
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 15
TIN AND ORGANOTIN COMPOUNDS
A Preliminary Review
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of the United Nations
Environment Programme and the World Health Organization
World Health Organization
Geneva, 1980
ISBN 92 4 154075 3
(c) World Health Organization 1980
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
TIN AND ORGANOTIN COMPOUNDS: A PRELIMINARY REVIEW
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1. Chemistry and uses of tin compounds
1.1.1. Inorganic tin
1.1.2. Organotin compounds
1.2. Analytical methods
1.3. Environmental concentrations and exposures
1.3.1. Environmental exposures
1.3.2. Occupational exposure
1.4. Metabolism
1.4.1. Inorganic tin
1.4.2. Organotin compounds
1.5. Effects on experimental animals
1.5.1. Inorganic tin
1.5.1.1 Local effects
1.5.1.2 Systemic effects
1.5.2. Organotin compounds
1.5.2.1 Local effects
1.5.2.2 Systemic effects
1.6. Effects in man
1.6.1. Inorganic tin
1.6.2. Organotin compounds
1.6.2.1 Local effects
1.6.2.2 Systemic effects
1.7. Recommendations for further studies
1.7.1. Analytical methods
1.7.2. Environmental data
1.7.3. Metabolism
1.7.4. Effects
2. CHEMISTRY AND ANALYTICAL METHODS
2.1. Elemental tin
2.2. Tin(II) compounds
2.3. Tin(IV) compounds
2.4. Organometallic compounds of tin
2.5. Analytical methods
2.5.1. Determination of inorganic tin
2.5.1.1 Atomic absorption spectrocopy
2.5.1.2 Emission spectroscopy
2.5.1.3 Neutron activation analysis
2.5.1.4 X-ray fluorescence
2.5.1.5 Miscellaneous analytical methods
2.5.2. Determination of organotin compounds
2.5.2.1 Diorganotin compounds
2.5.2.2 Triorganotin compounds
3. SOURCES OF ENVIRONMENTAL POLLUTION35
3.1. Natural occurrence
3.2. Industrial production
3.3. Tin consumption
3.4. Uses of tin
3.4.1. Tin and inorganic tin compounds
3.4.2. Organotin compounds
3.5. Tin-containing waste
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATIONS
4.1. Transport and bioconcentration
4.2. Environmental chemistry of tin
4.3. Degradation of organometallic tin compounds
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1. Ambient air
5.2. Soils and plants
5.3. Water and marine organisms
5.4. Food
5.5. Organotin residues
5.6. Working environment
5.7. Estimate of effective exposure of man through environmental
media
6. METABOLISM
6.1. Inorganic tin
6.1.1. Absorption
6.1.2. Distribution
6.1.2.1 Distribution in human tissues and
biological fluids
6.1.3. Excretion
6.1.4. Biological half-time
6.2. Organotin compounds
6.2.1. Absorption
6.2.2. Distribution
6.2.3. Excretion
6.2.4. Biotransformation
7. EFFECTS ON ANIMALS
7.1. Inorganic tin compounds
7.1.1. Effects on the skin
7.1.2. Respiratory system effects
7.1.3. Effects on the gastrointestinal system
7.1.4. Effects on the liver
7.1.5. Effects on the kidney
7.1.6. Effects on the blood-forming organs
7.1.7. Central nervous system effects
7.1.8. Effects on the reproductive system and the fetus
7.1.9. Carcinogenicity and mutagenicity
7.1.10. Other effects
7.1.11. Effective doses and dose rates
7.1.11.1 Lethal doses
7.1.11.2 Minimum effective and no-observed effects
doses
7.2. Organotin compounds
7.2.1. Effects on the skin and eyes
7.2.2. Respiratory system effects
7.2.3. Effects on the gastrointestinal system
7.2.4. Effects on the liver and bile duct
7.2.5. Effects on the kidney
7.2.6. Effects on lymphatic tissue and immunological
effects
7.2.7. Haematological effects
7.2.8. Central nervous system effects
7.2.9. Effects on reproduction and the fetus
7.2.10. Carcinogenicity
7.2.11. Effects on chromosomes
7.2.12. Other effects
7.2.13. Mechanisms of action
7.2.14. Effective doses and dose rates
7.2.14.1 Lethal doses
7.2.14.2 Minimum effective and no-observed-effect
doses
8. EFFECTS ON MAN
8.1. Inorganic tin compounds
8.1.1. Acute poisoning
8.1.2. Prolonged exposure
8.1.2.1 Effects of inhalation
8.1.2.2 Effects of ingestion
8.2. Organotin compounds
8.2.1. Local effects
8.2.2. Systemic effects
8.2.2.1 Effects of dermal exposure
8.2.2.2 Effects of inhalation
8.2.2.3 Effects of ingestion
8.3. Treatment of poisoning
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH ASPECTS OF TIN AND ORGANOTIN
COMPOUNDS
Members
Professor R. Lauwerys, Unité de Toxicologie industrielle et médicale,
Université catholique de Louvain, Bruxelles, Belgium
(Rapporteur)
Dr J. G. Noltes, Department of Organic and Organo-Element Chemistry,
Institute for Organic Chemistry, Organization for Applied
Scientific Research (TNO), Utrecht, Netherlands
Professor M. Piscator, Department of Environmental Hygiene, The
Karolinska Institute, Stockholm, Sweden
Professor A.B. Roscin, Department of Occupational Hygiene, Central
Institute for Advanced Medical Training, Moscow, USSR
Dr M. Sharratt, Division of Environmental Health & Chemical Hazards,
Department of Health & Social Security, London, England
(Chairman)
Professor M. Timar, State Institute of Occupational Health, Budapest,
Hungary (Vice-Chairman)
Dr L. Cemisanska, Department of Occupational Toxicology, Centre of
Hygiene, Sofia, Bulgaria
Secretariat
Dr L. Fishbein, National Centre for Toxicological Research, US Food &
Drug Administration, Jefferson, AR, USA (Temporary Adviser)
Dr Y. Hasegawa, Control of Environmental Pollution and Hazards,
Division of Environmental Health, WHO, Geneva, Switzerland
Dr J. E. Korneev, Control of Environmental Pollution and Hazards,
Division of Environmental Health, WHO, Geneva, Switzerland
Dr A. Stiles, Division of Vector Biology and Control, WHO, Geneva,
Switzerland
Dr V. B. Vouk, Control of Environmental Pollution and Hazards,
Division of Environmental Health, WHO, Geneva, Switzerland
(Secretary)
The following list of organotin compounds includes the trivial name of the compound used throughout the document, the Chemical Abstracts
Service (CAS) name and number, the molecular formula, and alternative names,
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Monosubstituted compounds
ethyltin trichloride stannane, trichloroethyl-(9Cl) (8Cl) 1066-57-5 C2H5Cl3Sn trichloroethylstannane;
trichloroethyltin;
ethyltrichlorostannane;
ethyltrichlorotin
ethyltin triiodide stannane, ethyltriiodo- (9Cl) (8Cl) 3646-94-46 C2H5I3Sn triiodoethyltin
butyltin trichloride stannane, butyltrichloro- (9Cl) (8Cl) 1118-46-3 C4H9Cl3Sn monobutyltin trichloride;
trichlorobutyltin;
butyltrichlorostannane;
butyltrichlorotin;
trichlorobutyltin;
trichlorobutylstannane;
stannane, trichlorobutyl-
butylstannoic acid stannane, butylhydroxyoxo- (9Cl) (8Cl) 2273-43-0 C4H10O2Sn 1-butanestannoic acid;
butyltin hydroxide
oxide; butylstannoic acid (VAN)
butylthiostannoic acid stannane, butylmercaptooxo- (8Cl) 26410-42-4 C4H10O S Sn
butyltin-S,S',S"-tris acetic acid, 2,2',2"- 25852-70-4 C34H66O6S3Sn butyltin tris(isooctyl
thioglycolate);
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Monosubstituted compounds cont'd.
(isooctylmercaptoacetate) ((butylstannylidyne)) tris (thio) stannane, butyltris
tris-, triisooctyl ester ((carboxymethyl) thio)-,
(9Cl); triisooctyl ester; butyltin
acetic acid, ((butylstannylidyne) tris(isooctyl thioglycollate);
trithio) tri,-triisooctylester (8Cl) monobutyltin tris
(isooctylthioglycolate);
monobutyltin tris(isooctyltin
thioglycolate); butyltin tris
(isooctyl mercaptoacetate);
triisooctyl(butylstannylidyne)
trithio) triacetate;
butylstannane tris(isooctyl
mercaptoacetate)
butyltin-S,S',S"-tris 8 oxa-3,5-dithia-4- 26864-37-9 C34H66O6S3Sn monobutyltin tris(2-ethylhexyl
(2-ethyl stannatetradecanoic acid, thioacetate), monobutyltin
hexylmercaptoacetate) 4 butyl-10-ethyl-4-((2-((2- tris(2-ethylhexyl thioglycolate)
ethylhexyl)oxyl)-2-oxoethyl)
thio)-7-oxo-, 2-ethylhexyl
ester (9Cl); acetic acid,
((butylstannylidyne)
trithio)tri-,tris(2-ethylhexyl)
ester (8Cl)
butyltin sulfide distannathiane, 15666-29-2 C8H18S3Sn2 butyl thiostannoic arthydride;
dibutyldithioxo-(9Cl); monobutyltin sulfide
distannthiane, 1,3-dibutyl-
1,3-dithioxo- (8Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Monosubstituted compounds cont'd.
octyltin trichloride stannanne, 3091-25-6 C8H17Cl3Sn trichlorooctylstannane;
trichlorooctyl-(9Cl) (8Cl) octyltrichlorostannane;
n-octyltin trichloride;
n-octyltri-chlorostannane;
mono-n-octyltin tri-chloride;
trichloro-n-octylstannane
octyltin tris(2-ethyl 8-oxa-3,5-dithia-4- 27107-89-7 C38H74O6S3Sn
hexylmercaptoacetate) stannatetradecanoic acid,
10-ethyl-4-((2-((2-ethylhexyl)oxy)
-2-oxoethyl) thio)-4-octyl-7-oxo-,
2-ethylhexylester (9Cl);
acetic acid, ((octylstannylidyne)
trithio)tri-, tris(2-ethylhexyl)
ester (8Cl)
cyclohexylstannoic acid stannano, cyclohexylhydroxyoxo- 22771-18-2 C6H12O2Sn cyclohexanestannoic acid
(9Cl) (8Cl)
Disubstituted compounds
dimethyltin dichloride stannane, dichlorodimethyl- 753-73-1 C2H6Cl2Sn dichlorodimethylstannane;
(9Cl) (8Cl) dichlorodimethyltin;
dimethyldichlorostannane
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dimethyltin S,S'-bis acetic acid, 2,2'-((dimethyl- 26636-01-1 C22H44O4S2Sn TM 181; diisooctyl
(isooctyl mercaptoacetate) stannylene) bis (thio))bis-, ((dimethylstannylene)
diisooctyl ester (9Cl) dithio)diacetate;
dimethylfin bis
(isooctylthioglycolate);
dimethyltin-S,S'-bis
(iso-octylthyoglycolate);
bis((((isooctyloxy)-
carbonyl)methyl)thio)
dimethylfin; T 40 (ester);
TM 181S; Advastab TM 181S;
Advastab TM 181 S
diethyltin dichloride stannane, dichlorodiethyl- 866-55-7 C4H10Cl2Sn tin, dichlorodiethyl-;
dichlorodiethyltin;
diethyldichlorotin;
dichlorodiethylstannane;
diethyldichlorostannane
diethyltin diiodide stannanne, diethyldiiodo- 2767-55-7 C4H10I2Sn tin, diethyldiiodo-;
diethyltin dioctanoate stannane, diethylbis((1-oxo 2641-56-7 C20H40O4Sn diethyldiiodostannane
octyl)oxy) (9Cl); diethyltin dicaprylate
stannane, diethylbis
(octanoyloxy) (8Cl)
dipropyltin dichloride stannane, dichlorodipropyl- 867-36-7 C6H14Cl2Sn dipropyltin chloride;
(9Cl) (8Cl) dichlorodipropylstannane;
di-n-propyltin dichloride
diisopropryltin dichloride stannane, dichlorobis 38802-82-3 C6H14Cl2Sn
(1-methylethyl)- (9Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dibutyltin dichloride stannane, dibutyldichloro- (9Cl) (8Cl) 683-18-1 C8H18Cl2Sn dichlorodibutyltin; dibutyltin
chloride;
dichlorodibutylstannane;
dibutyldichlorotin;
di-n-butyltin dichlorlde;
dibutyldi-chlorostannane
dibutyltin oxide stannane, dibutyloxo- (9Cl) (8Cl) 818-08-6 C8H18OSn di-n-butyltin oxide; tin,
dibutyloxo-;
dibutylstannane oxide;
dibutyloxotin;
dibutyloxostannane
dibutyltin diacetate stannane, bis(acetyloxy)dibutyl- (9Cl) 1067-33-0 C12H24OSn diacetoxydibutyltin;
T 1[catalyst]; T 1 (VAN);
Ba 2726
dibutyltin dilaurate stannane, dibutylbis 77-58-7 C32H64O4Sn Butynorate; DBTL; dibutylbis
((1-oxododecyl)oxy)- (lauroyloxy)-tin; Stabilizer
D-22; tin dibutyl dilaurate;
Stanclere DBTL; Davainex;
TVS Tin Lau;
dibutyltin didodecanoate;
dibutylbis-(laurato)tin;
Mark 1038; Tinostat; dibutyl-
tin n-dodecanoate; Stavinor
1200 SN; T 12 (catalyst);
dibutylstannylene dilaurate;
T 12 (VAN)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dibutyltin maleate 1,3,2-dioxastannepin-4,7-dione, 78-04-6 C12H20O4Sn dibutyl(maleoyldioxy)tin;
Advastab DBTM;
2,2'-dibutyl-(9Cl) (8Cl) Advastab T 290; Stavinor
1300 SN; dibutyl-stannylene
maleate; Advastab T 340;
Nuodex V 1525; Irgastab T 290
dibutyltin sulfide stannane, dibutylthioxo- 4253-22-9 C12H20O4Sn tin dibutyl mercaptide
(9Cl) (8Cl)
dibutyltin di stannane, dibutylbis 2781-10-4 C24H48O4Sn dibutyltin bis
(2-ethylhexoate) ((2-ethyl-1-oxohexyl)oxy)- (9Cl) (2-ethylhexanoate); dibutyltin
bis(alpha-ethylhexanoate)
dibutyltin dioctanoate stannane, dibutyl- 4731-77-5 C24H48O4Sn dibutyltin dioctoate;
bis((1-oxooctyl)oxy)- (9Cl); dibutyltin dicaprylate;
stannane, dibutyl dibutyltin octanoate
bis(octanoyloxy)- (8Cl)
dibutyltin di 5,7,12-trioxa-6-stannahexa 15546-16-4 C24H40O8Sn dibutyltin bis(monobutyl
(butyl maleate) deca-2,9-dienoic acid,6,6-dibutyl- maleate); maleic acid
4,8,11-trioxo, butyl ester, dibutyltin salt (2:1)
(Z,Z)- (9Cl) stannane, dibutylbis diisobutyl ester; B5
(3-carboxyacryloyl)oxy-, [stabilizer]; dibutyltin
dibutyl ester,(Z,Z)-(8Cl) bis(butyl maleate)
dibutyltin di stannane, dibutylbis 10584-97-1 C34H60O8Sn
(nonylmaleate) ((3-carboxyacryloyl)oxy)
-dinonyl ester, (z,z)-(8Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dibutyltin ß-mercapto 6H-1,3,2-oxathiastannin-6-one, 78-06-8 C11H22O2S Sn Thermolite 35; Advastab T-306;
propanoate 2,2-dibutyldihydro- Mark 488; dibutyltin
O,S'-mercaptopropionate,
dibutyltin
S,O-mercaptopropionate;
dibutyltin ß-mercaptopropionate
dibutyltin bis(lauryl stannane, dibutylbis 1185-81-5 C32H68S2Sn dibutylbis(dodecylthio)tin;
mercaptide) (dodecylthio)-(9Cl) (8Cl) Mellite 39; dibutyltin
bis(dodecylmercaptide);
bis-(dodecylthio)dibutyltin;
dibutylbis(dodecyl-thio)
dibutyltin; dibutylbis
(dodecylthio)-stannane;
Advastab TM 98; Mellite 139;
Thermolite 20; dibutyltin
S,S'-bis(dode-cylmercaptide)
dibutyltin "laurate- 2-butenoic acid, 4,4'- 73246-84-1 C32H64O4Sn solution of dibutyltin dilaurate
maleate" [(dibutylstannylene)bis(oxy)] C16H24O8Sn and dibutyltin maleate;
bis [4-oxo-, (Z,Z)- mixed with dibutyl Thermolite 17
bis [(1-oxododecyl)oxy] stannane (1-1)
(9Cl)
dibutyltin S,S'-bis acetic acid, 2,2' (dibutyl 25168-24-5 C28H56O4S2Sn dibutyltin bis(isooctyl
(isooctylthioglycolate) stannylene)bis(thio)bis-, mercaptoacetate); dibutyltin
diisooctyl ester (9Cl); S,S'-bis(isooctyl
acetic acid, ((dibutylstannylene) mercapto-acetate);
dithio)di, diisooctyl ester (8Cl) diisooctyl((dibutylstannylene)-
dithio)diacetate;
Thermolite 31; bis(iso-
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dibutyltin S,S'-bis octyioxycarbonylmethylthiolato)
(isooctylthioglycolate) dibutyibis((((isooctyloxy)
cont'd. carbonyl)methyl)-thio)tin;
BTS 70; T 101 (accelerator);
Irgastab 17M; T 101
dibutyltin S,S'-bis(2- 8-oxa-3,5-dithia-4-stannatetra 10584-98-2 C28H56O4S2Sn dibutyltin bis(2-ethyihexyl
ethylhexylmercaptoacetate) decanoic acid 4,4-dibutyl-10- thioglycolate); dibutyltin
ethyl-7-oxo-2-ethylhexyl ester (9Cl); S,S'-bis(2-ethylhexyl
acetic acid, ((dibutylstannylene) thio-glycolate)
dithio)di-, bis(2-ethylhexyl)
ester (9Cl)
dipentyitin dichloride stannane, dichlorodipentyl- (9Cl) 1118-42-9 C10H22Cl2Sn dichlorodipentyltin
dioctyltin dichloride stannane, dichlorodioctyl- (9Cl) (8Cl) 3542-36-7 C16H34Cl2Sn dichlorodioctyltin;
dichlorodioctylstannane
dioctyltin oxide stannane, dioctyloxo- (9Cl) (8Cl) 870-08-6 C16H34O Sn tin, dioctyloxo-; di-n-octyltin
oxide; dioctyloxostannane
dioctyltin acetate stannane, bis(acetyloxy)dioctyl- (9Cl) 17586-94-6 C20H40O4Sn dioctyldiacetoxytin
dioctyltin dilnurate stannane, dioctylbis 3648-18-8 C40H30O4Sn lauric acid, dioctyltin deriv;
((1-oxododecyl)oxy)-(9Cl); dioctyl-dilauroyloxytin;
stannane, bis(lauroyloxy) dioctyltin didodecanoate
dioctyl-(8Cl)
dioctyltin maleate 1,3,2-dioxastannepin-4,7-dione, 16091-18-2 C20H36O4Sn di-n-octyltin maleate;
2,2-dioctyl- (9Cl); Thermolite 813; Estabex U 18;
dioctylstannylene maleate;
Mellite 825
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dioctyltin dibutylmaleate 5,7,12-trioxa-6-stannahexadeca- 29575-02-8 C32H36O8Sn dioctyltinbis(butylmaleate)
2,9-dienoic acid, 6,6-dioctyl-
4,8,11-trioxo-, butyl ester,
(Z,Z)-(9Cl);
stannane, bis(3-carboxyacryloyl)
oxy)dioctyl-, dibutyl ester (Z,Z)-
(8Cl)
dioctyltin-S,S'-(ethylene 1,3-dioxa-6,9-d ithia-2-stanna 56875-68-4 C22H42O4S2Sn ethylenebisthioglycolate
glycol-bis-mercaptoacetate) cycloundecane-4,11-dione,2,2- dioctyltin
dioctyl-(9Cl)
dioctyltin-S,S'-bis acetic acid, 2,2'- 26401-97-8 C36H72O4S2Sn diisooctyl((dioctylstannylene)
(isooctyl-mercaptoacetate) ((dioctylstannylene)bis(thio) dithio)-diacetate; dioctyltin
bis-,diisooctyl ester (9Cl); bis(isooctylmercapto-acetate);
acetic acid, ((dioctylstannylene) Thermalite 831; dioctyltin
dithio)di-, diisooctyl ester (8Cl) di(iso- octylthyoglycolate);
di-n-octyltin bis(iso-
octylmercaptoacetate), bis
((((isooctyloxy)-carbonyl)
methyl)thio)dioctyltin; Mellite
831C; Irsastab 17 MOK;
dioctyltin bis
(iso-octylthyoglycolate);
dioctyltin bis
(diiso-octylthioglycolate)
dioctyltin mercaptoacetate 1,3,2-oxathiastannolan-5-one, 15535-79-2 C18H36O2S Sn dioctyltin S,O-mercaptoacetate
2,2-dioctyl-(9Cl) (8Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dioctyltin ß-mercapto 6H-1,3,2-oxathiastannin-6-one, 3033-29-2 C19H38O2S Sn dioctyltin
propanoate dihydro-2,2-dioctyl- (9Cl) (8Cl) S,O-3-mercaptopropionate;
dioctyltin mercaptopropionate
dioctyltin-S,S'-bis 8-oxa-3,5-dithia-4-stanna 27107-88-6 C28H56O4S2Sn
(butyl mercaptoacetate) dodecanoic acid, 4,4-dioctyi-7-oxo-,
butyl ester (9Cl)
acetic acid, (((dioctylstannylene)
dithio)di-, butyl ester (8Cl)
dioctyltin-S,S'-bis(2- 8-oxa-3,5-dithia-4-stannatetra 15571-58-1 C36H72O4S2Sn bis(2-ethylhexyl)
ethylhexylmercaptoacetate) decanoic acid, 10-ethyl-4,4- (dioctylstannylene)
dioctyl-7-oxo-, 2-ethylhexyl dithio)diacetate; dioctyltin
ester (9Cl); bis(2-ethyl-hexyl thioglycolate);
bis(2-ethylhexylthyo-glycolato)
dioctyltin; Advastab 17 MOK
acetic acid, ((dioctylstannylene)
dithio)di-, bis(2-ethylhexyl)
ester (8Cl)
dioctyltin-S,S'-bis 8-oxa-3,5-dithia-4-stannaeicosanoic 73246-85-2 C44H88O4S2Sn dioctyltin bis
(laurylmercaptoacetate) acid, 4,4-dioctyl-7-oxo, dodecyl ester (laurylthioglycolate)
(9Cl)
dioctyltin bis(2- 5,7,12-trioxa-6-stannaoctadeca-2,9- 10039-33-5 C40H72O8Sn di-n-octyltin bis
ethylhexylmaleate) dienoic acid, 14-ethyl-6,6- (2-ethylhexylmaleate)
dioctyl-4,8,11-trioxo, 2-ethylhexyl
ester, (Z,Z) (9Cl)
dioctyltin bis(dodecyl stannane, bis(dodecylthio) 22205-30-7 C40H84S2Sn bis(dodecylthio)dioctyltin;
mercaptide) dioctyl-(9Cl) (8Cl) dioctyltin bis(lauryl
mercaptide); dioctyltin,
dilauryl mercaptan salt
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
Disubstituted compounds cont'd.
dioctyltin-S,S'-(1,4- 1,9-dioxa-4,6-dithia-5- 69226-46-6 C24H46O4S2Sn
butanediol-bis-mercapto stannacyclotridecane-2,8-
acetate) dione, 5,5-dioctyl- (9Cl)
dioctyltin di(1,2- 1,3,8,11-tetraoxa-2- 69226-45-5 C27H44O8Sn
propyleneglycolmaleate) stannacyclo-pentadeca-5,13-diene
4,7,12,15-tetrone, 9-methyl-2,2-
dioctyl-, (Z,Z)-(9Cl)
dioctyltin bis(isobutyl stannane, bis[(3-carboxy- 15571-59-2 C32H56O8Sn dioctyltin bis(isobutylmaleato)
maleate) acryloyl)oxy]dioctyl-, diisobutyl tin; 5,7,12-triox-6-stannapenta-
ester, (Z,Z)- (9Cl) 2,9-dienoic acid, 6,6-dioctyl-
13-methyl-4,8,11-trioxo,
1-methyl-propyl ester
diphenyltin dichloride stannane, dichlorodiphenyl- (9Cl) (8Cl) 1135-99-5 C12H10Cl2Sn dichlorodiphenyltin;
diphenylstannyl dichloride;
diphenyldichlorotin;
diphenyl-tin chloride;
dichlorodiphenylstannane
dicyclohexyltin oxide stannane, dicyclohexyloxo- 22771-17-1 C12H22O Sn
(9Cl) (8Cl)
didodecyltin dibromide stannane, dibromodidodecyl- 65264-08-6 C24H50Br2Sn di-n-dodecyltin dibromide
(9Cl)
dioctadecyltin dibromide stannane, dibromodioctadecyl- 65264-09-7 C36H74Br2Sn di-n-octadecyltin dibromide
(9Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
TRISUBSTITUTED COMPOUNDS
triethyltin bromide stannane, bromotriethyl- (9Cl) (8Cl) 2767-54-6 C6H15Br Sn
triethyltin chloride stannane, chlorotriehyl- (9Cl) (8Cl) 994-31-0 C6H15Cl Sn chlorotriethyistannane;
chlorotriethyltin;
triethylstannyl chloride;
triethylchloro-stannane;
triethylchlorotin
triethyltin iodide stannane, triethyliodo- 2943-86-4 C6H15I Sn triethyliodostannane;
(9Cl) (8Cl) triethylstannyl iodide;
iodotriethylstannane
triethyltin suifate 4,6-dioxa-5-thia-3,7-distannanonane, 57-52-3 C12H30O4S Sn2 triethylhydroxytin sulfate;
3,3,7,7-tetraethyl- 5,5-dioxide (9Cl) bis(triethyltin)
stannane, triethylhydroxy- sulfate
sulfate (2:1) (8Cl)
triethyltin acetate stannane, (acetyloxy) 1907-13-7 C8H18O2Sn acetoxytriethylstannane
triethyl-(9Cl);
stannane, acetoxytriethyl-
(8Cl)
triethyltin hydroxide stannane, triethylhydroxy- 994-32-1 C6H16O Sn triethylstannanol;
(9Cl) (8Cl) triethylstannol;
triethyl-hydroxystannane;
hydroxytriethylstannane
trlethylstannylmethyl stannane, triethyl(3-methoxy 17869-84-0 C11H22O2Sn
(1-propynyl) formal methoxy)-l-propynyl-
triethylstannylphenyl stannane, trlethyl(phenylethynyl)- 1015-27-6 C14H20Sn
acetylene (9Cl) (8Cl)
1-triethylstannyl-3- silane, trimethyl((3-triethylstannyl)- 4628-88-0 C12H26O SiSn
trimethylsiloxi-1-propyne 2-propynyl)oxy)-
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
TRISUBSTITUTED COMPOUNDS
2-trichloro-1-(butine- stannane, (1-(3-butinyloxy) 17869-91-9 C12H21C13O2Sn
1'-oxide)-1-(triethyl -2,2,2-trichloroethoxy)triethyl-
stannyloxy)ethane
trivinyltin chloride stannane, chlorotriethenyl- 10008-90-9 C6H9Cl Sn chlorotrivinyltin;
(9Cl); chlorotrivinylstannane
stannane, chlorotrivinyl-
(8Cl)
tributyltin chloride stannane, tributylchloro- 1461-22-9 C12H27Cl Sn tributylchlorotin;
(9Cl) (8Cl) chlorotributylstannane;
tributylstannyl chloride
tributyltin fluoride stannane, tributylfluoro- 1983-10-4 C12H27F Sn tributylfluorostannane;
(9Cl) (8Cl) fluorotributyltin;
tri-n-butylstannyl fluoride
bis(tributyltin) oxide hexabutyldistannoxane 56-35-9 C24H54O2 Sn2 C-Sn-9; BioMeT TBTO; Bultinox;
distannoxane, hexabutyl- (9Cl) (8Cl) hexabutyl-distannoxane;
oxybis(tributyitin); TBTO;
6-oxa-5,7-distannaundecane,
5,5,7,7-tetra-butyl-;
Lastanox T; Vikol AF-25;
Vikol LO-25; BioMeT 66;
oxybis(tributylstannane);
BioMeT SRM; Lastanox T20;
bis(tri-butylstannyl)oxide;
Lastanox Q; Lastanox
F; Stannicide A; tributyltin
oxide; Myko-lastanox F
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
TRISUBSTITUTED COMPOUNDS
tributyltin acetate stannane, (acetyloxy)tributyl- (9Cl); 56-36-0 C14H30O2Sn acetoxytributyltin;
stannane, acetoxytributyl- (8Cl) tributylacetoxystannane;
tri-n-butyltin acetate;
acetoxytributyl-
tributyltin linoleate stannane, tributyl((1-oxo-9,12- 24124-25-2 C30H58O2Sn stannane; tributylstannylacetate
octadecad ienyl)oxy)-(Z,Z)-(9Cl);
stannane, tributyl(linoleoyloxy)-
(8Cl)
tributyltin benzoate stannane, (benzoyloxy)tributyl- 4342-36-3 C19H32O2Sn tri-n-butyltin benzoate; tin,
(9Cl) (8Cl) (benzoyloxy) tributyl-
tributyltin salicylate phenol, 2-[[(tributylstannyl)oxy] 4342-30-7 C19H32O3Sn tin, tributyl(salicyloyloxy)-
carbonyl]- (9Cl)
stannane, tributyl(salicyloyloxy)-
(8Cl)
tributyltin methacrylate stannane, tributyl((2-methyl-1- 2155-70-6 C16H32O2Sn tributylstannyl methacrylate;
oxo-2-propenyl)oxy)-(9Cl); tributyl- (methacryloxy)stannane;
stannane, tributyl(methacryloyloxy- tin, tributyl-
(8Cl) methacrylate; (methacryloyloxy)
tributyl-stannane;
tributylmethacryloyloxystannane
tributyltin laurate stannane, tributyl 3090-36-6 C24H50O2Sn tin, tributyl(lauroyloxy)-;
((1-oxo-dodecyl)oxy)-(9Cl) tributyltin laurate;
stannane, tributyl(lauroyloxy)- (8Cl) tributyltin dodecanoate
tributyltin oleate stannane, tributyl((1-oxo-9-octa 3090-35-5 C30H60O2Sn tin, tributyl(oleoyloxy)-;
decenyl)oxy)-, (Z)-(9Cl); N 5117 (Stauffer); N 5117
stannane, tributyl(oleoyloxy)- (8Cl)
trihexyltin acetate stannane, (acetyloxy)trihexyl- 2897-46-3 C20H42O2Sn tin, acetoxytrihexyl-;
(9Cl); acetoxytrihexyltin
stannane, acetoxytrihexyl- (8Cl)
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
TRISUBSTITUTED COMPOUNDS
tricyclohexyltin hydroxide stannane, tricyclohexylhydroxy- 13121-70-5 C18H34O Sn Plictran;
(9Cl) (8Cl) tricyclohexylhydroxystannane;
tricyclohexylhydroxytin;
Plyctran; M 3180; Cyhexatin;
hydroxytricyclohexylstannane;
Dowco 213; tricyclohexylstannanol
trioctyltin chloride stannane, chlotrioctyl- (9Cl) (8Cl) 2587-76-0 C24H51ClSn tin, chlorotrioctyl-;
chlorotrioctyltin; tri-n-
chlorotrioctylstannane
octyltin chloride;
triphenyltin chloride stannane, chlorotriphenyl- 639-58-7 C18H15ClSn LS 4442; GC 8993; General
(9Cl) (8Cl) Chemicals 8993; TPTC; HOE 2872;
Brestanoi; Fentin chloride;
chlorotriphenyltin;
chlorotri-phenylstannane;
triphenylchlorostannane;
triphenylchlorotin
triphenyltin hydroxide stannane, hydroxytriphenyl- 76-87-9 C18H16OSn hydroxytriphenyttin;
(9Cl) (8Cl) hydroxytriphenyl-stannane;
TPTH; triphenylstannanol; K 19
(VAN); Tenhide; Fenolovo; Du-Ter
Extra; Erithane; Vancide KS;
Dowco 186; ENT 28009; Du-Ter;
Fentin hydroxide
ORGANOTIN COMPOUNDS
Name used in text CAS Index name CAS number Molecular Synonyms
formula
TRISUBSTITUTED COMPOUNDS
triphenyltin acetate stannane, (acetyloxy)triphenyl- 900-95-8 C20H18O2Sn Brestan;
(9Cl); acetoxytriphenylstannane;
stannane, acetyloxytriphenyl- (8Cl) Batasan; Phentin acetate;
Suzu; acetato-triphenylstannane;
GC 6936; ENT 25208; Fentin
acetate; Lirostanol; tin
triphenyl acetate; TPTA;
triphenylacetostannane;
VP 19-40; Brestan 60; Liromatin
p-bromophenoxy triethyltin stannane, (p-bromophenoxy) 20961-09-5 C12H19BrOSn
triethyl- (8Cl)
TETRASUBSTITUTED COMPOUNDS
tetrarnethyltin stannane, tetramethyl- (9Cl) (8Cl) 594-27-4 C4H12Sn tetramethylstannane
tetraethyltin stannane, tetraethyl- (9Cl) (8Cl) 597-64-8 C8H20Sn tetraethylstannane
tetrabutyltin stannane, tetrabutyl- (9Cl) (8Cl) 1461-25-2 C16H36Sn tetra-n-butyltin;
tetrabutylstannane
tetraisobutyltin stannane, tetrakis(2-methylpropyl)- 3531-43-9 C16H36Sn tetraisobutylstannane
(9Cl)
stannane, tetraisobutyl- (8Cl)
tetraphenyltin stannane, tetraphenyl- (9Cl) (8Cl) 595-90-4 C24H20Sn tetraphenylstannane
tetraoctyltin stannane, tetraoctyl- (9Cl) (8Cl) 3590-84-9 C32H68Sn tetra-n-octyltin;
tetra-n-octylstannane
stannous octanoate octanoic acid, tin(2+) salt 1912-83-0 C8H16O2 1/2Sn tin octanoate; stannous
(9Cl) (8Cl) dioctanoate; tin(II)octanoate;
stannous caprylate;
stannous octcate
tin (II) cyclopentadienyl stannocene (9Cl) 1294-75-3 C10H10Sn tin, di-pi-cyclopentadienyl-
TIN AND ORGANOTIN COMPOUNDS: A PRELIMINARY REVIEW
This is the first volume in the UNEP/WHO Environmental Health
Criteria series containing a preliminary review of environmental
health aspects of a group of chemicals. Such reports are prepared in
accordance with the second objective of the WHO Environmental Health
Criteria Programme "to identify new or potential pollutants by
preparing preliminary reviews on health effects of agents likely to be
used in industry, agriculture, in the house, and elsewhere" (WHO,
1976). Organometallic tin compounds are being used in increasing
amounts for a variety of applications and the annual world production
has risen from less than 50 tonnes in 1950 to about 25 000 tonnes in
1975. One of the main applications is the use of dialkyland, to a much
lesser extent, monoalkytin compounds in the stabilization of
poly(vinyl chloride). Other applications include the use of
tributyltin compounds as industrial biocides and surface disinfectants
and the use of triphenyltin and tricylohexyltin compounds as
agricultural fungicides and agricultural acaricides.
Preliminary reviews differ from the criteria documents in that
they do not contain a separate section on health risk evaluation and
that the procedure for their preparation is simpler. Draft preliminary
reviews are not submitted for comments to the national focal points
for the WHO Environmental Health Criteria Programme. The first draft
is reviewed by a Task Group of experts, and on the basis of their
comments, a final draft is prepared and scientifically edited by the
WHO Secretariat. However, individual members of the Task Group and
other experts may be consulted during the scientific editing of the
documents.
The first draft of the present document was prepared by Dr L.
Fishbein, National Center for Toxicological Research, Jefferson, AR,
USA. Dr A. E. Martin, formerly Principal Medical Officer, Department
of Health and Social Security, London, England, assisted the
Secretariat in the preparation of a revised first draft, which was
circulated to the members of the Task Group prior to the meeting. The
Task Group on Environmental Health Aspects of Tin and Organotin
Compounds met in Geneva from 10-14 March 1975 to review and revise
this draft, and, on the basis of their comments, a final draft was
prepared by the Secretariat. The Secretariat wishes to acknowledge the
most valuable assistance in the final phases of preparation of the
document of Dr Renate Kimbrough, Center for Disease Control, Atlanta,
GA, USA, Professor Magnus Piscator, Department of Environmental
Hygiene, Karolinska Institute, Stockholm, Sweden, Dr Robert J. Horton,
US Environmental Protection Agency, Research Triangle Park, NC, and Dr
Warren T. Piver, National Institute of Environmental Health Sciences,
Research Triangle Park, NC, USA. The help is also gratefully
acknowledged of Dr H. Nordman, Institute of Occupational Health,
Helsinki, Finland, who assisted both in the preparation of the final
draft and in the final scientific editing of the document and of
Professor C. Schlatter and Dr R. Utzinger, Institué de Toxicologie,
Ecole Fédérale Polytechnique et Université de ZÜrich, Dr. D. S. Valley
Dr D. F. Walker, National Library of Medicine, Department of Health,
Education and Welfare, USA, and Dr A. Stiles, Consultant, Department
of Environmental Health, WHO, Geneva, who helped in compiling the list
of organotin compounds.
This document is based primarily on original publications listed
in the reference section. However, several publications reviewing the
health effects of inorganic and organotin compounds have also been
used. These include reviews by Barnes & Stoner (1959), Browning
(1969), FAO/WHO (1971), International Labour Office (1972), Kimbrough
(1976), MacIntosh (1969), National Institute of Occupational Safety
and Health (1976), and Piver (1973).
Details of the WHO Environmental Health Criteria Programme,
including definitions of some of the terms used in the documents, may
be found in the general introduction to the Environmental Health
Criteria Programme, published together with the environmental health
criteria document on mercury (Environmental Health Criteria I M
Mercury, Geneva, World Health Organization, 1976) and now available as
a reprint.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1 Chemistry and Uses of Tin Compounds
1.1.1 Inorganic tin
The annual world production of tin is around 225 000 tonnes, about
70% of which is obtained from ores, the remaining 30% being recovered
from scrap metal. Tin is mainly used in tinplated containers, but it
is also extensively used in solders, in alloys such as bronzes,
babbit, pewter, and type metal, and in more specialized alloys such as
dental amalgams and the titanium alloys used in aircraft engineering.
Inorganic tin compounds, in which the element may be present in
the oxidation states of +2 or +4 are used in a variety of industrial
processes for the strengthening of glass, as a base for colours, as
catalysts, as stabilizers in perfumes and soaps, and as dental
anticariogenic agents.
1.1.2 Organotin compounds
Organotin compounds are classified as R4Sn, R3SnX, R2SnX2, and
RSnX3. In compounds of industrial importance, R is usually a butyl,
octyl, or phenyl group and X, a chloride, fluoride, oxide, hydroxide,
carboxylate, or thiloate. So far, monosubstituted organotin compounds
(RSnX3) have had a very limited application, but they are used as
stabilizers in poly(vinyl chloride) films. Disubstituted organotin
compounds R2SnX2) are mainly used in the plastics industry,
particularly as stabilizers in poly(vinyl chloride). They are also
used as catalysts in the production of polyurethane foams and in the
room-temperature vulcanization of silicones. Trisubstituted organotin
compounds (R3SnX) have biocidal properties that are strongly
influenced by the R-groups. The most important of these compounds are
the tributyl-, triphenyl-, and tricyclohexyltin compounds, which are
used as agricultural and general fungicides, bactericides,
antihelminthics, miticides, herbicides, molluscicides, insecticides,
nematocides, ovicides, rodent repellents, and antifoulants in boat
paints. The tetrasubstituted organotin compounds (R4Sn) are mainly
used as intermediates in the preparation of other organotin compounds.
1.2 Analytical Methods
A wide variety of analytical methods is available for the
determination of tin at low concentrations.a However, these methods
have rarely been compared with regard to their suitability for
application to a particular problem and, on the basis of available
information, it is not possible to recommend a specific analytical
technique for a particular application.
Inorganic tin in food and biological materials is usually
determined by atomic absorption. Other spectroscopic methods have also
been used with satisfactory accuracy and precision, including emission
spectroscopy for air, water, and food samples and neutron activation
analysis for geological samples.
Many analytical methods have been used for the determination of
organotin compounds. Atomic absorption and other spectroscopic methods
combined with chromatography have been used for the estimation of
diorganotin compounds. Pesticide residues have been determined by
spectroscopic methods and gas-liquid or thin-layer chromatography.
However, reliable methods have still to be developed for the
quantitative extraction, separation, and determination of many
individual tin species in mixtures containing both inorganic tin and
organotin compounds that may occur in various media.
1.3 Environmental Concentrations and Exposures
1.3.1 Environmental exposures
On the whole, contamination of the environment by tin is only
slight. The levels of pollution arising from gases and fumes, waste
slag, and liquid wastes from tin processing are low because of the
high degree of recovery and reprocessing used in this industry.
Concentrations of tin in air are often below the detection limits
and, when detected, the levels are generally below 0.2-0.3 µg/m3,
except in the vicinity of industrial sources of emission, where
concentrations up to 5 µg/m3 may occur.
a Throughout the document the word concentration indicates mass
concentration unless otherwise stated.
Tin has not always been found in soils and plants; however, it is
possible that in some cases, concentrations have been below the
detection limits. Tin concentrations in soils are generally below
200 mg/kg but in regions of tin-containing minerals, the levels may
exceed 1000 mg/kg. The small amount of evidence available concerning
the uptake of tin by crops suggests that soil concentrations do not
markedly influence its uptake by plants.
Tin has been detected only occasionally in river and municipal
waters. Values exceeding 1 µg/litre are exceptional, although values
as high as 30 µg/litre have been found in drinking water. Sea water
concentrations are of the order of 3 µg/litre. Organotin compounds may
enter water, for example, from antifouling paints on the bottoms of
ships or from molluscicides, which, to be effective, should be present
at concentrations of about 1 mg/litre.
Food is the main source of tin for man. A diet composed
principally of fresh meat, cereals, and vegetables, is likely to
contain a mean tin concentration of about 1 mg/kg. Larger amounts of
tin exceeding 100 mg/kg may be found in foods stored in plain cans
and, occasionally, in foods stored in lacquered cans. Some foods such
as asparagus, tomatoes, fruits, and their juices tend to contain high
concentrations of tin if stored in unlaquered cans. Organotin
compounds may be introduced into foods through the use of such
compounds as pesticides and, to some extent, through migration of tin
from poly(vinyl chloride) materials. However, the levels of organotin
compounds in food are generally below 2 mg/kg.
Experimental studies have provided evidence of the
biotransformation of some triphenyl-, and tricyclohexyltin compounds.
There are also limited data suggesting methylation of tin by organisms
present in the environment. From the available information, it appears
that bioconcentration of tin and organotin compounds of a magnitude
that might endanger life or the environment is unlikely to occur.
The estimated mean total daily intake of tin by man ranges from
200 µg to 17 mg. A diet consisting of fresh foods probably provides
about 1-4 mg/day. The likely daily intake from water is estimated to
be less than 30 µg/day, and the daily amount entering the body from
air, less than 1 µg.
1.3.2 Occupational exposure
Several technological operations associated with the processing of
tin are known to cause excessive occupational inhalation exposure to
tin oxide which may result in a benign pneumoconiosis termed
stannosis, many cases of which have been reported in the past.
Workers involved in the processing of di- and trisubstituted
organotin compounds may be subject to excessive exposure from time to
time. Workers spraying fields or treating plants with trialkyl- or
triaryltin compounds may also run the risk of exposure to these
compounds.
1.4 Metabolism
1.4.1 Inorganic tin
The extent of absorption through the respiratory route has still
to be assessed. The absorption of ingested inorganic tin is likely to
be less than 5% although figures as high as 20% have been suggested.
Gastrointestinal absorption is influenced by the oxidation state,
tin(II) compounds being more readily absorbed than tin(IV) compounds.
The anion complement may also influence the rate of absorption.
Absorbed tin leaves the vascular system rapidly. Bone is the main
site of deposition and the highest concentrations of tin are found in
the lung, kidney, liver, and bone. Penetration of the blood-brain and
placental barriers appears to be very slight. With the exception of
the lungs, inorganic tin does not accumulate in organs with increasing
age.
Absorbed inorganic tin is mainly excreted in the urine. The
fraction excreted with the bile varies with the type of compound and
is probably below 15%.
1.4.2 Organotin compounds
In general, organotin compounds are more readily absorbed from the
gut than inorganic tin compounds; allowance must be made, however, for
the great variations found between different compounds and different
species. As a rule, tin compounds with a short alkyl chain are more
readily absorbed from the intestinal tract. The trialkyltin compounds
are usually well absorbed through the skin. As far as distribution is
concerned, the highest concentrations in rats, guineapigs, rabbits,
and hamsters have mostly been detected in the liver. Trisubstituted
organotin compounds have been found in the brain of various species
but the form of tin present in the brain has not been satisfactorily
identified.
Many organotin compounds are transformed, to some extent, in the
tissues. The dealkylation and dearylation of tetra-, tri-, and
disubstituted organotin compounds seem to occur in the liver, but the
dealkylation of diethyltin compounds appears to take place both in the
gut and in tissues of other organs. The mode of excretion of organotin
compounds largely depends on the type of the compound. For example,
ethyltin trichloride seems to be mainly excreted with the urine, but
diethyltin is eliminated with the faeces, urine, and the bile.
Triethyltin is not only excreted with the urine, but, at least in
lactating sheep, also with the milk. The route of excretion for many
compounds is not known. The biological half-time of different
organotin compounds varies and many compounds are slow to disappear
from the organs. Usually the biological half-time seems to be longer
in the brain than in other organs.
1.5 Effects on Experimental Animals
Although there is evidence that tin is essential for the normal
growth of rats, no evidence exists that it is essential for other
species including man.
1.5.1 Inorganic tin
1.5.1.1 Local effects
Many of the reported effects of inorganic tin are localized
because of its irritant properties. Vomiting and diarrhoea are typical
signs that follow oral intake of foods with a high tin content. In
cats, tin concentrations of 540 mg/litre or 1370 mg/litre in orange
juice caused vomiting in 1/11 animals and 3/10 animals, respectively.
However, these levels did not produce any effects in dogs. The only
adverse effect produced in guineapigs by both short-term and prolonged
exposure to 3 mg of tin tetrachloride per m3 of air was transient
irritation of the nose and eyes, but these findings have not been
corroborated. Application of 1% tin(II) chloride or 0.25% tin(II)
fluoride to the abraded skin of rabbits caused intradermal pustule
formation and epidermal destruction, but did not have any effect on
intact skin.
1.5.1.2 Systemic effects
The major systemic effects of inorganic tin salts in animals
include ataxia, twitching of the limbs, and fore-and hindleg weakness
progressing to paralysis. In rats, growth retardation and decreased
haemoglobin levels may follow administration of tin(II) chloride,
orthophosphate, sulfate, oxalate, and tartrate at a dietary level of
3 g/kg. However, administration of iron prevents the development of
anaemia. Higher dietary levels of tin (10 g/kg) over several weeks may
induce testicular degeneration, pancreatic atrophy, and a spongy state
of the white matter of the brain. Doses of pentafluorostannite of
100 mg/kg body weight may also affect growth, and a dose-related
decrease in haemoglobin levels may be seen with doses exceeding
100 mg/kg; no effect on growth was found at a dose of 20 mg/kg
administered orally to rats. A single intravenous injection of
pentafluorostannite at a concentration of 35 mg/kg body weight or
tin(II) chloride dihydrate (SnCl2-2H20) at 44.4 mg/kg in rats
produced extensive necrosis, mainly in the proximal tubules of the
kidney. A subcutaneous dose of tin(II) chloride at a concentration of
5.6 mg/kg body weight caused a 20-30 fold increase in the haem
oxidation activity in the kidney; this effect was dose-related.
Administration of tin(II) chloride at a concentration of 5 mg/litre,
from weaning to natural death, did not affect longevity in mice or in
male rats, but caused a decrease in longevity in female rats combined
with an increased incidence of fatty degeneration of the liver. There
is no conclusive evidence concerning the carcinogenicity or
teratogenicity of inorganic tin.
1.5.2 Organotin compounds
1.5.2.1 Local effects
Some butyltin compounds are known to produce gastrointestinal
irritation; submucosal, subserosal, and intraluminar haemorrhages were
seen in mice after a single oral dose of 4000 mg/kg body weight.
Dibutyltin dichloride administered at a dose of 50 mg/kg body weight
per day, for one week, produced gastroenteritis in rats.
Gastroenteritis was also produced in rats by administration of
tricyclhexyltin hydroxide (25 mg/kg body weight per day, for 19 days).
Dermal application of dibutyltin dichloride (10 mg/kg body weight
per day, for 12 days) caused severe local damage. Local irritation was
produced in rats by applications to the shaved skin of
bis(tributyltin) oxide in doses of 0.36-0.95 mg/kg; necrosis was
produced at doses of 1.4-185 mg/kg. Triphenyltin acetate also
irritated the skin of the rat, whereas triphenyltin hydroxide was
reported not to irritate the skin of the rabbit but to be extremely
irritating to the eyes.
1.5.2.2 Systemic effects
The systemic effects of monosubstituted, disubstituted, and
tri-substituted organotin compounds differ. In general, mono- and
di-organotin compounds are less toxic than triorganotin compounds. The
toxicity of trialkyltin compounds decreases as the number of carbon
atoms in the alkyl chain increases.
Dibutyltin compounds can produce inflammatory changes in the bile
duct. Single oral doses of dibutyltin dichloride at 50 mg/kg body
weight produced this effect in rats, and higher doses produced more
severe injury; necrotic changes were also produced in the liver of
mice and rats. Bile duct injury in rats and rabbits was seen following
dermal application of dibutyltin dichloride (10 mg/kg body weight).
Dioctyltin compounds produced slight changes in the germinal centres
of the spleen and steatosis of hepatocytes in mice at a single oral
dose of 4000 mg/kg body weight. Pulmonary oedema may be seen in rats
following intravenous administration of diethyl-, dipropyl-,
diisopropyl-, and dipentyltin compounds. Dibutyltin compounds can slow
down growth in rats. The no-observed-effect dietary level was reported
to be 40 mg/kg for a 3 month feeding period and 20 mg/kg for 6 months.
Recent studies showed that dioctyltin dichloride and dibutyltin
dichloride administered at dietary levels of 50 and 150 mg/kg,
respectively for 6 weeks, caused a dose-dependent atrophy of the
thymus and thymus-dependent organs and suppression of the
immunological response in rats, but not in mice and guineapigs.
Some trialkyltin compounds produce a characteristic lesion in the
central nervous system consisting of oedema throughout the white
matter. Orally administered trimethyl- and triethyltin compounds are
more potent in inducing this lesion than the higher homologues. The
first changes in the rat brain were visible after 3 days of
administration of triethyltin hydroxide at a dietary level of
20 mg/kg. Maximum changes were found after 2 weeks. Typical signs of
such intoxication included prostration and weakness of the progressing
to flaccid paralysis. The effects disappeared when exposure ceased.
Administration of triphenyltin compounds produced a reduction in
weight and in food intake in many species. Lethargy was a typical
symptom and histological changes in the liver and spleen were also
seen. A decreased immunological response with a reduction in the
number of leukocytes and of plasma cells in the lymph nodes of
guineapigs has been reported. A 2-year study indicated a
no-observed-effect level for triphenyltin acetate of 0.1 mg/kg body
weight per day.
A single intrarumenal dose of tricyclohexyltin hydroxide at
50 mg/kg body weight produced central nervous depression and diarrhoea
in sheep, whereas a dose of 15 mg/kg did not result in any adverse
effects. At higher doses, pulmonary congestion, tracheal haemorrhage,
enteritis, and eleotrocardiographic changes were seen.
No-observed-effect doses for long-term intake in the rat and dog were
given as 3 mg/kg body weight per day and 0.75 (mg/kg) per day,
respectively.
Tetraalkyltin compounds may produce muscular weakness, paralysis,
respiratory failure, tremors, and hyperexcitability as acute effects
in mice and dogs, while late effects are similar to those seen with
triorganotin poisoning.
There is no evidence that organotin compounds are carcinogenic or
teratogenic. Reported effects of triphenyltin hydroxide on the testes
and ovaries of rats require further confirmation.
Information concerning the mechanism of the toxic action of
organotin compounds is inadequate. Several dimethyl- and dioctyltin
compounds inhibit the oxidation of keto-acids and block mitochondrial
respiration. Trialkyltin compounds inhibit oxidative phosphorylation.
1.6 Effects in Man
1.6.1 Inorganic tin
Inhalation of elemental tin does not produce any effects in man,
whereas extended exposure to tin(IV) oxide dust and fumes can produce
a benign pneumoconiosis termed stannosis. This condition develops
after at least 3-5 years of exposure and is characterized by small
dense shadows in the pulmonary X-ray picture without impairment of
pulmonary function. Fibrosis is not seen. The generally-accepted
maximum allowable concentration of tin(IV) oxide in the air of work
rooms of 2 mg/m3 appears to give protection against this disorder.
Symptoms that have been reported following ingestion of food with
a high tin content include nausea, vomiting, diarrhoea, stomach
cramps, fatigue, and headache. The lowest concentration of tin
reported in association with such outbreaks was about 250 mg/litre in
canned orange and apple juice. Five human volunteers did not
experience any symptoms from the ingestion of fruit juices containing
concentrations of 500-730 mg/litre but all had gastrointestinal
disturbances at a level of 1370 mg/litre (corresponding to
4.4-6.7 mg/kg body weight). Ingestion of 50 mg of tin through eating
canned peaches that contained tin concentrations of about
300-600 mg/kg caused acute symptoms in 2 out of 7 persons. The
relative importance of, on one hand, the total amount of tin ingested
and, on the other hand, the concentration of tin in relation to the
development of symptoms has not been satisfactorily assessed.
1.6.2 Organotin compounds
1.6.2.1 Local effects
Dibutyl- and tributyltin compounds produced skin irritation in
workers 1-8 h after contact. Experimental application to the skin of
volunteers showed that some compounds (e.g., dibutyltin dichloride and
tributyltin chloride) produced this effect, whereas others such as
dibutyltin maleate and tetrabutyltin did not. Di- and tributyltin
compounds caused eye irritation after brief contact. A 20% solution of
triphenyltin acetate produced irritation of the skin and the mucous
membranes of the upper respiratory tract while tricyclohexyltin
hydroxide was reported not to cause skin irritation at a concentration
of 0.01 mg/kg body weight.
1.6.2.2 Systemic effects
The majority of accidental poisonings involving systemic effects
have been due to occupational exposure to triphenyltin acetate.
Systemic effects reported to have followed both dermal and inhalation
exposure include general malaise, nausea, gastric pain, dryness of the
mouth, vision disturbance, and shortness of breath. Hepatomegaly and
elevated levels of liver transaminase activity have been found in some
cases. Recovery has generally been complete but liver damage has been
known to persist for up to 2 years.
The hazard associated with the use of organotin compounds was
unmasked by an episode of intoxication in 1954 involving over 200
cases, 100 of which were fatal. The cause was the ingestion of an oral
preparation containing diethyltin diiodide at 15 mg/capsule. It was
suggested, however, that ethyltin triiodide, triethyltin iodide, and
tetraethyltin were present as impurities. Predominant symptoms and
signs included severe headache, nausea and vomiting, visual and
psychological disturbances, and sometimes loss of consciousness. At
autopsies and decompressive surgery, cerebral oedema of the white
matter was found. In many cases, symptoms lasted for at least 4 years;
follow-up information on the subjects involved is not available.
1.7 Recommendations for Further Studies
1.7.1 Analytical methods
More information is needed on the specificity, precision, and
accuracy of methods for the determination of inorganic tin compounds.
Data concerning interlaboratory comparisons of the methods used are
also lacking. In view of the variable results obtained in studies on
the tin contents of various materials and tissues, the use of
reference laboratories is recommended. Better methods are needed for
the quantitative extraction and separation of the various organotin
compounds present in environmental and biological samples. As
organotin compounds used as pesticides or stabilizers occur in foods
in minute amounts only, mare sensitive methods for their measurement
are needed.
1.7.2 Environmental data
More information regarding bioconcentration is needed. The fate of
organotin compounds entering water is largely unknown. The possibility
of the methylation of tin by organisms present in the environment is
of particular interest.
A wide variety of results concerning the daily intake of tin has
been reported. Although a certain range is to be expected, further
investigations of the concentrations of tin in food and water, and of
dietary intake are needed.
1.7.3 Metabolism
Information on the rate of absorption of tin from the
gastrointestinal tract is insufficient and little is known about the
absorption of various compounds through the respiratory tract which
may be of importance in occupational exposure. Furthermore, there is a
gap in information concerning the rate of absorption of organotin
compounds through the skin.
Many of the studies conducted on the distribution of tin in human
tissues may be unreliable because of the analytical methods available
at that time: thus, data on tissue contents should be obtained using
as sensitive methods as possible with emphasis on the precision,
specificity, and accuracy of the assays employed. Information on tin
concentrations in newborn infants compared with adults is lacking, and
data concerning tin concentrations in the tissues of occupationally-
exposed persons compared with unexposed populations are not available.
The increasing development and use of new organotin compounds will
necessitate further studies on the metabolism of such compounds.
At present, there is an obvious lack of information on the
bio-transformation of several organotin compounds. Data concerning the
accumulation and retention times of various compounds in animal
tissues are also desirable. Finally, the route or routes of excretion
of many organotin compounds are completely unknown.
1.7.4 Effects
Probably the most conspicuous lack of information concerns the
mechanism of action of various organotin compounds. More information
should be obtained on the carcinogenicity, teratogenicity, and
mutagenicity of these compounds. Compounds used industrially should be
studied with reference to possible allergenic properties. Recently
reported results suggest that the effects of various organotin
derivatives on the immune system should be studied in more detail.
Moreover, properly conducted studies on the effects on the sex glands
of different species using multigeneration studies seem urgent. The
importance of longitudinal epidemiological studies on
occupationally-exposed populations and the usefulness of information
that may be obtained through follow-up studies of accidental
intoxication should be emphasized.
2. CHEMISTRY AND ANALYTICAL METHODS
Tin can form a variety of both inorganic and organometallic
compounds. These two classes of compounds have different chemical and
physical properties which make them suitable for different
applications in industry, agriculture and elsewhere. They also have
different toxicities and require separate assessments of health risk.
The inorganic chemistry of tin has been described in standard texts on
inorganic chemistry such as those by Cotton & Wilkinson (1972, 1976)
and Heslop & Jones (1976). Sources of information on new developments
in the organometallic chemistry of tin include a review by van der
Verk (1972) and a collection of papers presented at a symposium of the
American Chemical Society (Zuckerman, 1976).
2.1 Elemental Tin
Tin (atomic number 50; relative atomic mass 118.70) is an element
of group IVb of the periodic system, together with carbon, silicon,
germanium, and lead. It exits in three allotropic modifications. At
room temperature, the stable form is a metallic form called ß- or
white tin. White tin is a silver-white, lustrous and soft metal with
considerable ductility, and can be rolled into very thin "tin foil".
Its density is 7.27, melting point 231.9°C, and boiling point 2507°C.
At ordinary temperatures, it is stable in both air and water. Below
13.3°C, the stable form of tin is the non-metallic grey tin
(alpha-tin). Above 161°C, the stable modification is the so-called
brittle tin, another metallic modification.
Metallic tin is normally covered with a thin protective film of
tin dioxide. Because tin is resistant to cold acids, a cohesive tin
layer will protect iron from corrosion (tin plate). However, if the
layer is damaged, the iron will rapidly corrode. Tin-plate used in the
food industry should not contain lead, not only because lead is toxic,
but also because its presence aids the corrosion of tin by dilute
organic acids.
Neutral aqueous salt solutions react slowly with metallic tin in
the presence of oxygen but solutions containing nitrates, iron(II)
chloride or sulfate, aluminium chloride or tin(IV) chloride dissolve
elemental tin.
Tin can form inorganic compounds in the oxidation state +2 (SnII,
tin(II) compounds, or stannous compounds), and in the oxidation state
+4 (SnIV, tin(IV) compounds, or stannic compounds). Because of their
different physieochemical properties, it is useful to discuss the 2
groups of compounds separately.
2.2 Tin(II) Compounds
Tin(II) compounds are generally more ionic than tin(IV) compounds.
They are unstable in dilute aqueous solutions, are easily oxidized,
and normally contain some SnIV; after some time, hydrolysis occurs
with the formation of the hydrated tin(II) oxide ion [Sn3(OH)4]2+.
Tin(II) chloride is readily soluble in small amounts of water. It
is a fairly good reducing agent, and has many uses in industry,
particularly as a mordant in dye printing. Aqueous solutions of
tin(II) chloride become turbid on dilution because a basic salt is
precipitated. The fluoride (SnF2) is slightly soluble in water. It is
used in fluoride-containing toothpastes. In aqueous solutions,
SnF3- is the major ion but other ions such as SnF+ and Sn3F5-
are also present. Tin(II) sulfate is a good source of SnII. Its
solubility decreases with temperature.
Tin(II) oxide (SnO) is a stable, blue-black crystalline solid. It
reacts with both mineral and organic acids, and dissolves in sodium
hydroxide solutions forming stannites, which probably contain the
SnO22- ion.
Other tin(II) compounds that have practical applications are
tin(II) acetate, tin(II) arsenate, tin(II) fluoroborate, tin(II)
pyrophosphate, and several tin(II) salts or organic acids, such as
tin(II) oxalate and tin(II)-2-ethylhexoate (tin(II)"octoate").
2.3 Tin(IV) Compounds
Tin in the oxidation state + 4 forms a large number of inorganic
compounds as well as organometallic compounds, which are discussed in
section 2.4. Some tin(IV) compounds, such as tin(IV) oxide (SnO2),
have long been used in industry; Others, e.g., tin(IV) chloride
(SnCl4), have found technological applications more recently. Also of
practical importance are the stannates, compounds in which the tin
atom is part of an anion. The structure of stannates can be
represented by MnSn(OH)6, where M is a metal ion.
The physical properties of tin tetrahalides, except those of
SnF4, correspond to the properties of covalent halides of carbon and
silicon. Tin(IV) chloride is a colourless liquid that fumes in moist
air and becomes turbid because of hydrolysis when complex ions such as
[SnCl3(OH)3]2- are formed. The addition of a limited amount, of
water to tin(IV) chloride results in the formation of a crystalline
hydrate, SnCl4.5H2O, the ionic character of which is probably due to
the presence of a complex ion [Sn(H2O4]4+.
Tin(IV) oxide occurs naturally as the mineral cassiterite. It has
a very high melting point (1127°C) and has wide application in
industry. The fusion of tin(IV) oxide with sodium or potassium
hydroxide yields stannates.
Other tin(IV) compounds that have found practical applications
include tin(IV) sulfide, tin(IV) vanadate, and tin(IV) molybdate.
2.4 Organometallic Compounds of Tin
Organometallic tin compounds or organotin compounds have one or
more carbon-tin covalent bonds that are responsible for the specific
properties of such molecules. Essentially all organometallic tin
compounds are of the SnIV type. The only well established compound
with tin in the oxidation state + 2 is the tin(II)cyclopentadienyl,
C10H10Sn. There are four series of organotin compounds depending on
the number of carbon-tin bonds. These series are designated as mono-,
di-, tri-, and tetraorganotin compounds with the general structure:
RnSn X4-n
where R = an alkyl or aryl group
Sn = the central tin atom in the oxidation state +4
X = a singly charged anion or an anionic organic group.
In the organotin compounds of practical importance, R is usually a
butyl, octyl, or phenyl group and X is commonly chloride, fluoride,
oxide, hydroxide, carboxylate, or thiolate.
Monoorganotin compounds, RSnX3, are known but so far have found
only limited application, for example, butyltin sulfide is used as a
stabilizer in poly(vinyl chloride) (PVC) film.
Diorganotin compounds, R2SnX2, are chemically reactive and most
of their applications are based on this property. They are used. as
stabilizers of PVC, as catalysts in the production of polyurethane
foams, and in the cold-curing of silicon elastomers.
Triorganotin compounds, R3SnX, are the most important class of
organotin chemicals. They are biologically very active and are widely
used as biocides. The chemical nature of the R group has a strong
influence on the biological properties of these compounds. The
X-group, on the other hand, influences their solubility and
volatility. The two most important groups of triorganotin compounds
are tributyltin and triphenyltin derivatives.
Tetraalkyl- and tetraaryltin compounds are primarily used as
intermediates in the preparation of other organotin compounds.
Tetraalkyltin compounds are colourless and the compounds of lower
molecular weight are liquids at room temperature. The tetraaryltin
compounds are solids. Tetraorganotin compounds possess typical
covalent bonds and are stable in the presence of air and water.
Tetrabutyltin, Sn(C4H9)4 is a colourless oily liquid with a
distinct odour. Tetraphenyltin, Sn(C6H5)4, is a white crystalline
powder, soluble in organic solvents and insoluble in water.
Since 1974, a new class of organotin compounds, called estertins,
has been developed for use as stabilizers in poly(vinyl chloride).
Their general structure is (R-O-CO-CH2-CH2)2SnX2 or
R-O-CO-CH2-CH2SnX3 where X may be, for example,
isooctylmercaptoacetate. They have a comparatively low volatility and
ex,tractability (Lanigon & Weinberg, 1976).
Solubility data for organotin compounds are incomplete. In
general, their solubility in water at ambient temperatures is of the
order of 5 to 50 mg/litre, but they are very soluble in many common
organic solvents, such as alcohol, ethers, and halogenated
hydrocarbons.
Commercial products are usually pure chemicals since, for
technological reasons, scrupulous care must be taken to avoid metal
contamination during manufacture. The impurities are primarily solvent
residues remaining from the product purification and separation
processes.
The carbon-tin bond is susceptible to nucleophilic and
electrophilic attack, e.g., hydrolysis, solvolysis, acidic and basic
attack, and halogenation. Water has little effect on symmetrical
saturated organotin compounds. Dialkyltin compounds react
spontaneously with moisture and air to form dialkyl hydrated oxides.
Photochemical reactions of organotin compounds are mentioned in
connexion with environmental transport and transformations (section
4). The physicochemical properties of some organotin compounds have
been listed by Weast (1976).
2.5 Analytical Methods
2.5.1 Determination of inorganic tin
2.5.1.1 Atomic absorption spectroscopy
Atomic absorption spectroscopy is the method most widely used for
the detection of low concentrations of tin. In general, the lowest
limit of detection is obtained with a fuel-rich, air-hydrogen flame,
e.g., about 1-1.5 mg/kg compared with 2-2.5, and 4-5 mg/kg for
air-ethylene and nitrous oxide-ethylene flames, respectively
(Christian & Feldman, 1970). The detection limits at the 3 absorption
lines, 2246.1, 2354.8, and 2863.3 nm do not differ much. Using some of
the procedures mentioned later, the detection limit may be reduced to
about 0.1-0.5 mg/kg.
Several atomic absorption techniques, modified to suit specific
purposes, have been reported. A detection limit of 0.5 µg/litre was
obtained when a hydride generation technique using sodium borohydride
and a flame-heated silica atomizing tube was used in the air-acetylene
atomic absorption determination of tin in solution (Thompson &
Thomerson, 1974). Capacho-Delgado & Manning (1966), using a high
intensity hollow cathode lamp as the source, reported a detection
limit of 0.1 mg/litre for water solutions of metallurgical samples,
while Schallis & Kann (1968) determined tin in lubricating oils with a
detection limit of about 0.5 mg/litre. Carbon filament atomic
absorption spectroscopy was employed by Everett et al. (1974) to
determine tin(II) chloride in aqueous and xylene solutions and tin
octoate in oil solution.
Atomic absorption spectroscopy has been used extensively for the
determination of tin in foods (Allan, 1962; Amos & Willis, 1966;
Capacho-Delgado & Manning, 1966; Christian & Feldman, 1970; Gatehouse
& Willis, 1961) and particularly in canned foods, including fruit
juice, fruits, and vegetables (Catala et al., 1971; Price & Roos,
1969; Sato et al., 1973; Shiraishi et al., 1972; Woidich &
Pfannhäuser, 1973). A detection limit of 0.5 mg/kg has been reported
for the determination of tin in canned fruit juice using a nitrous
oxide-acetylene flame (Price & Roos, 1969).
Engberg (1973) compared atomic absorption with a
spectrophotometric method using 2-(3,4,-dihydroxyphenyl)-3,5,7-
trihydroxy-4H-1-benzopyran-4-one (quercetin) for the determination
of tin in food. The methods gave similar results at concentrations of
tin generally found in canned food, but at very low concentrations
(e.g., organotin residues), the quercetin method was more suitable
because of its lower detection limit (section 2.5.1.5).
Atomic absorption spectroscopy has also been used for the
determination of tin in biological samples (Pearlman et al., 1970).
2.5.1.2 Emission spectroscopy
Emission spectroscopy is a rapid and specific method that has
frequently been used for the simultaneous determination of several
elements. Unfortunately, this method is exacting, demanding highly
qualified personnel, and the cost of the instrument is high. It has
been used for the determination of tin in atmospheric samples
(Hasegawa & Sugimae, 1971; Keenan & Byers, 1952; Laamanen et al.,
1971, Lee et al., 1972; Schroll & Krachsberger, 1970; Sugimae, 1974;
Tabor & Warren, 1958), tin in water (Ghafouri, 1970; Konovalov &
Kolesnikova, 1969; Rittenhouse et al, 1969), and for tin in various
foods including meats (Krylova & Balabuh, 1970), fruits, and
vegetables (Chisaka et al., 1973). A sensitivity of 0.04 mg/kg fresh
weight was reported by Tihonova & Zore (1968) for vegetables and
berries. Several investigators have used emission spectroscopy for the
determination of tin in biological samples (Avtandilov, 1967;
Geldmacher-von Mallinckrodt & Pooth, 1969; Kas'yanenko & Kul'skaya,
1969; Kehoe et al., 1940; Mulay et al., 1971; Saito & Endo, 1970
Tipton et al., 1963).
2.5.1.3 Neutron activation analysis
Although the limit of detection of tin by neutron activation is
comparatively high, the technique has been used to determine tin in
air samples (Bogen, 1973; Tuttle et al., 1970). It is also the most
commonly used method for the determination of tin in geological
samples (soils, sediments, rocks, off) (Johansen & Steinnes, 1969;
Obrusnik, 1969; Schramel et al, 1973). A neutron activation electron
probe was used by Kurosaki & Fusayama (1973) for the estimation of tin
in teeth. Disadvantages of neutron activation include the need for a
nuclear reactor and the possibility of interference from formation of
other isotopes.
2.5.1.4 X-ray fluorescence
X-ray fluorescence, a non-destructive method for the determination
of tin, has been used in the analysis of air for elements ranging from
titanium to caesium with a detection limit of 0.5 µg/m3 of air
(Dittrich & Cothern, 1971) and for river water with a detection limit
of 20-30 µg/litre for metals in the suspended or particulate form, and
0.25-0.4 mg/litre for ionic metals (Blasius et al., 1972).
2.5.1.5 Miscellaneous analytical methods
Among the spectrophotometric methods reported, Kirk & Pocklington
(1969) recommended the tin-quercetin method for the estimation of the
tin contents of foods at concentrations ranging from 10 to over
500 mg/kg. The smallest absolute amount of tin detectable with the
quercetin method has been reported to be about 1 µg (Engberg, 1973).
The pyrocatechol violet method has been used for the direct
determination of tin(II) and tin(IV) compounds at levels of about
5-50 mg/kg in fats and oils (Lowry & Tinsley, 1972), for the
determination of tin in metals, and for the determination of tin
concentrations ranging from 0.01-1.0 mg/kg in biological samples
(Corbin, 1973). Other spectrophotometric methods have been developed
for the determination of tin in food samples using phenylfluorone
(Bennet & Smith, 1959; Bergner & RÜdt, 1968; Luke, 1956; Nakamura &
Kamiwada, 1973; Smith, 1970) dithiol (Ljaskovskaja & Krasilnikova,
1961), salicyl-idenamino-2-thiophenol (Horio & Nakaseko, 1972) and
stilbazo (Kobayashi & Yada, 1968).
Infrared internal reflection spectroscopy was used in studies on
tooth enamel treated with tin(II) fluoride (SnF2) (Krutchkoff et al.,
1972).
A fluorometric method using 3'4'7-trihydroxyflavone with a
detection limit of 0.007 µg was reported for the determination of tin
in rock, soil, and biological materials (Filer, 1971). Other
fluorometric procedures have been reported using fiavonal (Coyle &
White, 1957), oxine-5-sulfonic acid (Pal & Ryan, 1956), and the
ammonium salt of 6-nitro-2-naphthylamine-8-sulfonic acid (Anderson &
Lowy, 1956).
Electroanalytical methods have also been employed for the
determination of tin in canned foods. Polarographic analysis was used
for the analysis of canned meat (Janitz, 1971; Jovanovic et al, 1967),
fruits (Miki & Fukui, 1971), and juices (Hayashi, 1969), and for the
estimation of tin in cans (Gruenwedel & Patnaik, 1973). Biston et al.
(1972) obtained good agreement between polarographic and
spectrophotometric (dithiol and phenylfluorone) methods for estimating
tin in vegetables at concentrations of 20-400 mg/kg. Anodic stripping
voltametry has been used for the determination of tin in water
(Portretnyj et al., 1973).
Tin estimations were made in biological samples by means of
spark-source mass spectroscopy (Evans & Morrison, 1968; Hamilton et
al., 1972/1973) and in canned vegetables and juices by titrimetric
analysis (Zohm, 1972).
2.5.2 Determination of organotin compounds
A number of techniques and procedures for the separation of mono-,
di-, and trisubstituted organotin compounds with their subsequent
determination have been described (Brinkman et al., 1977; Freitag &
Bock, 1974; Getzendaner & Corbin, 1972; Kumpulainen & Koivistoinen
1977; Meinema et al. 1978; Soderquist & Crosby, 1978; Woggon & Jehle,
1973, 1975). However, reliable methods have still to be developed for
the quantitative extraction and determination of many individual tin
species in mixtures containing inorganic tin (IV) and organotin
compounds that occur in various media including biological materials,
industrial effluents, and river sediments.
2.5.2.1 Diorganotin compounds
Compounded poly(vinyl chloride) formulations usually contain 1-2%
of dialkyltin compounds as stabilizers and various techniques have
been used for the determination of these organotin stabilizers in
foods. Organotin compounds were separated from inorganic tin
chromotographically and the inorganic tin was when determined
spectroscopically as its catechol violet complex. Koch & Figge (1971)
determined the extent of migration of dioctyltin dichloride and
di-octyltin bis(2-ethyldexylmercaptoacetate) from PVC bottles into
beer by the same method. The catechol violet complex was also used, by
Adamson (1962) and by Ross & White (1961), for the determination of
dialkyltin compounds in fats and olive oil. Spectroscopic methods
involving dithizone (Aldridge & Cremer, 1957; Chapman et al., 1959),
dip henylcarbazone (Skeel & Bricker, 1961) and
4-(2-pyridylazo)-resorcinol (Sawyer, 1967) have been described.
Dithizone and diphenylcarbazone were used for the determination of
diethyl- and dibutyltin compounds respectively, but some difficulties
may arise using either of these agents because of their inherent
instability in solution. Concentrations of diethyl- and triethyltin
compounds ranging up to 30 µg and 20 µg, respectively, could be
measured using dithizone (Aldridge & Cremer, 1957). The use of atomic
absorption spectroscopy for the determination of dibutyltin dilaurate
in animal feeds was described by George et al. (1973).
Neubert (1964) used thin-layer chromatography for the
determination of dialkyltin stabilizers obtaining a detection limit of
1 µg of organotin. Thin-layer chromatographic determination of
organotin stabilizers using their quercetin chelates yielded a
detection limit of 1.3/µg (Wieczorek, 1969). Udris (1971) described a
number of schemes for the analysis of commercial tin stabilizers
commonly used in poly(vinyl chloride) production. Methods were given
for the chemical breakdown of a sample and the subsequent separation
and identification of the degradation products. Schemes for the
analysis of dialkyltin thio-compounds and dialkyltin carboxylates and
hemiesters, respectively, were also presented.
2.5.2.2 Triorganotin compounds
A number of procedures have been used for the analysis of
organotin fungicide and miticide residues in food, including
spectrophotometry (Corbin, 1970; Getzendaner & Corbin, 1972; Trombette
& Maini, 1970), gas-liquid chromatography (Gauer et al., 1974), and
thin-layer chromatography (Wieczorek, 1969).
Corbin (1970) described a dithiol spectrophotometric method for
the determination of trace amounts of tin residues on fruits
previously treated with a miticide containing tricyclohexyltin
hydroxide as the active component. The detection limit with this
method was at least 0.2 kg tin at a concentration of 3 µg/kg. The
extraction method was tested for compatibility with 35 elements and
only arsenic and antimony seemed likely to interfere.
A sensitive fluorometric technique has recently been developed for
the determination of triphenyltin residues in potato samples (Vernon.
1974). Triphenyltin acetate deposits on potato leaves have also been
determined polarographically by Coussement (1972). The smallest
quantity of organotin fungicide detected by this method was
0.32 µg/cm2 leaf.
Freitag & Bock (1974) reported some methods for the extraction of
tri-, di-, and phenyltin compounds from mixtures containing these
compounds as well as inorganic tin(IV). The separated compounds were
determined by radiometric or photometric methods. Several thin-layer
chromatographic methods were also described.
Bönig & Heigener (1972) determined the tin contents of plants
treated with organotin fungicides by photometric estimation of the
phenylfluorone complex. In an alternative method, tin was extracted
with quercetin and the tin-quercetin complex determined
spectroscopically (Engberg, 1973). Akagi and his collaborators (1972)
separated some butyl- and phenylorganotin compounds in vinegar and
tomatoes by thin-layer chromatography.
A variety of trialkyl and triaryltin biocides have been determined
by a nonaqueous atomic absorption assay (Freeland & Hoskinson, 1970).
The limit of detection of this method appeared to be 2-12 mg/litre at
1% absorption. Studies have been reported by Woggon & Jehle (1973;
1975) and Woggon et al., (1972) in which anodic stripping was used for
the determination of a number of alkyl- and aryltin fungicides and
their degradation and decomposition products. The minimum detectable
amount was about 3.5 × 10-7 moles per litre for all the compounds
studied.
Polarography (Kockin et al., 1969; Tjurin & Flerov, 1970; Tjurin
et al., 1969) and oscillopolarography (Geyer & Rotermund, 1969; Shono
& Matsumura, 1970) have also been used for the determination of a
variety of organotin compounds.
Cenci & Cremonini (1969) described the thin-layer chromatographic
determination of 2 commercial organotin pesticides containing
triphenyltin acetate and triphenyltin hydroxide, respectively, and
their degradation products in various soils.
A gas-liquid chromatographic method was developed by Tonge (1965)
for the analysis of butyl-, octyl-, and phenyltin halides. A variety
of gas-liquid chromatographic procedures for the determination of a
large number of other organotin compounds has been reported (Devjatyh
et al., 1968; Dressler et al., 1971, 1975; Geissler & Kriegsmann 1964,
1965).
Gauer et al. (1974) reported a gas-liquid chromatographic method
for separating and determining tricyclohexyltin hydroxide and
dicyclohexyltin compounds formed by degradation (as the bromide) on
strawberries, apples, and grapes treated with a miticide. The
practical minimum limits of detection for tricyclohexyltin hydroxide
and dicyclohexyltin oxide were 0.1 and 1.0 mg/kg, respectively, in the
3 crops studied.
The quantitative determination of mono-, di-, tri- and
tetra-alkyltin compounds by gas-liquid chromatography after alkylation
was reported by Neubert & Wirth (1975); the same technique was applied
for the quantitative detection of tri- and dibutyltin species in
dilute aqueous solution by Neubert & Andreas (1976). Application of a
liquid-chromatograph coupled with a flameless atomic absorption
detector for speciation of trace amounts of triphenyl-and trialkyltin
compounds in aqueous solution has been reported by Brinkman et al.
(1977). Recently, Meinema et al. (1978) developed a combined gas
chromatography/mass spectrometry detection procedure for the
quantitative determination of trace amounts of tri-, di-, and butyltin
compounds in aqueous solutions.
3. SOURCES OF ENVIRONMENTAL POLLUTION
3.1 Natural Occurrence
Tin is not uniformly distributed over the earth's surface
(Goldschmidt, 1958; Schroeder et al., 1964) and, hence, it is not
found consistently in plants and soils (Schroeder et al., 1964). Most
samples of rock contain tin concentrations of approximately 2-50 mg/kg
(Johansen & Steinnes, 1969; Mason, 1966; Onishi & Sandell, 1957;
Vinogradov, 1956), although levels of about 260 and 1200 mg/kg have
been reported in Czechoslovakian and Norwegian samples of mica
(Johansen & Steinnes, 1969).
Of the 9 different tin-bearing minerals found in the earth's
crust, only cassiterite (tinstone, tin(IV) oxide) is of major
commercial importance (Heindl, 1970), although small quantities of tin
are recovered from the complex sulfides, e.g., stannite
(Cu2.FeS.SnS2); teallite (PbSnS2); cylindrite (PbSn4FeSb2S14)
and canfieldite (Ag8SnS6). Over 80% of the world's tin ore occurs in
low-grade deposits averaging about 240g of metallic tin per cubic
metre.
3.2 Industrial Production
The total world production of tin in 1975 was 236 000 tonnes,
about 92% of which was produced as primary tin. Six countries together
produced 72% of the total world production, i.e., China (10%,
estimated figure), Indonesia (8%), Malaysia (35%), Thailand (7%),
United Kingdom (6%), and the USSR (6%, estimated figure). The world
production of secondary tin was about 20 000 tonnes, almost 50% of
which was produced by France (United Nations, 1977).
The metallurgy of tin is simple, but its extraction from the ore
is complicated by the presence of reduced iron that forms "hard head"
with the tin, and by the high tin content of the slag produced. Thus,
smelting is carried out in 3 stages: (a) primary smelting in either
reverberatory or blast furnaces; (b) retreatment of slags, hard head,
and refinery dross; and (c) refining of the metallic tin to remove the
last traces of impurities. It should be noted that, because of the
high cost of tin, dust-collection equipment is necessary for
successful operation. Low fume production is one of the advantages of
electric smelting (MacIntosh, 1969).
During the last 10 years, the amount of tin recovered from
secondary sources has been practically constant (United Nations,
1977). The largest sources of scrap are clean tin plate clippings from
container manufacture; solder in the form of dross or sweepings; dross
from tinning pots; sludges from tinning lines; bronze rejects and used
parts; babbitt from discarded bearings; and type metal scrap
(Macintosh, 1969). Small quantities of tin are recovered at detinning
plants from used tin containers. (Heindl, 1970).
3.3 Tin Consumption
The USA is by far the largest consumer of tin, with Japan, the
United Kingdom, the Federal Republic of Germany, and France following
in that order. It has been estimated that, in the future, 30% of the
total demand for tin will be met by secondary recovery of the metal.
The total demand for primary tin from 1968 to the year 2000 has been
estimated to lie between 8.5 and 6.2 million tonnes, with a median
estimate of 7.5 million tonnes. The world reserve total is
approximately 6.5 million tonnes and it is considered likely that new
discoveries and increases in known reserves could result in sufficient
new tin to meet the median estimate for this period (Heindl, 1970).
Recently, nearly 40% of the total primary and secondary tin
consumption in the USA has been used in the production of tin-plate,
25% in solders, and 20% in bronze and brass, while smaller quantities
have been used in the production of babbitt and Chemicals
(approximately 3-4% each).
It is important to note the large potential for growth in the
consumption of organotin compounds in the manufacture of plastics.
This could result in a consumption of approximately 5000 tonnes of tin
in plastics in the year 2000 (Heindl, 1970).
3.4 Uses of Tin
3.4.1 Tin and inorganic tin compounds
Tin is mainly used by industries producing tin-plate, solder,
babbit, brasses and bronzes, pewter, printer's alloy (type metal),
plastics, and tin chemicals. The largest single use of tin is in
tin-plated steel, either by hot-dipping or by electroplating in a
continuous process in which thin layers are deposited, and a different
thickness can be applied on each side of the same sheet steel. In
addition to its use in food and beverage packaging, tin-plate is used
extensively in aerosol containers. Tin is also used in the
transportation, machinery, electrical, plumbing, and heating trades
and industries as solder, and in bearings and pipes.
Tin-lead solders contain from 2% tin for container-seaming to 63%
for electrical connexions. In lead-free solder alloys, tin is alloyed
with antimony, silver, zinc, or indium to obtain special properties
such as higher strength or corrosion resistance. The largest
quantities of solder are used in car radiators, air conditioners, heat
exchangers, plumbing and sheet metal joining, container seaming,
generating equipment, electronic equipment, and computers.
The copper-tin alloys are called bronzes. Phosphor-bronzes (5-10%)
are the most important of the tin bronzes, the major applications
being in marine and railway engineering.
Metals used for casting or lining bearing shells are classed as
white bearing alloys, but are better known as babbitt. Babbitt alloys
are used in bearings in marine propulsion, rail and road
transportation, compressors, motors, generators, and fans.
Table 1. Some applications of inorganic tin and its compounds
Compound Application
tin metal manufacture of tin plate, solders, bronzes,
pewter, alloys, amalgams, chemicals
tin(IV) oxide ceramic glaze opacifier, ceramic pigments
tin(IV) hydride gas-plate tin on metal, ceramics
tin(II) acetate catalyst
tin(II) chloride electrotinning of steel strip, tin coating of
sensitized paper antisludge agent for oils,
stabilizer of perfumes in soaps, additive for
drilling muds, electroplating, catalyst in
organic reactions
tin(II) fluoroborate tin-plating baths
tin(II) fluoride toothpaste and dental preparations
tin(II) 2-ethylhexoate catalyst for polyurethane foam production and
incurring silicone oil formulations
tin(II) oxalate catalyst for coal hydrogenation, catalyst for
acid-type esterification, transesterification
or polyesterification
tin(II) oxide manufacture of gold-tin and copper-tin ruby glass
tin(II) sulfate immersion plating of steel wire, electrotinning
strip, with copper sulfate for lacquer finishes
tin(II) tartrate dyeing and printing of textiles
tin(IV) chloride mordant in dyeing of silk, preparation of other
inorganic and organic tins, manufacture of
blueprint and other sensitized papers
sodium stannate alkaline electroplating tin baths
sodium pentafluorostannite dentifrice formulations
Type metals are lead-based alloys containing 1-25% antimony and
3-13% tin that are widely used in the printing trade.
Pewter, which contains 90-95% tin, 1-8% antimony, and 0.5-3%
copper, is used in the production of a wide variety of household
articles.
Special alloys using tin include dental amalgams, which are mainly
silver-tin-mercury alloys; alpha-type titanium alloys that are used in
aircraft, and zirconium alloys used in nuclear reactors.
Some of the manifold applications of inorganic tin compounds are
listed in Table 1.
3.4.2 Organotin compounds
Worldwide production of organotin compounds, the fourth largest
synthesis of organometallic compounds, was approximately 27 000 tonnes
in 1976 (Midwest Research Institute, 1977). This use of tin, however,
represents only about 0.8% of the total metallic tin consumed
globally.
The annual growth rate is expected to be 10% per year for the next
10 years, so that, by 1986, worldwide production of organotin
compounds would be approximately 63 000 tonnes (Midwest Research
Institute, 1977). This projected market outlet depends on 2 features.
Since 70% of the total production of organotin compounds is used to
heat-stabilize poly(vinyl chloride) plastic products, growth in PVC
production must continue to increase at a projected rate of 10-12% per
year for the next 10 years. Second, organotin compounds are expensive
in comparison with other heat stabilizers. Therefore, they must remain
competitive, at least as far as performance is concerned.
Of the 4 categories of organotin compounds, dialkyltin derivatives
are the most important commercially. They are used as heat and light
stabilizers for PVC plastics to prevent degradation of the polymer
during the melting and forming of the resin into its final products.
In addition, dialkyltin derivatives have the unique property of
protecting the plastic product from degradation during use. Other
important commercial uses of dialkyltin derivatives are as catalysts
in the production of polyurethane foam products and as vulcanizing
agents for silicone rubbers. The trialkyl-tin derivatives, which
account for approximately 10% of the total production of organotin
compounds, are used in agriculture as non-systemic fungicides and
acaricides. The tetraalkyltin derivatives are used as intermediates in
the manufacture of other organotin compounds. The monalkyltin
derivatives have a limited commercial use as heat stabilizers for PVC
plastic films.
About 0.5-2.0% by weight of dialkyltin derivatives are required in
the stabilization of rigid and flexible PVC plastics, including
food-grade PVC for wrapping and containers. In particular, dibutyl-
and dioctyltin compounds are important heat and light stabilizers for
PVC. In the USA, the Food and Drug Administration (1971) has set
specific levels for 2 dioctyltin derivatives that can be present in
food, packaged in food-grade PVC wrapping and containers. These 2
chemicals are dioctyltin maleate and dioctyltin
S,S'-bis(isooctylmercaptoacetate) (Piver, 1973).
Dialkyltin compounds such as dibutyltin diacetate or-dilaurate are
used as catalysts in the production of polyurethane foams. Recently,
dimethyltin compounds have also been found suitable for the
stabilization of PVC plastics.
Organotin compounds have many other applications: (a) as
antioxidants and anticracking agents to retard rubber deterioration
and to stabilize chlorinated rubbers in chlorinated paints; (b) in
transformers, capacitators, and cables as hydrochloric acid scavengers
to prevent corrosion when chlorinated diphenyls are present (e.g.,
tetraphenyltin); (c) as anti-oxidants for textile oils; (d) as
activators, stabilizers, and catalysts for polymers such as polyesters
and silicone elastomers and as catalysts for the polymerization of
olefins; (e) in the treatment of glass to increase crack resistance;
(f) in the treatment of fibreglass (with alkyl- and aryltin
compounds) for adhesion go resins; and (g) as curing catalysts in
the application of silicone to textiles and paper.
Organotin compounds have biocidal properties and are used; (a)
as agricultural fungicides (triphenyltin acetate, triphenyltin
hydroxide); (b) as general biocides (bis(tributyltin) oxide = TBTO)
in paints; in the preservation of manila and sisal ropes, leather,
textiles; to make fabrics mildew-resistant; for the protection of jute
and jute bags; in wood preservatives, slimicides, and in the
production of paper; (c) as bactericides and biostasis such as
disinfectants for use in hospitals and stables (tributyltin benzoate);
(d) as helminthicides in poultry (dibutyltin dilaurate,
tetraisobutyltin); (e) as nematocides ( p-bromophenoxy
triethyltin); (f) as herbicides (vinyl-tin compounds, e.g.,
trivinyltin chloride); (g) as rodent repellents (tributyltin
chloride, triphenyltin chloride and acetate); (h) as molluscicides
(triphenyl- and tributyltin compounds; (i) as ovicides (trialkyl-
and triaryltin chlorides in combination with DDT or pyrethrins); (j)
as antifoulants in ship paints and underwater coatings (triphenyl- and
tributyltin compounds); and (k) as miticides (tricyclohexyltin
hydroxide). Furthermore, triphenyltin compounds have been suggested as
insect chemosterilants. More detailed information concerning new uses
of organotin compounds can be found in the proceedings of a recent
symposium (Zuckerman, 1976). A schematic presentation of the various
uses of organotin compounds is given in Fig. 1.
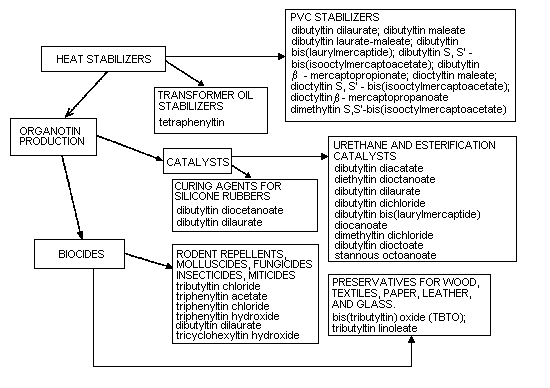 3.5 Tin-containing Wastes
Gases and fumes containing tin as well as sulfur dioxide and other
contaminants, and both soluble and insoluble tin-containing waste
materials such as water soluble salts, muds, and slags are produced
during various smelting, refining, and detinning operations. Solid
domestic and other wastes which may be dumped, incinerated, used for
land fills or composting, contain much tin, and used cans, aerosol
containers, and other miscellaneous tin-containing produces in solid
wastes account for 10-15% of tin-plated steel. The percentage of
plastics in solid wastes is increasing yearly and includes PVC
containing organotin compounds.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATIONS
4.1 Transport and Bioconcentration
Tin concentrations in air are usually low except in the
neighbourhood of some industrial sources (section 5.1). Similarly, it
has not been consistently detected in all soils, plants, and waters
(sections 5.2 and 5.3). This seems go support a geochemical
classification placing tin in a group of elements with a low rate of
migration in soils and waters (Perel'man, 1972; Bens et al., 1976).
However, it is widely used and Wood et al. (1975) have included it
among elements that are relatively accessible in the environment. A
biological cycle for tin has been recently proposed by Ridley et al.
(1977) (section 4.2).
There is a lack of information concerning the environmental
transport of organometallic tin compounds. It appears, however, that
the vapour pressures of some organotin compounds are high and that
environmental mobility is possible although there are no data to
confirm this deduction. Some organotin compounds seem to be well
adsorbed in the soil and they have a low solubility in water (Heron &
Sproul, 1958). A laboratory soil-leaching study (Barnes et al, 1973)
indicated that triphenyltin is strongly attached to the soil. In
natural waters, organotin compounds would be primarily adsorbed on the
suspended particles and sediments (Schramel et al., 1973). More recent
studies on the determination of tin and organotin compounds in natural
waters have been reported by Braman & Tompkins (1979) and Hodge et al.
(1979).
Little reliable information exists concerning the bioconcentration
of tin and its derivatives. Schroeder et al. (1964) reported that the
presence of tin in phosphate fertilizers originating from marine
phosphate suggested that marine animals in the Pleistocene era
absorbed tin from the sea. Although tin is present in seawater in
concentrations of up to about 3 µg/litre (Mason, 1966; Vinogradov,
1953), there are few reports of its occurrence in marine algae,
plankton, bacteria, flowering plants, protozoa, sponges,
coelenterates, echinoderms, crustacea, and most fishes. However, Bowen
(1966) noted tin levels of 0.2-20 mg/kg in certain marine organisms
and accumulation of tin by the sponge Terpios zeteki was also
reported by Bowen & Sutton (1951).
4.2 Environmental Chemistry of Tin
Because of the considerably higher toxicity of some
organo-metallic tin compounds compared with inorganic forms of tin
(section 7), the possibility of biomethylation of tin is obviously of
considerable interest (Wood, 1974). A possible mechanism of such
bio-methylation has been proposed (Ridley et al., 1977; Wood et al.,
1978). Recent laboratory studies have indicated that the methylation
of tin by methylcobalamina (CH3B12) requires a one electron
oxidation of SnII to a SnIII radical, which can take place in the
presence of FeIII (SnIV would, of course, require a single electron
reduction). The stannyl radical (SnIII) can then react with CH3-B12
(CoIII) to produce (under conditions of high chloride ion
concentration) CH3-SnCl2 and reduced cobalamin, containing CoII
(Wood et al., 1978). A demonstration that methylation of tin takes
place in a strain of Pseudomonas bacteria found in the Chesapeake
Bay, USA, is the laboratory work with these bacteria carried out by
Huey et al. (1974). However, the methylated tin species was not
identified. Incubation of mercury(II) in the presence of tin(IV)
resulted in enhanced formation of methylmercury, and the authors
considered that methylmercury might have been transferred from the
biologically methylated tin to the mercury(II) ion. Subsequently,
Brinckman & Iverson (1975) proposed a "mercury-tin cross-over" system,
which may have some real basis as indicated by Schramel et al. (1973),
who found that mercury and tin accumulated together in some water
plants in Bavarian rivers.
4.3 Degradation of Organometallic Tin Compounds
Organotin compounds may be degraded both chemically and
biochemically. Hydrolytic decomposition occurs at rather extreme pH
values (< 1 or > 13) unless there are other catalytic
influences. Nevertheless, some authors consider that it could occur
fairly rapidly in an aquatic environment, although the environmental
pH is usually between 4 and 10 (Sheldon, 1975, Vizgirda 1972). This
could be the case, if photochemical decomposition were also involved.
Indeed, photochemical decomposition of triphenyltin acetate by
ultraviolet irradiation has been demonstrated under laboratory
conditions by Chapman & Price (1972) and Barnes et al. (1973). Most
likely, under environmental conditions, the chemical and
photo-chemical degradation of organotin compounds is combined with
biochemical degradation, or degradation may be entirely biological.
Some available information, mainly on triphenyltin, tricyclohexyltin,
and tributyl tin compounds, is included in the following summary.
a A form of vitamin B12 (cobalamin) in which the methyl carbon
is directly bonded to the cobalt which is coordinated to the corrin
ring system in the vitamin B12 structure.
Bruggemann & Klimmer (1964) and BrÜggemann et al. (1964a,b)
reported that triphenyltin acetate on sugar beet leaves was rapidly
broken down during the silage process. Within 5 weeks, triphenyltin
acetate, originally present at a concentration of 2470 mg/kg of fresh
leaves, had degraded completely. It was not established whether
degradation was due to microbial action or resulted from the low pH
(3.5-4.0) of the silage process. The authors also found that
triphenyltin acetate was not broken down rapidly by microorganisms
during its passage through the rumen and intestines of cattle. Similar
results in feeding experiments with sheep were reported by Herok &
Götte (1963).
Using thin-layer chromatography, Cenci & Cremonini (1969) found
that triphenyltin acetate and hydroxide mixed into soil (80 mg/kg of
soil) disappeared in 3-10 days, and 3 days, respectively, but the
degradation products were not identified and the participation of
microorganisms in the process was not established.
Akagi & Sakagami (1971) reported that, under their experimental
conditions, ultraviolet irradiation of a solution of triphenyltin
chloride yielded a mixture of triphenyl-, diphenyl-, and phenyltin as
well as inorganic tin compounds, within 6 h. Similarly, irradiation of
trialkyltin compounds resulted in a mixture of di- and monoalkyltin
compounds and inorganic tin also within a period of 6 h. Total
degradation of triphenyltin to inorganic tin required irradiation for
more than 400 h. Chapman & Price (1972) investigated the degradation
of an agricultural fungicide containing triphenyltin acetate, which
had been found to disappear within a few days of spraying on crops,
probably as a result of weathering and sunlight. They exposed the
compound in thin layers on glass to ultraviolet light and found that
the rate of degradation was higher at lower wavelengths and was
independent of layer thickness (5-10 mµ). This was also true for the
intermediate diphenyl- and phenyltin compounds and for the parent
compound. When triphenyltin acetate has irradiated for 60 h
(wavelength above 235 nm: intensity 120 W/m2: thickness 10 µm), about
10% of the triphenyltin compound remained unchanged, about 30%
occurred as diphenyltin, 15% as phenyltin compounds, and approximately
45% was degraded to inorganic tin in the form of hydroxides or
hydrated oxides. Similar results were obtained by Barnes et al.
(1973), who also studied breakdown at concentrations of 5-10 mg/kg
using triphenyltin acetate in which the phenyl groups had been
labelled with 14C. Degradation was monitored by measuring the
evolution of radioactive carbon dioxide; a half-time of about 140 days
was determined. Since no 14CO2 was evolved when the loam was
heat-sterilized, it was concluded that the degradation process was due
to microbial action.
The decomposition of triphenyltin chloride on sugar beet leaves,
as reported by Freitag & Bock (1974b) gave the expected series of
degradation products, the final degradation product being tin oxide.
After 42 days, about 19% of triphenyltin chloride had undergone
degradation.
In studies by Starnes (unpublished data)a and by Smith et al.
(unpublished data)b quoted by Getzendaner & Corbin (1972),
tricyclohexyltin hydroxide (the active ingredient of a commercial
miticide) exposed on thin-layer chromatographic plates to a sun lamp
yielded dicyclohexyltin oxide and cyclohexylstannoic acid with further
degradation to inorganic tin. It has also been shown that tin residues
on apples and pears treated with tricyclohexyltin hydroxide
(commercial miticide) remained almost constant at a level of
0.1-0.2 mg/kg (Getzendaner & Corbin, 1972). This concentration was
relatively independent of the total number of applications and, within
a month of the final application, independent of the time between the
last application and harvest.
Mazaev et al. (1976) reported the degradation of bi(tributyltin)
oxide (TBTO), dibutyltin bis isooctylmercaptoacetate (BuIOMA),
tributyltin methacrylate, diethyltin dioctanoate and dioctyltin bis
(isobutylmaleate), and provided estimates for the half-times of these
compounds for different aqueous media. In distilled water at 20°C,
values ranged from 1.14 days (BuIOMA) to 18.2 days (TBTO). From this
report, it appears that dialkyltin compounds are more rapidly degraded
than trialkyltin compounds.
Triorganotin compounds such as TBTO and tributyltin fluoride used
as antifouling agents in paints for ship bottoms and in underwater
coatings appear to break down in a multislop process to give tin(IV)
oxide (Barnes et al., 1973; Sheldon, 1975).
The mechanism of the biological dealkylation of organotin
compounds is apparently more complicated than was considered by Blair
(1975), who thought that the basic process was the oxidative cleavage
of the carbon-tin bond (section 6.2.4).
a Starnes (1966) Unpublished report, The Dow Chemical Co.
(ALS 66-648).
b Smith et al. (1970) Unpublished report, The Dow Chemical Co.
(OL 30445 Feb. 1. 1970).
Available information shows that the persistence of organotin
compounds may vary considerably depending on the conditions and the
type of compound. Under extreme laboratory conditions, the solvolysis
may have a half-time ranging from about 1 min to about 100 days
(Bassindale et al., 1971; Roberts & El Kaissi, 1968). Under
environmental conditions, it is likely that the half-times for
triphenyltin compounds are somewhere between a few days and 140 days
(Freitag & Bock, 1974b). Diorganotin compounds probably have somewhat
shorter half-times (Mazaev et al., 1976).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1 Ambient Air
Tin is rarely detected in air, and, when detected, it is generally
in low concentrations (0.01 µg/m3), except in the proximity of some
industrial sources. Tin emission concentrations of 10-640 µg/m3 were
reported from electric furnaces at certain plants in Japan, in 1972.
At a distance of 700 metres, the atmospheric tin concentrations still
ranged from 3.8 to 4.4 µg/m3 (Environment Agency, Japan, 1971,
unpublished data).a
Concentrations from 0.003 to 0.3 µg/m3 were found in 60.6% of 754
samples tested from 22 cities in the USA. More than 50% of samples
from 3 urban and 3 rural sites were below the detectable level. The
highest concentration of tin (0.8 µg/m3) was found in a sample from a
Boston, USA, industrial site, which also contained the highest
concentration of lead together with relatively high concentrations of
zinc and cadmium (Tabor & Warren, 1958).
At stations of the US National Air Surveillance Networks during
1968 and 1969, concentrations ranged from below the minimum detectable
level to 0.23 µg/m3 in 1968 and 0.12 µg/m3 in 1969. Both these
concentrations were recorded in East Chicago (US Environmental
Protection Agency, 1973).
Concentrations ranging from 0.04 to 0.09 µg/m3, determined by
emission spectroscopy, were reported for Cincinnati and St Louis in
1970 by the US National Air Surveillance Cascade Impactor Network (Lee
et al., 1972). The size distributions of tin-containing particles
found in this study are given in Table 2.
Peak concentrations of tin were found in particles ranging from 1
to 3 µm in diameter. Bogen (1973) found that ambient air levels of tin
in the Heidelberg area of the Federal Republic of Germany between 30
April 1971 and 21 May 1971 ranged from 0.096 to 0.167 µg/m3.
The combustion of coal, oil, and lignite results in the discharge
of trace amounts of many elements into the atmosphere. Bertine &
Goldberg (1971) pointed out that the principal sites of fossil fuel
consumption are in the mid-latitudes of the Northern Hemisphere and
suggested a need for linking fossil fuel consumption with the
sedimentary cycles of trace metals from the atmosphere. They reported
that tin was present in coal and oil at average concentrations of 2
and 0.01 mg/kg, respectively.
a Japanese Background Paper No. 2, prepared for the WHO meeting
on the Effects on Health of Specific Air Pollutants from Industrial
Emissions, Geneva, November 4-9, 1974.
Table 2. Size distribution of tin-containing particles in urban aira
Cincinnatti St Louis
(A) (B)
4th Quarter 1st Quarter 4th Quarter
1970 1970 1970
Average concentration (µg/m3) 0.09 0.04 0.05
Average mass median diameter (µm) 0.93 1.40 1.53
% particles less than or equal to 1 µm 55 34 28
% particles less than or equal to 2 µm 86 68 65
a Adapted from Lee et al. (1972).
5.2 Soils and Plants
Tin has not been consistently found in all soils and plants;
however, allowance must be made for the possibility that the
concentrations present may have been below the limits of detection of
the methods employed. Bowen (1966) reported levels of tin in soil
ranging from about 2 to 200 mg/kg, the metal being strongly adsorbed
by the humus.
Schroeder et al., (1964) observed that concentrations of tin in
soils were localized and that it had not been detected in many areas.
In a limited study of tin in vegetation and foods, he found that
absorption by plants was erratic, even when the soil tin content was
high. A tin concentration of 157 mg/kg dry weight was recorded in
forest soil from southern Vermont, USA, but tin concentrations in
sections of an 100-year-old elm indicated that exposure of this tree
was not of recent origin.
Tin concentrations of 30-300 mg/kg have been reported in peat from
Finland by Gordon (1952), and high concentrations of tin were found by
Goldschmidt (1958) in forest litter and humus and also in coal. Tin
was present in all but 7 of 43 samples of foliage from 13 species of
ornamental plants and trees in New Jersey, USA (Hanna & Grant, 1962).
Lounamaa (1956), using a spectrographic method that had a limit of
detection of 10 mg tin/kg of ash, detected tin only sporadically in
higher plants in Finland. Lichens concentrated tin to exceptionally
high levels, considering the small amounts present in rocks. For
example, those growing on silicic rocks had tin concentrations of 72 ±
4.7 mg/kg ash (range: less than 10-100). Tin was undetected in 6 out
of 16 mosses while the remainder had concentrations of 10-60 mg/kg of
ash, concentrations being twice as high in those growing on silicic
rock as in those growing on calcareous rock. Pasture herbage growing
in Scotland was reported by Mitchell (1948) to contain tin
concentrations of only 0.3-0.4 mg/kg dry weight. He also quoted tin
concentrations in soils from north-east Scotland of up to 200 mg/kg.
Warren (1964, unpublished data) reported a tin concentration of
75 mg/kg in soils in unmineralized areas of Devon and Cornwall in
England, while soils from mineralized areas contained up to more than
1000 mg/kg.
5.3 Water and Marine Organisms
Tin has only been found occasionally in fresh water. It was found
by Durum & Haffty (1961) in 3 out of 59 samples from 15 major North
American rivers. No tin was detected by the National Water Quality
Network (1960) in 119 analyses at 71 locations on 28 major rivers in
the USA. Kleinkopf (1955, 1960) reported that Maine waters contained a
mean tin concentration of 0.038 µg/litre with a maximum concentration
of 2.50 µg/litre.
Tin has also only been found occasionally in municipal water
supplies (Durfor & Becker, 1964). Analyses by the US Public Health
Service showed concentrations of 0.0008-0.03 mg/litre in 32 out of 175
finished municipal waters (Taylor, 1964, personal communication).a
Although it is known that acidic or alkaline corrosive waters can
attack bronze fittings in pumping stations, presumably releasing some
tin, the relationship between corrosion and the aqueous release of tin
has not been established (Schroeder et al., 1964).
It has been reported by Vinogradov (1953) and Mason (1966) that
tin is present in sea water in amounts of about 0.003 mg/litre; thus,
its presence in marine organisms is to be expected. Tin levels of
0.2-20 mg/kg have been reported in marine animals by Bowen (1966) and
accumulation by the sponge Terpios zeteki has been noted (Bowen &
Sutton, 1951). Schroeder et al., (1964) reported that tin had not been
detected in plankton, bacteria, flowering plants, protozoa,
coelenterates, echinoderms, crustacea, or most fish. However,
Vinogradov (1953) reported the presence of small amounts of tin in a
marine worm, an oyster, and a dogfish.
a Taylor, F. B. (1964) Personal communication, cited by Shroeder,
H. A., Balassa, J. J., & Tipton, I. H. (1964) Abnormal trace metals
in man; Tin. J. chron. Dis., 17: 494.
Organotin compounds may enter water directly from antifouling
coatings on ship bottoms or they may be used as molluscicides and
added to vast areas of water for the control of snails, in which case
the effective concentration has to be about 1 mg/litre (WHO, 1973).
They may also be present indirectly as a result of industrial,
agricultural, and other uses of organotin compounds.
Tin(IV) oxide, suggested to be the final breakdown product of some
antifouling organotin agents (Shelemon, 1975), will eventually be
deposited in bottom sediments because of its insolubility. However, no
information is currently available concerning the rate and mechanism
of this degradation.
5.4 Food
Tin has been reported to occur in trace amounts in most natural
foods (de Groot et al., 1973). The tin content was determined by
atomic absorption spectroscopy in 11 known wheats or wheat blends, in
20 flours prepared commercially from these wheats, and in 25 products
specially prepared from the flours, as well as in 10 consumer products
from 10 different cities in the USA. Only one half of the tin in bread
could have been contributed by the flour. Tin concentrations in common
hard, common soft, and durum wheat were 5.6 ± 0.6, 7.9 ± 0.9, and 6.8
± 0.5 mg/kg respectively, while samples of flour and semolina from the
above sources of wheat contained tin at 4.1 ± 0.4, 3.7 ± 0.7, and 6.0
± 1.2 mg/kg, respectively. The tin concentrations in most products
exhibited significant regional differences (Zook et al., 1970).
Schroeder et al. (1964) calculated that a diet composed largely of
fresh meats, cereals, and vegetables would usually contain a tin
concentration of less than 1 mg/kg and would supply about 1 mg of tin
per day. In an area of southern Vermont, USA, where the soil contained
considerable amounts of tin (about 160 and 33 mg/kg, respectively, in
2 gardens), 10 out of 18 samples of fresh vegetables contained tin
levels of less than 1 mg/kg.
Hadzimicev (1971) measured the content of tin in foods from the
vegetable belt around Sofia, Bulgaria. Tin concentrations in wheat,
corn, beans, potatoes, tomatoes, cabbage, carrots, spinach, lettuce,
onions, apples, and peaches ranged from 0.02 to 1.02 mg/kg.
Larger amounts of tin may be present in processed foods and
drinks, because of corrosion and leaching of the metal from plain
unlacquered cans or from tin foil used for packaging. The corrosion of
tin-plate in food containers depends upon several factors including
the type of food product, the time and temperature of storage, the
acidity of the food and the quantity of air present in the headspace
of the can (Calloway & McMullen, 1966; Monier-Williams, 1949).
Oxidizing agents (eg., nitrates, ferric and cupric salts),and
anthocyanin pigments, methylamine, sulfur dioxide, and other sulfur
compounds accelerate corrosion, while fin salts in solution, sugars,
and colloids such as gelatine retard it (Monier & Williams, 1949). The
introduction of lacquered cans and the crimping of the tops minimizing
direct contact of food with solder has resulted in a general reduction
in corrosion and leaching.
A number of foods are unsuitable for packing in plain cans as they
promote corrosion. Thus, tin has been found in canned asparagus in
high concentrations varying from 120 to 550 mg/kg (Adam & Horner,
1937; Eyrich, 1972; Woidich & Pfannhäuser 1973). Tomato fruit can
accumulate nitrate when grown under combined conditions of high
temperature, high nitrogen fertilization levels, and low light
intensity. Such tomatoes caused excessive detinning of internal plain
can surfaces (Hoff & Wilcox, 1970). Iwamoto et al. (1968) also showed
a distinct detinning effect on unlacquered cans of both naturally
occurring and added nitrate in tomato juices; the authors suggested
that the concentrations of nitrate-nitrogen in juices should not
exceed 3 mg/kg. Tin contents exceeding 100 mg/kg have been found in
other foods in unlacquered cans including fish, orange, grape, and
mango juices, apricots, bananas, pineapple, and sugar syrup (Catala et
al., 1971; Eyrich, 1972; Mahadevaiak et al., 1969; Stanculescu et al.,
1972; Woidich & Pfannhäuser, 1973). In foodstuffs stored in
all-lacquered cans, the tin content was mainly below 25 mg/kg (Catala,
et al., 1971; Eyrich, 1972; Woidich & Pfannhäuser, 1973).
Several investigations have shown that high storage temperatures
increase the transfer of tin into canned foods (Catala et al., 1971;
Kimura et al, 1970; Zui, 1970). An increase of 2 mg/kg per month of
storage was reported by Zui (1970) for an increase of 1°C in
temperature.
Nishijama et al. (1971) showed an increase in the tin content from
50-77 to 260-300 mg/kg in pineapples stored at 8°C in unlacquered cans
for 72 h after opening the can. The transfer of tin into the fruits
was also increased by 0.5% aqueous citric or tartaric acid. The
content of tin in foods in lacquered cans was low and remained low
after opening. Klein et al. (1970) noted a similar effect of storage
of fruit juices in opened plain cans. Values exceeding 200 µg/kg were
found within 48 h of opening the cans.
A mean concentration of 0.0078 mg/litre was found in bottled
(glass) cow's milk compared with 16 mg/litre in evaporated milk in
unlacquered cans (diluted to the equivalent volume of cow's milk). In
some cases, concentrations of up to 110 mg/litre were found; however,
only milk from unlacquered cans contained significant amounts of tin,
while milk in lacquered cans contained less than 5 mg/litre (Hamilton
et al., 1972). The highest concentration of tin found by Wodsak (1967)
in canned condensed milk samples, less than 4 weeks old, was
40 mg/litre. The concentrations did not increase much during a further
5 months of storage but after 2 years of storage, concentrations up to
160 mg/litre were detected.
In addition to contamination by the containers, the presence of
tin in food may be due to the use of tin as an additive. Tin(II) ions
are used as additives in asparagus and peas packed in glass containers
or in lacquered cans, and also in soda water. The stannous ion is
added to prevent the migration of other heavy metals into the canned
foods and to inhibit the oxidation of ascorbic acid. Scott & Stewart
(1944) reported that Clostridium botulinum would not grow in
beetroot and carrots in unlacquered cans whereas normal growth
occurred in lacquered cans. For canned beetroot, the concentration of
added tin required for the prevention of growth was 150 mg/kg and for
carrots, 30-60 mg/kg.
High concentrations of tin have been reported in cheese packed in
tin foil (Dyer & Taylor, 1931, Elten, 1929).
5.5 Organotin Residues
Tin occurring in food and beverages may also result from the use
of organotin compounds as miticides in agriculture and as stabilizers
in PVC materials. In several experiments in various parts of the USA,
apples and pears from trees that had been sprayed 4 times with a
miticide, tricyclohexyltin hydroxide, had maximum organotin residues
of less than 2 mg/kg of whole fruit on the day of the final
application. These concentrations were reduced to about half within
3-5 weeks and, after 4 weeks, the residues consisted mainly of
tricyclohexyltin hydroxide with only small amounts of decomposition
products; inorganic tin concentrations were almost invariably below
0.2 mg/kg. The mean concentration of inorganic tin on apples and pears
after 1-5 applications of the miticide was 0.1 mg/kg (equivalent to
0.3 mg/kg, when calculated as tricyclohexyltin hydroxide); an
inorganic tin residue of 0.3 mg/kg (equivalent to 1.0 mg/kg of
tricyclohexyltin hydroxide) was found on pears by Getzendaner & Corbin
1972). In an appraisal of data on pesticide residues in food, the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues (FAO/WHO, 1971) concluded that residues of
tricyclohexyltin hydroxide on apples and pears decline by 50% in about
3 weeks due to photodegradation (section 4.3). A 20-50% reduction can
be achieved by washing and most of the residues can be removed by
peeling the fruit, after which only 0.1 mg/kg may be expected in the
fruit flesh. Data on tricyclohexyltin hydroxide on apples and pears
after 1-4 treatments showed mean values ranging from 0.4 to 2.0 mg/kg.
Similar concentrations on apples and pears have been reported from the
Netherlands (WHO, 1975). It has also been reported that the
concentrations of tricyclohexyltin hydroxide in treated products grown
under glasshouse conditions including cucumbers, tomatoes, and bell
peppers are unlikely to exceed 0.5 mg/kg (WHO, 1975).
The FAO/WHO joint meeting report (FAO/WHO, 1971) included data on
triphenyltin hydroxide, acetate, and chloride residues in various
foodstuffs such as potatoes, carrots, and sugar beets. Maximum
concentrations in potatoes and carrots only rarely exceeded 0.1 mg/kg.
Residues in food, fruit, and vegetables derived from direct contact
with the compound could be considerably reduced by washing.
When cows were fed with sugar beet leaves containing triphenyltin
acetate at 1 mg/kg a concentration of 0.004 mg/kg was found in the
milk.
In some countries, regulations permit the presence of 2 PVC
stabilizers in food, viz. dioctyltin S,S'-bis(isooctyl-
mercaptoacetate) and dioctyltin maleate polymer, up to a concentration
of total octyltin of 1 mg/kg (Department of National Health and
Welfare, Canada 1975; Food and Drug Administration, USA, 1971). The
migration of tin from PVC bottles into liquid foods contained in them
was studied by Carr (1969). The increase in the tin content of various
products during the storage period ranged from 0 to 0.07 mg/kg
(Table 3).
Table 3. Tin concentrations in foodstuffs after storage for 2 months in PVC bottles at 30 °C
Organotin
Tin content Tin content Tin extracted stabilizer
at beginning after ageing from bottle extracted from
Food product of experiment in PVC bottle (mg/kg) PVC bottle
(mg/kg) (mg/kg) (mg/kg)
mineral water 0.076 0.088 0.01 0.063
tomato juice 0.03 0.03 0 0
peanut oil 0.05 0.06 0.01 0.063
vegetable oil 0.08 0.09 0.01 0.063
apple juice 0 0.02 0.02 0.126
cherry soda 0 0.07 0.07 0.443
beer 0 0.01 0.01 0.063
milka 0.02 0.04 0.02 0.126
red wine 0 0 0 0
blended whisky 0.01 0.02 0.01 0.063
a Milk sample in PVC bottle aged for 2 weeks at 65 °C (Carr, 1969).
5.6 Working Environment
While most of the operations associated with the extraction and
treatment of tin ore are wet processes, tin dust or fumes may escape
during the bagging of concentrate in the ore rooms and during smelting
operations (mixing plant and furnace tapping), as well as during the
periodic cleaning of the bag filters used to remove particulate matter
from smelter furnace flue gas before release into the atmosphere (ILO,
1972). Exposure to dust and fumes of tin oxide may cause stannosis.
Additional exposure can occur at the final stage of upgrading the
cassiterite concentrate and during the roasting of sulfide ore.
Tin(II) chloride constitutes a hazard, when the rough molten tin is
separated from the rest of the charge during refining (ILO, 1972).
Other sources of industrial exposure include tinning of metals
(Duckering, 1968); the preparation and use of tin alloys and solders;
the use of tin(II) chloride as a reducing agent in calico printing;
the use of hydrated stannic acid, sodium metastannate, hydrated
tin(IV) chloride, and ammonium tin(IV) chloride as mordants in dyeing
and in the weighting of silk; the use of tin(II) fluoroborate
[Sn(BF4)2] in plating baths; and the production and use of organotin
compounds.
5.7 Estimate of Effective Exposure of Man through Environmental Media
Food is the main source of exposure to tin for man. Estimates of
human exposure from all environmental media are difficult to make
because of the lack of reliable data concerning environmental
concentrations (mainly in air and water), and because of variations in
the amounts of different foods and beverages consumed, especially
canned foods. Earlier estimates of the daily intake of tin by man have
been difficult to reconcile with more recent estimates. Kehoe et al.
(1940) reported that the mean daily intake of Gin by a normal adult in
the USA was 17 mg, while Tipton et al. (1966, 1969), in long-term
balance studies, reported average daily intakes of between 1.5 and
8.8 mg in 4 subjects. Schroeder et al. (1964) found 3.6 mg in a day's
institutional diet and considered that the major portion of the tin
probably came from canned fruit juices and fruits in the diet.
However, Schroeder and his colleagues pointed out that human intake
may vary considerably. They calculated that a 10 MJ (2400 kcal) diet
composed largely of fresh meats, grain products, and vegetables, which
usually contain less than 1 mg/kg of tin, would supply 1 mg/day.
However, a diet including a substantial proportion of canned
vegetables and fish could supply as much as 38 mg/day. A typical daily
tin balance for an adult in the USA, as estimated by Schroeder et al.
(1964), is shown in Table 4.
Hamilton et al. (1972) reported a daily intake of tin from food
and beverages by an adult in the United Kingdom of 0.187 ± 0.042 mg
(Table 5). It seems likely that this diet was composed of fresh foods,
which would account for the lower values obtained compared with the
North American data.
Table 4. Daily tin balance for an adult in the USAa
Intake (mg) Output (mg)
food 4.0 faeces (estimated) 3.98
(range, 1-40) (range, 1-40)
water 0.0 urine 0.023
(range, 0-0.03) (range, 0-0.04)
air 0.003
(range, 0-0.007)
total 4.003 (1-40) 4.003 (1-40)
a From: Schroeder et al. (1964).
Table 5. The concentration of tin in foods and total daily intake
for an adult in the United Kingdoma
Item Number of Concentration of tin (mg/kg)b
samples
cereals 3 4.7±1.5 × 10-2
meat 4 2.1±0.5 × 10-3
rats 4 3.7±0.8 × 10-2
fruits 5 0.5±0.1
root vegetables 3 2.2±1.0 × 10-2
green vegetables 4 2.3±10-2
milk (cow's) 17 7.8±1.2 × 10-3 (mg/litre)
total daily untake 0.187±0.042
a "From Hamilton et al. (1972).
b For prepared diet.
6. METABOLISM
Although a substantial amount of literature exists on the
absorption, distribution, excretion, and storage of tin, the results
of much of the early research are questionable in the light of modern
analytical techniques. For example, some studies depended on tissue
matrix destruction processes with the risk of loss of tin salts by
volatilization. In many cases, conditions were not controlled or
defined making it difficult to know whether pure valence states of
tin(II) or tin(IV) were being used, and whether the oxidation state of
the element affected its biological fate. In most cases, the purity of
the compound used was not indicated.
6.1 Inorganic Tin
6.1.1 Absorption
Evidence obtained from man and several animal species shows that
ingested inorganic tin is poorly absorbed. Most studies indicate that
less than 5% is absorbed from the gastrointestinal tract, although
values as high as 20% have been reported (Furchner & Drake, 1976;
Hiles, 1974; Kehoe et al., 1940; Kutzner & Brod, 1971,
Monier-Williams, 1949; Schroeder et al., 1964).
The influence of oxidation state and anion complement on the rate
of gastrointestinal absorption was studied by Hiles (1974) by
administering tin to rats in the form of tin(II) citrate, fluoride, or
pyrophosphate, and tin(IV) citrate or fluoride as a single oral dose
at 20 mg/kg body weight. The absorption of tin(II) citrate and
fluoride was calculated to be 2.8%, whereas only 0.6% of the dose of
tin(IV) citrate and fluoride was absorbed; the citrate and fluoride
were equally absorbed. Absorption of tin, when the anion was
pyrophosphate, was significantly lower than when the anion was citrate
or fluoride. The author considered that this was because pyrophosphate
had a greater tendency to form insoluble complexes with tin than
either fluoride or citrate.
6.1.2 Distribution
With both oral and parenteral administration of tin to animals,
the highest concentrations were found in the kidney, liver, and bone
(Durbin, 1960; Hiles, 1974; Moskalev, 1964), the principal site of
deposition being the bone (Furchner & Drake, 1976; Hiles, 1974).
The amounts of tin remaining in the organs of rats 48 h after
receiving a single oral dose of 20 mg of tin in the form of a
radioactive compound are shown in Table 6; the distribution 48 h after
a single intravenous administration of tin(II) or tin(IV) citrate is
shown in Table 7 (Hiles, 1974).
Rats given a daily oral dose of 20 mg of tin per kg of body weight
as tin(II) or tin(IV) fluoride did not show any detectable
trans-placental transfer at day 10 after conception, and at day 21,
the amounts of tin found in rats given the tin(II) compound were so
close to the limit of detection (= 0.1 µg of tin) that the
significance of the findings remained doubtful. In rats receiving the
same compounds at similar daily doses for 28 days, no significant
amounts of tin could be detected in the brain, heart, testes,
adrenals, reproductive tract, skeletal muscle, and spleen, while the
levels in kidney and liver were approximately the same as after a
single oral dose; however, the levels in bone were about 8 times
higher than those found after a single oral dose. Tissue
concentrations were higher following administration of tin(II)
fluoride than following tin(IV) fluoride administration (Hiles, 1974).
The results of distribution experiments performed by Hiles (1974)
suggested that tin is unlikely to be rapidly oxidized or reduced
during absorption and systemic transportation, although the actual
oxidation state of the tin in the tissues could not be determined.
Following intravenous injection of 113Sn(II) and 113Sn(IV)
citrates in rats, only small quantities of tin could be detected in
lung tissues (Hiles, 1974). However, tin is found in the human adult
lung and there is evidence indicating that the concentrations increase
with age often reaching higher levels than those in other tissues
(Schroeder et al., 1964). Thus, it seems probable that tin reaches the
lung primarily through inhalation.
Levels of 113Sn in the blood of rats, 2 days after oral or
intravenous administration, were extremely low and were only detected
in the erythrocytes (Hiles, 1974). Kehoe et al. (1940) found that 80%
of the tin in the blood of man was in the cells.
6.1.2.1. Distribution in human tissues and biological fluids
Tin concentrations found by Hamilton et al. (1972/1973) in some
tissues of healthy human adults are given in Table 8 together with
data reported by Kehoe et al. (1940) and Schroeder et al. (1964).
Comparatively high concentrations were found in lung, kidney, liver,
bone, and also in lymph nodes. Fairly high levels were reported by
Storozeva (1963) in the teeth of 24 persons, i.e., a range of
0.5-1.7 mg/kg of ash.
Table 6. 113Sn. distribution in rats after a single oral dose of solutions of various 113Sn compoundse
Percent of dosed radioactivity
Sample
SnF2 SnF4 Sn(II) Citrate Sn(IV) Citrate Sn2P2O7
Faeces and
gastrointestinal
wash 100.60±9.9 (10)a 98.40+3.6 (10) 98.00±2.1 (5) 95.40±10.3 (5) 100.0±2.8 (6)
Urine 1.02±0.58 (10)b,d 0.22±0.13 (10)b 0.90±0.23 (5)c 0.33±0.12 (5)c 0.41±0.19 (6)d
Kidneys 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.05±0.02 (5)c 0.01±0.00 (5)c 0.03±0.02 (6)d
Liver 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.07±0.02 (5) < 0.004 (5) 0.02±0.02 (6)d
Pancreas < 0.004 (5) 0.02±0.03 (5) < 0.004 (5) < 0.004 (5) < 0.004 (5)
Recovery 101.74±9.4 (10) 98.66+3.6 (10) 99.02±2.3 (5) 95.74±10.5 (5) 100.46±2.9 (6)
Percent of dosed 113Sn/g of sample
Bone (femur) 0.20±0.13 (10)b,d 0.04±0.02 (10)b 0.09±0.04 (5)c 0.03±0.02 (5)c 0.02±0.01 (6)d
Lymph nodes < 0.004 (5) 0.08±0.12 (9) < 0.004 (5) < 0.004 (5) 0.01±0.01 (5)
Blood clot 0.01±0.01 (10) < 0.004 0.01±0.00 (5) < 0.004 (5) < 0.004 (6)
Leg muscle < 0.004 (5) 0.01±0.01 (5) < 0.004 (5) < 0.004 (5) < 0.004 (6)
a % of dosed 113Sn±SD (number of animals) where 1% = 40 µg. Tissues containing < 0.010%, of the dose were not
normally included in the table.
b Significant difference at p < 0.05 between SnF2 and SnF4.
c Significant difference at p < 0.05 between Sn(II) citrate and Sn(IV) citrate.
d Significant difference at p < 0.05 between SnF2 and Sn2P2O7.
e From: Hiles (1974).
Table 7. Fate of inorganic tin in rats. Tin distribution after a single intravenous
dose of 113Sn(11) or 113Sn(IV) citratee
Percent of dosed radioactivity
Sample Sn(II) citrate Sn(IV) citrate
Bile-duct Bile-duct
Intact cannulated Intact cannulated
Urine 35.3±4.6a 23.3±12.8 39.8±10.8 24.8± 2.5
Faeces 12.1±1.8b,d 2.2±1.1d 3.1±1.4b 0.5±0.3
Bile -- 11.5±2.4c -- 0.1±0.1c
Injection site 3.3±1.8 6.2±4.7 4.1±1.5 3.9±3.3
Liver 2.0±0.2 5.1±1.6c,d 0.2±0.1d 0.2±0.1c
Kidney 5.9±0.9 8.3±1.3 5.3±3.4 4.2±2.5
Lungs 0.12±0.08 0.2±0.1 < 0.04 < 0.06
Spleen 0.4±0.0 -- < 0.04 --
Pancreas 0.2±0.1 -- < 0.04 --
Stomach 0.1±0.1a 0.2±0.0d < 0.04 < 0.06
Small intestine 0.5±0.1a 0.4±0.4d < 0.04 < 0.06
Large intestine 1.0±0.8b,d 0.4±0.3d 0.2±0.3b < 0.06
Carcass 33.4±5.3 -- 43.2±5.3 --
Femur (one) 1.6±0.2 1.9±0.1 3.2±0.6 2.0±0.1
Percent of dosed 113Sn/g
Bone (femur) 4.5±0.6b,d 8.4±1.0d 8.8±1.8b 7.4±0.9
Blood clot 0.6±0.2d 0.9±10.3d < 0.04 < 0.06
Leg muscle 0.06±0.03 -- < 0.04 --
a % of dosed 113Sn±SD where 1% = 4 µg tin.
b Significant difference at p < 0.05 between intact Sn(II) and Sn(IV) animals.
c Significant difference at p < 0.05 between bile-duct cannulated Sn(II) and
Sn(IV) animals.
d Significant difference at p < 0.05 between Sn(II) intact and bile-duct cannulated
animals.
e From: Hiles (1974).
Schroeder et al. (1964) reported a highly variable distribution of
tin in human tissues with significant differences related to age and
geographical location. Variations in the tissue contents of tin among
several geographical regions throughout the world were quite marked
but were less noticeable between 8 cities of the USA (Table 9 and 10).
All kidney, liver, and lung samples from Africa, Asia, and Europe had
lower mean and median tin contents than samples from the USA (the
difference being least in lungs). Tin in the tissue of Africans, where
exposure to tin would be expected to be much lower than in
industrialized North American and European areas, was, indeed,
noticeably low. Tin was rarely detected in the tissues of stillborn
infants, indicating that tin does not readily cross the placental
barrier. The lungs accumulated tin with advancing age but other organs
did not. Concentrations of tin in kidney, liver, lungs, and the ileum
according to decade of life are shown in Table 9. Small amount were
found in the heart, prostate, uterus, and trachea. Avtandilov (1967)
studied the trace element contents of normal and atherosclerotic
aortas. He reported a mean tin concentration of 0.08 mg/kg wet weight
in 20-39-year-old subjects with no difference between the two
categories of aorta.
Table 8. Tin content of human tissues, concentrations expressed as mg/kg wet weight.
Hamilton et al. (1972/73) Kehoe et al. (1940) Schroeder et al. (1964)
Tissue mean ± S.E. mean range (8 cities in
USA)
blood 0.009±0.002
brain 0.06±0.01 NDa
kidney 0.2±0.04 0.2 0.23-0.76
liver 0.4±0.08 0.35-1.0
lung 0.8±0.2 0.45 0.49-1.20
lymph node 1.5±0.6
muscle 0.07±0.01 0.1
bone 4.1±0.6b 0.5-0.8
a ND = not detected.
b mg of element per kg of ash.
Table 9. Tin in human tissues by age, mean US values (mg/kg ash)a
Age group (year) 0 0-1 1-10 11-20 21-30 31-40 41-50 51-60 81-70 71-84
kidney
concentration 0 57b 60b 33 20 34b 28b 22 34 32
occurrence 0/16 9/10 19/20 10/15 17/21 36/43 22/26 26/27 13/14 8/9
liver
concentration 0 48b 61b 42 34 33b 25 35 38b 34b
occurrence 0/21 11/12 22/23 14/14 20/21 39/43 27/28 25/25 13/13 9/9
lung
concentration (18) 35 34 45b 27 31b 39b 53b 58 64b
occurrence 1/10 5/6 5/6 10/13 18/21 34/35 27/27 28/29 13/13 9/9
ileum
concentration 0 101 98 80 174 116 97 53 172 140
occurrence 0/8 1/2 1/1 9/10 12/13 26/28 16/17 12/12 6/6 3/4
total occurrence
(%) 1.8 86.7 94.0 82.7 88.2 79.8 93.9 97.8 97.7 93.5
Note: Concentrations of positive samples.
Occurrence refers to the number positive/total cases.
a From: Schroeder et al. (1964).
b Excluding values > 150.
Table 10. Geographical distribution of tin in kidney, liver, and lung of man (mg/kg ash)a
No. of %
samples present Mean Range Median
kidney
United States of America 161 97 30 < 5-480 20
Switzerland 9 33 5 < 5-17 < 5
Africa 53 19 5 < 5-30 < 5
Middle East 43 42 8 < 5-55 < 5
Far East 57 40 11 < 5-110 < 5
liver
United States of America 163 96 35 < 5-300 24
Switzerland 9 78 6 < 5-20 Tc
Africa 49 25 4 < 5-20 < 5
Middle East 44 55 11 < 5-140 Tc
Far East 54 41 10 < 5-74 < 5
lung
United States of America 159 98 69 < 5-920 39
Switzerland 7 100 37 23-62 28
Africa 50 60 10 < 5-40 Tc
Middle East 45 91 33 < 5-200 17
Far East 57 93 65 < 5-1200 29
Note: Tc = trace, or barely detectable.
Mean and median values were obtained by assigning a value of one-half
the least detectable amount to those cases where tin was not detected.
a From: Schroeder et al. (1964).
Using a spark source mass spectrometric method with a detection
limit of 0.004 mg/kg wet weight, Hamilton et al. (1972/1973) reported
a mean concentration of 0.06 mg/kg (± 0.01) in 10 whole brain samples
from accidentally killed, presumably healthy adults. The concentration
found in the frontal lobe was 0.03 (± 0.07) and that in the basal
ganglia was 0.04 (± 0.02) mg/kg, measured in 2 samples. Tin was not
detected in human brain by Kehoe et al. (1940) and was found in only a
small percentage of subjects by Tipton (1960) and Schroeder et al.
(1964).
6.1.3 Excretion
The major route of excretion of absorbed inorganic tin is the
kidney although a small fraction is excreted into the bile (Hiles,
1974; Moskalev, 1964). The excretion of tin in the urine was studied
by Perry & Perry (1959) in 24 human adults. A mean of 16.6 µg/litre of
urine or 23.4 µg/day was reported. Kehoe et al. (1940) reported a mean
concentration of 18 µg/litre of urine in subjects in the USA. There
remains some doubt, however, as regards the accuracy of the analytical
technique used since, in the same paper, the concentration in the
urine of French males was reported to be zero.
In an experiment on rats, Hiles (1974) reported that after a
single oral dose of tin(II) or tin(IV)citrate or fluoride at 20 mg/kg
body weight (as the metal), about 50% of the absorbed tin was excreted
within 48 h. Following intravenous administration of 113Sn (2 mg of
tin per kg body weight), 12% of the 113Sn(II) and only 4% of the
113Sn(IV) appeared in the faeces of rats indicating that the biliary
route is probably more important in the elimination of tin(II) than of
tin(IV) compounds. Most of the biliary excretion (94%) took place
within 24 h.
6.1.4 Biological half-time
A half-time of 3-4 months was reported for 113Sn in the skeleton
of rats after intramuscular administration (Hamilton, 1948). However,
Hiles (1974) found a half-time of only 34-40 days for both tin(II) and
tin(IV) in the bone of rats following oral administration of tin(II)
fluoride or tin(IV) fluoride for 28 days. The half-time of tin(II) for
liver and kidney was reported to be 10-20 days. Furchner & Drake
(1976) using tin(II) chloride administered intraperitoneally and
intravenously in the mouse, rat, monkey, and dog, described the
elimination from the body as a 4 component-process that was similar in
all the species studied. The half-time for the longest component was
over 3 months.
6.2 Organotin Compounds
In this document, consideration will be limited to compounds of
the general type RSnX3, R3SnX2, R4SnX, and R4Sn where R is a
simple or complex action. Generally, little information is available
on the absorption, distribution, and excretion of these substances.
6.2.1 Absorption
Absorption from the intestinal tract of tin compounds with short
alkyl chains varies depending on the compound. There are also
considerable differences in absorption between species (Barnes &
Stoner, 1959).
Ethyltin trichloride administered orally was poorly absorbed by
rats, 92% of an oral dose of 25 mg/kg being eliminated in the faeces
within 2 days. It was not excreted in the bile (Bridges et al., 1967).
Triphenyltin acetate was almost completely eliminated in faeces within
a few days, when administered orally to sheep (Herok & Götte, 1964)
and cows (Bruggemann et al., 1964a). However, it was well absorbed,
when given orally to guineapigs and mice (Stoner, 1966). Results
obtained with rats were conflicting (Klimmer, 1963, 1964; Stoner,
1966). Tricyclohexyltin hydroxide was poorly absorbed in rats, only
about 2% being excreted in the urine after a single 25 mg/kg oral
dose. The remainder was recovered from the faeces and binary excretion
did not occur (FAO/WHO, 1971).
Trialkyltin compounds were well absorbed on contact with the skin,
since the dermal LD50s of various trimethyltin derivatives in mice
were of the order of 50-100 mg/kg (Hall & Ludwig, 1972) and that of
bis(tributyltin) oxide was below 200 mg/kg in rats and mice (Ascher &
Nissim, 1964; Elsea & Paynter, 1958). When a 20% fat solution of
various triethyltin derivatives was applied to the skin of rats and
mice for 10 min, all the animals died within 20-30 min (Ignatjeva et
al., 1968). In contrast, triphenyltin acetate did not penetrate
unbroken skin readily (Stoner, 1966).
6.2.2 Distribution
Following intravenous administration to mice and rats, the highest
concentrations of dibutyl- and diethyltin were found in liver and
kidney tissue; unchanged compounds were excreted in the bile (Barnes &
Magee, 1958). Rats were fed with 11 mg of triethyltin hydroxide over a
period of 89 days. At the end of this time, only 0.7 mg of the
compound could be recovered; 40% of this was in the blood, 28% in the
liver, and 29% in the skeletal muscle. Smaller amounts of triethyltin
were found in the kidney, brain, heart, and spleen (Cromer, 1957).
Species variation in the distribution of triethyltin has been
reported. When triethyltin (26 µg) was added to rat blood in vitro,
most of it (23 pg) was recovered from the erythrocytes and none was
found in the plasma, whereas in rabbit blood, triethyltin was more
equally distributed between erythrocytes (9 µg) and plasma (17 µg)
(Cromer, 1957). This could explain why triethyltin persists in the
blood of treated rats, whereas it quickly disappears from the blood of
treated rabbits (Barnes & Stoner, 1959). Triethyltin was found in the
brain of rats only 1-2 h after intraperitoneal injection of
triethyltin hydroxide (Cremer, 1957).
The tissue distribution of triethyltin following intravenous
administration of tetraethyltin resembled that following
administration of triethyltin (Cremer, 1957, 1958). Thus, a large
quantity of triethyltin was found in the liver, with smaller
quantities in the kidney, brain, and whole blood in a rabbit, 2 h
after administration.
After oral or intraperitoneal administration of 113Sn-labelled
triphenyltin to guineapigs, about 80% of the administered activity was
excreted in 10 days. The concentration of radioactive tin found in the
brain of rats and guineapigs after oral and intraperitoneal
administrations decreased with a half-life of several days. The form
of tin in the brain was not unequivocally identified, but it was not
in the form of free stannic ions nor was it necessarily triphenyltin
(Heath, 1967). Herok & Götte (1964) gave 3 sheep 10 mg of triphenyltin
acetate orally, every day for 20 days. The compound was labelled with
113Sn. The animals were killed at intervals ranging from 8 to 198 days
after the last dose. The highest concentration of 113Sn was found in
the liver but the brain also contained measurable amounts. In rats,
any absorbed triphenyltin chloride was rapidly distributed through the
tissues of the body including the brain. Tin was eliminated relatively
slowly and could be detected in the brain 38 days after a single dose
(Heath, 1963).
Only trace quantities of tricyclohexyltin hydroxide were found in
the tissues of rats and dogs fed up to 12 mg/kg bodyweight per day, in
the diet, for periods ranging from 45 days to 2 years (FAO/ WHO,
1971).
6.2.3 Excretion
When ethyltin trichloride was administered intraperitoneally to
rats, it was excreted almost exclusively in the urine; binary
excretion was negligible. When diethyltin was administered
intraperitoneally it was eliminated as diethyl- and ethyltin in both
faeces and urine. Diethyltin was excreted in the bile (Bridges et al.,
1967). Rats that had initially received a diet containing triethyltin
and had retained approximately 0.7 mg triethyltin in their tissues
were then given a normal diet; 12 days later, no triethyltin could be
detected in the tissues (Cremer, 1957). The route of excretion was not
known.
The elimination of triphenyltin from the body is slow (Stoner,
1966). Its persistence in guineapigs is suggested by the similarity
between the amounts consumed by those dying on diets containing 25 and
50 mg/kg and the acute oral LD50. In studies on guineapigs using
113Sn-labelled triphenyltin, the concentration of triphenyltin that
had entered the brain after a single oral dose did not decrease during
the first 10 days. In rats, triphenyltin disappeared more rapidly,
having a half-time of about 3 days in the brain (Heath, 1963, 1965).
Triphenyltin acetate labelled with 113Sn was slowly excreted in the
urine of lactating sheep given oral doses of the compound at the rate
of 10 mg/day for 20 days. The milk confined small amounts of tin
(about 1 µg/litre) in both organic and inorganic forms (Herok & Götte,
1964).
The biological half-time of tricyclohexyltin hydroxide in rats was
5-40 days, when the compound had been included in the diet over a
prolonged period. The brain was one of the tissues from which the
compound was removed most slowly (FAO/WHO, 1971).
6.2.4 Biotransformation
In some early studies on the metabolic degradation of organotin
compounds, it was suggested that destannylation (carbon-tin bond
cleavage) was the major result of this biotransformation. For example,
triethyl tin was detected in the tissues of rabbits and rats given
tetraethyltin intravenously (Cremer, 1957, 1958). The liver was the
organ most active in this conversion. Bridges et al. (1967) reported
that dealkylation of diethyltin occurred in both the gut and tissues
of the rat. Although Herok & Götte (1963) found that after
administration of triphenyltin acetate labelled with 113Sn small
amounts of inorganic tin were present in the milk of sheep, Stoner
(1966) considered that triphenyltin was not readily transformed in the
body. Dicyclohexyltin oxide and trace amounts of cyclohexylstannoic
acid were identified as metabolites in rats and dogs treated with
tricyclohexyltin hydroxide (FAO/WHO, 1971). Blair (1975) concluded
that tricyclohexyltin hydroxide undergoes metabolism in animals by
scission of cyclohexyl groups from the atom.
More recent studies using tributyltin acetate showed primary
biological oxidation reactions in the hydroxylation of carbon-hydrogen
bonds that are alpha, ß, gamma and delta to the tin atom (Fish et al.,
1976). This type of reaction was already assumed by Casida et al.
(1971), although no carbon-hydroxylated metabolites could be
identified when triethyltin derivatives were biologically oxidized
in vitro to monoethyl derivatives.
Fish et al. (1975) treated tributyltin acetate with rat liver
microsomes (a mono-oxygenase enzyme system) in the presence of NADPH,
and obtained alpha and ß hydroxylated metabolites in yields of 24% and
50%, respectively. An in vivo study with [1-14C]-tetrabutyltin
showed the occurrence of similar reactions in mice (Kimmel et al.,
1977). The alpha-hydroxyl metabolite is unstable and undergoes a
cleavage reaction at pH = 7.4 to give 1-butanol and a dibutyltin
derivative. A ß-elimination reaction in acidic media transforms the
ß-hydroxyl derivatives rapidly into 1-butane and a dibutyl derivative
(Fish et al., 1976).
Studies on the in vitro metabolism of tricyclohexyltin compounds
indicated that carbon-hydroxylation of the cyclohexyl group was a
major metabolic reaction (Fish et al., 1976; Kimmel et al., 1977).
When [113Sn] -triphenyltin acetate was subjected to in vitro
biological oxidation, the phenyl group was not biotransformed, but in
in vivo studies, rats were found to metabolize triphenyltin acetate
into diphenyl- and phenyltin derivatives (Kimmel et al., 1977).
7. EFFECTS ON ANIMALS
7.1 Inorganic Tin Compounds
Although tin is present in small amounts in most animal and human
tissues, it is uncertain whether it is an essential element for
mammals. However, recent results indicate that tin is an essential
nutrient for the growth of the rat. Both inorganic and organotin
compounds at concentrations similar to those present in feeds were
found to stimulate growth rate in rats maintained on purified amino
acid diets (Table 11) (Schwarz, 1971; 1974; Schwarz et al., 1970). The
authors concluded that tin, as an essential element, could have a
function at the active site of some metal-dependent enzymes; however,
this has still to be confirmed.
Compared with most organotin derivatives, inorganic tin and its
salts are not highly toxic, mainly because of their poor absorption
and rapid tissue turnover (Barnes & Stoner, 1959; Cheftel, 1967;
Hiles, 1974; National Academy of Sciences, Washington 1973). The
systemic toxicity of some simple tin salts is difficult to assess
because of the irritant properties of their solutions.
7.1.1 Effects on the Skin
The effects of 1% tin(II) chloride and 0.25% tin(II) fluoride
solutions, applied to the abraded skin of rabbits, were examined by
Stone & Willis (1968). Both compounds induced intraepidermal pustules
with complete destruction of the epidermis but the stratum corneum
remained intact. No injury occurred when the solutions were applied to
intact skin.
7.1.2 Respiratory system effects
According to Robertson (1960), intratracheal administration of
50 mg of metallic tin dust to rats was well tolerated and no fibrosis
was produced within one year of exposure.
Exposure of guineapigs by inhalation, to tin(IV) chloride (3 mg/
litre for 10 min, daily, for "several months") produced only transient
irritation of the nose and eyes (Pedley, 1927).
Table 11. The effect of tin(IV) sulfate on the growth of rats in a trace-element-controlled
environmenta
Dose level No. of Average daily Increase
Compound (µg of tin/kg) animals weight gain (%) p-value
control -- 5b 1.10±0.05c -- --
tin (IV) sulfate 500 8 1.37±0.10 2 .02
tin (IV) sulfate 1000 8 1.68±0.10 53 .001
tin (IV) sulfate 2000 8 1.75±0.10 59 .001
a From: Schwarz et al. (1970).
b 2 control rats died during the 26-29 days of the experiment.
c mean ± standard error.
7.1.3 Effects on the gastrointestinal system
Soluble tin salts are gastric irritants. This explains the signs
of acute poisoning occasionally observed in man and in experimental
animals following consumption of food containing high concentrations
of tin (de Groot et al, 1973). However, the concentrations required to
elicit an acute gastrointestinal reaction have not been determined
reliably. Beney et al. (1971) reported signs of illness in 3/10 cats
after ingestion of 5 ml/kg body weight of fruit juice containing a tin
concentration of 1370 mg/litre, and in 1/11 cats receiving the same
dose of fruit juice containing tin at a concentration of 540 mg/litre.
However, no adverse effects were recorded in cats receiving a similar
amount of juice containing a tin concentration of about 500 mg/litre
or in dogs given juice with a tin concentration of 1400 mg/litre.
7.1.4 Effects on the liver
A 3-fold increase in haem oxygenase (EC 1.14.99.3)a activity in
the liver of rats was observed 16 h after a single subcutaneous
injection of tin(II) chloride dihydrate (SnCl22H2O) at doses ranging
from 5.6 to 56.4 mg/kg body weight. Cytochrome P-450 mediated drug
metabolism and the content of cytochrome P-450 were reduced by one
third. These effects on the liver increased by about 15-20%, when the
compound was administered intraperitoneally (Kappas & Maines, 1976).
Tin chloride, oxalate, or sulfate added to the diet at a
concentration of 10 g/kg for 4 and 13 weeks resulted in homogenous
liver cell cytoplasm and hyperplasia of the bile duct in rats. The
liver changes were more distinct after 13 weeks administration.
Similar, although milder, hepatic alterations were seen after
administration of tin chloride, oxalate, or orthophosphate at a
concentration of 3 g/kg of dietb (de Groot et al., 1973).
Increased incidence of fatty degeneration in the liver was noted
by Schroeder et al. (1968) in female but not in male rats, when the
animals were given tin in the form of tin(II) chloride at a dose of
5 mg/litre in drinking water, from weaning until their natural death.
a The numbers within parentheses following the names of enzymes
are those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
b Tables for the approximate conversion of concentrations in diet
to mg/kg body weight per day are given in Nelson (1954).
7.1.5 Effects on the kidney
Renal damage was produced in rats by single intravenous and
intraperitoneal doses of sodium pentafluorostannite (NaSn2F5) and
tin(II) chloride dihydrate (SnCl22H2O) (Conine et al., 1973, 1975;
Yum et al, 1976). A single intraperitoneal injection of sodium
pentafluorostannite at 35 mg/kg body weight or of tin(II) chloride
dihydrate at 44.4 mg/kg produced extensive necrosis of epithelial
cells mainly involving proximal tubules. The extent of the necrosis
was thought to be related to the tin moiety of the compound because
damage of the same magnitude could not be produced by administration
of sodium fluoride (Yum et al., 1976).
Studies in which rats received single subcutanous injections of
tin(II) chloride dihydrate in doses ranging from 5.6-56.4 mg/kg body
weight revealed a 20 to 30-fold increase in the haem oxidation
activity in the kidney, 16 h after administration. This effect was
already noticeable at the lowest dose (5.6 mg/kg) and was found to be
dose-related (Kappas & Maines, 1976).
Conine et al. (1976) administered daily oral doses of sodium
pentafluorostannite for 15 or 30 days to rats at rates of 20, 100, and
175 mg/kg body weight. The highest dose caused degenerative changes in
the proximal epithelium of the kidneys that were similar to those
observed in chronic fluoride poisoning (Lindemann et al., 1959), and
affected about 15-20% of the 30 rats that were killed. Most of the 8
animals in the 175 mg/kg group that died spontaneously during the
experiment displayed necrosis of the proximal tubular epithelium
similar to that previously reported to be caused by tin (Yum et al.,
1976).
Exposure of rats to tin(II) chloride at a concentration of 5 mg of
tin/litre of drinking water, for life, produced vacuolar changes in
the renal tubules of animals of both sexes (Schroeder et al., 1968).
7.1.6 Effects on the blood-forming organs
When tin(II) (as chloride, orthophosphate, sulfate, oxalate, or
tartrate) was given in the diet to Wistar rats of both sexes at
concentrations of 3 and 10 g/kg for 4 weeks, food intake was reduced,
growth was retarded and slight anaemia developed. The signs of anaemia
included a reduction in haemoglobin levels of about 10% and
corresponding reductions in haematocrit values, erythrocyte counts,
and serum iron concentration (de Groot et al., 1973). Dietary
supplements of iron had a markedly protective effect (de Groot, 1973;
de Groot et al., 1973). The authors suggested that tin compounds might
inhibit haemopoiesis, possibly by interfering with the intestinal
absorption of iron. An alternative mechanism by which tin salts could
cause mild gastrointestinal bleeding was not investigated.
A dose-related decrease in haemoglobin concentrations was noted in
rats after daily oral treatment, for 15 days, with sodium
pentafluorostannite at 100 and 175 mg/kg body weight. Administration
of 20 mg/kg per day did not affect the haemoglobin level (Conine et
al., 1976).
7.1.7 Central nervous system effects
High doses of inorganic tin compounds seem to affect the central
nervous system producing such effects as ataxia, muscular weakness,
and the depression of the central nervous system. These signs were
observed by Conine et al., (1975) after oral administration to rats of
sodium pentafluorostannite and tin(II) chloride dihydrate in single
doses of the order of the median lethal dose (LD50). According to de
Groot et al. (1973), feeding rats with tin(II) chloride at a
concentration of 10 g/kg diet for 13 weeks, resulted in a spongy state
of the white matter of the brain. Ataxia, hind-leg paralysis, and
death were also observed in rats by Mamontova (1940) following daily
subcutaneous injections of 3 mg of tin(II) citrate for 5-6 months.
7.1.8 Effects on the reproductive system and the fetus
There is one report indicating that tin(II) chloride might have an
effect on the reproductive system. De Groot et al. (1973) noted
testicular degeneration in rats after prolonged feeding with this
compound (10 g/kg of food, for 13 weeks).
Inorganic tin compounds have not been shown to be fetotoxic.
Theuer et al. (1971) gave groups of pregnant rats sodium
pentafluorostannite, sodium pentachlorostannite, and tin(II) fluoride
corresponding to tin levels in the diet of 125, 250, and 500 mg/kg.
The rats were killed on day 20 of gestation. No effects were seen in
the fetuses; tin concentrations in the fetuses were about 1 mg/kg
compared with approximately 0.65 mg/kg in control fetuses, indicating
a rather low transplacental transfer.
7.1.9 Carcinogenicity and mutagenicity
Few reports are available concerning the carcinogenicity of
inorganic tin compounds. Walters & Roe (1965) administered either
sodium chlorostannate at 1 or 5 g/litre of drinking water or tin(II)
oleate at 5 g/kg of diet to mice, for up to one year; the survivors
were then killed. The incidence of lymphomas, hepatomas, or pulmonary
adenomas did not increase with any of the regimens.
Roe et al. (1965) reported 3 malignant tumours in 30 August rats
that survived for 1 year or more on a diet containing sodium
chlorostannate at a concentration of 20 g/kg, whereas a control group
of 33 rats did not exhibit any case of malignant tumours. One tumour
was an adenocarcinoma of mammary origin, another a pleomorphic sarcoma
in the uterus, and the third, an adenoma-carcinoma in the jaw region.
The difference was not statistically significant. No tumours were seen
in another group of 27 rats surviving on a diet containing tin(II)
2-ethylhexoate at a concentration of 5-10 g/kg.
Administration of tin(II) chloride to rats and mice at 5 mg/litre
in drinking water throughout their life-time did not produce any
increase in the incidence of tumours compared with a control group
consisting of an equal number of animals (Kanisawa & Schroeder, 1969).
Studies concerning the mutagenicity of inorganic tin compounds
were not available to the Task Group,
7.1.10 Other effects
Growth retardation in rats has been reported after the
administration of high doses of various tin compounds. Dietary levels
of tin as tin(II) oxalate, orthophosphate, chloride, sulfate, and
tartrate at 3 and 10 g/kg for 4 weeks caused inhibition of growth in
rats and oedema and atrophy of the pancreas (de Groot et al., 1973).
Daily administration, by garage, of sodium pentafluorostannite at 100
and 175 mg/kg body weight, for 30 days, resulted in a dose-related
retardation of growth in rats (Conine et al., 1976). Administration of
tin(II) chloride at a concentration of 5 mg of tin per litre of
drinking water reduced the life span of female rats, whereas the
longevity of male rats was unaffected (Schroeder et al., 1968).
7.1.11 Effective doses and dose rates
7.1.11.1 Lethal doses
The median lethal doses (LD50) for 2 inorganic tin compounds are
given in Table 12. A marked difference between the oral and parenteral
LD50 values can be explained by the low absorption of tin compounds.
Daily intravenous administration of 3.3-4.2 mg of sodium tin
citrate per kg body weight provoked death in rabbits in 7-8 days,
whereas at a dose of 2.5 mg/kg, death occurred only after 15 or more
days (Mamontova, 1940).
In studies on 6 rats, subcutaneous injection of 3 mg of tin
citrate/animal, per day, killed all the animals in 5-6 months. Similar
administration of the same compound in doses of 2 mg/kg body weight
produced vomiting, diarrhoea, weight loss, ataxia, hind-leg paralysis,
and death within 5 months (Mamontova, 1940).
Table 12. Median lethal doses (LD50) of tin(II) chloride dihydrate (SnCl22H2O) and sodium
pentafluorostannite (NaSn2F5)a
Sodium pentafluorostannite
Tin(II) chloride dihydrate
Route male mice male rats female rats male rats (LD50, mg/kg)
(LD50, mg/kg) (LD50, mg/kg) (LD50, mg/kg)
intravenous 18.9 12.9 12.9 29.3
intraperitoneal 80.9 75.4 65.0 258.4
oral (fasted) --b 223.1 218.7 2274.6
(fed) 592.9 573.1 --b 3190.1
a From: Conine et al. (1975).
b no data.
7.1.11.2 Minimum effective and no-observed-effect doses
Some information on effective doses has been given in the sections
describing the effects. However, the following information may also be
of interest.
De Groot et al. (1973) reported a normal growth rate in Wistar
rats that had received tin(IV) oxide, tin(II) sulfide, or tin(II)
oleate at dietary levels up to 10 g/kg for 4 weeks; haematological
data were also normal except for an increase in the haematocrit in
male rats fed with the highest concentration of tin(II) sulfide. The
weight and the gross and macroscopic appearance of the liver, kidney,
heart, and spleen were also normal.
When a similar experiment was conducted with tin(II) chloride for
13 weeks, the no-observed-effect concentration in food appeared to be
1 g/kg, if the diet were supplemented with iron (de Groot et al.,
1973). This is equivalent to a dose rate of about 20-30 mg tin/kg body
weight per day, for 90 days.
No effect on growth was observed by Conine et al. (1976), when
sodium pentafluorostannite was administered orally to rats at a dose
rate of 20 mg/kg body weight per day for 30 days.
Oral administration of sodium tin citrate in dose rates ranging
from 100-150 mg/kg body weight per day for 1 year did not produce any
noticeable signs of poisoning in rats (Mamontova, 1940). Similarly
rats fed a diet containing either sodium chlorostannate at 20 g/kg or
tin(II) 2-ethyl hexoate at 5-10 g/kg for one year did not show any
pathological changes in the gastrointestinal tract, kidneys, or liver
(Roe et al., 1965).
Mice that received tin in the form of sodium chlorostannate at a
concentration of 1 or 5 g/litre in drinking water or tin in the form
of tin(II) oleate in the diet at a concentration of 5 g/kg during
their lifetime did not show any adverse effects (Waiters & Roe, 1965).
Mice and rats (Schroeder & Balassa, 1961; Schroeder et al., 1968)
given tin(II) chloride in drinking water at a concentration of 5 mg of
tin/litre grew normally throughout their life. The life span of mice
of both sexes and of male rats was not affected but that of female
rats was shorter and there was an increased incidence of fatty
degeneration of the liver (section 7.1.4). Vacuolar changes in the
renal tubules were apparent in rats of both sexes (section 7.1.5).
7.2 Organotin Compounds
Distinction should be made between the effects of di-, tri-, and
tetrasubstituted organotin compounds. The principal toxicological
difference is that some trisubstituted compounds have a specific
effect on the central nervous system producing cerebral oedema (Barnes
& Stoner, 1958; Torack et al., 1969), whereas disubstituted compounds
do not produce this effect but are potent irritants that can induce an
inflammatory reaction in the bile duct (Barnes & Magee, 1958).
Toxicologically, the tetrasubstituted compounds resemble
trisubstituted compounds, which are, generally, more toxic than the
mono- and disubstituted derivatives.
7.2.1 Effects on the skin and eyes
Dermal application of dibutyltin dichloride at 10 mg/kg body
weight per day for a period of 12 days, caused severe local damage and
also bile duct injury in rats and mice. Guineapigs were more
resistant, showing little reaction to daily applications of 120 mg/kg
on 5 successive days (Barnes & Stoner, 1958).
An aqueous solution of bis(tributyltin) oxide applied to the
shaved skin (30 x 60 mm) of rats at concentrations of 0.36-0.95 mg/kg
body weight produced slight local irritation lasting 2-3 weeks
(Pelikan & Cerny, 1968b). At higher doses (1.40-185 mg/kg), marked
inflammation developed into skin necrosis. Severe effects on the eyes
were also observed with bis(tributyltin) oxide (Pelikan & Cerny,
1969).
Triphenyltin hydroxide was reported not to irritate rabbit skin or
sensitize the skin of guineapigs (Marks et al., 1969). However, it was
found to be extremely irritating to the eyes, even after brief
exposure, and could cause corneal opacity (Marks et al., 1969).
A solution of triphenyltin acetate in oil at a dose of 150 mg/kg
body weight caused a skin reaction in rats (Klimmer, 1964). Doses of
tricyclohexyltin hydroxide at 1.2-60 mg/kg body weight per day,
applied to the skin of rabbits over a 3-week period, produced distinct
reactions locally but did not result in any systemic ill effects
(FAO/WHO, 1971, p. 527)a
7.2.2 Respiratory system effects
Pulmonary congestion and oedema were observed in rats after single
intravenous administrations of solutions of diethyl-, di-propyl-,
diisopropyl-, and dipentyltin dichloride (20 mg/kg body weight), in
0.05 ml of polyoxyethylene sorbitin monoleate, whereas in the case of
dibutyltin dichloride, 10 mg/kg was enough to cause this effect
(Barnes & Stoner, 1958). Petechial and ecchymotic haemorrhages in the
trachea and larynx were observed in sheep after intrarumenal
administration of tricyclohexyltin hydroxide at 500 mg/kg body weight.
Congestion was present in the dorsal portion of all pulmonary lobes at
a dose as low as 150 mg/kg (Johnson et al., 1975).
7.2.3 Effects on the gastrointestinal system
A single dose of butyltin trichloride, butyltin- S,S',S"-tris
(2-ethylhexylmercaptoacetate), butylstannoic acid, and butyl
thiostannoic acid at 4000 mg/kg body weight, administered by stomach
tube to mice, produced various degrees of submucosal, subserosal, and
intra-luminar gastrointestinal hemorrhage within 24 h (Pelikan &
Cerny, 1970b). Similar administration of dioctyltin- S,S'-bis
(2-ethylhexyl-mercaptoacetate) produced dilatation of the stomach,
which was filled with gas. The stomach walls appeared ischaemic
whereas the intestinal walls were hyperaemic, and traces of blood were
found in the contents of the small stomach. Similar, but more
pronounced findings were recorded after administration of dioctyltin-
a Unpublished reports on triphenyltin compounds and tricyclohexyltin
hydroxide were abstracted in 1970 Evaluations of Some Pesticide
Residues in Food (FAO/WHO, 1971). The proprietary data were
submitted to the WHO by the manufacturers. Page numbers given in the
text refer to the FAO/WHO publication, which also contains
information with reference to authors and manufacturers.
bis-(butylmercaptoacetate), although no traces of blood were seen in
the stomach. Treatment with dioctyltin bis(2-ethylhexyl-
mercaptoacetate) resulted in large amounts of liquid in the stomach
contents, and slightly hyperaemic stomach walls; other findings were
similar to those produced by the other 2 dioctyltin compounds,
dioctyltin bis(dodecylmercaptide) and octyltin tris(2-ethylhexyl-
mercaptoacetate) (Pelikan & Cerny, 1970a). Ingestion of dibutyltin
dichloride at a dose of 50 mg/kg body weight per day for one week,
caused a temporary dilatation of the stomach in rats due to
accumulation of fluid, and diarrhoea (Barnes & Stoner, 1958).
Intrarumenal administration of tricyclohexyltin hydroxide at 150 mg/kg
body weight resulted in fluid diarrhoea in sheep which persisted up to
death. Necropsy findings included mild enteritis and colitis
characterized by severe hyperaemia and oedema (Johnson et al., 1975).
Gastroenteritis occurred in rats receiving an oral dose of
tricyclohexyltin hydroxide of 25 mg/kg body weight per day for 19 days
(FAO/WHO, 1971, pp. 527-528).
7.2.4 Effects on the liver and bile duct
Steatosis of hepatocytes and enlargement of the liver were seen
within 24 h of administration to mice through a stomach tube, of a
single dose of butylstannoic acid, butyltin trichloride, butyltin
tris(2-ethylhexylmercaptoacetate), or butylthiostannoic acid, each at
a dose of 4000 mg/kg body weight (Pelikan & Cerny, 1970b). In rats, a
single oral dose of dibutyl,tin dichloride at 50 mg/kg body weight
produced congestion and inflammation especially in the lower part of
the bile duct (Barnes & Magee, 1958; Gaunt et al., 1968). When this
dose of dibutyltin dichloride was given on 3 successive days, the
lesion was more severe involving also the proximal part of the bile
duct and the portal blood vessels. Necrotic areas were seen in the
liver. In some cases, bile escaped from the injured duct into pancreas
and peritoneum. The authors considered that death occurring 5 days
after the 3 successive doses was the result of a general toxic effect
of this compound, whereas death occurring later was secondary to bile
duct and liver damage. Changes in the pancreas were inflammatory and
confined to areas surrounding the bile duct. In surviving rats,
examined 6-12 months after receiving 3 daily doses of 50 mg/kg, the
bile duct was shorter and thicker than normal with fibrosis in the
wall. A similar reaction in the bile duct was seen after a single
intravenous administration of 5 mg/kg body weight, and after a dermal
application of 10 mg/kg body weight. The effects on mice of oral doses
of dibutyltin dichloride at 20-50 mg/kg body weight were similar to
those seen in rats, although liver damage appeared to be more
widespread. Repeated doses of this compound at 20-50 mg/kg body weight
killed rabbits, but did not cause bile duct or liver injury;
guineapigs, however, tolerated such doses without any signs of adverse
effects (Barnes & Magee, 1958). It has been suggested that bile duct
injury occurs only in species in which the bile duct and the
pancreatic duct have a common course (Kimbrough, 1976).
Daily doses of dibutyltin dichloride at 0.1 and 1.0 mg/kg body
weight, for 6 months, caused intoxication in rabbits with dystrophic
changes in the liver (Mazaev & Korolev, 1969). Dioctyltim- S,S'-bis
(2-ethylhexylmercaptoacetate), diootyltin- S,S'-bis
(butylmercaptoacetate), and diootyltin bis(dodecyl-mercaptide) at an
oral dose of 4000 mg/kg body weight produced steatosis of the
hepatocytes in mice (Pelikan & Cerny, 1970a). Nikonorow et al. (1973)
reported a significant increase in the mean liver weight of rats after
oral administration of dioctyltin- S,S'-bis(isoctylmercaptoacetate)
at 20 mg/kg body weight per day, for 3 months.
A single oral dose of tributyltin acetate, benzoate, chloride,
laurate, or oleate at 500 mg/kg body weight produced fatty
infiltration of the liver in mice (Pelikan & Cerny, 1968a) and
triphenyltin acetate at a dietary level of 10 mg/kg produced fatty
degeneration of the liver in guineapigs, when administered over a
2-year period (FAO/WHO, 1971, p. 341). Cholangitis was reported in
rats after administration of tricyclohexyltin hydroxide at a dietary
level of 400 mg/kg for 90 days (Shirasu, 1970). Similarly, both intra-
and extrahepatic cholangitis was noted in rats that had received
tri-cyclohexyltin hydroxide at 25 mg/kg body weight per day, for 19
days (FAO/WHO, 1971, pp. 527-528).
7.2.5 Effects on the kidney
Some degree of fatty degeneration of the renal cortical tubular
epithelium was seen on histological examination of mice that had
received a single oral dose (4000 mg/kg body weight) of butyltin
tin- S,S',S"-tris(2-ethylhexylmercalytoacetate), butylstannoic acid,
or butylthiostannoic acid, but not in animals given butyltin
trichloride. The structure of the renal tissue was unchanged.
Macroscopically, all compounds produced slight hyperaemia in the
kidneys (Pelikan & Cerny, 1970b).
Dioctyltin- S,S'-bis(2-ethylhexylmercaptoacetate) and
dioctyltin- S,S'-bis(butylmercaptoacetate) administered at the same
rate and by the same route produced a similar slight fatty
degeneration of the renal cortical tubular epithelium in mice (Pelikan
& Cerny, 1970a). Feeding rats with an organotin stabilizer,
dioctyltin- S,S'-bis(isooctyl mercaptoacetate) at a dietary level of
200 mg/kg for 12 months produced an increase in kidney weight in
female rats only (Nikonorow et al., 1973). Mazaev & Korolev (1969)
also reported dystrophic changes in the kidneys in rats treated with
dibutyltin dichloride at 0.1 and 1.0 mg/kg body weight.
Haemorrhages were reported in the kidneys of mice after single
administrations (gavage) of tributyltin benzoate, chloride, laurate,
and oleate at 500 mg/kg body weight. Hyperaemia of the kidney was seen
in all groups, whereas tubular cells containing lipids were observed
only in mice that had received laurate or oleate. Renal changes were
not reported in mice that were similarly dosed with tributyltin
acetate (Pelikan & Cerny, 1968a). Congestion of the glomeruli and mild
toxic nephrosis were observed in rats receiving a dietary level of
tricyclohexyltin hydroxide of 400 mg/kg for 3 months (Shirasu, 1970).
Similarly, toxic nephrosis was reported in rats after administration
of tricyclohexyltin hydroxide at a daily dose of 25 mg/kg body weight
for 19 days (FAO/WHO, 1971, pp. 527-528).
7.2.6 Effects on the lymphatic tissues and immunological effects
Small necrotic areas were observed in the germinal centres of the
splenic follicles of mice after a single administration, through a
stomach tube, of 4000 mg/kg body weight of butyltin- S,S',S"-tris-
(2-ethylhexylmercaptoacetate), butylthiostannoic acid, and
butyl-stannoic acid, (Pelikan & Cerny, 1970b); dioctyltin- S,S'-bis
(2-ethyl-hexylmercaptoacetate), and dioctyltin- S,S'-bis(butyl-
mercaptoacetate) (Pelikan & Cerny, 1970a).
Seinen & Willems (1976) reported that the main action of
di-octyltin dichloride, given to rats at dietary levels of 50 and
150 mg/ kg, was on the thymus, the weight of which was significantly
reduced during the experiment. In subsequent experiments, feeding rats
with dioctyltin dichloride or dibutyltin dichloride at dietary levels
of 50, or 150 mg/kg for 6 weeks, resulted in a dose-dependent
reduction in the weight of the thymus Land thymus-dependent peripheral
lymphoid organs (Seinen et al., 1977a). The delayed type
hypersensitivity was decreased and the allograft rejection was delayed
when the same compounds were given in the same way to rats.
Furthermore, it was reported that both these compounds decreased the
survival rate of rat and human thymocytes in in vitro studies
(Seinen et al., 1977b). The effects of diethyltin dichloride and
dipropyltin dichloride on rat lymphoid organs were similar but less
pronounced. However, other dialkyltin compounds such as dimethyltin
dichloride, didodecyltin dibromide, and dioctadecyltin dibromide, as
well as octyltin trichloride, trioctyltin chloride, and tetraoctyltin
did not cause atrophy of lymphoid tissues (Seinen et al., 1977a). The
rat proved more sensitive to the action of di-octyltin dichloride and
dibutyltin dichloride on lymphoid organs than the mouse and the
guineapig in which no atrophy of the lymphoid organs was produced.
(Seinen et al., 1977a).
Studies on triphenyltin acetate have been reviewed by the FAO/ WHO
(1971, pp. 327-366). In guineapigs, a dietary level of 15 mg/kg for 77
days produced a decrease in plasma cells in the spleen and in the
mesenteric, cervical, axillary, and popliteal lymph nodes. After
feeding the same dose to female guineapigs for 104 days, a reduction
in immune response to tetanus toxoid stimulation was observed. In
another experiment, a decrease in the number of lymphocytes and
leukocytes accompanied by histological changes in the lymphatic
tissues, such as atrophy of the white pulp of the spleen, was seen in
guineapigs at dietary levels of 5 mg/kg in females and 10 mg/kg in
males administered, for 12 weeks. A decrease in the number of
leukocytes was also reported in dogs after 8 weeks on a dietary level
of triphenyltin hydroxide of 25 mg/kg and 8 weeks on 50 mg/kg.
7.2.7 Haematological effects
Decreases in the numbers of erythrocytes and reticulocytes were
reported in rats after daily administration of an oral dose of
dibutyltin sulfide at 0.1 mg/kg body weight for 6 months (Mazaev &
Slepning, 1973). A mild anaemia was noted in rats that had received
dibutyltin dichloride for 3 months at a dietary level of 80 mg/kg, but
a level of 40 mg/kg did not produce this effect (Gaunt et al., 1968).
In vitro studies of the haemolytic activity of trialkyltin
compounds indicated that the most haemolytic organotin compounds were
the alkyltin derivatives with alkyl groups of 3-6 carbon atoms
(Byington et al, 1974). Decreases in the percentage haemoglobin and in
the number of erythrocytes were reported in guinea-pigs treated with
triphenyltin acetate at a dietary level of 10 mg/kg for 4 months
(FAO/WHO, 1971, p. 337). A reduction in haemoglobin and leukocytes was
also seen in rats after 12 weeks on a dietary level of triphenyltin
hydroxide of 50 mg/kg (FAO/WHO, 1971, pp. 337-333).
7.2.8 Central nervous system effects
Intoxication in animals by lower trialkyltin compounds is
manifested as generalized weakness progressing to paralysis, sometimes
accompanied by generalized tremor. In rabbits, typical reactions to a
lethal dose (5 mg/kg body weight) of triethyltin sulfate administered
intravenously are prostration, flaccid paralysis, and encephalopathy
(Stoner et al, 1955). These responses resemble those observed in human
subjects, accidentally poisoned with triethyltin compounds. Daily
intraperitoneal injections of triethyltin sulfate at 5 mg/kg body
weight in saline solution resulted in death in rats within 3 days.
Diffuse haemorrhagic encephalopathy was found in the brain (Suzuki,
1971). Rats maintained on a dietary level of triethyltin hydroxide of
20 mg/kg displayed weakness in the hind legs after one week. The
weakness reached a maximum at 3-4 weeks, when about half of the rats
died, but the signs disappeared in one week, when normal diet was
restored. Some of the rats showed signs of becoming resistant to the
substance. At a dietary level of 40 mg/kg, the recovery process was
unaltered but, at 80 mg/kg, the rats developed generalized muscular
tremors that resembled those seen in acute trimethyltin poisoning
(Stoner et al, 1955). This course of events, including the development
of resistance to triethyltin hydroxide at a dietary level of 20 mg/kg
was corroborated by Magee et al. (1957), who was also able to produce
a specific lesion of the central nervous system (CNS) by feeding rats
with a dietary level of triethyltin hydroxide of 20 mg/kg. The lesion
consisted of an oedema extending throughout the white matter. It was
microscopically visible after 3 days of exposure and progressed to a
maximum at about 2 weeks. After 4 months of normal diet, the changes
in the brain and spinal cord disappeared. Electronmicroscopic studies
of this lesion in rabbits disclosed that the myelin sheaths split to
form clefts and vacuoles in which the fluid responsible for the oedema
accumulated (Aleu et al., 1963). The triethyltin-induced lesion of the
CNS is different from those produced by alkyl derivatives of lead,
antimony, bismuth, and mercury, which cause damage to the nerve cells,
but appears to be similar to that produced in rats by exposure to
hexachlorophene (Kimbrough & Gaines, 1971). When 5 mg of triethyltin
sulfate per litre of drinking water was administered to newborn rats
and their mothers for 4 months, all young rats were asymptomatic,
whereas the mothers showed paralysis of posterior limbs, severe
cerebral oedema, and status spongiosus of the white matter (Suzuki,
1971). Hedges & Zaren (1969) described papilloedema in monkeys
following a single intraperitoneal dose of triethyltin acetate of
0.1 mg/kg, body weight administered as a 5% solution. Oedema of the
white matter of the brain extended through the chiasma and orbital
optic nerve to the retrolaminar area, but the papilloedema was not due
to extension of the intramyelinic tin oedema of the nerves through the
lamina. Papilloedema does not develop in the cat when similarly
treated, in spite of profound swelling of the intracerebral white
matter, chiasma, and the orbital optic nerve as far as the
retrolaminar portion.
Tricyclohexyltin hydroxide appears to be a depressant for the
central nervous system, although it is not as potent as triethyltin
acetate and cerebral oedema does not occur (FAO/WHO, 1971, p. 526).
Johnson et al. (1975) studied the acute toxicity of tricyclohexyltin
hydroxide in sheep by intrarumenal injection of doses ranging from 15
to 750 mg/kg body weight. Central nervous depression was observed at
50 mg/kg body weight.
Because of the enzymatic conversion of tetraalkyltin compounds by
hepatic microsomal enzymes (section 6.2.4), these compounds act in a
similar way to trialkyltin compounds. The toxicity of tetraalkyltin
compounds in mice and clogs was studied by Caujolle and his colleagues
(1954). Tetraethyltin was the most active, tetramethyltin slightly
less toxic, and for the higher members of the series, the toxicity
decreased with increasing molecular weight. The toxic effects of
tetramethyltin differed from those of the other members of the series
and the dominant findings were tremors and hyperexcitability. The
major effects of the other members of the tetraalkyltin series were
muscular weakness and paralysis followed by respiratory failure.
Development of the signs of poisoning were noticeably slow following
administration of tetraalkyltin compounds and death was often delayed.
Intravenous administration of tetraethyltin at 25 mg/kg produced a
slight increase in the respiratory rate and vasodilatation as
immediate effects, but after 1.5-2 h, prostration with muscular
weakness was noted. The later effects resembled those seen after
administration of triethyltin; the mode of death was also similar
(Stoner et al., 1955).
7.2.9. Effects on reproduction and the fetus
Few studies of the effects of disubstituted organotin compounds on
reproduction have been reported. Nikonorow et al. (1973) reported
toxic effects of dioctyltin- S,S'-bis(isooctylmercaptoacetate) on the
embryo and fetus at daily oral doses of 20 and 40 mg/kg body weight,
administered for about 3 months. A distinct increase in the incidence
of fetal deaths was observed. Teratogenic effects were not found.
Investigations on triphenyltin hydroxide have yielded variable
results. In 2 investigations on rats, oral dose rates of 20 mg/kg per
day for less than 4 weeks produced histologically evident
abnormalities in the testes and ovaries. Diminution in the size of the
testes and less advanced maturation of the germinal epithelium was
seen in rats receiving triphenyltin hydroxide at a rate of 5 mg/kg for
90 days, although a 3-generation reproduction study at this dietary
level failed to demonstrate any adverse effect on the reproductive
indices (FAO/WHO, 1971, pp. 332-333). In another experiment (Gaines &
Kimbrough, 1968), dietary levels of up to 200 mg/kg over a 276-day
period caused a reversible reduction in fertility in male rats which
was considered to be due to a pronounced decrease in food intake, that
was later reversed. No gross abnormalities were seen in the offspring
of these males when mated with untreated females. In 3 later
experiments (FAO/WHO, 1971, p. 333) in which weanling animals were fed
the compound in their diets at doses of up to 25 mg/kg, the previously
reported change in testicular development could not be confirmed.
In a 3-generation study in which rats produced 2 litters per
generation, animals continuously received a dietary level of
tricyclohexyltin hydroxide of up to 100 mg/kg (corresponding to 4-6 mg
test compound/kg body weight per day for an adult rat). No evidence of
any adverse effects on reproduction could be found and examination of
the fetuses did not reveal any indication of teratogenic effects
(FAO/WHO, 1971, p. 524). No evidence of teratogenic effects from
tricyclohexyltin hydroxide was obtained in a study in which pregnant
rabbits received up to 3 mg/kg body weight per day from the 8th to
16th day of gestation (FAO/WHO, 1971, p. 524). In a study in which
diets containing tricyclohexyltin hydroxide were fed to Japanese
quail, no evidence of ill effects on egg fertility or hatchability was
seen at a dietary level of 1 mg/kg.
Effects observed at a dietary level of 10 mg/kg were of doubtful
significance but a dietary level of 100 mg/kg had definite effects on
fertility, egg production, hatchability, and embryonic mortality
(FAO/WHO, 1971, pp. 523-524).
7.2.10 Carcinogenicity
In an 18-month study, mice were given an oral dose (stomach tube)
of 0.46 mg/kg body weight per day of triphenyltin acetate between the
ages of 7 and 28 days and thereafter a dietary level of 1206 mg/kg.
There was no statistically significant increase in tumours compared
with a control group (Innes et al., 1969). In a 2-year study on rats
receiving tricyclohexyltin hydroxide at concentrations up to 12 mg/kg
body weight (section 7.2.3), the pattern of tumour incidence
throughout both the control and test groups appeared to be random and
did not suggest a dose-response relationship (FAO/WHO, 1971, p. 527).
Reports of experiments specifically designed to investigate the
carcinogenicity of other compounds were net available to the Group.
7.2.11 Effects on chromosomes
Mazaev & Slepnina (1973) reported an increased percentage of
chromosomal aberrations in bone marrow cells and also an increased
mitotic index in cells of the small intestine mucosa of rats that had
received dibutyltin sulfide at 0.1 mg/kg body weight per day
administered by intubation over a period of 6 months. Doses of less
than 0.1 mg/kg body weight per day did not cause poisoning and did not
have any effect on the chromosomes or somatic cells.
7.2.12 Other effects
A reduction in weight gain, mainly related to reduced food intake,
has been recorded in various species given organotin compounds. In
rats, a dietary level of dibutyltin dichloride of 80 mg/kg
administered for 90 days caused growth retardation and decreased food
intake (Gaunt et al., 1968). Triphenyltin acetate given to guineapigs
at dietary levels exceeding 5 mg/kg for 2 years, caused a dose-related
inhibition of growth rate (FAO/WHO, 1971, p. 341). A dietary level of
tricyclohexyltin hydroxide of 25 mg/kg for 90 days, caused a slight
reduction in weight gain in female rats (Shirasu, 1970), and dogs
receiving the same compound in their diet at a level of 12 mg/kg for 6
months also lost weight; some animals that totally refused the food
died from starvation (FAO/WHO, 1971, pp. 526-527).
Severe cardiovascular changes were demonstrated electro-
cardiographically in sheep after intrarumenal administration of
tricyclohexyltin hydroxide at doses greater than 150 mg/kg body weight
(Johnson et al., 1975).
7.2.13 Mechanisms o[ action
There have been relatively few studies of the biochemical effects
of dialkyltin compounds, which do not cause cerebral oedema. In acute
toxicity experiments on the rat, diethyltin compounds did not affect
the sodium and potassium contents of the central nervous system but
caused a decrease in its water con, tent (Magee et al, 1957).
The action of a homologous series of disubstituted
organotin-compounds from dimethyl- to dioctyltin have been examined;
most of them inhibit mitochondrial respiration by preventing the
oxidation of keto acids, presumbly via the inhibition of alpha-keto
oxidase activity, leading to the accumulation of pyruvate (Aldridge,
1976; Piver, 1973).
Of the trisubstituted compounds, triethyltin compounds have been
the most closely examined in relation to the mechanism by which they
cause ill effects. Trimethyl- and triethyltin compounds are potent
inhibitors of oxidative phosphorylation in the mitochondria for which
these compounds have a high binding affinity (Aldridge, 1958; Aldridge
& Street, 1964, 1970, 1971). In a later study by Aldridge (1976),
triorganotin compounds were stated to derange mitochondrial function
in 3 different ways, namely by secondary responses caused by discharge
of a hydroxyl-chloride gradient across mitochondrial membranes, by
interaction with the basic energy conservation system involved in the
synthesis of ATP, and by an interaction with mitochondrial membranes
causing swelling and disruption.
Using a 113Sn-labelled triethyltin compound, Rose (1969)
identified a pair of histidine residues on guineapig liver
mitochondria as the binding site for triethyl compounds. Thus, one
molecule of rat haemoglobin binds 2 molecules of triethyltin; the
binding sites are located in the globin.
Stockdale et al. (1970) showed that the order of effectiveness for
the organotin compounds in inhibiting coupled respiration was tributyl
> tripropyl > triphenyl > trimethyl. Two separate effects were
suggested: (a) an oligomycin-like inhibition of coupled
phosphorylation; and (b) an alteration of hydroxide exchange across
lipid membranes producing uncoupling, swelling, and reduction of
intramitochondrial substrate and phosphate concentrations followed by
structural damage.
7.2.14 Effective doses and dose rates
7.2.14.1 Lethal doses
The median lethal doses for some mono-, di-, tri-, and
tetrasubstituted organotin compounds are given in Tables 13, 14, 15,
and 16.a Trimethyl and triethyltin compounds, administered orally,
are more toxic than the higher homologues of the trialkyltin group.
The oral toxicity diminishes progressively from tripropyltin to
trioctyltin compounds (Barnes & Stoner, 1958). This is probably
because of poorer absorption of higher trialkyltin compounds from the
gastrointestinal tract, as judged from the difference between the oral
and intraperitoneal toxicity of higher trisubstituted alkyltin
compounds (Stoner et al., 1955). The intraperitoneal toxicity of
various trialkyltin compounds does not differ to any large extent. In
rats, Stoner et al. (1955) found that triethyltin sulfate was equally
toxic after intravenous, intraperitoneal, and oral administration. The
lethal dose per kg body weight given intravenously or
intraperitoneally was 10 mg/kg, causing death within 4-5 days. A dose
of 40 mg/kg body weight killed the rat in 2 h. The oral LD50 for
tricyclohexyltin hydroxide (Table 15) for most species, appears to be
between 100 and 1000 mg/kg body weight, while the intraperitoneal and
intravenous routes yield LD50 values below 20 mg/kg. The difference
between oral and parenteral toxicity reflects the poor absorption of
tricyclohexyltin hydroxide from the gastrointestinal tract.
In rats and dogs fed with tricyclohexyltin hydroxide, the
metabolites, dicyclohexyltin oxide and traces of cyclohexylstannoic
acid have been identified. Dicyclohexyltin oxide and
cyclohexylstannoic acid also constitute a small portion of the
residues on fruit as a result of photodecomposition (FAO/WHO, 1971,
pp 522, 534). The median lethal dose of these metabolites may
therefore be of interest. The LD50 of dicyclohexyltin oxide in the
rat appears to be about 350 mg/kg body weight, whereas the LD50 value
of cyclohexylstannoic acid in this species is probably ten times
higher (FAO/WHO, 1971, pp. 524-526).
Only a small amount of data is available concerning the acute
toxicity of tetraalkyltin compounds. The median lethal dose for
tetraethyltin is given in Table 16.
a Additional data on the toxicity of organotin compounds may be
found in a proposal for the United Nations classification and hazard
grouping of organotin compounds submitted by the expert from the
Netherlands to the Committee of Experts on the Transport of Dangerous
Goods of the United Nations Economic and Social Council
(EN/CN.2/CONF.5/R.607 of November 1976).
Table 13. Acute oral toxicity of some mono-organotin compounds in various animal species
Medium lethal
Trivial name of compound dose Species Reference
(LD50, mg/kg)
butylstannoic acid > 6000 mouse Pelikan & Cerny (1970b)
butyltin trichloride 1400 mouse
2140 rat Marhold (1972)
butyltin-S,S'S"-tris 1520 mouse Pelikan & Cerny (1970b)
(isooethylmercaptoacetate)
octyltin trichloride 4600 mouse Klimmer (1969)
octyltin-S,S',S"-tris 1500 rat (male) Pelikan & Cerny (1970a)
(2-ethylexylmercaptoacetate)
7.2.14.2 Minimum effective and no-observed-effect doses
When dibutyltin dichloride was fed to rats for 90 days at dietary
levels of 10, 20, 40, and 80 mg/kg, the no-observed effect level was
40 mg/kg, equivalent to 2 mg/kg body weight per day (Gaunt et al.,
1968). When dibutyltin was administered to rats for 6 months, the
no-observed-effect level was 20 mg/kg diet (Barnes & Stoner, 1958).
Dibutyltin sulfide, administered to rats at oral doses of 1.0, 0.1,
0.01, and 0.001 mg/kg body weight, per day for 7 months, was tolerated
without any detected adverse effects up to a dose o.f 0.01 mg/kg per
day but the higher levels of 0.1 and 1.0 mg/kg per day caused
intoxication (Mazaev & Korolev, 1969). By comparison, the toxicity of
dioctyltin compounds was relatively low (Klimmer, 1969). Thus,
dioctyltin did not produce any injury in rats, mice, or guineapigs at
oral doses up to 400 mg/kg body weight per day when given for 3-4
successive days, nor were there any ill effects when it was added to
the daily diet of rats for 4 months at a rate of 200 mg/kg (Barnes &
Stoner, 1958).
Table 14. Acute toxicity of some diorganotin compounds in various animals
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dibutylin di(2-ethylhexoate) 200 (oral) rat (male) Calley et al. (1967)
dibutyltin di(butyl maleate) 120 (oral) rat Klimmer (1969)
dibutyltin di(nonylmaleate) 170 (oral)
dibutyltin dichlorlde 100 (oral) rat (male) Klimmer (1969)
182 (oral) rat (male) Mazaev et al. (1971)
112 (oral) rat (female)
35 (oral) mouse
190 (oral) guineapig
150 (oral) rat Marhold (1972)
dibutyltin-S,S'-bis 150 (oral) rat (male) Klimmer (1969)
(2-ethylhexylmercaptoacetate) 175 (oral) Klimmer (1969)
dibutyltin dilaurate 243 (oral) rat Marhold (1972)
45 (oral) rat Marhold (1972)
dibutyltin oxide 520 (oral) rat (male) Klimmer (1969)
39.9 (i.p.)a rat (female) Robinson (1969)
24 (oral) mouse Mazaev & Slepnina (1973)
dibutyltin sulfide 145 (oral) rat (male)
150 (oral) rabbit
180 (oral) rat (female)
800 (i.p.) rat (female) Robinson (1969)
> 800 (i.p.) rat (female) Robinson (1969)
dioctyltin acetate 2030 (oral) rat Klimmer (1969)
diocyltin dibutylmaleate 3750 (oral) mouse Pelikan et al. (1970)
dioctyltin-S,S'-bis
(butylmercaptoacetate) 1140 (oral) white mouse Pelikán & Cerny (1970a)
dioctyltin bis
(dodecylmercaptide) 4000 (oral) white mouse
dioctyltin bis
(2-ethylhexylmaleate) 2760/ (oral) rat (male) Klimmer (1969)
2750
Table 14. (contd).
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dioctyltin-S,S'-bis 2010 (oral) white mouse Pelikan & Cerny (1970a)
(2-ethylhexylmercaptoacetate)
dioctyltin-S,S'-bis 3700 (oral) rat (male) Klimmer (1969)
(laurylmercaptoacetate)
dioctyltin-S,S'-(1,4- 2950 (oral) rat (male)
butane-diol-bis-mercaptoacetate)
dioctyltin dichloride 5500/
8500 (oral) rat (male)
dioctyltin dilaurate 6450 (oral) rat (male)
800 (i.p.) rat (female) Robinson (1969)
dioctyltin di(1,2-
propylene-glycolmaleate) 4775 (oral) rat (male) Klimmer (1969)
dioctyltin-S,S'-
(ethyleneglycol-bis-
dimercaptoacetate) 880 (oral) rat (male)
dioctyltin maleate 4500 (oral) rat (male)
dioctyltin
ß-mercapto-propanoate 1850/
2050 (oral) rat (male)
dioctyltin oxide 2500 (oral) rat (male)
dioctyltin mercaptoacetate 945 (oral) rat (male)
a intraperitoneal
Table 15. Acute toxicity of some triorganotin compounds in various animals
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
2-trichloro-1-(butine-1'-
oxide)-1-triethyl- 9.8 (i.p.) rat Ignatjeva (1968)
stannyloxy)ethane 9.6 (i.p.) mouse Ignatjeva (1968)
triethylstannylmethyl-
(1-propynyl)formal 10.7 (i.p.) rat Ignatjeva (1968)
triethylstannylphenyl-
acetylene 9.9 (i.p.) mouse Ignatjeva (1968)
9.1 (i.p.) rat Ignatjeva (1968)
1-triethylstannyl-3-
trimethylsiloxi-1-
propyne 11.8 (i.p.) mouse Ignatjeva (1988)
11.4 (i.p.) rat Ignatjeva (1968)
triethyltin acetate 4 (oral) rat (female) Stoner (1966)
triethyltin chloride 5 (i.p.) rat (female) Robinson (1969)
triethyltin sulfate 5.7 (i.p.) rat (male) Stoner (1966)
5.3 (i.p.) guineapig Stoner (1966)
tributyltin acetate 46 (oral) white mouse Pelikan & Cerny (1968a)
99 (oral) J. Pharm. Sci. (1967)
133 (oral) rat (male) Klimmer (1969)
tributyltin benzoate 108 (oral) white mouse
Pelikan & Cerny (1968a)
132 (oral) rat Arzneimittelforsch. (1969)
tributyltin chloride 117 (oral) white mouse Pelikan & Cerny (1968a)
129 (oral) rat Manhold (1972)
tributyltin laurate 180 (oral) white mouse Pelikan & Cerny, (1968a)
tributyltin oleate 195 (oral) rat Klimmer (1969)
tributyltin salicylate 137 (oral) rat (male) Klimmer (1969)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
bis(tributyltin oxide) 112- (oral) rat (male) Klimmer (1969)
132
180 (oral) rat Truhaut et al. (1976)
234 (oral) rat Sheldon (1975)
194 (oral, rat (male) Elsea & Paynter (1958)
aqueous
solution)
148 (oral, rat (male) Elsea & Paynter (1958)
oil
solution)
11.7 (dermal) albino rabbit Elsea & Paynter (1958)
10.0 (oral) white mouse Pelikan & Cerny (1969)
trihexyltin acetate 1000 (oral) rat Barnes & Stoner (1958)
trioctyltin chloride 10 000 (oral) rat (male) Klimmer (1969)
triphenyltin acetate 21 (oral) guineapig Klimmer (1963)
24 (oral) guineapig Kimbrough (1976)
136 (oral) rat Klimmer (1963)
81 (oral) mouse (male) FAO/WHO (1971)
7.9 (i.p.) mouse (male) Stoner (1966)
136 (oral) rat (male) Klimmer (1964)
491 (oral) rat (female) Stoner (1966)
450 (dermal) rat (male) Klimmer (1963)
8.5 (i.p.) rat (female) Stoner (1966)
11.9 (i.p.) rat (female) Stoner (1966)
13.2 (i.p.) rat (male) Klimmer (1964)
21 (oral) guineapig
(male) Klimmer (1964)
3.7 (i.p.) guineapig
(male) Stoner (1966)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
triphenyltin acetate 5.3 (i.p.) guineapig
cont'd. (male) Klimmer (1964)
30- (oral) rabbit (male) Klimmer (1964)
50
triphenyltin chloride 80 (oral) rat (male) FAO/WHO (1971)
135 (oral) rat (female) FAO/WHO (1971)
triphenyltin hydroxide 245 (oral) mouse (male) FAO/WHO (1971)
209 (oral) mouse (female)
FAO/WHO (1971)
240 (oral) rat (male) Gaines & Kimbrough (1968)
360 (oral) rat (female) Gaines & Kimbrough (1968)
27.1 (oral) guineapig FAO/WHO (1971)
(male)
31.1 (oral) guineapig FAO/WHO (1971)
(female)
171 (oral) rat (male) Marks et al. (1969)
268 (oral) rat (female) Marks et al. (1969)
tricyclohexyltin 710a (oral) mouse- FAO/WHO (1971)
hydroxide peromyscus
1070a (oral) mouse-swiss FAO/WHO (1971)
white
540 (oral) rat FAO/WHO (1971)
13 (i.p.) rat FAO/WHO (1971)
780 (oral) guineapig FAO/WHO (1971)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
tricyclohexyltin 9 (i.p.) guineapig FAO/WHO (1971)
hydroxide 500- (oral) rabbit FAO/WHO (1971)
cont'd. 1000
> 126 (i.p.) rabbit FAO/WHO (1971)
150a (oral) sheep Johnson et al. (1975)
14 (i.v.)c dog FAO/WHO (1971)
6 (i.v.) cat FAO/WHO (1971)
a approximate lethal dose
b intraperitoneal
c intravenous
Table 16. Acute oral toxicity of some tetraorganotin compounds in various animal species
Trivial name Medium lethal dose Species Reference
of compound (LD50, mg/kg)
tetraethyltin 40.0 mouse Skackova (1967)
15.0 rat
40.0 guineapig
7.0 rabbit
40 mouse Mazaev et al. (1971)
9.0 rat
40.0 guineapig
7.0 rabbit
tetrabutyltin 6000 rat Skackova (1967)
Data on effective doses of trisubstituted organotin compounds are
available only for triphenyltin derivatives and tricyclohexyltin
hydroxide. Triphenyltin acetate was administered, by gavage, to groups
of rats at doses equivalent to dietary levels of 5, 10, 25, and
50 mg/kg for up to 17 days. While no adverse effects were found at the
25 mg/kg level, the 50 mg/kg level resulted in the death of animals,
mainly as a result of infection (Klimmer, 1964). In a 2-year study on
rats fed diets containing concentrations of triphenyltin hydroxide of
up to 10 mg/kg, the no-observed-effect level was about 2 mg/kg
(equivalent to about 0.1 mg/kg body weight per day) (FAO/WHO, 1971,
pp. 341-342). In another 2-year study in which groups of guineapigs
were fed diets containing concentrations of triphenyltin acetate, up
to 200 mg/kg, the no-observed-effect level was 5 mg/kg. At higher
levels there was a dose-related increase in mortality and the
occurrence of other effects such as inhibition of growth rate and
fatty degeneration of the liver and heart (FAO/ WHO, 1971, p. 341).
With respect to tricyclohexyltin hydroxide, no toxic effects were
noted in rats receiving 12.5 mg/kg body weight daily for 19 days,
whereas a rate of 25 mg/kg per day caused toxic effects (FAO/WHO,
1971, pp. 527-528). Rats were fed on diets providing up to 12 mg/kg
body weight per day of tricyclohexyltin hydroxide in a 2-year study;
the no-observed-effect level was 3 mg/kg body weight per day (FAO/WHO,
1971, p. 528). When dogs were fed tricyclohexyltin hydroxide in the
diet at concentrations of up to 12 mg/kg body weight per day for 6-24
months, many dogs refused the 12 mg/kg per day diet, causing weight
loss and death from starvation. The no-observed-effect level was found
to be about 0.75 mg/kg per day (FAO/WHO, 1971, pp. 526-527). Johnson
et al. (1975) also reported the effects of thoroughly wetting the skin
of yearling cattle, goats, and sheep with suspensions of
tricyclohexyltin hydroxide. They found that cattle tolerated
suspensions up to a concentration of 5 g/litre while anorexia was
registered in some animals after spraying with a suspension of
10 g/litre. Yearling goats and sheep tolerated suspensions of up to
1 g/litre. Transitory anorexia and eye irritation developed after
application of a suspension of 20 g/litre to the skin of goats.
Few data are available on the effective dose levels of
tetrasubstituted organotin compounds. Intravenous administration of
tetraethyltin at 25 mg/kg body weight produced a slight increase in
respiratory rate and vasodilatation as immediate effects, but, 1.5-2 h
later, prostration with muscular weakness occurred. (Stoner et al.,
1955).
8. EFFECTS ON MAN
There are comparatively few clinical observations and
epidemiological data concerning the effects of inorganic tin compounds
on man and even fewer on the effects of organotin compounds.
8.1 Inorganic Tin Compounds
8.1.1 Acute poisoning
Some episodes of acute poisoning have been reported, mainly in
association with the ingestion of fruit juices containing high
concentrations of tin. The major symptoms and signs noted were nausea,
vomiting, diarrhoea, fatigue, and headache. The concentrations of tin
in the products thought to have been associated with the incidents
were uncertain in many cases, but were probably in the range of
300-500 mg/kg (Horio et al, 1967). Orange and apple juice, containing
tin concentrations of 250-385 mg/kg were suspected of causing one
incident (Benoy et al., 1971). Nausea, vomiting, and diarrhoea were
recorded in individuals consuming-peach preserves that contained a tin
concentration of 563 mg/kg, zinc at 1.5 mg/kg, cadmium at 0.1 mg/kg,
lead at 0.16 mg/kg, copper at 1 mg/kg, nitrate at 93 mg/kg, nitrite at
1.7 mg/kg, and chloride at 115 mg/kg (Nehring, 1972). Acute
gastroenteritis followed ingestion of a fruit punch stored in a tin
can and containing a tin concentration of 2000 mg/litre. First
symptoms occurred after 1-2 h, the earliest and commonest being
bloatedness, followed by severe nausea, stomach cramps, vomiting and,
in one-third of the patients, mild diarrhoea (Warburton et al, 1962).
Other similar outbreaks have involved canned cherries (Luff & Metcalf,
1890), asparagus, herring (Schryver, 1909), and apricots (Savage,
1939) with tin concentrations ranging from 300 to about 1000 mg/kg.
An incident of acute intoxication after the ingestion of canned
peaches was reported by Sverrsson (1975). It was unique in that about
110 participants in a meeting received nothing else to eat or drink,
except for the peaches. The report was based on questionnaires
received from 85 persons, 76 (89%) of whom had fallen ill. About half
of these had developed symptoms such as nausea, vomiting, and
diarrhoea within 1 h. The majority became symptom-free after 24 h. The
fruit contained a mean tin concentration of 533 mg/kg (range
413-597 mg/kg) and the juice 369 mg/kg (range 298-405 mg/kg). Two out
of 7 persons who had consumed only one quarter of the contents fell
ill. The calculated intake of tin for these persons was 50 mg.
In another report, canned tomato juice was associated with 113
cases of acute gastroenteritis (Barker & Runte, 1972). In a small
number of these cases, superficial erosion of the mucosa of the mouth
was observed, while abdominal distension and cramps, and diarrhoea
were quite common. The cans containing the juice showed complete
corrosion of tin linings and this yielded a product containing a tin
concentration of approximately 400 mg/kg. The crops of tomatoes, used
to make this particular juice, had been grown in soil excessively
treated with nitrate fertilizer and contained high levels of nitrate;
complete corrosion of the lining of the cans occurred within
approximately 6 months.
Five human volunteers did not show any toxic signs after drinking
fruit juices containing about 500 or 730 mg/kg of tin, but all had
some gastrointestinal disturbance after drinking 5-7 ml/kg body weight
of fruit juice containing a tin concentration of about 1400 mg/litre
(Benoy et al., 1971). There was no evidence to indicate that the
effects were due to the absorption of tin, the likeliest cause being
local irritation of the mucous membranes of the alimentary tract.
8.1.2 Prolonged exposure
8.1.2.1 Effects of inhalation
The only available information on exposure by inhalation pertains
to pneumoconiosis caused by the inhalation of tin(IV) oxide. This
benign condition is termed stannosis.
More than 200 cases of stannosis have been described (Bartak
et al., 1948; Cutter et al., 1949; Dundon & Hughes, 1950; Oyanguren
et al., 1958; Pendergrass & Pryde, 1948; Robertson & Whitaker, 1957;
Schuler et al., 1958). The relative importance of exposure to tin
fumes in the etiology of this disorder was emphasized by Dundon &
Hughes (1950). The significance of the quantity of dust and the
duration of exposure were stressed by Robertson & Whitaker (1957), who
reviewed 121 cases.
Pendergrass & Pryde (1948) noted stannosis in a man who, for 15
years, had been bagging tin oxide material containing 96.5% tin(IV)
oxide and small amounts of aluminium, iron, and sodium but not silica.
Radiography revealed small dense shadows (denser than those of
silicosis) resembling those of barytosis in both lungs. Bartak et al.,
(1948) reported a similar case in a workman who had suffered from
asthma for many years and who attended a furnace in which metallic tin
was burnt to produce tin(IV) oxide. Necropsy of this man, who died
from gastric carcinoma, revealed deposits of tin(IV) oxide in the
lungs, lymph glands, liver, and spleen. Six other workmen with a
similar radiographic appearance of the lungs, did not have any
symptoms of asthma or signs of pulmonary dysfunction. Similar
radiological findings were reported by Cutter et al. (1949) in 2 cases
with nodules 1-2 mm in diameter unaccompanied by evidence of pulmonary
dysfunction. Both had been working in a tin recovery department for 20
years.
Oyanguren et al., (1958) reported 10 cases of stannosis in workers
involved in a process where tin ore concentrates were reduced to
metal. The workers were exposed to dust with a high tin and a low
silica content and to fumes rich in tin. None of the exposed workers
had any disability and the vital capacity, maximal breathing and
resting minute volume, and respiratory reserve were normal, as were
the findings on the blood and urine. Radiological examination of these
10 workers showed that the first alteration appeared to be increase of
bronchovascular markings, with hilar thickening in the early stages;
later, well-defined nodular elements appeared first in the middle
third of the right and then of the left lung. These appeared to
progress to the rest of the lung fields, and with continuous exposure,
accumulation of tin(IV) oxide gave a metallic density to a part of the
nodules, which subsequently acquired an appearance of lipoid droplets.
In the later stages, the bronchovascular markings disappeared as the
density of the shadows increased. The radiographic changes appeared
after some 3-5 years of exposure (Schuler et al., 1958). Hlebnikova
(1957) made a survey over a number of years of workers, who were
exposed to condensation aerosols, formed during the smelting of tin
and consisting mainly of tin(IV) oxide. The free silica concentration
in the aerosols was not more than 1% and the total silica did not
exceed 3%. The total dust concentration in air varied between 3 and
70 mg/m3. Workers developed pneumoconiosis after 6 to 8 years of
working at the smelter. The authors described 45 cases of
pneumoconiosis in workers, who were employed at the smelter from 6 to
20 years, Six of them were reported to have second degree
pneumoconiosis. X-ray examination showed small spotty shadows, but
there were no other symptoms or signs. The opacities seen on the
radiographs were thought to be due to the accumulation of
tin-containing dust and the development of connective tissue, This was
confirmed by experiments on rats exposed to tin(II) oxide. No cases of
pneumoconiosis were observed in 10 years, after the dust concentration
had been reduced to 10 mg/m3. An important feature of stannosis is
that fibrosis of the lung does not develop, providing that other
agents such as silica are not present.
8.1.2.2 Effects of ingestion
Packaged military rations were fed to 9 young male adult
volunteers for successive 24-day periods. The average tin content of a
control fresh diet was 13 mg/kg (in dry solids), while C-rations
stored at 1°C contained a tin concentration of 33 mg/kg, and rations
stored at 37°C contained 204 mg/kg. All the tin ingested was
accounted for by faecal elimination. No toxic effects were noted
(Calloway & McMullen, 1966). Dack (1955) reported a study in which 4
subjects ate canned pumpkin containing a tin concentration of about
380-480 mg/kg and canned asparagus containing a concentration of about
360 mg/kg, for 6 days, with no apparent illness. In an earlier study,
Mamontova (1940) did not observe any toxic manifestations in 4
volunteers who, for a period of 30 days, consumed 250 g per day of
canned fruit containing 212-250 mg of tin.
8.2 Organotin Compounds
8.2.1 Local effects
Toxic lesions among laboratory and process workers handling di-
and tributyltin compounds were reported by Lyle (1958). Most of the
lesions were typical acute skinburns, caused by the colourless di- or
tributyltin chlorides, which can come into contact with the skin
without exposed workers being aware of it. This type of lesion
developed 1 to 8 h following exposure. When the substance was washed
off immediately, no lesion developed. A more diffuse, but less rapidly
healing lesion, was caused by contact with clothes that had been
moistened by vapour or liquid compounds. This subacute irritation was
characterized by itching, affecting mostly the skin of the lower
abdomen, thighs, and groin. On examination, an easily distinguishable
erythematous eruption, was noted. Cessation of contact with the
compound was followed by rapid healing. Following contact with the
eye, lachrymation and severe suffusion of the conjunctivae appeared
within a few minutes and persisted for 4 days. Immediate lavage of the
eye did not prevent the development of the signs.
Lyle (1958) also studied the skin lesions induced by butyltin
compounds by applying various compounds on the skin of the back of the
hands of 5 volunteers. Skin lesions could be produced by a single
application of dibutyltin dichloride, and of the chloride, acetate,
and oxide of tributyltin, while the diacetate, dilaurate, oxide, and
maleate of dibutyltin and also tetrabutyltin failed to produce any
lesions. No visible changes occurred for 2 go 3 h after application,
although a swelling of the mouth of the hair follicles was noticeable.
The follicular inflammation progressed during the following 8 h with
only slight visible irritation of the skin between the openings of the
follicles. On the second day, sterile pustules developed over the
follicular openings, but remained small during the next 3 to 4 days.
After one week, the lesions had practically disappeared.
Symptoms experienced by female spray-painters working with a latex
paint, to which was added a fungicidal solution containing 20%
bis(tributyltin) oxide, ethylene oxide, ethanol, and water, were
described by Landa et al. (1973). The symptoms were reported to appear
"immediately" after the start of spraying and were experienced by all
the women engaged in the work. The first sensations were irritation of
the nasal mucosa and the conjunctivae. The exposure continued for
another fortnight during which the symptoms and signs became more
severe, and included bleeding from the nose and mucous discharges. An
otorhinolaryngological examination revealed rhinitis with distinct
hyperaemia and haemorrhages of the nasal septum. The workers reported
that the symptoms were less severe during weekends. When the addition
of the fungicidal solution was abandoned, the symptoms disappeared.
One year later, identical symptoms were reported by 4 spray-painters
using latex paint. An inquiry disclosed that the manufacturer had
begun to use bis(tributyltin) oxide as a fungicidal additive since the
banning of the use of mercury. The authors considered that the
trialkyltin compound was responsible for the symptoms. Measurements
performed later indicated that the concentrations of tin in the
breathing were below 0. 05 mg/m3 air.
Another description of local effects seen in workers handling
triphenyltin acetate was given by Markicevic & Turko (1967). These
workers were engaged in the formulation of a 20% solution of a
fungicide. During the hottest summer days, subjects working in the
dustiest places developed conjunctival irritation and irritation of
the mucous membranes of the upper respiratory tract as well as of the
skin, the hands and scrotum being particularly affected. Effects on
the central nervous system were not registered. The signs disappeared
rapidly after termination of exposure.
A wettable powder formation containing 50% tricyclohexyltin
hydroxide was examined for its irritation and sensitization potential.
No adverse reactions were observed in 53 females after sensitization
applications and a challenge application 20 days later of 0.5 ml of an
emulsion (10 g/litre). Tricyclohexyltin hydroxide was reported not to
be dermally irritating at a concentration of about 0.01 mg/kg body
weight (FAO/WHO, 1971).
8.2.2 Systemic effects
8.2.2.1 Effects of Dermal exposure
In a recent review (NIOSH, 1976), a fatal case was described in
which a 29-year-old woman was accidentally drenched in a slurry
containing triphenyltin chloride, diphenyltin dichloride, hexane, and
other unidentified compounds at a temperature of 79.4°C. On arrival at
hospital, first-degree thermal burns covered 10% of her body. Second-
and third-degree burns with 80-85% desquamated skin developed 12 h
later. Death from renal failure occurred 12 days after the accident.
However, the agent responsible for her symptoms and signs and for the
death could not be identified from the data available.
Mijatovic (1972) reported a case of systemic intoxication
resulting from dermal contact with a compound containing triphenyltin
acetate (60%) and manganese dithiocarbamate (15%). The subject,
engaged in agricultural aviation, spilt the compound on his hands and
chest while filling the aeroplane. The skin on his chest and abdomen
was endured after 3 h and vesicles developed the following day. He
experienced headache, nausea, epigastric pain, and general weakness.
Clinically the liver transaminases (SGPT) were elevated, reaching a
maximum one month later. Two months later the transaminases were
returning to normal, but the patient had pains over the liver, which
was tender and enlarged. The liver damage persisted for 2 years and
the case was labelled chronic hepatitis. No liver biopsy was
performed.
8.2.2.2 Effects of inhalation
Acute intoxication caused by the inhalation of triphenyltin
compounds has been reported in some instances. Three cases occurred in
farmers treating beetroot plants with triphenyltin acetate which was
inhaled (Guardascione & di Bosco, 1967). The symptoms started from a
few minutes to about 2 h after the first exposure. After 2 hours of
spraying, one subject felt a general malaise and severe headache and
eventually lost consciousness. On admission to the hospital he had a
diffuse tremor and a slightly depressed sensorium. All laboratory
investigations during the 2-week hospitalization were normal. When
mixing triphenyl tin acetate powder into a solution, a second subject
experienced repeated flushes, nausea, and shortness of breath, which
started only a few minutes after inhalation. During the 8-day hospital
surveillance, the only pathological finding was glucosuria. A third
subject suffered from severe headache, strong nausea, and epigastric
pains. However, laboratory investigations were normal. The headache
persisted for 2 days while the epigastric pains and the nausea
disappeared after one day. All 3 subjects recovered completely.
Horacek & Demcik (1970) reported a group poisoning involving 2
pilots and 3 mechanics following the spraying of a formulation
comprising 60% of triphenyltin acetate and 15° of manganese
dithiocarbamate. Protective measures were found to be deficient and
food was consumed with unwashed hands; thus, exposure by both
inhalation and ingestion could have occurred. Furthermore, other
pesticides containing copper, zinc, DDT, and organophosphates were
handled during exposure to the formulation, but the authors considered
these substances unlikely to be responsible for the symptoms
experienced. Exposure to the formulation continued for 2 weeks before
symptoms were recognized and continued for 2 further weeks. One pilot
experienced gastric pain, diarrhoea, and dryness of the mouth with
severe thirst that was not relieved by drinking. He also felt pressure
over the chest and slight shortness of breath. Blurred vision was
experienced after one week of exposure. Clinically hepatomegaly with a
painful liver was noted. Liver transaminases (SGPT) were elevated and
the highest recorded value was obtained 6 weeks later. Hyperglycaemia
and glycosuria were also present. Eight weeks afterwards, liver biopsy
revealed marked diffuse steathosis without any detected necrosis.
Monthly follow-ups showed that hepatomegaly and steathosis persisted
for one year. The second pilot suffered from heartburn, diarrhoea, and
vision disturbances. Clinically a hepatomegaly and a slight
hyperglycaemia were registered. The symptoms persisted for 4 weeks and
the glycosuria and hepatomegaly for 6 weeks. Recovery was complete.
The mechanics had symptoms that were less severe, comprising
diarrhoea, headache, eye pains, blurred vision, epigastric pain, and
thirst.
A worker engaged in the manufacture of butyltin compounds was
reported to suffer from a reduced sense of smell (Akatsuka et al.,
1959). It was first observed after an exposure period of 16 months,
and a further deterioration of the olfactory sense was established
during the following 8 months. The state persisted without any noted
improvement for 2 years. Other reported symptoms were headaches in the
occipital region, nasal haemorrhages, lassitude, and a feeling of
stiffness in the shoulders.
In four eases of acute poisoning due to exposure to organotin
vapours, patients were reported to have suffered from such symptoms as
vertigo, headaches, nausea and vomiting, and visual disturbances.
Clinically, stasis of the papilla was found and all patients displayed
pathological findings on the electroencephalograms. These were
reversible in 7-25 days, and all eases recovered clinically. The
organotin compound or compounds responsible for the intoxications were
not identified (Prüll & Rompel, 1976).
8.2.2.3 Effects of ingestion
A most serious episode involving organotin poisoning occurred in
1954 due to oral administration of a proprietary preparation used for
the treatment of furonculosis, osteomyelitis, anthrax, and acne. The
drug was responsible for about 100 deaths and a total of about 210
intoxications. These numbers vary to some extent depending on the
source of information: Alajouanine et al., (1958) reported a total of
210 deaths whereas another source quotes 102 deaths and at least 100
persons permanently affected ( Br. med. J., 1958). A total of about
400 000 capsules was sold ( Br. med. J., 1958), and about 1000
persons were believed to have taken the drug (Barnes & Stoner, 1959).
However, the capsules consumed by the intoxicated subjects amounted
only to about 7% of the lot distributed ( Br. med. J., 1958),
implying that the majority of the capsules were consumed without any
known adverse effects. The main ingredients of the preparation were
diethyltin diiodide (15 mg/capsule) and linoleic acid
(100 mg/capsule). It was suggested that ethyltin triiodide,
triethyltin iodide, or tetraethyltin could have been present as
impurities because of deficiencies in the manufacturing process or as
metabolites formed under the influence of various physical factors
(Rondepierre et al, 1958). A theory that diethyltin diiodide would
have reacted with the isolinoleic component producing tetraethyltin
was also presented (Lecoq, 1954). The symptoms and clinical findings
described in victims by Alajouanine et al. (1958) seem to favour the
hypothesis of a trialkyltin compound being the causal agent, probably
acting synergistically with other constituents. The clinical data of
201 cases including 98 deaths were reviewed by Alajouanine et al.
(1958). The dominating symptom, reported in about 98% of the cases,
was a diffuse headache, sometimes intolerably severe, and appearing a
few days after medication was started. Nausea and vomiting occurred in
73%, visual disturbances, mainly photophobia, but also double vision,
colour-vision disturbances and, in a few cases, blindness were
recorded in 33% of cases. Ophthalmoscopic findings included
congestion, papilloedema (Druault-Toufesco, 1955), and in some cases,
papillary stasis (Gayral et al., 1958; Pesme, 1955). Frequent symptoms
and signs were urinary incontinence, vertigo, loss of weight, and
abdominal pains. Absence of fever and a tendency towards hypothermia
were also noted. Psychological disturbances or stupor were reported in
70% of the cases. Other findings were meningeal irritation,
somnolence, insomnia, convulsions, constipation, and bradycardia.
Sometimes electroencephalograms were altered but did not suggest any
localized lesion. Death occurred during coma or from respiratory or
cardiac failure and in some cases during convulsions. It is probable
that most of the symptoms and signs could be attributed to a cerebral
oedema, the occurrence of which was established at autopsies and
decompressive surgery (Cossa et al., 1958, Fontan et al., 1958). This
cerebral oedema of the white matter appeared to be very similar to
that produced experimentally by administration of a proprietary
preparation containing an organotin compound, to mice and monkeys
(Gruner, 1958). Macroscopically, the brain was oedematic but the
microscopic findings were minor.
It has been reported that only 10 out of 103 subjects, who
survived, recovered completely: in the remainder, symptoms such as
headaches and asthenia persisted for at least 4 years (Barnes &
Stoner, 1959). Information on later, follow-up studies is not
available. The lethal dose of the preparation was in some instances
only about 25 capsules taken during one week. Ingestion of 3 capsules
was enough to cause intoxication in a 9-year-old child (Fontan et al.,
1955).
8.3 Treatment of Poisoning
Dimercaprol has been suggested by Stoner et al. (1945) to be an
effective antidote for dialkyltin poisoning. It has also been reported
to completely prevent the accumulation of alpha-keto acids produced by
dialkyltin compounds (Barnes & Stoner, 1959). Although dimercaprol
protected rats against the general toxic effects of these compounds,
it did not have any effect on the response to triethyltin compounds.
This seems to be due to the fact that dialkytin compounds, at least up
to the dihexyl derivatives, react readily with sulfhydryl groups and
the trialkyltin compounds do not (Barnes & Magos, 1968),
Studer et al. (1973) reported that steroid therapy (dexamethasone)
appeared to diminish mortality and the severity of brain oedema in
rats; there also appeared to be significant decreases in brain, liver,
and blood levels of triethyltin bromide. The authors suggested that
the beneficial effects of this steroid therapy might be partly due to
enhanced excretion or catabolism of triethyltin bromide.
Surgical decompression was considered to be the only treatment
that offered any benefit in human cases of cerebral oedema caused by
trialkyltin compounds (Alajouanine et al., 1958).
REFERENCES
ADAM, W. B. & HORNER, G. (1937) The tin content of English canned
fruits and vegetables. J. Soc. Chem. Ind., 56: 329-334.
ADAMSON, J. H. (1962) The colorimetric determination of dialkyltin
compounds dissolved in fats and olive oil. Analyst, 87: 597-598.
ADCOCK, L. H. & HOPE, W. G. (1970) A method for the determination of
tin in the range 0.2 to 1.6 µg, and its application to the
determination of organotin stabilizer in certain foodstuffs.
Analyst, 95: 868-874.
AKAGI, H. & SAKAGAMI, Y. (1971) [On degradation of organotin compounds
by ultraviolet ray.] Rep. Public Health Inst., 20: 1-4
(in Japanese).
AKAGI, H., FUJITA, M., & SAKAGAMI, Y. (1972) [Thin layer
chromatography of inorganotin and organotin compounds in foods.]
J. food Hyg., 13: 85-88 (in Japanese).
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A.,
KAWAME, N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S.,
YANAGIBASHI, H., NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA,
I., & IYO, T. (1959) [Experimental studies on disturbance of sense
of smell due to butyltin compounds.] J. Tokyo Med. Coll,
17: 1393-1402 (in Japanese).
ALAJOUANINE, T., DÉROBERT, L., & THIÉFRY, S. (1958) Etude clinique
d'ensemble de 210 cas d'intoxication par les sels organiques
d'étain. Rev. Neurol., 98: 85-96.
ALDRIDGE, W. N. (1958) The biochemistry of organotin compounds;
trialkyltins and oxidative phosphorylation. Biochem. J.,
69: 367-376.
ALDRIDGE, W. N. (1976) The influence of organotin compounds on
mitochondrial functions. In: Zuckerman, J. J., ed. Organotin
compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 186-196.
ALDRIDGE, W. N. & CREMER, J. E. (1957) Organotin dithizone complexes:
The colorimetric determination of diethyltin and triethyltin
compounds. Analyst, 82: 37-43.
ALDRIDGE, W. N. & STREET, B. W. (1964) Oxidative phosphorylation:
Biochemical effects and properties of trialkyltins. Biochem. J.,
91: 287-297.
ALDRIDGE, W. N. & STREET, B. W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver
mitochondria. Biochem. J., 118: 171-179.
ALDRIDGE, W. N. & STREET, B. W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and
triethyltin to mitochondria and their effects on various
mitochondrial functions. Biochem. J., 124: 221-234.
ALEU, F. P., KATZMAN, R., & TERRY, R. D. (1963) Fine structure and
electrolyte analysis of cerebral edema induced by alkyltin
intoxication. J. Neuropathol exp. Neurol., 22: 403-413.
ALLAN, J. E. (1962) Atomic absorption spectrophotometry absorption
lines and detection limits in the air-acetylene flame.
Spectrochim. Acta, 18: 259-263.
AMOS, M.D. & WILLIS, J. B. (1966) Use of high-temperature pre-mixed
flames in atomic absorption spectroscopy. Spectrochim. Acta,
22: 1325-1343.
ANALYTICAL METHODS COMMITTEE (1967) The determination of small amounts
of tin in organic matter. Analyst, 92: 320-323.
ANDERSON, S. R. A. & LOWY, S. L. (1956) A fluorometric determination
of tin. Anal. Chim. Acta, 15: 246-253.
ASCHER, K. R. S. & NISSIM, S. (1964) Organotin compounds and their
potential use in insect control. World Rev. Pest Control,
3: 188-211.
AVTANDILOV, G. G. (1967) [Trace element content in normal and
atherosclerotic portions of the human aorta in connection with
age.] Arh. Patol., 29: 40-42 (in Russian).
BARKER, W. H. & RUNTE, V. (1972) Tomato-juice-associated
gastroenteritis, Washington and Oregon, 1969. Am. J. Epidemiol.
96: 219-226.
BARNES, J. M. & MAGEE, P. N. (1958) The biliary and hepatic lesion
produced experimentally by dibutyltin salts. J. Pathol.
Bacteriol. 75: 267-279.
BARNES, J. M. & MAGOS, L. (1968) The toxicology of organometallic
compounds. Organometal. Chem. Rev., 3: 137-150.
BARNES, J. M. & STONER, H. B. (1958) Toxic properties of some dialkyl
and trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARNES, J. M. & STONER, H. B. (1959) The toxicology of tin compounds.
Pharmacol. Rev., 11: 211-231.
BARNES, R. D., BULL, A. T., & POLLER, R. C. (1973) Studies on the
persistence of the organotin fungicide, fentin acetate
(triphenyltin acetate) in the soil and on surfaces exposed to
light. Pestic. Sci., 4: 305-317.
BARTAK, F., TOMECKA, M, & TOMICEK, O. (1948) Stannosis. Cas. lék.
cesk., 87: 915-920 (Abstract in: J. ind. Hyg., (1949)
31 (5): 98-99)
BASSINDALE, A. R., EABORN, C., TAYLOR, R., THOMPSON, A. R., WALTON, D.
R. M, CRETNEY, J., & WRIGHT, G. J. (1971) Aromatic reactivity 46.
Interpretation of substituent effects in base-catalyzed cleavage
of aryltrimethylsilanes and aryltrimethyl stannanes. J. Chem.
Soc. B., 1155-1161.
BENNETT, R. L. & SMITH, H. A. (1959) Spectrophotometric determination
of tin with phenylfluorone. Anal. Chem., 31: 1441-1442.
BENOY, C. J., HOOPER, P. A., & SCHNEIDER, R. (1971) The toxicity of
tin in canned fruit juices or solid foods. Food Cosmet. Toxicol.,
9: 645-656.
BENS, A. A, GRABOVSKAJA, L. I., & TIHONOVA, I. V. (1976)
[ Geochemistry of the environment.] Moscow, Nedra (in Russian).
BERGNER, K. G. & RUDT, U. (1958) [On the absorption of lead and tin by
fat and food rich in fat in tin plate cans.] Z. Lebensm. Unters.
Forsch., 137: 337-351 (in German).
BERTINE, K. K. & GOLDBERG, E. D. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BISTON, R., VERSTRAETEN, E., & NANGNIOT, P. (1972) Dosage de l'étain
dans les conserves par polarographie oscillographique ŕ intensité
imposée: Comparaison avec les methodes colorimetriques au dithiol
et ŕ la phenyl-fluorone. Anal. Chim. Acta, 59: 453-460.
BLAIR E. H. (1975) Biodegradation of tricyclohexyltin hydroxide.
Environ. Qual. Sai., 3 (Suppl.): 406-409.
BLASIUS, M. B., KERKHOFF, S. J., WRIGHT, R. S., & COTHERN, C. R.
(1972) Use of X-ray fluorescence to determine trace metals in
water resources. Water Resour. Bull., 8: 704-714.
BOGEN, J. (1973) Trace elements in atmospheric aerosol in the
Heidelberg area, measured by instrumental neutron activation
analysis. Atmos. Environ., 7: 1117-1125.
BÖNIG, G. & HEIGENER, H. (1972) [Paper chromatography: Quantitative
determination of micro amounts of tin.] Landwirtsch. Forsch.,
25: 378-382 (in German).
BOWEN, H. J. M. (1966) Trace elements in biochemistry, New York,
Academic Press, pp. 203-204.
BOWEN, V. T. & SUTTON, D. (1951) Comparative studies of mineral
constituents of marine sponges. I. The Genera Dysidea,
Chondrilla, Terpios. J. mar. Res., 10: 153-167.
BRAMAN, R. S. & TOMPKINS, M. A. (1979) Separation and determination of
nanogram amounts of inorganic tin and methyltin compounds in the
environment. Anal. Chem. 51: 12-19.
BRIDGES, J. W., DAVIES, D. S., & WILLIAMS, R. T. (1967) The fate of
ethyltin and diethyltin derivates in the rat. Biochem. J.,
105: 1261-1266.
BRINCKMAN, F. E. & IVERSON, W. P. (1975) Marine chemistry in the
coastal environment. Paper presented at 169th American Chemical
Society Meeting. Philadelphia, PA., April 8-10.
BRINCKMAN, F. E., BLAIR, W. R., JEWETT, K. L., & IVERSON, W. P. (1977)
Application of a liquid chromatograph coupled with a flameless
atomic absorption detector for speciation of trace organometallic
compounds. J. Chromatogr. Sci., 15: 493-503.
BRITISH MEDICAL JOURNAL (1958) "Stalinon": A therapeutic disaster.
Br. med. J., March 1st, p. 515.
BROWNING, E. (1969) Tin. In: Toxicity ol industrial metals, 2nd ed.,
London Butterworths, pp. 323-330.
BRUGGEMANN, J. & KLIMMER, O. R. (1964) [Nutritional-physiological,
analytical and toxicological studies on fungicidal triphenyltin
acetate.] Zentralbl. Vet.-Med. A, 11: 1-3 (In German).
BRUGGEMANN, J., BARTH, K., & NIESAR, K.-H. (1964a) [Experimental
studies on the content of triphenyltin acetate residues in turnip
leaves, ensilage of turnip leaves, and in excrements of animals
fed with these products.] Zentralbl. Vet.-Med. A, 11: 4-19
(in German).
BRUGGEMANN, J., KLIMMER, O. R., & NIESAR, K.-H. (1964b) [The
importance of triphenytin acetate residues in plants and animals
from a hygienic-toxicologic standpoint.] Zentralbl. Vet.-Med. A,
11: 40-48 (in German).
BURGER, K. (1963) [Thin layer chromatographic method for the
detection, identification and separation of traces of mono-, di-,
tri- and zerovalent organotin compounds.] Z. anal. Chem.,
192: 280-286 (in German).
BYINGTON, K. H., YEH, R. Y., & FORTE, L. R. (1974) The hemolytic
activity of some trialkyltin and triphenyltin compounds. Toxicol.
appl. Pharmacol., 27: 230-240.
CALLEY, D. J., GUESS, W. L., & AUTIAN, J. (1967) Hepatotoxicity of a
series of organotin esters. J. Pharm. Sci., 56 (2): 240-243.
CALLOWAY, D. H. & MCMULLEN, J. J. (1966) Fecal excretion of iron and
tin by men fed stored canned foods. Amer. J. clin. Nutr.,
18: 1-6.
CALVERY, H. O. (1942) Trace elements in foods. Food Res.,
7: 313-331. CAPACHO-DELGADO, L. & MANNING, D.C. (1966)
Determination of tinby atomic absorption spectroscopy.
Spectrochim. Acta, 22: 1505-1513.
CARR, H. G. (1969) Interaction between PVC bottles and liquid foods.
Soc. Plast. Eng. 25: 72-74.
CASIDA, J. E., KIMMEL, E. C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and
trialkyleads by liver microsomes. Acta chem. Scand.,
25: 1497-1499.
CATALA, R., ROYO, I. J, & PRIOMO, E. (1971) Tin content of canned
orange juice. Rev. Agroquim. Tecnol. Aliment., 11: 409-417
( Chem. Abstr., (1972) 77: 370, Abstract no. 3974 w).
CAUJOLLE, E., LESBRE, M., & MEYNIER, D. (1954) Sur la toxicité du
tétraméthylstannane et du tétraéthylstannane. C. R. Acad. Sci
(Paris), 239: 556-558.
CENCI, P. & CREMONINI, B. (1969) [Thin-layer chromatography of two
organotin pesticides and their behavior in various types of soil.]
Ind. sac. Ital.. 69: 313-316 (in Italian).
CHAPMAN, A. H., & PRICE, J. W. (1972) Degradation of triphenyltin
acetate by ultraviolet light. Int. Pest Control, 14: 11-12.
CHAPMAN, A. H., DUCKWORTH, M. W., & PRICE, J. W. (1959) The
determination of dialkyltin compounds in polyvinylchloride.
Br. Plast., February, pp. 78, 87.
CHEFTEL, H. (1967) L'étain dans les aliments. Presented at the
Fourth Meeting of the FAO/WHO Codex Committee on Food Additives,
The Hague, 11-15 September 1967, Rome, Food and Agriculture
Organization of the United Nations, 10 pp.
CHISAKA, H., TANIZAKI, Y., & NAGATSUKA, S. (1973) [Determination of
tin and poisonous trace elements in canned juices by neutron
activation.] Radioisotopes, 22: 247-248 (in Japanese).
CHRISTIAN, G. D. & FELDMAN, F. T. (1970) Atomic absorption
spectrography. New York, Wiley, pp. 404-406.
CONINE, D. L., MUHLER, J. C., & FORNEY, R. B. (1972) Acute toxicity of
sodium pentafluorostannite in rats and mice. Toxicol. appl.
Pharmacol., 22: 303-304.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R.
B. (1973) Comparison of the metabolic and renal effects of sodium
pentafluorostannite, sodium fluoride, stannous chloride,
hydrochloric acid, and starvation in the rat. Pharmacologist,
15: 189.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., MUHLER, J.
C., & FORNEY, R. B. (1975) Toxicity of sodium pentafluorostannite,
a new anticariogenic agent. I. Comparison of the acute toxicity of
sodium pentafluorostannite, sodium fluoride and stannous chloride
in mice and/or rats. Toxicol. appl. Pharmacol., 33: 21-26.
CONINE, D. L., YUM, M., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R. B.
(1976) Toxicity of sodium pentafluorostannite. A new
anticariogenic agent. III. 30-day toxicity study in rats.
Toxicol. appl. Pharmacol., 35: 21-28.
CORBIN, H. B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Off. Anal.
Chem., 53: 140-146.
CORBIN, H. B. (1973) Rapid and selective pyrocatechol violet method
for tin. Anal. Chem., 45: 534-537.
COSSA, P., DUPLAY, FISCHGOLD, ARFEL-CAPDEVIELLE, LAFON, PASSOUANT,
MINVIELLE, & RADERMECKER, J. (1958) Encéphalopathies toxiques au
stalinon. Aspects anatomocliniques et alectroencéphalographiques.
Rev. Neurol., 95: 97-108.
COTTON, F. A. & WILKINSON, G. (1972) Advanced inorganic chemistry. A
comprehensive text, London, Wiley-Interscience, 3rd ed.
COTTON, F. A. & WILKINSON, G. (1976) Basic inorganic chemistry, New
York, Wiley.
COUSSEMENT, S. (1972) Analyse des dépôts de fentin acetate sur
feuilles de pomme de terre. Ann. Gembloux, 78: 41-46.
COYLE, C. F. & WHITE, C. E. (1957) Fluorometric determination of tin
with flavonol. Anal Chem, 29: 1486-1488.
CREMER, J. E. (1957) The metabolism in vitro of tissue slices from
rats given triethyltin compounds. Biochem. J., 67: 87-96.
CREMER, J. E. (1958) The biochemistry of organotin compounds: The
conversion of tetraethyltin into triethyltin in mammals.
Biochem. J., 65: 685-692.
CUTTER, H. C., FALLER, W. W., STOCKLEN, J. B., & WILSON, W. L. (1949)
Benign pneumoconiosis in a tin oxide recovery plant. J. And.
Hyg., 31: 139-141.
DACK, G. M. (1955) Chemical poisons in food. In: Food poisoning,
Chicago, University of Chicago Press, pp. 24-25.
DEPARTMENT OF NATIONAL HEALTH AND WELFARE, CANADA (1975) Food and
drug act and regulations office consolidation of the food and
drugs act and the food and drugs regulations with amendments to
December 18, 1975, B. 23.003, Ottawa, Queen's Printer and
Controller of Stationery, pp. 73B.
DEVJATYH, G. G., UMILIN, V. A., & CSINOVOI, Y. N. (1968) [Gas
chromatographic analysis of tetrabutyltin.] Tr. Himii i
Himiceskoj Tehnol., No. d, pp. 82-85 (in Russian).
DITTRICH, T. & COTHERN, C. R. (1971) Analysis of trace metal
particulates in atmospheric samples using X-ray fluorescence.
J. Air Pollut. Control Assoc., 21: 716-719.
DRESSLER, M., MARTINU, V., & JANAK, J. (1971) Response to the alkali
flame ionization detector to silicon, tin and lead compounds.
J. Chromatogr. 59: 429-433.
DRESSLER, M., BARTH, M., & VESPALEC, R. (1975) Chromatographic
determination of organotin compounds in fungistatic plaster.
Silikaty, 4: 301-310.
DRUAULT-TOUFESCO, M. N. (1955) A propos de deux cas d'intoxication
grave par le Stalinon. Bull. Soc. Ophtalmol. Fr., pp. 54-58.
DUCKERING, G. E. (1968) The cause of lead poisoning in the tinning of
metals. J. Hyg. (Lond.), 8: 474-503.
DUNDON, C. C. & HUGHES, J.P. (1950) Stannic oxide pneumoconiosis.
Am. J. Roentgenol., 63: 797-811.
DURBIN, P. W. (1960) Metabolic characteristics within a chemical
family. Health Phys., 2: 225-238.
DURFOR, C. N. & BECKER, E. (1964) Selected data on public supplies of
the 100 largest cities in the United States, 1962. J. Am. Water
Works Assoc., 56: 237-246.
DURUM, W. H. & HAFFTY, J. (1961) Occurrence of minor elements in
water, Washington, DC, pp. 1-11 (Geological Survey Circular
445).
DYER, B. & TAYLOR, G. (1931) Notes from the Report of Public Analysts.
Analyst, 56: 251-253.
ELSEA, J. R. & PAYNTER, O. E. (1958) Toxicological studies on bis
(tri- n-butyltin) oxide. Am. Med. Assoc. Arch. ind. Health,
18: 214-217.
ELTEN, R. (1929) [Study on tin foil used in cheese packeting; rindless
cheese.] Chem.-Ztg., 53: 586 (in German).
ENGBERG, A. (1973) A comparison of a spectrophotometric (Quercetin)
method and an atomic absorption method for the determination of
tin in food. Analyst, 98: 137-145.
EVANS, C. A. & MORRISON, G. H. (1968) Trace element survey analysis of
biological materials by spark source mass spectrometry. Anal.
Chem., 40: 869-875.
EVERETT, G. L, WEST, T. S., & WILLIAMS, R. W. (1974) The determination
of tin by carbon filament atomic absorption spectrometry. Anal.
Chim. Acta, 70: 291-298.
EYRICH, W. (1972) [Determination of tin in canned fruits and
vegetables.] Dtsch. Lebensm.-Rundsch, 9: 280-282 (in German).
FEY, R. (1969) [Heavy metal content in canned fruits and vegetables:
I. Tin and iron in canned asparagus.] Ind. Obst-Geinneseverwert.,
54: 27-33 (in German).
FILER, T. D. (1971) Fluorometric determination of submicrogram
quantities of tin. Anal. Chem., 43: 1753-1757.
FISCHER, H. W. & ZIMMERMAN, R. (1969) Long retention of stannic oxide:
Lack of tissue reaction in laboratory animals. Arch. Pathol.
88: 259-264.
FISH, R. H., KIMMEL, E. C., & CASIDA, J. E. (1975) Bioorganotin
chemistry. Biological oxidation of tributyltin derivatives.
J. Organometal Chem. 93 (1): C1-C4.
FISH, R. H., KIMMEL, E. C., & CASIDA, S. E. (1976) Bioorganotin
chemistry: Biological oxidation of organotin compounds. In:
Zuckerman, J. J., ed. Organotin compounds: New chemistry and
applications, Washington, DC, American Chemical Society,
pp. 197-203 (Advances in Chemistry Series 157).
FONTAN, M. M., VERGER, PERY, LOISEAU, & MULON (1955) Quatre cas dont
deux mortels d'intoxication par le "Stalinon" chez l'enfant. J.
Med. Bordeaux, 132: 399-405.
FAO/WHO (1971) 1970 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United
Nations/World Health Organization, pp. 327-366, 521-542.
FOOD AND DRUG ADMINISTRATION, USA (1971) Code of Federal Regulations,
Title 21 - Foods and Drugs, Washington DC, US Government
Printing Office, pp. 409-410 ( 121, 2602).
FREELAND, G. N. & HOSKINSON, R. M. (1970) Non-aqueous atomic
absorption spectrophotometric determination of organometallic
biocides. Analyst, 95: 579-582.
FREITAG, K-D. & BOCK, R. (1974a) [Analysis of mixtures of tri-, di-,
and monophenyltin compounds and inorganic tin (IV).] Z. anal.
Chem., 5: 337-346 (in German).
FREITAG, K.-D. & BOCK, R. (1974b) Degradation of triphenyltin chloride
on sugar beet plants. Pesticide Sci., 5: 731-739.
FURCHNER, J. E. & DRAKE, G. A. (1976) Comparative metabolism of
radionuclides in mammals - XI. Retention of 113Sn in the mouse,
rat, monkey, and dog. Health Phys., 31: 219-224.
GAINES, T. B. & KIMBROUGH, R. D. (1968) Toxicity of fentin hydroxide
to rats. Toxicol. appl. Pharmacol., 12: 397-403.
GATEHOUSE, B. M. & WILLIS, J. B. (1961) Performance of a simple atomic
absorption spectrometer. Spectrochim. Acta, 17: 710-718.
GAUER, W. O., SEIBER, J. N., & CROSBY, D. G. (1974) Determination of
organotin residues from Plictran in fruit crops by gas-liquid
chromatography. J. agric. food Chem., 22: 252-254.
GAUNT, I. F., COLLEY, J., GRASSO, P., & CREASEY, M., (1968) Acute and
short-term toxicity studies on di- n-butyltin dichloride in rats.
Food Cosmet. Toxicol., 6: 599-608.
GAYRAL, L., LAZORTHES, G., & PLANQUES, J. (1958) Méningo-encé-phalite
hypertensive par intoxication au Stalinon, guérie. Rev. neurol.,
98: 143-144.
GEISSLER, H. & KRIEGSMANN, H. (1964) [Analysis of butyltin compounds
(n-C4H9)nSnX4-n.] Z. Chem., 9: 354-355 (in German).
GEISSLER, H. & KRIEGSMANN, H. (1965) [Liquids used for separation in
some gas chromatographic investigations of some n-butyltin
compounds. Z. Chem., 11: 423-424 (in German).
GELDMACHER -- VON MALLINCKRODT, M. & POOTH, M. (1969) [Simultaneous
determination of twenty five metals and metalloids in biological
materials.] Arch. Toxicol., 25: 5-18 (in German).
GEORGE, G. M., ALBRECHT, M. A., FRAHM, L. J., & McDONNELL, J.P. (1973)
Atomic absorption spectrophotometric determination of dibutyltin
dilaurate in finished feeds. J. Assoc. Off. Anal. Chem.,
56: 1480-1482.
GETZENDANER, M. E. & CORBIN, H. (1972) Residues on apples and pears
from use of Plictran miticide. J. agric. food Chem.,
20: 881-885.
GEYER, R. & ROTERMUND, U. (1969) [Oscillopolarographic determination
of organotin compounds.] Acta chim. Hung., 59 (2): 201-210
(in German).
GHAFOURI, R. (1970) Etude des eaux minérales d'Ax-les-Thermes. Terres
Eaux, 64: 19-27.
GOLDSCHMIDT, V. M. (1958) Tin, In: Muir, A. ed. Geochemistry,
Oxford, Oxford University Press, pp. 391-397.
GORDON, M. (1952) [Trace elements in peat.] Torinachrichten,
3: 12 (in German).
DE GROOT, A. P. (1973) Subacute toxicity of inorganic tin as
influenced by dietary levels of iron and copper. Food Cosmet.
Toxicol., 11: 955-962.
DE GROOT, A. P., FERON, V. J., & TIL, H. P. (1973) Short-term toxicity
studies on some salts and oxides of tin in rats. Food Cosmet.
Toxicol., 11: 19-30.
GRUENWEDEL, D. W. & PATNAIK, R. K. (1973) Model studies regarding the
internal corrosion of tin-plated food cans. IV. Polarographie
investigation of the interaction of tin (II)-ions with L-cysteine.
Chem. Mikrobiol. Technol. Lebensm., 2: 97-101.
GRUNER, J-E. (1958) Lésions du névraxe secondaires ŕ l'ingestion
d'éthyl-étain (Stalinon). Rev. Neurol., 98: 109-116.
GUARDASCIONE, V. & DI BOSCO, M. M. (1967) [Contribution to the
knowledge of occupational disease due to pesticides; Three cases
of acute intoxication by triphenyltin acetate fungicides.]
Lay. Urn., 19: 307-313 (in Italian).
HADZIMICEV, P. (1971) [Natural content of heavy metals (copper, zinc,
tin) in foods from the vegetable belt around Sofia.] Khranit.
Prom., 20: 18-20 (in Bulgarian).
HALL, C. A. & LUDWIG, P. D. (1972) Evaluation of the potential use for
several organotin compounds against the sheep blowfly ( Lucilia
spp.). Vet. Rec., 90: 29-32.
HAMILTON, T. A. (1948) The metabolic properties of plutonium and
allied material Univ. Calif. Rad. Lab. Med. Health Phys. Q., pp.
4-16 (UCRL-270).
HAMILTON, E. I., MINSKI, M. J., CLEARY, J. J., & HALSEY, V. S. (1972)
Comments upon the chemical elements present in evaporated milk for
consumption by babies. Sci. total Environ., 1: 205-210.
HAMILTON, E. I., MINSKI, M. J., & CLEARY, J. J. (1972/1973) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study.
Sci. total Environ., 1: 341-374.
HANNA, W. J. & GRANT, C. L. (1962) Spectrochemical analysis of the
foliage of certain trees and ornamentals for 23 elements. Bull
Torrey Bot. Club, 89: 293-302.
HASEGAWA, T. & SUGIMAE, A. (1971) [Emission spectrographic
determination on trace metals in airborne particulates.]
Jpn. Anal., 20: 640-845 (in Japanese).
HAYASHI, H. (1969) [Square wave polarographic determination of
dissolved tin in canned juice.] Jpn. Anal., 18: 58-61
(in Japanese).
HEATH, D. F. (1963) Some problems in the determination of residues in
plants and animals. In: Proceedings of a symposium; Radiation and
Radioisotopes Applied to Insects of Agricultural Importance,
Athens, 22-26 April 1963, Vienna, International Atomic Energy
Agency, pp. 185-193.
HEATH, D. F., (1967) The retention or triphenyltin and dieldrin and
its relevance to the toxic effects of multiple dosing. In:
Radioisotopic detection of Pesticide Residues, Proceedings of a
Panel, Vienna 1965, pp. 18-26 (Abstract in: Chem. Abstr.,
67: 1828 W).
HEDGES, T. R. & ZAREN, H. A. (1969) Experimental papilledema; A study
of cats and monkeys intoxicated with triethyltin acetate.
Neurology, (Minneapolis), 19: 359-365.
HEINDL, R. A. (1970) Tin. In: Mineral facts and problems, 1970
edition, pp. 759-751. Reprint from: US Dept of the Interior,
Bureau of Mines, Bulletin 650.
HEROK, J. & GOTTE, H. (1963) [Radiometric analysis of triphenyltin
acetate in the metabolism of plants and animals.] Int. J. appl.
Radiat. Isot., 14: 461-479 (in German).
HEROK, J. & GÖTTE, H. (1964) [Radiometric study on the metabolism of
triphenyltin acetate in milk sheep.] Zentralbl. Vet.-Med. A,
11: 20-28 (in German).
HERON, P. N. & SPROULE, J. S. G. (1958) The chemical nature of
fungicidal agents employed in the pulp and paper industry. Indian
Pulp Pap., 12: 516-517.
HESLOP, R. B. & JONES, K. (1976) Inorganic chemistry. A guide to
advanced study. Amsterdam, Elsevier.
HILES, R. A. (1974) Absorption, distribution and excretion of
inorganic tin in rats. Toxicol. appl. Pharmacol., 27: 366-379.
HLEBNIKOVA, M. J. (1957) [Hygienic characteristics of dust in the
production of tin.] Sb. naucnyh Rab. voprogam Gig. Truda i
profpatol., No. 2, pp. 53-83 (in Russian).
HODGE, V. F., SEIDEL, S. L., & GOLDBERG, E. D. (1979) Determination of
tin (IV) and organotin compounds in natural waters, coastal
sediments and macro algae by atomic absorption spectrometry.
Anal. Chem., 51: 1259.
HOFF, J. E. & WILCOX, G. E. (1070) Accumulation of nitrate in tomato
fruit and its effect on derinning. J. Am. Soc. Hortic. Sci.,
95: 92-94.
HORACEK, V. & DEMCIK, K. (1970) [Group poisoning in spraying of field
cultures with Brestan-60 triphenyltin acetate.] Prac. Lék.,
22: 61-66 (in Czech).
HORIO, T. & NAKASEKO, M. (1972) [Spectrophotometric determination of
dissolved tin in canned foods using Salicylidenamion-2-
thiophenol.] Tokyo Inst. Food Technol. J., 17: 212-214
(in Japanese).
HORIO, T., IWAMOTO, Y., & SHIGA, I. (1967) The nitrate corrosion of
canned orange juice drinks in Japan. Communication presented at
the 5th International Congress on Canned Fools, Vienna 3-6,
October, 1967, 12 pp.
HUEY, C., BRINCKMAN, F. E., GRIM, S., & IVERSON, W. P. (1974) The role
of tin in bacterial methylation of mercury. Paper presented of the
International Conference on Transport of Persistent Chemicals in
Aquatic Ecosystems, Ottawa, Canada, 1-3 May, 1974.
IGNATJEVA, M. A., KUZNETSOV, I. G., MIRSKOV, R. G., & VLASOV, V. M.
(1968) [Toxicology of acetylene organotin compounds of the
triethylstannyl range.] Izv. sib. Otdel. Akad. Nauk SSSR,
1 (5): 118-119 (in Russian).
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M, MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumorigenicity
in mice: A preliminary note. J. Natl Cancer Inst.,
42: 1101-1114.
INTERNATIONAL LABOUR OFFICE (1972) Tin alloys and compounds. In:
Encyclopedia of Occupational Health and Safety, Geneva, ILO,
Vol. II, pp. 1407-1409.
IWAMOTO, Y., MIYAZAKI, M., KUNISATO, S., MAEDA, Y., HORIO, T., &
KOMURA, S. (1968) [Studies on abnormal detinning of tin can with
nitrate in foods: (II) Relationship between dissolution on tin and
pH of canned tomato juice.] J. Jpn Soc. Food Nutr., 21: 50-54
(in Japanese).
JANITZ, W. (1971) [Comments on the polarographic method of tin and
lead determination in tinned meat products.] Med. Weter.,
27: 43-45 (in Polish).
JOHANSEN, O. & STEINNES, E. (1969) Determination of tin in geological
material by neutron activation analysis. Analyst, 94: 976-978.
JOHNSON, J. H., YOUNGER, R. L., WITZEL, D. A., & RADELEFF, R. D.
(1975) Acute toxicity of tricyclohexyltin hydroxide to livestock.
Toxicol. appl. Pharmacol., 31: 66-71.
JOVANOVIC, D., STOJADINOVIC, L., & SPASOJEVIC, B. (1967) [Contribution
to the determination of tin in canned meat.] Vojno-sanit. Pregl.,
24: 405-408 (in Serbo-Croat).
KAHN, H. L. & SCHALLIS, J. E. (1968) Improvement of detection limits
for arsenic, selenium and other elements with an argon-hydrogen
flame. At. Absorpt. Newsl., 7: 5-9.
KANISAWA, M. & SCHROEDER, H. A. (1967) Life term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumors in mice. Cancer Res., 27: 1192-1195.
KANISAWA, M. & SCHROEDER, H. A. (1969). Life term studies on the
effect of trace elements on spontaneous rumours in mice and rats.
Cancer Res., 29: 892-895.
KAPPAS, A. & MAINES, M.D. (1976) Tin: A potent inducer of heme
oxygenase in kidney. Science, 192: 60-62.
KAS'YANENKO, I. V. & KUL'SKAYA, O. A. (1969) [Microelement content of
the blood of patients with cancer of stomach and chronic gastritis
secretory insufficiency.] Vopr. eksp. Onkol Respub. Mezhvedom,
SB, 4: 101-107 (in Russian).
KEENAN, R. G. & BYERS, D. H. (1952) Rapid analytical method for air
pollution surveys. Arch. ind. Hyg., 6: 226-230.
KEHOE, R. A., CHOLAK, J. & STORY, R. V. (1940) A spectrochemical study
of the normal ranges of concentration of certain trace metals in
biological materials. J. Nutr., 19: 579-592.
KERK, VAN DER, G. J. M. (1975) [The present use of organotin
compounds.] Chem. Zeitung, 99: 26-32 (in German).
KIMBROUGH, R. D. (1976) Toxicity and health effects of selected
organotin compounds: A review. Environ. Health Perspect.,
14: 51-56.
KIMBROUGH, R. D. & GAINES, T. B. (1971) Hexachlorophene effects on the
rat brain. Arch. environ. Health, 23: 114-118.
KIMMEL, E. C., FISH, R. H., & CASIDA, J. E. (1977) Bio-organotin
chemistry: Metabolism of organotin compounds in microsomal
monoxygenase systems and in mammals. J. Agric. Food Chem.,
25 (1): 1-8.
KIMURA, Y., UEDA, K., NISHIZIMA, M., MAKI, T., WADA, M., TAKAHASHI S.,
YAMADA, T., ARAI, M., & UEMATSU, T. (1970) [Studies on extractable
heavy metals in canned foods: I. Detection of tin and lead from
various sorts of canned baby foods after their opening.] Ann.
Rep. Tokyo Metrop. Inst. Hyg., 22: 95-100 (in Japanese).
KIRK, R. S. & POCKLINGTON, W. D. (1969) The determination of tin and
canned foods with quercetin. Analyst, 94: 71-74.
KLEIN, A., COP, R., CHATRNA, E., & BADALOVA, M. (1970) [The study of
vitamin C stability and growing of tin content in tinned juices.]
Cesk. Hyg., 15: 49-55 (in Czech).
KLEINKOPF, M.D. (1955) Trace element exploration of Maine lake water,
Academic dissertation, Columbia University, 153 pp. (University
Microfilm Pub. 12, 447).
KLEINKOPF, M.D. (1960) Spectrographic determination of trace elements
in lake waters of northern Maine. Geol. Soc. Am. Bull.,
71: 1231-1242.
KLIMMER, O. R. (1963) [Triphenyltin acetate and the toxicology of
organic tin compounds.] Arzneimittel-Forsch., 13: 432-436
(in German).
KLIMMER, O. R. (1964) [Toxicological studies on triphenyltin acetate
(TPZA).] Zentralbl. Vet.-Med., R.A 11: 29-39 (in German).
KLIMMER, O. R. (1969) [The application of organotin compounds reviewed
by the experimental toxicologist.] Arzneimittel-Forsch.,
19: 934-939 (in German).
KOBAYASHI, T. & YADA, M. (1968) [Spectrophotometric determination of
tin in foodstuffs with stilbazo.] J. Food Hyg. Soc. Jpn,
9: 465-468 (in Japanese).
KOCH, J. & FIGGE, K. (1971) [Migration of tin stabilizers from PVC
bottles into beer.] Z. Lebensmittel-Unters. 147: 8-10
(in German).
KOCKIN, D. A., SKORBATOVA, J. C., PEGUSOVA, L. D., & VORONKOV, N. A.
(1969) [Polarographic study on organotin compounds with an acid
content.] Zl. Obshch. Khim., 39: 1777-1781 (in Russian).
KONOVALOV, G. S. & KOLESNIKOVA, T. K. (1969) [Trace elements in
atmospheric precipitation around the Otkaznoe reservoir.]
Gidrokhim Mater., 49: 74-79 (in Russian).
KRUTCHKOFF, D. J., JORDAN, T. H., WEI, S. H. Y., & NORDQUIST, W. D.
(1972) Surface characterization of the stannous fluoride enamel
interaction. Arch. oral Biol., 17: 923-930.
KRYLOVA, N. & BALABUH, A. (1970) [Trace element level in meat.] Myas.
Ind. SSSR, 41: 39 (in Russian).
KUMPULAINEN, J. & KOIVISTOINEN, P. (1977) Advances in tin compound
analysis with special reference to organotin pesticide residues.
Res. Rev., 66: 1-18.
KUNISATO, S. & MIYAZAKI, M. (1972) [Application of plant growth
regulators on fruits and vegetables for processing: I. Nitrate
content in tomato fruits.] J. Hortic. Assoc. Jpn., 41: 411-420
(in Japanese).
KUROSAKI, N. & FUSAYAMA, T. (1973) Penetration of elements from
amalgam into dentin. J. dent. Res., 52: 309-317.
KUTZNER, J. & BROD, K. H. (1971) [Resorption and excretion of tin
after oral administration of tin-113.] Nucl. Med. (Stuttg.),
10: 286-297 (in German).
LAAMANEN, A., LOFGREN, A., & PARTANEN, T. J. (1971) Some trace metals
in particulates in the air of Helsinki and Turku, 1964-1969. Work
Environ. Health., 8: 63-69.
LANDA, K., FEJFUSOVA, J., & NEDOMLELOVA, R. (1973) [The hazards of
organic tin compounds used as fungicides in some industrial
applications.] Prac. Lék., 25: 391-394 (in Czech).
LANIGEN, D. & WEINBERG, E. L. (1976) The use of estertin stabilizers
in PVC. In: Zucherman, J. J., ed. Organotin compounds: New
chemistry and applications. Washington, DC, American Chemical
Society (Advances in Chemistry Series 157).
LECOQ, R. (1954) Contribution ŕ l'étude des effects toxiques du
tétraéthylétain C. R. Hebd. Acad. Sci. (Paris), 239: 678-680.
LEE, E. E., GORANSON, S.S., ENRIONE, R. E., & MORGAN, G. B. (1972)
National air surveillance cascade impactor network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol., 6: 1025-1030.
LINDEMANN, G., PINDEBORG, J. J., & PAULSEN, H. (1959) Recovery of the
rat kidney in fluorosis. Arch. Pathol., 67: 30-33.
LJASKOVSKAJA, Y., & KRASIL'NIKOVA, T. (1961) [Photometric
determination of tin in canned food by reaction with quercetin.]
Myasn. Ind. SSSR 4: 44-45 (in Russian) (English abstract in
Anal. Abstr. (1963) 10: 1177).
LOUNAMAA, J. (1956) Trace elements in plants growing wild on different
rocks in Finland: A semi-quantitative spectrographic survey. Ann.
Bot. Soc. "Vanamo" 29: 4.
LOWRY, R. R. & TINSLEY, I. J. (1972) Determination of tin salts in
fats. J. Am. Oil. Chem. Soc., 49: 508-509.
LUFF, A. P. & METCALFE, G. H. (1890) Four cases of tin poisoning
caused by tinned cherries. Br. med. J., 1: 833-834.
LUKE, C. L. (1956) Photometric determination of tin with
phenylfluorone: Determination of tin in lead and 1% antimony-lead
alloys. Anal. Chem., 28: 1276-1279.
LYLE, W. H. (1958) Lesions of the skin in process workers caused by
contact with butyltin compounds. Br. J. ind. Med., 15: 193-196.
MACINTOSH, R. M. (1968) Tin and tin alloys. In: Kirk-Othmer
encyclopedia of chemical technology, 2nd ed. New York,
Interscience pp. 273-325.
MAGEE, P. N., STONER, H. B., & BARNES, J. M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Pathol. Bacteriol., 73: 107-124.
MAHADEVAIAH, M., GOWRAMMA, R. V., RADAKRISHNAIAH SETTY, G., SASTRY, M.
V., SASTRY, L. V. L., & BHATNAGAR, H. C. (1969) Studies on
variation in tin content in canned mango nectar during storage.
J. food Sci. Technol., 6: 192-196.
MAMONTOVA, A. A. (1940) [Effects of tin on animal organism.] Vopr.
Pitan, 2: 13-25 (in Russian).
MARHOLD, J. V. (1972) [ A collection of results of toxicological
evaluation of substances and products.] Pro Vychovu Vedoucicn
Pracovniku Chemickeho Prumyclu Praha (in Czech).
MARKICEVIC, A. & TURKO, V. (1967) [Lesions due to triphenyltin acetate
"Brestan".] Arh. Hig. Rada, 18: 355-358 (in Serbo-Croat).
MARKS, M. J., WINEK, C. L., & SHANOR, S. P. (1969) Toxicity of
triphenyltin hydroxide. (Vancide KS). Toxicol. appl. Pharmacol.,
14: 627.
MASON, B. (1966) Principles of geochemistry, 3rd ed., New York,
Wiley.
MAZAEV, V. T. & KOROLEV, A. A. (1969) [Experimental standardization of
the maximum permissible concentration of the dichlorbutyltin in
surface water.] Prom. Zagrjaznenija Vodoemov, No 9, pp. 15-37
(in Russian).
MAZAEV, V. T. & SLEPNINA, T. G. (1973) [Experimental data on hygienic
standardization of dibutyltin sulfide in water bodies.] Gig. i.
Sanit., 8: 10-13 (in Russian).
MAZAEV, V. T., LOSEV, N. I., & VOINOV, V. A. (1968) [On some
vegetative reactions and on electrical activity of the brain
cortex after intoxication with organic derivatives of tin.] Bull.
exp. Biol. Med., 11: 72-74 (in Russian).
MAZAEV, V. T., KOROLEV, A. A., & SKACKOVA, I. N. (1971) Experimental
investigation of the toxic effect of tetraethyltin and
dichlorodibutyltin on the animal organism. J. Hyg. Epidemiol.
Microbiol. Immunol., 15: (1): 115-120.
MAZAEV, V. T., GOLOVANOV, O. V., IGUMOV, A. S., & TSAY, V. N. (1976)
[Problem of the transformation of organotin compounds in a water
medium.] Gig. i Sanit, 3: 17-20 (in Russian).
MEINEMA, H. A., BURGER-WIERSMA, T., VERSLUIS-DE-HAAN, G. & GEVERS, E.
Ch. (1978) Determination of trace amounts of butyltin compounds in
aqueous systems by gas chromatography/mass spectrometry. Environ.
Sci. Technol., 12 (3): 288-293.
MIDWEST RESEARCH INSTITUTE (1977) Assessment of the need for the
character of, and the impact resulting from limitations on
selected organotins. Phase I Assessment of the need for
limitations on organotins. Washington, DC, US Environmental
Protection Agency, EPA Contract No. 68-01-4313).
MIJATOVIC, M. (1972) [Chronic hepatitis due to exposure to Brestan.]
Jugosl. inostr. Dok. Zast. Radu, 8:3-9 (in Serbo-Croat).
MIKI, E. & FUKUI, Y. (1971) [Square wave polarographic determination
of tin in canned fruit.] Sci. Rep. Agric. Fac. Kagawa Univ.,
22: 35-40 (in Japanese).
MITCHELL, R. C. (1948) The spectrographic analysis of soils, plants,
and related materials. Bureau of Soil Science, pp. 1-173
(Technical Communication No. 44).
MONIER-WILLIAMS, G. W. (1949) Trace elements in food, London,
Chapman & Hall, pp. 138-161.
MOSKALEV, Ju. I. (1964) [Studies on the distribution of 113Sn. In:
Distribution, biological effects and rapid excretion of
radioactive isotopes.] Moscow, Medicina, pp. 78-82 (in Russian).
MULAY, I. L., RAY, R., KNOX, B. E., SUHR, N.H., & DELANEY, W. E.
(1971) Trace metal analysis of cancerous and non-cancerous human
tissues. J. Natl. Cancer Inst., 47: 1-13.
NAKAHARA, T., MUNEMORI, M., & MUSHA, S. (1972) Atomic absorption
spectrometric determination of tin in premixed inert gas
(entrained air) hydrogen flames. Anal. Chim. Acta, 62: 267-278.
NAKAMURA, Y. & KAMIWADA, S. (1973) [Colorimetric determination of tin
by decomposition and tin-phenylfluorene complex.] J. food Hyg.,
14: 352-356 (in Japanese).
NATIONAL ACADEMY OF SCIENCES. WASHINGTON (1973) Toxicants occurring
naturally in foods, 2nd ed., Washington, DC, NAS, pp. 63-64.
NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH (1976) Criteria
for a recommended standard ... occupational Exposure to organotin
compounds, Washington, DC, US Government Printing Office,
187 pp.
NATIONAL WATER QUALITY NETWORK (1960) Annual compilation ol data,
1 October 1959-30 September 1960, Washington, DC, US Department
of Health, Education and Welfare, Public Health Service,
pp. 353-356.
NEHRING, P. (1972) [Tin in peach preserves.] Ind. Obst.
Gemusseverwert., 57: 489-492 (in German).
NELSON, A. A. (1954) Approximate relation of parts per million in diet
to mg/kg/day. Assoc. Food Drug Off. Q. Bull, 18: 66.
NEUBERT, G. (1964) [Analysis of organotin stabilizers.] Z. anal.
Chem., 203: 265-272 (in German).
NEUBERT, G. & ANDREAS, H. (1976) [The analysis of organotin
compounds.] Z. anal. Chem., 280: 31 (in German).
NEUBERT, G. & WIRTH, H. O. (1975) [The analysis of organotin
stabilizers.] Z. anal. Chem., 273:19-23 (in German).
NEUMANN, W. P. (1970) The organic chemistry of tin, London, Wiley.
NEWMAN, E. J. & JONES, P. D. (1966) Separation and determination of
small amounts of tin. Analyst, 91: 406-410.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effects of orally
administered plasticizers in the rat. Toxicol. appl. Pharmacol.,
26: 253-259.
NISHIJIMA, M., UEDA, K., MAKI, T., WADA, M., TAKAHASHI, S., KIMURA,
Y., YAMADA, T., NAKAJIMA, K., & FUKUDA, T. (1971) [Studies on
heavy metals dissolved in canned foods. (II) Detection of tin and
lead from various canned fruits after their opening.] Ann. Rep.
Tokyo Metrop. Inst. Hyg., 23: 189-196, (in Japanese).
OBRUSNIK, I. (1969), Determination of indium and tin by activation
analysis using replacement substoichiometry. Talanta,
16: 563-566.
ONISHI, H. & SANDELL, E. B. (1957) Meteoritic and terrestrial
abundance of tin. Geochim. Cosmochim. Acta, 12: 262-270.
OYANGUREN, H., HADDAD, R., & MAASS, H. (1958) Stannosis. Benign
pneumoconiosis owing to inhalation of tin dust and fume. I.
Environmental and experimental studies. Ind. Med. Surg.,
27: 427-431.
PAL, B. K. & RYAN, D. E. (1969) Fluorescence and metallic valency
states. Part III. Determination of tin. Anal Chim. Acta,
48: 227-231.
PATE, B. D. & HAYS, R.L. (1968) Histological studies of testes in rats
treated with certain insect chemosterilants. J. econ. Entomol.
61: 32-34.
PEARLMAN, R. S., HEFFERREN, J. J., & LYON, H. W. (1970) Determination
of tin in enamel and dentin by atomic absorption spectroscopy.
J. dent. Res., 49: 1437-1443.
PEDLEY, F. G. (1927) Chronic poisoning by tin and its salts. J ind.
hyg., 9: 43-47
PELIKAN, Z. & CERNY, E. (1968a) [The toxic effect of tri-n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292
(in German).
PELIKAN, Z., & CERNY, E. (1968b) The effect of low doses of bis-
(tri-n-butyltin) oxide on the skin of rats. Berufsdermatosen,
16: 340-349.
PELIKAN, Z., & CERNY, E. (1969) Toxic effect of bis- (tri-n-butyltin)
oxide (TBTO) on the skin of rats. Berufsdermatosen, 17: 305-316.
PELIKAN, Z. & CERNY, E. (1970a) The toxic effects of some di- and
mono-n-octyltin compounds on white mice. Arch. Toxikol.,
26: 196-202.
PELIKAN, Z. & CERNY, E. (1970b) Toxic effects of some mono-n-butyltin
compounds on white mice. Arch. Toxikol., 27: 79-84.
PENDERGRASS, E. P. & PRYDE, A. W. (1948) Benign pneumoconiosis due to
tin oxide. J. ind. Hyg., 30: 119-123.
PEREL'MAN, A. I. (1972) [Geochemistry of elements in the zone of
hypergenesis.] Moscow, Nedra (in Russian).
PERRY, H. M. & PERRY, E. F. (1959) Normal concentrations of some trace
metals in human urine: Changes produced by ethylenediamine
tetraacetate. J. clin. Invest., 38: 1452-1463.
PESME, P. (1955) Complicationes oculaires observées chez quatre
enfants intoxiqués par le "Stalinon". Arch. Fr. Pediat.,
12: 327-328.
PIVER, W. T. (1973) Organotin compounds: Industrial applications and
biological investigation. Environ. Health. Perspect., 4: 61-79.
PORTRETNYJ, V. P., MALYUTA, V. F., & CHUIKO, V. T. (1973)
[Determination of tin in water, after concentration by
co-precipitation, by anodic stripping voltametry.] Zh. anal.
Khim., 28: 1337-1340 (in Russian).
PRICE, W. J. & ROOS, J. T. H. (1969) Analysis of fruit juice by atomic
absorption spectrophotometry. I -- The determination of iron and
tin in canned juice. J. Sci. food Agric., 20: 437-439.
PRULL, G. & ROMPEL, K. (1976) [Neurological and cerebro-electrical
disturbances in acute poisoning due to organotin compounds.]
Nervenarzt, 41: 516-520 (in German).
REMY, H. (1956) Treatise on inorganic chemistry, Amsterdam,
Elsevier, Vol. I.
RIDLEY, W. P., DIZIKES, L. J., & WOOD, J. M. (1977) Biomethylation of
toxic elements. Science, 197: 329-332.
RITTENHOUSE, G., FULTON, R. B., III GRABOWSKI, R. J., & BERNARD, J. L.
(1969) Minor elements in oil field waters. Chem. Geol., 4:
189-209.
ROBERTS, R. M. G. & EL KAISSI, F. (1968) Base-catalyzed clearage of
allylic derivatives of group IV A elements. J. Organometal.
Chem., 12 (1): 79-88.
ROBERTSON, A. J. (1960) Pneumoconiosis due to tin oxide. In: King, E.
J. & Fletcher, C. M. ed Symposium on industrial pulmonary
diseases, Boston, Little-Brown, pp. 168-184.
ROBERTSON, A. J. & WHITAKER, P. H. (1957) Radiological changes in
pneumoconiosis due to tin oxide. J. fac. Radiol., 6: 224-233.
ROBINSON, I. M. (1969) Effects of some organotin compounds on tissue
amine levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROE, F. J. C., BOYLAND, E., & MILLICAN, K. (1965) Effects of oral
administration of two tin compounds to rats over prolonged
periods. Food Cosmet. Toxicol., 3: 277-280.
RONDEPIERRE, J., TRUHAUT, R., GUILLY, P., HIVERT, P. E., & BARANDE, I.
(1958) Sur une intoxication aiguë par un dérivé organiqué de
l'étain. Rev. neurol., 98: 135-140.
ROSE, M. S. (1969) Evidence for histidine in the triethyltin-binding
site of rat haemoglobin. Biochem. J., 111: 129-137.
ROSS, W. J. & WHITE, J. C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33: 421-424.
SAITO, I. & ENDO, M. (1970) [Analytical studies on inorganic elements
of gall stones.] Hokkaido Igaku Zasshi, 14: 334-337
(in Japanese).
SATO, N., TSURUTA, K., KA1VIADA, I., NARITA, S., & ABUKAWA, H. (1973).
[Studies on dissolved tin in canned foods by atomic absorption
spectrophotometry.] J. Food Hyg Soc. Jpn, 14: 245-248
in Japanese).
SAVAGE, W. (1939) Canned foods in relation to health, Lancet
231 (2): 991-995.
SAWYER, R. (1967) Determination of dialkyltin stabilizers in aqueous
extracts from PVC and other plastics. Analyst, 92: 569-574.
SCHALLIS, J. E. & KAHN, H. L. (1968) The determination of tin in
lubricating oils with a nitrous oxide-acetylene flame.
At. Absorpt. Newsl., 7: 84.
SCHRAMEL, P., SAMSAHL, K., & PAVLU, J. (1973) Some determinations of
Hg, As, Se, Sb, Sn, and Br in water plants, sediments and fishes
in Bavarian rivers. Int. J. environ. Stud., 5: 37-40.
SCHROEDER, H. A. & BALASSA, J. J. (1961) Arsenic, germanium, tin and
vanadium in mice: Effects on growth, survival and tissue levels.
J. Nutr., 92: 245-253.
SCHROEDER, H. A., BALASSA, J. J., & TIPTON, I. H. (1964) Abnormal
trace metals in man: Tin. J. chron. Dis., 17: 483-502.
SCHROEDER, H. A., KANISAWA, M., FROST, D. V., & MITCHENER, M. (1968)
Germanium, tin and arsenic in rats: Effects on growth, survival,
pathological lesions and life span. J. Nutr., 96: 37-45.
SCHROLL, E. VON & KRACHSBERGER, H. (1970) [Study on the geochemistry
of impurities in atmospheric precipitates in the Vienna
metropolitan area.] Radex Rundsch., 5: 331-341 (in German).
SCHRYVER, S. B. (1901) Some investigations on the toxicology of tin,
with special reference to the metallic contamination of canned
foods. J. Hyg., 9: 253-263.
SCHULER, P., CRUZ, E., GUIJON, C., MATURANA, V., & VALENZUELA, A.
(1958) Stannosis. Benign pneumoconiosis owing to inhalation of tin
dust and fume. II. Clinical study. Ind. Med. Surg., 27: 432-435.
SCHWARZ, K. (1971) Tin as an essential growth factor for rats. In:
Mertz, W. & Comatzer, W. E., ed. Newer trace elements in
nutrition, New York, Marcel Dekker, pp. 313-325.
SCHWARZ, K. (1974) Recent dietary trace element research, exemplified
by tin, fluorine and silicon, Fed. Proc., 33: 1748-1757.
SCHWARZ, K., MILNE, D. B., & VINYARD, E. (1970) Growth effects of tin
compounds in rats maintained in a trace element-controlled
environment. Biochem. biophys. Res. Commun., 40: 22-29.
SCOTT, W. J. & STEWART, D. F. (1944) The influence of dissolved tin on
the growth of Clostridium botulinum in canned vegetables.
J. Counc. Sci. Ind. Res. Aust., 17: 16-22.
SEINEN, W. & WILLEMS, M. I. (1976) Toxicity of organotin compounds. I.
Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di-n-octyltin dichloride. Toxicol. appl. Pharmacol., 35: 63-75.
SEINEN, W., VOS, J. G., SPANJE, I. van, SNOEK, M., BRANDS, R., &
HOOYKAAS, H. (1977a) Toxicity of organotin compounds. II.
Comparative in vivo and in vitro studies with various
organotin and organolead compounds in different animal species
with special emphasis on lymphocyte cytotoxicity. Toxicol. appl.
Pharmacol., 42: 197-212.
SEINEN, W., VOS, J. G., KRIEKEN, R. van, PENNINKS, A., BRANDS, R., &
HOOYKAAS, H. (1977b) Toxicity of organotin compounds, III.
Suppression of thymus-dependent immunity in rats. by di-n-butyltin
dichloride and di-n-octyltin dichloride. Toxicol. appl.
Pharmacol., 42: 213-224.
SHELDON, A. W. (1975) Effects of organotin anti-fouling coatings on
man and his environment. J. Paint Technol., 47: 54-58.
SHIRAISHI, Y., KUZUHARA, Y., & SUENAGA, S. (1972) [Determination of
tin in food by atomic absorption spectrometry.] J. Food Hyg. Soc.
Jpn 13: 97-100 (in Japanese).
SHIRASU, Y. (1970) Acute and sub-chronic toxicology studies of Dowco
213 in mice and rats. Test report on the sub-acute toxicity of
an insecticide, Dowco 213, Tokyo, Animal Pharmacology Institute of
Physical and Chemical Research, 11 pp. (in Japanese).
SHONO, T. & MATSUMURA, Y. (1970) Organic synthesis by electrolysis VI.
Anodie oxidation of aryleyclopropanes. J. org. Chem.,
35: 4157-4160.
SKACKOVA, I. N. (1967) [Hygienic substantiation of the maximum
permissible concentration of tetraethyltin in water bodies.] Gig.
i Sanit., 4: 11-17 (in Russian).
SKEEL, RL., & BRICKER, C. E. (1961) Spectrophotometric determination
of dibutyltin dichloride. Anal. Chem., 33: 428-431.
SMITH, J. D. (1970) The spectrophotometric determination of microgram
amounts of tin with phenylfluorone. Analyst, 95: 347-350.
SODERQUIST, C. J. & CROSBY, D. G. (1978) Determination of triphenyltin
hydroxide and its degradation products in water. Anal. Chem.,
50: 1435-1439.
STANCULESCU, C., MARIN, E., SANDULESCU, C., ILLE, C., TRICIA, A.,
ILLIESCU, M., PETROVICI, C., & POPESCU, P. (1972) [Copper, lead,
tin and arsenic content in canned fish after storage. II. Canned
fresh water fish.] Lucr. Inst. Cercet. Projiect. Ailment.,
10: 295-309 (in Romanian).
STOCKDALE, M., DAWSON, A. P., & SELWYN, M. J. (1970) Effects of
trialkyltin and triphenyltin compounds on mitochondrial
respiration. Eur. J. Biochem., 15: 342-351.
STONE, O. J., & WILLIS, C. J. (1968) The effect of stannous fluoride
and stannous chloride on inflammation. Toxicol. appl. Pharmacol.,
13: 332-338.
STONER, H. B. (1966) Toxicity of triphenyltin. Br. J. ind. Med.,
23: 222-229.
STONER, H. B., BARNES, J. M., & DUFF, J. I. (1955) Studies on the
toxicity of alkyl tin compounds. Br. J. Pharmacol., 10: 16-25.
STOROZEVA, H. H. (1963) [Contents of lead and tin in human teeth in
normal and caries cases.] Stomatologia, 1: 44-48 (in Russian).
STUDER, R. K., SIEGEL, B. A., MORGAN, J., & POTCHEN, E. J. (1973)
Dexamethasone therapy of triethyltin induced cerebral edema. Exp.
Neurol., 38: 429-437.
SUGIMAE, A. (1974) Emission spectrographic determination of trace
elements in airborne particulate matter. Anal. Chem.
46: 1123-1125.
SUZUKI, K. (1971) Some new observations in triethyltin intoxication of
rats. Exp. Neurol., 31: 207-213.
SVENSSON, V. (1975) [Tin poisoning caused by canned peaches.] Hyg.
och Miljö, No. 6, 25-27. (in Swedish).
TABOR, E. C. & WARREN, W. V. (1958) Distribution of certain metals in
the atmosphere of some American cities. Arch. ind. Hyg.,
17: 145-151.
THEUER, R. C., MAHONEY, A. W., & SARETT, H. P. (1971) Placental
transfer of fluoride and tin in rats given various fluoride and
tin salts. J. Nutr., 101: 525-532.
THOMPSON, K. C. & THOMERSON, D. R. (1974) Atomic absorption studies on
the determination of antimony, arsenic, bismuth, germanium, lead,
selenium, tellurium and tin by utilizing the generation of
covalent hydrides. Analyst, 99: 595-601.
TIHONOVA, Z. I. & ZORE, V. A. (1968) [Spectroscopic determination of
manganese, copper, aluminium, lead and tin in certain vegetables
and berries.] Gig. i Sanit., 33: 384-386 (in Russian).
TIPTON, I. H. (1960) The distribution of trace metals in the human
body. In: Seven, M. T., ed. Metal binding in medicine,
Philadelphia, Montreal, Lippincott, pp. 27-42.
TIPTON, I. H., COOK, M. J. STEINER, R. L., BOYLE, C. A., PERRY, H. M.,
& SCHROEDER, H. A. (1963) Trace elements in human tissue. Part I
Methods. Health Phys., 9: 89-101.
TIPTON, I. H., STEWART, P. L., & MARTIN, P. G. (1966) Trace elements
in diets and excreta. Health Phys., 12: 1683-1689.
TIPTON, I. H., STEWART, P. L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys.,
16: 455-462.
TJURIN, Y. M. & FLEROV, V. N. (1970) [Study of adsorption effects
under electro-reducing of organotin compounds on mercury.]
Elektrochim., 6: 1548-1552, (in Russian).
TJURIN, Y. M., FLEROV, V. N., & NIKITINA, Z. A. (1969) [On the
mechanism of electro-reducing of the dialkyltin chloride on the
mercury drip electrode.] Elektrochim., 5: 903 (in Russian).
TONGE, B.C. (1965) The gas chromatographic analysis of butyl- octyl-,
and phenyltin halides. J. Chromatogr., 19: 182-184.
TORACK, R., GORDON, J., & PROKOP, J. (1969) Pathobiology of acute
tri-ethyltin intoxication. Int. Rev. Neurobiol., 12: 45-86.
TROMBETTI, G. & MAINI, P. (1970) Determination of tricyclohexyltin
hydroxide residues in apples and pears. Pestic. Sci.,
1: 144-149.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.P., NGUYEN PHU LICH, VAN DEN
DRIESSCHE, J., GUESNIER, L. R., & MORIN, N. (1976) Contribution to
the toxicological and pharmacological study of tributyltin oxide.
Eur. J. Toxicol. environ. Hyg., 9 (1): 31-40.
TUTTLE, R. F., VOGT, J. R., & PARKINSON, T. F. (1970) Neutron
activation analysis of trace elements in airborne particulates.
In: Proceedings of a Symposium on Nuclear Technology and
Environmental Pollution, Salzburg, Vienna, International Atomic
Energy Agency, pp. 119-137 (Paper No. IAEA-SM-142a/4).
UDRIS, J. (1971) The analysis of tin stabilizers used in
poly(vinylchloride) compositions. Analyst, 96: 130-139.
UNITED NATIONS (1977) United Nations yearbook of industrial
statistics: Commodity production data 1966-1975, Vol. II,
pp. 498-500.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Air quality data for
metals, 1968 and 1969 from the National Air Surveillance
Networks, Research Triangle Park, USEPA, June 1973.
VERNON, F. (1974) The fluorometric determination of triphenyltin
compounds. Anal. Chim. Acta, 71: 192-195.
VINOGRADOV, A. P. (1953) The elementary chemical composition of
marine organisms, Memoir Sears Foundation for Marine Research,
New Haver, Yale University, pp. 16, 211, 240, 266, 360, 366, 535.
VINOGRADOV, A. P. (1956) Regularity of distribution of chemical
elements in the earth's crust. Geokhimiya, 1: 6-52.
VINOGRADOV, A. P. (1962) Average content of chemical elements in the
principal types of igneous rocks of the earth's crust.
Geokhimiya, 555-541.
VIZGIRDA, R. J. (1972) Organotin antifoulants. Paint and varnish
Produc., 62 (12): 12-28.
WALTERS, M. & ROE, F. J. C. (1965) A study on the effects of zinc and
tin administered orally to mice over a prolonged period. Food
Cosmet. Toxicol., 3: 271-276.
WARBURTON, S., UDLER, W., EWERT, R. M., & HAYNES, W. S. (1962)
Outbreak of foodborne illness attributed to tin. Public Health
Rep. (Wash.), 77: 798-800.
WEAST, R. C., ed. (1974) Handbook of Chemistry and Physics, 55th ed.
Cleveland, CRC Press.
WHO Technical Report Series, No. 515, 1973 ( Shistosomiasis control:
report of a WHO Expert Committee). pp. 30-32.
WHO (1975) 1974 evaluations of some pesticide residues in food,
pp. 155-158 (WHO Pesticides Residues Series No. 4).
WHO (1976) Environmental Health Criteria 1: Mercury. Geneva, WHO,
132 pp.
WIECZOREK, H. (1969) [The quantitative determination of organotin
compounds by means of quercetin chelates.] Dtsch.
Lebensmitt-Rundsch., 3: 74-78 (in German).
WILLIAMS, D. J. & PRICE, J. W. (1960) Paper chromatography of some
organotin compounds. Analyst, 85: 579-582.
WODSAK, W. (1967) Tin content of condensed milk. Angew. Chem. Int.
Ed., 6: 885-886.
WOGGON, H. & JEHLE, D. (1973) [The inverse voltametric determination
of biocidal organotin compounds.] Die Nahrung, 17:739-748
(in German).
WOGGON, H. & JEHLE, D. (1975) [The residual analysis of biocidal
organo-tin compounds by means of inverse voltametric methods.]
Die Nahrung, 19:271-275 (in German).
WOGGON, H, SAUBERLICH, H., & UHDE, W. J. (1972) [A contribution to the
determination (anodic stripping method) of organotin compounds and
their degradation and decomposition products.] Z. anal. Chem.,
260: 268-274 (in German).
WOIDICH, H. & PFANNHAÜSER, W. (1973) [Distribution of tin between the
solid and liquid contents of cans of vegetables and fruits.]
Dtsch. Lebensmitt-Rundsch., 69:224-226 (in German).
WOOD, J. M. (1974) Biological cycles for toxic elements in the
environment. Science, 183: 1049-1052.
WOOD, J. M., SEGALL, H. J., RIDLEY, W. P., CHEH, A., CHUDYK, W., &
THAYER, J. S. (1975) Metabolic cycles for toxic elements in the
environment. In Hutchinson, T. C., ed. Symposium Proceedings Vol.
I, International Conference on Heavy Metals in the Environment.
Toronto, Canada, pp. 49-68.
WOOD, J. M., CHEH, A., DIZIKES, L. J., RIDLEY, W. P., RAKOW, S., &
LAKOWICZ, J. R. (1978) Mechanisms for biomethylation of metals and
metalloids. Fed. Proc., 37: 16-21.
YUM, N.M., CONINE, D. L., MARTZ, R. C., FORNEY, R. B., & STOOKEY, G.
K. (1976) Renal tubular injury in rats induced by sodium
pentalfluorostannite, a new anticariogenic agent. Toxicol. appl.
Pharmacol., 37: 363-370.
ZOHM, H. (1972) [A new method for determination of tin and iron in
canned vegetables and fruit juices.] Z. Lebensmitt. -Unters.,
149: 30-33 (in German).
3.5 Tin-containing Wastes
Gases and fumes containing tin as well as sulfur dioxide and other
contaminants, and both soluble and insoluble tin-containing waste
materials such as water soluble salts, muds, and slags are produced
during various smelting, refining, and detinning operations. Solid
domestic and other wastes which may be dumped, incinerated, used for
land fills or composting, contain much tin, and used cans, aerosol
containers, and other miscellaneous tin-containing produces in solid
wastes account for 10-15% of tin-plated steel. The percentage of
plastics in solid wastes is increasing yearly and includes PVC
containing organotin compounds.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATIONS
4.1 Transport and Bioconcentration
Tin concentrations in air are usually low except in the
neighbourhood of some industrial sources (section 5.1). Similarly, it
has not been consistently detected in all soils, plants, and waters
(sections 5.2 and 5.3). This seems go support a geochemical
classification placing tin in a group of elements with a low rate of
migration in soils and waters (Perel'man, 1972; Bens et al., 1976).
However, it is widely used and Wood et al. (1975) have included it
among elements that are relatively accessible in the environment. A
biological cycle for tin has been recently proposed by Ridley et al.
(1977) (section 4.2).
There is a lack of information concerning the environmental
transport of organometallic tin compounds. It appears, however, that
the vapour pressures of some organotin compounds are high and that
environmental mobility is possible although there are no data to
confirm this deduction. Some organotin compounds seem to be well
adsorbed in the soil and they have a low solubility in water (Heron &
Sproul, 1958). A laboratory soil-leaching study (Barnes et al, 1973)
indicated that triphenyltin is strongly attached to the soil. In
natural waters, organotin compounds would be primarily adsorbed on the
suspended particles and sediments (Schramel et al., 1973). More recent
studies on the determination of tin and organotin compounds in natural
waters have been reported by Braman & Tompkins (1979) and Hodge et al.
(1979).
Little reliable information exists concerning the bioconcentration
of tin and its derivatives. Schroeder et al. (1964) reported that the
presence of tin in phosphate fertilizers originating from marine
phosphate suggested that marine animals in the Pleistocene era
absorbed tin from the sea. Although tin is present in seawater in
concentrations of up to about 3 µg/litre (Mason, 1966; Vinogradov,
1953), there are few reports of its occurrence in marine algae,
plankton, bacteria, flowering plants, protozoa, sponges,
coelenterates, echinoderms, crustacea, and most fishes. However, Bowen
(1966) noted tin levels of 0.2-20 mg/kg in certain marine organisms
and accumulation of tin by the sponge Terpios zeteki was also
reported by Bowen & Sutton (1951).
4.2 Environmental Chemistry of Tin
Because of the considerably higher toxicity of some
organo-metallic tin compounds compared with inorganic forms of tin
(section 7), the possibility of biomethylation of tin is obviously of
considerable interest (Wood, 1974). A possible mechanism of such
bio-methylation has been proposed (Ridley et al., 1977; Wood et al.,
1978). Recent laboratory studies have indicated that the methylation
of tin by methylcobalamina (CH3B12) requires a one electron
oxidation of SnII to a SnIII radical, which can take place in the
presence of FeIII (SnIV would, of course, require a single electron
reduction). The stannyl radical (SnIII) can then react with CH3-B12
(CoIII) to produce (under conditions of high chloride ion
concentration) CH3-SnCl2 and reduced cobalamin, containing CoII
(Wood et al., 1978). A demonstration that methylation of tin takes
place in a strain of Pseudomonas bacteria found in the Chesapeake
Bay, USA, is the laboratory work with these bacteria carried out by
Huey et al. (1974). However, the methylated tin species was not
identified. Incubation of mercury(II) in the presence of tin(IV)
resulted in enhanced formation of methylmercury, and the authors
considered that methylmercury might have been transferred from the
biologically methylated tin to the mercury(II) ion. Subsequently,
Brinckman & Iverson (1975) proposed a "mercury-tin cross-over" system,
which may have some real basis as indicated by Schramel et al. (1973),
who found that mercury and tin accumulated together in some water
plants in Bavarian rivers.
4.3 Degradation of Organometallic Tin Compounds
Organotin compounds may be degraded both chemically and
biochemically. Hydrolytic decomposition occurs at rather extreme pH
values (< 1 or > 13) unless there are other catalytic
influences. Nevertheless, some authors consider that it could occur
fairly rapidly in an aquatic environment, although the environmental
pH is usually between 4 and 10 (Sheldon, 1975, Vizgirda 1972). This
could be the case, if photochemical decomposition were also involved.
Indeed, photochemical decomposition of triphenyltin acetate by
ultraviolet irradiation has been demonstrated under laboratory
conditions by Chapman & Price (1972) and Barnes et al. (1973). Most
likely, under environmental conditions, the chemical and
photo-chemical degradation of organotin compounds is combined with
biochemical degradation, or degradation may be entirely biological.
Some available information, mainly on triphenyltin, tricyclohexyltin,
and tributyl tin compounds, is included in the following summary.
a A form of vitamin B12 (cobalamin) in which the methyl carbon
is directly bonded to the cobalt which is coordinated to the corrin
ring system in the vitamin B12 structure.
Bruggemann & Klimmer (1964) and BrÜggemann et al. (1964a,b)
reported that triphenyltin acetate on sugar beet leaves was rapidly
broken down during the silage process. Within 5 weeks, triphenyltin
acetate, originally present at a concentration of 2470 mg/kg of fresh
leaves, had degraded completely. It was not established whether
degradation was due to microbial action or resulted from the low pH
(3.5-4.0) of the silage process. The authors also found that
triphenyltin acetate was not broken down rapidly by microorganisms
during its passage through the rumen and intestines of cattle. Similar
results in feeding experiments with sheep were reported by Herok &
Götte (1963).
Using thin-layer chromatography, Cenci & Cremonini (1969) found
that triphenyltin acetate and hydroxide mixed into soil (80 mg/kg of
soil) disappeared in 3-10 days, and 3 days, respectively, but the
degradation products were not identified and the participation of
microorganisms in the process was not established.
Akagi & Sakagami (1971) reported that, under their experimental
conditions, ultraviolet irradiation of a solution of triphenyltin
chloride yielded a mixture of triphenyl-, diphenyl-, and phenyltin as
well as inorganic tin compounds, within 6 h. Similarly, irradiation of
trialkyltin compounds resulted in a mixture of di- and monoalkyltin
compounds and inorganic tin also within a period of 6 h. Total
degradation of triphenyltin to inorganic tin required irradiation for
more than 400 h. Chapman & Price (1972) investigated the degradation
of an agricultural fungicide containing triphenyltin acetate, which
had been found to disappear within a few days of spraying on crops,
probably as a result of weathering and sunlight. They exposed the
compound in thin layers on glass to ultraviolet light and found that
the rate of degradation was higher at lower wavelengths and was
independent of layer thickness (5-10 mµ). This was also true for the
intermediate diphenyl- and phenyltin compounds and for the parent
compound. When triphenyltin acetate has irradiated for 60 h
(wavelength above 235 nm: intensity 120 W/m2: thickness 10 µm), about
10% of the triphenyltin compound remained unchanged, about 30%
occurred as diphenyltin, 15% as phenyltin compounds, and approximately
45% was degraded to inorganic tin in the form of hydroxides or
hydrated oxides. Similar results were obtained by Barnes et al.
(1973), who also studied breakdown at concentrations of 5-10 mg/kg
using triphenyltin acetate in which the phenyl groups had been
labelled with 14C. Degradation was monitored by measuring the
evolution of radioactive carbon dioxide; a half-time of about 140 days
was determined. Since no 14CO2 was evolved when the loam was
heat-sterilized, it was concluded that the degradation process was due
to microbial action.
The decomposition of triphenyltin chloride on sugar beet leaves,
as reported by Freitag & Bock (1974b) gave the expected series of
degradation products, the final degradation product being tin oxide.
After 42 days, about 19% of triphenyltin chloride had undergone
degradation.
In studies by Starnes (unpublished data)a and by Smith et al.
(unpublished data)b quoted by Getzendaner & Corbin (1972),
tricyclohexyltin hydroxide (the active ingredient of a commercial
miticide) exposed on thin-layer chromatographic plates to a sun lamp
yielded dicyclohexyltin oxide and cyclohexylstannoic acid with further
degradation to inorganic tin. It has also been shown that tin residues
on apples and pears treated with tricyclohexyltin hydroxide
(commercial miticide) remained almost constant at a level of
0.1-0.2 mg/kg (Getzendaner & Corbin, 1972). This concentration was
relatively independent of the total number of applications and, within
a month of the final application, independent of the time between the
last application and harvest.
Mazaev et al. (1976) reported the degradation of bi(tributyltin)
oxide (TBTO), dibutyltin bis isooctylmercaptoacetate (BuIOMA),
tributyltin methacrylate, diethyltin dioctanoate and dioctyltin bis
(isobutylmaleate), and provided estimates for the half-times of these
compounds for different aqueous media. In distilled water at 20°C,
values ranged from 1.14 days (BuIOMA) to 18.2 days (TBTO). From this
report, it appears that dialkyltin compounds are more rapidly degraded
than trialkyltin compounds.
Triorganotin compounds such as TBTO and tributyltin fluoride used
as antifouling agents in paints for ship bottoms and in underwater
coatings appear to break down in a multislop process to give tin(IV)
oxide (Barnes et al., 1973; Sheldon, 1975).
The mechanism of the biological dealkylation of organotin
compounds is apparently more complicated than was considered by Blair
(1975), who thought that the basic process was the oxidative cleavage
of the carbon-tin bond (section 6.2.4).
a Starnes (1966) Unpublished report, The Dow Chemical Co.
(ALS 66-648).
b Smith et al. (1970) Unpublished report, The Dow Chemical Co.
(OL 30445 Feb. 1. 1970).
Available information shows that the persistence of organotin
compounds may vary considerably depending on the conditions and the
type of compound. Under extreme laboratory conditions, the solvolysis
may have a half-time ranging from about 1 min to about 100 days
(Bassindale et al., 1971; Roberts & El Kaissi, 1968). Under
environmental conditions, it is likely that the half-times for
triphenyltin compounds are somewhere between a few days and 140 days
(Freitag & Bock, 1974b). Diorganotin compounds probably have somewhat
shorter half-times (Mazaev et al., 1976).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1 Ambient Air
Tin is rarely detected in air, and, when detected, it is generally
in low concentrations (0.01 µg/m3), except in the proximity of some
industrial sources. Tin emission concentrations of 10-640 µg/m3 were
reported from electric furnaces at certain plants in Japan, in 1972.
At a distance of 700 metres, the atmospheric tin concentrations still
ranged from 3.8 to 4.4 µg/m3 (Environment Agency, Japan, 1971,
unpublished data).a
Concentrations from 0.003 to 0.3 µg/m3 were found in 60.6% of 754
samples tested from 22 cities in the USA. More than 50% of samples
from 3 urban and 3 rural sites were below the detectable level. The
highest concentration of tin (0.8 µg/m3) was found in a sample from a
Boston, USA, industrial site, which also contained the highest
concentration of lead together with relatively high concentrations of
zinc and cadmium (Tabor & Warren, 1958).
At stations of the US National Air Surveillance Networks during
1968 and 1969, concentrations ranged from below the minimum detectable
level to 0.23 µg/m3 in 1968 and 0.12 µg/m3 in 1969. Both these
concentrations were recorded in East Chicago (US Environmental
Protection Agency, 1973).
Concentrations ranging from 0.04 to 0.09 µg/m3, determined by
emission spectroscopy, were reported for Cincinnati and St Louis in
1970 by the US National Air Surveillance Cascade Impactor Network (Lee
et al., 1972). The size distributions of tin-containing particles
found in this study are given in Table 2.
Peak concentrations of tin were found in particles ranging from 1
to 3 µm in diameter. Bogen (1973) found that ambient air levels of tin
in the Heidelberg area of the Federal Republic of Germany between 30
April 1971 and 21 May 1971 ranged from 0.096 to 0.167 µg/m3.
The combustion of coal, oil, and lignite results in the discharge
of trace amounts of many elements into the atmosphere. Bertine &
Goldberg (1971) pointed out that the principal sites of fossil fuel
consumption are in the mid-latitudes of the Northern Hemisphere and
suggested a need for linking fossil fuel consumption with the
sedimentary cycles of trace metals from the atmosphere. They reported
that tin was present in coal and oil at average concentrations of 2
and 0.01 mg/kg, respectively.
a Japanese Background Paper No. 2, prepared for the WHO meeting
on the Effects on Health of Specific Air Pollutants from Industrial
Emissions, Geneva, November 4-9, 1974.
Table 2. Size distribution of tin-containing particles in urban aira
Cincinnatti St Louis
(A) (B)
4th Quarter 1st Quarter 4th Quarter
1970 1970 1970
Average concentration (µg/m3) 0.09 0.04 0.05
Average mass median diameter (µm) 0.93 1.40 1.53
% particles less than or equal to 1 µm 55 34 28
% particles less than or equal to 2 µm 86 68 65
a Adapted from Lee et al. (1972).
5.2 Soils and Plants
Tin has not been consistently found in all soils and plants;
however, allowance must be made for the possibility that the
concentrations present may have been below the limits of detection of
the methods employed. Bowen (1966) reported levels of tin in soil
ranging from about 2 to 200 mg/kg, the metal being strongly adsorbed
by the humus.
Schroeder et al., (1964) observed that concentrations of tin in
soils were localized and that it had not been detected in many areas.
In a limited study of tin in vegetation and foods, he found that
absorption by plants was erratic, even when the soil tin content was
high. A tin concentration of 157 mg/kg dry weight was recorded in
forest soil from southern Vermont, USA, but tin concentrations in
sections of an 100-year-old elm indicated that exposure of this tree
was not of recent origin.
Tin concentrations of 30-300 mg/kg have been reported in peat from
Finland by Gordon (1952), and high concentrations of tin were found by
Goldschmidt (1958) in forest litter and humus and also in coal. Tin
was present in all but 7 of 43 samples of foliage from 13 species of
ornamental plants and trees in New Jersey, USA (Hanna & Grant, 1962).
Lounamaa (1956), using a spectrographic method that had a limit of
detection of 10 mg tin/kg of ash, detected tin only sporadically in
higher plants in Finland. Lichens concentrated tin to exceptionally
high levels, considering the small amounts present in rocks. For
example, those growing on silicic rocks had tin concentrations of 72 ±
4.7 mg/kg ash (range: less than 10-100). Tin was undetected in 6 out
of 16 mosses while the remainder had concentrations of 10-60 mg/kg of
ash, concentrations being twice as high in those growing on silicic
rock as in those growing on calcareous rock. Pasture herbage growing
in Scotland was reported by Mitchell (1948) to contain tin
concentrations of only 0.3-0.4 mg/kg dry weight. He also quoted tin
concentrations in soils from north-east Scotland of up to 200 mg/kg.
Warren (1964, unpublished data) reported a tin concentration of
75 mg/kg in soils in unmineralized areas of Devon and Cornwall in
England, while soils from mineralized areas contained up to more than
1000 mg/kg.
5.3 Water and Marine Organisms
Tin has only been found occasionally in fresh water. It was found
by Durum & Haffty (1961) in 3 out of 59 samples from 15 major North
American rivers. No tin was detected by the National Water Quality
Network (1960) in 119 analyses at 71 locations on 28 major rivers in
the USA. Kleinkopf (1955, 1960) reported that Maine waters contained a
mean tin concentration of 0.038 µg/litre with a maximum concentration
of 2.50 µg/litre.
Tin has also only been found occasionally in municipal water
supplies (Durfor & Becker, 1964). Analyses by the US Public Health
Service showed concentrations of 0.0008-0.03 mg/litre in 32 out of 175
finished municipal waters (Taylor, 1964, personal communication).a
Although it is known that acidic or alkaline corrosive waters can
attack bronze fittings in pumping stations, presumably releasing some
tin, the relationship between corrosion and the aqueous release of tin
has not been established (Schroeder et al., 1964).
It has been reported by Vinogradov (1953) and Mason (1966) that
tin is present in sea water in amounts of about 0.003 mg/litre; thus,
its presence in marine organisms is to be expected. Tin levels of
0.2-20 mg/kg have been reported in marine animals by Bowen (1966) and
accumulation by the sponge Terpios zeteki has been noted (Bowen &
Sutton, 1951). Schroeder et al., (1964) reported that tin had not been
detected in plankton, bacteria, flowering plants, protozoa,
coelenterates, echinoderms, crustacea, or most fish. However,
Vinogradov (1953) reported the presence of small amounts of tin in a
marine worm, an oyster, and a dogfish.
a Taylor, F. B. (1964) Personal communication, cited by Shroeder,
H. A., Balassa, J. J., & Tipton, I. H. (1964) Abnormal trace metals
in man; Tin. J. chron. Dis., 17: 494.
Organotin compounds may enter water directly from antifouling
coatings on ship bottoms or they may be used as molluscicides and
added to vast areas of water for the control of snails, in which case
the effective concentration has to be about 1 mg/litre (WHO, 1973).
They may also be present indirectly as a result of industrial,
agricultural, and other uses of organotin compounds.
Tin(IV) oxide, suggested to be the final breakdown product of some
antifouling organotin agents (Shelemon, 1975), will eventually be
deposited in bottom sediments because of its insolubility. However, no
information is currently available concerning the rate and mechanism
of this degradation.
5.4 Food
Tin has been reported to occur in trace amounts in most natural
foods (de Groot et al., 1973). The tin content was determined by
atomic absorption spectroscopy in 11 known wheats or wheat blends, in
20 flours prepared commercially from these wheats, and in 25 products
specially prepared from the flours, as well as in 10 consumer products
from 10 different cities in the USA. Only one half of the tin in bread
could have been contributed by the flour. Tin concentrations in common
hard, common soft, and durum wheat were 5.6 ± 0.6, 7.9 ± 0.9, and 6.8
± 0.5 mg/kg respectively, while samples of flour and semolina from the
above sources of wheat contained tin at 4.1 ± 0.4, 3.7 ± 0.7, and 6.0
± 1.2 mg/kg, respectively. The tin concentrations in most products
exhibited significant regional differences (Zook et al., 1970).
Schroeder et al. (1964) calculated that a diet composed largely of
fresh meats, cereals, and vegetables would usually contain a tin
concentration of less than 1 mg/kg and would supply about 1 mg of tin
per day. In an area of southern Vermont, USA, where the soil contained
considerable amounts of tin (about 160 and 33 mg/kg, respectively, in
2 gardens), 10 out of 18 samples of fresh vegetables contained tin
levels of less than 1 mg/kg.
Hadzimicev (1971) measured the content of tin in foods from the
vegetable belt around Sofia, Bulgaria. Tin concentrations in wheat,
corn, beans, potatoes, tomatoes, cabbage, carrots, spinach, lettuce,
onions, apples, and peaches ranged from 0.02 to 1.02 mg/kg.
Larger amounts of tin may be present in processed foods and
drinks, because of corrosion and leaching of the metal from plain
unlacquered cans or from tin foil used for packaging. The corrosion of
tin-plate in food containers depends upon several factors including
the type of food product, the time and temperature of storage, the
acidity of the food and the quantity of air present in the headspace
of the can (Calloway & McMullen, 1966; Monier-Williams, 1949).
Oxidizing agents (eg., nitrates, ferric and cupric salts),and
anthocyanin pigments, methylamine, sulfur dioxide, and other sulfur
compounds accelerate corrosion, while fin salts in solution, sugars,
and colloids such as gelatine retard it (Monier & Williams, 1949). The
introduction of lacquered cans and the crimping of the tops minimizing
direct contact of food with solder has resulted in a general reduction
in corrosion and leaching.
A number of foods are unsuitable for packing in plain cans as they
promote corrosion. Thus, tin has been found in canned asparagus in
high concentrations varying from 120 to 550 mg/kg (Adam & Horner,
1937; Eyrich, 1972; Woidich & Pfannhäuser 1973). Tomato fruit can
accumulate nitrate when grown under combined conditions of high
temperature, high nitrogen fertilization levels, and low light
intensity. Such tomatoes caused excessive detinning of internal plain
can surfaces (Hoff & Wilcox, 1970). Iwamoto et al. (1968) also showed
a distinct detinning effect on unlacquered cans of both naturally
occurring and added nitrate in tomato juices; the authors suggested
that the concentrations of nitrate-nitrogen in juices should not
exceed 3 mg/kg. Tin contents exceeding 100 mg/kg have been found in
other foods in unlacquered cans including fish, orange, grape, and
mango juices, apricots, bananas, pineapple, and sugar syrup (Catala et
al., 1971; Eyrich, 1972; Mahadevaiak et al., 1969; Stanculescu et al.,
1972; Woidich & Pfannhäuser, 1973). In foodstuffs stored in
all-lacquered cans, the tin content was mainly below 25 mg/kg (Catala,
et al., 1971; Eyrich, 1972; Woidich & Pfannhäuser, 1973).
Several investigations have shown that high storage temperatures
increase the transfer of tin into canned foods (Catala et al., 1971;
Kimura et al, 1970; Zui, 1970). An increase of 2 mg/kg per month of
storage was reported by Zui (1970) for an increase of 1°C in
temperature.
Nishijama et al. (1971) showed an increase in the tin content from
50-77 to 260-300 mg/kg in pineapples stored at 8°C in unlacquered cans
for 72 h after opening the can. The transfer of tin into the fruits
was also increased by 0.5% aqueous citric or tartaric acid. The
content of tin in foods in lacquered cans was low and remained low
after opening. Klein et al. (1970) noted a similar effect of storage
of fruit juices in opened plain cans. Values exceeding 200 µg/kg were
found within 48 h of opening the cans.
A mean concentration of 0.0078 mg/litre was found in bottled
(glass) cow's milk compared with 16 mg/litre in evaporated milk in
unlacquered cans (diluted to the equivalent volume of cow's milk). In
some cases, concentrations of up to 110 mg/litre were found; however,
only milk from unlacquered cans contained significant amounts of tin,
while milk in lacquered cans contained less than 5 mg/litre (Hamilton
et al., 1972). The highest concentration of tin found by Wodsak (1967)
in canned condensed milk samples, less than 4 weeks old, was
40 mg/litre. The concentrations did not increase much during a further
5 months of storage but after 2 years of storage, concentrations up to
160 mg/litre were detected.
In addition to contamination by the containers, the presence of
tin in food may be due to the use of tin as an additive. Tin(II) ions
are used as additives in asparagus and peas packed in glass containers
or in lacquered cans, and also in soda water. The stannous ion is
added to prevent the migration of other heavy metals into the canned
foods and to inhibit the oxidation of ascorbic acid. Scott & Stewart
(1944) reported that Clostridium botulinum would not grow in
beetroot and carrots in unlacquered cans whereas normal growth
occurred in lacquered cans. For canned beetroot, the concentration of
added tin required for the prevention of growth was 150 mg/kg and for
carrots, 30-60 mg/kg.
High concentrations of tin have been reported in cheese packed in
tin foil (Dyer & Taylor, 1931, Elten, 1929).
5.5 Organotin Residues
Tin occurring in food and beverages may also result from the use
of organotin compounds as miticides in agriculture and as stabilizers
in PVC materials. In several experiments in various parts of the USA,
apples and pears from trees that had been sprayed 4 times with a
miticide, tricyclohexyltin hydroxide, had maximum organotin residues
of less than 2 mg/kg of whole fruit on the day of the final
application. These concentrations were reduced to about half within
3-5 weeks and, after 4 weeks, the residues consisted mainly of
tricyclohexyltin hydroxide with only small amounts of decomposition
products; inorganic tin concentrations were almost invariably below
0.2 mg/kg. The mean concentration of inorganic tin on apples and pears
after 1-5 applications of the miticide was 0.1 mg/kg (equivalent to
0.3 mg/kg, when calculated as tricyclohexyltin hydroxide); an
inorganic tin residue of 0.3 mg/kg (equivalent to 1.0 mg/kg of
tricyclohexyltin hydroxide) was found on pears by Getzendaner & Corbin
1972). In an appraisal of data on pesticide residues in food, the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues (FAO/WHO, 1971) concluded that residues of
tricyclohexyltin hydroxide on apples and pears decline by 50% in about
3 weeks due to photodegradation (section 4.3). A 20-50% reduction can
be achieved by washing and most of the residues can be removed by
peeling the fruit, after which only 0.1 mg/kg may be expected in the
fruit flesh. Data on tricyclohexyltin hydroxide on apples and pears
after 1-4 treatments showed mean values ranging from 0.4 to 2.0 mg/kg.
Similar concentrations on apples and pears have been reported from the
Netherlands (WHO, 1975). It has also been reported that the
concentrations of tricyclohexyltin hydroxide in treated products grown
under glasshouse conditions including cucumbers, tomatoes, and bell
peppers are unlikely to exceed 0.5 mg/kg (WHO, 1975).
The FAO/WHO joint meeting report (FAO/WHO, 1971) included data on
triphenyltin hydroxide, acetate, and chloride residues in various
foodstuffs such as potatoes, carrots, and sugar beets. Maximum
concentrations in potatoes and carrots only rarely exceeded 0.1 mg/kg.
Residues in food, fruit, and vegetables derived from direct contact
with the compound could be considerably reduced by washing.
When cows were fed with sugar beet leaves containing triphenyltin
acetate at 1 mg/kg a concentration of 0.004 mg/kg was found in the
milk.
In some countries, regulations permit the presence of 2 PVC
stabilizers in food, viz. dioctyltin S,S'-bis(isooctyl-
mercaptoacetate) and dioctyltin maleate polymer, up to a concentration
of total octyltin of 1 mg/kg (Department of National Health and
Welfare, Canada 1975; Food and Drug Administration, USA, 1971). The
migration of tin from PVC bottles into liquid foods contained in them
was studied by Carr (1969). The increase in the tin content of various
products during the storage period ranged from 0 to 0.07 mg/kg
(Table 3).
Table 3. Tin concentrations in foodstuffs after storage for 2 months in PVC bottles at 30 °C
Organotin
Tin content Tin content Tin extracted stabilizer
at beginning after ageing from bottle extracted from
Food product of experiment in PVC bottle (mg/kg) PVC bottle
(mg/kg) (mg/kg) (mg/kg)
mineral water 0.076 0.088 0.01 0.063
tomato juice 0.03 0.03 0 0
peanut oil 0.05 0.06 0.01 0.063
vegetable oil 0.08 0.09 0.01 0.063
apple juice 0 0.02 0.02 0.126
cherry soda 0 0.07 0.07 0.443
beer 0 0.01 0.01 0.063
milka 0.02 0.04 0.02 0.126
red wine 0 0 0 0
blended whisky 0.01 0.02 0.01 0.063
a Milk sample in PVC bottle aged for 2 weeks at 65 °C (Carr, 1969).
5.6 Working Environment
While most of the operations associated with the extraction and
treatment of tin ore are wet processes, tin dust or fumes may escape
during the bagging of concentrate in the ore rooms and during smelting
operations (mixing plant and furnace tapping), as well as during the
periodic cleaning of the bag filters used to remove particulate matter
from smelter furnace flue gas before release into the atmosphere (ILO,
1972). Exposure to dust and fumes of tin oxide may cause stannosis.
Additional exposure can occur at the final stage of upgrading the
cassiterite concentrate and during the roasting of sulfide ore.
Tin(II) chloride constitutes a hazard, when the rough molten tin is
separated from the rest of the charge during refining (ILO, 1972).
Other sources of industrial exposure include tinning of metals
(Duckering, 1968); the preparation and use of tin alloys and solders;
the use of tin(II) chloride as a reducing agent in calico printing;
the use of hydrated stannic acid, sodium metastannate, hydrated
tin(IV) chloride, and ammonium tin(IV) chloride as mordants in dyeing
and in the weighting of silk; the use of tin(II) fluoroborate
[Sn(BF4)2] in plating baths; and the production and use of organotin
compounds.
5.7 Estimate of Effective Exposure of Man through Environmental Media
Food is the main source of exposure to tin for man. Estimates of
human exposure from all environmental media are difficult to make
because of the lack of reliable data concerning environmental
concentrations (mainly in air and water), and because of variations in
the amounts of different foods and beverages consumed, especially
canned foods. Earlier estimates of the daily intake of tin by man have
been difficult to reconcile with more recent estimates. Kehoe et al.
(1940) reported that the mean daily intake of Gin by a normal adult in
the USA was 17 mg, while Tipton et al. (1966, 1969), in long-term
balance studies, reported average daily intakes of between 1.5 and
8.8 mg in 4 subjects. Schroeder et al. (1964) found 3.6 mg in a day's
institutional diet and considered that the major portion of the tin
probably came from canned fruit juices and fruits in the diet.
However, Schroeder and his colleagues pointed out that human intake
may vary considerably. They calculated that a 10 MJ (2400 kcal) diet
composed largely of fresh meats, grain products, and vegetables, which
usually contain less than 1 mg/kg of tin, would supply 1 mg/day.
However, a diet including a substantial proportion of canned
vegetables and fish could supply as much as 38 mg/day. A typical daily
tin balance for an adult in the USA, as estimated by Schroeder et al.
(1964), is shown in Table 4.
Hamilton et al. (1972) reported a daily intake of tin from food
and beverages by an adult in the United Kingdom of 0.187 ± 0.042 mg
(Table 5). It seems likely that this diet was composed of fresh foods,
which would account for the lower values obtained compared with the
North American data.
Table 4. Daily tin balance for an adult in the USAa
Intake (mg) Output (mg)
food 4.0 faeces (estimated) 3.98
(range, 1-40) (range, 1-40)
water 0.0 urine 0.023
(range, 0-0.03) (range, 0-0.04)
air 0.003
(range, 0-0.007)
total 4.003 (1-40) 4.003 (1-40)
a From: Schroeder et al. (1964).
Table 5. The concentration of tin in foods and total daily intake
for an adult in the United Kingdoma
Item Number of Concentration of tin (mg/kg)b
samples
cereals 3 4.7±1.5 × 10-2
meat 4 2.1±0.5 × 10-3
rats 4 3.7±0.8 × 10-2
fruits 5 0.5±0.1
root vegetables 3 2.2±1.0 × 10-2
green vegetables 4 2.3±10-2
milk (cow's) 17 7.8±1.2 × 10-3 (mg/litre)
total daily untake 0.187±0.042
a "From Hamilton et al. (1972).
b For prepared diet.
6. METABOLISM
Although a substantial amount of literature exists on the
absorption, distribution, excretion, and storage of tin, the results
of much of the early research are questionable in the light of modern
analytical techniques. For example, some studies depended on tissue
matrix destruction processes with the risk of loss of tin salts by
volatilization. In many cases, conditions were not controlled or
defined making it difficult to know whether pure valence states of
tin(II) or tin(IV) were being used, and whether the oxidation state of
the element affected its biological fate. In most cases, the purity of
the compound used was not indicated.
6.1 Inorganic Tin
6.1.1 Absorption
Evidence obtained from man and several animal species shows that
ingested inorganic tin is poorly absorbed. Most studies indicate that
less than 5% is absorbed from the gastrointestinal tract, although
values as high as 20% have been reported (Furchner & Drake, 1976;
Hiles, 1974; Kehoe et al., 1940; Kutzner & Brod, 1971,
Monier-Williams, 1949; Schroeder et al., 1964).
The influence of oxidation state and anion complement on the rate
of gastrointestinal absorption was studied by Hiles (1974) by
administering tin to rats in the form of tin(II) citrate, fluoride, or
pyrophosphate, and tin(IV) citrate or fluoride as a single oral dose
at 20 mg/kg body weight. The absorption of tin(II) citrate and
fluoride was calculated to be 2.8%, whereas only 0.6% of the dose of
tin(IV) citrate and fluoride was absorbed; the citrate and fluoride
were equally absorbed. Absorption of tin, when the anion was
pyrophosphate, was significantly lower than when the anion was citrate
or fluoride. The author considered that this was because pyrophosphate
had a greater tendency to form insoluble complexes with tin than
either fluoride or citrate.
6.1.2 Distribution
With both oral and parenteral administration of tin to animals,
the highest concentrations were found in the kidney, liver, and bone
(Durbin, 1960; Hiles, 1974; Moskalev, 1964), the principal site of
deposition being the bone (Furchner & Drake, 1976; Hiles, 1974).
The amounts of tin remaining in the organs of rats 48 h after
receiving a single oral dose of 20 mg of tin in the form of a
radioactive compound are shown in Table 6; the distribution 48 h after
a single intravenous administration of tin(II) or tin(IV) citrate is
shown in Table 7 (Hiles, 1974).
Rats given a daily oral dose of 20 mg of tin per kg of body weight
as tin(II) or tin(IV) fluoride did not show any detectable
trans-placental transfer at day 10 after conception, and at day 21,
the amounts of tin found in rats given the tin(II) compound were so
close to the limit of detection (= 0.1 µg of tin) that the
significance of the findings remained doubtful. In rats receiving the
same compounds at similar daily doses for 28 days, no significant
amounts of tin could be detected in the brain, heart, testes,
adrenals, reproductive tract, skeletal muscle, and spleen, while the
levels in kidney and liver were approximately the same as after a
single oral dose; however, the levels in bone were about 8 times
higher than those found after a single oral dose. Tissue
concentrations were higher following administration of tin(II)
fluoride than following tin(IV) fluoride administration (Hiles, 1974).
The results of distribution experiments performed by Hiles (1974)
suggested that tin is unlikely to be rapidly oxidized or reduced
during absorption and systemic transportation, although the actual
oxidation state of the tin in the tissues could not be determined.
Following intravenous injection of 113Sn(II) and 113Sn(IV)
citrates in rats, only small quantities of tin could be detected in
lung tissues (Hiles, 1974). However, tin is found in the human adult
lung and there is evidence indicating that the concentrations increase
with age often reaching higher levels than those in other tissues
(Schroeder et al., 1964). Thus, it seems probable that tin reaches the
lung primarily through inhalation.
Levels of 113Sn in the blood of rats, 2 days after oral or
intravenous administration, were extremely low and were only detected
in the erythrocytes (Hiles, 1974). Kehoe et al. (1940) found that 80%
of the tin in the blood of man was in the cells.
6.1.2.1. Distribution in human tissues and biological fluids
Tin concentrations found by Hamilton et al. (1972/1973) in some
tissues of healthy human adults are given in Table 8 together with
data reported by Kehoe et al. (1940) and Schroeder et al. (1964).
Comparatively high concentrations were found in lung, kidney, liver,
bone, and also in lymph nodes. Fairly high levels were reported by
Storozeva (1963) in the teeth of 24 persons, i.e., a range of
0.5-1.7 mg/kg of ash.
Table 6. 113Sn. distribution in rats after a single oral dose of solutions of various 113Sn compoundse
Percent of dosed radioactivity
Sample
SnF2 SnF4 Sn(II) Citrate Sn(IV) Citrate Sn2P2O7
Faeces and
gastrointestinal
wash 100.60±9.9 (10)a 98.40+3.6 (10) 98.00±2.1 (5) 95.40±10.3 (5) 100.0±2.8 (6)
Urine 1.02±0.58 (10)b,d 0.22±0.13 (10)b 0.90±0.23 (5)c 0.33±0.12 (5)c 0.41±0.19 (6)d
Kidneys 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.05±0.02 (5)c 0.01±0.00 (5)c 0.03±0.02 (6)d
Liver 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.07±0.02 (5) < 0.004 (5) 0.02±0.02 (6)d
Pancreas < 0.004 (5) 0.02±0.03 (5) < 0.004 (5) < 0.004 (5) < 0.004 (5)
Recovery 101.74±9.4 (10) 98.66+3.6 (10) 99.02±2.3 (5) 95.74±10.5 (5) 100.46±2.9 (6)
Percent of dosed 113Sn/g of sample
Bone (femur) 0.20±0.13 (10)b,d 0.04±0.02 (10)b 0.09±0.04 (5)c 0.03±0.02 (5)c 0.02±0.01 (6)d
Lymph nodes < 0.004 (5) 0.08±0.12 (9) < 0.004 (5) < 0.004 (5) 0.01±0.01 (5)
Blood clot 0.01±0.01 (10) < 0.004 0.01±0.00 (5) < 0.004 (5) < 0.004 (6)
Leg muscle < 0.004 (5) 0.01±0.01 (5) < 0.004 (5) < 0.004 (5) < 0.004 (6)
a % of dosed 113Sn±SD (number of animals) where 1% = 40 µg. Tissues containing < 0.010%, of the dose were not
normally included in the table.
b Significant difference at p < 0.05 between SnF2 and SnF4.
c Significant difference at p < 0.05 between Sn(II) citrate and Sn(IV) citrate.
d Significant difference at p < 0.05 between SnF2 and Sn2P2O7.
e From: Hiles (1974).
Table 7. Fate of inorganic tin in rats. Tin distribution after a single intravenous
dose of 113Sn(11) or 113Sn(IV) citratee
Percent of dosed radioactivity
Sample Sn(II) citrate Sn(IV) citrate
Bile-duct Bile-duct
Intact cannulated Intact cannulated
Urine 35.3±4.6a 23.3±12.8 39.8±10.8 24.8± 2.5
Faeces 12.1±1.8b,d 2.2±1.1d 3.1±1.4b 0.5±0.3
Bile -- 11.5±2.4c -- 0.1±0.1c
Injection site 3.3±1.8 6.2±4.7 4.1±1.5 3.9±3.3
Liver 2.0±0.2 5.1±1.6c,d 0.2±0.1d 0.2±0.1c
Kidney 5.9±0.9 8.3±1.3 5.3±3.4 4.2±2.5
Lungs 0.12±0.08 0.2±0.1 < 0.04 < 0.06
Spleen 0.4±0.0 -- < 0.04 --
Pancreas 0.2±0.1 -- < 0.04 --
Stomach 0.1±0.1a 0.2±0.0d < 0.04 < 0.06
Small intestine 0.5±0.1a 0.4±0.4d < 0.04 < 0.06
Large intestine 1.0±0.8b,d 0.4±0.3d 0.2±0.3b < 0.06
Carcass 33.4±5.3 -- 43.2±5.3 --
Femur (one) 1.6±0.2 1.9±0.1 3.2±0.6 2.0±0.1
Percent of dosed 113Sn/g
Bone (femur) 4.5±0.6b,d 8.4±1.0d 8.8±1.8b 7.4±0.9
Blood clot 0.6±0.2d 0.9±10.3d < 0.04 < 0.06
Leg muscle 0.06±0.03 -- < 0.04 --
a % of dosed 113Sn±SD where 1% = 4 µg tin.
b Significant difference at p < 0.05 between intact Sn(II) and Sn(IV) animals.
c Significant difference at p < 0.05 between bile-duct cannulated Sn(II) and
Sn(IV) animals.
d Significant difference at p < 0.05 between Sn(II) intact and bile-duct cannulated
animals.
e From: Hiles (1974).
Schroeder et al. (1964) reported a highly variable distribution of
tin in human tissues with significant differences related to age and
geographical location. Variations in the tissue contents of tin among
several geographical regions throughout the world were quite marked
but were less noticeable between 8 cities of the USA (Table 9 and 10).
All kidney, liver, and lung samples from Africa, Asia, and Europe had
lower mean and median tin contents than samples from the USA (the
difference being least in lungs). Tin in the tissue of Africans, where
exposure to tin would be expected to be much lower than in
industrialized North American and European areas, was, indeed,
noticeably low. Tin was rarely detected in the tissues of stillborn
infants, indicating that tin does not readily cross the placental
barrier. The lungs accumulated tin with advancing age but other organs
did not. Concentrations of tin in kidney, liver, lungs, and the ileum
according to decade of life are shown in Table 9. Small amount were
found in the heart, prostate, uterus, and trachea. Avtandilov (1967)
studied the trace element contents of normal and atherosclerotic
aortas. He reported a mean tin concentration of 0.08 mg/kg wet weight
in 20-39-year-old subjects with no difference between the two
categories of aorta.
Table 8. Tin content of human tissues, concentrations expressed as mg/kg wet weight.
Hamilton et al. (1972/73) Kehoe et al. (1940) Schroeder et al. (1964)
Tissue mean ± S.E. mean range (8 cities in
USA)
blood 0.009±0.002
brain 0.06±0.01 NDa
kidney 0.2±0.04 0.2 0.23-0.76
liver 0.4±0.08 0.35-1.0
lung 0.8±0.2 0.45 0.49-1.20
lymph node 1.5±0.6
muscle 0.07±0.01 0.1
bone 4.1±0.6b 0.5-0.8
a ND = not detected.
b mg of element per kg of ash.
Table 9. Tin in human tissues by age, mean US values (mg/kg ash)a
Age group (year) 0 0-1 1-10 11-20 21-30 31-40 41-50 51-60 81-70 71-84
kidney
concentration 0 57b 60b 33 20 34b 28b 22 34 32
occurrence 0/16 9/10 19/20 10/15 17/21 36/43 22/26 26/27 13/14 8/9
liver
concentration 0 48b 61b 42 34 33b 25 35 38b 34b
occurrence 0/21 11/12 22/23 14/14 20/21 39/43 27/28 25/25 13/13 9/9
lung
concentration (18) 35 34 45b 27 31b 39b 53b 58 64b
occurrence 1/10 5/6 5/6 10/13 18/21 34/35 27/27 28/29 13/13 9/9
ileum
concentration 0 101 98 80 174 116 97 53 172 140
occurrence 0/8 1/2 1/1 9/10 12/13 26/28 16/17 12/12 6/6 3/4
total occurrence
(%) 1.8 86.7 94.0 82.7 88.2 79.8 93.9 97.8 97.7 93.5
Note: Concentrations of positive samples.
Occurrence refers to the number positive/total cases.
a From: Schroeder et al. (1964).
b Excluding values > 150.
Table 10. Geographical distribution of tin in kidney, liver, and lung of man (mg/kg ash)a
No. of %
samples present Mean Range Median
kidney
United States of America 161 97 30 < 5-480 20
Switzerland 9 33 5 < 5-17 < 5
Africa 53 19 5 < 5-30 < 5
Middle East 43 42 8 < 5-55 < 5
Far East 57 40 11 < 5-110 < 5
liver
United States of America 163 96 35 < 5-300 24
Switzerland 9 78 6 < 5-20 Tc
Africa 49 25 4 < 5-20 < 5
Middle East 44 55 11 < 5-140 Tc
Far East 54 41 10 < 5-74 < 5
lung
United States of America 159 98 69 < 5-920 39
Switzerland 7 100 37 23-62 28
Africa 50 60 10 < 5-40 Tc
Middle East 45 91 33 < 5-200 17
Far East 57 93 65 < 5-1200 29
Note: Tc = trace, or barely detectable.
Mean and median values were obtained by assigning a value of one-half
the least detectable amount to those cases where tin was not detected.
a From: Schroeder et al. (1964).
Using a spark source mass spectrometric method with a detection
limit of 0.004 mg/kg wet weight, Hamilton et al. (1972/1973) reported
a mean concentration of 0.06 mg/kg (± 0.01) in 10 whole brain samples
from accidentally killed, presumably healthy adults. The concentration
found in the frontal lobe was 0.03 (± 0.07) and that in the basal
ganglia was 0.04 (± 0.02) mg/kg, measured in 2 samples. Tin was not
detected in human brain by Kehoe et al. (1940) and was found in only a
small percentage of subjects by Tipton (1960) and Schroeder et al.
(1964).
6.1.3 Excretion
The major route of excretion of absorbed inorganic tin is the
kidney although a small fraction is excreted into the bile (Hiles,
1974; Moskalev, 1964). The excretion of tin in the urine was studied
by Perry & Perry (1959) in 24 human adults. A mean of 16.6 µg/litre of
urine or 23.4 µg/day was reported. Kehoe et al. (1940) reported a mean
concentration of 18 µg/litre of urine in subjects in the USA. There
remains some doubt, however, as regards the accuracy of the analytical
technique used since, in the same paper, the concentration in the
urine of French males was reported to be zero.
In an experiment on rats, Hiles (1974) reported that after a
single oral dose of tin(II) or tin(IV)citrate or fluoride at 20 mg/kg
body weight (as the metal), about 50% of the absorbed tin was excreted
within 48 h. Following intravenous administration of 113Sn (2 mg of
tin per kg body weight), 12% of the 113Sn(II) and only 4% of the
113Sn(IV) appeared in the faeces of rats indicating that the biliary
route is probably more important in the elimination of tin(II) than of
tin(IV) compounds. Most of the biliary excretion (94%) took place
within 24 h.
6.1.4 Biological half-time
A half-time of 3-4 months was reported for 113Sn in the skeleton
of rats after intramuscular administration (Hamilton, 1948). However,
Hiles (1974) found a half-time of only 34-40 days for both tin(II) and
tin(IV) in the bone of rats following oral administration of tin(II)
fluoride or tin(IV) fluoride for 28 days. The half-time of tin(II) for
liver and kidney was reported to be 10-20 days. Furchner & Drake
(1976) using tin(II) chloride administered intraperitoneally and
intravenously in the mouse, rat, monkey, and dog, described the
elimination from the body as a 4 component-process that was similar in
all the species studied. The half-time for the longest component was
over 3 months.
6.2 Organotin Compounds
In this document, consideration will be limited to compounds of
the general type RSnX3, R3SnX2, R4SnX, and R4Sn where R is a
simple or complex action. Generally, little information is available
on the absorption, distribution, and excretion of these substances.
6.2.1 Absorption
Absorption from the intestinal tract of tin compounds with short
alkyl chains varies depending on the compound. There are also
considerable differences in absorption between species (Barnes &
Stoner, 1959).
Ethyltin trichloride administered orally was poorly absorbed by
rats, 92% of an oral dose of 25 mg/kg being eliminated in the faeces
within 2 days. It was not excreted in the bile (Bridges et al., 1967).
Triphenyltin acetate was almost completely eliminated in faeces within
a few days, when administered orally to sheep (Herok & Götte, 1964)
and cows (Bruggemann et al., 1964a). However, it was well absorbed,
when given orally to guineapigs and mice (Stoner, 1966). Results
obtained with rats were conflicting (Klimmer, 1963, 1964; Stoner,
1966). Tricyclohexyltin hydroxide was poorly absorbed in rats, only
about 2% being excreted in the urine after a single 25 mg/kg oral
dose. The remainder was recovered from the faeces and binary excretion
did not occur (FAO/WHO, 1971).
Trialkyltin compounds were well absorbed on contact with the skin,
since the dermal LD50s of various trimethyltin derivatives in mice
were of the order of 50-100 mg/kg (Hall & Ludwig, 1972) and that of
bis(tributyltin) oxide was below 200 mg/kg in rats and mice (Ascher &
Nissim, 1964; Elsea & Paynter, 1958). When a 20% fat solution of
various triethyltin derivatives was applied to the skin of rats and
mice for 10 min, all the animals died within 20-30 min (Ignatjeva et
al., 1968). In contrast, triphenyltin acetate did not penetrate
unbroken skin readily (Stoner, 1966).
6.2.2 Distribution
Following intravenous administration to mice and rats, the highest
concentrations of dibutyl- and diethyltin were found in liver and
kidney tissue; unchanged compounds were excreted in the bile (Barnes &
Magee, 1958). Rats were fed with 11 mg of triethyltin hydroxide over a
period of 89 days. At the end of this time, only 0.7 mg of the
compound could be recovered; 40% of this was in the blood, 28% in the
liver, and 29% in the skeletal muscle. Smaller amounts of triethyltin
were found in the kidney, brain, heart, and spleen (Cromer, 1957).
Species variation in the distribution of triethyltin has been
reported. When triethyltin (26 µg) was added to rat blood in vitro,
most of it (23 pg) was recovered from the erythrocytes and none was
found in the plasma, whereas in rabbit blood, triethyltin was more
equally distributed between erythrocytes (9 µg) and plasma (17 µg)
(Cromer, 1957). This could explain why triethyltin persists in the
blood of treated rats, whereas it quickly disappears from the blood of
treated rabbits (Barnes & Stoner, 1959). Triethyltin was found in the
brain of rats only 1-2 h after intraperitoneal injection of
triethyltin hydroxide (Cremer, 1957).
The tissue distribution of triethyltin following intravenous
administration of tetraethyltin resembled that following
administration of triethyltin (Cremer, 1957, 1958). Thus, a large
quantity of triethyltin was found in the liver, with smaller
quantities in the kidney, brain, and whole blood in a rabbit, 2 h
after administration.
After oral or intraperitoneal administration of 113Sn-labelled
triphenyltin to guineapigs, about 80% of the administered activity was
excreted in 10 days. The concentration of radioactive tin found in the
brain of rats and guineapigs after oral and intraperitoneal
administrations decreased with a half-life of several days. The form
of tin in the brain was not unequivocally identified, but it was not
in the form of free stannic ions nor was it necessarily triphenyltin
(Heath, 1967). Herok & Götte (1964) gave 3 sheep 10 mg of triphenyltin
acetate orally, every day for 20 days. The compound was labelled with
113Sn. The animals were killed at intervals ranging from 8 to 198 days
after the last dose. The highest concentration of 113Sn was found in
the liver but the brain also contained measurable amounts. In rats,
any absorbed triphenyltin chloride was rapidly distributed through the
tissues of the body including the brain. Tin was eliminated relatively
slowly and could be detected in the brain 38 days after a single dose
(Heath, 1963).
Only trace quantities of tricyclohexyltin hydroxide were found in
the tissues of rats and dogs fed up to 12 mg/kg bodyweight per day, in
the diet, for periods ranging from 45 days to 2 years (FAO/ WHO,
1971).
6.2.3 Excretion
When ethyltin trichloride was administered intraperitoneally to
rats, it was excreted almost exclusively in the urine; binary
excretion was negligible. When diethyltin was administered
intraperitoneally it was eliminated as diethyl- and ethyltin in both
faeces and urine. Diethyltin was excreted in the bile (Bridges et al.,
1967). Rats that had initially received a diet containing triethyltin
and had retained approximately 0.7 mg triethyltin in their tissues
were then given a normal diet; 12 days later, no triethyltin could be
detected in the tissues (Cremer, 1957). The route of excretion was not
known.
The elimination of triphenyltin from the body is slow (Stoner,
1966). Its persistence in guineapigs is suggested by the similarity
between the amounts consumed by those dying on diets containing 25 and
50 mg/kg and the acute oral LD50. In studies on guineapigs using
113Sn-labelled triphenyltin, the concentration of triphenyltin that
had entered the brain after a single oral dose did not decrease during
the first 10 days. In rats, triphenyltin disappeared more rapidly,
having a half-time of about 3 days in the brain (Heath, 1963, 1965).
Triphenyltin acetate labelled with 113Sn was slowly excreted in the
urine of lactating sheep given oral doses of the compound at the rate
of 10 mg/day for 20 days. The milk confined small amounts of tin
(about 1 µg/litre) in both organic and inorganic forms (Herok & Götte,
1964).
The biological half-time of tricyclohexyltin hydroxide in rats was
5-40 days, when the compound had been included in the diet over a
prolonged period. The brain was one of the tissues from which the
compound was removed most slowly (FAO/WHO, 1971).
6.2.4 Biotransformation
In some early studies on the metabolic degradation of organotin
compounds, it was suggested that destannylation (carbon-tin bond
cleavage) was the major result of this biotransformation. For example,
triethyl tin was detected in the tissues of rabbits and rats given
tetraethyltin intravenously (Cremer, 1957, 1958). The liver was the
organ most active in this conversion. Bridges et al. (1967) reported
that dealkylation of diethyltin occurred in both the gut and tissues
of the rat. Although Herok & Götte (1963) found that after
administration of triphenyltin acetate labelled with 113Sn small
amounts of inorganic tin were present in the milk of sheep, Stoner
(1966) considered that triphenyltin was not readily transformed in the
body. Dicyclohexyltin oxide and trace amounts of cyclohexylstannoic
acid were identified as metabolites in rats and dogs treated with
tricyclohexyltin hydroxide (FAO/WHO, 1971). Blair (1975) concluded
that tricyclohexyltin hydroxide undergoes metabolism in animals by
scission of cyclohexyl groups from the atom.
More recent studies using tributyltin acetate showed primary
biological oxidation reactions in the hydroxylation of carbon-hydrogen
bonds that are alpha, ß, gamma and delta to the tin atom (Fish et al.,
1976). This type of reaction was already assumed by Casida et al.
(1971), although no carbon-hydroxylated metabolites could be
identified when triethyltin derivatives were biologically oxidized
in vitro to monoethyl derivatives.
Fish et al. (1975) treated tributyltin acetate with rat liver
microsomes (a mono-oxygenase enzyme system) in the presence of NADPH,
and obtained alpha and ß hydroxylated metabolites in yields of 24% and
50%, respectively. An in vivo study with [1-14C]-tetrabutyltin
showed the occurrence of similar reactions in mice (Kimmel et al.,
1977). The alpha-hydroxyl metabolite is unstable and undergoes a
cleavage reaction at pH = 7.4 to give 1-butanol and a dibutyltin
derivative. A ß-elimination reaction in acidic media transforms the
ß-hydroxyl derivatives rapidly into 1-butane and a dibutyl derivative
(Fish et al., 1976).
Studies on the in vitro metabolism of tricyclohexyltin compounds
indicated that carbon-hydroxylation of the cyclohexyl group was a
major metabolic reaction (Fish et al., 1976; Kimmel et al., 1977).
When [113Sn] -triphenyltin acetate was subjected to in vitro
biological oxidation, the phenyl group was not biotransformed, but in
in vivo studies, rats were found to metabolize triphenyltin acetate
into diphenyl- and phenyltin derivatives (Kimmel et al., 1977).
7. EFFECTS ON ANIMALS
7.1 Inorganic Tin Compounds
Although tin is present in small amounts in most animal and human
tissues, it is uncertain whether it is an essential element for
mammals. However, recent results indicate that tin is an essential
nutrient for the growth of the rat. Both inorganic and organotin
compounds at concentrations similar to those present in feeds were
found to stimulate growth rate in rats maintained on purified amino
acid diets (Table 11) (Schwarz, 1971; 1974; Schwarz et al., 1970). The
authors concluded that tin, as an essential element, could have a
function at the active site of some metal-dependent enzymes; however,
this has still to be confirmed.
Compared with most organotin derivatives, inorganic tin and its
salts are not highly toxic, mainly because of their poor absorption
and rapid tissue turnover (Barnes & Stoner, 1959; Cheftel, 1967;
Hiles, 1974; National Academy of Sciences, Washington 1973). The
systemic toxicity of some simple tin salts is difficult to assess
because of the irritant properties of their solutions.
7.1.1 Effects on the Skin
The effects of 1% tin(II) chloride and 0.25% tin(II) fluoride
solutions, applied to the abraded skin of rabbits, were examined by
Stone & Willis (1968). Both compounds induced intraepidermal pustules
with complete destruction of the epidermis but the stratum corneum
remained intact. No injury occurred when the solutions were applied to
intact skin.
7.1.2 Respiratory system effects
According to Robertson (1960), intratracheal administration of
50 mg of metallic tin dust to rats was well tolerated and no fibrosis
was produced within one year of exposure.
Exposure of guineapigs by inhalation, to tin(IV) chloride (3 mg/
litre for 10 min, daily, for "several months") produced only transient
irritation of the nose and eyes (Pedley, 1927).
Table 11. The effect of tin(IV) sulfate on the growth of rats in a trace-element-controlled
environmenta
Dose level No. of Average daily Increase
Compound (µg of tin/kg) animals weight gain (%) p-value
control -- 5b 1.10±0.05c -- --
tin (IV) sulfate 500 8 1.37±0.10 2 .02
tin (IV) sulfate 1000 8 1.68±0.10 53 .001
tin (IV) sulfate 2000 8 1.75±0.10 59 .001
a From: Schwarz et al. (1970).
b 2 control rats died during the 26-29 days of the experiment.
c mean ± standard error.
7.1.3 Effects on the gastrointestinal system
Soluble tin salts are gastric irritants. This explains the signs
of acute poisoning occasionally observed in man and in experimental
animals following consumption of food containing high concentrations
of tin (de Groot et al, 1973). However, the concentrations required to
elicit an acute gastrointestinal reaction have not been determined
reliably. Beney et al. (1971) reported signs of illness in 3/10 cats
after ingestion of 5 ml/kg body weight of fruit juice containing a tin
concentration of 1370 mg/litre, and in 1/11 cats receiving the same
dose of fruit juice containing tin at a concentration of 540 mg/litre.
However, no adverse effects were recorded in cats receiving a similar
amount of juice containing a tin concentration of about 500 mg/litre
or in dogs given juice with a tin concentration of 1400 mg/litre.
7.1.4 Effects on the liver
A 3-fold increase in haem oxygenase (EC 1.14.99.3)a activity in
the liver of rats was observed 16 h after a single subcutaneous
injection of tin(II) chloride dihydrate (SnCl22H2O) at doses ranging
from 5.6 to 56.4 mg/kg body weight. Cytochrome P-450 mediated drug
metabolism and the content of cytochrome P-450 were reduced by one
third. These effects on the liver increased by about 15-20%, when the
compound was administered intraperitoneally (Kappas & Maines, 1976).
Tin chloride, oxalate, or sulfate added to the diet at a
concentration of 10 g/kg for 4 and 13 weeks resulted in homogenous
liver cell cytoplasm and hyperplasia of the bile duct in rats. The
liver changes were more distinct after 13 weeks administration.
Similar, although milder, hepatic alterations were seen after
administration of tin chloride, oxalate, or orthophosphate at a
concentration of 3 g/kg of dietb (de Groot et al., 1973).
Increased incidence of fatty degeneration in the liver was noted
by Schroeder et al. (1968) in female but not in male rats, when the
animals were given tin in the form of tin(II) chloride at a dose of
5 mg/litre in drinking water, from weaning until their natural death.
a The numbers within parentheses following the names of enzymes
are those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
b Tables for the approximate conversion of concentrations in diet
to mg/kg body weight per day are given in Nelson (1954).
7.1.5 Effects on the kidney
Renal damage was produced in rats by single intravenous and
intraperitoneal doses of sodium pentafluorostannite (NaSn2F5) and
tin(II) chloride dihydrate (SnCl22H2O) (Conine et al., 1973, 1975;
Yum et al, 1976). A single intraperitoneal injection of sodium
pentafluorostannite at 35 mg/kg body weight or of tin(II) chloride
dihydrate at 44.4 mg/kg produced extensive necrosis of epithelial
cells mainly involving proximal tubules. The extent of the necrosis
was thought to be related to the tin moiety of the compound because
damage of the same magnitude could not be produced by administration
of sodium fluoride (Yum et al., 1976).
Studies in which rats received single subcutanous injections of
tin(II) chloride dihydrate in doses ranging from 5.6-56.4 mg/kg body
weight revealed a 20 to 30-fold increase in the haem oxidation
activity in the kidney, 16 h after administration. This effect was
already noticeable at the lowest dose (5.6 mg/kg) and was found to be
dose-related (Kappas & Maines, 1976).
Conine et al. (1976) administered daily oral doses of sodium
pentafluorostannite for 15 or 30 days to rats at rates of 20, 100, and
175 mg/kg body weight. The highest dose caused degenerative changes in
the proximal epithelium of the kidneys that were similar to those
observed in chronic fluoride poisoning (Lindemann et al., 1959), and
affected about 15-20% of the 30 rats that were killed. Most of the 8
animals in the 175 mg/kg group that died spontaneously during the
experiment displayed necrosis of the proximal tubular epithelium
similar to that previously reported to be caused by tin (Yum et al.,
1976).
Exposure of rats to tin(II) chloride at a concentration of 5 mg of
tin/litre of drinking water, for life, produced vacuolar changes in
the renal tubules of animals of both sexes (Schroeder et al., 1968).
7.1.6 Effects on the blood-forming organs
When tin(II) (as chloride, orthophosphate, sulfate, oxalate, or
tartrate) was given in the diet to Wistar rats of both sexes at
concentrations of 3 and 10 g/kg for 4 weeks, food intake was reduced,
growth was retarded and slight anaemia developed. The signs of anaemia
included a reduction in haemoglobin levels of about 10% and
corresponding reductions in haematocrit values, erythrocyte counts,
and serum iron concentration (de Groot et al., 1973). Dietary
supplements of iron had a markedly protective effect (de Groot, 1973;
de Groot et al., 1973). The authors suggested that tin compounds might
inhibit haemopoiesis, possibly by interfering with the intestinal
absorption of iron. An alternative mechanism by which tin salts could
cause mild gastrointestinal bleeding was not investigated.
A dose-related decrease in haemoglobin concentrations was noted in
rats after daily oral treatment, for 15 days, with sodium
pentafluorostannite at 100 and 175 mg/kg body weight. Administration
of 20 mg/kg per day did not affect the haemoglobin level (Conine et
al., 1976).
7.1.7 Central nervous system effects
High doses of inorganic tin compounds seem to affect the central
nervous system producing such effects as ataxia, muscular weakness,
and the depression of the central nervous system. These signs were
observed by Conine et al., (1975) after oral administration to rats of
sodium pentafluorostannite and tin(II) chloride dihydrate in single
doses of the order of the median lethal dose (LD50). According to de
Groot et al. (1973), feeding rats with tin(II) chloride at a
concentration of 10 g/kg diet for 13 weeks, resulted in a spongy state
of the white matter of the brain. Ataxia, hind-leg paralysis, and
death were also observed in rats by Mamontova (1940) following daily
subcutaneous injections of 3 mg of tin(II) citrate for 5-6 months.
7.1.8 Effects on the reproductive system and the fetus
There is one report indicating that tin(II) chloride might have an
effect on the reproductive system. De Groot et al. (1973) noted
testicular degeneration in rats after prolonged feeding with this
compound (10 g/kg of food, for 13 weeks).
Inorganic tin compounds have not been shown to be fetotoxic.
Theuer et al. (1971) gave groups of pregnant rats sodium
pentafluorostannite, sodium pentachlorostannite, and tin(II) fluoride
corresponding to tin levels in the diet of 125, 250, and 500 mg/kg.
The rats were killed on day 20 of gestation. No effects were seen in
the fetuses; tin concentrations in the fetuses were about 1 mg/kg
compared with approximately 0.65 mg/kg in control fetuses, indicating
a rather low transplacental transfer.
7.1.9 Carcinogenicity and mutagenicity
Few reports are available concerning the carcinogenicity of
inorganic tin compounds. Walters & Roe (1965) administered either
sodium chlorostannate at 1 or 5 g/litre of drinking water or tin(II)
oleate at 5 g/kg of diet to mice, for up to one year; the survivors
were then killed. The incidence of lymphomas, hepatomas, or pulmonary
adenomas did not increase with any of the regimens.
Roe et al. (1965) reported 3 malignant tumours in 30 August rats
that survived for 1 year or more on a diet containing sodium
chlorostannate at a concentration of 20 g/kg, whereas a control group
of 33 rats did not exhibit any case of malignant tumours. One tumour
was an adenocarcinoma of mammary origin, another a pleomorphic sarcoma
in the uterus, and the third, an adenoma-carcinoma in the jaw region.
The difference was not statistically significant. No tumours were seen
in another group of 27 rats surviving on a diet containing tin(II)
2-ethylhexoate at a concentration of 5-10 g/kg.
Administration of tin(II) chloride to rats and mice at 5 mg/litre
in drinking water throughout their life-time did not produce any
increase in the incidence of tumours compared with a control group
consisting of an equal number of animals (Kanisawa & Schroeder, 1969).
Studies concerning the mutagenicity of inorganic tin compounds
were not available to the Task Group,
7.1.10 Other effects
Growth retardation in rats has been reported after the
administration of high doses of various tin compounds. Dietary levels
of tin as tin(II) oxalate, orthophosphate, chloride, sulfate, and
tartrate at 3 and 10 g/kg for 4 weeks caused inhibition of growth in
rats and oedema and atrophy of the pancreas (de Groot et al., 1973).
Daily administration, by garage, of sodium pentafluorostannite at 100
and 175 mg/kg body weight, for 30 days, resulted in a dose-related
retardation of growth in rats (Conine et al., 1976). Administration of
tin(II) chloride at a concentration of 5 mg of tin per litre of
drinking water reduced the life span of female rats, whereas the
longevity of male rats was unaffected (Schroeder et al., 1968).
7.1.11 Effective doses and dose rates
7.1.11.1 Lethal doses
The median lethal doses (LD50) for 2 inorganic tin compounds are
given in Table 12. A marked difference between the oral and parenteral
LD50 values can be explained by the low absorption of tin compounds.
Daily intravenous administration of 3.3-4.2 mg of sodium tin
citrate per kg body weight provoked death in rabbits in 7-8 days,
whereas at a dose of 2.5 mg/kg, death occurred only after 15 or more
days (Mamontova, 1940).
In studies on 6 rats, subcutaneous injection of 3 mg of tin
citrate/animal, per day, killed all the animals in 5-6 months. Similar
administration of the same compound in doses of 2 mg/kg body weight
produced vomiting, diarrhoea, weight loss, ataxia, hind-leg paralysis,
and death within 5 months (Mamontova, 1940).
Table 12. Median lethal doses (LD50) of tin(II) chloride dihydrate (SnCl22H2O) and sodium
pentafluorostannite (NaSn2F5)a
Sodium pentafluorostannite
Tin(II) chloride dihydrate
Route male mice male rats female rats male rats (LD50, mg/kg)
(LD50, mg/kg) (LD50, mg/kg) (LD50, mg/kg)
intravenous 18.9 12.9 12.9 29.3
intraperitoneal 80.9 75.4 65.0 258.4
oral (fasted) --b 223.1 218.7 2274.6
(fed) 592.9 573.1 --b 3190.1
a From: Conine et al. (1975).
b no data.
7.1.11.2 Minimum effective and no-observed-effect doses
Some information on effective doses has been given in the sections
describing the effects. However, the following information may also be
of interest.
De Groot et al. (1973) reported a normal growth rate in Wistar
rats that had received tin(IV) oxide, tin(II) sulfide, or tin(II)
oleate at dietary levels up to 10 g/kg for 4 weeks; haematological
data were also normal except for an increase in the haematocrit in
male rats fed with the highest concentration of tin(II) sulfide. The
weight and the gross and macroscopic appearance of the liver, kidney,
heart, and spleen were also normal.
When a similar experiment was conducted with tin(II) chloride for
13 weeks, the no-observed-effect concentration in food appeared to be
1 g/kg, if the diet were supplemented with iron (de Groot et al.,
1973). This is equivalent to a dose rate of about 20-30 mg tin/kg body
weight per day, for 90 days.
No effect on growth was observed by Conine et al. (1976), when
sodium pentafluorostannite was administered orally to rats at a dose
rate of 20 mg/kg body weight per day for 30 days.
Oral administration of sodium tin citrate in dose rates ranging
from 100-150 mg/kg body weight per day for 1 year did not produce any
noticeable signs of poisoning in rats (Mamontova, 1940). Similarly
rats fed a diet containing either sodium chlorostannate at 20 g/kg or
tin(II) 2-ethyl hexoate at 5-10 g/kg for one year did not show any
pathological changes in the gastrointestinal tract, kidneys, or liver
(Roe et al., 1965).
Mice that received tin in the form of sodium chlorostannate at a
concentration of 1 or 5 g/litre in drinking water or tin in the form
of tin(II) oleate in the diet at a concentration of 5 g/kg during
their lifetime did not show any adverse effects (Waiters & Roe, 1965).
Mice and rats (Schroeder & Balassa, 1961; Schroeder et al., 1968)
given tin(II) chloride in drinking water at a concentration of 5 mg of
tin/litre grew normally throughout their life. The life span of mice
of both sexes and of male rats was not affected but that of female
rats was shorter and there was an increased incidence of fatty
degeneration of the liver (section 7.1.4). Vacuolar changes in the
renal tubules were apparent in rats of both sexes (section 7.1.5).
7.2 Organotin Compounds
Distinction should be made between the effects of di-, tri-, and
tetrasubstituted organotin compounds. The principal toxicological
difference is that some trisubstituted compounds have a specific
effect on the central nervous system producing cerebral oedema (Barnes
& Stoner, 1958; Torack et al., 1969), whereas disubstituted compounds
do not produce this effect but are potent irritants that can induce an
inflammatory reaction in the bile duct (Barnes & Magee, 1958).
Toxicologically, the tetrasubstituted compounds resemble
trisubstituted compounds, which are, generally, more toxic than the
mono- and disubstituted derivatives.
7.2.1 Effects on the skin and eyes
Dermal application of dibutyltin dichloride at 10 mg/kg body
weight per day for a period of 12 days, caused severe local damage and
also bile duct injury in rats and mice. Guineapigs were more
resistant, showing little reaction to daily applications of 120 mg/kg
on 5 successive days (Barnes & Stoner, 1958).
An aqueous solution of bis(tributyltin) oxide applied to the
shaved skin (30 x 60 mm) of rats at concentrations of 0.36-0.95 mg/kg
body weight produced slight local irritation lasting 2-3 weeks
(Pelikan & Cerny, 1968b). At higher doses (1.40-185 mg/kg), marked
inflammation developed into skin necrosis. Severe effects on the eyes
were also observed with bis(tributyltin) oxide (Pelikan & Cerny,
1969).
Triphenyltin hydroxide was reported not to irritate rabbit skin or
sensitize the skin of guineapigs (Marks et al., 1969). However, it was
found to be extremely irritating to the eyes, even after brief
exposure, and could cause corneal opacity (Marks et al., 1969).
A solution of triphenyltin acetate in oil at a dose of 150 mg/kg
body weight caused a skin reaction in rats (Klimmer, 1964). Doses of
tricyclohexyltin hydroxide at 1.2-60 mg/kg body weight per day,
applied to the skin of rabbits over a 3-week period, produced distinct
reactions locally but did not result in any systemic ill effects
(FAO/WHO, 1971, p. 527)a
7.2.2 Respiratory system effects
Pulmonary congestion and oedema were observed in rats after single
intravenous administrations of solutions of diethyl-, di-propyl-,
diisopropyl-, and dipentyltin dichloride (20 mg/kg body weight), in
0.05 ml of polyoxyethylene sorbitin monoleate, whereas in the case of
dibutyltin dichloride, 10 mg/kg was enough to cause this effect
(Barnes & Stoner, 1958). Petechial and ecchymotic haemorrhages in the
trachea and larynx were observed in sheep after intrarumenal
administration of tricyclohexyltin hydroxide at 500 mg/kg body weight.
Congestion was present in the dorsal portion of all pulmonary lobes at
a dose as low as 150 mg/kg (Johnson et al., 1975).
7.2.3 Effects on the gastrointestinal system
A single dose of butyltin trichloride, butyltin- S,S',S"-tris
(2-ethylhexylmercaptoacetate), butylstannoic acid, and butyl
thiostannoic acid at 4000 mg/kg body weight, administered by stomach
tube to mice, produced various degrees of submucosal, subserosal, and
intra-luminar gastrointestinal hemorrhage within 24 h (Pelikan &
Cerny, 1970b). Similar administration of dioctyltin- S,S'-bis
(2-ethylhexyl-mercaptoacetate) produced dilatation of the stomach,
which was filled with gas. The stomach walls appeared ischaemic
whereas the intestinal walls were hyperaemic, and traces of blood were
found in the contents of the small stomach. Similar, but more
pronounced findings were recorded after administration of dioctyltin-
a Unpublished reports on triphenyltin compounds and tricyclohexyltin
hydroxide were abstracted in 1970 Evaluations of Some Pesticide
Residues in Food (FAO/WHO, 1971). The proprietary data were
submitted to the WHO by the manufacturers. Page numbers given in the
text refer to the FAO/WHO publication, which also contains
information with reference to authors and manufacturers.
bis-(butylmercaptoacetate), although no traces of blood were seen in
the stomach. Treatment with dioctyltin bis(2-ethylhexyl-
mercaptoacetate) resulted in large amounts of liquid in the stomach
contents, and slightly hyperaemic stomach walls; other findings were
similar to those produced by the other 2 dioctyltin compounds,
dioctyltin bis(dodecylmercaptide) and octyltin tris(2-ethylhexyl-
mercaptoacetate) (Pelikan & Cerny, 1970a). Ingestion of dibutyltin
dichloride at a dose of 50 mg/kg body weight per day for one week,
caused a temporary dilatation of the stomach in rats due to
accumulation of fluid, and diarrhoea (Barnes & Stoner, 1958).
Intrarumenal administration of tricyclohexyltin hydroxide at 150 mg/kg
body weight resulted in fluid diarrhoea in sheep which persisted up to
death. Necropsy findings included mild enteritis and colitis
characterized by severe hyperaemia and oedema (Johnson et al., 1975).
Gastroenteritis occurred in rats receiving an oral dose of
tricyclohexyltin hydroxide of 25 mg/kg body weight per day for 19 days
(FAO/WHO, 1971, pp. 527-528).
7.2.4 Effects on the liver and bile duct
Steatosis of hepatocytes and enlargement of the liver were seen
within 24 h of administration to mice through a stomach tube, of a
single dose of butylstannoic acid, butyltin trichloride, butyltin
tris(2-ethylhexylmercaptoacetate), or butylthiostannoic acid, each at
a dose of 4000 mg/kg body weight (Pelikan & Cerny, 1970b). In rats, a
single oral dose of dibutyl,tin dichloride at 50 mg/kg body weight
produced congestion and inflammation especially in the lower part of
the bile duct (Barnes & Magee, 1958; Gaunt et al., 1968). When this
dose of dibutyltin dichloride was given on 3 successive days, the
lesion was more severe involving also the proximal part of the bile
duct and the portal blood vessels. Necrotic areas were seen in the
liver. In some cases, bile escaped from the injured duct into pancreas
and peritoneum. The authors considered that death occurring 5 days
after the 3 successive doses was the result of a general toxic effect
of this compound, whereas death occurring later was secondary to bile
duct and liver damage. Changes in the pancreas were inflammatory and
confined to areas surrounding the bile duct. In surviving rats,
examined 6-12 months after receiving 3 daily doses of 50 mg/kg, the
bile duct was shorter and thicker than normal with fibrosis in the
wall. A similar reaction in the bile duct was seen after a single
intravenous administration of 5 mg/kg body weight, and after a dermal
application of 10 mg/kg body weight. The effects on mice of oral doses
of dibutyltin dichloride at 20-50 mg/kg body weight were similar to
those seen in rats, although liver damage appeared to be more
widespread. Repeated doses of this compound at 20-50 mg/kg body weight
killed rabbits, but did not cause bile duct or liver injury;
guineapigs, however, tolerated such doses without any signs of adverse
effects (Barnes & Magee, 1958). It has been suggested that bile duct
injury occurs only in species in which the bile duct and the
pancreatic duct have a common course (Kimbrough, 1976).
Daily doses of dibutyltin dichloride at 0.1 and 1.0 mg/kg body
weight, for 6 months, caused intoxication in rabbits with dystrophic
changes in the liver (Mazaev & Korolev, 1969). Dioctyltim- S,S'-bis
(2-ethylhexylmercaptoacetate), diootyltin- S,S'-bis
(butylmercaptoacetate), and diootyltin bis(dodecyl-mercaptide) at an
oral dose of 4000 mg/kg body weight produced steatosis of the
hepatocytes in mice (Pelikan & Cerny, 1970a). Nikonorow et al. (1973)
reported a significant increase in the mean liver weight of rats after
oral administration of dioctyltin- S,S'-bis(isoctylmercaptoacetate)
at 20 mg/kg body weight per day, for 3 months.
A single oral dose of tributyltin acetate, benzoate, chloride,
laurate, or oleate at 500 mg/kg body weight produced fatty
infiltration of the liver in mice (Pelikan & Cerny, 1968a) and
triphenyltin acetate at a dietary level of 10 mg/kg produced fatty
degeneration of the liver in guineapigs, when administered over a
2-year period (FAO/WHO, 1971, p. 341). Cholangitis was reported in
rats after administration of tricyclohexyltin hydroxide at a dietary
level of 400 mg/kg for 90 days (Shirasu, 1970). Similarly, both intra-
and extrahepatic cholangitis was noted in rats that had received
tri-cyclohexyltin hydroxide at 25 mg/kg body weight per day, for 19
days (FAO/WHO, 1971, pp. 527-528).
7.2.5 Effects on the kidney
Some degree of fatty degeneration of the renal cortical tubular
epithelium was seen on histological examination of mice that had
received a single oral dose (4000 mg/kg body weight) of butyltin
tin- S,S',S"-tris(2-ethylhexylmercalytoacetate), butylstannoic acid,
or butylthiostannoic acid, but not in animals given butyltin
trichloride. The structure of the renal tissue was unchanged.
Macroscopically, all compounds produced slight hyperaemia in the
kidneys (Pelikan & Cerny, 1970b).
Dioctyltin- S,S'-bis(2-ethylhexylmercaptoacetate) and
dioctyltin- S,S'-bis(butylmercaptoacetate) administered at the same
rate and by the same route produced a similar slight fatty
degeneration of the renal cortical tubular epithelium in mice (Pelikan
& Cerny, 1970a). Feeding rats with an organotin stabilizer,
dioctyltin- S,S'-bis(isooctyl mercaptoacetate) at a dietary level of
200 mg/kg for 12 months produced an increase in kidney weight in
female rats only (Nikonorow et al., 1973). Mazaev & Korolev (1969)
also reported dystrophic changes in the kidneys in rats treated with
dibutyltin dichloride at 0.1 and 1.0 mg/kg body weight.
Haemorrhages were reported in the kidneys of mice after single
administrations (gavage) of tributyltin benzoate, chloride, laurate,
and oleate at 500 mg/kg body weight. Hyperaemia of the kidney was seen
in all groups, whereas tubular cells containing lipids were observed
only in mice that had received laurate or oleate. Renal changes were
not reported in mice that were similarly dosed with tributyltin
acetate (Pelikan & Cerny, 1968a). Congestion of the glomeruli and mild
toxic nephrosis were observed in rats receiving a dietary level of
tricyclohexyltin hydroxide of 400 mg/kg for 3 months (Shirasu, 1970).
Similarly, toxic nephrosis was reported in rats after administration
of tricyclohexyltin hydroxide at a daily dose of 25 mg/kg body weight
for 19 days (FAO/WHO, 1971, pp. 527-528).
7.2.6 Effects on the lymphatic tissues and immunological effects
Small necrotic areas were observed in the germinal centres of the
splenic follicles of mice after a single administration, through a
stomach tube, of 4000 mg/kg body weight of butyltin- S,S',S"-tris-
(2-ethylhexylmercaptoacetate), butylthiostannoic acid, and
butyl-stannoic acid, (Pelikan & Cerny, 1970b); dioctyltin- S,S'-bis
(2-ethyl-hexylmercaptoacetate), and dioctyltin- S,S'-bis(butyl-
mercaptoacetate) (Pelikan & Cerny, 1970a).
Seinen & Willems (1976) reported that the main action of
di-octyltin dichloride, given to rats at dietary levels of 50 and
150 mg/ kg, was on the thymus, the weight of which was significantly
reduced during the experiment. In subsequent experiments, feeding rats
with dioctyltin dichloride or dibutyltin dichloride at dietary levels
of 50, or 150 mg/kg for 6 weeks, resulted in a dose-dependent
reduction in the weight of the thymus Land thymus-dependent peripheral
lymphoid organs (Seinen et al., 1977a). The delayed type
hypersensitivity was decreased and the allograft rejection was delayed
when the same compounds were given in the same way to rats.
Furthermore, it was reported that both these compounds decreased the
survival rate of rat and human thymocytes in in vitro studies
(Seinen et al., 1977b). The effects of diethyltin dichloride and
dipropyltin dichloride on rat lymphoid organs were similar but less
pronounced. However, other dialkyltin compounds such as dimethyltin
dichloride, didodecyltin dibromide, and dioctadecyltin dibromide, as
well as octyltin trichloride, trioctyltin chloride, and tetraoctyltin
did not cause atrophy of lymphoid tissues (Seinen et al., 1977a). The
rat proved more sensitive to the action of di-octyltin dichloride and
dibutyltin dichloride on lymphoid organs than the mouse and the
guineapig in which no atrophy of the lymphoid organs was produced.
(Seinen et al., 1977a).
Studies on triphenyltin acetate have been reviewed by the FAO/ WHO
(1971, pp. 327-366). In guineapigs, a dietary level of 15 mg/kg for 77
days produced a decrease in plasma cells in the spleen and in the
mesenteric, cervical, axillary, and popliteal lymph nodes. After
feeding the same dose to female guineapigs for 104 days, a reduction
in immune response to tetanus toxoid stimulation was observed. In
another experiment, a decrease in the number of lymphocytes and
leukocytes accompanied by histological changes in the lymphatic
tissues, such as atrophy of the white pulp of the spleen, was seen in
guineapigs at dietary levels of 5 mg/kg in females and 10 mg/kg in
males administered, for 12 weeks. A decrease in the number of
leukocytes was also reported in dogs after 8 weeks on a dietary level
of triphenyltin hydroxide of 25 mg/kg and 8 weeks on 50 mg/kg.
7.2.7 Haematological effects
Decreases in the numbers of erythrocytes and reticulocytes were
reported in rats after daily administration of an oral dose of
dibutyltin sulfide at 0.1 mg/kg body weight for 6 months (Mazaev &
Slepning, 1973). A mild anaemia was noted in rats that had received
dibutyltin dichloride for 3 months at a dietary level of 80 mg/kg, but
a level of 40 mg/kg did not produce this effect (Gaunt et al., 1968).
In vitro studies of the haemolytic activity of trialkyltin
compounds indicated that the most haemolytic organotin compounds were
the alkyltin derivatives with alkyl groups of 3-6 carbon atoms
(Byington et al, 1974). Decreases in the percentage haemoglobin and in
the number of erythrocytes were reported in guinea-pigs treated with
triphenyltin acetate at a dietary level of 10 mg/kg for 4 months
(FAO/WHO, 1971, p. 337). A reduction in haemoglobin and leukocytes was
also seen in rats after 12 weeks on a dietary level of triphenyltin
hydroxide of 50 mg/kg (FAO/WHO, 1971, pp. 337-333).
7.2.8 Central nervous system effects
Intoxication in animals by lower trialkyltin compounds is
manifested as generalized weakness progressing to paralysis, sometimes
accompanied by generalized tremor. In rabbits, typical reactions to a
lethal dose (5 mg/kg body weight) of triethyltin sulfate administered
intravenously are prostration, flaccid paralysis, and encephalopathy
(Stoner et al, 1955). These responses resemble those observed in human
subjects, accidentally poisoned with triethyltin compounds. Daily
intraperitoneal injections of triethyltin sulfate at 5 mg/kg body
weight in saline solution resulted in death in rats within 3 days.
Diffuse haemorrhagic encephalopathy was found in the brain (Suzuki,
1971). Rats maintained on a dietary level of triethyltin hydroxide of
20 mg/kg displayed weakness in the hind legs after one week. The
weakness reached a maximum at 3-4 weeks, when about half of the rats
died, but the signs disappeared in one week, when normal diet was
restored. Some of the rats showed signs of becoming resistant to the
substance. At a dietary level of 40 mg/kg, the recovery process was
unaltered but, at 80 mg/kg, the rats developed generalized muscular
tremors that resembled those seen in acute trimethyltin poisoning
(Stoner et al, 1955). This course of events, including the development
of resistance to triethyltin hydroxide at a dietary level of 20 mg/kg
was corroborated by Magee et al. (1957), who was also able to produce
a specific lesion of the central nervous system (CNS) by feeding rats
with a dietary level of triethyltin hydroxide of 20 mg/kg. The lesion
consisted of an oedema extending throughout the white matter. It was
microscopically visible after 3 days of exposure and progressed to a
maximum at about 2 weeks. After 4 months of normal diet, the changes
in the brain and spinal cord disappeared. Electronmicroscopic studies
of this lesion in rabbits disclosed that the myelin sheaths split to
form clefts and vacuoles in which the fluid responsible for the oedema
accumulated (Aleu et al., 1963). The triethyltin-induced lesion of the
CNS is different from those produced by alkyl derivatives of lead,
antimony, bismuth, and mercury, which cause damage to the nerve cells,
but appears to be similar to that produced in rats by exposure to
hexachlorophene (Kimbrough & Gaines, 1971). When 5 mg of triethyltin
sulfate per litre of drinking water was administered to newborn rats
and their mothers for 4 months, all young rats were asymptomatic,
whereas the mothers showed paralysis of posterior limbs, severe
cerebral oedema, and status spongiosus of the white matter (Suzuki,
1971). Hedges & Zaren (1969) described papilloedema in monkeys
following a single intraperitoneal dose of triethyltin acetate of
0.1 mg/kg, body weight administered as a 5% solution. Oedema of the
white matter of the brain extended through the chiasma and orbital
optic nerve to the retrolaminar area, but the papilloedema was not due
to extension of the intramyelinic tin oedema of the nerves through the
lamina. Papilloedema does not develop in the cat when similarly
treated, in spite of profound swelling of the intracerebral white
matter, chiasma, and the orbital optic nerve as far as the
retrolaminar portion.
Tricyclohexyltin hydroxide appears to be a depressant for the
central nervous system, although it is not as potent as triethyltin
acetate and cerebral oedema does not occur (FAO/WHO, 1971, p. 526).
Johnson et al. (1975) studied the acute toxicity of tricyclohexyltin
hydroxide in sheep by intrarumenal injection of doses ranging from 15
to 750 mg/kg body weight. Central nervous depression was observed at
50 mg/kg body weight.
Because of the enzymatic conversion of tetraalkyltin compounds by
hepatic microsomal enzymes (section 6.2.4), these compounds act in a
similar way to trialkyltin compounds. The toxicity of tetraalkyltin
compounds in mice and clogs was studied by Caujolle and his colleagues
(1954). Tetraethyltin was the most active, tetramethyltin slightly
less toxic, and for the higher members of the series, the toxicity
decreased with increasing molecular weight. The toxic effects of
tetramethyltin differed from those of the other members of the series
and the dominant findings were tremors and hyperexcitability. The
major effects of the other members of the tetraalkyltin series were
muscular weakness and paralysis followed by respiratory failure.
Development of the signs of poisoning were noticeably slow following
administration of tetraalkyltin compounds and death was often delayed.
Intravenous administration of tetraethyltin at 25 mg/kg produced a
slight increase in the respiratory rate and vasodilatation as
immediate effects, but after 1.5-2 h, prostration with muscular
weakness was noted. The later effects resembled those seen after
administration of triethyltin; the mode of death was also similar
(Stoner et al., 1955).
7.2.9. Effects on reproduction and the fetus
Few studies of the effects of disubstituted organotin compounds on
reproduction have been reported. Nikonorow et al. (1973) reported
toxic effects of dioctyltin- S,S'-bis(isooctylmercaptoacetate) on the
embryo and fetus at daily oral doses of 20 and 40 mg/kg body weight,
administered for about 3 months. A distinct increase in the incidence
of fetal deaths was observed. Teratogenic effects were not found.
Investigations on triphenyltin hydroxide have yielded variable
results. In 2 investigations on rats, oral dose rates of 20 mg/kg per
day for less than 4 weeks produced histologically evident
abnormalities in the testes and ovaries. Diminution in the size of the
testes and less advanced maturation of the germinal epithelium was
seen in rats receiving triphenyltin hydroxide at a rate of 5 mg/kg for
90 days, although a 3-generation reproduction study at this dietary
level failed to demonstrate any adverse effect on the reproductive
indices (FAO/WHO, 1971, pp. 332-333). In another experiment (Gaines &
Kimbrough, 1968), dietary levels of up to 200 mg/kg over a 276-day
period caused a reversible reduction in fertility in male rats which
was considered to be due to a pronounced decrease in food intake, that
was later reversed. No gross abnormalities were seen in the offspring
of these males when mated with untreated females. In 3 later
experiments (FAO/WHO, 1971, p. 333) in which weanling animals were fed
the compound in their diets at doses of up to 25 mg/kg, the previously
reported change in testicular development could not be confirmed.
In a 3-generation study in which rats produced 2 litters per
generation, animals continuously received a dietary level of
tricyclohexyltin hydroxide of up to 100 mg/kg (corresponding to 4-6 mg
test compound/kg body weight per day for an adult rat). No evidence of
any adverse effects on reproduction could be found and examination of
the fetuses did not reveal any indication of teratogenic effects
(FAO/WHO, 1971, p. 524). No evidence of teratogenic effects from
tricyclohexyltin hydroxide was obtained in a study in which pregnant
rabbits received up to 3 mg/kg body weight per day from the 8th to
16th day of gestation (FAO/WHO, 1971, p. 524). In a study in which
diets containing tricyclohexyltin hydroxide were fed to Japanese
quail, no evidence of ill effects on egg fertility or hatchability was
seen at a dietary level of 1 mg/kg.
Effects observed at a dietary level of 10 mg/kg were of doubtful
significance but a dietary level of 100 mg/kg had definite effects on
fertility, egg production, hatchability, and embryonic mortality
(FAO/WHO, 1971, pp. 523-524).
7.2.10 Carcinogenicity
In an 18-month study, mice were given an oral dose (stomach tube)
of 0.46 mg/kg body weight per day of triphenyltin acetate between the
ages of 7 and 28 days and thereafter a dietary level of 1206 mg/kg.
There was no statistically significant increase in tumours compared
with a control group (Innes et al., 1969). In a 2-year study on rats
receiving tricyclohexyltin hydroxide at concentrations up to 12 mg/kg
body weight (section 7.2.3), the pattern of tumour incidence
throughout both the control and test groups appeared to be random and
did not suggest a dose-response relationship (FAO/WHO, 1971, p. 527).
Reports of experiments specifically designed to investigate the
carcinogenicity of other compounds were net available to the Group.
7.2.11 Effects on chromosomes
Mazaev & Slepnina (1973) reported an increased percentage of
chromosomal aberrations in bone marrow cells and also an increased
mitotic index in cells of the small intestine mucosa of rats that had
received dibutyltin sulfide at 0.1 mg/kg body weight per day
administered by intubation over a period of 6 months. Doses of less
than 0.1 mg/kg body weight per day did not cause poisoning and did not
have any effect on the chromosomes or somatic cells.
7.2.12 Other effects
A reduction in weight gain, mainly related to reduced food intake,
has been recorded in various species given organotin compounds. In
rats, a dietary level of dibutyltin dichloride of 80 mg/kg
administered for 90 days caused growth retardation and decreased food
intake (Gaunt et al., 1968). Triphenyltin acetate given to guineapigs
at dietary levels exceeding 5 mg/kg for 2 years, caused a dose-related
inhibition of growth rate (FAO/WHO, 1971, p. 341). A dietary level of
tricyclohexyltin hydroxide of 25 mg/kg for 90 days, caused a slight
reduction in weight gain in female rats (Shirasu, 1970), and dogs
receiving the same compound in their diet at a level of 12 mg/kg for 6
months also lost weight; some animals that totally refused the food
died from starvation (FAO/WHO, 1971, pp. 526-527).
Severe cardiovascular changes were demonstrated electro-
cardiographically in sheep after intrarumenal administration of
tricyclohexyltin hydroxide at doses greater than 150 mg/kg body weight
(Johnson et al., 1975).
7.2.13 Mechanisms o[ action
There have been relatively few studies of the biochemical effects
of dialkyltin compounds, which do not cause cerebral oedema. In acute
toxicity experiments on the rat, diethyltin compounds did not affect
the sodium and potassium contents of the central nervous system but
caused a decrease in its water con, tent (Magee et al, 1957).
The action of a homologous series of disubstituted
organotin-compounds from dimethyl- to dioctyltin have been examined;
most of them inhibit mitochondrial respiration by preventing the
oxidation of keto acids, presumbly via the inhibition of alpha-keto
oxidase activity, leading to the accumulation of pyruvate (Aldridge,
1976; Piver, 1973).
Of the trisubstituted compounds, triethyltin compounds have been
the most closely examined in relation to the mechanism by which they
cause ill effects. Trimethyl- and triethyltin compounds are potent
inhibitors of oxidative phosphorylation in the mitochondria for which
these compounds have a high binding affinity (Aldridge, 1958; Aldridge
& Street, 1964, 1970, 1971). In a later study by Aldridge (1976),
triorganotin compounds were stated to derange mitochondrial function
in 3 different ways, namely by secondary responses caused by discharge
of a hydroxyl-chloride gradient across mitochondrial membranes, by
interaction with the basic energy conservation system involved in the
synthesis of ATP, and by an interaction with mitochondrial membranes
causing swelling and disruption.
Using a 113Sn-labelled triethyltin compound, Rose (1969)
identified a pair of histidine residues on guineapig liver
mitochondria as the binding site for triethyl compounds. Thus, one
molecule of rat haemoglobin binds 2 molecules of triethyltin; the
binding sites are located in the globin.
Stockdale et al. (1970) showed that the order of effectiveness for
the organotin compounds in inhibiting coupled respiration was tributyl
> tripropyl > triphenyl > trimethyl. Two separate effects were
suggested: (a) an oligomycin-like inhibition of coupled
phosphorylation; and (b) an alteration of hydroxide exchange across
lipid membranes producing uncoupling, swelling, and reduction of
intramitochondrial substrate and phosphate concentrations followed by
structural damage.
7.2.14 Effective doses and dose rates
7.2.14.1 Lethal doses
The median lethal doses for some mono-, di-, tri-, and
tetrasubstituted organotin compounds are given in Tables 13, 14, 15,
and 16.a Trimethyl and triethyltin compounds, administered orally,
are more toxic than the higher homologues of the trialkyltin group.
The oral toxicity diminishes progressively from tripropyltin to
trioctyltin compounds (Barnes & Stoner, 1958). This is probably
because of poorer absorption of higher trialkyltin compounds from the
gastrointestinal tract, as judged from the difference between the oral
and intraperitoneal toxicity of higher trisubstituted alkyltin
compounds (Stoner et al., 1955). The intraperitoneal toxicity of
various trialkyltin compounds does not differ to any large extent. In
rats, Stoner et al. (1955) found that triethyltin sulfate was equally
toxic after intravenous, intraperitoneal, and oral administration. The
lethal dose per kg body weight given intravenously or
intraperitoneally was 10 mg/kg, causing death within 4-5 days. A dose
of 40 mg/kg body weight killed the rat in 2 h. The oral LD50 for
tricyclohexyltin hydroxide (Table 15) for most species, appears to be
between 100 and 1000 mg/kg body weight, while the intraperitoneal and
intravenous routes yield LD50 values below 20 mg/kg. The difference
between oral and parenteral toxicity reflects the poor absorption of
tricyclohexyltin hydroxide from the gastrointestinal tract.
In rats and dogs fed with tricyclohexyltin hydroxide, the
metabolites, dicyclohexyltin oxide and traces of cyclohexylstannoic
acid have been identified. Dicyclohexyltin oxide and
cyclohexylstannoic acid also constitute a small portion of the
residues on fruit as a result of photodecomposition (FAO/WHO, 1971,
pp 522, 534). The median lethal dose of these metabolites may
therefore be of interest. The LD50 of dicyclohexyltin oxide in the
rat appears to be about 350 mg/kg body weight, whereas the LD50 value
of cyclohexylstannoic acid in this species is probably ten times
higher (FAO/WHO, 1971, pp. 524-526).
Only a small amount of data is available concerning the acute
toxicity of tetraalkyltin compounds. The median lethal dose for
tetraethyltin is given in Table 16.
a Additional data on the toxicity of organotin compounds may be
found in a proposal for the United Nations classification and hazard
grouping of organotin compounds submitted by the expert from the
Netherlands to the Committee of Experts on the Transport of Dangerous
Goods of the United Nations Economic and Social Council
(EN/CN.2/CONF.5/R.607 of November 1976).
Table 13. Acute oral toxicity of some mono-organotin compounds in various animal species
Medium lethal
Trivial name of compound dose Species Reference
(LD50, mg/kg)
butylstannoic acid > 6000 mouse Pelikan & Cerny (1970b)
butyltin trichloride 1400 mouse
2140 rat Marhold (1972)
butyltin-S,S'S"-tris 1520 mouse Pelikan & Cerny (1970b)
(isooethylmercaptoacetate)
octyltin trichloride 4600 mouse Klimmer (1969)
octyltin-S,S',S"-tris 1500 rat (male) Pelikan & Cerny (1970a)
(2-ethylexylmercaptoacetate)
7.2.14.2 Minimum effective and no-observed-effect doses
When dibutyltin dichloride was fed to rats for 90 days at dietary
levels of 10, 20, 40, and 80 mg/kg, the no-observed effect level was
40 mg/kg, equivalent to 2 mg/kg body weight per day (Gaunt et al.,
1968). When dibutyltin was administered to rats for 6 months, the
no-observed-effect level was 20 mg/kg diet (Barnes & Stoner, 1958).
Dibutyltin sulfide, administered to rats at oral doses of 1.0, 0.1,
0.01, and 0.001 mg/kg body weight, per day for 7 months, was tolerated
without any detected adverse effects up to a dose o.f 0.01 mg/kg per
day but the higher levels of 0.1 and 1.0 mg/kg per day caused
intoxication (Mazaev & Korolev, 1969). By comparison, the toxicity of
dioctyltin compounds was relatively low (Klimmer, 1969). Thus,
dioctyltin did not produce any injury in rats, mice, or guineapigs at
oral doses up to 400 mg/kg body weight per day when given for 3-4
successive days, nor were there any ill effects when it was added to
the daily diet of rats for 4 months at a rate of 200 mg/kg (Barnes &
Stoner, 1958).
Table 14. Acute toxicity of some diorganotin compounds in various animals
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dibutylin di(2-ethylhexoate) 200 (oral) rat (male) Calley et al. (1967)
dibutyltin di(butyl maleate) 120 (oral) rat Klimmer (1969)
dibutyltin di(nonylmaleate) 170 (oral)
dibutyltin dichlorlde 100 (oral) rat (male) Klimmer (1969)
182 (oral) rat (male) Mazaev et al. (1971)
112 (oral) rat (female)
35 (oral) mouse
190 (oral) guineapig
150 (oral) rat Marhold (1972)
dibutyltin-S,S'-bis 150 (oral) rat (male) Klimmer (1969)
(2-ethylhexylmercaptoacetate) 175 (oral) Klimmer (1969)
dibutyltin dilaurate 243 (oral) rat Marhold (1972)
45 (oral) rat Marhold (1972)
dibutyltin oxide 520 (oral) rat (male) Klimmer (1969)
39.9 (i.p.)a rat (female) Robinson (1969)
24 (oral) mouse Mazaev & Slepnina (1973)
dibutyltin sulfide 145 (oral) rat (male)
150 (oral) rabbit
180 (oral) rat (female)
800 (i.p.) rat (female) Robinson (1969)
> 800 (i.p.) rat (female) Robinson (1969)
dioctyltin acetate 2030 (oral) rat Klimmer (1969)
diocyltin dibutylmaleate 3750 (oral) mouse Pelikan et al. (1970)
dioctyltin-S,S'-bis
(butylmercaptoacetate) 1140 (oral) white mouse Pelikán & Cerny (1970a)
dioctyltin bis
(dodecylmercaptide) 4000 (oral) white mouse
dioctyltin bis
(2-ethylhexylmaleate) 2760/ (oral) rat (male) Klimmer (1969)
2750
Table 14. (contd).
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dioctyltin-S,S'-bis 2010 (oral) white mouse Pelikan & Cerny (1970a)
(2-ethylhexylmercaptoacetate)
dioctyltin-S,S'-bis 3700 (oral) rat (male) Klimmer (1969)
(laurylmercaptoacetate)
dioctyltin-S,S'-(1,4- 2950 (oral) rat (male)
butane-diol-bis-mercaptoacetate)
dioctyltin dichloride 5500/
8500 (oral) rat (male)
dioctyltin dilaurate 6450 (oral) rat (male)
800 (i.p.) rat (female) Robinson (1969)
dioctyltin di(1,2-
propylene-glycolmaleate) 4775 (oral) rat (male) Klimmer (1969)
dioctyltin-S,S'-
(ethyleneglycol-bis-
dimercaptoacetate) 880 (oral) rat (male)
dioctyltin maleate 4500 (oral) rat (male)
dioctyltin
ß-mercapto-propanoate 1850/
2050 (oral) rat (male)
dioctyltin oxide 2500 (oral) rat (male)
dioctyltin mercaptoacetate 945 (oral) rat (male)
a intraperitoneal
Table 15. Acute toxicity of some triorganotin compounds in various animals
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
2-trichloro-1-(butine-1'-
oxide)-1-triethyl- 9.8 (i.p.) rat Ignatjeva (1968)
stannyloxy)ethane 9.6 (i.p.) mouse Ignatjeva (1968)
triethylstannylmethyl-
(1-propynyl)formal 10.7 (i.p.) rat Ignatjeva (1968)
triethylstannylphenyl-
acetylene 9.9 (i.p.) mouse Ignatjeva (1968)
9.1 (i.p.) rat Ignatjeva (1968)
1-triethylstannyl-3-
trimethylsiloxi-1-
propyne 11.8 (i.p.) mouse Ignatjeva (1988)
11.4 (i.p.) rat Ignatjeva (1968)
triethyltin acetate 4 (oral) rat (female) Stoner (1966)
triethyltin chloride 5 (i.p.) rat (female) Robinson (1969)
triethyltin sulfate 5.7 (i.p.) rat (male) Stoner (1966)
5.3 (i.p.) guineapig Stoner (1966)
tributyltin acetate 46 (oral) white mouse Pelikan & Cerny (1968a)
99 (oral) J. Pharm. Sci. (1967)
133 (oral) rat (male) Klimmer (1969)
tributyltin benzoate 108 (oral) white mouse
Pelikan & Cerny (1968a)
132 (oral) rat Arzneimittelforsch. (1969)
tributyltin chloride 117 (oral) white mouse Pelikan & Cerny (1968a)
129 (oral) rat Manhold (1972)
tributyltin laurate 180 (oral) white mouse Pelikan & Cerny, (1968a)
tributyltin oleate 195 (oral) rat Klimmer (1969)
tributyltin salicylate 137 (oral) rat (male) Klimmer (1969)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
bis(tributyltin oxide) 112- (oral) rat (male) Klimmer (1969)
132
180 (oral) rat Truhaut et al. (1976)
234 (oral) rat Sheldon (1975)
194 (oral, rat (male) Elsea & Paynter (1958)
aqueous
solution)
148 (oral, rat (male) Elsea & Paynter (1958)
oil
solution)
11.7 (dermal) albino rabbit Elsea & Paynter (1958)
10.0 (oral) white mouse Pelikan & Cerny (1969)
trihexyltin acetate 1000 (oral) rat Barnes & Stoner (1958)
trioctyltin chloride 10 000 (oral) rat (male) Klimmer (1969)
triphenyltin acetate 21 (oral) guineapig Klimmer (1963)
24 (oral) guineapig Kimbrough (1976)
136 (oral) rat Klimmer (1963)
81 (oral) mouse (male) FAO/WHO (1971)
7.9 (i.p.) mouse (male) Stoner (1966)
136 (oral) rat (male) Klimmer (1964)
491 (oral) rat (female) Stoner (1966)
450 (dermal) rat (male) Klimmer (1963)
8.5 (i.p.) rat (female) Stoner (1966)
11.9 (i.p.) rat (female) Stoner (1966)
13.2 (i.p.) rat (male) Klimmer (1964)
21 (oral) guineapig
(male) Klimmer (1964)
3.7 (i.p.) guineapig
(male) Stoner (1966)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
triphenyltin acetate 5.3 (i.p.) guineapig
cont'd. (male) Klimmer (1964)
30- (oral) rabbit (male) Klimmer (1964)
50
triphenyltin chloride 80 (oral) rat (male) FAO/WHO (1971)
135 (oral) rat (female) FAO/WHO (1971)
triphenyltin hydroxide 245 (oral) mouse (male) FAO/WHO (1971)
209 (oral) mouse (female)
FAO/WHO (1971)
240 (oral) rat (male) Gaines & Kimbrough (1968)
360 (oral) rat (female) Gaines & Kimbrough (1968)
27.1 (oral) guineapig FAO/WHO (1971)
(male)
31.1 (oral) guineapig FAO/WHO (1971)
(female)
171 (oral) rat (male) Marks et al. (1969)
268 (oral) rat (female) Marks et al. (1969)
tricyclohexyltin 710a (oral) mouse- FAO/WHO (1971)
hydroxide peromyscus
1070a (oral) mouse-swiss FAO/WHO (1971)
white
540 (oral) rat FAO/WHO (1971)
13 (i.p.) rat FAO/WHO (1971)
780 (oral) guineapig FAO/WHO (1971)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
tricyclohexyltin 9 (i.p.) guineapig FAO/WHO (1971)
hydroxide 500- (oral) rabbit FAO/WHO (1971)
cont'd. 1000
> 126 (i.p.) rabbit FAO/WHO (1971)
150a (oral) sheep Johnson et al. (1975)
14 (i.v.)c dog FAO/WHO (1971)
6 (i.v.) cat FAO/WHO (1971)
a approximate lethal dose
b intraperitoneal
c intravenous
Table 16. Acute oral toxicity of some tetraorganotin compounds in various animal species
Trivial name Medium lethal dose Species Reference
of compound (LD50, mg/kg)
tetraethyltin 40.0 mouse Skackova (1967)
15.0 rat
40.0 guineapig
7.0 rabbit
40 mouse Mazaev et al. (1971)
9.0 rat
40.0 guineapig
7.0 rabbit
tetrabutyltin 6000 rat Skackova (1967)
Data on effective doses of trisubstituted organotin compounds are
available only for triphenyltin derivatives and tricyclohexyltin
hydroxide. Triphenyltin acetate was administered, by gavage, to groups
of rats at doses equivalent to dietary levels of 5, 10, 25, and
50 mg/kg for up to 17 days. While no adverse effects were found at the
25 mg/kg level, the 50 mg/kg level resulted in the death of animals,
mainly as a result of infection (Klimmer, 1964). In a 2-year study on
rats fed diets containing concentrations of triphenyltin hydroxide of
up to 10 mg/kg, the no-observed-effect level was about 2 mg/kg
(equivalent to about 0.1 mg/kg body weight per day) (FAO/WHO, 1971,
pp. 341-342). In another 2-year study in which groups of guineapigs
were fed diets containing concentrations of triphenyltin acetate, up
to 200 mg/kg, the no-observed-effect level was 5 mg/kg. At higher
levels there was a dose-related increase in mortality and the
occurrence of other effects such as inhibition of growth rate and
fatty degeneration of the liver and heart (FAO/ WHO, 1971, p. 341).
With respect to tricyclohexyltin hydroxide, no toxic effects were
noted in rats receiving 12.5 mg/kg body weight daily for 19 days,
whereas a rate of 25 mg/kg per day caused toxic effects (FAO/WHO,
1971, pp. 527-528). Rats were fed on diets providing up to 12 mg/kg
body weight per day of tricyclohexyltin hydroxide in a 2-year study;
the no-observed-effect level was 3 mg/kg body weight per day (FAO/WHO,
1971, p. 528). When dogs were fed tricyclohexyltin hydroxide in the
diet at concentrations of up to 12 mg/kg body weight per day for 6-24
months, many dogs refused the 12 mg/kg per day diet, causing weight
loss and death from starvation. The no-observed-effect level was found
to be about 0.75 mg/kg per day (FAO/WHO, 1971, pp. 526-527). Johnson
et al. (1975) also reported the effects of thoroughly wetting the skin
of yearling cattle, goats, and sheep with suspensions of
tricyclohexyltin hydroxide. They found that cattle tolerated
suspensions up to a concentration of 5 g/litre while anorexia was
registered in some animals after spraying with a suspension of
10 g/litre. Yearling goats and sheep tolerated suspensions of up to
1 g/litre. Transitory anorexia and eye irritation developed after
application of a suspension of 20 g/litre to the skin of goats.
Few data are available on the effective dose levels of
tetrasubstituted organotin compounds. Intravenous administration of
tetraethyltin at 25 mg/kg body weight produced a slight increase in
respiratory rate and vasodilatation as immediate effects, but, 1.5-2 h
later, prostration with muscular weakness occurred. (Stoner et al.,
1955).
8. EFFECTS ON MAN
There are comparatively few clinical observations and
epidemiological data concerning the effects of inorganic tin compounds
on man and even fewer on the effects of organotin compounds.
8.1 Inorganic Tin Compounds
8.1.1 Acute poisoning
Some episodes of acute poisoning have been reported, mainly in
association with the ingestion of fruit juices containing high
concentrations of tin. The major symptoms and signs noted were nausea,
vomiting, diarrhoea, fatigue, and headache. The concentrations of tin
in the products thought to have been associated with the incidents
were uncertain in many cases, but were probably in the range of
300-500 mg/kg (Horio et al, 1967). Orange and apple juice, containing
tin concentrations of 250-385 mg/kg were suspected of causing one
incident (Benoy et al., 1971). Nausea, vomiting, and diarrhoea were
recorded in individuals consuming-peach preserves that contained a tin
concentration of 563 mg/kg, zinc at 1.5 mg/kg, cadmium at 0.1 mg/kg,
lead at 0.16 mg/kg, copper at 1 mg/kg, nitrate at 93 mg/kg, nitrite at
1.7 mg/kg, and chloride at 115 mg/kg (Nehring, 1972). Acute
gastroenteritis followed ingestion of a fruit punch stored in a tin
can and containing a tin concentration of 2000 mg/litre. First
symptoms occurred after 1-2 h, the earliest and commonest being
bloatedness, followed by severe nausea, stomach cramps, vomiting and,
in one-third of the patients, mild diarrhoea (Warburton et al, 1962).
Other similar outbreaks have involved canned cherries (Luff & Metcalf,
1890), asparagus, herring (Schryver, 1909), and apricots (Savage,
1939) with tin concentrations ranging from 300 to about 1000 mg/kg.
An incident of acute intoxication after the ingestion of canned
peaches was reported by Sverrsson (1975). It was unique in that about
110 participants in a meeting received nothing else to eat or drink,
except for the peaches. The report was based on questionnaires
received from 85 persons, 76 (89%) of whom had fallen ill. About half
of these had developed symptoms such as nausea, vomiting, and
diarrhoea within 1 h. The majority became symptom-free after 24 h. The
fruit contained a mean tin concentration of 533 mg/kg (range
413-597 mg/kg) and the juice 369 mg/kg (range 298-405 mg/kg). Two out
of 7 persons who had consumed only one quarter of the contents fell
ill. The calculated intake of tin for these persons was 50 mg.
In another report, canned tomato juice was associated with 113
cases of acute gastroenteritis (Barker & Runte, 1972). In a small
number of these cases, superficial erosion of the mucosa of the mouth
was observed, while abdominal distension and cramps, and diarrhoea
were quite common. The cans containing the juice showed complete
corrosion of tin linings and this yielded a product containing a tin
concentration of approximately 400 mg/kg. The crops of tomatoes, used
to make this particular juice, had been grown in soil excessively
treated with nitrate fertilizer and contained high levels of nitrate;
complete corrosion of the lining of the cans occurred within
approximately 6 months.
Five human volunteers did not show any toxic signs after drinking
fruit juices containing about 500 or 730 mg/kg of tin, but all had
some gastrointestinal disturbance after drinking 5-7 ml/kg body weight
of fruit juice containing a tin concentration of about 1400 mg/litre
(Benoy et al., 1971). There was no evidence to indicate that the
effects were due to the absorption of tin, the likeliest cause being
local irritation of the mucous membranes of the alimentary tract.
8.1.2 Prolonged exposure
8.1.2.1 Effects of inhalation
The only available information on exposure by inhalation pertains
to pneumoconiosis caused by the inhalation of tin(IV) oxide. This
benign condition is termed stannosis.
More than 200 cases of stannosis have been described (Bartak
et al., 1948; Cutter et al., 1949; Dundon & Hughes, 1950; Oyanguren
et al., 1958; Pendergrass & Pryde, 1948; Robertson & Whitaker, 1957;
Schuler et al., 1958). The relative importance of exposure to tin
fumes in the etiology of this disorder was emphasized by Dundon &
Hughes (1950). The significance of the quantity of dust and the
duration of exposure were stressed by Robertson & Whitaker (1957), who
reviewed 121 cases.
Pendergrass & Pryde (1948) noted stannosis in a man who, for 15
years, had been bagging tin oxide material containing 96.5% tin(IV)
oxide and small amounts of aluminium, iron, and sodium but not silica.
Radiography revealed small dense shadows (denser than those of
silicosis) resembling those of barytosis in both lungs. Bartak et al.,
(1948) reported a similar case in a workman who had suffered from
asthma for many years and who attended a furnace in which metallic tin
was burnt to produce tin(IV) oxide. Necropsy of this man, who died
from gastric carcinoma, revealed deposits of tin(IV) oxide in the
lungs, lymph glands, liver, and spleen. Six other workmen with a
similar radiographic appearance of the lungs, did not have any
symptoms of asthma or signs of pulmonary dysfunction. Similar
radiological findings were reported by Cutter et al. (1949) in 2 cases
with nodules 1-2 mm in diameter unaccompanied by evidence of pulmonary
dysfunction. Both had been working in a tin recovery department for 20
years.
Oyanguren et al., (1958) reported 10 cases of stannosis in workers
involved in a process where tin ore concentrates were reduced to
metal. The workers were exposed to dust with a high tin and a low
silica content and to fumes rich in tin. None of the exposed workers
had any disability and the vital capacity, maximal breathing and
resting minute volume, and respiratory reserve were normal, as were
the findings on the blood and urine. Radiological examination of these
10 workers showed that the first alteration appeared to be increase of
bronchovascular markings, with hilar thickening in the early stages;
later, well-defined nodular elements appeared first in the middle
third of the right and then of the left lung. These appeared to
progress to the rest of the lung fields, and with continuous exposure,
accumulation of tin(IV) oxide gave a metallic density to a part of the
nodules, which subsequently acquired an appearance of lipoid droplets.
In the later stages, the bronchovascular markings disappeared as the
density of the shadows increased. The radiographic changes appeared
after some 3-5 years of exposure (Schuler et al., 1958). Hlebnikova
(1957) made a survey over a number of years of workers, who were
exposed to condensation aerosols, formed during the smelting of tin
and consisting mainly of tin(IV) oxide. The free silica concentration
in the aerosols was not more than 1% and the total silica did not
exceed 3%. The total dust concentration in air varied between 3 and
70 mg/m3. Workers developed pneumoconiosis after 6 to 8 years of
working at the smelter. The authors described 45 cases of
pneumoconiosis in workers, who were employed at the smelter from 6 to
20 years, Six of them were reported to have second degree
pneumoconiosis. X-ray examination showed small spotty shadows, but
there were no other symptoms or signs. The opacities seen on the
radiographs were thought to be due to the accumulation of
tin-containing dust and the development of connective tissue, This was
confirmed by experiments on rats exposed to tin(II) oxide. No cases of
pneumoconiosis were observed in 10 years, after the dust concentration
had been reduced to 10 mg/m3. An important feature of stannosis is
that fibrosis of the lung does not develop, providing that other
agents such as silica are not present.
8.1.2.2 Effects of ingestion
Packaged military rations were fed to 9 young male adult
volunteers for successive 24-day periods. The average tin content of a
control fresh diet was 13 mg/kg (in dry solids), while C-rations
stored at 1°C contained a tin concentration of 33 mg/kg, and rations
stored at 37°C contained 204 mg/kg. All the tin ingested was
accounted for by faecal elimination. No toxic effects were noted
(Calloway & McMullen, 1966). Dack (1955) reported a study in which 4
subjects ate canned pumpkin containing a tin concentration of about
380-480 mg/kg and canned asparagus containing a concentration of about
360 mg/kg, for 6 days, with no apparent illness. In an earlier study,
Mamontova (1940) did not observe any toxic manifestations in 4
volunteers who, for a period of 30 days, consumed 250 g per day of
canned fruit containing 212-250 mg of tin.
8.2 Organotin Compounds
8.2.1 Local effects
Toxic lesions among laboratory and process workers handling di-
and tributyltin compounds were reported by Lyle (1958). Most of the
lesions were typical acute skinburns, caused by the colourless di- or
tributyltin chlorides, which can come into contact with the skin
without exposed workers being aware of it. This type of lesion
developed 1 to 8 h following exposure. When the substance was washed
off immediately, no lesion developed. A more diffuse, but less rapidly
healing lesion, was caused by contact with clothes that had been
moistened by vapour or liquid compounds. This subacute irritation was
characterized by itching, affecting mostly the skin of the lower
abdomen, thighs, and groin. On examination, an easily distinguishable
erythematous eruption, was noted. Cessation of contact with the
compound was followed by rapid healing. Following contact with the
eye, lachrymation and severe suffusion of the conjunctivae appeared
within a few minutes and persisted for 4 days. Immediate lavage of the
eye did not prevent the development of the signs.
Lyle (1958) also studied the skin lesions induced by butyltin
compounds by applying various compounds on the skin of the back of the
hands of 5 volunteers. Skin lesions could be produced by a single
application of dibutyltin dichloride, and of the chloride, acetate,
and oxide of tributyltin, while the diacetate, dilaurate, oxide, and
maleate of dibutyltin and also tetrabutyltin failed to produce any
lesions. No visible changes occurred for 2 go 3 h after application,
although a swelling of the mouth of the hair follicles was noticeable.
The follicular inflammation progressed during the following 8 h with
only slight visible irritation of the skin between the openings of the
follicles. On the second day, sterile pustules developed over the
follicular openings, but remained small during the next 3 to 4 days.
After one week, the lesions had practically disappeared.
Symptoms experienced by female spray-painters working with a latex
paint, to which was added a fungicidal solution containing 20%
bis(tributyltin) oxide, ethylene oxide, ethanol, and water, were
described by Landa et al. (1973). The symptoms were reported to appear
"immediately" after the start of spraying and were experienced by all
the women engaged in the work. The first sensations were irritation of
the nasal mucosa and the conjunctivae. The exposure continued for
another fortnight during which the symptoms and signs became more
severe, and included bleeding from the nose and mucous discharges. An
otorhinolaryngological examination revealed rhinitis with distinct
hyperaemia and haemorrhages of the nasal septum. The workers reported
that the symptoms were less severe during weekends. When the addition
of the fungicidal solution was abandoned, the symptoms disappeared.
One year later, identical symptoms were reported by 4 spray-painters
using latex paint. An inquiry disclosed that the manufacturer had
begun to use bis(tributyltin) oxide as a fungicidal additive since the
banning of the use of mercury. The authors considered that the
trialkyltin compound was responsible for the symptoms. Measurements
performed later indicated that the concentrations of tin in the
breathing were below 0. 05 mg/m3 air.
Another description of local effects seen in workers handling
triphenyltin acetate was given by Markicevic & Turko (1967). These
workers were engaged in the formulation of a 20% solution of a
fungicide. During the hottest summer days, subjects working in the
dustiest places developed conjunctival irritation and irritation of
the mucous membranes of the upper respiratory tract as well as of the
skin, the hands and scrotum being particularly affected. Effects on
the central nervous system were not registered. The signs disappeared
rapidly after termination of exposure.
A wettable powder formation containing 50% tricyclohexyltin
hydroxide was examined for its irritation and sensitization potential.
No adverse reactions were observed in 53 females after sensitization
applications and a challenge application 20 days later of 0.5 ml of an
emulsion (10 g/litre). Tricyclohexyltin hydroxide was reported not to
be dermally irritating at a concentration of about 0.01 mg/kg body
weight (FAO/WHO, 1971).
8.2.2 Systemic effects
8.2.2.1 Effects of Dermal exposure
In a recent review (NIOSH, 1976), a fatal case was described in
which a 29-year-old woman was accidentally drenched in a slurry
containing triphenyltin chloride, diphenyltin dichloride, hexane, and
other unidentified compounds at a temperature of 79.4°C. On arrival at
hospital, first-degree thermal burns covered 10% of her body. Second-
and third-degree burns with 80-85% desquamated skin developed 12 h
later. Death from renal failure occurred 12 days after the accident.
However, the agent responsible for her symptoms and signs and for the
death could not be identified from the data available.
Mijatovic (1972) reported a case of systemic intoxication
resulting from dermal contact with a compound containing triphenyltin
acetate (60%) and manganese dithiocarbamate (15%). The subject,
engaged in agricultural aviation, spilt the compound on his hands and
chest while filling the aeroplane. The skin on his chest and abdomen
was endured after 3 h and vesicles developed the following day. He
experienced headache, nausea, epigastric pain, and general weakness.
Clinically the liver transaminases (SGPT) were elevated, reaching a
maximum one month later. Two months later the transaminases were
returning to normal, but the patient had pains over the liver, which
was tender and enlarged. The liver damage persisted for 2 years and
the case was labelled chronic hepatitis. No liver biopsy was
performed.
8.2.2.2 Effects of inhalation
Acute intoxication caused by the inhalation of triphenyltin
compounds has been reported in some instances. Three cases occurred in
farmers treating beetroot plants with triphenyltin acetate which was
inhaled (Guardascione & di Bosco, 1967). The symptoms started from a
few minutes to about 2 h after the first exposure. After 2 hours of
spraying, one subject felt a general malaise and severe headache and
eventually lost consciousness. On admission to the hospital he had a
diffuse tremor and a slightly depressed sensorium. All laboratory
investigations during the 2-week hospitalization were normal. When
mixing triphenyl tin acetate powder into a solution, a second subject
experienced repeated flushes, nausea, and shortness of breath, which
started only a few minutes after inhalation. During the 8-day hospital
surveillance, the only pathological finding was glucosuria. A third
subject suffered from severe headache, strong nausea, and epigastric
pains. However, laboratory investigations were normal. The headache
persisted for 2 days while the epigastric pains and the nausea
disappeared after one day. All 3 subjects recovered completely.
Horacek & Demcik (1970) reported a group poisoning involving 2
pilots and 3 mechanics following the spraying of a formulation
comprising 60% of triphenyltin acetate and 15° of manganese
dithiocarbamate. Protective measures were found to be deficient and
food was consumed with unwashed hands; thus, exposure by both
inhalation and ingestion could have occurred. Furthermore, other
pesticides containing copper, zinc, DDT, and organophosphates were
handled during exposure to the formulation, but the authors considered
these substances unlikely to be responsible for the symptoms
experienced. Exposure to the formulation continued for 2 weeks before
symptoms were recognized and continued for 2 further weeks. One pilot
experienced gastric pain, diarrhoea, and dryness of the mouth with
severe thirst that was not relieved by drinking. He also felt pressure
over the chest and slight shortness of breath. Blurred vision was
experienced after one week of exposure. Clinically hepatomegaly with a
painful liver was noted. Liver transaminases (SGPT) were elevated and
the highest recorded value was obtained 6 weeks later. Hyperglycaemia
and glycosuria were also present. Eight weeks afterwards, liver biopsy
revealed marked diffuse steathosis without any detected necrosis.
Monthly follow-ups showed that hepatomegaly and steathosis persisted
for one year. The second pilot suffered from heartburn, diarrhoea, and
vision disturbances. Clinically a hepatomegaly and a slight
hyperglycaemia were registered. The symptoms persisted for 4 weeks and
the glycosuria and hepatomegaly for 6 weeks. Recovery was complete.
The mechanics had symptoms that were less severe, comprising
diarrhoea, headache, eye pains, blurred vision, epigastric pain, and
thirst.
A worker engaged in the manufacture of butyltin compounds was
reported to suffer from a reduced sense of smell (Akatsuka et al.,
1959). It was first observed after an exposure period of 16 months,
and a further deterioration of the olfactory sense was established
during the following 8 months. The state persisted without any noted
improvement for 2 years. Other reported symptoms were headaches in the
occipital region, nasal haemorrhages, lassitude, and a feeling of
stiffness in the shoulders.
In four eases of acute poisoning due to exposure to organotin
vapours, patients were reported to have suffered from such symptoms as
vertigo, headaches, nausea and vomiting, and visual disturbances.
Clinically, stasis of the papilla was found and all patients displayed
pathological findings on the electroencephalograms. These were
reversible in 7-25 days, and all eases recovered clinically. The
organotin compound or compounds responsible for the intoxications were
not identified (Prüll & Rompel, 1976).
8.2.2.3 Effects of ingestion
A most serious episode involving organotin poisoning occurred in
1954 due to oral administration of a proprietary preparation used for
the treatment of furonculosis, osteomyelitis, anthrax, and acne. The
drug was responsible for about 100 deaths and a total of about 210
intoxications. These numbers vary to some extent depending on the
source of information: Alajouanine et al., (1958) reported a total of
210 deaths whereas another source quotes 102 deaths and at least 100
persons permanently affected ( Br. med. J., 1958). A total of about
400 000 capsules was sold ( Br. med. J., 1958), and about 1000
persons were believed to have taken the drug (Barnes & Stoner, 1959).
However, the capsules consumed by the intoxicated subjects amounted
only to about 7% of the lot distributed ( Br. med. J., 1958),
implying that the majority of the capsules were consumed without any
known adverse effects. The main ingredients of the preparation were
diethyltin diiodide (15 mg/capsule) and linoleic acid
(100 mg/capsule). It was suggested that ethyltin triiodide,
triethyltin iodide, or tetraethyltin could have been present as
impurities because of deficiencies in the manufacturing process or as
metabolites formed under the influence of various physical factors
(Rondepierre et al, 1958). A theory that diethyltin diiodide would
have reacted with the isolinoleic component producing tetraethyltin
was also presented (Lecoq, 1954). The symptoms and clinical findings
described in victims by Alajouanine et al. (1958) seem to favour the
hypothesis of a trialkyltin compound being the causal agent, probably
acting synergistically with other constituents. The clinical data of
201 cases including 98 deaths were reviewed by Alajouanine et al.
(1958). The dominating symptom, reported in about 98% of the cases,
was a diffuse headache, sometimes intolerably severe, and appearing a
few days after medication was started. Nausea and vomiting occurred in
73%, visual disturbances, mainly photophobia, but also double vision,
colour-vision disturbances and, in a few cases, blindness were
recorded in 33% of cases. Ophthalmoscopic findings included
congestion, papilloedema (Druault-Toufesco, 1955), and in some cases,
papillary stasis (Gayral et al., 1958; Pesme, 1955). Frequent symptoms
and signs were urinary incontinence, vertigo, loss of weight, and
abdominal pains. Absence of fever and a tendency towards hypothermia
were also noted. Psychological disturbances or stupor were reported in
70% of the cases. Other findings were meningeal irritation,
somnolence, insomnia, convulsions, constipation, and bradycardia.
Sometimes electroencephalograms were altered but did not suggest any
localized lesion. Death occurred during coma or from respiratory or
cardiac failure and in some cases during convulsions. It is probable
that most of the symptoms and signs could be attributed to a cerebral
oedema, the occurrence of which was established at autopsies and
decompressive surgery (Cossa et al., 1958, Fontan et al., 1958). This
cerebral oedema of the white matter appeared to be very similar to
that produced experimentally by administration of a proprietary
preparation containing an organotin compound, to mice and monkeys
(Gruner, 1958). Macroscopically, the brain was oedematic but the
microscopic findings were minor.
It has been reported that only 10 out of 103 subjects, who
survived, recovered completely: in the remainder, symptoms such as
headaches and asthenia persisted for at least 4 years (Barnes &
Stoner, 1959). Information on later, follow-up studies is not
available. The lethal dose of the preparation was in some instances
only about 25 capsules taken during one week. Ingestion of 3 capsules
was enough to cause intoxication in a 9-year-old child (Fontan et al.,
1955).
8.3 Treatment of Poisoning
Dimercaprol has been suggested by Stoner et al. (1945) to be an
effective antidote for dialkyltin poisoning. It has also been reported
to completely prevent the accumulation of alpha-keto acids produced by
dialkyltin compounds (Barnes & Stoner, 1959). Although dimercaprol
protected rats against the general toxic effects of these compounds,
it did not have any effect on the response to triethyltin compounds.
This seems to be due to the fact that dialkytin compounds, at least up
to the dihexyl derivatives, react readily with sulfhydryl groups and
the trialkyltin compounds do not (Barnes & Magos, 1968),
Studer et al. (1973) reported that steroid therapy (dexamethasone)
appeared to diminish mortality and the severity of brain oedema in
rats; there also appeared to be significant decreases in brain, liver,
and blood levels of triethyltin bromide. The authors suggested that
the beneficial effects of this steroid therapy might be partly due to
enhanced excretion or catabolism of triethyltin bromide.
Surgical decompression was considered to be the only treatment
that offered any benefit in human cases of cerebral oedema caused by
trialkyltin compounds (Alajouanine et al., 1958).
REFERENCES
ADAM, W. B. & HORNER, G. (1937) The tin content of English canned
fruits and vegetables. J. Soc. Chem. Ind., 56: 329-334.
ADAMSON, J. H. (1962) The colorimetric determination of dialkyltin
compounds dissolved in fats and olive oil. Analyst, 87: 597-598.
ADCOCK, L. H. & HOPE, W. G. (1970) A method for the determination of
tin in the range 0.2 to 1.6 µg, and its application to the
determination of organotin stabilizer in certain foodstuffs.
Analyst, 95: 868-874.
AKAGI, H. & SAKAGAMI, Y. (1971) [On degradation of organotin compounds
by ultraviolet ray.] Rep. Public Health Inst., 20: 1-4
(in Japanese).
AKAGI, H., FUJITA, M., & SAKAGAMI, Y. (1972) [Thin layer
chromatography of inorganotin and organotin compounds in foods.]
J. food Hyg., 13: 85-88 (in Japanese).
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A.,
KAWAME, N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S.,
YANAGIBASHI, H., NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA,
I., & IYO, T. (1959) [Experimental studies on disturbance of sense
of smell due to butyltin compounds.] J. Tokyo Med. Coll,
17: 1393-1402 (in Japanese).
ALAJOUANINE, T., DÉROBERT, L., & THIÉFRY, S. (1958) Etude clinique
d'ensemble de 210 cas d'intoxication par les sels organiques
d'étain. Rev. Neurol., 98: 85-96.
ALDRIDGE, W. N. (1958) The biochemistry of organotin compounds;
trialkyltins and oxidative phosphorylation. Biochem. J.,
69: 367-376.
ALDRIDGE, W. N. (1976) The influence of organotin compounds on
mitochondrial functions. In: Zuckerman, J. J., ed. Organotin
compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 186-196.
ALDRIDGE, W. N. & CREMER, J. E. (1957) Organotin dithizone complexes:
The colorimetric determination of diethyltin and triethyltin
compounds. Analyst, 82: 37-43.
ALDRIDGE, W. N. & STREET, B. W. (1964) Oxidative phosphorylation:
Biochemical effects and properties of trialkyltins. Biochem. J.,
91: 287-297.
ALDRIDGE, W. N. & STREET, B. W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver
mitochondria. Biochem. J., 118: 171-179.
ALDRIDGE, W. N. & STREET, B. W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and
triethyltin to mitochondria and their effects on various
mitochondrial functions. Biochem. J., 124: 221-234.
ALEU, F. P., KATZMAN, R., & TERRY, R. D. (1963) Fine structure and
electrolyte analysis of cerebral edema induced by alkyltin
intoxication. J. Neuropathol exp. Neurol., 22: 403-413.
ALLAN, J. E. (1962) Atomic absorption spectrophotometry absorption
lines and detection limits in the air-acetylene flame.
Spectrochim. Acta, 18: 259-263.
AMOS, M.D. & WILLIS, J. B. (1966) Use of high-temperature pre-mixed
flames in atomic absorption spectroscopy. Spectrochim. Acta,
22: 1325-1343.
ANALYTICAL METHODS COMMITTEE (1967) The determination of small amounts
of tin in organic matter. Analyst, 92: 320-323.
ANDERSON, S. R. A. & LOWY, S. L. (1956) A fluorometric determination
of tin. Anal. Chim. Acta, 15: 246-253.
ASCHER, K. R. S. & NISSIM, S. (1964) Organotin compounds and their
potential use in insect control. World Rev. Pest Control,
3: 188-211.
AVTANDILOV, G. G. (1967) [Trace element content in normal and
atherosclerotic portions of the human aorta in connection with
age.] Arh. Patol., 29: 40-42 (in Russian).
BARKER, W. H. & RUNTE, V. (1972) Tomato-juice-associated
gastroenteritis, Washington and Oregon, 1969. Am. J. Epidemiol.
96: 219-226.
BARNES, J. M. & MAGEE, P. N. (1958) The biliary and hepatic lesion
produced experimentally by dibutyltin salts. J. Pathol.
Bacteriol. 75: 267-279.
BARNES, J. M. & MAGOS, L. (1968) The toxicology of organometallic
compounds. Organometal. Chem. Rev., 3: 137-150.
BARNES, J. M. & STONER, H. B. (1958) Toxic properties of some dialkyl
and trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARNES, J. M. & STONER, H. B. (1959) The toxicology of tin compounds.
Pharmacol. Rev., 11: 211-231.
BARNES, R. D., BULL, A. T., & POLLER, R. C. (1973) Studies on the
persistence of the organotin fungicide, fentin acetate
(triphenyltin acetate) in the soil and on surfaces exposed to
light. Pestic. Sci., 4: 305-317.
BARTAK, F., TOMECKA, M, & TOMICEK, O. (1948) Stannosis. Cas. lék.
cesk., 87: 915-920 (Abstract in: J. ind. Hyg., (1949)
31 (5): 98-99)
BASSINDALE, A. R., EABORN, C., TAYLOR, R., THOMPSON, A. R., WALTON, D.
R. M, CRETNEY, J., & WRIGHT, G. J. (1971) Aromatic reactivity 46.
Interpretation of substituent effects in base-catalyzed cleavage
of aryltrimethylsilanes and aryltrimethyl stannanes. J. Chem.
Soc. B., 1155-1161.
BENNETT, R. L. & SMITH, H. A. (1959) Spectrophotometric determination
of tin with phenylfluorone. Anal. Chem., 31: 1441-1442.
BENOY, C. J., HOOPER, P. A., & SCHNEIDER, R. (1971) The toxicity of
tin in canned fruit juices or solid foods. Food Cosmet. Toxicol.,
9: 645-656.
BENS, A. A, GRABOVSKAJA, L. I., & TIHONOVA, I. V. (1976)
[ Geochemistry of the environment.] Moscow, Nedra (in Russian).
BERGNER, K. G. & RUDT, U. (1958) [On the absorption of lead and tin by
fat and food rich in fat in tin plate cans.] Z. Lebensm. Unters.
Forsch., 137: 337-351 (in German).
BERTINE, K. K. & GOLDBERG, E. D. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BISTON, R., VERSTRAETEN, E., & NANGNIOT, P. (1972) Dosage de l'étain
dans les conserves par polarographie oscillographique ŕ intensité
imposée: Comparaison avec les methodes colorimetriques au dithiol
et ŕ la phenyl-fluorone. Anal. Chim. Acta, 59: 453-460.
BLAIR E. H. (1975) Biodegradation of tricyclohexyltin hydroxide.
Environ. Qual. Sai., 3 (Suppl.): 406-409.
BLASIUS, M. B., KERKHOFF, S. J., WRIGHT, R. S., & COTHERN, C. R.
(1972) Use of X-ray fluorescence to determine trace metals in
water resources. Water Resour. Bull., 8: 704-714.
BOGEN, J. (1973) Trace elements in atmospheric aerosol in the
Heidelberg area, measured by instrumental neutron activation
analysis. Atmos. Environ., 7: 1117-1125.
BÖNIG, G. & HEIGENER, H. (1972) [Paper chromatography: Quantitative
determination of micro amounts of tin.] Landwirtsch. Forsch.,
25: 378-382 (in German).
BOWEN, H. J. M. (1966) Trace elements in biochemistry, New York,
Academic Press, pp. 203-204.
BOWEN, V. T. & SUTTON, D. (1951) Comparative studies of mineral
constituents of marine sponges. I. The Genera Dysidea,
Chondrilla, Terpios. J. mar. Res., 10: 153-167.
BRAMAN, R. S. & TOMPKINS, M. A. (1979) Separation and determination of
nanogram amounts of inorganic tin and methyltin compounds in the
environment. Anal. Chem. 51: 12-19.
BRIDGES, J. W., DAVIES, D. S., & WILLIAMS, R. T. (1967) The fate of
ethyltin and diethyltin derivates in the rat. Biochem. J.,
105: 1261-1266.
BRINCKMAN, F. E. & IVERSON, W. P. (1975) Marine chemistry in the
coastal environment. Paper presented at 169th American Chemical
Society Meeting. Philadelphia, PA., April 8-10.
BRINCKMAN, F. E., BLAIR, W. R., JEWETT, K. L., & IVERSON, W. P. (1977)
Application of a liquid chromatograph coupled with a flameless
atomic absorption detector for speciation of trace organometallic
compounds. J. Chromatogr. Sci., 15: 493-503.
BRITISH MEDICAL JOURNAL (1958) "Stalinon": A therapeutic disaster.
Br. med. J., March 1st, p. 515.
BROWNING, E. (1969) Tin. In: Toxicity ol industrial metals, 2nd ed.,
London Butterworths, pp. 323-330.
BRUGGEMANN, J. & KLIMMER, O. R. (1964) [Nutritional-physiological,
analytical and toxicological studies on fungicidal triphenyltin
acetate.] Zentralbl. Vet.-Med. A, 11: 1-3 (In German).
BRUGGEMANN, J., BARTH, K., & NIESAR, K.-H. (1964a) [Experimental
studies on the content of triphenyltin acetate residues in turnip
leaves, ensilage of turnip leaves, and in excrements of animals
fed with these products.] Zentralbl. Vet.-Med. A, 11: 4-19
(in German).
BRUGGEMANN, J., KLIMMER, O. R., & NIESAR, K.-H. (1964b) [The
importance of triphenytin acetate residues in plants and animals
from a hygienic-toxicologic standpoint.] Zentralbl. Vet.-Med. A,
11: 40-48 (in German).
BURGER, K. (1963) [Thin layer chromatographic method for the
detection, identification and separation of traces of mono-, di-,
tri- and zerovalent organotin compounds.] Z. anal. Chem.,
192: 280-286 (in German).
BYINGTON, K. H., YEH, R. Y., & FORTE, L. R. (1974) The hemolytic
activity of some trialkyltin and triphenyltin compounds. Toxicol.
appl. Pharmacol., 27: 230-240.
CALLEY, D. J., GUESS, W. L., & AUTIAN, J. (1967) Hepatotoxicity of a
series of organotin esters. J. Pharm. Sci., 56 (2): 240-243.
CALLOWAY, D. H. & MCMULLEN, J. J. (1966) Fecal excretion of iron and
tin by men fed stored canned foods. Amer. J. clin. Nutr.,
18: 1-6.
CALVERY, H. O. (1942) Trace elements in foods. Food Res.,
7: 313-331. CAPACHO-DELGADO, L. & MANNING, D.C. (1966)
Determination of tinby atomic absorption spectroscopy.
Spectrochim. Acta, 22: 1505-1513.
CARR, H. G. (1969) Interaction between PVC bottles and liquid foods.
Soc. Plast. Eng. 25: 72-74.
CASIDA, J. E., KIMMEL, E. C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and
trialkyleads by liver microsomes. Acta chem. Scand.,
25: 1497-1499.
CATALA, R., ROYO, I. J, & PRIOMO, E. (1971) Tin content of canned
orange juice. Rev. Agroquim. Tecnol. Aliment., 11: 409-417
( Chem. Abstr., (1972) 77: 370, Abstract no. 3974 w).
CAUJOLLE, E., LESBRE, M., & MEYNIER, D. (1954) Sur la toxicité du
tétraméthylstannane et du tétraéthylstannane. C. R. Acad. Sci
(Paris), 239: 556-558.
CENCI, P. & CREMONINI, B. (1969) [Thin-layer chromatography of two
organotin pesticides and their behavior in various types of soil.]
Ind. sac. Ital.. 69: 313-316 (in Italian).
CHAPMAN, A. H., & PRICE, J. W. (1972) Degradation of triphenyltin
acetate by ultraviolet light. Int. Pest Control, 14: 11-12.
CHAPMAN, A. H., DUCKWORTH, M. W., & PRICE, J. W. (1959) The
determination of dialkyltin compounds in polyvinylchloride.
Br. Plast., February, pp. 78, 87.
CHEFTEL, H. (1967) L'étain dans les aliments. Presented at the
Fourth Meeting of the FAO/WHO Codex Committee on Food Additives,
The Hague, 11-15 September 1967, Rome, Food and Agriculture
Organization of the United Nations, 10 pp.
CHISAKA, H., TANIZAKI, Y., & NAGATSUKA, S. (1973) [Determination of
tin and poisonous trace elements in canned juices by neutron
activation.] Radioisotopes, 22: 247-248 (in Japanese).
CHRISTIAN, G. D. & FELDMAN, F. T. (1970) Atomic absorption
spectrography. New York, Wiley, pp. 404-406.
CONINE, D. L., MUHLER, J. C., & FORNEY, R. B. (1972) Acute toxicity of
sodium pentafluorostannite in rats and mice. Toxicol. appl.
Pharmacol., 22: 303-304.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R.
B. (1973) Comparison of the metabolic and renal effects of sodium
pentafluorostannite, sodium fluoride, stannous chloride,
hydrochloric acid, and starvation in the rat. Pharmacologist,
15: 189.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., MUHLER, J.
C., & FORNEY, R. B. (1975) Toxicity of sodium pentafluorostannite,
a new anticariogenic agent. I. Comparison of the acute toxicity of
sodium pentafluorostannite, sodium fluoride and stannous chloride
in mice and/or rats. Toxicol. appl. Pharmacol., 33: 21-26.
CONINE, D. L., YUM, M., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R. B.
(1976) Toxicity of sodium pentafluorostannite. A new
anticariogenic agent. III. 30-day toxicity study in rats.
Toxicol. appl. Pharmacol., 35: 21-28.
CORBIN, H. B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Off. Anal.
Chem., 53: 140-146.
CORBIN, H. B. (1973) Rapid and selective pyrocatechol violet method
for tin. Anal. Chem., 45: 534-537.
COSSA, P., DUPLAY, FISCHGOLD, ARFEL-CAPDEVIELLE, LAFON, PASSOUANT,
MINVIELLE, & RADERMECKER, J. (1958) Encéphalopathies toxiques au
stalinon. Aspects anatomocliniques et alectroencéphalographiques.
Rev. Neurol., 95: 97-108.
COTTON, F. A. & WILKINSON, G. (1972) Advanced inorganic chemistry. A
comprehensive text, London, Wiley-Interscience, 3rd ed.
COTTON, F. A. & WILKINSON, G. (1976) Basic inorganic chemistry, New
York, Wiley.
COUSSEMENT, S. (1972) Analyse des dépôts de fentin acetate sur
feuilles de pomme de terre. Ann. Gembloux, 78: 41-46.
COYLE, C. F. & WHITE, C. E. (1957) Fluorometric determination of tin
with flavonol. Anal Chem, 29: 1486-1488.
CREMER, J. E. (1957) The metabolism in vitro of tissue slices from
rats given triethyltin compounds. Biochem. J., 67: 87-96.
CREMER, J. E. (1958) The biochemistry of organotin compounds: The
conversion of tetraethyltin into triethyltin in mammals.
Biochem. J., 65: 685-692.
CUTTER, H. C., FALLER, W. W., STOCKLEN, J. B., & WILSON, W. L. (1949)
Benign pneumoconiosis in a tin oxide recovery plant. J. And.
Hyg., 31: 139-141.
DACK, G. M. (1955) Chemical poisons in food. In: Food poisoning,
Chicago, University of Chicago Press, pp. 24-25.
DEPARTMENT OF NATIONAL HEALTH AND WELFARE, CANADA (1975) Food and
drug act and regulations office consolidation of the food and
drugs act and the food and drugs regulations with amendments to
December 18, 1975, B. 23.003, Ottawa, Queen's Printer and
Controller of Stationery, pp. 73B.
DEVJATYH, G. G., UMILIN, V. A., & CSINOVOI, Y. N. (1968) [Gas
chromatographic analysis of tetrabutyltin.] Tr. Himii i
Himiceskoj Tehnol., No. d, pp. 82-85 (in Russian).
DITTRICH, T. & COTHERN, C. R. (1971) Analysis of trace metal
particulates in atmospheric samples using X-ray fluorescence.
J. Air Pollut. Control Assoc., 21: 716-719.
DRESSLER, M., MARTINU, V., & JANAK, J. (1971) Response to the alkali
flame ionization detector to silicon, tin and lead compounds.
J. Chromatogr. 59: 429-433.
DRESSLER, M., BARTH, M., & VESPALEC, R. (1975) Chromatographic
determination of organotin compounds in fungistatic plaster.
Silikaty, 4: 301-310.
DRUAULT-TOUFESCO, M. N. (1955) A propos de deux cas d'intoxication
grave par le Stalinon. Bull. Soc. Ophtalmol. Fr., pp. 54-58.
DUCKERING, G. E. (1968) The cause of lead poisoning in the tinning of
metals. J. Hyg. (Lond.), 8: 474-503.
DUNDON, C. C. & HUGHES, J.P. (1950) Stannic oxide pneumoconiosis.
Am. J. Roentgenol., 63: 797-811.
DURBIN, P. W. (1960) Metabolic characteristics within a chemical
family. Health Phys., 2: 225-238.
DURFOR, C. N. & BECKER, E. (1964) Selected data on public supplies of
the 100 largest cities in the United States, 1962. J. Am. Water
Works Assoc., 56: 237-246.
DURUM, W. H. & HAFFTY, J. (1961) Occurrence of minor elements in
water, Washington, DC, pp. 1-11 (Geological Survey Circular
445).
DYER, B. & TAYLOR, G. (1931) Notes from the Report of Public Analysts.
Analyst, 56: 251-253.
ELSEA, J. R. & PAYNTER, O. E. (1958) Toxicological studies on bis
(tri- n-butyltin) oxide. Am. Med. Assoc. Arch. ind. Health,
18: 214-217.
ELTEN, R. (1929) [Study on tin foil used in cheese packeting; rindless
cheese.] Chem.-Ztg., 53: 586 (in German).
ENGBERG, A. (1973) A comparison of a spectrophotometric (Quercetin)
method and an atomic absorption method for the determination of
tin in food. Analyst, 98: 137-145.
EVANS, C. A. & MORRISON, G. H. (1968) Trace element survey analysis of
biological materials by spark source mass spectrometry. Anal.
Chem., 40: 869-875.
EVERETT, G. L, WEST, T. S., & WILLIAMS, R. W. (1974) The determination
of tin by carbon filament atomic absorption spectrometry. Anal.
Chim. Acta, 70: 291-298.
EYRICH, W. (1972) [Determination of tin in canned fruits and
vegetables.] Dtsch. Lebensm.-Rundsch, 9: 280-282 (in German).
FEY, R. (1969) [Heavy metal content in canned fruits and vegetables:
I. Tin and iron in canned asparagus.] Ind. Obst-Geinneseverwert.,
54: 27-33 (in German).
FILER, T. D. (1971) Fluorometric determination of submicrogram
quantities of tin. Anal. Chem., 43: 1753-1757.
FISCHER, H. W. & ZIMMERMAN, R. (1969) Long retention of stannic oxide:
Lack of tissue reaction in laboratory animals. Arch. Pathol.
88: 259-264.
FISH, R. H., KIMMEL, E. C., & CASIDA, J. E. (1975) Bioorganotin
chemistry. Biological oxidation of tributyltin derivatives.
J. Organometal Chem. 93 (1): C1-C4.
FISH, R. H., KIMMEL, E. C., & CASIDA, S. E. (1976) Bioorganotin
chemistry: Biological oxidation of organotin compounds. In:
Zuckerman, J. J., ed. Organotin compounds: New chemistry and
applications, Washington, DC, American Chemical Society,
pp. 197-203 (Advances in Chemistry Series 157).
FONTAN, M. M., VERGER, PERY, LOISEAU, & MULON (1955) Quatre cas dont
deux mortels d'intoxication par le "Stalinon" chez l'enfant. J.
Med. Bordeaux, 132: 399-405.
FAO/WHO (1971) 1970 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United
Nations/World Health Organization, pp. 327-366, 521-542.
FOOD AND DRUG ADMINISTRATION, USA (1971) Code of Federal Regulations,
Title 21 - Foods and Drugs, Washington DC, US Government
Printing Office, pp. 409-410 ( 121, 2602).
FREELAND, G. N. & HOSKINSON, R. M. (1970) Non-aqueous atomic
absorption spectrophotometric determination of organometallic
biocides. Analyst, 95: 579-582.
FREITAG, K-D. & BOCK, R. (1974a) [Analysis of mixtures of tri-, di-,
and monophenyltin compounds and inorganic tin (IV).] Z. anal.
Chem., 5: 337-346 (in German).
FREITAG, K.-D. & BOCK, R. (1974b) Degradation of triphenyltin chloride
on sugar beet plants. Pesticide Sci., 5: 731-739.
FURCHNER, J. E. & DRAKE, G. A. (1976) Comparative metabolism of
radionuclides in mammals - XI. Retention of 113Sn in the mouse,
rat, monkey, and dog. Health Phys., 31: 219-224.
GAINES, T. B. & KIMBROUGH, R. D. (1968) Toxicity of fentin hydroxide
to rats. Toxicol. appl. Pharmacol., 12: 397-403.
GATEHOUSE, B. M. & WILLIS, J. B. (1961) Performance of a simple atomic
absorption spectrometer. Spectrochim. Acta, 17: 710-718.
GAUER, W. O., SEIBER, J. N., & CROSBY, D. G. (1974) Determination of
organotin residues from Plictran in fruit crops by gas-liquid
chromatography. J. agric. food Chem., 22: 252-254.
GAUNT, I. F., COLLEY, J., GRASSO, P., & CREASEY, M., (1968) Acute and
short-term toxicity studies on di- n-butyltin dichloride in rats.
Food Cosmet. Toxicol., 6: 599-608.
GAYRAL, L., LAZORTHES, G., & PLANQUES, J. (1958) Méningo-encé-phalite
hypertensive par intoxication au Stalinon, guérie. Rev. neurol.,
98: 143-144.
GEISSLER, H. & KRIEGSMANN, H. (1964) [Analysis of butyltin compounds
(n-C4H9)nSnX4-n.] Z. Chem., 9: 354-355 (in German).
GEISSLER, H. & KRIEGSMANN, H. (1965) [Liquids used for separation in
some gas chromatographic investigations of some n-butyltin
compounds. Z. Chem., 11: 423-424 (in German).
GELDMACHER -- VON MALLINCKRODT, M. & POOTH, M. (1969) [Simultaneous
determination of twenty five metals and metalloids in biological
materials.] Arch. Toxicol., 25: 5-18 (in German).
GEORGE, G. M., ALBRECHT, M. A., FRAHM, L. J., & McDONNELL, J.P. (1973)
Atomic absorption spectrophotometric determination of dibutyltin
dilaurate in finished feeds. J. Assoc. Off. Anal. Chem.,
56: 1480-1482.
GETZENDANER, M. E. & CORBIN, H. (1972) Residues on apples and pears
from use of Plictran miticide. J. agric. food Chem.,
20: 881-885.
GEYER, R. & ROTERMUND, U. (1969) [Oscillopolarographic determination
of organotin compounds.] Acta chim. Hung., 59 (2): 201-210
(in German).
GHAFOURI, R. (1970) Etude des eaux minérales d'Ax-les-Thermes. Terres
Eaux, 64: 19-27.
GOLDSCHMIDT, V. M. (1958) Tin, In: Muir, A. ed. Geochemistry,
Oxford, Oxford University Press, pp. 391-397.
GORDON, M. (1952) [Trace elements in peat.] Torinachrichten,
3: 12 (in German).
DE GROOT, A. P. (1973) Subacute toxicity of inorganic tin as
influenced by dietary levels of iron and copper. Food Cosmet.
Toxicol., 11: 955-962.
DE GROOT, A. P., FERON, V. J., & TIL, H. P. (1973) Short-term toxicity
studies on some salts and oxides of tin in rats. Food Cosmet.
Toxicol., 11: 19-30.
GRUENWEDEL, D. W. & PATNAIK, R. K. (1973) Model studies regarding the
internal corrosion of tin-plated food cans. IV. Polarographie
investigation of the interaction of tin (II)-ions with L-cysteine.
Chem. Mikrobiol. Technol. Lebensm., 2: 97-101.
GRUNER, J-E. (1958) Lésions du névraxe secondaires ŕ l'ingestion
d'éthyl-étain (Stalinon). Rev. Neurol., 98: 109-116.
GUARDASCIONE, V. & DI BOSCO, M. M. (1967) [Contribution to the
knowledge of occupational disease due to pesticides; Three cases
of acute intoxication by triphenyltin acetate fungicides.]
Lay. Urn., 19: 307-313 (in Italian).
HADZIMICEV, P. (1971) [Natural content of heavy metals (copper, zinc,
tin) in foods from the vegetable belt around Sofia.] Khranit.
Prom., 20: 18-20 (in Bulgarian).
HALL, C. A. & LUDWIG, P. D. (1972) Evaluation of the potential use for
several organotin compounds against the sheep blowfly ( Lucilia
spp.). Vet. Rec., 90: 29-32.
HAMILTON, T. A. (1948) The metabolic properties of plutonium and
allied material Univ. Calif. Rad. Lab. Med. Health Phys. Q., pp.
4-16 (UCRL-270).
HAMILTON, E. I., MINSKI, M. J., CLEARY, J. J., & HALSEY, V. S. (1972)
Comments upon the chemical elements present in evaporated milk for
consumption by babies. Sci. total Environ., 1: 205-210.
HAMILTON, E. I., MINSKI, M. J., & CLEARY, J. J. (1972/1973) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study.
Sci. total Environ., 1: 341-374.
HANNA, W. J. & GRANT, C. L. (1962) Spectrochemical analysis of the
foliage of certain trees and ornamentals for 23 elements. Bull
Torrey Bot. Club, 89: 293-302.
HASEGAWA, T. & SUGIMAE, A. (1971) [Emission spectrographic
determination on trace metals in airborne particulates.]
Jpn. Anal., 20: 640-845 (in Japanese).
HAYASHI, H. (1969) [Square wave polarographic determination of
dissolved tin in canned juice.] Jpn. Anal., 18: 58-61
(in Japanese).
HEATH, D. F. (1963) Some problems in the determination of residues in
plants and animals. In: Proceedings of a symposium; Radiation and
Radioisotopes Applied to Insects of Agricultural Importance,
Athens, 22-26 April 1963, Vienna, International Atomic Energy
Agency, pp. 185-193.
HEATH, D. F., (1967) The retention or triphenyltin and dieldrin and
its relevance to the toxic effects of multiple dosing. In:
Radioisotopic detection of Pesticide Residues, Proceedings of a
Panel, Vienna 1965, pp. 18-26 (Abstract in: Chem. Abstr.,
67: 1828 W).
HEDGES, T. R. & ZAREN, H. A. (1969) Experimental papilledema; A study
of cats and monkeys intoxicated with triethyltin acetate.
Neurology, (Minneapolis), 19: 359-365.
HEINDL, R. A. (1970) Tin. In: Mineral facts and problems, 1970
edition, pp. 759-751. Reprint from: US Dept of the Interior,
Bureau of Mines, Bulletin 650.
HEROK, J. & GOTTE, H. (1963) [Radiometric analysis of triphenyltin
acetate in the metabolism of plants and animals.] Int. J. appl.
Radiat. Isot., 14: 461-479 (in German).
HEROK, J. & GÖTTE, H. (1964) [Radiometric study on the metabolism of
triphenyltin acetate in milk sheep.] Zentralbl. Vet.-Med. A,
11: 20-28 (in German).
HERON, P. N. & SPROULE, J. S. G. (1958) The chemical nature of
fungicidal agents employed in the pulp and paper industry. Indian
Pulp Pap., 12: 516-517.
HESLOP, R. B. & JONES, K. (1976) Inorganic chemistry. A guide to
advanced study. Amsterdam, Elsevier.
HILES, R. A. (1974) Absorption, distribution and excretion of
inorganic tin in rats. Toxicol. appl. Pharmacol., 27: 366-379.
HLEBNIKOVA, M. J. (1957) [Hygienic characteristics of dust in the
production of tin.] Sb. naucnyh Rab. voprogam Gig. Truda i
profpatol., No. 2, pp. 53-83 (in Russian).
HODGE, V. F., SEIDEL, S. L., & GOLDBERG, E. D. (1979) Determination of
tin (IV) and organotin compounds in natural waters, coastal
sediments and macro algae by atomic absorption spectrometry.
Anal. Chem., 51: 1259.
HOFF, J. E. & WILCOX, G. E. (1070) Accumulation of nitrate in tomato
fruit and its effect on derinning. J. Am. Soc. Hortic. Sci.,
95: 92-94.
HORACEK, V. & DEMCIK, K. (1970) [Group poisoning in spraying of field
cultures with Brestan-60 triphenyltin acetate.] Prac. Lék.,
22: 61-66 (in Czech).
HORIO, T. & NAKASEKO, M. (1972) [Spectrophotometric determination of
dissolved tin in canned foods using Salicylidenamion-2-
thiophenol.] Tokyo Inst. Food Technol. J., 17: 212-214
(in Japanese).
HORIO, T., IWAMOTO, Y., & SHIGA, I. (1967) The nitrate corrosion of
canned orange juice drinks in Japan. Communication presented at
the 5th International Congress on Canned Fools, Vienna 3-6,
October, 1967, 12 pp.
HUEY, C., BRINCKMAN, F. E., GRIM, S., & IVERSON, W. P. (1974) The role
of tin in bacterial methylation of mercury. Paper presented of the
International Conference on Transport of Persistent Chemicals in
Aquatic Ecosystems, Ottawa, Canada, 1-3 May, 1974.
IGNATJEVA, M. A., KUZNETSOV, I. G., MIRSKOV, R. G., & VLASOV, V. M.
(1968) [Toxicology of acetylene organotin compounds of the
triethylstannyl range.] Izv. sib. Otdel. Akad. Nauk SSSR,
1 (5): 118-119 (in Russian).
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M, MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumorigenicity
in mice: A preliminary note. J. Natl Cancer Inst.,
42: 1101-1114.
INTERNATIONAL LABOUR OFFICE (1972) Tin alloys and compounds. In:
Encyclopedia of Occupational Health and Safety, Geneva, ILO,
Vol. II, pp. 1407-1409.
IWAMOTO, Y., MIYAZAKI, M., KUNISATO, S., MAEDA, Y., HORIO, T., &
KOMURA, S. (1968) [Studies on abnormal detinning of tin can with
nitrate in foods: (II) Relationship between dissolution on tin and
pH of canned tomato juice.] J. Jpn Soc. Food Nutr., 21: 50-54
(in Japanese).
JANITZ, W. (1971) [Comments on the polarographic method of tin and
lead determination in tinned meat products.] Med. Weter.,
27: 43-45 (in Polish).
JOHANSEN, O. & STEINNES, E. (1969) Determination of tin in geological
material by neutron activation analysis. Analyst, 94: 976-978.
JOHNSON, J. H., YOUNGER, R. L., WITZEL, D. A., & RADELEFF, R. D.
(1975) Acute toxicity of tricyclohexyltin hydroxide to livestock.
Toxicol. appl. Pharmacol., 31: 66-71.
JOVANOVIC, D., STOJADINOVIC, L., & SPASOJEVIC, B. (1967) [Contribution
to the determination of tin in canned meat.] Vojno-sanit. Pregl.,
24: 405-408 (in Serbo-Croat).
KAHN, H. L. & SCHALLIS, J. E. (1968) Improvement of detection limits
for arsenic, selenium and other elements with an argon-hydrogen
flame. At. Absorpt. Newsl., 7: 5-9.
KANISAWA, M. & SCHROEDER, H. A. (1967) Life term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumors in mice. Cancer Res., 27: 1192-1195.
KANISAWA, M. & SCHROEDER, H. A. (1969). Life term studies on the
effect of trace elements on spontaneous rumours in mice and rats.
Cancer Res., 29: 892-895.
KAPPAS, A. & MAINES, M.D. (1976) Tin: A potent inducer of heme
oxygenase in kidney. Science, 192: 60-62.
KAS'YANENKO, I. V. & KUL'SKAYA, O. A. (1969) [Microelement content of
the blood of patients with cancer of stomach and chronic gastritis
secretory insufficiency.] Vopr. eksp. Onkol Respub. Mezhvedom,
SB, 4: 101-107 (in Russian).
KEENAN, R. G. & BYERS, D. H. (1952) Rapid analytical method for air
pollution surveys. Arch. ind. Hyg., 6: 226-230.
KEHOE, R. A., CHOLAK, J. & STORY, R. V. (1940) A spectrochemical study
of the normal ranges of concentration of certain trace metals in
biological materials. J. Nutr., 19: 579-592.
KERK, VAN DER, G. J. M. (1975) [The present use of organotin
compounds.] Chem. Zeitung, 99: 26-32 (in German).
KIMBROUGH, R. D. (1976) Toxicity and health effects of selected
organotin compounds: A review. Environ. Health Perspect.,
14: 51-56.
KIMBROUGH, R. D. & GAINES, T. B. (1971) Hexachlorophene effects on the
rat brain. Arch. environ. Health, 23: 114-118.
KIMMEL, E. C., FISH, R. H., & CASIDA, J. E. (1977) Bio-organotin
chemistry: Metabolism of organotin compounds in microsomal
monoxygenase systems and in mammals. J. Agric. Food Chem.,
25 (1): 1-8.
KIMURA, Y., UEDA, K., NISHIZIMA, M., MAKI, T., WADA, M., TAKAHASHI S.,
YAMADA, T., ARAI, M., & UEMATSU, T. (1970) [Studies on extractable
heavy metals in canned foods: I. Detection of tin and lead from
various sorts of canned baby foods after their opening.] Ann.
Rep. Tokyo Metrop. Inst. Hyg., 22: 95-100 (in Japanese).
KIRK, R. S. & POCKLINGTON, W. D. (1969) The determination of tin and
canned foods with quercetin. Analyst, 94: 71-74.
KLEIN, A., COP, R., CHATRNA, E., & BADALOVA, M. (1970) [The study of
vitamin C stability and growing of tin content in tinned juices.]
Cesk. Hyg., 15: 49-55 (in Czech).
KLEINKOPF, M.D. (1955) Trace element exploration of Maine lake water,
Academic dissertation, Columbia University, 153 pp. (University
Microfilm Pub. 12, 447).
KLEINKOPF, M.D. (1960) Spectrographic determination of trace elements
in lake waters of northern Maine. Geol. Soc. Am. Bull.,
71: 1231-1242.
KLIMMER, O. R. (1963) [Triphenyltin acetate and the toxicology of
organic tin compounds.] Arzneimittel-Forsch., 13: 432-436
(in German).
KLIMMER, O. R. (1964) [Toxicological studies on triphenyltin acetate
(TPZA).] Zentralbl. Vet.-Med., R.A 11: 29-39 (in German).
KLIMMER, O. R. (1969) [The application of organotin compounds reviewed
by the experimental toxicologist.] Arzneimittel-Forsch.,
19: 934-939 (in German).
KOBAYASHI, T. & YADA, M. (1968) [Spectrophotometric determination of
tin in foodstuffs with stilbazo.] J. Food Hyg. Soc. Jpn,
9: 465-468 (in Japanese).
KOCH, J. & FIGGE, K. (1971) [Migration of tin stabilizers from PVC
bottles into beer.] Z. Lebensmittel-Unters. 147: 8-10
(in German).
KOCKIN, D. A., SKORBATOVA, J. C., PEGUSOVA, L. D., & VORONKOV, N. A.
(1969) [Polarographic study on organotin compounds with an acid
content.] Zl. Obshch. Khim., 39: 1777-1781 (in Russian).
KONOVALOV, G. S. & KOLESNIKOVA, T. K. (1969) [Trace elements in
atmospheric precipitation around the Otkaznoe reservoir.]
Gidrokhim Mater., 49: 74-79 (in Russian).
KRUTCHKOFF, D. J., JORDAN, T. H., WEI, S. H. Y., & NORDQUIST, W. D.
(1972) Surface characterization of the stannous fluoride enamel
interaction. Arch. oral Biol., 17: 923-930.
KRYLOVA, N. & BALABUH, A. (1970) [Trace element level in meat.] Myas.
Ind. SSSR, 41: 39 (in Russian).
KUMPULAINEN, J. & KOIVISTOINEN, P. (1977) Advances in tin compound
analysis with special reference to organotin pesticide residues.
Res. Rev., 66: 1-18.
KUNISATO, S. & MIYAZAKI, M. (1972) [Application of plant growth
regulators on fruits and vegetables for processing: I. Nitrate
content in tomato fruits.] J. Hortic. Assoc. Jpn., 41: 411-420
(in Japanese).
KUROSAKI, N. & FUSAYAMA, T. (1973) Penetration of elements from
amalgam into dentin. J. dent. Res., 52: 309-317.
KUTZNER, J. & BROD, K. H. (1971) [Resorption and excretion of tin
after oral administration of tin-113.] Nucl. Med. (Stuttg.),
10: 286-297 (in German).
LAAMANEN, A., LOFGREN, A., & PARTANEN, T. J. (1971) Some trace metals
in particulates in the air of Helsinki and Turku, 1964-1969. Work
Environ. Health., 8: 63-69.
LANDA, K., FEJFUSOVA, J., & NEDOMLELOVA, R. (1973) [The hazards of
organic tin compounds used as fungicides in some industrial
applications.] Prac. Lék., 25: 391-394 (in Czech).
LANIGEN, D. & WEINBERG, E. L. (1976) The use of estertin stabilizers
in PVC. In: Zucherman, J. J., ed. Organotin compounds: New
chemistry and applications. Washington, DC, American Chemical
Society (Advances in Chemistry Series 157).
LECOQ, R. (1954) Contribution ŕ l'étude des effects toxiques du
tétraéthylétain C. R. Hebd. Acad. Sci. (Paris), 239: 678-680.
LEE, E. E., GORANSON, S.S., ENRIONE, R. E., & MORGAN, G. B. (1972)
National air surveillance cascade impactor network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol., 6: 1025-1030.
LINDEMANN, G., PINDEBORG, J. J., & PAULSEN, H. (1959) Recovery of the
rat kidney in fluorosis. Arch. Pathol., 67: 30-33.
LJASKOVSKAJA, Y., & KRASIL'NIKOVA, T. (1961) [Photometric
determination of tin in canned food by reaction with quercetin.]
Myasn. Ind. SSSR 4: 44-45 (in Russian) (English abstract in
Anal. Abstr. (1963) 10: 1177).
LOUNAMAA, J. (1956) Trace elements in plants growing wild on different
rocks in Finland: A semi-quantitative spectrographic survey. Ann.
Bot. Soc. "Vanamo" 29: 4.
LOWRY, R. R. & TINSLEY, I. J. (1972) Determination of tin salts in
fats. J. Am. Oil. Chem. Soc., 49: 508-509.
LUFF, A. P. & METCALFE, G. H. (1890) Four cases of tin poisoning
caused by tinned cherries. Br. med. J., 1: 833-834.
LUKE, C. L. (1956) Photometric determination of tin with
phenylfluorone: Determination of tin in lead and 1% antimony-lead
alloys. Anal. Chem., 28: 1276-1279.
LYLE, W. H. (1958) Lesions of the skin in process workers caused by
contact with butyltin compounds. Br. J. ind. Med., 15: 193-196.
MACINTOSH, R. M. (1968) Tin and tin alloys. In: Kirk-Othmer
encyclopedia of chemical technology, 2nd ed. New York,
Interscience pp. 273-325.
MAGEE, P. N., STONER, H. B., & BARNES, J. M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Pathol. Bacteriol., 73: 107-124.
MAHADEVAIAH, M., GOWRAMMA, R. V., RADAKRISHNAIAH SETTY, G., SASTRY, M.
V., SASTRY, L. V. L., & BHATNAGAR, H. C. (1969) Studies on
variation in tin content in canned mango nectar during storage.
J. food Sci. Technol., 6: 192-196.
MAMONTOVA, A. A. (1940) [Effects of tin on animal organism.] Vopr.
Pitan, 2: 13-25 (in Russian).
MARHOLD, J. V. (1972) [ A collection of results of toxicological
evaluation of substances and products.] Pro Vychovu Vedoucicn
Pracovniku Chemickeho Prumyclu Praha (in Czech).
MARKICEVIC, A. & TURKO, V. (1967) [Lesions due to triphenyltin acetate
"Brestan".] Arh. Hig. Rada, 18: 355-358 (in Serbo-Croat).
MARKS, M. J., WINEK, C. L., & SHANOR, S. P. (1969) Toxicity of
triphenyltin hydroxide. (Vancide KS). Toxicol. appl. Pharmacol.,
14: 627.
MASON, B. (1966) Principles of geochemistry, 3rd ed., New York,
Wiley.
MAZAEV, V. T. & KOROLEV, A. A. (1969) [Experimental standardization of
the maximum permissible concentration of the dichlorbutyltin in
surface water.] Prom. Zagrjaznenija Vodoemov, No 9, pp. 15-37
(in Russian).
MAZAEV, V. T. & SLEPNINA, T. G. (1973) [Experimental data on hygienic
standardization of dibutyltin sulfide in water bodies.] Gig. i.
Sanit., 8: 10-13 (in Russian).
MAZAEV, V. T., LOSEV, N. I., & VOINOV, V. A. (1968) [On some
vegetative reactions and on electrical activity of the brain
cortex after intoxication with organic derivatives of tin.] Bull.
exp. Biol. Med., 11: 72-74 (in Russian).
MAZAEV, V. T., KOROLEV, A. A., & SKACKOVA, I. N. (1971) Experimental
investigation of the toxic effect of tetraethyltin and
dichlorodibutyltin on the animal organism. J. Hyg. Epidemiol.
Microbiol. Immunol., 15: (1): 115-120.
MAZAEV, V. T., GOLOVANOV, O. V., IGUMOV, A. S., & TSAY, V. N. (1976)
[Problem of the transformation of organotin compounds in a water
medium.] Gig. i Sanit, 3: 17-20 (in Russian).
MEINEMA, H. A., BURGER-WIERSMA, T., VERSLUIS-DE-HAAN, G. & GEVERS, E.
Ch. (1978) Determination of trace amounts of butyltin compounds in
aqueous systems by gas chromatography/mass spectrometry. Environ.
Sci. Technol., 12 (3): 288-293.
MIDWEST RESEARCH INSTITUTE (1977) Assessment of the need for the
character of, and the impact resulting from limitations on
selected organotins. Phase I Assessment of the need for
limitations on organotins. Washington, DC, US Environmental
Protection Agency, EPA Contract No. 68-01-4313).
MIJATOVIC, M. (1972) [Chronic hepatitis due to exposure to Brestan.]
Jugosl. inostr. Dok. Zast. Radu, 8:3-9 (in Serbo-Croat).
MIKI, E. & FUKUI, Y. (1971) [Square wave polarographic determination
of tin in canned fruit.] Sci. Rep. Agric. Fac. Kagawa Univ.,
22: 35-40 (in Japanese).
MITCHELL, R. C. (1948) The spectrographic analysis of soils, plants,
and related materials. Bureau of Soil Science, pp. 1-173
(Technical Communication No. 44).
MONIER-WILLIAMS, G. W. (1949) Trace elements in food, London,
Chapman & Hall, pp. 138-161.
MOSKALEV, Ju. I. (1964) [Studies on the distribution of 113Sn. In:
Distribution, biological effects and rapid excretion of
radioactive isotopes.] Moscow, Medicina, pp. 78-82 (in Russian).
MULAY, I. L., RAY, R., KNOX, B. E., SUHR, N.H., & DELANEY, W. E.
(1971) Trace metal analysis of cancerous and non-cancerous human
tissues. J. Natl. Cancer Inst., 47: 1-13.
NAKAHARA, T., MUNEMORI, M., & MUSHA, S. (1972) Atomic absorption
spectrometric determination of tin in premixed inert gas
(entrained air) hydrogen flames. Anal. Chim. Acta, 62: 267-278.
NAKAMURA, Y. & KAMIWADA, S. (1973) [Colorimetric determination of tin
by decomposition and tin-phenylfluorene complex.] J. food Hyg.,
14: 352-356 (in Japanese).
NATIONAL ACADEMY OF SCIENCES. WASHINGTON (1973) Toxicants occurring
naturally in foods, 2nd ed., Washington, DC, NAS, pp. 63-64.
NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH (1976) Criteria
for a recommended standard ... occupational Exposure to organotin
compounds, Washington, DC, US Government Printing Office,
187 pp.
NATIONAL WATER QUALITY NETWORK (1960) Annual compilation ol data,
1 October 1959-30 September 1960, Washington, DC, US Department
of Health, Education and Welfare, Public Health Service,
pp. 353-356.
NEHRING, P. (1972) [Tin in peach preserves.] Ind. Obst.
Gemusseverwert., 57: 489-492 (in German).
NELSON, A. A. (1954) Approximate relation of parts per million in diet
to mg/kg/day. Assoc. Food Drug Off. Q. Bull, 18: 66.
NEUBERT, G. (1964) [Analysis of organotin stabilizers.] Z. anal.
Chem., 203: 265-272 (in German).
NEUBERT, G. & ANDREAS, H. (1976) [The analysis of organotin
compounds.] Z. anal. Chem., 280: 31 (in German).
NEUBERT, G. & WIRTH, H. O. (1975) [The analysis of organotin
stabilizers.] Z. anal. Chem., 273:19-23 (in German).
NEUMANN, W. P. (1970) The organic chemistry of tin, London, Wiley.
NEWMAN, E. J. & JONES, P. D. (1966) Separation and determination of
small amounts of tin. Analyst, 91: 406-410.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effects of orally
administered plasticizers in the rat. Toxicol. appl. Pharmacol.,
26: 253-259.
NISHIJIMA, M., UEDA, K., MAKI, T., WADA, M., TAKAHASHI, S., KIMURA,
Y., YAMADA, T., NAKAJIMA, K., & FUKUDA, T. (1971) [Studies on
heavy metals dissolved in canned foods. (II) Detection of tin and
lead from various canned fruits after their opening.] Ann. Rep.
Tokyo Metrop. Inst. Hyg., 23: 189-196, (in Japanese).
OBRUSNIK, I. (1969), Determination of indium and tin by activation
analysis using replacement substoichiometry. Talanta,
16: 563-566.
ONISHI, H. & SANDELL, E. B. (1957) Meteoritic and terrestrial
abundance of tin. Geochim. Cosmochim. Acta, 12: 262-270.
OYANGUREN, H., HADDAD, R., & MAASS, H. (1958) Stannosis. Benign
pneumoconiosis owing to inhalation of tin dust and fume. I.
Environmental and experimental studies. Ind. Med. Surg.,
27: 427-431.
PAL, B. K. & RYAN, D. E. (1969) Fluorescence and metallic valency
states. Part III. Determination of tin. Anal Chim. Acta,
48: 227-231.
PATE, B. D. & HAYS, R.L. (1968) Histological studies of testes in rats
treated with certain insect chemosterilants. J. econ. Entomol.
61: 32-34.
PEARLMAN, R. S., HEFFERREN, J. J., & LYON, H. W. (1970) Determination
of tin in enamel and dentin by atomic absorption spectroscopy.
J. dent. Res., 49: 1437-1443.
PEDLEY, F. G. (1927) Chronic poisoning by tin and its salts. J ind.
hyg., 9: 43-47
PELIKAN, Z. & CERNY, E. (1968a) [The toxic effect of tri-n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292
(in German).
PELIKAN, Z., & CERNY, E. (1968b) The effect of low doses of bis-
(tri-n-butyltin) oxide on the skin of rats. Berufsdermatosen,
16: 340-349.
PELIKAN, Z., & CERNY, E. (1969) Toxic effect of bis- (tri-n-butyltin)
oxide (TBTO) on the skin of rats. Berufsdermatosen, 17: 305-316.
PELIKAN, Z. & CERNY, E. (1970a) The toxic effects of some di- and
mono-n-octyltin compounds on white mice. Arch. Toxikol.,
26: 196-202.
PELIKAN, Z. & CERNY, E. (1970b) Toxic effects of some mono-n-butyltin
compounds on white mice. Arch. Toxikol., 27: 79-84.
PENDERGRASS, E. P. & PRYDE, A. W. (1948) Benign pneumoconiosis due to
tin oxide. J. ind. Hyg., 30: 119-123.
PEREL'MAN, A. I. (1972) [Geochemistry of elements in the zone of
hypergenesis.] Moscow, Nedra (in Russian).
PERRY, H. M. & PERRY, E. F. (1959) Normal concentrations of some trace
metals in human urine: Changes produced by ethylenediamine
tetraacetate. J. clin. Invest., 38: 1452-1463.
PESME, P. (1955) Complicationes oculaires observées chez quatre
enfants intoxiqués par le "Stalinon". Arch. Fr. Pediat.,
12: 327-328.
PIVER, W. T. (1973) Organotin compounds: Industrial applications and
biological investigation. Environ. Health. Perspect., 4: 61-79.
PORTRETNYJ, V. P., MALYUTA, V. F., & CHUIKO, V. T. (1973)
[Determination of tin in water, after concentration by
co-precipitation, by anodic stripping voltametry.] Zh. anal.
Khim., 28: 1337-1340 (in Russian).
PRICE, W. J. & ROOS, J. T. H. (1969) Analysis of fruit juice by atomic
absorption spectrophotometry. I -- The determination of iron and
tin in canned juice. J. Sci. food Agric., 20: 437-439.
PRULL, G. & ROMPEL, K. (1976) [Neurological and cerebro-electrical
disturbances in acute poisoning due to organotin compounds.]
Nervenarzt, 41: 516-520 (in German).
REMY, H. (1956) Treatise on inorganic chemistry, Amsterdam,
Elsevier, Vol. I.
RIDLEY, W. P., DIZIKES, L. J., & WOOD, J. M. (1977) Biomethylation of
toxic elements. Science, 197: 329-332.
RITTENHOUSE, G., FULTON, R. B., III GRABOWSKI, R. J., & BERNARD, J. L.
(1969) Minor elements in oil field waters. Chem. Geol., 4:
189-209.
ROBERTS, R. M. G. & EL KAISSI, F. (1968) Base-catalyzed clearage of
allylic derivatives of group IV A elements. J. Organometal.
Chem., 12 (1): 79-88.
ROBERTSON, A. J. (1960) Pneumoconiosis due to tin oxide. In: King, E.
J. & Fletcher, C. M. ed Symposium on industrial pulmonary
diseases, Boston, Little-Brown, pp. 168-184.
ROBERTSON, A. J. & WHITAKER, P. H. (1957) Radiological changes in
pneumoconiosis due to tin oxide. J. fac. Radiol., 6: 224-233.
ROBINSON, I. M. (1969) Effects of some organotin compounds on tissue
amine levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROE, F. J. C., BOYLAND, E., & MILLICAN, K. (1965) Effects of oral
administration of two tin compounds to rats over prolonged
periods. Food Cosmet. Toxicol., 3: 277-280.
RONDEPIERRE, J., TRUHAUT, R., GUILLY, P., HIVERT, P. E., & BARANDE, I.
(1958) Sur une intoxication aiguë par un dérivé organiqué de
l'étain. Rev. neurol., 98: 135-140.
ROSE, M. S. (1969) Evidence for histidine in the triethyltin-binding
site of rat haemoglobin. Biochem. J., 111: 129-137.
ROSS, W. J. & WHITE, J. C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33: 421-424.
SAITO, I. & ENDO, M. (1970) [Analytical studies on inorganic elements
of gall stones.] Hokkaido Igaku Zasshi, 14: 334-337
(in Japanese).
SATO, N., TSURUTA, K., KA1VIADA, I., NARITA, S., & ABUKAWA, H. (1973).
[Studies on dissolved tin in canned foods by atomic absorption
spectrophotometry.] J. Food Hyg Soc. Jpn, 14: 245-248
in Japanese).
SAVAGE, W. (1939) Canned foods in relation to health, Lancet
231 (2): 991-995.
SAWYER, R. (1967) Determination of dialkyltin stabilizers in aqueous
extracts from PVC and other plastics. Analyst, 92: 569-574.
SCHALLIS, J. E. & KAHN, H. L. (1968) The determination of tin in
lubricating oils with a nitrous oxide-acetylene flame.
At. Absorpt. Newsl., 7: 84.
SCHRAMEL, P., SAMSAHL, K., & PAVLU, J. (1973) Some determinations of
Hg, As, Se, Sb, Sn, and Br in water plants, sediments and fishes
in Bavarian rivers. Int. J. environ. Stud., 5: 37-40.
SCHROEDER, H. A. & BALASSA, J. J. (1961) Arsenic, germanium, tin and
vanadium in mice: Effects on growth, survival and tissue levels.
J. Nutr., 92: 245-253.
SCHROEDER, H. A., BALASSA, J. J., & TIPTON, I. H. (1964) Abnormal
trace metals in man: Tin. J. chron. Dis., 17: 483-502.
SCHROEDER, H. A., KANISAWA, M., FROST, D. V., & MITCHENER, M. (1968)
Germanium, tin and arsenic in rats: Effects on growth, survival,
pathological lesions and life span. J. Nutr., 96: 37-45.
SCHROLL, E. VON & KRACHSBERGER, H. (1970) [Study on the geochemistry
of impurities in atmospheric precipitates in the Vienna
metropolitan area.] Radex Rundsch., 5: 331-341 (in German).
SCHRYVER, S. B. (1901) Some investigations on the toxicology of tin,
with special reference to the metallic contamination of canned
foods. J. Hyg., 9: 253-263.
SCHULER, P., CRUZ, E., GUIJON, C., MATURANA, V., & VALENZUELA, A.
(1958) Stannosis. Benign pneumoconiosis owing to inhalation of tin
dust and fume. II. Clinical study. Ind. Med. Surg., 27: 432-435.
SCHWARZ, K. (1971) Tin as an essential growth factor for rats. In:
Mertz, W. & Comatzer, W. E., ed. Newer trace elements in
nutrition, New York, Marcel Dekker, pp. 313-325.
SCHWARZ, K. (1974) Recent dietary trace element research, exemplified
by tin, fluorine and silicon, Fed. Proc., 33: 1748-1757.
SCHWARZ, K., MILNE, D. B., & VINYARD, E. (1970) Growth effects of tin
compounds in rats maintained in a trace element-controlled
environment. Biochem. biophys. Res. Commun., 40: 22-29.
SCOTT, W. J. & STEWART, D. F. (1944) The influence of dissolved tin on
the growth of Clostridium botulinum in canned vegetables.
J. Counc. Sci. Ind. Res. Aust., 17: 16-22.
SEINEN, W. & WILLEMS, M. I. (1976) Toxicity of organotin compounds. I.
Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di-n-octyltin dichloride. Toxicol. appl. Pharmacol., 35: 63-75.
SEINEN, W., VOS, J. G., SPANJE, I. van, SNOEK, M., BRANDS, R., &
HOOYKAAS, H. (1977a) Toxicity of organotin compounds. II.
Comparative in vivo and in vitro studies with various
organotin and organolead compounds in different animal species
with special emphasis on lymphocyte cytotoxicity. Toxicol. appl.
Pharmacol., 42: 197-212.
SEINEN, W., VOS, J. G., KRIEKEN, R. van, PENNINKS, A., BRANDS, R., &
HOOYKAAS, H. (1977b) Toxicity of organotin compounds, III.
Suppression of thymus-dependent immunity in rats. by di-n-butyltin
dichloride and di-n-octyltin dichloride. Toxicol. appl.
Pharmacol., 42: 213-224.
SHELDON, A. W. (1975) Effects of organotin anti-fouling coatings on
man and his environment. J. Paint Technol., 47: 54-58.
SHIRAISHI, Y., KUZUHARA, Y., & SUENAGA, S. (1972) [Determination of
tin in food by atomic absorption spectrometry.] J. Food Hyg. Soc.
Jpn 13: 97-100 (in Japanese).
SHIRASU, Y. (1970) Acute and sub-chronic toxicology studies of Dowco
213 in mice and rats. Test report on the sub-acute toxicity of
an insecticide, Dowco 213, Tokyo, Animal Pharmacology Institute of
Physical and Chemical Research, 11 pp. (in Japanese).
SHONO, T. & MATSUMURA, Y. (1970) Organic synthesis by electrolysis VI.
Anodie oxidation of aryleyclopropanes. J. org. Chem.,
35: 4157-4160.
SKACKOVA, I. N. (1967) [Hygienic substantiation of the maximum
permissible concentration of tetraethyltin in water bodies.] Gig.
i Sanit., 4: 11-17 (in Russian).
SKEEL, RL., & BRICKER, C. E. (1961) Spectrophotometric determination
of dibutyltin dichloride. Anal. Chem., 33: 428-431.
SMITH, J. D. (1970) The spectrophotometric determination of microgram
amounts of tin with phenylfluorone. Analyst, 95: 347-350.
SODERQUIST, C. J. & CROSBY, D. G. (1978) Determination of triphenyltin
hydroxide and its degradation products in water. Anal. Chem.,
50: 1435-1439.
STANCULESCU, C., MARIN, E., SANDULESCU, C., ILLE, C., TRICIA, A.,
ILLIESCU, M., PETROVICI, C., & POPESCU, P. (1972) [Copper, lead,
tin and arsenic content in canned fish after storage. II. Canned
fresh water fish.] Lucr. Inst. Cercet. Projiect. Ailment.,
10: 295-309 (in Romanian).
STOCKDALE, M., DAWSON, A. P., & SELWYN, M. J. (1970) Effects of
trialkyltin and triphenyltin compounds on mitochondrial
respiration. Eur. J. Biochem., 15: 342-351.
STONE, O. J., & WILLIS, C. J. (1968) The effect of stannous fluoride
and stannous chloride on inflammation. Toxicol. appl. Pharmacol.,
13: 332-338.
STONER, H. B. (1966) Toxicity of triphenyltin. Br. J. ind. Med.,
23: 222-229.
STONER, H. B., BARNES, J. M., & DUFF, J. I. (1955) Studies on the
toxicity of alkyl tin compounds. Br. J. Pharmacol., 10: 16-25.
STOROZEVA, H. H. (1963) [Contents of lead and tin in human teeth in
normal and caries cases.] Stomatologia, 1: 44-48 (in Russian).
STUDER, R. K., SIEGEL, B. A., MORGAN, J., & POTCHEN, E. J. (1973)
Dexamethasone therapy of triethyltin induced cerebral edema. Exp.
Neurol., 38: 429-437.
SUGIMAE, A. (1974) Emission spectrographic determination of trace
elements in airborne particulate matter. Anal. Chem.
46: 1123-1125.
SUZUKI, K. (1971) Some new observations in triethyltin intoxication of
rats. Exp. Neurol., 31: 207-213.
SVENSSON, V. (1975) [Tin poisoning caused by canned peaches.] Hyg.
och Miljö, No. 6, 25-27. (in Swedish).
TABOR, E. C. & WARREN, W. V. (1958) Distribution of certain metals in
the atmosphere of some American cities. Arch. ind. Hyg.,
17: 145-151.
THEUER, R. C., MAHONEY, A. W., & SARETT, H. P. (1971) Placental
transfer of fluoride and tin in rats given various fluoride and
tin salts. J. Nutr., 101: 525-532.
THOMPSON, K. C. & THOMERSON, D. R. (1974) Atomic absorption studies on
the determination of antimony, arsenic, bismuth, germanium, lead,
selenium, tellurium and tin by utilizing the generation of
covalent hydrides. Analyst, 99: 595-601.
TIHONOVA, Z. I. & ZORE, V. A. (1968) [Spectroscopic determination of
manganese, copper, aluminium, lead and tin in certain vegetables
and berries.] Gig. i Sanit., 33: 384-386 (in Russian).
TIPTON, I. H. (1960) The distribution of trace metals in the human
body. In: Seven, M. T., ed. Metal binding in medicine,
Philadelphia, Montreal, Lippincott, pp. 27-42.
TIPTON, I. H., COOK, M. J. STEINER, R. L., BOYLE, C. A., PERRY, H. M.,
& SCHROEDER, H. A. (1963) Trace elements in human tissue. Part I
Methods. Health Phys., 9: 89-101.
TIPTON, I. H., STEWART, P. L., & MARTIN, P. G. (1966) Trace elements
in diets and excreta. Health Phys., 12: 1683-1689.
TIPTON, I. H., STEWART, P. L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys.,
16: 455-462.
TJURIN, Y. M. & FLEROV, V. N. (1970) [Study of adsorption effects
under electro-reducing of organotin compounds on mercury.]
Elektrochim., 6: 1548-1552, (in Russian).
TJURIN, Y. M., FLEROV, V. N., & NIKITINA, Z. A. (1969) [On the
mechanism of electro-reducing of the dialkyltin chloride on the
mercury drip electrode.] Elektrochim., 5: 903 (in Russian).
TONGE, B.C. (1965) The gas chromatographic analysis of butyl- octyl-,
and phenyltin halides. J. Chromatogr., 19: 182-184.
TORACK, R., GORDON, J., & PROKOP, J. (1969) Pathobiology of acute
tri-ethyltin intoxication. Int. Rev. Neurobiol., 12: 45-86.
TROMBETTI, G. & MAINI, P. (1970) Determination of tricyclohexyltin
hydroxide residues in apples and pears. Pestic. Sci.,
1: 144-149.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.P., NGUYEN PHU LICH, VAN DEN
DRIESSCHE, J., GUESNIER, L. R., & MORIN, N. (1976) Contribution to
the toxicological and pharmacological study of tributyltin oxide.
Eur. J. Toxicol. environ. Hyg., 9 (1): 31-40.
TUTTLE, R. F., VOGT, J. R., & PARKINSON, T. F. (1970) Neutron
activation analysis of trace elements in airborne particulates.
In: Proceedings of a Symposium on Nuclear Technology and
Environmental Pollution, Salzburg, Vienna, International Atomic
Energy Agency, pp. 119-137 (Paper No. IAEA-SM-142a/4).
UDRIS, J. (1971) The analysis of tin stabilizers used in
poly(vinylchloride) compositions. Analyst, 96: 130-139.
UNITED NATIONS (1977) United Nations yearbook of industrial
statistics: Commodity production data 1966-1975, Vol. II,
pp. 498-500.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Air quality data for
metals, 1968 and 1969 from the National Air Surveillance
Networks, Research Triangle Park, USEPA, June 1973.
VERNON, F. (1974) The fluorometric determination of triphenyltin
compounds. Anal. Chim. Acta, 71: 192-195.
VINOGRADOV, A. P. (1953) The elementary chemical composition of
marine organisms, Memoir Sears Foundation for Marine Research,
New Haver, Yale University, pp. 16, 211, 240, 266, 360, 366, 535.
VINOGRADOV, A. P. (1956) Regularity of distribution of chemical
elements in the earth's crust. Geokhimiya, 1: 6-52.
VINOGRADOV, A. P. (1962) Average content of chemical elements in the
principal types of igneous rocks of the earth's crust.
Geokhimiya, 555-541.
VIZGIRDA, R. J. (1972) Organotin antifoulants. Paint and varnish
Produc., 62 (12): 12-28.
WALTERS, M. & ROE, F. J. C. (1965) A study on the effects of zinc and
tin administered orally to mice over a prolonged period. Food
Cosmet. Toxicol., 3: 271-276.
WARBURTON, S., UDLER, W., EWERT, R. M., & HAYNES, W. S. (1962)
Outbreak of foodborne illness attributed to tin. Public Health
Rep. (Wash.), 77: 798-800.
WEAST, R. C., ed. (1974) Handbook of Chemistry and Physics, 55th ed.
Cleveland, CRC Press.
WHO Technical Report Series, No. 515, 1973 ( Shistosomiasis control:
report of a WHO Expert Committee). pp. 30-32.
WHO (1975) 1974 evaluations of some pesticide residues in food,
pp. 155-158 (WHO Pesticides Residues Series No. 4).
WHO (1976) Environmental Health Criteria 1: Mercury. Geneva, WHO,
132 pp.
WIECZOREK, H. (1969) [The quantitative determination of organotin
compounds by means of quercetin chelates.] Dtsch.
Lebensmitt-Rundsch., 3: 74-78 (in German).
WILLIAMS, D. J. & PRICE, J. W. (1960) Paper chromatography of some
organotin compounds. Analyst, 85: 579-582.
WODSAK, W. (1967) Tin content of condensed milk. Angew. Chem. Int.
Ed., 6: 885-886.
WOGGON, H. & JEHLE, D. (1973) [The inverse voltametric determination
of biocidal organotin compounds.] Die Nahrung, 17:739-748
(in German).
WOGGON, H. & JEHLE, D. (1975) [The residual analysis of biocidal
organo-tin compounds by means of inverse voltametric methods.]
Die Nahrung, 19:271-275 (in German).
WOGGON, H, SAUBERLICH, H., & UHDE, W. J. (1972) [A contribution to the
determination (anodic stripping method) of organotin compounds and
their degradation and decomposition products.] Z. anal. Chem.,
260: 268-274 (in German).
WOIDICH, H. & PFANNHAÜSER, W. (1973) [Distribution of tin between the
solid and liquid contents of cans of vegetables and fruits.]
Dtsch. Lebensmitt-Rundsch., 69:224-226 (in German).
WOOD, J. M. (1974) Biological cycles for toxic elements in the
environment. Science, 183: 1049-1052.
WOOD, J. M., SEGALL, H. J., RIDLEY, W. P., CHEH, A., CHUDYK, W., &
THAYER, J. S. (1975) Metabolic cycles for toxic elements in the
environment. In Hutchinson, T. C., ed. Symposium Proceedings Vol.
I, International Conference on Heavy Metals in the Environment.
Toronto, Canada, pp. 49-68.
WOOD, J. M., CHEH, A., DIZIKES, L. J., RIDLEY, W. P., RAKOW, S., &
LAKOWICZ, J. R. (1978) Mechanisms for biomethylation of metals and
metalloids. Fed. Proc., 37: 16-21.
YUM, N.M., CONINE, D. L., MARTZ, R. C., FORNEY, R. B., & STOOKEY, G.
K. (1976) Renal tubular injury in rats induced by sodium
pentalfluorostannite, a new anticariogenic agent. Toxicol. appl.
Pharmacol., 37: 363-370.
ZOHM, H. (1972) [A new method for determination of tin and iron in
canned vegetables and fruit juices.] Z. Lebensmitt. -Unters.,
149: 30-33 (in German).
 3.5 Tin-containing Wastes
Gases and fumes containing tin as well as sulfur dioxide and other
contaminants, and both soluble and insoluble tin-containing waste
materials such as water soluble salts, muds, and slags are produced
during various smelting, refining, and detinning operations. Solid
domestic and other wastes which may be dumped, incinerated, used for
land fills or composting, contain much tin, and used cans, aerosol
containers, and other miscellaneous tin-containing produces in solid
wastes account for 10-15% of tin-plated steel. The percentage of
plastics in solid wastes is increasing yearly and includes PVC
containing organotin compounds.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATIONS
4.1 Transport and Bioconcentration
Tin concentrations in air are usually low except in the
neighbourhood of some industrial sources (section 5.1). Similarly, it
has not been consistently detected in all soils, plants, and waters
(sections 5.2 and 5.3). This seems go support a geochemical
classification placing tin in a group of elements with a low rate of
migration in soils and waters (Perel'man, 1972; Bens et al., 1976).
However, it is widely used and Wood et al. (1975) have included it
among elements that are relatively accessible in the environment. A
biological cycle for tin has been recently proposed by Ridley et al.
(1977) (section 4.2).
There is a lack of information concerning the environmental
transport of organometallic tin compounds. It appears, however, that
the vapour pressures of some organotin compounds are high and that
environmental mobility is possible although there are no data to
confirm this deduction. Some organotin compounds seem to be well
adsorbed in the soil and they have a low solubility in water (Heron &
Sproul, 1958). A laboratory soil-leaching study (Barnes et al, 1973)
indicated that triphenyltin is strongly attached to the soil. In
natural waters, organotin compounds would be primarily adsorbed on the
suspended particles and sediments (Schramel et al., 1973). More recent
studies on the determination of tin and organotin compounds in natural
waters have been reported by Braman & Tompkins (1979) and Hodge et al.
(1979).
Little reliable information exists concerning the bioconcentration
of tin and its derivatives. Schroeder et al. (1964) reported that the
presence of tin in phosphate fertilizers originating from marine
phosphate suggested that marine animals in the Pleistocene era
absorbed tin from the sea. Although tin is present in seawater in
concentrations of up to about 3 µg/litre (Mason, 1966; Vinogradov,
1953), there are few reports of its occurrence in marine algae,
plankton, bacteria, flowering plants, protozoa, sponges,
coelenterates, echinoderms, crustacea, and most fishes. However, Bowen
(1966) noted tin levels of 0.2-20 mg/kg in certain marine organisms
and accumulation of tin by the sponge Terpios zeteki was also
reported by Bowen & Sutton (1951).
4.2 Environmental Chemistry of Tin
Because of the considerably higher toxicity of some
organo-metallic tin compounds compared with inorganic forms of tin
(section 7), the possibility of biomethylation of tin is obviously of
considerable interest (Wood, 1974). A possible mechanism of such
bio-methylation has been proposed (Ridley et al., 1977; Wood et al.,
1978). Recent laboratory studies have indicated that the methylation
of tin by methylcobalamina (CH3B12) requires a one electron
oxidation of SnII to a SnIII radical, which can take place in the
presence of FeIII (SnIV would, of course, require a single electron
reduction). The stannyl radical (SnIII) can then react with CH3-B12
(CoIII) to produce (under conditions of high chloride ion
concentration) CH3-SnCl2 and reduced cobalamin, containing CoII
(Wood et al., 1978). A demonstration that methylation of tin takes
place in a strain of Pseudomonas bacteria found in the Chesapeake
Bay, USA, is the laboratory work with these bacteria carried out by
Huey et al. (1974). However, the methylated tin species was not
identified. Incubation of mercury(II) in the presence of tin(IV)
resulted in enhanced formation of methylmercury, and the authors
considered that methylmercury might have been transferred from the
biologically methylated tin to the mercury(II) ion. Subsequently,
Brinckman & Iverson (1975) proposed a "mercury-tin cross-over" system,
which may have some real basis as indicated by Schramel et al. (1973),
who found that mercury and tin accumulated together in some water
plants in Bavarian rivers.
4.3 Degradation of Organometallic Tin Compounds
Organotin compounds may be degraded both chemically and
biochemically. Hydrolytic decomposition occurs at rather extreme pH
values (< 1 or > 13) unless there are other catalytic
influences. Nevertheless, some authors consider that it could occur
fairly rapidly in an aquatic environment, although the environmental
pH is usually between 4 and 10 (Sheldon, 1975, Vizgirda 1972). This
could be the case, if photochemical decomposition were also involved.
Indeed, photochemical decomposition of triphenyltin acetate by
ultraviolet irradiation has been demonstrated under laboratory
conditions by Chapman & Price (1972) and Barnes et al. (1973). Most
likely, under environmental conditions, the chemical and
photo-chemical degradation of organotin compounds is combined with
biochemical degradation, or degradation may be entirely biological.
Some available information, mainly on triphenyltin, tricyclohexyltin,
and tributyl tin compounds, is included in the following summary.
a A form of vitamin B12 (cobalamin) in which the methyl carbon
is directly bonded to the cobalt which is coordinated to the corrin
ring system in the vitamin B12 structure.
Bruggemann & Klimmer (1964) and BrÜggemann et al. (1964a,b)
reported that triphenyltin acetate on sugar beet leaves was rapidly
broken down during the silage process. Within 5 weeks, triphenyltin
acetate, originally present at a concentration of 2470 mg/kg of fresh
leaves, had degraded completely. It was not established whether
degradation was due to microbial action or resulted from the low pH
(3.5-4.0) of the silage process. The authors also found that
triphenyltin acetate was not broken down rapidly by microorganisms
during its passage through the rumen and intestines of cattle. Similar
results in feeding experiments with sheep were reported by Herok &
Götte (1963).
Using thin-layer chromatography, Cenci & Cremonini (1969) found
that triphenyltin acetate and hydroxide mixed into soil (80 mg/kg of
soil) disappeared in 3-10 days, and 3 days, respectively, but the
degradation products were not identified and the participation of
microorganisms in the process was not established.
Akagi & Sakagami (1971) reported that, under their experimental
conditions, ultraviolet irradiation of a solution of triphenyltin
chloride yielded a mixture of triphenyl-, diphenyl-, and phenyltin as
well as inorganic tin compounds, within 6 h. Similarly, irradiation of
trialkyltin compounds resulted in a mixture of di- and monoalkyltin
compounds and inorganic tin also within a period of 6 h. Total
degradation of triphenyltin to inorganic tin required irradiation for
more than 400 h. Chapman & Price (1972) investigated the degradation
of an agricultural fungicide containing triphenyltin acetate, which
had been found to disappear within a few days of spraying on crops,
probably as a result of weathering and sunlight. They exposed the
compound in thin layers on glass to ultraviolet light and found that
the rate of degradation was higher at lower wavelengths and was
independent of layer thickness (5-10 mµ). This was also true for the
intermediate diphenyl- and phenyltin compounds and for the parent
compound. When triphenyltin acetate has irradiated for 60 h
(wavelength above 235 nm: intensity 120 W/m2: thickness 10 µm), about
10% of the triphenyltin compound remained unchanged, about 30%
occurred as diphenyltin, 15% as phenyltin compounds, and approximately
45% was degraded to inorganic tin in the form of hydroxides or
hydrated oxides. Similar results were obtained by Barnes et al.
(1973), who also studied breakdown at concentrations of 5-10 mg/kg
using triphenyltin acetate in which the phenyl groups had been
labelled with 14C. Degradation was monitored by measuring the
evolution of radioactive carbon dioxide; a half-time of about 140 days
was determined. Since no 14CO2 was evolved when the loam was
heat-sterilized, it was concluded that the degradation process was due
to microbial action.
The decomposition of triphenyltin chloride on sugar beet leaves,
as reported by Freitag & Bock (1974b) gave the expected series of
degradation products, the final degradation product being tin oxide.
After 42 days, about 19% of triphenyltin chloride had undergone
degradation.
In studies by Starnes (unpublished data)a and by Smith et al.
(unpublished data)b quoted by Getzendaner & Corbin (1972),
tricyclohexyltin hydroxide (the active ingredient of a commercial
miticide) exposed on thin-layer chromatographic plates to a sun lamp
yielded dicyclohexyltin oxide and cyclohexylstannoic acid with further
degradation to inorganic tin. It has also been shown that tin residues
on apples and pears treated with tricyclohexyltin hydroxide
(commercial miticide) remained almost constant at a level of
0.1-0.2 mg/kg (Getzendaner & Corbin, 1972). This concentration was
relatively independent of the total number of applications and, within
a month of the final application, independent of the time between the
last application and harvest.
Mazaev et al. (1976) reported the degradation of bi(tributyltin)
oxide (TBTO), dibutyltin bis isooctylmercaptoacetate (BuIOMA),
tributyltin methacrylate, diethyltin dioctanoate and dioctyltin bis
(isobutylmaleate), and provided estimates for the half-times of these
compounds for different aqueous media. In distilled water at 20°C,
values ranged from 1.14 days (BuIOMA) to 18.2 days (TBTO). From this
report, it appears that dialkyltin compounds are more rapidly degraded
than trialkyltin compounds.
Triorganotin compounds such as TBTO and tributyltin fluoride used
as antifouling agents in paints for ship bottoms and in underwater
coatings appear to break down in a multislop process to give tin(IV)
oxide (Barnes et al., 1973; Sheldon, 1975).
The mechanism of the biological dealkylation of organotin
compounds is apparently more complicated than was considered by Blair
(1975), who thought that the basic process was the oxidative cleavage
of the carbon-tin bond (section 6.2.4).
a Starnes (1966) Unpublished report, The Dow Chemical Co.
(ALS 66-648).
b Smith et al. (1970) Unpublished report, The Dow Chemical Co.
(OL 30445 Feb. 1. 1970).
Available information shows that the persistence of organotin
compounds may vary considerably depending on the conditions and the
type of compound. Under extreme laboratory conditions, the solvolysis
may have a half-time ranging from about 1 min to about 100 days
(Bassindale et al., 1971; Roberts & El Kaissi, 1968). Under
environmental conditions, it is likely that the half-times for
triphenyltin compounds are somewhere between a few days and 140 days
(Freitag & Bock, 1974b). Diorganotin compounds probably have somewhat
shorter half-times (Mazaev et al., 1976).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1 Ambient Air
Tin is rarely detected in air, and, when detected, it is generally
in low concentrations (0.01 µg/m3), except in the proximity of some
industrial sources. Tin emission concentrations of 10-640 µg/m3 were
reported from electric furnaces at certain plants in Japan, in 1972.
At a distance of 700 metres, the atmospheric tin concentrations still
ranged from 3.8 to 4.4 µg/m3 (Environment Agency, Japan, 1971,
unpublished data).a
Concentrations from 0.003 to 0.3 µg/m3 were found in 60.6% of 754
samples tested from 22 cities in the USA. More than 50% of samples
from 3 urban and 3 rural sites were below the detectable level. The
highest concentration of tin (0.8 µg/m3) was found in a sample from a
Boston, USA, industrial site, which also contained the highest
concentration of lead together with relatively high concentrations of
zinc and cadmium (Tabor & Warren, 1958).
At stations of the US National Air Surveillance Networks during
1968 and 1969, concentrations ranged from below the minimum detectable
level to 0.23 µg/m3 in 1968 and 0.12 µg/m3 in 1969. Both these
concentrations were recorded in East Chicago (US Environmental
Protection Agency, 1973).
Concentrations ranging from 0.04 to 0.09 µg/m3, determined by
emission spectroscopy, were reported for Cincinnati and St Louis in
1970 by the US National Air Surveillance Cascade Impactor Network (Lee
et al., 1972). The size distributions of tin-containing particles
found in this study are given in Table 2.
Peak concentrations of tin were found in particles ranging from 1
to 3 µm in diameter. Bogen (1973) found that ambient air levels of tin
in the Heidelberg area of the Federal Republic of Germany between 30
April 1971 and 21 May 1971 ranged from 0.096 to 0.167 µg/m3.
The combustion of coal, oil, and lignite results in the discharge
of trace amounts of many elements into the atmosphere. Bertine &
Goldberg (1971) pointed out that the principal sites of fossil fuel
consumption are in the mid-latitudes of the Northern Hemisphere and
suggested a need for linking fossil fuel consumption with the
sedimentary cycles of trace metals from the atmosphere. They reported
that tin was present in coal and oil at average concentrations of 2
and 0.01 mg/kg, respectively.
a Japanese Background Paper No. 2, prepared for the WHO meeting
on the Effects on Health of Specific Air Pollutants from Industrial
Emissions, Geneva, November 4-9, 1974.
Table 2. Size distribution of tin-containing particles in urban aira
Cincinnatti St Louis
(A) (B)
4th Quarter 1st Quarter 4th Quarter
1970 1970 1970
Average concentration (µg/m3) 0.09 0.04 0.05
Average mass median diameter (µm) 0.93 1.40 1.53
% particles less than or equal to 1 µm 55 34 28
% particles less than or equal to 2 µm 86 68 65
a Adapted from Lee et al. (1972).
5.2 Soils and Plants
Tin has not been consistently found in all soils and plants;
however, allowance must be made for the possibility that the
concentrations present may have been below the limits of detection of
the methods employed. Bowen (1966) reported levels of tin in soil
ranging from about 2 to 200 mg/kg, the metal being strongly adsorbed
by the humus.
Schroeder et al., (1964) observed that concentrations of tin in
soils were localized and that it had not been detected in many areas.
In a limited study of tin in vegetation and foods, he found that
absorption by plants was erratic, even when the soil tin content was
high. A tin concentration of 157 mg/kg dry weight was recorded in
forest soil from southern Vermont, USA, but tin concentrations in
sections of an 100-year-old elm indicated that exposure of this tree
was not of recent origin.
Tin concentrations of 30-300 mg/kg have been reported in peat from
Finland by Gordon (1952), and high concentrations of tin were found by
Goldschmidt (1958) in forest litter and humus and also in coal. Tin
was present in all but 7 of 43 samples of foliage from 13 species of
ornamental plants and trees in New Jersey, USA (Hanna & Grant, 1962).
Lounamaa (1956), using a spectrographic method that had a limit of
detection of 10 mg tin/kg of ash, detected tin only sporadically in
higher plants in Finland. Lichens concentrated tin to exceptionally
high levels, considering the small amounts present in rocks. For
example, those growing on silicic rocks had tin concentrations of 72 ±
4.7 mg/kg ash (range: less than 10-100). Tin was undetected in 6 out
of 16 mosses while the remainder had concentrations of 10-60 mg/kg of
ash, concentrations being twice as high in those growing on silicic
rock as in those growing on calcareous rock. Pasture herbage growing
in Scotland was reported by Mitchell (1948) to contain tin
concentrations of only 0.3-0.4 mg/kg dry weight. He also quoted tin
concentrations in soils from north-east Scotland of up to 200 mg/kg.
Warren (1964, unpublished data) reported a tin concentration of
75 mg/kg in soils in unmineralized areas of Devon and Cornwall in
England, while soils from mineralized areas contained up to more than
1000 mg/kg.
5.3 Water and Marine Organisms
Tin has only been found occasionally in fresh water. It was found
by Durum & Haffty (1961) in 3 out of 59 samples from 15 major North
American rivers. No tin was detected by the National Water Quality
Network (1960) in 119 analyses at 71 locations on 28 major rivers in
the USA. Kleinkopf (1955, 1960) reported that Maine waters contained a
mean tin concentration of 0.038 µg/litre with a maximum concentration
of 2.50 µg/litre.
Tin has also only been found occasionally in municipal water
supplies (Durfor & Becker, 1964). Analyses by the US Public Health
Service showed concentrations of 0.0008-0.03 mg/litre in 32 out of 175
finished municipal waters (Taylor, 1964, personal communication).a
Although it is known that acidic or alkaline corrosive waters can
attack bronze fittings in pumping stations, presumably releasing some
tin, the relationship between corrosion and the aqueous release of tin
has not been established (Schroeder et al., 1964).
It has been reported by Vinogradov (1953) and Mason (1966) that
tin is present in sea water in amounts of about 0.003 mg/litre; thus,
its presence in marine organisms is to be expected. Tin levels of
0.2-20 mg/kg have been reported in marine animals by Bowen (1966) and
accumulation by the sponge Terpios zeteki has been noted (Bowen &
Sutton, 1951). Schroeder et al., (1964) reported that tin had not been
detected in plankton, bacteria, flowering plants, protozoa,
coelenterates, echinoderms, crustacea, or most fish. However,
Vinogradov (1953) reported the presence of small amounts of tin in a
marine worm, an oyster, and a dogfish.
a Taylor, F. B. (1964) Personal communication, cited by Shroeder,
H. A., Balassa, J. J., & Tipton, I. H. (1964) Abnormal trace metals
in man; Tin. J. chron. Dis., 17: 494.
Organotin compounds may enter water directly from antifouling
coatings on ship bottoms or they may be used as molluscicides and
added to vast areas of water for the control of snails, in which case
the effective concentration has to be about 1 mg/litre (WHO, 1973).
They may also be present indirectly as a result of industrial,
agricultural, and other uses of organotin compounds.
Tin(IV) oxide, suggested to be the final breakdown product of some
antifouling organotin agents (Shelemon, 1975), will eventually be
deposited in bottom sediments because of its insolubility. However, no
information is currently available concerning the rate and mechanism
of this degradation.
5.4 Food
Tin has been reported to occur in trace amounts in most natural
foods (de Groot et al., 1973). The tin content was determined by
atomic absorption spectroscopy in 11 known wheats or wheat blends, in
20 flours prepared commercially from these wheats, and in 25 products
specially prepared from the flours, as well as in 10 consumer products
from 10 different cities in the USA. Only one half of the tin in bread
could have been contributed by the flour. Tin concentrations in common
hard, common soft, and durum wheat were 5.6 ± 0.6, 7.9 ± 0.9, and 6.8
± 0.5 mg/kg respectively, while samples of flour and semolina from the
above sources of wheat contained tin at 4.1 ± 0.4, 3.7 ± 0.7, and 6.0
± 1.2 mg/kg, respectively. The tin concentrations in most products
exhibited significant regional differences (Zook et al., 1970).
Schroeder et al. (1964) calculated that a diet composed largely of
fresh meats, cereals, and vegetables would usually contain a tin
concentration of less than 1 mg/kg and would supply about 1 mg of tin
per day. In an area of southern Vermont, USA, where the soil contained
considerable amounts of tin (about 160 and 33 mg/kg, respectively, in
2 gardens), 10 out of 18 samples of fresh vegetables contained tin
levels of less than 1 mg/kg.
Hadzimicev (1971) measured the content of tin in foods from the
vegetable belt around Sofia, Bulgaria. Tin concentrations in wheat,
corn, beans, potatoes, tomatoes, cabbage, carrots, spinach, lettuce,
onions, apples, and peaches ranged from 0.02 to 1.02 mg/kg.
Larger amounts of tin may be present in processed foods and
drinks, because of corrosion and leaching of the metal from plain
unlacquered cans or from tin foil used for packaging. The corrosion of
tin-plate in food containers depends upon several factors including
the type of food product, the time and temperature of storage, the
acidity of the food and the quantity of air present in the headspace
of the can (Calloway & McMullen, 1966; Monier-Williams, 1949).
Oxidizing agents (eg., nitrates, ferric and cupric salts),and
anthocyanin pigments, methylamine, sulfur dioxide, and other sulfur
compounds accelerate corrosion, while fin salts in solution, sugars,
and colloids such as gelatine retard it (Monier & Williams, 1949). The
introduction of lacquered cans and the crimping of the tops minimizing
direct contact of food with solder has resulted in a general reduction
in corrosion and leaching.
A number of foods are unsuitable for packing in plain cans as they
promote corrosion. Thus, tin has been found in canned asparagus in
high concentrations varying from 120 to 550 mg/kg (Adam & Horner,
1937; Eyrich, 1972; Woidich & Pfannhäuser 1973). Tomato fruit can
accumulate nitrate when grown under combined conditions of high
temperature, high nitrogen fertilization levels, and low light
intensity. Such tomatoes caused excessive detinning of internal plain
can surfaces (Hoff & Wilcox, 1970). Iwamoto et al. (1968) also showed
a distinct detinning effect on unlacquered cans of both naturally
occurring and added nitrate in tomato juices; the authors suggested
that the concentrations of nitrate-nitrogen in juices should not
exceed 3 mg/kg. Tin contents exceeding 100 mg/kg have been found in
other foods in unlacquered cans including fish, orange, grape, and
mango juices, apricots, bananas, pineapple, and sugar syrup (Catala et
al., 1971; Eyrich, 1972; Mahadevaiak et al., 1969; Stanculescu et al.,
1972; Woidich & Pfannhäuser, 1973). In foodstuffs stored in
all-lacquered cans, the tin content was mainly below 25 mg/kg (Catala,
et al., 1971; Eyrich, 1972; Woidich & Pfannhäuser, 1973).
Several investigations have shown that high storage temperatures
increase the transfer of tin into canned foods (Catala et al., 1971;
Kimura et al, 1970; Zui, 1970). An increase of 2 mg/kg per month of
storage was reported by Zui (1970) for an increase of 1°C in
temperature.
Nishijama et al. (1971) showed an increase in the tin content from
50-77 to 260-300 mg/kg in pineapples stored at 8°C in unlacquered cans
for 72 h after opening the can. The transfer of tin into the fruits
was also increased by 0.5% aqueous citric or tartaric acid. The
content of tin in foods in lacquered cans was low and remained low
after opening. Klein et al. (1970) noted a similar effect of storage
of fruit juices in opened plain cans. Values exceeding 200 µg/kg were
found within 48 h of opening the cans.
A mean concentration of 0.0078 mg/litre was found in bottled
(glass) cow's milk compared with 16 mg/litre in evaporated milk in
unlacquered cans (diluted to the equivalent volume of cow's milk). In
some cases, concentrations of up to 110 mg/litre were found; however,
only milk from unlacquered cans contained significant amounts of tin,
while milk in lacquered cans contained less than 5 mg/litre (Hamilton
et al., 1972). The highest concentration of tin found by Wodsak (1967)
in canned condensed milk samples, less than 4 weeks old, was
40 mg/litre. The concentrations did not increase much during a further
5 months of storage but after 2 years of storage, concentrations up to
160 mg/litre were detected.
In addition to contamination by the containers, the presence of
tin in food may be due to the use of tin as an additive. Tin(II) ions
are used as additives in asparagus and peas packed in glass containers
or in lacquered cans, and also in soda water. The stannous ion is
added to prevent the migration of other heavy metals into the canned
foods and to inhibit the oxidation of ascorbic acid. Scott & Stewart
(1944) reported that Clostridium botulinum would not grow in
beetroot and carrots in unlacquered cans whereas normal growth
occurred in lacquered cans. For canned beetroot, the concentration of
added tin required for the prevention of growth was 150 mg/kg and for
carrots, 30-60 mg/kg.
High concentrations of tin have been reported in cheese packed in
tin foil (Dyer & Taylor, 1931, Elten, 1929).
5.5 Organotin Residues
Tin occurring in food and beverages may also result from the use
of organotin compounds as miticides in agriculture and as stabilizers
in PVC materials. In several experiments in various parts of the USA,
apples and pears from trees that had been sprayed 4 times with a
miticide, tricyclohexyltin hydroxide, had maximum organotin residues
of less than 2 mg/kg of whole fruit on the day of the final
application. These concentrations were reduced to about half within
3-5 weeks and, after 4 weeks, the residues consisted mainly of
tricyclohexyltin hydroxide with only small amounts of decomposition
products; inorganic tin concentrations were almost invariably below
0.2 mg/kg. The mean concentration of inorganic tin on apples and pears
after 1-5 applications of the miticide was 0.1 mg/kg (equivalent to
0.3 mg/kg, when calculated as tricyclohexyltin hydroxide); an
inorganic tin residue of 0.3 mg/kg (equivalent to 1.0 mg/kg of
tricyclohexyltin hydroxide) was found on pears by Getzendaner & Corbin
1972). In an appraisal of data on pesticide residues in food, the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues (FAO/WHO, 1971) concluded that residues of
tricyclohexyltin hydroxide on apples and pears decline by 50% in about
3 weeks due to photodegradation (section 4.3). A 20-50% reduction can
be achieved by washing and most of the residues can be removed by
peeling the fruit, after which only 0.1 mg/kg may be expected in the
fruit flesh. Data on tricyclohexyltin hydroxide on apples and pears
after 1-4 treatments showed mean values ranging from 0.4 to 2.0 mg/kg.
Similar concentrations on apples and pears have been reported from the
Netherlands (WHO, 1975). It has also been reported that the
concentrations of tricyclohexyltin hydroxide in treated products grown
under glasshouse conditions including cucumbers, tomatoes, and bell
peppers are unlikely to exceed 0.5 mg/kg (WHO, 1975).
The FAO/WHO joint meeting report (FAO/WHO, 1971) included data on
triphenyltin hydroxide, acetate, and chloride residues in various
foodstuffs such as potatoes, carrots, and sugar beets. Maximum
concentrations in potatoes and carrots only rarely exceeded 0.1 mg/kg.
Residues in food, fruit, and vegetables derived from direct contact
with the compound could be considerably reduced by washing.
When cows were fed with sugar beet leaves containing triphenyltin
acetate at 1 mg/kg a concentration of 0.004 mg/kg was found in the
milk.
In some countries, regulations permit the presence of 2 PVC
stabilizers in food, viz. dioctyltin S,S'-bis(isooctyl-
mercaptoacetate) and dioctyltin maleate polymer, up to a concentration
of total octyltin of 1 mg/kg (Department of National Health and
Welfare, Canada 1975; Food and Drug Administration, USA, 1971). The
migration of tin from PVC bottles into liquid foods contained in them
was studied by Carr (1969). The increase in the tin content of various
products during the storage period ranged from 0 to 0.07 mg/kg
(Table 3).
Table 3. Tin concentrations in foodstuffs after storage for 2 months in PVC bottles at 30 °C
Organotin
Tin content Tin content Tin extracted stabilizer
at beginning after ageing from bottle extracted from
Food product of experiment in PVC bottle (mg/kg) PVC bottle
(mg/kg) (mg/kg) (mg/kg)
mineral water 0.076 0.088 0.01 0.063
tomato juice 0.03 0.03 0 0
peanut oil 0.05 0.06 0.01 0.063
vegetable oil 0.08 0.09 0.01 0.063
apple juice 0 0.02 0.02 0.126
cherry soda 0 0.07 0.07 0.443
beer 0 0.01 0.01 0.063
milka 0.02 0.04 0.02 0.126
red wine 0 0 0 0
blended whisky 0.01 0.02 0.01 0.063
a Milk sample in PVC bottle aged for 2 weeks at 65 °C (Carr, 1969).
5.6 Working Environment
While most of the operations associated with the extraction and
treatment of tin ore are wet processes, tin dust or fumes may escape
during the bagging of concentrate in the ore rooms and during smelting
operations (mixing plant and furnace tapping), as well as during the
periodic cleaning of the bag filters used to remove particulate matter
from smelter furnace flue gas before release into the atmosphere (ILO,
1972). Exposure to dust and fumes of tin oxide may cause stannosis.
Additional exposure can occur at the final stage of upgrading the
cassiterite concentrate and during the roasting of sulfide ore.
Tin(II) chloride constitutes a hazard, when the rough molten tin is
separated from the rest of the charge during refining (ILO, 1972).
Other sources of industrial exposure include tinning of metals
(Duckering, 1968); the preparation and use of tin alloys and solders;
the use of tin(II) chloride as a reducing agent in calico printing;
the use of hydrated stannic acid, sodium metastannate, hydrated
tin(IV) chloride, and ammonium tin(IV) chloride as mordants in dyeing
and in the weighting of silk; the use of tin(II) fluoroborate
[Sn(BF4)2] in plating baths; and the production and use of organotin
compounds.
5.7 Estimate of Effective Exposure of Man through Environmental Media
Food is the main source of exposure to tin for man. Estimates of
human exposure from all environmental media are difficult to make
because of the lack of reliable data concerning environmental
concentrations (mainly in air and water), and because of variations in
the amounts of different foods and beverages consumed, especially
canned foods. Earlier estimates of the daily intake of tin by man have
been difficult to reconcile with more recent estimates. Kehoe et al.
(1940) reported that the mean daily intake of Gin by a normal adult in
the USA was 17 mg, while Tipton et al. (1966, 1969), in long-term
balance studies, reported average daily intakes of between 1.5 and
8.8 mg in 4 subjects. Schroeder et al. (1964) found 3.6 mg in a day's
institutional diet and considered that the major portion of the tin
probably came from canned fruit juices and fruits in the diet.
However, Schroeder and his colleagues pointed out that human intake
may vary considerably. They calculated that a 10 MJ (2400 kcal) diet
composed largely of fresh meats, grain products, and vegetables, which
usually contain less than 1 mg/kg of tin, would supply 1 mg/day.
However, a diet including a substantial proportion of canned
vegetables and fish could supply as much as 38 mg/day. A typical daily
tin balance for an adult in the USA, as estimated by Schroeder et al.
(1964), is shown in Table 4.
Hamilton et al. (1972) reported a daily intake of tin from food
and beverages by an adult in the United Kingdom of 0.187 ± 0.042 mg
(Table 5). It seems likely that this diet was composed of fresh foods,
which would account for the lower values obtained compared with the
North American data.
Table 4. Daily tin balance for an adult in the USAa
Intake (mg) Output (mg)
food 4.0 faeces (estimated) 3.98
(range, 1-40) (range, 1-40)
water 0.0 urine 0.023
(range, 0-0.03) (range, 0-0.04)
air 0.003
(range, 0-0.007)
total 4.003 (1-40) 4.003 (1-40)
a From: Schroeder et al. (1964).
Table 5. The concentration of tin in foods and total daily intake
for an adult in the United Kingdoma
Item Number of Concentration of tin (mg/kg)b
samples
cereals 3 4.7±1.5 × 10-2
meat 4 2.1±0.5 × 10-3
rats 4 3.7±0.8 × 10-2
fruits 5 0.5±0.1
root vegetables 3 2.2±1.0 × 10-2
green vegetables 4 2.3±10-2
milk (cow's) 17 7.8±1.2 × 10-3 (mg/litre)
total daily untake 0.187±0.042
a "From Hamilton et al. (1972).
b For prepared diet.
6. METABOLISM
Although a substantial amount of literature exists on the
absorption, distribution, excretion, and storage of tin, the results
of much of the early research are questionable in the light of modern
analytical techniques. For example, some studies depended on tissue
matrix destruction processes with the risk of loss of tin salts by
volatilization. In many cases, conditions were not controlled or
defined making it difficult to know whether pure valence states of
tin(II) or tin(IV) were being used, and whether the oxidation state of
the element affected its biological fate. In most cases, the purity of
the compound used was not indicated.
6.1 Inorganic Tin
6.1.1 Absorption
Evidence obtained from man and several animal species shows that
ingested inorganic tin is poorly absorbed. Most studies indicate that
less than 5% is absorbed from the gastrointestinal tract, although
values as high as 20% have been reported (Furchner & Drake, 1976;
Hiles, 1974; Kehoe et al., 1940; Kutzner & Brod, 1971,
Monier-Williams, 1949; Schroeder et al., 1964).
The influence of oxidation state and anion complement on the rate
of gastrointestinal absorption was studied by Hiles (1974) by
administering tin to rats in the form of tin(II) citrate, fluoride, or
pyrophosphate, and tin(IV) citrate or fluoride as a single oral dose
at 20 mg/kg body weight. The absorption of tin(II) citrate and
fluoride was calculated to be 2.8%, whereas only 0.6% of the dose of
tin(IV) citrate and fluoride was absorbed; the citrate and fluoride
were equally absorbed. Absorption of tin, when the anion was
pyrophosphate, was significantly lower than when the anion was citrate
or fluoride. The author considered that this was because pyrophosphate
had a greater tendency to form insoluble complexes with tin than
either fluoride or citrate.
6.1.2 Distribution
With both oral and parenteral administration of tin to animals,
the highest concentrations were found in the kidney, liver, and bone
(Durbin, 1960; Hiles, 1974; Moskalev, 1964), the principal site of
deposition being the bone (Furchner & Drake, 1976; Hiles, 1974).
The amounts of tin remaining in the organs of rats 48 h after
receiving a single oral dose of 20 mg of tin in the form of a
radioactive compound are shown in Table 6; the distribution 48 h after
a single intravenous administration of tin(II) or tin(IV) citrate is
shown in Table 7 (Hiles, 1974).
Rats given a daily oral dose of 20 mg of tin per kg of body weight
as tin(II) or tin(IV) fluoride did not show any detectable
trans-placental transfer at day 10 after conception, and at day 21,
the amounts of tin found in rats given the tin(II) compound were so
close to the limit of detection (= 0.1 µg of tin) that the
significance of the findings remained doubtful. In rats receiving the
same compounds at similar daily doses for 28 days, no significant
amounts of tin could be detected in the brain, heart, testes,
adrenals, reproductive tract, skeletal muscle, and spleen, while the
levels in kidney and liver were approximately the same as after a
single oral dose; however, the levels in bone were about 8 times
higher than those found after a single oral dose. Tissue
concentrations were higher following administration of tin(II)
fluoride than following tin(IV) fluoride administration (Hiles, 1974).
The results of distribution experiments performed by Hiles (1974)
suggested that tin is unlikely to be rapidly oxidized or reduced
during absorption and systemic transportation, although the actual
oxidation state of the tin in the tissues could not be determined.
Following intravenous injection of 113Sn(II) and 113Sn(IV)
citrates in rats, only small quantities of tin could be detected in
lung tissues (Hiles, 1974). However, tin is found in the human adult
lung and there is evidence indicating that the concentrations increase
with age often reaching higher levels than those in other tissues
(Schroeder et al., 1964). Thus, it seems probable that tin reaches the
lung primarily through inhalation.
Levels of 113Sn in the blood of rats, 2 days after oral or
intravenous administration, were extremely low and were only detected
in the erythrocytes (Hiles, 1974). Kehoe et al. (1940) found that 80%
of the tin in the blood of man was in the cells.
6.1.2.1. Distribution in human tissues and biological fluids
Tin concentrations found by Hamilton et al. (1972/1973) in some
tissues of healthy human adults are given in Table 8 together with
data reported by Kehoe et al. (1940) and Schroeder et al. (1964).
Comparatively high concentrations were found in lung, kidney, liver,
bone, and also in lymph nodes. Fairly high levels were reported by
Storozeva (1963) in the teeth of 24 persons, i.e., a range of
0.5-1.7 mg/kg of ash.
Table 6. 113Sn. distribution in rats after a single oral dose of solutions of various 113Sn compoundse
Percent of dosed radioactivity
Sample
SnF2 SnF4 Sn(II) Citrate Sn(IV) Citrate Sn2P2O7
Faeces and
gastrointestinal
wash 100.60±9.9 (10)a 98.40+3.6 (10) 98.00±2.1 (5) 95.40±10.3 (5) 100.0±2.8 (6)
Urine 1.02±0.58 (10)b,d 0.22±0.13 (10)b 0.90±0.23 (5)c 0.33±0.12 (5)c 0.41±0.19 (6)d
Kidneys 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.05±0.02 (5)c 0.01±0.00 (5)c 0.03±0.02 (6)d
Liver 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.07±0.02 (5) < 0.004 (5) 0.02±0.02 (6)d
Pancreas < 0.004 (5) 0.02±0.03 (5) < 0.004 (5) < 0.004 (5) < 0.004 (5)
Recovery 101.74±9.4 (10) 98.66+3.6 (10) 99.02±2.3 (5) 95.74±10.5 (5) 100.46±2.9 (6)
Percent of dosed 113Sn/g of sample
Bone (femur) 0.20±0.13 (10)b,d 0.04±0.02 (10)b 0.09±0.04 (5)c 0.03±0.02 (5)c 0.02±0.01 (6)d
Lymph nodes < 0.004 (5) 0.08±0.12 (9) < 0.004 (5) < 0.004 (5) 0.01±0.01 (5)
Blood clot 0.01±0.01 (10) < 0.004 0.01±0.00 (5) < 0.004 (5) < 0.004 (6)
Leg muscle < 0.004 (5) 0.01±0.01 (5) < 0.004 (5) < 0.004 (5) < 0.004 (6)
a % of dosed 113Sn±SD (number of animals) where 1% = 40 µg. Tissues containing < 0.010%, of the dose were not
normally included in the table.
b Significant difference at p < 0.05 between SnF2 and SnF4.
c Significant difference at p < 0.05 between Sn(II) citrate and Sn(IV) citrate.
d Significant difference at p < 0.05 between SnF2 and Sn2P2O7.
e From: Hiles (1974).
Table 7. Fate of inorganic tin in rats. Tin distribution after a single intravenous
dose of 113Sn(11) or 113Sn(IV) citratee
Percent of dosed radioactivity
Sample Sn(II) citrate Sn(IV) citrate
Bile-duct Bile-duct
Intact cannulated Intact cannulated
Urine 35.3±4.6a 23.3±12.8 39.8±10.8 24.8± 2.5
Faeces 12.1±1.8b,d 2.2±1.1d 3.1±1.4b 0.5±0.3
Bile -- 11.5±2.4c -- 0.1±0.1c
Injection site 3.3±1.8 6.2±4.7 4.1±1.5 3.9±3.3
Liver 2.0±0.2 5.1±1.6c,d 0.2±0.1d 0.2±0.1c
Kidney 5.9±0.9 8.3±1.3 5.3±3.4 4.2±2.5
Lungs 0.12±0.08 0.2±0.1 < 0.04 < 0.06
Spleen 0.4±0.0 -- < 0.04 --
Pancreas 0.2±0.1 -- < 0.04 --
Stomach 0.1±0.1a 0.2±0.0d < 0.04 < 0.06
Small intestine 0.5±0.1a 0.4±0.4d < 0.04 < 0.06
Large intestine 1.0±0.8b,d 0.4±0.3d 0.2±0.3b < 0.06
Carcass 33.4±5.3 -- 43.2±5.3 --
Femur (one) 1.6±0.2 1.9±0.1 3.2±0.6 2.0±0.1
Percent of dosed 113Sn/g
Bone (femur) 4.5±0.6b,d 8.4±1.0d 8.8±1.8b 7.4±0.9
Blood clot 0.6±0.2d 0.9±10.3d < 0.04 < 0.06
Leg muscle 0.06±0.03 -- < 0.04 --
a % of dosed 113Sn±SD where 1% = 4 µg tin.
b Significant difference at p < 0.05 between intact Sn(II) and Sn(IV) animals.
c Significant difference at p < 0.05 between bile-duct cannulated Sn(II) and
Sn(IV) animals.
d Significant difference at p < 0.05 between Sn(II) intact and bile-duct cannulated
animals.
e From: Hiles (1974).
Schroeder et al. (1964) reported a highly variable distribution of
tin in human tissues with significant differences related to age and
geographical location. Variations in the tissue contents of tin among
several geographical regions throughout the world were quite marked
but were less noticeable between 8 cities of the USA (Table 9 and 10).
All kidney, liver, and lung samples from Africa, Asia, and Europe had
lower mean and median tin contents than samples from the USA (the
difference being least in lungs). Tin in the tissue of Africans, where
exposure to tin would be expected to be much lower than in
industrialized North American and European areas, was, indeed,
noticeably low. Tin was rarely detected in the tissues of stillborn
infants, indicating that tin does not readily cross the placental
barrier. The lungs accumulated tin with advancing age but other organs
did not. Concentrations of tin in kidney, liver, lungs, and the ileum
according to decade of life are shown in Table 9. Small amount were
found in the heart, prostate, uterus, and trachea. Avtandilov (1967)
studied the trace element contents of normal and atherosclerotic
aortas. He reported a mean tin concentration of 0.08 mg/kg wet weight
in 20-39-year-old subjects with no difference between the two
categories of aorta.
Table 8. Tin content of human tissues, concentrations expressed as mg/kg wet weight.
Hamilton et al. (1972/73) Kehoe et al. (1940) Schroeder et al. (1964)
Tissue mean ± S.E. mean range (8 cities in
USA)
blood 0.009±0.002
brain 0.06±0.01 NDa
kidney 0.2±0.04 0.2 0.23-0.76
liver 0.4±0.08 0.35-1.0
lung 0.8±0.2 0.45 0.49-1.20
lymph node 1.5±0.6
muscle 0.07±0.01 0.1
bone 4.1±0.6b 0.5-0.8
a ND = not detected.
b mg of element per kg of ash.
Table 9. Tin in human tissues by age, mean US values (mg/kg ash)a
Age group (year) 0 0-1 1-10 11-20 21-30 31-40 41-50 51-60 81-70 71-84
kidney
concentration 0 57b 60b 33 20 34b 28b 22 34 32
occurrence 0/16 9/10 19/20 10/15 17/21 36/43 22/26 26/27 13/14 8/9
liver
concentration 0 48b 61b 42 34 33b 25 35 38b 34b
occurrence 0/21 11/12 22/23 14/14 20/21 39/43 27/28 25/25 13/13 9/9
lung
concentration (18) 35 34 45b 27 31b 39b 53b 58 64b
occurrence 1/10 5/6 5/6 10/13 18/21 34/35 27/27 28/29 13/13 9/9
ileum
concentration 0 101 98 80 174 116 97 53 172 140
occurrence 0/8 1/2 1/1 9/10 12/13 26/28 16/17 12/12 6/6 3/4
total occurrence
(%) 1.8 86.7 94.0 82.7 88.2 79.8 93.9 97.8 97.7 93.5
Note: Concentrations of positive samples.
Occurrence refers to the number positive/total cases.
a From: Schroeder et al. (1964).
b Excluding values > 150.
Table 10. Geographical distribution of tin in kidney, liver, and lung of man (mg/kg ash)a
No. of %
samples present Mean Range Median
kidney
United States of America 161 97 30 < 5-480 20
Switzerland 9 33 5 < 5-17 < 5
Africa 53 19 5 < 5-30 < 5
Middle East 43 42 8 < 5-55 < 5
Far East 57 40 11 < 5-110 < 5
liver
United States of America 163 96 35 < 5-300 24
Switzerland 9 78 6 < 5-20 Tc
Africa 49 25 4 < 5-20 < 5
Middle East 44 55 11 < 5-140 Tc
Far East 54 41 10 < 5-74 < 5
lung
United States of America 159 98 69 < 5-920 39
Switzerland 7 100 37 23-62 28
Africa 50 60 10 < 5-40 Tc
Middle East 45 91 33 < 5-200 17
Far East 57 93 65 < 5-1200 29
Note: Tc = trace, or barely detectable.
Mean and median values were obtained by assigning a value of one-half
the least detectable amount to those cases where tin was not detected.
a From: Schroeder et al. (1964).
Using a spark source mass spectrometric method with a detection
limit of 0.004 mg/kg wet weight, Hamilton et al. (1972/1973) reported
a mean concentration of 0.06 mg/kg (± 0.01) in 10 whole brain samples
from accidentally killed, presumably healthy adults. The concentration
found in the frontal lobe was 0.03 (± 0.07) and that in the basal
ganglia was 0.04 (± 0.02) mg/kg, measured in 2 samples. Tin was not
detected in human brain by Kehoe et al. (1940) and was found in only a
small percentage of subjects by Tipton (1960) and Schroeder et al.
(1964).
6.1.3 Excretion
The major route of excretion of absorbed inorganic tin is the
kidney although a small fraction is excreted into the bile (Hiles,
1974; Moskalev, 1964). The excretion of tin in the urine was studied
by Perry & Perry (1959) in 24 human adults. A mean of 16.6 µg/litre of
urine or 23.4 µg/day was reported. Kehoe et al. (1940) reported a mean
concentration of 18 µg/litre of urine in subjects in the USA. There
remains some doubt, however, as regards the accuracy of the analytical
technique used since, in the same paper, the concentration in the
urine of French males was reported to be zero.
In an experiment on rats, Hiles (1974) reported that after a
single oral dose of tin(II) or tin(IV)citrate or fluoride at 20 mg/kg
body weight (as the metal), about 50% of the absorbed tin was excreted
within 48 h. Following intravenous administration of 113Sn (2 mg of
tin per kg body weight), 12% of the 113Sn(II) and only 4% of the
113Sn(IV) appeared in the faeces of rats indicating that the biliary
route is probably more important in the elimination of tin(II) than of
tin(IV) compounds. Most of the biliary excretion (94%) took place
within 24 h.
6.1.4 Biological half-time
A half-time of 3-4 months was reported for 113Sn in the skeleton
of rats after intramuscular administration (Hamilton, 1948). However,
Hiles (1974) found a half-time of only 34-40 days for both tin(II) and
tin(IV) in the bone of rats following oral administration of tin(II)
fluoride or tin(IV) fluoride for 28 days. The half-time of tin(II) for
liver and kidney was reported to be 10-20 days. Furchner & Drake
(1976) using tin(II) chloride administered intraperitoneally and
intravenously in the mouse, rat, monkey, and dog, described the
elimination from the body as a 4 component-process that was similar in
all the species studied. The half-time for the longest component was
over 3 months.
6.2 Organotin Compounds
In this document, consideration will be limited to compounds of
the general type RSnX3, R3SnX2, R4SnX, and R4Sn where R is a
simple or complex action. Generally, little information is available
on the absorption, distribution, and excretion of these substances.
6.2.1 Absorption
Absorption from the intestinal tract of tin compounds with short
alkyl chains varies depending on the compound. There are also
considerable differences in absorption between species (Barnes &
Stoner, 1959).
Ethyltin trichloride administered orally was poorly absorbed by
rats, 92% of an oral dose of 25 mg/kg being eliminated in the faeces
within 2 days. It was not excreted in the bile (Bridges et al., 1967).
Triphenyltin acetate was almost completely eliminated in faeces within
a few days, when administered orally to sheep (Herok & Götte, 1964)
and cows (Bruggemann et al., 1964a). However, it was well absorbed,
when given orally to guineapigs and mice (Stoner, 1966). Results
obtained with rats were conflicting (Klimmer, 1963, 1964; Stoner,
1966). Tricyclohexyltin hydroxide was poorly absorbed in rats, only
about 2% being excreted in the urine after a single 25 mg/kg oral
dose. The remainder was recovered from the faeces and binary excretion
did not occur (FAO/WHO, 1971).
Trialkyltin compounds were well absorbed on contact with the skin,
since the dermal LD50s of various trimethyltin derivatives in mice
were of the order of 50-100 mg/kg (Hall & Ludwig, 1972) and that of
bis(tributyltin) oxide was below 200 mg/kg in rats and mice (Ascher &
Nissim, 1964; Elsea & Paynter, 1958). When a 20% fat solution of
various triethyltin derivatives was applied to the skin of rats and
mice for 10 min, all the animals died within 20-30 min (Ignatjeva et
al., 1968). In contrast, triphenyltin acetate did not penetrate
unbroken skin readily (Stoner, 1966).
6.2.2 Distribution
Following intravenous administration to mice and rats, the highest
concentrations of dibutyl- and diethyltin were found in liver and
kidney tissue; unchanged compounds were excreted in the bile (Barnes &
Magee, 1958). Rats were fed with 11 mg of triethyltin hydroxide over a
period of 89 days. At the end of this time, only 0.7 mg of the
compound could be recovered; 40% of this was in the blood, 28% in the
liver, and 29% in the skeletal muscle. Smaller amounts of triethyltin
were found in the kidney, brain, heart, and spleen (Cromer, 1957).
Species variation in the distribution of triethyltin has been
reported. When triethyltin (26 µg) was added to rat blood in vitro,
most of it (23 pg) was recovered from the erythrocytes and none was
found in the plasma, whereas in rabbit blood, triethyltin was more
equally distributed between erythrocytes (9 µg) and plasma (17 µg)
(Cromer, 1957). This could explain why triethyltin persists in the
blood of treated rats, whereas it quickly disappears from the blood of
treated rabbits (Barnes & Stoner, 1959). Triethyltin was found in the
brain of rats only 1-2 h after intraperitoneal injection of
triethyltin hydroxide (Cremer, 1957).
The tissue distribution of triethyltin following intravenous
administration of tetraethyltin resembled that following
administration of triethyltin (Cremer, 1957, 1958). Thus, a large
quantity of triethyltin was found in the liver, with smaller
quantities in the kidney, brain, and whole blood in a rabbit, 2 h
after administration.
After oral or intraperitoneal administration of 113Sn-labelled
triphenyltin to guineapigs, about 80% of the administered activity was
excreted in 10 days. The concentration of radioactive tin found in the
brain of rats and guineapigs after oral and intraperitoneal
administrations decreased with a half-life of several days. The form
of tin in the brain was not unequivocally identified, but it was not
in the form of free stannic ions nor was it necessarily triphenyltin
(Heath, 1967). Herok & Götte (1964) gave 3 sheep 10 mg of triphenyltin
acetate orally, every day for 20 days. The compound was labelled with
113Sn. The animals were killed at intervals ranging from 8 to 198 days
after the last dose. The highest concentration of 113Sn was found in
the liver but the brain also contained measurable amounts. In rats,
any absorbed triphenyltin chloride was rapidly distributed through the
tissues of the body including the brain. Tin was eliminated relatively
slowly and could be detected in the brain 38 days after a single dose
(Heath, 1963).
Only trace quantities of tricyclohexyltin hydroxide were found in
the tissues of rats and dogs fed up to 12 mg/kg bodyweight per day, in
the diet, for periods ranging from 45 days to 2 years (FAO/ WHO,
1971).
6.2.3 Excretion
When ethyltin trichloride was administered intraperitoneally to
rats, it was excreted almost exclusively in the urine; binary
excretion was negligible. When diethyltin was administered
intraperitoneally it was eliminated as diethyl- and ethyltin in both
faeces and urine. Diethyltin was excreted in the bile (Bridges et al.,
1967). Rats that had initially received a diet containing triethyltin
and had retained approximately 0.7 mg triethyltin in their tissues
were then given a normal diet; 12 days later, no triethyltin could be
detected in the tissues (Cremer, 1957). The route of excretion was not
known.
The elimination of triphenyltin from the body is slow (Stoner,
1966). Its persistence in guineapigs is suggested by the similarity
between the amounts consumed by those dying on diets containing 25 and
50 mg/kg and the acute oral LD50. In studies on guineapigs using
113Sn-labelled triphenyltin, the concentration of triphenyltin that
had entered the brain after a single oral dose did not decrease during
the first 10 days. In rats, triphenyltin disappeared more rapidly,
having a half-time of about 3 days in the brain (Heath, 1963, 1965).
Triphenyltin acetate labelled with 113Sn was slowly excreted in the
urine of lactating sheep given oral doses of the compound at the rate
of 10 mg/day for 20 days. The milk confined small amounts of tin
(about 1 µg/litre) in both organic and inorganic forms (Herok & Götte,
1964).
The biological half-time of tricyclohexyltin hydroxide in rats was
5-40 days, when the compound had been included in the diet over a
prolonged period. The brain was one of the tissues from which the
compound was removed most slowly (FAO/WHO, 1971).
6.2.4 Biotransformation
In some early studies on the metabolic degradation of organotin
compounds, it was suggested that destannylation (carbon-tin bond
cleavage) was the major result of this biotransformation. For example,
triethyl tin was detected in the tissues of rabbits and rats given
tetraethyltin intravenously (Cremer, 1957, 1958). The liver was the
organ most active in this conversion. Bridges et al. (1967) reported
that dealkylation of diethyltin occurred in both the gut and tissues
of the rat. Although Herok & Götte (1963) found that after
administration of triphenyltin acetate labelled with 113Sn small
amounts of inorganic tin were present in the milk of sheep, Stoner
(1966) considered that triphenyltin was not readily transformed in the
body. Dicyclohexyltin oxide and trace amounts of cyclohexylstannoic
acid were identified as metabolites in rats and dogs treated with
tricyclohexyltin hydroxide (FAO/WHO, 1971). Blair (1975) concluded
that tricyclohexyltin hydroxide undergoes metabolism in animals by
scission of cyclohexyl groups from the atom.
More recent studies using tributyltin acetate showed primary
biological oxidation reactions in the hydroxylation of carbon-hydrogen
bonds that are alpha, ß, gamma and delta to the tin atom (Fish et al.,
1976). This type of reaction was already assumed by Casida et al.
(1971), although no carbon-hydroxylated metabolites could be
identified when triethyltin derivatives were biologically oxidized
in vitro to monoethyl derivatives.
Fish et al. (1975) treated tributyltin acetate with rat liver
microsomes (a mono-oxygenase enzyme system) in the presence of NADPH,
and obtained alpha and ß hydroxylated metabolites in yields of 24% and
50%, respectively. An in vivo study with [1-14C]-tetrabutyltin
showed the occurrence of similar reactions in mice (Kimmel et al.,
1977). The alpha-hydroxyl metabolite is unstable and undergoes a
cleavage reaction at pH = 7.4 to give 1-butanol and a dibutyltin
derivative. A ß-elimination reaction in acidic media transforms the
ß-hydroxyl derivatives rapidly into 1-butane and a dibutyl derivative
(Fish et al., 1976).
Studies on the in vitro metabolism of tricyclohexyltin compounds
indicated that carbon-hydroxylation of the cyclohexyl group was a
major metabolic reaction (Fish et al., 1976; Kimmel et al., 1977).
When [113Sn] -triphenyltin acetate was subjected to in vitro
biological oxidation, the phenyl group was not biotransformed, but in
in vivo studies, rats were found to metabolize triphenyltin acetate
into diphenyl- and phenyltin derivatives (Kimmel et al., 1977).
7. EFFECTS ON ANIMALS
7.1 Inorganic Tin Compounds
Although tin is present in small amounts in most animal and human
tissues, it is uncertain whether it is an essential element for
mammals. However, recent results indicate that tin is an essential
nutrient for the growth of the rat. Both inorganic and organotin
compounds at concentrations similar to those present in feeds were
found to stimulate growth rate in rats maintained on purified amino
acid diets (Table 11) (Schwarz, 1971; 1974; Schwarz et al., 1970). The
authors concluded that tin, as an essential element, could have a
function at the active site of some metal-dependent enzymes; however,
this has still to be confirmed.
Compared with most organotin derivatives, inorganic tin and its
salts are not highly toxic, mainly because of their poor absorption
and rapid tissue turnover (Barnes & Stoner, 1959; Cheftel, 1967;
Hiles, 1974; National Academy of Sciences, Washington 1973). The
systemic toxicity of some simple tin salts is difficult to assess
because of the irritant properties of their solutions.
7.1.1 Effects on the Skin
The effects of 1% tin(II) chloride and 0.25% tin(II) fluoride
solutions, applied to the abraded skin of rabbits, were examined by
Stone & Willis (1968). Both compounds induced intraepidermal pustules
with complete destruction of the epidermis but the stratum corneum
remained intact. No injury occurred when the solutions were applied to
intact skin.
7.1.2 Respiratory system effects
According to Robertson (1960), intratracheal administration of
50 mg of metallic tin dust to rats was well tolerated and no fibrosis
was produced within one year of exposure.
Exposure of guineapigs by inhalation, to tin(IV) chloride (3 mg/
litre for 10 min, daily, for "several months") produced only transient
irritation of the nose and eyes (Pedley, 1927).
Table 11. The effect of tin(IV) sulfate on the growth of rats in a trace-element-controlled
environmenta
Dose level No. of Average daily Increase
Compound (µg of tin/kg) animals weight gain (%) p-value
control -- 5b 1.10±0.05c -- --
tin (IV) sulfate 500 8 1.37±0.10 2 .02
tin (IV) sulfate 1000 8 1.68±0.10 53 .001
tin (IV) sulfate 2000 8 1.75±0.10 59 .001
a From: Schwarz et al. (1970).
b 2 control rats died during the 26-29 days of the experiment.
c mean ± standard error.
7.1.3 Effects on the gastrointestinal system
Soluble tin salts are gastric irritants. This explains the signs
of acute poisoning occasionally observed in man and in experimental
animals following consumption of food containing high concentrations
of tin (de Groot et al, 1973). However, the concentrations required to
elicit an acute gastrointestinal reaction have not been determined
reliably. Beney et al. (1971) reported signs of illness in 3/10 cats
after ingestion of 5 ml/kg body weight of fruit juice containing a tin
concentration of 1370 mg/litre, and in 1/11 cats receiving the same
dose of fruit juice containing tin at a concentration of 540 mg/litre.
However, no adverse effects were recorded in cats receiving a similar
amount of juice containing a tin concentration of about 500 mg/litre
or in dogs given juice with a tin concentration of 1400 mg/litre.
7.1.4 Effects on the liver
A 3-fold increase in haem oxygenase (EC 1.14.99.3)a activity in
the liver of rats was observed 16 h after a single subcutaneous
injection of tin(II) chloride dihydrate (SnCl22H2O) at doses ranging
from 5.6 to 56.4 mg/kg body weight. Cytochrome P-450 mediated drug
metabolism and the content of cytochrome P-450 were reduced by one
third. These effects on the liver increased by about 15-20%, when the
compound was administered intraperitoneally (Kappas & Maines, 1976).
Tin chloride, oxalate, or sulfate added to the diet at a
concentration of 10 g/kg for 4 and 13 weeks resulted in homogenous
liver cell cytoplasm and hyperplasia of the bile duct in rats. The
liver changes were more distinct after 13 weeks administration.
Similar, although milder, hepatic alterations were seen after
administration of tin chloride, oxalate, or orthophosphate at a
concentration of 3 g/kg of dietb (de Groot et al., 1973).
Increased incidence of fatty degeneration in the liver was noted
by Schroeder et al. (1968) in female but not in male rats, when the
animals were given tin in the form of tin(II) chloride at a dose of
5 mg/litre in drinking water, from weaning until their natural death.
a The numbers within parentheses following the names of enzymes
are those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
b Tables for the approximate conversion of concentrations in diet
to mg/kg body weight per day are given in Nelson (1954).
7.1.5 Effects on the kidney
Renal damage was produced in rats by single intravenous and
intraperitoneal doses of sodium pentafluorostannite (NaSn2F5) and
tin(II) chloride dihydrate (SnCl22H2O) (Conine et al., 1973, 1975;
Yum et al, 1976). A single intraperitoneal injection of sodium
pentafluorostannite at 35 mg/kg body weight or of tin(II) chloride
dihydrate at 44.4 mg/kg produced extensive necrosis of epithelial
cells mainly involving proximal tubules. The extent of the necrosis
was thought to be related to the tin moiety of the compound because
damage of the same magnitude could not be produced by administration
of sodium fluoride (Yum et al., 1976).
Studies in which rats received single subcutanous injections of
tin(II) chloride dihydrate in doses ranging from 5.6-56.4 mg/kg body
weight revealed a 20 to 30-fold increase in the haem oxidation
activity in the kidney, 16 h after administration. This effect was
already noticeable at the lowest dose (5.6 mg/kg) and was found to be
dose-related (Kappas & Maines, 1976).
Conine et al. (1976) administered daily oral doses of sodium
pentafluorostannite for 15 or 30 days to rats at rates of 20, 100, and
175 mg/kg body weight. The highest dose caused degenerative changes in
the proximal epithelium of the kidneys that were similar to those
observed in chronic fluoride poisoning (Lindemann et al., 1959), and
affected about 15-20% of the 30 rats that were killed. Most of the 8
animals in the 175 mg/kg group that died spontaneously during the
experiment displayed necrosis of the proximal tubular epithelium
similar to that previously reported to be caused by tin (Yum et al.,
1976).
Exposure of rats to tin(II) chloride at a concentration of 5 mg of
tin/litre of drinking water, for life, produced vacuolar changes in
the renal tubules of animals of both sexes (Schroeder et al., 1968).
7.1.6 Effects on the blood-forming organs
When tin(II) (as chloride, orthophosphate, sulfate, oxalate, or
tartrate) was given in the diet to Wistar rats of both sexes at
concentrations of 3 and 10 g/kg for 4 weeks, food intake was reduced,
growth was retarded and slight anaemia developed. The signs of anaemia
included a reduction in haemoglobin levels of about 10% and
corresponding reductions in haematocrit values, erythrocyte counts,
and serum iron concentration (de Groot et al., 1973). Dietary
supplements of iron had a markedly protective effect (de Groot, 1973;
de Groot et al., 1973). The authors suggested that tin compounds might
inhibit haemopoiesis, possibly by interfering with the intestinal
absorption of iron. An alternative mechanism by which tin salts could
cause mild gastrointestinal bleeding was not investigated.
A dose-related decrease in haemoglobin concentrations was noted in
rats after daily oral treatment, for 15 days, with sodium
pentafluorostannite at 100 and 175 mg/kg body weight. Administration
of 20 mg/kg per day did not affect the haemoglobin level (Conine et
al., 1976).
7.1.7 Central nervous system effects
High doses of inorganic tin compounds seem to affect the central
nervous system producing such effects as ataxia, muscular weakness,
and the depression of the central nervous system. These signs were
observed by Conine et al., (1975) after oral administration to rats of
sodium pentafluorostannite and tin(II) chloride dihydrate in single
doses of the order of the median lethal dose (LD50). According to de
Groot et al. (1973), feeding rats with tin(II) chloride at a
concentration of 10 g/kg diet for 13 weeks, resulted in a spongy state
of the white matter of the brain. Ataxia, hind-leg paralysis, and
death were also observed in rats by Mamontova (1940) following daily
subcutaneous injections of 3 mg of tin(II) citrate for 5-6 months.
7.1.8 Effects on the reproductive system and the fetus
There is one report indicating that tin(II) chloride might have an
effect on the reproductive system. De Groot et al. (1973) noted
testicular degeneration in rats after prolonged feeding with this
compound (10 g/kg of food, for 13 weeks).
Inorganic tin compounds have not been shown to be fetotoxic.
Theuer et al. (1971) gave groups of pregnant rats sodium
pentafluorostannite, sodium pentachlorostannite, and tin(II) fluoride
corresponding to tin levels in the diet of 125, 250, and 500 mg/kg.
The rats were killed on day 20 of gestation. No effects were seen in
the fetuses; tin concentrations in the fetuses were about 1 mg/kg
compared with approximately 0.65 mg/kg in control fetuses, indicating
a rather low transplacental transfer.
7.1.9 Carcinogenicity and mutagenicity
Few reports are available concerning the carcinogenicity of
inorganic tin compounds. Walters & Roe (1965) administered either
sodium chlorostannate at 1 or 5 g/litre of drinking water or tin(II)
oleate at 5 g/kg of diet to mice, for up to one year; the survivors
were then killed. The incidence of lymphomas, hepatomas, or pulmonary
adenomas did not increase with any of the regimens.
Roe et al. (1965) reported 3 malignant tumours in 30 August rats
that survived for 1 year or more on a diet containing sodium
chlorostannate at a concentration of 20 g/kg, whereas a control group
of 33 rats did not exhibit any case of malignant tumours. One tumour
was an adenocarcinoma of mammary origin, another a pleomorphic sarcoma
in the uterus, and the third, an adenoma-carcinoma in the jaw region.
The difference was not statistically significant. No tumours were seen
in another group of 27 rats surviving on a diet containing tin(II)
2-ethylhexoate at a concentration of 5-10 g/kg.
Administration of tin(II) chloride to rats and mice at 5 mg/litre
in drinking water throughout their life-time did not produce any
increase in the incidence of tumours compared with a control group
consisting of an equal number of animals (Kanisawa & Schroeder, 1969).
Studies concerning the mutagenicity of inorganic tin compounds
were not available to the Task Group,
7.1.10 Other effects
Growth retardation in rats has been reported after the
administration of high doses of various tin compounds. Dietary levels
of tin as tin(II) oxalate, orthophosphate, chloride, sulfate, and
tartrate at 3 and 10 g/kg for 4 weeks caused inhibition of growth in
rats and oedema and atrophy of the pancreas (de Groot et al., 1973).
Daily administration, by garage, of sodium pentafluorostannite at 100
and 175 mg/kg body weight, for 30 days, resulted in a dose-related
retardation of growth in rats (Conine et al., 1976). Administration of
tin(II) chloride at a concentration of 5 mg of tin per litre of
drinking water reduced the life span of female rats, whereas the
longevity of male rats was unaffected (Schroeder et al., 1968).
7.1.11 Effective doses and dose rates
7.1.11.1 Lethal doses
The median lethal doses (LD50) for 2 inorganic tin compounds are
given in Table 12. A marked difference between the oral and parenteral
LD50 values can be explained by the low absorption of tin compounds.
Daily intravenous administration of 3.3-4.2 mg of sodium tin
citrate per kg body weight provoked death in rabbits in 7-8 days,
whereas at a dose of 2.5 mg/kg, death occurred only after 15 or more
days (Mamontova, 1940).
In studies on 6 rats, subcutaneous injection of 3 mg of tin
citrate/animal, per day, killed all the animals in 5-6 months. Similar
administration of the same compound in doses of 2 mg/kg body weight
produced vomiting, diarrhoea, weight loss, ataxia, hind-leg paralysis,
and death within 5 months (Mamontova, 1940).
Table 12. Median lethal doses (LD50) of tin(II) chloride dihydrate (SnCl22H2O) and sodium
pentafluorostannite (NaSn2F5)a
Sodium pentafluorostannite
Tin(II) chloride dihydrate
Route male mice male rats female rats male rats (LD50, mg/kg)
(LD50, mg/kg) (LD50, mg/kg) (LD50, mg/kg)
intravenous 18.9 12.9 12.9 29.3
intraperitoneal 80.9 75.4 65.0 258.4
oral (fasted) --b 223.1 218.7 2274.6
(fed) 592.9 573.1 --b 3190.1
a From: Conine et al. (1975).
b no data.
7.1.11.2 Minimum effective and no-observed-effect doses
Some information on effective doses has been given in the sections
describing the effects. However, the following information may also be
of interest.
De Groot et al. (1973) reported a normal growth rate in Wistar
rats that had received tin(IV) oxide, tin(II) sulfide, or tin(II)
oleate at dietary levels up to 10 g/kg for 4 weeks; haematological
data were also normal except for an increase in the haematocrit in
male rats fed with the highest concentration of tin(II) sulfide. The
weight and the gross and macroscopic appearance of the liver, kidney,
heart, and spleen were also normal.
When a similar experiment was conducted with tin(II) chloride for
13 weeks, the no-observed-effect concentration in food appeared to be
1 g/kg, if the diet were supplemented with iron (de Groot et al.,
1973). This is equivalent to a dose rate of about 20-30 mg tin/kg body
weight per day, for 90 days.
No effect on growth was observed by Conine et al. (1976), when
sodium pentafluorostannite was administered orally to rats at a dose
rate of 20 mg/kg body weight per day for 30 days.
Oral administration of sodium tin citrate in dose rates ranging
from 100-150 mg/kg body weight per day for 1 year did not produce any
noticeable signs of poisoning in rats (Mamontova, 1940). Similarly
rats fed a diet containing either sodium chlorostannate at 20 g/kg or
tin(II) 2-ethyl hexoate at 5-10 g/kg for one year did not show any
pathological changes in the gastrointestinal tract, kidneys, or liver
(Roe et al., 1965).
Mice that received tin in the form of sodium chlorostannate at a
concentration of 1 or 5 g/litre in drinking water or tin in the form
of tin(II) oleate in the diet at a concentration of 5 g/kg during
their lifetime did not show any adverse effects (Waiters & Roe, 1965).
Mice and rats (Schroeder & Balassa, 1961; Schroeder et al., 1968)
given tin(II) chloride in drinking water at a concentration of 5 mg of
tin/litre grew normally throughout their life. The life span of mice
of both sexes and of male rats was not affected but that of female
rats was shorter and there was an increased incidence of fatty
degeneration of the liver (section 7.1.4). Vacuolar changes in the
renal tubules were apparent in rats of both sexes (section 7.1.5).
7.2 Organotin Compounds
Distinction should be made between the effects of di-, tri-, and
tetrasubstituted organotin compounds. The principal toxicological
difference is that some trisubstituted compounds have a specific
effect on the central nervous system producing cerebral oedema (Barnes
& Stoner, 1958; Torack et al., 1969), whereas disubstituted compounds
do not produce this effect but are potent irritants that can induce an
inflammatory reaction in the bile duct (Barnes & Magee, 1958).
Toxicologically, the tetrasubstituted compounds resemble
trisubstituted compounds, which are, generally, more toxic than the
mono- and disubstituted derivatives.
7.2.1 Effects on the skin and eyes
Dermal application of dibutyltin dichloride at 10 mg/kg body
weight per day for a period of 12 days, caused severe local damage and
also bile duct injury in rats and mice. Guineapigs were more
resistant, showing little reaction to daily applications of 120 mg/kg
on 5 successive days (Barnes & Stoner, 1958).
An aqueous solution of bis(tributyltin) oxide applied to the
shaved skin (30 x 60 mm) of rats at concentrations of 0.36-0.95 mg/kg
body weight produced slight local irritation lasting 2-3 weeks
(Pelikan & Cerny, 1968b). At higher doses (1.40-185 mg/kg), marked
inflammation developed into skin necrosis. Severe effects on the eyes
were also observed with bis(tributyltin) oxide (Pelikan & Cerny,
1969).
Triphenyltin hydroxide was reported not to irritate rabbit skin or
sensitize the skin of guineapigs (Marks et al., 1969). However, it was
found to be extremely irritating to the eyes, even after brief
exposure, and could cause corneal opacity (Marks et al., 1969).
A solution of triphenyltin acetate in oil at a dose of 150 mg/kg
body weight caused a skin reaction in rats (Klimmer, 1964). Doses of
tricyclohexyltin hydroxide at 1.2-60 mg/kg body weight per day,
applied to the skin of rabbits over a 3-week period, produced distinct
reactions locally but did not result in any systemic ill effects
(FAO/WHO, 1971, p. 527)a
7.2.2 Respiratory system effects
Pulmonary congestion and oedema were observed in rats after single
intravenous administrations of solutions of diethyl-, di-propyl-,
diisopropyl-, and dipentyltin dichloride (20 mg/kg body weight), in
0.05 ml of polyoxyethylene sorbitin monoleate, whereas in the case of
dibutyltin dichloride, 10 mg/kg was enough to cause this effect
(Barnes & Stoner, 1958). Petechial and ecchymotic haemorrhages in the
trachea and larynx were observed in sheep after intrarumenal
administration of tricyclohexyltin hydroxide at 500 mg/kg body weight.
Congestion was present in the dorsal portion of all pulmonary lobes at
a dose as low as 150 mg/kg (Johnson et al., 1975).
7.2.3 Effects on the gastrointestinal system
A single dose of butyltin trichloride, butyltin- S,S',S"-tris
(2-ethylhexylmercaptoacetate), butylstannoic acid, and butyl
thiostannoic acid at 4000 mg/kg body weight, administered by stomach
tube to mice, produced various degrees of submucosal, subserosal, and
intra-luminar gastrointestinal hemorrhage within 24 h (Pelikan &
Cerny, 1970b). Similar administration of dioctyltin- S,S'-bis
(2-ethylhexyl-mercaptoacetate) produced dilatation of the stomach,
which was filled with gas. The stomach walls appeared ischaemic
whereas the intestinal walls were hyperaemic, and traces of blood were
found in the contents of the small stomach. Similar, but more
pronounced findings were recorded after administration of dioctyltin-
a Unpublished reports on triphenyltin compounds and tricyclohexyltin
hydroxide were abstracted in 1970 Evaluations of Some Pesticide
Residues in Food (FAO/WHO, 1971). The proprietary data were
submitted to the WHO by the manufacturers. Page numbers given in the
text refer to the FAO/WHO publication, which also contains
information with reference to authors and manufacturers.
bis-(butylmercaptoacetate), although no traces of blood were seen in
the stomach. Treatment with dioctyltin bis(2-ethylhexyl-
mercaptoacetate) resulted in large amounts of liquid in the stomach
contents, and slightly hyperaemic stomach walls; other findings were
similar to those produced by the other 2 dioctyltin compounds,
dioctyltin bis(dodecylmercaptide) and octyltin tris(2-ethylhexyl-
mercaptoacetate) (Pelikan & Cerny, 1970a). Ingestion of dibutyltin
dichloride at a dose of 50 mg/kg body weight per day for one week,
caused a temporary dilatation of the stomach in rats due to
accumulation of fluid, and diarrhoea (Barnes & Stoner, 1958).
Intrarumenal administration of tricyclohexyltin hydroxide at 150 mg/kg
body weight resulted in fluid diarrhoea in sheep which persisted up to
death. Necropsy findings included mild enteritis and colitis
characterized by severe hyperaemia and oedema (Johnson et al., 1975).
Gastroenteritis occurred in rats receiving an oral dose of
tricyclohexyltin hydroxide of 25 mg/kg body weight per day for 19 days
(FAO/WHO, 1971, pp. 527-528).
7.2.4 Effects on the liver and bile duct
Steatosis of hepatocytes and enlargement of the liver were seen
within 24 h of administration to mice through a stomach tube, of a
single dose of butylstannoic acid, butyltin trichloride, butyltin
tris(2-ethylhexylmercaptoacetate), or butylthiostannoic acid, each at
a dose of 4000 mg/kg body weight (Pelikan & Cerny, 1970b). In rats, a
single oral dose of dibutyl,tin dichloride at 50 mg/kg body weight
produced congestion and inflammation especially in the lower part of
the bile duct (Barnes & Magee, 1958; Gaunt et al., 1968). When this
dose of dibutyltin dichloride was given on 3 successive days, the
lesion was more severe involving also the proximal part of the bile
duct and the portal blood vessels. Necrotic areas were seen in the
liver. In some cases, bile escaped from the injured duct into pancreas
and peritoneum. The authors considered that death occurring 5 days
after the 3 successive doses was the result of a general toxic effect
of this compound, whereas death occurring later was secondary to bile
duct and liver damage. Changes in the pancreas were inflammatory and
confined to areas surrounding the bile duct. In surviving rats,
examined 6-12 months after receiving 3 daily doses of 50 mg/kg, the
bile duct was shorter and thicker than normal with fibrosis in the
wall. A similar reaction in the bile duct was seen after a single
intravenous administration of 5 mg/kg body weight, and after a dermal
application of 10 mg/kg body weight. The effects on mice of oral doses
of dibutyltin dichloride at 20-50 mg/kg body weight were similar to
those seen in rats, although liver damage appeared to be more
widespread. Repeated doses of this compound at 20-50 mg/kg body weight
killed rabbits, but did not cause bile duct or liver injury;
guineapigs, however, tolerated such doses without any signs of adverse
effects (Barnes & Magee, 1958). It has been suggested that bile duct
injury occurs only in species in which the bile duct and the
pancreatic duct have a common course (Kimbrough, 1976).
Daily doses of dibutyltin dichloride at 0.1 and 1.0 mg/kg body
weight, for 6 months, caused intoxication in rabbits with dystrophic
changes in the liver (Mazaev & Korolev, 1969). Dioctyltim- S,S'-bis
(2-ethylhexylmercaptoacetate), diootyltin- S,S'-bis
(butylmercaptoacetate), and diootyltin bis(dodecyl-mercaptide) at an
oral dose of 4000 mg/kg body weight produced steatosis of the
hepatocytes in mice (Pelikan & Cerny, 1970a). Nikonorow et al. (1973)
reported a significant increase in the mean liver weight of rats after
oral administration of dioctyltin- S,S'-bis(isoctylmercaptoacetate)
at 20 mg/kg body weight per day, for 3 months.
A single oral dose of tributyltin acetate, benzoate, chloride,
laurate, or oleate at 500 mg/kg body weight produced fatty
infiltration of the liver in mice (Pelikan & Cerny, 1968a) and
triphenyltin acetate at a dietary level of 10 mg/kg produced fatty
degeneration of the liver in guineapigs, when administered over a
2-year period (FAO/WHO, 1971, p. 341). Cholangitis was reported in
rats after administration of tricyclohexyltin hydroxide at a dietary
level of 400 mg/kg for 90 days (Shirasu, 1970). Similarly, both intra-
and extrahepatic cholangitis was noted in rats that had received
tri-cyclohexyltin hydroxide at 25 mg/kg body weight per day, for 19
days (FAO/WHO, 1971, pp. 527-528).
7.2.5 Effects on the kidney
Some degree of fatty degeneration of the renal cortical tubular
epithelium was seen on histological examination of mice that had
received a single oral dose (4000 mg/kg body weight) of butyltin
tin- S,S',S"-tris(2-ethylhexylmercalytoacetate), butylstannoic acid,
or butylthiostannoic acid, but not in animals given butyltin
trichloride. The structure of the renal tissue was unchanged.
Macroscopically, all compounds produced slight hyperaemia in the
kidneys (Pelikan & Cerny, 1970b).
Dioctyltin- S,S'-bis(2-ethylhexylmercaptoacetate) and
dioctyltin- S,S'-bis(butylmercaptoacetate) administered at the same
rate and by the same route produced a similar slight fatty
degeneration of the renal cortical tubular epithelium in mice (Pelikan
& Cerny, 1970a). Feeding rats with an organotin stabilizer,
dioctyltin- S,S'-bis(isooctyl mercaptoacetate) at a dietary level of
200 mg/kg for 12 months produced an increase in kidney weight in
female rats only (Nikonorow et al., 1973). Mazaev & Korolev (1969)
also reported dystrophic changes in the kidneys in rats treated with
dibutyltin dichloride at 0.1 and 1.0 mg/kg body weight.
Haemorrhages were reported in the kidneys of mice after single
administrations (gavage) of tributyltin benzoate, chloride, laurate,
and oleate at 500 mg/kg body weight. Hyperaemia of the kidney was seen
in all groups, whereas tubular cells containing lipids were observed
only in mice that had received laurate or oleate. Renal changes were
not reported in mice that were similarly dosed with tributyltin
acetate (Pelikan & Cerny, 1968a). Congestion of the glomeruli and mild
toxic nephrosis were observed in rats receiving a dietary level of
tricyclohexyltin hydroxide of 400 mg/kg for 3 months (Shirasu, 1970).
Similarly, toxic nephrosis was reported in rats after administration
of tricyclohexyltin hydroxide at a daily dose of 25 mg/kg body weight
for 19 days (FAO/WHO, 1971, pp. 527-528).
7.2.6 Effects on the lymphatic tissues and immunological effects
Small necrotic areas were observed in the germinal centres of the
splenic follicles of mice after a single administration, through a
stomach tube, of 4000 mg/kg body weight of butyltin- S,S',S"-tris-
(2-ethylhexylmercaptoacetate), butylthiostannoic acid, and
butyl-stannoic acid, (Pelikan & Cerny, 1970b); dioctyltin- S,S'-bis
(2-ethyl-hexylmercaptoacetate), and dioctyltin- S,S'-bis(butyl-
mercaptoacetate) (Pelikan & Cerny, 1970a).
Seinen & Willems (1976) reported that the main action of
di-octyltin dichloride, given to rats at dietary levels of 50 and
150 mg/ kg, was on the thymus, the weight of which was significantly
reduced during the experiment. In subsequent experiments, feeding rats
with dioctyltin dichloride or dibutyltin dichloride at dietary levels
of 50, or 150 mg/kg for 6 weeks, resulted in a dose-dependent
reduction in the weight of the thymus Land thymus-dependent peripheral
lymphoid organs (Seinen et al., 1977a). The delayed type
hypersensitivity was decreased and the allograft rejection was delayed
when the same compounds were given in the same way to rats.
Furthermore, it was reported that both these compounds decreased the
survival rate of rat and human thymocytes in in vitro studies
(Seinen et al., 1977b). The effects of diethyltin dichloride and
dipropyltin dichloride on rat lymphoid organs were similar but less
pronounced. However, other dialkyltin compounds such as dimethyltin
dichloride, didodecyltin dibromide, and dioctadecyltin dibromide, as
well as octyltin trichloride, trioctyltin chloride, and tetraoctyltin
did not cause atrophy of lymphoid tissues (Seinen et al., 1977a). The
rat proved more sensitive to the action of di-octyltin dichloride and
dibutyltin dichloride on lymphoid organs than the mouse and the
guineapig in which no atrophy of the lymphoid organs was produced.
(Seinen et al., 1977a).
Studies on triphenyltin acetate have been reviewed by the FAO/ WHO
(1971, pp. 327-366). In guineapigs, a dietary level of 15 mg/kg for 77
days produced a decrease in plasma cells in the spleen and in the
mesenteric, cervical, axillary, and popliteal lymph nodes. After
feeding the same dose to female guineapigs for 104 days, a reduction
in immune response to tetanus toxoid stimulation was observed. In
another experiment, a decrease in the number of lymphocytes and
leukocytes accompanied by histological changes in the lymphatic
tissues, such as atrophy of the white pulp of the spleen, was seen in
guineapigs at dietary levels of 5 mg/kg in females and 10 mg/kg in
males administered, for 12 weeks. A decrease in the number of
leukocytes was also reported in dogs after 8 weeks on a dietary level
of triphenyltin hydroxide of 25 mg/kg and 8 weeks on 50 mg/kg.
7.2.7 Haematological effects
Decreases in the numbers of erythrocytes and reticulocytes were
reported in rats after daily administration of an oral dose of
dibutyltin sulfide at 0.1 mg/kg body weight for 6 months (Mazaev &
Slepning, 1973). A mild anaemia was noted in rats that had received
dibutyltin dichloride for 3 months at a dietary level of 80 mg/kg, but
a level of 40 mg/kg did not produce this effect (Gaunt et al., 1968).
In vitro studies of the haemolytic activity of trialkyltin
compounds indicated that the most haemolytic organotin compounds were
the alkyltin derivatives with alkyl groups of 3-6 carbon atoms
(Byington et al, 1974). Decreases in the percentage haemoglobin and in
the number of erythrocytes were reported in guinea-pigs treated with
triphenyltin acetate at a dietary level of 10 mg/kg for 4 months
(FAO/WHO, 1971, p. 337). A reduction in haemoglobin and leukocytes was
also seen in rats after 12 weeks on a dietary level of triphenyltin
hydroxide of 50 mg/kg (FAO/WHO, 1971, pp. 337-333).
7.2.8 Central nervous system effects
Intoxication in animals by lower trialkyltin compounds is
manifested as generalized weakness progressing to paralysis, sometimes
accompanied by generalized tremor. In rabbits, typical reactions to a
lethal dose (5 mg/kg body weight) of triethyltin sulfate administered
intravenously are prostration, flaccid paralysis, and encephalopathy
(Stoner et al, 1955). These responses resemble those observed in human
subjects, accidentally poisoned with triethyltin compounds. Daily
intraperitoneal injections of triethyltin sulfate at 5 mg/kg body
weight in saline solution resulted in death in rats within 3 days.
Diffuse haemorrhagic encephalopathy was found in the brain (Suzuki,
1971). Rats maintained on a dietary level of triethyltin hydroxide of
20 mg/kg displayed weakness in the hind legs after one week. The
weakness reached a maximum at 3-4 weeks, when about half of the rats
died, but the signs disappeared in one week, when normal diet was
restored. Some of the rats showed signs of becoming resistant to the
substance. At a dietary level of 40 mg/kg, the recovery process was
unaltered but, at 80 mg/kg, the rats developed generalized muscular
tremors that resembled those seen in acute trimethyltin poisoning
(Stoner et al, 1955). This course of events, including the development
of resistance to triethyltin hydroxide at a dietary level of 20 mg/kg
was corroborated by Magee et al. (1957), who was also able to produce
a specific lesion of the central nervous system (CNS) by feeding rats
with a dietary level of triethyltin hydroxide of 20 mg/kg. The lesion
consisted of an oedema extending throughout the white matter. It was
microscopically visible after 3 days of exposure and progressed to a
maximum at about 2 weeks. After 4 months of normal diet, the changes
in the brain and spinal cord disappeared. Electronmicroscopic studies
of this lesion in rabbits disclosed that the myelin sheaths split to
form clefts and vacuoles in which the fluid responsible for the oedema
accumulated (Aleu et al., 1963). The triethyltin-induced lesion of the
CNS is different from those produced by alkyl derivatives of lead,
antimony, bismuth, and mercury, which cause damage to the nerve cells,
but appears to be similar to that produced in rats by exposure to
hexachlorophene (Kimbrough & Gaines, 1971). When 5 mg of triethyltin
sulfate per litre of drinking water was administered to newborn rats
and their mothers for 4 months, all young rats were asymptomatic,
whereas the mothers showed paralysis of posterior limbs, severe
cerebral oedema, and status spongiosus of the white matter (Suzuki,
1971). Hedges & Zaren (1969) described papilloedema in monkeys
following a single intraperitoneal dose of triethyltin acetate of
0.1 mg/kg, body weight administered as a 5% solution. Oedema of the
white matter of the brain extended through the chiasma and orbital
optic nerve to the retrolaminar area, but the papilloedema was not due
to extension of the intramyelinic tin oedema of the nerves through the
lamina. Papilloedema does not develop in the cat when similarly
treated, in spite of profound swelling of the intracerebral white
matter, chiasma, and the orbital optic nerve as far as the
retrolaminar portion.
Tricyclohexyltin hydroxide appears to be a depressant for the
central nervous system, although it is not as potent as triethyltin
acetate and cerebral oedema does not occur (FAO/WHO, 1971, p. 526).
Johnson et al. (1975) studied the acute toxicity of tricyclohexyltin
hydroxide in sheep by intrarumenal injection of doses ranging from 15
to 750 mg/kg body weight. Central nervous depression was observed at
50 mg/kg body weight.
Because of the enzymatic conversion of tetraalkyltin compounds by
hepatic microsomal enzymes (section 6.2.4), these compounds act in a
similar way to trialkyltin compounds. The toxicity of tetraalkyltin
compounds in mice and clogs was studied by Caujolle and his colleagues
(1954). Tetraethyltin was the most active, tetramethyltin slightly
less toxic, and for the higher members of the series, the toxicity
decreased with increasing molecular weight. The toxic effects of
tetramethyltin differed from those of the other members of the series
and the dominant findings were tremors and hyperexcitability. The
major effects of the other members of the tetraalkyltin series were
muscular weakness and paralysis followed by respiratory failure.
Development of the signs of poisoning were noticeably slow following
administration of tetraalkyltin compounds and death was often delayed.
Intravenous administration of tetraethyltin at 25 mg/kg produced a
slight increase in the respiratory rate and vasodilatation as
immediate effects, but after 1.5-2 h, prostration with muscular
weakness was noted. The later effects resembled those seen after
administration of triethyltin; the mode of death was also similar
(Stoner et al., 1955).
7.2.9. Effects on reproduction and the fetus
Few studies of the effects of disubstituted organotin compounds on
reproduction have been reported. Nikonorow et al. (1973) reported
toxic effects of dioctyltin- S,S'-bis(isooctylmercaptoacetate) on the
embryo and fetus at daily oral doses of 20 and 40 mg/kg body weight,
administered for about 3 months. A distinct increase in the incidence
of fetal deaths was observed. Teratogenic effects were not found.
Investigations on triphenyltin hydroxide have yielded variable
results. In 2 investigations on rats, oral dose rates of 20 mg/kg per
day for less than 4 weeks produced histologically evident
abnormalities in the testes and ovaries. Diminution in the size of the
testes and less advanced maturation of the germinal epithelium was
seen in rats receiving triphenyltin hydroxide at a rate of 5 mg/kg for
90 days, although a 3-generation reproduction study at this dietary
level failed to demonstrate any adverse effect on the reproductive
indices (FAO/WHO, 1971, pp. 332-333). In another experiment (Gaines &
Kimbrough, 1968), dietary levels of up to 200 mg/kg over a 276-day
period caused a reversible reduction in fertility in male rats which
was considered to be due to a pronounced decrease in food intake, that
was later reversed. No gross abnormalities were seen in the offspring
of these males when mated with untreated females. In 3 later
experiments (FAO/WHO, 1971, p. 333) in which weanling animals were fed
the compound in their diets at doses of up to 25 mg/kg, the previously
reported change in testicular development could not be confirmed.
In a 3-generation study in which rats produced 2 litters per
generation, animals continuously received a dietary level of
tricyclohexyltin hydroxide of up to 100 mg/kg (corresponding to 4-6 mg
test compound/kg body weight per day for an adult rat). No evidence of
any adverse effects on reproduction could be found and examination of
the fetuses did not reveal any indication of teratogenic effects
(FAO/WHO, 1971, p. 524). No evidence of teratogenic effects from
tricyclohexyltin hydroxide was obtained in a study in which pregnant
rabbits received up to 3 mg/kg body weight per day from the 8th to
16th day of gestation (FAO/WHO, 1971, p. 524). In a study in which
diets containing tricyclohexyltin hydroxide were fed to Japanese
quail, no evidence of ill effects on egg fertility or hatchability was
seen at a dietary level of 1 mg/kg.
Effects observed at a dietary level of 10 mg/kg were of doubtful
significance but a dietary level of 100 mg/kg had definite effects on
fertility, egg production, hatchability, and embryonic mortality
(FAO/WHO, 1971, pp. 523-524).
7.2.10 Carcinogenicity
In an 18-month study, mice were given an oral dose (stomach tube)
of 0.46 mg/kg body weight per day of triphenyltin acetate between the
ages of 7 and 28 days and thereafter a dietary level of 1206 mg/kg.
There was no statistically significant increase in tumours compared
with a control group (Innes et al., 1969). In a 2-year study on rats
receiving tricyclohexyltin hydroxide at concentrations up to 12 mg/kg
body weight (section 7.2.3), the pattern of tumour incidence
throughout both the control and test groups appeared to be random and
did not suggest a dose-response relationship (FAO/WHO, 1971, p. 527).
Reports of experiments specifically designed to investigate the
carcinogenicity of other compounds were net available to the Group.
7.2.11 Effects on chromosomes
Mazaev & Slepnina (1973) reported an increased percentage of
chromosomal aberrations in bone marrow cells and also an increased
mitotic index in cells of the small intestine mucosa of rats that had
received dibutyltin sulfide at 0.1 mg/kg body weight per day
administered by intubation over a period of 6 months. Doses of less
than 0.1 mg/kg body weight per day did not cause poisoning and did not
have any effect on the chromosomes or somatic cells.
7.2.12 Other effects
A reduction in weight gain, mainly related to reduced food intake,
has been recorded in various species given organotin compounds. In
rats, a dietary level of dibutyltin dichloride of 80 mg/kg
administered for 90 days caused growth retardation and decreased food
intake (Gaunt et al., 1968). Triphenyltin acetate given to guineapigs
at dietary levels exceeding 5 mg/kg for 2 years, caused a dose-related
inhibition of growth rate (FAO/WHO, 1971, p. 341). A dietary level of
tricyclohexyltin hydroxide of 25 mg/kg for 90 days, caused a slight
reduction in weight gain in female rats (Shirasu, 1970), and dogs
receiving the same compound in their diet at a level of 12 mg/kg for 6
months also lost weight; some animals that totally refused the food
died from starvation (FAO/WHO, 1971, pp. 526-527).
Severe cardiovascular changes were demonstrated electro-
cardiographically in sheep after intrarumenal administration of
tricyclohexyltin hydroxide at doses greater than 150 mg/kg body weight
(Johnson et al., 1975).
7.2.13 Mechanisms o[ action
There have been relatively few studies of the biochemical effects
of dialkyltin compounds, which do not cause cerebral oedema. In acute
toxicity experiments on the rat, diethyltin compounds did not affect
the sodium and potassium contents of the central nervous system but
caused a decrease in its water con, tent (Magee et al, 1957).
The action of a homologous series of disubstituted
organotin-compounds from dimethyl- to dioctyltin have been examined;
most of them inhibit mitochondrial respiration by preventing the
oxidation of keto acids, presumbly via the inhibition of alpha-keto
oxidase activity, leading to the accumulation of pyruvate (Aldridge,
1976; Piver, 1973).
Of the trisubstituted compounds, triethyltin compounds have been
the most closely examined in relation to the mechanism by which they
cause ill effects. Trimethyl- and triethyltin compounds are potent
inhibitors of oxidative phosphorylation in the mitochondria for which
these compounds have a high binding affinity (Aldridge, 1958; Aldridge
& Street, 1964, 1970, 1971). In a later study by Aldridge (1976),
triorganotin compounds were stated to derange mitochondrial function
in 3 different ways, namely by secondary responses caused by discharge
of a hydroxyl-chloride gradient across mitochondrial membranes, by
interaction with the basic energy conservation system involved in the
synthesis of ATP, and by an interaction with mitochondrial membranes
causing swelling and disruption.
Using a 113Sn-labelled triethyltin compound, Rose (1969)
identified a pair of histidine residues on guineapig liver
mitochondria as the binding site for triethyl compounds. Thus, one
molecule of rat haemoglobin binds 2 molecules of triethyltin; the
binding sites are located in the globin.
Stockdale et al. (1970) showed that the order of effectiveness for
the organotin compounds in inhibiting coupled respiration was tributyl
> tripropyl > triphenyl > trimethyl. Two separate effects were
suggested: (a) an oligomycin-like inhibition of coupled
phosphorylation; and (b) an alteration of hydroxide exchange across
lipid membranes producing uncoupling, swelling, and reduction of
intramitochondrial substrate and phosphate concentrations followed by
structural damage.
7.2.14 Effective doses and dose rates
7.2.14.1 Lethal doses
The median lethal doses for some mono-, di-, tri-, and
tetrasubstituted organotin compounds are given in Tables 13, 14, 15,
and 16.a Trimethyl and triethyltin compounds, administered orally,
are more toxic than the higher homologues of the trialkyltin group.
The oral toxicity diminishes progressively from tripropyltin to
trioctyltin compounds (Barnes & Stoner, 1958). This is probably
because of poorer absorption of higher trialkyltin compounds from the
gastrointestinal tract, as judged from the difference between the oral
and intraperitoneal toxicity of higher trisubstituted alkyltin
compounds (Stoner et al., 1955). The intraperitoneal toxicity of
various trialkyltin compounds does not differ to any large extent. In
rats, Stoner et al. (1955) found that triethyltin sulfate was equally
toxic after intravenous, intraperitoneal, and oral administration. The
lethal dose per kg body weight given intravenously or
intraperitoneally was 10 mg/kg, causing death within 4-5 days. A dose
of 40 mg/kg body weight killed the rat in 2 h. The oral LD50 for
tricyclohexyltin hydroxide (Table 15) for most species, appears to be
between 100 and 1000 mg/kg body weight, while the intraperitoneal and
intravenous routes yield LD50 values below 20 mg/kg. The difference
between oral and parenteral toxicity reflects the poor absorption of
tricyclohexyltin hydroxide from the gastrointestinal tract.
In rats and dogs fed with tricyclohexyltin hydroxide, the
metabolites, dicyclohexyltin oxide and traces of cyclohexylstannoic
acid have been identified. Dicyclohexyltin oxide and
cyclohexylstannoic acid also constitute a small portion of the
residues on fruit as a result of photodecomposition (FAO/WHO, 1971,
pp 522, 534). The median lethal dose of these metabolites may
therefore be of interest. The LD50 of dicyclohexyltin oxide in the
rat appears to be about 350 mg/kg body weight, whereas the LD50 value
of cyclohexylstannoic acid in this species is probably ten times
higher (FAO/WHO, 1971, pp. 524-526).
Only a small amount of data is available concerning the acute
toxicity of tetraalkyltin compounds. The median lethal dose for
tetraethyltin is given in Table 16.
a Additional data on the toxicity of organotin compounds may be
found in a proposal for the United Nations classification and hazard
grouping of organotin compounds submitted by the expert from the
Netherlands to the Committee of Experts on the Transport of Dangerous
Goods of the United Nations Economic and Social Council
(EN/CN.2/CONF.5/R.607 of November 1976).
Table 13. Acute oral toxicity of some mono-organotin compounds in various animal species
Medium lethal
Trivial name of compound dose Species Reference
(LD50, mg/kg)
butylstannoic acid > 6000 mouse Pelikan & Cerny (1970b)
butyltin trichloride 1400 mouse
2140 rat Marhold (1972)
butyltin-S,S'S"-tris 1520 mouse Pelikan & Cerny (1970b)
(isooethylmercaptoacetate)
octyltin trichloride 4600 mouse Klimmer (1969)
octyltin-S,S',S"-tris 1500 rat (male) Pelikan & Cerny (1970a)
(2-ethylexylmercaptoacetate)
7.2.14.2 Minimum effective and no-observed-effect doses
When dibutyltin dichloride was fed to rats for 90 days at dietary
levels of 10, 20, 40, and 80 mg/kg, the no-observed effect level was
40 mg/kg, equivalent to 2 mg/kg body weight per day (Gaunt et al.,
1968). When dibutyltin was administered to rats for 6 months, the
no-observed-effect level was 20 mg/kg diet (Barnes & Stoner, 1958).
Dibutyltin sulfide, administered to rats at oral doses of 1.0, 0.1,
0.01, and 0.001 mg/kg body weight, per day for 7 months, was tolerated
without any detected adverse effects up to a dose o.f 0.01 mg/kg per
day but the higher levels of 0.1 and 1.0 mg/kg per day caused
intoxication (Mazaev & Korolev, 1969). By comparison, the toxicity of
dioctyltin compounds was relatively low (Klimmer, 1969). Thus,
dioctyltin did not produce any injury in rats, mice, or guineapigs at
oral doses up to 400 mg/kg body weight per day when given for 3-4
successive days, nor were there any ill effects when it was added to
the daily diet of rats for 4 months at a rate of 200 mg/kg (Barnes &
Stoner, 1958).
Table 14. Acute toxicity of some diorganotin compounds in various animals
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dibutylin di(2-ethylhexoate) 200 (oral) rat (male) Calley et al. (1967)
dibutyltin di(butyl maleate) 120 (oral) rat Klimmer (1969)
dibutyltin di(nonylmaleate) 170 (oral)
dibutyltin dichlorlde 100 (oral) rat (male) Klimmer (1969)
182 (oral) rat (male) Mazaev et al. (1971)
112 (oral) rat (female)
35 (oral) mouse
190 (oral) guineapig
150 (oral) rat Marhold (1972)
dibutyltin-S,S'-bis 150 (oral) rat (male) Klimmer (1969)
(2-ethylhexylmercaptoacetate) 175 (oral) Klimmer (1969)
dibutyltin dilaurate 243 (oral) rat Marhold (1972)
45 (oral) rat Marhold (1972)
dibutyltin oxide 520 (oral) rat (male) Klimmer (1969)
39.9 (i.p.)a rat (female) Robinson (1969)
24 (oral) mouse Mazaev & Slepnina (1973)
dibutyltin sulfide 145 (oral) rat (male)
150 (oral) rabbit
180 (oral) rat (female)
800 (i.p.) rat (female) Robinson (1969)
> 800 (i.p.) rat (female) Robinson (1969)
dioctyltin acetate 2030 (oral) rat Klimmer (1969)
diocyltin dibutylmaleate 3750 (oral) mouse Pelikan et al. (1970)
dioctyltin-S,S'-bis
(butylmercaptoacetate) 1140 (oral) white mouse Pelikán & Cerny (1970a)
dioctyltin bis
(dodecylmercaptide) 4000 (oral) white mouse
dioctyltin bis
(2-ethylhexylmaleate) 2760/ (oral) rat (male) Klimmer (1969)
2750
Table 14. (contd).
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dioctyltin-S,S'-bis 2010 (oral) white mouse Pelikan & Cerny (1970a)
(2-ethylhexylmercaptoacetate)
dioctyltin-S,S'-bis 3700 (oral) rat (male) Klimmer (1969)
(laurylmercaptoacetate)
dioctyltin-S,S'-(1,4- 2950 (oral) rat (male)
butane-diol-bis-mercaptoacetate)
dioctyltin dichloride 5500/
8500 (oral) rat (male)
dioctyltin dilaurate 6450 (oral) rat (male)
800 (i.p.) rat (female) Robinson (1969)
dioctyltin di(1,2-
propylene-glycolmaleate) 4775 (oral) rat (male) Klimmer (1969)
dioctyltin-S,S'-
(ethyleneglycol-bis-
dimercaptoacetate) 880 (oral) rat (male)
dioctyltin maleate 4500 (oral) rat (male)
dioctyltin
ß-mercapto-propanoate 1850/
2050 (oral) rat (male)
dioctyltin oxide 2500 (oral) rat (male)
dioctyltin mercaptoacetate 945 (oral) rat (male)
a intraperitoneal
Table 15. Acute toxicity of some triorganotin compounds in various animals
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
2-trichloro-1-(butine-1'-
oxide)-1-triethyl- 9.8 (i.p.) rat Ignatjeva (1968)
stannyloxy)ethane 9.6 (i.p.) mouse Ignatjeva (1968)
triethylstannylmethyl-
(1-propynyl)formal 10.7 (i.p.) rat Ignatjeva (1968)
triethylstannylphenyl-
acetylene 9.9 (i.p.) mouse Ignatjeva (1968)
9.1 (i.p.) rat Ignatjeva (1968)
1-triethylstannyl-3-
trimethylsiloxi-1-
propyne 11.8 (i.p.) mouse Ignatjeva (1988)
11.4 (i.p.) rat Ignatjeva (1968)
triethyltin acetate 4 (oral) rat (female) Stoner (1966)
triethyltin chloride 5 (i.p.) rat (female) Robinson (1969)
triethyltin sulfate 5.7 (i.p.) rat (male) Stoner (1966)
5.3 (i.p.) guineapig Stoner (1966)
tributyltin acetate 46 (oral) white mouse Pelikan & Cerny (1968a)
99 (oral) J. Pharm. Sci. (1967)
133 (oral) rat (male) Klimmer (1969)
tributyltin benzoate 108 (oral) white mouse
Pelikan & Cerny (1968a)
132 (oral) rat Arzneimittelforsch. (1969)
tributyltin chloride 117 (oral) white mouse Pelikan & Cerny (1968a)
129 (oral) rat Manhold (1972)
tributyltin laurate 180 (oral) white mouse Pelikan & Cerny, (1968a)
tributyltin oleate 195 (oral) rat Klimmer (1969)
tributyltin salicylate 137 (oral) rat (male) Klimmer (1969)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
bis(tributyltin oxide) 112- (oral) rat (male) Klimmer (1969)
132
180 (oral) rat Truhaut et al. (1976)
234 (oral) rat Sheldon (1975)
194 (oral, rat (male) Elsea & Paynter (1958)
aqueous
solution)
148 (oral, rat (male) Elsea & Paynter (1958)
oil
solution)
11.7 (dermal) albino rabbit Elsea & Paynter (1958)
10.0 (oral) white mouse Pelikan & Cerny (1969)
trihexyltin acetate 1000 (oral) rat Barnes & Stoner (1958)
trioctyltin chloride 10 000 (oral) rat (male) Klimmer (1969)
triphenyltin acetate 21 (oral) guineapig Klimmer (1963)
24 (oral) guineapig Kimbrough (1976)
136 (oral) rat Klimmer (1963)
81 (oral) mouse (male) FAO/WHO (1971)
7.9 (i.p.) mouse (male) Stoner (1966)
136 (oral) rat (male) Klimmer (1964)
491 (oral) rat (female) Stoner (1966)
450 (dermal) rat (male) Klimmer (1963)
8.5 (i.p.) rat (female) Stoner (1966)
11.9 (i.p.) rat (female) Stoner (1966)
13.2 (i.p.) rat (male) Klimmer (1964)
21 (oral) guineapig
(male) Klimmer (1964)
3.7 (i.p.) guineapig
(male) Stoner (1966)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
triphenyltin acetate 5.3 (i.p.) guineapig
cont'd. (male) Klimmer (1964)
30- (oral) rabbit (male) Klimmer (1964)
50
triphenyltin chloride 80 (oral) rat (male) FAO/WHO (1971)
135 (oral) rat (female) FAO/WHO (1971)
triphenyltin hydroxide 245 (oral) mouse (male) FAO/WHO (1971)
209 (oral) mouse (female)
FAO/WHO (1971)
240 (oral) rat (male) Gaines & Kimbrough (1968)
360 (oral) rat (female) Gaines & Kimbrough (1968)
27.1 (oral) guineapig FAO/WHO (1971)
(male)
31.1 (oral) guineapig FAO/WHO (1971)
(female)
171 (oral) rat (male) Marks et al. (1969)
268 (oral) rat (female) Marks et al. (1969)
tricyclohexyltin 710a (oral) mouse- FAO/WHO (1971)
hydroxide peromyscus
1070a (oral) mouse-swiss FAO/WHO (1971)
white
540 (oral) rat FAO/WHO (1971)
13 (i.p.) rat FAO/WHO (1971)
780 (oral) guineapig FAO/WHO (1971)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
tricyclohexyltin 9 (i.p.) guineapig FAO/WHO (1971)
hydroxide 500- (oral) rabbit FAO/WHO (1971)
cont'd. 1000
> 126 (i.p.) rabbit FAO/WHO (1971)
150a (oral) sheep Johnson et al. (1975)
14 (i.v.)c dog FAO/WHO (1971)
6 (i.v.) cat FAO/WHO (1971)
a approximate lethal dose
b intraperitoneal
c intravenous
Table 16. Acute oral toxicity of some tetraorganotin compounds in various animal species
Trivial name Medium lethal dose Species Reference
of compound (LD50, mg/kg)
tetraethyltin 40.0 mouse Skackova (1967)
15.0 rat
40.0 guineapig
7.0 rabbit
40 mouse Mazaev et al. (1971)
9.0 rat
40.0 guineapig
7.0 rabbit
tetrabutyltin 6000 rat Skackova (1967)
Data on effective doses of trisubstituted organotin compounds are
available only for triphenyltin derivatives and tricyclohexyltin
hydroxide. Triphenyltin acetate was administered, by gavage, to groups
of rats at doses equivalent to dietary levels of 5, 10, 25, and
50 mg/kg for up to 17 days. While no adverse effects were found at the
25 mg/kg level, the 50 mg/kg level resulted in the death of animals,
mainly as a result of infection (Klimmer, 1964). In a 2-year study on
rats fed diets containing concentrations of triphenyltin hydroxide of
up to 10 mg/kg, the no-observed-effect level was about 2 mg/kg
(equivalent to about 0.1 mg/kg body weight per day) (FAO/WHO, 1971,
pp. 341-342). In another 2-year study in which groups of guineapigs
were fed diets containing concentrations of triphenyltin acetate, up
to 200 mg/kg, the no-observed-effect level was 5 mg/kg. At higher
levels there was a dose-related increase in mortality and the
occurrence of other effects such as inhibition of growth rate and
fatty degeneration of the liver and heart (FAO/ WHO, 1971, p. 341).
With respect to tricyclohexyltin hydroxide, no toxic effects were
noted in rats receiving 12.5 mg/kg body weight daily for 19 days,
whereas a rate of 25 mg/kg per day caused toxic effects (FAO/WHO,
1971, pp. 527-528). Rats were fed on diets providing up to 12 mg/kg
body weight per day of tricyclohexyltin hydroxide in a 2-year study;
the no-observed-effect level was 3 mg/kg body weight per day (FAO/WHO,
1971, p. 528). When dogs were fed tricyclohexyltin hydroxide in the
diet at concentrations of up to 12 mg/kg body weight per day for 6-24
months, many dogs refused the 12 mg/kg per day diet, causing weight
loss and death from starvation. The no-observed-effect level was found
to be about 0.75 mg/kg per day (FAO/WHO, 1971, pp. 526-527). Johnson
et al. (1975) also reported the effects of thoroughly wetting the skin
of yearling cattle, goats, and sheep with suspensions of
tricyclohexyltin hydroxide. They found that cattle tolerated
suspensions up to a concentration of 5 g/litre while anorexia was
registered in some animals after spraying with a suspension of
10 g/litre. Yearling goats and sheep tolerated suspensions of up to
1 g/litre. Transitory anorexia and eye irritation developed after
application of a suspension of 20 g/litre to the skin of goats.
Few data are available on the effective dose levels of
tetrasubstituted organotin compounds. Intravenous administration of
tetraethyltin at 25 mg/kg body weight produced a slight increase in
respiratory rate and vasodilatation as immediate effects, but, 1.5-2 h
later, prostration with muscular weakness occurred. (Stoner et al.,
1955).
8. EFFECTS ON MAN
There are comparatively few clinical observations and
epidemiological data concerning the effects of inorganic tin compounds
on man and even fewer on the effects of organotin compounds.
8.1 Inorganic Tin Compounds
8.1.1 Acute poisoning
Some episodes of acute poisoning have been reported, mainly in
association with the ingestion of fruit juices containing high
concentrations of tin. The major symptoms and signs noted were nausea,
vomiting, diarrhoea, fatigue, and headache. The concentrations of tin
in the products thought to have been associated with the incidents
were uncertain in many cases, but were probably in the range of
300-500 mg/kg (Horio et al, 1967). Orange and apple juice, containing
tin concentrations of 250-385 mg/kg were suspected of causing one
incident (Benoy et al., 1971). Nausea, vomiting, and diarrhoea were
recorded in individuals consuming-peach preserves that contained a tin
concentration of 563 mg/kg, zinc at 1.5 mg/kg, cadmium at 0.1 mg/kg,
lead at 0.16 mg/kg, copper at 1 mg/kg, nitrate at 93 mg/kg, nitrite at
1.7 mg/kg, and chloride at 115 mg/kg (Nehring, 1972). Acute
gastroenteritis followed ingestion of a fruit punch stored in a tin
can and containing a tin concentration of 2000 mg/litre. First
symptoms occurred after 1-2 h, the earliest and commonest being
bloatedness, followed by severe nausea, stomach cramps, vomiting and,
in one-third of the patients, mild diarrhoea (Warburton et al, 1962).
Other similar outbreaks have involved canned cherries (Luff & Metcalf,
1890), asparagus, herring (Schryver, 1909), and apricots (Savage,
1939) with tin concentrations ranging from 300 to about 1000 mg/kg.
An incident of acute intoxication after the ingestion of canned
peaches was reported by Sverrsson (1975). It was unique in that about
110 participants in a meeting received nothing else to eat or drink,
except for the peaches. The report was based on questionnaires
received from 85 persons, 76 (89%) of whom had fallen ill. About half
of these had developed symptoms such as nausea, vomiting, and
diarrhoea within 1 h. The majority became symptom-free after 24 h. The
fruit contained a mean tin concentration of 533 mg/kg (range
413-597 mg/kg) and the juice 369 mg/kg (range 298-405 mg/kg). Two out
of 7 persons who had consumed only one quarter of the contents fell
ill. The calculated intake of tin for these persons was 50 mg.
In another report, canned tomato juice was associated with 113
cases of acute gastroenteritis (Barker & Runte, 1972). In a small
number of these cases, superficial erosion of the mucosa of the mouth
was observed, while abdominal distension and cramps, and diarrhoea
were quite common. The cans containing the juice showed complete
corrosion of tin linings and this yielded a product containing a tin
concentration of approximately 400 mg/kg. The crops of tomatoes, used
to make this particular juice, had been grown in soil excessively
treated with nitrate fertilizer and contained high levels of nitrate;
complete corrosion of the lining of the cans occurred within
approximately 6 months.
Five human volunteers did not show any toxic signs after drinking
fruit juices containing about 500 or 730 mg/kg of tin, but all had
some gastrointestinal disturbance after drinking 5-7 ml/kg body weight
of fruit juice containing a tin concentration of about 1400 mg/litre
(Benoy et al., 1971). There was no evidence to indicate that the
effects were due to the absorption of tin, the likeliest cause being
local irritation of the mucous membranes of the alimentary tract.
8.1.2 Prolonged exposure
8.1.2.1 Effects of inhalation
The only available information on exposure by inhalation pertains
to pneumoconiosis caused by the inhalation of tin(IV) oxide. This
benign condition is termed stannosis.
More than 200 cases of stannosis have been described (Bartak
et al., 1948; Cutter et al., 1949; Dundon & Hughes, 1950; Oyanguren
et al., 1958; Pendergrass & Pryde, 1948; Robertson & Whitaker, 1957;
Schuler et al., 1958). The relative importance of exposure to tin
fumes in the etiology of this disorder was emphasized by Dundon &
Hughes (1950). The significance of the quantity of dust and the
duration of exposure were stressed by Robertson & Whitaker (1957), who
reviewed 121 cases.
Pendergrass & Pryde (1948) noted stannosis in a man who, for 15
years, had been bagging tin oxide material containing 96.5% tin(IV)
oxide and small amounts of aluminium, iron, and sodium but not silica.
Radiography revealed small dense shadows (denser than those of
silicosis) resembling those of barytosis in both lungs. Bartak et al.,
(1948) reported a similar case in a workman who had suffered from
asthma for many years and who attended a furnace in which metallic tin
was burnt to produce tin(IV) oxide. Necropsy of this man, who died
from gastric carcinoma, revealed deposits of tin(IV) oxide in the
lungs, lymph glands, liver, and spleen. Six other workmen with a
similar radiographic appearance of the lungs, did not have any
symptoms of asthma or signs of pulmonary dysfunction. Similar
radiological findings were reported by Cutter et al. (1949) in 2 cases
with nodules 1-2 mm in diameter unaccompanied by evidence of pulmonary
dysfunction. Both had been working in a tin recovery department for 20
years.
Oyanguren et al., (1958) reported 10 cases of stannosis in workers
involved in a process where tin ore concentrates were reduced to
metal. The workers were exposed to dust with a high tin and a low
silica content and to fumes rich in tin. None of the exposed workers
had any disability and the vital capacity, maximal breathing and
resting minute volume, and respiratory reserve were normal, as were
the findings on the blood and urine. Radiological examination of these
10 workers showed that the first alteration appeared to be increase of
bronchovascular markings, with hilar thickening in the early stages;
later, well-defined nodular elements appeared first in the middle
third of the right and then of the left lung. These appeared to
progress to the rest of the lung fields, and with continuous exposure,
accumulation of tin(IV) oxide gave a metallic density to a part of the
nodules, which subsequently acquired an appearance of lipoid droplets.
In the later stages, the bronchovascular markings disappeared as the
density of the shadows increased. The radiographic changes appeared
after some 3-5 years of exposure (Schuler et al., 1958). Hlebnikova
(1957) made a survey over a number of years of workers, who were
exposed to condensation aerosols, formed during the smelting of tin
and consisting mainly of tin(IV) oxide. The free silica concentration
in the aerosols was not more than 1% and the total silica did not
exceed 3%. The total dust concentration in air varied between 3 and
70 mg/m3. Workers developed pneumoconiosis after 6 to 8 years of
working at the smelter. The authors described 45 cases of
pneumoconiosis in workers, who were employed at the smelter from 6 to
20 years, Six of them were reported to have second degree
pneumoconiosis. X-ray examination showed small spotty shadows, but
there were no other symptoms or signs. The opacities seen on the
radiographs were thought to be due to the accumulation of
tin-containing dust and the development of connective tissue, This was
confirmed by experiments on rats exposed to tin(II) oxide. No cases of
pneumoconiosis were observed in 10 years, after the dust concentration
had been reduced to 10 mg/m3. An important feature of stannosis is
that fibrosis of the lung does not develop, providing that other
agents such as silica are not present.
8.1.2.2 Effects of ingestion
Packaged military rations were fed to 9 young male adult
volunteers for successive 24-day periods. The average tin content of a
control fresh diet was 13 mg/kg (in dry solids), while C-rations
stored at 1°C contained a tin concentration of 33 mg/kg, and rations
stored at 37°C contained 204 mg/kg. All the tin ingested was
accounted for by faecal elimination. No toxic effects were noted
(Calloway & McMullen, 1966). Dack (1955) reported a study in which 4
subjects ate canned pumpkin containing a tin concentration of about
380-480 mg/kg and canned asparagus containing a concentration of about
360 mg/kg, for 6 days, with no apparent illness. In an earlier study,
Mamontova (1940) did not observe any toxic manifestations in 4
volunteers who, for a period of 30 days, consumed 250 g per day of
canned fruit containing 212-250 mg of tin.
8.2 Organotin Compounds
8.2.1 Local effects
Toxic lesions among laboratory and process workers handling di-
and tributyltin compounds were reported by Lyle (1958). Most of the
lesions were typical acute skinburns, caused by the colourless di- or
tributyltin chlorides, which can come into contact with the skin
without exposed workers being aware of it. This type of lesion
developed 1 to 8 h following exposure. When the substance was washed
off immediately, no lesion developed. A more diffuse, but less rapidly
healing lesion, was caused by contact with clothes that had been
moistened by vapour or liquid compounds. This subacute irritation was
characterized by itching, affecting mostly the skin of the lower
abdomen, thighs, and groin. On examination, an easily distinguishable
erythematous eruption, was noted. Cessation of contact with the
compound was followed by rapid healing. Following contact with the
eye, lachrymation and severe suffusion of the conjunctivae appeared
within a few minutes and persisted for 4 days. Immediate lavage of the
eye did not prevent the development of the signs.
Lyle (1958) also studied the skin lesions induced by butyltin
compounds by applying various compounds on the skin of the back of the
hands of 5 volunteers. Skin lesions could be produced by a single
application of dibutyltin dichloride, and of the chloride, acetate,
and oxide of tributyltin, while the diacetate, dilaurate, oxide, and
maleate of dibutyltin and also tetrabutyltin failed to produce any
lesions. No visible changes occurred for 2 go 3 h after application,
although a swelling of the mouth of the hair follicles was noticeable.
The follicular inflammation progressed during the following 8 h with
only slight visible irritation of the skin between the openings of the
follicles. On the second day, sterile pustules developed over the
follicular openings, but remained small during the next 3 to 4 days.
After one week, the lesions had practically disappeared.
Symptoms experienced by female spray-painters working with a latex
paint, to which was added a fungicidal solution containing 20%
bis(tributyltin) oxide, ethylene oxide, ethanol, and water, were
described by Landa et al. (1973). The symptoms were reported to appear
"immediately" after the start of spraying and were experienced by all
the women engaged in the work. The first sensations were irritation of
the nasal mucosa and the conjunctivae. The exposure continued for
another fortnight during which the symptoms and signs became more
severe, and included bleeding from the nose and mucous discharges. An
otorhinolaryngological examination revealed rhinitis with distinct
hyperaemia and haemorrhages of the nasal septum. The workers reported
that the symptoms were less severe during weekends. When the addition
of the fungicidal solution was abandoned, the symptoms disappeared.
One year later, identical symptoms were reported by 4 spray-painters
using latex paint. An inquiry disclosed that the manufacturer had
begun to use bis(tributyltin) oxide as a fungicidal additive since the
banning of the use of mercury. The authors considered that the
trialkyltin compound was responsible for the symptoms. Measurements
performed later indicated that the concentrations of tin in the
breathing were below 0. 05 mg/m3 air.
Another description of local effects seen in workers handling
triphenyltin acetate was given by Markicevic & Turko (1967). These
workers were engaged in the formulation of a 20% solution of a
fungicide. During the hottest summer days, subjects working in the
dustiest places developed conjunctival irritation and irritation of
the mucous membranes of the upper respiratory tract as well as of the
skin, the hands and scrotum being particularly affected. Effects on
the central nervous system were not registered. The signs disappeared
rapidly after termination of exposure.
A wettable powder formation containing 50% tricyclohexyltin
hydroxide was examined for its irritation and sensitization potential.
No adverse reactions were observed in 53 females after sensitization
applications and a challenge application 20 days later of 0.5 ml of an
emulsion (10 g/litre). Tricyclohexyltin hydroxide was reported not to
be dermally irritating at a concentration of about 0.01 mg/kg body
weight (FAO/WHO, 1971).
8.2.2 Systemic effects
8.2.2.1 Effects of Dermal exposure
In a recent review (NIOSH, 1976), a fatal case was described in
which a 29-year-old woman was accidentally drenched in a slurry
containing triphenyltin chloride, diphenyltin dichloride, hexane, and
other unidentified compounds at a temperature of 79.4°C. On arrival at
hospital, first-degree thermal burns covered 10% of her body. Second-
and third-degree burns with 80-85% desquamated skin developed 12 h
later. Death from renal failure occurred 12 days after the accident.
However, the agent responsible for her symptoms and signs and for the
death could not be identified from the data available.
Mijatovic (1972) reported a case of systemic intoxication
resulting from dermal contact with a compound containing triphenyltin
acetate (60%) and manganese dithiocarbamate (15%). The subject,
engaged in agricultural aviation, spilt the compound on his hands and
chest while filling the aeroplane. The skin on his chest and abdomen
was endured after 3 h and vesicles developed the following day. He
experienced headache, nausea, epigastric pain, and general weakness.
Clinically the liver transaminases (SGPT) were elevated, reaching a
maximum one month later. Two months later the transaminases were
returning to normal, but the patient had pains over the liver, which
was tender and enlarged. The liver damage persisted for 2 years and
the case was labelled chronic hepatitis. No liver biopsy was
performed.
8.2.2.2 Effects of inhalation
Acute intoxication caused by the inhalation of triphenyltin
compounds has been reported in some instances. Three cases occurred in
farmers treating beetroot plants with triphenyltin acetate which was
inhaled (Guardascione & di Bosco, 1967). The symptoms started from a
few minutes to about 2 h after the first exposure. After 2 hours of
spraying, one subject felt a general malaise and severe headache and
eventually lost consciousness. On admission to the hospital he had a
diffuse tremor and a slightly depressed sensorium. All laboratory
investigations during the 2-week hospitalization were normal. When
mixing triphenyl tin acetate powder into a solution, a second subject
experienced repeated flushes, nausea, and shortness of breath, which
started only a few minutes after inhalation. During the 8-day hospital
surveillance, the only pathological finding was glucosuria. A third
subject suffered from severe headache, strong nausea, and epigastric
pains. However, laboratory investigations were normal. The headache
persisted for 2 days while the epigastric pains and the nausea
disappeared after one day. All 3 subjects recovered completely.
Horacek & Demcik (1970) reported a group poisoning involving 2
pilots and 3 mechanics following the spraying of a formulation
comprising 60% of triphenyltin acetate and 15° of manganese
dithiocarbamate. Protective measures were found to be deficient and
food was consumed with unwashed hands; thus, exposure by both
inhalation and ingestion could have occurred. Furthermore, other
pesticides containing copper, zinc, DDT, and organophosphates were
handled during exposure to the formulation, but the authors considered
these substances unlikely to be responsible for the symptoms
experienced. Exposure to the formulation continued for 2 weeks before
symptoms were recognized and continued for 2 further weeks. One pilot
experienced gastric pain, diarrhoea, and dryness of the mouth with
severe thirst that was not relieved by drinking. He also felt pressure
over the chest and slight shortness of breath. Blurred vision was
experienced after one week of exposure. Clinically hepatomegaly with a
painful liver was noted. Liver transaminases (SGPT) were elevated and
the highest recorded value was obtained 6 weeks later. Hyperglycaemia
and glycosuria were also present. Eight weeks afterwards, liver biopsy
revealed marked diffuse steathosis without any detected necrosis.
Monthly follow-ups showed that hepatomegaly and steathosis persisted
for one year. The second pilot suffered from heartburn, diarrhoea, and
vision disturbances. Clinically a hepatomegaly and a slight
hyperglycaemia were registered. The symptoms persisted for 4 weeks and
the glycosuria and hepatomegaly for 6 weeks. Recovery was complete.
The mechanics had symptoms that were less severe, comprising
diarrhoea, headache, eye pains, blurred vision, epigastric pain, and
thirst.
A worker engaged in the manufacture of butyltin compounds was
reported to suffer from a reduced sense of smell (Akatsuka et al.,
1959). It was first observed after an exposure period of 16 months,
and a further deterioration of the olfactory sense was established
during the following 8 months. The state persisted without any noted
improvement for 2 years. Other reported symptoms were headaches in the
occipital region, nasal haemorrhages, lassitude, and a feeling of
stiffness in the shoulders.
In four eases of acute poisoning due to exposure to organotin
vapours, patients were reported to have suffered from such symptoms as
vertigo, headaches, nausea and vomiting, and visual disturbances.
Clinically, stasis of the papilla was found and all patients displayed
pathological findings on the electroencephalograms. These were
reversible in 7-25 days, and all eases recovered clinically. The
organotin compound or compounds responsible for the intoxications were
not identified (Prüll & Rompel, 1976).
8.2.2.3 Effects of ingestion
A most serious episode involving organotin poisoning occurred in
1954 due to oral administration of a proprietary preparation used for
the treatment of furonculosis, osteomyelitis, anthrax, and acne. The
drug was responsible for about 100 deaths and a total of about 210
intoxications. These numbers vary to some extent depending on the
source of information: Alajouanine et al., (1958) reported a total of
210 deaths whereas another source quotes 102 deaths and at least 100
persons permanently affected ( Br. med. J., 1958). A total of about
400 000 capsules was sold ( Br. med. J., 1958), and about 1000
persons were believed to have taken the drug (Barnes & Stoner, 1959).
However, the capsules consumed by the intoxicated subjects amounted
only to about 7% of the lot distributed ( Br. med. J., 1958),
implying that the majority of the capsules were consumed without any
known adverse effects. The main ingredients of the preparation were
diethyltin diiodide (15 mg/capsule) and linoleic acid
(100 mg/capsule). It was suggested that ethyltin triiodide,
triethyltin iodide, or tetraethyltin could have been present as
impurities because of deficiencies in the manufacturing process or as
metabolites formed under the influence of various physical factors
(Rondepierre et al, 1958). A theory that diethyltin diiodide would
have reacted with the isolinoleic component producing tetraethyltin
was also presented (Lecoq, 1954). The symptoms and clinical findings
described in victims by Alajouanine et al. (1958) seem to favour the
hypothesis of a trialkyltin compound being the causal agent, probably
acting synergistically with other constituents. The clinical data of
201 cases including 98 deaths were reviewed by Alajouanine et al.
(1958). The dominating symptom, reported in about 98% of the cases,
was a diffuse headache, sometimes intolerably severe, and appearing a
few days after medication was started. Nausea and vomiting occurred in
73%, visual disturbances, mainly photophobia, but also double vision,
colour-vision disturbances and, in a few cases, blindness were
recorded in 33% of cases. Ophthalmoscopic findings included
congestion, papilloedema (Druault-Toufesco, 1955), and in some cases,
papillary stasis (Gayral et al., 1958; Pesme, 1955). Frequent symptoms
and signs were urinary incontinence, vertigo, loss of weight, and
abdominal pains. Absence of fever and a tendency towards hypothermia
were also noted. Psychological disturbances or stupor were reported in
70% of the cases. Other findings were meningeal irritation,
somnolence, insomnia, convulsions, constipation, and bradycardia.
Sometimes electroencephalograms were altered but did not suggest any
localized lesion. Death occurred during coma or from respiratory or
cardiac failure and in some cases during convulsions. It is probable
that most of the symptoms and signs could be attributed to a cerebral
oedema, the occurrence of which was established at autopsies and
decompressive surgery (Cossa et al., 1958, Fontan et al., 1958). This
cerebral oedema of the white matter appeared to be very similar to
that produced experimentally by administration of a proprietary
preparation containing an organotin compound, to mice and monkeys
(Gruner, 1958). Macroscopically, the brain was oedematic but the
microscopic findings were minor.
It has been reported that only 10 out of 103 subjects, who
survived, recovered completely: in the remainder, symptoms such as
headaches and asthenia persisted for at least 4 years (Barnes &
Stoner, 1959). Information on later, follow-up studies is not
available. The lethal dose of the preparation was in some instances
only about 25 capsules taken during one week. Ingestion of 3 capsules
was enough to cause intoxication in a 9-year-old child (Fontan et al.,
1955).
8.3 Treatment of Poisoning
Dimercaprol has been suggested by Stoner et al. (1945) to be an
effective antidote for dialkyltin poisoning. It has also been reported
to completely prevent the accumulation of alpha-keto acids produced by
dialkyltin compounds (Barnes & Stoner, 1959). Although dimercaprol
protected rats against the general toxic effects of these compounds,
it did not have any effect on the response to triethyltin compounds.
This seems to be due to the fact that dialkytin compounds, at least up
to the dihexyl derivatives, react readily with sulfhydryl groups and
the trialkyltin compounds do not (Barnes & Magos, 1968),
Studer et al. (1973) reported that steroid therapy (dexamethasone)
appeared to diminish mortality and the severity of brain oedema in
rats; there also appeared to be significant decreases in brain, liver,
and blood levels of triethyltin bromide. The authors suggested that
the beneficial effects of this steroid therapy might be partly due to
enhanced excretion or catabolism of triethyltin bromide.
Surgical decompression was considered to be the only treatment
that offered any benefit in human cases of cerebral oedema caused by
trialkyltin compounds (Alajouanine et al., 1958).
REFERENCES
ADAM, W. B. & HORNER, G. (1937) The tin content of English canned
fruits and vegetables. J. Soc. Chem. Ind., 56: 329-334.
ADAMSON, J. H. (1962) The colorimetric determination of dialkyltin
compounds dissolved in fats and olive oil. Analyst, 87: 597-598.
ADCOCK, L. H. & HOPE, W. G. (1970) A method for the determination of
tin in the range 0.2 to 1.6 µg, and its application to the
determination of organotin stabilizer in certain foodstuffs.
Analyst, 95: 868-874.
AKAGI, H. & SAKAGAMI, Y. (1971) [On degradation of organotin compounds
by ultraviolet ray.] Rep. Public Health Inst., 20: 1-4
(in Japanese).
AKAGI, H., FUJITA, M., & SAKAGAMI, Y. (1972) [Thin layer
chromatography of inorganotin and organotin compounds in foods.]
J. food Hyg., 13: 85-88 (in Japanese).
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A.,
KAWAME, N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S.,
YANAGIBASHI, H., NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA,
I., & IYO, T. (1959) [Experimental studies on disturbance of sense
of smell due to butyltin compounds.] J. Tokyo Med. Coll,
17: 1393-1402 (in Japanese).
ALAJOUANINE, T., DÉROBERT, L., & THIÉFRY, S. (1958) Etude clinique
d'ensemble de 210 cas d'intoxication par les sels organiques
d'étain. Rev. Neurol., 98: 85-96.
ALDRIDGE, W. N. (1958) The biochemistry of organotin compounds;
trialkyltins and oxidative phosphorylation. Biochem. J.,
69: 367-376.
ALDRIDGE, W. N. (1976) The influence of organotin compounds on
mitochondrial functions. In: Zuckerman, J. J., ed. Organotin
compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 186-196.
ALDRIDGE, W. N. & CREMER, J. E. (1957) Organotin dithizone complexes:
The colorimetric determination of diethyltin and triethyltin
compounds. Analyst, 82: 37-43.
ALDRIDGE, W. N. & STREET, B. W. (1964) Oxidative phosphorylation:
Biochemical effects and properties of trialkyltins. Biochem. J.,
91: 287-297.
ALDRIDGE, W. N. & STREET, B. W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver
mitochondria. Biochem. J., 118: 171-179.
ALDRIDGE, W. N. & STREET, B. W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and
triethyltin to mitochondria and their effects on various
mitochondrial functions. Biochem. J., 124: 221-234.
ALEU, F. P., KATZMAN, R., & TERRY, R. D. (1963) Fine structure and
electrolyte analysis of cerebral edema induced by alkyltin
intoxication. J. Neuropathol exp. Neurol., 22: 403-413.
ALLAN, J. E. (1962) Atomic absorption spectrophotometry absorption
lines and detection limits in the air-acetylene flame.
Spectrochim. Acta, 18: 259-263.
AMOS, M.D. & WILLIS, J. B. (1966) Use of high-temperature pre-mixed
flames in atomic absorption spectroscopy. Spectrochim. Acta,
22: 1325-1343.
ANALYTICAL METHODS COMMITTEE (1967) The determination of small amounts
of tin in organic matter. Analyst, 92: 320-323.
ANDERSON, S. R. A. & LOWY, S. L. (1956) A fluorometric determination
of tin. Anal. Chim. Acta, 15: 246-253.
ASCHER, K. R. S. & NISSIM, S. (1964) Organotin compounds and their
potential use in insect control. World Rev. Pest Control,
3: 188-211.
AVTANDILOV, G. G. (1967) [Trace element content in normal and
atherosclerotic portions of the human aorta in connection with
age.] Arh. Patol., 29: 40-42 (in Russian).
BARKER, W. H. & RUNTE, V. (1972) Tomato-juice-associated
gastroenteritis, Washington and Oregon, 1969. Am. J. Epidemiol.
96: 219-226.
BARNES, J. M. & MAGEE, P. N. (1958) The biliary and hepatic lesion
produced experimentally by dibutyltin salts. J. Pathol.
Bacteriol. 75: 267-279.
BARNES, J. M. & MAGOS, L. (1968) The toxicology of organometallic
compounds. Organometal. Chem. Rev., 3: 137-150.
BARNES, J. M. & STONER, H. B. (1958) Toxic properties of some dialkyl
and trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARNES, J. M. & STONER, H. B. (1959) The toxicology of tin compounds.
Pharmacol. Rev., 11: 211-231.
BARNES, R. D., BULL, A. T., & POLLER, R. C. (1973) Studies on the
persistence of the organotin fungicide, fentin acetate
(triphenyltin acetate) in the soil and on surfaces exposed to
light. Pestic. Sci., 4: 305-317.
BARTAK, F., TOMECKA, M, & TOMICEK, O. (1948) Stannosis. Cas. lék.
cesk., 87: 915-920 (Abstract in: J. ind. Hyg., (1949)
31 (5): 98-99)
BASSINDALE, A. R., EABORN, C., TAYLOR, R., THOMPSON, A. R., WALTON, D.
R. M, CRETNEY, J., & WRIGHT, G. J. (1971) Aromatic reactivity 46.
Interpretation of substituent effects in base-catalyzed cleavage
of aryltrimethylsilanes and aryltrimethyl stannanes. J. Chem.
Soc. B., 1155-1161.
BENNETT, R. L. & SMITH, H. A. (1959) Spectrophotometric determination
of tin with phenylfluorone. Anal. Chem., 31: 1441-1442.
BENOY, C. J., HOOPER, P. A., & SCHNEIDER, R. (1971) The toxicity of
tin in canned fruit juices or solid foods. Food Cosmet. Toxicol.,
9: 645-656.
BENS, A. A, GRABOVSKAJA, L. I., & TIHONOVA, I. V. (1976)
[ Geochemistry of the environment.] Moscow, Nedra (in Russian).
BERGNER, K. G. & RUDT, U. (1958) [On the absorption of lead and tin by
fat and food rich in fat in tin plate cans.] Z. Lebensm. Unters.
Forsch., 137: 337-351 (in German).
BERTINE, K. K. & GOLDBERG, E. D. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BISTON, R., VERSTRAETEN, E., & NANGNIOT, P. (1972) Dosage de l'étain
dans les conserves par polarographie oscillographique ŕ intensité
imposée: Comparaison avec les methodes colorimetriques au dithiol
et ŕ la phenyl-fluorone. Anal. Chim. Acta, 59: 453-460.
BLAIR E. H. (1975) Biodegradation of tricyclohexyltin hydroxide.
Environ. Qual. Sai., 3 (Suppl.): 406-409.
BLASIUS, M. B., KERKHOFF, S. J., WRIGHT, R. S., & COTHERN, C. R.
(1972) Use of X-ray fluorescence to determine trace metals in
water resources. Water Resour. Bull., 8: 704-714.
BOGEN, J. (1973) Trace elements in atmospheric aerosol in the
Heidelberg area, measured by instrumental neutron activation
analysis. Atmos. Environ., 7: 1117-1125.
BÖNIG, G. & HEIGENER, H. (1972) [Paper chromatography: Quantitative
determination of micro amounts of tin.] Landwirtsch. Forsch.,
25: 378-382 (in German).
BOWEN, H. J. M. (1966) Trace elements in biochemistry, New York,
Academic Press, pp. 203-204.
BOWEN, V. T. & SUTTON, D. (1951) Comparative studies of mineral
constituents of marine sponges. I. The Genera Dysidea,
Chondrilla, Terpios. J. mar. Res., 10: 153-167.
BRAMAN, R. S. & TOMPKINS, M. A. (1979) Separation and determination of
nanogram amounts of inorganic tin and methyltin compounds in the
environment. Anal. Chem. 51: 12-19.
BRIDGES, J. W., DAVIES, D. S., & WILLIAMS, R. T. (1967) The fate of
ethyltin and diethyltin derivates in the rat. Biochem. J.,
105: 1261-1266.
BRINCKMAN, F. E. & IVERSON, W. P. (1975) Marine chemistry in the
coastal environment. Paper presented at 169th American Chemical
Society Meeting. Philadelphia, PA., April 8-10.
BRINCKMAN, F. E., BLAIR, W. R., JEWETT, K. L., & IVERSON, W. P. (1977)
Application of a liquid chromatograph coupled with a flameless
atomic absorption detector for speciation of trace organometallic
compounds. J. Chromatogr. Sci., 15: 493-503.
BRITISH MEDICAL JOURNAL (1958) "Stalinon": A therapeutic disaster.
Br. med. J., March 1st, p. 515.
BROWNING, E. (1969) Tin. In: Toxicity ol industrial metals, 2nd ed.,
London Butterworths, pp. 323-330.
BRUGGEMANN, J. & KLIMMER, O. R. (1964) [Nutritional-physiological,
analytical and toxicological studies on fungicidal triphenyltin
acetate.] Zentralbl. Vet.-Med. A, 11: 1-3 (In German).
BRUGGEMANN, J., BARTH, K., & NIESAR, K.-H. (1964a) [Experimental
studies on the content of triphenyltin acetate residues in turnip
leaves, ensilage of turnip leaves, and in excrements of animals
fed with these products.] Zentralbl. Vet.-Med. A, 11: 4-19
(in German).
BRUGGEMANN, J., KLIMMER, O. R., & NIESAR, K.-H. (1964b) [The
importance of triphenytin acetate residues in plants and animals
from a hygienic-toxicologic standpoint.] Zentralbl. Vet.-Med. A,
11: 40-48 (in German).
BURGER, K. (1963) [Thin layer chromatographic method for the
detection, identification and separation of traces of mono-, di-,
tri- and zerovalent organotin compounds.] Z. anal. Chem.,
192: 280-286 (in German).
BYINGTON, K. H., YEH, R. Y., & FORTE, L. R. (1974) The hemolytic
activity of some trialkyltin and triphenyltin compounds. Toxicol.
appl. Pharmacol., 27: 230-240.
CALLEY, D. J., GUESS, W. L., & AUTIAN, J. (1967) Hepatotoxicity of a
series of organotin esters. J. Pharm. Sci., 56 (2): 240-243.
CALLOWAY, D. H. & MCMULLEN, J. J. (1966) Fecal excretion of iron and
tin by men fed stored canned foods. Amer. J. clin. Nutr.,
18: 1-6.
CALVERY, H. O. (1942) Trace elements in foods. Food Res.,
7: 313-331. CAPACHO-DELGADO, L. & MANNING, D.C. (1966)
Determination of tinby atomic absorption spectroscopy.
Spectrochim. Acta, 22: 1505-1513.
CARR, H. G. (1969) Interaction between PVC bottles and liquid foods.
Soc. Plast. Eng. 25: 72-74.
CASIDA, J. E., KIMMEL, E. C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and
trialkyleads by liver microsomes. Acta chem. Scand.,
25: 1497-1499.
CATALA, R., ROYO, I. J, & PRIOMO, E. (1971) Tin content of canned
orange juice. Rev. Agroquim. Tecnol. Aliment., 11: 409-417
( Chem. Abstr., (1972) 77: 370, Abstract no. 3974 w).
CAUJOLLE, E., LESBRE, M., & MEYNIER, D. (1954) Sur la toxicité du
tétraméthylstannane et du tétraéthylstannane. C. R. Acad. Sci
(Paris), 239: 556-558.
CENCI, P. & CREMONINI, B. (1969) [Thin-layer chromatography of two
organotin pesticides and their behavior in various types of soil.]
Ind. sac. Ital.. 69: 313-316 (in Italian).
CHAPMAN, A. H., & PRICE, J. W. (1972) Degradation of triphenyltin
acetate by ultraviolet light. Int. Pest Control, 14: 11-12.
CHAPMAN, A. H., DUCKWORTH, M. W., & PRICE, J. W. (1959) The
determination of dialkyltin compounds in polyvinylchloride.
Br. Plast., February, pp. 78, 87.
CHEFTEL, H. (1967) L'étain dans les aliments. Presented at the
Fourth Meeting of the FAO/WHO Codex Committee on Food Additives,
The Hague, 11-15 September 1967, Rome, Food and Agriculture
Organization of the United Nations, 10 pp.
CHISAKA, H., TANIZAKI, Y., & NAGATSUKA, S. (1973) [Determination of
tin and poisonous trace elements in canned juices by neutron
activation.] Radioisotopes, 22: 247-248 (in Japanese).
CHRISTIAN, G. D. & FELDMAN, F. T. (1970) Atomic absorption
spectrography. New York, Wiley, pp. 404-406.
CONINE, D. L., MUHLER, J. C., & FORNEY, R. B. (1972) Acute toxicity of
sodium pentafluorostannite in rats and mice. Toxicol. appl.
Pharmacol., 22: 303-304.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R.
B. (1973) Comparison of the metabolic and renal effects of sodium
pentafluorostannite, sodium fluoride, stannous chloride,
hydrochloric acid, and starvation in the rat. Pharmacologist,
15: 189.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., MUHLER, J.
C., & FORNEY, R. B. (1975) Toxicity of sodium pentafluorostannite,
a new anticariogenic agent. I. Comparison of the acute toxicity of
sodium pentafluorostannite, sodium fluoride and stannous chloride
in mice and/or rats. Toxicol. appl. Pharmacol., 33: 21-26.
CONINE, D. L., YUM, M., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R. B.
(1976) Toxicity of sodium pentafluorostannite. A new
anticariogenic agent. III. 30-day toxicity study in rats.
Toxicol. appl. Pharmacol., 35: 21-28.
CORBIN, H. B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Off. Anal.
Chem., 53: 140-146.
CORBIN, H. B. (1973) Rapid and selective pyrocatechol violet method
for tin. Anal. Chem., 45: 534-537.
COSSA, P., DUPLAY, FISCHGOLD, ARFEL-CAPDEVIELLE, LAFON, PASSOUANT,
MINVIELLE, & RADERMECKER, J. (1958) Encéphalopathies toxiques au
stalinon. Aspects anatomocliniques et alectroencéphalographiques.
Rev. Neurol., 95: 97-108.
COTTON, F. A. & WILKINSON, G. (1972) Advanced inorganic chemistry. A
comprehensive text, London, Wiley-Interscience, 3rd ed.
COTTON, F. A. & WILKINSON, G. (1976) Basic inorganic chemistry, New
York, Wiley.
COUSSEMENT, S. (1972) Analyse des dépôts de fentin acetate sur
feuilles de pomme de terre. Ann. Gembloux, 78: 41-46.
COYLE, C. F. & WHITE, C. E. (1957) Fluorometric determination of tin
with flavonol. Anal Chem, 29: 1486-1488.
CREMER, J. E. (1957) The metabolism in vitro of tissue slices from
rats given triethyltin compounds. Biochem. J., 67: 87-96.
CREMER, J. E. (1958) The biochemistry of organotin compounds: The
conversion of tetraethyltin into triethyltin in mammals.
Biochem. J., 65: 685-692.
CUTTER, H. C., FALLER, W. W., STOCKLEN, J. B., & WILSON, W. L. (1949)
Benign pneumoconiosis in a tin oxide recovery plant. J. And.
Hyg., 31: 139-141.
DACK, G. M. (1955) Chemical poisons in food. In: Food poisoning,
Chicago, University of Chicago Press, pp. 24-25.
DEPARTMENT OF NATIONAL HEALTH AND WELFARE, CANADA (1975) Food and
drug act and regulations office consolidation of the food and
drugs act and the food and drugs regulations with amendments to
December 18, 1975, B. 23.003, Ottawa, Queen's Printer and
Controller of Stationery, pp. 73B.
DEVJATYH, G. G., UMILIN, V. A., & CSINOVOI, Y. N. (1968) [Gas
chromatographic analysis of tetrabutyltin.] Tr. Himii i
Himiceskoj Tehnol., No. d, pp. 82-85 (in Russian).
DITTRICH, T. & COTHERN, C. R. (1971) Analysis of trace metal
particulates in atmospheric samples using X-ray fluorescence.
J. Air Pollut. Control Assoc., 21: 716-719.
DRESSLER, M., MARTINU, V., & JANAK, J. (1971) Response to the alkali
flame ionization detector to silicon, tin and lead compounds.
J. Chromatogr. 59: 429-433.
DRESSLER, M., BARTH, M., & VESPALEC, R. (1975) Chromatographic
determination of organotin compounds in fungistatic plaster.
Silikaty, 4: 301-310.
DRUAULT-TOUFESCO, M. N. (1955) A propos de deux cas d'intoxication
grave par le Stalinon. Bull. Soc. Ophtalmol. Fr., pp. 54-58.
DUCKERING, G. E. (1968) The cause of lead poisoning in the tinning of
metals. J. Hyg. (Lond.), 8: 474-503.
DUNDON, C. C. & HUGHES, J.P. (1950) Stannic oxide pneumoconiosis.
Am. J. Roentgenol., 63: 797-811.
DURBIN, P. W. (1960) Metabolic characteristics within a chemical
family. Health Phys., 2: 225-238.
DURFOR, C. N. & BECKER, E. (1964) Selected data on public supplies of
the 100 largest cities in the United States, 1962. J. Am. Water
Works Assoc., 56: 237-246.
DURUM, W. H. & HAFFTY, J. (1961) Occurrence of minor elements in
water, Washington, DC, pp. 1-11 (Geological Survey Circular
445).
DYER, B. & TAYLOR, G. (1931) Notes from the Report of Public Analysts.
Analyst, 56: 251-253.
ELSEA, J. R. & PAYNTER, O. E. (1958) Toxicological studies on bis
(tri- n-butyltin) oxide. Am. Med. Assoc. Arch. ind. Health,
18: 214-217.
ELTEN, R. (1929) [Study on tin foil used in cheese packeting; rindless
cheese.] Chem.-Ztg., 53: 586 (in German).
ENGBERG, A. (1973) A comparison of a spectrophotometric (Quercetin)
method and an atomic absorption method for the determination of
tin in food. Analyst, 98: 137-145.
EVANS, C. A. & MORRISON, G. H. (1968) Trace element survey analysis of
biological materials by spark source mass spectrometry. Anal.
Chem., 40: 869-875.
EVERETT, G. L, WEST, T. S., & WILLIAMS, R. W. (1974) The determination
of tin by carbon filament atomic absorption spectrometry. Anal.
Chim. Acta, 70: 291-298.
EYRICH, W. (1972) [Determination of tin in canned fruits and
vegetables.] Dtsch. Lebensm.-Rundsch, 9: 280-282 (in German).
FEY, R. (1969) [Heavy metal content in canned fruits and vegetables:
I. Tin and iron in canned asparagus.] Ind. Obst-Geinneseverwert.,
54: 27-33 (in German).
FILER, T. D. (1971) Fluorometric determination of submicrogram
quantities of tin. Anal. Chem., 43: 1753-1757.
FISCHER, H. W. & ZIMMERMAN, R. (1969) Long retention of stannic oxide:
Lack of tissue reaction in laboratory animals. Arch. Pathol.
88: 259-264.
FISH, R. H., KIMMEL, E. C., & CASIDA, J. E. (1975) Bioorganotin
chemistry. Biological oxidation of tributyltin derivatives.
J. Organometal Chem. 93 (1): C1-C4.
FISH, R. H., KIMMEL, E. C., & CASIDA, S. E. (1976) Bioorganotin
chemistry: Biological oxidation of organotin compounds. In:
Zuckerman, J. J., ed. Organotin compounds: New chemistry and
applications, Washington, DC, American Chemical Society,
pp. 197-203 (Advances in Chemistry Series 157).
FONTAN, M. M., VERGER, PERY, LOISEAU, & MULON (1955) Quatre cas dont
deux mortels d'intoxication par le "Stalinon" chez l'enfant. J.
Med. Bordeaux, 132: 399-405.
FAO/WHO (1971) 1970 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United
Nations/World Health Organization, pp. 327-366, 521-542.
FOOD AND DRUG ADMINISTRATION, USA (1971) Code of Federal Regulations,
Title 21 - Foods and Drugs, Washington DC, US Government
Printing Office, pp. 409-410 ( 121, 2602).
FREELAND, G. N. & HOSKINSON, R. M. (1970) Non-aqueous atomic
absorption spectrophotometric determination of organometallic
biocides. Analyst, 95: 579-582.
FREITAG, K-D. & BOCK, R. (1974a) [Analysis of mixtures of tri-, di-,
and monophenyltin compounds and inorganic tin (IV).] Z. anal.
Chem., 5: 337-346 (in German).
FREITAG, K.-D. & BOCK, R. (1974b) Degradation of triphenyltin chloride
on sugar beet plants. Pesticide Sci., 5: 731-739.
FURCHNER, J. E. & DRAKE, G. A. (1976) Comparative metabolism of
radionuclides in mammals - XI. Retention of 113Sn in the mouse,
rat, monkey, and dog. Health Phys., 31: 219-224.
GAINES, T. B. & KIMBROUGH, R. D. (1968) Toxicity of fentin hydroxide
to rats. Toxicol. appl. Pharmacol., 12: 397-403.
GATEHOUSE, B. M. & WILLIS, J. B. (1961) Performance of a simple atomic
absorption spectrometer. Spectrochim. Acta, 17: 710-718.
GAUER, W. O., SEIBER, J. N., & CROSBY, D. G. (1974) Determination of
organotin residues from Plictran in fruit crops by gas-liquid
chromatography. J. agric. food Chem., 22: 252-254.
GAUNT, I. F., COLLEY, J., GRASSO, P., & CREASEY, M., (1968) Acute and
short-term toxicity studies on di- n-butyltin dichloride in rats.
Food Cosmet. Toxicol., 6: 599-608.
GAYRAL, L., LAZORTHES, G., & PLANQUES, J. (1958) Méningo-encé-phalite
hypertensive par intoxication au Stalinon, guérie. Rev. neurol.,
98: 143-144.
GEISSLER, H. & KRIEGSMANN, H. (1964) [Analysis of butyltin compounds
(n-C4H9)nSnX4-n.] Z. Chem., 9: 354-355 (in German).
GEISSLER, H. & KRIEGSMANN, H. (1965) [Liquids used for separation in
some gas chromatographic investigations of some n-butyltin
compounds. Z. Chem., 11: 423-424 (in German).
GELDMACHER -- VON MALLINCKRODT, M. & POOTH, M. (1969) [Simultaneous
determination of twenty five metals and metalloids in biological
materials.] Arch. Toxicol., 25: 5-18 (in German).
GEORGE, G. M., ALBRECHT, M. A., FRAHM, L. J., & McDONNELL, J.P. (1973)
Atomic absorption spectrophotometric determination of dibutyltin
dilaurate in finished feeds. J. Assoc. Off. Anal. Chem.,
56: 1480-1482.
GETZENDANER, M. E. & CORBIN, H. (1972) Residues on apples and pears
from use of Plictran miticide. J. agric. food Chem.,
20: 881-885.
GEYER, R. & ROTERMUND, U. (1969) [Oscillopolarographic determination
of organotin compounds.] Acta chim. Hung., 59 (2): 201-210
(in German).
GHAFOURI, R. (1970) Etude des eaux minérales d'Ax-les-Thermes. Terres
Eaux, 64: 19-27.
GOLDSCHMIDT, V. M. (1958) Tin, In: Muir, A. ed. Geochemistry,
Oxford, Oxford University Press, pp. 391-397.
GORDON, M. (1952) [Trace elements in peat.] Torinachrichten,
3: 12 (in German).
DE GROOT, A. P. (1973) Subacute toxicity of inorganic tin as
influenced by dietary levels of iron and copper. Food Cosmet.
Toxicol., 11: 955-962.
DE GROOT, A. P., FERON, V. J., & TIL, H. P. (1973) Short-term toxicity
studies on some salts and oxides of tin in rats. Food Cosmet.
Toxicol., 11: 19-30.
GRUENWEDEL, D. W. & PATNAIK, R. K. (1973) Model studies regarding the
internal corrosion of tin-plated food cans. IV. Polarographie
investigation of the interaction of tin (II)-ions with L-cysteine.
Chem. Mikrobiol. Technol. Lebensm., 2: 97-101.
GRUNER, J-E. (1958) Lésions du névraxe secondaires ŕ l'ingestion
d'éthyl-étain (Stalinon). Rev. Neurol., 98: 109-116.
GUARDASCIONE, V. & DI BOSCO, M. M. (1967) [Contribution to the
knowledge of occupational disease due to pesticides; Three cases
of acute intoxication by triphenyltin acetate fungicides.]
Lay. Urn., 19: 307-313 (in Italian).
HADZIMICEV, P. (1971) [Natural content of heavy metals (copper, zinc,
tin) in foods from the vegetable belt around Sofia.] Khranit.
Prom., 20: 18-20 (in Bulgarian).
HALL, C. A. & LUDWIG, P. D. (1972) Evaluation of the potential use for
several organotin compounds against the sheep blowfly ( Lucilia
spp.). Vet. Rec., 90: 29-32.
HAMILTON, T. A. (1948) The metabolic properties of plutonium and
allied material Univ. Calif. Rad. Lab. Med. Health Phys. Q., pp.
4-16 (UCRL-270).
HAMILTON, E. I., MINSKI, M. J., CLEARY, J. J., & HALSEY, V. S. (1972)
Comments upon the chemical elements present in evaporated milk for
consumption by babies. Sci. total Environ., 1: 205-210.
HAMILTON, E. I., MINSKI, M. J., & CLEARY, J. J. (1972/1973) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study.
Sci. total Environ., 1: 341-374.
HANNA, W. J. & GRANT, C. L. (1962) Spectrochemical analysis of the
foliage of certain trees and ornamentals for 23 elements. Bull
Torrey Bot. Club, 89: 293-302.
HASEGAWA, T. & SUGIMAE, A. (1971) [Emission spectrographic
determination on trace metals in airborne particulates.]
Jpn. Anal., 20: 640-845 (in Japanese).
HAYASHI, H. (1969) [Square wave polarographic determination of
dissolved tin in canned juice.] Jpn. Anal., 18: 58-61
(in Japanese).
HEATH, D. F. (1963) Some problems in the determination of residues in
plants and animals. In: Proceedings of a symposium; Radiation and
Radioisotopes Applied to Insects of Agricultural Importance,
Athens, 22-26 April 1963, Vienna, International Atomic Energy
Agency, pp. 185-193.
HEATH, D. F., (1967) The retention or triphenyltin and dieldrin and
its relevance to the toxic effects of multiple dosing. In:
Radioisotopic detection of Pesticide Residues, Proceedings of a
Panel, Vienna 1965, pp. 18-26 (Abstract in: Chem. Abstr.,
67: 1828 W).
HEDGES, T. R. & ZAREN, H. A. (1969) Experimental papilledema; A study
of cats and monkeys intoxicated with triethyltin acetate.
Neurology, (Minneapolis), 19: 359-365.
HEINDL, R. A. (1970) Tin. In: Mineral facts and problems, 1970
edition, pp. 759-751. Reprint from: US Dept of the Interior,
Bureau of Mines, Bulletin 650.
HEROK, J. & GOTTE, H. (1963) [Radiometric analysis of triphenyltin
acetate in the metabolism of plants and animals.] Int. J. appl.
Radiat. Isot., 14: 461-479 (in German).
HEROK, J. & GÖTTE, H. (1964) [Radiometric study on the metabolism of
triphenyltin acetate in milk sheep.] Zentralbl. Vet.-Med. A,
11: 20-28 (in German).
HERON, P. N. & SPROULE, J. S. G. (1958) The chemical nature of
fungicidal agents employed in the pulp and paper industry. Indian
Pulp Pap., 12: 516-517.
HESLOP, R. B. & JONES, K. (1976) Inorganic chemistry. A guide to
advanced study. Amsterdam, Elsevier.
HILES, R. A. (1974) Absorption, distribution and excretion of
inorganic tin in rats. Toxicol. appl. Pharmacol., 27: 366-379.
HLEBNIKOVA, M. J. (1957) [Hygienic characteristics of dust in the
production of tin.] Sb. naucnyh Rab. voprogam Gig. Truda i
profpatol., No. 2, pp. 53-83 (in Russian).
HODGE, V. F., SEIDEL, S. L., & GOLDBERG, E. D. (1979) Determination of
tin (IV) and organotin compounds in natural waters, coastal
sediments and macro algae by atomic absorption spectrometry.
Anal. Chem., 51: 1259.
HOFF, J. E. & WILCOX, G. E. (1070) Accumulation of nitrate in tomato
fruit and its effect on derinning. J. Am. Soc. Hortic. Sci.,
95: 92-94.
HORACEK, V. & DEMCIK, K. (1970) [Group poisoning in spraying of field
cultures with Brestan-60 triphenyltin acetate.] Prac. Lék.,
22: 61-66 (in Czech).
HORIO, T. & NAKASEKO, M. (1972) [Spectrophotometric determination of
dissolved tin in canned foods using Salicylidenamion-2-
thiophenol.] Tokyo Inst. Food Technol. J., 17: 212-214
(in Japanese).
HORIO, T., IWAMOTO, Y., & SHIGA, I. (1967) The nitrate corrosion of
canned orange juice drinks in Japan. Communication presented at
the 5th International Congress on Canned Fools, Vienna 3-6,
October, 1967, 12 pp.
HUEY, C., BRINCKMAN, F. E., GRIM, S., & IVERSON, W. P. (1974) The role
of tin in bacterial methylation of mercury. Paper presented of the
International Conference on Transport of Persistent Chemicals in
Aquatic Ecosystems, Ottawa, Canada, 1-3 May, 1974.
IGNATJEVA, M. A., KUZNETSOV, I. G., MIRSKOV, R. G., & VLASOV, V. M.
(1968) [Toxicology of acetylene organotin compounds of the
triethylstannyl range.] Izv. sib. Otdel. Akad. Nauk SSSR,
1 (5): 118-119 (in Russian).
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M, MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumorigenicity
in mice: A preliminary note. J. Natl Cancer Inst.,
42: 1101-1114.
INTERNATIONAL LABOUR OFFICE (1972) Tin alloys and compounds. In:
Encyclopedia of Occupational Health and Safety, Geneva, ILO,
Vol. II, pp. 1407-1409.
IWAMOTO, Y., MIYAZAKI, M., KUNISATO, S., MAEDA, Y., HORIO, T., &
KOMURA, S. (1968) [Studies on abnormal detinning of tin can with
nitrate in foods: (II) Relationship between dissolution on tin and
pH of canned tomato juice.] J. Jpn Soc. Food Nutr., 21: 50-54
(in Japanese).
JANITZ, W. (1971) [Comments on the polarographic method of tin and
lead determination in tinned meat products.] Med. Weter.,
27: 43-45 (in Polish).
JOHANSEN, O. & STEINNES, E. (1969) Determination of tin in geological
material by neutron activation analysis. Analyst, 94: 976-978.
JOHNSON, J. H., YOUNGER, R. L., WITZEL, D. A., & RADELEFF, R. D.
(1975) Acute toxicity of tricyclohexyltin hydroxide to livestock.
Toxicol. appl. Pharmacol., 31: 66-71.
JOVANOVIC, D., STOJADINOVIC, L., & SPASOJEVIC, B. (1967) [Contribution
to the determination of tin in canned meat.] Vojno-sanit. Pregl.,
24: 405-408 (in Serbo-Croat).
KAHN, H. L. & SCHALLIS, J. E. (1968) Improvement of detection limits
for arsenic, selenium and other elements with an argon-hydrogen
flame. At. Absorpt. Newsl., 7: 5-9.
KANISAWA, M. & SCHROEDER, H. A. (1967) Life term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumors in mice. Cancer Res., 27: 1192-1195.
KANISAWA, M. & SCHROEDER, H. A. (1969). Life term studies on the
effect of trace elements on spontaneous rumours in mice and rats.
Cancer Res., 29: 892-895.
KAPPAS, A. & MAINES, M.D. (1976) Tin: A potent inducer of heme
oxygenase in kidney. Science, 192: 60-62.
KAS'YANENKO, I. V. & KUL'SKAYA, O. A. (1969) [Microelement content of
the blood of patients with cancer of stomach and chronic gastritis
secretory insufficiency.] Vopr. eksp. Onkol Respub. Mezhvedom,
SB, 4: 101-107 (in Russian).
KEENAN, R. G. & BYERS, D. H. (1952) Rapid analytical method for air
pollution surveys. Arch. ind. Hyg., 6: 226-230.
KEHOE, R. A., CHOLAK, J. & STORY, R. V. (1940) A spectrochemical study
of the normal ranges of concentration of certain trace metals in
biological materials. J. Nutr., 19: 579-592.
KERK, VAN DER, G. J. M. (1975) [The present use of organotin
compounds.] Chem. Zeitung, 99: 26-32 (in German).
KIMBROUGH, R. D. (1976) Toxicity and health effects of selected
organotin compounds: A review. Environ. Health Perspect.,
14: 51-56.
KIMBROUGH, R. D. & GAINES, T. B. (1971) Hexachlorophene effects on the
rat brain. Arch. environ. Health, 23: 114-118.
KIMMEL, E. C., FISH, R. H., & CASIDA, J. E. (1977) Bio-organotin
chemistry: Metabolism of organotin compounds in microsomal
monoxygenase systems and in mammals. J. Agric. Food Chem.,
25 (1): 1-8.
KIMURA, Y., UEDA, K., NISHIZIMA, M., MAKI, T., WADA, M., TAKAHASHI S.,
YAMADA, T., ARAI, M., & UEMATSU, T. (1970) [Studies on extractable
heavy metals in canned foods: I. Detection of tin and lead from
various sorts of canned baby foods after their opening.] Ann.
Rep. Tokyo Metrop. Inst. Hyg., 22: 95-100 (in Japanese).
KIRK, R. S. & POCKLINGTON, W. D. (1969) The determination of tin and
canned foods with quercetin. Analyst, 94: 71-74.
KLEIN, A., COP, R., CHATRNA, E., & BADALOVA, M. (1970) [The study of
vitamin C stability and growing of tin content in tinned juices.]
Cesk. Hyg., 15: 49-55 (in Czech).
KLEINKOPF, M.D. (1955) Trace element exploration of Maine lake water,
Academic dissertation, Columbia University, 153 pp. (University
Microfilm Pub. 12, 447).
KLEINKOPF, M.D. (1960) Spectrographic determination of trace elements
in lake waters of northern Maine. Geol. Soc. Am. Bull.,
71: 1231-1242.
KLIMMER, O. R. (1963) [Triphenyltin acetate and the toxicology of
organic tin compounds.] Arzneimittel-Forsch., 13: 432-436
(in German).
KLIMMER, O. R. (1964) [Toxicological studies on triphenyltin acetate
(TPZA).] Zentralbl. Vet.-Med., R.A 11: 29-39 (in German).
KLIMMER, O. R. (1969) [The application of organotin compounds reviewed
by the experimental toxicologist.] Arzneimittel-Forsch.,
19: 934-939 (in German).
KOBAYASHI, T. & YADA, M. (1968) [Spectrophotometric determination of
tin in foodstuffs with stilbazo.] J. Food Hyg. Soc. Jpn,
9: 465-468 (in Japanese).
KOCH, J. & FIGGE, K. (1971) [Migration of tin stabilizers from PVC
bottles into beer.] Z. Lebensmittel-Unters. 147: 8-10
(in German).
KOCKIN, D. A., SKORBATOVA, J. C., PEGUSOVA, L. D., & VORONKOV, N. A.
(1969) [Polarographic study on organotin compounds with an acid
content.] Zl. Obshch. Khim., 39: 1777-1781 (in Russian).
KONOVALOV, G. S. & KOLESNIKOVA, T. K. (1969) [Trace elements in
atmospheric precipitation around the Otkaznoe reservoir.]
Gidrokhim Mater., 49: 74-79 (in Russian).
KRUTCHKOFF, D. J., JORDAN, T. H., WEI, S. H. Y., & NORDQUIST, W. D.
(1972) Surface characterization of the stannous fluoride enamel
interaction. Arch. oral Biol., 17: 923-930.
KRYLOVA, N. & BALABUH, A. (1970) [Trace element level in meat.] Myas.
Ind. SSSR, 41: 39 (in Russian).
KUMPULAINEN, J. & KOIVISTOINEN, P. (1977) Advances in tin compound
analysis with special reference to organotin pesticide residues.
Res. Rev., 66: 1-18.
KUNISATO, S. & MIYAZAKI, M. (1972) [Application of plant growth
regulators on fruits and vegetables for processing: I. Nitrate
content in tomato fruits.] J. Hortic. Assoc. Jpn., 41: 411-420
(in Japanese).
KUROSAKI, N. & FUSAYAMA, T. (1973) Penetration of elements from
amalgam into dentin. J. dent. Res., 52: 309-317.
KUTZNER, J. & BROD, K. H. (1971) [Resorption and excretion of tin
after oral administration of tin-113.] Nucl. Med. (Stuttg.),
10: 286-297 (in German).
LAAMANEN, A., LOFGREN, A., & PARTANEN, T. J. (1971) Some trace metals
in particulates in the air of Helsinki and Turku, 1964-1969. Work
Environ. Health., 8: 63-69.
LANDA, K., FEJFUSOVA, J., & NEDOMLELOVA, R. (1973) [The hazards of
organic tin compounds used as fungicides in some industrial
applications.] Prac. Lék., 25: 391-394 (in Czech).
LANIGEN, D. & WEINBERG, E. L. (1976) The use of estertin stabilizers
in PVC. In: Zucherman, J. J., ed. Organotin compounds: New
chemistry and applications. Washington, DC, American Chemical
Society (Advances in Chemistry Series 157).
LECOQ, R. (1954) Contribution ŕ l'étude des effects toxiques du
tétraéthylétain C. R. Hebd. Acad. Sci. (Paris), 239: 678-680.
LEE, E. E., GORANSON, S.S., ENRIONE, R. E., & MORGAN, G. B. (1972)
National air surveillance cascade impactor network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol., 6: 1025-1030.
LINDEMANN, G., PINDEBORG, J. J., & PAULSEN, H. (1959) Recovery of the
rat kidney in fluorosis. Arch. Pathol., 67: 30-33.
LJASKOVSKAJA, Y., & KRASIL'NIKOVA, T. (1961) [Photometric
determination of tin in canned food by reaction with quercetin.]
Myasn. Ind. SSSR 4: 44-45 (in Russian) (English abstract in
Anal. Abstr. (1963) 10: 1177).
LOUNAMAA, J. (1956) Trace elements in plants growing wild on different
rocks in Finland: A semi-quantitative spectrographic survey. Ann.
Bot. Soc. "Vanamo" 29: 4.
LOWRY, R. R. & TINSLEY, I. J. (1972) Determination of tin salts in
fats. J. Am. Oil. Chem. Soc., 49: 508-509.
LUFF, A. P. & METCALFE, G. H. (1890) Four cases of tin poisoning
caused by tinned cherries. Br. med. J., 1: 833-834.
LUKE, C. L. (1956) Photometric determination of tin with
phenylfluorone: Determination of tin in lead and 1% antimony-lead
alloys. Anal. Chem., 28: 1276-1279.
LYLE, W. H. (1958) Lesions of the skin in process workers caused by
contact with butyltin compounds. Br. J. ind. Med., 15: 193-196.
MACINTOSH, R. M. (1968) Tin and tin alloys. In: Kirk-Othmer
encyclopedia of chemical technology, 2nd ed. New York,
Interscience pp. 273-325.
MAGEE, P. N., STONER, H. B., & BARNES, J. M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Pathol. Bacteriol., 73: 107-124.
MAHADEVAIAH, M., GOWRAMMA, R. V., RADAKRISHNAIAH SETTY, G., SASTRY, M.
V., SASTRY, L. V. L., & BHATNAGAR, H. C. (1969) Studies on
variation in tin content in canned mango nectar during storage.
J. food Sci. Technol., 6: 192-196.
MAMONTOVA, A. A. (1940) [Effects of tin on animal organism.] Vopr.
Pitan, 2: 13-25 (in Russian).
MARHOLD, J. V. (1972) [ A collection of results of toxicological
evaluation of substances and products.] Pro Vychovu Vedoucicn
Pracovniku Chemickeho Prumyclu Praha (in Czech).
MARKICEVIC, A. & TURKO, V. (1967) [Lesions due to triphenyltin acetate
"Brestan".] Arh. Hig. Rada, 18: 355-358 (in Serbo-Croat).
MARKS, M. J., WINEK, C. L., & SHANOR, S. P. (1969) Toxicity of
triphenyltin hydroxide. (Vancide KS). Toxicol. appl. Pharmacol.,
14: 627.
MASON, B. (1966) Principles of geochemistry, 3rd ed., New York,
Wiley.
MAZAEV, V. T. & KOROLEV, A. A. (1969) [Experimental standardization of
the maximum permissible concentration of the dichlorbutyltin in
surface water.] Prom. Zagrjaznenija Vodoemov, No 9, pp. 15-37
(in Russian).
MAZAEV, V. T. & SLEPNINA, T. G. (1973) [Experimental data on hygienic
standardization of dibutyltin sulfide in water bodies.] Gig. i.
Sanit., 8: 10-13 (in Russian).
MAZAEV, V. T., LOSEV, N. I., & VOINOV, V. A. (1968) [On some
vegetative reactions and on electrical activity of the brain
cortex after intoxication with organic derivatives of tin.] Bull.
exp. Biol. Med., 11: 72-74 (in Russian).
MAZAEV, V. T., KOROLEV, A. A., & SKACKOVA, I. N. (1971) Experimental
investigation of the toxic effect of tetraethyltin and
dichlorodibutyltin on the animal organism. J. Hyg. Epidemiol.
Microbiol. Immunol., 15: (1): 115-120.
MAZAEV, V. T., GOLOVANOV, O. V., IGUMOV, A. S., & TSAY, V. N. (1976)
[Problem of the transformation of organotin compounds in a water
medium.] Gig. i Sanit, 3: 17-20 (in Russian).
MEINEMA, H. A., BURGER-WIERSMA, T., VERSLUIS-DE-HAAN, G. & GEVERS, E.
Ch. (1978) Determination of trace amounts of butyltin compounds in
aqueous systems by gas chromatography/mass spectrometry. Environ.
Sci. Technol., 12 (3): 288-293.
MIDWEST RESEARCH INSTITUTE (1977) Assessment of the need for the
character of, and the impact resulting from limitations on
selected organotins. Phase I Assessment of the need for
limitations on organotins. Washington, DC, US Environmental
Protection Agency, EPA Contract No. 68-01-4313).
MIJATOVIC, M. (1972) [Chronic hepatitis due to exposure to Brestan.]
Jugosl. inostr. Dok. Zast. Radu, 8:3-9 (in Serbo-Croat).
MIKI, E. & FUKUI, Y. (1971) [Square wave polarographic determination
of tin in canned fruit.] Sci. Rep. Agric. Fac. Kagawa Univ.,
22: 35-40 (in Japanese).
MITCHELL, R. C. (1948) The spectrographic analysis of soils, plants,
and related materials. Bureau of Soil Science, pp. 1-173
(Technical Communication No. 44).
MONIER-WILLIAMS, G. W. (1949) Trace elements in food, London,
Chapman & Hall, pp. 138-161.
MOSKALEV, Ju. I. (1964) [Studies on the distribution of 113Sn. In:
Distribution, biological effects and rapid excretion of
radioactive isotopes.] Moscow, Medicina, pp. 78-82 (in Russian).
MULAY, I. L., RAY, R., KNOX, B. E., SUHR, N.H., & DELANEY, W. E.
(1971) Trace metal analysis of cancerous and non-cancerous human
tissues. J. Natl. Cancer Inst., 47: 1-13.
NAKAHARA, T., MUNEMORI, M., & MUSHA, S. (1972) Atomic absorption
spectrometric determination of tin in premixed inert gas
(entrained air) hydrogen flames. Anal. Chim. Acta, 62: 267-278.
NAKAMURA, Y. & KAMIWADA, S. (1973) [Colorimetric determination of tin
by decomposition and tin-phenylfluorene complex.] J. food Hyg.,
14: 352-356 (in Japanese).
NATIONAL ACADEMY OF SCIENCES. WASHINGTON (1973) Toxicants occurring
naturally in foods, 2nd ed., Washington, DC, NAS, pp. 63-64.
NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH (1976) Criteria
for a recommended standard ... occupational Exposure to organotin
compounds, Washington, DC, US Government Printing Office,
187 pp.
NATIONAL WATER QUALITY NETWORK (1960) Annual compilation ol data,
1 October 1959-30 September 1960, Washington, DC, US Department
of Health, Education and Welfare, Public Health Service,
pp. 353-356.
NEHRING, P. (1972) [Tin in peach preserves.] Ind. Obst.
Gemusseverwert., 57: 489-492 (in German).
NELSON, A. A. (1954) Approximate relation of parts per million in diet
to mg/kg/day. Assoc. Food Drug Off. Q. Bull, 18: 66.
NEUBERT, G. (1964) [Analysis of organotin stabilizers.] Z. anal.
Chem., 203: 265-272 (in German).
NEUBERT, G. & ANDREAS, H. (1976) [The analysis of organotin
compounds.] Z. anal. Chem., 280: 31 (in German).
NEUBERT, G. & WIRTH, H. O. (1975) [The analysis of organotin
stabilizers.] Z. anal. Chem., 273:19-23 (in German).
NEUMANN, W. P. (1970) The organic chemistry of tin, London, Wiley.
NEWMAN, E. J. & JONES, P. D. (1966) Separation and determination of
small amounts of tin. Analyst, 91: 406-410.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effects of orally
administered plasticizers in the rat. Toxicol. appl. Pharmacol.,
26: 253-259.
NISHIJIMA, M., UEDA, K., MAKI, T., WADA, M., TAKAHASHI, S., KIMURA,
Y., YAMADA, T., NAKAJIMA, K., & FUKUDA, T. (1971) [Studies on
heavy metals dissolved in canned foods. (II) Detection of tin and
lead from various canned fruits after their opening.] Ann. Rep.
Tokyo Metrop. Inst. Hyg., 23: 189-196, (in Japanese).
OBRUSNIK, I. (1969), Determination of indium and tin by activation
analysis using replacement substoichiometry. Talanta,
16: 563-566.
ONISHI, H. & SANDELL, E. B. (1957) Meteoritic and terrestrial
abundance of tin. Geochim. Cosmochim. Acta, 12: 262-270.
OYANGUREN, H., HADDAD, R., & MAASS, H. (1958) Stannosis. Benign
pneumoconiosis owing to inhalation of tin dust and fume. I.
Environmental and experimental studies. Ind. Med. Surg.,
27: 427-431.
PAL, B. K. & RYAN, D. E. (1969) Fluorescence and metallic valency
states. Part III. Determination of tin. Anal Chim. Acta,
48: 227-231.
PATE, B. D. & HAYS, R.L. (1968) Histological studies of testes in rats
treated with certain insect chemosterilants. J. econ. Entomol.
61: 32-34.
PEARLMAN, R. S., HEFFERREN, J. J., & LYON, H. W. (1970) Determination
of tin in enamel and dentin by atomic absorption spectroscopy.
J. dent. Res., 49: 1437-1443.
PEDLEY, F. G. (1927) Chronic poisoning by tin and its salts. J ind.
hyg., 9: 43-47
PELIKAN, Z. & CERNY, E. (1968a) [The toxic effect of tri-n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292
(in German).
PELIKAN, Z., & CERNY, E. (1968b) The effect of low doses of bis-
(tri-n-butyltin) oxide on the skin of rats. Berufsdermatosen,
16: 340-349.
PELIKAN, Z., & CERNY, E. (1969) Toxic effect of bis- (tri-n-butyltin)
oxide (TBTO) on the skin of rats. Berufsdermatosen, 17: 305-316.
PELIKAN, Z. & CERNY, E. (1970a) The toxic effects of some di- and
mono-n-octyltin compounds on white mice. Arch. Toxikol.,
26: 196-202.
PELIKAN, Z. & CERNY, E. (1970b) Toxic effects of some mono-n-butyltin
compounds on white mice. Arch. Toxikol., 27: 79-84.
PENDERGRASS, E. P. & PRYDE, A. W. (1948) Benign pneumoconiosis due to
tin oxide. J. ind. Hyg., 30: 119-123.
PEREL'MAN, A. I. (1972) [Geochemistry of elements in the zone of
hypergenesis.] Moscow, Nedra (in Russian).
PERRY, H. M. & PERRY, E. F. (1959) Normal concentrations of some trace
metals in human urine: Changes produced by ethylenediamine
tetraacetate. J. clin. Invest., 38: 1452-1463.
PESME, P. (1955) Complicationes oculaires observées chez quatre
enfants intoxiqués par le "Stalinon". Arch. Fr. Pediat.,
12: 327-328.
PIVER, W. T. (1973) Organotin compounds: Industrial applications and
biological investigation. Environ. Health. Perspect., 4: 61-79.
PORTRETNYJ, V. P., MALYUTA, V. F., & CHUIKO, V. T. (1973)
[Determination of tin in water, after concentration by
co-precipitation, by anodic stripping voltametry.] Zh. anal.
Khim., 28: 1337-1340 (in Russian).
PRICE, W. J. & ROOS, J. T. H. (1969) Analysis of fruit juice by atomic
absorption spectrophotometry. I -- The determination of iron and
tin in canned juice. J. Sci. food Agric., 20: 437-439.
PRULL, G. & ROMPEL, K. (1976) [Neurological and cerebro-electrical
disturbances in acute poisoning due to organotin compounds.]
Nervenarzt, 41: 516-520 (in German).
REMY, H. (1956) Treatise on inorganic chemistry, Amsterdam,
Elsevier, Vol. I.
RIDLEY, W. P., DIZIKES, L. J., & WOOD, J. M. (1977) Biomethylation of
toxic elements. Science, 197: 329-332.
RITTENHOUSE, G., FULTON, R. B., III GRABOWSKI, R. J., & BERNARD, J. L.
(1969) Minor elements in oil field waters. Chem. Geol., 4:
189-209.
ROBERTS, R. M. G. & EL KAISSI, F. (1968) Base-catalyzed clearage of
allylic derivatives of group IV A elements. J. Organometal.
Chem., 12 (1): 79-88.
ROBERTSON, A. J. (1960) Pneumoconiosis due to tin oxide. In: King, E.
J. & Fletcher, C. M. ed Symposium on industrial pulmonary
diseases, Boston, Little-Brown, pp. 168-184.
ROBERTSON, A. J. & WHITAKER, P. H. (1957) Radiological changes in
pneumoconiosis due to tin oxide. J. fac. Radiol., 6: 224-233.
ROBINSON, I. M. (1969) Effects of some organotin compounds on tissue
amine levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROE, F. J. C., BOYLAND, E., & MILLICAN, K. (1965) Effects of oral
administration of two tin compounds to rats over prolonged
periods. Food Cosmet. Toxicol., 3: 277-280.
RONDEPIERRE, J., TRUHAUT, R., GUILLY, P., HIVERT, P. E., & BARANDE, I.
(1958) Sur une intoxication aiguë par un dérivé organiqué de
l'étain. Rev. neurol., 98: 135-140.
ROSE, M. S. (1969) Evidence for histidine in the triethyltin-binding
site of rat haemoglobin. Biochem. J., 111: 129-137.
ROSS, W. J. & WHITE, J. C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33: 421-424.
SAITO, I. & ENDO, M. (1970) [Analytical studies on inorganic elements
of gall stones.] Hokkaido Igaku Zasshi, 14: 334-337
(in Japanese).
SATO, N., TSURUTA, K., KA1VIADA, I., NARITA, S., & ABUKAWA, H. (1973).
[Studies on dissolved tin in canned foods by atomic absorption
spectrophotometry.] J. Food Hyg Soc. Jpn, 14: 245-248
in Japanese).
SAVAGE, W. (1939) Canned foods in relation to health, Lancet
231 (2): 991-995.
SAWYER, R. (1967) Determination of dialkyltin stabilizers in aqueous
extracts from PVC and other plastics. Analyst, 92: 569-574.
SCHALLIS, J. E. & KAHN, H. L. (1968) The determination of tin in
lubricating oils with a nitrous oxide-acetylene flame.
At. Absorpt. Newsl., 7: 84.
SCHRAMEL, P., SAMSAHL, K., & PAVLU, J. (1973) Some determinations of
Hg, As, Se, Sb, Sn, and Br in water plants, sediments and fishes
in Bavarian rivers. Int. J. environ. Stud., 5: 37-40.
SCHROEDER, H. A. & BALASSA, J. J. (1961) Arsenic, germanium, tin and
vanadium in mice: Effects on growth, survival and tissue levels.
J. Nutr., 92: 245-253.
SCHROEDER, H. A., BALASSA, J. J., & TIPTON, I. H. (1964) Abnormal
trace metals in man: Tin. J. chron. Dis., 17: 483-502.
SCHROEDER, H. A., KANISAWA, M., FROST, D. V., & MITCHENER, M. (1968)
Germanium, tin and arsenic in rats: Effects on growth, survival,
pathological lesions and life span. J. Nutr., 96: 37-45.
SCHROLL, E. VON & KRACHSBERGER, H. (1970) [Study on the geochemistry
of impurities in atmospheric precipitates in the Vienna
metropolitan area.] Radex Rundsch., 5: 331-341 (in German).
SCHRYVER, S. B. (1901) Some investigations on the toxicology of tin,
with special reference to the metallic contamination of canned
foods. J. Hyg., 9: 253-263.
SCHULER, P., CRUZ, E., GUIJON, C., MATURANA, V., & VALENZUELA, A.
(1958) Stannosis. Benign pneumoconiosis owing to inhalation of tin
dust and fume. II. Clinical study. Ind. Med. Surg., 27: 432-435.
SCHWARZ, K. (1971) Tin as an essential growth factor for rats. In:
Mertz, W. & Comatzer, W. E., ed. Newer trace elements in
nutrition, New York, Marcel Dekker, pp. 313-325.
SCHWARZ, K. (1974) Recent dietary trace element research, exemplified
by tin, fluorine and silicon, Fed. Proc., 33: 1748-1757.
SCHWARZ, K., MILNE, D. B., & VINYARD, E. (1970) Growth effects of tin
compounds in rats maintained in a trace element-controlled
environment. Biochem. biophys. Res. Commun., 40: 22-29.
SCOTT, W. J. & STEWART, D. F. (1944) The influence of dissolved tin on
the growth of Clostridium botulinum in canned vegetables.
J. Counc. Sci. Ind. Res. Aust., 17: 16-22.
SEINEN, W. & WILLEMS, M. I. (1976) Toxicity of organotin compounds. I.
Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di-n-octyltin dichloride. Toxicol. appl. Pharmacol., 35: 63-75.
SEINEN, W., VOS, J. G., SPANJE, I. van, SNOEK, M., BRANDS, R., &
HOOYKAAS, H. (1977a) Toxicity of organotin compounds. II.
Comparative in vivo and in vitro studies with various
organotin and organolead compounds in different animal species
with special emphasis on lymphocyte cytotoxicity. Toxicol. appl.
Pharmacol., 42: 197-212.
SEINEN, W., VOS, J. G., KRIEKEN, R. van, PENNINKS, A., BRANDS, R., &
HOOYKAAS, H. (1977b) Toxicity of organotin compounds, III.
Suppression of thymus-dependent immunity in rats. by di-n-butyltin
dichloride and di-n-octyltin dichloride. Toxicol. appl.
Pharmacol., 42: 213-224.
SHELDON, A. W. (1975) Effects of organotin anti-fouling coatings on
man and his environment. J. Paint Technol., 47: 54-58.
SHIRAISHI, Y., KUZUHARA, Y., & SUENAGA, S. (1972) [Determination of
tin in food by atomic absorption spectrometry.] J. Food Hyg. Soc.
Jpn 13: 97-100 (in Japanese).
SHIRASU, Y. (1970) Acute and sub-chronic toxicology studies of Dowco
213 in mice and rats. Test report on the sub-acute toxicity of
an insecticide, Dowco 213, Tokyo, Animal Pharmacology Institute of
Physical and Chemical Research, 11 pp. (in Japanese).
SHONO, T. & MATSUMURA, Y. (1970) Organic synthesis by electrolysis VI.
Anodie oxidation of aryleyclopropanes. J. org. Chem.,
35: 4157-4160.
SKACKOVA, I. N. (1967) [Hygienic substantiation of the maximum
permissible concentration of tetraethyltin in water bodies.] Gig.
i Sanit., 4: 11-17 (in Russian).
SKEEL, RL., & BRICKER, C. E. (1961) Spectrophotometric determination
of dibutyltin dichloride. Anal. Chem., 33: 428-431.
SMITH, J. D. (1970) The spectrophotometric determination of microgram
amounts of tin with phenylfluorone. Analyst, 95: 347-350.
SODERQUIST, C. J. & CROSBY, D. G. (1978) Determination of triphenyltin
hydroxide and its degradation products in water. Anal. Chem.,
50: 1435-1439.
STANCULESCU, C., MARIN, E., SANDULESCU, C., ILLE, C., TRICIA, A.,
ILLIESCU, M., PETROVICI, C., & POPESCU, P. (1972) [Copper, lead,
tin and arsenic content in canned fish after storage. II. Canned
fresh water fish.] Lucr. Inst. Cercet. Projiect. Ailment.,
10: 295-309 (in Romanian).
STOCKDALE, M., DAWSON, A. P., & SELWYN, M. J. (1970) Effects of
trialkyltin and triphenyltin compounds on mitochondrial
respiration. Eur. J. Biochem., 15: 342-351.
STONE, O. J., & WILLIS, C. J. (1968) The effect of stannous fluoride
and stannous chloride on inflammation. Toxicol. appl. Pharmacol.,
13: 332-338.
STONER, H. B. (1966) Toxicity of triphenyltin. Br. J. ind. Med.,
23: 222-229.
STONER, H. B., BARNES, J. M., & DUFF, J. I. (1955) Studies on the
toxicity of alkyl tin compounds. Br. J. Pharmacol., 10: 16-25.
STOROZEVA, H. H. (1963) [Contents of lead and tin in human teeth in
normal and caries cases.] Stomatologia, 1: 44-48 (in Russian).
STUDER, R. K., SIEGEL, B. A., MORGAN, J., & POTCHEN, E. J. (1973)
Dexamethasone therapy of triethyltin induced cerebral edema. Exp.
Neurol., 38: 429-437.
SUGIMAE, A. (1974) Emission spectrographic determination of trace
elements in airborne particulate matter. Anal. Chem.
46: 1123-1125.
SUZUKI, K. (1971) Some new observations in triethyltin intoxication of
rats. Exp. Neurol., 31: 207-213.
SVENSSON, V. (1975) [Tin poisoning caused by canned peaches.] Hyg.
och Miljö, No. 6, 25-27. (in Swedish).
TABOR, E. C. & WARREN, W. V. (1958) Distribution of certain metals in
the atmosphere of some American cities. Arch. ind. Hyg.,
17: 145-151.
THEUER, R. C., MAHONEY, A. W., & SARETT, H. P. (1971) Placental
transfer of fluoride and tin in rats given various fluoride and
tin salts. J. Nutr., 101: 525-532.
THOMPSON, K. C. & THOMERSON, D. R. (1974) Atomic absorption studies on
the determination of antimony, arsenic, bismuth, germanium, lead,
selenium, tellurium and tin by utilizing the generation of
covalent hydrides. Analyst, 99: 595-601.
TIHONOVA, Z. I. & ZORE, V. A. (1968) [Spectroscopic determination of
manganese, copper, aluminium, lead and tin in certain vegetables
and berries.] Gig. i Sanit., 33: 384-386 (in Russian).
TIPTON, I. H. (1960) The distribution of trace metals in the human
body. In: Seven, M. T., ed. Metal binding in medicine,
Philadelphia, Montreal, Lippincott, pp. 27-42.
TIPTON, I. H., COOK, M. J. STEINER, R. L., BOYLE, C. A., PERRY, H. M.,
& SCHROEDER, H. A. (1963) Trace elements in human tissue. Part I
Methods. Health Phys., 9: 89-101.
TIPTON, I. H., STEWART, P. L., & MARTIN, P. G. (1966) Trace elements
in diets and excreta. Health Phys., 12: 1683-1689.
TIPTON, I. H., STEWART, P. L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys.,
16: 455-462.
TJURIN, Y. M. & FLEROV, V. N. (1970) [Study of adsorption effects
under electro-reducing of organotin compounds on mercury.]
Elektrochim., 6: 1548-1552, (in Russian).
TJURIN, Y. M., FLEROV, V. N., & NIKITINA, Z. A. (1969) [On the
mechanism of electro-reducing of the dialkyltin chloride on the
mercury drip electrode.] Elektrochim., 5: 903 (in Russian).
TONGE, B.C. (1965) The gas chromatographic analysis of butyl- octyl-,
and phenyltin halides. J. Chromatogr., 19: 182-184.
TORACK, R., GORDON, J., & PROKOP, J. (1969) Pathobiology of acute
tri-ethyltin intoxication. Int. Rev. Neurobiol., 12: 45-86.
TROMBETTI, G. & MAINI, P. (1970) Determination of tricyclohexyltin
hydroxide residues in apples and pears. Pestic. Sci.,
1: 144-149.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.P., NGUYEN PHU LICH, VAN DEN
DRIESSCHE, J., GUESNIER, L. R., & MORIN, N. (1976) Contribution to
the toxicological and pharmacological study of tributyltin oxide.
Eur. J. Toxicol. environ. Hyg., 9 (1): 31-40.
TUTTLE, R. F., VOGT, J. R., & PARKINSON, T. F. (1970) Neutron
activation analysis of trace elements in airborne particulates.
In: Proceedings of a Symposium on Nuclear Technology and
Environmental Pollution, Salzburg, Vienna, International Atomic
Energy Agency, pp. 119-137 (Paper No. IAEA-SM-142a/4).
UDRIS, J. (1971) The analysis of tin stabilizers used in
poly(vinylchloride) compositions. Analyst, 96: 130-139.
UNITED NATIONS (1977) United Nations yearbook of industrial
statistics: Commodity production data 1966-1975, Vol. II,
pp. 498-500.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Air quality data for
metals, 1968 and 1969 from the National Air Surveillance
Networks, Research Triangle Park, USEPA, June 1973.
VERNON, F. (1974) The fluorometric determination of triphenyltin
compounds. Anal. Chim. Acta, 71: 192-195.
VINOGRADOV, A. P. (1953) The elementary chemical composition of
marine organisms, Memoir Sears Foundation for Marine Research,
New Haver, Yale University, pp. 16, 211, 240, 266, 360, 366, 535.
VINOGRADOV, A. P. (1956) Regularity of distribution of chemical
elements in the earth's crust. Geokhimiya, 1: 6-52.
VINOGRADOV, A. P. (1962) Average content of chemical elements in the
principal types of igneous rocks of the earth's crust.
Geokhimiya, 555-541.
VIZGIRDA, R. J. (1972) Organotin antifoulants. Paint and varnish
Produc., 62 (12): 12-28.
WALTERS, M. & ROE, F. J. C. (1965) A study on the effects of zinc and
tin administered orally to mice over a prolonged period. Food
Cosmet. Toxicol., 3: 271-276.
WARBURTON, S., UDLER, W., EWERT, R. M., & HAYNES, W. S. (1962)
Outbreak of foodborne illness attributed to tin. Public Health
Rep. (Wash.), 77: 798-800.
WEAST, R. C., ed. (1974) Handbook of Chemistry and Physics, 55th ed.
Cleveland, CRC Press.
WHO Technical Report Series, No. 515, 1973 ( Shistosomiasis control:
report of a WHO Expert Committee). pp. 30-32.
WHO (1975) 1974 evaluations of some pesticide residues in food,
pp. 155-158 (WHO Pesticides Residues Series No. 4).
WHO (1976) Environmental Health Criteria 1: Mercury. Geneva, WHO,
132 pp.
WIECZOREK, H. (1969) [The quantitative determination of organotin
compounds by means of quercetin chelates.] Dtsch.
Lebensmitt-Rundsch., 3: 74-78 (in German).
WILLIAMS, D. J. & PRICE, J. W. (1960) Paper chromatography of some
organotin compounds. Analyst, 85: 579-582.
WODSAK, W. (1967) Tin content of condensed milk. Angew. Chem. Int.
Ed., 6: 885-886.
WOGGON, H. & JEHLE, D. (1973) [The inverse voltametric determination
of biocidal organotin compounds.] Die Nahrung, 17:739-748
(in German).
WOGGON, H. & JEHLE, D. (1975) [The residual analysis of biocidal
organo-tin compounds by means of inverse voltametric methods.]
Die Nahrung, 19:271-275 (in German).
WOGGON, H, SAUBERLICH, H., & UHDE, W. J. (1972) [A contribution to the
determination (anodic stripping method) of organotin compounds and
their degradation and decomposition products.] Z. anal. Chem.,
260: 268-274 (in German).
WOIDICH, H. & PFANNHAÜSER, W. (1973) [Distribution of tin between the
solid and liquid contents of cans of vegetables and fruits.]
Dtsch. Lebensmitt-Rundsch., 69:224-226 (in German).
WOOD, J. M. (1974) Biological cycles for toxic elements in the
environment. Science, 183: 1049-1052.
WOOD, J. M., SEGALL, H. J., RIDLEY, W. P., CHEH, A., CHUDYK, W., &
THAYER, J. S. (1975) Metabolic cycles for toxic elements in the
environment. In Hutchinson, T. C., ed. Symposium Proceedings Vol.
I, International Conference on Heavy Metals in the Environment.
Toronto, Canada, pp. 49-68.
WOOD, J. M., CHEH, A., DIZIKES, L. J., RIDLEY, W. P., RAKOW, S., &
LAKOWICZ, J. R. (1978) Mechanisms for biomethylation of metals and
metalloids. Fed. Proc., 37: 16-21.
YUM, N.M., CONINE, D. L., MARTZ, R. C., FORNEY, R. B., & STOOKEY, G.
K. (1976) Renal tubular injury in rats induced by sodium
pentalfluorostannite, a new anticariogenic agent. Toxicol. appl.
Pharmacol., 37: 363-370.
ZOHM, H. (1972) [A new method for determination of tin and iron in
canned vegetables and fruit juices.] Z. Lebensmitt. -Unters.,
149: 30-33 (in German).
3.5 Tin-containing Wastes
Gases and fumes containing tin as well as sulfur dioxide and other
contaminants, and both soluble and insoluble tin-containing waste
materials such as water soluble salts, muds, and slags are produced
during various smelting, refining, and detinning operations. Solid
domestic and other wastes which may be dumped, incinerated, used for
land fills or composting, contain much tin, and used cans, aerosol
containers, and other miscellaneous tin-containing produces in solid
wastes account for 10-15% of tin-plated steel. The percentage of
plastics in solid wastes is increasing yearly and includes PVC
containing organotin compounds.
4. ENVIRONMENTAL TRANSPORT AND TRANSFORMATIONS
4.1 Transport and Bioconcentration
Tin concentrations in air are usually low except in the
neighbourhood of some industrial sources (section 5.1). Similarly, it
has not been consistently detected in all soils, plants, and waters
(sections 5.2 and 5.3). This seems go support a geochemical
classification placing tin in a group of elements with a low rate of
migration in soils and waters (Perel'man, 1972; Bens et al., 1976).
However, it is widely used and Wood et al. (1975) have included it
among elements that are relatively accessible in the environment. A
biological cycle for tin has been recently proposed by Ridley et al.
(1977) (section 4.2).
There is a lack of information concerning the environmental
transport of organometallic tin compounds. It appears, however, that
the vapour pressures of some organotin compounds are high and that
environmental mobility is possible although there are no data to
confirm this deduction. Some organotin compounds seem to be well
adsorbed in the soil and they have a low solubility in water (Heron &
Sproul, 1958). A laboratory soil-leaching study (Barnes et al, 1973)
indicated that triphenyltin is strongly attached to the soil. In
natural waters, organotin compounds would be primarily adsorbed on the
suspended particles and sediments (Schramel et al., 1973). More recent
studies on the determination of tin and organotin compounds in natural
waters have been reported by Braman & Tompkins (1979) and Hodge et al.
(1979).
Little reliable information exists concerning the bioconcentration
of tin and its derivatives. Schroeder et al. (1964) reported that the
presence of tin in phosphate fertilizers originating from marine
phosphate suggested that marine animals in the Pleistocene era
absorbed tin from the sea. Although tin is present in seawater in
concentrations of up to about 3 µg/litre (Mason, 1966; Vinogradov,
1953), there are few reports of its occurrence in marine algae,
plankton, bacteria, flowering plants, protozoa, sponges,
coelenterates, echinoderms, crustacea, and most fishes. However, Bowen
(1966) noted tin levels of 0.2-20 mg/kg in certain marine organisms
and accumulation of tin by the sponge Terpios zeteki was also
reported by Bowen & Sutton (1951).
4.2 Environmental Chemistry of Tin
Because of the considerably higher toxicity of some
organo-metallic tin compounds compared with inorganic forms of tin
(section 7), the possibility of biomethylation of tin is obviously of
considerable interest (Wood, 1974). A possible mechanism of such
bio-methylation has been proposed (Ridley et al., 1977; Wood et al.,
1978). Recent laboratory studies have indicated that the methylation
of tin by methylcobalamina (CH3B12) requires a one electron
oxidation of SnII to a SnIII radical, which can take place in the
presence of FeIII (SnIV would, of course, require a single electron
reduction). The stannyl radical (SnIII) can then react with CH3-B12
(CoIII) to produce (under conditions of high chloride ion
concentration) CH3-SnCl2 and reduced cobalamin, containing CoII
(Wood et al., 1978). A demonstration that methylation of tin takes
place in a strain of Pseudomonas bacteria found in the Chesapeake
Bay, USA, is the laboratory work with these bacteria carried out by
Huey et al. (1974). However, the methylated tin species was not
identified. Incubation of mercury(II) in the presence of tin(IV)
resulted in enhanced formation of methylmercury, and the authors
considered that methylmercury might have been transferred from the
biologically methylated tin to the mercury(II) ion. Subsequently,
Brinckman & Iverson (1975) proposed a "mercury-tin cross-over" system,
which may have some real basis as indicated by Schramel et al. (1973),
who found that mercury and tin accumulated together in some water
plants in Bavarian rivers.
4.3 Degradation of Organometallic Tin Compounds
Organotin compounds may be degraded both chemically and
biochemically. Hydrolytic decomposition occurs at rather extreme pH
values (< 1 or > 13) unless there are other catalytic
influences. Nevertheless, some authors consider that it could occur
fairly rapidly in an aquatic environment, although the environmental
pH is usually between 4 and 10 (Sheldon, 1975, Vizgirda 1972). This
could be the case, if photochemical decomposition were also involved.
Indeed, photochemical decomposition of triphenyltin acetate by
ultraviolet irradiation has been demonstrated under laboratory
conditions by Chapman & Price (1972) and Barnes et al. (1973). Most
likely, under environmental conditions, the chemical and
photo-chemical degradation of organotin compounds is combined with
biochemical degradation, or degradation may be entirely biological.
Some available information, mainly on triphenyltin, tricyclohexyltin,
and tributyl tin compounds, is included in the following summary.
a A form of vitamin B12 (cobalamin) in which the methyl carbon
is directly bonded to the cobalt which is coordinated to the corrin
ring system in the vitamin B12 structure.
Bruggemann & Klimmer (1964) and BrÜggemann et al. (1964a,b)
reported that triphenyltin acetate on sugar beet leaves was rapidly
broken down during the silage process. Within 5 weeks, triphenyltin
acetate, originally present at a concentration of 2470 mg/kg of fresh
leaves, had degraded completely. It was not established whether
degradation was due to microbial action or resulted from the low pH
(3.5-4.0) of the silage process. The authors also found that
triphenyltin acetate was not broken down rapidly by microorganisms
during its passage through the rumen and intestines of cattle. Similar
results in feeding experiments with sheep were reported by Herok &
Götte (1963).
Using thin-layer chromatography, Cenci & Cremonini (1969) found
that triphenyltin acetate and hydroxide mixed into soil (80 mg/kg of
soil) disappeared in 3-10 days, and 3 days, respectively, but the
degradation products were not identified and the participation of
microorganisms in the process was not established.
Akagi & Sakagami (1971) reported that, under their experimental
conditions, ultraviolet irradiation of a solution of triphenyltin
chloride yielded a mixture of triphenyl-, diphenyl-, and phenyltin as
well as inorganic tin compounds, within 6 h. Similarly, irradiation of
trialkyltin compounds resulted in a mixture of di- and monoalkyltin
compounds and inorganic tin also within a period of 6 h. Total
degradation of triphenyltin to inorganic tin required irradiation for
more than 400 h. Chapman & Price (1972) investigated the degradation
of an agricultural fungicide containing triphenyltin acetate, which
had been found to disappear within a few days of spraying on crops,
probably as a result of weathering and sunlight. They exposed the
compound in thin layers on glass to ultraviolet light and found that
the rate of degradation was higher at lower wavelengths and was
independent of layer thickness (5-10 mµ). This was also true for the
intermediate diphenyl- and phenyltin compounds and for the parent
compound. When triphenyltin acetate has irradiated for 60 h
(wavelength above 235 nm: intensity 120 W/m2: thickness 10 µm), about
10% of the triphenyltin compound remained unchanged, about 30%
occurred as diphenyltin, 15% as phenyltin compounds, and approximately
45% was degraded to inorganic tin in the form of hydroxides or
hydrated oxides. Similar results were obtained by Barnes et al.
(1973), who also studied breakdown at concentrations of 5-10 mg/kg
using triphenyltin acetate in which the phenyl groups had been
labelled with 14C. Degradation was monitored by measuring the
evolution of radioactive carbon dioxide; a half-time of about 140 days
was determined. Since no 14CO2 was evolved when the loam was
heat-sterilized, it was concluded that the degradation process was due
to microbial action.
The decomposition of triphenyltin chloride on sugar beet leaves,
as reported by Freitag & Bock (1974b) gave the expected series of
degradation products, the final degradation product being tin oxide.
After 42 days, about 19% of triphenyltin chloride had undergone
degradation.
In studies by Starnes (unpublished data)a and by Smith et al.
(unpublished data)b quoted by Getzendaner & Corbin (1972),
tricyclohexyltin hydroxide (the active ingredient of a commercial
miticide) exposed on thin-layer chromatographic plates to a sun lamp
yielded dicyclohexyltin oxide and cyclohexylstannoic acid with further
degradation to inorganic tin. It has also been shown that tin residues
on apples and pears treated with tricyclohexyltin hydroxide
(commercial miticide) remained almost constant at a level of
0.1-0.2 mg/kg (Getzendaner & Corbin, 1972). This concentration was
relatively independent of the total number of applications and, within
a month of the final application, independent of the time between the
last application and harvest.
Mazaev et al. (1976) reported the degradation of bi(tributyltin)
oxide (TBTO), dibutyltin bis isooctylmercaptoacetate (BuIOMA),
tributyltin methacrylate, diethyltin dioctanoate and dioctyltin bis
(isobutylmaleate), and provided estimates for the half-times of these
compounds for different aqueous media. In distilled water at 20°C,
values ranged from 1.14 days (BuIOMA) to 18.2 days (TBTO). From this
report, it appears that dialkyltin compounds are more rapidly degraded
than trialkyltin compounds.
Triorganotin compounds such as TBTO and tributyltin fluoride used
as antifouling agents in paints for ship bottoms and in underwater
coatings appear to break down in a multislop process to give tin(IV)
oxide (Barnes et al., 1973; Sheldon, 1975).
The mechanism of the biological dealkylation of organotin
compounds is apparently more complicated than was considered by Blair
(1975), who thought that the basic process was the oxidative cleavage
of the carbon-tin bond (section 6.2.4).
a Starnes (1966) Unpublished report, The Dow Chemical Co.
(ALS 66-648).
b Smith et al. (1970) Unpublished report, The Dow Chemical Co.
(OL 30445 Feb. 1. 1970).
Available information shows that the persistence of organotin
compounds may vary considerably depending on the conditions and the
type of compound. Under extreme laboratory conditions, the solvolysis
may have a half-time ranging from about 1 min to about 100 days
(Bassindale et al., 1971; Roberts & El Kaissi, 1968). Under
environmental conditions, it is likely that the half-times for
triphenyltin compounds are somewhere between a few days and 140 days
(Freitag & Bock, 1974b). Diorganotin compounds probably have somewhat
shorter half-times (Mazaev et al., 1976).
5. ENVIRONMENTAL CONCENTRATIONS AND EXPOSURES
5.1 Ambient Air
Tin is rarely detected in air, and, when detected, it is generally
in low concentrations (0.01 µg/m3), except in the proximity of some
industrial sources. Tin emission concentrations of 10-640 µg/m3 were
reported from electric furnaces at certain plants in Japan, in 1972.
At a distance of 700 metres, the atmospheric tin concentrations still
ranged from 3.8 to 4.4 µg/m3 (Environment Agency, Japan, 1971,
unpublished data).a
Concentrations from 0.003 to 0.3 µg/m3 were found in 60.6% of 754
samples tested from 22 cities in the USA. More than 50% of samples
from 3 urban and 3 rural sites were below the detectable level. The
highest concentration of tin (0.8 µg/m3) was found in a sample from a
Boston, USA, industrial site, which also contained the highest
concentration of lead together with relatively high concentrations of
zinc and cadmium (Tabor & Warren, 1958).
At stations of the US National Air Surveillance Networks during
1968 and 1969, concentrations ranged from below the minimum detectable
level to 0.23 µg/m3 in 1968 and 0.12 µg/m3 in 1969. Both these
concentrations were recorded in East Chicago (US Environmental
Protection Agency, 1973).
Concentrations ranging from 0.04 to 0.09 µg/m3, determined by
emission spectroscopy, were reported for Cincinnati and St Louis in
1970 by the US National Air Surveillance Cascade Impactor Network (Lee
et al., 1972). The size distributions of tin-containing particles
found in this study are given in Table 2.
Peak concentrations of tin were found in particles ranging from 1
to 3 µm in diameter. Bogen (1973) found that ambient air levels of tin
in the Heidelberg area of the Federal Republic of Germany between 30
April 1971 and 21 May 1971 ranged from 0.096 to 0.167 µg/m3.
The combustion of coal, oil, and lignite results in the discharge
of trace amounts of many elements into the atmosphere. Bertine &
Goldberg (1971) pointed out that the principal sites of fossil fuel
consumption are in the mid-latitudes of the Northern Hemisphere and
suggested a need for linking fossil fuel consumption with the
sedimentary cycles of trace metals from the atmosphere. They reported
that tin was present in coal and oil at average concentrations of 2
and 0.01 mg/kg, respectively.
a Japanese Background Paper No. 2, prepared for the WHO meeting
on the Effects on Health of Specific Air Pollutants from Industrial
Emissions, Geneva, November 4-9, 1974.
Table 2. Size distribution of tin-containing particles in urban aira
Cincinnatti St Louis
(A) (B)
4th Quarter 1st Quarter 4th Quarter
1970 1970 1970
Average concentration (µg/m3) 0.09 0.04 0.05
Average mass median diameter (µm) 0.93 1.40 1.53
% particles less than or equal to 1 µm 55 34 28
% particles less than or equal to 2 µm 86 68 65
a Adapted from Lee et al. (1972).
5.2 Soils and Plants
Tin has not been consistently found in all soils and plants;
however, allowance must be made for the possibility that the
concentrations present may have been below the limits of detection of
the methods employed. Bowen (1966) reported levels of tin in soil
ranging from about 2 to 200 mg/kg, the metal being strongly adsorbed
by the humus.
Schroeder et al., (1964) observed that concentrations of tin in
soils were localized and that it had not been detected in many areas.
In a limited study of tin in vegetation and foods, he found that
absorption by plants was erratic, even when the soil tin content was
high. A tin concentration of 157 mg/kg dry weight was recorded in
forest soil from southern Vermont, USA, but tin concentrations in
sections of an 100-year-old elm indicated that exposure of this tree
was not of recent origin.
Tin concentrations of 30-300 mg/kg have been reported in peat from
Finland by Gordon (1952), and high concentrations of tin were found by
Goldschmidt (1958) in forest litter and humus and also in coal. Tin
was present in all but 7 of 43 samples of foliage from 13 species of
ornamental plants and trees in New Jersey, USA (Hanna & Grant, 1962).
Lounamaa (1956), using a spectrographic method that had a limit of
detection of 10 mg tin/kg of ash, detected tin only sporadically in
higher plants in Finland. Lichens concentrated tin to exceptionally
high levels, considering the small amounts present in rocks. For
example, those growing on silicic rocks had tin concentrations of 72 ±
4.7 mg/kg ash (range: less than 10-100). Tin was undetected in 6 out
of 16 mosses while the remainder had concentrations of 10-60 mg/kg of
ash, concentrations being twice as high in those growing on silicic
rock as in those growing on calcareous rock. Pasture herbage growing
in Scotland was reported by Mitchell (1948) to contain tin
concentrations of only 0.3-0.4 mg/kg dry weight. He also quoted tin
concentrations in soils from north-east Scotland of up to 200 mg/kg.
Warren (1964, unpublished data) reported a tin concentration of
75 mg/kg in soils in unmineralized areas of Devon and Cornwall in
England, while soils from mineralized areas contained up to more than
1000 mg/kg.
5.3 Water and Marine Organisms
Tin has only been found occasionally in fresh water. It was found
by Durum & Haffty (1961) in 3 out of 59 samples from 15 major North
American rivers. No tin was detected by the National Water Quality
Network (1960) in 119 analyses at 71 locations on 28 major rivers in
the USA. Kleinkopf (1955, 1960) reported that Maine waters contained a
mean tin concentration of 0.038 µg/litre with a maximum concentration
of 2.50 µg/litre.
Tin has also only been found occasionally in municipal water
supplies (Durfor & Becker, 1964). Analyses by the US Public Health
Service showed concentrations of 0.0008-0.03 mg/litre in 32 out of 175
finished municipal waters (Taylor, 1964, personal communication).a
Although it is known that acidic or alkaline corrosive waters can
attack bronze fittings in pumping stations, presumably releasing some
tin, the relationship between corrosion and the aqueous release of tin
has not been established (Schroeder et al., 1964).
It has been reported by Vinogradov (1953) and Mason (1966) that
tin is present in sea water in amounts of about 0.003 mg/litre; thus,
its presence in marine organisms is to be expected. Tin levels of
0.2-20 mg/kg have been reported in marine animals by Bowen (1966) and
accumulation by the sponge Terpios zeteki has been noted (Bowen &
Sutton, 1951). Schroeder et al., (1964) reported that tin had not been
detected in plankton, bacteria, flowering plants, protozoa,
coelenterates, echinoderms, crustacea, or most fish. However,
Vinogradov (1953) reported the presence of small amounts of tin in a
marine worm, an oyster, and a dogfish.
a Taylor, F. B. (1964) Personal communication, cited by Shroeder,
H. A., Balassa, J. J., & Tipton, I. H. (1964) Abnormal trace metals
in man; Tin. J. chron. Dis., 17: 494.
Organotin compounds may enter water directly from antifouling
coatings on ship bottoms or they may be used as molluscicides and
added to vast areas of water for the control of snails, in which case
the effective concentration has to be about 1 mg/litre (WHO, 1973).
They may also be present indirectly as a result of industrial,
agricultural, and other uses of organotin compounds.
Tin(IV) oxide, suggested to be the final breakdown product of some
antifouling organotin agents (Shelemon, 1975), will eventually be
deposited in bottom sediments because of its insolubility. However, no
information is currently available concerning the rate and mechanism
of this degradation.
5.4 Food
Tin has been reported to occur in trace amounts in most natural
foods (de Groot et al., 1973). The tin content was determined by
atomic absorption spectroscopy in 11 known wheats or wheat blends, in
20 flours prepared commercially from these wheats, and in 25 products
specially prepared from the flours, as well as in 10 consumer products
from 10 different cities in the USA. Only one half of the tin in bread
could have been contributed by the flour. Tin concentrations in common
hard, common soft, and durum wheat were 5.6 ± 0.6, 7.9 ± 0.9, and 6.8
± 0.5 mg/kg respectively, while samples of flour and semolina from the
above sources of wheat contained tin at 4.1 ± 0.4, 3.7 ± 0.7, and 6.0
± 1.2 mg/kg, respectively. The tin concentrations in most products
exhibited significant regional differences (Zook et al., 1970).
Schroeder et al. (1964) calculated that a diet composed largely of
fresh meats, cereals, and vegetables would usually contain a tin
concentration of less than 1 mg/kg and would supply about 1 mg of tin
per day. In an area of southern Vermont, USA, where the soil contained
considerable amounts of tin (about 160 and 33 mg/kg, respectively, in
2 gardens), 10 out of 18 samples of fresh vegetables contained tin
levels of less than 1 mg/kg.
Hadzimicev (1971) measured the content of tin in foods from the
vegetable belt around Sofia, Bulgaria. Tin concentrations in wheat,
corn, beans, potatoes, tomatoes, cabbage, carrots, spinach, lettuce,
onions, apples, and peaches ranged from 0.02 to 1.02 mg/kg.
Larger amounts of tin may be present in processed foods and
drinks, because of corrosion and leaching of the metal from plain
unlacquered cans or from tin foil used for packaging. The corrosion of
tin-plate in food containers depends upon several factors including
the type of food product, the time and temperature of storage, the
acidity of the food and the quantity of air present in the headspace
of the can (Calloway & McMullen, 1966; Monier-Williams, 1949).
Oxidizing agents (eg., nitrates, ferric and cupric salts),and
anthocyanin pigments, methylamine, sulfur dioxide, and other sulfur
compounds accelerate corrosion, while fin salts in solution, sugars,
and colloids such as gelatine retard it (Monier & Williams, 1949). The
introduction of lacquered cans and the crimping of the tops minimizing
direct contact of food with solder has resulted in a general reduction
in corrosion and leaching.
A number of foods are unsuitable for packing in plain cans as they
promote corrosion. Thus, tin has been found in canned asparagus in
high concentrations varying from 120 to 550 mg/kg (Adam & Horner,
1937; Eyrich, 1972; Woidich & Pfannhäuser 1973). Tomato fruit can
accumulate nitrate when grown under combined conditions of high
temperature, high nitrogen fertilization levels, and low light
intensity. Such tomatoes caused excessive detinning of internal plain
can surfaces (Hoff & Wilcox, 1970). Iwamoto et al. (1968) also showed
a distinct detinning effect on unlacquered cans of both naturally
occurring and added nitrate in tomato juices; the authors suggested
that the concentrations of nitrate-nitrogen in juices should not
exceed 3 mg/kg. Tin contents exceeding 100 mg/kg have been found in
other foods in unlacquered cans including fish, orange, grape, and
mango juices, apricots, bananas, pineapple, and sugar syrup (Catala et
al., 1971; Eyrich, 1972; Mahadevaiak et al., 1969; Stanculescu et al.,
1972; Woidich & Pfannhäuser, 1973). In foodstuffs stored in
all-lacquered cans, the tin content was mainly below 25 mg/kg (Catala,
et al., 1971; Eyrich, 1972; Woidich & Pfannhäuser, 1973).
Several investigations have shown that high storage temperatures
increase the transfer of tin into canned foods (Catala et al., 1971;
Kimura et al, 1970; Zui, 1970). An increase of 2 mg/kg per month of
storage was reported by Zui (1970) for an increase of 1°C in
temperature.
Nishijama et al. (1971) showed an increase in the tin content from
50-77 to 260-300 mg/kg in pineapples stored at 8°C in unlacquered cans
for 72 h after opening the can. The transfer of tin into the fruits
was also increased by 0.5% aqueous citric or tartaric acid. The
content of tin in foods in lacquered cans was low and remained low
after opening. Klein et al. (1970) noted a similar effect of storage
of fruit juices in opened plain cans. Values exceeding 200 µg/kg were
found within 48 h of opening the cans.
A mean concentration of 0.0078 mg/litre was found in bottled
(glass) cow's milk compared with 16 mg/litre in evaporated milk in
unlacquered cans (diluted to the equivalent volume of cow's milk). In
some cases, concentrations of up to 110 mg/litre were found; however,
only milk from unlacquered cans contained significant amounts of tin,
while milk in lacquered cans contained less than 5 mg/litre (Hamilton
et al., 1972). The highest concentration of tin found by Wodsak (1967)
in canned condensed milk samples, less than 4 weeks old, was
40 mg/litre. The concentrations did not increase much during a further
5 months of storage but after 2 years of storage, concentrations up to
160 mg/litre were detected.
In addition to contamination by the containers, the presence of
tin in food may be due to the use of tin as an additive. Tin(II) ions
are used as additives in asparagus and peas packed in glass containers
or in lacquered cans, and also in soda water. The stannous ion is
added to prevent the migration of other heavy metals into the canned
foods and to inhibit the oxidation of ascorbic acid. Scott & Stewart
(1944) reported that Clostridium botulinum would not grow in
beetroot and carrots in unlacquered cans whereas normal growth
occurred in lacquered cans. For canned beetroot, the concentration of
added tin required for the prevention of growth was 150 mg/kg and for
carrots, 30-60 mg/kg.
High concentrations of tin have been reported in cheese packed in
tin foil (Dyer & Taylor, 1931, Elten, 1929).
5.5 Organotin Residues
Tin occurring in food and beverages may also result from the use
of organotin compounds as miticides in agriculture and as stabilizers
in PVC materials. In several experiments in various parts of the USA,
apples and pears from trees that had been sprayed 4 times with a
miticide, tricyclohexyltin hydroxide, had maximum organotin residues
of less than 2 mg/kg of whole fruit on the day of the final
application. These concentrations were reduced to about half within
3-5 weeks and, after 4 weeks, the residues consisted mainly of
tricyclohexyltin hydroxide with only small amounts of decomposition
products; inorganic tin concentrations were almost invariably below
0.2 mg/kg. The mean concentration of inorganic tin on apples and pears
after 1-5 applications of the miticide was 0.1 mg/kg (equivalent to
0.3 mg/kg, when calculated as tricyclohexyltin hydroxide); an
inorganic tin residue of 0.3 mg/kg (equivalent to 1.0 mg/kg of
tricyclohexyltin hydroxide) was found on pears by Getzendaner & Corbin
1972). In an appraisal of data on pesticide residues in food, the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues (FAO/WHO, 1971) concluded that residues of
tricyclohexyltin hydroxide on apples and pears decline by 50% in about
3 weeks due to photodegradation (section 4.3). A 20-50% reduction can
be achieved by washing and most of the residues can be removed by
peeling the fruit, after which only 0.1 mg/kg may be expected in the
fruit flesh. Data on tricyclohexyltin hydroxide on apples and pears
after 1-4 treatments showed mean values ranging from 0.4 to 2.0 mg/kg.
Similar concentrations on apples and pears have been reported from the
Netherlands (WHO, 1975). It has also been reported that the
concentrations of tricyclohexyltin hydroxide in treated products grown
under glasshouse conditions including cucumbers, tomatoes, and bell
peppers are unlikely to exceed 0.5 mg/kg (WHO, 1975).
The FAO/WHO joint meeting report (FAO/WHO, 1971) included data on
triphenyltin hydroxide, acetate, and chloride residues in various
foodstuffs such as potatoes, carrots, and sugar beets. Maximum
concentrations in potatoes and carrots only rarely exceeded 0.1 mg/kg.
Residues in food, fruit, and vegetables derived from direct contact
with the compound could be considerably reduced by washing.
When cows were fed with sugar beet leaves containing triphenyltin
acetate at 1 mg/kg a concentration of 0.004 mg/kg was found in the
milk.
In some countries, regulations permit the presence of 2 PVC
stabilizers in food, viz. dioctyltin S,S'-bis(isooctyl-
mercaptoacetate) and dioctyltin maleate polymer, up to a concentration
of total octyltin of 1 mg/kg (Department of National Health and
Welfare, Canada 1975; Food and Drug Administration, USA, 1971). The
migration of tin from PVC bottles into liquid foods contained in them
was studied by Carr (1969). The increase in the tin content of various
products during the storage period ranged from 0 to 0.07 mg/kg
(Table 3).
Table 3. Tin concentrations in foodstuffs after storage for 2 months in PVC bottles at 30 °C
Organotin
Tin content Tin content Tin extracted stabilizer
at beginning after ageing from bottle extracted from
Food product of experiment in PVC bottle (mg/kg) PVC bottle
(mg/kg) (mg/kg) (mg/kg)
mineral water 0.076 0.088 0.01 0.063
tomato juice 0.03 0.03 0 0
peanut oil 0.05 0.06 0.01 0.063
vegetable oil 0.08 0.09 0.01 0.063
apple juice 0 0.02 0.02 0.126
cherry soda 0 0.07 0.07 0.443
beer 0 0.01 0.01 0.063
milka 0.02 0.04 0.02 0.126
red wine 0 0 0 0
blended whisky 0.01 0.02 0.01 0.063
a Milk sample in PVC bottle aged for 2 weeks at 65 °C (Carr, 1969).
5.6 Working Environment
While most of the operations associated with the extraction and
treatment of tin ore are wet processes, tin dust or fumes may escape
during the bagging of concentrate in the ore rooms and during smelting
operations (mixing plant and furnace tapping), as well as during the
periodic cleaning of the bag filters used to remove particulate matter
from smelter furnace flue gas before release into the atmosphere (ILO,
1972). Exposure to dust and fumes of tin oxide may cause stannosis.
Additional exposure can occur at the final stage of upgrading the
cassiterite concentrate and during the roasting of sulfide ore.
Tin(II) chloride constitutes a hazard, when the rough molten tin is
separated from the rest of the charge during refining (ILO, 1972).
Other sources of industrial exposure include tinning of metals
(Duckering, 1968); the preparation and use of tin alloys and solders;
the use of tin(II) chloride as a reducing agent in calico printing;
the use of hydrated stannic acid, sodium metastannate, hydrated
tin(IV) chloride, and ammonium tin(IV) chloride as mordants in dyeing
and in the weighting of silk; the use of tin(II) fluoroborate
[Sn(BF4)2] in plating baths; and the production and use of organotin
compounds.
5.7 Estimate of Effective Exposure of Man through Environmental Media
Food is the main source of exposure to tin for man. Estimates of
human exposure from all environmental media are difficult to make
because of the lack of reliable data concerning environmental
concentrations (mainly in air and water), and because of variations in
the amounts of different foods and beverages consumed, especially
canned foods. Earlier estimates of the daily intake of tin by man have
been difficult to reconcile with more recent estimates. Kehoe et al.
(1940) reported that the mean daily intake of Gin by a normal adult in
the USA was 17 mg, while Tipton et al. (1966, 1969), in long-term
balance studies, reported average daily intakes of between 1.5 and
8.8 mg in 4 subjects. Schroeder et al. (1964) found 3.6 mg in a day's
institutional diet and considered that the major portion of the tin
probably came from canned fruit juices and fruits in the diet.
However, Schroeder and his colleagues pointed out that human intake
may vary considerably. They calculated that a 10 MJ (2400 kcal) diet
composed largely of fresh meats, grain products, and vegetables, which
usually contain less than 1 mg/kg of tin, would supply 1 mg/day.
However, a diet including a substantial proportion of canned
vegetables and fish could supply as much as 38 mg/day. A typical daily
tin balance for an adult in the USA, as estimated by Schroeder et al.
(1964), is shown in Table 4.
Hamilton et al. (1972) reported a daily intake of tin from food
and beverages by an adult in the United Kingdom of 0.187 ± 0.042 mg
(Table 5). It seems likely that this diet was composed of fresh foods,
which would account for the lower values obtained compared with the
North American data.
Table 4. Daily tin balance for an adult in the USAa
Intake (mg) Output (mg)
food 4.0 faeces (estimated) 3.98
(range, 1-40) (range, 1-40)
water 0.0 urine 0.023
(range, 0-0.03) (range, 0-0.04)
air 0.003
(range, 0-0.007)
total 4.003 (1-40) 4.003 (1-40)
a From: Schroeder et al. (1964).
Table 5. The concentration of tin in foods and total daily intake
for an adult in the United Kingdoma
Item Number of Concentration of tin (mg/kg)b
samples
cereals 3 4.7±1.5 × 10-2
meat 4 2.1±0.5 × 10-3
rats 4 3.7±0.8 × 10-2
fruits 5 0.5±0.1
root vegetables 3 2.2±1.0 × 10-2
green vegetables 4 2.3±10-2
milk (cow's) 17 7.8±1.2 × 10-3 (mg/litre)
total daily untake 0.187±0.042
a "From Hamilton et al. (1972).
b For prepared diet.
6. METABOLISM
Although a substantial amount of literature exists on the
absorption, distribution, excretion, and storage of tin, the results
of much of the early research are questionable in the light of modern
analytical techniques. For example, some studies depended on tissue
matrix destruction processes with the risk of loss of tin salts by
volatilization. In many cases, conditions were not controlled or
defined making it difficult to know whether pure valence states of
tin(II) or tin(IV) were being used, and whether the oxidation state of
the element affected its biological fate. In most cases, the purity of
the compound used was not indicated.
6.1 Inorganic Tin
6.1.1 Absorption
Evidence obtained from man and several animal species shows that
ingested inorganic tin is poorly absorbed. Most studies indicate that
less than 5% is absorbed from the gastrointestinal tract, although
values as high as 20% have been reported (Furchner & Drake, 1976;
Hiles, 1974; Kehoe et al., 1940; Kutzner & Brod, 1971,
Monier-Williams, 1949; Schroeder et al., 1964).
The influence of oxidation state and anion complement on the rate
of gastrointestinal absorption was studied by Hiles (1974) by
administering tin to rats in the form of tin(II) citrate, fluoride, or
pyrophosphate, and tin(IV) citrate or fluoride as a single oral dose
at 20 mg/kg body weight. The absorption of tin(II) citrate and
fluoride was calculated to be 2.8%, whereas only 0.6% of the dose of
tin(IV) citrate and fluoride was absorbed; the citrate and fluoride
were equally absorbed. Absorption of tin, when the anion was
pyrophosphate, was significantly lower than when the anion was citrate
or fluoride. The author considered that this was because pyrophosphate
had a greater tendency to form insoluble complexes with tin than
either fluoride or citrate.
6.1.2 Distribution
With both oral and parenteral administration of tin to animals,
the highest concentrations were found in the kidney, liver, and bone
(Durbin, 1960; Hiles, 1974; Moskalev, 1964), the principal site of
deposition being the bone (Furchner & Drake, 1976; Hiles, 1974).
The amounts of tin remaining in the organs of rats 48 h after
receiving a single oral dose of 20 mg of tin in the form of a
radioactive compound are shown in Table 6; the distribution 48 h after
a single intravenous administration of tin(II) or tin(IV) citrate is
shown in Table 7 (Hiles, 1974).
Rats given a daily oral dose of 20 mg of tin per kg of body weight
as tin(II) or tin(IV) fluoride did not show any detectable
trans-placental transfer at day 10 after conception, and at day 21,
the amounts of tin found in rats given the tin(II) compound were so
close to the limit of detection (= 0.1 µg of tin) that the
significance of the findings remained doubtful. In rats receiving the
same compounds at similar daily doses for 28 days, no significant
amounts of tin could be detected in the brain, heart, testes,
adrenals, reproductive tract, skeletal muscle, and spleen, while the
levels in kidney and liver were approximately the same as after a
single oral dose; however, the levels in bone were about 8 times
higher than those found after a single oral dose. Tissue
concentrations were higher following administration of tin(II)
fluoride than following tin(IV) fluoride administration (Hiles, 1974).
The results of distribution experiments performed by Hiles (1974)
suggested that tin is unlikely to be rapidly oxidized or reduced
during absorption and systemic transportation, although the actual
oxidation state of the tin in the tissues could not be determined.
Following intravenous injection of 113Sn(II) and 113Sn(IV)
citrates in rats, only small quantities of tin could be detected in
lung tissues (Hiles, 1974). However, tin is found in the human adult
lung and there is evidence indicating that the concentrations increase
with age often reaching higher levels than those in other tissues
(Schroeder et al., 1964). Thus, it seems probable that tin reaches the
lung primarily through inhalation.
Levels of 113Sn in the blood of rats, 2 days after oral or
intravenous administration, were extremely low and were only detected
in the erythrocytes (Hiles, 1974). Kehoe et al. (1940) found that 80%
of the tin in the blood of man was in the cells.
6.1.2.1. Distribution in human tissues and biological fluids
Tin concentrations found by Hamilton et al. (1972/1973) in some
tissues of healthy human adults are given in Table 8 together with
data reported by Kehoe et al. (1940) and Schroeder et al. (1964).
Comparatively high concentrations were found in lung, kidney, liver,
bone, and also in lymph nodes. Fairly high levels were reported by
Storozeva (1963) in the teeth of 24 persons, i.e., a range of
0.5-1.7 mg/kg of ash.
Table 6. 113Sn. distribution in rats after a single oral dose of solutions of various 113Sn compoundse
Percent of dosed radioactivity
Sample
SnF2 SnF4 Sn(II) Citrate Sn(IV) Citrate Sn2P2O7
Faeces and
gastrointestinal
wash 100.60±9.9 (10)a 98.40+3.6 (10) 98.00±2.1 (5) 95.40±10.3 (5) 100.0±2.8 (6)
Urine 1.02±0.58 (10)b,d 0.22±0.13 (10)b 0.90±0.23 (5)c 0.33±0.12 (5)c 0.41±0.19 (6)d
Kidneys 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.05±0.02 (5)c 0.01±0.00 (5)c 0.03±0.02 (6)d
Liver 0.08±0.04 (10)b,d 0.01±0.01 (10)b 0.07±0.02 (5) < 0.004 (5) 0.02±0.02 (6)d
Pancreas < 0.004 (5) 0.02±0.03 (5) < 0.004 (5) < 0.004 (5) < 0.004 (5)
Recovery 101.74±9.4 (10) 98.66+3.6 (10) 99.02±2.3 (5) 95.74±10.5 (5) 100.46±2.9 (6)
Percent of dosed 113Sn/g of sample
Bone (femur) 0.20±0.13 (10)b,d 0.04±0.02 (10)b 0.09±0.04 (5)c 0.03±0.02 (5)c 0.02±0.01 (6)d
Lymph nodes < 0.004 (5) 0.08±0.12 (9) < 0.004 (5) < 0.004 (5) 0.01±0.01 (5)
Blood clot 0.01±0.01 (10) < 0.004 0.01±0.00 (5) < 0.004 (5) < 0.004 (6)
Leg muscle < 0.004 (5) 0.01±0.01 (5) < 0.004 (5) < 0.004 (5) < 0.004 (6)
a % of dosed 113Sn±SD (number of animals) where 1% = 40 µg. Tissues containing < 0.010%, of the dose were not
normally included in the table.
b Significant difference at p < 0.05 between SnF2 and SnF4.
c Significant difference at p < 0.05 between Sn(II) citrate and Sn(IV) citrate.
d Significant difference at p < 0.05 between SnF2 and Sn2P2O7.
e From: Hiles (1974).
Table 7. Fate of inorganic tin in rats. Tin distribution after a single intravenous
dose of 113Sn(11) or 113Sn(IV) citratee
Percent of dosed radioactivity
Sample Sn(II) citrate Sn(IV) citrate
Bile-duct Bile-duct
Intact cannulated Intact cannulated
Urine 35.3±4.6a 23.3±12.8 39.8±10.8 24.8± 2.5
Faeces 12.1±1.8b,d 2.2±1.1d 3.1±1.4b 0.5±0.3
Bile -- 11.5±2.4c -- 0.1±0.1c
Injection site 3.3±1.8 6.2±4.7 4.1±1.5 3.9±3.3
Liver 2.0±0.2 5.1±1.6c,d 0.2±0.1d 0.2±0.1c
Kidney 5.9±0.9 8.3±1.3 5.3±3.4 4.2±2.5
Lungs 0.12±0.08 0.2±0.1 < 0.04 < 0.06
Spleen 0.4±0.0 -- < 0.04 --
Pancreas 0.2±0.1 -- < 0.04 --
Stomach 0.1±0.1a 0.2±0.0d < 0.04 < 0.06
Small intestine 0.5±0.1a 0.4±0.4d < 0.04 < 0.06
Large intestine 1.0±0.8b,d 0.4±0.3d 0.2±0.3b < 0.06
Carcass 33.4±5.3 -- 43.2±5.3 --
Femur (one) 1.6±0.2 1.9±0.1 3.2±0.6 2.0±0.1
Percent of dosed 113Sn/g
Bone (femur) 4.5±0.6b,d 8.4±1.0d 8.8±1.8b 7.4±0.9
Blood clot 0.6±0.2d 0.9±10.3d < 0.04 < 0.06
Leg muscle 0.06±0.03 -- < 0.04 --
a % of dosed 113Sn±SD where 1% = 4 µg tin.
b Significant difference at p < 0.05 between intact Sn(II) and Sn(IV) animals.
c Significant difference at p < 0.05 between bile-duct cannulated Sn(II) and
Sn(IV) animals.
d Significant difference at p < 0.05 between Sn(II) intact and bile-duct cannulated
animals.
e From: Hiles (1974).
Schroeder et al. (1964) reported a highly variable distribution of
tin in human tissues with significant differences related to age and
geographical location. Variations in the tissue contents of tin among
several geographical regions throughout the world were quite marked
but were less noticeable between 8 cities of the USA (Table 9 and 10).
All kidney, liver, and lung samples from Africa, Asia, and Europe had
lower mean and median tin contents than samples from the USA (the
difference being least in lungs). Tin in the tissue of Africans, where
exposure to tin would be expected to be much lower than in
industrialized North American and European areas, was, indeed,
noticeably low. Tin was rarely detected in the tissues of stillborn
infants, indicating that tin does not readily cross the placental
barrier. The lungs accumulated tin with advancing age but other organs
did not. Concentrations of tin in kidney, liver, lungs, and the ileum
according to decade of life are shown in Table 9. Small amount were
found in the heart, prostate, uterus, and trachea. Avtandilov (1967)
studied the trace element contents of normal and atherosclerotic
aortas. He reported a mean tin concentration of 0.08 mg/kg wet weight
in 20-39-year-old subjects with no difference between the two
categories of aorta.
Table 8. Tin content of human tissues, concentrations expressed as mg/kg wet weight.
Hamilton et al. (1972/73) Kehoe et al. (1940) Schroeder et al. (1964)
Tissue mean ± S.E. mean range (8 cities in
USA)
blood 0.009±0.002
brain 0.06±0.01 NDa
kidney 0.2±0.04 0.2 0.23-0.76
liver 0.4±0.08 0.35-1.0
lung 0.8±0.2 0.45 0.49-1.20
lymph node 1.5±0.6
muscle 0.07±0.01 0.1
bone 4.1±0.6b 0.5-0.8
a ND = not detected.
b mg of element per kg of ash.
Table 9. Tin in human tissues by age, mean US values (mg/kg ash)a
Age group (year) 0 0-1 1-10 11-20 21-30 31-40 41-50 51-60 81-70 71-84
kidney
concentration 0 57b 60b 33 20 34b 28b 22 34 32
occurrence 0/16 9/10 19/20 10/15 17/21 36/43 22/26 26/27 13/14 8/9
liver
concentration 0 48b 61b 42 34 33b 25 35 38b 34b
occurrence 0/21 11/12 22/23 14/14 20/21 39/43 27/28 25/25 13/13 9/9
lung
concentration (18) 35 34 45b 27 31b 39b 53b 58 64b
occurrence 1/10 5/6 5/6 10/13 18/21 34/35 27/27 28/29 13/13 9/9
ileum
concentration 0 101 98 80 174 116 97 53 172 140
occurrence 0/8 1/2 1/1 9/10 12/13 26/28 16/17 12/12 6/6 3/4
total occurrence
(%) 1.8 86.7 94.0 82.7 88.2 79.8 93.9 97.8 97.7 93.5
Note: Concentrations of positive samples.
Occurrence refers to the number positive/total cases.
a From: Schroeder et al. (1964).
b Excluding values > 150.
Table 10. Geographical distribution of tin in kidney, liver, and lung of man (mg/kg ash)a
No. of %
samples present Mean Range Median
kidney
United States of America 161 97 30 < 5-480 20
Switzerland 9 33 5 < 5-17 < 5
Africa 53 19 5 < 5-30 < 5
Middle East 43 42 8 < 5-55 < 5
Far East 57 40 11 < 5-110 < 5
liver
United States of America 163 96 35 < 5-300 24
Switzerland 9 78 6 < 5-20 Tc
Africa 49 25 4 < 5-20 < 5
Middle East 44 55 11 < 5-140 Tc
Far East 54 41 10 < 5-74 < 5
lung
United States of America 159 98 69 < 5-920 39
Switzerland 7 100 37 23-62 28
Africa 50 60 10 < 5-40 Tc
Middle East 45 91 33 < 5-200 17
Far East 57 93 65 < 5-1200 29
Note: Tc = trace, or barely detectable.
Mean and median values were obtained by assigning a value of one-half
the least detectable amount to those cases where tin was not detected.
a From: Schroeder et al. (1964).
Using a spark source mass spectrometric method with a detection
limit of 0.004 mg/kg wet weight, Hamilton et al. (1972/1973) reported
a mean concentration of 0.06 mg/kg (± 0.01) in 10 whole brain samples
from accidentally killed, presumably healthy adults. The concentration
found in the frontal lobe was 0.03 (± 0.07) and that in the basal
ganglia was 0.04 (± 0.02) mg/kg, measured in 2 samples. Tin was not
detected in human brain by Kehoe et al. (1940) and was found in only a
small percentage of subjects by Tipton (1960) and Schroeder et al.
(1964).
6.1.3 Excretion
The major route of excretion of absorbed inorganic tin is the
kidney although a small fraction is excreted into the bile (Hiles,
1974; Moskalev, 1964). The excretion of tin in the urine was studied
by Perry & Perry (1959) in 24 human adults. A mean of 16.6 µg/litre of
urine or 23.4 µg/day was reported. Kehoe et al. (1940) reported a mean
concentration of 18 µg/litre of urine in subjects in the USA. There
remains some doubt, however, as regards the accuracy of the analytical
technique used since, in the same paper, the concentration in the
urine of French males was reported to be zero.
In an experiment on rats, Hiles (1974) reported that after a
single oral dose of tin(II) or tin(IV)citrate or fluoride at 20 mg/kg
body weight (as the metal), about 50% of the absorbed tin was excreted
within 48 h. Following intravenous administration of 113Sn (2 mg of
tin per kg body weight), 12% of the 113Sn(II) and only 4% of the
113Sn(IV) appeared in the faeces of rats indicating that the biliary
route is probably more important in the elimination of tin(II) than of
tin(IV) compounds. Most of the biliary excretion (94%) took place
within 24 h.
6.1.4 Biological half-time
A half-time of 3-4 months was reported for 113Sn in the skeleton
of rats after intramuscular administration (Hamilton, 1948). However,
Hiles (1974) found a half-time of only 34-40 days for both tin(II) and
tin(IV) in the bone of rats following oral administration of tin(II)
fluoride or tin(IV) fluoride for 28 days. The half-time of tin(II) for
liver and kidney was reported to be 10-20 days. Furchner & Drake
(1976) using tin(II) chloride administered intraperitoneally and
intravenously in the mouse, rat, monkey, and dog, described the
elimination from the body as a 4 component-process that was similar in
all the species studied. The half-time for the longest component was
over 3 months.
6.2 Organotin Compounds
In this document, consideration will be limited to compounds of
the general type RSnX3, R3SnX2, R4SnX, and R4Sn where R is a
simple or complex action. Generally, little information is available
on the absorption, distribution, and excretion of these substances.
6.2.1 Absorption
Absorption from the intestinal tract of tin compounds with short
alkyl chains varies depending on the compound. There are also
considerable differences in absorption between species (Barnes &
Stoner, 1959).
Ethyltin trichloride administered orally was poorly absorbed by
rats, 92% of an oral dose of 25 mg/kg being eliminated in the faeces
within 2 days. It was not excreted in the bile (Bridges et al., 1967).
Triphenyltin acetate was almost completely eliminated in faeces within
a few days, when administered orally to sheep (Herok & Götte, 1964)
and cows (Bruggemann et al., 1964a). However, it was well absorbed,
when given orally to guineapigs and mice (Stoner, 1966). Results
obtained with rats were conflicting (Klimmer, 1963, 1964; Stoner,
1966). Tricyclohexyltin hydroxide was poorly absorbed in rats, only
about 2% being excreted in the urine after a single 25 mg/kg oral
dose. The remainder was recovered from the faeces and binary excretion
did not occur (FAO/WHO, 1971).
Trialkyltin compounds were well absorbed on contact with the skin,
since the dermal LD50s of various trimethyltin derivatives in mice
were of the order of 50-100 mg/kg (Hall & Ludwig, 1972) and that of
bis(tributyltin) oxide was below 200 mg/kg in rats and mice (Ascher &
Nissim, 1964; Elsea & Paynter, 1958). When a 20% fat solution of
various triethyltin derivatives was applied to the skin of rats and
mice for 10 min, all the animals died within 20-30 min (Ignatjeva et
al., 1968). In contrast, triphenyltin acetate did not penetrate
unbroken skin readily (Stoner, 1966).
6.2.2 Distribution
Following intravenous administration to mice and rats, the highest
concentrations of dibutyl- and diethyltin were found in liver and
kidney tissue; unchanged compounds were excreted in the bile (Barnes &
Magee, 1958). Rats were fed with 11 mg of triethyltin hydroxide over a
period of 89 days. At the end of this time, only 0.7 mg of the
compound could be recovered; 40% of this was in the blood, 28% in the
liver, and 29% in the skeletal muscle. Smaller amounts of triethyltin
were found in the kidney, brain, heart, and spleen (Cromer, 1957).
Species variation in the distribution of triethyltin has been
reported. When triethyltin (26 µg) was added to rat blood in vitro,
most of it (23 pg) was recovered from the erythrocytes and none was
found in the plasma, whereas in rabbit blood, triethyltin was more
equally distributed between erythrocytes (9 µg) and plasma (17 µg)
(Cromer, 1957). This could explain why triethyltin persists in the
blood of treated rats, whereas it quickly disappears from the blood of
treated rabbits (Barnes & Stoner, 1959). Triethyltin was found in the
brain of rats only 1-2 h after intraperitoneal injection of
triethyltin hydroxide (Cremer, 1957).
The tissue distribution of triethyltin following intravenous
administration of tetraethyltin resembled that following
administration of triethyltin (Cremer, 1957, 1958). Thus, a large
quantity of triethyltin was found in the liver, with smaller
quantities in the kidney, brain, and whole blood in a rabbit, 2 h
after administration.
After oral or intraperitoneal administration of 113Sn-labelled
triphenyltin to guineapigs, about 80% of the administered activity was
excreted in 10 days. The concentration of radioactive tin found in the
brain of rats and guineapigs after oral and intraperitoneal
administrations decreased with a half-life of several days. The form
of tin in the brain was not unequivocally identified, but it was not
in the form of free stannic ions nor was it necessarily triphenyltin
(Heath, 1967). Herok & Götte (1964) gave 3 sheep 10 mg of triphenyltin
acetate orally, every day for 20 days. The compound was labelled with
113Sn. The animals were killed at intervals ranging from 8 to 198 days
after the last dose. The highest concentration of 113Sn was found in
the liver but the brain also contained measurable amounts. In rats,
any absorbed triphenyltin chloride was rapidly distributed through the
tissues of the body including the brain. Tin was eliminated relatively
slowly and could be detected in the brain 38 days after a single dose
(Heath, 1963).
Only trace quantities of tricyclohexyltin hydroxide were found in
the tissues of rats and dogs fed up to 12 mg/kg bodyweight per day, in
the diet, for periods ranging from 45 days to 2 years (FAO/ WHO,
1971).
6.2.3 Excretion
When ethyltin trichloride was administered intraperitoneally to
rats, it was excreted almost exclusively in the urine; binary
excretion was negligible. When diethyltin was administered
intraperitoneally it was eliminated as diethyl- and ethyltin in both
faeces and urine. Diethyltin was excreted in the bile (Bridges et al.,
1967). Rats that had initially received a diet containing triethyltin
and had retained approximately 0.7 mg triethyltin in their tissues
were then given a normal diet; 12 days later, no triethyltin could be
detected in the tissues (Cremer, 1957). The route of excretion was not
known.
The elimination of triphenyltin from the body is slow (Stoner,
1966). Its persistence in guineapigs is suggested by the similarity
between the amounts consumed by those dying on diets containing 25 and
50 mg/kg and the acute oral LD50. In studies on guineapigs using
113Sn-labelled triphenyltin, the concentration of triphenyltin that
had entered the brain after a single oral dose did not decrease during
the first 10 days. In rats, triphenyltin disappeared more rapidly,
having a half-time of about 3 days in the brain (Heath, 1963, 1965).
Triphenyltin acetate labelled with 113Sn was slowly excreted in the
urine of lactating sheep given oral doses of the compound at the rate
of 10 mg/day for 20 days. The milk confined small amounts of tin
(about 1 µg/litre) in both organic and inorganic forms (Herok & Götte,
1964).
The biological half-time of tricyclohexyltin hydroxide in rats was
5-40 days, when the compound had been included in the diet over a
prolonged period. The brain was one of the tissues from which the
compound was removed most slowly (FAO/WHO, 1971).
6.2.4 Biotransformation
In some early studies on the metabolic degradation of organotin
compounds, it was suggested that destannylation (carbon-tin bond
cleavage) was the major result of this biotransformation. For example,
triethyl tin was detected in the tissues of rabbits and rats given
tetraethyltin intravenously (Cremer, 1957, 1958). The liver was the
organ most active in this conversion. Bridges et al. (1967) reported
that dealkylation of diethyltin occurred in both the gut and tissues
of the rat. Although Herok & Götte (1963) found that after
administration of triphenyltin acetate labelled with 113Sn small
amounts of inorganic tin were present in the milk of sheep, Stoner
(1966) considered that triphenyltin was not readily transformed in the
body. Dicyclohexyltin oxide and trace amounts of cyclohexylstannoic
acid were identified as metabolites in rats and dogs treated with
tricyclohexyltin hydroxide (FAO/WHO, 1971). Blair (1975) concluded
that tricyclohexyltin hydroxide undergoes metabolism in animals by
scission of cyclohexyl groups from the atom.
More recent studies using tributyltin acetate showed primary
biological oxidation reactions in the hydroxylation of carbon-hydrogen
bonds that are alpha, ß, gamma and delta to the tin atom (Fish et al.,
1976). This type of reaction was already assumed by Casida et al.
(1971), although no carbon-hydroxylated metabolites could be
identified when triethyltin derivatives were biologically oxidized
in vitro to monoethyl derivatives.
Fish et al. (1975) treated tributyltin acetate with rat liver
microsomes (a mono-oxygenase enzyme system) in the presence of NADPH,
and obtained alpha and ß hydroxylated metabolites in yields of 24% and
50%, respectively. An in vivo study with [1-14C]-tetrabutyltin
showed the occurrence of similar reactions in mice (Kimmel et al.,
1977). The alpha-hydroxyl metabolite is unstable and undergoes a
cleavage reaction at pH = 7.4 to give 1-butanol and a dibutyltin
derivative. A ß-elimination reaction in acidic media transforms the
ß-hydroxyl derivatives rapidly into 1-butane and a dibutyl derivative
(Fish et al., 1976).
Studies on the in vitro metabolism of tricyclohexyltin compounds
indicated that carbon-hydroxylation of the cyclohexyl group was a
major metabolic reaction (Fish et al., 1976; Kimmel et al., 1977).
When [113Sn] -triphenyltin acetate was subjected to in vitro
biological oxidation, the phenyl group was not biotransformed, but in
in vivo studies, rats were found to metabolize triphenyltin acetate
into diphenyl- and phenyltin derivatives (Kimmel et al., 1977).
7. EFFECTS ON ANIMALS
7.1 Inorganic Tin Compounds
Although tin is present in small amounts in most animal and human
tissues, it is uncertain whether it is an essential element for
mammals. However, recent results indicate that tin is an essential
nutrient for the growth of the rat. Both inorganic and organotin
compounds at concentrations similar to those present in feeds were
found to stimulate growth rate in rats maintained on purified amino
acid diets (Table 11) (Schwarz, 1971; 1974; Schwarz et al., 1970). The
authors concluded that tin, as an essential element, could have a
function at the active site of some metal-dependent enzymes; however,
this has still to be confirmed.
Compared with most organotin derivatives, inorganic tin and its
salts are not highly toxic, mainly because of their poor absorption
and rapid tissue turnover (Barnes & Stoner, 1959; Cheftel, 1967;
Hiles, 1974; National Academy of Sciences, Washington 1973). The
systemic toxicity of some simple tin salts is difficult to assess
because of the irritant properties of their solutions.
7.1.1 Effects on the Skin
The effects of 1% tin(II) chloride and 0.25% tin(II) fluoride
solutions, applied to the abraded skin of rabbits, were examined by
Stone & Willis (1968). Both compounds induced intraepidermal pustules
with complete destruction of the epidermis but the stratum corneum
remained intact. No injury occurred when the solutions were applied to
intact skin.
7.1.2 Respiratory system effects
According to Robertson (1960), intratracheal administration of
50 mg of metallic tin dust to rats was well tolerated and no fibrosis
was produced within one year of exposure.
Exposure of guineapigs by inhalation, to tin(IV) chloride (3 mg/
litre for 10 min, daily, for "several months") produced only transient
irritation of the nose and eyes (Pedley, 1927).
Table 11. The effect of tin(IV) sulfate on the growth of rats in a trace-element-controlled
environmenta
Dose level No. of Average daily Increase
Compound (µg of tin/kg) animals weight gain (%) p-value
control -- 5b 1.10±0.05c -- --
tin (IV) sulfate 500 8 1.37±0.10 2 .02
tin (IV) sulfate 1000 8 1.68±0.10 53 .001
tin (IV) sulfate 2000 8 1.75±0.10 59 .001
a From: Schwarz et al. (1970).
b 2 control rats died during the 26-29 days of the experiment.
c mean ± standard error.
7.1.3 Effects on the gastrointestinal system
Soluble tin salts are gastric irritants. This explains the signs
of acute poisoning occasionally observed in man and in experimental
animals following consumption of food containing high concentrations
of tin (de Groot et al, 1973). However, the concentrations required to
elicit an acute gastrointestinal reaction have not been determined
reliably. Beney et al. (1971) reported signs of illness in 3/10 cats
after ingestion of 5 ml/kg body weight of fruit juice containing a tin
concentration of 1370 mg/litre, and in 1/11 cats receiving the same
dose of fruit juice containing tin at a concentration of 540 mg/litre.
However, no adverse effects were recorded in cats receiving a similar
amount of juice containing a tin concentration of about 500 mg/litre
or in dogs given juice with a tin concentration of 1400 mg/litre.
7.1.4 Effects on the liver
A 3-fold increase in haem oxygenase (EC 1.14.99.3)a activity in
the liver of rats was observed 16 h after a single subcutaneous
injection of tin(II) chloride dihydrate (SnCl22H2O) at doses ranging
from 5.6 to 56.4 mg/kg body weight. Cytochrome P-450 mediated drug
metabolism and the content of cytochrome P-450 were reduced by one
third. These effects on the liver increased by about 15-20%, when the
compound was administered intraperitoneally (Kappas & Maines, 1976).
Tin chloride, oxalate, or sulfate added to the diet at a
concentration of 10 g/kg for 4 and 13 weeks resulted in homogenous
liver cell cytoplasm and hyperplasia of the bile duct in rats. The
liver changes were more distinct after 13 weeks administration.
Similar, although milder, hepatic alterations were seen after
administration of tin chloride, oxalate, or orthophosphate at a
concentration of 3 g/kg of dietb (de Groot et al., 1973).
Increased incidence of fatty degeneration in the liver was noted
by Schroeder et al. (1968) in female but not in male rats, when the
animals were given tin in the form of tin(II) chloride at a dose of
5 mg/litre in drinking water, from weaning until their natural death.
a The numbers within parentheses following the names of enzymes
are those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
b Tables for the approximate conversion of concentrations in diet
to mg/kg body weight per day are given in Nelson (1954).
7.1.5 Effects on the kidney
Renal damage was produced in rats by single intravenous and
intraperitoneal doses of sodium pentafluorostannite (NaSn2F5) and
tin(II) chloride dihydrate (SnCl22H2O) (Conine et al., 1973, 1975;
Yum et al, 1976). A single intraperitoneal injection of sodium
pentafluorostannite at 35 mg/kg body weight or of tin(II) chloride
dihydrate at 44.4 mg/kg produced extensive necrosis of epithelial
cells mainly involving proximal tubules. The extent of the necrosis
was thought to be related to the tin moiety of the compound because
damage of the same magnitude could not be produced by administration
of sodium fluoride (Yum et al., 1976).
Studies in which rats received single subcutanous injections of
tin(II) chloride dihydrate in doses ranging from 5.6-56.4 mg/kg body
weight revealed a 20 to 30-fold increase in the haem oxidation
activity in the kidney, 16 h after administration. This effect was
already noticeable at the lowest dose (5.6 mg/kg) and was found to be
dose-related (Kappas & Maines, 1976).
Conine et al. (1976) administered daily oral doses of sodium
pentafluorostannite for 15 or 30 days to rats at rates of 20, 100, and
175 mg/kg body weight. The highest dose caused degenerative changes in
the proximal epithelium of the kidneys that were similar to those
observed in chronic fluoride poisoning (Lindemann et al., 1959), and
affected about 15-20% of the 30 rats that were killed. Most of the 8
animals in the 175 mg/kg group that died spontaneously during the
experiment displayed necrosis of the proximal tubular epithelium
similar to that previously reported to be caused by tin (Yum et al.,
1976).
Exposure of rats to tin(II) chloride at a concentration of 5 mg of
tin/litre of drinking water, for life, produced vacuolar changes in
the renal tubules of animals of both sexes (Schroeder et al., 1968).
7.1.6 Effects on the blood-forming organs
When tin(II) (as chloride, orthophosphate, sulfate, oxalate, or
tartrate) was given in the diet to Wistar rats of both sexes at
concentrations of 3 and 10 g/kg for 4 weeks, food intake was reduced,
growth was retarded and slight anaemia developed. The signs of anaemia
included a reduction in haemoglobin levels of about 10% and
corresponding reductions in haematocrit values, erythrocyte counts,
and serum iron concentration (de Groot et al., 1973). Dietary
supplements of iron had a markedly protective effect (de Groot, 1973;
de Groot et al., 1973). The authors suggested that tin compounds might
inhibit haemopoiesis, possibly by interfering with the intestinal
absorption of iron. An alternative mechanism by which tin salts could
cause mild gastrointestinal bleeding was not investigated.
A dose-related decrease in haemoglobin concentrations was noted in
rats after daily oral treatment, for 15 days, with sodium
pentafluorostannite at 100 and 175 mg/kg body weight. Administration
of 20 mg/kg per day did not affect the haemoglobin level (Conine et
al., 1976).
7.1.7 Central nervous system effects
High doses of inorganic tin compounds seem to affect the central
nervous system producing such effects as ataxia, muscular weakness,
and the depression of the central nervous system. These signs were
observed by Conine et al., (1975) after oral administration to rats of
sodium pentafluorostannite and tin(II) chloride dihydrate in single
doses of the order of the median lethal dose (LD50). According to de
Groot et al. (1973), feeding rats with tin(II) chloride at a
concentration of 10 g/kg diet for 13 weeks, resulted in a spongy state
of the white matter of the brain. Ataxia, hind-leg paralysis, and
death were also observed in rats by Mamontova (1940) following daily
subcutaneous injections of 3 mg of tin(II) citrate for 5-6 months.
7.1.8 Effects on the reproductive system and the fetus
There is one report indicating that tin(II) chloride might have an
effect on the reproductive system. De Groot et al. (1973) noted
testicular degeneration in rats after prolonged feeding with this
compound (10 g/kg of food, for 13 weeks).
Inorganic tin compounds have not been shown to be fetotoxic.
Theuer et al. (1971) gave groups of pregnant rats sodium
pentafluorostannite, sodium pentachlorostannite, and tin(II) fluoride
corresponding to tin levels in the diet of 125, 250, and 500 mg/kg.
The rats were killed on day 20 of gestation. No effects were seen in
the fetuses; tin concentrations in the fetuses were about 1 mg/kg
compared with approximately 0.65 mg/kg in control fetuses, indicating
a rather low transplacental transfer.
7.1.9 Carcinogenicity and mutagenicity
Few reports are available concerning the carcinogenicity of
inorganic tin compounds. Walters & Roe (1965) administered either
sodium chlorostannate at 1 or 5 g/litre of drinking water or tin(II)
oleate at 5 g/kg of diet to mice, for up to one year; the survivors
were then killed. The incidence of lymphomas, hepatomas, or pulmonary
adenomas did not increase with any of the regimens.
Roe et al. (1965) reported 3 malignant tumours in 30 August rats
that survived for 1 year or more on a diet containing sodium
chlorostannate at a concentration of 20 g/kg, whereas a control group
of 33 rats did not exhibit any case of malignant tumours. One tumour
was an adenocarcinoma of mammary origin, another a pleomorphic sarcoma
in the uterus, and the third, an adenoma-carcinoma in the jaw region.
The difference was not statistically significant. No tumours were seen
in another group of 27 rats surviving on a diet containing tin(II)
2-ethylhexoate at a concentration of 5-10 g/kg.
Administration of tin(II) chloride to rats and mice at 5 mg/litre
in drinking water throughout their life-time did not produce any
increase in the incidence of tumours compared with a control group
consisting of an equal number of animals (Kanisawa & Schroeder, 1969).
Studies concerning the mutagenicity of inorganic tin compounds
were not available to the Task Group,
7.1.10 Other effects
Growth retardation in rats has been reported after the
administration of high doses of various tin compounds. Dietary levels
of tin as tin(II) oxalate, orthophosphate, chloride, sulfate, and
tartrate at 3 and 10 g/kg for 4 weeks caused inhibition of growth in
rats and oedema and atrophy of the pancreas (de Groot et al., 1973).
Daily administration, by garage, of sodium pentafluorostannite at 100
and 175 mg/kg body weight, for 30 days, resulted in a dose-related
retardation of growth in rats (Conine et al., 1976). Administration of
tin(II) chloride at a concentration of 5 mg of tin per litre of
drinking water reduced the life span of female rats, whereas the
longevity of male rats was unaffected (Schroeder et al., 1968).
7.1.11 Effective doses and dose rates
7.1.11.1 Lethal doses
The median lethal doses (LD50) for 2 inorganic tin compounds are
given in Table 12. A marked difference between the oral and parenteral
LD50 values can be explained by the low absorption of tin compounds.
Daily intravenous administration of 3.3-4.2 mg of sodium tin
citrate per kg body weight provoked death in rabbits in 7-8 days,
whereas at a dose of 2.5 mg/kg, death occurred only after 15 or more
days (Mamontova, 1940).
In studies on 6 rats, subcutaneous injection of 3 mg of tin
citrate/animal, per day, killed all the animals in 5-6 months. Similar
administration of the same compound in doses of 2 mg/kg body weight
produced vomiting, diarrhoea, weight loss, ataxia, hind-leg paralysis,
and death within 5 months (Mamontova, 1940).
Table 12. Median lethal doses (LD50) of tin(II) chloride dihydrate (SnCl22H2O) and sodium
pentafluorostannite (NaSn2F5)a
Sodium pentafluorostannite
Tin(II) chloride dihydrate
Route male mice male rats female rats male rats (LD50, mg/kg)
(LD50, mg/kg) (LD50, mg/kg) (LD50, mg/kg)
intravenous 18.9 12.9 12.9 29.3
intraperitoneal 80.9 75.4 65.0 258.4
oral (fasted) --b 223.1 218.7 2274.6
(fed) 592.9 573.1 --b 3190.1
a From: Conine et al. (1975).
b no data.
7.1.11.2 Minimum effective and no-observed-effect doses
Some information on effective doses has been given in the sections
describing the effects. However, the following information may also be
of interest.
De Groot et al. (1973) reported a normal growth rate in Wistar
rats that had received tin(IV) oxide, tin(II) sulfide, or tin(II)
oleate at dietary levels up to 10 g/kg for 4 weeks; haematological
data were also normal except for an increase in the haematocrit in
male rats fed with the highest concentration of tin(II) sulfide. The
weight and the gross and macroscopic appearance of the liver, kidney,
heart, and spleen were also normal.
When a similar experiment was conducted with tin(II) chloride for
13 weeks, the no-observed-effect concentration in food appeared to be
1 g/kg, if the diet were supplemented with iron (de Groot et al.,
1973). This is equivalent to a dose rate of about 20-30 mg tin/kg body
weight per day, for 90 days.
No effect on growth was observed by Conine et al. (1976), when
sodium pentafluorostannite was administered orally to rats at a dose
rate of 20 mg/kg body weight per day for 30 days.
Oral administration of sodium tin citrate in dose rates ranging
from 100-150 mg/kg body weight per day for 1 year did not produce any
noticeable signs of poisoning in rats (Mamontova, 1940). Similarly
rats fed a diet containing either sodium chlorostannate at 20 g/kg or
tin(II) 2-ethyl hexoate at 5-10 g/kg for one year did not show any
pathological changes in the gastrointestinal tract, kidneys, or liver
(Roe et al., 1965).
Mice that received tin in the form of sodium chlorostannate at a
concentration of 1 or 5 g/litre in drinking water or tin in the form
of tin(II) oleate in the diet at a concentration of 5 g/kg during
their lifetime did not show any adverse effects (Waiters & Roe, 1965).
Mice and rats (Schroeder & Balassa, 1961; Schroeder et al., 1968)
given tin(II) chloride in drinking water at a concentration of 5 mg of
tin/litre grew normally throughout their life. The life span of mice
of both sexes and of male rats was not affected but that of female
rats was shorter and there was an increased incidence of fatty
degeneration of the liver (section 7.1.4). Vacuolar changes in the
renal tubules were apparent in rats of both sexes (section 7.1.5).
7.2 Organotin Compounds
Distinction should be made between the effects of di-, tri-, and
tetrasubstituted organotin compounds. The principal toxicological
difference is that some trisubstituted compounds have a specific
effect on the central nervous system producing cerebral oedema (Barnes
& Stoner, 1958; Torack et al., 1969), whereas disubstituted compounds
do not produce this effect but are potent irritants that can induce an
inflammatory reaction in the bile duct (Barnes & Magee, 1958).
Toxicologically, the tetrasubstituted compounds resemble
trisubstituted compounds, which are, generally, more toxic than the
mono- and disubstituted derivatives.
7.2.1 Effects on the skin and eyes
Dermal application of dibutyltin dichloride at 10 mg/kg body
weight per day for a period of 12 days, caused severe local damage and
also bile duct injury in rats and mice. Guineapigs were more
resistant, showing little reaction to daily applications of 120 mg/kg
on 5 successive days (Barnes & Stoner, 1958).
An aqueous solution of bis(tributyltin) oxide applied to the
shaved skin (30 x 60 mm) of rats at concentrations of 0.36-0.95 mg/kg
body weight produced slight local irritation lasting 2-3 weeks
(Pelikan & Cerny, 1968b). At higher doses (1.40-185 mg/kg), marked
inflammation developed into skin necrosis. Severe effects on the eyes
were also observed with bis(tributyltin) oxide (Pelikan & Cerny,
1969).
Triphenyltin hydroxide was reported not to irritate rabbit skin or
sensitize the skin of guineapigs (Marks et al., 1969). However, it was
found to be extremely irritating to the eyes, even after brief
exposure, and could cause corneal opacity (Marks et al., 1969).
A solution of triphenyltin acetate in oil at a dose of 150 mg/kg
body weight caused a skin reaction in rats (Klimmer, 1964). Doses of
tricyclohexyltin hydroxide at 1.2-60 mg/kg body weight per day,
applied to the skin of rabbits over a 3-week period, produced distinct
reactions locally but did not result in any systemic ill effects
(FAO/WHO, 1971, p. 527)a
7.2.2 Respiratory system effects
Pulmonary congestion and oedema were observed in rats after single
intravenous administrations of solutions of diethyl-, di-propyl-,
diisopropyl-, and dipentyltin dichloride (20 mg/kg body weight), in
0.05 ml of polyoxyethylene sorbitin monoleate, whereas in the case of
dibutyltin dichloride, 10 mg/kg was enough to cause this effect
(Barnes & Stoner, 1958). Petechial and ecchymotic haemorrhages in the
trachea and larynx were observed in sheep after intrarumenal
administration of tricyclohexyltin hydroxide at 500 mg/kg body weight.
Congestion was present in the dorsal portion of all pulmonary lobes at
a dose as low as 150 mg/kg (Johnson et al., 1975).
7.2.3 Effects on the gastrointestinal system
A single dose of butyltin trichloride, butyltin- S,S',S"-tris
(2-ethylhexylmercaptoacetate), butylstannoic acid, and butyl
thiostannoic acid at 4000 mg/kg body weight, administered by stomach
tube to mice, produced various degrees of submucosal, subserosal, and
intra-luminar gastrointestinal hemorrhage within 24 h (Pelikan &
Cerny, 1970b). Similar administration of dioctyltin- S,S'-bis
(2-ethylhexyl-mercaptoacetate) produced dilatation of the stomach,
which was filled with gas. The stomach walls appeared ischaemic
whereas the intestinal walls were hyperaemic, and traces of blood were
found in the contents of the small stomach. Similar, but more
pronounced findings were recorded after administration of dioctyltin-
a Unpublished reports on triphenyltin compounds and tricyclohexyltin
hydroxide were abstracted in 1970 Evaluations of Some Pesticide
Residues in Food (FAO/WHO, 1971). The proprietary data were
submitted to the WHO by the manufacturers. Page numbers given in the
text refer to the FAO/WHO publication, which also contains
information with reference to authors and manufacturers.
bis-(butylmercaptoacetate), although no traces of blood were seen in
the stomach. Treatment with dioctyltin bis(2-ethylhexyl-
mercaptoacetate) resulted in large amounts of liquid in the stomach
contents, and slightly hyperaemic stomach walls; other findings were
similar to those produced by the other 2 dioctyltin compounds,
dioctyltin bis(dodecylmercaptide) and octyltin tris(2-ethylhexyl-
mercaptoacetate) (Pelikan & Cerny, 1970a). Ingestion of dibutyltin
dichloride at a dose of 50 mg/kg body weight per day for one week,
caused a temporary dilatation of the stomach in rats due to
accumulation of fluid, and diarrhoea (Barnes & Stoner, 1958).
Intrarumenal administration of tricyclohexyltin hydroxide at 150 mg/kg
body weight resulted in fluid diarrhoea in sheep which persisted up to
death. Necropsy findings included mild enteritis and colitis
characterized by severe hyperaemia and oedema (Johnson et al., 1975).
Gastroenteritis occurred in rats receiving an oral dose of
tricyclohexyltin hydroxide of 25 mg/kg body weight per day for 19 days
(FAO/WHO, 1971, pp. 527-528).
7.2.4 Effects on the liver and bile duct
Steatosis of hepatocytes and enlargement of the liver were seen
within 24 h of administration to mice through a stomach tube, of a
single dose of butylstannoic acid, butyltin trichloride, butyltin
tris(2-ethylhexylmercaptoacetate), or butylthiostannoic acid, each at
a dose of 4000 mg/kg body weight (Pelikan & Cerny, 1970b). In rats, a
single oral dose of dibutyl,tin dichloride at 50 mg/kg body weight
produced congestion and inflammation especially in the lower part of
the bile duct (Barnes & Magee, 1958; Gaunt et al., 1968). When this
dose of dibutyltin dichloride was given on 3 successive days, the
lesion was more severe involving also the proximal part of the bile
duct and the portal blood vessels. Necrotic areas were seen in the
liver. In some cases, bile escaped from the injured duct into pancreas
and peritoneum. The authors considered that death occurring 5 days
after the 3 successive doses was the result of a general toxic effect
of this compound, whereas death occurring later was secondary to bile
duct and liver damage. Changes in the pancreas were inflammatory and
confined to areas surrounding the bile duct. In surviving rats,
examined 6-12 months after receiving 3 daily doses of 50 mg/kg, the
bile duct was shorter and thicker than normal with fibrosis in the
wall. A similar reaction in the bile duct was seen after a single
intravenous administration of 5 mg/kg body weight, and after a dermal
application of 10 mg/kg body weight. The effects on mice of oral doses
of dibutyltin dichloride at 20-50 mg/kg body weight were similar to
those seen in rats, although liver damage appeared to be more
widespread. Repeated doses of this compound at 20-50 mg/kg body weight
killed rabbits, but did not cause bile duct or liver injury;
guineapigs, however, tolerated such doses without any signs of adverse
effects (Barnes & Magee, 1958). It has been suggested that bile duct
injury occurs only in species in which the bile duct and the
pancreatic duct have a common course (Kimbrough, 1976).
Daily doses of dibutyltin dichloride at 0.1 and 1.0 mg/kg body
weight, for 6 months, caused intoxication in rabbits with dystrophic
changes in the liver (Mazaev & Korolev, 1969). Dioctyltim- S,S'-bis
(2-ethylhexylmercaptoacetate), diootyltin- S,S'-bis
(butylmercaptoacetate), and diootyltin bis(dodecyl-mercaptide) at an
oral dose of 4000 mg/kg body weight produced steatosis of the
hepatocytes in mice (Pelikan & Cerny, 1970a). Nikonorow et al. (1973)
reported a significant increase in the mean liver weight of rats after
oral administration of dioctyltin- S,S'-bis(isoctylmercaptoacetate)
at 20 mg/kg body weight per day, for 3 months.
A single oral dose of tributyltin acetate, benzoate, chloride,
laurate, or oleate at 500 mg/kg body weight produced fatty
infiltration of the liver in mice (Pelikan & Cerny, 1968a) and
triphenyltin acetate at a dietary level of 10 mg/kg produced fatty
degeneration of the liver in guineapigs, when administered over a
2-year period (FAO/WHO, 1971, p. 341). Cholangitis was reported in
rats after administration of tricyclohexyltin hydroxide at a dietary
level of 400 mg/kg for 90 days (Shirasu, 1970). Similarly, both intra-
and extrahepatic cholangitis was noted in rats that had received
tri-cyclohexyltin hydroxide at 25 mg/kg body weight per day, for 19
days (FAO/WHO, 1971, pp. 527-528).
7.2.5 Effects on the kidney
Some degree of fatty degeneration of the renal cortical tubular
epithelium was seen on histological examination of mice that had
received a single oral dose (4000 mg/kg body weight) of butyltin
tin- S,S',S"-tris(2-ethylhexylmercalytoacetate), butylstannoic acid,
or butylthiostannoic acid, but not in animals given butyltin
trichloride. The structure of the renal tissue was unchanged.
Macroscopically, all compounds produced slight hyperaemia in the
kidneys (Pelikan & Cerny, 1970b).
Dioctyltin- S,S'-bis(2-ethylhexylmercaptoacetate) and
dioctyltin- S,S'-bis(butylmercaptoacetate) administered at the same
rate and by the same route produced a similar slight fatty
degeneration of the renal cortical tubular epithelium in mice (Pelikan
& Cerny, 1970a). Feeding rats with an organotin stabilizer,
dioctyltin- S,S'-bis(isooctyl mercaptoacetate) at a dietary level of
200 mg/kg for 12 months produced an increase in kidney weight in
female rats only (Nikonorow et al., 1973). Mazaev & Korolev (1969)
also reported dystrophic changes in the kidneys in rats treated with
dibutyltin dichloride at 0.1 and 1.0 mg/kg body weight.
Haemorrhages were reported in the kidneys of mice after single
administrations (gavage) of tributyltin benzoate, chloride, laurate,
and oleate at 500 mg/kg body weight. Hyperaemia of the kidney was seen
in all groups, whereas tubular cells containing lipids were observed
only in mice that had received laurate or oleate. Renal changes were
not reported in mice that were similarly dosed with tributyltin
acetate (Pelikan & Cerny, 1968a). Congestion of the glomeruli and mild
toxic nephrosis were observed in rats receiving a dietary level of
tricyclohexyltin hydroxide of 400 mg/kg for 3 months (Shirasu, 1970).
Similarly, toxic nephrosis was reported in rats after administration
of tricyclohexyltin hydroxide at a daily dose of 25 mg/kg body weight
for 19 days (FAO/WHO, 1971, pp. 527-528).
7.2.6 Effects on the lymphatic tissues and immunological effects
Small necrotic areas were observed in the germinal centres of the
splenic follicles of mice after a single administration, through a
stomach tube, of 4000 mg/kg body weight of butyltin- S,S',S"-tris-
(2-ethylhexylmercaptoacetate), butylthiostannoic acid, and
butyl-stannoic acid, (Pelikan & Cerny, 1970b); dioctyltin- S,S'-bis
(2-ethyl-hexylmercaptoacetate), and dioctyltin- S,S'-bis(butyl-
mercaptoacetate) (Pelikan & Cerny, 1970a).
Seinen & Willems (1976) reported that the main action of
di-octyltin dichloride, given to rats at dietary levels of 50 and
150 mg/ kg, was on the thymus, the weight of which was significantly
reduced during the experiment. In subsequent experiments, feeding rats
with dioctyltin dichloride or dibutyltin dichloride at dietary levels
of 50, or 150 mg/kg for 6 weeks, resulted in a dose-dependent
reduction in the weight of the thymus Land thymus-dependent peripheral
lymphoid organs (Seinen et al., 1977a). The delayed type
hypersensitivity was decreased and the allograft rejection was delayed
when the same compounds were given in the same way to rats.
Furthermore, it was reported that both these compounds decreased the
survival rate of rat and human thymocytes in in vitro studies
(Seinen et al., 1977b). The effects of diethyltin dichloride and
dipropyltin dichloride on rat lymphoid organs were similar but less
pronounced. However, other dialkyltin compounds such as dimethyltin
dichloride, didodecyltin dibromide, and dioctadecyltin dibromide, as
well as octyltin trichloride, trioctyltin chloride, and tetraoctyltin
did not cause atrophy of lymphoid tissues (Seinen et al., 1977a). The
rat proved more sensitive to the action of di-octyltin dichloride and
dibutyltin dichloride on lymphoid organs than the mouse and the
guineapig in which no atrophy of the lymphoid organs was produced.
(Seinen et al., 1977a).
Studies on triphenyltin acetate have been reviewed by the FAO/ WHO
(1971, pp. 327-366). In guineapigs, a dietary level of 15 mg/kg for 77
days produced a decrease in plasma cells in the spleen and in the
mesenteric, cervical, axillary, and popliteal lymph nodes. After
feeding the same dose to female guineapigs for 104 days, a reduction
in immune response to tetanus toxoid stimulation was observed. In
another experiment, a decrease in the number of lymphocytes and
leukocytes accompanied by histological changes in the lymphatic
tissues, such as atrophy of the white pulp of the spleen, was seen in
guineapigs at dietary levels of 5 mg/kg in females and 10 mg/kg in
males administered, for 12 weeks. A decrease in the number of
leukocytes was also reported in dogs after 8 weeks on a dietary level
of triphenyltin hydroxide of 25 mg/kg and 8 weeks on 50 mg/kg.
7.2.7 Haematological effects
Decreases in the numbers of erythrocytes and reticulocytes were
reported in rats after daily administration of an oral dose of
dibutyltin sulfide at 0.1 mg/kg body weight for 6 months (Mazaev &
Slepning, 1973). A mild anaemia was noted in rats that had received
dibutyltin dichloride for 3 months at a dietary level of 80 mg/kg, but
a level of 40 mg/kg did not produce this effect (Gaunt et al., 1968).
In vitro studies of the haemolytic activity of trialkyltin
compounds indicated that the most haemolytic organotin compounds were
the alkyltin derivatives with alkyl groups of 3-6 carbon atoms
(Byington et al, 1974). Decreases in the percentage haemoglobin and in
the number of erythrocytes were reported in guinea-pigs treated with
triphenyltin acetate at a dietary level of 10 mg/kg for 4 months
(FAO/WHO, 1971, p. 337). A reduction in haemoglobin and leukocytes was
also seen in rats after 12 weeks on a dietary level of triphenyltin
hydroxide of 50 mg/kg (FAO/WHO, 1971, pp. 337-333).
7.2.8 Central nervous system effects
Intoxication in animals by lower trialkyltin compounds is
manifested as generalized weakness progressing to paralysis, sometimes
accompanied by generalized tremor. In rabbits, typical reactions to a
lethal dose (5 mg/kg body weight) of triethyltin sulfate administered
intravenously are prostration, flaccid paralysis, and encephalopathy
(Stoner et al, 1955). These responses resemble those observed in human
subjects, accidentally poisoned with triethyltin compounds. Daily
intraperitoneal injections of triethyltin sulfate at 5 mg/kg body
weight in saline solution resulted in death in rats within 3 days.
Diffuse haemorrhagic encephalopathy was found in the brain (Suzuki,
1971). Rats maintained on a dietary level of triethyltin hydroxide of
20 mg/kg displayed weakness in the hind legs after one week. The
weakness reached a maximum at 3-4 weeks, when about half of the rats
died, but the signs disappeared in one week, when normal diet was
restored. Some of the rats showed signs of becoming resistant to the
substance. At a dietary level of 40 mg/kg, the recovery process was
unaltered but, at 80 mg/kg, the rats developed generalized muscular
tremors that resembled those seen in acute trimethyltin poisoning
(Stoner et al, 1955). This course of events, including the development
of resistance to triethyltin hydroxide at a dietary level of 20 mg/kg
was corroborated by Magee et al. (1957), who was also able to produce
a specific lesion of the central nervous system (CNS) by feeding rats
with a dietary level of triethyltin hydroxide of 20 mg/kg. The lesion
consisted of an oedema extending throughout the white matter. It was
microscopically visible after 3 days of exposure and progressed to a
maximum at about 2 weeks. After 4 months of normal diet, the changes
in the brain and spinal cord disappeared. Electronmicroscopic studies
of this lesion in rabbits disclosed that the myelin sheaths split to
form clefts and vacuoles in which the fluid responsible for the oedema
accumulated (Aleu et al., 1963). The triethyltin-induced lesion of the
CNS is different from those produced by alkyl derivatives of lead,
antimony, bismuth, and mercury, which cause damage to the nerve cells,
but appears to be similar to that produced in rats by exposure to
hexachlorophene (Kimbrough & Gaines, 1971). When 5 mg of triethyltin
sulfate per litre of drinking water was administered to newborn rats
and their mothers for 4 months, all young rats were asymptomatic,
whereas the mothers showed paralysis of posterior limbs, severe
cerebral oedema, and status spongiosus of the white matter (Suzuki,
1971). Hedges & Zaren (1969) described papilloedema in monkeys
following a single intraperitoneal dose of triethyltin acetate of
0.1 mg/kg, body weight administered as a 5% solution. Oedema of the
white matter of the brain extended through the chiasma and orbital
optic nerve to the retrolaminar area, but the papilloedema was not due
to extension of the intramyelinic tin oedema of the nerves through the
lamina. Papilloedema does not develop in the cat when similarly
treated, in spite of profound swelling of the intracerebral white
matter, chiasma, and the orbital optic nerve as far as the
retrolaminar portion.
Tricyclohexyltin hydroxide appears to be a depressant for the
central nervous system, although it is not as potent as triethyltin
acetate and cerebral oedema does not occur (FAO/WHO, 1971, p. 526).
Johnson et al. (1975) studied the acute toxicity of tricyclohexyltin
hydroxide in sheep by intrarumenal injection of doses ranging from 15
to 750 mg/kg body weight. Central nervous depression was observed at
50 mg/kg body weight.
Because of the enzymatic conversion of tetraalkyltin compounds by
hepatic microsomal enzymes (section 6.2.4), these compounds act in a
similar way to trialkyltin compounds. The toxicity of tetraalkyltin
compounds in mice and clogs was studied by Caujolle and his colleagues
(1954). Tetraethyltin was the most active, tetramethyltin slightly
less toxic, and for the higher members of the series, the toxicity
decreased with increasing molecular weight. The toxic effects of
tetramethyltin differed from those of the other members of the series
and the dominant findings were tremors and hyperexcitability. The
major effects of the other members of the tetraalkyltin series were
muscular weakness and paralysis followed by respiratory failure.
Development of the signs of poisoning were noticeably slow following
administration of tetraalkyltin compounds and death was often delayed.
Intravenous administration of tetraethyltin at 25 mg/kg produced a
slight increase in the respiratory rate and vasodilatation as
immediate effects, but after 1.5-2 h, prostration with muscular
weakness was noted. The later effects resembled those seen after
administration of triethyltin; the mode of death was also similar
(Stoner et al., 1955).
7.2.9. Effects on reproduction and the fetus
Few studies of the effects of disubstituted organotin compounds on
reproduction have been reported. Nikonorow et al. (1973) reported
toxic effects of dioctyltin- S,S'-bis(isooctylmercaptoacetate) on the
embryo and fetus at daily oral doses of 20 and 40 mg/kg body weight,
administered for about 3 months. A distinct increase in the incidence
of fetal deaths was observed. Teratogenic effects were not found.
Investigations on triphenyltin hydroxide have yielded variable
results. In 2 investigations on rats, oral dose rates of 20 mg/kg per
day for less than 4 weeks produced histologically evident
abnormalities in the testes and ovaries. Diminution in the size of the
testes and less advanced maturation of the germinal epithelium was
seen in rats receiving triphenyltin hydroxide at a rate of 5 mg/kg for
90 days, although a 3-generation reproduction study at this dietary
level failed to demonstrate any adverse effect on the reproductive
indices (FAO/WHO, 1971, pp. 332-333). In another experiment (Gaines &
Kimbrough, 1968), dietary levels of up to 200 mg/kg over a 276-day
period caused a reversible reduction in fertility in male rats which
was considered to be due to a pronounced decrease in food intake, that
was later reversed. No gross abnormalities were seen in the offspring
of these males when mated with untreated females. In 3 later
experiments (FAO/WHO, 1971, p. 333) in which weanling animals were fed
the compound in their diets at doses of up to 25 mg/kg, the previously
reported change in testicular development could not be confirmed.
In a 3-generation study in which rats produced 2 litters per
generation, animals continuously received a dietary level of
tricyclohexyltin hydroxide of up to 100 mg/kg (corresponding to 4-6 mg
test compound/kg body weight per day for an adult rat). No evidence of
any adverse effects on reproduction could be found and examination of
the fetuses did not reveal any indication of teratogenic effects
(FAO/WHO, 1971, p. 524). No evidence of teratogenic effects from
tricyclohexyltin hydroxide was obtained in a study in which pregnant
rabbits received up to 3 mg/kg body weight per day from the 8th to
16th day of gestation (FAO/WHO, 1971, p. 524). In a study in which
diets containing tricyclohexyltin hydroxide were fed to Japanese
quail, no evidence of ill effects on egg fertility or hatchability was
seen at a dietary level of 1 mg/kg.
Effects observed at a dietary level of 10 mg/kg were of doubtful
significance but a dietary level of 100 mg/kg had definite effects on
fertility, egg production, hatchability, and embryonic mortality
(FAO/WHO, 1971, pp. 523-524).
7.2.10 Carcinogenicity
In an 18-month study, mice were given an oral dose (stomach tube)
of 0.46 mg/kg body weight per day of triphenyltin acetate between the
ages of 7 and 28 days and thereafter a dietary level of 1206 mg/kg.
There was no statistically significant increase in tumours compared
with a control group (Innes et al., 1969). In a 2-year study on rats
receiving tricyclohexyltin hydroxide at concentrations up to 12 mg/kg
body weight (section 7.2.3), the pattern of tumour incidence
throughout both the control and test groups appeared to be random and
did not suggest a dose-response relationship (FAO/WHO, 1971, p. 527).
Reports of experiments specifically designed to investigate the
carcinogenicity of other compounds were net available to the Group.
7.2.11 Effects on chromosomes
Mazaev & Slepnina (1973) reported an increased percentage of
chromosomal aberrations in bone marrow cells and also an increased
mitotic index in cells of the small intestine mucosa of rats that had
received dibutyltin sulfide at 0.1 mg/kg body weight per day
administered by intubation over a period of 6 months. Doses of less
than 0.1 mg/kg body weight per day did not cause poisoning and did not
have any effect on the chromosomes or somatic cells.
7.2.12 Other effects
A reduction in weight gain, mainly related to reduced food intake,
has been recorded in various species given organotin compounds. In
rats, a dietary level of dibutyltin dichloride of 80 mg/kg
administered for 90 days caused growth retardation and decreased food
intake (Gaunt et al., 1968). Triphenyltin acetate given to guineapigs
at dietary levels exceeding 5 mg/kg for 2 years, caused a dose-related
inhibition of growth rate (FAO/WHO, 1971, p. 341). A dietary level of
tricyclohexyltin hydroxide of 25 mg/kg for 90 days, caused a slight
reduction in weight gain in female rats (Shirasu, 1970), and dogs
receiving the same compound in their diet at a level of 12 mg/kg for 6
months also lost weight; some animals that totally refused the food
died from starvation (FAO/WHO, 1971, pp. 526-527).
Severe cardiovascular changes were demonstrated electro-
cardiographically in sheep after intrarumenal administration of
tricyclohexyltin hydroxide at doses greater than 150 mg/kg body weight
(Johnson et al., 1975).
7.2.13 Mechanisms o[ action
There have been relatively few studies of the biochemical effects
of dialkyltin compounds, which do not cause cerebral oedema. In acute
toxicity experiments on the rat, diethyltin compounds did not affect
the sodium and potassium contents of the central nervous system but
caused a decrease in its water con, tent (Magee et al, 1957).
The action of a homologous series of disubstituted
organotin-compounds from dimethyl- to dioctyltin have been examined;
most of them inhibit mitochondrial respiration by preventing the
oxidation of keto acids, presumbly via the inhibition of alpha-keto
oxidase activity, leading to the accumulation of pyruvate (Aldridge,
1976; Piver, 1973).
Of the trisubstituted compounds, triethyltin compounds have been
the most closely examined in relation to the mechanism by which they
cause ill effects. Trimethyl- and triethyltin compounds are potent
inhibitors of oxidative phosphorylation in the mitochondria for which
these compounds have a high binding affinity (Aldridge, 1958; Aldridge
& Street, 1964, 1970, 1971). In a later study by Aldridge (1976),
triorganotin compounds were stated to derange mitochondrial function
in 3 different ways, namely by secondary responses caused by discharge
of a hydroxyl-chloride gradient across mitochondrial membranes, by
interaction with the basic energy conservation system involved in the
synthesis of ATP, and by an interaction with mitochondrial membranes
causing swelling and disruption.
Using a 113Sn-labelled triethyltin compound, Rose (1969)
identified a pair of histidine residues on guineapig liver
mitochondria as the binding site for triethyl compounds. Thus, one
molecule of rat haemoglobin binds 2 molecules of triethyltin; the
binding sites are located in the globin.
Stockdale et al. (1970) showed that the order of effectiveness for
the organotin compounds in inhibiting coupled respiration was tributyl
> tripropyl > triphenyl > trimethyl. Two separate effects were
suggested: (a) an oligomycin-like inhibition of coupled
phosphorylation; and (b) an alteration of hydroxide exchange across
lipid membranes producing uncoupling, swelling, and reduction of
intramitochondrial substrate and phosphate concentrations followed by
structural damage.
7.2.14 Effective doses and dose rates
7.2.14.1 Lethal doses
The median lethal doses for some mono-, di-, tri-, and
tetrasubstituted organotin compounds are given in Tables 13, 14, 15,
and 16.a Trimethyl and triethyltin compounds, administered orally,
are more toxic than the higher homologues of the trialkyltin group.
The oral toxicity diminishes progressively from tripropyltin to
trioctyltin compounds (Barnes & Stoner, 1958). This is probably
because of poorer absorption of higher trialkyltin compounds from the
gastrointestinal tract, as judged from the difference between the oral
and intraperitoneal toxicity of higher trisubstituted alkyltin
compounds (Stoner et al., 1955). The intraperitoneal toxicity of
various trialkyltin compounds does not differ to any large extent. In
rats, Stoner et al. (1955) found that triethyltin sulfate was equally
toxic after intravenous, intraperitoneal, and oral administration. The
lethal dose per kg body weight given intravenously or
intraperitoneally was 10 mg/kg, causing death within 4-5 days. A dose
of 40 mg/kg body weight killed the rat in 2 h. The oral LD50 for
tricyclohexyltin hydroxide (Table 15) for most species, appears to be
between 100 and 1000 mg/kg body weight, while the intraperitoneal and
intravenous routes yield LD50 values below 20 mg/kg. The difference
between oral and parenteral toxicity reflects the poor absorption of
tricyclohexyltin hydroxide from the gastrointestinal tract.
In rats and dogs fed with tricyclohexyltin hydroxide, the
metabolites, dicyclohexyltin oxide and traces of cyclohexylstannoic
acid have been identified. Dicyclohexyltin oxide and
cyclohexylstannoic acid also constitute a small portion of the
residues on fruit as a result of photodecomposition (FAO/WHO, 1971,
pp 522, 534). The median lethal dose of these metabolites may
therefore be of interest. The LD50 of dicyclohexyltin oxide in the
rat appears to be about 350 mg/kg body weight, whereas the LD50 value
of cyclohexylstannoic acid in this species is probably ten times
higher (FAO/WHO, 1971, pp. 524-526).
Only a small amount of data is available concerning the acute
toxicity of tetraalkyltin compounds. The median lethal dose for
tetraethyltin is given in Table 16.
a Additional data on the toxicity of organotin compounds may be
found in a proposal for the United Nations classification and hazard
grouping of organotin compounds submitted by the expert from the
Netherlands to the Committee of Experts on the Transport of Dangerous
Goods of the United Nations Economic and Social Council
(EN/CN.2/CONF.5/R.607 of November 1976).
Table 13. Acute oral toxicity of some mono-organotin compounds in various animal species
Medium lethal
Trivial name of compound dose Species Reference
(LD50, mg/kg)
butylstannoic acid > 6000 mouse Pelikan & Cerny (1970b)
butyltin trichloride 1400 mouse
2140 rat Marhold (1972)
butyltin-S,S'S"-tris 1520 mouse Pelikan & Cerny (1970b)
(isooethylmercaptoacetate)
octyltin trichloride 4600 mouse Klimmer (1969)
octyltin-S,S',S"-tris 1500 rat (male) Pelikan & Cerny (1970a)
(2-ethylexylmercaptoacetate)
7.2.14.2 Minimum effective and no-observed-effect doses
When dibutyltin dichloride was fed to rats for 90 days at dietary
levels of 10, 20, 40, and 80 mg/kg, the no-observed effect level was
40 mg/kg, equivalent to 2 mg/kg body weight per day (Gaunt et al.,
1968). When dibutyltin was administered to rats for 6 months, the
no-observed-effect level was 20 mg/kg diet (Barnes & Stoner, 1958).
Dibutyltin sulfide, administered to rats at oral doses of 1.0, 0.1,
0.01, and 0.001 mg/kg body weight, per day for 7 months, was tolerated
without any detected adverse effects up to a dose o.f 0.01 mg/kg per
day but the higher levels of 0.1 and 1.0 mg/kg per day caused
intoxication (Mazaev & Korolev, 1969). By comparison, the toxicity of
dioctyltin compounds was relatively low (Klimmer, 1969). Thus,
dioctyltin did not produce any injury in rats, mice, or guineapigs at
oral doses up to 400 mg/kg body weight per day when given for 3-4
successive days, nor were there any ill effects when it was added to
the daily diet of rats for 4 months at a rate of 200 mg/kg (Barnes &
Stoner, 1958).
Table 14. Acute toxicity of some diorganotin compounds in various animals
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dibutylin di(2-ethylhexoate) 200 (oral) rat (male) Calley et al. (1967)
dibutyltin di(butyl maleate) 120 (oral) rat Klimmer (1969)
dibutyltin di(nonylmaleate) 170 (oral)
dibutyltin dichlorlde 100 (oral) rat (male) Klimmer (1969)
182 (oral) rat (male) Mazaev et al. (1971)
112 (oral) rat (female)
35 (oral) mouse
190 (oral) guineapig
150 (oral) rat Marhold (1972)
dibutyltin-S,S'-bis 150 (oral) rat (male) Klimmer (1969)
(2-ethylhexylmercaptoacetate) 175 (oral) Klimmer (1969)
dibutyltin dilaurate 243 (oral) rat Marhold (1972)
45 (oral) rat Marhold (1972)
dibutyltin oxide 520 (oral) rat (male) Klimmer (1969)
39.9 (i.p.)a rat (female) Robinson (1969)
24 (oral) mouse Mazaev & Slepnina (1973)
dibutyltin sulfide 145 (oral) rat (male)
150 (oral) rabbit
180 (oral) rat (female)
800 (i.p.) rat (female) Robinson (1969)
> 800 (i.p.) rat (female) Robinson (1969)
dioctyltin acetate 2030 (oral) rat Klimmer (1969)
diocyltin dibutylmaleate 3750 (oral) mouse Pelikan et al. (1970)
dioctyltin-S,S'-bis
(butylmercaptoacetate) 1140 (oral) white mouse Pelikán & Cerny (1970a)
dioctyltin bis
(dodecylmercaptide) 4000 (oral) white mouse
dioctyltin bis
(2-ethylhexylmaleate) 2760/ (oral) rat (male) Klimmer (1969)
2750
Table 14. (contd).
Medium lethal dose
Trivial name of compound (LD50, mg/kg) Species Reference
dioctyltin-S,S'-bis 2010 (oral) white mouse Pelikan & Cerny (1970a)
(2-ethylhexylmercaptoacetate)
dioctyltin-S,S'-bis 3700 (oral) rat (male) Klimmer (1969)
(laurylmercaptoacetate)
dioctyltin-S,S'-(1,4- 2950 (oral) rat (male)
butane-diol-bis-mercaptoacetate)
dioctyltin dichloride 5500/
8500 (oral) rat (male)
dioctyltin dilaurate 6450 (oral) rat (male)
800 (i.p.) rat (female) Robinson (1969)
dioctyltin di(1,2-
propylene-glycolmaleate) 4775 (oral) rat (male) Klimmer (1969)
dioctyltin-S,S'-
(ethyleneglycol-bis-
dimercaptoacetate) 880 (oral) rat (male)
dioctyltin maleate 4500 (oral) rat (male)
dioctyltin
ß-mercapto-propanoate 1850/
2050 (oral) rat (male)
dioctyltin oxide 2500 (oral) rat (male)
dioctyltin mercaptoacetate 945 (oral) rat (male)
a intraperitoneal
Table 15. Acute toxicity of some triorganotin compounds in various animals
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
2-trichloro-1-(butine-1'-
oxide)-1-triethyl- 9.8 (i.p.) rat Ignatjeva (1968)
stannyloxy)ethane 9.6 (i.p.) mouse Ignatjeva (1968)
triethylstannylmethyl-
(1-propynyl)formal 10.7 (i.p.) rat Ignatjeva (1968)
triethylstannylphenyl-
acetylene 9.9 (i.p.) mouse Ignatjeva (1968)
9.1 (i.p.) rat Ignatjeva (1968)
1-triethylstannyl-3-
trimethylsiloxi-1-
propyne 11.8 (i.p.) mouse Ignatjeva (1988)
11.4 (i.p.) rat Ignatjeva (1968)
triethyltin acetate 4 (oral) rat (female) Stoner (1966)
triethyltin chloride 5 (i.p.) rat (female) Robinson (1969)
triethyltin sulfate 5.7 (i.p.) rat (male) Stoner (1966)
5.3 (i.p.) guineapig Stoner (1966)
tributyltin acetate 46 (oral) white mouse Pelikan & Cerny (1968a)
99 (oral) J. Pharm. Sci. (1967)
133 (oral) rat (male) Klimmer (1969)
tributyltin benzoate 108 (oral) white mouse
Pelikan & Cerny (1968a)
132 (oral) rat Arzneimittelforsch. (1969)
tributyltin chloride 117 (oral) white mouse Pelikan & Cerny (1968a)
129 (oral) rat Manhold (1972)
tributyltin laurate 180 (oral) white mouse Pelikan & Cerny, (1968a)
tributyltin oleate 195 (oral) rat Klimmer (1969)
tributyltin salicylate 137 (oral) rat (male) Klimmer (1969)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
bis(tributyltin oxide) 112- (oral) rat (male) Klimmer (1969)
132
180 (oral) rat Truhaut et al. (1976)
234 (oral) rat Sheldon (1975)
194 (oral, rat (male) Elsea & Paynter (1958)
aqueous
solution)
148 (oral, rat (male) Elsea & Paynter (1958)
oil
solution)
11.7 (dermal) albino rabbit Elsea & Paynter (1958)
10.0 (oral) white mouse Pelikan & Cerny (1969)
trihexyltin acetate 1000 (oral) rat Barnes & Stoner (1958)
trioctyltin chloride 10 000 (oral) rat (male) Klimmer (1969)
triphenyltin acetate 21 (oral) guineapig Klimmer (1963)
24 (oral) guineapig Kimbrough (1976)
136 (oral) rat Klimmer (1963)
81 (oral) mouse (male) FAO/WHO (1971)
7.9 (i.p.) mouse (male) Stoner (1966)
136 (oral) rat (male) Klimmer (1964)
491 (oral) rat (female) Stoner (1966)
450 (dermal) rat (male) Klimmer (1963)
8.5 (i.p.) rat (female) Stoner (1966)
11.9 (i.p.) rat (female) Stoner (1966)
13.2 (i.p.) rat (male) Klimmer (1964)
21 (oral) guineapig
(male) Klimmer (1964)
3.7 (i.p.) guineapig
(male) Stoner (1966)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
triphenyltin acetate 5.3 (i.p.) guineapig
cont'd. (male) Klimmer (1964)
30- (oral) rabbit (male) Klimmer (1964)
50
triphenyltin chloride 80 (oral) rat (male) FAO/WHO (1971)
135 (oral) rat (female) FAO/WHO (1971)
triphenyltin hydroxide 245 (oral) mouse (male) FAO/WHO (1971)
209 (oral) mouse (female)
FAO/WHO (1971)
240 (oral) rat (male) Gaines & Kimbrough (1968)
360 (oral) rat (female) Gaines & Kimbrough (1968)
27.1 (oral) guineapig FAO/WHO (1971)
(male)
31.1 (oral) guineapig FAO/WHO (1971)
(female)
171 (oral) rat (male) Marks et al. (1969)
268 (oral) rat (female) Marks et al. (1969)
tricyclohexyltin 710a (oral) mouse- FAO/WHO (1971)
hydroxide peromyscus
1070a (oral) mouse-swiss FAO/WHO (1971)
white
540 (oral) rat FAO/WHO (1971)
13 (i.p.) rat FAO/WHO (1971)
780 (oral) guineapig FAO/WHO (1971)
Table 15. (contd).
Trivial name Medium lethal dose
of compound (LD50, mg/kg) Species Reference
tricyclohexyltin 9 (i.p.) guineapig FAO/WHO (1971)
hydroxide 500- (oral) rabbit FAO/WHO (1971)
cont'd. 1000
> 126 (i.p.) rabbit FAO/WHO (1971)
150a (oral) sheep Johnson et al. (1975)
14 (i.v.)c dog FAO/WHO (1971)
6 (i.v.) cat FAO/WHO (1971)
a approximate lethal dose
b intraperitoneal
c intravenous
Table 16. Acute oral toxicity of some tetraorganotin compounds in various animal species
Trivial name Medium lethal dose Species Reference
of compound (LD50, mg/kg)
tetraethyltin 40.0 mouse Skackova (1967)
15.0 rat
40.0 guineapig
7.0 rabbit
40 mouse Mazaev et al. (1971)
9.0 rat
40.0 guineapig
7.0 rabbit
tetrabutyltin 6000 rat Skackova (1967)
Data on effective doses of trisubstituted organotin compounds are
available only for triphenyltin derivatives and tricyclohexyltin
hydroxide. Triphenyltin acetate was administered, by gavage, to groups
of rats at doses equivalent to dietary levels of 5, 10, 25, and
50 mg/kg for up to 17 days. While no adverse effects were found at the
25 mg/kg level, the 50 mg/kg level resulted in the death of animals,
mainly as a result of infection (Klimmer, 1964). In a 2-year study on
rats fed diets containing concentrations of triphenyltin hydroxide of
up to 10 mg/kg, the no-observed-effect level was about 2 mg/kg
(equivalent to about 0.1 mg/kg body weight per day) (FAO/WHO, 1971,
pp. 341-342). In another 2-year study in which groups of guineapigs
were fed diets containing concentrations of triphenyltin acetate, up
to 200 mg/kg, the no-observed-effect level was 5 mg/kg. At higher
levels there was a dose-related increase in mortality and the
occurrence of other effects such as inhibition of growth rate and
fatty degeneration of the liver and heart (FAO/ WHO, 1971, p. 341).
With respect to tricyclohexyltin hydroxide, no toxic effects were
noted in rats receiving 12.5 mg/kg body weight daily for 19 days,
whereas a rate of 25 mg/kg per day caused toxic effects (FAO/WHO,
1971, pp. 527-528). Rats were fed on diets providing up to 12 mg/kg
body weight per day of tricyclohexyltin hydroxide in a 2-year study;
the no-observed-effect level was 3 mg/kg body weight per day (FAO/WHO,
1971, p. 528). When dogs were fed tricyclohexyltin hydroxide in the
diet at concentrations of up to 12 mg/kg body weight per day for 6-24
months, many dogs refused the 12 mg/kg per day diet, causing weight
loss and death from starvation. The no-observed-effect level was found
to be about 0.75 mg/kg per day (FAO/WHO, 1971, pp. 526-527). Johnson
et al. (1975) also reported the effects of thoroughly wetting the skin
of yearling cattle, goats, and sheep with suspensions of
tricyclohexyltin hydroxide. They found that cattle tolerated
suspensions up to a concentration of 5 g/litre while anorexia was
registered in some animals after spraying with a suspension of
10 g/litre. Yearling goats and sheep tolerated suspensions of up to
1 g/litre. Transitory anorexia and eye irritation developed after
application of a suspension of 20 g/litre to the skin of goats.
Few data are available on the effective dose levels of
tetrasubstituted organotin compounds. Intravenous administration of
tetraethyltin at 25 mg/kg body weight produced a slight increase in
respiratory rate and vasodilatation as immediate effects, but, 1.5-2 h
later, prostration with muscular weakness occurred. (Stoner et al.,
1955).
8. EFFECTS ON MAN
There are comparatively few clinical observations and
epidemiological data concerning the effects of inorganic tin compounds
on man and even fewer on the effects of organotin compounds.
8.1 Inorganic Tin Compounds
8.1.1 Acute poisoning
Some episodes of acute poisoning have been reported, mainly in
association with the ingestion of fruit juices containing high
concentrations of tin. The major symptoms and signs noted were nausea,
vomiting, diarrhoea, fatigue, and headache. The concentrations of tin
in the products thought to have been associated with the incidents
were uncertain in many cases, but were probably in the range of
300-500 mg/kg (Horio et al, 1967). Orange and apple juice, containing
tin concentrations of 250-385 mg/kg were suspected of causing one
incident (Benoy et al., 1971). Nausea, vomiting, and diarrhoea were
recorded in individuals consuming-peach preserves that contained a tin
concentration of 563 mg/kg, zinc at 1.5 mg/kg, cadmium at 0.1 mg/kg,
lead at 0.16 mg/kg, copper at 1 mg/kg, nitrate at 93 mg/kg, nitrite at
1.7 mg/kg, and chloride at 115 mg/kg (Nehring, 1972). Acute
gastroenteritis followed ingestion of a fruit punch stored in a tin
can and containing a tin concentration of 2000 mg/litre. First
symptoms occurred after 1-2 h, the earliest and commonest being
bloatedness, followed by severe nausea, stomach cramps, vomiting and,
in one-third of the patients, mild diarrhoea (Warburton et al, 1962).
Other similar outbreaks have involved canned cherries (Luff & Metcalf,
1890), asparagus, herring (Schryver, 1909), and apricots (Savage,
1939) with tin concentrations ranging from 300 to about 1000 mg/kg.
An incident of acute intoxication after the ingestion of canned
peaches was reported by Sverrsson (1975). It was unique in that about
110 participants in a meeting received nothing else to eat or drink,
except for the peaches. The report was based on questionnaires
received from 85 persons, 76 (89%) of whom had fallen ill. About half
of these had developed symptoms such as nausea, vomiting, and
diarrhoea within 1 h. The majority became symptom-free after 24 h. The
fruit contained a mean tin concentration of 533 mg/kg (range
413-597 mg/kg) and the juice 369 mg/kg (range 298-405 mg/kg). Two out
of 7 persons who had consumed only one quarter of the contents fell
ill. The calculated intake of tin for these persons was 50 mg.
In another report, canned tomato juice was associated with 113
cases of acute gastroenteritis (Barker & Runte, 1972). In a small
number of these cases, superficial erosion of the mucosa of the mouth
was observed, while abdominal distension and cramps, and diarrhoea
were quite common. The cans containing the juice showed complete
corrosion of tin linings and this yielded a product containing a tin
concentration of approximately 400 mg/kg. The crops of tomatoes, used
to make this particular juice, had been grown in soil excessively
treated with nitrate fertilizer and contained high levels of nitrate;
complete corrosion of the lining of the cans occurred within
approximately 6 months.
Five human volunteers did not show any toxic signs after drinking
fruit juices containing about 500 or 730 mg/kg of tin, but all had
some gastrointestinal disturbance after drinking 5-7 ml/kg body weight
of fruit juice containing a tin concentration of about 1400 mg/litre
(Benoy et al., 1971). There was no evidence to indicate that the
effects were due to the absorption of tin, the likeliest cause being
local irritation of the mucous membranes of the alimentary tract.
8.1.2 Prolonged exposure
8.1.2.1 Effects of inhalation
The only available information on exposure by inhalation pertains
to pneumoconiosis caused by the inhalation of tin(IV) oxide. This
benign condition is termed stannosis.
More than 200 cases of stannosis have been described (Bartak
et al., 1948; Cutter et al., 1949; Dundon & Hughes, 1950; Oyanguren
et al., 1958; Pendergrass & Pryde, 1948; Robertson & Whitaker, 1957;
Schuler et al., 1958). The relative importance of exposure to tin
fumes in the etiology of this disorder was emphasized by Dundon &
Hughes (1950). The significance of the quantity of dust and the
duration of exposure were stressed by Robertson & Whitaker (1957), who
reviewed 121 cases.
Pendergrass & Pryde (1948) noted stannosis in a man who, for 15
years, had been bagging tin oxide material containing 96.5% tin(IV)
oxide and small amounts of aluminium, iron, and sodium but not silica.
Radiography revealed small dense shadows (denser than those of
silicosis) resembling those of barytosis in both lungs. Bartak et al.,
(1948) reported a similar case in a workman who had suffered from
asthma for many years and who attended a furnace in which metallic tin
was burnt to produce tin(IV) oxide. Necropsy of this man, who died
from gastric carcinoma, revealed deposits of tin(IV) oxide in the
lungs, lymph glands, liver, and spleen. Six other workmen with a
similar radiographic appearance of the lungs, did not have any
symptoms of asthma or signs of pulmonary dysfunction. Similar
radiological findings were reported by Cutter et al. (1949) in 2 cases
with nodules 1-2 mm in diameter unaccompanied by evidence of pulmonary
dysfunction. Both had been working in a tin recovery department for 20
years.
Oyanguren et al., (1958) reported 10 cases of stannosis in workers
involved in a process where tin ore concentrates were reduced to
metal. The workers were exposed to dust with a high tin and a low
silica content and to fumes rich in tin. None of the exposed workers
had any disability and the vital capacity, maximal breathing and
resting minute volume, and respiratory reserve were normal, as were
the findings on the blood and urine. Radiological examination of these
10 workers showed that the first alteration appeared to be increase of
bronchovascular markings, with hilar thickening in the early stages;
later, well-defined nodular elements appeared first in the middle
third of the right and then of the left lung. These appeared to
progress to the rest of the lung fields, and with continuous exposure,
accumulation of tin(IV) oxide gave a metallic density to a part of the
nodules, which subsequently acquired an appearance of lipoid droplets.
In the later stages, the bronchovascular markings disappeared as the
density of the shadows increased. The radiographic changes appeared
after some 3-5 years of exposure (Schuler et al., 1958). Hlebnikova
(1957) made a survey over a number of years of workers, who were
exposed to condensation aerosols, formed during the smelting of tin
and consisting mainly of tin(IV) oxide. The free silica concentration
in the aerosols was not more than 1% and the total silica did not
exceed 3%. The total dust concentration in air varied between 3 and
70 mg/m3. Workers developed pneumoconiosis after 6 to 8 years of
working at the smelter. The authors described 45 cases of
pneumoconiosis in workers, who were employed at the smelter from 6 to
20 years, Six of them were reported to have second degree
pneumoconiosis. X-ray examination showed small spotty shadows, but
there were no other symptoms or signs. The opacities seen on the
radiographs were thought to be due to the accumulation of
tin-containing dust and the development of connective tissue, This was
confirmed by experiments on rats exposed to tin(II) oxide. No cases of
pneumoconiosis were observed in 10 years, after the dust concentration
had been reduced to 10 mg/m3. An important feature of stannosis is
that fibrosis of the lung does not develop, providing that other
agents such as silica are not present.
8.1.2.2 Effects of ingestion
Packaged military rations were fed to 9 young male adult
volunteers for successive 24-day periods. The average tin content of a
control fresh diet was 13 mg/kg (in dry solids), while C-rations
stored at 1°C contained a tin concentration of 33 mg/kg, and rations
stored at 37°C contained 204 mg/kg. All the tin ingested was
accounted for by faecal elimination. No toxic effects were noted
(Calloway & McMullen, 1966). Dack (1955) reported a study in which 4
subjects ate canned pumpkin containing a tin concentration of about
380-480 mg/kg and canned asparagus containing a concentration of about
360 mg/kg, for 6 days, with no apparent illness. In an earlier study,
Mamontova (1940) did not observe any toxic manifestations in 4
volunteers who, for a period of 30 days, consumed 250 g per day of
canned fruit containing 212-250 mg of tin.
8.2 Organotin Compounds
8.2.1 Local effects
Toxic lesions among laboratory and process workers handling di-
and tributyltin compounds were reported by Lyle (1958). Most of the
lesions were typical acute skinburns, caused by the colourless di- or
tributyltin chlorides, which can come into contact with the skin
without exposed workers being aware of it. This type of lesion
developed 1 to 8 h following exposure. When the substance was washed
off immediately, no lesion developed. A more diffuse, but less rapidly
healing lesion, was caused by contact with clothes that had been
moistened by vapour or liquid compounds. This subacute irritation was
characterized by itching, affecting mostly the skin of the lower
abdomen, thighs, and groin. On examination, an easily distinguishable
erythematous eruption, was noted. Cessation of contact with the
compound was followed by rapid healing. Following contact with the
eye, lachrymation and severe suffusion of the conjunctivae appeared
within a few minutes and persisted for 4 days. Immediate lavage of the
eye did not prevent the development of the signs.
Lyle (1958) also studied the skin lesions induced by butyltin
compounds by applying various compounds on the skin of the back of the
hands of 5 volunteers. Skin lesions could be produced by a single
application of dibutyltin dichloride, and of the chloride, acetate,
and oxide of tributyltin, while the diacetate, dilaurate, oxide, and
maleate of dibutyltin and also tetrabutyltin failed to produce any
lesions. No visible changes occurred for 2 go 3 h after application,
although a swelling of the mouth of the hair follicles was noticeable.
The follicular inflammation progressed during the following 8 h with
only slight visible irritation of the skin between the openings of the
follicles. On the second day, sterile pustules developed over the
follicular openings, but remained small during the next 3 to 4 days.
After one week, the lesions had practically disappeared.
Symptoms experienced by female spray-painters working with a latex
paint, to which was added a fungicidal solution containing 20%
bis(tributyltin) oxide, ethylene oxide, ethanol, and water, were
described by Landa et al. (1973). The symptoms were reported to appear
"immediately" after the start of spraying and were experienced by all
the women engaged in the work. The first sensations were irritation of
the nasal mucosa and the conjunctivae. The exposure continued for
another fortnight during which the symptoms and signs became more
severe, and included bleeding from the nose and mucous discharges. An
otorhinolaryngological examination revealed rhinitis with distinct
hyperaemia and haemorrhages of the nasal septum. The workers reported
that the symptoms were less severe during weekends. When the addition
of the fungicidal solution was abandoned, the symptoms disappeared.
One year later, identical symptoms were reported by 4 spray-painters
using latex paint. An inquiry disclosed that the manufacturer had
begun to use bis(tributyltin) oxide as a fungicidal additive since the
banning of the use of mercury. The authors considered that the
trialkyltin compound was responsible for the symptoms. Measurements
performed later indicated that the concentrations of tin in the
breathing were below 0. 05 mg/m3 air.
Another description of local effects seen in workers handling
triphenyltin acetate was given by Markicevic & Turko (1967). These
workers were engaged in the formulation of a 20% solution of a
fungicide. During the hottest summer days, subjects working in the
dustiest places developed conjunctival irritation and irritation of
the mucous membranes of the upper respiratory tract as well as of the
skin, the hands and scrotum being particularly affected. Effects on
the central nervous system were not registered. The signs disappeared
rapidly after termination of exposure.
A wettable powder formation containing 50% tricyclohexyltin
hydroxide was examined for its irritation and sensitization potential.
No adverse reactions were observed in 53 females after sensitization
applications and a challenge application 20 days later of 0.5 ml of an
emulsion (10 g/litre). Tricyclohexyltin hydroxide was reported not to
be dermally irritating at a concentration of about 0.01 mg/kg body
weight (FAO/WHO, 1971).
8.2.2 Systemic effects
8.2.2.1 Effects of Dermal exposure
In a recent review (NIOSH, 1976), a fatal case was described in
which a 29-year-old woman was accidentally drenched in a slurry
containing triphenyltin chloride, diphenyltin dichloride, hexane, and
other unidentified compounds at a temperature of 79.4°C. On arrival at
hospital, first-degree thermal burns covered 10% of her body. Second-
and third-degree burns with 80-85% desquamated skin developed 12 h
later. Death from renal failure occurred 12 days after the accident.
However, the agent responsible for her symptoms and signs and for the
death could not be identified from the data available.
Mijatovic (1972) reported a case of systemic intoxication
resulting from dermal contact with a compound containing triphenyltin
acetate (60%) and manganese dithiocarbamate (15%). The subject,
engaged in agricultural aviation, spilt the compound on his hands and
chest while filling the aeroplane. The skin on his chest and abdomen
was endured after 3 h and vesicles developed the following day. He
experienced headache, nausea, epigastric pain, and general weakness.
Clinically the liver transaminases (SGPT) were elevated, reaching a
maximum one month later. Two months later the transaminases were
returning to normal, but the patient had pains over the liver, which
was tender and enlarged. The liver damage persisted for 2 years and
the case was labelled chronic hepatitis. No liver biopsy was
performed.
8.2.2.2 Effects of inhalation
Acute intoxication caused by the inhalation of triphenyltin
compounds has been reported in some instances. Three cases occurred in
farmers treating beetroot plants with triphenyltin acetate which was
inhaled (Guardascione & di Bosco, 1967). The symptoms started from a
few minutes to about 2 h after the first exposure. After 2 hours of
spraying, one subject felt a general malaise and severe headache and
eventually lost consciousness. On admission to the hospital he had a
diffuse tremor and a slightly depressed sensorium. All laboratory
investigations during the 2-week hospitalization were normal. When
mixing triphenyl tin acetate powder into a solution, a second subject
experienced repeated flushes, nausea, and shortness of breath, which
started only a few minutes after inhalation. During the 8-day hospital
surveillance, the only pathological finding was glucosuria. A third
subject suffered from severe headache, strong nausea, and epigastric
pains. However, laboratory investigations were normal. The headache
persisted for 2 days while the epigastric pains and the nausea
disappeared after one day. All 3 subjects recovered completely.
Horacek & Demcik (1970) reported a group poisoning involving 2
pilots and 3 mechanics following the spraying of a formulation
comprising 60% of triphenyltin acetate and 15° of manganese
dithiocarbamate. Protective measures were found to be deficient and
food was consumed with unwashed hands; thus, exposure by both
inhalation and ingestion could have occurred. Furthermore, other
pesticides containing copper, zinc, DDT, and organophosphates were
handled during exposure to the formulation, but the authors considered
these substances unlikely to be responsible for the symptoms
experienced. Exposure to the formulation continued for 2 weeks before
symptoms were recognized and continued for 2 further weeks. One pilot
experienced gastric pain, diarrhoea, and dryness of the mouth with
severe thirst that was not relieved by drinking. He also felt pressure
over the chest and slight shortness of breath. Blurred vision was
experienced after one week of exposure. Clinically hepatomegaly with a
painful liver was noted. Liver transaminases (SGPT) were elevated and
the highest recorded value was obtained 6 weeks later. Hyperglycaemia
and glycosuria were also present. Eight weeks afterwards, liver biopsy
revealed marked diffuse steathosis without any detected necrosis.
Monthly follow-ups showed that hepatomegaly and steathosis persisted
for one year. The second pilot suffered from heartburn, diarrhoea, and
vision disturbances. Clinically a hepatomegaly and a slight
hyperglycaemia were registered. The symptoms persisted for 4 weeks and
the glycosuria and hepatomegaly for 6 weeks. Recovery was complete.
The mechanics had symptoms that were less severe, comprising
diarrhoea, headache, eye pains, blurred vision, epigastric pain, and
thirst.
A worker engaged in the manufacture of butyltin compounds was
reported to suffer from a reduced sense of smell (Akatsuka et al.,
1959). It was first observed after an exposure period of 16 months,
and a further deterioration of the olfactory sense was established
during the following 8 months. The state persisted without any noted
improvement for 2 years. Other reported symptoms were headaches in the
occipital region, nasal haemorrhages, lassitude, and a feeling of
stiffness in the shoulders.
In four eases of acute poisoning due to exposure to organotin
vapours, patients were reported to have suffered from such symptoms as
vertigo, headaches, nausea and vomiting, and visual disturbances.
Clinically, stasis of the papilla was found and all patients displayed
pathological findings on the electroencephalograms. These were
reversible in 7-25 days, and all eases recovered clinically. The
organotin compound or compounds responsible for the intoxications were
not identified (Prüll & Rompel, 1976).
8.2.2.3 Effects of ingestion
A most serious episode involving organotin poisoning occurred in
1954 due to oral administration of a proprietary preparation used for
the treatment of furonculosis, osteomyelitis, anthrax, and acne. The
drug was responsible for about 100 deaths and a total of about 210
intoxications. These numbers vary to some extent depending on the
source of information: Alajouanine et al., (1958) reported a total of
210 deaths whereas another source quotes 102 deaths and at least 100
persons permanently affected ( Br. med. J., 1958). A total of about
400 000 capsules was sold ( Br. med. J., 1958), and about 1000
persons were believed to have taken the drug (Barnes & Stoner, 1959).
However, the capsules consumed by the intoxicated subjects amounted
only to about 7% of the lot distributed ( Br. med. J., 1958),
implying that the majority of the capsules were consumed without any
known adverse effects. The main ingredients of the preparation were
diethyltin diiodide (15 mg/capsule) and linoleic acid
(100 mg/capsule). It was suggested that ethyltin triiodide,
triethyltin iodide, or tetraethyltin could have been present as
impurities because of deficiencies in the manufacturing process or as
metabolites formed under the influence of various physical factors
(Rondepierre et al, 1958). A theory that diethyltin diiodide would
have reacted with the isolinoleic component producing tetraethyltin
was also presented (Lecoq, 1954). The symptoms and clinical findings
described in victims by Alajouanine et al. (1958) seem to favour the
hypothesis of a trialkyltin compound being the causal agent, probably
acting synergistically with other constituents. The clinical data of
201 cases including 98 deaths were reviewed by Alajouanine et al.
(1958). The dominating symptom, reported in about 98% of the cases,
was a diffuse headache, sometimes intolerably severe, and appearing a
few days after medication was started. Nausea and vomiting occurred in
73%, visual disturbances, mainly photophobia, but also double vision,
colour-vision disturbances and, in a few cases, blindness were
recorded in 33% of cases. Ophthalmoscopic findings included
congestion, papilloedema (Druault-Toufesco, 1955), and in some cases,
papillary stasis (Gayral et al., 1958; Pesme, 1955). Frequent symptoms
and signs were urinary incontinence, vertigo, loss of weight, and
abdominal pains. Absence of fever and a tendency towards hypothermia
were also noted. Psychological disturbances or stupor were reported in
70% of the cases. Other findings were meningeal irritation,
somnolence, insomnia, convulsions, constipation, and bradycardia.
Sometimes electroencephalograms were altered but did not suggest any
localized lesion. Death occurred during coma or from respiratory or
cardiac failure and in some cases during convulsions. It is probable
that most of the symptoms and signs could be attributed to a cerebral
oedema, the occurrence of which was established at autopsies and
decompressive surgery (Cossa et al., 1958, Fontan et al., 1958). This
cerebral oedema of the white matter appeared to be very similar to
that produced experimentally by administration of a proprietary
preparation containing an organotin compound, to mice and monkeys
(Gruner, 1958). Macroscopically, the brain was oedematic but the
microscopic findings were minor.
It has been reported that only 10 out of 103 subjects, who
survived, recovered completely: in the remainder, symptoms such as
headaches and asthenia persisted for at least 4 years (Barnes &
Stoner, 1959). Information on later, follow-up studies is not
available. The lethal dose of the preparation was in some instances
only about 25 capsules taken during one week. Ingestion of 3 capsules
was enough to cause intoxication in a 9-year-old child (Fontan et al.,
1955).
8.3 Treatment of Poisoning
Dimercaprol has been suggested by Stoner et al. (1945) to be an
effective antidote for dialkyltin poisoning. It has also been reported
to completely prevent the accumulation of alpha-keto acids produced by
dialkyltin compounds (Barnes & Stoner, 1959). Although dimercaprol
protected rats against the general toxic effects of these compounds,
it did not have any effect on the response to triethyltin compounds.
This seems to be due to the fact that dialkytin compounds, at least up
to the dihexyl derivatives, react readily with sulfhydryl groups and
the trialkyltin compounds do not (Barnes & Magos, 1968),
Studer et al. (1973) reported that steroid therapy (dexamethasone)
appeared to diminish mortality and the severity of brain oedema in
rats; there also appeared to be significant decreases in brain, liver,
and blood levels of triethyltin bromide. The authors suggested that
the beneficial effects of this steroid therapy might be partly due to
enhanced excretion or catabolism of triethyltin bromide.
Surgical decompression was considered to be the only treatment
that offered any benefit in human cases of cerebral oedema caused by
trialkyltin compounds (Alajouanine et al., 1958).
REFERENCES
ADAM, W. B. & HORNER, G. (1937) The tin content of English canned
fruits and vegetables. J. Soc. Chem. Ind., 56: 329-334.
ADAMSON, J. H. (1962) The colorimetric determination of dialkyltin
compounds dissolved in fats and olive oil. Analyst, 87: 597-598.
ADCOCK, L. H. & HOPE, W. G. (1970) A method for the determination of
tin in the range 0.2 to 1.6 µg, and its application to the
determination of organotin stabilizer in certain foodstuffs.
Analyst, 95: 868-874.
AKAGI, H. & SAKAGAMI, Y. (1971) [On degradation of organotin compounds
by ultraviolet ray.] Rep. Public Health Inst., 20: 1-4
(in Japanese).
AKAGI, H., FUJITA, M., & SAKAGAMI, Y. (1972) [Thin layer
chromatography of inorganotin and organotin compounds in foods.]
J. food Hyg., 13: 85-88 (in Japanese).
AKATSUKA, K., MIYAZAWA, J., IGARASHI, I., MORISHITA, M., HANDA, A.,
KAWAME, N., IWAMOTO, I., MORITO, F., MURAYAMA, K., NAKANO, S.,
YANAGIBASHI, H., NAGASAKI, T., KOTANI, Y., MATSUTANI, W., FUKUDA,
I., & IYO, T. (1959) [Experimental studies on disturbance of sense
of smell due to butyltin compounds.] J. Tokyo Med. Coll,
17: 1393-1402 (in Japanese).
ALAJOUANINE, T., DÉROBERT, L., & THIÉFRY, S. (1958) Etude clinique
d'ensemble de 210 cas d'intoxication par les sels organiques
d'étain. Rev. Neurol., 98: 85-96.
ALDRIDGE, W. N. (1958) The biochemistry of organotin compounds;
trialkyltins and oxidative phosphorylation. Biochem. J.,
69: 367-376.
ALDRIDGE, W. N. (1976) The influence of organotin compounds on
mitochondrial functions. In: Zuckerman, J. J., ed. Organotin
compounds: New chemistry and applications, Washington, DC,
American Chemical Society, pp. 186-196.
ALDRIDGE, W. N. & CREMER, J. E. (1957) Organotin dithizone complexes:
The colorimetric determination of diethyltin and triethyltin
compounds. Analyst, 82: 37-43.
ALDRIDGE, W. N. & STREET, B. W. (1964) Oxidative phosphorylation:
Biochemical effects and properties of trialkyltins. Biochem. J.,
91: 287-297.
ALDRIDGE, W. N. & STREET, B. W. (1970) Oxidative phosphorylation: The
specific binding of trimethyltin and triethyltin to rat liver
mitochondria. Biochem. J., 118: 171-179.
ALDRIDGE, W. N. & STREET, B. W. (1971) Oxidative phosphorylation: The
relation between the specific binding of trimethyltin and
triethyltin to mitochondria and their effects on various
mitochondrial functions. Biochem. J., 124: 221-234.
ALEU, F. P., KATZMAN, R., & TERRY, R. D. (1963) Fine structure and
electrolyte analysis of cerebral edema induced by alkyltin
intoxication. J. Neuropathol exp. Neurol., 22: 403-413.
ALLAN, J. E. (1962) Atomic absorption spectrophotometry absorption
lines and detection limits in the air-acetylene flame.
Spectrochim. Acta, 18: 259-263.
AMOS, M.D. & WILLIS, J. B. (1966) Use of high-temperature pre-mixed
flames in atomic absorption spectroscopy. Spectrochim. Acta,
22: 1325-1343.
ANALYTICAL METHODS COMMITTEE (1967) The determination of small amounts
of tin in organic matter. Analyst, 92: 320-323.
ANDERSON, S. R. A. & LOWY, S. L. (1956) A fluorometric determination
of tin. Anal. Chim. Acta, 15: 246-253.
ASCHER, K. R. S. & NISSIM, S. (1964) Organotin compounds and their
potential use in insect control. World Rev. Pest Control,
3: 188-211.
AVTANDILOV, G. G. (1967) [Trace element content in normal and
atherosclerotic portions of the human aorta in connection with
age.] Arh. Patol., 29: 40-42 (in Russian).
BARKER, W. H. & RUNTE, V. (1972) Tomato-juice-associated
gastroenteritis, Washington and Oregon, 1969. Am. J. Epidemiol.
96: 219-226.
BARNES, J. M. & MAGEE, P. N. (1958) The biliary and hepatic lesion
produced experimentally by dibutyltin salts. J. Pathol.
Bacteriol. 75: 267-279.
BARNES, J. M. & MAGOS, L. (1968) The toxicology of organometallic
compounds. Organometal. Chem. Rev., 3: 137-150.
BARNES, J. M. & STONER, H. B. (1958) Toxic properties of some dialkyl
and trialkyl tin salts. Br. J. ind. Med., 15: 15-22.
BARNES, J. M. & STONER, H. B. (1959) The toxicology of tin compounds.
Pharmacol. Rev., 11: 211-231.
BARNES, R. D., BULL, A. T., & POLLER, R. C. (1973) Studies on the
persistence of the organotin fungicide, fentin acetate
(triphenyltin acetate) in the soil and on surfaces exposed to
light. Pestic. Sci., 4: 305-317.
BARTAK, F., TOMECKA, M, & TOMICEK, O. (1948) Stannosis. Cas. lék.
cesk., 87: 915-920 (Abstract in: J. ind. Hyg., (1949)
31 (5): 98-99)
BASSINDALE, A. R., EABORN, C., TAYLOR, R., THOMPSON, A. R., WALTON, D.
R. M, CRETNEY, J., & WRIGHT, G. J. (1971) Aromatic reactivity 46.
Interpretation of substituent effects in base-catalyzed cleavage
of aryltrimethylsilanes and aryltrimethyl stannanes. J. Chem.
Soc. B., 1155-1161.
BENNETT, R. L. & SMITH, H. A. (1959) Spectrophotometric determination
of tin with phenylfluorone. Anal. Chem., 31: 1441-1442.
BENOY, C. J., HOOPER, P. A., & SCHNEIDER, R. (1971) The toxicity of
tin in canned fruit juices or solid foods. Food Cosmet. Toxicol.,
9: 645-656.
BENS, A. A, GRABOVSKAJA, L. I., & TIHONOVA, I. V. (1976)
[ Geochemistry of the environment.] Moscow, Nedra (in Russian).
BERGNER, K. G. & RUDT, U. (1958) [On the absorption of lead and tin by
fat and food rich in fat in tin plate cans.] Z. Lebensm. Unters.
Forsch., 137: 337-351 (in German).
BERTINE, K. K. & GOLDBERG, E. D. (1971) Fossil fuel combustion and the
major sedimentary cycle. Science, 173: 233-235.
BISTON, R., VERSTRAETEN, E., & NANGNIOT, P. (1972) Dosage de l'étain
dans les conserves par polarographie oscillographique ŕ intensité
imposée: Comparaison avec les methodes colorimetriques au dithiol
et ŕ la phenyl-fluorone. Anal. Chim. Acta, 59: 453-460.
BLAIR E. H. (1975) Biodegradation of tricyclohexyltin hydroxide.
Environ. Qual. Sai., 3 (Suppl.): 406-409.
BLASIUS, M. B., KERKHOFF, S. J., WRIGHT, R. S., & COTHERN, C. R.
(1972) Use of X-ray fluorescence to determine trace metals in
water resources. Water Resour. Bull., 8: 704-714.
BOGEN, J. (1973) Trace elements in atmospheric aerosol in the
Heidelberg area, measured by instrumental neutron activation
analysis. Atmos. Environ., 7: 1117-1125.
BÖNIG, G. & HEIGENER, H. (1972) [Paper chromatography: Quantitative
determination of micro amounts of tin.] Landwirtsch. Forsch.,
25: 378-382 (in German).
BOWEN, H. J. M. (1966) Trace elements in biochemistry, New York,
Academic Press, pp. 203-204.
BOWEN, V. T. & SUTTON, D. (1951) Comparative studies of mineral
constituents of marine sponges. I. The Genera Dysidea,
Chondrilla, Terpios. J. mar. Res., 10: 153-167.
BRAMAN, R. S. & TOMPKINS, M. A. (1979) Separation and determination of
nanogram amounts of inorganic tin and methyltin compounds in the
environment. Anal. Chem. 51: 12-19.
BRIDGES, J. W., DAVIES, D. S., & WILLIAMS, R. T. (1967) The fate of
ethyltin and diethyltin derivates in the rat. Biochem. J.,
105: 1261-1266.
BRINCKMAN, F. E. & IVERSON, W. P. (1975) Marine chemistry in the
coastal environment. Paper presented at 169th American Chemical
Society Meeting. Philadelphia, PA., April 8-10.
BRINCKMAN, F. E., BLAIR, W. R., JEWETT, K. L., & IVERSON, W. P. (1977)
Application of a liquid chromatograph coupled with a flameless
atomic absorption detector for speciation of trace organometallic
compounds. J. Chromatogr. Sci., 15: 493-503.
BRITISH MEDICAL JOURNAL (1958) "Stalinon": A therapeutic disaster.
Br. med. J., March 1st, p. 515.
BROWNING, E. (1969) Tin. In: Toxicity ol industrial metals, 2nd ed.,
London Butterworths, pp. 323-330.
BRUGGEMANN, J. & KLIMMER, O. R. (1964) [Nutritional-physiological,
analytical and toxicological studies on fungicidal triphenyltin
acetate.] Zentralbl. Vet.-Med. A, 11: 1-3 (In German).
BRUGGEMANN, J., BARTH, K., & NIESAR, K.-H. (1964a) [Experimental
studies on the content of triphenyltin acetate residues in turnip
leaves, ensilage of turnip leaves, and in excrements of animals
fed with these products.] Zentralbl. Vet.-Med. A, 11: 4-19
(in German).
BRUGGEMANN, J., KLIMMER, O. R., & NIESAR, K.-H. (1964b) [The
importance of triphenytin acetate residues in plants and animals
from a hygienic-toxicologic standpoint.] Zentralbl. Vet.-Med. A,
11: 40-48 (in German).
BURGER, K. (1963) [Thin layer chromatographic method for the
detection, identification and separation of traces of mono-, di-,
tri- and zerovalent organotin compounds.] Z. anal. Chem.,
192: 280-286 (in German).
BYINGTON, K. H., YEH, R. Y., & FORTE, L. R. (1974) The hemolytic
activity of some trialkyltin and triphenyltin compounds. Toxicol.
appl. Pharmacol., 27: 230-240.
CALLEY, D. J., GUESS, W. L., & AUTIAN, J. (1967) Hepatotoxicity of a
series of organotin esters. J. Pharm. Sci., 56 (2): 240-243.
CALLOWAY, D. H. & MCMULLEN, J. J. (1966) Fecal excretion of iron and
tin by men fed stored canned foods. Amer. J. clin. Nutr.,
18: 1-6.
CALVERY, H. O. (1942) Trace elements in foods. Food Res.,
7: 313-331. CAPACHO-DELGADO, L. & MANNING, D.C. (1966)
Determination of tinby atomic absorption spectroscopy.
Spectrochim. Acta, 22: 1505-1513.
CARR, H. G. (1969) Interaction between PVC bottles and liquid foods.
Soc. Plast. Eng. 25: 72-74.
CASIDA, J. E., KIMMEL, E. C., HOLM, B., & WIDMARK, G. (1971) Oxidative
dealkylation of tetra-, tri-, and dialkyltin and tetra- and
trialkyleads by liver microsomes. Acta chem. Scand.,
25: 1497-1499.
CATALA, R., ROYO, I. J, & PRIOMO, E. (1971) Tin content of canned
orange juice. Rev. Agroquim. Tecnol. Aliment., 11: 409-417
( Chem. Abstr., (1972) 77: 370, Abstract no. 3974 w).
CAUJOLLE, E., LESBRE, M., & MEYNIER, D. (1954) Sur la toxicité du
tétraméthylstannane et du tétraéthylstannane. C. R. Acad. Sci
(Paris), 239: 556-558.
CENCI, P. & CREMONINI, B. (1969) [Thin-layer chromatography of two
organotin pesticides and their behavior in various types of soil.]
Ind. sac. Ital.. 69: 313-316 (in Italian).
CHAPMAN, A. H., & PRICE, J. W. (1972) Degradation of triphenyltin
acetate by ultraviolet light. Int. Pest Control, 14: 11-12.
CHAPMAN, A. H., DUCKWORTH, M. W., & PRICE, J. W. (1959) The
determination of dialkyltin compounds in polyvinylchloride.
Br. Plast., February, pp. 78, 87.
CHEFTEL, H. (1967) L'étain dans les aliments. Presented at the
Fourth Meeting of the FAO/WHO Codex Committee on Food Additives,
The Hague, 11-15 September 1967, Rome, Food and Agriculture
Organization of the United Nations, 10 pp.
CHISAKA, H., TANIZAKI, Y., & NAGATSUKA, S. (1973) [Determination of
tin and poisonous trace elements in canned juices by neutron
activation.] Radioisotopes, 22: 247-248 (in Japanese).
CHRISTIAN, G. D. & FELDMAN, F. T. (1970) Atomic absorption
spectrography. New York, Wiley, pp. 404-406.
CONINE, D. L., MUHLER, J. C., & FORNEY, R. B. (1972) Acute toxicity of
sodium pentafluorostannite in rats and mice. Toxicol. appl.
Pharmacol., 22: 303-304.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R.
B. (1973) Comparison of the metabolic and renal effects of sodium
pentafluorostannite, sodium fluoride, stannous chloride,
hydrochloric acid, and starvation in the rat. Pharmacologist,
15: 189.
CONINE, D. L., YUM, M. N., MARTZ, R. C., STOOKEY, G. K., MUHLER, J.
C., & FORNEY, R. B. (1975) Toxicity of sodium pentafluorostannite,
a new anticariogenic agent. I. Comparison of the acute toxicity of
sodium pentafluorostannite, sodium fluoride and stannous chloride
in mice and/or rats. Toxicol. appl. Pharmacol., 33: 21-26.
CONINE, D. L., YUM, M., MARTZ, R. C., STOOKEY, G. K., & FORNEY, R. B.
(1976) Toxicity of sodium pentafluorostannite. A new
anticariogenic agent. III. 30-day toxicity study in rats.
Toxicol. appl. Pharmacol., 35: 21-28.
CORBIN, H. B. (1970) Separation and determination of trace amounts of
tin present as organotin residues on fruits. J. Assoc. Off. Anal.
Chem., 53: 140-146.
CORBIN, H. B. (1973) Rapid and selective pyrocatechol violet method
for tin. Anal. Chem., 45: 534-537.
COSSA, P., DUPLAY, FISCHGOLD, ARFEL-CAPDEVIELLE, LAFON, PASSOUANT,
MINVIELLE, & RADERMECKER, J. (1958) Encéphalopathies toxiques au
stalinon. Aspects anatomocliniques et alectroencéphalographiques.
Rev. Neurol., 95: 97-108.
COTTON, F. A. & WILKINSON, G. (1972) Advanced inorganic chemistry. A
comprehensive text, London, Wiley-Interscience, 3rd ed.
COTTON, F. A. & WILKINSON, G. (1976) Basic inorganic chemistry, New
York, Wiley.
COUSSEMENT, S. (1972) Analyse des dépôts de fentin acetate sur
feuilles de pomme de terre. Ann. Gembloux, 78: 41-46.
COYLE, C. F. & WHITE, C. E. (1957) Fluorometric determination of tin
with flavonol. Anal Chem, 29: 1486-1488.
CREMER, J. E. (1957) The metabolism in vitro of tissue slices from
rats given triethyltin compounds. Biochem. J., 67: 87-96.
CREMER, J. E. (1958) The biochemistry of organotin compounds: The
conversion of tetraethyltin into triethyltin in mammals.
Biochem. J., 65: 685-692.
CUTTER, H. C., FALLER, W. W., STOCKLEN, J. B., & WILSON, W. L. (1949)
Benign pneumoconiosis in a tin oxide recovery plant. J. And.
Hyg., 31: 139-141.
DACK, G. M. (1955) Chemical poisons in food. In: Food poisoning,
Chicago, University of Chicago Press, pp. 24-25.
DEPARTMENT OF NATIONAL HEALTH AND WELFARE, CANADA (1975) Food and
drug act and regulations office consolidation of the food and
drugs act and the food and drugs regulations with amendments to
December 18, 1975, B. 23.003, Ottawa, Queen's Printer and
Controller of Stationery, pp. 73B.
DEVJATYH, G. G., UMILIN, V. A., & CSINOVOI, Y. N. (1968) [Gas
chromatographic analysis of tetrabutyltin.] Tr. Himii i
Himiceskoj Tehnol., No. d, pp. 82-85 (in Russian).
DITTRICH, T. & COTHERN, C. R. (1971) Analysis of trace metal
particulates in atmospheric samples using X-ray fluorescence.
J. Air Pollut. Control Assoc., 21: 716-719.
DRESSLER, M., MARTINU, V., & JANAK, J. (1971) Response to the alkali
flame ionization detector to silicon, tin and lead compounds.
J. Chromatogr. 59: 429-433.
DRESSLER, M., BARTH, M., & VESPALEC, R. (1975) Chromatographic
determination of organotin compounds in fungistatic plaster.
Silikaty, 4: 301-310.
DRUAULT-TOUFESCO, M. N. (1955) A propos de deux cas d'intoxication
grave par le Stalinon. Bull. Soc. Ophtalmol. Fr., pp. 54-58.
DUCKERING, G. E. (1968) The cause of lead poisoning in the tinning of
metals. J. Hyg. (Lond.), 8: 474-503.
DUNDON, C. C. & HUGHES, J.P. (1950) Stannic oxide pneumoconiosis.
Am. J. Roentgenol., 63: 797-811.
DURBIN, P. W. (1960) Metabolic characteristics within a chemical
family. Health Phys., 2: 225-238.
DURFOR, C. N. & BECKER, E. (1964) Selected data on public supplies of
the 100 largest cities in the United States, 1962. J. Am. Water
Works Assoc., 56: 237-246.
DURUM, W. H. & HAFFTY, J. (1961) Occurrence of minor elements in
water, Washington, DC, pp. 1-11 (Geological Survey Circular
445).
DYER, B. & TAYLOR, G. (1931) Notes from the Report of Public Analysts.
Analyst, 56: 251-253.
ELSEA, J. R. & PAYNTER, O. E. (1958) Toxicological studies on bis
(tri- n-butyltin) oxide. Am. Med. Assoc. Arch. ind. Health,
18: 214-217.
ELTEN, R. (1929) [Study on tin foil used in cheese packeting; rindless
cheese.] Chem.-Ztg., 53: 586 (in German).
ENGBERG, A. (1973) A comparison of a spectrophotometric (Quercetin)
method and an atomic absorption method for the determination of
tin in food. Analyst, 98: 137-145.
EVANS, C. A. & MORRISON, G. H. (1968) Trace element survey analysis of
biological materials by spark source mass spectrometry. Anal.
Chem., 40: 869-875.
EVERETT, G. L, WEST, T. S., & WILLIAMS, R. W. (1974) The determination
of tin by carbon filament atomic absorption spectrometry. Anal.
Chim. Acta, 70: 291-298.
EYRICH, W. (1972) [Determination of tin in canned fruits and
vegetables.] Dtsch. Lebensm.-Rundsch, 9: 280-282 (in German).
FEY, R. (1969) [Heavy metal content in canned fruits and vegetables:
I. Tin and iron in canned asparagus.] Ind. Obst-Geinneseverwert.,
54: 27-33 (in German).
FILER, T. D. (1971) Fluorometric determination of submicrogram
quantities of tin. Anal. Chem., 43: 1753-1757.
FISCHER, H. W. & ZIMMERMAN, R. (1969) Long retention of stannic oxide:
Lack of tissue reaction in laboratory animals. Arch. Pathol.
88: 259-264.
FISH, R. H., KIMMEL, E. C., & CASIDA, J. E. (1975) Bioorganotin
chemistry. Biological oxidation of tributyltin derivatives.
J. Organometal Chem. 93 (1): C1-C4.
FISH, R. H., KIMMEL, E. C., & CASIDA, S. E. (1976) Bioorganotin
chemistry: Biological oxidation of organotin compounds. In:
Zuckerman, J. J., ed. Organotin compounds: New chemistry and
applications, Washington, DC, American Chemical Society,
pp. 197-203 (Advances in Chemistry Series 157).
FONTAN, M. M., VERGER, PERY, LOISEAU, & MULON (1955) Quatre cas dont
deux mortels d'intoxication par le "Stalinon" chez l'enfant. J.
Med. Bordeaux, 132: 399-405.
FAO/WHO (1971) 1970 Evaluations of some pesticide residues in food.
Rome, Food and Agriculture Organization of the United
Nations/World Health Organization, pp. 327-366, 521-542.
FOOD AND DRUG ADMINISTRATION, USA (1971) Code of Federal Regulations,
Title 21 - Foods and Drugs, Washington DC, US Government
Printing Office, pp. 409-410 ( 121, 2602).
FREELAND, G. N. & HOSKINSON, R. M. (1970) Non-aqueous atomic
absorption spectrophotometric determination of organometallic
biocides. Analyst, 95: 579-582.
FREITAG, K-D. & BOCK, R. (1974a) [Analysis of mixtures of tri-, di-,
and monophenyltin compounds and inorganic tin (IV).] Z. anal.
Chem., 5: 337-346 (in German).
FREITAG, K.-D. & BOCK, R. (1974b) Degradation of triphenyltin chloride
on sugar beet plants. Pesticide Sci., 5: 731-739.
FURCHNER, J. E. & DRAKE, G. A. (1976) Comparative metabolism of
radionuclides in mammals - XI. Retention of 113Sn in the mouse,
rat, monkey, and dog. Health Phys., 31: 219-224.
GAINES, T. B. & KIMBROUGH, R. D. (1968) Toxicity of fentin hydroxide
to rats. Toxicol. appl. Pharmacol., 12: 397-403.
GATEHOUSE, B. M. & WILLIS, J. B. (1961) Performance of a simple atomic
absorption spectrometer. Spectrochim. Acta, 17: 710-718.
GAUER, W. O., SEIBER, J. N., & CROSBY, D. G. (1974) Determination of
organotin residues from Plictran in fruit crops by gas-liquid
chromatography. J. agric. food Chem., 22: 252-254.
GAUNT, I. F., COLLEY, J., GRASSO, P., & CREASEY, M., (1968) Acute and
short-term toxicity studies on di- n-butyltin dichloride in rats.
Food Cosmet. Toxicol., 6: 599-608.
GAYRAL, L., LAZORTHES, G., & PLANQUES, J. (1958) Méningo-encé-phalite
hypertensive par intoxication au Stalinon, guérie. Rev. neurol.,
98: 143-144.
GEISSLER, H. & KRIEGSMANN, H. (1964) [Analysis of butyltin compounds
(n-C4H9)nSnX4-n.] Z. Chem., 9: 354-355 (in German).
GEISSLER, H. & KRIEGSMANN, H. (1965) [Liquids used for separation in
some gas chromatographic investigations of some n-butyltin
compounds. Z. Chem., 11: 423-424 (in German).
GELDMACHER -- VON MALLINCKRODT, M. & POOTH, M. (1969) [Simultaneous
determination of twenty five metals and metalloids in biological
materials.] Arch. Toxicol., 25: 5-18 (in German).
GEORGE, G. M., ALBRECHT, M. A., FRAHM, L. J., & McDONNELL, J.P. (1973)
Atomic absorption spectrophotometric determination of dibutyltin
dilaurate in finished feeds. J. Assoc. Off. Anal. Chem.,
56: 1480-1482.
GETZENDANER, M. E. & CORBIN, H. (1972) Residues on apples and pears
from use of Plictran miticide. J. agric. food Chem.,
20: 881-885.
GEYER, R. & ROTERMUND, U. (1969) [Oscillopolarographic determination
of organotin compounds.] Acta chim. Hung., 59 (2): 201-210
(in German).
GHAFOURI, R. (1970) Etude des eaux minérales d'Ax-les-Thermes. Terres
Eaux, 64: 19-27.
GOLDSCHMIDT, V. M. (1958) Tin, In: Muir, A. ed. Geochemistry,
Oxford, Oxford University Press, pp. 391-397.
GORDON, M. (1952) [Trace elements in peat.] Torinachrichten,
3: 12 (in German).
DE GROOT, A. P. (1973) Subacute toxicity of inorganic tin as
influenced by dietary levels of iron and copper. Food Cosmet.
Toxicol., 11: 955-962.
DE GROOT, A. P., FERON, V. J., & TIL, H. P. (1973) Short-term toxicity
studies on some salts and oxides of tin in rats. Food Cosmet.
Toxicol., 11: 19-30.
GRUENWEDEL, D. W. & PATNAIK, R. K. (1973) Model studies regarding the
internal corrosion of tin-plated food cans. IV. Polarographie
investigation of the interaction of tin (II)-ions with L-cysteine.
Chem. Mikrobiol. Technol. Lebensm., 2: 97-101.
GRUNER, J-E. (1958) Lésions du névraxe secondaires ŕ l'ingestion
d'éthyl-étain (Stalinon). Rev. Neurol., 98: 109-116.
GUARDASCIONE, V. & DI BOSCO, M. M. (1967) [Contribution to the
knowledge of occupational disease due to pesticides; Three cases
of acute intoxication by triphenyltin acetate fungicides.]
Lay. Urn., 19: 307-313 (in Italian).
HADZIMICEV, P. (1971) [Natural content of heavy metals (copper, zinc,
tin) in foods from the vegetable belt around Sofia.] Khranit.
Prom., 20: 18-20 (in Bulgarian).
HALL, C. A. & LUDWIG, P. D. (1972) Evaluation of the potential use for
several organotin compounds against the sheep blowfly ( Lucilia
spp.). Vet. Rec., 90: 29-32.
HAMILTON, T. A. (1948) The metabolic properties of plutonium and
allied material Univ. Calif. Rad. Lab. Med. Health Phys. Q., pp.
4-16 (UCRL-270).
HAMILTON, E. I., MINSKI, M. J., CLEARY, J. J., & HALSEY, V. S. (1972)
Comments upon the chemical elements present in evaporated milk for
consumption by babies. Sci. total Environ., 1: 205-210.
HAMILTON, E. I., MINSKI, M. J., & CLEARY, J. J. (1972/1973) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study.
Sci. total Environ., 1: 341-374.
HANNA, W. J. & GRANT, C. L. (1962) Spectrochemical analysis of the
foliage of certain trees and ornamentals for 23 elements. Bull
Torrey Bot. Club, 89: 293-302.
HASEGAWA, T. & SUGIMAE, A. (1971) [Emission spectrographic
determination on trace metals in airborne particulates.]
Jpn. Anal., 20: 640-845 (in Japanese).
HAYASHI, H. (1969) [Square wave polarographic determination of
dissolved tin in canned juice.] Jpn. Anal., 18: 58-61
(in Japanese).
HEATH, D. F. (1963) Some problems in the determination of residues in
plants and animals. In: Proceedings of a symposium; Radiation and
Radioisotopes Applied to Insects of Agricultural Importance,
Athens, 22-26 April 1963, Vienna, International Atomic Energy
Agency, pp. 185-193.
HEATH, D. F., (1967) The retention or triphenyltin and dieldrin and
its relevance to the toxic effects of multiple dosing. In:
Radioisotopic detection of Pesticide Residues, Proceedings of a
Panel, Vienna 1965, pp. 18-26 (Abstract in: Chem. Abstr.,
67: 1828 W).
HEDGES, T. R. & ZAREN, H. A. (1969) Experimental papilledema; A study
of cats and monkeys intoxicated with triethyltin acetate.
Neurology, (Minneapolis), 19: 359-365.
HEINDL, R. A. (1970) Tin. In: Mineral facts and problems, 1970
edition, pp. 759-751. Reprint from: US Dept of the Interior,
Bureau of Mines, Bulletin 650.
HEROK, J. & GOTTE, H. (1963) [Radiometric analysis of triphenyltin
acetate in the metabolism of plants and animals.] Int. J. appl.
Radiat. Isot., 14: 461-479 (in German).
HEROK, J. & GÖTTE, H. (1964) [Radiometric study on the metabolism of
triphenyltin acetate in milk sheep.] Zentralbl. Vet.-Med. A,
11: 20-28 (in German).
HERON, P. N. & SPROULE, J. S. G. (1958) The chemical nature of
fungicidal agents employed in the pulp and paper industry. Indian
Pulp Pap., 12: 516-517.
HESLOP, R. B. & JONES, K. (1976) Inorganic chemistry. A guide to
advanced study. Amsterdam, Elsevier.
HILES, R. A. (1974) Absorption, distribution and excretion of
inorganic tin in rats. Toxicol. appl. Pharmacol., 27: 366-379.
HLEBNIKOVA, M. J. (1957) [Hygienic characteristics of dust in the
production of tin.] Sb. naucnyh Rab. voprogam Gig. Truda i
profpatol., No. 2, pp. 53-83 (in Russian).
HODGE, V. F., SEIDEL, S. L., & GOLDBERG, E. D. (1979) Determination of
tin (IV) and organotin compounds in natural waters, coastal
sediments and macro algae by atomic absorption spectrometry.
Anal. Chem., 51: 1259.
HOFF, J. E. & WILCOX, G. E. (1070) Accumulation of nitrate in tomato
fruit and its effect on derinning. J. Am. Soc. Hortic. Sci.,
95: 92-94.
HORACEK, V. & DEMCIK, K. (1970) [Group poisoning in spraying of field
cultures with Brestan-60 triphenyltin acetate.] Prac. Lék.,
22: 61-66 (in Czech).
HORIO, T. & NAKASEKO, M. (1972) [Spectrophotometric determination of
dissolved tin in canned foods using Salicylidenamion-2-
thiophenol.] Tokyo Inst. Food Technol. J., 17: 212-214
(in Japanese).
HORIO, T., IWAMOTO, Y., & SHIGA, I. (1967) The nitrate corrosion of
canned orange juice drinks in Japan. Communication presented at
the 5th International Congress on Canned Fools, Vienna 3-6,
October, 1967, 12 pp.
HUEY, C., BRINCKMAN, F. E., GRIM, S., & IVERSON, W. P. (1974) The role
of tin in bacterial methylation of mercury. Paper presented of the
International Conference on Transport of Persistent Chemicals in
Aquatic Ecosystems, Ottawa, Canada, 1-3 May, 1974.
IGNATJEVA, M. A., KUZNETSOV, I. G., MIRSKOV, R. G., & VLASOV, V. M.
(1968) [Toxicology of acetylene organotin compounds of the
triethylstannyl range.] Izv. sib. Otdel. Akad. Nauk SSSR,
1 (5): 118-119 (in Russian).
INNES, J. R. M., ULLAND, B. M., VALERIO, M. G., PETRUCELLI, L.,
FISHBEIN, L., HART, E. R., PALLOTTA, A. J., BATES, R. R., FALK, H.
L., GART, J. J., KLEIN, M, MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumorigenicity
in mice: A preliminary note. J. Natl Cancer Inst.,
42: 1101-1114.
INTERNATIONAL LABOUR OFFICE (1972) Tin alloys and compounds. In:
Encyclopedia of Occupational Health and Safety, Geneva, ILO,
Vol. II, pp. 1407-1409.
IWAMOTO, Y., MIYAZAKI, M., KUNISATO, S., MAEDA, Y., HORIO, T., &
KOMURA, S. (1968) [Studies on abnormal detinning of tin can with
nitrate in foods: (II) Relationship between dissolution on tin and
pH of canned tomato juice.] J. Jpn Soc. Food Nutr., 21: 50-54
(in Japanese).
JANITZ, W. (1971) [Comments on the polarographic method of tin and
lead determination in tinned meat products.] Med. Weter.,
27: 43-45 (in Polish).
JOHANSEN, O. & STEINNES, E. (1969) Determination of tin in geological
material by neutron activation analysis. Analyst, 94: 976-978.
JOHNSON, J. H., YOUNGER, R. L., WITZEL, D. A., & RADELEFF, R. D.
(1975) Acute toxicity of tricyclohexyltin hydroxide to livestock.
Toxicol. appl. Pharmacol., 31: 66-71.
JOVANOVIC, D., STOJADINOVIC, L., & SPASOJEVIC, B. (1967) [Contribution
to the determination of tin in canned meat.] Vojno-sanit. Pregl.,
24: 405-408 (in Serbo-Croat).
KAHN, H. L. & SCHALLIS, J. E. (1968) Improvement of detection limits
for arsenic, selenium and other elements with an argon-hydrogen
flame. At. Absorpt. Newsl., 7: 5-9.
KANISAWA, M. & SCHROEDER, H. A. (1967) Life term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumors in mice. Cancer Res., 27: 1192-1195.
KANISAWA, M. & SCHROEDER, H. A. (1969). Life term studies on the
effect of trace elements on spontaneous rumours in mice and rats.
Cancer Res., 29: 892-895.
KAPPAS, A. & MAINES, M.D. (1976) Tin: A potent inducer of heme
oxygenase in kidney. Science, 192: 60-62.
KAS'YANENKO, I. V. & KUL'SKAYA, O. A. (1969) [Microelement content of
the blood of patients with cancer of stomach and chronic gastritis
secretory insufficiency.] Vopr. eksp. Onkol Respub. Mezhvedom,
SB, 4: 101-107 (in Russian).
KEENAN, R. G. & BYERS, D. H. (1952) Rapid analytical method for air
pollution surveys. Arch. ind. Hyg., 6: 226-230.
KEHOE, R. A., CHOLAK, J. & STORY, R. V. (1940) A spectrochemical study
of the normal ranges of concentration of certain trace metals in
biological materials. J. Nutr., 19: 579-592.
KERK, VAN DER, G. J. M. (1975) [The present use of organotin
compounds.] Chem. Zeitung, 99: 26-32 (in German).
KIMBROUGH, R. D. (1976) Toxicity and health effects of selected
organotin compounds: A review. Environ. Health Perspect.,
14: 51-56.
KIMBROUGH, R. D. & GAINES, T. B. (1971) Hexachlorophene effects on the
rat brain. Arch. environ. Health, 23: 114-118.
KIMMEL, E. C., FISH, R. H., & CASIDA, J. E. (1977) Bio-organotin
chemistry: Metabolism of organotin compounds in microsomal
monoxygenase systems and in mammals. J. Agric. Food Chem.,
25 (1): 1-8.
KIMURA, Y., UEDA, K., NISHIZIMA, M., MAKI, T., WADA, M., TAKAHASHI S.,
YAMADA, T., ARAI, M., & UEMATSU, T. (1970) [Studies on extractable
heavy metals in canned foods: I. Detection of tin and lead from
various sorts of canned baby foods after their opening.] Ann.
Rep. Tokyo Metrop. Inst. Hyg., 22: 95-100 (in Japanese).
KIRK, R. S. & POCKLINGTON, W. D. (1969) The determination of tin and
canned foods with quercetin. Analyst, 94: 71-74.
KLEIN, A., COP, R., CHATRNA, E., & BADALOVA, M. (1970) [The study of
vitamin C stability and growing of tin content in tinned juices.]
Cesk. Hyg., 15: 49-55 (in Czech).
KLEINKOPF, M.D. (1955) Trace element exploration of Maine lake water,
Academic dissertation, Columbia University, 153 pp. (University
Microfilm Pub. 12, 447).
KLEINKOPF, M.D. (1960) Spectrographic determination of trace elements
in lake waters of northern Maine. Geol. Soc. Am. Bull.,
71: 1231-1242.
KLIMMER, O. R. (1963) [Triphenyltin acetate and the toxicology of
organic tin compounds.] Arzneimittel-Forsch., 13: 432-436
(in German).
KLIMMER, O. R. (1964) [Toxicological studies on triphenyltin acetate
(TPZA).] Zentralbl. Vet.-Med., R.A 11: 29-39 (in German).
KLIMMER, O. R. (1969) [The application of organotin compounds reviewed
by the experimental toxicologist.] Arzneimittel-Forsch.,
19: 934-939 (in German).
KOBAYASHI, T. & YADA, M. (1968) [Spectrophotometric determination of
tin in foodstuffs with stilbazo.] J. Food Hyg. Soc. Jpn,
9: 465-468 (in Japanese).
KOCH, J. & FIGGE, K. (1971) [Migration of tin stabilizers from PVC
bottles into beer.] Z. Lebensmittel-Unters. 147: 8-10
(in German).
KOCKIN, D. A., SKORBATOVA, J. C., PEGUSOVA, L. D., & VORONKOV, N. A.
(1969) [Polarographic study on organotin compounds with an acid
content.] Zl. Obshch. Khim., 39: 1777-1781 (in Russian).
KONOVALOV, G. S. & KOLESNIKOVA, T. K. (1969) [Trace elements in
atmospheric precipitation around the Otkaznoe reservoir.]
Gidrokhim Mater., 49: 74-79 (in Russian).
KRUTCHKOFF, D. J., JORDAN, T. H., WEI, S. H. Y., & NORDQUIST, W. D.
(1972) Surface characterization of the stannous fluoride enamel
interaction. Arch. oral Biol., 17: 923-930.
KRYLOVA, N. & BALABUH, A. (1970) [Trace element level in meat.] Myas.
Ind. SSSR, 41: 39 (in Russian).
KUMPULAINEN, J. & KOIVISTOINEN, P. (1977) Advances in tin compound
analysis with special reference to organotin pesticide residues.
Res. Rev., 66: 1-18.
KUNISATO, S. & MIYAZAKI, M. (1972) [Application of plant growth
regulators on fruits and vegetables for processing: I. Nitrate
content in tomato fruits.] J. Hortic. Assoc. Jpn., 41: 411-420
(in Japanese).
KUROSAKI, N. & FUSAYAMA, T. (1973) Penetration of elements from
amalgam into dentin. J. dent. Res., 52: 309-317.
KUTZNER, J. & BROD, K. H. (1971) [Resorption and excretion of tin
after oral administration of tin-113.] Nucl. Med. (Stuttg.),
10: 286-297 (in German).
LAAMANEN, A., LOFGREN, A., & PARTANEN, T. J. (1971) Some trace metals
in particulates in the air of Helsinki and Turku, 1964-1969. Work
Environ. Health., 8: 63-69.
LANDA, K., FEJFUSOVA, J., & NEDOMLELOVA, R. (1973) [The hazards of
organic tin compounds used as fungicides in some industrial
applications.] Prac. Lék., 25: 391-394 (in Czech).
LANIGEN, D. & WEINBERG, E. L. (1976) The use of estertin stabilizers
in PVC. In: Zucherman, J. J., ed. Organotin compounds: New
chemistry and applications. Washington, DC, American Chemical
Society (Advances in Chemistry Series 157).
LECOQ, R. (1954) Contribution ŕ l'étude des effects toxiques du
tétraéthylétain C. R. Hebd. Acad. Sci. (Paris), 239: 678-680.
LEE, E. E., GORANSON, S.S., ENRIONE, R. E., & MORGAN, G. B. (1972)
National air surveillance cascade impactor network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol., 6: 1025-1030.
LINDEMANN, G., PINDEBORG, J. J., & PAULSEN, H. (1959) Recovery of the
rat kidney in fluorosis. Arch. Pathol., 67: 30-33.
LJASKOVSKAJA, Y., & KRASIL'NIKOVA, T. (1961) [Photometric
determination of tin in canned food by reaction with quercetin.]
Myasn. Ind. SSSR 4: 44-45 (in Russian) (English abstract in
Anal. Abstr. (1963) 10: 1177).
LOUNAMAA, J. (1956) Trace elements in plants growing wild on different
rocks in Finland: A semi-quantitative spectrographic survey. Ann.
Bot. Soc. "Vanamo" 29: 4.
LOWRY, R. R. & TINSLEY, I. J. (1972) Determination of tin salts in
fats. J. Am. Oil. Chem. Soc., 49: 508-509.
LUFF, A. P. & METCALFE, G. H. (1890) Four cases of tin poisoning
caused by tinned cherries. Br. med. J., 1: 833-834.
LUKE, C. L. (1956) Photometric determination of tin with
phenylfluorone: Determination of tin in lead and 1% antimony-lead
alloys. Anal. Chem., 28: 1276-1279.
LYLE, W. H. (1958) Lesions of the skin in process workers caused by
contact with butyltin compounds. Br. J. ind. Med., 15: 193-196.
MACINTOSH, R. M. (1968) Tin and tin alloys. In: Kirk-Othmer
encyclopedia of chemical technology, 2nd ed. New York,
Interscience pp. 273-325.
MAGEE, P. N., STONER, H. B., & BARNES, J. M. (1957) The experimental
production of oedema in the central nervous system of the rat by
triethyltin compounds. J. Pathol. Bacteriol., 73: 107-124.
MAHADEVAIAH, M., GOWRAMMA, R. V., RADAKRISHNAIAH SETTY, G., SASTRY, M.
V., SASTRY, L. V. L., & BHATNAGAR, H. C. (1969) Studies on
variation in tin content in canned mango nectar during storage.
J. food Sci. Technol., 6: 192-196.
MAMONTOVA, A. A. (1940) [Effects of tin on animal organism.] Vopr.
Pitan, 2: 13-25 (in Russian).
MARHOLD, J. V. (1972) [ A collection of results of toxicological
evaluation of substances and products.] Pro Vychovu Vedoucicn
Pracovniku Chemickeho Prumyclu Praha (in Czech).
MARKICEVIC, A. & TURKO, V. (1967) [Lesions due to triphenyltin acetate
"Brestan".] Arh. Hig. Rada, 18: 355-358 (in Serbo-Croat).
MARKS, M. J., WINEK, C. L., & SHANOR, S. P. (1969) Toxicity of
triphenyltin hydroxide. (Vancide KS). Toxicol. appl. Pharmacol.,
14: 627.
MASON, B. (1966) Principles of geochemistry, 3rd ed., New York,
Wiley.
MAZAEV, V. T. & KOROLEV, A. A. (1969) [Experimental standardization of
the maximum permissible concentration of the dichlorbutyltin in
surface water.] Prom. Zagrjaznenija Vodoemov, No 9, pp. 15-37
(in Russian).
MAZAEV, V. T. & SLEPNINA, T. G. (1973) [Experimental data on hygienic
standardization of dibutyltin sulfide in water bodies.] Gig. i.
Sanit., 8: 10-13 (in Russian).
MAZAEV, V. T., LOSEV, N. I., & VOINOV, V. A. (1968) [On some
vegetative reactions and on electrical activity of the brain
cortex after intoxication with organic derivatives of tin.] Bull.
exp. Biol. Med., 11: 72-74 (in Russian).
MAZAEV, V. T., KOROLEV, A. A., & SKACKOVA, I. N. (1971) Experimental
investigation of the toxic effect of tetraethyltin and
dichlorodibutyltin on the animal organism. J. Hyg. Epidemiol.
Microbiol. Immunol., 15: (1): 115-120.
MAZAEV, V. T., GOLOVANOV, O. V., IGUMOV, A. S., & TSAY, V. N. (1976)
[Problem of the transformation of organotin compounds in a water
medium.] Gig. i Sanit, 3: 17-20 (in Russian).
MEINEMA, H. A., BURGER-WIERSMA, T., VERSLUIS-DE-HAAN, G. & GEVERS, E.
Ch. (1978) Determination of trace amounts of butyltin compounds in
aqueous systems by gas chromatography/mass spectrometry. Environ.
Sci. Technol., 12 (3): 288-293.
MIDWEST RESEARCH INSTITUTE (1977) Assessment of the need for the
character of, and the impact resulting from limitations on
selected organotins. Phase I Assessment of the need for
limitations on organotins. Washington, DC, US Environmental
Protection Agency, EPA Contract No. 68-01-4313).
MIJATOVIC, M. (1972) [Chronic hepatitis due to exposure to Brestan.]
Jugosl. inostr. Dok. Zast. Radu, 8:3-9 (in Serbo-Croat).
MIKI, E. & FUKUI, Y. (1971) [Square wave polarographic determination
of tin in canned fruit.] Sci. Rep. Agric. Fac. Kagawa Univ.,
22: 35-40 (in Japanese).
MITCHELL, R. C. (1948) The spectrographic analysis of soils, plants,
and related materials. Bureau of Soil Science, pp. 1-173
(Technical Communication No. 44).
MONIER-WILLIAMS, G. W. (1949) Trace elements in food, London,
Chapman & Hall, pp. 138-161.
MOSKALEV, Ju. I. (1964) [Studies on the distribution of 113Sn. In:
Distribution, biological effects and rapid excretion of
radioactive isotopes.] Moscow, Medicina, pp. 78-82 (in Russian).
MULAY, I. L., RAY, R., KNOX, B. E., SUHR, N.H., & DELANEY, W. E.
(1971) Trace metal analysis of cancerous and non-cancerous human
tissues. J. Natl. Cancer Inst., 47: 1-13.
NAKAHARA, T., MUNEMORI, M., & MUSHA, S. (1972) Atomic absorption
spectrometric determination of tin in premixed inert gas
(entrained air) hydrogen flames. Anal. Chim. Acta, 62: 267-278.
NAKAMURA, Y. & KAMIWADA, S. (1973) [Colorimetric determination of tin
by decomposition and tin-phenylfluorene complex.] J. food Hyg.,
14: 352-356 (in Japanese).
NATIONAL ACADEMY OF SCIENCES. WASHINGTON (1973) Toxicants occurring
naturally in foods, 2nd ed., Washington, DC, NAS, pp. 63-64.
NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH (1976) Criteria
for a recommended standard ... occupational Exposure to organotin
compounds, Washington, DC, US Government Printing Office,
187 pp.
NATIONAL WATER QUALITY NETWORK (1960) Annual compilation ol data,
1 October 1959-30 September 1960, Washington, DC, US Department
of Health, Education and Welfare, Public Health Service,
pp. 353-356.
NEHRING, P. (1972) [Tin in peach preserves.] Ind. Obst.
Gemusseverwert., 57: 489-492 (in German).
NELSON, A. A. (1954) Approximate relation of parts per million in diet
to mg/kg/day. Assoc. Food Drug Off. Q. Bull, 18: 66.
NEUBERT, G. (1964) [Analysis of organotin stabilizers.] Z. anal.
Chem., 203: 265-272 (in German).
NEUBERT, G. & ANDREAS, H. (1976) [The analysis of organotin
compounds.] Z. anal. Chem., 280: 31 (in German).
NEUBERT, G. & WIRTH, H. O. (1975) [The analysis of organotin
stabilizers.] Z. anal. Chem., 273:19-23 (in German).
NEUMANN, W. P. (1970) The organic chemistry of tin, London, Wiley.
NEWMAN, E. J. & JONES, P. D. (1966) Separation and determination of
small amounts of tin. Analyst, 91: 406-410.
NIKONOROW, M., MAZUR, H., & PIEKACZ, H. (1973) Effects of orally
administered plasticizers in the rat. Toxicol. appl. Pharmacol.,
26: 253-259.
NISHIJIMA, M., UEDA, K., MAKI, T., WADA, M., TAKAHASHI, S., KIMURA,
Y., YAMADA, T., NAKAJIMA, K., & FUKUDA, T. (1971) [Studies on
heavy metals dissolved in canned foods. (II) Detection of tin and
lead from various canned fruits after their opening.] Ann. Rep.
Tokyo Metrop. Inst. Hyg., 23: 189-196, (in Japanese).
OBRUSNIK, I. (1969), Determination of indium and tin by activation
analysis using replacement substoichiometry. Talanta,
16: 563-566.
ONISHI, H. & SANDELL, E. B. (1957) Meteoritic and terrestrial
abundance of tin. Geochim. Cosmochim. Acta, 12: 262-270.
OYANGUREN, H., HADDAD, R., & MAASS, H. (1958) Stannosis. Benign
pneumoconiosis owing to inhalation of tin dust and fume. I.
Environmental and experimental studies. Ind. Med. Surg.,
27: 427-431.
PAL, B. K. & RYAN, D. E. (1969) Fluorescence and metallic valency
states. Part III. Determination of tin. Anal Chim. Acta,
48: 227-231.
PATE, B. D. & HAYS, R.L. (1968) Histological studies of testes in rats
treated with certain insect chemosterilants. J. econ. Entomol.
61: 32-34.
PEARLMAN, R. S., HEFFERREN, J. J., & LYON, H. W. (1970) Determination
of tin in enamel and dentin by atomic absorption spectroscopy.
J. dent. Res., 49: 1437-1443.
PEDLEY, F. G. (1927) Chronic poisoning by tin and its salts. J ind.
hyg., 9: 43-47
PELIKAN, Z. & CERNY, E. (1968a) [The toxic effect of tri-n-butyltin
compounds on white mice.] Arch. Toxikol., 23: 283-292
(in German).
PELIKAN, Z., & CERNY, E. (1968b) The effect of low doses of bis-
(tri-n-butyltin) oxide on the skin of rats. Berufsdermatosen,
16: 340-349.
PELIKAN, Z., & CERNY, E. (1969) Toxic effect of bis- (tri-n-butyltin)
oxide (TBTO) on the skin of rats. Berufsdermatosen, 17: 305-316.
PELIKAN, Z. & CERNY, E. (1970a) The toxic effects of some di- and
mono-n-octyltin compounds on white mice. Arch. Toxikol.,
26: 196-202.
PELIKAN, Z. & CERNY, E. (1970b) Toxic effects of some mono-n-butyltin
compounds on white mice. Arch. Toxikol., 27: 79-84.
PENDERGRASS, E. P. & PRYDE, A. W. (1948) Benign pneumoconiosis due to
tin oxide. J. ind. Hyg., 30: 119-123.
PEREL'MAN, A. I. (1972) [Geochemistry of elements in the zone of
hypergenesis.] Moscow, Nedra (in Russian).
PERRY, H. M. & PERRY, E. F. (1959) Normal concentrations of some trace
metals in human urine: Changes produced by ethylenediamine
tetraacetate. J. clin. Invest., 38: 1452-1463.
PESME, P. (1955) Complicationes oculaires observées chez quatre
enfants intoxiqués par le "Stalinon". Arch. Fr. Pediat.,
12: 327-328.
PIVER, W. T. (1973) Organotin compounds: Industrial applications and
biological investigation. Environ. Health. Perspect., 4: 61-79.
PORTRETNYJ, V. P., MALYUTA, V. F., & CHUIKO, V. T. (1973)
[Determination of tin in water, after concentration by
co-precipitation, by anodic stripping voltametry.] Zh. anal.
Khim., 28: 1337-1340 (in Russian).
PRICE, W. J. & ROOS, J. T. H. (1969) Analysis of fruit juice by atomic
absorption spectrophotometry. I -- The determination of iron and
tin in canned juice. J. Sci. food Agric., 20: 437-439.
PRULL, G. & ROMPEL, K. (1976) [Neurological and cerebro-electrical
disturbances in acute poisoning due to organotin compounds.]
Nervenarzt, 41: 516-520 (in German).
REMY, H. (1956) Treatise on inorganic chemistry, Amsterdam,
Elsevier, Vol. I.
RIDLEY, W. P., DIZIKES, L. J., & WOOD, J. M. (1977) Biomethylation of
toxic elements. Science, 197: 329-332.
RITTENHOUSE, G., FULTON, R. B., III GRABOWSKI, R. J., & BERNARD, J. L.
(1969) Minor elements in oil field waters. Chem. Geol., 4:
189-209.
ROBERTS, R. M. G. & EL KAISSI, F. (1968) Base-catalyzed clearage of
allylic derivatives of group IV A elements. J. Organometal.
Chem., 12 (1): 79-88.
ROBERTSON, A. J. (1960) Pneumoconiosis due to tin oxide. In: King, E.
J. & Fletcher, C. M. ed Symposium on industrial pulmonary
diseases, Boston, Little-Brown, pp. 168-184.
ROBERTSON, A. J. & WHITAKER, P. H. (1957) Radiological changes in
pneumoconiosis due to tin oxide. J. fac. Radiol., 6: 224-233.
ROBINSON, I. M. (1969) Effects of some organotin compounds on tissue
amine levels in rats. Food Cosmet. Toxicol., 7: 47-52.
ROE, F. J. C., BOYLAND, E., & MILLICAN, K. (1965) Effects of oral
administration of two tin compounds to rats over prolonged
periods. Food Cosmet. Toxicol., 3: 277-280.
RONDEPIERRE, J., TRUHAUT, R., GUILLY, P., HIVERT, P. E., & BARANDE, I.
(1958) Sur une intoxication aiguë par un dérivé organiqué de
l'étain. Rev. neurol., 98: 135-140.
ROSE, M. S. (1969) Evidence for histidine in the triethyltin-binding
site of rat haemoglobin. Biochem. J., 111: 129-137.
ROSS, W. J. & WHITE, J. C. (1961) Application of pyrocatechol violet
as a colorimetric reagent for tin. Anal. Chem., 33: 421-424.
SAITO, I. & ENDO, M. (1970) [Analytical studies on inorganic elements
of gall stones.] Hokkaido Igaku Zasshi, 14: 334-337
(in Japanese).
SATO, N., TSURUTA, K., KA1VIADA, I., NARITA, S., & ABUKAWA, H. (1973).
[Studies on dissolved tin in canned foods by atomic absorption
spectrophotometry.] J. Food Hyg Soc. Jpn, 14: 245-248
in Japanese).
SAVAGE, W. (1939) Canned foods in relation to health, Lancet
231 (2): 991-995.
SAWYER, R. (1967) Determination of dialkyltin stabilizers in aqueous
extracts from PVC and other plastics. Analyst, 92: 569-574.
SCHALLIS, J. E. & KAHN, H. L. (1968) The determination of tin in
lubricating oils with a nitrous oxide-acetylene flame.
At. Absorpt. Newsl., 7: 84.
SCHRAMEL, P., SAMSAHL, K., & PAVLU, J. (1973) Some determinations of
Hg, As, Se, Sb, Sn, and Br in water plants, sediments and fishes
in Bavarian rivers. Int. J. environ. Stud., 5: 37-40.
SCHROEDER, H. A. & BALASSA, J. J. (1961) Arsenic, germanium, tin and
vanadium in mice: Effects on growth, survival and tissue levels.
J. Nutr., 92: 245-253.
SCHROEDER, H. A., BALASSA, J. J., & TIPTON, I. H. (1964) Abnormal
trace metals in man: Tin. J. chron. Dis., 17: 483-502.
SCHROEDER, H. A., KANISAWA, M., FROST, D. V., & MITCHENER, M. (1968)
Germanium, tin and arsenic in rats: Effects on growth, survival,
pathological lesions and life span. J. Nutr., 96: 37-45.
SCHROLL, E. VON & KRACHSBERGER, H. (1970) [Study on the geochemistry
of impurities in atmospheric precipitates in the Vienna
metropolitan area.] Radex Rundsch., 5: 331-341 (in German).
SCHRYVER, S. B. (1901) Some investigations on the toxicology of tin,
with special reference to the metallic contamination of canned
foods. J. Hyg., 9: 253-263.
SCHULER, P., CRUZ, E., GUIJON, C., MATURANA, V., & VALENZUELA, A.
(1958) Stannosis. Benign pneumoconiosis owing to inhalation of tin
dust and fume. II. Clinical study. Ind. Med. Surg., 27: 432-435.
SCHWARZ, K. (1971) Tin as an essential growth factor for rats. In:
Mertz, W. & Comatzer, W. E., ed. Newer trace elements in
nutrition, New York, Marcel Dekker, pp. 313-325.
SCHWARZ, K. (1974) Recent dietary trace element research, exemplified
by tin, fluorine and silicon, Fed. Proc., 33: 1748-1757.
SCHWARZ, K., MILNE, D. B., & VINYARD, E. (1970) Growth effects of tin
compounds in rats maintained in a trace element-controlled
environment. Biochem. biophys. Res. Commun., 40: 22-29.
SCOTT, W. J. & STEWART, D. F. (1944) The influence of dissolved tin on
the growth of Clostridium botulinum in canned vegetables.
J. Counc. Sci. Ind. Res. Aust., 17: 16-22.
SEINEN, W. & WILLEMS, M. I. (1976) Toxicity of organotin compounds. I.
Atrophy of thymus and thymus-dependent lymphoid tissue in rats fed
di-n-octyltin dichloride. Toxicol. appl. Pharmacol., 35: 63-75.
SEINEN, W., VOS, J. G., SPANJE, I. van, SNOEK, M., BRANDS, R., &
HOOYKAAS, H. (1977a) Toxicity of organotin compounds. II.
Comparative in vivo and in vitro studies with various
organotin and organolead compounds in different animal species
with special emphasis on lymphocyte cytotoxicity. Toxicol. appl.
Pharmacol., 42: 197-212.
SEINEN, W., VOS, J. G., KRIEKEN, R. van, PENNINKS, A., BRANDS, R., &
HOOYKAAS, H. (1977b) Toxicity of organotin compounds, III.
Suppression of thymus-dependent immunity in rats. by di-n-butyltin
dichloride and di-n-octyltin dichloride. Toxicol. appl.
Pharmacol., 42: 213-224.
SHELDON, A. W. (1975) Effects of organotin anti-fouling coatings on
man and his environment. J. Paint Technol., 47: 54-58.
SHIRAISHI, Y., KUZUHARA, Y., & SUENAGA, S. (1972) [Determination of
tin in food by atomic absorption spectrometry.] J. Food Hyg. Soc.
Jpn 13: 97-100 (in Japanese).
SHIRASU, Y. (1970) Acute and sub-chronic toxicology studies of Dowco
213 in mice and rats. Test report on the sub-acute toxicity of
an insecticide, Dowco 213, Tokyo, Animal Pharmacology Institute of
Physical and Chemical Research, 11 pp. (in Japanese).
SHONO, T. & MATSUMURA, Y. (1970) Organic synthesis by electrolysis VI.
Anodie oxidation of aryleyclopropanes. J. org. Chem.,
35: 4157-4160.
SKACKOVA, I. N. (1967) [Hygienic substantiation of the maximum
permissible concentration of tetraethyltin in water bodies.] Gig.
i Sanit., 4: 11-17 (in Russian).
SKEEL, RL., & BRICKER, C. E. (1961) Spectrophotometric determination
of dibutyltin dichloride. Anal. Chem., 33: 428-431.
SMITH, J. D. (1970) The spectrophotometric determination of microgram
amounts of tin with phenylfluorone. Analyst, 95: 347-350.
SODERQUIST, C. J. & CROSBY, D. G. (1978) Determination of triphenyltin
hydroxide and its degradation products in water. Anal. Chem.,
50: 1435-1439.
STANCULESCU, C., MARIN, E., SANDULESCU, C., ILLE, C., TRICIA, A.,
ILLIESCU, M., PETROVICI, C., & POPESCU, P. (1972) [Copper, lead,
tin and arsenic content in canned fish after storage. II. Canned
fresh water fish.] Lucr. Inst. Cercet. Projiect. Ailment.,
10: 295-309 (in Romanian).
STOCKDALE, M., DAWSON, A. P., & SELWYN, M. J. (1970) Effects of
trialkyltin and triphenyltin compounds on mitochondrial
respiration. Eur. J. Biochem., 15: 342-351.
STONE, O. J., & WILLIS, C. J. (1968) The effect of stannous fluoride
and stannous chloride on inflammation. Toxicol. appl. Pharmacol.,
13: 332-338.
STONER, H. B. (1966) Toxicity of triphenyltin. Br. J. ind. Med.,
23: 222-229.
STONER, H. B., BARNES, J. M., & DUFF, J. I. (1955) Studies on the
toxicity of alkyl tin compounds. Br. J. Pharmacol., 10: 16-25.
STOROZEVA, H. H. (1963) [Contents of lead and tin in human teeth in
normal and caries cases.] Stomatologia, 1: 44-48 (in Russian).
STUDER, R. K., SIEGEL, B. A., MORGAN, J., & POTCHEN, E. J. (1973)
Dexamethasone therapy of triethyltin induced cerebral edema. Exp.
Neurol., 38: 429-437.
SUGIMAE, A. (1974) Emission spectrographic determination of trace
elements in airborne particulate matter. Anal. Chem.
46: 1123-1125.
SUZUKI, K. (1971) Some new observations in triethyltin intoxication of
rats. Exp. Neurol., 31: 207-213.
SVENSSON, V. (1975) [Tin poisoning caused by canned peaches.] Hyg.
och Miljö, No. 6, 25-27. (in Swedish).
TABOR, E. C. & WARREN, W. V. (1958) Distribution of certain metals in
the atmosphere of some American cities. Arch. ind. Hyg.,
17: 145-151.
THEUER, R. C., MAHONEY, A. W., & SARETT, H. P. (1971) Placental
transfer of fluoride and tin in rats given various fluoride and
tin salts. J. Nutr., 101: 525-532.
THOMPSON, K. C. & THOMERSON, D. R. (1974) Atomic absorption studies on
the determination of antimony, arsenic, bismuth, germanium, lead,
selenium, tellurium and tin by utilizing the generation of
covalent hydrides. Analyst, 99: 595-601.
TIHONOVA, Z. I. & ZORE, V. A. (1968) [Spectroscopic determination of
manganese, copper, aluminium, lead and tin in certain vegetables
and berries.] Gig. i Sanit., 33: 384-386 (in Russian).
TIPTON, I. H. (1960) The distribution of trace metals in the human
body. In: Seven, M. T., ed. Metal binding in medicine,
Philadelphia, Montreal, Lippincott, pp. 27-42.
TIPTON, I. H., COOK, M. J. STEINER, R. L., BOYLE, C. A., PERRY, H. M.,
& SCHROEDER, H. A. (1963) Trace elements in human tissue. Part I
Methods. Health Phys., 9: 89-101.
TIPTON, I. H., STEWART, P. L., & MARTIN, P. G. (1966) Trace elements
in diets and excreta. Health Phys., 12: 1683-1689.
TIPTON, I. H., STEWART, P. L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys.,
16: 455-462.
TJURIN, Y. M. & FLEROV, V. N. (1970) [Study of adsorption effects
under electro-reducing of organotin compounds on mercury.]
Elektrochim., 6: 1548-1552, (in Russian).
TJURIN, Y. M., FLEROV, V. N., & NIKITINA, Z. A. (1969) [On the
mechanism of electro-reducing of the dialkyltin chloride on the
mercury drip electrode.] Elektrochim., 5: 903 (in Russian).
TONGE, B.C. (1965) The gas chromatographic analysis of butyl- octyl-,
and phenyltin halides. J. Chromatogr., 19: 182-184.
TORACK, R., GORDON, J., & PROKOP, J. (1969) Pathobiology of acute
tri-ethyltin intoxication. Int. Rev. Neurobiol., 12: 45-86.
TROMBETTI, G. & MAINI, P. (1970) Determination of tricyclohexyltin
hydroxide residues in apples and pears. Pestic. Sci.,
1: 144-149.
TRUHAUT, R., CHAUVEL, Y., ANGER, J.P., NGUYEN PHU LICH, VAN DEN
DRIESSCHE, J., GUESNIER, L. R., & MORIN, N. (1976) Contribution to
the toxicological and pharmacological study of tributyltin oxide.
Eur. J. Toxicol. environ. Hyg., 9 (1): 31-40.
TUTTLE, R. F., VOGT, J. R., & PARKINSON, T. F. (1970) Neutron
activation analysis of trace elements in airborne particulates.
In: Proceedings of a Symposium on Nuclear Technology and
Environmental Pollution, Salzburg, Vienna, International Atomic
Energy Agency, pp. 119-137 (Paper No. IAEA-SM-142a/4).
UDRIS, J. (1971) The analysis of tin stabilizers used in
poly(vinylchloride) compositions. Analyst, 96: 130-139.
UNITED NATIONS (1977) United Nations yearbook of industrial
statistics: Commodity production data 1966-1975, Vol. II,
pp. 498-500.
US ENVIRONMENTAL PROTECTION AGENCY (1973) Air quality data for
metals, 1968 and 1969 from the National Air Surveillance
Networks, Research Triangle Park, USEPA, June 1973.
VERNON, F. (1974) The fluorometric determination of triphenyltin
compounds. Anal. Chim. Acta, 71: 192-195.
VINOGRADOV, A. P. (1953) The elementary chemical composition of
marine organisms, Memoir Sears Foundation for Marine Research,
New Haver, Yale University, pp. 16, 211, 240, 266, 360, 366, 535.
VINOGRADOV, A. P. (1956) Regularity of distribution of chemical
elements in the earth's crust. Geokhimiya, 1: 6-52.
VINOGRADOV, A. P. (1962) Average content of chemical elements in the
principal types of igneous rocks of the earth's crust.
Geokhimiya, 555-541.
VIZGIRDA, R. J. (1972) Organotin antifoulants. Paint and varnish
Produc., 62 (12): 12-28.
WALTERS, M. & ROE, F. J. C. (1965) A study on the effects of zinc and
tin administered orally to mice over a prolonged period. Food
Cosmet. Toxicol., 3: 271-276.
WARBURTON, S., UDLER, W., EWERT, R. M., & HAYNES, W. S. (1962)
Outbreak of foodborne illness attributed to tin. Public Health
Rep. (Wash.), 77: 798-800.
WEAST, R. C., ed. (1974) Handbook of Chemistry and Physics, 55th ed.
Cleveland, CRC Press.
WHO Technical Report Series, No. 515, 1973 ( Shistosomiasis control:
report of a WHO Expert Committee). pp. 30-32.
WHO (1975) 1974 evaluations of some pesticide residues in food,
pp. 155-158 (WHO Pesticides Residues Series No. 4).
WHO (1976) Environmental Health Criteria 1: Mercury. Geneva, WHO,
132 pp.
WIECZOREK, H. (1969) [The quantitative determination of organotin
compounds by means of quercetin chelates.] Dtsch.
Lebensmitt-Rundsch., 3: 74-78 (in German).
WILLIAMS, D. J. & PRICE, J. W. (1960) Paper chromatography of some
organotin compounds. Analyst, 85: 579-582.
WODSAK, W. (1967) Tin content of condensed milk. Angew. Chem. Int.
Ed., 6: 885-886.
WOGGON, H. & JEHLE, D. (1973) [The inverse voltametric determination
of biocidal organotin compounds.] Die Nahrung, 17:739-748
(in German).
WOGGON, H. & JEHLE, D. (1975) [The residual analysis of biocidal
organo-tin compounds by means of inverse voltametric methods.]
Die Nahrung, 19:271-275 (in German).
WOGGON, H, SAUBERLICH, H., & UHDE, W. J. (1972) [A contribution to the
determination (anodic stripping method) of organotin compounds and
their degradation and decomposition products.] Z. anal. Chem.,
260: 268-274 (in German).
WOIDICH, H. & PFANNHAÜSER, W. (1973) [Distribution of tin between the
solid and liquid contents of cans of vegetables and fruits.]
Dtsch. Lebensmitt-Rundsch., 69:224-226 (in German).
WOOD, J. M. (1974) Biological cycles for toxic elements in the
environment. Science, 183: 1049-1052.
WOOD, J. M., SEGALL, H. J., RIDLEY, W. P., CHEH, A., CHUDYK, W., &
THAYER, J. S. (1975) Metabolic cycles for toxic elements in the
environment. In Hutchinson, T. C., ed. Symposium Proceedings Vol.
I, International Conference on Heavy Metals in the Environment.
Toronto, Canada, pp. 49-68.
WOOD, J. M., CHEH, A., DIZIKES, L. J., RIDLEY, W. P., RAKOW, S., &
LAKOWICZ, J. R. (1978) Mechanisms for biomethylation of metals and
metalloids. Fed. Proc., 37: 16-21.
YUM, N.M., CONINE, D. L., MARTZ, R. C., FORNEY, R. B., & STOOKEY, G.
K. (1976) Renal tubular injury in rats induced by sodium
pentalfluorostannite, a new anticariogenic agent. Toxicol. appl.
Pharmacol., 37: 363-370.
ZOHM, H. (1972) [A new method for determination of tin and iron in
canned vegetables and fruit juices.] Z. Lebensmitt. -Unters.,
149: 30-33 (in German).
