potential mutagenic activity of selected petroleum products
was assessed using a battery of in vivo and in vitro bioassays,
including the dominant lethal test, the Ames' test (with and without
activation), the mouse lymphoma cell transformation test, and
observations on cytogenicity. The following products elicited negative
responses in one or more tests: VM&P Naphtha, Stoddard Solvent, mixed
xylenes, "60 Solvent", "70 Solvent", 140° Flash Aliphatic Solvent, "50
Thinner", kerosene, toluene, high solvency naphtha- unleaded petrol,
and No. 2 heating oil (40% aromatic compounds). Positive mouse
lumphoma cell transformation tests were elicited by benzene, diesel
fuel, No. 2 heating oil, and jet A fuel; positive results in
cytogenetic tests (clastogenic responses) were obtained with rubber
solvents, "60 Solvent", high aromatic solvent, No. 2 heating oil, and
jet A fuel. For each of these products the Ames bacterial bioassay was
negative (API, 1974, 1975b, c, 1977a, 1978a, d, f, 1979d).
3.6.3.2 Teratogenicity
Tests for teratogenicity induced by inhalation of high and low
doses of benzene, Stoddard Solvent, toluene, mixed xylenes, unleaded
petrol, high aromatic solvent, n-hexane, diesel fuel, VM&P naphtha,
kerosene, rubber solvent, jet fuel A, and No. 2 heating oil were all
negative. However, benzene exposure at 120 mg/m3 induced a
statistically significant increase in fetal resorptions in rats (API,
1974, 1975a, b, c, 1977 a, b, 1978a-f, 1979a-d).
3.6.3.3 Carcinogenicity
Carcinogenicity tests have only been conducted on the group of
high-boiling aromatic extracts derived from the solvent refining of
lubricating base oils. These studies will be considered together with
long-term studies on lubricating base oils, but, in short, practically
the whole of the carcinogenic polynuclear aromatic hydrocarbons were
found in the extracts from the base oils. However, the carcinogenic
activity of the extracts was considerably less than that of
coal-tar-derived products.
Similar carcinogenicity studies have not been carried out with
the lower boiling aromatic solvents (white spirits). Such studies
would be indicated, though the content of potentially carcinogenic 4,
5 and 6 condensed ring polynuclear aromatic hydrocarbons in these
aromatic solvents is probably much lower than in high boiling aromatic
solvents, because of the lower boiling range. Lijinsky & Raha (1961)
mention, however, that no commercial distillation procedure will
completely remove all traces of polynuclear aromatic hydrocarbons from
petroleum solvents and that special treatment is necessary to achieve
this. Though, in most cases, the authors found very low concentrations
(in the µg/m3-range), they are of the opinion that these levels
should be investigated, particularly in food-grade material.
3.7 Effects on Man
3.7.1 Controlled exposures
3.7.1.1 Effects of dermal exposure
Klauder & Brille (1947) patch-tested petroleum solvents of
various boiling ranges on the skin of human volunteers. They found a
correlation between the boiling ranges of the petroleum products of
paraffinic origin and their irritant and defatting action on the skin.
Both effects decreased, the higher the boiling range. Petroleum
solvents with boiling ranges up to and including that of kerosene
(approximately 230°C) were found to be primary irritants. Petroleum
solvents of naphthenic origin or with a high aromatic content were
more irritant than solvents of paraffinic origin of the same boiling
range. The skin of Negroes showed a higher tolerance than that of
Caucasians.
Pre-existing skin disease may increase the susceptibility of the
skin to the effects of contact with petroleum solvents and will also
facilitate uptake by this route (Klauder & Brille, 1947; Riihimäki &
Pfäffli, 1978).
The effects of various solvents on the horny layer of the skin
were examined by Malten et al. (1968) and Spruit et al. (1970). They
found that petroleum ether (SBP 40/65) caused serious irritation of
human forearm skin, when applied for periods of 10-30 min. When
applied for 15 min on 6 successive days, injury occurred in the horny
layer. Recovery -- as measured by water vapour loss -- could take up
to 6 weeks. The skin irritation and the changes in the composition of
the horny layer were independent phenomena.
Tagami & Ogino (1973) applied undiluted refined kerosene to the
arm of a volunteer in an occluded patch-test. After 1 h, a burning
sensation developed, slight erythema appeared after 2 h, and after
7 h, the skin was tender and very red, even beyond the patch-test
site. After 12 h, the burning sensation had subsided but a large tense
bulla had appeared surrounded by small scattered vesicles. This
changed into a large flaccid purulent bulla 24 h later, which easily
broke, leaving a raw surface. The main differences between the test
situations just described were: (a) a marked difference in boiling
range (volatility and skin penetration) of the products tested; and
(b) the length of exposure, the effect increasing with increasing
duration of exposure.
The same authors then applied 85%, 70%, 55%, and 40% dilutions of
the kerosene in mineral oil in covered skin test on 34-adult male
Negro and Caucasian volunteers. The skin of all the subjects reacted
to 85% kerosene solution, the 70% solution caused skin irritation in
29 subjects, the 55% solution in 8, and the 40% solution was
completely without effect. The skin of Negroes appeared to be less
irritated by kerosene than that of Caucasians; the influence of age
was not clear. No definite correlation was found between individual
response to kerosene and the permeability of the horny layer of the
skin (Tagami & Ogino, 1973).
3.7.1.2 Effects of inhalation
Inhalation of air containing petrol at concentrations of
1350-3150 mg/m3 for 18 min by human volunteers did not cause any
symptoms; a 14-min exposure to 12 600-31 500 mg/m3 induced dizziness
(Fieldner et al., 1921). Davis et al. (1960) exposed human volunteers
for 30 min to concentrations of petrol of 900, 2250, or 4500 mg/m3 in
air. Very few symptoms were noted. Itching and burning of the eyes was
apparent in most subjects in the highest exposure group.
The human odour- and sensory irritation thresholds were measured
as part of the toxicity evaluation of solvents carried out by
Carpenter et al. (1977a) (Table 6). It can be seen that vapours of
hydrocarbon solvents can be detected at rather low concentrations, but
that unpleasant odour and irritation only become apparent at much
higher concentrations. Nevertheless, in establishing an exposure limit
for each solvent, human sensory data were often limiting factors.
In volunteer studies, psychological functions were affected by a
50-min exposure to a concentration of white spirit of 4000 mg/m3
resulting in a prolonged reaction time and impaired short-term memory.
Lower concentrations did not have any effect (Gamberale et al., 1975).
3.7.2 Epidemiological studies
A distinction between epidemiological and clinical studies has
always been difficult in view of the inadequacy of reporting. The
various aspects will therefore be discussed under headings relevant to
the information available.
3.7.2.1 Occupational exposure
(a) Haematological effects
Benzene is unquestionably the most dangerous hydrocarbon used in
industry and the benzene content of some petroleum solvents presents a
major long-term hazard for man. In particular, special boiling
solvents in the lower-boiling range may contain a considerable
percentage of benzene (Gerarde, 1960). In this review, it is
impossible to consider in detail the effects of benzene, which
basically causes bone-marrow depression and has a leukaemogenic
action. Excellent reports on all aspects of benzene toxicity can be
found in: ILO (1968); Deutsche Forschungsgemeinschaft (1970); IARC
(1974); and Laskin & Goldstein (1977).
In the past, benzene-like effects have also been ascribed to
other low-boiling petroleum solvents. However, there now seems to be
general agreement that these effects only occur, when benzene is
present in the mixture (Browning, 1959, 1965).
(b) Neuropathological effects
The neuropathological effects of inhalation of petroleum solvents
in man have been discussed in a number of reviews including those of
Cavanagh (1973), Allen (1975), Seppäläinen (1975), Comstock (1977),
and Savolainen (1977). The reviews include discussions of methods used
to evaluate human neuropathy.
Over the past 15 years, an increasing number of cases of
poly-neuropathy and other neurotoxic effects have been described in
workers exposed to hydrocarbon solvents. Many of these cases have been
associated with prolonged and repeated exposure to high concentrations
of n-hexane. Frequently, such exposure has resulted from poor
ventilation in a workroom in which solvents containing high
concentrations of hexane have been used. Miyagaki (1967) reported
hexane concentrations in air of 1800-3600 mg/m3 in the workroom and
7200 mg/m3 near the source in a work-place where 17 cases of
peripheral neuropathy occurred. Yamamura (1967) and Inoue et al.
(1970) found concentrations ranging from 1800-9000 mg/m3 in a work
place where, over a 9-year period, 93 out of the 1662 workers suffered
from polyneuritis. These workers used glue in the manufacture of vinyl
sandals for 8-14 h daily, probably for more than 5 days each week,
over long periods, in poorly ventilated rooms. The presence of hexane
in glues is reported to have caused similar incidents in Italy and
Iran (Capellini et al., 1968; Scrima & de Rosa, 1973; Ghazai, 1974).
In most cases, the extent of exposure to hexane was uncertain, either
because air concentrations were not measured or because excessive skin
contamination occurred; workers frequently handled the glues and
washed residues off the skin with hexane-containing solvents. In other
cases, air concentrations were measured. Hexane concentrations of
approximately 2340 mg/m3 (but at times up to 4680 mg/m3) were found
in a small, poorly ventilated workroom in which 3 women, who developed
polyneuropathy, had worked; again excessive skin contact took place
(Herskowitz et al, 1971; Ishii et al., 1972). Glue was also the source
of hexane concentrations of 1000-4000 mg/m3 found by Paulson &
Waylonis (1976) in the air of a work-place where 8 out of 50 employees
developed mild neuropathy.
The main clinical manifestation in these cases was polyneuropathy
of the glove-and-stocking type with sensations of numbness and cold.
This was accompanied by muscular weakness and headaches. Gradual
recovery was usual, when exposure ceased, with the exception of some
subjects who had severe muscular atrophy. Peripheral nerve biopsy
showed demyelination with relative preservation of the axon. Iida
et al. (1969) reported that in 44 cases there was a reasonable, but
not exact, correlation between clinical findings and electromyography
and nerve conduction velocity data.
Optic involvement was observed by Inoue et al. (1970). The
neuro-ophthalmological function was studied by Raitta et al. (1978) in
15 workers who had been exposed at work over periods of 5-21 years to
hexane concentrations of 1800-3600 mg/m3, with occasional levels of
10 800 mg/m3. Defective colour discrimination was found in 12 of the
workers and slight macular changes in 11 out of 15. The visually
evoked potentials and electroretinograms were interpreted by
Seppäläinen et al. (1979) as indicating cerebral dysfunction, probably
a conduction block in intracerebral axons.
Polyneuropathy attributable to the inhalation of hexane has also
been observed in "glue sniffers" -- subjects who inhale solvent
vapours to induce elation or other states of mind. Cases including
very rare cases following medicinal use, were described by Schwarz
(1933), Browning (1965), Karani (1966), Gonzales & Downey (1972),
Matsumura et al. (1972), Taher et al. (1974) and Korobkin et al.
(1975). Other incidents have been reported or reviewed by Shirabe et
al. (1974), and Poklis & Burkett (1977). While hexane appears to have
played a major role, a direct or synergistic action of other
components cannot be ruled out.
Some cases have been described that were thought to arise from
the long-term, continuous domestic use of kerosene stoves in poorly
ventilated rooms (Contamin et al., 1960). Under simulated conditions,
hexane concentrations of about 1440 mg/m3 were produced in
experimental rooms by Lièvre et al. (1967).
The possibility of the production of peripheral neuropathy by
components other than hexane, or the intensification of the activity
of hexane, is suggested by some clinical reports. Cargill (1972)
(cited by Gaultier et al., 1973) described peripheral neuritis in
workers using glue containing cyclohexane, gasoline "c", and methyl
ketone, and Franco et al. (1979) reported sensory and peripheral motor
conduction disturbances, where exposure to cyclohexane had occurred.
The condition was described by Gaultier et al. (1973) in subjects who
had been in prolonged contact with a glue solvent containing 80%
pentane, 5% hexane, and 14% heptane. Gasoline concentrations of
2250 mg/m3 were found in the workshop atmosphere. Atmospheric
gasoline concentrations of up to 5625 mg/m3 (mainly n-pentane,
n-hexane, and n-heptane) were found in workshops where workers had
developed polyneuropathy and had complained of insomnia, irritability,
and other non-specific CNS symptoms. White spirit has also been
implicated as a cause of peripheral neuropathy (Gaultier et al.,
1973). Heavy exposure to jet fuel vapours was reported in workers who
experienced dizziness, palpitations, nausea, and headaches, later
followed by signs and symptoms of polyneuropathy; higher vibration
thresholds, compared with unexposed controls were also found. (Knave
et al., 1976).
Other symptoms besides those of polyneuropathy have been
described in subjects exposed to hydrocarbon solvents. Sterner (1941)
reported headaches, nausea, mental depression, anorexia, inability to
concentrate and sustain activity, and slight anaemia, in workers
exposed to gasoline vapours (used to dilute spray paint) containing
5-10% aromatic hydrocarbons and producing concentrations of total
aromatic hydrocarbons in air of 300-800 ppma. Knave et al. (1978)
compared 30 workers, occupationally exposed to jet fuel, with
unexposed controls. The average period of exposure was 17 years and
the estimated TWA exposure was 300 mg/m3. Significant differences
were found between exposed and unexposed groups in the incidence and
prevalence of psychiatric symptoms, psychological test results,
especially attention and sensorimotor speed, and in
electroencephalograms. Exposure of car painters, over many years, to
low concentrations (31.8% of the Finnish TLV on average) of solvent
mixtures containing toluene, xylene, butyl acetate, and "white
spirit", was found to be associated with an increased incidence of
sleep disturbance, absentmindedness, falling asleep while watching the
television, and headaches. Lower peripheral nerve conduction
velocities, psychomotor impairment, and personality changes were more
common in exposed than in control subjects. Further studies are needed
to elucidate the significance of such findings (Hänninen et al., 1976;
Seppäläinen et al., 1978).
In summary, the most serious adverse neurological effect of
hydrocarbon solvents in man is the production of peripheral
neuropathy. Observations in man and studies on experimental animals
support the view that exposure to n-hexane is the principal cause.
However, the synergistic activity of other hydrocarbons is possible;
such a phenomenon has been reported following the sniffing of a glue
thinner containing both the neuropathic solvent, methyl- n-butyl
ketone, and the non-neuropathic solvent, methyl ethyl ketone
(Altenkirch et al., 1977). At present, the possibility that other
hydrocarbons also have some neurotoxic activity cannot be ruled out.
Peripheral neuropathy has occurred only in conditions of prolonged and
repeated exposure to high concentrations of hydro-carbon solvent
vapour in air; in many cases, there was also excessive skin contact.
Atmospheric exposure levels in excess of 2 g/m3 are usually
encountered where peripheral neuropathy is seen, but more studies are
required to clarify the situation, and until this is done, close
supervision is needed to ensure that the TLV for solvents is not
exceeded. Further studies are also necessary to determine the
significance of psychological disturbances and of neurophysiological
findings in workers exposed to lower levels of the hydrocarbons.
(c) Effects on reproductive functions
Examination of 408 female workers in petroleum refineries, who
had been subjected to long-term exposure to hydrocarbons, hydrogen
sulfide, and other products related to the treatment of sulfurous
crude, revealed disturbances in the menstrual function, mainly in the
form of hypomenstruation and pre-menstrual syndromes. According to
Suhanova & Melnikova (1974), such disturbance of the menstrual
function in female workers in refineries and in persons suffering from
chronic intoxication from petroleum products (36 persons in the age
bracket 30-39 years) is caused by the hypo-functioning of the ovaries.
a Since the composition is not given, it is not possible to
transform this concentration into mg/m3, the adopted SI unit.
Beskrovnaja et al. (1979) studied the gynaecological disease rate
in more than 5000 female operators in plants producing rubber articles
(petroleum solvent vapour concentration in the air of 250-350 mg/m3).
They observed disturbances in the menstrual cycle in workers with more
than 5 years' service and a high frequency of metrorrhagia. As the
period of service increased, a reduction in the frequency of
miscarriages was noticed, which was interpreted by the authors as
possible adaptation. A disturbance of the ovarian function was noted
in 24.4% of the workers examined, mostly in the form of a functional
deficiency of corpus luteum.
Investigations of vaginal smears of 184 female gluers in the
rubber industry in the age bracket 18-38 years revealed a disturbance
of the ovarian function with reduced estrogen stimulation in 21.7% of
the women (10.4% in the control group). This figure was related to the
period of service. After 10 years service, the percentage was twice as
high as after 5 years' service.
Women who had been in contact with petroleum solvents were found
to have a reduced estrogen level in the blood (22.2 µg/day compared
with 29.6 µg/day in the control group). Essentially, no changes were
observed in the excretion of the follicle-stimulating and luteinizing
hormone pregnanediol. The authors of this study assumed that the
sensitivity of the ovaries towards the stimulating effect of the
gonadotrophins was reduced (Hrustaleva et al., 1979).
Novikov et. al. (1979) studied lactation in 332 nursing mothers
288 of whom worked in the rubber industry (vulcanizers, pressers,
gluers). The concentration of petroleum solvents (the physico-chemical
properties of which are not described) in the air of the operating
premises was predominantly 300 mg/m3. Hypolactation, found in 23.8%
of the women compared with 6.7% in the control group was related to
length of service. Hydrocarbon solvents were found in the milk of all
the persons examined (71) in concentrations of 0.50 ± 0.05 mg to
0.60 ± 0.09 mg/litre. The serotonin content of the blood of these
women was significantly lower than in the control group. It is assumed
that hypolactation was the result of the effect of solvents on the
lactation control mechanism via the hypothalamus and the
serotoninergic system.
(d) Effects on the skin
Skin can also be affected by exposure to solvents. In a study on
skin conditions and other diseases in 54 gasoline and diesel station
workers, skin conditions related to exposure to gasoline and diesel
fuel were minimal and did not interfere with capacity to work. Only
dryness, chapping, and reddening of the skin were found. The dark skin
of Indonesians seemed to be more resistant to these effects. The
gasoline vapour in the working environment caused some non-specific
symptoms in addition to those resulting from heavy out-door work
(Suma'mur & Susianti Wenas, 1979).
3.7.2.2 General population exposure
Data are not available concerning the exposure of the general
population to petroleum solvents with the exception of that of
"sniffers" and addicts already mentioned in section 3.7.2.1.
3.7.3 Clinical studies
It should be noted that literature surveys of clinical studies
and clinical effects have been published by Gerarde (1960), Browning
(1965), and Levina (1976).
3.7.3.1 Effects of dermal exposure
In general, petroleum solvents have a defatting action on the
skin and, on repeated contact, cause injury to the horny layer
(section 3.7.1.1). This makes the skin more susceptible to other
irritants, sensitizing agents, and bacteria. It may also result in
progressive dermatitis, characterized successively by dryness,
redness, chapping and scaling, which could lead, by sensitization, to
eczema. These stages of dermatitis may be seen in workers in garages
or automobile repair shops, who wash their hands with solvents, petrol
or kerosene (Tagami & Ogino 1973). The more aromatic solvents, in
particular, can cause a significant degree of primary skin irritation.
The defatting action and primary irritation caused by petroleum
solvents decrease, the higher the boiling range section 3.6.1).
Cases in which gasoline or kerosene remains in contact with the
skin for prolonged periods mainly occur in children or in unconscious
accident-cases, when clothing has become soaked with the solvent. In
such cases, the lesions start with a burning sensation and erythema,
followed by the formation of small or large vesicles, blisters, or
even extensive epidermolysis. The vesicles and blisters may become
mucopurulent in a few days. The acute picture is that of a chemical
burn, (Helbling, 1950; Aidin, 1958; Ainsworth, 1960; Stewart, 1960;
Browning, 1965; Hunter, 1968; Tagami & Ogino 1973).
Gasoline may occasionally be absorbed through the skin in toxic
quantities if large areas of skin such as the hands and forearms are
regularly exposed (Hayhurst, 1936), or in cases of extensive
epidermolysis in contact with gasoline-soaked clothing. However
inhalation of vapour plays a significant additional role in all these
cases and generally is the main route of absorption (Browning, 1965).
3.7.3.2 Effects of inhalation
The acute effect of massive overexposure to gasoline vapour is
mainly narcosis with loss of consciousness and possibly convulsions,
which may be fatal (Browning, 1965). Octane causes rapid and deep
narcosis, pentane and hexane are less powerful narcotics, but they and
heptane exert a paralytic effect on the central nervous system and its
respiratory centre.
In more gradual overexposure, the symptoms just described may be
preceded by eye irritation, irritation of the respiratory tract,
dizziness, headache, and a sense of drunkenness.
A gasoline concentration of 9000 mg/m3 can be breathed without
significant ill-effects by most people but susceptible subjects may
show symptoms after exposure to 1350-2250 mg/m3 (Ainsworth, 1960). At
31 500 mg/m3, dizziness and symptoms of drunkenness may appear.
Exposures in excess of 45 000 mg/m3 soon become intolerable and may
rapidly prove fatal (Machle, 1941, Aidin, 1958). Lower and higher
values have been quoted, obviously depending on the composition of the
gasoline (Browning, 1965). The margin of safety between narcosis and
respiratory arrest is very narrow in exposures to high concentrations
of gasoline (Wang, 1961).
Absorption of gasoline vapour by inhalation may be very rapid if
the concentration is high, especially with the lower members of the
paraffinic range, and symptoms can appear within a few minutes.
Excretion, probably of unchanged vapours, takes place mainly via the
lungs (Browning, 1965).
Acute occupational poisoning by gasoline vapour is mostly caused
by entering unpurged gasoline tanks or other premises where high
concentrations of gasoline may have accumulated. Exposure to high
concentrations may also occur in car accidents, when victims are
trapped and/or unconscious.
Histopathological changes found in subjects who have died after
exposure to high concentrations of gasoline vapour include: hyperaemia
and petechial haemorrhages in the lungs, and, sometimes, necrosis of
the alveolar walls. There may be haemorrhages or effusions in internal
organs and serous cavities (Helbling, 1950; Ainsworth, 1960; Browning,
1965). Liver and kidney may show fatty degeneration. Hyperaemia and
oedema of the brain are common in this condition and myelin swelling
may occur (Machle, 1941).
Long-term exposure to low (unspecified) vapour concentrations may
cause nonspecific symptoms of the nervous system and digestive tract
(Zielhuis, 1961; Browning, 1965; Muhametova & Podrez, 1975; Sehtman et
al., 1979), including changes in liver function and in the visual
organ. In women, the reproductive organs may be affected (Muhametova &
Podrez, 1975; Sehtman et al., 1979). Neurological disturbances, as
described in section 3.7.2.1 may develop (Hayhurst, 1936; Machle,
1941; Browning, 1965). Browning (1965) considered that significant
changes observed in the blood count were caused by the presence of
benzene in the solvent.
This chronic form of toxicity may be found in small,
insufficiently ventilated workshops where solvents, or products
containing them, are handled in an unsatisfactory way (section
3.7.2.1) and when these materials are used in substantial amounts in
enclosed spaces, particularly for cleaning or degreasing (Anon.,
1974).
3.7.3.3 Effects of ingestion
Accidental ingestion of petroleum distillates in the range of
petroleum solvents is an important cause of poisoning in children
(Waring, 1933; Nunn & Martin, 1934; Lesser et al., 1943; Carithers,
1955; Daeschner et al., 1957; Gerarde, 1959, 1963). Most cases,
however, are caused by gasoline and kerosene and fewer by petroleum
solvents. The symptomatology is the same in all cases.
Coughing, choking, and gagging are often noted at the time of
ingestion of these substances. Respiratory embarrassment may be
present early, indicating that aspiration has taken place. Epigastric
discomfort may develop, followed by vomiting with a further risk of
aspiration. Aspiration by one mechanism or another is reported to
occur in up to 95% of cases in children, but this depends on the
situation and on the type (boiling-range) of the solvent involved.
Aspiration is less common in adults, but may occur when trying to
siphon gasoline from a tank. Small amounts of kerosene of the order of
1-2 ml, if aspirated, can cause severe and even fatal pulmonary
changes. On the other hand, if aspiration does not occur, much larger
quantities can be tolerated (Daeschner et al., 1957; Hensen, 1959;
Browning, 1965). Browning (1965) quotes a value of 7.5 g/kg body
weight, but this may depend on the type of solvent. Children appear to
be more susceptible to the toxic effects than adults (Siwe, 1932). In
cases where aspiration does not take place, and especially with the
lower-boiling solvents, central nervous system symptoms may develop
such as lethargy, convulsions, and coma. With smaller doses, the
symptoms include vertigo, headache, and signs of drunkenness. Nausea,
vomiting, and diarrhoea may occur and the stools may be blood-tainted.
In uncomplicated cases, the gastrointestinal symptoms will
disappear within 48 h. Pulmonary symptomatology will not develop, if
aspiration has not occurred and if there was no massive exposure to
vapours.
The syndrome of acute chemical aspiration pneumonitis will be
described in a wider context in section 4.8. Human experience is in
accordance with the results of experimental animal studies (section
3.6.1). Chemical pneumonitis with pulmonary oedema and haemorrhagic
frothy sputum may develop extremely rapidly following aspiration of
petroleum solvents. Roentgenographic changes may be seen within a few
hours, especially at the lung bases. Later, bacterial pneumonia can
complicate the situation (Daeschner et al., 1957; Gerarde, 1963).
The prognosis of aspiration of hydrocarbon solvents appears to
have improved with better treatment in the course of time. Siwe (1932)
reported that 50% of the cases he reported were fatal, Nunn & Martin
(1934) reported 28% mortality following the ingestion of gasoline and
9.2% following kerosene-ingestion, and Blattner (1951) reported that
death resulted in 10-14% of cases of kerosene poisoning. Recently, 3
cases have been described, where exposure to diesel oil seemed to
cause acute renal failure with oliguria (Reidenberg et al., 1964;
Barrientos et al., 1977; Crisp et al., 1979). However, in 2 cases,
absorption seemed to have been mainly through the skin (section
3.5.1).
4. LUBRICATING BASE OILS AND RELATED OILS,
GREASES, AND WAXES
4.1 Properties and Analytical Methods
4.1.1 Chemical and physical properties
Base oils are a limited group of petroleum products in the
boiling-range of 300-700°C, normally derived from the high-vacuum
distillation of the residues of the crude-distilling process. These
oils undergo a further refining process before being used.
Lubricating oils, metal-working oils, and related products are
produced by blending base oils in order to obtain the desired physical
properties. Chemical additives are frequently added, usually in small
amounts (a few mg to a few g/kg) to improve the performance of the
lubricant. In some special cases, higher concentrations are necessary.
Additives belong to the following general categories: viscosity-index
improver, emulsifiers, wetting agents, antioxidants, dispersants,
antiwear additives, extreme pressure additives, rust-inhibitors,
antifoam agents, pourpoint depressants, and germicides.
Greases based on mineral oil consist of solid or semisolid
dispersions of metallic soaps and other thickeners in a mineral oil
base.
Petroleum waxes are crystalline solids at normal temperature.
"Slack wax", a soft, impure paraffin-wax is obtained in the
manufacture of lubricating base oils from paraffinic crude oils by a
dewaxing process. Slack wax can be refined into 2 types of commercial
wax: paraffin wax and microcrystalline wax.
Base oils are very complex mixtures of hundreds to thousands of
different hydrocarbons, containing straight-chain and branched
paraffins, cycloparaffins, naphthenic, aromatic, and polynuclear
aromatic hydrocarbons in the range of C-17 and higher. The separate
molecules are so large, that any molecule may, for instance, contain
one or more aromatic rings with one or more long side-chains.
The actual composition depends on the source of the crude oil,
from which the product is derived, and the manufacturing and treating
processes used. They range from thin, easily flowing "spindle oils" to
thick "cylinder oils". A limited number of base oils are used for
blending to obtain the commercial products. As the relation between
viscosity and temperature is an important factor in this field, base
oil grades are characterized by their viscosity and viscosity index
(VI). The higher the viscosity index, the less the change in viscosity
with temperature.
Low viscosity index (LVI) oils are used whenever the viscosity
temperature characteristics and oxidation stability are of less
importance. In general, they are derived from naphthenic oils and
undergo treatment with sulfuric acid and clay or are given a
hydro-finishing treatment.
Medium viscosity index (MVI) oils, can be used as a base for
general-purpose lubricants. They can be derived from naphthenic (MVIN)
or paraffinic (MVIP) feedstocks. MVIP has to be dewaxed and can also
be solvent refined, acid- and clay-treated or hydro-treated.
High viscosity index (HVI) oils, are prepared by both solvent
refining and dewaxing of paraffinic feedstocks. They are used for
gasoline and diesel engine oils and for turbine lubricants, because
they are, in general, more oxidation-stable than other base oils and
have appropriate viscosity/temperature characteristics.
White oils are generally produced by more drastic refining of MVI
oils, in order to remove unsaturated compounds, aromatic compounds,
and other constituents that influence colour, odour, taste, and
acceptability as food-grade material. Solvent extraction followed by
repeated treatment with oleum and alkali is used. Hydrogenation is
another means of producing such oils.
Medicinal oil is the highest purified grade, which complies with
the requirements of the various national pharmacopoeias and
regulations on food-grade material. As liquid paraffin, the same grade
is used as a lubricant for food-handling machines and as an ingredient
in pharmaceutical preparations and cosmetics.
Technical white oils, less rigidly purified than medicinal oil,
are non-carcinogenic oils that can be used for the lubrication of
textile machinery, spinning mules, etc., but are mainly used in the
cosmetic industry in the manufacture of hair-oils, creams, etc.
Aromatic extracts, which are obtained in the solvent-refining
process, have already been discussed in section 3.1.1.
Petroleum waxes consist essentially of high relative molecular
mass paraffinic hydrocarbons with approximately 20-40 carbon atoms per
molecule. Paraffin waxes consist mainly of normal paraffins together
with some iso and cycloparaffins. They are macrocrystalline with a
melting-point of 43-71°C.
Microcrystalline waxes on the other hand, consist mainly of iso
and cycloparaffins with some alkylated aromatic hydrocarbons. They are
mostly soft materials, but may sometimes be a hard brittle solid.
When highly purified, the colour may be white, however, it is
usually yellow or amber, sometimes even black. The melting-point is
60-90°C. The paraffin oil content ranges from 5 to 50 g/kg.
Petrolatum is also known as petroleum jelly and is a
micro-crystalline wax with a high oil content.
4.1.1.1 Purity of Product
As these oils and waxes are complex mixtures, the nature and
proportion of potentially undesirable "impurities" depends to a large
extent on the definition of the term and on the degree of refining. In
the range from the highest refined oil -- for example, white medicinal
oil -- to the least refined oil, "impurities" could include
polynuclear aromatic hydrocarbons and unsaturated hydrocarbon
compounds.
It is clear that highly purified products can be obtained at the
cost of extra refining and treating processes. Paraffin waxes are an
example of progressive refining: fully refined paraffin wax is a white
solid material containing an oil concentration of less than 5 g/kg. It
is odourless and tasteless and has a melting-point of 50-70°C. Candle
waxes range from fully refined paraffin wax to less refined waxes
containing oil levels of up to 15 g/kg. This type of wax is not
completely colourless and odourless. Scale wax and match wax contain
an oil residue of up to 30 g/kg.
4.1.2 Methods of sampling and analysis
See section 2.1.2.
4.2 Sources of Environmental Pollution
4.2.1 Natural occurrence
Base oils occur in nature only as components of the crude oils
from which they are derived.
4.2.2 Man-made sources
4.2.2.1 Production
As mentioned earlier, lubricating base oils are a group of
petroleum distillates and residues in the boiling-range 300-700°C,
derived by high-vacuum distillation of the residues obtained in crude
oil distillation (section 4.1.1). In order to obtain base oils with
the qualities and specifications needed for various applications,
further refining and treatment of the various distillate fractions is
needed. The main processes used are:
(a) Solvent extraction
This is a process by which aromatic hydrocarbons can be extracted
from oil fractions, thus obtaining a low aromatic or aromatic-free
raffinate, and a high aromatic extract. Liquid sulfur dioxide,
sulfolane, benzene, phenol, or furfural can be used as solvents for
this purpose. The raffinate can be used for producing a lubricating
base oil, the extract can be used as a solvent (see under petroleum
solvents).
(b) Dewaxing process
In this process, "slack wax" is separated (crystallized) from
base oil fractions obtained from paraffinic crude oil residues. This
is done by cooling, followed by filter-pressing. The separation of wax
and oil under chilled conditions is facilitated by the addition of
solvent. The resulting wax may still have an oil content of up to
100-400 g/kg.
(c) Acid treatment
The oil is mixed with 98% sulfuric acid and the acid sludge is
centrifuged off. This is usually followed by clay treatment in which
the oil is mixed with absorbent clay, followed by filtration. This
neutralizes the oil and improves colour, odour, and stability.
(d) Hydrofinishing
This is a catalytic hydrogen treatment at elevated temperature
and pressure. Improved stability and colour of the oil is obtained by
the hydrogenation of unstable compounds, which removes sulfur and
saturated olefins, diolefins, and aromatic components.
As the definition and classification of lubricating base oils are
not clear, it is difficult to give precise production data for this
group of products. The best current estimate is 35-38 million tonnes
per year. Though there was a decrease in the annual production after
1973, lubricant production has subsequently increased, but at a lower
rate.
4.2.2.2 Uses
In general, the main functions of lubricating oils and
metal-working oils are: to reduce friction, to remove generated heat,
to remove debris from the contact area, and to protect against
corrosion. In addition, mineral oils are used as hydraulic media in a
wide variety of applications. Very general remarks have already been
made concerning the composition of, and the various additives in,
lubrication oils, metal-working oils, and greases. The following is a
list of the most important categories of products on the market, from
which it is clear that the great majority are handled in industrial
situations, and that the general population has regular contact with
only a few such products:
(a) Industrial lubricating oils, which can vary from thin spindle-oil
to the very viscous oils used in steam engines;
(b) Lubricants for internal combustion engines of various types;
(c) Crankcase, compressor, gear, and turbine oils;
(d) Greases for bearings and other purposes;
(e) Hydraulic, transformer, insulating, heat-transmission oils;
(f) Cooling, quenching, anticorrosion, and mould oils;
(g) Metal-working oils: cutting, grinding, rolling, drilling,
drawing-oil, a multitude of products for specific purposes. They
consist in general of complex mixtures with various additives,
for use in the neat state and also as water extendible fluids or
emulsions;
(h) Textile oils: spindle, hatching, technical white lubricating oil;
(i) Process oils: used in printing-inks, as rubber extenders, and as
technical white oils in cosmetics, etc;
(j) Waxes of various grades and purity;
(k) Medicinal white oil used for medicinal and food-grade
applications.
The manufacturing, treating, and purification processes used in
the preparation of the base oils vary according to the future
application of the commercial product.
Many of the products can undergo considerable changes during use.
This should be borne in mind in extrapolating from used oils to the
original product in the case of adverse health effects, e.g., in the
case of skin sensitization. The following are a few examples of
changes that may occur:
(a) During use, the chemical and physical characteristics of
lubricating oils change, mainly because of contamination, oxidation,
and polymerization. Metal particles, airborne dust, water, and, in the
case of internal combustion engine oils, small quantities of fuel,
acids, and soot, are the main contaminants. All this can result in the
formation of sludge.
(b) Metal-working oils become contaminated in use with a wide
variety of foreign matter, in particular metal particles (and ions).
Water-based metal-working emulsions can also be affected by bacteria,
yeasts, and fungi. Oxidation and heat cracking may occur at the
application point, causing smoke, steam, and oil mist to be emitted
into the working environment. Nitrite may be added to most water-based
cutting oils, thus these sometimes contain carcinogenic nitrosamines
(Zingmark & Rappe, 1977).
Cutting oils, lubricating oils, and greases can also contain
diethanolamine, which may react with nitrites and nitrogen oxides in
the air to form nitrosamines.
(c) Quenching oils are used for hardening steel. Oxidation and
cracking of the oil can occur because of the high temperatures
involved. The amount of benzo (alpha) pyrene in lubricating oils
increases considerably when they are exposed to heat during use, as in
motor-car engines, metal quenching, and other processes (Thony et al.,
1975).
4.2.2.3 Disposal of waste
Without a proper method for the disposal of used lubricating and
other oils, severe environmental contamination and hazards may occur.
There is a vast literature on methods for the disposal, reuse,
and recovery of industrial lubricants and much research is being done
in this field. It would be out of context in this document to go into
a detailed discussion of the various aspects. For this, the reader is
referred to Concawe Report 9/73 (Concawe Task Force, 1973) and API
publication No. 4036 (API, 1969), which contain discussions on the
situations in Western Europe and the USA, respectively, as well as on
more general aspects.
At present, the main ways of disposal are:
(a) dumping in sewage systems, waterways, etc; this is illegal in
many countries and ecologically undesirable;
(b) dumping into the ground, in garbage dumps, in dry wells, if
approved by local authorities;
(c) using as road oils, etc;
(d) controlled incineration or burning for heat value;
(e) re-refining and other recycling methods.
4.3 Environmental Exposure Levels
Specific data are not available concerning levels of this group
of products in air, water, food, or other environmental media, levels
of possible contamination, and uptake by man.
Depending on occupation and on the hygiene precautions adopted,
skin exposure in workers can vary from minimal to very high. No
systematic measurements have been carried out in this field. Exposure
to oil mists occurs in certain manufacturing processes and
applications, such as the operation of automatic lathes. This type of
exposure has been measured by various authors and will be discussed in
section 4.7.1.2. Secondary oral intake of unknown quantities of
mineral oil can take place under these conditions.
When "sulphofrezol" (approximate composition: 50-60% "goudron"
(?flux oil), 40-50% spindle oil distillate, and 1.7% sulfur) was used
as a lubricating and cooling liquid on metal-working lathes, the air
of the operating premises was found to contain an oily aerosol in
concentrations of 1-50 mg/m3, hydrocarbons at 26- 150 mg/m3, carbon
monoxide at 8-12 mg/m3, formaldehyde at 0.05-1.2 mg/m3, sulfur
dioxide at 2-20 mg/ms, and benzo(alpha)pyrene at 0.01-0.2 mg/m3.
When multicomponent lubricating-cooling liquids containing chlorine
and fluorine compounds are used as additives, hydrogen chloride and
other substances may also find their way into the workroom air. It is
therefore essential to provide the metal-working lathes with
vapour-extracting facilities (Medved' et al., 1976).
4.4 Environmental Distribution and Transformation
Data on lubricating base oils concerned with distribution among
media, environmental transformation and degradation, interaction with
physical, chemical, or biological factors, and bioconcentration are
not available. However, as mentioned in section 3.4, there is a lot of
information concerning the microbial degradation of individual
petroleum hydrocarbons. For example, it has been shown that certain
polynuclear aromatic hydrocarbons in lubricating oils can be oxidized
by bacteria; benzo(alpha)pyrene and benzo(alpha)anthracene have been
shown to be oxidized to cis-dihydrodiols. The oxidation is not thought
to reach the stage of carcinogenic activation found in mammals, as
this would result in a trans-configuration (Gibson et al., 1975).
4.5 Metabolism
Because of the complex and variable composition of compounds in
this group, only a few factual data relating to their metabolism can
be given.
The skin barrier is permeable only to hydrocarbons of a certain
relative molecular mass and structure. For paraffinic substances this
appears to be up to 20 carbon atoms. However, from their studies on
guineapigs, Hoekstra & Phillips (1963) reported that, because aromatic
compounds have a more compact structure, compounds in this group with
a higher number of carbon atoms might still pass through the skin
barrier.
In studies on mice, rats, hamsters, rabbits, and dogs, mineral
oil droplets were phagocytosed in the lungs and clinical observations
in man have shown that deposition takes place in the hilar nodes, from
where they may be transported to other organs such as the spleen
(Sante, 1949; Proudfit et al., 1950; Shoshkes et al., 1950; Wagner et
al., 1964). Almost all (95-99%) of ingested food-grade mineral oil
leaves the body unchanged in the faeces, 1-5% being absorbed as such
via the intestinal mucosa. Phagocytosis may play a part in this. The
ingested part of the oil is transported throughout the body via the
lymphatics and the bloodstream. Storage takes place in adipose tissue
or in the fat in organs. After excessive exposure, mineral oil
droplets have been identified in mesenteric and portal lymph nodes,
and also in liver, spleen, and adipose tissue in man (Stryker, 1941;
Ebert et al., 1966; Boitnott & Margolis, 1966b).
Data relating to food-grade mineral oil have been reviewed in WHO
(1974). Very few data are available on the biotransformation and rate
of elimination of this group of products but data concerning some of
the components are referred to in section 3.5.
Injections of 0.5 ml of various mineral oils were made in the
peritoneal cavity of mice and the oil recovered after 7-24 days (Twort
et al., 1937). Both the refractive index and density of the oil had
decreased proportionally, probably as the result of chemical
transformation in the body. Treatment of mineral oil with sulfuric
acid and clay, or solvent extraction had a similar effect. The solvent
extracts, however, showed increased refractive indices and densities.
The authors suggested that both parameters might be useful indicators
of the carcinogenicity of the mineral oil.
4.6 Effects on Experimental Animals
4.6.1 Short-term exposure
Data are not available concerning the LD50s of base oils but the
oral and dermal LD50 data available for commercial lubricating oils
indicate that, in general, these products are only moderately or
slightly toxic.
Acute no-observed-adverse-effect levels have not been determined.
Oral studies with food-grade mineral oils show that these are
laxatives. The same applies to non-food-grade commercial mineral oils,
though it is impossible to predict whether in a certain case a toxic
or laxative effect will prevail.
4.6.1.1 Effects of dermal exposure
More factual information is available on the acute or short-term
effects of mineral base oils on the skin. In a very thorough study
Hoekstra & Phillips (1963) examined the effects of mineral oils and
their fractions on the skin of guineapigs. It appeared that very
short-chain paraffins had a mainly defatting action on the skin and
that the effects of longer-chain and aromatic hydrocarbons were
closely related to the permeability of the skin to these compounds. We
can do no better than cite the summary of this work:
"A number of light mineral oils applied topically to the skin of
guinea-pigs caused a marked epidermal hypertrophy, hyperplasia,
hyperkeratosis, and subsequent depilation. This dermatoxic effect
could not be closely related to source of the crude oil or its
viscosity, degree of refinement, or the acid used in refinement.
" n-Paraffin, isoparaffin, naphthene and aromatic fractions
separated from light mineral oil each produced the dermatoxic effect
as did highly purified individual paraffins from C-12 to C-18. Oleic
acid caused only a slight dermatoxic effect. Fractional distillation
of an aromatic-free mineral oil demonstrated that while all
lower-boiling fractions were dermatoxic, a distillation range was
reached at which the fractions were innocuous to the skin. This was
also true for n-paraffin-, isoparaffin-, monocyclic naphthene-, and
polycyclic naphthene-rich fractions derived from a mineral oil.
Fractional distillation of the aromatic hydrocarbons from mineral oil
representing the same distillation range did not yield fractions
without skin-damaging effect. Crude estimates of the molecular size of
the n-paraffins, isoparaffins, monocyclic naphthenes, and polycyclic
naphthenes by comparison with the boiling-temperatures of known,
homologous series of hydrocarbons indicated that the maximum skin
damage resulted from hydrocarbons of about 14 to 19 carbon atoms. The
transition point to non-dermatoxic hydrocarbons occurred at about
21-23 carbon atoms. This was verified with purified n-paraffins.
Variations may exist for the different types of hydrocarbons.
"Simultaneous application of the innocuous "higher-boiling"
mineral oil fractions with dermatoxic "lower-boiling" fractions or
with hexadecane greatly reduced or eliminated the skin-damaging
effects. The alleviation of the skin damage from aromatic fractions by
simultaneous application of the "higher-boiling", non-aromatic
fractions was much less pronounced.
"The hyperkeratotic reaction of the skin to petroleum
hydrocarbons appears to he a very general response to lipid solvents
and is not related to any specific reactive group or type of
structure. Molecular size seems to be very important in determining
the dermatoxic properties of hydrocarbons, the larger molecules being
innocuous. Under the conditions of the experiments the very
low-molecular weight hydrocarbons also had little or no dermatoxic
properties, presumably because of their volatility. It is postulated
that the skin barrier is permeable only to hydrocarbons of a certain
maximal effective size and that penetration into or through the
barrier is essential for the initiation of the hyperkeratotic
response. The alleviation of the dermatoxic effect of hydrocarbons by
admixture with innocuous, higher molecular weight hydrocarbons, is
likewise explained by a reduction in penetration of the dermatoxic
component through the Skin barrier" (Hoekstra & Phillips, 1963)
4.6.2 Long-term exposure
Most long-term studies with mineral oils have been concerned with
the carcinogenicity of the compounds. Data concerning mutagenicity,
embryotoxicity, and teratogenicity are lacking.
4.6.2.1 Carcinogenic effects
The carcinogenic activity of certain polynuclear aromatic
hydrocarbons is attributed to electronic structural features of the
molecule. The various theories on these mechanisms are discussed in
detail by Arcos & Argus (1974), Bergel (1974), and Jerina & Daly
(1974). Carcinogenic polynuclear aromatic hydrocarbons have regions of
high-electron density in their molecules, the so-called K-regions that
are readily epoxidized by tissue mixed-function oxidases and more
specifically by the enzyme aryl hydrocarbon hydroxylase.
Polynuclear aromatic hydrocarbons are in fact precarcinogens,
which, though harmless in themselves, are metabolized in the body, by
the combined action of mixed function oxidase and epoxide hydratase
(4.2.1.63), to biologically reactive intermediates that constitute the
ultimate carcinogens (Sims et al., 1974). The enzymes responsible are
active in the liver, and in other organs such as the skin, lungs, and
intestines.
Whether, in fact this metabolism leads to detoxication or to
"lethal synthesis" may differ with various polynuclear aromatic
hydrocarbons and may depend on many other factors (Anon., 1975). The
short-lived epoxides resulting from the metabolism of at least some of
the polynuclear aromatic hydrocarbons are probably the activated
metabolites with the real carcinogenic effects.
Evidence is now accumulating that it is the 7,8 dihydroxy-
benzpyrene - 9,10 oxide of 3,4 benzo(alpha)pyrene that is the
ultimate carcinogen and, consequently, this has now superseded the
K-region epoxide in biological importance with regard to
carcinogenicity (Yang et al., 1976; Thakker et al., 1977; Yagi et al.,
1977; Koreeda et al., 1978). The enzyme aryl hydrocarbon monooxygenase
(according to recent international rules of nomenclature) in the case
of 3,4 benzo(alpha)pyrene, is referred to as benzopyrene
3-monooxy-genase (EC 1.14.14.2) (de Pierre & Ernster, 1978).
There are indications that the tissue concentrations of the
enzyme aryl hydrocarbon hydroxylase vary considerably with the age,
sex, species, strain, and environment of the animal (Nebert & Gelboin,
1969). Outside agents, such as polynuclear aromatic hydrocarbons, can
induce the activity of this enzyme (Wattenberg, 1972). It seems that
the inducibility depends on genetic factors in both mice and human
subjects (Nebert et al., 1972; Kellerman et al., 1973). In mice, it
seems to depend on a single dominant autosomal gene (Nebert et al,
1972).
4.6.2.2 Effects of dermal exposure and subcutaneous
administration
Leitch (1922) repeatedly painted crude shale oil on the skin of
mice. He obtained skin tumours in a substantial percentage of the
mice. Similar tests were carried out by Twort & Ing (1928) and Twort &
Twort (1931) using a range of crude oil as well as more refined
products of both shale oil and petroleum oil. They found that
petroleum oils had a lower and sometimes negligible carcinogenic
activity compared with shale oils. Naphthenic oils were less active
than oils with a high aromatic content. The more potent fractions
could be extracted with solvents from the oils.
Acid treatment of the oils decreased the carcinogenic activity
and heavier-grade oils were less potent than spindle oils.
Oils with a boiling-point above 370°C, derived from fluid
catalytic cracking, appeared to be carcinogenic to the skin of mice.
The most potent fraction distilled between 430 and 550°C, and the
carcinogenic activity was contained in the aromatic components of
these oils.
Woodhouse (1934) compared the carcinogenicity of sulfur-
dioxide-solvent-extracts of spindle distillates, derived from
crude oils from different parts of the world, with that of the
unrefined spindle oils and coal-tar, using a mouse-skin-painting
technique. He found that in all cases, the carcinogenic activity,
which was more or less characterized by its UV-fluorescence spectrum,
was concentrated in the solvent extract. Potency varied according to
the source of the crude oil. The potency of the most active extract,
however, was lower than the activity of coal-tar. By thorough
sulfur-dioxide-solvent refining, the carcinogenicity of the spindle
oil was almost completely removed. The same author (Woodhouse, 1950)
reported studies on the carcinogenic activity of various petroleum
fractions and extracts. Results were in line with his previous
findings and with those of other workers. Three white oils including
medicinal liquid paraffin gave negative results.
More recent studies have been aimed at identifying the
carcinogenic substances.
Dilution of carcinogenic oils with non-carcinogenic oils more
than proportionally reduced the carcinogenic activity, which at times
disappeared completely. Mild hydrogenation or treatment with active
absorbents reduced, but did not abolish carcinogenicity. Mild acid
treatment, on the other hand, did not have such an effect (Smith et
al., 1951). Eight samples of unrefined "slack waxes" obtained from
pressing operations -- still containing 12-29% of oil -- were painted
3 times a week on the skin of white mice (Smith et al., 1951).
Aromatic extracts, obtained by the solvent refining of these waxes,
were examined in the same way. After skin-painting for almost the life
span of the animals, some benign rumours were found with all samples
and in a few cases, skin carcinomas developed. The aromatic extracts,
however, were much more active. The authors concluded that the
aromatic components which were removed by further refining of the
waxes, were the cause of the carcinogenic activity of "slack waxes".
Fischer et al. (1951) described methods for the determination of
polynuclear aromatic hydrocarbons: the ultraviolet absorption method,
the caffein extraction method, the chromatographic ref-ractometric
method, and the maleic anhydride method. Levels, determined by any of
these methods, were fairly well correlated with the tumorigenic
potency of these oils as established in animal experiments. These
analytical techniques are relatively simple and much more rapid than
long-term animal experiments. They are considered to have some
predictive value. A further development is the DMSO
extraction/refractive index method.
The correlation between the analytical data and the carcinogenic
activity in skin-painting studies on mice is now under investigation
(Shell Toxicology Laboratory -- private communication) and encouraging
results have been obtained with the lower- and higher- range
polynuclear hydrocarbons. The compounds in the middle range are still
under investigation.
Auld (1940) considered that it was not the general
UV-fluorescence that was characteristic of carcinogenicity but the
pattern of the spectrum and its classification. However, in a report
by the Analytical Subcommittee of the Institute of Petroleum
(Catchpole et al., 1971a), it was concluded that, at the time of the
report, there was no suitable, easy and quick method for determining
either the total polynuclear aromatic hydrocarbons or individual
carcinogens in them.
The tumorigenic activity of various concentrations of a large
number of pure aromatic hydrocarbons was examined in different strains
and species of animals by Hartwell (1951), who found that the potent
substances contained 4,5, or 6 condensed aromatic rings with
relative-molecular masses ranging from 230 to 320.
Cook et al. (1958) tried to isolate individual carcinogens from 3
Kuwait crude oils by fractional distillation and various treatment
processes. They tested the fractions obtained on the skin of mice and
rabbits and found that more of the carcinogenic activity was contained
in the fraction distilling between 350 and 400°C and that this
activity was associated with the aromatic constituents. Solvent
extraction with aqueous acetone or furfural removed the activity,
which passed into the extract (20% of the original fraction). Several
polynuclear aromatic hydrocarbons such as di-, tri-, and tetramethyl-
phenanthrenes, tetramethylfluorene, 1-methylpyrene, 1,2-benzofluorene
and 8-methyl-1,2-benzofluorene could be identified in these extracts
as well as some complex organic sulfur compounds such as
dibenzothiophen derivatives and polycyclic thiophens, a number of
which are known to have carcinogenic activity. Pentamethyl-carbazole
was identified as an example of a complex organic nitrogen compound.
Similar findings were obtained by Bogovsky et al. (1960) on
fractionating Estonian shale oil.
Approximately 100 fractions were separated from a catalytically
cracked oil by various techniques (Tye et al., 1966). Selected
fractions were chemically analysed and assayed for carcinogenicity by
repeated application to the skin of C3H mice. In the highest-boiling
fractions (438-455°C), the major carcinogens were 4 and 5 ringed
polynuclear aromatic hydrocarbons, benzo(alpha)pyrene as a
characteristic carcinogen was present at a concentration of 0.4%. In
the intermediate boiling range (404-438°C), unsubstituted and
methyl-substituted 4-ringed polynuclear aromatic hydrocarbons formed
the major carcinogens, benz (a)anthracene and its alkyl homologues
being present at a concentration of 0.4%. In the low-boiling fraction
(339-404°C), assorted smaller molecules including alkyl-3-ringed
polynuclear aromatic hydrocarbons were present.
Benzo (c)phenanthrenes, the most dominant carcinogens in this
fraction, were present at a concentration of 0.01%. The carcinogenic
potency in the bioassay was found in the intermediate- and
high-boiling fraction (boiling-range 404-455°C); fractions boiling at
under 349°C did not appear to be carcinogenic.
Generally, where the alkyl-substituted 4-6 condensed-ring
polynuclear aromatic hydrocarbons were concerned, the longer the
side-chain, the lower the carcinogenic activity (Auld, 1950).
These findings were confirmed by the United Kingdom Medical
Research Council (1968), who reported similar studies. Uncracked crude
oil fractions boiling at above 350°C, which had the highest
carcinogenic activity in skin tests on mice and rabbits, contained the
same or similar polynuclear aromatic hydrocarbons; no other new
carcinogens were found. After solvent extraction, the carcinogenic
activity of the extract was much higher than that of the raffinate,
which was very low.
The carcinogenicity of individual polynuclear aromatic
hydrocarbons will not be discussed in this document. For this, the
reader should refer to IARC (1973).
The fact that various cracking processes considerably increase
the contents of polynuclear aromatic hydrocarbons in mineral oils has
been demonstrated by Kennaway (1925), Twort & Fulton (1930), Twort &
Twort (1935), Pates (1952), and Dietz et al. (1952). Kennaway (1925)
discovered that skin cancer of mice could be produced with synthetic
tars obtained by pyrolising substances like acetylene, isoprene,
yeast, human skin, and a non-carcinogenic petroleum. Badger (1962)
pyrolysed a whole range of aliphatic and simple aromatic hydrocarbons
at 700°C. It was concluded from an analysis of the resulting tars
that, at high temperatures, polycyclic aromatic hydrocarbons were
formed from simpler hydrocarbons via primary radicals formed by
carbon-hydrogen and carbon-carbon fission. The same process occurred
at 550°C, but to a much lesser extent.
Skin tests on C3H-mice using 15 base oils with known content of
polynuclear aromatic hydrocarbons, which, in some cases, only differed
in theft refining history, revealed that solvent-refining removed the
carcinogenic components to such an extent that none of the oils
treated in this manner induced carcinomas (Bingham et al., 1965).
Solvent extraction was carried out with the usual solvents including
phenol, cresol, and furfural. Conventional acid and clay treatment
only removed certain of the polynuclear aromatic hydrocarbons and all
oils treated in this way still retained some carcinogenic activity. In
these tests, a general correlation was again found between the content
of 4 and 5 ring polynuclear aromatic hydrocarbons and the
carcinogenicity of the base oils examined.
Catchpole et al. (1971b) compared mass spectrometry analysis of
an untreated distillate with its solvent-treated and its hydrogenated
derivatives. The decrease in polynuclear aromatic hydrocarbons
thiophenes, and sulfur in both treated samples compared with the
untreated sample was quite marked. Smaller differences were found
between the levels in the two refined samples.
In studies by Boehme & Huehnermann (1966), the relative amounts
of aromatic compounds present in some of the more purified mineral oil
products were determined from their UV absorption spectra. The
relative concentrations were:
medicinal white oil 1
paraffin waxes 100
micro crystalline waxes 300
white petrolatum 1000
yellow petrolatum 10 000
The carcinogenicity of a sample of amber petrolatum was tested on
the skin of mice. A 15% solution of the petrolatum in iso-octane, did
not show any significant carcinogenicity. The solvent-extracted
aromatic fraction (1.2% of the original material) tested in iso-octane
solution at 50 times its concentration in the petrolatum did not
produce any carcinogenic effects on mouse skin (Lijinski et al.,
1966). Studies are reported by Oser et al. (1965) in which 3 kinds of
pharmaceutical and food-grade petrolatum were administered to mice in
the form of a single subcutaneous injection of 100 mg of petrolatum
per mouse. Observations over 18 months did not reveal any carcinogenic
or other toxic effects.
Liquid paraffin was found to be non-carcinogenic in long-term skin
testing on mice by Twort & Twort (1931) and Woodhouse (1934). White
mineral oil was also found to be non-carcinogenic in mice after
long-term skin application (WHO, 1974).
Shubik et al. (1962) examined 36 samples of petroleum wax. Eight
samples contained identifiable polynuclear aromatic hydrocarbons; the
highest concentration was 0.64 mg/kg. The results of skin and
subcutaneous testing follow in the author's words:
"Five petroleum waxes were tested by repeated skin application in
benzene solution to mice and rabbits. In addition one of the test
waxes was fractionated and its aromatic and non-aromatic components
were tested separately on mice, also by repeated skin applications.
Solvent-treated controls were kept. No carcinogenic effects were
detected. Five petroleum waxes were tested in mice by subcutaneous
implantation in disc form. Fibrosarcomas developed around the implants
with incidences correlated to the melting points of the waxes. In
addition, one of the test waxes was fractionated and its aromatic and
non-aromatic components tested separately; the same wax was also
tested subcutaneously in powdered form. The findings indicate that the
subcutaneous sarcomas occurred as a result of the physical rather than
the chemical properties of the materials."
From the various studies, it would seen that there is no relation
between the carcinogenicity of a lubricating base oil and its
potential to cause dermatitis.
In all the previously mentioned studies and others by Horton et
al. (1963) and Bingham & Horton (1966), there is general agreement
that the carcinogenicity of a mineral oil is largely related to the
aromatic fraction with a boiling-point above 370°C, and more
particularly to the polynuclear aromatic hydrocarbons containing 4-6
condensed benzene rings. However, some long-chain, aliphatic,
alicyclic, and alkyl-aromatic hydrocarbons from a lower boiling-range
with 10-20 carbon atoms, such as n-dodecane, cyclohexyl-decane, and
dodecyl-benzene may act as accelerators or co-carcinogens. Though
completely non-carcinogenic themselves, they were found to increase
tumour incidence and reduce the time of appearance, when carcinogenic
fractions dissolved in them were applied, to the skin of mice (Horton
et al., 1963; Bingham & Horton, 1966). However, in order to act as a
cocarcinogen, n-dodecane must be present in a concentration of
20-30% or more. Such solutions in accelerating solvents show an
unusual capacity to spread upon the skin (Horton et al., 1957, 1965).
In a further study on the effects of co-carcinogenic compounds on
the carcinogenic action of benzo(alpha)pyrene and benz (a)anthracene
on the skin of mice (Bingham & Falk, 1969), the co-carcinogenicity of
n-dodecane was confirmed. However, when the authors claim "that
there is a 1000-fold increase in the enhancement of potency of low
concentrations of benzo(alpha)pyrene and benz (a)anthracene when
dodecane is the diluent", this statement may not be fully justified by
the results obtained in relatively small numbers of animals per test
group. Nevertheless 50% n-dodecane in the solvent decaline doubled
or trebled the tumour incidence in the highest dose-group, and tumours
were found at much higher dilutions than when decaline alone was used
as a solvent. When 2-dodecanol and 2-phenyldodecane were used in
various concentrations in the solvent of benzo(alpha)pyrene, higher
concentrations of these 2 substances shortened the interval of
appearance of tumours rather than increasing the total incidence of
rumours.
From this it would appear that these accelerators would not have
any effect, when present in an intrinsically non-carcinogenic oil, and
that they would only have an effect in other oils, when present in
appreciable concentrations. This effect seems to be due, at least in
part, to the spreading power of the solvent causing more intimate
contact with a greater skin area. On the other hand, a more plausible
explanation could be an increase in P-450 enzyme induced by dodecane,
which possibly does not occur with 2-dodecanol and 2-phenyldodecane.
3,4 Benzo(alpha)pyrene can induce P-450 microsomal mixed function
oxidase and this can also catalyse arylhydrocarbon (AH) monooxygenase
activity (de Pierre & Ernster, 1978). The increase in P-450 induced by
dodecane will accelerate and increase the formation of the ultimate
carcinogen 3,4 benzo(alpha)pyrene and thus increase the total
incidence of tumours. However, these substances will not normally be
present in base oils and related products, because they fall into a
different boiling range.
Apart from this, some frequently used additives - which in
themselves are non-carcinogenic - such as elemental sulfur and some
sulfur-containing compounds such as benzyldisulfide, ditertiary
butylpolysulfide and ditertiary octylpolysulfide (Horton et al., 1965;
Bingham et al., 1965; Bingham & Horton 1966) as well as certain
phenols (Boutwell & Bosch 1959) have also been shown to enhance the
carcinogenic activity of polynuclear aromatic hydrocarbons on the
mouse skin. Addition of sulfur to a non-carcinogenic oil did not, of
course, have any effect on the carcinogenicity (Bingham et al., 1965).
Baldwin et al. (1964) reported that additives such as lead naphthenate
did not have an enhancing effect and there are indications that other
components of complex mineral oils with a boiling-range above 370°C
act as inhibitors of the carcinogenic effect of polynuclear aromatic
hydrocarbons, e.g., the saturated (probably cyclic) hydrocarbons
(Bingham & Horton, 1966).
Sunderland et al. (1951) reported skin tests of mineral oil
fractions on mice, under various conditions. They found that washing
the skin with soap and water, after the application of the oil,
reduced both the number of tumours and the rate of appearance. The
reduction was related to the length of the interval between oil
application and washing. Painting once, instead of twice, weekly
greatly reduced the rate of tumour appearance.
From human experience (section 4.7.1.1), it is known that the
ultraviolet radiation of sunshine has a potentiating effect on the
carcinogenicity of mineral oils. Similar indications exist from animal
studies (Emmett, 1975). It has also been demonstrated that certain
co-carcinogenic n-alkanes may increase the carcinogenic potential of
certain wavelengths of UV light on mouse skin (Bingham & Word, 1977).
In comparative studies with various mineral oil fractions on the
skin of mice and rabbits, it became apparent that the rabbit skin was
more sensitive than the mouse skin according to the type, number, and
growth rate of tumours (Cruickshank & Squire 1950; Hieger & Woodhouse
1952; Antonov & Lints 1960; UK Medical Research Council, 1968). In
some instances, mineral oil fractions were non-carcinogenic to mouse
skin, but positive on the skin of rabbits (Shubik & Saffiotti), 1954).
While, rats and guineapigs are apparently less sensitive than mice
(Sugiura et al., 1949; Desoille et al., 1973) Rhesus monkeys appear to
be fairly sensitive (Sugiura et al., 1949).
As to the suspected causes of intra- and inter- species
differences in susceptibility to polynuclear aromatic hydrocarbons,
the levels of the enzymes forming and detoxifying the biological
reactive intermediates, the ultimate carcinogens, in the tissue of the
various strains and species might be one of the important factors
(section 4.6.2.1). Clear intra-species differences in tumorigenic
response have been reported by, for example, Smith & Sunderland (1951)
and Gilman & Vesselinovitch (1955).
In addition to these studies on "pure" mineral oil fractions and
various additives, some studies with metal working oils and textile
oils have been published.
Two cutting oils based on sulfurized mineral oils, were diluted
with water and were tested in various concentrations on the skin of 3
strains of mice. Both were found to be carcinogenic. A marked
reduction in incidence and an increase in induction time of tumours
was found at a dilution of 1:8 compared with 1:4 (Gilman &
Vesselinovitch, 1955). The same authors (Gilman & Vesselinovitch,
1956) compared a straight cutting oil and a water-soluble cutting
fluid in 2 different strains of mice. They found a consistent,
comparable but low incidence of skin tumours in 3 separate trials.
In a later study, 3 commercial additive-containing cutting oils,
one of which was an emulsifiable oil, were repeatedly applied to mouse
skin for up to 31 weeks. Carcinogenic skin changes were observed with
all these oils but were, possibly, less marked with the emulsifiable
oil. In addition to these carcinogenic effects, focal necrosis of the
liver associated with amyloid deposition and amyloidosis of the skin,
spleen, and kidneys were observed. The additives may have had a
contributory effect in some of the pathological changes observed
(Jepson et al., 1977).
In the case of cutting oils, the temperatures to which the oils
are exposed at the cutting edges of the tools are such that cracking
of the oil might conceivably occur and theoretically a
non-carcinogenic cutting oil might become carcinogenic during use. An
unspecified cutting oil from the sump of a machine was tested on the
skin of mice and rabbits. It induced benign tumours in rabbits only
(Cruickshank & Squire, 1950). As the fresh oil was not examined, no
conclusion can be drawn concerning the previously-mentioned
assumption.
Unused cutting oil, used cutting oil, and residue from the sump
were compared in skin tests on 2 strains of mice by Dargent et al.
(1967). Both used and unused cutting oil caused a high incidence of
skin ulceration. Hyperkeratosis and papillomas were more frequent in
the case of used oils and a single case of skin cancer occurred in the
groups of mice treated with used oil and sump residue.
Cutting and quenching oils, fresh and used, were tested on the
skin of rats, guineapigs, and mice. All tests on rats and guineapigs
were negative. In mice, skin tumours were more numerous and more
malignant with used oils than with fresh oil. Only used oils produced
tumours in organs distant from the site of application. The unused
quenching oil was found to contain a benzo(alpha)pyrene level of
0.6-0.8 mg/kg (ppm); in the used oil, this fraction had increased to
20 mg/kg, which is in agreement with the finding of its increased
carcinogenicity. The increase in carcinogenic poly-nuclear aromatic
hydrocarbons may be the result of thermal cracking due to the heat of
the process (Desoille et al., 1973). The studies of Thony et al.
(1975) indicated that the increase in poly-nuclear aromatic
hydrocarbons in cutting oils was very small and considerably less than
for quenching oils and engine oils. The benzo(alpha)pyrene content of
new cutting oils ranged from 0 to 150 mg/kg, compared with 0 to
250 mg/kg for used cutting oils.
A sample of commercial jute batching oil of unspecified origin and
composition, containing a concentration of 3,4-benzopyrene of less
than 1 mg/kg, was tested for carcinogenic activity on the skin of
mice. The oil in question induced malignant skin tumours in 6 out of
24 test animals and proved to be a potent tumour promotor in mice
pre-treated with 7,12-dimethylbenz (a)anthracene (DMBA) (Roe et al.,
1967).
To summarize, it may be concluded that: (a) the carcinogenic
activity of mineral oils seems to be related mainly to the presence
and concentration of certain polynuclear aromatic hydrocarbons,
containing 4,5, or 6 condensed rings; and (b) cracking processes
tend to increase the polynuclear aromatic hydrocarbon contents in
petroleum products. For example such compounds may accumulate in a
metal working oil in contact with the host cutting edge of a tool.
The polynuclear aromatic hydrocarbon content of mineral oils can
be decreased by solvent extraction and/or by hydrogenation. Acid
treatment is less effective for this purpose.
Analytical methods for the determination of total polynuclear
aromatic hydrocarbons should provide a useful, quick, and cheap tool
for predictive screening of mineral oils for carcinogenicity. These
methods should be biologically validated. The correlation will not
always be good, because the carcinogenicity of polynuclear aromatic
hydrocarbons varies considerably and because of the unpredictable
effects of cocarcinogens, inhibitors, and accelerators.
Various substances that are used as additives such as sulfur
compounds, as well as substances that may normally be present in
mineral oils, such as n-dodecane, may act as cocarcinogens. Other
saturated hydrocarbons, normally present, may act as inhibitors of the
same effect.
It is clear from animal studies that washing with soap and water
substantially reduces the hazard to the skin of repeated and long
contact with potentially carcinogenic oils.
4.6.2.3 Effects of inhalation and intratracheal exposures
The aspiration hazard and toxicity for animals of a number of
hydrocarbons and hydrocarbon mixtures was determined by Gerarde
(1963). This study has been discussed in more detail in section 3.6.1.
He found that the aspiration hazard decreased with increased viscosity
of the product and with mixtures containing low viscosity products.
Mice were exposed to mists of various mineral and vegetable oils
with an average droplet size of 2.5 µm, 80% of the particles retained
in all areas of the lungs were 2.5 µm or less in diameter. The highest
concentration of retained particles was found around the terminal
bronchioles and alveolar ducts in all parts of both lungs. Oil
particles were immediately phagocytosed, a process that was
essentially completed within 48 h, unless prolonged exposures (2-4
weeks) had been given. The initial concentration of retained oil
droplets was similar for all types of oil mists. During a 90-h
follow-up, however, the concentration of vegetable oil droplets
decreased progressively, whereas the concentration of mineral oil
droplets remained practically unchanged. After 2-4 weeks of exposure,
mineral oil droplet retention gave rise to localized slight
foreign-body reactions as well as to a few patches of lipoid
pneumonia. Of the other oils, only cod liver oil caused a moderate
foreign-body reaction (Shoshkes et al., 1950).
The effects of prolonged inhalation of oil mists (mineral oil
levels in air of 63-132 mg/m3) were observed in mice, rats, rabbits,
and monkeys. Ordinary automobile lubricating oil and a smoke-screening
oil, were tested for periods varying from 100 to 365 consecutive days
(Lushbaugh et al., 1950) The authors found that surprisingly little
oil accumulated in the lungs and that whatever was retained was
rapidly phagocytosed and transferred to the pulmonary connective
tissue and the hilar lymph nodes. Lipoid pneumonia was not found to be
a hazard at these dosages, though the incidence of infectious
pneumonia in the exposed monkeys greatly increased. Many exposed
monkeys died of a hyperplastic gastritis, probably because much of the
inhaled oil was initially deposited in the nasal passages and
subsequently swallowed. Hyper-plastic gastritis was especially evident
with the shale oil (which is different from petroleum oil) used as
smoke screening oil (Lushbaugh, 1947). No significant increase in
tumour incidence or reduction in the latent period of tumour
production was found in the mouse study.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Rats and guineapigs were exposed for 6 h/day, 4 days a
week, for up to 2 years, to a cutting oil mist consisting of a 10:1
mixture of neutral paraffin and prime lard oil. In some cases, this
caused multi-focal adenomatosis in the lungs of the animals. A
carcinoma of the lung was found in only 1 of 105 exposed rats and not
in any of 65 guineapigs.
In tests on mice, Wagner et al. (1961) found that inhalation of
either mineral or motor oil mist reduced the acute lethal effects of
respired oxidants such as ozone and nitrogen dioxide. The effect was
demonstrable only after a latent period of up to 8-9 days following
oil mist exposure and was thought to result from the formation of a
thin film of oil on the alveolar surfaces.
Long-term inhalation toxicity studies using a highly purified
white mineral oil, composed of naphthene-based saturated hydrocarbons,
were reported by Wagner et al. (1964). Five species of laboratory
animals (dog, rabbit, rat, hamster, mouse) were exposed daily, for
periods from one year to 26 months, to a petroleum-base mineral oil
mist at concentrations of 5 mg/m3, the current USA threshold limit
value, and 100 mg/m3. Histological evaluation of tissues of the dogs
and rats exposed to 100 mg/m3 showed significant pulmonary alveolar
and hilar lymph node oil deposition and/or lipid granuloma formation
after 12 months of exposure. In addition, these animals showed
significant increases in the activities of basic and
magnesium-activated phosphatases.
Body-weight gain, haematological variables, and respiratory
function values did not deviate significantly from the control data at
any of the exposure levels. Studies with a spontaneous
pulmonary-tumour-susceptible strain of mouse presented equivocal
evidence of an increased rate of tumour formation at the 100 mg/m3
concentration. These findings suggest that prolonged exposure to a
mineral oil mist concentration of 5 mg/m3 would not present any toxic
hazard. It would appear, however, that protracted exposure at
approximately 100 mg/m3 would, in time, produce harmful physiological
effects.
The toxicity of aerosols of various petroleum oils (industrial,
transformer, and compressor distillates) differed only slightly in a
long-term experiment by Lutov (1974). White rats were exposed to
concentrations of 13, 30, and 60 mg/m3 for 5 h/day, for 6 months.
Changes in the electrocardiogram, and reductions in arterial pressure,
respiratory frequency, and immunological reactivity were seen even at
12-13 mg/m3, which was considered to be just above the threshold
level. A concentration of 5 mg/m3 is recommended as a maximum
permissible concentration for petroleum oil aerosols without
additives.
In a further long-term study on white rats, Lutov et al. (1976)
studied the toxicity of petroleum oil aerosols in concentrations
ranging from 11.4 ± 1.5 to 61.4 ± 5.2 mg/m3 in combination with
products obtained by thermo-decomposition, i.e., hydrocarbons in
concentrations ranging from 200.0 ± 5.8 to 410.0 ± 12.8 mg/m3 and
carbon monoxide in concentrations ranging from 11.2 ± 1.5 to
35.4 ± 4.6 mg/m3. White female rats were exposed for, 5 h/day over a
6-month period. The combined exposure was found to produce more marked
effects on the parameters tested in the earlier experiment.
The minimum concentrations of oil aerosol that caused functional
changes in the respiratory system with a single inhalation were in the
range of 860-1200 mg/m3. When concentrations of 53 and 60 mg/m3 were
used in the long-term experiment, physical growth was impaired and the
functional condition of the nervous system and the liver changed.
There were morphological changes in the lungs including a
catarrhal-desquamative bronchitis, a swelling of the alveolar
membranes, formation of oleogranules, and the presence of a large
quantity of oleophages and oil droplets in the lymphatic vessels and
the peribronchial and bronchopulmonary lymphatic nodules. At
concentrations of 10-17 mg/m3, the changes were slight and
reversible. A maximum admissible concentration in the air of a work
place of 5 mg/m3 is the official USSR exposure limit (Ivanov et al.,
1978).
In tests on rabbits, Laughlen (1925) demonstrated that mineral oil
given by mouth or nose can enter the trachea. Corper & Freed (1922),
injected olive oil and medicinal mineral oil intratracheally into
rabbits in amounts of 0.5-1.0 ml. The oil was readily aspirated into
the finest pulmonary divisions and into the alveoli. It was retained
for months and caused a mild proliferative reaction typical of a
foreign body reaction. In studies by Pinkerton (1928), rabbits and
puppies were injected intratracheally with animal, vegetable, and
medicinal oil. Complete removal of the oil from the lungs took several
months in all cases. Neutral vegetable oil produced practically no
reaction, animal oils caused giant cell formation and rapid and marked
pulmonary fibrosis. Mineral oil was rapidly phagocytosed, giant cell
formation and slight fibrosis were apparent after 2-3 months, and
storage of the oil took place in the bronchial lymph nodes.
4.6.2.4 Dietary studies
Long-term oral studies have not been conducted with lubricating
base oils.
Lushbaugh & Hackett (1948/1949) carried out a study in which rats
received an average of 0.2 ml/rat per day of a highly refined
diesel-engine lubricating oil, used as smoke screening oil, in theft
diet for a period of 14 months. In this group of 40 rats, 2 developed
foci of colonic mucosal hyperplasia and one developed colonic
adeno-carcinoma.
Results of various dietary studies using food-grade and
pharma-ceutical-grade materials, such as medicinal and food-grade
mineral oil, and food-grade paraffin waxes are included in the
following summary, though these studies with highly purified material
are not representative for the less purified grades.
Schmahl & Reiter (1933) fed 2% (w/v) mineral oil in the diet of
rats for 500 days without adverse effects. In studies by Daniel et al.
(1953), rats were kept for 15 months on diets supplemented with 10%
liquid paraffin, without adverse effects. Five kinds of petroleum
waxes were tested by feeding rats a diet containing 10% of the wax for
2 years. The rats were then observed until natural death. No toxic or
carcinogenic effects were found (Shubik et al., 1962). In the studies
by Oser et al. (1965), already reported in section 4.6.2.2, 3 kinds of
petrolatum were also administered to rats in the diet at 50 mg/kg
diet. Observations over 2 years did not reveal any carcinogenic or
other toxic effects.
4.7 Effects on Man
The general population does not have any contact with base oils;
contact with the commercial products derived from them will, at most,
be occasional and of a lower order of magnitude than occupational
exposure. Cases of accidental ingestion are exceptions.
However, pharmaceutical grade liquid paraffin in the form of
medicinal oil is widely used as a laxative (faecal softener).
Occupational exposure to base oils is restricted to the small
group of workers who manufacture them in oil refineries and to workers
in blending units, who mix and blend them with additives in order to
produce commercial products such as lubricating oils, greases,
metal-working oils, and textile oils. These activities are normally
carried out in closed systems in which case occupational contact is
minimal and discontinuous, usually accidental. This also applies, when
the finished product is put into containers.
4.7.1 Occupational exposure
Epidemiological and clinical data concerning base oils are almost
non-existent. Thus, in this section, it is only possible to analyse
epidemiological studies of workers exposed to products containing base
oils, such as lubricating oils, metal-working oils, and textile oils.
From the list of lubricating oil products given in section 4.2.2,
it is clear that various groups of workers are occupationally exposed
to these products, the most widely-used being the metal-working oils
and textile oils.
The magnitude of occupational exposure varies considerably. In the
case of some lubricants and transformer oils, handling is only
occasional, and, even then, exposure is minimal. In other situations,
such as in the earlier years of mule spinning and in work with
automatic lathes of old design, extremely high exposure did - and
sometimes still does occur. Not only is there continuous direct
contamination of clothes and exposed parts of the skin, but the rapid
movement of the machinery may turn the oil into an aerosol and thus
generate an oil mist that can be inhaled and further contaminate skin
and clothing. Equipment and floors may become covered with an oil-film
and situations have been described where oil even dripped from the
roof.
A certain degree of oil mist generation may also occur in the
printing and rubber industries and with pneumatic equipment (e.g.,
drills), especially under conditions of limited ventilation such as
are found underground.
Even under the best technical conditions, it may sometimes be
difficult to completely avoid contamination. For example, in the case
of toolsetters, the wearing of protective clothing impedes easy
movement, and clothing, including underwear, may become soaked with
oil.
Henry (1946/1947) gave an excellent insight into the types and
magnitude of occupational exposure up to the time of the report. It is
known that some of these conditions had continued for a period of
about 20 years. However, in many countries, great improvements have
been made since then. An understanding of the range of such exposures
is needed to put epidemiological studies of later years into
perspective, especially in view of the long latency of carcinogenic
effects. In the evaluation of such studies, it has to be kept in mind
that today's findings may reflect the industrial hygiene situation of
20-30 years ago.
4.7.1.1 Skin disorders
A great variety of skin disorders attributed to working with
mineral oil or with products based on mineral oils, such as metal
working fluids, are mentioned in the medical literature. This is not
surprising considering the many factors that influence theft
development (van Raalte, 1963; Key et al., 1966; Kipling, 1968;
Hodgson, 1970, 1973).
The main factors include: (a) the degree of intrinsic potential
of a mineral oil product to damage the skin; (b) the integrity of
the skin; (c) the degree and continuity of contact between oil and
skin; and (d) individual predisposition.
The situation, however, is more complex than this, for, in actual
practice, a multitude of other factors may influence the main
conditions to such an extent and in such a complex way, that a
cause-and-effect relationship may become obscured. Most authors agree
that practically all skin disorders attributed to exposure to mineral
oil products can be prevented entirely by adequate industrial and
personal hygiene practices, and that the majority of cases have
occurred in workshops where inadequate conditions prevailed.
Some of the more important factors in the complex interplay are:
(a) Factors related to the mineral oil product used:
(i) Base oil:
the lower the boiling-point of the oil, the more
pronounced the solvent action of the product; this causes
defatting of the skin and leads to dryness, chapping,
scaling, and cracking;
the higher the boiling-point, the more blocking of skin
pores occurs, giving rise to acne formation;
some mineral oils are more irritant than others; the
lower-boiling fractions are sometimes, but not always,
more irritant than higher-boiling fractions (section
4.6.1);
mineral oils themselves do not appear to be sensitizers.
(ii) Additives:
these may be primary irritants or sensitizers in theft own
right (e.g., chromium salts), though manufacturers try to
avoid the use of such additives;
solvents and detergents increase the defatting effect of
the lower boiling-range oils;
some chlorinated additives, such as chlorinated
naphthalenes, may cause chloracne. The use of chlorinated
naphthalenes was discontinued in most countries, many
years ago.
(iii) Changes in the composition of the oil that may occur
during its use:
cracking of oil fractions may occur due to heat;
reactions may occur between components of the mixture or
between them and materials that are added at a later
stage;
metal salts or ions may be formed in the mixture or
solution in the case of metal-working oils; in the case
of, for example, chromium and nickel, this can cause skin
reactions in sensitized workers.
(iv) Impurities of all kinds may accumulate in, for instance,
metal-working oil baths in which the oil is not regularly
changed. Important among these impurities are: metal
particles, which may cause microtraumata of the skin; and
microorganisms, which may cause inflammation of the skin
by way of infection or exotoxins; bacteria, yeasts, or
fungi may be present in mono- or mixed culture, and some
of these organisms can degrade an oil and form potentially
irritating compounds.
(b) Factors related to the work situation:
(i) design of workshop and equipment determine to a large
extent the magnitude and duration of exposure to the oil,
and whether this exposure is continuous or intermittent;
(ii) availability of general and exhaust ventilation of
sufficient design and capacity may determine whether there
is exposure to oil mist or not;
(iii) use of protective clothing such as gloves, will diminish
duration and intensity of skin contact;
(iv) general hygienic facilities such as wash basins near the
work-place, shower facilities, frequent changing and
laundering of work clothing, all of which help in limiting
the extent and duration of exposure;
(v) hands and contaminated parts of the body should be washed
with fatty toilet soap and, after washing the skin should
be treated with a suitable emollient cream; use of
solvents, and alkaline or abrasive soaps for washing can
damage the skin and contribute to the occurrence of skin
diseases.
(c) Individual factors:
(i) general and hereditary conditions of the skin may
predispose to adverse skin effects from all sorts of
chemicals;
(ii) work discipline and safety-mindedness can influence the
duration and intensity of the exposure;
(iii) proper use of safety equipment and general hygiene
facilities, as well as personal hygiene in the form of
frequent bathing and changing of underwear, will help to
avoid over- and prolonged exposure.
Contamination of the skin may often occur despite all precautions
taken, particularly in the case of metal-working oils. Wiping with
oil-soaked rags is an additional source of contamination and may cause
skin lesions. Continuous wearing of oil-contaminated clothing is an
important factor in the etiology of scrotal cancer. Rapidly rotating
machinery may generate oil foams and mists.
The most common form of skin disorder is acute or chronic contact
irritative (toxic) dermatitis caused by the irritative action of
various components, additives, and/or impurities in mineral oils.
Dermatitis may be preceded by defatting and/or maceration of the skin.
Mechanical irritation, microtraumata, and skin cuts may play a role in
its origin. Clinical signs of contact irritative dermatitis are, in
order of severity, erythema, oedema, bullae or necrosis, and sharp
demarcation of the affected areas from unaffected ones.
The other type of dermatitis is contact allergic dermatitis
(synonym: contact eczema) caused by allergic sensitization to various
allergens. Additives or impurities are the most allergenic components
of oil formulations. The signs of eczema are more variable then those
of toxic dermatitis and include erythema, papulae, vesiculae, bullae,
scales, hyperkeratoses, and rhagades. These lesions have a tendency to
spread into areas that have not been in direct contact with the
allergen. Eczema may be preceded by contact irritative dermatitis,
from which it develops by secondary sensitization.
Oil folliculitis, or oil acne is characterized by the triad
comedones, folliculitis, and follicular scars. The lesions are found
on the parts of the body with the greatest exposure. Friction from
clothing and machinery rubbing the oil into the exposed parts of the
skin, is an important additional factor. First there is plugging of
the hair follicles and pores of the skin by follicular hyperkeratosis,
cell debris, and oil with its impurities, followed by blackheads and
secondary infection. Poor personal hygiene is the main cause. Oil acne
occurs more often in the earlier years of exposure to oil. Later,
there appears to be a gradual change in the reaction of the skin to
the oil (Kinnear et al., 1954). Chlorinated naphthalenes and related
compounds have given rise to chloracne.
Photosensitivity is an abnormal sensitivity of the skin to
sunlight caused by certain constituents of coal-tar, but also
sometimes by mineral oil constituents. Related to this is melanosis,
the general darkening of the skin that may follow acute
photodermatitis, as well as toxic melanoderma, which develop after
long-term exposure to oils containing certain anthracene fractions.
Both photosensitivity and melanosis rarely result from exposure to
mineral oil.
Hyperkeratosis may occur either together with dermatitis and oil
acne of long standing, or in isolation -- mostly on the forearms or
other heavily exposed parts of the body. Two forms of hyperkeratosis
can be distinguished: (a) circular, white and flat hyperkeratotic
areas of a few mm in diameter, sometimes in the form of smooth
plaques; these may occur in small clusters and are slightly raised
above the level of the surrounding skin; and (b) a second form,
which may occur at the same time, and consists of rugose, pigmented
warts that are considerably raised above the surrounding tissue level.
The pattern is generally irregular, but may be round or oval.
Precarcinogenic changes may be present in the hyperkeratotic
plaques in the form of rough, slightly raised patches, which sometimes
may take the form of horns or warts. In themselves these forms are
still harmless, but they have a tendency to become malignant. As soon
as these forms contain some malignant cells they are called
keratoacanthomata. After growing for a certain period, they may be
shed from the skin. Another form of precarcinogenic change that may be
encountered is the shark- or shagreen-skin, a pigmented, atrophic
skin, beset with small horns and warts.
A basal cell carcinoma (basalioma) is a very slowly enlarging
tumour of the skin. It may ulcerate, or invade the area round it, but,
in general, it does not metastasize. The most common form of malignant
tumour is the squamous cell carcinoma (spinalioma), starting as a
small tumour, that may arise from a keratosis or in apparently healthy
skin. It continues to grow, starts ulcerating, invades surrounding
tissues and eventually may metastasize. None of these conditions is
limited to mineral oil exposure. Similar changes may occur as a result
of UV-light exposure, excessive doses of X-rays, exposure to pitch
coal-tar, or ingestion of arsenic. A specific localization, however,
is the epithelium of the scrotum. In this case "it is reasonable to
assume that it could be caused by occupational exposure to soot, tar,
pitch, or oil" (Kipling, 1968).
A combination of various potentiating factors, such as mineral oil
contact and exposure to sunlight (UV-radiation) increases the tendency
to develop skin cancer (Schwartz et al., 1947; Smiley, 1951; Kinnear
et al., 1954; Emmett, 1975). This is especially pronounced in persons
with fair hair.
4.7.1.2 Skin carcinogenicity
The history of the development of skin cancer as a result of
exposure to mineral oil, as well as the major epidemiological
literature related to the subject have been reviewed by the
International Agency for Research on Cancer (IARC, 1973). To quote
theft findings:
"Volkmann (1875) described scrotal cancers among workers producing
paraffin by the distillation of coal-tar. Subsequently, Liebe (1892)
noted the absence of such hazard among workers exposed to pure
paraffin. Several investigators have since shown that cancers among
paraffin workers are not due to the paraffin but to impurities in oils
produced during processing (Leitch, 1922; Hendricks et al., 1959).
Refined paraffin is free of PAH and does not induce skin cancer in
mice (Shubik et al., 1962).
"Bell (1876) first described cancer of the scrotum in a Scottish
shale oil worker. In a 23-year period, 49 Scottish paraffin workers
developed skin cancer of which 13 were scrotal (Henry, 1946).
"The cotton mule spinning industry in Great Britain originally
used shale oil for the lubrication of the spindles (Henry, 1946). The
first case of death from scrotal cancer in a worker who used shale oil
in mule spinning occurred in 1923 (Bridge & Henry, 1928). In the years
1920 to 1943, there were 1303 legally notified cases of skin cancer in
the British mule spinning industry, including 824 of the scrotum.
There were 575 fatal cases of scrotal cancer recorded between 1911 and
1938 (Henry, 1946).
"In Great Britain, the Mule Spinning Regulations have ensured that
since 1953 only oil drastically refined with sulphuric acid shall be
used in mule spinning and that mule spinners shall be medically
examined every six months. These measures, together with the marked
decline of the process of mule spinning, have produced a sustained
fall in the incidence of cancer of the scrotum in Great Britain.
"Cutting oils used by workers to cut metals were found to increase
the risk of skin cancer in Birmingham, England (Cruickshank & Squire,
1950; Cruickshank & Gourevitch, 1952), particularly among workers in
automatic machine shops. Between 1950 and 1967, 187 cases of scrotal
cancer occurred in this region, of which at least two-thirds could be
attributed to oil (Waterhouse, 1971).
"At the present time toolsetters and setter operators in automatic
shops who use neat cutting oil have an increased risk of cancer. The
work requires constant contact with the machines and consequent
contamination with the oil. In the Birmingham area of England, a high
frequency of skin and scrotal cancer from oil has occurred,
particularly among bar automatic machine workers; but other
engineering practices also present a cancer hazard, e.g., metal
rolling, tube drawing, metal hardening and machine operating. Although
the major risk is from exposure to undiluted oils, emulsions have been
incriminated occasionally. The industries most affected are those with
automatic shops, such as nut and bolt manufacturers. Workers have also
been affected after exposure during the changing of transformer oil in
electrical sub-stations and during the painting or spraying of mould
oil for brick- and tile-making or concrete moulding, in drop forging,
rubber mixing, wire drawing, rope making and in the jute industry and
from grease in metal working (Kipling, 1968).
"In France, in the valley of the river Arve in the Savoy Alps,
there have occurred since 1955 at least 60 cases of cancer of the
scrotum together with many cases of cancer of the skin among the bar
automatic machine workers (décolleteurs). The very high frequency in
the relatively small population of the valley was observed mainly
among the self-employed and workers in small premises (Thony & Thony,
1970). They were in contact with undiluted cutting oils.
"Cancers of the larynx, lung and stomach have also been attributed
to oil mist (Southam, 1928); and recently evidence has been produced
that persons who developed cancer of the scrotum are significantly
more liable to develop cancers at other sites, e.g., in the
respiratory tract or upper digestive tract (Holmes et al., 1970)."
Kinnear et al. (1954, 1955) published the results of an extensive
epidemiological study of skin disease in jute workers in relation to
mineral oil exposure. They found a high incidence of premalignant
changes on the skin of the exposed parts of the body in long-term,
older workers and isolated cases of scrotal carcinoma.
Bingham & Horton (1966) estimated the latent period for skin
cancer caused by mineral oil exposure to be 50-54 years (range 4-75
years). In the case of crude paraffin oil, they mentioned an average
of 15-18 years (range 3-35 years). In these cases, the range may be
more important than the average, though the average indicates that, in
general, the latent period is very long. A considerable proportion of
the cases of skin epithelioma caused by mineral oil exposure had such
a long latency period that the disease only appeared after retirement
from active work (Cruickshank & Gourevitch, 1952; Kinnear et al.,
1955).
Five cases of squamous cell carcinoma of the hand and forearm and
one of the scrotum occurred in machine operators at a plant in
Ontario, Canada (Mastromatteo, 1955). These workers had been exposed,
for an average of 21 years, to cutting fluids that were subsequently
demonstrated to be carcinogenic in animal tests (Gilman &
Vesselinovitch, 1955).
Milne (1970) traced 5 cases of carcinoma of the scrotum, as
registered in the Central Cancer Registry of Victoria, Australia, and
found that, in all cases, the carcinomas occurred during or after the
seventh decade of life. Three of the subjects had had intensive
contact with mineral oils throughout theft working life, one was a
stoker in a gas-works, and the fifth had always been involved in
administrative work.
In the Netherlands, a recent survey demonstrated that scrotal
cancer occurred only sporadically and was not correlated with
occupational exposure to mineral oil (Pruyn & Reijnierse, 1972;
Fokkens et al., 1972; van Raalte, 1972).
Eight cases of scrotal cancer were discovered by Avellan et al.
(1967), over a period of 24 years, among 250 automatic lathe operators
in Gothenburg, Sweden. Diagnosis was made when the operators were
between 54 and 66 years of age and after periods of exposure to
mineral oil ranging from 19 to 43 years. In the words of the authors:
"All of the cases occurred among operators who began their work
during the era when the exposure, as a result of the prevailing
machine construction, was considerable and before the regulations and
the controls, which were instituted after the discovery of the first
cases, had been set up."
Wahlberg (1974) analysed 34 cases of scrotal cancer reported to
the Swedish Cancer Registry between 1958 and 1970. Seven cases (21%)
had been heavily exposed, occupationally, in the past to oil. and oil
mist, e.g., as automatic lathe operators. One of this group developed
a primary lung cancer.
A retrospective study of 298 cases of scrotal cancer registered in
the Birmingham region in the United Kingdom between 1936 and 1972 was
reported by Brown et al. (1975). The incidence of scrotal cancer was
5-6 cases per million males per year, whereas for the whole of the
United Kingdom, the incidence is 1-2 cases per million males per year.
The patients or relatives were interviewed in 109 cases: 94 had been
exposed to mineral oil (mainly tool-setters and machine operators, and
all cases had been exposed to cutting oil, 14 had been exposed to
pitch or tar, and, in 7 cases, there was no apparent occupational
exposure. In 298 cases of scrotal cancer, 52 other primary rumours
were noted; of these, 42 arose following the scrotal cancer including
15 skin tumours and 12 bronchial tumours. The incidence of both these
types of cancers was much in excess of what would be expected
statistically. The majority of the group with second primary tumours
were machine operators and tool-setters (75% in the case of bronchial
carcinoma). The authors postulated that in these cases both primary
rumours were initiated by the same carcinogen.
After studying all the available literature, Desoille et al.
(1973) concluded that, in general, and with the exception of certain
extreme situations, the number of tumours caused by exposure to
mineral oils was low. According to Auld (1950) and Eckhardt (1957),
though certain cutting oils and other mineral oils are carcinogenic to
the skin, the degree of carcinogenicity is very low compared with that
of coal-tar, pitch, and shale oil. In their opinion, and that of most
other authors, e.g., Avellan et al. (1967), even this level of
carcinogenicity would disappear, if a minimum of industrial and
personal hygiene measures were adhered to. On the other hand, Auld
(1950), Desoille et al. (1973), Thony et al. (1975) and many others
urge that alternative products should be developed that do not expose
the workers to a tumorigenic hazard.
In a further report on the skin cancer epidemic in the French Arve
Valley, Thony et al. (1975) recorded 133 epithelioma cases in 15
years, mostly of the scrotum. The incidence in these workers was 36
times that expected in the general population. In workers in small
workshops with poor industrial and personal hygiene, the incidence was
found to be 3 times higher than in the larger plants. The average age
was 54 years (range 35-75 years) and the average exposure 30 years
(range 15-50 years), at the time of clinical diagnosis. In addition,
an increased incidence of bronchopulmonary tumours was found,
especially in the décolleteurs. Officially until 1947, but in
practice possibly until 1950, coaltar-derived anthracene oils were
used, which originally contained benzo(alpha)pyrene levels of up to
1000 mg/kg. The level was later reduced to 10 mg/kg. These oils may be
responsible for many of the previously mentioned cases, but skin
cancer also occurred in some of the workers exposed only to the
petroleum-derived oils that were used exclusively later on. Fresh
cutting oils, as delivered to the users, contained benzo(alpha)pyrene
levels ranging from 0.5 to 145 µg/litre and the concentration of
carcinogens was found to increase during use, though not to the same
extent as that in quenching oils (2-100 times with an average of 30
times), and in lubricating oils for internal combustion engines.
4.7.1.3 Effects of oil mist exposure
Southam (1928) was the first to attribute cancers of the larynx,
lung, and stomach to oil mist exposure.
In a report by Huguenin et al. (1950), 32 out of 144 patients with
lung cancer had been in prolonged and intense contact with oil mist in
the past. Old metal-working machines with improper protection against
oil mist, processes where oil vapour arose from contact with hot
metal, and high-pressure cleaning of machines with oil, were found in
the places where these patients had worked. Hendricks et al. (1962)
considered the observations of Huguenin et al. (1950) to be of
doubtful significance in view of the high incidence of this disease in
non-oil-mist-exposed groups. Nevertheless, the conditions in these
cases may have been such, that a causal relationship, in at least some
of them, cannot altogether be excluded.
More printing industry workers were found among cases of bronchial
carcinoma in a Stockholm clinic (8 out of 125) than would have been
expected (Ask-Upmark, 1955). Certain industrial processes where oil
mist might occur were studied by Hendricks et al. (1962), who gave an
indication of possible exposures in their table of actual exposures
measured in various industries (Table 7).
The average particle size in the oil mists varied according to the
generating process and was found to be about 1.0-5.0 µm.
In 241 Kodak workers exposed to oil mist (Ely et al., 1970), no
significant differences were found, either in mortality, respiratory
symptoms, and disease, or in lung function compared with a control
group.
In the same year, Holmes et al. (1970) produced evidence, that
persons who developed cancer of the scrotum were significantly more
liable to develop cancers of the respiratory tract and the upper
digestive tract.
Similar findings have been reported in a preliminary paper by
Waterhouse (1972). An excess of primary tumours at other sites (skin,
respiratory and upper alimentary tracts) was found in men with scrotal
epitheliomas.
From animal studies, it appears that oil mist particles larger
than 5 µm will not easily penetrate into the lungs, but will be
retained mainly in the nasopharynx and upper respiratory tract.
Smaller particles, especially those of 2.5 µm and less, will readily
pass into the alveoli, where they will be phagocytosed and passed on
to the lymph nodes. When this mechanism cannot cope with the
situation, in cases of continuously repeated high exposure to oil
mists, a chemical pneumonitis and chronic lipid pneumonia may develop
(Proudfit et al., 1950; Foe & Bigham, 1954), but this appears to be an
extremely exceptional condition. Clinical details and examples of this
will be discussed in section 4.8.
TABLE 7. Exposure to oil mist in selected industries
Type of industry Observations Exposure range
oil mist mg/m3
brass and aluminium production 5 1.4-20.7
copper mining 7 5.4-22.0
automobile manufacture 37 1.0-56.5
manufacture of steel products 33 0.8-50.0
newspaper (press room) 8 2.0-16.6
screw manufacture 6 1.0-14.2
From: Hendricks et al. (1962).
In 12 out of 19 workers with oil mist exposures ranging from 9 to
18 years, Jones (1961) found a marked linear and reticular pattern in
the radiograms of the lungs. Exposures ranged from 1 to 9 mg/m3 with
70% of the particles of the order of 1 µm.
Various groups of research workers have studied different aspects
of the printing industry. Printing-ink consists chiefly of a
suspension of carbon black in mineral oil or aromatic extracts of
mineral oil. Modern high-speed newsprint presses can generate fairly
high concentrations of ink-mist aerosol. Most particles, however, are
outside the respirable range and may end up in the stomach, rather
than in the lungs of exposed workers. Lippmann & Goldstein (1970)
found an average droplet size of 14 µm (ranging up to 30 µm) in the
press rooms of the New York Times. The time-weighted average
concentration of respirable mist particles was found to be 1.4 mg/m3
against a total time-weighted average mist concentration of
8.6 mg/m3. In a parallel epidemiological study in the same firm,
mortality and morbidity data over a 15-year period were compared for
pressmen and compositors. No significant differences in respiratory
mortality or morbidity were found (Goldstein & Benoit, 1970).
In a similar study by Pasternak & Ehrlich (1972), there was no
increase in respiratory symptoms or decrement in respiratory
performance in 778 New York pressmen compared with 1207 compositors.
No significant differences in death rates, were found in those who
were less than 40 years old when first employed, even if they had
worked for more than 20 years. In those first employed when they were
more than 40 years old and with more than 20 years of employment,
there was a significantly higher death rate in pressmen compared with
compositors. The reason for this finding was not clear. The mean oil
mist concentration measured in the 3 workshops concerned was
3.7-5.2 mg/m3.
Moss et al. (1972) analysed the causes of death of 3485 former
full-time printing industry workers from London and Manchester, who
died in the period 1952-66; Greenberg (1972) studied the death
certificates of 670 male printing workers who died between 1954 and
1966. In both studies, an excess number of cancers of the lung and
bronchus were found. This excess was more marked in Manchester and
greatest in machine-room men. The authors concluded that the slight
excess found might or might not be due to occupation. They considered
that an occupational cause was more likely in the case of the greater
excess in the Manchester machine-room men.
These studies in the printing industry have been mentioned in
relation to mineral oil exposure. However, the same data could be
related to carbon black and even to lead exposure. Similar
considerations should be kept in mind in the evaluation of the data in
other occupations where many other factors might also play a role,
either individually or combined with the mineral oil used, as, for
instance, chromium in the metal-working industry.
In studies in 34 metal-working firms in Baden-Würtemberg (FGR),
the frequency of respiratory complaints (cough, expectoration, and
dyspnoea) in 443 workers exposed to oil mist for long periods was
compared with that in 398 unexposed controls, matched for age. Mineral
oil mist exposures from cutting oils had ranged from 40 to 150 mg/m3
over long periods. The highest incidence of complaints was found among
unexposed smokers, the lowest incidence amongst non-smoking,
oil-mist-exposed workers. The authors did not find any signs of
irritative effects from oil-mist exposure, but a significant
protective effect against the well-known irritant effects of smoking
(Drasche et al., 1974). This finding is in line with findings in
experimental animals, that previous exposure to oil mist reduces the
lethal effects of respiratory oxidants such as ozone and nitrogen
dioxide in mice (Wagner et al., 1961). The authors stated that
conclusions could not be drawn from this study in relation to the
carcinogenicity of these oil mists in the respiratory tract.
During the cold processing of metal, the concentration of mineral
oil mist in the air (spindle oil aerosol) fluctuated from 3 to
40 mg/m3 (on average 10 mg/m3). During an examination of 77 lathe
operators (men and women), functional disturbances found to occur in
the respiratory system, in particular after a period of service of
more than 10 years, included a reduction in the active volume and the
maximum ventilation of the lungs and an increase in oxygen requirement
and in its coefficient of utilization (Bruskin & Demcenko, 1975).
A 30-year retrospective cancer mortality study was carried out by
Decoufle (1976) on 5189 workers engaged in metal-working for at least
one year. No significant differences in cancer mortality were
observed, when compared with the general population. Indications of
increased incidences of respiratory and digestive cancer, observed
when comparison was made on an age group or exposure basis, were not
statistically significant.
Decoufle (1978) published a further study on a group of 2485 male
workers employed between 1938 and 1967 in jobs exposing them to
various levels of cutting oil mists. Compared with the total death
rate of the US male population, no significant differences were
observed for 15 cancer site categories. However, a 2-fold risk of
cancers of the stomach and large intestine (combined) was seen after
20 years of follow-up in the subgroup of men with 5 or more years'
exposure to cutting oil mists, prior to 1938. Deaths from nonmalignant
respiratory disease were significantly fewer than expected. These
results suggested that occupational exposure to soluble and insoluble
cutting oil mists, during various metal machining processes, did not
pose a health hazard in terms of respiratory cancer and fatal
nonmalignant respiratory disease, but might be associated with certain
forms of gastrointestinal cancer.
To summarize, it can be concluded from the literature that
oil-mist exposure can give rise to pulmonary disease, but only after
prolonged exposure in workplaces with unsatisfactory hygienic
conditions. If oils with a low content of polynuclear aromatic
hydrocarbons are used in situations where oil mists can be generated
and the TLV for oil mists is not exceeded, this problem is unlikely to
arise.
4.8 Clinical Studies
Clinical studies on the effects of mineral base oils and products
derived from them on the skin have been discussed together with
epidemiological studies in sections 4.7.1.1 and 4.7.1.2. It was felt,
that compilation of individual case-histories from the literature on
this subject was superfluous. Adverse effects resulting from the
surgical use of paraffin for cosmetic purposes, and those following
grease-gun accidents have not been considered in this review.
Clinical studies on the effects of these products after ingestion
show that the main effects are caused by aspiration, which may be a
complication of ingestion, and usually occurs during subsequent
spontaneous or induced vomiting. Otherwise, there is general agreement
that mineral base oils, lubricating oils, and greases have a low order
of toxicity, when ingested (Gerarde, 1960). At the most, some
gastrointestinal symptoms, such as abdominal cramps and diarrhoea,
result.
There was evidence from a clinical case study that prolonged
ingestion of mineral oil over a number of years could result in oil
deposition in the small intestine, abdominal lymph nodes, liver, and
spleen and lungs. This produced significant structural and functional
abnormalities that were considered to have contributed to the
patient's death (Nochomovitz et al., 1975).
White mineral oil (pharmaceutical grade) is a base oil
specification intended for oral use. It is a highly purified base oil
distillate, mainly containing saturated paraffinic fractions, and it
has to be free of polynuclear aromatic hydrocarbons. It is used as a
laxative, in pharmaceutical formulations, and as a food-grade
lubricant. From studies with radio-opaque oil, it appears possible
that a small amount of mineral oil, when taken orally as a laxative
just before retiring, may gain entrance into the lungs. Though this is
not of any consequence if it occurs only once, a cumulative effect in
the lungs might result, if it occurs repeatedly, day after day, over
several years (Sante, 1949; Miller et al, 1962).
Clinical experience of the effects of inhalation of mineral oil in
the lungs is in agreement with the results of animal studies (section
4.6.2.3). In man, mineral oil is rapidly phagocytosed and transferred
to the regional lymph nodes. In contrast with vegetable oil and oil of
animal origin, however, mineral oil cannot be metabolized and is
deposited in the interstitial tissue and in the lymph nodes, where it
induces a cellular reaction, with the appearance of giant cells that
may result in fibrosis of more or less extensive lung areas. The
resulting clinical and pathological picture depends to a large extent
on whether there has been massive, acute over-exposure, with the
body's defence mechanisms overwhelmed (see (a)), or whether long-term,
low-level exposure has occurred, where the defence mechanisms can cope
with the daily exposure and no disease will become apparent, until the
signs and symptoms of the secondary fibrotic reaction appear (see
(b)), (Pinkerton, 1927, 1928; Cannon, 1940; Freiman et al., 1940; Moel
& Taylor, 1943).
A few typical clinical entities can be recognized:
(a) Diffuse acute mineral oil pneumonia is practically always
the result of an accidental massive aspiration of mineral oil (Sante,
1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham, 1954;
Gerarde, 1960). It is, in fact, a chemical pneumonitis frequently with
a superimposed secondary infection, progressing into an interstitial
proliferative inflammation. Cases resulting from aspiration following
accidental ingestion of products containing gasoline, kerosene, or
other petroleum solvents in the same boiling-range are well-known from
the clinical literature. Many cases have occurred as a result of
aspiration of seawater contaminated with diesel oil by survivors of
sinking ships (Weissman, 1951).
Aspiration of mineral oils in the boiling-range under discussion
may have similar results. This can happen when choking occurs, while
taking white medicinal oil. This acute type of mineral oil pneumonitis
is not likely to occur as a result of occupational exposure to oil
mist. Clinically, all signs and symptoms of an acute massive
pneumonitis are present with elevated temperature and a chest X-ray
typical for this condition. Oil droplets may be found in the sputum
and, histologically, the condition is characterized by intra-alveolar
accumulation of oil-laden phagocytes and inflammatory cells. Acute
mineral oil pneumonia is a special form of lipoid pneumonia, which
presents a similar clinical picture and is the result of aspiration of
vegetable or animal oil (mainly cod liver oil, but also egg-yolk and
milk). Though in these cases a similar severe acute pneumonia may
develop, the oil can be metabolized and disappears completely after a
certain time (Sante, 1949). With oils of animal origin, such as cod
liver oil, however, the reaction of the body (lungs and other internal
organs) can be much more serious than with mineral oils (Young et al.,
1939).
(b) Diffuse chronic mineral oil pneumonia occurs as a result of
gradually developing fibrotic and proliferative changes in both lungs.
It may follow years after the acute form, or without an acute
beginning after a long, practically asymptomatic period as a result of
repeated smaller "aspirations", such as those resulting from regular
massive use of mineral-oil-based laxatives, nose-drops, or nose-sprays
(Pinkerton, 1927; Bishop, 1940; Freiman et al., 1940). The apparently
rare cases of mineral oil pneumonitis following prolonged occupational
exposure to excessive oil mist concentrations fall into this category
(Sante, 1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham,
1954; Gerarde, 1960). Reports from the literature suggest that in some
of these cases, at least, a predisposing factor was present in the
form of a pre-existent or concomitant lung disease (Freiman et al.,
1940; Weissman, 1951; Forbes & Markham, 1967). Increasing dyspnoea and
productive cough are the most important symptoms. The chest X-ray
generally shows increased perihilar opacities with signs of lung
fibrosis and diffuse patchy opacities. Oil droplets or oil-laden
phagocytes may be found in sputum or in tissue derived from lung
biopsy (Goodwin, 1934; Bishop, 1940; Freiman et al., 1940; Rossier &
Bühlmann, 1949; Sante, 1949; Proudfit et al., 1950; Borrie & Gwynne,
1973).
(c) Paraffinomas are large fibrous nodules or globules of liquid
mineral oil embedded in dense hyaline fibrous tissue. They represent a
separate form of the chronic mineral oil pneumonitis in which
oil-laden phagocytes, destroyed by pressure, atrophy in the fibrous
scar tissue formed. Paraffinomas may be found singly or in clusters
around the large bronchial branches or at the site of the hilar lymph
nodes. It may be difficult to differentiate a paraffinoma from a lung
tumour radiologically (Brown & Biskind, 1941; Wood, 1943; Sante, 1949;
Proudfit et al., 1950; Weissman, 1951; Bryan & Boitnott, 1969; Borrie
& Gwynne, 1973). Aspiration biopsy may help in the differential
diagnosis (Nathanson et al., 1943).
Boitnott & Margolis (1966a) have described analytical methods for
the identification of the various oils in human tissues. Mineral oil
droplets may pass from the hilar nodes via the thoracic duct into the
systemic circulation (Pinkerton, 1927; Young et al., 1939; Freiman et
al., 1940; Boitnott & Margolis, 1966b). As a result of this
transmission, oil droplets have been found in the liver, spleen, and
other organs (Pinkerton & Moragues, 1940; Rewell, 1947).
The general pattern found in young children is slightly different
from that found in adults. Pinkerton (1927) described 6 cases of
lipoid pneumonia in children and data on 25 cases in children have
been summarized by Goodwin (1934). Seven cases have been reported by
Ikeda (1935) and 27 cases by Bromer & Wolman (1939). There is general
agreement that fats and oils of animal origin play a more important
role than mineral oil in lipoid pneumonia in infants and young
children. Furthermore, the acute massive aspiration type is more
frequently seen in children than the chronic form. The main causal
factors in these observations are: false passage of a gavage tube,
false deglutition in bottle-feeding with the baby lying on its back --
especially in cases of debilitating disease, and also aspiration
following forced administration of milk or cod liver oil with or
without vomiting or choking (Freiman et al, 1949).
With regard to adults, Ikeda (1937) summarized 106 cases from the
literature, Graef (1939) 22 cases, Bishop (1940) 136 cases, Freiman et
al. (1940) 58 cases, and Moel & Taylor (1943) 20 cases. They concluded
that lipid pneumonia, especially the diffuse chronic form, occurs more
frequently in adults than is generally believed. Liquid paraffin is by
far the most important etiological agent in the adult. Debilitated
states, dysphagia and impaired cough reflexes, because of neurological
or other disorders, are important predisposing factors. The authors
stress, however, that mineral oil is widely used without evident harm,
even in elderly persons. Wherever there is a real indication for this
type of medication, they see no reason to discontinue it. Extensive
use, especially self-medicated, of liquid paraffin intranasally or via
the oral route by debilitated or dysphagic patients should, however,
be discouraged (Bishop, 1940; Freiman et al., 1940).
As a matter of interest, a few cases of lipoid pneumonia have been
described in relation to the intratracheal administration of
mineral-oil-based mixtures by opera singers, prior to every
performance on the stage, in order to improve the quality of the voice
(Even, 1947; Facquet & Langeard, 1947; Meyer, 1976). A similar case
was described by Garvin (1939) as a result of intratracheal
self-medication.
From the literature, it is apparent that with the recognition of
the causal factors and the change from oil-based to water-based
nose-drops, the incidence of the type of lipid pneumonia just
described has drastically decreased, since the end of the forties.
The following more or less typical cases, in which there was -- or
might have been -- a relation between occupational exposure to oil
mist and the occurrence of lipid pneumonia, were found in the
literature. Proudfit et al. (1950) reported a case of chronic lipid
pneumonia in a 40-year-old man who had been spraying mineral oil for
17 years; he had a typical chest X-ray. The chief complaints were
cough, shortness of breath, and fatigue. Mineral oil droplets were
identified in the sputum; 3 years later the condition had progressed
slightly. A case of lipid pneumonia was described by Weissman (1951)
in which the disease apparently developed on the basis of
long-standing pulmonary fibrosis as the result of blast-spraying of
machine parts with mineral oil; no mask had been used as protection
against the inhalation of nebulized oil. Foe & Bigham (1954) reported
the case of a 30-year-old aircraft mechanic who complained of fatigue,
shortness of breath on exertion, and frequent chest colds. Lipid
pneumonia was diagnosed from a lung biopsy. The mechanic had been
spray-cleaning aircraft engines with a mixture of 50% kerosene and 50%
vegetable-oil-soap.
Two cases of progressive respiratory disease, which developed in
the fifth decade of life were described by Forbes & Markham (1967).
Both patients had a moderate to heavy smoking history; one, in
addition to this, had a family history of asthma; dyspnoea and
wheezing were the major signs in each case. Both reacted well to
treatment, but recurrence of signs and symptoms was related to working
with cutting oils, the composition of which was not mentioned.
The epidemiological data on the possible relationship between
cancer of the respiratory tract and long-term occupational exposure to
oil mist has been discussed in section 4.7.1.2. There is some evidence
that in cases where an unsatisfactory industrial hygiene situation
coincided with the use of an oil with probable carcinogenic
properties, such a causal relationship might exist. On the other hand,
various extensive studies have shown that, in general, it is certainly
not a major problem.
However, Wood (1943) reported a fatal case of extensive lipid
pneumonia in a house-maid who had used oily nose-drops in large
quantities for recurrent sinusitis over a period of 10 years. At
postmortem, an alveolar-cell carcinoma was found in the lungs. The
author suggested that there might have been a causal relationship
between the 2 diseases, though he assumed that this occurrence would
be rare. Two cases of bronchogenic carcinoma were described by Sante
(1949). In the first case, the malignant epithelial tumour was
situated in an area of dense fibrotic tissue, typical of a
paraffinoma. In the other case, a squamous cell carcinoma was found
together with lipid pneumonia in an early stage of organization. The
author felt that there might have been an etiological relationship in
the first case, but considered this less likely in the second case.
Both patients had taken a tablespoon of mineral oil as a laxative,
just before retiring, for years. Volk (1964) studied a series of more
than 100 cases of mineral oil pneumonia and noted that not one case of
bronchogenic carcinoma occurred in this group. In addition, he
reviewed 114 consecutive autopsies of adenocarcinoma of the lung in
which he did not find any lesions that might be associated with
mineral oil pneumonia.
A rapidly lethal case of multifocal alveolar cell carcinoma of the
lung was reported in a 63-year-old man who had centrifuged used
cutting oil for 23 years and had been exposed continuously to oil mist
of this type during that period (Despierres et al, 1965). Wahlberg
(1974) described one case of primary lung cancer in 7 men who
developed scrotal cancer following heavy occupational exposure to oil
and oil mist in, for example, automatic lathe operation. A case in
which achalasia led to chronic mineral oil pneumonia was reported by
Bryan & Boitnott (1969). An adenocarcinoma developed in the area of
scarring resulting from the mineral oil and caused death. On the basis
of a literature study and theft own findings, the authors concluded
that the pathogenesis might be related to the pulmonary scarring
rather than directly to the mineral oil (see also Yokoo & Suckow,
1961). However, the authors also considered that there was no reason
to suppose that carcinomas were more likely to arise in a scar induced
by mineral oil than in a scar of a different origin (Bryan & Boitnott,
1969).
5. BITUMEN
5.1 Properties and Analytical Methods
5.1.1 Chemical and physical properties
The term bitumen is applied to solid and semi-solid residues from
the distillation of suitable crude oils. This product is known as
"asphalt" in the USA. In most other countries, the term asphalt is
reserved for certain natural deposits and for mechanically made
mixtures of bitumen and mineral matter.
Bitumen is the residue obtained by atmospheric and vacuum
distillation of certain types of crude oil. It is generally a black or
dark-brown material, ranging from a highly viscous liquid to a solid
and brittle substance at normal ambient temperatures, depending on the
proportion of light fractions removed. On heating, bitumen softens
gradually and eventually becomes fluid. Grades are characterized by
their "penetration" and "softening" point. According to the Petroleum
Handbook (1966), bitumen can be considered as a colloidal system of
highly condensed aromatic particles in an oil with ring-type
molecules. From this statement, it is clear that bitumen is a very
complex mixture of mainly high-boiling hydrocarbons. Its composition
varies widely with the geographical source of the crude oil and the
process of manufacture. For example, a mixture of 6 samples was found
to contain:
(a) 32% asphaltenes: high-relative-molecular-mass aromatic compounds
and heterocyclic hydrocarbons of which some are unsaturated. They
are soluble in carbon disulfide but insoluble in petroleum
naphtha;
(b) 32% resins: polymers resulting from the processing of unsaturated
hydrocarbons;
(c) 14% saturated hydrocarbons: hydrocarbons in which the carbon atoms
are connected by a single bond; and
(d) 22% aromatic hydrocarbons: hydrocarbons containing one or more
benzene rings per molecule, including condensed polycyclic
aromatic hydrocarbons (Simmers et al., 1959; Simmers, 1964).
While the appearance and engineering applications of bitumens and
asphalts are similar to those of coal-tars and pitches, fundamental
differences exist between these 2 classes of materials (Puzinauskas &
Corbett, 1978). Bitumen is generally derived from crude oil by a
process that does not involve cracking or thermal conversion, and
coal-tars and pitches are obtained by high-temperature carbonization
of bituminous coal. Chemically, coal-tar materials are mainly composed
of highly condensed-ring aromatic and heterocyclic hydrocarbons.
Bitumens, on the other hand, contain a much higher proportion of high
relative molecular mass paraffinic and naphthenic hydrocarbons and
their derivatives. Under comparable heating during application and
use, coal-tars generate substantially higher emissions of polynuclear
aromatic hydrocarbons than bitumen. While epidemiological surveys of
workers engaged in the production of coal-tar have revealed an
increased incidence of lung cancer, no increases in cancer or other
adverse effects have been observed in studies on workers involved in
the manufacture and application of asphalt.
The benzo(alpha)pyrene content of petroleum bitumens derived from
various Russian crude oils was determined by Janyseva et al. (1963).
They demonstrated that the benzo(alpha)pyrene content of straight-run
bitumen was considerably lower (of the order of 0.6 mg/kg) than that
of bitumens derived from cracking residues (of the order of
4-272 mg/kg). Schamp & van Wassenhove (1972) reported
benzo(alpha)-pyrene levels of 3-5 mg/kg in bitumens.
5.1.2 Methods of sampling and analysis
See section 2.1.2.
5.2 Sources of Environmental Pollution
5.2.1 Natural sources
Natural bitumen and asphalt deposits occur in various parts of the
world, mainly as a result of mineral oil seepage from the ground. The
most well-known natural asphalt deposit is the Trinidad Lake, which
contains a mixture of about 39% bitumen, 32% mineral matter, and 29%
water and gas.
5.2.2 Man-made sources
5.2.2.1 Production
Total world-wide bitumen production reached 90 million tonnes in
1973. As with crude oil, this was approximately 10 times the immediate
pre-war level. Bitumen production rose to 100 million tonnes in 1979
and is expected to continue to increase in the future, although at a
lower rate of growth than in the past.
The following types of bitumen are produced by refining and
treatment:
"Straight" bitumen
The residue of atmospheric or vacuum distillation of
asphaltic-based crude oils. For special applications, very hard
pitch-type bitumen residues can be obtained by distilling cracked
oils.
"Blown" bitumen
Manufactured by feeding air-bubbles countercurrent through a
column of hot molten straight bitumen. Oxidation reactions occur
leading to dehydrogenation and polymerization of the unsaturated and
aromatic components. In this process, large condensed aromatic nuclei
may also formed.
"Cutback" bitumen (or more fluid bitumen grades)
Obtained by mixing bitumen with petroleum solvents or mineral oil,
sometimes with coal-tar or high aromatic extracts.
Bitumen emulsion
Made by emulsifying 50-65% of bitumen in water in the presence of
0.5-1.0% of an emulsifier, usually soap and generally used cold for
both roadmaking and industrial purposes.
5.2.2.2 Uses
The main use of bitumen is for paving roads. It is also used in:
(a) lining irrigation canals, water reservoirs, dams, and dykes;
(b) mastic asphalt for industrial flooring;
(c) bituminized felts for roofing;
(d) protective coatings, for walls, motor-cars, water mains;
(e) adhesives for the building industry;
(f) coal briquetting;
(g) electrical insulation; and
(h) battery-making.
5.3 Environmental Exposure Levels
Apart from walking or riding on bituminous pavements and roads,
the general population will not normally come into contact with
bitumen, except in the form of protective coatings and coal
briquettes. On the other hand, the general population can, on
occasion, be exposed to fumes from heated bitumens for short periods
of time during road-building or the covering of roofs. Emissions from
asphalt roads have been mentioned in the literature as a possible
source of exposure for the general population, but such exposure is
considered to be as negligible. Recent evidence from the Federal
Republic of Germany and the USA confirms this (Hettche, 1963).
Occupational exposure to bitumen may be much more intensive in
certain professions and may range from accidental splashing with hot
bitumen to repeated and prolonged contact of the skin with the more
liquid bitumen grades or to exposure to fumes from heated bitumen.
5.4 Environmental Distribution and Transformation
No specific data relating to bitumens are available in relation to
distribution among media, environmental transformation and
degradation, interaction with physical, chemical, or biological
factors, and bioconcentration. On the other hand, there is a lot of
information on the microbial degradation of individual petroleum
hydrocarbons (section 3.4).
5.5 Metabolism
Uptake and storage of the light fractions contained in bitumens
may occur; however, no specific data exist on this subject.
Bitumens occur in a variety of commercial products. The
composition of these products may vary widely depending on the
geographical origin of the crude oil used and the manufacturing
process applied. These facts may influence the results of metabolic
studies, all of which are concerned with the exposure of experimental
animals. Human data are lacking.
5.6 Effects on Experimental Animals
5.6.1 Short-term exposure
No data are available on acute toxicity, exposures related to
adverse effects, interactions, and species comparisons. It is
generally accepted that the acute toxicity of bitumens is low.
5.6.2 Long-term exposure
No data appear to have been published on toxic effects in specific
organs, teratogenicity, or reproduction. The data on mutagenicity are
limited. On the other hand, substantial experimental work has been
carried out concerning the carcinogenic effects of bitumens on skin.
Twort & Fulton (1930) examined the carcinogenic effects of various
synthetic tars and their fractions on the skin of mice. The
carcinogenic activity varied according to the compound used and with
the temperature of pyrolysis. Results of these studies confirmed the
earlier finding of Kennaway (1925), that the temperature at which the
tar formed was an important factor in the production of carcinogenic
substances. Cancer of the skin of mice was induced by applications of
the synthetic residues obtained from heating substances such as
acetylene, isoprene, a noncarcinogenic petroleum, yeast, and human
skin. The yield of carcinogens decreased when carbonization had
occurred at temperatures above 950°C. The yield was greatest between
850 and 870°C; less between 600 and 750°C, and negligible at 500°C.
Furthermore, the authors found that the carcinogenic activity of the
synthetic residues could be reduced considerably by oxidation or
reduction (by various methods) or by dilution with oleic acid.
A wide range of aliphatic and simple aromatic hydrocarbons were
pyrolysed by Badger (1962), who showed that polynuclear aromatic
hydrocarbons were formed from simpler hydrocarbons at 700°C via
primary radicals formed by carbon-hydrogen and carbon-carbon fission
(cracking) at this elevated temperature. The same process occurred to
a much lesser extent at 550°C.
According to Bogovski et al. (1963), thermal distillation of a tar
to coke decreased the carcinogenic activity by the formation of
irreversible condensation products from polynuclear aromatic
hydrocarbons, together with other high molecular compounds. In
long-term, skin-painting experiments on white mice, the authors showed
that such a coking process reduced the tumour incidence from 68% to
3.7% in the case of shale-oil tar. As shale-oil tars have a much
higher carcinogenic activity than petroleum residues (Twort & Twort,
1931), it might be expected that coking of petroleum residues would
reduce the carcinogenicity of the material still further.
The carcinogenic effects of bitumen on the skin of C-57 black mice
was studied by Simmers and co-workers (1959). In a first series of
tests, they used a mixture of 6 samples from Southern Californian
refineries, in which both steam- and aft-blown bitumens were mixed.
Painted twice weekly on the skin for a lifetime, the mixture caused 12
dermoid carcinomas in 68 animals, compared with none in the untreated
control group. Formation of cancer was preceded by hair loss, dryness
and scaling of the skin, and papilloma formation. After subcutaneous
injection, 8 sarcomas occurred in 62 animals at the site of injection
compared with none in the control group. However, the relevance of
results from such subcutaneous studies is questionable.
In an inhalation study on the same pooled sample and using the
same strain of mice, the animals inhaled an aerosol of bitumen
droplets suspended in moist air for 30 min/day, 5 days per week, for
up to nearly 17 months (Simmers, 1964). Changes found on microscopic
examination were minimal and included occasional congestion, acute
bronchitis, pneumonitis, bronchial dilatation, and some peribronchial
round-cell infiltration. In a second inhalation study, the animals
were exposed to cooled smoke from bitumen at 120°C for 6-7´ h/day, 5
days a week, for up to 21 months. In this study, peribronchial
round-cell infiltration, bronchitis, pneumonitis, abscess formation,
loss of cilia, epithelial atrophy, and necrosis were more common.
Squamous cell metaplasia was rare, but hyperplasia was more commonly
seen. The changes in both experiments were patchy, rather
non-specific, and similar to those described as a result of exposure
to other air pollutants.
In his next series of tests, Simmers (1965a) compared the
carcinogenicity of straightrun and air-blown bitumen in prolonged
regular skin application tests and after single or repeated
subcutaneous injection. Undiluted air-blown bitumen did not induce
tumours, when applied to the skin, probably because the bitumen was
too hard; when dissolved in toluene the incidence of skin cancers
increased to 45%. This may be the result of better contact with, or
penetration into the skin. On the other hand, the polynuclear aromatic
hydrocarbons might be concentrated by the solvent. In a similar
skin-painting study with straight-run bitumen, skin cancers occurred
in only 14%. The same trend was apparent after subcutaneous injection,
where 0 and 13% tumours were found at the site of injection with
straight-run and air-blown bitumen, respectively. The author presumed
that this difference in response resulted from a difference in
chemical composition. Although the aromatic fraction of air-blown
bitumen was lower than that of straight-run bitumen, it contained more
complex aromatic hydrocarbons due to polymerization and condensation
caused by the air-blowing.
Straight-run bitumen was separated into 4 fractions, which were
painted 3 times a week, continuously, on the skin of the same strain
of mice (Simmers 1965b). The fraction containing most of the saturated
compounds and aromatic hydrocarbons -- which also showed practically
all the UV light fluorescence -- induced considerably more skin
rumours (43.3%) than straight-run and air-blown bitumens had done in
earlier studies. The author pointed out that, though fluorescence was
not a guarantee of carcinogenic activity, polynuclear aromatic
hydrocarbons known to be carcinogenic are fluorescent. In this
context, both Kennaway & Heiger (1930) and Berenblum et al. (1947)
suggested that these compounds -- even in very small quantities --
might be discovered by this method. The 4 bitumen fractions used in
the previous study were injected subcutaneously -- once or repeatedly
-- in the same strain of mice, at various doses (Simmers, 1966). A
variety of benign and malignant tumours resulted, both at the
injection site and in distant organs. The dose seemed to be more
important for tumour formation than the duration of exposure.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Four road-bitumens of different geographical and
manufacturing origin were applied to the skin of, or injected
intra-muscularly into, mice and rats. In some of the test-animals,
tumours were found at the site of application. Fumes from heated
coal-tar and from a blown bitumen used as a roofing bitumen did not
induce cancers of the lungs in rats and guineapigs in inhalation
experiments lasting up to 2 years. However, the general typical
reactions found in lung-tissue were the same as those described by
Simmers (1964). Condensates of the coal-tar fumes were highly
carcinogenic when applied to the skin and intramuscularly in mice;
condensates of the blown bitumens fumes were non-carcinogenic to the
skin of mice and rabbits in similar tests.
Petroleum residues derived from cracking were painted 3 times a
week for a lifetime on the skin of albino mice. Depending on the
cracking process, the residues exhibited various degrees of
carcinogenic activity, but none were as active as a higher temperature
generated coal-tar. Only those fractions distilled from the tar above
370°C showed carcinogenic activity. Blending with a noncarcinogenic
oil increased the carcinogenic activity of one bitumen, possibly
because of better skin penetration (Smith et al., 1951).
Kireeva (1968) painted groups of white SS-57 mice, once a week
throughout the life span, with 40% solutions in benzene of various
bitumens derived from Ukrainian crudes and coal-tar pitch. The
control-group was painted with benzene only. With coal-tar pitch, skin
tumours appeared in 88.4% of the animals; with bitumens prepared from
cracking residues, they occurred in 9.5-18.4%, and with straight-run
bitumens, depending on its origin, in only 0.0-4.6% of the animals.
Benzene solutions of 8 bitumens of different geographical origin
and 2 coal-tar pitches were applied twice weekly to the skin of Swiss
albino mice throughout their lifetime. Benzene alone was applied to
control animals (Wallcave et al., 1971). The polynuclear aromatic
hydrocarbon content of the coal-tar pitches was found to be several
orders of magnitude greater than that of the bitumens examined. Only
one carcinoma and 5 papillomas were observed in 218 mice treated with
bitumens, whereas over 90% of the coal-tar pitch treated animals
developed such tumours. Again, it was suggested that the tumour
incidence depended on the polynuclear aromatic hydrocarbon content of
the product tested.
Benzene solutions of the bitumen or polynuclear aromatic fractions
of Athabasca tar sands were not mutagenic for Salmonella typhimurium.
This may have been a reflection of the complex interactions occurring
with such hydrocarbon mixtures (Shahin & Fournier, 1978).
To summarize, in animal studies, some bitumens have been shown to
possess some carcinogenic activity, when applied to the skin, while
inhalation studies with bitumen vapours have proved to be negative.
The carcinogenicity of bitumens depends to a certain degree on the
method of production (cracking, blowing) or mixing. "Cutback" with
high aromatic oil or coal-tar facilitates skin-contact and increases
the carcinogenicity of the mixture. The carcinogenic activity,
however, is low in comparison with that of coal-tar. Moreover, as Siou
(1972) in a literature study on bitumens concluded, it is difficult to
extrapolate from these animal data to man, because the contact with
bitumen, even in occupational exposures, is of a quite different order
to regularly repeated skin application throughout the life span of a
test animal.
5.7 Effects on Man
5.7.1 Epidemiological studies
5.7.1.1 Occupational exposure
Henry (1947), in his analysis of 3753 cases of skin cancer, found
only one case in which bitumen might have been involved. The man had
worked for 23 years as a road-worker and had been exposed to coal-tar
in the earlier years.
The health and the causes of death of 96 workers who had worked in
a bitumen plant since it opened, including those who had left the
company was reviewed by Hoogendam (1962). Exposures ranged up to 40
years. Thirty-nine workers had been exposed for more than 20 years and
at least 15 had been exposed for more than 30 years. No significant
differences were found in theft general health pattern and no cases of
skin tumours were found. One man, who had worked for 40 years in this
plant died at the age of 55 of bronchial carcinoma. It is impossible
to determine whether there was a relation with his work in this case.
Vital capacities and forced expiratory volume (FEV) of those still
working did not differ from those of a control group.
The mortality rate of refinery workers was compared with those of
other workers in a study on 15 437 employees of an oil refinery over
the 29-year period 1935-63. A comparison was also made with data from
a similar group of the outside population. The incidence of lung
cancer was no greater in the refinery operators than in the other
groups (Baird, 1967).
Baylor & Weaver (1968) compared the health of 462 asphalt workers
in 25 oil refineries with that of 379 controls. Each of the asphalt
workers had been engaged in this type of work for at least 5 years
with an average of 15.1 years, service. No significant differences in
health were found between the 2 groups. The authors also studied the
medical literature of the 20 years preceding theft publication and
were unable to find one single case of lung or skin cancer that could
be attributed to petroleum bitumens. Furthermore, an extensive search
for information on the health of workers was carried out with road
construction firms (31 companies in 24 states with 11 478 man-years of
asphalt working and 15 boards of health of the state highway
commissions), roofing industries (3 firms with over 1100 long-term
bitumen workers), and bitumen trucking firms (with over 5000 drivers).
This gave no indication that bitumen constituted a health hazard or
that there were cancers of the skin or lungs in these groups that
could be attributed to working with bitumen. Similar indirect
information was obtained from 6 large insurance companies. The authors
concluded that the bitumens in current use did not present a
significant health hazard and that the study indicated that the
carcinogenic or other harmful properties of most commercial asphalts
under the present commercial usage -- if present at all -- are likely
to be of a very low order.
Studies conducted on behalf of the API did not reveal any
occupational health hazards for a "medium-exposure group" of petroleum
refinery workers. This group included asphalt workers, grease, and
lubricant workers (Tabershaw/Cooper Associates Inc. 1974/75).
Hermann (1975) and Hettche (1963) drew similar conclusions to
those of Baylor & Weaver (1968) in inferring that bitumens including
blown grades, were biologically inactive and that vapours from hot mix
plants were not carcinogenic.
5.7.1.2 General population exposure
Measurement of emissions of polynuclear aromatic hydrocarbons from
various industrial processes, including bitumen "blowing" and the
manufacture of asphalt hot-road-mixture, revealed that these
industrial processes were not major sources of benzo(alpha)pyrene
emissions (used as an indicator for polynuclear aromatic hydrocarbons
emission) and certainly emitted less than residential and small
industrial coal-burning furnaces (Von Lehmden et al., 1965).
In the literature, the possibility is mentioned that vapours
emanating from asphalt, or dust arising from it might contribute to
the overall incidence o f cancer of the respiratory tract (Hueper,
1961; Berge, 1969). There are not, however, any data to substantiate
this assumption and, in extrapolating the results of animal testing on
bitumen-vapour inhalation, the chances of such an effect occurring
among the general population seem quite remote. It can be presumed
that a lot of the confusion has been generated by the former use of
coaltar and mixed grades for this purpose, a fact which clearly has
not always been taken into consideration by those working in the
field.
In the USSR, however, the use of "carcinogenic" road material
(that is material containing benzo (a) pyrene) as the upper layer of
roads is forbidden in heavily populated areas. In practice, this means
that coal-tar must not be used for this purpose and that petroleum
bitumen should be used instead (Gorbov & Fomenko, 1962).
5.7.1.3 High (accidental) exposure
Burrell (1957) found a very high incidence of oesophageal cancer
in a group of Bantu confined to one location in East London (South
Africa). The author related this to the long-term preparation and use
of the soporific alcoholic concoction "cidiviki", which was locally
fermented in drums still containing a ¨-inch coating of"cutback"
bitumen. The author presumed that enough carcinogenic material could
have leached out into the brew to have at least acted as a
cocarcinogen.
The most common accidental over-exposure in handling bitumen is
the occurrence of burns from hot bitumen splashes. A few cases,
however, have been described, in which a skin tumour developed within
1-3 months of a burn caused by hot bitumen, coal-tar, or crude oil
(Bang, 1923; Huguenin, 1925; Gunsett, 1930; Sträuli 1957). Considering
the total number of such bums, only a very small proportion develop
into rumours and these cases relate to combinations of such burns with
fresh scar tissue and/or lesions of the mucous membranes. Tumours have
been described in similar situations following heat burns by wood,
welding electrodes, etc. (Gunsett, 1930; Sträuli, 1957). Such skin
tumours can occur in burn scars and are often multifactorial (Emmett,
1975).
In relation to the vast amount of bitumen that has been used for
many decades, the reports of tumours are extremely scarce. In fact, it
can be postulated that, though, from animal testing, it is known that
some bitumens are weak carcinogens, there is no evidence from normal
occupational exposure to indicate that these compounds are a
carcinogenic hazard to the skin or the respiratory tract. This would
also indicate that the present pattern of use of bitumens would not
cause any hazard, whatsoever, for the general population.
The Asphalt Institute studied emissions from the hot-mix process
for the manufacture of paving asphalts (Puzinauskas & Corbett, 1975).
The concentration of polynuclear aromatic hydrocarbons in the
particulate emission was low at 340 mg/m3 and the average
concentration of benzo(alpha)pyrene was very low at 13 ng/m3.
5.7.2 Clinical studies
These relate mainly to bitumen skin burns and will not be
considered here. Other clinical data have been included in the
description of the epidemiological data.
The skin of fair-haired persons might be more prone to react
adversely to repeated prolonged exposure to UV-radiation in sunlight
in combination with bitumen exposure (Smiley, 1951; Kinnear et al.
1954; Emmett, 1975).
6. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO CRUDE OILS AND
SELECTED PETROLEUM PRODUCTS
6.1 Crude Oils
Crude oil is normally handled in a closed system from the oil
well, via storage tanks, pipelines, and shipment by tankers to the
refineries. Under these conditions, health hazards to, and death of
workers involved in these operations will occur only when a serious
breakdown or leakage occurs. Volatile components escaping at well
heads, at pump glands, or through vents in storage tanks and ships'
tanks may, under certain conditions, constitute a similar health
hazard; hydrogen sulfide, if present, is the most acutely toxic
component but detailed consideration is outside the scope of this
review. These volatile components may also contribute to the pollution
of the atmosphere in storage or pumping areas. To the general
population, this is mainly a nuisance problem, because of the odour
from the hydrogen sulfide and mercaptans involved. Crude oil pollution
of seas and inland waterways as a result of accidents with crude oil
tankers or pipelines may, at times, cause a major and sudden
environmental hazard. Tank washings from tankers not using the
"load-on-top" system are another source of pollution of the sea with
crude oils. However, this particular aspect falls outside the scope of
this review.
Atmospheric concentrations of the volatile components of crude oil
were found to be lower near marine drilling rigs than land-based rigs.
Differences in temperature and air movement have been shown to be the
main causes.
The lower temperature of marine pipelines encourages
solidification of waxy compounds of the crude oil on their inner
surfaces. During their repair, the clothes and skin of workers may be
contaminated by these compounds (Alekperov et al., 1974).
6.2 Petroleum Solvents
Though petroleum products are widely used, they do not generally
present a health risk for the general population, as their volatility
prevents them accumulating in the ambient air in concentrations high
enough to cause adverse health effects. However, if improperly used in
closed, poorly ventilated rooms, they may become a cause of accidental
acute poisoning. Under these conditions, the lower-boiling solvents
may also present a fire hazard.
Cases of accidental ingestion may occur, especially in children.
Serious and even fatal lung disease may develop when aspiration in the
lungs occurs. Absorption from the gut, however, is generally not a
serious health hazard.
Substances with a strong odour may cause a nuisance, when present
in the ambient air, even in very low concentrations. The same applies
to the possible contamination of drinking-water through leakage of
petroleum solvents from containers.
Health impairment due to occupational exposure to petroleum
solvents occurs only infrequently in normal work practice. Repeated
skin contact may result in contact irritative dermatitis and only
rarely in contact allergic dermatitis. Continuous day-to-day exposure
to excessive vapour concentrations (which usually occur in poorly
ventilated workshops) may give rise to general non-specific symptoms
of ill-health. Some solvents, however, have a specific systemic
effect, e.g., benzene (bone marrow depression, leukaemogenesis) and
n-hexane (polyneuropathies). Polyneuropathies also occur in cases of
abusive use of petroleum solvents by "sniffers" and addicts.
Accidental exposure to excessive vapour concentration may cause
narcosis, which can be followed very rapidly by respiratory arrest and
death. Accidental over-exposure of the skin, especially if the solvent
is allowed to remain in contact with the skin, may cause skin
irritation, possibly leading to chemical burns.
Blood dyscrasia does not occur through exposure to petroleum
solvents that do not contain benzene. The specific long-term health
hazards of exposure to benzene and petroleum solvents that contain a
substantial percentage of benzene will not be discussed in this
document.
The high aromatic residues that are still used, occasionally, as
solvents for specific purposes may pose a carcinogenic risk to the
skin or on inhalation, if such contact is intensive and prolonged.
6.3 Lubricating Base Oils, Greases, and Waxes
This group of products is unlikely to present a health risk for
the general population, even with gross accidental over-exposure.
Unrestricted and indiscriminate oral or nasal administration of white
medicinal oil can occasionally give rise to the occurrence of lipid
pneumonia, when pre-existent disease or other conditions predispose to
the entry of mineral oil into the respiratory tract.
Prolonged and intensive contact of the skin with metal-working
oils during occupational exposure results in a high incidence of skin
disorders and relatively more cases of skin cancer have occurred in
such occupations. Improvements in both industrial and personal hygiene
have reduced the incidence of malignant and non-malignant diseases of
the skin. Results from animal studies indicate that substitution of
the oils, formerly used, by more refined products may significantly
contribute to the prevention of skin cancer caused by exposure to oil
under poor standards of industrial hygiene. Because chronic lung
disease following exposure to oil mist is extremely rare and an
increase in the incidence of pulmonary carcinoma has only been
reported under exposure conditions where skin cancer occurs, it seems
reasonable to assume that these occupational diseases can be prevented
by suitable preventive measures.
6.4 Bitumen
The health risks associated with bitumen appear to be minimal,
because contact is unlikely. Occupational exposure is not associated
with an increase in cancer of the skin or the respiratory tract. The
most significant occupational hazard is burning of the skin from
splashes of heated bitumen.
Certain bitumen grades, however, such as those derived from
cracked oils, and those mixed with coal-tar or high aromatic extracts,
may have a carcinogenic potential and, therefore, require special
precautions in handling.
7. CONTROL MEASURES
7.1 General
Every effort should be made not to contaminate workers, the work
place, or the general environment with petroleum products. Proper
design of machinery, equipment and the workshop, enclosure of
processes, adequate ventilation, provision of protective clothing and
adequate facilities for personal hygiene, suitable education and
supervision, and development of safe working procedures are essential
basic requirements; good hygienic work practices can achieve a great
deal in protecting the worker.
Care should be taken, when developing products, to ensure that
levels of the most toxic components, e.g., benzene, n-hexane, and
polynuclear aromatic hydrocarbons are known, so that proper controls
can be devised for the use of the products.
Products containing such highly toxic components should, when
possible, be avoided and alternatives sought, where exposure is
unavoidable or is likely to occur. Products which, during use, may
generate more and highly toxic contaminants should be identified and
efforts made to reduce contamination. Care should be exercised when
handling and using such products and consideration given to the need
for renewal of products at appropriate intervals. In relation to
metal-working, the danger of repeatedly "topping-up" machinery over
long periods with a product, and of mixing different products in one
machine, is emphasized.
Where contact is unavoidable, suitable protective equipment should
be used, although this should always be used as the last resort. Where
skin contact is inevitable, efforts should be made to limit or avoid
not only benzene, n-hexane, and polynuclear aromatic hydrocarbons
but also any additives that may have an adverse effect on the skin.
The use of abrasives and solvents in cleansing the skin should be
discouraged.
In the less developed countries, there is often a lack of
awareness concerning the need for proper control measures when
handling petroleum products. In these countries, the health education
of employers and workers should be promoted with reference to the
products. Adequate control programmes should be implemented using
known techniques. Whenever necessary, these should be modified to fit
the particular circumstances of the country.
In addition, proper planning is necessary to control the disposal
of the many types of waste oil products so that environmental
contamination is avoided. Thought must also be given to the need to
control the siting of housing and amenities in relation to petroleum
refineries and petrochemical plants.
7.2 Petroleum Solvents
Excessive exposures to these products should be avoided. Proper
education of manufacturers and users and appropriate labelling of
consumer products are important. Regular control of workroom
atmospheric concentrations of petroleum solvents is indicated,
especially in smaller workshops.
Petroleum solvents are, with the exception of benzene and
n-hexane, less toxic than most other solvents and, with suitable
precautions, can be safely used. For this reason, and because they are
cheap, they are widely used in industry and their replacement by other
solvents is unlikely in the foreseeable future.
7.3 Lubricating Base Oils, Greases, and Waxes
The only way in which members of the general population could,
under normal circumstances, be over-exposed to any of this group of
compounds would be in the indiscriminate use of medicinal liquid
paraffin. Excessive use -- especially self-administered -- of this
material, intranasally or via the oral route, by debilitated or
dysphagic patients should be discouraged.
The main possibilities of over-exposure occur in the occupational
situation. Without going into detail, the following factors should be
considered with the aim of avoiding hazardous exposures:
(a) Avoid contact by technological means including: proper design
of equipment, machinery, and workshop; proper design and operation of
general and exhaust ventilation systems in places where oil mist
generation cannot be avoided; use of base oils with the lowest content
of polynuclear aromatic hydrocarbons that can practically be achieved
by suitable refining processes; limiting of the concent of additives
that have adverse effects on the skin; frequent changing of oil in
situations where carcinogenic compounds might be generated by heat;
avoidance of the reuse of used oils, without refining, for other
purposes where they might cause a health hazard; education and
instruction in all these safe-working procedures, and supervision of
their proper execution;
(b) Use of safe-working procedures and, where required, protective
clothing and proper protective equipment; availability of general
hygienic facilities including wash-basins in or near the work-place,
shower facilities, lockers for private clothing outside the
contaminated area; regular changing and laundering of working clothes
and underwear; the use of suitable emollient skin creams and in some
instances barrier creams. Abrasives and solvents should not be used
for cleaning the skin.
(c) Periodic medical examinations.
These precautions have been compiled and adapted from the
following references: Kipling (1968); UK Medical Research Council
(1968); Cruickshank (1969); Desoille et al. (1973); BRMA (undated).
Manufacturer can contribute by ensuring that these precautions are
observed in their own premises and by giving the proper information
and guidance to the consumer (see also Eckardt, 1967).
Government actions vary from country to country depending on local
legislation and practice. They may range from supervision and control
of the measures taken by industry to the issue of directives or codes
of practice. Cautionary notices can be issued (e.g., HMSO, 1979),
which have to be displayed in the working place, as well as leaflets
for the workers (e.g., HMSO, 1967). Diseases caused by mineral oil may
be notifiable as occupational diseases, according to national
legislation.
Consumer education on the possible hazards of the products used
and their safe handling can best be done by the manufacturing
industries, when possible in cooperation with relevant Government
agencies.
Medical action in this field would consist of cooperating in the
team supervising the industrial hygiene of the operation, in
pre-placement examination and selection, and later in the periodic
medical follow-up of the exposed workers.
Various other organic liquids meet requirements for lubricating
oils and greases. Carboxylic esters are generally used. These
synthetic lubricants, however, are not in abundant supply, are
expensive, and are not likely to be widely used outside their specific
field of application. Replacement products are sometimes more
hazardous. For instance, cutting oils may be replaced by mixtures
containing ethanolamine that give rise, under certain conditions, to
the formation of nitrosamines.
When safe lubricating base oils are used there is, on the other
hand, no reason from an occupational health point of view to use such
alternatives, not even in places where long-term intensive skin
contact and/or oil mist exposure might take place. In these cases,
industrial hygiene should be improved and personal hygiene should be
closely supervised, regardless of the product used, to avoid excess
exposure.
7.4 Bitumen
In road-building which is the major application of bitumen,
alternative materials such as plastics and resins are far too
expensive for general use. Coal-tar, which was used previously,
presents a greater environmental health hazard than bitumen. Other
alternatives would be concrete and bricks.
In various other applications, such as flooring, roofing,
protective coatings, and adhesives, alternative materials are
available, but these are not always superior in quality and/or
competitive in price.
REFERENCES
ABBRITTI, G., SIRACUSA, A., CIANCHETTI, C., COLI, C. A., CURRADI, F.,
PERTICONI, G. F., & De ROSA, F. (1976) Shoe-makers' polyneuropathy
in Italy: The aetiological problem. Br. J. ind. Med., 33: 92-99.
ACGIH (1971a) Documentation of the threshold limit values,
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 19-20.
ACGIH (1971b) Documentation for TLV -- Supplements for those
substances added or changed since 1971, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists, p. 341.
ACGIH (1974) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1974, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978a) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1978, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978b) Air sampling instruments for evaluation of atmospheric
contaminants. 5th ed., Cincinnatti, OH, USA, American Conference
of Governmental Industrial Hygienists.
ADLARD, E. R. (1972) A review of the methods for the identification of
persistent hydrocarbon pollutants on seas and beaches. J. Inst.
Petrol., 58: 63-74.
AIDIN, R. (1958) Petrol-vapour poisoning. Br. med. J., ii: 369-370.
AIHA (1976) Direct reading colorimetric indicator tubes manual,
Akron, OH, USA, American Industrial Hygiene Association.
AINSWORTH, R. W. (1960) Petrol-vapour poisoning. Br. med. J.,
i: 1547-1548.
ALEKPEROV, I. I., AMIROV, R. O., POVOVAROV, V. D., & LOSEVA, I. E.
(1974) [Hygiene of work at exploitation of marine oil deposits.]
Moscow, Idz. "Nedra" (in Russian).
ALLEN, N. (1975) Chemical neurotoxins in industry and the environment.
In: Tower, D. B., ed. The nervous system, Vol. 2, The clinical
neurosciences, New York, Raven, pp. 235-248.
ALTENKIRCH, H., MAGER, J., STOLTENBURG, G., & HELMBREHT, J. (1977)
Toxic polyneuropathies after sniffing a glue thinner. J. Neurol.,
214: 137.
ANDRÉ, M. L. & O'NEAL, M. J. (1959) Mass spectrometric analyses of
medium viscosity lubricating oils. Anal. Chem., 31: 164-169.
ANON, (1973) Evaluations of world's important crudes. Oklahoma, USA,
The Petroleum Publishing Company (Reprinted from Oil Gas J.).
ANON (1974) Exposure to petrol fumes. Br. med. J., ii: 115.
ANON (1975) Benzpyrene hydroxylase - innate determinant of PAH
toxicity? Food cosmet. Toxicol., 12: 264-268.
ANTONOV, A.M. & LINTS, A.M. (1960) The blastomogenic action of natural
Saratov oil. Problems Oncol., 6: 1629-1634.
API (1965) Toxicological review. Aromatic petroleum naphtha. 2nd
ed., New York, American Petroleum Institute, pp. 5.
API (1967a) Toxicological review. Gasoline, 1st ed., New York,
American Petroleum Institute, pp. 5.
API (1967b) Toxicological review. Kerosine, 2nd ed., New York,
American Petroleum Institute, pp. 4.
API (1969a) Final Report of the task force on used oil disposal.
Washington, DC, American Petroleum Institute, pp. 44.
API (1969b) Toxicological review. Petroleum naphthas, 1st ed., New
York, American Petroleum Institute, pp. 7.
API (1974) Mutagenicity study of thirteen petroleum fractions,
Washington, DC, American Petroleum Institute (API U-150-14, The
Hine Laboratories Inc. Report No. 4).
API (1975a) Review of analytical methods for determination of oil and
grease in waters produced from oil and gas extraction operations.
Prepared by the Task Group on Analytical Methods July 14th 1975,
New York, American Petroleum Institute, pp. 38.
API (1975b) Mutagenic evaluation of rubber solvent (CHF-32-263),
W/filtered rubber solvent (Summary and analysis), Washington,
DC, American Petroleum Institute (Litton Bionetics Inc. Report).
API (1975c) Research report. Mutagenic evaluation of 60 solvent
(CHF-33-148) (Summary and Analysis), Washington, DC, American
Petroleum Institute (Litton Bionetics Inc. Report).
API (1977a) Research report. Mutagenicity evaluation of kerosene.
Final report (Summary and Analysis), Washington, DC, American
Petroleum Institute, 3 pp. (Litton Bionetics Inc. Project
No. 2697).
API (1977b) Research report. Teratology study in rats: Stoddard
solvent. Final report (Summary and Analysis), Washington, DC,
American Petroleum Institute, 5 pp. (Litton Bionetics Inc. Project
No. 20698-2).
API (1978a) Mutagenicity evaluation of Stoddard solvent (Summary),
Washington, DC, American Petroleum Institute (Litton Bionetics
Inc. Report no. API-PS-4).
API (1978b) Research report - Teratology study in rats: Rubber
solvent. Final report. Litton, API (Full Report and Analyses).
API (1978c) Teratology study in rats: Xylene. Final Report,
Washington, DC, American Petroleum Institute, 39 pp. (Litton
Bionetics Inc. Project No. 20698-5).
API (1978d) Research report. Mutagenicity of xylene. Final report
(Full report and Analytical).
API (1978e) Teratology study in rats: Toluene. Final report (Summary
and Analysis), Washington, DC, American Petroleum Institute, 2 pp.
(Litton Bionetics Inc. Project No, 20698-4).
(1978f) Mutagenicity evaluation of toluene (Summary and Analysis),
Washington, DC, American Petroleum Institute, 3 pp. (Litton
Bionetics Inc. Project No. 20847).
API (1979a) Research report. Teratology study in rats: Kerosene.
Final report (Summary and Analytical), Washington, DC, American
Petroleum Institute 2 pp. (Litton Bionetics Inc. Project
No. 20698-10).
API (1979b) Research report. Inhalation teratology study in rats: VM
and P naphtha, Final report, Washington, DC, American Petroleum
Institute, 18 pp. (Litton Bionetics Inc. Project No. 21035-02).
API (1979c) Research report. Teratology study in rats: n-Hexane.
Final report, Washington, DC, American Petroleum Institute,
24 pp. (Litton Bionetics Inc. Project No. 20698-9).
API (1979d) In vitro and in vivo mutagenicity studies: New oil
composite. Final report, Washington, DC, American Petroleum
Institute (Hazleton report).
ARCOS, J. C. & ARGUS, M. F. (1974) Chemical induction of
cancer-structural bases and biological mechanisms, Vol. 11A,
New York and London, Academic Press.
ASK-UPMARK, E. (1955) Bronchial carcinoma in printing workers. Dis.
Chest., 27: 427-235.
ASTM D2820 (1972) Standard method of test for Cl through C5
hydrocarbons in the atmosphere by gas chromatography,
Philadelphia, Am. Soc. for Testing and Materials.
ASTM D1605-60 (1973) Standard recommended practices for sampling
atmospheres for analysis of gases and vapours, Philadelphia,
Am. Soc. For Testing and Materials.
ASTM D510 (1974) Standard methods of sampling water, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2682 (1974) Standard method of test for polynuclear
aromatic hydrocarbons in air particulate matter, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2778 (1974) Standard method of test for solvent extraction or
organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ASTM D3370 (1976) Standard method of test for solvent extraction
of organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ÅSTRAND, I., KILBOM, A., & ÖVRUM, P. (1975) Exposure to white spirit.
I. Concentration in alveolar, air and blood during rest and
exercise. Scand. J. Work environ. Health, 1: 15-30.
ÅSTRAND, L., ENGSTRÖM, J., & OVRUM, P. (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man.
Scand. J. Work Environ. Health, 4: 185-194.
AULD, S. J. M. (1950) Environmental cancer and petroleum. J. Inst.
Petrol., 36: 235-253.
AVELLAN, L., JACOBSON, B., & JOHANSON, B. (1967) Carcinoma of scrotum
induced by mineral oil. Scand. J. plast. reconstr. Surg.,
1: 135-140.
AXELSON, O., HANE, M., & HOGSTEDT, C. (1976) [Case descriptions of
chronic psycho-organic syndrome in house painters.]
Läkartidningen, 73: 317-318 (in Swedish).
BADGER, G. M. (1962) Mode of formation of carcinogens in human
environment. Nat. Cancer Inst. Monogr., 9: 1-16.
BAIRD, V. C. (1967) Effects of atmospheric contamination on cancer
mortality in petroleum refinery employees. J. occup. Med.,
9: 415-420.
BALDWIN, R. W., CUNNIGHAM, G. J., & PRATT, D. (1964) Carcinogenic
action of motor engine oil additives. Br. J. Cancer,
18: 503-507.
BANG, F. (1923) Contribution a l'étude de la cancérisation de la
cellule et du temps d'éclosion des tumeurs malignes. A propos d'un
cas de "cancer aigu" du goudron chez un ouvrier. Bull. Assoc. Fr.
Étud. Cancer, 12: 184-199.
BARANOVA, V. G. & LOGINOVA, N. K. (1977) [Determining cyclopentadiene
in the air of industrial premises.] Gig. Tr. prof. Zabol.,
No. 3, pp. 54-55 (in Russian).
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of ethyl
benzene, styrene and alpha-methylstyrene in man. Am. Ind. Hyg.
Assoc. J., 31: 206-209.
BARRIENTOS, A., ORTUNO, M. T., MORALES, J. M., TELLO, F. M., &
RODICIO, J. L. (1977) Acute renal failure after use of diesel fuel
as shampoo. Arch. intern. Med., 137: 1217.
BATT-NEAL, L. & WOLMAN, M. (1977) Tumors and amyloidosis in mice
painted with crude oil found on bathing beaches. Bull. environ.
Contam. Toxicol., 18: 385-391.
BAYLOR, C. H. & WEAVER, N. K. (1968) A health survey of petroleum
asphalt workers. Arch. environ. Health, 17: 210-214.
BELINKY, B. R. (1980) In: Dolberg, D. D. & Verstuyft, ed. Analytical
techniques in occupational health chemistry. Washington, DC,
ACS, (ACS Symposium Series 120).
BELL, J. (1876) Paraffin epithelioma of the scrotum. Edin. med. J.,
22: 135.
BERENBLUM, I., HOLIDAY, E. R., & JOPE, E. M. (1947) Some physical
methods of investigating carcinogenic hydrocarbons. Br. med.
Bull., 4: 326-331.
BERGE, H. (1969) [Harmful emissions in and around cities.]
Vitalstoffe, 14: 161-163 (in German).
BERGEL, F. (1974) Carcinogenic hazards in natural and man-made
environments. Proc. R. Soc. (London), 185: 165-181.
BERTINE, K. K. & GOLDBERG, E. E. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BESKROVNAJA, N. I., HRUSTALEVA, G. F., ZIGULINA, G. A., & DAVYDKINA,
T. I. (1979) [The gynaecological sick rate of female workers in
the rubber industry.] Gig. Tr. prof. Zabol., No. 8, pp. 36-38
(in Russian).
BILIMAIER, D., YEE, H. T., ALLEN, N., CRAFT, B., WILLIAMS, N.,
EPSTEIN, S., & FONTAINE, R. (1947) Peripheral neuropathy in a
coated fabrics plant. J. occup. Med., 16: 665-671.
BINGHAM, E. & FALK, H. L. (1969) Environmental carcinogens. The
modifying effect of co-carcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BINGHAM, E. & HORTON, A. W. (1966) Environmental carcinogenesis:
experimental observations related to occupational cancer.
Adv. Biol. Skin, 7: 183-193.
BINGHAM, E. & WORD, P. J. (1977) Co-carcinogenic effects of n-alkanes
and ultraviolet light on mice. J. Natl Cancer Inst.,
38(4): 1099-1101.
BINGHAM, E., HORTON, A. W, & TYE, R. (1965) The carcinogenic potency
of certain oils. Arch. environ. Health, 10: 449-451.
BISHOP, P. G. C. (1940) Oil aspiration pneumonia and pneumolipoidosis.
Ann. int. Med., 13: 1327-1359.
BLATTNER, R. J. (1951) Kerosene poisoning. J. Pediatr., 39: 391-392.
BOEHME, H. & HUEHNERMANN, W. (1966) Examination of mineral oil
products in the (West) German Pharmacopoeia, 7th edition. Arch.
Pharm., 299: 368-80D.
BOENHEIM, A. F. & PEARSON, A. J. (1973) Petroleum hydrocarbon
solvents. In: Modern petroleum technology, 4th ed., Barking,
Applied Science Publishers Ltd.
BOGOVSKI, P. A., EISEN, O. G., & ARRO, I. H. (1960) [On the
carcinogenic action of some chromatographic fractions of
chamber-oven tar obtained from Estonian oil-shale.] Vop. Onkol.,
6: 34-42 (in Russian).
BOGOVSKI, P. A., GORTALUM, G. M., & KOZHEVNIKOV, A. V. (1963)
Decancerigenization of some shale processing products. Acta Un.
Int. Cancer, 19: 481-482.
BOHLEN, P., SCHLUNEGGER, U. P., & LÄUPPI, E. (1973) Uptake and
distribution of hexane in tissues. Toxicol. appl. Pharmacol.,
25: 242.
BOITNOTT, J. K. & MARGOLIS, S. (1966a) Mineral oil in human tissues.
I. Detection of saturated hydrocarbons using thin-layer
chromatography. Bull. John Hopkins Hosp., 118: 402-713.
BOITNOTT, J. K. & MARGOLIS, S. (1966b) Mineral oils in human tissues,
II. Oil droplets in lymphnodes of the porta hepatis. Bull. John
Hopkins Hosp., 118: 414-422.
BORRIE, J. & GWYNNE, J. F. (1973) Paraffinoma of lung: Lipoid
pneumonia. Report of two cases. Thorax, 28: 214-221.
BOURNE, L. B. (1967) Skin diseases from oils - causes and prevention,
London, British Safety Council, 11 pp.
BOUTWELL, R. K. & BOSCH, D. K. (1959) The tumor-promoting action of
phenol and related compounds for mouse skin. Cancer Res.,
19: 413-424.
BP (1973) Health guide to BP petroleum products, London, The British
Petroleum Company Ltd, pp. 26.
BP (1975) Trace elements and other elements in crude oil:
A literature review, Sunbury, The British Petroleum Company Ltd.
BRIDGE, J. C. & HENRY, S. A. (1928) Industrial cancers. In: Report of
the International Conference on Cancer, London, Bristol, John
Wright, pp. 258-268.
BRMA (undated) Mineral oils, London, British Rubber Manufacturers
Association Ltd.
BROMER, R. S. & WOLMAN, I. J. (1939) Lipoid pneumonia in infants and
children. Radiology, 32: 1-7.
BROWN, A. J., WALDRON, H. A., & WATERHOUSE, J. A. H. (1975) Report on
a study of occupational skin cancer with special reference to
scrotal cancer, Birmingham, UK, University of Birmingham.
BROWN, A. L. & BISKIND, G. R. (1941) Differential diagnosis between
lipid pneumonia and pulmonary neoplasm. J. Am. Med. Assoc.,
117: 4-6.
BROWNING, E. (1959) Toxic solvents: a review. Br. J. ind. Med.,
16: 23-39.
BROWNING, E. (1965) Toxicity and metabolism of industrial solvents,
Amsterdam, London, New York, Elsevier Publishing Company.
BRUSKIN, Z. Z. & DEMCENKO, V. G. (1975) [External respiration and gas
exchange of workers who are subjected to the effect of lubricating
oil aerosols.] Gig. Tr. prof. Zabol., No. 4, pp. 28-30
(in Russian).
BRYAN, C. S. & BOITNOTT, J. K. (1969) Adenocarcinoma of the lung with
chronic mineral oil pneumonia. Am. Rev. Respir. Dis.,
99: 272-274.
BUKOVSKIJ, M. I., ZUKOV, V. L, KOZUHOVA, T. V., SANOCKIJ, I. V., &
SIDOROV, K. K., ed. (1978) [Maximum permissible concentrations
and guidelines for safety levels of hazardous substances in the
environment.] Severodoneck, All-Union Research Institute of
Safety Technique in the Chemical Industry, and Research Institute
of Labour Hygiene and Occupational Diseases, Academy of Medical
Sciences of USSR (in Russian).
BURRELL, R. J. W. (1957) Oesophageal cancer in the Bantu. S. A.
Tydskrif vir Geneeskunde, 31: 401-409.
BÜTTNER, G. (1974) Benzene in the work environment. Deutsche
Forschungsgemeinschaft, Bonn, FRG, Harald Boldt Verlag K. G.
Boppard, 63 pp.
CANNON, P. R. (1940) The problem of lipid pneumonia. J. Am. Med.
Assoc., 115: 2156-2179.
CAPELLINI, A., CHIAPPINO, G., & ZURIO, N. (1968) [Clinical and
experimental observations on so-called tricresylphosphate
polyneuritis.] Med. Lav., 59: 721-759 (in Italian with English
summary).
CARGILL, C. (1972) Polynévrites dans l'industrie du cuir et de la
chaussure: toxicité du n-hexane, Thèse, Paris VII.
CARITHERS, H. A. (1955) Accident prevention in childhood -- the
kerosene hazard. J. Am. Med. Assoc., 159: 109-11.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977a) Petroleum hydrocarbon
toxicity studies. XIV. Animal and human response to vapors of
"high aromatic solvent". Toxicol. appl. Pharmacol.,
41: 235-241.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977b) Petroleum hydrocarbon
toxicity studies. XV. Animal response to vapors of "high
naphthenic solvent". Toxicol. appl. Pharmacol., 41: 251-260.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977c) Petroleum hydrocarbon
toxicity studies. XVI. Animal response to vapours of Naphthenic
Aromatic Solvent. Toxicol. appl. Pharmacol., 41: 261-270
(contains references to the other studies in this series).
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971a) Specifications
for cutting oils with special reference to carcinogenicity. Ann.
occup. Hyg., 14: 171-179.
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971b) Specifications
for cutting oils with special reference to carcinogenicity.
J. Inst. Petrol., 57: 247-260.
CAVANAGH, J. B. (1973) Peripheral neuropathy caused by chemical
agents. In: C.R.C. Crit. Rev. Toxicol., 2: 365-417.
CIANCHETTI, C., ABBRITTI, G., PERTICONI, G., SIRACUSA, A., & CURRADI,
A. (1976) Toxic polyneuropathy of shoe-industry workers: A study
of 122 cases. J. Neurol. Neurosurg. Psychiatr., 39: 1151-1161.
CLAYTON, G. D. & CLAYTON F. E. (1978) Patty's industrial hygiene and
toxicology. 3rd revised ed., New York, Wiley & Sons.
COHR, K.-H. & STOCKHOLM, J. (1979) Toluene. A toxicological review.
Scand. J. Work Environ. Health, 5: 71-90.
COMSTOCK, B. S. (1977) A review of psychological measurements relevant
to central nervous system toxicity with special reference to
solvent inhalation. Clin. Toxicol, 11: 317-324.
CONCAWE (1981a) Exposure to atmospheric benzene vapour associated
with motor gasoline. The Hague (Concawe report No. 2/81).
CONCAWE (1981b) Guidelines for the determination of atmospheric
concentrations of oil-mists. The Hague (Concawe report
No. 1/81).
CONCAWE STUDY GROUP (1972) Methods for the analysis of oil in water
and soil, The Hague, Stichting Concawe, pp. 66 (Concawe report
No. 9/72).
CONCAWE TASK FORCE (1973) Disposal of used lubricating oil in Western
Europe, The Hague, Stichting Concawe, 59 pp. (Concawe report
No. 9/73).
CONCAWE TASK FORCE (1976) Methods for the determination of
hydro-carbons in the atmosphere, The Hague, Stichting Concawe,
pp. 23 (Concawe report No. 3/76).
CONTAMIN, F., GOULON, M., & MARGAIRAZ, A. (1960) Polynévrites
observées chez des sujets utilisant comme moyen de chauffage des
appareils à combusion catalytique de l'essence. Rev Neurol.,
103: 341-345.
COOK, J., CARRUTHERS, W., & WOODHOUSE, D. L. (1958) Carcinogenicity of
mineral oil fractions. Br. med. Bull., 14: 132-135.
CORPER, H. J. & FREED, H. (1922) The intratracheal injection of oils
for diagnostic and therapeutic purposes. J. Am. Med. Assoc.,
79: 1739-1743.
CRISP, A. J, BHALLA, A. K., & HOFFBRAND, B. I. (1979) Acute tubular
necrosis after exposure to diesel oil. Br. med. J.,
2(6183): 177.
CRUICKSHANK, C. N. D. (1969) Occupational cancer of the scrotum.
Br. med. j., 26: 249-250.
CRUICKSHANK, C. N. D. & GOUREVITCH, A. (1952) Skin cancer of the hand
and forearm. Br. J. ind. Med., 9: 74-79.
CRUICKSHANK, C. N. D. & SQUIRE, J. R. (1950) Skin cancer in the
engineering industry from the use of mineral oil. Br. J. ind.
Med., 7: 1-11.
DAESCHNER, C. W. Jr, BLATTNER, R. J., & COLLINGS, V. P. (1957)
Hydrocarbon pneumonitis. Pediatr. Clin. North Am., resp.
Disorders. February: 243-253, Philadelphia.
DANIEL, J. W., FRAZER, A. C., FRENCH, J. M., & SAMMONS, H. G. (1953)
The intestinal absorption of liquid paraffin in the rat.
Biochem. J., 54: 37p.
DARGENT, M., MARCEAU, M., JOSEPH, P., & FAUCON, M. (1967) Etude
experimentale sur les effets tumorigènes des lubrifiants utilisés
dans le décolletage des métaux. Rev. Lyonnaise Méd.,
16: 409-420.
DAS, B. S. & THOMAS, G. H. (1978) Fluorescence detection in high
performance liquid chromatographic determination of polycyclic
aromatic hydrocarbons. Anal. Chem., 50: 967-973.
DAVIS, A., SCHAFER, L. J., & BELL, Z. G. (1960) The effect in human
volunteers of exposure to air containing gasoline vapour. Arch.
environ. Health, 1: 548-554.
DECOUFLE, P. (1976) Cancer mortality among workers exposed to cutting
oil mist. Ann. N.Y. Acad. Sci., 271: 94-101.
DECOUFLE, P. (1978) Further analysis of cancer mortality patterns
among workers exposed to cutting oil mist. J. Natl Cancer Inst.,
61: 1025-1030.
De PIERRE, J. W. & ERNSTER, L. (1978) The metabolism of polycyclic
hydrocarbons and its relationship to cancer. Biochim. Biophys.
Acta., 473: 149-186.
DESOILLE, H., PHILBERT, M., RIPAULT, G., CAVIGNEAUX, A., & ROSSIGNOLI,
H. (1973) Action cancérogène des huiles minérales utilisées en
métallurgie. Arch. Mal. prof. 34: 669-680.
DESPIERRES, G., BENNET, P. A., PHELIP, H., & PLANTE, M. (1965) Cancer
alveolaire et inhalation d'huiles industrielles. J. Fr. Med.
Chir. Thorac., 19: 561-567.
Deutsche Forschungsgemeinschaft (1970) [Health hazardous substances
in the workplace.] Weinheim, Verlag. Chemie (in German).
DIETZ, W. A., KING, W. H. Jr, PRIESTLEY, W. Jr, & REHNER, J. Jr (1952)
Properties of high boiling petroleum products. Ind. Eng. Chem.,
44: 1818-1827.
DRASCHE, H., FINZEL, L., MARTSCHEI, H., & MEYER, R. (1974)
[Occupational health considerations in persons exposed to oil
mist.] Zbl. Arbeitsmed., 24: 305-312 (in German).
EBERT, A. G., SCHLEIFER, C. R., & HESS, S. M. (1966) Absorption,
disposition, and excretion of 3H-mineral oil in rats. J. Pharm.
Sci., 55: 923 -929.
ECKARDT, R. E. (1957) Carcinogenicity of petroleum products with
particular reference to the automotive industry. Ind. med. Surg.,
26: 396 -398.
ECKARDT, R. E. (1967) Cancer Prevention in the petroleum industry.
Int. J. Cancer, 2: 656-661.
EHRHARDT, M. & HEINEMAN, J. (1975) Hydrocarbons in blue mussels from
the Kiel Bight. Environ. Pollut., 9: 263-281.
ELY, T. S., PEDLEY, S. F., HEARNE, F. T., & STILLE, W. T. (1970) A
study of mortality symptoms and respiratory function in humans
occupationally exposed to oil mist. J. occup. Med.,
12: 253-261.
EMMETT, E. A. (1975) Occupational skin cancer: A review. J. occup.
Med., 17: 44-49.
ENGSTROM, K., HUSMAN, K., & RIIHIMÄKI, V. (1977) Percutaneous
absorption of m-xylene in man. Int. Arch. occup. environ. Health,
39: 181-189.
ESSO RESEARCH (1960) Unpublished report in: Toxicological evaluation
of some food additives including anticaking agents, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series,
No. 5, World Health Organization, Geneva.
ESSO (undated) This is about health-lubricating oils and cutting
fluids, London, Esso.
EVEN, R. (1947) Les pneumopathies huileuses de l'adulte. Soc. Med.
Hop. Paris, 63: 78-80.
EYRES, A. R. (1973) Codes of practice relating to metal working
fluids: the oil suppliers' view. J. Inst. Petrol., 59: 9-14.
FACQUET, M. J. & LANGEARD, Mme (1947) Deux cas d'opacités pulmonaires
du lobe moyen chez des chanteurs (pneumonie huileuse probable).
Bull. Mém. Soc. Méd. Hop. Paris, 63: 200-204.
FAO/WHO (1970) Toxicological evaluation of some extraction solvents
and certain other substances (Nutrition Meetings Report Series
No. 48a; WHO/Food Add./70.39).
FAO/WHO (1971) Specifications for the identity and purity of some
extraction solvents and certain other substances (Nutrition
Meetings Report Series No. 48b; WHO/Food Add./70.40).
FIELDNER, A. C., KATZ, S. H., & KINNEY, S. P. (1921) Permeation of
oxygen breathing apparatus by gases and vapours, Washington,
United States Bureau of Mines (Technical Paper 272).
FISCHER, H. G. M., PRIESTLEY, W. Jr, EBY, L. T., WANLESS, G. G., &
REHNER, J. Jr (1951) Properties of high boiling petroleum
products. Arch. ind. Hyg. occup. Med., 4: 315-324.
FLOODGATE, G. D. (1972) Microbiol degradation of oil. Mar. Pollut.
Bull., 3: 41-43.
FOE, R. B. & BIGHAM, R. S. (1954) Lipid pneumonia following
occupational exposure to oil spray. J. Am. Med. Assoc.,
155: 33-34.
FOKKENS, W., HOUWEN, J. W., HUENDER, H. G. P., KARPIAK-VAN-DRIEL, J.,
LIEM, T. H., & De WAARD, F. (1972) [Scrotal cancer and mineral
oils.] T. Soc. Geneeskd., 50: 343 (in Dutch).
FORBES, J. D. & MARKHAM, T. N. (1967) Cutting and grinding fluids in
chronic pulmonary airway disease. J. occup. Med., 9: 421-423.
FOURNAS, D. W. & HINE, C. H. (1958) Neurotoxicity of some selected
hydrocarbons. Arch. ind. Health, 18: 9-15.
FRANCO, G., MOGLIA, A., & GHITTORI, S. (1979) [Occupational neuropathy
due to cyclohexane.] Med. Lav., 70(2): 118-124
(in Italian).
FREIMAN, D. G., ENGELBERG, H., & MERRIT, W. H. (1940) Oil aspiration
(lipoid) pneumonia in adults -- A clinicopathologic study of
forty-seven cases. Arch. int. Med., 66: 11-38.
FÜHNER, H. (1921) [Narcotic action of gasoline and its components
(pentane, hexane, octane).] Biochem. Zeitschr., 115: 235-261
(in German).
GAMBERALE, F., ANNWALL, G., & HULTENGREN, M. (1975) Exposure to white
spirit. II. Psychological functions. Scand. J. Work Enrivon.
Health, 1: 31-39.
GARVIN, C. G. (1939) Lipoid pneumonia. Arch. int. Med., 64: 586-589.
GAULTIER, M., RANCUREL, G., PIRA, C., & EFTHYMIOU, M.-L. (1973)
Polynévrites et hydrocarbures aliphatiques. J. eur. Toxicol.,
6: 294-296.
GERARDE, H. W. (1959) Toxicological studies on hydrocarbons. V.
Kerosine. Toxicol. appl. Pharmacol., 1: 462-474.
GERARDE, H. W. (1960) Toxicology and biochemistry of aromatic
hydrocarbons in commercial solvents, Amsterdam, London, New
York, Princeton, Elsevier Publishing Company.
GERARDE, H. W. (1963) Toxicological studies on hydrocarbons. IX. The
aspiration hazard and toxicity of hydrocarbons and hydrocarbon
mixtures. Arch. environ. Health, 6: 329-341.
GERARDE, H. W., & GERARDE, D. F. (1961/1962) The ubiquitous
hydrocarbons (Reprinted from Assoc. Food Drug Off. United
States, Vols XXV and XXVI, 1961/1962).
GHAZAI, S. (1974) Sept cas de polynévrite dans une usine de chaussures
à Teheran. Arch. Mal. prof., 35: 552-553.
GIBSON, D. T., MAHADERAN, V., JERINA, D. M., YAGI, H., & YEH, H. J. C.
(1975) Oxidation of the carcinogens benzo(a)pyrene and
benzo(a)-anthracene to dihydrodiols by a bacterium. Science,
189: 295-297.
GILCHRIST, C. A., LYNES, A., STEEL, G., & WHITHAM, B. T. (1972) The
determination of polycyclic aromatic hydrocarbons in mineral oils
by thin-layer chromatography and mass spectrometry. Analyst,
97: 880-888.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1955) Cutting oils and
squamous-cell carcinoma. Part II: An experimental study of the
carcinogenicity of two types of cutting oils. Br. J. ind. Med,
12: 244-248.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1956) An evaluation of the
relative carcinogenicity of two types of cutting oils. A.M.A.
Arch. ind. Health, 14: 341-345.
GOLDSTEIN, D. H. & BENOIT, J. N. (1970) An epidemiologic study of an
oil mist exposure. Arch. environ. Health, 21: 600-603.
GONZALEZ, E.G. & DOWNEY, J. A. (1972) Polyneuropathy in a glue
sniffer. Arch. Phys. Med. Rehabil., 53: 333-337.
GOODWIN, T. C. (1934) Lipoid cell pneumonia. Am. J. Dis. Child.,
48: 309 -326.
GORBOV, V. A. & FOMENKO, V. N. (1962) [On the hygienic evaluation of
asphalt coverings as a possible source of air pollution with
carcinogens.] Gig. i Sanit., 27: 100-101 (in Russian).
GRAEF, I. (1939) Studies in lipid pneumonia. Arch. Pathol.,
28: 613-667.
GREENBERG, M. (1972) A proportional mortality study of a group of
newspaper workers. Br. J. in Med., 29: 15-20.
GRIMMER, G. (1972) [The quantitative determination of polycyclic
aromatics by capillary gas chromatography.] Erdöl Kohle,
25: 339-343 (in German).
GRIMMER, G. (1979) Distribution of polycylic dromatic hydrocarbons in
environmental samples. In: Egan, H., ed. Environmental
carcinogens, selected methods of analysis. Vol. 3 - Analysis
of polycyclic aromatic hydrocarbons in environmental samples,
Lyons, IARC pp. 55-61 (WHO/IARC Publication No. 29).
GRIMMER, G. & BÖHNKE, H. (1976) [Enrichment and gas chromatographic
profile analysis of polycyclic aromatic hydrocarbons in
lubricating oils.] Chromatographia, 9: 30-40 (in German).
GRIMMER, G. & BÖHNKE, H. (1978) [Polycyclic aromatic hydrocarbons and
heterocyclic compounds.] Erdöl Koble, 31: 272-277 (in German).
GRIMMER, G. & BÖHNKE, H. (1972) [Determination of total content of
polycyclic aromatic hydrocarbons in air dust and automobile
exhaust by capillary GC.] Z. anal. Chem., 261: 310-314 (in
German).
GUNSETT, A. (1930) Un cas de cancer aigu, épithélioma
spino-cellulaire, developpé après traumatisme par de l'asphalte
enflammé. Bull. Assoc. Etud. Cancer, 19: 459-462.
GUSEIN-ZADE, K. M. (1975) [Reactions of the skin on the effect of
Apsheron commercial oils.] Gig. Tr. prof. Zabol., No. 7,
pp. 51-52 (in Russian).
HAENNI, E. O. & HALL, M. A. (1960) Tentative ultraviolet absorption
limits for mineral oils. J.A.O.A.C., 43: 92-95.
HAENNI, E. O., JOE, F. L. Jr, HOWARD, J. W. & LEIBEL, R. L. (1962a) A
more sensitive and selective ultraviolet absorption criterion for
mineral oil. J.A.O.A.C., 45:(1): 59-66.
HAENNI, E. O., HOWARD, J. W., & JOE, F. L. Jr (1962b) Dimethyl
sulfoxide: A superior analytical extraction solvent for
polynuclear hydrocarbons and for some highly chlorinated
hydrocarbons. J.A.O.A.C., 45: 67 -70.
HAGGARD, H. W. (1921) The anesthetic and convulsant effects of
gasoline vapour. J. Pharmacol. Exper. Ther., 16: 401-404.
HAINES, J. R. & ALEXANDER, M. (1974) Microbial degradation of high
molecularweight alkanes. Appl. Microbiol., 28(6): 1084-1085.
HÄNNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioral effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HARTWELL, J. L. (1951) Survey of compounds which have been tested for
carcinogenic activity. 2nd ed. Washington, DC, Federal Security
Agency (Public Health Service Pub. No. 149).
HAYHURST, E. R. (1936) Poisoning by petroleum distillates. Ind. med.
Surg., 5: 53.
HELBLING, V. (1950) [A case of acute lethal percutaneous and
inhallatory poisoning by petrol.] Z. Unfallmed. Berufskr.,
43: 218-228 (in German).
HELLMANN, H. & ZEHLE, H. (1972) [Long-term investigation on
behavioural of crude oil on water.] Dtsch. Gewaesserkd. Mitt.,
16: 46-52 (in German).
HENDRICKS, N. V., BERRY, C. M., LIONE, J. G., & THORPE, J. J. (1959)
Cancer of the scrotum in wax pressman. I. Epidemiology. Arch.
ind. Health, 19: 524-529.
HENDRICKS, N. V., COLLINGS, G. H., DOOLEY, A. E., GARRETT, J. T., &
RATHER, J. B. Jr (1962) A review of exposure to oil mist. Arch.
environ. Health, 4: 139-145.
HENRY, S. A. (1946) Cancer of the scrotum in relation to occupation,
London, Oxford Medical Publications, Oxford University Press,
100 pp.
HENRY, S. A. (1946/1947) Occupational cutaneous cancer attributable to
certain chemicals in industry. Br. reed. Bull., 4: 389-401.
HENRY, S. A. (1947) Symposium on industrial skin cancer. Br. J.
Radiol., 20: 149-157.
HENSEN, E. V. (1959) Toxicology of some of the aliphatic and alicyclic
hydrocarbons. J. occup. Med., 1: 105-112.
HERMANN, H. (1975) Handling of crudes and petroleum products ...
industrial hygiene. Erdoel Kohle, Erdgas, Petrochem.
Brennst.-Chem., 28(6): 282-286.
HERSKOWITZ, A., ISHI, N., & SCHAUMBERG, H. (1971) n-Hexane
neuropathy; a syndrome occurring as a result of industrial
exposure. New Engl. J. Med., 285-2: 82-85.
HETTCHE, H. O. (1963) [The effects of bitumen and its vapours on human
health.] Städtehygiene, 14: 65-68 (in German).
HIEGER, I. & WOODHOUSE, D. L. (1952) The value of the rabbit for
carcinogenicity tests on petroleum fractions. Br. J. Cancer,
6: 293-299.
HINE, C. H. & ZUIDEMA, H. H. (1970) The toxicological properties of
hydrocarbon solvents. Ind. Med., 39(5): 215-220.
HMSO (1967) Cancer of the skin caused by oil, London, Her Majesty's
Stationary Office, pp. (SHW295/295A).
HMSO (1979) Cautionary Notice: Effects of mineral oil on the skin,
London, HMSO, (SHW397 revised 1979).
HODGSON, G. (1970) Cutaneous hazards of lubricants. Ind. Med.,
39(2): 68-73.
HODGSON, G. (1973) Health problems arising from contact and exposure
of workers to metal working fluids. J. Inst. Petr.,
59(565): 1-8.
HOEKSTRA, W. G. & PHILLIPS, P. H. (1963) Effects of topically applied
mineral oil fractions on the skin of guinea-pigs. J. invest.
Dermatol., 40(2): 79-88.
HOLMES, J. G., KIPLING, M.D., & WATERHOUSE, J. A. H. (1970) Subsequent
malignancies in men with scrotal epithelioma. Lancet,
ii: 214-215.
HOOGENDAM, I. (1962) Health checks on asphalt workers. In:
Proceedings of the Shell Industrial Doctors Meeting, 22-25 May,
1962, The Hague, Med. Div. Shell Int. Petrol. Co. Ltd. (Internal
Report).
HORTON, A. W., DENMAN, D. T., & TROSSET, R. P. (1957) Carcinogenesis
of the skin. II. The accelerating properties of aliphatic and
related hydrocarbons. Cancer Res., 17: 758-766.
HORTON, A. W., BURTON, M. J., TYE, R., & BINGHAM, E. L. (1963)
Composition versus carcinogenicity of distillate oils. Am. Chem.
Soc. Div. Pet. Chem. Prepr., 8: No. 4-C: 59-65.
HORTON, A. W., BINGHAM, E. L., BURTON, M. J. G., & TYE, R. (1965)
Carcinogenesis of the skin. III. The contribution of elemental
sulfur and of organic sulfur compounds. Cancer Res.,
25: 1759-1763.
HRUSTALEVA, G. F., CEMBARTSEVA, A. N., SVECNIKOVA, F. A., BESKROVNAJA,
N. I., ROTIN, V. V., & LESEHEV, S. S. (1979) [Hypophyseal-ovarian
relationships of women coming into contact with petroleum solvents
in industry.] Gig. Tr. prof. Zabol., No. 7, pp. 31-33
(in Russian).
HUEPER, W. C. (1961) Carcinogens in the human environment. Arch.
Pathol., 71: 355-380.
HUEPER, W. C. & PAYNE, W. W. (1960) Carcinogenic studies on petroleum
asphalt, cooling oil, and coal tar. Arch. Pathol., 70: 372-384.
HUGUENIN, R. (1925) Cancer aigu consécutif à une brûlure par le
mazout. Bull. Assoc. Fr. Etud. Cancer, 14: 403-404.
HUGUENIN, R., FAUVET, F., & MAZABRAUD, M. (1950) Rôle éventuel des
nébulisations d'huiles industrielles dans la pathogénie des
cancers broncho-pulmonaires. Arch. Mal. prof., 11: 48-51.
HUNTER, G. A. (1968) Chemical burns of the skin after contact with
petrol. Br. J. plast, Surg., 21: 337-341.
IARC (1973) Certain polycyclic aromatic hydrocarbons and heterocyclic
compounds. In: IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Lyons, International Agency for
Research on Cancer, Vol. 3, pp. 30-42.
IARC (1974) Some anti-thyroid and related substances, nitrofurans and
industrial chemicals. In: IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International
Agency for Research on Cancer, Vol. 7,326 pp.
ICHIHARA, K., KUSUNOSE, E., & KUSUNOSE, M. (1969) Microsomal
hydroxylation of decane. Biochim. Biophys. Acta, 176: 713-719.
IIDA, M., YAMAMURA, Y., & SOBUE, I. (1969) Electromyographic findings
and conduction velocity in n-hexane polyneuropathy.
Electromyography, 9: 247-261.
IKEDA, K. (1935) Oil aspiration pneumonia (lipoid pneumonia).
Am. J. Dis. Child. 49: 985-1006.
IKEDA K. (1937) Lipoid pneumonia of the adult type (paraffinoma of the
lung). Arch. Pathol., 23: 471-492.
ILO (1968) Benzene: uses, toxic effects, substitutes. Report of
expert meeting 1967, Geneva, International Labour Office, 287 pp.
INOUE, T., TAKEUCHI, Y., TAKEUCHI, S., YAMADE, S, SUZUKI, H.,
MATSUSHITE, T., MIYAGAKI, H., MAEDE, K., & MOTSUMOTO, T. (1970) A
health survey on vinyl sandal manufacturers where a high incidence
of n-hexane intoxication occurred. Jpn. J. ind. Health,
12: 5-16.
INSTITUTE OF PETROLEUM (1974) Petroleum statistics with 1973 totals,
London, Institute of Petroleum.
ISHII, N., HERSKOWITZ, A., & SCHAUMBERG, H. (1972) n-Hexane
poly-neuropathy: a clinical and experimental study.
J. Neuropathol. exp. Neurol., 31: 198.
IVANOV, N. G., POZDNJAKOV, V. S., & ROZOVA, T. A. (1978) [The maximum
admissible content of mineral (petroleum) oil aerosol in air of
working premises.] Gig. Tr. prof. Zabol., No. 5, pp. 28-31
(in Russian).
JANYSEVA, N. I., KIREEVA, I. S., & SERZANTOVA, N. N. (1963) [A propos
the content of 3,4-benzopyrene in petroleum bitumen.] Gig. i
Sanit., 28: 71-73 (in Russian).
JEPSON, J. R., STOVANOV, S., UNGER, M., CLAUSEN, J., & CHRISTENSEN, H.
E. (1977) Cutting fluids and their effects on the skin of mice.
Acta Pathol. Microbiol. Scand. (Section A), 85: 731-738.
JERINA, D. M. & DALY, J. W. (1974) Arene oxides: A new aspect of drug
metabolism. Science, 185: 573-582.
JONES, J. G. (1961) An investigation into the effects of exposure to
an oil mist on workers in a mill for the cold reduction of steel
strip. Ann. occup. Hyg., 3: 264-271.
KARANI, V. (1966) Peripheral neuritis after addiction to petrol.
Br. med. J., i: 216.
KELLERMAN, G., SHAW, C. R, & LUYTEN-KELLERMAN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KENNAWAY, E. L. (1925) Experiments on cancer-producing substances.
Br. meal J., ii: 1-6.
KENNAWAY, E. L. & HIEGER, I. (1930) Carcinogenic substances and their
fluorescence spectra. Br. med. J., 2: 1044-1046.
KEY, M. M., RITTER, E. J., & ARNDT, K. A. (1966) Cutting and grinding
fluids and their effects on the skin. Am. Ind. Hyg. Assoc. J.,
27: 423-427.
KINNEAR, J., ROGERS, J., & FINN, O. A. (1954) Degenerative changes in
the skin with special reference to jute-workers. Br. J.
Dermatol., 46: 344-349.
KINNEAR, J., ROGERS, J., FINN, O. A., & MAIR, A. (1955) Dermatoses in
jute-workers. Br. J. ind. Med., 12: 36-42.
KIPLING, M. D. (1968) Oil and the skin. Ann. Rep. of H.M. Chief
Inspector of Factories, 1967, London, HMSO, pp. 105-120.
KIREEVA, I. S. (1968) Carcinogenic properties of coal-tar pitch and
petroleum asphalts used as binders for coal briquettes. Hyg.
Sanit., 33(4, 5, 6): 180-186.
KLAUDER, J. V. & BRILLE, F. A. (1947) Correlation of boiling ranges of
some petroleum solvents with irritant action on the skin. Arch.
Dermatol. Syph., 56: 197-215.
KNAVE, B., PERSSON, H. E., GOLDBERG, J. M., & WESTERHOLM, P. (1976)
Long-term exposure to jet fuel. An investigation on
occupationally-exposed workers with special reference to the
nervous system. Scand. J. Work Environ. Health, 3: 152-164.
KNAVE, B., OLSEN, B. A., ELOFSON, S., GAMBERALE, F., ISAKSSON, A.,
MINDUS, P., PERSSON, H. E., STRUWE, G., WENNBERG, A., &
WESTERHOLM, P. (1978) Long-term exposure to jet fuel II. A
cross-sectional epidemiologic investigation on
occupationally-exposed industrial workers with special reference
to the nervous system. Scand. J. Work Environ. Health,
4: 19-45.
KOREEDA, M., MOORE, P. D., WISLOCKI, P. G., LEVIN, W., CONNEZ, A. H.,
YAGI, H., & JERINA, D. M. (1978) Binding of benzo (a)pyrene 7,8
diol -- 9,10 epoxides to DNA, RNA and protein of mouse skin occurs
with high stereoselectivity. Science, 199: 778-781.
KOROBKIN, R., ASBURY, A. K., SUMNER, A. J., & NIELSON, S. L. (1975)
Glue-sniffing neuropathy. Arch. Neurol., 32: 158-162.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KRASSOVITSKAJA, M. L. (1976) [Health protection of atmospheric air in
regions in which oil processing and petrochemical enterprises are
located. In: Guide to hygiene of the atmospheric air.] Moscow,
The Medicine Publishing House, pp. 273-304 (in Russian).
KURITA, H. (1967) Experimental studies on the effects of n-hexane on
albino rats. Jpn. J. ind. Health, 9: 24-36.
LASKIN, S. & GOLDSTEIN, B. D., ed. (1977) Benzene toxicity. A critical
evaluation Washington and London, Hemisphere Publishing Co.
LAUGHLEN, G. F. (1925) Studies on pneumonia following naso-pharyngeal
injections of oil. Am. J. Pathol., 1: 407-414.
LAZAREW, N. W. (1929) [The toxicology of benzenes.] Arch. Hyg.
Bakteriol., 102: 227-239 (in German).
LAZAREV, N. N. & LEVINA, E. A. (1976) [Harmful substances in
industry.] 7th ed., Leningrad, Publishing House "Khimia"
(in Russian).
LAZARINI, H.-J., DOIGNON, J., L'ÉPÉE, P., & MERILLON, H. (1974) Une
nouvelle observation de polynévrite par le n-hexane. Arch. Mal
prof., 35: 920-921.
LEE, M. L., BARTLE, K. D., & NOVOTNY, M. V. (1975) Profiles of the
polynuclear aromatic fraction from engine oils obtained by
capillary column gas -- liquid chromatography and
nitrogen-selective detection. Anal. Chem., 47(3): 540-543.
LEITCH, A. (1922) Paraffin cancer and its experimental production.
Br. med. J., ii: 1104-1009.
LEITCH, A. (1924) Mule spinners cancer and mineral oils. Br. med. J.,
ii: 941-943.
LEITHE, W. (1970) The analysis of air pollutants. Ann Arbor, Ann
Arbor-Humphrey Science Publishers, pp. 78-93.
LEITHE, W. (1972) [The analysis of organic pollutants in drinking,
industrially-used, and sewage water.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft MBH, pp. 119-132
(in German).
LESSER, L. I., WEENS, H. S., & McKEY, J. D. (1943) Pulmonary
manifestations following ingestion of kerosene. J. Pediatr.,
23: 352-364.
LEVINA, E. N. (1976) [Hydrocarbons.] In: Lazarev, N. V. & Levina, E.
N., ed. [Harmful substances in industry Volume I, Organic
substances.] 7th ed., Leningrad, Publishing House Himia, pp.
9-121 (in Russian).
LIEBE, G. (1892) [On tar or paraffin cancer.] Med. Jahrb., 236: 65
(in German).
LIEVRE, J., BENICHOU, C., & DESROY, M. (1967) Polynévrite par poêle à
combustion catalytique (trots sujets atteints dans une famille).
Bull. Soc. Méd. Hop. Paris, 118: 91-99.
LIJINSKY, W. (1960) Separation of polycyclic aromatic hydrocarbons in
complex mixtures: Chromatographic determination of trace amounts
in petroleum waxes. Anal. Chem., 32: 684-687.
LIJINSKY, W. & RAHA, C. R. (1961) Polycyclic aromatic hydrocarbons in
commercial solvents. Toxicol. appl. Pharmacol., 3: 469-473.
LIJINSKY, W., RAHA, C. R., & KEELING, J. (1961) Comparison of
procedures for the determination of polycyclic aromatic
hydrocarbons in waxes. Anal. Chem., 33: 810-812.
LIJINSKY, W, DOMSKY, I., MASON, G., RAMAHI, H. Y., & SAFARI, T. (1963)
The chromatographic determination of trace amounts of polynuclear
hydrocarbons in petrolatum, mineral oil, and coal-tar. Anal.
Chem., 33: 952-956.
LIJINSKY, W., SAFFIOTTI, U, & SHUBIK, P. (1966) Skin tumorigenesis by
an extract of amber petrolatum. Toxicol. appl. Pharm.,
8: 113-117.
LIPOVSKIJ, S. M. (1978) [The serotonin content in the tissue of uterus
and particulars on its contracting activity under the influence of
petroleum solvent vapours.] Gig. Tr. prof. Zabol., No. 7,
pp. 37-40 (in Russian).
LIPOVSKIJ, S. I., ELKIN, V. I., TOMAEVA, L. V., & ZORINA, V. V. (1977)
[The solvents content in the blood of workers in the rubber
industry.] Gig. Tr. prof. Zabol., No. 5, pp. 50-51 (in Russian).
LIPOVSKIJ, C. M., TOMAEVA, L. V., VARFOLOMEEV, D. I., FEDOSEEV, Ja.
E., & KARGANOVA, E. V. (1979) [The accumulation of solvents in the
tissues and organs of pregnant women.] Gig. Tr. prof. Zabol.,
No. 3, pp. 25-28 (in Russian).
LIPPMANN, M. & GOLDSTEIN, D. H. (1970) Oil.mist studies, environmental
evaluation and control. Arch. environ. Health, 21: 591-599.
LUSHBAUGH, C. C. (1947) Experimental hyperplastic gastritis and
gastric polyposis in monkeys. J. Natl Cancer Inst., 7: 313-320.
LUSHBAUGH, C. C. & HACKETT, A. (1948/1949) An infiltrating
aden-omatous lesion of the colon of rats ingesting motor
lubricating oil (S.F.G. No. 1 oil). J. Natl Cancer Inst.,
9: 159-172.
LUSHBAUGH, C. C., GREEN, J. W., & REDEMANN, C. E. (1950) Effects of
prolonged inhalation of oil fogs on experimental animals. Arch.
ind. Hyg. occup. Med., 1: 237-247.
LUTOV, V. A. (1974) [Materials for establishing the maximum admissible
concentration of petroleum oil aerosols without additives, used as
lubricating and cooling liquids.] Gig. Tr. prof. Zabol., No 10,
pp. 49-52 (in Russian).
LUTOV, V. Ya., CIRKIN, A. A., GRJADITSKIJ, Ju. E., & VASILENKO, N. I.
(1976) [The effect of petroleum oil aerosols and thermo-oxidizing
degradation products on the functional condition and immunologic
reactivity of the organism of experimental animals.] Gig. Tr.
Prof. Zabol., No. 2, pp. 33-37 (in Russian).
LYNCH, P. F. & BROWN, C. W. (1973) Identifying source of petroleum by
infrared spectrometry. Environ. Sci. Technol., 7: 1123-1127.
MACHLE, W. (1941) Gasoline intoxication. J. Am. Med. Assoc.,
117: 1965-1971.
MALTEN, K. E., SPRUIT, D., BOEMAARS, H. G. M., & De KEIZER, M. J. M.
(1968) Horny layer injury by solvents. Berufsdermatosen,
16: 135-147.
MASON, B. (1966) The concentration of minor elements in biogenic
deposits. Principles of geochemistry, 3rd ed., New York, John
Wiley & Sons Inc., pp. 241-243.
MASTROMATTEO, E. (1955) Cutting oils and squamous-cell carcinoma. Part
I. Incidence in a plant with a report of six cases. Br. J. ind.
Med., 12: 240-243.
MATHER, R. N. (1973) Codes of practice relating to metal-working
fluids -- the users' views. J. Inst. Petrol., 59: 15-17.
MATSUMOTO, T. (1971) Experimental studies on toluene poisoning, II.
Jpn. J. ind. Health, 13: 399-407.
MATSUMURA, M., INOUE, N., OHNISHI, A., SANTA, T., & GOTO, I. (1972)
Toxic polyneuropathy due to glue-sniffing. Clin. Neurol. (Japan),
12: 290-296.
MAZEE, W. M., GERSMANN, H. R., & VAN DER WIEL, A. (1966) Analysis of
polycyclic aromatic hydrocarbons in petroleum waxes and white
mineral oils with appraisal of merits of different methods of
analysis. Food cosmet. Toxicol., 4: 17-33.
McDERMOTT, H. J. & KILLIANY, S. E. Jr (1978) Quest for a gasoline TLV.
Am. Ind. Hyg. Assoc. J., 39(2): 110-117.
McKAY, J. F. & LATHAM, D. R. (1972) Fluorescence spectrometry in the
characterization of high boiling petroleum distillates. Anal.
Chem., 44(13): 2132-2137.
McKAY, J. F. & LATHAM, D. R. (1973) Polyaromatic hydrocarbons in high
boiling petroleum distillates. Anal. Chem., 45: 1050-1055.
Medical Research Council (1968) The carcinogenic action of mineral
oils. A chemical and biological study. London HMSO (Special Report
Series No. 306).
MEDVED', R. A., KUZINA, V. F, & KRIGMAN, B. I. (1976) [The hygienic
assessment of lubricating and cooling liquids introduced instead
of sulphofrezol for the cold processing of metal by cutting.]
Gig. Tr. prof. Zabol., No. 6, pp. 1-5 (in Russian).
MENDELL, J. R., SAIDA, K, GANANSIA, M. F., JACKSON, D. B., WEISS, H.,
GARDIER, R. W., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J.,
MARKS, B., & HETLAND, L. (1974) Toxic polyneuropathy produced by
methyl n-butyl ketone. Science, 185: 787-789.
MEYER, A. (1976) Huilome pulmonaire chez les chanteurs. Arch. Mal.
prof., 37(9): 629-631.
MILLER, A., BADER, R. A., BADER, M. E., TEIRSTEIN, A. S., & SELIKOFF,
I. J. (1962) Mineral oil pneumonia. Ann. intern. Med.,
57: 627-634.
MILNE, J. E. (1970) Carcinoma of the scrotum. Med. J. Aust.,
1: 13-16.
MINER, S. (1969) Air pollution aspects of hydrogen sulfide,
Bethesda, MD, Litton Systems Inc. (prepared for the United States
Department of Health, Education and Welfare Contract No.
PH-22-68025).
MIYAGAKI, H. (1967) Electrophysiological studies on the peripheral
neurotoxicity of n-hexane. Jpn. J. ind. Health, 9: 12-23.
MOEL, M. & TAYLOR, H. K. (1943) Oil aspiration pneumonia. Am. J.
Röntgenol. rad. Ther., 49(2): 177-184.
MOSS, E., SCOTT, T. S., & ATHERLEY, G. R. C. (1972) Mortality of
newspaper workers from lung cancer and bronchitis 1952-1966.
Br. J. ind. Med., 29(1): 1-14.
MUHAMETOVA, G. M. & PODREZ, Z. G. (1975) [Problems of the course,
pathogenesis and therapy of chronic patrol poisoning.] Ufa,
Baskirskoe kniznoe izdatelstvo (in Russian).
NATHANSON, L., FRANKEL, D., & JACOBI, M. (1943) Diagnosis of lipoid
pneumonia by aspiration biopsy. Arch. int. Med., 72: 627-634.
NEBERT, D. W. & GELBOIN, H. V. (1969) The in vivo and in vitro
induction of aryl hydrocarbon hydroxylase in mammalian cells of
different species, tissues, strains, and developmental and
hormonal states. Arch. Biochem, Biophys., 134: 76-89.
NEBERT, D. W., GOUJON, F. M., & GIELEN, J, E. (1972)Aryl hydrocarbon
hydroxylase induction by polycyclic hydrocarbons: simple autosomal
dominant trait in the mouse. Nature New Biology, 236: 107-109.
NELSON, L. B. & STORMONT, D. J. (1960) Determination of polynuclear
aromatic hydrocarbons in petroleum waxes. Chem. Ind., 21: 561.
NIOSH (1973) The industrial environment -- its evaluation and
control. Washington, DC, US Dept of Health, Education and
Welfare.
NIOSH (1977A) Occupational exposure sampling strategy manual.
Washington, DC, US Dept of Health, Education and Welfare (DHEW
(NIOSH) Publication No. 77-173).
NIOSH (1977B) Criteria for a recommended standard ... occupational
exposure to asphalt fumes, Washington DC, US Department of
Health, Education and Welfare (DHEW (NIOSH) Publ. No. 78-106).
NIOSH (1977C) Criteria for a recommended standard ... occupational
exposure to hydrogen sulphide, Washington DC, US Department of
Health, Education and Welfare.
NIOSH (1977D) Criteria for a recommended standard ... occupational
exposure to refined petroleum solvents, Washington DC, US
Department of Health, Education and Welfare, 247 pp. (DHEW (NIOSH)
Publ. No. 77-192).
NIOSH (1977-79) NIOSH manual of analytical methods. 2nd ed.
Cincinnati, OH, US Dept of Health, Education and Welfare, USA.
NIOSH (1979) Summary of NIOSH recommendations for occupational health
standards, Washington DC, US Department of Health and Welfare,
9 pp.
NOCHOMOVITZ, L. E., UYS, C. J., & EPSTEIN, S. (1975) Massive
deposition of mineral oil after prolonged ingestion. S.A. med.J.,
13 Dec., 2187-2190.
NOMIYAMA, K. & NOMIYAMA, H. (1974) Respiratory retention, uptake and
excretion of organic solvents in man. Int. Arch. Arbeitsmed.,
32: 75-91.
NOVIKOV, K. I., KOLODINA, L. N., LIPOVSKIJ, S. M., & BADALOVA, L. V.
(1979) [Lactation particulars in female workers of the chemical
(rubber) industry.] Gig. Tr. prof. Zabol., No. 2, pp. 45-48
(in Russian).
NUNN, J. A. & MARTIN, F. M. (1934) [Gasoline (petrolether) and
kerosine (petroleum) poisonings in children.] Samml.
Vergiftungsf., 5: 183-185 (in German).
OGATA, M., ASAHARA, H., & SAEKI, T. (1975) Sampling and analysis of
some aromatic aliphatic and chlorinated hydrocarbon vapours in
air, a gas-liquid chromatographic and colorimetric method.
Int. Arch. Arbeitsmed., 34: 25-37.
OSER, B. L., OSER, M., & CARSON, S. (1965) Toxicological studies of
petrolatum in mice and rats. Toxicol. appl. Pharmacol.,
7: 382-401.
PASTERNACK, B. & EHRLICH, L. (1972) Occupational exposure to an oil
mist atmosphere. A 12-year mortality study. Arch. environ.
Health, 25: 286-294.
PATES, M. M. (1952) [Blastomogenic effect of pyrolyse tars from
petroleum.] Tr. Akad. Med. Nauk., SSSR, 21: 157-163
(in Russian).
PAULSON, G. W. & WAYLONIS, G. W. (1976) Polyneuropathy due to
n-hexane. Arch. intern. Med., 136: 880-882.
PERBELLINI, L., BRUGNONE, F. & FAGGIONATO, G. (1981) Urinary excretion
of the metabolites of n-hexane and its isomers during
occupational exposure. Br. J. ind. Med., 38: 20-26.
PETROLEUM HANDBOOK (1966) London, Shell International Petroleum
Company Limited.
PINKERTON, H. (1927) Oils and fats. Their entrance into and fate in
the lungs of infants and children: A clinical and pathologic
report. Am. J. Dis. Child., 33: 259-285.
PINKERTON, H. (1928) The reaction to oils and fats in the lung. Arch.
Pathol, 5: 380-401.
PINKERTON, H. & MORAGUES, V. (1940) Paraffinoma of the lung with
secondary tuberclelike lesions in liver and spleen. Arch.
Pathol., 29: 691-699.
PIOTROWSKI, J. K. (1977) Exposure tests for organic compounds in
industrial toxicology. Cincinnati, OH, NIOSH, (DHEW (NIOSH)
Publication No. 77-144).
POKLIS, A. & BURKETT, C. D. (1977) Gasoline sniffing: A review. Clin.
Toxicol., 11: 35-41.
POLIANSKY, V. A. & MUSERSKAJA, A. N. (1971) [Pollution of the air
environment at modern oil processing plants and evaluation of the
implementation of recommendating.] In: [Occupational hygiene and
the protection of the health of workers in oil and petrochemical
industries.] Ufa, pp. 50-54 (in Russian).
PROCKOP, L. D. (1977) Multifocal nervous system damage from inhalation
of volatile hydrocarbons. J. occup. Med., 19: 139-140.
PROUDFIT, J.P., ORDSTRAND, H. S. VAN, & MILLER, C. W. (1950) Chronic
lipid pneumonia following occupational exposure. Arch. ind. Hyg.,
1: 105-111.
PRUYN, F. H. A.M. & REIJNIERSE, K. (1972) [Carcinoma of the scrotum
through mineral oils.] T. Soc. Geneesk., 50: 182-187 (in Dutch).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1975) Report on emissions from
asphalt hot mixes, Maryland, USA, The Asphalt Institute
(Research Report RR-75-IA).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1978) Differences between
petroleum asphalt, coal-tar pitch and road tar, Maryland, USA,
The Asphalt Institute (Research report RR-78-1).
RAALTE, H. G. S. VAN (1963) [Medico-toxicological aspects of oil used
in metal processing.] Tijdschr. Soc. Geneesk., 41: 611-617
(in Dutch).
RAALTE, H. G. S. VAN (1972) [Carcinoma of the scrotum through mineral
oils.] Tijdschr. Soc. Geneesk, 50: 604-605 (in Dutch).
RAITTA, C., SEPPÄLÄINEN, A. M., & HUUSKONEN, M. (1978) N-hexane
maculopathy in industried workers. Graefes Arch. klin. exp.
Ophthalmol., 209: 99-110.
REID, F. H. & HALPIN, W. R. (1968) Determination of halogenated and
aromatic hydrocarbons in air by charcoal tube and gas
chromatography. Am. Ind. Hyg. Assoc. J., 29: 390.
REIDENBERG, M. M., POWERS, D. V., & BELLO, C. T. (1964) Acute renal
failure due to nephrotoxins. Am. J. med. Sci., 59: 25-63.
REWELL, R. E. (1947) Lipoid pneumonia: A pitfall in diagnosis.
Br. med. J., 29: 409-411.
RIIHIMÄKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
Environ. Health, 5: 143-150.
RIIHIMÄKI, V. & PFÄFFLI, P. (1978) Percutaneous absorption of solvent
vapours in man. Scand. J. Work Environ. Health, 4: 73-85.
RIIHIMÄKI, V., PFÄFFLI, P., SAVOLAINEN, K., & PEKARI, K. (1979)
Kinetics of m-xylene in man. General features of absorption,
distribution, biotransformation and excretion in repetitive
inhalation exposure. Scand. J. Work Environ. Health,
5: 217-231.
ROBINSON, E. & ROBBINS, R. C. (1968) Sources, abundance, and fate of
gaseous atmospheric pollutants. Chapter VI; Hydrocarbons and
organics in the atmosphere, Huntsville, Alabama, Stanford
Research Institute (Report PR-6755 prepared for the American
Petroleum Institute).
ROE, F. J. C., CARTER, R. L., & TAYLOR, W. (1967) Cancer hazard from
mineral oil used in the processing of jute. Br. J. Cancer,
21: 694-702.
ROSSIER, P. H. & BÜHLMANN, A. (1949) [Clinical and laboratory studies.
An oil-aspiration pneumonia following the use of liquid paraffin
as nose drops for some years.] Schweiz. Med. Wochenschr.,
79: 685-686.
SANTE, L. R. (1949) The fate of oil particles in the lung and their
possible relationships to the development of bronchogenic
carcinoma. Am. J. Röntgenol., 62: 788-797.
SAVOLAINEN, H. (1977) Some aspects of the mechanisms by which
industrial solvents produce neurotoxic effects. Chem. biol.
Interactions, 18: 1-10.
SCHAMP, N. & VAN WASSENHOVE, F. (1972) Determination of
benzo(alpha)-pyrene in bitumen and plants. J. Chromatogr.,
69: 421-425.
SCHAUMBURG, H. H. & SPENCER, P.S. (1976) Degeneration in central and
peripheral nervous systems produced by n-hexane: An experimental
study. Brain, 99: 183-192.
SCHEUPLEIN, R. J. & BLANK, I. H. (1971) Permeability of the skin.
Physiol. Rev., 51: 702-747.
SCHMAHL, D. & REITER, A. (1933) [Experiments on cancer production by
liquid paraffin yellow vaseline and lanolin.] Arzneim. Forsch.,
3: 403-406 (in German).
SCHWARZ, H. G. (1933) [Petrol poisoning, chronic, iatrogenic.] Samml.
Vergiftungsf., 4: 247-248 (in German).
SCHWARTZ, L., JULIPAN, L., & PECK, S. (1947) Dermatoses caused by
petroleum. In: Occupational diseases of the skin, 2nd ed.,
Kimpton, London, pp. 232-258.
SCRIMA, M. & De ROSA, F. (1973) [Polyneuritis in upper shoe factories:
Environmental and concomitant causes.] Lav. Um., 25: 191-198
(in Italian).
SEDIVEC, V. & FLEK, J. (1976) The absorption, metabolism and excretion
of xylenes in man. Int. Arch. occup. Environ. Health,
37: 205-217.
SEHTMAN, B. A, SAMEDOR, I. G., & MUHAMETOVA, G. M. (1979)
[Work hygiene in the petroleum industry.] Moscow, Medicina Publ.
House (in Russian).
SEPPÄLÄINEN, A. M. (1975) Applications of neurophysiological methods
in occupational medicine: A review. Scand. J. Work Environ.
Health, 1: 1-14.
SEPPÄLÄINEN, A. M., HUSMAN, K., & MARTENSSON, C. (1978)
Neuro-physiological effects of long-term exposure to a mixture of
organic solvents. Scand. J. Work Environ. Health, 4: 304-314.
SEPPÄLÄINEN, A.M., RAITTA, C., & HUUSKONEN, M. (1979)
n-Hexane-induced changes in visual evoked potentials and
electroretinograms of industrial workers. Electroencephalogr.
clin. Neurophysiol., 47(4): 492-498.
SERRATRICE, G., CORREARD, R.-P., ARLAUD, J.-A., & JOUGLARD, J. (1973)
Polynévrite et manipulation de peinture pour bateaux. J. eur.
Toxicol., 6: 304-305.
SHAHIN, M. M. & FOURNIER, F. (1978) Suppression of mutation induction
and failure to detect mutagenic activity with Athabasca Tar sand
fractions. Mutat. Res., 58: 29-34.
SHIRABE, T., TSUDA, T., TERAO, A., & ARAKI, S. (1974) Toxic
poly-neuropathy due to glue-sniffing; report of two cases with
light and electron-microscopic study of the peripheral nerves and
muscles. J. Neurol. Sci., 21: 101-113.
SHOSHKES, M., BANFIELD, W., & ROSENBAUM, S. J. (1950) Distribution,
effect and fate of oil aerosol particles retained in the lungs of
mice. Arch. ind. Hyg. occup. Med., 1: 20-35.
SHUBIK, P. & SAFFIOTTI, U. (1954) Carcinogenic and promoting action of
low-boiling catalytically cracked oils. Proc. Am. Assoc. Cancer
Res., 1: 45.
SHUBIK, P., SAFFIOTTI, U. LIJINSKY, W., PIETRA, G., RAPPAPORT, H.,
TOTH, B., RAHA, C. R., TOMATIS, L., FELDMAN, R., & RAMAHI, H.
(1962) Studies on the toxicity of petroleum waxes, Toxicol. appl.
Pharmacol., 4 (Suppl,, Nov.): 1-62.
SIMMERS, M. H. (1964) Petroleum asphalt inhalation by mice, Arch,
environ, Hearth, 9(6): 727-734.
SIMMERS, M. H. (1965A) Cancers from air.refined and steam refined
asphalt. Ind. Med. Surg., 34(3): 244-261.
SIMMERS, M. H. (1965B) Cancers in mice from asphalt fractions,
Ind. Med. Surg., 34(7): 573-577.
SIMMERS, M. H. (1966) Tumors from asphalt fractions injected into
mice. Ind. Med. Surg., 35(10): 889-894.
SIMMERS, M. H., PODOLAK, E., & KINOSITA, R. (1959) Carcinogenic
effects of petroleum asphalt. Proc. Soc. exp. Blot. Med.,
10: 266-268.
SIMS, P., GROVER, P. L., SWAISLAND, A., PAL, K., & HEWER, A. (1974)
Metabolic activation of benzo(a)pyrene precedes by a diol-epoxide.
Nature (Lond.), 252: 326-328.
SIOU, G. (1972) Existe-t-il un risque de cancérisation par les
bitumes. Rev. Path. comp. Méd. exp., 72: 65-75.
SIWE, S. A. (1932) [The signs and symptoms of acute poisoning by
liquid hydrocarbons in the aliphatic series.] Monatschr.
Kinderheilkd., 55: 146-153 (in German).
SMILEY, J. A. (1951) The hazards of rope making. Br. J. ind. Med.,
8: 265-270.
SMITH, W. E. & SUNDERLAND, D. A. (1951) Responses of mice to
carcinogenic derivatives of petroleum. Cancer Res., 11: 281.
SMITH, W. E., SUNDERLAND, D. A., & SUGIURA, K. (1951) Experimental
analysis of the carcinogenic activity of certain petroleum
products. Arch. ind. Hyg. occup. Med., 4: 299-314.
SMS 2337-1 (1972) Determination of low concentrations of benzene in
air, The Hague, Shell Int. Petr. My. (Shell Method Series).
SMYTH, H. F. & SMYTH, H. F. (1928) Inhalation experiments with certain
lacquer solvents. J. ind. Hyg., 10: 261-271.
SOUTHAM, A. (1928) Mule-spinners' cancer. In: Report of the
International Conference on Cancer, London, Bristol, John
Wright, p. 28C.
SPENCER, P. S., BISCHOFF, M. C., & SCHAUMBURG, H. H. (1978) On the
specific molecular configuration of neurotoxic aliphatic
hexacarbon compounds causing peripheral distal axonopathy.
Toxicol. appl. Pharmacol., 44: 17-28.
SPRUIT, D., MALTEN, K. E., LIPMANN, E. W. R. M., & LIANG, T. P. (1970)
Horny layer injury by solvents. II. Can the irritancy of petroleum
either be diminished by pretreatment. Berufsdermatosen,
18: 269-280.
STERNER, J. H. (1941) Study of hazards in spray-painting with gasoline
as a diluent. J. ind. Hyg. Toxicol., 23: 437-448.
STEWART, I. (1960) Petrol vapour poisoning. Br. med. J.,
i: 1739-1740.
STRÄULI, P. (1957) [Acute tar cancer.] Oncologia, 10: 72-75
(in German).
STRYKER, W. A. (1941) Absorption of liquid petrolatum (mineral oil)
from the intestine. Arch. Pathol., 31: 670-692.
SUGIURA, K., SMITH, W. E., & SUNDERLAND, D. (1949) Carcinogenic action
of certain catalytically cracked oils with high boiling points.
Cancer Res., 9: 631.
SUHANOVA, V. A. & MEL'NIKOVA, V. V. (1974) [The menstruation function
of female workers at refineries and patients suffering from
chronic intoxication by petroleum products.] Gig. Tr. prof.
Zabol., No. 4, pp. 30-41 (in Russian).
SULLIVAN, R. J. (1969) Air pollution aspects of odorous compounds,
Bethesda, Maryland, Litton Systems Inc. (prepared for the US Dept.
of Health, Education and Welfare, Contract No. PH-22-68-25).
SUMA'MUR, P. K. & SUSIANTI WENAS. (1978/1979) Skin and other
affections in gasoline and/or diesel oil pump workers. Indones.
J. ind. Hyg. occup. Health, 11-12: 20-22 (XI No. 1, 2, 3, 4 and
XII No. 1 and 2).
SUNDERLAND, D. A., SMITH, W. E., & SUGIURA, K. (1951) The pathology of
growth behaviour of experimental tumours induced by certain
petroleum products. Cancer, 4: 1232-1245.
SUZUKI, T., SHIMBO, S., & NISHITANI, H. (1974) Muscular atrophy due to
glue sniffing. Int. Arch. Arbeitsmed., 33: 115-123.
SWANN, H. E. Jr., KWONN, B. K., HOGAN, G. K., & SNELLINGS, W. M.
(1974) Acute inhalation toxicology of volatile hydrocarbons.
Am. Ind. Hyg. Assoc. J., 35(9): 511-518.
Tabershaw/Cooper Association Inc. (1974/75) Morbidity study of
petroleum refinery workers. (Project OH-4, Medical Research
Report prepared for API).
TAGAMI, H. & OGINO, A. (1973) Kerosene dermatitis; factors affecting
skin irritability to kerosene. Dermatologica, 146: 123-131.
TAHER, S. M., ANDERSON, R. J., McCARTNEY, R, POPOVTZER, M. M., &
SCHRIER, R. W. (1974) Renal tubular acidosis associated with
toluene «sniffing». New Engl. J. Med., 290: 765-768.
TAKEUCHI, Y., MABUCHI, C., & TAKAGI, S. (1975) Polyneuropathy caused
by petroleum benzine. Int. Arch. Arbeitsmed., 34: 185-197.
THAKKER, D. R., YAGI, H, AKAGI, H, KOREEDA, M., LU, A. Y.-H., LERIM,
W., WOOD, A. W., CONNEY, A. H., & JERINA, D. M. (1977) Metabolism
of benzo(alpha)pyrene VI. Stereoselective metabolism of
benzo(alpha)-pyrene and benzo(alpha)pyrene 7,8-dihydrodiol to
diolepoxides. Chem. biol. Interaction 16: 281-300.
THONY, C. & THONY, J. (1970) Enquête épidémiologique sur le cancer des
huiles de coupe. In: Proceedings of the 16th International
Congress on Occupational Health, Tokyo, 1969, pp. 655-657.
THONY, C., THONY, J., LAFONTAINE, M., & LIMASSET, J. C. (1975)
Concentrations en hydrocarbures polycycliques aromatiques
cancérogènes de quelques huiles minerale étude due risque
correspondant. Arch. Mal. prof., 36: 37-52.
TRUHAUT, R. (1978) Introductory remarks. In: Meeting on MACs, XIX In.
International Congress on Occupational Health, 26 September 1978.
TRUHAUT, R. & MURRAY, R. (1978) International workshop on toxicology
of benzene, Paris, 9-11 November 1976. Int. Arch. Occup. Env,
41: 65-76.
TRUHAUT, R., LAGET, P., PIAT, G., DUTERTRE-CATELLA, P.-L. H., & HUYEN,
V. N. (1973) Premiers résultats électrophysiologiques après
intoxications experimentales par l'hexane et par l'heptane
techniques chez le rat blanc. Arch. Mal. prof.,
34(7-8): 417-426.
TWORT, C. C. & FULTON, J. D. (1930) Further experiments on the
carcinogenicity of synthetic tars and their fractions. J. Path.
Bacteriol., 32: 119-143.
TWORT, C. C. & ING, H. R. (1928) [Investigations on carcinogenic
agents.] Z. Krebsforsch., 27: 308-351 (in German).
TWORT, C. C. & TWORT, J. M. (1931) The carcinogenic potency of mineral
oils. J. And. Hyg. Toxicol., 13: 204-226.
TWORT, C. C. & TWORT, J. M. (1935) Induction of cancer by cracked
mineral oils. Lancet, November 30: 1226-1228.
TWORT, C. C., LYTH, R., & TWORT, J. M. (1937) The significance of
density, refractive index and viscosity of mineral oils in
relation to the type and degree of animal reaction. J. Hyg.,
37: 14-29.
TYE, R., BURTON, M. J., BINGHAM, E., BELL, Z., & HORTON, A. W. (1966)
Carcinogens in a cracked petroleum residuum. Arch. environ.
Health, 13: 202-207.
UK MEDICAL RESEARCH COUNCIL (1968) The carcinogenic action of mineral
oils. A chemical and biological study, London, Her Majesty's
Stationary Office, 8 pp. (Special Report Series No. 306).
UNGAR, H., HINE, C. H., KODAMA, J. K., & ANDERSON, H. H. (1955)
Neuropathology of rats experimentally poisoned with
p-tertiary-butyl-toluene. A.M.A. Arch. Pathol., 69: 139-149.
US DHEW (1970) Air quality criteria for hydrocarbons, Washington,
DC, United States Department of Health, Education and Welfare.
USSR (1972) [Sanitary norms for the planning of industrial
enterprises.] Moscow (in Russian).
VAN DER LINDEN, A. C. & THYSSE, G. J. E. (1965) The mechanisms of
microbial oxidations of petroleum hydrocarbons. Adv. Enzymol.,
27: 469-546.
VAUGHAN, C. G., WHEALS, B. B., & WHITEHOUSE, M. J. (1973) The use of
pressure-assisted liquid chromatography in the separation of
polynuclear hydrocarbons. J. Chromatogr., 78: 203-210.
VIGALJUK, R. V., ZAMALEEVA, Z. S., RUBINSKIJ, L. Ya., SOLODOVA, N. L.,
& LJUTAJA, T. I. (1977) [The chromatographic determination of the
total content of C1 C10 paraffin hydrocarbons and C6-C8
aromatics in the air of industrial plants.] Gig. Tr. prof.
Zabol., No. 6, pp. 57-57 (in Russian).
VOBORSKY, R. C. (1980 In: Dolberg, D. D. & Verstuyft, A. W, ed.
Analytical techniques in occupational health chemistry,
Washington, DC, ACS (ACS Symposium Series 120).
VOLK, B. W. (1964) Lipoid pneumonia: Clinical, pathologic and chemical
aspects. Biochem. Clin., 4: 187.
VOLKMANN, R. (1875) [On tar, paraffin, and soot cancer (chimneysweep's
cancer.] In: Beiträge zur Chirurgie, Leipzig, Breitkopf und
Hartel (in German).
VON LEHMDEN, D. J., VON HANGERBRAUCK, R. P., & MEEKER, J. E. (1965)
Polynuclear hydrocarbon emissions from selected industrial
process. J. Air. Pollut. Control Assoc., 15: 306-312.
WAGNER, W. D., DOBROGORSKI, O. J., & STOKINGER, H. E. (1961)
Antagonistic action of oil mists on air pollutants. Arch.
environ. Health, 2: 523-534.
WAGNER, W. D., WRIGHT, P. G., & STOKINGER, H. E. (1964) Inhalation
toxicology of oil mists. I. Chronic effects of white mineral oil.
Am. Ind. Hyg. Assoc. J., 25: 158-167.
WAHLBERG, J. E. (1974) Occupational and non-occupational scrotal
cancer in Sweden, 1958-1970. Acta Dermatovenerol., 54: 471-474.
WALKER, J. D, COLWELL, R. R., & PETRAKIS, I. (1975) A study of the
biodegradation of a South Louisiana crude oil employing
computerized mass spectrometry. In: Joint Conference on the
Prevention and Control of Oil Pollution, San Francisco, p. 197.
WALLCAVE, L, GARCIA, H., FELDMAN, R., LIJINSKY, W., & SHUBIK, P.
(1971) Skin tumorigenesis in mice by petroleum asphalts and
coaltar pitches of known polynuclear aromatic hydrocarbon content.
Toxicol. appl. Pharmacol., 18: 41-52.
WANG, C. C. (1961) Acute gasoline intoxication. Arch. environ.
Health, 2: 714-716.
WARING, J. I. (1933) Pneumonia in kerosene poisoning. Am. J. med.
Sci., 185: 325-330.
WATERHOUSE, J. A. H. (1971) Cutting oils and cancer. Ann. occup.
Hyg., 14: 161-170.
WATERHOUSE, J. A. H. (1972) Lung cancer and gastro-intestinal cancer
in mineral oil workers. Ann. occup. Hyg. 15: 43-44.
WATTENBERG, L. W. (1972) Dietary modification of intestinal and
pulmonary aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEISS, F. T. (1975) Activities of standardization groups in the
identification of waterborne oils. Paper read at National Bureau
of Standards Workshop - Santa Barbara, California, 6-7 October,
1975, 17 pp.
WEISSMAN, H. (1951) Lipoid pneumonia. Am. Rev. Tuberc., 64: 572-576.
WHO (1974) Toxicological evaluation of some food additives
including anti-caking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents, Geneva, World Health
Organization, pp. 417-422 (WHO Food Additive Series No. 5).
WHO (1982) Environmental health criteria 19: Hydrogen sulfide.
Geneva, World Health Organization.
WIEL, A. VAN DER (1975) Collaborative determination of precision of
DMSO extraction-refractive index method, Amsterdam,
Koninklijke/Shell Laboratory (Memorandum LFA/200/75).
WIEL, A. VAN DER (1979) Determination of potentially hazardous
poly-nuclear aromatics in petroleum fractions, Amsterdam,
Koninklijke/Shell Laboratory (Amsterdam Method Series, AMS 642-2).
WILLIAMS, R. T. (1959) Detoxication mechanisms, 2nd ed., New York,
John Wiley & Sons Inc.
WOOD, E. H. (1943) Unusual case of carcinoma of both lungs associated
with lipoid pneumonia. Radiology, 40: 193-195.
WOODHOUSE, D. L. (1934) The non-carcinogenicity of certain synthetic
lubricating oils and the action of some commercial spindle-oils,
solvent fractionated spindle oil distillates and tar oils, tested
under similar conditions. J. Inst. Petr. Technical.,
20: 1057-1063.
WOODHOUSE, D. L. (1950) The carcinogenic activity of some petroleum
fractions and extracts. J. Hyg. Camb., 48: 121-134.
YAGI, H., THAKKER, D. R., HERNANDEZ, O., KOREEDA, M., & JERINA, D. M.
(1977) Synthesis and reactions of the highly mutagenic 7, 8-diol
9, 10-epoxides of the carcinogen benzo-(a) pyrene. J. Am. Chem.
Soc., 99: 1604-1611.
YAMAMURA, Y. (1967) n-Hexane neuropathy. Folia psychiatr. neurol.
Jpn., 23: 45-57.
YANG, S. K., McCOURT, D. W., ROLLER, P. R., & GELBOIN, H. V. (1976)
Enzymatic conversion of benzo(alpha)pyrene leading predominantly
to the diol-epoxide r-7, t-8-dihydroxy-t-9, 10-oxy-7,8,9,10-
tetrahydrobenzo (a) pyrene through a single emantiomer of r-7,
t-8-dihydroxy-7, 8-dihydrobenzo-pyrene. Proc. Natl Acad. Sci.
US, 73: 2594-2598.
YOKOO, H. & SUCKOW, E. E. (1961) Peripheral lung cancers arising in
scars. Cancer, 14: 1205-1215.
YOUNG, A.M., APPLEBAUM, H. S., & WASSERMAN, P. B. (1939) Lipoid
pneumonia. J. Am. Med. Assoc., 112: 2406-2409.
ZANGGER, H. (1933) [Occupational accidents and hazards at work inside
enclosed spaces (reservoirs, tanks, and transport lorries.]
Arch. Gewerbepathol. Gewerbehyg., 4: 117-140 (in German).
ZIELHUIS, R. L. (1961) [Early complaints occurring on exposure to
organic solvents.] Tijdschr. Soc. Geneesk., 39: 595-600
(in Dutch).
ZINGMARK, P. A. & RAPPE, C. (1977) On the formation of
N-nitrosodi-ethanolamine in a grinding fluid concentrate after
storage. Ambio, 6: 237-238.
2.2.2.3 Disposal of waste
In a refining process, the release of oil into refinery effluents
is practically negligible and of a lower order of magnitude than
tanker washings in tankers that do not use the "load-on-top" system.
Waste gas in production fields is generally burnt on the spot. In
refineries and chemical plants, it may be necessary to burn some gas
at a flare for reasons of safety, and some oil and gas is consumed as
refinery fuel. Atmospheric pollutants in and around refineries
basically consist of saturated and unsaturated hydrocarbons, carbon
monoxide, hydrogen sulfide, and sulfur dioxide (Poliansky &
Musserskaja, 1971; Krasovitskaja, 1976). Sulfur dioxide, hydrogen
sulfide, and mercaptan emissions are not discussed in this review and
emissions of hydrocarbon vapours into the atmosphere from storage
terminals, filling stations, and cars will be covered in another
document.
2.3 Toxicological Effects of Crude Oils
As, in this document, crude oils are discussed only to provide
background information for the petroleum solvents, lubricating base
oils, and bitumens derived from them, no detailed discussion will
follow concerning environmental exposure levels, environmental
distribution and transport, physiological factors relating to
mammalian uptake, dose-response relationships, and maximum permissible
levels. Most of the relevant aspects will, however, be covered in the
sections on fractions derived from the crude oils. This also applies
to toxicological effects on experimental animals and man, with the
exception of a very few studies that are related to crude oil exposure
only.
The toxicological and nuisance aspects of hydrogen sulfide and
mercaptans have been reviewed in detail by Miner (1969) and Sullivan
(1969). A review on hydrogen sulfide has been prepared by NIOSH
(1977c) and an Environmental Health Criteria document on hydrogen
sulfide has recently been published (WHO, 1982).
2.3.1 Effects on experimental animals
Leitch (1924) examined 16 untreated crude oils from various parts
of the world for their carcinogenicity by applying them 3 times a week
to the skin of mice and found significant differences in
tumorigenicity among these oils. Similar results were reported by
Hieger & Woodhouse (1952) in skin tests on mice and rabbits. The
tumorigenicity of the crude oils they examined was low in comparison
with that of some of the distilled fractions. Skin tests were also
carried out on mice and rabbits by Antonov & Lints (1960), who found
that Saratov oil possessed weak carcinogenic properties. The main
causes of death in these tests, however, were pneumonia and general
intoxication, probably from absorption of oil components through the
skin. The authors found that rabbits were more sensitive than mice, as
did Hieger & Woodhouse (1952).
Batt-Neal & Wolman (1977) demonstrated skin tumorigenicity and
amyloid deposition following skin exposure of mice to saturated
acetone extracts of various oils collected from beaches.
2.3.2 Effects on man
Examination of 743 oilfield workers exposed to California crude
oil and excessive sunlight revealed that 7 of them had epitheliomas on
exposed parts of the body and that nearly 20% had keratotic changes on
the hands, forearms, face, and neck. Five of the 7 subjects, who
developed epitheliomas, were blonds, though blonds were in the
minority in this group of workers (Schwartz et al., 1947).
During 1938-39, Schwartz saw 189 cases of carcinomas on exposed
parts of the skin; 128 were in males, 71 of whom were oilfield workers
20 others being workers exposed to excessive sunlight only. Emmett
(1975) mentions the strong potentiating effect of UV radiation on
other potentially carcinogenic exposures. In southern Texas, however,
the incidence of skin carcinomas in 330 oilfield workers was low,
which underlines the fact that Texas and Pennsylvania oils are known
to be less carcinogenic than California oil (Twort & Ing, 1928).
In a study on 50 volunteer operators, who had not previously been
in contact with oil and petroleum products, crude oil was applied to
the skin of the inner surface of the forearm, for periods of 3-6 h. An
inflammatory reaction of the skin developed with moderate erythema,
oedema, and slight burning. Changes in the thermosensitive threshold
were noted, as well as an increase in the permeability of the
epidermis (Gusein-Zade, 1975).
3. PETROLEUM SOLVENTS
3.1 Properties and Analytical Methods
3.1.1 Chemical composition and properties
Only solvents consisting of hydrogen and carbon alone and
produced from petroleum will be considered in this review. It should
be noted, however, that similar solvents are also produced from coal.
Petroleum solvents consist of complex mixtures of hydrocarbons
reflecting the hydrocarbon constituents of the crude oil or, more
usually, the intermediate refinery streams from which they are
distilled. Because of their complex nature, classification is a
problem and no standard, worldwide-accepted nomenclature exists.
However, providing that it is recognized that considerable overlapping
and many exceptions occur, they can be classified into 3 broad
subdivisions, based on distillation ranges:
(a) special-boiling-point solvents (SBPs) - grades with narrow or
wide distillation ranges within the main limits of 30-160°C;
(b) white spirits - grades distilling within the main range
150-220°C, the boiling-points of individual grades usually
ranging over more than 20°C;
(c) high-boiling aromatic solvents - grades distilling in the range
160-300°C with final boiling-points above 220°C.
Benzene, toluene, and the xylene isomers occur as components of
petroleum solvents, but as they fall more naturally into the category
of chemical intermediates, they will be referred to here only in so
far as they are important as components of the mixtures being
discussed.
Two further clarifications can be made. Firstly, it is common
industrial practice to ascribe the name of the predominant isomer
present to the petroleum solvent; thus the descriptions pentane,
isopentane, hexane, isohexane, and heptane are commonly met. However,
in almost all cases, the amount of the named isomer present in an
industrial scale product will not exceed 95% v/v of the solvent and
may be as small as 30% v/v.
Second, most petroleum solvents are marketed on the basis of
typical physical properties rather than on chemical specifications,
because of the limitations during refining of controlling the complex
mixtures of isomers that make up the petroleum solvents. As production
techniques become more sophisticated, greater control is possible and
more properties can be specified within narrower limits. However, even
when such narrow limits are met, the mixture of components present may
vary, because of variations in the types of crude oil being processed
and alterations in conditions in processing units.
To meet the wide range of properties required by the market,
several different processes are used. Distillation is the common
process setting the volatility range. Chemical conversion techniques,
including reforming, alkylation, and hydrogenation, alter the chemical
composition and hence the solvency, as do physical conversion
techniques such as solvent extraction and molecular sieve separation.
Specific treatments such as caustic soda and sulfuric acid washing and
clay percolation are frequently applied to remove odourous substances,
chiefly sulfur compounds.
The reader is referred to Boenheim & Pearson (1973) for detailed
discussions of the chemical and physical composition and uses of
petroleum solvents.
3.1.1.1 Special-boiling-point solvents (SBPs)
These are highly purified naphtha fractions with specially
selected boiling ranges. The boiling range may be narrow or wide, and
generally falls within the limits of 30-160°C: SBPs are classified
according to their boiling range, e.g., SBP 62/82. Petroleum ether,
lighter fluid, spot remover, and rubber solvent are consumer products
in this range. Generally, SBPs consist of a mixture of hydrocarbons in
the C-5 to C-9 range: normal and branched paraffins, cycloparaffins,
and aromatic compounds. They contain only traces of olefins. An
example of the composition of a typical sample of straight run (i.e.,
non-dearomatized) SBP 80/110 is given in Table 2.
3.1.1.2 White spirits
The boiling-range of this group of solvents falls within the
limits 150-220°C (intermediate between gasoline and kerosene). These
solvents can be classified into low-aromatic grades (approximately
15-20% aromatic hydrocarbons) and high-aromatic grades (45% or more
aromatic hydrocarbons). They generally consist of hydrocarbons in the
C-7 to C-12 range, again including normal and branched paraffins as
well as naphthenic (cycloparaffins) and aromatic compounds. Olefins
are present in trace amounts only. Stoddard solvent, mineral spirits,
low-aromatic white spirits (LAWS) and turpentine-substitute are
well-known examples from this range.
3.1.1.3 High-boiling aromatic solvents
Aromatic hydrocarbons occur naturally in certain crude oils in
widely varying concentrations. They are also formed during secondary
processes such as thermal and catalytic reforming. They can be
concentrated and extracted by solvent extraction.
Apart from benzene, toluene, and xylene, which will not be
discussed separately in this review, this group includes solvents with
an aromatic content of 80-100%, and a wide boiling-range from 160 to
300°C. High-boiling aromatic solvents are obtained by distillation or
solvent extraction from refinery fractions such as kerosene and
lubricating base oils, and consist of very complex mixtures of
hydrocarbons with more than 9 carbon atoms per molecule. The
composition of a typical sample of one of these aromatic hydrocarbons
from the middle range (distillation range approximately 192-203°C) is
given in Table 3.
Most aromatic solvents are highly purified "white" solvents.
Those in the higher boiling range, derived from lubricating base oil
stocks by solvent extraction, may be less pure and coloured. They are
often by-products and are used as solvents for various technical
purposes. In many cases, they are referred to as "processing oils"
instead of solvents, and considered under lubricating oils.
3.1.2 Purity of petroleum solvents
In these complex mixtures, impurity is, of course, a matter of
definition. Components that are taken out in the course of the various
refining and treating processes used to obtain the more pure solvents
could be regarded as such. The major impurities would then be sulfur
compounds such as hydrogen sulfide, mercaptans, and thiophens, as well
as, olefins and other reactive unsaturated hydrocarbons.
A second category of impurities includes the hydrocarbons that
have been demonstrated to be carcinogenic in animals and man, such as
benzene, the polynuclear hydrocarbons and related heterocyclic
compounds containing nitrogen or sulfur.
TABLE 2. Composition of typical sample of SBP 80/110a
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
normal n-pentane 0.2 36.2
paraffins n-hexane 8.2 69.0
n-heptane 17.2 98.4
branched 2 methyl butane Tc 0.1 27.9
paraffins 2,2 dimethyl butane T trace 49.7
2,3 dimethyl butane T 0.3 58.0
2 methyl pentane 1.5 60.3
3 methyl pentane 1.6 63.3
2,2 dimethyl pentane 1.0 79.2
2,4 dimethyl pentane 1.3 80.5
2,2,3 trimethyl butane T 0.3 80.9
2,3 dimethyl pentane 9.7 89.8
3 methyl hexane 9.2 91.9
3 ethyl pentane 3.1 93.5
2,2,4 trimethyl pentane trace 99.2
2,2 dimethyl hexane trace 106.8
2,5 dimethyl hexane 0.6 109.1
3,3 dimethyl hexane T trace 112.0
2,3 dimethyl hexane 0.8 115.66
3,4 dimethyl hexane trace 117.7
3 methyl heptane 0.5 118.9
cyclo C-6 cyclohexane 8.4 80.7
paraffins methyl cyclohexane 14.2 100.9
cyclo C-5 cyclopentane T trace 49.3
paraffins methyl cyclopentane 4.7 71.8
1,1 dimethyl cyclopentane T 2.9 87.9
1-cis-3-dimethyl 1.9 90.8
cyclopentane T
TABLE 2. (contd).
Hydrocarbon Hydrocarbon % mass present Boiling
type in sampleb point °C
cyclo C-5 1-trans-3-dimethyl 2.7 91.7
paraffins cyclopentane T
contd. 1-trans-2-dimethyl 0.5 91.9
cyclopentane T
1-cis-2-dimethyl cyclopentane T 0.5 99.5
ethyl cyclopentane 0.6 103.5
1,1,3 trimethyl cyclopentane T 0.8 104.9
1-trans-2-cis-4-trimethyl 0.4 109.3
cyclopentane T
1-trans-2-cis-3-trimethyl 0.4 110.2
cyclopentane T
1,1,2 trimethyl cyclopentane T 0.3 113.7
unidentified Probably
paraffins 1.1 110.0
aromatic benzene 0.7 80.1
compounds toluene 3.9 110.6
olefins 0.4
a From: Shell International Petroleum Co., London (unpublished data).
b Average of duplicate analyses.
c T = tentative identification.
TABLE 3. Composition of typical sample of Solvesso 150a
Hydrocarbon % v/v of solvent
n-butylbenzene 2.47
sec-butylbenzene 0.08
tert-butylbenzene 0.05
m-cymene 0.13
o-cymene 0.01
p-cymene 0.52
1.2-diethylbenzene 1.72
1.3-diethylbenzene 1.10
1.4-diethylbenzene 0.56
1.2-dimethyl-3-ethylbenzene 2.86
1.2-dimethyl-4-ethylbenzene 6.64
1.3-dimethyl-2-ethylbenzene 0.71
1.3-dimethyl-4-ethylbenzene 4.17
1.3-dimethyl-5-ethylbenzene 2.80
1.4-dimethyl-2-ethylbenzene 3.26
m-ethyltoluene 0.37
o-ethyltoluene 0.02
p-ethyltoluene 0.01
indane 0.46
isobutylbenzene 0.32
isopropylbenzene 0.01
1-methyl-3-t-butylbenzene 0.76
1-methyl-2-n-propylbenzene 1.26
1-methyl-3-n-propylbenzene 2.08
1-methyl-4-n-propylbenzene 1.93
1-methylindane 0.91
2-methylindane 2.43
4-methylindane 9.28
5-methylindane 2.02
naphthalene 4.03
n-propylbenzene 0.00
1.2,3,4-tetramethylbenzene 3.66
1.2,3,5-tetramethylbenzene 8.84
1.2,4,5-tetramethylbenzene 5.53
toluene 0.02
1.2,3-trimethylbenzene 0.10
1.2,4-trimethylbenzene 0.05
1,3,5-trimethylbenzene 0.01
a Courtesy Esso Standard Oil Company, New York, N.Y.,
USA (From: Gerarde, 1960).
TABLE 3. (contd).
Hydrocarbon % v/v of solvent
m-xylene 0.05
o-xylene 0.03
p-xylene 0.03
C-11-naphthalenes 0.31
C-11-indanes 3.58
C-11-alkylbenzene 18.27
C-12-alkylbenzene 0.73
C-12-indanes 0.08
C-13-alkylbenzene 0.02
C-10-indenes 0.10
C-11-indenes 0.07
C-12-naphthalenes trace
C-13-naphthalenes
C-12-indenes 0.10
aromatic compounds Total 94.55
Generally, the total sulfur content, the olefin content, and the
total aromatic content are specified for commercial petroleum
solvents. Where special products such as food-grade materials are
concerned, the benzene content is specified as well as the UV
absorption limits at certain wavelengths, as a measure of the
polynuclear aromatic hydrocarbon content.
3.1.3 Methods of sampling and analysis
See section 2.1.2.
3.2 Sources of Environmental Pollution
3.2.1 Natural occurrence
Petroleum solvents do not occur in nature as such, but only as
components of the crude oils from which they are derived.
Environmental pollution is always man-made and related to the use of
the solvents.
3.2.2 Man-made sources
3.2.2.1 Production
Because there is no uniform system of definition and
classification of petroleum solvents, firm statistics concerning the
magnitude of production of this group of materials do not exist. The
best estimate of the world-wide production of the group of solvents
would be 9 million tonnes for the year 1979.
3.2.2.2 Uses
It is not feasible to give more than a general outline of the
uses of the range of petroleum solvents.
(a) SpeciaL-boiling-point solvents (SBPs)
SBPs are mainly used as: solvents and thinners in lacquers and
paints; extraction solvents for perfumes, for vegetable oils and oil
and fats of animal origin; quick-drying solvents in printing-ink,
coatings, and adhesives; lighter fuel; and for dry-cleaning and
degreasing purposes.
(b) White spirits
White spirits are mainly used as: solvents and thinners for
lacquers, paints, resins, and printing-ink; solvents in formulations
of chemical products, e.g., pesticides; and for metal degreasing, wool
degreasing, and dry-cleaning.
(c) Aromatic extracts
The higher-boiling and less-purified aromatic extracts have very
good solvent properties for many polymers and are used as
ex-tender-oils in rubber, plastics, and bitumens, and also as solvents
in printing-ink and pesticide formulations. Furthermore, they can be
used as base-materials in the manufacture of carbon black.
3.3 Environmental Exposure Levels
Specific data are not available concerning levels of petroleum
solvents in air, water, food, or other environmental media. However,
low concentrations of hydrocarbons found in mussels have probably been
derived from petroleum hydrocarbons present in the environment
(Ehrhardt & Heineman, 1975).
Because of the relatively low boiling-range of these solvents,
industrial exposure to vapour may sometimes be high. This is known to
occur, especially in small workshops with insufficient ventilation,
where, for example, adhesives are used routinely. Although a lot of
consumer products may contain these solvents, excessive domestic
exposure would not normally be expected unless neat solvent were used
for cleaning purposes, indoors. Very limited, indirect exposure of the
general population is possible following the use of these solvents as
extractants in the production of food-grade vegetable oils.
Exposure to the higher-boiling and less-purified aromatic
extracts is mainly confined to occupational situations, where
excessive skin-contact may occur, or exposure to vapour in processes
carried out at elevated temperatures or with high-speed machines that
could give rise to fumes or mists. This will be considered in detail
under lubricating base oils.
3.4 Environmental Distribution and Transformation
Data on the distribution between media, environmental
transformation and degradation, interaction with physical, chemical,
or biological factors and bioconcentration, are not available for
petroleum solvents.
However some information exists concerning the behaviour and
degradation of crude oil in water (Floodgate, 1972, Hellmann & Zehle,
1972), and of hydrocarbons in general (Walker et al., 1975), and there
is much information on the microbial degradation of individual
petroleum hydrocarbons (Van der Linden & Thysse, 1965; Haines &
Alexander, 1974).
From these publications it can be seen that the subject is highly
complex and many factors have to be taken into account, such as the
composition of the oil product, the extent of dispersion into the
medium, and climatic conditions.
3.5 Metabolism
3.5.1 Absorption
The kinetics are determined by diffusion rates, solubility in
fat, and the concentration gradients in the individual compartments of
the body.
The highly volatile C-5, C-6, and C-7 paraffins, cycloparaffins,
and aromatic hydrocarbons readily pass across the alveolar membrane
into the bloodstream and are transported within minutes to the central
nervous system. Longer-chain homologues can, to a certain extent, also
pass the alveolar membrane, but their principal effect is local. This
was shown by Gerarde (1963) in studies on rats.
The alveolar air and blood concentrations of white spirit have
been measured in man following inhalation (Åstrand et al., 1975).
Aromatic hydrocarbons were absorbed to a greater extent into the
bloodstream than aliphatic hydrocarbons (approximate values being 62%
and 50%, respectively). Similar uptake values in man were shown for
the aromatic hydrocarbons, benzene and toluene, by Nomiyama & Nomiyama
(1974), for xylene by Sedivec & Flek (1976), Åstrand et al. (1978),
and Riihimäki et al. (1979), and for ethylbenzene by Bardodej &
Bardodejová (1970). Nomiyama & Nomiyama (1974) demonstrated a much
lower pulmonary absorption for n-hexane, the only aliphatic compound
that they tested; it was also rapidly excreted.
The skin is only permeable to hydrocarbons of a certain size.
With paraffinic substances, the maximum chain length appeared to be up
to 14 C-atoms (Scheuplein & Blank, 1971). Aromatic compounds have a
more compact structure and, in studies on guineapigs, Hoekstra &
Phillips (1967) showed that compounds from this group with a higher
number of C atoms could still pass the skin barrier.
The absorption of vapours through the skin is of minor
importance. For example, in man, whole body skin exposure to
2250 mg/m3 (600 ppm) of toluene was equivalent to an inhalation
exposure of less than 37.5 mg/m3 (10 ppm) (Riihimäki & Pfäffli,
1978). However, absorption during immersion in liquid solvents may be
considerable. Percutaneous absorption during immersion of both hands
in pure xylene was equal to an inhalation exposure of 435 mg/m3
(100 ppm) (Engström et al., 1977). The permeation of xylene is thus
about 20 nmol/min per cm2 (Engström et al., 1977; Riihimäki, 1979)
and that for toluene, 3 µmol/min per cm2 (Cohr & Stockholm, 1979).
Cutaneous exposure was probably a major route of absorption in 2 cases
of acute renal failure with oliguria, caused by exposure to diesel oil
(Barrientos et al., 1977; Crisp et al., 1979).
Data for absorption in the intestinal tract are not available,
but it is presumed that it would resemble absorption in the alveoli
rather than that through the skin.
3.5.2 Distribution in the body
Tissue hexane levels in rats, following inhalation of anaesthetic
concentrations, were measured by Böhlen et al. (1973). The tissue
distribution generally depended on exposure time and was proportional
to the lipid content of an organ until saturation occurred. The liver
was a special case for, as its lipid level changed rapidly, the
saturation level varied. Hexane was also apparently bound to some
blood components.
Women working at conveyor belts gluing parts of rubber footwear
had concentrations of petroleum solvents (no details on
physicochemical properties given) in the blood ranging from 2 . 35 ±
0.4 up to 4.6 ± 0.6 mg/litre at concentrations in the air of
100-300 mg/ m3. The solvent concentration in the blood increased with
increasing length of the working period from 1.6 mg/litre in the first
year to 2.5 mg/litre after 3.5 years and 3.4 mg/litre after 7-8 years
of service.
Wistar rats were exposed to the solvents used in the factory at
concentrations in air of 300-1000 mg/m3 for 30-45 days, 4 h/day. The
concentration of solvent in the blood amounted to 0.45 ± 0.05 -
1.2 ± 0.01 mg/litre (Lipovskij et al., 1977a).
Transfer of petroleum solvents through the placenta was studied
in 85 pregnant women workers in the rubber industry, who came into
contact with petroleum solvents during work (physicochemical
properties of the solvents not defined, concentration in the air of
the operating premises 300 ± 10 mg/m3). The average level of solvents
in the blood of 46 pregnant women, on whom abortion was performed, was
1.27 ± 0.3 mg/litre. A level of 3.29 ± 0.6 mg/kg was found in the
tissue of the embryo. Women giving birth to a child (39 women) had a
level of solvents in the blood of 2.5 ± 0.3 g/litre, while the content
in the blood of the umbilical cord was 3.5 ± 0.3 g/litre. The
concentration of solvents in the blood of the newborn infants was
twice that of the mothers.
Pregnant Wistar rats were exposed to the same solvent at a
concentration of 300 ± 10 mg/m3, for 48 days, 4 h per day. The
solvent was present in the blood, brain, liver, placenta, uterus, and
fetal tissues (Lipovskij et al., 1979).
3.5.3 Biotransformation
In both man and animals, the aliphatic hydrocarbons are generally
considered to be biochemically inert and excreted in the same form
(Williams, 1959). However, it has been shown that some normal alkanes
will, at least in part, be oxidized by the mammalian organism. For
example, Ichihara et al. (1969) demonstrated the oxidation of decane
in animals such as mice and rats, and the oxidative pathway of
n-hexane to hexane-2,5-dione and hexane-2,5-diol via
methyl- n-butylketone has been well established (see for example
Spencer et al., 1978).
As far as the metabolism of the cycloparaffins and aromatic
hydrocarbons is concerned, the half-life, form, and rate of excretion
of each component of the solvent has to be considered. It should be
mentioned, however, that the metabolism of individual compounds will
not be discussed in this document and readers are referred to the
reviews by Williams (1959) and Gerarde (1960).
The carcinogenicity of the solvents is thought to be due to the
presence of benzene and some of the polynuclear aromatic compounds.
3.5.4 Elimination
The elimination of the lower-boiling solvents (SBP type) in both
animals and man is usually rapid and mainly occurs via the respiratory
tract. However, in the case of ingestion of the heavier solvents
(white spirits), elimination mainly takes place with the faeces
(Browning, 1965).
3.6 Effects on Experimental Animals
It has been mentioned in section 3.1.1, that the petroleum
solvents under discussion in this document are more or less complex
mixtures of a range of hydrocarbons. For the commercial products, the
specification given generally includes the specific gravity,
boiling-range, and total content of aromatic hydrocarbons. The
concentrations of individual components vary, within certain limits,
with the source of the crude oil from which the solvent is derived,
and with the processes by which it is produced. These facts should be
kept in mind because:
(a) the toxicity data developed for a certain solvent-specification
indicate the order of magnitude of the toxicity of this type of
product;
(b) in practice it would be impossible and impracticable to carry out
complete toxicity testing on every single solvent on the market.
It is only sensible to develop toxicity data for typical
representative samples of a certain boiling range and within a
certain specification of aromatic content. In the evaluation of
the results, however, the analytical composition of the material
- especially its contents of n-hexane, benzene, and polynuclear
aromatic hydrocarbons should be taken into account.
3.6.1 Short-term exposure
Hine & Zuidema (1970) examined various aspects of the acute
toxicity of 10 samples of petroleum solvents that contained components
representative of the range of hydrocarbons found in commercial
petroleum solvents. Four were aromatic solvents containing at least
98% aromatic hydrocarbons (coded A) and 6 were non-aromatic solvents
containing less than 1% aromatic hydrocarbons (coded S). The boiling
ranges and principal components of the samples examined are given in
Table 4.
Acute oral, inhalation, and percutaneous toxicity and skin and
eye irritancy were examined for all samples. Intratracheal aspiration
was simulated with 2 samples and repeated skin irritation tests were
carried out using 5 of the samples. Undiluted samples were used for
the investigations, all of which were carried out on rats with the
exception of skin and eye irritancy and skin toxicity rests in which
rabbits were used.
TABLE 4. The boiling-range and principal components of solvents examined for
acute toxicitya
Sample Boiling-range Principal components
A-1 281-286°F (138-141°C) C-8 aromatic compounds (ortho, meta, and
paraxylene; ethyl benzene)
A-2 362-398°F (163-203°C) C-9, C-10 and C-11 aromatic compounds
A-3 364-408°F (188-209°C) C-10 and C-11 aromatic compounds
A-4 384-507°F (196-264°C) C-11 to C-14 aromatic compounds
S-1 149-166°F (65-75°C) C-6 normal and isoparaffins (hexanes) and
naphthenes (cyclohexane, methylcyclopentane)
S-2 196-220°F (91-104°C) C-7 normal and isoparaffins (heptanes) and naphthenes
(methylcyclohexane, dimethylcyclopentane)
S-3 313-356°F (156-180°C) C-9 and C-10 normal and isoparaffins and naphthenes
S-4 368-395°F (187-212°C) C-11 and C-12 normal and isoparaffins and naphthenes
S-5 345-402°F (174-216°C) C-12 isoparaffins
S-6 384-500°F (195-260°C) C-13 to C-16 normal and isoparaffins and naphthenes
a From: Hine & Zuidema (1970).
The findings of Hine & Zuidema (1970) which are summarized in
Table 5, showed that all the solvents tested could be considered of
low hazard to health unless aspirated or inhaled in extremely high
concentrations. Aromatic solvents were more toxic than non-aromatic
materials, the dose of solvent required to kill 50% of rats, when
administered orally or percutaneously, being lower for aromatic than
for non-aromatic solvents. Skin and eye irritancy were also greater
with aromatic solvents. The toxicity of the vapours could not be
compared, because the volatility of samples varied greatly. All
solvents induced similar toxic effects, whatever the route of
administration, including central nervous system depression
(characterized by incoordination, prostration, and coma) followed by
death. Convulsions sometimes occurred. All solvents caused skin and
eye irritation though, in general, as the chain length of the
non-aromatic solvents increased their irritant properties decreased.
Repeated skin exposure led to skin irritation and necrosis with all
solvents.
Hoekstra & Phillips (1963) found that light mineral oils, when
applied topically to the skin of guineapigs, caused epidermal
hypertrophy, hyperplasia, hyperkeratosis, and depilation. Examination
of the effects of various oil fractions demonstrated that the main
effect of the short-chain volatile paraffins was to defat the skin,
while longer-chain and aromatic hydrocarbons had a dermatoxic effect
that was related to the permeability of the skin to these compounds.
The maximum dermatoxic effect was seen with hydrocarbons containing
14-19 carbon atoms, while a transition to non-dermatoxicity occurred
around 21-23 carbon atoms. This was confirmed with pure n-paraffins,
but variations may exist with other types of hydrocarbons.
Simultaneous application of innocuous long-chain substances together
with irritant short-chain substances greatly reduced their toxicity,
though this effect was less marked with aromatic solvents.
In further studies on the effects of inhaling the vapours of
hydrocarbon solvents (Carpenter et al. 1977a, b, c), the acute (4-h
exposure) LC50 and no-observed-adverse-effect concentrations were
studied in rats cats, and dogs. Results are summarized in Table 6.
These studies confirmed the occurrence of central nervous system
depression and there was also evidence of respiratory tract irritancy.
There were no marked or consistent differences between the species
examined. The major factor determining the acute inhalation hazard was
the volatility of the solvent, those containing 9 or more carbon atoms
tending to be insufficiently volatile to produce concentrations high
enough to be lethal over a short period of exposure. One exception was
a "high naphthenic" solvent, which was peculiar also in that
depression was not preceded by signs of irritation of the respiratory
tract, so that there was no warning of overexposure. Increased
aromatic content did not consistently result in increased inhalation
toxicity, though earlier work (Lazarew, 1929) suggested that the acute
inhalation toxicity of gasoline vapours increased with increasing
contents of cycloparaffins and aromatic hydrocarbons. The narcotic
action was also found to increase in each step by a factor of 3 in the
series - pentane, hexane, heptane, and octane (Fühner, 1921). Swann et
al (1974) found that anaesthesia occurred with these compounds at
concentrations of 32 000 ppm or more and that respiratory tract
irritation increased with chain length. Full anaesthesia can be
produced with gasoline (Haggard, 1921), but anaesthetic concentrations
are little lower than those that cause convulsions and death
(Browning, 1965).
TABLE 5. Toxicity of solvents. Summary of resultsa
Test Sample Result Classification
Oral A-1 10.0(7.5-13.3) practically non-toxic
LD50 A-2 4.5(3.0-6.8) slightly toxic
(ml/kg) A-3 13.3(7.5-23.7) practically non-toxic
A-4 12.3(8.1-18.7) practically non-toxic
S-1 >25.0b relatively harmless
S-2 >25.0b relatively harmless
S-3 >25.0b relatively harmless
S-4 >25.0b relatively harmless
S-5 >25.0b relatively harmless
S-6 >25.0b relatively harmless
Vapour A-1 6 350(4 670-8 640) slightly toxice
exposure A-2 >2 450b SVNTARTd
LC50 in ppm A-3 >580c SVNTART
for 4 h A-4 >553c SVNTART
S-1 73 680(66 310-79 940) practically non-toxic
S-2 14 000-16 000 practically non-toxic
S-3 2 000-2 600 slightly toxic
S-4 >710 SVNTART
S-5 >792 SVNTART
S-6 >263 SVNTART
Aspiration A-4 5/10 hazardous
(mortality) S-6 5/10 hazardous
Primary A-1 2.21 moderately irritating
skin A-2 2.04 moderately irritating
irritation A-3 2.17 moderately irritating
A-4 2.79 moderately irritating
S-1 1.92 slightly irritating
S-2 1.13 slightly irritating
S-3 2.38 moderately irritating
S-4 1.04 slightly irritating
S-5 1.29 slightly irritating
S-6 0.75 minimally irritating
Eye A-1 6.33 moderately irritating
irritation A-2 6.0 moderately irritating
A-3 4.33 moderately irritating
A-4 3.67 slightly irritating
S-1 0.33 minimally irritating
S-2 1.0 minimally irritating
S-3 2.0 minimally irritating
S-4 0 minimally irritating
S-5 0 minimally irritating
S-6 0 minimally irritating
TABLE 5. (contd).
Test Sample Result Classification
4-h A-1 approx. 5.0 practically non-toxic
percutaneous A-2 approx. 5.0 practically non-toxic
LD50 rangefind A-3 approx. 5.0 practically non-toxic
(ml/kg) A-4 approx. 5.0 practically non-toxic
S-1 >5.0 practically non-toxic
S-2 >5.0 practically non-toxice
S-3 >5.0 practically non-toxice
S-4 approx. 5.0 practically non-toxice
S-5 5.0 practically non-toxice
S-6 5.0 practically non-toxice
Repeated benzene 3.6
skin toluene 3.5
irritationf A-1 3.3
a From: Hine & Zuidema (1970).
b Doses above this amount not practical for testing.
c Maximum concentration obtainable at 25 °C.
d SVNTART = Saturated vapours not toxic at room temperature.
e Lowest toxicity classification may be "relatively harmless"
f Scored according to the method of Draize.
The greatest health hazard arises when hydrocarbon solvents are
aspirated into the lungs. This rapidly induces acute chemical
pneumonitis, which is characterized by pulmonary oedema and
haemorrhage, and is generally fatal (Waring, 1933; Lesser et al.,
1943; Gerarde, 1959). Gerarde (1959) demonstrated that the ratio of
the oral and intratracheal LD50s was 140:1 for kerosene, the
intra-tracheal LD50 being 0.2 ml for rats. This and other evidence
demonstrated that pulmonary injury was caused by direct contact with
the solvent and not by solvent present in the blood, following its
absorption through the gastrointestinal tract.
TABLE 6. Toxicity of solventsa
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
V.M. & P. 118-151 55.4 32.7 11.9 C-7 to C-10 16 >8 >19 2.8 2.8 0.7-7 2.1 2.0
Naphtha (3400) (>1700) (>4100) (600) (800) (0.15- 1.5) (450) (430)
Stoddard 153-194 47.7 37.6 14.7 C-8 to C-12 >8.2 >2.9 > 10 1.1 1.9 0.5 - 5 7.85 1.15
solvent (>1400) (>510) (>1700) (190) (330) (09-.9) (>150) (200)
Rubber 75-112 41.4 53.6 4.9 C-6 to C-7 61 >5.9 >49 7.9 7.9 40 1.7 1.7
solvent (15000) (>1500) (>12000) (2000) (2000) (10) (430) (430)
Mixed 138-141 - - 100 C-8 29 >5.4 <41 3.5 3.5 0.6-6 >0.46 0.46
xylenes (6700) (>1200) (<9500) (810) (180) (.14-1.4 (>110) (110)
'60' 128-159 c28.8 c21.4 c49.5 C-8 to C-9 24 >9.5 <20 0.44 1.4 10 C. 1.3 0.44
Solvent (4900) (>1900) (<4100) (90) (280) (2) (260) (90)
'70' 157-211 16.5 15.7 67.8 C-9 to C-11 >4.4 >2.4 >2 1.1 1.1 4 0.32 0.32
Solvent (>810) (>440) (>370) (200) (200) (0.7) (59) (59)
'140' 184-205 60.8 35.7 3.4 C-5 to C-12 >0.27 >0.21> > >0.23 >0.23 4 0.31 0.23
Flash (sic) (>43) (>33) saturated (>37) (>37) (0.6) (49) (37)
aliphatic vapour
solvent'
'80' 96-142 9.7 18.9 71.4 C-6 to C-8 27 >2.1 >24 > 1.7 > 1.7 4 0.45 0.45
Thinner (6200) (>480) (>5500) (>390) (>390) (0.9) (100) (100)
'50' 98- 105 66.3 0.6 33.1 C-7 33 >3.4 >30 2.4 2.4 10 1.7 1.7
Thinner (8300) (>600) (>7600) (600) (600) (2.5) (430) (430)
Deodorised 208-272 55.2 40.~9 3.9 not stated >0.1 This value applies for all toxicity evaluation and is 0.1
kerosene probably (14) a saturated atmosphere at room temperature. (14)
C 12 to
C-15
TABLE 6. (contd)
Coined Boiling Compositionb Major 4-8h LC50c 13-wk Inhalation Human data Recommended
name range °C % Constituents mg/litre (ppm) NEL mg/litre (ppm) hygiene
carbon Odour Sensory limit
P N A number rat dog cat rat dog threshold threshold mg/litre
mg/m3 mg/litre (ppm)
(ppm) (ppm)
'40' 187-231 35.4 32.9 31.5 C-9 to C-13 >0.2 >0.25 >7 0.22 0,22 1 0.21 0.15
Thinner (>33) (>41) (aerosol) (36) (36) (0.17) (35) (25)
Toluene 95-110 38.7 15.4 45.9 C-6 to C-7 35 >3 >31 3.9 3.9 10 1.9 1.9
concentrate (8800) (>760) (>7800) (980) (980) (2.5) (480) (480)
'High 184-206 0.3 0.8 98.9 C-9 to C-12 >0.38 This value applies for all animal 0.4 0.15 0.15
aromatic (>66) toxicity evaluations and is a (0.07) (26) (26)
solvent saturated atmosphere at room temperature
'High 157- 183 29.0 69.9 1.1 not stated 5.3 3.8 - 2.1 - - - 2.1
naphthenic probably (960) (>690) (380) (380)
solvent' C-9 to
C-11
'Naphthenic 151-200 24.7 37.2 38.1 C-9 to C-11 >10 - - 2.2 - - - 2.2
aromatic (some (380) (380)
solvent' aerosol
present)
a Summary of data from Carpenter et al. (1977a,b,c).
b p = paraffins, N = naphthenes, A = aromatic compounds.
c For details of actual length of LC50 exposure, see original papers.
d NEL/No-ill effect/exposures 6th/day, 5 days/week for 13 weeks.
The same author found that viscosity appeared to be the property
that determined the aspiration hazard of liquid hydrocarbons.
Lower-boiling-point solvents (B.P. up to 100°C) evaporated so rapidly
in the mouth of anaesthetized rats that death was due to CNS
depression following absorption of the vapours. Higher-boiling-point
solvents tended to induce chemical aspiration pneumonitis. With
alkanes, the aspiration hazard decreased sharply with solvents
containing 16 or more carbon atoms, probably because the viscosity of
such solvents prevented aspiration into the alveoli. In the aromatic
series, side-chains containing more than 6 carbon atoms also tended to
diminish the aspiration hazard, as did blending with more viscous
lubricants (Gerarde, 1963).
3.6.2 Long-term exposure
The repeated skin tests of Hoekstra & Phillips (1963) and Hine &
Zuidema (1970) have already been mentioned in section 3.6.1.
Smyth & Smyth (1928) exposed guineapigs for 4 h/day, 6 days/
week, for a total of 65 exposures to a gasoline-type solvent
(boiling-range 145-183°C) at a concentration of 6750 mg/m3. During
the earlier exposures, the animals appeared to be restless. This was
followed by slight narcotic effects. Diarrhoea and albuminuria
developed temporarily, but blood, lung, and other changes were absent.
In a recent series of inhalation studies on various hydrocarbon
fractions, Carpenter et al. (1977a, b, c) provided much new
information on the toxicity of a wide range of solvents. Physical
properties and the general chemical constitution are detailed in the
papers and the approximate compositions are given in Table 6. The
no-observed-adverse-effect levels of individual solvents following
inhalation studies over 13 weeks in rats and dogs is also tabulated,
together with the human sensory response (section 3.7.1.2). In
general, these studies were remarkable in the lack of toxicity that
they indicated. The few toxic effects that did occur, were usually
kidney damage in rats and minor haematological variations.
Other long-term animal studies have been carried out with the
lower-boiling fractions and more specifically with technical hexane
and technical heptane, because of their possible neurotoxic effects
and to establish a safe level of industrial exposure (TLV).
Mature female Wistar rats were exposed to petroleum solvent
vapour (physicochemical properties not given) at a concentration of
300 ± 8.2 mg/m3 for 30-45 days, for 4 h/day. The serotonin content of
the myometrium in exposed rats equalled 75.7 ± 2.6 µg/kg compared with
68.47 ± 2.5 µg/kg in the control group. Uterine contractions were more
numerous and stronger in exposed animals. The level of solvent in the
venous blood was 2.0 ± 0.4 mg/litre. In the uterine tissues it was
almost twice as high (3.8 ± 0.6 mg/kg). The increase in serotonin
content in the organism could cause disturbances in the transport of
the fertilized egg cell and the nidation, and subsequently, early
abortion (Lipovskij, 1978).
Miyagaki (1967) exposed 5 groups of 10 male mice to vapour
concentrations of technical hexane of 360, 900, 1800, 3600, and
7200 mg/m3. There was a sixth control group. The exposures lasted
24 h/day, 6 days/week, over a period of one year. The composition of
the hexane used is uncertain. The measured vapour concentrations
correlated well with those calculated. Electromyographical studies
were carried out and strength-duration curves, electrical reaction
time, flexor/extensor ratios, and gait-posture were observed. The
muscular atrophy of the hind legs was measured and in some of the
animals, histological examination of the distal mucles of the hind
legs was carried out. Evidence of neurogenic muscular atrophy was only
seen in the group receiving the highest exposure. At the lowest
exposure level (360 mg/m3), no changes were found in any of the
variables studied. At the other levels, changes were found related to
the severity and duration of exposure. Based on their results, the
authors proposed a reduction in the TLV to 360 mg/m3.
In a different study, which included a group of 5 rats (sex and
age not stated) exposed to a hexane concentration of 3060 mg/m3, over
a period of 143 days (no other details given) and a control group, no
significant differences were found in average body weights and blood
values (haematocrit, total serum protein, and protein fractions)
between treated and control animals. Histological examination of
"many" organs revealed only a "slight reaction of the RES in the
spleen" in the exposed animals. In the sciatic nerve and its
ramifications, injuries such as degeneration of myelin and axon
cylinders were seen. The myoneural junction remained unaffected. The
exposed group manifested some decrease in nocturnal activity (Kurita,
1967).
Truhaut et al. (1973) published the results of
electrophysiological studies on rats exposed to technical hexane or
technical heptane. The air concentration of hexane was 7200 ±
720 mg/m3 and that of heptane, 6000 ± 600 mg/m3 (calculated and
expressed as n-hexane and n-heptane). The duration of exposure was
5 h/day, 5 days/week for 1-6 months. The initial weight of the male
and female rats was 150g. No data on the growth-weight curves were
included. The composition of technical hexane was given as:
n-pentane 0.3%
methyl-2-pentane and cyclopentane 25.1%
methyl-3-pentane 18.4%
n-hexane 45.8%
methylcyclopentane 8 %
methylhexanes 1.2%
benzene 1.2%
and that of technical heptane as:
methyl-2-hexane plus dimethyl 2,3 pentane plus cyclohexane 9.8%
methyl-3-hexane 16.2%
n-heptane 52.4%
dimethyl 2,4 hexane plus methylcyclohexane 15.4%
methylheptane 3.3%
benzene 0.1%
toluene 2.8%
Electrophysiological studies were carried out on isolated sciatic
and saphenous nerves taken from anaesthetized rats. During the first 2
months of exposure, no changes were found in any of the treatments
but, in the succeeding months, symptoms of neural involvement were
detected, in general, increasing with the duration of exposure. The
signs were: decrease in conduction-velocity, increase in refractory
period, and decrease in excitability. Histopathological examination
did not reveal clear-cut demyelination, but early indications of this
type of change were certainly found. Truhaut has recently cast doubt
on these findings, stating that his experimental method was not
sufficiently rigorous to allow his earlier conclusions to be made
(Truhaut, 1978).
Numerous studies on the neuropathological effects of n-hexane
have been published including a review by Schaumburg & Spencer (1976).
Rats exposed for 24 h/day to an n-hexane concentration of
1440-2160 mg/m3 for about 20 weeks developed peripheral neuropathy.
Toxicity data on the presumptive in vivo neurotoxic oxidation
products of n-hexane have also been published (Spencer et al.,
1978). It should be mentioned that many authors have used the term
" n-hexane" loosely; the tested material often really consisted of
various C-6 hydrocarbons.
Some other hydrocarbon solvents have been implicated as possibly
causing neuropathy, following prolonged exposure to high
concentrations. As these reports are generally related to human
exposure, they will be considered in section 3.7.2.1. Of relevance is
the observation that toluene inhaled by rats over a period of one year
induced electrophysiological changes at concentrations of 7500 mg/m3
and 750 mg/m3 but not at 375 mg/m3 (Matsumoto, 1971). Fournas & Hine
(1958) exposed rats to high concentrations of various alkyl aromatic
hydrocarbons and found some clinical evidence of neurotoxicity with
most of the compounds tested; p-t-butyltoluene was shown to induce
CNS damage in rats by Ungar et al. (1955).
3.6.3 Mutagenicity, teratogenicity, and carcinogenicity
3.6.3.1 Mutagenicity
The potential mutagenic activity of selected petroleum products
was assessed using a battery of in vivo and in vitro bioassays,
including the dominant lethal test, the Ames' test (with and without
activation), the mouse lymphoma cell transformation test, and
observations on cytogenicity. The following products elicited negative
responses in one or more tests: VM&P Naphtha, Stoddard Solvent, mixed
xylenes, "60 Solvent", "70 Solvent", 140° Flash Aliphatic Solvent, "50
Thinner", kerosene, toluene, high solvency naphtha- unleaded petrol,
and No. 2 heating oil (40% aromatic compounds). Positive mouse
lumphoma cell transformation tests were elicited by benzene, diesel
fuel, No. 2 heating oil, and jet A fuel; positive results in
cytogenetic tests (clastogenic responses) were obtained with rubber
solvents, "60 Solvent", high aromatic solvent, No. 2 heating oil, and
jet A fuel. For each of these products the Ames bacterial bioassay was
negative (API, 1974, 1975b, c, 1977a, 1978a, d, f, 1979d).
3.6.3.2 Teratogenicity
Tests for teratogenicity induced by inhalation of high and low
doses of benzene, Stoddard Solvent, toluene, mixed xylenes, unleaded
petrol, high aromatic solvent, n-hexane, diesel fuel, VM&P naphtha,
kerosene, rubber solvent, jet fuel A, and No. 2 heating oil were all
negative. However, benzene exposure at 120 mg/m3 induced a
statistically significant increase in fetal resorptions in rats (API,
1974, 1975a, b, c, 1977 a, b, 1978a-f, 1979a-d).
3.6.3.3 Carcinogenicity
Carcinogenicity tests have only been conducted on the group of
high-boiling aromatic extracts derived from the solvent refining of
lubricating base oils. These studies will be considered together with
long-term studies on lubricating base oils, but, in short, practically
the whole of the carcinogenic polynuclear aromatic hydrocarbons were
found in the extracts from the base oils. However, the carcinogenic
activity of the extracts was considerably less than that of
coal-tar-derived products.
Similar carcinogenicity studies have not been carried out with
the lower boiling aromatic solvents (white spirits). Such studies
would be indicated, though the content of potentially carcinogenic 4,
5 and 6 condensed ring polynuclear aromatic hydrocarbons in these
aromatic solvents is probably much lower than in high boiling aromatic
solvents, because of the lower boiling range. Lijinsky & Raha (1961)
mention, however, that no commercial distillation procedure will
completely remove all traces of polynuclear aromatic hydrocarbons from
petroleum solvents and that special treatment is necessary to achieve
this. Though, in most cases, the authors found very low concentrations
(in the µg/m3-range), they are of the opinion that these levels
should be investigated, particularly in food-grade material.
3.7 Effects on Man
3.7.1 Controlled exposures
3.7.1.1 Effects of dermal exposure
Klauder & Brille (1947) patch-tested petroleum solvents of
various boiling ranges on the skin of human volunteers. They found a
correlation between the boiling ranges of the petroleum products of
paraffinic origin and their irritant and defatting action on the skin.
Both effects decreased, the higher the boiling range. Petroleum
solvents with boiling ranges up to and including that of kerosene
(approximately 230°C) were found to be primary irritants. Petroleum
solvents of naphthenic origin or with a high aromatic content were
more irritant than solvents of paraffinic origin of the same boiling
range. The skin of Negroes showed a higher tolerance than that of
Caucasians.
Pre-existing skin disease may increase the susceptibility of the
skin to the effects of contact with petroleum solvents and will also
facilitate uptake by this route (Klauder & Brille, 1947; Riihimäki &
Pfäffli, 1978).
The effects of various solvents on the horny layer of the skin
were examined by Malten et al. (1968) and Spruit et al. (1970). They
found that petroleum ether (SBP 40/65) caused serious irritation of
human forearm skin, when applied for periods of 10-30 min. When
applied for 15 min on 6 successive days, injury occurred in the horny
layer. Recovery -- as measured by water vapour loss -- could take up
to 6 weeks. The skin irritation and the changes in the composition of
the horny layer were independent phenomena.
Tagami & Ogino (1973) applied undiluted refined kerosene to the
arm of a volunteer in an occluded patch-test. After 1 h, a burning
sensation developed, slight erythema appeared after 2 h, and after
7 h, the skin was tender and very red, even beyond the patch-test
site. After 12 h, the burning sensation had subsided but a large tense
bulla had appeared surrounded by small scattered vesicles. This
changed into a large flaccid purulent bulla 24 h later, which easily
broke, leaving a raw surface. The main differences between the test
situations just described were: (a) a marked difference in boiling
range (volatility and skin penetration) of the products tested; and
(b) the length of exposure, the effect increasing with increasing
duration of exposure.
The same authors then applied 85%, 70%, 55%, and 40% dilutions of
the kerosene in mineral oil in covered skin test on 34-adult male
Negro and Caucasian volunteers. The skin of all the subjects reacted
to 85% kerosene solution, the 70% solution caused skin irritation in
29 subjects, the 55% solution in 8, and the 40% solution was
completely without effect. The skin of Negroes appeared to be less
irritated by kerosene than that of Caucasians; the influence of age
was not clear. No definite correlation was found between individual
response to kerosene and the permeability of the horny layer of the
skin (Tagami & Ogino, 1973).
3.7.1.2 Effects of inhalation
Inhalation of air containing petrol at concentrations of
1350-3150 mg/m3 for 18 min by human volunteers did not cause any
symptoms; a 14-min exposure to 12 600-31 500 mg/m3 induced dizziness
(Fieldner et al., 1921). Davis et al. (1960) exposed human volunteers
for 30 min to concentrations of petrol of 900, 2250, or 4500 mg/m3 in
air. Very few symptoms were noted. Itching and burning of the eyes was
apparent in most subjects in the highest exposure group.
The human odour- and sensory irritation thresholds were measured
as part of the toxicity evaluation of solvents carried out by
Carpenter et al. (1977a) (Table 6). It can be seen that vapours of
hydrocarbon solvents can be detected at rather low concentrations, but
that unpleasant odour and irritation only become apparent at much
higher concentrations. Nevertheless, in establishing an exposure limit
for each solvent, human sensory data were often limiting factors.
In volunteer studies, psychological functions were affected by a
50-min exposure to a concentration of white spirit of 4000 mg/m3
resulting in a prolonged reaction time and impaired short-term memory.
Lower concentrations did not have any effect (Gamberale et al., 1975).
3.7.2 Epidemiological studies
A distinction between epidemiological and clinical studies has
always been difficult in view of the inadequacy of reporting. The
various aspects will therefore be discussed under headings relevant to
the information available.
3.7.2.1 Occupational exposure
(a) Haematological effects
Benzene is unquestionably the most dangerous hydrocarbon used in
industry and the benzene content of some petroleum solvents presents a
major long-term hazard for man. In particular, special boiling
solvents in the lower-boiling range may contain a considerable
percentage of benzene (Gerarde, 1960). In this review, it is
impossible to consider in detail the effects of benzene, which
basically causes bone-marrow depression and has a leukaemogenic
action. Excellent reports on all aspects of benzene toxicity can be
found in: ILO (1968); Deutsche Forschungsgemeinschaft (1970); IARC
(1974); and Laskin & Goldstein (1977).
In the past, benzene-like effects have also been ascribed to
other low-boiling petroleum solvents. However, there now seems to be
general agreement that these effects only occur, when benzene is
present in the mixture (Browning, 1959, 1965).
(b) Neuropathological effects
The neuropathological effects of inhalation of petroleum solvents
in man have been discussed in a number of reviews including those of
Cavanagh (1973), Allen (1975), Seppäläinen (1975), Comstock (1977),
and Savolainen (1977). The reviews include discussions of methods used
to evaluate human neuropathy.
Over the past 15 years, an increasing number of cases of
poly-neuropathy and other neurotoxic effects have been described in
workers exposed to hydrocarbon solvents. Many of these cases have been
associated with prolonged and repeated exposure to high concentrations
of n-hexane. Frequently, such exposure has resulted from poor
ventilation in a workroom in which solvents containing high
concentrations of hexane have been used. Miyagaki (1967) reported
hexane concentrations in air of 1800-3600 mg/m3 in the workroom and
7200 mg/m3 near the source in a work-place where 17 cases of
peripheral neuropathy occurred. Yamamura (1967) and Inoue et al.
(1970) found concentrations ranging from 1800-9000 mg/m3 in a work
place where, over a 9-year period, 93 out of the 1662 workers suffered
from polyneuritis. These workers used glue in the manufacture of vinyl
sandals for 8-14 h daily, probably for more than 5 days each week,
over long periods, in poorly ventilated rooms. The presence of hexane
in glues is reported to have caused similar incidents in Italy and
Iran (Capellini et al., 1968; Scrima & de Rosa, 1973; Ghazai, 1974).
In most cases, the extent of exposure to hexane was uncertain, either
because air concentrations were not measured or because excessive skin
contamination occurred; workers frequently handled the glues and
washed residues off the skin with hexane-containing solvents. In other
cases, air concentrations were measured. Hexane concentrations of
approximately 2340 mg/m3 (but at times up to 4680 mg/m3) were found
in a small, poorly ventilated workroom in which 3 women, who developed
polyneuropathy, had worked; again excessive skin contact took place
(Herskowitz et al, 1971; Ishii et al., 1972). Glue was also the source
of hexane concentrations of 1000-4000 mg/m3 found by Paulson &
Waylonis (1976) in the air of a work-place where 8 out of 50 employees
developed mild neuropathy.
The main clinical manifestation in these cases was polyneuropathy
of the glove-and-stocking type with sensations of numbness and cold.
This was accompanied by muscular weakness and headaches. Gradual
recovery was usual, when exposure ceased, with the exception of some
subjects who had severe muscular atrophy. Peripheral nerve biopsy
showed demyelination with relative preservation of the axon. Iida
et al. (1969) reported that in 44 cases there was a reasonable, but
not exact, correlation between clinical findings and electromyography
and nerve conduction velocity data.
Optic involvement was observed by Inoue et al. (1970). The
neuro-ophthalmological function was studied by Raitta et al. (1978) in
15 workers who had been exposed at work over periods of 5-21 years to
hexane concentrations of 1800-3600 mg/m3, with occasional levels of
10 800 mg/m3. Defective colour discrimination was found in 12 of the
workers and slight macular changes in 11 out of 15. The visually
evoked potentials and electroretinograms were interpreted by
Seppäläinen et al. (1979) as indicating cerebral dysfunction, probably
a conduction block in intracerebral axons.
Polyneuropathy attributable to the inhalation of hexane has also
been observed in "glue sniffers" -- subjects who inhale solvent
vapours to induce elation or other states of mind. Cases including
very rare cases following medicinal use, were described by Schwarz
(1933), Browning (1965), Karani (1966), Gonzales & Downey (1972),
Matsumura et al. (1972), Taher et al. (1974) and Korobkin et al.
(1975). Other incidents have been reported or reviewed by Shirabe et
al. (1974), and Poklis & Burkett (1977). While hexane appears to have
played a major role, a direct or synergistic action of other
components cannot be ruled out.
Some cases have been described that were thought to arise from
the long-term, continuous domestic use of kerosene stoves in poorly
ventilated rooms (Contamin et al., 1960). Under simulated conditions,
hexane concentrations of about 1440 mg/m3 were produced in
experimental rooms by Lièvre et al. (1967).
The possibility of the production of peripheral neuropathy by
components other than hexane, or the intensification of the activity
of hexane, is suggested by some clinical reports. Cargill (1972)
(cited by Gaultier et al., 1973) described peripheral neuritis in
workers using glue containing cyclohexane, gasoline "c", and methyl
ketone, and Franco et al. (1979) reported sensory and peripheral motor
conduction disturbances, where exposure to cyclohexane had occurred.
The condition was described by Gaultier et al. (1973) in subjects who
had been in prolonged contact with a glue solvent containing 80%
pentane, 5% hexane, and 14% heptane. Gasoline concentrations of
2250 mg/m3 were found in the workshop atmosphere. Atmospheric
gasoline concentrations of up to 5625 mg/m3 (mainly n-pentane,
n-hexane, and n-heptane) were found in workshops where workers had
developed polyneuropathy and had complained of insomnia, irritability,
and other non-specific CNS symptoms. White spirit has also been
implicated as a cause of peripheral neuropathy (Gaultier et al.,
1973). Heavy exposure to jet fuel vapours was reported in workers who
experienced dizziness, palpitations, nausea, and headaches, later
followed by signs and symptoms of polyneuropathy; higher vibration
thresholds, compared with unexposed controls were also found. (Knave
et al., 1976).
Other symptoms besides those of polyneuropathy have been
described in subjects exposed to hydrocarbon solvents. Sterner (1941)
reported headaches, nausea, mental depression, anorexia, inability to
concentrate and sustain activity, and slight anaemia, in workers
exposed to gasoline vapours (used to dilute spray paint) containing
5-10% aromatic hydrocarbons and producing concentrations of total
aromatic hydrocarbons in air of 300-800 ppma. Knave et al. (1978)
compared 30 workers, occupationally exposed to jet fuel, with
unexposed controls. The average period of exposure was 17 years and
the estimated TWA exposure was 300 mg/m3. Significant differences
were found between exposed and unexposed groups in the incidence and
prevalence of psychiatric symptoms, psychological test results,
especially attention and sensorimotor speed, and in
electroencephalograms. Exposure of car painters, over many years, to
low concentrations (31.8% of the Finnish TLV on average) of solvent
mixtures containing toluene, xylene, butyl acetate, and "white
spirit", was found to be associated with an increased incidence of
sleep disturbance, absentmindedness, falling asleep while watching the
television, and headaches. Lower peripheral nerve conduction
velocities, psychomotor impairment, and personality changes were more
common in exposed than in control subjects. Further studies are needed
to elucidate the significance of such findings (Hänninen et al., 1976;
Seppäläinen et al., 1978).
In summary, the most serious adverse neurological effect of
hydrocarbon solvents in man is the production of peripheral
neuropathy. Observations in man and studies on experimental animals
support the view that exposure to n-hexane is the principal cause.
However, the synergistic activity of other hydrocarbons is possible;
such a phenomenon has been reported following the sniffing of a glue
thinner containing both the neuropathic solvent, methyl- n-butyl
ketone, and the non-neuropathic solvent, methyl ethyl ketone
(Altenkirch et al., 1977). At present, the possibility that other
hydrocarbons also have some neurotoxic activity cannot be ruled out.
Peripheral neuropathy has occurred only in conditions of prolonged and
repeated exposure to high concentrations of hydro-carbon solvent
vapour in air; in many cases, there was also excessive skin contact.
Atmospheric exposure levels in excess of 2 g/m3 are usually
encountered where peripheral neuropathy is seen, but more studies are
required to clarify the situation, and until this is done, close
supervision is needed to ensure that the TLV for solvents is not
exceeded. Further studies are also necessary to determine the
significance of psychological disturbances and of neurophysiological
findings in workers exposed to lower levels of the hydrocarbons.
(c) Effects on reproductive functions
Examination of 408 female workers in petroleum refineries, who
had been subjected to long-term exposure to hydrocarbons, hydrogen
sulfide, and other products related to the treatment of sulfurous
crude, revealed disturbances in the menstrual function, mainly in the
form of hypomenstruation and pre-menstrual syndromes. According to
Suhanova & Melnikova (1974), such disturbance of the menstrual
function in female workers in refineries and in persons suffering from
chronic intoxication from petroleum products (36 persons in the age
bracket 30-39 years) is caused by the hypo-functioning of the ovaries.
a Since the composition is not given, it is not possible to
transform this concentration into mg/m3, the adopted SI unit.
Beskrovnaja et al. (1979) studied the gynaecological disease rate
in more than 5000 female operators in plants producing rubber articles
(petroleum solvent vapour concentration in the air of 250-350 mg/m3).
They observed disturbances in the menstrual cycle in workers with more
than 5 years' service and a high frequency of metrorrhagia. As the
period of service increased, a reduction in the frequency of
miscarriages was noticed, which was interpreted by the authors as
possible adaptation. A disturbance of the ovarian function was noted
in 24.4% of the workers examined, mostly in the form of a functional
deficiency of corpus luteum.
Investigations of vaginal smears of 184 female gluers in the
rubber industry in the age bracket 18-38 years revealed a disturbance
of the ovarian function with reduced estrogen stimulation in 21.7% of
the women (10.4% in the control group). This figure was related to the
period of service. After 10 years service, the percentage was twice as
high as after 5 years' service.
Women who had been in contact with petroleum solvents were found
to have a reduced estrogen level in the blood (22.2 µg/day compared
with 29.6 µg/day in the control group). Essentially, no changes were
observed in the excretion of the follicle-stimulating and luteinizing
hormone pregnanediol. The authors of this study assumed that the
sensitivity of the ovaries towards the stimulating effect of the
gonadotrophins was reduced (Hrustaleva et al., 1979).
Novikov et. al. (1979) studied lactation in 332 nursing mothers
288 of whom worked in the rubber industry (vulcanizers, pressers,
gluers). The concentration of petroleum solvents (the physico-chemical
properties of which are not described) in the air of the operating
premises was predominantly 300 mg/m3. Hypolactation, found in 23.8%
of the women compared with 6.7% in the control group was related to
length of service. Hydrocarbon solvents were found in the milk of all
the persons examined (71) in concentrations of 0.50 ± 0.05 mg to
0.60 ± 0.09 mg/litre. The serotonin content of the blood of these
women was significantly lower than in the control group. It is assumed
that hypolactation was the result of the effect of solvents on the
lactation control mechanism via the hypothalamus and the
serotoninergic system.
(d) Effects on the skin
Skin can also be affected by exposure to solvents. In a study on
skin conditions and other diseases in 54 gasoline and diesel station
workers, skin conditions related to exposure to gasoline and diesel
fuel were minimal and did not interfere with capacity to work. Only
dryness, chapping, and reddening of the skin were found. The dark skin
of Indonesians seemed to be more resistant to these effects. The
gasoline vapour in the working environment caused some non-specific
symptoms in addition to those resulting from heavy out-door work
(Suma'mur & Susianti Wenas, 1979).
3.7.2.2 General population exposure
Data are not available concerning the exposure of the general
population to petroleum solvents with the exception of that of
"sniffers" and addicts already mentioned in section 3.7.2.1.
3.7.3 Clinical studies
It should be noted that literature surveys of clinical studies
and clinical effects have been published by Gerarde (1960), Browning
(1965), and Levina (1976).
3.7.3.1 Effects of dermal exposure
In general, petroleum solvents have a defatting action on the
skin and, on repeated contact, cause injury to the horny layer
(section 3.7.1.1). This makes the skin more susceptible to other
irritants, sensitizing agents, and bacteria. It may also result in
progressive dermatitis, characterized successively by dryness,
redness, chapping and scaling, which could lead, by sensitization, to
eczema. These stages of dermatitis may be seen in workers in garages
or automobile repair shops, who wash their hands with solvents, petrol
or kerosene (Tagami & Ogino 1973). The more aromatic solvents, in
particular, can cause a significant degree of primary skin irritation.
The defatting action and primary irritation caused by petroleum
solvents decrease, the higher the boiling range section 3.6.1).
Cases in which gasoline or kerosene remains in contact with the
skin for prolonged periods mainly occur in children or in unconscious
accident-cases, when clothing has become soaked with the solvent. In
such cases, the lesions start with a burning sensation and erythema,
followed by the formation of small or large vesicles, blisters, or
even extensive epidermolysis. The vesicles and blisters may become
mucopurulent in a few days. The acute picture is that of a chemical
burn, (Helbling, 1950; Aidin, 1958; Ainsworth, 1960; Stewart, 1960;
Browning, 1965; Hunter, 1968; Tagami & Ogino 1973).
Gasoline may occasionally be absorbed through the skin in toxic
quantities if large areas of skin such as the hands and forearms are
regularly exposed (Hayhurst, 1936), or in cases of extensive
epidermolysis in contact with gasoline-soaked clothing. However
inhalation of vapour plays a significant additional role in all these
cases and generally is the main route of absorption (Browning, 1965).
3.7.3.2 Effects of inhalation
The acute effect of massive overexposure to gasoline vapour is
mainly narcosis with loss of consciousness and possibly convulsions,
which may be fatal (Browning, 1965). Octane causes rapid and deep
narcosis, pentane and hexane are less powerful narcotics, but they and
heptane exert a paralytic effect on the central nervous system and its
respiratory centre.
In more gradual overexposure, the symptoms just described may be
preceded by eye irritation, irritation of the respiratory tract,
dizziness, headache, and a sense of drunkenness.
A gasoline concentration of 9000 mg/m3 can be breathed without
significant ill-effects by most people but susceptible subjects may
show symptoms after exposure to 1350-2250 mg/m3 (Ainsworth, 1960). At
31 500 mg/m3, dizziness and symptoms of drunkenness may appear.
Exposures in excess of 45 000 mg/m3 soon become intolerable and may
rapidly prove fatal (Machle, 1941, Aidin, 1958). Lower and higher
values have been quoted, obviously depending on the composition of the
gasoline (Browning, 1965). The margin of safety between narcosis and
respiratory arrest is very narrow in exposures to high concentrations
of gasoline (Wang, 1961).
Absorption of gasoline vapour by inhalation may be very rapid if
the concentration is high, especially with the lower members of the
paraffinic range, and symptoms can appear within a few minutes.
Excretion, probably of unchanged vapours, takes place mainly via the
lungs (Browning, 1965).
Acute occupational poisoning by gasoline vapour is mostly caused
by entering unpurged gasoline tanks or other premises where high
concentrations of gasoline may have accumulated. Exposure to high
concentrations may also occur in car accidents, when victims are
trapped and/or unconscious.
Histopathological changes found in subjects who have died after
exposure to high concentrations of gasoline vapour include: hyperaemia
and petechial haemorrhages in the lungs, and, sometimes, necrosis of
the alveolar walls. There may be haemorrhages or effusions in internal
organs and serous cavities (Helbling, 1950; Ainsworth, 1960; Browning,
1965). Liver and kidney may show fatty degeneration. Hyperaemia and
oedema of the brain are common in this condition and myelin swelling
may occur (Machle, 1941).
Long-term exposure to low (unspecified) vapour concentrations may
cause nonspecific symptoms of the nervous system and digestive tract
(Zielhuis, 1961; Browning, 1965; Muhametova & Podrez, 1975; Sehtman et
al., 1979), including changes in liver function and in the visual
organ. In women, the reproductive organs may be affected (Muhametova &
Podrez, 1975; Sehtman et al., 1979). Neurological disturbances, as
described in section 3.7.2.1 may develop (Hayhurst, 1936; Machle,
1941; Browning, 1965). Browning (1965) considered that significant
changes observed in the blood count were caused by the presence of
benzene in the solvent.
This chronic form of toxicity may be found in small,
insufficiently ventilated workshops where solvents, or products
containing them, are handled in an unsatisfactory way (section
3.7.2.1) and when these materials are used in substantial amounts in
enclosed spaces, particularly for cleaning or degreasing (Anon.,
1974).
3.7.3.3 Effects of ingestion
Accidental ingestion of petroleum distillates in the range of
petroleum solvents is an important cause of poisoning in children
(Waring, 1933; Nunn & Martin, 1934; Lesser et al., 1943; Carithers,
1955; Daeschner et al., 1957; Gerarde, 1959, 1963). Most cases,
however, are caused by gasoline and kerosene and fewer by petroleum
solvents. The symptomatology is the same in all cases.
Coughing, choking, and gagging are often noted at the time of
ingestion of these substances. Respiratory embarrassment may be
present early, indicating that aspiration has taken place. Epigastric
discomfort may develop, followed by vomiting with a further risk of
aspiration. Aspiration by one mechanism or another is reported to
occur in up to 95% of cases in children, but this depends on the
situation and on the type (boiling-range) of the solvent involved.
Aspiration is less common in adults, but may occur when trying to
siphon gasoline from a tank. Small amounts of kerosene of the order of
1-2 ml, if aspirated, can cause severe and even fatal pulmonary
changes. On the other hand, if aspiration does not occur, much larger
quantities can be tolerated (Daeschner et al., 1957; Hensen, 1959;
Browning, 1965). Browning (1965) quotes a value of 7.5 g/kg body
weight, but this may depend on the type of solvent. Children appear to
be more susceptible to the toxic effects than adults (Siwe, 1932). In
cases where aspiration does not take place, and especially with the
lower-boiling solvents, central nervous system symptoms may develop
such as lethargy, convulsions, and coma. With smaller doses, the
symptoms include vertigo, headache, and signs of drunkenness. Nausea,
vomiting, and diarrhoea may occur and the stools may be blood-tainted.
In uncomplicated cases, the gastrointestinal symptoms will
disappear within 48 h. Pulmonary symptomatology will not develop, if
aspiration has not occurred and if there was no massive exposure to
vapours.
The syndrome of acute chemical aspiration pneumonitis will be
described in a wider context in section 4.8. Human experience is in
accordance with the results of experimental animal studies (section
3.6.1). Chemical pneumonitis with pulmonary oedema and haemorrhagic
frothy sputum may develop extremely rapidly following aspiration of
petroleum solvents. Roentgenographic changes may be seen within a few
hours, especially at the lung bases. Later, bacterial pneumonia can
complicate the situation (Daeschner et al., 1957; Gerarde, 1963).
The prognosis of aspiration of hydrocarbon solvents appears to
have improved with better treatment in the course of time. Siwe (1932)
reported that 50% of the cases he reported were fatal, Nunn & Martin
(1934) reported 28% mortality following the ingestion of gasoline and
9.2% following kerosene-ingestion, and Blattner (1951) reported that
death resulted in 10-14% of cases of kerosene poisoning. Recently, 3
cases have been described, where exposure to diesel oil seemed to
cause acute renal failure with oliguria (Reidenberg et al., 1964;
Barrientos et al., 1977; Crisp et al., 1979). However, in 2 cases,
absorption seemed to have been mainly through the skin (section
3.5.1).
4. LUBRICATING BASE OILS AND RELATED OILS,
GREASES, AND WAXES
4.1 Properties and Analytical Methods
4.1.1 Chemical and physical properties
Base oils are a limited group of petroleum products in the
boiling-range of 300-700°C, normally derived from the high-vacuum
distillation of the residues of the crude-distilling process. These
oils undergo a further refining process before being used.
Lubricating oils, metal-working oils, and related products are
produced by blending base oils in order to obtain the desired physical
properties. Chemical additives are frequently added, usually in small
amounts (a few mg to a few g/kg) to improve the performance of the
lubricant. In some special cases, higher concentrations are necessary.
Additives belong to the following general categories: viscosity-index
improver, emulsifiers, wetting agents, antioxidants, dispersants,
antiwear additives, extreme pressure additives, rust-inhibitors,
antifoam agents, pourpoint depressants, and germicides.
Greases based on mineral oil consist of solid or semisolid
dispersions of metallic soaps and other thickeners in a mineral oil
base.
Petroleum waxes are crystalline solids at normal temperature.
"Slack wax", a soft, impure paraffin-wax is obtained in the
manufacture of lubricating base oils from paraffinic crude oils by a
dewaxing process. Slack wax can be refined into 2 types of commercial
wax: paraffin wax and microcrystalline wax.
Base oils are very complex mixtures of hundreds to thousands of
different hydrocarbons, containing straight-chain and branched
paraffins, cycloparaffins, naphthenic, aromatic, and polynuclear
aromatic hydrocarbons in the range of C-17 and higher. The separate
molecules are so large, that any molecule may, for instance, contain
one or more aromatic rings with one or more long side-chains.
The actual composition depends on the source of the crude oil,
from which the product is derived, and the manufacturing and treating
processes used. They range from thin, easily flowing "spindle oils" to
thick "cylinder oils". A limited number of base oils are used for
blending to obtain the commercial products. As the relation between
viscosity and temperature is an important factor in this field, base
oil grades are characterized by their viscosity and viscosity index
(VI). The higher the viscosity index, the less the change in viscosity
with temperature.
Low viscosity index (LVI) oils are used whenever the viscosity
temperature characteristics and oxidation stability are of less
importance. In general, they are derived from naphthenic oils and
undergo treatment with sulfuric acid and clay or are given a
hydro-finishing treatment.
Medium viscosity index (MVI) oils, can be used as a base for
general-purpose lubricants. They can be derived from naphthenic (MVIN)
or paraffinic (MVIP) feedstocks. MVIP has to be dewaxed and can also
be solvent refined, acid- and clay-treated or hydro-treated.
High viscosity index (HVI) oils, are prepared by both solvent
refining and dewaxing of paraffinic feedstocks. They are used for
gasoline and diesel engine oils and for turbine lubricants, because
they are, in general, more oxidation-stable than other base oils and
have appropriate viscosity/temperature characteristics.
White oils are generally produced by more drastic refining of MVI
oils, in order to remove unsaturated compounds, aromatic compounds,
and other constituents that influence colour, odour, taste, and
acceptability as food-grade material. Solvent extraction followed by
repeated treatment with oleum and alkali is used. Hydrogenation is
another means of producing such oils.
Medicinal oil is the highest purified grade, which complies with
the requirements of the various national pharmacopoeias and
regulations on food-grade material. As liquid paraffin, the same grade
is used as a lubricant for food-handling machines and as an ingredient
in pharmaceutical preparations and cosmetics.
Technical white oils, less rigidly purified than medicinal oil,
are non-carcinogenic oils that can be used for the lubrication of
textile machinery, spinning mules, etc., but are mainly used in the
cosmetic industry in the manufacture of hair-oils, creams, etc.
Aromatic extracts, which are obtained in the solvent-refining
process, have already been discussed in section 3.1.1.
Petroleum waxes consist essentially of high relative molecular
mass paraffinic hydrocarbons with approximately 20-40 carbon atoms per
molecule. Paraffin waxes consist mainly of normal paraffins together
with some iso and cycloparaffins. They are macrocrystalline with a
melting-point of 43-71°C.
Microcrystalline waxes on the other hand, consist mainly of iso
and cycloparaffins with some alkylated aromatic hydrocarbons. They are
mostly soft materials, but may sometimes be a hard brittle solid.
When highly purified, the colour may be white, however, it is
usually yellow or amber, sometimes even black. The melting-point is
60-90°C. The paraffin oil content ranges from 5 to 50 g/kg.
Petrolatum is also known as petroleum jelly and is a
micro-crystalline wax with a high oil content.
4.1.1.1 Purity of Product
As these oils and waxes are complex mixtures, the nature and
proportion of potentially undesirable "impurities" depends to a large
extent on the definition of the term and on the degree of refining. In
the range from the highest refined oil -- for example, white medicinal
oil -- to the least refined oil, "impurities" could include
polynuclear aromatic hydrocarbons and unsaturated hydrocarbon
compounds.
It is clear that highly purified products can be obtained at the
cost of extra refining and treating processes. Paraffin waxes are an
example of progressive refining: fully refined paraffin wax is a white
solid material containing an oil concentration of less than 5 g/kg. It
is odourless and tasteless and has a melting-point of 50-70°C. Candle
waxes range from fully refined paraffin wax to less refined waxes
containing oil levels of up to 15 g/kg. This type of wax is not
completely colourless and odourless. Scale wax and match wax contain
an oil residue of up to 30 g/kg.
4.1.2 Methods of sampling and analysis
See section 2.1.2.
4.2 Sources of Environmental Pollution
4.2.1 Natural occurrence
Base oils occur in nature only as components of the crude oils
from which they are derived.
4.2.2 Man-made sources
4.2.2.1 Production
As mentioned earlier, lubricating base oils are a group of
petroleum distillates and residues in the boiling-range 300-700°C,
derived by high-vacuum distillation of the residues obtained in crude
oil distillation (section 4.1.1). In order to obtain base oils with
the qualities and specifications needed for various applications,
further refining and treatment of the various distillate fractions is
needed. The main processes used are:
(a) Solvent extraction
This is a process by which aromatic hydrocarbons can be extracted
from oil fractions, thus obtaining a low aromatic or aromatic-free
raffinate, and a high aromatic extract. Liquid sulfur dioxide,
sulfolane, benzene, phenol, or furfural can be used as solvents for
this purpose. The raffinate can be used for producing a lubricating
base oil, the extract can be used as a solvent (see under petroleum
solvents).
(b) Dewaxing process
In this process, "slack wax" is separated (crystallized) from
base oil fractions obtained from paraffinic crude oil residues. This
is done by cooling, followed by filter-pressing. The separation of wax
and oil under chilled conditions is facilitated by the addition of
solvent. The resulting wax may still have an oil content of up to
100-400 g/kg.
(c) Acid treatment
The oil is mixed with 98% sulfuric acid and the acid sludge is
centrifuged off. This is usually followed by clay treatment in which
the oil is mixed with absorbent clay, followed by filtration. This
neutralizes the oil and improves colour, odour, and stability.
(d) Hydrofinishing
This is a catalytic hydrogen treatment at elevated temperature
and pressure. Improved stability and colour of the oil is obtained by
the hydrogenation of unstable compounds, which removes sulfur and
saturated olefins, diolefins, and aromatic components.
As the definition and classification of lubricating base oils are
not clear, it is difficult to give precise production data for this
group of products. The best current estimate is 35-38 million tonnes
per year. Though there was a decrease in the annual production after
1973, lubricant production has subsequently increased, but at a lower
rate.
4.2.2.2 Uses
In general, the main functions of lubricating oils and
metal-working oils are: to reduce friction, to remove generated heat,
to remove debris from the contact area, and to protect against
corrosion. In addition, mineral oils are used as hydraulic media in a
wide variety of applications. Very general remarks have already been
made concerning the composition of, and the various additives in,
lubrication oils, metal-working oils, and greases. The following is a
list of the most important categories of products on the market, from
which it is clear that the great majority are handled in industrial
situations, and that the general population has regular contact with
only a few such products:
(a) Industrial lubricating oils, which can vary from thin spindle-oil
to the very viscous oils used in steam engines;
(b) Lubricants for internal combustion engines of various types;
(c) Crankcase, compressor, gear, and turbine oils;
(d) Greases for bearings and other purposes;
(e) Hydraulic, transformer, insulating, heat-transmission oils;
(f) Cooling, quenching, anticorrosion, and mould oils;
(g) Metal-working oils: cutting, grinding, rolling, drilling,
drawing-oil, a multitude of products for specific purposes. They
consist in general of complex mixtures with various additives,
for use in the neat state and also as water extendible fluids or
emulsions;
(h) Textile oils: spindle, hatching, technical white lubricating oil;
(i) Process oils: used in printing-inks, as rubber extenders, and as
technical white oils in cosmetics, etc;
(j) Waxes of various grades and purity;
(k) Medicinal white oil used for medicinal and food-grade
applications.
The manufacturing, treating, and purification processes used in
the preparation of the base oils vary according to the future
application of the commercial product.
Many of the products can undergo considerable changes during use.
This should be borne in mind in extrapolating from used oils to the
original product in the case of adverse health effects, e.g., in the
case of skin sensitization. The following are a few examples of
changes that may occur:
(a) During use, the chemical and physical characteristics of
lubricating oils change, mainly because of contamination, oxidation,
and polymerization. Metal particles, airborne dust, water, and, in the
case of internal combustion engine oils, small quantities of fuel,
acids, and soot, are the main contaminants. All this can result in the
formation of sludge.
(b) Metal-working oils become contaminated in use with a wide
variety of foreign matter, in particular metal particles (and ions).
Water-based metal-working emulsions can also be affected by bacteria,
yeasts, and fungi. Oxidation and heat cracking may occur at the
application point, causing smoke, steam, and oil mist to be emitted
into the working environment. Nitrite may be added to most water-based
cutting oils, thus these sometimes contain carcinogenic nitrosamines
(Zingmark & Rappe, 1977).
Cutting oils, lubricating oils, and greases can also contain
diethanolamine, which may react with nitrites and nitrogen oxides in
the air to form nitrosamines.
(c) Quenching oils are used for hardening steel. Oxidation and
cracking of the oil can occur because of the high temperatures
involved. The amount of benzo (alpha) pyrene in lubricating oils
increases considerably when they are exposed to heat during use, as in
motor-car engines, metal quenching, and other processes (Thony et al.,
1975).
4.2.2.3 Disposal of waste
Without a proper method for the disposal of used lubricating and
other oils, severe environmental contamination and hazards may occur.
There is a vast literature on methods for the disposal, reuse,
and recovery of industrial lubricants and much research is being done
in this field. It would be out of context in this document to go into
a detailed discussion of the various aspects. For this, the reader is
referred to Concawe Report 9/73 (Concawe Task Force, 1973) and API
publication No. 4036 (API, 1969), which contain discussions on the
situations in Western Europe and the USA, respectively, as well as on
more general aspects.
At present, the main ways of disposal are:
(a) dumping in sewage systems, waterways, etc; this is illegal in
many countries and ecologically undesirable;
(b) dumping into the ground, in garbage dumps, in dry wells, if
approved by local authorities;
(c) using as road oils, etc;
(d) controlled incineration or burning for heat value;
(e) re-refining and other recycling methods.
4.3 Environmental Exposure Levels
Specific data are not available concerning levels of this group
of products in air, water, food, or other environmental media, levels
of possible contamination, and uptake by man.
Depending on occupation and on the hygiene precautions adopted,
skin exposure in workers can vary from minimal to very high. No
systematic measurements have been carried out in this field. Exposure
to oil mists occurs in certain manufacturing processes and
applications, such as the operation of automatic lathes. This type of
exposure has been measured by various authors and will be discussed in
section 4.7.1.2. Secondary oral intake of unknown quantities of
mineral oil can take place under these conditions.
When "sulphofrezol" (approximate composition: 50-60% "goudron"
(?flux oil), 40-50% spindle oil distillate, and 1.7% sulfur) was used
as a lubricating and cooling liquid on metal-working lathes, the air
of the operating premises was found to contain an oily aerosol in
concentrations of 1-50 mg/m3, hydrocarbons at 26- 150 mg/m3, carbon
monoxide at 8-12 mg/m3, formaldehyde at 0.05-1.2 mg/m3, sulfur
dioxide at 2-20 mg/ms, and benzo(alpha)pyrene at 0.01-0.2 mg/m3.
When multicomponent lubricating-cooling liquids containing chlorine
and fluorine compounds are used as additives, hydrogen chloride and
other substances may also find their way into the workroom air. It is
therefore essential to provide the metal-working lathes with
vapour-extracting facilities (Medved' et al., 1976).
4.4 Environmental Distribution and Transformation
Data on lubricating base oils concerned with distribution among
media, environmental transformation and degradation, interaction with
physical, chemical, or biological factors, and bioconcentration are
not available. However, as mentioned in section 3.4, there is a lot of
information concerning the microbial degradation of individual
petroleum hydrocarbons. For example, it has been shown that certain
polynuclear aromatic hydrocarbons in lubricating oils can be oxidized
by bacteria; benzo(alpha)pyrene and benzo(alpha)anthracene have been
shown to be oxidized to cis-dihydrodiols. The oxidation is not thought
to reach the stage of carcinogenic activation found in mammals, as
this would result in a trans-configuration (Gibson et al., 1975).
4.5 Metabolism
Because of the complex and variable composition of compounds in
this group, only a few factual data relating to their metabolism can
be given.
The skin barrier is permeable only to hydrocarbons of a certain
relative molecular mass and structure. For paraffinic substances this
appears to be up to 20 carbon atoms. However, from their studies on
guineapigs, Hoekstra & Phillips (1963) reported that, because aromatic
compounds have a more compact structure, compounds in this group with
a higher number of carbon atoms might still pass through the skin
barrier.
In studies on mice, rats, hamsters, rabbits, and dogs, mineral
oil droplets were phagocytosed in the lungs and clinical observations
in man have shown that deposition takes place in the hilar nodes, from
where they may be transported to other organs such as the spleen
(Sante, 1949; Proudfit et al., 1950; Shoshkes et al., 1950; Wagner et
al., 1964). Almost all (95-99%) of ingested food-grade mineral oil
leaves the body unchanged in the faeces, 1-5% being absorbed as such
via the intestinal mucosa. Phagocytosis may play a part in this. The
ingested part of the oil is transported throughout the body via the
lymphatics and the bloodstream. Storage takes place in adipose tissue
or in the fat in organs. After excessive exposure, mineral oil
droplets have been identified in mesenteric and portal lymph nodes,
and also in liver, spleen, and adipose tissue in man (Stryker, 1941;
Ebert et al., 1966; Boitnott & Margolis, 1966b).
Data relating to food-grade mineral oil have been reviewed in WHO
(1974). Very few data are available on the biotransformation and rate
of elimination of this group of products but data concerning some of
the components are referred to in section 3.5.
Injections of 0.5 ml of various mineral oils were made in the
peritoneal cavity of mice and the oil recovered after 7-24 days (Twort
et al., 1937). Both the refractive index and density of the oil had
decreased proportionally, probably as the result of chemical
transformation in the body. Treatment of mineral oil with sulfuric
acid and clay, or solvent extraction had a similar effect. The solvent
extracts, however, showed increased refractive indices and densities.
The authors suggested that both parameters might be useful indicators
of the carcinogenicity of the mineral oil.
4.6 Effects on Experimental Animals
4.6.1 Short-term exposure
Data are not available concerning the LD50s of base oils but the
oral and dermal LD50 data available for commercial lubricating oils
indicate that, in general, these products are only moderately or
slightly toxic.
Acute no-observed-adverse-effect levels have not been determined.
Oral studies with food-grade mineral oils show that these are
laxatives. The same applies to non-food-grade commercial mineral oils,
though it is impossible to predict whether in a certain case a toxic
or laxative effect will prevail.
4.6.1.1 Effects of dermal exposure
More factual information is available on the acute or short-term
effects of mineral base oils on the skin. In a very thorough study
Hoekstra & Phillips (1963) examined the effects of mineral oils and
their fractions on the skin of guineapigs. It appeared that very
short-chain paraffins had a mainly defatting action on the skin and
that the effects of longer-chain and aromatic hydrocarbons were
closely related to the permeability of the skin to these compounds. We
can do no better than cite the summary of this work:
"A number of light mineral oils applied topically to the skin of
guinea-pigs caused a marked epidermal hypertrophy, hyperplasia,
hyperkeratosis, and subsequent depilation. This dermatoxic effect
could not be closely related to source of the crude oil or its
viscosity, degree of refinement, or the acid used in refinement.
" n-Paraffin, isoparaffin, naphthene and aromatic fractions
separated from light mineral oil each produced the dermatoxic effect
as did highly purified individual paraffins from C-12 to C-18. Oleic
acid caused only a slight dermatoxic effect. Fractional distillation
of an aromatic-free mineral oil demonstrated that while all
lower-boiling fractions were dermatoxic, a distillation range was
reached at which the fractions were innocuous to the skin. This was
also true for n-paraffin-, isoparaffin-, monocyclic naphthene-, and
polycyclic naphthene-rich fractions derived from a mineral oil.
Fractional distillation of the aromatic hydrocarbons from mineral oil
representing the same distillation range did not yield fractions
without skin-damaging effect. Crude estimates of the molecular size of
the n-paraffins, isoparaffins, monocyclic naphthenes, and polycyclic
naphthenes by comparison with the boiling-temperatures of known,
homologous series of hydrocarbons indicated that the maximum skin
damage resulted from hydrocarbons of about 14 to 19 carbon atoms. The
transition point to non-dermatoxic hydrocarbons occurred at about
21-23 carbon atoms. This was verified with purified n-paraffins.
Variations may exist for the different types of hydrocarbons.
"Simultaneous application of the innocuous "higher-boiling"
mineral oil fractions with dermatoxic "lower-boiling" fractions or
with hexadecane greatly reduced or eliminated the skin-damaging
effects. The alleviation of the skin damage from aromatic fractions by
simultaneous application of the "higher-boiling", non-aromatic
fractions was much less pronounced.
"The hyperkeratotic reaction of the skin to petroleum
hydrocarbons appears to he a very general response to lipid solvents
and is not related to any specific reactive group or type of
structure. Molecular size seems to be very important in determining
the dermatoxic properties of hydrocarbons, the larger molecules being
innocuous. Under the conditions of the experiments the very
low-molecular weight hydrocarbons also had little or no dermatoxic
properties, presumably because of their volatility. It is postulated
that the skin barrier is permeable only to hydrocarbons of a certain
maximal effective size and that penetration into or through the
barrier is essential for the initiation of the hyperkeratotic
response. The alleviation of the dermatoxic effect of hydrocarbons by
admixture with innocuous, higher molecular weight hydrocarbons, is
likewise explained by a reduction in penetration of the dermatoxic
component through the Skin barrier" (Hoekstra & Phillips, 1963)
4.6.2 Long-term exposure
Most long-term studies with mineral oils have been concerned with
the carcinogenicity of the compounds. Data concerning mutagenicity,
embryotoxicity, and teratogenicity are lacking.
4.6.2.1 Carcinogenic effects
The carcinogenic activity of certain polynuclear aromatic
hydrocarbons is attributed to electronic structural features of the
molecule. The various theories on these mechanisms are discussed in
detail by Arcos & Argus (1974), Bergel (1974), and Jerina & Daly
(1974). Carcinogenic polynuclear aromatic hydrocarbons have regions of
high-electron density in their molecules, the so-called K-regions that
are readily epoxidized by tissue mixed-function oxidases and more
specifically by the enzyme aryl hydrocarbon hydroxylase.
Polynuclear aromatic hydrocarbons are in fact precarcinogens,
which, though harmless in themselves, are metabolized in the body, by
the combined action of mixed function oxidase and epoxide hydratase
(4.2.1.63), to biologically reactive intermediates that constitute the
ultimate carcinogens (Sims et al., 1974). The enzymes responsible are
active in the liver, and in other organs such as the skin, lungs, and
intestines.
Whether, in fact this metabolism leads to detoxication or to
"lethal synthesis" may differ with various polynuclear aromatic
hydrocarbons and may depend on many other factors (Anon., 1975). The
short-lived epoxides resulting from the metabolism of at least some of
the polynuclear aromatic hydrocarbons are probably the activated
metabolites with the real carcinogenic effects.
Evidence is now accumulating that it is the 7,8 dihydroxy-
benzpyrene - 9,10 oxide of 3,4 benzo(alpha)pyrene that is the
ultimate carcinogen and, consequently, this has now superseded the
K-region epoxide in biological importance with regard to
carcinogenicity (Yang et al., 1976; Thakker et al., 1977; Yagi et al.,
1977; Koreeda et al., 1978). The enzyme aryl hydrocarbon monooxygenase
(according to recent international rules of nomenclature) in the case
of 3,4 benzo(alpha)pyrene, is referred to as benzopyrene
3-monooxy-genase (EC 1.14.14.2) (de Pierre & Ernster, 1978).
There are indications that the tissue concentrations of the
enzyme aryl hydrocarbon hydroxylase vary considerably with the age,
sex, species, strain, and environment of the animal (Nebert & Gelboin,
1969). Outside agents, such as polynuclear aromatic hydrocarbons, can
induce the activity of this enzyme (Wattenberg, 1972). It seems that
the inducibility depends on genetic factors in both mice and human
subjects (Nebert et al., 1972; Kellerman et al., 1973). In mice, it
seems to depend on a single dominant autosomal gene (Nebert et al,
1972).
4.6.2.2 Effects of dermal exposure and subcutaneous
administration
Leitch (1922) repeatedly painted crude shale oil on the skin of
mice. He obtained skin tumours in a substantial percentage of the
mice. Similar tests were carried out by Twort & Ing (1928) and Twort &
Twort (1931) using a range of crude oil as well as more refined
products of both shale oil and petroleum oil. They found that
petroleum oils had a lower and sometimes negligible carcinogenic
activity compared with shale oils. Naphthenic oils were less active
than oils with a high aromatic content. The more potent fractions
could be extracted with solvents from the oils.
Acid treatment of the oils decreased the carcinogenic activity
and heavier-grade oils were less potent than spindle oils.
Oils with a boiling-point above 370°C, derived from fluid
catalytic cracking, appeared to be carcinogenic to the skin of mice.
The most potent fraction distilled between 430 and 550°C, and the
carcinogenic activity was contained in the aromatic components of
these oils.
Woodhouse (1934) compared the carcinogenicity of sulfur-
dioxide-solvent-extracts of spindle distillates, derived from
crude oils from different parts of the world, with that of the
unrefined spindle oils and coal-tar, using a mouse-skin-painting
technique. He found that in all cases, the carcinogenic activity,
which was more or less characterized by its UV-fluorescence spectrum,
was concentrated in the solvent extract. Potency varied according to
the source of the crude oil. The potency of the most active extract,
however, was lower than the activity of coal-tar. By thorough
sulfur-dioxide-solvent refining, the carcinogenicity of the spindle
oil was almost completely removed. The same author (Woodhouse, 1950)
reported studies on the carcinogenic activity of various petroleum
fractions and extracts. Results were in line with his previous
findings and with those of other workers. Three white oils including
medicinal liquid paraffin gave negative results.
More recent studies have been aimed at identifying the
carcinogenic substances.
Dilution of carcinogenic oils with non-carcinogenic oils more
than proportionally reduced the carcinogenic activity, which at times
disappeared completely. Mild hydrogenation or treatment with active
absorbents reduced, but did not abolish carcinogenicity. Mild acid
treatment, on the other hand, did not have such an effect (Smith et
al., 1951). Eight samples of unrefined "slack waxes" obtained from
pressing operations -- still containing 12-29% of oil -- were painted
3 times a week on the skin of white mice (Smith et al., 1951).
Aromatic extracts, obtained by the solvent refining of these waxes,
were examined in the same way. After skin-painting for almost the life
span of the animals, some benign rumours were found with all samples
and in a few cases, skin carcinomas developed. The aromatic extracts,
however, were much more active. The authors concluded that the
aromatic components which were removed by further refining of the
waxes, were the cause of the carcinogenic activity of "slack waxes".
Fischer et al. (1951) described methods for the determination of
polynuclear aromatic hydrocarbons: the ultraviolet absorption method,
the caffein extraction method, the chromatographic ref-ractometric
method, and the maleic anhydride method. Levels, determined by any of
these methods, were fairly well correlated with the tumorigenic
potency of these oils as established in animal experiments. These
analytical techniques are relatively simple and much more rapid than
long-term animal experiments. They are considered to have some
predictive value. A further development is the DMSO
extraction/refractive index method.
The correlation between the analytical data and the carcinogenic
activity in skin-painting studies on mice is now under investigation
(Shell Toxicology Laboratory -- private communication) and encouraging
results have been obtained with the lower- and higher- range
polynuclear hydrocarbons. The compounds in the middle range are still
under investigation.
Auld (1940) considered that it was not the general
UV-fluorescence that was characteristic of carcinogenicity but the
pattern of the spectrum and its classification. However, in a report
by the Analytical Subcommittee of the Institute of Petroleum
(Catchpole et al., 1971a), it was concluded that, at the time of the
report, there was no suitable, easy and quick method for determining
either the total polynuclear aromatic hydrocarbons or individual
carcinogens in them.
The tumorigenic activity of various concentrations of a large
number of pure aromatic hydrocarbons was examined in different strains
and species of animals by Hartwell (1951), who found that the potent
substances contained 4,5, or 6 condensed aromatic rings with
relative-molecular masses ranging from 230 to 320.
Cook et al. (1958) tried to isolate individual carcinogens from 3
Kuwait crude oils by fractional distillation and various treatment
processes. They tested the fractions obtained on the skin of mice and
rabbits and found that more of the carcinogenic activity was contained
in the fraction distilling between 350 and 400°C and that this
activity was associated with the aromatic constituents. Solvent
extraction with aqueous acetone or furfural removed the activity,
which passed into the extract (20% of the original fraction). Several
polynuclear aromatic hydrocarbons such as di-, tri-, and tetramethyl-
phenanthrenes, tetramethylfluorene, 1-methylpyrene, 1,2-benzofluorene
and 8-methyl-1,2-benzofluorene could be identified in these extracts
as well as some complex organic sulfur compounds such as
dibenzothiophen derivatives and polycyclic thiophens, a number of
which are known to have carcinogenic activity. Pentamethyl-carbazole
was identified as an example of a complex organic nitrogen compound.
Similar findings were obtained by Bogovsky et al. (1960) on
fractionating Estonian shale oil.
Approximately 100 fractions were separated from a catalytically
cracked oil by various techniques (Tye et al., 1966). Selected
fractions were chemically analysed and assayed for carcinogenicity by
repeated application to the skin of C3H mice. In the highest-boiling
fractions (438-455°C), the major carcinogens were 4 and 5 ringed
polynuclear aromatic hydrocarbons, benzo(alpha)pyrene as a
characteristic carcinogen was present at a concentration of 0.4%. In
the intermediate boiling range (404-438°C), unsubstituted and
methyl-substituted 4-ringed polynuclear aromatic hydrocarbons formed
the major carcinogens, benz (a)anthracene and its alkyl homologues
being present at a concentration of 0.4%. In the low-boiling fraction
(339-404°C), assorted smaller molecules including alkyl-3-ringed
polynuclear aromatic hydrocarbons were present.
Benzo (c)phenanthrenes, the most dominant carcinogens in this
fraction, were present at a concentration of 0.01%. The carcinogenic
potency in the bioassay was found in the intermediate- and
high-boiling fraction (boiling-range 404-455°C); fractions boiling at
under 349°C did not appear to be carcinogenic.
Generally, where the alkyl-substituted 4-6 condensed-ring
polynuclear aromatic hydrocarbons were concerned, the longer the
side-chain, the lower the carcinogenic activity (Auld, 1950).
These findings were confirmed by the United Kingdom Medical
Research Council (1968), who reported similar studies. Uncracked crude
oil fractions boiling at above 350°C, which had the highest
carcinogenic activity in skin tests on mice and rabbits, contained the
same or similar polynuclear aromatic hydrocarbons; no other new
carcinogens were found. After solvent extraction, the carcinogenic
activity of the extract was much higher than that of the raffinate,
which was very low.
The carcinogenicity of individual polynuclear aromatic
hydrocarbons will not be discussed in this document. For this, the
reader should refer to IARC (1973).
The fact that various cracking processes considerably increase
the contents of polynuclear aromatic hydrocarbons in mineral oils has
been demonstrated by Kennaway (1925), Twort & Fulton (1930), Twort &
Twort (1935), Pates (1952), and Dietz et al. (1952). Kennaway (1925)
discovered that skin cancer of mice could be produced with synthetic
tars obtained by pyrolising substances like acetylene, isoprene,
yeast, human skin, and a non-carcinogenic petroleum. Badger (1962)
pyrolysed a whole range of aliphatic and simple aromatic hydrocarbons
at 700°C. It was concluded from an analysis of the resulting tars
that, at high temperatures, polycyclic aromatic hydrocarbons were
formed from simpler hydrocarbons via primary radicals formed by
carbon-hydrogen and carbon-carbon fission. The same process occurred
at 550°C, but to a much lesser extent.
Skin tests on C3H-mice using 15 base oils with known content of
polynuclear aromatic hydrocarbons, which, in some cases, only differed
in theft refining history, revealed that solvent-refining removed the
carcinogenic components to such an extent that none of the oils
treated in this manner induced carcinomas (Bingham et al., 1965).
Solvent extraction was carried out with the usual solvents including
phenol, cresol, and furfural. Conventional acid and clay treatment
only removed certain of the polynuclear aromatic hydrocarbons and all
oils treated in this way still retained some carcinogenic activity. In
these tests, a general correlation was again found between the content
of 4 and 5 ring polynuclear aromatic hydrocarbons and the
carcinogenicity of the base oils examined.
Catchpole et al. (1971b) compared mass spectrometry analysis of
an untreated distillate with its solvent-treated and its hydrogenated
derivatives. The decrease in polynuclear aromatic hydrocarbons
thiophenes, and sulfur in both treated samples compared with the
untreated sample was quite marked. Smaller differences were found
between the levels in the two refined samples.
In studies by Boehme & Huehnermann (1966), the relative amounts
of aromatic compounds present in some of the more purified mineral oil
products were determined from their UV absorption spectra. The
relative concentrations were:
medicinal white oil 1
paraffin waxes 100
micro crystalline waxes 300
white petrolatum 1000
yellow petrolatum 10 000
The carcinogenicity of a sample of amber petrolatum was tested on
the skin of mice. A 15% solution of the petrolatum in iso-octane, did
not show any significant carcinogenicity. The solvent-extracted
aromatic fraction (1.2% of the original material) tested in iso-octane
solution at 50 times its concentration in the petrolatum did not
produce any carcinogenic effects on mouse skin (Lijinski et al.,
1966). Studies are reported by Oser et al. (1965) in which 3 kinds of
pharmaceutical and food-grade petrolatum were administered to mice in
the form of a single subcutaneous injection of 100 mg of petrolatum
per mouse. Observations over 18 months did not reveal any carcinogenic
or other toxic effects.
Liquid paraffin was found to be non-carcinogenic in long-term skin
testing on mice by Twort & Twort (1931) and Woodhouse (1934). White
mineral oil was also found to be non-carcinogenic in mice after
long-term skin application (WHO, 1974).
Shubik et al. (1962) examined 36 samples of petroleum wax. Eight
samples contained identifiable polynuclear aromatic hydrocarbons; the
highest concentration was 0.64 mg/kg. The results of skin and
subcutaneous testing follow in the author's words:
"Five petroleum waxes were tested by repeated skin application in
benzene solution to mice and rabbits. In addition one of the test
waxes was fractionated and its aromatic and non-aromatic components
were tested separately on mice, also by repeated skin applications.
Solvent-treated controls were kept. No carcinogenic effects were
detected. Five petroleum waxes were tested in mice by subcutaneous
implantation in disc form. Fibrosarcomas developed around the implants
with incidences correlated to the melting points of the waxes. In
addition, one of the test waxes was fractionated and its aromatic and
non-aromatic components tested separately; the same wax was also
tested subcutaneously in powdered form. The findings indicate that the
subcutaneous sarcomas occurred as a result of the physical rather than
the chemical properties of the materials."
From the various studies, it would seen that there is no relation
between the carcinogenicity of a lubricating base oil and its
potential to cause dermatitis.
In all the previously mentioned studies and others by Horton et
al. (1963) and Bingham & Horton (1966), there is general agreement
that the carcinogenicity of a mineral oil is largely related to the
aromatic fraction with a boiling-point above 370°C, and more
particularly to the polynuclear aromatic hydrocarbons containing 4-6
condensed benzene rings. However, some long-chain, aliphatic,
alicyclic, and alkyl-aromatic hydrocarbons from a lower boiling-range
with 10-20 carbon atoms, such as n-dodecane, cyclohexyl-decane, and
dodecyl-benzene may act as accelerators or co-carcinogens. Though
completely non-carcinogenic themselves, they were found to increase
tumour incidence and reduce the time of appearance, when carcinogenic
fractions dissolved in them were applied, to the skin of mice (Horton
et al., 1963; Bingham & Horton, 1966). However, in order to act as a
cocarcinogen, n-dodecane must be present in a concentration of
20-30% or more. Such solutions in accelerating solvents show an
unusual capacity to spread upon the skin (Horton et al., 1957, 1965).
In a further study on the effects of co-carcinogenic compounds on
the carcinogenic action of benzo(alpha)pyrene and benz (a)anthracene
on the skin of mice (Bingham & Falk, 1969), the co-carcinogenicity of
n-dodecane was confirmed. However, when the authors claim "that
there is a 1000-fold increase in the enhancement of potency of low
concentrations of benzo(alpha)pyrene and benz (a)anthracene when
dodecane is the diluent", this statement may not be fully justified by
the results obtained in relatively small numbers of animals per test
group. Nevertheless 50% n-dodecane in the solvent decaline doubled
or trebled the tumour incidence in the highest dose-group, and tumours
were found at much higher dilutions than when decaline alone was used
as a solvent. When 2-dodecanol and 2-phenyldodecane were used in
various concentrations in the solvent of benzo(alpha)pyrene, higher
concentrations of these 2 substances shortened the interval of
appearance of tumours rather than increasing the total incidence of
rumours.
From this it would appear that these accelerators would not have
any effect, when present in an intrinsically non-carcinogenic oil, and
that they would only have an effect in other oils, when present in
appreciable concentrations. This effect seems to be due, at least in
part, to the spreading power of the solvent causing more intimate
contact with a greater skin area. On the other hand, a more plausible
explanation could be an increase in P-450 enzyme induced by dodecane,
which possibly does not occur with 2-dodecanol and 2-phenyldodecane.
3,4 Benzo(alpha)pyrene can induce P-450 microsomal mixed function
oxidase and this can also catalyse arylhydrocarbon (AH) monooxygenase
activity (de Pierre & Ernster, 1978). The increase in P-450 induced by
dodecane will accelerate and increase the formation of the ultimate
carcinogen 3,4 benzo(alpha)pyrene and thus increase the total
incidence of tumours. However, these substances will not normally be
present in base oils and related products, because they fall into a
different boiling range.
Apart from this, some frequently used additives - which in
themselves are non-carcinogenic - such as elemental sulfur and some
sulfur-containing compounds such as benzyldisulfide, ditertiary
butylpolysulfide and ditertiary octylpolysulfide (Horton et al., 1965;
Bingham et al., 1965; Bingham & Horton 1966) as well as certain
phenols (Boutwell & Bosch 1959) have also been shown to enhance the
carcinogenic activity of polynuclear aromatic hydrocarbons on the
mouse skin. Addition of sulfur to a non-carcinogenic oil did not, of
course, have any effect on the carcinogenicity (Bingham et al., 1965).
Baldwin et al. (1964) reported that additives such as lead naphthenate
did not have an enhancing effect and there are indications that other
components of complex mineral oils with a boiling-range above 370°C
act as inhibitors of the carcinogenic effect of polynuclear aromatic
hydrocarbons, e.g., the saturated (probably cyclic) hydrocarbons
(Bingham & Horton, 1966).
Sunderland et al. (1951) reported skin tests of mineral oil
fractions on mice, under various conditions. They found that washing
the skin with soap and water, after the application of the oil,
reduced both the number of tumours and the rate of appearance. The
reduction was related to the length of the interval between oil
application and washing. Painting once, instead of twice, weekly
greatly reduced the rate of tumour appearance.
From human experience (section 4.7.1.1), it is known that the
ultraviolet radiation of sunshine has a potentiating effect on the
carcinogenicity of mineral oils. Similar indications exist from animal
studies (Emmett, 1975). It has also been demonstrated that certain
co-carcinogenic n-alkanes may increase the carcinogenic potential of
certain wavelengths of UV light on mouse skin (Bingham & Word, 1977).
In comparative studies with various mineral oil fractions on the
skin of mice and rabbits, it became apparent that the rabbit skin was
more sensitive than the mouse skin according to the type, number, and
growth rate of tumours (Cruickshank & Squire 1950; Hieger & Woodhouse
1952; Antonov & Lints 1960; UK Medical Research Council, 1968). In
some instances, mineral oil fractions were non-carcinogenic to mouse
skin, but positive on the skin of rabbits (Shubik & Saffiotti), 1954).
While, rats and guineapigs are apparently less sensitive than mice
(Sugiura et al., 1949; Desoille et al., 1973) Rhesus monkeys appear to
be fairly sensitive (Sugiura et al., 1949).
As to the suspected causes of intra- and inter- species
differences in susceptibility to polynuclear aromatic hydrocarbons,
the levels of the enzymes forming and detoxifying the biological
reactive intermediates, the ultimate carcinogens, in the tissue of the
various strains and species might be one of the important factors
(section 4.6.2.1). Clear intra-species differences in tumorigenic
response have been reported by, for example, Smith & Sunderland (1951)
and Gilman & Vesselinovitch (1955).
In addition to these studies on "pure" mineral oil fractions and
various additives, some studies with metal working oils and textile
oils have been published.
Two cutting oils based on sulfurized mineral oils, were diluted
with water and were tested in various concentrations on the skin of 3
strains of mice. Both were found to be carcinogenic. A marked
reduction in incidence and an increase in induction time of tumours
was found at a dilution of 1:8 compared with 1:4 (Gilman &
Vesselinovitch, 1955). The same authors (Gilman & Vesselinovitch,
1956) compared a straight cutting oil and a water-soluble cutting
fluid in 2 different strains of mice. They found a consistent,
comparable but low incidence of skin tumours in 3 separate trials.
In a later study, 3 commercial additive-containing cutting oils,
one of which was an emulsifiable oil, were repeatedly applied to mouse
skin for up to 31 weeks. Carcinogenic skin changes were observed with
all these oils but were, possibly, less marked with the emulsifiable
oil. In addition to these carcinogenic effects, focal necrosis of the
liver associated with amyloid deposition and amyloidosis of the skin,
spleen, and kidneys were observed. The additives may have had a
contributory effect in some of the pathological changes observed
(Jepson et al., 1977).
In the case of cutting oils, the temperatures to which the oils
are exposed at the cutting edges of the tools are such that cracking
of the oil might conceivably occur and theoretically a
non-carcinogenic cutting oil might become carcinogenic during use. An
unspecified cutting oil from the sump of a machine was tested on the
skin of mice and rabbits. It induced benign tumours in rabbits only
(Cruickshank & Squire, 1950). As the fresh oil was not examined, no
conclusion can be drawn concerning the previously-mentioned
assumption.
Unused cutting oil, used cutting oil, and residue from the sump
were compared in skin tests on 2 strains of mice by Dargent et al.
(1967). Both used and unused cutting oil caused a high incidence of
skin ulceration. Hyperkeratosis and papillomas were more frequent in
the case of used oils and a single case of skin cancer occurred in the
groups of mice treated with used oil and sump residue.
Cutting and quenching oils, fresh and used, were tested on the
skin of rats, guineapigs, and mice. All tests on rats and guineapigs
were negative. In mice, skin tumours were more numerous and more
malignant with used oils than with fresh oil. Only used oils produced
tumours in organs distant from the site of application. The unused
quenching oil was found to contain a benzo(alpha)pyrene level of
0.6-0.8 mg/kg (ppm); in the used oil, this fraction had increased to
20 mg/kg, which is in agreement with the finding of its increased
carcinogenicity. The increase in carcinogenic poly-nuclear aromatic
hydrocarbons may be the result of thermal cracking due to the heat of
the process (Desoille et al., 1973). The studies of Thony et al.
(1975) indicated that the increase in poly-nuclear aromatic
hydrocarbons in cutting oils was very small and considerably less than
for quenching oils and engine oils. The benzo(alpha)pyrene content of
new cutting oils ranged from 0 to 150 mg/kg, compared with 0 to
250 mg/kg for used cutting oils.
A sample of commercial jute batching oil of unspecified origin and
composition, containing a concentration of 3,4-benzopyrene of less
than 1 mg/kg, was tested for carcinogenic activity on the skin of
mice. The oil in question induced malignant skin tumours in 6 out of
24 test animals and proved to be a potent tumour promotor in mice
pre-treated with 7,12-dimethylbenz (a)anthracene (DMBA) (Roe et al.,
1967).
To summarize, it may be concluded that: (a) the carcinogenic
activity of mineral oils seems to be related mainly to the presence
and concentration of certain polynuclear aromatic hydrocarbons,
containing 4,5, or 6 condensed rings; and (b) cracking processes
tend to increase the polynuclear aromatic hydrocarbon contents in
petroleum products. For example such compounds may accumulate in a
metal working oil in contact with the host cutting edge of a tool.
The polynuclear aromatic hydrocarbon content of mineral oils can
be decreased by solvent extraction and/or by hydrogenation. Acid
treatment is less effective for this purpose.
Analytical methods for the determination of total polynuclear
aromatic hydrocarbons should provide a useful, quick, and cheap tool
for predictive screening of mineral oils for carcinogenicity. These
methods should be biologically validated. The correlation will not
always be good, because the carcinogenicity of polynuclear aromatic
hydrocarbons varies considerably and because of the unpredictable
effects of cocarcinogens, inhibitors, and accelerators.
Various substances that are used as additives such as sulfur
compounds, as well as substances that may normally be present in
mineral oils, such as n-dodecane, may act as cocarcinogens. Other
saturated hydrocarbons, normally present, may act as inhibitors of the
same effect.
It is clear from animal studies that washing with soap and water
substantially reduces the hazard to the skin of repeated and long
contact with potentially carcinogenic oils.
4.6.2.3 Effects of inhalation and intratracheal exposures
The aspiration hazard and toxicity for animals of a number of
hydrocarbons and hydrocarbon mixtures was determined by Gerarde
(1963). This study has been discussed in more detail in section 3.6.1.
He found that the aspiration hazard decreased with increased viscosity
of the product and with mixtures containing low viscosity products.
Mice were exposed to mists of various mineral and vegetable oils
with an average droplet size of 2.5 µm, 80% of the particles retained
in all areas of the lungs were 2.5 µm or less in diameter. The highest
concentration of retained particles was found around the terminal
bronchioles and alveolar ducts in all parts of both lungs. Oil
particles were immediately phagocytosed, a process that was
essentially completed within 48 h, unless prolonged exposures (2-4
weeks) had been given. The initial concentration of retained oil
droplets was similar for all types of oil mists. During a 90-h
follow-up, however, the concentration of vegetable oil droplets
decreased progressively, whereas the concentration of mineral oil
droplets remained practically unchanged. After 2-4 weeks of exposure,
mineral oil droplet retention gave rise to localized slight
foreign-body reactions as well as to a few patches of lipoid
pneumonia. Of the other oils, only cod liver oil caused a moderate
foreign-body reaction (Shoshkes et al., 1950).
The effects of prolonged inhalation of oil mists (mineral oil
levels in air of 63-132 mg/m3) were observed in mice, rats, rabbits,
and monkeys. Ordinary automobile lubricating oil and a smoke-screening
oil, were tested for periods varying from 100 to 365 consecutive days
(Lushbaugh et al., 1950) The authors found that surprisingly little
oil accumulated in the lungs and that whatever was retained was
rapidly phagocytosed and transferred to the pulmonary connective
tissue and the hilar lymph nodes. Lipoid pneumonia was not found to be
a hazard at these dosages, though the incidence of infectious
pneumonia in the exposed monkeys greatly increased. Many exposed
monkeys died of a hyperplastic gastritis, probably because much of the
inhaled oil was initially deposited in the nasal passages and
subsequently swallowed. Hyper-plastic gastritis was especially evident
with the shale oil (which is different from petroleum oil) used as
smoke screening oil (Lushbaugh, 1947). No significant increase in
tumour incidence or reduction in the latent period of tumour
production was found in the mouse study.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Rats and guineapigs were exposed for 6 h/day, 4 days a
week, for up to 2 years, to a cutting oil mist consisting of a 10:1
mixture of neutral paraffin and prime lard oil. In some cases, this
caused multi-focal adenomatosis in the lungs of the animals. A
carcinoma of the lung was found in only 1 of 105 exposed rats and not
in any of 65 guineapigs.
In tests on mice, Wagner et al. (1961) found that inhalation of
either mineral or motor oil mist reduced the acute lethal effects of
respired oxidants such as ozone and nitrogen dioxide. The effect was
demonstrable only after a latent period of up to 8-9 days following
oil mist exposure and was thought to result from the formation of a
thin film of oil on the alveolar surfaces.
Long-term inhalation toxicity studies using a highly purified
white mineral oil, composed of naphthene-based saturated hydrocarbons,
were reported by Wagner et al. (1964). Five species of laboratory
animals (dog, rabbit, rat, hamster, mouse) were exposed daily, for
periods from one year to 26 months, to a petroleum-base mineral oil
mist at concentrations of 5 mg/m3, the current USA threshold limit
value, and 100 mg/m3. Histological evaluation of tissues of the dogs
and rats exposed to 100 mg/m3 showed significant pulmonary alveolar
and hilar lymph node oil deposition and/or lipid granuloma formation
after 12 months of exposure. In addition, these animals showed
significant increases in the activities of basic and
magnesium-activated phosphatases.
Body-weight gain, haematological variables, and respiratory
function values did not deviate significantly from the control data at
any of the exposure levels. Studies with a spontaneous
pulmonary-tumour-susceptible strain of mouse presented equivocal
evidence of an increased rate of tumour formation at the 100 mg/m3
concentration. These findings suggest that prolonged exposure to a
mineral oil mist concentration of 5 mg/m3 would not present any toxic
hazard. It would appear, however, that protracted exposure at
approximately 100 mg/m3 would, in time, produce harmful physiological
effects.
The toxicity of aerosols of various petroleum oils (industrial,
transformer, and compressor distillates) differed only slightly in a
long-term experiment by Lutov (1974). White rats were exposed to
concentrations of 13, 30, and 60 mg/m3 for 5 h/day, for 6 months.
Changes in the electrocardiogram, and reductions in arterial pressure,
respiratory frequency, and immunological reactivity were seen even at
12-13 mg/m3, which was considered to be just above the threshold
level. A concentration of 5 mg/m3 is recommended as a maximum
permissible concentration for petroleum oil aerosols without
additives.
In a further long-term study on white rats, Lutov et al. (1976)
studied the toxicity of petroleum oil aerosols in concentrations
ranging from 11.4 ± 1.5 to 61.4 ± 5.2 mg/m3 in combination with
products obtained by thermo-decomposition, i.e., hydrocarbons in
concentrations ranging from 200.0 ± 5.8 to 410.0 ± 12.8 mg/m3 and
carbon monoxide in concentrations ranging from 11.2 ± 1.5 to
35.4 ± 4.6 mg/m3. White female rats were exposed for, 5 h/day over a
6-month period. The combined exposure was found to produce more marked
effects on the parameters tested in the earlier experiment.
The minimum concentrations of oil aerosol that caused functional
changes in the respiratory system with a single inhalation were in the
range of 860-1200 mg/m3. When concentrations of 53 and 60 mg/m3 were
used in the long-term experiment, physical growth was impaired and the
functional condition of the nervous system and the liver changed.
There were morphological changes in the lungs including a
catarrhal-desquamative bronchitis, a swelling of the alveolar
membranes, formation of oleogranules, and the presence of a large
quantity of oleophages and oil droplets in the lymphatic vessels and
the peribronchial and bronchopulmonary lymphatic nodules. At
concentrations of 10-17 mg/m3, the changes were slight and
reversible. A maximum admissible concentration in the air of a work
place of 5 mg/m3 is the official USSR exposure limit (Ivanov et al.,
1978).
In tests on rabbits, Laughlen (1925) demonstrated that mineral oil
given by mouth or nose can enter the trachea. Corper & Freed (1922),
injected olive oil and medicinal mineral oil intratracheally into
rabbits in amounts of 0.5-1.0 ml. The oil was readily aspirated into
the finest pulmonary divisions and into the alveoli. It was retained
for months and caused a mild proliferative reaction typical of a
foreign body reaction. In studies by Pinkerton (1928), rabbits and
puppies were injected intratracheally with animal, vegetable, and
medicinal oil. Complete removal of the oil from the lungs took several
months in all cases. Neutral vegetable oil produced practically no
reaction, animal oils caused giant cell formation and rapid and marked
pulmonary fibrosis. Mineral oil was rapidly phagocytosed, giant cell
formation and slight fibrosis were apparent after 2-3 months, and
storage of the oil took place in the bronchial lymph nodes.
4.6.2.4 Dietary studies
Long-term oral studies have not been conducted with lubricating
base oils.
Lushbaugh & Hackett (1948/1949) carried out a study in which rats
received an average of 0.2 ml/rat per day of a highly refined
diesel-engine lubricating oil, used as smoke screening oil, in theft
diet for a period of 14 months. In this group of 40 rats, 2 developed
foci of colonic mucosal hyperplasia and one developed colonic
adeno-carcinoma.
Results of various dietary studies using food-grade and
pharma-ceutical-grade materials, such as medicinal and food-grade
mineral oil, and food-grade paraffin waxes are included in the
following summary, though these studies with highly purified material
are not representative for the less purified grades.
Schmahl & Reiter (1933) fed 2% (w/v) mineral oil in the diet of
rats for 500 days without adverse effects. In studies by Daniel et al.
(1953), rats were kept for 15 months on diets supplemented with 10%
liquid paraffin, without adverse effects. Five kinds of petroleum
waxes were tested by feeding rats a diet containing 10% of the wax for
2 years. The rats were then observed until natural death. No toxic or
carcinogenic effects were found (Shubik et al., 1962). In the studies
by Oser et al. (1965), already reported in section 4.6.2.2, 3 kinds of
petrolatum were also administered to rats in the diet at 50 mg/kg
diet. Observations over 2 years did not reveal any carcinogenic or
other toxic effects.
4.7 Effects on Man
The general population does not have any contact with base oils;
contact with the commercial products derived from them will, at most,
be occasional and of a lower order of magnitude than occupational
exposure. Cases of accidental ingestion are exceptions.
However, pharmaceutical grade liquid paraffin in the form of
medicinal oil is widely used as a laxative (faecal softener).
Occupational exposure to base oils is restricted to the small
group of workers who manufacture them in oil refineries and to workers
in blending units, who mix and blend them with additives in order to
produce commercial products such as lubricating oils, greases,
metal-working oils, and textile oils. These activities are normally
carried out in closed systems in which case occupational contact is
minimal and discontinuous, usually accidental. This also applies, when
the finished product is put into containers.
4.7.1 Occupational exposure
Epidemiological and clinical data concerning base oils are almost
non-existent. Thus, in this section, it is only possible to analyse
epidemiological studies of workers exposed to products containing base
oils, such as lubricating oils, metal-working oils, and textile oils.
From the list of lubricating oil products given in section 4.2.2,
it is clear that various groups of workers are occupationally exposed
to these products, the most widely-used being the metal-working oils
and textile oils.
The magnitude of occupational exposure varies considerably. In the
case of some lubricants and transformer oils, handling is only
occasional, and, even then, exposure is minimal. In other situations,
such as in the earlier years of mule spinning and in work with
automatic lathes of old design, extremely high exposure did - and
sometimes still does occur. Not only is there continuous direct
contamination of clothes and exposed parts of the skin, but the rapid
movement of the machinery may turn the oil into an aerosol and thus
generate an oil mist that can be inhaled and further contaminate skin
and clothing. Equipment and floors may become covered with an oil-film
and situations have been described where oil even dripped from the
roof.
A certain degree of oil mist generation may also occur in the
printing and rubber industries and with pneumatic equipment (e.g.,
drills), especially under conditions of limited ventilation such as
are found underground.
Even under the best technical conditions, it may sometimes be
difficult to completely avoid contamination. For example, in the case
of toolsetters, the wearing of protective clothing impedes easy
movement, and clothing, including underwear, may become soaked with
oil.
Henry (1946/1947) gave an excellent insight into the types and
magnitude of occupational exposure up to the time of the report. It is
known that some of these conditions had continued for a period of
about 20 years. However, in many countries, great improvements have
been made since then. An understanding of the range of such exposures
is needed to put epidemiological studies of later years into
perspective, especially in view of the long latency of carcinogenic
effects. In the evaluation of such studies, it has to be kept in mind
that today's findings may reflect the industrial hygiene situation of
20-30 years ago.
4.7.1.1 Skin disorders
A great variety of skin disorders attributed to working with
mineral oil or with products based on mineral oils, such as metal
working fluids, are mentioned in the medical literature. This is not
surprising considering the many factors that influence theft
development (van Raalte, 1963; Key et al., 1966; Kipling, 1968;
Hodgson, 1970, 1973).
The main factors include: (a) the degree of intrinsic potential
of a mineral oil product to damage the skin; (b) the integrity of
the skin; (c) the degree and continuity of contact between oil and
skin; and (d) individual predisposition.
The situation, however, is more complex than this, for, in actual
practice, a multitude of other factors may influence the main
conditions to such an extent and in such a complex way, that a
cause-and-effect relationship may become obscured. Most authors agree
that practically all skin disorders attributed to exposure to mineral
oil products can be prevented entirely by adequate industrial and
personal hygiene practices, and that the majority of cases have
occurred in workshops where inadequate conditions prevailed.
Some of the more important factors in the complex interplay are:
(a) Factors related to the mineral oil product used:
(i) Base oil:
the lower the boiling-point of the oil, the more
pronounced the solvent action of the product; this causes
defatting of the skin and leads to dryness, chapping,
scaling, and cracking;
the higher the boiling-point, the more blocking of skin
pores occurs, giving rise to acne formation;
some mineral oils are more irritant than others; the
lower-boiling fractions are sometimes, but not always,
more irritant than higher-boiling fractions (section
4.6.1);
mineral oils themselves do not appear to be sensitizers.
(ii) Additives:
these may be primary irritants or sensitizers in theft own
right (e.g., chromium salts), though manufacturers try to
avoid the use of such additives;
solvents and detergents increase the defatting effect of
the lower boiling-range oils;
some chlorinated additives, such as chlorinated
naphthalenes, may cause chloracne. The use of chlorinated
naphthalenes was discontinued in most countries, many
years ago.
(iii) Changes in the composition of the oil that may occur
during its use:
cracking of oil fractions may occur due to heat;
reactions may occur between components of the mixture or
between them and materials that are added at a later
stage;
metal salts or ions may be formed in the mixture or
solution in the case of metal-working oils; in the case
of, for example, chromium and nickel, this can cause skin
reactions in sensitized workers.
(iv) Impurities of all kinds may accumulate in, for instance,
metal-working oil baths in which the oil is not regularly
changed. Important among these impurities are: metal
particles, which may cause microtraumata of the skin; and
microorganisms, which may cause inflammation of the skin
by way of infection or exotoxins; bacteria, yeasts, or
fungi may be present in mono- or mixed culture, and some
of these organisms can degrade an oil and form potentially
irritating compounds.
(b) Factors related to the work situation:
(i) design of workshop and equipment determine to a large
extent the magnitude and duration of exposure to the oil,
and whether this exposure is continuous or intermittent;
(ii) availability of general and exhaust ventilation of
sufficient design and capacity may determine whether there
is exposure to oil mist or not;
(iii) use of protective clothing such as gloves, will diminish
duration and intensity of skin contact;
(iv) general hygienic facilities such as wash basins near the
work-place, shower facilities, frequent changing and
laundering of work clothing, all of which help in limiting
the extent and duration of exposure;
(v) hands and contaminated parts of the body should be washed
with fatty toilet soap and, after washing the skin should
be treated with a suitable emollient cream; use of
solvents, and alkaline or abrasive soaps for washing can
damage the skin and contribute to the occurrence of skin
diseases.
(c) Individual factors:
(i) general and hereditary conditions of the skin may
predispose to adverse skin effects from all sorts of
chemicals;
(ii) work discipline and safety-mindedness can influence the
duration and intensity of the exposure;
(iii) proper use of safety equipment and general hygiene
facilities, as well as personal hygiene in the form of
frequent bathing and changing of underwear, will help to
avoid over- and prolonged exposure.
Contamination of the skin may often occur despite all precautions
taken, particularly in the case of metal-working oils. Wiping with
oil-soaked rags is an additional source of contamination and may cause
skin lesions. Continuous wearing of oil-contaminated clothing is an
important factor in the etiology of scrotal cancer. Rapidly rotating
machinery may generate oil foams and mists.
The most common form of skin disorder is acute or chronic contact
irritative (toxic) dermatitis caused by the irritative action of
various components, additives, and/or impurities in mineral oils.
Dermatitis may be preceded by defatting and/or maceration of the skin.
Mechanical irritation, microtraumata, and skin cuts may play a role in
its origin. Clinical signs of contact irritative dermatitis are, in
order of severity, erythema, oedema, bullae or necrosis, and sharp
demarcation of the affected areas from unaffected ones.
The other type of dermatitis is contact allergic dermatitis
(synonym: contact eczema) caused by allergic sensitization to various
allergens. Additives or impurities are the most allergenic components
of oil formulations. The signs of eczema are more variable then those
of toxic dermatitis and include erythema, papulae, vesiculae, bullae,
scales, hyperkeratoses, and rhagades. These lesions have a tendency to
spread into areas that have not been in direct contact with the
allergen. Eczema may be preceded by contact irritative dermatitis,
from which it develops by secondary sensitization.
Oil folliculitis, or oil acne is characterized by the triad
comedones, folliculitis, and follicular scars. The lesions are found
on the parts of the body with the greatest exposure. Friction from
clothing and machinery rubbing the oil into the exposed parts of the
skin, is an important additional factor. First there is plugging of
the hair follicles and pores of the skin by follicular hyperkeratosis,
cell debris, and oil with its impurities, followed by blackheads and
secondary infection. Poor personal hygiene is the main cause. Oil acne
occurs more often in the earlier years of exposure to oil. Later,
there appears to be a gradual change in the reaction of the skin to
the oil (Kinnear et al., 1954). Chlorinated naphthalenes and related
compounds have given rise to chloracne.
Photosensitivity is an abnormal sensitivity of the skin to
sunlight caused by certain constituents of coal-tar, but also
sometimes by mineral oil constituents. Related to this is melanosis,
the general darkening of the skin that may follow acute
photodermatitis, as well as toxic melanoderma, which develop after
long-term exposure to oils containing certain anthracene fractions.
Both photosensitivity and melanosis rarely result from exposure to
mineral oil.
Hyperkeratosis may occur either together with dermatitis and oil
acne of long standing, or in isolation -- mostly on the forearms or
other heavily exposed parts of the body. Two forms of hyperkeratosis
can be distinguished: (a) circular, white and flat hyperkeratotic
areas of a few mm in diameter, sometimes in the form of smooth
plaques; these may occur in small clusters and are slightly raised
above the level of the surrounding skin; and (b) a second form,
which may occur at the same time, and consists of rugose, pigmented
warts that are considerably raised above the surrounding tissue level.
The pattern is generally irregular, but may be round or oval.
Precarcinogenic changes may be present in the hyperkeratotic
plaques in the form of rough, slightly raised patches, which sometimes
may take the form of horns or warts. In themselves these forms are
still harmless, but they have a tendency to become malignant. As soon
as these forms contain some malignant cells they are called
keratoacanthomata. After growing for a certain period, they may be
shed from the skin. Another form of precarcinogenic change that may be
encountered is the shark- or shagreen-skin, a pigmented, atrophic
skin, beset with small horns and warts.
A basal cell carcinoma (basalioma) is a very slowly enlarging
tumour of the skin. It may ulcerate, or invade the area round it, but,
in general, it does not metastasize. The most common form of malignant
tumour is the squamous cell carcinoma (spinalioma), starting as a
small tumour, that may arise from a keratosis or in apparently healthy
skin. It continues to grow, starts ulcerating, invades surrounding
tissues and eventually may metastasize. None of these conditions is
limited to mineral oil exposure. Similar changes may occur as a result
of UV-light exposure, excessive doses of X-rays, exposure to pitch
coal-tar, or ingestion of arsenic. A specific localization, however,
is the epithelium of the scrotum. In this case "it is reasonable to
assume that it could be caused by occupational exposure to soot, tar,
pitch, or oil" (Kipling, 1968).
A combination of various potentiating factors, such as mineral oil
contact and exposure to sunlight (UV-radiation) increases the tendency
to develop skin cancer (Schwartz et al., 1947; Smiley, 1951; Kinnear
et al., 1954; Emmett, 1975). This is especially pronounced in persons
with fair hair.
4.7.1.2 Skin carcinogenicity
The history of the development of skin cancer as a result of
exposure to mineral oil, as well as the major epidemiological
literature related to the subject have been reviewed by the
International Agency for Research on Cancer (IARC, 1973). To quote
theft findings:
"Volkmann (1875) described scrotal cancers among workers producing
paraffin by the distillation of coal-tar. Subsequently, Liebe (1892)
noted the absence of such hazard among workers exposed to pure
paraffin. Several investigators have since shown that cancers among
paraffin workers are not due to the paraffin but to impurities in oils
produced during processing (Leitch, 1922; Hendricks et al., 1959).
Refined paraffin is free of PAH and does not induce skin cancer in
mice (Shubik et al., 1962).
"Bell (1876) first described cancer of the scrotum in a Scottish
shale oil worker. In a 23-year period, 49 Scottish paraffin workers
developed skin cancer of which 13 were scrotal (Henry, 1946).
"The cotton mule spinning industry in Great Britain originally
used shale oil for the lubrication of the spindles (Henry, 1946). The
first case of death from scrotal cancer in a worker who used shale oil
in mule spinning occurred in 1923 (Bridge & Henry, 1928). In the years
1920 to 1943, there were 1303 legally notified cases of skin cancer in
the British mule spinning industry, including 824 of the scrotum.
There were 575 fatal cases of scrotal cancer recorded between 1911 and
1938 (Henry, 1946).
"In Great Britain, the Mule Spinning Regulations have ensured that
since 1953 only oil drastically refined with sulphuric acid shall be
used in mule spinning and that mule spinners shall be medically
examined every six months. These measures, together with the marked
decline of the process of mule spinning, have produced a sustained
fall in the incidence of cancer of the scrotum in Great Britain.
"Cutting oils used by workers to cut metals were found to increase
the risk of skin cancer in Birmingham, England (Cruickshank & Squire,
1950; Cruickshank & Gourevitch, 1952), particularly among workers in
automatic machine shops. Between 1950 and 1967, 187 cases of scrotal
cancer occurred in this region, of which at least two-thirds could be
attributed to oil (Waterhouse, 1971).
"At the present time toolsetters and setter operators in automatic
shops who use neat cutting oil have an increased risk of cancer. The
work requires constant contact with the machines and consequent
contamination with the oil. In the Birmingham area of England, a high
frequency of skin and scrotal cancer from oil has occurred,
particularly among bar automatic machine workers; but other
engineering practices also present a cancer hazard, e.g., metal
rolling, tube drawing, metal hardening and machine operating. Although
the major risk is from exposure to undiluted oils, emulsions have been
incriminated occasionally. The industries most affected are those with
automatic shops, such as nut and bolt manufacturers. Workers have also
been affected after exposure during the changing of transformer oil in
electrical sub-stations and during the painting or spraying of mould
oil for brick- and tile-making or concrete moulding, in drop forging,
rubber mixing, wire drawing, rope making and in the jute industry and
from grease in metal working (Kipling, 1968).
"In France, in the valley of the river Arve in the Savoy Alps,
there have occurred since 1955 at least 60 cases of cancer of the
scrotum together with many cases of cancer of the skin among the bar
automatic machine workers (décolleteurs). The very high frequency in
the relatively small population of the valley was observed mainly
among the self-employed and workers in small premises (Thony & Thony,
1970). They were in contact with undiluted cutting oils.
"Cancers of the larynx, lung and stomach have also been attributed
to oil mist (Southam, 1928); and recently evidence has been produced
that persons who developed cancer of the scrotum are significantly
more liable to develop cancers at other sites, e.g., in the
respiratory tract or upper digestive tract (Holmes et al., 1970)."
Kinnear et al. (1954, 1955) published the results of an extensive
epidemiological study of skin disease in jute workers in relation to
mineral oil exposure. They found a high incidence of premalignant
changes on the skin of the exposed parts of the body in long-term,
older workers and isolated cases of scrotal carcinoma.
Bingham & Horton (1966) estimated the latent period for skin
cancer caused by mineral oil exposure to be 50-54 years (range 4-75
years). In the case of crude paraffin oil, they mentioned an average
of 15-18 years (range 3-35 years). In these cases, the range may be
more important than the average, though the average indicates that, in
general, the latent period is very long. A considerable proportion of
the cases of skin epithelioma caused by mineral oil exposure had such
a long latency period that the disease only appeared after retirement
from active work (Cruickshank & Gourevitch, 1952; Kinnear et al.,
1955).
Five cases of squamous cell carcinoma of the hand and forearm and
one of the scrotum occurred in machine operators at a plant in
Ontario, Canada (Mastromatteo, 1955). These workers had been exposed,
for an average of 21 years, to cutting fluids that were subsequently
demonstrated to be carcinogenic in animal tests (Gilman &
Vesselinovitch, 1955).
Milne (1970) traced 5 cases of carcinoma of the scrotum, as
registered in the Central Cancer Registry of Victoria, Australia, and
found that, in all cases, the carcinomas occurred during or after the
seventh decade of life. Three of the subjects had had intensive
contact with mineral oils throughout theft working life, one was a
stoker in a gas-works, and the fifth had always been involved in
administrative work.
In the Netherlands, a recent survey demonstrated that scrotal
cancer occurred only sporadically and was not correlated with
occupational exposure to mineral oil (Pruyn & Reijnierse, 1972;
Fokkens et al., 1972; van Raalte, 1972).
Eight cases of scrotal cancer were discovered by Avellan et al.
(1967), over a period of 24 years, among 250 automatic lathe operators
in Gothenburg, Sweden. Diagnosis was made when the operators were
between 54 and 66 years of age and after periods of exposure to
mineral oil ranging from 19 to 43 years. In the words of the authors:
"All of the cases occurred among operators who began their work
during the era when the exposure, as a result of the prevailing
machine construction, was considerable and before the regulations and
the controls, which were instituted after the discovery of the first
cases, had been set up."
Wahlberg (1974) analysed 34 cases of scrotal cancer reported to
the Swedish Cancer Registry between 1958 and 1970. Seven cases (21%)
had been heavily exposed, occupationally, in the past to oil. and oil
mist, e.g., as automatic lathe operators. One of this group developed
a primary lung cancer.
A retrospective study of 298 cases of scrotal cancer registered in
the Birmingham region in the United Kingdom between 1936 and 1972 was
reported by Brown et al. (1975). The incidence of scrotal cancer was
5-6 cases per million males per year, whereas for the whole of the
United Kingdom, the incidence is 1-2 cases per million males per year.
The patients or relatives were interviewed in 109 cases: 94 had been
exposed to mineral oil (mainly tool-setters and machine operators, and
all cases had been exposed to cutting oil, 14 had been exposed to
pitch or tar, and, in 7 cases, there was no apparent occupational
exposure. In 298 cases of scrotal cancer, 52 other primary rumours
were noted; of these, 42 arose following the scrotal cancer including
15 skin tumours and 12 bronchial tumours. The incidence of both these
types of cancers was much in excess of what would be expected
statistically. The majority of the group with second primary tumours
were machine operators and tool-setters (75% in the case of bronchial
carcinoma). The authors postulated that in these cases both primary
rumours were initiated by the same carcinogen.
After studying all the available literature, Desoille et al.
(1973) concluded that, in general, and with the exception of certain
extreme situations, the number of tumours caused by exposure to
mineral oils was low. According to Auld (1950) and Eckhardt (1957),
though certain cutting oils and other mineral oils are carcinogenic to
the skin, the degree of carcinogenicity is very low compared with that
of coal-tar, pitch, and shale oil. In their opinion, and that of most
other authors, e.g., Avellan et al. (1967), even this level of
carcinogenicity would disappear, if a minimum of industrial and
personal hygiene measures were adhered to. On the other hand, Auld
(1950), Desoille et al. (1973), Thony et al. (1975) and many others
urge that alternative products should be developed that do not expose
the workers to a tumorigenic hazard.
In a further report on the skin cancer epidemic in the French Arve
Valley, Thony et al. (1975) recorded 133 epithelioma cases in 15
years, mostly of the scrotum. The incidence in these workers was 36
times that expected in the general population. In workers in small
workshops with poor industrial and personal hygiene, the incidence was
found to be 3 times higher than in the larger plants. The average age
was 54 years (range 35-75 years) and the average exposure 30 years
(range 15-50 years), at the time of clinical diagnosis. In addition,
an increased incidence of bronchopulmonary tumours was found,
especially in the décolleteurs. Officially until 1947, but in
practice possibly until 1950, coaltar-derived anthracene oils were
used, which originally contained benzo(alpha)pyrene levels of up to
1000 mg/kg. The level was later reduced to 10 mg/kg. These oils may be
responsible for many of the previously mentioned cases, but skin
cancer also occurred in some of the workers exposed only to the
petroleum-derived oils that were used exclusively later on. Fresh
cutting oils, as delivered to the users, contained benzo(alpha)pyrene
levels ranging from 0.5 to 145 µg/litre and the concentration of
carcinogens was found to increase during use, though not to the same
extent as that in quenching oils (2-100 times with an average of 30
times), and in lubricating oils for internal combustion engines.
4.7.1.3 Effects of oil mist exposure
Southam (1928) was the first to attribute cancers of the larynx,
lung, and stomach to oil mist exposure.
In a report by Huguenin et al. (1950), 32 out of 144 patients with
lung cancer had been in prolonged and intense contact with oil mist in
the past. Old metal-working machines with improper protection against
oil mist, processes where oil vapour arose from contact with hot
metal, and high-pressure cleaning of machines with oil, were found in
the places where these patients had worked. Hendricks et al. (1962)
considered the observations of Huguenin et al. (1950) to be of
doubtful significance in view of the high incidence of this disease in
non-oil-mist-exposed groups. Nevertheless, the conditions in these
cases may have been such, that a causal relationship, in at least some
of them, cannot altogether be excluded.
More printing industry workers were found among cases of bronchial
carcinoma in a Stockholm clinic (8 out of 125) than would have been
expected (Ask-Upmark, 1955). Certain industrial processes where oil
mist might occur were studied by Hendricks et al. (1962), who gave an
indication of possible exposures in their table of actual exposures
measured in various industries (Table 7).
The average particle size in the oil mists varied according to the
generating process and was found to be about 1.0-5.0 µm.
In 241 Kodak workers exposed to oil mist (Ely et al., 1970), no
significant differences were found, either in mortality, respiratory
symptoms, and disease, or in lung function compared with a control
group.
In the same year, Holmes et al. (1970) produced evidence, that
persons who developed cancer of the scrotum were significantly more
liable to develop cancers of the respiratory tract and the upper
digestive tract.
Similar findings have been reported in a preliminary paper by
Waterhouse (1972). An excess of primary tumours at other sites (skin,
respiratory and upper alimentary tracts) was found in men with scrotal
epitheliomas.
From animal studies, it appears that oil mist particles larger
than 5 µm will not easily penetrate into the lungs, but will be
retained mainly in the nasopharynx and upper respiratory tract.
Smaller particles, especially those of 2.5 µm and less, will readily
pass into the alveoli, where they will be phagocytosed and passed on
to the lymph nodes. When this mechanism cannot cope with the
situation, in cases of continuously repeated high exposure to oil
mists, a chemical pneumonitis and chronic lipid pneumonia may develop
(Proudfit et al., 1950; Foe & Bigham, 1954), but this appears to be an
extremely exceptional condition. Clinical details and examples of this
will be discussed in section 4.8.
TABLE 7. Exposure to oil mist in selected industries
Type of industry Observations Exposure range
oil mist mg/m3
brass and aluminium production 5 1.4-20.7
copper mining 7 5.4-22.0
automobile manufacture 37 1.0-56.5
manufacture of steel products 33 0.8-50.0
newspaper (press room) 8 2.0-16.6
screw manufacture 6 1.0-14.2
From: Hendricks et al. (1962).
In 12 out of 19 workers with oil mist exposures ranging from 9 to
18 years, Jones (1961) found a marked linear and reticular pattern in
the radiograms of the lungs. Exposures ranged from 1 to 9 mg/m3 with
70% of the particles of the order of 1 µm.
Various groups of research workers have studied different aspects
of the printing industry. Printing-ink consists chiefly of a
suspension of carbon black in mineral oil or aromatic extracts of
mineral oil. Modern high-speed newsprint presses can generate fairly
high concentrations of ink-mist aerosol. Most particles, however, are
outside the respirable range and may end up in the stomach, rather
than in the lungs of exposed workers. Lippmann & Goldstein (1970)
found an average droplet size of 14 µm (ranging up to 30 µm) in the
press rooms of the New York Times. The time-weighted average
concentration of respirable mist particles was found to be 1.4 mg/m3
against a total time-weighted average mist concentration of
8.6 mg/m3. In a parallel epidemiological study in the same firm,
mortality and morbidity data over a 15-year period were compared for
pressmen and compositors. No significant differences in respiratory
mortality or morbidity were found (Goldstein & Benoit, 1970).
In a similar study by Pasternak & Ehrlich (1972), there was no
increase in respiratory symptoms or decrement in respiratory
performance in 778 New York pressmen compared with 1207 compositors.
No significant differences in death rates, were found in those who
were less than 40 years old when first employed, even if they had
worked for more than 20 years. In those first employed when they were
more than 40 years old and with more than 20 years of employment,
there was a significantly higher death rate in pressmen compared with
compositors. The reason for this finding was not clear. The mean oil
mist concentration measured in the 3 workshops concerned was
3.7-5.2 mg/m3.
Moss et al. (1972) analysed the causes of death of 3485 former
full-time printing industry workers from London and Manchester, who
died in the period 1952-66; Greenberg (1972) studied the death
certificates of 670 male printing workers who died between 1954 and
1966. In both studies, an excess number of cancers of the lung and
bronchus were found. This excess was more marked in Manchester and
greatest in machine-room men. The authors concluded that the slight
excess found might or might not be due to occupation. They considered
that an occupational cause was more likely in the case of the greater
excess in the Manchester machine-room men.
These studies in the printing industry have been mentioned in
relation to mineral oil exposure. However, the same data could be
related to carbon black and even to lead exposure. Similar
considerations should be kept in mind in the evaluation of the data in
other occupations where many other factors might also play a role,
either individually or combined with the mineral oil used, as, for
instance, chromium in the metal-working industry.
In studies in 34 metal-working firms in Baden-Würtemberg (FGR),
the frequency of respiratory complaints (cough, expectoration, and
dyspnoea) in 443 workers exposed to oil mist for long periods was
compared with that in 398 unexposed controls, matched for age. Mineral
oil mist exposures from cutting oils had ranged from 40 to 150 mg/m3
over long periods. The highest incidence of complaints was found among
unexposed smokers, the lowest incidence amongst non-smoking,
oil-mist-exposed workers. The authors did not find any signs of
irritative effects from oil-mist exposure, but a significant
protective effect against the well-known irritant effects of smoking
(Drasche et al., 1974). This finding is in line with findings in
experimental animals, that previous exposure to oil mist reduces the
lethal effects of respiratory oxidants such as ozone and nitrogen
dioxide in mice (Wagner et al., 1961). The authors stated that
conclusions could not be drawn from this study in relation to the
carcinogenicity of these oil mists in the respiratory tract.
During the cold processing of metal, the concentration of mineral
oil mist in the air (spindle oil aerosol) fluctuated from 3 to
40 mg/m3 (on average 10 mg/m3). During an examination of 77 lathe
operators (men and women), functional disturbances found to occur in
the respiratory system, in particular after a period of service of
more than 10 years, included a reduction in the active volume and the
maximum ventilation of the lungs and an increase in oxygen requirement
and in its coefficient of utilization (Bruskin & Demcenko, 1975).
A 30-year retrospective cancer mortality study was carried out by
Decoufle (1976) on 5189 workers engaged in metal-working for at least
one year. No significant differences in cancer mortality were
observed, when compared with the general population. Indications of
increased incidences of respiratory and digestive cancer, observed
when comparison was made on an age group or exposure basis, were not
statistically significant.
Decoufle (1978) published a further study on a group of 2485 male
workers employed between 1938 and 1967 in jobs exposing them to
various levels of cutting oil mists. Compared with the total death
rate of the US male population, no significant differences were
observed for 15 cancer site categories. However, a 2-fold risk of
cancers of the stomach and large intestine (combined) was seen after
20 years of follow-up in the subgroup of men with 5 or more years'
exposure to cutting oil mists, prior to 1938. Deaths from nonmalignant
respiratory disease were significantly fewer than expected. These
results suggested that occupational exposure to soluble and insoluble
cutting oil mists, during various metal machining processes, did not
pose a health hazard in terms of respiratory cancer and fatal
nonmalignant respiratory disease, but might be associated with certain
forms of gastrointestinal cancer.
To summarize, it can be concluded from the literature that
oil-mist exposure can give rise to pulmonary disease, but only after
prolonged exposure in workplaces with unsatisfactory hygienic
conditions. If oils with a low content of polynuclear aromatic
hydrocarbons are used in situations where oil mists can be generated
and the TLV for oil mists is not exceeded, this problem is unlikely to
arise.
4.8 Clinical Studies
Clinical studies on the effects of mineral base oils and products
derived from them on the skin have been discussed together with
epidemiological studies in sections 4.7.1.1 and 4.7.1.2. It was felt,
that compilation of individual case-histories from the literature on
this subject was superfluous. Adverse effects resulting from the
surgical use of paraffin for cosmetic purposes, and those following
grease-gun accidents have not been considered in this review.
Clinical studies on the effects of these products after ingestion
show that the main effects are caused by aspiration, which may be a
complication of ingestion, and usually occurs during subsequent
spontaneous or induced vomiting. Otherwise, there is general agreement
that mineral base oils, lubricating oils, and greases have a low order
of toxicity, when ingested (Gerarde, 1960). At the most, some
gastrointestinal symptoms, such as abdominal cramps and diarrhoea,
result.
There was evidence from a clinical case study that prolonged
ingestion of mineral oil over a number of years could result in oil
deposition in the small intestine, abdominal lymph nodes, liver, and
spleen and lungs. This produced significant structural and functional
abnormalities that were considered to have contributed to the
patient's death (Nochomovitz et al., 1975).
White mineral oil (pharmaceutical grade) is a base oil
specification intended for oral use. It is a highly purified base oil
distillate, mainly containing saturated paraffinic fractions, and it
has to be free of polynuclear aromatic hydrocarbons. It is used as a
laxative, in pharmaceutical formulations, and as a food-grade
lubricant. From studies with radio-opaque oil, it appears possible
that a small amount of mineral oil, when taken orally as a laxative
just before retiring, may gain entrance into the lungs. Though this is
not of any consequence if it occurs only once, a cumulative effect in
the lungs might result, if it occurs repeatedly, day after day, over
several years (Sante, 1949; Miller et al, 1962).
Clinical experience of the effects of inhalation of mineral oil in
the lungs is in agreement with the results of animal studies (section
4.6.2.3). In man, mineral oil is rapidly phagocytosed and transferred
to the regional lymph nodes. In contrast with vegetable oil and oil of
animal origin, however, mineral oil cannot be metabolized and is
deposited in the interstitial tissue and in the lymph nodes, where it
induces a cellular reaction, with the appearance of giant cells that
may result in fibrosis of more or less extensive lung areas. The
resulting clinical and pathological picture depends to a large extent
on whether there has been massive, acute over-exposure, with the
body's defence mechanisms overwhelmed (see (a)), or whether long-term,
low-level exposure has occurred, where the defence mechanisms can cope
with the daily exposure and no disease will become apparent, until the
signs and symptoms of the secondary fibrotic reaction appear (see
(b)), (Pinkerton, 1927, 1928; Cannon, 1940; Freiman et al., 1940; Moel
& Taylor, 1943).
A few typical clinical entities can be recognized:
(a) Diffuse acute mineral oil pneumonia is practically always
the result of an accidental massive aspiration of mineral oil (Sante,
1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham, 1954;
Gerarde, 1960). It is, in fact, a chemical pneumonitis frequently with
a superimposed secondary infection, progressing into an interstitial
proliferative inflammation. Cases resulting from aspiration following
accidental ingestion of products containing gasoline, kerosene, or
other petroleum solvents in the same boiling-range are well-known from
the clinical literature. Many cases have occurred as a result of
aspiration of seawater contaminated with diesel oil by survivors of
sinking ships (Weissman, 1951).
Aspiration of mineral oils in the boiling-range under discussion
may have similar results. This can happen when choking occurs, while
taking white medicinal oil. This acute type of mineral oil pneumonitis
is not likely to occur as a result of occupational exposure to oil
mist. Clinically, all signs and symptoms of an acute massive
pneumonitis are present with elevated temperature and a chest X-ray
typical for this condition. Oil droplets may be found in the sputum
and, histologically, the condition is characterized by intra-alveolar
accumulation of oil-laden phagocytes and inflammatory cells. Acute
mineral oil pneumonia is a special form of lipoid pneumonia, which
presents a similar clinical picture and is the result of aspiration of
vegetable or animal oil (mainly cod liver oil, but also egg-yolk and
milk). Though in these cases a similar severe acute pneumonia may
develop, the oil can be metabolized and disappears completely after a
certain time (Sante, 1949). With oils of animal origin, such as cod
liver oil, however, the reaction of the body (lungs and other internal
organs) can be much more serious than with mineral oils (Young et al.,
1939).
(b) Diffuse chronic mineral oil pneumonia occurs as a result of
gradually developing fibrotic and proliferative changes in both lungs.
It may follow years after the acute form, or without an acute
beginning after a long, practically asymptomatic period as a result of
repeated smaller "aspirations", such as those resulting from regular
massive use of mineral-oil-based laxatives, nose-drops, or nose-sprays
(Pinkerton, 1927; Bishop, 1940; Freiman et al., 1940). The apparently
rare cases of mineral oil pneumonitis following prolonged occupational
exposure to excessive oil mist concentrations fall into this category
(Sante, 1949; Proudfit et al., 1950; Weissman, 1951; Foe & Bigham,
1954; Gerarde, 1960). Reports from the literature suggest that in some
of these cases, at least, a predisposing factor was present in the
form of a pre-existent or concomitant lung disease (Freiman et al.,
1940; Weissman, 1951; Forbes & Markham, 1967). Increasing dyspnoea and
productive cough are the most important symptoms. The chest X-ray
generally shows increased perihilar opacities with signs of lung
fibrosis and diffuse patchy opacities. Oil droplets or oil-laden
phagocytes may be found in sputum or in tissue derived from lung
biopsy (Goodwin, 1934; Bishop, 1940; Freiman et al., 1940; Rossier &
Bühlmann, 1949; Sante, 1949; Proudfit et al., 1950; Borrie & Gwynne,
1973).
(c) Paraffinomas are large fibrous nodules or globules of liquid
mineral oil embedded in dense hyaline fibrous tissue. They represent a
separate form of the chronic mineral oil pneumonitis in which
oil-laden phagocytes, destroyed by pressure, atrophy in the fibrous
scar tissue formed. Paraffinomas may be found singly or in clusters
around the large bronchial branches or at the site of the hilar lymph
nodes. It may be difficult to differentiate a paraffinoma from a lung
tumour radiologically (Brown & Biskind, 1941; Wood, 1943; Sante, 1949;
Proudfit et al., 1950; Weissman, 1951; Bryan & Boitnott, 1969; Borrie
& Gwynne, 1973). Aspiration biopsy may help in the differential
diagnosis (Nathanson et al., 1943).
Boitnott & Margolis (1966a) have described analytical methods for
the identification of the various oils in human tissues. Mineral oil
droplets may pass from the hilar nodes via the thoracic duct into the
systemic circulation (Pinkerton, 1927; Young et al., 1939; Freiman et
al., 1940; Boitnott & Margolis, 1966b). As a result of this
transmission, oil droplets have been found in the liver, spleen, and
other organs (Pinkerton & Moragues, 1940; Rewell, 1947).
The general pattern found in young children is slightly different
from that found in adults. Pinkerton (1927) described 6 cases of
lipoid pneumonia in children and data on 25 cases in children have
been summarized by Goodwin (1934). Seven cases have been reported by
Ikeda (1935) and 27 cases by Bromer & Wolman (1939). There is general
agreement that fats and oils of animal origin play a more important
role than mineral oil in lipoid pneumonia in infants and young
children. Furthermore, the acute massive aspiration type is more
frequently seen in children than the chronic form. The main causal
factors in these observations are: false passage of a gavage tube,
false deglutition in bottle-feeding with the baby lying on its back --
especially in cases of debilitating disease, and also aspiration
following forced administration of milk or cod liver oil with or
without vomiting or choking (Freiman et al, 1949).
With regard to adults, Ikeda (1937) summarized 106 cases from the
literature, Graef (1939) 22 cases, Bishop (1940) 136 cases, Freiman et
al. (1940) 58 cases, and Moel & Taylor (1943) 20 cases. They concluded
that lipid pneumonia, especially the diffuse chronic form, occurs more
frequently in adults than is generally believed. Liquid paraffin is by
far the most important etiological agent in the adult. Debilitated
states, dysphagia and impaired cough reflexes, because of neurological
or other disorders, are important predisposing factors. The authors
stress, however, that mineral oil is widely used without evident harm,
even in elderly persons. Wherever there is a real indication for this
type of medication, they see no reason to discontinue it. Extensive
use, especially self-medicated, of liquid paraffin intranasally or via
the oral route by debilitated or dysphagic patients should, however,
be discouraged (Bishop, 1940; Freiman et al., 1940).
As a matter of interest, a few cases of lipoid pneumonia have been
described in relation to the intratracheal administration of
mineral-oil-based mixtures by opera singers, prior to every
performance on the stage, in order to improve the quality of the voice
(Even, 1947; Facquet & Langeard, 1947; Meyer, 1976). A similar case
was described by Garvin (1939) as a result of intratracheal
self-medication.
From the literature, it is apparent that with the recognition of
the causal factors and the change from oil-based to water-based
nose-drops, the incidence of the type of lipid pneumonia just
described has drastically decreased, since the end of the forties.
The following more or less typical cases, in which there was -- or
might have been -- a relation between occupational exposure to oil
mist and the occurrence of lipid pneumonia, were found in the
literature. Proudfit et al. (1950) reported a case of chronic lipid
pneumonia in a 40-year-old man who had been spraying mineral oil for
17 years; he had a typical chest X-ray. The chief complaints were
cough, shortness of breath, and fatigue. Mineral oil droplets were
identified in the sputum; 3 years later the condition had progressed
slightly. A case of lipid pneumonia was described by Weissman (1951)
in which the disease apparently developed on the basis of
long-standing pulmonary fibrosis as the result of blast-spraying of
machine parts with mineral oil; no mask had been used as protection
against the inhalation of nebulized oil. Foe & Bigham (1954) reported
the case of a 30-year-old aircraft mechanic who complained of fatigue,
shortness of breath on exertion, and frequent chest colds. Lipid
pneumonia was diagnosed from a lung biopsy. The mechanic had been
spray-cleaning aircraft engines with a mixture of 50% kerosene and 50%
vegetable-oil-soap.
Two cases of progressive respiratory disease, which developed in
the fifth decade of life were described by Forbes & Markham (1967).
Both patients had a moderate to heavy smoking history; one, in
addition to this, had a family history of asthma; dyspnoea and
wheezing were the major signs in each case. Both reacted well to
treatment, but recurrence of signs and symptoms was related to working
with cutting oils, the composition of which was not mentioned.
The epidemiological data on the possible relationship between
cancer of the respiratory tract and long-term occupational exposure to
oil mist has been discussed in section 4.7.1.2. There is some evidence
that in cases where an unsatisfactory industrial hygiene situation
coincided with the use of an oil with probable carcinogenic
properties, such a causal relationship might exist. On the other hand,
various extensive studies have shown that, in general, it is certainly
not a major problem.
However, Wood (1943) reported a fatal case of extensive lipid
pneumonia in a house-maid who had used oily nose-drops in large
quantities for recurrent sinusitis over a period of 10 years. At
postmortem, an alveolar-cell carcinoma was found in the lungs. The
author suggested that there might have been a causal relationship
between the 2 diseases, though he assumed that this occurrence would
be rare. Two cases of bronchogenic carcinoma were described by Sante
(1949). In the first case, the malignant epithelial tumour was
situated in an area of dense fibrotic tissue, typical of a
paraffinoma. In the other case, a squamous cell carcinoma was found
together with lipid pneumonia in an early stage of organization. The
author felt that there might have been an etiological relationship in
the first case, but considered this less likely in the second case.
Both patients had taken a tablespoon of mineral oil as a laxative,
just before retiring, for years. Volk (1964) studied a series of more
than 100 cases of mineral oil pneumonia and noted that not one case of
bronchogenic carcinoma occurred in this group. In addition, he
reviewed 114 consecutive autopsies of adenocarcinoma of the lung in
which he did not find any lesions that might be associated with
mineral oil pneumonia.
A rapidly lethal case of multifocal alveolar cell carcinoma of the
lung was reported in a 63-year-old man who had centrifuged used
cutting oil for 23 years and had been exposed continuously to oil mist
of this type during that period (Despierres et al, 1965). Wahlberg
(1974) described one case of primary lung cancer in 7 men who
developed scrotal cancer following heavy occupational exposure to oil
and oil mist in, for example, automatic lathe operation. A case in
which achalasia led to chronic mineral oil pneumonia was reported by
Bryan & Boitnott (1969). An adenocarcinoma developed in the area of
scarring resulting from the mineral oil and caused death. On the basis
of a literature study and theft own findings, the authors concluded
that the pathogenesis might be related to the pulmonary scarring
rather than directly to the mineral oil (see also Yokoo & Suckow,
1961). However, the authors also considered that there was no reason
to suppose that carcinomas were more likely to arise in a scar induced
by mineral oil than in a scar of a different origin (Bryan & Boitnott,
1969).
5. BITUMEN
5.1 Properties and Analytical Methods
5.1.1 Chemical and physical properties
The term bitumen is applied to solid and semi-solid residues from
the distillation of suitable crude oils. This product is known as
"asphalt" in the USA. In most other countries, the term asphalt is
reserved for certain natural deposits and for mechanically made
mixtures of bitumen and mineral matter.
Bitumen is the residue obtained by atmospheric and vacuum
distillation of certain types of crude oil. It is generally a black or
dark-brown material, ranging from a highly viscous liquid to a solid
and brittle substance at normal ambient temperatures, depending on the
proportion of light fractions removed. On heating, bitumen softens
gradually and eventually becomes fluid. Grades are characterized by
their "penetration" and "softening" point. According to the Petroleum
Handbook (1966), bitumen can be considered as a colloidal system of
highly condensed aromatic particles in an oil with ring-type
molecules. From this statement, it is clear that bitumen is a very
complex mixture of mainly high-boiling hydrocarbons. Its composition
varies widely with the geographical source of the crude oil and the
process of manufacture. For example, a mixture of 6 samples was found
to contain:
(a) 32% asphaltenes: high-relative-molecular-mass aromatic compounds
and heterocyclic hydrocarbons of which some are unsaturated. They
are soluble in carbon disulfide but insoluble in petroleum
naphtha;
(b) 32% resins: polymers resulting from the processing of unsaturated
hydrocarbons;
(c) 14% saturated hydrocarbons: hydrocarbons in which the carbon atoms
are connected by a single bond; and
(d) 22% aromatic hydrocarbons: hydrocarbons containing one or more
benzene rings per molecule, including condensed polycyclic
aromatic hydrocarbons (Simmers et al., 1959; Simmers, 1964).
While the appearance and engineering applications of bitumens and
asphalts are similar to those of coal-tars and pitches, fundamental
differences exist between these 2 classes of materials (Puzinauskas &
Corbett, 1978). Bitumen is generally derived from crude oil by a
process that does not involve cracking or thermal conversion, and
coal-tars and pitches are obtained by high-temperature carbonization
of bituminous coal. Chemically, coal-tar materials are mainly composed
of highly condensed-ring aromatic and heterocyclic hydrocarbons.
Bitumens, on the other hand, contain a much higher proportion of high
relative molecular mass paraffinic and naphthenic hydrocarbons and
their derivatives. Under comparable heating during application and
use, coal-tars generate substantially higher emissions of polynuclear
aromatic hydrocarbons than bitumen. While epidemiological surveys of
workers engaged in the production of coal-tar have revealed an
increased incidence of lung cancer, no increases in cancer or other
adverse effects have been observed in studies on workers involved in
the manufacture and application of asphalt.
The benzo(alpha)pyrene content of petroleum bitumens derived from
various Russian crude oils was determined by Janyseva et al. (1963).
They demonstrated that the benzo(alpha)pyrene content of straight-run
bitumen was considerably lower (of the order of 0.6 mg/kg) than that
of bitumens derived from cracking residues (of the order of
4-272 mg/kg). Schamp & van Wassenhove (1972) reported
benzo(alpha)-pyrene levels of 3-5 mg/kg in bitumens.
5.1.2 Methods of sampling and analysis
See section 2.1.2.
5.2 Sources of Environmental Pollution
5.2.1 Natural sources
Natural bitumen and asphalt deposits occur in various parts of the
world, mainly as a result of mineral oil seepage from the ground. The
most well-known natural asphalt deposit is the Trinidad Lake, which
contains a mixture of about 39% bitumen, 32% mineral matter, and 29%
water and gas.
5.2.2 Man-made sources
5.2.2.1 Production
Total world-wide bitumen production reached 90 million tonnes in
1973. As with crude oil, this was approximately 10 times the immediate
pre-war level. Bitumen production rose to 100 million tonnes in 1979
and is expected to continue to increase in the future, although at a
lower rate of growth than in the past.
The following types of bitumen are produced by refining and
treatment:
"Straight" bitumen
The residue of atmospheric or vacuum distillation of
asphaltic-based crude oils. For special applications, very hard
pitch-type bitumen residues can be obtained by distilling cracked
oils.
"Blown" bitumen
Manufactured by feeding air-bubbles countercurrent through a
column of hot molten straight bitumen. Oxidation reactions occur
leading to dehydrogenation and polymerization of the unsaturated and
aromatic components. In this process, large condensed aromatic nuclei
may also formed.
"Cutback" bitumen (or more fluid bitumen grades)
Obtained by mixing bitumen with petroleum solvents or mineral oil,
sometimes with coal-tar or high aromatic extracts.
Bitumen emulsion
Made by emulsifying 50-65% of bitumen in water in the presence of
0.5-1.0% of an emulsifier, usually soap and generally used cold for
both roadmaking and industrial purposes.
5.2.2.2 Uses
The main use of bitumen is for paving roads. It is also used in:
(a) lining irrigation canals, water reservoirs, dams, and dykes;
(b) mastic asphalt for industrial flooring;
(c) bituminized felts for roofing;
(d) protective coatings, for walls, motor-cars, water mains;
(e) adhesives for the building industry;
(f) coal briquetting;
(g) electrical insulation; and
(h) battery-making.
5.3 Environmental Exposure Levels
Apart from walking or riding on bituminous pavements and roads,
the general population will not normally come into contact with
bitumen, except in the form of protective coatings and coal
briquettes. On the other hand, the general population can, on
occasion, be exposed to fumes from heated bitumens for short periods
of time during road-building or the covering of roofs. Emissions from
asphalt roads have been mentioned in the literature as a possible
source of exposure for the general population, but such exposure is
considered to be as negligible. Recent evidence from the Federal
Republic of Germany and the USA confirms this (Hettche, 1963).
Occupational exposure to bitumen may be much more intensive in
certain professions and may range from accidental splashing with hot
bitumen to repeated and prolonged contact of the skin with the more
liquid bitumen grades or to exposure to fumes from heated bitumen.
5.4 Environmental Distribution and Transformation
No specific data relating to bitumens are available in relation to
distribution among media, environmental transformation and
degradation, interaction with physical, chemical, or biological
factors, and bioconcentration. On the other hand, there is a lot of
information on the microbial degradation of individual petroleum
hydrocarbons (section 3.4).
5.5 Metabolism
Uptake and storage of the light fractions contained in bitumens
may occur; however, no specific data exist on this subject.
Bitumens occur in a variety of commercial products. The
composition of these products may vary widely depending on the
geographical origin of the crude oil used and the manufacturing
process applied. These facts may influence the results of metabolic
studies, all of which are concerned with the exposure of experimental
animals. Human data are lacking.
5.6 Effects on Experimental Animals
5.6.1 Short-term exposure
No data are available on acute toxicity, exposures related to
adverse effects, interactions, and species comparisons. It is
generally accepted that the acute toxicity of bitumens is low.
5.6.2 Long-term exposure
No data appear to have been published on toxic effects in specific
organs, teratogenicity, or reproduction. The data on mutagenicity are
limited. On the other hand, substantial experimental work has been
carried out concerning the carcinogenic effects of bitumens on skin.
Twort & Fulton (1930) examined the carcinogenic effects of various
synthetic tars and their fractions on the skin of mice. The
carcinogenic activity varied according to the compound used and with
the temperature of pyrolysis. Results of these studies confirmed the
earlier finding of Kennaway (1925), that the temperature at which the
tar formed was an important factor in the production of carcinogenic
substances. Cancer of the skin of mice was induced by applications of
the synthetic residues obtained from heating substances such as
acetylene, isoprene, a noncarcinogenic petroleum, yeast, and human
skin. The yield of carcinogens decreased when carbonization had
occurred at temperatures above 950°C. The yield was greatest between
850 and 870°C; less between 600 and 750°C, and negligible at 500°C.
Furthermore, the authors found that the carcinogenic activity of the
synthetic residues could be reduced considerably by oxidation or
reduction (by various methods) or by dilution with oleic acid.
A wide range of aliphatic and simple aromatic hydrocarbons were
pyrolysed by Badger (1962), who showed that polynuclear aromatic
hydrocarbons were formed from simpler hydrocarbons at 700°C via
primary radicals formed by carbon-hydrogen and carbon-carbon fission
(cracking) at this elevated temperature. The same process occurred to
a much lesser extent at 550°C.
According to Bogovski et al. (1963), thermal distillation of a tar
to coke decreased the carcinogenic activity by the formation of
irreversible condensation products from polynuclear aromatic
hydrocarbons, together with other high molecular compounds. In
long-term, skin-painting experiments on white mice, the authors showed
that such a coking process reduced the tumour incidence from 68% to
3.7% in the case of shale-oil tar. As shale-oil tars have a much
higher carcinogenic activity than petroleum residues (Twort & Twort,
1931), it might be expected that coking of petroleum residues would
reduce the carcinogenicity of the material still further.
The carcinogenic effects of bitumen on the skin of C-57 black mice
was studied by Simmers and co-workers (1959). In a first series of
tests, they used a mixture of 6 samples from Southern Californian
refineries, in which both steam- and aft-blown bitumens were mixed.
Painted twice weekly on the skin for a lifetime, the mixture caused 12
dermoid carcinomas in 68 animals, compared with none in the untreated
control group. Formation of cancer was preceded by hair loss, dryness
and scaling of the skin, and papilloma formation. After subcutaneous
injection, 8 sarcomas occurred in 62 animals at the site of injection
compared with none in the control group. However, the relevance of
results from such subcutaneous studies is questionable.
In an inhalation study on the same pooled sample and using the
same strain of mice, the animals inhaled an aerosol of bitumen
droplets suspended in moist air for 30 min/day, 5 days per week, for
up to nearly 17 months (Simmers, 1964). Changes found on microscopic
examination were minimal and included occasional congestion, acute
bronchitis, pneumonitis, bronchial dilatation, and some peribronchial
round-cell infiltration. In a second inhalation study, the animals
were exposed to cooled smoke from bitumen at 120°C for 6-7´ h/day, 5
days a week, for up to 21 months. In this study, peribronchial
round-cell infiltration, bronchitis, pneumonitis, abscess formation,
loss of cilia, epithelial atrophy, and necrosis were more common.
Squamous cell metaplasia was rare, but hyperplasia was more commonly
seen. The changes in both experiments were patchy, rather
non-specific, and similar to those described as a result of exposure
to other air pollutants.
In his next series of tests, Simmers (1965a) compared the
carcinogenicity of straightrun and air-blown bitumen in prolonged
regular skin application tests and after single or repeated
subcutaneous injection. Undiluted air-blown bitumen did not induce
tumours, when applied to the skin, probably because the bitumen was
too hard; when dissolved in toluene the incidence of skin cancers
increased to 45%. This may be the result of better contact with, or
penetration into the skin. On the other hand, the polynuclear aromatic
hydrocarbons might be concentrated by the solvent. In a similar
skin-painting study with straight-run bitumen, skin cancers occurred
in only 14%. The same trend was apparent after subcutaneous injection,
where 0 and 13% tumours were found at the site of injection with
straight-run and air-blown bitumen, respectively. The author presumed
that this difference in response resulted from a difference in
chemical composition. Although the aromatic fraction of air-blown
bitumen was lower than that of straight-run bitumen, it contained more
complex aromatic hydrocarbons due to polymerization and condensation
caused by the air-blowing.
Straight-run bitumen was separated into 4 fractions, which were
painted 3 times a week, continuously, on the skin of the same strain
of mice (Simmers 1965b). The fraction containing most of the saturated
compounds and aromatic hydrocarbons -- which also showed practically
all the UV light fluorescence -- induced considerably more skin
rumours (43.3%) than straight-run and air-blown bitumens had done in
earlier studies. The author pointed out that, though fluorescence was
not a guarantee of carcinogenic activity, polynuclear aromatic
hydrocarbons known to be carcinogenic are fluorescent. In this
context, both Kennaway & Heiger (1930) and Berenblum et al. (1947)
suggested that these compounds -- even in very small quantities --
might be discovered by this method. The 4 bitumen fractions used in
the previous study were injected subcutaneously -- once or repeatedly
-- in the same strain of mice, at various doses (Simmers, 1966). A
variety of benign and malignant tumours resulted, both at the
injection site and in distant organs. The dose seemed to be more
important for tumour formation than the duration of exposure.
Hueper & Payne (1960) compared the carcinogenicity of various
petroleum products and coal-tar in long-term tests on mice, rats, and
guineapigs. Four road-bitumens of different geographical and
manufacturing origin were applied to the skin of, or injected
intra-muscularly into, mice and rats. In some of the test-animals,
tumours were found at the site of application. Fumes from heated
coal-tar and from a blown bitumen used as a roofing bitumen did not
induce cancers of the lungs in rats and guineapigs in inhalation
experiments lasting up to 2 years. However, the general typical
reactions found in lung-tissue were the same as those described by
Simmers (1964). Condensates of the coal-tar fumes were highly
carcinogenic when applied to the skin and intramuscularly in mice;
condensates of the blown bitumens fumes were non-carcinogenic to the
skin of mice and rabbits in similar tests.
Petroleum residues derived from cracking were painted 3 times a
week for a lifetime on the skin of albino mice. Depending on the
cracking process, the residues exhibited various degrees of
carcinogenic activity, but none were as active as a higher temperature
generated coal-tar. Only those fractions distilled from the tar above
370°C showed carcinogenic activity. Blending with a noncarcinogenic
oil increased the carcinogenic activity of one bitumen, possibly
because of better skin penetration (Smith et al., 1951).
Kireeva (1968) painted groups of white SS-57 mice, once a week
throughout the life span, with 40% solutions in benzene of various
bitumens derived from Ukrainian crudes and coal-tar pitch. The
control-group was painted with benzene only. With coal-tar pitch, skin
tumours appeared in 88.4% of the animals; with bitumens prepared from
cracking residues, they occurred in 9.5-18.4%, and with straight-run
bitumens, depending on its origin, in only 0.0-4.6% of the animals.
Benzene solutions of 8 bitumens of different geographical origin
and 2 coal-tar pitches were applied twice weekly to the skin of Swiss
albino mice throughout their lifetime. Benzene alone was applied to
control animals (Wallcave et al., 1971). The polynuclear aromatic
hydrocarbon content of the coal-tar pitches was found to be several
orders of magnitude greater than that of the bitumens examined. Only
one carcinoma and 5 papillomas were observed in 218 mice treated with
bitumens, whereas over 90% of the coal-tar pitch treated animals
developed such tumours. Again, it was suggested that the tumour
incidence depended on the polynuclear aromatic hydrocarbon content of
the product tested.
Benzene solutions of the bitumen or polynuclear aromatic fractions
of Athabasca tar sands were not mutagenic for Salmonella typhimurium.
This may have been a reflection of the complex interactions occurring
with such hydrocarbon mixtures (Shahin & Fournier, 1978).
To summarize, in animal studies, some bitumens have been shown to
possess some carcinogenic activity, when applied to the skin, while
inhalation studies with bitumen vapours have proved to be negative.
The carcinogenicity of bitumens depends to a certain degree on the
method of production (cracking, blowing) or mixing. "Cutback" with
high aromatic oil or coal-tar facilitates skin-contact and increases
the carcinogenicity of the mixture. The carcinogenic activity,
however, is low in comparison with that of coal-tar. Moreover, as Siou
(1972) in a literature study on bitumens concluded, it is difficult to
extrapolate from these animal data to man, because the contact with
bitumen, even in occupational exposures, is of a quite different order
to regularly repeated skin application throughout the life span of a
test animal.
5.7 Effects on Man
5.7.1 Epidemiological studies
5.7.1.1 Occupational exposure
Henry (1947), in his analysis of 3753 cases of skin cancer, found
only one case in which bitumen might have been involved. The man had
worked for 23 years as a road-worker and had been exposed to coal-tar
in the earlier years.
The health and the causes of death of 96 workers who had worked in
a bitumen plant since it opened, including those who had left the
company was reviewed by Hoogendam (1962). Exposures ranged up to 40
years. Thirty-nine workers had been exposed for more than 20 years and
at least 15 had been exposed for more than 30 years. No significant
differences were found in theft general health pattern and no cases of
skin tumours were found. One man, who had worked for 40 years in this
plant died at the age of 55 of bronchial carcinoma. It is impossible
to determine whether there was a relation with his work in this case.
Vital capacities and forced expiratory volume (FEV) of those still
working did not differ from those of a control group.
The mortality rate of refinery workers was compared with those of
other workers in a study on 15 437 employees of an oil refinery over
the 29-year period 1935-63. A comparison was also made with data from
a similar group of the outside population. The incidence of lung
cancer was no greater in the refinery operators than in the other
groups (Baird, 1967).
Baylor & Weaver (1968) compared the health of 462 asphalt workers
in 25 oil refineries with that of 379 controls. Each of the asphalt
workers had been engaged in this type of work for at least 5 years
with an average of 15.1 years, service. No significant differences in
health were found between the 2 groups. The authors also studied the
medical literature of the 20 years preceding theft publication and
were unable to find one single case of lung or skin cancer that could
be attributed to petroleum bitumens. Furthermore, an extensive search
for information on the health of workers was carried out with road
construction firms (31 companies in 24 states with 11 478 man-years of
asphalt working and 15 boards of health of the state highway
commissions), roofing industries (3 firms with over 1100 long-term
bitumen workers), and bitumen trucking firms (with over 5000 drivers).
This gave no indication that bitumen constituted a health hazard or
that there were cancers of the skin or lungs in these groups that
could be attributed to working with bitumen. Similar indirect
information was obtained from 6 large insurance companies. The authors
concluded that the bitumens in current use did not present a
significant health hazard and that the study indicated that the
carcinogenic or other harmful properties of most commercial asphalts
under the present commercial usage -- if present at all -- are likely
to be of a very low order.
Studies conducted on behalf of the API did not reveal any
occupational health hazards for a "medium-exposure group" of petroleum
refinery workers. This group included asphalt workers, grease, and
lubricant workers (Tabershaw/Cooper Associates Inc. 1974/75).
Hermann (1975) and Hettche (1963) drew similar conclusions to
those of Baylor & Weaver (1968) in inferring that bitumens including
blown grades, were biologically inactive and that vapours from hot mix
plants were not carcinogenic.
5.7.1.2 General population exposure
Measurement of emissions of polynuclear aromatic hydrocarbons from
various industrial processes, including bitumen "blowing" and the
manufacture of asphalt hot-road-mixture, revealed that these
industrial processes were not major sources of benzo(alpha)pyrene
emissions (used as an indicator for polynuclear aromatic hydrocarbons
emission) and certainly emitted less than residential and small
industrial coal-burning furnaces (Von Lehmden et al., 1965).
In the literature, the possibility is mentioned that vapours
emanating from asphalt, or dust arising from it might contribute to
the overall incidence o f cancer of the respiratory tract (Hueper,
1961; Berge, 1969). There are not, however, any data to substantiate
this assumption and, in extrapolating the results of animal testing on
bitumen-vapour inhalation, the chances of such an effect occurring
among the general population seem quite remote. It can be presumed
that a lot of the confusion has been generated by the former use of
coaltar and mixed grades for this purpose, a fact which clearly has
not always been taken into consideration by those working in the
field.
In the USSR, however, the use of "carcinogenic" road material
(that is material containing benzo (a) pyrene) as the upper layer of
roads is forbidden in heavily populated areas. In practice, this means
that coal-tar must not be used for this purpose and that petroleum
bitumen should be used instead (Gorbov & Fomenko, 1962).
5.7.1.3 High (accidental) exposure
Burrell (1957) found a very high incidence of oesophageal cancer
in a group of Bantu confined to one location in East London (South
Africa). The author related this to the long-term preparation and use
of the soporific alcoholic concoction "cidiviki", which was locally
fermented in drums still containing a ¨-inch coating of"cutback"
bitumen. The author presumed that enough carcinogenic material could
have leached out into the brew to have at least acted as a
cocarcinogen.
The most common accidental over-exposure in handling bitumen is
the occurrence of burns from hot bitumen splashes. A few cases,
however, have been described, in which a skin tumour developed within
1-3 months of a burn caused by hot bitumen, coal-tar, or crude oil
(Bang, 1923; Huguenin, 1925; Gunsett, 1930; Sträuli 1957). Considering
the total number of such bums, only a very small proportion develop
into rumours and these cases relate to combinations of such burns with
fresh scar tissue and/or lesions of the mucous membranes. Tumours have
been described in similar situations following heat burns by wood,
welding electrodes, etc. (Gunsett, 1930; Sträuli, 1957). Such skin
tumours can occur in burn scars and are often multifactorial (Emmett,
1975).
In relation to the vast amount of bitumen that has been used for
many decades, the reports of tumours are extremely scarce. In fact, it
can be postulated that, though, from animal testing, it is known that
some bitumens are weak carcinogens, there is no evidence from normal
occupational exposure to indicate that these compounds are a
carcinogenic hazard to the skin or the respiratory tract. This would
also indicate that the present pattern of use of bitumens would not
cause any hazard, whatsoever, for the general population.
The Asphalt Institute studied emissions from the hot-mix process
for the manufacture of paving asphalts (Puzinauskas & Corbett, 1975).
The concentration of polynuclear aromatic hydrocarbons in the
particulate emission was low at 340 mg/m3 and the average
concentration of benzo(alpha)pyrene was very low at 13 ng/m3.
5.7.2 Clinical studies
These relate mainly to bitumen skin burns and will not be
considered here. Other clinical data have been included in the
description of the epidemiological data.
The skin of fair-haired persons might be more prone to react
adversely to repeated prolonged exposure to UV-radiation in sunlight
in combination with bitumen exposure (Smiley, 1951; Kinnear et al.
1954; Emmett, 1975).
6. EVALUATION OF HEALTH RISKS FROM EXPOSURE TO CRUDE OILS AND
SELECTED PETROLEUM PRODUCTS
6.1 Crude Oils
Crude oil is normally handled in a closed system from the oil
well, via storage tanks, pipelines, and shipment by tankers to the
refineries. Under these conditions, health hazards to, and death of
workers involved in these operations will occur only when a serious
breakdown or leakage occurs. Volatile components escaping at well
heads, at pump glands, or through vents in storage tanks and ships'
tanks may, under certain conditions, constitute a similar health
hazard; hydrogen sulfide, if present, is the most acutely toxic
component but detailed consideration is outside the scope of this
review. These volatile components may also contribute to the pollution
of the atmosphere in storage or pumping areas. To the general
population, this is mainly a nuisance problem, because of the odour
from the hydrogen sulfide and mercaptans involved. Crude oil pollution
of seas and inland waterways as a result of accidents with crude oil
tankers or pipelines may, at times, cause a major and sudden
environmental hazard. Tank washings from tankers not using the
"load-on-top" system are another source of pollution of the sea with
crude oils. However, this particular aspect falls outside the scope of
this review.
Atmospheric concentrations of the volatile components of crude oil
were found to be lower near marine drilling rigs than land-based rigs.
Differences in temperature and air movement have been shown to be the
main causes.
The lower temperature of marine pipelines encourages
solidification of waxy compounds of the crude oil on their inner
surfaces. During their repair, the clothes and skin of workers may be
contaminated by these compounds (Alekperov et al., 1974).
6.2 Petroleum Solvents
Though petroleum products are widely used, they do not generally
present a health risk for the general population, as their volatility
prevents them accumulating in the ambient air in concentrations high
enough to cause adverse health effects. However, if improperly used in
closed, poorly ventilated rooms, they may become a cause of accidental
acute poisoning. Under these conditions, the lower-boiling solvents
may also present a fire hazard.
Cases of accidental ingestion may occur, especially in children.
Serious and even fatal lung disease may develop when aspiration in the
lungs occurs. Absorption from the gut, however, is generally not a
serious health hazard.
Substances with a strong odour may cause a nuisance, when present
in the ambient air, even in very low concentrations. The same applies
to the possible contamination of drinking-water through leakage of
petroleum solvents from containers.
Health impairment due to occupational exposure to petroleum
solvents occurs only infrequently in normal work practice. Repeated
skin contact may result in contact irritative dermatitis and only
rarely in contact allergic dermatitis. Continuous day-to-day exposure
to excessive vapour concentrations (which usually occur in poorly
ventilated workshops) may give rise to general non-specific symptoms
of ill-health. Some solvents, however, have a specific systemic
effect, e.g., benzene (bone marrow depression, leukaemogenesis) and
n-hexane (polyneuropathies). Polyneuropathies also occur in cases of
abusive use of petroleum solvents by "sniffers" and addicts.
Accidental exposure to excessive vapour concentration may cause
narcosis, which can be followed very rapidly by respiratory arrest and
death. Accidental over-exposure of the skin, especially if the solvent
is allowed to remain in contact with the skin, may cause skin
irritation, possibly leading to chemical burns.
Blood dyscrasia does not occur through exposure to petroleum
solvents that do not contain benzene. The specific long-term health
hazards of exposure to benzene and petroleum solvents that contain a
substantial percentage of benzene will not be discussed in this
document.
The high aromatic residues that are still used, occasionally, as
solvents for specific purposes may pose a carcinogenic risk to the
skin or on inhalation, if such contact is intensive and prolonged.
6.3 Lubricating Base Oils, Greases, and Waxes
This group of products is unlikely to present a health risk for
the general population, even with gross accidental over-exposure.
Unrestricted and indiscriminate oral or nasal administration of white
medicinal oil can occasionally give rise to the occurrence of lipid
pneumonia, when pre-existent disease or other conditions predispose to
the entry of mineral oil into the respiratory tract.
Prolonged and intensive contact of the skin with metal-working
oils during occupational exposure results in a high incidence of skin
disorders and relatively more cases of skin cancer have occurred in
such occupations. Improvements in both industrial and personal hygiene
have reduced the incidence of malignant and non-malignant diseases of
the skin. Results from animal studies indicate that substitution of
the oils, formerly used, by more refined products may significantly
contribute to the prevention of skin cancer caused by exposure to oil
under poor standards of industrial hygiene. Because chronic lung
disease following exposure to oil mist is extremely rare and an
increase in the incidence of pulmonary carcinoma has only been
reported under exposure conditions where skin cancer occurs, it seems
reasonable to assume that these occupational diseases can be prevented
by suitable preventive measures.
6.4 Bitumen
The health risks associated with bitumen appear to be minimal,
because contact is unlikely. Occupational exposure is not associated
with an increase in cancer of the skin or the respiratory tract. The
most significant occupational hazard is burning of the skin from
splashes of heated bitumen.
Certain bitumen grades, however, such as those derived from
cracked oils, and those mixed with coal-tar or high aromatic extracts,
may have a carcinogenic potential and, therefore, require special
precautions in handling.
7. CONTROL MEASURES
7.1 General
Every effort should be made not to contaminate workers, the work
place, or the general environment with petroleum products. Proper
design of machinery, equipment and the workshop, enclosure of
processes, adequate ventilation, provision of protective clothing and
adequate facilities for personal hygiene, suitable education and
supervision, and development of safe working procedures are essential
basic requirements; good hygienic work practices can achieve a great
deal in protecting the worker.
Care should be taken, when developing products, to ensure that
levels of the most toxic components, e.g., benzene, n-hexane, and
polynuclear aromatic hydrocarbons are known, so that proper controls
can be devised for the use of the products.
Products containing such highly toxic components should, when
possible, be avoided and alternatives sought, where exposure is
unavoidable or is likely to occur. Products which, during use, may
generate more and highly toxic contaminants should be identified and
efforts made to reduce contamination. Care should be exercised when
handling and using such products and consideration given to the need
for renewal of products at appropriate intervals. In relation to
metal-working, the danger of repeatedly "topping-up" machinery over
long periods with a product, and of mixing different products in one
machine, is emphasized.
Where contact is unavoidable, suitable protective equipment should
be used, although this should always be used as the last resort. Where
skin contact is inevitable, efforts should be made to limit or avoid
not only benzene, n-hexane, and polynuclear aromatic hydrocarbons
but also any additives that may have an adverse effect on the skin.
The use of abrasives and solvents in cleansing the skin should be
discouraged.
In the less developed countries, there is often a lack of
awareness concerning the need for proper control measures when
handling petroleum products. In these countries, the health education
of employers and workers should be promoted with reference to the
products. Adequate control programmes should be implemented using
known techniques. Whenever necessary, these should be modified to fit
the particular circumstances of the country.
In addition, proper planning is necessary to control the disposal
of the many types of waste oil products so that environmental
contamination is avoided. Thought must also be given to the need to
control the siting of housing and amenities in relation to petroleum
refineries and petrochemical plants.
7.2 Petroleum Solvents
Excessive exposures to these products should be avoided. Proper
education of manufacturers and users and appropriate labelling of
consumer products are important. Regular control of workroom
atmospheric concentrations of petroleum solvents is indicated,
especially in smaller workshops.
Petroleum solvents are, with the exception of benzene and
n-hexane, less toxic than most other solvents and, with suitable
precautions, can be safely used. For this reason, and because they are
cheap, they are widely used in industry and their replacement by other
solvents is unlikely in the foreseeable future.
7.3 Lubricating Base Oils, Greases, and Waxes
The only way in which members of the general population could,
under normal circumstances, be over-exposed to any of this group of
compounds would be in the indiscriminate use of medicinal liquid
paraffin. Excessive use -- especially self-administered -- of this
material, intranasally or via the oral route, by debilitated or
dysphagic patients should be discouraged.
The main possibilities of over-exposure occur in the occupational
situation. Without going into detail, the following factors should be
considered with the aim of avoiding hazardous exposures:
(a) Avoid contact by technological means including: proper design
of equipment, machinery, and workshop; proper design and operation of
general and exhaust ventilation systems in places where oil mist
generation cannot be avoided; use of base oils with the lowest content
of polynuclear aromatic hydrocarbons that can practically be achieved
by suitable refining processes; limiting of the concent of additives
that have adverse effects on the skin; frequent changing of oil in
situations where carcinogenic compounds might be generated by heat;
avoidance of the reuse of used oils, without refining, for other
purposes where they might cause a health hazard; education and
instruction in all these safe-working procedures, and supervision of
their proper execution;
(b) Use of safe-working procedures and, where required, protective
clothing and proper protective equipment; availability of general
hygienic facilities including wash-basins in or near the work-place,
shower facilities, lockers for private clothing outside the
contaminated area; regular changing and laundering of working clothes
and underwear; the use of suitable emollient skin creams and in some
instances barrier creams. Abrasives and solvents should not be used
for cleaning the skin.
(c) Periodic medical examinations.
These precautions have been compiled and adapted from the
following references: Kipling (1968); UK Medical Research Council
(1968); Cruickshank (1969); Desoille et al. (1973); BRMA (undated).
Manufacturer can contribute by ensuring that these precautions are
observed in their own premises and by giving the proper information
and guidance to the consumer (see also Eckardt, 1967).
Government actions vary from country to country depending on local
legislation and practice. They may range from supervision and control
of the measures taken by industry to the issue of directives or codes
of practice. Cautionary notices can be issued (e.g., HMSO, 1979),
which have to be displayed in the working place, as well as leaflets
for the workers (e.g., HMSO, 1967). Diseases caused by mineral oil may
be notifiable as occupational diseases, according to national
legislation.
Consumer education on the possible hazards of the products used
and their safe handling can best be done by the manufacturing
industries, when possible in cooperation with relevant Government
agencies.
Medical action in this field would consist of cooperating in the
team supervising the industrial hygiene of the operation, in
pre-placement examination and selection, and later in the periodic
medical follow-up of the exposed workers.
Various other organic liquids meet requirements for lubricating
oils and greases. Carboxylic esters are generally used. These
synthetic lubricants, however, are not in abundant supply, are
expensive, and are not likely to be widely used outside their specific
field of application. Replacement products are sometimes more
hazardous. For instance, cutting oils may be replaced by mixtures
containing ethanolamine that give rise, under certain conditions, to
the formation of nitrosamines.
When safe lubricating base oils are used there is, on the other
hand, no reason from an occupational health point of view to use such
alternatives, not even in places where long-term intensive skin
contact and/or oil mist exposure might take place. In these cases,
industrial hygiene should be improved and personal hygiene should be
closely supervised, regardless of the product used, to avoid excess
exposure.
7.4 Bitumen
In road-building which is the major application of bitumen,
alternative materials such as plastics and resins are far too
expensive for general use. Coal-tar, which was used previously,
presents a greater environmental health hazard than bitumen. Other
alternatives would be concrete and bricks.
In various other applications, such as flooring, roofing,
protective coatings, and adhesives, alternative materials are
available, but these are not always superior in quality and/or
competitive in price.
REFERENCES
ABBRITTI, G., SIRACUSA, A., CIANCHETTI, C., COLI, C. A., CURRADI, F.,
PERTICONI, G. F., & De ROSA, F. (1976) Shoe-makers' polyneuropathy
in Italy: The aetiological problem. Br. J. ind. Med., 33: 92-99.
ACGIH (1971a) Documentation of the threshold limit values,
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 19-20.
ACGIH (1971b) Documentation for TLV -- Supplements for those
substances added or changed since 1971, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists, p. 341.
ACGIH (1974) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1974, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978a) Threshold limit values for chemical substances in
workroom air adopted by ACGIH for 1978, Cincinnati, OH, American
Conference of Governmental Industrial Hygienists.
ACGIH (1978b) Air sampling instruments for evaluation of atmospheric
contaminants. 5th ed., Cincinnatti, OH, USA, American Conference
of Governmental Industrial Hygienists.
ADLARD, E. R. (1972) A review of the methods for the identification of
persistent hydrocarbon pollutants on seas and beaches. J. Inst.
Petrol., 58: 63-74.
AIDIN, R. (1958) Petrol-vapour poisoning. Br. med. J., ii: 369-370.
AIHA (1976) Direct reading colorimetric indicator tubes manual,
Akron, OH, USA, American Industrial Hygiene Association.
AINSWORTH, R. W. (1960) Petrol-vapour poisoning. Br. med. J.,
i: 1547-1548.
ALEKPEROV, I. I., AMIROV, R. O., POVOVAROV, V. D., & LOSEVA, I. E.
(1974) [Hygiene of work at exploitation of marine oil deposits.]
Moscow, Idz. "Nedra" (in Russian).
ALLEN, N. (1975) Chemical neurotoxins in industry and the environment.
In: Tower, D. B., ed. The nervous system, Vol. 2, The clinical
neurosciences, New York, Raven, pp. 235-248.
ALTENKIRCH, H., MAGER, J., STOLTENBURG, G., & HELMBREHT, J. (1977)
Toxic polyneuropathies after sniffing a glue thinner. J. Neurol.,
214: 137.
ANDRÉ, M. L. & O'NEAL, M. J. (1959) Mass spectrometric analyses of
medium viscosity lubricating oils. Anal. Chem., 31: 164-169.
ANON, (1973) Evaluations of world's important crudes. Oklahoma, USA,
The Petroleum Publishing Company (Reprinted from Oil Gas J.).
ANON (1974) Exposure to petrol fumes. Br. med. J., ii: 115.
ANON (1975) Benzpyrene hydroxylase - innate determinant of PAH
toxicity? Food cosmet. Toxicol., 12: 264-268.
ANTONOV, A.M. & LINTS, A.M. (1960) The blastomogenic action of natural
Saratov oil. Problems Oncol., 6: 1629-1634.
API (1965) Toxicological review. Aromatic petroleum naphtha. 2nd
ed., New York, American Petroleum Institute, pp. 5.
API (1967a) Toxicological review. Gasoline, 1st ed., New York,
American Petroleum Institute, pp. 5.
API (1967b) Toxicological review. Kerosine, 2nd ed., New York,
American Petroleum Institute, pp. 4.
API (1969a) Final Report of the task force on used oil disposal.
Washington, DC, American Petroleum Institute, pp. 44.
API (1969b) Toxicological review. Petroleum naphthas, 1st ed., New
York, American Petroleum Institute, pp. 7.
API (1974) Mutagenicity study of thirteen petroleum fractions,
Washington, DC, American Petroleum Institute (API U-150-14, The
Hine Laboratories Inc. Report No. 4).
API (1975a) Review of analytical methods for determination of oil and
grease in waters produced from oil and gas extraction operations.
Prepared by the Task Group on Analytical Methods July 14th 1975,
New York, American Petroleum Institute, pp. 38.
API (1975b) Mutagenic evaluation of rubber solvent (CHF-32-263),
W/filtered rubber solvent (Summary and analysis), Washington,
DC, American Petroleum Institute (Litton Bionetics Inc. Report).
API (1975c) Research report. Mutagenic evaluation of 60 solvent
(CHF-33-148) (Summary and Analysis), Washington, DC, American
Petroleum Institute (Litton Bionetics Inc. Report).
API (1977a) Research report. Mutagenicity evaluation of kerosene.
Final report (Summary and Analysis), Washington, DC, American
Petroleum Institute, 3 pp. (Litton Bionetics Inc. Project
No. 2697).
API (1977b) Research report. Teratology study in rats: Stoddard
solvent. Final report (Summary and Analysis), Washington, DC,
American Petroleum Institute, 5 pp. (Litton Bionetics Inc. Project
No. 20698-2).
API (1978a) Mutagenicity evaluation of Stoddard solvent (Summary),
Washington, DC, American Petroleum Institute (Litton Bionetics
Inc. Report no. API-PS-4).
API (1978b) Research report - Teratology study in rats: Rubber
solvent. Final report. Litton, API (Full Report and Analyses).
API (1978c) Teratology study in rats: Xylene. Final Report,
Washington, DC, American Petroleum Institute, 39 pp. (Litton
Bionetics Inc. Project No. 20698-5).
API (1978d) Research report. Mutagenicity of xylene. Final report
(Full report and Analytical).
API (1978e) Teratology study in rats: Toluene. Final report (Summary
and Analysis), Washington, DC, American Petroleum Institute, 2 pp.
(Litton Bionetics Inc. Project No, 20698-4).
(1978f) Mutagenicity evaluation of toluene (Summary and Analysis),
Washington, DC, American Petroleum Institute, 3 pp. (Litton
Bionetics Inc. Project No. 20847).
API (1979a) Research report. Teratology study in rats: Kerosene.
Final report (Summary and Analytical), Washington, DC, American
Petroleum Institute 2 pp. (Litton Bionetics Inc. Project
No. 20698-10).
API (1979b) Research report. Inhalation teratology study in rats: VM
and P naphtha, Final report, Washington, DC, American Petroleum
Institute, 18 pp. (Litton Bionetics Inc. Project No. 21035-02).
API (1979c) Research report. Teratology study in rats: n-Hexane.
Final report, Washington, DC, American Petroleum Institute,
24 pp. (Litton Bionetics Inc. Project No. 20698-9).
API (1979d) In vitro and in vivo mutagenicity studies: New oil
composite. Final report, Washington, DC, American Petroleum
Institute (Hazleton report).
ARCOS, J. C. & ARGUS, M. F. (1974) Chemical induction of
cancer-structural bases and biological mechanisms, Vol. 11A,
New York and London, Academic Press.
ASK-UPMARK, E. (1955) Bronchial carcinoma in printing workers. Dis.
Chest., 27: 427-235.
ASTM D2820 (1972) Standard method of test for Cl through C5
hydrocarbons in the atmosphere by gas chromatography,
Philadelphia, Am. Soc. for Testing and Materials.
ASTM D1605-60 (1973) Standard recommended practices for sampling
atmospheres for analysis of gases and vapours, Philadelphia,
Am. Soc. For Testing and Materials.
ASTM D510 (1974) Standard methods of sampling water, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2682 (1974) Standard method of test for polynuclear
aromatic hydrocarbons in air particulate matter, Philadelphia,
Am. Soc. for Testing and Materials.
ASTM D2778 (1974) Standard method of test for solvent extraction or
organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ASTM D3370 (1976) Standard method of test for solvent extraction
of organic matter from water, Philadelphia, Am. Soc. for Testing
and Materials.
ÅSTRAND, I., KILBOM, A., & ÖVRUM, P. (1975) Exposure to white spirit.
I. Concentration in alveolar, air and blood during rest and
exercise. Scand. J. Work environ. Health, 1: 15-30.
ÅSTRAND, L., ENGSTRÖM, J., & OVRUM, P. (1978) Exposure to xylene and
ethylbenzene. I. Uptake, distribution and elimination in man.
Scand. J. Work Environ. Health, 4: 185-194.
AULD, S. J. M. (1950) Environmental cancer and petroleum. J. Inst.
Petrol., 36: 235-253.
AVELLAN, L., JACOBSON, B., & JOHANSON, B. (1967) Carcinoma of scrotum
induced by mineral oil. Scand. J. plast. reconstr. Surg.,
1: 135-140.
AXELSON, O., HANE, M., & HOGSTEDT, C. (1976) [Case descriptions of
chronic psycho-organic syndrome in house painters.]
Läkartidningen, 73: 317-318 (in Swedish).
BADGER, G. M. (1962) Mode of formation of carcinogens in human
environment. Nat. Cancer Inst. Monogr., 9: 1-16.
BAIRD, V. C. (1967) Effects of atmospheric contamination on cancer
mortality in petroleum refinery employees. J. occup. Med.,
9: 415-420.
BALDWIN, R. W., CUNNIGHAM, G. J., & PRATT, D. (1964) Carcinogenic
action of motor engine oil additives. Br. J. Cancer,
18: 503-507.
BANG, F. (1923) Contribution a l'étude de la cancérisation de la
cellule et du temps d'éclosion des tumeurs malignes. A propos d'un
cas de "cancer aigu" du goudron chez un ouvrier. Bull. Assoc. Fr.
Étud. Cancer, 12: 184-199.
BARANOVA, V. G. & LOGINOVA, N. K. (1977) [Determining cyclopentadiene
in the air of industrial premises.] Gig. Tr. prof. Zabol.,
No. 3, pp. 54-55 (in Russian).
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of ethyl
benzene, styrene and alpha-methylstyrene in man. Am. Ind. Hyg.
Assoc. J., 31: 206-209.
BARRIENTOS, A., ORTUNO, M. T., MORALES, J. M., TELLO, F. M., &
RODICIO, J. L. (1977) Acute renal failure after use of diesel fuel
as shampoo. Arch. intern. Med., 137: 1217.
BATT-NEAL, L. & WOLMAN, M. (1977) Tumors and amyloidosis in mice
painted with crude oil found on bathing beaches. Bull. environ.
Contam. Toxicol., 18: 385-391.
BAYLOR, C. H. & WEAVER, N. K. (1968) A health survey of petroleum
asphalt workers. Arch. environ. Health, 17: 210-214.
BELINKY, B. R. (1980) In: Dolberg, D. D. & Verstuyft, ed. Analytical
techniques in occupational health chemistry. Washington, DC,
ACS, (ACS Symposium Series 120).
BELL, J. (1876) Paraffin epithelioma of the scrotum. Edin. med. J.,
22: 135.
BERENBLUM, I., HOLIDAY, E. R., & JOPE, E. M. (1947) Some physical
methods of investigating carcinogenic hydrocarbons. Br. med.
Bull., 4: 326-331.
BERGE, H. (1969) [Harmful emissions in and around cities.]
Vitalstoffe, 14: 161-163 (in German).
BERGEL, F. (1974) Carcinogenic hazards in natural and man-made
environments. Proc. R. Soc. (London), 185: 165-181.
BERTINE, K. K. & GOLDBERG, E. E. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BESKROVNAJA, N. I., HRUSTALEVA, G. F., ZIGULINA, G. A., & DAVYDKINA,
T. I. (1979) [The gynaecological sick rate of female workers in
the rubber industry.] Gig. Tr. prof. Zabol., No. 8, pp. 36-38
(in Russian).
BILIMAIER, D., YEE, H. T., ALLEN, N., CRAFT, B., WILLIAMS, N.,
EPSTEIN, S., & FONTAINE, R. (1947) Peripheral neuropathy in a
coated fabrics plant. J. occup. Med., 16: 665-671.
BINGHAM, E. & FALK, H. L. (1969) Environmental carcinogens. The
modifying effect of co-carcinogens on the threshold response.
Arch. environ. Health, 19: 779-783.
BINGHAM, E. & HORTON, A. W. (1966) Environmental carcinogenesis:
experimental observations related to occupational cancer.
Adv. Biol. Skin, 7: 183-193.
BINGHAM, E. & WORD, P. J. (1977) Co-carcinogenic effects of n-alkanes
and ultraviolet light on mice. J. Natl Cancer Inst.,
38(4): 1099-1101.
BINGHAM, E., HORTON, A. W, & TYE, R. (1965) The carcinogenic potency
of certain oils. Arch. environ. Health, 10: 449-451.
BISHOP, P. G. C. (1940) Oil aspiration pneumonia and pneumolipoidosis.
Ann. int. Med., 13: 1327-1359.
BLATTNER, R. J. (1951) Kerosene poisoning. J. Pediatr., 39: 391-392.
BOEHME, H. & HUEHNERMANN, W. (1966) Examination of mineral oil
products in the (West) German Pharmacopoeia, 7th edition. Arch.
Pharm., 299: 368-80D.
BOENHEIM, A. F. & PEARSON, A. J. (1973) Petroleum hydrocarbon
solvents. In: Modern petroleum technology, 4th ed., Barking,
Applied Science Publishers Ltd.
BOGOVSKI, P. A., EISEN, O. G., & ARRO, I. H. (1960) [On the
carcinogenic action of some chromatographic fractions of
chamber-oven tar obtained from Estonian oil-shale.] Vop. Onkol.,
6: 34-42 (in Russian).
BOGOVSKI, P. A., GORTALUM, G. M., & KOZHEVNIKOV, A. V. (1963)
Decancerigenization of some shale processing products. Acta Un.
Int. Cancer, 19: 481-482.
BOHLEN, P., SCHLUNEGGER, U. P., & LÄUPPI, E. (1973) Uptake and
distribution of hexane in tissues. Toxicol. appl. Pharmacol.,
25: 242.
BOITNOTT, J. K. & MARGOLIS, S. (1966a) Mineral oil in human tissues.
I. Detection of saturated hydrocarbons using thin-layer
chromatography. Bull. John Hopkins Hosp., 118: 402-713.
BOITNOTT, J. K. & MARGOLIS, S. (1966b) Mineral oils in human tissues,
II. Oil droplets in lymphnodes of the porta hepatis. Bull. John
Hopkins Hosp., 118: 414-422.
BORRIE, J. & GWYNNE, J. F. (1973) Paraffinoma of lung: Lipoid
pneumonia. Report of two cases. Thorax, 28: 214-221.
BOURNE, L. B. (1967) Skin diseases from oils - causes and prevention,
London, British Safety Council, 11 pp.
BOUTWELL, R. K. & BOSCH, D. K. (1959) The tumor-promoting action of
phenol and related compounds for mouse skin. Cancer Res.,
19: 413-424.
BP (1973) Health guide to BP petroleum products, London, The British
Petroleum Company Ltd, pp. 26.
BP (1975) Trace elements and other elements in crude oil:
A literature review, Sunbury, The British Petroleum Company Ltd.
BRIDGE, J. C. & HENRY, S. A. (1928) Industrial cancers. In: Report of
the International Conference on Cancer, London, Bristol, John
Wright, pp. 258-268.
BRMA (undated) Mineral oils, London, British Rubber Manufacturers
Association Ltd.
BROMER, R. S. & WOLMAN, I. J. (1939) Lipoid pneumonia in infants and
children. Radiology, 32: 1-7.
BROWN, A. J., WALDRON, H. A., & WATERHOUSE, J. A. H. (1975) Report on
a study of occupational skin cancer with special reference to
scrotal cancer, Birmingham, UK, University of Birmingham.
BROWN, A. L. & BISKIND, G. R. (1941) Differential diagnosis between
lipid pneumonia and pulmonary neoplasm. J. Am. Med. Assoc.,
117: 4-6.
BROWNING, E. (1959) Toxic solvents: a review. Br. J. ind. Med.,
16: 23-39.
BROWNING, E. (1965) Toxicity and metabolism of industrial solvents,
Amsterdam, London, New York, Elsevier Publishing Company.
BRUSKIN, Z. Z. & DEMCENKO, V. G. (1975) [External respiration and gas
exchange of workers who are subjected to the effect of lubricating
oil aerosols.] Gig. Tr. prof. Zabol., No. 4, pp. 28-30
(in Russian).
BRYAN, C. S. & BOITNOTT, J. K. (1969) Adenocarcinoma of the lung with
chronic mineral oil pneumonia. Am. Rev. Respir. Dis.,
99: 272-274.
BUKOVSKIJ, M. I., ZUKOV, V. L, KOZUHOVA, T. V., SANOCKIJ, I. V., &
SIDOROV, K. K., ed. (1978) [Maximum permissible concentrations
and guidelines for safety levels of hazardous substances in the
environment.] Severodoneck, All-Union Research Institute of
Safety Technique in the Chemical Industry, and Research Institute
of Labour Hygiene and Occupational Diseases, Academy of Medical
Sciences of USSR (in Russian).
BURRELL, R. J. W. (1957) Oesophageal cancer in the Bantu. S. A.
Tydskrif vir Geneeskunde, 31: 401-409.
BÜTTNER, G. (1974) Benzene in the work environment. Deutsche
Forschungsgemeinschaft, Bonn, FRG, Harald Boldt Verlag K. G.
Boppard, 63 pp.
CANNON, P. R. (1940) The problem of lipid pneumonia. J. Am. Med.
Assoc., 115: 2156-2179.
CAPELLINI, A., CHIAPPINO, G., & ZURIO, N. (1968) [Clinical and
experimental observations on so-called tricresylphosphate
polyneuritis.] Med. Lav., 59: 721-759 (in Italian with English
summary).
CARGILL, C. (1972) Polynévrites dans l'industrie du cuir et de la
chaussure: toxicité du n-hexane, Thèse, Paris VII.
CARITHERS, H. A. (1955) Accident prevention in childhood -- the
kerosene hazard. J. Am. Med. Assoc., 159: 109-11.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977a) Petroleum hydrocarbon
toxicity studies. XIV. Animal and human response to vapors of
"high aromatic solvent". Toxicol. appl. Pharmacol.,
41: 235-241.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977b) Petroleum hydrocarbon
toxicity studies. XV. Animal response to vapors of "high
naphthenic solvent". Toxicol. appl. Pharmacol., 41: 251-260.
CARPENTER, C. P., GEARY, D. L., Jr, MYERS, R. C., NACHREINER, D. J.,
SULLIVAN, L. J., & KING, J. M. (1977c) Petroleum hydrocarbon
toxicity studies. XVI. Animal response to vapours of Naphthenic
Aromatic Solvent. Toxicol. appl. Pharmacol., 41: 261-270
(contains references to the other studies in this series).
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971a) Specifications
for cutting oils with special reference to carcinogenicity. Ann.
occup. Hyg., 14: 171-179.
CATCHPOLE, W. M., MACMILLAN, E., & POWELL, H. (1971b) Specifications
for cutting oils with special reference to carcinogenicity.
J. Inst. Petrol., 57: 247-260.
CAVANAGH, J. B. (1973) Peripheral neuropathy caused by chemical
agents. In: C.R.C. Crit. Rev. Toxicol., 2: 365-417.
CIANCHETTI, C., ABBRITTI, G., PERTICONI, G., SIRACUSA, A., & CURRADI,
A. (1976) Toxic polyneuropathy of shoe-industry workers: A study
of 122 cases. J. Neurol. Neurosurg. Psychiatr., 39: 1151-1161.
CLAYTON, G. D. & CLAYTON F. E. (1978) Patty's industrial hygiene and
toxicology. 3rd revised ed., New York, Wiley & Sons.
COHR, K.-H. & STOCKHOLM, J. (1979) Toluene. A toxicological review.
Scand. J. Work Environ. Health, 5: 71-90.
COMSTOCK, B. S. (1977) A review of psychological measurements relevant
to central nervous system toxicity with special reference to
solvent inhalation. Clin. Toxicol, 11: 317-324.
CONCAWE (1981a) Exposure to atmospheric benzene vapour associated
with motor gasoline. The Hague (Concawe report No. 2/81).
CONCAWE (1981b) Guidelines for the determination of atmospheric
concentrations of oil-mists. The Hague (Concawe report
No. 1/81).
CONCAWE STUDY GROUP (1972) Methods for the analysis of oil in water
and soil, The Hague, Stichting Concawe, pp. 66 (Concawe report
No. 9/72).
CONCAWE TASK FORCE (1973) Disposal of used lubricating oil in Western
Europe, The Hague, Stichting Concawe, 59 pp. (Concawe report
No. 9/73).
CONCAWE TASK FORCE (1976) Methods for the determination of
hydro-carbons in the atmosphere, The Hague, Stichting Concawe,
pp. 23 (Concawe report No. 3/76).
CONTAMIN, F., GOULON, M., & MARGAIRAZ, A. (1960) Polynévrites
observées chez des sujets utilisant comme moyen de chauffage des
appareils à combusion catalytique de l'essence. Rev Neurol.,
103: 341-345.
COOK, J., CARRUTHERS, W., & WOODHOUSE, D. L. (1958) Carcinogenicity of
mineral oil fractions. Br. med. Bull., 14: 132-135.
CORPER, H. J. & FREED, H. (1922) The intratracheal injection of oils
for diagnostic and therapeutic purposes. J. Am. Med. Assoc.,
79: 1739-1743.
CRISP, A. J, BHALLA, A. K., & HOFFBRAND, B. I. (1979) Acute tubular
necrosis after exposure to diesel oil. Br. med. J.,
2(6183): 177.
CRUICKSHANK, C. N. D. (1969) Occupational cancer of the scrotum.
Br. med. j., 26: 249-250.
CRUICKSHANK, C. N. D. & GOUREVITCH, A. (1952) Skin cancer of the hand
and forearm. Br. J. ind. Med., 9: 74-79.
CRUICKSHANK, C. N. D. & SQUIRE, J. R. (1950) Skin cancer in the
engineering industry from the use of mineral oil. Br. J. ind.
Med., 7: 1-11.
DAESCHNER, C. W. Jr, BLATTNER, R. J., & COLLINGS, V. P. (1957)
Hydrocarbon pneumonitis. Pediatr. Clin. North Am., resp.
Disorders. February: 243-253, Philadelphia.
DANIEL, J. W., FRAZER, A. C., FRENCH, J. M., & SAMMONS, H. G. (1953)
The intestinal absorption of liquid paraffin in the rat.
Biochem. J., 54: 37p.
DARGENT, M., MARCEAU, M., JOSEPH, P., & FAUCON, M. (1967) Etude
experimentale sur les effets tumorigènes des lubrifiants utilisés
dans le décolletage des métaux. Rev. Lyonnaise Méd.,
16: 409-420.
DAS, B. S. & THOMAS, G. H. (1978) Fluorescence detection in high
performance liquid chromatographic determination of polycyclic
aromatic hydrocarbons. Anal. Chem., 50: 967-973.
DAVIS, A., SCHAFER, L. J., & BELL, Z. G. (1960) The effect in human
volunteers of exposure to air containing gasoline vapour. Arch.
environ. Health, 1: 548-554.
DECOUFLE, P. (1976) Cancer mortality among workers exposed to cutting
oil mist. Ann. N.Y. Acad. Sci., 271: 94-101.
DECOUFLE, P. (1978) Further analysis of cancer mortality patterns
among workers exposed to cutting oil mist. J. Natl Cancer Inst.,
61: 1025-1030.
De PIERRE, J. W. & ERNSTER, L. (1978) The metabolism of polycyclic
hydrocarbons and its relationship to cancer. Biochim. Biophys.
Acta., 473: 149-186.
DESOILLE, H., PHILBERT, M., RIPAULT, G., CAVIGNEAUX, A., & ROSSIGNOLI,
H. (1973) Action cancérogène des huiles minérales utilisées en
métallurgie. Arch. Mal. prof. 34: 669-680.
DESPIERRES, G., BENNET, P. A., PHELIP, H., & PLANTE, M. (1965) Cancer
alveolaire et inhalation d'huiles industrielles. J. Fr. Med.
Chir. Thorac., 19: 561-567.
Deutsche Forschungsgemeinschaft (1970) [Health hazardous substances
in the workplace.] Weinheim, Verlag. Chemie (in German).
DIETZ, W. A., KING, W. H. Jr, PRIESTLEY, W. Jr, & REHNER, J. Jr (1952)
Properties of high boiling petroleum products. Ind. Eng. Chem.,
44: 1818-1827.
DRASCHE, H., FINZEL, L., MARTSCHEI, H., & MEYER, R. (1974)
[Occupational health considerations in persons exposed to oil
mist.] Zbl. Arbeitsmed., 24: 305-312 (in German).
EBERT, A. G., SCHLEIFER, C. R., & HESS, S. M. (1966) Absorption,
disposition, and excretion of 3H-mineral oil in rats. J. Pharm.
Sci., 55: 923 -929.
ECKARDT, R. E. (1957) Carcinogenicity of petroleum products with
particular reference to the automotive industry. Ind. med. Surg.,
26: 396 -398.
ECKARDT, R. E. (1967) Cancer Prevention in the petroleum industry.
Int. J. Cancer, 2: 656-661.
EHRHARDT, M. & HEINEMAN, J. (1975) Hydrocarbons in blue mussels from
the Kiel Bight. Environ. Pollut., 9: 263-281.
ELY, T. S., PEDLEY, S. F., HEARNE, F. T., & STILLE, W. T. (1970) A
study of mortality symptoms and respiratory function in humans
occupationally exposed to oil mist. J. occup. Med.,
12: 253-261.
EMMETT, E. A. (1975) Occupational skin cancer: A review. J. occup.
Med., 17: 44-49.
ENGSTROM, K., HUSMAN, K., & RIIHIMÄKI, V. (1977) Percutaneous
absorption of m-xylene in man. Int. Arch. occup. environ. Health,
39: 181-189.
ESSO RESEARCH (1960) Unpublished report in: Toxicological evaluation
of some food additives including anticaking agents, antioxidants,
emulsifiers and thickening agents. WHO Food Additive Series,
No. 5, World Health Organization, Geneva.
ESSO (undated) This is about health-lubricating oils and cutting
fluids, London, Esso.
EVEN, R. (1947) Les pneumopathies huileuses de l'adulte. Soc. Med.
Hop. Paris, 63: 78-80.
EYRES, A. R. (1973) Codes of practice relating to metal working
fluids: the oil suppliers' view. J. Inst. Petrol., 59: 9-14.
FACQUET, M. J. & LANGEARD, Mme (1947) Deux cas d'opacités pulmonaires
du lobe moyen chez des chanteurs (pneumonie huileuse probable).
Bull. Mém. Soc. Méd. Hop. Paris, 63: 200-204.
FAO/WHO (1970) Toxicological evaluation of some extraction solvents
and certain other substances (Nutrition Meetings Report Series
No. 48a; WHO/Food Add./70.39).
FAO/WHO (1971) Specifications for the identity and purity of some
extraction solvents and certain other substances (Nutrition
Meetings Report Series No. 48b; WHO/Food Add./70.40).
FIELDNER, A. C., KATZ, S. H., & KINNEY, S. P. (1921) Permeation of
oxygen breathing apparatus by gases and vapours, Washington,
United States Bureau of Mines (Technical Paper 272).
FISCHER, H. G. M., PRIESTLEY, W. Jr, EBY, L. T., WANLESS, G. G., &
REHNER, J. Jr (1951) Properties of high boiling petroleum
products. Arch. ind. Hyg. occup. Med., 4: 315-324.
FLOODGATE, G. D. (1972) Microbiol degradation of oil. Mar. Pollut.
Bull., 3: 41-43.
FOE, R. B. & BIGHAM, R. S. (1954) Lipid pneumonia following
occupational exposure to oil spray. J. Am. Med. Assoc.,
155: 33-34.
FOKKENS, W., HOUWEN, J. W., HUENDER, H. G. P., KARPIAK-VAN-DRIEL, J.,
LIEM, T. H., & De WAARD, F. (1972) [Scrotal cancer and mineral
oils.] T. Soc. Geneeskd., 50: 343 (in Dutch).
FORBES, J. D. & MARKHAM, T. N. (1967) Cutting and grinding fluids in
chronic pulmonary airway disease. J. occup. Med., 9: 421-423.
FOURNAS, D. W. & HINE, C. H. (1958) Neurotoxicity of some selected
hydrocarbons. Arch. ind. Health, 18: 9-15.
FRANCO, G., MOGLIA, A., & GHITTORI, S. (1979) [Occupational neuropathy
due to cyclohexane.] Med. Lav., 70(2): 118-124
(in Italian).
FREIMAN, D. G., ENGELBERG, H., & MERRIT, W. H. (1940) Oil aspiration
(lipoid) pneumonia in adults -- A clinicopathologic study of
forty-seven cases. Arch. int. Med., 66: 11-38.
FÜHNER, H. (1921) [Narcotic action of gasoline and its components
(pentane, hexane, octane).] Biochem. Zeitschr., 115: 235-261
(in German).
GAMBERALE, F., ANNWALL, G., & HULTENGREN, M. (1975) Exposure to white
spirit. II. Psychological functions. Scand. J. Work Enrivon.
Health, 1: 31-39.
GARVIN, C. G. (1939) Lipoid pneumonia. Arch. int. Med., 64: 586-589.
GAULTIER, M., RANCUREL, G., PIRA, C., & EFTHYMIOU, M.-L. (1973)
Polynévrites et hydrocarbures aliphatiques. J. eur. Toxicol.,
6: 294-296.
GERARDE, H. W. (1959) Toxicological studies on hydrocarbons. V.
Kerosine. Toxicol. appl. Pharmacol., 1: 462-474.
GERARDE, H. W. (1960) Toxicology and biochemistry of aromatic
hydrocarbons in commercial solvents, Amsterdam, London, New
York, Princeton, Elsevier Publishing Company.
GERARDE, H. W. (1963) Toxicological studies on hydrocarbons. IX. The
aspiration hazard and toxicity of hydrocarbons and hydrocarbon
mixtures. Arch. environ. Health, 6: 329-341.
GERARDE, H. W., & GERARDE, D. F. (1961/1962) The ubiquitous
hydrocarbons (Reprinted from Assoc. Food Drug Off. United
States, Vols XXV and XXVI, 1961/1962).
GHAZAI, S. (1974) Sept cas de polynévrite dans une usine de chaussures
à Teheran. Arch. Mal. prof., 35: 552-553.
GIBSON, D. T., MAHADERAN, V., JERINA, D. M., YAGI, H., & YEH, H. J. C.
(1975) Oxidation of the carcinogens benzo(a)pyrene and
benzo(a)-anthracene to dihydrodiols by a bacterium. Science,
189: 295-297.
GILCHRIST, C. A., LYNES, A., STEEL, G., & WHITHAM, B. T. (1972) The
determination of polycyclic aromatic hydrocarbons in mineral oils
by thin-layer chromatography and mass spectrometry. Analyst,
97: 880-888.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1955) Cutting oils and
squamous-cell carcinoma. Part II: An experimental study of the
carcinogenicity of two types of cutting oils. Br. J. ind. Med,
12: 244-248.
GILMAN, J. P. W. & VESSELINOVITCH, S. D. (1956) An evaluation of the
relative carcinogenicity of two types of cutting oils. A.M.A.
Arch. ind. Health, 14: 341-345.
GOLDSTEIN, D. H. & BENOIT, J. N. (1970) An epidemiologic study of an
oil mist exposure. Arch. environ. Health, 21: 600-603.
GONZALEZ, E.G. & DOWNEY, J. A. (1972) Polyneuropathy in a glue
sniffer. Arch. Phys. Med. Rehabil., 53: 333-337.
GOODWIN, T. C. (1934) Lipoid cell pneumonia. Am. J. Dis. Child.,
48: 309 -326.
GORBOV, V. A. & FOMENKO, V. N. (1962) [On the hygienic evaluation of
asphalt coverings as a possible source of air pollution with
carcinogens.] Gig. i Sanit., 27: 100-101 (in Russian).
GRAEF, I. (1939) Studies in lipid pneumonia. Arch. Pathol.,
28: 613-667.
GREENBERG, M. (1972) A proportional mortality study of a group of
newspaper workers. Br. J. in Med., 29: 15-20.
GRIMMER, G. (1972) [The quantitative determination of polycyclic
aromatics by capillary gas chromatography.] Erdöl Kohle,
25: 339-343 (in German).
GRIMMER, G. (1979) Distribution of polycylic dromatic hydrocarbons in
environmental samples. In: Egan, H., ed. Environmental
carcinogens, selected methods of analysis. Vol. 3 - Analysis
of polycyclic aromatic hydrocarbons in environmental samples,
Lyons, IARC pp. 55-61 (WHO/IARC Publication No. 29).
GRIMMER, G. & BÖHNKE, H. (1976) [Enrichment and gas chromatographic
profile analysis of polycyclic aromatic hydrocarbons in
lubricating oils.] Chromatographia, 9: 30-40 (in German).
GRIMMER, G. & BÖHNKE, H. (1978) [Polycyclic aromatic hydrocarbons and
heterocyclic compounds.] Erdöl Koble, 31: 272-277 (in German).
GRIMMER, G. & BÖHNKE, H. (1972) [Determination of total content of
polycyclic aromatic hydrocarbons in air dust and automobile
exhaust by capillary GC.] Z. anal. Chem., 261: 310-314 (in
German).
GUNSETT, A. (1930) Un cas de cancer aigu, épithélioma
spino-cellulaire, developpé après traumatisme par de l'asphalte
enflammé. Bull. Assoc. Etud. Cancer, 19: 459-462.
GUSEIN-ZADE, K. M. (1975) [Reactions of the skin on the effect of
Apsheron commercial oils.] Gig. Tr. prof. Zabol., No. 7,
pp. 51-52 (in Russian).
HAENNI, E. O. & HALL, M. A. (1960) Tentative ultraviolet absorption
limits for mineral oils. J.A.O.A.C., 43: 92-95.
HAENNI, E. O., JOE, F. L. Jr, HOWARD, J. W. & LEIBEL, R. L. (1962a) A
more sensitive and selective ultraviolet absorption criterion for
mineral oil. J.A.O.A.C., 45:(1): 59-66.
HAENNI, E. O., HOWARD, J. W., & JOE, F. L. Jr (1962b) Dimethyl
sulfoxide: A superior analytical extraction solvent for
polynuclear hydrocarbons and for some highly chlorinated
hydrocarbons. J.A.O.A.C., 45: 67 -70.
HAGGARD, H. W. (1921) The anesthetic and convulsant effects of
gasoline vapour. J. Pharmacol. Exper. Ther., 16: 401-404.
HAINES, J. R. & ALEXANDER, M. (1974) Microbial degradation of high
molecularweight alkanes. Appl. Microbiol., 28(6): 1084-1085.
HÄNNINEN, H., ESKELINEN, L., HUSMAN, K., & NURMINEN, M. (1976)
Behavioral effects of long-term exposure to a mixture of organic
solvents. Scand. J. Work Environ. Health, 4: 240-255.
HARTWELL, J. L. (1951) Survey of compounds which have been tested for
carcinogenic activity. 2nd ed. Washington, DC, Federal Security
Agency (Public Health Service Pub. No. 149).
HAYHURST, E. R. (1936) Poisoning by petroleum distillates. Ind. med.
Surg., 5: 53.
HELBLING, V. (1950) [A case of acute lethal percutaneous and
inhallatory poisoning by petrol.] Z. Unfallmed. Berufskr.,
43: 218-228 (in German).
HELLMANN, H. & ZEHLE, H. (1972) [Long-term investigation on
behavioural of crude oil on water.] Dtsch. Gewaesserkd. Mitt.,
16: 46-52 (in German).
HENDRICKS, N. V., BERRY, C. M., LIONE, J. G., & THORPE, J. J. (1959)
Cancer of the scrotum in wax pressman. I. Epidemiology. Arch.
ind. Health, 19: 524-529.
HENDRICKS, N. V., COLLINGS, G. H., DOOLEY, A. E., GARRETT, J. T., &
RATHER, J. B. Jr (1962) A review of exposure to oil mist. Arch.
environ. Health, 4: 139-145.
HENRY, S. A. (1946) Cancer of the scrotum in relation to occupation,
London, Oxford Medical Publications, Oxford University Press,
100 pp.
HENRY, S. A. (1946/1947) Occupational cutaneous cancer attributable to
certain chemicals in industry. Br. reed. Bull., 4: 389-401.
HENRY, S. A. (1947) Symposium on industrial skin cancer. Br. J.
Radiol., 20: 149-157.
HENSEN, E. V. (1959) Toxicology of some of the aliphatic and alicyclic
hydrocarbons. J. occup. Med., 1: 105-112.
HERMANN, H. (1975) Handling of crudes and petroleum products ...
industrial hygiene. Erdoel Kohle, Erdgas, Petrochem.
Brennst.-Chem., 28(6): 282-286.
HERSKOWITZ, A., ISHI, N., & SCHAUMBERG, H. (1971) n-Hexane
neuropathy; a syndrome occurring as a result of industrial
exposure. New Engl. J. Med., 285-2: 82-85.
HETTCHE, H. O. (1963) [The effects of bitumen and its vapours on human
health.] Städtehygiene, 14: 65-68 (in German).
HIEGER, I. & WOODHOUSE, D. L. (1952) The value of the rabbit for
carcinogenicity tests on petroleum fractions. Br. J. Cancer,
6: 293-299.
HINE, C. H. & ZUIDEMA, H. H. (1970) The toxicological properties of
hydrocarbon solvents. Ind. Med., 39(5): 215-220.
HMSO (1967) Cancer of the skin caused by oil, London, Her Majesty's
Stationary Office, pp. (SHW295/295A).
HMSO (1979) Cautionary Notice: Effects of mineral oil on the skin,
London, HMSO, (SHW397 revised 1979).
HODGSON, G. (1970) Cutaneous hazards of lubricants. Ind. Med.,
39(2): 68-73.
HODGSON, G. (1973) Health problems arising from contact and exposure
of workers to metal working fluids. J. Inst. Petr.,
59(565): 1-8.
HOEKSTRA, W. G. & PHILLIPS, P. H. (1963) Effects of topically applied
mineral oil fractions on the skin of guinea-pigs. J. invest.
Dermatol., 40(2): 79-88.
HOLMES, J. G., KIPLING, M.D., & WATERHOUSE, J. A. H. (1970) Subsequent
malignancies in men with scrotal epithelioma. Lancet,
ii: 214-215.
HOOGENDAM, I. (1962) Health checks on asphalt workers. In:
Proceedings of the Shell Industrial Doctors Meeting, 22-25 May,
1962, The Hague, Med. Div. Shell Int. Petrol. Co. Ltd. (Internal
Report).
HORTON, A. W., DENMAN, D. T., & TROSSET, R. P. (1957) Carcinogenesis
of the skin. II. The accelerating properties of aliphatic and
related hydrocarbons. Cancer Res., 17: 758-766.
HORTON, A. W., BURTON, M. J., TYE, R., & BINGHAM, E. L. (1963)
Composition versus carcinogenicity of distillate oils. Am. Chem.
Soc. Div. Pet. Chem. Prepr., 8: No. 4-C: 59-65.
HORTON, A. W., BINGHAM, E. L., BURTON, M. J. G., & TYE, R. (1965)
Carcinogenesis of the skin. III. The contribution of elemental
sulfur and of organic sulfur compounds. Cancer Res.,
25: 1759-1763.
HRUSTALEVA, G. F., CEMBARTSEVA, A. N., SVECNIKOVA, F. A., BESKROVNAJA,
N. I., ROTIN, V. V., & LESEHEV, S. S. (1979) [Hypophyseal-ovarian
relationships of women coming into contact with petroleum solvents
in industry.] Gig. Tr. prof. Zabol., No. 7, pp. 31-33
(in Russian).
HUEPER, W. C. (1961) Carcinogens in the human environment. Arch.
Pathol., 71: 355-380.
HUEPER, W. C. & PAYNE, W. W. (1960) Carcinogenic studies on petroleum
asphalt, cooling oil, and coal tar. Arch. Pathol., 70: 372-384.
HUGUENIN, R. (1925) Cancer aigu consécutif à une brûlure par le
mazout. Bull. Assoc. Fr. Etud. Cancer, 14: 403-404.
HUGUENIN, R., FAUVET, F., & MAZABRAUD, M. (1950) Rôle éventuel des
nébulisations d'huiles industrielles dans la pathogénie des
cancers broncho-pulmonaires. Arch. Mal. prof., 11: 48-51.
HUNTER, G. A. (1968) Chemical burns of the skin after contact with
petrol. Br. J. plast, Surg., 21: 337-341.
IARC (1973) Certain polycyclic aromatic hydrocarbons and heterocyclic
compounds. In: IARC monographs on the evaluation of carcinogenic
risk of chemicals to man, Lyons, International Agency for
Research on Cancer, Vol. 3, pp. 30-42.
IARC (1974) Some anti-thyroid and related substances, nitrofurans and
industrial chemicals. In: IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyons, International
Agency for Research on Cancer, Vol. 7,326 pp.
ICHIHARA, K., KUSUNOSE, E., & KUSUNOSE, M. (1969) Microsomal
hydroxylation of decane. Biochim. Biophys. Acta, 176: 713-719.
IIDA, M., YAMAMURA, Y., & SOBUE, I. (1969) Electromyographic findings
and conduction velocity in n-hexane polyneuropathy.
Electromyography, 9: 247-261.
IKEDA, K. (1935) Oil aspiration pneumonia (lipoid pneumonia).
Am. J. Dis. Child. 49: 985-1006.
IKEDA K. (1937) Lipoid pneumonia of the adult type (paraffinoma of the
lung). Arch. Pathol., 23: 471-492.
ILO (1968) Benzene: uses, toxic effects, substitutes. Report of
expert meeting 1967, Geneva, International Labour Office, 287 pp.
INOUE, T., TAKEUCHI, Y., TAKEUCHI, S., YAMADE, S, SUZUKI, H.,
MATSUSHITE, T., MIYAGAKI, H., MAEDE, K., & MOTSUMOTO, T. (1970) A
health survey on vinyl sandal manufacturers where a high incidence
of n-hexane intoxication occurred. Jpn. J. ind. Health,
12: 5-16.
INSTITUTE OF PETROLEUM (1974) Petroleum statistics with 1973 totals,
London, Institute of Petroleum.
ISHII, N., HERSKOWITZ, A., & SCHAUMBERG, H. (1972) n-Hexane
poly-neuropathy: a clinical and experimental study.
J. Neuropathol. exp. Neurol., 31: 198.
IVANOV, N. G., POZDNJAKOV, V. S., & ROZOVA, T. A. (1978) [The maximum
admissible content of mineral (petroleum) oil aerosol in air of
working premises.] Gig. Tr. prof. Zabol., No. 5, pp. 28-31
(in Russian).
JANYSEVA, N. I., KIREEVA, I. S., & SERZANTOVA, N. N. (1963) [A propos
the content of 3,4-benzopyrene in petroleum bitumen.] Gig. i
Sanit., 28: 71-73 (in Russian).
JEPSON, J. R., STOVANOV, S., UNGER, M., CLAUSEN, J., & CHRISTENSEN, H.
E. (1977) Cutting fluids and their effects on the skin of mice.
Acta Pathol. Microbiol. Scand. (Section A), 85: 731-738.
JERINA, D. M. & DALY, J. W. (1974) Arene oxides: A new aspect of drug
metabolism. Science, 185: 573-582.
JONES, J. G. (1961) An investigation into the effects of exposure to
an oil mist on workers in a mill for the cold reduction of steel
strip. Ann. occup. Hyg., 3: 264-271.
KARANI, V. (1966) Peripheral neuritis after addiction to petrol.
Br. med. J., i: 216.
KELLERMAN, G., SHAW, C. R, & LUYTEN-KELLERMAN, M. (1973) Aryl
hydrocarbon hydroxylase inducibility and bronchogenic carcinoma.
New Engl. J. Med., 289: 934-937.
KENNAWAY, E. L. (1925) Experiments on cancer-producing substances.
Br. meal J., ii: 1-6.
KENNAWAY, E. L. & HIEGER, I. (1930) Carcinogenic substances and their
fluorescence spectra. Br. med. J., 2: 1044-1046.
KEY, M. M., RITTER, E. J., & ARNDT, K. A. (1966) Cutting and grinding
fluids and their effects on the skin. Am. Ind. Hyg. Assoc. J.,
27: 423-427.
KINNEAR, J., ROGERS, J., & FINN, O. A. (1954) Degenerative changes in
the skin with special reference to jute-workers. Br. J.
Dermatol., 46: 344-349.
KINNEAR, J., ROGERS, J., FINN, O. A., & MAIR, A. (1955) Dermatoses in
jute-workers. Br. J. ind. Med., 12: 36-42.
KIPLING, M. D. (1968) Oil and the skin. Ann. Rep. of H.M. Chief
Inspector of Factories, 1967, London, HMSO, pp. 105-120.
KIREEVA, I. S. (1968) Carcinogenic properties of coal-tar pitch and
petroleum asphalts used as binders for coal briquettes. Hyg.
Sanit., 33(4, 5, 6): 180-186.
KLAUDER, J. V. & BRILLE, F. A. (1947) Correlation of boiling ranges of
some petroleum solvents with irritant action on the skin. Arch.
Dermatol. Syph., 56: 197-215.
KNAVE, B., PERSSON, H. E., GOLDBERG, J. M., & WESTERHOLM, P. (1976)
Long-term exposure to jet fuel. An investigation on
occupationally-exposed workers with special reference to the
nervous system. Scand. J. Work Environ. Health, 3: 152-164.
KNAVE, B., OLSEN, B. A., ELOFSON, S., GAMBERALE, F., ISAKSSON, A.,
MINDUS, P., PERSSON, H. E., STRUWE, G., WENNBERG, A., &
WESTERHOLM, P. (1978) Long-term exposure to jet fuel II. A
cross-sectional epidemiologic investigation on
occupationally-exposed industrial workers with special reference
to the nervous system. Scand. J. Work Environ. Health,
4: 19-45.
KOREEDA, M., MOORE, P. D., WISLOCKI, P. G., LEVIN, W., CONNEZ, A. H.,
YAGI, H., & JERINA, D. M. (1978) Binding of benzo (a)pyrene 7,8
diol -- 9,10 epoxides to DNA, RNA and protein of mouse skin occurs
with high stereoselectivity. Science, 199: 778-781.
KOROBKIN, R., ASBURY, A. K., SUMNER, A. J., & NIELSON, S. L. (1975)
Glue-sniffing neuropathy. Arch. Neurol., 32: 158-162.
KOTIN, P. & FALK, H. L. (1956) The experimental induction of pulmonary
tumors in strain-A mice after their exposure to an atmosphere of
ozonized gasoline. Cancer, 9: 910-917.
KRASSOVITSKAJA, M. L. (1976) [Health protection of atmospheric air in
regions in which oil processing and petrochemical enterprises are
located. In: Guide to hygiene of the atmospheric air.] Moscow,
The Medicine Publishing House, pp. 273-304 (in Russian).
KURITA, H. (1967) Experimental studies on the effects of n-hexane on
albino rats. Jpn. J. ind. Health, 9: 24-36.
LASKIN, S. & GOLDSTEIN, B. D., ed. (1977) Benzene toxicity. A critical
evaluation Washington and London, Hemisphere Publishing Co.
LAUGHLEN, G. F. (1925) Studies on pneumonia following naso-pharyngeal
injections of oil. Am. J. Pathol., 1: 407-414.
LAZAREW, N. W. (1929) [The toxicology of benzenes.] Arch. Hyg.
Bakteriol., 102: 227-239 (in German).
LAZAREV, N. N. & LEVINA, E. A. (1976) [Harmful substances in
industry.] 7th ed., Leningrad, Publishing House "Khimia"
(in Russian).
LAZARINI, H.-J., DOIGNON, J., L'ÉPÉE, P., & MERILLON, H. (1974) Une
nouvelle observation de polynévrite par le n-hexane. Arch. Mal
prof., 35: 920-921.
LEE, M. L., BARTLE, K. D., & NOVOTNY, M. V. (1975) Profiles of the
polynuclear aromatic fraction from engine oils obtained by
capillary column gas -- liquid chromatography and
nitrogen-selective detection. Anal. Chem., 47(3): 540-543.
LEITCH, A. (1922) Paraffin cancer and its experimental production.
Br. med. J., ii: 1104-1009.
LEITCH, A. (1924) Mule spinners cancer and mineral oils. Br. med. J.,
ii: 941-943.
LEITHE, W. (1970) The analysis of air pollutants. Ann Arbor, Ann
Arbor-Humphrey Science Publishers, pp. 78-93.
LEITHE, W. (1972) [The analysis of organic pollutants in drinking,
industrially-used, and sewage water.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft MBH, pp. 119-132
(in German).
LESSER, L. I., WEENS, H. S., & McKEY, J. D. (1943) Pulmonary
manifestations following ingestion of kerosene. J. Pediatr.,
23: 352-364.
LEVINA, E. N. (1976) [Hydrocarbons.] In: Lazarev, N. V. & Levina, E.
N., ed. [Harmful substances in industry Volume I, Organic
substances.] 7th ed., Leningrad, Publishing House Himia, pp.
9-121 (in Russian).
LIEBE, G. (1892) [On tar or paraffin cancer.] Med. Jahrb., 236: 65
(in German).
LIEVRE, J., BENICHOU, C., & DESROY, M. (1967) Polynévrite par poêle à
combustion catalytique (trots sujets atteints dans une famille).
Bull. Soc. Méd. Hop. Paris, 118: 91-99.
LIJINSKY, W. (1960) Separation of polycyclic aromatic hydrocarbons in
complex mixtures: Chromatographic determination of trace amounts
in petroleum waxes. Anal. Chem., 32: 684-687.
LIJINSKY, W. & RAHA, C. R. (1961) Polycyclic aromatic hydrocarbons in
commercial solvents. Toxicol. appl. Pharmacol., 3: 469-473.
LIJINSKY, W., RAHA, C. R., & KEELING, J. (1961) Comparison of
procedures for the determination of polycyclic aromatic
hydrocarbons in waxes. Anal. Chem., 33: 810-812.
LIJINSKY, W, DOMSKY, I., MASON, G., RAMAHI, H. Y., & SAFARI, T. (1963)
The chromatographic determination of trace amounts of polynuclear
hydrocarbons in petrolatum, mineral oil, and coal-tar. Anal.
Chem., 33: 952-956.
LIJINSKY, W., SAFFIOTTI, U, & SHUBIK, P. (1966) Skin tumorigenesis by
an extract of amber petrolatum. Toxicol. appl. Pharm.,
8: 113-117.
LIPOVSKIJ, S. M. (1978) [The serotonin content in the tissue of uterus
and particulars on its contracting activity under the influence of
petroleum solvent vapours.] Gig. Tr. prof. Zabol., No. 7,
pp. 37-40 (in Russian).
LIPOVSKIJ, S. I., ELKIN, V. I., TOMAEVA, L. V., & ZORINA, V. V. (1977)
[The solvents content in the blood of workers in the rubber
industry.] Gig. Tr. prof. Zabol., No. 5, pp. 50-51 (in Russian).
LIPOVSKIJ, C. M., TOMAEVA, L. V., VARFOLOMEEV, D. I., FEDOSEEV, Ja.
E., & KARGANOVA, E. V. (1979) [The accumulation of solvents in the
tissues and organs of pregnant women.] Gig. Tr. prof. Zabol.,
No. 3, pp. 25-28 (in Russian).
LIPPMANN, M. & GOLDSTEIN, D. H. (1970) Oil.mist studies, environmental
evaluation and control. Arch. environ. Health, 21: 591-599.
LUSHBAUGH, C. C. (1947) Experimental hyperplastic gastritis and
gastric polyposis in monkeys. J. Natl Cancer Inst., 7: 313-320.
LUSHBAUGH, C. C. & HACKETT, A. (1948/1949) An infiltrating
aden-omatous lesion of the colon of rats ingesting motor
lubricating oil (S.F.G. No. 1 oil). J. Natl Cancer Inst.,
9: 159-172.
LUSHBAUGH, C. C., GREEN, J. W., & REDEMANN, C. E. (1950) Effects of
prolonged inhalation of oil fogs on experimental animals. Arch.
ind. Hyg. occup. Med., 1: 237-247.
LUTOV, V. A. (1974) [Materials for establishing the maximum admissible
concentration of petroleum oil aerosols without additives, used as
lubricating and cooling liquids.] Gig. Tr. prof. Zabol., No 10,
pp. 49-52 (in Russian).
LUTOV, V. Ya., CIRKIN, A. A., GRJADITSKIJ, Ju. E., & VASILENKO, N. I.
(1976) [The effect of petroleum oil aerosols and thermo-oxidizing
degradation products on the functional condition and immunologic
reactivity of the organism of experimental animals.] Gig. Tr.
Prof. Zabol., No. 2, pp. 33-37 (in Russian).
LYNCH, P. F. & BROWN, C. W. (1973) Identifying source of petroleum by
infrared spectrometry. Environ. Sci. Technol., 7: 1123-1127.
MACHLE, W. (1941) Gasoline intoxication. J. Am. Med. Assoc.,
117: 1965-1971.
MALTEN, K. E., SPRUIT, D., BOEMAARS, H. G. M., & De KEIZER, M. J. M.
(1968) Horny layer injury by solvents. Berufsdermatosen,
16: 135-147.
MASON, B. (1966) The concentration of minor elements in biogenic
deposits. Principles of geochemistry, 3rd ed., New York, John
Wiley & Sons Inc., pp. 241-243.
MASTROMATTEO, E. (1955) Cutting oils and squamous-cell carcinoma. Part
I. Incidence in a plant with a report of six cases. Br. J. ind.
Med., 12: 240-243.
MATHER, R. N. (1973) Codes of practice relating to metal-working
fluids -- the users' views. J. Inst. Petrol., 59: 15-17.
MATSUMOTO, T. (1971) Experimental studies on toluene poisoning, II.
Jpn. J. ind. Health, 13: 399-407.
MATSUMURA, M., INOUE, N., OHNISHI, A., SANTA, T., & GOTO, I. (1972)
Toxic polyneuropathy due to glue-sniffing. Clin. Neurol. (Japan),
12: 290-296.
MAZEE, W. M., GERSMANN, H. R., & VAN DER WIEL, A. (1966) Analysis of
polycyclic aromatic hydrocarbons in petroleum waxes and white
mineral oils with appraisal of merits of different methods of
analysis. Food cosmet. Toxicol., 4: 17-33.
McDERMOTT, H. J. & KILLIANY, S. E. Jr (1978) Quest for a gasoline TLV.
Am. Ind. Hyg. Assoc. J., 39(2): 110-117.
McKAY, J. F. & LATHAM, D. R. (1972) Fluorescence spectrometry in the
characterization of high boiling petroleum distillates. Anal.
Chem., 44(13): 2132-2137.
McKAY, J. F. & LATHAM, D. R. (1973) Polyaromatic hydrocarbons in high
boiling petroleum distillates. Anal. Chem., 45: 1050-1055.
Medical Research Council (1968) The carcinogenic action of mineral
oils. A chemical and biological study. London HMSO (Special Report
Series No. 306).
MEDVED', R. A., KUZINA, V. F, & KRIGMAN, B. I. (1976) [The hygienic
assessment of lubricating and cooling liquids introduced instead
of sulphofrezol for the cold processing of metal by cutting.]
Gig. Tr. prof. Zabol., No. 6, pp. 1-5 (in Russian).
MENDELL, J. R., SAIDA, K, GANANSIA, M. F., JACKSON, D. B., WEISS, H.,
GARDIER, R. W., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J.,
MARKS, B., & HETLAND, L. (1974) Toxic polyneuropathy produced by
methyl n-butyl ketone. Science, 185: 787-789.
MEYER, A. (1976) Huilome pulmonaire chez les chanteurs. Arch. Mal.
prof., 37(9): 629-631.
MILLER, A., BADER, R. A., BADER, M. E., TEIRSTEIN, A. S., & SELIKOFF,
I. J. (1962) Mineral oil pneumonia. Ann. intern. Med.,
57: 627-634.
MILNE, J. E. (1970) Carcinoma of the scrotum. Med. J. Aust.,
1: 13-16.
MINER, S. (1969) Air pollution aspects of hydrogen sulfide,
Bethesda, MD, Litton Systems Inc. (prepared for the United States
Department of Health, Education and Welfare Contract No.
PH-22-68025).
MIYAGAKI, H. (1967) Electrophysiological studies on the peripheral
neurotoxicity of n-hexane. Jpn. J. ind. Health, 9: 12-23.
MOEL, M. & TAYLOR, H. K. (1943) Oil aspiration pneumonia. Am. J.
Röntgenol. rad. Ther., 49(2): 177-184.
MOSS, E., SCOTT, T. S., & ATHERLEY, G. R. C. (1972) Mortality of
newspaper workers from lung cancer and bronchitis 1952-1966.
Br. J. ind. Med., 29(1): 1-14.
MUHAMETOVA, G. M. & PODREZ, Z. G. (1975) [Problems of the course,
pathogenesis and therapy of chronic patrol poisoning.] Ufa,
Baskirskoe kniznoe izdatelstvo (in Russian).
NATHANSON, L., FRANKEL, D., & JACOBI, M. (1943) Diagnosis of lipoid
pneumonia by aspiration biopsy. Arch. int. Med., 72: 627-634.
NEBERT, D. W. & GELBOIN, H. V. (1969) The in vivo and in vitro
induction of aryl hydrocarbon hydroxylase in mammalian cells of
different species, tissues, strains, and developmental and
hormonal states. Arch. Biochem, Biophys., 134: 76-89.
NEBERT, D. W., GOUJON, F. M., & GIELEN, J, E. (1972)Aryl hydrocarbon
hydroxylase induction by polycyclic hydrocarbons: simple autosomal
dominant trait in the mouse. Nature New Biology, 236: 107-109.
NELSON, L. B. & STORMONT, D. J. (1960) Determination of polynuclear
aromatic hydrocarbons in petroleum waxes. Chem. Ind., 21: 561.
NIOSH (1973) The industrial environment -- its evaluation and
control. Washington, DC, US Dept of Health, Education and
Welfare.
NIOSH (1977A) Occupational exposure sampling strategy manual.
Washington, DC, US Dept of Health, Education and Welfare (DHEW
(NIOSH) Publication No. 77-173).
NIOSH (1977B) Criteria for a recommended standard ... occupational
exposure to asphalt fumes, Washington DC, US Department of
Health, Education and Welfare (DHEW (NIOSH) Publ. No. 78-106).
NIOSH (1977C) Criteria for a recommended standard ... occupational
exposure to hydrogen sulphide, Washington DC, US Department of
Health, Education and Welfare.
NIOSH (1977D) Criteria for a recommended standard ... occupational
exposure to refined petroleum solvents, Washington DC, US
Department of Health, Education and Welfare, 247 pp. (DHEW (NIOSH)
Publ. No. 77-192).
NIOSH (1977-79) NIOSH manual of analytical methods. 2nd ed.
Cincinnati, OH, US Dept of Health, Education and Welfare, USA.
NIOSH (1979) Summary of NIOSH recommendations for occupational health
standards, Washington DC, US Department of Health and Welfare,
9 pp.
NOCHOMOVITZ, L. E., UYS, C. J., & EPSTEIN, S. (1975) Massive
deposition of mineral oil after prolonged ingestion. S.A. med.J.,
13 Dec., 2187-2190.
NOMIYAMA, K. & NOMIYAMA, H. (1974) Respiratory retention, uptake and
excretion of organic solvents in man. Int. Arch. Arbeitsmed.,
32: 75-91.
NOVIKOV, K. I., KOLODINA, L. N., LIPOVSKIJ, S. M., & BADALOVA, L. V.
(1979) [Lactation particulars in female workers of the chemical
(rubber) industry.] Gig. Tr. prof. Zabol., No. 2, pp. 45-48
(in Russian).
NUNN, J. A. & MARTIN, F. M. (1934) [Gasoline (petrolether) and
kerosine (petroleum) poisonings in children.] Samml.
Vergiftungsf., 5: 183-185 (in German).
OGATA, M., ASAHARA, H., & SAEKI, T. (1975) Sampling and analysis of
some aromatic aliphatic and chlorinated hydrocarbon vapours in
air, a gas-liquid chromatographic and colorimetric method.
Int. Arch. Arbeitsmed., 34: 25-37.
OSER, B. L., OSER, M., & CARSON, S. (1965) Toxicological studies of
petrolatum in mice and rats. Toxicol. appl. Pharmacol.,
7: 382-401.
PASTERNACK, B. & EHRLICH, L. (1972) Occupational exposure to an oil
mist atmosphere. A 12-year mortality study. Arch. environ.
Health, 25: 286-294.
PATES, M. M. (1952) [Blastomogenic effect of pyrolyse tars from
petroleum.] Tr. Akad. Med. Nauk., SSSR, 21: 157-163
(in Russian).
PAULSON, G. W. & WAYLONIS, G. W. (1976) Polyneuropathy due to
n-hexane. Arch. intern. Med., 136: 880-882.
PERBELLINI, L., BRUGNONE, F. & FAGGIONATO, G. (1981) Urinary excretion
of the metabolites of n-hexane and its isomers during
occupational exposure. Br. J. ind. Med., 38: 20-26.
PETROLEUM HANDBOOK (1966) London, Shell International Petroleum
Company Limited.
PINKERTON, H. (1927) Oils and fats. Their entrance into and fate in
the lungs of infants and children: A clinical and pathologic
report. Am. J. Dis. Child., 33: 259-285.
PINKERTON, H. (1928) The reaction to oils and fats in the lung. Arch.
Pathol, 5: 380-401.
PINKERTON, H. & MORAGUES, V. (1940) Paraffinoma of the lung with
secondary tuberclelike lesions in liver and spleen. Arch.
Pathol., 29: 691-699.
PIOTROWSKI, J. K. (1977) Exposure tests for organic compounds in
industrial toxicology. Cincinnati, OH, NIOSH, (DHEW (NIOSH)
Publication No. 77-144).
POKLIS, A. & BURKETT, C. D. (1977) Gasoline sniffing: A review. Clin.
Toxicol., 11: 35-41.
POLIANSKY, V. A. & MUSERSKAJA, A. N. (1971) [Pollution of the air
environment at modern oil processing plants and evaluation of the
implementation of recommendating.] In: [Occupational hygiene and
the protection of the health of workers in oil and petrochemical
industries.] Ufa, pp. 50-54 (in Russian).
PROCKOP, L. D. (1977) Multifocal nervous system damage from inhalation
of volatile hydrocarbons. J. occup. Med., 19: 139-140.
PROUDFIT, J.P., ORDSTRAND, H. S. VAN, & MILLER, C. W. (1950) Chronic
lipid pneumonia following occupational exposure. Arch. ind. Hyg.,
1: 105-111.
PRUYN, F. H. A.M. & REIJNIERSE, K. (1972) [Carcinoma of the scrotum
through mineral oils.] T. Soc. Geneesk., 50: 182-187 (in Dutch).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1975) Report on emissions from
asphalt hot mixes, Maryland, USA, The Asphalt Institute
(Research Report RR-75-IA).
PUZINAUSKAS, V. P. & CORBETT, L. W. (1978) Differences between
petroleum asphalt, coal-tar pitch and road tar, Maryland, USA,
The Asphalt Institute (Research report RR-78-1).
RAALTE, H. G. S. VAN (1963) [Medico-toxicological aspects of oil used
in metal processing.] Tijdschr. Soc. Geneesk., 41: 611-617
(in Dutch).
RAALTE, H. G. S. VAN (1972) [Carcinoma of the scrotum through mineral
oils.] Tijdschr. Soc. Geneesk, 50: 604-605 (in Dutch).
RAITTA, C., SEPPÄLÄINEN, A. M., & HUUSKONEN, M. (1978) N-hexane
maculopathy in industried workers. Graefes Arch. klin. exp.
Ophthalmol., 209: 99-110.
REID, F. H. & HALPIN, W. R. (1968) Determination of halogenated and
aromatic hydrocarbons in air by charcoal tube and gas
chromatography. Am. Ind. Hyg. Assoc. J., 29: 390.
REIDENBERG, M. M., POWERS, D. V., & BELLO, C. T. (1964) Acute renal
failure due to nephrotoxins. Am. J. med. Sci., 59: 25-63.
REWELL, R. E. (1947) Lipoid pneumonia: A pitfall in diagnosis.
Br. med. J., 29: 409-411.
RIIHIMÄKI, V. (1979) Percutaneous absorption of m-xylene from a
mixture of m-xylene and isobutyl alcohol in man. Scand. J. Work
Environ. Health, 5: 143-150.
RIIHIMÄKI, V. & PFÄFFLI, P. (1978) Percutaneous absorption of solvent
vapours in man. Scand. J. Work Environ. Health, 4: 73-85.
RIIHIMÄKI, V., PFÄFFLI, P., SAVOLAINEN, K., & PEKARI, K. (1979)
Kinetics of m-xylene in man. General features of absorption,
distribution, biotransformation and excretion in repetitive
inhalation exposure. Scand. J. Work Environ. Health,
5: 217-231.
ROBINSON, E. & ROBBINS, R. C. (1968) Sources, abundance, and fate of
gaseous atmospheric pollutants. Chapter VI; Hydrocarbons and
organics in the atmosphere, Huntsville, Alabama, Stanford
Research Institute (Report PR-6755 prepared for the American
Petroleum Institute).
ROE, F. J. C., CARTER, R. L., & TAYLOR, W. (1967) Cancer hazard from
mineral oil used in the processing of jute. Br. J. Cancer,
21: 694-702.
ROSSIER, P. H. & BÜHLMANN, A. (1949) [Clinical and laboratory studies.
An oil-aspiration pneumonia following the use of liquid paraffin
as nose drops for some years.] Schweiz. Med. Wochenschr.,
79: 685-686.
SANTE, L. R. (1949) The fate of oil particles in the lung and their
possible relationships to the development of bronchogenic
carcinoma. Am. J. Röntgenol., 62: 788-797.
SAVOLAINEN, H. (1977) Some aspects of the mechanisms by which
industrial solvents produce neurotoxic effects. Chem. biol.
Interactions, 18: 1-10.
SCHAMP, N. & VAN WASSENHOVE, F. (1972) Determination of
benzo(alpha)-pyrene in bitumen and plants. J. Chromatogr.,
69: 421-425.
SCHAUMBURG, H. H. & SPENCER, P.S. (1976) Degeneration in central and
peripheral nervous systems produced by n-hexane: An experimental
study. Brain, 99: 183-192.
SCHEUPLEIN, R. J. & BLANK, I. H. (1971) Permeability of the skin.
Physiol. Rev., 51: 702-747.
SCHMAHL, D. & REITER, A. (1933) [Experiments on cancer production by
liquid paraffin yellow vaseline and lanolin.] Arzneim. Forsch.,
3: 403-406 (in German).
SCHWARZ, H. G. (1933) [Petrol poisoning, chronic, iatrogenic.] Samml.
Vergiftungsf., 4: 247-248 (in German).
SCHWARTZ, L., JULIPAN, L., & PECK, S. (1947) Dermatoses caused by
petroleum. In: Occupational diseases of the skin, 2nd ed.,
Kimpton, London, pp. 232-258.
SCRIMA, M. & De ROSA, F. (1973) [Polyneuritis in upper shoe factories:
Environmental and concomitant causes.] Lav. Um., 25: 191-198
(in Italian).
SEDIVEC, V. & FLEK, J. (1976) The absorption, metabolism and excretion
of xylenes in man. Int. Arch. occup. Environ. Health,
37: 205-217.
SEHTMAN, B. A, SAMEDOR, I. G., & MUHAMETOVA, G. M. (1979)
[Work hygiene in the petroleum industry.] Moscow, Medicina Publ.
House (in Russian).
SEPPÄLÄINEN, A. M. (1975) Applications of neurophysiological methods
in occupational medicine: A review. Scand. J. Work Environ.
Health, 1: 1-14.
SEPPÄLÄINEN, A. M., HUSMAN, K., & MARTENSSON, C. (1978)
Neuro-physiological effects of long-term exposure to a mixture of
organic solvents. Scand. J. Work Environ. Health, 4: 304-314.
SEPPÄLÄINEN, A.M., RAITTA, C., & HUUSKONEN, M. (1979)
n-Hexane-induced changes in visual evoked potentials and
electroretinograms of industrial workers. Electroencephalogr.
clin. Neurophysiol., 47(4): 492-498.
SERRATRICE, G., CORREARD, R.-P., ARLAUD, J.-A., & JOUGLARD, J. (1973)
Polynévrite et manipulation de peinture pour bateaux. J. eur.
Toxicol., 6: 304-305.
SHAHIN, M. M. & FOURNIER, F. (1978) Suppression of mutation induction
and failure to detect mutagenic activity with Athabasca Tar sand
fractions. Mutat. Res., 58: 29-34.
SHIRABE, T., TSUDA, T., TERAO, A., & ARAKI, S. (1974) Toxic
poly-neuropathy due to glue-sniffing; report of two cases with
light and electron-microscopic study of the peripheral nerves and
muscles. J. Neurol. Sci., 21: 101-113.
SHOSHKES, M., BANFIELD, W., & ROSENBAUM, S. J. (1950) Distribution,
effect and fate of oil aerosol particles retained in the lungs of
mice. Arch. ind. Hyg. occup. Med., 1: 20-35.
SHUBIK, P. & SAFFIOTTI, U. (1954) Carcinogenic and promoting action of
low-boiling catalytically cracked oils. Proc. Am. Assoc. Cancer
Res., 1: 45.
SHUBIK, P., SAFFIOTTI, U. LIJINSKY, W., PIETRA, G., RAPPAPORT, H.,
TOTH, B., RAHA, C. R., TOMATIS, L., FELDMAN, R., & RAMAHI, H.
(1962) Studies on the toxicity of petroleum waxes, Toxicol. appl.
Pharmacol., 4 (Suppl,, Nov.): 1-62.
SIMMERS, M. H. (1964) Petroleum asphalt inhalation by mice, Arch,
environ, Hearth, 9(6): 727-734.
SIMMERS, M. H. (1965A) Cancers from air.refined and steam refined
asphalt. Ind. Med. Surg., 34(3): 244-261.
SIMMERS, M. H. (1965B) Cancers in mice from asphalt fractions,
Ind. Med. Surg., 34(7): 573-577.
SIMMERS, M. H. (1966) Tumors from asphalt fractions injected into
mice. Ind. Med. Surg., 35(10): 889-894.
SIMMERS, M. H., PODOLAK, E., & KINOSITA, R. (1959) Carcinogenic
effects of petroleum asphalt. Proc. Soc. exp. Blot. Med.,
10: 266-268.
SIMS, P., GROVER, P. L., SWAISLAND, A., PAL, K., & HEWER, A. (1974)
Metabolic activation of benzo(a)pyrene precedes by a diol-epoxide.
Nature (Lond.), 252: 326-328.
SIOU, G. (1972) Existe-t-il un risque de cancérisation par les
bitumes. Rev. Path. comp. Méd. exp., 72: 65-75.
SIWE, S. A. (1932) [The signs and symptoms of acute poisoning by
liquid hydrocarbons in the aliphatic series.] Monatschr.
Kinderheilkd., 55: 146-153 (in German).
SMILEY, J. A. (1951) The hazards of rope making. Br. J. ind. Med.,
8: 265-270.
SMITH, W. E. & SUNDERLAND, D. A. (1951) Responses of mice to
carcinogenic derivatives of petroleum. Cancer Res., 11: 281.
SMITH, W. E., SUNDERLAND, D. A., & SUGIURA, K. (1951) Experimental
analysis of the carcinogenic activity of certain petroleum
products. Arch. ind. Hyg. occup. Med., 4: 299-314.
SMS 2337-1 (1972) Determination of low concentrations of benzene in
air, The Hague, Shell Int. Petr. My. (Shell Method Series).
SMYTH, H. F. & SMYTH, H. F. (1928) Inhalation experiments with certain
lacquer solvents. J. ind. Hyg., 10: 261-271.
SOUTHAM, A. (1928) Mule-spinners' cancer. In: Report of the
International Conference on Cancer, London, Bristol, John
Wright, p. 28C.
SPENCER, P. S., BISCHOFF, M. C., & SCHAUMBURG, H. H. (1978) On the
specific molecular configuration of neurotoxic aliphatic
hexacarbon compounds causing peripheral distal axonopathy.
Toxicol. appl. Pharmacol., 44: 17-28.
SPRUIT, D., MALTEN, K. E., LIPMANN, E. W. R. M., & LIANG, T. P. (1970)
Horny layer injury by solvents. II. Can the irritancy of petroleum
either be diminished by pretreatment. Berufsdermatosen,
18: 269-280.
STERNER, J. H. (1941) Study of hazards in spray-painting with gasoline
as a diluent. J. ind. Hyg. Toxicol., 23: 437-448.
STEWART, I. (1960) Petrol vapour poisoning. Br. med. J.,
i: 1739-1740.
STRÄULI, P. (1957) [Acute tar cancer.] Oncologia, 10: 72-75
(in German).
STRYKER, W. A. (1941) Absorption of liquid petrolatum (mineral oil)
from the intestine. Arch. Pathol., 31: 670-692.
SUGIURA, K., SMITH, W. E., & SUNDERLAND, D. (1949) Carcinogenic action
of certain catalytically cracked oils with high boiling points.
Cancer Res., 9: 631.
SUHANOVA, V. A. & MEL'NIKOVA, V. V. (1974) [The menstruation function
of female workers at refineries and patients suffering from
chronic intoxication by petroleum products.] Gig. Tr. prof.
Zabol., No. 4, pp. 30-41 (in Russian).
SULLIVAN, R. J. (1969) Air pollution aspects of odorous compounds,
Bethesda, Maryland, Litton Systems Inc. (prepared for the US Dept.
of Health, Education and Welfare, Contract No. PH-22-68-25).
SUMA'MUR, P. K. & SUSIANTI WENAS. (1978/1979) Skin and other
affections in gasoline and/or diesel oil pump workers. Indones.
J. ind. Hyg. occup. Health, 11-12: 20-22 (XI No. 1, 2, 3, 4 and
XII No. 1 and 2).
SUNDERLAND, D. A., SMITH, W. E., & SUGIURA, K. (1951) The pathology of
growth behaviour of experimental tumours induced by certain
petroleum products. Cancer, 4: 1232-1245.
SUZUKI, T., SHIMBO, S., & NISHITANI, H. (1974) Muscular atrophy due to
glue sniffing. Int. Arch. Arbeitsmed., 33: 115-123.
SWANN, H. E. Jr., KWONN, B. K., HOGAN, G. K., & SNELLINGS, W. M.
(1974) Acute inhalation toxicology of volatile hydrocarbons.
Am. Ind. Hyg. Assoc. J., 35(9): 511-518.
Tabershaw/Cooper Association Inc. (1974/75) Morbidity study of
petroleum refinery workers. (Project OH-4, Medical Research
Report prepared for API).
TAGAMI, H. & OGINO, A. (1973) Kerosene dermatitis; factors affecting
skin irritability to kerosene. Dermatologica, 146: 123-131.
TAHER, S. M., ANDERSON, R. J., McCARTNEY, R, POPOVTZER, M. M., &
SCHRIER, R. W. (1974) Renal tubular acidosis associated with
toluene «sniffing». New Engl. J. Med., 290: 765-768.
TAKEUCHI, Y., MABUCHI, C., & TAKAGI, S. (1975) Polyneuropathy caused
by petroleum benzine. Int. Arch. Arbeitsmed., 34: 185-197.
THAKKER, D. R., YAGI, H, AKAGI, H, KOREEDA, M., LU, A. Y.-H., LERIM,
W., WOOD, A. W., CONNEY, A. H., & JERINA, D. M. (1977) Metabolism
of benzo(alpha)pyrene VI. Stereoselective metabolism of
benzo(alpha)-pyrene and benzo(alpha)pyrene 7,8-dihydrodiol to
diolepoxides. Chem. biol. Interaction 16: 281-300.
THONY, C. & THONY, J. (1970) Enquête épidémiologique sur le cancer des
huiles de coupe. In: Proceedings of the 16th International
Congress on Occupational Health, Tokyo, 1969, pp. 655-657.
THONY, C., THONY, J., LAFONTAINE, M., & LIMASSET, J. C. (1975)
Concentrations en hydrocarbures polycycliques aromatiques
cancérogènes de quelques huiles minerale étude due risque
correspondant. Arch. Mal. prof., 36: 37-52.
TRUHAUT, R. (1978) Introductory remarks. In: Meeting on MACs, XIX In.
International Congress on Occupational Health, 26 September 1978.
TRUHAUT, R. & MURRAY, R. (1978) International workshop on toxicology
of benzene, Paris, 9-11 November 1976. Int. Arch. Occup. Env,
41: 65-76.
TRUHAUT, R., LAGET, P., PIAT, G., DUTERTRE-CATELLA, P.-L. H., & HUYEN,
V. N. (1973) Premiers résultats électrophysiologiques après
intoxications experimentales par l'hexane et par l'heptane
techniques chez le rat blanc. Arch. Mal. prof.,
34(7-8): 417-426.
TWORT, C. C. & FULTON, J. D. (1930) Further experiments on the
carcinogenicity of synthetic tars and their fractions. J. Path.
Bacteriol., 32: 119-143.
TWORT, C. C. & ING, H. R. (1928) [Investigations on carcinogenic
agents.] Z. Krebsforsch., 27: 308-351 (in German).
TWORT, C. C. & TWORT, J. M. (1931) The carcinogenic potency of mineral
oils. J. And. Hyg. Toxicol., 13: 204-226.
TWORT, C. C. & TWORT, J. M. (1935) Induction of cancer by cracked
mineral oils. Lancet, November 30: 1226-1228.
TWORT, C. C., LYTH, R., & TWORT, J. M. (1937) The significance of
density, refractive index and viscosity of mineral oils in
relation to the type and degree of animal reaction. J. Hyg.,
37: 14-29.
TYE, R., BURTON, M. J., BINGHAM, E., BELL, Z., & HORTON, A. W. (1966)
Carcinogens in a cracked petroleum residuum. Arch. environ.
Health, 13: 202-207.
UK MEDICAL RESEARCH COUNCIL (1968) The carcinogenic action of mineral
oils. A chemical and biological study, London, Her Majesty's
Stationary Office, 8 pp. (Special Report Series No. 306).
UNGAR, H., HINE, C. H., KODAMA, J. K., & ANDERSON, H. H. (1955)
Neuropathology of rats experimentally poisoned with
p-tertiary-butyl-toluene. A.M.A. Arch. Pathol., 69: 139-149.
US DHEW (1970) Air quality criteria for hydrocarbons, Washington,
DC, United States Department of Health, Education and Welfare.
USSR (1972) [Sanitary norms for the planning of industrial
enterprises.] Moscow (in Russian).
VAN DER LINDEN, A. C. & THYSSE, G. J. E. (1965) The mechanisms of
microbial oxidations of petroleum hydrocarbons. Adv. Enzymol.,
27: 469-546.
VAUGHAN, C. G., WHEALS, B. B., & WHITEHOUSE, M. J. (1973) The use of
pressure-assisted liquid chromatography in the separation of
polynuclear hydrocarbons. J. Chromatogr., 78: 203-210.
VIGALJUK, R. V., ZAMALEEVA, Z. S., RUBINSKIJ, L. Ya., SOLODOVA, N. L.,
& LJUTAJA, T. I. (1977) [The chromatographic determination of the
total content of C1 C10 paraffin hydrocarbons and C6-C8
aromatics in the air of industrial plants.] Gig. Tr. prof.
Zabol., No. 6, pp. 57-57 (in Russian).
VOBORSKY, R. C. (1980 In: Dolberg, D. D. & Verstuyft, A. W, ed.
Analytical techniques in occupational health chemistry,
Washington, DC, ACS (ACS Symposium Series 120).
VOLK, B. W. (1964) Lipoid pneumonia: Clinical, pathologic and chemical
aspects. Biochem. Clin., 4: 187.
VOLKMANN, R. (1875) [On tar, paraffin, and soot cancer (chimneysweep's
cancer.] In: Beiträge zur Chirurgie, Leipzig, Breitkopf und
Hartel (in German).
VON LEHMDEN, D. J., VON HANGERBRAUCK, R. P., & MEEKER, J. E. (1965)
Polynuclear hydrocarbon emissions from selected industrial
process. J. Air. Pollut. Control Assoc., 15: 306-312.
WAGNER, W. D., DOBROGORSKI, O. J., & STOKINGER, H. E. (1961)
Antagonistic action of oil mists on air pollutants. Arch.
environ. Health, 2: 523-534.
WAGNER, W. D., WRIGHT, P. G., & STOKINGER, H. E. (1964) Inhalation
toxicology of oil mists. I. Chronic effects of white mineral oil.
Am. Ind. Hyg. Assoc. J., 25: 158-167.
WAHLBERG, J. E. (1974) Occupational and non-occupational scrotal
cancer in Sweden, 1958-1970. Acta Dermatovenerol., 54: 471-474.
WALKER, J. D, COLWELL, R. R., & PETRAKIS, I. (1975) A study of the
biodegradation of a South Louisiana crude oil employing
computerized mass spectrometry. In: Joint Conference on the
Prevention and Control of Oil Pollution, San Francisco, p. 197.
WALLCAVE, L, GARCIA, H., FELDMAN, R., LIJINSKY, W., & SHUBIK, P.
(1971) Skin tumorigenesis in mice by petroleum asphalts and
coaltar pitches of known polynuclear aromatic hydrocarbon content.
Toxicol. appl. Pharmacol., 18: 41-52.
WANG, C. C. (1961) Acute gasoline intoxication. Arch. environ.
Health, 2: 714-716.
WARING, J. I. (1933) Pneumonia in kerosene poisoning. Am. J. med.
Sci., 185: 325-330.
WATERHOUSE, J. A. H. (1971) Cutting oils and cancer. Ann. occup.
Hyg., 14: 161-170.
WATERHOUSE, J. A. H. (1972) Lung cancer and gastro-intestinal cancer
in mineral oil workers. Ann. occup. Hyg. 15: 43-44.
WATTENBERG, L. W. (1972) Dietary modification of intestinal and
pulmonary aryl hydrocarbon hydroxylase activity. Toxicol. appl.
Pharmacol., 23: 741-748.
WEISS, F. T. (1975) Activities of standardization groups in the
identification of waterborne oils. Paper read at National Bureau
of Standards Workshop - Santa Barbara, California, 6-7 October,
1975, 17 pp.
WEISSMAN, H. (1951) Lipoid pneumonia. Am. Rev. Tuberc., 64: 572-576.
WHO (1974) Toxicological evaluation of some food additives
including anti-caking agents, antimicrobials, antioxidants,
emulsifiers and thickening agents, Geneva, World Health
Organization, pp. 417-422 (WHO Food Additive Series No. 5).
WHO (1982) Environmental health criteria 19: Hydrogen sulfide.
Geneva, World Health Organization.
WIEL, A. VAN DER (1975) Collaborative determination of precision of
DMSO extraction-refractive index method, Amsterdam,
Koninklijke/Shell Laboratory (Memorandum LFA/200/75).
WIEL, A. VAN DER (1979) Determination of potentially hazardous
poly-nuclear aromatics in petroleum fractions, Amsterdam,
Koninklijke/Shell Laboratory (Amsterdam Method Series, AMS 642-2).
WILLIAMS, R. T. (1959) Detoxication mechanisms, 2nd ed., New York,
John Wiley & Sons Inc.
WOOD, E. H. (1943) Unusual case of carcinoma of both lungs associated
with lipoid pneumonia. Radiology, 40: 193-195.
WOODHOUSE, D. L. (1934) The non-carcinogenicity of certain synthetic
lubricating oils and the action of some commercial spindle-oils,
solvent fractionated spindle oil distillates and tar oils, tested
under similar conditions. J. Inst. Petr. Technical.,
20: 1057-1063.
WOODHOUSE, D. L. (1950) The carcinogenic activity of some petroleum
fractions and extracts. J. Hyg. Camb., 48: 121-134.
YAGI, H., THAKKER, D. R., HERNANDEZ, O., KOREEDA, M., & JERINA, D. M.
(1977) Synthesis and reactions of the highly mutagenic 7, 8-diol
9, 10-epoxides of the carcinogen benzo-(a) pyrene. J. Am. Chem.
Soc., 99: 1604-1611.
YAMAMURA, Y. (1967) n-Hexane neuropathy. Folia psychiatr. neurol.
Jpn., 23: 45-57.
YANG, S. K., McCOURT, D. W., ROLLER, P. R., & GELBOIN, H. V. (1976)
Enzymatic conversion of benzo(alpha)pyrene leading predominantly
to the diol-epoxide r-7, t-8-dihydroxy-t-9, 10-oxy-7,8,9,10-
tetrahydrobenzo (a) pyrene through a single emantiomer of r-7,
t-8-dihydroxy-7, 8-dihydrobenzo-pyrene. Proc. Natl Acad. Sci.
US, 73: 2594-2598.
YOKOO, H. & SUCKOW, E. E. (1961) Peripheral lung cancers arising in
scars. Cancer, 14: 1205-1215.
YOUNG, A.M., APPLEBAUM, H. S., & WASSERMAN, P. B. (1939) Lipoid
pneumonia. J. Am. Med. Assoc., 112: 2406-2409.
ZANGGER, H. (1933) [Occupational accidents and hazards at work inside
enclosed spaces (reservoirs, tanks, and transport lorries.]
Arch. Gewerbepathol. Gewerbehyg., 4: 117-140 (in German).
ZIELHUIS, R. L. (1961) [Early complaints occurring on exposure to
organic solvents.] Tijdschr. Soc. Geneesk., 39: 595-600
(in Dutch).
ZINGMARK, P. A. & RAPPE, C. (1977) On the formation of
N-nitrosodi-ethanolamine in a grinding fluid concentrate after
storage. Ambio, 6: 237-238.
