
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 11
MYCOTOXINS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of
the United Nations Environment Programme,
and the World Health Organization
World Health Organization
Geneva, 1979
ISBN 92 4 154071 0
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the impression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city, or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR MYCOTOXINS
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1 Summary
1.1.1 Aflatoxins
1.1.1.1 Sources and occurrence
1.1.1.2 Effects and associated exposures
1.1.2 Other mycotoxins
1.1.2.1 Ochratoxins
1.1.2.2 Zearalenone
1.1.2.3 Trichothecenes
1.2 Recommendations for further studies
1.2.1 General recommendations
1.2.2 Recommendations for aflatoxins
1.2.3 Recommendations for other mycotoxins
2. MYCOTOXINS AND HUMAN HEALTH
3. AFLATOXINS
3.1 Properties and analytical methods
3.1.1 Chemical properties
3.1.2 Methods of analysis for aflatoxins in foodstuffs
3.1.2.1 Sampling
3.1.2.2 Methods of analysis
3.2 Sources and occurrence
3.2.1 Formation by fungi
3.2.1.1 Moisture content and temperature
3.2.1.2 Invasion of field crops by A. flavus
3.2.2 Occurrence in foodstuffs
3.2.2.1 Maize
3.2.2.2 Wheat, barley, oats, rye, rice, and
sorghum
3.2.2.3 Groundnuts (peanuts)
3.2.2.4 Soybeans and common beans
3.2.2.5 Tree nuts
3.2.2.6 Copra
3.2.2.7 Cottonseed
3.2.2.8 Spices and condiments
3.2.2.9 Animal feeds
3.2.2.10 Animal products
3.2.3 Fate of aflatoxins during the handling and
processing of food
3.2.4 Pathways and levels of exposure
3.3 Metabolism
3.3.1 Absorption
3.3.2 Tissue distribution
3.3.2.1 Animal studies
3.3.2.2 Studies in man
3.3.3 Metabolic transformation and activation
3.3.4 Excretion
3.3.4.1 Animal studies
3.3.4.2 Studies in man
3.4 Effects in animals
3.4.1 Field observations
3.4.2 Experimental studies
3.4.2.1 Acute and chronic effects:
hepatotoxicity
3.4.2.2 Hepatotoxicity connected with
extrahepatic effects
3.4.2.3 Carcinogenesis
3.4.2.4 Teratogenicity
3.4.2.5 Mutagenicity
3.4.2.6 Biochemical effects and mode of action
3.4.2.7 Factors modifying the effects
and dose-response relationships of
aflatoxins
3.5 Effects in man -- epidemiological and clinical studies
3.5.1 General population studies
3.5.1.1 Liver carcinogenesis
3.5.1.2 Other effects reported to be
associated with aflatoxins
3.5.2 Occupational exposure
3.6 Evaluation of the health risks of exposure to aflatoxins
3.6.1 Human exposure conditions
3.6.1.1 Sources and levels of aflatoxins in
food
3.6.1.2 Dietary intake and levels in human
tissues
3.6.2 Acute effects of exposure
3.6.2.1 Acute liver disease
3.6.2.2 Reye's syndrome
3.6.3 Chronic effects of aflatoxin exposure
3.6.3.1 Cancer of the liver
3.6.3.2 Juvenile cirrhosis in India
3.6.4 Guidelines for health protection
4. OTHER MYCOTOXINS
4.1 Ochratoxins
4.1.1 Properties and analytical methods
4.1.1.1 Chemical properties
4.1.1.2 Methods for the analysis of foodstuffs
4.1.2 Sources and occurrence
4.1.2.1 Fungal formation
4.1.2.2 Occurrence in foodstuffs
4.1.3 Metabolism
4.1.3.1 Absorption
4.1.3.2 Tissue distribution and metabolic
conversion
4.1.3.3 Excretion
4.1.4 Effects in animals
4.1.4.1 Field observations
4.1.4.2 Experimental studies
4.1.5 Effects in man
4.1.5.1 Ochratoxin A and Balkan nephropathy
4.1.6 Conclusions and evaluation of the health risks
to man of ochratoxins
4.1.6.1 Experimental animal studies
4.1.6.2 Studies in man
4.1.6.3 Evaluation of health risks
4.2 Zearalenone
4.2.1 Properties, analytical methods, and sources
4.2.2 Occurrence
4.2.3 Effects in animals
4.2.3.1 Field observations
4.2.3.2 Experimental studies
4.2.4 Conclusions and evaluation of health risks to
man of zearalenone
4.2.4.1 Animal studies
4.2.4.2 Evaluation of health risks
4.3 Trichothecenes
4.3.1 Properties and sources
4.3.2 Occurrence
4.3.3 Effects in animals
4.3.3.1 Field observations
4.3.3.2 Experimental studies
4.3.4 Alimentary toxic aleukia
4.3.5 Conclusions and evaluations of the health risks
to man of trichothecenes
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Division of
Environmental Health, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda which
will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR MYCOTOXINS
Members
Dr B. K. Armstrong, University Department of Medicine, Perth Medical
Centre, Nedlands, Australiaa
Dr A.D. Campbell, Food and Drug Administration, US Department of
Health, Education and Welfare, Washington, DC, USAb
Dr T. Denizel, Department of Agriculture Microbiology, Faculty of
Agriculture, University of Ankara, Ankara, Turkeya
Dr M. Jemmali, Service Mycotoxines de l'INRA, Station de Biochimie
et Physico-Chimie des Céréales, Institut National de la
Recherche Agronomique, Paris, Francea
Professor P. Krogh, The University Institute of Pathological
Anatomy, Copenhagen, Denmark,a,b,c
Professor V. Kusak, Institute of Experimental Medicine,
Czechoslovak Academy of Sciences, Prague, Czechoslovakia
(Vice-Chairman)a,b
Dr V. Nagarajan, National Institute of Nutrition, Jamai-Osmania,
Hyderabad, Indiaa
Dr M. F. Nesterin, Institute of Nutrition, Academy of Medical
Sciences of the USSR, Moscow, USSRb
Professor P. Newberne, Department of Nutrition and Food Science,
Massachusetts Institute of Technology, Cambridge, MA, USAa
Dr D. S. P. Patterson, Central Veterinary Laboratory, Ministry of
Agriculture, Fisheries and Food, Weybridge, England
(Chairman)a,b
Dr F. G. Peers, Tropical Products Institute, London, Englanda,b
Professor A. C. Sarkisov, Laboratory of Antibiotics and Mycology,
All-Union Institute of Experimental Veterinary Science, Moscow,
USSRa
Dr P. L. Schuller, Laboratory of Chemical Analysis of Foodstuffs,
National Institute of Public Health, Bilthoven, Netherlands
(Vice-Chairman)a
Dr A. Rogers, Department of Nutrition and Food Science,
Massachusetts Institute of Technology, Cambridge, MA, USA
(Rapporteur)b
Professor H. D. Tendon, All-India Institute of Medical Sciences,
New Delhi, Indiaa
Professor A. Wasunna, Department of Surgery, University of
Nairobi, Kenyaa
Representatives of other International Organizations
Dr O. Alozie, United Nations Environment Programme,
Nairobi, Kenyaa,b
Dr D. Djordjevic, Occupational Safety and Health Branch,
International Labour Office, Geneva, Switzerlanda
Dr G. D. Kouthon, Food and Agriculture Organization of the
United Nations, Rome, Italya
Professor D. Reymond, Coordinating Committee on Food Chemistry,
International Union of Pure and Applied Chemistry, La Tour
de Peilz, Switzerlandb
Mrs. M. Th. van der Venne, Commission of the European Communities,
Health Protection Directorate, Luxembourga,b
WHO Secretariat
Dr C. Agthe, Environmental Health Criteria arid Standards, Division
of Environmental Health, WHO, Geneva, Switzerland
(Co-Secretary)a,b
Dr L. Fishbein, US Public Health Service, National Centre for
Toxicological Research, Chemistry Division, Jefferson, AR, USA
(Temporary Adviser)a
Dr J. Korneev, Environmental Health Criteria and Standards, Division
of Environmental Health, WHO, Geneva, Switzerlanda,b
Professor E. Lillehoj, US Department of Agriculture, Northern
Research Laboratory, Peoria, IL, USA (Temporary Adviser)a
Dr C. A. Linsell, Interdisciplinary Programme and International
Liaison, IARC, Lyons, Francea,b
R. Lunt, Cancer Unit, Division of Noncommunicable Diseases, WHO,
Geneva, Switzerlandb
Dr Z. Matyas, Veterinary Public Health, Division of Communicable
Diseases, WHO, Geneva, Switzerlanda,b
Professor C. J. Mirocha, Department of Plant Pathology, University
of Minnesota, St Paul, MA, USA (Temporary adviser)a
Dr J. Parizek, Environmental Health Criteria and Standards,
Division of Environmental Health, WHO, Geneva, Switzerland
(Co-Secretary)a,b
Dr V. B. Vouk, Environmental Health Criteria and Standards, Division
of Environmental Health, WHO, Geneva, Switzerlanda,b
a Attended the first meeting of the Task Group.
b Attended the second meeting of the Task Group.
c Present address: Department of Veterinary Microbiology,
Pathology and Public Health, School of Veterinary Medicine,
Purdue University, West Layayette, IN, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR MYCOTOXINS
Members of the Task Group on Environmental Health Criteria for
Mycotoxins met in Geneva from 1 to 7 March 1977 and from 19 to 23
June 1978.
The first meeting was opened on behalf of the Director-General
by Dr B. H. Dieterich, Director, Division of Environmental Health,
and the second by Dr C. Agthe, Division of Environmental Health.
The first and second draft criteria documents were prepared by
Professor C. J. Mirocha. The comments on which the second draft was
based were received from the national focal points for the WHO
Environmental Health Criteria Programme in Belgium, Czechoslovakia,
Federal Republic of Germany, India, New Zealand, Poland, Sweden,
Thailand, USSR, and USA, and from the International Agency for
Research on Cancer (IARC), Lyons, the United Nations Industrial
Development Organization (UNIDO), Vienna, and the Food and
Agriculture Organization of the United Nations (FAO), Rome. Comments
were also received from the Tropical Products Institute, London.
Dr P. Krogh, Dr D. S. P. Patterson, and Dr P. L. Schuler
assisted in the preparation of the third draft criteria document,
which was submitted for review to all the members of the Task Group,
and to Dr R. Plestina of the Institute for Medical Research and
Occupational Health, Yugoslav Academy of Sciences and Arts, Zagreb,
Yugoslavia before the second meeting of the Task Group. The final
edited draft was kindly reviewed by Dr D. S. P. Patterson. The
collaboration of these national institutions, international
organizations, WHO collaborating centres, and individual experts is
gratefully acknowledged.
The document is based primarily on original publications listed
in the reference section. However, several recent publications
reviewing the occurrence, health effects, and other aspects of
mycotoxins have also been used including monographs prepared by
Purchase (1974), Pokrovskij et al. (1977) and Wyllie & Morehouse
(1977), and the report on the joint FAO/ WHO/UNEP Conference on
Mycotoxins in Nairobi 1977 (FAO, 1977). In addition, comprehensive
data have been obtained from the proceedings of several symposia and
meetings including the Conference on Mycotoxins in Human and Animal
Health, held in Maryland, USA, in 1976 (Rodricks et al., 1977).
Details of the WHO Environmental Health Criteria Programme
including some of the terms frequently used in the documents, may be
found in the general introduction to the Environmental Health
Criteria Programme published together with the environmental health
criteria document on mercury (Environmental Health Criteria 1,
Mercury, Geneva, World Health Organization, 1976), and now available
as a reprint.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER RESEARCH
1.1 Summary
The ingestion of food containing mycotoxins, the toxic products
of microscopic fungi (moulds), may have serious adverse health
effects in man. Occasionally, occupational exposure to airborne
mycotoxins may also occur.
The occurrence of mycotoxins in foodstuffs depends on their
formation by specific strains of fungi and is influenced by
environmental factors such as humidity and temperature. Thus,
mycotoxin contamination of foodstuffs may vary with geographical
conditions;, production and storage methods, and also with the type
of food, since some food products are more suitable substrates for
fungal growth than others.
The present document contains an evaluation of health risks
associated with four classes of mycotoxins. Aflatoxins are treated
in most detail because more is known about them than about the other
mycotoxins and because there is epidemiological evidence associating
health effects in man with exposure to aflatoxins.
For the other 3 classes (ochratoxins, zearalenone, and
trichothecenes), toxic effects in animals have been established and
there is well-documented evidence that human exposure may occur, at
least for the first two classes.
1.1.1 Aflatoxins
1.1.1.1 Sources and occurrence
Aflatoxins are produced by certain strains of Aspergillus
flavus and Aspergillus parasiticus. These fungi are ubiquitous
and the potential for contamination of foodstuffs and animal feeds
is widespread. The occurrence and magnitude of aflatoxin
contamination varies with geographical and seasonal factors, and
also with the conditions under which a crop is grown, harvested, and
stored. Crops in tropical and subtropical areas are more subject to
contamination than those in temperate regions, since optimal
conditions for toxin formation are prevalent in areas with high
humidity and temperature. Toxin-producing fungi can infect growing
crops as a consequence of insect or other damage, and may produce
toxins prior to harvest, or during harvesting and storage.
The chemical structures of aflatoxins have been elucidated, and
analytical techniques are available for their identification and
determination in foodstuffs and body tissues at the µg/kg level and
lower. Four aflatoxins (B1, G1, B2, G2, often occurring
simultaneously, have been detected in foods of plant origin
including maize, groundnuts (peanuts), and tree nuts as well as many
other foodstuffs and feeds.
In animals, ingested aflatoxins may be metabolically degraded.
Aflatoxin B1 may be converted into aflatoxin M1 which may occur
in the milk. The concentration of aflatoxin M1 in the milk of cows
is about 300 times lower than the concentration of aflatoxin B1
consumed in the feed. In certain experimental animals, only small
amounts of administered aflatoxins have been found in tissues, 24 h
after injection.
In studies on pigs, aflatoxin residues were detected in the
liver, kidney, and muscle tissues of animals given aflatoxins in the
feed for several months. There do not appear to be any published
works on aflatoxin residues in the tissues of slaughtered animals.
The use of resistant varieties of seed and of pesticides, and
careful drying and storing procedures can reduce fungal infestation
and thus diminish food contamination by aflatoxins. The toxin is not
eliminated from foodstuffs or animal feeds by ordinary cooking or
processing practices and, since pre-and post-harvest procedures do
not ensure total protection from aflatoxin contamination, techniques
for decontamination have been developed. The toxin is generally
concentrated in a small proportion of seeds that are often different
in colour. Segregation of discoloured seeds by sorting can
significantly reduce the aflatoxin levels in some crops, such as
groundnuts. Visual inspection for mould growth before processing can
serve as an initial screening technique but toxin-producing fungi
can be present without detectable aflatoxins and vice versa. Because
aflatoxin distribution in a contaminated, unprocessed commodity is
uneven, adequate sampling is essential for effective monitoring. As
aflatoxins can be chemically degraded in vitro by several oxidizing
agents and alkalis, hydrogen peroxide and ammonia are currently used
for the chemical decontamination of animal feeds.
1.1.1.2 Effects and associated exposures
Outbreaks of aflatoxicosis in farm animals have been reported
from many areas of the world. The liver is mainly affected in such
outbreaks and also in experimental studies on animals, including
nonhuman primates. The acute liver lesions are characterized by
necrosis of the hepatocytes and biliary proliferation, and chronic
manifestations may include fibrosis. A feed level of aflatoxin as
low as 300 µg/kg can induce chronic aflatoxicosis in pigs within
3-4 months.
Aflatoxin B1 is a liver carcinogen in air least 8 species
including nonhuman primates. Dose-response relationships have been
established in studies on rats and rainbow trout, with a 10% tumour
incidence estimated to occur at feed levels of aflatoxin B1 of
1 µg/kg, and 0.1 µg/kg, respectively. In some studies, carcinomas of
the colon and kidney have been observed in rats treated with
aflatoxins. Aflatoxin B1 causes chromosomal aberrations and DNA
breakage in plant and animal cells, and, after microsomal
activation, gene mutations in several bacterial test systems. In
high doses, it may be teratogenic.
The acute toxicity and carcinogenicity of aflatoxins are greater
in male than in female rats; hormonal involvement may be responsible
for this sex-linked difference. Nutritional status in animals,
particularly with respect to lipotropes, proteins, vitamin A, and
lipids (including cyclopropenoid fatty acids), can modify the
expression of acute toxicity or carcinogenicity or both.
There is little information on the association of acute
hepatoxicity in man with exposure to aflatoxins but cases of acute
liver damage have been encountered that could possibly be attributed
to acute aflatoxicosis. A recent outbreak of acute hepatitis in
adjacent districts of two neighbouring states in north-west India,
which affected several hundred people, was apparently associated
with the ingestion of heavily contaminated maize, some samples of
which contained aflatoxin levels in the mg/kg range, the highest
reported level being 15 mg/kg.
Liver cancer is more common in some regions of Africa and
southeastern Asia than in other parts of the: world and, when local
epidemiological information is considered together with experimental
animal data, it appears that increased exposure to aflatoxins may
increase the risk of primary liver cancer. Pooled data from Kenya,
Mozambique, Swaziland, and Thailand, show a positive correlation
between daily dietary aflatoxin intake (in the range of 3.5 to
222.4 ng/kg body weight per day) and the crude incidence rate of
primary liver cancer (ranging from 1.2 to 13.0 cases per 100 000
people per year). There is also some evidence of a vital involvement
in the etiology of the disease.
In view of the evidence concerning the effects, particularly the
carcinogenic effects, of aflatoxins in several animal species, and
in view of the association between aflatoxin exposure levels and
human liver cancer incidence observed in some parts of the world,
exposure to aflatoxins should be kept as low as practically
achievable. The tolerance levels for food products established in
several countries should be understood as management tools intended
to facilitate the implementation of aflatoxin control programmes,
and not as exposure limits that necessarily ensure health
protection.
1.1.2 Other mycotoxins
1.1.2.1 Ochratoxins
Ochratoxins are produced by several species of the fungal genera
Aspergillus and Pencillium. These fungi are ubiquitous and the
potential for contamination of foodstuffs and animal feed is
widespread. Ochratoxin A, the major compound, has been found in more
than 10 countries in Europe and the USA. Ochratoxin formation by
Aspergillus species appears to be limited to conditions of high
humidity and temperature, whereas at least some Pencillium species
may produce ochratoxin at temperatures as low as 5°C.
Analytical techniques have been developed for the identification
and quantitative determination of ochratoxin levels in the µg/kg
range.
Ochratoxin A has been found in maize, barley, wheat, and oats, as
well as in many other food products, but the occurrence of
ochratoxin B is rare. Residues of ochratoxin A have been identified
in the tissues of pigs in slaughterhouses, and it has been shown,
under experimental conditions, that residues can still be detected
in pig tissues one month after the termination of exposure.
Field cases of ochratoxicosis in farm animals (pigs, poultry)
have been reported from several areas of the world, the primary
manifestation being chronic nephropathy. The lesions include tubular
atrophy, interstitial fibrosis, and, at later stages, hyalinized
glomeruli. Ochratoxin A has been found to be nephrotoxic in all
species of animals studied so far, even at the lowest level tested
(200 µg/kg feed in rats and pigs). It has also been reported to
produce teratogenic effects in mice, rats, and hamsters.
Human endemic nephropathy is a kidney disease of unknown
etiology that has so far only been encountered in some areas of the
Balkan Peninsula. The renal changes observed with this disease are
comparable to those seen in ochratoxin A-associated nephropathy in
pigs. High ochratoxin A exposure through diet has been found in some
of the areas of the Balkan Peninsula, where endemic nephropathy is
prevalent.
1.1.2.2 Zearalenone
Zearalenone, a metabolite produced by various species of
Fusarium, has been observed as a natural contaminant of cereals,
in particular maize, in many countries in Africa and Europe, and in
the USA.
It has been shown to produce estrogenic effects in animals, and
field cases of a specific estrogenic syndrome in pigs and of
infertility in cattle have been encountered in association with feed
levels of zearalenone of 0.1-6.8 mg/kg and 14 mg/kg, respectively.
The compound has also produced congenital malformations in the rat
skeleton.
In some countries, zearalenone has been found in samples of
cornmeal and cornflakes destined for human consumption, at levels up
to 70 µg/kg, corresponding to doses 400-600 times lower than those
causing effects in monkeys or mice under experimental conditions. In
certain areas of Africa, substantially higher levels have
occasionally been found in beer and sour porridge prepared from
contaminated maize and sorghum.
No adverse effects due to zearalenone intake have been reported
in man, so far, but a possible health hazard connected with the
dally intake of zearalenone at levels such as those reported for
African fermented preparations needs further attention.
1.1.2.3 Trichothecenes
Trichothecene toxins belong to a group of closely related
chemical compounds produced by several species of Fusarium,
Cephalosporium, Myrothecium, Trichoderma, and Stachybotrys.
Four trichothecenes (T-2 toxin, nivalenol, deoxynivalenol, and
diacetoxyscirpenol) have been detected as natural contaminants in a
small number of food samples.
Alimentary toxic aleukia, a disease diagnosed in man about 40
years ago, was apparently associated with the ingestion of grains
invaded by Fusarium species. No cases have been reported since the
end of the Second World War and the disappearance of the disease is
probably due to improved food production and storage conditions.
There is no firm evidence connecting the recently identified
trichothecenes with alimentary toxic aleukia occurring in the past,
or with other human disease.
1.2 Recommendations for Further Studies
1.2.1 General recommendations
(a) There is a need for more information concerning the
occurrence of mycotoxins in various parts of the world and
the possible daily intake of mycotoxins by man.
(b) Further studies should be undertaken on factors affecting
fungal growth and mycotoxin formation in foodstuffs, under
preharvest, postharvest, and storage conditions.
(c) The effects of various cooking processes on the levels of
mycotoxins in foods should be elucidated.
(d) Better methods should be developed for the rapid detection
and measurement of mycotoxin levels in foodcrops.
(e) Sampling has proved to be the most difficult step in the
surveillance of food commodities. The development of
reliable, internationally accepted sampling procedures is
strongly recommended.
(f) Better methods should be developed for the identification
and measurement of mycotoxins in human tissues, body fluids,
and excreta.
(g) A network of reference centres should be established to
assist Member States in confirming the identity of
individual mycotoxins found in human foods and tissues.
These reference centres should also provide mycotoxin
reference samples, upon request, to reinforce the
inter-comparability of analytical results obtained in
different parts of the world.
(h) Better understanding is needed of the role of mycotoxins in
human diseases. Where association between exposure to
mycotoxins and the incidence of certain diseases is
suspected, detailed epidemiological studies should be
carried out.
(i) Improved diagnostic methods for the effects on health of
mycotoxins are needed, particularly methods for the
detection of early changes that occur before the development
of irreversible effects.
(j) Attempts should be made to monitor exposure levels and to
search for effects in workers handling pure mycotoxins or
contaminated materials. This could provide important
information on the effects of chronic exposure to mycotoxins
and also indicate the need for safety measures.
1.2.2 Recommendations for aflatoxins
(a) The validity of the assumption of a causal relationship
between aflatoxin ingestion and primary liver cancer should
be examined further by introducing control measures to
reduce aflatoxin exposure in areas of high liver cancer
incidence and high aflatoxin exposure. This should be
followed by the monitoring of liver cancer incidence in
these areas and in comparable areas where the aflatoxin
exposure has been low.
(b) The prevalence of hepatitis B antigen should be determined
in areas with various levels of aflatoxin exposure and a
high incidence of primary liver cancer.
(c) Suspected outbreaks of acute aflatoxicosis should be
studied in detail. Such studies should include measurements
of the exposure to aflatoxins through foods and other
routes. The presence of aflatoxins and their derivatives in
the tissues and excreta of individuals including both those
affected and those apparently unaffected by the disease,
should be investigated.
(d) A prolonged, continuous surveillance of the health status
of exposed populations is considered essential in
localities, where outbreaks of acute aflatoxicosis have
occurred. Such follow-up studies are important to fill the
gaps in knowledge on the late effects of short-term exposure
to high levels of aflatoxin in man. Recently reported
outbreaks of aflatoxin-associated hepatitis in southeastern
Asia may provide an ideal opportunity for such studies.
(e) Reports from the various countries on the presence of
aflatoxins in human tissues, body fluids, and excreta should
be confirmed using specific assay methods with adequate
limits of detection. The frequency of such events should be
studied in appropriate samples of the general population of
various countries and a search made for the sources of
aflatoxin exposure.
(f) The implication of aflatoxin as a contributing factor in
the development of Reye's syndrome should be further
investigated using the case-control approach. Data should be
obtained on the presence of aflatoxins in tissues, body
fluids, and excretion products for each case and its
control, and attempts should be made to identify dietary
sources of aflatoxins.
(g) More information is needed on the gastrointestinal
absorption of aflatoxins in animals and human subjects, as
well as on the rate of disappearance of aflatoxins from
farm-animal and human tissues. This is important both for
the evaluation of aflatoxin residues in food of animal
origin, and for the evaluation of aflatoxin levels in human
tissues as a means of assessing exposure.
(h) The modifying effect of the dietary intake of lipotropes,
protein, or vitamin A on aflatoxin-related carcinogenesis
should be further studied in experimental animals. This
aspect should also be included in epidemiological studies on
the association between human liver cancer incidence and
aflatoxin intake.
1.2.3 Recommendations for other mycotoxins
(a) The levels of ochratoxin A and possibly citrinin should be
measured in "food-on-the-plate" in areas of the Balkan
Peninsula with different incidence rates of Balkan
nephropathy.
(b) Further systematic investigations are needed on the levels
of ochratoxin in foodstuffs and animal feeds in different
parts of the world, and their association with nephropathy
in farm animals. More work is needed in various parts of the
world to confirm or exclude the strictly localized
occurrence of endemic nephropathy affecting human subjects
and considered so far to be confined to certain areas of the
Balkan Peninsula.
(c) Further studies are required on the mechanisms of ochratoxin
toxicity, and on possible interactions with other nephrotoxic
agents.
(d) Studies should be made in different countries of the levels
of zearalenone in human food and on total daily intake.
(e) More information is needed on the levels of zearalenone in
foods prepared from fermented maize and sorghum, such as
those found in certain parts of Africa, and on the possible
adverse effects of the daily consumption of these products,
particularly in view of the estrogen-like effects of
zearalenone observed in animals.
2. MYCOTOXINS AND HUMAN HEALTH
The toxicity of certain mushrooms has been known for a long
time. However, the potential human hazard of the toxic products of
other fungi was not recognized until the 1850s when an association
between the ingestion of rye infected with Claviceps purpurea and
the clinical features of ergotism was discovered. This was followed
by reports of other mycotoxicoses that affected man such as the
identification of a syndrome associated with the ingestion of bread
infected by Fusarium graminearum, recognition of human
stachybotryotoxicosis, and studies on the association between
alimentary toxic aleukia (ATA) and the ingestion of over-wintered
grains infested with Fusarium poae and Fusarium sporotrichioides
(Sarkisov, 1954).
Recognition of the association of ATA with the consumption of
food contaminated by moulds and the corresponding preventive
measures taken, resulted in the eradication of the disease (Leonov,
1977) showing that, even before the isolation of the first
mycotoxins, fungi-related foodborne diseases could be prevented.
The discovery of the hepatotoxic and hepatocarcinogenic
properties of Aspergillus flavus in the early 1960s quickly followed
by the elucidation of the structure of the aflatoxins changed the
control strategy in the whole field of mycotoxins. A more
quantitative approach is now possible, based primarily upon the
chemical determination of the toxins and on studies of their effects
in relation to dose.
In spite of increasing knowledge concerning human mycotoxicoses,
the majority of data available on mycotoxins and mycotoxicoses have
been obtained from veterinary medicine. Field studies, as well as
studies on experimental animals indicate that the potential toxicity
of mycotoxins is great. Future investigations may well establish a
causal role of mycotoxins in other human diseases besides those
considered so far.
Almost all plant products can serve as substrates for fungal
growth and subsequent mycotoxin formation, thus providing the
potential for direct contamination of human food. When farm animals
used for food production, ingest feed contaminated with mycotoxins,
not only may a direct toxic effect on the animals occur but there
may also be a carry-over of the toxins into milk and meat, thus
creating a further avenue for human exposure to mycotoxins.
Furthermore, occupational exposure may occur through other media
such as air.
In this document, the risks of health effects have been
considered only for those mycotoxins for which there is evidence of
human exposure and of well defined adverse effects, at least in
animals. This category includes the aflatoxins, ochratoxins, and
zearalenone. The trichothecenes have also been included, as these
have been shown, more recently, to be produced by fungi, that were
reported to be associated with outbreaks of human illness several
decades ago (ATA).
During recent years many other mycotoxins have been discovered
such as: citreoviridin; citrinin; cyclochlorotine; luteoskyrin;
maltoryzine; patulin; P R toxin; rubratoxin; rugulosin;
sterigmatocystine; and tremorgens.
Some of these toxins, which are not discussed in this document,
have been suggested to be related to disease outbreaks in farm
animals (Pier et al., 1977). Certain human diseases, suspected of
being associated with mycotoxins (van Rensburg, 1977), have not been
discussed in this document as the causative agents have not been
identified.
Of the four groups of mycotoxins considered only aflatoxins have
been shown to be associated with well recognized human health
effects. For this reason, they are treated separately from the other
mycotoxins.
3. AFLATOXINS
3.1 Properties and Analytical Methods
3.1.1 Chemical properties
Although 17 compounds, all designated aflatoxins, have been
isolated, the term aflatoxins usually refers to 4 compounds of the
group of bis-furanocoumarin metabolites produced by Aspergillus
flavus and A. parasiticus, named B1, B2, G1 and G2,
which occur naturally in plant products. The 4 substances are
distinguished on the basis of their fluorescent colour, B standing
for blue and G for green with subscripts relating to the relative
chromatographic mobility. Cows fed rations containing aflatoxin B1
and B2 excrete metabolites in the milk called aflatoxin M1 and
aflatoxin M2 (see section 3.3.4.1); M stands for milk, and again
the subscripts relate to the relative chromatographic mobility.
(Aflatoxin M1 is also a fungal metabolite.) Of the 4 major
aflatoxins, B1 is usually found in the highest concentrations,
followed by G1 while B2 and G2 occur in lower concentrations.
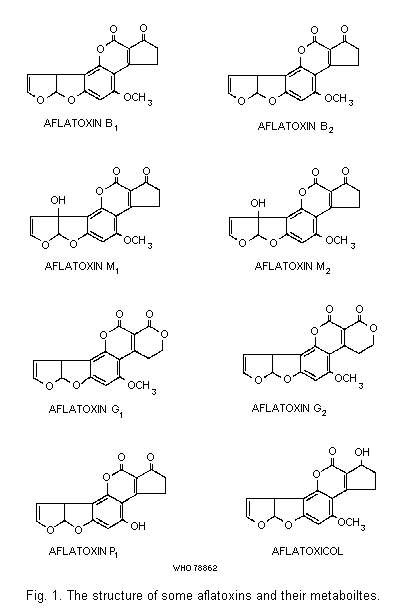
The structures of a number of aflatoxins and of aflatoxin
B1-related metabolites (see section 3.3.3) are illustrated in
Fig. 1. The structure of aflatoxins B1 and G1 were determined
by Asao et al. (1963, 1965) and that of B2 by Chang et al. (1963).
Aflatoxins B2 and G2 are dihydroderivatives of the parent
compounds (Hartley et al., 1963). Aflatoxins M1 and M2 are the
hydroxylated metabolites of B1 and B2, respectively (Holzapfel
et al., 1966; Masri et al., 1967; Buchi & Weinreb, 1969). Chemical
properties of some naturally-occurring aflatoxins and metabolites
are summarized in Table 1.
The aflatoxins are intensely fluorescent, when exposed to
long-wave ultraviolet (UV) light. This makes it possible to detect
these compounds at extremely low levels (ca. 0.5 ng or less per spot
on thin-layer chromatograms) and provides the basis for practically
all the physicochemical methods for their detection and
quantification. A concentration of aflatoxin M1 of 0.02 µg/litre
can be detected in liquid milk (Schuller et al., 1977).
Aflatoxins are freely soluble in moderately polar solvents
(e.g., chloroform and methanol) and especially in dimethylsulfoxide
(the solvent usually used as a vehicle in the administration of
aflatoxins to experimental animals); the solubility of aflatoxins in
water ranges from 10-20 mg/litre.
As pure substances, the aflatoxins are very stable at high
temperatures, when heated in air. However, they are relatively
unstable, when exposed to light, and particularly to UV radiation,
and air on a TLC plate and especially when dissolved in highly polar
solvents. Chloroform and benzene solutions are stable for years if
kept in the: dark and cold.
 Little or no destruction of aflatoxins occurs under ordinary
cooking conditions, and heating for pasteurization. However,
roasting groundnuts appreciably reduces the levels of aflatoxins
(see section 3.2.3) and they can be totally destroyed by drastic
treatment such as autoclaving in the presence of ammonia or by
treatment with hypochlorite.
Table 1. Physical and chemical properties of some aflatoxins and their metabolites
Aflatoxin Molecular Relative Melting Ultraviolet absorption (epsilon)c Fluorescence Reference
formula molecular point emission
mass °C 265 nm 360-362 nm nm
B1a C17H12O6 312 268-269 12 400 21 800 425 Asao et al. (1965)
B2a C17H14O6 314 286-289 12 100 24 000 425 Chang et al. (1963)
G1a C17H12O6 328 244-246 9 600 17 700 450 Asao et al. (1965)
G2a C17H14O7 330d 237-240d 8 200 17 100 450 Hartley et al. (1963)
M1a C17H12O7 328 299 14 150 21 250 (357 nm) 425 Holzapfel et al. (1966)
M2a C17H14O7 330 293 12 100 (264 nm) 22 900 (357 nm) f Holzapfel et al. (1966)
P1b C16H10O6 298 >320 11 200 (267 nm) 15 400 (362 nm) f Dalezios et al. (1971 ) and
14 900 (342 nm) Buchi et al. (1973)
Q1 C17H12O7 328 e 11 450 (267 nm) 17 500 (366 nm) f Masri et al. (1974a,b)
Aflatoxicol C17H14O6 314 230--234d 10 800 (261 nm) 14 100 (375 nm) 425 Detroy & Hesseltine (1970)
a Molar absorption coefficient for aflatoxins B1, B2, G1, and G2 obtained from Rodricks et al. (1970) and those for M1 and M2
from Stubblefield et al, (1972).
b P stands for phenolic products of O-demethylation of aflatoxin B1.
c Compounds dissolved in methanol except for aflatoxin P1 which in this case was dissolved in ethanol. Data on molar absorption
coefficients for other peaks and on the ultraviolet absorption characteristics of aflatoxins in other solvents can be found
in the original papers.
d Data from Butler (1974).
e Not available.
f Violet fluorescence of aflatoxin M2 and yellow-green fluorescence of aflatoxins P1 and Q1 reported in original papers.
The presence of a lactone ring in the aflatoxin molecule makes
them susceptible to alkaline hydrolysis (De Iongh et al., 1962).
This characteristic is important in that any food processing
involving alkali treatment can decrease the contamination of the
products (section 3.2.3) although the presence of protein, the pH,
and the duration of treatment may modify the results (Beckwith et
al., 1975). However, if the alkaline treatment is mild,
acidification will reverse the reaction to reform the original
aflatoxin.
The chemistry of the aflatoxins has recently been reviewed by
Roberts (1974).
3.1.2 Methods of analysis for aflatoxins in foodstuffs
3.1.2.1 Sampling
Sampling is an integral part of the analytical procedure and the
sample drawn should be representative of the lot. The total error
made in an analytical procedure consists of the sampling error, the
subsampling error, and the error in analysis (Whitaker, 1977). The
difficulty in sampling for aflatoxins arises because of the
heterogeneity of aflatoxin distribution in contaminated unprocessed
commodities. On the basis of a large number of analyses, Whitaker et
al. (1974a) were able to calculate the contribution of each error to
the total error. The total variance of the analytical procedure is
primarily caused by sampling variability, whereas the subsampling
variability and the analysis variability are more or less
independent of aflatoxin concentration. The coefficient of variation
associated with sampling is about 115% at a level of contamination
of 20 µg/kg and about 145% at a level of contamination of 10 µg/kg.
Whitaker et al. (1974b) and Whitaker (1977) have summarized a
procedure for sampling and have developed a number of sampling plans
used in the USA for the control of aflatoxin contamination in
shelled groundnuts (peanuts). Other recent publications deal with
aflatoxin-testing programmes for maize (Whitaker et al., 1978) and
cottonseed (Whitaker & Whitten, 1977).
The sampling of small grains, oilseed cakes, foodstuffs, and
feeds is also difficult, although in most cases the aflatoxin
distribution within a batch is not likely to be as uneven as in the
case of groundnuts. Fluids and well-mixed processed products such as
milk and milk products, beer, and cider do not present such a
sampling problem.
Peanut butter, flours, and cornmeal do not present the same
problems as the original raw materials because a finely divided
product is formed during processing from which it is much easier to
obtain a representative analytical sample.
Some practical aspects of sampling are dealt with in Chapter 26
of "Official methods of analysis" of the Association of Official
Analytical Chemists (Horwitz et al., 1975). For survey purposes,
1-5 kg samples are usually taken and the size of the sample for
analysis ranges from 20 to 100 g. A sample of 50 g ensures both a
representative sample and solvent economy.
3.1.2.2 Methods of analysis
Biological and chemical procedure have been developed for the
detection and determination of aflatoxins and other mycotoxins. The
bioassay techniques that are currently available are not suitable
for routine screening purposes and their detection levels are not
low enough. The chemical assay techniques, although more accurate
and faster, are not always specific. The presence of a certain toxin
is usually confirmed by derivative formation and its toxicity
verified by bioassay.
Biological methods. In the original biological test (Carnaghan
et al., 1963), one-day-old ducklings were used as test animals for
determining the presence of aflatoxins in suspect food by measuring
the degree of biliary proliferation as a semiquantitative index (see
section 3.4.2.1). The lowest dose level of 0.4 µg/day administered
for 5 days represents the minimum intake required to induce a
detectable biliary proliferation. The test is also effective for
detecting aflatoxin M1 in both liquid and powdered milk (Purchase,
1967). Little information is available on the sensitivity of this
test (and other biological methods) to aflatoxins other than B1.
A commonly used method in regulatory actions is the chicken
embryo bioassay in which 0.1-0.2 µg of aflatoxin B1 is applied to
the egg membrane and the mortality rate recorded during the 23-day
period of hatching (Horwitz, 1975).
Several other biological procedures have been developed, using
maize seedlings, zebra fish larvae, brine shrimps, bacteria etc.
Detailed descriptions can he found in the reviews by Goldblatt
(1969) and Ciegler et al. (1971).
Chemical methods. Although procedures are continually
changing, the basic steps remain; extraction, lipid removal,
cleanup, separation, and quantification. Since there is considerable
overlap in the various methods, most of the published reviews
(Jones, 1972; Stoloff, 1972) examine the different procedures by
these basic steps. Depending on the nature of the commodity, methods
can sometimes be simplified by omitting unnecessary steps. The
presence of specific interferences such as theobromine in cacao and
gossypol in cottonseed, may require additional steps.
Numerous methods of analysis have been reported for the
determination of aflatoxins in human and animal foodstuffs. Many of
them are minor modifications of the basic steps adapted to special
commodities or problems.
Collaborative studies designed to assess the performances of
different laboratories give information on the accuracy, precision,
and specificity of the method under consideration, as well as on the
occurrence of false negative and false positive results. Only
methods that have been subjected to such studies are reported in
this document.
Chemical methods have mainly been developed for such commodities
as groundnuts. In the first method for the analysis of groundnuts,
the aflatoxins were extracted from the contaminated sample using
methanol; this was later replaced by chloroform. An improvement was
made by Lee (1965) who showed that the addition of water to
hydrophilic plant tissues during extraction with chloroform resulted
in more effective removal of aflatoxin. The combination of
liquid-liquid extraction techniques and partition chromatography led
to a method, which is now one of the most widely used, known as the
Contamination Branch (CB) method (Eppley, 1966). The sample is
extracted with water and the water extracted with chloroform, the
lipids and aflatoxins are transferred to a silica-gel column where
the lipids are selectively eluted with hexane and the pigments and
other interfering material eluted with absolute diethylether;
finally the aflatoxins are eluted from the column with 3% methanol
in chloroform.
Because the CB method is time-consuming, attempts have been made
to simplify it. Waltking et al. (1968) drew attention to the fact
that a separation funnel was simpler and faster for liquid-liquid
partition than the silica-gel column, and that centrifuging was a
faster method of separating a solid than filtration. Thus the Best
Foods (BF) method was developed, which is faster and more economical
in terms of the amounts of solvents used but provides a poorer
cleanup. The sample is extracted and defatted with a two-phase
aqueous methanol-hexane system, the aflatoxins are then partitioned
from the aqueous phase into chloroform, leaving lipids and pigments
in the hexane and aqueous methanol.
In both the CB and BF methods, the aflatoxins are concentrated
by evaporation of the chloroform, and then separated by thin-layer
chromatography (TLC). Aflatoxins are intensely fluorescent when
exposed to long-wave ultraviolet radiation, which makes it possible
to determine these compounds at extremely low levels. An analyst
experienced in this field can detect 0.5 ng aflatoxin B1 on a TLC
plate. In most methods, the intensity of fluorescence of the sample
is compared with that of a standard. Under ideal conditions this
technique; has a coefficient of variation of about 20% which can be
reduced to 5%-9% by the use of a fluorodensitometer.
It should be pointed out that quantification and confirmation of
identity can only be obtained if pure authentic standards are
available for reference. Current sources of aflatoxin standards and
methods for the determination of mass concentration and purity cart
be found in Chapter 26 of the AOAC "Official methods of analysis"
(Horwitz et al., 1975).
When the CB and BF methods were compared in a collaborative
study (Waltking, 1970), the methods were found to be equivalent in
accuracy and precision with a recovery of about 70% of added
aflatoxin and an overall coefficient of variation of about 35% for
total aflatoxin levels down to about 20 µg/kg. This result has been
confirmed by the latest International Aflatoxin Check Sample Study
(Coon et al., 1972) in which 129 laboratories participated. However,
the coefficient of variation was about twice as high as in the
original collaborative studies. This illustrates the inadequacies of
many laboratories.
Another collaborative study was conducted (Stack, 1974) in which
the CB and BF methods were compared at levels down to the 2-10 µg/kg
range, Again both methods proved to be equally accurate (about 80%
recovery) for total aflatoxins at the 5-10 µg/kg levels. The CB
method, however, was as precise (coefficient of variation = 30%) at
the lowest level of 2 µg/kg as at the highest levels. The BF method
lost precision at these low levels and the coefficient of variation
was of the order of 100%.
In spite of cleanup and separation procedures, there might still
be problems with compounds that have fluorescent and chromatographic
properties similar to those of the aflatoxins. Thus, the presence of
a spot on a TLC plate is only presumptive evidence of identity and
additional confirmatory tests are necessary. Probably the first step
in confirming the presence of aflatoxins is to use additional
solvent systems in the TLC. The developed TLC plate can be sprayed
with 25% sulfuric acid (Schuller et al., 1967), which changes the
fluorescence colour of the aflatoxin spots to yellow. This test, if
negative, would rule out the presence of aflatoxins but does not
provide confirmatory evidence. The formation of chemical derivatives
was first described for aflatoxins B1 and G1 (Andrellos & Reid,
1964). The reagents used were formic acid-thionyl chloride, acetic
acid-thionyl chloride, and trifluoroacetic acid, the acid-catalysed
addition products formed were a dimeric acetate and an addition
product with water, respectively. The characteristic mobilities and
fluorescent properties on thin-layer chromatograms can be compared
with those of standard derivatives. Pohland et al. (1970) simplified
the preparation of the derivatives by using a mixture of
hydrochloric acid and acetic anhydride, and hydrochloric acid alone.
Further improvement, by elimination of the preparative
chromatography step in these procedures, has been achieved by
Przybylski (1975). The water adduct is formed directly on a TLC
plate from as little as 0.5 ng of aflatoxin B1 or G2.
In addition to the procedures for groundnuts, methods of
analysis have been developed for cottonseed, copra, maize, various
tree nuts (pistachio, walnut, Brazil nuts, etc.) and for animal
feeds. Many of these methods are modifications of the CB and BF
methods. Milk and dairy products require a far greater sensitivity
for the determination of M1 and M2 because these animal
metabolites are usually only found at sub µg/kg levels; additional
cleanup to eliminate interferences and sometimes two-dimensional TLC
techniques (Schuller et al., 1973) are necessary to attain
satisfactory performance. These methods are described in Chapter 26
of the AOAC "Official methods of analysis" (Horwitz et al., 1975).
Several analytical methods for the detection of aflatoxin
residues in animal tissues have been developed, and their detection
limits evaluated (Jemmali & Murphy, 1976).
Column detection methods are being used for control purposes in
the field because of their simplicity. The method of Romer (1975) is
of particular value because it combines column detection, TLC
quantification, and TLC plate chemical derivative confirmation in a
method that has a wide application for a number of foodstuffs and
feeds including mixed feeds.
It appears that methods using high-pressure liquid
chromatography will become the methods of choice for mycotoxin
analyses in the near future because of their sensitivity and
improved accuracy, and because they can be applied to a number of
mycotoxins including aflatoxins B1, B2, G1, and G2 (Panalaks
& Scott, 1977).
3.2 Sources and Occurrence
3.2.1 Formation by fungi
The ability to produce aflatoxins seems to be confined to
strains of the two species Aspergillus flavus Link and
A. parasiticus Speare, both members of the A. flavus group.
Aflatoxin-producing strains of A. flavus are common and
widespread, and have been isolated from a host of different
materials. As indicated in Table 2, a high proportion (from 20% to
98%) of isolated strains of A. flavus is able to produce
aflatoxins.
Table 2. Aflatoxin-producing strains of A. flavus isolated from
four field cropsa
Source Isolates Isolates producing Maximum yield
tested aflatoxin of aflatoxin B1
(No.) (%) (µg/flask)
groundnut 100 98 3300
cottonseed 59 81 3200
rice 127 20 1100
sorghum 63 24 3300
a Data from Schroeder & Boller quoted by Hesseltine (1976) in
"Mycotoxins and other fungal related food problems".
3.2.1.1 Moisture content and temperature
The moisture content of the substrate and temperature are the
main factors regulating fungal growth and mycotoxin formation.
Koehler (1938) established that a moisture content of 18.3% on a
wet weight basis, was the lower limit for the growth of A. flavus
in shelled corn. Extensive studies under precisely controlled
conditions (Sanders et al., 1968; Diener & Davis, 1969; Davis &
Diener, 1970) established a moisture content in equilibrium with a
relative humidity of 85% (or water activity (aw) = 0.85) as the
lower limit for growth of A. flavus and for the production of
aflatoxins. In starchy, cereal grain such as wheat, oats, barley,
rice, sorghum, and maize, the lower limit is a moisture content of
18.3%-18.5% on a wet weight basis and in groundnuts, Brazil nuts,
other nuts, copra, and sunflower and safflower seeds, all of which
have a high oil content, it is a moisture content of 9%-10%.
The minimum, optimum, and maximum temperatures for aflatoxin
production are 12° C, 27° C, and 40-42° C, respectively (Davis &
Diener, 1970). Northolt et al. (1976) studied the effect of water
activity and temperature on the growth and aflatoxin production of
A. parasiticus and came to the conclusion that no detectable
quantities of aflatoxin B1 were formed at an aw value below 0.83
and at temperatures below 10°C.
3.2.1.2 Invasion of field crops by A. flavus
Groundnut seeds may be invaded by A. flavus before harvest but
are more likely to be invaded very rapidly after the plants have
been pulled and piled for preliminary drying before the nuts are
removed. This postharvest period is the "high hazard" time for
aflatoxin production. On the other hand, studies of aflatoxin
contamination in North Carolina (USA) (Dickens & Satterwhite, 1973)
under conditions of drought, suggest that drought after groundnuts
are formed but before they are dug is conducive to their infection
with A. flavus. Damage caused by the lesser cornstalk borer (LCB)
might also aid in the infection process because insects may carry
fungal spores, although many drought area fields infested with LCB
did not produce groundnuts with a high aflatoxin content. Drought
alone does not result in high levels of aflatoxin contamination. It
must coincide with, or promote infestation by insects which in turn
infect the groundnut. The LCB may act as a vector for A. flavus.
Pettit et al. (1971) reported that groundnuts grown under dry
land conditions (drought stress) accumulated more aflatoxin before
digging than those grown under irrigation. Dry land fresh-dug
kernels contained a maximum aflatoxin level of 35 800 µg/kg while a
maximum of 50 µg/kg was detected in kernels from irrigated plots.
Apparently, the higher kernel moisture content occurring under
irrigated conditions reduced the aflatoxin production potential,
whereas a moisture content of about 31% under drought conditions was
near optimum. Similar observations have been reported from West
Africa.
In some irrigated regions with moist weather at harvest time,
cottonseed may be invaded by A. flavus while still on the plant
and after the bolls open and may contain large amounts of aflatoxins
(Marsh et al., 1973). Stephenson & Russell (1974) related the high
aflatoxin contamination in the field (in USA) to invasion by insects
that provided a site of injury and served as vectors for A. flavus.
Insect injury in ears of maize in the field may also be
accompanied or followed by infection with A. flavus and by
aflatoxin formation before harvest (Lillehoj et al., 1976). To what
extent this constitutes a contamination problem in many regions of
the world, where maize is an important crop, is not known.
Aflatoxins have also been reported in heads of sorghum heavily
infected with mould in India (Tripathi, 1973). Pistachio nuts can
become contaminated with Aflatoxins prior to harvest but the cause
of infection with aflatoxin-producing strains of fungi has not yet
been found. The contamination of almonds and of walnuts has been
traced to specific types of insect damage in the orchard (Stoloff,
1977).
3.2.2 Occurrence in foodstuffs
This subject has been reviewed recently by Stoloff (1976). Of
the four major aflatoxins (B1, B2, G1, G2) B1 is usually
found in the greatest concentrations. Measurements of toxin
concentration are based on the wet weight of the commodity in
question. The four toxins may occur together, although they need
not, and their concentrations in relation to each other and their
occurrence may vary depending on the fungal strain and substrate.
For example, Hesseltine et al. (1970) found that most fungal strains
that produced aflatoxin G1 also produced aflatoxin B1, but that
not all strains that produced aflatoxin B1 produced aflatoxin
G1. One strain of A. flavus from black pepper produced only
aflatoxin B2 on 2 natural substrates tested (Schroeder & Carlton,
1973).
Although aflatoxins have been found in a variety of foodstuffs,
the most pronounced contamination has been encountered in groundnuts
and other oilseeds including cottonseed and maize. The most
frequently contaminated tree nuts are Brazil nuts and pistachios.
3.2.2.1 Maize
Surveys in the USA of more than 1500 samples of maize collected
in crop years 1964-67, mainly from commercial channels, revealed
that 2%-3% of the samples contained aflatoxins (total aflatoxin B1
and G1) in the range of 3-37 µg/kg (Shotwell et al., 1969a, 1970).
In a subsequent survey of 293 samples, 8 samples (2.7%) contained
aflatoxin B1 levels in the range of 6-25 µg/kg, one of the samples
containing aflatoxin G1 (25 µg/kg) as well as aflatoxin B1
(Shotwell et al., 1971). Aflatoxin B2 was also detected in some of
the samples. In a further study of 60 samples from south-east USA
(Shotwell et al., 1973), aflatoxin B1 was found in 21 samples
(35%) at levels ranging from 6-308 µg/kg in the 1969-70 period.
Aflatoxin B2 at levels ranging from a trace to 40 µg/kg was found
in 15 of these samples and aflatoxin G1 was found in 5 samples at
levels of a trace to 10 µg/kg. In 2 samples, aflatoxin G2 (1 and
<2 µg/kg) was detected in addition to aflatoxin B1. In some of
the southeastern states of the USA a high frequency of aflatoxin
contamination was also found in maize in the field (Anderson et al.,
1975; Lillehoj et al., 1976). In some of the field samples, the
levels of aflatoxin B1 ranged up to several thousand µg/kg or
more. Most of the field contamination was associated with damage
caused by insects such as the European corn borer, corn ear worms,
or weevils, and it seems likely that wherever such damage is
prevalent, field contamination with aflatoxins will occur. In
Thailand, 35% of maize samples contained aflatoxin B1 (average
level 400 µg/kg) while 40% contamination by aflatoxin B1 (average
level 133 µg/kg) was found in Uganda (Stoloff, 1976), and 97% in the
Philippine island of Sebu (average 213 µg/kg) (Alpert et al., 1971;
Campbell & Salamat, 1971; Shank et al., 1972a; Campbell & Stoloff,
1974; Stoloff, 1976).
Aflatoxin levels found in household maize samples in connexion
with the outbreak of acute toxic hepatitis in north-west India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977) are discussed
in section 3.5.1.2.
3.2.2.2 Wheat, barley, oats, rye, rice, and sorghum
Shotwell et al. (1968b) reported the presence of aflatoxins at
levels of less than 19 µg/kg in 9/1368 samples of wheat, sorghum,
and oats in the USA. Shotwell et al. (1976b) did not detect any
aflatoxin B1 (detection limit 1-3 µg/kg) in 848 samples of wheat
from various districts of the USA. The presence of aflatoxins B1,
B2, G1, and G2 was reported by Tripathi (1973) in heads of
sorghum heavily infected with mould, in field samples in India, but
he did not apply any confirmatory tests. Aflatoxins were also found
in sorghum in Uganda (Alpert et al., 1971) and, in a survey in the
USA, aflatoxins were detected in 2/66 samples of sorghum grain (13
and 50 µg/kg) (Stoloff, 1976). Aflatoxins have been detected in less
than 2% of more than 400 samples of rice from markets in Africa, the
Philippines, and Thailand (Alpert et al., 1971; Campbell & Salamat,
1971; Shank et al., 1972a). However, Lucas et al. (1970-71) reported
that out of 139 samples of rice obtained from the Ho Chi Minh
(Saigon) area in Viet Nam, 31% were found positive for aflatoxins,
no confirmatory tests were included in this study. In surveys of
wheat and other cereals in the USSR, Lvova et al. (1976) found
aflatoxin B1 at a level of 100 µg/kg in 1/169 samples (0.6%) in
the 1972 crop. Aflatoxins (aflatoxin B1 at levels ranging from 20
to 444 µg/kg and aflatoxin G1 at levels of 10-333 µg/kg) were
found in 24/138 samples (17.4%) in the 1973 crop year. In this year,
the samples to be analysed were specially selected from those that
were mouldy or had undergone heating or both. In a survey of wheat
in southern USSR (Kazakstan), Bucarbaeva & Nikov (1977) found
aflatoxin B1 in 2/50 samples (4%) from one district and 3/50
samples (6%) from another (levels ranging from 5 to 10 µg/kg).
3.2.2.3 Groundnuts (peanuts)
In the 1973 survey in the USA of shelled consumer groundnuts,
15% of 361 samples contained aflatoxins in the range of trace to
50 µg/kg (Stoloff, 1976). Krogh & Hald (1969) found aflatoxins in
86.5% of 52 samples of groundnut products imported into Denmark for
feed, one sample contained 3465 µg/kg. Aflatoxins were found in 41%
of 173 samples of groundnuts in the Sudan, 16% of the samples
containing more than 250 µg/kg, and 9%, more than 1000 µg/kg (Habish
et al., 1971). In the Philippines, all the samples of peanut butter,
tested in 1967-69, contained aflatoxins with a median value of
155 µg/kg and a mean value of 500 µg/kg. The highest level detected
was 8600 µg/kg (Campbell & Salamat, 1971). In Thailand, 49% of
market samples contained an average level of aflatoxins of
1530 µg/kg (Shank et al., 1972a).
3.2.2.4 Soybeans and common beans
No significant degree of aflatoxin contamination has been found
in soybeans or common beans in commerce in the USA (Stoloff, 1976),
although aflatoxin contamination sufficient to be of public health
concern has been found in various types of edible beans in Thailand
(Shank et al., 1972a) and in Africa (Alpert et al., 1971).
3.2.2.5 Tree nuts
Aflatoxin has been found occasionally in Brazil nuts, almonds,
walnuts, pistachio nuts, pecans, and filberts. In some of these,
contamination occurs when the nuts are still on the tree and is
usually associated with damage of one sort or another. However,
apparently sound, undamaged pecans may contain aflatoxins (Stoloff,
1976). Yndestad & Underdal (1975)in Norway found 66% of Brazil nuts
contaminated with aflatoxin B1 and Nilsson et al. (1974) found
that all of 23 batches of Brazil nuts intended for importation to
Sweden were contaminated. Fourteen percent of 74 samples of
California almonds were contaminated with aflatoxin B1 with levels
of less than 20 µg/kg in 90% of the contaminated samples (Schade et
al., 1975).
3.2.2.6 Copra
Aflatoxins were found in 88% of 72 samples of copra and copra
meal (Stoloff, 1976) in amounts ranging from a trace to 30 µg/kg,
and similar contamination was found by Krogh et al. (1970) in copra
imported into Finland.
3.2.2.7 Cottonseed
In 3 successive crop years (1964-67), aflatoxin B1 was
detected in 6.5%-8.8% of more than 3000 cottonseed samples and in
12.8%-21.5% of more than 3000 samples of cottonseed meal (Stoloff,
1976). In contrast, aflatoxin was not detected in cottonseed hulls
(Whitten, 1969). Relatively high levels of aflatoxin contamination
were found in an area in southern California. Aflatoxin levels
increased from 1735 µg/kg in some samples of seed harvested in
November to 2578 µg/kg in some samples going into storage in late
January. The amount present in the stored seeds did not increase
with time, even though fungi, including A. flavus, could be seen
growing on some of the seeds. Marsh et al. (1973) tested cottonseeds
from 13 locations across the USA cotton belt in 1969 and from 11
locations in 1970. Aflatoxins B1 and B2 were found in one or
more samples from 3 regions in areas where boll rot caused by
A. flavus had been repeatedly observed in previous years. Seeds
from individual lots contained aflatoxin B1 levels ranging from
200 000 to 300 000 µg/kg indicating the high potential hazard that
might occur from cottonseed.
3.2.2.8 Spices and condiments
Scott & Kennedy (1973) did not find any aflatoxins in 24 samples
of ground black or white pepper. Low concentrations (up to 8 µg/kg)
were found in 10/33 samples of cayenne pepper and 6/6 samples of
Indian chili powder, mainly as trace amounts.
3.2.2.9 Animal feeds
In studies by Strzelecki & Gasiorowska (1974), aflatoxins
occurred in 12.7% of 306 samples of animal feed and feed components
in Poland, 4.2% of the samples containing more than 100 µg/kg and
2.6% of the samples containing more than 1000 µg/kg. Feed
components, mainly groundnut meals, were contaminated by aflatoxins
more frequently and with higher levels. On the other hand, aflatoxin
was detected in only one sample (2.7%) of cattle and sheep feeds
(300 µg/kg) and in one sample (1.7%)of poultry feeds (30 µg/kg).
Swine feeds contained aflatoxins in 11.4% of samples, with 6 samples
(5.7%) exceeding 250 µg/kg. Two recent surveys of mixed feeds in the
Federal Republic of Germany revealed that 1 in 60 samples contained
aflatoxin B1 levels exceeding 20 µg/kg (Seibold & Ruch, 1977); 45
out of another 105 samples contained levels of between 7 and
300 µg/kg (Kiermeier et al., 1977). Similar results were obtained in
the United Kingdom (Patterson, personal communication) where 95/172
samples of dairy feed were contaminated with aflatoxin B1 levels
of 1-350 µg/kg, and 92.4% contained no more than 30 µg/kg.
3.2.2.10 Animal products
Surveys in several countries have shown that aflatoxin M1 may
be present in liquid or dried milk (Table 3) and in milk products
(Kiermeier, 1977). In addition, highly exceptional aflatoxin levels
in the range of 50-500 µg/litre were reported by Suzanger et al.
(1976) in half of the samples of cow's milk collected in villages
around Isfahan, Iran (15/30 samples collected in 1973 and 21/37
samples in 1974). In contrast, no aflatoxins were detected in 8
samples of milk obtained from large-scale producers in the same area
in 1974 and only 10% of such samples (2/20) contained aflatoxin M1
(in the range 8-10 µg/litre) in 1973. Aflatoxin M1 was identified
in all the positive samples. Eight of the 36 village samples
containing aflatoxin M1 also contained aflatoxin M2 and 2
samples contained aflatoxin B1. Considerable differences in the
handling and storage of animal feeds were thought by the authors to
be responsible for the differences in the aflatoxin M1 contents of
milk samples from villages and large-scale producers in this area.
However, levels of aflatoxins in animal feeds were not reported in
this paper, but they must have been exceptionally high.
Table 3. Selected surveys of aflatoxin M1 in cow's milk
Milk Country Total no. No. containing Range of concentrations Reference
samples of samples aflatoxin M1 in the positive samples
analysed (µg/litre or µg/kg)
Liquid Belgium 68 42 0.02-0.2 Van Pee et al. (1977)
German Democratic Republic 36 4a 1.7-6.5c Fritz et al. (1977)
Germany, Federal Republic of 61 28 0.01-0.25 Kiermeier (1973)
Germany, Federal Republic of 419 79 trace-0.54 Kiermeier et al. ( 1977)
Germany, Federal Republic of 260 118a 0.05-0.33 Polzhofer (1977)
India 21 3 up to 13.3 Paul et al. (1976)
Netherlands 95 74 0.09-0.5 Schuller et al. (1977)
United Kingdom 278 85a 0.03-0.52a Patterson et al. (in press)
Dried German Democratic Republic 18 0f --- Fritz et al. (1977)
Germany, Federal Republic of 166 8 0.67-2.0 Neumann-Kleinpaul & Terplan (1972)
Germany, Federal Republic of 52 35 trace-4.0 Hanssen & Jung (1972)
Germany, Federal Republic of 120 7a 0.05-0.13 Jung & Hanssen (1974)
South Africa 56 0 --- Luck et al. (1977)
USA 320 24 0.1-0.4 FDA (1977) 1973 survey
USA 302 192 trace-3.9e FDA (1977) 1977 survey
a Seasonal effect observed, i.e., concentration obviously, dependent upon level of concentrate feeding.
b Samples collected in retail outlets in 4 southeast States of the USA; it has been estimated that approximately two-thirds
of the crops in these areas contained aflatoxin concentrations exceeding 20 µg/kg because of unusual drought, insect
damage, and high temperature conditions that occurred in 1977.
c Values for 4 positive samples collected in winter; aflatoxin was not detected in the other 32 winter milk samples as well as
in 12 milk samples collected in summer (detection limit = 0.1 µg/kg).
d 92.5% samples contained aflatoxin M1 concentrations of less than 0.1 µg/litre.
e Levels ranging from 0.1 to 0.4 µg/kg reported in 158 samples; levels exceeding 0.5 µg/kg reported in 19 samples.
f Aflatoxin B1 contamination detected in one sample.
Aflatoxin residues have been found in animal tissues, eggs, and
poultry following the experimental ingestion of aflatoxin-
contaminated feed and this subject has been reviewed by Rodricks &
Stoloff (1977). However, the toxins have not yet been found in these
products on the market.
3.2.3 Fate of aflatoxins during the handling and processing
of food
Aflatoxins are affected by some ordinary food processing
procedures. In the roasting of groundnuts, approximately 50% of the
aflatoxins are altered to such an extent that they can no longer be
detected (Lee et al., 1969b; Waltking, 1971). The chemical nature of
the alteration products has not been fully elucidated.
The usual methods of processing groundnuts to make peanut butter
and some nuts for confections may appreciably reduce aflatoxin
contamination. The removal of undersized nuts (shrivels and pegs);
the removal of nuts that resist splitting and blanching; and the
removal of discoloured nuts by hand or electronic sorting are
effective means of reducing contamination (Rodricks et al., 1977).
In the removal of oil from oilseeds, most of the aflatoxins are
found in the oilseed meal. Small amounts remaining in the crude
vegetable oil are mainly taken out in the soap stock, the byproduct
from the alkali refining step. The remaining traces of aflatoxins
are removed in the bleaching refining steps to give aflatoxin-free
refined oil (Parker & Melnick, 1966).
The normal alkali processing of maize to produce tortilla-type
foods, a common practice in some areas of the world including some
Latin American countries, effectively reduces the levels of
aflatoxins in contaminated maize (Ulloa-Sosa & Schroeder, 1969).
Although the mechanism of this reduction has not been clarified,
some of the aflatoxin is most likely washed out by the initial
soaking of the maize in lye water and some is undoubtedly chemically
changed by the alkali; although this process is reported to produce
a substantial reduction in aflatoxin contamination, it is not enough
to give a safe product, when highly contaminated maize is being
processed.
Jemmali & Lafont (1972) reported only partial destruction of
aflatoxins during bread making, indicating the importance of the
contamination of wheat with A. flavus.
The above treatments are normal steps in the processing of
particular foods. In addition to such steps, procedures have been
developed specifically for the destruction or removal of aflatoxins
from grains, nuts, oilseeds, and oilseed meals (cake) (FAO, 1977).
Treatments with ammonia or hydrogen peroxide (H2O2) have been
the most effective procedures developed to date for the
detoxification of foodstuffs and animal feeds (Goldblatt & Dollear,
1977). The treatments with ammonia, developed for the industrial
decontamination of aflatoxin-contaminated groundnut and cottonseed
meal (cake) and maize, are limited to the production of animal
feeds. These procedures have recently been discussed in detail at
the joint FAO/WHO/ UNEP conference on mycotoxins in Nairobi (FAO,
1977). The process of treating groundnut protein isolate with
hydrogen peroxide to obtain a product suitable for use as a human
food supplement was also discussed. This process has been developed
in India and is reported to be operating on a small commercial
scale.
The feasibility of methods combining the physical separation of
contaminated portions of produce (for detailed descriptions of
segregation techniques see, for example, Rodricks et al., 1977) with
chemical decontamination, was considered at the same conference
(FAO, 1977).
3.2.4 Pathways and levels of exposure
From the previous discussion, it can be seen that a range of
commodities may become contaminated with trace amounts of
aflatoxins. In vegetable foods, this contamination results directly
from fungal spoilage, maize and nuts being particularly susceptible.
On the other hand, milk, and possibly meat and eggs can become
indirectly contaminated through the absorption by farm animals of
aflatoxins from contaminated feed resulting in residues of the
parent toxin or its metabolites in body fluids or tissues.
Thus, the level of man's exposure to dietary aflatoxins depends
upon the food available and on eating habits and will vary from
country to country according to the local conditions, including the
traditions of different ethnic groups, and amongst individuals.
Where contaminated groundnuts or maize make a significant
contribution to the diet, the level of exposure will be relatively
higher than where less commonly contaminated commodities take their
place as the staple food or when milk is the sole
aflatoxin-containing constituent of the diet.
In this connexion, the Task Group felt it was important to
identify the infant as being potentially at risk because: (a) baby
food products may be made from dried milk or even maize, commodities
known to be prone to contamination by aflatoxins; and (b) in terms
of larger amounts of food consumed per kg body weight, any level of
aflatoxin contamination is more significant for the child than for
the adult.
Attempts to quantify dietary exposure to aflatoxins are
discussed in detail in section 3.6.1.1.
Occupational exposure (section 3.5.2) to aflatoxins with its
attendant high risks, concerns two groups of individuals: those who
handle grain, animal feedstuffs, groundnuts, groundnut meal etc.
(where exposure could occur largely through inhalation of
contaminated dust), and those who work with toxins in experiments or
with pure toxins as analytical standards.
In one paper (van Nieuwenhuize et al., 1973) available to the
Task Group, an attempt was made to quantify occupational exposure to
airborne aflatoxins in an oil-mill crushing groundnuts and other
oil-seeds. Based on airborne dust determinations (mean aflatoxin
concentrations of 250 and 410 µg/kg airborne dust), the estimated
airborne aflatoxin levels ranged from 0.87 to 72 ng/m3 of air.
3.3 Metabolism
3.3.1 Absorption
Although quantitative data on absorption are not available at
present, there is no doubt that most of the field cases of
aflatoxin-induced diseases in animals and man have been associated
with ingestion of aflatoxin-contaminated foodstuffs and thus with
the absorption of aflatoxins in the alimentary tract. In spite of
reports of respiratory exposure (sections 3.2.4 and 3.5.2), there is
no quantitative information available on aflatoxin resorption from
the respiratory tract or on percutaneous absorption.
3.3.2 Tissue distribution
3.3.2.1 Animal studies
Experiments with 14C-ring-labelled aflatoxin B1 have shown
that rats retain about 20% of the 14C activity 24 h after a single
intraperitoneal dose of 0.07 mg/kg body weight (Wogan et al., 1967).
The highest concentration was found in the liver, which contained
amounts of radioactivity equivalent to the entire remainder of the
carcass (about 5%-8% of the total 14C recovered).
When poultry were fed rations containing aflatoxins at
concentrations ranging from 25 to 15 000 µg/kg for 8 weeks, residues
of aflatoxin B1 were found in the liver and in muscle tissue
(Mintzlaff et al., 1974). The liver contained the highest
concentration, with a mean value of 15 µg/kg at the highest exposure
level. Similarly the highest concentration of aflatoxin B1 was
found in the livers of pigs (range: trace-137 µg/kg) fed rations
containing aflatoxins (both aflatoxin B1 and B2) at levels of
300 and 500 µg/kg for 4 months (Krogh et al., 1973a). Aflatoxin
residues were also detected in kidneys, and muscle and adipose
tissue.
3.3.2.2 Studies in man
Levels of aflatoxins in the tissues of children with Reye's
syndrome are discussed in section 3.6.2.2. In a liver biopsy from a
subject with carcinoma of the rectum and liver in the USA, Phillips
et al. (1976) found 520 µg/kg of aflatoxin B1. In France, Richir
et al. (1976) found aflatoxin B1 in liver biopsies in 6 out of 100
subjects suffering from various diseases. Concentrations observed
ranged from 1.6-8 µg/kg.
3.3.3 Metabolic transformation and activation
With one exception, all primary biotransformations of aflatoxin
B1 involve its conversion to hydroxylated metabolites but only one
such derivative, aflatoxin M1 has appreciable oral toxicity
(Holzapfel et al., 1966). Even so, this metabolite may be detoxified
by conjugation with taurocholic and glucuronic acids prior to
excretion in the bile or urine (Bassir & Osiyemi, 1967). In this
respect, two recently discovered metabolites, P1 (Dalezios et al.,
1971; Buchi et al., 1973) and Q1 (Masri et al., 1974a,b) are
similar in that they also undergo this type of detoxification
(Dalezios et al., 1971; Dalezios & Wogan, 1972).
The conversion in the liver (Fig. 2) of aflatoxin B1 to
aflatoxicol (Patterson & Roberts, 1971) and to aflatoxicol H1 via
aflatoxin Q1 (Salhab & Hsieh, 1975) is unusual in that, unlike
other biotransformations that are catalysed by liver microsomal
enzymes, a cytoplasmic NADH-dependent dehydrogenase is involved.
Furthermore, the formation of aflatoxicol can be inhibited by
17-ketosteroid sex hormones (Patterson & Roberts, 1972a) and this is
the only metabolic transformation of aflatoxin in vitro known to
be sensitive to hormones.
Liver homogenates of certain avian and rodent species are
particularly active in converting aflatoxins B1 and G1 to their
2-hydroxy, 2,3-dihydro derivatives or hemiacetals called also
aflatoxins B2a and G2a (Patterson & Roberts, 1970). These
metabolites bind strongly to protein and are probably sufficiently
reactive, when formed in vivo, to cause many of the acute effects
of aflatoxin poisoning (Patterson & Roberts, 1972b; Patterson, 1973,
1977).
At present, there is only indirect evidence for the formation of
the epoxides of aflatoxins B1 and G1 but this is probably the
more important form of metabolic activation. When either of the
parent toxins is incubated with microsomes prepared from the livers
of many animal species including man, a metabolite is formed which
appears to have only a transient existence, is highly reactive,
binds covalently to DNA, and induces mutation in a bacterial
in vitro test system (Garner et al., 1971, 1972; Ames et al.,
1973). The metabolite of B1 has not been isolated but the
2,3-dihydrodiol has been recovered following mild acid hydrolysis of
an adduct formed when the microsomal metabolite was generated in the
presence of added DNA or RNA (Swenson et al., 1,974) and, more
recently, after in vivo intraperitoneal injection of aflatoxin
B1 (Swenson et al., 1977). This has been assumed to be indirect
evidence of the formation of the 2,3-epoxide and, in view of the
interaction with DNA, it is now generally accepted that the epoxide
of aflatoxin B1 is the bacterial mutagen and the proximal
carcinogen.
Little or no destruction of aflatoxins occurs under ordinary
cooking conditions, and heating for pasteurization. However,
roasting groundnuts appreciably reduces the levels of aflatoxins
(see section 3.2.3) and they can be totally destroyed by drastic
treatment such as autoclaving in the presence of ammonia or by
treatment with hypochlorite.
Table 1. Physical and chemical properties of some aflatoxins and their metabolites
Aflatoxin Molecular Relative Melting Ultraviolet absorption (epsilon)c Fluorescence Reference
formula molecular point emission
mass °C 265 nm 360-362 nm nm
B1a C17H12O6 312 268-269 12 400 21 800 425 Asao et al. (1965)
B2a C17H14O6 314 286-289 12 100 24 000 425 Chang et al. (1963)
G1a C17H12O6 328 244-246 9 600 17 700 450 Asao et al. (1965)
G2a C17H14O7 330d 237-240d 8 200 17 100 450 Hartley et al. (1963)
M1a C17H12O7 328 299 14 150 21 250 (357 nm) 425 Holzapfel et al. (1966)
M2a C17H14O7 330 293 12 100 (264 nm) 22 900 (357 nm) f Holzapfel et al. (1966)
P1b C16H10O6 298 >320 11 200 (267 nm) 15 400 (362 nm) f Dalezios et al. (1971 ) and
14 900 (342 nm) Buchi et al. (1973)
Q1 C17H12O7 328 e 11 450 (267 nm) 17 500 (366 nm) f Masri et al. (1974a,b)
Aflatoxicol C17H14O6 314 230--234d 10 800 (261 nm) 14 100 (375 nm) 425 Detroy & Hesseltine (1970)
a Molar absorption coefficient for aflatoxins B1, B2, G1, and G2 obtained from Rodricks et al. (1970) and those for M1 and M2
from Stubblefield et al, (1972).
b P stands for phenolic products of O-demethylation of aflatoxin B1.
c Compounds dissolved in methanol except for aflatoxin P1 which in this case was dissolved in ethanol. Data on molar absorption
coefficients for other peaks and on the ultraviolet absorption characteristics of aflatoxins in other solvents can be found
in the original papers.
d Data from Butler (1974).
e Not available.
f Violet fluorescence of aflatoxin M2 and yellow-green fluorescence of aflatoxins P1 and Q1 reported in original papers.
The presence of a lactone ring in the aflatoxin molecule makes
them susceptible to alkaline hydrolysis (De Iongh et al., 1962).
This characteristic is important in that any food processing
involving alkali treatment can decrease the contamination of the
products (section 3.2.3) although the presence of protein, the pH,
and the duration of treatment may modify the results (Beckwith et
al., 1975). However, if the alkaline treatment is mild,
acidification will reverse the reaction to reform the original
aflatoxin.
The chemistry of the aflatoxins has recently been reviewed by
Roberts (1974).
3.1.2 Methods of analysis for aflatoxins in foodstuffs
3.1.2.1 Sampling
Sampling is an integral part of the analytical procedure and the
sample drawn should be representative of the lot. The total error
made in an analytical procedure consists of the sampling error, the
subsampling error, and the error in analysis (Whitaker, 1977). The
difficulty in sampling for aflatoxins arises because of the
heterogeneity of aflatoxin distribution in contaminated unprocessed
commodities. On the basis of a large number of analyses, Whitaker et
al. (1974a) were able to calculate the contribution of each error to
the total error. The total variance of the analytical procedure is
primarily caused by sampling variability, whereas the subsampling
variability and the analysis variability are more or less
independent of aflatoxin concentration. The coefficient of variation
associated with sampling is about 115% at a level of contamination
of 20 µg/kg and about 145% at a level of contamination of 10 µg/kg.
Whitaker et al. (1974b) and Whitaker (1977) have summarized a
procedure for sampling and have developed a number of sampling plans
used in the USA for the control of aflatoxin contamination in
shelled groundnuts (peanuts). Other recent publications deal with
aflatoxin-testing programmes for maize (Whitaker et al., 1978) and
cottonseed (Whitaker & Whitten, 1977).
The sampling of small grains, oilseed cakes, foodstuffs, and
feeds is also difficult, although in most cases the aflatoxin
distribution within a batch is not likely to be as uneven as in the
case of groundnuts. Fluids and well-mixed processed products such as
milk and milk products, beer, and cider do not present such a
sampling problem.
Peanut butter, flours, and cornmeal do not present the same
problems as the original raw materials because a finely divided
product is formed during processing from which it is much easier to
obtain a representative analytical sample.
Some practical aspects of sampling are dealt with in Chapter 26
of "Official methods of analysis" of the Association of Official
Analytical Chemists (Horwitz et al., 1975). For survey purposes,
1-5 kg samples are usually taken and the size of the sample for
analysis ranges from 20 to 100 g. A sample of 50 g ensures both a
representative sample and solvent economy.
3.1.2.2 Methods of analysis
Biological and chemical procedure have been developed for the
detection and determination of aflatoxins and other mycotoxins. The
bioassay techniques that are currently available are not suitable
for routine screening purposes and their detection levels are not
low enough. The chemical assay techniques, although more accurate
and faster, are not always specific. The presence of a certain toxin
is usually confirmed by derivative formation and its toxicity
verified by bioassay.
Biological methods. In the original biological test (Carnaghan
et al., 1963), one-day-old ducklings were used as test animals for
determining the presence of aflatoxins in suspect food by measuring
the degree of biliary proliferation as a semiquantitative index (see
section 3.4.2.1). The lowest dose level of 0.4 µg/day administered
for 5 days represents the minimum intake required to induce a
detectable biliary proliferation. The test is also effective for
detecting aflatoxin M1 in both liquid and powdered milk (Purchase,
1967). Little information is available on the sensitivity of this
test (and other biological methods) to aflatoxins other than B1.
A commonly used method in regulatory actions is the chicken
embryo bioassay in which 0.1-0.2 µg of aflatoxin B1 is applied to
the egg membrane and the mortality rate recorded during the 23-day
period of hatching (Horwitz, 1975).
Several other biological procedures have been developed, using
maize seedlings, zebra fish larvae, brine shrimps, bacteria etc.
Detailed descriptions can he found in the reviews by Goldblatt
(1969) and Ciegler et al. (1971).
Chemical methods. Although procedures are continually
changing, the basic steps remain; extraction, lipid removal,
cleanup, separation, and quantification. Since there is considerable
overlap in the various methods, most of the published reviews
(Jones, 1972; Stoloff, 1972) examine the different procedures by
these basic steps. Depending on the nature of the commodity, methods
can sometimes be simplified by omitting unnecessary steps. The
presence of specific interferences such as theobromine in cacao and
gossypol in cottonseed, may require additional steps.
Numerous methods of analysis have been reported for the
determination of aflatoxins in human and animal foodstuffs. Many of
them are minor modifications of the basic steps adapted to special
commodities or problems.
Collaborative studies designed to assess the performances of
different laboratories give information on the accuracy, precision,
and specificity of the method under consideration, as well as on the
occurrence of false negative and false positive results. Only
methods that have been subjected to such studies are reported in
this document.
Chemical methods have mainly been developed for such commodities
as groundnuts. In the first method for the analysis of groundnuts,
the aflatoxins were extracted from the contaminated sample using
methanol; this was later replaced by chloroform. An improvement was
made by Lee (1965) who showed that the addition of water to
hydrophilic plant tissues during extraction with chloroform resulted
in more effective removal of aflatoxin. The combination of
liquid-liquid extraction techniques and partition chromatography led
to a method, which is now one of the most widely used, known as the
Contamination Branch (CB) method (Eppley, 1966). The sample is
extracted with water and the water extracted with chloroform, the
lipids and aflatoxins are transferred to a silica-gel column where
the lipids are selectively eluted with hexane and the pigments and
other interfering material eluted with absolute diethylether;
finally the aflatoxins are eluted from the column with 3% methanol
in chloroform.
Because the CB method is time-consuming, attempts have been made
to simplify it. Waltking et al. (1968) drew attention to the fact
that a separation funnel was simpler and faster for liquid-liquid
partition than the silica-gel column, and that centrifuging was a
faster method of separating a solid than filtration. Thus the Best
Foods (BF) method was developed, which is faster and more economical
in terms of the amounts of solvents used but provides a poorer
cleanup. The sample is extracted and defatted with a two-phase
aqueous methanol-hexane system, the aflatoxins are then partitioned
from the aqueous phase into chloroform, leaving lipids and pigments
in the hexane and aqueous methanol.
In both the CB and BF methods, the aflatoxins are concentrated
by evaporation of the chloroform, and then separated by thin-layer
chromatography (TLC). Aflatoxins are intensely fluorescent when
exposed to long-wave ultraviolet radiation, which makes it possible
to determine these compounds at extremely low levels. An analyst
experienced in this field can detect 0.5 ng aflatoxin B1 on a TLC
plate. In most methods, the intensity of fluorescence of the sample
is compared with that of a standard. Under ideal conditions this
technique; has a coefficient of variation of about 20% which can be
reduced to 5%-9% by the use of a fluorodensitometer.
It should be pointed out that quantification and confirmation of
identity can only be obtained if pure authentic standards are
available for reference. Current sources of aflatoxin standards and
methods for the determination of mass concentration and purity cart
be found in Chapter 26 of the AOAC "Official methods of analysis"
(Horwitz et al., 1975).
When the CB and BF methods were compared in a collaborative
study (Waltking, 1970), the methods were found to be equivalent in
accuracy and precision with a recovery of about 70% of added
aflatoxin and an overall coefficient of variation of about 35% for
total aflatoxin levels down to about 20 µg/kg. This result has been
confirmed by the latest International Aflatoxin Check Sample Study
(Coon et al., 1972) in which 129 laboratories participated. However,
the coefficient of variation was about twice as high as in the
original collaborative studies. This illustrates the inadequacies of
many laboratories.
Another collaborative study was conducted (Stack, 1974) in which
the CB and BF methods were compared at levels down to the 2-10 µg/kg
range, Again both methods proved to be equally accurate (about 80%
recovery) for total aflatoxins at the 5-10 µg/kg levels. The CB
method, however, was as precise (coefficient of variation = 30%) at
the lowest level of 2 µg/kg as at the highest levels. The BF method
lost precision at these low levels and the coefficient of variation
was of the order of 100%.
In spite of cleanup and separation procedures, there might still
be problems with compounds that have fluorescent and chromatographic
properties similar to those of the aflatoxins. Thus, the presence of
a spot on a TLC plate is only presumptive evidence of identity and
additional confirmatory tests are necessary. Probably the first step
in confirming the presence of aflatoxins is to use additional
solvent systems in the TLC. The developed TLC plate can be sprayed
with 25% sulfuric acid (Schuller et al., 1967), which changes the
fluorescence colour of the aflatoxin spots to yellow. This test, if
negative, would rule out the presence of aflatoxins but does not
provide confirmatory evidence. The formation of chemical derivatives
was first described for aflatoxins B1 and G1 (Andrellos & Reid,
1964). The reagents used were formic acid-thionyl chloride, acetic
acid-thionyl chloride, and trifluoroacetic acid, the acid-catalysed
addition products formed were a dimeric acetate and an addition
product with water, respectively. The characteristic mobilities and
fluorescent properties on thin-layer chromatograms can be compared
with those of standard derivatives. Pohland et al. (1970) simplified
the preparation of the derivatives by using a mixture of
hydrochloric acid and acetic anhydride, and hydrochloric acid alone.
Further improvement, by elimination of the preparative
chromatography step in these procedures, has been achieved by
Przybylski (1975). The water adduct is formed directly on a TLC
plate from as little as 0.5 ng of aflatoxin B1 or G2.
In addition to the procedures for groundnuts, methods of
analysis have been developed for cottonseed, copra, maize, various
tree nuts (pistachio, walnut, Brazil nuts, etc.) and for animal
feeds. Many of these methods are modifications of the CB and BF
methods. Milk and dairy products require a far greater sensitivity
for the determination of M1 and M2 because these animal
metabolites are usually only found at sub µg/kg levels; additional
cleanup to eliminate interferences and sometimes two-dimensional TLC
techniques (Schuller et al., 1973) are necessary to attain
satisfactory performance. These methods are described in Chapter 26
of the AOAC "Official methods of analysis" (Horwitz et al., 1975).
Several analytical methods for the detection of aflatoxin
residues in animal tissues have been developed, and their detection
limits evaluated (Jemmali & Murphy, 1976).
Column detection methods are being used for control purposes in
the field because of their simplicity. The method of Romer (1975) is
of particular value because it combines column detection, TLC
quantification, and TLC plate chemical derivative confirmation in a
method that has a wide application for a number of foodstuffs and
feeds including mixed feeds.
It appears that methods using high-pressure liquid
chromatography will become the methods of choice for mycotoxin
analyses in the near future because of their sensitivity and
improved accuracy, and because they can be applied to a number of
mycotoxins including aflatoxins B1, B2, G1, and G2 (Panalaks
& Scott, 1977).
3.2 Sources and Occurrence
3.2.1 Formation by fungi
The ability to produce aflatoxins seems to be confined to
strains of the two species Aspergillus flavus Link and
A. parasiticus Speare, both members of the A. flavus group.
Aflatoxin-producing strains of A. flavus are common and
widespread, and have been isolated from a host of different
materials. As indicated in Table 2, a high proportion (from 20% to
98%) of isolated strains of A. flavus is able to produce
aflatoxins.
Table 2. Aflatoxin-producing strains of A. flavus isolated from
four field cropsa
Source Isolates Isolates producing Maximum yield
tested aflatoxin of aflatoxin B1
(No.) (%) (µg/flask)
groundnut 100 98 3300
cottonseed 59 81 3200
rice 127 20 1100
sorghum 63 24 3300
a Data from Schroeder & Boller quoted by Hesseltine (1976) in
"Mycotoxins and other fungal related food problems".
3.2.1.1 Moisture content and temperature
The moisture content of the substrate and temperature are the
main factors regulating fungal growth and mycotoxin formation.
Koehler (1938) established that a moisture content of 18.3% on a
wet weight basis, was the lower limit for the growth of A. flavus
in shelled corn. Extensive studies under precisely controlled
conditions (Sanders et al., 1968; Diener & Davis, 1969; Davis &
Diener, 1970) established a moisture content in equilibrium with a
relative humidity of 85% (or water activity (aw) = 0.85) as the
lower limit for growth of A. flavus and for the production of
aflatoxins. In starchy, cereal grain such as wheat, oats, barley,
rice, sorghum, and maize, the lower limit is a moisture content of
18.3%-18.5% on a wet weight basis and in groundnuts, Brazil nuts,
other nuts, copra, and sunflower and safflower seeds, all of which
have a high oil content, it is a moisture content of 9%-10%.
The minimum, optimum, and maximum temperatures for aflatoxin
production are 12° C, 27° C, and 40-42° C, respectively (Davis &
Diener, 1970). Northolt et al. (1976) studied the effect of water
activity and temperature on the growth and aflatoxin production of
A. parasiticus and came to the conclusion that no detectable
quantities of aflatoxin B1 were formed at an aw value below 0.83
and at temperatures below 10°C.
3.2.1.2 Invasion of field crops by A. flavus
Groundnut seeds may be invaded by A. flavus before harvest but
are more likely to be invaded very rapidly after the plants have
been pulled and piled for preliminary drying before the nuts are
removed. This postharvest period is the "high hazard" time for
aflatoxin production. On the other hand, studies of aflatoxin
contamination in North Carolina (USA) (Dickens & Satterwhite, 1973)
under conditions of drought, suggest that drought after groundnuts
are formed but before they are dug is conducive to their infection
with A. flavus. Damage caused by the lesser cornstalk borer (LCB)
might also aid in the infection process because insects may carry
fungal spores, although many drought area fields infested with LCB
did not produce groundnuts with a high aflatoxin content. Drought
alone does not result in high levels of aflatoxin contamination. It
must coincide with, or promote infestation by insects which in turn
infect the groundnut. The LCB may act as a vector for A. flavus.
Pettit et al. (1971) reported that groundnuts grown under dry
land conditions (drought stress) accumulated more aflatoxin before
digging than those grown under irrigation. Dry land fresh-dug
kernels contained a maximum aflatoxin level of 35 800 µg/kg while a
maximum of 50 µg/kg was detected in kernels from irrigated plots.
Apparently, the higher kernel moisture content occurring under
irrigated conditions reduced the aflatoxin production potential,
whereas a moisture content of about 31% under drought conditions was
near optimum. Similar observations have been reported from West
Africa.
In some irrigated regions with moist weather at harvest time,
cottonseed may be invaded by A. flavus while still on the plant
and after the bolls open and may contain large amounts of aflatoxins
(Marsh et al., 1973). Stephenson & Russell (1974) related the high
aflatoxin contamination in the field (in USA) to invasion by insects
that provided a site of injury and served as vectors for A. flavus.
Insect injury in ears of maize in the field may also be
accompanied or followed by infection with A. flavus and by
aflatoxin formation before harvest (Lillehoj et al., 1976). To what
extent this constitutes a contamination problem in many regions of
the world, where maize is an important crop, is not known.
Aflatoxins have also been reported in heads of sorghum heavily
infected with mould in India (Tripathi, 1973). Pistachio nuts can
become contaminated with Aflatoxins prior to harvest but the cause
of infection with aflatoxin-producing strains of fungi has not yet
been found. The contamination of almonds and of walnuts has been
traced to specific types of insect damage in the orchard (Stoloff,
1977).
3.2.2 Occurrence in foodstuffs
This subject has been reviewed recently by Stoloff (1976). Of
the four major aflatoxins (B1, B2, G1, G2) B1 is usually
found in the greatest concentrations. Measurements of toxin
concentration are based on the wet weight of the commodity in
question. The four toxins may occur together, although they need
not, and their concentrations in relation to each other and their
occurrence may vary depending on the fungal strain and substrate.
For example, Hesseltine et al. (1970) found that most fungal strains
that produced aflatoxin G1 also produced aflatoxin B1, but that
not all strains that produced aflatoxin B1 produced aflatoxin
G1. One strain of A. flavus from black pepper produced only
aflatoxin B2 on 2 natural substrates tested (Schroeder & Carlton,
1973).
Although aflatoxins have been found in a variety of foodstuffs,
the most pronounced contamination has been encountered in groundnuts
and other oilseeds including cottonseed and maize. The most
frequently contaminated tree nuts are Brazil nuts and pistachios.
3.2.2.1 Maize
Surveys in the USA of more than 1500 samples of maize collected
in crop years 1964-67, mainly from commercial channels, revealed
that 2%-3% of the samples contained aflatoxins (total aflatoxin B1
and G1) in the range of 3-37 µg/kg (Shotwell et al., 1969a, 1970).
In a subsequent survey of 293 samples, 8 samples (2.7%) contained
aflatoxin B1 levels in the range of 6-25 µg/kg, one of the samples
containing aflatoxin G1 (25 µg/kg) as well as aflatoxin B1
(Shotwell et al., 1971). Aflatoxin B2 was also detected in some of
the samples. In a further study of 60 samples from south-east USA
(Shotwell et al., 1973), aflatoxin B1 was found in 21 samples
(35%) at levels ranging from 6-308 µg/kg in the 1969-70 period.
Aflatoxin B2 at levels ranging from a trace to 40 µg/kg was found
in 15 of these samples and aflatoxin G1 was found in 5 samples at
levels of a trace to 10 µg/kg. In 2 samples, aflatoxin G2 (1 and
<2 µg/kg) was detected in addition to aflatoxin B1. In some of
the southeastern states of the USA a high frequency of aflatoxin
contamination was also found in maize in the field (Anderson et al.,
1975; Lillehoj et al., 1976). In some of the field samples, the
levels of aflatoxin B1 ranged up to several thousand µg/kg or
more. Most of the field contamination was associated with damage
caused by insects such as the European corn borer, corn ear worms,
or weevils, and it seems likely that wherever such damage is
prevalent, field contamination with aflatoxins will occur. In
Thailand, 35% of maize samples contained aflatoxin B1 (average
level 400 µg/kg) while 40% contamination by aflatoxin B1 (average
level 133 µg/kg) was found in Uganda (Stoloff, 1976), and 97% in the
Philippine island of Sebu (average 213 µg/kg) (Alpert et al., 1971;
Campbell & Salamat, 1971; Shank et al., 1972a; Campbell & Stoloff,
1974; Stoloff, 1976).
Aflatoxin levels found in household maize samples in connexion
with the outbreak of acute toxic hepatitis in north-west India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977) are discussed
in section 3.5.1.2.
3.2.2.2 Wheat, barley, oats, rye, rice, and sorghum
Shotwell et al. (1968b) reported the presence of aflatoxins at
levels of less than 19 µg/kg in 9/1368 samples of wheat, sorghum,
and oats in the USA. Shotwell et al. (1976b) did not detect any
aflatoxin B1 (detection limit 1-3 µg/kg) in 848 samples of wheat
from various districts of the USA. The presence of aflatoxins B1,
B2, G1, and G2 was reported by Tripathi (1973) in heads of
sorghum heavily infected with mould, in field samples in India, but
he did not apply any confirmatory tests. Aflatoxins were also found
in sorghum in Uganda (Alpert et al., 1971) and, in a survey in the
USA, aflatoxins were detected in 2/66 samples of sorghum grain (13
and 50 µg/kg) (Stoloff, 1976). Aflatoxins have been detected in less
than 2% of more than 400 samples of rice from markets in Africa, the
Philippines, and Thailand (Alpert et al., 1971; Campbell & Salamat,
1971; Shank et al., 1972a). However, Lucas et al. (1970-71) reported
that out of 139 samples of rice obtained from the Ho Chi Minh
(Saigon) area in Viet Nam, 31% were found positive for aflatoxins,
no confirmatory tests were included in this study. In surveys of
wheat and other cereals in the USSR, Lvova et al. (1976) found
aflatoxin B1 at a level of 100 µg/kg in 1/169 samples (0.6%) in
the 1972 crop. Aflatoxins (aflatoxin B1 at levels ranging from 20
to 444 µg/kg and aflatoxin G1 at levels of 10-333 µg/kg) were
found in 24/138 samples (17.4%) in the 1973 crop year. In this year,
the samples to be analysed were specially selected from those that
were mouldy or had undergone heating or both. In a survey of wheat
in southern USSR (Kazakstan), Bucarbaeva & Nikov (1977) found
aflatoxin B1 in 2/50 samples (4%) from one district and 3/50
samples (6%) from another (levels ranging from 5 to 10 µg/kg).
3.2.2.3 Groundnuts (peanuts)
In the 1973 survey in the USA of shelled consumer groundnuts,
15% of 361 samples contained aflatoxins in the range of trace to
50 µg/kg (Stoloff, 1976). Krogh & Hald (1969) found aflatoxins in
86.5% of 52 samples of groundnut products imported into Denmark for
feed, one sample contained 3465 µg/kg. Aflatoxins were found in 41%
of 173 samples of groundnuts in the Sudan, 16% of the samples
containing more than 250 µg/kg, and 9%, more than 1000 µg/kg (Habish
et al., 1971). In the Philippines, all the samples of peanut butter,
tested in 1967-69, contained aflatoxins with a median value of
155 µg/kg and a mean value of 500 µg/kg. The highest level detected
was 8600 µg/kg (Campbell & Salamat, 1971). In Thailand, 49% of
market samples contained an average level of aflatoxins of
1530 µg/kg (Shank et al., 1972a).
3.2.2.4 Soybeans and common beans
No significant degree of aflatoxin contamination has been found
in soybeans or common beans in commerce in the USA (Stoloff, 1976),
although aflatoxin contamination sufficient to be of public health
concern has been found in various types of edible beans in Thailand
(Shank et al., 1972a) and in Africa (Alpert et al., 1971).
3.2.2.5 Tree nuts
Aflatoxin has been found occasionally in Brazil nuts, almonds,
walnuts, pistachio nuts, pecans, and filberts. In some of these,
contamination occurs when the nuts are still on the tree and is
usually associated with damage of one sort or another. However,
apparently sound, undamaged pecans may contain aflatoxins (Stoloff,
1976). Yndestad & Underdal (1975)in Norway found 66% of Brazil nuts
contaminated with aflatoxin B1 and Nilsson et al. (1974) found
that all of 23 batches of Brazil nuts intended for importation to
Sweden were contaminated. Fourteen percent of 74 samples of
California almonds were contaminated with aflatoxin B1 with levels
of less than 20 µg/kg in 90% of the contaminated samples (Schade et
al., 1975).
3.2.2.6 Copra
Aflatoxins were found in 88% of 72 samples of copra and copra
meal (Stoloff, 1976) in amounts ranging from a trace to 30 µg/kg,
and similar contamination was found by Krogh et al. (1970) in copra
imported into Finland.
3.2.2.7 Cottonseed
In 3 successive crop years (1964-67), aflatoxin B1 was
detected in 6.5%-8.8% of more than 3000 cottonseed samples and in
12.8%-21.5% of more than 3000 samples of cottonseed meal (Stoloff,
1976). In contrast, aflatoxin was not detected in cottonseed hulls
(Whitten, 1969). Relatively high levels of aflatoxin contamination
were found in an area in southern California. Aflatoxin levels
increased from 1735 µg/kg in some samples of seed harvested in
November to 2578 µg/kg in some samples going into storage in late
January. The amount present in the stored seeds did not increase
with time, even though fungi, including A. flavus, could be seen
growing on some of the seeds. Marsh et al. (1973) tested cottonseeds
from 13 locations across the USA cotton belt in 1969 and from 11
locations in 1970. Aflatoxins B1 and B2 were found in one or
more samples from 3 regions in areas where boll rot caused by
A. flavus had been repeatedly observed in previous years. Seeds
from individual lots contained aflatoxin B1 levels ranging from
200 000 to 300 000 µg/kg indicating the high potential hazard that
might occur from cottonseed.
3.2.2.8 Spices and condiments
Scott & Kennedy (1973) did not find any aflatoxins in 24 samples
of ground black or white pepper. Low concentrations (up to 8 µg/kg)
were found in 10/33 samples of cayenne pepper and 6/6 samples of
Indian chili powder, mainly as trace amounts.
3.2.2.9 Animal feeds
In studies by Strzelecki & Gasiorowska (1974), aflatoxins
occurred in 12.7% of 306 samples of animal feed and feed components
in Poland, 4.2% of the samples containing more than 100 µg/kg and
2.6% of the samples containing more than 1000 µg/kg. Feed
components, mainly groundnut meals, were contaminated by aflatoxins
more frequently and with higher levels. On the other hand, aflatoxin
was detected in only one sample (2.7%) of cattle and sheep feeds
(300 µg/kg) and in one sample (1.7%)of poultry feeds (30 µg/kg).
Swine feeds contained aflatoxins in 11.4% of samples, with 6 samples
(5.7%) exceeding 250 µg/kg. Two recent surveys of mixed feeds in the
Federal Republic of Germany revealed that 1 in 60 samples contained
aflatoxin B1 levels exceeding 20 µg/kg (Seibold & Ruch, 1977); 45
out of another 105 samples contained levels of between 7 and
300 µg/kg (Kiermeier et al., 1977). Similar results were obtained in
the United Kingdom (Patterson, personal communication) where 95/172
samples of dairy feed were contaminated with aflatoxin B1 levels
of 1-350 µg/kg, and 92.4% contained no more than 30 µg/kg.
3.2.2.10 Animal products
Surveys in several countries have shown that aflatoxin M1 may
be present in liquid or dried milk (Table 3) and in milk products
(Kiermeier, 1977). In addition, highly exceptional aflatoxin levels
in the range of 50-500 µg/litre were reported by Suzanger et al.
(1976) in half of the samples of cow's milk collected in villages
around Isfahan, Iran (15/30 samples collected in 1973 and 21/37
samples in 1974). In contrast, no aflatoxins were detected in 8
samples of milk obtained from large-scale producers in the same area
in 1974 and only 10% of such samples (2/20) contained aflatoxin M1
(in the range 8-10 µg/litre) in 1973. Aflatoxin M1 was identified
in all the positive samples. Eight of the 36 village samples
containing aflatoxin M1 also contained aflatoxin M2 and 2
samples contained aflatoxin B1. Considerable differences in the
handling and storage of animal feeds were thought by the authors to
be responsible for the differences in the aflatoxin M1 contents of
milk samples from villages and large-scale producers in this area.
However, levels of aflatoxins in animal feeds were not reported in
this paper, but they must have been exceptionally high.
Table 3. Selected surveys of aflatoxin M1 in cow's milk
Milk Country Total no. No. containing Range of concentrations Reference
samples of samples aflatoxin M1 in the positive samples
analysed (µg/litre or µg/kg)
Liquid Belgium 68 42 0.02-0.2 Van Pee et al. (1977)
German Democratic Republic 36 4a 1.7-6.5c Fritz et al. (1977)
Germany, Federal Republic of 61 28 0.01-0.25 Kiermeier (1973)
Germany, Federal Republic of 419 79 trace-0.54 Kiermeier et al. ( 1977)
Germany, Federal Republic of 260 118a 0.05-0.33 Polzhofer (1977)
India 21 3 up to 13.3 Paul et al. (1976)
Netherlands 95 74 0.09-0.5 Schuller et al. (1977)
United Kingdom 278 85a 0.03-0.52a Patterson et al. (in press)
Dried German Democratic Republic 18 0f --- Fritz et al. (1977)
Germany, Federal Republic of 166 8 0.67-2.0 Neumann-Kleinpaul & Terplan (1972)
Germany, Federal Republic of 52 35 trace-4.0 Hanssen & Jung (1972)
Germany, Federal Republic of 120 7a 0.05-0.13 Jung & Hanssen (1974)
South Africa 56 0 --- Luck et al. (1977)
USA 320 24 0.1-0.4 FDA (1977) 1973 survey
USA 302 192 trace-3.9e FDA (1977) 1977 survey
a Seasonal effect observed, i.e., concentration obviously, dependent upon level of concentrate feeding.
b Samples collected in retail outlets in 4 southeast States of the USA; it has been estimated that approximately two-thirds
of the crops in these areas contained aflatoxin concentrations exceeding 20 µg/kg because of unusual drought, insect
damage, and high temperature conditions that occurred in 1977.
c Values for 4 positive samples collected in winter; aflatoxin was not detected in the other 32 winter milk samples as well as
in 12 milk samples collected in summer (detection limit = 0.1 µg/kg).
d 92.5% samples contained aflatoxin M1 concentrations of less than 0.1 µg/litre.
e Levels ranging from 0.1 to 0.4 µg/kg reported in 158 samples; levels exceeding 0.5 µg/kg reported in 19 samples.
f Aflatoxin B1 contamination detected in one sample.
Aflatoxin residues have been found in animal tissues, eggs, and
poultry following the experimental ingestion of aflatoxin-
contaminated feed and this subject has been reviewed by Rodricks &
Stoloff (1977). However, the toxins have not yet been found in these
products on the market.
3.2.3 Fate of aflatoxins during the handling and processing
of food
Aflatoxins are affected by some ordinary food processing
procedures. In the roasting of groundnuts, approximately 50% of the
aflatoxins are altered to such an extent that they can no longer be
detected (Lee et al., 1969b; Waltking, 1971). The chemical nature of
the alteration products has not been fully elucidated.
The usual methods of processing groundnuts to make peanut butter
and some nuts for confections may appreciably reduce aflatoxin
contamination. The removal of undersized nuts (shrivels and pegs);
the removal of nuts that resist splitting and blanching; and the
removal of discoloured nuts by hand or electronic sorting are
effective means of reducing contamination (Rodricks et al., 1977).
In the removal of oil from oilseeds, most of the aflatoxins are
found in the oilseed meal. Small amounts remaining in the crude
vegetable oil are mainly taken out in the soap stock, the byproduct
from the alkali refining step. The remaining traces of aflatoxins
are removed in the bleaching refining steps to give aflatoxin-free
refined oil (Parker & Melnick, 1966).
The normal alkali processing of maize to produce tortilla-type
foods, a common practice in some areas of the world including some
Latin American countries, effectively reduces the levels of
aflatoxins in contaminated maize (Ulloa-Sosa & Schroeder, 1969).
Although the mechanism of this reduction has not been clarified,
some of the aflatoxin is most likely washed out by the initial
soaking of the maize in lye water and some is undoubtedly chemically
changed by the alkali; although this process is reported to produce
a substantial reduction in aflatoxin contamination, it is not enough
to give a safe product, when highly contaminated maize is being
processed.
Jemmali & Lafont (1972) reported only partial destruction of
aflatoxins during bread making, indicating the importance of the
contamination of wheat with A. flavus.
The above treatments are normal steps in the processing of
particular foods. In addition to such steps, procedures have been
developed specifically for the destruction or removal of aflatoxins
from grains, nuts, oilseeds, and oilseed meals (cake) (FAO, 1977).
Treatments with ammonia or hydrogen peroxide (H2O2) have been
the most effective procedures developed to date for the
detoxification of foodstuffs and animal feeds (Goldblatt & Dollear,
1977). The treatments with ammonia, developed for the industrial
decontamination of aflatoxin-contaminated groundnut and cottonseed
meal (cake) and maize, are limited to the production of animal
feeds. These procedures have recently been discussed in detail at
the joint FAO/WHO/ UNEP conference on mycotoxins in Nairobi (FAO,
1977). The process of treating groundnut protein isolate with
hydrogen peroxide to obtain a product suitable for use as a human
food supplement was also discussed. This process has been developed
in India and is reported to be operating on a small commercial
scale.
The feasibility of methods combining the physical separation of
contaminated portions of produce (for detailed descriptions of
segregation techniques see, for example, Rodricks et al., 1977) with
chemical decontamination, was considered at the same conference
(FAO, 1977).
3.2.4 Pathways and levels of exposure
From the previous discussion, it can be seen that a range of
commodities may become contaminated with trace amounts of
aflatoxins. In vegetable foods, this contamination results directly
from fungal spoilage, maize and nuts being particularly susceptible.
On the other hand, milk, and possibly meat and eggs can become
indirectly contaminated through the absorption by farm animals of
aflatoxins from contaminated feed resulting in residues of the
parent toxin or its metabolites in body fluids or tissues.
Thus, the level of man's exposure to dietary aflatoxins depends
upon the food available and on eating habits and will vary from
country to country according to the local conditions, including the
traditions of different ethnic groups, and amongst individuals.
Where contaminated groundnuts or maize make a significant
contribution to the diet, the level of exposure will be relatively
higher than where less commonly contaminated commodities take their
place as the staple food or when milk is the sole
aflatoxin-containing constituent of the diet.
In this connexion, the Task Group felt it was important to
identify the infant as being potentially at risk because: (a) baby
food products may be made from dried milk or even maize, commodities
known to be prone to contamination by aflatoxins; and (b) in terms
of larger amounts of food consumed per kg body weight, any level of
aflatoxin contamination is more significant for the child than for
the adult.
Attempts to quantify dietary exposure to aflatoxins are
discussed in detail in section 3.6.1.1.
Occupational exposure (section 3.5.2) to aflatoxins with its
attendant high risks, concerns two groups of individuals: those who
handle grain, animal feedstuffs, groundnuts, groundnut meal etc.
(where exposure could occur largely through inhalation of
contaminated dust), and those who work with toxins in experiments or
with pure toxins as analytical standards.
In one paper (van Nieuwenhuize et al., 1973) available to the
Task Group, an attempt was made to quantify occupational exposure to
airborne aflatoxins in an oil-mill crushing groundnuts and other
oil-seeds. Based on airborne dust determinations (mean aflatoxin
concentrations of 250 and 410 µg/kg airborne dust), the estimated
airborne aflatoxin levels ranged from 0.87 to 72 ng/m3 of air.
3.3 Metabolism
3.3.1 Absorption
Although quantitative data on absorption are not available at
present, there is no doubt that most of the field cases of
aflatoxin-induced diseases in animals and man have been associated
with ingestion of aflatoxin-contaminated foodstuffs and thus with
the absorption of aflatoxins in the alimentary tract. In spite of
reports of respiratory exposure (sections 3.2.4 and 3.5.2), there is
no quantitative information available on aflatoxin resorption from
the respiratory tract or on percutaneous absorption.
3.3.2 Tissue distribution
3.3.2.1 Animal studies
Experiments with 14C-ring-labelled aflatoxin B1 have shown
that rats retain about 20% of the 14C activity 24 h after a single
intraperitoneal dose of 0.07 mg/kg body weight (Wogan et al., 1967).
The highest concentration was found in the liver, which contained
amounts of radioactivity equivalent to the entire remainder of the
carcass (about 5%-8% of the total 14C recovered).
When poultry were fed rations containing aflatoxins at
concentrations ranging from 25 to 15 000 µg/kg for 8 weeks, residues
of aflatoxin B1 were found in the liver and in muscle tissue
(Mintzlaff et al., 1974). The liver contained the highest
concentration, with a mean value of 15 µg/kg at the highest exposure
level. Similarly the highest concentration of aflatoxin B1 was
found in the livers of pigs (range: trace-137 µg/kg) fed rations
containing aflatoxins (both aflatoxin B1 and B2) at levels of
300 and 500 µg/kg for 4 months (Krogh et al., 1973a). Aflatoxin
residues were also detected in kidneys, and muscle and adipose
tissue.
3.3.2.2 Studies in man
Levels of aflatoxins in the tissues of children with Reye's
syndrome are discussed in section 3.6.2.2. In a liver biopsy from a
subject with carcinoma of the rectum and liver in the USA, Phillips
et al. (1976) found 520 µg/kg of aflatoxin B1. In France, Richir
et al. (1976) found aflatoxin B1 in liver biopsies in 6 out of 100
subjects suffering from various diseases. Concentrations observed
ranged from 1.6-8 µg/kg.
3.3.3 Metabolic transformation and activation
With one exception, all primary biotransformations of aflatoxin
B1 involve its conversion to hydroxylated metabolites but only one
such derivative, aflatoxin M1 has appreciable oral toxicity
(Holzapfel et al., 1966). Even so, this metabolite may be detoxified
by conjugation with taurocholic and glucuronic acids prior to
excretion in the bile or urine (Bassir & Osiyemi, 1967). In this
respect, two recently discovered metabolites, P1 (Dalezios et al.,
1971; Buchi et al., 1973) and Q1 (Masri et al., 1974a,b) are
similar in that they also undergo this type of detoxification
(Dalezios et al., 1971; Dalezios & Wogan, 1972).
The conversion in the liver (Fig. 2) of aflatoxin B1 to
aflatoxicol (Patterson & Roberts, 1971) and to aflatoxicol H1 via
aflatoxin Q1 (Salhab & Hsieh, 1975) is unusual in that, unlike
other biotransformations that are catalysed by liver microsomal
enzymes, a cytoplasmic NADH-dependent dehydrogenase is involved.
Furthermore, the formation of aflatoxicol can be inhibited by
17-ketosteroid sex hormones (Patterson & Roberts, 1972a) and this is
the only metabolic transformation of aflatoxin in vitro known to
be sensitive to hormones.
Liver homogenates of certain avian and rodent species are
particularly active in converting aflatoxins B1 and G1 to their
2-hydroxy, 2,3-dihydro derivatives or hemiacetals called also
aflatoxins B2a and G2a (Patterson & Roberts, 1970). These
metabolites bind strongly to protein and are probably sufficiently
reactive, when formed in vivo, to cause many of the acute effects
of aflatoxin poisoning (Patterson & Roberts, 1972b; Patterson, 1973,
1977).
At present, there is only indirect evidence for the formation of
the epoxides of aflatoxins B1 and G1 but this is probably the
more important form of metabolic activation. When either of the
parent toxins is incubated with microsomes prepared from the livers
of many animal species including man, a metabolite is formed which
appears to have only a transient existence, is highly reactive,
binds covalently to DNA, and induces mutation in a bacterial
in vitro test system (Garner et al., 1971, 1972; Ames et al.,
1973). The metabolite of B1 has not been isolated but the
2,3-dihydrodiol has been recovered following mild acid hydrolysis of
an adduct formed when the microsomal metabolite was generated in the
presence of added DNA or RNA (Swenson et al., 1,974) and, more
recently, after in vivo intraperitoneal injection of aflatoxin
B1 (Swenson et al., 1977). This has been assumed to be indirect
evidence of the formation of the 2,3-epoxide and, in view of the
interaction with DNA, it is now generally accepted that the epoxide
of aflatoxin B1 is the bacterial mutagen and the proximal
carcinogen.
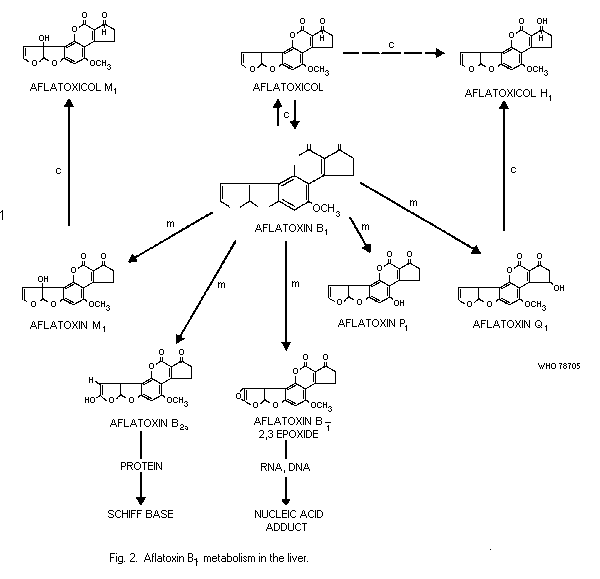 Certain of these biotransformations are better developed in some
animal species than others (Patterson, 1977) and attempts have been
made to correlate liver metabolism of aflatoxins with toxicity. In
the first such attempt (Patterson, 1973), it was proposed that rapid
in vitro formation of aflatoxin hemiacetal was correlated with
susceptibility to acute aflatoxin poisoning. More recently (Hsieh et
al., 1977), it has been suggested that the reversible formation of
aflatoxicol, which is thought to provide a "metabolic reservoir" of
aflatoxin (Patterson & Roberts, 1972b), is correlated with
susceptibility to liver tumour induction. On the basis of this, it
has been tentatively suggested (Hsieh, 1977; Salhab & Edwards, 1977)
that the human liver might be relatively more resistant to aflatoxin
carcinogenesis than that of some other species, particularly the
rat.
3.3.4 Excretion
3.3.4.1 Animal studies
Excretory pathways. Using aflatoxin B1, ring-labelled or
methoxy-labelled with 14C, Wogan et al. (1967) have shown that
rats excrete 7096-80% of a single intraperitoneal dose within 24 h.
A major excretory route of the ring-labelled toxin was through
biliary excretion into the faeces, accounting for about 60% of the
administered dose; approximately 20% of administered radioactivity
was excreted in the urine, and only negligible amounts in expired
air in the form of 14CO2. In contrast, approximately 25% of
radioactivity from methoxy-labelled material appeared in expired air
as 14CO2 with a concomitant decrease in the faeces, indicating
that O-demethylation is a significant metabolic pathway for
aflatoxin B1 in the rat.
Excretion in the milk of farm animals. Several reviews deal
with the excretion of aflatoxins in the milk of farm animals
(Allcroft, 1969; Kiermeier, 1973, 1977; Patterson, 1977; Rodricks &
Stoloff, 1977). When cattle (Allcroft et al., 1968), sheep (Nabney
et al., 1967) or goats (Vesely, et al., 1978) are given feed
contaminated with aflatoxin B1 their milk contains aflatoxin M1.
In the cow, there is a linear relationship between the amount of
aflatoxin B1 ingested daily and the level of aflatoxin M1 in the
milk (Allcroft & Roberts, 1968; Purchase, 1972; Patterson, 1977; see
Fig. 3), indicating that about 1.5% of aflatoxin B1 is excreted as
the metabolite M1 (Kiermeier, 1973), and that the concentration of
aflatoxin B1 in milk is approximately 1/300 of the concentration
of aflatoxin B1 in the dairy ration (Rodricks & Stoloff, 1977).
Smaller quantities of unmetabolized aflatoxin B1 have been found
in cow's and sheep's milk (Nabney et al., 1967; Allcroft et al.,
1968; Wogan, 1969).
Certain of these biotransformations are better developed in some
animal species than others (Patterson, 1977) and attempts have been
made to correlate liver metabolism of aflatoxins with toxicity. In
the first such attempt (Patterson, 1973), it was proposed that rapid
in vitro formation of aflatoxin hemiacetal was correlated with
susceptibility to acute aflatoxin poisoning. More recently (Hsieh et
al., 1977), it has been suggested that the reversible formation of
aflatoxicol, which is thought to provide a "metabolic reservoir" of
aflatoxin (Patterson & Roberts, 1972b), is correlated with
susceptibility to liver tumour induction. On the basis of this, it
has been tentatively suggested (Hsieh, 1977; Salhab & Edwards, 1977)
that the human liver might be relatively more resistant to aflatoxin
carcinogenesis than that of some other species, particularly the
rat.
3.3.4 Excretion
3.3.4.1 Animal studies
Excretory pathways. Using aflatoxin B1, ring-labelled or
methoxy-labelled with 14C, Wogan et al. (1967) have shown that
rats excrete 7096-80% of a single intraperitoneal dose within 24 h.
A major excretory route of the ring-labelled toxin was through
biliary excretion into the faeces, accounting for about 60% of the
administered dose; approximately 20% of administered radioactivity
was excreted in the urine, and only negligible amounts in expired
air in the form of 14CO2. In contrast, approximately 25% of
radioactivity from methoxy-labelled material appeared in expired air
as 14CO2 with a concomitant decrease in the faeces, indicating
that O-demethylation is a significant metabolic pathway for
aflatoxin B1 in the rat.
Excretion in the milk of farm animals. Several reviews deal
with the excretion of aflatoxins in the milk of farm animals
(Allcroft, 1969; Kiermeier, 1973, 1977; Patterson, 1977; Rodricks &
Stoloff, 1977). When cattle (Allcroft et al., 1968), sheep (Nabney
et al., 1967) or goats (Vesely, et al., 1978) are given feed
contaminated with aflatoxin B1 their milk contains aflatoxin M1.
In the cow, there is a linear relationship between the amount of
aflatoxin B1 ingested daily and the level of aflatoxin M1 in the
milk (Allcroft & Roberts, 1968; Purchase, 1972; Patterson, 1977; see
Fig. 3), indicating that about 1.5% of aflatoxin B1 is excreted as
the metabolite M1 (Kiermeier, 1973), and that the concentration of
aflatoxin B1 in milk is approximately 1/300 of the concentration
of aflatoxin B1 in the dairy ration (Rodricks & Stoloff, 1977).
Smaller quantities of unmetabolized aflatoxin B1 have been found
in cow's and sheep's milk (Nabney et al., 1967; Allcroft et al.,
1968; Wogan, 1969).
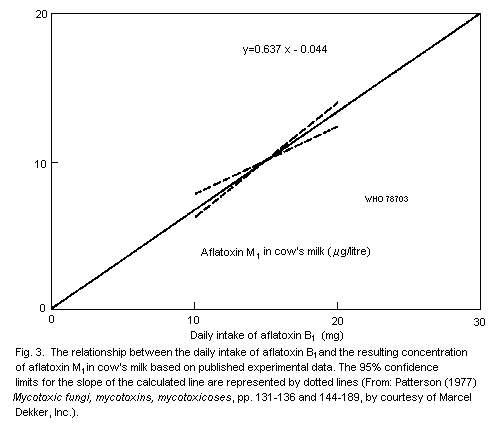 3.3.4.2 Studies in man
In the Philippines, aflatoxin M1 has been found (not
measured)in the urine of human subjects known to have ingested
aflatoxin-contaminated peanut butter (Campbell et al., 1970).
Claims concerning an aflatoxin involvement in the etiology of
juvenile cirrhosis in India (section 3.6.3.2) based on a
blue-fluorescent B1 spot in the breast milk of mothers and the
urine of children with the disease (Robinson, 1967) are largely
discounted by the later studies of Yadgiri et al. (1970) who
produced spectrophotometric evidence that, although such a spot
could be identified in the urine of children with the overt disease,
this was not aflatoxin B1 For other reports see section 3.5.1.
3.4 Effects in Animals
The effects of aflatoxins in animals have., been reviewed by
Allcroft (1969), Newberne & Butler (1969) and Butler (1974).
3.4.1 Field observations
When foodstuffs are affected by microbial deterioration, man
normally eats the less affected parts, whereas domestic animals may
be exposed to more contaminated rations. This explains why the
discovery of several mycotoxins has been based on field observations
in domestic animals.
The first observation of a disease in animals subsequently
associated with aflatoxins was an acute outbreak of a lethal disease
in turkey poults in England in 1960 causing an estimated loss of at
least 100 000 birds. Extensive research eventually revealed that the
disease was caused by aflatoxins contained in a batch of Brazilian
groundnut meal. The concentration of aflatoxin B1 in the original
groundnut meal was later estimated to be about 10 mg/kg. The disease
was characterized by rapid deterioration in the condition of the
birds, subcutaneous haemorrhages, and death. At postmortem, the
livers of the birds were pale, fatty, and showed extensive necrosis
and biliary proliferation (Butler, 1974). A similar case of acute
disease was observed in day-old ducklings fed "toxic" groundnut meal
(Asplin & Carnaghan, 1961), where the liver changes described were
followed by cirrhosis. Outbreaks of liver disease in chickens have
also been associated with aflatoxin-contaminated feed (Asplin &
Carnaghan, 1961).
Loosmore & Harding (1961) noted outbreaks in pigs fed groundnut
meal in which the toxic factor was later identified as aflatoxins.
The lesions in the pigs included haemorrhages, and liver damage
characterized by dissecting fibrosis and biliary proliferation.
Calves fed rations containing 15% toxic groundnut meal also
developed liver lesions characterized by fibrosis and biliary
proliferation (Loosmore & Markson, 1961). Outbreaks associated with
"toxic" groundnut meal and characterized by similar liver lesions
have been reported in older cattle even though they are more
resistant (Clegg & Bryston, 1962); there was also a drop in milk
production.
A liver disease "hepatitis X" has been reported in dogs in
southeastern USA (Seibold & Bailey, 1952; Newberne et al., 1955).
Icterus and in some cases ascites were observed and the liver
lesions included fatty changes with centrilobular parenchymal
necrosis and biliary proliferation. Commercial dog food thought to
be the cause of toxicity was later found to contain aflatoxin B1
(up to 1.75 mg/kg) (Newberne et al., 1966a). Similar lesions have
been reproduced in dogs by peroral administration of aflatoxins, and
it has been suggested that the "hepatitis X" in dogs could be
causally associated with aflatoxins in the diet (Newberne et al.,
1966a). During an outbreak of toxic hepatitis affecting several
hundred people in north-west India and considered to be possibly
associated with the consumption of maize heavily contaminated by
aflatoxins (section 3.5.2 and 3.6.2.1), dogs fed food remnants from
households in affected villages manifested a disease characterized
by jaundice, ascites, and frequently death (Krisnamachari et al.,
1975a, b; Tandon et al., 1977). A nonportal type of micronodular
cirrhosis, with less conspicuous parenchymal and cholangiolar
changes was found on histological examination of the livers of two
dogs (Tandon et al., 1977).
3.4.2 Experimental studies
3.4.2.1 Acute and chronic effects: hepatotoxicity
Different species vary in their susceptibility to acute
poisoning by aflatoxins, with LD50 values ranging from 0.3 to
17.9 mg/kg body weight (Table 4). In all the animals studied, the
liver was the principal target organ (see for example Butler, 1974).
Table 4. Acute toxicity of aflatoxin B1a
Species LD50 Zone of
(mg/kg body weight) liver lesion
chick embryo 0.025d
rabbit 0.3 midzonal
duckling 0.335 periportal
cat 0.55 periportal
pig 0.62 centrilobular
dog 0.5-1.0 centrilobular
sheep 1.0 centrilobular
guineapig 1.4 centrilobular
baboonb 2.0 centrilobular
rat (male) 7.2 periportal
macaque femalec 7.8 centrilobular
mouse 9.0
hamster 10.2
rat (female) 17.9 periportal
a Adapted from: Newberne & Butler (1969) and Butler (1974).
b From: Peers & Linsell (1976).
c From: Shank et al. (1971 b).
d µg/embryo.
The lesions observed in field cases (section 3.4.1) in poultry,
pigs, cattle, and dogs have all been reproduced in the same animal
species by feeding experiments during periods of time ranging from a
few weeks to a few months, using diets containing aflatoxins, or
pure aflatoxins ranging from 0.3 to several mg/kg (Newberne &
Butler, 1969).
In the study by Carnaghan et al. (1966), chickens were fed a
diet containing aflatoxin B1 at a level of 1.5 mg/kg. Groups of 3
control and 3 test chicks were killed after 3´ days, 7 days, and
then at weekly intervals for 8 weeks. After 4 weeks, the liver
lesions included fatty change, biliary proliferation, and fibrosis.
In 20 pigs, aflatoxins (aflatoxins B1 and B2) at a feed
level as low as 300 µg/kg resulted in the development of
centrilobular necrosis and fibrosis of the liver as well as growth
depression, during a normal feeding period of 3-4 months (Krogh et
al., 1973a). In cattle, the liver lesions (centrilobular
degeneration, fibrosis, biliary proliferation) occurred in all 4
animals after 4 months on a feed containing an aflatoxin level of
2 mg/kg (Allcroft & Lewis, 1963). The hepatic lesions induced in the
duckling by the aflatoxins formed the basis of a bioassay originally
described by Sargeant et al. (1961). At sublethal doses of
aflatoxins, the bioassay depends upon an assessment of the degree of
biliary proliferation. Liver lesions similar to those observed in
farm animals have been experimentally induced by the administration
of aflatoxins in a number of laboratory animals, including the rat,
cat, guinea-pig, and rabbit (Newberne & Butler, 1969).
In a study of Madhavan et al. (1965b), 2 rhesus monkeys
(Macaca mulatta) were given daily oral doses of aflatoxins at
500 µg/animal for 18 days (corresponding approximately to 250 µg/kg
body weight per day) and then 1 mg/animal per day (corresponding
approximately to 500 µg/kg body weight per day) until death occurred
after 32 and 34 days. Three rhesus monkeys were given 1 mg each,
daily, until death occurred after 19, 20, and 27 days respectively.
The liver lesions included fatty infiltration, biliary
proliferation, and portal fibrosis. Death or similar lesions were
not observed in the 2 control monkeys.
Deo et al. (1970) studied the effect on male rhesus monkeys of
repeated administration by gastric tube of 3 different levels of
aflatoxins (B1 + G1). At the highest dose level (1 mg/kg body
weight daily for 3 weeks), 35/35 animals died within 22 days with
extensive haemorrhagic necrosis of the liver. A dose level of
0.25 mg/kg body weight, twice a week, for 5 months induced various
degrees of liver changes in 24/24 animals characterized by biliary
proliferation and focal appearance of the liver cells with multiple
nuclei and giant-sized liver cells with enlarged hyperchromatic
nuclei. At the lowest dose, 5 animals were given 62 µg/kg body
weight once a week for periods ranging from a few days to 2 years.
Liver changes were similar to the changes seen in the second group
but in a milder form.
Cynomolgus monkeys (Macaca fascicularis = M. irus) fed a
dietary level of aflatoxin B1 of 5 mg/kg rapidly developed liver
damage with biliary proliferation and all 6 animals died within 2
months. When fed aflatoxin B1 at a dietary level of 1.8 mg/kg, 5
animals died within 3 months showing liver damage characterized by
centrilobular necrosis, biliary proliferation, and fibrosis. Two
animals survived and were killed after 3 years; the liver of one
animal had the appearance of nodular cirrhosis. Two groups of 4
animals each were fed lower levels of aflatoxin B1 (0.07 and
0.36 mg/kg, respectively) for 3 years without showing any signs of
liver lesions (Cuthbertson et al., 1967).
The relationship between the chemical structure of different
aflatoxins and their biological activity (discussed also in section
3.4.2.3) was investigated in a small number of experiments; more
extensive studies were not possible because of the limited
quantifies available of some of the pure aflatoxins. Carnaghan et
al. (1963) compared 6-day mortality following single doses of
different aflatoxins, administered by intubation to one-day-old
Khaki Cambell ducklings, and concluded that both aflatoxins B2 and
G2 were less toxic than aflatoxins B1 and G1, the ratio of
LD50 values being 1:4.7 for B1:B2 and 1:4.4 for G1:G2.
Aflatoxins G1 and G2 were less toxic than the corresponding
aflatoxins B1 and B2, the ratio of the LD50 values being
1:2.15 for B1:G1 and 1:2.03 for B2:G2. The corresponding
LD50 values for aflatoxins B1, B2, G1, and G2 were 0.36,
1.70, 0.78, and 3.45 mg/kg, respectively. Comparable results were
obtained by Wogan et al. (1971) who recorded 14 day mortality after
intubation of male Pekin ducklings and reported LD50 values of
0.73, 1.76, 1.18 and 2.83 mg/kg for aflatoxins B1. B2, G1, and
G2, respectively. In the same paper, a study was reported on the
14-day mortality of male Fischer rats after a single
(intra-peritoneal) dose of aflatoxin. The LD50 value for aflatoxin
B1 was 1.16 mg/kg body weight (95% confidence interval 0.91 to
1.48 mg/kg) whereas the LD50 for aflatoxin G1 was between 1.5
and 2.0 mg/kg body weight. On the other hand, no deaths occurred in
20 rats given 12-200 mg of aflatoxin B2 per kg body weight, and
all 4 rats given 170-200 mg of aflatoxin G2 per kg body weight
survived. A similar difference in the toxicity of aflatoxins was
observed when male Fischer rats were given repeated doses of
aflatoxins by stomach tube over a 4-week period. The 4-week
mortality in rats given a total dose of 1 mg of aflatoxin B1 per
rat was 8/10 whereas all 10 rats given the same dose of aflatoxin
G1 survived and only 4/10 animals given double this dose of
aflatoxin G1 died. In another trial, all 11 rats survived
intragastric administration of 3.75 mg of aflatoxin B2 per rat
repeated every second day for 4 weeks to give a total dose of
52.5 mg per rat.
Holzapfel et al. (1966) and Purchase (1967) reported 7-day
mortality alter oral dosing of one-day-old Pekin ducklings with
aflatoxins B1, M1, and M2. Five groups of 2-3 ducklings (body
weight 40-50 g) were used for each of the aflatoxins tested, and the
following LD50s were calculated (with 95% confidence limits given
in brackets): aflatoxin B1, 12 (3.9-37.2) µg per duckling,
aflatoxin M1, 16 (5.4-51.5) µg per duckling, and aflatoxin M2,
61.4 (37-100) µg per duckling. Ducklings receiving aflatoxin M2
showed characteristic liver lesions indistinguishable from those
observed after a similar dose of aflatoxin B1. Higher doses of
aflatoxin M2 produced similar effects (Purchase, 1967). A study
comparing the acute toxicity of synthetic (racetalc) aflatoxins B1
and M1 and the natural optical isomer of aflatoxin B1 was
reported by Pong & Wogan (1971), suggesting that only one isomer of
each synthetized racemic mixture was biologically active.
Fourteen-day mortality rates observed after a single intraperitoneal
dose of 1.5 mg/kg body weight of synthetic aflatoxin B1 and
synthetic aflatoxin M1 were 1/1 and 1/2, respectively. However, no
deaths occurred in groups of rats (each consisting of 4 animals)
given these synthetic aflatoxins at doses of 1, 0.8, 0.6, or
0.4 mg/kg body weight. With the natural aflatoxin B1, the observed
mortalities at these dose levels were 4/4, 2/4, 2/4, and 0/4
respectively.
For information on the toxicity of certain other aflatoxin
metabolites or derivatives, see Wogan et al. (1971) and Patterson
(1976).
3.4.2.2 Hepatotoxicity connected with extrahepatic effects
Many other organs besides the liver are more or less severely
affected in acute experiments with high doses of aflatoxins (Butler,
1964): in male and female rats, a single dose of aflatoxin B1
proved lethal in half of the animals (7.2 mg/kg body weight in the
male and 17.9 mg/kg body weight in the female, by garage). Frequent
bilateral adrenal haemorrhages, petechial haemorrhages in many
organs, particularly in the congested lungs, and occasionally patchy
necroses in the myocardium and in other organs (kidney, spleen) were
observed during the first few days following administration. These
changes were not detected in male or female rats given aflatoxin B1
at 3.5 mg/kg body weight. With higher doses, the haemorrhages seen
in the lungs, kidneys, and adrenals were more extensive. Animals
dying within the first few days often had altered blood in the whole
of the small intestine and in the colon. Ascites and oedema of the
omentum were observed in some of the animals a week or more (but not
one month) after aflatoxin administration. After a month, with the
exception of the liver damage, all the other organs appeared normal
in surviving animals. Histologically, certain renal changes were
detected in the loops of Henle at this stage, consisting of a few
cells with large irregular hyperchromatic nuclei, very similar to
those seen in the liver (Butler, 1964).
Congested lungs with small petechial haemorrhages, haemorrhagic
necroses in the adrenals (localized in the inner zone of the
reticularis) and patchy necroses in the kidneys, pancreas, and
spleen were observed in guineapigs 2-3 days after a single
intraperitoneal injection of aflatoxin B1 at 1.4 mg/kg body weight
(lethal in half of the males and females). Even at this dose, the
small intestine was frequently filled with altered blood. At higher
doses, the haemorrhagic disease was more marked, with pleural,
pericardial, and peritoneal haemorrhages. The only change seen in
the heart of the guineapig, 2-3 days after aflatoxin administration,
was an occasional small area of fatty degeneration of the
myocardium. Many animals showed marked ascites and oedema of the
omentum and subcutaneous tissue during the first week after
injection (Butler, 1966).
Bourgeois et al. (1971) reported a special syndrome induced by
oral administration of aflatoxin B1 in the macaque (Macaca
fascicularis). In 2 groups of 4 young females, each receiving a
single oral dose of aflatoxin B1 at 13.5 or 40.5 mg/kg body
weight, all animals died within 149 h. Death occurred in 1 out of 4
other animals receiving a dose of 4.5 mg/kg body weight. Doses of
toxin of 1.5 mg/kg or 0.5 mg/kg (4 animals in each group) did not
result in death or unusual clinical signs. Cough, vomiting,
diarrhoea, and coma were characteristic clinical findings in animals
exposed to toxic doses. Analysis of blood serum revealed a
dose-dependent decrease in serum levels of phospholipids within 24 h
of administration of the aflatoxin. A dose-dependent decrease in
serum levels of glucose and an increase in nonesterified fatty acids
occurred within 72 h of aflatoxin administration. The liver lesions
included centrilobular necrosis, some biliary proliferation, and
massive fatty degeneration which was also observed in the heart and
kidneys. Cerebral oedema with neuronal degeneration was seen. Some
of these findings resemble those associated with Reye's syndrome in
children (see section 3.5.1.2).
3.4.2.3 Carcinogenesis
The carcinogenesis of aflatoxins has been reviewed by Wogan
(1973, 1977) and re-evaluated by IARC (1976).
Hepatic and renal tumours. Orally administered aflatoxins,
mainly B1, have been hepatocarcinogenic in all species of test
animals studied so far (including nonhuman primates), with the
exception of the mouse, in which carcinogenic effects have been
demonstrated only following intraperitoneal administration of
aflatoxin B1 to neonates (Tables 5 and 6). These studies were
concerned with repeated or long-term exposure to aflatoxins. In a
study by Carnaghan (1967), 2 groups consisting of 16 and 18 weanling
female Wistar rats, respectively, were given single oral doses of
crystalline aflatoxin B1 or a mixture of aflatoxins containing
about 40% aflatoxin B1 and 60% aflatoxin G1, at the rate of
0.5 mg/rat in 0.1 ml dimethylformamide. These doses corresponded to
averages of 7.65 mg aflatoxin B1/kg body weight and 2.7 mg
aflatoxin B1 plus 4.0 mg aflatoxin G1/kg body weight,
respectively. Within 21-32 months, 7 rats out of each group
developed hepatic tumours with metastases in half the cases. Hepatic
rumours were not observed in 19 control rats given the solvent only.
No hepatocellular carcinomas were found in 22 male Fischer rats
killed successively 16 weeks (3 rats), 25 weeks (5 rats), 38 weeks
(5 rats), 55 weeks (4 rats), and 69 weeks (5 rats) after a single
dose of aflatoxin B1 at 5.0 mg/kg body weight, administered by
garage (Wogan & Newberne, 1967).
A linear dose-response relationship was observed by Wogan et al.
(1974) for the development of liver-cell carcinomas in male Fischer
rats fed dietary concentrations of aflatoxin B1 ranging from
1-100 µg/kg (Table 7). At 1 µg/kg, a 10% tumour incidence was found,
compared with no rumours in the control group and at 100 µg/kg the
tumour incidence was 100%. A linear log (dose)-response relationship
has been demonstrated in trout fed dietary levels of aflatoxin B1
ranging from 0.5 to 20.0 µg/kg, for 20 months. Extrapolating this
relationship to lower exposure levels, an incidence of approximately
10% would be expected with a dietary concentration of 0.1 µg/kg.
Table 5. Hepatocarcinogenicity of aflatoxin B1 in rodentsa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
rat, Fischer 1.0 mg/kg diet 33 weeks 52 weeks 3/6 Svoboda et al. (1966)
rat, Fischer 1.0 mg/kg diet 41-64 weeks 41-64 weeks 18/21 Wogan & Newberne (1967)
rat, Porton 1.0 mg/kg diet 20 weeks 90 weeks 19/30 Butler (1969)
rat, Wistar 1.0 mg/kg diet 21 weeks 87 weeks 12/14 Epstein et al. (1969)
mouse, Swiss 150 mg/kg dietb 80 weeks 80 weeks 0/60 Wogan (1973)
mouse, C57Bl/6NB 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, C3HfB/HEN 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, hybrid F1, 4 days old 6.0 µg/g body weight 3 doses (i.p.) 80 weeks 16/16 Vesselinovitch et al. (1972)
a From: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
Table 6. Hepatocarcinogenicity of aflatoxin B1 in nonrodent speciesa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
monkey, rhesus (M) 1.655 g totalb 5.5 years 8.0 years 1/1 Gopalan et al. (1972)
monkey, rhesus (F) 1.855 g total 5.5 years 10.75 years 1/1 Tilak (1975)
monkey, rhesus (F) 0.504 g total 6.0 years 8.0 years 1/1 Adamson et al. (1973)
marmoset 3.0 mg total 50-55 weeks 50-55 weeks 1/3 Lin et al. (1974)
5.04-5.84 mg totalc 87-94 weeks 87-94 weeks 2/3 Lin et al, (1974)
tree shrew (M & F) 24-66 mg total 74-172 weeks 74-172 weeks 9/12 Reddy et al, (1976)
ferret 0.3-2.0 µg/kg 28-37 months 28-37 months 7/9 Butler (1969)
duck 30 µg/kg 14 months 14 months 8/11 Carnaghan (1965)
rainbow trout 4 µg/kg in diet 12 months 12 months 15% Sinnhuber et al, (1968b)
8 µg/kg in diet 12 months 12 months 40% Sinnhuber et al. (1968b)
rainbow trout embryos 0.5 mg/kg in water 1 h 296-321 days 38% Sinnhuber & Wales (1974)
salmon 12µg/kg in dietd 20 months 20 months 50% Wales & Sinnhuber (1972)
guppy 6 mg/kg in diet 11 months 11 months 7/11 Sato et al. (1973)
a Modified from: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
c These animals were infected simultaneously with hepatitis virus.
d This diet also contained 50 mg/kg cyclopropenoid fatty acids.
Table 7. Dose-response characteristics of aflatoxin B1 carcinogenesis
in male Fischer strain ratsa
Dietary Duration Liver Time of appearance
aflatoxin of feeding carcinoma of earliest tumour
level (week) incidenceb (week)
(µg/kg)
0 74--109 0/18c --
1 78--105 2/22 104
5 65--93 1/22 93
15 69--96 4/21 96
50 71--97 20/25d 82
100 54--88 28/28e 54
a From: Wogan et al. (1974).
b In animals at risk (surviving longer than 50 weeks).
c Animals surviving for maximum period.
d Two animals had pulmonary metastases.
e Four animals had pulmonary metastases.
In a recent study (FDA, 1978), an attempt was made to calculate
the lifetime liver cancer risk in rats, which could be connected
with aflatoxin feed levels lower than those directly tested in
animal experiments. Estimates of life-time liver cancer incidence
rates corresponding to aflatoxin feed levels of 0.1 and 0.3 µg/kg
were derived and compared for several selected rat studies using the
mathematical procedure developed by Mantel & Bryan (1961) and
modified by Mantel et al. (1975). A more detailed description of
this procedure can be found in other publications including WHO
(1978) and Hoel et al. (1975). Thus, the estimated life-time liver
cancer risk derived from the experimental results reported by Wogan
et al. (1974) (see Table 7) corresponded to life-time liver cancer
incidence rates of 70 (600) per 105 rats for an aflatoxin dietary
level of 0.1 µg/kg and 360 (2300) for a level of 0.3 µg/kg. (Numbers
in parentheses; are upper 99% confidence limits.) Estimates derived
from different rat studies varied considerably. The lifetime
incidence rates calculated for the combined studies were 240 (470)
and 1100 (1900) per 105 rats for aflatoxin feed levels of 0.1 and
0.3 µg/kg, respectively (FDA, 1978).
The studies in primates are included in Table 6. No attempts
have been made to establish dose-response relationships in primates,
but they are susceptible to aflatoxin hepatocarcinogenesis.
The carcinogenic effects of different purified aflatoxins have
been compared in a limited number of studies. The results of a study
by Butler et al. (1969) in which 8-9 week old, male (M) and female
(F) MRC rats were given aflatoxins B1, B2, or G1 in drinking
water for 10 or 20 weeks, are shown in Table 8. The earliest renal
neoplasm was seen 54 weeks after the discontinuation of aflatoxin
treatment. Renal tumours were detected only in males. The 2 rats
treated with aflatoxin B1 that developed renal tumours did not
have hepatic carcinomas, but 5 of the 11 rats receiving aflatoxin
G1 developed both renal and hepatic carcinomas.
Wogan et al. (1971) studied the relationship between the
chemical structures of aflatoxins and their hepatocarcinogenicity in
male Fischer rats, and concluded that aflatoxin B1 was apparently
more carcinogenic than aflatoxin G1 and that both were much more
active than aflatoxin B2. A total intraperitoneal dose of
aflatoxin B2 of 150 mg per rat given in 40 equal doses over 8
weeks induced hepatocellular carcinomas in 3/9 rats. A similar
regimen, containing a total dose of aflatoxin B1 of 1.3 mg,
induced liver tumours in 9/9 animals. In other experiments to
compare the carcinogenicity of aflatoxins G1 and B1 given by
stomach tube to rats, aflatoxin G1 in a total dose of 1.4 mg per
animal (divided into 14 equal doses over 2.5 weeks) induced
hepatocellular carcinoma in 3/5 rats within 68 weeks. Hepatocellular
carcinomas were observed in all 18 animals given a total dose of
2 mg of aflatoxin G1 (divided in 40 equal doses over 8 weeks) and
killed within 45-64 weeks. Six of these rats had pulmonary
metastases.
Hepatocellular carcinomas were also found in 7/7 rats given
aflatoxin B1 in a total dose of 0.5 mg per animal (divided in 20
equal doses over 4 weeks) and sacrificed within 74 weeks, in 18/18
rats given 1 mg per animal (divided into 40 equal doses over 8
weeks) and killed within 42-58 weeks, and in 17/17 given 1.5 mg per
animal (divided into 40 equal doses over 8 weeks) and killed within
42-46 weeks. With the 2 higher doses, 2 and 7 animals, respectively
had pulmonary metastases. Renal adenocarcinomas were found in 4/26
rats given aflatoxin G1.
Synthetic racemic aflatoxin M1 induced hepatocarcinomas at 100
weeks in 1/29 male Fischer rats given 1 mg of the compound
intragastrically in divided doses over a period of 8 weeks. The
incidence of hepatocarcinomas in 9 rats given the same dose of
natural aflatoxin B1 was 100%, 1 year after treatment (Wogan &
Paglialunga, 1974). Natural aflatoxin M1 was less effective than
natural aflatoxin B1 in inducing tumours in trout, particularly in
the males. Only 14% of male trout, fed natural aflatoxin M1 for a
year at a dietary level of 4 µg/kg, developed liver tumours compared
with 68% of those receiving a similar feed level of aflatoxin B1
Table 8. Carcinogenesis in rats due to ingestion of aflatoxin B1, G1 or B2 in drinking watera
Compound Concentration Daily Duration Total No. and sex No. of animals No. of animals
(µg/ml) dose (µg) weeks dose (mg) of animals with tumours with other
treated neoplasms
liver kidney
aflatoxin B1 1 20 20 2 15 M 8 2 4
15 F 11 0 1
aflatoxin B1 1 20 10 1 10 M 3 0 2
aflatoxin G1 3 60 20 6 11 M 9 6 5
15 F 12 0 3
aflatoxin G1 1 20 20 2 15 M 2 5 2
15 F 1 0 7
aflatoxin G1 1 20 10 1 10 M 1 0 1
aflatoxin B2 1 20 10 1 10 M 0 0 2
controls 0 0 0 0 15 M 0 0 6
15 F 0 0
a From: Butler et al. (1969).
(the experiment was terminated 8 months after the discontinuation of
aflatoxin feeding). In females, the difference was less pronounced,
liver tumours occurring in 48% of aflatoxin B1-treated trout and
78% of aflatoxin B1-treated trout (see Table 9) (Sinnhuber et al.,
1974). Haemorrhages within the cancerous liver resulting in death,
which were observed in most females receiving dietary levels of
aflatoxin M1 of 16-64 µg/kg, were not observed in similarly
treated males.
Table 9. Aflatoxin M1 liver carcinogenesis in rainbow trout
(Salmo gairdneri)a
Aflatoxin in the diet Liver tumour incidence
males females
M1 4 µg/kg 4/28 (14%) 13/27 (48%)
M1 16 µg/kg 22/27 (81%) 11/14 (79%)
M1 32 µg/kg 24/25 (96%) 13/14 (93%)
M1 64 µg/kg 21/24 (88%) 9/10 (90%)
B1 4 µg/kg 15/22 (68%) 18/23 (78%)
a From: Sinnhuber et al. (1974).
A probable effect of rat strain on aflatoxin carcinogenesis was
seen in the high incidence of renal epithelial neoplasms reported in
male Wistar strain rats fed diets containing aflatoxin B1, for 147
days, and then maintained on a basal diet until death (Epstein et
al., 1969). Renal tumours developed in 57% (8/14), 28% (5/18), and
23% (3/13) of male rats exposed to diets containing aflatoxin B1
levels of 1.0, 0.5, and 0.25 mg/kg feed, respectively. The
incidences of malignant hepatomas in corresponding groups were 86%,
72%, and 62% respectively. No renal tumours or malignant hepatomas
were detected in a control group (24 animals). Approximately
one-third of the aflatoxin-exposed rats with renal rumours did not
have hepatomas. The first malignant hepatoma and the first renal
tumour were detected 463 and 468 days after initiation of the
experiment, respectively; both these tumours occurred in the group
with the highest exposure. Approximately half of the renal tumours
were bilateral.
Early hepatic lesions possibly related to carcinogenesis.
Aflatoxin B1 given to rats in repeated doses of 15-25 µg/day, for
3.5 weeks, making a total dose of 375 µg, elicited increased DNA
synthesis and mitosis in clusters of liver cells that could be
distinguished from the surrounding cells by their histological
appearance and biochemical activity (Newberne & Wogan, 1968a; Rogers
& Newberne, 1969). Development of the abnormal foci was more
prominent in rats fed a high-fat, lipotrope-deficient diet, which
enhances aflatoxin carcinogenesis (section 3.4.2.7), than in rats
fed a nutritionally adequate diet. The abnormal foci were already
present in deficient rats at the end of carcinogen administration.
Similar foci are found in the livers of rats exposed to other
hepatic carcinogens and may be useful in studying pathogenesis or
metabolic aspects of liver cancer, since they are thought to be the
possible precursors of tumours. However, many of the foci disappear
with time after treatment, and progression is not inevitable.
Other tumours. Carcinoma of the colon have occasionally been
reported following aflatoxin exposure (Newberne & Butler, 1969).
Increased incidence of tumours in the distal half of the colon was
observed in vitamin A-deficient rats fed aflatoxin B1 (section
3.4.2.7) (Newberne & Rogers, 1973; Newberne & Suphakarn, 1977).
Tumours of the colon were also found in more than 20% (12/53) of
F344 rats (NIH) exposed, either from conception (7/34), or from 6-7
weeks of age (5/19), to a diet containing an aflatoxin B1 level of
2 mg/kg and an unspecified amount of vitamin A (Ward et al., 1975).
The tumours developed in both males and females at 42-64 weeks of
age and were primarily polyploid neoplasms in the ascending colon,
although 2 rats had tumours in the descending colon. No colon
tumours were found in 18 control rats.
Carcinomas of the glandular stomach were observed in 1/6 young
rats given a diet of groundnut meal containing an aflatoxin level of
3-4 mg/kg for 3 weeks and in 1/16 animals given the same diet at the
age of one year (Butler & Barnes, 1966). Two definite and one
probable adenocarcinomas of the stomach had previously been observed
in rats fed a diet prepared from the same batch of groundnut meal
(Butler & Barnes, 1963).
Other extrahepatic tumours have occasionally been reported after
oral aflatoxin exposure, e.g., tumours of the lacrimal glands
(Dickens et al., 1966; Goodall & Butler, 1969; Butler et al., 1969),
squamous cell carcinoma of the tongue (Ward et al., 1975) and
oesophagus (Butler et al., 1969). Tumours at various sites have been
induced in several species of test animals by intratracheal,
subcutaneous, or intraperitoneal administration of aflatoxins. The
experimental results obtained by intratracheal administration are of
particular interest in connexion with reported effects in man
associated with airborne aflatoxins (section 3.5.2). Squamous-cell
carcinoma of the trachea developed within 37-62 weeks in 3/6 rats
given a mixture of aflatoxins (containing B1, B2, G1, and
G2) at 300 µg per animal intra-tracheally twice weekly, for 30
weeks. Four of these rats also developed hepatomas within 49-62
weeks (Dickens et al., 1966).
Prenatal and early postnatal exposure. In a study of these
effects, 6 groups of 10 female, Wistar rats were each fed a diet
containing 25% or 50% groundnut meal contaminated with aflatoxin
B1 at 10 mg/kg and aflatoxin B2 at 0.2 mg/kg. Dams received this
diet from day 10 of pregnancy to parturition (exposure of offspring
in utero); from 1 day post partum to 10 days post partum (exposure
via milk); or from day 10 of pregnancy to 10 days post partum
(exposure in utero and via milk). Among 113 male and 95 female
offspring observed for up to 36 months, 1 male exposed in utero, 1
female exposed via milk, and 2 females exposed in utero
and via milk developed malignant liver tumours (Grice et al., 1973).
No liver tumours were seen in control offspring (50 male and 50
female rats).
3.4.2.4 Teratogenicity
The teratogenicity of aflatoxins has been reviewed by Ong
(1975). The effect of aflatoxin B1 on embryos in hamsters was
studied by Elis & Di Paolo (1967) who reported that a single
intraperitoneal injection of aflatoxin B1 at 4 mg/kg body weight,
given on day 8 of pregnancy, resulted in a high proportion of
malformed and dead or reabsorbed fetuses. Approximately 50% of the
fetuses in aflatoxin-treated mothers and over 85% in control mothers
were normal. A dose of 2 mg/kg did not have any effects. In studies
by Di Paolo et al. (1967), no teratogenic effects were observed when
12 pregnant C3H mice were given repeated dally intraperitoneal
injections of aflatoxin B1 at 4 mg/kg body weight. However, a high
proportion of dead or reabsorbed fetuses was observed in
aflatoxin-treated mice in comparison with controls.
3.4.2.5 Mutagenicity
Aflatoxin B1 causes chromosomal aberrations (chromosomal
fragments with occasional bridges, chromatid bridges, chromatid
breakage) and DNA breakage in plant and animal cells (Ong, 1975). It
has also been shown to cause gene mutations in bacterial test
systems (Ames test), when activated by microsomal preparations from
rat and human liver (Wong & Hsieh, 1976). However, no mutagenic
effects were observed in mice exposed intra-peritoneally to
aflatoxin B1 at 5 mg/kg body weight (Leonard et al., 1975).
3.4.2.6 Biochemical effects and mode of action
Individual aflatoxins (B1, B2, G1, G2 etc.) with
slightly different chemical structures affect experimental animals
(sections 3.4.2.1 and 3.4.2.3) and interact with in vitro test
systems to different degrees (Wogan et al., 1971; Patterson, 1976).
However, as aflatoxin B1 is the commonest and the most potent, it
has been studied extensively and the majority of reported
biochemical effects are specifically related to this toxin. As
discussed in section 3.3.3, the aflatoxin molecule appears to be
metabolically activated before exerting its acute and chronic
effects.
Interaction between these activated molecular species and the
liver cell apparently occurs at several loci. In the nucleus,
DNA-dependent RNA polymerase (EC 2.7.7.6)a is inhibited (Pong &
Wogan, 1970), the toxin binds covalently to DNA in vitro and
in vivo (Clifford & Rees, 1967; Lijinsky et al., 1970; Gamer,
1973, 1975; Swenson et al., 1974, 1977), DNA repair is stimulated
(Seegers & Pitout, 1973; Stich & Laishes, 1975), and the aflatoxin
is activated on the outer nuclear membrane to a form that inhibits
RNA synthesis (Neal & Godoy, 1976).
The permeability of mitochondria increases (Bababunmi & Bassir,
1972; Doherty & Campbell, 1973) and electron transport is
interrupted with a decline in respiration (Doherty & Campbell, 1972,
1973). Lysosomal membranes are also rendered permeable and unbound
acid hydrolases leak out (Tung et al., 1970; Pokrovsky et al., 1972;
Adekunle & Elegbe, 1974). Activation of lysosomal enzymes and their
effects on cellular structures may be a component of the toxic
mechanism of aflatoxins (Pokrovsky et al., 1977).
Aflatoxin is metabolized in the endoplasmic reticulum (section
3.3.3) and the unmetabolized toxin competes with steroid sex
hormones for polysome-binding sites (Williams & Rabin, 1971). The
reticulum degranulates (Theron, 1965; Theron et al., 1965; Butler,
1971, 1972) with the breakdown of polysome profiles (Villa-Trevino &
Leaver, 1968; Pong & Wogan, 1969; Godoy et al., 1976) and the
formation of helical polysome forms (Sarasin & Moule, 1976). RNA
polymerase is inhibited (Pong & Wogan, 1970) and the toxin binds
covalently to RNA (Swenson et al., 1977). Many metabolic functions
are inhibited, including protein synthesis, enzyme induction (Wogan
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
& Friedman, 1968; John & Miller, 1969; Kato et al., 1970) and the
synthesis of blood clotting factors II and VII (Bassir & Bababunmi,
1969). Glucose metabolism via the 6-phosphate pathway (Brown &
Abrams, 1965; Feuer et al., 1965;; Shankaran et al., 1970), and the
synthesis of fatty acids and phospholipids are also depressed
(Clifford & Rees, 1969; Kato et al., 1969; Black et al., 1970;
Donaldson et al., 1972; Lo & Black, 1972). Furthermore, feed-back
control of cholesterol synthesis is lost (Horton et al., 1972), a
change considered characteristic of the pre-cancerous state.
It has been shown that aflatoxins have immunosuppressive
properties, probably related to their inhibitory effect on protein
synthesis. Thus, at levels in poultry feed of 0.25-0.5 mg/kg,
aflatoxins have been found to reduce resistance to infection by
Pasteurella multocida, Salmonella spp., Marek's disease virus,
coccidia, and Candida albicans (Brown & Abrams, 1965; Smith et al.,
1969; Pier & Heddleston, 1970; Hamilton & Harris, 1971; Edds et al.,
1973).
In the liver cytoplasm, there is a transient stimulation
followed by a depression of glycogenolysis and the pentose shunt
pathway of glucose metabolism (Shankaran et al., 1970). Aflatoxins
also compete with further steroid binding sites in the cytoplasm,
notably NADP-linked 17-hydroxy steroid dehydrogenase (EC 1.1.1.148)
(Patterson & Roberts, 1971).
Thus, acute hepatocellular necrosis appears to result from the
interaction of aflatoxins at a number of intercellular sites whereas
the mutagenic (section 3.4.2.5) and carcinogenic (section 3.4.2.3)
properties of aflatoxins probably depend upon metabolic activation
to a DNA alkylating agent presumably the 2,3-epoxide (section
3.3.3).
3.4.2.7 Factors modifying the effects and dose-response
relationships of aflatoxins
Numerous reports have dealt with factors of various types that
modify the carcinogenic and other toxic effects of aflatoxins in
experimental animals. These include host factors, particularly the
sex-linked and endocrine characteristics, and interactions with
other environmental factors. The effects of nutrients deserve
particular attention in view of nutritional deficiencies occurring
in certain parts of the world, where aflatoxins exposure can be
considerable. The more recently discovered effect of exposure to
(artificial) sunlight might also be of interest in this respect.
The mechanisms by which hormones, nutrition, and other factors
influence aflatoxin carcinogenesis are not known but are thought to
include effects on DNA synthesis and cell division and
differentiation, and/or effects on aflatoxin metabolism, and
excretion. Animals with a severely restricted energy food intake do
not grow and are less susceptible to the action of many carcinogens
than normal animals. The retardation of growth induced by severe
protein deficiency and also by hypophysectomy may explain reduced
tumour incidence under these experimental conditions. Conversion of
aflatoxin B1 to a bacterial mutagen is different in microsomal
liver preparations from rats fed a marginal lipotrope diet compared
with those from normal rats. Excretion of mutagens in the urine is
also different in lipotrope-deficient rats (Suit et al., 1977).
Changes in aflatoxin B1 metabolism and reduced levels of
hepatic macro-molecule-bound aflatoxin B1 adducts were reported in
rats pretreated with phenobarbital (Garner, 1975; Swenson et al.,
1977), and also in hypophysectomized animals (Swenson et al., 1977).
Sex-linked differences and endocrine status. Several studies
indicate that in comparison with males, female rats are more
resistant to both acute toxic and carcinogenic effects of
aflatoxins. Thus, with a single administration of aflatoxin B1 by
gavage, the LD50 was estimated to be 7.2 mg/kg body weight
(fiducial limits 5.36-8.23) in male rats and 17.9 mg/kg body weight
(fiducial limits 14.4-22.5) in female rats (Butler, 1964). The
sex-dependent influence of vitamin A deficiency on the acute
toxicity of aflatoxins is discussed later in this section.
In another study (Newberne & Wogan, 1968a), Fischer rats of both
sexes were kept on diets containing aflatoxin B1 levels of 0.015,
0.3, or 1.0 mg/kg and killed successively for histological
examination. The early hepatic lesions, considered as precancerous,
appeared with almost the same rate of incidence and at approximately
the same time in both sexes; however, there was a considerably
longer period between the appearance of the pre-cancerous lesions
and progression to liver carcinomas in females than in males. At a
dietary level of aflatoxin B1 of 1 mg/kg, males developed
carcinomas after 35 weeks of exposure while tumours in females were
observed only after 64 weeks. A similar, if much less pronounced,
sex-linked difference was observed at the 2 lower aflatoxin levels.
Calculations of the approximate total intake of aflatoxin B1
before the appearance of tumours (based on average intake of food
containing a known quantity of aflatoxin) and the time over which
these total amounts were consumed are given in Table 10. In a study
by Ward et al. (1975), male rats (F344, NIH) kept on a diet
containing aflatoxin B1 at 2 mg/kg, died with malignant
haemorrhagic liver tumours significantly earlier than females.
Kidney tumours observed in male but not female rats exposed to
aflatoxins (Butler et al., 1969) have already been discussed in
section 3.4.2.3.
Newberne & Williams (1969) reported that fewer male rats
(Charles River CD) fed for more than a year on a diet containing
aflatoxin B1 at 0.2 mg/kg and diethylstilboestrol at 4 mg/kg
developed liver tumours (8/40) than those fed the same aflatoxin
diet without the estrogen (25/35).
In a study on the influence of hypophysectomy on aflatoxin
carcinogenesis, male albino (MRC) rats were fed a diet containing an
aflatoxin B1 level of 4 mg/kg. All of 14 control rats developed
liver tumours in 49 weeks whereas none of 14 hypophysectomized rats
developed liver tumours in the same period. However, tumours of
extrahepatic tissues (4/14 carcinomas of retro-orbital lacrimal
glands-see also section 3.4.2.3) were observed in the
hypophysectomized aflatoxin-treated rats (Goodall & Butler, 1969).
Table 10. Sex-linked difference in the carcinogenicity (liver cell
carcinoma) of dietary aflatoxin B1a
Dietary level Approximate total intake of Average time (days over
of aflatoxin B1 aflatoxin B1 before the which the total amount was
appearance of tumours consumed
males females males females
1 mg/kg 2.9 mg/rat 5.9 mg/rat 245 days 448 days
0.015 mg/kg 95 µg/rat 11 5 µg/rat 476 days 560 days
a Data from Newberne & Wogan (1968a).
Nutritional factors (food components). The influence of
nutritional factors on the effects and dose-response relationships
of aflatoxins has been reviewed by Wogan (1973, 1977), Newberne
(1974, 1976), Newberne & Rogers (1976), and Newberne & Gross (1977).
(a) Dietary protein and lipotropic agents. Rhesus monkeys were
given daily doses of 100 µg aflatoxin per animal by stomach tube.
Two animals, fed a severely protein-deficient ration (1% casein) for
8 weeks before and during aflatoxin administration, developed fatty
liver and biliary proliferation and fibrosis and died with
gastrointestinal haemorrhage within 30 days of aflatoxin treatment.
Two animals fed a control ration (16% casein) survived in apparent
good health up to the termination of the experiment (35 days of
aflatoxin treatment) (Madhavan et al., 1965a).
In a study on weanling male rats given 50 µg aflatoxin per
animal per day for 20 days, 2/6 animals, fed a diet containing 4%
casein, died on days 18 and 19 of the experiment. Extensive liver
damage was found in these rats and in others in the 4%a casein
group within 20 days of aflatoxin treatment. All 12 rats fed a diet
containing 20% casein survived similar aflatoxin dosing with only
mild changes in the liver (Madhavan & Gopalan, 1965). In another
study by Madhavan & Gopalan (1968) 2 groups of 12 male rats were fed
the 2 diets (5%a or 20% casein) for 2 years from weaning and were
given, from the beginning of the experiment, 232 daily doses of
5 µg aflatoxin per animal or 225 daily doses of 10 µg aflatoxin per
animal. All 12 animals fed the 20% casein diet survived the period
of aflatoxin dosing and 50% developed hepatomas; lung metastases
were observed in 2 of these rats. Five of the 12 animals fed on the
5% casein diet died during the period of aflatoxin dosing. No
hepatomas but one renal cell carcinoma were found in the remaining 7
rats. Both diets used in these experiments were supplemented only
with 0.01% choline.
In experiments by Newberne & Wogan (1968b), rats fed a diet
containing 9% protein developed a higher incidence of liver tumours
(11/15) in a shorter period of time (8 months) than rats fed a diet
containing 22% protein (incidence 7/14 after 10 months). Both groups
of rats were given a total dose of 375 µg of aflatoxin B1 per
animal by gastric intubation, over 3 weeks, at the beginning of the
experiment.
In studies examining lipotropic effects on aflatoxin activity, a
diet marginal in methionine and choline, deficient in folate, and
high in fat protected male rats against the acute toxicity of a
single dose of aflatoxin B1; however, susceptibility to the toxic
effects of repeated doses of aflatoxin B1 increased, and the
carcinogenicity of aflatoxin B1 was enhanced. The experimental
diet (Rogers & Newberne, 1971; Rogers, 1975) contained peanut meal
(12%, alcohol extracted), gelatine (6%), casein (3%, vitamin free)
and fibrin (1%) as sources of protein, with a supplement of
L-cystine (0.5%). This diet is marginally but not severely deficient
in threonine, tryptophan, and arginine as well as in methionine. The
diet contained choline chloride (0.2%), and was high in fat (beef
fat 30%; corn oil 2%). The control, nutritionally complete diet
contained casein (22%, vitamin free) as the protein source, 15% or
16% oil (corn or mixed vegetable oil) and 0.3% choline chloride.
Both diets were adequate in other essential nutrients.
a According to Madhavan & Gopalan (1968) the composition of the
diets used in both studies was the same. However, their 1965 paper
gives 4% whereas that of 1968 gives 5% as the level of casein in the
diet.
As shown in Table 11, the marginal lipotrope diet fed for 2
weeks protected rats against acute aflatoxin B11 toxicity. The
rats that died had haemorrhagic necrosis of the liver with various
degrees of bile duct proliferation and, occasionally, haemorrhagic
necrosis in the adrenals and kidneys. The surviving rats (killed 2
weeks after aflatoxin administration) had focal necrosis of
hepatocytes, bile duct proliferation, and an increase in the size of
periportal hepatocytes. Approximately 20% of the rats fed the
marginal lipotrope diet and given a single dose of aflatoxin B1
had focal areas of abnormal hepatocytes which showed an increased
uptake of 3H thymidine (section 3.4.2.3) (Rogers & Newberne,
1971).
Table 11. Effect of a marginal lipotrope diet on the acute toxicity of
aflatoxin B1 in rata
Aflatoxin B1 Route of Diet No. of Mortality
(mg/kg) administration rats at 2 weeks
(%)
Sprague-Dawley (males)
7 Intragastric control 5 60
marginal lipotrope 5 0
9 Intragastric control 5 80
marginal lipotrope 10 0
7 Intraperitoneal control 5 100
marginal lipotrope 5 0
Fischer (males)
7 intragastric control 10 100
marginal lipotrope 10 0
a From: Rogers & Newberne (1971).
Although resistant to the toxicity of a single dose of aflatoxin
B1, rats fed the marginal lipotrope diet for 2 weeks before and
also during repeated aflatoxin exposure (136 males) were highly
sensitive to repeated daily doses of aflatoxin B1 at 25 µg per
animal. One half of these rats died, most of them after having
received 8 or 9 doses of aflatoxin B1 (200 or 225 µg total).
Various degrees of necrosis were observed in the livers with
extensive proliferation of bile duct cells. The mortality in rats
fed the control diet (66 males) was only 4% during the
administration of the total dose of 350 lag of aflatoxin B1
(Rogers & Newberne, 1971).
Enhanced aflatoxin B1 carcinogenicity in rats fed marginal
lipotrope diets, observed repeatedly in several experiments reviewed
by Newberne & Gross (1977), is demonstrated in Fig. 4 (Rogers,
1975). Cumulative probability of death from a tumour was calculated
here by the method described by Saffiotti et al. (1972) i.e., from
the number of animals at risk and the number of deaths from tumours
each week. A total dose of aflatoxin B1 of 375 µg, divided in 25
intragastric doses of 15 µg/animal per day over 7 weeks, was given
to male Fischer rats. Hepatocarcinomas developed in 87% of 52
animals fed the marginal lipotrope diet and in only 11% of 27 rats
fed the nutritionally complete diet ( P < 0.001). Twenty-seven
percent of tumours in rats fed the marginal diet metastasized to
other abdominal organs or the lung; no metastases were detected in
rats fed the control diet (Rogers, 1975). The groundnut meal fed in
these experiments did not contain detectable aflatoxins.
3.3.4.2 Studies in man
In the Philippines, aflatoxin M1 has been found (not
measured)in the urine of human subjects known to have ingested
aflatoxin-contaminated peanut butter (Campbell et al., 1970).
Claims concerning an aflatoxin involvement in the etiology of
juvenile cirrhosis in India (section 3.6.3.2) based on a
blue-fluorescent B1 spot in the breast milk of mothers and the
urine of children with the disease (Robinson, 1967) are largely
discounted by the later studies of Yadgiri et al. (1970) who
produced spectrophotometric evidence that, although such a spot
could be identified in the urine of children with the overt disease,
this was not aflatoxin B1 For other reports see section 3.5.1.
3.4 Effects in Animals
The effects of aflatoxins in animals have., been reviewed by
Allcroft (1969), Newberne & Butler (1969) and Butler (1974).
3.4.1 Field observations
When foodstuffs are affected by microbial deterioration, man
normally eats the less affected parts, whereas domestic animals may
be exposed to more contaminated rations. This explains why the
discovery of several mycotoxins has been based on field observations
in domestic animals.
The first observation of a disease in animals subsequently
associated with aflatoxins was an acute outbreak of a lethal disease
in turkey poults in England in 1960 causing an estimated loss of at
least 100 000 birds. Extensive research eventually revealed that the
disease was caused by aflatoxins contained in a batch of Brazilian
groundnut meal. The concentration of aflatoxin B1 in the original
groundnut meal was later estimated to be about 10 mg/kg. The disease
was characterized by rapid deterioration in the condition of the
birds, subcutaneous haemorrhages, and death. At postmortem, the
livers of the birds were pale, fatty, and showed extensive necrosis
and biliary proliferation (Butler, 1974). A similar case of acute
disease was observed in day-old ducklings fed "toxic" groundnut meal
(Asplin & Carnaghan, 1961), where the liver changes described were
followed by cirrhosis. Outbreaks of liver disease in chickens have
also been associated with aflatoxin-contaminated feed (Asplin &
Carnaghan, 1961).
Loosmore & Harding (1961) noted outbreaks in pigs fed groundnut
meal in which the toxic factor was later identified as aflatoxins.
The lesions in the pigs included haemorrhages, and liver damage
characterized by dissecting fibrosis and biliary proliferation.
Calves fed rations containing 15% toxic groundnut meal also
developed liver lesions characterized by fibrosis and biliary
proliferation (Loosmore & Markson, 1961). Outbreaks associated with
"toxic" groundnut meal and characterized by similar liver lesions
have been reported in older cattle even though they are more
resistant (Clegg & Bryston, 1962); there was also a drop in milk
production.
A liver disease "hepatitis X" has been reported in dogs in
southeastern USA (Seibold & Bailey, 1952; Newberne et al., 1955).
Icterus and in some cases ascites were observed and the liver
lesions included fatty changes with centrilobular parenchymal
necrosis and biliary proliferation. Commercial dog food thought to
be the cause of toxicity was later found to contain aflatoxin B1
(up to 1.75 mg/kg) (Newberne et al., 1966a). Similar lesions have
been reproduced in dogs by peroral administration of aflatoxins, and
it has been suggested that the "hepatitis X" in dogs could be
causally associated with aflatoxins in the diet (Newberne et al.,
1966a). During an outbreak of toxic hepatitis affecting several
hundred people in north-west India and considered to be possibly
associated with the consumption of maize heavily contaminated by
aflatoxins (section 3.5.2 and 3.6.2.1), dogs fed food remnants from
households in affected villages manifested a disease characterized
by jaundice, ascites, and frequently death (Krisnamachari et al.,
1975a, b; Tandon et al., 1977). A nonportal type of micronodular
cirrhosis, with less conspicuous parenchymal and cholangiolar
changes was found on histological examination of the livers of two
dogs (Tandon et al., 1977).
3.4.2 Experimental studies
3.4.2.1 Acute and chronic effects: hepatotoxicity
Different species vary in their susceptibility to acute
poisoning by aflatoxins, with LD50 values ranging from 0.3 to
17.9 mg/kg body weight (Table 4). In all the animals studied, the
liver was the principal target organ (see for example Butler, 1974).
Table 4. Acute toxicity of aflatoxin B1a
Species LD50 Zone of
(mg/kg body weight) liver lesion
chick embryo 0.025d
rabbit 0.3 midzonal
duckling 0.335 periportal
cat 0.55 periportal
pig 0.62 centrilobular
dog 0.5-1.0 centrilobular
sheep 1.0 centrilobular
guineapig 1.4 centrilobular
baboonb 2.0 centrilobular
rat (male) 7.2 periportal
macaque femalec 7.8 centrilobular
mouse 9.0
hamster 10.2
rat (female) 17.9 periportal
a Adapted from: Newberne & Butler (1969) and Butler (1974).
b From: Peers & Linsell (1976).
c From: Shank et al. (1971 b).
d µg/embryo.
The lesions observed in field cases (section 3.4.1) in poultry,
pigs, cattle, and dogs have all been reproduced in the same animal
species by feeding experiments during periods of time ranging from a
few weeks to a few months, using diets containing aflatoxins, or
pure aflatoxins ranging from 0.3 to several mg/kg (Newberne &
Butler, 1969).
In the study by Carnaghan et al. (1966), chickens were fed a
diet containing aflatoxin B1 at a level of 1.5 mg/kg. Groups of 3
control and 3 test chicks were killed after 3´ days, 7 days, and
then at weekly intervals for 8 weeks. After 4 weeks, the liver
lesions included fatty change, biliary proliferation, and fibrosis.
In 20 pigs, aflatoxins (aflatoxins B1 and B2) at a feed
level as low as 300 µg/kg resulted in the development of
centrilobular necrosis and fibrosis of the liver as well as growth
depression, during a normal feeding period of 3-4 months (Krogh et
al., 1973a). In cattle, the liver lesions (centrilobular
degeneration, fibrosis, biliary proliferation) occurred in all 4
animals after 4 months on a feed containing an aflatoxin level of
2 mg/kg (Allcroft & Lewis, 1963). The hepatic lesions induced in the
duckling by the aflatoxins formed the basis of a bioassay originally
described by Sargeant et al. (1961). At sublethal doses of
aflatoxins, the bioassay depends upon an assessment of the degree of
biliary proliferation. Liver lesions similar to those observed in
farm animals have been experimentally induced by the administration
of aflatoxins in a number of laboratory animals, including the rat,
cat, guinea-pig, and rabbit (Newberne & Butler, 1969).
In a study of Madhavan et al. (1965b), 2 rhesus monkeys
(Macaca mulatta) were given daily oral doses of aflatoxins at
500 µg/animal for 18 days (corresponding approximately to 250 µg/kg
body weight per day) and then 1 mg/animal per day (corresponding
approximately to 500 µg/kg body weight per day) until death occurred
after 32 and 34 days. Three rhesus monkeys were given 1 mg each,
daily, until death occurred after 19, 20, and 27 days respectively.
The liver lesions included fatty infiltration, biliary
proliferation, and portal fibrosis. Death or similar lesions were
not observed in the 2 control monkeys.
Deo et al. (1970) studied the effect on male rhesus monkeys of
repeated administration by gastric tube of 3 different levels of
aflatoxins (B1 + G1). At the highest dose level (1 mg/kg body
weight daily for 3 weeks), 35/35 animals died within 22 days with
extensive haemorrhagic necrosis of the liver. A dose level of
0.25 mg/kg body weight, twice a week, for 5 months induced various
degrees of liver changes in 24/24 animals characterized by biliary
proliferation and focal appearance of the liver cells with multiple
nuclei and giant-sized liver cells with enlarged hyperchromatic
nuclei. At the lowest dose, 5 animals were given 62 µg/kg body
weight once a week for periods ranging from a few days to 2 years.
Liver changes were similar to the changes seen in the second group
but in a milder form.
Cynomolgus monkeys (Macaca fascicularis = M. irus) fed a
dietary level of aflatoxin B1 of 5 mg/kg rapidly developed liver
damage with biliary proliferation and all 6 animals died within 2
months. When fed aflatoxin B1 at a dietary level of 1.8 mg/kg, 5
animals died within 3 months showing liver damage characterized by
centrilobular necrosis, biliary proliferation, and fibrosis. Two
animals survived and were killed after 3 years; the liver of one
animal had the appearance of nodular cirrhosis. Two groups of 4
animals each were fed lower levels of aflatoxin B1 (0.07 and
0.36 mg/kg, respectively) for 3 years without showing any signs of
liver lesions (Cuthbertson et al., 1967).
The relationship between the chemical structure of different
aflatoxins and their biological activity (discussed also in section
3.4.2.3) was investigated in a small number of experiments; more
extensive studies were not possible because of the limited
quantifies available of some of the pure aflatoxins. Carnaghan et
al. (1963) compared 6-day mortality following single doses of
different aflatoxins, administered by intubation to one-day-old
Khaki Cambell ducklings, and concluded that both aflatoxins B2 and
G2 were less toxic than aflatoxins B1 and G1, the ratio of
LD50 values being 1:4.7 for B1:B2 and 1:4.4 for G1:G2.
Aflatoxins G1 and G2 were less toxic than the corresponding
aflatoxins B1 and B2, the ratio of the LD50 values being
1:2.15 for B1:G1 and 1:2.03 for B2:G2. The corresponding
LD50 values for aflatoxins B1, B2, G1, and G2 were 0.36,
1.70, 0.78, and 3.45 mg/kg, respectively. Comparable results were
obtained by Wogan et al. (1971) who recorded 14 day mortality after
intubation of male Pekin ducklings and reported LD50 values of
0.73, 1.76, 1.18 and 2.83 mg/kg for aflatoxins B1. B2, G1, and
G2, respectively. In the same paper, a study was reported on the
14-day mortality of male Fischer rats after a single
(intra-peritoneal) dose of aflatoxin. The LD50 value for aflatoxin
B1 was 1.16 mg/kg body weight (95% confidence interval 0.91 to
1.48 mg/kg) whereas the LD50 for aflatoxin G1 was between 1.5
and 2.0 mg/kg body weight. On the other hand, no deaths occurred in
20 rats given 12-200 mg of aflatoxin B2 per kg body weight, and
all 4 rats given 170-200 mg of aflatoxin G2 per kg body weight
survived. A similar difference in the toxicity of aflatoxins was
observed when male Fischer rats were given repeated doses of
aflatoxins by stomach tube over a 4-week period. The 4-week
mortality in rats given a total dose of 1 mg of aflatoxin B1 per
rat was 8/10 whereas all 10 rats given the same dose of aflatoxin
G1 survived and only 4/10 animals given double this dose of
aflatoxin G1 died. In another trial, all 11 rats survived
intragastric administration of 3.75 mg of aflatoxin B2 per rat
repeated every second day for 4 weeks to give a total dose of
52.5 mg per rat.
Holzapfel et al. (1966) and Purchase (1967) reported 7-day
mortality alter oral dosing of one-day-old Pekin ducklings with
aflatoxins B1, M1, and M2. Five groups of 2-3 ducklings (body
weight 40-50 g) were used for each of the aflatoxins tested, and the
following LD50s were calculated (with 95% confidence limits given
in brackets): aflatoxin B1, 12 (3.9-37.2) µg per duckling,
aflatoxin M1, 16 (5.4-51.5) µg per duckling, and aflatoxin M2,
61.4 (37-100) µg per duckling. Ducklings receiving aflatoxin M2
showed characteristic liver lesions indistinguishable from those
observed after a similar dose of aflatoxin B1. Higher doses of
aflatoxin M2 produced similar effects (Purchase, 1967). A study
comparing the acute toxicity of synthetic (racetalc) aflatoxins B1
and M1 and the natural optical isomer of aflatoxin B1 was
reported by Pong & Wogan (1971), suggesting that only one isomer of
each synthetized racemic mixture was biologically active.
Fourteen-day mortality rates observed after a single intraperitoneal
dose of 1.5 mg/kg body weight of synthetic aflatoxin B1 and
synthetic aflatoxin M1 were 1/1 and 1/2, respectively. However, no
deaths occurred in groups of rats (each consisting of 4 animals)
given these synthetic aflatoxins at doses of 1, 0.8, 0.6, or
0.4 mg/kg body weight. With the natural aflatoxin B1, the observed
mortalities at these dose levels were 4/4, 2/4, 2/4, and 0/4
respectively.
For information on the toxicity of certain other aflatoxin
metabolites or derivatives, see Wogan et al. (1971) and Patterson
(1976).
3.4.2.2 Hepatotoxicity connected with extrahepatic effects
Many other organs besides the liver are more or less severely
affected in acute experiments with high doses of aflatoxins (Butler,
1964): in male and female rats, a single dose of aflatoxin B1
proved lethal in half of the animals (7.2 mg/kg body weight in the
male and 17.9 mg/kg body weight in the female, by garage). Frequent
bilateral adrenal haemorrhages, petechial haemorrhages in many
organs, particularly in the congested lungs, and occasionally patchy
necroses in the myocardium and in other organs (kidney, spleen) were
observed during the first few days following administration. These
changes were not detected in male or female rats given aflatoxin B1
at 3.5 mg/kg body weight. With higher doses, the haemorrhages seen
in the lungs, kidneys, and adrenals were more extensive. Animals
dying within the first few days often had altered blood in the whole
of the small intestine and in the colon. Ascites and oedema of the
omentum were observed in some of the animals a week or more (but not
one month) after aflatoxin administration. After a month, with the
exception of the liver damage, all the other organs appeared normal
in surviving animals. Histologically, certain renal changes were
detected in the loops of Henle at this stage, consisting of a few
cells with large irregular hyperchromatic nuclei, very similar to
those seen in the liver (Butler, 1964).
Congested lungs with small petechial haemorrhages, haemorrhagic
necroses in the adrenals (localized in the inner zone of the
reticularis) and patchy necroses in the kidneys, pancreas, and
spleen were observed in guineapigs 2-3 days after a single
intraperitoneal injection of aflatoxin B1 at 1.4 mg/kg body weight
(lethal in half of the males and females). Even at this dose, the
small intestine was frequently filled with altered blood. At higher
doses, the haemorrhagic disease was more marked, with pleural,
pericardial, and peritoneal haemorrhages. The only change seen in
the heart of the guineapig, 2-3 days after aflatoxin administration,
was an occasional small area of fatty degeneration of the
myocardium. Many animals showed marked ascites and oedema of the
omentum and subcutaneous tissue during the first week after
injection (Butler, 1966).
Bourgeois et al. (1971) reported a special syndrome induced by
oral administration of aflatoxin B1 in the macaque (Macaca
fascicularis). In 2 groups of 4 young females, each receiving a
single oral dose of aflatoxin B1 at 13.5 or 40.5 mg/kg body
weight, all animals died within 149 h. Death occurred in 1 out of 4
other animals receiving a dose of 4.5 mg/kg body weight. Doses of
toxin of 1.5 mg/kg or 0.5 mg/kg (4 animals in each group) did not
result in death or unusual clinical signs. Cough, vomiting,
diarrhoea, and coma were characteristic clinical findings in animals
exposed to toxic doses. Analysis of blood serum revealed a
dose-dependent decrease in serum levels of phospholipids within 24 h
of administration of the aflatoxin. A dose-dependent decrease in
serum levels of glucose and an increase in nonesterified fatty acids
occurred within 72 h of aflatoxin administration. The liver lesions
included centrilobular necrosis, some biliary proliferation, and
massive fatty degeneration which was also observed in the heart and
kidneys. Cerebral oedema with neuronal degeneration was seen. Some
of these findings resemble those associated with Reye's syndrome in
children (see section 3.5.1.2).
3.4.2.3 Carcinogenesis
The carcinogenesis of aflatoxins has been reviewed by Wogan
(1973, 1977) and re-evaluated by IARC (1976).
Hepatic and renal tumours. Orally administered aflatoxins,
mainly B1, have been hepatocarcinogenic in all species of test
animals studied so far (including nonhuman primates), with the
exception of the mouse, in which carcinogenic effects have been
demonstrated only following intraperitoneal administration of
aflatoxin B1 to neonates (Tables 5 and 6). These studies were
concerned with repeated or long-term exposure to aflatoxins. In a
study by Carnaghan (1967), 2 groups consisting of 16 and 18 weanling
female Wistar rats, respectively, were given single oral doses of
crystalline aflatoxin B1 or a mixture of aflatoxins containing
about 40% aflatoxin B1 and 60% aflatoxin G1, at the rate of
0.5 mg/rat in 0.1 ml dimethylformamide. These doses corresponded to
averages of 7.65 mg aflatoxin B1/kg body weight and 2.7 mg
aflatoxin B1 plus 4.0 mg aflatoxin G1/kg body weight,
respectively. Within 21-32 months, 7 rats out of each group
developed hepatic tumours with metastases in half the cases. Hepatic
rumours were not observed in 19 control rats given the solvent only.
No hepatocellular carcinomas were found in 22 male Fischer rats
killed successively 16 weeks (3 rats), 25 weeks (5 rats), 38 weeks
(5 rats), 55 weeks (4 rats), and 69 weeks (5 rats) after a single
dose of aflatoxin B1 at 5.0 mg/kg body weight, administered by
garage (Wogan & Newberne, 1967).
A linear dose-response relationship was observed by Wogan et al.
(1974) for the development of liver-cell carcinomas in male Fischer
rats fed dietary concentrations of aflatoxin B1 ranging from
1-100 µg/kg (Table 7). At 1 µg/kg, a 10% tumour incidence was found,
compared with no rumours in the control group and at 100 µg/kg the
tumour incidence was 100%. A linear log (dose)-response relationship
has been demonstrated in trout fed dietary levels of aflatoxin B1
ranging from 0.5 to 20.0 µg/kg, for 20 months. Extrapolating this
relationship to lower exposure levels, an incidence of approximately
10% would be expected with a dietary concentration of 0.1 µg/kg.
Table 5. Hepatocarcinogenicity of aflatoxin B1 in rodentsa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
rat, Fischer 1.0 mg/kg diet 33 weeks 52 weeks 3/6 Svoboda et al. (1966)
rat, Fischer 1.0 mg/kg diet 41-64 weeks 41-64 weeks 18/21 Wogan & Newberne (1967)
rat, Porton 1.0 mg/kg diet 20 weeks 90 weeks 19/30 Butler (1969)
rat, Wistar 1.0 mg/kg diet 21 weeks 87 weeks 12/14 Epstein et al. (1969)
mouse, Swiss 150 mg/kg dietb 80 weeks 80 weeks 0/60 Wogan (1973)
mouse, C57Bl/6NB 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, C3HfB/HEN 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, hybrid F1, 4 days old 6.0 µg/g body weight 3 doses (i.p.) 80 weeks 16/16 Vesselinovitch et al. (1972)
a From: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
Table 6. Hepatocarcinogenicity of aflatoxin B1 in nonrodent speciesa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
monkey, rhesus (M) 1.655 g totalb 5.5 years 8.0 years 1/1 Gopalan et al. (1972)
monkey, rhesus (F) 1.855 g total 5.5 years 10.75 years 1/1 Tilak (1975)
monkey, rhesus (F) 0.504 g total 6.0 years 8.0 years 1/1 Adamson et al. (1973)
marmoset 3.0 mg total 50-55 weeks 50-55 weeks 1/3 Lin et al. (1974)
5.04-5.84 mg totalc 87-94 weeks 87-94 weeks 2/3 Lin et al, (1974)
tree shrew (M & F) 24-66 mg total 74-172 weeks 74-172 weeks 9/12 Reddy et al, (1976)
ferret 0.3-2.0 µg/kg 28-37 months 28-37 months 7/9 Butler (1969)
duck 30 µg/kg 14 months 14 months 8/11 Carnaghan (1965)
rainbow trout 4 µg/kg in diet 12 months 12 months 15% Sinnhuber et al, (1968b)
8 µg/kg in diet 12 months 12 months 40% Sinnhuber et al. (1968b)
rainbow trout embryos 0.5 mg/kg in water 1 h 296-321 days 38% Sinnhuber & Wales (1974)
salmon 12µg/kg in dietd 20 months 20 months 50% Wales & Sinnhuber (1972)
guppy 6 mg/kg in diet 11 months 11 months 7/11 Sato et al. (1973)
a Modified from: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
c These animals were infected simultaneously with hepatitis virus.
d This diet also contained 50 mg/kg cyclopropenoid fatty acids.
Table 7. Dose-response characteristics of aflatoxin B1 carcinogenesis
in male Fischer strain ratsa
Dietary Duration Liver Time of appearance
aflatoxin of feeding carcinoma of earliest tumour
level (week) incidenceb (week)
(µg/kg)
0 74--109 0/18c --
1 78--105 2/22 104
5 65--93 1/22 93
15 69--96 4/21 96
50 71--97 20/25d 82
100 54--88 28/28e 54
a From: Wogan et al. (1974).
b In animals at risk (surviving longer than 50 weeks).
c Animals surviving for maximum period.
d Two animals had pulmonary metastases.
e Four animals had pulmonary metastases.
In a recent study (FDA, 1978), an attempt was made to calculate
the lifetime liver cancer risk in rats, which could be connected
with aflatoxin feed levels lower than those directly tested in
animal experiments. Estimates of life-time liver cancer incidence
rates corresponding to aflatoxin feed levels of 0.1 and 0.3 µg/kg
were derived and compared for several selected rat studies using the
mathematical procedure developed by Mantel & Bryan (1961) and
modified by Mantel et al. (1975). A more detailed description of
this procedure can be found in other publications including WHO
(1978) and Hoel et al. (1975). Thus, the estimated life-time liver
cancer risk derived from the experimental results reported by Wogan
et al. (1974) (see Table 7) corresponded to life-time liver cancer
incidence rates of 70 (600) per 105 rats for an aflatoxin dietary
level of 0.1 µg/kg and 360 (2300) for a level of 0.3 µg/kg. (Numbers
in parentheses; are upper 99% confidence limits.) Estimates derived
from different rat studies varied considerably. The lifetime
incidence rates calculated for the combined studies were 240 (470)
and 1100 (1900) per 105 rats for aflatoxin feed levels of 0.1 and
0.3 µg/kg, respectively (FDA, 1978).
The studies in primates are included in Table 6. No attempts
have been made to establish dose-response relationships in primates,
but they are susceptible to aflatoxin hepatocarcinogenesis.
The carcinogenic effects of different purified aflatoxins have
been compared in a limited number of studies. The results of a study
by Butler et al. (1969) in which 8-9 week old, male (M) and female
(F) MRC rats were given aflatoxins B1, B2, or G1 in drinking
water for 10 or 20 weeks, are shown in Table 8. The earliest renal
neoplasm was seen 54 weeks after the discontinuation of aflatoxin
treatment. Renal tumours were detected only in males. The 2 rats
treated with aflatoxin B1 that developed renal tumours did not
have hepatic carcinomas, but 5 of the 11 rats receiving aflatoxin
G1 developed both renal and hepatic carcinomas.
Wogan et al. (1971) studied the relationship between the
chemical structures of aflatoxins and their hepatocarcinogenicity in
male Fischer rats, and concluded that aflatoxin B1 was apparently
more carcinogenic than aflatoxin G1 and that both were much more
active than aflatoxin B2. A total intraperitoneal dose of
aflatoxin B2 of 150 mg per rat given in 40 equal doses over 8
weeks induced hepatocellular carcinomas in 3/9 rats. A similar
regimen, containing a total dose of aflatoxin B1 of 1.3 mg,
induced liver tumours in 9/9 animals. In other experiments to
compare the carcinogenicity of aflatoxins G1 and B1 given by
stomach tube to rats, aflatoxin G1 in a total dose of 1.4 mg per
animal (divided into 14 equal doses over 2.5 weeks) induced
hepatocellular carcinoma in 3/5 rats within 68 weeks. Hepatocellular
carcinomas were observed in all 18 animals given a total dose of
2 mg of aflatoxin G1 (divided in 40 equal doses over 8 weeks) and
killed within 45-64 weeks. Six of these rats had pulmonary
metastases.
Hepatocellular carcinomas were also found in 7/7 rats given
aflatoxin B1 in a total dose of 0.5 mg per animal (divided in 20
equal doses over 4 weeks) and sacrificed within 74 weeks, in 18/18
rats given 1 mg per animal (divided into 40 equal doses over 8
weeks) and killed within 42-58 weeks, and in 17/17 given 1.5 mg per
animal (divided into 40 equal doses over 8 weeks) and killed within
42-46 weeks. With the 2 higher doses, 2 and 7 animals, respectively
had pulmonary metastases. Renal adenocarcinomas were found in 4/26
rats given aflatoxin G1.
Synthetic racemic aflatoxin M1 induced hepatocarcinomas at 100
weeks in 1/29 male Fischer rats given 1 mg of the compound
intragastrically in divided doses over a period of 8 weeks. The
incidence of hepatocarcinomas in 9 rats given the same dose of
natural aflatoxin B1 was 100%, 1 year after treatment (Wogan &
Paglialunga, 1974). Natural aflatoxin M1 was less effective than
natural aflatoxin B1 in inducing tumours in trout, particularly in
the males. Only 14% of male trout, fed natural aflatoxin M1 for a
year at a dietary level of 4 µg/kg, developed liver tumours compared
with 68% of those receiving a similar feed level of aflatoxin B1
Table 8. Carcinogenesis in rats due to ingestion of aflatoxin B1, G1 or B2 in drinking watera
Compound Concentration Daily Duration Total No. and sex No. of animals No. of animals
(µg/ml) dose (µg) weeks dose (mg) of animals with tumours with other
treated neoplasms
liver kidney
aflatoxin B1 1 20 20 2 15 M 8 2 4
15 F 11 0 1
aflatoxin B1 1 20 10 1 10 M 3 0 2
aflatoxin G1 3 60 20 6 11 M 9 6 5
15 F 12 0 3
aflatoxin G1 1 20 20 2 15 M 2 5 2
15 F 1 0 7
aflatoxin G1 1 20 10 1 10 M 1 0 1
aflatoxin B2 1 20 10 1 10 M 0 0 2
controls 0 0 0 0 15 M 0 0 6
15 F 0 0
a From: Butler et al. (1969).
(the experiment was terminated 8 months after the discontinuation of
aflatoxin feeding). In females, the difference was less pronounced,
liver tumours occurring in 48% of aflatoxin B1-treated trout and
78% of aflatoxin B1-treated trout (see Table 9) (Sinnhuber et al.,
1974). Haemorrhages within the cancerous liver resulting in death,
which were observed in most females receiving dietary levels of
aflatoxin M1 of 16-64 µg/kg, were not observed in similarly
treated males.
Table 9. Aflatoxin M1 liver carcinogenesis in rainbow trout
(Salmo gairdneri)a
Aflatoxin in the diet Liver tumour incidence
males females
M1 4 µg/kg 4/28 (14%) 13/27 (48%)
M1 16 µg/kg 22/27 (81%) 11/14 (79%)
M1 32 µg/kg 24/25 (96%) 13/14 (93%)
M1 64 µg/kg 21/24 (88%) 9/10 (90%)
B1 4 µg/kg 15/22 (68%) 18/23 (78%)
a From: Sinnhuber et al. (1974).
A probable effect of rat strain on aflatoxin carcinogenesis was
seen in the high incidence of renal epithelial neoplasms reported in
male Wistar strain rats fed diets containing aflatoxin B1, for 147
days, and then maintained on a basal diet until death (Epstein et
al., 1969). Renal tumours developed in 57% (8/14), 28% (5/18), and
23% (3/13) of male rats exposed to diets containing aflatoxin B1
levels of 1.0, 0.5, and 0.25 mg/kg feed, respectively. The
incidences of malignant hepatomas in corresponding groups were 86%,
72%, and 62% respectively. No renal tumours or malignant hepatomas
were detected in a control group (24 animals). Approximately
one-third of the aflatoxin-exposed rats with renal rumours did not
have hepatomas. The first malignant hepatoma and the first renal
tumour were detected 463 and 468 days after initiation of the
experiment, respectively; both these tumours occurred in the group
with the highest exposure. Approximately half of the renal tumours
were bilateral.
Early hepatic lesions possibly related to carcinogenesis.
Aflatoxin B1 given to rats in repeated doses of 15-25 µg/day, for
3.5 weeks, making a total dose of 375 µg, elicited increased DNA
synthesis and mitosis in clusters of liver cells that could be
distinguished from the surrounding cells by their histological
appearance and biochemical activity (Newberne & Wogan, 1968a; Rogers
& Newberne, 1969). Development of the abnormal foci was more
prominent in rats fed a high-fat, lipotrope-deficient diet, which
enhances aflatoxin carcinogenesis (section 3.4.2.7), than in rats
fed a nutritionally adequate diet. The abnormal foci were already
present in deficient rats at the end of carcinogen administration.
Similar foci are found in the livers of rats exposed to other
hepatic carcinogens and may be useful in studying pathogenesis or
metabolic aspects of liver cancer, since they are thought to be the
possible precursors of tumours. However, many of the foci disappear
with time after treatment, and progression is not inevitable.
Other tumours. Carcinoma of the colon have occasionally been
reported following aflatoxin exposure (Newberne & Butler, 1969).
Increased incidence of tumours in the distal half of the colon was
observed in vitamin A-deficient rats fed aflatoxin B1 (section
3.4.2.7) (Newberne & Rogers, 1973; Newberne & Suphakarn, 1977).
Tumours of the colon were also found in more than 20% (12/53) of
F344 rats (NIH) exposed, either from conception (7/34), or from 6-7
weeks of age (5/19), to a diet containing an aflatoxin B1 level of
2 mg/kg and an unspecified amount of vitamin A (Ward et al., 1975).
The tumours developed in both males and females at 42-64 weeks of
age and were primarily polyploid neoplasms in the ascending colon,
although 2 rats had tumours in the descending colon. No colon
tumours were found in 18 control rats.
Carcinomas of the glandular stomach were observed in 1/6 young
rats given a diet of groundnut meal containing an aflatoxin level of
3-4 mg/kg for 3 weeks and in 1/16 animals given the same diet at the
age of one year (Butler & Barnes, 1966). Two definite and one
probable adenocarcinomas of the stomach had previously been observed
in rats fed a diet prepared from the same batch of groundnut meal
(Butler & Barnes, 1963).
Other extrahepatic tumours have occasionally been reported after
oral aflatoxin exposure, e.g., tumours of the lacrimal glands
(Dickens et al., 1966; Goodall & Butler, 1969; Butler et al., 1969),
squamous cell carcinoma of the tongue (Ward et al., 1975) and
oesophagus (Butler et al., 1969). Tumours at various sites have been
induced in several species of test animals by intratracheal,
subcutaneous, or intraperitoneal administration of aflatoxins. The
experimental results obtained by intratracheal administration are of
particular interest in connexion with reported effects in man
associated with airborne aflatoxins (section 3.5.2). Squamous-cell
carcinoma of the trachea developed within 37-62 weeks in 3/6 rats
given a mixture of aflatoxins (containing B1, B2, G1, and
G2) at 300 µg per animal intra-tracheally twice weekly, for 30
weeks. Four of these rats also developed hepatomas within 49-62
weeks (Dickens et al., 1966).
Prenatal and early postnatal exposure. In a study of these
effects, 6 groups of 10 female, Wistar rats were each fed a diet
containing 25% or 50% groundnut meal contaminated with aflatoxin
B1 at 10 mg/kg and aflatoxin B2 at 0.2 mg/kg. Dams received this
diet from day 10 of pregnancy to parturition (exposure of offspring
in utero); from 1 day post partum to 10 days post partum (exposure
via milk); or from day 10 of pregnancy to 10 days post partum
(exposure in utero and via milk). Among 113 male and 95 female
offspring observed for up to 36 months, 1 male exposed in utero, 1
female exposed via milk, and 2 females exposed in utero
and via milk developed malignant liver tumours (Grice et al., 1973).
No liver tumours were seen in control offspring (50 male and 50
female rats).
3.4.2.4 Teratogenicity
The teratogenicity of aflatoxins has been reviewed by Ong
(1975). The effect of aflatoxin B1 on embryos in hamsters was
studied by Elis & Di Paolo (1967) who reported that a single
intraperitoneal injection of aflatoxin B1 at 4 mg/kg body weight,
given on day 8 of pregnancy, resulted in a high proportion of
malformed and dead or reabsorbed fetuses. Approximately 50% of the
fetuses in aflatoxin-treated mothers and over 85% in control mothers
were normal. A dose of 2 mg/kg did not have any effects. In studies
by Di Paolo et al. (1967), no teratogenic effects were observed when
12 pregnant C3H mice were given repeated dally intraperitoneal
injections of aflatoxin B1 at 4 mg/kg body weight. However, a high
proportion of dead or reabsorbed fetuses was observed in
aflatoxin-treated mice in comparison with controls.
3.4.2.5 Mutagenicity
Aflatoxin B1 causes chromosomal aberrations (chromosomal
fragments with occasional bridges, chromatid bridges, chromatid
breakage) and DNA breakage in plant and animal cells (Ong, 1975). It
has also been shown to cause gene mutations in bacterial test
systems (Ames test), when activated by microsomal preparations from
rat and human liver (Wong & Hsieh, 1976). However, no mutagenic
effects were observed in mice exposed intra-peritoneally to
aflatoxin B1 at 5 mg/kg body weight (Leonard et al., 1975).
3.4.2.6 Biochemical effects and mode of action
Individual aflatoxins (B1, B2, G1, G2 etc.) with
slightly different chemical structures affect experimental animals
(sections 3.4.2.1 and 3.4.2.3) and interact with in vitro test
systems to different degrees (Wogan et al., 1971; Patterson, 1976).
However, as aflatoxin B1 is the commonest and the most potent, it
has been studied extensively and the majority of reported
biochemical effects are specifically related to this toxin. As
discussed in section 3.3.3, the aflatoxin molecule appears to be
metabolically activated before exerting its acute and chronic
effects.
Interaction between these activated molecular species and the
liver cell apparently occurs at several loci. In the nucleus,
DNA-dependent RNA polymerase (EC 2.7.7.6)a is inhibited (Pong &
Wogan, 1970), the toxin binds covalently to DNA in vitro and
in vivo (Clifford & Rees, 1967; Lijinsky et al., 1970; Gamer,
1973, 1975; Swenson et al., 1974, 1977), DNA repair is stimulated
(Seegers & Pitout, 1973; Stich & Laishes, 1975), and the aflatoxin
is activated on the outer nuclear membrane to a form that inhibits
RNA synthesis (Neal & Godoy, 1976).
The permeability of mitochondria increases (Bababunmi & Bassir,
1972; Doherty & Campbell, 1973) and electron transport is
interrupted with a decline in respiration (Doherty & Campbell, 1972,
1973). Lysosomal membranes are also rendered permeable and unbound
acid hydrolases leak out (Tung et al., 1970; Pokrovsky et al., 1972;
Adekunle & Elegbe, 1974). Activation of lysosomal enzymes and their
effects on cellular structures may be a component of the toxic
mechanism of aflatoxins (Pokrovsky et al., 1977).
Aflatoxin is metabolized in the endoplasmic reticulum (section
3.3.3) and the unmetabolized toxin competes with steroid sex
hormones for polysome-binding sites (Williams & Rabin, 1971). The
reticulum degranulates (Theron, 1965; Theron et al., 1965; Butler,
1971, 1972) with the breakdown of polysome profiles (Villa-Trevino &
Leaver, 1968; Pong & Wogan, 1969; Godoy et al., 1976) and the
formation of helical polysome forms (Sarasin & Moule, 1976). RNA
polymerase is inhibited (Pong & Wogan, 1970) and the toxin binds
covalently to RNA (Swenson et al., 1977). Many metabolic functions
are inhibited, including protein synthesis, enzyme induction (Wogan
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
& Friedman, 1968; John & Miller, 1969; Kato et al., 1970) and the
synthesis of blood clotting factors II and VII (Bassir & Bababunmi,
1969). Glucose metabolism via the 6-phosphate pathway (Brown &
Abrams, 1965; Feuer et al., 1965;; Shankaran et al., 1970), and the
synthesis of fatty acids and phospholipids are also depressed
(Clifford & Rees, 1969; Kato et al., 1969; Black et al., 1970;
Donaldson et al., 1972; Lo & Black, 1972). Furthermore, feed-back
control of cholesterol synthesis is lost (Horton et al., 1972), a
change considered characteristic of the pre-cancerous state.
It has been shown that aflatoxins have immunosuppressive
properties, probably related to their inhibitory effect on protein
synthesis. Thus, at levels in poultry feed of 0.25-0.5 mg/kg,
aflatoxins have been found to reduce resistance to infection by
Pasteurella multocida, Salmonella spp., Marek's disease virus,
coccidia, and Candida albicans (Brown & Abrams, 1965; Smith et al.,
1969; Pier & Heddleston, 1970; Hamilton & Harris, 1971; Edds et al.,
1973).
In the liver cytoplasm, there is a transient stimulation
followed by a depression of glycogenolysis and the pentose shunt
pathway of glucose metabolism (Shankaran et al., 1970). Aflatoxins
also compete with further steroid binding sites in the cytoplasm,
notably NADP-linked 17-hydroxy steroid dehydrogenase (EC 1.1.1.148)
(Patterson & Roberts, 1971).
Thus, acute hepatocellular necrosis appears to result from the
interaction of aflatoxins at a number of intercellular sites whereas
the mutagenic (section 3.4.2.5) and carcinogenic (section 3.4.2.3)
properties of aflatoxins probably depend upon metabolic activation
to a DNA alkylating agent presumably the 2,3-epoxide (section
3.3.3).
3.4.2.7 Factors modifying the effects and dose-response
relationships of aflatoxins
Numerous reports have dealt with factors of various types that
modify the carcinogenic and other toxic effects of aflatoxins in
experimental animals. These include host factors, particularly the
sex-linked and endocrine characteristics, and interactions with
other environmental factors. The effects of nutrients deserve
particular attention in view of nutritional deficiencies occurring
in certain parts of the world, where aflatoxins exposure can be
considerable. The more recently discovered effect of exposure to
(artificial) sunlight might also be of interest in this respect.
The mechanisms by which hormones, nutrition, and other factors
influence aflatoxin carcinogenesis are not known but are thought to
include effects on DNA synthesis and cell division and
differentiation, and/or effects on aflatoxin metabolism, and
excretion. Animals with a severely restricted energy food intake do
not grow and are less susceptible to the action of many carcinogens
than normal animals. The retardation of growth induced by severe
protein deficiency and also by hypophysectomy may explain reduced
tumour incidence under these experimental conditions. Conversion of
aflatoxin B1 to a bacterial mutagen is different in microsomal
liver preparations from rats fed a marginal lipotrope diet compared
with those from normal rats. Excretion of mutagens in the urine is
also different in lipotrope-deficient rats (Suit et al., 1977).
Changes in aflatoxin B1 metabolism and reduced levels of
hepatic macro-molecule-bound aflatoxin B1 adducts were reported in
rats pretreated with phenobarbital (Garner, 1975; Swenson et al.,
1977), and also in hypophysectomized animals (Swenson et al., 1977).
Sex-linked differences and endocrine status. Several studies
indicate that in comparison with males, female rats are more
resistant to both acute toxic and carcinogenic effects of
aflatoxins. Thus, with a single administration of aflatoxin B1 by
gavage, the LD50 was estimated to be 7.2 mg/kg body weight
(fiducial limits 5.36-8.23) in male rats and 17.9 mg/kg body weight
(fiducial limits 14.4-22.5) in female rats (Butler, 1964). The
sex-dependent influence of vitamin A deficiency on the acute
toxicity of aflatoxins is discussed later in this section.
In another study (Newberne & Wogan, 1968a), Fischer rats of both
sexes were kept on diets containing aflatoxin B1 levels of 0.015,
0.3, or 1.0 mg/kg and killed successively for histological
examination. The early hepatic lesions, considered as precancerous,
appeared with almost the same rate of incidence and at approximately
the same time in both sexes; however, there was a considerably
longer period between the appearance of the pre-cancerous lesions
and progression to liver carcinomas in females than in males. At a
dietary level of aflatoxin B1 of 1 mg/kg, males developed
carcinomas after 35 weeks of exposure while tumours in females were
observed only after 64 weeks. A similar, if much less pronounced,
sex-linked difference was observed at the 2 lower aflatoxin levels.
Calculations of the approximate total intake of aflatoxin B1
before the appearance of tumours (based on average intake of food
containing a known quantity of aflatoxin) and the time over which
these total amounts were consumed are given in Table 10. In a study
by Ward et al. (1975), male rats (F344, NIH) kept on a diet
containing aflatoxin B1 at 2 mg/kg, died with malignant
haemorrhagic liver tumours significantly earlier than females.
Kidney tumours observed in male but not female rats exposed to
aflatoxins (Butler et al., 1969) have already been discussed in
section 3.4.2.3.
Newberne & Williams (1969) reported that fewer male rats
(Charles River CD) fed for more than a year on a diet containing
aflatoxin B1 at 0.2 mg/kg and diethylstilboestrol at 4 mg/kg
developed liver tumours (8/40) than those fed the same aflatoxin
diet without the estrogen (25/35).
In a study on the influence of hypophysectomy on aflatoxin
carcinogenesis, male albino (MRC) rats were fed a diet containing an
aflatoxin B1 level of 4 mg/kg. All of 14 control rats developed
liver tumours in 49 weeks whereas none of 14 hypophysectomized rats
developed liver tumours in the same period. However, tumours of
extrahepatic tissues (4/14 carcinomas of retro-orbital lacrimal
glands-see also section 3.4.2.3) were observed in the
hypophysectomized aflatoxin-treated rats (Goodall & Butler, 1969).
Table 10. Sex-linked difference in the carcinogenicity (liver cell
carcinoma) of dietary aflatoxin B1a
Dietary level Approximate total intake of Average time (days over
of aflatoxin B1 aflatoxin B1 before the which the total amount was
appearance of tumours consumed
males females males females
1 mg/kg 2.9 mg/rat 5.9 mg/rat 245 days 448 days
0.015 mg/kg 95 µg/rat 11 5 µg/rat 476 days 560 days
a Data from Newberne & Wogan (1968a).
Nutritional factors (food components). The influence of
nutritional factors on the effects and dose-response relationships
of aflatoxins has been reviewed by Wogan (1973, 1977), Newberne
(1974, 1976), Newberne & Rogers (1976), and Newberne & Gross (1977).
(a) Dietary protein and lipotropic agents. Rhesus monkeys were
given daily doses of 100 µg aflatoxin per animal by stomach tube.
Two animals, fed a severely protein-deficient ration (1% casein) for
8 weeks before and during aflatoxin administration, developed fatty
liver and biliary proliferation and fibrosis and died with
gastrointestinal haemorrhage within 30 days of aflatoxin treatment.
Two animals fed a control ration (16% casein) survived in apparent
good health up to the termination of the experiment (35 days of
aflatoxin treatment) (Madhavan et al., 1965a).
In a study on weanling male rats given 50 µg aflatoxin per
animal per day for 20 days, 2/6 animals, fed a diet containing 4%
casein, died on days 18 and 19 of the experiment. Extensive liver
damage was found in these rats and in others in the 4%a casein
group within 20 days of aflatoxin treatment. All 12 rats fed a diet
containing 20% casein survived similar aflatoxin dosing with only
mild changes in the liver (Madhavan & Gopalan, 1965). In another
study by Madhavan & Gopalan (1968) 2 groups of 12 male rats were fed
the 2 diets (5%a or 20% casein) for 2 years from weaning and were
given, from the beginning of the experiment, 232 daily doses of
5 µg aflatoxin per animal or 225 daily doses of 10 µg aflatoxin per
animal. All 12 animals fed the 20% casein diet survived the period
of aflatoxin dosing and 50% developed hepatomas; lung metastases
were observed in 2 of these rats. Five of the 12 animals fed on the
5% casein diet died during the period of aflatoxin dosing. No
hepatomas but one renal cell carcinoma were found in the remaining 7
rats. Both diets used in these experiments were supplemented only
with 0.01% choline.
In experiments by Newberne & Wogan (1968b), rats fed a diet
containing 9% protein developed a higher incidence of liver tumours
(11/15) in a shorter period of time (8 months) than rats fed a diet
containing 22% protein (incidence 7/14 after 10 months). Both groups
of rats were given a total dose of 375 µg of aflatoxin B1 per
animal by gastric intubation, over 3 weeks, at the beginning of the
experiment.
In studies examining lipotropic effects on aflatoxin activity, a
diet marginal in methionine and choline, deficient in folate, and
high in fat protected male rats against the acute toxicity of a
single dose of aflatoxin B1; however, susceptibility to the toxic
effects of repeated doses of aflatoxin B1 increased, and the
carcinogenicity of aflatoxin B1 was enhanced. The experimental
diet (Rogers & Newberne, 1971; Rogers, 1975) contained peanut meal
(12%, alcohol extracted), gelatine (6%), casein (3%, vitamin free)
and fibrin (1%) as sources of protein, with a supplement of
L-cystine (0.5%). This diet is marginally but not severely deficient
in threonine, tryptophan, and arginine as well as in methionine. The
diet contained choline chloride (0.2%), and was high in fat (beef
fat 30%; corn oil 2%). The control, nutritionally complete diet
contained casein (22%, vitamin free) as the protein source, 15% or
16% oil (corn or mixed vegetable oil) and 0.3% choline chloride.
Both diets were adequate in other essential nutrients.
a According to Madhavan & Gopalan (1968) the composition of the
diets used in both studies was the same. However, their 1965 paper
gives 4% whereas that of 1968 gives 5% as the level of casein in the
diet.
As shown in Table 11, the marginal lipotrope diet fed for 2
weeks protected rats against acute aflatoxin B11 toxicity. The
rats that died had haemorrhagic necrosis of the liver with various
degrees of bile duct proliferation and, occasionally, haemorrhagic
necrosis in the adrenals and kidneys. The surviving rats (killed 2
weeks after aflatoxin administration) had focal necrosis of
hepatocytes, bile duct proliferation, and an increase in the size of
periportal hepatocytes. Approximately 20% of the rats fed the
marginal lipotrope diet and given a single dose of aflatoxin B1
had focal areas of abnormal hepatocytes which showed an increased
uptake of 3H thymidine (section 3.4.2.3) (Rogers & Newberne,
1971).
Table 11. Effect of a marginal lipotrope diet on the acute toxicity of
aflatoxin B1 in rata
Aflatoxin B1 Route of Diet No. of Mortality
(mg/kg) administration rats at 2 weeks
(%)
Sprague-Dawley (males)
7 Intragastric control 5 60
marginal lipotrope 5 0
9 Intragastric control 5 80
marginal lipotrope 10 0
7 Intraperitoneal control 5 100
marginal lipotrope 5 0
Fischer (males)
7 intragastric control 10 100
marginal lipotrope 10 0
a From: Rogers & Newberne (1971).
Although resistant to the toxicity of a single dose of aflatoxin
B1, rats fed the marginal lipotrope diet for 2 weeks before and
also during repeated aflatoxin exposure (136 males) were highly
sensitive to repeated daily doses of aflatoxin B1 at 25 µg per
animal. One half of these rats died, most of them after having
received 8 or 9 doses of aflatoxin B1 (200 or 225 µg total).
Various degrees of necrosis were observed in the livers with
extensive proliferation of bile duct cells. The mortality in rats
fed the control diet (66 males) was only 4% during the
administration of the total dose of 350 lag of aflatoxin B1
(Rogers & Newberne, 1971).
Enhanced aflatoxin B1 carcinogenicity in rats fed marginal
lipotrope diets, observed repeatedly in several experiments reviewed
by Newberne & Gross (1977), is demonstrated in Fig. 4 (Rogers,
1975). Cumulative probability of death from a tumour was calculated
here by the method described by Saffiotti et al. (1972) i.e., from
the number of animals at risk and the number of deaths from tumours
each week. A total dose of aflatoxin B1 of 375 µg, divided in 25
intragastric doses of 15 µg/animal per day over 7 weeks, was given
to male Fischer rats. Hepatocarcinomas developed in 87% of 52
animals fed the marginal lipotrope diet and in only 11% of 27 rats
fed the nutritionally complete diet ( P < 0.001). Twenty-seven
percent of tumours in rats fed the marginal diet metastasized to
other abdominal organs or the lung; no metastases were detected in
rats fed the control diet (Rogers, 1975). The groundnut meal fed in
these experiments did not contain detectable aflatoxins.
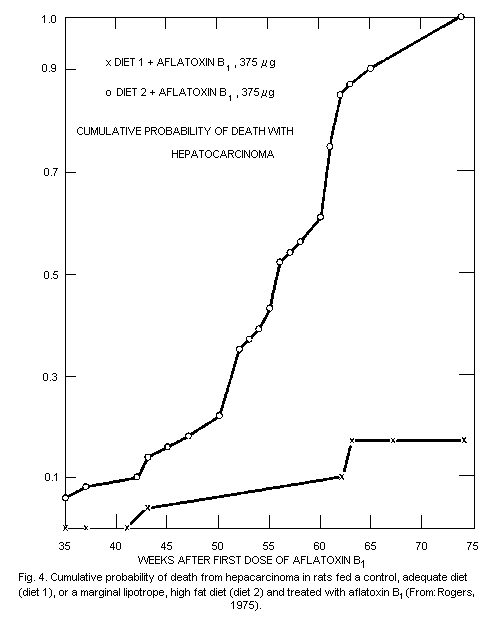 Further studies were carried out to determine how far the high
fat content of the marginal lipotrope diet contributed to the
enhancement of carcinogenesis. Male Fischer rats were fed the
marginal lipotrope diet, the nutritionally complete control diet, or
the control diet with substitution of the fat from the marginal
lipotrope diet (30% beef fat, 2% corn oil) for the fat in the
control diet (15% mixed vegetable oils). Each rat was given a total
of 375 µg of aflatoxin B1 intragastrically, in divided doses over
7 weeks and kept until moribund or dead, or until 90 weeks after
treatment, and then necropsied. Hepatocarcinoma incidence was based
on the number of rats that survived until the first death with
hepatocarcinoma, i.e., 27-34 rats per group. Incidences were found
of 39% in rats fed the marginal lipotrope diet, 15% in control rats,
and zero in rats fed the control diet with beef fat (30%) and corn
oil (2%) substituted for vegetable oil (15%) (Rogers et al.,
unpublished data). Thus the high fat content of the deficient diet
inhibited, rather than contributed to the enhancement of aflatoxin
carcinogenesis.
An enhancing effect on aflatoxin carcinogenicity was observed in
the experiments in which male rats (Charles River CD Sprague-Dawley)
were fed a low lipotrope diet containing 20% isolated soybean
protein and supplemented with 0.1% DL-methionine and 0.1% choline
chloride. Aflatoxin B1 was given intragastrically during the early
weeks of the experiment in a total dose of 240 µg/animal (divided in
24 daily doses of 10 µg, 5 days a week). Liver cell carcinomas were
observed 5/17 animals fed this diet. No tumours were found in rats
given the same dose of aflatoxin B1 and fed the same basal diet
supplemented with 0.6% DL-methionine, 0.6% choline chloride, and
vitamin B12 (50 µg/kg diet) (Newberne et al., 1968).
It should be noted that a more severe lipotrope deficiency may
decrease rather than increase the incidence of liver carcinoma in
aflatoxin-treated rats. A decrease in liver carcinoma was observed
in aflatoxin-treated, male, Sprague-Dawley rats with severe
lipotrope deficiency particularly if penicillin, at a level of 0.1%
were added to the diet. The expected penicillin-induced inhibition
of cirrhosis development was not observed and the interactions
between penicillin and aflatoxins have not been elucidated. Both
diets used in these experiments, the control, adequate, and the
highly lipo-trope-deficient contained alcohol-extracted groundnut
meal (25%) and casein (6%) as protein source. No cystine or
methionine was added, and choline and vitamin B12 were added to
the control diet at levels of 0.3% and 50 µg/kg, respectively. When
rats were killed 12 months after receiving a total dose of 375 tag
aflatoxin B1 per animal (divided into 15 daily intra-gastric doses
of 25 µg per animal), hepatomas were found in 64% (9/14) control
animals and in 41% (7/17) lipotrope-deficient animals. In rats fed
diets containing penicillin, hepatomas were found in 70% (14/20) of
the controls and in only 17% (3/18) of the lipotrope-deficient
animals (Newberne & Rogers, 1971).
The different effects of marginal and severe lipotrope
deficiencies on aflatoxin carcinogenesis are of interest in
connexion with apparent discrepancies between different studies on
aflatoxin carcinogenesis in rats fed protein-deficient diets
containing different levels of lipotropic agents discussed earlier.
Vitamin B12 is a weakly lipotropic factor that has recently
been found to increase tumour incidence in aflatoxin-treated rats.
In a study by Temcharoen et at. (1978), male Fischer rats were fed
diets containing 20% or 5% casein with or without vitamin B12
(50 µg/kg diet) for 33 weeks. The composition of the diets, with the
exception of the contents of casein (and dextrin) and vitamin B12,
was similar to that used in previous studies by Wogan & Newberne
(1967) and Newberne & Wogan (1968a). Choline chloride was added at
the level 0.036%. The casein content was similar to that in diets
used in the study by Madhavan & Gopalan (1968) mentioned earlier. A
mixture of crystalline aflatoxins (containing aflatoxins B1 and
G1 approximately in the proportion of 1: 1 and about 5% of
aflatoxins B2 and G2) was added to the diets at the level of
1 mg/kg. As shown in Table 12, vitamin B12 supplementation
increased liver rumour incidence in rats fed a diet containing
aflatoxin and 20% casein. Severe protein deficiency affected the
growth of the animals, the body weight of rats fed 5% casein being
reduced to about one third of the controls at the termination of the
experiment. Liver cirrhosis was observed only in the
aflatoxin-treated, protein-deficient rats. As suggested by
Temcharoen et al. (1978), a high incidence of hyperplastic nodules
and cholangiofibrosis in the protein-depleted, aflatoxin-treated
animals may indicate that the carcinogenic process was retarded but
not entirely absent.
(b) Vitamin A. In a study by Reddy et al. (1973), male and
female albino rats fed a vitamin A-deficient diet for 9 weeks after
weaning or fed the same diet with a daily oral supplement of 30 µg
(100 IU) of vitamin A/ rat were given a preparation of crystalline
aflatoxins containing aflatoxins B1 (44%), G1 (44%), and B2
and G2 (2%)in a single intraperitoneal dose of 3.5 mg/kg body
weight. High mortality was observed in vitamin A-deficient males
(Table 13). The vitamin A-deficient females and all the vitamin
A-supplemented animals did not show any adverse reactions 40 h after
aflatoxin injection. Histologically, severe liver damage was
observed in vitamin A-deficient, aflatoxin-treated male rats, in
contrast to minimal liver damage in female rats and male rats given
vitamin A.
Table 12. The effects of dietary protein and vitamin B12 on aflatoxin-induced liver changesa
Experimental diet No. of animals Average bodyb No. of animals with
(for details see text) at the beginning/ weight (g) at
termination of the termination cholangiofibrosis cirrhosis hyperplastic hepatoma
the experiment of the nodules
experiment
1. 5% casein 12/6 85 0 0 0 0
2. 5%casein + B12 12/12 91 0 0 0 0
3. 5% casein + aflatoxins 25/23 125 9 21 17 3
4. 5% casein + B12 + 25/24 129 8 12 15 1
aflatoxins
5. 20% casein 12/9 328 0 0 0 0
6. 20% casein + B12 12/10 369 0 0 0 0
7. 20% casein + aflatoxins 25/24 409 0 0 0 1
8. 20% casein + B12 + 25/25 375 0 0 4 6
aflatoxins
a Modified from: Temcharoen et al. (1978).
b Calculated from data of the authors on average liver weight and liver weight/100 g body weight.
In a study on rats fed diets containing aflatoxin B1 in the
range of 15-100 µg/kg and vitamin A in deficient, adequate, or
excessive levels over a 2-year period, the vitamin A-deficient
animals developed a similar incidence of liver tumours to the other
2 groups but had an increased incidence of colon carcinomas
(Newberne & Rogers, 1973). Thus, in a group of 50 male rats (Charles
River CD Sprague-Dawley) exposed to a dietary level of aflatoxin
B1 of 100 µg/kg and 5 µg vitamin A (retinyl palmitate) per animal
per day, colon tumours and liver tumours were observed in 6 and 11
rats, respectively, whereas with daily intakes of 50 or 500 µg
retinyl palmitate per rat, no colon cancers were observed and the
incidence of liver tumours was 24/50 or 19/50, respectively. The
results of a further study (Newberne & Suphakarn, 1977) are shown in
Table 14. Charles River CD Sprague-Dawley male (M) and female (F)
rats were fed a diet containing an aflatoxin B1 level of 1 mg/kg
(AFB1) and 3 different dietary levels of vitamin A (retinyl
acetate). Again, there was an increased incidence of colon
carcinomas in vitamin A-deficient rats. Excessive vitamin A did not
give protection against aflatoxin carcinogenesis in either the liver
or the colon.
Table 13. The effect of vitamin A status on the acute toxicity of aflatoxins
in male and female ratsa
Sex Vitamin A No. of Mortality Vitamin A
daily supplement animals 40 h after liver content
(IU/rat per day) aflatoxin (IU/whole liver)
injection means ± SEMc
Male 0 6 100% 36.5 ± 6.16b
100d 6 0% 2128.1 ± 153.56
Female 0 6 0% 18.6 ± 3.42
100d 6 0% 2299.1 ± 111.49
a From: Reddy et al. (1973).
b Vitamin A values for 5 animals only.
c SEM = standard error of the mean.
d 30µg.
Table 14. Vitamin A status, aflatoxin B1, and liver and colon
tumors in rats
Dietary AFB1 No. Sex Tumour incidence (%)
retinyl rats at
acetate risk liver colon both
(mg/kg)
control
3.0 0 24 M 0.0 0.0 0.0
3.0 0 26 F 0.0 0.0 0.0
3.0 + 24 M 87.5 4.1 4.1
3.0 + 24 F 79.1 8.3 8.3
low
0.3 0 10 M 0.0 0.0 0.0
0.3 0 12 F 0.0 0.0 0.0
0.3 + 66 M 89.4 28.8 25.7
0.3 + 42 F 76.2 28.6 11.9
high
30.0 0 23 M 0.0 0.0 0.0
30.0 0 20 F 0.0 0.0 0.0
30.0 + 26 M 92.3 7.7 7.7
30.0 + 31 F 83.9 9.7 6.4
From: Newberne & Suphakarn (1977).
(c) Selenium. A single oral dose of aflatoxin B1 given to
rats at 7 mg/kg bodyweight was less toxic in animals fed a high
selenium (selenite) diet containing selenium at 1 mg/kg (2-week
mortality 7/28) than in animals fed diets adequate or marginal in
selenium, containing selenium (selenite) at 0.1 or 0.03 mg/kg feed,
respectively (2-week mortalities 20/20 and 28/29). However, a
further increase in selenium intake (5 mg/kg feed) reaching toxic
levels predisposed the liver to aflatoxin injury and together with
aflatoxin exposure resulted in kidney lesions (tubular necrosis at
the cortico-medullary junction) (Newberne & Conner, 1974). The
incidence of liver tumours in Sprague-Dawley rats given a total of
500 µg of aflatoxin B1 intragastrically, over a 4-week period, was
not influenced by dietary selenium (as selenite) contents ranging
from 0.03 to 5.0 mg/kg (Grant et al., 1977).
(d) Cyclopropenoid fatty acids (CPFA). Cyclopropenoid fatty
acids (CPFA) which occur for example in cottonseed oil, enhanced
tumour induction in trout by both aflatoxin B1 and aflatoxin M1
(Sinnhuber et al., 1968, 1974). Young trout were fed a purified diet
which contained an aflatoxin B1 concentration of 4 µg/kg with or
without the addition of CPFA at 220 mg/kg diet. Hepatomas were found
in 27/30 fish fed CPFA and necropsied after 6 months; at 9 months,
20/20 bore hepatomas. Corresponding incidences of hepatomas in fish
that did not receive CPFA were 0/30 and 4/20, respectively
(Sinnhuber et al., 1968). In later experiments, fish were fed
aflatoxin M1 at the rate of 4 µg/kg diet, with or without the
addition of CPFA at 100 mg/kg. The incidences of hepatomas in
CPFA-fed fish were 6/40 at 4 months and 42/63 at 12 months.
Corresponding incidences in fish not fed CPFA were 2/40 and 6/40
respectively (Sinnhuber et al., 1974).
On the other hand, the enhancing effect of CPFA on aflatoxin
hepato-carcinogenesis was not clearly evident in several studies on
rats (Friedman & Molar, 1968; Lee et al., 1969a; Nixon et al.,
1974). No significant increase in liver tumours was observed in
Wistar male (M) and female (F) rats when sources of CPFA such as;
food grade cottonseed oil (CSO) or Sterculia foetida oil (SFO)
were added at levels of 10% and 0.04%, respectively, to diets
containing aflatoxin B1 at concentrations of 20 or 100 µg/kg.
Feeding these diets for different periods (generally exceeding 500
days) at the aflatoxin level of 20 µg/kg resulted in hepatomas in
4/36 rats M: 2/17; F: 2/19) with CSO exposure, in 1/37 rats
(F: 1/19) with SFO exposure, and in 0/38 rats without CSO or SFO in
the diet. With an aflatoxin B1 exposure level of 100 µg/kg diet,
the incidences of hepatomas in the CSO group, SFO group, and the
group without CSO or SFO were 15/36 (M: 12/17; F: 3/19), 17/37
(M: 10/17; F: 7/18), and 15/35 (M: 7/17; F: 8/18), respectively. In
Fischer rats, CSO was tested only in combination with the lower
concentration of aflatoxin; with an aflatoxin B1 level of
20 µg/kg diet, the hepatoma incidence was 6/31 (M: 4/15; F: 2/16)
with CSO, and 6/28 (M: 5/13; F: 1/15) without CSO exposure (Nixon et
al., 1974).
Other chemicals. Sodium phenobarbital given to rats in the
drinking water (1 g/litre) for 9 weeks, together with a diet
contaminated with aflatoxin B1 (at the level of approximately
5 mg/kg) resulted in a lower incidence (11/20) and delayed
appearance of liver tumours within the following 2 years compared
with the incidence in rats fed aflatoxin alone (17/20) (McLean &
Marshall, 1971). The effects of phenobarbital were confirmed in
experiments by Swenson et al. (1977) in which 2 groups of 18 male
Fischer rats were each given a diet containing aflatoxin B1 at a
level of 0.3 mg/kg and drinking water with or without sodium
phenobarbital (1 g/litre) for a period of 15 months. Examination by
laparotomy at the end of aflatoxin exposure revealed liver tumours
in 11 aflatoxin-treated controls (61%) and in only 2 (15%) rats
exposed to aflatoxin with phenobarbital. When the experiment was
terminated 5 months later, hepatocellular carcinomas were detected
in 18 (100%) aflatoxin-exposed rats and in 12 (67%) rats given
aflatoxin and phenobarbital. On the other hand, no effect on
aflatoxin hepatocarcinogenesis was observed in rats fed a similar
aflatoxin diet with the addition of benz (a)anthracene (70 mg/kg)
or ascorbic acid (25 g/kg) (Swenson et al., 1977).
Viral infection. Liver tumours were observed in 2/7 marmosets
fed aflatoxin B1 at a concentration of 2 mg/kg feed and injected
with hepatitis-type candidate virus (G. Barker strain) during
aflatoxin exposure. The animals survived 3-94 weeks of treatment.
Tumours were found in 3/9 marmosets given the aflatoxin diet only
(Lin et al., 1974). Exposure to both agents produced more severe
effects on the liver (cirrhosis) than exposure to aflatoxin B1
alone.
Exposure to artificial sunlight. The effects of exposure to
artificial sunlight on acute aflatoxin toxicity (Newberne et al.,
1974) and carcinogenicity (Joseph-Bravo et al., 1976) were studied
recently, using a long-arc xenon source and filter combination,
which had a spectral distribution in the ultraviolet and visible
ranges closely approximating the 6000 K colour temperature of
natural light. The rats, located 1.5 metres from the source, were
kept at an illumination level of about 29 600 1x corresponding to an
irradiance level of approximately 160 W/m2 over a 2-h period. In
groups consisting of 40 Sprague-Dawley male rats each, 18 control
rats died within 2 weeks of a single intragastric dose of aflatoxin
B1 at 7 mg/kg body weight whereas 23 of the rats exposed to
artificial sunlight for 2 h after aflatoxin administration died.
When an excessive dose of riboflavin was given intra-gastrically 30
min before aflatoxin, the corresponding 2-week mortalities in
animals unexposed and exposed to artificial sunlight were 20/40 and
30/40, respectively (Newberne et al., 1974). In the second study, a
total dose of 375 µg of aflatoxin B1 was given to male
Sprague-Dawley Charles River CD rats, in the form of 15 doses of
25 µg per rat, administered intra-gastrically over a 3-week period.
Thirty min after each aflatoxin administration, half of the animals
were exposed for 2 h to artificial sunlight as described earlier.
When the animals were killed 53 weeks after the last aflatoxin dose,
benign or malignant liver tumours were found in all 11
non-irradiated animals and in only 5/12 of the irradiated group
(Joseph-Bravo et al., 1976).
3.5 Effects in Man---Epidemiological and Clinical Studies
3.5.1 General population studies
3.5.1.1 Liver carcinogenesis
The data on aflatoxins and human cancer available before October
1975 were reviewed by IARC (1976) and several other reviews have
been published more recently (Linsell & Peers, 1977; Shank, 1977;
Van Rensburg, 1977). A positive association between aflatoxin
ingestion and liver cancer in man has been found in population
studies in which estimates of aflatoxin intake and the incidence of
primary liver cancer were made concurrently.
In a study in different parts of Uganda, it was found that
increased frequencies of detectable aflatoxin contamination of food
samples (range: 10.8%-43%) were associated with increased incidence
of primary liver cancer (range: 1.4-15.0 cases per 100 000 total
population per year) (Alpert et al., 1971). Four hundred and eighty
samples of foods were analysed from 8 areas in Uganda; the total
intake was not calculated. Within Swaziland, Keen & Martin
(1971 a, b) showed regional differences in liver cancer frequencies
consistent with regional differences in the frequency of aflatoxin
contamination of groundnuts. From the results of a questionnaire
study, they also suggested that tribal differences in the
preparation of groundnuts for food, and in eating habits, resulting
in higher aflatoxin exposure, could explain the apparently higher
liver cancer rate in the Shangaans living in Swaziland compared with
the Swazis.a
In the studies conducted by Shank et al. (1972a,b) in Thailand,
Peers & Linsell (1973) in Kenya, Van Rensburg et al. (1974) in
Mozambique, and Peers et al. (1976) in Swaziland, actual
concentrations of aflatoxin in meals about to be eaten (food on the
plate) were related to the incidence of primary liver cancer in the
areas where the meal samples were collected. These studies are
summarized in Table 15. A linear regression between the incidence of
liver cancer and the logarithm to the base 10 of the estimated
dietary intake of aflatoxin was found within the range of the
aflatoxin exposure levels and the liver cancer incidence rates
existing in the areas studied? Within Kenya and Swaziland, Peers &
Linsell (1977) demonstrated a steeper rise in liver cancer incidence
with increasing aflatoxin intake in men than in women (Fig. 5). A
similar difference seems to exist also in the other areas studied
(Shank, 1977).
a A positive association of fiver cancer incidence variations with
the availability of aflatoxin contaminated staple foods was also
reported recently from the Philippines (Bulatao-Jayme et al., 1976).
Table 15. Summary of available data on aflatoxin ingestion levels and primary
liver cancer incidencea
Country Area Aflatoxin Liver cancer
Estimated average
daily intakeb in No. of Incidence
adults--ng/kg body cases per 105
weight per day registered of total
population
per year
Kenya High altitude 3.5 4 1.2
Thailand Songkhla 5.0 2 2.0
Swaziland High veld 5.1 11 2.2
Kenya Middle altitude 5.9 33 2.5
Swaziland Mid veld 8.9 29 3.8
Kenya Low altitude 10.0 49 4.0
Swaziland Lebombo 15.4 4 4.3
Thailand Ratburi 45.0 6 6.0
Swaziland Low veld 43.1 42 9.2
Mozambique Inhambanec 222.1 --d 13.0
a From: Peers & Linsell (1977).
b Excluding any aflatoxin present in native beers.
c Revised incidence estimate taken from Van Rensburg (1977).
d Number of cases not available, probably >100.
The possibility that hepatitis B virus infection may confound
the relationship between aflatoxin ingestion and liver cancer
incidence has been considered (Linsell & Peers, 1977). Hepatitis B
infection is common in countries with a high incidence of primary
liver cancer and evidence of prior exposure to hepatitis B virus is
more common in individuals with liver cancer in these countries than
in normal subjects (Vogel et al., 1970; Reys & Sequeira, 1974;
Prince et al., 1975; Chainuvati et al., 1975). Nevertheless, the
present evidence favours aflatoxin as a possible major disease
determinant in primary liver cancer but hepatitis B virus may well
be a cofactor in the etiology (Peers & Linsell, 1977).
a When Stoloff & Friedman (1976) compared published reports on the
incidence of cancer in rural and urban areas of southeastern USA,
and in southeast states compared with other areas in the USA, they
found lower incidences of liver cancer in the rural areas and in the
southeast states, respectively, even though they expected that
long-term exposure to aflatoxins would be higher in these areas.
Further studies were carried out to determine how far the high
fat content of the marginal lipotrope diet contributed to the
enhancement of carcinogenesis. Male Fischer rats were fed the
marginal lipotrope diet, the nutritionally complete control diet, or
the control diet with substitution of the fat from the marginal
lipotrope diet (30% beef fat, 2% corn oil) for the fat in the
control diet (15% mixed vegetable oils). Each rat was given a total
of 375 µg of aflatoxin B1 intragastrically, in divided doses over
7 weeks and kept until moribund or dead, or until 90 weeks after
treatment, and then necropsied. Hepatocarcinoma incidence was based
on the number of rats that survived until the first death with
hepatocarcinoma, i.e., 27-34 rats per group. Incidences were found
of 39% in rats fed the marginal lipotrope diet, 15% in control rats,
and zero in rats fed the control diet with beef fat (30%) and corn
oil (2%) substituted for vegetable oil (15%) (Rogers et al.,
unpublished data). Thus the high fat content of the deficient diet
inhibited, rather than contributed to the enhancement of aflatoxin
carcinogenesis.
An enhancing effect on aflatoxin carcinogenicity was observed in
the experiments in which male rats (Charles River CD Sprague-Dawley)
were fed a low lipotrope diet containing 20% isolated soybean
protein and supplemented with 0.1% DL-methionine and 0.1% choline
chloride. Aflatoxin B1 was given intragastrically during the early
weeks of the experiment in a total dose of 240 µg/animal (divided in
24 daily doses of 10 µg, 5 days a week). Liver cell carcinomas were
observed 5/17 animals fed this diet. No tumours were found in rats
given the same dose of aflatoxin B1 and fed the same basal diet
supplemented with 0.6% DL-methionine, 0.6% choline chloride, and
vitamin B12 (50 µg/kg diet) (Newberne et al., 1968).
It should be noted that a more severe lipotrope deficiency may
decrease rather than increase the incidence of liver carcinoma in
aflatoxin-treated rats. A decrease in liver carcinoma was observed
in aflatoxin-treated, male, Sprague-Dawley rats with severe
lipotrope deficiency particularly if penicillin, at a level of 0.1%
were added to the diet. The expected penicillin-induced inhibition
of cirrhosis development was not observed and the interactions
between penicillin and aflatoxins have not been elucidated. Both
diets used in these experiments, the control, adequate, and the
highly lipo-trope-deficient contained alcohol-extracted groundnut
meal (25%) and casein (6%) as protein source. No cystine or
methionine was added, and choline and vitamin B12 were added to
the control diet at levels of 0.3% and 50 µg/kg, respectively. When
rats were killed 12 months after receiving a total dose of 375 tag
aflatoxin B1 per animal (divided into 15 daily intra-gastric doses
of 25 µg per animal), hepatomas were found in 64% (9/14) control
animals and in 41% (7/17) lipotrope-deficient animals. In rats fed
diets containing penicillin, hepatomas were found in 70% (14/20) of
the controls and in only 17% (3/18) of the lipotrope-deficient
animals (Newberne & Rogers, 1971).
The different effects of marginal and severe lipotrope
deficiencies on aflatoxin carcinogenesis are of interest in
connexion with apparent discrepancies between different studies on
aflatoxin carcinogenesis in rats fed protein-deficient diets
containing different levels of lipotropic agents discussed earlier.
Vitamin B12 is a weakly lipotropic factor that has recently
been found to increase tumour incidence in aflatoxin-treated rats.
In a study by Temcharoen et at. (1978), male Fischer rats were fed
diets containing 20% or 5% casein with or without vitamin B12
(50 µg/kg diet) for 33 weeks. The composition of the diets, with the
exception of the contents of casein (and dextrin) and vitamin B12,
was similar to that used in previous studies by Wogan & Newberne
(1967) and Newberne & Wogan (1968a). Choline chloride was added at
the level 0.036%. The casein content was similar to that in diets
used in the study by Madhavan & Gopalan (1968) mentioned earlier. A
mixture of crystalline aflatoxins (containing aflatoxins B1 and
G1 approximately in the proportion of 1: 1 and about 5% of
aflatoxins B2 and G2) was added to the diets at the level of
1 mg/kg. As shown in Table 12, vitamin B12 supplementation
increased liver rumour incidence in rats fed a diet containing
aflatoxin and 20% casein. Severe protein deficiency affected the
growth of the animals, the body weight of rats fed 5% casein being
reduced to about one third of the controls at the termination of the
experiment. Liver cirrhosis was observed only in the
aflatoxin-treated, protein-deficient rats. As suggested by
Temcharoen et al. (1978), a high incidence of hyperplastic nodules
and cholangiofibrosis in the protein-depleted, aflatoxin-treated
animals may indicate that the carcinogenic process was retarded but
not entirely absent.
(b) Vitamin A. In a study by Reddy et al. (1973), male and
female albino rats fed a vitamin A-deficient diet for 9 weeks after
weaning or fed the same diet with a daily oral supplement of 30 µg
(100 IU) of vitamin A/ rat were given a preparation of crystalline
aflatoxins containing aflatoxins B1 (44%), G1 (44%), and B2
and G2 (2%)in a single intraperitoneal dose of 3.5 mg/kg body
weight. High mortality was observed in vitamin A-deficient males
(Table 13). The vitamin A-deficient females and all the vitamin
A-supplemented animals did not show any adverse reactions 40 h after
aflatoxin injection. Histologically, severe liver damage was
observed in vitamin A-deficient, aflatoxin-treated male rats, in
contrast to minimal liver damage in female rats and male rats given
vitamin A.
Table 12. The effects of dietary protein and vitamin B12 on aflatoxin-induced liver changesa
Experimental diet No. of animals Average bodyb No. of animals with
(for details see text) at the beginning/ weight (g) at
termination of the termination cholangiofibrosis cirrhosis hyperplastic hepatoma
the experiment of the nodules
experiment
1. 5% casein 12/6 85 0 0 0 0
2. 5%casein + B12 12/12 91 0 0 0 0
3. 5% casein + aflatoxins 25/23 125 9 21 17 3
4. 5% casein + B12 + 25/24 129 8 12 15 1
aflatoxins
5. 20% casein 12/9 328 0 0 0 0
6. 20% casein + B12 12/10 369 0 0 0 0
7. 20% casein + aflatoxins 25/24 409 0 0 0 1
8. 20% casein + B12 + 25/25 375 0 0 4 6
aflatoxins
a Modified from: Temcharoen et al. (1978).
b Calculated from data of the authors on average liver weight and liver weight/100 g body weight.
In a study on rats fed diets containing aflatoxin B1 in the
range of 15-100 µg/kg and vitamin A in deficient, adequate, or
excessive levels over a 2-year period, the vitamin A-deficient
animals developed a similar incidence of liver tumours to the other
2 groups but had an increased incidence of colon carcinomas
(Newberne & Rogers, 1973). Thus, in a group of 50 male rats (Charles
River CD Sprague-Dawley) exposed to a dietary level of aflatoxin
B1 of 100 µg/kg and 5 µg vitamin A (retinyl palmitate) per animal
per day, colon tumours and liver tumours were observed in 6 and 11
rats, respectively, whereas with daily intakes of 50 or 500 µg
retinyl palmitate per rat, no colon cancers were observed and the
incidence of liver tumours was 24/50 or 19/50, respectively. The
results of a further study (Newberne & Suphakarn, 1977) are shown in
Table 14. Charles River CD Sprague-Dawley male (M) and female (F)
rats were fed a diet containing an aflatoxin B1 level of 1 mg/kg
(AFB1) and 3 different dietary levels of vitamin A (retinyl
acetate). Again, there was an increased incidence of colon
carcinomas in vitamin A-deficient rats. Excessive vitamin A did not
give protection against aflatoxin carcinogenesis in either the liver
or the colon.
Table 13. The effect of vitamin A status on the acute toxicity of aflatoxins
in male and female ratsa
Sex Vitamin A No. of Mortality Vitamin A
daily supplement animals 40 h after liver content
(IU/rat per day) aflatoxin (IU/whole liver)
injection means ± SEMc
Male 0 6 100% 36.5 ± 6.16b
100d 6 0% 2128.1 ± 153.56
Female 0 6 0% 18.6 ± 3.42
100d 6 0% 2299.1 ± 111.49
a From: Reddy et al. (1973).
b Vitamin A values for 5 animals only.
c SEM = standard error of the mean.
d 30µg.
Table 14. Vitamin A status, aflatoxin B1, and liver and colon
tumors in rats
Dietary AFB1 No. Sex Tumour incidence (%)
retinyl rats at
acetate risk liver colon both
(mg/kg)
control
3.0 0 24 M 0.0 0.0 0.0
3.0 0 26 F 0.0 0.0 0.0
3.0 + 24 M 87.5 4.1 4.1
3.0 + 24 F 79.1 8.3 8.3
low
0.3 0 10 M 0.0 0.0 0.0
0.3 0 12 F 0.0 0.0 0.0
0.3 + 66 M 89.4 28.8 25.7
0.3 + 42 F 76.2 28.6 11.9
high
30.0 0 23 M 0.0 0.0 0.0
30.0 0 20 F 0.0 0.0 0.0
30.0 + 26 M 92.3 7.7 7.7
30.0 + 31 F 83.9 9.7 6.4
From: Newberne & Suphakarn (1977).
(c) Selenium. A single oral dose of aflatoxin B1 given to
rats at 7 mg/kg bodyweight was less toxic in animals fed a high
selenium (selenite) diet containing selenium at 1 mg/kg (2-week
mortality 7/28) than in animals fed diets adequate or marginal in
selenium, containing selenium (selenite) at 0.1 or 0.03 mg/kg feed,
respectively (2-week mortalities 20/20 and 28/29). However, a
further increase in selenium intake (5 mg/kg feed) reaching toxic
levels predisposed the liver to aflatoxin injury and together with
aflatoxin exposure resulted in kidney lesions (tubular necrosis at
the cortico-medullary junction) (Newberne & Conner, 1974). The
incidence of liver tumours in Sprague-Dawley rats given a total of
500 µg of aflatoxin B1 intragastrically, over a 4-week period, was
not influenced by dietary selenium (as selenite) contents ranging
from 0.03 to 5.0 mg/kg (Grant et al., 1977).
(d) Cyclopropenoid fatty acids (CPFA). Cyclopropenoid fatty
acids (CPFA) which occur for example in cottonseed oil, enhanced
tumour induction in trout by both aflatoxin B1 and aflatoxin M1
(Sinnhuber et al., 1968, 1974). Young trout were fed a purified diet
which contained an aflatoxin B1 concentration of 4 µg/kg with or
without the addition of CPFA at 220 mg/kg diet. Hepatomas were found
in 27/30 fish fed CPFA and necropsied after 6 months; at 9 months,
20/20 bore hepatomas. Corresponding incidences of hepatomas in fish
that did not receive CPFA were 0/30 and 4/20, respectively
(Sinnhuber et al., 1968). In later experiments, fish were fed
aflatoxin M1 at the rate of 4 µg/kg diet, with or without the
addition of CPFA at 100 mg/kg. The incidences of hepatomas in
CPFA-fed fish were 6/40 at 4 months and 42/63 at 12 months.
Corresponding incidences in fish not fed CPFA were 2/40 and 6/40
respectively (Sinnhuber et al., 1974).
On the other hand, the enhancing effect of CPFA on aflatoxin
hepato-carcinogenesis was not clearly evident in several studies on
rats (Friedman & Molar, 1968; Lee et al., 1969a; Nixon et al.,
1974). No significant increase in liver tumours was observed in
Wistar male (M) and female (F) rats when sources of CPFA such as;
food grade cottonseed oil (CSO) or Sterculia foetida oil (SFO)
were added at levels of 10% and 0.04%, respectively, to diets
containing aflatoxin B1 at concentrations of 20 or 100 µg/kg.
Feeding these diets for different periods (generally exceeding 500
days) at the aflatoxin level of 20 µg/kg resulted in hepatomas in
4/36 rats M: 2/17; F: 2/19) with CSO exposure, in 1/37 rats
(F: 1/19) with SFO exposure, and in 0/38 rats without CSO or SFO in
the diet. With an aflatoxin B1 exposure level of 100 µg/kg diet,
the incidences of hepatomas in the CSO group, SFO group, and the
group without CSO or SFO were 15/36 (M: 12/17; F: 3/19), 17/37
(M: 10/17; F: 7/18), and 15/35 (M: 7/17; F: 8/18), respectively. In
Fischer rats, CSO was tested only in combination with the lower
concentration of aflatoxin; with an aflatoxin B1 level of
20 µg/kg diet, the hepatoma incidence was 6/31 (M: 4/15; F: 2/16)
with CSO, and 6/28 (M: 5/13; F: 1/15) without CSO exposure (Nixon et
al., 1974).
Other chemicals. Sodium phenobarbital given to rats in the
drinking water (1 g/litre) for 9 weeks, together with a diet
contaminated with aflatoxin B1 (at the level of approximately
5 mg/kg) resulted in a lower incidence (11/20) and delayed
appearance of liver tumours within the following 2 years compared
with the incidence in rats fed aflatoxin alone (17/20) (McLean &
Marshall, 1971). The effects of phenobarbital were confirmed in
experiments by Swenson et al. (1977) in which 2 groups of 18 male
Fischer rats were each given a diet containing aflatoxin B1 at a
level of 0.3 mg/kg and drinking water with or without sodium
phenobarbital (1 g/litre) for a period of 15 months. Examination by
laparotomy at the end of aflatoxin exposure revealed liver tumours
in 11 aflatoxin-treated controls (61%) and in only 2 (15%) rats
exposed to aflatoxin with phenobarbital. When the experiment was
terminated 5 months later, hepatocellular carcinomas were detected
in 18 (100%) aflatoxin-exposed rats and in 12 (67%) rats given
aflatoxin and phenobarbital. On the other hand, no effect on
aflatoxin hepatocarcinogenesis was observed in rats fed a similar
aflatoxin diet with the addition of benz (a)anthracene (70 mg/kg)
or ascorbic acid (25 g/kg) (Swenson et al., 1977).
Viral infection. Liver tumours were observed in 2/7 marmosets
fed aflatoxin B1 at a concentration of 2 mg/kg feed and injected
with hepatitis-type candidate virus (G. Barker strain) during
aflatoxin exposure. The animals survived 3-94 weeks of treatment.
Tumours were found in 3/9 marmosets given the aflatoxin diet only
(Lin et al., 1974). Exposure to both agents produced more severe
effects on the liver (cirrhosis) than exposure to aflatoxin B1
alone.
Exposure to artificial sunlight. The effects of exposure to
artificial sunlight on acute aflatoxin toxicity (Newberne et al.,
1974) and carcinogenicity (Joseph-Bravo et al., 1976) were studied
recently, using a long-arc xenon source and filter combination,
which had a spectral distribution in the ultraviolet and visible
ranges closely approximating the 6000 K colour temperature of
natural light. The rats, located 1.5 metres from the source, were
kept at an illumination level of about 29 600 1x corresponding to an
irradiance level of approximately 160 W/m2 over a 2-h period. In
groups consisting of 40 Sprague-Dawley male rats each, 18 control
rats died within 2 weeks of a single intragastric dose of aflatoxin
B1 at 7 mg/kg body weight whereas 23 of the rats exposed to
artificial sunlight for 2 h after aflatoxin administration died.
When an excessive dose of riboflavin was given intra-gastrically 30
min before aflatoxin, the corresponding 2-week mortalities in
animals unexposed and exposed to artificial sunlight were 20/40 and
30/40, respectively (Newberne et al., 1974). In the second study, a
total dose of 375 µg of aflatoxin B1 was given to male
Sprague-Dawley Charles River CD rats, in the form of 15 doses of
25 µg per rat, administered intra-gastrically over a 3-week period.
Thirty min after each aflatoxin administration, half of the animals
were exposed for 2 h to artificial sunlight as described earlier.
When the animals were killed 53 weeks after the last aflatoxin dose,
benign or malignant liver tumours were found in all 11
non-irradiated animals and in only 5/12 of the irradiated group
(Joseph-Bravo et al., 1976).
3.5 Effects in Man---Epidemiological and Clinical Studies
3.5.1 General population studies
3.5.1.1 Liver carcinogenesis
The data on aflatoxins and human cancer available before October
1975 were reviewed by IARC (1976) and several other reviews have
been published more recently (Linsell & Peers, 1977; Shank, 1977;
Van Rensburg, 1977). A positive association between aflatoxin
ingestion and liver cancer in man has been found in population
studies in which estimates of aflatoxin intake and the incidence of
primary liver cancer were made concurrently.
In a study in different parts of Uganda, it was found that
increased frequencies of detectable aflatoxin contamination of food
samples (range: 10.8%-43%) were associated with increased incidence
of primary liver cancer (range: 1.4-15.0 cases per 100 000 total
population per year) (Alpert et al., 1971). Four hundred and eighty
samples of foods were analysed from 8 areas in Uganda; the total
intake was not calculated. Within Swaziland, Keen & Martin
(1971 a, b) showed regional differences in liver cancer frequencies
consistent with regional differences in the frequency of aflatoxin
contamination of groundnuts. From the results of a questionnaire
study, they also suggested that tribal differences in the
preparation of groundnuts for food, and in eating habits, resulting
in higher aflatoxin exposure, could explain the apparently higher
liver cancer rate in the Shangaans living in Swaziland compared with
the Swazis.a
In the studies conducted by Shank et al. (1972a,b) in Thailand,
Peers & Linsell (1973) in Kenya, Van Rensburg et al. (1974) in
Mozambique, and Peers et al. (1976) in Swaziland, actual
concentrations of aflatoxin in meals about to be eaten (food on the
plate) were related to the incidence of primary liver cancer in the
areas where the meal samples were collected. These studies are
summarized in Table 15. A linear regression between the incidence of
liver cancer and the logarithm to the base 10 of the estimated
dietary intake of aflatoxin was found within the range of the
aflatoxin exposure levels and the liver cancer incidence rates
existing in the areas studied? Within Kenya and Swaziland, Peers &
Linsell (1977) demonstrated a steeper rise in liver cancer incidence
with increasing aflatoxin intake in men than in women (Fig. 5). A
similar difference seems to exist also in the other areas studied
(Shank, 1977).
a A positive association of fiver cancer incidence variations with
the availability of aflatoxin contaminated staple foods was also
reported recently from the Philippines (Bulatao-Jayme et al., 1976).
Table 15. Summary of available data on aflatoxin ingestion levels and primary
liver cancer incidencea
Country Area Aflatoxin Liver cancer
Estimated average
daily intakeb in No. of Incidence
adults--ng/kg body cases per 105
weight per day registered of total
population
per year
Kenya High altitude 3.5 4 1.2
Thailand Songkhla 5.0 2 2.0
Swaziland High veld 5.1 11 2.2
Kenya Middle altitude 5.9 33 2.5
Swaziland Mid veld 8.9 29 3.8
Kenya Low altitude 10.0 49 4.0
Swaziland Lebombo 15.4 4 4.3
Thailand Ratburi 45.0 6 6.0
Swaziland Low veld 43.1 42 9.2
Mozambique Inhambanec 222.1 --d 13.0
a From: Peers & Linsell (1977).
b Excluding any aflatoxin present in native beers.
c Revised incidence estimate taken from Van Rensburg (1977).
d Number of cases not available, probably >100.
The possibility that hepatitis B virus infection may confound
the relationship between aflatoxin ingestion and liver cancer
incidence has been considered (Linsell & Peers, 1977). Hepatitis B
infection is common in countries with a high incidence of primary
liver cancer and evidence of prior exposure to hepatitis B virus is
more common in individuals with liver cancer in these countries than
in normal subjects (Vogel et al., 1970; Reys & Sequeira, 1974;
Prince et al., 1975; Chainuvati et al., 1975). Nevertheless, the
present evidence favours aflatoxin as a possible major disease
determinant in primary liver cancer but hepatitis B virus may well
be a cofactor in the etiology (Peers & Linsell, 1977).
a When Stoloff & Friedman (1976) compared published reports on the
incidence of cancer in rural and urban areas of southeastern USA,
and in southeast states compared with other areas in the USA, they
found lower incidences of liver cancer in the rural areas and in the
southeast states, respectively, even though they expected that
long-term exposure to aflatoxins would be higher in these areas.
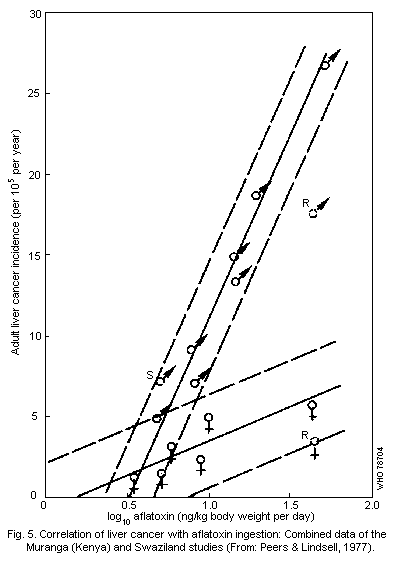 Two studies discussed by the Task Group reported the presence of
aflatoxins in the tissues of cancer patients.
Pang et al. unpublished dataa reported the results of a 2-year
study in Indonesia in which the aflatoxins contents were determined
in liver tissue biopsy specimens from 71 patients with primary
cancer of the liver (histologically verified hepatocellular
carcinoma in 62 patients and cholangiohepatocellular cancer in 7
patients). Dietary history indicated consumption of contaminated
food, many patients having eaten groundnuts, almost daily, since
childhood. Great variation was found in the aflatoxin contents of
food samples (type of food and number of analyses not given) with
aflatoxin B1 levels ranging from 17 to 1190 µg/kg, and aflatoxin
G1 levels ranging from 5 to 690 µg/kg. In extracts of liver tissue
biopsy samples obtained soon after the first visit to the hospital,
spots corresponding to aflatoxins were chromatographically detected
for 41 patients (57.7%) but not in extracts of liver tissues from 15
patients without liver cancer serving as controls. The authors also
reported the more frequent presence of aflatoxins in the urine of
liver cancer patients compared with the controls. Aflatoxin B1 at
an estimated level of 520 µg/kg fresh weight was detected in the
liver of a resident of the USA, suffering from carcinoma of the
liver and the rectum (Phillips et al., 1976). No attempt was made to
associate the aflatoxin with cancer in this case.
3.5.1.2 Other effects reported to be associated with aflatoxins
Reye's syndrome. The possibility that some cases of Reye's
syndrome (encephalopathy with fatty degeneration of the viscera)
(Reye et al., 1963), might be due to aflatoxin ingestion was first
suggested by Becroft (1966), who subsequently reported the presence
of aflatoxins B1 and G1 in the livers of 2 children who had died
from Reye's syndrome in New Zealand (Becroft & Webster, 1972).
Following this report, Dvorackova et al. (1974) in Czechoslovakia
and Chaves-Caballo et al. (1976) in the USA detected aflatoxins in
the livers of patients with Reye's syndrome. More recently, in the
USA, Hogan et al. (1978) detected aflatoxin B1 in the blood serum
of 2 patients with Reye's syndrome.
A dietary source of aflatoxin was not identified in any of these
case reports. Aflatoxins were not found in the livers of 5 further
subjects with Reye's syndrome in the USA (Shank, 1976).
a PANG, R. T. L., HUSAINI, S. N., & KARYADI, D. (1974) Aflatoxin
and primary hepatic cancer in Indonesia. Paper presented at the
Vth World Congress of Gastroenterology, 1319 October 1974, Mexico.
Clustering of Reye's syndrome cases, observed in north-east
Thailand, occurred mainly in the villages but not within families
and was geographically and seasonally related to high levels of
aflatoxin contamination of market food samples (Olson et al., 1971;
Bourgeois, 1975). In 2 cases, the presence of heavy aflatoxin
contamination in food, eaten 2 or 3 days before death, was
demonstrated (Bourgeois et al., 1971; Bourgeois, 1975).
Shank et al. (1971a) reported trace amounts of aflatoxin B1 in
tissues, body fluids, gastrointestinal contents or stools of 22/23
Thai patients who had died from Reye's syndrome and 11/15 who had
died from other causes. More than trace amounts of both aflatoxin
B1 and B2 were found in at least 2 liver specimens (47 and 93
lag aflatoxin B1/kg, respectively) from 2 of the 23 patients who
had died from Reye's syndrome but not in any of the specimens from
patients dying from other causes. These 2 series of patients are not
entirely comparable, however, because of differences in the
frequencies with which the various tissues and body contents were
examined.
Twenty-seven cases of Reye's syndrome collected over a 5-year
period (age range: 3 days to 8 years) were investigated by
Dvorackova et al. (1977). Aflatoxin B1 was found in the liver in
all cases and aflatoxin M1 in 4 cases. No aflatoxin was found in
the livers of 25 children, who had died from other causes.
Contamination of milk powder with aflatoxin B1 in the home was
suggested to be the source of exposure in 5 of the cases. No
aflatoxin M1 was found in this milk.
With the exception of the previously mentioned studies from
Thailand (Shank et al., 1971 a; Shank, 1977), cases of Reye's
syndrome have not been reported from other countries in which food
is commonly contaminated with more than traces of aflatoxins. As
Reye et al. (1963) initially suggested, encephalopathy with fatty
infiltration of the viscera is probably a syndrome of varied
etiology.
Other liver diseases. Instances of human liver disease, other
than Reye's syndrome and cancer, that had apparently followed
presumed dietary exposure to aflatoxins are summarized in Table 16.
This table also gives the levels of aflatoxins found in the
suspected foods. In these reports, the data are insufficient to
establish a definite association or causal relationship. In the
studies of Ling et al. (1967), however, there was a geographical and
temporal association between the availability of mouldy food for
consumption and the development of disease.
Table 16. Reports of liver disease (other than Reye's syndrome and cancer) in individuals exposed to aflatoxins in food
Country No. of Age Suspected Estimated Nature and outcome of liver Reference
cases group aflatoxin aflatoxin disease
of liver vehicle concentration
disease (mg/kg)
Senegal 2 4-6 years groundnut meal 0.5-1 Hepatitis leading to hepatatic Payet et al. (1966)
fibrosis in one case.
China (Province 26 all ages rice 0.2 Acute liver disease, 3 deaths in Ling et al. (1967)
of Taiwan) children.
Uganda 1 15 years cassava 1.7 Acute hepatitis leading to death. Serck-Hansen (1970)
India 20 1.5-5 years groundnut meal 0.3 Hepatomegaly. Hepatic failure and Amla et al. (1971 )
death in 3 cases. Subsequent
cirrhosis in some cases (juvenile
cirrhosis).
India several infants not maize 0.25-15 Acute toxic hepatitis; more than Krishnamachari et al.
hundred affected 100 deaths. (1975a,b);
Tandon et al. (1977)
The recent outbreak of acute toxic hepatitis in India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977, 1978) is
described in detail, because of the number of people affected and
because of the evaluation of this outbreak by the Task Group (see
section 3.6.2).
During the last 2 months of 1974 an outbreak of epidemic
jaundice with a high mortality rate affected more than 150 villages
in adjacent districts of 2 neighbouring states, Gujarat and
Rajasthan, in north-west India. Three reports from 2 independent
studies of this outbreak were available for evaluation by the Task
Group (section 3.6.2.1). The first, preliminary report
(Krishnamachari et al., 1975a) mentioned 397 patients in both
affected states with 106 deaths. In a later more detailed paper
(Krishnamachari et al., 1975b), the same group reported 277 cases in
the Panchamahals district of the state of Gujarat with 75 deaths,
and 126 hospitalized patients with 38 deaths in the Banswada
district of the state Rajasthan. In an even later study, a different
group (Tandon et al., 1977) reinvestigating the outbreak in
Rajasthan, reported 994 affected individuals with 97 deaths in the
Banswada and Dungarpur districts of Rajasthan. As reported by
Krishnamachari et al. (1975b), the outbreak started almost
simultaneously in all affected villages, with only a few households
affected in each village and several members of the same household
becoming ill in some instances. Cases were confined to rural areas
and to tribal populations whose staple food, particularly during the
period October-February, was locally grown maize. The outbreak
commenced with the consumption of recently harvested, badly stored
maize, which had been affected by unusual rainfalls in October 1974.
Although the maize was visibly spoiled, it was consumed, leaving
relatively better cobs for seed purposes and for later use.
Suspecting that the outbreak could have been caused by the massive
consumption of maize heavily contaminated with fungi, Krishnamachari
et al. (1975b) determined the mycoflora and the aflatoxin contents
of 10 food samples. A. flavus was detected in all 5 samples of
maize that were obtained from households affected with the disease,
and the aflatoxin B1 levels in these samples ranged from
0.25 mg/kg to 15.6 mg/kg. In contrast, only traces of aflatoxin were
found in maize supplied to a hostel by local shops in one affected
village and no aflatoxin was detected in 4 samples of other
foodstuffs from the same source. A. flavus was not found in these
5 food samples of commercial origin. Assuming a dally local
consumption of maize of up to 400 g per adult per day, and with
aflatoxin contamination up to 15 mg/kg, Krishnamachari et al.
(1975b) concluded that the affected people could have been exposed
to considerable quantities of aflatoxins (up to 6 mg/day), for
several weeks.
One liver sample obtained at necropsy and 7 blood serum and 7
urine samples collected from affected persons were analysed for
aflatoxin content. No information is given on the stage of the
disease at which the samples were collected or on the time that had
elapsed since the last exposure to food suspected to be contaminated
by aflatoxins. Traces of aflatoxin B1 were reported in only 2
blood serum samples, with negative results for all the other human
tissue and fluid samples (Krishnamachari, 1975b).
Tandon et al. (1977) reinvestigated the outbreak in Rajasthan,
presenting results of a retrospective epidemiological survey in an
area, where the largest number of patients with jaundice had been
reported. Statements on dietary history obtained from members of 47
affected families (304 household members, 70 of whom had manifested
the disease) and 29 other families (185 members with no case
reported) did not indicate any difference in the reported
consumption of mouldy maize between affected and non-affected
families or between affected or non-affected members of the same
household. However, A. flavus was detected in 85% of mouldy maize
samples collected from 14 affected families compared with 12% in
samples obtained from 17 families without manifestation of the
disease and 3% in samples obtained from 2 grain dealers. Aflatoxins
B1 and G1 were detected in 13 out of 14 samples from affected
families and in 17 out of 19 samples from the other families
investigated in this area. Aflatoxin B1 levels ranged from 0.1 to
0.6 mg/kg in all positive maize samples, with the exception of 2
from affected families where levels of 0.9 and 1.1 mg/kg were found.
Information is not given in the paper concerning the time at which
the samples were collected in relation to the occurrence of the
disease, whether the samples were analysed for Aspergillus and
aflatoxin contamination, and how far the aflatoxin levels found
reflected the levels that could have occurred in maize actually
consumed before and during the outbreak.
According to Krishnamachari et al. (1975b), all the cases
occurred among subjects whose staple food was maize. Even if maize
were also the staple food in the community studied by Tandon et al.
(1977), statements obtained from members of families studied
indicated that 31% of the members of non-affected families and 16%
of members of affected families reportedly did not consume maize.
From 70 cases of illness, 10 patients (14%) were reported not to
have consumed maize at all but the paper does not indicate the
staple food that they consumed instead.
In another part of the study by Tandon et al. (1977), clinical
data on 200 hospitalized patients were analysed, using hospital
records (176 cases) or direct clinical observations (24 patients).
The disease had a subacute onset starting with fever (in 86%
patients) followed by rapidly developing jaundice (98% cases) and
ascites (74%). In the patients where ascites was not massive it was
possible to detect hepatosplenomegaly. Vomiting, at the onset or at
the time of reporting to the hospital, was present in 46% of cases.
Leukocytosis (mainly an increase in polymorphonuclear leukocytes)
was detected in the initial stage in 87% of patients. Raised levels
of predominantly direct reacting bilirubin and alkaline phosphatase
(EC 3.1.3.1) were found in blood serum; transaminase elevation was
only mild or moderate and even normal levels were observed in blood
samples collected from 11 patients. From the 200 hospitalized
patients studied, 10% died in hospital, usually within 6 weeks of
the onset of illness.
This description of the principal signs and symptoms is in good
agreement with cases reported in the same outbreak by Krishnamachari
et al. (1975b). Both Tandon et al. (1977) and Krishnamachari et al.
(1975a,b) pointed out that two-thirds of affected people were males.
The disease was not reported in infants at all, in the study of
Krishnamachari (1975a,b). The youngest patient reported by Tandon et
al. (1977) was 2´ years old but very few cases were observed below
the age of 5 years. Both studies pointed out the concurrent liver
disease with jaundice and ascites observed in village dogs fed food
remnants from households (see also section 3.4.1).
Results of histopathological liver examinations are available
from 10 patients in this outbreak. In one necropsy sample described
by Krishnamachari et al. (1975a,b), microscopic examination revealed
extensive bile duct proliferation with periductal fibrosis and
cholestasis. Apparently normal liver cells were observed over wide
areas, occasionally replaced in some areas by multinucleated giant
cells or hepatocytes with foamy cytoplasm. Tandon et al. (1977,
1978), who examined liver biopsy specimens obtained from 8 patients
and one liver specimen from autopsy, pointed out that several
histopathological liver changes were characteristic. These included:
(a) oedema and collagenization of the central veins (thrombosis
was not observed); (b) cholangiolar proliferation; (c) moderate
to severe ballooning of the hepatocytes (giant cell transformation
of the liver cells); (d) perisinusoidal fibrosis;
(e) cholestasis; and (f) cirrhosis with reverse lobulation. On
the basis of liver histopathology, Tandon et al. (1977, 1978)
excluded any possibility of vital hepatitis and pointed out that the
cholangiolar proliferation and syncytial giant cell transformation
of hepatocytes (as well as the high prevalence of icterus in
affected people) were also not pathognomonic of the veno-occlusive
disease of the liver.
In one study by Maleki et al. (1976), an attempt was made to
assess the urinary excretion of aflatoxins in patients suffering
from cirrhosis of the liver, who came from a rural area of Iran
where this disease is considered to be frequent without any clear
association with high consumption of alcohol or hepatotoxic spices.
The authors reported that the urine of 6/25 patients with a clinical
diagnosis of cirrhosis contained aflatoxin M, whereas no aflatoxin
M, was detected in the urine of 30 non-cirrhotic patients. The urine
for aflatoxin analysis was collected within 2 days of admission to
hospital. The patients came from villages near Isfahan, Iran where
exceptionally high levels of aflatoxin M, in cow's milk had been
reported (section 3.2.2.10).
3.5.2 Occupational exposure
Three available papers deal with occupational exposure to
aflatoxins. Eleven out of a group of 55 workers, exposed for 2-9
years to dust containing aflatoxins in a mill crushing groundnuts
and other oil seed, developed cancer of various organs within the
observation period of up to 11 years. Primary liver cancer
(cholangiocarcinoma) was reported in one of these patients. Two
other workers were diagnosed to have died of another liver disease.
On the basis of airborne dust determinations at various work places
and dust analysis for aflatoxins (see section 3.2.4), the authors
calculated that airborne aflatoxin levels could have ranged between
0.87 ng/m3 and 72 ng/m3. Assuming respiratory exposure to
airborne aflatoxins in the range of 39 ng to 3.2 µg per worker per
week, the authors concluded that, depending on the length of
employment, the total amount of airborne aflatoxins, to which the
patients had been exposed during the whole period of work in the
mill, could have ranged in individual cases from 160 to 395 µg. In
an age-matched group of 55 workers from a different factory in the
same area, 4 cancer patients were found and no cases of liver cancer
or death from other liver diseases were recorded (Van Nieuwenhuize
et al., 1973).
Dvorackova (1976) reported that 2 men, who had previously been
carrying out the same type of work on a method of sterilizing
Brazilian groundnut meal contaminated by A. flavus, died with a
diagnosis of pulmonary adenomatosis. Analysis of lung samples
obtained at autopsy from one of these patients suggested the
presence of aflatoxin B1. Deger (1976) observed that carcinoma of
the colon developed in 2 research workers, who, for several years,
had been involved in the same type of work in the same laboratory,
purifying substantial amounts of aflatoxins for research purposes.
No other people were involved in this work in the institute.
3.6 Evaluation of the Health Risks of Exposure to Aflatoxins
3.6.1 Human exposure conditions
The main source of human exposure to aflatoxins is contaminated
food. Two pathways of dietary exposure have been identified:
(a) direct ingestion of aflatoxins (mainly B1) in contaminated
foods of plant origin such as maize and nuts and their products; and
(b) ingestion of aflatoxins carried over from feed into milk and
milk products including cheese and powdered milk, where they appear
mainly as aflatoxin M1.
Exposure by pathway (a) is likely to be much greater than by
pathway (b) irrespective of some toxicological differences between
aflatoxins B1 and M1. In tropical countries, where optimal
conditions for fungal growth exist, many components of the diet may
become contaminated. In the surveillance of the first of these
pathways of exposure, sampling is very important, as errors from
this source are much greater than those from the analytical methods
used. Since surveillance programmes have been established in only
very few countries (section 3.6.1.2), information on dietary
exposure to aflatoxins is not yet available on a worldwide basis.
Occasionally, workers may be exposed to the dust of agricultural
commodities that contain aflatoxins. The only available quantitative
data on such exposure (section 3.2.4) indicate that the
concentration of aflatoxins in air under these conditions may be of
the order of 0.1 µg/m3.
3.6.1.1 Sources and levels of aflatoxins in food
Aflatoxins are fungal products of some moulds that belong to two
species: Aspergillus flavus and A. parasiticus. These moulds are
found all over the word, except in polar regions, but their growth
and the formation of aflatoxins require humidity and temperature
conditions (section 3.2.1.1) that are prevalent in tropical and
subtropical areas, but may occasionally be found in colder regions
such as Northern Europe. The formation of aflatoxins takes place
mainly during harvesting and storage, but there is evidence that
attacks by insects carrying fungal spores can result in the
pre-harvest formation of aflatoxins (section 3.2.1.2).
Although aflatoxin-producing moulds can grow on a large variety
of foodstuffs, particularly on plant products, it appears that
certain foodstuffs are more suitable substrates for aflatoxin
formation than others and, thus, may be contaminated more frequently
and with higher levels. These include oil seeds (groundnuts, some
other nuts, cottonseed) and some cereals (maize) (section 3.2.2).
Existing data on the contamination of foodstuffs by aflatoxins
have been obtained by means of various analytical procedures
(section 3.1.2). Collaboratively tested methods are now available,
and as more countries develop adequate laboratory facilities and
make use of these methods, more comparable survey data should become
available.
Several species of cereals and nuts can be contaminated with
aflatoxins and, from published survey reports, it appears that
contamination is highest in groundnuts, Brazil nuts, maize, and
maize products (section 3.2.2). An extremely high value of about
3500 µg/kg was reported in a single sample of groundnuts imported
into Europe for feed, and in a survey in Thailand, an average
concentration of about 1500 µg/kg was recorded. However, average
values of 5 µg/kg or less are more common in countries where control
measures have been implemented. Peanut butter can also contain
aflatoxins, depending on the quality of the groundnuts used in its
manufacture. Roasting of groundnuts reduces but does not eliminate
aflatoxins contamination (section 3.2.3); in general, cooking of
food is not a safeguard against aflatoxin exposure.
There is reliable evidence showing that the level of aflatoxin M1
in milk is directly related to the daily intake of aflatoxin B1 in
dairy feeds, (section 3.3.4.1) and it is generally recognized that
groundnut and cottonseed meals, and maize are the major sources of
this contaminant. However, the level of aflatoxin M1 in milk is
approximately 300 times lower than the level of aflatoxin B1 in
the feed consumed. Several surveys of liquid and dried milk powder
have been carried out throughout the world (section 3.2.2.10). The
highest reported level of aflatoxin M1 in cow's milk exceeds
10 µg/litre, but, in countries where the quality of dairy products
is strictly controlled, levels of 0.1 µg/litre or less are commoner.
Animal experiments indicate that, in addition to the carry-over
into milk, residues of aflatoxins may be present in the tissues of
animals that consume contaminated feed (section 3.2.2.10). However,
the Task Group was not aware of any data from surveys for aflatoxin
residues in meat and meat products.
3.6.1.2 Dietary intake and levels in human tissues
Dietary exposure will depend on the levels of aflatoxins in food
and on food consumption patterns. It is evident that the
contamination of staple foods is of major concern, and that
population segments exposed to monotonous diets based on such staple
foods are at particular risk. Two methods are available for
assessing dietary exposure to aflatoxins: (a) determination of
contamination levels in major food commodities, combined with
nutritional surveys; and (b) analysis of food eaten
("food-on-the-plate" analysis).
In principle, the second method provides a better estimate of
aflatoxin intake but involves many practical difficulties.
Even though there are many reports from different parts of the
world on the presence of aflatoxins in individual food items
(section 3.2.2), the use of food consumption data for the assessment
of aflatoxin intake seems to be limited, at present, to certain
parts of the USA. "Food-on-the-plate" analysis data on aflatoxins
are available only for certain restricted areas in the regions of
the world where the incidence: of liver cancer is high (section
3.5.1.1).
A comprehensive nutritional and commodity survey conducted in
the southeastern states of the USA gave an estimated average level
of aflatoxin B1 in groundnuts and groundnut products of 2 µg/kg,
and an average level of 5-10 µg/kg for maize products. Based on
these data, the estimated average daily intake of aflatoxin B1 in
these areas of the USA was reported (FDA, 1978) to amount to
2.73 ng/kg body weight (maximum 9.03 ng/kg). In certain areas of
Thailand and East Africa, the estimated average daily intake based
on "food-on-the-plate" data ranged from 3.5 to 222.4 ng/kg body
weight (see section 3.5.1.1). However, during the outbreaks of acute
liver disease in south-east Asia, much higher estimates of daily
intake were obtained (up to 120 µg/kg body weight), and levels of
aflatoxin B1 up to 15 mg/kg were found in the contaminated maize
consumed (section 3.5.1.2).
Aflatoxin B1 has been found in the liver and other tissues of
human subjects at levels up to 500 µg/kg or more (section 3.3.2.2).
Some of these cases occurred in Europe and North America indicating
that, at least in some individuals, significant intake of aflatoxins
might occur in these areas.
3.6.2 Acute effects of exposure
Cases of acute human intoxication (section 3.5.1.2) have been
reportedly associated with dietary aflatoxin levels substantially
higher (in the mg/kg range) than the levels thought to be associated
with liver cancer (µg/kg total food range). The Task Group was not
aware of any long-term follow-up study of human populations in which
acute intoxication was reported to have occurred.
3.6.2.1 Acute liver disease
The association of an outbreak of liver disease in turkeys with
aflatoxins (section 3.4.1) was of basic importance in the
recognition of aflatoxins as an environmental hazard.
Similar outbreaks of acute liver disease associated with the
ingestion of aflatoxins were also observed in other species. The
hepatotoxicity of aflatoxins has been confirmed by animal
experiments and dose-response relationships have been obtained for
different species (section 3.4.2).
Acute aflatoxicosis in man has rarely been reported but such
cases may not always have been recognized. Apart from the death of 3
children in the Province of Taiwan, China and one child in Uganda,
where acute liver necrosis was associated with the ingestion of rice
and cassava contaminated with aflatoxins at levels of 200 µg/kg and
1700 µg/kg, respectively, the most convincing case of association of
aflatoxins with acute liver disease was an epidemic of toxic
hepatitis in north-west India in 1974 (section 3.5.1.2). In this
epidemic, several hundred villagers who consumed maize, presumably
contaminated with aflatoxins at levels up to 15 mg/kg, exhibited
signs and symptoms of poisoning and more than one hundred people
died. Estimated daily ingestion of levels up to 6 mg per person were
reported, corresponding approximately to dose rates up to 120 µg/kg
body weight per day. Such dose rates exceed those required to
produce liver damage in non-human primates and this provides
additional support for the assumption that this epidemic was indeed
related to aflatoxin ingestion. Although the role of aflatoxin was
not unequivocally demonstrated, the Task Group agreed that this
incident represented the most acceptable evidence to date of acute
human aflatoxicosis, supported by the information on liver
histology, the space-time clustering of the cases, and the deaths
among village dogs due to a similar form of acute toxic hepatitis.
Examination of tissues and body fluids for aflatoxins was limited to
15 samples (1 necropsy liver sample, 7 urine samples, and 7 blood
samples); aflatoxin was detected only in 2 blood samples. However,
available animal data suggest that the detectable residues of
mycotoxins may remain in tissues and body fluids for only a
relatively short time after ingestion and that, therefore, the
absence of residues does not exclude the possibility of prior
exposure.
In the light of two earlier similar occurrences of aflatoxin
intoxication associated specifically with children, it is somewhat
surprising that, in the Indian epidemic, infants were completely
spared and children under the age of 5 years were less commonly
affected than adults.
Similar epidemics could be expected in the future if the unusual
harvesting circumstances, considered in this case to be responsible
for the high contamination of the staple diet, recurred. The paucity
of reports of epidemics of this type would suggest that massive
contamination of human staple food is a rare occurrence.
3.6.2.2 Reye's syndrome
This syndrome (section 3.5.1.2) is found in many countries of the
world and unlike liver cancer does not show a geographical
association with areas of high aflatoxin intake. Out of four
countries in which the relationship between aflatoxins and Reye's
syndrome has been studied (Czechoslovakia, New Zealand, Thailand,
and the USA) only Thailand belongs to an area with high aflatoxin
levels in food. With the exception of the Thailand cases, the Task
Group was not aware of any other similar reports of Reye's syndrome
in association with aflatoxins in countries thought to be at
increased risk from aflatoxin exposure.
A disease showing many similarities to Reye's syndrome has been
experimentally demonstrated in macaques (section 3.4.2.2). This
resulted from single doses of aflatoxin B1 ranging from 4.5 to
40.5 mg/kg body weight. Reports on numerous experimental studies in
different animal species, including other nonhuman primates, did not
mention brain lesions; it is, however, possible that brains were not
examined for abnormalities.
Among the reports on the presence of aflatoxins in the tissues
of patients with Reye's syndrome, two studies deserve; attention
because of the number of cases included, and because control
subjects were available (section 3.5.1.2).
In Thailand, appreciable amounts of aflatoxins were detected in
autopsy specimens of 6 out of 23 cases of Reye's syndrome and trace
amounts (1-4 µg/kg) were found in a further 16 cases. Similar trace
amounts of aflatoxins were detected in specimens from 11 out of 15
children who had died from other causes.
In a systematic study over several years in Czechoslovakia,
aflatoxin B1 was unequivocally demonstrated in the liver tissue of
26/27 cases, and M1 in 4 cases (in one case the liver tissue was
not examined for aflatoxins). No aflatoxin was found in the liver
tissue of 25 children who died from other causes.
Cases where aflatoxins have been identified in the tissues or
body fluids are sporadic, and the Task Group had no indication of
the number of symptomatic cases in which it was not possible to
demonstrate the association with aflatoxin exposure. As regards
cases in which aflatoxin was detected in the tissues, it cannot be
excluded that pathological changes connected with Reye's syndrome
could have decreased the clearance of aflatoxins from tissues.
In view of these considerations, aflatoxins cannot be excluded
as a contributing factor to Reye's syndrome in some areas, although
an exclusive causal relationship cannot be accepted. There is
evidence that other factors, particularly influenza B virus, may be
associated with this syndrome.
3.6.3 Chronic effects of aflatoxin exposure
3.6.3.1 Cancer of the liver
Epidemiological data indicate an association between the level
of daily aflatoxin ingestion and the incidence of primary liver cell
cancer in certain areas of Kenya, Mozambique, Swaziland, and
Thailand (section 3.5.1.1). This relationship is strongly supported
by studies in experimental animals. In at least 8 species of
experimental animals, aflatoxin has been shown to increase the
incidence of liver cancer (section 3.4.2.3).
If the data from the 4 epidemiological studies (section 3.5.1.1)
are combined, the best fit to the data points is obtained by a
linear regression of the crude liver cancer incidence rates on the
logarithms of the dietary aflatoxin intake. The regression has been
estimated for dietary intakes ranging from 3.5 to 222.4 ng/kg body
weight per day and for crude liver cancer incidence rates from 1.2
to 13 cases/100 000 population per year. At the lower ranges of
aflatoxin intake, liver cancer rates are of the magnitude
encountered in parts of the world in which liver cancer frequency is
considered to be low.
It may be recalled that the liver cancer incidence in rats can
be increased by ingestion of diets containing aflatoxin B1 at a
level of 1 µg/kg (Table 7). The estimated levels of exposure to
aflatoxins in the USA (see section 3.6.1.2) have been used in the
assessment of corresponding life-time liver cancer risks in rats
(section 3.4.2.3) (FDA, 1978). For combined rat studies, these risk
estimates amounted to 240 and 1100 per 10 000 for aflatoxin exposure
levels of 0.1 and 0.3 µg/kg feed, respectively; the estimated
lifetime risks of primary liver cancer in the human population of
the USA from all causes is approximately 161 per 100 000 (FDA,
1978). Comparison of epidemiological and experimental data would
seem to indicate that man is not more but probably less susceptible
to aflatoxins than the rat. This conclusion also seems to be
supported by studies on the metabolic transformation of aflatoxins
(see section 3.3.3).
An association between aflatoxin intake and human liver cancer
incidence was established in surveys that included areas with high
estimated aflatoxin exposure and high liver cancer incidence. These
studies compared the current average aflatoxin intake and the crude
liver cancer incidence rate and did not allow for the period of
latency between the beginning of exposure and cancer manifestation.
The length of this latent period and factors that may modify its
length are not well known. However, the studies were conducted in
rural areas with stable populations and conditions of exposure
thought not to have changed substantially. Data from intervention
studies, in which populations are followed up after a reduction in
exposure levels has been achieved, are not available.
Published epidemiological studies have been limited in scope and
only a single possible etiological factor, i.e., aflatoxin exposure
has been examined. Other factors for which there is some evidence of
an etiological or modifying role in liver cancer, such as
nutritional status, cirrhosis, or viral hepatitis, or the
possibility of interactions between these and still other factors
have not been considered.
In rats, dietary intake of lipotropes, protein, and vitamin A
modified the carcinogenic potential of aflatoxins (section 3.4.2.7).
Diet, marginally deficient in lipotropes (choline, methionine,
folate), enhanced liver cancer induction by aflatoxin B1. A diet
marginally deficient in protein (9% casein) also increased liver
cancer incidence in aflatoxin-treated rats. Severe lipotrope
deficiency or severe protein deficiency (4% casein, combined with
lipotrope deficiency) decreased cancer incidence in
aflatoxin-treated rats. Severe deficiencies which markedly inhibit
the growth of experimental animals can reduce the cancer incidence
after exposure to different carcinogens. This probably represents a
general effect on growth rather than a specific effect on
carcinogenesis. Vitamin A deficiency did not influence the incidence
of liver cancer in aflatoxin-treated rats but altered the effect of
aflatoxin so that the incidence of colon cancers increased. In view
of the importance of these results in relation to human health and
the shortcomings in some of the experiments reported, further animal
studies are necessary to quantify these effects. Future
epidemiological studies should consider such interactions.
Other questions raised by the data from animal studies but not
demonstrated in the epidemiological studies, and thought by the Task
Group to require further study before they can be applied to the
human risk assessment, are the possible induction of extrahepatic
cancers by aflatoxin (section 3.4.2.3), and the possibility that
cancer could be induced by short-time exposure to high
concentrations of aflatoxin.
In spite of the existing gaps in knowledge, it should be
recognized that in animal experiments there is "strong evidence"
of carcinogenicitya for aflatoxins with established dose-response
relationships, and that epidemiological studies in some parts of the
world, where liver cancer is more frequent, have indicated a highly
significant positive correlation between the crude incidence rate of
liver cancer and the estimated current ingestion of aflatoxin in
these areas.b The Task Group, therefore, concluded that aflatoxin
ingestion may increase the risk of liver cancer, that the risk depends
on the amount of aflatoxin ingested, and that reduction in daily
aflatoxin exposure could be expected to reduce the liver cancer risk.
3.6.3.2 Juvenile cirrhosis in India
The Task Group concluded that the postulated involvement of
aflatoxins in juvenile cirrhosis in India has not been substantiated
(section 3.3.4.2 and Table 16 in section 3.5.1.2) and that it is
unlikely in view of the epidemiological and morphological evidence
available. Preliminary data suggesting the involvement of aflatoxins
in the etiology of this disease were not supported by later
measurements of aflatoxin exposure and examination of urine and
liver specimens.
3.6.4 Guidelines for health protection
The effect of aflatoxins which is of greatest concern is the
possible induction of liver cancer in man. Even if it is not
possible, at present, to quantify individual risk corresponding to a
given exposure to aflatoxins, it is nevertheless prudent to attempt
to reduce exposure as much as is practically achievable. Reduction
of food contamination by aflatoxins, sufficient to significantly
reduce liver cancer risk, would of course significantly reduce the
risk of acute toxic effects.
Although there are several aspects of the relationship between
aflatoxin exposure and carcinogenic risks in man that require
elucidation by further experimental and epidemiological studies,
there is, at present, sufficient evidence to justify the
implementation or strengthening of national aflatoxin control
programmes. It is impractical to insist that staple foodstuffs be
aflatoxin-free, but the level of aflatoxin contamination should be
reduced gradually by programmes involving the following components:
education of farmers to improve crop quality and storage;
surveillance of foodstuffs and animal feeds for the presence of
aflatoxins; and application of appropriate food-processing
technology to separate contaminated, from noncontaminated food
elements. These and other measures have recently been discussed
elsewhere (FAO, 1977).
a "Strong evidence" of carcinogenicity is considered to exist when
a chemical has been shown unequivocally to produce malignant
neoplasms. Chemicals for which the evidence of carcinogenicity is
based solely on the appearance of such neoplastic lesions as lung
adenomas or hepatomas in mice belong to the class of chemicals for
which there is "weak evidence" of carcinogenicity (IARC, 1977).
b Of course, this association between liver cancer incidence and
aflatoxin does not necessarily mean that these two variables are
causally related. However, the existing animal data tend to support
a causal relationship, although there may be other factors that
contribute to the development of liver cancer in these areas.
Several countries have established tolerance levels for
aflatoxins in specific food items (for review see Stoloff, 1977;
Krogh, 1978). It should be clearly understood that these tolerance
limits are only management tools intended to facilitate the
implementation of aflatoxin control programmes, and that adherance
to these tolerance limits does not provide an absolute protection
against the increased liver cancer risk associated with aflatoxin
exposure.
4. OTHER MYCOTOXINS
4.1 Ochratoxins
4.1.1 Properties and analytical methods
4.1.1.1 Chemical properties
Two studies discussed by the Task Group reported the presence of
aflatoxins in the tissues of cancer patients.
Pang et al. unpublished dataa reported the results of a 2-year
study in Indonesia in which the aflatoxins contents were determined
in liver tissue biopsy specimens from 71 patients with primary
cancer of the liver (histologically verified hepatocellular
carcinoma in 62 patients and cholangiohepatocellular cancer in 7
patients). Dietary history indicated consumption of contaminated
food, many patients having eaten groundnuts, almost daily, since
childhood. Great variation was found in the aflatoxin contents of
food samples (type of food and number of analyses not given) with
aflatoxin B1 levels ranging from 17 to 1190 µg/kg, and aflatoxin
G1 levels ranging from 5 to 690 µg/kg. In extracts of liver tissue
biopsy samples obtained soon after the first visit to the hospital,
spots corresponding to aflatoxins were chromatographically detected
for 41 patients (57.7%) but not in extracts of liver tissues from 15
patients without liver cancer serving as controls. The authors also
reported the more frequent presence of aflatoxins in the urine of
liver cancer patients compared with the controls. Aflatoxin B1 at
an estimated level of 520 µg/kg fresh weight was detected in the
liver of a resident of the USA, suffering from carcinoma of the
liver and the rectum (Phillips et al., 1976). No attempt was made to
associate the aflatoxin with cancer in this case.
3.5.1.2 Other effects reported to be associated with aflatoxins
Reye's syndrome. The possibility that some cases of Reye's
syndrome (encephalopathy with fatty degeneration of the viscera)
(Reye et al., 1963), might be due to aflatoxin ingestion was first
suggested by Becroft (1966), who subsequently reported the presence
of aflatoxins B1 and G1 in the livers of 2 children who had died
from Reye's syndrome in New Zealand (Becroft & Webster, 1972).
Following this report, Dvorackova et al. (1974) in Czechoslovakia
and Chaves-Caballo et al. (1976) in the USA detected aflatoxins in
the livers of patients with Reye's syndrome. More recently, in the
USA, Hogan et al. (1978) detected aflatoxin B1 in the blood serum
of 2 patients with Reye's syndrome.
A dietary source of aflatoxin was not identified in any of these
case reports. Aflatoxins were not found in the livers of 5 further
subjects with Reye's syndrome in the USA (Shank, 1976).
a PANG, R. T. L., HUSAINI, S. N., & KARYADI, D. (1974) Aflatoxin
and primary hepatic cancer in Indonesia. Paper presented at the
Vth World Congress of Gastroenterology, 1319 October 1974, Mexico.
Clustering of Reye's syndrome cases, observed in north-east
Thailand, occurred mainly in the villages but not within families
and was geographically and seasonally related to high levels of
aflatoxin contamination of market food samples (Olson et al., 1971;
Bourgeois, 1975). In 2 cases, the presence of heavy aflatoxin
contamination in food, eaten 2 or 3 days before death, was
demonstrated (Bourgeois et al., 1971; Bourgeois, 1975).
Shank et al. (1971a) reported trace amounts of aflatoxin B1 in
tissues, body fluids, gastrointestinal contents or stools of 22/23
Thai patients who had died from Reye's syndrome and 11/15 who had
died from other causes. More than trace amounts of both aflatoxin
B1 and B2 were found in at least 2 liver specimens (47 and 93
lag aflatoxin B1/kg, respectively) from 2 of the 23 patients who
had died from Reye's syndrome but not in any of the specimens from
patients dying from other causes. These 2 series of patients are not
entirely comparable, however, because of differences in the
frequencies with which the various tissues and body contents were
examined.
Twenty-seven cases of Reye's syndrome collected over a 5-year
period (age range: 3 days to 8 years) were investigated by
Dvorackova et al. (1977). Aflatoxin B1 was found in the liver in
all cases and aflatoxin M1 in 4 cases. No aflatoxin was found in
the livers of 25 children, who had died from other causes.
Contamination of milk powder with aflatoxin B1 in the home was
suggested to be the source of exposure in 5 of the cases. No
aflatoxin M1 was found in this milk.
With the exception of the previously mentioned studies from
Thailand (Shank et al., 1971 a; Shank, 1977), cases of Reye's
syndrome have not been reported from other countries in which food
is commonly contaminated with more than traces of aflatoxins. As
Reye et al. (1963) initially suggested, encephalopathy with fatty
infiltration of the viscera is probably a syndrome of varied
etiology.
Other liver diseases. Instances of human liver disease, other
than Reye's syndrome and cancer, that had apparently followed
presumed dietary exposure to aflatoxins are summarized in Table 16.
This table also gives the levels of aflatoxins found in the
suspected foods. In these reports, the data are insufficient to
establish a definite association or causal relationship. In the
studies of Ling et al. (1967), however, there was a geographical and
temporal association between the availability of mouldy food for
consumption and the development of disease.
Table 16. Reports of liver disease (other than Reye's syndrome and cancer) in individuals exposed to aflatoxins in food
Country No. of Age Suspected Estimated Nature and outcome of liver Reference
cases group aflatoxin aflatoxin disease
of liver vehicle concentration
disease (mg/kg)
Senegal 2 4-6 years groundnut meal 0.5-1 Hepatitis leading to hepatatic Payet et al. (1966)
fibrosis in one case.
China (Province 26 all ages rice 0.2 Acute liver disease, 3 deaths in Ling et al. (1967)
of Taiwan) children.
Uganda 1 15 years cassava 1.7 Acute hepatitis leading to death. Serck-Hansen (1970)
India 20 1.5-5 years groundnut meal 0.3 Hepatomegaly. Hepatic failure and Amla et al. (1971 )
death in 3 cases. Subsequent
cirrhosis in some cases (juvenile
cirrhosis).
India several infants not maize 0.25-15 Acute toxic hepatitis; more than Krishnamachari et al.
hundred affected 100 deaths. (1975a,b);
Tandon et al. (1977)
The recent outbreak of acute toxic hepatitis in India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977, 1978) is
described in detail, because of the number of people affected and
because of the evaluation of this outbreak by the Task Group (see
section 3.6.2).
During the last 2 months of 1974 an outbreak of epidemic
jaundice with a high mortality rate affected more than 150 villages
in adjacent districts of 2 neighbouring states, Gujarat and
Rajasthan, in north-west India. Three reports from 2 independent
studies of this outbreak were available for evaluation by the Task
Group (section 3.6.2.1). The first, preliminary report
(Krishnamachari et al., 1975a) mentioned 397 patients in both
affected states with 106 deaths. In a later more detailed paper
(Krishnamachari et al., 1975b), the same group reported 277 cases in
the Panchamahals district of the state of Gujarat with 75 deaths,
and 126 hospitalized patients with 38 deaths in the Banswada
district of the state Rajasthan. In an even later study, a different
group (Tandon et al., 1977) reinvestigating the outbreak in
Rajasthan, reported 994 affected individuals with 97 deaths in the
Banswada and Dungarpur districts of Rajasthan. As reported by
Krishnamachari et al. (1975b), the outbreak started almost
simultaneously in all affected villages, with only a few households
affected in each village and several members of the same household
becoming ill in some instances. Cases were confined to rural areas
and to tribal populations whose staple food, particularly during the
period October-February, was locally grown maize. The outbreak
commenced with the consumption of recently harvested, badly stored
maize, which had been affected by unusual rainfalls in October 1974.
Although the maize was visibly spoiled, it was consumed, leaving
relatively better cobs for seed purposes and for later use.
Suspecting that the outbreak could have been caused by the massive
consumption of maize heavily contaminated with fungi, Krishnamachari
et al. (1975b) determined the mycoflora and the aflatoxin contents
of 10 food samples. A. flavus was detected in all 5 samples of
maize that were obtained from households affected with the disease,
and the aflatoxin B1 levels in these samples ranged from
0.25 mg/kg to 15.6 mg/kg. In contrast, only traces of aflatoxin were
found in maize supplied to a hostel by local shops in one affected
village and no aflatoxin was detected in 4 samples of other
foodstuffs from the same source. A. flavus was not found in these
5 food samples of commercial origin. Assuming a dally local
consumption of maize of up to 400 g per adult per day, and with
aflatoxin contamination up to 15 mg/kg, Krishnamachari et al.
(1975b) concluded that the affected people could have been exposed
to considerable quantities of aflatoxins (up to 6 mg/day), for
several weeks.
One liver sample obtained at necropsy and 7 blood serum and 7
urine samples collected from affected persons were analysed for
aflatoxin content. No information is given on the stage of the
disease at which the samples were collected or on the time that had
elapsed since the last exposure to food suspected to be contaminated
by aflatoxins. Traces of aflatoxin B1 were reported in only 2
blood serum samples, with negative results for all the other human
tissue and fluid samples (Krishnamachari, 1975b).
Tandon et al. (1977) reinvestigated the outbreak in Rajasthan,
presenting results of a retrospective epidemiological survey in an
area, where the largest number of patients with jaundice had been
reported. Statements on dietary history obtained from members of 47
affected families (304 household members, 70 of whom had manifested
the disease) and 29 other families (185 members with no case
reported) did not indicate any difference in the reported
consumption of mouldy maize between affected and non-affected
families or between affected or non-affected members of the same
household. However, A. flavus was detected in 85% of mouldy maize
samples collected from 14 affected families compared with 12% in
samples obtained from 17 families without manifestation of the
disease and 3% in samples obtained from 2 grain dealers. Aflatoxins
B1 and G1 were detected in 13 out of 14 samples from affected
families and in 17 out of 19 samples from the other families
investigated in this area. Aflatoxin B1 levels ranged from 0.1 to
0.6 mg/kg in all positive maize samples, with the exception of 2
from affected families where levels of 0.9 and 1.1 mg/kg were found.
Information is not given in the paper concerning the time at which
the samples were collected in relation to the occurrence of the
disease, whether the samples were analysed for Aspergillus and
aflatoxin contamination, and how far the aflatoxin levels found
reflected the levels that could have occurred in maize actually
consumed before and during the outbreak.
According to Krishnamachari et al. (1975b), all the cases
occurred among subjects whose staple food was maize. Even if maize
were also the staple food in the community studied by Tandon et al.
(1977), statements obtained from members of families studied
indicated that 31% of the members of non-affected families and 16%
of members of affected families reportedly did not consume maize.
From 70 cases of illness, 10 patients (14%) were reported not to
have consumed maize at all but the paper does not indicate the
staple food that they consumed instead.
In another part of the study by Tandon et al. (1977), clinical
data on 200 hospitalized patients were analysed, using hospital
records (176 cases) or direct clinical observations (24 patients).
The disease had a subacute onset starting with fever (in 86%
patients) followed by rapidly developing jaundice (98% cases) and
ascites (74%). In the patients where ascites was not massive it was
possible to detect hepatosplenomegaly. Vomiting, at the onset or at
the time of reporting to the hospital, was present in 46% of cases.
Leukocytosis (mainly an increase in polymorphonuclear leukocytes)
was detected in the initial stage in 87% of patients. Raised levels
of predominantly direct reacting bilirubin and alkaline phosphatase
(EC 3.1.3.1) were found in blood serum; transaminase elevation was
only mild or moderate and even normal levels were observed in blood
samples collected from 11 patients. From the 200 hospitalized
patients studied, 10% died in hospital, usually within 6 weeks of
the onset of illness.
This description of the principal signs and symptoms is in good
agreement with cases reported in the same outbreak by Krishnamachari
et al. (1975b). Both Tandon et al. (1977) and Krishnamachari et al.
(1975a,b) pointed out that two-thirds of affected people were males.
The disease was not reported in infants at all, in the study of
Krishnamachari (1975a,b). The youngest patient reported by Tandon et
al. (1977) was 2´ years old but very few cases were observed below
the age of 5 years. Both studies pointed out the concurrent liver
disease with jaundice and ascites observed in village dogs fed food
remnants from households (see also section 3.4.1).
Results of histopathological liver examinations are available
from 10 patients in this outbreak. In one necropsy sample described
by Krishnamachari et al. (1975a,b), microscopic examination revealed
extensive bile duct proliferation with periductal fibrosis and
cholestasis. Apparently normal liver cells were observed over wide
areas, occasionally replaced in some areas by multinucleated giant
cells or hepatocytes with foamy cytoplasm. Tandon et al. (1977,
1978), who examined liver biopsy specimens obtained from 8 patients
and one liver specimen from autopsy, pointed out that several
histopathological liver changes were characteristic. These included:
(a) oedema and collagenization of the central veins (thrombosis
was not observed); (b) cholangiolar proliferation; (c) moderate
to severe ballooning of the hepatocytes (giant cell transformation
of the liver cells); (d) perisinusoidal fibrosis;
(e) cholestasis; and (f) cirrhosis with reverse lobulation. On
the basis of liver histopathology, Tandon et al. (1977, 1978)
excluded any possibility of vital hepatitis and pointed out that the
cholangiolar proliferation and syncytial giant cell transformation
of hepatocytes (as well as the high prevalence of icterus in
affected people) were also not pathognomonic of the veno-occlusive
disease of the liver.
In one study by Maleki et al. (1976), an attempt was made to
assess the urinary excretion of aflatoxins in patients suffering
from cirrhosis of the liver, who came from a rural area of Iran
where this disease is considered to be frequent without any clear
association with high consumption of alcohol or hepatotoxic spices.
The authors reported that the urine of 6/25 patients with a clinical
diagnosis of cirrhosis contained aflatoxin M, whereas no aflatoxin
M, was detected in the urine of 30 non-cirrhotic patients. The urine
for aflatoxin analysis was collected within 2 days of admission to
hospital. The patients came from villages near Isfahan, Iran where
exceptionally high levels of aflatoxin M, in cow's milk had been
reported (section 3.2.2.10).
3.5.2 Occupational exposure
Three available papers deal with occupational exposure to
aflatoxins. Eleven out of a group of 55 workers, exposed for 2-9
years to dust containing aflatoxins in a mill crushing groundnuts
and other oil seed, developed cancer of various organs within the
observation period of up to 11 years. Primary liver cancer
(cholangiocarcinoma) was reported in one of these patients. Two
other workers were diagnosed to have died of another liver disease.
On the basis of airborne dust determinations at various work places
and dust analysis for aflatoxins (see section 3.2.4), the authors
calculated that airborne aflatoxin levels could have ranged between
0.87 ng/m3 and 72 ng/m3. Assuming respiratory exposure to
airborne aflatoxins in the range of 39 ng to 3.2 µg per worker per
week, the authors concluded that, depending on the length of
employment, the total amount of airborne aflatoxins, to which the
patients had been exposed during the whole period of work in the
mill, could have ranged in individual cases from 160 to 395 µg. In
an age-matched group of 55 workers from a different factory in the
same area, 4 cancer patients were found and no cases of liver cancer
or death from other liver diseases were recorded (Van Nieuwenhuize
et al., 1973).
Dvorackova (1976) reported that 2 men, who had previously been
carrying out the same type of work on a method of sterilizing
Brazilian groundnut meal contaminated by A. flavus, died with a
diagnosis of pulmonary adenomatosis. Analysis of lung samples
obtained at autopsy from one of these patients suggested the
presence of aflatoxin B1. Deger (1976) observed that carcinoma of
the colon developed in 2 research workers, who, for several years,
had been involved in the same type of work in the same laboratory,
purifying substantial amounts of aflatoxins for research purposes.
No other people were involved in this work in the institute.
3.6 Evaluation of the Health Risks of Exposure to Aflatoxins
3.6.1 Human exposure conditions
The main source of human exposure to aflatoxins is contaminated
food. Two pathways of dietary exposure have been identified:
(a) direct ingestion of aflatoxins (mainly B1) in contaminated
foods of plant origin such as maize and nuts and their products; and
(b) ingestion of aflatoxins carried over from feed into milk and
milk products including cheese and powdered milk, where they appear
mainly as aflatoxin M1.
Exposure by pathway (a) is likely to be much greater than by
pathway (b) irrespective of some toxicological differences between
aflatoxins B1 and M1. In tropical countries, where optimal
conditions for fungal growth exist, many components of the diet may
become contaminated. In the surveillance of the first of these
pathways of exposure, sampling is very important, as errors from
this source are much greater than those from the analytical methods
used. Since surveillance programmes have been established in only
very few countries (section 3.6.1.2), information on dietary
exposure to aflatoxins is not yet available on a worldwide basis.
Occasionally, workers may be exposed to the dust of agricultural
commodities that contain aflatoxins. The only available quantitative
data on such exposure (section 3.2.4) indicate that the
concentration of aflatoxins in air under these conditions may be of
the order of 0.1 µg/m3.
3.6.1.1 Sources and levels of aflatoxins in food
Aflatoxins are fungal products of some moulds that belong to two
species: Aspergillus flavus and A. parasiticus. These moulds are
found all over the word, except in polar regions, but their growth
and the formation of aflatoxins require humidity and temperature
conditions (section 3.2.1.1) that are prevalent in tropical and
subtropical areas, but may occasionally be found in colder regions
such as Northern Europe. The formation of aflatoxins takes place
mainly during harvesting and storage, but there is evidence that
attacks by insects carrying fungal spores can result in the
pre-harvest formation of aflatoxins (section 3.2.1.2).
Although aflatoxin-producing moulds can grow on a large variety
of foodstuffs, particularly on plant products, it appears that
certain foodstuffs are more suitable substrates for aflatoxin
formation than others and, thus, may be contaminated more frequently
and with higher levels. These include oil seeds (groundnuts, some
other nuts, cottonseed) and some cereals (maize) (section 3.2.2).
Existing data on the contamination of foodstuffs by aflatoxins
have been obtained by means of various analytical procedures
(section 3.1.2). Collaboratively tested methods are now available,
and as more countries develop adequate laboratory facilities and
make use of these methods, more comparable survey data should become
available.
Several species of cereals and nuts can be contaminated with
aflatoxins and, from published survey reports, it appears that
contamination is highest in groundnuts, Brazil nuts, maize, and
maize products (section 3.2.2). An extremely high value of about
3500 µg/kg was reported in a single sample of groundnuts imported
into Europe for feed, and in a survey in Thailand, an average
concentration of about 1500 µg/kg was recorded. However, average
values of 5 µg/kg or less are more common in countries where control
measures have been implemented. Peanut butter can also contain
aflatoxins, depending on the quality of the groundnuts used in its
manufacture. Roasting of groundnuts reduces but does not eliminate
aflatoxins contamination (section 3.2.3); in general, cooking of
food is not a safeguard against aflatoxin exposure.
There is reliable evidence showing that the level of aflatoxin M1
in milk is directly related to the daily intake of aflatoxin B1 in
dairy feeds, (section 3.3.4.1) and it is generally recognized that
groundnut and cottonseed meals, and maize are the major sources of
this contaminant. However, the level of aflatoxin M1 in milk is
approximately 300 times lower than the level of aflatoxin B1 in
the feed consumed. Several surveys of liquid and dried milk powder
have been carried out throughout the world (section 3.2.2.10). The
highest reported level of aflatoxin M1 in cow's milk exceeds
10 µg/litre, but, in countries where the quality of dairy products
is strictly controlled, levels of 0.1 µg/litre or less are commoner.
Animal experiments indicate that, in addition to the carry-over
into milk, residues of aflatoxins may be present in the tissues of
animals that consume contaminated feed (section 3.2.2.10). However,
the Task Group was not aware of any data from surveys for aflatoxin
residues in meat and meat products.
3.6.1.2 Dietary intake and levels in human tissues
Dietary exposure will depend on the levels of aflatoxins in food
and on food consumption patterns. It is evident that the
contamination of staple foods is of major concern, and that
population segments exposed to monotonous diets based on such staple
foods are at particular risk. Two methods are available for
assessing dietary exposure to aflatoxins: (a) determination of
contamination levels in major food commodities, combined with
nutritional surveys; and (b) analysis of food eaten
("food-on-the-plate" analysis).
In principle, the second method provides a better estimate of
aflatoxin intake but involves many practical difficulties.
Even though there are many reports from different parts of the
world on the presence of aflatoxins in individual food items
(section 3.2.2), the use of food consumption data for the assessment
of aflatoxin intake seems to be limited, at present, to certain
parts of the USA. "Food-on-the-plate" analysis data on aflatoxins
are available only for certain restricted areas in the regions of
the world where the incidence: of liver cancer is high (section
3.5.1.1).
A comprehensive nutritional and commodity survey conducted in
the southeastern states of the USA gave an estimated average level
of aflatoxin B1 in groundnuts and groundnut products of 2 µg/kg,
and an average level of 5-10 µg/kg for maize products. Based on
these data, the estimated average daily intake of aflatoxin B1 in
these areas of the USA was reported (FDA, 1978) to amount to
2.73 ng/kg body weight (maximum 9.03 ng/kg). In certain areas of
Thailand and East Africa, the estimated average daily intake based
on "food-on-the-plate" data ranged from 3.5 to 222.4 ng/kg body
weight (see section 3.5.1.1). However, during the outbreaks of acute
liver disease in south-east Asia, much higher estimates of daily
intake were obtained (up to 120 µg/kg body weight), and levels of
aflatoxin B1 up to 15 mg/kg were found in the contaminated maize
consumed (section 3.5.1.2).
Aflatoxin B1 has been found in the liver and other tissues of
human subjects at levels up to 500 µg/kg or more (section 3.3.2.2).
Some of these cases occurred in Europe and North America indicating
that, at least in some individuals, significant intake of aflatoxins
might occur in these areas.
3.6.2 Acute effects of exposure
Cases of acute human intoxication (section 3.5.1.2) have been
reportedly associated with dietary aflatoxin levels substantially
higher (in the mg/kg range) than the levels thought to be associated
with liver cancer (µg/kg total food range). The Task Group was not
aware of any long-term follow-up study of human populations in which
acute intoxication was reported to have occurred.
3.6.2.1 Acute liver disease
The association of an outbreak of liver disease in turkeys with
aflatoxins (section 3.4.1) was of basic importance in the
recognition of aflatoxins as an environmental hazard.
Similar outbreaks of acute liver disease associated with the
ingestion of aflatoxins were also observed in other species. The
hepatotoxicity of aflatoxins has been confirmed by animal
experiments and dose-response relationships have been obtained for
different species (section 3.4.2).
Acute aflatoxicosis in man has rarely been reported but such
cases may not always have been recognized. Apart from the death of 3
children in the Province of Taiwan, China and one child in Uganda,
where acute liver necrosis was associated with the ingestion of rice
and cassava contaminated with aflatoxins at levels of 200 µg/kg and
1700 µg/kg, respectively, the most convincing case of association of
aflatoxins with acute liver disease was an epidemic of toxic
hepatitis in north-west India in 1974 (section 3.5.1.2). In this
epidemic, several hundred villagers who consumed maize, presumably
contaminated with aflatoxins at levels up to 15 mg/kg, exhibited
signs and symptoms of poisoning and more than one hundred people
died. Estimated daily ingestion of levels up to 6 mg per person were
reported, corresponding approximately to dose rates up to 120 µg/kg
body weight per day. Such dose rates exceed those required to
produce liver damage in non-human primates and this provides
additional support for the assumption that this epidemic was indeed
related to aflatoxin ingestion. Although the role of aflatoxin was
not unequivocally demonstrated, the Task Group agreed that this
incident represented the most acceptable evidence to date of acute
human aflatoxicosis, supported by the information on liver
histology, the space-time clustering of the cases, and the deaths
among village dogs due to a similar form of acute toxic hepatitis.
Examination of tissues and body fluids for aflatoxins was limited to
15 samples (1 necropsy liver sample, 7 urine samples, and 7 blood
samples); aflatoxin was detected only in 2 blood samples. However,
available animal data suggest that the detectable residues of
mycotoxins may remain in tissues and body fluids for only a
relatively short time after ingestion and that, therefore, the
absence of residues does not exclude the possibility of prior
exposure.
In the light of two earlier similar occurrences of aflatoxin
intoxication associated specifically with children, it is somewhat
surprising that, in the Indian epidemic, infants were completely
spared and children under the age of 5 years were less commonly
affected than adults.
Similar epidemics could be expected in the future if the unusual
harvesting circumstances, considered in this case to be responsible
for the high contamination of the staple diet, recurred. The paucity
of reports of epidemics of this type would suggest that massive
contamination of human staple food is a rare occurrence.
3.6.2.2 Reye's syndrome
This syndrome (section 3.5.1.2) is found in many countries of the
world and unlike liver cancer does not show a geographical
association with areas of high aflatoxin intake. Out of four
countries in which the relationship between aflatoxins and Reye's
syndrome has been studied (Czechoslovakia, New Zealand, Thailand,
and the USA) only Thailand belongs to an area with high aflatoxin
levels in food. With the exception of the Thailand cases, the Task
Group was not aware of any other similar reports of Reye's syndrome
in association with aflatoxins in countries thought to be at
increased risk from aflatoxin exposure.
A disease showing many similarities to Reye's syndrome has been
experimentally demonstrated in macaques (section 3.4.2.2). This
resulted from single doses of aflatoxin B1 ranging from 4.5 to
40.5 mg/kg body weight. Reports on numerous experimental studies in
different animal species, including other nonhuman primates, did not
mention brain lesions; it is, however, possible that brains were not
examined for abnormalities.
Among the reports on the presence of aflatoxins in the tissues
of patients with Reye's syndrome, two studies deserve; attention
because of the number of cases included, and because control
subjects were available (section 3.5.1.2).
In Thailand, appreciable amounts of aflatoxins were detected in
autopsy specimens of 6 out of 23 cases of Reye's syndrome and trace
amounts (1-4 µg/kg) were found in a further 16 cases. Similar trace
amounts of aflatoxins were detected in specimens from 11 out of 15
children who had died from other causes.
In a systematic study over several years in Czechoslovakia,
aflatoxin B1 was unequivocally demonstrated in the liver tissue of
26/27 cases, and M1 in 4 cases (in one case the liver tissue was
not examined for aflatoxins). No aflatoxin was found in the liver
tissue of 25 children who died from other causes.
Cases where aflatoxins have been identified in the tissues or
body fluids are sporadic, and the Task Group had no indication of
the number of symptomatic cases in which it was not possible to
demonstrate the association with aflatoxin exposure. As regards
cases in which aflatoxin was detected in the tissues, it cannot be
excluded that pathological changes connected with Reye's syndrome
could have decreased the clearance of aflatoxins from tissues.
In view of these considerations, aflatoxins cannot be excluded
as a contributing factor to Reye's syndrome in some areas, although
an exclusive causal relationship cannot be accepted. There is
evidence that other factors, particularly influenza B virus, may be
associated with this syndrome.
3.6.3 Chronic effects of aflatoxin exposure
3.6.3.1 Cancer of the liver
Epidemiological data indicate an association between the level
of daily aflatoxin ingestion and the incidence of primary liver cell
cancer in certain areas of Kenya, Mozambique, Swaziland, and
Thailand (section 3.5.1.1). This relationship is strongly supported
by studies in experimental animals. In at least 8 species of
experimental animals, aflatoxin has been shown to increase the
incidence of liver cancer (section 3.4.2.3).
If the data from the 4 epidemiological studies (section 3.5.1.1)
are combined, the best fit to the data points is obtained by a
linear regression of the crude liver cancer incidence rates on the
logarithms of the dietary aflatoxin intake. The regression has been
estimated for dietary intakes ranging from 3.5 to 222.4 ng/kg body
weight per day and for crude liver cancer incidence rates from 1.2
to 13 cases/100 000 population per year. At the lower ranges of
aflatoxin intake, liver cancer rates are of the magnitude
encountered in parts of the world in which liver cancer frequency is
considered to be low.
It may be recalled that the liver cancer incidence in rats can
be increased by ingestion of diets containing aflatoxin B1 at a
level of 1 µg/kg (Table 7). The estimated levels of exposure to
aflatoxins in the USA (see section 3.6.1.2) have been used in the
assessment of corresponding life-time liver cancer risks in rats
(section 3.4.2.3) (FDA, 1978). For combined rat studies, these risk
estimates amounted to 240 and 1100 per 10 000 for aflatoxin exposure
levels of 0.1 and 0.3 µg/kg feed, respectively; the estimated
lifetime risks of primary liver cancer in the human population of
the USA from all causes is approximately 161 per 100 000 (FDA,
1978). Comparison of epidemiological and experimental data would
seem to indicate that man is not more but probably less susceptible
to aflatoxins than the rat. This conclusion also seems to be
supported by studies on the metabolic transformation of aflatoxins
(see section 3.3.3).
An association between aflatoxin intake and human liver cancer
incidence was established in surveys that included areas with high
estimated aflatoxin exposure and high liver cancer incidence. These
studies compared the current average aflatoxin intake and the crude
liver cancer incidence rate and did not allow for the period of
latency between the beginning of exposure and cancer manifestation.
The length of this latent period and factors that may modify its
length are not well known. However, the studies were conducted in
rural areas with stable populations and conditions of exposure
thought not to have changed substantially. Data from intervention
studies, in which populations are followed up after a reduction in
exposure levels has been achieved, are not available.
Published epidemiological studies have been limited in scope and
only a single possible etiological factor, i.e., aflatoxin exposure
has been examined. Other factors for which there is some evidence of
an etiological or modifying role in liver cancer, such as
nutritional status, cirrhosis, or viral hepatitis, or the
possibility of interactions between these and still other factors
have not been considered.
In rats, dietary intake of lipotropes, protein, and vitamin A
modified the carcinogenic potential of aflatoxins (section 3.4.2.7).
Diet, marginally deficient in lipotropes (choline, methionine,
folate), enhanced liver cancer induction by aflatoxin B1. A diet
marginally deficient in protein (9% casein) also increased liver
cancer incidence in aflatoxin-treated rats. Severe lipotrope
deficiency or severe protein deficiency (4% casein, combined with
lipotrope deficiency) decreased cancer incidence in
aflatoxin-treated rats. Severe deficiencies which markedly inhibit
the growth of experimental animals can reduce the cancer incidence
after exposure to different carcinogens. This probably represents a
general effect on growth rather than a specific effect on
carcinogenesis. Vitamin A deficiency did not influence the incidence
of liver cancer in aflatoxin-treated rats but altered the effect of
aflatoxin so that the incidence of colon cancers increased. In view
of the importance of these results in relation to human health and
the shortcomings in some of the experiments reported, further animal
studies are necessary to quantify these effects. Future
epidemiological studies should consider such interactions.
Other questions raised by the data from animal studies but not
demonstrated in the epidemiological studies, and thought by the Task
Group to require further study before they can be applied to the
human risk assessment, are the possible induction of extrahepatic
cancers by aflatoxin (section 3.4.2.3), and the possibility that
cancer could be induced by short-time exposure to high
concentrations of aflatoxin.
In spite of the existing gaps in knowledge, it should be
recognized that in animal experiments there is "strong evidence"
of carcinogenicitya for aflatoxins with established dose-response
relationships, and that epidemiological studies in some parts of the
world, where liver cancer is more frequent, have indicated a highly
significant positive correlation between the crude incidence rate of
liver cancer and the estimated current ingestion of aflatoxin in
these areas.b The Task Group, therefore, concluded that aflatoxin
ingestion may increase the risk of liver cancer, that the risk depends
on the amount of aflatoxin ingested, and that reduction in daily
aflatoxin exposure could be expected to reduce the liver cancer risk.
3.6.3.2 Juvenile cirrhosis in India
The Task Group concluded that the postulated involvement of
aflatoxins in juvenile cirrhosis in India has not been substantiated
(section 3.3.4.2 and Table 16 in section 3.5.1.2) and that it is
unlikely in view of the epidemiological and morphological evidence
available. Preliminary data suggesting the involvement of aflatoxins
in the etiology of this disease were not supported by later
measurements of aflatoxin exposure and examination of urine and
liver specimens.
3.6.4 Guidelines for health protection
The effect of aflatoxins which is of greatest concern is the
possible induction of liver cancer in man. Even if it is not
possible, at present, to quantify individual risk corresponding to a
given exposure to aflatoxins, it is nevertheless prudent to attempt
to reduce exposure as much as is practically achievable. Reduction
of food contamination by aflatoxins, sufficient to significantly
reduce liver cancer risk, would of course significantly reduce the
risk of acute toxic effects.
Although there are several aspects of the relationship between
aflatoxin exposure and carcinogenic risks in man that require
elucidation by further experimental and epidemiological studies,
there is, at present, sufficient evidence to justify the
implementation or strengthening of national aflatoxin control
programmes. It is impractical to insist that staple foodstuffs be
aflatoxin-free, but the level of aflatoxin contamination should be
reduced gradually by programmes involving the following components:
education of farmers to improve crop quality and storage;
surveillance of foodstuffs and animal feeds for the presence of
aflatoxins; and application of appropriate food-processing
technology to separate contaminated, from noncontaminated food
elements. These and other measures have recently been discussed
elsewhere (FAO, 1977).
a "Strong evidence" of carcinogenicity is considered to exist when
a chemical has been shown unequivocally to produce malignant
neoplasms. Chemicals for which the evidence of carcinogenicity is
based solely on the appearance of such neoplastic lesions as lung
adenomas or hepatomas in mice belong to the class of chemicals for
which there is "weak evidence" of carcinogenicity (IARC, 1977).
b Of course, this association between liver cancer incidence and
aflatoxin does not necessarily mean that these two variables are
causally related. However, the existing animal data tend to support
a causal relationship, although there may be other factors that
contribute to the development of liver cancer in these areas.
Several countries have established tolerance levels for
aflatoxins in specific food items (for review see Stoloff, 1977;
Krogh, 1978). It should be clearly understood that these tolerance
limits are only management tools intended to facilitate the
implementation of aflatoxin control programmes, and that adherance
to these tolerance limits does not provide an absolute protection
against the increased liver cancer risk associated with aflatoxin
exposure.
4. OTHER MYCOTOXINS
4.1 Ochratoxins
4.1.1 Properties and analytical methods
4.1.1.1 Chemical properties
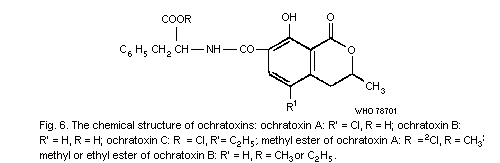 The ochratoxins are a group of structurally-related compounds
(Fig. 6), classified according to biosynthetic origin as
pentaketides within the group polyketides (Turner, 1971). The first
compound discovered, ochratoxin A, was isolated from a strain of
Aspergillus ochraceus (van der Merwe et al., 1965). It is a
colourless, crystalline compound, exhibiting blue fluorescence under
UV-light. The sodium salt of ochratoxin A is soluble in water; as an
acid, it is moderately soluble in polar organic solvents (e.g.,
chloroform and methanol). Some of the chemical and physical
properties of three ochratoxins are summarized in Table 17. Only
ochratoxin A, and very rarely ochratoxin B, have been encountered as
natural contaminants of foodstuffs, the remaining ochratoxins listed
in Fig. 6 have been isolated only from fungal cultures, under
laboratory conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine and an optically active lactone acid, ochratoxin
alpha, a metabolite which has been found in the urine of test
animals ingesting ochratoxin A-contaminated feed. This subject has
been reviewed by Chu (1974) and Harwig (1974), and the
spectroanalytical parameters have been reviewed by Neely & West
(1972).
Table 17. Chemical and physical properties of some ochratoxins
Ochratoxin Molecular Relative Melting Absorption maxima (nm) Reference
formula molecular mass point °C absorption coefficient (Epsilon)
A C20H18CINO6 403 169 (xylene) 213(36 800); 322(6400) Steyn & Holzapfel
89-95 (benzene) (1967)
B C20H19NO6 369 221 218(37 200); 318(6900) van der Merwe et al.
(1965)
Alpha C11H9CIO5 256 229 212(30 000); 338(5600) van der Merwe et al.
(1965)
4.1.1.2 Methods for the analysis of foodstuffs
Methods of analysing foodstuffs for ochratoxins have been
reviewed by Nesheim (1976). The distribution of ochratoxins in
commodities has not been studied in detail and no specific sampling
plans have been developed. However, the general principles of
sampling, outlined in section 3.1.2.1 for aflatoxins, are also
applicable to ochratoxin.
Several chemical methods have been developed, with limits of
detection as low as 2 µg/kg (Nesheim, 1976). Ochratoxin A in
acidified commodities is readily soluble in many organic solvents,
and this characteristic has been used as the principle of extraction
in several methods. The most widely used method, for cereals in
particular, includes extraction with chloroformaqueous phosphoric
acid followed by cleanup on an aqueous bicarbonate-diatomaceous
earth column, and quantitative determination using thin-layer
chromatography (Nesheim et al., 1973). This procedure has been
recommended by the International Union of Pure and Applied Chemistry
(IUPAC) as an international method (IUPAC, 1976), and has a limit of
detection of a few µg/kg, when improved by ammoniation.
Minicolumn methods for screening purposes have been developed
(Hald & Krogh, 1975; Holaday, 1976), as well as a spectrophotometric
procedure based on cleavage of ochratoxin A to form ochratoxin a and
phenylalanine (Hult & Gatenbeck, 1976).
A number of bioassays involving zebra fish larvae, brine
shrimps, and bacteria have been developed, but none of the assays
has been used routinely, so far (Harwig, 1974).
4.1.2 Sources and occurrence
4.1.2.1 Fungal formation
Ochratoxin A was first obtained from A. ochraceus, but
subsequent investigations have revealed that a variety of moulds
included in the fungal genera Aspergillus and Penicillium are able
to produce ochratoxins (Table 18). The main producers appear to be
A. ochraceus and P. viridicatum. This subject has been reviewed by
Krogh (1976a).
Moisture content and temperature. In studies of ochratoxin A
production by A. ochraceus, optimal production occurred between 20
and 30° C (Schindler & Nesheim, 1970; Bacon et al., 1973). Maximum
production was observed at 30° C and a water activity (%) of 0.953
(39% of water content, % dry weight). At lower temperatures, such as
15° C, the moisture requirement was higher (aw = 0.997, or 52%
moisture) (Table 19).
The genus Penicillium includes psychrophilic species, and
investigations of the influence of low incubation temperatures have
revealed that strains of P. viridicatum are able to produce
ochratoxin A at 5-10° C (Harwig & Chen, 1974) (Table 19). This
indicates that the heavy ochtratoxin contamination observed in
countries with cold climates such as Canada and the Scandinavian
countries is mainly produced by the Penicillia.
Table 18. Ochratoxin-producing fungia
Penicillium Link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica-Lanata:
P. commune series: P. commune Thom
Asymmetrica-Fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata-Symmetrica:
P. purpurogenum series: P. variabile Sopp
Aspergillus Micheli
Aspergillus ochraceus group:
A. sulphureus (Fres.) Thom and Church
A. sclerotiorum Huber
A. alliaceus Thom and Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Voros
a From: Krogh (1978).
Table 19. Production of ochratoxin A by A. ochraceus and
P. viridicatum at various aw and temperatures
(a) A. ochraceus (after 2 weeks of incubation)a
aw 15° C 22° C 30° C
mg ochratoxin A/kg
0.852 0 0 60
0.901 0 46 111
0.953 36 201 302
0.997 81 156 218
(b) P. viridicatum (after 3 weeks of incubation)b
aw 5° C 12° C 25° C
mg ochratoxin A/kg
0.85-0.86 4000 11 000
0.90-0.93 160 000 280 000
0.95-0.97 17 000
(after
5 weeks)
a Adapted from: Bacon et al. (1973).
b Adapted from: Harwig & Chen (1974).
4.1.2.2 Occurrence in foodstuffs
Plant products. The occurrence of ochratoxins in foodstuffs
has been reviewed by Chu (1974a), Krogh (1976a, 1977a), and Stoloff
(1976). Naturally occurring ochratoxin A was first reported at a
concentration of 110-150 µg/kg in one sample of maize included in a
survey of 283 samples from commercial markets in the USA (Shotwell
et al., 1969c). Data from subsequent surveys of plant products in
various areas of the world are summarized in Table 20.
Residues in food of animal origin. In 1971, a farm was traced
where pigs had been fed ochratoxin A-contaminated feed. When the
bacon pigs, some of which suffered from nephropathy, were delivered
to the slaughterhouse, samples of kidney, liver, and adipose tissue
were collected for analysis. Residues of ochratoxin A were detected
in 18/19 investigated kidneys, at levels up to 67 µg/kg (Hald &
Krogh, 1972). Residues were also detected in 7/8 livers, and in all
8 samples of adipose tissue analysed.
Table 20. Natural occurrence of ochratoxin A in plant products
Commodity Country No. of Percentage Range of Reference
samples contaminated contamination
analysed (µg/kg)
wheat, oats, barley, rye (feed) Canada 32 56.3 30-27 000c Scott et al. (1972)
barley, oats Denmark 33 57.6 28-27 500b,c Krogh et al. (1973b)
malt barley Denmark 50 6.0 9-189 Krogh (1978)
maize France 463 2.6 15-200 Galtier (1975)
barley, wheat, oats, rye, maize (feed) Poland 150 5.3 50-200 Juszkiewicz & Piskorska-Pliszczynska (1976)
mixed feed Poland 203 4.9 10-50 Juszkiewicz & Piskorska-Pliszczynska (1977)
barley, oats (feed) Sweden 84 8.3 16-409 Krogh et al. (1974)
maize, wheat, barley Yugoslavia 47 12.8 5-90 Krogh et al. (1977)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize USA 283 0.4 110-150 Shotwell et al. (1969c)
maize USA 293 1.0 83-166a Shotwell et al. (1971 )
wheat (red winter) USA 291 1.0 5-115 Shotwell et al. (1976a)
wheat (red spring) USA 286 2.8 5-115 Shotwell et al. (1976a)
a Two of these samples also contained ochratoxin B.
b Ochratoxin B, as well as ochratoxin A detected in 2 additional samples of barley.
c Most of the samples containing high levels had undergone "heating".
Table 21. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
Tissue Regression equation r
kidney y = 2.15 + 0.0123x 0.86
liver y = 0.35 + 0.0095x 0.82
adipose y = 2.51 + 0.0099x 0.78
a Modified from: Krogh et al. (1974).
x = ochratoxin A in feed (µg/kg)
y = ochratoxin A residue (µg/kg tissue)
r = correlation coefficient
The regression is calculated on feed levels of ochratoxin A in
the range of 200-4000 µg/kg.
Surveillance studies based on data from meat inspection in
Denmark have revealed prevalence rates of porcine nephropathy
ranging from 10-80 cases per 100 000 slaughtered pigs (Krogh,
1976b). A survey of kidneys from pigs with the disease collected at
various slaughterhouses, showed that 35% of the affected kidneys
contained residues of ochratoxin A, ranging from 2-68 µg/kg (Krogh,
1977b). A similar survey of porcine nephropathy in Sweden revealed
that 25% of the affected kidneys contained ochratoxin A at levels
ranging from 2 to 104 µg/kg (Rutqvist et al., 1977). The carry-over
of ochratoxin A from feed to animal tissues has been elucidated in
studies in which groups of pigs were exposed for 3-4 months to
dietary levels of ochratoxin A of 200, 1000, and 4000 µg/kg (Krogh
et al., 1974). At termination (slaughter), the highest levels of
ochratoxin A residues were found in the kidneys (mean level 50 µg/kg
at the 4000 µg/kg feed level) with slightly lower levels in the
liver, and even lower levels in muscle and adipose tissue. Other
tissues were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 21). In another study on pigs (Krogh et al.,
1976a), a high correlation ( r = 0.74-0.94) was found between
ochratoxin A levels in the kidney and in other organs and tissues
including the liver, muscle, and adipose tissue (Table 22 and
section 4.1.3.3).
Table 22. Correlation between ochratoxin A mass concentration
residues in the kidney and certain other tissuesa
Tissue Regression equation r
liver y = --0.650 + 0.706x 0.937
muscle y = --0.603 + 0.438x 0.888
adipose y = --0.775 + 0.309x 0.739
a From: Krogh et al. (1976a).
x = ochratoxin A mass concentration (µg/kg) in the kidney
y = ochratoxin A mass concentration (µg/kg) in the other tissues
r = correlation coefficient
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected in one slaughterhouse (Elling et al.,
1975). The birds had been condemned because of nephropathy. In
another study, groups of hens were exposed for 1-2 years to dietary
levels of ochratoxin A of 0.3 and 1 mg/kg (Krogh et al., 1976c). The
kidneys contained the highest residues, with a mean value of
19 µg/kg tissue in the group fed ochratoxin A at 1 mg/kg: the liver
and muscle contained lower levels of ochratoxin A residues.
Ochratoxins were not detected in the eggs.
4.1.3 Metabolism
4.1.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged ochratoxin
A, and it was concluded that ochratoxin A was absorbed mainly in the
stomach. In the caecum and the large intestine, small amounts (1-3%
of the total dose), were detected as the isocoumarin moiety
(ochratoxin a) most likely as the result of the hydrolysing action
of the intestinal microflora (Galtier & Alvinerie, 1976; Hult et
al., 1976).
In in vitro studies, Pitout (1969) showed that ochratoxin
alpha could also be formed from the hydrolysis of ochratoxin A by
carboxypeptidase A (EC 3.4.12.2) and alpha-chymotrypsin. No
quantitative information is available on the rate of absorption of
ochratoxin A and ochratoxin a from the gastrointestinal tract.
4.1.3.2 Tissue distribution and metabolic conversion
In slaughterhouse cases of mycotoxic porcine nephropathy studied
by Hald & Krogh (1972), residues of unchanged ochratoxin A were
found in all tissues investigated (kidney, liver and muscle), the
highest levels (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin A,
residues of this toxin were found in all 4 tissues in the decreasing
order of kidney, liver, muscle, adipose tissue (Krogh et al., 1974).
When rats were exposed perorally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered dose
in the whole kidneys, 0.9% in the whole liver, and 0.6% in the total
muscle tissue, 96 h after exposure. Chang & Chu (1977), using a
single intraperitoneal injection of I mg ochratoxin A per rat
(labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
0.5 h, amounting to 4-5% of the total dose.
It has been shown by in vitro studies that ochratoxin A binds to
serum albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies of rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin it has been detected in the urine and faeces of
rats intraperitoneally injected with ochratoxin A (Nel & Purchase,
1968; Chang & Chu, 1977), indicating the cleavage of ochratoxin A
into ochratoxin alpha and phenylalanine under these conditions.
Studies with 14C-labelled ochratoxin A indicated that some other,
not yet identified, metabolities are formed in the body. Less than
half of the radioactivity excreted in the urine within 24 h of a
single intra-peritoneal injection of 14C-phenylalanine-labelled
ochratoxin A was identified as ochratoxin A (Chang & Chu, 1977).
4.1.3.3 Excretion
Using 14C-labelled ochratoxin A in studies on rats, it has
been demonstrated that this toxin is excreted primarily in the urine
(Chang & Chu, 1977) although faecal excretion also occurs to some
extent (Galtier, 1974b; Chang & Chu, 1977). Ochratoxin A has been
detected in the urine of bacon pigs suffering from nephropathy
(Krogh, personal communication).
In a study of the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for 1 month and then kept on a toxin-free diet for another month
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 23) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue), with residual life values (RL50)a in the range of
3.3-4.5 days; the toxin could still be detected in kidneys one month
after termination of exposure. When the level in the kidney is
known, the ochratoxin A residues in the 3 other tissues can be
calculated (Table 22). No data are available on ochratoxin levels in
human tissues, urine, or faeces.
Table 23. The rate of disappearance of ochratoxin A residues from pig
tissues after termination of one month exposure to
ochratoxin A at 1 mg/kg feeda
Tissue Ochratoxin A (µg/kg tissue)
at time t (days) after
termination of exposure
kidney 28.22 exp (--0.1522t)
liver 19.49 exp (--0.1598t)
muscle 12.94 exp (--0.2096t)
adipose 4.62 exp (--0.0565t)
a From: Krogh et al. (1976a),
4.1.4 Effects in animals
4.1.4.1 Field observations
Pigs. The effects of ochratoxins in animals have been reviewed
by Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy, have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, although unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs and epidemics were encountered in 1963 and 1971, associated
with a high moisture content in the grain caused by unusual climatic
conditions (Krogh, 1976b). Extensive etiological studies have
a RL50 = half-time of residues calculated from the exponential
equations shown in Table 23.
revealed that ochratoxin A is a major disease determinant of porcine
nephropathy, although other factors such as citrinin are also
involved as causal determinants (for review see Krogh, 1976a).
Analyses of kidneys from cases of porcine nephropathy collected at
slaughterhouses have revealed that 35% of these kidneys contained
ochratoxin A in concentrations ranging from 2-68 µg/kg (Krogh,
1977b). As this compound has not been detected in healthy kidneys,
it is indicated that it may play a causal role in the disease.
The morphological changes in the kidneys in cases of mycotoxic
porcine nephropathy are characterized by degeneration of the
proximal tubules, followed by atrophy of the tubular epithelium,
interstitial fibrosis in the renal cortex, and hyalinization of some
glomeruli (Elling & Moller, 1973). Although mycotoxic porcine
nephropathy has only been reported from one other European country
besides Scandinavia (Buckley, 1971), there are indications that this
disease also occurs in other countries in Europe and North America.
Poultry. In a preliminary study in Denmark of chickens and
hens condemned by meat inspectors because of renal lesions, 29% of
14 birds were suffering from nephopathy associated with ingestion of
ochratoxin A (Elling et al., 1975). The morphological renal lesions
were characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons, and interstitial fibrosis.
4.1.4.2 Experimental studies
Acute and chronic effects. The acute and chronic effects of
ochratoxins in experimental animals have been reviewed by Chu
(1974a), Harwig (1974), and Krogh (1976a). Different species vary in
their susceptibility to acute poisoning by ochratoxin A, with LD50
values ranging from 3.4 to 30.3 mg/kg (Table 24). When administered
orally to rats, the female is more sensitive to ochratoxin A than
the male. The kidney is the target organ, but changes in the liver
have also been noted during studies of acute effects.
Table 24. Acute toxicity of ochratoxin A
Animal LD50 mg/kg Route of Reference
body weight administration
mouse (female) 22 intraperitoneal Sansing et al. (1976)
rat, male 30.3 peroral Galtier et al. (1974)
rat, female 21.4 peroral Galtier et al. (1974)
rat, male 12.6 intraperitoneal Galtier et al. (1974)
rat, female 14.3 intraperitoneal Galtier et al. (1974)
guineapig, male 9.1 peroral Thacker (1976)
guineapig, female 8.1 peroral Thacker (1976)
white leghorn 3.4 peroral Prior et al. (1976)
turkey 5.9 peroral Prior et al. (1976)
Japanese quail 16.5 peroral Prior et al. (1976)
rainbow trout 4.7 intraperitoneal Doster et al. (1972)
beagle dog, male < 9 (total dose) perorala Szczech et al. (1973a)
pig, female < 6 (total dose) peroralb Szczech et al. (1973b)
a All 3 dogs, dosed daily with 3 mg/kg, died within 3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed within 3 days, and
both pigs receiving 1 mg/kg daily were moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic porcine
nephropathy (section 4.1.4.1) have been reproduced by feeding diets
containing levels of ochratoxin A identical to those encountered in
naturally contaminated products (section 4.1.2.2). Thus 39 pigs fed
rations containing ochratoxin A at levels ranging from
200-4000 µg/kg developed nephropathy after 4 months at all levels of
exposure (Krogh et al., 1974). Changes in renal function were
characterized by impairment of tubular function, indicated
particularly by a decrease in TmPAH/CIna and reduced ability
to produce concentrated urine. These functional changes corresponded
well with the changes in renal structure observed at all exposure
levels including atrophy of the proximal tubules, and interstitial
cortical fibrosis. Sclerotized glomeruli were also observed in the
group receiving the highest dose of ochratoxin A of 4000 µg/kg feed.
No other organ or tissue exhibited any changes.
a TmPAH = transport maximum for para-aminohippuric acid, CIn =
clearance of inulin.
Kidney damage, identical to the naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to a
feed level of 1 mg/kg for 3 months. Similar damage was not observed
in 9 controls. Significant renal tubular impairment was detected
after only 5 weeks of ochratoxin exposure (Krogh et al., 1976b).
In pigs and dogs given high peroral doses, corresponding to feed
levels of more than 5-10 mg/kg (levels rarely found in nature)
extrarenal effects, in addition to renal lesions, were observed,
involving the liver, intestine, spleen, lymphoid tissue, and
leukocytes (Szczech et al., 1973a,b,c). Three groups of rats, each
consisting of 15 animals were exposed to feed levels of ochratoxin A
ranging from 0.2 to 5 mg/kg for 3 months. Renal damage in the form
of tubular degeneration was observed at all dose levels (Munro et
al., 1974).
Avian nephropathy similar to spontaneously occurring cases
(section 4.1.4.1) developed in chickens and hens exposed to dietary
levels of 0.3 and 1 mg/kg for 1 year (Krogh et al., 1976c). The
renal changes included degeneration of the tubular epithelium,
mainly confined to the proximal and distal tubules of both reptilian
and mammalian nephrons; impairment of glomerular and tubular
function was also observed. Acute necrosis and "visceral gout" was
observed in chickens exposed to high levels of ochratoxin A (LD50
values) (Peckham et al., 1971). The same authors reported that
ochratoxin B, the other naturally occurring ochratoxin was not
highly toxic to chickens (LD50: 54 mg/kg); no toxic effects have
been reported in other animals.
The toxic effects of ochratoxin A on the renal epithelial cells
of the monkey were demonstrated in in vitro studies, in the form
of abnormal mitotic cells (Steyn et al., 1975).
Teratogenic effects. Intraperitoneal injection of pregnant
mice with ochratoxin A at 5 mg/kg body weight on one of gestation
days 7-12 resulted in increased prenatal mortality, decreased fetal
weight, and various fetal malformations including exencephaly and
anomalies of the eyes, face, digits, and tail (Hayes et al., 1974).
When rats were treated perorally with ochratoxin A at 0.75 and
1.0 mg/kg body weight on gestation days 6-15, fetuses taken on day
20 showed decreased weight and various anomalies (e.g., open eyes,
wavy ribs, and agenesis of vertebrae) (Brown et al., 1976). In
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9, increased
prenatal mortality and malformations were observed, including
hydrocephalus, micrognathia, and heart defects (Hood et al., 1976).
Mutagenicity. No data were available on the mutagenicity of
ochratoxins.
Carcinogenesis. There have not been any recent data that would
change the conclusion that an evaluation of the carcinogenic risk of
ochratoxins cannot be made because of the inadequacy of available
studies in terms of the numbers of animals used and survival rates
(IARC, 1976).
Biochemical effects. Ochratoxin A affects the carbohydrate
metabolism in rats. Thus, a single oral dose of ochratoxin A at
15 mg/kg body weight caused a decrease in the glycogen level in the
liver and an increase in the heart glycogen level 4 h later (Suzuki
& Satoh, 1973). In a more extensive study on rats, the decrease in
liver glycogen level, 4 h after a single oral dose of ochratoxin A
at 15 mg/kg body weight, was associated with an increase in serum
glucose levels and a decrease in liver glucose-6-phosphate (Suzuki
et al., 1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin A
at 5 mg/kg body weight caused a decrease in liver glycogen
concentration, measured on the fourth day. The decrease was
attributed to inhibition of the active transport of glucose into the
liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies of rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further experiments with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that ochratoxin
A inhibited intramitochondrial phosphate transport, resulting in
deterioration of the mitochondria (Meisner, 1976). This might
explain the degeneration of liver mitochondria observed by Purchase
& Theron (1968) in rats exposed perorally to a single dose of
ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975) who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. Ochratoxin A acted synergistically, in this
respect, with another nephro-toxic mycotoxin, citrinin.
4.1.5 Effects in man
4.1.5.1 Ochratoxin A and Balkan nephropathy
Balkan endemic nephropathy is a kidney disease only observed so
far in rural populations in Bulgaria, Romania, and Yugoslavia. In
the past 2 decades, etiological investigations covering bacteria,
viruses, toxic metals, genetic factors, etc. have been conducted but
with unconvincing results (reviewed Puchlev, 1973, 1974). Balkan
endemic nephropathy is a chronic disease that is commonest between
30 and 50 years of age and progresses slowly up to death. The
kidneys are remarkably reduced in size. Histologically, the renal
disease is characterized by tubular degeneration, interstitial
fibrosis, and hyalization of glomeruli in the more superficial part
of the cortex (Heptinstall, 1966). Impairment of tubular function,
indicated by a decrease in TmPAH, is a prominent and early sign
(Dotchev, 1973).
The disease occurs endemically and affects females more often
than males (Hrabar et al., 1976, Chernozemsky et al., 1977). In
Bulgaria and Yugoslavia, a high incidence of urinary tract tumours
has been found to be closely correlated with the incidence and
mortality rates of Balkan endemic nephropathy (Ceovic et al., 1976;
Chernozemsky et al., 1977).
Fungal growth in foodstuffs and subsequent mycotoxin formation
is influenced by the water content of the foodstuffs, and can be
changed by climatic conditions such as heavy rainfalls during
harvest. Thus, the observation (Austwick, 1975) of a positive
correlation ( r = 0.80) between excess rainfall and the number of
people who died of nephropathy during the succeeding 2 years in the
Balkan peninsula might be interpreted as suggesting a fungal
involvement in the etiology of endemic nephropathy.
Attention has been called to the striking similarities in the
changes of renal structure and function found in Balkan endemic
nephropathy and in ochratoxin A-induced porcine nephropathy,
suggesting common causal relationships (Krogh, 1974). Furthermore,
epidemiological similarities have been noted, in particular, the
endemic occurrence (Krogh, 1976b). Preliminary results of a survey
of foodstuff's indicate that exposure to food-borne ochratoxin A
seems to be higher (12.8% contamination) in an area of Yugoslavia
with a high prevalence of human endemic nephropathy than in
nonendemic (control) areas (1.6% contamination) (Krogh et al.,
1977).
4.1.6 Conclusions and evaluation of the health risks to man
of ochratoxins
4.1.6.1 Experimental animal studies
The toxic effects of ochratoxin A have been studied extensively
in a variety of experimental animals. All the animals studied so far
have been susceptible to orally administered ochratoxin A, but to
various degrees, as indicated by the range of LD50 values
(Table 24). At high levels of ochratoxin A, changes were found in
the kidneys and also in other organs and tissues. However, only
renal lesions were observed at exposure levels identical to those
occurring environmentally. The renal lesions included degeneration
of the tubules, interstitial fibrosis, and, at later stages,
hyalinization of glomeruli, with impairment of tubular function as a
prime manifestation. Feed levels as low as 200 µg/kg produced renal
changes in the course of 3 months in rats and pigs. Field cases of
ochratoxin A-induced nephropathy are regularly encountered in pigs
and poultry. Ochratoxin A is teratogenic in the mouse, rat, and
hamster.
Ochratoxin B, rarely found as a natural contaminant, is much
less toxic; the other ochratoxins have never been encountered in
natural products.
4.1.6.2 Studies in man
The ochratoxin A-induced nephropathy in farm animals is similar
to Balkan endemic nephropathy in several aspects. In a preliminary
study in an area where Balkan endemic nephropathy is prevalent, the
ochratoxin A contamination of food appeared to be more frequent than
in control areas. However, the hypothesis that ochratoxin A may be a
causal determinant in this disease, needs further support.
4.1.6.3 Evaluation of health risks
The nephrotoxic potential of ochratoxin A is well documented
from all experimental studies, with a feed level of 200 µg/kg
causing nephropathy in pigs and rats. Lower levels have not been
tested. Field eases of ochratoxin A-induced nephropathy in farm
animals have long been recognized. The toxin has been found in a
variety of foodstuffs, with levels in commodities used as feed
ranging up to 27 mg/kg, and with levels in foodstuffs used for human
consumption in the range of trace to about 100 µg/kg. In one area
where endemic nephropathy was prevalent in the human population,
home produced foodstuffs were more frequently contaminated with
ochratoxin A than those from control areas. However, the total
intake of ochratoxin A by man has not been assessed so far, and
there is, at present, no proof that ochratoxin A is causally
involved in human diseases.
4.2 Zearalenone
4.2.1 Properties, analytical methods, and sources
The properties, analytical methods, sources, and occurrence of
zearalenone have been reviewed by Mirocha & Christensen (1974),
Pathre & Mirocha (1976), and Mirocha et al. (1977). Zearalenone is a
phenolic resorcylic acid lactone (Fig. 5), classified, according to
biosynthetic origin, as a nonaketide within the group polyketides
(Turner, 1971). Zearalenone (C18H22O5) is a white crystalline
compound with a relative molecular mass of 318, melting point
164-165 °C, and absorption maxima (and absorption coefficient) at
236 nm (29 700), 274 nm (13 909) and 316 nm (6020).
Zearalenone exhibits blue-green fluorescence when excited by
long wavelength (360 nm) UV-light, and a more intense green
fluorescence when excited with short wavelength (260 nm) UV-light.
A number of derivatives of zearalenone have been isolated from
fungal cultures (Fig. 7), but none of these derivatives has been
encountered, so far, as a natural contaminant of foodstuffs.
A multiple detection method for aflatoxin, ochratoxin, and
zearalenone has been developed (Eppley, 1968) and tested
collaboratively (Shotwell et al., 1976b). The procedure consists of
water-chloroform extraction combined with sequential elution of the
mycotoxins from a silica-gel column and the detection limit is in
the range of 50-100 µg/kg. A versatile method of analysis for
zearalenone has been described by Mirocha et al. (1974) using
thin-layer chromatography (TLC), gas-liquid chromatography (GLC),
gas-liquid chromatography-mass spectrometry, or a combination of all
these methods; the limit of detection is about 50 µg/kg. The
derivatives dimethoxyzearalenone and methyl oxime-di-TMS-zearalenone
are used to confirm the identity of zearalenone. Two methods using
high pressure liquid chromatography (HPLC) are now available (Scott
et al., 1978; Ware & Thorpe, 1978). With HPLC, a concentration of
zearalenone in cornflakes of 5 µg/kg could be determined (Scott et
al., 1978). Zearalenone is produced by strains of Fusarium
graminearum, F. tricinctum, F. oxysporum, F. sporotrichioides, and
F. moniliforme, and a period of low temperature ( 12°-14°C) during
fungal formation seems essential for high yield.
The ochratoxins are a group of structurally-related compounds
(Fig. 6), classified according to biosynthetic origin as
pentaketides within the group polyketides (Turner, 1971). The first
compound discovered, ochratoxin A, was isolated from a strain of
Aspergillus ochraceus (van der Merwe et al., 1965). It is a
colourless, crystalline compound, exhibiting blue fluorescence under
UV-light. The sodium salt of ochratoxin A is soluble in water; as an
acid, it is moderately soluble in polar organic solvents (e.g.,
chloroform and methanol). Some of the chemical and physical
properties of three ochratoxins are summarized in Table 17. Only
ochratoxin A, and very rarely ochratoxin B, have been encountered as
natural contaminants of foodstuffs, the remaining ochratoxins listed
in Fig. 6 have been isolated only from fungal cultures, under
laboratory conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine and an optically active lactone acid, ochratoxin
alpha, a metabolite which has been found in the urine of test
animals ingesting ochratoxin A-contaminated feed. This subject has
been reviewed by Chu (1974) and Harwig (1974), and the
spectroanalytical parameters have been reviewed by Neely & West
(1972).
Table 17. Chemical and physical properties of some ochratoxins
Ochratoxin Molecular Relative Melting Absorption maxima (nm) Reference
formula molecular mass point °C absorption coefficient (Epsilon)
A C20H18CINO6 403 169 (xylene) 213(36 800); 322(6400) Steyn & Holzapfel
89-95 (benzene) (1967)
B C20H19NO6 369 221 218(37 200); 318(6900) van der Merwe et al.
(1965)
Alpha C11H9CIO5 256 229 212(30 000); 338(5600) van der Merwe et al.
(1965)
4.1.1.2 Methods for the analysis of foodstuffs
Methods of analysing foodstuffs for ochratoxins have been
reviewed by Nesheim (1976). The distribution of ochratoxins in
commodities has not been studied in detail and no specific sampling
plans have been developed. However, the general principles of
sampling, outlined in section 3.1.2.1 for aflatoxins, are also
applicable to ochratoxin.
Several chemical methods have been developed, with limits of
detection as low as 2 µg/kg (Nesheim, 1976). Ochratoxin A in
acidified commodities is readily soluble in many organic solvents,
and this characteristic has been used as the principle of extraction
in several methods. The most widely used method, for cereals in
particular, includes extraction with chloroformaqueous phosphoric
acid followed by cleanup on an aqueous bicarbonate-diatomaceous
earth column, and quantitative determination using thin-layer
chromatography (Nesheim et al., 1973). This procedure has been
recommended by the International Union of Pure and Applied Chemistry
(IUPAC) as an international method (IUPAC, 1976), and has a limit of
detection of a few µg/kg, when improved by ammoniation.
Minicolumn methods for screening purposes have been developed
(Hald & Krogh, 1975; Holaday, 1976), as well as a spectrophotometric
procedure based on cleavage of ochratoxin A to form ochratoxin a and
phenylalanine (Hult & Gatenbeck, 1976).
A number of bioassays involving zebra fish larvae, brine
shrimps, and bacteria have been developed, but none of the assays
has been used routinely, so far (Harwig, 1974).
4.1.2 Sources and occurrence
4.1.2.1 Fungal formation
Ochratoxin A was first obtained from A. ochraceus, but
subsequent investigations have revealed that a variety of moulds
included in the fungal genera Aspergillus and Penicillium are able
to produce ochratoxins (Table 18). The main producers appear to be
A. ochraceus and P. viridicatum. This subject has been reviewed by
Krogh (1976a).
Moisture content and temperature. In studies of ochratoxin A
production by A. ochraceus, optimal production occurred between 20
and 30° C (Schindler & Nesheim, 1970; Bacon et al., 1973). Maximum
production was observed at 30° C and a water activity (%) of 0.953
(39% of water content, % dry weight). At lower temperatures, such as
15° C, the moisture requirement was higher (aw = 0.997, or 52%
moisture) (Table 19).
The genus Penicillium includes psychrophilic species, and
investigations of the influence of low incubation temperatures have
revealed that strains of P. viridicatum are able to produce
ochratoxin A at 5-10° C (Harwig & Chen, 1974) (Table 19). This
indicates that the heavy ochtratoxin contamination observed in
countries with cold climates such as Canada and the Scandinavian
countries is mainly produced by the Penicillia.
Table 18. Ochratoxin-producing fungia
Penicillium Link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica-Lanata:
P. commune series: P. commune Thom
Asymmetrica-Fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata-Symmetrica:
P. purpurogenum series: P. variabile Sopp
Aspergillus Micheli
Aspergillus ochraceus group:
A. sulphureus (Fres.) Thom and Church
A. sclerotiorum Huber
A. alliaceus Thom and Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Voros
a From: Krogh (1978).
Table 19. Production of ochratoxin A by A. ochraceus and
P. viridicatum at various aw and temperatures
(a) A. ochraceus (after 2 weeks of incubation)a
aw 15° C 22° C 30° C
mg ochratoxin A/kg
0.852 0 0 60
0.901 0 46 111
0.953 36 201 302
0.997 81 156 218
(b) P. viridicatum (after 3 weeks of incubation)b
aw 5° C 12° C 25° C
mg ochratoxin A/kg
0.85-0.86 4000 11 000
0.90-0.93 160 000 280 000
0.95-0.97 17 000
(after
5 weeks)
a Adapted from: Bacon et al. (1973).
b Adapted from: Harwig & Chen (1974).
4.1.2.2 Occurrence in foodstuffs
Plant products. The occurrence of ochratoxins in foodstuffs
has been reviewed by Chu (1974a), Krogh (1976a, 1977a), and Stoloff
(1976). Naturally occurring ochratoxin A was first reported at a
concentration of 110-150 µg/kg in one sample of maize included in a
survey of 283 samples from commercial markets in the USA (Shotwell
et al., 1969c). Data from subsequent surveys of plant products in
various areas of the world are summarized in Table 20.
Residues in food of animal origin. In 1971, a farm was traced
where pigs had been fed ochratoxin A-contaminated feed. When the
bacon pigs, some of which suffered from nephropathy, were delivered
to the slaughterhouse, samples of kidney, liver, and adipose tissue
were collected for analysis. Residues of ochratoxin A were detected
in 18/19 investigated kidneys, at levels up to 67 µg/kg (Hald &
Krogh, 1972). Residues were also detected in 7/8 livers, and in all
8 samples of adipose tissue analysed.
Table 20. Natural occurrence of ochratoxin A in plant products
Commodity Country No. of Percentage Range of Reference
samples contaminated contamination
analysed (µg/kg)
wheat, oats, barley, rye (feed) Canada 32 56.3 30-27 000c Scott et al. (1972)
barley, oats Denmark 33 57.6 28-27 500b,c Krogh et al. (1973b)
malt barley Denmark 50 6.0 9-189 Krogh (1978)
maize France 463 2.6 15-200 Galtier (1975)
barley, wheat, oats, rye, maize (feed) Poland 150 5.3 50-200 Juszkiewicz & Piskorska-Pliszczynska (1976)
mixed feed Poland 203 4.9 10-50 Juszkiewicz & Piskorska-Pliszczynska (1977)
barley, oats (feed) Sweden 84 8.3 16-409 Krogh et al. (1974)
maize, wheat, barley Yugoslavia 47 12.8 5-90 Krogh et al. (1977)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize USA 283 0.4 110-150 Shotwell et al. (1969c)
maize USA 293 1.0 83-166a Shotwell et al. (1971 )
wheat (red winter) USA 291 1.0 5-115 Shotwell et al. (1976a)
wheat (red spring) USA 286 2.8 5-115 Shotwell et al. (1976a)
a Two of these samples also contained ochratoxin B.
b Ochratoxin B, as well as ochratoxin A detected in 2 additional samples of barley.
c Most of the samples containing high levels had undergone "heating".
Table 21. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
Tissue Regression equation r
kidney y = 2.15 + 0.0123x 0.86
liver y = 0.35 + 0.0095x 0.82
adipose y = 2.51 + 0.0099x 0.78
a Modified from: Krogh et al. (1974).
x = ochratoxin A in feed (µg/kg)
y = ochratoxin A residue (µg/kg tissue)
r = correlation coefficient
The regression is calculated on feed levels of ochratoxin A in
the range of 200-4000 µg/kg.
Surveillance studies based on data from meat inspection in
Denmark have revealed prevalence rates of porcine nephropathy
ranging from 10-80 cases per 100 000 slaughtered pigs (Krogh,
1976b). A survey of kidneys from pigs with the disease collected at
various slaughterhouses, showed that 35% of the affected kidneys
contained residues of ochratoxin A, ranging from 2-68 µg/kg (Krogh,
1977b). A similar survey of porcine nephropathy in Sweden revealed
that 25% of the affected kidneys contained ochratoxin A at levels
ranging from 2 to 104 µg/kg (Rutqvist et al., 1977). The carry-over
of ochratoxin A from feed to animal tissues has been elucidated in
studies in which groups of pigs were exposed for 3-4 months to
dietary levels of ochratoxin A of 200, 1000, and 4000 µg/kg (Krogh
et al., 1974). At termination (slaughter), the highest levels of
ochratoxin A residues were found in the kidneys (mean level 50 µg/kg
at the 4000 µg/kg feed level) with slightly lower levels in the
liver, and even lower levels in muscle and adipose tissue. Other
tissues were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 21). In another study on pigs (Krogh et al.,
1976a), a high correlation ( r = 0.74-0.94) was found between
ochratoxin A levels in the kidney and in other organs and tissues
including the liver, muscle, and adipose tissue (Table 22 and
section 4.1.3.3).
Table 22. Correlation between ochratoxin A mass concentration
residues in the kidney and certain other tissuesa
Tissue Regression equation r
liver y = --0.650 + 0.706x 0.937
muscle y = --0.603 + 0.438x 0.888
adipose y = --0.775 + 0.309x 0.739
a From: Krogh et al. (1976a).
x = ochratoxin A mass concentration (µg/kg) in the kidney
y = ochratoxin A mass concentration (µg/kg) in the other tissues
r = correlation coefficient
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected in one slaughterhouse (Elling et al.,
1975). The birds had been condemned because of nephropathy. In
another study, groups of hens were exposed for 1-2 years to dietary
levels of ochratoxin A of 0.3 and 1 mg/kg (Krogh et al., 1976c). The
kidneys contained the highest residues, with a mean value of
19 µg/kg tissue in the group fed ochratoxin A at 1 mg/kg: the liver
and muscle contained lower levels of ochratoxin A residues.
Ochratoxins were not detected in the eggs.
4.1.3 Metabolism
4.1.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged ochratoxin
A, and it was concluded that ochratoxin A was absorbed mainly in the
stomach. In the caecum and the large intestine, small amounts (1-3%
of the total dose), were detected as the isocoumarin moiety
(ochratoxin a) most likely as the result of the hydrolysing action
of the intestinal microflora (Galtier & Alvinerie, 1976; Hult et
al., 1976).
In in vitro studies, Pitout (1969) showed that ochratoxin
alpha could also be formed from the hydrolysis of ochratoxin A by
carboxypeptidase A (EC 3.4.12.2) and alpha-chymotrypsin. No
quantitative information is available on the rate of absorption of
ochratoxin A and ochratoxin a from the gastrointestinal tract.
4.1.3.2 Tissue distribution and metabolic conversion
In slaughterhouse cases of mycotoxic porcine nephropathy studied
by Hald & Krogh (1972), residues of unchanged ochratoxin A were
found in all tissues investigated (kidney, liver and muscle), the
highest levels (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin A,
residues of this toxin were found in all 4 tissues in the decreasing
order of kidney, liver, muscle, adipose tissue (Krogh et al., 1974).
When rats were exposed perorally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered dose
in the whole kidneys, 0.9% in the whole liver, and 0.6% in the total
muscle tissue, 96 h after exposure. Chang & Chu (1977), using a
single intraperitoneal injection of I mg ochratoxin A per rat
(labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
0.5 h, amounting to 4-5% of the total dose.
It has been shown by in vitro studies that ochratoxin A binds to
serum albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies of rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin it has been detected in the urine and faeces of
rats intraperitoneally injected with ochratoxin A (Nel & Purchase,
1968; Chang & Chu, 1977), indicating the cleavage of ochratoxin A
into ochratoxin alpha and phenylalanine under these conditions.
Studies with 14C-labelled ochratoxin A indicated that some other,
not yet identified, metabolities are formed in the body. Less than
half of the radioactivity excreted in the urine within 24 h of a
single intra-peritoneal injection of 14C-phenylalanine-labelled
ochratoxin A was identified as ochratoxin A (Chang & Chu, 1977).
4.1.3.3 Excretion
Using 14C-labelled ochratoxin A in studies on rats, it has
been demonstrated that this toxin is excreted primarily in the urine
(Chang & Chu, 1977) although faecal excretion also occurs to some
extent (Galtier, 1974b; Chang & Chu, 1977). Ochratoxin A has been
detected in the urine of bacon pigs suffering from nephropathy
(Krogh, personal communication).
In a study of the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for 1 month and then kept on a toxin-free diet for another month
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 23) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue), with residual life values (RL50)a in the range of
3.3-4.5 days; the toxin could still be detected in kidneys one month
after termination of exposure. When the level in the kidney is
known, the ochratoxin A residues in the 3 other tissues can be
calculated (Table 22). No data are available on ochratoxin levels in
human tissues, urine, or faeces.
Table 23. The rate of disappearance of ochratoxin A residues from pig
tissues after termination of one month exposure to
ochratoxin A at 1 mg/kg feeda
Tissue Ochratoxin A (µg/kg tissue)
at time t (days) after
termination of exposure
kidney 28.22 exp (--0.1522t)
liver 19.49 exp (--0.1598t)
muscle 12.94 exp (--0.2096t)
adipose 4.62 exp (--0.0565t)
a From: Krogh et al. (1976a),
4.1.4 Effects in animals
4.1.4.1 Field observations
Pigs. The effects of ochratoxins in animals have been reviewed
by Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy, have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, although unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs and epidemics were encountered in 1963 and 1971, associated
with a high moisture content in the grain caused by unusual climatic
conditions (Krogh, 1976b). Extensive etiological studies have
a RL50 = half-time of residues calculated from the exponential
equations shown in Table 23.
revealed that ochratoxin A is a major disease determinant of porcine
nephropathy, although other factors such as citrinin are also
involved as causal determinants (for review see Krogh, 1976a).
Analyses of kidneys from cases of porcine nephropathy collected at
slaughterhouses have revealed that 35% of these kidneys contained
ochratoxin A in concentrations ranging from 2-68 µg/kg (Krogh,
1977b). As this compound has not been detected in healthy kidneys,
it is indicated that it may play a causal role in the disease.
The morphological changes in the kidneys in cases of mycotoxic
porcine nephropathy are characterized by degeneration of the
proximal tubules, followed by atrophy of the tubular epithelium,
interstitial fibrosis in the renal cortex, and hyalinization of some
glomeruli (Elling & Moller, 1973). Although mycotoxic porcine
nephropathy has only been reported from one other European country
besides Scandinavia (Buckley, 1971), there are indications that this
disease also occurs in other countries in Europe and North America.
Poultry. In a preliminary study in Denmark of chickens and
hens condemned by meat inspectors because of renal lesions, 29% of
14 birds were suffering from nephopathy associated with ingestion of
ochratoxin A (Elling et al., 1975). The morphological renal lesions
were characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons, and interstitial fibrosis.
4.1.4.2 Experimental studies
Acute and chronic effects. The acute and chronic effects of
ochratoxins in experimental animals have been reviewed by Chu
(1974a), Harwig (1974), and Krogh (1976a). Different species vary in
their susceptibility to acute poisoning by ochratoxin A, with LD50
values ranging from 3.4 to 30.3 mg/kg (Table 24). When administered
orally to rats, the female is more sensitive to ochratoxin A than
the male. The kidney is the target organ, but changes in the liver
have also been noted during studies of acute effects.
Table 24. Acute toxicity of ochratoxin A
Animal LD50 mg/kg Route of Reference
body weight administration
mouse (female) 22 intraperitoneal Sansing et al. (1976)
rat, male 30.3 peroral Galtier et al. (1974)
rat, female 21.4 peroral Galtier et al. (1974)
rat, male 12.6 intraperitoneal Galtier et al. (1974)
rat, female 14.3 intraperitoneal Galtier et al. (1974)
guineapig, male 9.1 peroral Thacker (1976)
guineapig, female 8.1 peroral Thacker (1976)
white leghorn 3.4 peroral Prior et al. (1976)
turkey 5.9 peroral Prior et al. (1976)
Japanese quail 16.5 peroral Prior et al. (1976)
rainbow trout 4.7 intraperitoneal Doster et al. (1972)
beagle dog, male < 9 (total dose) perorala Szczech et al. (1973a)
pig, female < 6 (total dose) peroralb Szczech et al. (1973b)
a All 3 dogs, dosed daily with 3 mg/kg, died within 3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed within 3 days, and
both pigs receiving 1 mg/kg daily were moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic porcine
nephropathy (section 4.1.4.1) have been reproduced by feeding diets
containing levels of ochratoxin A identical to those encountered in
naturally contaminated products (section 4.1.2.2). Thus 39 pigs fed
rations containing ochratoxin A at levels ranging from
200-4000 µg/kg developed nephropathy after 4 months at all levels of
exposure (Krogh et al., 1974). Changes in renal function were
characterized by impairment of tubular function, indicated
particularly by a decrease in TmPAH/CIna and reduced ability
to produce concentrated urine. These functional changes corresponded
well with the changes in renal structure observed at all exposure
levels including atrophy of the proximal tubules, and interstitial
cortical fibrosis. Sclerotized glomeruli were also observed in the
group receiving the highest dose of ochratoxin A of 4000 µg/kg feed.
No other organ or tissue exhibited any changes.
a TmPAH = transport maximum for para-aminohippuric acid, CIn =
clearance of inulin.
Kidney damage, identical to the naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to a
feed level of 1 mg/kg for 3 months. Similar damage was not observed
in 9 controls. Significant renal tubular impairment was detected
after only 5 weeks of ochratoxin exposure (Krogh et al., 1976b).
In pigs and dogs given high peroral doses, corresponding to feed
levels of more than 5-10 mg/kg (levels rarely found in nature)
extrarenal effects, in addition to renal lesions, were observed,
involving the liver, intestine, spleen, lymphoid tissue, and
leukocytes (Szczech et al., 1973a,b,c). Three groups of rats, each
consisting of 15 animals were exposed to feed levels of ochratoxin A
ranging from 0.2 to 5 mg/kg for 3 months. Renal damage in the form
of tubular degeneration was observed at all dose levels (Munro et
al., 1974).
Avian nephropathy similar to spontaneously occurring cases
(section 4.1.4.1) developed in chickens and hens exposed to dietary
levels of 0.3 and 1 mg/kg for 1 year (Krogh et al., 1976c). The
renal changes included degeneration of the tubular epithelium,
mainly confined to the proximal and distal tubules of both reptilian
and mammalian nephrons; impairment of glomerular and tubular
function was also observed. Acute necrosis and "visceral gout" was
observed in chickens exposed to high levels of ochratoxin A (LD50
values) (Peckham et al., 1971). The same authors reported that
ochratoxin B, the other naturally occurring ochratoxin was not
highly toxic to chickens (LD50: 54 mg/kg); no toxic effects have
been reported in other animals.
The toxic effects of ochratoxin A on the renal epithelial cells
of the monkey were demonstrated in in vitro studies, in the form
of abnormal mitotic cells (Steyn et al., 1975).
Teratogenic effects. Intraperitoneal injection of pregnant
mice with ochratoxin A at 5 mg/kg body weight on one of gestation
days 7-12 resulted in increased prenatal mortality, decreased fetal
weight, and various fetal malformations including exencephaly and
anomalies of the eyes, face, digits, and tail (Hayes et al., 1974).
When rats were treated perorally with ochratoxin A at 0.75 and
1.0 mg/kg body weight on gestation days 6-15, fetuses taken on day
20 showed decreased weight and various anomalies (e.g., open eyes,
wavy ribs, and agenesis of vertebrae) (Brown et al., 1976). In
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9, increased
prenatal mortality and malformations were observed, including
hydrocephalus, micrognathia, and heart defects (Hood et al., 1976).
Mutagenicity. No data were available on the mutagenicity of
ochratoxins.
Carcinogenesis. There have not been any recent data that would
change the conclusion that an evaluation of the carcinogenic risk of
ochratoxins cannot be made because of the inadequacy of available
studies in terms of the numbers of animals used and survival rates
(IARC, 1976).
Biochemical effects. Ochratoxin A affects the carbohydrate
metabolism in rats. Thus, a single oral dose of ochratoxin A at
15 mg/kg body weight caused a decrease in the glycogen level in the
liver and an increase in the heart glycogen level 4 h later (Suzuki
& Satoh, 1973). In a more extensive study on rats, the decrease in
liver glycogen level, 4 h after a single oral dose of ochratoxin A
at 15 mg/kg body weight, was associated with an increase in serum
glucose levels and a decrease in liver glucose-6-phosphate (Suzuki
et al., 1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin A
at 5 mg/kg body weight caused a decrease in liver glycogen
concentration, measured on the fourth day. The decrease was
attributed to inhibition of the active transport of glucose into the
liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies of rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further experiments with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that ochratoxin
A inhibited intramitochondrial phosphate transport, resulting in
deterioration of the mitochondria (Meisner, 1976). This might
explain the degeneration of liver mitochondria observed by Purchase
& Theron (1968) in rats exposed perorally to a single dose of
ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975) who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. Ochratoxin A acted synergistically, in this
respect, with another nephro-toxic mycotoxin, citrinin.
4.1.5 Effects in man
4.1.5.1 Ochratoxin A and Balkan nephropathy
Balkan endemic nephropathy is a kidney disease only observed so
far in rural populations in Bulgaria, Romania, and Yugoslavia. In
the past 2 decades, etiological investigations covering bacteria,
viruses, toxic metals, genetic factors, etc. have been conducted but
with unconvincing results (reviewed Puchlev, 1973, 1974). Balkan
endemic nephropathy is a chronic disease that is commonest between
30 and 50 years of age and progresses slowly up to death. The
kidneys are remarkably reduced in size. Histologically, the renal
disease is characterized by tubular degeneration, interstitial
fibrosis, and hyalization of glomeruli in the more superficial part
of the cortex (Heptinstall, 1966). Impairment of tubular function,
indicated by a decrease in TmPAH, is a prominent and early sign
(Dotchev, 1973).
The disease occurs endemically and affects females more often
than males (Hrabar et al., 1976, Chernozemsky et al., 1977). In
Bulgaria and Yugoslavia, a high incidence of urinary tract tumours
has been found to be closely correlated with the incidence and
mortality rates of Balkan endemic nephropathy (Ceovic et al., 1976;
Chernozemsky et al., 1977).
Fungal growth in foodstuffs and subsequent mycotoxin formation
is influenced by the water content of the foodstuffs, and can be
changed by climatic conditions such as heavy rainfalls during
harvest. Thus, the observation (Austwick, 1975) of a positive
correlation ( r = 0.80) between excess rainfall and the number of
people who died of nephropathy during the succeeding 2 years in the
Balkan peninsula might be interpreted as suggesting a fungal
involvement in the etiology of endemic nephropathy.
Attention has been called to the striking similarities in the
changes of renal structure and function found in Balkan endemic
nephropathy and in ochratoxin A-induced porcine nephropathy,
suggesting common causal relationships (Krogh, 1974). Furthermore,
epidemiological similarities have been noted, in particular, the
endemic occurrence (Krogh, 1976b). Preliminary results of a survey
of foodstuff's indicate that exposure to food-borne ochratoxin A
seems to be higher (12.8% contamination) in an area of Yugoslavia
with a high prevalence of human endemic nephropathy than in
nonendemic (control) areas (1.6% contamination) (Krogh et al.,
1977).
4.1.6 Conclusions and evaluation of the health risks to man
of ochratoxins
4.1.6.1 Experimental animal studies
The toxic effects of ochratoxin A have been studied extensively
in a variety of experimental animals. All the animals studied so far
have been susceptible to orally administered ochratoxin A, but to
various degrees, as indicated by the range of LD50 values
(Table 24). At high levels of ochratoxin A, changes were found in
the kidneys and also in other organs and tissues. However, only
renal lesions were observed at exposure levels identical to those
occurring environmentally. The renal lesions included degeneration
of the tubules, interstitial fibrosis, and, at later stages,
hyalinization of glomeruli, with impairment of tubular function as a
prime manifestation. Feed levels as low as 200 µg/kg produced renal
changes in the course of 3 months in rats and pigs. Field cases of
ochratoxin A-induced nephropathy are regularly encountered in pigs
and poultry. Ochratoxin A is teratogenic in the mouse, rat, and
hamster.
Ochratoxin B, rarely found as a natural contaminant, is much
less toxic; the other ochratoxins have never been encountered in
natural products.
4.1.6.2 Studies in man
The ochratoxin A-induced nephropathy in farm animals is similar
to Balkan endemic nephropathy in several aspects. In a preliminary
study in an area where Balkan endemic nephropathy is prevalent, the
ochratoxin A contamination of food appeared to be more frequent than
in control areas. However, the hypothesis that ochratoxin A may be a
causal determinant in this disease, needs further support.
4.1.6.3 Evaluation of health risks
The nephrotoxic potential of ochratoxin A is well documented
from all experimental studies, with a feed level of 200 µg/kg
causing nephropathy in pigs and rats. Lower levels have not been
tested. Field eases of ochratoxin A-induced nephropathy in farm
animals have long been recognized. The toxin has been found in a
variety of foodstuffs, with levels in commodities used as feed
ranging up to 27 mg/kg, and with levels in foodstuffs used for human
consumption in the range of trace to about 100 µg/kg. In one area
where endemic nephropathy was prevalent in the human population,
home produced foodstuffs were more frequently contaminated with
ochratoxin A than those from control areas. However, the total
intake of ochratoxin A by man has not been assessed so far, and
there is, at present, no proof that ochratoxin A is causally
involved in human diseases.
4.2 Zearalenone
4.2.1 Properties, analytical methods, and sources
The properties, analytical methods, sources, and occurrence of
zearalenone have been reviewed by Mirocha & Christensen (1974),
Pathre & Mirocha (1976), and Mirocha et al. (1977). Zearalenone is a
phenolic resorcylic acid lactone (Fig. 5), classified, according to
biosynthetic origin, as a nonaketide within the group polyketides
(Turner, 1971). Zearalenone (C18H22O5) is a white crystalline
compound with a relative molecular mass of 318, melting point
164-165 °C, and absorption maxima (and absorption coefficient) at
236 nm (29 700), 274 nm (13 909) and 316 nm (6020).
Zearalenone exhibits blue-green fluorescence when excited by
long wavelength (360 nm) UV-light, and a more intense green
fluorescence when excited with short wavelength (260 nm) UV-light.
A number of derivatives of zearalenone have been isolated from
fungal cultures (Fig. 7), but none of these derivatives has been
encountered, so far, as a natural contaminant of foodstuffs.
A multiple detection method for aflatoxin, ochratoxin, and
zearalenone has been developed (Eppley, 1968) and tested
collaboratively (Shotwell et al., 1976b). The procedure consists of
water-chloroform extraction combined with sequential elution of the
mycotoxins from a silica-gel column and the detection limit is in
the range of 50-100 µg/kg. A versatile method of analysis for
zearalenone has been described by Mirocha et al. (1974) using
thin-layer chromatography (TLC), gas-liquid chromatography (GLC),
gas-liquid chromatography-mass spectrometry, or a combination of all
these methods; the limit of detection is about 50 µg/kg. The
derivatives dimethoxyzearalenone and methyl oxime-di-TMS-zearalenone
are used to confirm the identity of zearalenone. Two methods using
high pressure liquid chromatography (HPLC) are now available (Scott
et al., 1978; Ware & Thorpe, 1978). With HPLC, a concentration of
zearalenone in cornflakes of 5 µg/kg could be determined (Scott et
al., 1978). Zearalenone is produced by strains of Fusarium
graminearum, F. tricinctum, F. oxysporum, F. sporotrichioides, and
F. moniliforme, and a period of low temperature ( 12°-14°C) during
fungal formation seems essential for high yield.
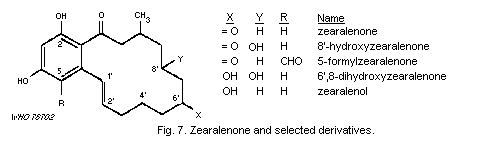 4.2.2 Occurrence
The occurrence of zearalenone in foodstuffs has been reviewed by
Stoloff (1976). Zearalenone has been encountered as a natural
contaminant, particularly in maize, but occasionally in other
cereals and in feedstuffs. In a survey of maize in the USA during
the period 1968-69, zearalenone was found in 6 out of 576 (1%)
samples at levels ranging from 450 to 800 µg/kg (Shotwell et al.,
1971). In 1972, when conditions in the USA were conducive to
Fusarium ear rot, zearalenone was found in 17% of 223 samples of
maize at levels ranging from 0.1 to 5.0 mg/kg (Eppley et al., 1974).
It has also been detected in maize in France and in barley and mixed
feed in England, Finland, and Yugoslavia (Stoloff, 1976).
Nine out of 11 commercial corn meal samples in the USA contained
zearalenone at levels ranging from 12 to 69 µg/kg (Ware & Thorpe,
1977). The compound has been found in one sample of cornflakes
(13 µg/kg) (Scott et al., 1978), and in maize beer, in Zambia, in
the range of 0.01-4.6 mg/litre (Lovelace & Nyathi, 1977).
Zearalenone was detected in 11% of 55 samples of Swazi sour
drinks, sour porridges, and beers (range: 8-53 mg/kg) and in 12% of
140 beer samples from Lesotho (range: 0.3-2 mg/kg), but such high
figures have not been reported elsewhere. No data on consumption
were given (Martin & Keen, 1978).
4.2.3 Effects in animals
4.2.3.1 Field observations
The effects of zearalenone in animals have been reviewed by
Mirocha & Christensen (1974). Field cases of the estrogenic syndrome
in pigs, associated with the use of mouldy feed, were first observed
half a century ago. The disease was characterized by enlarged
oedematous vulvae and mammary glands (McNutt et al., 1928).
Subsequently, this syndrome has been encountered in a number of
countries in North America and Europe and in Australia (Table 25),
and in most cases zemralenone has been identified in associated
feeds, indicating together with the result of experimental studies
(4.2.2.2) a causal role of this compound. Zearalenone feed levels
reported to be associated with the syndrome in swine (Mirocha et
al., 1977) are listed in Table 26. Cases of reduced fertility in
cattle indicated by an increase in the artificial insemination index
have been reported to be associated with a feed (hay) content of
zearalenone of 14 mg/kg (Mirocha et al., 1968b). Fertility
disturbances and prolonged heat in a herd of cattle suggested to be
associated with zearalenone-contaminated feed were reported by Roine
et al. (1971).
Table 25. Occurrence of the estrogenic syndrome in pigs in
various countriesa
Year Country Feedstuff
1928 USA maize
1937 Australia maize
1952 Ireland barley
1962 France maize
1962 Italy maize
1963 Yugoslavia maize and barley
1967 Romania maize
1968 Hungary maize
1968 Denmark barley
1971 Canada maize
a From: Mirocha & Christenson (1974).
Table 26. Natural occurrence of zearalenone in feeds associated with
hyperestrogenism in swinea
Feed sample Level of
contamination
(mg/kg)
maize kernels (Minnesota)b 0.1-0.15
dry sow ration (Vancouver) 0.15
farrowing ration (Vancouver)c 0.066
dry sow ration (Vancouver) 0.15
corn kernels (Vancouver) 0.20
dry sow ration (Vancouver)c 0.25
lactation ration (Vancouver) 1.00
gestation ration (Vancouver) 0.6
milo (Minnesota) 2.5-5.6
sesame meal (Univ. of Minn.)d 1.5
corn kernels (Ohio) 0.12
mixed feed corn (Ohio)b 0.12
corn kernels (Minnesota) 6.4
commercial pelletted mixed feed 6.8
(Minnesota)
a From Mirocha et al. (1977).
b Rectal prolapse in gilts.
c Diethylstilbestrol was also present in these samples.
d Associated with hyperestrogenism in turkey poults.
4.2.3.2 Experimental studies
Under experimental conditions, pigs (6-week-old-gilts) exposed
perorally to zearalenone at 5 mg per animal per day for 5 days
developed enlarged vulvae and mammae, and prolapse of the vagina
within a few days; effects were reversible on termination of
exposure. In another experiment, oral administration of 8 mg of pure
crystalline zearalenone to 6-week-old, pre-pubertal gilts (8 doses
of 1 mg/day per animal) induced pronounced tumefaction of the vulva
(Mirocha & Christensen, 1974). Histological changes in the genital
tract of pigs exposed to zearalenone included metaplasia of the
epithelium in the cervix and vagina, and oedema in the wall of the
uterus (Kurtz et al., 1969).
Observations on pigs (Miller et al., 197.3) suggested that
ingestion of grain contaminated with zearalenone during late
gestation might be related to still-birth and splayleg. Splayleg was
observed in the offspring of one sow and one gilt given dally
intramuscular injections of zearalenone at 5 mg per animal
throughout the last month of pregnancy. However, in the experiment
of Patterson et al. (1977), all 7 gilts fed zearalenone at a level
of 2 mg/kg feed throughout pregnancy remained clinically normal and
embryonic survival rates were not affected by the toxin. Splayleg
was diagnosed in only 1/63 piglets and the condition appeared to
have resolved within 24 h. Results in the 2 groups of 3 pigs fed the
lower levels were inconclusive (unpublished data, Chang & Kurtz,
quoted by Mirocha et al., 1977).
No effects on egg production were observed when laying hens were
fed rations containing zearalenone levels of 250 and 500 mg/kg
(Speers et al., 1971).
In a 2-generation study on rats exposed to dietary levels of
zearalenone corresponding to daily intakes of 0.1, 1.0, and 10 mg/kg
body weight, no teratogenic effects were observed at any level of
intake but impaired fertility and resorptions and stillbirths
occurred in animals receiving 10 mg/kg body weight daily with 56% of
dams showing complete litter resorption (Bailey et al., 1976).
Ruddick et al. (1976) found fetal skeleton anomalies in rats
exposed orally to zearalenone at doses ranging from 1-10 mg/kg body
weight during the gestation period, with defect incidences of 12.8%
(11/86) at the 1 mg/kg level, 26.1% (18/69) at the 5 mg/kg level,
and 36.8% (28/76) at the 10 mg/kg level. No effect was observed with
exposure to zearalenone at a dose of 0.075-0.30 mg/kg body weight.
The results of a study by Mirocha et al. (1968a) in which the
dose-effect relationships for zearalenone and estrone were compared
with regard to increase in uterus weight, are given in Table 27. The
results of a study comparing the effects of estrogens and
zearalenone in 24 adult female castrated rhesus monkeys with a
previous history of regular menstrual cycles are recorded in Table
28.
Table 27. Effect of peroral dosing of zearalenone and estrone in micea
Material Total dose No. of Mean uterine
administered (µg) mice ratio ± S.E.
control 0 10 0.76 ± 0.06
estrone 0.5 10 1.21 ± 0.12
1 9 1.19 ± 0.10
2 9 2.12 ± 0.23
4 10 2.93 ± 0.21
zearalenone 12.5 10 1.11 ± 0.04
25 10 1.48 ± 0,18
50 10 1.80 ± 0.12
100 9 2.35 ± 0.17
a From: Mirocha et al. (1968a).
4.2.4 Conclusions and evaluation of the health risks to man of
zearalenone
4.2.4.1 Animal studies
Field cases of the estrogenic syndrome in pigs have been
encountered in many countries in association with zearalenone in
feeds at levels ranging from 0.1 to 6.8 mg/kg (Table 26), in samples
not known to be contaminated with other estrogens. The condition has
been reproduced experimentally in pigs, with a dally dose of
1 mg/animal for 8 days. Infertility, sporadically encountered in
cattle, has been suggested to be causally associated with
zearalenone in feed at a reported level of 14 mg/kg of hay.
In one of two studies on teratogenic effects in rats, skeletal
defects were detected in fetuses at a daily oral dose of 1 mg/kg
body weight.
Table 28. The estimated minimum dose of estrogens that will depress
serum gonadotropin (FSH or LH) in castrated rhesus monkeysa
Estrogen administered Estimated minimum dose
(µg/kg)
Subcutaneousb Oralc
LHd FSHd LH FSH
estradiol-17ß 4 1 5 --
diethylstilboestrol 0.5 2 2.5 --
zearalenone 14 56 400 --
a From: Hobson et al. (1977).
b Two injections given in oil.
c Given on 4 consecutive days,
d FSH = follicle-stimulating hormone; LH = luteinizing hormone.
4.2.4.2 Evaluation of health risks
There are no reports on the adverse effects of zearalenone in
man. With the exception of 2 reports from Africa, levels of
zearalenone ranging from 12 to 69 µg/kg have been found in a limited
number of maize products destined for human consumption. Even
assuming that 1 kg of these products is consumed dally, it can be
estimated that a 70 kg man would not receive more than 1 µg
zearalenone per kg body weight in food. This level of exposure is
400 times lower than the lowest peroral dose causing effects in
tests on monkeys (Table 28) and more than 600 times lower than the
lowest dose (µg/kg) used in assessing the estrogenic potency of
perorally administered zearalenone in mice (Table 27).
However, in the 2 reports from certain parts of Africa, high
levels of zearalenone were found in beer and sour porridge prepared
from maize and sorghum. Long-term exposure to such contaminated
drinks could represent a health hazard.
4.3 Trichothecenes
4.3.1 Properties and sources
The properties and sources of trichothecenes have been reviewed
by Bamburg & Strong (1971), Smalley & Strong (1974), and Bamburg
(1976). The trichothecenes possess the tetracyclic
12,13-epoxytrichothec-9-ene skeleton. More than 30 trichothecene
derivatives have been isolated from fungal cultures, but, so far,
only 4 have been identified as natural contaminants of foodstuffs
(Table 29). The compounds have been detected by various methods
including thin-layer chromatography, gas chromatography, and
bioassays, in particular the rabbit skin test.
One or more of the trichothecenes have been isolated from
strains of the following Fusarium species: F. episphae,
F. lateritium, F. nivale, F. oxysporum, F. rigidisculum,
F. solani, F. roseum, and F. tricinctum (syn.
F. sporotrichioides). In addition, species of Cephalosporium,
Myrothecium, Trichoderma, and Stachybotrys have been found to
produce trichothecenes.
Table 29. Natural occurrence of trichothecenes
Compound Concentration Commodity Reference
(µg/kg)
T-2 toxin 2 maize Hsu et al. (1972)
T-2 toxin 25 barley Puls & Greenway (1976)
T-2 toxin 0.076 mixed feed Mirocha et al. (1976)
nivalenol n.q.a barley Morooka et al. (1972)
deoxynivalenol 7.3 barley Morooka et al, (1972)
deoxynivalenol n.m.b maize Vesonder et al. (1973)
deoxynivalenol 0.1, 1.0, 1.8 maize Mirocha et al. (1976)
(3 samples)
deoxynivalenol 1.0, 1.0, 0.06 mixed feed Mirocha et al. (1976)
(3 samples)
diacetoxyscirpenol 0.38, 0.5 mixed feed Mirocha et al. (1976)
(2 samples)
a n.q. = not quoted
b n.m. = not measured
4.3.2 Occurrence
So far, the trichothecenes have only been found very
sporadically in natural products. Naturally occurring trichothecenes
include the following, based on chemical identification: T-2 toxin,
nivalenol, deoxynivalenol, (vomitoxin), and diacetoxyscirpenol. In
available studies, these 4 compounds have only been found in a total
of 14 samples (Table 29), sometimes (as in the case of
deoxynivalenol) concomitantly with zearalenone. No information is
available concerning the number of samples analysed in these studies
and therefore on the frequency of positive results.
4.3.3 Effects in animals
4.3.3.1 Field observations
A field case involving the death of 20% of a dairy herd was
suggested to be associated with ingestion of mouldy corn in the feed
containing a concentration of T-2 toxin of approximately 2 mg/kg dry
weight (Hsu et al., 1972). The lesions in the cattle included
extensive haemorrhages on the serosal surface of all internal
viscera.
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was suggested to be associated with mouldy barley
containing T-2 toxin at approximately 25 mg/kg (Greenway & Puls,
1976). The lesions in the geese included necrosis of the mucosa of
the oesophagus, proventriculus, and gizzards.
Deoxynivalenol (vomitoxin) was isolated from a batch of maize
that had caused vomiting in pigs (Vesonder et al., 1973).
4.3.3.2 Experimental studies
Acute effects of trichothecenes determined as LD50 values, are
listed in Table 30. Sato et al. (1975) studied tile effects in cats
of T-2 toxin, administered in several ways. Two cats weighing 1.5
and 4.0 kg were given purified T-2 toxin in single subcutaneous
doses of 0.5 and 1.0 mg/kg, respectively. Nausea and vomiting
appeared after 1 h and the animals died 20 h after the injection.
The autopsy revealed extensive necrosis of the mucosa in the small
and large intestine, marked karyorrhexis in the germ centre of the
lymph follicles of the spleen and lymph nodes, and diffuse vacuolar
degeneration of the renal tubules. Damage to bone marrow cells was
observed in the cervical, thoracic, and lumbar vertebrae. In two
cats subcutaneously injected with T-2 toxin (repeated doses of 0.1
and 0.05 mg/kg body weight, given over a 4-week period) a decrease
in the white blood cell (WBC) count was observed and the WBC value
remained low until death occurred during the fourth week of
exposure. The clinical signs in the cats were nausea and vomiting a
few hours after each injection and ataxia of the hind legs. At the
postmortem examination, hypoplasia was observed in the thymus,
spleen, lymph nodes, and bone marrow. Other changes included marked
meningeal haemorrhage, extensive bleeding in the lung, and vacuolar
degeneration of the renal tubular epithelium. A marked decrease in
the WBC values was also observed in 3 cats given T-2 toxin
subcutaneously at a daily dose of 0.05 mg/kg for 12 days (i.e.,
total dose of 0.60 mg/kg); 2 of these cats died, 4 and 35 days,
respectively, after the last injection and one cat survived with the
WBC count returning to the normal level 17 days after the last
injection. Leukopenia and death were also observed in 3 cats (body
weight 2-3 kg) receiving a crude preparation containing 4% T-2 toxin
and 1% neosolaniol. This crude preparation was first administered
subcutaneously once a week for 5 weeks in repeated doses of 1 mg/kg
body weight (corresponding to a repeated dose of T-2 toxin of
0.04 mg/kg). This subcutaneous administration was then followed by
daily oral dosing (15 mg of the crude preparation corresponding to
0.6 mg of T-2 toxin per animal per day) for 17 days.
Table 30. Acute toxicity of naturally occurring trichothecenesa
Compound LD50 Route of Animal
(mg/kg body weight) administration
T-2 toxin 3.04 intraperitoneal mouse
T-2 toxin 3.8 peroral rat
T-2 toxin 5.1 peroral trout
T-2 toxin 5.25 peroral one-day old chickb
nivalenol 4.0 intraperitoneal mouse
diacetoxyscirpenol 10.0 intravenous mouse
diacetoxyscirpenol 0.75 intraperitoneal rat
diacetoxyscirpenol 7.3 peroral rat
a From: Bamburg & Strong (1971 ).
b From: Chi at al. (1977).
More recently Yagen et al. (1978) have mentioned an experiment,
in which oral administration of gelatin capsules containing purified
T-2 toxin to 10 cats resulted in vomiting, leukopenia, haemorrhagic
diathasis, neurological disturbances, and death. The dose and
frequency of administration are not given in the paper.
The effects observed in these experiments, in particular
leukopenia, resemble those produced when cats are fed cultures of
F. sporotrichioides, which is thought to be causally associated
with alimentary toxic aleukia (ATA) in man (section 4.3.4).
Feeding laying hens T-2 toxin at a dietary level of 20 mg/kg for
3 weeks, resulted in oral necrotic lesions, decreased leukocyte
count, and reduced egg production (Wyatt et al., 1975). In mice,
intraperitoneal injection with T-2 toxin at doses of 1.0 and
1.5 mg/kg body weight on one of the days 7-11 of gestation resulted
in a number of maternal deaths as well as in an increase in prenatal
mortality. Malformations of the fetuses were observed including tail
and limb malformations, exencephaly, and retarded jaw development
(Stanford et al., 1975).
Daily oral doses of crude or purified T-2 toxin (0.2 mg/kg body
weight) continued for 79 days failed to produce ill effects in
calves, although the total amount of toxin ingested by one calf was
almost 1.8 g (Matthews et al., 1977).
4.3.4 Alimentary toxic aleukia
Studies on alimentary toxic aleukia (ATA), a disease encountered
in man in the period 1931-43, have been reviewed by Sarkisov (1954)
and more recently by Bilai (1977) and Leonov (1977). The dominant
pathological changes were necrotic lesions of the oral cavity, the
oesophagus, and stomach, and in particular a pronounced leukopenia.
The disease was lethal in a high proportion of cases. An association
was established with ingestion of grain invaded by some moulds, in
particular Fusarium poae and F. sporotrichioides. Effects similar
to ATA have been reproduced in cats by feeding cultures of these
species (Bilai, 1977). T-2 toxin and other trichothecenes have been
identified in a submerged culture of F. sporotrichioides by
Mirocha & Pathre (1973).
4.3.5 Conclusions and evaluation of the health risks to man of
trichothecenes
In recent years, a group of mycotoxins, the trichothecenes, has
been isolated under experimental conditions from many fungi,
including Fusarium species. Isolated field cases of intoxication
in farm animals have been suggested to be related to some of the
trichothecenes.
About 40 years ago, a disease in man known as alimentary toxic
aleukia (ATA), occurred that was suggested to be related to the
presence of toxic Fusarium species in mouldy over-wintered grain.
With improved harvesting, food production, and storage conditions,
the disease disappeared and no new outbreaks have occurred. However,
present knowledge is not sufficient to establish a causal
relationship between any of the isolated trichothecenes and this
outbreak. There are no reports on the exposure of man to
trichothecenes.
REFERENCES
ADAMSON, R. H., CORREA, P., & DALGARD, D. W. (1973) Brief
communication: Occurrence of a primary liver carcinoma in a rhesus
monkey fed aflatoxin B1. J. Natl Cancer Inst., 50:549-551.
ADAMSON, R. H., CORREA, P., SIEBER, S. M., MCINTIRE, K. R., &
DALGARD, D. W. (1976) Carcinogenicity of aflatoxin B1 in rhesus
monkeys: Two additional cases of primary liver cancer. J. Natl
Cancer Inst., 57: 67-78.
ADENKUNLE, A. A. & ELEGBE, R. A. (1974.) Lysosomal activity in
aflatoxin-B1-treated avian embryos. Lancet, 1: 991.
ALLCROFT, R. (1969) Aflatoxicosis in farm animals. In: Goldblatt,
L.A., ed. Aflatoxin scientific background, control and
implications, New York, Academic Press, pp. 237-264.
ALLCROFT, R. & LEWIS, G. (1963) Groundnut toxicity in cattle:
Experimental poisoning of calves and a report on clinical effects in
older cattle. Vet. Rec., 75: 487-493.
ALLCROFT, R. & ROBERTS, B. A. (1968) Toxic groundnut meal: The
relationship between aflatoxin B1 intake by cows and excretion of
aflatoxin M1 in milk. Vet. Rec., 82: 116-118.
ALLCROFT, R., ROBERTS, B. A., & LLOYD, M. K. (1968) Excretion of
aflatoxin in a lactating cow. Food Cosmet. Toxicol., 6: 619-625.
ALPERT, g. E., HUTT, g. S. R., WOGAN, G. N., & DAVIDSON, C. S.
(1971) Association between aflatoxin content of food and hepatoma
frequency in Uganda. Cancer, 28: 253-260.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973)
Carcinogens are mutagens: A simple test system combining liver
homogenates for activation and bacteria for detection. Proc. Natl
Acad. Sci. USA, 70: 2281-2285.
AMLA, I., KAMALA, C. S., GOPALAKRISHNA, G. S., JAYARAJ, A. P.,
SREENIVASAMURTHY, V., & PARPIA, H. A. B. (1971) Cirrhosis in
children from peanut meal contaminated by aflatoxin. Am. J. clin.
Nutr., 24: 609-614.
ANDERSON, H. W., NEHRING, E. W., & WICHSER, W. R. (1975) Aflatoxin
contamination of corn in the field. J. agric. food Chem., 23:
775-782.
ANDRELLOS, P. J. & REID, G. R. (1964) Confirmatory tests for
aflatoxin B1. J. Assoc. Off. Anal. Chem., 47: 801-803.
AMBRECHT, B. H., SHALKOP, W. T., ROLLINS, L. D., POHLAND, A. E., &
STOLOFF, L. (1970) Acute toxicity of aflatoxin B1 in wethers.
Nature (Lond.), 225: 1062.
ASAO, T., BUCHI, G., ABDEL-KADER, M., CHANG, S. B., WICK, E. L., &
WOGAN, G. N. (1963) Aflatoxins B and G. J. Am. Chem. Soc., 85:
1706-1707.
ASAO, T., BUCHI, G., ABDEL-KADER, M. M., CHANG, S. B., WICK, E. L.,
& WOGAN, G. N. (1965) The structures of aflatoxins B and G1. J.
Am. Chem. Soc., 87: 882-886.
ASHWORTH, L. J., JR, MCMEANS, J. L., HOUSTON, B. R., WITTEN, M. E.,
& BROWN, C. M. (1971) Mycoflora, aflatoxins and free fatty acids in
California cottonseed during 1967-1968. J. Am. Oil Chem. Soc., 48:
129-133.
ASPLIN, F. D. & CARNAGHAN, R. B. A. (1961) The toxicity of certain
groundnut meals for poultry with special reference to their effect
on ducklings and chickens. Vet. Rec., 73: 1215-1218.
AUSTWICK, P. K. C. (1975) Balkan nephropathy. Proc. R. Soc. Med.,
68: 219-221.
BABABUNMI, E. A. & BASSIR, O. (1972) Effects of aflatoxin B1 on
the swelling and adenosine triphosphatase activities of mitochondria
isolated from different tissues of the rat. Febs Lett., 26:
102-104.
BACON, C. W., SWEENEY, J. G., ROBBINS, J. D., & BURDICK, D. (1973)
Production of penicillic acid and ochratoxin A on poultry feed by
Aspergillus ochraceus: Temperature and moisture requirements.
Appl. Microbiol., 26: 155-160.
BAGSHAWE, A. F., GACENGI, D. M., CAMERON, C. H., DORMAN, J., & DANE,
D. S. (1975) Hepatitis Bs antigen and liver cancer in Kenya.
Br. J. Cancer, 31: 581-584.
BAILEY, D. E., Cox, G. E., MORGAREIDGE, K., & TAYLOR, J. (1976)
Acute and subacute toxicity of zearalenone in the rat. TAP, 37:
144.
BAMBURG, J. R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American
Chemical Society, pp. 144-162 (Advances in Chemistry Series 149).
BAMBURG, J. R. & STRONG, F. M. (1971) 12,13-epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S. J., ed. Microbial toxins, New
York, Academic Press, Vol. VII, pp. 207-289.
BASSIR, O. & BABABUNMI, E. A. (1969) Inhibition of blood clotting
factors by aflatoxin. West Aft. J. biol. appl. Chem., 12: 28-32.
BASSIR, O. & OSIYEMI, F. (1967) Biliary excretion of aflatoxin in
the rat after a single dose. Nature (Lond.), 215: 882.
BECKWITH, A. C., VESONDER, R. F., & CIEGLER, A. (1975) Action of
weak bases upon aflatoxin B1 in contact with macromolecular
reactants. J. agric. food Chem., 23: 582-587.
BECROFT, D. M. O. (1966) Syndrome of encephalopathy and fatty
degeneration of viscera in New Zealand children. Br. med. J., 2:
135-140.
BECROFT, D. M. O. & WEBSTER, D. R. (1972) Aflatoxins and Reye's
disease. Br. med. J., 14 Oct., p. 117.
BILAI, V. I. (1977) [Fuzarii.] Kiev, Naukova Dumka, pp. 360-394 (in
Russian).
BLACK, H. S., SMITH, J. D., AUSTIN, B. J., & RAUSCHKOLB, E. W.
(1970) Effect of aflatoxin B1 on the in vitro incorporation of
14C-acetate into human skin lipids. Experientia (Basel), 26:
1292-1293.
BOURGEOIS, C. H. (1975) Encephalopathy and fatty viscera: A possible
response to acute aflatoxin poisoning. In: Pollack, J. D., ed.
Reye's Syndrome, Grune & Stratton, New York, pp. 131-134.
BOURGEOIS, C. H., SHANK, R. C., GROSSMAN, R. A., JOHNSEN, D. O.,
WOODING, W. L., & CHANDAVIMOL, P. (1971) Acute aflatoxin B1
toxicity in the macaque and its similarities to Reye's Syndrome.
Lab. Invest., 24: 206-216.
BROWN, J. M. M. & ABRAMS, L. (1965)Biochemical studies on
aflatoxicosis. Onderstepoort J. vet. Res., 32: 119-146.
BROWN, M. H., SZCZECH G. M., & PURMALIS, B. P. (1976) Teratogenic
and toxic effects of ochratoxin A in rats. Toxicol. appl.
Pharmacol., 37: 331-338.
BUCARBAEVA, A. S. & NIKOV, P.S. (1977) [Aflatoxin sources in
foodstuffs from different regions of Kazachstan.] In: [ Mycotoxins
(sources, chemistry, biosynthesis, analysis and effects on the
organism), Scientific Papers of a Regional Symposium, 28-30
September 1977.] Orenburg, Nutrition Institute of the USSR Academy
of Medical Sciences, pp. 29-31 (in Russian).
BUCHI, G. & WEINREB, S. M. (1969) The total synthesis of racemic
aflatoxin-M1 (milk toxin). J. Am. Chem. Soc., 91: 5408-5409.
BUCHI, G., SPITZNER, D., PAGLIALUNGA, S., & WOGAN, G. N. (1973)
Synthesis and toxicity evaluation of aflatoxin P1. Life Sci.,
13: 1143-1149.
BUCKLEY, H. G. (1971) Fungal nephrotoxicity in swine. Irish
vet. J., 25: 194-196.
BULATAO-JAYME, J., ALMERO, E. M., & SALAMAT, L,. (1976) Epidemiology
of primary liver cancer in the Philippines with special
consideration of a possible aflatoxin factor. J. Philipp. Med.
Assoc., 52: 129-150.
BUTLER, W. H. (1964) Acute toxicity of aflatoxin B1 in rats.
Br. J. Cancer, 18: 756-762.
BUTLER, W. H. (1966) Acute toxicity of aflatoxin B1 in guinea
pigs. J. Pathol. Bacteriol., 91: 277-280.
BUTLER, W. H. (1969) Aflatoxicosis in laboratory animals. In:
Goldblatt, L.A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 223-236.
BUTLER, W. H. (1971-1972) Further ultrastructural observations on
injury of rat hepatic parenchymal cells induced by aflatoxin B1.
Chem. biol. Interactions, 4: 49-65.
BUTLER, W. H. (1974) Aflatoxin. In: Purchase, I. F. H., ed.
Mycotoxins, Amsterdam, Elsevier, pp. 1-28.
BUTLER, W. H. & BARNES, J. M. (1963) Toxic effects of groundnut meal
containing aflatoxin to rats and guinea pigs. Br. J. Cancer, 17:
699-710.
BUTLER, W. H. & BARNES, J. M. (1966) Carcinoma of the glandular
stomach in rats given diets containing aflatoxin. Nature (Lond.),
209: 90.
BUTLER, W. H. & WIGGLESWORTH, J. S. (1966) The effects of aflatoxin
B1 on the pregnant rat. Br. J. exp. Pathol., 47: 242-247.
BUTLER, W. H., GREENBLATT, M., & LIJINSKY W. (1969) Carcinogenesis
in rats by aflatoxins B1, G1, and B2. Cancer Res., 29:
2206-2211.
CAMPBELL, T. C. & SALAMAT, L. (1971) Aflatoxin ingestion and
excretion by humans. In: Purchase, I. F. H., ed. Mycotoxins in human
health, London, Macmillan Press, pp. 271-280.
CAMPBELL, T. C. & STOLOFF, L. (1974) Implication of mycotoxins for
human health. J. agric. food Chem., 22: 1006-1015.
CAMPBELL, T. C., CAEDO, J.P., JR, BULATAO-JAYME, J., SALAMAT, L., &
ENGEL, R. W. (1970) Aflatoxin M1 in human urine. Nature (Lond.),
227: 403-404.
CARNAGHAN, R. B. A. (1965) Hepatic tumours in ducks fed on low level
of toxic ground-nut meal. Nature (Lond.), 208, 308.
CARNAGHAN, R. B. A. (1967) Hepatic tumours and other chronic liver
changes in rats following a single oral administration of aflatoxin.
Br. J. Cancer, 21: 811-814.
CARNAGHAN, R. B. A., HARTLEY, R. D., & O'KELLY, J. (1963) Toxicity
and fluorescence properties of the aflatoxins. Nature (Lond.),
200: 1101.
CARNAGHAN, R. B. A., LEWIS, G., PATTERSON, D. S. P., & ALLCROFT, R.
(1966) Biochemical and pathological aspects of groundnut poisoning
in chickens. Pathol. Vet., 3: 601-615.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijee vjesn., 98: 301-304 (in Croatian).
CHAINUVATI, T., VIRANUVATT, V., & PONGPIPAT, D. (1975) Relationship
of hepatitis B antigen in cirrhosis and hepatoma in Thailand. An
etiological significance. Gastroenterology, 68: 1261-1264.
CHANG, F. C. & Car, F. S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, S. B., ABDEL-KADER, M. M., WICK, E. L., & WOGAN, G. N. (1963)
Aflatoxin B2: Chemical identity and biological activity.
Science, 142: 1191-1192.
CHAVES-CARBALLO, E., ELLEFSON, R. D., & GOMEZ, M. R. (1976) An
aflatoxin in the liver of a patient with Reye-Johnson syndrome.
Mayo Clin. Proc., 51: 48-50.
CHERNOZEMSKY, I. N., STOYANOV, I. S., PETKOVA-BOCHAROVA, T. K.,
NICOLOV, I. G., DRAGANOV, I. V., STOICHEV, I. I., TANCHEV, Y.,
NAIDENOV, D., & KALCHEVA, W. D. (1977) Geographic correlation
between the occurrence of endemic nephropathy and urinary tract
tumours in Vratza district, Bulgaria. Int. J. Cancer, 19: 1-11.
CHI, M. S., MIROCHA, C. J., KURTZ, M. Y., WEAVER, G., BATES, F., SHIMODA,
W., & BURMEISTER, H. R. (1977) Acute toxicity of T-2 toxin in
broiler chicks and laying hens. Poult. Sci., 56: 103-116.
CHU, F. S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F. S. (1974a) Studies on ochratoxins. CRC crit. Rev.
Toxicol., 2: 499-524.
CHU, F. S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol.,
23: 1105-1113.
CIEGLER, A., KADIS, S., & AJL, S. J., ed. (1971) Microbial toxins,
fungal toxins, New York, Academic Press, Vol. VI, p. 563.
CLEGG, F. G. & BRYSON, H. (1962) An outbreak of poisoning in store
cattle attributed to Brazilian groundnut meal. Vet. Rec., 37:
992-994.
CLIFFORD, J. I. & REES, K. R. (1966) Aflatoxin: A site of action in
the rat liver cell. Nature (Lond.), 209: 312-313.
CLIFFORD, J. I. & REES, K. R. (1967) The action of aflatoxin B, on
the rat liver. Biochem. J., 102: 65-75.
COON, F. B., BAUR, F. J., & SYMMES, L. R. L. (1972) International
aflatoxin check sample program: 1971 study. J. Assoc. Off. Anal.
Chem., 55: 315-327.
CUTHBERTSON, W. F. J., LAURSEN, A. C., & PRATT, D. A. H. (1967)
Effect of groundnut meal containing aflatoxin on Cynomolgus monkeys.
Br. J. Nutr., 21: 893-908.
DALEZIOS, J. I. & WOGAN, G. N. (1972) Metabolism of aflatoxin B1
in rhesus monkeys. Cancer Res., 32: 2297-2303.
DALEZIOS, J. I., WOGAN, G. N., & WEINREB, S. M. (1971) Aflatoxin
P1: A new aflatoxin metabolite in monkeys. Science, 171:
584-585.
DAVIS, N. D. & DIENER, U. L. (1970) Environmental factors affecting
the production of aflatoxin. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on "Toxic Microorganisms",
Washington, DC, US Govt Printing Office, pp. 43-47.
DEGER, G. E. (1976) Aflatoxin-Human colon carcinogenesis? Ann.
int. Med., 85: 204.
DE IONGH, H., BEERTHUIS, R. K., VLES, R. O., BARRETT, C. B., & ORD,
W. O. (1962) Investigation of the factor in groundnut meal
responsible for turkey X disease. Biochim. Biophys. Acta, 65:
548-551.
DEO, M. G., DAYAL, Y., & RAMALINGASWAMI, V (1970) Aflatoxins and
liver injury in the rhesus monkey. J. Pathol., 101 47-56.
DETROY, R. W. & HESSELTINE, C. W. (1970) Aflatoxicol: Structure of a
new transformation product of aflatoxin B1 Can. J. Biochem.,
48: 830-832.
DICKENS, J. W. & SATTERWHITE, J. B. (1973) Aflatoxin-contaminated
peanuts produced on North Carolina farms in 1968. J. Am. Peanut
Res. Educ. Assoc. Inc., 5: 11 pp.
DICKENS, F., JONES, H. E. H., & WAYNFORTH, H B. (1966) Oral
subcutaneous and intra-tracheal administration of carcinogenic
lactones and related substances: The intratracheal administration of
cigarette tar in the rat. Br. J. Cancer, 20: 134-144.
DIENER, U. L. & DAVIS, N. D. (1969) Production of aflatoxins on
peanuts under controlled environments. J. stored Prod. Res.,
5: 251-258;.
DI PAOLO, J. A., ELIS, J., & ERWIN, H. (1967) Teratogenic response
by hamsters, rats and mice to aflatoxin B1. Nature (Lond.), 215:
638-639.
DOHERTY, W. P. & CAMPBELL, T. C. (1972) Inhibition of rat-liver
mitochondria electron-transport flow by aflatoxin B1. Res.
Commun. chem. Path. Pharmacol., 3: 601-612.
DOHERTY, W. P. & CAMPBELL, T. C. (1973) Aflatoxin inhibition of rat
liver mitochondria. Chem. biol. Interactions, 7: 63-77.
DONALDSON, W. E., TUNG, H-T., & HAMILTON, P. B. (1972) Depression of
fatty acid synthesis in chick liver (Gallus domesticus) aflatoxin.
Comp. Biochem. Physiol., 41: 843-847.
DOSTER, R. C., SINNHUBER, R. O., & WALES, J. H. (1972) Acute
intraperitoneal toxicity of ochratoxins A and B in rainbow trout
(Salmo gairdneri). Food Cosmet. Toxicol, 10: 85-92.
DOTCHEV, V. D. (1973) [The endemic (Balkan) nephropathy in
Bulgaria.] Muench. med. Woenschr., 115: 537-541 (in German).
DVORACKOVA I. (1976) Aflatoxin inhalation and alveolar cell
carcinoma. Br. med. J., 20: 691.
DVORACKOVA I., BRODSKY, F., & GERMAN, J. (1974) Aflatoxin and
encephalitic syndrome with fatty degeneration of viscera. Nutr.
Rep. Int., 10: 89-102.
DVORACKOVA I., KUSAK, V., & NESNIDAL, P. (1977) Aflatoxin and
encephalopathy with fatty degeneration of viscera (Reye). Ann.
Nutr. Ailment., 31: 977-989.
EDDS, G. T., NAIR, K. P. C., & SIMPSON, C. F. (1973) Effects of
aflatoxin B1 on resistance in poultry against cecal coccidiosis
and Marek's disease. Am. J. vet. Res., 34: 819-826.
ELIS, J. & DI PAOLO, J. A. (1967) Aflatoxin B1. Arch. Pathol., 83:
53-57.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
Worm Health Organ., 49: 411-418.
ELLING, F., HALO, B., JACOBSEN, CHR., & KROGH, P. (1975) Spontaneous
toxic nephropathy in poultry associated with ochratoxin A. Acta
path. microbiol. Scand. Sect. A, 85: 739-741.
EPPLEY, R. M. (1966) A versatile procedure for assay and preparatory
separation of aflatoxins from peanut products. J. Assoc. Off.
Anal. Chem, 49: 1218-1223.
EPPLEY, R. M. (1968) Screening method for zearalenone, aflatoxin,
and ochratoxin. J. Assoc. Off. Anal. Chem, 51: 74-78.
EPPLEY, R. M., STOLOFF, L., TRUCKSESS, M. W., & CHUNG, C. W. (1974)
Survey of corn for Fusarium toxins. J. Assoc. Off. Anal. Chem,
57: 632-635.
EPSTEIN, S. M., BARTUS, B., & FARBER, E. (1969) Renal epithelial
neoplasms induced in male Wistar rats by oral aflatoxin B1. Cancer
Res., 29: 1045-1050.
FAO (1977) Mycotoxins. Report of the joint FAO/WHO/UNEP Conference
on Mycotoxins, Nairobi, 19-27 September, 1977, Rome, Food and
Agriculture Organization of the United Nations, 105 pp. (FAO Food
and Nutrition Paper 2).
FDA (1977) Report on action level for aflatoxin M1 in milk.
Washington DC, 21 November, Bureau of Foods, US Food and Drug
Administration.
FDA (1978) Assessment of estimated risk resulting from aflatoxins
in consumer peanut products and other food commodities.
Washington, DC, 19 January, Bureau of Foods, US Food and Drug
Administration.
FEUER, G, GOLBERG, L., & LEPELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose-6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FRIEDMAN, L. & MOHR, H. (1968) Absence of interaction between
cyclopropenoid fatty acids and aflatoxin in rats. Fed. Proc., 27:
551 (Abstract no. 1882).
FRITZ, W., DONATH, R., & ENGST, R. (1977) [Determination and
occurrence of aflatoxin M1 and B1 in milk and dairy produce.]
Nahrung, 21: 79-84 (in German).
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport san Ruin de la toxine chez le rat. Ann. Rech.
vet., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal II. Distribution tissulaire et élimination chez le rat. Ann.
Rech. vet., 5: 319-328.
GALTIER, P. (1975) Metabolism et mode d'action d'ochratoxine A. In:
Biquet, J. & Jemmali, M., ed. Les Mycotoxines, Paris, Editions
INSERM, pp. 69-78.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vet., 7:
91-98.
GALTIER, P., MORÉ., J., & BODIN, G. (1974) Toxine d' Aspergillus
ochraceus Wilhelm. III. Toxicité aigue de l'ochratoxine A chez le
rat et la souris adultes. Ann. Rech. vet., 5: 233-247.
GARNER, R. C. (1973) Chemical evidence for the formation of a
reactive aflatoxin B1 metabolite, by hamster liver microsomes.
Febs Lett., 36: 261-264.
GARNER, R. C. (1975) Reduction in binding of [14C]aflatoxin B1
to rat liver macromolecules by phenobarbitone pretreatment.
Biochem. Pharmacol., 24: 1553-1556.
GARNER, R. C., MILLER, E. C., MILLER, J. A., GARNER, J. V., &
HANSON, R. S. (1971) Formation of a factor lethal for S. typhimurium
TA 1530 and TA 1531 on incubation of aflatoxin B1 with rat liver
microsomes. Biochem. biophys. res. Comm., 45: 774-780.
GARNER, R. C., MILLER, E. C., & MILLER, J. A. (1972) Liver
microsomal metabolism of aflatoxin B1 to a reactive derivative
toxic to Salmonella typhimurium TA 1530. Cancer Res., 32:
2058-2066.
GODOY, H. M., JUDAH, D. J., ARORA, H. L., NEAL, G. E., & JONES, G.
(1976) The effects of prolonged feeding with aflatoxin B1 on adult
rat liver. Cancer Res., 36: 2399-2407.
GOLDBLATT, L. E., ed. (1969) Aflatoxin; scientific background,
control, and implications, New York, Academic Press, pp. 472.
GOLDBLATT, L. A. & DOLLEAR, F. G. (1977) Detoxification of
contaminated crops. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publishers Inc., pp. 139-150.
GOODAL, C. M. & BUTLER, W. H. (1969) Aflatoxin carcinogenesis:
Inhibition of liver cancer induction in hypophysectomized rats.
Int. J. Cancer, 4: 422-429.
GOPALAN; C., TULPULE, P. G., & KRISHNAMURTHI, D. (1972) Induction of
hepatic carcinoma with aflatoxin in the rhesus monkey. Food
Cosmet. Toxicol., 10: 519-521.
GRANT, K. E., COMER, M. W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small doses
of aflatoxin B1. Toxicol. appl. Pharmacol, 41: 166 (Abstract no.
84).
GREENWAY, J. A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Columbia. I. Natural occurrence and diagnosis. Can. J.
comp. Med., 40: 12-15.
GRICE, M. C., MOODIE, C. A., & SMITH, B. C. (1973) The carcinogenic
potential of aflatoxin or its metabolites in rats from dams fed
aflatoxin pre- and postpartum. Cancer Res., 33: 262-268.
HABISH, H. A., ABDULLA, M. H., & BROADBENT, J. H. (1971) The
incidence of aflatoxin in Sudanese groundnuts. Trop. Sci., 8:
279-287.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs. In:
Abstracts, IUPAC-Sponsored Symposium, Control of Mycotoxins,
Kungalr, Goteborg, Sweden, 21-22 Aug., 1972, Goteborg, Sweden,
Swedish Institute for Food Preservation Research, p. 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A in barley,
using silica gel mini-columns. J. Assoc. Off. Anal. Chem.,
58:156-158.
HALVER, J. E. (1969) Aflatoxicosis and trout hepatoma. In:
Goldblatt, L. A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 265-306.
HAMILTON, P. B. & HARRIS, J. R. (1971)Interaction of aflatoxicosis
with Candida albicans infections and other stresses in chickens.
Poult. Sci., 50: 906-912.
HANSSEN, E. & JUNG, M. (1972) [On the presence of aflatoxin in
non-mouldy food and proposals for sampling.]
Z. Lebensmitt.-Unters.-Forsch., 150: 141-145 (in German).
HARTLEY, R. D., NESBITT, B. F., & O'KELLY, J. (1963) Toxic
metabolites of Aspergillus flavus. Nature (Load.), 198: 1056-1058.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
345-367.
HARWIG, J. & CHEN, Y.-K. (1974) Some conditions favoring production
of ochratoxin A and citrinin by Penicillium viridicatum in wheat
and barley. Can. J. Plant. Sci., 54: 17-22.
HAYES, A. W., HOOD, R. D., & LEE, H. L. (1974) Teratogenic effects
of ochratoxin A in mice. Teratology, 9: 93-98.
HEPTINSTALL, R. H. (1966) Pathology of the kidney, London,
Churchill, pp. 457-464.
HESSELTINE, C. W. (1976) Conditions leading to mycotoxin
contamination of foods and feeds. In: Rodricks, J. V., ed.
Mycotoxins and other fungal related food problems, Washington, DC,
American Chemical Society, pp. 1-22 (Advances in Chemistry Series
149).
HESSELTINE, C. W., SORENSEN, W. G., & SMITH, M. (1970) Taxonomic
studies of the aflatoxin producing strains in the Aspergillus
flavus group. Mycologia, 62: 123-132.
HOBSON. W., BAILEY, J., & FULLER, G. B. (1970 Hormone effects of
zearalenone in non-human primates. J. Toxicol. environ. Health, 3:
43-57.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1: 133-151.
HOGAN, G. R., RYAN, N.J., & HAYES, A. W. (1978) Aflatoxin B1 and
Reye's syndrome. Lancet, March 11, p. 561.
HOLADAY, C. E. (1976) A rapid screening method For the aflatoxins
and ochratoxin A. J. Am. Oil Chem. Soc., 53: 603-605.
HORWITZ, W, SENZEL, A., REYNOLDS, H., & PARK, D. L., ed. (1975)
Natural poisons. In: Chapter 26, Official Methods of analysis of
the Association of Official Analytical Chemists, Washington, DC,
AOAC, p. 24.
HRABAR, A., SUJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Morbidity and mortality from endemic
nephropathy in the village of Kaniza.] Arh. hig. rada, 27: 137-145
(in Serbo-Croat).
HSIEH, D. P. H., WONG, Z. A, WONt, J. J., MICHAS, C., & RUEBNER, B.
H. (1977) Comparative metabolism of aflatoxin. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers Inc.,
pp. 37-50.
HSU I-CH., SMALLEY, E. B., STRONG, F. M., & RIBELIN, W. E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. MicrobioL., 24: 684-690.
HULT, K. & GATENECK, S. (1976) A spectrophotometric procedure using
carboxy-peptidase A, for the quantitative measurement of ochratoxin
A. J. Assoc. Off. Anal. Chem., 59: 128-129.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32:
443-444.
IARC (1976) Aflatoxins. In: IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man: Some naturally occurring
substances. Lyons, International Agency for Research on Cancer,
Vol. 10, pp. 51-72.
IARC (1977) IARC Monograph Programme on the evaluation of the
carcinogenic risk of chemicals to humans. Preamble. Lyons,
International Agency for Research on Cancer, 31 pp. (IARC Internal
Technical Report No. 77/002).
IUPAC (1976) Recommended methods of ochratoxins A and B in barley,
Oxford, International Union of Pure & Applied Chemistry Secretariat,
pp. 1-7 (Technical Reports-No. 14).
JEMMALI, M. & LAFONT, P. (1972) Evolution de l'aflatoxine B1 au
tours de la panification. Cah. Nutr. Diét., 7: 319-322.
JEMMALI, M. & MURTHY, T. R. K. (1976) A chemical assay method for
the determination of aflatoxin residues in animal tissues.
Z. Lebensm.-Unters.-Forsch., 161: 13-17.
JOHN, D. W. & MILLER, L. L. (1969) Effect of aflatoxin B1 on net
synthesis of albumin, fibrinogen and alpha1-acid glycoprotein by
the isolated perfused rat liver. Biochem. Pharmacol., 18:
1135-1146.
JONES, B. D. (1972) Methods of aflatoxin analysis. London,
Tropical Products Institute, 58 pp. (Report G 70).
JOSEPH-BRAVO, P. I., FINDLEY, M., & NEWBERNE P.M. (1976) Some
interactions of light, riboflavin, and aflatoxin B1 in vivo and
in vitro. J. Toxicol. environ. Health, 1: 353-376.
JUNG, M. & HANSSEN, E. (1974) [The occurrence of aflatoxin M1 in
dried milk products.] Food Cosmet. Toxicol., 12: 131-138 (in
German).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZNSKA, J. (1976) [Occurrence of
aflatoxin B1, B2, G1, and G2, ochratoxin A and B,
sterigmatocystin and zearalenone in cereals.] Med. Weter., 32:
617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feed and concentrates.] Med. Weter., 53: 193-196
(in Polish).
KATO, R., ONODA, K., & OMORI, Y. (1969) The effect of aflatoxin B1
on incorporation of 14C-acetate into cholesterol by rat liver.
Experientia (Basel), 25: 1026.
KATO, R., TAKANAKA, A., ONODA, K., & OMORI, Y. (1970) Different
effect of aflatoxin on the induction of tryptophan oxygenase and of
microsomal drug-hydroxylase system. J. Biochem. (Tokyo), 68:
589-592.
KEEN, P. & MARTIN, P. (1971a) The toxicity and fungal infestation of
foodstuffs in Swaziland in relation to harvesting and storage.
Trop. geogr. Med., 23-35-43.
KEEN, P. & MARTIN, P. (1971b) Is aflatoxin carcinogenic in man? The
evidence in Swaziland. Trop. geogr. Med., 23: 44-53.
KIERMEIER, F. ( 1973) Aflatoxin residues in fluid milk. Pure appl.
Chem., 35: 271-274.
KIERMEIER, R. (1977) The significance of aflatoxins in the dairy
Industry. Annu. Bull. Int. Dairy Fed., 98: 1-23, 25-43.
KIERMEIER, F., WEISS, G., BEHNRINGER, G., MILLER, M., & RANFFT, K.
(1977) [On the presence and the content of aflatoxin M1 in milk
shipped to a dairy plant.] Z. Lebensmitt.- Unters.-Forsch., 163:
171-174 (in German).
KOEHLER, B. (1938) Fungus growth in shelled corn as affected by
moisture J. agric. Res., 56: 291-307.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975a) Investigations into an outbreak of hepatitis in parts
of Western India. Indian J. med. Res., 63: 1036-1048.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975b) Hepatitis due to aflatoxicosis. Lancet, 1: 1061.
KROGH, P. (1974) Mycotoxic porcine nephropathy: A possible model for
Balkan endemic nephropathy. In: Puchlev, A., ed. Endemic
Nephropathy. Proceedings of the Second International Symposium on
Endemic Nephropathy, Sofia, November 9-11, 1972. Sofia, Bulgarian
Academy of Sciences, pp. 266-270.
KROGH, P. (1976a) Mycotoxic nephropathy. In: Advances in
veterinary science and comparative medicine, New York, Academic
Press, Vol. 20, pp. 147-170.
KROGH, P. (1976b) Epidemiology of mycotoxic porcine nephropathy.
Nord. Vet.-Med., 28: 452-458.
KROGH, P. (1977a) Ochratoxins. In: Rodricks, J. V., Hesseltine, C.
W., & Mehlman, M. A., ed. Mycotoxins in human and animal health,
Park Forest South, IL, USA, Pathotox Publishers, Inc., pp. 489-498.
KROGH, P. (1977b) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. Vet.-Med., 29: 402-405.
KROGH, P. (1977c) Mycotoxin tolerances in foodstuffs. Pure appl.
Chem., 49: 1719-1721.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy.
Acta path. microbiol. Scand. Sect. A, Suppl. no. 269.
KROGH, P. & ELLING, F. (1977) Mycotoxic nephropathy. Vet. Sci.
Commun., 1: 51-63.
KROGH, P. & HALD, B. (1969) [Occurrence of aflatoxin in imported
groundnut products.] Nord. Vet.-Med., 21: 398-407 (in Danish).
KROGH, P., HALD, B., & KORPINEN, E.-L. (1970) [Occurrence of
aflatoxin in groundnut- and copra-products imported to Finland.]
Nord. Vet.-Med., 22: 584-589 (in Norwegian).
KROGH, P., HALD, B., HASSELAGER, E., MADSEN, A., MORTENSEN, H. P.,
LARSEN, A. E., & CAMPBELL, A.D. (1973a) Aflatoxin residues in bacon
pigs. Pure appl. Chem., 35: 275-282.
KROGH, P., HALD, B., & PEDERSEN, E. J. (1973b) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta path. microbiol. Scand. Sect. B, 81:
689-695.
KROGH, P., AXELSEN, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A. E., MADSEN, A., MORTENSEN, H. P.,
MOLLER, T., PETERSEN, O. K., RAVNSKOV, U., ROSTGAARD, M., & AALUND,
O. (1974) Experimental porcine nephropathy: Changes of renal
function and structure induced by ochratoxin A- contaminated feed.
Acta path. microbiol. Scand. Sect. A, Suppl. No. 246, 9 pp.
KROGH, P., ELLING, F., BALD, B., LARSEN, A. E., LILLEHOJ, E. B.,
MADSEN, A., & MORTENSEN, H. P. (1976a) Time-dependent disappearance
of ochratoxin A residues in tissues of bacon pigs. Toxicology, 6:
235-242.
KROGH, P., ELLING, F., GYRD-HANSEN, H., HALD, B., LARSEN, A. E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H. P., & RAVNSKOV, U.
(1976b) Experimental porcine nephropathy: Changes of renal function
and structure perorally induced by crystalline ochratoxin A. Acta.
pathol. microbiol. Scand. Sect. A, 84: 429-434.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V. E.,
SKADHAUGE, E., & SVENDSEN, C. K. (1976c) Experimental avian
nephropathy: Changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta path. microbiol. Scand. Sect.
4, 84: 215-221.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and foodborne ochratoxin A: Preliminary
results of a survey of foodstuffs. Acta path. microbiol. Scand.
Sect. B, 85. 238-240.
KURTZ, M. J., NAIRN, M. E., NELSON, G. H., CHRISTENSEN, C. M., &
MIROCHA, C. J. (1969) Histologic changes in the genital tracts of
swine fed estrogenic mycotoxin. Am. J. vet. Res., 30: 551-556.
LARSEN, S. (1928-1929) [On chronic degeneration of the kidneys
caused by mouldy rye.] Maanedsskr. Dyrl., 40: 259-284 & 289-300
(in Danish).
LE BRETON, E., FRAYSSINET, C., LAFARGE, C., & DE RECONDO, A.M.
(1964) Aflatoxine-mechanisme de l'action. Food Cosmet. Toxicol.,
2: 675-676.
LEE, D. J., WALES, J. H., & SINNHUBER, R. O. (1969a) Hepatoma and
renal tubule adenoma in rats fed aflatoxin and cyclopropenoid fatty
acids. J. Natl Cancer Inst., 43: 1037-1044.
LEE, L. S., CUCULLU, A. F., FRANZ, A. O., JR, & PONS, W. A., JR
(1969b) Destruction of aflatoxins in peanuts during dry and oil
roasting. J. agric. food Chem., 17: 451-453.
LEE, W. V. (1965) Quantitative determination of aflatoxin in
groundnut products. Analyst, 90: 305-307.
LEONARD, A., DEKNUDT, GH., & LINDEN, G. (1975) Mutagenicity tests
with aflatoxins in the mouse. Mutat. Res., 28: 137-139.
LEONOV, A. N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 323-328.
LEVI, C. P., TRENK, H. L., & MOHR, H. K. (1974) A study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LIJINSKY, W. & BUTLER, W. H. (1966) Purification and toxicity of
aflatoxin G1. Proc. Soc. Exp. Biol. Med., 123: 151-154.
LIJINSKY, W., LEE, K. Y., & GALLAGHER, C. H. (1970) Interaction of
aflatoxin B1 and G1 with tissues of the rat. Cancer Res., 30:
2280-2283.
LILLEHOJ, E. B., FENNELL, D. I., & KWOLEK, W. F. (1976)
Aspergillus flavus and aflatoxin in Iowa corn before harvest.
Science, 193: 195-496.
LIN, J. J., LIU, C., & SVOBODA, D. J. (1974) Long-term effects of
aflatoxin B1 and viral hepatitis on marmoset liver. Lab. Invest.,
30: 267-278.
LING, K. H., WANG, J. J., WU, R., TUNG, T. C., LIN, C. K., LIN,
S.S., & LIN, T. M. (1967) Intoxication possibly caused by aflatoxin
B1 in mouldy rice in Shuang-Chih township. J. Formosan Med.
Assoc., 66: 517-525.
LINSELL, C. A. & PEERS, F. G. (1977) Field studies on liver cell
cancer. In: Hiatt, H. H., Watson, J. D., & Wingsten, J. A., ed.
Origins of human cancer. Cold Spring Harbor, NY, Cold Spring
Harbor Laboratory, pp. 549-556.
LO, W-B. & BLACK, H. S. (1972) Effects of aflatoxin B1 on human
skin lipids metabolism-a site of action on 14C-acetate
incorporation. Experientia (Basel), 28: 1278-1279.
LOOSMORE, R. M. & HARDING, J. D. J. (1961) A toxic factor in
Brazilian groundnut causing liver damage in pigs. Vet. Rec., 73:
1362-11364.
LOOSMORE, R. M. & MARKSON, L. M. (1961) Poisoning of cattle by
Brazilian groundnut meal. Vet. Rec., 73: 813-814.
LOVELACE, C. E. A. & NYATHI, C. B. (1977) Estimation of the fungal
toxins, zearalenone and aflatoxin, contaminating opaque maize beer
in Zambia. J. Sci. Food Agric., 28: 288-292.
LUCAS, F. V., JR., MONROE, P., NGA, P. V., TOWNSEND, J. F., & LUCAS,
F. V. (1970-1971) Mycotoxin contamination of South Vietnamese rice.
J. trop. Med. Hyg., 74: 182-183.
LUCK. H., STEYN, M., & WEHNER, F. C. (1917) A survey of milk powder
for aflatoxin content. S. Afr. J. dairy Technol., 8: 85-86.
LVOVA, L. S., SOSEDOV, N. I., GARALL, W., SCHWARZMAN, M. I.,
SHATILOVA, T. I., & SHULGINA, A. P. (1976) [Formation of aflatoxins
in wheat grain induced by self-heating and change in its chemical
composition by the development of storage moulds.] Prikl. Biochem.
Mikrobiol., 12: 741-749 (in Russian).
MADHAVAN, T. V. & GOPALAN, C. (1975) Effect of dietary protein on
aflatoxin injury in weanling rats. Arch. Pathol.,
80: 123-126.
MADHAVAN, T. V. & GOPALAN, C. (1968) The effect of dietary protein
on carcinogenesis of aflatoxin. Arch. Pathol., 85: 133-137.
MADHAVAN, T. V., SURYANARAYANA RAO, K., The effect of dietary
protein level on susceptibility of monkeys to aflatoxin liver
injury. Indian J. med. Res., 53: 984-989.
MADHAVAN, T. V., TULPULE, P. G, & GOPALAN, C. (1965b)
Aflatoxin-induced hepatic fibrosis in rhesus monkeys. Arch.
Pathol., 79: 466-469.
MALEKI, M. F., SUZANGAR, M., JAMSHIDI, CH., TAHMASEBI, A., &
BARNETT, R. (1976) Urinary aflatoxin detected in cirrhotic patients
in Ispahan, Iran. Clin. Res., 24: 630A.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MARSH, P. B., SIMPSON, M. E., CRAIG, G. O., DONOSO, J., & RAMEY, H.
H., JR (1973) Occurrence of aflatoxins in cotton seeds at harvest in
relation to location of growth and field temperatures. J. environ.
Qual., 2: 276-281.
MARTIN, P.M. D. & KEEN, P. (1978) The occurrence of zearalenone in
raw and fermented products from Swaziland and Lesotho. Sabrouaudia,
16: 15,22.
MASRI, M. S., LUNDIN, R. E., PAGE, J. R., & GARCIA, V. V. (1967)
Crystalline aflatoxin M1 from urine and milk. Nature (Lond.),
215: 753-755.
MASRI, M. S., HADDON, W. F., LUNDIN, R. E., & HSIEH, D. P. H.
(1974a) Aflatoxin Q1: A newly identified major metabolite of
aflatoxin B1 in monkey liver. J. agric. food Chem., 22: 512-514.
MASRI, M. S., BOOTH, A. N., & HSIEH, D. P. H. (1974b) Comparative
metabolic conversion of aflatoxin B1 to aflatoxin M1 and
aflatoxin Q1 by monkey, rat and chicken liver. Life Sci., 15:
203-212.
MATTHEWS, J. G., PATTERSON, D. S. P., ROBERTS, B. A., & SHREEVE, B.
J. (1977) T-2 toxin and haemorrhagic syndromes in cattle. Vet.
Rec., 101 (19): 391.
MCLEAN, A. E. M. & MARSHALL, A. (1971) Reduced carcinogenic effects
of aflatoxin in rats given phenobarbitone. Br. d. exp. Pathol.,
52: 322-329.
MCNUTT, S. H., PURWIN, P., & MURRAY, C. (1928) Vulvovaginitis in
swine J. Am. Vet. Med. Assoc., 73: 484;-492.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MERWE, K. J. van der, STEYNE, P.S., FOURIE, L., SCOTT, DE B., &
THERON, J. J. (1965) Ochratoxin A, a toxic metabolite produced by
Aspergillus ochraceus Wilh. Nature (Lond.), 205: 1112-1113.
MILLER, J. K., HACKING, A., & GROSS, V. J. (I[973) Stillbirths,
neonatal mortality and small litters in pigs associated with the
ingestion of Fusarium toxin by pregnant sows. Vet. Rec., 93:
555-559.
MINTZLAFF, H.-J., LOTZSCH, R., TAUCHMANN, F., MEYER, W., & LEISTNER,
L. (1974) [Aflatoxin residues in the liver and muscle of broiler
chicken given aflatoxin containing feed.] Fleischwirtschaft, 54:
774-778 (in German).
MIROCHA, C. J. & CHRISTENSEN, C. M. (1974) Oestrogenic mycotoxins
synthesized by Fusarium. In: Purchase, I. F. H, ed. Mycotoxins,
Amsterdam, Elsevier, pp. 129-148.
MIROCHA, C. J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poaefusarin. Appl. Microbiol., 26:
719-724.
MIROCHA, C. J., CHRISTENSEN, C. M., & NELSON, G. H. (1968a) Toxic
metabolites produced by fungi implicated in mycotoxicoses.
Biotech. Bioeng., 10: 469-482.
MIROCHA, C. J., HARRISON, J., NICHOLS, A. A.., & MCCLINTOCK, M.
(1968b) Detection of a fungal estrogen (F-2) in hay associated with
infertility in dairy cattle. Appl. Microbiol., 16: 797-798.
MIROCHA, C. J., SCHAUERHAMER, B., & PATHRE, S. V. (1974) Isolation,
detection and quantitation of zearalenone in maize and barley.
J. Assoc. Off. Anal. Chem., 75: 1104-1110.
MIROCHA, C. J., PATHRE, S. V., SCHAUERHAMER, B., & CHRISTENSEN, C.
M. (1976) Natural occurrence of Fusarium toxins in feedstuff. Appl
environ. Microbiol., 32: 553-556.
MIROCHA, C. J., PATHRE, S. W., & CHRISTENSEN, C. M. (1977)
Zearalenone. In: Rodricks, J. V., Hesseltine, C. W., & Mehlman, M.
A., ed. Mycotoxins in human and animal health, Park Forest South,
IL, USA, Pathotox Publishers Inc., pp. 345-364.
MOROOKA, N., URATSUJI, N., YOSHIZAWA, T., & YAMAMOTO, H. (1972)
[Studies on the toxic substances in barley infected with Fusarium
spp.] Jpn. J. food Hyg, 13: 368-375 (in Japanese).
MUNRO, I. C, MOODIE, C. A, KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H. C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol, 28: 180-188.
NABNEY, J., BURBAGE, M. B., ALLCROFT, R., & LEWIS, G. (1967)
Metabolism of aflatoxin in sheep: Excretion pattern in the lactating
ewe. Food Cosmet. Toxicol., 5: 11-17.
NEAL, G. E. & GODOY, H. M. (1976) The effect of pre-treatment with
phenobarbitone on the activation of aflatoxin B1 by rat liver.
Chem. biol. Interactions, 14: 279-289.
NEELY, W. C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55: 1305-1309.
NEL, W. & PURCHASE, I. F. M. (1968) Note. The fate of ochratoxin A
in rats. J.S. Afr. Chem. Inst., 21: 87-88.
NESHEIM, S. (1971) Report on ochratoxins: Occurrence, production,
analysis and toxicity. In: Abstracts of the 85th Annual meeting of
the Association of Official Analytical Chemists, Washington, DC,
Oct. 11-14, 1971, p. 23.
NESHEIM, S. (1976) The ochratoxins and other related compounds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 276-295
(Advance.,; in Chemistry Series 149).
NESHEIM, S., HARDIN, N. F., FRANCIS, O. J., JR, & LANGHAM, W. S.
(1973) Analysis of ochratoxins A and B and their esters in barley,
using partition and thin layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NEUMANN-KLEINPAUL, A. & TERPLAN, G. (1972) [The presence of
aflatoxin M1 in dried milk products.] Arch. Lebensmittelhyg.,
23: 128-132 (in German).
NEWBERNE, P.M. (1974) Mycotoxins: Toxicity, carcinogenicity and the
influence of various nutritional conditions. Environ. Health
Perspect., 9: 1-32.
NEWBERNE, P.M. (1976) Environmental modifiers of susceptibility to
carcinogenesis. Cancer Detect. Prev., 1: 129-173.
NEWBERNE, P.M. & BUTLER, W. H. (1969) Acute and chronic effects of
aflatoxin on the liver of domestic and laboratory animals: A review.
Cancer Res., 29: 236-250.
NEWBERNE, P.M. & CONNER, M. W. (1974) Effect of selenium on acute
response to aflatoxin B1. In: Hemphill, D. D., ed. A Symposium.
Trace Substances in Environmental Health-VIII, Columbia, MO,
University of Missouri, pp. 323-328.
NEWBERNE, P.M. & GROSS, R. L. (1977) The role of nutrition in
aflatoxin injury. In: Rodricks, J. V., Hesseltine, C. W, & Mehlman,
M. A., ed. Mycotoxins in human and animal health, Park Forest
South, IL, USA, Pathotox Publishers Inc., pp. 51-65.
NEWBERNE, P.M. & ROGERS, A. E. (1971) Aflatoxin carcinogenesis in
rats: Dietary effects. In: Purchase, I. F. H., ed. Mycotoxins in
human health, London, Macmillan, pp. 195-208.
NEWBERNE, P.M. & ROGERS, A. E. (1973) Rat colon carcinomas
associated with aflatoxin and marginal vitamin A. J. Natl Cancer
Inst., 50: 439-448.
NEWBERNE, P.M. & ROGERS, A. E. (1976) Nutritional modulation of
carcinogenesis. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. of Tokyo Press, & Baltimore, Univ.
Park Press, pp. 15-40.
NEWBERNE, P.M. & SUPHAKARN, V. (1977) Preventive role of vitamin A
in colon carcinogenesis in rats. Cancer, 40: 2553-2556.
NEWBERNE, P.M. & WILLIAMS, G. (1969) Inhibition of aflatoxin
carcinogenesis by diethyl-stilbestrol in male rats. Arch. environ.
Health, 19: 489-498.
NEWBERNE, P.M. & WOGAN, G. N. (1968a) Sequential morphologic changes
in aflatoxin B1 carcinogenesis in the rat. Cancer Res., 28:
770-781.
NEWBERNE, P.M. & WOGAN, G. N. (1968b) Potentiating effects of
low-protein diets on effect of aflatoxin in rats. Toxicol. appl.
Pharmacol., 12: 309-310.
NEWBERNE, J. W., BAILEY, W. S., & SEIBOLD, H. R. (1955) Notes on a
recent outbreak and experimental reproduction of hepatitis X in
dogs. J. Am. Vet. Med. Assoc., 127: 59-62.
NEWBERNE, P.M., RUSSO, R., & WOGAN, G. N. (1966a) Acute toxicity of
aflatoxin B1 in the dog. Pathol. vet., 3: 331-340.
NEWBERNE, P.M., HARRINGTON, D. H., & WOGAN, G. N. (1966b) Effects of
cirrhosis and other liver insults on induction of liver tumours by
aflatoxin. Lab. Invest., 15: 962-969.
NEWBERNE, P.M., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal
lesions in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
NEWBERNE, P.M., CHAN, W-CH. M., & ROGERS, A. E. (1974) Influence of
light, riboflavin, and carotene on the response of rats to the acute
toxicity of aflatoxin and mono-crotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NILSSON, G. (1974) [Aflatoxin in nuts.] Vor Foda, 26: 56-62 (in
Swedish).
NIXON, J. E., SINNHUBER, R. O., LEE, D. J., LANDERS, M. K., & HARR,
J. R. (1974) Effect of cyclopropenoid compounds on the carcinogenic
activity of diethylnitrosamine and aflatoxin B1 in rats. J. Natl
Cancer Inst., 53: 453-458.
NORTHOLT, M.D., VERHULSDONK, C. A. H., SOENTORO, P.S. S., & PAULSCH,
W. E. (1976) Effect of water activity and temperature on aflatoxin
production by Aspergillus parasiticus. J. milk food Technol., 39:
170-174.
OLSON, L. C., BOURGEOIS, C. H., JR, COTTON, R. B., HARIKUL, S.,
GROSSMAN, R. A., & SMITH, T. J. (1971) Encephalopathy and fatty
degeneration of the viscera in northeastern Thailand. Clinical
syndrome and epidemiology. Pediatrics, 47: 707-716.
ONG, T-M. (1975) Aflatoxin mutagenesis. Mutat. Res., 32: 35-53.
PAG FAG/WHO/UNICEF PROTEIN ADVISORY GROUP (1969) PAG Recommendation
on aflatoxin. PAG Statement No. 2. New York, United Nations, 2 pp.
PANALAKS, T. & SCOTT, P.M. (1977) Sensitive silica gel-packed
flowcell for fluorometric detection of aflatoxins by high pressure
liquid chromatography. J. Assoc. Off. Anal. Chem., 60: 583-589.
PARKER, W. A. & MELNICK, D. (1966) Absence of aflatoxin from refined
vegetable oils. J. Am. Oil Chem. Soc., 43: 635-638.
PATHRE, S. V. & MIROCHA, C. J. (1976) Zearalenone and related
compounds. In: Rodricks, J. V., ed. Mycotoxins and other fungal
related food problems, Washington, DC, American Chemical Society
pp. 178-227 (Advances in Chemistry Series 149).
PATTERSON, D. S. P. (1973) Metabolism as a factor in determining the
toxic action of the aflatoxin in different animal species. Food
Cosmet. Toxicol., 11: 287-294.
PATTERSON, D. S. P. (1976) Structure, metabolism and toxicity of the
aflatoxins: A review. Cah. Nutr. Diét., 11 (Suppl. 2): 71-76.
PATTERSON, D. S. P. (1977) Aflatoxin and related compounds. In:
Wyllie, T.D. & Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: An encyclopedic handbook, New York & Basel, Marcel
Dekker, pp. 131-136 & 144-189.
PATTERSON, D. S. P. & ROBERTS, B. A. (1970) The formation of
aflatoxin B2a and G2a and their degradation products during the
in vitro detoxification of aflatoxin by livers of certain avian
and mammalian species. Food Cosmet. Toxicol., 8: 527-538.
PATTERSON, D. S. P. & ROBERTS, B. A. (1971) The in vitro reduction
of aflatoxins B1 and B2 by soluble avian liver enzymes. Food
Cosmet. Toxicol., 9: 829-837.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972a) Steroid sex hormones as
inhibitors of aflatoxin metabolism in liver homogenates.
Experientia (Basel), 28: 929-930.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972b) Aflatoxin metabolism in
duck-liver homogenates: The relative importance of reversible
cyclopentenone reduction and hemiacetal formation. Food Cosmet.
Toxicol., 10: 501-512.
PATTERSON, D. S. P., ROBERTS, B. A., SHREEVE, B. J., WRATHALL, A.
E., & GITTER, M. (1977) Aflatoxin, ochratoxin and zearalenone in
animal feedstuffs: Some clinical and experimental observations. Ann
Nutr. Aliment., 31: 643-650.
PATTERSON, D. S. P., SHREEVE, B. J., & ROBERTS, B. A. (in press)
Mycotoxin residues in body fluids and tissues of food-producing
animals. In: Proceedings of the 12th International Congress
Microbiology, Munchen, Federal Republic of Germany, 3-8 September
1978.
PAUL, R., KALRA, M. S., & SINGH, A. (1976) Incidence of aflatoxins
in milk and milk products. Indian J. dairy Sol., 29: 318-322.
PAYET, M., CROS, J., QUENUM, C., SANKALE, M., & MOULANIER, M. (1966)
Deux observations d'enfants avant consommé de façon prolongée des
farines souillées par "Aspergillus flavus". Presse med.,
13: 649-651.
PECKHAM, J. C., DOUPNIK, B., JR, & JONES, O. H., JR (1971) Acute
toxicity of ochratoxins A and B in chicks. Appl. Microbiol., 21:
492-494.
PEERS, F. G. & LINSELL, C. A. (1973) Dietary aflatoxins and liver
cancer--a population based study in Kenya. Br. J. Cancer, 27:
73-484.
PEERS, F. G. & LINSELL, C. A. (1976) Acute toxicity of aflatoxin
B1 for baboons. Food Cosmet. Toxicol., 14: 227-228.
PEERS, F. G. & LINSELL, C. A. (1977) Dietary aflatoxins and human
primary liver cancer. Gaucher, A., ed. Ann. Nutr. Aliment., 31:
1005-1018.
PEERS, F. G., GILMAN, G. A., & LINSELL, C. A. (1976) Dietary
aflatoxins and human liver cancer. A study in Swaziland. Int. J.
Cancer, 17: 167-176.
PETTIT, R. E., TABER, R. A., SCHROEDER, H. W., & HARRISON, A. L.
(1971) Influence of fungicides and irrigation practice on aflatoxin
in peanuts before digging. Appl. Microbiol., 22: 629-634.
PHILLIPS, D. L., YOURTEE, D. M., & SEARLES, S. (1976) Presence of
aflatoxin B1 in human liver in the United States. Toxicol. appl.
Pharmacol., 36: 403-406.
PIER, A. C. & HEDDLESTON, K. L. (1970) The effect of aflatoxin on
immunity in turkeys. I. Impairment of actively acquired resistance
to bacterial challenge. Avian Dis., 14: 797-809.
PIER, A. C., CYSEWSKI, S. J., RICHARD, J. L., & THURSTON, J. R.
(1977) Mycotoxins as a veterinary problem. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 745-750.
PITOUT, M. J. (1969) The hydrolysis of ochratoxin A by some
proteolytic enzymes. Biochem. Pharmacol., 18: 485-491.
POHLAND, A. E., YIN, L., & DANTZMAN, J. G. (1970) Rapid chemical
confirmatory method for aflatoxin B1. I. Development of the
method. J. Asoc. Off. Anal. Chem., 53: 101-102.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELJAN, V. A. (1972) Effect
of aflatoxin on rat liver lysosomes. Toxicon, 10: 25-30.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELIAN, V. A. (1977)
[Aflatoxins.] Toksikologija, Moskva, Viniti, Vol. 8, 108 pp. (in
Russian).
POLZHOFER, K. (1977) [Determination of aflatoxins in milk and milk
products.] Z. Lebensmitt.-Unters.-Forsch., 163: 175-177 (in
German).
PONG, R. S. & WOGAN, G. N. (1969) Time course of alterations of rat
liver polysome profiles induced by aflatoxin B1. Biochem.
Pharmacol., 18: 2357-2361.
PONG, R. S. & WOGAN, G. N. (1970) Time course and dose-response
characteristics of aflatoxin B1 effects on rat liver RNA
polymerase and ultrastructure. Cancer Res., 30: 294-304.
PONG, R. S. & WOGAN, G. N. (1971) Toxicity and biochemical and fine
structural effects of synthetic aflatoxins M1 and B1 in rat
liver. J. Natl Cancer Inst., 47: 585-592.
PRINCE, A.M., SZMUNESS, W., MICHON, J, DEMAILLE, J., DIEBOLT, G.,
LINHARD, J., QUENUM, C., & SANKALE, M. (1975) A case/control study
of the association between primary liver cancer and hepatitis B
infection in Senegal. Int. J. Cancer, 16: 376-383.
PRIOR, M. G., SISODIA, C. S., & O'NEIL, J. B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese
quail. Poult. Sci., 55: 786-790.
PRZYBYLSKI, W. (1975) Formation of aflatoxin derivatives on thin
layer chromatographic plates. J. Assoc. Off. Anal. Chem., 58:
163-164.
PUCHLEV, A. (1973) La néphropathie endémique en Bulgarie. Extrait
du Symposium sur la néphropathie endémique de l'Académie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A. (1974) Basic problems in endemic nephropathy. In:
Puchlev, A., ed. Endemic Nephropathy, Proceedings of the Second
International Symposium on Endemic Nephropathy, Sofia, 9-11
November, 1972, Sofia, Publishing House of the Bulgarian Academy
of Sciences, pp. 15-18.
PULS, R. & GREENWAY, J. A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis and toxicity of suspected barley.
Can. J. comp. Med., 40: 16-19.
PURCHASE, I. F. H. (1967) Acute toxicity of aflatoxins M1 and M2
in one-day-old ducklings. Food Cosmet. Toxicol., 5: 339-342.
PURCHASE, I. F. H. (1972) Aflatoxin residues in food of animal
origin. Food Cosmet. Toxicol., 10: 531-544.
PURCHASE, I. F. H. (1974) Mycotoxins, Amsterdam, Elsevier.
PURCHASE, I. F. H. & THERUN, J. J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
PURCHASE, I. F. H. & VORSTER, L. J. (1968) Aflatoxin in commercial
milk samples. S. Afr. med. J., 42: 219.
REDDY, G. S., TILAK, T. B. G., & KRISHNAMURTHI, D. (1973)
Susceptibility of vitamin A- deficient rats to aflatoxin. Food
Cosmet. Toxicol., 11: 467-470.
REDDY, J. K. & SVOBOOA, D. J. (1975) Aflatoxin B1-induced liver
tumors in Tupaia glis (tree shrews) a nonhuman primate. Fed.
Proc., 34: 827 (Abstract no. 3432).
REDDY, J. K., SVOBODA, D. J., & RAO, M. S. (1976) Induction of liver
tumors by aflatoxin B1 in the tree shrew (Tupaia glis), a
nonhuman primate. Cancer Res., 36: 151-160.
REYE, R. D. K., MORGAN, G., & BARAL, J. (1963) Encephalopathy and
fatty degeneration of the viscera: A disease entity in childhood.
Lancet, 2: 749-752.
REYS, L. L. & SEQUEIRA, O. A. (1974) Detection of Australia antigen
(HBAg) in blood donors and hepatoma patients in Mozambique.
S. Aft. med. J., 48: 267-269.
RICHIR, CL., PACCALIN, J., LARCEBEAU, S., FAUGERES, J., MORARD,
J.-L., & LAMANT, M. (1976) Présence d'aflatoxine B1 dans le toie
humain. Cah. Nutr. Diet., 11: 223-226.
ROBERTS, J. C. (1974) Aflatoxins and sterigmatocystins. Fortschr.
Chem. Org. Naturst., 31: 119-151.
ROBINSON, P. (1967) Infantile cirrhosis of the liver in India. With
special reference to probable aflatoxin etiology. Clin. Pediatr.,
6: 57-62.
RODRICKS, J. V. & STOLOFF, L. (1970) Determination of concentration
and purity of aflatoxin standards. J. Assoc. Off. Anal. Chem., 53:
92-95.
RODRICKS, J. V. & STOLOFF, L. (1977) Food producing animals. In:
Rodricks, J. V., Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins
in human and animal health, Park Forest South, IL, USA, Pathotox
Publishers Inc., pp. 67-79.
RODRICKS, J. V., STOLOFF, L., PONS, W. A., JR,. ROBERTSON, J. A., &
GOLDBLATT, L. A. (1970) Molar absorptivity values for aflatoxins and
justification for their use as criteria of purity of analytical
standards. J. Assoc. Off. Anal. Chem., 53: 96-101.
RODRICKS, J. V., HESSELTINE, C. W., & MEHLMAN, M. A., ed. (1977)
Mycotoxins in human and animal health, Park Forest South, IL, USA,
Pathotox Publishers Inc., 807 pp.
ROGERS, A. E. (1975) Variable effects of a lipotrope-deficient, high
fat diet on chemical carcinogenesis in rats. Cancer Res., 35:
2469-2474.
ROGERS, A. E. & NEWBERNE, P.M. (1969) Aflatoxin B1 carcinogenesis
in lipotrope-deficient rats. Cancer Res., 29: 1965-1972.
ROGERS, A. E. & NEWBERNE, P.M. (1971) Diet and aflatoxin B1
toxicity in rats. Toxicol. appl. Pharmacol., 20: 113-121.
ROINE, K., KORPINEN, E.-L., & KALLELA, K. (1971) Mycotoxicosis as a
probable cause of infertility in dairy cows. Nord. Vet.-Med., 23:
628-633.
ROMER, T. R. (1975) Screening method for the detection of aflatoxins
in mixed feeds and other agricultural commodities with subsequent
confirmation and quantitative measurement of aflatoxins in positive
samples. J. Assoc. Off. Anal. Chem., 58: 500-506.
RUDDICK, J. A., SCOTT, P. M., & HARWIG, J. (1976) Teratological
evaluation of zearalenone administered orally to the rat. Bull.
environ. Contain. Toxicol., 15: 678-681.
RUTQVlST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of
fattening pigs. Zentralbl. Vet.-Med. A, 24: 402-408.
SAFFIOTTI, U., MONTESANO, R., SELLAKUMAR, A. R., CEFIS, F., &
KAUFMAN, D. G. (1972) Respiratory tract carcinogenesis in hamsters
induced by different numbers of administrations of benzo (a) pyrene
and ferric oxide. Cancer Res., 32: 1073-1081.
SALHAB, A. S. & EDWARDS, G. S. (1977) Comparative in vitro
metabolism of aflatoxicol by liver preparations from animals and
humans. Cancer Res., 37: 1016-1021.
SALHAB, A. S. & HSIEH, D. P. H. (1975) Aflatoxicol H1: A major
metabolite of aflatoxin B1 produced by human and rhesus monkey
livers in vitro. Res. Commun. chem. Pathol. Pharmacol.,
10: 419-431.
SANDERS, T. H, DAVIS, N. D., & DIENER, U. L. (1968) Effect of carbon
dioxide, temperature, and relative humidity on production of
aflatoxin in peanuts. J. Am. Oil Chem. Soc., 45: 683-685.
SANSING, G. A., LILLEHOJ, E. B., DETROY, R. W., & MILLER, M. A.
(1976) Synergistic toxic effect of citrinin ochratoxin A and
penicillic acid in mice. Toxicon, 14: 213-220.
SARASIN, A. & MOULE, Y. (1976) Helical polysomes induced by
aflatoxin B1 in vivo. A new hypothesis for helix formation by
chemicals and carcinogens. Exp. cell Res., 97: 346-358.
SARGEANT, K., O'KELLY, J., CARNAGHAN, R. B. A., & ALLCROFT, R.
(1961) The assay of a toxic principle in certain groundnut meals.
Vet. Rec., 73: 1219-1223.
SARKISOV, A. C. (1954) [Mycoses.] Moscow, State Publishing House
for Agricultural Literature, 216 pp. (in Russian).
SATO, S., MATSUSHIMA, T., TANAKA, N., SUGIMURA, T., & TAKASHIMA, F.
(1973) Hepatic tumors in the guppy (Lebistes reticulatus) induced
by aflatoxin B1, dimethylnitrosamine, and 2-acetylaminofluorene.
J. Natl Cancer Inst., 50: 765-778.
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches to
the toxic metabolites of Fusaria. VIII. Acute and sub-acute
toxicities of T-2 toxins in cats. Jpn. J. Pharmacol., 25: 263-270.
SCHADE, J. E., MCGREEVY, K., KING, A.D., MACKEY, B., & FULLER, G.
(1975) Incidence of aflatoxin in California almonds. Appl.
Microbiol., 29: 48-53.
SCHINDLER, A. F. & NESHEIM, S. (1970) Effect of moisture and
incubation time on ochratoxin A production by an isolate of
Aspergillus ochraceus. J. Assoc. Off. Anal. Chem., 53: 89-91.
SCHROEDER, H. W. & CARLTON, W. W. (1973) Accumulation of only
aflatoxin B2 by a strain of Aspergillus flavus. Appl.
Microbiol., 25: 146-148.
SCHULLER, P. L., OCKHUIZEN, TH., WERRINGLOER, J., & MARQUARDT, P.
(1967) [Aflatoxin B1 and histamine in wine.]
Arzneimitteforschung, 17: 888-890 (in German).
SCHULLER, P. L., VERHOLSHONK, C. A. H., & PAULSCH, W. E. (1973)
Analysis of aflatoxin M1 in liquid and powdered milk. Pure appl.
Chem., 35: 291-296.
SCHULLER, P. L., VERHULSDONK, C. A. H, & PAULSCH, W. E. (1967)
Aflatoxin M1 in liquid and powdered milk (evaluation of methods
and collaborative study). Zesz. Probl. Postepow Nauk Roln.,
189: 255-261.
SCOTT, P.M. & KENNEDY, B. P. C. (1973) Analysis and survey of ground
black, white, and capsicum peppers for aflatoxins. J. Assoc. Off.
Anal. Chem., 56: 1452-1457.
SCOTT, P.M., VAN WALBEEK, W., KENNEDY, B, & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. Agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., PANALAKS, T., KANHERE, S., & MILES, W. F. (1978)
Determination of zearalenone in cornflakes and other corn-based
foods by thin layer chromatography, high pressure liquid
chromatography, and gas-liquid chromatography/high resolution mass
spectrometry. J. Assoc. Off. Anal. Chem., 61: 593-600.
SEEGERS, J. C. & PITOUT, M. J. (1973) DNA repair synthesis in cell
cultures exposed to low doses of aflatoxin B1 or sterigmatocystin
S. Aft. lab. clin. Med., 19: 961 (abstr. 23).
SEIBOLD, H. R. & BAILEY, W. S. (1952) An epizootic of hepatitis in
the dog. J. Am. Vet. Med. Assoc., 121: 201-206.
SEIBOLD, R. & RUCH, W. (1977) [Aflatoxin content of mixed feeds of
dairy cows.] Kraftfutter, 60: 182-185 (in German).
SERCK-HANSSEN, A. (1970) Aflatoxin-induced fatal hepatitis? A case
report from Uganda. Arch. environ. Health, 20: 729-731.
SHANK, R. C. (1976) The role of aflatoxin in human disease. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems. Washington, DC, American Chemical Society, pp. 51-57
(Advances in Chemistry Series 149).
SHANK, R. C. (1977) Epidemiology of aflatoxin carcinogenesis. In:
Kraybill, H. F. & Mehlman, M. M., ed. Environmental cancer.
Advances in modern toxicology, Vol. 3, John Wiley and Sons, New
York, pp. 291-318.
SHANK, R. C., BOURGEOIS, C. H., KESCHAMRAS, N., & CHANDAVIMOL, P.
(1971a) Aflatoxins in autopsy specimens from Thai children with an
acute disease of unknown actiology. Food Cosmet. Toxicol., 9:
501-507.
SHANK, R. C., JOHNSEN, D. O., TANTICHAROENYOS, P., WOODING, W. L., &
BOURGEOIS, C. H. (1971b) Acute toxicity of aflatoxin B, in the
macaque monkey. Toxicol. appl. Pharmacol., 20: 227-231.
SHANK, R. C., WOGAN, G. N., GIBSON, J. B., & NONDASUTA, A. (1972a)
Dietary aflatoxins and human liver cancer. II. Aflatoxins in market
foods and foodstuffs of Thailand and Hong Kong. Food Cosmet.
Toxicol., 10: 61-69.
SHANK, R. C., GORDON, J. E., WOGAN, G. N., NONDASUTA, A., &
SUBHAMANI, B. (1972b) Dietary aflatoxins and human liver cancer.
III. Field survey of rural Thai families for ingested aflatoxins.
Food Cosmet. Toxicol., 10: 71-84.
SHANK, R. C., BHAMARAPRAVATI, N., GORDON, J. E., & WOGAN, O. N.
(1972c) Dietary aflatoxins and human liver cancer. IV. Incidence of
primary cancer in two municipal populations of Thailand. Food
Cosmet. Toxicol, 10: 171-179.
SHANKARAN, R., RAJ, H. G., & VENKITASUBRAMANIAN, T. A. (1970) Effect
of aflatoxin on carbohydrate metabolism in chick liver.
Enzymology, 39: 371-378.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969a) Survey of cereal grains
and soybeans for the presence of aflatoxin: II. Corn and soybeans.
Cereal Chem., 46. 454-463.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969b) Survey of cereal grains
and soybeans for the presence of aflatoxin: I. Wheat, grain sorghum,
and oats. Cereal Chem., 46: 446-454.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1969)
Ochratoxin A: Occurrence as natural contaminant of a corn sample.
Appl. Microbiol., 17: 765-766.
SHOTWELL, O. L., HESSELTINE, C. W., GOULDEN, M. L., & VANDEGRAFT, E.
E. (1970) Survey of corn for aflatoxin, zearalenone and ochratoxin.
Cereal Chem., 47: 700-707.
SHOTWELL, O. L., HESSELTINE, C. W., VANDEGRAFT, E. E., & GOULDEN, M.
L. (1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16: 266-273.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1973)
Incidence of aflatoxin in southern corn, 1969-1970. Cereal Sci.
Today, 18, 192-195.
SHOTWELL, O. L., GOULDEN, M. L., & HESSELTINE, C. W. (1976a) Survey
of US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal.
Chem., 59: 122-124.
SHOTWELL, O. L., GOULDEN, M. L., & BENNETT, G. A. (1976b)
Determination of zearalenone in corn: Collaborative study.
J. Assoc. Off. Anal. Chem., 59: 666-670.
SINNHUBER, R. O. & WALES, J. H. (1974) Aflatoxin B1
hepatocarcinogenicity in rainbow trout embryos. Fed. Proc., 33:
247 (Abstract no. 253).
SINNHUBER, R. O., LEE, D. J., WALES, J. H., & AYRES, J. L. (1968a)
Dietary factors and hepatoma in rainbow trout (Salmo gairdneri).
II. Cocarcinogenesis by cyclopropenoid fatty acids and the effect of
gossypol and altered lipids on aflatoxin-induced liver cancer.
J. Natl Cancer Inst., 41: 1293-1301.
SINNHUBER, R. O., WALES, J. H., AYRES, J. L., ENGEBRECHT, R. H., &
AMEND, D. L. (1968b) Dietary factors and hepatoma in rainbow trout
(Salmo gairdneri) I. Aflatoxins in vegetable protein feedstuffs.
J. Natl Cancer Inst., 41: 711-718.
SINNHUBER, R. O., LEE, D. J., WALES, J. H., LANDERS, M. K., KEYL, A.
C. (1974) Hepatic carcinogenesis of aflatoxin M1 in rainbow trout
(Salmo gairdneri) and its enchancement by cyclopropene fatty
acids. J. Natl Cancer Inst., 53: 1285-1288.
SMALLEY, E. B. & STRONG, F. M. (1974) Toxic trichothecenes. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
199-228.
SMITH, J. W., PRINCE, W. R., & HAMILTON, P. B. (1969) Relationship
of aflatoxicosis to Salmonella gallinarum infections of chickens.
Appl. Microbiol., 18: 946-947.
SPEERS, G. M., MERONUCK, R. A., BARNES, D. M., & MIROCHA, C. J.
(1971) Effect of feeding Fusarium roseum f. sp. graminearum
contaminated corn and the mycotoxin F-2 on the growing chick and
laying hen. Poult. Sci., 50: 627-633.
STACK, M. E. (1974) Collaborative study of AOAC methods I and III
for the determination of aflatoxins in peanut butter. J. Assoc.
Off. Anal. Chem., 57: 871-874.
STANFORD, G. K., HOOD, R. D., & HAVES, A. W. (1975) Effect of
prenatal administration of T-2 toxin to mice. Res. Commun. chem.
Path. Pharmacol., 10: 743-746.
STEPHENSON, L. W. & RUSSELL, T. E. (1974) The association of
Aspergillus flavus with hemipterous and other insects infesting
cotton bracts and foliage. Phytopathology, 64: 1502-1506.
STERN, P.S., & HOLZAPFEL, C. W. (1967) The synthesis of ochratoxins
A and B, metabolites of Aspergillus ochraceus Wilh. Tetrahedron
Lett., 23: 4449-4461.
STEYN, P.S., VLEGGAAR, R., Du PREEZ, N. P., BLYTH, A. A., & SEEGERS,
J. C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STICH, H. F. & LAISHES, B. A. (1975) The response of Xeroderma
pigmentosum cells and controls to the activated mycotoxins,
aflatoxins and sterigmatocystin. Int. J. Cancer, 16: 266-274.
STOLOFF, L. (1972) Analytical methods for mycotoxins. Clin.
Toxicol., 5: 465-494.
STOLOFF, L. (1976) Occurrence of mycotoxins in foods and feeds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 23-50
(Advances in Chemistry Series 149).
STOLOFF, L. (1977) Aflatoxins-an overview. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 7-28.
STOLOFF, L. & FRIEDMAN, L. (1976) Information bearing on the
evaluation of the hazard to man from aflatoxin ingestion.
PAG Bulletin, 6: 21-32.
STRZELECKI, E. L. & GASIOROWSKA, U. W. (1974) Aflatoxin B1 in
feedstuffs. Zentralbl. Vet.-Med. B, 21: 395-400.
STUBBLEFIELD, R.D., SHOTWELL, O. L., & SHANNON, O. H. (1972)
Aflatoxins M1 and M2 and parasiticol: Thin layer chromatography
and physical and chemical properties. J. Assoc. Off. Anal. Chem.,
55: 762-767.
SUIT, J. L., ROGERS, A. E., JETTEN, M. E. R., & LURIA, S. E. (1977)
Effects of diet on conversion of aflatoxin B1 to bacterial
mutagen(s) by rats in vivo and by rat hepatic microsomes
in vitro. Mutat. Res., 46: 313-323.
SUZANGAR, M., EMAMI, A., & BARNETT, R. (1976) Aflatoxin
contamination of village milk in Isfahan, Iran. Trop. Sci., 18:
155-159.
SUZUKI, S. & SATOH, T. (1973) Effects of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Pharmacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl.
Pharmacol., 32: 116-122.
SVOBODA, D., GRADY, H. J, & HIGGINSON, J. (1966) Aflatoxin B1
injury in rat and monkey liver. Am. J. Pathol., 49: 1023-1051.
SWENSON, D. H., MILLER, E. C., & MILLER, J. A. (1974) Aflatoxin
B1-2, 3-oxide: Evidence for its formation in rat liver in vivo
and by human liver microsomes in vitro. Biochem. Biophys. res.
Comm., 60: 1036-1043.
SWENSON, D. H., LIN, J.-K, MILLER, E. C., & MILLER, J. A. (1977)
Aflatoxin B1-2, 3-oxide as a probable intermediate in the covalent
binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal
RNA in vivo. Cancer Res., 37: 172-181.
SZCZECH, G. M., CARLTON, W. W., & TUITE, J. (1973a) Ochratoxicosis
in beagle dogs. I. Clinical and clinicopathological features.
Vet. Pathol., 10: 135-154.
SZCZECH, G. M., CARLTON, W. W., TUITE, J., & CALDWELL, R. (1973b)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10: 347-364.
SZCZECH, G. M., CARLTON, W. W, & TUITE, J. (1973c) Ochratoxicosis in
beagle dogs. II. Pathology. Vet. Pathol., 10: 219-231.
TANDON, B. N., KRISHNAMURTHY, L., KOSHY, A., TANDON, H. D.,
RAMALINGASWAMI, V., BHANDARI, J. R., MATHUR, M. M., & MATHUR, P. D.
(1977) Study of an epidemic of jaundice, presumably due to toxic
hepatits, in northwest India. Gastroenterology, 72: 488-494.
TANDON, H. D., TANDON, B. N., & RAMALINGASWAMI, V. (1978) Epidemic
of toxic hepatitis in India of possible mycotoxic origin. Arch.
Pathol. lab. Med., 102. 372-376.
TEMCHAROEN, P., ANUKARAHANONTA, T., & BHAMARAPRAVATI, N. (1978) The
influence of dietary protein and vitamin B12 on the toxicity and
carcinogenicity of aflatoxins in rat liver. Cancer Res.,
38: 2185-2190.
THACKER, H. L. (1976) Citrinin and ochratoxin A mycotoxicosis in
guinea pigs, Lafayette, Indiana, Purdue University (A thesis).
THERON, J. J. (1965) Acute liver injury in ducklings as a result of
aflatoxin poisoning. Lab. Invest., 14: 1586-1603.
THERON, J. J., LIEBENBERG, N., & JOUBERT, H. J. B. (1965)
Histochemistry and electron microscopy of acute liver lesions
induced by aflatoxin B1 in ducklings. Nature (Lond.), 206:
908-909.
TILAK, T. B. (1975) Induction of cholangiocarcinoma following
treatment of a rhesus monkey with aflatoxin. Food Cosmet.
Toxicol., 13: 247-249.
TRIPATHI, R. K. (1973) Aflatoxins in sorghum grains infested with
head moulds. Indian J. exp. Biol., 11: 361-362.
TUNG, H.-T., DONALDSON, W. E., & HAMILTON, P. B. (1970) Effects of
aflatoxin on some marker enzymes of lysosomes. Biochem. Biophys.
Acta, 222. 665-667.
TURNER, W. B. (1971) Fungal metabolites, New York, Academic Press,
446 pp.
ULLOA-SOSA, M. & SCHROEDER, H. W. (1969) Note on aflatoxin
decomposition in the process of making tortillas from corn.
Cereal Chem., 46: 397-400.
VAN NIEUWENHUIZE, J.P., HERBER, F. M., DE BRUIN, A., MEYER, P. B., &
DUBA, W. C. (1973) [Cancers in men following a long-term low-level
exposure to aflatoxins in indoor air.] T. Soc. Geneeskd., 51:
754-760 (in Dutch).
VAN PÉE, W., VAN BRABANT, J., & JOOSTENS, J. (1977) La détection et
le dosage de l'aflatoxine M1 dans le lait et le lait en poudre.
Rev. Agrie. (Bruxelles), 30: 403-415.
VAN RENSBURG, S. J. (1977) Role of epidemiology in the elucidation
of mycotoxin health risks. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publ., pp. 699-711.
VAN RENSBURG, S. J., VAN DER WATT, J. J., PURCHASE, I. F. H.,
PEREIRA COUTINHO, L., & MARKHAM, R. (1974) Primary liver cancer rate
and aflatoxin intake in a high cancer area. S. Afr. Med. J., 48:
2508a-2508d.
VANYI, A. & SZAILER, E. (1974) Investigation of the cytotoxic effect
of F-2 toxin (zearalenone) in various monolayer cell cultures.
Acta Vet. Acad. Sci. Hung., 24: 407-412.
VERRETT, M. J., MARLIAC, J.-P., & MCLAUGHLIN, J., JR (1964) Use of
the chicken embryo in the assay of aflatoxin toxicity.
J. Assoc. Off. Anal. Chem., 47: 1003-1006.
VESELY, D., VESELA, D., KUSAK, V., & NESNIDAL, P. (1978)
[Distribution of perorally administered aflatoxin B1 in tissues
and organs of the goat.] Veterinarni medicina, 23 (LI): 555-558 (in
Czech).
VESONDER, R. F., CIEGLER, A., & JENSEN, A. H. (1973) Isolation of
the emetic principle from Fusarium-infected corn.
Appl. Microbiol., 26: 1008-1010.
VESSELINOVITCH, S. D., MIHAILOVICH, N., WOGAN, G. N., LOMBARD, L.
S., & RAO, K. V. N. (1972) Aflatoxin B1, a hepatocarcinogen in the
infant mouse. Cancer Res., 32: 2289-2291.
VILLA-TREVINO, S. & LEAVER, D. D. (1968) Effects of the hepatotoxic
agents retrorsine and aflatoxin B1 on hepatic protein synthesis in
the rat. Biochem. J., 109: 87-91.
VOGEL, C. L., ANTHONY, P. P., MODY, N., & BARKER, L. F. (1970)
Hepatitis-associated antigen in Ugandan patients with hepatocellular
carcinoma. Lancet, 2: 621-624.
WALES, J. H. & SINNHUBER, R. O. (1972) Hepatomas induced by
aflatoxin in the sockeye salmon (Oncorhynchus nerka). J. Natl
Cancer Inst., 48: 1529-1530.
WALTKING, A. E. (1970) Collaborative study of three methods for
determination of aflatoxin in peanuts and peanut products.
J. Assoc. Off. Anal. Chem., 53: 104-113.
WALTKING, A. E. (1971) Fate of aflatoxin during roasting and storage
of contaminated peanut products. J. Assoc. Off. Anal. Chem., 54:
533-539.
WALTKING, A. E., BLEFFERT, G., & KIERNAN, M. (1968) An improved
rapid physio-chemical assay method for aflatoxin in peanuts and
peanut products. J. Am. Oil Chem. Soc., 45: 880-884.
WARD, J. M., SONTAG, J. M., WEISBURGER, E. K., & BROWN, C. A. (1975)
Effect of lifetime exposure to aflatoxin B1 in rats.
J. Natl Cancer Inst., 55: 107-113.
WARE, G. M. & THORPE, C. W. (1977) Analysis for zearalenone in corn
by high pressure liquid chromatography with a fluorescence detector.
Abstracts 91st Annual Meeting, Association of Official Analytical
Chemists, Washington, DC, p. 47 (Abstract no. 148).
WARE, G. M. & THORPE, C. W. (1978) Determination of zearalenone in
corn by high-pressure liquid chromatography and fluorescence
detection. J. Assoc. Off. Anal, Chem., 61 (5): 1058-1062.
WHITAKER, T. B. (1977)Sampling granular foodstuffs for aflatoxin.
Pure appl. Chem., 49: 1709-1717.
WHITAKER, T. B. & WHITTEN, M. E. (1977) Evaluation of cottonseed
aflatoxin testing programs. J. Am. Oil Chem. Soc., 54: 436-441.
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (1974a) Variability
of aflatoxin test results. J. Am. Oil Chem. Soc., 51: 214-218.
WHITAKER, T. B., DICKENS, J. W., WISER, E. H., & MONROE, R. J.
(1974b) Development of a method to evaluate sampling plans used to
estimate aflatoxin concentrations in lots of shelled peanuts.
I.U.P.A.C. Information Bulletin, Oxford, International Union of Pure
& Applied Chemistry Secretariat, pp. 1-14 (Technical Report-No. 10).
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (in press)
Variability associated with testing corn for aflatoxin. J. Am. Oil
Chem. Soc.
WHITTEN, M. E. (1969) Aflatoxins in cottonseed hulls. J. Am. Oil
Chem. Soc., 47: 5-6.
WHO (1978) Environmental Health Criteria 6-- Principles and methods
for evaluating the toxicity of chemicals. Part I, Geneva, World
Health Organization, 272 pp.
WILLIAMS, D. J. & RABIN, B. R. (1971) Disruption by carcinogens of
the hormone dependent association of membranes with polysomes.
Nature (Lond.), 232: 102-105.
WOGAN, G. N. (1966) Chemical nature and biological effects of the
aflatoxins. Bacteriol. Rev., 30: 460-470.
WOGAN, G. N. (1969) Metabolism and biochemical effects of
aflatoxins. In: Goldblatt, L. A., ed. Aflatoxin: Scientific
background, control and implications, New York, Academic Press,
pp. 151-186.
WOGAN, G. N. (1973)Aflatoxin carcinogenesis. In: Bush, M., ed.
Methods in cancer research, New York, Academic Press, pp. 309-344.
WOGAN, G. N. (1977) Mycotoxins and other naturally occurring
carcinogens. In: Kraybill, H. F. & Mehlman, M. A., ed.
Environmental cancer, Advances in modern toxicology, Washington,
DC, Hemisphere Publishing Corporation, Vol. 3, pp. 263-290.
WOGAN, G. N. & FRIEDMAN, M. A. (1968) Inhibition by aflatoxin B1
of hydrocortisone induction of rat liver tryptophan pyrrolase and
tyrosine transaminase. Arch. Biochem. Biophys., 128: 509-516.
WOGAN, G. N. & NEWBERNE, P.M. (1967) Dose-response characteristics
of aflatoxin B1 carcinogenesis in the rat. Cancer Res., 27:
2370-2376.
WOGAN, G. N. & PAGLIALUNGA, S. (1974) Carcinogenicity of synthetic
aflatoxin M1 in rats. Food Cosmet. Toxicol., 12: 381-384.
WOGAN, G. N., EDWARDS, G. S., & SHANK, R. C. (1967) Excretion and
tissue distribution of radioactivity from aflatoxin B1-14C in
rats. Cancer Res., 27: 1729-1736.
WOGAN, G. N., EDWARDS, G. S., & NEWBERNE, P.M. (1971)
Structure-activity relationships in toxicity and carcinogenicity of
aflatoxins and analogs. Cancer Res., 31: 1936-1942.
WOGAN, G. N., PAGLIALUNGA, S., & NEWBERNE, P.M. (1974) Carcinogenic
effects of low dietary levels of aflatoxin B1 in rats. Food
Cosmet. Toxicol., 12: 681-685.
WONG, J. J. & HSIEH, D. P. H. (1976) Mutagenicity of aflatoxins
related to their metabolism and carcinogenic potential. Proc. Natl
Acad. Sci. (USA), 73: 2241-2244.
WYATT, R. D., DOERR, J. A., HAMILTON, P. B., & BURMEISTER, H. R.
(1975) Egg production, shell thickness and other physiological
parameters of laying hens affected by T-2 toxin. Appl. Microbiol.,
29: 641-645.
WYLLIE, W. D. & MOREHOUSE, L. D., ed. (1977) Mycotoxic fungi,
mycotoxins, mycotoxicosis: An encyclopaedic handbook. New York and
Basel, Marcel Dekker.
YADGIRI, B., REDDY, V., TULPULE, P. G., SRIKANTIA, S. G., & GOPALAN,
C. (1970) Aflatoxin and Indian childhood cirrhosis; Am. J. clin.
Nutr., 23: 94-98.
YAGEN, B., JOFFE, A. Z., HORN, P., MOR, N., & LUTSKY, I. I. (1977)
Toxins from a strain involved in ATA. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 329-336.
YNDESTAD, M. & UNDERDAL, B. (1975) [Aflatoxin in foods on the
Norwegian market.] Nord. Vet.-Med., 27: 42-48 (in Norwegian).
4.2.2 Occurrence
The occurrence of zearalenone in foodstuffs has been reviewed by
Stoloff (1976). Zearalenone has been encountered as a natural
contaminant, particularly in maize, but occasionally in other
cereals and in feedstuffs. In a survey of maize in the USA during
the period 1968-69, zearalenone was found in 6 out of 576 (1%)
samples at levels ranging from 450 to 800 µg/kg (Shotwell et al.,
1971). In 1972, when conditions in the USA were conducive to
Fusarium ear rot, zearalenone was found in 17% of 223 samples of
maize at levels ranging from 0.1 to 5.0 mg/kg (Eppley et al., 1974).
It has also been detected in maize in France and in barley and mixed
feed in England, Finland, and Yugoslavia (Stoloff, 1976).
Nine out of 11 commercial corn meal samples in the USA contained
zearalenone at levels ranging from 12 to 69 µg/kg (Ware & Thorpe,
1977). The compound has been found in one sample of cornflakes
(13 µg/kg) (Scott et al., 1978), and in maize beer, in Zambia, in
the range of 0.01-4.6 mg/litre (Lovelace & Nyathi, 1977).
Zearalenone was detected in 11% of 55 samples of Swazi sour
drinks, sour porridges, and beers (range: 8-53 mg/kg) and in 12% of
140 beer samples from Lesotho (range: 0.3-2 mg/kg), but such high
figures have not been reported elsewhere. No data on consumption
were given (Martin & Keen, 1978).
4.2.3 Effects in animals
4.2.3.1 Field observations
The effects of zearalenone in animals have been reviewed by
Mirocha & Christensen (1974). Field cases of the estrogenic syndrome
in pigs, associated with the use of mouldy feed, were first observed
half a century ago. The disease was characterized by enlarged
oedematous vulvae and mammary glands (McNutt et al., 1928).
Subsequently, this syndrome has been encountered in a number of
countries in North America and Europe and in Australia (Table 25),
and in most cases zemralenone has been identified in associated
feeds, indicating together with the result of experimental studies
(4.2.2.2) a causal role of this compound. Zearalenone feed levels
reported to be associated with the syndrome in swine (Mirocha et
al., 1977) are listed in Table 26. Cases of reduced fertility in
cattle indicated by an increase in the artificial insemination index
have been reported to be associated with a feed (hay) content of
zearalenone of 14 mg/kg (Mirocha et al., 1968b). Fertility
disturbances and prolonged heat in a herd of cattle suggested to be
associated with zearalenone-contaminated feed were reported by Roine
et al. (1971).
Table 25. Occurrence of the estrogenic syndrome in pigs in
various countriesa
Year Country Feedstuff
1928 USA maize
1937 Australia maize
1952 Ireland barley
1962 France maize
1962 Italy maize
1963 Yugoslavia maize and barley
1967 Romania maize
1968 Hungary maize
1968 Denmark barley
1971 Canada maize
a From: Mirocha & Christenson (1974).
Table 26. Natural occurrence of zearalenone in feeds associated with
hyperestrogenism in swinea
Feed sample Level of
contamination
(mg/kg)
maize kernels (Minnesota)b 0.1-0.15
dry sow ration (Vancouver) 0.15
farrowing ration (Vancouver)c 0.066
dry sow ration (Vancouver) 0.15
corn kernels (Vancouver) 0.20
dry sow ration (Vancouver)c 0.25
lactation ration (Vancouver) 1.00
gestation ration (Vancouver) 0.6
milo (Minnesota) 2.5-5.6
sesame meal (Univ. of Minn.)d 1.5
corn kernels (Ohio) 0.12
mixed feed corn (Ohio)b 0.12
corn kernels (Minnesota) 6.4
commercial pelletted mixed feed 6.8
(Minnesota)
a From Mirocha et al. (1977).
b Rectal prolapse in gilts.
c Diethylstilbestrol was also present in these samples.
d Associated with hyperestrogenism in turkey poults.
4.2.3.2 Experimental studies
Under experimental conditions, pigs (6-week-old-gilts) exposed
perorally to zearalenone at 5 mg per animal per day for 5 days
developed enlarged vulvae and mammae, and prolapse of the vagina
within a few days; effects were reversible on termination of
exposure. In another experiment, oral administration of 8 mg of pure
crystalline zearalenone to 6-week-old, pre-pubertal gilts (8 doses
of 1 mg/day per animal) induced pronounced tumefaction of the vulva
(Mirocha & Christensen, 1974). Histological changes in the genital
tract of pigs exposed to zearalenone included metaplasia of the
epithelium in the cervix and vagina, and oedema in the wall of the
uterus (Kurtz et al., 1969).
Observations on pigs (Miller et al., 197.3) suggested that
ingestion of grain contaminated with zearalenone during late
gestation might be related to still-birth and splayleg. Splayleg was
observed in the offspring of one sow and one gilt given dally
intramuscular injections of zearalenone at 5 mg per animal
throughout the last month of pregnancy. However, in the experiment
of Patterson et al. (1977), all 7 gilts fed zearalenone at a level
of 2 mg/kg feed throughout pregnancy remained clinically normal and
embryonic survival rates were not affected by the toxin. Splayleg
was diagnosed in only 1/63 piglets and the condition appeared to
have resolved within 24 h. Results in the 2 groups of 3 pigs fed the
lower levels were inconclusive (unpublished data, Chang & Kurtz,
quoted by Mirocha et al., 1977).
No effects on egg production were observed when laying hens were
fed rations containing zearalenone levels of 250 and 500 mg/kg
(Speers et al., 1971).
In a 2-generation study on rats exposed to dietary levels of
zearalenone corresponding to daily intakes of 0.1, 1.0, and 10 mg/kg
body weight, no teratogenic effects were observed at any level of
intake but impaired fertility and resorptions and stillbirths
occurred in animals receiving 10 mg/kg body weight daily with 56% of
dams showing complete litter resorption (Bailey et al., 1976).
Ruddick et al. (1976) found fetal skeleton anomalies in rats
exposed orally to zearalenone at doses ranging from 1-10 mg/kg body
weight during the gestation period, with defect incidences of 12.8%
(11/86) at the 1 mg/kg level, 26.1% (18/69) at the 5 mg/kg level,
and 36.8% (28/76) at the 10 mg/kg level. No effect was observed with
exposure to zearalenone at a dose of 0.075-0.30 mg/kg body weight.
The results of a study by Mirocha et al. (1968a) in which the
dose-effect relationships for zearalenone and estrone were compared
with regard to increase in uterus weight, are given in Table 27. The
results of a study comparing the effects of estrogens and
zearalenone in 24 adult female castrated rhesus monkeys with a
previous history of regular menstrual cycles are recorded in Table
28.
Table 27. Effect of peroral dosing of zearalenone and estrone in micea
Material Total dose No. of Mean uterine
administered (µg) mice ratio ± S.E.
control 0 10 0.76 ± 0.06
estrone 0.5 10 1.21 ± 0.12
1 9 1.19 ± 0.10
2 9 2.12 ± 0.23
4 10 2.93 ± 0.21
zearalenone 12.5 10 1.11 ± 0.04
25 10 1.48 ± 0,18
50 10 1.80 ± 0.12
100 9 2.35 ± 0.17
a From: Mirocha et al. (1968a).
4.2.4 Conclusions and evaluation of the health risks to man of
zearalenone
4.2.4.1 Animal studies
Field cases of the estrogenic syndrome in pigs have been
encountered in many countries in association with zearalenone in
feeds at levels ranging from 0.1 to 6.8 mg/kg (Table 26), in samples
not known to be contaminated with other estrogens. The condition has
been reproduced experimentally in pigs, with a dally dose of
1 mg/animal for 8 days. Infertility, sporadically encountered in
cattle, has been suggested to be causally associated with
zearalenone in feed at a reported level of 14 mg/kg of hay.
In one of two studies on teratogenic effects in rats, skeletal
defects were detected in fetuses at a daily oral dose of 1 mg/kg
body weight.
Table 28. The estimated minimum dose of estrogens that will depress
serum gonadotropin (FSH or LH) in castrated rhesus monkeysa
Estrogen administered Estimated minimum dose
(µg/kg)
Subcutaneousb Oralc
LHd FSHd LH FSH
estradiol-17ß 4 1 5 --
diethylstilboestrol 0.5 2 2.5 --
zearalenone 14 56 400 --
a From: Hobson et al. (1977).
b Two injections given in oil.
c Given on 4 consecutive days,
d FSH = follicle-stimulating hormone; LH = luteinizing hormone.
4.2.4.2 Evaluation of health risks
There are no reports on the adverse effects of zearalenone in
man. With the exception of 2 reports from Africa, levels of
zearalenone ranging from 12 to 69 µg/kg have been found in a limited
number of maize products destined for human consumption. Even
assuming that 1 kg of these products is consumed dally, it can be
estimated that a 70 kg man would not receive more than 1 µg
zearalenone per kg body weight in food. This level of exposure is
400 times lower than the lowest peroral dose causing effects in
tests on monkeys (Table 28) and more than 600 times lower than the
lowest dose (µg/kg) used in assessing the estrogenic potency of
perorally administered zearalenone in mice (Table 27).
However, in the 2 reports from certain parts of Africa, high
levels of zearalenone were found in beer and sour porridge prepared
from maize and sorghum. Long-term exposure to such contaminated
drinks could represent a health hazard.
4.3 Trichothecenes
4.3.1 Properties and sources
The properties and sources of trichothecenes have been reviewed
by Bamburg & Strong (1971), Smalley & Strong (1974), and Bamburg
(1976). The trichothecenes possess the tetracyclic
12,13-epoxytrichothec-9-ene skeleton. More than 30 trichothecene
derivatives have been isolated from fungal cultures, but, so far,
only 4 have been identified as natural contaminants of foodstuffs
(Table 29). The compounds have been detected by various methods
including thin-layer chromatography, gas chromatography, and
bioassays, in particular the rabbit skin test.
One or more of the trichothecenes have been isolated from
strains of the following Fusarium species: F. episphae,
F. lateritium, F. nivale, F. oxysporum, F. rigidisculum,
F. solani, F. roseum, and F. tricinctum (syn.
F. sporotrichioides). In addition, species of Cephalosporium,
Myrothecium, Trichoderma, and Stachybotrys have been found to
produce trichothecenes.
Table 29. Natural occurrence of trichothecenes
Compound Concentration Commodity Reference
(µg/kg)
T-2 toxin 2 maize Hsu et al. (1972)
T-2 toxin 25 barley Puls & Greenway (1976)
T-2 toxin 0.076 mixed feed Mirocha et al. (1976)
nivalenol n.q.a barley Morooka et al. (1972)
deoxynivalenol 7.3 barley Morooka et al, (1972)
deoxynivalenol n.m.b maize Vesonder et al. (1973)
deoxynivalenol 0.1, 1.0, 1.8 maize Mirocha et al. (1976)
(3 samples)
deoxynivalenol 1.0, 1.0, 0.06 mixed feed Mirocha et al. (1976)
(3 samples)
diacetoxyscirpenol 0.38, 0.5 mixed feed Mirocha et al. (1976)
(2 samples)
a n.q. = not quoted
b n.m. = not measured
4.3.2 Occurrence
So far, the trichothecenes have only been found very
sporadically in natural products. Naturally occurring trichothecenes
include the following, based on chemical identification: T-2 toxin,
nivalenol, deoxynivalenol, (vomitoxin), and diacetoxyscirpenol. In
available studies, these 4 compounds have only been found in a total
of 14 samples (Table 29), sometimes (as in the case of
deoxynivalenol) concomitantly with zearalenone. No information is
available concerning the number of samples analysed in these studies
and therefore on the frequency of positive results.
4.3.3 Effects in animals
4.3.3.1 Field observations
A field case involving the death of 20% of a dairy herd was
suggested to be associated with ingestion of mouldy corn in the feed
containing a concentration of T-2 toxin of approximately 2 mg/kg dry
weight (Hsu et al., 1972). The lesions in the cattle included
extensive haemorrhages on the serosal surface of all internal
viscera.
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was suggested to be associated with mouldy barley
containing T-2 toxin at approximately 25 mg/kg (Greenway & Puls,
1976). The lesions in the geese included necrosis of the mucosa of
the oesophagus, proventriculus, and gizzards.
Deoxynivalenol (vomitoxin) was isolated from a batch of maize
that had caused vomiting in pigs (Vesonder et al., 1973).
4.3.3.2 Experimental studies
Acute effects of trichothecenes determined as LD50 values, are
listed in Table 30. Sato et al. (1975) studied tile effects in cats
of T-2 toxin, administered in several ways. Two cats weighing 1.5
and 4.0 kg were given purified T-2 toxin in single subcutaneous
doses of 0.5 and 1.0 mg/kg, respectively. Nausea and vomiting
appeared after 1 h and the animals died 20 h after the injection.
The autopsy revealed extensive necrosis of the mucosa in the small
and large intestine, marked karyorrhexis in the germ centre of the
lymph follicles of the spleen and lymph nodes, and diffuse vacuolar
degeneration of the renal tubules. Damage to bone marrow cells was
observed in the cervical, thoracic, and lumbar vertebrae. In two
cats subcutaneously injected with T-2 toxin (repeated doses of 0.1
and 0.05 mg/kg body weight, given over a 4-week period) a decrease
in the white blood cell (WBC) count was observed and the WBC value
remained low until death occurred during the fourth week of
exposure. The clinical signs in the cats were nausea and vomiting a
few hours after each injection and ataxia of the hind legs. At the
postmortem examination, hypoplasia was observed in the thymus,
spleen, lymph nodes, and bone marrow. Other changes included marked
meningeal haemorrhage, extensive bleeding in the lung, and vacuolar
degeneration of the renal tubular epithelium. A marked decrease in
the WBC values was also observed in 3 cats given T-2 toxin
subcutaneously at a daily dose of 0.05 mg/kg for 12 days (i.e.,
total dose of 0.60 mg/kg); 2 of these cats died, 4 and 35 days,
respectively, after the last injection and one cat survived with the
WBC count returning to the normal level 17 days after the last
injection. Leukopenia and death were also observed in 3 cats (body
weight 2-3 kg) receiving a crude preparation containing 4% T-2 toxin
and 1% neosolaniol. This crude preparation was first administered
subcutaneously once a week for 5 weeks in repeated doses of 1 mg/kg
body weight (corresponding to a repeated dose of T-2 toxin of
0.04 mg/kg). This subcutaneous administration was then followed by
daily oral dosing (15 mg of the crude preparation corresponding to
0.6 mg of T-2 toxin per animal per day) for 17 days.
Table 30. Acute toxicity of naturally occurring trichothecenesa
Compound LD50 Route of Animal
(mg/kg body weight) administration
T-2 toxin 3.04 intraperitoneal mouse
T-2 toxin 3.8 peroral rat
T-2 toxin 5.1 peroral trout
T-2 toxin 5.25 peroral one-day old chickb
nivalenol 4.0 intraperitoneal mouse
diacetoxyscirpenol 10.0 intravenous mouse
diacetoxyscirpenol 0.75 intraperitoneal rat
diacetoxyscirpenol 7.3 peroral rat
a From: Bamburg & Strong (1971 ).
b From: Chi at al. (1977).
More recently Yagen et al. (1978) have mentioned an experiment,
in which oral administration of gelatin capsules containing purified
T-2 toxin to 10 cats resulted in vomiting, leukopenia, haemorrhagic
diathasis, neurological disturbances, and death. The dose and
frequency of administration are not given in the paper.
The effects observed in these experiments, in particular
leukopenia, resemble those produced when cats are fed cultures of
F. sporotrichioides, which is thought to be causally associated
with alimentary toxic aleukia (ATA) in man (section 4.3.4).
Feeding laying hens T-2 toxin at a dietary level of 20 mg/kg for
3 weeks, resulted in oral necrotic lesions, decreased leukocyte
count, and reduced egg production (Wyatt et al., 1975). In mice,
intraperitoneal injection with T-2 toxin at doses of 1.0 and
1.5 mg/kg body weight on one of the days 7-11 of gestation resulted
in a number of maternal deaths as well as in an increase in prenatal
mortality. Malformations of the fetuses were observed including tail
and limb malformations, exencephaly, and retarded jaw development
(Stanford et al., 1975).
Daily oral doses of crude or purified T-2 toxin (0.2 mg/kg body
weight) continued for 79 days failed to produce ill effects in
calves, although the total amount of toxin ingested by one calf was
almost 1.8 g (Matthews et al., 1977).
4.3.4 Alimentary toxic aleukia
Studies on alimentary toxic aleukia (ATA), a disease encountered
in man in the period 1931-43, have been reviewed by Sarkisov (1954)
and more recently by Bilai (1977) and Leonov (1977). The dominant
pathological changes were necrotic lesions of the oral cavity, the
oesophagus, and stomach, and in particular a pronounced leukopenia.
The disease was lethal in a high proportion of cases. An association
was established with ingestion of grain invaded by some moulds, in
particular Fusarium poae and F. sporotrichioides. Effects similar
to ATA have been reproduced in cats by feeding cultures of these
species (Bilai, 1977). T-2 toxin and other trichothecenes have been
identified in a submerged culture of F. sporotrichioides by
Mirocha & Pathre (1973).
4.3.5 Conclusions and evaluation of the health risks to man of
trichothecenes
In recent years, a group of mycotoxins, the trichothecenes, has
been isolated under experimental conditions from many fungi,
including Fusarium species. Isolated field cases of intoxication
in farm animals have been suggested to be related to some of the
trichothecenes.
About 40 years ago, a disease in man known as alimentary toxic
aleukia (ATA), occurred that was suggested to be related to the
presence of toxic Fusarium species in mouldy over-wintered grain.
With improved harvesting, food production, and storage conditions,
the disease disappeared and no new outbreaks have occurred. However,
present knowledge is not sufficient to establish a causal
relationship between any of the isolated trichothecenes and this
outbreak. There are no reports on the exposure of man to
trichothecenes.
REFERENCES
ADAMSON, R. H., CORREA, P., & DALGARD, D. W. (1973) Brief
communication: Occurrence of a primary liver carcinoma in a rhesus
monkey fed aflatoxin B1. J. Natl Cancer Inst., 50:549-551.
ADAMSON, R. H., CORREA, P., SIEBER, S. M., MCINTIRE, K. R., &
DALGARD, D. W. (1976) Carcinogenicity of aflatoxin B1 in rhesus
monkeys: Two additional cases of primary liver cancer. J. Natl
Cancer Inst., 57: 67-78.
ADENKUNLE, A. A. & ELEGBE, R. A. (1974.) Lysosomal activity in
aflatoxin-B1-treated avian embryos. Lancet, 1: 991.
ALLCROFT, R. (1969) Aflatoxicosis in farm animals. In: Goldblatt,
L.A., ed. Aflatoxin scientific background, control and
implications, New York, Academic Press, pp. 237-264.
ALLCROFT, R. & LEWIS, G. (1963) Groundnut toxicity in cattle:
Experimental poisoning of calves and a report on clinical effects in
older cattle. Vet. Rec., 75: 487-493.
ALLCROFT, R. & ROBERTS, B. A. (1968) Toxic groundnut meal: The
relationship between aflatoxin B1 intake by cows and excretion of
aflatoxin M1 in milk. Vet. Rec., 82: 116-118.
ALLCROFT, R., ROBERTS, B. A., & LLOYD, M. K. (1968) Excretion of
aflatoxin in a lactating cow. Food Cosmet. Toxicol., 6: 619-625.
ALPERT, g. E., HUTT, g. S. R., WOGAN, G. N., & DAVIDSON, C. S.
(1971) Association between aflatoxin content of food and hepatoma
frequency in Uganda. Cancer, 28: 253-260.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973)
Carcinogens are mutagens: A simple test system combining liver
homogenates for activation and bacteria for detection. Proc. Natl
Acad. Sci. USA, 70: 2281-2285.
AMLA, I., KAMALA, C. S., GOPALAKRISHNA, G. S., JAYARAJ, A. P.,
SREENIVASAMURTHY, V., & PARPIA, H. A. B. (1971) Cirrhosis in
children from peanut meal contaminated by aflatoxin. Am. J. clin.
Nutr., 24: 609-614.
ANDERSON, H. W., NEHRING, E. W., & WICHSER, W. R. (1975) Aflatoxin
contamination of corn in the field. J. agric. food Chem., 23:
775-782.
ANDRELLOS, P. J. & REID, G. R. (1964) Confirmatory tests for
aflatoxin B1. J. Assoc. Off. Anal. Chem., 47: 801-803.
AMBRECHT, B. H., SHALKOP, W. T., ROLLINS, L. D., POHLAND, A. E., &
STOLOFF, L. (1970) Acute toxicity of aflatoxin B1 in wethers.
Nature (Lond.), 225: 1062.
ASAO, T., BUCHI, G., ABDEL-KADER, M., CHANG, S. B., WICK, E. L., &
WOGAN, G. N. (1963) Aflatoxins B and G. J. Am. Chem. Soc., 85:
1706-1707.
ASAO, T., BUCHI, G., ABDEL-KADER, M. M., CHANG, S. B., WICK, E. L.,
& WOGAN, G. N. (1965) The structures of aflatoxins B and G1. J.
Am. Chem. Soc., 87: 882-886.
ASHWORTH, L. J., JR, MCMEANS, J. L., HOUSTON, B. R., WITTEN, M. E.,
& BROWN, C. M. (1971) Mycoflora, aflatoxins and free fatty acids in
California cottonseed during 1967-1968. J. Am. Oil Chem. Soc., 48:
129-133.
ASPLIN, F. D. & CARNAGHAN, R. B. A. (1961) The toxicity of certain
groundnut meals for poultry with special reference to their effect
on ducklings and chickens. Vet. Rec., 73: 1215-1218.
AUSTWICK, P. K. C. (1975) Balkan nephropathy. Proc. R. Soc. Med.,
68: 219-221.
BABABUNMI, E. A. & BASSIR, O. (1972) Effects of aflatoxin B1 on
the swelling and adenosine triphosphatase activities of mitochondria
isolated from different tissues of the rat. Febs Lett., 26:
102-104.
BACON, C. W., SWEENEY, J. G., ROBBINS, J. D., & BURDICK, D. (1973)
Production of penicillic acid and ochratoxin A on poultry feed by
Aspergillus ochraceus: Temperature and moisture requirements.
Appl. Microbiol., 26: 155-160.
BAGSHAWE, A. F., GACENGI, D. M., CAMERON, C. H., DORMAN, J., & DANE,
D. S. (1975) Hepatitis Bs antigen and liver cancer in Kenya.
Br. J. Cancer, 31: 581-584.
BAILEY, D. E., Cox, G. E., MORGAREIDGE, K., & TAYLOR, J. (1976)
Acute and subacute toxicity of zearalenone in the rat. TAP, 37:
144.
BAMBURG, J. R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American
Chemical Society, pp. 144-162 (Advances in Chemistry Series 149).
BAMBURG, J. R. & STRONG, F. M. (1971) 12,13-epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S. J., ed. Microbial toxins, New
York, Academic Press, Vol. VII, pp. 207-289.
BASSIR, O. & BABABUNMI, E. A. (1969) Inhibition of blood clotting
factors by aflatoxin. West Aft. J. biol. appl. Chem., 12: 28-32.
BASSIR, O. & OSIYEMI, F. (1967) Biliary excretion of aflatoxin in
the rat after a single dose. Nature (Lond.), 215: 882.
BECKWITH, A. C., VESONDER, R. F., & CIEGLER, A. (1975) Action of
weak bases upon aflatoxin B1 in contact with macromolecular
reactants. J. agric. food Chem., 23: 582-587.
BECROFT, D. M. O. (1966) Syndrome of encephalopathy and fatty
degeneration of viscera in New Zealand children. Br. med. J., 2:
135-140.
BECROFT, D. M. O. & WEBSTER, D. R. (1972) Aflatoxins and Reye's
disease. Br. med. J., 14 Oct., p. 117.
BILAI, V. I. (1977) [Fuzarii.] Kiev, Naukova Dumka, pp. 360-394 (in
Russian).
BLACK, H. S., SMITH, J. D., AUSTIN, B. J., & RAUSCHKOLB, E. W.
(1970) Effect of aflatoxin B1 on the in vitro incorporation of
14C-acetate into human skin lipids. Experientia (Basel), 26:
1292-1293.
BOURGEOIS, C. H. (1975) Encephalopathy and fatty viscera: A possible
response to acute aflatoxin poisoning. In: Pollack, J. D., ed.
Reye's Syndrome, Grune & Stratton, New York, pp. 131-134.
BOURGEOIS, C. H., SHANK, R. C., GROSSMAN, R. A., JOHNSEN, D. O.,
WOODING, W. L., & CHANDAVIMOL, P. (1971) Acute aflatoxin B1
toxicity in the macaque and its similarities to Reye's Syndrome.
Lab. Invest., 24: 206-216.
BROWN, J. M. M. & ABRAMS, L. (1965)Biochemical studies on
aflatoxicosis. Onderstepoort J. vet. Res., 32: 119-146.
BROWN, M. H., SZCZECH G. M., & PURMALIS, B. P. (1976) Teratogenic
and toxic effects of ochratoxin A in rats. Toxicol. appl.
Pharmacol., 37: 331-338.
BUCARBAEVA, A. S. & NIKOV, P.S. (1977) [Aflatoxin sources in
foodstuffs from different regions of Kazachstan.] In: [ Mycotoxins
(sources, chemistry, biosynthesis, analysis and effects on the
organism), Scientific Papers of a Regional Symposium, 28-30
September 1977.] Orenburg, Nutrition Institute of the USSR Academy
of Medical Sciences, pp. 29-31 (in Russian).
BUCHI, G. & WEINREB, S. M. (1969) The total synthesis of racemic
aflatoxin-M1 (milk toxin). J. Am. Chem. Soc., 91: 5408-5409.
BUCHI, G., SPITZNER, D., PAGLIALUNGA, S., & WOGAN, G. N. (1973)
Synthesis and toxicity evaluation of aflatoxin P1. Life Sci.,
13: 1143-1149.
BUCKLEY, H. G. (1971) Fungal nephrotoxicity in swine. Irish
vet. J., 25: 194-196.
BULATAO-JAYME, J., ALMERO, E. M., & SALAMAT, L,. (1976) Epidemiology
of primary liver cancer in the Philippines with special
consideration of a possible aflatoxin factor. J. Philipp. Med.
Assoc., 52: 129-150.
BUTLER, W. H. (1964) Acute toxicity of aflatoxin B1 in rats.
Br. J. Cancer, 18: 756-762.
BUTLER, W. H. (1966) Acute toxicity of aflatoxin B1 in guinea
pigs. J. Pathol. Bacteriol., 91: 277-280.
BUTLER, W. H. (1969) Aflatoxicosis in laboratory animals. In:
Goldblatt, L.A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 223-236.
BUTLER, W. H. (1971-1972) Further ultrastructural observations on
injury of rat hepatic parenchymal cells induced by aflatoxin B1.
Chem. biol. Interactions, 4: 49-65.
BUTLER, W. H. (1974) Aflatoxin. In: Purchase, I. F. H., ed.
Mycotoxins, Amsterdam, Elsevier, pp. 1-28.
BUTLER, W. H. & BARNES, J. M. (1963) Toxic effects of groundnut meal
containing aflatoxin to rats and guinea pigs. Br. J. Cancer, 17:
699-710.
BUTLER, W. H. & BARNES, J. M. (1966) Carcinoma of the glandular
stomach in rats given diets containing aflatoxin. Nature (Lond.),
209: 90.
BUTLER, W. H. & WIGGLESWORTH, J. S. (1966) The effects of aflatoxin
B1 on the pregnant rat. Br. J. exp. Pathol., 47: 242-247.
BUTLER, W. H., GREENBLATT, M., & LIJINSKY W. (1969) Carcinogenesis
in rats by aflatoxins B1, G1, and B2. Cancer Res., 29:
2206-2211.
CAMPBELL, T. C. & SALAMAT, L. (1971) Aflatoxin ingestion and
excretion by humans. In: Purchase, I. F. H., ed. Mycotoxins in human
health, London, Macmillan Press, pp. 271-280.
CAMPBELL, T. C. & STOLOFF, L. (1974) Implication of mycotoxins for
human health. J. agric. food Chem., 22: 1006-1015.
CAMPBELL, T. C., CAEDO, J.P., JR, BULATAO-JAYME, J., SALAMAT, L., &
ENGEL, R. W. (1970) Aflatoxin M1 in human urine. Nature (Lond.),
227: 403-404.
CARNAGHAN, R. B. A. (1965) Hepatic tumours in ducks fed on low level
of toxic ground-nut meal. Nature (Lond.), 208, 308.
CARNAGHAN, R. B. A. (1967) Hepatic tumours and other chronic liver
changes in rats following a single oral administration of aflatoxin.
Br. J. Cancer, 21: 811-814.
CARNAGHAN, R. B. A., HARTLEY, R. D., & O'KELLY, J. (1963) Toxicity
and fluorescence properties of the aflatoxins. Nature (Lond.),
200: 1101.
CARNAGHAN, R. B. A., LEWIS, G., PATTERSON, D. S. P., & ALLCROFT, R.
(1966) Biochemical and pathological aspects of groundnut poisoning
in chickens. Pathol. Vet., 3: 601-615.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijee vjesn., 98: 301-304 (in Croatian).
CHAINUVATI, T., VIRANUVATT, V., & PONGPIPAT, D. (1975) Relationship
of hepatitis B antigen in cirrhosis and hepatoma in Thailand. An
etiological significance. Gastroenterology, 68: 1261-1264.
CHANG, F. C. & Car, F. S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, S. B., ABDEL-KADER, M. M., WICK, E. L., & WOGAN, G. N. (1963)
Aflatoxin B2: Chemical identity and biological activity.
Science, 142: 1191-1192.
CHAVES-CARBALLO, E., ELLEFSON, R. D., & GOMEZ, M. R. (1976) An
aflatoxin in the liver of a patient with Reye-Johnson syndrome.
Mayo Clin. Proc., 51: 48-50.
CHERNOZEMSKY, I. N., STOYANOV, I. S., PETKOVA-BOCHAROVA, T. K.,
NICOLOV, I. G., DRAGANOV, I. V., STOICHEV, I. I., TANCHEV, Y.,
NAIDENOV, D., & KALCHEVA, W. D. (1977) Geographic correlation
between the occurrence of endemic nephropathy and urinary tract
tumours in Vratza district, Bulgaria. Int. J. Cancer, 19: 1-11.
CHI, M. S., MIROCHA, C. J., KURTZ, M. Y., WEAVER, G., BATES, F., SHIMODA,
W., & BURMEISTER, H. R. (1977) Acute toxicity of T-2 toxin in
broiler chicks and laying hens. Poult. Sci., 56: 103-116.
CHU, F. S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F. S. (1974a) Studies on ochratoxins. CRC crit. Rev.
Toxicol., 2: 499-524.
CHU, F. S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol.,
23: 1105-1113.
CIEGLER, A., KADIS, S., & AJL, S. J., ed. (1971) Microbial toxins,
fungal toxins, New York, Academic Press, Vol. VI, p. 563.
CLEGG, F. G. & BRYSON, H. (1962) An outbreak of poisoning in store
cattle attributed to Brazilian groundnut meal. Vet. Rec., 37:
992-994.
CLIFFORD, J. I. & REES, K. R. (1966) Aflatoxin: A site of action in
the rat liver cell. Nature (Lond.), 209: 312-313.
CLIFFORD, J. I. & REES, K. R. (1967) The action of aflatoxin B, on
the rat liver. Biochem. J., 102: 65-75.
COON, F. B., BAUR, F. J., & SYMMES, L. R. L. (1972) International
aflatoxin check sample program: 1971 study. J. Assoc. Off. Anal.
Chem., 55: 315-327.
CUTHBERTSON, W. F. J., LAURSEN, A. C., & PRATT, D. A. H. (1967)
Effect of groundnut meal containing aflatoxin on Cynomolgus monkeys.
Br. J. Nutr., 21: 893-908.
DALEZIOS, J. I. & WOGAN, G. N. (1972) Metabolism of aflatoxin B1
in rhesus monkeys. Cancer Res., 32: 2297-2303.
DALEZIOS, J. I., WOGAN, G. N., & WEINREB, S. M. (1971) Aflatoxin
P1: A new aflatoxin metabolite in monkeys. Science, 171:
584-585.
DAVIS, N. D. & DIENER, U. L. (1970) Environmental factors affecting
the production of aflatoxin. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on "Toxic Microorganisms",
Washington, DC, US Govt Printing Office, pp. 43-47.
DEGER, G. E. (1976) Aflatoxin-Human colon carcinogenesis? Ann.
int. Med., 85: 204.
DE IONGH, H., BEERTHUIS, R. K., VLES, R. O., BARRETT, C. B., & ORD,
W. O. (1962) Investigation of the factor in groundnut meal
responsible for turkey X disease. Biochim. Biophys. Acta, 65:
548-551.
DEO, M. G., DAYAL, Y., & RAMALINGASWAMI, V (1970) Aflatoxins and
liver injury in the rhesus monkey. J. Pathol., 101 47-56.
DETROY, R. W. & HESSELTINE, C. W. (1970) Aflatoxicol: Structure of a
new transformation product of aflatoxin B1 Can. J. Biochem.,
48: 830-832.
DICKENS, J. W. & SATTERWHITE, J. B. (1973) Aflatoxin-contaminated
peanuts produced on North Carolina farms in 1968. J. Am. Peanut
Res. Educ. Assoc. Inc., 5: 11 pp.
DICKENS, F., JONES, H. E. H., & WAYNFORTH, H B. (1966) Oral
subcutaneous and intra-tracheal administration of carcinogenic
lactones and related substances: The intratracheal administration of
cigarette tar in the rat. Br. J. Cancer, 20: 134-144.
DIENER, U. L. & DAVIS, N. D. (1969) Production of aflatoxins on
peanuts under controlled environments. J. stored Prod. Res.,
5: 251-258;.
DI PAOLO, J. A., ELIS, J., & ERWIN, H. (1967) Teratogenic response
by hamsters, rats and mice to aflatoxin B1. Nature (Lond.), 215:
638-639.
DOHERTY, W. P. & CAMPBELL, T. C. (1972) Inhibition of rat-liver
mitochondria electron-transport flow by aflatoxin B1. Res.
Commun. chem. Path. Pharmacol., 3: 601-612.
DOHERTY, W. P. & CAMPBELL, T. C. (1973) Aflatoxin inhibition of rat
liver mitochondria. Chem. biol. Interactions, 7: 63-77.
DONALDSON, W. E., TUNG, H-T., & HAMILTON, P. B. (1972) Depression of
fatty acid synthesis in chick liver (Gallus domesticus) aflatoxin.
Comp. Biochem. Physiol., 41: 843-847.
DOSTER, R. C., SINNHUBER, R. O., & WALES, J. H. (1972) Acute
intraperitoneal toxicity of ochratoxins A and B in rainbow trout
(Salmo gairdneri). Food Cosmet. Toxicol, 10: 85-92.
DOTCHEV, V. D. (1973) [The endemic (Balkan) nephropathy in
Bulgaria.] Muench. med. Woenschr., 115: 537-541 (in German).
DVORACKOVA I. (1976) Aflatoxin inhalation and alveolar cell
carcinoma. Br. med. J., 20: 691.
DVORACKOVA I., BRODSKY, F., & GERMAN, J. (1974) Aflatoxin and
encephalitic syndrome with fatty degeneration of viscera. Nutr.
Rep. Int., 10: 89-102.
DVORACKOVA I., KUSAK, V., & NESNIDAL, P. (1977) Aflatoxin and
encephalopathy with fatty degeneration of viscera (Reye). Ann.
Nutr. Ailment., 31: 977-989.
EDDS, G. T., NAIR, K. P. C., & SIMPSON, C. F. (1973) Effects of
aflatoxin B1 on resistance in poultry against cecal coccidiosis
and Marek's disease. Am. J. vet. Res., 34: 819-826.
ELIS, J. & DI PAOLO, J. A. (1967) Aflatoxin B1. Arch. Pathol., 83:
53-57.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
Worm Health Organ., 49: 411-418.
ELLING, F., HALO, B., JACOBSEN, CHR., & KROGH, P. (1975) Spontaneous
toxic nephropathy in poultry associated with ochratoxin A. Acta
path. microbiol. Scand. Sect. A, 85: 739-741.
EPPLEY, R. M. (1966) A versatile procedure for assay and preparatory
separation of aflatoxins from peanut products. J. Assoc. Off.
Anal. Chem, 49: 1218-1223.
EPPLEY, R. M. (1968) Screening method for zearalenone, aflatoxin,
and ochratoxin. J. Assoc. Off. Anal. Chem, 51: 74-78.
EPPLEY, R. M., STOLOFF, L., TRUCKSESS, M. W., & CHUNG, C. W. (1974)
Survey of corn for Fusarium toxins. J. Assoc. Off. Anal. Chem,
57: 632-635.
EPSTEIN, S. M., BARTUS, B., & FARBER, E. (1969) Renal epithelial
neoplasms induced in male Wistar rats by oral aflatoxin B1. Cancer
Res., 29: 1045-1050.
FAO (1977) Mycotoxins. Report of the joint FAO/WHO/UNEP Conference
on Mycotoxins, Nairobi, 19-27 September, 1977, Rome, Food and
Agriculture Organization of the United Nations, 105 pp. (FAO Food
and Nutrition Paper 2).
FDA (1977) Report on action level for aflatoxin M1 in milk.
Washington DC, 21 November, Bureau of Foods, US Food and Drug
Administration.
FDA (1978) Assessment of estimated risk resulting from aflatoxins
in consumer peanut products and other food commodities.
Washington, DC, 19 January, Bureau of Foods, US Food and Drug
Administration.
FEUER, G, GOLBERG, L., & LEPELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose-6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FRIEDMAN, L. & MOHR, H. (1968) Absence of interaction between
cyclopropenoid fatty acids and aflatoxin in rats. Fed. Proc., 27:
551 (Abstract no. 1882).
FRITZ, W., DONATH, R., & ENGST, R. (1977) [Determination and
occurrence of aflatoxin M1 and B1 in milk and dairy produce.]
Nahrung, 21: 79-84 (in German).
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport san Ruin de la toxine chez le rat. Ann. Rech.
vet., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal II. Distribution tissulaire et élimination chez le rat. Ann.
Rech. vet., 5: 319-328.
GALTIER, P. (1975) Metabolism et mode d'action d'ochratoxine A. In:
Biquet, J. & Jemmali, M., ed. Les Mycotoxines, Paris, Editions
INSERM, pp. 69-78.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vet., 7:
91-98.
GALTIER, P., MORÉ., J., & BODIN, G. (1974) Toxine d' Aspergillus
ochraceus Wilhelm. III. Toxicité aigue de l'ochratoxine A chez le
rat et la souris adultes. Ann. Rech. vet., 5: 233-247.
GARNER, R. C. (1973) Chemical evidence for the formation of a
reactive aflatoxin B1 metabolite, by hamster liver microsomes.
Febs Lett., 36: 261-264.
GARNER, R. C. (1975) Reduction in binding of [14C]aflatoxin B1
to rat liver macromolecules by phenobarbitone pretreatment.
Biochem. Pharmacol., 24: 1553-1556.
GARNER, R. C., MILLER, E. C., MILLER, J. A., GARNER, J. V., &
HANSON, R. S. (1971) Formation of a factor lethal for S. typhimurium
TA 1530 and TA 1531 on incubation of aflatoxin B1 with rat liver
microsomes. Biochem. biophys. res. Comm., 45: 774-780.
GARNER, R. C., MILLER, E. C., & MILLER, J. A. (1972) Liver
microsomal metabolism of aflatoxin B1 to a reactive derivative
toxic to Salmonella typhimurium TA 1530. Cancer Res., 32:
2058-2066.
GODOY, H. M., JUDAH, D. J., ARORA, H. L., NEAL, G. E., & JONES, G.
(1976) The effects of prolonged feeding with aflatoxin B1 on adult
rat liver. Cancer Res., 36: 2399-2407.
GOLDBLATT, L. E., ed. (1969) Aflatoxin; scientific background,
control, and implications, New York, Academic Press, pp. 472.
GOLDBLATT, L. A. & DOLLEAR, F. G. (1977) Detoxification of
contaminated crops. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publishers Inc., pp. 139-150.
GOODAL, C. M. & BUTLER, W. H. (1969) Aflatoxin carcinogenesis:
Inhibition of liver cancer induction in hypophysectomized rats.
Int. J. Cancer, 4: 422-429.
GOPALAN; C., TULPULE, P. G., & KRISHNAMURTHI, D. (1972) Induction of
hepatic carcinoma with aflatoxin in the rhesus monkey. Food
Cosmet. Toxicol., 10: 519-521.
GRANT, K. E., COMER, M. W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small doses
of aflatoxin B1. Toxicol. appl. Pharmacol, 41: 166 (Abstract no.
84).
GREENWAY, J. A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Columbia. I. Natural occurrence and diagnosis. Can. J.
comp. Med., 40: 12-15.
GRICE, M. C., MOODIE, C. A., & SMITH, B. C. (1973) The carcinogenic
potential of aflatoxin or its metabolites in rats from dams fed
aflatoxin pre- and postpartum. Cancer Res., 33: 262-268.
HABISH, H. A., ABDULLA, M. H., & BROADBENT, J. H. (1971) The
incidence of aflatoxin in Sudanese groundnuts. Trop. Sci., 8:
279-287.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs. In:
Abstracts, IUPAC-Sponsored Symposium, Control of Mycotoxins,
Kungalr, Goteborg, Sweden, 21-22 Aug., 1972, Goteborg, Sweden,
Swedish Institute for Food Preservation Research, p. 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A in barley,
using silica gel mini-columns. J. Assoc. Off. Anal. Chem.,
58:156-158.
HALVER, J. E. (1969) Aflatoxicosis and trout hepatoma. In:
Goldblatt, L. A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 265-306.
HAMILTON, P. B. & HARRIS, J. R. (1971)Interaction of aflatoxicosis
with Candida albicans infections and other stresses in chickens.
Poult. Sci., 50: 906-912.
HANSSEN, E. & JUNG, M. (1972) [On the presence of aflatoxin in
non-mouldy food and proposals for sampling.]
Z. Lebensmitt.-Unters.-Forsch., 150: 141-145 (in German).
HARTLEY, R. D., NESBITT, B. F., & O'KELLY, J. (1963) Toxic
metabolites of Aspergillus flavus. Nature (Load.), 198: 1056-1058.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
345-367.
HARWIG, J. & CHEN, Y.-K. (1974) Some conditions favoring production
of ochratoxin A and citrinin by Penicillium viridicatum in wheat
and barley. Can. J. Plant. Sci., 54: 17-22.
HAYES, A. W., HOOD, R. D., & LEE, H. L. (1974) Teratogenic effects
of ochratoxin A in mice. Teratology, 9: 93-98.
HEPTINSTALL, R. H. (1966) Pathology of the kidney, London,
Churchill, pp. 457-464.
HESSELTINE, C. W. (1976) Conditions leading to mycotoxin
contamination of foods and feeds. In: Rodricks, J. V., ed.
Mycotoxins and other fungal related food problems, Washington, DC,
American Chemical Society, pp. 1-22 (Advances in Chemistry Series
149).
HESSELTINE, C. W., SORENSEN, W. G., & SMITH, M. (1970) Taxonomic
studies of the aflatoxin producing strains in the Aspergillus
flavus group. Mycologia, 62: 123-132.
HOBSON. W., BAILEY, J., & FULLER, G. B. (1970 Hormone effects of
zearalenone in non-human primates. J. Toxicol. environ. Health, 3:
43-57.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1: 133-151.
HOGAN, G. R., RYAN, N.J., & HAYES, A. W. (1978) Aflatoxin B1 and
Reye's syndrome. Lancet, March 11, p. 561.
HOLADAY, C. E. (1976) A rapid screening method For the aflatoxins
and ochratoxin A. J. Am. Oil Chem. Soc., 53: 603-605.
HORWITZ, W, SENZEL, A., REYNOLDS, H., & PARK, D. L., ed. (1975)
Natural poisons. In: Chapter 26, Official Methods of analysis of
the Association of Official Analytical Chemists, Washington, DC,
AOAC, p. 24.
HRABAR, A., SUJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Morbidity and mortality from endemic
nephropathy in the village of Kaniza.] Arh. hig. rada, 27: 137-145
(in Serbo-Croat).
HSIEH, D. P. H., WONG, Z. A, WONt, J. J., MICHAS, C., & RUEBNER, B.
H. (1977) Comparative metabolism of aflatoxin. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers Inc.,
pp. 37-50.
HSU I-CH., SMALLEY, E. B., STRONG, F. M., & RIBELIN, W. E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. MicrobioL., 24: 684-690.
HULT, K. & GATENECK, S. (1976) A spectrophotometric procedure using
carboxy-peptidase A, for the quantitative measurement of ochratoxin
A. J. Assoc. Off. Anal. Chem., 59: 128-129.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32:
443-444.
IARC (1976) Aflatoxins. In: IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man: Some naturally occurring
substances. Lyons, International Agency for Research on Cancer,
Vol. 10, pp. 51-72.
IARC (1977) IARC Monograph Programme on the evaluation of the
carcinogenic risk of chemicals to humans. Preamble. Lyons,
International Agency for Research on Cancer, 31 pp. (IARC Internal
Technical Report No. 77/002).
IUPAC (1976) Recommended methods of ochratoxins A and B in barley,
Oxford, International Union of Pure & Applied Chemistry Secretariat,
pp. 1-7 (Technical Reports-No. 14).
JEMMALI, M. & LAFONT, P. (1972) Evolution de l'aflatoxine B1 au
tours de la panification. Cah. Nutr. Diét., 7: 319-322.
JEMMALI, M. & MURTHY, T. R. K. (1976) A chemical assay method for
the determination of aflatoxin residues in animal tissues.
Z. Lebensm.-Unters.-Forsch., 161: 13-17.
JOHN, D. W. & MILLER, L. L. (1969) Effect of aflatoxin B1 on net
synthesis of albumin, fibrinogen and alpha1-acid glycoprotein by
the isolated perfused rat liver. Biochem. Pharmacol., 18:
1135-1146.
JONES, B. D. (1972) Methods of aflatoxin analysis. London,
Tropical Products Institute, 58 pp. (Report G 70).
JOSEPH-BRAVO, P. I., FINDLEY, M., & NEWBERNE P.M. (1976) Some
interactions of light, riboflavin, and aflatoxin B1 in vivo and
in vitro. J. Toxicol. environ. Health, 1: 353-376.
JUNG, M. & HANSSEN, E. (1974) [The occurrence of aflatoxin M1 in
dried milk products.] Food Cosmet. Toxicol., 12: 131-138 (in
German).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZNSKA, J. (1976) [Occurrence of
aflatoxin B1, B2, G1, and G2, ochratoxin A and B,
sterigmatocystin and zearalenone in cereals.] Med. Weter., 32:
617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feed and concentrates.] Med. Weter., 53: 193-196
(in Polish).
KATO, R., ONODA, K., & OMORI, Y. (1969) The effect of aflatoxin B1
on incorporation of 14C-acetate into cholesterol by rat liver.
Experientia (Basel), 25: 1026.
KATO, R., TAKANAKA, A., ONODA, K., & OMORI, Y. (1970) Different
effect of aflatoxin on the induction of tryptophan oxygenase and of
microsomal drug-hydroxylase system. J. Biochem. (Tokyo), 68:
589-592.
KEEN, P. & MARTIN, P. (1971a) The toxicity and fungal infestation of
foodstuffs in Swaziland in relation to harvesting and storage.
Trop. geogr. Med., 23-35-43.
KEEN, P. & MARTIN, P. (1971b) Is aflatoxin carcinogenic in man? The
evidence in Swaziland. Trop. geogr. Med., 23: 44-53.
KIERMEIER, F. ( 1973) Aflatoxin residues in fluid milk. Pure appl.
Chem., 35: 271-274.
KIERMEIER, R. (1977) The significance of aflatoxins in the dairy
Industry. Annu. Bull. Int. Dairy Fed., 98: 1-23, 25-43.
KIERMEIER, F., WEISS, G., BEHNRINGER, G., MILLER, M., & RANFFT, K.
(1977) [On the presence and the content of aflatoxin M1 in milk
shipped to a dairy plant.] Z. Lebensmitt.- Unters.-Forsch., 163:
171-174 (in German).
KOEHLER, B. (1938) Fungus growth in shelled corn as affected by
moisture J. agric. Res., 56: 291-307.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975a) Investigations into an outbreak of hepatitis in parts
of Western India. Indian J. med. Res., 63: 1036-1048.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975b) Hepatitis due to aflatoxicosis. Lancet, 1: 1061.
KROGH, P. (1974) Mycotoxic porcine nephropathy: A possible model for
Balkan endemic nephropathy. In: Puchlev, A., ed. Endemic
Nephropathy. Proceedings of the Second International Symposium on
Endemic Nephropathy, Sofia, November 9-11, 1972. Sofia, Bulgarian
Academy of Sciences, pp. 266-270.
KROGH, P. (1976a) Mycotoxic nephropathy. In: Advances in
veterinary science and comparative medicine, New York, Academic
Press, Vol. 20, pp. 147-170.
KROGH, P. (1976b) Epidemiology of mycotoxic porcine nephropathy.
Nord. Vet.-Med., 28: 452-458.
KROGH, P. (1977a) Ochratoxins. In: Rodricks, J. V., Hesseltine, C.
W., & Mehlman, M. A., ed. Mycotoxins in human and animal health,
Park Forest South, IL, USA, Pathotox Publishers, Inc., pp. 489-498.
KROGH, P. (1977b) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. Vet.-Med., 29: 402-405.
KROGH, P. (1977c) Mycotoxin tolerances in foodstuffs. Pure appl.
Chem., 49: 1719-1721.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy.
Acta path. microbiol. Scand. Sect. A, Suppl. no. 269.
KROGH, P. & ELLING, F. (1977) Mycotoxic nephropathy. Vet. Sci.
Commun., 1: 51-63.
KROGH, P. & HALD, B. (1969) [Occurrence of aflatoxin in imported
groundnut products.] Nord. Vet.-Med., 21: 398-407 (in Danish).
KROGH, P., HALD, B., & KORPINEN, E.-L. (1970) [Occurrence of
aflatoxin in groundnut- and copra-products imported to Finland.]
Nord. Vet.-Med., 22: 584-589 (in Norwegian).
KROGH, P., HALD, B., HASSELAGER, E., MADSEN, A., MORTENSEN, H. P.,
LARSEN, A. E., & CAMPBELL, A.D. (1973a) Aflatoxin residues in bacon
pigs. Pure appl. Chem., 35: 275-282.
KROGH, P., HALD, B., & PEDERSEN, E. J. (1973b) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta path. microbiol. Scand. Sect. B, 81:
689-695.
KROGH, P., AXELSEN, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A. E., MADSEN, A., MORTENSEN, H. P.,
MOLLER, T., PETERSEN, O. K., RAVNSKOV, U., ROSTGAARD, M., & AALUND,
O. (1974) Experimental porcine nephropathy: Changes of renal
function and structure induced by ochratoxin A- contaminated feed.
Acta path. microbiol. Scand. Sect. A, Suppl. No. 246, 9 pp.
KROGH, P., ELLING, F., BALD, B., LARSEN, A. E., LILLEHOJ, E. B.,
MADSEN, A., & MORTENSEN, H. P. (1976a) Time-dependent disappearance
of ochratoxin A residues in tissues of bacon pigs. Toxicology, 6:
235-242.
KROGH, P., ELLING, F., GYRD-HANSEN, H., HALD, B., LARSEN, A. E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H. P., & RAVNSKOV, U.
(1976b) Experimental porcine nephropathy: Changes of renal function
and structure perorally induced by crystalline ochratoxin A. Acta.
pathol. microbiol. Scand. Sect. A, 84: 429-434.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V. E.,
SKADHAUGE, E., & SVENDSEN, C. K. (1976c) Experimental avian
nephropathy: Changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta path. microbiol. Scand. Sect.
4, 84: 215-221.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and foodborne ochratoxin A: Preliminary
results of a survey of foodstuffs. Acta path. microbiol. Scand.
Sect. B, 85. 238-240.
KURTZ, M. J., NAIRN, M. E., NELSON, G. H., CHRISTENSEN, C. M., &
MIROCHA, C. J. (1969) Histologic changes in the genital tracts of
swine fed estrogenic mycotoxin. Am. J. vet. Res., 30: 551-556.
LARSEN, S. (1928-1929) [On chronic degeneration of the kidneys
caused by mouldy rye.] Maanedsskr. Dyrl., 40: 259-284 & 289-300
(in Danish).
LE BRETON, E., FRAYSSINET, C., LAFARGE, C., & DE RECONDO, A.M.
(1964) Aflatoxine-mechanisme de l'action. Food Cosmet. Toxicol.,
2: 675-676.
LEE, D. J., WALES, J. H., & SINNHUBER, R. O. (1969a) Hepatoma and
renal tubule adenoma in rats fed aflatoxin and cyclopropenoid fatty
acids. J. Natl Cancer Inst., 43: 1037-1044.
LEE, L. S., CUCULLU, A. F., FRANZ, A. O., JR, & PONS, W. A., JR
(1969b) Destruction of aflatoxins in peanuts during dry and oil
roasting. J. agric. food Chem., 17: 451-453.
LEE, W. V. (1965) Quantitative determination of aflatoxin in
groundnut products. Analyst, 90: 305-307.
LEONARD, A., DEKNUDT, GH., & LINDEN, G. (1975) Mutagenicity tests
with aflatoxins in the mouse. Mutat. Res., 28: 137-139.
LEONOV, A. N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 323-328.
LEVI, C. P., TRENK, H. L., & MOHR, H. K. (1974) A study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LIJINSKY, W. & BUTLER, W. H. (1966) Purification and toxicity of
aflatoxin G1. Proc. Soc. Exp. Biol. Med., 123: 151-154.
LIJINSKY, W., LEE, K. Y., & GALLAGHER, C. H. (1970) Interaction of
aflatoxin B1 and G1 with tissues of the rat. Cancer Res., 30:
2280-2283.
LILLEHOJ, E. B., FENNELL, D. I., & KWOLEK, W. F. (1976)
Aspergillus flavus and aflatoxin in Iowa corn before harvest.
Science, 193: 195-496.
LIN, J. J., LIU, C., & SVOBODA, D. J. (1974) Long-term effects of
aflatoxin B1 and viral hepatitis on marmoset liver. Lab. Invest.,
30: 267-278.
LING, K. H., WANG, J. J., WU, R., TUNG, T. C., LIN, C. K., LIN,
S.S., & LIN, T. M. (1967) Intoxication possibly caused by aflatoxin
B1 in mouldy rice in Shuang-Chih township. J. Formosan Med.
Assoc., 66: 517-525.
LINSELL, C. A. & PEERS, F. G. (1977) Field studies on liver cell
cancer. In: Hiatt, H. H., Watson, J. D., & Wingsten, J. A., ed.
Origins of human cancer. Cold Spring Harbor, NY, Cold Spring
Harbor Laboratory, pp. 549-556.
LO, W-B. & BLACK, H. S. (1972) Effects of aflatoxin B1 on human
skin lipids metabolism-a site of action on 14C-acetate
incorporation. Experientia (Basel), 28: 1278-1279.
LOOSMORE, R. M. & HARDING, J. D. J. (1961) A toxic factor in
Brazilian groundnut causing liver damage in pigs. Vet. Rec., 73:
1362-11364.
LOOSMORE, R. M. & MARKSON, L. M. (1961) Poisoning of cattle by
Brazilian groundnut meal. Vet. Rec., 73: 813-814.
LOVELACE, C. E. A. & NYATHI, C. B. (1977) Estimation of the fungal
toxins, zearalenone and aflatoxin, contaminating opaque maize beer
in Zambia. J. Sci. Food Agric., 28: 288-292.
LUCAS, F. V., JR., MONROE, P., NGA, P. V., TOWNSEND, J. F., & LUCAS,
F. V. (1970-1971) Mycotoxin contamination of South Vietnamese rice.
J. trop. Med. Hyg., 74: 182-183.
LUCK. H., STEYN, M., & WEHNER, F. C. (1917) A survey of milk powder
for aflatoxin content. S. Afr. J. dairy Technol., 8: 85-86.
LVOVA, L. S., SOSEDOV, N. I., GARALL, W., SCHWARZMAN, M. I.,
SHATILOVA, T. I., & SHULGINA, A. P. (1976) [Formation of aflatoxins
in wheat grain induced by self-heating and change in its chemical
composition by the development of storage moulds.] Prikl. Biochem.
Mikrobiol., 12: 741-749 (in Russian).
MADHAVAN, T. V. & GOPALAN, C. (1975) Effect of dietary protein on
aflatoxin injury in weanling rats. Arch. Pathol.,
80: 123-126.
MADHAVAN, T. V. & GOPALAN, C. (1968) The effect of dietary protein
on carcinogenesis of aflatoxin. Arch. Pathol., 85: 133-137.
MADHAVAN, T. V., SURYANARAYANA RAO, K., The effect of dietary
protein level on susceptibility of monkeys to aflatoxin liver
injury. Indian J. med. Res., 53: 984-989.
MADHAVAN, T. V., TULPULE, P. G, & GOPALAN, C. (1965b)
Aflatoxin-induced hepatic fibrosis in rhesus monkeys. Arch.
Pathol., 79: 466-469.
MALEKI, M. F., SUZANGAR, M., JAMSHIDI, CH., TAHMASEBI, A., &
BARNETT, R. (1976) Urinary aflatoxin detected in cirrhotic patients
in Ispahan, Iran. Clin. Res., 24: 630A.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MARSH, P. B., SIMPSON, M. E., CRAIG, G. O., DONOSO, J., & RAMEY, H.
H., JR (1973) Occurrence of aflatoxins in cotton seeds at harvest in
relation to location of growth and field temperatures. J. environ.
Qual., 2: 276-281.
MARTIN, P.M. D. & KEEN, P. (1978) The occurrence of zearalenone in
raw and fermented products from Swaziland and Lesotho. Sabrouaudia,
16: 15,22.
MASRI, M. S., LUNDIN, R. E., PAGE, J. R., & GARCIA, V. V. (1967)
Crystalline aflatoxin M1 from urine and milk. Nature (Lond.),
215: 753-755.
MASRI, M. S., HADDON, W. F., LUNDIN, R. E., & HSIEH, D. P. H.
(1974a) Aflatoxin Q1: A newly identified major metabolite of
aflatoxin B1 in monkey liver. J. agric. food Chem., 22: 512-514.
MASRI, M. S., BOOTH, A. N., & HSIEH, D. P. H. (1974b) Comparative
metabolic conversion of aflatoxin B1 to aflatoxin M1 and
aflatoxin Q1 by monkey, rat and chicken liver. Life Sci., 15:
203-212.
MATTHEWS, J. G., PATTERSON, D. S. P., ROBERTS, B. A., & SHREEVE, B.
J. (1977) T-2 toxin and haemorrhagic syndromes in cattle. Vet.
Rec., 101 (19): 391.
MCLEAN, A. E. M. & MARSHALL, A. (1971) Reduced carcinogenic effects
of aflatoxin in rats given phenobarbitone. Br. d. exp. Pathol.,
52: 322-329.
MCNUTT, S. H., PURWIN, P., & MURRAY, C. (1928) Vulvovaginitis in
swine J. Am. Vet. Med. Assoc., 73: 484;-492.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MERWE, K. J. van der, STEYNE, P.S., FOURIE, L., SCOTT, DE B., &
THERON, J. J. (1965) Ochratoxin A, a toxic metabolite produced by
Aspergillus ochraceus Wilh. Nature (Lond.), 205: 1112-1113.
MILLER, J. K., HACKING, A., & GROSS, V. J. (I[973) Stillbirths,
neonatal mortality and small litters in pigs associated with the
ingestion of Fusarium toxin by pregnant sows. Vet. Rec., 93:
555-559.
MINTZLAFF, H.-J., LOTZSCH, R., TAUCHMANN, F., MEYER, W., & LEISTNER,
L. (1974) [Aflatoxin residues in the liver and muscle of broiler
chicken given aflatoxin containing feed.] Fleischwirtschaft, 54:
774-778 (in German).
MIROCHA, C. J. & CHRISTENSEN, C. M. (1974) Oestrogenic mycotoxins
synthesized by Fusarium. In: Purchase, I. F. H, ed. Mycotoxins,
Amsterdam, Elsevier, pp. 129-148.
MIROCHA, C. J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poaefusarin. Appl. Microbiol., 26:
719-724.
MIROCHA, C. J., CHRISTENSEN, C. M., & NELSON, G. H. (1968a) Toxic
metabolites produced by fungi implicated in mycotoxicoses.
Biotech. Bioeng., 10: 469-482.
MIROCHA, C. J., HARRISON, J., NICHOLS, A. A.., & MCCLINTOCK, M.
(1968b) Detection of a fungal estrogen (F-2) in hay associated with
infertility in dairy cattle. Appl. Microbiol., 16: 797-798.
MIROCHA, C. J., SCHAUERHAMER, B., & PATHRE, S. V. (1974) Isolation,
detection and quantitation of zearalenone in maize and barley.
J. Assoc. Off. Anal. Chem., 75: 1104-1110.
MIROCHA, C. J., PATHRE, S. V., SCHAUERHAMER, B., & CHRISTENSEN, C.
M. (1976) Natural occurrence of Fusarium toxins in feedstuff. Appl
environ. Microbiol., 32: 553-556.
MIROCHA, C. J., PATHRE, S. W., & CHRISTENSEN, C. M. (1977)
Zearalenone. In: Rodricks, J. V., Hesseltine, C. W., & Mehlman, M.
A., ed. Mycotoxins in human and animal health, Park Forest South,
IL, USA, Pathotox Publishers Inc., pp. 345-364.
MOROOKA, N., URATSUJI, N., YOSHIZAWA, T., & YAMAMOTO, H. (1972)
[Studies on the toxic substances in barley infected with Fusarium
spp.] Jpn. J. food Hyg, 13: 368-375 (in Japanese).
MUNRO, I. C, MOODIE, C. A, KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H. C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol, 28: 180-188.
NABNEY, J., BURBAGE, M. B., ALLCROFT, R., & LEWIS, G. (1967)
Metabolism of aflatoxin in sheep: Excretion pattern in the lactating
ewe. Food Cosmet. Toxicol., 5: 11-17.
NEAL, G. E. & GODOY, H. M. (1976) The effect of pre-treatment with
phenobarbitone on the activation of aflatoxin B1 by rat liver.
Chem. biol. Interactions, 14: 279-289.
NEELY, W. C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55: 1305-1309.
NEL, W. & PURCHASE, I. F. M. (1968) Note. The fate of ochratoxin A
in rats. J.S. Afr. Chem. Inst., 21: 87-88.
NESHEIM, S. (1971) Report on ochratoxins: Occurrence, production,
analysis and toxicity. In: Abstracts of the 85th Annual meeting of
the Association of Official Analytical Chemists, Washington, DC,
Oct. 11-14, 1971, p. 23.
NESHEIM, S. (1976) The ochratoxins and other related compounds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 276-295
(Advance.,; in Chemistry Series 149).
NESHEIM, S., HARDIN, N. F., FRANCIS, O. J., JR, & LANGHAM, W. S.
(1973) Analysis of ochratoxins A and B and their esters in barley,
using partition and thin layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NEUMANN-KLEINPAUL, A. & TERPLAN, G. (1972) [The presence of
aflatoxin M1 in dried milk products.] Arch. Lebensmittelhyg.,
23: 128-132 (in German).
NEWBERNE, P.M. (1974) Mycotoxins: Toxicity, carcinogenicity and the
influence of various nutritional conditions. Environ. Health
Perspect., 9: 1-32.
NEWBERNE, P.M. (1976) Environmental modifiers of susceptibility to
carcinogenesis. Cancer Detect. Prev., 1: 129-173.
NEWBERNE, P.M. & BUTLER, W. H. (1969) Acute and chronic effects of
aflatoxin on the liver of domestic and laboratory animals: A review.
Cancer Res., 29: 236-250.
NEWBERNE, P.M. & CONNER, M. W. (1974) Effect of selenium on acute
response to aflatoxin B1. In: Hemphill, D. D., ed. A Symposium.
Trace Substances in Environmental Health-VIII, Columbia, MO,
University of Missouri, pp. 323-328.
NEWBERNE, P.M. & GROSS, R. L. (1977) The role of nutrition in
aflatoxin injury. In: Rodricks, J. V., Hesseltine, C. W, & Mehlman,
M. A., ed. Mycotoxins in human and animal health, Park Forest
South, IL, USA, Pathotox Publishers Inc., pp. 51-65.
NEWBERNE, P.M. & ROGERS, A. E. (1971) Aflatoxin carcinogenesis in
rats: Dietary effects. In: Purchase, I. F. H., ed. Mycotoxins in
human health, London, Macmillan, pp. 195-208.
NEWBERNE, P.M. & ROGERS, A. E. (1973) Rat colon carcinomas
associated with aflatoxin and marginal vitamin A. J. Natl Cancer
Inst., 50: 439-448.
NEWBERNE, P.M. & ROGERS, A. E. (1976) Nutritional modulation of
carcinogenesis. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. of Tokyo Press, & Baltimore, Univ.
Park Press, pp. 15-40.
NEWBERNE, P.M. & SUPHAKARN, V. (1977) Preventive role of vitamin A
in colon carcinogenesis in rats. Cancer, 40: 2553-2556.
NEWBERNE, P.M. & WILLIAMS, G. (1969) Inhibition of aflatoxin
carcinogenesis by diethyl-stilbestrol in male rats. Arch. environ.
Health, 19: 489-498.
NEWBERNE, P.M. & WOGAN, G. N. (1968a) Sequential morphologic changes
in aflatoxin B1 carcinogenesis in the rat. Cancer Res., 28:
770-781.
NEWBERNE, P.M. & WOGAN, G. N. (1968b) Potentiating effects of
low-protein diets on effect of aflatoxin in rats. Toxicol. appl.
Pharmacol., 12: 309-310.
NEWBERNE, J. W., BAILEY, W. S., & SEIBOLD, H. R. (1955) Notes on a
recent outbreak and experimental reproduction of hepatitis X in
dogs. J. Am. Vet. Med. Assoc., 127: 59-62.
NEWBERNE, P.M., RUSSO, R., & WOGAN, G. N. (1966a) Acute toxicity of
aflatoxin B1 in the dog. Pathol. vet., 3: 331-340.
NEWBERNE, P.M., HARRINGTON, D. H., & WOGAN, G. N. (1966b) Effects of
cirrhosis and other liver insults on induction of liver tumours by
aflatoxin. Lab. Invest., 15: 962-969.
NEWBERNE, P.M., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal
lesions in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
NEWBERNE, P.M., CHAN, W-CH. M., & ROGERS, A. E. (1974) Influence of
light, riboflavin, and carotene on the response of rats to the acute
toxicity of aflatoxin and mono-crotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NILSSON, G. (1974) [Aflatoxin in nuts.] Vor Foda, 26: 56-62 (in
Swedish).
NIXON, J. E., SINNHUBER, R. O., LEE, D. J., LANDERS, M. K., & HARR,
J. R. (1974) Effect of cyclopropenoid compounds on the carcinogenic
activity of diethylnitrosamine and aflatoxin B1 in rats. J. Natl
Cancer Inst., 53: 453-458.
NORTHOLT, M.D., VERHULSDONK, C. A. H., SOENTORO, P.S. S., & PAULSCH,
W. E. (1976) Effect of water activity and temperature on aflatoxin
production by Aspergillus parasiticus. J. milk food Technol., 39:
170-174.
OLSON, L. C., BOURGEOIS, C. H., JR, COTTON, R. B., HARIKUL, S.,
GROSSMAN, R. A., & SMITH, T. J. (1971) Encephalopathy and fatty
degeneration of the viscera in northeastern Thailand. Clinical
syndrome and epidemiology. Pediatrics, 47: 707-716.
ONG, T-M. (1975) Aflatoxin mutagenesis. Mutat. Res., 32: 35-53.
PAG FAG/WHO/UNICEF PROTEIN ADVISORY GROUP (1969) PAG Recommendation
on aflatoxin. PAG Statement No. 2. New York, United Nations, 2 pp.
PANALAKS, T. & SCOTT, P.M. (1977) Sensitive silica gel-packed
flowcell for fluorometric detection of aflatoxins by high pressure
liquid chromatography. J. Assoc. Off. Anal. Chem., 60: 583-589.
PARKER, W. A. & MELNICK, D. (1966) Absence of aflatoxin from refined
vegetable oils. J. Am. Oil Chem. Soc., 43: 635-638.
PATHRE, S. V. & MIROCHA, C. J. (1976) Zearalenone and related
compounds. In: Rodricks, J. V., ed. Mycotoxins and other fungal
related food problems, Washington, DC, American Chemical Society
pp. 178-227 (Advances in Chemistry Series 149).
PATTERSON, D. S. P. (1973) Metabolism as a factor in determining the
toxic action of the aflatoxin in different animal species. Food
Cosmet. Toxicol., 11: 287-294.
PATTERSON, D. S. P. (1976) Structure, metabolism and toxicity of the
aflatoxins: A review. Cah. Nutr. Diét., 11 (Suppl. 2): 71-76.
PATTERSON, D. S. P. (1977) Aflatoxin and related compounds. In:
Wyllie, T.D. & Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: An encyclopedic handbook, New York & Basel, Marcel
Dekker, pp. 131-136 & 144-189.
PATTERSON, D. S. P. & ROBERTS, B. A. (1970) The formation of
aflatoxin B2a and G2a and their degradation products during the
in vitro detoxification of aflatoxin by livers of certain avian
and mammalian species. Food Cosmet. Toxicol., 8: 527-538.
PATTERSON, D. S. P. & ROBERTS, B. A. (1971) The in vitro reduction
of aflatoxins B1 and B2 by soluble avian liver enzymes. Food
Cosmet. Toxicol., 9: 829-837.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972a) Steroid sex hormones as
inhibitors of aflatoxin metabolism in liver homogenates.
Experientia (Basel), 28: 929-930.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972b) Aflatoxin metabolism in
duck-liver homogenates: The relative importance of reversible
cyclopentenone reduction and hemiacetal formation. Food Cosmet.
Toxicol., 10: 501-512.
PATTERSON, D. S. P., ROBERTS, B. A., SHREEVE, B. J., WRATHALL, A.
E., & GITTER, M. (1977) Aflatoxin, ochratoxin and zearalenone in
animal feedstuffs: Some clinical and experimental observations. Ann
Nutr. Aliment., 31: 643-650.
PATTERSON, D. S. P., SHREEVE, B. J., & ROBERTS, B. A. (in press)
Mycotoxin residues in body fluids and tissues of food-producing
animals. In: Proceedings of the 12th International Congress
Microbiology, Munchen, Federal Republic of Germany, 3-8 September
1978.
PAUL, R., KALRA, M. S., & SINGH, A. (1976) Incidence of aflatoxins
in milk and milk products. Indian J. dairy Sol., 29: 318-322.
PAYET, M., CROS, J., QUENUM, C., SANKALE, M., & MOULANIER, M. (1966)
Deux observations d'enfants avant consommé de façon prolongée des
farines souillées par "Aspergillus flavus". Presse med.,
13: 649-651.
PECKHAM, J. C., DOUPNIK, B., JR, & JONES, O. H., JR (1971) Acute
toxicity of ochratoxins A and B in chicks. Appl. Microbiol., 21:
492-494.
PEERS, F. G. & LINSELL, C. A. (1973) Dietary aflatoxins and liver
cancer--a population based study in Kenya. Br. J. Cancer, 27:
73-484.
PEERS, F. G. & LINSELL, C. A. (1976) Acute toxicity of aflatoxin
B1 for baboons. Food Cosmet. Toxicol., 14: 227-228.
PEERS, F. G. & LINSELL, C. A. (1977) Dietary aflatoxins and human
primary liver cancer. Gaucher, A., ed. Ann. Nutr. Aliment., 31:
1005-1018.
PEERS, F. G., GILMAN, G. A., & LINSELL, C. A. (1976) Dietary
aflatoxins and human liver cancer. A study in Swaziland. Int. J.
Cancer, 17: 167-176.
PETTIT, R. E., TABER, R. A., SCHROEDER, H. W., & HARRISON, A. L.
(1971) Influence of fungicides and irrigation practice on aflatoxin
in peanuts before digging. Appl. Microbiol., 22: 629-634.
PHILLIPS, D. L., YOURTEE, D. M., & SEARLES, S. (1976) Presence of
aflatoxin B1 in human liver in the United States. Toxicol. appl.
Pharmacol., 36: 403-406.
PIER, A. C. & HEDDLESTON, K. L. (1970) The effect of aflatoxin on
immunity in turkeys. I. Impairment of actively acquired resistance
to bacterial challenge. Avian Dis., 14: 797-809.
PIER, A. C., CYSEWSKI, S. J., RICHARD, J. L., & THURSTON, J. R.
(1977) Mycotoxins as a veterinary problem. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 745-750.
PITOUT, M. J. (1969) The hydrolysis of ochratoxin A by some
proteolytic enzymes. Biochem. Pharmacol., 18: 485-491.
POHLAND, A. E., YIN, L., & DANTZMAN, J. G. (1970) Rapid chemical
confirmatory method for aflatoxin B1. I. Development of the
method. J. Asoc. Off. Anal. Chem., 53: 101-102.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELJAN, V. A. (1972) Effect
of aflatoxin on rat liver lysosomes. Toxicon, 10: 25-30.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELIAN, V. A. (1977)
[Aflatoxins.] Toksikologija, Moskva, Viniti, Vol. 8, 108 pp. (in
Russian).
POLZHOFER, K. (1977) [Determination of aflatoxins in milk and milk
products.] Z. Lebensmitt.-Unters.-Forsch., 163: 175-177 (in
German).
PONG, R. S. & WOGAN, G. N. (1969) Time course of alterations of rat
liver polysome profiles induced by aflatoxin B1. Biochem.
Pharmacol., 18: 2357-2361.
PONG, R. S. & WOGAN, G. N. (1970) Time course and dose-response
characteristics of aflatoxin B1 effects on rat liver RNA
polymerase and ultrastructure. Cancer Res., 30: 294-304.
PONG, R. S. & WOGAN, G. N. (1971) Toxicity and biochemical and fine
structural effects of synthetic aflatoxins M1 and B1 in rat
liver. J. Natl Cancer Inst., 47: 585-592.
PRINCE, A.M., SZMUNESS, W., MICHON, J, DEMAILLE, J., DIEBOLT, G.,
LINHARD, J., QUENUM, C., & SANKALE, M. (1975) A case/control study
of the association between primary liver cancer and hepatitis B
infection in Senegal. Int. J. Cancer, 16: 376-383.
PRIOR, M. G., SISODIA, C. S., & O'NEIL, J. B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese
quail. Poult. Sci., 55: 786-790.
PRZYBYLSKI, W. (1975) Formation of aflatoxin derivatives on thin
layer chromatographic plates. J. Assoc. Off. Anal. Chem., 58:
163-164.
PUCHLEV, A. (1973) La néphropathie endémique en Bulgarie. Extrait
du Symposium sur la néphropathie endémique de l'Académie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A. (1974) Basic problems in endemic nephropathy. In:
Puchlev, A., ed. Endemic Nephropathy, Proceedings of the Second
International Symposium on Endemic Nephropathy, Sofia, 9-11
November, 1972, Sofia, Publishing House of the Bulgarian Academy
of Sciences, pp. 15-18.
PULS, R. & GREENWAY, J. A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis and toxicity of suspected barley.
Can. J. comp. Med., 40: 16-19.
PURCHASE, I. F. H. (1967) Acute toxicity of aflatoxins M1 and M2
in one-day-old ducklings. Food Cosmet. Toxicol., 5: 339-342.
PURCHASE, I. F. H. (1972) Aflatoxin residues in food of animal
origin. Food Cosmet. Toxicol., 10: 531-544.
PURCHASE, I. F. H. (1974) Mycotoxins, Amsterdam, Elsevier.
PURCHASE, I. F. H. & THERUN, J. J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
PURCHASE, I. F. H. & VORSTER, L. J. (1968) Aflatoxin in commercial
milk samples. S. Afr. med. J., 42: 219.
REDDY, G. S., TILAK, T. B. G., & KRISHNAMURTHI, D. (1973)
Susceptibility of vitamin A- deficient rats to aflatoxin. Food
Cosmet. Toxicol., 11: 467-470.
REDDY, J. K. & SVOBOOA, D. J. (1975) Aflatoxin B1-induced liver
tumors in Tupaia glis (tree shrews) a nonhuman primate. Fed.
Proc., 34: 827 (Abstract no. 3432).
REDDY, J. K., SVOBODA, D. J., & RAO, M. S. (1976) Induction of liver
tumors by aflatoxin B1 in the tree shrew (Tupaia glis), a
nonhuman primate. Cancer Res., 36: 151-160.
REYE, R. D. K., MORGAN, G., & BARAL, J. (1963) Encephalopathy and
fatty degeneration of the viscera: A disease entity in childhood.
Lancet, 2: 749-752.
REYS, L. L. & SEQUEIRA, O. A. (1974) Detection of Australia antigen
(HBAg) in blood donors and hepatoma patients in Mozambique.
S. Aft. med. J., 48: 267-269.
RICHIR, CL., PACCALIN, J., LARCEBEAU, S., FAUGERES, J., MORARD,
J.-L., & LAMANT, M. (1976) Présence d'aflatoxine B1 dans le toie
humain. Cah. Nutr. Diet., 11: 223-226.
ROBERTS, J. C. (1974) Aflatoxins and sterigmatocystins. Fortschr.
Chem. Org. Naturst., 31: 119-151.
ROBINSON, P. (1967) Infantile cirrhosis of the liver in India. With
special reference to probable aflatoxin etiology. Clin. Pediatr.,
6: 57-62.
RODRICKS, J. V. & STOLOFF, L. (1970) Determination of concentration
and purity of aflatoxin standards. J. Assoc. Off. Anal. Chem., 53:
92-95.
RODRICKS, J. V. & STOLOFF, L. (1977) Food producing animals. In:
Rodricks, J. V., Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins
in human and animal health, Park Forest South, IL, USA, Pathotox
Publishers Inc., pp. 67-79.
RODRICKS, J. V., STOLOFF, L., PONS, W. A., JR,. ROBERTSON, J. A., &
GOLDBLATT, L. A. (1970) Molar absorptivity values for aflatoxins and
justification for their use as criteria of purity of analytical
standards. J. Assoc. Off. Anal. Chem., 53: 96-101.
RODRICKS, J. V., HESSELTINE, C. W., & MEHLMAN, M. A., ed. (1977)
Mycotoxins in human and animal health, Park Forest South, IL, USA,
Pathotox Publishers Inc., 807 pp.
ROGERS, A. E. (1975) Variable effects of a lipotrope-deficient, high
fat diet on chemical carcinogenesis in rats. Cancer Res., 35:
2469-2474.
ROGERS, A. E. & NEWBERNE, P.M. (1969) Aflatoxin B1 carcinogenesis
in lipotrope-deficient rats. Cancer Res., 29: 1965-1972.
ROGERS, A. E. & NEWBERNE, P.M. (1971) Diet and aflatoxin B1
toxicity in rats. Toxicol. appl. Pharmacol., 20: 113-121.
ROINE, K., KORPINEN, E.-L., & KALLELA, K. (1971) Mycotoxicosis as a
probable cause of infertility in dairy cows. Nord. Vet.-Med., 23:
628-633.
ROMER, T. R. (1975) Screening method for the detection of aflatoxins
in mixed feeds and other agricultural commodities with subsequent
confirmation and quantitative measurement of aflatoxins in positive
samples. J. Assoc. Off. Anal. Chem., 58: 500-506.
RUDDICK, J. A., SCOTT, P. M., & HARWIG, J. (1976) Teratological
evaluation of zearalenone administered orally to the rat. Bull.
environ. Contain. Toxicol., 15: 678-681.
RUTQVlST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of
fattening pigs. Zentralbl. Vet.-Med. A, 24: 402-408.
SAFFIOTTI, U., MONTESANO, R., SELLAKUMAR, A. R., CEFIS, F., &
KAUFMAN, D. G. (1972) Respiratory tract carcinogenesis in hamsters
induced by different numbers of administrations of benzo (a) pyrene
and ferric oxide. Cancer Res., 32: 1073-1081.
SALHAB, A. S. & EDWARDS, G. S. (1977) Comparative in vitro
metabolism of aflatoxicol by liver preparations from animals and
humans. Cancer Res., 37: 1016-1021.
SALHAB, A. S. & HSIEH, D. P. H. (1975) Aflatoxicol H1: A major
metabolite of aflatoxin B1 produced by human and rhesus monkey
livers in vitro. Res. Commun. chem. Pathol. Pharmacol.,
10: 419-431.
SANDERS, T. H, DAVIS, N. D., & DIENER, U. L. (1968) Effect of carbon
dioxide, temperature, and relative humidity on production of
aflatoxin in peanuts. J. Am. Oil Chem. Soc., 45: 683-685.
SANSING, G. A., LILLEHOJ, E. B., DETROY, R. W., & MILLER, M. A.
(1976) Synergistic toxic effect of citrinin ochratoxin A and
penicillic acid in mice. Toxicon, 14: 213-220.
SARASIN, A. & MOULE, Y. (1976) Helical polysomes induced by
aflatoxin B1 in vivo. A new hypothesis for helix formation by
chemicals and carcinogens. Exp. cell Res., 97: 346-358.
SARGEANT, K., O'KELLY, J., CARNAGHAN, R. B. A., & ALLCROFT, R.
(1961) The assay of a toxic principle in certain groundnut meals.
Vet. Rec., 73: 1219-1223.
SARKISOV, A. C. (1954) [Mycoses.] Moscow, State Publishing House
for Agricultural Literature, 216 pp. (in Russian).
SATO, S., MATSUSHIMA, T., TANAKA, N., SUGIMURA, T., & TAKASHIMA, F.
(1973) Hepatic tumors in the guppy (Lebistes reticulatus) induced
by aflatoxin B1, dimethylnitrosamine, and 2-acetylaminofluorene.
J. Natl Cancer Inst., 50: 765-778.
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches to
the toxic metabolites of Fusaria. VIII. Acute and sub-acute
toxicities of T-2 toxins in cats. Jpn. J. Pharmacol., 25: 263-270.
SCHADE, J. E., MCGREEVY, K., KING, A.D., MACKEY, B., & FULLER, G.
(1975) Incidence of aflatoxin in California almonds. Appl.
Microbiol., 29: 48-53.
SCHINDLER, A. F. & NESHEIM, S. (1970) Effect of moisture and
incubation time on ochratoxin A production by an isolate of
Aspergillus ochraceus. J. Assoc. Off. Anal. Chem., 53: 89-91.
SCHROEDER, H. W. & CARLTON, W. W. (1973) Accumulation of only
aflatoxin B2 by a strain of Aspergillus flavus. Appl.
Microbiol., 25: 146-148.
SCHULLER, P. L., OCKHUIZEN, TH., WERRINGLOER, J., & MARQUARDT, P.
(1967) [Aflatoxin B1 and histamine in wine.]
Arzneimitteforschung, 17: 888-890 (in German).
SCHULLER, P. L., VERHOLSHONK, C. A. H., & PAULSCH, W. E. (1973)
Analysis of aflatoxin M1 in liquid and powdered milk. Pure appl.
Chem., 35: 291-296.
SCHULLER, P. L., VERHULSDONK, C. A. H, & PAULSCH, W. E. (1967)
Aflatoxin M1 in liquid and powdered milk (evaluation of methods
and collaborative study). Zesz. Probl. Postepow Nauk Roln.,
189: 255-261.
SCOTT, P.M. & KENNEDY, B. P. C. (1973) Analysis and survey of ground
black, white, and capsicum peppers for aflatoxins. J. Assoc. Off.
Anal. Chem., 56: 1452-1457.
SCOTT, P.M., VAN WALBEEK, W., KENNEDY, B, & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. Agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., PANALAKS, T., KANHERE, S., & MILES, W. F. (1978)
Determination of zearalenone in cornflakes and other corn-based
foods by thin layer chromatography, high pressure liquid
chromatography, and gas-liquid chromatography/high resolution mass
spectrometry. J. Assoc. Off. Anal. Chem., 61: 593-600.
SEEGERS, J. C. & PITOUT, M. J. (1973) DNA repair synthesis in cell
cultures exposed to low doses of aflatoxin B1 or sterigmatocystin
S. Aft. lab. clin. Med., 19: 961 (abstr. 23).
SEIBOLD, H. R. & BAILEY, W. S. (1952) An epizootic of hepatitis in
the dog. J. Am. Vet. Med. Assoc., 121: 201-206.
SEIBOLD, R. & RUCH, W. (1977) [Aflatoxin content of mixed feeds of
dairy cows.] Kraftfutter, 60: 182-185 (in German).
SERCK-HANSSEN, A. (1970) Aflatoxin-induced fatal hepatitis? A case
report from Uganda. Arch. environ. Health, 20: 729-731.
SHANK, R. C. (1976) The role of aflatoxin in human disease. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems. Washington, DC, American Chemical Society, pp. 51-57
(Advances in Chemistry Series 149).
SHANK, R. C. (1977) Epidemiology of aflatoxin carcinogenesis. In:
Kraybill, H. F. & Mehlman, M. M., ed. Environmental cancer.
Advances in modern toxicology, Vol. 3, John Wiley and Sons, New
York, pp. 291-318.
SHANK, R. C., BOURGEOIS, C. H., KESCHAMRAS, N., & CHANDAVIMOL, P.
(1971a) Aflatoxins in autopsy specimens from Thai children with an
acute disease of unknown actiology. Food Cosmet. Toxicol., 9:
501-507.
SHANK, R. C., JOHNSEN, D. O., TANTICHAROENYOS, P., WOODING, W. L., &
BOURGEOIS, C. H. (1971b) Acute toxicity of aflatoxin B, in the
macaque monkey. Toxicol. appl. Pharmacol., 20: 227-231.
SHANK, R. C., WOGAN, G. N., GIBSON, J. B., & NONDASUTA, A. (1972a)
Dietary aflatoxins and human liver cancer. II. Aflatoxins in market
foods and foodstuffs of Thailand and Hong Kong. Food Cosmet.
Toxicol., 10: 61-69.
SHANK, R. C., GORDON, J. E., WOGAN, G. N., NONDASUTA, A., &
SUBHAMANI, B. (1972b) Dietary aflatoxins and human liver cancer.
III. Field survey of rural Thai families for ingested aflatoxins.
Food Cosmet. Toxicol., 10: 71-84.
SHANK, R. C., BHAMARAPRAVATI, N., GORDON, J. E., & WOGAN, O. N.
(1972c) Dietary aflatoxins and human liver cancer. IV. Incidence of
primary cancer in two municipal populations of Thailand. Food
Cosmet. Toxicol, 10: 171-179.
SHANKARAN, R., RAJ, H. G., & VENKITASUBRAMANIAN, T. A. (1970) Effect
of aflatoxin on carbohydrate metabolism in chick liver.
Enzymology, 39: 371-378.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969a) Survey of cereal grains
and soybeans for the presence of aflatoxin: II. Corn and soybeans.
Cereal Chem., 46. 454-463.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969b) Survey of cereal grains
and soybeans for the presence of aflatoxin: I. Wheat, grain sorghum,
and oats. Cereal Chem., 46: 446-454.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1969)
Ochratoxin A: Occurrence as natural contaminant of a corn sample.
Appl. Microbiol., 17: 765-766.
SHOTWELL, O. L., HESSELTINE, C. W., GOULDEN, M. L., & VANDEGRAFT, E.
E. (1970) Survey of corn for aflatoxin, zearalenone and ochratoxin.
Cereal Chem., 47: 700-707.
SHOTWELL, O. L., HESSELTINE, C. W., VANDEGRAFT, E. E., & GOULDEN, M.
L. (1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16: 266-273.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1973)
Incidence of aflatoxin in southern corn, 1969-1970. Cereal Sci.
Today, 18, 192-195.
SHOTWELL, O. L., GOULDEN, M. L., & HESSELTINE, C. W. (1976a) Survey
of US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal.
Chem., 59: 122-124.
SHOTWELL, O. L., GOULDEN, M. L., & BENNETT, G. A. (1976b)
Determination of zearalenone in corn: Collaborative study.
J. Assoc. Off. Anal. Chem., 59: 666-670.
SINNHUBER, R. O. & WALES, J. H. (1974) Aflatoxin B1
hepatocarcinogenicity in rainbow trout embryos. Fed. Proc., 33:
247 (Abstract no. 253).
SINNHUBER, R. O., LEE, D. J., WALES, J. H., & AYRES, J. L. (1968a)
Dietary factors and hepatoma in rainbow trout (Salmo gairdneri).
II. Cocarcinogenesis by cyclopropenoid fatty acids and the effect of
gossypol and altered lipids on aflatoxin-induced liver cancer.
J. Natl Cancer Inst., 41: 1293-1301.
SINNHUBER, R. O., WALES, J. H., AYRES, J. L., ENGEBRECHT, R. H., &
AMEND, D. L. (1968b) Dietary factors and hepatoma in rainbow trout
(Salmo gairdneri) I. Aflatoxins in vegetable protein feedstuffs.
J. Natl Cancer Inst., 41: 711-718.
SINNHUBER, R. O., LEE, D. J., WALES, J. H., LANDERS, M. K., KEYL, A.
C. (1974) Hepatic carcinogenesis of aflatoxin M1 in rainbow trout
(Salmo gairdneri) and its enchancement by cyclopropene fatty
acids. J. Natl Cancer Inst., 53: 1285-1288.
SMALLEY, E. B. & STRONG, F. M. (1974) Toxic trichothecenes. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
199-228.
SMITH, J. W., PRINCE, W. R., & HAMILTON, P. B. (1969) Relationship
of aflatoxicosis to Salmonella gallinarum infections of chickens.
Appl. Microbiol., 18: 946-947.
SPEERS, G. M., MERONUCK, R. A., BARNES, D. M., & MIROCHA, C. J.
(1971) Effect of feeding Fusarium roseum f. sp. graminearum
contaminated corn and the mycotoxin F-2 on the growing chick and
laying hen. Poult. Sci., 50: 627-633.
STACK, M. E. (1974) Collaborative study of AOAC methods I and III
for the determination of aflatoxins in peanut butter. J. Assoc.
Off. Anal. Chem., 57: 871-874.
STANFORD, G. K., HOOD, R. D., & HAVES, A. W. (1975) Effect of
prenatal administration of T-2 toxin to mice. Res. Commun. chem.
Path. Pharmacol., 10: 743-746.
STEPHENSON, L. W. & RUSSELL, T. E. (1974) The association of
Aspergillus flavus with hemipterous and other insects infesting
cotton bracts and foliage. Phytopathology, 64: 1502-1506.
STERN, P.S., & HOLZAPFEL, C. W. (1967) The synthesis of ochratoxins
A and B, metabolites of Aspergillus ochraceus Wilh. Tetrahedron
Lett., 23: 4449-4461.
STEYN, P.S., VLEGGAAR, R., Du PREEZ, N. P., BLYTH, A. A., & SEEGERS,
J. C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STICH, H. F. & LAISHES, B. A. (1975) The response of Xeroderma
pigmentosum cells and controls to the activated mycotoxins,
aflatoxins and sterigmatocystin. Int. J. Cancer, 16: 266-274.
STOLOFF, L. (1972) Analytical methods for mycotoxins. Clin.
Toxicol., 5: 465-494.
STOLOFF, L. (1976) Occurrence of mycotoxins in foods and feeds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 23-50
(Advances in Chemistry Series 149).
STOLOFF, L. (1977) Aflatoxins-an overview. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 7-28.
STOLOFF, L. & FRIEDMAN, L. (1976) Information bearing on the
evaluation of the hazard to man from aflatoxin ingestion.
PAG Bulletin, 6: 21-32.
STRZELECKI, E. L. & GASIOROWSKA, U. W. (1974) Aflatoxin B1 in
feedstuffs. Zentralbl. Vet.-Med. B, 21: 395-400.
STUBBLEFIELD, R.D., SHOTWELL, O. L., & SHANNON, O. H. (1972)
Aflatoxins M1 and M2 and parasiticol: Thin layer chromatography
and physical and chemical properties. J. Assoc. Off. Anal. Chem.,
55: 762-767.
SUIT, J. L., ROGERS, A. E., JETTEN, M. E. R., & LURIA, S. E. (1977)
Effects of diet on conversion of aflatoxin B1 to bacterial
mutagen(s) by rats in vivo and by rat hepatic microsomes
in vitro. Mutat. Res., 46: 313-323.
SUZANGAR, M., EMAMI, A., & BARNETT, R. (1976) Aflatoxin
contamination of village milk in Isfahan, Iran. Trop. Sci., 18:
155-159.
SUZUKI, S. & SATOH, T. (1973) Effects of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Pharmacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl.
Pharmacol., 32: 116-122.
SVOBODA, D., GRADY, H. J, & HIGGINSON, J. (1966) Aflatoxin B1
injury in rat and monkey liver. Am. J. Pathol., 49: 1023-1051.
SWENSON, D. H., MILLER, E. C., & MILLER, J. A. (1974) Aflatoxin
B1-2, 3-oxide: Evidence for its formation in rat liver in vivo
and by human liver microsomes in vitro. Biochem. Biophys. res.
Comm., 60: 1036-1043.
SWENSON, D. H., LIN, J.-K, MILLER, E. C., & MILLER, J. A. (1977)
Aflatoxin B1-2, 3-oxide as a probable intermediate in the covalent
binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal
RNA in vivo. Cancer Res., 37: 172-181.
SZCZECH, G. M., CARLTON, W. W., & TUITE, J. (1973a) Ochratoxicosis
in beagle dogs. I. Clinical and clinicopathological features.
Vet. Pathol., 10: 135-154.
SZCZECH, G. M., CARLTON, W. W., TUITE, J., & CALDWELL, R. (1973b)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10: 347-364.
SZCZECH, G. M., CARLTON, W. W, & TUITE, J. (1973c) Ochratoxicosis in
beagle dogs. II. Pathology. Vet. Pathol., 10: 219-231.
TANDON, B. N., KRISHNAMURTHY, L., KOSHY, A., TANDON, H. D.,
RAMALINGASWAMI, V., BHANDARI, J. R., MATHUR, M. M., & MATHUR, P. D.
(1977) Study of an epidemic of jaundice, presumably due to toxic
hepatits, in northwest India. Gastroenterology, 72: 488-494.
TANDON, H. D., TANDON, B. N., & RAMALINGASWAMI, V. (1978) Epidemic
of toxic hepatitis in India of possible mycotoxic origin. Arch.
Pathol. lab. Med., 102. 372-376.
TEMCHAROEN, P., ANUKARAHANONTA, T., & BHAMARAPRAVATI, N. (1978) The
influence of dietary protein and vitamin B12 on the toxicity and
carcinogenicity of aflatoxins in rat liver. Cancer Res.,
38: 2185-2190.
THACKER, H. L. (1976) Citrinin and ochratoxin A mycotoxicosis in
guinea pigs, Lafayette, Indiana, Purdue University (A thesis).
THERON, J. J. (1965) Acute liver injury in ducklings as a result of
aflatoxin poisoning. Lab. Invest., 14: 1586-1603.
THERON, J. J., LIEBENBERG, N., & JOUBERT, H. J. B. (1965)
Histochemistry and electron microscopy of acute liver lesions
induced by aflatoxin B1 in ducklings. Nature (Lond.), 206:
908-909.
TILAK, T. B. (1975) Induction of cholangiocarcinoma following
treatment of a rhesus monkey with aflatoxin. Food Cosmet.
Toxicol., 13: 247-249.
TRIPATHI, R. K. (1973) Aflatoxins in sorghum grains infested with
head moulds. Indian J. exp. Biol., 11: 361-362.
TUNG, H.-T., DONALDSON, W. E., & HAMILTON, P. B. (1970) Effects of
aflatoxin on some marker enzymes of lysosomes. Biochem. Biophys.
Acta, 222. 665-667.
TURNER, W. B. (1971) Fungal metabolites, New York, Academic Press,
446 pp.
ULLOA-SOSA, M. & SCHROEDER, H. W. (1969) Note on aflatoxin
decomposition in the process of making tortillas from corn.
Cereal Chem., 46: 397-400.
VAN NIEUWENHUIZE, J.P., HERBER, F. M., DE BRUIN, A., MEYER, P. B., &
DUBA, W. C. (1973) [Cancers in men following a long-term low-level
exposure to aflatoxins in indoor air.] T. Soc. Geneeskd., 51:
754-760 (in Dutch).
VAN PÉE, W., VAN BRABANT, J., & JOOSTENS, J. (1977) La détection et
le dosage de l'aflatoxine M1 dans le lait et le lait en poudre.
Rev. Agrie. (Bruxelles), 30: 403-415.
VAN RENSBURG, S. J. (1977) Role of epidemiology in the elucidation
of mycotoxin health risks. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publ., pp. 699-711.
VAN RENSBURG, S. J., VAN DER WATT, J. J., PURCHASE, I. F. H.,
PEREIRA COUTINHO, L., & MARKHAM, R. (1974) Primary liver cancer rate
and aflatoxin intake in a high cancer area. S. Afr. Med. J., 48:
2508a-2508d.
VANYI, A. & SZAILER, E. (1974) Investigation of the cytotoxic effect
of F-2 toxin (zearalenone) in various monolayer cell cultures.
Acta Vet. Acad. Sci. Hung., 24: 407-412.
VERRETT, M. J., MARLIAC, J.-P., & MCLAUGHLIN, J., JR (1964) Use of
the chicken embryo in the assay of aflatoxin toxicity.
J. Assoc. Off. Anal. Chem., 47: 1003-1006.
VESELY, D., VESELA, D., KUSAK, V., & NESNIDAL, P. (1978)
[Distribution of perorally administered aflatoxin B1 in tissues
and organs of the goat.] Veterinarni medicina, 23 (LI): 555-558 (in
Czech).
VESONDER, R. F., CIEGLER, A., & JENSEN, A. H. (1973) Isolation of
the emetic principle from Fusarium-infected corn.
Appl. Microbiol., 26: 1008-1010.
VESSELINOVITCH, S. D., MIHAILOVICH, N., WOGAN, G. N., LOMBARD, L.
S., & RAO, K. V. N. (1972) Aflatoxin B1, a hepatocarcinogen in the
infant mouse. Cancer Res., 32: 2289-2291.
VILLA-TREVINO, S. & LEAVER, D. D. (1968) Effects of the hepatotoxic
agents retrorsine and aflatoxin B1 on hepatic protein synthesis in
the rat. Biochem. J., 109: 87-91.
VOGEL, C. L., ANTHONY, P. P., MODY, N., & BARKER, L. F. (1970)
Hepatitis-associated antigen in Ugandan patients with hepatocellular
carcinoma. Lancet, 2: 621-624.
WALES, J. H. & SINNHUBER, R. O. (1972) Hepatomas induced by
aflatoxin in the sockeye salmon (Oncorhynchus nerka). J. Natl
Cancer Inst., 48: 1529-1530.
WALTKING, A. E. (1970) Collaborative study of three methods for
determination of aflatoxin in peanuts and peanut products.
J. Assoc. Off. Anal. Chem., 53: 104-113.
WALTKING, A. E. (1971) Fate of aflatoxin during roasting and storage
of contaminated peanut products. J. Assoc. Off. Anal. Chem., 54:
533-539.
WALTKING, A. E., BLEFFERT, G., & KIERNAN, M. (1968) An improved
rapid physio-chemical assay method for aflatoxin in peanuts and
peanut products. J. Am. Oil Chem. Soc., 45: 880-884.
WARD, J. M., SONTAG, J. M., WEISBURGER, E. K., & BROWN, C. A. (1975)
Effect of lifetime exposure to aflatoxin B1 in rats.
J. Natl Cancer Inst., 55: 107-113.
WARE, G. M. & THORPE, C. W. (1977) Analysis for zearalenone in corn
by high pressure liquid chromatography with a fluorescence detector.
Abstracts 91st Annual Meeting, Association of Official Analytical
Chemists, Washington, DC, p. 47 (Abstract no. 148).
WARE, G. M. & THORPE, C. W. (1978) Determination of zearalenone in
corn by high-pressure liquid chromatography and fluorescence
detection. J. Assoc. Off. Anal, Chem., 61 (5): 1058-1062.
WHITAKER, T. B. (1977)Sampling granular foodstuffs for aflatoxin.
Pure appl. Chem., 49: 1709-1717.
WHITAKER, T. B. & WHITTEN, M. E. (1977) Evaluation of cottonseed
aflatoxin testing programs. J. Am. Oil Chem. Soc., 54: 436-441.
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (1974a) Variability
of aflatoxin test results. J. Am. Oil Chem. Soc., 51: 214-218.
WHITAKER, T. B., DICKENS, J. W., WISER, E. H., & MONROE, R. J.
(1974b) Development of a method to evaluate sampling plans used to
estimate aflatoxin concentrations in lots of shelled peanuts.
I.U.P.A.C. Information Bulletin, Oxford, International Union of Pure
& Applied Chemistry Secretariat, pp. 1-14 (Technical Report-No. 10).
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (in press)
Variability associated with testing corn for aflatoxin. J. Am. Oil
Chem. Soc.
WHITTEN, M. E. (1969) Aflatoxins in cottonseed hulls. J. Am. Oil
Chem. Soc., 47: 5-6.
WHO (1978) Environmental Health Criteria 6-- Principles and methods
for evaluating the toxicity of chemicals. Part I, Geneva, World
Health Organization, 272 pp.
WILLIAMS, D. J. & RABIN, B. R. (1971) Disruption by carcinogens of
the hormone dependent association of membranes with polysomes.
Nature (Lond.), 232: 102-105.
WOGAN, G. N. (1966) Chemical nature and biological effects of the
aflatoxins. Bacteriol. Rev., 30: 460-470.
WOGAN, G. N. (1969) Metabolism and biochemical effects of
aflatoxins. In: Goldblatt, L. A., ed. Aflatoxin: Scientific
background, control and implications, New York, Academic Press,
pp. 151-186.
WOGAN, G. N. (1973)Aflatoxin carcinogenesis. In: Bush, M., ed.
Methods in cancer research, New York, Academic Press, pp. 309-344.
WOGAN, G. N. (1977) Mycotoxins and other naturally occurring
carcinogens. In: Kraybill, H. F. & Mehlman, M. A., ed.
Environmental cancer, Advances in modern toxicology, Washington,
DC, Hemisphere Publishing Corporation, Vol. 3, pp. 263-290.
WOGAN, G. N. & FRIEDMAN, M. A. (1968) Inhibition by aflatoxin B1
of hydrocortisone induction of rat liver tryptophan pyrrolase and
tyrosine transaminase. Arch. Biochem. Biophys., 128: 509-516.
WOGAN, G. N. & NEWBERNE, P.M. (1967) Dose-response characteristics
of aflatoxin B1 carcinogenesis in the rat. Cancer Res., 27:
2370-2376.
WOGAN, G. N. & PAGLIALUNGA, S. (1974) Carcinogenicity of synthetic
aflatoxin M1 in rats. Food Cosmet. Toxicol., 12: 381-384.
WOGAN, G. N., EDWARDS, G. S., & SHANK, R. C. (1967) Excretion and
tissue distribution of radioactivity from aflatoxin B1-14C in
rats. Cancer Res., 27: 1729-1736.
WOGAN, G. N., EDWARDS, G. S., & NEWBERNE, P.M. (1971)
Structure-activity relationships in toxicity and carcinogenicity of
aflatoxins and analogs. Cancer Res., 31: 1936-1942.
WOGAN, G. N., PAGLIALUNGA, S., & NEWBERNE, P.M. (1974) Carcinogenic
effects of low dietary levels of aflatoxin B1 in rats. Food
Cosmet. Toxicol., 12: 681-685.
WONG, J. J. & HSIEH, D. P. H. (1976) Mutagenicity of aflatoxins
related to their metabolism and carcinogenic potential. Proc. Natl
Acad. Sci. (USA), 73: 2241-2244.
WYATT, R. D., DOERR, J. A., HAMILTON, P. B., & BURMEISTER, H. R.
(1975) Egg production, shell thickness and other physiological
parameters of laying hens affected by T-2 toxin. Appl. Microbiol.,
29: 641-645.
WYLLIE, W. D. & MOREHOUSE, L. D., ed. (1977) Mycotoxic fungi,
mycotoxins, mycotoxicosis: An encyclopaedic handbook. New York and
Basel, Marcel Dekker.
YADGIRI, B., REDDY, V., TULPULE, P. G., SRIKANTIA, S. G., & GOPALAN,
C. (1970) Aflatoxin and Indian childhood cirrhosis; Am. J. clin.
Nutr., 23: 94-98.
YAGEN, B., JOFFE, A. Z., HORN, P., MOR, N., & LUTSKY, I. I. (1977)
Toxins from a strain involved in ATA. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 329-336.
YNDESTAD, M. & UNDERDAL, B. (1975) [Aflatoxin in foods on the
Norwegian market.] Nord. Vet.-Med., 27: 42-48 (in Norwegian).
See Also:
Toxicological Abbreviations
 Little or no destruction of aflatoxins occurs under ordinary
cooking conditions, and heating for pasteurization. However,
roasting groundnuts appreciably reduces the levels of aflatoxins
(see section 3.2.3) and they can be totally destroyed by drastic
treatment such as autoclaving in the presence of ammonia or by
treatment with hypochlorite.
Table 1. Physical and chemical properties of some aflatoxins and their metabolites
Aflatoxin Molecular Relative Melting Ultraviolet absorption (epsilon)c Fluorescence Reference
formula molecular point emission
mass °C 265 nm 360-362 nm nm
B1a C17H12O6 312 268-269 12 400 21 800 425 Asao et al. (1965)
B2a C17H14O6 314 286-289 12 100 24 000 425 Chang et al. (1963)
G1a C17H12O6 328 244-246 9 600 17 700 450 Asao et al. (1965)
G2a C17H14O7 330d 237-240d 8 200 17 100 450 Hartley et al. (1963)
M1a C17H12O7 328 299 14 150 21 250 (357 nm) 425 Holzapfel et al. (1966)
M2a C17H14O7 330 293 12 100 (264 nm) 22 900 (357 nm) f Holzapfel et al. (1966)
P1b C16H10O6 298 >320 11 200 (267 nm) 15 400 (362 nm) f Dalezios et al. (1971 ) and
14 900 (342 nm) Buchi et al. (1973)
Q1 C17H12O7 328 e 11 450 (267 nm) 17 500 (366 nm) f Masri et al. (1974a,b)
Aflatoxicol C17H14O6 314 230--234d 10 800 (261 nm) 14 100 (375 nm) 425 Detroy & Hesseltine (1970)
a Molar absorption coefficient for aflatoxins B1, B2, G1, and G2 obtained from Rodricks et al. (1970) and those for M1 and M2
from Stubblefield et al, (1972).
b P stands for phenolic products of O-demethylation of aflatoxin B1.
c Compounds dissolved in methanol except for aflatoxin P1 which in this case was dissolved in ethanol. Data on molar absorption
coefficients for other peaks and on the ultraviolet absorption characteristics of aflatoxins in other solvents can be found
in the original papers.
d Data from Butler (1974).
e Not available.
f Violet fluorescence of aflatoxin M2 and yellow-green fluorescence of aflatoxins P1 and Q1 reported in original papers.
The presence of a lactone ring in the aflatoxin molecule makes
them susceptible to alkaline hydrolysis (De Iongh et al., 1962).
This characteristic is important in that any food processing
involving alkali treatment can decrease the contamination of the
products (section 3.2.3) although the presence of protein, the pH,
and the duration of treatment may modify the results (Beckwith et
al., 1975). However, if the alkaline treatment is mild,
acidification will reverse the reaction to reform the original
aflatoxin.
The chemistry of the aflatoxins has recently been reviewed by
Roberts (1974).
3.1.2 Methods of analysis for aflatoxins in foodstuffs
3.1.2.1 Sampling
Sampling is an integral part of the analytical procedure and the
sample drawn should be representative of the lot. The total error
made in an analytical procedure consists of the sampling error, the
subsampling error, and the error in analysis (Whitaker, 1977). The
difficulty in sampling for aflatoxins arises because of the
heterogeneity of aflatoxin distribution in contaminated unprocessed
commodities. On the basis of a large number of analyses, Whitaker et
al. (1974a) were able to calculate the contribution of each error to
the total error. The total variance of the analytical procedure is
primarily caused by sampling variability, whereas the subsampling
variability and the analysis variability are more or less
independent of aflatoxin concentration. The coefficient of variation
associated with sampling is about 115% at a level of contamination
of 20 µg/kg and about 145% at a level of contamination of 10 µg/kg.
Whitaker et al. (1974b) and Whitaker (1977) have summarized a
procedure for sampling and have developed a number of sampling plans
used in the USA for the control of aflatoxin contamination in
shelled groundnuts (peanuts). Other recent publications deal with
aflatoxin-testing programmes for maize (Whitaker et al., 1978) and
cottonseed (Whitaker & Whitten, 1977).
The sampling of small grains, oilseed cakes, foodstuffs, and
feeds is also difficult, although in most cases the aflatoxin
distribution within a batch is not likely to be as uneven as in the
case of groundnuts. Fluids and well-mixed processed products such as
milk and milk products, beer, and cider do not present such a
sampling problem.
Peanut butter, flours, and cornmeal do not present the same
problems as the original raw materials because a finely divided
product is formed during processing from which it is much easier to
obtain a representative analytical sample.
Some practical aspects of sampling are dealt with in Chapter 26
of "Official methods of analysis" of the Association of Official
Analytical Chemists (Horwitz et al., 1975). For survey purposes,
1-5 kg samples are usually taken and the size of the sample for
analysis ranges from 20 to 100 g. A sample of 50 g ensures both a
representative sample and solvent economy.
3.1.2.2 Methods of analysis
Biological and chemical procedure have been developed for the
detection and determination of aflatoxins and other mycotoxins. The
bioassay techniques that are currently available are not suitable
for routine screening purposes and their detection levels are not
low enough. The chemical assay techniques, although more accurate
and faster, are not always specific. The presence of a certain toxin
is usually confirmed by derivative formation and its toxicity
verified by bioassay.
Biological methods. In the original biological test (Carnaghan
et al., 1963), one-day-old ducklings were used as test animals for
determining the presence of aflatoxins in suspect food by measuring
the degree of biliary proliferation as a semiquantitative index (see
section 3.4.2.1). The lowest dose level of 0.4 µg/day administered
for 5 days represents the minimum intake required to induce a
detectable biliary proliferation. The test is also effective for
detecting aflatoxin M1 in both liquid and powdered milk (Purchase,
1967). Little information is available on the sensitivity of this
test (and other biological methods) to aflatoxins other than B1.
A commonly used method in regulatory actions is the chicken
embryo bioassay in which 0.1-0.2 µg of aflatoxin B1 is applied to
the egg membrane and the mortality rate recorded during the 23-day
period of hatching (Horwitz, 1975).
Several other biological procedures have been developed, using
maize seedlings, zebra fish larvae, brine shrimps, bacteria etc.
Detailed descriptions can he found in the reviews by Goldblatt
(1969) and Ciegler et al. (1971).
Chemical methods. Although procedures are continually
changing, the basic steps remain; extraction, lipid removal,
cleanup, separation, and quantification. Since there is considerable
overlap in the various methods, most of the published reviews
(Jones, 1972; Stoloff, 1972) examine the different procedures by
these basic steps. Depending on the nature of the commodity, methods
can sometimes be simplified by omitting unnecessary steps. The
presence of specific interferences such as theobromine in cacao and
gossypol in cottonseed, may require additional steps.
Numerous methods of analysis have been reported for the
determination of aflatoxins in human and animal foodstuffs. Many of
them are minor modifications of the basic steps adapted to special
commodities or problems.
Collaborative studies designed to assess the performances of
different laboratories give information on the accuracy, precision,
and specificity of the method under consideration, as well as on the
occurrence of false negative and false positive results. Only
methods that have been subjected to such studies are reported in
this document.
Chemical methods have mainly been developed for such commodities
as groundnuts. In the first method for the analysis of groundnuts,
the aflatoxins were extracted from the contaminated sample using
methanol; this was later replaced by chloroform. An improvement was
made by Lee (1965) who showed that the addition of water to
hydrophilic plant tissues during extraction with chloroform resulted
in more effective removal of aflatoxin. The combination of
liquid-liquid extraction techniques and partition chromatography led
to a method, which is now one of the most widely used, known as the
Contamination Branch (CB) method (Eppley, 1966). The sample is
extracted with water and the water extracted with chloroform, the
lipids and aflatoxins are transferred to a silica-gel column where
the lipids are selectively eluted with hexane and the pigments and
other interfering material eluted with absolute diethylether;
finally the aflatoxins are eluted from the column with 3% methanol
in chloroform.
Because the CB method is time-consuming, attempts have been made
to simplify it. Waltking et al. (1968) drew attention to the fact
that a separation funnel was simpler and faster for liquid-liquid
partition than the silica-gel column, and that centrifuging was a
faster method of separating a solid than filtration. Thus the Best
Foods (BF) method was developed, which is faster and more economical
in terms of the amounts of solvents used but provides a poorer
cleanup. The sample is extracted and defatted with a two-phase
aqueous methanol-hexane system, the aflatoxins are then partitioned
from the aqueous phase into chloroform, leaving lipids and pigments
in the hexane and aqueous methanol.
In both the CB and BF methods, the aflatoxins are concentrated
by evaporation of the chloroform, and then separated by thin-layer
chromatography (TLC). Aflatoxins are intensely fluorescent when
exposed to long-wave ultraviolet radiation, which makes it possible
to determine these compounds at extremely low levels. An analyst
experienced in this field can detect 0.5 ng aflatoxin B1 on a TLC
plate. In most methods, the intensity of fluorescence of the sample
is compared with that of a standard. Under ideal conditions this
technique; has a coefficient of variation of about 20% which can be
reduced to 5%-9% by the use of a fluorodensitometer.
It should be pointed out that quantification and confirmation of
identity can only be obtained if pure authentic standards are
available for reference. Current sources of aflatoxin standards and
methods for the determination of mass concentration and purity cart
be found in Chapter 26 of the AOAC "Official methods of analysis"
(Horwitz et al., 1975).
When the CB and BF methods were compared in a collaborative
study (Waltking, 1970), the methods were found to be equivalent in
accuracy and precision with a recovery of about 70% of added
aflatoxin and an overall coefficient of variation of about 35% for
total aflatoxin levels down to about 20 µg/kg. This result has been
confirmed by the latest International Aflatoxin Check Sample Study
(Coon et al., 1972) in which 129 laboratories participated. However,
the coefficient of variation was about twice as high as in the
original collaborative studies. This illustrates the inadequacies of
many laboratories.
Another collaborative study was conducted (Stack, 1974) in which
the CB and BF methods were compared at levels down to the 2-10 µg/kg
range, Again both methods proved to be equally accurate (about 80%
recovery) for total aflatoxins at the 5-10 µg/kg levels. The CB
method, however, was as precise (coefficient of variation = 30%) at
the lowest level of 2 µg/kg as at the highest levels. The BF method
lost precision at these low levels and the coefficient of variation
was of the order of 100%.
In spite of cleanup and separation procedures, there might still
be problems with compounds that have fluorescent and chromatographic
properties similar to those of the aflatoxins. Thus, the presence of
a spot on a TLC plate is only presumptive evidence of identity and
additional confirmatory tests are necessary. Probably the first step
in confirming the presence of aflatoxins is to use additional
solvent systems in the TLC. The developed TLC plate can be sprayed
with 25% sulfuric acid (Schuller et al., 1967), which changes the
fluorescence colour of the aflatoxin spots to yellow. This test, if
negative, would rule out the presence of aflatoxins but does not
provide confirmatory evidence. The formation of chemical derivatives
was first described for aflatoxins B1 and G1 (Andrellos & Reid,
1964). The reagents used were formic acid-thionyl chloride, acetic
acid-thionyl chloride, and trifluoroacetic acid, the acid-catalysed
addition products formed were a dimeric acetate and an addition
product with water, respectively. The characteristic mobilities and
fluorescent properties on thin-layer chromatograms can be compared
with those of standard derivatives. Pohland et al. (1970) simplified
the preparation of the derivatives by using a mixture of
hydrochloric acid and acetic anhydride, and hydrochloric acid alone.
Further improvement, by elimination of the preparative
chromatography step in these procedures, has been achieved by
Przybylski (1975). The water adduct is formed directly on a TLC
plate from as little as 0.5 ng of aflatoxin B1 or G2.
In addition to the procedures for groundnuts, methods of
analysis have been developed for cottonseed, copra, maize, various
tree nuts (pistachio, walnut, Brazil nuts, etc.) and for animal
feeds. Many of these methods are modifications of the CB and BF
methods. Milk and dairy products require a far greater sensitivity
for the determination of M1 and M2 because these animal
metabolites are usually only found at sub µg/kg levels; additional
cleanup to eliminate interferences and sometimes two-dimensional TLC
techniques (Schuller et al., 1973) are necessary to attain
satisfactory performance. These methods are described in Chapter 26
of the AOAC "Official methods of analysis" (Horwitz et al., 1975).
Several analytical methods for the detection of aflatoxin
residues in animal tissues have been developed, and their detection
limits evaluated (Jemmali & Murphy, 1976).
Column detection methods are being used for control purposes in
the field because of their simplicity. The method of Romer (1975) is
of particular value because it combines column detection, TLC
quantification, and TLC plate chemical derivative confirmation in a
method that has a wide application for a number of foodstuffs and
feeds including mixed feeds.
It appears that methods using high-pressure liquid
chromatography will become the methods of choice for mycotoxin
analyses in the near future because of their sensitivity and
improved accuracy, and because they can be applied to a number of
mycotoxins including aflatoxins B1, B2, G1, and G2 (Panalaks
& Scott, 1977).
3.2 Sources and Occurrence
3.2.1 Formation by fungi
The ability to produce aflatoxins seems to be confined to
strains of the two species Aspergillus flavus Link and
A. parasiticus Speare, both members of the A. flavus group.
Aflatoxin-producing strains of A. flavus are common and
widespread, and have been isolated from a host of different
materials. As indicated in Table 2, a high proportion (from 20% to
98%) of isolated strains of A. flavus is able to produce
aflatoxins.
Table 2. Aflatoxin-producing strains of A. flavus isolated from
four field cropsa
Source Isolates Isolates producing Maximum yield
tested aflatoxin of aflatoxin B1
(No.) (%) (µg/flask)
groundnut 100 98 3300
cottonseed 59 81 3200
rice 127 20 1100
sorghum 63 24 3300
a Data from Schroeder & Boller quoted by Hesseltine (1976) in
"Mycotoxins and other fungal related food problems".
3.2.1.1 Moisture content and temperature
The moisture content of the substrate and temperature are the
main factors regulating fungal growth and mycotoxin formation.
Koehler (1938) established that a moisture content of 18.3% on a
wet weight basis, was the lower limit for the growth of A. flavus
in shelled corn. Extensive studies under precisely controlled
conditions (Sanders et al., 1968; Diener & Davis, 1969; Davis &
Diener, 1970) established a moisture content in equilibrium with a
relative humidity of 85% (or water activity (aw) = 0.85) as the
lower limit for growth of A. flavus and for the production of
aflatoxins. In starchy, cereal grain such as wheat, oats, barley,
rice, sorghum, and maize, the lower limit is a moisture content of
18.3%-18.5% on a wet weight basis and in groundnuts, Brazil nuts,
other nuts, copra, and sunflower and safflower seeds, all of which
have a high oil content, it is a moisture content of 9%-10%.
The minimum, optimum, and maximum temperatures for aflatoxin
production are 12° C, 27° C, and 40-42° C, respectively (Davis &
Diener, 1970). Northolt et al. (1976) studied the effect of water
activity and temperature on the growth and aflatoxin production of
A. parasiticus and came to the conclusion that no detectable
quantities of aflatoxin B1 were formed at an aw value below 0.83
and at temperatures below 10°C.
3.2.1.2 Invasion of field crops by A. flavus
Groundnut seeds may be invaded by A. flavus before harvest but
are more likely to be invaded very rapidly after the plants have
been pulled and piled for preliminary drying before the nuts are
removed. This postharvest period is the "high hazard" time for
aflatoxin production. On the other hand, studies of aflatoxin
contamination in North Carolina (USA) (Dickens & Satterwhite, 1973)
under conditions of drought, suggest that drought after groundnuts
are formed but before they are dug is conducive to their infection
with A. flavus. Damage caused by the lesser cornstalk borer (LCB)
might also aid in the infection process because insects may carry
fungal spores, although many drought area fields infested with LCB
did not produce groundnuts with a high aflatoxin content. Drought
alone does not result in high levels of aflatoxin contamination. It
must coincide with, or promote infestation by insects which in turn
infect the groundnut. The LCB may act as a vector for A. flavus.
Pettit et al. (1971) reported that groundnuts grown under dry
land conditions (drought stress) accumulated more aflatoxin before
digging than those grown under irrigation. Dry land fresh-dug
kernels contained a maximum aflatoxin level of 35 800 µg/kg while a
maximum of 50 µg/kg was detected in kernels from irrigated plots.
Apparently, the higher kernel moisture content occurring under
irrigated conditions reduced the aflatoxin production potential,
whereas a moisture content of about 31% under drought conditions was
near optimum. Similar observations have been reported from West
Africa.
In some irrigated regions with moist weather at harvest time,
cottonseed may be invaded by A. flavus while still on the plant
and after the bolls open and may contain large amounts of aflatoxins
(Marsh et al., 1973). Stephenson & Russell (1974) related the high
aflatoxin contamination in the field (in USA) to invasion by insects
that provided a site of injury and served as vectors for A. flavus.
Insect injury in ears of maize in the field may also be
accompanied or followed by infection with A. flavus and by
aflatoxin formation before harvest (Lillehoj et al., 1976). To what
extent this constitutes a contamination problem in many regions of
the world, where maize is an important crop, is not known.
Aflatoxins have also been reported in heads of sorghum heavily
infected with mould in India (Tripathi, 1973). Pistachio nuts can
become contaminated with Aflatoxins prior to harvest but the cause
of infection with aflatoxin-producing strains of fungi has not yet
been found. The contamination of almonds and of walnuts has been
traced to specific types of insect damage in the orchard (Stoloff,
1977).
3.2.2 Occurrence in foodstuffs
This subject has been reviewed recently by Stoloff (1976). Of
the four major aflatoxins (B1, B2, G1, G2) B1 is usually
found in the greatest concentrations. Measurements of toxin
concentration are based on the wet weight of the commodity in
question. The four toxins may occur together, although they need
not, and their concentrations in relation to each other and their
occurrence may vary depending on the fungal strain and substrate.
For example, Hesseltine et al. (1970) found that most fungal strains
that produced aflatoxin G1 also produced aflatoxin B1, but that
not all strains that produced aflatoxin B1 produced aflatoxin
G1. One strain of A. flavus from black pepper produced only
aflatoxin B2 on 2 natural substrates tested (Schroeder & Carlton,
1973).
Although aflatoxins have been found in a variety of foodstuffs,
the most pronounced contamination has been encountered in groundnuts
and other oilseeds including cottonseed and maize. The most
frequently contaminated tree nuts are Brazil nuts and pistachios.
3.2.2.1 Maize
Surveys in the USA of more than 1500 samples of maize collected
in crop years 1964-67, mainly from commercial channels, revealed
that 2%-3% of the samples contained aflatoxins (total aflatoxin B1
and G1) in the range of 3-37 µg/kg (Shotwell et al., 1969a, 1970).
In a subsequent survey of 293 samples, 8 samples (2.7%) contained
aflatoxin B1 levels in the range of 6-25 µg/kg, one of the samples
containing aflatoxin G1 (25 µg/kg) as well as aflatoxin B1
(Shotwell et al., 1971). Aflatoxin B2 was also detected in some of
the samples. In a further study of 60 samples from south-east USA
(Shotwell et al., 1973), aflatoxin B1 was found in 21 samples
(35%) at levels ranging from 6-308 µg/kg in the 1969-70 period.
Aflatoxin B2 at levels ranging from a trace to 40 µg/kg was found
in 15 of these samples and aflatoxin G1 was found in 5 samples at
levels of a trace to 10 µg/kg. In 2 samples, aflatoxin G2 (1 and
<2 µg/kg) was detected in addition to aflatoxin B1. In some of
the southeastern states of the USA a high frequency of aflatoxin
contamination was also found in maize in the field (Anderson et al.,
1975; Lillehoj et al., 1976). In some of the field samples, the
levels of aflatoxin B1 ranged up to several thousand µg/kg or
more. Most of the field contamination was associated with damage
caused by insects such as the European corn borer, corn ear worms,
or weevils, and it seems likely that wherever such damage is
prevalent, field contamination with aflatoxins will occur. In
Thailand, 35% of maize samples contained aflatoxin B1 (average
level 400 µg/kg) while 40% contamination by aflatoxin B1 (average
level 133 µg/kg) was found in Uganda (Stoloff, 1976), and 97% in the
Philippine island of Sebu (average 213 µg/kg) (Alpert et al., 1971;
Campbell & Salamat, 1971; Shank et al., 1972a; Campbell & Stoloff,
1974; Stoloff, 1976).
Aflatoxin levels found in household maize samples in connexion
with the outbreak of acute toxic hepatitis in north-west India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977) are discussed
in section 3.5.1.2.
3.2.2.2 Wheat, barley, oats, rye, rice, and sorghum
Shotwell et al. (1968b) reported the presence of aflatoxins at
levels of less than 19 µg/kg in 9/1368 samples of wheat, sorghum,
and oats in the USA. Shotwell et al. (1976b) did not detect any
aflatoxin B1 (detection limit 1-3 µg/kg) in 848 samples of wheat
from various districts of the USA. The presence of aflatoxins B1,
B2, G1, and G2 was reported by Tripathi (1973) in heads of
sorghum heavily infected with mould, in field samples in India, but
he did not apply any confirmatory tests. Aflatoxins were also found
in sorghum in Uganda (Alpert et al., 1971) and, in a survey in the
USA, aflatoxins were detected in 2/66 samples of sorghum grain (13
and 50 µg/kg) (Stoloff, 1976). Aflatoxins have been detected in less
than 2% of more than 400 samples of rice from markets in Africa, the
Philippines, and Thailand (Alpert et al., 1971; Campbell & Salamat,
1971; Shank et al., 1972a). However, Lucas et al. (1970-71) reported
that out of 139 samples of rice obtained from the Ho Chi Minh
(Saigon) area in Viet Nam, 31% were found positive for aflatoxins,
no confirmatory tests were included in this study. In surveys of
wheat and other cereals in the USSR, Lvova et al. (1976) found
aflatoxin B1 at a level of 100 µg/kg in 1/169 samples (0.6%) in
the 1972 crop. Aflatoxins (aflatoxin B1 at levels ranging from 20
to 444 µg/kg and aflatoxin G1 at levels of 10-333 µg/kg) were
found in 24/138 samples (17.4%) in the 1973 crop year. In this year,
the samples to be analysed were specially selected from those that
were mouldy or had undergone heating or both. In a survey of wheat
in southern USSR (Kazakstan), Bucarbaeva & Nikov (1977) found
aflatoxin B1 in 2/50 samples (4%) from one district and 3/50
samples (6%) from another (levels ranging from 5 to 10 µg/kg).
3.2.2.3 Groundnuts (peanuts)
In the 1973 survey in the USA of shelled consumer groundnuts,
15% of 361 samples contained aflatoxins in the range of trace to
50 µg/kg (Stoloff, 1976). Krogh & Hald (1969) found aflatoxins in
86.5% of 52 samples of groundnut products imported into Denmark for
feed, one sample contained 3465 µg/kg. Aflatoxins were found in 41%
of 173 samples of groundnuts in the Sudan, 16% of the samples
containing more than 250 µg/kg, and 9%, more than 1000 µg/kg (Habish
et al., 1971). In the Philippines, all the samples of peanut butter,
tested in 1967-69, contained aflatoxins with a median value of
155 µg/kg and a mean value of 500 µg/kg. The highest level detected
was 8600 µg/kg (Campbell & Salamat, 1971). In Thailand, 49% of
market samples contained an average level of aflatoxins of
1530 µg/kg (Shank et al., 1972a).
3.2.2.4 Soybeans and common beans
No significant degree of aflatoxin contamination has been found
in soybeans or common beans in commerce in the USA (Stoloff, 1976),
although aflatoxin contamination sufficient to be of public health
concern has been found in various types of edible beans in Thailand
(Shank et al., 1972a) and in Africa (Alpert et al., 1971).
3.2.2.5 Tree nuts
Aflatoxin has been found occasionally in Brazil nuts, almonds,
walnuts, pistachio nuts, pecans, and filberts. In some of these,
contamination occurs when the nuts are still on the tree and is
usually associated with damage of one sort or another. However,
apparently sound, undamaged pecans may contain aflatoxins (Stoloff,
1976). Yndestad & Underdal (1975)in Norway found 66% of Brazil nuts
contaminated with aflatoxin B1 and Nilsson et al. (1974) found
that all of 23 batches of Brazil nuts intended for importation to
Sweden were contaminated. Fourteen percent of 74 samples of
California almonds were contaminated with aflatoxin B1 with levels
of less than 20 µg/kg in 90% of the contaminated samples (Schade et
al., 1975).
3.2.2.6 Copra
Aflatoxins were found in 88% of 72 samples of copra and copra
meal (Stoloff, 1976) in amounts ranging from a trace to 30 µg/kg,
and similar contamination was found by Krogh et al. (1970) in copra
imported into Finland.
3.2.2.7 Cottonseed
In 3 successive crop years (1964-67), aflatoxin B1 was
detected in 6.5%-8.8% of more than 3000 cottonseed samples and in
12.8%-21.5% of more than 3000 samples of cottonseed meal (Stoloff,
1976). In contrast, aflatoxin was not detected in cottonseed hulls
(Whitten, 1969). Relatively high levels of aflatoxin contamination
were found in an area in southern California. Aflatoxin levels
increased from 1735 µg/kg in some samples of seed harvested in
November to 2578 µg/kg in some samples going into storage in late
January. The amount present in the stored seeds did not increase
with time, even though fungi, including A. flavus, could be seen
growing on some of the seeds. Marsh et al. (1973) tested cottonseeds
from 13 locations across the USA cotton belt in 1969 and from 11
locations in 1970. Aflatoxins B1 and B2 were found in one or
more samples from 3 regions in areas where boll rot caused by
A. flavus had been repeatedly observed in previous years. Seeds
from individual lots contained aflatoxin B1 levels ranging from
200 000 to 300 000 µg/kg indicating the high potential hazard that
might occur from cottonseed.
3.2.2.8 Spices and condiments
Scott & Kennedy (1973) did not find any aflatoxins in 24 samples
of ground black or white pepper. Low concentrations (up to 8 µg/kg)
were found in 10/33 samples of cayenne pepper and 6/6 samples of
Indian chili powder, mainly as trace amounts.
3.2.2.9 Animal feeds
In studies by Strzelecki & Gasiorowska (1974), aflatoxins
occurred in 12.7% of 306 samples of animal feed and feed components
in Poland, 4.2% of the samples containing more than 100 µg/kg and
2.6% of the samples containing more than 1000 µg/kg. Feed
components, mainly groundnut meals, were contaminated by aflatoxins
more frequently and with higher levels. On the other hand, aflatoxin
was detected in only one sample (2.7%) of cattle and sheep feeds
(300 µg/kg) and in one sample (1.7%)of poultry feeds (30 µg/kg).
Swine feeds contained aflatoxins in 11.4% of samples, with 6 samples
(5.7%) exceeding 250 µg/kg. Two recent surveys of mixed feeds in the
Federal Republic of Germany revealed that 1 in 60 samples contained
aflatoxin B1 levels exceeding 20 µg/kg (Seibold & Ruch, 1977); 45
out of another 105 samples contained levels of between 7 and
300 µg/kg (Kiermeier et al., 1977). Similar results were obtained in
the United Kingdom (Patterson, personal communication) where 95/172
samples of dairy feed were contaminated with aflatoxin B1 levels
of 1-350 µg/kg, and 92.4% contained no more than 30 µg/kg.
3.2.2.10 Animal products
Surveys in several countries have shown that aflatoxin M1 may
be present in liquid or dried milk (Table 3) and in milk products
(Kiermeier, 1977). In addition, highly exceptional aflatoxin levels
in the range of 50-500 µg/litre were reported by Suzanger et al.
(1976) in half of the samples of cow's milk collected in villages
around Isfahan, Iran (15/30 samples collected in 1973 and 21/37
samples in 1974). In contrast, no aflatoxins were detected in 8
samples of milk obtained from large-scale producers in the same area
in 1974 and only 10% of such samples (2/20) contained aflatoxin M1
(in the range 8-10 µg/litre) in 1973. Aflatoxin M1 was identified
in all the positive samples. Eight of the 36 village samples
containing aflatoxin M1 also contained aflatoxin M2 and 2
samples contained aflatoxin B1. Considerable differences in the
handling and storage of animal feeds were thought by the authors to
be responsible for the differences in the aflatoxin M1 contents of
milk samples from villages and large-scale producers in this area.
However, levels of aflatoxins in animal feeds were not reported in
this paper, but they must have been exceptionally high.
Table 3. Selected surveys of aflatoxin M1 in cow's milk
Milk Country Total no. No. containing Range of concentrations Reference
samples of samples aflatoxin M1 in the positive samples
analysed (µg/litre or µg/kg)
Liquid Belgium 68 42 0.02-0.2 Van Pee et al. (1977)
German Democratic Republic 36 4a 1.7-6.5c Fritz et al. (1977)
Germany, Federal Republic of 61 28 0.01-0.25 Kiermeier (1973)
Germany, Federal Republic of 419 79 trace-0.54 Kiermeier et al. ( 1977)
Germany, Federal Republic of 260 118a 0.05-0.33 Polzhofer (1977)
India 21 3 up to 13.3 Paul et al. (1976)
Netherlands 95 74 0.09-0.5 Schuller et al. (1977)
United Kingdom 278 85a 0.03-0.52a Patterson et al. (in press)
Dried German Democratic Republic 18 0f --- Fritz et al. (1977)
Germany, Federal Republic of 166 8 0.67-2.0 Neumann-Kleinpaul & Terplan (1972)
Germany, Federal Republic of 52 35 trace-4.0 Hanssen & Jung (1972)
Germany, Federal Republic of 120 7a 0.05-0.13 Jung & Hanssen (1974)
South Africa 56 0 --- Luck et al. (1977)
USA 320 24 0.1-0.4 FDA (1977) 1973 survey
USA 302 192 trace-3.9e FDA (1977) 1977 survey
a Seasonal effect observed, i.e., concentration obviously, dependent upon level of concentrate feeding.
b Samples collected in retail outlets in 4 southeast States of the USA; it has been estimated that approximately two-thirds
of the crops in these areas contained aflatoxin concentrations exceeding 20 µg/kg because of unusual drought, insect
damage, and high temperature conditions that occurred in 1977.
c Values for 4 positive samples collected in winter; aflatoxin was not detected in the other 32 winter milk samples as well as
in 12 milk samples collected in summer (detection limit = 0.1 µg/kg).
d 92.5% samples contained aflatoxin M1 concentrations of less than 0.1 µg/litre.
e Levels ranging from 0.1 to 0.4 µg/kg reported in 158 samples; levels exceeding 0.5 µg/kg reported in 19 samples.
f Aflatoxin B1 contamination detected in one sample.
Aflatoxin residues have been found in animal tissues, eggs, and
poultry following the experimental ingestion of aflatoxin-
contaminated feed and this subject has been reviewed by Rodricks &
Stoloff (1977). However, the toxins have not yet been found in these
products on the market.
3.2.3 Fate of aflatoxins during the handling and processing
of food
Aflatoxins are affected by some ordinary food processing
procedures. In the roasting of groundnuts, approximately 50% of the
aflatoxins are altered to such an extent that they can no longer be
detected (Lee et al., 1969b; Waltking, 1971). The chemical nature of
the alteration products has not been fully elucidated.
The usual methods of processing groundnuts to make peanut butter
and some nuts for confections may appreciably reduce aflatoxin
contamination. The removal of undersized nuts (shrivels and pegs);
the removal of nuts that resist splitting and blanching; and the
removal of discoloured nuts by hand or electronic sorting are
effective means of reducing contamination (Rodricks et al., 1977).
In the removal of oil from oilseeds, most of the aflatoxins are
found in the oilseed meal. Small amounts remaining in the crude
vegetable oil are mainly taken out in the soap stock, the byproduct
from the alkali refining step. The remaining traces of aflatoxins
are removed in the bleaching refining steps to give aflatoxin-free
refined oil (Parker & Melnick, 1966).
The normal alkali processing of maize to produce tortilla-type
foods, a common practice in some areas of the world including some
Latin American countries, effectively reduces the levels of
aflatoxins in contaminated maize (Ulloa-Sosa & Schroeder, 1969).
Although the mechanism of this reduction has not been clarified,
some of the aflatoxin is most likely washed out by the initial
soaking of the maize in lye water and some is undoubtedly chemically
changed by the alkali; although this process is reported to produce
a substantial reduction in aflatoxin contamination, it is not enough
to give a safe product, when highly contaminated maize is being
processed.
Jemmali & Lafont (1972) reported only partial destruction of
aflatoxins during bread making, indicating the importance of the
contamination of wheat with A. flavus.
The above treatments are normal steps in the processing of
particular foods. In addition to such steps, procedures have been
developed specifically for the destruction or removal of aflatoxins
from grains, nuts, oilseeds, and oilseed meals (cake) (FAO, 1977).
Treatments with ammonia or hydrogen peroxide (H2O2) have been
the most effective procedures developed to date for the
detoxification of foodstuffs and animal feeds (Goldblatt & Dollear,
1977). The treatments with ammonia, developed for the industrial
decontamination of aflatoxin-contaminated groundnut and cottonseed
meal (cake) and maize, are limited to the production of animal
feeds. These procedures have recently been discussed in detail at
the joint FAO/WHO/ UNEP conference on mycotoxins in Nairobi (FAO,
1977). The process of treating groundnut protein isolate with
hydrogen peroxide to obtain a product suitable for use as a human
food supplement was also discussed. This process has been developed
in India and is reported to be operating on a small commercial
scale.
The feasibility of methods combining the physical separation of
contaminated portions of produce (for detailed descriptions of
segregation techniques see, for example, Rodricks et al., 1977) with
chemical decontamination, was considered at the same conference
(FAO, 1977).
3.2.4 Pathways and levels of exposure
From the previous discussion, it can be seen that a range of
commodities may become contaminated with trace amounts of
aflatoxins. In vegetable foods, this contamination results directly
from fungal spoilage, maize and nuts being particularly susceptible.
On the other hand, milk, and possibly meat and eggs can become
indirectly contaminated through the absorption by farm animals of
aflatoxins from contaminated feed resulting in residues of the
parent toxin or its metabolites in body fluids or tissues.
Thus, the level of man's exposure to dietary aflatoxins depends
upon the food available and on eating habits and will vary from
country to country according to the local conditions, including the
traditions of different ethnic groups, and amongst individuals.
Where contaminated groundnuts or maize make a significant
contribution to the diet, the level of exposure will be relatively
higher than where less commonly contaminated commodities take their
place as the staple food or when milk is the sole
aflatoxin-containing constituent of the diet.
In this connexion, the Task Group felt it was important to
identify the infant as being potentially at risk because: (a) baby
food products may be made from dried milk or even maize, commodities
known to be prone to contamination by aflatoxins; and (b) in terms
of larger amounts of food consumed per kg body weight, any level of
aflatoxin contamination is more significant for the child than for
the adult.
Attempts to quantify dietary exposure to aflatoxins are
discussed in detail in section 3.6.1.1.
Occupational exposure (section 3.5.2) to aflatoxins with its
attendant high risks, concerns two groups of individuals: those who
handle grain, animal feedstuffs, groundnuts, groundnut meal etc.
(where exposure could occur largely through inhalation of
contaminated dust), and those who work with toxins in experiments or
with pure toxins as analytical standards.
In one paper (van Nieuwenhuize et al., 1973) available to the
Task Group, an attempt was made to quantify occupational exposure to
airborne aflatoxins in an oil-mill crushing groundnuts and other
oil-seeds. Based on airborne dust determinations (mean aflatoxin
concentrations of 250 and 410 µg/kg airborne dust), the estimated
airborne aflatoxin levels ranged from 0.87 to 72 ng/m3 of air.
3.3 Metabolism
3.3.1 Absorption
Although quantitative data on absorption are not available at
present, there is no doubt that most of the field cases of
aflatoxin-induced diseases in animals and man have been associated
with ingestion of aflatoxin-contaminated foodstuffs and thus with
the absorption of aflatoxins in the alimentary tract. In spite of
reports of respiratory exposure (sections 3.2.4 and 3.5.2), there is
no quantitative information available on aflatoxin resorption from
the respiratory tract or on percutaneous absorption.
3.3.2 Tissue distribution
3.3.2.1 Animal studies
Experiments with 14C-ring-labelled aflatoxin B1 have shown
that rats retain about 20% of the 14C activity 24 h after a single
intraperitoneal dose of 0.07 mg/kg body weight (Wogan et al., 1967).
The highest concentration was found in the liver, which contained
amounts of radioactivity equivalent to the entire remainder of the
carcass (about 5%-8% of the total 14C recovered).
When poultry were fed rations containing aflatoxins at
concentrations ranging from 25 to 15 000 µg/kg for 8 weeks, residues
of aflatoxin B1 were found in the liver and in muscle tissue
(Mintzlaff et al., 1974). The liver contained the highest
concentration, with a mean value of 15 µg/kg at the highest exposure
level. Similarly the highest concentration of aflatoxin B1 was
found in the livers of pigs (range: trace-137 µg/kg) fed rations
containing aflatoxins (both aflatoxin B1 and B2) at levels of
300 and 500 µg/kg for 4 months (Krogh et al., 1973a). Aflatoxin
residues were also detected in kidneys, and muscle and adipose
tissue.
3.3.2.2 Studies in man
Levels of aflatoxins in the tissues of children with Reye's
syndrome are discussed in section 3.6.2.2. In a liver biopsy from a
subject with carcinoma of the rectum and liver in the USA, Phillips
et al. (1976) found 520 µg/kg of aflatoxin B1. In France, Richir
et al. (1976) found aflatoxin B1 in liver biopsies in 6 out of 100
subjects suffering from various diseases. Concentrations observed
ranged from 1.6-8 µg/kg.
3.3.3 Metabolic transformation and activation
With one exception, all primary biotransformations of aflatoxin
B1 involve its conversion to hydroxylated metabolites but only one
such derivative, aflatoxin M1 has appreciable oral toxicity
(Holzapfel et al., 1966). Even so, this metabolite may be detoxified
by conjugation with taurocholic and glucuronic acids prior to
excretion in the bile or urine (Bassir & Osiyemi, 1967). In this
respect, two recently discovered metabolites, P1 (Dalezios et al.,
1971; Buchi et al., 1973) and Q1 (Masri et al., 1974a,b) are
similar in that they also undergo this type of detoxification
(Dalezios et al., 1971; Dalezios & Wogan, 1972).
The conversion in the liver (Fig. 2) of aflatoxin B1 to
aflatoxicol (Patterson & Roberts, 1971) and to aflatoxicol H1 via
aflatoxin Q1 (Salhab & Hsieh, 1975) is unusual in that, unlike
other biotransformations that are catalysed by liver microsomal
enzymes, a cytoplasmic NADH-dependent dehydrogenase is involved.
Furthermore, the formation of aflatoxicol can be inhibited by
17-ketosteroid sex hormones (Patterson & Roberts, 1972a) and this is
the only metabolic transformation of aflatoxin in vitro known to
be sensitive to hormones.
Liver homogenates of certain avian and rodent species are
particularly active in converting aflatoxins B1 and G1 to their
2-hydroxy, 2,3-dihydro derivatives or hemiacetals called also
aflatoxins B2a and G2a (Patterson & Roberts, 1970). These
metabolites bind strongly to protein and are probably sufficiently
reactive, when formed in vivo, to cause many of the acute effects
of aflatoxin poisoning (Patterson & Roberts, 1972b; Patterson, 1973,
1977).
At present, there is only indirect evidence for the formation of
the epoxides of aflatoxins B1 and G1 but this is probably the
more important form of metabolic activation. When either of the
parent toxins is incubated with microsomes prepared from the livers
of many animal species including man, a metabolite is formed which
appears to have only a transient existence, is highly reactive,
binds covalently to DNA, and induces mutation in a bacterial
in vitro test system (Garner et al., 1971, 1972; Ames et al.,
1973). The metabolite of B1 has not been isolated but the
2,3-dihydrodiol has been recovered following mild acid hydrolysis of
an adduct formed when the microsomal metabolite was generated in the
presence of added DNA or RNA (Swenson et al., 1,974) and, more
recently, after in vivo intraperitoneal injection of aflatoxin
B1 (Swenson et al., 1977). This has been assumed to be indirect
evidence of the formation of the 2,3-epoxide and, in view of the
interaction with DNA, it is now generally accepted that the epoxide
of aflatoxin B1 is the bacterial mutagen and the proximal
carcinogen.
Little or no destruction of aflatoxins occurs under ordinary
cooking conditions, and heating for pasteurization. However,
roasting groundnuts appreciably reduces the levels of aflatoxins
(see section 3.2.3) and they can be totally destroyed by drastic
treatment such as autoclaving in the presence of ammonia or by
treatment with hypochlorite.
Table 1. Physical and chemical properties of some aflatoxins and their metabolites
Aflatoxin Molecular Relative Melting Ultraviolet absorption (epsilon)c Fluorescence Reference
formula molecular point emission
mass °C 265 nm 360-362 nm nm
B1a C17H12O6 312 268-269 12 400 21 800 425 Asao et al. (1965)
B2a C17H14O6 314 286-289 12 100 24 000 425 Chang et al. (1963)
G1a C17H12O6 328 244-246 9 600 17 700 450 Asao et al. (1965)
G2a C17H14O7 330d 237-240d 8 200 17 100 450 Hartley et al. (1963)
M1a C17H12O7 328 299 14 150 21 250 (357 nm) 425 Holzapfel et al. (1966)
M2a C17H14O7 330 293 12 100 (264 nm) 22 900 (357 nm) f Holzapfel et al. (1966)
P1b C16H10O6 298 >320 11 200 (267 nm) 15 400 (362 nm) f Dalezios et al. (1971 ) and
14 900 (342 nm) Buchi et al. (1973)
Q1 C17H12O7 328 e 11 450 (267 nm) 17 500 (366 nm) f Masri et al. (1974a,b)
Aflatoxicol C17H14O6 314 230--234d 10 800 (261 nm) 14 100 (375 nm) 425 Detroy & Hesseltine (1970)
a Molar absorption coefficient for aflatoxins B1, B2, G1, and G2 obtained from Rodricks et al. (1970) and those for M1 and M2
from Stubblefield et al, (1972).
b P stands for phenolic products of O-demethylation of aflatoxin B1.
c Compounds dissolved in methanol except for aflatoxin P1 which in this case was dissolved in ethanol. Data on molar absorption
coefficients for other peaks and on the ultraviolet absorption characteristics of aflatoxins in other solvents can be found
in the original papers.
d Data from Butler (1974).
e Not available.
f Violet fluorescence of aflatoxin M2 and yellow-green fluorescence of aflatoxins P1 and Q1 reported in original papers.
The presence of a lactone ring in the aflatoxin molecule makes
them susceptible to alkaline hydrolysis (De Iongh et al., 1962).
This characteristic is important in that any food processing
involving alkali treatment can decrease the contamination of the
products (section 3.2.3) although the presence of protein, the pH,
and the duration of treatment may modify the results (Beckwith et
al., 1975). However, if the alkaline treatment is mild,
acidification will reverse the reaction to reform the original
aflatoxin.
The chemistry of the aflatoxins has recently been reviewed by
Roberts (1974).
3.1.2 Methods of analysis for aflatoxins in foodstuffs
3.1.2.1 Sampling
Sampling is an integral part of the analytical procedure and the
sample drawn should be representative of the lot. The total error
made in an analytical procedure consists of the sampling error, the
subsampling error, and the error in analysis (Whitaker, 1977). The
difficulty in sampling for aflatoxins arises because of the
heterogeneity of aflatoxin distribution in contaminated unprocessed
commodities. On the basis of a large number of analyses, Whitaker et
al. (1974a) were able to calculate the contribution of each error to
the total error. The total variance of the analytical procedure is
primarily caused by sampling variability, whereas the subsampling
variability and the analysis variability are more or less
independent of aflatoxin concentration. The coefficient of variation
associated with sampling is about 115% at a level of contamination
of 20 µg/kg and about 145% at a level of contamination of 10 µg/kg.
Whitaker et al. (1974b) and Whitaker (1977) have summarized a
procedure for sampling and have developed a number of sampling plans
used in the USA for the control of aflatoxin contamination in
shelled groundnuts (peanuts). Other recent publications deal with
aflatoxin-testing programmes for maize (Whitaker et al., 1978) and
cottonseed (Whitaker & Whitten, 1977).
The sampling of small grains, oilseed cakes, foodstuffs, and
feeds is also difficult, although in most cases the aflatoxin
distribution within a batch is not likely to be as uneven as in the
case of groundnuts. Fluids and well-mixed processed products such as
milk and milk products, beer, and cider do not present such a
sampling problem.
Peanut butter, flours, and cornmeal do not present the same
problems as the original raw materials because a finely divided
product is formed during processing from which it is much easier to
obtain a representative analytical sample.
Some practical aspects of sampling are dealt with in Chapter 26
of "Official methods of analysis" of the Association of Official
Analytical Chemists (Horwitz et al., 1975). For survey purposes,
1-5 kg samples are usually taken and the size of the sample for
analysis ranges from 20 to 100 g. A sample of 50 g ensures both a
representative sample and solvent economy.
3.1.2.2 Methods of analysis
Biological and chemical procedure have been developed for the
detection and determination of aflatoxins and other mycotoxins. The
bioassay techniques that are currently available are not suitable
for routine screening purposes and their detection levels are not
low enough. The chemical assay techniques, although more accurate
and faster, are not always specific. The presence of a certain toxin
is usually confirmed by derivative formation and its toxicity
verified by bioassay.
Biological methods. In the original biological test (Carnaghan
et al., 1963), one-day-old ducklings were used as test animals for
determining the presence of aflatoxins in suspect food by measuring
the degree of biliary proliferation as a semiquantitative index (see
section 3.4.2.1). The lowest dose level of 0.4 µg/day administered
for 5 days represents the minimum intake required to induce a
detectable biliary proliferation. The test is also effective for
detecting aflatoxin M1 in both liquid and powdered milk (Purchase,
1967). Little information is available on the sensitivity of this
test (and other biological methods) to aflatoxins other than B1.
A commonly used method in regulatory actions is the chicken
embryo bioassay in which 0.1-0.2 µg of aflatoxin B1 is applied to
the egg membrane and the mortality rate recorded during the 23-day
period of hatching (Horwitz, 1975).
Several other biological procedures have been developed, using
maize seedlings, zebra fish larvae, brine shrimps, bacteria etc.
Detailed descriptions can he found in the reviews by Goldblatt
(1969) and Ciegler et al. (1971).
Chemical methods. Although procedures are continually
changing, the basic steps remain; extraction, lipid removal,
cleanup, separation, and quantification. Since there is considerable
overlap in the various methods, most of the published reviews
(Jones, 1972; Stoloff, 1972) examine the different procedures by
these basic steps. Depending on the nature of the commodity, methods
can sometimes be simplified by omitting unnecessary steps. The
presence of specific interferences such as theobromine in cacao and
gossypol in cottonseed, may require additional steps.
Numerous methods of analysis have been reported for the
determination of aflatoxins in human and animal foodstuffs. Many of
them are minor modifications of the basic steps adapted to special
commodities or problems.
Collaborative studies designed to assess the performances of
different laboratories give information on the accuracy, precision,
and specificity of the method under consideration, as well as on the
occurrence of false negative and false positive results. Only
methods that have been subjected to such studies are reported in
this document.
Chemical methods have mainly been developed for such commodities
as groundnuts. In the first method for the analysis of groundnuts,
the aflatoxins were extracted from the contaminated sample using
methanol; this was later replaced by chloroform. An improvement was
made by Lee (1965) who showed that the addition of water to
hydrophilic plant tissues during extraction with chloroform resulted
in more effective removal of aflatoxin. The combination of
liquid-liquid extraction techniques and partition chromatography led
to a method, which is now one of the most widely used, known as the
Contamination Branch (CB) method (Eppley, 1966). The sample is
extracted with water and the water extracted with chloroform, the
lipids and aflatoxins are transferred to a silica-gel column where
the lipids are selectively eluted with hexane and the pigments and
other interfering material eluted with absolute diethylether;
finally the aflatoxins are eluted from the column with 3% methanol
in chloroform.
Because the CB method is time-consuming, attempts have been made
to simplify it. Waltking et al. (1968) drew attention to the fact
that a separation funnel was simpler and faster for liquid-liquid
partition than the silica-gel column, and that centrifuging was a
faster method of separating a solid than filtration. Thus the Best
Foods (BF) method was developed, which is faster and more economical
in terms of the amounts of solvents used but provides a poorer
cleanup. The sample is extracted and defatted with a two-phase
aqueous methanol-hexane system, the aflatoxins are then partitioned
from the aqueous phase into chloroform, leaving lipids and pigments
in the hexane and aqueous methanol.
In both the CB and BF methods, the aflatoxins are concentrated
by evaporation of the chloroform, and then separated by thin-layer
chromatography (TLC). Aflatoxins are intensely fluorescent when
exposed to long-wave ultraviolet radiation, which makes it possible
to determine these compounds at extremely low levels. An analyst
experienced in this field can detect 0.5 ng aflatoxin B1 on a TLC
plate. In most methods, the intensity of fluorescence of the sample
is compared with that of a standard. Under ideal conditions this
technique; has a coefficient of variation of about 20% which can be
reduced to 5%-9% by the use of a fluorodensitometer.
It should be pointed out that quantification and confirmation of
identity can only be obtained if pure authentic standards are
available for reference. Current sources of aflatoxin standards and
methods for the determination of mass concentration and purity cart
be found in Chapter 26 of the AOAC "Official methods of analysis"
(Horwitz et al., 1975).
When the CB and BF methods were compared in a collaborative
study (Waltking, 1970), the methods were found to be equivalent in
accuracy and precision with a recovery of about 70% of added
aflatoxin and an overall coefficient of variation of about 35% for
total aflatoxin levels down to about 20 µg/kg. This result has been
confirmed by the latest International Aflatoxin Check Sample Study
(Coon et al., 1972) in which 129 laboratories participated. However,
the coefficient of variation was about twice as high as in the
original collaborative studies. This illustrates the inadequacies of
many laboratories.
Another collaborative study was conducted (Stack, 1974) in which
the CB and BF methods were compared at levels down to the 2-10 µg/kg
range, Again both methods proved to be equally accurate (about 80%
recovery) for total aflatoxins at the 5-10 µg/kg levels. The CB
method, however, was as precise (coefficient of variation = 30%) at
the lowest level of 2 µg/kg as at the highest levels. The BF method
lost precision at these low levels and the coefficient of variation
was of the order of 100%.
In spite of cleanup and separation procedures, there might still
be problems with compounds that have fluorescent and chromatographic
properties similar to those of the aflatoxins. Thus, the presence of
a spot on a TLC plate is only presumptive evidence of identity and
additional confirmatory tests are necessary. Probably the first step
in confirming the presence of aflatoxins is to use additional
solvent systems in the TLC. The developed TLC plate can be sprayed
with 25% sulfuric acid (Schuller et al., 1967), which changes the
fluorescence colour of the aflatoxin spots to yellow. This test, if
negative, would rule out the presence of aflatoxins but does not
provide confirmatory evidence. The formation of chemical derivatives
was first described for aflatoxins B1 and G1 (Andrellos & Reid,
1964). The reagents used were formic acid-thionyl chloride, acetic
acid-thionyl chloride, and trifluoroacetic acid, the acid-catalysed
addition products formed were a dimeric acetate and an addition
product with water, respectively. The characteristic mobilities and
fluorescent properties on thin-layer chromatograms can be compared
with those of standard derivatives. Pohland et al. (1970) simplified
the preparation of the derivatives by using a mixture of
hydrochloric acid and acetic anhydride, and hydrochloric acid alone.
Further improvement, by elimination of the preparative
chromatography step in these procedures, has been achieved by
Przybylski (1975). The water adduct is formed directly on a TLC
plate from as little as 0.5 ng of aflatoxin B1 or G2.
In addition to the procedures for groundnuts, methods of
analysis have been developed for cottonseed, copra, maize, various
tree nuts (pistachio, walnut, Brazil nuts, etc.) and for animal
feeds. Many of these methods are modifications of the CB and BF
methods. Milk and dairy products require a far greater sensitivity
for the determination of M1 and M2 because these animal
metabolites are usually only found at sub µg/kg levels; additional
cleanup to eliminate interferences and sometimes two-dimensional TLC
techniques (Schuller et al., 1973) are necessary to attain
satisfactory performance. These methods are described in Chapter 26
of the AOAC "Official methods of analysis" (Horwitz et al., 1975).
Several analytical methods for the detection of aflatoxin
residues in animal tissues have been developed, and their detection
limits evaluated (Jemmali & Murphy, 1976).
Column detection methods are being used for control purposes in
the field because of their simplicity. The method of Romer (1975) is
of particular value because it combines column detection, TLC
quantification, and TLC plate chemical derivative confirmation in a
method that has a wide application for a number of foodstuffs and
feeds including mixed feeds.
It appears that methods using high-pressure liquid
chromatography will become the methods of choice for mycotoxin
analyses in the near future because of their sensitivity and
improved accuracy, and because they can be applied to a number of
mycotoxins including aflatoxins B1, B2, G1, and G2 (Panalaks
& Scott, 1977).
3.2 Sources and Occurrence
3.2.1 Formation by fungi
The ability to produce aflatoxins seems to be confined to
strains of the two species Aspergillus flavus Link and
A. parasiticus Speare, both members of the A. flavus group.
Aflatoxin-producing strains of A. flavus are common and
widespread, and have been isolated from a host of different
materials. As indicated in Table 2, a high proportion (from 20% to
98%) of isolated strains of A. flavus is able to produce
aflatoxins.
Table 2. Aflatoxin-producing strains of A. flavus isolated from
four field cropsa
Source Isolates Isolates producing Maximum yield
tested aflatoxin of aflatoxin B1
(No.) (%) (µg/flask)
groundnut 100 98 3300
cottonseed 59 81 3200
rice 127 20 1100
sorghum 63 24 3300
a Data from Schroeder & Boller quoted by Hesseltine (1976) in
"Mycotoxins and other fungal related food problems".
3.2.1.1 Moisture content and temperature
The moisture content of the substrate and temperature are the
main factors regulating fungal growth and mycotoxin formation.
Koehler (1938) established that a moisture content of 18.3% on a
wet weight basis, was the lower limit for the growth of A. flavus
in shelled corn. Extensive studies under precisely controlled
conditions (Sanders et al., 1968; Diener & Davis, 1969; Davis &
Diener, 1970) established a moisture content in equilibrium with a
relative humidity of 85% (or water activity (aw) = 0.85) as the
lower limit for growth of A. flavus and for the production of
aflatoxins. In starchy, cereal grain such as wheat, oats, barley,
rice, sorghum, and maize, the lower limit is a moisture content of
18.3%-18.5% on a wet weight basis and in groundnuts, Brazil nuts,
other nuts, copra, and sunflower and safflower seeds, all of which
have a high oil content, it is a moisture content of 9%-10%.
The minimum, optimum, and maximum temperatures for aflatoxin
production are 12° C, 27° C, and 40-42° C, respectively (Davis &
Diener, 1970). Northolt et al. (1976) studied the effect of water
activity and temperature on the growth and aflatoxin production of
A. parasiticus and came to the conclusion that no detectable
quantities of aflatoxin B1 were formed at an aw value below 0.83
and at temperatures below 10°C.
3.2.1.2 Invasion of field crops by A. flavus
Groundnut seeds may be invaded by A. flavus before harvest but
are more likely to be invaded very rapidly after the plants have
been pulled and piled for preliminary drying before the nuts are
removed. This postharvest period is the "high hazard" time for
aflatoxin production. On the other hand, studies of aflatoxin
contamination in North Carolina (USA) (Dickens & Satterwhite, 1973)
under conditions of drought, suggest that drought after groundnuts
are formed but before they are dug is conducive to their infection
with A. flavus. Damage caused by the lesser cornstalk borer (LCB)
might also aid in the infection process because insects may carry
fungal spores, although many drought area fields infested with LCB
did not produce groundnuts with a high aflatoxin content. Drought
alone does not result in high levels of aflatoxin contamination. It
must coincide with, or promote infestation by insects which in turn
infect the groundnut. The LCB may act as a vector for A. flavus.
Pettit et al. (1971) reported that groundnuts grown under dry
land conditions (drought stress) accumulated more aflatoxin before
digging than those grown under irrigation. Dry land fresh-dug
kernels contained a maximum aflatoxin level of 35 800 µg/kg while a
maximum of 50 µg/kg was detected in kernels from irrigated plots.
Apparently, the higher kernel moisture content occurring under
irrigated conditions reduced the aflatoxin production potential,
whereas a moisture content of about 31% under drought conditions was
near optimum. Similar observations have been reported from West
Africa.
In some irrigated regions with moist weather at harvest time,
cottonseed may be invaded by A. flavus while still on the plant
and after the bolls open and may contain large amounts of aflatoxins
(Marsh et al., 1973). Stephenson & Russell (1974) related the high
aflatoxin contamination in the field (in USA) to invasion by insects
that provided a site of injury and served as vectors for A. flavus.
Insect injury in ears of maize in the field may also be
accompanied or followed by infection with A. flavus and by
aflatoxin formation before harvest (Lillehoj et al., 1976). To what
extent this constitutes a contamination problem in many regions of
the world, where maize is an important crop, is not known.
Aflatoxins have also been reported in heads of sorghum heavily
infected with mould in India (Tripathi, 1973). Pistachio nuts can
become contaminated with Aflatoxins prior to harvest but the cause
of infection with aflatoxin-producing strains of fungi has not yet
been found. The contamination of almonds and of walnuts has been
traced to specific types of insect damage in the orchard (Stoloff,
1977).
3.2.2 Occurrence in foodstuffs
This subject has been reviewed recently by Stoloff (1976). Of
the four major aflatoxins (B1, B2, G1, G2) B1 is usually
found in the greatest concentrations. Measurements of toxin
concentration are based on the wet weight of the commodity in
question. The four toxins may occur together, although they need
not, and their concentrations in relation to each other and their
occurrence may vary depending on the fungal strain and substrate.
For example, Hesseltine et al. (1970) found that most fungal strains
that produced aflatoxin G1 also produced aflatoxin B1, but that
not all strains that produced aflatoxin B1 produced aflatoxin
G1. One strain of A. flavus from black pepper produced only
aflatoxin B2 on 2 natural substrates tested (Schroeder & Carlton,
1973).
Although aflatoxins have been found in a variety of foodstuffs,
the most pronounced contamination has been encountered in groundnuts
and other oilseeds including cottonseed and maize. The most
frequently contaminated tree nuts are Brazil nuts and pistachios.
3.2.2.1 Maize
Surveys in the USA of more than 1500 samples of maize collected
in crop years 1964-67, mainly from commercial channels, revealed
that 2%-3% of the samples contained aflatoxins (total aflatoxin B1
and G1) in the range of 3-37 µg/kg (Shotwell et al., 1969a, 1970).
In a subsequent survey of 293 samples, 8 samples (2.7%) contained
aflatoxin B1 levels in the range of 6-25 µg/kg, one of the samples
containing aflatoxin G1 (25 µg/kg) as well as aflatoxin B1
(Shotwell et al., 1971). Aflatoxin B2 was also detected in some of
the samples. In a further study of 60 samples from south-east USA
(Shotwell et al., 1973), aflatoxin B1 was found in 21 samples
(35%) at levels ranging from 6-308 µg/kg in the 1969-70 period.
Aflatoxin B2 at levels ranging from a trace to 40 µg/kg was found
in 15 of these samples and aflatoxin G1 was found in 5 samples at
levels of a trace to 10 µg/kg. In 2 samples, aflatoxin G2 (1 and
<2 µg/kg) was detected in addition to aflatoxin B1. In some of
the southeastern states of the USA a high frequency of aflatoxin
contamination was also found in maize in the field (Anderson et al.,
1975; Lillehoj et al., 1976). In some of the field samples, the
levels of aflatoxin B1 ranged up to several thousand µg/kg or
more. Most of the field contamination was associated with damage
caused by insects such as the European corn borer, corn ear worms,
or weevils, and it seems likely that wherever such damage is
prevalent, field contamination with aflatoxins will occur. In
Thailand, 35% of maize samples contained aflatoxin B1 (average
level 400 µg/kg) while 40% contamination by aflatoxin B1 (average
level 133 µg/kg) was found in Uganda (Stoloff, 1976), and 97% in the
Philippine island of Sebu (average 213 µg/kg) (Alpert et al., 1971;
Campbell & Salamat, 1971; Shank et al., 1972a; Campbell & Stoloff,
1974; Stoloff, 1976).
Aflatoxin levels found in household maize samples in connexion
with the outbreak of acute toxic hepatitis in north-west India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977) are discussed
in section 3.5.1.2.
3.2.2.2 Wheat, barley, oats, rye, rice, and sorghum
Shotwell et al. (1968b) reported the presence of aflatoxins at
levels of less than 19 µg/kg in 9/1368 samples of wheat, sorghum,
and oats in the USA. Shotwell et al. (1976b) did not detect any
aflatoxin B1 (detection limit 1-3 µg/kg) in 848 samples of wheat
from various districts of the USA. The presence of aflatoxins B1,
B2, G1, and G2 was reported by Tripathi (1973) in heads of
sorghum heavily infected with mould, in field samples in India, but
he did not apply any confirmatory tests. Aflatoxins were also found
in sorghum in Uganda (Alpert et al., 1971) and, in a survey in the
USA, aflatoxins were detected in 2/66 samples of sorghum grain (13
and 50 µg/kg) (Stoloff, 1976). Aflatoxins have been detected in less
than 2% of more than 400 samples of rice from markets in Africa, the
Philippines, and Thailand (Alpert et al., 1971; Campbell & Salamat,
1971; Shank et al., 1972a). However, Lucas et al. (1970-71) reported
that out of 139 samples of rice obtained from the Ho Chi Minh
(Saigon) area in Viet Nam, 31% were found positive for aflatoxins,
no confirmatory tests were included in this study. In surveys of
wheat and other cereals in the USSR, Lvova et al. (1976) found
aflatoxin B1 at a level of 100 µg/kg in 1/169 samples (0.6%) in
the 1972 crop. Aflatoxins (aflatoxin B1 at levels ranging from 20
to 444 µg/kg and aflatoxin G1 at levels of 10-333 µg/kg) were
found in 24/138 samples (17.4%) in the 1973 crop year. In this year,
the samples to be analysed were specially selected from those that
were mouldy or had undergone heating or both. In a survey of wheat
in southern USSR (Kazakstan), Bucarbaeva & Nikov (1977) found
aflatoxin B1 in 2/50 samples (4%) from one district and 3/50
samples (6%) from another (levels ranging from 5 to 10 µg/kg).
3.2.2.3 Groundnuts (peanuts)
In the 1973 survey in the USA of shelled consumer groundnuts,
15% of 361 samples contained aflatoxins in the range of trace to
50 µg/kg (Stoloff, 1976). Krogh & Hald (1969) found aflatoxins in
86.5% of 52 samples of groundnut products imported into Denmark for
feed, one sample contained 3465 µg/kg. Aflatoxins were found in 41%
of 173 samples of groundnuts in the Sudan, 16% of the samples
containing more than 250 µg/kg, and 9%, more than 1000 µg/kg (Habish
et al., 1971). In the Philippines, all the samples of peanut butter,
tested in 1967-69, contained aflatoxins with a median value of
155 µg/kg and a mean value of 500 µg/kg. The highest level detected
was 8600 µg/kg (Campbell & Salamat, 1971). In Thailand, 49% of
market samples contained an average level of aflatoxins of
1530 µg/kg (Shank et al., 1972a).
3.2.2.4 Soybeans and common beans
No significant degree of aflatoxin contamination has been found
in soybeans or common beans in commerce in the USA (Stoloff, 1976),
although aflatoxin contamination sufficient to be of public health
concern has been found in various types of edible beans in Thailand
(Shank et al., 1972a) and in Africa (Alpert et al., 1971).
3.2.2.5 Tree nuts
Aflatoxin has been found occasionally in Brazil nuts, almonds,
walnuts, pistachio nuts, pecans, and filberts. In some of these,
contamination occurs when the nuts are still on the tree and is
usually associated with damage of one sort or another. However,
apparently sound, undamaged pecans may contain aflatoxins (Stoloff,
1976). Yndestad & Underdal (1975)in Norway found 66% of Brazil nuts
contaminated with aflatoxin B1 and Nilsson et al. (1974) found
that all of 23 batches of Brazil nuts intended for importation to
Sweden were contaminated. Fourteen percent of 74 samples of
California almonds were contaminated with aflatoxin B1 with levels
of less than 20 µg/kg in 90% of the contaminated samples (Schade et
al., 1975).
3.2.2.6 Copra
Aflatoxins were found in 88% of 72 samples of copra and copra
meal (Stoloff, 1976) in amounts ranging from a trace to 30 µg/kg,
and similar contamination was found by Krogh et al. (1970) in copra
imported into Finland.
3.2.2.7 Cottonseed
In 3 successive crop years (1964-67), aflatoxin B1 was
detected in 6.5%-8.8% of more than 3000 cottonseed samples and in
12.8%-21.5% of more than 3000 samples of cottonseed meal (Stoloff,
1976). In contrast, aflatoxin was not detected in cottonseed hulls
(Whitten, 1969). Relatively high levels of aflatoxin contamination
were found in an area in southern California. Aflatoxin levels
increased from 1735 µg/kg in some samples of seed harvested in
November to 2578 µg/kg in some samples going into storage in late
January. The amount present in the stored seeds did not increase
with time, even though fungi, including A. flavus, could be seen
growing on some of the seeds. Marsh et al. (1973) tested cottonseeds
from 13 locations across the USA cotton belt in 1969 and from 11
locations in 1970. Aflatoxins B1 and B2 were found in one or
more samples from 3 regions in areas where boll rot caused by
A. flavus had been repeatedly observed in previous years. Seeds
from individual lots contained aflatoxin B1 levels ranging from
200 000 to 300 000 µg/kg indicating the high potential hazard that
might occur from cottonseed.
3.2.2.8 Spices and condiments
Scott & Kennedy (1973) did not find any aflatoxins in 24 samples
of ground black or white pepper. Low concentrations (up to 8 µg/kg)
were found in 10/33 samples of cayenne pepper and 6/6 samples of
Indian chili powder, mainly as trace amounts.
3.2.2.9 Animal feeds
In studies by Strzelecki & Gasiorowska (1974), aflatoxins
occurred in 12.7% of 306 samples of animal feed and feed components
in Poland, 4.2% of the samples containing more than 100 µg/kg and
2.6% of the samples containing more than 1000 µg/kg. Feed
components, mainly groundnut meals, were contaminated by aflatoxins
more frequently and with higher levels. On the other hand, aflatoxin
was detected in only one sample (2.7%) of cattle and sheep feeds
(300 µg/kg) and in one sample (1.7%)of poultry feeds (30 µg/kg).
Swine feeds contained aflatoxins in 11.4% of samples, with 6 samples
(5.7%) exceeding 250 µg/kg. Two recent surveys of mixed feeds in the
Federal Republic of Germany revealed that 1 in 60 samples contained
aflatoxin B1 levels exceeding 20 µg/kg (Seibold & Ruch, 1977); 45
out of another 105 samples contained levels of between 7 and
300 µg/kg (Kiermeier et al., 1977). Similar results were obtained in
the United Kingdom (Patterson, personal communication) where 95/172
samples of dairy feed were contaminated with aflatoxin B1 levels
of 1-350 µg/kg, and 92.4% contained no more than 30 µg/kg.
3.2.2.10 Animal products
Surveys in several countries have shown that aflatoxin M1 may
be present in liquid or dried milk (Table 3) and in milk products
(Kiermeier, 1977). In addition, highly exceptional aflatoxin levels
in the range of 50-500 µg/litre were reported by Suzanger et al.
(1976) in half of the samples of cow's milk collected in villages
around Isfahan, Iran (15/30 samples collected in 1973 and 21/37
samples in 1974). In contrast, no aflatoxins were detected in 8
samples of milk obtained from large-scale producers in the same area
in 1974 and only 10% of such samples (2/20) contained aflatoxin M1
(in the range 8-10 µg/litre) in 1973. Aflatoxin M1 was identified
in all the positive samples. Eight of the 36 village samples
containing aflatoxin M1 also contained aflatoxin M2 and 2
samples contained aflatoxin B1. Considerable differences in the
handling and storage of animal feeds were thought by the authors to
be responsible for the differences in the aflatoxin M1 contents of
milk samples from villages and large-scale producers in this area.
However, levels of aflatoxins in animal feeds were not reported in
this paper, but they must have been exceptionally high.
Table 3. Selected surveys of aflatoxin M1 in cow's milk
Milk Country Total no. No. containing Range of concentrations Reference
samples of samples aflatoxin M1 in the positive samples
analysed (µg/litre or µg/kg)
Liquid Belgium 68 42 0.02-0.2 Van Pee et al. (1977)
German Democratic Republic 36 4a 1.7-6.5c Fritz et al. (1977)
Germany, Federal Republic of 61 28 0.01-0.25 Kiermeier (1973)
Germany, Federal Republic of 419 79 trace-0.54 Kiermeier et al. ( 1977)
Germany, Federal Republic of 260 118a 0.05-0.33 Polzhofer (1977)
India 21 3 up to 13.3 Paul et al. (1976)
Netherlands 95 74 0.09-0.5 Schuller et al. (1977)
United Kingdom 278 85a 0.03-0.52a Patterson et al. (in press)
Dried German Democratic Republic 18 0f --- Fritz et al. (1977)
Germany, Federal Republic of 166 8 0.67-2.0 Neumann-Kleinpaul & Terplan (1972)
Germany, Federal Republic of 52 35 trace-4.0 Hanssen & Jung (1972)
Germany, Federal Republic of 120 7a 0.05-0.13 Jung & Hanssen (1974)
South Africa 56 0 --- Luck et al. (1977)
USA 320 24 0.1-0.4 FDA (1977) 1973 survey
USA 302 192 trace-3.9e FDA (1977) 1977 survey
a Seasonal effect observed, i.e., concentration obviously, dependent upon level of concentrate feeding.
b Samples collected in retail outlets in 4 southeast States of the USA; it has been estimated that approximately two-thirds
of the crops in these areas contained aflatoxin concentrations exceeding 20 µg/kg because of unusual drought, insect
damage, and high temperature conditions that occurred in 1977.
c Values for 4 positive samples collected in winter; aflatoxin was not detected in the other 32 winter milk samples as well as
in 12 milk samples collected in summer (detection limit = 0.1 µg/kg).
d 92.5% samples contained aflatoxin M1 concentrations of less than 0.1 µg/litre.
e Levels ranging from 0.1 to 0.4 µg/kg reported in 158 samples; levels exceeding 0.5 µg/kg reported in 19 samples.
f Aflatoxin B1 contamination detected in one sample.
Aflatoxin residues have been found in animal tissues, eggs, and
poultry following the experimental ingestion of aflatoxin-
contaminated feed and this subject has been reviewed by Rodricks &
Stoloff (1977). However, the toxins have not yet been found in these
products on the market.
3.2.3 Fate of aflatoxins during the handling and processing
of food
Aflatoxins are affected by some ordinary food processing
procedures. In the roasting of groundnuts, approximately 50% of the
aflatoxins are altered to such an extent that they can no longer be
detected (Lee et al., 1969b; Waltking, 1971). The chemical nature of
the alteration products has not been fully elucidated.
The usual methods of processing groundnuts to make peanut butter
and some nuts for confections may appreciably reduce aflatoxin
contamination. The removal of undersized nuts (shrivels and pegs);
the removal of nuts that resist splitting and blanching; and the
removal of discoloured nuts by hand or electronic sorting are
effective means of reducing contamination (Rodricks et al., 1977).
In the removal of oil from oilseeds, most of the aflatoxins are
found in the oilseed meal. Small amounts remaining in the crude
vegetable oil are mainly taken out in the soap stock, the byproduct
from the alkali refining step. The remaining traces of aflatoxins
are removed in the bleaching refining steps to give aflatoxin-free
refined oil (Parker & Melnick, 1966).
The normal alkali processing of maize to produce tortilla-type
foods, a common practice in some areas of the world including some
Latin American countries, effectively reduces the levels of
aflatoxins in contaminated maize (Ulloa-Sosa & Schroeder, 1969).
Although the mechanism of this reduction has not been clarified,
some of the aflatoxin is most likely washed out by the initial
soaking of the maize in lye water and some is undoubtedly chemically
changed by the alkali; although this process is reported to produce
a substantial reduction in aflatoxin contamination, it is not enough
to give a safe product, when highly contaminated maize is being
processed.
Jemmali & Lafont (1972) reported only partial destruction of
aflatoxins during bread making, indicating the importance of the
contamination of wheat with A. flavus.
The above treatments are normal steps in the processing of
particular foods. In addition to such steps, procedures have been
developed specifically for the destruction or removal of aflatoxins
from grains, nuts, oilseeds, and oilseed meals (cake) (FAO, 1977).
Treatments with ammonia or hydrogen peroxide (H2O2) have been
the most effective procedures developed to date for the
detoxification of foodstuffs and animal feeds (Goldblatt & Dollear,
1977). The treatments with ammonia, developed for the industrial
decontamination of aflatoxin-contaminated groundnut and cottonseed
meal (cake) and maize, are limited to the production of animal
feeds. These procedures have recently been discussed in detail at
the joint FAO/WHO/ UNEP conference on mycotoxins in Nairobi (FAO,
1977). The process of treating groundnut protein isolate with
hydrogen peroxide to obtain a product suitable for use as a human
food supplement was also discussed. This process has been developed
in India and is reported to be operating on a small commercial
scale.
The feasibility of methods combining the physical separation of
contaminated portions of produce (for detailed descriptions of
segregation techniques see, for example, Rodricks et al., 1977) with
chemical decontamination, was considered at the same conference
(FAO, 1977).
3.2.4 Pathways and levels of exposure
From the previous discussion, it can be seen that a range of
commodities may become contaminated with trace amounts of
aflatoxins. In vegetable foods, this contamination results directly
from fungal spoilage, maize and nuts being particularly susceptible.
On the other hand, milk, and possibly meat and eggs can become
indirectly contaminated through the absorption by farm animals of
aflatoxins from contaminated feed resulting in residues of the
parent toxin or its metabolites in body fluids or tissues.
Thus, the level of man's exposure to dietary aflatoxins depends
upon the food available and on eating habits and will vary from
country to country according to the local conditions, including the
traditions of different ethnic groups, and amongst individuals.
Where contaminated groundnuts or maize make a significant
contribution to the diet, the level of exposure will be relatively
higher than where less commonly contaminated commodities take their
place as the staple food or when milk is the sole
aflatoxin-containing constituent of the diet.
In this connexion, the Task Group felt it was important to
identify the infant as being potentially at risk because: (a) baby
food products may be made from dried milk or even maize, commodities
known to be prone to contamination by aflatoxins; and (b) in terms
of larger amounts of food consumed per kg body weight, any level of
aflatoxin contamination is more significant for the child than for
the adult.
Attempts to quantify dietary exposure to aflatoxins are
discussed in detail in section 3.6.1.1.
Occupational exposure (section 3.5.2) to aflatoxins with its
attendant high risks, concerns two groups of individuals: those who
handle grain, animal feedstuffs, groundnuts, groundnut meal etc.
(where exposure could occur largely through inhalation of
contaminated dust), and those who work with toxins in experiments or
with pure toxins as analytical standards.
In one paper (van Nieuwenhuize et al., 1973) available to the
Task Group, an attempt was made to quantify occupational exposure to
airborne aflatoxins in an oil-mill crushing groundnuts and other
oil-seeds. Based on airborne dust determinations (mean aflatoxin
concentrations of 250 and 410 µg/kg airborne dust), the estimated
airborne aflatoxin levels ranged from 0.87 to 72 ng/m3 of air.
3.3 Metabolism
3.3.1 Absorption
Although quantitative data on absorption are not available at
present, there is no doubt that most of the field cases of
aflatoxin-induced diseases in animals and man have been associated
with ingestion of aflatoxin-contaminated foodstuffs and thus with
the absorption of aflatoxins in the alimentary tract. In spite of
reports of respiratory exposure (sections 3.2.4 and 3.5.2), there is
no quantitative information available on aflatoxin resorption from
the respiratory tract or on percutaneous absorption.
3.3.2 Tissue distribution
3.3.2.1 Animal studies
Experiments with 14C-ring-labelled aflatoxin B1 have shown
that rats retain about 20% of the 14C activity 24 h after a single
intraperitoneal dose of 0.07 mg/kg body weight (Wogan et al., 1967).
The highest concentration was found in the liver, which contained
amounts of radioactivity equivalent to the entire remainder of the
carcass (about 5%-8% of the total 14C recovered).
When poultry were fed rations containing aflatoxins at
concentrations ranging from 25 to 15 000 µg/kg for 8 weeks, residues
of aflatoxin B1 were found in the liver and in muscle tissue
(Mintzlaff et al., 1974). The liver contained the highest
concentration, with a mean value of 15 µg/kg at the highest exposure
level. Similarly the highest concentration of aflatoxin B1 was
found in the livers of pigs (range: trace-137 µg/kg) fed rations
containing aflatoxins (both aflatoxin B1 and B2) at levels of
300 and 500 µg/kg for 4 months (Krogh et al., 1973a). Aflatoxin
residues were also detected in kidneys, and muscle and adipose
tissue.
3.3.2.2 Studies in man
Levels of aflatoxins in the tissues of children with Reye's
syndrome are discussed in section 3.6.2.2. In a liver biopsy from a
subject with carcinoma of the rectum and liver in the USA, Phillips
et al. (1976) found 520 µg/kg of aflatoxin B1. In France, Richir
et al. (1976) found aflatoxin B1 in liver biopsies in 6 out of 100
subjects suffering from various diseases. Concentrations observed
ranged from 1.6-8 µg/kg.
3.3.3 Metabolic transformation and activation
With one exception, all primary biotransformations of aflatoxin
B1 involve its conversion to hydroxylated metabolites but only one
such derivative, aflatoxin M1 has appreciable oral toxicity
(Holzapfel et al., 1966). Even so, this metabolite may be detoxified
by conjugation with taurocholic and glucuronic acids prior to
excretion in the bile or urine (Bassir & Osiyemi, 1967). In this
respect, two recently discovered metabolites, P1 (Dalezios et al.,
1971; Buchi et al., 1973) and Q1 (Masri et al., 1974a,b) are
similar in that they also undergo this type of detoxification
(Dalezios et al., 1971; Dalezios & Wogan, 1972).
The conversion in the liver (Fig. 2) of aflatoxin B1 to
aflatoxicol (Patterson & Roberts, 1971) and to aflatoxicol H1 via
aflatoxin Q1 (Salhab & Hsieh, 1975) is unusual in that, unlike
other biotransformations that are catalysed by liver microsomal
enzymes, a cytoplasmic NADH-dependent dehydrogenase is involved.
Furthermore, the formation of aflatoxicol can be inhibited by
17-ketosteroid sex hormones (Patterson & Roberts, 1972a) and this is
the only metabolic transformation of aflatoxin in vitro known to
be sensitive to hormones.
Liver homogenates of certain avian and rodent species are
particularly active in converting aflatoxins B1 and G1 to their
2-hydroxy, 2,3-dihydro derivatives or hemiacetals called also
aflatoxins B2a and G2a (Patterson & Roberts, 1970). These
metabolites bind strongly to protein and are probably sufficiently
reactive, when formed in vivo, to cause many of the acute effects
of aflatoxin poisoning (Patterson & Roberts, 1972b; Patterson, 1973,
1977).
At present, there is only indirect evidence for the formation of
the epoxides of aflatoxins B1 and G1 but this is probably the
more important form of metabolic activation. When either of the
parent toxins is incubated with microsomes prepared from the livers
of many animal species including man, a metabolite is formed which
appears to have only a transient existence, is highly reactive,
binds covalently to DNA, and induces mutation in a bacterial
in vitro test system (Garner et al., 1971, 1972; Ames et al.,
1973). The metabolite of B1 has not been isolated but the
2,3-dihydrodiol has been recovered following mild acid hydrolysis of
an adduct formed when the microsomal metabolite was generated in the
presence of added DNA or RNA (Swenson et al., 1,974) and, more
recently, after in vivo intraperitoneal injection of aflatoxin
B1 (Swenson et al., 1977). This has been assumed to be indirect
evidence of the formation of the 2,3-epoxide and, in view of the
interaction with DNA, it is now generally accepted that the epoxide
of aflatoxin B1 is the bacterial mutagen and the proximal
carcinogen.
 Certain of these biotransformations are better developed in some
animal species than others (Patterson, 1977) and attempts have been
made to correlate liver metabolism of aflatoxins with toxicity. In
the first such attempt (Patterson, 1973), it was proposed that rapid
in vitro formation of aflatoxin hemiacetal was correlated with
susceptibility to acute aflatoxin poisoning. More recently (Hsieh et
al., 1977), it has been suggested that the reversible formation of
aflatoxicol, which is thought to provide a "metabolic reservoir" of
aflatoxin (Patterson & Roberts, 1972b), is correlated with
susceptibility to liver tumour induction. On the basis of this, it
has been tentatively suggested (Hsieh, 1977; Salhab & Edwards, 1977)
that the human liver might be relatively more resistant to aflatoxin
carcinogenesis than that of some other species, particularly the
rat.
3.3.4 Excretion
3.3.4.1 Animal studies
Excretory pathways. Using aflatoxin B1, ring-labelled or
methoxy-labelled with 14C, Wogan et al. (1967) have shown that
rats excrete 7096-80% of a single intraperitoneal dose within 24 h.
A major excretory route of the ring-labelled toxin was through
biliary excretion into the faeces, accounting for about 60% of the
administered dose; approximately 20% of administered radioactivity
was excreted in the urine, and only negligible amounts in expired
air in the form of 14CO2. In contrast, approximately 25% of
radioactivity from methoxy-labelled material appeared in expired air
as 14CO2 with a concomitant decrease in the faeces, indicating
that O-demethylation is a significant metabolic pathway for
aflatoxin B1 in the rat.
Excretion in the milk of farm animals. Several reviews deal
with the excretion of aflatoxins in the milk of farm animals
(Allcroft, 1969; Kiermeier, 1973, 1977; Patterson, 1977; Rodricks &
Stoloff, 1977). When cattle (Allcroft et al., 1968), sheep (Nabney
et al., 1967) or goats (Vesely, et al., 1978) are given feed
contaminated with aflatoxin B1 their milk contains aflatoxin M1.
In the cow, there is a linear relationship between the amount of
aflatoxin B1 ingested daily and the level of aflatoxin M1 in the
milk (Allcroft & Roberts, 1968; Purchase, 1972; Patterson, 1977; see
Fig. 3), indicating that about 1.5% of aflatoxin B1 is excreted as
the metabolite M1 (Kiermeier, 1973), and that the concentration of
aflatoxin B1 in milk is approximately 1/300 of the concentration
of aflatoxin B1 in the dairy ration (Rodricks & Stoloff, 1977).
Smaller quantities of unmetabolized aflatoxin B1 have been found
in cow's and sheep's milk (Nabney et al., 1967; Allcroft et al.,
1968; Wogan, 1969).
Certain of these biotransformations are better developed in some
animal species than others (Patterson, 1977) and attempts have been
made to correlate liver metabolism of aflatoxins with toxicity. In
the first such attempt (Patterson, 1973), it was proposed that rapid
in vitro formation of aflatoxin hemiacetal was correlated with
susceptibility to acute aflatoxin poisoning. More recently (Hsieh et
al., 1977), it has been suggested that the reversible formation of
aflatoxicol, which is thought to provide a "metabolic reservoir" of
aflatoxin (Patterson & Roberts, 1972b), is correlated with
susceptibility to liver tumour induction. On the basis of this, it
has been tentatively suggested (Hsieh, 1977; Salhab & Edwards, 1977)
that the human liver might be relatively more resistant to aflatoxin
carcinogenesis than that of some other species, particularly the
rat.
3.3.4 Excretion
3.3.4.1 Animal studies
Excretory pathways. Using aflatoxin B1, ring-labelled or
methoxy-labelled with 14C, Wogan et al. (1967) have shown that
rats excrete 7096-80% of a single intraperitoneal dose within 24 h.
A major excretory route of the ring-labelled toxin was through
biliary excretion into the faeces, accounting for about 60% of the
administered dose; approximately 20% of administered radioactivity
was excreted in the urine, and only negligible amounts in expired
air in the form of 14CO2. In contrast, approximately 25% of
radioactivity from methoxy-labelled material appeared in expired air
as 14CO2 with a concomitant decrease in the faeces, indicating
that O-demethylation is a significant metabolic pathway for
aflatoxin B1 in the rat.
Excretion in the milk of farm animals. Several reviews deal
with the excretion of aflatoxins in the milk of farm animals
(Allcroft, 1969; Kiermeier, 1973, 1977; Patterson, 1977; Rodricks &
Stoloff, 1977). When cattle (Allcroft et al., 1968), sheep (Nabney
et al., 1967) or goats (Vesely, et al., 1978) are given feed
contaminated with aflatoxin B1 their milk contains aflatoxin M1.
In the cow, there is a linear relationship between the amount of
aflatoxin B1 ingested daily and the level of aflatoxin M1 in the
milk (Allcroft & Roberts, 1968; Purchase, 1972; Patterson, 1977; see
Fig. 3), indicating that about 1.5% of aflatoxin B1 is excreted as
the metabolite M1 (Kiermeier, 1973), and that the concentration of
aflatoxin B1 in milk is approximately 1/300 of the concentration
of aflatoxin B1 in the dairy ration (Rodricks & Stoloff, 1977).
Smaller quantities of unmetabolized aflatoxin B1 have been found
in cow's and sheep's milk (Nabney et al., 1967; Allcroft et al.,
1968; Wogan, 1969).
 3.3.4.2 Studies in man
In the Philippines, aflatoxin M1 has been found (not
measured)in the urine of human subjects known to have ingested
aflatoxin-contaminated peanut butter (Campbell et al., 1970).
Claims concerning an aflatoxin involvement in the etiology of
juvenile cirrhosis in India (section 3.6.3.2) based on a
blue-fluorescent B1 spot in the breast milk of mothers and the
urine of children with the disease (Robinson, 1967) are largely
discounted by the later studies of Yadgiri et al. (1970) who
produced spectrophotometric evidence that, although such a spot
could be identified in the urine of children with the overt disease,
this was not aflatoxin B1 For other reports see section 3.5.1.
3.4 Effects in Animals
The effects of aflatoxins in animals have., been reviewed by
Allcroft (1969), Newberne & Butler (1969) and Butler (1974).
3.4.1 Field observations
When foodstuffs are affected by microbial deterioration, man
normally eats the less affected parts, whereas domestic animals may
be exposed to more contaminated rations. This explains why the
discovery of several mycotoxins has been based on field observations
in domestic animals.
The first observation of a disease in animals subsequently
associated with aflatoxins was an acute outbreak of a lethal disease
in turkey poults in England in 1960 causing an estimated loss of at
least 100 000 birds. Extensive research eventually revealed that the
disease was caused by aflatoxins contained in a batch of Brazilian
groundnut meal. The concentration of aflatoxin B1 in the original
groundnut meal was later estimated to be about 10 mg/kg. The disease
was characterized by rapid deterioration in the condition of the
birds, subcutaneous haemorrhages, and death. At postmortem, the
livers of the birds were pale, fatty, and showed extensive necrosis
and biliary proliferation (Butler, 1974). A similar case of acute
disease was observed in day-old ducklings fed "toxic" groundnut meal
(Asplin & Carnaghan, 1961), where the liver changes described were
followed by cirrhosis. Outbreaks of liver disease in chickens have
also been associated with aflatoxin-contaminated feed (Asplin &
Carnaghan, 1961).
Loosmore & Harding (1961) noted outbreaks in pigs fed groundnut
meal in which the toxic factor was later identified as aflatoxins.
The lesions in the pigs included haemorrhages, and liver damage
characterized by dissecting fibrosis and biliary proliferation.
Calves fed rations containing 15% toxic groundnut meal also
developed liver lesions characterized by fibrosis and biliary
proliferation (Loosmore & Markson, 1961). Outbreaks associated with
"toxic" groundnut meal and characterized by similar liver lesions
have been reported in older cattle even though they are more
resistant (Clegg & Bryston, 1962); there was also a drop in milk
production.
A liver disease "hepatitis X" has been reported in dogs in
southeastern USA (Seibold & Bailey, 1952; Newberne et al., 1955).
Icterus and in some cases ascites were observed and the liver
lesions included fatty changes with centrilobular parenchymal
necrosis and biliary proliferation. Commercial dog food thought to
be the cause of toxicity was later found to contain aflatoxin B1
(up to 1.75 mg/kg) (Newberne et al., 1966a). Similar lesions have
been reproduced in dogs by peroral administration of aflatoxins, and
it has been suggested that the "hepatitis X" in dogs could be
causally associated with aflatoxins in the diet (Newberne et al.,
1966a). During an outbreak of toxic hepatitis affecting several
hundred people in north-west India and considered to be possibly
associated with the consumption of maize heavily contaminated by
aflatoxins (section 3.5.2 and 3.6.2.1), dogs fed food remnants from
households in affected villages manifested a disease characterized
by jaundice, ascites, and frequently death (Krisnamachari et al.,
1975a, b; Tandon et al., 1977). A nonportal type of micronodular
cirrhosis, with less conspicuous parenchymal and cholangiolar
changes was found on histological examination of the livers of two
dogs (Tandon et al., 1977).
3.4.2 Experimental studies
3.4.2.1 Acute and chronic effects: hepatotoxicity
Different species vary in their susceptibility to acute
poisoning by aflatoxins, with LD50 values ranging from 0.3 to
17.9 mg/kg body weight (Table 4). In all the animals studied, the
liver was the principal target organ (see for example Butler, 1974).
Table 4. Acute toxicity of aflatoxin B1a
Species LD50 Zone of
(mg/kg body weight) liver lesion
chick embryo 0.025d
rabbit 0.3 midzonal
duckling 0.335 periportal
cat 0.55 periportal
pig 0.62 centrilobular
dog 0.5-1.0 centrilobular
sheep 1.0 centrilobular
guineapig 1.4 centrilobular
baboonb 2.0 centrilobular
rat (male) 7.2 periportal
macaque femalec 7.8 centrilobular
mouse 9.0
hamster 10.2
rat (female) 17.9 periportal
a Adapted from: Newberne & Butler (1969) and Butler (1974).
b From: Peers & Linsell (1976).
c From: Shank et al. (1971 b).
d µg/embryo.
The lesions observed in field cases (section 3.4.1) in poultry,
pigs, cattle, and dogs have all been reproduced in the same animal
species by feeding experiments during periods of time ranging from a
few weeks to a few months, using diets containing aflatoxins, or
pure aflatoxins ranging from 0.3 to several mg/kg (Newberne &
Butler, 1969).
In the study by Carnaghan et al. (1966), chickens were fed a
diet containing aflatoxin B1 at a level of 1.5 mg/kg. Groups of 3
control and 3 test chicks were killed after 3´ days, 7 days, and
then at weekly intervals for 8 weeks. After 4 weeks, the liver
lesions included fatty change, biliary proliferation, and fibrosis.
In 20 pigs, aflatoxins (aflatoxins B1 and B2) at a feed
level as low as 300 µg/kg resulted in the development of
centrilobular necrosis and fibrosis of the liver as well as growth
depression, during a normal feeding period of 3-4 months (Krogh et
al., 1973a). In cattle, the liver lesions (centrilobular
degeneration, fibrosis, biliary proliferation) occurred in all 4
animals after 4 months on a feed containing an aflatoxin level of
2 mg/kg (Allcroft & Lewis, 1963). The hepatic lesions induced in the
duckling by the aflatoxins formed the basis of a bioassay originally
described by Sargeant et al. (1961). At sublethal doses of
aflatoxins, the bioassay depends upon an assessment of the degree of
biliary proliferation. Liver lesions similar to those observed in
farm animals have been experimentally induced by the administration
of aflatoxins in a number of laboratory animals, including the rat,
cat, guinea-pig, and rabbit (Newberne & Butler, 1969).
In a study of Madhavan et al. (1965b), 2 rhesus monkeys
(Macaca mulatta) were given daily oral doses of aflatoxins at
500 µg/animal for 18 days (corresponding approximately to 250 µg/kg
body weight per day) and then 1 mg/animal per day (corresponding
approximately to 500 µg/kg body weight per day) until death occurred
after 32 and 34 days. Three rhesus monkeys were given 1 mg each,
daily, until death occurred after 19, 20, and 27 days respectively.
The liver lesions included fatty infiltration, biliary
proliferation, and portal fibrosis. Death or similar lesions were
not observed in the 2 control monkeys.
Deo et al. (1970) studied the effect on male rhesus monkeys of
repeated administration by gastric tube of 3 different levels of
aflatoxins (B1 + G1). At the highest dose level (1 mg/kg body
weight daily for 3 weeks), 35/35 animals died within 22 days with
extensive haemorrhagic necrosis of the liver. A dose level of
0.25 mg/kg body weight, twice a week, for 5 months induced various
degrees of liver changes in 24/24 animals characterized by biliary
proliferation and focal appearance of the liver cells with multiple
nuclei and giant-sized liver cells with enlarged hyperchromatic
nuclei. At the lowest dose, 5 animals were given 62 µg/kg body
weight once a week for periods ranging from a few days to 2 years.
Liver changes were similar to the changes seen in the second group
but in a milder form.
Cynomolgus monkeys (Macaca fascicularis = M. irus) fed a
dietary level of aflatoxin B1 of 5 mg/kg rapidly developed liver
damage with biliary proliferation and all 6 animals died within 2
months. When fed aflatoxin B1 at a dietary level of 1.8 mg/kg, 5
animals died within 3 months showing liver damage characterized by
centrilobular necrosis, biliary proliferation, and fibrosis. Two
animals survived and were killed after 3 years; the liver of one
animal had the appearance of nodular cirrhosis. Two groups of 4
animals each were fed lower levels of aflatoxin B1 (0.07 and
0.36 mg/kg, respectively) for 3 years without showing any signs of
liver lesions (Cuthbertson et al., 1967).
The relationship between the chemical structure of different
aflatoxins and their biological activity (discussed also in section
3.4.2.3) was investigated in a small number of experiments; more
extensive studies were not possible because of the limited
quantifies available of some of the pure aflatoxins. Carnaghan et
al. (1963) compared 6-day mortality following single doses of
different aflatoxins, administered by intubation to one-day-old
Khaki Cambell ducklings, and concluded that both aflatoxins B2 and
G2 were less toxic than aflatoxins B1 and G1, the ratio of
LD50 values being 1:4.7 for B1:B2 and 1:4.4 for G1:G2.
Aflatoxins G1 and G2 were less toxic than the corresponding
aflatoxins B1 and B2, the ratio of the LD50 values being
1:2.15 for B1:G1 and 1:2.03 for B2:G2. The corresponding
LD50 values for aflatoxins B1, B2, G1, and G2 were 0.36,
1.70, 0.78, and 3.45 mg/kg, respectively. Comparable results were
obtained by Wogan et al. (1971) who recorded 14 day mortality after
intubation of male Pekin ducklings and reported LD50 values of
0.73, 1.76, 1.18 and 2.83 mg/kg for aflatoxins B1. B2, G1, and
G2, respectively. In the same paper, a study was reported on the
14-day mortality of male Fischer rats after a single
(intra-peritoneal) dose of aflatoxin. The LD50 value for aflatoxin
B1 was 1.16 mg/kg body weight (95% confidence interval 0.91 to
1.48 mg/kg) whereas the LD50 for aflatoxin G1 was between 1.5
and 2.0 mg/kg body weight. On the other hand, no deaths occurred in
20 rats given 12-200 mg of aflatoxin B2 per kg body weight, and
all 4 rats given 170-200 mg of aflatoxin G2 per kg body weight
survived. A similar difference in the toxicity of aflatoxins was
observed when male Fischer rats were given repeated doses of
aflatoxins by stomach tube over a 4-week period. The 4-week
mortality in rats given a total dose of 1 mg of aflatoxin B1 per
rat was 8/10 whereas all 10 rats given the same dose of aflatoxin
G1 survived and only 4/10 animals given double this dose of
aflatoxin G1 died. In another trial, all 11 rats survived
intragastric administration of 3.75 mg of aflatoxin B2 per rat
repeated every second day for 4 weeks to give a total dose of
52.5 mg per rat.
Holzapfel et al. (1966) and Purchase (1967) reported 7-day
mortality alter oral dosing of one-day-old Pekin ducklings with
aflatoxins B1, M1, and M2. Five groups of 2-3 ducklings (body
weight 40-50 g) were used for each of the aflatoxins tested, and the
following LD50s were calculated (with 95% confidence limits given
in brackets): aflatoxin B1, 12 (3.9-37.2) µg per duckling,
aflatoxin M1, 16 (5.4-51.5) µg per duckling, and aflatoxin M2,
61.4 (37-100) µg per duckling. Ducklings receiving aflatoxin M2
showed characteristic liver lesions indistinguishable from those
observed after a similar dose of aflatoxin B1. Higher doses of
aflatoxin M2 produced similar effects (Purchase, 1967). A study
comparing the acute toxicity of synthetic (racetalc) aflatoxins B1
and M1 and the natural optical isomer of aflatoxin B1 was
reported by Pong & Wogan (1971), suggesting that only one isomer of
each synthetized racemic mixture was biologically active.
Fourteen-day mortality rates observed after a single intraperitoneal
dose of 1.5 mg/kg body weight of synthetic aflatoxin B1 and
synthetic aflatoxin M1 were 1/1 and 1/2, respectively. However, no
deaths occurred in groups of rats (each consisting of 4 animals)
given these synthetic aflatoxins at doses of 1, 0.8, 0.6, or
0.4 mg/kg body weight. With the natural aflatoxin B1, the observed
mortalities at these dose levels were 4/4, 2/4, 2/4, and 0/4
respectively.
For information on the toxicity of certain other aflatoxin
metabolites or derivatives, see Wogan et al. (1971) and Patterson
(1976).
3.4.2.2 Hepatotoxicity connected with extrahepatic effects
Many other organs besides the liver are more or less severely
affected in acute experiments with high doses of aflatoxins (Butler,
1964): in male and female rats, a single dose of aflatoxin B1
proved lethal in half of the animals (7.2 mg/kg body weight in the
male and 17.9 mg/kg body weight in the female, by garage). Frequent
bilateral adrenal haemorrhages, petechial haemorrhages in many
organs, particularly in the congested lungs, and occasionally patchy
necroses in the myocardium and in other organs (kidney, spleen) were
observed during the first few days following administration. These
changes were not detected in male or female rats given aflatoxin B1
at 3.5 mg/kg body weight. With higher doses, the haemorrhages seen
in the lungs, kidneys, and adrenals were more extensive. Animals
dying within the first few days often had altered blood in the whole
of the small intestine and in the colon. Ascites and oedema of the
omentum were observed in some of the animals a week or more (but not
one month) after aflatoxin administration. After a month, with the
exception of the liver damage, all the other organs appeared normal
in surviving animals. Histologically, certain renal changes were
detected in the loops of Henle at this stage, consisting of a few
cells with large irregular hyperchromatic nuclei, very similar to
those seen in the liver (Butler, 1964).
Congested lungs with small petechial haemorrhages, haemorrhagic
necroses in the adrenals (localized in the inner zone of the
reticularis) and patchy necroses in the kidneys, pancreas, and
spleen were observed in guineapigs 2-3 days after a single
intraperitoneal injection of aflatoxin B1 at 1.4 mg/kg body weight
(lethal in half of the males and females). Even at this dose, the
small intestine was frequently filled with altered blood. At higher
doses, the haemorrhagic disease was more marked, with pleural,
pericardial, and peritoneal haemorrhages. The only change seen in
the heart of the guineapig, 2-3 days after aflatoxin administration,
was an occasional small area of fatty degeneration of the
myocardium. Many animals showed marked ascites and oedema of the
omentum and subcutaneous tissue during the first week after
injection (Butler, 1966).
Bourgeois et al. (1971) reported a special syndrome induced by
oral administration of aflatoxin B1 in the macaque (Macaca
fascicularis). In 2 groups of 4 young females, each receiving a
single oral dose of aflatoxin B1 at 13.5 or 40.5 mg/kg body
weight, all animals died within 149 h. Death occurred in 1 out of 4
other animals receiving a dose of 4.5 mg/kg body weight. Doses of
toxin of 1.5 mg/kg or 0.5 mg/kg (4 animals in each group) did not
result in death or unusual clinical signs. Cough, vomiting,
diarrhoea, and coma were characteristic clinical findings in animals
exposed to toxic doses. Analysis of blood serum revealed a
dose-dependent decrease in serum levels of phospholipids within 24 h
of administration of the aflatoxin. A dose-dependent decrease in
serum levels of glucose and an increase in nonesterified fatty acids
occurred within 72 h of aflatoxin administration. The liver lesions
included centrilobular necrosis, some biliary proliferation, and
massive fatty degeneration which was also observed in the heart and
kidneys. Cerebral oedema with neuronal degeneration was seen. Some
of these findings resemble those associated with Reye's syndrome in
children (see section 3.5.1.2).
3.4.2.3 Carcinogenesis
The carcinogenesis of aflatoxins has been reviewed by Wogan
(1973, 1977) and re-evaluated by IARC (1976).
Hepatic and renal tumours. Orally administered aflatoxins,
mainly B1, have been hepatocarcinogenic in all species of test
animals studied so far (including nonhuman primates), with the
exception of the mouse, in which carcinogenic effects have been
demonstrated only following intraperitoneal administration of
aflatoxin B1 to neonates (Tables 5 and 6). These studies were
concerned with repeated or long-term exposure to aflatoxins. In a
study by Carnaghan (1967), 2 groups consisting of 16 and 18 weanling
female Wistar rats, respectively, were given single oral doses of
crystalline aflatoxin B1 or a mixture of aflatoxins containing
about 40% aflatoxin B1 and 60% aflatoxin G1, at the rate of
0.5 mg/rat in 0.1 ml dimethylformamide. These doses corresponded to
averages of 7.65 mg aflatoxin B1/kg body weight and 2.7 mg
aflatoxin B1 plus 4.0 mg aflatoxin G1/kg body weight,
respectively. Within 21-32 months, 7 rats out of each group
developed hepatic tumours with metastases in half the cases. Hepatic
rumours were not observed in 19 control rats given the solvent only.
No hepatocellular carcinomas were found in 22 male Fischer rats
killed successively 16 weeks (3 rats), 25 weeks (5 rats), 38 weeks
(5 rats), 55 weeks (4 rats), and 69 weeks (5 rats) after a single
dose of aflatoxin B1 at 5.0 mg/kg body weight, administered by
garage (Wogan & Newberne, 1967).
A linear dose-response relationship was observed by Wogan et al.
(1974) for the development of liver-cell carcinomas in male Fischer
rats fed dietary concentrations of aflatoxin B1 ranging from
1-100 µg/kg (Table 7). At 1 µg/kg, a 10% tumour incidence was found,
compared with no rumours in the control group and at 100 µg/kg the
tumour incidence was 100%. A linear log (dose)-response relationship
has been demonstrated in trout fed dietary levels of aflatoxin B1
ranging from 0.5 to 20.0 µg/kg, for 20 months. Extrapolating this
relationship to lower exposure levels, an incidence of approximately
10% would be expected with a dietary concentration of 0.1 µg/kg.
Table 5. Hepatocarcinogenicity of aflatoxin B1 in rodentsa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
rat, Fischer 1.0 mg/kg diet 33 weeks 52 weeks 3/6 Svoboda et al. (1966)
rat, Fischer 1.0 mg/kg diet 41-64 weeks 41-64 weeks 18/21 Wogan & Newberne (1967)
rat, Porton 1.0 mg/kg diet 20 weeks 90 weeks 19/30 Butler (1969)
rat, Wistar 1.0 mg/kg diet 21 weeks 87 weeks 12/14 Epstein et al. (1969)
mouse, Swiss 150 mg/kg dietb 80 weeks 80 weeks 0/60 Wogan (1973)
mouse, C57Bl/6NB 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, C3HfB/HEN 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, hybrid F1, 4 days old 6.0 µg/g body weight 3 doses (i.p.) 80 weeks 16/16 Vesselinovitch et al. (1972)
a From: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
Table 6. Hepatocarcinogenicity of aflatoxin B1 in nonrodent speciesa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
monkey, rhesus (M) 1.655 g totalb 5.5 years 8.0 years 1/1 Gopalan et al. (1972)
monkey, rhesus (F) 1.855 g total 5.5 years 10.75 years 1/1 Tilak (1975)
monkey, rhesus (F) 0.504 g total 6.0 years 8.0 years 1/1 Adamson et al. (1973)
marmoset 3.0 mg total 50-55 weeks 50-55 weeks 1/3 Lin et al. (1974)
5.04-5.84 mg totalc 87-94 weeks 87-94 weeks 2/3 Lin et al, (1974)
tree shrew (M & F) 24-66 mg total 74-172 weeks 74-172 weeks 9/12 Reddy et al, (1976)
ferret 0.3-2.0 µg/kg 28-37 months 28-37 months 7/9 Butler (1969)
duck 30 µg/kg 14 months 14 months 8/11 Carnaghan (1965)
rainbow trout 4 µg/kg in diet 12 months 12 months 15% Sinnhuber et al, (1968b)
8 µg/kg in diet 12 months 12 months 40% Sinnhuber et al. (1968b)
rainbow trout embryos 0.5 mg/kg in water 1 h 296-321 days 38% Sinnhuber & Wales (1974)
salmon 12µg/kg in dietd 20 months 20 months 50% Wales & Sinnhuber (1972)
guppy 6 mg/kg in diet 11 months 11 months 7/11 Sato et al. (1973)
a Modified from: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
c These animals were infected simultaneously with hepatitis virus.
d This diet also contained 50 mg/kg cyclopropenoid fatty acids.
Table 7. Dose-response characteristics of aflatoxin B1 carcinogenesis
in male Fischer strain ratsa
Dietary Duration Liver Time of appearance
aflatoxin of feeding carcinoma of earliest tumour
level (week) incidenceb (week)
(µg/kg)
0 74--109 0/18c --
1 78--105 2/22 104
5 65--93 1/22 93
15 69--96 4/21 96
50 71--97 20/25d 82
100 54--88 28/28e 54
a From: Wogan et al. (1974).
b In animals at risk (surviving longer than 50 weeks).
c Animals surviving for maximum period.
d Two animals had pulmonary metastases.
e Four animals had pulmonary metastases.
In a recent study (FDA, 1978), an attempt was made to calculate
the lifetime liver cancer risk in rats, which could be connected
with aflatoxin feed levels lower than those directly tested in
animal experiments. Estimates of life-time liver cancer incidence
rates corresponding to aflatoxin feed levels of 0.1 and 0.3 µg/kg
were derived and compared for several selected rat studies using the
mathematical procedure developed by Mantel & Bryan (1961) and
modified by Mantel et al. (1975). A more detailed description of
this procedure can be found in other publications including WHO
(1978) and Hoel et al. (1975). Thus, the estimated life-time liver
cancer risk derived from the experimental results reported by Wogan
et al. (1974) (see Table 7) corresponded to life-time liver cancer
incidence rates of 70 (600) per 105 rats for an aflatoxin dietary
level of 0.1 µg/kg and 360 (2300) for a level of 0.3 µg/kg. (Numbers
in parentheses; are upper 99% confidence limits.) Estimates derived
from different rat studies varied considerably. The lifetime
incidence rates calculated for the combined studies were 240 (470)
and 1100 (1900) per 105 rats for aflatoxin feed levels of 0.1 and
0.3 µg/kg, respectively (FDA, 1978).
The studies in primates are included in Table 6. No attempts
have been made to establish dose-response relationships in primates,
but they are susceptible to aflatoxin hepatocarcinogenesis.
The carcinogenic effects of different purified aflatoxins have
been compared in a limited number of studies. The results of a study
by Butler et al. (1969) in which 8-9 week old, male (M) and female
(F) MRC rats were given aflatoxins B1, B2, or G1 in drinking
water for 10 or 20 weeks, are shown in Table 8. The earliest renal
neoplasm was seen 54 weeks after the discontinuation of aflatoxin
treatment. Renal tumours were detected only in males. The 2 rats
treated with aflatoxin B1 that developed renal tumours did not
have hepatic carcinomas, but 5 of the 11 rats receiving aflatoxin
G1 developed both renal and hepatic carcinomas.
Wogan et al. (1971) studied the relationship between the
chemical structures of aflatoxins and their hepatocarcinogenicity in
male Fischer rats, and concluded that aflatoxin B1 was apparently
more carcinogenic than aflatoxin G1 and that both were much more
active than aflatoxin B2. A total intraperitoneal dose of
aflatoxin B2 of 150 mg per rat given in 40 equal doses over 8
weeks induced hepatocellular carcinomas in 3/9 rats. A similar
regimen, containing a total dose of aflatoxin B1 of 1.3 mg,
induced liver tumours in 9/9 animals. In other experiments to
compare the carcinogenicity of aflatoxins G1 and B1 given by
stomach tube to rats, aflatoxin G1 in a total dose of 1.4 mg per
animal (divided into 14 equal doses over 2.5 weeks) induced
hepatocellular carcinoma in 3/5 rats within 68 weeks. Hepatocellular
carcinomas were observed in all 18 animals given a total dose of
2 mg of aflatoxin G1 (divided in 40 equal doses over 8 weeks) and
killed within 45-64 weeks. Six of these rats had pulmonary
metastases.
Hepatocellular carcinomas were also found in 7/7 rats given
aflatoxin B1 in a total dose of 0.5 mg per animal (divided in 20
equal doses over 4 weeks) and sacrificed within 74 weeks, in 18/18
rats given 1 mg per animal (divided into 40 equal doses over 8
weeks) and killed within 42-58 weeks, and in 17/17 given 1.5 mg per
animal (divided into 40 equal doses over 8 weeks) and killed within
42-46 weeks. With the 2 higher doses, 2 and 7 animals, respectively
had pulmonary metastases. Renal adenocarcinomas were found in 4/26
rats given aflatoxin G1.
Synthetic racemic aflatoxin M1 induced hepatocarcinomas at 100
weeks in 1/29 male Fischer rats given 1 mg of the compound
intragastrically in divided doses over a period of 8 weeks. The
incidence of hepatocarcinomas in 9 rats given the same dose of
natural aflatoxin B1 was 100%, 1 year after treatment (Wogan &
Paglialunga, 1974). Natural aflatoxin M1 was less effective than
natural aflatoxin B1 in inducing tumours in trout, particularly in
the males. Only 14% of male trout, fed natural aflatoxin M1 for a
year at a dietary level of 4 µg/kg, developed liver tumours compared
with 68% of those receiving a similar feed level of aflatoxin B1
Table 8. Carcinogenesis in rats due to ingestion of aflatoxin B1, G1 or B2 in drinking watera
Compound Concentration Daily Duration Total No. and sex No. of animals No. of animals
(µg/ml) dose (µg) weeks dose (mg) of animals with tumours with other
treated neoplasms
liver kidney
aflatoxin B1 1 20 20 2 15 M 8 2 4
15 F 11 0 1
aflatoxin B1 1 20 10 1 10 M 3 0 2
aflatoxin G1 3 60 20 6 11 M 9 6 5
15 F 12 0 3
aflatoxin G1 1 20 20 2 15 M 2 5 2
15 F 1 0 7
aflatoxin G1 1 20 10 1 10 M 1 0 1
aflatoxin B2 1 20 10 1 10 M 0 0 2
controls 0 0 0 0 15 M 0 0 6
15 F 0 0
a From: Butler et al. (1969).
(the experiment was terminated 8 months after the discontinuation of
aflatoxin feeding). In females, the difference was less pronounced,
liver tumours occurring in 48% of aflatoxin B1-treated trout and
78% of aflatoxin B1-treated trout (see Table 9) (Sinnhuber et al.,
1974). Haemorrhages within the cancerous liver resulting in death,
which were observed in most females receiving dietary levels of
aflatoxin M1 of 16-64 µg/kg, were not observed in similarly
treated males.
Table 9. Aflatoxin M1 liver carcinogenesis in rainbow trout
(Salmo gairdneri)a
Aflatoxin in the diet Liver tumour incidence
males females
M1 4 µg/kg 4/28 (14%) 13/27 (48%)
M1 16 µg/kg 22/27 (81%) 11/14 (79%)
M1 32 µg/kg 24/25 (96%) 13/14 (93%)
M1 64 µg/kg 21/24 (88%) 9/10 (90%)
B1 4 µg/kg 15/22 (68%) 18/23 (78%)
a From: Sinnhuber et al. (1974).
A probable effect of rat strain on aflatoxin carcinogenesis was
seen in the high incidence of renal epithelial neoplasms reported in
male Wistar strain rats fed diets containing aflatoxin B1, for 147
days, and then maintained on a basal diet until death (Epstein et
al., 1969). Renal tumours developed in 57% (8/14), 28% (5/18), and
23% (3/13) of male rats exposed to diets containing aflatoxin B1
levels of 1.0, 0.5, and 0.25 mg/kg feed, respectively. The
incidences of malignant hepatomas in corresponding groups were 86%,
72%, and 62% respectively. No renal tumours or malignant hepatomas
were detected in a control group (24 animals). Approximately
one-third of the aflatoxin-exposed rats with renal rumours did not
have hepatomas. The first malignant hepatoma and the first renal
tumour were detected 463 and 468 days after initiation of the
experiment, respectively; both these tumours occurred in the group
with the highest exposure. Approximately half of the renal tumours
were bilateral.
Early hepatic lesions possibly related to carcinogenesis.
Aflatoxin B1 given to rats in repeated doses of 15-25 µg/day, for
3.5 weeks, making a total dose of 375 µg, elicited increased DNA
synthesis and mitosis in clusters of liver cells that could be
distinguished from the surrounding cells by their histological
appearance and biochemical activity (Newberne & Wogan, 1968a; Rogers
& Newberne, 1969). Development of the abnormal foci was more
prominent in rats fed a high-fat, lipotrope-deficient diet, which
enhances aflatoxin carcinogenesis (section 3.4.2.7), than in rats
fed a nutritionally adequate diet. The abnormal foci were already
present in deficient rats at the end of carcinogen administration.
Similar foci are found in the livers of rats exposed to other
hepatic carcinogens and may be useful in studying pathogenesis or
metabolic aspects of liver cancer, since they are thought to be the
possible precursors of tumours. However, many of the foci disappear
with time after treatment, and progression is not inevitable.
Other tumours. Carcinoma of the colon have occasionally been
reported following aflatoxin exposure (Newberne & Butler, 1969).
Increased incidence of tumours in the distal half of the colon was
observed in vitamin A-deficient rats fed aflatoxin B1 (section
3.4.2.7) (Newberne & Rogers, 1973; Newberne & Suphakarn, 1977).
Tumours of the colon were also found in more than 20% (12/53) of
F344 rats (NIH) exposed, either from conception (7/34), or from 6-7
weeks of age (5/19), to a diet containing an aflatoxin B1 level of
2 mg/kg and an unspecified amount of vitamin A (Ward et al., 1975).
The tumours developed in both males and females at 42-64 weeks of
age and were primarily polyploid neoplasms in the ascending colon,
although 2 rats had tumours in the descending colon. No colon
tumours were found in 18 control rats.
Carcinomas of the glandular stomach were observed in 1/6 young
rats given a diet of groundnut meal containing an aflatoxin level of
3-4 mg/kg for 3 weeks and in 1/16 animals given the same diet at the
age of one year (Butler & Barnes, 1966). Two definite and one
probable adenocarcinomas of the stomach had previously been observed
in rats fed a diet prepared from the same batch of groundnut meal
(Butler & Barnes, 1963).
Other extrahepatic tumours have occasionally been reported after
oral aflatoxin exposure, e.g., tumours of the lacrimal glands
(Dickens et al., 1966; Goodall & Butler, 1969; Butler et al., 1969),
squamous cell carcinoma of the tongue (Ward et al., 1975) and
oesophagus (Butler et al., 1969). Tumours at various sites have been
induced in several species of test animals by intratracheal,
subcutaneous, or intraperitoneal administration of aflatoxins. The
experimental results obtained by intratracheal administration are of
particular interest in connexion with reported effects in man
associated with airborne aflatoxins (section 3.5.2). Squamous-cell
carcinoma of the trachea developed within 37-62 weeks in 3/6 rats
given a mixture of aflatoxins (containing B1, B2, G1, and
G2) at 300 µg per animal intra-tracheally twice weekly, for 30
weeks. Four of these rats also developed hepatomas within 49-62
weeks (Dickens et al., 1966).
Prenatal and early postnatal exposure. In a study of these
effects, 6 groups of 10 female, Wistar rats were each fed a diet
containing 25% or 50% groundnut meal contaminated with aflatoxin
B1 at 10 mg/kg and aflatoxin B2 at 0.2 mg/kg. Dams received this
diet from day 10 of pregnancy to parturition (exposure of offspring
in utero); from 1 day post partum to 10 days post partum (exposure
via milk); or from day 10 of pregnancy to 10 days post partum
(exposure in utero and via milk). Among 113 male and 95 female
offspring observed for up to 36 months, 1 male exposed in utero, 1
female exposed via milk, and 2 females exposed in utero
and via milk developed malignant liver tumours (Grice et al., 1973).
No liver tumours were seen in control offspring (50 male and 50
female rats).
3.4.2.4 Teratogenicity
The teratogenicity of aflatoxins has been reviewed by Ong
(1975). The effect of aflatoxin B1 on embryos in hamsters was
studied by Elis & Di Paolo (1967) who reported that a single
intraperitoneal injection of aflatoxin B1 at 4 mg/kg body weight,
given on day 8 of pregnancy, resulted in a high proportion of
malformed and dead or reabsorbed fetuses. Approximately 50% of the
fetuses in aflatoxin-treated mothers and over 85% in control mothers
were normal. A dose of 2 mg/kg did not have any effects. In studies
by Di Paolo et al. (1967), no teratogenic effects were observed when
12 pregnant C3H mice were given repeated dally intraperitoneal
injections of aflatoxin B1 at 4 mg/kg body weight. However, a high
proportion of dead or reabsorbed fetuses was observed in
aflatoxin-treated mice in comparison with controls.
3.4.2.5 Mutagenicity
Aflatoxin B1 causes chromosomal aberrations (chromosomal
fragments with occasional bridges, chromatid bridges, chromatid
breakage) and DNA breakage in plant and animal cells (Ong, 1975). It
has also been shown to cause gene mutations in bacterial test
systems (Ames test), when activated by microsomal preparations from
rat and human liver (Wong & Hsieh, 1976). However, no mutagenic
effects were observed in mice exposed intra-peritoneally to
aflatoxin B1 at 5 mg/kg body weight (Leonard et al., 1975).
3.4.2.6 Biochemical effects and mode of action
Individual aflatoxins (B1, B2, G1, G2 etc.) with
slightly different chemical structures affect experimental animals
(sections 3.4.2.1 and 3.4.2.3) and interact with in vitro test
systems to different degrees (Wogan et al., 1971; Patterson, 1976).
However, as aflatoxin B1 is the commonest and the most potent, it
has been studied extensively and the majority of reported
biochemical effects are specifically related to this toxin. As
discussed in section 3.3.3, the aflatoxin molecule appears to be
metabolically activated before exerting its acute and chronic
effects.
Interaction between these activated molecular species and the
liver cell apparently occurs at several loci. In the nucleus,
DNA-dependent RNA polymerase (EC 2.7.7.6)a is inhibited (Pong &
Wogan, 1970), the toxin binds covalently to DNA in vitro and
in vivo (Clifford & Rees, 1967; Lijinsky et al., 1970; Gamer,
1973, 1975; Swenson et al., 1974, 1977), DNA repair is stimulated
(Seegers & Pitout, 1973; Stich & Laishes, 1975), and the aflatoxin
is activated on the outer nuclear membrane to a form that inhibits
RNA synthesis (Neal & Godoy, 1976).
The permeability of mitochondria increases (Bababunmi & Bassir,
1972; Doherty & Campbell, 1973) and electron transport is
interrupted with a decline in respiration (Doherty & Campbell, 1972,
1973). Lysosomal membranes are also rendered permeable and unbound
acid hydrolases leak out (Tung et al., 1970; Pokrovsky et al., 1972;
Adekunle & Elegbe, 1974). Activation of lysosomal enzymes and their
effects on cellular structures may be a component of the toxic
mechanism of aflatoxins (Pokrovsky et al., 1977).
Aflatoxin is metabolized in the endoplasmic reticulum (section
3.3.3) and the unmetabolized toxin competes with steroid sex
hormones for polysome-binding sites (Williams & Rabin, 1971). The
reticulum degranulates (Theron, 1965; Theron et al., 1965; Butler,
1971, 1972) with the breakdown of polysome profiles (Villa-Trevino &
Leaver, 1968; Pong & Wogan, 1969; Godoy et al., 1976) and the
formation of helical polysome forms (Sarasin & Moule, 1976). RNA
polymerase is inhibited (Pong & Wogan, 1970) and the toxin binds
covalently to RNA (Swenson et al., 1977). Many metabolic functions
are inhibited, including protein synthesis, enzyme induction (Wogan
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
& Friedman, 1968; John & Miller, 1969; Kato et al., 1970) and the
synthesis of blood clotting factors II and VII (Bassir & Bababunmi,
1969). Glucose metabolism via the 6-phosphate pathway (Brown &
Abrams, 1965; Feuer et al., 1965;; Shankaran et al., 1970), and the
synthesis of fatty acids and phospholipids are also depressed
(Clifford & Rees, 1969; Kato et al., 1969; Black et al., 1970;
Donaldson et al., 1972; Lo & Black, 1972). Furthermore, feed-back
control of cholesterol synthesis is lost (Horton et al., 1972), a
change considered characteristic of the pre-cancerous state.
It has been shown that aflatoxins have immunosuppressive
properties, probably related to their inhibitory effect on protein
synthesis. Thus, at levels in poultry feed of 0.25-0.5 mg/kg,
aflatoxins have been found to reduce resistance to infection by
Pasteurella multocida, Salmonella spp., Marek's disease virus,
coccidia, and Candida albicans (Brown & Abrams, 1965; Smith et al.,
1969; Pier & Heddleston, 1970; Hamilton & Harris, 1971; Edds et al.,
1973).
In the liver cytoplasm, there is a transient stimulation
followed by a depression of glycogenolysis and the pentose shunt
pathway of glucose metabolism (Shankaran et al., 1970). Aflatoxins
also compete with further steroid binding sites in the cytoplasm,
notably NADP-linked 17-hydroxy steroid dehydrogenase (EC 1.1.1.148)
(Patterson & Roberts, 1971).
Thus, acute hepatocellular necrosis appears to result from the
interaction of aflatoxins at a number of intercellular sites whereas
the mutagenic (section 3.4.2.5) and carcinogenic (section 3.4.2.3)
properties of aflatoxins probably depend upon metabolic activation
to a DNA alkylating agent presumably the 2,3-epoxide (section
3.3.3).
3.4.2.7 Factors modifying the effects and dose-response
relationships of aflatoxins
Numerous reports have dealt with factors of various types that
modify the carcinogenic and other toxic effects of aflatoxins in
experimental animals. These include host factors, particularly the
sex-linked and endocrine characteristics, and interactions with
other environmental factors. The effects of nutrients deserve
particular attention in view of nutritional deficiencies occurring
in certain parts of the world, where aflatoxins exposure can be
considerable. The more recently discovered effect of exposure to
(artificial) sunlight might also be of interest in this respect.
The mechanisms by which hormones, nutrition, and other factors
influence aflatoxin carcinogenesis are not known but are thought to
include effects on DNA synthesis and cell division and
differentiation, and/or effects on aflatoxin metabolism, and
excretion. Animals with a severely restricted energy food intake do
not grow and are less susceptible to the action of many carcinogens
than normal animals. The retardation of growth induced by severe
protein deficiency and also by hypophysectomy may explain reduced
tumour incidence under these experimental conditions. Conversion of
aflatoxin B1 to a bacterial mutagen is different in microsomal
liver preparations from rats fed a marginal lipotrope diet compared
with those from normal rats. Excretion of mutagens in the urine is
also different in lipotrope-deficient rats (Suit et al., 1977).
Changes in aflatoxin B1 metabolism and reduced levels of
hepatic macro-molecule-bound aflatoxin B1 adducts were reported in
rats pretreated with phenobarbital (Garner, 1975; Swenson et al.,
1977), and also in hypophysectomized animals (Swenson et al., 1977).
Sex-linked differences and endocrine status. Several studies
indicate that in comparison with males, female rats are more
resistant to both acute toxic and carcinogenic effects of
aflatoxins. Thus, with a single administration of aflatoxin B1 by
gavage, the LD50 was estimated to be 7.2 mg/kg body weight
(fiducial limits 5.36-8.23) in male rats and 17.9 mg/kg body weight
(fiducial limits 14.4-22.5) in female rats (Butler, 1964). The
sex-dependent influence of vitamin A deficiency on the acute
toxicity of aflatoxins is discussed later in this section.
In another study (Newberne & Wogan, 1968a), Fischer rats of both
sexes were kept on diets containing aflatoxin B1 levels of 0.015,
0.3, or 1.0 mg/kg and killed successively for histological
examination. The early hepatic lesions, considered as precancerous,
appeared with almost the same rate of incidence and at approximately
the same time in both sexes; however, there was a considerably
longer period between the appearance of the pre-cancerous lesions
and progression to liver carcinomas in females than in males. At a
dietary level of aflatoxin B1 of 1 mg/kg, males developed
carcinomas after 35 weeks of exposure while tumours in females were
observed only after 64 weeks. A similar, if much less pronounced,
sex-linked difference was observed at the 2 lower aflatoxin levels.
Calculations of the approximate total intake of aflatoxin B1
before the appearance of tumours (based on average intake of food
containing a known quantity of aflatoxin) and the time over which
these total amounts were consumed are given in Table 10. In a study
by Ward et al. (1975), male rats (F344, NIH) kept on a diet
containing aflatoxin B1 at 2 mg/kg, died with malignant
haemorrhagic liver tumours significantly earlier than females.
Kidney tumours observed in male but not female rats exposed to
aflatoxins (Butler et al., 1969) have already been discussed in
section 3.4.2.3.
Newberne & Williams (1969) reported that fewer male rats
(Charles River CD) fed for more than a year on a diet containing
aflatoxin B1 at 0.2 mg/kg and diethylstilboestrol at 4 mg/kg
developed liver tumours (8/40) than those fed the same aflatoxin
diet without the estrogen (25/35).
In a study on the influence of hypophysectomy on aflatoxin
carcinogenesis, male albino (MRC) rats were fed a diet containing an
aflatoxin B1 level of 4 mg/kg. All of 14 control rats developed
liver tumours in 49 weeks whereas none of 14 hypophysectomized rats
developed liver tumours in the same period. However, tumours of
extrahepatic tissues (4/14 carcinomas of retro-orbital lacrimal
glands-see also section 3.4.2.3) were observed in the
hypophysectomized aflatoxin-treated rats (Goodall & Butler, 1969).
Table 10. Sex-linked difference in the carcinogenicity (liver cell
carcinoma) of dietary aflatoxin B1a
Dietary level Approximate total intake of Average time (days over
of aflatoxin B1 aflatoxin B1 before the which the total amount was
appearance of tumours consumed
males females males females
1 mg/kg 2.9 mg/rat 5.9 mg/rat 245 days 448 days
0.015 mg/kg 95 µg/rat 11 5 µg/rat 476 days 560 days
a Data from Newberne & Wogan (1968a).
Nutritional factors (food components). The influence of
nutritional factors on the effects and dose-response relationships
of aflatoxins has been reviewed by Wogan (1973, 1977), Newberne
(1974, 1976), Newberne & Rogers (1976), and Newberne & Gross (1977).
(a) Dietary protein and lipotropic agents. Rhesus monkeys were
given daily doses of 100 µg aflatoxin per animal by stomach tube.
Two animals, fed a severely protein-deficient ration (1% casein) for
8 weeks before and during aflatoxin administration, developed fatty
liver and biliary proliferation and fibrosis and died with
gastrointestinal haemorrhage within 30 days of aflatoxin treatment.
Two animals fed a control ration (16% casein) survived in apparent
good health up to the termination of the experiment (35 days of
aflatoxin treatment) (Madhavan et al., 1965a).
In a study on weanling male rats given 50 µg aflatoxin per
animal per day for 20 days, 2/6 animals, fed a diet containing 4%
casein, died on days 18 and 19 of the experiment. Extensive liver
damage was found in these rats and in others in the 4%a casein
group within 20 days of aflatoxin treatment. All 12 rats fed a diet
containing 20% casein survived similar aflatoxin dosing with only
mild changes in the liver (Madhavan & Gopalan, 1965). In another
study by Madhavan & Gopalan (1968) 2 groups of 12 male rats were fed
the 2 diets (5%a or 20% casein) for 2 years from weaning and were
given, from the beginning of the experiment, 232 daily doses of
5 µg aflatoxin per animal or 225 daily doses of 10 µg aflatoxin per
animal. All 12 animals fed the 20% casein diet survived the period
of aflatoxin dosing and 50% developed hepatomas; lung metastases
were observed in 2 of these rats. Five of the 12 animals fed on the
5% casein diet died during the period of aflatoxin dosing. No
hepatomas but one renal cell carcinoma were found in the remaining 7
rats. Both diets used in these experiments were supplemented only
with 0.01% choline.
In experiments by Newberne & Wogan (1968b), rats fed a diet
containing 9% protein developed a higher incidence of liver tumours
(11/15) in a shorter period of time (8 months) than rats fed a diet
containing 22% protein (incidence 7/14 after 10 months). Both groups
of rats were given a total dose of 375 µg of aflatoxin B1 per
animal by gastric intubation, over 3 weeks, at the beginning of the
experiment.
In studies examining lipotropic effects on aflatoxin activity, a
diet marginal in methionine and choline, deficient in folate, and
high in fat protected male rats against the acute toxicity of a
single dose of aflatoxin B1; however, susceptibility to the toxic
effects of repeated doses of aflatoxin B1 increased, and the
carcinogenicity of aflatoxin B1 was enhanced. The experimental
diet (Rogers & Newberne, 1971; Rogers, 1975) contained peanut meal
(12%, alcohol extracted), gelatine (6%), casein (3%, vitamin free)
and fibrin (1%) as sources of protein, with a supplement of
L-cystine (0.5%). This diet is marginally but not severely deficient
in threonine, tryptophan, and arginine as well as in methionine. The
diet contained choline chloride (0.2%), and was high in fat (beef
fat 30%; corn oil 2%). The control, nutritionally complete diet
contained casein (22%, vitamin free) as the protein source, 15% or
16% oil (corn or mixed vegetable oil) and 0.3% choline chloride.
Both diets were adequate in other essential nutrients.
a According to Madhavan & Gopalan (1968) the composition of the
diets used in both studies was the same. However, their 1965 paper
gives 4% whereas that of 1968 gives 5% as the level of casein in the
diet.
As shown in Table 11, the marginal lipotrope diet fed for 2
weeks protected rats against acute aflatoxin B11 toxicity. The
rats that died had haemorrhagic necrosis of the liver with various
degrees of bile duct proliferation and, occasionally, haemorrhagic
necrosis in the adrenals and kidneys. The surviving rats (killed 2
weeks after aflatoxin administration) had focal necrosis of
hepatocytes, bile duct proliferation, and an increase in the size of
periportal hepatocytes. Approximately 20% of the rats fed the
marginal lipotrope diet and given a single dose of aflatoxin B1
had focal areas of abnormal hepatocytes which showed an increased
uptake of 3H thymidine (section 3.4.2.3) (Rogers & Newberne,
1971).
Table 11. Effect of a marginal lipotrope diet on the acute toxicity of
aflatoxin B1 in rata
Aflatoxin B1 Route of Diet No. of Mortality
(mg/kg) administration rats at 2 weeks
(%)
Sprague-Dawley (males)
7 Intragastric control 5 60
marginal lipotrope 5 0
9 Intragastric control 5 80
marginal lipotrope 10 0
7 Intraperitoneal control 5 100
marginal lipotrope 5 0
Fischer (males)
7 intragastric control 10 100
marginal lipotrope 10 0
a From: Rogers & Newberne (1971).
Although resistant to the toxicity of a single dose of aflatoxin
B1, rats fed the marginal lipotrope diet for 2 weeks before and
also during repeated aflatoxin exposure (136 males) were highly
sensitive to repeated daily doses of aflatoxin B1 at 25 µg per
animal. One half of these rats died, most of them after having
received 8 or 9 doses of aflatoxin B1 (200 or 225 µg total).
Various degrees of necrosis were observed in the livers with
extensive proliferation of bile duct cells. The mortality in rats
fed the control diet (66 males) was only 4% during the
administration of the total dose of 350 lag of aflatoxin B1
(Rogers & Newberne, 1971).
Enhanced aflatoxin B1 carcinogenicity in rats fed marginal
lipotrope diets, observed repeatedly in several experiments reviewed
by Newberne & Gross (1977), is demonstrated in Fig. 4 (Rogers,
1975). Cumulative probability of death from a tumour was calculated
here by the method described by Saffiotti et al. (1972) i.e., from
the number of animals at risk and the number of deaths from tumours
each week. A total dose of aflatoxin B1 of 375 µg, divided in 25
intragastric doses of 15 µg/animal per day over 7 weeks, was given
to male Fischer rats. Hepatocarcinomas developed in 87% of 52
animals fed the marginal lipotrope diet and in only 11% of 27 rats
fed the nutritionally complete diet ( P < 0.001). Twenty-seven
percent of tumours in rats fed the marginal diet metastasized to
other abdominal organs or the lung; no metastases were detected in
rats fed the control diet (Rogers, 1975). The groundnut meal fed in
these experiments did not contain detectable aflatoxins.
3.3.4.2 Studies in man
In the Philippines, aflatoxin M1 has been found (not
measured)in the urine of human subjects known to have ingested
aflatoxin-contaminated peanut butter (Campbell et al., 1970).
Claims concerning an aflatoxin involvement in the etiology of
juvenile cirrhosis in India (section 3.6.3.2) based on a
blue-fluorescent B1 spot in the breast milk of mothers and the
urine of children with the disease (Robinson, 1967) are largely
discounted by the later studies of Yadgiri et al. (1970) who
produced spectrophotometric evidence that, although such a spot
could be identified in the urine of children with the overt disease,
this was not aflatoxin B1 For other reports see section 3.5.1.
3.4 Effects in Animals
The effects of aflatoxins in animals have., been reviewed by
Allcroft (1969), Newberne & Butler (1969) and Butler (1974).
3.4.1 Field observations
When foodstuffs are affected by microbial deterioration, man
normally eats the less affected parts, whereas domestic animals may
be exposed to more contaminated rations. This explains why the
discovery of several mycotoxins has been based on field observations
in domestic animals.
The first observation of a disease in animals subsequently
associated with aflatoxins was an acute outbreak of a lethal disease
in turkey poults in England in 1960 causing an estimated loss of at
least 100 000 birds. Extensive research eventually revealed that the
disease was caused by aflatoxins contained in a batch of Brazilian
groundnut meal. The concentration of aflatoxin B1 in the original
groundnut meal was later estimated to be about 10 mg/kg. The disease
was characterized by rapid deterioration in the condition of the
birds, subcutaneous haemorrhages, and death. At postmortem, the
livers of the birds were pale, fatty, and showed extensive necrosis
and biliary proliferation (Butler, 1974). A similar case of acute
disease was observed in day-old ducklings fed "toxic" groundnut meal
(Asplin & Carnaghan, 1961), where the liver changes described were
followed by cirrhosis. Outbreaks of liver disease in chickens have
also been associated with aflatoxin-contaminated feed (Asplin &
Carnaghan, 1961).
Loosmore & Harding (1961) noted outbreaks in pigs fed groundnut
meal in which the toxic factor was later identified as aflatoxins.
The lesions in the pigs included haemorrhages, and liver damage
characterized by dissecting fibrosis and biliary proliferation.
Calves fed rations containing 15% toxic groundnut meal also
developed liver lesions characterized by fibrosis and biliary
proliferation (Loosmore & Markson, 1961). Outbreaks associated with
"toxic" groundnut meal and characterized by similar liver lesions
have been reported in older cattle even though they are more
resistant (Clegg & Bryston, 1962); there was also a drop in milk
production.
A liver disease "hepatitis X" has been reported in dogs in
southeastern USA (Seibold & Bailey, 1952; Newberne et al., 1955).
Icterus and in some cases ascites were observed and the liver
lesions included fatty changes with centrilobular parenchymal
necrosis and biliary proliferation. Commercial dog food thought to
be the cause of toxicity was later found to contain aflatoxin B1
(up to 1.75 mg/kg) (Newberne et al., 1966a). Similar lesions have
been reproduced in dogs by peroral administration of aflatoxins, and
it has been suggested that the "hepatitis X" in dogs could be
causally associated with aflatoxins in the diet (Newberne et al.,
1966a). During an outbreak of toxic hepatitis affecting several
hundred people in north-west India and considered to be possibly
associated with the consumption of maize heavily contaminated by
aflatoxins (section 3.5.2 and 3.6.2.1), dogs fed food remnants from
households in affected villages manifested a disease characterized
by jaundice, ascites, and frequently death (Krisnamachari et al.,
1975a, b; Tandon et al., 1977). A nonportal type of micronodular
cirrhosis, with less conspicuous parenchymal and cholangiolar
changes was found on histological examination of the livers of two
dogs (Tandon et al., 1977).
3.4.2 Experimental studies
3.4.2.1 Acute and chronic effects: hepatotoxicity
Different species vary in their susceptibility to acute
poisoning by aflatoxins, with LD50 values ranging from 0.3 to
17.9 mg/kg body weight (Table 4). In all the animals studied, the
liver was the principal target organ (see for example Butler, 1974).
Table 4. Acute toxicity of aflatoxin B1a
Species LD50 Zone of
(mg/kg body weight) liver lesion
chick embryo 0.025d
rabbit 0.3 midzonal
duckling 0.335 periportal
cat 0.55 periportal
pig 0.62 centrilobular
dog 0.5-1.0 centrilobular
sheep 1.0 centrilobular
guineapig 1.4 centrilobular
baboonb 2.0 centrilobular
rat (male) 7.2 periportal
macaque femalec 7.8 centrilobular
mouse 9.0
hamster 10.2
rat (female) 17.9 periportal
a Adapted from: Newberne & Butler (1969) and Butler (1974).
b From: Peers & Linsell (1976).
c From: Shank et al. (1971 b).
d µg/embryo.
The lesions observed in field cases (section 3.4.1) in poultry,
pigs, cattle, and dogs have all been reproduced in the same animal
species by feeding experiments during periods of time ranging from a
few weeks to a few months, using diets containing aflatoxins, or
pure aflatoxins ranging from 0.3 to several mg/kg (Newberne &
Butler, 1969).
In the study by Carnaghan et al. (1966), chickens were fed a
diet containing aflatoxin B1 at a level of 1.5 mg/kg. Groups of 3
control and 3 test chicks were killed after 3´ days, 7 days, and
then at weekly intervals for 8 weeks. After 4 weeks, the liver
lesions included fatty change, biliary proliferation, and fibrosis.
In 20 pigs, aflatoxins (aflatoxins B1 and B2) at a feed
level as low as 300 µg/kg resulted in the development of
centrilobular necrosis and fibrosis of the liver as well as growth
depression, during a normal feeding period of 3-4 months (Krogh et
al., 1973a). In cattle, the liver lesions (centrilobular
degeneration, fibrosis, biliary proliferation) occurred in all 4
animals after 4 months on a feed containing an aflatoxin level of
2 mg/kg (Allcroft & Lewis, 1963). The hepatic lesions induced in the
duckling by the aflatoxins formed the basis of a bioassay originally
described by Sargeant et al. (1961). At sublethal doses of
aflatoxins, the bioassay depends upon an assessment of the degree of
biliary proliferation. Liver lesions similar to those observed in
farm animals have been experimentally induced by the administration
of aflatoxins in a number of laboratory animals, including the rat,
cat, guinea-pig, and rabbit (Newberne & Butler, 1969).
In a study of Madhavan et al. (1965b), 2 rhesus monkeys
(Macaca mulatta) were given daily oral doses of aflatoxins at
500 µg/animal for 18 days (corresponding approximately to 250 µg/kg
body weight per day) and then 1 mg/animal per day (corresponding
approximately to 500 µg/kg body weight per day) until death occurred
after 32 and 34 days. Three rhesus monkeys were given 1 mg each,
daily, until death occurred after 19, 20, and 27 days respectively.
The liver lesions included fatty infiltration, biliary
proliferation, and portal fibrosis. Death or similar lesions were
not observed in the 2 control monkeys.
Deo et al. (1970) studied the effect on male rhesus monkeys of
repeated administration by gastric tube of 3 different levels of
aflatoxins (B1 + G1). At the highest dose level (1 mg/kg body
weight daily for 3 weeks), 35/35 animals died within 22 days with
extensive haemorrhagic necrosis of the liver. A dose level of
0.25 mg/kg body weight, twice a week, for 5 months induced various
degrees of liver changes in 24/24 animals characterized by biliary
proliferation and focal appearance of the liver cells with multiple
nuclei and giant-sized liver cells with enlarged hyperchromatic
nuclei. At the lowest dose, 5 animals were given 62 µg/kg body
weight once a week for periods ranging from a few days to 2 years.
Liver changes were similar to the changes seen in the second group
but in a milder form.
Cynomolgus monkeys (Macaca fascicularis = M. irus) fed a
dietary level of aflatoxin B1 of 5 mg/kg rapidly developed liver
damage with biliary proliferation and all 6 animals died within 2
months. When fed aflatoxin B1 at a dietary level of 1.8 mg/kg, 5
animals died within 3 months showing liver damage characterized by
centrilobular necrosis, biliary proliferation, and fibrosis. Two
animals survived and were killed after 3 years; the liver of one
animal had the appearance of nodular cirrhosis. Two groups of 4
animals each were fed lower levels of aflatoxin B1 (0.07 and
0.36 mg/kg, respectively) for 3 years without showing any signs of
liver lesions (Cuthbertson et al., 1967).
The relationship between the chemical structure of different
aflatoxins and their biological activity (discussed also in section
3.4.2.3) was investigated in a small number of experiments; more
extensive studies were not possible because of the limited
quantifies available of some of the pure aflatoxins. Carnaghan et
al. (1963) compared 6-day mortality following single doses of
different aflatoxins, administered by intubation to one-day-old
Khaki Cambell ducklings, and concluded that both aflatoxins B2 and
G2 were less toxic than aflatoxins B1 and G1, the ratio of
LD50 values being 1:4.7 for B1:B2 and 1:4.4 for G1:G2.
Aflatoxins G1 and G2 were less toxic than the corresponding
aflatoxins B1 and B2, the ratio of the LD50 values being
1:2.15 for B1:G1 and 1:2.03 for B2:G2. The corresponding
LD50 values for aflatoxins B1, B2, G1, and G2 were 0.36,
1.70, 0.78, and 3.45 mg/kg, respectively. Comparable results were
obtained by Wogan et al. (1971) who recorded 14 day mortality after
intubation of male Pekin ducklings and reported LD50 values of
0.73, 1.76, 1.18 and 2.83 mg/kg for aflatoxins B1. B2, G1, and
G2, respectively. In the same paper, a study was reported on the
14-day mortality of male Fischer rats after a single
(intra-peritoneal) dose of aflatoxin. The LD50 value for aflatoxin
B1 was 1.16 mg/kg body weight (95% confidence interval 0.91 to
1.48 mg/kg) whereas the LD50 for aflatoxin G1 was between 1.5
and 2.0 mg/kg body weight. On the other hand, no deaths occurred in
20 rats given 12-200 mg of aflatoxin B2 per kg body weight, and
all 4 rats given 170-200 mg of aflatoxin G2 per kg body weight
survived. A similar difference in the toxicity of aflatoxins was
observed when male Fischer rats were given repeated doses of
aflatoxins by stomach tube over a 4-week period. The 4-week
mortality in rats given a total dose of 1 mg of aflatoxin B1 per
rat was 8/10 whereas all 10 rats given the same dose of aflatoxin
G1 survived and only 4/10 animals given double this dose of
aflatoxin G1 died. In another trial, all 11 rats survived
intragastric administration of 3.75 mg of aflatoxin B2 per rat
repeated every second day for 4 weeks to give a total dose of
52.5 mg per rat.
Holzapfel et al. (1966) and Purchase (1967) reported 7-day
mortality alter oral dosing of one-day-old Pekin ducklings with
aflatoxins B1, M1, and M2. Five groups of 2-3 ducklings (body
weight 40-50 g) were used for each of the aflatoxins tested, and the
following LD50s were calculated (with 95% confidence limits given
in brackets): aflatoxin B1, 12 (3.9-37.2) µg per duckling,
aflatoxin M1, 16 (5.4-51.5) µg per duckling, and aflatoxin M2,
61.4 (37-100) µg per duckling. Ducklings receiving aflatoxin M2
showed characteristic liver lesions indistinguishable from those
observed after a similar dose of aflatoxin B1. Higher doses of
aflatoxin M2 produced similar effects (Purchase, 1967). A study
comparing the acute toxicity of synthetic (racetalc) aflatoxins B1
and M1 and the natural optical isomer of aflatoxin B1 was
reported by Pong & Wogan (1971), suggesting that only one isomer of
each synthetized racemic mixture was biologically active.
Fourteen-day mortality rates observed after a single intraperitoneal
dose of 1.5 mg/kg body weight of synthetic aflatoxin B1 and
synthetic aflatoxin M1 were 1/1 and 1/2, respectively. However, no
deaths occurred in groups of rats (each consisting of 4 animals)
given these synthetic aflatoxins at doses of 1, 0.8, 0.6, or
0.4 mg/kg body weight. With the natural aflatoxin B1, the observed
mortalities at these dose levels were 4/4, 2/4, 2/4, and 0/4
respectively.
For information on the toxicity of certain other aflatoxin
metabolites or derivatives, see Wogan et al. (1971) and Patterson
(1976).
3.4.2.2 Hepatotoxicity connected with extrahepatic effects
Many other organs besides the liver are more or less severely
affected in acute experiments with high doses of aflatoxins (Butler,
1964): in male and female rats, a single dose of aflatoxin B1
proved lethal in half of the animals (7.2 mg/kg body weight in the
male and 17.9 mg/kg body weight in the female, by garage). Frequent
bilateral adrenal haemorrhages, petechial haemorrhages in many
organs, particularly in the congested lungs, and occasionally patchy
necroses in the myocardium and in other organs (kidney, spleen) were
observed during the first few days following administration. These
changes were not detected in male or female rats given aflatoxin B1
at 3.5 mg/kg body weight. With higher doses, the haemorrhages seen
in the lungs, kidneys, and adrenals were more extensive. Animals
dying within the first few days often had altered blood in the whole
of the small intestine and in the colon. Ascites and oedema of the
omentum were observed in some of the animals a week or more (but not
one month) after aflatoxin administration. After a month, with the
exception of the liver damage, all the other organs appeared normal
in surviving animals. Histologically, certain renal changes were
detected in the loops of Henle at this stage, consisting of a few
cells with large irregular hyperchromatic nuclei, very similar to
those seen in the liver (Butler, 1964).
Congested lungs with small petechial haemorrhages, haemorrhagic
necroses in the adrenals (localized in the inner zone of the
reticularis) and patchy necroses in the kidneys, pancreas, and
spleen were observed in guineapigs 2-3 days after a single
intraperitoneal injection of aflatoxin B1 at 1.4 mg/kg body weight
(lethal in half of the males and females). Even at this dose, the
small intestine was frequently filled with altered blood. At higher
doses, the haemorrhagic disease was more marked, with pleural,
pericardial, and peritoneal haemorrhages. The only change seen in
the heart of the guineapig, 2-3 days after aflatoxin administration,
was an occasional small area of fatty degeneration of the
myocardium. Many animals showed marked ascites and oedema of the
omentum and subcutaneous tissue during the first week after
injection (Butler, 1966).
Bourgeois et al. (1971) reported a special syndrome induced by
oral administration of aflatoxin B1 in the macaque (Macaca
fascicularis). In 2 groups of 4 young females, each receiving a
single oral dose of aflatoxin B1 at 13.5 or 40.5 mg/kg body
weight, all animals died within 149 h. Death occurred in 1 out of 4
other animals receiving a dose of 4.5 mg/kg body weight. Doses of
toxin of 1.5 mg/kg or 0.5 mg/kg (4 animals in each group) did not
result in death or unusual clinical signs. Cough, vomiting,
diarrhoea, and coma were characteristic clinical findings in animals
exposed to toxic doses. Analysis of blood serum revealed a
dose-dependent decrease in serum levels of phospholipids within 24 h
of administration of the aflatoxin. A dose-dependent decrease in
serum levels of glucose and an increase in nonesterified fatty acids
occurred within 72 h of aflatoxin administration. The liver lesions
included centrilobular necrosis, some biliary proliferation, and
massive fatty degeneration which was also observed in the heart and
kidneys. Cerebral oedema with neuronal degeneration was seen. Some
of these findings resemble those associated with Reye's syndrome in
children (see section 3.5.1.2).
3.4.2.3 Carcinogenesis
The carcinogenesis of aflatoxins has been reviewed by Wogan
(1973, 1977) and re-evaluated by IARC (1976).
Hepatic and renal tumours. Orally administered aflatoxins,
mainly B1, have been hepatocarcinogenic in all species of test
animals studied so far (including nonhuman primates), with the
exception of the mouse, in which carcinogenic effects have been
demonstrated only following intraperitoneal administration of
aflatoxin B1 to neonates (Tables 5 and 6). These studies were
concerned with repeated or long-term exposure to aflatoxins. In a
study by Carnaghan (1967), 2 groups consisting of 16 and 18 weanling
female Wistar rats, respectively, were given single oral doses of
crystalline aflatoxin B1 or a mixture of aflatoxins containing
about 40% aflatoxin B1 and 60% aflatoxin G1, at the rate of
0.5 mg/rat in 0.1 ml dimethylformamide. These doses corresponded to
averages of 7.65 mg aflatoxin B1/kg body weight and 2.7 mg
aflatoxin B1 plus 4.0 mg aflatoxin G1/kg body weight,
respectively. Within 21-32 months, 7 rats out of each group
developed hepatic tumours with metastases in half the cases. Hepatic
rumours were not observed in 19 control rats given the solvent only.
No hepatocellular carcinomas were found in 22 male Fischer rats
killed successively 16 weeks (3 rats), 25 weeks (5 rats), 38 weeks
(5 rats), 55 weeks (4 rats), and 69 weeks (5 rats) after a single
dose of aflatoxin B1 at 5.0 mg/kg body weight, administered by
garage (Wogan & Newberne, 1967).
A linear dose-response relationship was observed by Wogan et al.
(1974) for the development of liver-cell carcinomas in male Fischer
rats fed dietary concentrations of aflatoxin B1 ranging from
1-100 µg/kg (Table 7). At 1 µg/kg, a 10% tumour incidence was found,
compared with no rumours in the control group and at 100 µg/kg the
tumour incidence was 100%. A linear log (dose)-response relationship
has been demonstrated in trout fed dietary levels of aflatoxin B1
ranging from 0.5 to 20.0 µg/kg, for 20 months. Extrapolating this
relationship to lower exposure levels, an incidence of approximately
10% would be expected with a dietary concentration of 0.1 µg/kg.
Table 5. Hepatocarcinogenicity of aflatoxin B1 in rodentsa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
rat, Fischer 1.0 mg/kg diet 33 weeks 52 weeks 3/6 Svoboda et al. (1966)
rat, Fischer 1.0 mg/kg diet 41-64 weeks 41-64 weeks 18/21 Wogan & Newberne (1967)
rat, Porton 1.0 mg/kg diet 20 weeks 90 weeks 19/30 Butler (1969)
rat, Wistar 1.0 mg/kg diet 21 weeks 87 weeks 12/14 Epstein et al. (1969)
mouse, Swiss 150 mg/kg dietb 80 weeks 80 weeks 0/60 Wogan (1973)
mouse, C57Bl/6NB 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, C3HfB/HEN 1.0 mg/kg diet 80 weeks 80 weeks 0/30 Wogan (1973)
mouse, hybrid F1, 4 days old 6.0 µg/g body weight 3 doses (i.p.) 80 weeks 16/16 Vesselinovitch et al. (1972)
a From: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
Table 6. Hepatocarcinogenicity of aflatoxin B1 in nonrodent speciesa
Species Dosing regimen Duration of Period of Liver Reference
treatment observation tumour
incidence
monkey, rhesus (M) 1.655 g totalb 5.5 years 8.0 years 1/1 Gopalan et al. (1972)
monkey, rhesus (F) 1.855 g total 5.5 years 10.75 years 1/1 Tilak (1975)
monkey, rhesus (F) 0.504 g total 6.0 years 8.0 years 1/1 Adamson et al. (1973)
marmoset 3.0 mg total 50-55 weeks 50-55 weeks 1/3 Lin et al. (1974)
5.04-5.84 mg totalc 87-94 weeks 87-94 weeks 2/3 Lin et al, (1974)
tree shrew (M & F) 24-66 mg total 74-172 weeks 74-172 weeks 9/12 Reddy et al, (1976)
ferret 0.3-2.0 µg/kg 28-37 months 28-37 months 7/9 Butler (1969)
duck 30 µg/kg 14 months 14 months 8/11 Carnaghan (1965)
rainbow trout 4 µg/kg in diet 12 months 12 months 15% Sinnhuber et al, (1968b)
8 µg/kg in diet 12 months 12 months 40% Sinnhuber et al. (1968b)
rainbow trout embryos 0.5 mg/kg in water 1 h 296-321 days 38% Sinnhuber & Wales (1974)
salmon 12µg/kg in dietd 20 months 20 months 50% Wales & Sinnhuber (1972)
guppy 6 mg/kg in diet 11 months 11 months 7/11 Sato et al. (1973)
a Modified from: Wogan (1977).
b A mixture of aflatoxins B1 and G1 was used in this experiment.
c These animals were infected simultaneously with hepatitis virus.
d This diet also contained 50 mg/kg cyclopropenoid fatty acids.
Table 7. Dose-response characteristics of aflatoxin B1 carcinogenesis
in male Fischer strain ratsa
Dietary Duration Liver Time of appearance
aflatoxin of feeding carcinoma of earliest tumour
level (week) incidenceb (week)
(µg/kg)
0 74--109 0/18c --
1 78--105 2/22 104
5 65--93 1/22 93
15 69--96 4/21 96
50 71--97 20/25d 82
100 54--88 28/28e 54
a From: Wogan et al. (1974).
b In animals at risk (surviving longer than 50 weeks).
c Animals surviving for maximum period.
d Two animals had pulmonary metastases.
e Four animals had pulmonary metastases.
In a recent study (FDA, 1978), an attempt was made to calculate
the lifetime liver cancer risk in rats, which could be connected
with aflatoxin feed levels lower than those directly tested in
animal experiments. Estimates of life-time liver cancer incidence
rates corresponding to aflatoxin feed levels of 0.1 and 0.3 µg/kg
were derived and compared for several selected rat studies using the
mathematical procedure developed by Mantel & Bryan (1961) and
modified by Mantel et al. (1975). A more detailed description of
this procedure can be found in other publications including WHO
(1978) and Hoel et al. (1975). Thus, the estimated life-time liver
cancer risk derived from the experimental results reported by Wogan
et al. (1974) (see Table 7) corresponded to life-time liver cancer
incidence rates of 70 (600) per 105 rats for an aflatoxin dietary
level of 0.1 µg/kg and 360 (2300) for a level of 0.3 µg/kg. (Numbers
in parentheses; are upper 99% confidence limits.) Estimates derived
from different rat studies varied considerably. The lifetime
incidence rates calculated for the combined studies were 240 (470)
and 1100 (1900) per 105 rats for aflatoxin feed levels of 0.1 and
0.3 µg/kg, respectively (FDA, 1978).
The studies in primates are included in Table 6. No attempts
have been made to establish dose-response relationships in primates,
but they are susceptible to aflatoxin hepatocarcinogenesis.
The carcinogenic effects of different purified aflatoxins have
been compared in a limited number of studies. The results of a study
by Butler et al. (1969) in which 8-9 week old, male (M) and female
(F) MRC rats were given aflatoxins B1, B2, or G1 in drinking
water for 10 or 20 weeks, are shown in Table 8. The earliest renal
neoplasm was seen 54 weeks after the discontinuation of aflatoxin
treatment. Renal tumours were detected only in males. The 2 rats
treated with aflatoxin B1 that developed renal tumours did not
have hepatic carcinomas, but 5 of the 11 rats receiving aflatoxin
G1 developed both renal and hepatic carcinomas.
Wogan et al. (1971) studied the relationship between the
chemical structures of aflatoxins and their hepatocarcinogenicity in
male Fischer rats, and concluded that aflatoxin B1 was apparently
more carcinogenic than aflatoxin G1 and that both were much more
active than aflatoxin B2. A total intraperitoneal dose of
aflatoxin B2 of 150 mg per rat given in 40 equal doses over 8
weeks induced hepatocellular carcinomas in 3/9 rats. A similar
regimen, containing a total dose of aflatoxin B1 of 1.3 mg,
induced liver tumours in 9/9 animals. In other experiments to
compare the carcinogenicity of aflatoxins G1 and B1 given by
stomach tube to rats, aflatoxin G1 in a total dose of 1.4 mg per
animal (divided into 14 equal doses over 2.5 weeks) induced
hepatocellular carcinoma in 3/5 rats within 68 weeks. Hepatocellular
carcinomas were observed in all 18 animals given a total dose of
2 mg of aflatoxin G1 (divided in 40 equal doses over 8 weeks) and
killed within 45-64 weeks. Six of these rats had pulmonary
metastases.
Hepatocellular carcinomas were also found in 7/7 rats given
aflatoxin B1 in a total dose of 0.5 mg per animal (divided in 20
equal doses over 4 weeks) and sacrificed within 74 weeks, in 18/18
rats given 1 mg per animal (divided into 40 equal doses over 8
weeks) and killed within 42-58 weeks, and in 17/17 given 1.5 mg per
animal (divided into 40 equal doses over 8 weeks) and killed within
42-46 weeks. With the 2 higher doses, 2 and 7 animals, respectively
had pulmonary metastases. Renal adenocarcinomas were found in 4/26
rats given aflatoxin G1.
Synthetic racemic aflatoxin M1 induced hepatocarcinomas at 100
weeks in 1/29 male Fischer rats given 1 mg of the compound
intragastrically in divided doses over a period of 8 weeks. The
incidence of hepatocarcinomas in 9 rats given the same dose of
natural aflatoxin B1 was 100%, 1 year after treatment (Wogan &
Paglialunga, 1974). Natural aflatoxin M1 was less effective than
natural aflatoxin B1 in inducing tumours in trout, particularly in
the males. Only 14% of male trout, fed natural aflatoxin M1 for a
year at a dietary level of 4 µg/kg, developed liver tumours compared
with 68% of those receiving a similar feed level of aflatoxin B1
Table 8. Carcinogenesis in rats due to ingestion of aflatoxin B1, G1 or B2 in drinking watera
Compound Concentration Daily Duration Total No. and sex No. of animals No. of animals
(µg/ml) dose (µg) weeks dose (mg) of animals with tumours with other
treated neoplasms
liver kidney
aflatoxin B1 1 20 20 2 15 M 8 2 4
15 F 11 0 1
aflatoxin B1 1 20 10 1 10 M 3 0 2
aflatoxin G1 3 60 20 6 11 M 9 6 5
15 F 12 0 3
aflatoxin G1 1 20 20 2 15 M 2 5 2
15 F 1 0 7
aflatoxin G1 1 20 10 1 10 M 1 0 1
aflatoxin B2 1 20 10 1 10 M 0 0 2
controls 0 0 0 0 15 M 0 0 6
15 F 0 0
a From: Butler et al. (1969).
(the experiment was terminated 8 months after the discontinuation of
aflatoxin feeding). In females, the difference was less pronounced,
liver tumours occurring in 48% of aflatoxin B1-treated trout and
78% of aflatoxin B1-treated trout (see Table 9) (Sinnhuber et al.,
1974). Haemorrhages within the cancerous liver resulting in death,
which were observed in most females receiving dietary levels of
aflatoxin M1 of 16-64 µg/kg, were not observed in similarly
treated males.
Table 9. Aflatoxin M1 liver carcinogenesis in rainbow trout
(Salmo gairdneri)a
Aflatoxin in the diet Liver tumour incidence
males females
M1 4 µg/kg 4/28 (14%) 13/27 (48%)
M1 16 µg/kg 22/27 (81%) 11/14 (79%)
M1 32 µg/kg 24/25 (96%) 13/14 (93%)
M1 64 µg/kg 21/24 (88%) 9/10 (90%)
B1 4 µg/kg 15/22 (68%) 18/23 (78%)
a From: Sinnhuber et al. (1974).
A probable effect of rat strain on aflatoxin carcinogenesis was
seen in the high incidence of renal epithelial neoplasms reported in
male Wistar strain rats fed diets containing aflatoxin B1, for 147
days, and then maintained on a basal diet until death (Epstein et
al., 1969). Renal tumours developed in 57% (8/14), 28% (5/18), and
23% (3/13) of male rats exposed to diets containing aflatoxin B1
levels of 1.0, 0.5, and 0.25 mg/kg feed, respectively. The
incidences of malignant hepatomas in corresponding groups were 86%,
72%, and 62% respectively. No renal tumours or malignant hepatomas
were detected in a control group (24 animals). Approximately
one-third of the aflatoxin-exposed rats with renal rumours did not
have hepatomas. The first malignant hepatoma and the first renal
tumour were detected 463 and 468 days after initiation of the
experiment, respectively; both these tumours occurred in the group
with the highest exposure. Approximately half of the renal tumours
were bilateral.
Early hepatic lesions possibly related to carcinogenesis.
Aflatoxin B1 given to rats in repeated doses of 15-25 µg/day, for
3.5 weeks, making a total dose of 375 µg, elicited increased DNA
synthesis and mitosis in clusters of liver cells that could be
distinguished from the surrounding cells by their histological
appearance and biochemical activity (Newberne & Wogan, 1968a; Rogers
& Newberne, 1969). Development of the abnormal foci was more
prominent in rats fed a high-fat, lipotrope-deficient diet, which
enhances aflatoxin carcinogenesis (section 3.4.2.7), than in rats
fed a nutritionally adequate diet. The abnormal foci were already
present in deficient rats at the end of carcinogen administration.
Similar foci are found in the livers of rats exposed to other
hepatic carcinogens and may be useful in studying pathogenesis or
metabolic aspects of liver cancer, since they are thought to be the
possible precursors of tumours. However, many of the foci disappear
with time after treatment, and progression is not inevitable.
Other tumours. Carcinoma of the colon have occasionally been
reported following aflatoxin exposure (Newberne & Butler, 1969).
Increased incidence of tumours in the distal half of the colon was
observed in vitamin A-deficient rats fed aflatoxin B1 (section
3.4.2.7) (Newberne & Rogers, 1973; Newberne & Suphakarn, 1977).
Tumours of the colon were also found in more than 20% (12/53) of
F344 rats (NIH) exposed, either from conception (7/34), or from 6-7
weeks of age (5/19), to a diet containing an aflatoxin B1 level of
2 mg/kg and an unspecified amount of vitamin A (Ward et al., 1975).
The tumours developed in both males and females at 42-64 weeks of
age and were primarily polyploid neoplasms in the ascending colon,
although 2 rats had tumours in the descending colon. No colon
tumours were found in 18 control rats.
Carcinomas of the glandular stomach were observed in 1/6 young
rats given a diet of groundnut meal containing an aflatoxin level of
3-4 mg/kg for 3 weeks and in 1/16 animals given the same diet at the
age of one year (Butler & Barnes, 1966). Two definite and one
probable adenocarcinomas of the stomach had previously been observed
in rats fed a diet prepared from the same batch of groundnut meal
(Butler & Barnes, 1963).
Other extrahepatic tumours have occasionally been reported after
oral aflatoxin exposure, e.g., tumours of the lacrimal glands
(Dickens et al., 1966; Goodall & Butler, 1969; Butler et al., 1969),
squamous cell carcinoma of the tongue (Ward et al., 1975) and
oesophagus (Butler et al., 1969). Tumours at various sites have been
induced in several species of test animals by intratracheal,
subcutaneous, or intraperitoneal administration of aflatoxins. The
experimental results obtained by intratracheal administration are of
particular interest in connexion with reported effects in man
associated with airborne aflatoxins (section 3.5.2). Squamous-cell
carcinoma of the trachea developed within 37-62 weeks in 3/6 rats
given a mixture of aflatoxins (containing B1, B2, G1, and
G2) at 300 µg per animal intra-tracheally twice weekly, for 30
weeks. Four of these rats also developed hepatomas within 49-62
weeks (Dickens et al., 1966).
Prenatal and early postnatal exposure. In a study of these
effects, 6 groups of 10 female, Wistar rats were each fed a diet
containing 25% or 50% groundnut meal contaminated with aflatoxin
B1 at 10 mg/kg and aflatoxin B2 at 0.2 mg/kg. Dams received this
diet from day 10 of pregnancy to parturition (exposure of offspring
in utero); from 1 day post partum to 10 days post partum (exposure
via milk); or from day 10 of pregnancy to 10 days post partum
(exposure in utero and via milk). Among 113 male and 95 female
offspring observed for up to 36 months, 1 male exposed in utero, 1
female exposed via milk, and 2 females exposed in utero
and via milk developed malignant liver tumours (Grice et al., 1973).
No liver tumours were seen in control offspring (50 male and 50
female rats).
3.4.2.4 Teratogenicity
The teratogenicity of aflatoxins has been reviewed by Ong
(1975). The effect of aflatoxin B1 on embryos in hamsters was
studied by Elis & Di Paolo (1967) who reported that a single
intraperitoneal injection of aflatoxin B1 at 4 mg/kg body weight,
given on day 8 of pregnancy, resulted in a high proportion of
malformed and dead or reabsorbed fetuses. Approximately 50% of the
fetuses in aflatoxin-treated mothers and over 85% in control mothers
were normal. A dose of 2 mg/kg did not have any effects. In studies
by Di Paolo et al. (1967), no teratogenic effects were observed when
12 pregnant C3H mice were given repeated dally intraperitoneal
injections of aflatoxin B1 at 4 mg/kg body weight. However, a high
proportion of dead or reabsorbed fetuses was observed in
aflatoxin-treated mice in comparison with controls.
3.4.2.5 Mutagenicity
Aflatoxin B1 causes chromosomal aberrations (chromosomal
fragments with occasional bridges, chromatid bridges, chromatid
breakage) and DNA breakage in plant and animal cells (Ong, 1975). It
has also been shown to cause gene mutations in bacterial test
systems (Ames test), when activated by microsomal preparations from
rat and human liver (Wong & Hsieh, 1976). However, no mutagenic
effects were observed in mice exposed intra-peritoneally to
aflatoxin B1 at 5 mg/kg body weight (Leonard et al., 1975).
3.4.2.6 Biochemical effects and mode of action
Individual aflatoxins (B1, B2, G1, G2 etc.) with
slightly different chemical structures affect experimental animals
(sections 3.4.2.1 and 3.4.2.3) and interact with in vitro test
systems to different degrees (Wogan et al., 1971; Patterson, 1976).
However, as aflatoxin B1 is the commonest and the most potent, it
has been studied extensively and the majority of reported
biochemical effects are specifically related to this toxin. As
discussed in section 3.3.3, the aflatoxin molecule appears to be
metabolically activated before exerting its acute and chronic
effects.
Interaction between these activated molecular species and the
liver cell apparently occurs at several loci. In the nucleus,
DNA-dependent RNA polymerase (EC 2.7.7.6)a is inhibited (Pong &
Wogan, 1970), the toxin binds covalently to DNA in vitro and
in vivo (Clifford & Rees, 1967; Lijinsky et al., 1970; Gamer,
1973, 1975; Swenson et al., 1974, 1977), DNA repair is stimulated
(Seegers & Pitout, 1973; Stich & Laishes, 1975), and the aflatoxin
is activated on the outer nuclear membrane to a form that inhibits
RNA synthesis (Neal & Godoy, 1976).
The permeability of mitochondria increases (Bababunmi & Bassir,
1972; Doherty & Campbell, 1973) and electron transport is
interrupted with a decline in respiration (Doherty & Campbell, 1972,
1973). Lysosomal membranes are also rendered permeable and unbound
acid hydrolases leak out (Tung et al., 1970; Pokrovsky et al., 1972;
Adekunle & Elegbe, 1974). Activation of lysosomal enzymes and their
effects on cellular structures may be a component of the toxic
mechanism of aflatoxins (Pokrovsky et al., 1977).
Aflatoxin is metabolized in the endoplasmic reticulum (section
3.3.3) and the unmetabolized toxin competes with steroid sex
hormones for polysome-binding sites (Williams & Rabin, 1971). The
reticulum degranulates (Theron, 1965; Theron et al., 1965; Butler,
1971, 1972) with the breakdown of polysome profiles (Villa-Trevino &
Leaver, 1968; Pong & Wogan, 1969; Godoy et al., 1976) and the
formation of helical polysome forms (Sarasin & Moule, 1976). RNA
polymerase is inhibited (Pong & Wogan, 1970) and the toxin binds
covalently to RNA (Swenson et al., 1977). Many metabolic functions
are inhibited, including protein synthesis, enzyme induction (Wogan
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
& Friedman, 1968; John & Miller, 1969; Kato et al., 1970) and the
synthesis of blood clotting factors II and VII (Bassir & Bababunmi,
1969). Glucose metabolism via the 6-phosphate pathway (Brown &
Abrams, 1965; Feuer et al., 1965;; Shankaran et al., 1970), and the
synthesis of fatty acids and phospholipids are also depressed
(Clifford & Rees, 1969; Kato et al., 1969; Black et al., 1970;
Donaldson et al., 1972; Lo & Black, 1972). Furthermore, feed-back
control of cholesterol synthesis is lost (Horton et al., 1972), a
change considered characteristic of the pre-cancerous state.
It has been shown that aflatoxins have immunosuppressive
properties, probably related to their inhibitory effect on protein
synthesis. Thus, at levels in poultry feed of 0.25-0.5 mg/kg,
aflatoxins have been found to reduce resistance to infection by
Pasteurella multocida, Salmonella spp., Marek's disease virus,
coccidia, and Candida albicans (Brown & Abrams, 1965; Smith et al.,
1969; Pier & Heddleston, 1970; Hamilton & Harris, 1971; Edds et al.,
1973).
In the liver cytoplasm, there is a transient stimulation
followed by a depression of glycogenolysis and the pentose shunt
pathway of glucose metabolism (Shankaran et al., 1970). Aflatoxins
also compete with further steroid binding sites in the cytoplasm,
notably NADP-linked 17-hydroxy steroid dehydrogenase (EC 1.1.1.148)
(Patterson & Roberts, 1971).
Thus, acute hepatocellular necrosis appears to result from the
interaction of aflatoxins at a number of intercellular sites whereas
the mutagenic (section 3.4.2.5) and carcinogenic (section 3.4.2.3)
properties of aflatoxins probably depend upon metabolic activation
to a DNA alkylating agent presumably the 2,3-epoxide (section
3.3.3).
3.4.2.7 Factors modifying the effects and dose-response
relationships of aflatoxins
Numerous reports have dealt with factors of various types that
modify the carcinogenic and other toxic effects of aflatoxins in
experimental animals. These include host factors, particularly the
sex-linked and endocrine characteristics, and interactions with
other environmental factors. The effects of nutrients deserve
particular attention in view of nutritional deficiencies occurring
in certain parts of the world, where aflatoxins exposure can be
considerable. The more recently discovered effect of exposure to
(artificial) sunlight might also be of interest in this respect.
The mechanisms by which hormones, nutrition, and other factors
influence aflatoxin carcinogenesis are not known but are thought to
include effects on DNA synthesis and cell division and
differentiation, and/or effects on aflatoxin metabolism, and
excretion. Animals with a severely restricted energy food intake do
not grow and are less susceptible to the action of many carcinogens
than normal animals. The retardation of growth induced by severe
protein deficiency and also by hypophysectomy may explain reduced
tumour incidence under these experimental conditions. Conversion of
aflatoxin B1 to a bacterial mutagen is different in microsomal
liver preparations from rats fed a marginal lipotrope diet compared
with those from normal rats. Excretion of mutagens in the urine is
also different in lipotrope-deficient rats (Suit et al., 1977).
Changes in aflatoxin B1 metabolism and reduced levels of
hepatic macro-molecule-bound aflatoxin B1 adducts were reported in
rats pretreated with phenobarbital (Garner, 1975; Swenson et al.,
1977), and also in hypophysectomized animals (Swenson et al., 1977).
Sex-linked differences and endocrine status. Several studies
indicate that in comparison with males, female rats are more
resistant to both acute toxic and carcinogenic effects of
aflatoxins. Thus, with a single administration of aflatoxin B1 by
gavage, the LD50 was estimated to be 7.2 mg/kg body weight
(fiducial limits 5.36-8.23) in male rats and 17.9 mg/kg body weight
(fiducial limits 14.4-22.5) in female rats (Butler, 1964). The
sex-dependent influence of vitamin A deficiency on the acute
toxicity of aflatoxins is discussed later in this section.
In another study (Newberne & Wogan, 1968a), Fischer rats of both
sexes were kept on diets containing aflatoxin B1 levels of 0.015,
0.3, or 1.0 mg/kg and killed successively for histological
examination. The early hepatic lesions, considered as precancerous,
appeared with almost the same rate of incidence and at approximately
the same time in both sexes; however, there was a considerably
longer period between the appearance of the pre-cancerous lesions
and progression to liver carcinomas in females than in males. At a
dietary level of aflatoxin B1 of 1 mg/kg, males developed
carcinomas after 35 weeks of exposure while tumours in females were
observed only after 64 weeks. A similar, if much less pronounced,
sex-linked difference was observed at the 2 lower aflatoxin levels.
Calculations of the approximate total intake of aflatoxin B1
before the appearance of tumours (based on average intake of food
containing a known quantity of aflatoxin) and the time over which
these total amounts were consumed are given in Table 10. In a study
by Ward et al. (1975), male rats (F344, NIH) kept on a diet
containing aflatoxin B1 at 2 mg/kg, died with malignant
haemorrhagic liver tumours significantly earlier than females.
Kidney tumours observed in male but not female rats exposed to
aflatoxins (Butler et al., 1969) have already been discussed in
section 3.4.2.3.
Newberne & Williams (1969) reported that fewer male rats
(Charles River CD) fed for more than a year on a diet containing
aflatoxin B1 at 0.2 mg/kg and diethylstilboestrol at 4 mg/kg
developed liver tumours (8/40) than those fed the same aflatoxin
diet without the estrogen (25/35).
In a study on the influence of hypophysectomy on aflatoxin
carcinogenesis, male albino (MRC) rats were fed a diet containing an
aflatoxin B1 level of 4 mg/kg. All of 14 control rats developed
liver tumours in 49 weeks whereas none of 14 hypophysectomized rats
developed liver tumours in the same period. However, tumours of
extrahepatic tissues (4/14 carcinomas of retro-orbital lacrimal
glands-see also section 3.4.2.3) were observed in the
hypophysectomized aflatoxin-treated rats (Goodall & Butler, 1969).
Table 10. Sex-linked difference in the carcinogenicity (liver cell
carcinoma) of dietary aflatoxin B1a
Dietary level Approximate total intake of Average time (days over
of aflatoxin B1 aflatoxin B1 before the which the total amount was
appearance of tumours consumed
males females males females
1 mg/kg 2.9 mg/rat 5.9 mg/rat 245 days 448 days
0.015 mg/kg 95 µg/rat 11 5 µg/rat 476 days 560 days
a Data from Newberne & Wogan (1968a).
Nutritional factors (food components). The influence of
nutritional factors on the effects and dose-response relationships
of aflatoxins has been reviewed by Wogan (1973, 1977), Newberne
(1974, 1976), Newberne & Rogers (1976), and Newberne & Gross (1977).
(a) Dietary protein and lipotropic agents. Rhesus monkeys were
given daily doses of 100 µg aflatoxin per animal by stomach tube.
Two animals, fed a severely protein-deficient ration (1% casein) for
8 weeks before and during aflatoxin administration, developed fatty
liver and biliary proliferation and fibrosis and died with
gastrointestinal haemorrhage within 30 days of aflatoxin treatment.
Two animals fed a control ration (16% casein) survived in apparent
good health up to the termination of the experiment (35 days of
aflatoxin treatment) (Madhavan et al., 1965a).
In a study on weanling male rats given 50 µg aflatoxin per
animal per day for 20 days, 2/6 animals, fed a diet containing 4%
casein, died on days 18 and 19 of the experiment. Extensive liver
damage was found in these rats and in others in the 4%a casein
group within 20 days of aflatoxin treatment. All 12 rats fed a diet
containing 20% casein survived similar aflatoxin dosing with only
mild changes in the liver (Madhavan & Gopalan, 1965). In another
study by Madhavan & Gopalan (1968) 2 groups of 12 male rats were fed
the 2 diets (5%a or 20% casein) for 2 years from weaning and were
given, from the beginning of the experiment, 232 daily doses of
5 µg aflatoxin per animal or 225 daily doses of 10 µg aflatoxin per
animal. All 12 animals fed the 20% casein diet survived the period
of aflatoxin dosing and 50% developed hepatomas; lung metastases
were observed in 2 of these rats. Five of the 12 animals fed on the
5% casein diet died during the period of aflatoxin dosing. No
hepatomas but one renal cell carcinoma were found in the remaining 7
rats. Both diets used in these experiments were supplemented only
with 0.01% choline.
In experiments by Newberne & Wogan (1968b), rats fed a diet
containing 9% protein developed a higher incidence of liver tumours
(11/15) in a shorter period of time (8 months) than rats fed a diet
containing 22% protein (incidence 7/14 after 10 months). Both groups
of rats were given a total dose of 375 µg of aflatoxin B1 per
animal by gastric intubation, over 3 weeks, at the beginning of the
experiment.
In studies examining lipotropic effects on aflatoxin activity, a
diet marginal in methionine and choline, deficient in folate, and
high in fat protected male rats against the acute toxicity of a
single dose of aflatoxin B1; however, susceptibility to the toxic
effects of repeated doses of aflatoxin B1 increased, and the
carcinogenicity of aflatoxin B1 was enhanced. The experimental
diet (Rogers & Newberne, 1971; Rogers, 1975) contained peanut meal
(12%, alcohol extracted), gelatine (6%), casein (3%, vitamin free)
and fibrin (1%) as sources of protein, with a supplement of
L-cystine (0.5%). This diet is marginally but not severely deficient
in threonine, tryptophan, and arginine as well as in methionine. The
diet contained choline chloride (0.2%), and was high in fat (beef
fat 30%; corn oil 2%). The control, nutritionally complete diet
contained casein (22%, vitamin free) as the protein source, 15% or
16% oil (corn or mixed vegetable oil) and 0.3% choline chloride.
Both diets were adequate in other essential nutrients.
a According to Madhavan & Gopalan (1968) the composition of the
diets used in both studies was the same. However, their 1965 paper
gives 4% whereas that of 1968 gives 5% as the level of casein in the
diet.
As shown in Table 11, the marginal lipotrope diet fed for 2
weeks protected rats against acute aflatoxin B11 toxicity. The
rats that died had haemorrhagic necrosis of the liver with various
degrees of bile duct proliferation and, occasionally, haemorrhagic
necrosis in the adrenals and kidneys. The surviving rats (killed 2
weeks after aflatoxin administration) had focal necrosis of
hepatocytes, bile duct proliferation, and an increase in the size of
periportal hepatocytes. Approximately 20% of the rats fed the
marginal lipotrope diet and given a single dose of aflatoxin B1
had focal areas of abnormal hepatocytes which showed an increased
uptake of 3H thymidine (section 3.4.2.3) (Rogers & Newberne,
1971).
Table 11. Effect of a marginal lipotrope diet on the acute toxicity of
aflatoxin B1 in rata
Aflatoxin B1 Route of Diet No. of Mortality
(mg/kg) administration rats at 2 weeks
(%)
Sprague-Dawley (males)
7 Intragastric control 5 60
marginal lipotrope 5 0
9 Intragastric control 5 80
marginal lipotrope 10 0
7 Intraperitoneal control 5 100
marginal lipotrope 5 0
Fischer (males)
7 intragastric control 10 100
marginal lipotrope 10 0
a From: Rogers & Newberne (1971).
Although resistant to the toxicity of a single dose of aflatoxin
B1, rats fed the marginal lipotrope diet for 2 weeks before and
also during repeated aflatoxin exposure (136 males) were highly
sensitive to repeated daily doses of aflatoxin B1 at 25 µg per
animal. One half of these rats died, most of them after having
received 8 or 9 doses of aflatoxin B1 (200 or 225 µg total).
Various degrees of necrosis were observed in the livers with
extensive proliferation of bile duct cells. The mortality in rats
fed the control diet (66 males) was only 4% during the
administration of the total dose of 350 lag of aflatoxin B1
(Rogers & Newberne, 1971).
Enhanced aflatoxin B1 carcinogenicity in rats fed marginal
lipotrope diets, observed repeatedly in several experiments reviewed
by Newberne & Gross (1977), is demonstrated in Fig. 4 (Rogers,
1975). Cumulative probability of death from a tumour was calculated
here by the method described by Saffiotti et al. (1972) i.e., from
the number of animals at risk and the number of deaths from tumours
each week. A total dose of aflatoxin B1 of 375 µg, divided in 25
intragastric doses of 15 µg/animal per day over 7 weeks, was given
to male Fischer rats. Hepatocarcinomas developed in 87% of 52
animals fed the marginal lipotrope diet and in only 11% of 27 rats
fed the nutritionally complete diet ( P < 0.001). Twenty-seven
percent of tumours in rats fed the marginal diet metastasized to
other abdominal organs or the lung; no metastases were detected in
rats fed the control diet (Rogers, 1975). The groundnut meal fed in
these experiments did not contain detectable aflatoxins.
 Further studies were carried out to determine how far the high
fat content of the marginal lipotrope diet contributed to the
enhancement of carcinogenesis. Male Fischer rats were fed the
marginal lipotrope diet, the nutritionally complete control diet, or
the control diet with substitution of the fat from the marginal
lipotrope diet (30% beef fat, 2% corn oil) for the fat in the
control diet (15% mixed vegetable oils). Each rat was given a total
of 375 µg of aflatoxin B1 intragastrically, in divided doses over
7 weeks and kept until moribund or dead, or until 90 weeks after
treatment, and then necropsied. Hepatocarcinoma incidence was based
on the number of rats that survived until the first death with
hepatocarcinoma, i.e., 27-34 rats per group. Incidences were found
of 39% in rats fed the marginal lipotrope diet, 15% in control rats,
and zero in rats fed the control diet with beef fat (30%) and corn
oil (2%) substituted for vegetable oil (15%) (Rogers et al.,
unpublished data). Thus the high fat content of the deficient diet
inhibited, rather than contributed to the enhancement of aflatoxin
carcinogenesis.
An enhancing effect on aflatoxin carcinogenicity was observed in
the experiments in which male rats (Charles River CD Sprague-Dawley)
were fed a low lipotrope diet containing 20% isolated soybean
protein and supplemented with 0.1% DL-methionine and 0.1% choline
chloride. Aflatoxin B1 was given intragastrically during the early
weeks of the experiment in a total dose of 240 µg/animal (divided in
24 daily doses of 10 µg, 5 days a week). Liver cell carcinomas were
observed 5/17 animals fed this diet. No tumours were found in rats
given the same dose of aflatoxin B1 and fed the same basal diet
supplemented with 0.6% DL-methionine, 0.6% choline chloride, and
vitamin B12 (50 µg/kg diet) (Newberne et al., 1968).
It should be noted that a more severe lipotrope deficiency may
decrease rather than increase the incidence of liver carcinoma in
aflatoxin-treated rats. A decrease in liver carcinoma was observed
in aflatoxin-treated, male, Sprague-Dawley rats with severe
lipotrope deficiency particularly if penicillin, at a level of 0.1%
were added to the diet. The expected penicillin-induced inhibition
of cirrhosis development was not observed and the interactions
between penicillin and aflatoxins have not been elucidated. Both
diets used in these experiments, the control, adequate, and the
highly lipo-trope-deficient contained alcohol-extracted groundnut
meal (25%) and casein (6%) as protein source. No cystine or
methionine was added, and choline and vitamin B12 were added to
the control diet at levels of 0.3% and 50 µg/kg, respectively. When
rats were killed 12 months after receiving a total dose of 375 tag
aflatoxin B1 per animal (divided into 15 daily intra-gastric doses
of 25 µg per animal), hepatomas were found in 64% (9/14) control
animals and in 41% (7/17) lipotrope-deficient animals. In rats fed
diets containing penicillin, hepatomas were found in 70% (14/20) of
the controls and in only 17% (3/18) of the lipotrope-deficient
animals (Newberne & Rogers, 1971).
The different effects of marginal and severe lipotrope
deficiencies on aflatoxin carcinogenesis are of interest in
connexion with apparent discrepancies between different studies on
aflatoxin carcinogenesis in rats fed protein-deficient diets
containing different levels of lipotropic agents discussed earlier.
Vitamin B12 is a weakly lipotropic factor that has recently
been found to increase tumour incidence in aflatoxin-treated rats.
In a study by Temcharoen et at. (1978), male Fischer rats were fed
diets containing 20% or 5% casein with or without vitamin B12
(50 µg/kg diet) for 33 weeks. The composition of the diets, with the
exception of the contents of casein (and dextrin) and vitamin B12,
was similar to that used in previous studies by Wogan & Newberne
(1967) and Newberne & Wogan (1968a). Choline chloride was added at
the level 0.036%. The casein content was similar to that in diets
used in the study by Madhavan & Gopalan (1968) mentioned earlier. A
mixture of crystalline aflatoxins (containing aflatoxins B1 and
G1 approximately in the proportion of 1: 1 and about 5% of
aflatoxins B2 and G2) was added to the diets at the level of
1 mg/kg. As shown in Table 12, vitamin B12 supplementation
increased liver rumour incidence in rats fed a diet containing
aflatoxin and 20% casein. Severe protein deficiency affected the
growth of the animals, the body weight of rats fed 5% casein being
reduced to about one third of the controls at the termination of the
experiment. Liver cirrhosis was observed only in the
aflatoxin-treated, protein-deficient rats. As suggested by
Temcharoen et al. (1978), a high incidence of hyperplastic nodules
and cholangiofibrosis in the protein-depleted, aflatoxin-treated
animals may indicate that the carcinogenic process was retarded but
not entirely absent.
(b) Vitamin A. In a study by Reddy et al. (1973), male and
female albino rats fed a vitamin A-deficient diet for 9 weeks after
weaning or fed the same diet with a daily oral supplement of 30 µg
(100 IU) of vitamin A/ rat were given a preparation of crystalline
aflatoxins containing aflatoxins B1 (44%), G1 (44%), and B2
and G2 (2%)in a single intraperitoneal dose of 3.5 mg/kg body
weight. High mortality was observed in vitamin A-deficient males
(Table 13). The vitamin A-deficient females and all the vitamin
A-supplemented animals did not show any adverse reactions 40 h after
aflatoxin injection. Histologically, severe liver damage was
observed in vitamin A-deficient, aflatoxin-treated male rats, in
contrast to minimal liver damage in female rats and male rats given
vitamin A.
Table 12. The effects of dietary protein and vitamin B12 on aflatoxin-induced liver changesa
Experimental diet No. of animals Average bodyb No. of animals with
(for details see text) at the beginning/ weight (g) at
termination of the termination cholangiofibrosis cirrhosis hyperplastic hepatoma
the experiment of the nodules
experiment
1. 5% casein 12/6 85 0 0 0 0
2. 5%casein + B12 12/12 91 0 0 0 0
3. 5% casein + aflatoxins 25/23 125 9 21 17 3
4. 5% casein + B12 + 25/24 129 8 12 15 1
aflatoxins
5. 20% casein 12/9 328 0 0 0 0
6. 20% casein + B12 12/10 369 0 0 0 0
7. 20% casein + aflatoxins 25/24 409 0 0 0 1
8. 20% casein + B12 + 25/25 375 0 0 4 6
aflatoxins
a Modified from: Temcharoen et al. (1978).
b Calculated from data of the authors on average liver weight and liver weight/100 g body weight.
In a study on rats fed diets containing aflatoxin B1 in the
range of 15-100 µg/kg and vitamin A in deficient, adequate, or
excessive levels over a 2-year period, the vitamin A-deficient
animals developed a similar incidence of liver tumours to the other
2 groups but had an increased incidence of colon carcinomas
(Newberne & Rogers, 1973). Thus, in a group of 50 male rats (Charles
River CD Sprague-Dawley) exposed to a dietary level of aflatoxin
B1 of 100 µg/kg and 5 µg vitamin A (retinyl palmitate) per animal
per day, colon tumours and liver tumours were observed in 6 and 11
rats, respectively, whereas with daily intakes of 50 or 500 µg
retinyl palmitate per rat, no colon cancers were observed and the
incidence of liver tumours was 24/50 or 19/50, respectively. The
results of a further study (Newberne & Suphakarn, 1977) are shown in
Table 14. Charles River CD Sprague-Dawley male (M) and female (F)
rats were fed a diet containing an aflatoxin B1 level of 1 mg/kg
(AFB1) and 3 different dietary levels of vitamin A (retinyl
acetate). Again, there was an increased incidence of colon
carcinomas in vitamin A-deficient rats. Excessive vitamin A did not
give protection against aflatoxin carcinogenesis in either the liver
or the colon.
Table 13. The effect of vitamin A status on the acute toxicity of aflatoxins
in male and female ratsa
Sex Vitamin A No. of Mortality Vitamin A
daily supplement animals 40 h after liver content
(IU/rat per day) aflatoxin (IU/whole liver)
injection means ± SEMc
Male 0 6 100% 36.5 ± 6.16b
100d 6 0% 2128.1 ± 153.56
Female 0 6 0% 18.6 ± 3.42
100d 6 0% 2299.1 ± 111.49
a From: Reddy et al. (1973).
b Vitamin A values for 5 animals only.
c SEM = standard error of the mean.
d 30µg.
Table 14. Vitamin A status, aflatoxin B1, and liver and colon
tumors in rats
Dietary AFB1 No. Sex Tumour incidence (%)
retinyl rats at
acetate risk liver colon both
(mg/kg)
control
3.0 0 24 M 0.0 0.0 0.0
3.0 0 26 F 0.0 0.0 0.0
3.0 + 24 M 87.5 4.1 4.1
3.0 + 24 F 79.1 8.3 8.3
low
0.3 0 10 M 0.0 0.0 0.0
0.3 0 12 F 0.0 0.0 0.0
0.3 + 66 M 89.4 28.8 25.7
0.3 + 42 F 76.2 28.6 11.9
high
30.0 0 23 M 0.0 0.0 0.0
30.0 0 20 F 0.0 0.0 0.0
30.0 + 26 M 92.3 7.7 7.7
30.0 + 31 F 83.9 9.7 6.4
From: Newberne & Suphakarn (1977).
(c) Selenium. A single oral dose of aflatoxin B1 given to
rats at 7 mg/kg bodyweight was less toxic in animals fed a high
selenium (selenite) diet containing selenium at 1 mg/kg (2-week
mortality 7/28) than in animals fed diets adequate or marginal in
selenium, containing selenium (selenite) at 0.1 or 0.03 mg/kg feed,
respectively (2-week mortalities 20/20 and 28/29). However, a
further increase in selenium intake (5 mg/kg feed) reaching toxic
levels predisposed the liver to aflatoxin injury and together with
aflatoxin exposure resulted in kidney lesions (tubular necrosis at
the cortico-medullary junction) (Newberne & Conner, 1974). The
incidence of liver tumours in Sprague-Dawley rats given a total of
500 µg of aflatoxin B1 intragastrically, over a 4-week period, was
not influenced by dietary selenium (as selenite) contents ranging
from 0.03 to 5.0 mg/kg (Grant et al., 1977).
(d) Cyclopropenoid fatty acids (CPFA). Cyclopropenoid fatty
acids (CPFA) which occur for example in cottonseed oil, enhanced
tumour induction in trout by both aflatoxin B1 and aflatoxin M1
(Sinnhuber et al., 1968, 1974). Young trout were fed a purified diet
which contained an aflatoxin B1 concentration of 4 µg/kg with or
without the addition of CPFA at 220 mg/kg diet. Hepatomas were found
in 27/30 fish fed CPFA and necropsied after 6 months; at 9 months,
20/20 bore hepatomas. Corresponding incidences of hepatomas in fish
that did not receive CPFA were 0/30 and 4/20, respectively
(Sinnhuber et al., 1968). In later experiments, fish were fed
aflatoxin M1 at the rate of 4 µg/kg diet, with or without the
addition of CPFA at 100 mg/kg. The incidences of hepatomas in
CPFA-fed fish were 6/40 at 4 months and 42/63 at 12 months.
Corresponding incidences in fish not fed CPFA were 2/40 and 6/40
respectively (Sinnhuber et al., 1974).
On the other hand, the enhancing effect of CPFA on aflatoxin
hepato-carcinogenesis was not clearly evident in several studies on
rats (Friedman & Molar, 1968; Lee et al., 1969a; Nixon et al.,
1974). No significant increase in liver tumours was observed in
Wistar male (M) and female (F) rats when sources of CPFA such as;
food grade cottonseed oil (CSO) or Sterculia foetida oil (SFO)
were added at levels of 10% and 0.04%, respectively, to diets
containing aflatoxin B1 at concentrations of 20 or 100 µg/kg.
Feeding these diets for different periods (generally exceeding 500
days) at the aflatoxin level of 20 µg/kg resulted in hepatomas in
4/36 rats M: 2/17; F: 2/19) with CSO exposure, in 1/37 rats
(F: 1/19) with SFO exposure, and in 0/38 rats without CSO or SFO in
the diet. With an aflatoxin B1 exposure level of 100 µg/kg diet,
the incidences of hepatomas in the CSO group, SFO group, and the
group without CSO or SFO were 15/36 (M: 12/17; F: 3/19), 17/37
(M: 10/17; F: 7/18), and 15/35 (M: 7/17; F: 8/18), respectively. In
Fischer rats, CSO was tested only in combination with the lower
concentration of aflatoxin; with an aflatoxin B1 level of
20 µg/kg diet, the hepatoma incidence was 6/31 (M: 4/15; F: 2/16)
with CSO, and 6/28 (M: 5/13; F: 1/15) without CSO exposure (Nixon et
al., 1974).
Other chemicals. Sodium phenobarbital given to rats in the
drinking water (1 g/litre) for 9 weeks, together with a diet
contaminated with aflatoxin B1 (at the level of approximately
5 mg/kg) resulted in a lower incidence (11/20) and delayed
appearance of liver tumours within the following 2 years compared
with the incidence in rats fed aflatoxin alone (17/20) (McLean &
Marshall, 1971). The effects of phenobarbital were confirmed in
experiments by Swenson et al. (1977) in which 2 groups of 18 male
Fischer rats were each given a diet containing aflatoxin B1 at a
level of 0.3 mg/kg and drinking water with or without sodium
phenobarbital (1 g/litre) for a period of 15 months. Examination by
laparotomy at the end of aflatoxin exposure revealed liver tumours
in 11 aflatoxin-treated controls (61%) and in only 2 (15%) rats
exposed to aflatoxin with phenobarbital. When the experiment was
terminated 5 months later, hepatocellular carcinomas were detected
in 18 (100%) aflatoxin-exposed rats and in 12 (67%) rats given
aflatoxin and phenobarbital. On the other hand, no effect on
aflatoxin hepatocarcinogenesis was observed in rats fed a similar
aflatoxin diet with the addition of benz (a)anthracene (70 mg/kg)
or ascorbic acid (25 g/kg) (Swenson et al., 1977).
Viral infection. Liver tumours were observed in 2/7 marmosets
fed aflatoxin B1 at a concentration of 2 mg/kg feed and injected
with hepatitis-type candidate virus (G. Barker strain) during
aflatoxin exposure. The animals survived 3-94 weeks of treatment.
Tumours were found in 3/9 marmosets given the aflatoxin diet only
(Lin et al., 1974). Exposure to both agents produced more severe
effects on the liver (cirrhosis) than exposure to aflatoxin B1
alone.
Exposure to artificial sunlight. The effects of exposure to
artificial sunlight on acute aflatoxin toxicity (Newberne et al.,
1974) and carcinogenicity (Joseph-Bravo et al., 1976) were studied
recently, using a long-arc xenon source and filter combination,
which had a spectral distribution in the ultraviolet and visible
ranges closely approximating the 6000 K colour temperature of
natural light. The rats, located 1.5 metres from the source, were
kept at an illumination level of about 29 600 1x corresponding to an
irradiance level of approximately 160 W/m2 over a 2-h period. In
groups consisting of 40 Sprague-Dawley male rats each, 18 control
rats died within 2 weeks of a single intragastric dose of aflatoxin
B1 at 7 mg/kg body weight whereas 23 of the rats exposed to
artificial sunlight for 2 h after aflatoxin administration died.
When an excessive dose of riboflavin was given intra-gastrically 30
min before aflatoxin, the corresponding 2-week mortalities in
animals unexposed and exposed to artificial sunlight were 20/40 and
30/40, respectively (Newberne et al., 1974). In the second study, a
total dose of 375 µg of aflatoxin B1 was given to male
Sprague-Dawley Charles River CD rats, in the form of 15 doses of
25 µg per rat, administered intra-gastrically over a 3-week period.
Thirty min after each aflatoxin administration, half of the animals
were exposed for 2 h to artificial sunlight as described earlier.
When the animals were killed 53 weeks after the last aflatoxin dose,
benign or malignant liver tumours were found in all 11
non-irradiated animals and in only 5/12 of the irradiated group
(Joseph-Bravo et al., 1976).
3.5 Effects in Man---Epidemiological and Clinical Studies
3.5.1 General population studies
3.5.1.1 Liver carcinogenesis
The data on aflatoxins and human cancer available before October
1975 were reviewed by IARC (1976) and several other reviews have
been published more recently (Linsell & Peers, 1977; Shank, 1977;
Van Rensburg, 1977). A positive association between aflatoxin
ingestion and liver cancer in man has been found in population
studies in which estimates of aflatoxin intake and the incidence of
primary liver cancer were made concurrently.
In a study in different parts of Uganda, it was found that
increased frequencies of detectable aflatoxin contamination of food
samples (range: 10.8%-43%) were associated with increased incidence
of primary liver cancer (range: 1.4-15.0 cases per 100 000 total
population per year) (Alpert et al., 1971). Four hundred and eighty
samples of foods were analysed from 8 areas in Uganda; the total
intake was not calculated. Within Swaziland, Keen & Martin
(1971 a, b) showed regional differences in liver cancer frequencies
consistent with regional differences in the frequency of aflatoxin
contamination of groundnuts. From the results of a questionnaire
study, they also suggested that tribal differences in the
preparation of groundnuts for food, and in eating habits, resulting
in higher aflatoxin exposure, could explain the apparently higher
liver cancer rate in the Shangaans living in Swaziland compared with
the Swazis.a
In the studies conducted by Shank et al. (1972a,b) in Thailand,
Peers & Linsell (1973) in Kenya, Van Rensburg et al. (1974) in
Mozambique, and Peers et al. (1976) in Swaziland, actual
concentrations of aflatoxin in meals about to be eaten (food on the
plate) were related to the incidence of primary liver cancer in the
areas where the meal samples were collected. These studies are
summarized in Table 15. A linear regression between the incidence of
liver cancer and the logarithm to the base 10 of the estimated
dietary intake of aflatoxin was found within the range of the
aflatoxin exposure levels and the liver cancer incidence rates
existing in the areas studied? Within Kenya and Swaziland, Peers &
Linsell (1977) demonstrated a steeper rise in liver cancer incidence
with increasing aflatoxin intake in men than in women (Fig. 5). A
similar difference seems to exist also in the other areas studied
(Shank, 1977).
a A positive association of fiver cancer incidence variations with
the availability of aflatoxin contaminated staple foods was also
reported recently from the Philippines (Bulatao-Jayme et al., 1976).
Table 15. Summary of available data on aflatoxin ingestion levels and primary
liver cancer incidencea
Country Area Aflatoxin Liver cancer
Estimated average
daily intakeb in No. of Incidence
adults--ng/kg body cases per 105
weight per day registered of total
population
per year
Kenya High altitude 3.5 4 1.2
Thailand Songkhla 5.0 2 2.0
Swaziland High veld 5.1 11 2.2
Kenya Middle altitude 5.9 33 2.5
Swaziland Mid veld 8.9 29 3.8
Kenya Low altitude 10.0 49 4.0
Swaziland Lebombo 15.4 4 4.3
Thailand Ratburi 45.0 6 6.0
Swaziland Low veld 43.1 42 9.2
Mozambique Inhambanec 222.1 --d 13.0
a From: Peers & Linsell (1977).
b Excluding any aflatoxin present in native beers.
c Revised incidence estimate taken from Van Rensburg (1977).
d Number of cases not available, probably >100.
The possibility that hepatitis B virus infection may confound
the relationship between aflatoxin ingestion and liver cancer
incidence has been considered (Linsell & Peers, 1977). Hepatitis B
infection is common in countries with a high incidence of primary
liver cancer and evidence of prior exposure to hepatitis B virus is
more common in individuals with liver cancer in these countries than
in normal subjects (Vogel et al., 1970; Reys & Sequeira, 1974;
Prince et al., 1975; Chainuvati et al., 1975). Nevertheless, the
present evidence favours aflatoxin as a possible major disease
determinant in primary liver cancer but hepatitis B virus may well
be a cofactor in the etiology (Peers & Linsell, 1977).
a When Stoloff & Friedman (1976) compared published reports on the
incidence of cancer in rural and urban areas of southeastern USA,
and in southeast states compared with other areas in the USA, they
found lower incidences of liver cancer in the rural areas and in the
southeast states, respectively, even though they expected that
long-term exposure to aflatoxins would be higher in these areas.
Further studies were carried out to determine how far the high
fat content of the marginal lipotrope diet contributed to the
enhancement of carcinogenesis. Male Fischer rats were fed the
marginal lipotrope diet, the nutritionally complete control diet, or
the control diet with substitution of the fat from the marginal
lipotrope diet (30% beef fat, 2% corn oil) for the fat in the
control diet (15% mixed vegetable oils). Each rat was given a total
of 375 µg of aflatoxin B1 intragastrically, in divided doses over
7 weeks and kept until moribund or dead, or until 90 weeks after
treatment, and then necropsied. Hepatocarcinoma incidence was based
on the number of rats that survived until the first death with
hepatocarcinoma, i.e., 27-34 rats per group. Incidences were found
of 39% in rats fed the marginal lipotrope diet, 15% in control rats,
and zero in rats fed the control diet with beef fat (30%) and corn
oil (2%) substituted for vegetable oil (15%) (Rogers et al.,
unpublished data). Thus the high fat content of the deficient diet
inhibited, rather than contributed to the enhancement of aflatoxin
carcinogenesis.
An enhancing effect on aflatoxin carcinogenicity was observed in
the experiments in which male rats (Charles River CD Sprague-Dawley)
were fed a low lipotrope diet containing 20% isolated soybean
protein and supplemented with 0.1% DL-methionine and 0.1% choline
chloride. Aflatoxin B1 was given intragastrically during the early
weeks of the experiment in a total dose of 240 µg/animal (divided in
24 daily doses of 10 µg, 5 days a week). Liver cell carcinomas were
observed 5/17 animals fed this diet. No tumours were found in rats
given the same dose of aflatoxin B1 and fed the same basal diet
supplemented with 0.6% DL-methionine, 0.6% choline chloride, and
vitamin B12 (50 µg/kg diet) (Newberne et al., 1968).
It should be noted that a more severe lipotrope deficiency may
decrease rather than increase the incidence of liver carcinoma in
aflatoxin-treated rats. A decrease in liver carcinoma was observed
in aflatoxin-treated, male, Sprague-Dawley rats with severe
lipotrope deficiency particularly if penicillin, at a level of 0.1%
were added to the diet. The expected penicillin-induced inhibition
of cirrhosis development was not observed and the interactions
between penicillin and aflatoxins have not been elucidated. Both
diets used in these experiments, the control, adequate, and the
highly lipo-trope-deficient contained alcohol-extracted groundnut
meal (25%) and casein (6%) as protein source. No cystine or
methionine was added, and choline and vitamin B12 were added to
the control diet at levels of 0.3% and 50 µg/kg, respectively. When
rats were killed 12 months after receiving a total dose of 375 tag
aflatoxin B1 per animal (divided into 15 daily intra-gastric doses
of 25 µg per animal), hepatomas were found in 64% (9/14) control
animals and in 41% (7/17) lipotrope-deficient animals. In rats fed
diets containing penicillin, hepatomas were found in 70% (14/20) of
the controls and in only 17% (3/18) of the lipotrope-deficient
animals (Newberne & Rogers, 1971).
The different effects of marginal and severe lipotrope
deficiencies on aflatoxin carcinogenesis are of interest in
connexion with apparent discrepancies between different studies on
aflatoxin carcinogenesis in rats fed protein-deficient diets
containing different levels of lipotropic agents discussed earlier.
Vitamin B12 is a weakly lipotropic factor that has recently
been found to increase tumour incidence in aflatoxin-treated rats.
In a study by Temcharoen et at. (1978), male Fischer rats were fed
diets containing 20% or 5% casein with or without vitamin B12
(50 µg/kg diet) for 33 weeks. The composition of the diets, with the
exception of the contents of casein (and dextrin) and vitamin B12,
was similar to that used in previous studies by Wogan & Newberne
(1967) and Newberne & Wogan (1968a). Choline chloride was added at
the level 0.036%. The casein content was similar to that in diets
used in the study by Madhavan & Gopalan (1968) mentioned earlier. A
mixture of crystalline aflatoxins (containing aflatoxins B1 and
G1 approximately in the proportion of 1: 1 and about 5% of
aflatoxins B2 and G2) was added to the diets at the level of
1 mg/kg. As shown in Table 12, vitamin B12 supplementation
increased liver rumour incidence in rats fed a diet containing
aflatoxin and 20% casein. Severe protein deficiency affected the
growth of the animals, the body weight of rats fed 5% casein being
reduced to about one third of the controls at the termination of the
experiment. Liver cirrhosis was observed only in the
aflatoxin-treated, protein-deficient rats. As suggested by
Temcharoen et al. (1978), a high incidence of hyperplastic nodules
and cholangiofibrosis in the protein-depleted, aflatoxin-treated
animals may indicate that the carcinogenic process was retarded but
not entirely absent.
(b) Vitamin A. In a study by Reddy et al. (1973), male and
female albino rats fed a vitamin A-deficient diet for 9 weeks after
weaning or fed the same diet with a daily oral supplement of 30 µg
(100 IU) of vitamin A/ rat were given a preparation of crystalline
aflatoxins containing aflatoxins B1 (44%), G1 (44%), and B2
and G2 (2%)in a single intraperitoneal dose of 3.5 mg/kg body
weight. High mortality was observed in vitamin A-deficient males
(Table 13). The vitamin A-deficient females and all the vitamin
A-supplemented animals did not show any adverse reactions 40 h after
aflatoxin injection. Histologically, severe liver damage was
observed in vitamin A-deficient, aflatoxin-treated male rats, in
contrast to minimal liver damage in female rats and male rats given
vitamin A.
Table 12. The effects of dietary protein and vitamin B12 on aflatoxin-induced liver changesa
Experimental diet No. of animals Average bodyb No. of animals with
(for details see text) at the beginning/ weight (g) at
termination of the termination cholangiofibrosis cirrhosis hyperplastic hepatoma
the experiment of the nodules
experiment
1. 5% casein 12/6 85 0 0 0 0
2. 5%casein + B12 12/12 91 0 0 0 0
3. 5% casein + aflatoxins 25/23 125 9 21 17 3
4. 5% casein + B12 + 25/24 129 8 12 15 1
aflatoxins
5. 20% casein 12/9 328 0 0 0 0
6. 20% casein + B12 12/10 369 0 0 0 0
7. 20% casein + aflatoxins 25/24 409 0 0 0 1
8. 20% casein + B12 + 25/25 375 0 0 4 6
aflatoxins
a Modified from: Temcharoen et al. (1978).
b Calculated from data of the authors on average liver weight and liver weight/100 g body weight.
In a study on rats fed diets containing aflatoxin B1 in the
range of 15-100 µg/kg and vitamin A in deficient, adequate, or
excessive levels over a 2-year period, the vitamin A-deficient
animals developed a similar incidence of liver tumours to the other
2 groups but had an increased incidence of colon carcinomas
(Newberne & Rogers, 1973). Thus, in a group of 50 male rats (Charles
River CD Sprague-Dawley) exposed to a dietary level of aflatoxin
B1 of 100 µg/kg and 5 µg vitamin A (retinyl palmitate) per animal
per day, colon tumours and liver tumours were observed in 6 and 11
rats, respectively, whereas with daily intakes of 50 or 500 µg
retinyl palmitate per rat, no colon cancers were observed and the
incidence of liver tumours was 24/50 or 19/50, respectively. The
results of a further study (Newberne & Suphakarn, 1977) are shown in
Table 14. Charles River CD Sprague-Dawley male (M) and female (F)
rats were fed a diet containing an aflatoxin B1 level of 1 mg/kg
(AFB1) and 3 different dietary levels of vitamin A (retinyl
acetate). Again, there was an increased incidence of colon
carcinomas in vitamin A-deficient rats. Excessive vitamin A did not
give protection against aflatoxin carcinogenesis in either the liver
or the colon.
Table 13. The effect of vitamin A status on the acute toxicity of aflatoxins
in male and female ratsa
Sex Vitamin A No. of Mortality Vitamin A
daily supplement animals 40 h after liver content
(IU/rat per day) aflatoxin (IU/whole liver)
injection means ± SEMc
Male 0 6 100% 36.5 ± 6.16b
100d 6 0% 2128.1 ± 153.56
Female 0 6 0% 18.6 ± 3.42
100d 6 0% 2299.1 ± 111.49
a From: Reddy et al. (1973).
b Vitamin A values for 5 animals only.
c SEM = standard error of the mean.
d 30µg.
Table 14. Vitamin A status, aflatoxin B1, and liver and colon
tumors in rats
Dietary AFB1 No. Sex Tumour incidence (%)
retinyl rats at
acetate risk liver colon both
(mg/kg)
control
3.0 0 24 M 0.0 0.0 0.0
3.0 0 26 F 0.0 0.0 0.0
3.0 + 24 M 87.5 4.1 4.1
3.0 + 24 F 79.1 8.3 8.3
low
0.3 0 10 M 0.0 0.0 0.0
0.3 0 12 F 0.0 0.0 0.0
0.3 + 66 M 89.4 28.8 25.7
0.3 + 42 F 76.2 28.6 11.9
high
30.0 0 23 M 0.0 0.0 0.0
30.0 0 20 F 0.0 0.0 0.0
30.0 + 26 M 92.3 7.7 7.7
30.0 + 31 F 83.9 9.7 6.4
From: Newberne & Suphakarn (1977).
(c) Selenium. A single oral dose of aflatoxin B1 given to
rats at 7 mg/kg bodyweight was less toxic in animals fed a high
selenium (selenite) diet containing selenium at 1 mg/kg (2-week
mortality 7/28) than in animals fed diets adequate or marginal in
selenium, containing selenium (selenite) at 0.1 or 0.03 mg/kg feed,
respectively (2-week mortalities 20/20 and 28/29). However, a
further increase in selenium intake (5 mg/kg feed) reaching toxic
levels predisposed the liver to aflatoxin injury and together with
aflatoxin exposure resulted in kidney lesions (tubular necrosis at
the cortico-medullary junction) (Newberne & Conner, 1974). The
incidence of liver tumours in Sprague-Dawley rats given a total of
500 µg of aflatoxin B1 intragastrically, over a 4-week period, was
not influenced by dietary selenium (as selenite) contents ranging
from 0.03 to 5.0 mg/kg (Grant et al., 1977).
(d) Cyclopropenoid fatty acids (CPFA). Cyclopropenoid fatty
acids (CPFA) which occur for example in cottonseed oil, enhanced
tumour induction in trout by both aflatoxin B1 and aflatoxin M1
(Sinnhuber et al., 1968, 1974). Young trout were fed a purified diet
which contained an aflatoxin B1 concentration of 4 µg/kg with or
without the addition of CPFA at 220 mg/kg diet. Hepatomas were found
in 27/30 fish fed CPFA and necropsied after 6 months; at 9 months,
20/20 bore hepatomas. Corresponding incidences of hepatomas in fish
that did not receive CPFA were 0/30 and 4/20, respectively
(Sinnhuber et al., 1968). In later experiments, fish were fed
aflatoxin M1 at the rate of 4 µg/kg diet, with or without the
addition of CPFA at 100 mg/kg. The incidences of hepatomas in
CPFA-fed fish were 6/40 at 4 months and 42/63 at 12 months.
Corresponding incidences in fish not fed CPFA were 2/40 and 6/40
respectively (Sinnhuber et al., 1974).
On the other hand, the enhancing effect of CPFA on aflatoxin
hepato-carcinogenesis was not clearly evident in several studies on
rats (Friedman & Molar, 1968; Lee et al., 1969a; Nixon et al.,
1974). No significant increase in liver tumours was observed in
Wistar male (M) and female (F) rats when sources of CPFA such as;
food grade cottonseed oil (CSO) or Sterculia foetida oil (SFO)
were added at levels of 10% and 0.04%, respectively, to diets
containing aflatoxin B1 at concentrations of 20 or 100 µg/kg.
Feeding these diets for different periods (generally exceeding 500
days) at the aflatoxin level of 20 µg/kg resulted in hepatomas in
4/36 rats M: 2/17; F: 2/19) with CSO exposure, in 1/37 rats
(F: 1/19) with SFO exposure, and in 0/38 rats without CSO or SFO in
the diet. With an aflatoxin B1 exposure level of 100 µg/kg diet,
the incidences of hepatomas in the CSO group, SFO group, and the
group without CSO or SFO were 15/36 (M: 12/17; F: 3/19), 17/37
(M: 10/17; F: 7/18), and 15/35 (M: 7/17; F: 8/18), respectively. In
Fischer rats, CSO was tested only in combination with the lower
concentration of aflatoxin; with an aflatoxin B1 level of
20 µg/kg diet, the hepatoma incidence was 6/31 (M: 4/15; F: 2/16)
with CSO, and 6/28 (M: 5/13; F: 1/15) without CSO exposure (Nixon et
al., 1974).
Other chemicals. Sodium phenobarbital given to rats in the
drinking water (1 g/litre) for 9 weeks, together with a diet
contaminated with aflatoxin B1 (at the level of approximately
5 mg/kg) resulted in a lower incidence (11/20) and delayed
appearance of liver tumours within the following 2 years compared
with the incidence in rats fed aflatoxin alone (17/20) (McLean &
Marshall, 1971). The effects of phenobarbital were confirmed in
experiments by Swenson et al. (1977) in which 2 groups of 18 male
Fischer rats were each given a diet containing aflatoxin B1 at a
level of 0.3 mg/kg and drinking water with or without sodium
phenobarbital (1 g/litre) for a period of 15 months. Examination by
laparotomy at the end of aflatoxin exposure revealed liver tumours
in 11 aflatoxin-treated controls (61%) and in only 2 (15%) rats
exposed to aflatoxin with phenobarbital. When the experiment was
terminated 5 months later, hepatocellular carcinomas were detected
in 18 (100%) aflatoxin-exposed rats and in 12 (67%) rats given
aflatoxin and phenobarbital. On the other hand, no effect on
aflatoxin hepatocarcinogenesis was observed in rats fed a similar
aflatoxin diet with the addition of benz (a)anthracene (70 mg/kg)
or ascorbic acid (25 g/kg) (Swenson et al., 1977).
Viral infection. Liver tumours were observed in 2/7 marmosets
fed aflatoxin B1 at a concentration of 2 mg/kg feed and injected
with hepatitis-type candidate virus (G. Barker strain) during
aflatoxin exposure. The animals survived 3-94 weeks of treatment.
Tumours were found in 3/9 marmosets given the aflatoxin diet only
(Lin et al., 1974). Exposure to both agents produced more severe
effects on the liver (cirrhosis) than exposure to aflatoxin B1
alone.
Exposure to artificial sunlight. The effects of exposure to
artificial sunlight on acute aflatoxin toxicity (Newberne et al.,
1974) and carcinogenicity (Joseph-Bravo et al., 1976) were studied
recently, using a long-arc xenon source and filter combination,
which had a spectral distribution in the ultraviolet and visible
ranges closely approximating the 6000 K colour temperature of
natural light. The rats, located 1.5 metres from the source, were
kept at an illumination level of about 29 600 1x corresponding to an
irradiance level of approximately 160 W/m2 over a 2-h period. In
groups consisting of 40 Sprague-Dawley male rats each, 18 control
rats died within 2 weeks of a single intragastric dose of aflatoxin
B1 at 7 mg/kg body weight whereas 23 of the rats exposed to
artificial sunlight for 2 h after aflatoxin administration died.
When an excessive dose of riboflavin was given intra-gastrically 30
min before aflatoxin, the corresponding 2-week mortalities in
animals unexposed and exposed to artificial sunlight were 20/40 and
30/40, respectively (Newberne et al., 1974). In the second study, a
total dose of 375 µg of aflatoxin B1 was given to male
Sprague-Dawley Charles River CD rats, in the form of 15 doses of
25 µg per rat, administered intra-gastrically over a 3-week period.
Thirty min after each aflatoxin administration, half of the animals
were exposed for 2 h to artificial sunlight as described earlier.
When the animals were killed 53 weeks after the last aflatoxin dose,
benign or malignant liver tumours were found in all 11
non-irradiated animals and in only 5/12 of the irradiated group
(Joseph-Bravo et al., 1976).
3.5 Effects in Man---Epidemiological and Clinical Studies
3.5.1 General population studies
3.5.1.1 Liver carcinogenesis
The data on aflatoxins and human cancer available before October
1975 were reviewed by IARC (1976) and several other reviews have
been published more recently (Linsell & Peers, 1977; Shank, 1977;
Van Rensburg, 1977). A positive association between aflatoxin
ingestion and liver cancer in man has been found in population
studies in which estimates of aflatoxin intake and the incidence of
primary liver cancer were made concurrently.
In a study in different parts of Uganda, it was found that
increased frequencies of detectable aflatoxin contamination of food
samples (range: 10.8%-43%) were associated with increased incidence
of primary liver cancer (range: 1.4-15.0 cases per 100 000 total
population per year) (Alpert et al., 1971). Four hundred and eighty
samples of foods were analysed from 8 areas in Uganda; the total
intake was not calculated. Within Swaziland, Keen & Martin
(1971 a, b) showed regional differences in liver cancer frequencies
consistent with regional differences in the frequency of aflatoxin
contamination of groundnuts. From the results of a questionnaire
study, they also suggested that tribal differences in the
preparation of groundnuts for food, and in eating habits, resulting
in higher aflatoxin exposure, could explain the apparently higher
liver cancer rate in the Shangaans living in Swaziland compared with
the Swazis.a
In the studies conducted by Shank et al. (1972a,b) in Thailand,
Peers & Linsell (1973) in Kenya, Van Rensburg et al. (1974) in
Mozambique, and Peers et al. (1976) in Swaziland, actual
concentrations of aflatoxin in meals about to be eaten (food on the
plate) were related to the incidence of primary liver cancer in the
areas where the meal samples were collected. These studies are
summarized in Table 15. A linear regression between the incidence of
liver cancer and the logarithm to the base 10 of the estimated
dietary intake of aflatoxin was found within the range of the
aflatoxin exposure levels and the liver cancer incidence rates
existing in the areas studied? Within Kenya and Swaziland, Peers &
Linsell (1977) demonstrated a steeper rise in liver cancer incidence
with increasing aflatoxin intake in men than in women (Fig. 5). A
similar difference seems to exist also in the other areas studied
(Shank, 1977).
a A positive association of fiver cancer incidence variations with
the availability of aflatoxin contaminated staple foods was also
reported recently from the Philippines (Bulatao-Jayme et al., 1976).
Table 15. Summary of available data on aflatoxin ingestion levels and primary
liver cancer incidencea
Country Area Aflatoxin Liver cancer
Estimated average
daily intakeb in No. of Incidence
adults--ng/kg body cases per 105
weight per day registered of total
population
per year
Kenya High altitude 3.5 4 1.2
Thailand Songkhla 5.0 2 2.0
Swaziland High veld 5.1 11 2.2
Kenya Middle altitude 5.9 33 2.5
Swaziland Mid veld 8.9 29 3.8
Kenya Low altitude 10.0 49 4.0
Swaziland Lebombo 15.4 4 4.3
Thailand Ratburi 45.0 6 6.0
Swaziland Low veld 43.1 42 9.2
Mozambique Inhambanec 222.1 --d 13.0
a From: Peers & Linsell (1977).
b Excluding any aflatoxin present in native beers.
c Revised incidence estimate taken from Van Rensburg (1977).
d Number of cases not available, probably >100.
The possibility that hepatitis B virus infection may confound
the relationship between aflatoxin ingestion and liver cancer
incidence has been considered (Linsell & Peers, 1977). Hepatitis B
infection is common in countries with a high incidence of primary
liver cancer and evidence of prior exposure to hepatitis B virus is
more common in individuals with liver cancer in these countries than
in normal subjects (Vogel et al., 1970; Reys & Sequeira, 1974;
Prince et al., 1975; Chainuvati et al., 1975). Nevertheless, the
present evidence favours aflatoxin as a possible major disease
determinant in primary liver cancer but hepatitis B virus may well
be a cofactor in the etiology (Peers & Linsell, 1977).
a When Stoloff & Friedman (1976) compared published reports on the
incidence of cancer in rural and urban areas of southeastern USA,
and in southeast states compared with other areas in the USA, they
found lower incidences of liver cancer in the rural areas and in the
southeast states, respectively, even though they expected that
long-term exposure to aflatoxins would be higher in these areas.
 Two studies discussed by the Task Group reported the presence of
aflatoxins in the tissues of cancer patients.
Pang et al. unpublished dataa reported the results of a 2-year
study in Indonesia in which the aflatoxins contents were determined
in liver tissue biopsy specimens from 71 patients with primary
cancer of the liver (histologically verified hepatocellular
carcinoma in 62 patients and cholangiohepatocellular cancer in 7
patients). Dietary history indicated consumption of contaminated
food, many patients having eaten groundnuts, almost daily, since
childhood. Great variation was found in the aflatoxin contents of
food samples (type of food and number of analyses not given) with
aflatoxin B1 levels ranging from 17 to 1190 µg/kg, and aflatoxin
G1 levels ranging from 5 to 690 µg/kg. In extracts of liver tissue
biopsy samples obtained soon after the first visit to the hospital,
spots corresponding to aflatoxins were chromatographically detected
for 41 patients (57.7%) but not in extracts of liver tissues from 15
patients without liver cancer serving as controls. The authors also
reported the more frequent presence of aflatoxins in the urine of
liver cancer patients compared with the controls. Aflatoxin B1 at
an estimated level of 520 µg/kg fresh weight was detected in the
liver of a resident of the USA, suffering from carcinoma of the
liver and the rectum (Phillips et al., 1976). No attempt was made to
associate the aflatoxin with cancer in this case.
3.5.1.2 Other effects reported to be associated with aflatoxins
Reye's syndrome. The possibility that some cases of Reye's
syndrome (encephalopathy with fatty degeneration of the viscera)
(Reye et al., 1963), might be due to aflatoxin ingestion was first
suggested by Becroft (1966), who subsequently reported the presence
of aflatoxins B1 and G1 in the livers of 2 children who had died
from Reye's syndrome in New Zealand (Becroft & Webster, 1972).
Following this report, Dvorackova et al. (1974) in Czechoslovakia
and Chaves-Caballo et al. (1976) in the USA detected aflatoxins in
the livers of patients with Reye's syndrome. More recently, in the
USA, Hogan et al. (1978) detected aflatoxin B1 in the blood serum
of 2 patients with Reye's syndrome.
A dietary source of aflatoxin was not identified in any of these
case reports. Aflatoxins were not found in the livers of 5 further
subjects with Reye's syndrome in the USA (Shank, 1976).
a PANG, R. T. L., HUSAINI, S. N., & KARYADI, D. (1974) Aflatoxin
and primary hepatic cancer in Indonesia. Paper presented at the
Vth World Congress of Gastroenterology, 1319 October 1974, Mexico.
Clustering of Reye's syndrome cases, observed in north-east
Thailand, occurred mainly in the villages but not within families
and was geographically and seasonally related to high levels of
aflatoxin contamination of market food samples (Olson et al., 1971;
Bourgeois, 1975). In 2 cases, the presence of heavy aflatoxin
contamination in food, eaten 2 or 3 days before death, was
demonstrated (Bourgeois et al., 1971; Bourgeois, 1975).
Shank et al. (1971a) reported trace amounts of aflatoxin B1 in
tissues, body fluids, gastrointestinal contents or stools of 22/23
Thai patients who had died from Reye's syndrome and 11/15 who had
died from other causes. More than trace amounts of both aflatoxin
B1 and B2 were found in at least 2 liver specimens (47 and 93
lag aflatoxin B1/kg, respectively) from 2 of the 23 patients who
had died from Reye's syndrome but not in any of the specimens from
patients dying from other causes. These 2 series of patients are not
entirely comparable, however, because of differences in the
frequencies with which the various tissues and body contents were
examined.
Twenty-seven cases of Reye's syndrome collected over a 5-year
period (age range: 3 days to 8 years) were investigated by
Dvorackova et al. (1977). Aflatoxin B1 was found in the liver in
all cases and aflatoxin M1 in 4 cases. No aflatoxin was found in
the livers of 25 children, who had died from other causes.
Contamination of milk powder with aflatoxin B1 in the home was
suggested to be the source of exposure in 5 of the cases. No
aflatoxin M1 was found in this milk.
With the exception of the previously mentioned studies from
Thailand (Shank et al., 1971 a; Shank, 1977), cases of Reye's
syndrome have not been reported from other countries in which food
is commonly contaminated with more than traces of aflatoxins. As
Reye et al. (1963) initially suggested, encephalopathy with fatty
infiltration of the viscera is probably a syndrome of varied
etiology.
Other liver diseases. Instances of human liver disease, other
than Reye's syndrome and cancer, that had apparently followed
presumed dietary exposure to aflatoxins are summarized in Table 16.
This table also gives the levels of aflatoxins found in the
suspected foods. In these reports, the data are insufficient to
establish a definite association or causal relationship. In the
studies of Ling et al. (1967), however, there was a geographical and
temporal association between the availability of mouldy food for
consumption and the development of disease.
Table 16. Reports of liver disease (other than Reye's syndrome and cancer) in individuals exposed to aflatoxins in food
Country No. of Age Suspected Estimated Nature and outcome of liver Reference
cases group aflatoxin aflatoxin disease
of liver vehicle concentration
disease (mg/kg)
Senegal 2 4-6 years groundnut meal 0.5-1 Hepatitis leading to hepatatic Payet et al. (1966)
fibrosis in one case.
China (Province 26 all ages rice 0.2 Acute liver disease, 3 deaths in Ling et al. (1967)
of Taiwan) children.
Uganda 1 15 years cassava 1.7 Acute hepatitis leading to death. Serck-Hansen (1970)
India 20 1.5-5 years groundnut meal 0.3 Hepatomegaly. Hepatic failure and Amla et al. (1971 )
death in 3 cases. Subsequent
cirrhosis in some cases (juvenile
cirrhosis).
India several infants not maize 0.25-15 Acute toxic hepatitis; more than Krishnamachari et al.
hundred affected 100 deaths. (1975a,b);
Tandon et al. (1977)
The recent outbreak of acute toxic hepatitis in India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977, 1978) is
described in detail, because of the number of people affected and
because of the evaluation of this outbreak by the Task Group (see
section 3.6.2).
During the last 2 months of 1974 an outbreak of epidemic
jaundice with a high mortality rate affected more than 150 villages
in adjacent districts of 2 neighbouring states, Gujarat and
Rajasthan, in north-west India. Three reports from 2 independent
studies of this outbreak were available for evaluation by the Task
Group (section 3.6.2.1). The first, preliminary report
(Krishnamachari et al., 1975a) mentioned 397 patients in both
affected states with 106 deaths. In a later more detailed paper
(Krishnamachari et al., 1975b), the same group reported 277 cases in
the Panchamahals district of the state of Gujarat with 75 deaths,
and 126 hospitalized patients with 38 deaths in the Banswada
district of the state Rajasthan. In an even later study, a different
group (Tandon et al., 1977) reinvestigating the outbreak in
Rajasthan, reported 994 affected individuals with 97 deaths in the
Banswada and Dungarpur districts of Rajasthan. As reported by
Krishnamachari et al. (1975b), the outbreak started almost
simultaneously in all affected villages, with only a few households
affected in each village and several members of the same household
becoming ill in some instances. Cases were confined to rural areas
and to tribal populations whose staple food, particularly during the
period October-February, was locally grown maize. The outbreak
commenced with the consumption of recently harvested, badly stored
maize, which had been affected by unusual rainfalls in October 1974.
Although the maize was visibly spoiled, it was consumed, leaving
relatively better cobs for seed purposes and for later use.
Suspecting that the outbreak could have been caused by the massive
consumption of maize heavily contaminated with fungi, Krishnamachari
et al. (1975b) determined the mycoflora and the aflatoxin contents
of 10 food samples. A. flavus was detected in all 5 samples of
maize that were obtained from households affected with the disease,
and the aflatoxin B1 levels in these samples ranged from
0.25 mg/kg to 15.6 mg/kg. In contrast, only traces of aflatoxin were
found in maize supplied to a hostel by local shops in one affected
village and no aflatoxin was detected in 4 samples of other
foodstuffs from the same source. A. flavus was not found in these
5 food samples of commercial origin. Assuming a dally local
consumption of maize of up to 400 g per adult per day, and with
aflatoxin contamination up to 15 mg/kg, Krishnamachari et al.
(1975b) concluded that the affected people could have been exposed
to considerable quantities of aflatoxins (up to 6 mg/day), for
several weeks.
One liver sample obtained at necropsy and 7 blood serum and 7
urine samples collected from affected persons were analysed for
aflatoxin content. No information is given on the stage of the
disease at which the samples were collected or on the time that had
elapsed since the last exposure to food suspected to be contaminated
by aflatoxins. Traces of aflatoxin B1 were reported in only 2
blood serum samples, with negative results for all the other human
tissue and fluid samples (Krishnamachari, 1975b).
Tandon et al. (1977) reinvestigated the outbreak in Rajasthan,
presenting results of a retrospective epidemiological survey in an
area, where the largest number of patients with jaundice had been
reported. Statements on dietary history obtained from members of 47
affected families (304 household members, 70 of whom had manifested
the disease) and 29 other families (185 members with no case
reported) did not indicate any difference in the reported
consumption of mouldy maize between affected and non-affected
families or between affected or non-affected members of the same
household. However, A. flavus was detected in 85% of mouldy maize
samples collected from 14 affected families compared with 12% in
samples obtained from 17 families without manifestation of the
disease and 3% in samples obtained from 2 grain dealers. Aflatoxins
B1 and G1 were detected in 13 out of 14 samples from affected
families and in 17 out of 19 samples from the other families
investigated in this area. Aflatoxin B1 levels ranged from 0.1 to
0.6 mg/kg in all positive maize samples, with the exception of 2
from affected families where levels of 0.9 and 1.1 mg/kg were found.
Information is not given in the paper concerning the time at which
the samples were collected in relation to the occurrence of the
disease, whether the samples were analysed for Aspergillus and
aflatoxin contamination, and how far the aflatoxin levels found
reflected the levels that could have occurred in maize actually
consumed before and during the outbreak.
According to Krishnamachari et al. (1975b), all the cases
occurred among subjects whose staple food was maize. Even if maize
were also the staple food in the community studied by Tandon et al.
(1977), statements obtained from members of families studied
indicated that 31% of the members of non-affected families and 16%
of members of affected families reportedly did not consume maize.
From 70 cases of illness, 10 patients (14%) were reported not to
have consumed maize at all but the paper does not indicate the
staple food that they consumed instead.
In another part of the study by Tandon et al. (1977), clinical
data on 200 hospitalized patients were analysed, using hospital
records (176 cases) or direct clinical observations (24 patients).
The disease had a subacute onset starting with fever (in 86%
patients) followed by rapidly developing jaundice (98% cases) and
ascites (74%). In the patients where ascites was not massive it was
possible to detect hepatosplenomegaly. Vomiting, at the onset or at
the time of reporting to the hospital, was present in 46% of cases.
Leukocytosis (mainly an increase in polymorphonuclear leukocytes)
was detected in the initial stage in 87% of patients. Raised levels
of predominantly direct reacting bilirubin and alkaline phosphatase
(EC 3.1.3.1) were found in blood serum; transaminase elevation was
only mild or moderate and even normal levels were observed in blood
samples collected from 11 patients. From the 200 hospitalized
patients studied, 10% died in hospital, usually within 6 weeks of
the onset of illness.
This description of the principal signs and symptoms is in good
agreement with cases reported in the same outbreak by Krishnamachari
et al. (1975b). Both Tandon et al. (1977) and Krishnamachari et al.
(1975a,b) pointed out that two-thirds of affected people were males.
The disease was not reported in infants at all, in the study of
Krishnamachari (1975a,b). The youngest patient reported by Tandon et
al. (1977) was 2´ years old but very few cases were observed below
the age of 5 years. Both studies pointed out the concurrent liver
disease with jaundice and ascites observed in village dogs fed food
remnants from households (see also section 3.4.1).
Results of histopathological liver examinations are available
from 10 patients in this outbreak. In one necropsy sample described
by Krishnamachari et al. (1975a,b), microscopic examination revealed
extensive bile duct proliferation with periductal fibrosis and
cholestasis. Apparently normal liver cells were observed over wide
areas, occasionally replaced in some areas by multinucleated giant
cells or hepatocytes with foamy cytoplasm. Tandon et al. (1977,
1978), who examined liver biopsy specimens obtained from 8 patients
and one liver specimen from autopsy, pointed out that several
histopathological liver changes were characteristic. These included:
(a) oedema and collagenization of the central veins (thrombosis
was not observed); (b) cholangiolar proliferation; (c) moderate
to severe ballooning of the hepatocytes (giant cell transformation
of the liver cells); (d) perisinusoidal fibrosis;
(e) cholestasis; and (f) cirrhosis with reverse lobulation. On
the basis of liver histopathology, Tandon et al. (1977, 1978)
excluded any possibility of vital hepatitis and pointed out that the
cholangiolar proliferation and syncytial giant cell transformation
of hepatocytes (as well as the high prevalence of icterus in
affected people) were also not pathognomonic of the veno-occlusive
disease of the liver.
In one study by Maleki et al. (1976), an attempt was made to
assess the urinary excretion of aflatoxins in patients suffering
from cirrhosis of the liver, who came from a rural area of Iran
where this disease is considered to be frequent without any clear
association with high consumption of alcohol or hepatotoxic spices.
The authors reported that the urine of 6/25 patients with a clinical
diagnosis of cirrhosis contained aflatoxin M, whereas no aflatoxin
M, was detected in the urine of 30 non-cirrhotic patients. The urine
for aflatoxin analysis was collected within 2 days of admission to
hospital. The patients came from villages near Isfahan, Iran where
exceptionally high levels of aflatoxin M, in cow's milk had been
reported (section 3.2.2.10).
3.5.2 Occupational exposure
Three available papers deal with occupational exposure to
aflatoxins. Eleven out of a group of 55 workers, exposed for 2-9
years to dust containing aflatoxins in a mill crushing groundnuts
and other oil seed, developed cancer of various organs within the
observation period of up to 11 years. Primary liver cancer
(cholangiocarcinoma) was reported in one of these patients. Two
other workers were diagnosed to have died of another liver disease.
On the basis of airborne dust determinations at various work places
and dust analysis for aflatoxins (see section 3.2.4), the authors
calculated that airborne aflatoxin levels could have ranged between
0.87 ng/m3 and 72 ng/m3. Assuming respiratory exposure to
airborne aflatoxins in the range of 39 ng to 3.2 µg per worker per
week, the authors concluded that, depending on the length of
employment, the total amount of airborne aflatoxins, to which the
patients had been exposed during the whole period of work in the
mill, could have ranged in individual cases from 160 to 395 µg. In
an age-matched group of 55 workers from a different factory in the
same area, 4 cancer patients were found and no cases of liver cancer
or death from other liver diseases were recorded (Van Nieuwenhuize
et al., 1973).
Dvorackova (1976) reported that 2 men, who had previously been
carrying out the same type of work on a method of sterilizing
Brazilian groundnut meal contaminated by A. flavus, died with a
diagnosis of pulmonary adenomatosis. Analysis of lung samples
obtained at autopsy from one of these patients suggested the
presence of aflatoxin B1. Deger (1976) observed that carcinoma of
the colon developed in 2 research workers, who, for several years,
had been involved in the same type of work in the same laboratory,
purifying substantial amounts of aflatoxins for research purposes.
No other people were involved in this work in the institute.
3.6 Evaluation of the Health Risks of Exposure to Aflatoxins
3.6.1 Human exposure conditions
The main source of human exposure to aflatoxins is contaminated
food. Two pathways of dietary exposure have been identified:
(a) direct ingestion of aflatoxins (mainly B1) in contaminated
foods of plant origin such as maize and nuts and their products; and
(b) ingestion of aflatoxins carried over from feed into milk and
milk products including cheese and powdered milk, where they appear
mainly as aflatoxin M1.
Exposure by pathway (a) is likely to be much greater than by
pathway (b) irrespective of some toxicological differences between
aflatoxins B1 and M1. In tropical countries, where optimal
conditions for fungal growth exist, many components of the diet may
become contaminated. In the surveillance of the first of these
pathways of exposure, sampling is very important, as errors from
this source are much greater than those from the analytical methods
used. Since surveillance programmes have been established in only
very few countries (section 3.6.1.2), information on dietary
exposure to aflatoxins is not yet available on a worldwide basis.
Occasionally, workers may be exposed to the dust of agricultural
commodities that contain aflatoxins. The only available quantitative
data on such exposure (section 3.2.4) indicate that the
concentration of aflatoxins in air under these conditions may be of
the order of 0.1 µg/m3.
3.6.1.1 Sources and levels of aflatoxins in food
Aflatoxins are fungal products of some moulds that belong to two
species: Aspergillus flavus and A. parasiticus. These moulds are
found all over the word, except in polar regions, but their growth
and the formation of aflatoxins require humidity and temperature
conditions (section 3.2.1.1) that are prevalent in tropical and
subtropical areas, but may occasionally be found in colder regions
such as Northern Europe. The formation of aflatoxins takes place
mainly during harvesting and storage, but there is evidence that
attacks by insects carrying fungal spores can result in the
pre-harvest formation of aflatoxins (section 3.2.1.2).
Although aflatoxin-producing moulds can grow on a large variety
of foodstuffs, particularly on plant products, it appears that
certain foodstuffs are more suitable substrates for aflatoxin
formation than others and, thus, may be contaminated more frequently
and with higher levels. These include oil seeds (groundnuts, some
other nuts, cottonseed) and some cereals (maize) (section 3.2.2).
Existing data on the contamination of foodstuffs by aflatoxins
have been obtained by means of various analytical procedures
(section 3.1.2). Collaboratively tested methods are now available,
and as more countries develop adequate laboratory facilities and
make use of these methods, more comparable survey data should become
available.
Several species of cereals and nuts can be contaminated with
aflatoxins and, from published survey reports, it appears that
contamination is highest in groundnuts, Brazil nuts, maize, and
maize products (section 3.2.2). An extremely high value of about
3500 µg/kg was reported in a single sample of groundnuts imported
into Europe for feed, and in a survey in Thailand, an average
concentration of about 1500 µg/kg was recorded. However, average
values of 5 µg/kg or less are more common in countries where control
measures have been implemented. Peanut butter can also contain
aflatoxins, depending on the quality of the groundnuts used in its
manufacture. Roasting of groundnuts reduces but does not eliminate
aflatoxins contamination (section 3.2.3); in general, cooking of
food is not a safeguard against aflatoxin exposure.
There is reliable evidence showing that the level of aflatoxin M1
in milk is directly related to the daily intake of aflatoxin B1 in
dairy feeds, (section 3.3.4.1) and it is generally recognized that
groundnut and cottonseed meals, and maize are the major sources of
this contaminant. However, the level of aflatoxin M1 in milk is
approximately 300 times lower than the level of aflatoxin B1 in
the feed consumed. Several surveys of liquid and dried milk powder
have been carried out throughout the world (section 3.2.2.10). The
highest reported level of aflatoxin M1 in cow's milk exceeds
10 µg/litre, but, in countries where the quality of dairy products
is strictly controlled, levels of 0.1 µg/litre or less are commoner.
Animal experiments indicate that, in addition to the carry-over
into milk, residues of aflatoxins may be present in the tissues of
animals that consume contaminated feed (section 3.2.2.10). However,
the Task Group was not aware of any data from surveys for aflatoxin
residues in meat and meat products.
3.6.1.2 Dietary intake and levels in human tissues
Dietary exposure will depend on the levels of aflatoxins in food
and on food consumption patterns. It is evident that the
contamination of staple foods is of major concern, and that
population segments exposed to monotonous diets based on such staple
foods are at particular risk. Two methods are available for
assessing dietary exposure to aflatoxins: (a) determination of
contamination levels in major food commodities, combined with
nutritional surveys; and (b) analysis of food eaten
("food-on-the-plate" analysis).
In principle, the second method provides a better estimate of
aflatoxin intake but involves many practical difficulties.
Even though there are many reports from different parts of the
world on the presence of aflatoxins in individual food items
(section 3.2.2), the use of food consumption data for the assessment
of aflatoxin intake seems to be limited, at present, to certain
parts of the USA. "Food-on-the-plate" analysis data on aflatoxins
are available only for certain restricted areas in the regions of
the world where the incidence: of liver cancer is high (section
3.5.1.1).
A comprehensive nutritional and commodity survey conducted in
the southeastern states of the USA gave an estimated average level
of aflatoxin B1 in groundnuts and groundnut products of 2 µg/kg,
and an average level of 5-10 µg/kg for maize products. Based on
these data, the estimated average daily intake of aflatoxin B1 in
these areas of the USA was reported (FDA, 1978) to amount to
2.73 ng/kg body weight (maximum 9.03 ng/kg). In certain areas of
Thailand and East Africa, the estimated average daily intake based
on "food-on-the-plate" data ranged from 3.5 to 222.4 ng/kg body
weight (see section 3.5.1.1). However, during the outbreaks of acute
liver disease in south-east Asia, much higher estimates of daily
intake were obtained (up to 120 µg/kg body weight), and levels of
aflatoxin B1 up to 15 mg/kg were found in the contaminated maize
consumed (section 3.5.1.2).
Aflatoxin B1 has been found in the liver and other tissues of
human subjects at levels up to 500 µg/kg or more (section 3.3.2.2).
Some of these cases occurred in Europe and North America indicating
that, at least in some individuals, significant intake of aflatoxins
might occur in these areas.
3.6.2 Acute effects of exposure
Cases of acute human intoxication (section 3.5.1.2) have been
reportedly associated with dietary aflatoxin levels substantially
higher (in the mg/kg range) than the levels thought to be associated
with liver cancer (µg/kg total food range). The Task Group was not
aware of any long-term follow-up study of human populations in which
acute intoxication was reported to have occurred.
3.6.2.1 Acute liver disease
The association of an outbreak of liver disease in turkeys with
aflatoxins (section 3.4.1) was of basic importance in the
recognition of aflatoxins as an environmental hazard.
Similar outbreaks of acute liver disease associated with the
ingestion of aflatoxins were also observed in other species. The
hepatotoxicity of aflatoxins has been confirmed by animal
experiments and dose-response relationships have been obtained for
different species (section 3.4.2).
Acute aflatoxicosis in man has rarely been reported but such
cases may not always have been recognized. Apart from the death of 3
children in the Province of Taiwan, China and one child in Uganda,
where acute liver necrosis was associated with the ingestion of rice
and cassava contaminated with aflatoxins at levels of 200 µg/kg and
1700 µg/kg, respectively, the most convincing case of association of
aflatoxins with acute liver disease was an epidemic of toxic
hepatitis in north-west India in 1974 (section 3.5.1.2). In this
epidemic, several hundred villagers who consumed maize, presumably
contaminated with aflatoxins at levels up to 15 mg/kg, exhibited
signs and symptoms of poisoning and more than one hundred people
died. Estimated daily ingestion of levels up to 6 mg per person were
reported, corresponding approximately to dose rates up to 120 µg/kg
body weight per day. Such dose rates exceed those required to
produce liver damage in non-human primates and this provides
additional support for the assumption that this epidemic was indeed
related to aflatoxin ingestion. Although the role of aflatoxin was
not unequivocally demonstrated, the Task Group agreed that this
incident represented the most acceptable evidence to date of acute
human aflatoxicosis, supported by the information on liver
histology, the space-time clustering of the cases, and the deaths
among village dogs due to a similar form of acute toxic hepatitis.
Examination of tissues and body fluids for aflatoxins was limited to
15 samples (1 necropsy liver sample, 7 urine samples, and 7 blood
samples); aflatoxin was detected only in 2 blood samples. However,
available animal data suggest that the detectable residues of
mycotoxins may remain in tissues and body fluids for only a
relatively short time after ingestion and that, therefore, the
absence of residues does not exclude the possibility of prior
exposure.
In the light of two earlier similar occurrences of aflatoxin
intoxication associated specifically with children, it is somewhat
surprising that, in the Indian epidemic, infants were completely
spared and children under the age of 5 years were less commonly
affected than adults.
Similar epidemics could be expected in the future if the unusual
harvesting circumstances, considered in this case to be responsible
for the high contamination of the staple diet, recurred. The paucity
of reports of epidemics of this type would suggest that massive
contamination of human staple food is a rare occurrence.
3.6.2.2 Reye's syndrome
This syndrome (section 3.5.1.2) is found in many countries of the
world and unlike liver cancer does not show a geographical
association with areas of high aflatoxin intake. Out of four
countries in which the relationship between aflatoxins and Reye's
syndrome has been studied (Czechoslovakia, New Zealand, Thailand,
and the USA) only Thailand belongs to an area with high aflatoxin
levels in food. With the exception of the Thailand cases, the Task
Group was not aware of any other similar reports of Reye's syndrome
in association with aflatoxins in countries thought to be at
increased risk from aflatoxin exposure.
A disease showing many similarities to Reye's syndrome has been
experimentally demonstrated in macaques (section 3.4.2.2). This
resulted from single doses of aflatoxin B1 ranging from 4.5 to
40.5 mg/kg body weight. Reports on numerous experimental studies in
different animal species, including other nonhuman primates, did not
mention brain lesions; it is, however, possible that brains were not
examined for abnormalities.
Among the reports on the presence of aflatoxins in the tissues
of patients with Reye's syndrome, two studies deserve; attention
because of the number of cases included, and because control
subjects were available (section 3.5.1.2).
In Thailand, appreciable amounts of aflatoxins were detected in
autopsy specimens of 6 out of 23 cases of Reye's syndrome and trace
amounts (1-4 µg/kg) were found in a further 16 cases. Similar trace
amounts of aflatoxins were detected in specimens from 11 out of 15
children who had died from other causes.
In a systematic study over several years in Czechoslovakia,
aflatoxin B1 was unequivocally demonstrated in the liver tissue of
26/27 cases, and M1 in 4 cases (in one case the liver tissue was
not examined for aflatoxins). No aflatoxin was found in the liver
tissue of 25 children who died from other causes.
Cases where aflatoxins have been identified in the tissues or
body fluids are sporadic, and the Task Group had no indication of
the number of symptomatic cases in which it was not possible to
demonstrate the association with aflatoxin exposure. As regards
cases in which aflatoxin was detected in the tissues, it cannot be
excluded that pathological changes connected with Reye's syndrome
could have decreased the clearance of aflatoxins from tissues.
In view of these considerations, aflatoxins cannot be excluded
as a contributing factor to Reye's syndrome in some areas, although
an exclusive causal relationship cannot be accepted. There is
evidence that other factors, particularly influenza B virus, may be
associated with this syndrome.
3.6.3 Chronic effects of aflatoxin exposure
3.6.3.1 Cancer of the liver
Epidemiological data indicate an association between the level
of daily aflatoxin ingestion and the incidence of primary liver cell
cancer in certain areas of Kenya, Mozambique, Swaziland, and
Thailand (section 3.5.1.1). This relationship is strongly supported
by studies in experimental animals. In at least 8 species of
experimental animals, aflatoxin has been shown to increase the
incidence of liver cancer (section 3.4.2.3).
If the data from the 4 epidemiological studies (section 3.5.1.1)
are combined, the best fit to the data points is obtained by a
linear regression of the crude liver cancer incidence rates on the
logarithms of the dietary aflatoxin intake. The regression has been
estimated for dietary intakes ranging from 3.5 to 222.4 ng/kg body
weight per day and for crude liver cancer incidence rates from 1.2
to 13 cases/100 000 population per year. At the lower ranges of
aflatoxin intake, liver cancer rates are of the magnitude
encountered in parts of the world in which liver cancer frequency is
considered to be low.
It may be recalled that the liver cancer incidence in rats can
be increased by ingestion of diets containing aflatoxin B1 at a
level of 1 µg/kg (Table 7). The estimated levels of exposure to
aflatoxins in the USA (see section 3.6.1.2) have been used in the
assessment of corresponding life-time liver cancer risks in rats
(section 3.4.2.3) (FDA, 1978). For combined rat studies, these risk
estimates amounted to 240 and 1100 per 10 000 for aflatoxin exposure
levels of 0.1 and 0.3 µg/kg feed, respectively; the estimated
lifetime risks of primary liver cancer in the human population of
the USA from all causes is approximately 161 per 100 000 (FDA,
1978). Comparison of epidemiological and experimental data would
seem to indicate that man is not more but probably less susceptible
to aflatoxins than the rat. This conclusion also seems to be
supported by studies on the metabolic transformation of aflatoxins
(see section 3.3.3).
An association between aflatoxin intake and human liver cancer
incidence was established in surveys that included areas with high
estimated aflatoxin exposure and high liver cancer incidence. These
studies compared the current average aflatoxin intake and the crude
liver cancer incidence rate and did not allow for the period of
latency between the beginning of exposure and cancer manifestation.
The length of this latent period and factors that may modify its
length are not well known. However, the studies were conducted in
rural areas with stable populations and conditions of exposure
thought not to have changed substantially. Data from intervention
studies, in which populations are followed up after a reduction in
exposure levels has been achieved, are not available.
Published epidemiological studies have been limited in scope and
only a single possible etiological factor, i.e., aflatoxin exposure
has been examined. Other factors for which there is some evidence of
an etiological or modifying role in liver cancer, such as
nutritional status, cirrhosis, or viral hepatitis, or the
possibility of interactions between these and still other factors
have not been considered.
In rats, dietary intake of lipotropes, protein, and vitamin A
modified the carcinogenic potential of aflatoxins (section 3.4.2.7).
Diet, marginally deficient in lipotropes (choline, methionine,
folate), enhanced liver cancer induction by aflatoxin B1. A diet
marginally deficient in protein (9% casein) also increased liver
cancer incidence in aflatoxin-treated rats. Severe lipotrope
deficiency or severe protein deficiency (4% casein, combined with
lipotrope deficiency) decreased cancer incidence in
aflatoxin-treated rats. Severe deficiencies which markedly inhibit
the growth of experimental animals can reduce the cancer incidence
after exposure to different carcinogens. This probably represents a
general effect on growth rather than a specific effect on
carcinogenesis. Vitamin A deficiency did not influence the incidence
of liver cancer in aflatoxin-treated rats but altered the effect of
aflatoxin so that the incidence of colon cancers increased. In view
of the importance of these results in relation to human health and
the shortcomings in some of the experiments reported, further animal
studies are necessary to quantify these effects. Future
epidemiological studies should consider such interactions.
Other questions raised by the data from animal studies but not
demonstrated in the epidemiological studies, and thought by the Task
Group to require further study before they can be applied to the
human risk assessment, are the possible induction of extrahepatic
cancers by aflatoxin (section 3.4.2.3), and the possibility that
cancer could be induced by short-time exposure to high
concentrations of aflatoxin.
In spite of the existing gaps in knowledge, it should be
recognized that in animal experiments there is "strong evidence"
of carcinogenicitya for aflatoxins with established dose-response
relationships, and that epidemiological studies in some parts of the
world, where liver cancer is more frequent, have indicated a highly
significant positive correlation between the crude incidence rate of
liver cancer and the estimated current ingestion of aflatoxin in
these areas.b The Task Group, therefore, concluded that aflatoxin
ingestion may increase the risk of liver cancer, that the risk depends
on the amount of aflatoxin ingested, and that reduction in daily
aflatoxin exposure could be expected to reduce the liver cancer risk.
3.6.3.2 Juvenile cirrhosis in India
The Task Group concluded that the postulated involvement of
aflatoxins in juvenile cirrhosis in India has not been substantiated
(section 3.3.4.2 and Table 16 in section 3.5.1.2) and that it is
unlikely in view of the epidemiological and morphological evidence
available. Preliminary data suggesting the involvement of aflatoxins
in the etiology of this disease were not supported by later
measurements of aflatoxin exposure and examination of urine and
liver specimens.
3.6.4 Guidelines for health protection
The effect of aflatoxins which is of greatest concern is the
possible induction of liver cancer in man. Even if it is not
possible, at present, to quantify individual risk corresponding to a
given exposure to aflatoxins, it is nevertheless prudent to attempt
to reduce exposure as much as is practically achievable. Reduction
of food contamination by aflatoxins, sufficient to significantly
reduce liver cancer risk, would of course significantly reduce the
risk of acute toxic effects.
Although there are several aspects of the relationship between
aflatoxin exposure and carcinogenic risks in man that require
elucidation by further experimental and epidemiological studies,
there is, at present, sufficient evidence to justify the
implementation or strengthening of national aflatoxin control
programmes. It is impractical to insist that staple foodstuffs be
aflatoxin-free, but the level of aflatoxin contamination should be
reduced gradually by programmes involving the following components:
education of farmers to improve crop quality and storage;
surveillance of foodstuffs and animal feeds for the presence of
aflatoxins; and application of appropriate food-processing
technology to separate contaminated, from noncontaminated food
elements. These and other measures have recently been discussed
elsewhere (FAO, 1977).
a "Strong evidence" of carcinogenicity is considered to exist when
a chemical has been shown unequivocally to produce malignant
neoplasms. Chemicals for which the evidence of carcinogenicity is
based solely on the appearance of such neoplastic lesions as lung
adenomas or hepatomas in mice belong to the class of chemicals for
which there is "weak evidence" of carcinogenicity (IARC, 1977).
b Of course, this association between liver cancer incidence and
aflatoxin does not necessarily mean that these two variables are
causally related. However, the existing animal data tend to support
a causal relationship, although there may be other factors that
contribute to the development of liver cancer in these areas.
Several countries have established tolerance levels for
aflatoxins in specific food items (for review see Stoloff, 1977;
Krogh, 1978). It should be clearly understood that these tolerance
limits are only management tools intended to facilitate the
implementation of aflatoxin control programmes, and that adherance
to these tolerance limits does not provide an absolute protection
against the increased liver cancer risk associated with aflatoxin
exposure.
4. OTHER MYCOTOXINS
4.1 Ochratoxins
4.1.1 Properties and analytical methods
4.1.1.1 Chemical properties
Two studies discussed by the Task Group reported the presence of
aflatoxins in the tissues of cancer patients.
Pang et al. unpublished dataa reported the results of a 2-year
study in Indonesia in which the aflatoxins contents were determined
in liver tissue biopsy specimens from 71 patients with primary
cancer of the liver (histologically verified hepatocellular
carcinoma in 62 patients and cholangiohepatocellular cancer in 7
patients). Dietary history indicated consumption of contaminated
food, many patients having eaten groundnuts, almost daily, since
childhood. Great variation was found in the aflatoxin contents of
food samples (type of food and number of analyses not given) with
aflatoxin B1 levels ranging from 17 to 1190 µg/kg, and aflatoxin
G1 levels ranging from 5 to 690 µg/kg. In extracts of liver tissue
biopsy samples obtained soon after the first visit to the hospital,
spots corresponding to aflatoxins were chromatographically detected
for 41 patients (57.7%) but not in extracts of liver tissues from 15
patients without liver cancer serving as controls. The authors also
reported the more frequent presence of aflatoxins in the urine of
liver cancer patients compared with the controls. Aflatoxin B1 at
an estimated level of 520 µg/kg fresh weight was detected in the
liver of a resident of the USA, suffering from carcinoma of the
liver and the rectum (Phillips et al., 1976). No attempt was made to
associate the aflatoxin with cancer in this case.
3.5.1.2 Other effects reported to be associated with aflatoxins
Reye's syndrome. The possibility that some cases of Reye's
syndrome (encephalopathy with fatty degeneration of the viscera)
(Reye et al., 1963), might be due to aflatoxin ingestion was first
suggested by Becroft (1966), who subsequently reported the presence
of aflatoxins B1 and G1 in the livers of 2 children who had died
from Reye's syndrome in New Zealand (Becroft & Webster, 1972).
Following this report, Dvorackova et al. (1974) in Czechoslovakia
and Chaves-Caballo et al. (1976) in the USA detected aflatoxins in
the livers of patients with Reye's syndrome. More recently, in the
USA, Hogan et al. (1978) detected aflatoxin B1 in the blood serum
of 2 patients with Reye's syndrome.
A dietary source of aflatoxin was not identified in any of these
case reports. Aflatoxins were not found in the livers of 5 further
subjects with Reye's syndrome in the USA (Shank, 1976).
a PANG, R. T. L., HUSAINI, S. N., & KARYADI, D. (1974) Aflatoxin
and primary hepatic cancer in Indonesia. Paper presented at the
Vth World Congress of Gastroenterology, 1319 October 1974, Mexico.
Clustering of Reye's syndrome cases, observed in north-east
Thailand, occurred mainly in the villages but not within families
and was geographically and seasonally related to high levels of
aflatoxin contamination of market food samples (Olson et al., 1971;
Bourgeois, 1975). In 2 cases, the presence of heavy aflatoxin
contamination in food, eaten 2 or 3 days before death, was
demonstrated (Bourgeois et al., 1971; Bourgeois, 1975).
Shank et al. (1971a) reported trace amounts of aflatoxin B1 in
tissues, body fluids, gastrointestinal contents or stools of 22/23
Thai patients who had died from Reye's syndrome and 11/15 who had
died from other causes. More than trace amounts of both aflatoxin
B1 and B2 were found in at least 2 liver specimens (47 and 93
lag aflatoxin B1/kg, respectively) from 2 of the 23 patients who
had died from Reye's syndrome but not in any of the specimens from
patients dying from other causes. These 2 series of patients are not
entirely comparable, however, because of differences in the
frequencies with which the various tissues and body contents were
examined.
Twenty-seven cases of Reye's syndrome collected over a 5-year
period (age range: 3 days to 8 years) were investigated by
Dvorackova et al. (1977). Aflatoxin B1 was found in the liver in
all cases and aflatoxin M1 in 4 cases. No aflatoxin was found in
the livers of 25 children, who had died from other causes.
Contamination of milk powder with aflatoxin B1 in the home was
suggested to be the source of exposure in 5 of the cases. No
aflatoxin M1 was found in this milk.
With the exception of the previously mentioned studies from
Thailand (Shank et al., 1971 a; Shank, 1977), cases of Reye's
syndrome have not been reported from other countries in which food
is commonly contaminated with more than traces of aflatoxins. As
Reye et al. (1963) initially suggested, encephalopathy with fatty
infiltration of the viscera is probably a syndrome of varied
etiology.
Other liver diseases. Instances of human liver disease, other
than Reye's syndrome and cancer, that had apparently followed
presumed dietary exposure to aflatoxins are summarized in Table 16.
This table also gives the levels of aflatoxins found in the
suspected foods. In these reports, the data are insufficient to
establish a definite association or causal relationship. In the
studies of Ling et al. (1967), however, there was a geographical and
temporal association between the availability of mouldy food for
consumption and the development of disease.
Table 16. Reports of liver disease (other than Reye's syndrome and cancer) in individuals exposed to aflatoxins in food
Country No. of Age Suspected Estimated Nature and outcome of liver Reference
cases group aflatoxin aflatoxin disease
of liver vehicle concentration
disease (mg/kg)
Senegal 2 4-6 years groundnut meal 0.5-1 Hepatitis leading to hepatatic Payet et al. (1966)
fibrosis in one case.
China (Province 26 all ages rice 0.2 Acute liver disease, 3 deaths in Ling et al. (1967)
of Taiwan) children.
Uganda 1 15 years cassava 1.7 Acute hepatitis leading to death. Serck-Hansen (1970)
India 20 1.5-5 years groundnut meal 0.3 Hepatomegaly. Hepatic failure and Amla et al. (1971 )
death in 3 cases. Subsequent
cirrhosis in some cases (juvenile
cirrhosis).
India several infants not maize 0.25-15 Acute toxic hepatitis; more than Krishnamachari et al.
hundred affected 100 deaths. (1975a,b);
Tandon et al. (1977)
The recent outbreak of acute toxic hepatitis in India
(Krishnamachari et al., 1975a,b; Tandon et al., 1977, 1978) is
described in detail, because of the number of people affected and
because of the evaluation of this outbreak by the Task Group (see
section 3.6.2).
During the last 2 months of 1974 an outbreak of epidemic
jaundice with a high mortality rate affected more than 150 villages
in adjacent districts of 2 neighbouring states, Gujarat and
Rajasthan, in north-west India. Three reports from 2 independent
studies of this outbreak were available for evaluation by the Task
Group (section 3.6.2.1). The first, preliminary report
(Krishnamachari et al., 1975a) mentioned 397 patients in both
affected states with 106 deaths. In a later more detailed paper
(Krishnamachari et al., 1975b), the same group reported 277 cases in
the Panchamahals district of the state of Gujarat with 75 deaths,
and 126 hospitalized patients with 38 deaths in the Banswada
district of the state Rajasthan. In an even later study, a different
group (Tandon et al., 1977) reinvestigating the outbreak in
Rajasthan, reported 994 affected individuals with 97 deaths in the
Banswada and Dungarpur districts of Rajasthan. As reported by
Krishnamachari et al. (1975b), the outbreak started almost
simultaneously in all affected villages, with only a few households
affected in each village and several members of the same household
becoming ill in some instances. Cases were confined to rural areas
and to tribal populations whose staple food, particularly during the
period October-February, was locally grown maize. The outbreak
commenced with the consumption of recently harvested, badly stored
maize, which had been affected by unusual rainfalls in October 1974.
Although the maize was visibly spoiled, it was consumed, leaving
relatively better cobs for seed purposes and for later use.
Suspecting that the outbreak could have been caused by the massive
consumption of maize heavily contaminated with fungi, Krishnamachari
et al. (1975b) determined the mycoflora and the aflatoxin contents
of 10 food samples. A. flavus was detected in all 5 samples of
maize that were obtained from households affected with the disease,
and the aflatoxin B1 levels in these samples ranged from
0.25 mg/kg to 15.6 mg/kg. In contrast, only traces of aflatoxin were
found in maize supplied to a hostel by local shops in one affected
village and no aflatoxin was detected in 4 samples of other
foodstuffs from the same source. A. flavus was not found in these
5 food samples of commercial origin. Assuming a dally local
consumption of maize of up to 400 g per adult per day, and with
aflatoxin contamination up to 15 mg/kg, Krishnamachari et al.
(1975b) concluded that the affected people could have been exposed
to considerable quantities of aflatoxins (up to 6 mg/day), for
several weeks.
One liver sample obtained at necropsy and 7 blood serum and 7
urine samples collected from affected persons were analysed for
aflatoxin content. No information is given on the stage of the
disease at which the samples were collected or on the time that had
elapsed since the last exposure to food suspected to be contaminated
by aflatoxins. Traces of aflatoxin B1 were reported in only 2
blood serum samples, with negative results for all the other human
tissue and fluid samples (Krishnamachari, 1975b).
Tandon et al. (1977) reinvestigated the outbreak in Rajasthan,
presenting results of a retrospective epidemiological survey in an
area, where the largest number of patients with jaundice had been
reported. Statements on dietary history obtained from members of 47
affected families (304 household members, 70 of whom had manifested
the disease) and 29 other families (185 members with no case
reported) did not indicate any difference in the reported
consumption of mouldy maize between affected and non-affected
families or between affected or non-affected members of the same
household. However, A. flavus was detected in 85% of mouldy maize
samples collected from 14 affected families compared with 12% in
samples obtained from 17 families without manifestation of the
disease and 3% in samples obtained from 2 grain dealers. Aflatoxins
B1 and G1 were detected in 13 out of 14 samples from affected
families and in 17 out of 19 samples from the other families
investigated in this area. Aflatoxin B1 levels ranged from 0.1 to
0.6 mg/kg in all positive maize samples, with the exception of 2
from affected families where levels of 0.9 and 1.1 mg/kg were found.
Information is not given in the paper concerning the time at which
the samples were collected in relation to the occurrence of the
disease, whether the samples were analysed for Aspergillus and
aflatoxin contamination, and how far the aflatoxin levels found
reflected the levels that could have occurred in maize actually
consumed before and during the outbreak.
According to Krishnamachari et al. (1975b), all the cases
occurred among subjects whose staple food was maize. Even if maize
were also the staple food in the community studied by Tandon et al.
(1977), statements obtained from members of families studied
indicated that 31% of the members of non-affected families and 16%
of members of affected families reportedly did not consume maize.
From 70 cases of illness, 10 patients (14%) were reported not to
have consumed maize at all but the paper does not indicate the
staple food that they consumed instead.
In another part of the study by Tandon et al. (1977), clinical
data on 200 hospitalized patients were analysed, using hospital
records (176 cases) or direct clinical observations (24 patients).
The disease had a subacute onset starting with fever (in 86%
patients) followed by rapidly developing jaundice (98% cases) and
ascites (74%). In the patients where ascites was not massive it was
possible to detect hepatosplenomegaly. Vomiting, at the onset or at
the time of reporting to the hospital, was present in 46% of cases.
Leukocytosis (mainly an increase in polymorphonuclear leukocytes)
was detected in the initial stage in 87% of patients. Raised levels
of predominantly direct reacting bilirubin and alkaline phosphatase
(EC 3.1.3.1) were found in blood serum; transaminase elevation was
only mild or moderate and even normal levels were observed in blood
samples collected from 11 patients. From the 200 hospitalized
patients studied, 10% died in hospital, usually within 6 weeks of
the onset of illness.
This description of the principal signs and symptoms is in good
agreement with cases reported in the same outbreak by Krishnamachari
et al. (1975b). Both Tandon et al. (1977) and Krishnamachari et al.
(1975a,b) pointed out that two-thirds of affected people were males.
The disease was not reported in infants at all, in the study of
Krishnamachari (1975a,b). The youngest patient reported by Tandon et
al. (1977) was 2´ years old but very few cases were observed below
the age of 5 years. Both studies pointed out the concurrent liver
disease with jaundice and ascites observed in village dogs fed food
remnants from households (see also section 3.4.1).
Results of histopathological liver examinations are available
from 10 patients in this outbreak. In one necropsy sample described
by Krishnamachari et al. (1975a,b), microscopic examination revealed
extensive bile duct proliferation with periductal fibrosis and
cholestasis. Apparently normal liver cells were observed over wide
areas, occasionally replaced in some areas by multinucleated giant
cells or hepatocytes with foamy cytoplasm. Tandon et al. (1977,
1978), who examined liver biopsy specimens obtained from 8 patients
and one liver specimen from autopsy, pointed out that several
histopathological liver changes were characteristic. These included:
(a) oedema and collagenization of the central veins (thrombosis
was not observed); (b) cholangiolar proliferation; (c) moderate
to severe ballooning of the hepatocytes (giant cell transformation
of the liver cells); (d) perisinusoidal fibrosis;
(e) cholestasis; and (f) cirrhosis with reverse lobulation. On
the basis of liver histopathology, Tandon et al. (1977, 1978)
excluded any possibility of vital hepatitis and pointed out that the
cholangiolar proliferation and syncytial giant cell transformation
of hepatocytes (as well as the high prevalence of icterus in
affected people) were also not pathognomonic of the veno-occlusive
disease of the liver.
In one study by Maleki et al. (1976), an attempt was made to
assess the urinary excretion of aflatoxins in patients suffering
from cirrhosis of the liver, who came from a rural area of Iran
where this disease is considered to be frequent without any clear
association with high consumption of alcohol or hepatotoxic spices.
The authors reported that the urine of 6/25 patients with a clinical
diagnosis of cirrhosis contained aflatoxin M, whereas no aflatoxin
M, was detected in the urine of 30 non-cirrhotic patients. The urine
for aflatoxin analysis was collected within 2 days of admission to
hospital. The patients came from villages near Isfahan, Iran where
exceptionally high levels of aflatoxin M, in cow's milk had been
reported (section 3.2.2.10).
3.5.2 Occupational exposure
Three available papers deal with occupational exposure to
aflatoxins. Eleven out of a group of 55 workers, exposed for 2-9
years to dust containing aflatoxins in a mill crushing groundnuts
and other oil seed, developed cancer of various organs within the
observation period of up to 11 years. Primary liver cancer
(cholangiocarcinoma) was reported in one of these patients. Two
other workers were diagnosed to have died of another liver disease.
On the basis of airborne dust determinations at various work places
and dust analysis for aflatoxins (see section 3.2.4), the authors
calculated that airborne aflatoxin levels could have ranged between
0.87 ng/m3 and 72 ng/m3. Assuming respiratory exposure to
airborne aflatoxins in the range of 39 ng to 3.2 µg per worker per
week, the authors concluded that, depending on the length of
employment, the total amount of airborne aflatoxins, to which the
patients had been exposed during the whole period of work in the
mill, could have ranged in individual cases from 160 to 395 µg. In
an age-matched group of 55 workers from a different factory in the
same area, 4 cancer patients were found and no cases of liver cancer
or death from other liver diseases were recorded (Van Nieuwenhuize
et al., 1973).
Dvorackova (1976) reported that 2 men, who had previously been
carrying out the same type of work on a method of sterilizing
Brazilian groundnut meal contaminated by A. flavus, died with a
diagnosis of pulmonary adenomatosis. Analysis of lung samples
obtained at autopsy from one of these patients suggested the
presence of aflatoxin B1. Deger (1976) observed that carcinoma of
the colon developed in 2 research workers, who, for several years,
had been involved in the same type of work in the same laboratory,
purifying substantial amounts of aflatoxins for research purposes.
No other people were involved in this work in the institute.
3.6 Evaluation of the Health Risks of Exposure to Aflatoxins
3.6.1 Human exposure conditions
The main source of human exposure to aflatoxins is contaminated
food. Two pathways of dietary exposure have been identified:
(a) direct ingestion of aflatoxins (mainly B1) in contaminated
foods of plant origin such as maize and nuts and their products; and
(b) ingestion of aflatoxins carried over from feed into milk and
milk products including cheese and powdered milk, where they appear
mainly as aflatoxin M1.
Exposure by pathway (a) is likely to be much greater than by
pathway (b) irrespective of some toxicological differences between
aflatoxins B1 and M1. In tropical countries, where optimal
conditions for fungal growth exist, many components of the diet may
become contaminated. In the surveillance of the first of these
pathways of exposure, sampling is very important, as errors from
this source are much greater than those from the analytical methods
used. Since surveillance programmes have been established in only
very few countries (section 3.6.1.2), information on dietary
exposure to aflatoxins is not yet available on a worldwide basis.
Occasionally, workers may be exposed to the dust of agricultural
commodities that contain aflatoxins. The only available quantitative
data on such exposure (section 3.2.4) indicate that the
concentration of aflatoxins in air under these conditions may be of
the order of 0.1 µg/m3.
3.6.1.1 Sources and levels of aflatoxins in food
Aflatoxins are fungal products of some moulds that belong to two
species: Aspergillus flavus and A. parasiticus. These moulds are
found all over the word, except in polar regions, but their growth
and the formation of aflatoxins require humidity and temperature
conditions (section 3.2.1.1) that are prevalent in tropical and
subtropical areas, but may occasionally be found in colder regions
such as Northern Europe. The formation of aflatoxins takes place
mainly during harvesting and storage, but there is evidence that
attacks by insects carrying fungal spores can result in the
pre-harvest formation of aflatoxins (section 3.2.1.2).
Although aflatoxin-producing moulds can grow on a large variety
of foodstuffs, particularly on plant products, it appears that
certain foodstuffs are more suitable substrates for aflatoxin
formation than others and, thus, may be contaminated more frequently
and with higher levels. These include oil seeds (groundnuts, some
other nuts, cottonseed) and some cereals (maize) (section 3.2.2).
Existing data on the contamination of foodstuffs by aflatoxins
have been obtained by means of various analytical procedures
(section 3.1.2). Collaboratively tested methods are now available,
and as more countries develop adequate laboratory facilities and
make use of these methods, more comparable survey data should become
available.
Several species of cereals and nuts can be contaminated with
aflatoxins and, from published survey reports, it appears that
contamination is highest in groundnuts, Brazil nuts, maize, and
maize products (section 3.2.2). An extremely high value of about
3500 µg/kg was reported in a single sample of groundnuts imported
into Europe for feed, and in a survey in Thailand, an average
concentration of about 1500 µg/kg was recorded. However, average
values of 5 µg/kg or less are more common in countries where control
measures have been implemented. Peanut butter can also contain
aflatoxins, depending on the quality of the groundnuts used in its
manufacture. Roasting of groundnuts reduces but does not eliminate
aflatoxins contamination (section 3.2.3); in general, cooking of
food is not a safeguard against aflatoxin exposure.
There is reliable evidence showing that the level of aflatoxin M1
in milk is directly related to the daily intake of aflatoxin B1 in
dairy feeds, (section 3.3.4.1) and it is generally recognized that
groundnut and cottonseed meals, and maize are the major sources of
this contaminant. However, the level of aflatoxin M1 in milk is
approximately 300 times lower than the level of aflatoxin B1 in
the feed consumed. Several surveys of liquid and dried milk powder
have been carried out throughout the world (section 3.2.2.10). The
highest reported level of aflatoxin M1 in cow's milk exceeds
10 µg/litre, but, in countries where the quality of dairy products
is strictly controlled, levels of 0.1 µg/litre or less are commoner.
Animal experiments indicate that, in addition to the carry-over
into milk, residues of aflatoxins may be present in the tissues of
animals that consume contaminated feed (section 3.2.2.10). However,
the Task Group was not aware of any data from surveys for aflatoxin
residues in meat and meat products.
3.6.1.2 Dietary intake and levels in human tissues
Dietary exposure will depend on the levels of aflatoxins in food
and on food consumption patterns. It is evident that the
contamination of staple foods is of major concern, and that
population segments exposed to monotonous diets based on such staple
foods are at particular risk. Two methods are available for
assessing dietary exposure to aflatoxins: (a) determination of
contamination levels in major food commodities, combined with
nutritional surveys; and (b) analysis of food eaten
("food-on-the-plate" analysis).
In principle, the second method provides a better estimate of
aflatoxin intake but involves many practical difficulties.
Even though there are many reports from different parts of the
world on the presence of aflatoxins in individual food items
(section 3.2.2), the use of food consumption data for the assessment
of aflatoxin intake seems to be limited, at present, to certain
parts of the USA. "Food-on-the-plate" analysis data on aflatoxins
are available only for certain restricted areas in the regions of
the world where the incidence: of liver cancer is high (section
3.5.1.1).
A comprehensive nutritional and commodity survey conducted in
the southeastern states of the USA gave an estimated average level
of aflatoxin B1 in groundnuts and groundnut products of 2 µg/kg,
and an average level of 5-10 µg/kg for maize products. Based on
these data, the estimated average daily intake of aflatoxin B1 in
these areas of the USA was reported (FDA, 1978) to amount to
2.73 ng/kg body weight (maximum 9.03 ng/kg). In certain areas of
Thailand and East Africa, the estimated average daily intake based
on "food-on-the-plate" data ranged from 3.5 to 222.4 ng/kg body
weight (see section 3.5.1.1). However, during the outbreaks of acute
liver disease in south-east Asia, much higher estimates of daily
intake were obtained (up to 120 µg/kg body weight), and levels of
aflatoxin B1 up to 15 mg/kg were found in the contaminated maize
consumed (section 3.5.1.2).
Aflatoxin B1 has been found in the liver and other tissues of
human subjects at levels up to 500 µg/kg or more (section 3.3.2.2).
Some of these cases occurred in Europe and North America indicating
that, at least in some individuals, significant intake of aflatoxins
might occur in these areas.
3.6.2 Acute effects of exposure
Cases of acute human intoxication (section 3.5.1.2) have been
reportedly associated with dietary aflatoxin levels substantially
higher (in the mg/kg range) than the levels thought to be associated
with liver cancer (µg/kg total food range). The Task Group was not
aware of any long-term follow-up study of human populations in which
acute intoxication was reported to have occurred.
3.6.2.1 Acute liver disease
The association of an outbreak of liver disease in turkeys with
aflatoxins (section 3.4.1) was of basic importance in the
recognition of aflatoxins as an environmental hazard.
Similar outbreaks of acute liver disease associated with the
ingestion of aflatoxins were also observed in other species. The
hepatotoxicity of aflatoxins has been confirmed by animal
experiments and dose-response relationships have been obtained for
different species (section 3.4.2).
Acute aflatoxicosis in man has rarely been reported but such
cases may not always have been recognized. Apart from the death of 3
children in the Province of Taiwan, China and one child in Uganda,
where acute liver necrosis was associated with the ingestion of rice
and cassava contaminated with aflatoxins at levels of 200 µg/kg and
1700 µg/kg, respectively, the most convincing case of association of
aflatoxins with acute liver disease was an epidemic of toxic
hepatitis in north-west India in 1974 (section 3.5.1.2). In this
epidemic, several hundred villagers who consumed maize, presumably
contaminated with aflatoxins at levels up to 15 mg/kg, exhibited
signs and symptoms of poisoning and more than one hundred people
died. Estimated daily ingestion of levels up to 6 mg per person were
reported, corresponding approximately to dose rates up to 120 µg/kg
body weight per day. Such dose rates exceed those required to
produce liver damage in non-human primates and this provides
additional support for the assumption that this epidemic was indeed
related to aflatoxin ingestion. Although the role of aflatoxin was
not unequivocally demonstrated, the Task Group agreed that this
incident represented the most acceptable evidence to date of acute
human aflatoxicosis, supported by the information on liver
histology, the space-time clustering of the cases, and the deaths
among village dogs due to a similar form of acute toxic hepatitis.
Examination of tissues and body fluids for aflatoxins was limited to
15 samples (1 necropsy liver sample, 7 urine samples, and 7 blood
samples); aflatoxin was detected only in 2 blood samples. However,
available animal data suggest that the detectable residues of
mycotoxins may remain in tissues and body fluids for only a
relatively short time after ingestion and that, therefore, the
absence of residues does not exclude the possibility of prior
exposure.
In the light of two earlier similar occurrences of aflatoxin
intoxication associated specifically with children, it is somewhat
surprising that, in the Indian epidemic, infants were completely
spared and children under the age of 5 years were less commonly
affected than adults.
Similar epidemics could be expected in the future if the unusual
harvesting circumstances, considered in this case to be responsible
for the high contamination of the staple diet, recurred. The paucity
of reports of epidemics of this type would suggest that massive
contamination of human staple food is a rare occurrence.
3.6.2.2 Reye's syndrome
This syndrome (section 3.5.1.2) is found in many countries of the
world and unlike liver cancer does not show a geographical
association with areas of high aflatoxin intake. Out of four
countries in which the relationship between aflatoxins and Reye's
syndrome has been studied (Czechoslovakia, New Zealand, Thailand,
and the USA) only Thailand belongs to an area with high aflatoxin
levels in food. With the exception of the Thailand cases, the Task
Group was not aware of any other similar reports of Reye's syndrome
in association with aflatoxins in countries thought to be at
increased risk from aflatoxin exposure.
A disease showing many similarities to Reye's syndrome has been
experimentally demonstrated in macaques (section 3.4.2.2). This
resulted from single doses of aflatoxin B1 ranging from 4.5 to
40.5 mg/kg body weight. Reports on numerous experimental studies in
different animal species, including other nonhuman primates, did not
mention brain lesions; it is, however, possible that brains were not
examined for abnormalities.
Among the reports on the presence of aflatoxins in the tissues
of patients with Reye's syndrome, two studies deserve; attention
because of the number of cases included, and because control
subjects were available (section 3.5.1.2).
In Thailand, appreciable amounts of aflatoxins were detected in
autopsy specimens of 6 out of 23 cases of Reye's syndrome and trace
amounts (1-4 µg/kg) were found in a further 16 cases. Similar trace
amounts of aflatoxins were detected in specimens from 11 out of 15
children who had died from other causes.
In a systematic study over several years in Czechoslovakia,
aflatoxin B1 was unequivocally demonstrated in the liver tissue of
26/27 cases, and M1 in 4 cases (in one case the liver tissue was
not examined for aflatoxins). No aflatoxin was found in the liver
tissue of 25 children who died from other causes.
Cases where aflatoxins have been identified in the tissues or
body fluids are sporadic, and the Task Group had no indication of
the number of symptomatic cases in which it was not possible to
demonstrate the association with aflatoxin exposure. As regards
cases in which aflatoxin was detected in the tissues, it cannot be
excluded that pathological changes connected with Reye's syndrome
could have decreased the clearance of aflatoxins from tissues.
In view of these considerations, aflatoxins cannot be excluded
as a contributing factor to Reye's syndrome in some areas, although
an exclusive causal relationship cannot be accepted. There is
evidence that other factors, particularly influenza B virus, may be
associated with this syndrome.
3.6.3 Chronic effects of aflatoxin exposure
3.6.3.1 Cancer of the liver
Epidemiological data indicate an association between the level
of daily aflatoxin ingestion and the incidence of primary liver cell
cancer in certain areas of Kenya, Mozambique, Swaziland, and
Thailand (section 3.5.1.1). This relationship is strongly supported
by studies in experimental animals. In at least 8 species of
experimental animals, aflatoxin has been shown to increase the
incidence of liver cancer (section 3.4.2.3).
If the data from the 4 epidemiological studies (section 3.5.1.1)
are combined, the best fit to the data points is obtained by a
linear regression of the crude liver cancer incidence rates on the
logarithms of the dietary aflatoxin intake. The regression has been
estimated for dietary intakes ranging from 3.5 to 222.4 ng/kg body
weight per day and for crude liver cancer incidence rates from 1.2
to 13 cases/100 000 population per year. At the lower ranges of
aflatoxin intake, liver cancer rates are of the magnitude
encountered in parts of the world in which liver cancer frequency is
considered to be low.
It may be recalled that the liver cancer incidence in rats can
be increased by ingestion of diets containing aflatoxin B1 at a
level of 1 µg/kg (Table 7). The estimated levels of exposure to
aflatoxins in the USA (see section 3.6.1.2) have been used in the
assessment of corresponding life-time liver cancer risks in rats
(section 3.4.2.3) (FDA, 1978). For combined rat studies, these risk
estimates amounted to 240 and 1100 per 10 000 for aflatoxin exposure
levels of 0.1 and 0.3 µg/kg feed, respectively; the estimated
lifetime risks of primary liver cancer in the human population of
the USA from all causes is approximately 161 per 100 000 (FDA,
1978). Comparison of epidemiological and experimental data would
seem to indicate that man is not more but probably less susceptible
to aflatoxins than the rat. This conclusion also seems to be
supported by studies on the metabolic transformation of aflatoxins
(see section 3.3.3).
An association between aflatoxin intake and human liver cancer
incidence was established in surveys that included areas with high
estimated aflatoxin exposure and high liver cancer incidence. These
studies compared the current average aflatoxin intake and the crude
liver cancer incidence rate and did not allow for the period of
latency between the beginning of exposure and cancer manifestation.
The length of this latent period and factors that may modify its
length are not well known. However, the studies were conducted in
rural areas with stable populations and conditions of exposure
thought not to have changed substantially. Data from intervention
studies, in which populations are followed up after a reduction in
exposure levels has been achieved, are not available.
Published epidemiological studies have been limited in scope and
only a single possible etiological factor, i.e., aflatoxin exposure
has been examined. Other factors for which there is some evidence of
an etiological or modifying role in liver cancer, such as
nutritional status, cirrhosis, or viral hepatitis, or the
possibility of interactions between these and still other factors
have not been considered.
In rats, dietary intake of lipotropes, protein, and vitamin A
modified the carcinogenic potential of aflatoxins (section 3.4.2.7).
Diet, marginally deficient in lipotropes (choline, methionine,
folate), enhanced liver cancer induction by aflatoxin B1. A diet
marginally deficient in protein (9% casein) also increased liver
cancer incidence in aflatoxin-treated rats. Severe lipotrope
deficiency or severe protein deficiency (4% casein, combined with
lipotrope deficiency) decreased cancer incidence in
aflatoxin-treated rats. Severe deficiencies which markedly inhibit
the growth of experimental animals can reduce the cancer incidence
after exposure to different carcinogens. This probably represents a
general effect on growth rather than a specific effect on
carcinogenesis. Vitamin A deficiency did not influence the incidence
of liver cancer in aflatoxin-treated rats but altered the effect of
aflatoxin so that the incidence of colon cancers increased. In view
of the importance of these results in relation to human health and
the shortcomings in some of the experiments reported, further animal
studies are necessary to quantify these effects. Future
epidemiological studies should consider such interactions.
Other questions raised by the data from animal studies but not
demonstrated in the epidemiological studies, and thought by the Task
Group to require further study before they can be applied to the
human risk assessment, are the possible induction of extrahepatic
cancers by aflatoxin (section 3.4.2.3), and the possibility that
cancer could be induced by short-time exposure to high
concentrations of aflatoxin.
In spite of the existing gaps in knowledge, it should be
recognized that in animal experiments there is "strong evidence"
of carcinogenicitya for aflatoxins with established dose-response
relationships, and that epidemiological studies in some parts of the
world, where liver cancer is more frequent, have indicated a highly
significant positive correlation between the crude incidence rate of
liver cancer and the estimated current ingestion of aflatoxin in
these areas.b The Task Group, therefore, concluded that aflatoxin
ingestion may increase the risk of liver cancer, that the risk depends
on the amount of aflatoxin ingested, and that reduction in daily
aflatoxin exposure could be expected to reduce the liver cancer risk.
3.6.3.2 Juvenile cirrhosis in India
The Task Group concluded that the postulated involvement of
aflatoxins in juvenile cirrhosis in India has not been substantiated
(section 3.3.4.2 and Table 16 in section 3.5.1.2) and that it is
unlikely in view of the epidemiological and morphological evidence
available. Preliminary data suggesting the involvement of aflatoxins
in the etiology of this disease were not supported by later
measurements of aflatoxin exposure and examination of urine and
liver specimens.
3.6.4 Guidelines for health protection
The effect of aflatoxins which is of greatest concern is the
possible induction of liver cancer in man. Even if it is not
possible, at present, to quantify individual risk corresponding to a
given exposure to aflatoxins, it is nevertheless prudent to attempt
to reduce exposure as much as is practically achievable. Reduction
of food contamination by aflatoxins, sufficient to significantly
reduce liver cancer risk, would of course significantly reduce the
risk of acute toxic effects.
Although there are several aspects of the relationship between
aflatoxin exposure and carcinogenic risks in man that require
elucidation by further experimental and epidemiological studies,
there is, at present, sufficient evidence to justify the
implementation or strengthening of national aflatoxin control
programmes. It is impractical to insist that staple foodstuffs be
aflatoxin-free, but the level of aflatoxin contamination should be
reduced gradually by programmes involving the following components:
education of farmers to improve crop quality and storage;
surveillance of foodstuffs and animal feeds for the presence of
aflatoxins; and application of appropriate food-processing
technology to separate contaminated, from noncontaminated food
elements. These and other measures have recently been discussed
elsewhere (FAO, 1977).
a "Strong evidence" of carcinogenicity is considered to exist when
a chemical has been shown unequivocally to produce malignant
neoplasms. Chemicals for which the evidence of carcinogenicity is
based solely on the appearance of such neoplastic lesions as lung
adenomas or hepatomas in mice belong to the class of chemicals for
which there is "weak evidence" of carcinogenicity (IARC, 1977).
b Of course, this association between liver cancer incidence and
aflatoxin does not necessarily mean that these two variables are
causally related. However, the existing animal data tend to support
a causal relationship, although there may be other factors that
contribute to the development of liver cancer in these areas.
Several countries have established tolerance levels for
aflatoxins in specific food items (for review see Stoloff, 1977;
Krogh, 1978). It should be clearly understood that these tolerance
limits are only management tools intended to facilitate the
implementation of aflatoxin control programmes, and that adherance
to these tolerance limits does not provide an absolute protection
against the increased liver cancer risk associated with aflatoxin
exposure.
4. OTHER MYCOTOXINS
4.1 Ochratoxins
4.1.1 Properties and analytical methods
4.1.1.1 Chemical properties
 The ochratoxins are a group of structurally-related compounds
(Fig. 6), classified according to biosynthetic origin as
pentaketides within the group polyketides (Turner, 1971). The first
compound discovered, ochratoxin A, was isolated from a strain of
Aspergillus ochraceus (van der Merwe et al., 1965). It is a
colourless, crystalline compound, exhibiting blue fluorescence under
UV-light. The sodium salt of ochratoxin A is soluble in water; as an
acid, it is moderately soluble in polar organic solvents (e.g.,
chloroform and methanol). Some of the chemical and physical
properties of three ochratoxins are summarized in Table 17. Only
ochratoxin A, and very rarely ochratoxin B, have been encountered as
natural contaminants of foodstuffs, the remaining ochratoxins listed
in Fig. 6 have been isolated only from fungal cultures, under
laboratory conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine and an optically active lactone acid, ochratoxin
alpha, a metabolite which has been found in the urine of test
animals ingesting ochratoxin A-contaminated feed. This subject has
been reviewed by Chu (1974) and Harwig (1974), and the
spectroanalytical parameters have been reviewed by Neely & West
(1972).
Table 17. Chemical and physical properties of some ochratoxins
Ochratoxin Molecular Relative Melting Absorption maxima (nm) Reference
formula molecular mass point °C absorption coefficient (Epsilon)
A C20H18CINO6 403 169 (xylene) 213(36 800); 322(6400) Steyn & Holzapfel
89-95 (benzene) (1967)
B C20H19NO6 369 221 218(37 200); 318(6900) van der Merwe et al.
(1965)
Alpha C11H9CIO5 256 229 212(30 000); 338(5600) van der Merwe et al.
(1965)
4.1.1.2 Methods for the analysis of foodstuffs
Methods of analysing foodstuffs for ochratoxins have been
reviewed by Nesheim (1976). The distribution of ochratoxins in
commodities has not been studied in detail and no specific sampling
plans have been developed. However, the general principles of
sampling, outlined in section 3.1.2.1 for aflatoxins, are also
applicable to ochratoxin.
Several chemical methods have been developed, with limits of
detection as low as 2 µg/kg (Nesheim, 1976). Ochratoxin A in
acidified commodities is readily soluble in many organic solvents,
and this characteristic has been used as the principle of extraction
in several methods. The most widely used method, for cereals in
particular, includes extraction with chloroformaqueous phosphoric
acid followed by cleanup on an aqueous bicarbonate-diatomaceous
earth column, and quantitative determination using thin-layer
chromatography (Nesheim et al., 1973). This procedure has been
recommended by the International Union of Pure and Applied Chemistry
(IUPAC) as an international method (IUPAC, 1976), and has a limit of
detection of a few µg/kg, when improved by ammoniation.
Minicolumn methods for screening purposes have been developed
(Hald & Krogh, 1975; Holaday, 1976), as well as a spectrophotometric
procedure based on cleavage of ochratoxin A to form ochratoxin a and
phenylalanine (Hult & Gatenbeck, 1976).
A number of bioassays involving zebra fish larvae, brine
shrimps, and bacteria have been developed, but none of the assays
has been used routinely, so far (Harwig, 1974).
4.1.2 Sources and occurrence
4.1.2.1 Fungal formation
Ochratoxin A was first obtained from A. ochraceus, but
subsequent investigations have revealed that a variety of moulds
included in the fungal genera Aspergillus and Penicillium are able
to produce ochratoxins (Table 18). The main producers appear to be
A. ochraceus and P. viridicatum. This subject has been reviewed by
Krogh (1976a).
Moisture content and temperature. In studies of ochratoxin A
production by A. ochraceus, optimal production occurred between 20
and 30° C (Schindler & Nesheim, 1970; Bacon et al., 1973). Maximum
production was observed at 30° C and a water activity (%) of 0.953
(39% of water content, % dry weight). At lower temperatures, such as
15° C, the moisture requirement was higher (aw = 0.997, or 52%
moisture) (Table 19).
The genus Penicillium includes psychrophilic species, and
investigations of the influence of low incubation temperatures have
revealed that strains of P. viridicatum are able to produce
ochratoxin A at 5-10° C (Harwig & Chen, 1974) (Table 19). This
indicates that the heavy ochtratoxin contamination observed in
countries with cold climates such as Canada and the Scandinavian
countries is mainly produced by the Penicillia.
Table 18. Ochratoxin-producing fungia
Penicillium Link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica-Lanata:
P. commune series: P. commune Thom
Asymmetrica-Fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata-Symmetrica:
P. purpurogenum series: P. variabile Sopp
Aspergillus Micheli
Aspergillus ochraceus group:
A. sulphureus (Fres.) Thom and Church
A. sclerotiorum Huber
A. alliaceus Thom and Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Voros
a From: Krogh (1978).
Table 19. Production of ochratoxin A by A. ochraceus and
P. viridicatum at various aw and temperatures
(a) A. ochraceus (after 2 weeks of incubation)a
aw 15° C 22° C 30° C
mg ochratoxin A/kg
0.852 0 0 60
0.901 0 46 111
0.953 36 201 302
0.997 81 156 218
(b) P. viridicatum (after 3 weeks of incubation)b
aw 5° C 12° C 25° C
mg ochratoxin A/kg
0.85-0.86 4000 11 000
0.90-0.93 160 000 280 000
0.95-0.97 17 000
(after
5 weeks)
a Adapted from: Bacon et al. (1973).
b Adapted from: Harwig & Chen (1974).
4.1.2.2 Occurrence in foodstuffs
Plant products. The occurrence of ochratoxins in foodstuffs
has been reviewed by Chu (1974a), Krogh (1976a, 1977a), and Stoloff
(1976). Naturally occurring ochratoxin A was first reported at a
concentration of 110-150 µg/kg in one sample of maize included in a
survey of 283 samples from commercial markets in the USA (Shotwell
et al., 1969c). Data from subsequent surveys of plant products in
various areas of the world are summarized in Table 20.
Residues in food of animal origin. In 1971, a farm was traced
where pigs had been fed ochratoxin A-contaminated feed. When the
bacon pigs, some of which suffered from nephropathy, were delivered
to the slaughterhouse, samples of kidney, liver, and adipose tissue
were collected for analysis. Residues of ochratoxin A were detected
in 18/19 investigated kidneys, at levels up to 67 µg/kg (Hald &
Krogh, 1972). Residues were also detected in 7/8 livers, and in all
8 samples of adipose tissue analysed.
Table 20. Natural occurrence of ochratoxin A in plant products
Commodity Country No. of Percentage Range of Reference
samples contaminated contamination
analysed (µg/kg)
wheat, oats, barley, rye (feed) Canada 32 56.3 30-27 000c Scott et al. (1972)
barley, oats Denmark 33 57.6 28-27 500b,c Krogh et al. (1973b)
malt barley Denmark 50 6.0 9-189 Krogh (1978)
maize France 463 2.6 15-200 Galtier (1975)
barley, wheat, oats, rye, maize (feed) Poland 150 5.3 50-200 Juszkiewicz & Piskorska-Pliszczynska (1976)
mixed feed Poland 203 4.9 10-50 Juszkiewicz & Piskorska-Pliszczynska (1977)
barley, oats (feed) Sweden 84 8.3 16-409 Krogh et al. (1974)
maize, wheat, barley Yugoslavia 47 12.8 5-90 Krogh et al. (1977)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize USA 283 0.4 110-150 Shotwell et al. (1969c)
maize USA 293 1.0 83-166a Shotwell et al. (1971 )
wheat (red winter) USA 291 1.0 5-115 Shotwell et al. (1976a)
wheat (red spring) USA 286 2.8 5-115 Shotwell et al. (1976a)
a Two of these samples also contained ochratoxin B.
b Ochratoxin B, as well as ochratoxin A detected in 2 additional samples of barley.
c Most of the samples containing high levels had undergone "heating".
Table 21. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
Tissue Regression equation r
kidney y = 2.15 + 0.0123x 0.86
liver y = 0.35 + 0.0095x 0.82
adipose y = 2.51 + 0.0099x 0.78
a Modified from: Krogh et al. (1974).
x = ochratoxin A in feed (µg/kg)
y = ochratoxin A residue (µg/kg tissue)
r = correlation coefficient
The regression is calculated on feed levels of ochratoxin A in
the range of 200-4000 µg/kg.
Surveillance studies based on data from meat inspection in
Denmark have revealed prevalence rates of porcine nephropathy
ranging from 10-80 cases per 100 000 slaughtered pigs (Krogh,
1976b). A survey of kidneys from pigs with the disease collected at
various slaughterhouses, showed that 35% of the affected kidneys
contained residues of ochratoxin A, ranging from 2-68 µg/kg (Krogh,
1977b). A similar survey of porcine nephropathy in Sweden revealed
that 25% of the affected kidneys contained ochratoxin A at levels
ranging from 2 to 104 µg/kg (Rutqvist et al., 1977). The carry-over
of ochratoxin A from feed to animal tissues has been elucidated in
studies in which groups of pigs were exposed for 3-4 months to
dietary levels of ochratoxin A of 200, 1000, and 4000 µg/kg (Krogh
et al., 1974). At termination (slaughter), the highest levels of
ochratoxin A residues were found in the kidneys (mean level 50 µg/kg
at the 4000 µg/kg feed level) with slightly lower levels in the
liver, and even lower levels in muscle and adipose tissue. Other
tissues were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 21). In another study on pigs (Krogh et al.,
1976a), a high correlation ( r = 0.74-0.94) was found between
ochratoxin A levels in the kidney and in other organs and tissues
including the liver, muscle, and adipose tissue (Table 22 and
section 4.1.3.3).
Table 22. Correlation between ochratoxin A mass concentration
residues in the kidney and certain other tissuesa
Tissue Regression equation r
liver y = --0.650 + 0.706x 0.937
muscle y = --0.603 + 0.438x 0.888
adipose y = --0.775 + 0.309x 0.739
a From: Krogh et al. (1976a).
x = ochratoxin A mass concentration (µg/kg) in the kidney
y = ochratoxin A mass concentration (µg/kg) in the other tissues
r = correlation coefficient
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected in one slaughterhouse (Elling et al.,
1975). The birds had been condemned because of nephropathy. In
another study, groups of hens were exposed for 1-2 years to dietary
levels of ochratoxin A of 0.3 and 1 mg/kg (Krogh et al., 1976c). The
kidneys contained the highest residues, with a mean value of
19 µg/kg tissue in the group fed ochratoxin A at 1 mg/kg: the liver
and muscle contained lower levels of ochratoxin A residues.
Ochratoxins were not detected in the eggs.
4.1.3 Metabolism
4.1.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged ochratoxin
A, and it was concluded that ochratoxin A was absorbed mainly in the
stomach. In the caecum and the large intestine, small amounts (1-3%
of the total dose), were detected as the isocoumarin moiety
(ochratoxin a) most likely as the result of the hydrolysing action
of the intestinal microflora (Galtier & Alvinerie, 1976; Hult et
al., 1976).
In in vitro studies, Pitout (1969) showed that ochratoxin
alpha could also be formed from the hydrolysis of ochratoxin A by
carboxypeptidase A (EC 3.4.12.2) and alpha-chymotrypsin. No
quantitative information is available on the rate of absorption of
ochratoxin A and ochratoxin a from the gastrointestinal tract.
4.1.3.2 Tissue distribution and metabolic conversion
In slaughterhouse cases of mycotoxic porcine nephropathy studied
by Hald & Krogh (1972), residues of unchanged ochratoxin A were
found in all tissues investigated (kidney, liver and muscle), the
highest levels (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin A,
residues of this toxin were found in all 4 tissues in the decreasing
order of kidney, liver, muscle, adipose tissue (Krogh et al., 1974).
When rats were exposed perorally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered dose
in the whole kidneys, 0.9% in the whole liver, and 0.6% in the total
muscle tissue, 96 h after exposure. Chang & Chu (1977), using a
single intraperitoneal injection of I mg ochratoxin A per rat
(labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
0.5 h, amounting to 4-5% of the total dose.
It has been shown by in vitro studies that ochratoxin A binds to
serum albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies of rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin it has been detected in the urine and faeces of
rats intraperitoneally injected with ochratoxin A (Nel & Purchase,
1968; Chang & Chu, 1977), indicating the cleavage of ochratoxin A
into ochratoxin alpha and phenylalanine under these conditions.
Studies with 14C-labelled ochratoxin A indicated that some other,
not yet identified, metabolities are formed in the body. Less than
half of the radioactivity excreted in the urine within 24 h of a
single intra-peritoneal injection of 14C-phenylalanine-labelled
ochratoxin A was identified as ochratoxin A (Chang & Chu, 1977).
4.1.3.3 Excretion
Using 14C-labelled ochratoxin A in studies on rats, it has
been demonstrated that this toxin is excreted primarily in the urine
(Chang & Chu, 1977) although faecal excretion also occurs to some
extent (Galtier, 1974b; Chang & Chu, 1977). Ochratoxin A has been
detected in the urine of bacon pigs suffering from nephropathy
(Krogh, personal communication).
In a study of the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for 1 month and then kept on a toxin-free diet for another month
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 23) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue), with residual life values (RL50)a in the range of
3.3-4.5 days; the toxin could still be detected in kidneys one month
after termination of exposure. When the level in the kidney is
known, the ochratoxin A residues in the 3 other tissues can be
calculated (Table 22). No data are available on ochratoxin levels in
human tissues, urine, or faeces.
Table 23. The rate of disappearance of ochratoxin A residues from pig
tissues after termination of one month exposure to
ochratoxin A at 1 mg/kg feeda
Tissue Ochratoxin A (µg/kg tissue)
at time t (days) after
termination of exposure
kidney 28.22 exp (--0.1522t)
liver 19.49 exp (--0.1598t)
muscle 12.94 exp (--0.2096t)
adipose 4.62 exp (--0.0565t)
a From: Krogh et al. (1976a),
4.1.4 Effects in animals
4.1.4.1 Field observations
Pigs. The effects of ochratoxins in animals have been reviewed
by Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy, have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, although unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs and epidemics were encountered in 1963 and 1971, associated
with a high moisture content in the grain caused by unusual climatic
conditions (Krogh, 1976b). Extensive etiological studies have
a RL50 = half-time of residues calculated from the exponential
equations shown in Table 23.
revealed that ochratoxin A is a major disease determinant of porcine
nephropathy, although other factors such as citrinin are also
involved as causal determinants (for review see Krogh, 1976a).
Analyses of kidneys from cases of porcine nephropathy collected at
slaughterhouses have revealed that 35% of these kidneys contained
ochratoxin A in concentrations ranging from 2-68 µg/kg (Krogh,
1977b). As this compound has not been detected in healthy kidneys,
it is indicated that it may play a causal role in the disease.
The morphological changes in the kidneys in cases of mycotoxic
porcine nephropathy are characterized by degeneration of the
proximal tubules, followed by atrophy of the tubular epithelium,
interstitial fibrosis in the renal cortex, and hyalinization of some
glomeruli (Elling & Moller, 1973). Although mycotoxic porcine
nephropathy has only been reported from one other European country
besides Scandinavia (Buckley, 1971), there are indications that this
disease also occurs in other countries in Europe and North America.
Poultry. In a preliminary study in Denmark of chickens and
hens condemned by meat inspectors because of renal lesions, 29% of
14 birds were suffering from nephopathy associated with ingestion of
ochratoxin A (Elling et al., 1975). The morphological renal lesions
were characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons, and interstitial fibrosis.
4.1.4.2 Experimental studies
Acute and chronic effects. The acute and chronic effects of
ochratoxins in experimental animals have been reviewed by Chu
(1974a), Harwig (1974), and Krogh (1976a). Different species vary in
their susceptibility to acute poisoning by ochratoxin A, with LD50
values ranging from 3.4 to 30.3 mg/kg (Table 24). When administered
orally to rats, the female is more sensitive to ochratoxin A than
the male. The kidney is the target organ, but changes in the liver
have also been noted during studies of acute effects.
Table 24. Acute toxicity of ochratoxin A
Animal LD50 mg/kg Route of Reference
body weight administration
mouse (female) 22 intraperitoneal Sansing et al. (1976)
rat, male 30.3 peroral Galtier et al. (1974)
rat, female 21.4 peroral Galtier et al. (1974)
rat, male 12.6 intraperitoneal Galtier et al. (1974)
rat, female 14.3 intraperitoneal Galtier et al. (1974)
guineapig, male 9.1 peroral Thacker (1976)
guineapig, female 8.1 peroral Thacker (1976)
white leghorn 3.4 peroral Prior et al. (1976)
turkey 5.9 peroral Prior et al. (1976)
Japanese quail 16.5 peroral Prior et al. (1976)
rainbow trout 4.7 intraperitoneal Doster et al. (1972)
beagle dog, male < 9 (total dose) perorala Szczech et al. (1973a)
pig, female < 6 (total dose) peroralb Szczech et al. (1973b)
a All 3 dogs, dosed daily with 3 mg/kg, died within 3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed within 3 days, and
both pigs receiving 1 mg/kg daily were moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic porcine
nephropathy (section 4.1.4.1) have been reproduced by feeding diets
containing levels of ochratoxin A identical to those encountered in
naturally contaminated products (section 4.1.2.2). Thus 39 pigs fed
rations containing ochratoxin A at levels ranging from
200-4000 µg/kg developed nephropathy after 4 months at all levels of
exposure (Krogh et al., 1974). Changes in renal function were
characterized by impairment of tubular function, indicated
particularly by a decrease in TmPAH/CIna and reduced ability
to produce concentrated urine. These functional changes corresponded
well with the changes in renal structure observed at all exposure
levels including atrophy of the proximal tubules, and interstitial
cortical fibrosis. Sclerotized glomeruli were also observed in the
group receiving the highest dose of ochratoxin A of 4000 µg/kg feed.
No other organ or tissue exhibited any changes.
a TmPAH = transport maximum for para-aminohippuric acid, CIn =
clearance of inulin.
Kidney damage, identical to the naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to a
feed level of 1 mg/kg for 3 months. Similar damage was not observed
in 9 controls. Significant renal tubular impairment was detected
after only 5 weeks of ochratoxin exposure (Krogh et al., 1976b).
In pigs and dogs given high peroral doses, corresponding to feed
levels of more than 5-10 mg/kg (levels rarely found in nature)
extrarenal effects, in addition to renal lesions, were observed,
involving the liver, intestine, spleen, lymphoid tissue, and
leukocytes (Szczech et al., 1973a,b,c). Three groups of rats, each
consisting of 15 animals were exposed to feed levels of ochratoxin A
ranging from 0.2 to 5 mg/kg for 3 months. Renal damage in the form
of tubular degeneration was observed at all dose levels (Munro et
al., 1974).
Avian nephropathy similar to spontaneously occurring cases
(section 4.1.4.1) developed in chickens and hens exposed to dietary
levels of 0.3 and 1 mg/kg for 1 year (Krogh et al., 1976c). The
renal changes included degeneration of the tubular epithelium,
mainly confined to the proximal and distal tubules of both reptilian
and mammalian nephrons; impairment of glomerular and tubular
function was also observed. Acute necrosis and "visceral gout" was
observed in chickens exposed to high levels of ochratoxin A (LD50
values) (Peckham et al., 1971). The same authors reported that
ochratoxin B, the other naturally occurring ochratoxin was not
highly toxic to chickens (LD50: 54 mg/kg); no toxic effects have
been reported in other animals.
The toxic effects of ochratoxin A on the renal epithelial cells
of the monkey were demonstrated in in vitro studies, in the form
of abnormal mitotic cells (Steyn et al., 1975).
Teratogenic effects. Intraperitoneal injection of pregnant
mice with ochratoxin A at 5 mg/kg body weight on one of gestation
days 7-12 resulted in increased prenatal mortality, decreased fetal
weight, and various fetal malformations including exencephaly and
anomalies of the eyes, face, digits, and tail (Hayes et al., 1974).
When rats were treated perorally with ochratoxin A at 0.75 and
1.0 mg/kg body weight on gestation days 6-15, fetuses taken on day
20 showed decreased weight and various anomalies (e.g., open eyes,
wavy ribs, and agenesis of vertebrae) (Brown et al., 1976). In
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9, increased
prenatal mortality and malformations were observed, including
hydrocephalus, micrognathia, and heart defects (Hood et al., 1976).
Mutagenicity. No data were available on the mutagenicity of
ochratoxins.
Carcinogenesis. There have not been any recent data that would
change the conclusion that an evaluation of the carcinogenic risk of
ochratoxins cannot be made because of the inadequacy of available
studies in terms of the numbers of animals used and survival rates
(IARC, 1976).
Biochemical effects. Ochratoxin A affects the carbohydrate
metabolism in rats. Thus, a single oral dose of ochratoxin A at
15 mg/kg body weight caused a decrease in the glycogen level in the
liver and an increase in the heart glycogen level 4 h later (Suzuki
& Satoh, 1973). In a more extensive study on rats, the decrease in
liver glycogen level, 4 h after a single oral dose of ochratoxin A
at 15 mg/kg body weight, was associated with an increase in serum
glucose levels and a decrease in liver glucose-6-phosphate (Suzuki
et al., 1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin A
at 5 mg/kg body weight caused a decrease in liver glycogen
concentration, measured on the fourth day. The decrease was
attributed to inhibition of the active transport of glucose into the
liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies of rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further experiments with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that ochratoxin
A inhibited intramitochondrial phosphate transport, resulting in
deterioration of the mitochondria (Meisner, 1976). This might
explain the degeneration of liver mitochondria observed by Purchase
& Theron (1968) in rats exposed perorally to a single dose of
ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975) who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. Ochratoxin A acted synergistically, in this
respect, with another nephro-toxic mycotoxin, citrinin.
4.1.5 Effects in man
4.1.5.1 Ochratoxin A and Balkan nephropathy
Balkan endemic nephropathy is a kidney disease only observed so
far in rural populations in Bulgaria, Romania, and Yugoslavia. In
the past 2 decades, etiological investigations covering bacteria,
viruses, toxic metals, genetic factors, etc. have been conducted but
with unconvincing results (reviewed Puchlev, 1973, 1974). Balkan
endemic nephropathy is a chronic disease that is commonest between
30 and 50 years of age and progresses slowly up to death. The
kidneys are remarkably reduced in size. Histologically, the renal
disease is characterized by tubular degeneration, interstitial
fibrosis, and hyalization of glomeruli in the more superficial part
of the cortex (Heptinstall, 1966). Impairment of tubular function,
indicated by a decrease in TmPAH, is a prominent and early sign
(Dotchev, 1973).
The disease occurs endemically and affects females more often
than males (Hrabar et al., 1976, Chernozemsky et al., 1977). In
Bulgaria and Yugoslavia, a high incidence of urinary tract tumours
has been found to be closely correlated with the incidence and
mortality rates of Balkan endemic nephropathy (Ceovic et al., 1976;
Chernozemsky et al., 1977).
Fungal growth in foodstuffs and subsequent mycotoxin formation
is influenced by the water content of the foodstuffs, and can be
changed by climatic conditions such as heavy rainfalls during
harvest. Thus, the observation (Austwick, 1975) of a positive
correlation ( r = 0.80) between excess rainfall and the number of
people who died of nephropathy during the succeeding 2 years in the
Balkan peninsula might be interpreted as suggesting a fungal
involvement in the etiology of endemic nephropathy.
Attention has been called to the striking similarities in the
changes of renal structure and function found in Balkan endemic
nephropathy and in ochratoxin A-induced porcine nephropathy,
suggesting common causal relationships (Krogh, 1974). Furthermore,
epidemiological similarities have been noted, in particular, the
endemic occurrence (Krogh, 1976b). Preliminary results of a survey
of foodstuff's indicate that exposure to food-borne ochratoxin A
seems to be higher (12.8% contamination) in an area of Yugoslavia
with a high prevalence of human endemic nephropathy than in
nonendemic (control) areas (1.6% contamination) (Krogh et al.,
1977).
4.1.6 Conclusions and evaluation of the health risks to man
of ochratoxins
4.1.6.1 Experimental animal studies
The toxic effects of ochratoxin A have been studied extensively
in a variety of experimental animals. All the animals studied so far
have been susceptible to orally administered ochratoxin A, but to
various degrees, as indicated by the range of LD50 values
(Table 24). At high levels of ochratoxin A, changes were found in
the kidneys and also in other organs and tissues. However, only
renal lesions were observed at exposure levels identical to those
occurring environmentally. The renal lesions included degeneration
of the tubules, interstitial fibrosis, and, at later stages,
hyalinization of glomeruli, with impairment of tubular function as a
prime manifestation. Feed levels as low as 200 µg/kg produced renal
changes in the course of 3 months in rats and pigs. Field cases of
ochratoxin A-induced nephropathy are regularly encountered in pigs
and poultry. Ochratoxin A is teratogenic in the mouse, rat, and
hamster.
Ochratoxin B, rarely found as a natural contaminant, is much
less toxic; the other ochratoxins have never been encountered in
natural products.
4.1.6.2 Studies in man
The ochratoxin A-induced nephropathy in farm animals is similar
to Balkan endemic nephropathy in several aspects. In a preliminary
study in an area where Balkan endemic nephropathy is prevalent, the
ochratoxin A contamination of food appeared to be more frequent than
in control areas. However, the hypothesis that ochratoxin A may be a
causal determinant in this disease, needs further support.
4.1.6.3 Evaluation of health risks
The nephrotoxic potential of ochratoxin A is well documented
from all experimental studies, with a feed level of 200 µg/kg
causing nephropathy in pigs and rats. Lower levels have not been
tested. Field eases of ochratoxin A-induced nephropathy in farm
animals have long been recognized. The toxin has been found in a
variety of foodstuffs, with levels in commodities used as feed
ranging up to 27 mg/kg, and with levels in foodstuffs used for human
consumption in the range of trace to about 100 µg/kg. In one area
where endemic nephropathy was prevalent in the human population,
home produced foodstuffs were more frequently contaminated with
ochratoxin A than those from control areas. However, the total
intake of ochratoxin A by man has not been assessed so far, and
there is, at present, no proof that ochratoxin A is causally
involved in human diseases.
4.2 Zearalenone
4.2.1 Properties, analytical methods, and sources
The properties, analytical methods, sources, and occurrence of
zearalenone have been reviewed by Mirocha & Christensen (1974),
Pathre & Mirocha (1976), and Mirocha et al. (1977). Zearalenone is a
phenolic resorcylic acid lactone (Fig. 5), classified, according to
biosynthetic origin, as a nonaketide within the group polyketides
(Turner, 1971). Zearalenone (C18H22O5) is a white crystalline
compound with a relative molecular mass of 318, melting point
164-165 °C, and absorption maxima (and absorption coefficient) at
236 nm (29 700), 274 nm (13 909) and 316 nm (6020).
Zearalenone exhibits blue-green fluorescence when excited by
long wavelength (360 nm) UV-light, and a more intense green
fluorescence when excited with short wavelength (260 nm) UV-light.
A number of derivatives of zearalenone have been isolated from
fungal cultures (Fig. 7), but none of these derivatives has been
encountered, so far, as a natural contaminant of foodstuffs.
A multiple detection method for aflatoxin, ochratoxin, and
zearalenone has been developed (Eppley, 1968) and tested
collaboratively (Shotwell et al., 1976b). The procedure consists of
water-chloroform extraction combined with sequential elution of the
mycotoxins from a silica-gel column and the detection limit is in
the range of 50-100 µg/kg. A versatile method of analysis for
zearalenone has been described by Mirocha et al. (1974) using
thin-layer chromatography (TLC), gas-liquid chromatography (GLC),
gas-liquid chromatography-mass spectrometry, or a combination of all
these methods; the limit of detection is about 50 µg/kg. The
derivatives dimethoxyzearalenone and methyl oxime-di-TMS-zearalenone
are used to confirm the identity of zearalenone. Two methods using
high pressure liquid chromatography (HPLC) are now available (Scott
et al., 1978; Ware & Thorpe, 1978). With HPLC, a concentration of
zearalenone in cornflakes of 5 µg/kg could be determined (Scott et
al., 1978). Zearalenone is produced by strains of Fusarium
graminearum, F. tricinctum, F. oxysporum, F. sporotrichioides, and
F. moniliforme, and a period of low temperature ( 12°-14°C) during
fungal formation seems essential for high yield.
The ochratoxins are a group of structurally-related compounds
(Fig. 6), classified according to biosynthetic origin as
pentaketides within the group polyketides (Turner, 1971). The first
compound discovered, ochratoxin A, was isolated from a strain of
Aspergillus ochraceus (van der Merwe et al., 1965). It is a
colourless, crystalline compound, exhibiting blue fluorescence under
UV-light. The sodium salt of ochratoxin A is soluble in water; as an
acid, it is moderately soluble in polar organic solvents (e.g.,
chloroform and methanol). Some of the chemical and physical
properties of three ochratoxins are summarized in Table 17. Only
ochratoxin A, and very rarely ochratoxin B, have been encountered as
natural contaminants of foodstuffs, the remaining ochratoxins listed
in Fig. 6 have been isolated only from fungal cultures, under
laboratory conditions. On acid hydrolysis, ochratoxin A yields
phenylalanine and an optically active lactone acid, ochratoxin
alpha, a metabolite which has been found in the urine of test
animals ingesting ochratoxin A-contaminated feed. This subject has
been reviewed by Chu (1974) and Harwig (1974), and the
spectroanalytical parameters have been reviewed by Neely & West
(1972).
Table 17. Chemical and physical properties of some ochratoxins
Ochratoxin Molecular Relative Melting Absorption maxima (nm) Reference
formula molecular mass point °C absorption coefficient (Epsilon)
A C20H18CINO6 403 169 (xylene) 213(36 800); 322(6400) Steyn & Holzapfel
89-95 (benzene) (1967)
B C20H19NO6 369 221 218(37 200); 318(6900) van der Merwe et al.
(1965)
Alpha C11H9CIO5 256 229 212(30 000); 338(5600) van der Merwe et al.
(1965)
4.1.1.2 Methods for the analysis of foodstuffs
Methods of analysing foodstuffs for ochratoxins have been
reviewed by Nesheim (1976). The distribution of ochratoxins in
commodities has not been studied in detail and no specific sampling
plans have been developed. However, the general principles of
sampling, outlined in section 3.1.2.1 for aflatoxins, are also
applicable to ochratoxin.
Several chemical methods have been developed, with limits of
detection as low as 2 µg/kg (Nesheim, 1976). Ochratoxin A in
acidified commodities is readily soluble in many organic solvents,
and this characteristic has been used as the principle of extraction
in several methods. The most widely used method, for cereals in
particular, includes extraction with chloroformaqueous phosphoric
acid followed by cleanup on an aqueous bicarbonate-diatomaceous
earth column, and quantitative determination using thin-layer
chromatography (Nesheim et al., 1973). This procedure has been
recommended by the International Union of Pure and Applied Chemistry
(IUPAC) as an international method (IUPAC, 1976), and has a limit of
detection of a few µg/kg, when improved by ammoniation.
Minicolumn methods for screening purposes have been developed
(Hald & Krogh, 1975; Holaday, 1976), as well as a spectrophotometric
procedure based on cleavage of ochratoxin A to form ochratoxin a and
phenylalanine (Hult & Gatenbeck, 1976).
A number of bioassays involving zebra fish larvae, brine
shrimps, and bacteria have been developed, but none of the assays
has been used routinely, so far (Harwig, 1974).
4.1.2 Sources and occurrence
4.1.2.1 Fungal formation
Ochratoxin A was first obtained from A. ochraceus, but
subsequent investigations have revealed that a variety of moulds
included in the fungal genera Aspergillus and Penicillium are able
to produce ochratoxins (Table 18). The main producers appear to be
A. ochraceus and P. viridicatum. This subject has been reviewed by
Krogh (1976a).
Moisture content and temperature. In studies of ochratoxin A
production by A. ochraceus, optimal production occurred between 20
and 30° C (Schindler & Nesheim, 1970; Bacon et al., 1973). Maximum
production was observed at 30° C and a water activity (%) of 0.953
(39% of water content, % dry weight). At lower temperatures, such as
15° C, the moisture requirement was higher (aw = 0.997, or 52%
moisture) (Table 19).
The genus Penicillium includes psychrophilic species, and
investigations of the influence of low incubation temperatures have
revealed that strains of P. viridicatum are able to produce
ochratoxin A at 5-10° C (Harwig & Chen, 1974) (Table 19). This
indicates that the heavy ochtratoxin contamination observed in
countries with cold climates such as Canada and the Scandinavian
countries is mainly produced by the Penicillia.
Table 18. Ochratoxin-producing fungia
Penicillium Link
Monoverticillata:
P. frequentans series: P. purpurrescens Sopp
Asymmetrica-Lanata:
P. commune series: P. commune Thom
Asymmetrica-Fasciculata:
P. viridicatum series: P. viridicatum Westling
P. palitans Westling
P. cyclopium series: P. cyclopium Westling
Biverticillata-Symmetrica:
P. purpurogenum series: P. variabile Sopp
Aspergillus Micheli
Aspergillus ochraceus group:
A. sulphureus (Fres.) Thom and Church
A. sclerotiorum Huber
A. alliaceus Thom and Church
A. melleus Yukawa
A. ochraceus Wilhelm
A. ostianus Wehmer
A. petrakii Voros
a From: Krogh (1978).
Table 19. Production of ochratoxin A by A. ochraceus and
P. viridicatum at various aw and temperatures
(a) A. ochraceus (after 2 weeks of incubation)a
aw 15° C 22° C 30° C
mg ochratoxin A/kg
0.852 0 0 60
0.901 0 46 111
0.953 36 201 302
0.997 81 156 218
(b) P. viridicatum (after 3 weeks of incubation)b
aw 5° C 12° C 25° C
mg ochratoxin A/kg
0.85-0.86 4000 11 000
0.90-0.93 160 000 280 000
0.95-0.97 17 000
(after
5 weeks)
a Adapted from: Bacon et al. (1973).
b Adapted from: Harwig & Chen (1974).
4.1.2.2 Occurrence in foodstuffs
Plant products. The occurrence of ochratoxins in foodstuffs
has been reviewed by Chu (1974a), Krogh (1976a, 1977a), and Stoloff
(1976). Naturally occurring ochratoxin A was first reported at a
concentration of 110-150 µg/kg in one sample of maize included in a
survey of 283 samples from commercial markets in the USA (Shotwell
et al., 1969c). Data from subsequent surveys of plant products in
various areas of the world are summarized in Table 20.
Residues in food of animal origin. In 1971, a farm was traced
where pigs had been fed ochratoxin A-contaminated feed. When the
bacon pigs, some of which suffered from nephropathy, were delivered
to the slaughterhouse, samples of kidney, liver, and adipose tissue
were collected for analysis. Residues of ochratoxin A were detected
in 18/19 investigated kidneys, at levels up to 67 µg/kg (Hald &
Krogh, 1972). Residues were also detected in 7/8 livers, and in all
8 samples of adipose tissue analysed.
Table 20. Natural occurrence of ochratoxin A in plant products
Commodity Country No. of Percentage Range of Reference
samples contaminated contamination
analysed (µg/kg)
wheat, oats, barley, rye (feed) Canada 32 56.3 30-27 000c Scott et al. (1972)
barley, oats Denmark 33 57.6 28-27 500b,c Krogh et al. (1973b)
malt barley Denmark 50 6.0 9-189 Krogh (1978)
maize France 463 2.6 15-200 Galtier (1975)
barley, wheat, oats, rye, maize (feed) Poland 150 5.3 50-200 Juszkiewicz & Piskorska-Pliszczynska (1976)
mixed feed Poland 203 4.9 10-50 Juszkiewicz & Piskorska-Pliszczynska (1977)
barley, oats (feed) Sweden 84 8.3 16-409 Krogh et al. (1974)
maize, wheat, barley Yugoslavia 47 12.8 5-90 Krogh et al. (1977)
barley USA 127 14.2 10-40 Nesheim (1971)
coffee beans USA 267 7.1 20-360 Levi et al. (1974)
maize USA 283 0.4 110-150 Shotwell et al. (1969c)
maize USA 293 1.0 83-166a Shotwell et al. (1971 )
wheat (red winter) USA 291 1.0 5-115 Shotwell et al. (1976a)
wheat (red spring) USA 286 2.8 5-115 Shotwell et al. (1976a)
a Two of these samples also contained ochratoxin B.
b Ochratoxin B, as well as ochratoxin A detected in 2 additional samples of barley.
c Most of the samples containing high levels had undergone "heating".
Table 21. Correlation between feed level and tissue levels
(residues) of ochratoxin A in pigsa
Tissue Regression equation r
kidney y = 2.15 + 0.0123x 0.86
liver y = 0.35 + 0.0095x 0.82
adipose y = 2.51 + 0.0099x 0.78
a Modified from: Krogh et al. (1974).
x = ochratoxin A in feed (µg/kg)
y = ochratoxin A residue (µg/kg tissue)
r = correlation coefficient
The regression is calculated on feed levels of ochratoxin A in
the range of 200-4000 µg/kg.
Surveillance studies based on data from meat inspection in
Denmark have revealed prevalence rates of porcine nephropathy
ranging from 10-80 cases per 100 000 slaughtered pigs (Krogh,
1976b). A survey of kidneys from pigs with the disease collected at
various slaughterhouses, showed that 35% of the affected kidneys
contained residues of ochratoxin A, ranging from 2-68 µg/kg (Krogh,
1977b). A similar survey of porcine nephropathy in Sweden revealed
that 25% of the affected kidneys contained ochratoxin A at levels
ranging from 2 to 104 µg/kg (Rutqvist et al., 1977). The carry-over
of ochratoxin A from feed to animal tissues has been elucidated in
studies in which groups of pigs were exposed for 3-4 months to
dietary levels of ochratoxin A of 200, 1000, and 4000 µg/kg (Krogh
et al., 1974). At termination (slaughter), the highest levels of
ochratoxin A residues were found in the kidneys (mean level 50 µg/kg
at the 4000 µg/kg feed level) with slightly lower levels in the
liver, and even lower levels in muscle and adipose tissue. Other
tissues were not analysed. There was a high correlation between the
feed level of ochratoxin A and the residue levels in the 4 tissues
investigated (Table 21). In another study on pigs (Krogh et al.,
1976a), a high correlation ( r = 0.74-0.94) was found between
ochratoxin A levels in the kidney and in other organs and tissues
including the liver, muscle, and adipose tissue (Table 22 and
section 4.1.3.3).
Table 22. Correlation between ochratoxin A mass concentration
residues in the kidney and certain other tissuesa
Tissue Regression equation r
liver y = --0.650 + 0.706x 0.937
muscle y = --0.603 + 0.438x 0.888
adipose y = --0.775 + 0.309x 0.739
a From: Krogh et al. (1976a).
x = ochratoxin A mass concentration (µg/kg) in the kidney
y = ochratoxin A mass concentration (µg/kg) in the other tissues
r = correlation coefficient
Ochratoxin A levels of up to 29 µg/kg were found in the muscle
of hens and chickens collected in one slaughterhouse (Elling et al.,
1975). The birds had been condemned because of nephropathy. In
another study, groups of hens were exposed for 1-2 years to dietary
levels of ochratoxin A of 0.3 and 1 mg/kg (Krogh et al., 1976c). The
kidneys contained the highest residues, with a mean value of
19 µg/kg tissue in the group fed ochratoxin A at 1 mg/kg: the liver
and muscle contained lower levels of ochratoxin A residues.
Ochratoxins were not detected in the eggs.
4.1.3 Metabolism
4.1.3.1 Absorption
In a study on rats exposed by gavage to a single dose of
ochratoxin A at 10 mg/kg body weight, Galtier (1974b) found the
highest tissue level of unchanged ochratoxin A in the stomach wall
during the first 4 h following administration. The small and large
intestine and caecum contained small amounts of unchanged ochratoxin
A, and it was concluded that ochratoxin A was absorbed mainly in the
stomach. In the caecum and the large intestine, small amounts (1-3%
of the total dose), were detected as the isocoumarin moiety
(ochratoxin a) most likely as the result of the hydrolysing action
of the intestinal microflora (Galtier & Alvinerie, 1976; Hult et
al., 1976).
In in vitro studies, Pitout (1969) showed that ochratoxin
alpha could also be formed from the hydrolysis of ochratoxin A by
carboxypeptidase A (EC 3.4.12.2) and alpha-chymotrypsin. No
quantitative information is available on the rate of absorption of
ochratoxin A and ochratoxin a from the gastrointestinal tract.
4.1.3.2 Tissue distribution and metabolic conversion
In slaughterhouse cases of mycotoxic porcine nephropathy studied
by Hald & Krogh (1972), residues of unchanged ochratoxin A were
found in all tissues investigated (kidney, liver and muscle), the
highest levels (up to 67 µg/kg) occurring in the kidney. In
experimental studies on pigs ingesting feed containing ochratoxin A,
residues of this toxin were found in all 4 tissues in the decreasing
order of kidney, liver, muscle, adipose tissue (Krogh et al., 1974).
When rats were exposed perorally to an ochratoxin A dose of 10 mg/kg
body weight, Galtier (1974b) recovered 0.3% of the administered dose
in the whole kidneys, 0.9% in the whole liver, and 0.6% in the total
muscle tissue, 96 h after exposure. Chang & Chu (1977), using a
single intraperitoneal injection of I mg ochratoxin A per rat
(labelled with 14C in phenylalanine), found that the kidney
contained twice as much unchanged ochratoxin A as the liver after
0.5 h, amounting to 4-5% of the total dose.
It has been shown by in vitro studies that ochratoxin A binds to
serum albumin (Chu, 1971, 1974b); this binding has also been
observed in in vivo studies of rats (Galtier, 1974a; Chang & Chu,
1977). Ochratoxin it has been detected in the urine and faeces of
rats intraperitoneally injected with ochratoxin A (Nel & Purchase,
1968; Chang & Chu, 1977), indicating the cleavage of ochratoxin A
into ochratoxin alpha and phenylalanine under these conditions.
Studies with 14C-labelled ochratoxin A indicated that some other,
not yet identified, metabolities are formed in the body. Less than
half of the radioactivity excreted in the urine within 24 h of a
single intra-peritoneal injection of 14C-phenylalanine-labelled
ochratoxin A was identified as ochratoxin A (Chang & Chu, 1977).
4.1.3.3 Excretion
Using 14C-labelled ochratoxin A in studies on rats, it has
been demonstrated that this toxin is excreted primarily in the urine
(Chang & Chu, 1977) although faecal excretion also occurs to some
extent (Galtier, 1974b; Chang & Chu, 1977). Ochratoxin A has been
detected in the urine of bacon pigs suffering from nephropathy
(Krogh, personal communication).
In a study of the disappearance rates for various tissues,
female bacon pigs were fed ochratoxin A at a level of 1 mg/kg feed
for 1 month and then kept on a toxin-free diet for another month
during which animals were sacrificed at regular intervals (Krogh et
al., 1976a). Ochratoxin A disappeared exponentially (Table 23) from
the 4 tissues investigated (kidney, liver, muscle, and adipose
tissue), with residual life values (RL50)a in the range of
3.3-4.5 days; the toxin could still be detected in kidneys one month
after termination of exposure. When the level in the kidney is
known, the ochratoxin A residues in the 3 other tissues can be
calculated (Table 22). No data are available on ochratoxin levels in
human tissues, urine, or faeces.
Table 23. The rate of disappearance of ochratoxin A residues from pig
tissues after termination of one month exposure to
ochratoxin A at 1 mg/kg feeda
Tissue Ochratoxin A (µg/kg tissue)
at time t (days) after
termination of exposure
kidney 28.22 exp (--0.1522t)
liver 19.49 exp (--0.1598t)
muscle 12.94 exp (--0.2096t)
adipose 4.62 exp (--0.0565t)
a From: Krogh et al. (1976a),
4.1.4 Effects in animals
4.1.4.1 Field observations
Pigs. The effects of ochratoxins in animals have been reviewed
by Krogh (1976a, 1978). Cases of mycotoxic porcine nephropathy, have
been regularly encountered in studies in Denmark since the disease
was first discovered 50 years ago (Larsen, 1928). The disease is
endemic in all areas of the country, although unevenly distributed.
Prevalence rates in 1971 varied from 0.6 to 65.9 cases per 10 000
pigs and epidemics were encountered in 1963 and 1971, associated
with a high moisture content in the grain caused by unusual climatic
conditions (Krogh, 1976b). Extensive etiological studies have
a RL50 = half-time of residues calculated from the exponential
equations shown in Table 23.
revealed that ochratoxin A is a major disease determinant of porcine
nephropathy, although other factors such as citrinin are also
involved as causal determinants (for review see Krogh, 1976a).
Analyses of kidneys from cases of porcine nephropathy collected at
slaughterhouses have revealed that 35% of these kidneys contained
ochratoxin A in concentrations ranging from 2-68 µg/kg (Krogh,
1977b). As this compound has not been detected in healthy kidneys,
it is indicated that it may play a causal role in the disease.
The morphological changes in the kidneys in cases of mycotoxic
porcine nephropathy are characterized by degeneration of the
proximal tubules, followed by atrophy of the tubular epithelium,
interstitial fibrosis in the renal cortex, and hyalinization of some
glomeruli (Elling & Moller, 1973). Although mycotoxic porcine
nephropathy has only been reported from one other European country
besides Scandinavia (Buckley, 1971), there are indications that this
disease also occurs in other countries in Europe and North America.
Poultry. In a preliminary study in Denmark of chickens and
hens condemned by meat inspectors because of renal lesions, 29% of
14 birds were suffering from nephopathy associated with ingestion of
ochratoxin A (Elling et al., 1975). The morphological renal lesions
were characterized by degeneration of proximal and distal tubules of
both reptilian and mammalian nephrons, and interstitial fibrosis.
4.1.4.2 Experimental studies
Acute and chronic effects. The acute and chronic effects of
ochratoxins in experimental animals have been reviewed by Chu
(1974a), Harwig (1974), and Krogh (1976a). Different species vary in
their susceptibility to acute poisoning by ochratoxin A, with LD50
values ranging from 3.4 to 30.3 mg/kg (Table 24). When administered
orally to rats, the female is more sensitive to ochratoxin A than
the male. The kidney is the target organ, but changes in the liver
have also been noted during studies of acute effects.
Table 24. Acute toxicity of ochratoxin A
Animal LD50 mg/kg Route of Reference
body weight administration
mouse (female) 22 intraperitoneal Sansing et al. (1976)
rat, male 30.3 peroral Galtier et al. (1974)
rat, female 21.4 peroral Galtier et al. (1974)
rat, male 12.6 intraperitoneal Galtier et al. (1974)
rat, female 14.3 intraperitoneal Galtier et al. (1974)
guineapig, male 9.1 peroral Thacker (1976)
guineapig, female 8.1 peroral Thacker (1976)
white leghorn 3.4 peroral Prior et al. (1976)
turkey 5.9 peroral Prior et al. (1976)
Japanese quail 16.5 peroral Prior et al. (1976)
rainbow trout 4.7 intraperitoneal Doster et al. (1972)
beagle dog, male < 9 (total dose) perorala Szczech et al. (1973a)
pig, female < 6 (total dose) peroralb Szczech et al. (1973b)
a All 3 dogs, dosed daily with 3 mg/kg, died within 3 days.
b Both pigs receiving 2 mg/kg daily were moribund and killed within 3 days, and
both pigs receiving 1 mg/kg daily were moribund and killed within 6 days.
The lesions observed in field cases of mycotoxic porcine
nephropathy (section 4.1.4.1) have been reproduced by feeding diets
containing levels of ochratoxin A identical to those encountered in
naturally contaminated products (section 4.1.2.2). Thus 39 pigs fed
rations containing ochratoxin A at levels ranging from
200-4000 µg/kg developed nephropathy after 4 months at all levels of
exposure (Krogh et al., 1974). Changes in renal function were
characterized by impairment of tubular function, indicated
particularly by a decrease in TmPAH/CIna and reduced ability
to produce concentrated urine. These functional changes corresponded
well with the changes in renal structure observed at all exposure
levels including atrophy of the proximal tubules, and interstitial
cortical fibrosis. Sclerotized glomeruli were also observed in the
group receiving the highest dose of ochratoxin A of 4000 µg/kg feed.
No other organ or tissue exhibited any changes.
a TmPAH = transport maximum for para-aminohippuric acid, CIn =
clearance of inulin.
Kidney damage, identical to the naturally occurring porcine
nephropathy, was produced in another study by feeding pigs (9
animals) with crystalline ochratoxin A in amounts corresponding to a
feed level of 1 mg/kg for 3 months. Similar damage was not observed
in 9 controls. Significant renal tubular impairment was detected
after only 5 weeks of ochratoxin exposure (Krogh et al., 1976b).
In pigs and dogs given high peroral doses, corresponding to feed
levels of more than 5-10 mg/kg (levels rarely found in nature)
extrarenal effects, in addition to renal lesions, were observed,
involving the liver, intestine, spleen, lymphoid tissue, and
leukocytes (Szczech et al., 1973a,b,c). Three groups of rats, each
consisting of 15 animals were exposed to feed levels of ochratoxin A
ranging from 0.2 to 5 mg/kg for 3 months. Renal damage in the form
of tubular degeneration was observed at all dose levels (Munro et
al., 1974).
Avian nephropathy similar to spontaneously occurring cases
(section 4.1.4.1) developed in chickens and hens exposed to dietary
levels of 0.3 and 1 mg/kg for 1 year (Krogh et al., 1976c). The
renal changes included degeneration of the tubular epithelium,
mainly confined to the proximal and distal tubules of both reptilian
and mammalian nephrons; impairment of glomerular and tubular
function was also observed. Acute necrosis and "visceral gout" was
observed in chickens exposed to high levels of ochratoxin A (LD50
values) (Peckham et al., 1971). The same authors reported that
ochratoxin B, the other naturally occurring ochratoxin was not
highly toxic to chickens (LD50: 54 mg/kg); no toxic effects have
been reported in other animals.
The toxic effects of ochratoxin A on the renal epithelial cells
of the monkey were demonstrated in in vitro studies, in the form
of abnormal mitotic cells (Steyn et al., 1975).
Teratogenic effects. Intraperitoneal injection of pregnant
mice with ochratoxin A at 5 mg/kg body weight on one of gestation
days 7-12 resulted in increased prenatal mortality, decreased fetal
weight, and various fetal malformations including exencephaly and
anomalies of the eyes, face, digits, and tail (Hayes et al., 1974).
When rats were treated perorally with ochratoxin A at 0.75 and
1.0 mg/kg body weight on gestation days 6-15, fetuses taken on day
20 showed decreased weight and various anomalies (e.g., open eyes,
wavy ribs, and agenesis of vertebrae) (Brown et al., 1976). In
hamsters injected intraperitoneally with ochratoxin A at doses of
5-20 mg/kg body weight on one of gestation days 7-9, increased
prenatal mortality and malformations were observed, including
hydrocephalus, micrognathia, and heart defects (Hood et al., 1976).
Mutagenicity. No data were available on the mutagenicity of
ochratoxins.
Carcinogenesis. There have not been any recent data that would
change the conclusion that an evaluation of the carcinogenic risk of
ochratoxins cannot be made because of the inadequacy of available
studies in terms of the numbers of animals used and survival rates
(IARC, 1976).
Biochemical effects. Ochratoxin A affects the carbohydrate
metabolism in rats. Thus, a single oral dose of ochratoxin A at
15 mg/kg body weight caused a decrease in the glycogen level in the
liver and an increase in the heart glycogen level 4 h later (Suzuki
& Satoh, 1973). In a more extensive study on rats, the decrease in
liver glycogen level, 4 h after a single oral dose of ochratoxin A
at 15 mg/kg body weight, was associated with an increase in serum
glucose levels and a decrease in liver glucose-6-phosphate (Suzuki
et al., 1975). At the same time, the liver glycogen synthetase (EC
2.7.1.37) activity decreased and the liver phosphorylase (EC
2.4.1.1) activity increased. Three daily oral doses of ochratoxin A
at 5 mg/kg body weight caused a decrease in liver glycogen
concentration, measured on the fourth day. The decrease was
attributed to inhibition of the active transport of glucose into the
liver, suppression of glycogen synthesis from glucose, and
acceleration of glycogen decomposition.
During in vitro studies of rat liver mitochondria, it was
observed that ochratoxin A inhibited the respiration of whole
mitochondria by acting as a competitive inhibitor of transport
carrier proteins located in the inner mitochondrial membrane
(Meisner & Chan, 1974). Further experiments with mitochondrial
preparations revealed that the mitochondrial uptake of ochratoxin A
was an energy-using process that resulted in depletion of
intramitochondrial adenosine triphosphate (ATP), and that ochratoxin
A inhibited intramitochondrial phosphate transport, resulting in
deterioration of the mitochondria (Meisner, 1976). This might
explain the degeneration of liver mitochondria observed by Purchase
& Theron (1968) in rats exposed perorally to a single dose of
ochratoxin A at 10 mg/kg body weight. These authors observed
accumulation of glycogen in the cytoplasm of the rat liver cells
microscopically. This was in contrast to the previously discussed
observations of Suzuki et al. (1975) who found a decrease in
glycogen levels.
In a study on mice, Sansing et al. (1976) found that ochratoxin
A, administered intraperitoneally at 6 mg/kg body weight, inhibited
orotic acid incorporation into both liver and kidney RNA, 6 h after
toxin injection. Ochratoxin A acted synergistically, in this
respect, with another nephro-toxic mycotoxin, citrinin.
4.1.5 Effects in man
4.1.5.1 Ochratoxin A and Balkan nephropathy
Balkan endemic nephropathy is a kidney disease only observed so
far in rural populations in Bulgaria, Romania, and Yugoslavia. In
the past 2 decades, etiological investigations covering bacteria,
viruses, toxic metals, genetic factors, etc. have been conducted but
with unconvincing results (reviewed Puchlev, 1973, 1974). Balkan
endemic nephropathy is a chronic disease that is commonest between
30 and 50 years of age and progresses slowly up to death. The
kidneys are remarkably reduced in size. Histologically, the renal
disease is characterized by tubular degeneration, interstitial
fibrosis, and hyalization of glomeruli in the more superficial part
of the cortex (Heptinstall, 1966). Impairment of tubular function,
indicated by a decrease in TmPAH, is a prominent and early sign
(Dotchev, 1973).
The disease occurs endemically and affects females more often
than males (Hrabar et al., 1976, Chernozemsky et al., 1977). In
Bulgaria and Yugoslavia, a high incidence of urinary tract tumours
has been found to be closely correlated with the incidence and
mortality rates of Balkan endemic nephropathy (Ceovic et al., 1976;
Chernozemsky et al., 1977).
Fungal growth in foodstuffs and subsequent mycotoxin formation
is influenced by the water content of the foodstuffs, and can be
changed by climatic conditions such as heavy rainfalls during
harvest. Thus, the observation (Austwick, 1975) of a positive
correlation ( r = 0.80) between excess rainfall and the number of
people who died of nephropathy during the succeeding 2 years in the
Balkan peninsula might be interpreted as suggesting a fungal
involvement in the etiology of endemic nephropathy.
Attention has been called to the striking similarities in the
changes of renal structure and function found in Balkan endemic
nephropathy and in ochratoxin A-induced porcine nephropathy,
suggesting common causal relationships (Krogh, 1974). Furthermore,
epidemiological similarities have been noted, in particular, the
endemic occurrence (Krogh, 1976b). Preliminary results of a survey
of foodstuff's indicate that exposure to food-borne ochratoxin A
seems to be higher (12.8% contamination) in an area of Yugoslavia
with a high prevalence of human endemic nephropathy than in
nonendemic (control) areas (1.6% contamination) (Krogh et al.,
1977).
4.1.6 Conclusions and evaluation of the health risks to man
of ochratoxins
4.1.6.1 Experimental animal studies
The toxic effects of ochratoxin A have been studied extensively
in a variety of experimental animals. All the animals studied so far
have been susceptible to orally administered ochratoxin A, but to
various degrees, as indicated by the range of LD50 values
(Table 24). At high levels of ochratoxin A, changes were found in
the kidneys and also in other organs and tissues. However, only
renal lesions were observed at exposure levels identical to those
occurring environmentally. The renal lesions included degeneration
of the tubules, interstitial fibrosis, and, at later stages,
hyalinization of glomeruli, with impairment of tubular function as a
prime manifestation. Feed levels as low as 200 µg/kg produced renal
changes in the course of 3 months in rats and pigs. Field cases of
ochratoxin A-induced nephropathy are regularly encountered in pigs
and poultry. Ochratoxin A is teratogenic in the mouse, rat, and
hamster.
Ochratoxin B, rarely found as a natural contaminant, is much
less toxic; the other ochratoxins have never been encountered in
natural products.
4.1.6.2 Studies in man
The ochratoxin A-induced nephropathy in farm animals is similar
to Balkan endemic nephropathy in several aspects. In a preliminary
study in an area where Balkan endemic nephropathy is prevalent, the
ochratoxin A contamination of food appeared to be more frequent than
in control areas. However, the hypothesis that ochratoxin A may be a
causal determinant in this disease, needs further support.
4.1.6.3 Evaluation of health risks
The nephrotoxic potential of ochratoxin A is well documented
from all experimental studies, with a feed level of 200 µg/kg
causing nephropathy in pigs and rats. Lower levels have not been
tested. Field eases of ochratoxin A-induced nephropathy in farm
animals have long been recognized. The toxin has been found in a
variety of foodstuffs, with levels in commodities used as feed
ranging up to 27 mg/kg, and with levels in foodstuffs used for human
consumption in the range of trace to about 100 µg/kg. In one area
where endemic nephropathy was prevalent in the human population,
home produced foodstuffs were more frequently contaminated with
ochratoxin A than those from control areas. However, the total
intake of ochratoxin A by man has not been assessed so far, and
there is, at present, no proof that ochratoxin A is causally
involved in human diseases.
4.2 Zearalenone
4.2.1 Properties, analytical methods, and sources
The properties, analytical methods, sources, and occurrence of
zearalenone have been reviewed by Mirocha & Christensen (1974),
Pathre & Mirocha (1976), and Mirocha et al. (1977). Zearalenone is a
phenolic resorcylic acid lactone (Fig. 5), classified, according to
biosynthetic origin, as a nonaketide within the group polyketides
(Turner, 1971). Zearalenone (C18H22O5) is a white crystalline
compound with a relative molecular mass of 318, melting point
164-165 °C, and absorption maxima (and absorption coefficient) at
236 nm (29 700), 274 nm (13 909) and 316 nm (6020).
Zearalenone exhibits blue-green fluorescence when excited by
long wavelength (360 nm) UV-light, and a more intense green
fluorescence when excited with short wavelength (260 nm) UV-light.
A number of derivatives of zearalenone have been isolated from
fungal cultures (Fig. 7), but none of these derivatives has been
encountered, so far, as a natural contaminant of foodstuffs.
A multiple detection method for aflatoxin, ochratoxin, and
zearalenone has been developed (Eppley, 1968) and tested
collaboratively (Shotwell et al., 1976b). The procedure consists of
water-chloroform extraction combined with sequential elution of the
mycotoxins from a silica-gel column and the detection limit is in
the range of 50-100 µg/kg. A versatile method of analysis for
zearalenone has been described by Mirocha et al. (1974) using
thin-layer chromatography (TLC), gas-liquid chromatography (GLC),
gas-liquid chromatography-mass spectrometry, or a combination of all
these methods; the limit of detection is about 50 µg/kg. The
derivatives dimethoxyzearalenone and methyl oxime-di-TMS-zearalenone
are used to confirm the identity of zearalenone. Two methods using
high pressure liquid chromatography (HPLC) are now available (Scott
et al., 1978; Ware & Thorpe, 1978). With HPLC, a concentration of
zearalenone in cornflakes of 5 µg/kg could be determined (Scott et
al., 1978). Zearalenone is produced by strains of Fusarium
graminearum, F. tricinctum, F. oxysporum, F. sporotrichioides, and
F. moniliforme, and a period of low temperature ( 12°-14°C) during
fungal formation seems essential for high yield.
 4.2.2 Occurrence
The occurrence of zearalenone in foodstuffs has been reviewed by
Stoloff (1976). Zearalenone has been encountered as a natural
contaminant, particularly in maize, but occasionally in other
cereals and in feedstuffs. In a survey of maize in the USA during
the period 1968-69, zearalenone was found in 6 out of 576 (1%)
samples at levels ranging from 450 to 800 µg/kg (Shotwell et al.,
1971). In 1972, when conditions in the USA were conducive to
Fusarium ear rot, zearalenone was found in 17% of 223 samples of
maize at levels ranging from 0.1 to 5.0 mg/kg (Eppley et al., 1974).
It has also been detected in maize in France and in barley and mixed
feed in England, Finland, and Yugoslavia (Stoloff, 1976).
Nine out of 11 commercial corn meal samples in the USA contained
zearalenone at levels ranging from 12 to 69 µg/kg (Ware & Thorpe,
1977). The compound has been found in one sample of cornflakes
(13 µg/kg) (Scott et al., 1978), and in maize beer, in Zambia, in
the range of 0.01-4.6 mg/litre (Lovelace & Nyathi, 1977).
Zearalenone was detected in 11% of 55 samples of Swazi sour
drinks, sour porridges, and beers (range: 8-53 mg/kg) and in 12% of
140 beer samples from Lesotho (range: 0.3-2 mg/kg), but such high
figures have not been reported elsewhere. No data on consumption
were given (Martin & Keen, 1978).
4.2.3 Effects in animals
4.2.3.1 Field observations
The effects of zearalenone in animals have been reviewed by
Mirocha & Christensen (1974). Field cases of the estrogenic syndrome
in pigs, associated with the use of mouldy feed, were first observed
half a century ago. The disease was characterized by enlarged
oedematous vulvae and mammary glands (McNutt et al., 1928).
Subsequently, this syndrome has been encountered in a number of
countries in North America and Europe and in Australia (Table 25),
and in most cases zemralenone has been identified in associated
feeds, indicating together with the result of experimental studies
(4.2.2.2) a causal role of this compound. Zearalenone feed levels
reported to be associated with the syndrome in swine (Mirocha et
al., 1977) are listed in Table 26. Cases of reduced fertility in
cattle indicated by an increase in the artificial insemination index
have been reported to be associated with a feed (hay) content of
zearalenone of 14 mg/kg (Mirocha et al., 1968b). Fertility
disturbances and prolonged heat in a herd of cattle suggested to be
associated with zearalenone-contaminated feed were reported by Roine
et al. (1971).
Table 25. Occurrence of the estrogenic syndrome in pigs in
various countriesa
Year Country Feedstuff
1928 USA maize
1937 Australia maize
1952 Ireland barley
1962 France maize
1962 Italy maize
1963 Yugoslavia maize and barley
1967 Romania maize
1968 Hungary maize
1968 Denmark barley
1971 Canada maize
a From: Mirocha & Christenson (1974).
Table 26. Natural occurrence of zearalenone in feeds associated with
hyperestrogenism in swinea
Feed sample Level of
contamination
(mg/kg)
maize kernels (Minnesota)b 0.1-0.15
dry sow ration (Vancouver) 0.15
farrowing ration (Vancouver)c 0.066
dry sow ration (Vancouver) 0.15
corn kernels (Vancouver) 0.20
dry sow ration (Vancouver)c 0.25
lactation ration (Vancouver) 1.00
gestation ration (Vancouver) 0.6
milo (Minnesota) 2.5-5.6
sesame meal (Univ. of Minn.)d 1.5
corn kernels (Ohio) 0.12
mixed feed corn (Ohio)b 0.12
corn kernels (Minnesota) 6.4
commercial pelletted mixed feed 6.8
(Minnesota)
a From Mirocha et al. (1977).
b Rectal prolapse in gilts.
c Diethylstilbestrol was also present in these samples.
d Associated with hyperestrogenism in turkey poults.
4.2.3.2 Experimental studies
Under experimental conditions, pigs (6-week-old-gilts) exposed
perorally to zearalenone at 5 mg per animal per day for 5 days
developed enlarged vulvae and mammae, and prolapse of the vagina
within a few days; effects were reversible on termination of
exposure. In another experiment, oral administration of 8 mg of pure
crystalline zearalenone to 6-week-old, pre-pubertal gilts (8 doses
of 1 mg/day per animal) induced pronounced tumefaction of the vulva
(Mirocha & Christensen, 1974). Histological changes in the genital
tract of pigs exposed to zearalenone included metaplasia of the
epithelium in the cervix and vagina, and oedema in the wall of the
uterus (Kurtz et al., 1969).
Observations on pigs (Miller et al., 197.3) suggested that
ingestion of grain contaminated with zearalenone during late
gestation might be related to still-birth and splayleg. Splayleg was
observed in the offspring of one sow and one gilt given dally
intramuscular injections of zearalenone at 5 mg per animal
throughout the last month of pregnancy. However, in the experiment
of Patterson et al. (1977), all 7 gilts fed zearalenone at a level
of 2 mg/kg feed throughout pregnancy remained clinically normal and
embryonic survival rates were not affected by the toxin. Splayleg
was diagnosed in only 1/63 piglets and the condition appeared to
have resolved within 24 h. Results in the 2 groups of 3 pigs fed the
lower levels were inconclusive (unpublished data, Chang & Kurtz,
quoted by Mirocha et al., 1977).
No effects on egg production were observed when laying hens were
fed rations containing zearalenone levels of 250 and 500 mg/kg
(Speers et al., 1971).
In a 2-generation study on rats exposed to dietary levels of
zearalenone corresponding to daily intakes of 0.1, 1.0, and 10 mg/kg
body weight, no teratogenic effects were observed at any level of
intake but impaired fertility and resorptions and stillbirths
occurred in animals receiving 10 mg/kg body weight daily with 56% of
dams showing complete litter resorption (Bailey et al., 1976).
Ruddick et al. (1976) found fetal skeleton anomalies in rats
exposed orally to zearalenone at doses ranging from 1-10 mg/kg body
weight during the gestation period, with defect incidences of 12.8%
(11/86) at the 1 mg/kg level, 26.1% (18/69) at the 5 mg/kg level,
and 36.8% (28/76) at the 10 mg/kg level. No effect was observed with
exposure to zearalenone at a dose of 0.075-0.30 mg/kg body weight.
The results of a study by Mirocha et al. (1968a) in which the
dose-effect relationships for zearalenone and estrone were compared
with regard to increase in uterus weight, are given in Table 27. The
results of a study comparing the effects of estrogens and
zearalenone in 24 adult female castrated rhesus monkeys with a
previous history of regular menstrual cycles are recorded in Table
28.
Table 27. Effect of peroral dosing of zearalenone and estrone in micea
Material Total dose No. of Mean uterine
administered (µg) mice ratio ± S.E.
control 0 10 0.76 ± 0.06
estrone 0.5 10 1.21 ± 0.12
1 9 1.19 ± 0.10
2 9 2.12 ± 0.23
4 10 2.93 ± 0.21
zearalenone 12.5 10 1.11 ± 0.04
25 10 1.48 ± 0,18
50 10 1.80 ± 0.12
100 9 2.35 ± 0.17
a From: Mirocha et al. (1968a).
4.2.4 Conclusions and evaluation of the health risks to man of
zearalenone
4.2.4.1 Animal studies
Field cases of the estrogenic syndrome in pigs have been
encountered in many countries in association with zearalenone in
feeds at levels ranging from 0.1 to 6.8 mg/kg (Table 26), in samples
not known to be contaminated with other estrogens. The condition has
been reproduced experimentally in pigs, with a dally dose of
1 mg/animal for 8 days. Infertility, sporadically encountered in
cattle, has been suggested to be causally associated with
zearalenone in feed at a reported level of 14 mg/kg of hay.
In one of two studies on teratogenic effects in rats, skeletal
defects were detected in fetuses at a daily oral dose of 1 mg/kg
body weight.
Table 28. The estimated minimum dose of estrogens that will depress
serum gonadotropin (FSH or LH) in castrated rhesus monkeysa
Estrogen administered Estimated minimum dose
(µg/kg)
Subcutaneousb Oralc
LHd FSHd LH FSH
estradiol-17ß 4 1 5 --
diethylstilboestrol 0.5 2 2.5 --
zearalenone 14 56 400 --
a From: Hobson et al. (1977).
b Two injections given in oil.
c Given on 4 consecutive days,
d FSH = follicle-stimulating hormone; LH = luteinizing hormone.
4.2.4.2 Evaluation of health risks
There are no reports on the adverse effects of zearalenone in
man. With the exception of 2 reports from Africa, levels of
zearalenone ranging from 12 to 69 µg/kg have been found in a limited
number of maize products destined for human consumption. Even
assuming that 1 kg of these products is consumed dally, it can be
estimated that a 70 kg man would not receive more than 1 µg
zearalenone per kg body weight in food. This level of exposure is
400 times lower than the lowest peroral dose causing effects in
tests on monkeys (Table 28) and more than 600 times lower than the
lowest dose (µg/kg) used in assessing the estrogenic potency of
perorally administered zearalenone in mice (Table 27).
However, in the 2 reports from certain parts of Africa, high
levels of zearalenone were found in beer and sour porridge prepared
from maize and sorghum. Long-term exposure to such contaminated
drinks could represent a health hazard.
4.3 Trichothecenes
4.3.1 Properties and sources
The properties and sources of trichothecenes have been reviewed
by Bamburg & Strong (1971), Smalley & Strong (1974), and Bamburg
(1976). The trichothecenes possess the tetracyclic
12,13-epoxytrichothec-9-ene skeleton. More than 30 trichothecene
derivatives have been isolated from fungal cultures, but, so far,
only 4 have been identified as natural contaminants of foodstuffs
(Table 29). The compounds have been detected by various methods
including thin-layer chromatography, gas chromatography, and
bioassays, in particular the rabbit skin test.
One or more of the trichothecenes have been isolated from
strains of the following Fusarium species: F. episphae,
F. lateritium, F. nivale, F. oxysporum, F. rigidisculum,
F. solani, F. roseum, and F. tricinctum (syn.
F. sporotrichioides). In addition, species of Cephalosporium,
Myrothecium, Trichoderma, and Stachybotrys have been found to
produce trichothecenes.
Table 29. Natural occurrence of trichothecenes
Compound Concentration Commodity Reference
(µg/kg)
T-2 toxin 2 maize Hsu et al. (1972)
T-2 toxin 25 barley Puls & Greenway (1976)
T-2 toxin 0.076 mixed feed Mirocha et al. (1976)
nivalenol n.q.a barley Morooka et al. (1972)
deoxynivalenol 7.3 barley Morooka et al, (1972)
deoxynivalenol n.m.b maize Vesonder et al. (1973)
deoxynivalenol 0.1, 1.0, 1.8 maize Mirocha et al. (1976)
(3 samples)
deoxynivalenol 1.0, 1.0, 0.06 mixed feed Mirocha et al. (1976)
(3 samples)
diacetoxyscirpenol 0.38, 0.5 mixed feed Mirocha et al. (1976)
(2 samples)
a n.q. = not quoted
b n.m. = not measured
4.3.2 Occurrence
So far, the trichothecenes have only been found very
sporadically in natural products. Naturally occurring trichothecenes
include the following, based on chemical identification: T-2 toxin,
nivalenol, deoxynivalenol, (vomitoxin), and diacetoxyscirpenol. In
available studies, these 4 compounds have only been found in a total
of 14 samples (Table 29), sometimes (as in the case of
deoxynivalenol) concomitantly with zearalenone. No information is
available concerning the number of samples analysed in these studies
and therefore on the frequency of positive results.
4.3.3 Effects in animals
4.3.3.1 Field observations
A field case involving the death of 20% of a dairy herd was
suggested to be associated with ingestion of mouldy corn in the feed
containing a concentration of T-2 toxin of approximately 2 mg/kg dry
weight (Hsu et al., 1972). The lesions in the cattle included
extensive haemorrhages on the serosal surface of all internal
viscera.
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was suggested to be associated with mouldy barley
containing T-2 toxin at approximately 25 mg/kg (Greenway & Puls,
1976). The lesions in the geese included necrosis of the mucosa of
the oesophagus, proventriculus, and gizzards.
Deoxynivalenol (vomitoxin) was isolated from a batch of maize
that had caused vomiting in pigs (Vesonder et al., 1973).
4.3.3.2 Experimental studies
Acute effects of trichothecenes determined as LD50 values, are
listed in Table 30. Sato et al. (1975) studied tile effects in cats
of T-2 toxin, administered in several ways. Two cats weighing 1.5
and 4.0 kg were given purified T-2 toxin in single subcutaneous
doses of 0.5 and 1.0 mg/kg, respectively. Nausea and vomiting
appeared after 1 h and the animals died 20 h after the injection.
The autopsy revealed extensive necrosis of the mucosa in the small
and large intestine, marked karyorrhexis in the germ centre of the
lymph follicles of the spleen and lymph nodes, and diffuse vacuolar
degeneration of the renal tubules. Damage to bone marrow cells was
observed in the cervical, thoracic, and lumbar vertebrae. In two
cats subcutaneously injected with T-2 toxin (repeated doses of 0.1
and 0.05 mg/kg body weight, given over a 4-week period) a decrease
in the white blood cell (WBC) count was observed and the WBC value
remained low until death occurred during the fourth week of
exposure. The clinical signs in the cats were nausea and vomiting a
few hours after each injection and ataxia of the hind legs. At the
postmortem examination, hypoplasia was observed in the thymus,
spleen, lymph nodes, and bone marrow. Other changes included marked
meningeal haemorrhage, extensive bleeding in the lung, and vacuolar
degeneration of the renal tubular epithelium. A marked decrease in
the WBC values was also observed in 3 cats given T-2 toxin
subcutaneously at a daily dose of 0.05 mg/kg for 12 days (i.e.,
total dose of 0.60 mg/kg); 2 of these cats died, 4 and 35 days,
respectively, after the last injection and one cat survived with the
WBC count returning to the normal level 17 days after the last
injection. Leukopenia and death were also observed in 3 cats (body
weight 2-3 kg) receiving a crude preparation containing 4% T-2 toxin
and 1% neosolaniol. This crude preparation was first administered
subcutaneously once a week for 5 weeks in repeated doses of 1 mg/kg
body weight (corresponding to a repeated dose of T-2 toxin of
0.04 mg/kg). This subcutaneous administration was then followed by
daily oral dosing (15 mg of the crude preparation corresponding to
0.6 mg of T-2 toxin per animal per day) for 17 days.
Table 30. Acute toxicity of naturally occurring trichothecenesa
Compound LD50 Route of Animal
(mg/kg body weight) administration
T-2 toxin 3.04 intraperitoneal mouse
T-2 toxin 3.8 peroral rat
T-2 toxin 5.1 peroral trout
T-2 toxin 5.25 peroral one-day old chickb
nivalenol 4.0 intraperitoneal mouse
diacetoxyscirpenol 10.0 intravenous mouse
diacetoxyscirpenol 0.75 intraperitoneal rat
diacetoxyscirpenol 7.3 peroral rat
a From: Bamburg & Strong (1971 ).
b From: Chi at al. (1977).
More recently Yagen et al. (1978) have mentioned an experiment,
in which oral administration of gelatin capsules containing purified
T-2 toxin to 10 cats resulted in vomiting, leukopenia, haemorrhagic
diathasis, neurological disturbances, and death. The dose and
frequency of administration are not given in the paper.
The effects observed in these experiments, in particular
leukopenia, resemble those produced when cats are fed cultures of
F. sporotrichioides, which is thought to be causally associated
with alimentary toxic aleukia (ATA) in man (section 4.3.4).
Feeding laying hens T-2 toxin at a dietary level of 20 mg/kg for
3 weeks, resulted in oral necrotic lesions, decreased leukocyte
count, and reduced egg production (Wyatt et al., 1975). In mice,
intraperitoneal injection with T-2 toxin at doses of 1.0 and
1.5 mg/kg body weight on one of the days 7-11 of gestation resulted
in a number of maternal deaths as well as in an increase in prenatal
mortality. Malformations of the fetuses were observed including tail
and limb malformations, exencephaly, and retarded jaw development
(Stanford et al., 1975).
Daily oral doses of crude or purified T-2 toxin (0.2 mg/kg body
weight) continued for 79 days failed to produce ill effects in
calves, although the total amount of toxin ingested by one calf was
almost 1.8 g (Matthews et al., 1977).
4.3.4 Alimentary toxic aleukia
Studies on alimentary toxic aleukia (ATA), a disease encountered
in man in the period 1931-43, have been reviewed by Sarkisov (1954)
and more recently by Bilai (1977) and Leonov (1977). The dominant
pathological changes were necrotic lesions of the oral cavity, the
oesophagus, and stomach, and in particular a pronounced leukopenia.
The disease was lethal in a high proportion of cases. An association
was established with ingestion of grain invaded by some moulds, in
particular Fusarium poae and F. sporotrichioides. Effects similar
to ATA have been reproduced in cats by feeding cultures of these
species (Bilai, 1977). T-2 toxin and other trichothecenes have been
identified in a submerged culture of F. sporotrichioides by
Mirocha & Pathre (1973).
4.3.5 Conclusions and evaluation of the health risks to man of
trichothecenes
In recent years, a group of mycotoxins, the trichothecenes, has
been isolated under experimental conditions from many fungi,
including Fusarium species. Isolated field cases of intoxication
in farm animals have been suggested to be related to some of the
trichothecenes.
About 40 years ago, a disease in man known as alimentary toxic
aleukia (ATA), occurred that was suggested to be related to the
presence of toxic Fusarium species in mouldy over-wintered grain.
With improved harvesting, food production, and storage conditions,
the disease disappeared and no new outbreaks have occurred. However,
present knowledge is not sufficient to establish a causal
relationship between any of the isolated trichothecenes and this
outbreak. There are no reports on the exposure of man to
trichothecenes.
REFERENCES
ADAMSON, R. H., CORREA, P., & DALGARD, D. W. (1973) Brief
communication: Occurrence of a primary liver carcinoma in a rhesus
monkey fed aflatoxin B1. J. Natl Cancer Inst., 50:549-551.
ADAMSON, R. H., CORREA, P., SIEBER, S. M., MCINTIRE, K. R., &
DALGARD, D. W. (1976) Carcinogenicity of aflatoxin B1 in rhesus
monkeys: Two additional cases of primary liver cancer. J. Natl
Cancer Inst., 57: 67-78.
ADENKUNLE, A. A. & ELEGBE, R. A. (1974.) Lysosomal activity in
aflatoxin-B1-treated avian embryos. Lancet, 1: 991.
ALLCROFT, R. (1969) Aflatoxicosis in farm animals. In: Goldblatt,
L.A., ed. Aflatoxin scientific background, control and
implications, New York, Academic Press, pp. 237-264.
ALLCROFT, R. & LEWIS, G. (1963) Groundnut toxicity in cattle:
Experimental poisoning of calves and a report on clinical effects in
older cattle. Vet. Rec., 75: 487-493.
ALLCROFT, R. & ROBERTS, B. A. (1968) Toxic groundnut meal: The
relationship between aflatoxin B1 intake by cows and excretion of
aflatoxin M1 in milk. Vet. Rec., 82: 116-118.
ALLCROFT, R., ROBERTS, B. A., & LLOYD, M. K. (1968) Excretion of
aflatoxin in a lactating cow. Food Cosmet. Toxicol., 6: 619-625.
ALPERT, g. E., HUTT, g. S. R., WOGAN, G. N., & DAVIDSON, C. S.
(1971) Association between aflatoxin content of food and hepatoma
frequency in Uganda. Cancer, 28: 253-260.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973)
Carcinogens are mutagens: A simple test system combining liver
homogenates for activation and bacteria for detection. Proc. Natl
Acad. Sci. USA, 70: 2281-2285.
AMLA, I., KAMALA, C. S., GOPALAKRISHNA, G. S., JAYARAJ, A. P.,
SREENIVASAMURTHY, V., & PARPIA, H. A. B. (1971) Cirrhosis in
children from peanut meal contaminated by aflatoxin. Am. J. clin.
Nutr., 24: 609-614.
ANDERSON, H. W., NEHRING, E. W., & WICHSER, W. R. (1975) Aflatoxin
contamination of corn in the field. J. agric. food Chem., 23:
775-782.
ANDRELLOS, P. J. & REID, G. R. (1964) Confirmatory tests for
aflatoxin B1. J. Assoc. Off. Anal. Chem., 47: 801-803.
AMBRECHT, B. H., SHALKOP, W. T., ROLLINS, L. D., POHLAND, A. E., &
STOLOFF, L. (1970) Acute toxicity of aflatoxin B1 in wethers.
Nature (Lond.), 225: 1062.
ASAO, T., BUCHI, G., ABDEL-KADER, M., CHANG, S. B., WICK, E. L., &
WOGAN, G. N. (1963) Aflatoxins B and G. J. Am. Chem. Soc., 85:
1706-1707.
ASAO, T., BUCHI, G., ABDEL-KADER, M. M., CHANG, S. B., WICK, E. L.,
& WOGAN, G. N. (1965) The structures of aflatoxins B and G1. J.
Am. Chem. Soc., 87: 882-886.
ASHWORTH, L. J., JR, MCMEANS, J. L., HOUSTON, B. R., WITTEN, M. E.,
& BROWN, C. M. (1971) Mycoflora, aflatoxins and free fatty acids in
California cottonseed during 1967-1968. J. Am. Oil Chem. Soc., 48:
129-133.
ASPLIN, F. D. & CARNAGHAN, R. B. A. (1961) The toxicity of certain
groundnut meals for poultry with special reference to their effect
on ducklings and chickens. Vet. Rec., 73: 1215-1218.
AUSTWICK, P. K. C. (1975) Balkan nephropathy. Proc. R. Soc. Med.,
68: 219-221.
BABABUNMI, E. A. & BASSIR, O. (1972) Effects of aflatoxin B1 on
the swelling and adenosine triphosphatase activities of mitochondria
isolated from different tissues of the rat. Febs Lett., 26:
102-104.
BACON, C. W., SWEENEY, J. G., ROBBINS, J. D., & BURDICK, D. (1973)
Production of penicillic acid and ochratoxin A on poultry feed by
Aspergillus ochraceus: Temperature and moisture requirements.
Appl. Microbiol., 26: 155-160.
BAGSHAWE, A. F., GACENGI, D. M., CAMERON, C. H., DORMAN, J., & DANE,
D. S. (1975) Hepatitis Bs antigen and liver cancer in Kenya.
Br. J. Cancer, 31: 581-584.
BAILEY, D. E., Cox, G. E., MORGAREIDGE, K., & TAYLOR, J. (1976)
Acute and subacute toxicity of zearalenone in the rat. TAP, 37:
144.
BAMBURG, J. R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American
Chemical Society, pp. 144-162 (Advances in Chemistry Series 149).
BAMBURG, J. R. & STRONG, F. M. (1971) 12,13-epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S. J., ed. Microbial toxins, New
York, Academic Press, Vol. VII, pp. 207-289.
BASSIR, O. & BABABUNMI, E. A. (1969) Inhibition of blood clotting
factors by aflatoxin. West Aft. J. biol. appl. Chem., 12: 28-32.
BASSIR, O. & OSIYEMI, F. (1967) Biliary excretion of aflatoxin in
the rat after a single dose. Nature (Lond.), 215: 882.
BECKWITH, A. C., VESONDER, R. F., & CIEGLER, A. (1975) Action of
weak bases upon aflatoxin B1 in contact with macromolecular
reactants. J. agric. food Chem., 23: 582-587.
BECROFT, D. M. O. (1966) Syndrome of encephalopathy and fatty
degeneration of viscera in New Zealand children. Br. med. J., 2:
135-140.
BECROFT, D. M. O. & WEBSTER, D. R. (1972) Aflatoxins and Reye's
disease. Br. med. J., 14 Oct., p. 117.
BILAI, V. I. (1977) [Fuzarii.] Kiev, Naukova Dumka, pp. 360-394 (in
Russian).
BLACK, H. S., SMITH, J. D., AUSTIN, B. J., & RAUSCHKOLB, E. W.
(1970) Effect of aflatoxin B1 on the in vitro incorporation of
14C-acetate into human skin lipids. Experientia (Basel), 26:
1292-1293.
BOURGEOIS, C. H. (1975) Encephalopathy and fatty viscera: A possible
response to acute aflatoxin poisoning. In: Pollack, J. D., ed.
Reye's Syndrome, Grune & Stratton, New York, pp. 131-134.
BOURGEOIS, C. H., SHANK, R. C., GROSSMAN, R. A., JOHNSEN, D. O.,
WOODING, W. L., & CHANDAVIMOL, P. (1971) Acute aflatoxin B1
toxicity in the macaque and its similarities to Reye's Syndrome.
Lab. Invest., 24: 206-216.
BROWN, J. M. M. & ABRAMS, L. (1965)Biochemical studies on
aflatoxicosis. Onderstepoort J. vet. Res., 32: 119-146.
BROWN, M. H., SZCZECH G. M., & PURMALIS, B. P. (1976) Teratogenic
and toxic effects of ochratoxin A in rats. Toxicol. appl.
Pharmacol., 37: 331-338.
BUCARBAEVA, A. S. & NIKOV, P.S. (1977) [Aflatoxin sources in
foodstuffs from different regions of Kazachstan.] In: [ Mycotoxins
(sources, chemistry, biosynthesis, analysis and effects on the
organism), Scientific Papers of a Regional Symposium, 28-30
September 1977.] Orenburg, Nutrition Institute of the USSR Academy
of Medical Sciences, pp. 29-31 (in Russian).
BUCHI, G. & WEINREB, S. M. (1969) The total synthesis of racemic
aflatoxin-M1 (milk toxin). J. Am. Chem. Soc., 91: 5408-5409.
BUCHI, G., SPITZNER, D., PAGLIALUNGA, S., & WOGAN, G. N. (1973)
Synthesis and toxicity evaluation of aflatoxin P1. Life Sci.,
13: 1143-1149.
BUCKLEY, H. G. (1971) Fungal nephrotoxicity in swine. Irish
vet. J., 25: 194-196.
BULATAO-JAYME, J., ALMERO, E. M., & SALAMAT, L,. (1976) Epidemiology
of primary liver cancer in the Philippines with special
consideration of a possible aflatoxin factor. J. Philipp. Med.
Assoc., 52: 129-150.
BUTLER, W. H. (1964) Acute toxicity of aflatoxin B1 in rats.
Br. J. Cancer, 18: 756-762.
BUTLER, W. H. (1966) Acute toxicity of aflatoxin B1 in guinea
pigs. J. Pathol. Bacteriol., 91: 277-280.
BUTLER, W. H. (1969) Aflatoxicosis in laboratory animals. In:
Goldblatt, L.A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 223-236.
BUTLER, W. H. (1971-1972) Further ultrastructural observations on
injury of rat hepatic parenchymal cells induced by aflatoxin B1.
Chem. biol. Interactions, 4: 49-65.
BUTLER, W. H. (1974) Aflatoxin. In: Purchase, I. F. H., ed.
Mycotoxins, Amsterdam, Elsevier, pp. 1-28.
BUTLER, W. H. & BARNES, J. M. (1963) Toxic effects of groundnut meal
containing aflatoxin to rats and guinea pigs. Br. J. Cancer, 17:
699-710.
BUTLER, W. H. & BARNES, J. M. (1966) Carcinoma of the glandular
stomach in rats given diets containing aflatoxin. Nature (Lond.),
209: 90.
BUTLER, W. H. & WIGGLESWORTH, J. S. (1966) The effects of aflatoxin
B1 on the pregnant rat. Br. J. exp. Pathol., 47: 242-247.
BUTLER, W. H., GREENBLATT, M., & LIJINSKY W. (1969) Carcinogenesis
in rats by aflatoxins B1, G1, and B2. Cancer Res., 29:
2206-2211.
CAMPBELL, T. C. & SALAMAT, L. (1971) Aflatoxin ingestion and
excretion by humans. In: Purchase, I. F. H., ed. Mycotoxins in human
health, London, Macmillan Press, pp. 271-280.
CAMPBELL, T. C. & STOLOFF, L. (1974) Implication of mycotoxins for
human health. J. agric. food Chem., 22: 1006-1015.
CAMPBELL, T. C., CAEDO, J.P., JR, BULATAO-JAYME, J., SALAMAT, L., &
ENGEL, R. W. (1970) Aflatoxin M1 in human urine. Nature (Lond.),
227: 403-404.
CARNAGHAN, R. B. A. (1965) Hepatic tumours in ducks fed on low level
of toxic ground-nut meal. Nature (Lond.), 208, 308.
CARNAGHAN, R. B. A. (1967) Hepatic tumours and other chronic liver
changes in rats following a single oral administration of aflatoxin.
Br. J. Cancer, 21: 811-814.
CARNAGHAN, R. B. A., HARTLEY, R. D., & O'KELLY, J. (1963) Toxicity
and fluorescence properties of the aflatoxins. Nature (Lond.),
200: 1101.
CARNAGHAN, R. B. A., LEWIS, G., PATTERSON, D. S. P., & ALLCROFT, R.
(1966) Biochemical and pathological aspects of groundnut poisoning
in chickens. Pathol. Vet., 3: 601-615.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijee vjesn., 98: 301-304 (in Croatian).
CHAINUVATI, T., VIRANUVATT, V., & PONGPIPAT, D. (1975) Relationship
of hepatitis B antigen in cirrhosis and hepatoma in Thailand. An
etiological significance. Gastroenterology, 68: 1261-1264.
CHANG, F. C. & Car, F. S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, S. B., ABDEL-KADER, M. M., WICK, E. L., & WOGAN, G. N. (1963)
Aflatoxin B2: Chemical identity and biological activity.
Science, 142: 1191-1192.
CHAVES-CARBALLO, E., ELLEFSON, R. D., & GOMEZ, M. R. (1976) An
aflatoxin in the liver of a patient with Reye-Johnson syndrome.
Mayo Clin. Proc., 51: 48-50.
CHERNOZEMSKY, I. N., STOYANOV, I. S., PETKOVA-BOCHAROVA, T. K.,
NICOLOV, I. G., DRAGANOV, I. V., STOICHEV, I. I., TANCHEV, Y.,
NAIDENOV, D., & KALCHEVA, W. D. (1977) Geographic correlation
between the occurrence of endemic nephropathy and urinary tract
tumours in Vratza district, Bulgaria. Int. J. Cancer, 19: 1-11.
CHI, M. S., MIROCHA, C. J., KURTZ, M. Y., WEAVER, G., BATES, F., SHIMODA,
W., & BURMEISTER, H. R. (1977) Acute toxicity of T-2 toxin in
broiler chicks and laying hens. Poult. Sci., 56: 103-116.
CHU, F. S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F. S. (1974a) Studies on ochratoxins. CRC crit. Rev.
Toxicol., 2: 499-524.
CHU, F. S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol.,
23: 1105-1113.
CIEGLER, A., KADIS, S., & AJL, S. J., ed. (1971) Microbial toxins,
fungal toxins, New York, Academic Press, Vol. VI, p. 563.
CLEGG, F. G. & BRYSON, H. (1962) An outbreak of poisoning in store
cattle attributed to Brazilian groundnut meal. Vet. Rec., 37:
992-994.
CLIFFORD, J. I. & REES, K. R. (1966) Aflatoxin: A site of action in
the rat liver cell. Nature (Lond.), 209: 312-313.
CLIFFORD, J. I. & REES, K. R. (1967) The action of aflatoxin B, on
the rat liver. Biochem. J., 102: 65-75.
COON, F. B., BAUR, F. J., & SYMMES, L. R. L. (1972) International
aflatoxin check sample program: 1971 study. J. Assoc. Off. Anal.
Chem., 55: 315-327.
CUTHBERTSON, W. F. J., LAURSEN, A. C., & PRATT, D. A. H. (1967)
Effect of groundnut meal containing aflatoxin on Cynomolgus monkeys.
Br. J. Nutr., 21: 893-908.
DALEZIOS, J. I. & WOGAN, G. N. (1972) Metabolism of aflatoxin B1
in rhesus monkeys. Cancer Res., 32: 2297-2303.
DALEZIOS, J. I., WOGAN, G. N., & WEINREB, S. M. (1971) Aflatoxin
P1: A new aflatoxin metabolite in monkeys. Science, 171:
584-585.
DAVIS, N. D. & DIENER, U. L. (1970) Environmental factors affecting
the production of aflatoxin. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on "Toxic Microorganisms",
Washington, DC, US Govt Printing Office, pp. 43-47.
DEGER, G. E. (1976) Aflatoxin-Human colon carcinogenesis? Ann.
int. Med., 85: 204.
DE IONGH, H., BEERTHUIS, R. K., VLES, R. O., BARRETT, C. B., & ORD,
W. O. (1962) Investigation of the factor in groundnut meal
responsible for turkey X disease. Biochim. Biophys. Acta, 65:
548-551.
DEO, M. G., DAYAL, Y., & RAMALINGASWAMI, V (1970) Aflatoxins and
liver injury in the rhesus monkey. J. Pathol., 101 47-56.
DETROY, R. W. & HESSELTINE, C. W. (1970) Aflatoxicol: Structure of a
new transformation product of aflatoxin B1 Can. J. Biochem.,
48: 830-832.
DICKENS, J. W. & SATTERWHITE, J. B. (1973) Aflatoxin-contaminated
peanuts produced on North Carolina farms in 1968. J. Am. Peanut
Res. Educ. Assoc. Inc., 5: 11 pp.
DICKENS, F., JONES, H. E. H., & WAYNFORTH, H B. (1966) Oral
subcutaneous and intra-tracheal administration of carcinogenic
lactones and related substances: The intratracheal administration of
cigarette tar in the rat. Br. J. Cancer, 20: 134-144.
DIENER, U. L. & DAVIS, N. D. (1969) Production of aflatoxins on
peanuts under controlled environments. J. stored Prod. Res.,
5: 251-258;.
DI PAOLO, J. A., ELIS, J., & ERWIN, H. (1967) Teratogenic response
by hamsters, rats and mice to aflatoxin B1. Nature (Lond.), 215:
638-639.
DOHERTY, W. P. & CAMPBELL, T. C. (1972) Inhibition of rat-liver
mitochondria electron-transport flow by aflatoxin B1. Res.
Commun. chem. Path. Pharmacol., 3: 601-612.
DOHERTY, W. P. & CAMPBELL, T. C. (1973) Aflatoxin inhibition of rat
liver mitochondria. Chem. biol. Interactions, 7: 63-77.
DONALDSON, W. E., TUNG, H-T., & HAMILTON, P. B. (1972) Depression of
fatty acid synthesis in chick liver (Gallus domesticus) aflatoxin.
Comp. Biochem. Physiol., 41: 843-847.
DOSTER, R. C., SINNHUBER, R. O., & WALES, J. H. (1972) Acute
intraperitoneal toxicity of ochratoxins A and B in rainbow trout
(Salmo gairdneri). Food Cosmet. Toxicol, 10: 85-92.
DOTCHEV, V. D. (1973) [The endemic (Balkan) nephropathy in
Bulgaria.] Muench. med. Woenschr., 115: 537-541 (in German).
DVORACKOVA I. (1976) Aflatoxin inhalation and alveolar cell
carcinoma. Br. med. J., 20: 691.
DVORACKOVA I., BRODSKY, F., & GERMAN, J. (1974) Aflatoxin and
encephalitic syndrome with fatty degeneration of viscera. Nutr.
Rep. Int., 10: 89-102.
DVORACKOVA I., KUSAK, V., & NESNIDAL, P. (1977) Aflatoxin and
encephalopathy with fatty degeneration of viscera (Reye). Ann.
Nutr. Ailment., 31: 977-989.
EDDS, G. T., NAIR, K. P. C., & SIMPSON, C. F. (1973) Effects of
aflatoxin B1 on resistance in poultry against cecal coccidiosis
and Marek's disease. Am. J. vet. Res., 34: 819-826.
ELIS, J. & DI PAOLO, J. A. (1967) Aflatoxin B1. Arch. Pathol., 83:
53-57.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
Worm Health Organ., 49: 411-418.
ELLING, F., HALO, B., JACOBSEN, CHR., & KROGH, P. (1975) Spontaneous
toxic nephropathy in poultry associated with ochratoxin A. Acta
path. microbiol. Scand. Sect. A, 85: 739-741.
EPPLEY, R. M. (1966) A versatile procedure for assay and preparatory
separation of aflatoxins from peanut products. J. Assoc. Off.
Anal. Chem, 49: 1218-1223.
EPPLEY, R. M. (1968) Screening method for zearalenone, aflatoxin,
and ochratoxin. J. Assoc. Off. Anal. Chem, 51: 74-78.
EPPLEY, R. M., STOLOFF, L., TRUCKSESS, M. W., & CHUNG, C. W. (1974)
Survey of corn for Fusarium toxins. J. Assoc. Off. Anal. Chem,
57: 632-635.
EPSTEIN, S. M., BARTUS, B., & FARBER, E. (1969) Renal epithelial
neoplasms induced in male Wistar rats by oral aflatoxin B1. Cancer
Res., 29: 1045-1050.
FAO (1977) Mycotoxins. Report of the joint FAO/WHO/UNEP Conference
on Mycotoxins, Nairobi, 19-27 September, 1977, Rome, Food and
Agriculture Organization of the United Nations, 105 pp. (FAO Food
and Nutrition Paper 2).
FDA (1977) Report on action level for aflatoxin M1 in milk.
Washington DC, 21 November, Bureau of Foods, US Food and Drug
Administration.
FDA (1978) Assessment of estimated risk resulting from aflatoxins
in consumer peanut products and other food commodities.
Washington, DC, 19 January, Bureau of Foods, US Food and Drug
Administration.
FEUER, G, GOLBERG, L., & LEPELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose-6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FRIEDMAN, L. & MOHR, H. (1968) Absence of interaction between
cyclopropenoid fatty acids and aflatoxin in rats. Fed. Proc., 27:
551 (Abstract no. 1882).
FRITZ, W., DONATH, R., & ENGST, R. (1977) [Determination and
occurrence of aflatoxin M1 and B1 in milk and dairy produce.]
Nahrung, 21: 79-84 (in German).
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport san Ruin de la toxine chez le rat. Ann. Rech.
vet., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal II. Distribution tissulaire et élimination chez le rat. Ann.
Rech. vet., 5: 319-328.
GALTIER, P. (1975) Metabolism et mode d'action d'ochratoxine A. In:
Biquet, J. & Jemmali, M., ed. Les Mycotoxines, Paris, Editions
INSERM, pp. 69-78.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vet., 7:
91-98.
GALTIER, P., MORÉ., J., & BODIN, G. (1974) Toxine d' Aspergillus
ochraceus Wilhelm. III. Toxicité aigue de l'ochratoxine A chez le
rat et la souris adultes. Ann. Rech. vet., 5: 233-247.
GARNER, R. C. (1973) Chemical evidence for the formation of a
reactive aflatoxin B1 metabolite, by hamster liver microsomes.
Febs Lett., 36: 261-264.
GARNER, R. C. (1975) Reduction in binding of [14C]aflatoxin B1
to rat liver macromolecules by phenobarbitone pretreatment.
Biochem. Pharmacol., 24: 1553-1556.
GARNER, R. C., MILLER, E. C., MILLER, J. A., GARNER, J. V., &
HANSON, R. S. (1971) Formation of a factor lethal for S. typhimurium
TA 1530 and TA 1531 on incubation of aflatoxin B1 with rat liver
microsomes. Biochem. biophys. res. Comm., 45: 774-780.
GARNER, R. C., MILLER, E. C., & MILLER, J. A. (1972) Liver
microsomal metabolism of aflatoxin B1 to a reactive derivative
toxic to Salmonella typhimurium TA 1530. Cancer Res., 32:
2058-2066.
GODOY, H. M., JUDAH, D. J., ARORA, H. L., NEAL, G. E., & JONES, G.
(1976) The effects of prolonged feeding with aflatoxin B1 on adult
rat liver. Cancer Res., 36: 2399-2407.
GOLDBLATT, L. E., ed. (1969) Aflatoxin; scientific background,
control, and implications, New York, Academic Press, pp. 472.
GOLDBLATT, L. A. & DOLLEAR, F. G. (1977) Detoxification of
contaminated crops. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publishers Inc., pp. 139-150.
GOODAL, C. M. & BUTLER, W. H. (1969) Aflatoxin carcinogenesis:
Inhibition of liver cancer induction in hypophysectomized rats.
Int. J. Cancer, 4: 422-429.
GOPALAN; C., TULPULE, P. G., & KRISHNAMURTHI, D. (1972) Induction of
hepatic carcinoma with aflatoxin in the rhesus monkey. Food
Cosmet. Toxicol., 10: 519-521.
GRANT, K. E., COMER, M. W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small doses
of aflatoxin B1. Toxicol. appl. Pharmacol, 41: 166 (Abstract no.
84).
GREENWAY, J. A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Columbia. I. Natural occurrence and diagnosis. Can. J.
comp. Med., 40: 12-15.
GRICE, M. C., MOODIE, C. A., & SMITH, B. C. (1973) The carcinogenic
potential of aflatoxin or its metabolites in rats from dams fed
aflatoxin pre- and postpartum. Cancer Res., 33: 262-268.
HABISH, H. A., ABDULLA, M. H., & BROADBENT, J. H. (1971) The
incidence of aflatoxin in Sudanese groundnuts. Trop. Sci., 8:
279-287.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs. In:
Abstracts, IUPAC-Sponsored Symposium, Control of Mycotoxins,
Kungalr, Goteborg, Sweden, 21-22 Aug., 1972, Goteborg, Sweden,
Swedish Institute for Food Preservation Research, p. 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A in barley,
using silica gel mini-columns. J. Assoc. Off. Anal. Chem.,
58:156-158.
HALVER, J. E. (1969) Aflatoxicosis and trout hepatoma. In:
Goldblatt, L. A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 265-306.
HAMILTON, P. B. & HARRIS, J. R. (1971)Interaction of aflatoxicosis
with Candida albicans infections and other stresses in chickens.
Poult. Sci., 50: 906-912.
HANSSEN, E. & JUNG, M. (1972) [On the presence of aflatoxin in
non-mouldy food and proposals for sampling.]
Z. Lebensmitt.-Unters.-Forsch., 150: 141-145 (in German).
HARTLEY, R. D., NESBITT, B. F., & O'KELLY, J. (1963) Toxic
metabolites of Aspergillus flavus. Nature (Load.), 198: 1056-1058.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
345-367.
HARWIG, J. & CHEN, Y.-K. (1974) Some conditions favoring production
of ochratoxin A and citrinin by Penicillium viridicatum in wheat
and barley. Can. J. Plant. Sci., 54: 17-22.
HAYES, A. W., HOOD, R. D., & LEE, H. L. (1974) Teratogenic effects
of ochratoxin A in mice. Teratology, 9: 93-98.
HEPTINSTALL, R. H. (1966) Pathology of the kidney, London,
Churchill, pp. 457-464.
HESSELTINE, C. W. (1976) Conditions leading to mycotoxin
contamination of foods and feeds. In: Rodricks, J. V., ed.
Mycotoxins and other fungal related food problems, Washington, DC,
American Chemical Society, pp. 1-22 (Advances in Chemistry Series
149).
HESSELTINE, C. W., SORENSEN, W. G., & SMITH, M. (1970) Taxonomic
studies of the aflatoxin producing strains in the Aspergillus
flavus group. Mycologia, 62: 123-132.
HOBSON. W., BAILEY, J., & FULLER, G. B. (1970 Hormone effects of
zearalenone in non-human primates. J. Toxicol. environ. Health, 3:
43-57.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1: 133-151.
HOGAN, G. R., RYAN, N.J., & HAYES, A. W. (1978) Aflatoxin B1 and
Reye's syndrome. Lancet, March 11, p. 561.
HOLADAY, C. E. (1976) A rapid screening method For the aflatoxins
and ochratoxin A. J. Am. Oil Chem. Soc., 53: 603-605.
HORWITZ, W, SENZEL, A., REYNOLDS, H., & PARK, D. L., ed. (1975)
Natural poisons. In: Chapter 26, Official Methods of analysis of
the Association of Official Analytical Chemists, Washington, DC,
AOAC, p. 24.
HRABAR, A., SUJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Morbidity and mortality from endemic
nephropathy in the village of Kaniza.] Arh. hig. rada, 27: 137-145
(in Serbo-Croat).
HSIEH, D. P. H., WONG, Z. A, WONt, J. J., MICHAS, C., & RUEBNER, B.
H. (1977) Comparative metabolism of aflatoxin. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers Inc.,
pp. 37-50.
HSU I-CH., SMALLEY, E. B., STRONG, F. M., & RIBELIN, W. E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. MicrobioL., 24: 684-690.
HULT, K. & GATENECK, S. (1976) A spectrophotometric procedure using
carboxy-peptidase A, for the quantitative measurement of ochratoxin
A. J. Assoc. Off. Anal. Chem., 59: 128-129.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32:
443-444.
IARC (1976) Aflatoxins. In: IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man: Some naturally occurring
substances. Lyons, International Agency for Research on Cancer,
Vol. 10, pp. 51-72.
IARC (1977) IARC Monograph Programme on the evaluation of the
carcinogenic risk of chemicals to humans. Preamble. Lyons,
International Agency for Research on Cancer, 31 pp. (IARC Internal
Technical Report No. 77/002).
IUPAC (1976) Recommended methods of ochratoxins A and B in barley,
Oxford, International Union of Pure & Applied Chemistry Secretariat,
pp. 1-7 (Technical Reports-No. 14).
JEMMALI, M. & LAFONT, P. (1972) Evolution de l'aflatoxine B1 au
tours de la panification. Cah. Nutr. Diét., 7: 319-322.
JEMMALI, M. & MURTHY, T. R. K. (1976) A chemical assay method for
the determination of aflatoxin residues in animal tissues.
Z. Lebensm.-Unters.-Forsch., 161: 13-17.
JOHN, D. W. & MILLER, L. L. (1969) Effect of aflatoxin B1 on net
synthesis of albumin, fibrinogen and alpha1-acid glycoprotein by
the isolated perfused rat liver. Biochem. Pharmacol., 18:
1135-1146.
JONES, B. D. (1972) Methods of aflatoxin analysis. London,
Tropical Products Institute, 58 pp. (Report G 70).
JOSEPH-BRAVO, P. I., FINDLEY, M., & NEWBERNE P.M. (1976) Some
interactions of light, riboflavin, and aflatoxin B1 in vivo and
in vitro. J. Toxicol. environ. Health, 1: 353-376.
JUNG, M. & HANSSEN, E. (1974) [The occurrence of aflatoxin M1 in
dried milk products.] Food Cosmet. Toxicol., 12: 131-138 (in
German).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZNSKA, J. (1976) [Occurrence of
aflatoxin B1, B2, G1, and G2, ochratoxin A and B,
sterigmatocystin and zearalenone in cereals.] Med. Weter., 32:
617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feed and concentrates.] Med. Weter., 53: 193-196
(in Polish).
KATO, R., ONODA, K., & OMORI, Y. (1969) The effect of aflatoxin B1
on incorporation of 14C-acetate into cholesterol by rat liver.
Experientia (Basel), 25: 1026.
KATO, R., TAKANAKA, A., ONODA, K., & OMORI, Y. (1970) Different
effect of aflatoxin on the induction of tryptophan oxygenase and of
microsomal drug-hydroxylase system. J. Biochem. (Tokyo), 68:
589-592.
KEEN, P. & MARTIN, P. (1971a) The toxicity and fungal infestation of
foodstuffs in Swaziland in relation to harvesting and storage.
Trop. geogr. Med., 23-35-43.
KEEN, P. & MARTIN, P. (1971b) Is aflatoxin carcinogenic in man? The
evidence in Swaziland. Trop. geogr. Med., 23: 44-53.
KIERMEIER, F. ( 1973) Aflatoxin residues in fluid milk. Pure appl.
Chem., 35: 271-274.
KIERMEIER, R. (1977) The significance of aflatoxins in the dairy
Industry. Annu. Bull. Int. Dairy Fed., 98: 1-23, 25-43.
KIERMEIER, F., WEISS, G., BEHNRINGER, G., MILLER, M., & RANFFT, K.
(1977) [On the presence and the content of aflatoxin M1 in milk
shipped to a dairy plant.] Z. Lebensmitt.- Unters.-Forsch., 163:
171-174 (in German).
KOEHLER, B. (1938) Fungus growth in shelled corn as affected by
moisture J. agric. Res., 56: 291-307.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975a) Investigations into an outbreak of hepatitis in parts
of Western India. Indian J. med. Res., 63: 1036-1048.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975b) Hepatitis due to aflatoxicosis. Lancet, 1: 1061.
KROGH, P. (1974) Mycotoxic porcine nephropathy: A possible model for
Balkan endemic nephropathy. In: Puchlev, A., ed. Endemic
Nephropathy. Proceedings of the Second International Symposium on
Endemic Nephropathy, Sofia, November 9-11, 1972. Sofia, Bulgarian
Academy of Sciences, pp. 266-270.
KROGH, P. (1976a) Mycotoxic nephropathy. In: Advances in
veterinary science and comparative medicine, New York, Academic
Press, Vol. 20, pp. 147-170.
KROGH, P. (1976b) Epidemiology of mycotoxic porcine nephropathy.
Nord. Vet.-Med., 28: 452-458.
KROGH, P. (1977a) Ochratoxins. In: Rodricks, J. V., Hesseltine, C.
W., & Mehlman, M. A., ed. Mycotoxins in human and animal health,
Park Forest South, IL, USA, Pathotox Publishers, Inc., pp. 489-498.
KROGH, P. (1977b) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. Vet.-Med., 29: 402-405.
KROGH, P. (1977c) Mycotoxin tolerances in foodstuffs. Pure appl.
Chem., 49: 1719-1721.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy.
Acta path. microbiol. Scand. Sect. A, Suppl. no. 269.
KROGH, P. & ELLING, F. (1977) Mycotoxic nephropathy. Vet. Sci.
Commun., 1: 51-63.
KROGH, P. & HALD, B. (1969) [Occurrence of aflatoxin in imported
groundnut products.] Nord. Vet.-Med., 21: 398-407 (in Danish).
KROGH, P., HALD, B., & KORPINEN, E.-L. (1970) [Occurrence of
aflatoxin in groundnut- and copra-products imported to Finland.]
Nord. Vet.-Med., 22: 584-589 (in Norwegian).
KROGH, P., HALD, B., HASSELAGER, E., MADSEN, A., MORTENSEN, H. P.,
LARSEN, A. E., & CAMPBELL, A.D. (1973a) Aflatoxin residues in bacon
pigs. Pure appl. Chem., 35: 275-282.
KROGH, P., HALD, B., & PEDERSEN, E. J. (1973b) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta path. microbiol. Scand. Sect. B, 81:
689-695.
KROGH, P., AXELSEN, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A. E., MADSEN, A., MORTENSEN, H. P.,
MOLLER, T., PETERSEN, O. K., RAVNSKOV, U., ROSTGAARD, M., & AALUND,
O. (1974) Experimental porcine nephropathy: Changes of renal
function and structure induced by ochratoxin A- contaminated feed.
Acta path. microbiol. Scand. Sect. A, Suppl. No. 246, 9 pp.
KROGH, P., ELLING, F., BALD, B., LARSEN, A. E., LILLEHOJ, E. B.,
MADSEN, A., & MORTENSEN, H. P. (1976a) Time-dependent disappearance
of ochratoxin A residues in tissues of bacon pigs. Toxicology, 6:
235-242.
KROGH, P., ELLING, F., GYRD-HANSEN, H., HALD, B., LARSEN, A. E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H. P., & RAVNSKOV, U.
(1976b) Experimental porcine nephropathy: Changes of renal function
and structure perorally induced by crystalline ochratoxin A. Acta.
pathol. microbiol. Scand. Sect. A, 84: 429-434.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V. E.,
SKADHAUGE, E., & SVENDSEN, C. K. (1976c) Experimental avian
nephropathy: Changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta path. microbiol. Scand. Sect.
4, 84: 215-221.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and foodborne ochratoxin A: Preliminary
results of a survey of foodstuffs. Acta path. microbiol. Scand.
Sect. B, 85. 238-240.
KURTZ, M. J., NAIRN, M. E., NELSON, G. H., CHRISTENSEN, C. M., &
MIROCHA, C. J. (1969) Histologic changes in the genital tracts of
swine fed estrogenic mycotoxin. Am. J. vet. Res., 30: 551-556.
LARSEN, S. (1928-1929) [On chronic degeneration of the kidneys
caused by mouldy rye.] Maanedsskr. Dyrl., 40: 259-284 & 289-300
(in Danish).
LE BRETON, E., FRAYSSINET, C., LAFARGE, C., & DE RECONDO, A.M.
(1964) Aflatoxine-mechanisme de l'action. Food Cosmet. Toxicol.,
2: 675-676.
LEE, D. J., WALES, J. H., & SINNHUBER, R. O. (1969a) Hepatoma and
renal tubule adenoma in rats fed aflatoxin and cyclopropenoid fatty
acids. J. Natl Cancer Inst., 43: 1037-1044.
LEE, L. S., CUCULLU, A. F., FRANZ, A. O., JR, & PONS, W. A., JR
(1969b) Destruction of aflatoxins in peanuts during dry and oil
roasting. J. agric. food Chem., 17: 451-453.
LEE, W. V. (1965) Quantitative determination of aflatoxin in
groundnut products. Analyst, 90: 305-307.
LEONARD, A., DEKNUDT, GH., & LINDEN, G. (1975) Mutagenicity tests
with aflatoxins in the mouse. Mutat. Res., 28: 137-139.
LEONOV, A. N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 323-328.
LEVI, C. P., TRENK, H. L., & MOHR, H. K. (1974) A study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LIJINSKY, W. & BUTLER, W. H. (1966) Purification and toxicity of
aflatoxin G1. Proc. Soc. Exp. Biol. Med., 123: 151-154.
LIJINSKY, W., LEE, K. Y., & GALLAGHER, C. H. (1970) Interaction of
aflatoxin B1 and G1 with tissues of the rat. Cancer Res., 30:
2280-2283.
LILLEHOJ, E. B., FENNELL, D. I., & KWOLEK, W. F. (1976)
Aspergillus flavus and aflatoxin in Iowa corn before harvest.
Science, 193: 195-496.
LIN, J. J., LIU, C., & SVOBODA, D. J. (1974) Long-term effects of
aflatoxin B1 and viral hepatitis on marmoset liver. Lab. Invest.,
30: 267-278.
LING, K. H., WANG, J. J., WU, R., TUNG, T. C., LIN, C. K., LIN,
S.S., & LIN, T. M. (1967) Intoxication possibly caused by aflatoxin
B1 in mouldy rice in Shuang-Chih township. J. Formosan Med.
Assoc., 66: 517-525.
LINSELL, C. A. & PEERS, F. G. (1977) Field studies on liver cell
cancer. In: Hiatt, H. H., Watson, J. D., & Wingsten, J. A., ed.
Origins of human cancer. Cold Spring Harbor, NY, Cold Spring
Harbor Laboratory, pp. 549-556.
LO, W-B. & BLACK, H. S. (1972) Effects of aflatoxin B1 on human
skin lipids metabolism-a site of action on 14C-acetate
incorporation. Experientia (Basel), 28: 1278-1279.
LOOSMORE, R. M. & HARDING, J. D. J. (1961) A toxic factor in
Brazilian groundnut causing liver damage in pigs. Vet. Rec., 73:
1362-11364.
LOOSMORE, R. M. & MARKSON, L. M. (1961) Poisoning of cattle by
Brazilian groundnut meal. Vet. Rec., 73: 813-814.
LOVELACE, C. E. A. & NYATHI, C. B. (1977) Estimation of the fungal
toxins, zearalenone and aflatoxin, contaminating opaque maize beer
in Zambia. J. Sci. Food Agric., 28: 288-292.
LUCAS, F. V., JR., MONROE, P., NGA, P. V., TOWNSEND, J. F., & LUCAS,
F. V. (1970-1971) Mycotoxin contamination of South Vietnamese rice.
J. trop. Med. Hyg., 74: 182-183.
LUCK. H., STEYN, M., & WEHNER, F. C. (1917) A survey of milk powder
for aflatoxin content. S. Afr. J. dairy Technol., 8: 85-86.
LVOVA, L. S., SOSEDOV, N. I., GARALL, W., SCHWARZMAN, M. I.,
SHATILOVA, T. I., & SHULGINA, A. P. (1976) [Formation of aflatoxins
in wheat grain induced by self-heating and change in its chemical
composition by the development of storage moulds.] Prikl. Biochem.
Mikrobiol., 12: 741-749 (in Russian).
MADHAVAN, T. V. & GOPALAN, C. (1975) Effect of dietary protein on
aflatoxin injury in weanling rats. Arch. Pathol.,
80: 123-126.
MADHAVAN, T. V. & GOPALAN, C. (1968) The effect of dietary protein
on carcinogenesis of aflatoxin. Arch. Pathol., 85: 133-137.
MADHAVAN, T. V., SURYANARAYANA RAO, K., The effect of dietary
protein level on susceptibility of monkeys to aflatoxin liver
injury. Indian J. med. Res., 53: 984-989.
MADHAVAN, T. V., TULPULE, P. G, & GOPALAN, C. (1965b)
Aflatoxin-induced hepatic fibrosis in rhesus monkeys. Arch.
Pathol., 79: 466-469.
MALEKI, M. F., SUZANGAR, M., JAMSHIDI, CH., TAHMASEBI, A., &
BARNETT, R. (1976) Urinary aflatoxin detected in cirrhotic patients
in Ispahan, Iran. Clin. Res., 24: 630A.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MARSH, P. B., SIMPSON, M. E., CRAIG, G. O., DONOSO, J., & RAMEY, H.
H., JR (1973) Occurrence of aflatoxins in cotton seeds at harvest in
relation to location of growth and field temperatures. J. environ.
Qual., 2: 276-281.
MARTIN, P.M. D. & KEEN, P. (1978) The occurrence of zearalenone in
raw and fermented products from Swaziland and Lesotho. Sabrouaudia,
16: 15,22.
MASRI, M. S., LUNDIN, R. E., PAGE, J. R., & GARCIA, V. V. (1967)
Crystalline aflatoxin M1 from urine and milk. Nature (Lond.),
215: 753-755.
MASRI, M. S., HADDON, W. F., LUNDIN, R. E., & HSIEH, D. P. H.
(1974a) Aflatoxin Q1: A newly identified major metabolite of
aflatoxin B1 in monkey liver. J. agric. food Chem., 22: 512-514.
MASRI, M. S., BOOTH, A. N., & HSIEH, D. P. H. (1974b) Comparative
metabolic conversion of aflatoxin B1 to aflatoxin M1 and
aflatoxin Q1 by monkey, rat and chicken liver. Life Sci., 15:
203-212.
MATTHEWS, J. G., PATTERSON, D. S. P., ROBERTS, B. A., & SHREEVE, B.
J. (1977) T-2 toxin and haemorrhagic syndromes in cattle. Vet.
Rec., 101 (19): 391.
MCLEAN, A. E. M. & MARSHALL, A. (1971) Reduced carcinogenic effects
of aflatoxin in rats given phenobarbitone. Br. d. exp. Pathol.,
52: 322-329.
MCNUTT, S. H., PURWIN, P., & MURRAY, C. (1928) Vulvovaginitis in
swine J. Am. Vet. Med. Assoc., 73: 484;-492.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MERWE, K. J. van der, STEYNE, P.S., FOURIE, L., SCOTT, DE B., &
THERON, J. J. (1965) Ochratoxin A, a toxic metabolite produced by
Aspergillus ochraceus Wilh. Nature (Lond.), 205: 1112-1113.
MILLER, J. K., HACKING, A., & GROSS, V. J. (I[973) Stillbirths,
neonatal mortality and small litters in pigs associated with the
ingestion of Fusarium toxin by pregnant sows. Vet. Rec., 93:
555-559.
MINTZLAFF, H.-J., LOTZSCH, R., TAUCHMANN, F., MEYER, W., & LEISTNER,
L. (1974) [Aflatoxin residues in the liver and muscle of broiler
chicken given aflatoxin containing feed.] Fleischwirtschaft, 54:
774-778 (in German).
MIROCHA, C. J. & CHRISTENSEN, C. M. (1974) Oestrogenic mycotoxins
synthesized by Fusarium. In: Purchase, I. F. H, ed. Mycotoxins,
Amsterdam, Elsevier, pp. 129-148.
MIROCHA, C. J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poaefusarin. Appl. Microbiol., 26:
719-724.
MIROCHA, C. J., CHRISTENSEN, C. M., & NELSON, G. H. (1968a) Toxic
metabolites produced by fungi implicated in mycotoxicoses.
Biotech. Bioeng., 10: 469-482.
MIROCHA, C. J., HARRISON, J., NICHOLS, A. A.., & MCCLINTOCK, M.
(1968b) Detection of a fungal estrogen (F-2) in hay associated with
infertility in dairy cattle. Appl. Microbiol., 16: 797-798.
MIROCHA, C. J., SCHAUERHAMER, B., & PATHRE, S. V. (1974) Isolation,
detection and quantitation of zearalenone in maize and barley.
J. Assoc. Off. Anal. Chem., 75: 1104-1110.
MIROCHA, C. J., PATHRE, S. V., SCHAUERHAMER, B., & CHRISTENSEN, C.
M. (1976) Natural occurrence of Fusarium toxins in feedstuff. Appl
environ. Microbiol., 32: 553-556.
MIROCHA, C. J., PATHRE, S. W., & CHRISTENSEN, C. M. (1977)
Zearalenone. In: Rodricks, J. V., Hesseltine, C. W., & Mehlman, M.
A., ed. Mycotoxins in human and animal health, Park Forest South,
IL, USA, Pathotox Publishers Inc., pp. 345-364.
MOROOKA, N., URATSUJI, N., YOSHIZAWA, T., & YAMAMOTO, H. (1972)
[Studies on the toxic substances in barley infected with Fusarium
spp.] Jpn. J. food Hyg, 13: 368-375 (in Japanese).
MUNRO, I. C, MOODIE, C. A, KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H. C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol, 28: 180-188.
NABNEY, J., BURBAGE, M. B., ALLCROFT, R., & LEWIS, G. (1967)
Metabolism of aflatoxin in sheep: Excretion pattern in the lactating
ewe. Food Cosmet. Toxicol., 5: 11-17.
NEAL, G. E. & GODOY, H. M. (1976) The effect of pre-treatment with
phenobarbitone on the activation of aflatoxin B1 by rat liver.
Chem. biol. Interactions, 14: 279-289.
NEELY, W. C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55: 1305-1309.
NEL, W. & PURCHASE, I. F. M. (1968) Note. The fate of ochratoxin A
in rats. J.S. Afr. Chem. Inst., 21: 87-88.
NESHEIM, S. (1971) Report on ochratoxins: Occurrence, production,
analysis and toxicity. In: Abstracts of the 85th Annual meeting of
the Association of Official Analytical Chemists, Washington, DC,
Oct. 11-14, 1971, p. 23.
NESHEIM, S. (1976) The ochratoxins and other related compounds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 276-295
(Advance.,; in Chemistry Series 149).
NESHEIM, S., HARDIN, N. F., FRANCIS, O. J., JR, & LANGHAM, W. S.
(1973) Analysis of ochratoxins A and B and their esters in barley,
using partition and thin layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NEUMANN-KLEINPAUL, A. & TERPLAN, G. (1972) [The presence of
aflatoxin M1 in dried milk products.] Arch. Lebensmittelhyg.,
23: 128-132 (in German).
NEWBERNE, P.M. (1974) Mycotoxins: Toxicity, carcinogenicity and the
influence of various nutritional conditions. Environ. Health
Perspect., 9: 1-32.
NEWBERNE, P.M. (1976) Environmental modifiers of susceptibility to
carcinogenesis. Cancer Detect. Prev., 1: 129-173.
NEWBERNE, P.M. & BUTLER, W. H. (1969) Acute and chronic effects of
aflatoxin on the liver of domestic and laboratory animals: A review.
Cancer Res., 29: 236-250.
NEWBERNE, P.M. & CONNER, M. W. (1974) Effect of selenium on acute
response to aflatoxin B1. In: Hemphill, D. D., ed. A Symposium.
Trace Substances in Environmental Health-VIII, Columbia, MO,
University of Missouri, pp. 323-328.
NEWBERNE, P.M. & GROSS, R. L. (1977) The role of nutrition in
aflatoxin injury. In: Rodricks, J. V., Hesseltine, C. W, & Mehlman,
M. A., ed. Mycotoxins in human and animal health, Park Forest
South, IL, USA, Pathotox Publishers Inc., pp. 51-65.
NEWBERNE, P.M. & ROGERS, A. E. (1971) Aflatoxin carcinogenesis in
rats: Dietary effects. In: Purchase, I. F. H., ed. Mycotoxins in
human health, London, Macmillan, pp. 195-208.
NEWBERNE, P.M. & ROGERS, A. E. (1973) Rat colon carcinomas
associated with aflatoxin and marginal vitamin A. J. Natl Cancer
Inst., 50: 439-448.
NEWBERNE, P.M. & ROGERS, A. E. (1976) Nutritional modulation of
carcinogenesis. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. of Tokyo Press, & Baltimore, Univ.
Park Press, pp. 15-40.
NEWBERNE, P.M. & SUPHAKARN, V. (1977) Preventive role of vitamin A
in colon carcinogenesis in rats. Cancer, 40: 2553-2556.
NEWBERNE, P.M. & WILLIAMS, G. (1969) Inhibition of aflatoxin
carcinogenesis by diethyl-stilbestrol in male rats. Arch. environ.
Health, 19: 489-498.
NEWBERNE, P.M. & WOGAN, G. N. (1968a) Sequential morphologic changes
in aflatoxin B1 carcinogenesis in the rat. Cancer Res., 28:
770-781.
NEWBERNE, P.M. & WOGAN, G. N. (1968b) Potentiating effects of
low-protein diets on effect of aflatoxin in rats. Toxicol. appl.
Pharmacol., 12: 309-310.
NEWBERNE, J. W., BAILEY, W. S., & SEIBOLD, H. R. (1955) Notes on a
recent outbreak and experimental reproduction of hepatitis X in
dogs. J. Am. Vet. Med. Assoc., 127: 59-62.
NEWBERNE, P.M., RUSSO, R., & WOGAN, G. N. (1966a) Acute toxicity of
aflatoxin B1 in the dog. Pathol. vet., 3: 331-340.
NEWBERNE, P.M., HARRINGTON, D. H., & WOGAN, G. N. (1966b) Effects of
cirrhosis and other liver insults on induction of liver tumours by
aflatoxin. Lab. Invest., 15: 962-969.
NEWBERNE, P.M., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal
lesions in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
NEWBERNE, P.M., CHAN, W-CH. M., & ROGERS, A. E. (1974) Influence of
light, riboflavin, and carotene on the response of rats to the acute
toxicity of aflatoxin and mono-crotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NILSSON, G. (1974) [Aflatoxin in nuts.] Vor Foda, 26: 56-62 (in
Swedish).
NIXON, J. E., SINNHUBER, R. O., LEE, D. J., LANDERS, M. K., & HARR,
J. R. (1974) Effect of cyclopropenoid compounds on the carcinogenic
activity of diethylnitrosamine and aflatoxin B1 in rats. J. Natl
Cancer Inst., 53: 453-458.
NORTHOLT, M.D., VERHULSDONK, C. A. H., SOENTORO, P.S. S., & PAULSCH,
W. E. (1976) Effect of water activity and temperature on aflatoxin
production by Aspergillus parasiticus. J. milk food Technol., 39:
170-174.
OLSON, L. C., BOURGEOIS, C. H., JR, COTTON, R. B., HARIKUL, S.,
GROSSMAN, R. A., & SMITH, T. J. (1971) Encephalopathy and fatty
degeneration of the viscera in northeastern Thailand. Clinical
syndrome and epidemiology. Pediatrics, 47: 707-716.
ONG, T-M. (1975) Aflatoxin mutagenesis. Mutat. Res., 32: 35-53.
PAG FAG/WHO/UNICEF PROTEIN ADVISORY GROUP (1969) PAG Recommendation
on aflatoxin. PAG Statement No. 2. New York, United Nations, 2 pp.
PANALAKS, T. & SCOTT, P.M. (1977) Sensitive silica gel-packed
flowcell for fluorometric detection of aflatoxins by high pressure
liquid chromatography. J. Assoc. Off. Anal. Chem., 60: 583-589.
PARKER, W. A. & MELNICK, D. (1966) Absence of aflatoxin from refined
vegetable oils. J. Am. Oil Chem. Soc., 43: 635-638.
PATHRE, S. V. & MIROCHA, C. J. (1976) Zearalenone and related
compounds. In: Rodricks, J. V., ed. Mycotoxins and other fungal
related food problems, Washington, DC, American Chemical Society
pp. 178-227 (Advances in Chemistry Series 149).
PATTERSON, D. S. P. (1973) Metabolism as a factor in determining the
toxic action of the aflatoxin in different animal species. Food
Cosmet. Toxicol., 11: 287-294.
PATTERSON, D. S. P. (1976) Structure, metabolism and toxicity of the
aflatoxins: A review. Cah. Nutr. Diét., 11 (Suppl. 2): 71-76.
PATTERSON, D. S. P. (1977) Aflatoxin and related compounds. In:
Wyllie, T.D. & Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: An encyclopedic handbook, New York & Basel, Marcel
Dekker, pp. 131-136 & 144-189.
PATTERSON, D. S. P. & ROBERTS, B. A. (1970) The formation of
aflatoxin B2a and G2a and their degradation products during the
in vitro detoxification of aflatoxin by livers of certain avian
and mammalian species. Food Cosmet. Toxicol., 8: 527-538.
PATTERSON, D. S. P. & ROBERTS, B. A. (1971) The in vitro reduction
of aflatoxins B1 and B2 by soluble avian liver enzymes. Food
Cosmet. Toxicol., 9: 829-837.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972a) Steroid sex hormones as
inhibitors of aflatoxin metabolism in liver homogenates.
Experientia (Basel), 28: 929-930.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972b) Aflatoxin metabolism in
duck-liver homogenates: The relative importance of reversible
cyclopentenone reduction and hemiacetal formation. Food Cosmet.
Toxicol., 10: 501-512.
PATTERSON, D. S. P., ROBERTS, B. A., SHREEVE, B. J., WRATHALL, A.
E., & GITTER, M. (1977) Aflatoxin, ochratoxin and zearalenone in
animal feedstuffs: Some clinical and experimental observations. Ann
Nutr. Aliment., 31: 643-650.
PATTERSON, D. S. P., SHREEVE, B. J., & ROBERTS, B. A. (in press)
Mycotoxin residues in body fluids and tissues of food-producing
animals. In: Proceedings of the 12th International Congress
Microbiology, Munchen, Federal Republic of Germany, 3-8 September
1978.
PAUL, R., KALRA, M. S., & SINGH, A. (1976) Incidence of aflatoxins
in milk and milk products. Indian J. dairy Sol., 29: 318-322.
PAYET, M., CROS, J., QUENUM, C., SANKALE, M., & MOULANIER, M. (1966)
Deux observations d'enfants avant consommé de façon prolongée des
farines souillées par "Aspergillus flavus". Presse med.,
13: 649-651.
PECKHAM, J. C., DOUPNIK, B., JR, & JONES, O. H., JR (1971) Acute
toxicity of ochratoxins A and B in chicks. Appl. Microbiol., 21:
492-494.
PEERS, F. G. & LINSELL, C. A. (1973) Dietary aflatoxins and liver
cancer--a population based study in Kenya. Br. J. Cancer, 27:
73-484.
PEERS, F. G. & LINSELL, C. A. (1976) Acute toxicity of aflatoxin
B1 for baboons. Food Cosmet. Toxicol., 14: 227-228.
PEERS, F. G. & LINSELL, C. A. (1977) Dietary aflatoxins and human
primary liver cancer. Gaucher, A., ed. Ann. Nutr. Aliment., 31:
1005-1018.
PEERS, F. G., GILMAN, G. A., & LINSELL, C. A. (1976) Dietary
aflatoxins and human liver cancer. A study in Swaziland. Int. J.
Cancer, 17: 167-176.
PETTIT, R. E., TABER, R. A., SCHROEDER, H. W., & HARRISON, A. L.
(1971) Influence of fungicides and irrigation practice on aflatoxin
in peanuts before digging. Appl. Microbiol., 22: 629-634.
PHILLIPS, D. L., YOURTEE, D. M., & SEARLES, S. (1976) Presence of
aflatoxin B1 in human liver in the United States. Toxicol. appl.
Pharmacol., 36: 403-406.
PIER, A. C. & HEDDLESTON, K. L. (1970) The effect of aflatoxin on
immunity in turkeys. I. Impairment of actively acquired resistance
to bacterial challenge. Avian Dis., 14: 797-809.
PIER, A. C., CYSEWSKI, S. J., RICHARD, J. L., & THURSTON, J. R.
(1977) Mycotoxins as a veterinary problem. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 745-750.
PITOUT, M. J. (1969) The hydrolysis of ochratoxin A by some
proteolytic enzymes. Biochem. Pharmacol., 18: 485-491.
POHLAND, A. E., YIN, L., & DANTZMAN, J. G. (1970) Rapid chemical
confirmatory method for aflatoxin B1. I. Development of the
method. J. Asoc. Off. Anal. Chem., 53: 101-102.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELJAN, V. A. (1972) Effect
of aflatoxin on rat liver lysosomes. Toxicon, 10: 25-30.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELIAN, V. A. (1977)
[Aflatoxins.] Toksikologija, Moskva, Viniti, Vol. 8, 108 pp. (in
Russian).
POLZHOFER, K. (1977) [Determination of aflatoxins in milk and milk
products.] Z. Lebensmitt.-Unters.-Forsch., 163: 175-177 (in
German).
PONG, R. S. & WOGAN, G. N. (1969) Time course of alterations of rat
liver polysome profiles induced by aflatoxin B1. Biochem.
Pharmacol., 18: 2357-2361.
PONG, R. S. & WOGAN, G. N. (1970) Time course and dose-response
characteristics of aflatoxin B1 effects on rat liver RNA
polymerase and ultrastructure. Cancer Res., 30: 294-304.
PONG, R. S. & WOGAN, G. N. (1971) Toxicity and biochemical and fine
structural effects of synthetic aflatoxins M1 and B1 in rat
liver. J. Natl Cancer Inst., 47: 585-592.
PRINCE, A.M., SZMUNESS, W., MICHON, J, DEMAILLE, J., DIEBOLT, G.,
LINHARD, J., QUENUM, C., & SANKALE, M. (1975) A case/control study
of the association between primary liver cancer and hepatitis B
infection in Senegal. Int. J. Cancer, 16: 376-383.
PRIOR, M. G., SISODIA, C. S., & O'NEIL, J. B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese
quail. Poult. Sci., 55: 786-790.
PRZYBYLSKI, W. (1975) Formation of aflatoxin derivatives on thin
layer chromatographic plates. J. Assoc. Off. Anal. Chem., 58:
163-164.
PUCHLEV, A. (1973) La néphropathie endémique en Bulgarie. Extrait
du Symposium sur la néphropathie endémique de l'Académie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A. (1974) Basic problems in endemic nephropathy. In:
Puchlev, A., ed. Endemic Nephropathy, Proceedings of the Second
International Symposium on Endemic Nephropathy, Sofia, 9-11
November, 1972, Sofia, Publishing House of the Bulgarian Academy
of Sciences, pp. 15-18.
PULS, R. & GREENWAY, J. A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis and toxicity of suspected barley.
Can. J. comp. Med., 40: 16-19.
PURCHASE, I. F. H. (1967) Acute toxicity of aflatoxins M1 and M2
in one-day-old ducklings. Food Cosmet. Toxicol., 5: 339-342.
PURCHASE, I. F. H. (1972) Aflatoxin residues in food of animal
origin. Food Cosmet. Toxicol., 10: 531-544.
PURCHASE, I. F. H. (1974) Mycotoxins, Amsterdam, Elsevier.
PURCHASE, I. F. H. & THERUN, J. J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
PURCHASE, I. F. H. & VORSTER, L. J. (1968) Aflatoxin in commercial
milk samples. S. Afr. med. J., 42: 219.
REDDY, G. S., TILAK, T. B. G., & KRISHNAMURTHI, D. (1973)
Susceptibility of vitamin A- deficient rats to aflatoxin. Food
Cosmet. Toxicol., 11: 467-470.
REDDY, J. K. & SVOBOOA, D. J. (1975) Aflatoxin B1-induced liver
tumors in Tupaia glis (tree shrews) a nonhuman primate. Fed.
Proc., 34: 827 (Abstract no. 3432).
REDDY, J. K., SVOBODA, D. J., & RAO, M. S. (1976) Induction of liver
tumors by aflatoxin B1 in the tree shrew (Tupaia glis), a
nonhuman primate. Cancer Res., 36: 151-160.
REYE, R. D. K., MORGAN, G., & BARAL, J. (1963) Encephalopathy and
fatty degeneration of the viscera: A disease entity in childhood.
Lancet, 2: 749-752.
REYS, L. L. & SEQUEIRA, O. A. (1974) Detection of Australia antigen
(HBAg) in blood donors and hepatoma patients in Mozambique.
S. Aft. med. J., 48: 267-269.
RICHIR, CL., PACCALIN, J., LARCEBEAU, S., FAUGERES, J., MORARD,
J.-L., & LAMANT, M. (1976) Présence d'aflatoxine B1 dans le toie
humain. Cah. Nutr. Diet., 11: 223-226.
ROBERTS, J. C. (1974) Aflatoxins and sterigmatocystins. Fortschr.
Chem. Org. Naturst., 31: 119-151.
ROBINSON, P. (1967) Infantile cirrhosis of the liver in India. With
special reference to probable aflatoxin etiology. Clin. Pediatr.,
6: 57-62.
RODRICKS, J. V. & STOLOFF, L. (1970) Determination of concentration
and purity of aflatoxin standards. J. Assoc. Off. Anal. Chem., 53:
92-95.
RODRICKS, J. V. & STOLOFF, L. (1977) Food producing animals. In:
Rodricks, J. V., Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins
in human and animal health, Park Forest South, IL, USA, Pathotox
Publishers Inc., pp. 67-79.
RODRICKS, J. V., STOLOFF, L., PONS, W. A., JR,. ROBERTSON, J. A., &
GOLDBLATT, L. A. (1970) Molar absorptivity values for aflatoxins and
justification for their use as criteria of purity of analytical
standards. J. Assoc. Off. Anal. Chem., 53: 96-101.
RODRICKS, J. V., HESSELTINE, C. W., & MEHLMAN, M. A., ed. (1977)
Mycotoxins in human and animal health, Park Forest South, IL, USA,
Pathotox Publishers Inc., 807 pp.
ROGERS, A. E. (1975) Variable effects of a lipotrope-deficient, high
fat diet on chemical carcinogenesis in rats. Cancer Res., 35:
2469-2474.
ROGERS, A. E. & NEWBERNE, P.M. (1969) Aflatoxin B1 carcinogenesis
in lipotrope-deficient rats. Cancer Res., 29: 1965-1972.
ROGERS, A. E. & NEWBERNE, P.M. (1971) Diet and aflatoxin B1
toxicity in rats. Toxicol. appl. Pharmacol., 20: 113-121.
ROINE, K., KORPINEN, E.-L., & KALLELA, K. (1971) Mycotoxicosis as a
probable cause of infertility in dairy cows. Nord. Vet.-Med., 23:
628-633.
ROMER, T. R. (1975) Screening method for the detection of aflatoxins
in mixed feeds and other agricultural commodities with subsequent
confirmation and quantitative measurement of aflatoxins in positive
samples. J. Assoc. Off. Anal. Chem., 58: 500-506.
RUDDICK, J. A., SCOTT, P. M., & HARWIG, J. (1976) Teratological
evaluation of zearalenone administered orally to the rat. Bull.
environ. Contain. Toxicol., 15: 678-681.
RUTQVlST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of
fattening pigs. Zentralbl. Vet.-Med. A, 24: 402-408.
SAFFIOTTI, U., MONTESANO, R., SELLAKUMAR, A. R., CEFIS, F., &
KAUFMAN, D. G. (1972) Respiratory tract carcinogenesis in hamsters
induced by different numbers of administrations of benzo (a) pyrene
and ferric oxide. Cancer Res., 32: 1073-1081.
SALHAB, A. S. & EDWARDS, G. S. (1977) Comparative in vitro
metabolism of aflatoxicol by liver preparations from animals and
humans. Cancer Res., 37: 1016-1021.
SALHAB, A. S. & HSIEH, D. P. H. (1975) Aflatoxicol H1: A major
metabolite of aflatoxin B1 produced by human and rhesus monkey
livers in vitro. Res. Commun. chem. Pathol. Pharmacol.,
10: 419-431.
SANDERS, T. H, DAVIS, N. D., & DIENER, U. L. (1968) Effect of carbon
dioxide, temperature, and relative humidity on production of
aflatoxin in peanuts. J. Am. Oil Chem. Soc., 45: 683-685.
SANSING, G. A., LILLEHOJ, E. B., DETROY, R. W., & MILLER, M. A.
(1976) Synergistic toxic effect of citrinin ochratoxin A and
penicillic acid in mice. Toxicon, 14: 213-220.
SARASIN, A. & MOULE, Y. (1976) Helical polysomes induced by
aflatoxin B1 in vivo. A new hypothesis for helix formation by
chemicals and carcinogens. Exp. cell Res., 97: 346-358.
SARGEANT, K., O'KELLY, J., CARNAGHAN, R. B. A., & ALLCROFT, R.
(1961) The assay of a toxic principle in certain groundnut meals.
Vet. Rec., 73: 1219-1223.
SARKISOV, A. C. (1954) [Mycoses.] Moscow, State Publishing House
for Agricultural Literature, 216 pp. (in Russian).
SATO, S., MATSUSHIMA, T., TANAKA, N., SUGIMURA, T., & TAKASHIMA, F.
(1973) Hepatic tumors in the guppy (Lebistes reticulatus) induced
by aflatoxin B1, dimethylnitrosamine, and 2-acetylaminofluorene.
J. Natl Cancer Inst., 50: 765-778.
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches to
the toxic metabolites of Fusaria. VIII. Acute and sub-acute
toxicities of T-2 toxins in cats. Jpn. J. Pharmacol., 25: 263-270.
SCHADE, J. E., MCGREEVY, K., KING, A.D., MACKEY, B., & FULLER, G.
(1975) Incidence of aflatoxin in California almonds. Appl.
Microbiol., 29: 48-53.
SCHINDLER, A. F. & NESHEIM, S. (1970) Effect of moisture and
incubation time on ochratoxin A production by an isolate of
Aspergillus ochraceus. J. Assoc. Off. Anal. Chem., 53: 89-91.
SCHROEDER, H. W. & CARLTON, W. W. (1973) Accumulation of only
aflatoxin B2 by a strain of Aspergillus flavus. Appl.
Microbiol., 25: 146-148.
SCHULLER, P. L., OCKHUIZEN, TH., WERRINGLOER, J., & MARQUARDT, P.
(1967) [Aflatoxin B1 and histamine in wine.]
Arzneimitteforschung, 17: 888-890 (in German).
SCHULLER, P. L., VERHOLSHONK, C. A. H., & PAULSCH, W. E. (1973)
Analysis of aflatoxin M1 in liquid and powdered milk. Pure appl.
Chem., 35: 291-296.
SCHULLER, P. L., VERHULSDONK, C. A. H, & PAULSCH, W. E. (1967)
Aflatoxin M1 in liquid and powdered milk (evaluation of methods
and collaborative study). Zesz. Probl. Postepow Nauk Roln.,
189: 255-261.
SCOTT, P.M. & KENNEDY, B. P. C. (1973) Analysis and survey of ground
black, white, and capsicum peppers for aflatoxins. J. Assoc. Off.
Anal. Chem., 56: 1452-1457.
SCOTT, P.M., VAN WALBEEK, W., KENNEDY, B, & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. Agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., PANALAKS, T., KANHERE, S., & MILES, W. F. (1978)
Determination of zearalenone in cornflakes and other corn-based
foods by thin layer chromatography, high pressure liquid
chromatography, and gas-liquid chromatography/high resolution mass
spectrometry. J. Assoc. Off. Anal. Chem., 61: 593-600.
SEEGERS, J. C. & PITOUT, M. J. (1973) DNA repair synthesis in cell
cultures exposed to low doses of aflatoxin B1 or sterigmatocystin
S. Aft. lab. clin. Med., 19: 961 (abstr. 23).
SEIBOLD, H. R. & BAILEY, W. S. (1952) An epizootic of hepatitis in
the dog. J. Am. Vet. Med. Assoc., 121: 201-206.
SEIBOLD, R. & RUCH, W. (1977) [Aflatoxin content of mixed feeds of
dairy cows.] Kraftfutter, 60: 182-185 (in German).
SERCK-HANSSEN, A. (1970) Aflatoxin-induced fatal hepatitis? A case
report from Uganda. Arch. environ. Health, 20: 729-731.
SHANK, R. C. (1976) The role of aflatoxin in human disease. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems. Washington, DC, American Chemical Society, pp. 51-57
(Advances in Chemistry Series 149).
SHANK, R. C. (1977) Epidemiology of aflatoxin carcinogenesis. In:
Kraybill, H. F. & Mehlman, M. M., ed. Environmental cancer.
Advances in modern toxicology, Vol. 3, John Wiley and Sons, New
York, pp. 291-318.
SHANK, R. C., BOURGEOIS, C. H., KESCHAMRAS, N., & CHANDAVIMOL, P.
(1971a) Aflatoxins in autopsy specimens from Thai children with an
acute disease of unknown actiology. Food Cosmet. Toxicol., 9:
501-507.
SHANK, R. C., JOHNSEN, D. O., TANTICHAROENYOS, P., WOODING, W. L., &
BOURGEOIS, C. H. (1971b) Acute toxicity of aflatoxin B, in the
macaque monkey. Toxicol. appl. Pharmacol., 20: 227-231.
SHANK, R. C., WOGAN, G. N., GIBSON, J. B., & NONDASUTA, A. (1972a)
Dietary aflatoxins and human liver cancer. II. Aflatoxins in market
foods and foodstuffs of Thailand and Hong Kong. Food Cosmet.
Toxicol., 10: 61-69.
SHANK, R. C., GORDON, J. E., WOGAN, G. N., NONDASUTA, A., &
SUBHAMANI, B. (1972b) Dietary aflatoxins and human liver cancer.
III. Field survey of rural Thai families for ingested aflatoxins.
Food Cosmet. Toxicol., 10: 71-84.
SHANK, R. C., BHAMARAPRAVATI, N., GORDON, J. E., & WOGAN, O. N.
(1972c) Dietary aflatoxins and human liver cancer. IV. Incidence of
primary cancer in two municipal populations of Thailand. Food
Cosmet. Toxicol, 10: 171-179.
SHANKARAN, R., RAJ, H. G., & VENKITASUBRAMANIAN, T. A. (1970) Effect
of aflatoxin on carbohydrate metabolism in chick liver.
Enzymology, 39: 371-378.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969a) Survey of cereal grains
and soybeans for the presence of aflatoxin: II. Corn and soybeans.
Cereal Chem., 46. 454-463.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969b) Survey of cereal grains
and soybeans for the presence of aflatoxin: I. Wheat, grain sorghum,
and oats. Cereal Chem., 46: 446-454.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1969)
Ochratoxin A: Occurrence as natural contaminant of a corn sample.
Appl. Microbiol., 17: 765-766.
SHOTWELL, O. L., HESSELTINE, C. W., GOULDEN, M. L., & VANDEGRAFT, E.
E. (1970) Survey of corn for aflatoxin, zearalenone and ochratoxin.
Cereal Chem., 47: 700-707.
SHOTWELL, O. L., HESSELTINE, C. W., VANDEGRAFT, E. E., & GOULDEN, M.
L. (1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16: 266-273.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1973)
Incidence of aflatoxin in southern corn, 1969-1970. Cereal Sci.
Today, 18, 192-195.
SHOTWELL, O. L., GOULDEN, M. L., & HESSELTINE, C. W. (1976a) Survey
of US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal.
Chem., 59: 122-124.
SHOTWELL, O. L., GOULDEN, M. L., & BENNETT, G. A. (1976b)
Determination of zearalenone in corn: Collaborative study.
J. Assoc. Off. Anal. Chem., 59: 666-670.
SINNHUBER, R. O. & WALES, J. H. (1974) Aflatoxin B1
hepatocarcinogenicity in rainbow trout embryos. Fed. Proc., 33:
247 (Abstract no. 253).
SINNHUBER, R. O., LEE, D. J., WALES, J. H., & AYRES, J. L. (1968a)
Dietary factors and hepatoma in rainbow trout (Salmo gairdneri).
II. Cocarcinogenesis by cyclopropenoid fatty acids and the effect of
gossypol and altered lipids on aflatoxin-induced liver cancer.
J. Natl Cancer Inst., 41: 1293-1301.
SINNHUBER, R. O., WALES, J. H., AYRES, J. L., ENGEBRECHT, R. H., &
AMEND, D. L. (1968b) Dietary factors and hepatoma in rainbow trout
(Salmo gairdneri) I. Aflatoxins in vegetable protein feedstuffs.
J. Natl Cancer Inst., 41: 711-718.
SINNHUBER, R. O., LEE, D. J., WALES, J. H., LANDERS, M. K., KEYL, A.
C. (1974) Hepatic carcinogenesis of aflatoxin M1 in rainbow trout
(Salmo gairdneri) and its enchancement by cyclopropene fatty
acids. J. Natl Cancer Inst., 53: 1285-1288.
SMALLEY, E. B. & STRONG, F. M. (1974) Toxic trichothecenes. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
199-228.
SMITH, J. W., PRINCE, W. R., & HAMILTON, P. B. (1969) Relationship
of aflatoxicosis to Salmonella gallinarum infections of chickens.
Appl. Microbiol., 18: 946-947.
SPEERS, G. M., MERONUCK, R. A., BARNES, D. M., & MIROCHA, C. J.
(1971) Effect of feeding Fusarium roseum f. sp. graminearum
contaminated corn and the mycotoxin F-2 on the growing chick and
laying hen. Poult. Sci., 50: 627-633.
STACK, M. E. (1974) Collaborative study of AOAC methods I and III
for the determination of aflatoxins in peanut butter. J. Assoc.
Off. Anal. Chem., 57: 871-874.
STANFORD, G. K., HOOD, R. D., & HAVES, A. W. (1975) Effect of
prenatal administration of T-2 toxin to mice. Res. Commun. chem.
Path. Pharmacol., 10: 743-746.
STEPHENSON, L. W. & RUSSELL, T. E. (1974) The association of
Aspergillus flavus with hemipterous and other insects infesting
cotton bracts and foliage. Phytopathology, 64: 1502-1506.
STERN, P.S., & HOLZAPFEL, C. W. (1967) The synthesis of ochratoxins
A and B, metabolites of Aspergillus ochraceus Wilh. Tetrahedron
Lett., 23: 4449-4461.
STEYN, P.S., VLEGGAAR, R., Du PREEZ, N. P., BLYTH, A. A., & SEEGERS,
J. C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STICH, H. F. & LAISHES, B. A. (1975) The response of Xeroderma
pigmentosum cells and controls to the activated mycotoxins,
aflatoxins and sterigmatocystin. Int. J. Cancer, 16: 266-274.
STOLOFF, L. (1972) Analytical methods for mycotoxins. Clin.
Toxicol., 5: 465-494.
STOLOFF, L. (1976) Occurrence of mycotoxins in foods and feeds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 23-50
(Advances in Chemistry Series 149).
STOLOFF, L. (1977) Aflatoxins-an overview. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 7-28.
STOLOFF, L. & FRIEDMAN, L. (1976) Information bearing on the
evaluation of the hazard to man from aflatoxin ingestion.
PAG Bulletin, 6: 21-32.
STRZELECKI, E. L. & GASIOROWSKA, U. W. (1974) Aflatoxin B1 in
feedstuffs. Zentralbl. Vet.-Med. B, 21: 395-400.
STUBBLEFIELD, R.D., SHOTWELL, O. L., & SHANNON, O. H. (1972)
Aflatoxins M1 and M2 and parasiticol: Thin layer chromatography
and physical and chemical properties. J. Assoc. Off. Anal. Chem.,
55: 762-767.
SUIT, J. L., ROGERS, A. E., JETTEN, M. E. R., & LURIA, S. E. (1977)
Effects of diet on conversion of aflatoxin B1 to bacterial
mutagen(s) by rats in vivo and by rat hepatic microsomes
in vitro. Mutat. Res., 46: 313-323.
SUZANGAR, M., EMAMI, A., & BARNETT, R. (1976) Aflatoxin
contamination of village milk in Isfahan, Iran. Trop. Sci., 18:
155-159.
SUZUKI, S. & SATOH, T. (1973) Effects of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Pharmacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl.
Pharmacol., 32: 116-122.
SVOBODA, D., GRADY, H. J, & HIGGINSON, J. (1966) Aflatoxin B1
injury in rat and monkey liver. Am. J. Pathol., 49: 1023-1051.
SWENSON, D. H., MILLER, E. C., & MILLER, J. A. (1974) Aflatoxin
B1-2, 3-oxide: Evidence for its formation in rat liver in vivo
and by human liver microsomes in vitro. Biochem. Biophys. res.
Comm., 60: 1036-1043.
SWENSON, D. H., LIN, J.-K, MILLER, E. C., & MILLER, J. A. (1977)
Aflatoxin B1-2, 3-oxide as a probable intermediate in the covalent
binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal
RNA in vivo. Cancer Res., 37: 172-181.
SZCZECH, G. M., CARLTON, W. W., & TUITE, J. (1973a) Ochratoxicosis
in beagle dogs. I. Clinical and clinicopathological features.
Vet. Pathol., 10: 135-154.
SZCZECH, G. M., CARLTON, W. W., TUITE, J., & CALDWELL, R. (1973b)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10: 347-364.
SZCZECH, G. M., CARLTON, W. W, & TUITE, J. (1973c) Ochratoxicosis in
beagle dogs. II. Pathology. Vet. Pathol., 10: 219-231.
TANDON, B. N., KRISHNAMURTHY, L., KOSHY, A., TANDON, H. D.,
RAMALINGASWAMI, V., BHANDARI, J. R., MATHUR, M. M., & MATHUR, P. D.
(1977) Study of an epidemic of jaundice, presumably due to toxic
hepatits, in northwest India. Gastroenterology, 72: 488-494.
TANDON, H. D., TANDON, B. N., & RAMALINGASWAMI, V. (1978) Epidemic
of toxic hepatitis in India of possible mycotoxic origin. Arch.
Pathol. lab. Med., 102. 372-376.
TEMCHAROEN, P., ANUKARAHANONTA, T., & BHAMARAPRAVATI, N. (1978) The
influence of dietary protein and vitamin B12 on the toxicity and
carcinogenicity of aflatoxins in rat liver. Cancer Res.,
38: 2185-2190.
THACKER, H. L. (1976) Citrinin and ochratoxin A mycotoxicosis in
guinea pigs, Lafayette, Indiana, Purdue University (A thesis).
THERON, J. J. (1965) Acute liver injury in ducklings as a result of
aflatoxin poisoning. Lab. Invest., 14: 1586-1603.
THERON, J. J., LIEBENBERG, N., & JOUBERT, H. J. B. (1965)
Histochemistry and electron microscopy of acute liver lesions
induced by aflatoxin B1 in ducklings. Nature (Lond.), 206:
908-909.
TILAK, T. B. (1975) Induction of cholangiocarcinoma following
treatment of a rhesus monkey with aflatoxin. Food Cosmet.
Toxicol., 13: 247-249.
TRIPATHI, R. K. (1973) Aflatoxins in sorghum grains infested with
head moulds. Indian J. exp. Biol., 11: 361-362.
TUNG, H.-T., DONALDSON, W. E., & HAMILTON, P. B. (1970) Effects of
aflatoxin on some marker enzymes of lysosomes. Biochem. Biophys.
Acta, 222. 665-667.
TURNER, W. B. (1971) Fungal metabolites, New York, Academic Press,
446 pp.
ULLOA-SOSA, M. & SCHROEDER, H. W. (1969) Note on aflatoxin
decomposition in the process of making tortillas from corn.
Cereal Chem., 46: 397-400.
VAN NIEUWENHUIZE, J.P., HERBER, F. M., DE BRUIN, A., MEYER, P. B., &
DUBA, W. C. (1973) [Cancers in men following a long-term low-level
exposure to aflatoxins in indoor air.] T. Soc. Geneeskd., 51:
754-760 (in Dutch).
VAN PÉE, W., VAN BRABANT, J., & JOOSTENS, J. (1977) La détection et
le dosage de l'aflatoxine M1 dans le lait et le lait en poudre.
Rev. Agrie. (Bruxelles), 30: 403-415.
VAN RENSBURG, S. J. (1977) Role of epidemiology in the elucidation
of mycotoxin health risks. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publ., pp. 699-711.
VAN RENSBURG, S. J., VAN DER WATT, J. J., PURCHASE, I. F. H.,
PEREIRA COUTINHO, L., & MARKHAM, R. (1974) Primary liver cancer rate
and aflatoxin intake in a high cancer area. S. Afr. Med. J., 48:
2508a-2508d.
VANYI, A. & SZAILER, E. (1974) Investigation of the cytotoxic effect
of F-2 toxin (zearalenone) in various monolayer cell cultures.
Acta Vet. Acad. Sci. Hung., 24: 407-412.
VERRETT, M. J., MARLIAC, J.-P., & MCLAUGHLIN, J., JR (1964) Use of
the chicken embryo in the assay of aflatoxin toxicity.
J. Assoc. Off. Anal. Chem., 47: 1003-1006.
VESELY, D., VESELA, D., KUSAK, V., & NESNIDAL, P. (1978)
[Distribution of perorally administered aflatoxin B1 in tissues
and organs of the goat.] Veterinarni medicina, 23 (LI): 555-558 (in
Czech).
VESONDER, R. F., CIEGLER, A., & JENSEN, A. H. (1973) Isolation of
the emetic principle from Fusarium-infected corn.
Appl. Microbiol., 26: 1008-1010.
VESSELINOVITCH, S. D., MIHAILOVICH, N., WOGAN, G. N., LOMBARD, L.
S., & RAO, K. V. N. (1972) Aflatoxin B1, a hepatocarcinogen in the
infant mouse. Cancer Res., 32: 2289-2291.
VILLA-TREVINO, S. & LEAVER, D. D. (1968) Effects of the hepatotoxic
agents retrorsine and aflatoxin B1 on hepatic protein synthesis in
the rat. Biochem. J., 109: 87-91.
VOGEL, C. L., ANTHONY, P. P., MODY, N., & BARKER, L. F. (1970)
Hepatitis-associated antigen in Ugandan patients with hepatocellular
carcinoma. Lancet, 2: 621-624.
WALES, J. H. & SINNHUBER, R. O. (1972) Hepatomas induced by
aflatoxin in the sockeye salmon (Oncorhynchus nerka). J. Natl
Cancer Inst., 48: 1529-1530.
WALTKING, A. E. (1970) Collaborative study of three methods for
determination of aflatoxin in peanuts and peanut products.
J. Assoc. Off. Anal. Chem., 53: 104-113.
WALTKING, A. E. (1971) Fate of aflatoxin during roasting and storage
of contaminated peanut products. J. Assoc. Off. Anal. Chem., 54:
533-539.
WALTKING, A. E., BLEFFERT, G., & KIERNAN, M. (1968) An improved
rapid physio-chemical assay method for aflatoxin in peanuts and
peanut products. J. Am. Oil Chem. Soc., 45: 880-884.
WARD, J. M., SONTAG, J. M., WEISBURGER, E. K., & BROWN, C. A. (1975)
Effect of lifetime exposure to aflatoxin B1 in rats.
J. Natl Cancer Inst., 55: 107-113.
WARE, G. M. & THORPE, C. W. (1977) Analysis for zearalenone in corn
by high pressure liquid chromatography with a fluorescence detector.
Abstracts 91st Annual Meeting, Association of Official Analytical
Chemists, Washington, DC, p. 47 (Abstract no. 148).
WARE, G. M. & THORPE, C. W. (1978) Determination of zearalenone in
corn by high-pressure liquid chromatography and fluorescence
detection. J. Assoc. Off. Anal, Chem., 61 (5): 1058-1062.
WHITAKER, T. B. (1977)Sampling granular foodstuffs for aflatoxin.
Pure appl. Chem., 49: 1709-1717.
WHITAKER, T. B. & WHITTEN, M. E. (1977) Evaluation of cottonseed
aflatoxin testing programs. J. Am. Oil Chem. Soc., 54: 436-441.
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (1974a) Variability
of aflatoxin test results. J. Am. Oil Chem. Soc., 51: 214-218.
WHITAKER, T. B., DICKENS, J. W., WISER, E. H., & MONROE, R. J.
(1974b) Development of a method to evaluate sampling plans used to
estimate aflatoxin concentrations in lots of shelled peanuts.
I.U.P.A.C. Information Bulletin, Oxford, International Union of Pure
& Applied Chemistry Secretariat, pp. 1-14 (Technical Report-No. 10).
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (in press)
Variability associated with testing corn for aflatoxin. J. Am. Oil
Chem. Soc.
WHITTEN, M. E. (1969) Aflatoxins in cottonseed hulls. J. Am. Oil
Chem. Soc., 47: 5-6.
WHO (1978) Environmental Health Criteria 6-- Principles and methods
for evaluating the toxicity of chemicals. Part I, Geneva, World
Health Organization, 272 pp.
WILLIAMS, D. J. & RABIN, B. R. (1971) Disruption by carcinogens of
the hormone dependent association of membranes with polysomes.
Nature (Lond.), 232: 102-105.
WOGAN, G. N. (1966) Chemical nature and biological effects of the
aflatoxins. Bacteriol. Rev., 30: 460-470.
WOGAN, G. N. (1969) Metabolism and biochemical effects of
aflatoxins. In: Goldblatt, L. A., ed. Aflatoxin: Scientific
background, control and implications, New York, Academic Press,
pp. 151-186.
WOGAN, G. N. (1973)Aflatoxin carcinogenesis. In: Bush, M., ed.
Methods in cancer research, New York, Academic Press, pp. 309-344.
WOGAN, G. N. (1977) Mycotoxins and other naturally occurring
carcinogens. In: Kraybill, H. F. & Mehlman, M. A., ed.
Environmental cancer, Advances in modern toxicology, Washington,
DC, Hemisphere Publishing Corporation, Vol. 3, pp. 263-290.
WOGAN, G. N. & FRIEDMAN, M. A. (1968) Inhibition by aflatoxin B1
of hydrocortisone induction of rat liver tryptophan pyrrolase and
tyrosine transaminase. Arch. Biochem. Biophys., 128: 509-516.
WOGAN, G. N. & NEWBERNE, P.M. (1967) Dose-response characteristics
of aflatoxin B1 carcinogenesis in the rat. Cancer Res., 27:
2370-2376.
WOGAN, G. N. & PAGLIALUNGA, S. (1974) Carcinogenicity of synthetic
aflatoxin M1 in rats. Food Cosmet. Toxicol., 12: 381-384.
WOGAN, G. N., EDWARDS, G. S., & SHANK, R. C. (1967) Excretion and
tissue distribution of radioactivity from aflatoxin B1-14C in
rats. Cancer Res., 27: 1729-1736.
WOGAN, G. N., EDWARDS, G. S., & NEWBERNE, P.M. (1971)
Structure-activity relationships in toxicity and carcinogenicity of
aflatoxins and analogs. Cancer Res., 31: 1936-1942.
WOGAN, G. N., PAGLIALUNGA, S., & NEWBERNE, P.M. (1974) Carcinogenic
effects of low dietary levels of aflatoxin B1 in rats. Food
Cosmet. Toxicol., 12: 681-685.
WONG, J. J. & HSIEH, D. P. H. (1976) Mutagenicity of aflatoxins
related to their metabolism and carcinogenic potential. Proc. Natl
Acad. Sci. (USA), 73: 2241-2244.
WYATT, R. D., DOERR, J. A., HAMILTON, P. B., & BURMEISTER, H. R.
(1975) Egg production, shell thickness and other physiological
parameters of laying hens affected by T-2 toxin. Appl. Microbiol.,
29: 641-645.
WYLLIE, W. D. & MOREHOUSE, L. D., ed. (1977) Mycotoxic fungi,
mycotoxins, mycotoxicosis: An encyclopaedic handbook. New York and
Basel, Marcel Dekker.
YADGIRI, B., REDDY, V., TULPULE, P. G., SRIKANTIA, S. G., & GOPALAN,
C. (1970) Aflatoxin and Indian childhood cirrhosis; Am. J. clin.
Nutr., 23: 94-98.
YAGEN, B., JOFFE, A. Z., HORN, P., MOR, N., & LUTSKY, I. I. (1977)
Toxins from a strain involved in ATA. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 329-336.
YNDESTAD, M. & UNDERDAL, B. (1975) [Aflatoxin in foods on the
Norwegian market.] Nord. Vet.-Med., 27: 42-48 (in Norwegian).
4.2.2 Occurrence
The occurrence of zearalenone in foodstuffs has been reviewed by
Stoloff (1976). Zearalenone has been encountered as a natural
contaminant, particularly in maize, but occasionally in other
cereals and in feedstuffs. In a survey of maize in the USA during
the period 1968-69, zearalenone was found in 6 out of 576 (1%)
samples at levels ranging from 450 to 800 µg/kg (Shotwell et al.,
1971). In 1972, when conditions in the USA were conducive to
Fusarium ear rot, zearalenone was found in 17% of 223 samples of
maize at levels ranging from 0.1 to 5.0 mg/kg (Eppley et al., 1974).
It has also been detected in maize in France and in barley and mixed
feed in England, Finland, and Yugoslavia (Stoloff, 1976).
Nine out of 11 commercial corn meal samples in the USA contained
zearalenone at levels ranging from 12 to 69 µg/kg (Ware & Thorpe,
1977). The compound has been found in one sample of cornflakes
(13 µg/kg) (Scott et al., 1978), and in maize beer, in Zambia, in
the range of 0.01-4.6 mg/litre (Lovelace & Nyathi, 1977).
Zearalenone was detected in 11% of 55 samples of Swazi sour
drinks, sour porridges, and beers (range: 8-53 mg/kg) and in 12% of
140 beer samples from Lesotho (range: 0.3-2 mg/kg), but such high
figures have not been reported elsewhere. No data on consumption
were given (Martin & Keen, 1978).
4.2.3 Effects in animals
4.2.3.1 Field observations
The effects of zearalenone in animals have been reviewed by
Mirocha & Christensen (1974). Field cases of the estrogenic syndrome
in pigs, associated with the use of mouldy feed, were first observed
half a century ago. The disease was characterized by enlarged
oedematous vulvae and mammary glands (McNutt et al., 1928).
Subsequently, this syndrome has been encountered in a number of
countries in North America and Europe and in Australia (Table 25),
and in most cases zemralenone has been identified in associated
feeds, indicating together with the result of experimental studies
(4.2.2.2) a causal role of this compound. Zearalenone feed levels
reported to be associated with the syndrome in swine (Mirocha et
al., 1977) are listed in Table 26. Cases of reduced fertility in
cattle indicated by an increase in the artificial insemination index
have been reported to be associated with a feed (hay) content of
zearalenone of 14 mg/kg (Mirocha et al., 1968b). Fertility
disturbances and prolonged heat in a herd of cattle suggested to be
associated with zearalenone-contaminated feed were reported by Roine
et al. (1971).
Table 25. Occurrence of the estrogenic syndrome in pigs in
various countriesa
Year Country Feedstuff
1928 USA maize
1937 Australia maize
1952 Ireland barley
1962 France maize
1962 Italy maize
1963 Yugoslavia maize and barley
1967 Romania maize
1968 Hungary maize
1968 Denmark barley
1971 Canada maize
a From: Mirocha & Christenson (1974).
Table 26. Natural occurrence of zearalenone in feeds associated with
hyperestrogenism in swinea
Feed sample Level of
contamination
(mg/kg)
maize kernels (Minnesota)b 0.1-0.15
dry sow ration (Vancouver) 0.15
farrowing ration (Vancouver)c 0.066
dry sow ration (Vancouver) 0.15
corn kernels (Vancouver) 0.20
dry sow ration (Vancouver)c 0.25
lactation ration (Vancouver) 1.00
gestation ration (Vancouver) 0.6
milo (Minnesota) 2.5-5.6
sesame meal (Univ. of Minn.)d 1.5
corn kernels (Ohio) 0.12
mixed feed corn (Ohio)b 0.12
corn kernels (Minnesota) 6.4
commercial pelletted mixed feed 6.8
(Minnesota)
a From Mirocha et al. (1977).
b Rectal prolapse in gilts.
c Diethylstilbestrol was also present in these samples.
d Associated with hyperestrogenism in turkey poults.
4.2.3.2 Experimental studies
Under experimental conditions, pigs (6-week-old-gilts) exposed
perorally to zearalenone at 5 mg per animal per day for 5 days
developed enlarged vulvae and mammae, and prolapse of the vagina
within a few days; effects were reversible on termination of
exposure. In another experiment, oral administration of 8 mg of pure
crystalline zearalenone to 6-week-old, pre-pubertal gilts (8 doses
of 1 mg/day per animal) induced pronounced tumefaction of the vulva
(Mirocha & Christensen, 1974). Histological changes in the genital
tract of pigs exposed to zearalenone included metaplasia of the
epithelium in the cervix and vagina, and oedema in the wall of the
uterus (Kurtz et al., 1969).
Observations on pigs (Miller et al., 197.3) suggested that
ingestion of grain contaminated with zearalenone during late
gestation might be related to still-birth and splayleg. Splayleg was
observed in the offspring of one sow and one gilt given dally
intramuscular injections of zearalenone at 5 mg per animal
throughout the last month of pregnancy. However, in the experiment
of Patterson et al. (1977), all 7 gilts fed zearalenone at a level
of 2 mg/kg feed throughout pregnancy remained clinically normal and
embryonic survival rates were not affected by the toxin. Splayleg
was diagnosed in only 1/63 piglets and the condition appeared to
have resolved within 24 h. Results in the 2 groups of 3 pigs fed the
lower levels were inconclusive (unpublished data, Chang & Kurtz,
quoted by Mirocha et al., 1977).
No effects on egg production were observed when laying hens were
fed rations containing zearalenone levels of 250 and 500 mg/kg
(Speers et al., 1971).
In a 2-generation study on rats exposed to dietary levels of
zearalenone corresponding to daily intakes of 0.1, 1.0, and 10 mg/kg
body weight, no teratogenic effects were observed at any level of
intake but impaired fertility and resorptions and stillbirths
occurred in animals receiving 10 mg/kg body weight daily with 56% of
dams showing complete litter resorption (Bailey et al., 1976).
Ruddick et al. (1976) found fetal skeleton anomalies in rats
exposed orally to zearalenone at doses ranging from 1-10 mg/kg body
weight during the gestation period, with defect incidences of 12.8%
(11/86) at the 1 mg/kg level, 26.1% (18/69) at the 5 mg/kg level,
and 36.8% (28/76) at the 10 mg/kg level. No effect was observed with
exposure to zearalenone at a dose of 0.075-0.30 mg/kg body weight.
The results of a study by Mirocha et al. (1968a) in which the
dose-effect relationships for zearalenone and estrone were compared
with regard to increase in uterus weight, are given in Table 27. The
results of a study comparing the effects of estrogens and
zearalenone in 24 adult female castrated rhesus monkeys with a
previous history of regular menstrual cycles are recorded in Table
28.
Table 27. Effect of peroral dosing of zearalenone and estrone in micea
Material Total dose No. of Mean uterine
administered (µg) mice ratio ± S.E.
control 0 10 0.76 ± 0.06
estrone 0.5 10 1.21 ± 0.12
1 9 1.19 ± 0.10
2 9 2.12 ± 0.23
4 10 2.93 ± 0.21
zearalenone 12.5 10 1.11 ± 0.04
25 10 1.48 ± 0,18
50 10 1.80 ± 0.12
100 9 2.35 ± 0.17
a From: Mirocha et al. (1968a).
4.2.4 Conclusions and evaluation of the health risks to man of
zearalenone
4.2.4.1 Animal studies
Field cases of the estrogenic syndrome in pigs have been
encountered in many countries in association with zearalenone in
feeds at levels ranging from 0.1 to 6.8 mg/kg (Table 26), in samples
not known to be contaminated with other estrogens. The condition has
been reproduced experimentally in pigs, with a dally dose of
1 mg/animal for 8 days. Infertility, sporadically encountered in
cattle, has been suggested to be causally associated with
zearalenone in feed at a reported level of 14 mg/kg of hay.
In one of two studies on teratogenic effects in rats, skeletal
defects were detected in fetuses at a daily oral dose of 1 mg/kg
body weight.
Table 28. The estimated minimum dose of estrogens that will depress
serum gonadotropin (FSH or LH) in castrated rhesus monkeysa
Estrogen administered Estimated minimum dose
(µg/kg)
Subcutaneousb Oralc
LHd FSHd LH FSH
estradiol-17ß 4 1 5 --
diethylstilboestrol 0.5 2 2.5 --
zearalenone 14 56 400 --
a From: Hobson et al. (1977).
b Two injections given in oil.
c Given on 4 consecutive days,
d FSH = follicle-stimulating hormone; LH = luteinizing hormone.
4.2.4.2 Evaluation of health risks
There are no reports on the adverse effects of zearalenone in
man. With the exception of 2 reports from Africa, levels of
zearalenone ranging from 12 to 69 µg/kg have been found in a limited
number of maize products destined for human consumption. Even
assuming that 1 kg of these products is consumed dally, it can be
estimated that a 70 kg man would not receive more than 1 µg
zearalenone per kg body weight in food. This level of exposure is
400 times lower than the lowest peroral dose causing effects in
tests on monkeys (Table 28) and more than 600 times lower than the
lowest dose (µg/kg) used in assessing the estrogenic potency of
perorally administered zearalenone in mice (Table 27).
However, in the 2 reports from certain parts of Africa, high
levels of zearalenone were found in beer and sour porridge prepared
from maize and sorghum. Long-term exposure to such contaminated
drinks could represent a health hazard.
4.3 Trichothecenes
4.3.1 Properties and sources
The properties and sources of trichothecenes have been reviewed
by Bamburg & Strong (1971), Smalley & Strong (1974), and Bamburg
(1976). The trichothecenes possess the tetracyclic
12,13-epoxytrichothec-9-ene skeleton. More than 30 trichothecene
derivatives have been isolated from fungal cultures, but, so far,
only 4 have been identified as natural contaminants of foodstuffs
(Table 29). The compounds have been detected by various methods
including thin-layer chromatography, gas chromatography, and
bioassays, in particular the rabbit skin test.
One or more of the trichothecenes have been isolated from
strains of the following Fusarium species: F. episphae,
F. lateritium, F. nivale, F. oxysporum, F. rigidisculum,
F. solani, F. roseum, and F. tricinctum (syn.
F. sporotrichioides). In addition, species of Cephalosporium,
Myrothecium, Trichoderma, and Stachybotrys have been found to
produce trichothecenes.
Table 29. Natural occurrence of trichothecenes
Compound Concentration Commodity Reference
(µg/kg)
T-2 toxin 2 maize Hsu et al. (1972)
T-2 toxin 25 barley Puls & Greenway (1976)
T-2 toxin 0.076 mixed feed Mirocha et al. (1976)
nivalenol n.q.a barley Morooka et al. (1972)
deoxynivalenol 7.3 barley Morooka et al, (1972)
deoxynivalenol n.m.b maize Vesonder et al. (1973)
deoxynivalenol 0.1, 1.0, 1.8 maize Mirocha et al. (1976)
(3 samples)
deoxynivalenol 1.0, 1.0, 0.06 mixed feed Mirocha et al. (1976)
(3 samples)
diacetoxyscirpenol 0.38, 0.5 mixed feed Mirocha et al. (1976)
(2 samples)
a n.q. = not quoted
b n.m. = not measured
4.3.2 Occurrence
So far, the trichothecenes have only been found very
sporadically in natural products. Naturally occurring trichothecenes
include the following, based on chemical identification: T-2 toxin,
nivalenol, deoxynivalenol, (vomitoxin), and diacetoxyscirpenol. In
available studies, these 4 compounds have only been found in a total
of 14 samples (Table 29), sometimes (as in the case of
deoxynivalenol) concomitantly with zearalenone. No information is
available concerning the number of samples analysed in these studies
and therefore on the frequency of positive results.
4.3.3 Effects in animals
4.3.3.1 Field observations
A field case involving the death of 20% of a dairy herd was
suggested to be associated with ingestion of mouldy corn in the feed
containing a concentration of T-2 toxin of approximately 2 mg/kg dry
weight (Hsu et al., 1972). The lesions in the cattle included
extensive haemorrhages on the serosal surface of all internal
viscera.
An outbreak of a disease, observed in poultry (ducks, geese),
horses, and pigs, was suggested to be associated with mouldy barley
containing T-2 toxin at approximately 25 mg/kg (Greenway & Puls,
1976). The lesions in the geese included necrosis of the mucosa of
the oesophagus, proventriculus, and gizzards.
Deoxynivalenol (vomitoxin) was isolated from a batch of maize
that had caused vomiting in pigs (Vesonder et al., 1973).
4.3.3.2 Experimental studies
Acute effects of trichothecenes determined as LD50 values, are
listed in Table 30. Sato et al. (1975) studied tile effects in cats
of T-2 toxin, administered in several ways. Two cats weighing 1.5
and 4.0 kg were given purified T-2 toxin in single subcutaneous
doses of 0.5 and 1.0 mg/kg, respectively. Nausea and vomiting
appeared after 1 h and the animals died 20 h after the injection.
The autopsy revealed extensive necrosis of the mucosa in the small
and large intestine, marked karyorrhexis in the germ centre of the
lymph follicles of the spleen and lymph nodes, and diffuse vacuolar
degeneration of the renal tubules. Damage to bone marrow cells was
observed in the cervical, thoracic, and lumbar vertebrae. In two
cats subcutaneously injected with T-2 toxin (repeated doses of 0.1
and 0.05 mg/kg body weight, given over a 4-week period) a decrease
in the white blood cell (WBC) count was observed and the WBC value
remained low until death occurred during the fourth week of
exposure. The clinical signs in the cats were nausea and vomiting a
few hours after each injection and ataxia of the hind legs. At the
postmortem examination, hypoplasia was observed in the thymus,
spleen, lymph nodes, and bone marrow. Other changes included marked
meningeal haemorrhage, extensive bleeding in the lung, and vacuolar
degeneration of the renal tubular epithelium. A marked decrease in
the WBC values was also observed in 3 cats given T-2 toxin
subcutaneously at a daily dose of 0.05 mg/kg for 12 days (i.e.,
total dose of 0.60 mg/kg); 2 of these cats died, 4 and 35 days,
respectively, after the last injection and one cat survived with the
WBC count returning to the normal level 17 days after the last
injection. Leukopenia and death were also observed in 3 cats (body
weight 2-3 kg) receiving a crude preparation containing 4% T-2 toxin
and 1% neosolaniol. This crude preparation was first administered
subcutaneously once a week for 5 weeks in repeated doses of 1 mg/kg
body weight (corresponding to a repeated dose of T-2 toxin of
0.04 mg/kg). This subcutaneous administration was then followed by
daily oral dosing (15 mg of the crude preparation corresponding to
0.6 mg of T-2 toxin per animal per day) for 17 days.
Table 30. Acute toxicity of naturally occurring trichothecenesa
Compound LD50 Route of Animal
(mg/kg body weight) administration
T-2 toxin 3.04 intraperitoneal mouse
T-2 toxin 3.8 peroral rat
T-2 toxin 5.1 peroral trout
T-2 toxin 5.25 peroral one-day old chickb
nivalenol 4.0 intraperitoneal mouse
diacetoxyscirpenol 10.0 intravenous mouse
diacetoxyscirpenol 0.75 intraperitoneal rat
diacetoxyscirpenol 7.3 peroral rat
a From: Bamburg & Strong (1971 ).
b From: Chi at al. (1977).
More recently Yagen et al. (1978) have mentioned an experiment,
in which oral administration of gelatin capsules containing purified
T-2 toxin to 10 cats resulted in vomiting, leukopenia, haemorrhagic
diathasis, neurological disturbances, and death. The dose and
frequency of administration are not given in the paper.
The effects observed in these experiments, in particular
leukopenia, resemble those produced when cats are fed cultures of
F. sporotrichioides, which is thought to be causally associated
with alimentary toxic aleukia (ATA) in man (section 4.3.4).
Feeding laying hens T-2 toxin at a dietary level of 20 mg/kg for
3 weeks, resulted in oral necrotic lesions, decreased leukocyte
count, and reduced egg production (Wyatt et al., 1975). In mice,
intraperitoneal injection with T-2 toxin at doses of 1.0 and
1.5 mg/kg body weight on one of the days 7-11 of gestation resulted
in a number of maternal deaths as well as in an increase in prenatal
mortality. Malformations of the fetuses were observed including tail
and limb malformations, exencephaly, and retarded jaw development
(Stanford et al., 1975).
Daily oral doses of crude or purified T-2 toxin (0.2 mg/kg body
weight) continued for 79 days failed to produce ill effects in
calves, although the total amount of toxin ingested by one calf was
almost 1.8 g (Matthews et al., 1977).
4.3.4 Alimentary toxic aleukia
Studies on alimentary toxic aleukia (ATA), a disease encountered
in man in the period 1931-43, have been reviewed by Sarkisov (1954)
and more recently by Bilai (1977) and Leonov (1977). The dominant
pathological changes were necrotic lesions of the oral cavity, the
oesophagus, and stomach, and in particular a pronounced leukopenia.
The disease was lethal in a high proportion of cases. An association
was established with ingestion of grain invaded by some moulds, in
particular Fusarium poae and F. sporotrichioides. Effects similar
to ATA have been reproduced in cats by feeding cultures of these
species (Bilai, 1977). T-2 toxin and other trichothecenes have been
identified in a submerged culture of F. sporotrichioides by
Mirocha & Pathre (1973).
4.3.5 Conclusions and evaluation of the health risks to man of
trichothecenes
In recent years, a group of mycotoxins, the trichothecenes, has
been isolated under experimental conditions from many fungi,
including Fusarium species. Isolated field cases of intoxication
in farm animals have been suggested to be related to some of the
trichothecenes.
About 40 years ago, a disease in man known as alimentary toxic
aleukia (ATA), occurred that was suggested to be related to the
presence of toxic Fusarium species in mouldy over-wintered grain.
With improved harvesting, food production, and storage conditions,
the disease disappeared and no new outbreaks have occurred. However,
present knowledge is not sufficient to establish a causal
relationship between any of the isolated trichothecenes and this
outbreak. There are no reports on the exposure of man to
trichothecenes.
REFERENCES
ADAMSON, R. H., CORREA, P., & DALGARD, D. W. (1973) Brief
communication: Occurrence of a primary liver carcinoma in a rhesus
monkey fed aflatoxin B1. J. Natl Cancer Inst., 50:549-551.
ADAMSON, R. H., CORREA, P., SIEBER, S. M., MCINTIRE, K. R., &
DALGARD, D. W. (1976) Carcinogenicity of aflatoxin B1 in rhesus
monkeys: Two additional cases of primary liver cancer. J. Natl
Cancer Inst., 57: 67-78.
ADENKUNLE, A. A. & ELEGBE, R. A. (1974.) Lysosomal activity in
aflatoxin-B1-treated avian embryos. Lancet, 1: 991.
ALLCROFT, R. (1969) Aflatoxicosis in farm animals. In: Goldblatt,
L.A., ed. Aflatoxin scientific background, control and
implications, New York, Academic Press, pp. 237-264.
ALLCROFT, R. & LEWIS, G. (1963) Groundnut toxicity in cattle:
Experimental poisoning of calves and a report on clinical effects in
older cattle. Vet. Rec., 75: 487-493.
ALLCROFT, R. & ROBERTS, B. A. (1968) Toxic groundnut meal: The
relationship between aflatoxin B1 intake by cows and excretion of
aflatoxin M1 in milk. Vet. Rec., 82: 116-118.
ALLCROFT, R., ROBERTS, B. A., & LLOYD, M. K. (1968) Excretion of
aflatoxin in a lactating cow. Food Cosmet. Toxicol., 6: 619-625.
ALPERT, g. E., HUTT, g. S. R., WOGAN, G. N., & DAVIDSON, C. S.
(1971) Association between aflatoxin content of food and hepatoma
frequency in Uganda. Cancer, 28: 253-260.
AMES, B. N., DURSTON, W. E., YAMASAKI, E., & LEE, F. D. (1973)
Carcinogens are mutagens: A simple test system combining liver
homogenates for activation and bacteria for detection. Proc. Natl
Acad. Sci. USA, 70: 2281-2285.
AMLA, I., KAMALA, C. S., GOPALAKRISHNA, G. S., JAYARAJ, A. P.,
SREENIVASAMURTHY, V., & PARPIA, H. A. B. (1971) Cirrhosis in
children from peanut meal contaminated by aflatoxin. Am. J. clin.
Nutr., 24: 609-614.
ANDERSON, H. W., NEHRING, E. W., & WICHSER, W. R. (1975) Aflatoxin
contamination of corn in the field. J. agric. food Chem., 23:
775-782.
ANDRELLOS, P. J. & REID, G. R. (1964) Confirmatory tests for
aflatoxin B1. J. Assoc. Off. Anal. Chem., 47: 801-803.
AMBRECHT, B. H., SHALKOP, W. T., ROLLINS, L. D., POHLAND, A. E., &
STOLOFF, L. (1970) Acute toxicity of aflatoxin B1 in wethers.
Nature (Lond.), 225: 1062.
ASAO, T., BUCHI, G., ABDEL-KADER, M., CHANG, S. B., WICK, E. L., &
WOGAN, G. N. (1963) Aflatoxins B and G. J. Am. Chem. Soc., 85:
1706-1707.
ASAO, T., BUCHI, G., ABDEL-KADER, M. M., CHANG, S. B., WICK, E. L.,
& WOGAN, G. N. (1965) The structures of aflatoxins B and G1. J.
Am. Chem. Soc., 87: 882-886.
ASHWORTH, L. J., JR, MCMEANS, J. L., HOUSTON, B. R., WITTEN, M. E.,
& BROWN, C. M. (1971) Mycoflora, aflatoxins and free fatty acids in
California cottonseed during 1967-1968. J. Am. Oil Chem. Soc., 48:
129-133.
ASPLIN, F. D. & CARNAGHAN, R. B. A. (1961) The toxicity of certain
groundnut meals for poultry with special reference to their effect
on ducklings and chickens. Vet. Rec., 73: 1215-1218.
AUSTWICK, P. K. C. (1975) Balkan nephropathy. Proc. R. Soc. Med.,
68: 219-221.
BABABUNMI, E. A. & BASSIR, O. (1972) Effects of aflatoxin B1 on
the swelling and adenosine triphosphatase activities of mitochondria
isolated from different tissues of the rat. Febs Lett., 26:
102-104.
BACON, C. W., SWEENEY, J. G., ROBBINS, J. D., & BURDICK, D. (1973)
Production of penicillic acid and ochratoxin A on poultry feed by
Aspergillus ochraceus: Temperature and moisture requirements.
Appl. Microbiol., 26: 155-160.
BAGSHAWE, A. F., GACENGI, D. M., CAMERON, C. H., DORMAN, J., & DANE,
D. S. (1975) Hepatitis Bs antigen and liver cancer in Kenya.
Br. J. Cancer, 31: 581-584.
BAILEY, D. E., Cox, G. E., MORGAREIDGE, K., & TAYLOR, J. (1976)
Acute and subacute toxicity of zearalenone in the rat. TAP, 37:
144.
BAMBURG, J. R. (1976) Chemical and biochemical studies of the
trichothecene mycotoxins. In: Rodricks, J.V., ed. Mycotoxins and
other fungal related food problems, Washington, DC, American
Chemical Society, pp. 144-162 (Advances in Chemistry Series 149).
BAMBURG, J. R. & STRONG, F. M. (1971) 12,13-epoxytrichothecenes. In:
Kadis, S., Ciegler, A., & Ajl, S. J., ed. Microbial toxins, New
York, Academic Press, Vol. VII, pp. 207-289.
BASSIR, O. & BABABUNMI, E. A. (1969) Inhibition of blood clotting
factors by aflatoxin. West Aft. J. biol. appl. Chem., 12: 28-32.
BASSIR, O. & OSIYEMI, F. (1967) Biliary excretion of aflatoxin in
the rat after a single dose. Nature (Lond.), 215: 882.
BECKWITH, A. C., VESONDER, R. F., & CIEGLER, A. (1975) Action of
weak bases upon aflatoxin B1 in contact with macromolecular
reactants. J. agric. food Chem., 23: 582-587.
BECROFT, D. M. O. (1966) Syndrome of encephalopathy and fatty
degeneration of viscera in New Zealand children. Br. med. J., 2:
135-140.
BECROFT, D. M. O. & WEBSTER, D. R. (1972) Aflatoxins and Reye's
disease. Br. med. J., 14 Oct., p. 117.
BILAI, V. I. (1977) [Fuzarii.] Kiev, Naukova Dumka, pp. 360-394 (in
Russian).
BLACK, H. S., SMITH, J. D., AUSTIN, B. J., & RAUSCHKOLB, E. W.
(1970) Effect of aflatoxin B1 on the in vitro incorporation of
14C-acetate into human skin lipids. Experientia (Basel), 26:
1292-1293.
BOURGEOIS, C. H. (1975) Encephalopathy and fatty viscera: A possible
response to acute aflatoxin poisoning. In: Pollack, J. D., ed.
Reye's Syndrome, Grune & Stratton, New York, pp. 131-134.
BOURGEOIS, C. H., SHANK, R. C., GROSSMAN, R. A., JOHNSEN, D. O.,
WOODING, W. L., & CHANDAVIMOL, P. (1971) Acute aflatoxin B1
toxicity in the macaque and its similarities to Reye's Syndrome.
Lab. Invest., 24: 206-216.
BROWN, J. M. M. & ABRAMS, L. (1965)Biochemical studies on
aflatoxicosis. Onderstepoort J. vet. Res., 32: 119-146.
BROWN, M. H., SZCZECH G. M., & PURMALIS, B. P. (1976) Teratogenic
and toxic effects of ochratoxin A in rats. Toxicol. appl.
Pharmacol., 37: 331-338.
BUCARBAEVA, A. S. & NIKOV, P.S. (1977) [Aflatoxin sources in
foodstuffs from different regions of Kazachstan.] In: [ Mycotoxins
(sources, chemistry, biosynthesis, analysis and effects on the
organism), Scientific Papers of a Regional Symposium, 28-30
September 1977.] Orenburg, Nutrition Institute of the USSR Academy
of Medical Sciences, pp. 29-31 (in Russian).
BUCHI, G. & WEINREB, S. M. (1969) The total synthesis of racemic
aflatoxin-M1 (milk toxin). J. Am. Chem. Soc., 91: 5408-5409.
BUCHI, G., SPITZNER, D., PAGLIALUNGA, S., & WOGAN, G. N. (1973)
Synthesis and toxicity evaluation of aflatoxin P1. Life Sci.,
13: 1143-1149.
BUCKLEY, H. G. (1971) Fungal nephrotoxicity in swine. Irish
vet. J., 25: 194-196.
BULATAO-JAYME, J., ALMERO, E. M., & SALAMAT, L,. (1976) Epidemiology
of primary liver cancer in the Philippines with special
consideration of a possible aflatoxin factor. J. Philipp. Med.
Assoc., 52: 129-150.
BUTLER, W. H. (1964) Acute toxicity of aflatoxin B1 in rats.
Br. J. Cancer, 18: 756-762.
BUTLER, W. H. (1966) Acute toxicity of aflatoxin B1 in guinea
pigs. J. Pathol. Bacteriol., 91: 277-280.
BUTLER, W. H. (1969) Aflatoxicosis in laboratory animals. In:
Goldblatt, L.A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 223-236.
BUTLER, W. H. (1971-1972) Further ultrastructural observations on
injury of rat hepatic parenchymal cells induced by aflatoxin B1.
Chem. biol. Interactions, 4: 49-65.
BUTLER, W. H. (1974) Aflatoxin. In: Purchase, I. F. H., ed.
Mycotoxins, Amsterdam, Elsevier, pp. 1-28.
BUTLER, W. H. & BARNES, J. M. (1963) Toxic effects of groundnut meal
containing aflatoxin to rats and guinea pigs. Br. J. Cancer, 17:
699-710.
BUTLER, W. H. & BARNES, J. M. (1966) Carcinoma of the glandular
stomach in rats given diets containing aflatoxin. Nature (Lond.),
209: 90.
BUTLER, W. H. & WIGGLESWORTH, J. S. (1966) The effects of aflatoxin
B1 on the pregnant rat. Br. J. exp. Pathol., 47: 242-247.
BUTLER, W. H., GREENBLATT, M., & LIJINSKY W. (1969) Carcinogenesis
in rats by aflatoxins B1, G1, and B2. Cancer Res., 29:
2206-2211.
CAMPBELL, T. C. & SALAMAT, L. (1971) Aflatoxin ingestion and
excretion by humans. In: Purchase, I. F. H., ed. Mycotoxins in human
health, London, Macmillan Press, pp. 271-280.
CAMPBELL, T. C. & STOLOFF, L. (1974) Implication of mycotoxins for
human health. J. agric. food Chem., 22: 1006-1015.
CAMPBELL, T. C., CAEDO, J.P., JR, BULATAO-JAYME, J., SALAMAT, L., &
ENGEL, R. W. (1970) Aflatoxin M1 in human urine. Nature (Lond.),
227: 403-404.
CARNAGHAN, R. B. A. (1965) Hepatic tumours in ducks fed on low level
of toxic ground-nut meal. Nature (Lond.), 208, 308.
CARNAGHAN, R. B. A. (1967) Hepatic tumours and other chronic liver
changes in rats following a single oral administration of aflatoxin.
Br. J. Cancer, 21: 811-814.
CARNAGHAN, R. B. A., HARTLEY, R. D., & O'KELLY, J. (1963) Toxicity
and fluorescence properties of the aflatoxins. Nature (Lond.),
200: 1101.
CARNAGHAN, R. B. A., LEWIS, G., PATTERSON, D. S. P., & ALLCROFT, R.
(1966) Biochemical and pathological aspects of groundnut poisoning
in chickens. Pathol. Vet., 3: 601-615.
CEOVIC, S., GRIMS, P., & MITAR, J. (1976) [The incidence of tumours
of the urinary organs in a region of endemic nephropathy and in a
control region.] Lijee vjesn., 98: 301-304 (in Croatian).
CHAINUVATI, T., VIRANUVATT, V., & PONGPIPAT, D. (1975) Relationship
of hepatitis B antigen in cirrhosis and hepatoma in Thailand. An
etiological significance. Gastroenterology, 68: 1261-1264.
CHANG, F. C. & Car, F. S. (1977) The fate of ochratoxin A in rats.
Food Cosmet. Toxicol., 15: 199-204.
CHANG, S. B., ABDEL-KADER, M. M., WICK, E. L., & WOGAN, G. N. (1963)
Aflatoxin B2: Chemical identity and biological activity.
Science, 142: 1191-1192.
CHAVES-CARBALLO, E., ELLEFSON, R. D., & GOMEZ, M. R. (1976) An
aflatoxin in the liver of a patient with Reye-Johnson syndrome.
Mayo Clin. Proc., 51: 48-50.
CHERNOZEMSKY, I. N., STOYANOV, I. S., PETKOVA-BOCHAROVA, T. K.,
NICOLOV, I. G., DRAGANOV, I. V., STOICHEV, I. I., TANCHEV, Y.,
NAIDENOV, D., & KALCHEVA, W. D. (1977) Geographic correlation
between the occurrence of endemic nephropathy and urinary tract
tumours in Vratza district, Bulgaria. Int. J. Cancer, 19: 1-11.
CHI, M. S., MIROCHA, C. J., KURTZ, M. Y., WEAVER, G., BATES, F., SHIMODA,
W., & BURMEISTER, H. R. (1977) Acute toxicity of T-2 toxin in
broiler chicks and laying hens. Poult. Sci., 56: 103-116.
CHU, F. S. (1971) Interaction of ochratoxin A with bovine serum
albumin. Arch. Biochem. Biophys., 147: 359-366.
CHU, F. S. (1974a) Studies on ochratoxins. CRC crit. Rev.
Toxicol., 2: 499-524.
CHU, F. S. (1974b) A comparative study of the interaction of
ochratoxins with bovine serum albumin. Biochem. Pharmacol.,
23: 1105-1113.
CIEGLER, A., KADIS, S., & AJL, S. J., ed. (1971) Microbial toxins,
fungal toxins, New York, Academic Press, Vol. VI, p. 563.
CLEGG, F. G. & BRYSON, H. (1962) An outbreak of poisoning in store
cattle attributed to Brazilian groundnut meal. Vet. Rec., 37:
992-994.
CLIFFORD, J. I. & REES, K. R. (1966) Aflatoxin: A site of action in
the rat liver cell. Nature (Lond.), 209: 312-313.
CLIFFORD, J. I. & REES, K. R. (1967) The action of aflatoxin B, on
the rat liver. Biochem. J., 102: 65-75.
COON, F. B., BAUR, F. J., & SYMMES, L. R. L. (1972) International
aflatoxin check sample program: 1971 study. J. Assoc. Off. Anal.
Chem., 55: 315-327.
CUTHBERTSON, W. F. J., LAURSEN, A. C., & PRATT, D. A. H. (1967)
Effect of groundnut meal containing aflatoxin on Cynomolgus monkeys.
Br. J. Nutr., 21: 893-908.
DALEZIOS, J. I. & WOGAN, G. N. (1972) Metabolism of aflatoxin B1
in rhesus monkeys. Cancer Res., 32: 2297-2303.
DALEZIOS, J. I., WOGAN, G. N., & WEINREB, S. M. (1971) Aflatoxin
P1: A new aflatoxin metabolite in monkeys. Science, 171:
584-585.
DAVIS, N. D. & DIENER, U. L. (1970) Environmental factors affecting
the production of aflatoxin. In: Herzberg, M., ed. Proceedings of
the First US-Japan Conference on "Toxic Microorganisms",
Washington, DC, US Govt Printing Office, pp. 43-47.
DEGER, G. E. (1976) Aflatoxin-Human colon carcinogenesis? Ann.
int. Med., 85: 204.
DE IONGH, H., BEERTHUIS, R. K., VLES, R. O., BARRETT, C. B., & ORD,
W. O. (1962) Investigation of the factor in groundnut meal
responsible for turkey X disease. Biochim. Biophys. Acta, 65:
548-551.
DEO, M. G., DAYAL, Y., & RAMALINGASWAMI, V (1970) Aflatoxins and
liver injury in the rhesus monkey. J. Pathol., 101 47-56.
DETROY, R. W. & HESSELTINE, C. W. (1970) Aflatoxicol: Structure of a
new transformation product of aflatoxin B1 Can. J. Biochem.,
48: 830-832.
DICKENS, J. W. & SATTERWHITE, J. B. (1973) Aflatoxin-contaminated
peanuts produced on North Carolina farms in 1968. J. Am. Peanut
Res. Educ. Assoc. Inc., 5: 11 pp.
DICKENS, F., JONES, H. E. H., & WAYNFORTH, H B. (1966) Oral
subcutaneous and intra-tracheal administration of carcinogenic
lactones and related substances: The intratracheal administration of
cigarette tar in the rat. Br. J. Cancer, 20: 134-144.
DIENER, U. L. & DAVIS, N. D. (1969) Production of aflatoxins on
peanuts under controlled environments. J. stored Prod. Res.,
5: 251-258;.
DI PAOLO, J. A., ELIS, J., & ERWIN, H. (1967) Teratogenic response
by hamsters, rats and mice to aflatoxin B1. Nature (Lond.), 215:
638-639.
DOHERTY, W. P. & CAMPBELL, T. C. (1972) Inhibition of rat-liver
mitochondria electron-transport flow by aflatoxin B1. Res.
Commun. chem. Path. Pharmacol., 3: 601-612.
DOHERTY, W. P. & CAMPBELL, T. C. (1973) Aflatoxin inhibition of rat
liver mitochondria. Chem. biol. Interactions, 7: 63-77.
DONALDSON, W. E., TUNG, H-T., & HAMILTON, P. B. (1972) Depression of
fatty acid synthesis in chick liver (Gallus domesticus) aflatoxin.
Comp. Biochem. Physiol., 41: 843-847.
DOSTER, R. C., SINNHUBER, R. O., & WALES, J. H. (1972) Acute
intraperitoneal toxicity of ochratoxins A and B in rainbow trout
(Salmo gairdneri). Food Cosmet. Toxicol, 10: 85-92.
DOTCHEV, V. D. (1973) [The endemic (Balkan) nephropathy in
Bulgaria.] Muench. med. Woenschr., 115: 537-541 (in German).
DVORACKOVA I. (1976) Aflatoxin inhalation and alveolar cell
carcinoma. Br. med. J., 20: 691.
DVORACKOVA I., BRODSKY, F., & GERMAN, J. (1974) Aflatoxin and
encephalitic syndrome with fatty degeneration of viscera. Nutr.
Rep. Int., 10: 89-102.
DVORACKOVA I., KUSAK, V., & NESNIDAL, P. (1977) Aflatoxin and
encephalopathy with fatty degeneration of viscera (Reye). Ann.
Nutr. Ailment., 31: 977-989.
EDDS, G. T., NAIR, K. P. C., & SIMPSON, C. F. (1973) Effects of
aflatoxin B1 on resistance in poultry against cecal coccidiosis
and Marek's disease. Am. J. vet. Res., 34: 819-826.
ELIS, J. & DI PAOLO, J. A. (1967) Aflatoxin B1. Arch. Pathol., 83:
53-57.
ELLING, F. & MOLLER, T. (1973) Mycotoxic nephropathy in pigs. Bull.
Worm Health Organ., 49: 411-418.
ELLING, F., HALO, B., JACOBSEN, CHR., & KROGH, P. (1975) Spontaneous
toxic nephropathy in poultry associated with ochratoxin A. Acta
path. microbiol. Scand. Sect. A, 85: 739-741.
EPPLEY, R. M. (1966) A versatile procedure for assay and preparatory
separation of aflatoxins from peanut products. J. Assoc. Off.
Anal. Chem, 49: 1218-1223.
EPPLEY, R. M. (1968) Screening method for zearalenone, aflatoxin,
and ochratoxin. J. Assoc. Off. Anal. Chem, 51: 74-78.
EPPLEY, R. M., STOLOFF, L., TRUCKSESS, M. W., & CHUNG, C. W. (1974)
Survey of corn for Fusarium toxins. J. Assoc. Off. Anal. Chem,
57: 632-635.
EPSTEIN, S. M., BARTUS, B., & FARBER, E. (1969) Renal epithelial
neoplasms induced in male Wistar rats by oral aflatoxin B1. Cancer
Res., 29: 1045-1050.
FAO (1977) Mycotoxins. Report of the joint FAO/WHO/UNEP Conference
on Mycotoxins, Nairobi, 19-27 September, 1977, Rome, Food and
Agriculture Organization of the United Nations, 105 pp. (FAO Food
and Nutrition Paper 2).
FDA (1977) Report on action level for aflatoxin M1 in milk.
Washington DC, 21 November, Bureau of Foods, US Food and Drug
Administration.
FDA (1978) Assessment of estimated risk resulting from aflatoxins
in consumer peanut products and other food commodities.
Washington, DC, 19 January, Bureau of Foods, US Food and Drug
Administration.
FEUER, G, GOLBERG, L., & LEPELLEY, J. R. (1965) Liver response
tests. I. Exploratory studies on glucose-6-phosphatase and other
liver enzymes. Food Cosmet. Toxicol., 3: 235-249.
FRIEDMAN, L. & MOHR, H. (1968) Absence of interaction between
cyclopropenoid fatty acids and aflatoxin in rats. Fed. Proc., 27:
551 (Abstract no. 1882).
FRITZ, W., DONATH, R., & ENGST, R. (1977) [Determination and
occurrence of aflatoxin M1 and B1 in milk and dairy produce.]
Nahrung, 21: 79-84 (in German).
GALTIER, P. (1974a) Devenir de l'ochratoxine A dans l'organisme
animal. I. Transport san Ruin de la toxine chez le rat. Ann. Rech.
vet., 5: 311-318.
GALTIER, P. (1974b) Devenir de l'ochratoxine A dans l'organisme
animal II. Distribution tissulaire et élimination chez le rat. Ann.
Rech. vet., 5: 319-328.
GALTIER, P. (1975) Metabolism et mode d'action d'ochratoxine A. In:
Biquet, J. & Jemmali, M., ed. Les Mycotoxines, Paris, Editions
INSERM, pp. 69-78.
GALTIER, P. & ALVINERIE, M. (1976) In vitro transformation of
ochratoxin A by animal microbial floras. Ann. Rech. vet., 7:
91-98.
GALTIER, P., MORÉ., J., & BODIN, G. (1974) Toxine d' Aspergillus
ochraceus Wilhelm. III. Toxicité aigue de l'ochratoxine A chez le
rat et la souris adultes. Ann. Rech. vet., 5: 233-247.
GARNER, R. C. (1973) Chemical evidence for the formation of a
reactive aflatoxin B1 metabolite, by hamster liver microsomes.
Febs Lett., 36: 261-264.
GARNER, R. C. (1975) Reduction in binding of [14C]aflatoxin B1
to rat liver macromolecules by phenobarbitone pretreatment.
Biochem. Pharmacol., 24: 1553-1556.
GARNER, R. C., MILLER, E. C., MILLER, J. A., GARNER, J. V., &
HANSON, R. S. (1971) Formation of a factor lethal for S. typhimurium
TA 1530 and TA 1531 on incubation of aflatoxin B1 with rat liver
microsomes. Biochem. biophys. res. Comm., 45: 774-780.
GARNER, R. C., MILLER, E. C., & MILLER, J. A. (1972) Liver
microsomal metabolism of aflatoxin B1 to a reactive derivative
toxic to Salmonella typhimurium TA 1530. Cancer Res., 32:
2058-2066.
GODOY, H. M., JUDAH, D. J., ARORA, H. L., NEAL, G. E., & JONES, G.
(1976) The effects of prolonged feeding with aflatoxin B1 on adult
rat liver. Cancer Res., 36: 2399-2407.
GOLDBLATT, L. E., ed. (1969) Aflatoxin; scientific background,
control, and implications, New York, Academic Press, pp. 472.
GOLDBLATT, L. A. & DOLLEAR, F. G. (1977) Detoxification of
contaminated crops. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publishers Inc., pp. 139-150.
GOODAL, C. M. & BUTLER, W. H. (1969) Aflatoxin carcinogenesis:
Inhibition of liver cancer induction in hypophysectomized rats.
Int. J. Cancer, 4: 422-429.
GOPALAN; C., TULPULE, P. G., & KRISHNAMURTHI, D. (1972) Induction of
hepatic carcinoma with aflatoxin in the rhesus monkey. Food
Cosmet. Toxicol., 10: 519-521.
GRANT, K. E., COMER, M. W., & NEWBERNE, P.M. (1977) Effect of
dietary sodium selenite upon lesions induced by repeated small doses
of aflatoxin B1. Toxicol. appl. Pharmacol, 41: 166 (Abstract no.
84).
GREENWAY, J. A. & PULS, R. (1976) Fusariotoxicosis from barley in
British Columbia. I. Natural occurrence and diagnosis. Can. J.
comp. Med., 40: 12-15.
GRICE, M. C., MOODIE, C. A., & SMITH, B. C. (1973) The carcinogenic
potential of aflatoxin or its metabolites in rats from dams fed
aflatoxin pre- and postpartum. Cancer Res., 33: 262-268.
HABISH, H. A., ABDULLA, M. H., & BROADBENT, J. H. (1971) The
incidence of aflatoxin in Sudanese groundnuts. Trop. Sci., 8:
279-287.
HALD, B. & KROGH, P. (1972) Ochratoxin residues in bacon pigs. In:
Abstracts, IUPAC-Sponsored Symposium, Control of Mycotoxins,
Kungalr, Goteborg, Sweden, 21-22 Aug., 1972, Goteborg, Sweden,
Swedish Institute for Food Preservation Research, p. 18.
HALD, B. & KROGH, P. (1975) Detection of ochratoxin A in barley,
using silica gel mini-columns. J. Assoc. Off. Anal. Chem.,
58:156-158.
HALVER, J. E. (1969) Aflatoxicosis and trout hepatoma. In:
Goldblatt, L. A., ed. Aflatoxin: Scientific background, control
and implications, New York, Academic Press, pp. 265-306.
HAMILTON, P. B. & HARRIS, J. R. (1971)Interaction of aflatoxicosis
with Candida albicans infections and other stresses in chickens.
Poult. Sci., 50: 906-912.
HANSSEN, E. & JUNG, M. (1972) [On the presence of aflatoxin in
non-mouldy food and proposals for sampling.]
Z. Lebensmitt.-Unters.-Forsch., 150: 141-145 (in German).
HARTLEY, R. D., NESBITT, B. F., & O'KELLY, J. (1963) Toxic
metabolites of Aspergillus flavus. Nature (Load.), 198: 1056-1058.
HARWIG, J. (1974) Ochratoxin A and related metabolites. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
345-367.
HARWIG, J. & CHEN, Y.-K. (1974) Some conditions favoring production
of ochratoxin A and citrinin by Penicillium viridicatum in wheat
and barley. Can. J. Plant. Sci., 54: 17-22.
HAYES, A. W., HOOD, R. D., & LEE, H. L. (1974) Teratogenic effects
of ochratoxin A in mice. Teratology, 9: 93-98.
HEPTINSTALL, R. H. (1966) Pathology of the kidney, London,
Churchill, pp. 457-464.
HESSELTINE, C. W. (1976) Conditions leading to mycotoxin
contamination of foods and feeds. In: Rodricks, J. V., ed.
Mycotoxins and other fungal related food problems, Washington, DC,
American Chemical Society, pp. 1-22 (Advances in Chemistry Series
149).
HESSELTINE, C. W., SORENSEN, W. G., & SMITH, M. (1970) Taxonomic
studies of the aflatoxin producing strains in the Aspergillus
flavus group. Mycologia, 62: 123-132.
HOBSON. W., BAILEY, J., & FULLER, G. B. (1970 Hormone effects of
zearalenone in non-human primates. J. Toxicol. environ. Health, 3:
43-57.
HOEL, D. G., GAYLOR, D. W., KIRSCHSTEIN, R. L., SAFFIOTTI, U., &
SCHNEIDERMAN, M. S. (1975) Estimation of risks of irreversible
delayed toxicity. J. Toxicol. environ. Health, 1: 133-151.
HOGAN, G. R., RYAN, N.J., & HAYES, A. W. (1978) Aflatoxin B1 and
Reye's syndrome. Lancet, March 11, p. 561.
HOLADAY, C. E. (1976) A rapid screening method For the aflatoxins
and ochratoxin A. J. Am. Oil Chem. Soc., 53: 603-605.
HORWITZ, W, SENZEL, A., REYNOLDS, H., & PARK, D. L., ed. (1975)
Natural poisons. In: Chapter 26, Official Methods of analysis of
the Association of Official Analytical Chemists, Washington, DC,
AOAC, p. 24.
HRABAR, A., SUJAGA, K., BORCIC, B., ALERAJ, B., CEOVIC, S., &
CVORISCEC, D. (1976) [Morbidity and mortality from endemic
nephropathy in the village of Kaniza.] Arh. hig. rada, 27: 137-145
(in Serbo-Croat).
HSIEH, D. P. H., WONG, Z. A, WONt, J. J., MICHAS, C., & RUEBNER, B.
H. (1977) Comparative metabolism of aflatoxin. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers Inc.,
pp. 37-50.
HSU I-CH., SMALLEY, E. B., STRONG, F. M., & RIBELIN, W. E. (1972)
Identification of T-2 toxin in moldy corn associated with a lethal
toxicosis in dairy cattle. Appl. MicrobioL., 24: 684-690.
HULT, K. & GATENECK, S. (1976) A spectrophotometric procedure using
carboxy-peptidase A, for the quantitative measurement of ochratoxin
A. J. Assoc. Off. Anal. Chem., 59: 128-129.
HULT, K., TEILING, A., & GATENBECK, S. (1976) Degradation of
ochratoxin A by a ruminant. Appl. environ. Microbiol., 32:
443-444.
IARC (1976) Aflatoxins. In: IARC Monographs on the evaluation of
carcinogenic risk of chemicals to man: Some naturally occurring
substances. Lyons, International Agency for Research on Cancer,
Vol. 10, pp. 51-72.
IARC (1977) IARC Monograph Programme on the evaluation of the
carcinogenic risk of chemicals to humans. Preamble. Lyons,
International Agency for Research on Cancer, 31 pp. (IARC Internal
Technical Report No. 77/002).
IUPAC (1976) Recommended methods of ochratoxins A and B in barley,
Oxford, International Union of Pure & Applied Chemistry Secretariat,
pp. 1-7 (Technical Reports-No. 14).
JEMMALI, M. & LAFONT, P. (1972) Evolution de l'aflatoxine B1 au
tours de la panification. Cah. Nutr. Diét., 7: 319-322.
JEMMALI, M. & MURTHY, T. R. K. (1976) A chemical assay method for
the determination of aflatoxin residues in animal tissues.
Z. Lebensm.-Unters.-Forsch., 161: 13-17.
JOHN, D. W. & MILLER, L. L. (1969) Effect of aflatoxin B1 on net
synthesis of albumin, fibrinogen and alpha1-acid glycoprotein by
the isolated perfused rat liver. Biochem. Pharmacol., 18:
1135-1146.
JONES, B. D. (1972) Methods of aflatoxin analysis. London,
Tropical Products Institute, 58 pp. (Report G 70).
JOSEPH-BRAVO, P. I., FINDLEY, M., & NEWBERNE P.M. (1976) Some
interactions of light, riboflavin, and aflatoxin B1 in vivo and
in vitro. J. Toxicol. environ. Health, 1: 353-376.
JUNG, M. & HANSSEN, E. (1974) [The occurrence of aflatoxin M1 in
dried milk products.] Food Cosmet. Toxicol., 12: 131-138 (in
German).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZNSKA, J. (1976) [Occurrence of
aflatoxin B1, B2, G1, and G2, ochratoxin A and B,
sterigmatocystin and zearalenone in cereals.] Med. Weter., 32:
617-619 (in Polish).
JUSZKIEWICZ, T. & PISKORSKA-PLISZCZYNSKA, J. (1977) [Occurrence of
mycotoxins in mixed feed and concentrates.] Med. Weter., 53: 193-196
(in Polish).
KATO, R., ONODA, K., & OMORI, Y. (1969) The effect of aflatoxin B1
on incorporation of 14C-acetate into cholesterol by rat liver.
Experientia (Basel), 25: 1026.
KATO, R., TAKANAKA, A., ONODA, K., & OMORI, Y. (1970) Different
effect of aflatoxin on the induction of tryptophan oxygenase and of
microsomal drug-hydroxylase system. J. Biochem. (Tokyo), 68:
589-592.
KEEN, P. & MARTIN, P. (1971a) The toxicity and fungal infestation of
foodstuffs in Swaziland in relation to harvesting and storage.
Trop. geogr. Med., 23-35-43.
KEEN, P. & MARTIN, P. (1971b) Is aflatoxin carcinogenic in man? The
evidence in Swaziland. Trop. geogr. Med., 23: 44-53.
KIERMEIER, F. ( 1973) Aflatoxin residues in fluid milk. Pure appl.
Chem., 35: 271-274.
KIERMEIER, R. (1977) The significance of aflatoxins in the dairy
Industry. Annu. Bull. Int. Dairy Fed., 98: 1-23, 25-43.
KIERMEIER, F., WEISS, G., BEHNRINGER, G., MILLER, M., & RANFFT, K.
(1977) [On the presence and the content of aflatoxin M1 in milk
shipped to a dairy plant.] Z. Lebensmitt.- Unters.-Forsch., 163:
171-174 (in German).
KOEHLER, B. (1938) Fungus growth in shelled corn as affected by
moisture J. agric. Res., 56: 291-307.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975a) Investigations into an outbreak of hepatitis in parts
of Western India. Indian J. med. Res., 63: 1036-1048.
KRISHNAMACHARI, K. A. V. R., BHAT, R. V., NAGARAJAN, V., & TILAK, T.
B. G. (1975b) Hepatitis due to aflatoxicosis. Lancet, 1: 1061.
KROGH, P. (1974) Mycotoxic porcine nephropathy: A possible model for
Balkan endemic nephropathy. In: Puchlev, A., ed. Endemic
Nephropathy. Proceedings of the Second International Symposium on
Endemic Nephropathy, Sofia, November 9-11, 1972. Sofia, Bulgarian
Academy of Sciences, pp. 266-270.
KROGH, P. (1976a) Mycotoxic nephropathy. In: Advances in
veterinary science and comparative medicine, New York, Academic
Press, Vol. 20, pp. 147-170.
KROGH, P. (1976b) Epidemiology of mycotoxic porcine nephropathy.
Nord. Vet.-Med., 28: 452-458.
KROGH, P. (1977a) Ochratoxins. In: Rodricks, J. V., Hesseltine, C.
W., & Mehlman, M. A., ed. Mycotoxins in human and animal health,
Park Forest South, IL, USA, Pathotox Publishers, Inc., pp. 489-498.
KROGH, P. (1977b) Ochratoxin A residues in tissues of slaughter pigs
with nephropathy. Nord. Vet.-Med., 29: 402-405.
KROGH, P. (1977c) Mycotoxin tolerances in foodstuffs. Pure appl.
Chem., 49: 1719-1721.
KROGH, P. (1978) Causal associations of mycotoxic nephropathy.
Acta path. microbiol. Scand. Sect. A, Suppl. no. 269.
KROGH, P. & ELLING, F. (1977) Mycotoxic nephropathy. Vet. Sci.
Commun., 1: 51-63.
KROGH, P. & HALD, B. (1969) [Occurrence of aflatoxin in imported
groundnut products.] Nord. Vet.-Med., 21: 398-407 (in Danish).
KROGH, P., HALD, B., & KORPINEN, E.-L. (1970) [Occurrence of
aflatoxin in groundnut- and copra-products imported to Finland.]
Nord. Vet.-Med., 22: 584-589 (in Norwegian).
KROGH, P., HALD, B., HASSELAGER, E., MADSEN, A., MORTENSEN, H. P.,
LARSEN, A. E., & CAMPBELL, A.D. (1973a) Aflatoxin residues in bacon
pigs. Pure appl. Chem., 35: 275-282.
KROGH, P., HALD, B., & PEDERSEN, E. J. (1973b) Occurrence of
ochratoxin A and citrinin in cereals associated with mycotoxic
porcine nephropathy. Acta path. microbiol. Scand. Sect. B, 81:
689-695.
KROGH, P., AXELSEN, N.H., ELLING, F., GYRD-HANSEN, N., HALD, B.,
HYLDGAARD-JENSEN, J., LARSEN, A. E., MADSEN, A., MORTENSEN, H. P.,
MOLLER, T., PETERSEN, O. K., RAVNSKOV, U., ROSTGAARD, M., & AALUND,
O. (1974) Experimental porcine nephropathy: Changes of renal
function and structure induced by ochratoxin A- contaminated feed.
Acta path. microbiol. Scand. Sect. A, Suppl. No. 246, 9 pp.
KROGH, P., ELLING, F., BALD, B., LARSEN, A. E., LILLEHOJ, E. B.,
MADSEN, A., & MORTENSEN, H. P. (1976a) Time-dependent disappearance
of ochratoxin A residues in tissues of bacon pigs. Toxicology, 6:
235-242.
KROGH, P., ELLING, F., GYRD-HANSEN, H., HALD, B., LARSEN, A. E.,
LILLEHOJ, E. B., MADSEN, A., MORTENSEN, H. P., & RAVNSKOV, U.
(1976b) Experimental porcine nephropathy: Changes of renal function
and structure perorally induced by crystalline ochratoxin A. Acta.
pathol. microbiol. Scand. Sect. A, 84: 429-434.
KROGH, P., ELLING, F., HALD, B., JYLLING, B., PETERSEN, V. E.,
SKADHAUGE, E., & SVENDSEN, C. K. (1976c) Experimental avian
nephropathy: Changes of renal function and structure induced by
ochratoxin A-contaminated feed. Acta path. microbiol. Scand. Sect.
4, 84: 215-221.
KROGH, P., HALD, B., PLESTINA, R., & CEOVIC, S. (1977) Balkan
(endemic) nephropathy and foodborne ochratoxin A: Preliminary
results of a survey of foodstuffs. Acta path. microbiol. Scand.
Sect. B, 85. 238-240.
KURTZ, M. J., NAIRN, M. E., NELSON, G. H., CHRISTENSEN, C. M., &
MIROCHA, C. J. (1969) Histologic changes in the genital tracts of
swine fed estrogenic mycotoxin. Am. J. vet. Res., 30: 551-556.
LARSEN, S. (1928-1929) [On chronic degeneration of the kidneys
caused by mouldy rye.] Maanedsskr. Dyrl., 40: 259-284 & 289-300
(in Danish).
LE BRETON, E., FRAYSSINET, C., LAFARGE, C., & DE RECONDO, A.M.
(1964) Aflatoxine-mechanisme de l'action. Food Cosmet. Toxicol.,
2: 675-676.
LEE, D. J., WALES, J. H., & SINNHUBER, R. O. (1969a) Hepatoma and
renal tubule adenoma in rats fed aflatoxin and cyclopropenoid fatty
acids. J. Natl Cancer Inst., 43: 1037-1044.
LEE, L. S., CUCULLU, A. F., FRANZ, A. O., JR, & PONS, W. A., JR
(1969b) Destruction of aflatoxins in peanuts during dry and oil
roasting. J. agric. food Chem., 17: 451-453.
LEE, W. V. (1965) Quantitative determination of aflatoxin in
groundnut products. Analyst, 90: 305-307.
LEONARD, A., DEKNUDT, GH., & LINDEN, G. (1975) Mutagenicity tests
with aflatoxins in the mouse. Mutat. Res., 28: 137-139.
LEONOV, A. N. (1977) Current view of the chemical nature of factors
responsible for alimentary toxic aleukia. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 323-328.
LEVI, C. P., TRENK, H. L., & MOHR, H. K. (1974) A study of the
occurrence of ochratoxin A in green coffee beans. J. Assoc. Off.
Anal. Chem., 57: 866-870.
LIJINSKY, W. & BUTLER, W. H. (1966) Purification and toxicity of
aflatoxin G1. Proc. Soc. Exp. Biol. Med., 123: 151-154.
LIJINSKY, W., LEE, K. Y., & GALLAGHER, C. H. (1970) Interaction of
aflatoxin B1 and G1 with tissues of the rat. Cancer Res., 30:
2280-2283.
LILLEHOJ, E. B., FENNELL, D. I., & KWOLEK, W. F. (1976)
Aspergillus flavus and aflatoxin in Iowa corn before harvest.
Science, 193: 195-496.
LIN, J. J., LIU, C., & SVOBODA, D. J. (1974) Long-term effects of
aflatoxin B1 and viral hepatitis on marmoset liver. Lab. Invest.,
30: 267-278.
LING, K. H., WANG, J. J., WU, R., TUNG, T. C., LIN, C. K., LIN,
S.S., & LIN, T. M. (1967) Intoxication possibly caused by aflatoxin
B1 in mouldy rice in Shuang-Chih township. J. Formosan Med.
Assoc., 66: 517-525.
LINSELL, C. A. & PEERS, F. G. (1977) Field studies on liver cell
cancer. In: Hiatt, H. H., Watson, J. D., & Wingsten, J. A., ed.
Origins of human cancer. Cold Spring Harbor, NY, Cold Spring
Harbor Laboratory, pp. 549-556.
LO, W-B. & BLACK, H. S. (1972) Effects of aflatoxin B1 on human
skin lipids metabolism-a site of action on 14C-acetate
incorporation. Experientia (Basel), 28: 1278-1279.
LOOSMORE, R. M. & HARDING, J. D. J. (1961) A toxic factor in
Brazilian groundnut causing liver damage in pigs. Vet. Rec., 73:
1362-11364.
LOOSMORE, R. M. & MARKSON, L. M. (1961) Poisoning of cattle by
Brazilian groundnut meal. Vet. Rec., 73: 813-814.
LOVELACE, C. E. A. & NYATHI, C. B. (1977) Estimation of the fungal
toxins, zearalenone and aflatoxin, contaminating opaque maize beer
in Zambia. J. Sci. Food Agric., 28: 288-292.
LUCAS, F. V., JR., MONROE, P., NGA, P. V., TOWNSEND, J. F., & LUCAS,
F. V. (1970-1971) Mycotoxin contamination of South Vietnamese rice.
J. trop. Med. Hyg., 74: 182-183.
LUCK. H., STEYN, M., & WEHNER, F. C. (1917) A survey of milk powder
for aflatoxin content. S. Afr. J. dairy Technol., 8: 85-86.
LVOVA, L. S., SOSEDOV, N. I., GARALL, W., SCHWARZMAN, M. I.,
SHATILOVA, T. I., & SHULGINA, A. P. (1976) [Formation of aflatoxins
in wheat grain induced by self-heating and change in its chemical
composition by the development of storage moulds.] Prikl. Biochem.
Mikrobiol., 12: 741-749 (in Russian).
MADHAVAN, T. V. & GOPALAN, C. (1975) Effect of dietary protein on
aflatoxin injury in weanling rats. Arch. Pathol.,
80: 123-126.
MADHAVAN, T. V. & GOPALAN, C. (1968) The effect of dietary protein
on carcinogenesis of aflatoxin. Arch. Pathol., 85: 133-137.
MADHAVAN, T. V., SURYANARAYANA RAO, K., The effect of dietary
protein level on susceptibility of monkeys to aflatoxin liver
injury. Indian J. med. Res., 53: 984-989.
MADHAVAN, T. V., TULPULE, P. G, & GOPALAN, C. (1965b)
Aflatoxin-induced hepatic fibrosis in rhesus monkeys. Arch.
Pathol., 79: 466-469.
MALEKI, M. F., SUZANGAR, M., JAMSHIDI, CH., TAHMASEBI, A., &
BARNETT, R. (1976) Urinary aflatoxin detected in cirrhotic patients
in Ispahan, Iran. Clin. Res., 24: 630A.
MANTEL, N. & BRYAN, W. R. (1961) Safety testing of carcinogenic
agents. J. Natl Cancer Inst., 27: 455-470.
MANTEL, N., BOHIDAR, N., BROWN, C., CIMINERA, J., & TUKEY, J. (1975)
An improved "Mantel-Bryan" procedure for "safety" testing of
carcinogens. Cancer Res., 35: 865-872.
MARSH, P. B., SIMPSON, M. E., CRAIG, G. O., DONOSO, J., & RAMEY, H.
H., JR (1973) Occurrence of aflatoxins in cotton seeds at harvest in
relation to location of growth and field temperatures. J. environ.
Qual., 2: 276-281.
MARTIN, P.M. D. & KEEN, P. (1978) The occurrence of zearalenone in
raw and fermented products from Swaziland and Lesotho. Sabrouaudia,
16: 15,22.
MASRI, M. S., LUNDIN, R. E., PAGE, J. R., & GARCIA, V. V. (1967)
Crystalline aflatoxin M1 from urine and milk. Nature (Lond.),
215: 753-755.
MASRI, M. S., HADDON, W. F., LUNDIN, R. E., & HSIEH, D. P. H.
(1974a) Aflatoxin Q1: A newly identified major metabolite of
aflatoxin B1 in monkey liver. J. agric. food Chem., 22: 512-514.
MASRI, M. S., BOOTH, A. N., & HSIEH, D. P. H. (1974b) Comparative
metabolic conversion of aflatoxin B1 to aflatoxin M1 and
aflatoxin Q1 by monkey, rat and chicken liver. Life Sci., 15:
203-212.
MATTHEWS, J. G., PATTERSON, D. S. P., ROBERTS, B. A., & SHREEVE, B.
J. (1977) T-2 toxin and haemorrhagic syndromes in cattle. Vet.
Rec., 101 (19): 391.
MCLEAN, A. E. M. & MARSHALL, A. (1971) Reduced carcinogenic effects
of aflatoxin in rats given phenobarbitone. Br. d. exp. Pathol.,
52: 322-329.
MCNUTT, S. H., PURWIN, P., & MURRAY, C. (1928) Vulvovaginitis in
swine J. Am. Vet. Med. Assoc., 73: 484;-492.
MEISNER, H. (1976) Energy-dependent uptake of ochratoxin A by
mitochondria. Arch. Biochem. Biophys., 173: 132-140.
MEISNER, H. & CHAN, S. (1974) Ochratoxin A, an inhibitor of
mitochondrial transport systems. Biochemistry, 13: 2795-2800.
MERWE, K. J. van der, STEYNE, P.S., FOURIE, L., SCOTT, DE B., &
THERON, J. J. (1965) Ochratoxin A, a toxic metabolite produced by
Aspergillus ochraceus Wilh. Nature (Lond.), 205: 1112-1113.
MILLER, J. K., HACKING, A., & GROSS, V. J. (I[973) Stillbirths,
neonatal mortality and small litters in pigs associated with the
ingestion of Fusarium toxin by pregnant sows. Vet. Rec., 93:
555-559.
MINTZLAFF, H.-J., LOTZSCH, R., TAUCHMANN, F., MEYER, W., & LEISTNER,
L. (1974) [Aflatoxin residues in the liver and muscle of broiler
chicken given aflatoxin containing feed.] Fleischwirtschaft, 54:
774-778 (in German).
MIROCHA, C. J. & CHRISTENSEN, C. M. (1974) Oestrogenic mycotoxins
synthesized by Fusarium. In: Purchase, I. F. H, ed. Mycotoxins,
Amsterdam, Elsevier, pp. 129-148.
MIROCHA, C. J. & PATHRE, S. (1973) Identification of the toxic
principle in a sample of poaefusarin. Appl. Microbiol., 26:
719-724.
MIROCHA, C. J., CHRISTENSEN, C. M., & NELSON, G. H. (1968a) Toxic
metabolites produced by fungi implicated in mycotoxicoses.
Biotech. Bioeng., 10: 469-482.
MIROCHA, C. J., HARRISON, J., NICHOLS, A. A.., & MCCLINTOCK, M.
(1968b) Detection of a fungal estrogen (F-2) in hay associated with
infertility in dairy cattle. Appl. Microbiol., 16: 797-798.
MIROCHA, C. J., SCHAUERHAMER, B., & PATHRE, S. V. (1974) Isolation,
detection and quantitation of zearalenone in maize and barley.
J. Assoc. Off. Anal. Chem., 75: 1104-1110.
MIROCHA, C. J., PATHRE, S. V., SCHAUERHAMER, B., & CHRISTENSEN, C.
M. (1976) Natural occurrence of Fusarium toxins in feedstuff. Appl
environ. Microbiol., 32: 553-556.
MIROCHA, C. J., PATHRE, S. W., & CHRISTENSEN, C. M. (1977)
Zearalenone. In: Rodricks, J. V., Hesseltine, C. W., & Mehlman, M.
A., ed. Mycotoxins in human and animal health, Park Forest South,
IL, USA, Pathotox Publishers Inc., pp. 345-364.
MOROOKA, N., URATSUJI, N., YOSHIZAWA, T., & YAMAMOTO, H. (1972)
[Studies on the toxic substances in barley infected with Fusarium
spp.] Jpn. J. food Hyg, 13: 368-375 (in Japanese).
MUNRO, I. C, MOODIE, C. A, KUIPER-GOODMAN, T., SCOTT, P.M., & GRICE,
H. C. (1974) Toxicologic changes in rats fed graded dietary levels
of ochratoxin A. Toxicol. appl. Pharmacol, 28: 180-188.
NABNEY, J., BURBAGE, M. B., ALLCROFT, R., & LEWIS, G. (1967)
Metabolism of aflatoxin in sheep: Excretion pattern in the lactating
ewe. Food Cosmet. Toxicol., 5: 11-17.
NEAL, G. E. & GODOY, H. M. (1976) The effect of pre-treatment with
phenobarbitone on the activation of aflatoxin B1 by rat liver.
Chem. biol. Interactions, 14: 279-289.
NEELY, W. C. & WEST, A.D. (1972) Spectroanalytical parameters of
fungal metabolites. III. Ochratoxin A. J. Assoc. Off. Anal. Chem.,
55: 1305-1309.
NEL, W. & PURCHASE, I. F. M. (1968) Note. The fate of ochratoxin A
in rats. J.S. Afr. Chem. Inst., 21: 87-88.
NESHEIM, S. (1971) Report on ochratoxins: Occurrence, production,
analysis and toxicity. In: Abstracts of the 85th Annual meeting of
the Association of Official Analytical Chemists, Washington, DC,
Oct. 11-14, 1971, p. 23.
NESHEIM, S. (1976) The ochratoxins and other related compounds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 276-295
(Advance.,; in Chemistry Series 149).
NESHEIM, S., HARDIN, N. F., FRANCIS, O. J., JR, & LANGHAM, W. S.
(1973) Analysis of ochratoxins A and B and their esters in barley,
using partition and thin layer chromatography. I. Development of the
method. J. Assoc. Off. Anal. Chem., 56: 817-821.
NEUMANN-KLEINPAUL, A. & TERPLAN, G. (1972) [The presence of
aflatoxin M1 in dried milk products.] Arch. Lebensmittelhyg.,
23: 128-132 (in German).
NEWBERNE, P.M. (1974) Mycotoxins: Toxicity, carcinogenicity and the
influence of various nutritional conditions. Environ. Health
Perspect., 9: 1-32.
NEWBERNE, P.M. (1976) Environmental modifiers of susceptibility to
carcinogenesis. Cancer Detect. Prev., 1: 129-173.
NEWBERNE, P.M. & BUTLER, W. H. (1969) Acute and chronic effects of
aflatoxin on the liver of domestic and laboratory animals: A review.
Cancer Res., 29: 236-250.
NEWBERNE, P.M. & CONNER, M. W. (1974) Effect of selenium on acute
response to aflatoxin B1. In: Hemphill, D. D., ed. A Symposium.
Trace Substances in Environmental Health-VIII, Columbia, MO,
University of Missouri, pp. 323-328.
NEWBERNE, P.M. & GROSS, R. L. (1977) The role of nutrition in
aflatoxin injury. In: Rodricks, J. V., Hesseltine, C. W, & Mehlman,
M. A., ed. Mycotoxins in human and animal health, Park Forest
South, IL, USA, Pathotox Publishers Inc., pp. 51-65.
NEWBERNE, P.M. & ROGERS, A. E. (1971) Aflatoxin carcinogenesis in
rats: Dietary effects. In: Purchase, I. F. H., ed. Mycotoxins in
human health, London, Macmillan, pp. 195-208.
NEWBERNE, P.M. & ROGERS, A. E. (1973) Rat colon carcinomas
associated with aflatoxin and marginal vitamin A. J. Natl Cancer
Inst., 50: 439-448.
NEWBERNE, P.M. & ROGERS, A. E. (1976) Nutritional modulation of
carcinogenesis. In: Magee, P. N. et al., ed. Fundamentals in
cancer prevention, Tokyo, Univ. of Tokyo Press, & Baltimore, Univ.
Park Press, pp. 15-40.
NEWBERNE, P.M. & SUPHAKARN, V. (1977) Preventive role of vitamin A
in colon carcinogenesis in rats. Cancer, 40: 2553-2556.
NEWBERNE, P.M. & WILLIAMS, G. (1969) Inhibition of aflatoxin
carcinogenesis by diethyl-stilbestrol in male rats. Arch. environ.
Health, 19: 489-498.
NEWBERNE, P.M. & WOGAN, G. N. (1968a) Sequential morphologic changes
in aflatoxin B1 carcinogenesis in the rat. Cancer Res., 28:
770-781.
NEWBERNE, P.M. & WOGAN, G. N. (1968b) Potentiating effects of
low-protein diets on effect of aflatoxin in rats. Toxicol. appl.
Pharmacol., 12: 309-310.
NEWBERNE, J. W., BAILEY, W. S., & SEIBOLD, H. R. (1955) Notes on a
recent outbreak and experimental reproduction of hepatitis X in
dogs. J. Am. Vet. Med. Assoc., 127: 59-62.
NEWBERNE, P.M., RUSSO, R., & WOGAN, G. N. (1966a) Acute toxicity of
aflatoxin B1 in the dog. Pathol. vet., 3: 331-340.
NEWBERNE, P.M., HARRINGTON, D. H., & WOGAN, G. N. (1966b) Effects of
cirrhosis and other liver insults on induction of liver tumours by
aflatoxin. Lab. Invest., 15: 962-969.
NEWBERNE, P.M., ROGERS, A. E., & WOGAN, G. N. (1968) Hepatorenal
lesions in rats fed a low lipotrope diet and exposed to aflatoxin.
J. Nutr., 94: 331-343.
NEWBERNE, P.M., CHAN, W-CH. M., & ROGERS, A. E. (1974) Influence of
light, riboflavin, and carotene on the response of rats to the acute
toxicity of aflatoxin and mono-crotaline. Toxicol. appl. Pharmacol.,
28: 200-208.
NILSSON, G. (1974) [Aflatoxin in nuts.] Vor Foda, 26: 56-62 (in
Swedish).
NIXON, J. E., SINNHUBER, R. O., LEE, D. J., LANDERS, M. K., & HARR,
J. R. (1974) Effect of cyclopropenoid compounds on the carcinogenic
activity of diethylnitrosamine and aflatoxin B1 in rats. J. Natl
Cancer Inst., 53: 453-458.
NORTHOLT, M.D., VERHULSDONK, C. A. H., SOENTORO, P.S. S., & PAULSCH,
W. E. (1976) Effect of water activity and temperature on aflatoxin
production by Aspergillus parasiticus. J. milk food Technol., 39:
170-174.
OLSON, L. C., BOURGEOIS, C. H., JR, COTTON, R. B., HARIKUL, S.,
GROSSMAN, R. A., & SMITH, T. J. (1971) Encephalopathy and fatty
degeneration of the viscera in northeastern Thailand. Clinical
syndrome and epidemiology. Pediatrics, 47: 707-716.
ONG, T-M. (1975) Aflatoxin mutagenesis. Mutat. Res., 32: 35-53.
PAG FAG/WHO/UNICEF PROTEIN ADVISORY GROUP (1969) PAG Recommendation
on aflatoxin. PAG Statement No. 2. New York, United Nations, 2 pp.
PANALAKS, T. & SCOTT, P.M. (1977) Sensitive silica gel-packed
flowcell for fluorometric detection of aflatoxins by high pressure
liquid chromatography. J. Assoc. Off. Anal. Chem., 60: 583-589.
PARKER, W. A. & MELNICK, D. (1966) Absence of aflatoxin from refined
vegetable oils. J. Am. Oil Chem. Soc., 43: 635-638.
PATHRE, S. V. & MIROCHA, C. J. (1976) Zearalenone and related
compounds. In: Rodricks, J. V., ed. Mycotoxins and other fungal
related food problems, Washington, DC, American Chemical Society
pp. 178-227 (Advances in Chemistry Series 149).
PATTERSON, D. S. P. (1973) Metabolism as a factor in determining the
toxic action of the aflatoxin in different animal species. Food
Cosmet. Toxicol., 11: 287-294.
PATTERSON, D. S. P. (1976) Structure, metabolism and toxicity of the
aflatoxins: A review. Cah. Nutr. Diét., 11 (Suppl. 2): 71-76.
PATTERSON, D. S. P. (1977) Aflatoxin and related compounds. In:
Wyllie, T.D. & Morehouse, L.G., ed. Mycotoxic fungi, mycotoxins,
mycotoxicoses: An encyclopedic handbook, New York & Basel, Marcel
Dekker, pp. 131-136 & 144-189.
PATTERSON, D. S. P. & ROBERTS, B. A. (1970) The formation of
aflatoxin B2a and G2a and their degradation products during the
in vitro detoxification of aflatoxin by livers of certain avian
and mammalian species. Food Cosmet. Toxicol., 8: 527-538.
PATTERSON, D. S. P. & ROBERTS, B. A. (1971) The in vitro reduction
of aflatoxins B1 and B2 by soluble avian liver enzymes. Food
Cosmet. Toxicol., 9: 829-837.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972a) Steroid sex hormones as
inhibitors of aflatoxin metabolism in liver homogenates.
Experientia (Basel), 28: 929-930.
PATTERSON, D. S. P. & ROBERTS, B. A. (1972b) Aflatoxin metabolism in
duck-liver homogenates: The relative importance of reversible
cyclopentenone reduction and hemiacetal formation. Food Cosmet.
Toxicol., 10: 501-512.
PATTERSON, D. S. P., ROBERTS, B. A., SHREEVE, B. J., WRATHALL, A.
E., & GITTER, M. (1977) Aflatoxin, ochratoxin and zearalenone in
animal feedstuffs: Some clinical and experimental observations. Ann
Nutr. Aliment., 31: 643-650.
PATTERSON, D. S. P., SHREEVE, B. J., & ROBERTS, B. A. (in press)
Mycotoxin residues in body fluids and tissues of food-producing
animals. In: Proceedings of the 12th International Congress
Microbiology, Munchen, Federal Republic of Germany, 3-8 September
1978.
PAUL, R., KALRA, M. S., & SINGH, A. (1976) Incidence of aflatoxins
in milk and milk products. Indian J. dairy Sol., 29: 318-322.
PAYET, M., CROS, J., QUENUM, C., SANKALE, M., & MOULANIER, M. (1966)
Deux observations d'enfants avant consommé de façon prolongée des
farines souillées par "Aspergillus flavus". Presse med.,
13: 649-651.
PECKHAM, J. C., DOUPNIK, B., JR, & JONES, O. H., JR (1971) Acute
toxicity of ochratoxins A and B in chicks. Appl. Microbiol., 21:
492-494.
PEERS, F. G. & LINSELL, C. A. (1973) Dietary aflatoxins and liver
cancer--a population based study in Kenya. Br. J. Cancer, 27:
73-484.
PEERS, F. G. & LINSELL, C. A. (1976) Acute toxicity of aflatoxin
B1 for baboons. Food Cosmet. Toxicol., 14: 227-228.
PEERS, F. G. & LINSELL, C. A. (1977) Dietary aflatoxins and human
primary liver cancer. Gaucher, A., ed. Ann. Nutr. Aliment., 31:
1005-1018.
PEERS, F. G., GILMAN, G. A., & LINSELL, C. A. (1976) Dietary
aflatoxins and human liver cancer. A study in Swaziland. Int. J.
Cancer, 17: 167-176.
PETTIT, R. E., TABER, R. A., SCHROEDER, H. W., & HARRISON, A. L.
(1971) Influence of fungicides and irrigation practice on aflatoxin
in peanuts before digging. Appl. Microbiol., 22: 629-634.
PHILLIPS, D. L., YOURTEE, D. M., & SEARLES, S. (1976) Presence of
aflatoxin B1 in human liver in the United States. Toxicol. appl.
Pharmacol., 36: 403-406.
PIER, A. C. & HEDDLESTON, K. L. (1970) The effect of aflatoxin on
immunity in turkeys. I. Impairment of actively acquired resistance
to bacterial challenge. Avian Dis., 14: 797-809.
PIER, A. C., CYSEWSKI, S. J., RICHARD, J. L., & THURSTON, J. R.
(1977) Mycotoxins as a veterinary problem. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 745-750.
PITOUT, M. J. (1969) The hydrolysis of ochratoxin A by some
proteolytic enzymes. Biochem. Pharmacol., 18: 485-491.
POHLAND, A. E., YIN, L., & DANTZMAN, J. G. (1970) Rapid chemical
confirmatory method for aflatoxin B1. I. Development of the
method. J. Asoc. Off. Anal. Chem., 53: 101-102.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELJAN, V. A. (1972) Effect
of aflatoxin on rat liver lysosomes. Toxicon, 10: 25-30.
POKROVSKY, A. A., KRAVCENKO, L. V., & TUTELIAN, V. A. (1977)
[Aflatoxins.] Toksikologija, Moskva, Viniti, Vol. 8, 108 pp. (in
Russian).
POLZHOFER, K. (1977) [Determination of aflatoxins in milk and milk
products.] Z. Lebensmitt.-Unters.-Forsch., 163: 175-177 (in
German).
PONG, R. S. & WOGAN, G. N. (1969) Time course of alterations of rat
liver polysome profiles induced by aflatoxin B1. Biochem.
Pharmacol., 18: 2357-2361.
PONG, R. S. & WOGAN, G. N. (1970) Time course and dose-response
characteristics of aflatoxin B1 effects on rat liver RNA
polymerase and ultrastructure. Cancer Res., 30: 294-304.
PONG, R. S. & WOGAN, G. N. (1971) Toxicity and biochemical and fine
structural effects of synthetic aflatoxins M1 and B1 in rat
liver. J. Natl Cancer Inst., 47: 585-592.
PRINCE, A.M., SZMUNESS, W., MICHON, J, DEMAILLE, J., DIEBOLT, G.,
LINHARD, J., QUENUM, C., & SANKALE, M. (1975) A case/control study
of the association between primary liver cancer and hepatitis B
infection in Senegal. Int. J. Cancer, 16: 376-383.
PRIOR, M. G., SISODIA, C. S., & O'NEIL, J. B. (1976) Acute oral
ochratoxicosis in day-old white leghorns, turkeys and Japanese
quail. Poult. Sci., 55: 786-790.
PRZYBYLSKI, W. (1975) Formation of aflatoxin derivatives on thin
layer chromatographic plates. J. Assoc. Off. Anal. Chem., 58:
163-164.
PUCHLEV, A. (1973) La néphropathie endémique en Bulgarie. Extrait
du Symposium sur la néphropathie endémique de l'Académie serbe des
sciences et des arts, Belgrade, pp. 15-27.
PUCHLEV, A. (1974) Basic problems in endemic nephropathy. In:
Puchlev, A., ed. Endemic Nephropathy, Proceedings of the Second
International Symposium on Endemic Nephropathy, Sofia, 9-11
November, 1972, Sofia, Publishing House of the Bulgarian Academy
of Sciences, pp. 15-18.
PULS, R. & GREENWAY, J. A. (1976) Fusariotoxicosis from barley in
British Columbia. II. Analysis and toxicity of suspected barley.
Can. J. comp. Med., 40: 16-19.
PURCHASE, I. F. H. (1967) Acute toxicity of aflatoxins M1 and M2
in one-day-old ducklings. Food Cosmet. Toxicol., 5: 339-342.
PURCHASE, I. F. H. (1972) Aflatoxin residues in food of animal
origin. Food Cosmet. Toxicol., 10: 531-544.
PURCHASE, I. F. H. (1974) Mycotoxins, Amsterdam, Elsevier.
PURCHASE, I. F. H. & THERUN, J. J. (1968) The acute toxicity of
ochratoxin A to rats. Food Cosmet. Toxicol., 6: 479-483.
PURCHASE, I. F. H. & VORSTER, L. J. (1968) Aflatoxin in commercial
milk samples. S. Afr. med. J., 42: 219.
REDDY, G. S., TILAK, T. B. G., & KRISHNAMURTHI, D. (1973)
Susceptibility of vitamin A- deficient rats to aflatoxin. Food
Cosmet. Toxicol., 11: 467-470.
REDDY, J. K. & SVOBOOA, D. J. (1975) Aflatoxin B1-induced liver
tumors in Tupaia glis (tree shrews) a nonhuman primate. Fed.
Proc., 34: 827 (Abstract no. 3432).
REDDY, J. K., SVOBODA, D. J., & RAO, M. S. (1976) Induction of liver
tumors by aflatoxin B1 in the tree shrew (Tupaia glis), a
nonhuman primate. Cancer Res., 36: 151-160.
REYE, R. D. K., MORGAN, G., & BARAL, J. (1963) Encephalopathy and
fatty degeneration of the viscera: A disease entity in childhood.
Lancet, 2: 749-752.
REYS, L. L. & SEQUEIRA, O. A. (1974) Detection of Australia antigen
(HBAg) in blood donors and hepatoma patients in Mozambique.
S. Aft. med. J., 48: 267-269.
RICHIR, CL., PACCALIN, J., LARCEBEAU, S., FAUGERES, J., MORARD,
J.-L., & LAMANT, M. (1976) Présence d'aflatoxine B1 dans le toie
humain. Cah. Nutr. Diet., 11: 223-226.
ROBERTS, J. C. (1974) Aflatoxins and sterigmatocystins. Fortschr.
Chem. Org. Naturst., 31: 119-151.
ROBINSON, P. (1967) Infantile cirrhosis of the liver in India. With
special reference to probable aflatoxin etiology. Clin. Pediatr.,
6: 57-62.
RODRICKS, J. V. & STOLOFF, L. (1970) Determination of concentration
and purity of aflatoxin standards. J. Assoc. Off. Anal. Chem., 53:
92-95.
RODRICKS, J. V. & STOLOFF, L. (1977) Food producing animals. In:
Rodricks, J. V., Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins
in human and animal health, Park Forest South, IL, USA, Pathotox
Publishers Inc., pp. 67-79.
RODRICKS, J. V., STOLOFF, L., PONS, W. A., JR,. ROBERTSON, J. A., &
GOLDBLATT, L. A. (1970) Molar absorptivity values for aflatoxins and
justification for their use as criteria of purity of analytical
standards. J. Assoc. Off. Anal. Chem., 53: 96-101.
RODRICKS, J. V., HESSELTINE, C. W., & MEHLMAN, M. A., ed. (1977)
Mycotoxins in human and animal health, Park Forest South, IL, USA,
Pathotox Publishers Inc., 807 pp.
ROGERS, A. E. (1975) Variable effects of a lipotrope-deficient, high
fat diet on chemical carcinogenesis in rats. Cancer Res., 35:
2469-2474.
ROGERS, A. E. & NEWBERNE, P.M. (1969) Aflatoxin B1 carcinogenesis
in lipotrope-deficient rats. Cancer Res., 29: 1965-1972.
ROGERS, A. E. & NEWBERNE, P.M. (1971) Diet and aflatoxin B1
toxicity in rats. Toxicol. appl. Pharmacol., 20: 113-121.
ROINE, K., KORPINEN, E.-L., & KALLELA, K. (1971) Mycotoxicosis as a
probable cause of infertility in dairy cows. Nord. Vet.-Med., 23:
628-633.
ROMER, T. R. (1975) Screening method for the detection of aflatoxins
in mixed feeds and other agricultural commodities with subsequent
confirmation and quantitative measurement of aflatoxins in positive
samples. J. Assoc. Off. Anal. Chem., 58: 500-506.
RUDDICK, J. A., SCOTT, P. M., & HARWIG, J. (1976) Teratological
evaluation of zearalenone administered orally to the rat. Bull.
environ. Contain. Toxicol., 15: 678-681.
RUTQVlST, L., BJORKLUND, N.-E., HULT, K., & GATENBECK, S. (1977)
Spontaneous occurrence of ochratoxin residues in kidneys of
fattening pigs. Zentralbl. Vet.-Med. A, 24: 402-408.
SAFFIOTTI, U., MONTESANO, R., SELLAKUMAR, A. R., CEFIS, F., &
KAUFMAN, D. G. (1972) Respiratory tract carcinogenesis in hamsters
induced by different numbers of administrations of benzo (a) pyrene
and ferric oxide. Cancer Res., 32: 1073-1081.
SALHAB, A. S. & EDWARDS, G. S. (1977) Comparative in vitro
metabolism of aflatoxicol by liver preparations from animals and
humans. Cancer Res., 37: 1016-1021.
SALHAB, A. S. & HSIEH, D. P. H. (1975) Aflatoxicol H1: A major
metabolite of aflatoxin B1 produced by human and rhesus monkey
livers in vitro. Res. Commun. chem. Pathol. Pharmacol.,
10: 419-431.
SANDERS, T. H, DAVIS, N. D., & DIENER, U. L. (1968) Effect of carbon
dioxide, temperature, and relative humidity on production of
aflatoxin in peanuts. J. Am. Oil Chem. Soc., 45: 683-685.
SANSING, G. A., LILLEHOJ, E. B., DETROY, R. W., & MILLER, M. A.
(1976) Synergistic toxic effect of citrinin ochratoxin A and
penicillic acid in mice. Toxicon, 14: 213-220.
SARASIN, A. & MOULE, Y. (1976) Helical polysomes induced by
aflatoxin B1 in vivo. A new hypothesis for helix formation by
chemicals and carcinogens. Exp. cell Res., 97: 346-358.
SARGEANT, K., O'KELLY, J., CARNAGHAN, R. B. A., & ALLCROFT, R.
(1961) The assay of a toxic principle in certain groundnut meals.
Vet. Rec., 73: 1219-1223.
SARKISOV, A. C. (1954) [Mycoses.] Moscow, State Publishing House
for Agricultural Literature, 216 pp. (in Russian).
SATO, S., MATSUSHIMA, T., TANAKA, N., SUGIMURA, T., & TAKASHIMA, F.
(1973) Hepatic tumors in the guppy (Lebistes reticulatus) induced
by aflatoxin B1, dimethylnitrosamine, and 2-acetylaminofluorene.
J. Natl Cancer Inst., 50: 765-778.
SATO, N., UENO, Y., & ENOMOTO, M. (1975) Toxicological approaches to
the toxic metabolites of Fusaria. VIII. Acute and sub-acute
toxicities of T-2 toxins in cats. Jpn. J. Pharmacol., 25: 263-270.
SCHADE, J. E., MCGREEVY, K., KING, A.D., MACKEY, B., & FULLER, G.
(1975) Incidence of aflatoxin in California almonds. Appl.
Microbiol., 29: 48-53.
SCHINDLER, A. F. & NESHEIM, S. (1970) Effect of moisture and
incubation time on ochratoxin A production by an isolate of
Aspergillus ochraceus. J. Assoc. Off. Anal. Chem., 53: 89-91.
SCHROEDER, H. W. & CARLTON, W. W. (1973) Accumulation of only
aflatoxin B2 by a strain of Aspergillus flavus. Appl.
Microbiol., 25: 146-148.
SCHULLER, P. L., OCKHUIZEN, TH., WERRINGLOER, J., & MARQUARDT, P.
(1967) [Aflatoxin B1 and histamine in wine.]
Arzneimitteforschung, 17: 888-890 (in German).
SCHULLER, P. L., VERHOLSHONK, C. A. H., & PAULSCH, W. E. (1973)
Analysis of aflatoxin M1 in liquid and powdered milk. Pure appl.
Chem., 35: 291-296.
SCHULLER, P. L., VERHULSDONK, C. A. H, & PAULSCH, W. E. (1967)
Aflatoxin M1 in liquid and powdered milk (evaluation of methods
and collaborative study). Zesz. Probl. Postepow Nauk Roln.,
189: 255-261.
SCOTT, P.M. & KENNEDY, B. P. C. (1973) Analysis and survey of ground
black, white, and capsicum peppers for aflatoxins. J. Assoc. Off.
Anal. Chem., 56: 1452-1457.
SCOTT, P.M., VAN WALBEEK, W., KENNEDY, B, & ANYETI, D. (1972)
Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and
toxigenic fungi in grains and other agricultural products. Agric.
food Chem., 20: 1103-1109.
SCOTT, P.M., PANALAKS, T., KANHERE, S., & MILES, W. F. (1978)
Determination of zearalenone in cornflakes and other corn-based
foods by thin layer chromatography, high pressure liquid
chromatography, and gas-liquid chromatography/high resolution mass
spectrometry. J. Assoc. Off. Anal. Chem., 61: 593-600.
SEEGERS, J. C. & PITOUT, M. J. (1973) DNA repair synthesis in cell
cultures exposed to low doses of aflatoxin B1 or sterigmatocystin
S. Aft. lab. clin. Med., 19: 961 (abstr. 23).
SEIBOLD, H. R. & BAILEY, W. S. (1952) An epizootic of hepatitis in
the dog. J. Am. Vet. Med. Assoc., 121: 201-206.
SEIBOLD, R. & RUCH, W. (1977) [Aflatoxin content of mixed feeds of
dairy cows.] Kraftfutter, 60: 182-185 (in German).
SERCK-HANSSEN, A. (1970) Aflatoxin-induced fatal hepatitis? A case
report from Uganda. Arch. environ. Health, 20: 729-731.
SHANK, R. C. (1976) The role of aflatoxin in human disease. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems. Washington, DC, American Chemical Society, pp. 51-57
(Advances in Chemistry Series 149).
SHANK, R. C. (1977) Epidemiology of aflatoxin carcinogenesis. In:
Kraybill, H. F. & Mehlman, M. M., ed. Environmental cancer.
Advances in modern toxicology, Vol. 3, John Wiley and Sons, New
York, pp. 291-318.
SHANK, R. C., BOURGEOIS, C. H., KESCHAMRAS, N., & CHANDAVIMOL, P.
(1971a) Aflatoxins in autopsy specimens from Thai children with an
acute disease of unknown actiology. Food Cosmet. Toxicol., 9:
501-507.
SHANK, R. C., JOHNSEN, D. O., TANTICHAROENYOS, P., WOODING, W. L., &
BOURGEOIS, C. H. (1971b) Acute toxicity of aflatoxin B, in the
macaque monkey. Toxicol. appl. Pharmacol., 20: 227-231.
SHANK, R. C., WOGAN, G. N., GIBSON, J. B., & NONDASUTA, A. (1972a)
Dietary aflatoxins and human liver cancer. II. Aflatoxins in market
foods and foodstuffs of Thailand and Hong Kong. Food Cosmet.
Toxicol., 10: 61-69.
SHANK, R. C., GORDON, J. E., WOGAN, G. N., NONDASUTA, A., &
SUBHAMANI, B. (1972b) Dietary aflatoxins and human liver cancer.
III. Field survey of rural Thai families for ingested aflatoxins.
Food Cosmet. Toxicol., 10: 71-84.
SHANK, R. C., BHAMARAPRAVATI, N., GORDON, J. E., & WOGAN, O. N.
(1972c) Dietary aflatoxins and human liver cancer. IV. Incidence of
primary cancer in two municipal populations of Thailand. Food
Cosmet. Toxicol, 10: 171-179.
SHANKARAN, R., RAJ, H. G., & VENKITASUBRAMANIAN, T. A. (1970) Effect
of aflatoxin on carbohydrate metabolism in chick liver.
Enzymology, 39: 371-378.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969a) Survey of cereal grains
and soybeans for the presence of aflatoxin: II. Corn and soybeans.
Cereal Chem., 46. 454-463.
SHOTWELL, O. L., HESSELTINE, C. W., BURMEISTER, H. R., KWOLEK, W.
F., SHANNON, G. M., & HALL, H. H. (1969b) Survey of cereal grains
and soybeans for the presence of aflatoxin: I. Wheat, grain sorghum,
and oats. Cereal Chem., 46: 446-454.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1969)
Ochratoxin A: Occurrence as natural contaminant of a corn sample.
Appl. Microbiol., 17: 765-766.
SHOTWELL, O. L., HESSELTINE, C. W., GOULDEN, M. L., & VANDEGRAFT, E.
E. (1970) Survey of corn for aflatoxin, zearalenone and ochratoxin.
Cereal Chem., 47: 700-707.
SHOTWELL, O. L., HESSELTINE, C. W., VANDEGRAFT, E. E., & GOULDEN, M.
L. (1971) Survey of corn from different regions for aflatoxin,
ochratoxin, and zearalenone. Cereal Sci. Today, 16: 266-273.
SHOTWELL, O. L., HESSELTINE, C. W., & GOULDEN, M. L. (1973)
Incidence of aflatoxin in southern corn, 1969-1970. Cereal Sci.
Today, 18, 192-195.
SHOTWELL, O. L., GOULDEN, M. L., & HESSELTINE, C. W. (1976a) Survey
of US wheat for ochratoxin and aflatoxin. J. Assoc. Off. Anal.
Chem., 59: 122-124.
SHOTWELL, O. L., GOULDEN, M. L., & BENNETT, G. A. (1976b)
Determination of zearalenone in corn: Collaborative study.
J. Assoc. Off. Anal. Chem., 59: 666-670.
SINNHUBER, R. O. & WALES, J. H. (1974) Aflatoxin B1
hepatocarcinogenicity in rainbow trout embryos. Fed. Proc., 33:
247 (Abstract no. 253).
SINNHUBER, R. O., LEE, D. J., WALES, J. H., & AYRES, J. L. (1968a)
Dietary factors and hepatoma in rainbow trout (Salmo gairdneri).
II. Cocarcinogenesis by cyclopropenoid fatty acids and the effect of
gossypol and altered lipids on aflatoxin-induced liver cancer.
J. Natl Cancer Inst., 41: 1293-1301.
SINNHUBER, R. O., WALES, J. H., AYRES, J. L., ENGEBRECHT, R. H., &
AMEND, D. L. (1968b) Dietary factors and hepatoma in rainbow trout
(Salmo gairdneri) I. Aflatoxins in vegetable protein feedstuffs.
J. Natl Cancer Inst., 41: 711-718.
SINNHUBER, R. O., LEE, D. J., WALES, J. H., LANDERS, M. K., KEYL, A.
C. (1974) Hepatic carcinogenesis of aflatoxin M1 in rainbow trout
(Salmo gairdneri) and its enchancement by cyclopropene fatty
acids. J. Natl Cancer Inst., 53: 1285-1288.
SMALLEY, E. B. & STRONG, F. M. (1974) Toxic trichothecenes. In:
Purchase, I. F. H., ed. Mycotoxins, Amsterdam, Elsevier, pp.
199-228.
SMITH, J. W., PRINCE, W. R., & HAMILTON, P. B. (1969) Relationship
of aflatoxicosis to Salmonella gallinarum infections of chickens.
Appl. Microbiol., 18: 946-947.
SPEERS, G. M., MERONUCK, R. A., BARNES, D. M., & MIROCHA, C. J.
(1971) Effect of feeding Fusarium roseum f. sp. graminearum
contaminated corn and the mycotoxin F-2 on the growing chick and
laying hen. Poult. Sci., 50: 627-633.
STACK, M. E. (1974) Collaborative study of AOAC methods I and III
for the determination of aflatoxins in peanut butter. J. Assoc.
Off. Anal. Chem., 57: 871-874.
STANFORD, G. K., HOOD, R. D., & HAVES, A. W. (1975) Effect of
prenatal administration of T-2 toxin to mice. Res. Commun. chem.
Path. Pharmacol., 10: 743-746.
STEPHENSON, L. W. & RUSSELL, T. E. (1974) The association of
Aspergillus flavus with hemipterous and other insects infesting
cotton bracts and foliage. Phytopathology, 64: 1502-1506.
STERN, P.S., & HOLZAPFEL, C. W. (1967) The synthesis of ochratoxins
A and B, metabolites of Aspergillus ochraceus Wilh. Tetrahedron
Lett., 23: 4449-4461.
STEYN, P.S., VLEGGAAR, R., Du PREEZ, N. P., BLYTH, A. A., & SEEGERS,
J. C. (1975) The in vitro toxicity of analogs of ochratoxin A in
monkey kidney epithelial cells. Toxicol. appl. Pharmacol., 32:
198-203.
STICH, H. F. & LAISHES, B. A. (1975) The response of Xeroderma
pigmentosum cells and controls to the activated mycotoxins,
aflatoxins and sterigmatocystin. Int. J. Cancer, 16: 266-274.
STOLOFF, L. (1972) Analytical methods for mycotoxins. Clin.
Toxicol., 5: 465-494.
STOLOFF, L. (1976) Occurrence of mycotoxins in foods and feeds. In:
Rodricks, J. V., ed. Mycotoxins and other fungal related food
problems, Washington, DC, American Chemical Society, pp. 23-50
(Advances in Chemistry Series 149).
STOLOFF, L. (1977) Aflatoxins-an overview. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 7-28.
STOLOFF, L. & FRIEDMAN, L. (1976) Information bearing on the
evaluation of the hazard to man from aflatoxin ingestion.
PAG Bulletin, 6: 21-32.
STRZELECKI, E. L. & GASIOROWSKA, U. W. (1974) Aflatoxin B1 in
feedstuffs. Zentralbl. Vet.-Med. B, 21: 395-400.
STUBBLEFIELD, R.D., SHOTWELL, O. L., & SHANNON, O. H. (1972)
Aflatoxins M1 and M2 and parasiticol: Thin layer chromatography
and physical and chemical properties. J. Assoc. Off. Anal. Chem.,
55: 762-767.
SUIT, J. L., ROGERS, A. E., JETTEN, M. E. R., & LURIA, S. E. (1977)
Effects of diet on conversion of aflatoxin B1 to bacterial
mutagen(s) by rats in vivo and by rat hepatic microsomes
in vitro. Mutat. Res., 46: 313-323.
SUZANGAR, M., EMAMI, A., & BARNETT, R. (1976) Aflatoxin
contamination of village milk in Isfahan, Iran. Trop. Sci., 18:
155-159.
SUZUKI, S. & SATOH, T. (1973) Effects of ochratoxin A on tissue
glycogen levels in rats. Jpn. J. Pharmacol., 23: 415-419.
SUZUKI, S., SATOH, T., & YAMAZAKI, M. (1975) Effect of ochratoxin A
on carbohydrate metabolism in rat liver. Toxicol. appl.
Pharmacol., 32: 116-122.
SVOBODA, D., GRADY, H. J, & HIGGINSON, J. (1966) Aflatoxin B1
injury in rat and monkey liver. Am. J. Pathol., 49: 1023-1051.
SWENSON, D. H., MILLER, E. C., & MILLER, J. A. (1974) Aflatoxin
B1-2, 3-oxide: Evidence for its formation in rat liver in vivo
and by human liver microsomes in vitro. Biochem. Biophys. res.
Comm., 60: 1036-1043.
SWENSON, D. H., LIN, J.-K, MILLER, E. C., & MILLER, J. A. (1977)
Aflatoxin B1-2, 3-oxide as a probable intermediate in the covalent
binding of aflatoxins B1 and B2 to rat liver DNA and ribosomal
RNA in vivo. Cancer Res., 37: 172-181.
SZCZECH, G. M., CARLTON, W. W., & TUITE, J. (1973a) Ochratoxicosis
in beagle dogs. I. Clinical and clinicopathological features.
Vet. Pathol., 10: 135-154.
SZCZECH, G. M., CARLTON, W. W., TUITE, J., & CALDWELL, R. (1973b)
Ochratoxin A toxicosis in swine. Vet. Pathol., 10: 347-364.
SZCZECH, G. M., CARLTON, W. W, & TUITE, J. (1973c) Ochratoxicosis in
beagle dogs. II. Pathology. Vet. Pathol., 10: 219-231.
TANDON, B. N., KRISHNAMURTHY, L., KOSHY, A., TANDON, H. D.,
RAMALINGASWAMI, V., BHANDARI, J. R., MATHUR, M. M., & MATHUR, P. D.
(1977) Study of an epidemic of jaundice, presumably due to toxic
hepatits, in northwest India. Gastroenterology, 72: 488-494.
TANDON, H. D., TANDON, B. N., & RAMALINGASWAMI, V. (1978) Epidemic
of toxic hepatitis in India of possible mycotoxic origin. Arch.
Pathol. lab. Med., 102. 372-376.
TEMCHAROEN, P., ANUKARAHANONTA, T., & BHAMARAPRAVATI, N. (1978) The
influence of dietary protein and vitamin B12 on the toxicity and
carcinogenicity of aflatoxins in rat liver. Cancer Res.,
38: 2185-2190.
THACKER, H. L. (1976) Citrinin and ochratoxin A mycotoxicosis in
guinea pigs, Lafayette, Indiana, Purdue University (A thesis).
THERON, J. J. (1965) Acute liver injury in ducklings as a result of
aflatoxin poisoning. Lab. Invest., 14: 1586-1603.
THERON, J. J., LIEBENBERG, N., & JOUBERT, H. J. B. (1965)
Histochemistry and electron microscopy of acute liver lesions
induced by aflatoxin B1 in ducklings. Nature (Lond.), 206:
908-909.
TILAK, T. B. (1975) Induction of cholangiocarcinoma following
treatment of a rhesus monkey with aflatoxin. Food Cosmet.
Toxicol., 13: 247-249.
TRIPATHI, R. K. (1973) Aflatoxins in sorghum grains infested with
head moulds. Indian J. exp. Biol., 11: 361-362.
TUNG, H.-T., DONALDSON, W. E., & HAMILTON, P. B. (1970) Effects of
aflatoxin on some marker enzymes of lysosomes. Biochem. Biophys.
Acta, 222. 665-667.
TURNER, W. B. (1971) Fungal metabolites, New York, Academic Press,
446 pp.
ULLOA-SOSA, M. & SCHROEDER, H. W. (1969) Note on aflatoxin
decomposition in the process of making tortillas from corn.
Cereal Chem., 46: 397-400.
VAN NIEUWENHUIZE, J.P., HERBER, F. M., DE BRUIN, A., MEYER, P. B., &
DUBA, W. C. (1973) [Cancers in men following a long-term low-level
exposure to aflatoxins in indoor air.] T. Soc. Geneeskd., 51:
754-760 (in Dutch).
VAN PÉE, W., VAN BRABANT, J., & JOOSTENS, J. (1977) La détection et
le dosage de l'aflatoxine M1 dans le lait et le lait en poudre.
Rev. Agrie. (Bruxelles), 30: 403-415.
VAN RENSBURG, S. J. (1977) Role of epidemiology in the elucidation
of mycotoxin health risks. In: Rodricks, J. V., Hesseltine, C. W., &
Mehlman, M. A., ed. Mycotoxins in human and animal health, Park
Forest South, IL, USA, Pathotox Publ., pp. 699-711.
VAN RENSBURG, S. J., VAN DER WATT, J. J., PURCHASE, I. F. H.,
PEREIRA COUTINHO, L., & MARKHAM, R. (1974) Primary liver cancer rate
and aflatoxin intake in a high cancer area. S. Afr. Med. J., 48:
2508a-2508d.
VANYI, A. & SZAILER, E. (1974) Investigation of the cytotoxic effect
of F-2 toxin (zearalenone) in various monolayer cell cultures.
Acta Vet. Acad. Sci. Hung., 24: 407-412.
VERRETT, M. J., MARLIAC, J.-P., & MCLAUGHLIN, J., JR (1964) Use of
the chicken embryo in the assay of aflatoxin toxicity.
J. Assoc. Off. Anal. Chem., 47: 1003-1006.
VESELY, D., VESELA, D., KUSAK, V., & NESNIDAL, P. (1978)
[Distribution of perorally administered aflatoxin B1 in tissues
and organs of the goat.] Veterinarni medicina, 23 (LI): 555-558 (in
Czech).
VESONDER, R. F., CIEGLER, A., & JENSEN, A. H. (1973) Isolation of
the emetic principle from Fusarium-infected corn.
Appl. Microbiol., 26: 1008-1010.
VESSELINOVITCH, S. D., MIHAILOVICH, N., WOGAN, G. N., LOMBARD, L.
S., & RAO, K. V. N. (1972) Aflatoxin B1, a hepatocarcinogen in the
infant mouse. Cancer Res., 32: 2289-2291.
VILLA-TREVINO, S. & LEAVER, D. D. (1968) Effects of the hepatotoxic
agents retrorsine and aflatoxin B1 on hepatic protein synthesis in
the rat. Biochem. J., 109: 87-91.
VOGEL, C. L., ANTHONY, P. P., MODY, N., & BARKER, L. F. (1970)
Hepatitis-associated antigen in Ugandan patients with hepatocellular
carcinoma. Lancet, 2: 621-624.
WALES, J. H. & SINNHUBER, R. O. (1972) Hepatomas induced by
aflatoxin in the sockeye salmon (Oncorhynchus nerka). J. Natl
Cancer Inst., 48: 1529-1530.
WALTKING, A. E. (1970) Collaborative study of three methods for
determination of aflatoxin in peanuts and peanut products.
J. Assoc. Off. Anal. Chem., 53: 104-113.
WALTKING, A. E. (1971) Fate of aflatoxin during roasting and storage
of contaminated peanut products. J. Assoc. Off. Anal. Chem., 54:
533-539.
WALTKING, A. E., BLEFFERT, G., & KIERNAN, M. (1968) An improved
rapid physio-chemical assay method for aflatoxin in peanuts and
peanut products. J. Am. Oil Chem. Soc., 45: 880-884.
WARD, J. M., SONTAG, J. M., WEISBURGER, E. K., & BROWN, C. A. (1975)
Effect of lifetime exposure to aflatoxin B1 in rats.
J. Natl Cancer Inst., 55: 107-113.
WARE, G. M. & THORPE, C. W. (1977) Analysis for zearalenone in corn
by high pressure liquid chromatography with a fluorescence detector.
Abstracts 91st Annual Meeting, Association of Official Analytical
Chemists, Washington, DC, p. 47 (Abstract no. 148).
WARE, G. M. & THORPE, C. W. (1978) Determination of zearalenone in
corn by high-pressure liquid chromatography and fluorescence
detection. J. Assoc. Off. Anal, Chem., 61 (5): 1058-1062.
WHITAKER, T. B. (1977)Sampling granular foodstuffs for aflatoxin.
Pure appl. Chem., 49: 1709-1717.
WHITAKER, T. B. & WHITTEN, M. E. (1977) Evaluation of cottonseed
aflatoxin testing programs. J. Am. Oil Chem. Soc., 54: 436-441.
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (1974a) Variability
of aflatoxin test results. J. Am. Oil Chem. Soc., 51: 214-218.
WHITAKER, T. B., DICKENS, J. W., WISER, E. H., & MONROE, R. J.
(1974b) Development of a method to evaluate sampling plans used to
estimate aflatoxin concentrations in lots of shelled peanuts.
I.U.P.A.C. Information Bulletin, Oxford, International Union of Pure
& Applied Chemistry Secretariat, pp. 1-14 (Technical Report-No. 10).
WHITAKER, T. B., DICKENS, J. W., & MONROE, R. J. (in press)
Variability associated with testing corn for aflatoxin. J. Am. Oil
Chem. Soc.
WHITTEN, M. E. (1969) Aflatoxins in cottonseed hulls. J. Am. Oil
Chem. Soc., 47: 5-6.
WHO (1978) Environmental Health Criteria 6-- Principles and methods
for evaluating the toxicity of chemicals. Part I, Geneva, World
Health Organization, 272 pp.
WILLIAMS, D. J. & RABIN, B. R. (1971) Disruption by carcinogens of
the hormone dependent association of membranes with polysomes.
Nature (Lond.), 232: 102-105.
WOGAN, G. N. (1966) Chemical nature and biological effects of the
aflatoxins. Bacteriol. Rev., 30: 460-470.
WOGAN, G. N. (1969) Metabolism and biochemical effects of
aflatoxins. In: Goldblatt, L. A., ed. Aflatoxin: Scientific
background, control and implications, New York, Academic Press,
pp. 151-186.
WOGAN, G. N. (1973)Aflatoxin carcinogenesis. In: Bush, M., ed.
Methods in cancer research, New York, Academic Press, pp. 309-344.
WOGAN, G. N. (1977) Mycotoxins and other naturally occurring
carcinogens. In: Kraybill, H. F. & Mehlman, M. A., ed.
Environmental cancer, Advances in modern toxicology, Washington,
DC, Hemisphere Publishing Corporation, Vol. 3, pp. 263-290.
WOGAN, G. N. & FRIEDMAN, M. A. (1968) Inhibition by aflatoxin B1
of hydrocortisone induction of rat liver tryptophan pyrrolase and
tyrosine transaminase. Arch. Biochem. Biophys., 128: 509-516.
WOGAN, G. N. & NEWBERNE, P.M. (1967) Dose-response characteristics
of aflatoxin B1 carcinogenesis in the rat. Cancer Res., 27:
2370-2376.
WOGAN, G. N. & PAGLIALUNGA, S. (1974) Carcinogenicity of synthetic
aflatoxin M1 in rats. Food Cosmet. Toxicol., 12: 381-384.
WOGAN, G. N., EDWARDS, G. S., & SHANK, R. C. (1967) Excretion and
tissue distribution of radioactivity from aflatoxin B1-14C in
rats. Cancer Res., 27: 1729-1736.
WOGAN, G. N., EDWARDS, G. S., & NEWBERNE, P.M. (1971)
Structure-activity relationships in toxicity and carcinogenicity of
aflatoxins and analogs. Cancer Res., 31: 1936-1942.
WOGAN, G. N., PAGLIALUNGA, S., & NEWBERNE, P.M. (1974) Carcinogenic
effects of low dietary levels of aflatoxin B1 in rats. Food
Cosmet. Toxicol., 12: 681-685.
WONG, J. J. & HSIEH, D. P. H. (1976) Mutagenicity of aflatoxins
related to their metabolism and carcinogenic potential. Proc. Natl
Acad. Sci. (USA), 73: 2241-2244.
WYATT, R. D., DOERR, J. A., HAMILTON, P. B., & BURMEISTER, H. R.
(1975) Egg production, shell thickness and other physiological
parameters of laying hens affected by T-2 toxin. Appl. Microbiol.,
29: 641-645.
WYLLIE, W. D. & MOREHOUSE, L. D., ed. (1977) Mycotoxic fungi,
mycotoxins, mycotoxicosis: An encyclopaedic handbook. New York and
Basel, Marcel Dekker.
YADGIRI, B., REDDY, V., TULPULE, P. G., SRIKANTIA, S. G., & GOPALAN,
C. (1970) Aflatoxin and Indian childhood cirrhosis; Am. J. clin.
Nutr., 23: 94-98.
YAGEN, B., JOFFE, A. Z., HORN, P., MOR, N., & LUTSKY, I. I. (1977)
Toxins from a strain involved in ATA. In: Rodricks, J. V.,
Hesseltine, C. W., & Mehlman, M. A., ed. Mycotoxins in human and
animal health, Park Forest South, IL, USA, Pathotox Publishers
Inc., pp. 329-336.
YNDESTAD, M. & UNDERDAL, B. (1975) [Aflatoxin in foods on the
Norwegian market.] Nord. Vet.-Med., 27: 42-48 (in Norwegian).
